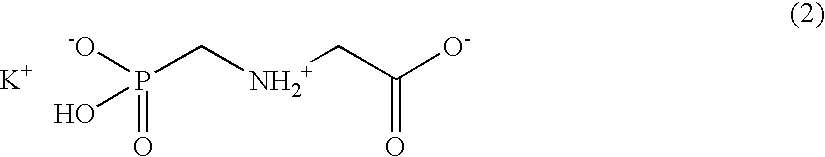
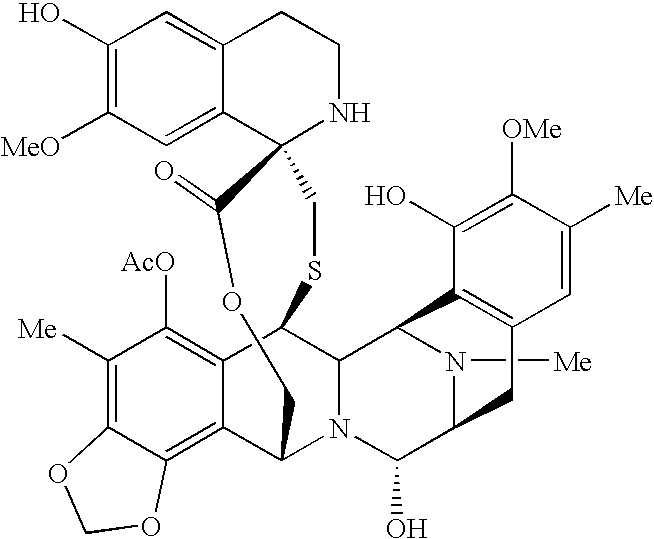
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1040results about How to "Increased toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Insect-resistant transgenic plants
The invention provides transgenic plants and transformed host cells which express modified cry 3B genes with enhanced toxicity to Coleopteran insects. Also disclosed are methods of making and using these transgenic plants, methods of making recombinant host cells expressing these delta -endotoxins, and methods of killing insects such as Colorado potato beetle (Leptinotarsa decemlineata), southern corn rootworm (Diabrotica undecimpunctata howardi Barber) and western corn rootworm (Diabrotica virgifera virgifera LeConte.
Owner:MONSANTO CO (MONSANTO CY)
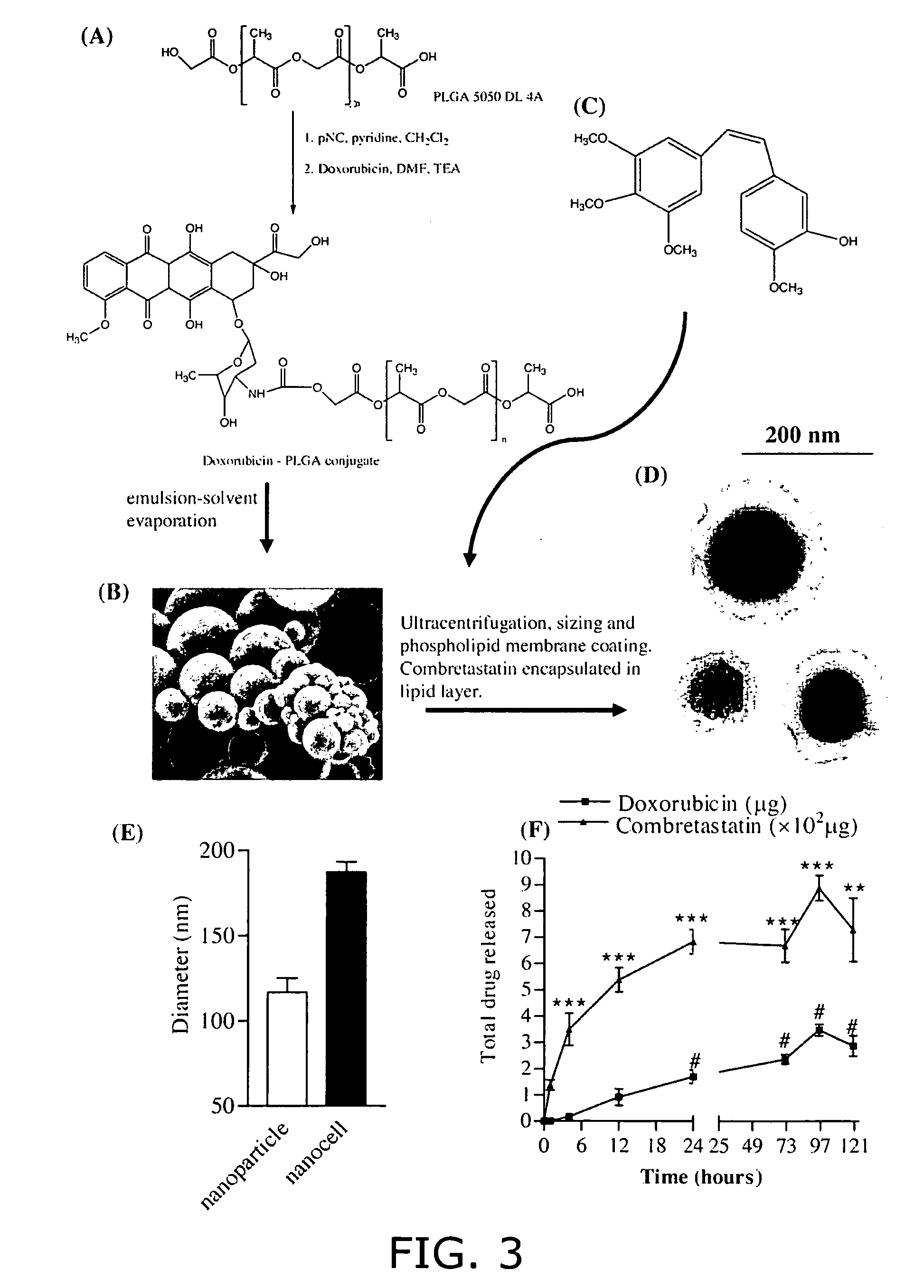
Nanocell drug delivery system
InactiveUS20050266067A1Avoid flowIncreased toxicityAntibacterial agentsOrganic active ingredientsLipid formationAntigen
Nanocells allow the sequential delivery of two different therapeutic agents with different modes of action or different pharmacokinetics. A nanocell is formed by encapsulating a nanocore with a first agent inside a lipid vesicle containing a second agent. The agent in the outer lipid compartment is released first and may exert its effect before the agent in the nanocore is released. The nanocell delivery system may be formulated in pharmaceutical composition for delivery to patients suffering from diseases such as cancer, inflammatory diseases such as asthma, autoimmune diseases such as rheumatoid arthritis, infectious diseases, and neurological diseases such as epilepsy. In treating cancer, a traditional antineoplastic agent is contained in the outer lipid vesicle of the nanocell, and an antiangiogenic agent is loaded into the nanocore. This arrangement allows the antineoplastic agent to be released first and delivered to the tumor before the tumor's blood supply is cut off by the antianiogenic agent.
Owner:MASSACHUSETTS INST OF TECH
Nanocell drug delivery system
InactiveUS20070053845A1Lose efficacyIncreased toxicityPowder deliveryPeptide/protein ingredientsNervous systemAsthma
Nanocells allow the sequential delivery of two different therapeutic agents with different modes of action or different pharmacokinetics. A nanocell is formed by encapsulating a nanocore with a first agent inside a lipid vesicle containing a second agent. The agent in the outer lipid compartment is released first and may exert its effect before the agent in the nanocore is released. The nanocell delivery system may be formulated in pharmaceutical composition for delivery to patients suffering from diseases such as cancer, inflammatory diseases such as asthma, autoimmune diseases such as rheumatoid arthritis, infectious diseases, and neurological diseases such as epilepsy. In treating cancer, a traditional antineoplastic agent is contained in the outer lipid vesicle of the nanocell, and an antiangiogenic agent is loaded into the nanocore. This arrangement allows the antineoplastic agent to be released first and delivered to the tumor before the tumor's blood supply is cut off by the antianiogenic agent.
Owner:MASSACHUSETTS INST OF TECH
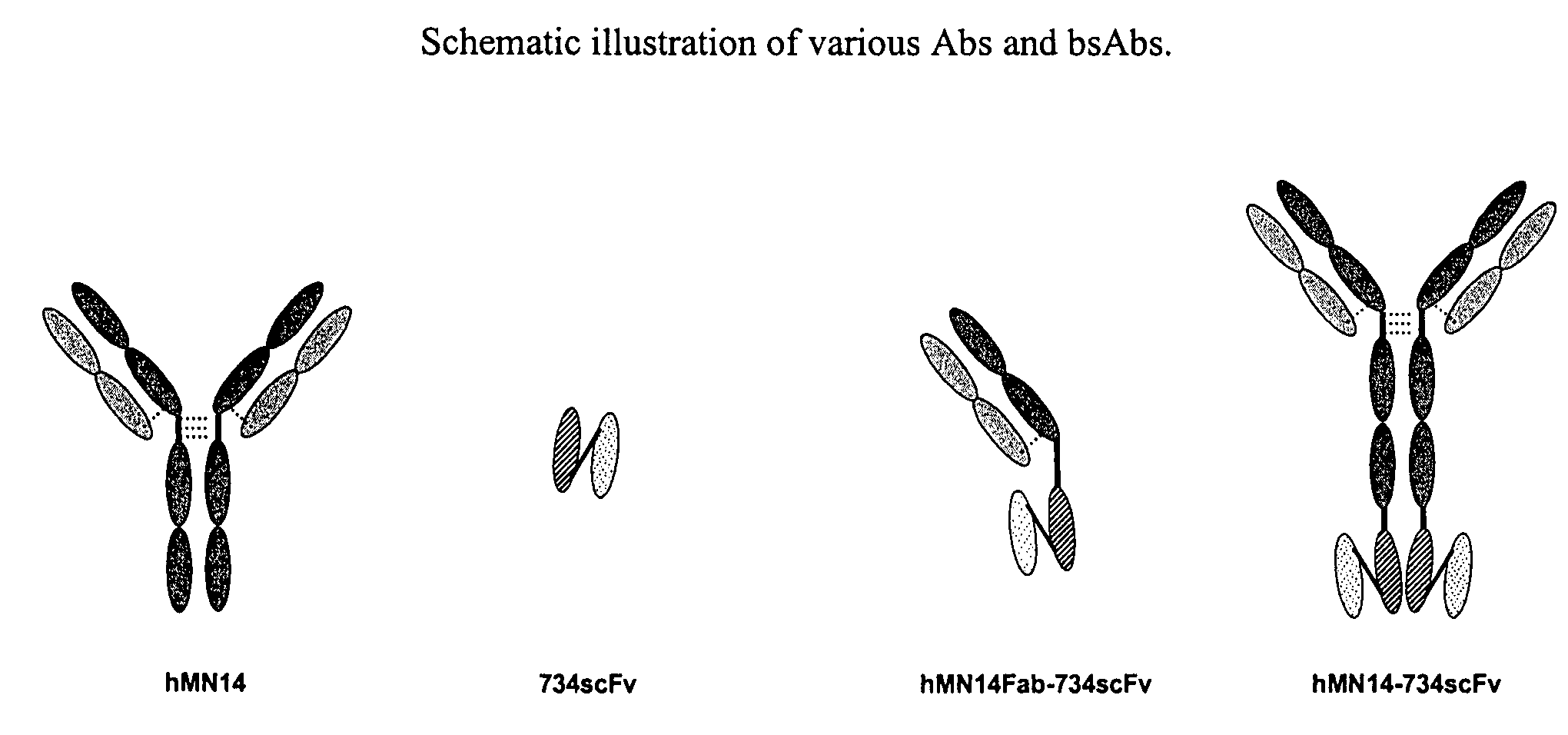
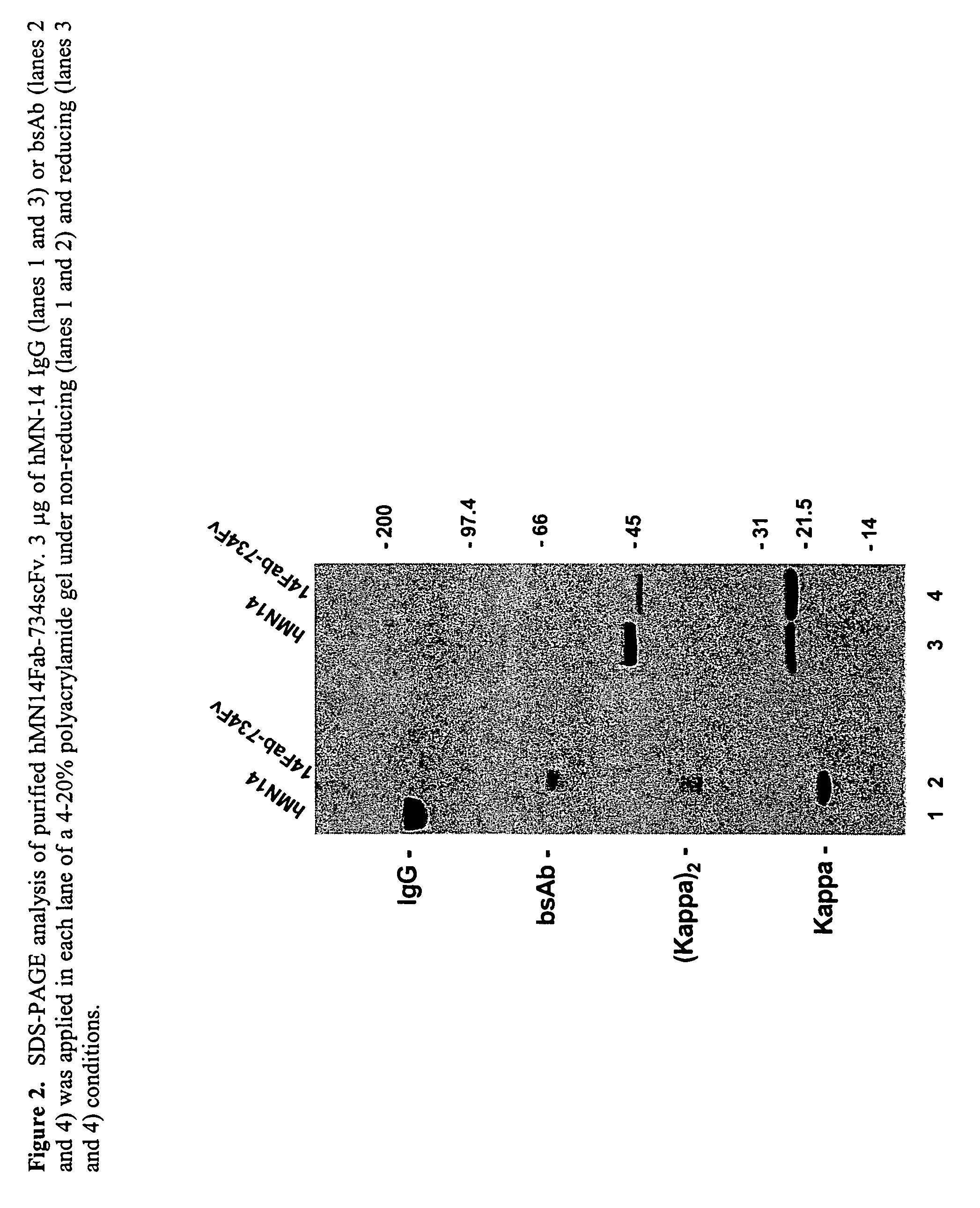
Use of bi-specific antibodies for pre-targeting diagnosis and therapy
InactiveUS7074405B1Increased toxicityLow toxicityHybrid immunoglobulinsInorganic boron active ingredientsEpitopeDiagnostic agent
The present invention relates to a bi-specific antibody or antibody fragment having at least one arm that specifically binds a targeted tissue and at least one other arm that specifically binds a targetable conjugate. The targetable conjugate comprises a carrier portion which comprises or bears at least one epitope recognizable by at least one arm of said bi-specific antibody or antibody fragment. The targetable conjugate further comprises one or more therapeutic or diagnostic agents or enzymes. The invention provides constructs and methods for producing the bi-specific antibodies or antibody fragments, as well as methods for using them.
Owner:IMMUNOMEDICS INC
Bi-specific antibodies for pre-targeting diagnosis and therapy
InactiveUS7052872B1Increased toxicityLow toxicityIn-vivo radioactive preparationsSugar derivativesEpitopeDiagnostic agent
The present invention relates to a bi-specific antibody or antibody fragment having at least one arm that specifically binds a targeted tissue and at least one other arm that specifically binds a targetable conjugate. The targetable conjugate comprises a carrier portion which comprises or bears at least one epitope recognizable by at least one arm of said bi-specific antibody or antibody fragment. The targetable conjugate further comprises one or more therapeutic or diagnostic agents or enzymes. The invention provides constructs and methods for producing the bi-specific antibodies or antibody fragments, as well as methods for using them.
Owner:IMMUNOMEDICS INC
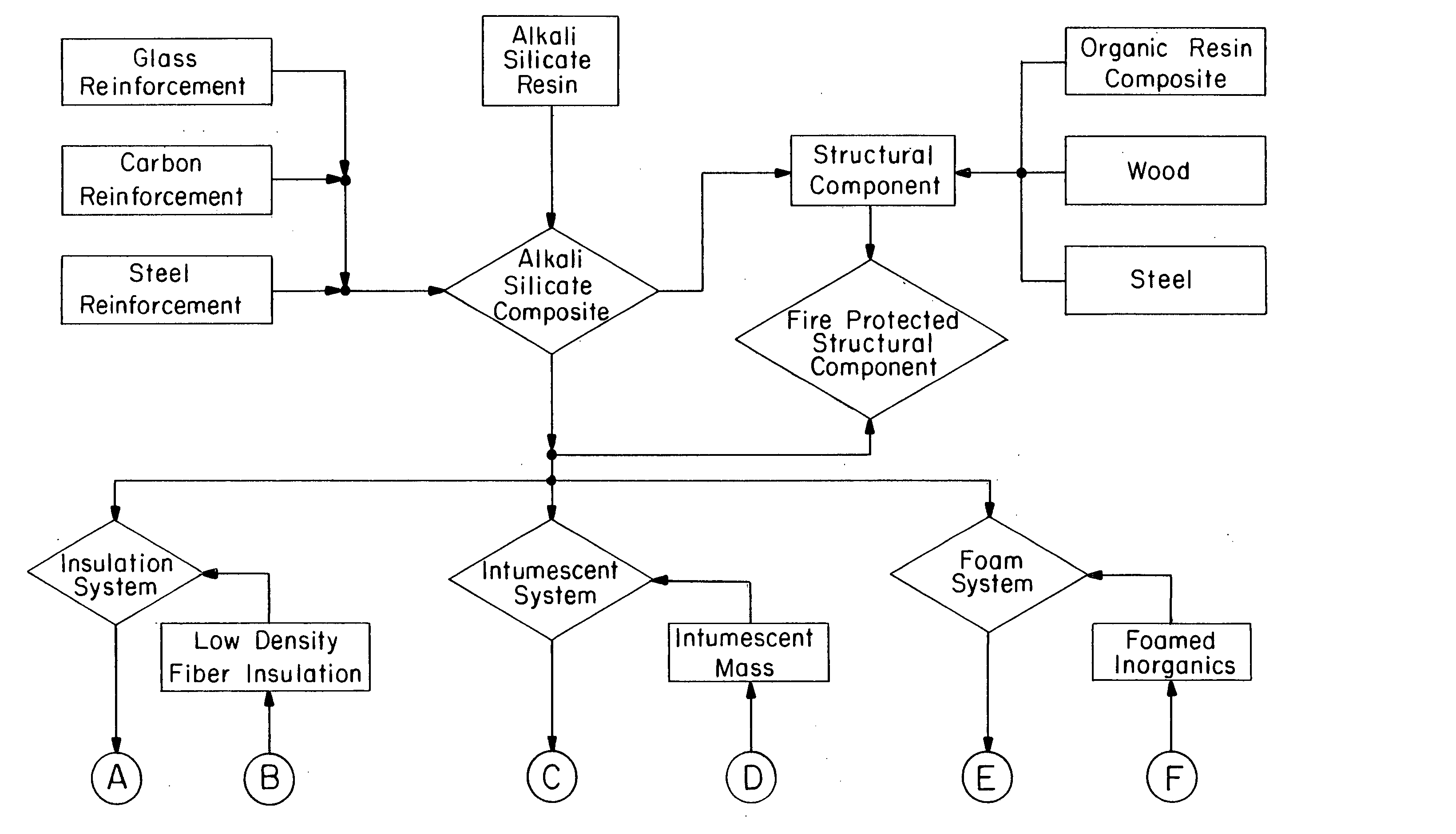
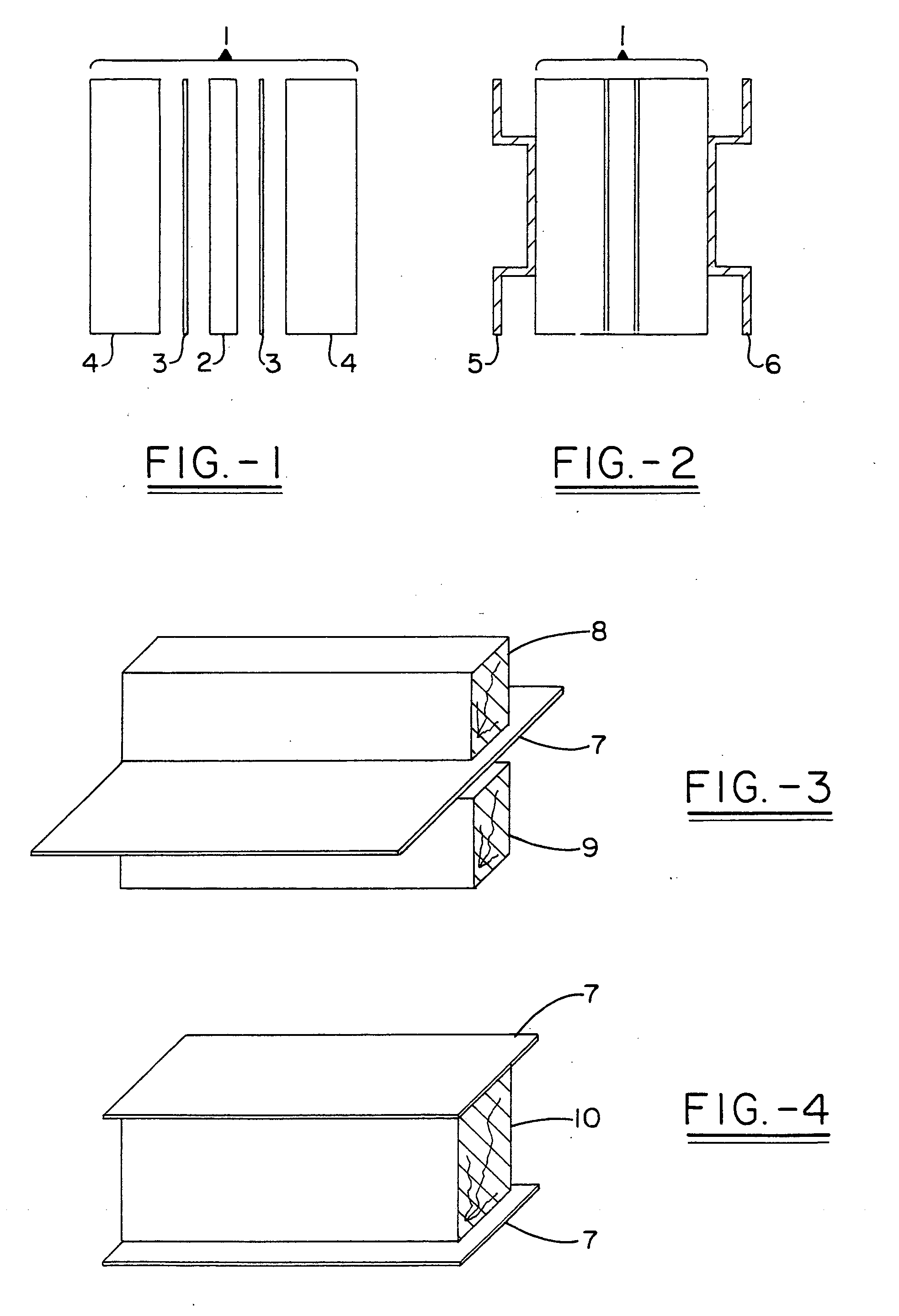
Multi-layer fire barrier systems
InactiveUS20050031843A1Good thermal and electrical insulating characteristicMaintain good propertiesWood working apparatusCement productionInsulation layerCombined use
A fire-barrier system comprises at least one alkali silicate resin composition layer and at least one layer of any of the following: an insulation layer, an intumescent layer, a foam layer, a corrugated layer, a reflective surface layer, and a reinforcing material layer. The fire-barrier system when utilize in association with a substrate such as wood, a polymer, etc. provides enhanced fire resistance performance, thermal barrier, an oxidation barrier, and the like.
Owner:THE BF GOODRICH CO
Essential oil compositions for killing or repelling ectoparasites and pests and methods for use thereof
InactiveUS20080193387A1Pleasant scentWithout burdensome safety precautionBiocideCosmetic preparationsTickFlea
Essential-oil compositions comprising Lippia javanica essential oil in combination with one, two, three, four, five, six or more essential oils are provided. The Lippia javanica essential-oil compositions are effective for killing and / or repelling ectoparasites and / or pests, including lice, ticks, mosquitoes, mites, ants and fleas. Methods of using the compositions comprising Lippia javanica essential oil in combination with one, two, three, four, five, six or more essential oils for killing or repelling ectoparasites and / or pests also are provided. Also provided are articles of manufacture and kits that include the Lippia javanica essential-oil compositions.
Owner:RICKI DE WOLFFE
Agents for controlling biological fluids and methods of use thereof
InactiveUS20070059350A1Good hemostasisHigh viscosityOrganic active ingredientsBiocideMedicineIncision Site
Therapeutic formulations adapted for positive-pressure application for controlling biological fluid at a desired site in a subject, absorbent articles comprising therapeutic formulations, and anti-infective devices coated with therapeutic formulations, said formulations comprising about 25% to about 99% by weight liquid-crystal forming compound and 0% to about 75% by weight solvent. In addition, methods of using said formulations including methods for controlling biological fluid at a desired site in a subject, methods for controlling blood loss, and methods for facilitating effective closure of a vascular wound or incision site at a desired site in a subject are disclosed, the methods comprising administering particular formulations comprising liquid-crystal forming compounds and solvents that are described herein.
Owner:SOUTHEASTERN MEDICAL TECH
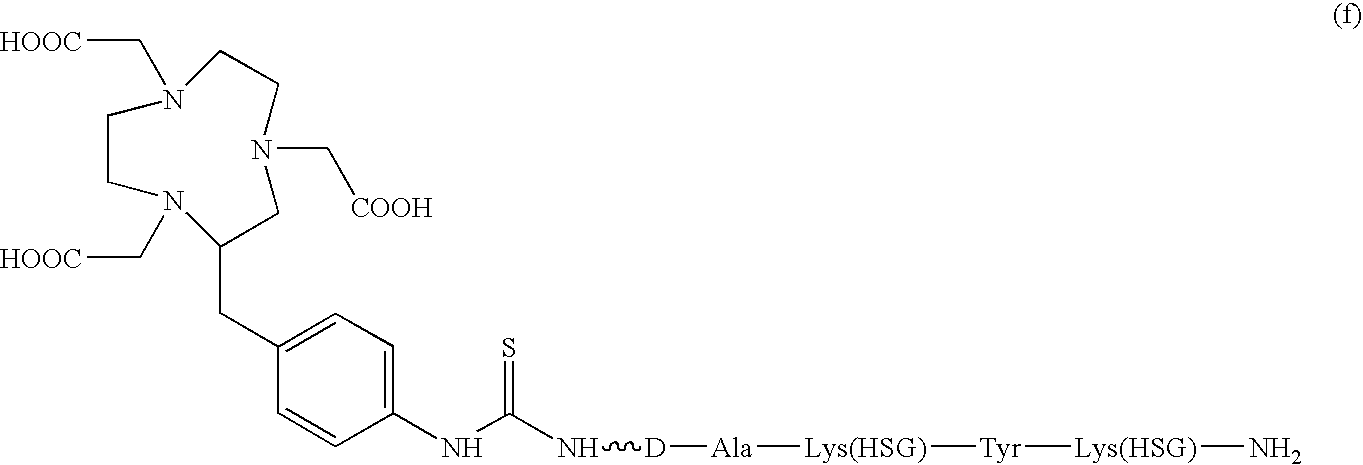
D-amino acid peptides
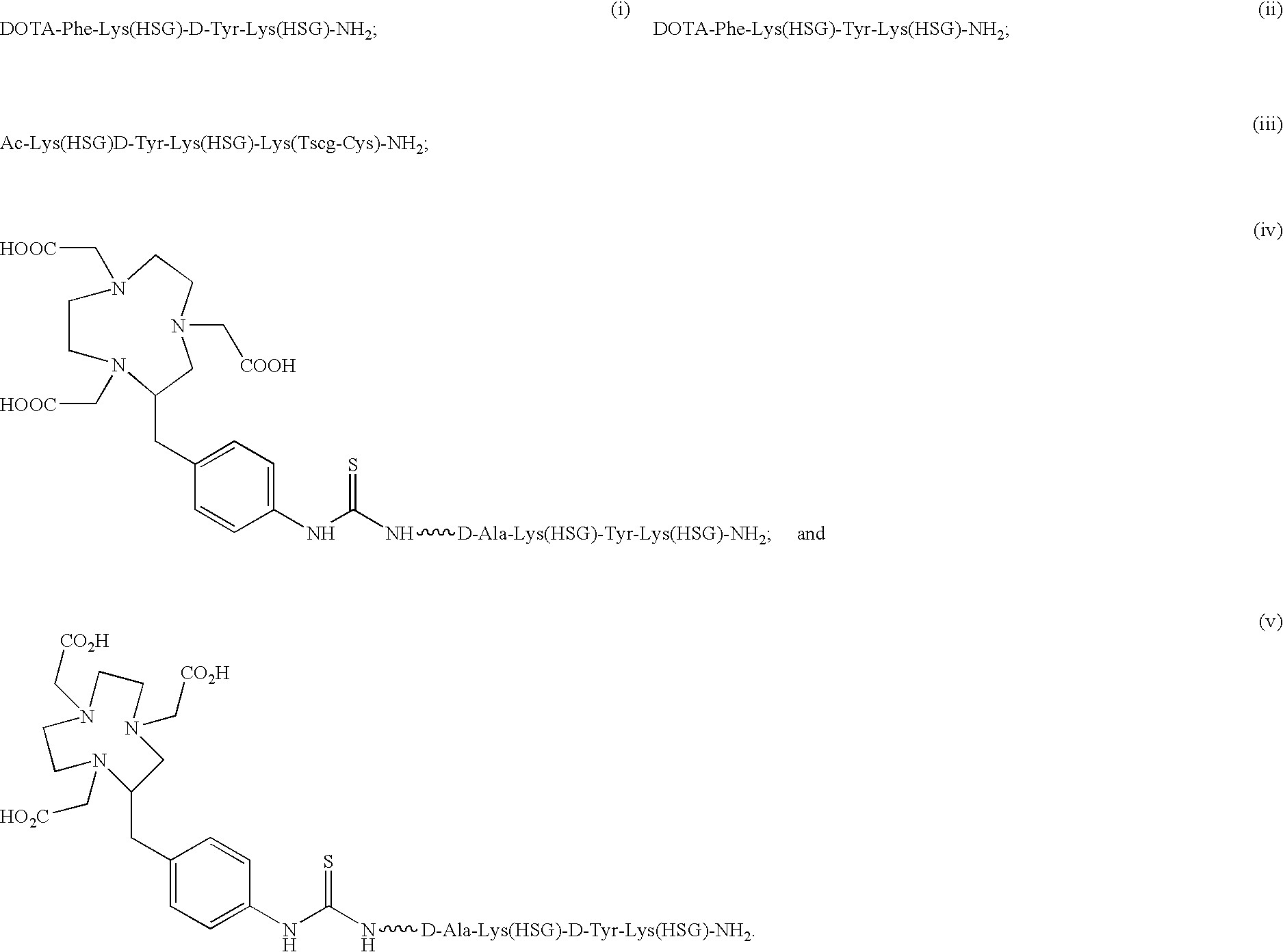
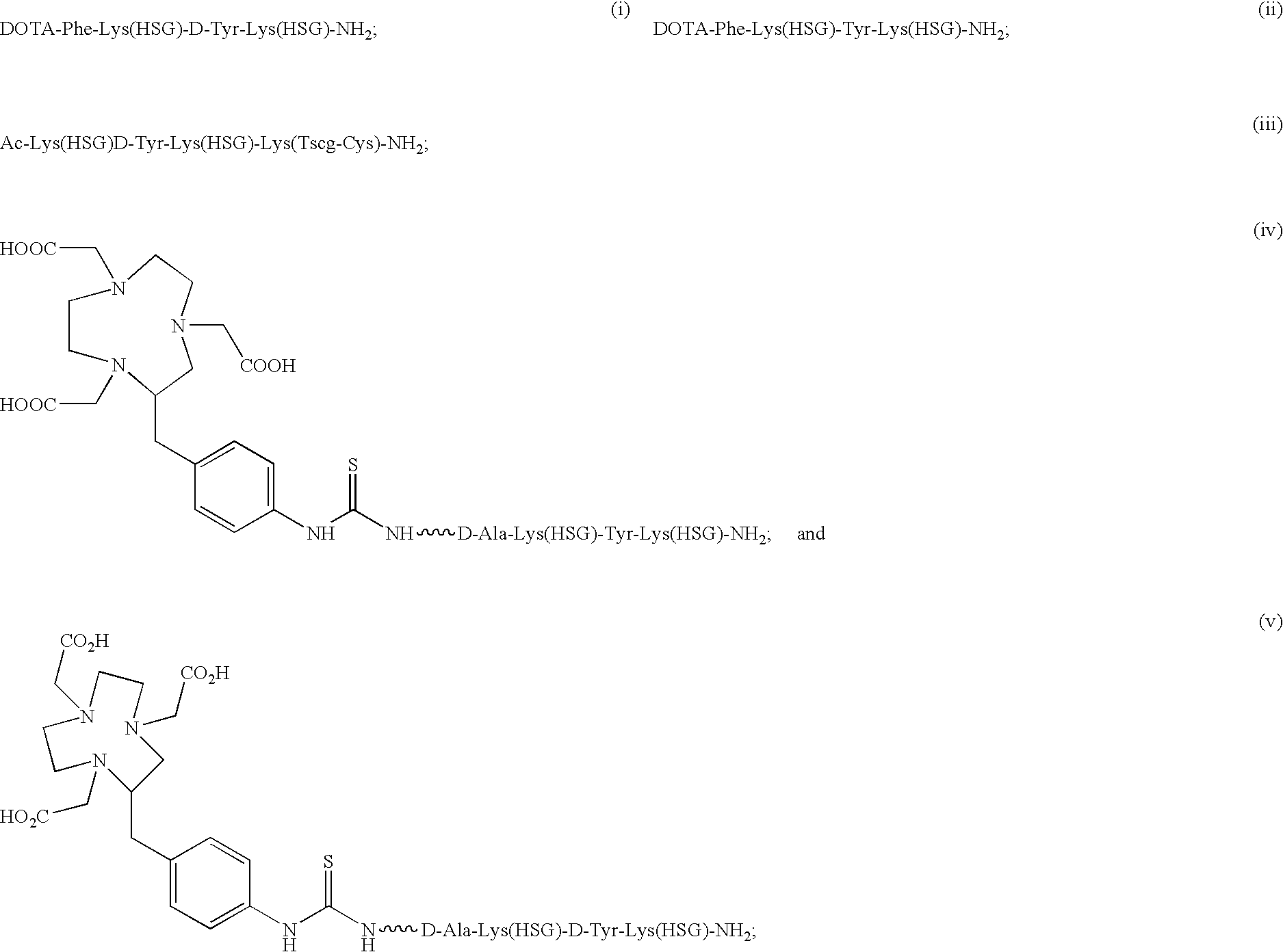
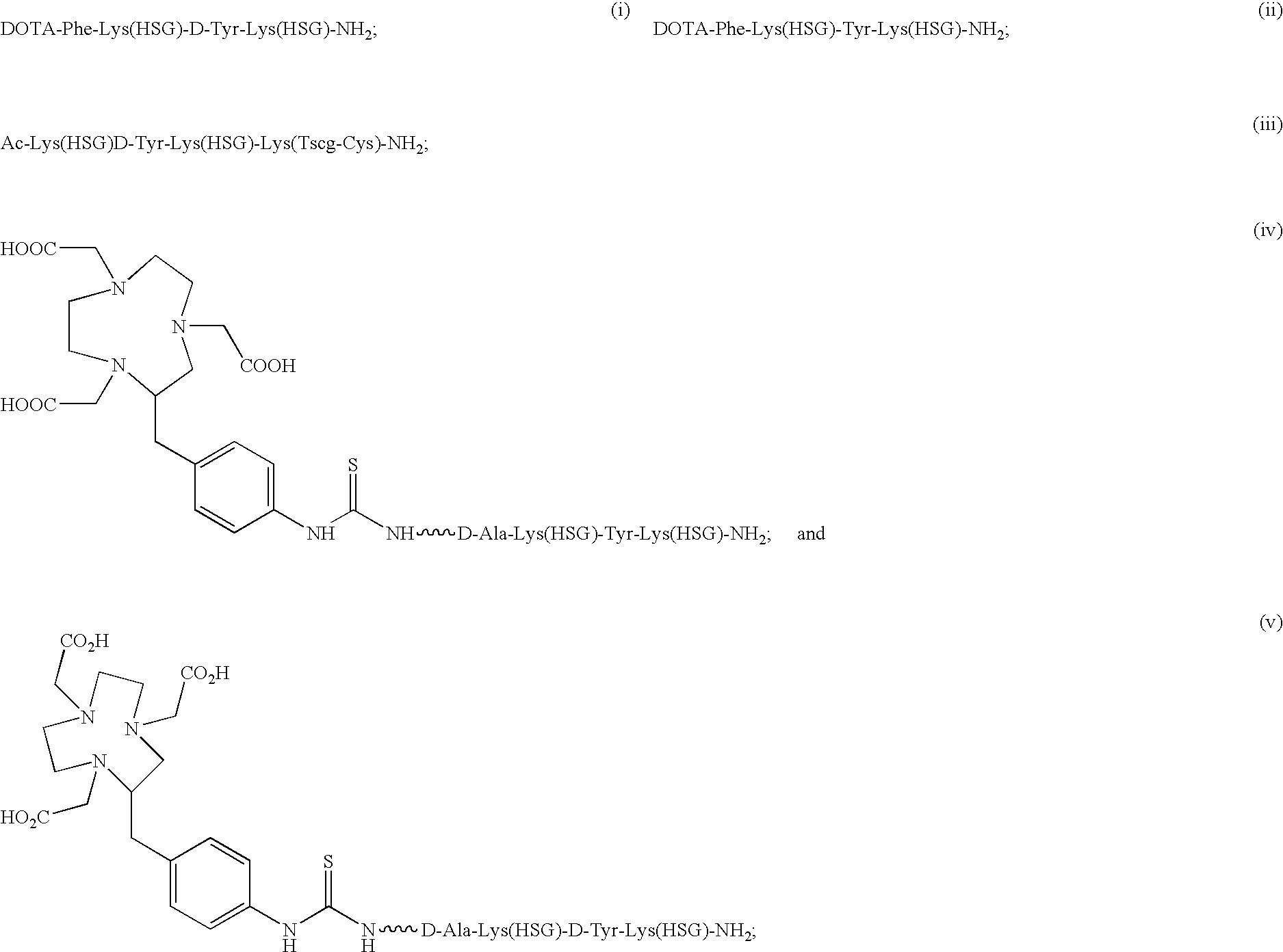
The present invention provides compounds of the formula X—R1-D-[Dpr, Orn or Lys](A)-R2(Z)-D-[Dpr, Orn or Lys](B)—R3(Y)—NR4R5; or R1(X)-D-[Dpr, Orn or Lys](A)-R2(Z)-D-[Dpr, Orn or Lys](B)—R3(Y)—NR4R5, in which X is a hard acid cation chelator, a soft acid cation chelator or Ac—, R1, R2 and R3 are independently selected from a covalent bond or one or more D-amino acids that can be the same or different, Y is a hard acid cation chelator, a soft acid cation chelator or absent, Z is a hard acid cation chelator, a soft acid cation chelator or absent, and A and B are haptens or hard acid cation chelators and can be the same or different, and R4 and R5 are independently selected from the group consisting of hard acid cation chelators, soft acid cation chelators, enzymes, therapeutic agents, diagnostic agents and H. The present invention also provides methods of using these compounds and kits containing the compounds.
Owner:IMMUNOMEDICS INC
Stabilized biodegradable neurotoxin implants
InactiveUS20050232966A1Patient compliance is goodReduce complicationsAntibacterial agentsBacterial antigen ingredientsOligomerTherapeutic effect
Biodegradable neurotoxin implants and methods of making and using such implants are provided. Biodegradable neurotoxin implants include a neurotoxin, a biodegradable polymer component, and an acidity regulating component. The biodegradable polymer component is effective in controlling the release of the neurotoxin from the implant when the implant is located in a patient's body. The acidity regulating component is effective in maintaining a pH of the implant in a desired range that may be effective in stabilizing the neurotoxin as the implant biodegrades when the implant is located in a patient's body. In one embodiment, an implant includes a botulinum toxin, a biodegradable polymer, and either monomers from which a biodegradable polymer is derived or oligomers including monomeric units substantially identical to a monomer from which a biodegradable polymer is derived, or a combination of such monomers and oligomers. The oligomers and biodegradable polymer may be derived from a single type of monomer. The implants disclosed herein may be administered to a human or animal patient in which a therapeutic effect is desired for prolonged periods of time.
Owner:ALLERGAN INC
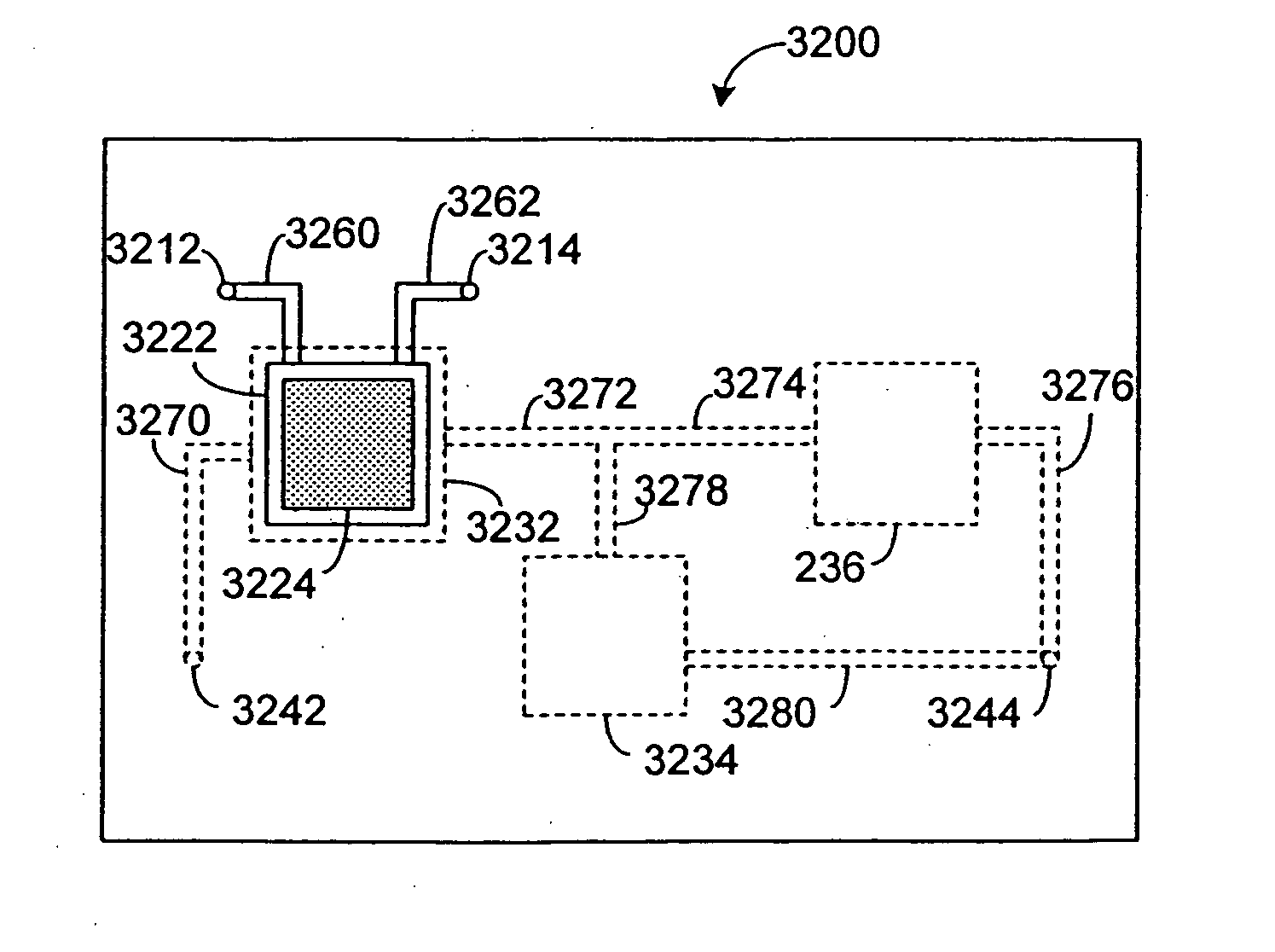
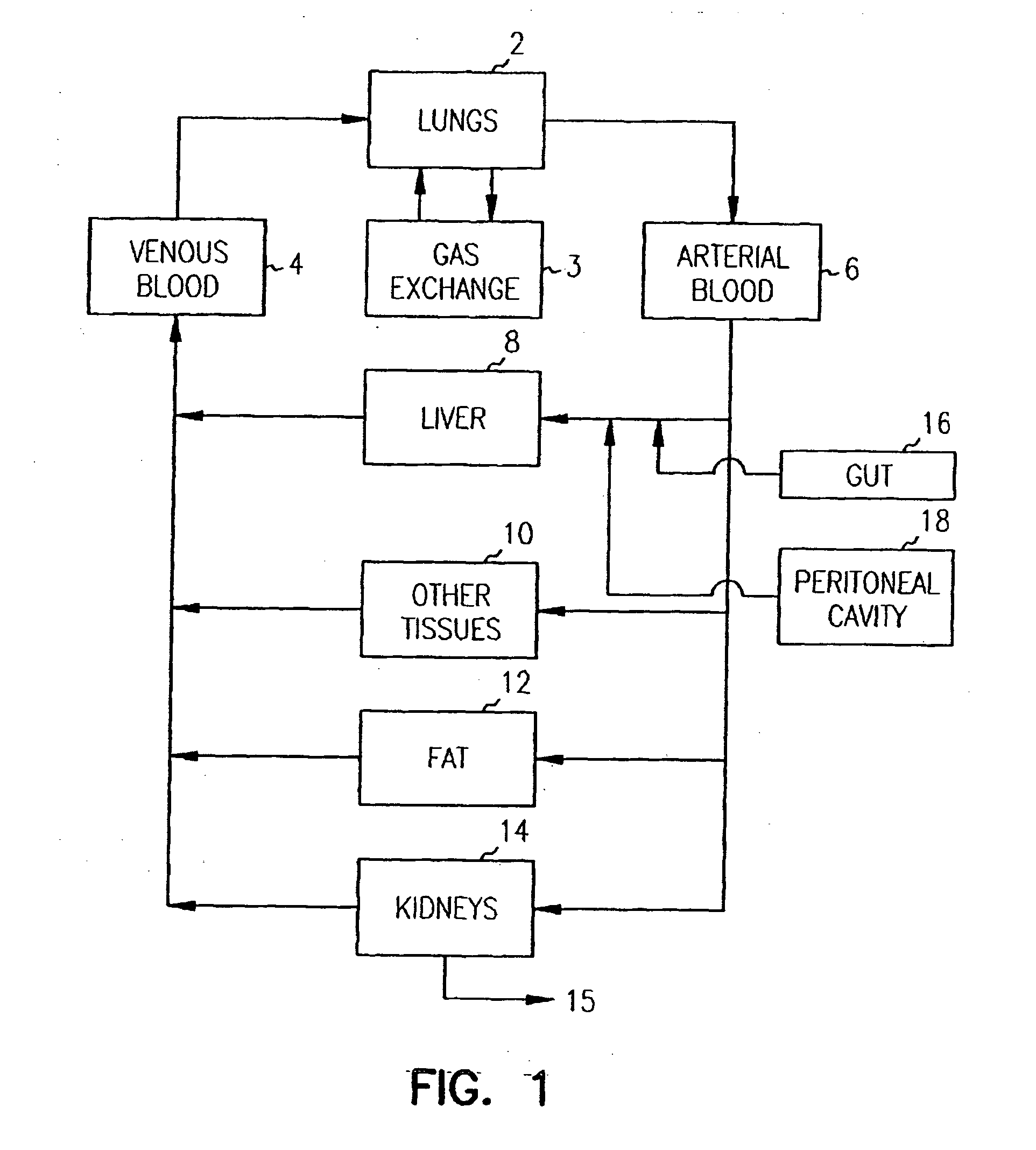
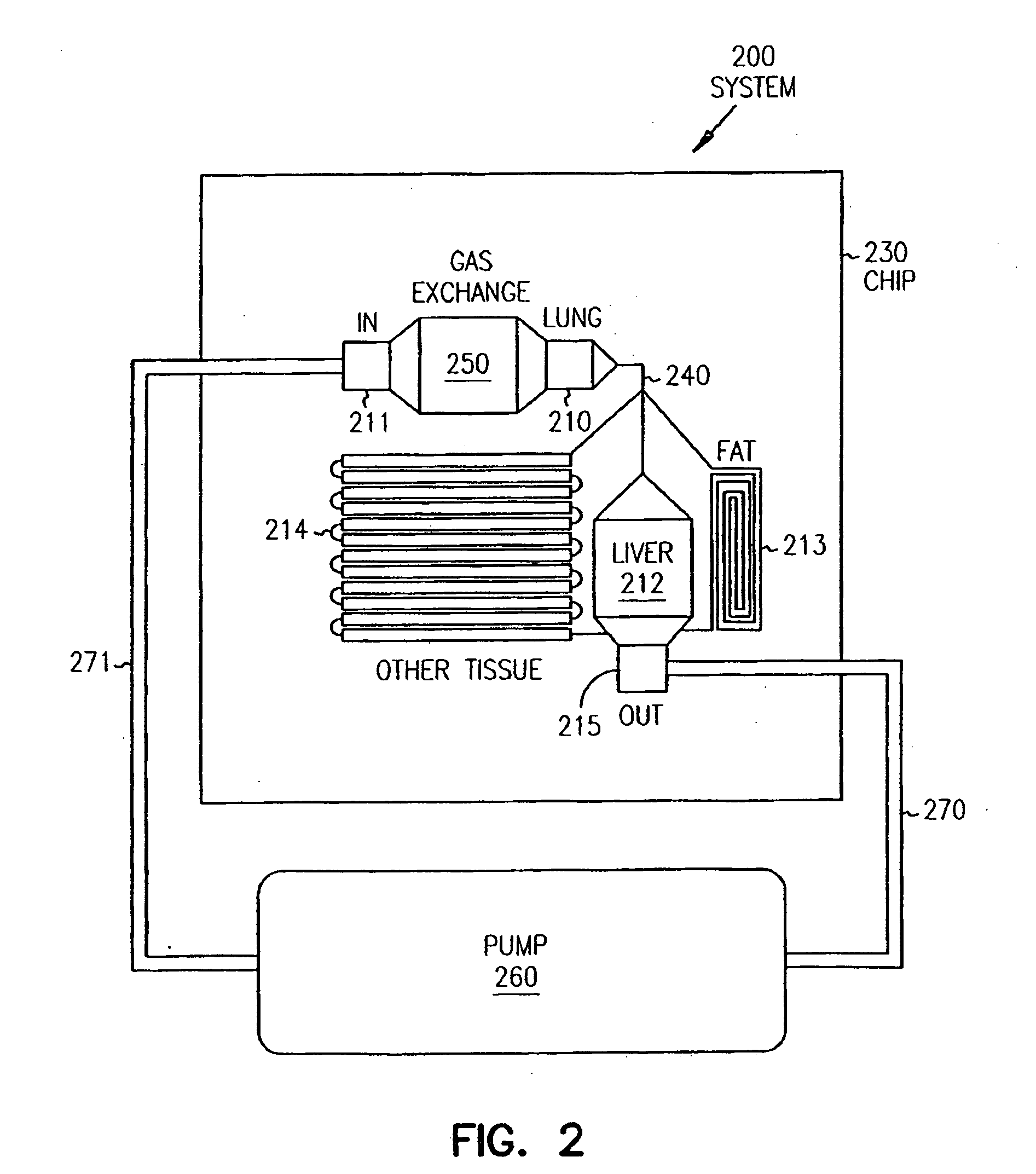
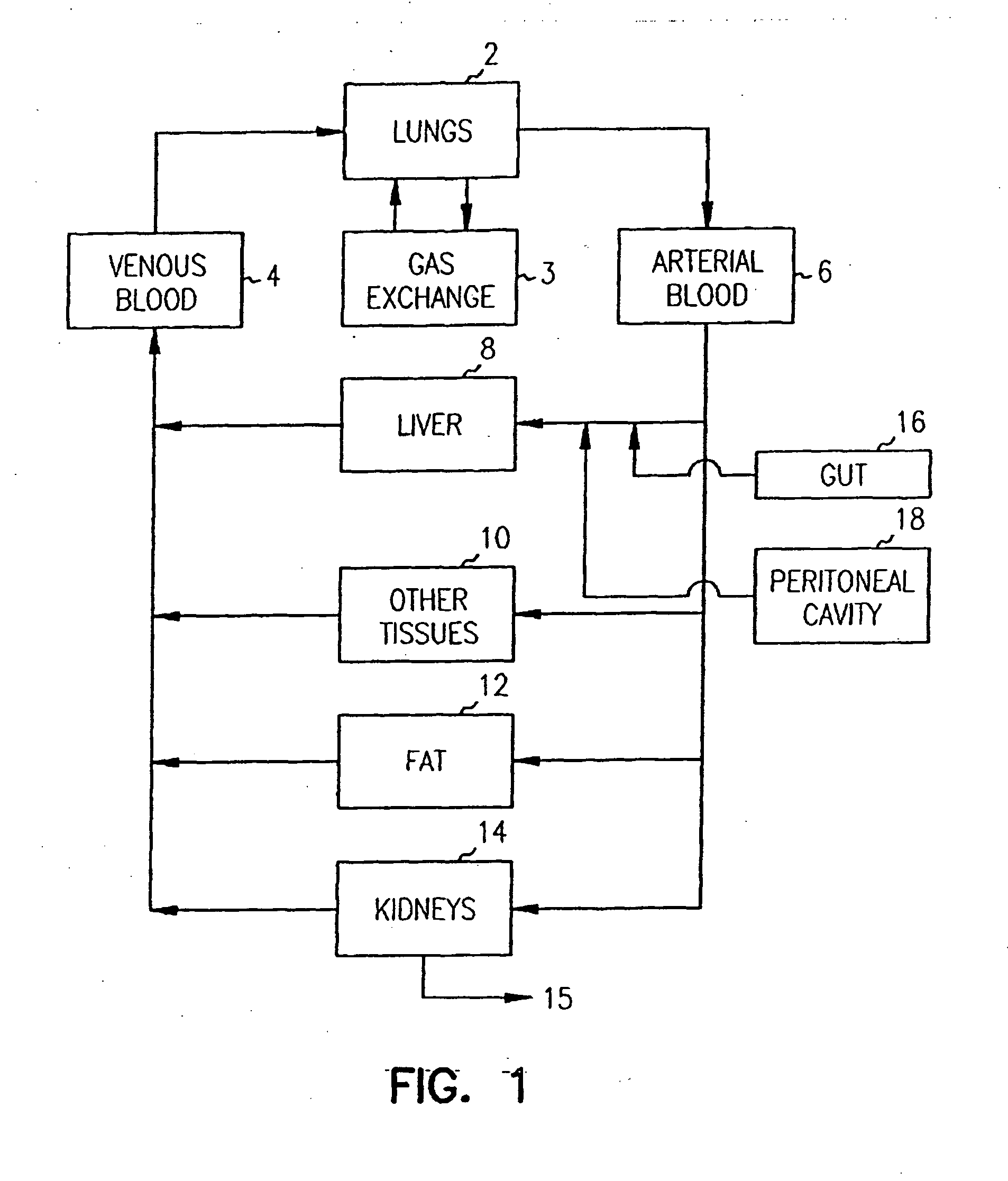
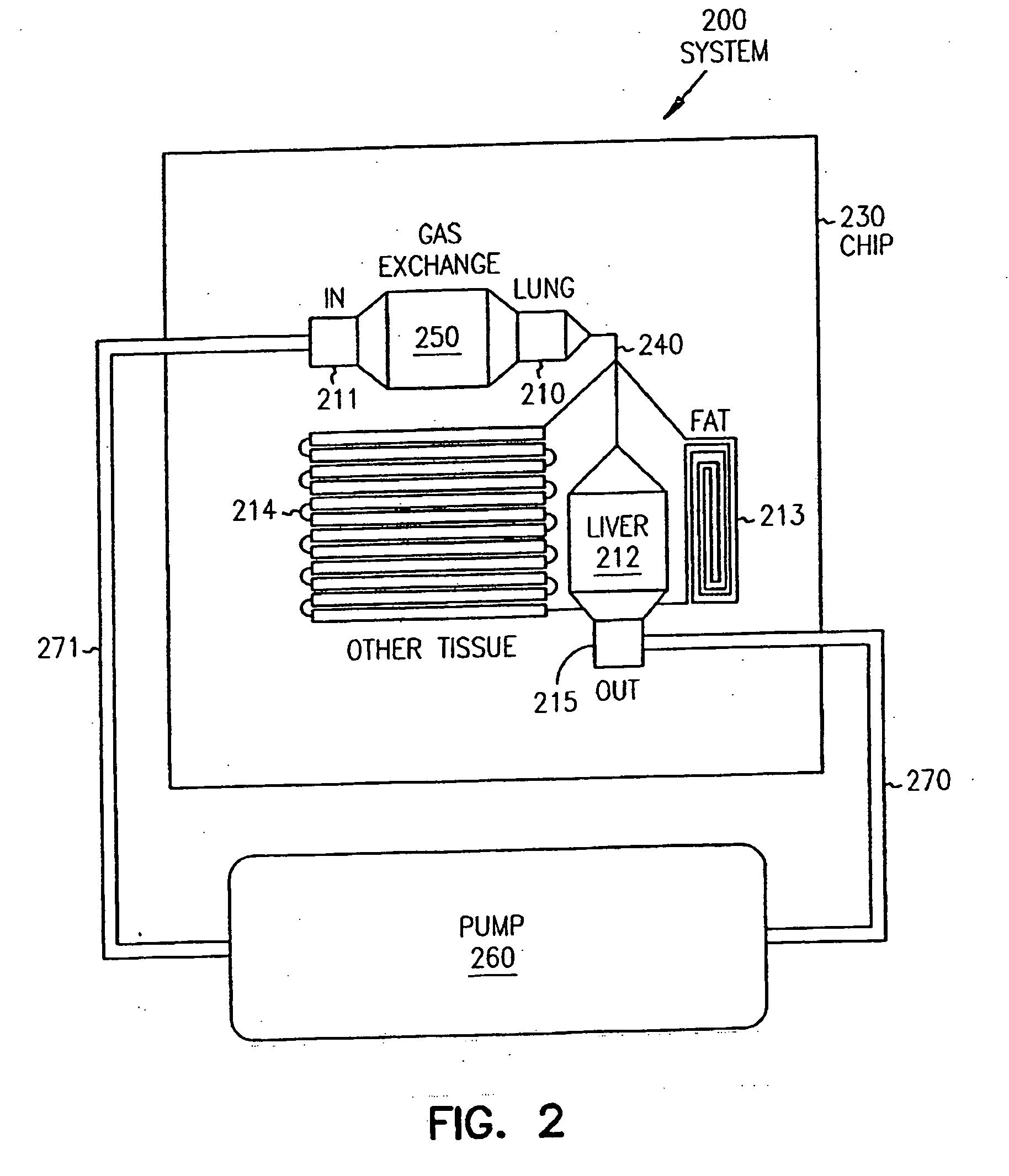
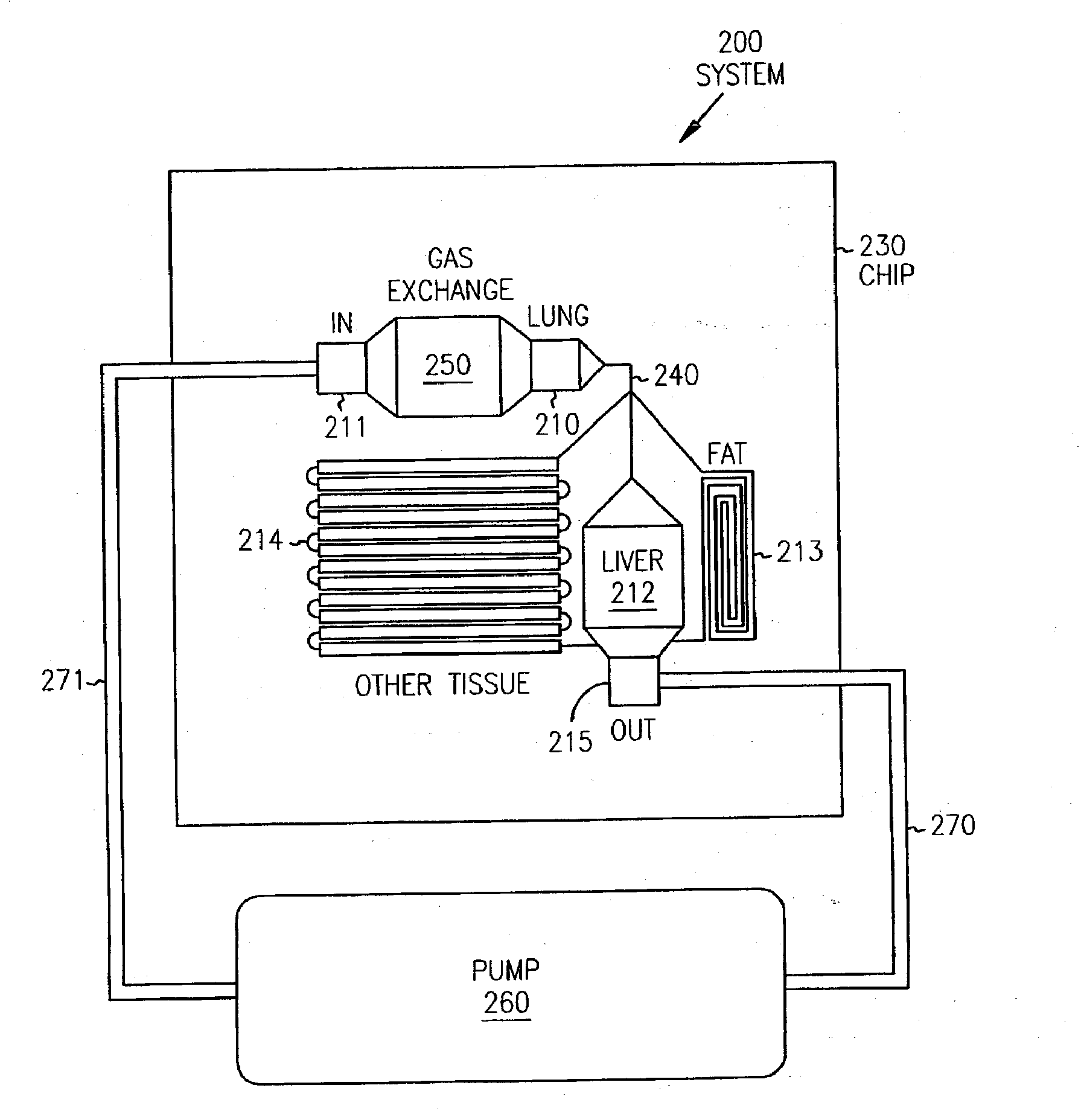
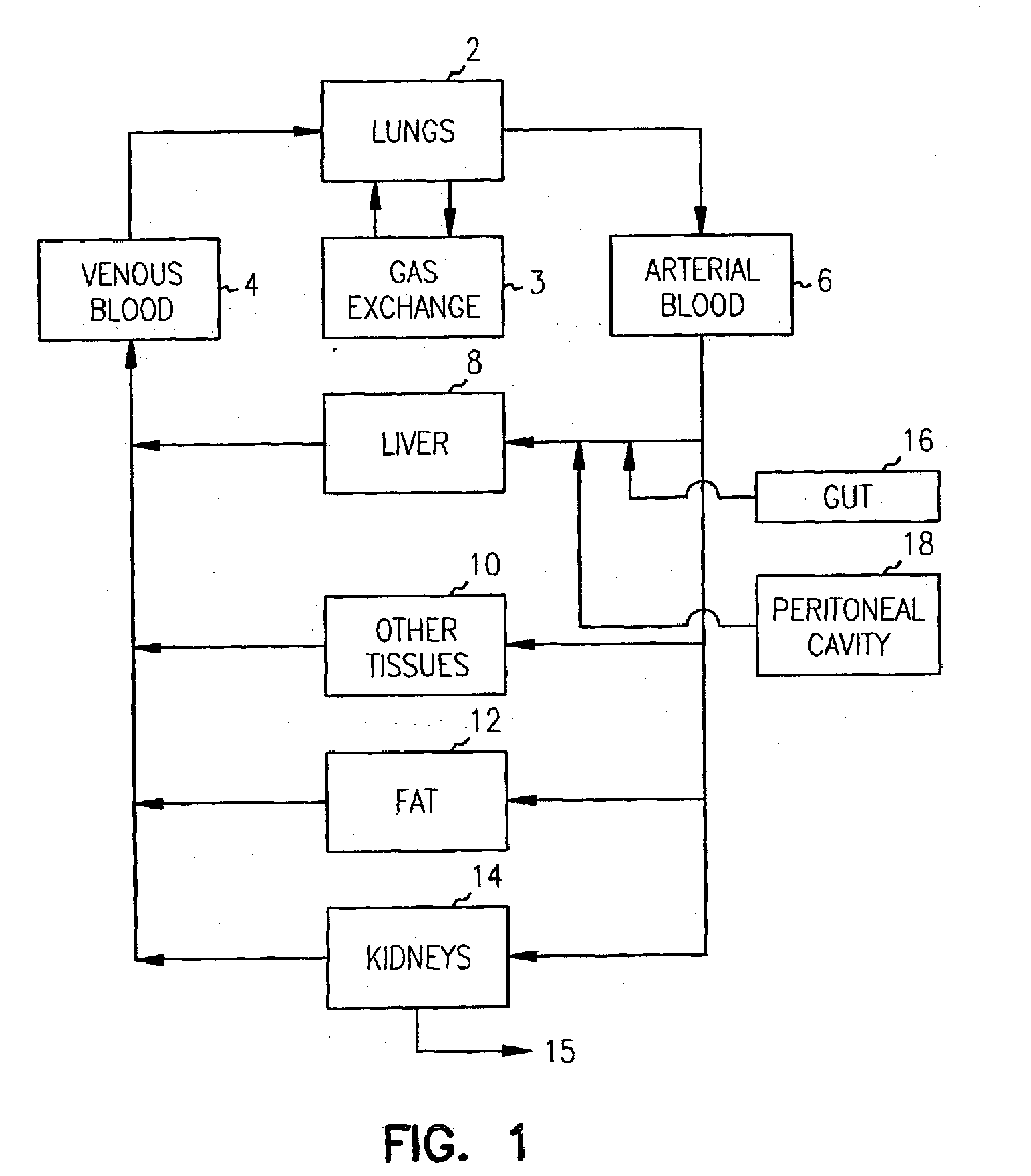
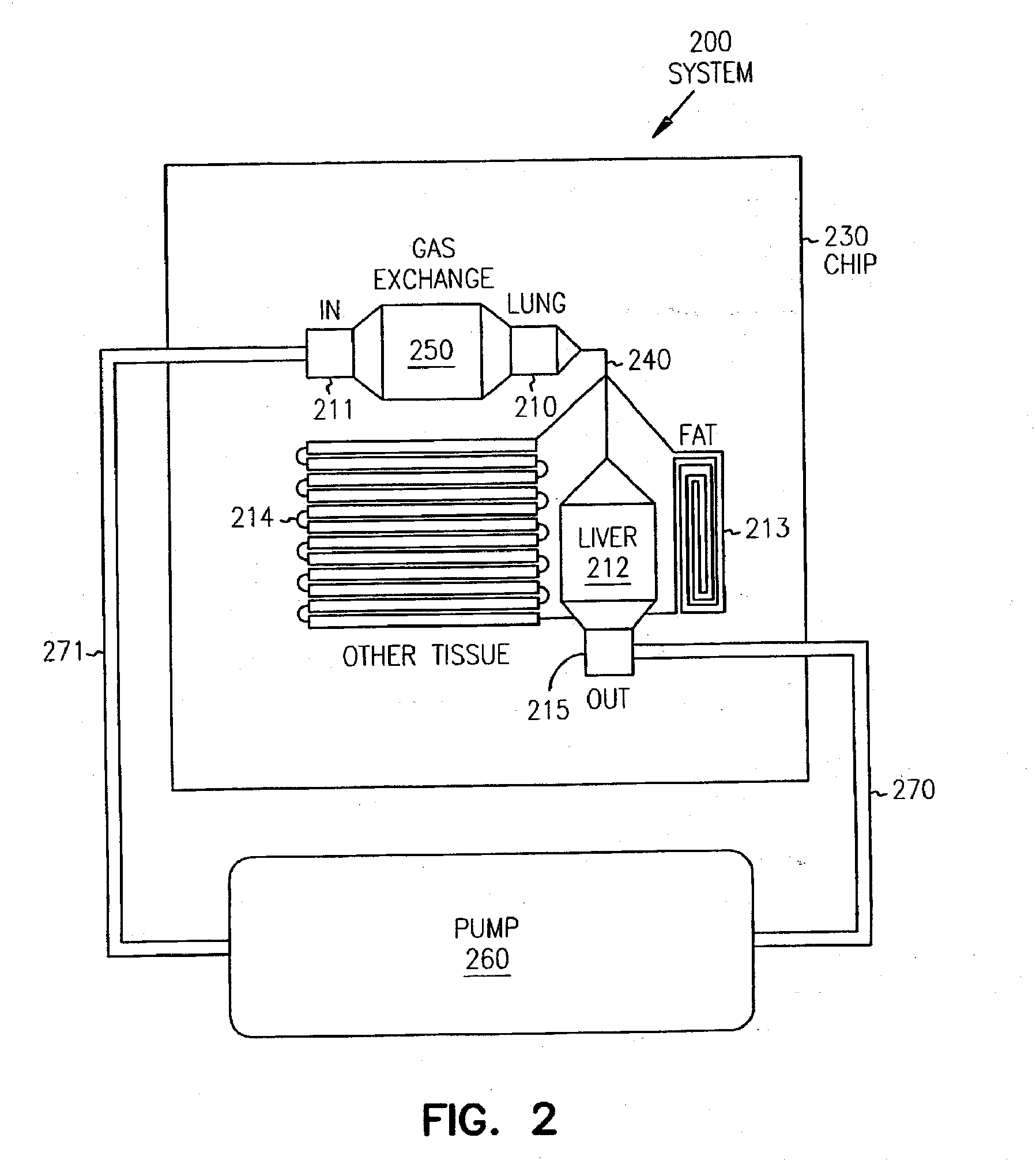
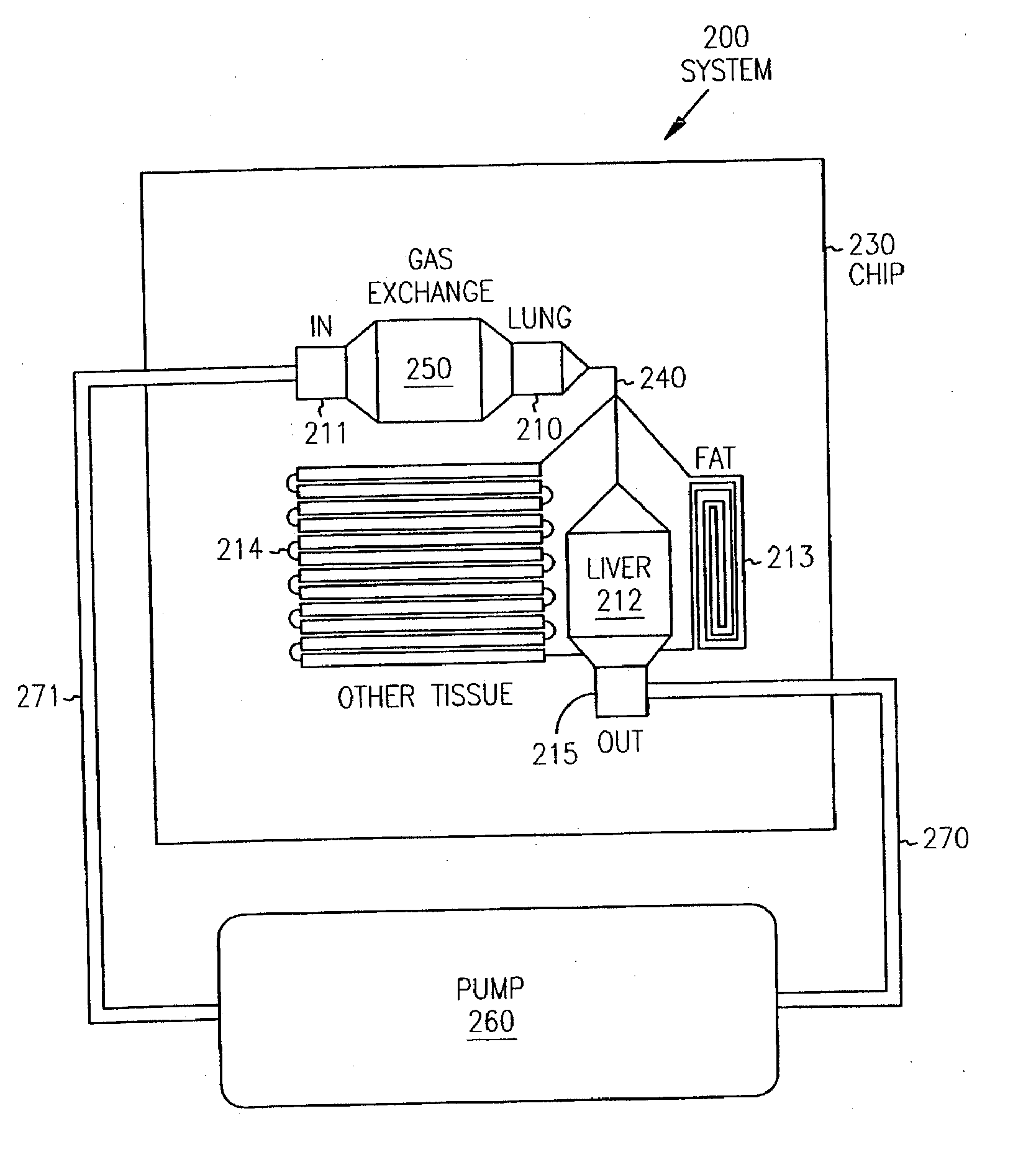
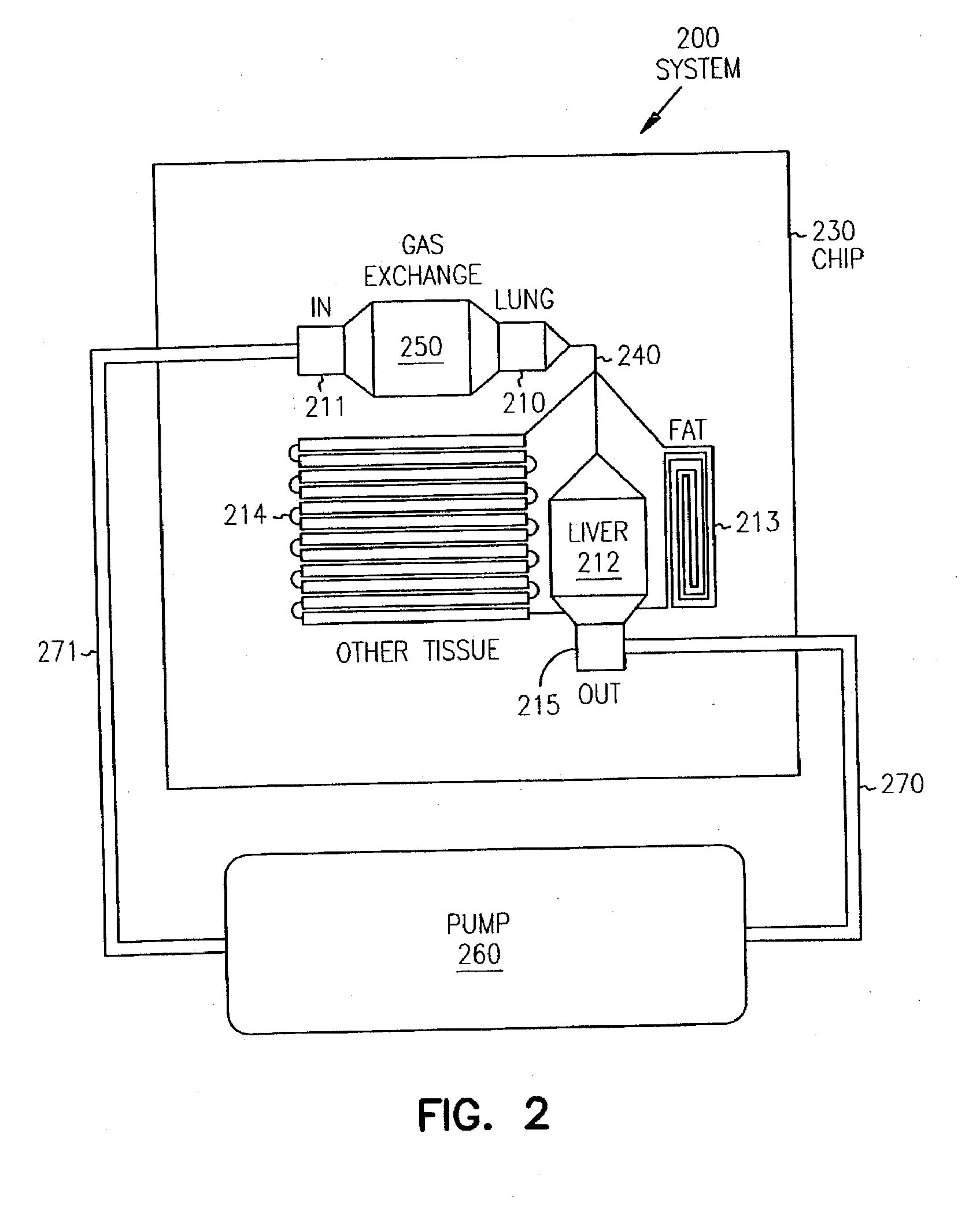
Pharmacokinetic-based culture system with biological barriers
InactiveUS20070037277A1Increase valueIncreased toxicityBioreactor/fermenter combinationsBiological substance pretreatmentsBlood flowDrug compound
Systems and methods are disclosed for microscale pharmacokinetics. Various organs and their interactions with drug compounds can be simulated in vitro by use of microscale compartments that can be interconnected by microscale channels. Cells or cellular materials associated with the organs can be cultured in such compartments to allow interactions with drug compounds in one or more fluidic flows. Such fluidic systems can include, by way of examples, gastrointestinal flow, blood flow, bile flow, urinary flow, and brain fluid flow. Interactions between fluidic systems can be simulated by a microscale permeable member. In one example, blood-biliary interaction can be simulated by a microscale permeable material having hepatocytes bound to a permeable substrate via a binder.
Owner:CORNELL RES FOUNDATION INC
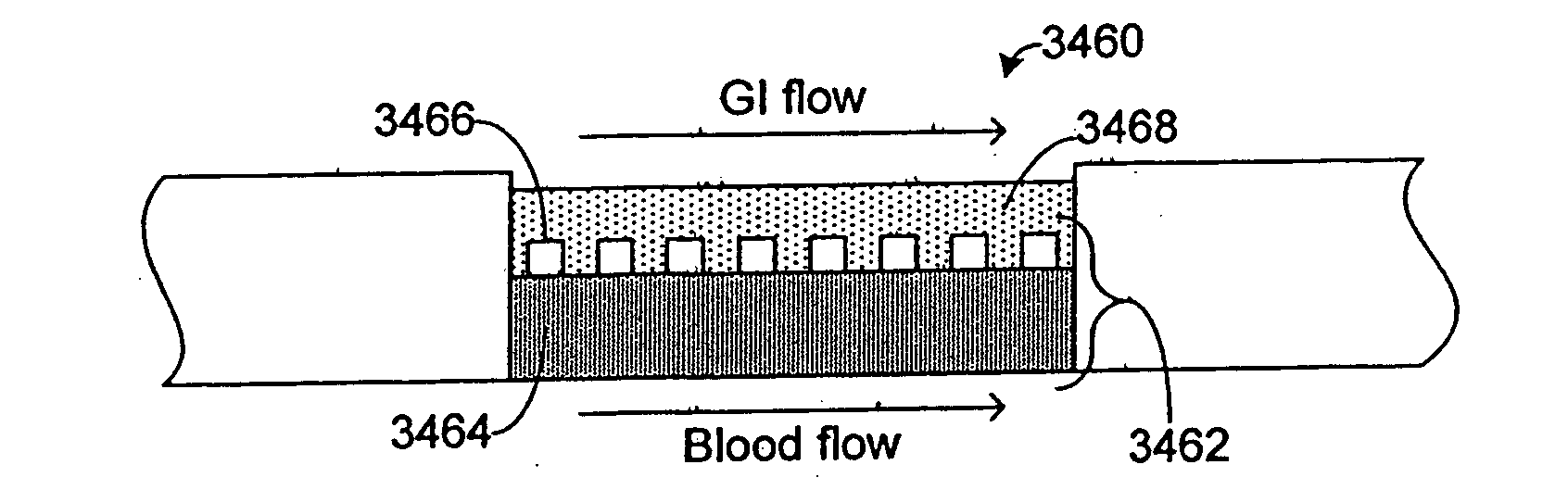
Biliary barrier
InactiveUS20070048727A1Increased toxicityIncrease valueBioreactor/fermenter combinationsBiological substance pretreatmentsDrug compoundMedicine
Systems and methods are disclosed for microscale pharmacokinetics. Various organs and their interactions with drug compounds can be simulated in vitro by use of microscale compartments that can be interconnected by microscale channels. Cells or cellular materials associated with the organs can be cultured in such compartments to allow interactions with drug compounds in one or more fluidic flows. Such fluidic systems can include, by way of examples, gastrointestinal flow, blood flow, bile flow, urinary flow, and brain fluid flow. Interactions between fluidic systems can be simulated by a microscale permeable member. In one example, blood-biliary interaction can be simulated by a microscale permeable material having hepatocytes bound to a permeable substrate via a binder.
Owner:CORNELL RES FOUNDATION INC
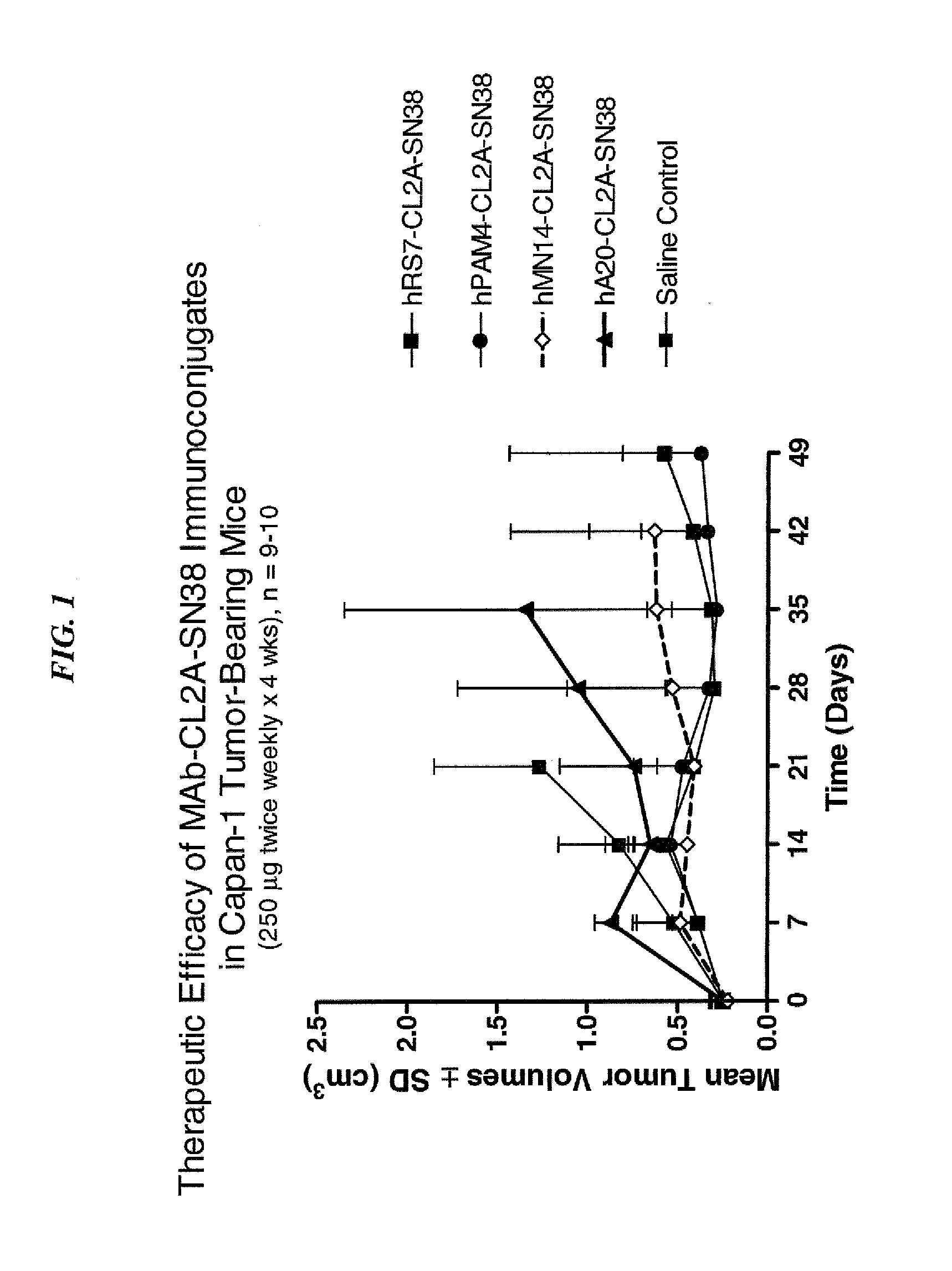
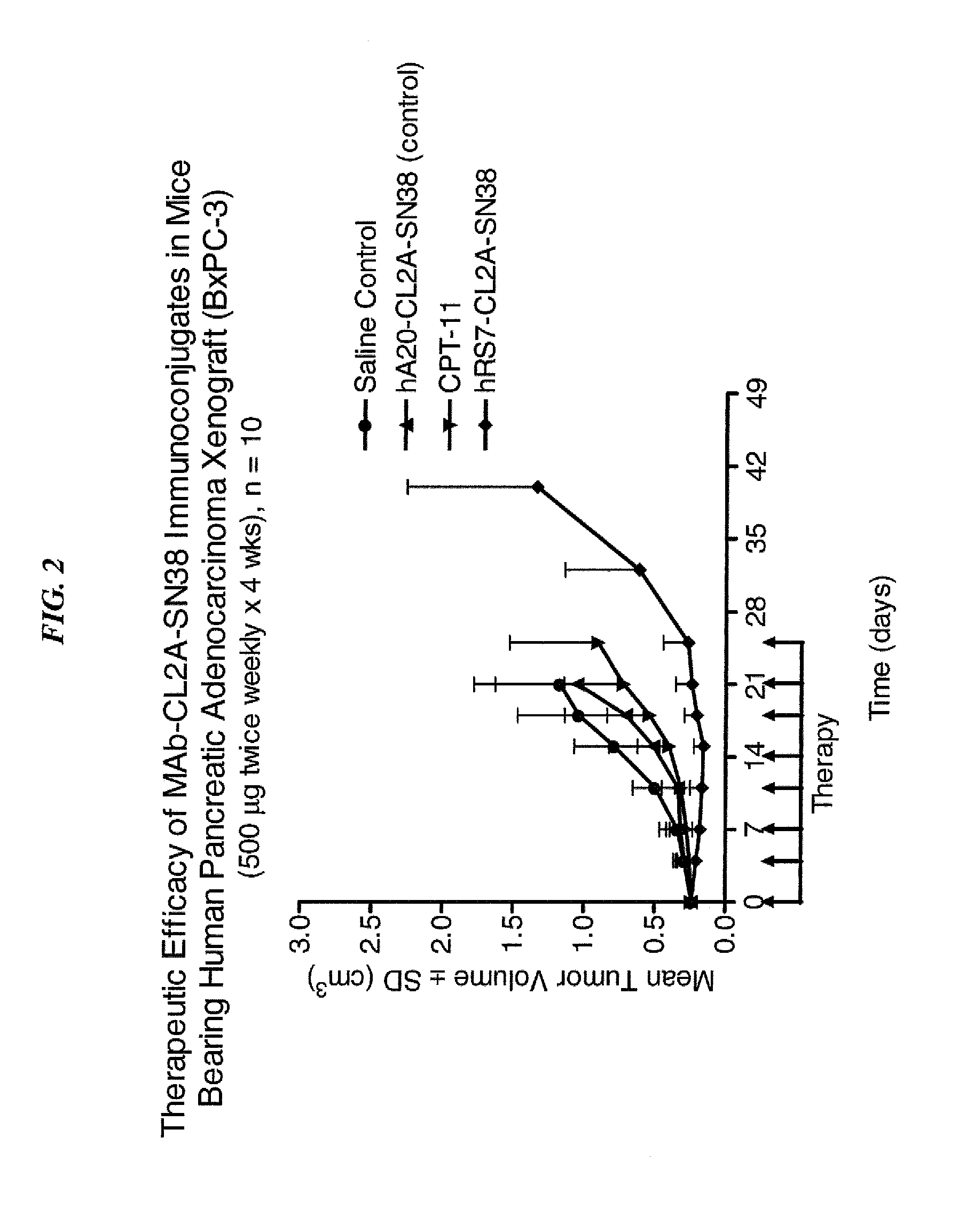
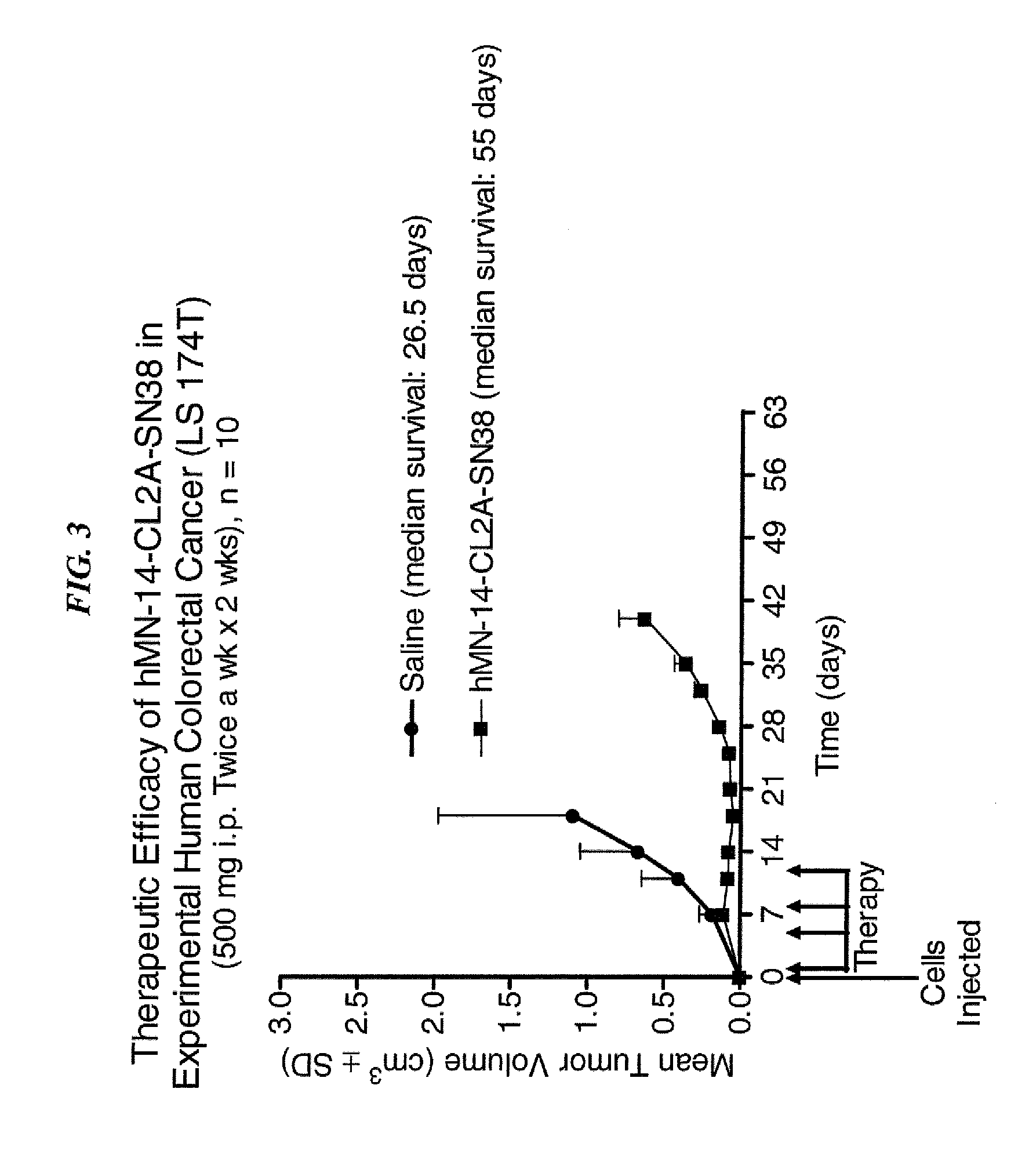
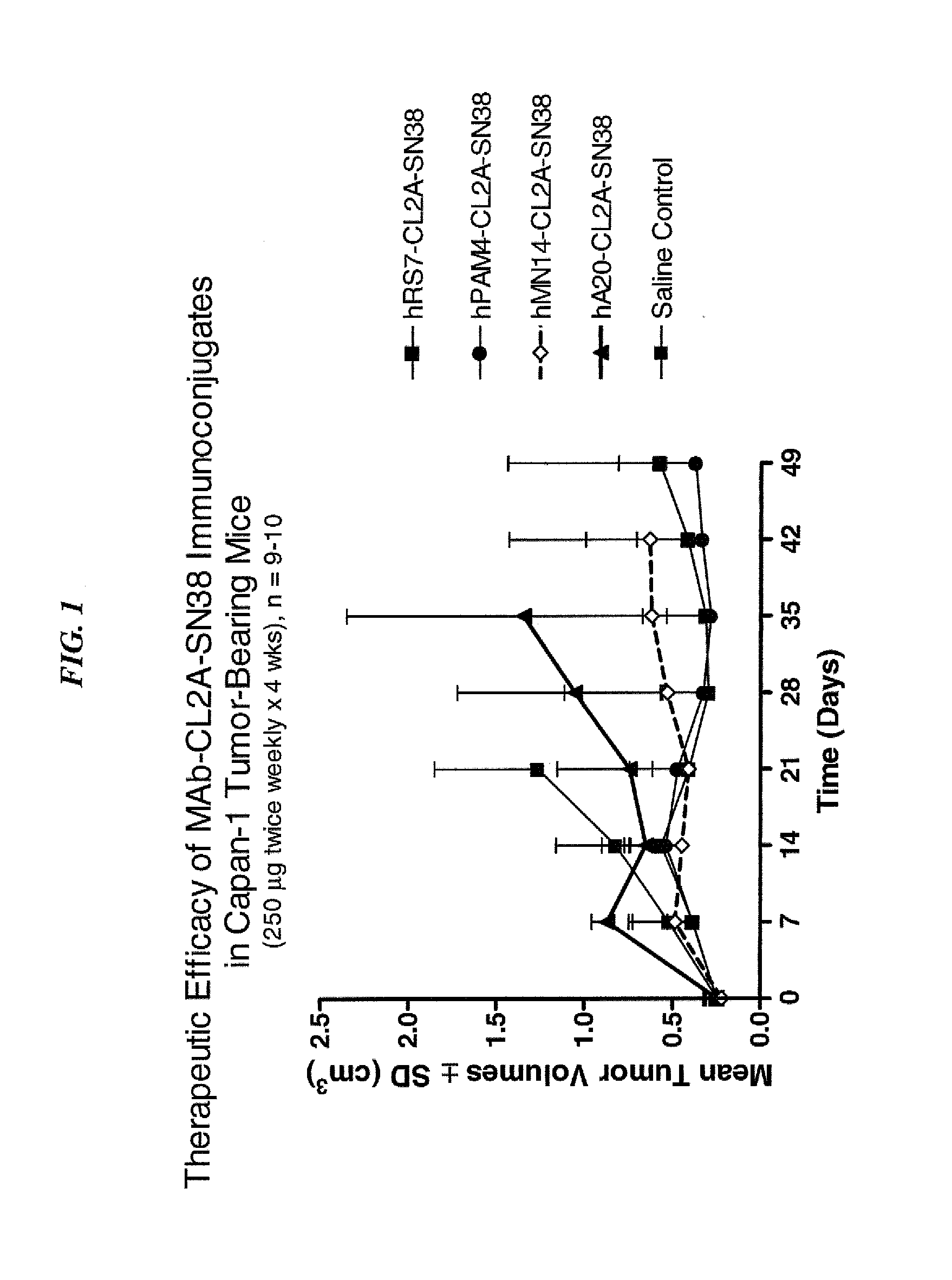
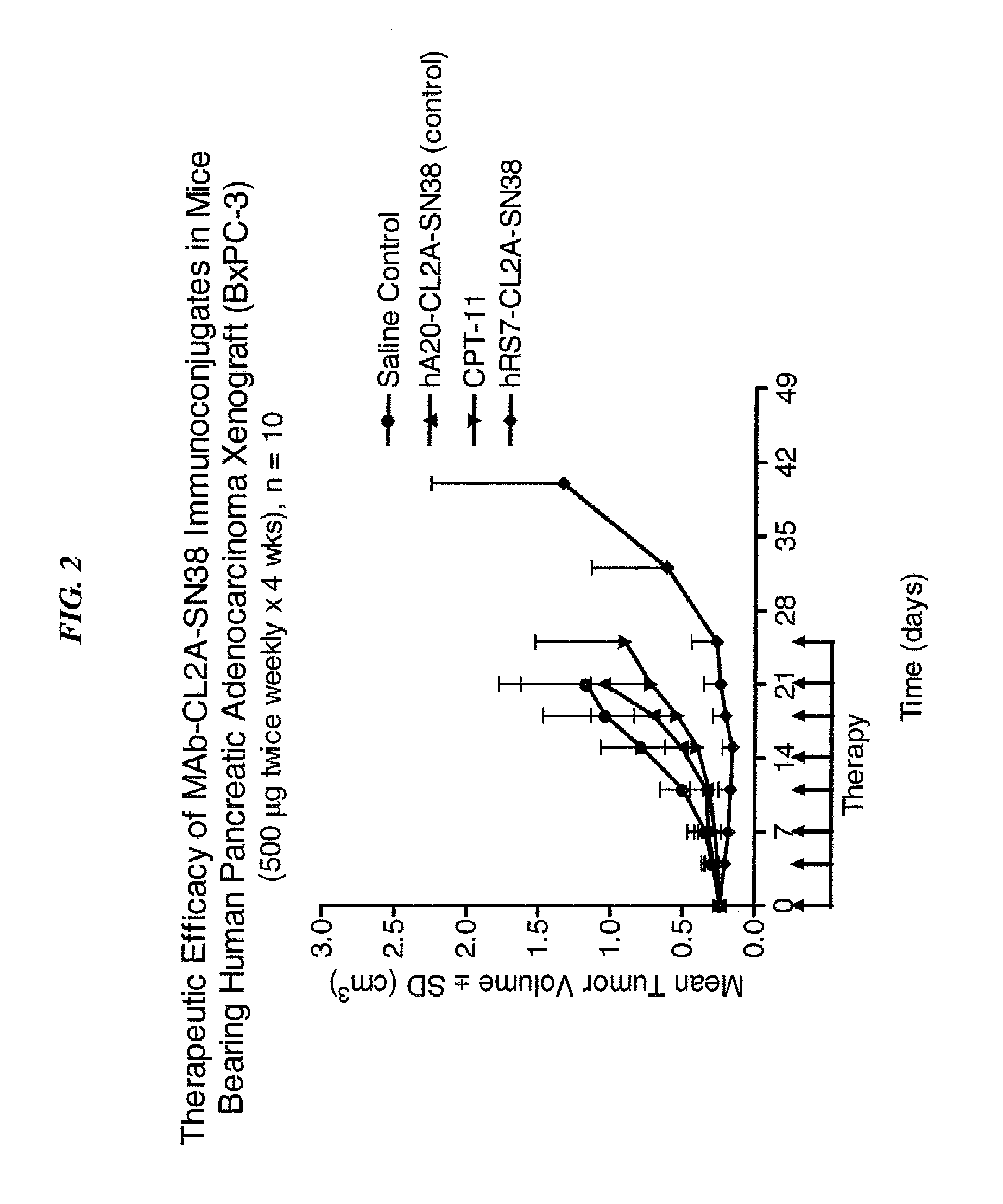
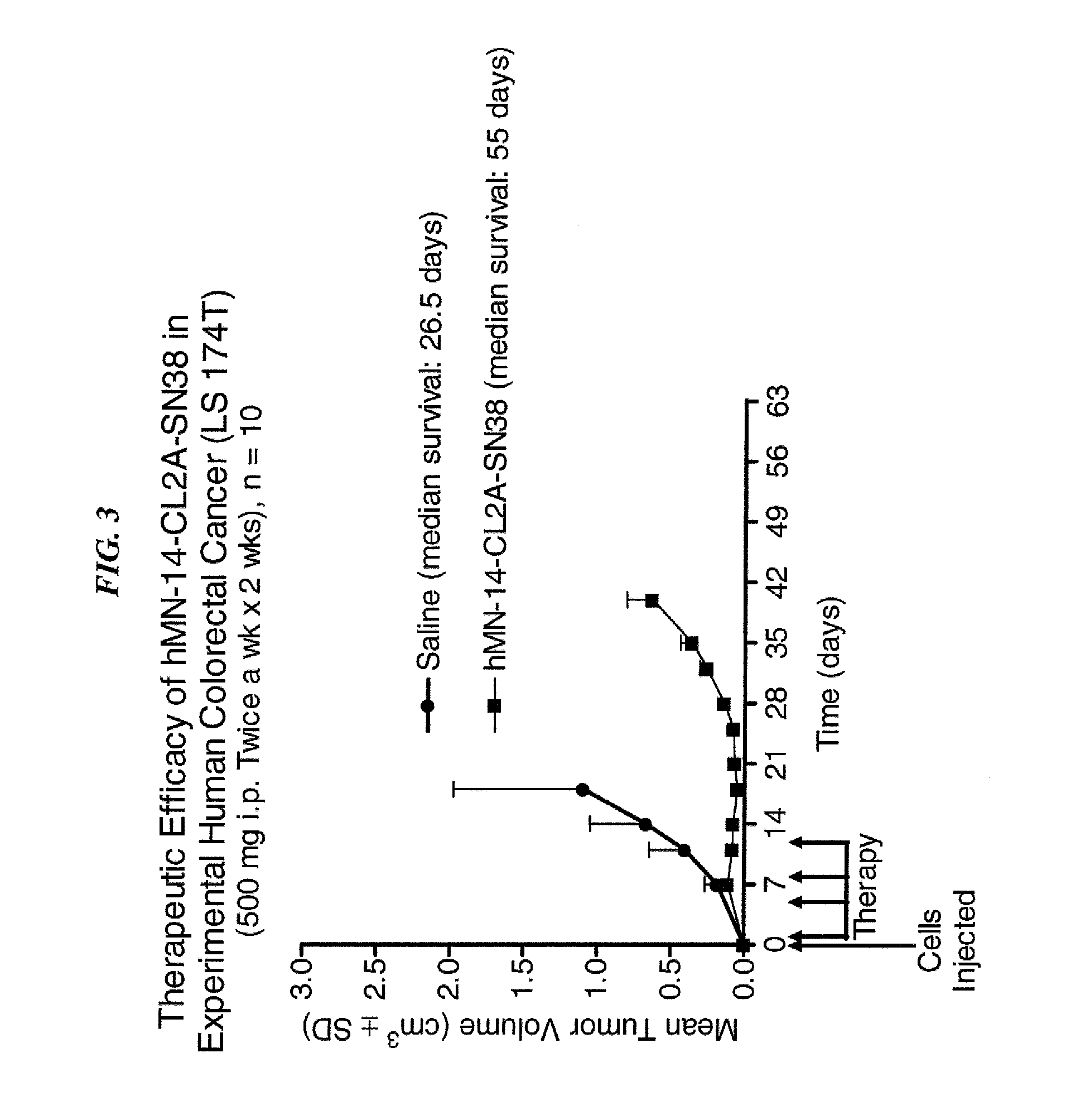
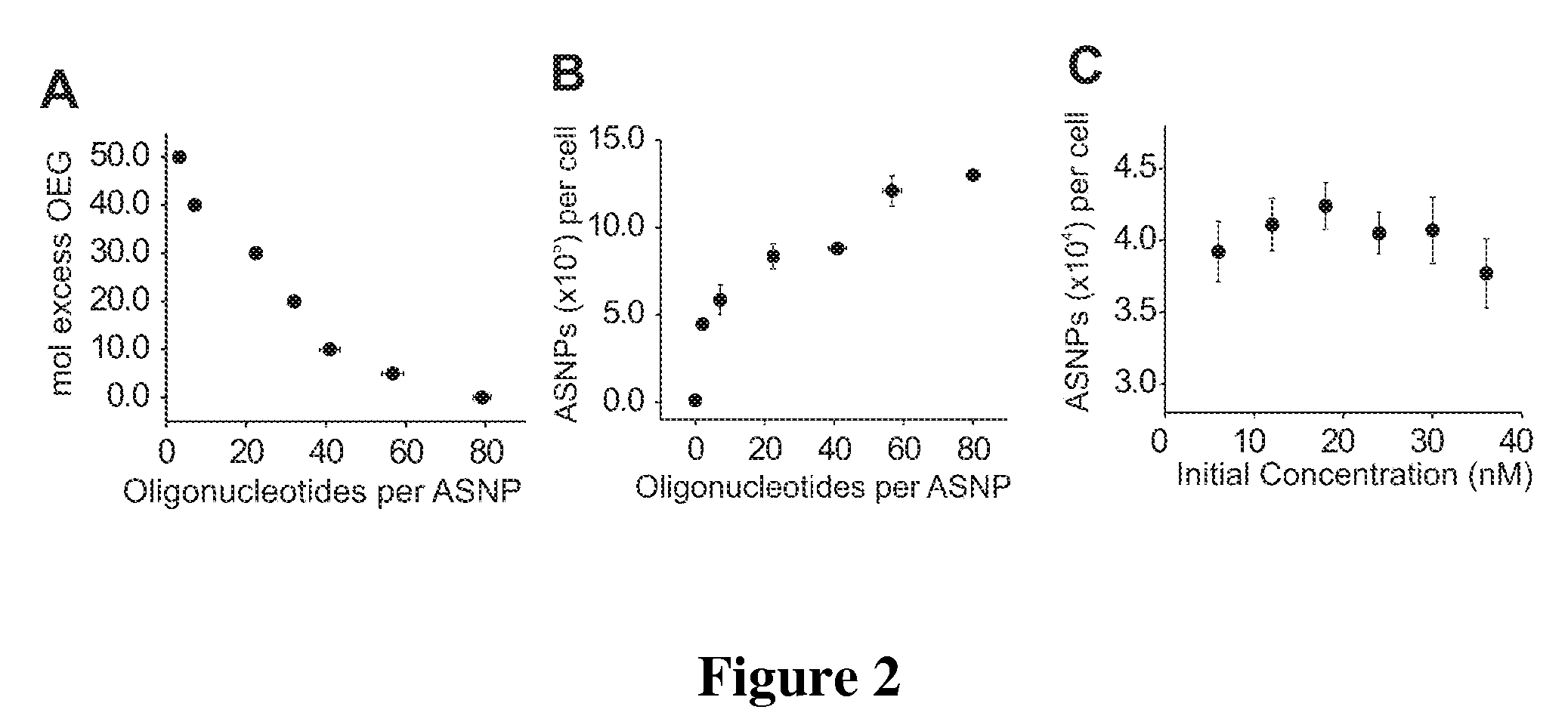
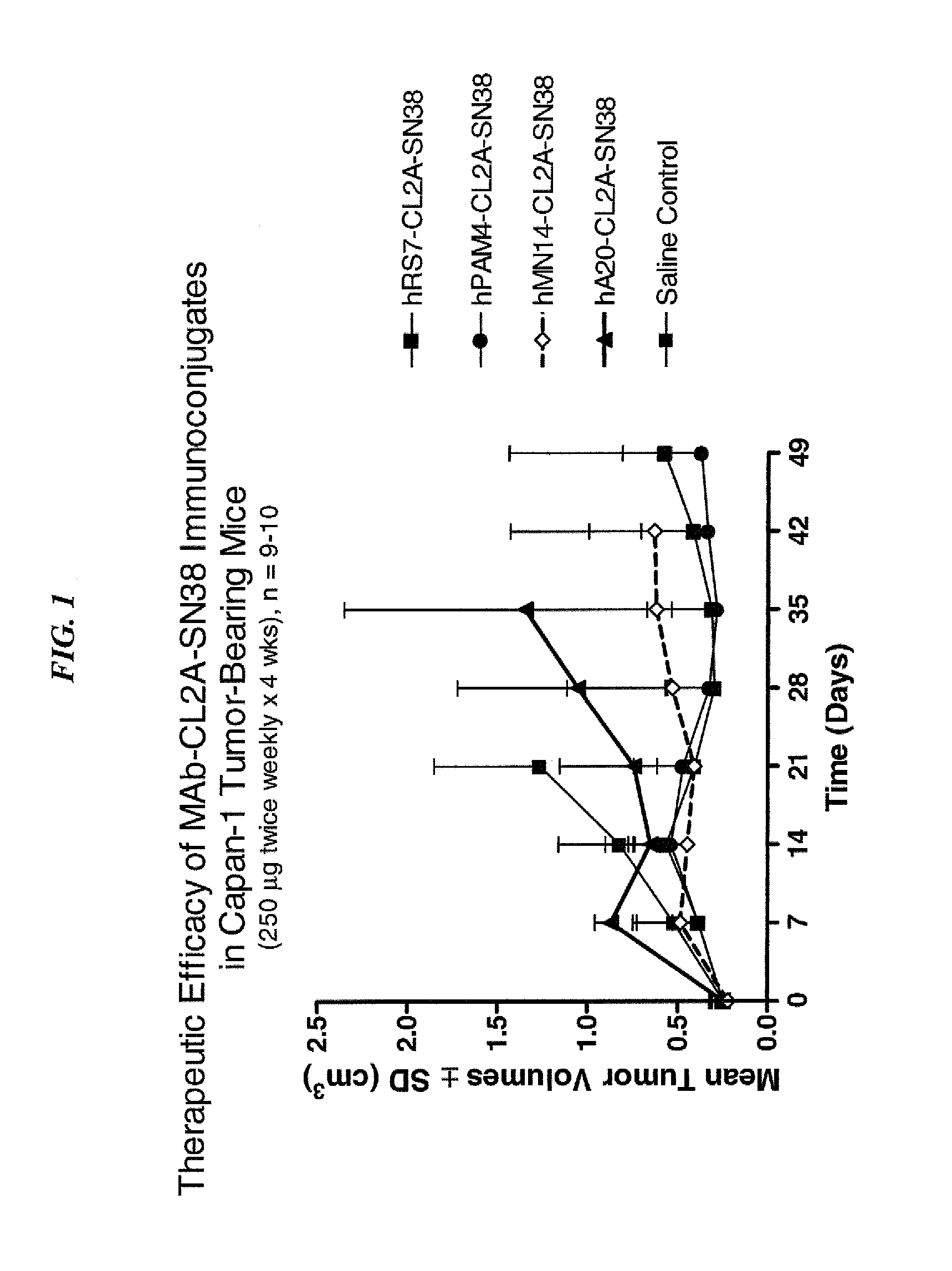
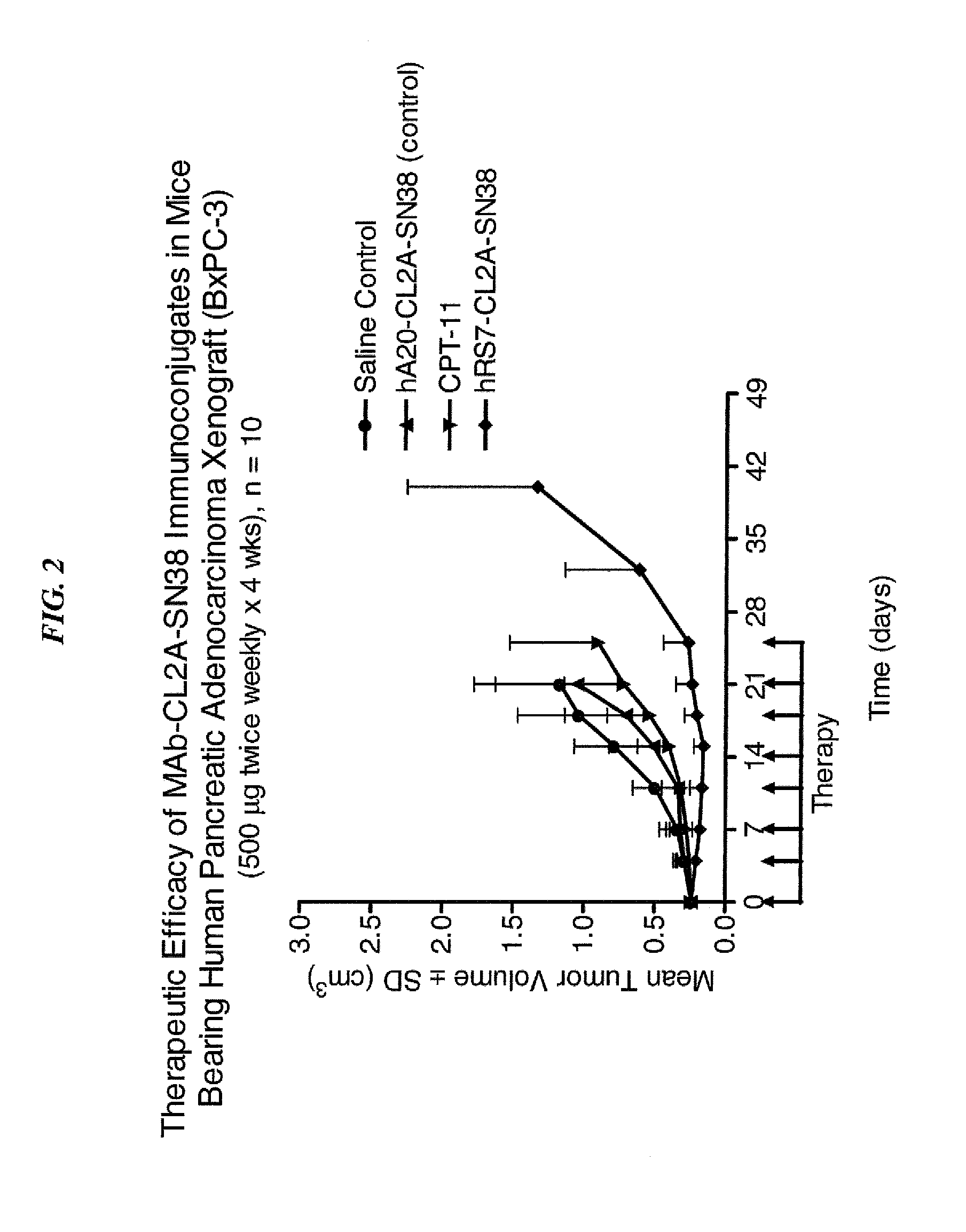
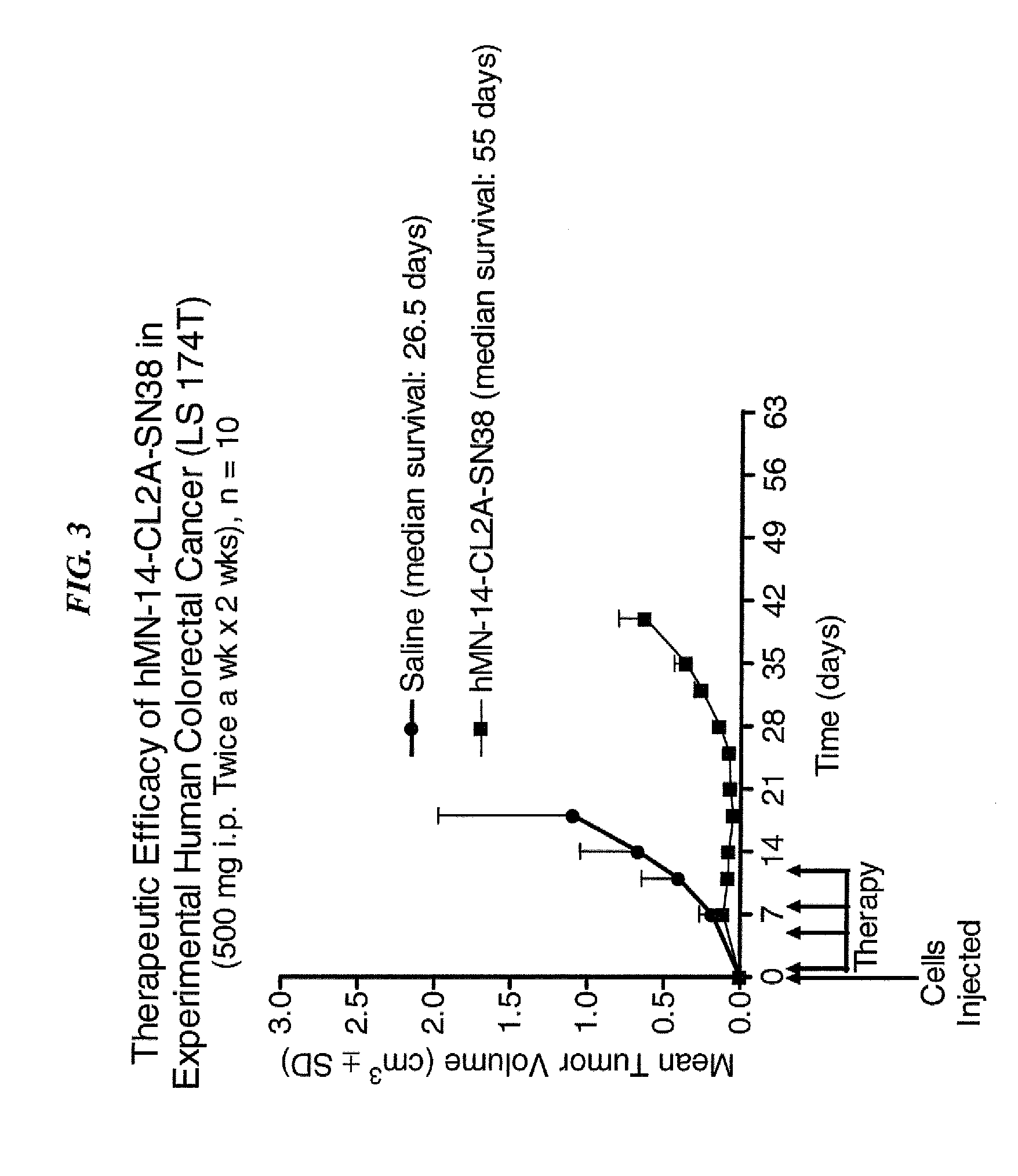
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140170063A1Overcome tumorImprove targetingHeavy metal active ingredientsOrganic active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 ROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140219914A1Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsRadioactive preparation carriersCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
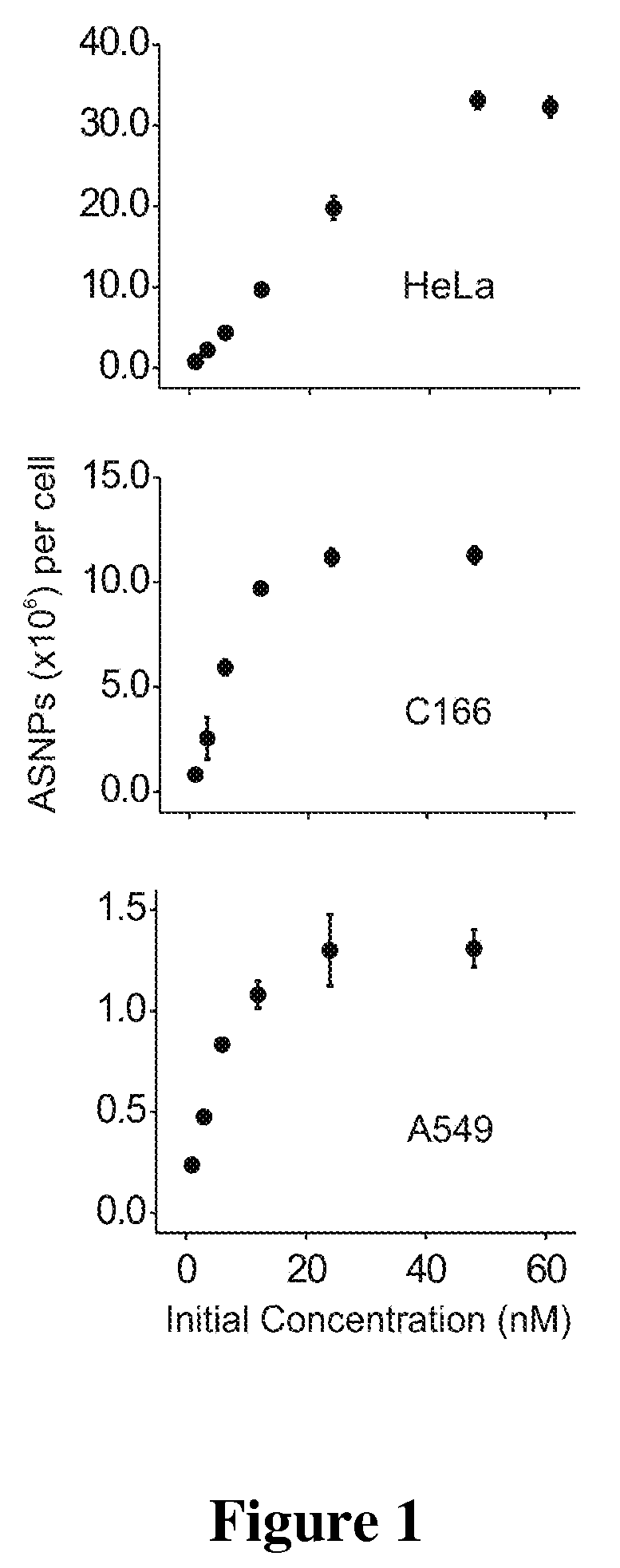
Nucleic Acid Functionalized Nanoparticles for Therapeutic Applications
InactiveUS20080306016A1Enhance cellular uptakeIncreased cellular uptakeOrganic active ingredientsSpecial deliveryFunctionalized nanoparticlesBiology
Materials and Methods for modulating cellular uptake of functionalized nanoparticles are provided. Also provided are materials and methods for modulating the effectiveness of a therapeutic agent with a functionalized nanoparticle.
Owner:NORTHWESTERN UNIV
Dosages of immunoconjugates of antibodies and SN-38 for improved efficacy and decreased toxicity
ActiveUS9028833B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
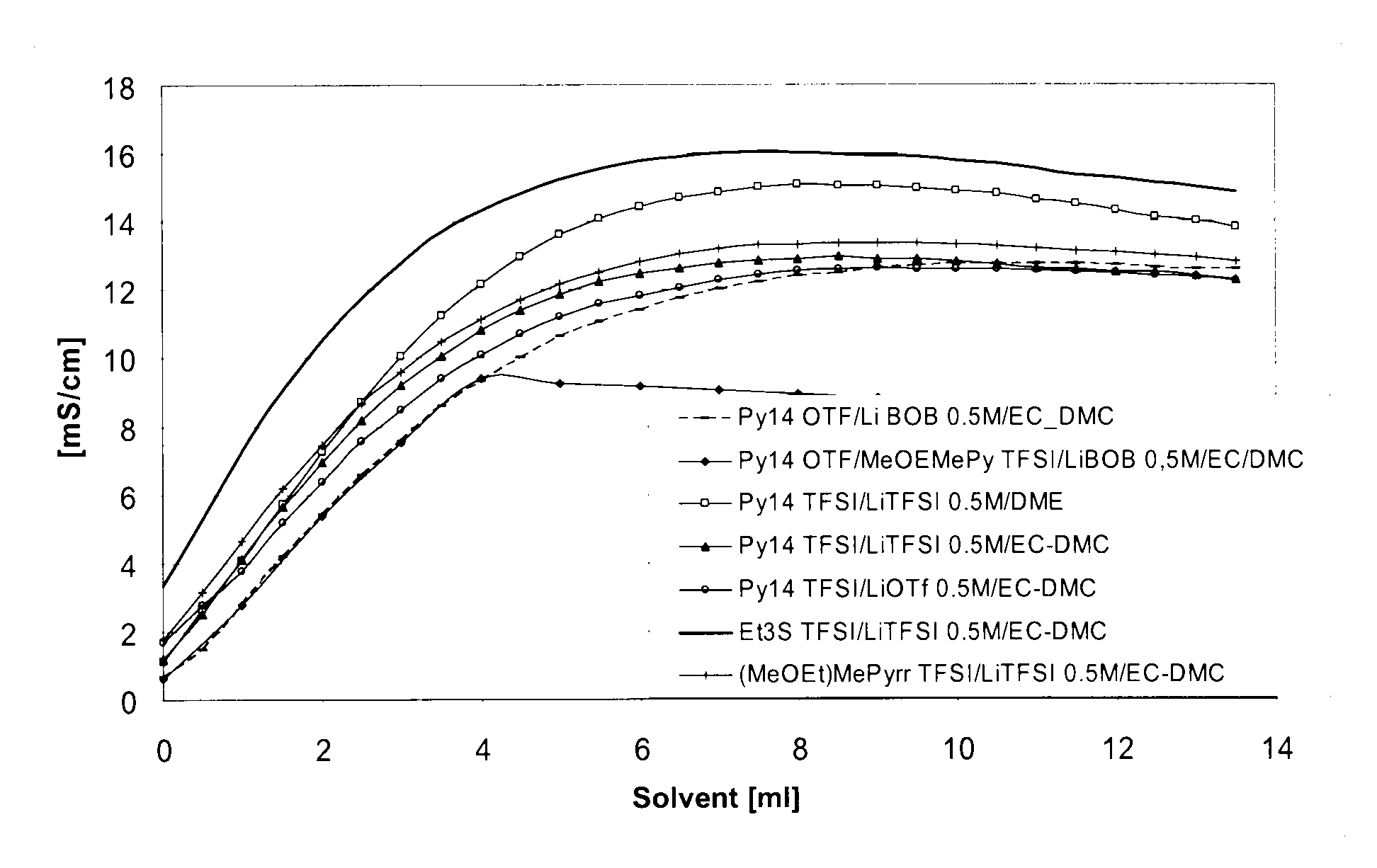
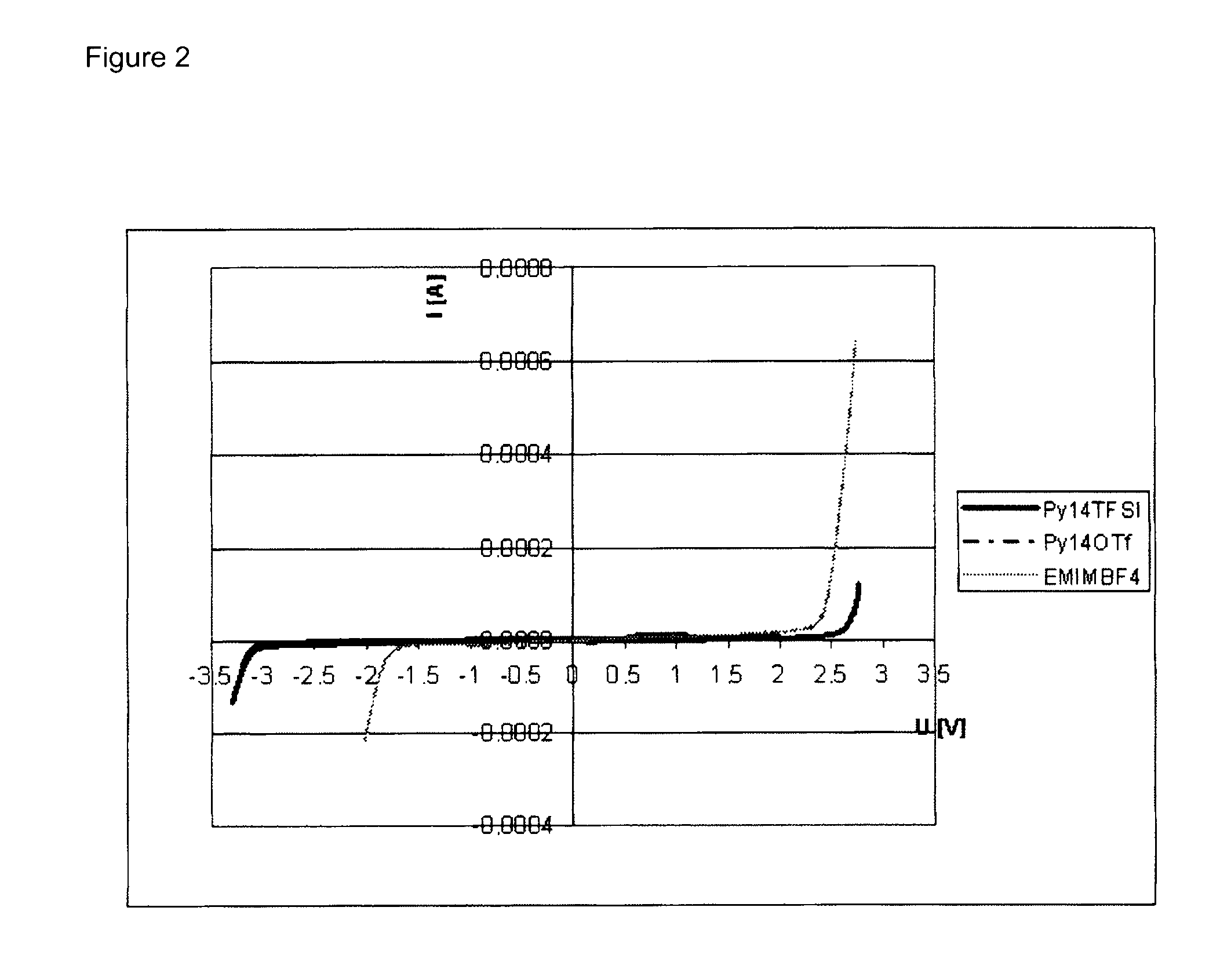
Electrolyte formulations for energy storage devices based on ionic liquids
InactiveUS20100304225A1Cost advantageImprove performanceAlkaline accumulatorsHybrid capacitor electrolytesChemical physicsElectrochemistry
The invention relates to an electrolyte formulation comprising a) an ionic liquid which is electrochemically stable over a range of at least 4.5 V, has a viscosity of less than 300 mPa·s at 20° C. and has a conductivity of at least 1 mS / cm at 20° C., and b) an aprotic, dipolar solvent in an amount of 20 to 60% by volume based on the electrolyte formulation, wherein the conductivity of the electrolyte formulation is greater at least by a factor of 2 than the conductivity of the ionic liquid.
Owner:EVONIK DEGUSSA GMBH
Devices and methods for pharmacokinetic-based cell culture system
InactiveUS20080064088A1Increase valueIncreased toxicityBioreactor/fermenter combinationsBiological substance pretreatmentsIn vitro cell cultureBiophysics
Owner:CORNELL RES FOUNDATION INC
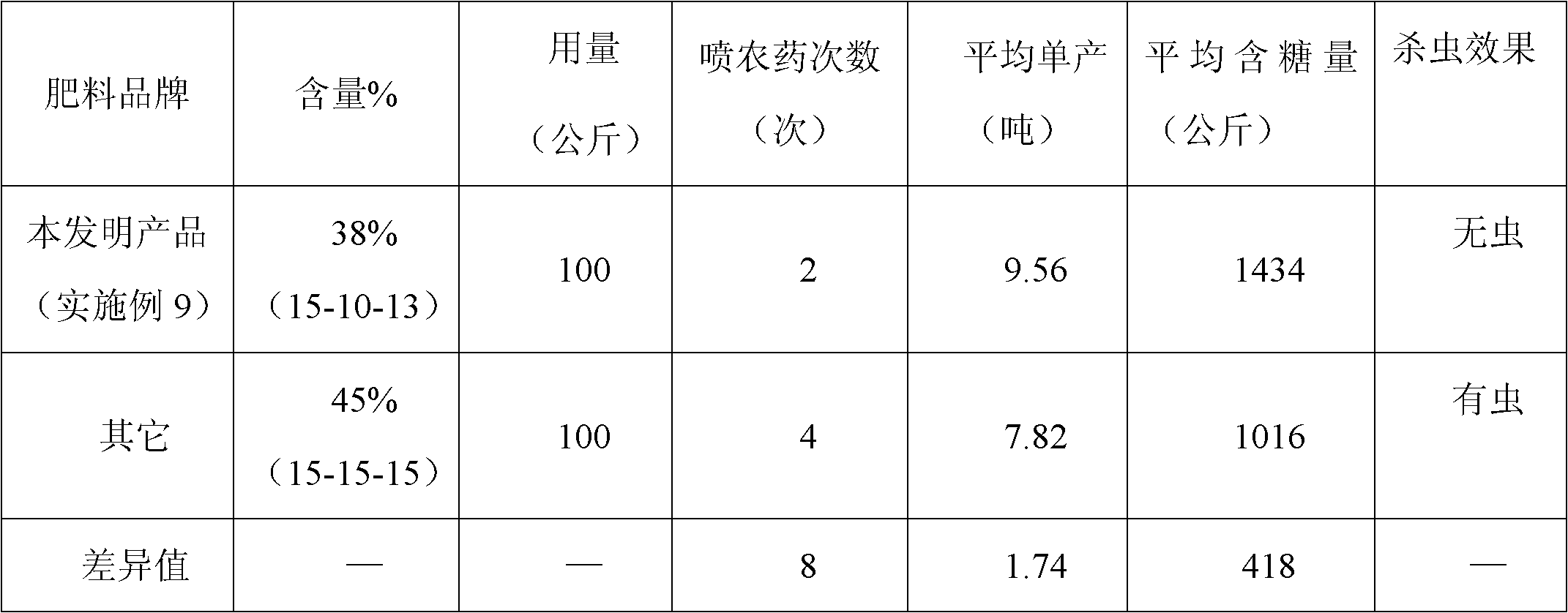
Sugarcane fertilizer for preventing and treating soil insects and production method thereof
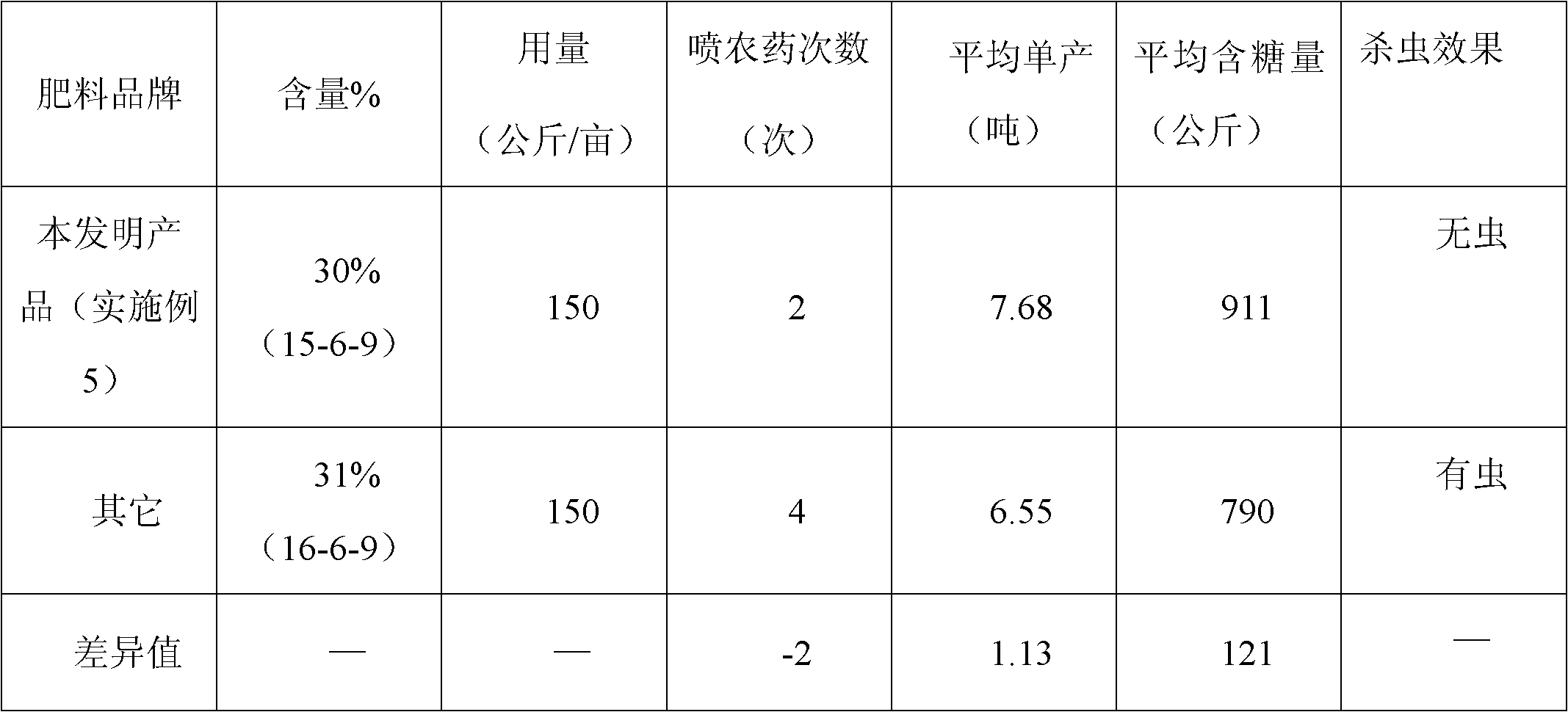
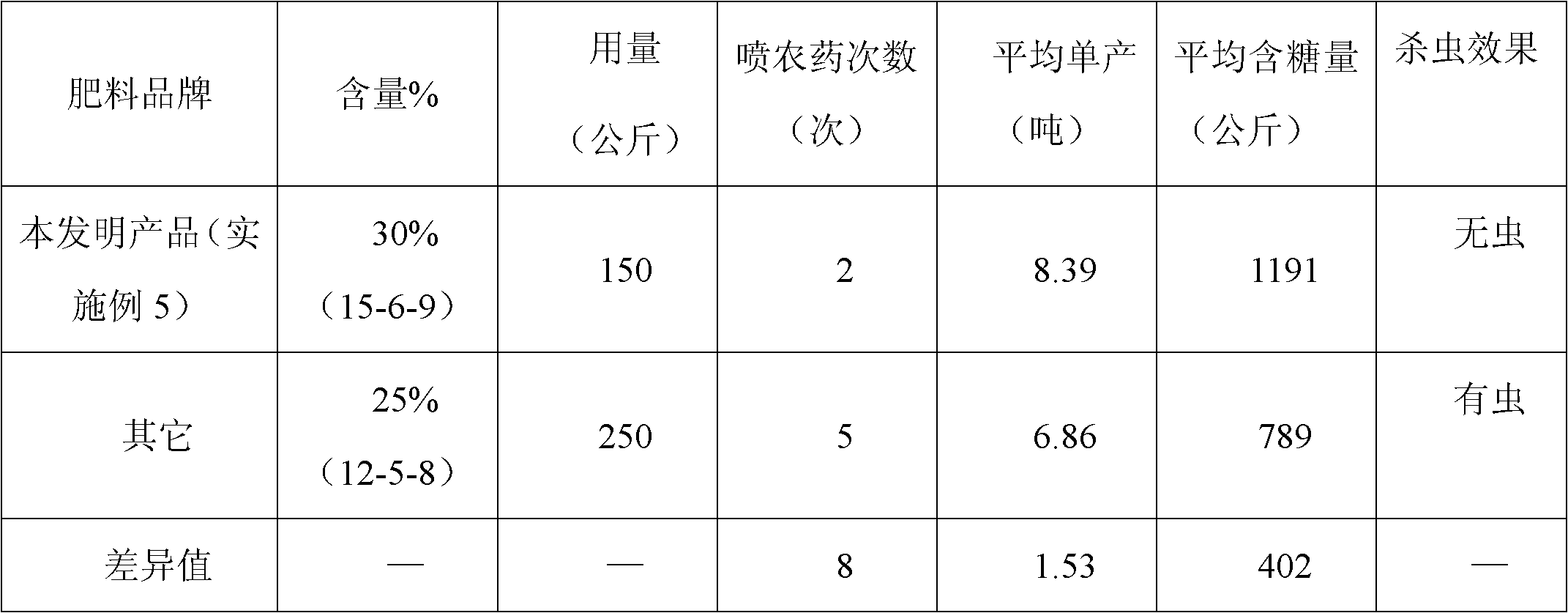
The invention discloses a sugarcane fertilizer for preventing and treating soil insects and a production method of the sugarcane fertilizer. The sugarcane fertilizer comprises the following ingredients: inorganic fertilizer, organic fertilizer, reagents, plant growth regulating agents and bonding agents, wherein the inorganic fertilizer accounts for 45 to 92 weight percent of the total weight of the sugarcane fertilizer, the organic fertilizer accounts for 7 to 50 weight percent of the total weight of the sugarcane fertilizer, the reagents account for 0.25 to 3.5 weight percent of the total weight of the sugarcane fertilizer, the plant growth regulating agents account for 0.04 to 0.05 weight percent of the total weight of the sugarcane fertilizer, and the bonding agents account for 0.075 to 3.0 weight percent of the total weight of the sugarcane fertilizer. The reagents are combination of phoxim, chlorpyrifos and imidacloprid or dinotefuran according to a weight ratio of (1-2):1:(0.1-4.5), and the plant growth regulating agents are sodium nitrophenolate. Products of the sugarcane fertilizer fully meet the requirement on the nutrition in the sugarcane growth process, simultaneously, the effects of preventing and treating sugarcane insects such as stem borers, sugarcane turtles and grubs and treating insects such as cotton aphids, longicorns and the like can be realized, the yield and the sugar content are improved, and the production cost is also reduced.
Owner:GUANGXI LIYUANBAO SCI & TECH
Inhibitors of DNA Methyltransferase
InactiveUS20080132525A1Poor absorptionFast excretionBiocideOrganic chemistryDNA methyltransferaseMolecular biology
The invention relates to the inhibition of DNA methyltransferase isoforms DNMT1 and DNMT3b2. The invention provides compounds and methods for inhibiting DNMT1 and DNMT3b2.
Owner:METHYLGENE
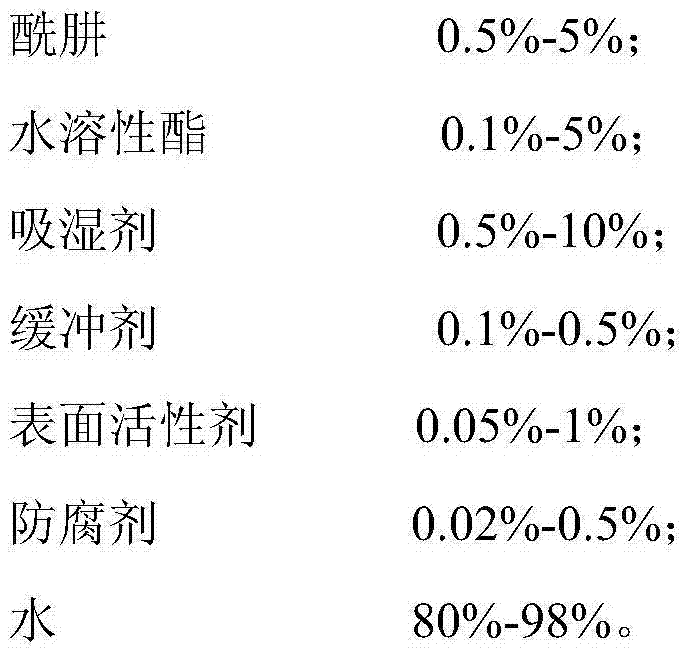
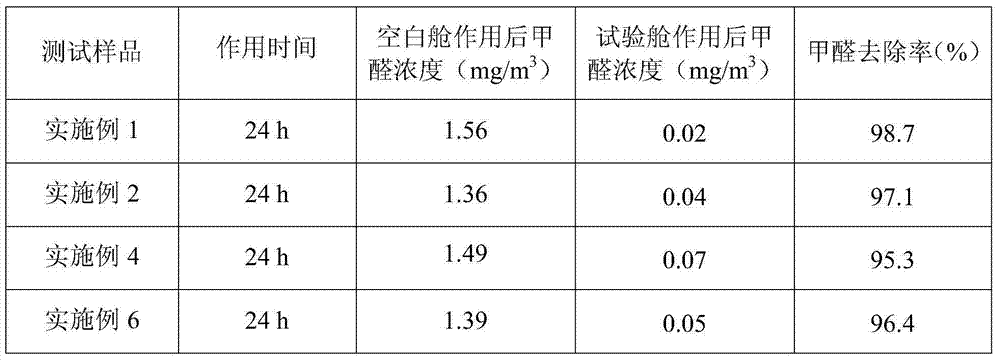
Efficient low-toxicity formaldehyde scavenger
ActiveCN104324583ANon-toxic or low toxicityNon-oxidizingDispersed particle separationScavengerPreservative
The invention discloses an efficient low-toxicity formaldehyde scavenger containing the following raw material components by weight: 0.5%-5% of hydrazide, 0.1%-5% of a water-soluble ester, 0.5%-10% of a moisture absorbent, 0.1%-0.5% and of a buffer, 0.05%-1% of a surface active agent, 0.02%-0.5% of a preservative and 80%-98% of water. The free hydrazine content in the efficient low-toxicity formaldehyde scavenger product is controlled in the range as low as possible by the manner of fine selection of the hydrazide raw material, control of free hydrazine content, adding of the water-soluble ester, adding of the buffer, and the like, and the efficient low-toxicity formaldehyde scavenger product has better security. The efficient low-toxicity formaldehyde scavenger product is good in formaldehyde removal effect, safe to people and objects, and simple in production process, and can be used for governance of the problem of formaldehyde exceeding the standard in new decorated residence or furniture.
Owner:广州超威生物科技有限公司
Crosslinkable macromers
InactiveUS20060240072A1Increased toxicityImprove bindingBone implantAbsorbent padsSolubilityFormamide
A crosslinkable macromer system and related methods of preparing the system and using the system in the form of a crosslinked matrix between a tissue site and an implant article such as a tissue implant or on the porous surface of a prosthetic device. The macromer system includes two or more polymer-pendent polymerizable groups and one or more multifunctional initiator groups. The polymerizable groups and the initiator group(s), when polymer-pendent, can be pendent on the same or different polymeric backbones. The macromer system provides advantages over the use of polymerizable macromers and separate, low molecular weight initiators, including advantages with respect to such properties as nontoxicity, efficiency, and solubility. A macromer system of the invention can be used as an interface between the tissue site and implant article in a manner sufficient to permit tissue growth through the crosslinked matrix and between the tissue site and implant. In a preferred embodiment, polymers with pendent polymerizable groups, for use in the macromer system, are prepared by reacting a polysaccharide polymer with a reactive moiety in an organic, polar solvent such as formamide.
Owner:SURMODICS INC
Production and use of novel peptide-based agents with bispecific antibodies
InactiveUS20060034759A1Increased toxicityUltrasonic/sonic/infrasonic diagnosticsAntibacterial agentsEpitopeDiagnostic agent
The present invention relates to a bi-specific antibody or antibody fragment having at least one arm that specifically binds a targeted tissue and at least one other arm that specifically binds a targetable construct. The targetable construct comprises a carrier portion which comprises or bears at least one epitope recognizable by at least one arm of said bi-specific antibody or antibody fragment. The targetable construct further comprises one or more therapeutic or diagnostic agents or enzymes. The invention provides constructs and methods for producing the bi-specific antibodies or antibody fragments, as well as methods for using them.
Owner:IMMUNOMEDICS INC
Devices and methods for pharmacokinetic-based cell culture systems
InactiveUS20070015275A1Increase valueIncreased toxicityBioreactor/fermenter combinationsBiological substance pretreatmentsIn vitro cell cultureBiophysics
Owner:CORNELL RES FOUNDATION INC
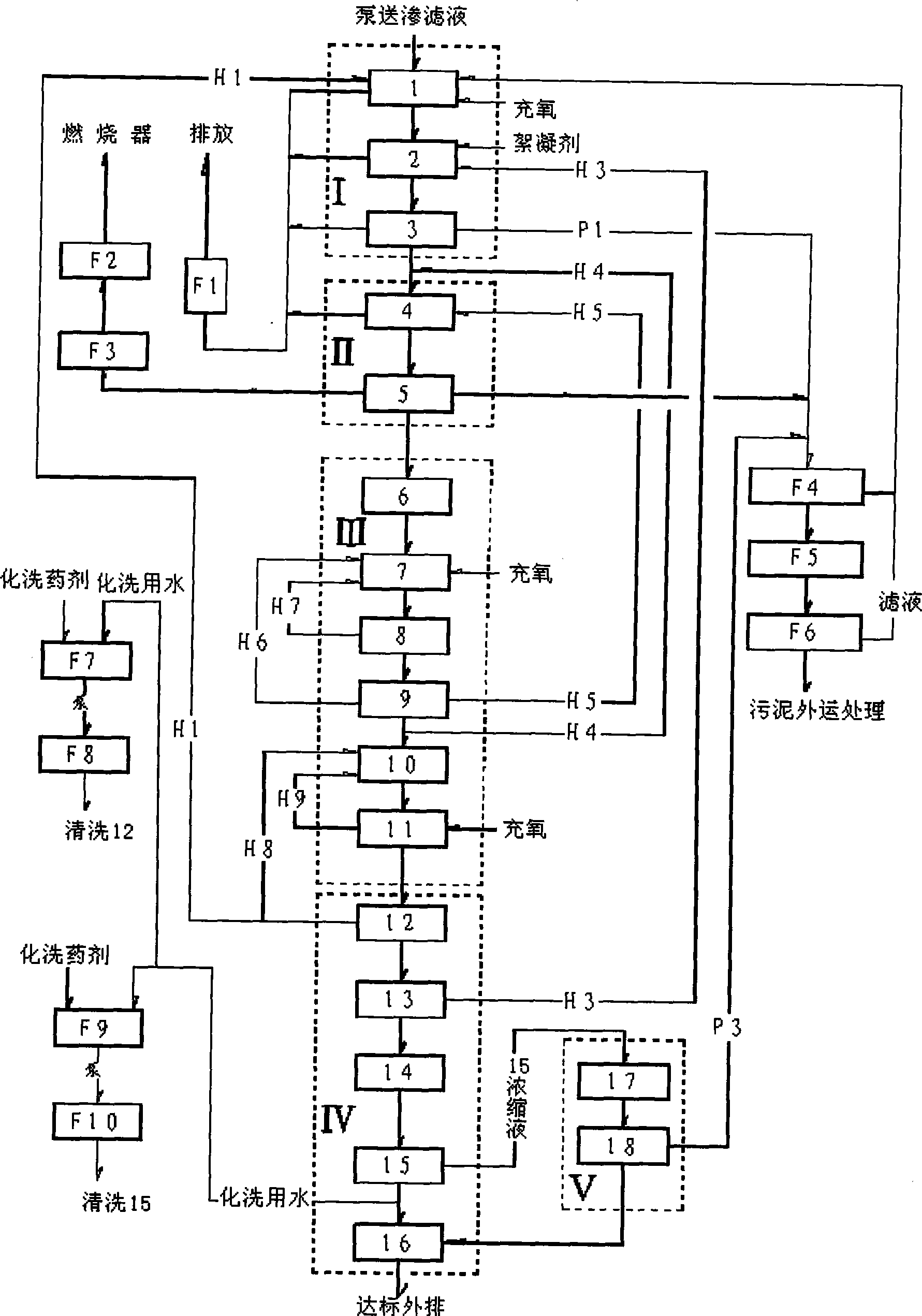
Treatment process for garbage leachate
ActiveCN101428938AImprove biodegradabilityPrevent rancidityTreatment with aerobic and anaerobic processesWater/sewage treatment bu osmosis/dialysisFlocculationHydrolysis
The invention discloses a method for treating landfill leachate, which orderly comprises flocculation precipitation pretreatment, hydrolysis pre-acidification treatment, anaerobic treatment, aerobic treatment comprising shortcut nitrification-denitrification and secondary nitrification-denitrification, membrane separation treatment and oxidation flocculation treatment. The method has the advantages of having higher removal rate of ammonia nitrogen and lower energy consumption, realizing the up-to-standard discharge of leachate concentrated solution, ensuring sound operation of the whole system, greatly lowering the oxidation-reduction potential in the anaerobic reaction, and improving the anaerobic treatment effect, thereby making the landfill leachate fully meet the first-level discharge standard of the Integrated Wastewater Discharge Standard.
Owner:浙江永峰环保科技股份有限公司 +1
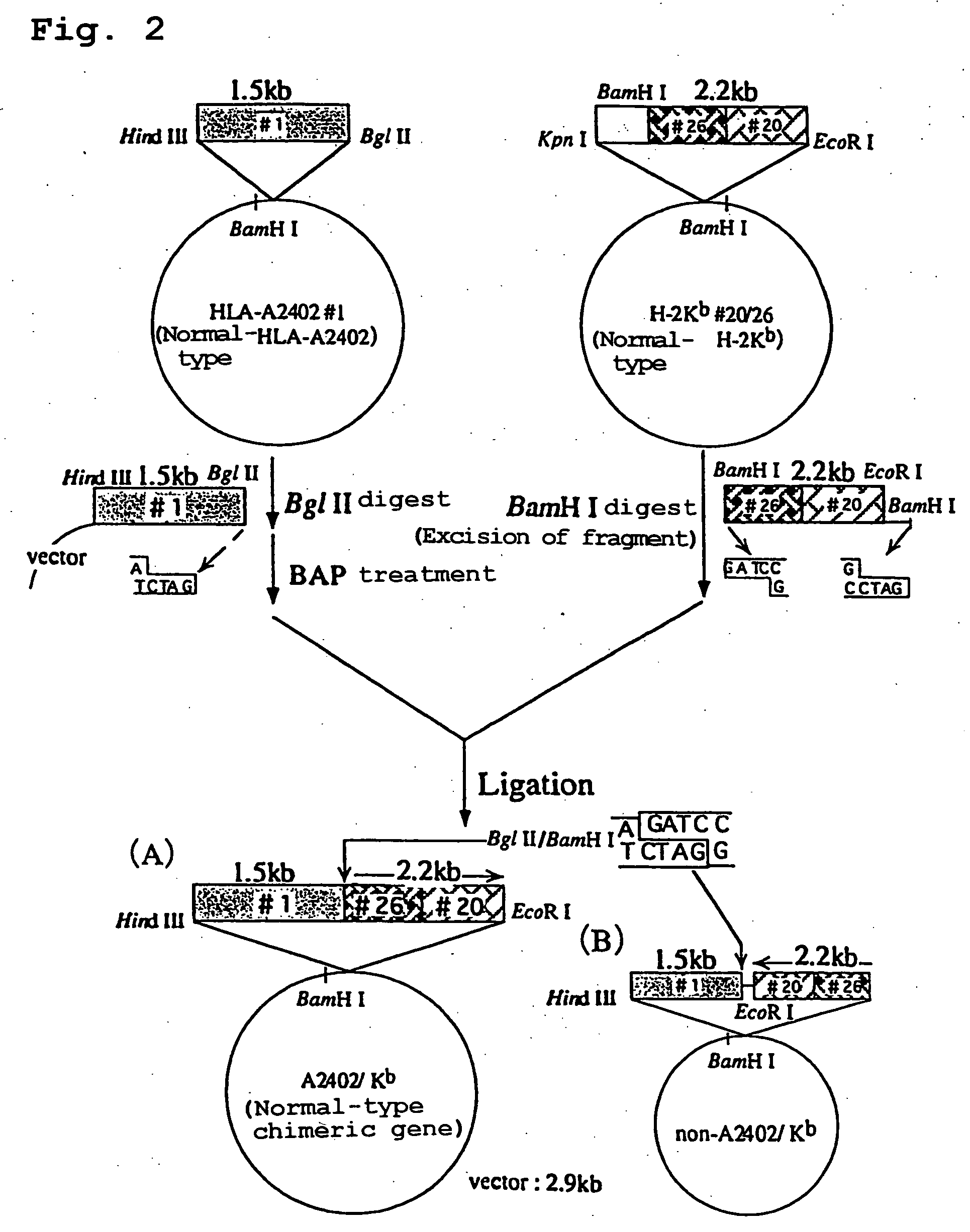
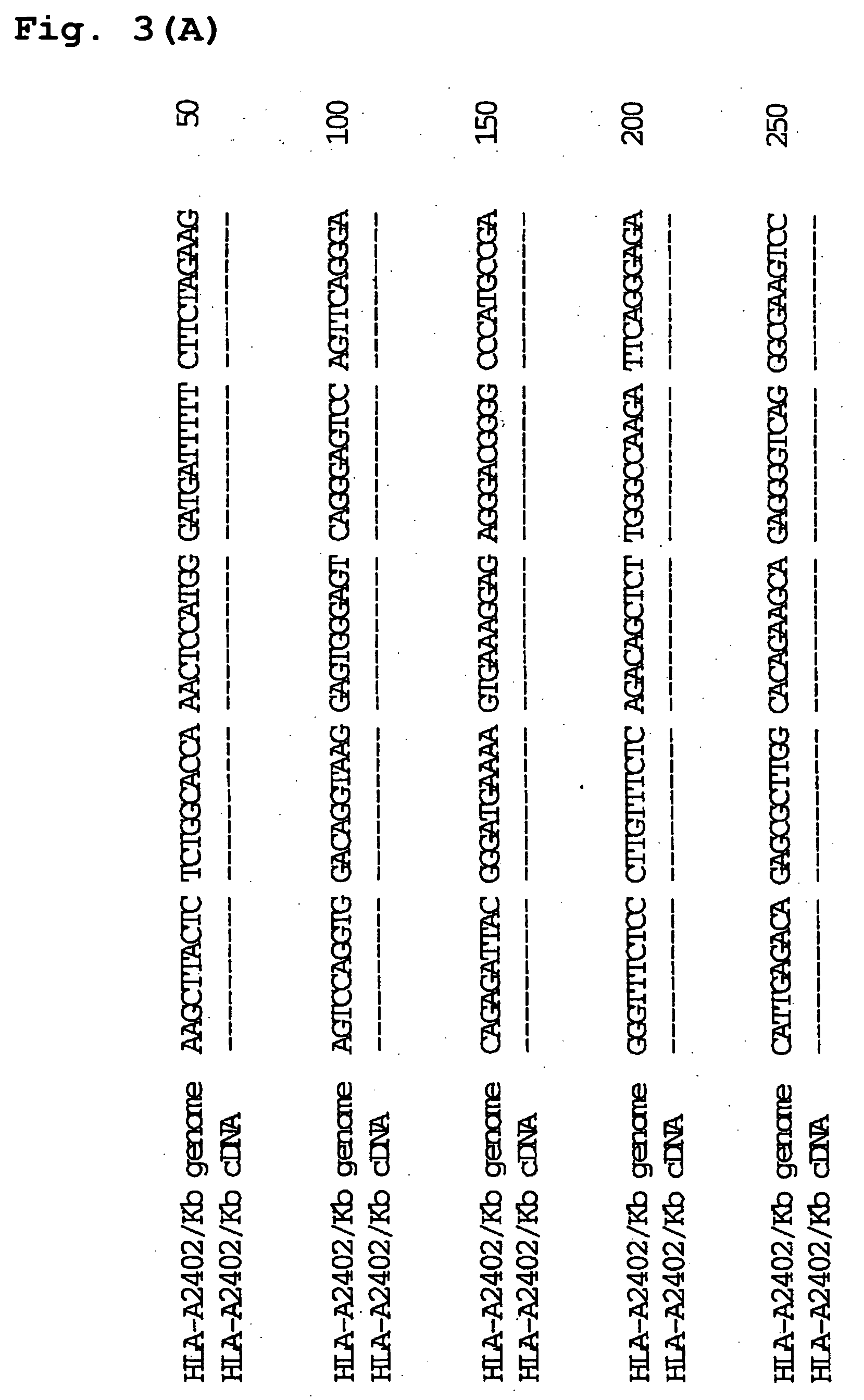
Transgenic animal expressing hla-a24 and utilization thereof
InactiveUS20050050580A1Effectively useIncreased toxicityAntibody mimetics/scaffoldsAntiviralsTumour-associated antigenScreening method
The present invention relates to a non-human transgenic mammal which has had an HLA-A24 gene introduced and in which CTLs are induced when stimulated by an HLA-A24-binding antigen, a method of screening therapeutic or preventive agents for tumors or virus infections comprising administering a test substance to said transgenic non-human mammal and assaying and evaluating whether CTLs specific for the test substance are induced, an HLA-A24-binding tumor antigen peptide of PSA origin selected by said screening method, a chimera DNA (DNA construct) useful in the generation of said non-human transgenic mammal, a host cell transformed by said chimera gene and use thereof, and the like.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Chimeric, human and humanized anti-CSAP monoclonal antibodies
InactiveUS20050169926A1Increased toxicityAntibody mimetics/scaffoldsMilk immunoglobulinsCancerMonoclonal antibody
Owner:IMMUNOMEDICS INC
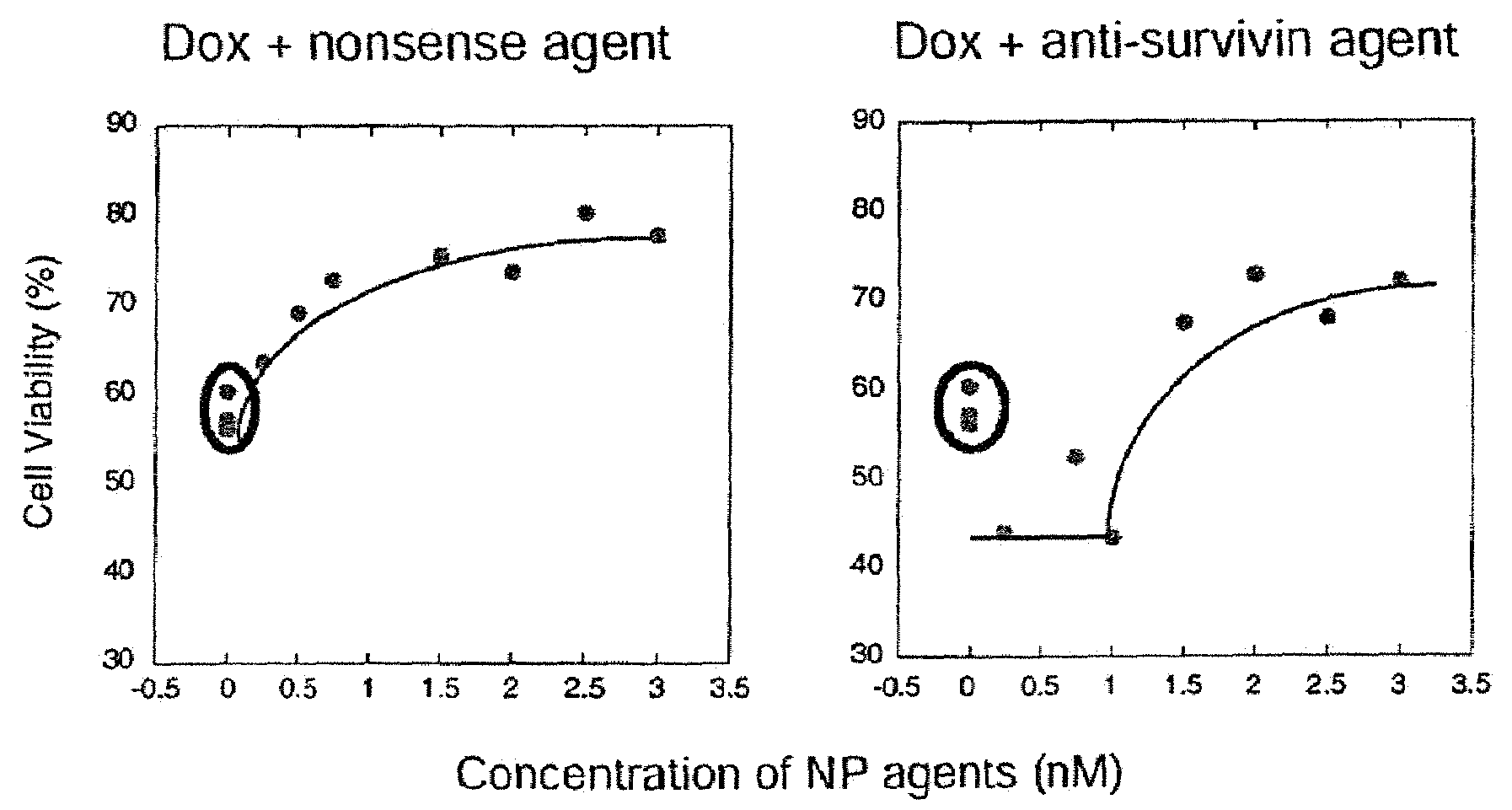
Pharmaceutical compositions comprising antisense oligonucleotides and methods of using same
ActiveUS20110281787A1Improve efficiencyIncreased toxicityAntibacterial agentsOrganic active ingredientsGenetic disorderCharged polymers
The invention provides compositions and associated methods for the antisense treatment of genetic disorders, infections and various other medical conditions. In particular, embodiments of the present invention are directed to pharmaceutical compositions comprising a combination of an antisense oligonucleotide compound conjugated with a positively charged polymer (“ON-PCP”) and a negatively charged polymer. Pharmaceutical compositions in accordance with the present invention have demonstrated improved antisense efficiency and reductions in cell toxicity compared to compositions that contain an oligonucleotide compound conjugated with a positively charged polymer.
Owner:CHARLOTTE MECKLENBURG HOSPITAL AUTHORITY
Pesticide compositions containing oxalic acid
InactiveUS6992045B2Easy to controlIncreased toxicityBiocideDead animal preservationOxalateHydroxyproline
Pesticidal concentrate and spray compositions are described which exhibit enhanced efficacy due to the addition thereto of a compound which increases cell membrane permeability, suppresses oxidative burst, or increases expression of hydroxyproline-rich glycoproteins.
Owner:MONSANTO TECH LLC
Combined use of ecteinascidin-743 and platinum antineoplastic compounds
InactiveUS20070128201A1Reduce resistanceOvercome resistanceHeavy metal active ingredientsAntibody ingredientsToxicityHuman cancer
ET-743 can be used to mitigate resistance to and potentiate the cytotoxic effects of a platinum coordination complex anti-neoplastic agent in a human cancer patient.
Owner:PHARMA MAR U
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com