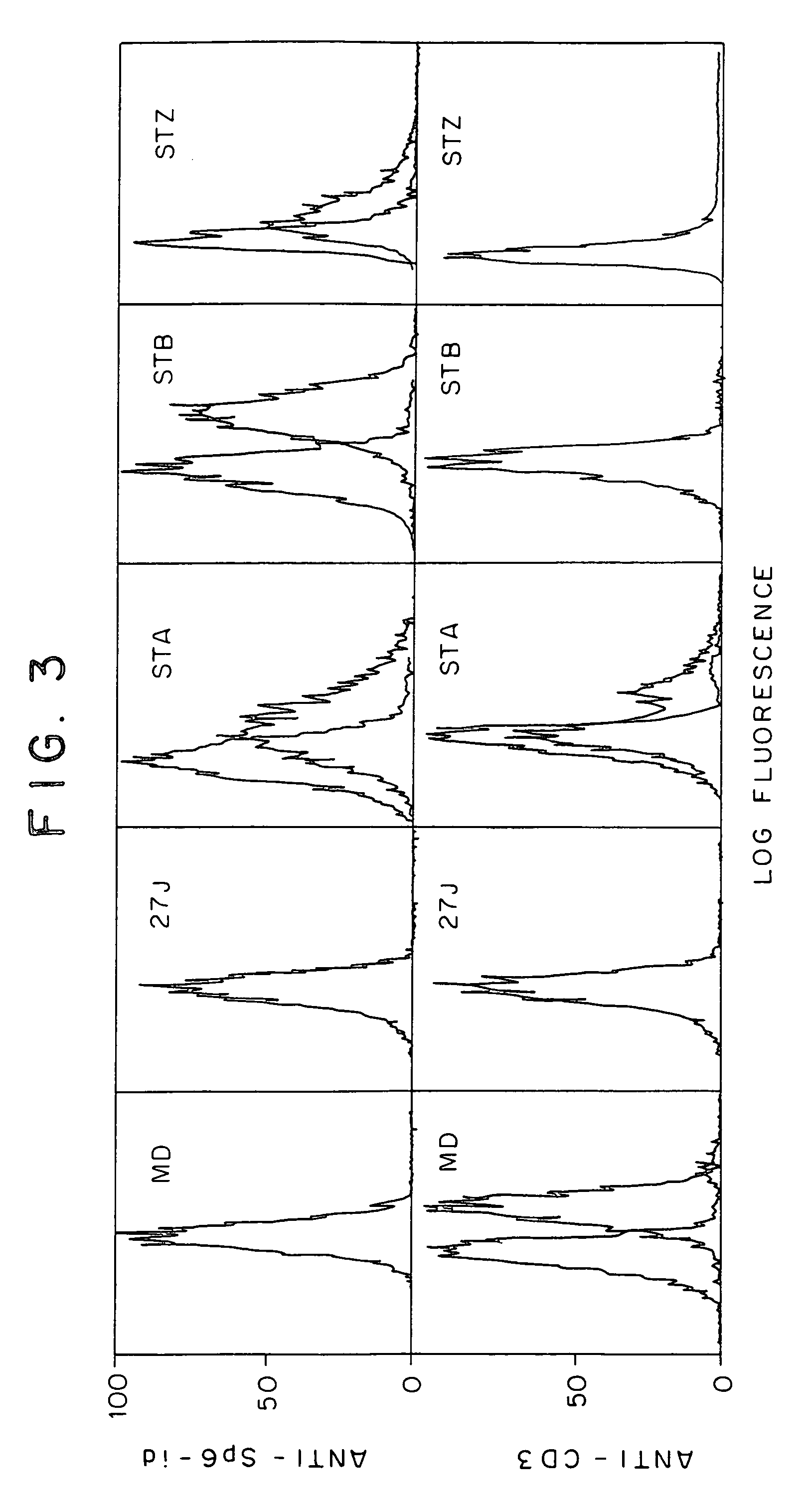
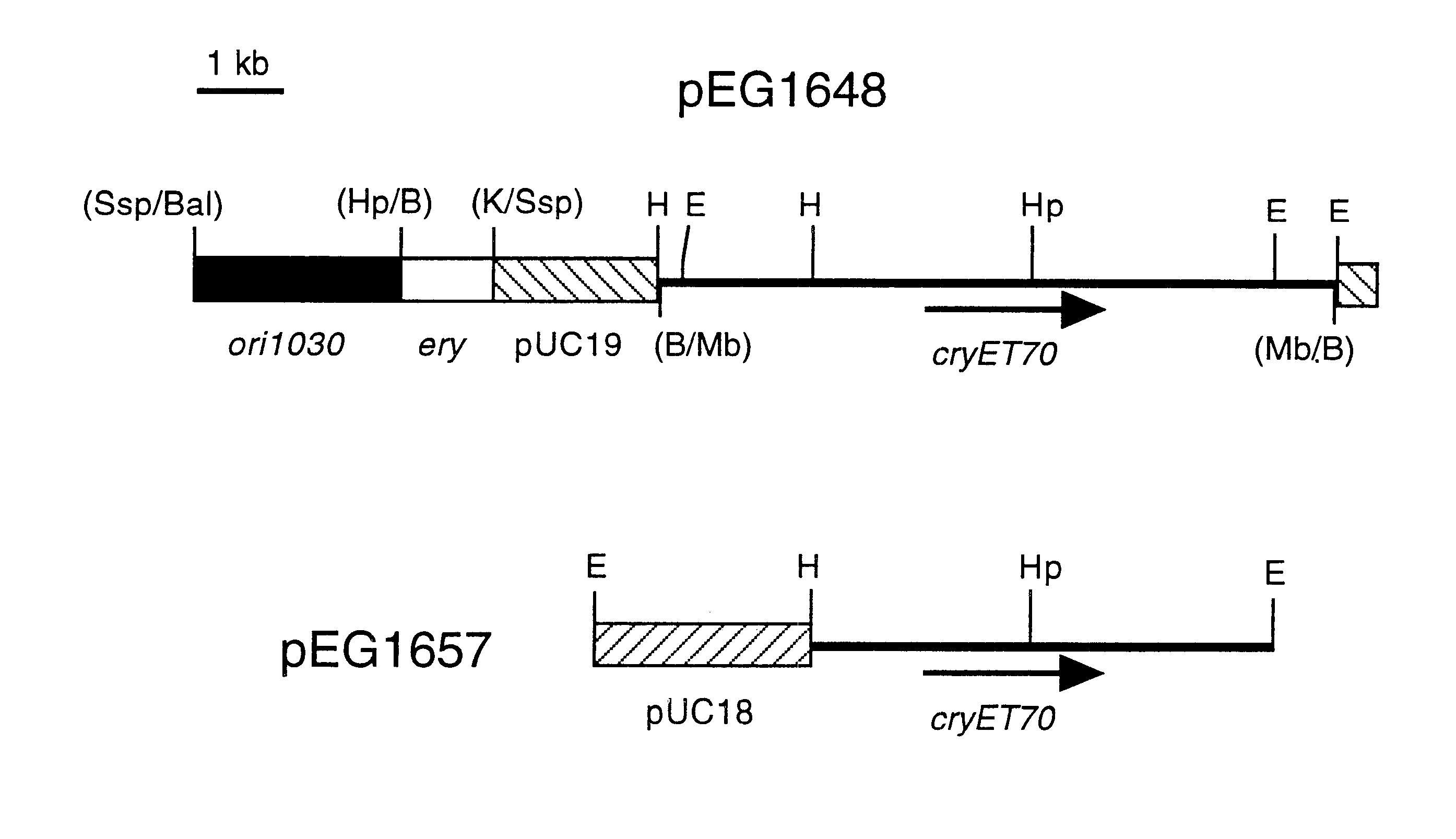
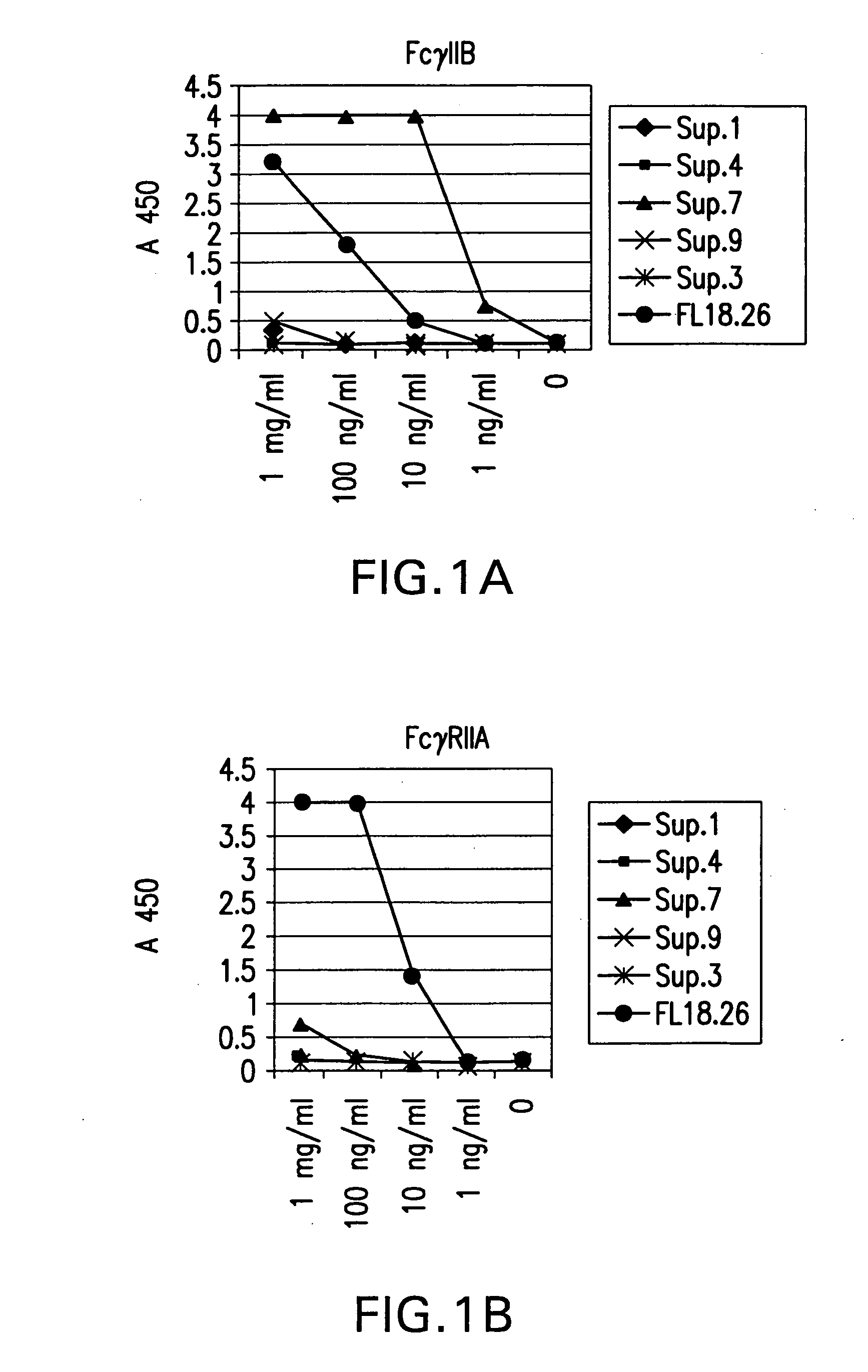
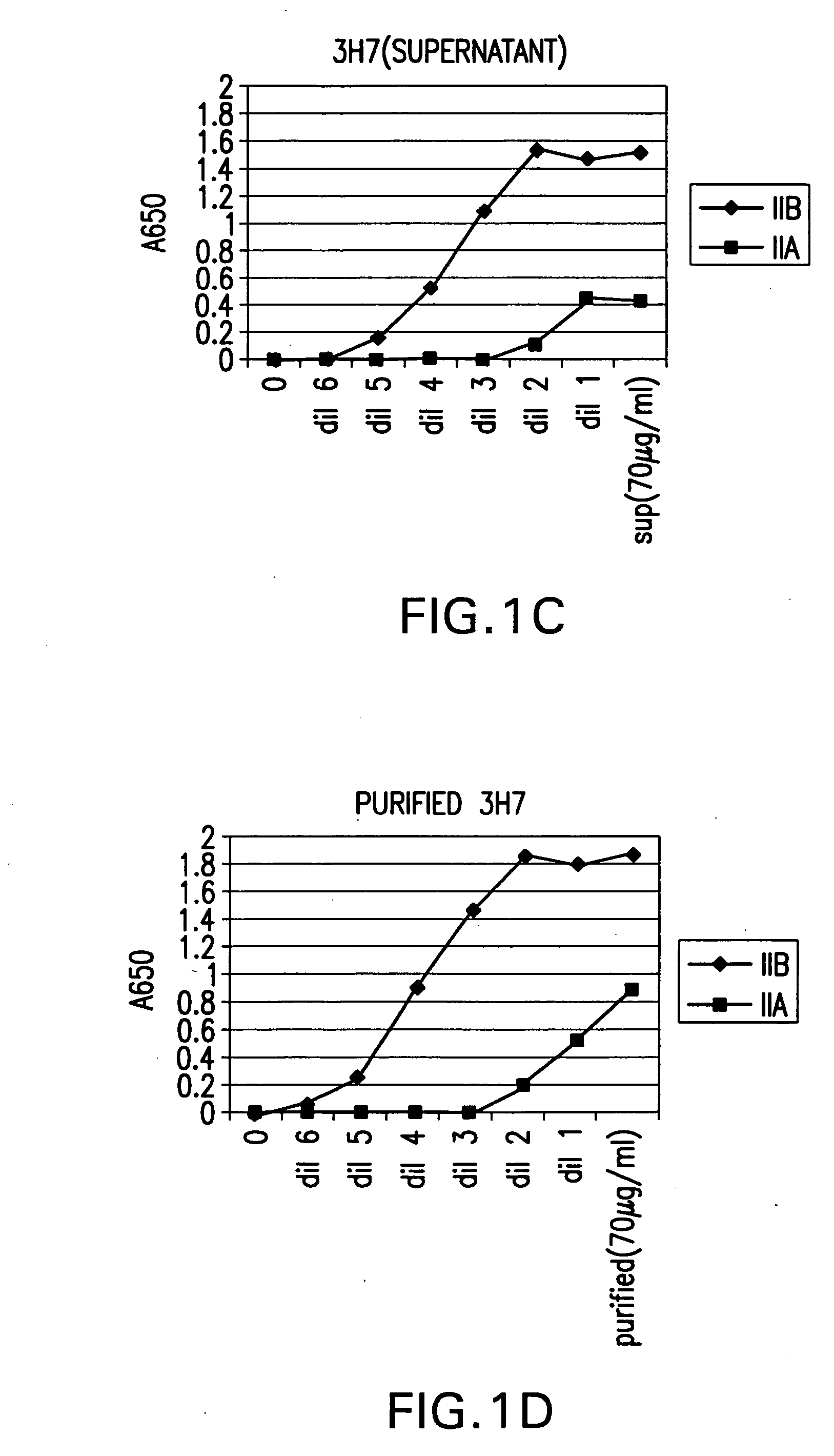
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3884 results about "Specific antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
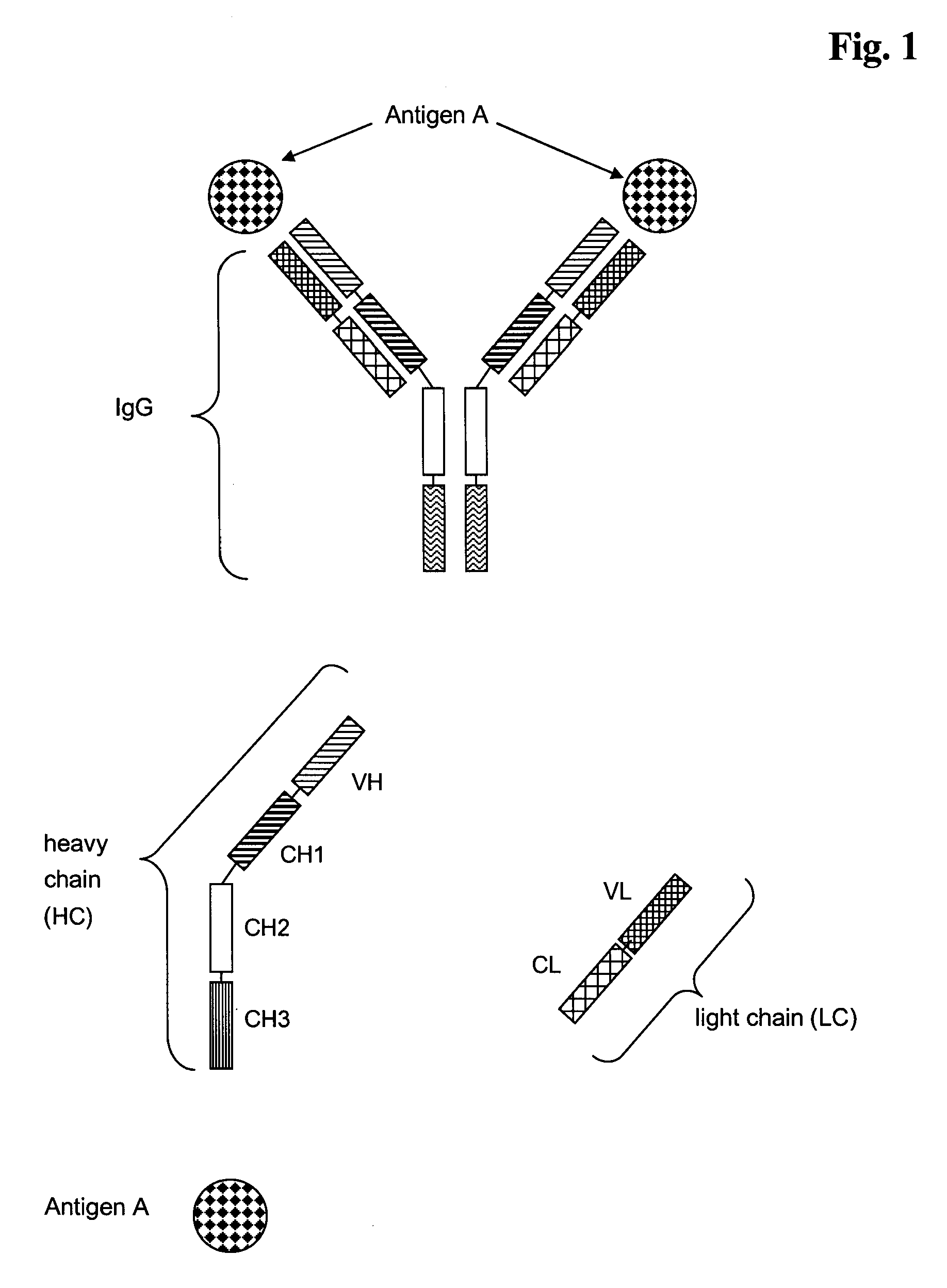
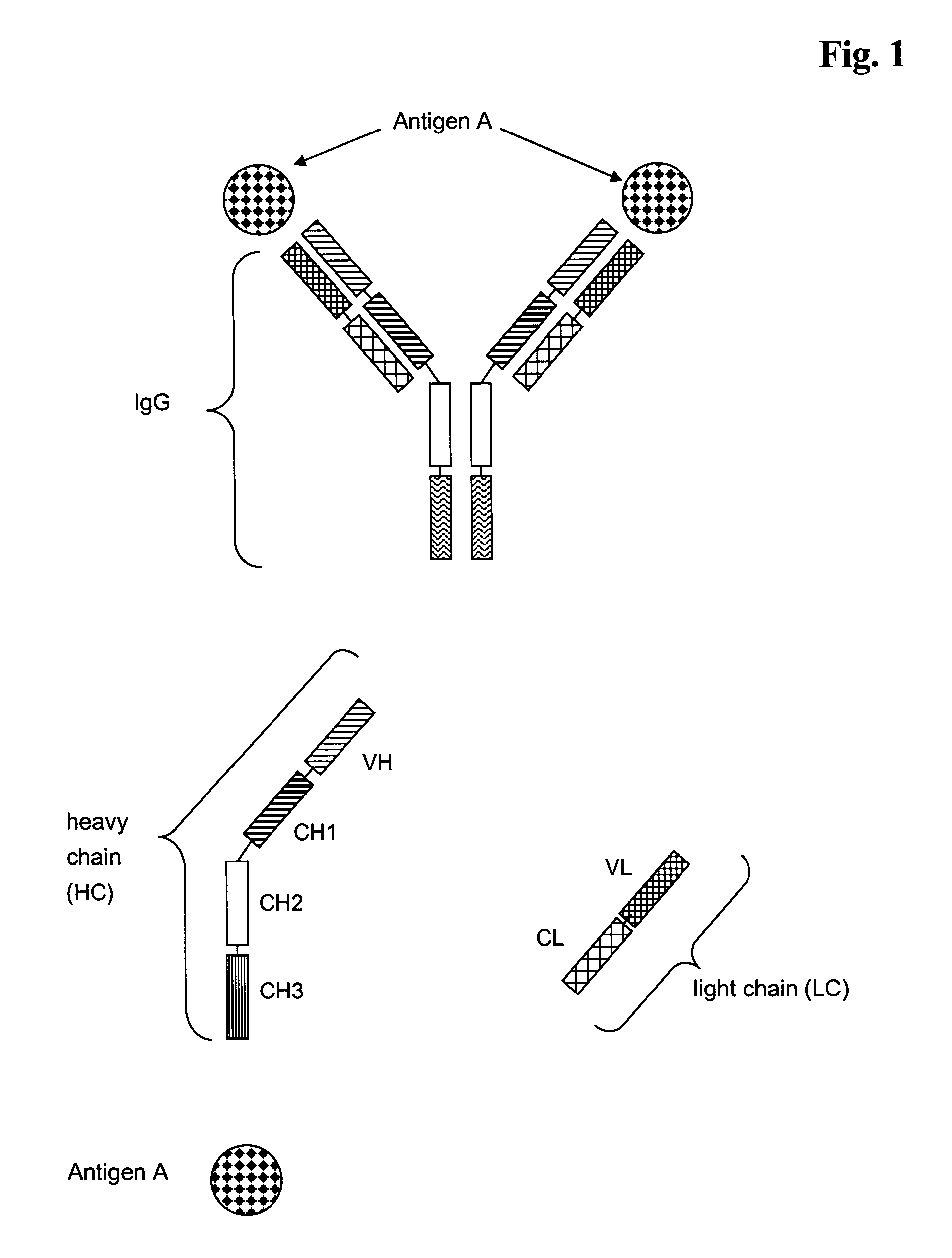
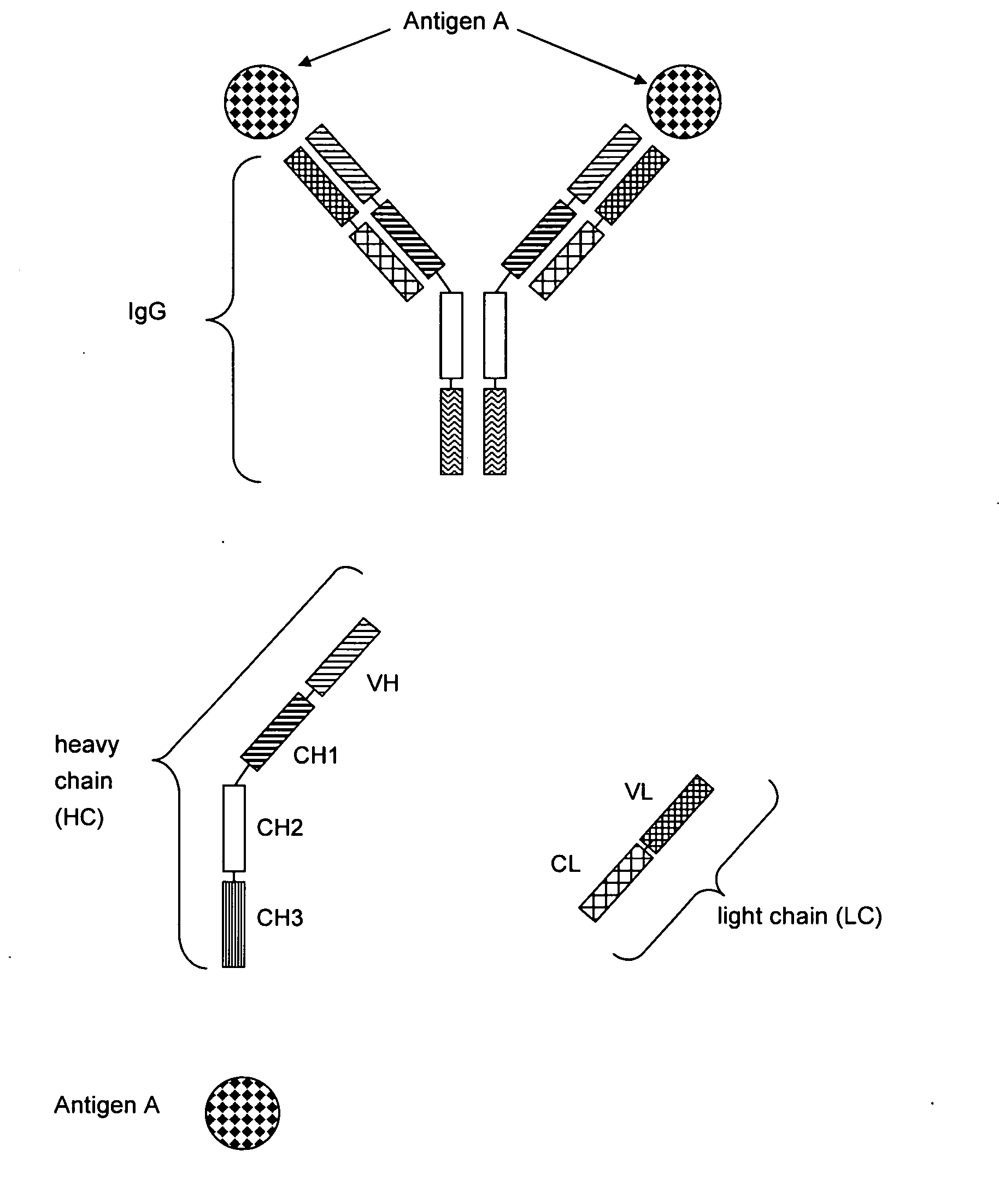
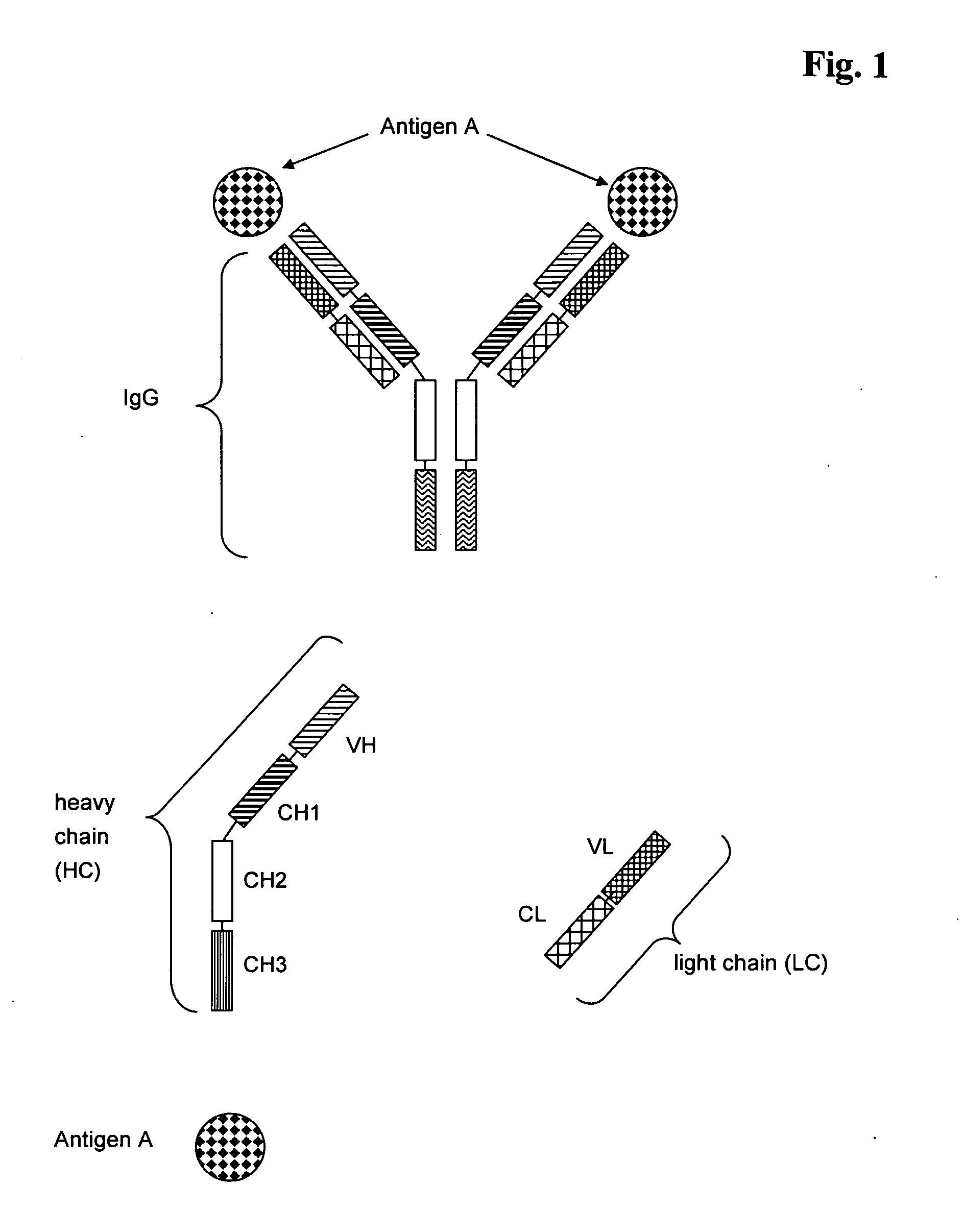
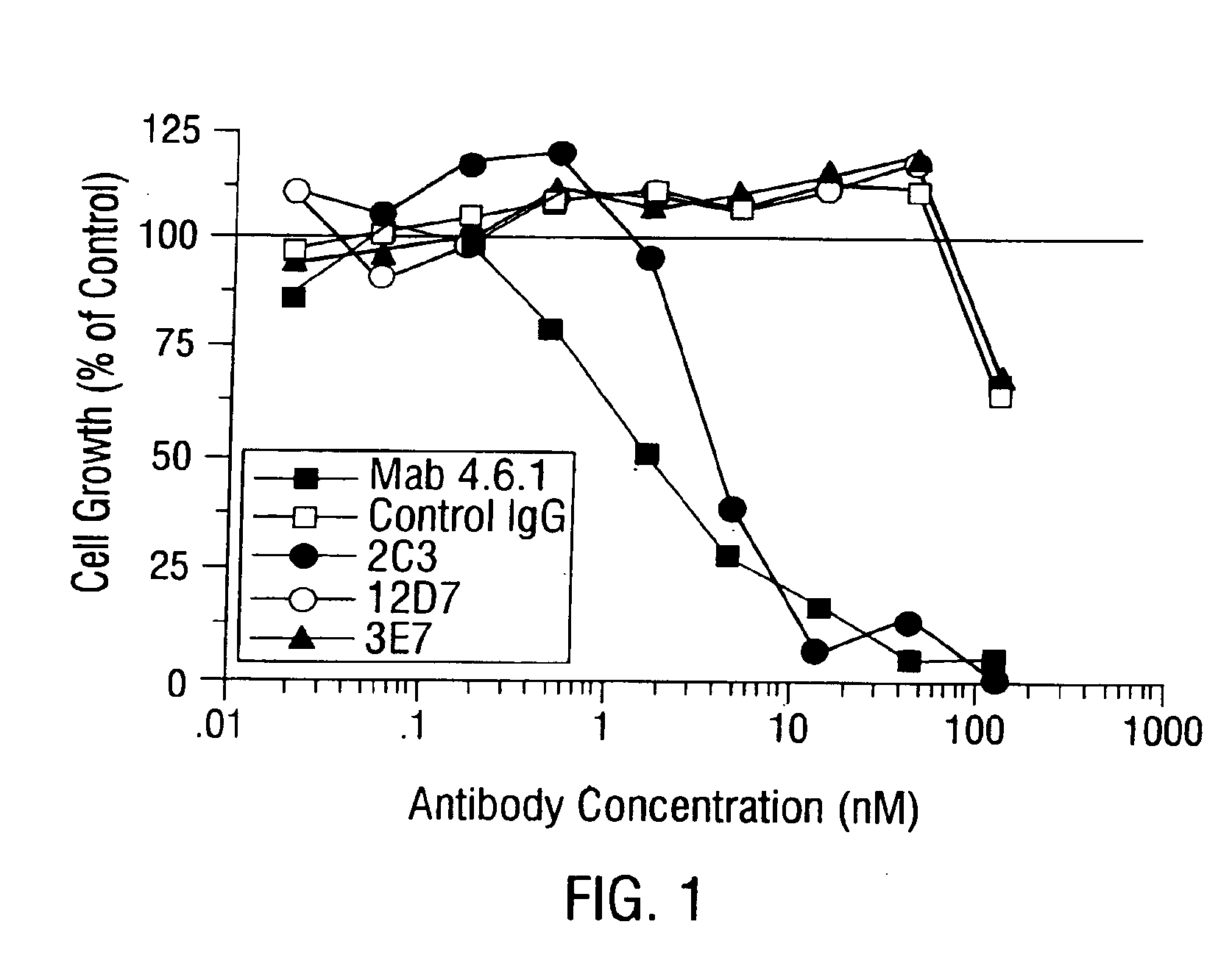
Antibodies recognize specific antigens by identifying certain areas on the surface of the antigen known as antigenic determinants. Once the specific antigenic determinant is recognized, the antibody will bind to the determinant. The antigen is tagged as an intruder and labeled for destruction by other immune cells.
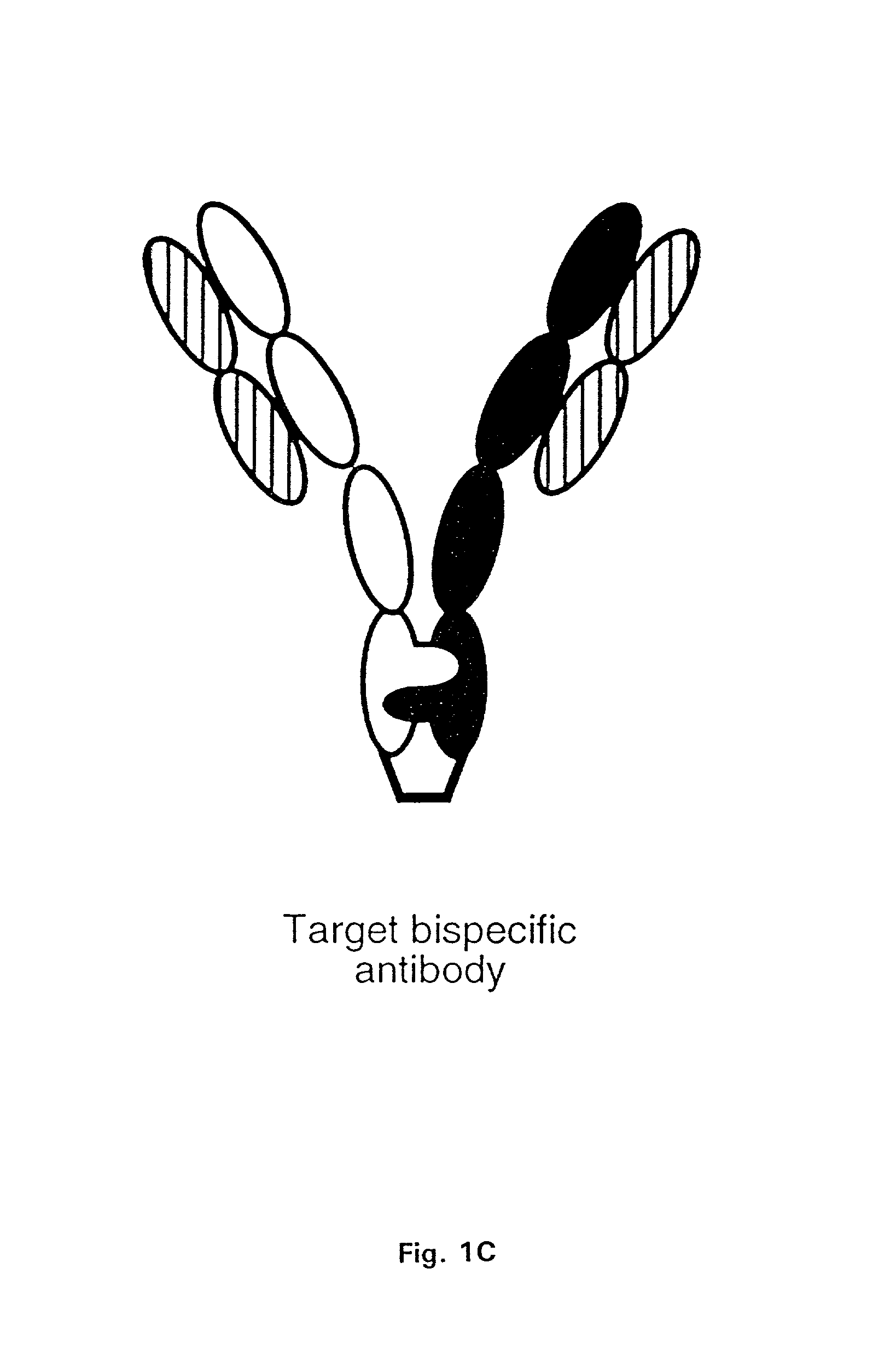
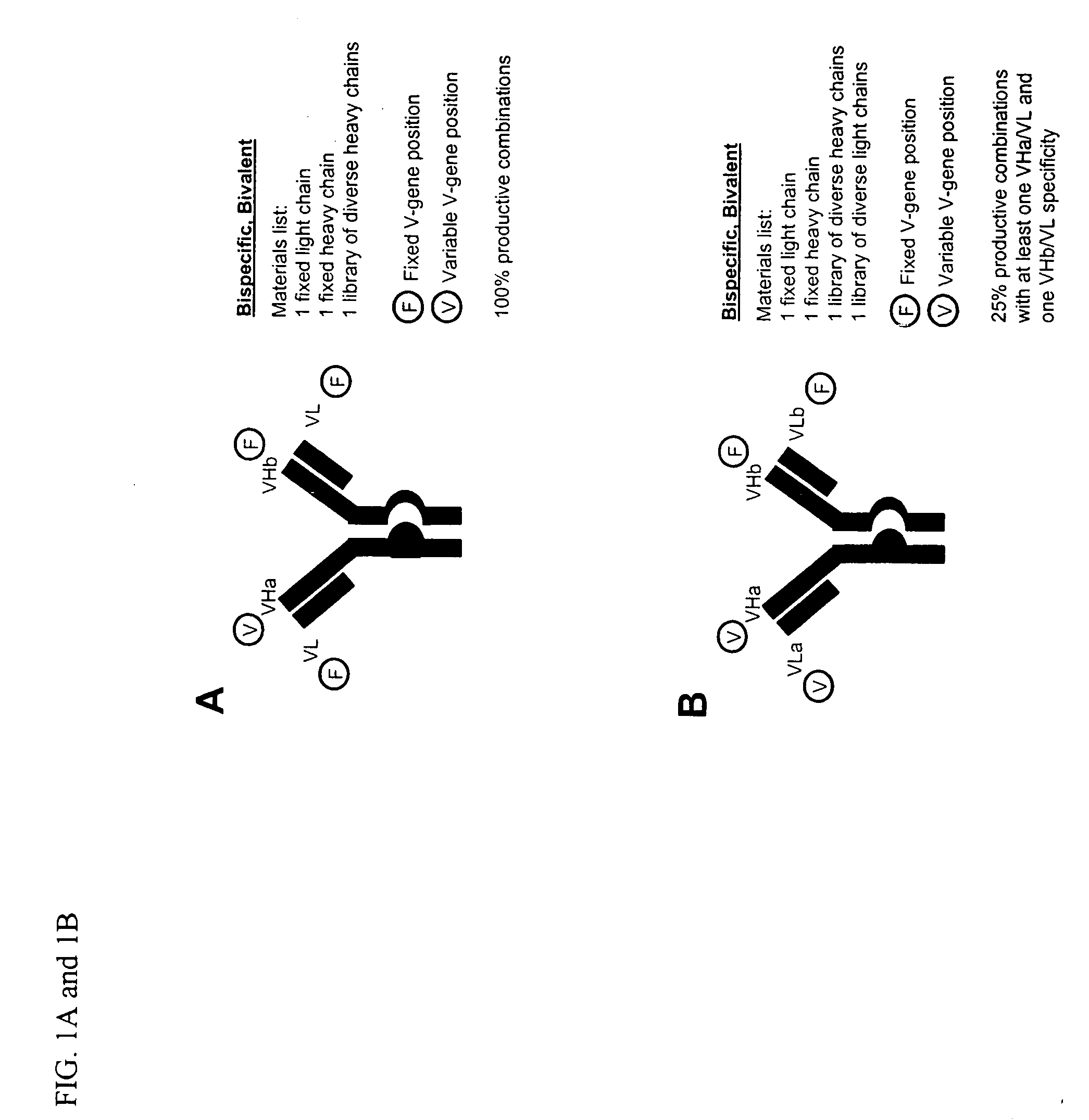
Multispecific antibodies
ActiveUS20080069820A1High binding affinityHigh affinityImmunoglobulins against growth factorsAntibody ingredientsSpecific antibody
Owner:GENENTECH INC
Method for making multispecific antibodies having heteromultimeric and common components
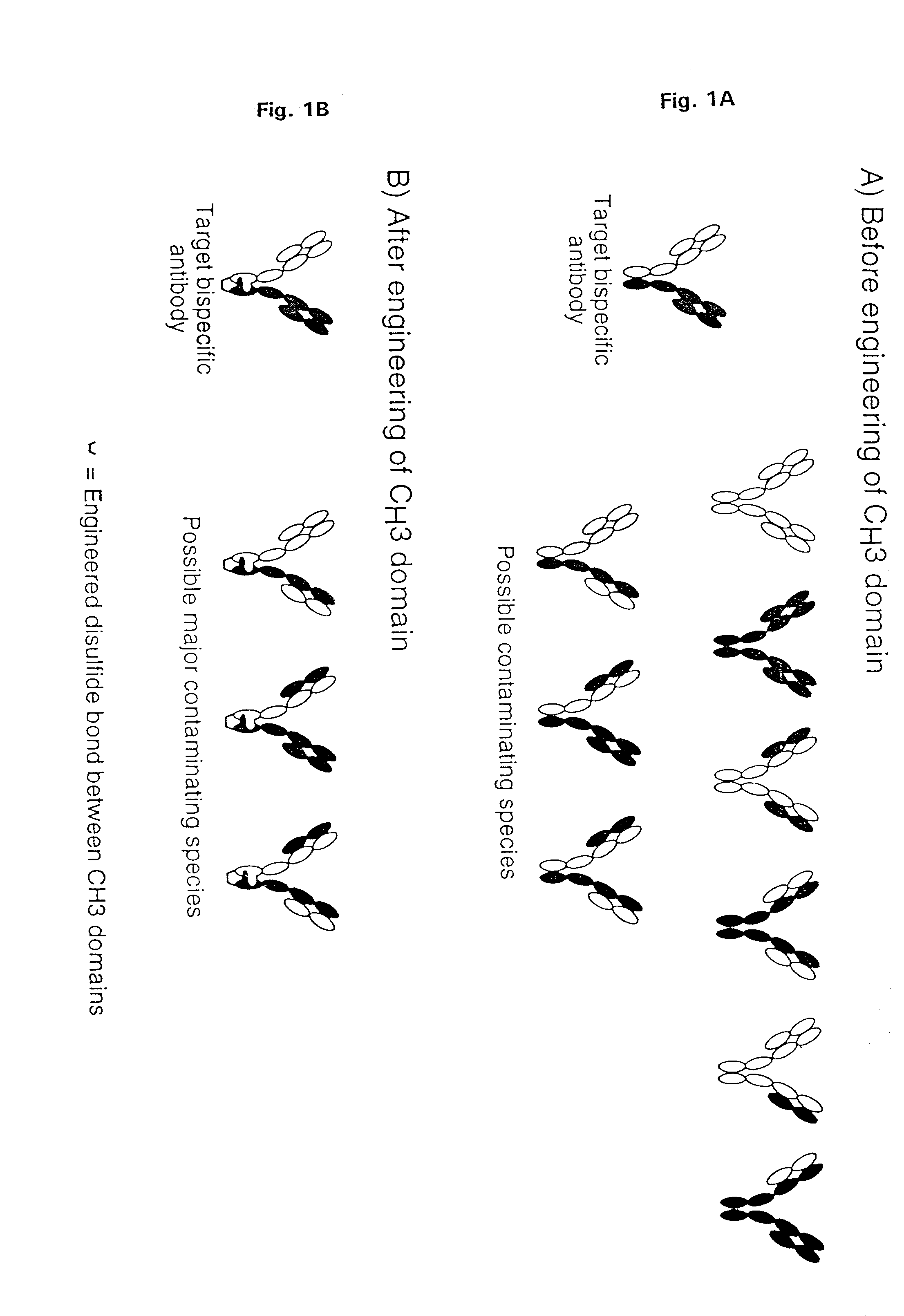
InactiveUS7183076B2Increased formationIncrease productionAnimal cellsAntibody mimetics/scaffoldsSpecific immunityBispecific antibody
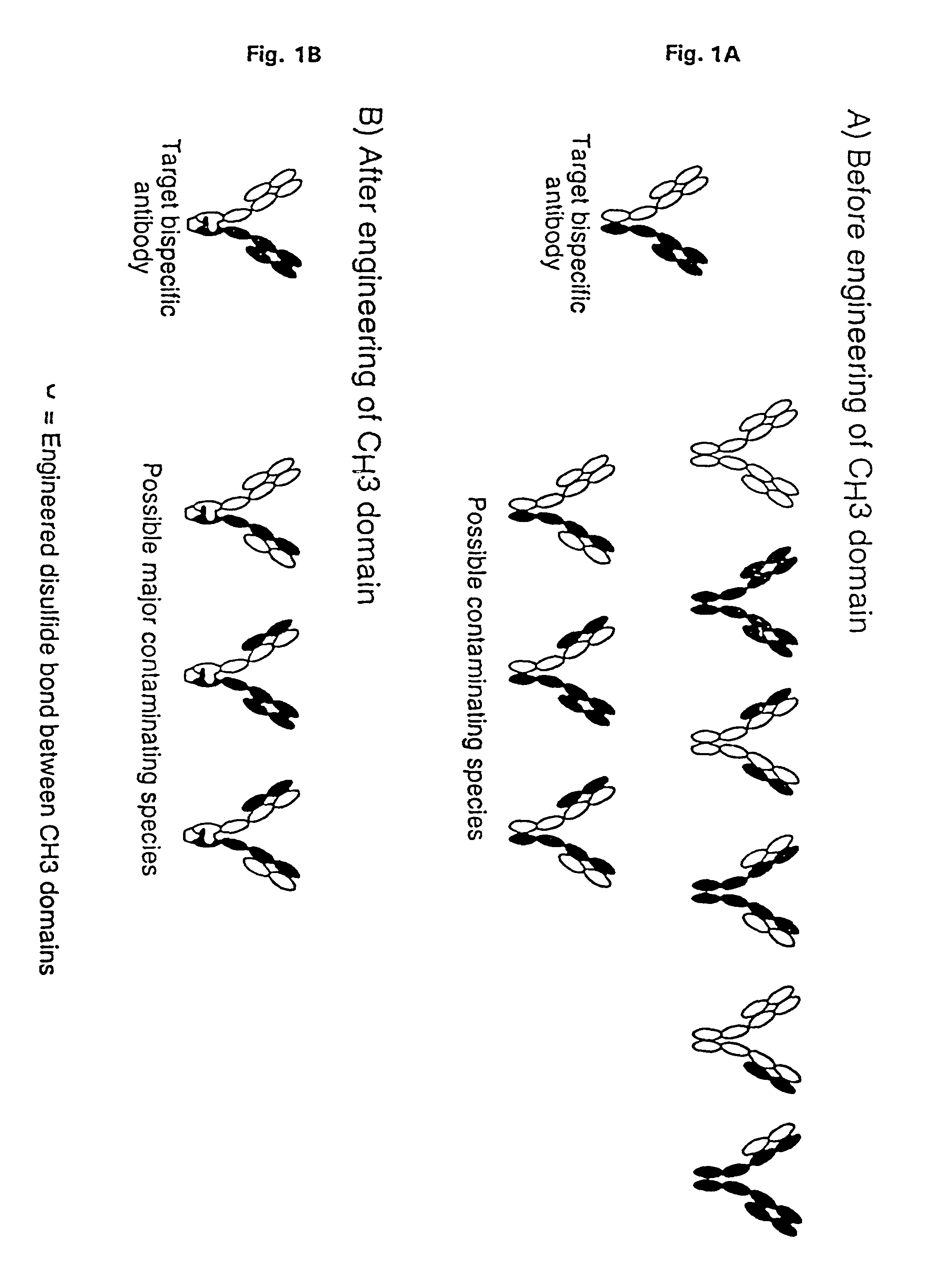
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method provides a multispecific antibody having a common light chain associated with each heteromeric polypeptide having an antibody binding domain. Additionally the method futher involves introducing into the multispecific antibody a specific and complementary interaction at the interface of a first polypeptide and the interface of a second polypeptide, so as to promote heteromultimer formation and hinder homomultimer formation; and / or a free thiol-containing residue at the interface of a first polypeptide and a corresponding free thiol-containing residue in the interface of a second polypeptide, such that a non-naturally occurring disulfide bond is formed between the first and second polypeptide. The method allows for the enhanced formation of the desired heteromultimer relative to undesired heteromultimers and homomultimers.
Owner:GENENTECH INC
Chimeric receptor genes and cells transformed therewith
ActiveUS7741465B1Limit acquisitionMicroorganismsGenetic material ingredientsAntibody typesLymphocyte
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF +1
Polypeptide compositions toxic to diabrotic insects, and methods of use
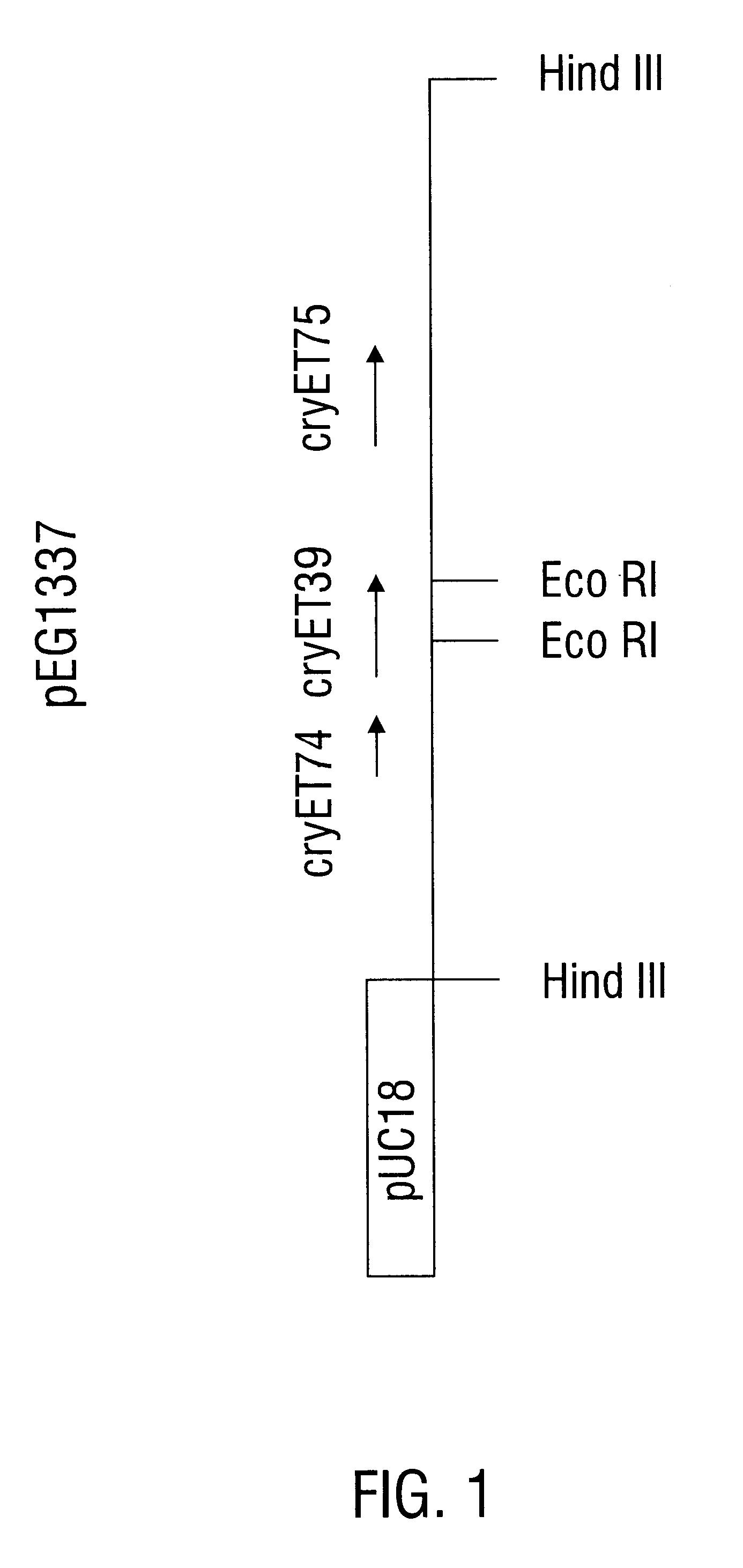
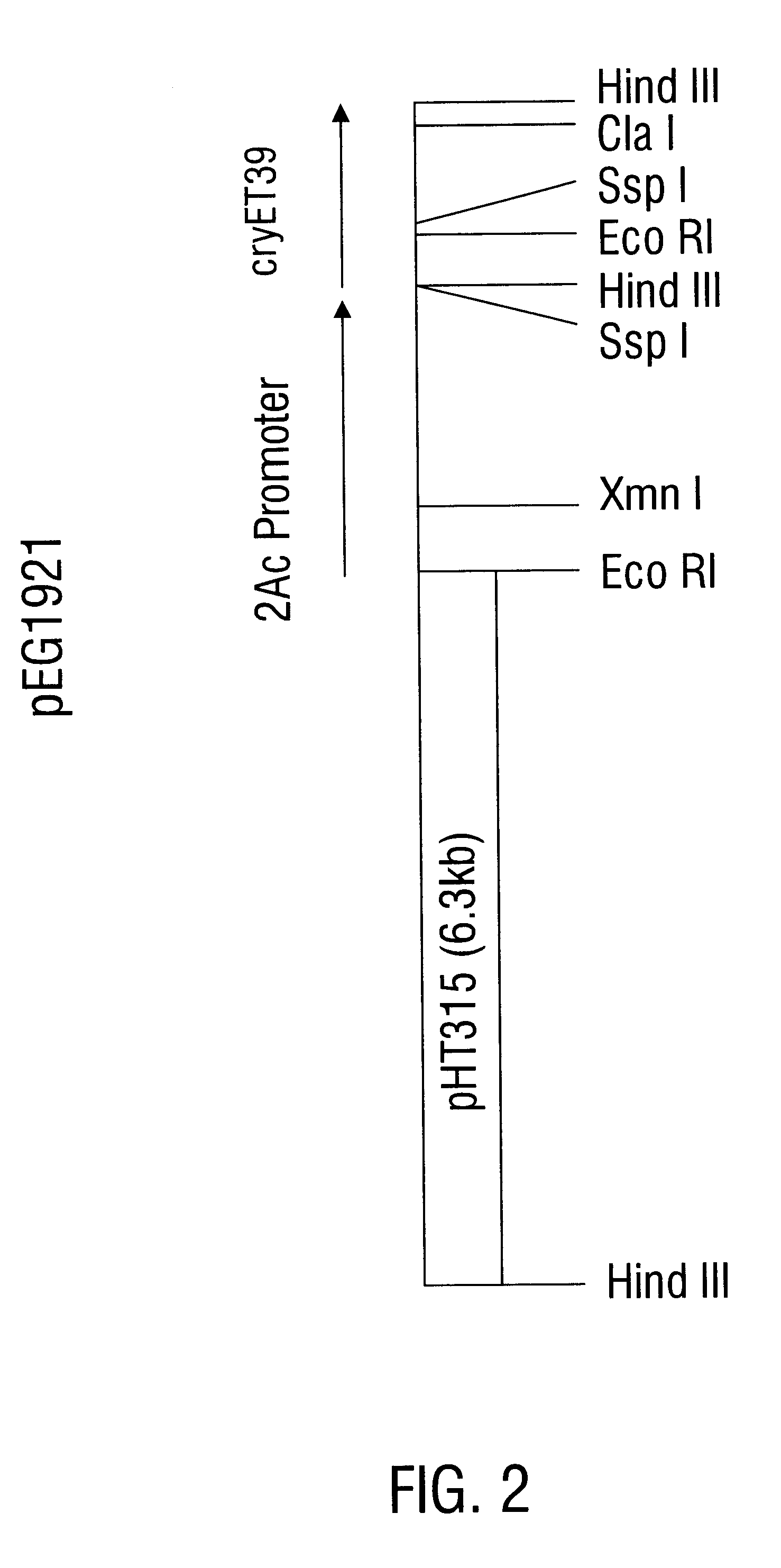
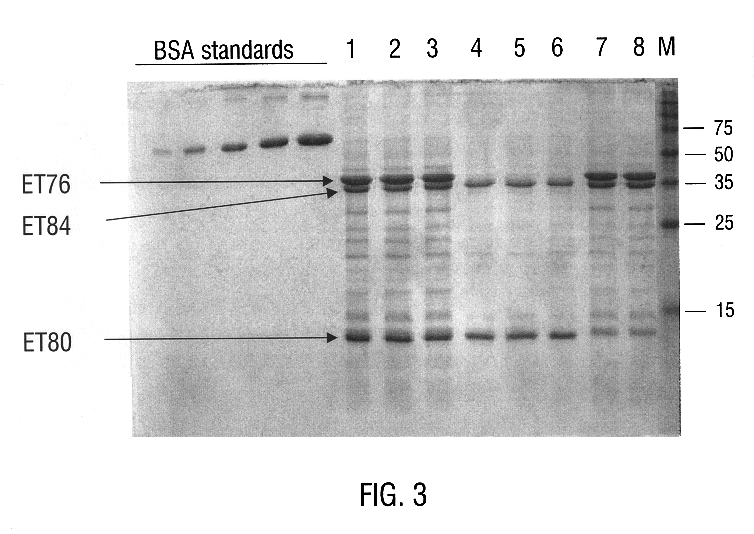
InactiveUS6468523B1Easy to storeInhibit microbial growthBiocidePeptide/protein ingredientsDelta endotoxinPolynucleotide
Disclosed is a novel Lepidopteran- and Coleopteran-active delta-endotoxin polypeptide, and compositions comprising the polypeptide, peptide fragments thereof, and antibodies specific therefor. Also disclosed are vectors, transformed host cells, and transgenic plants that comprise nucleic acid segments encoding the polypeptide. Also disclosed are methods of identifying related polypeptides and polynucleotides, methods of making and using transgenic cells comprising the novel sequences of the invention, as well as methods for controlling an insect population, such as the Western Corn Rootworm and Colorado potato beetle, and for conferring to a plant population resistance to the target insect species.
Owner:MONSANTO TECH LLC
Mammalian cell surface antigens; related reagents
InactiveUS7025962B1Abnormal immune responseFacilitated DiffusionPeptide/protein ingredientsAntibody mimetics/scaffoldsMammalT cell
Owner:MERCK SHARP & DOHME CORP
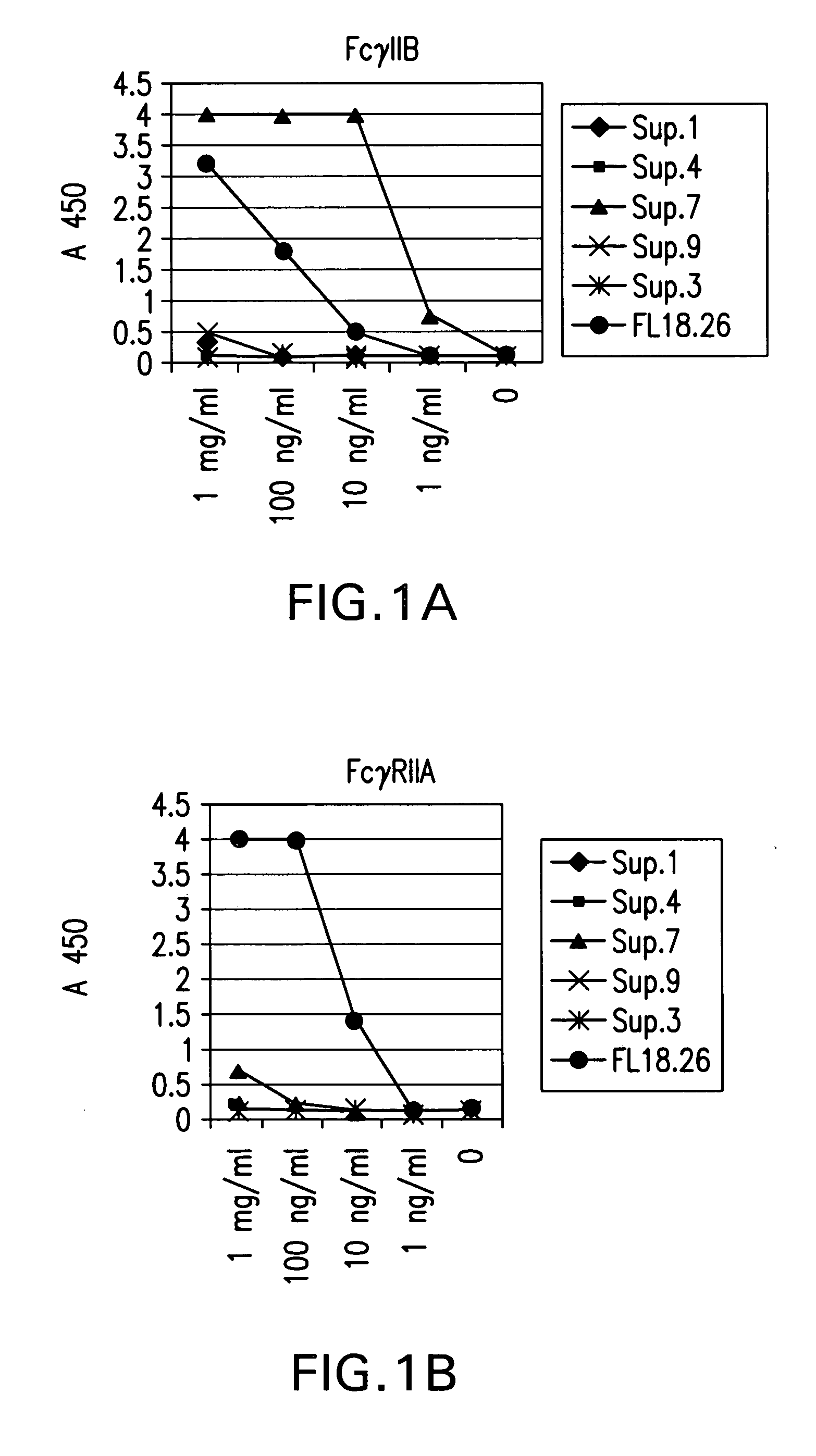
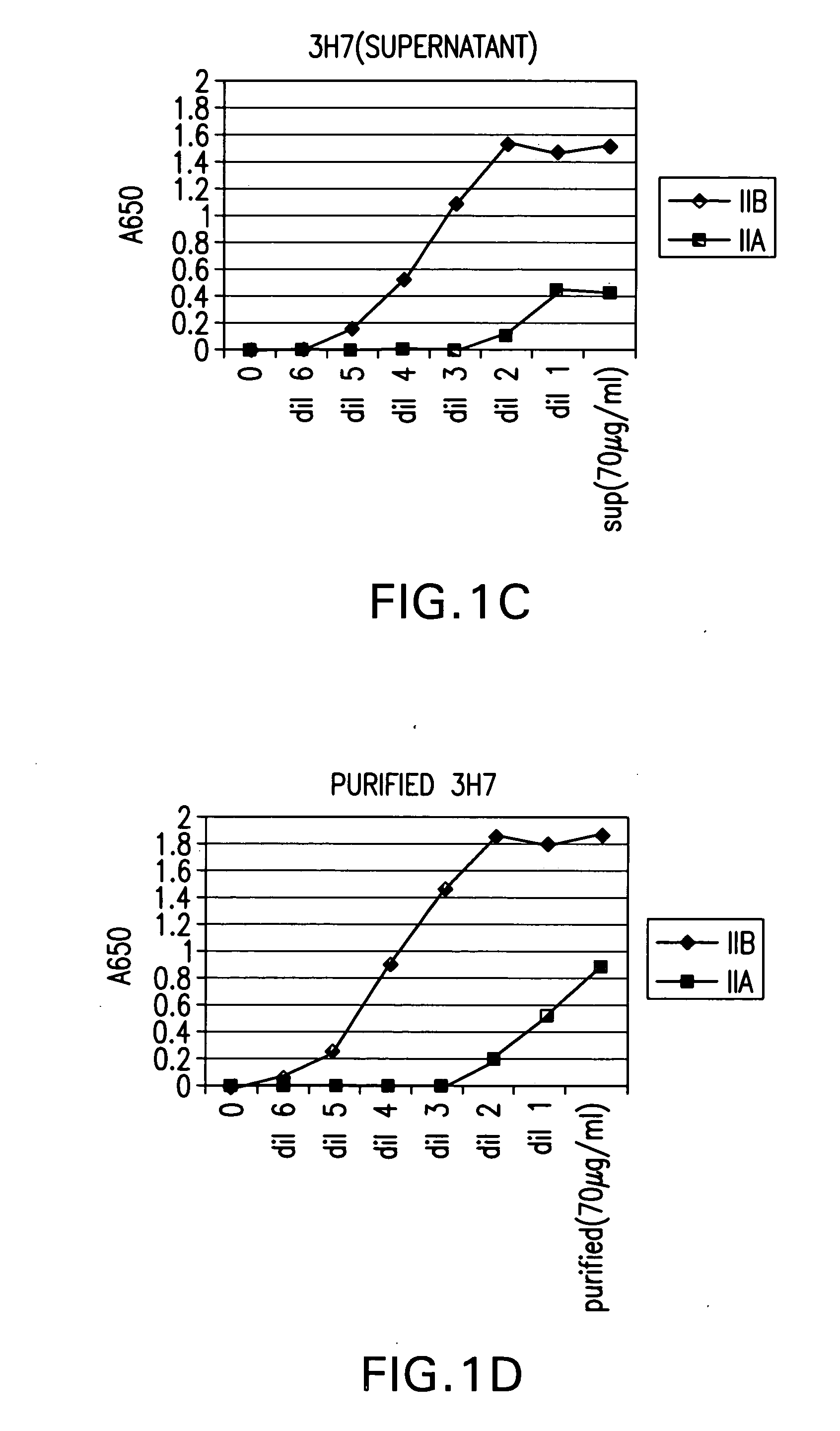
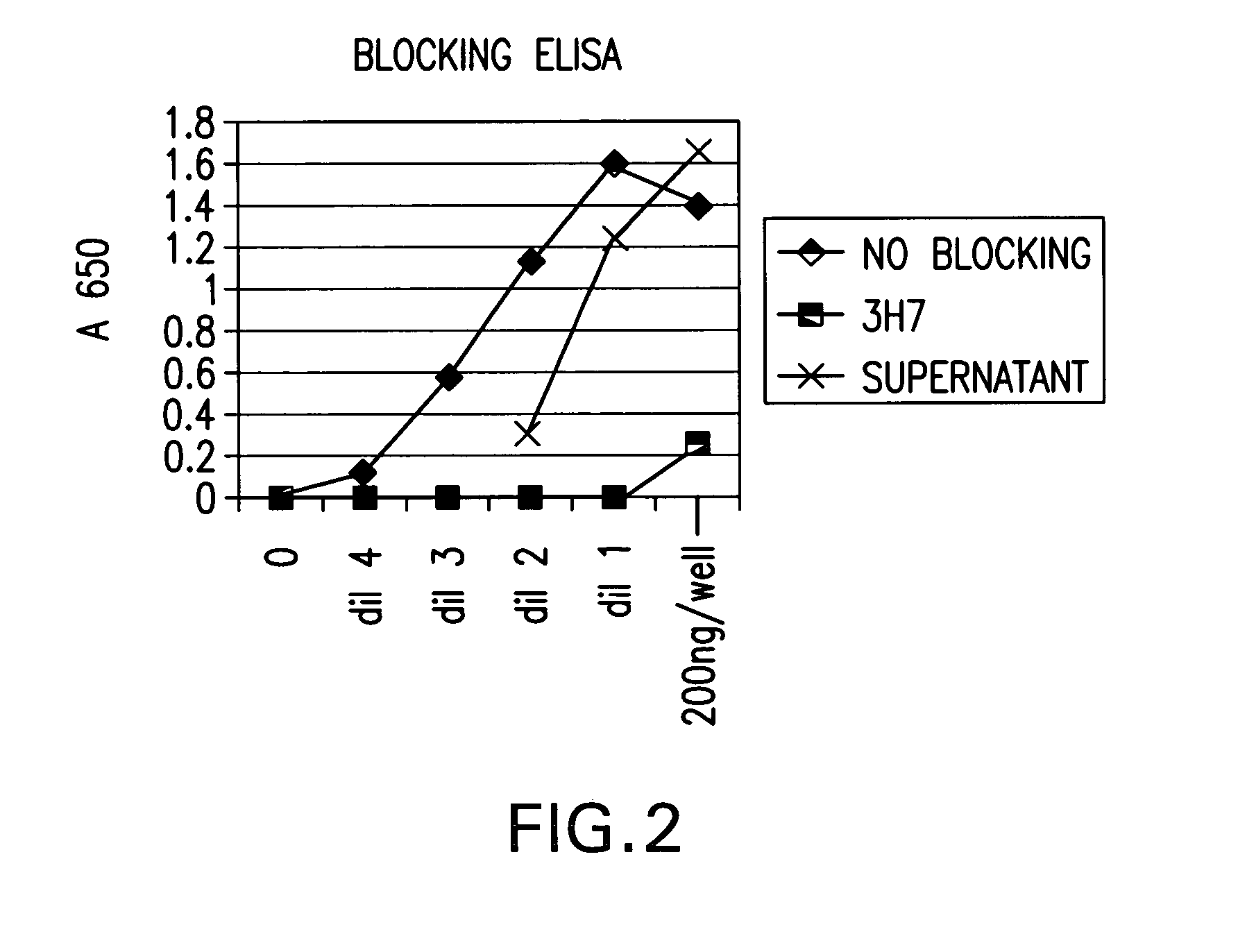
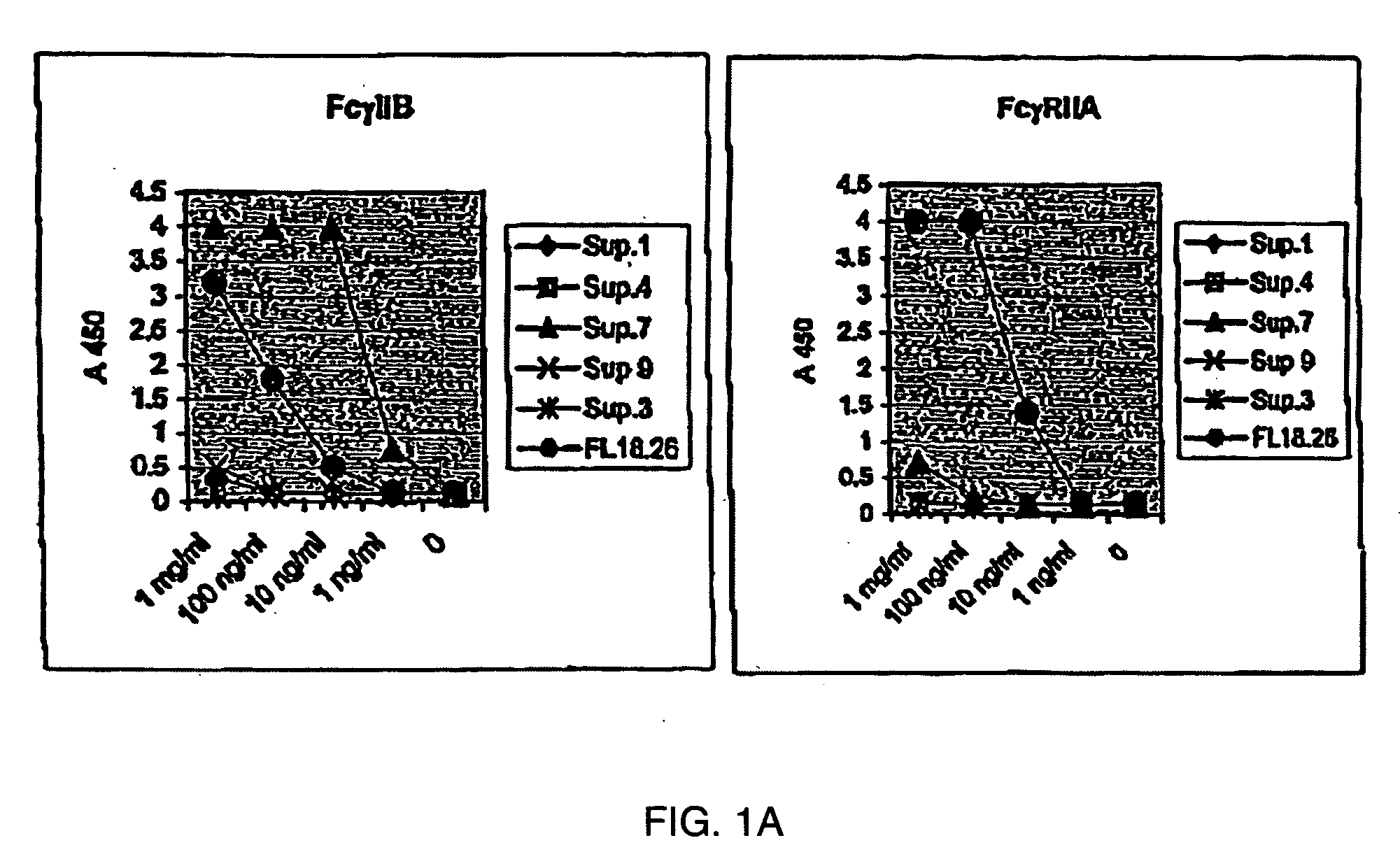
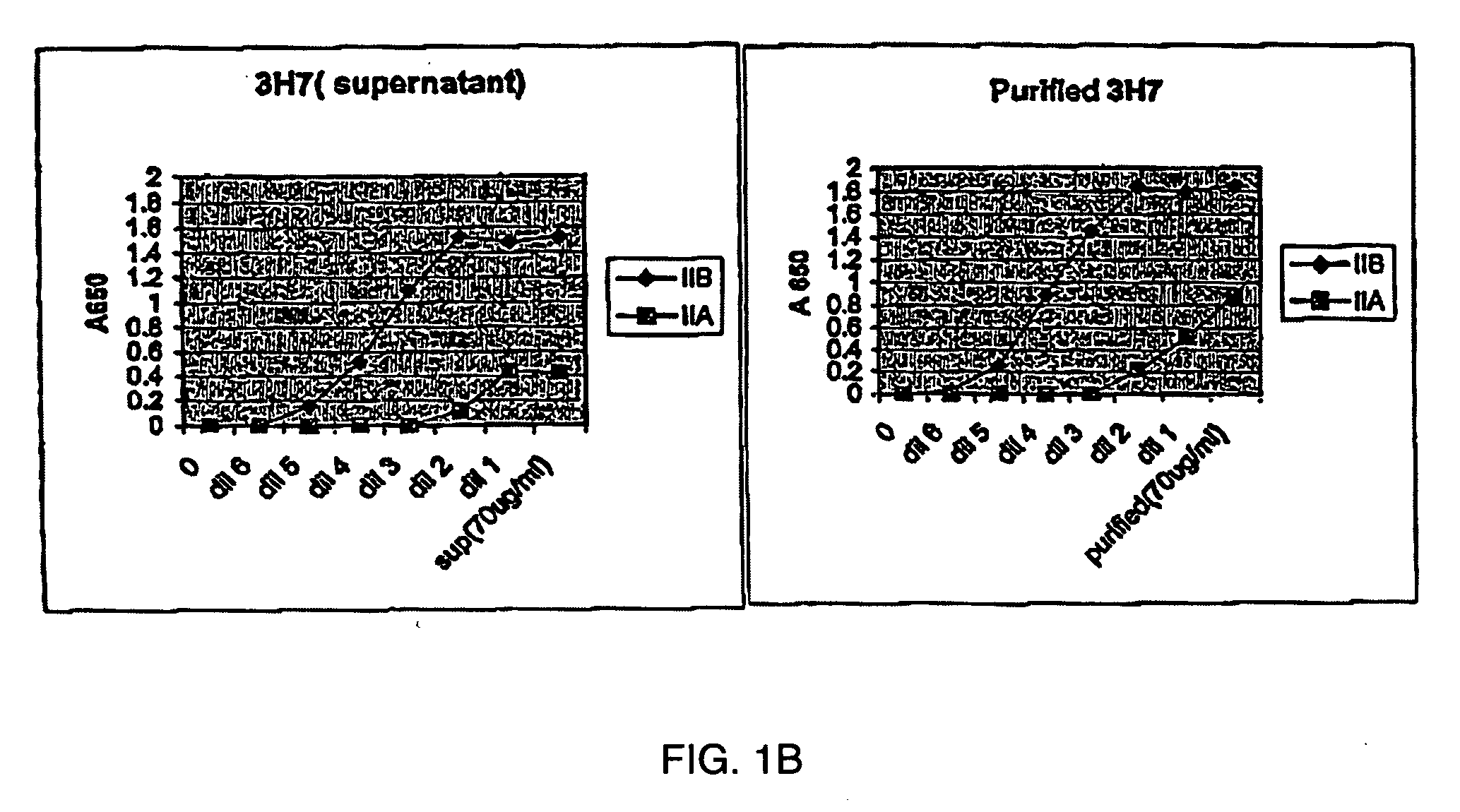
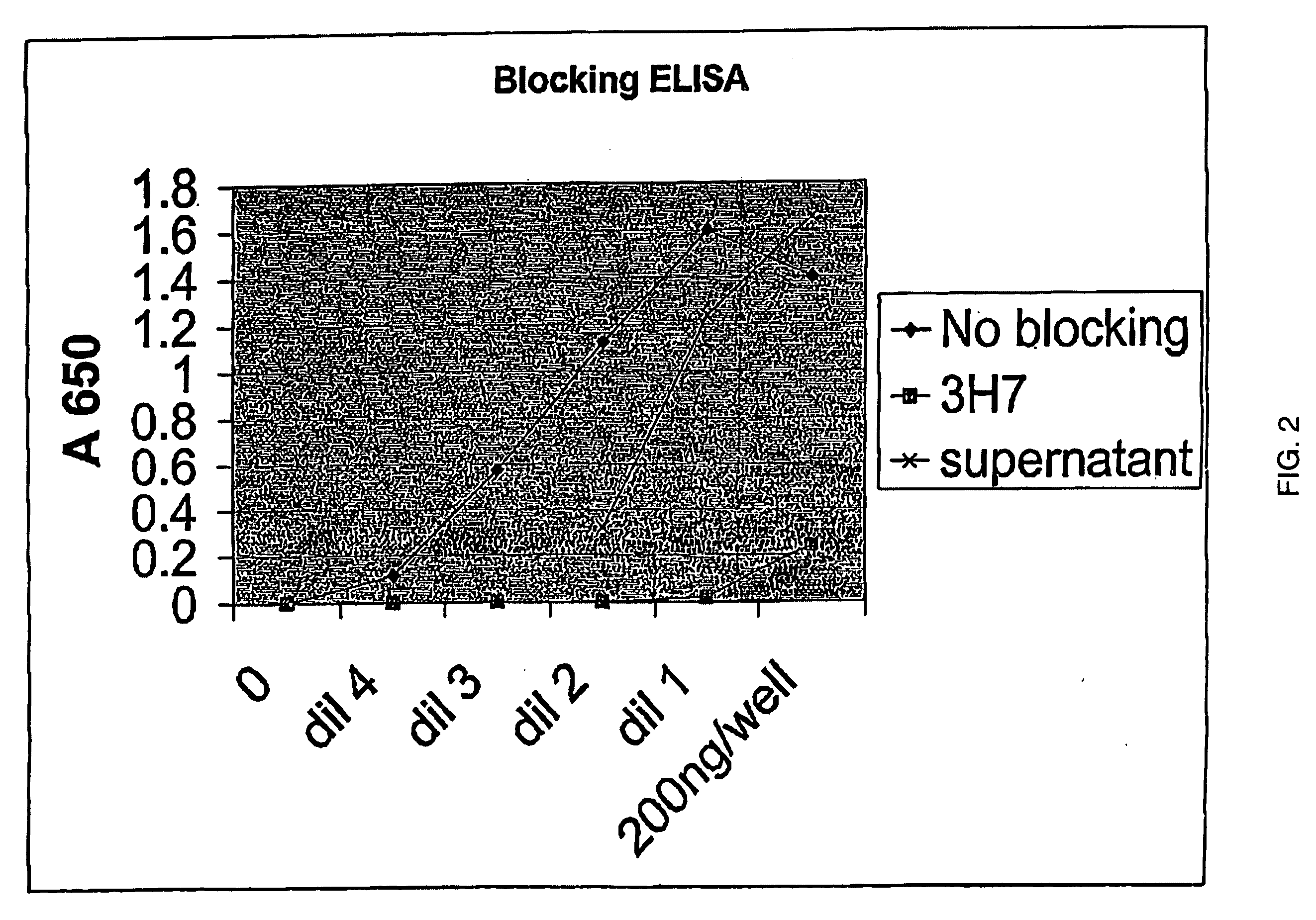
FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20040185045A1Strong therapeutic activityEnhancing antibody-mediated effector functionSenses disorderAntipyreticTherapeutic antibodyTreatment effect
The present invention relates to antibodies or fragments thereof that specifically bind FcgammaRIIB, particularly human FcgammaRIIB, with greater affinity than said antibodies or fragments thereof bind FcgammaRIIA, particularly human FcgammaRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Cyclic single-chain trispecific antibody
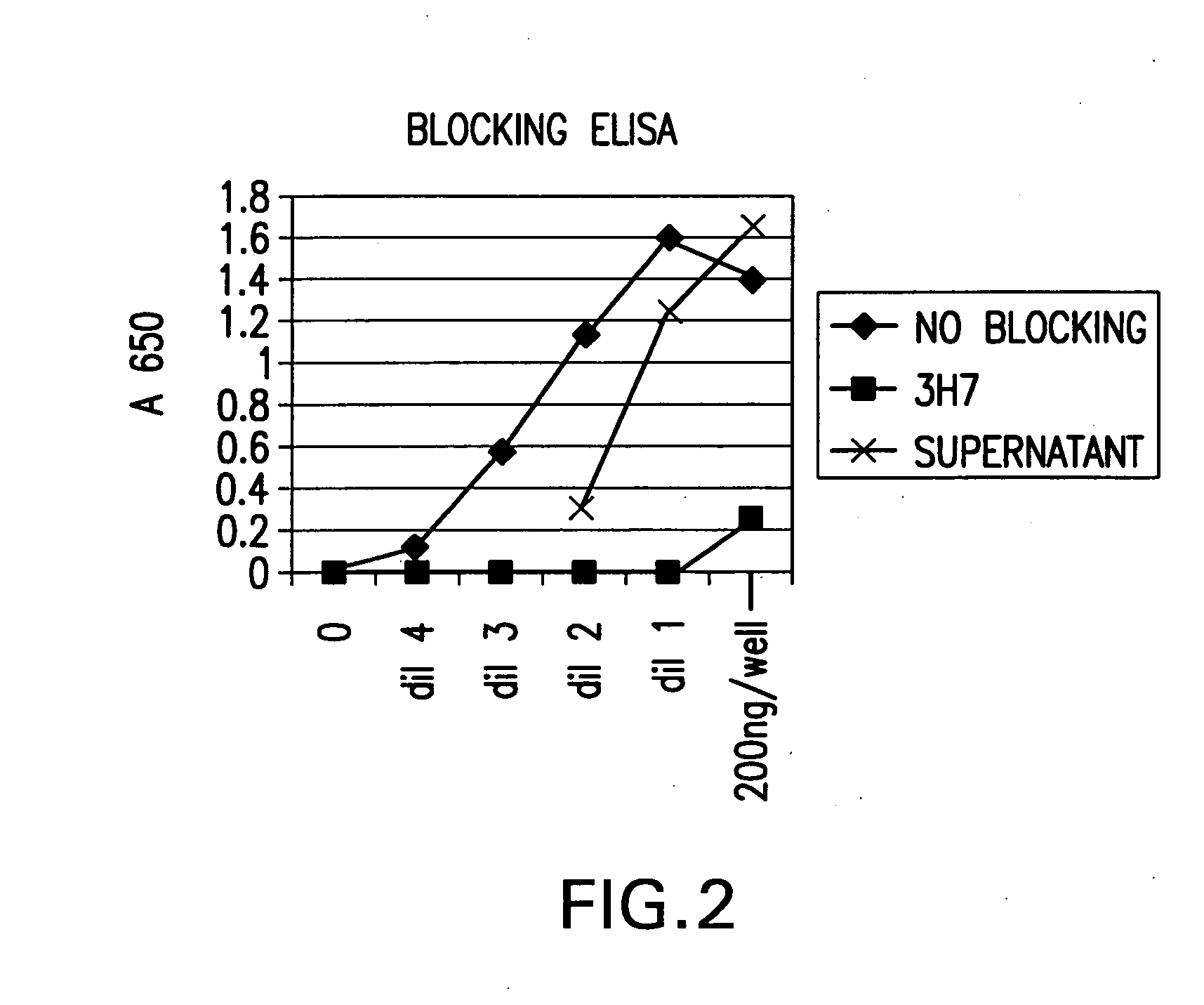
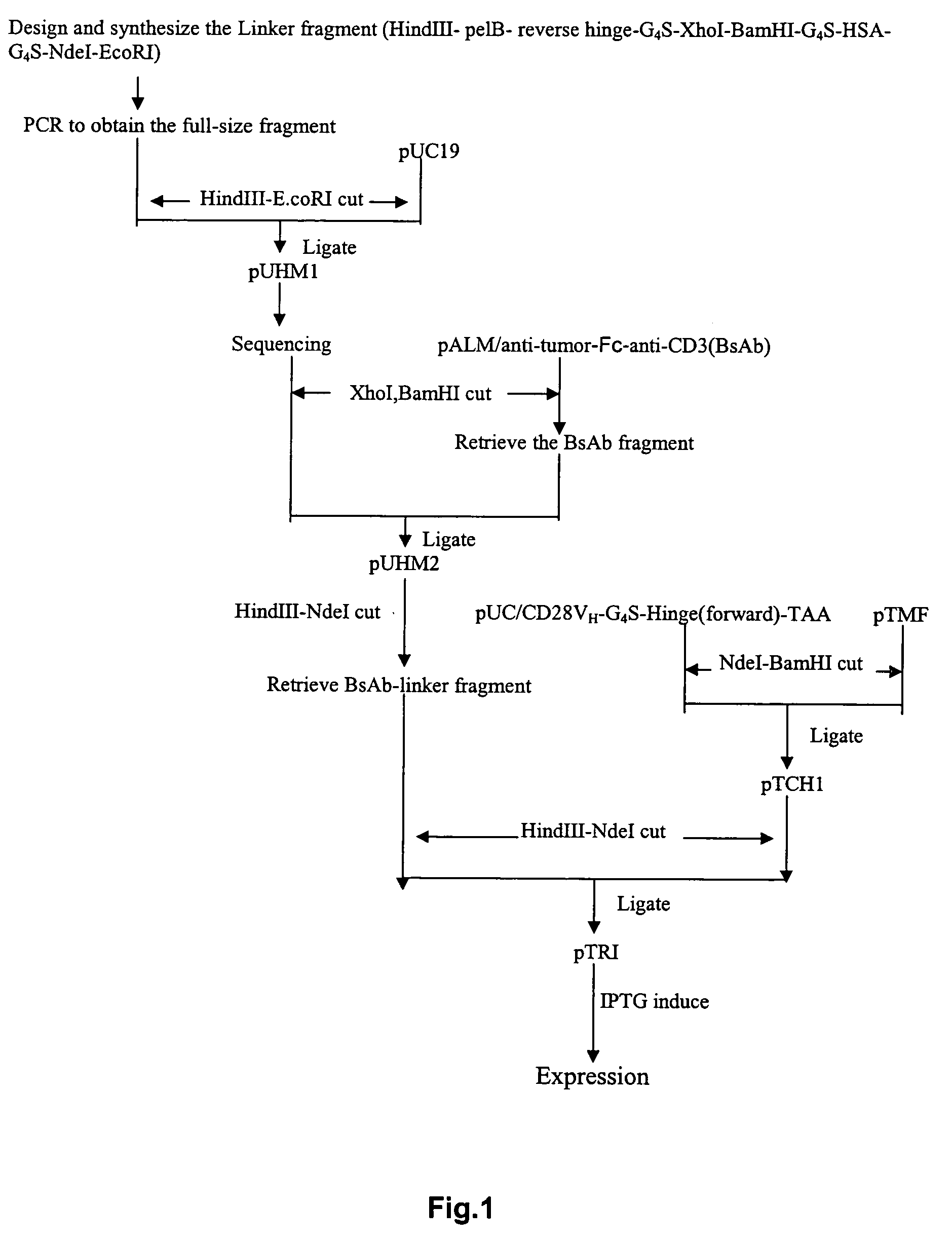
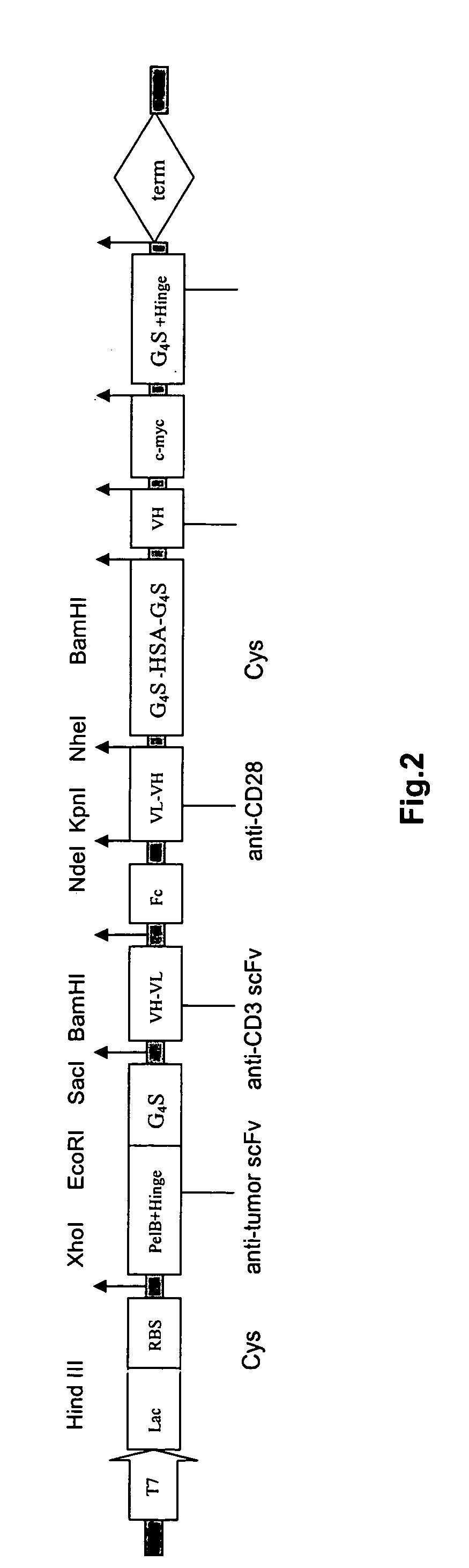
The invention provides a cyclic single-chain trispecific antibody against human tumor. It comprises three parts. The first part is an anti-tumor Fab antibody, an anti-tumor single-domain antibody or an scFv. The second part is a reshaped Fab antibody against human CD3, a reshaped single-domain antibody against human CD3 or a reshaped scFv against human CD3. The third part is a reshaped Fab antibody against human CD28, a reshaped single-domain antibody against human CD28 or a reshaped scFv against human CD28. The present invention also offers the DNA sequence coding for this trispecific antibody, expression vectors containing this DNA sequence and host cells (E. coli) containing the vectors.
Owner:DONGGUAN HAOFA BIOTECH DEVAL +2
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Owner:GLAXO SMITHKLINE LLC
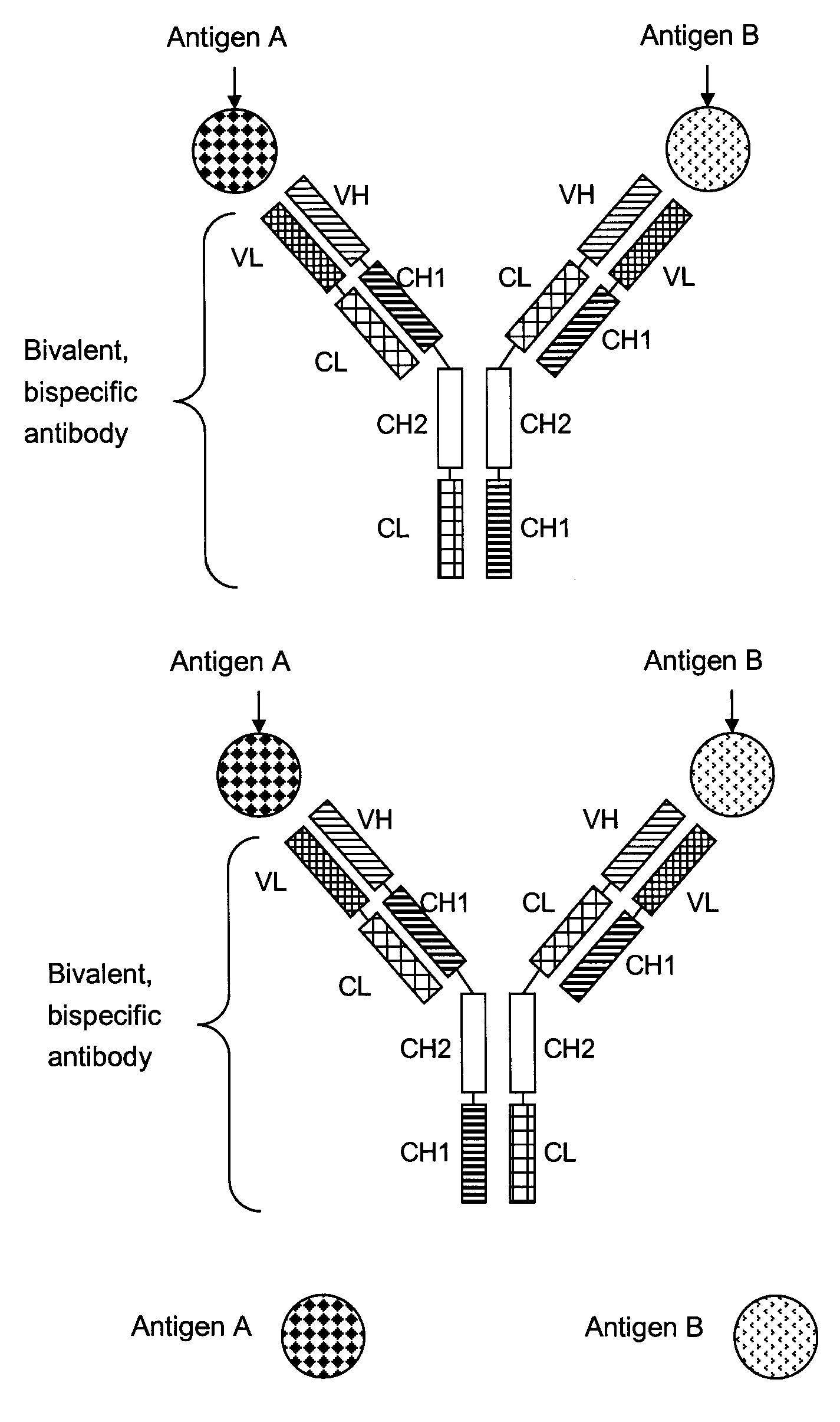
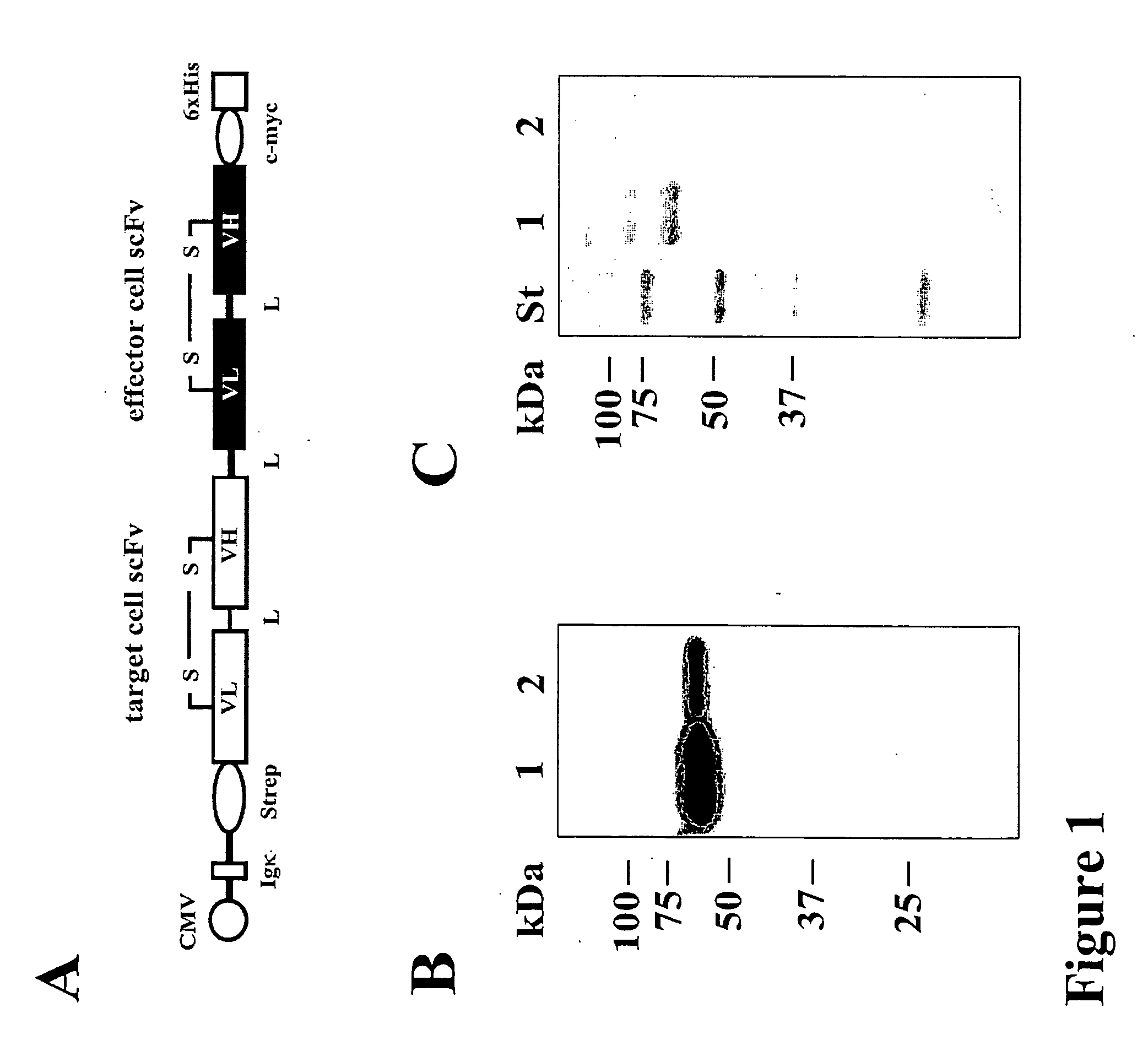
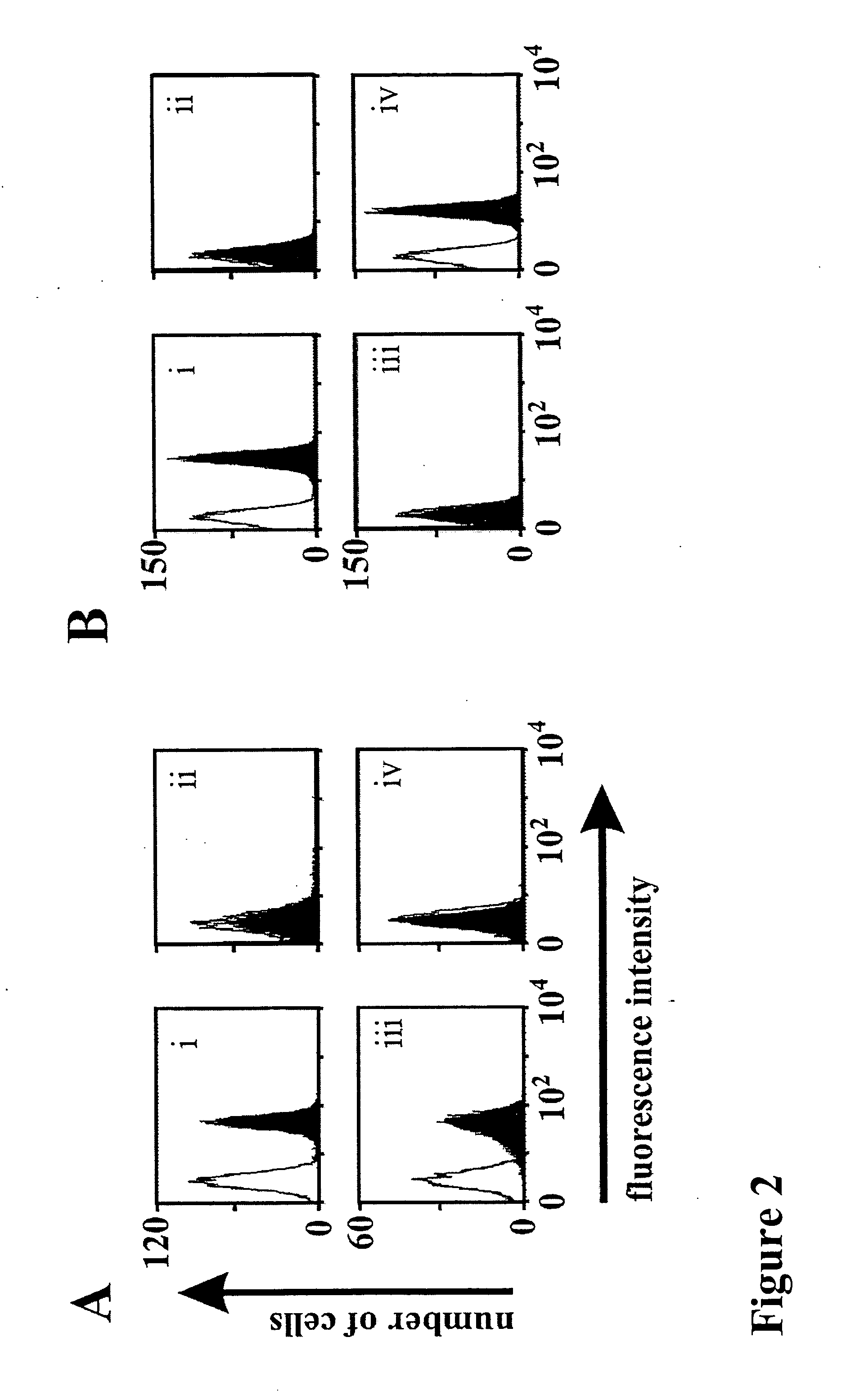
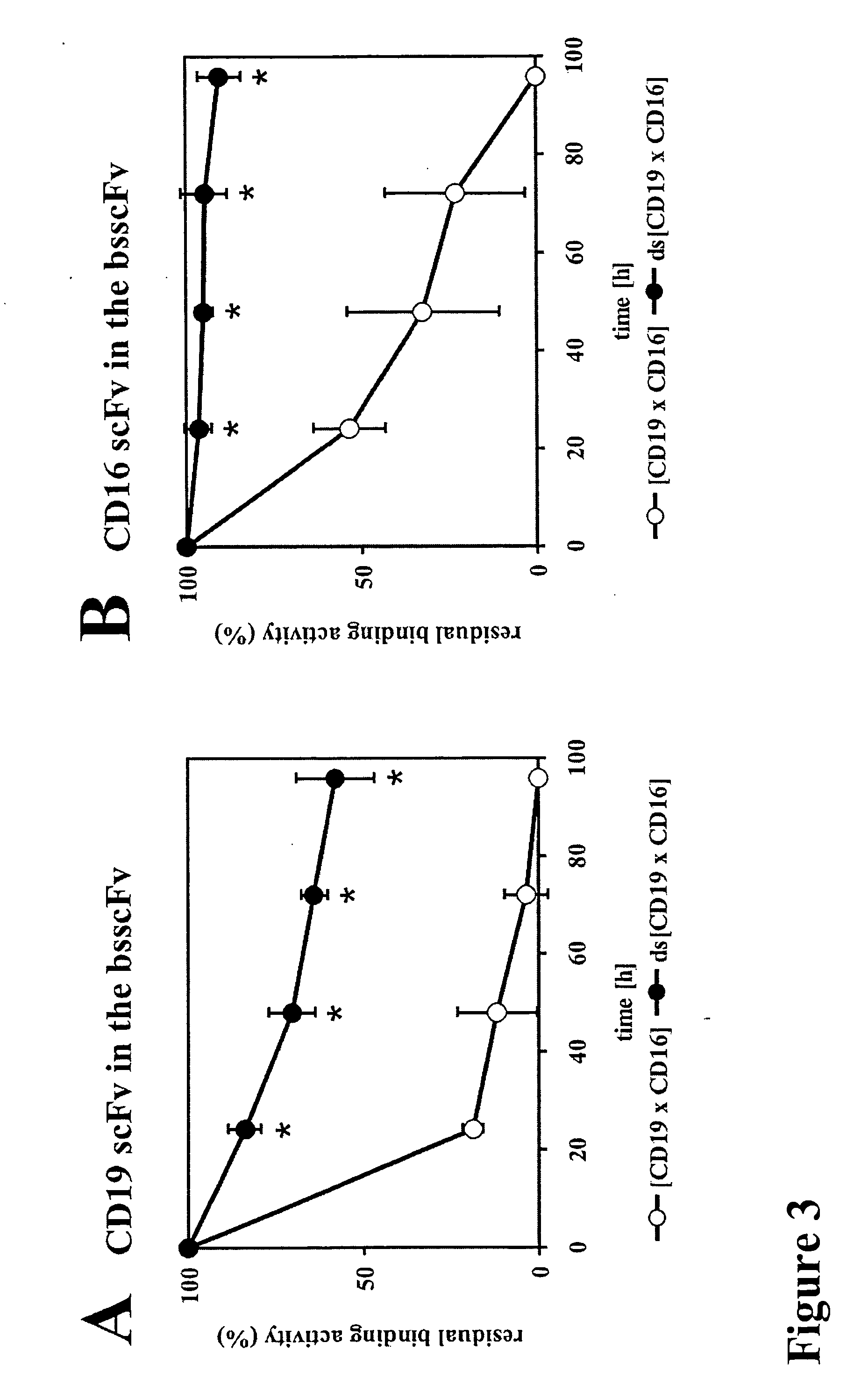
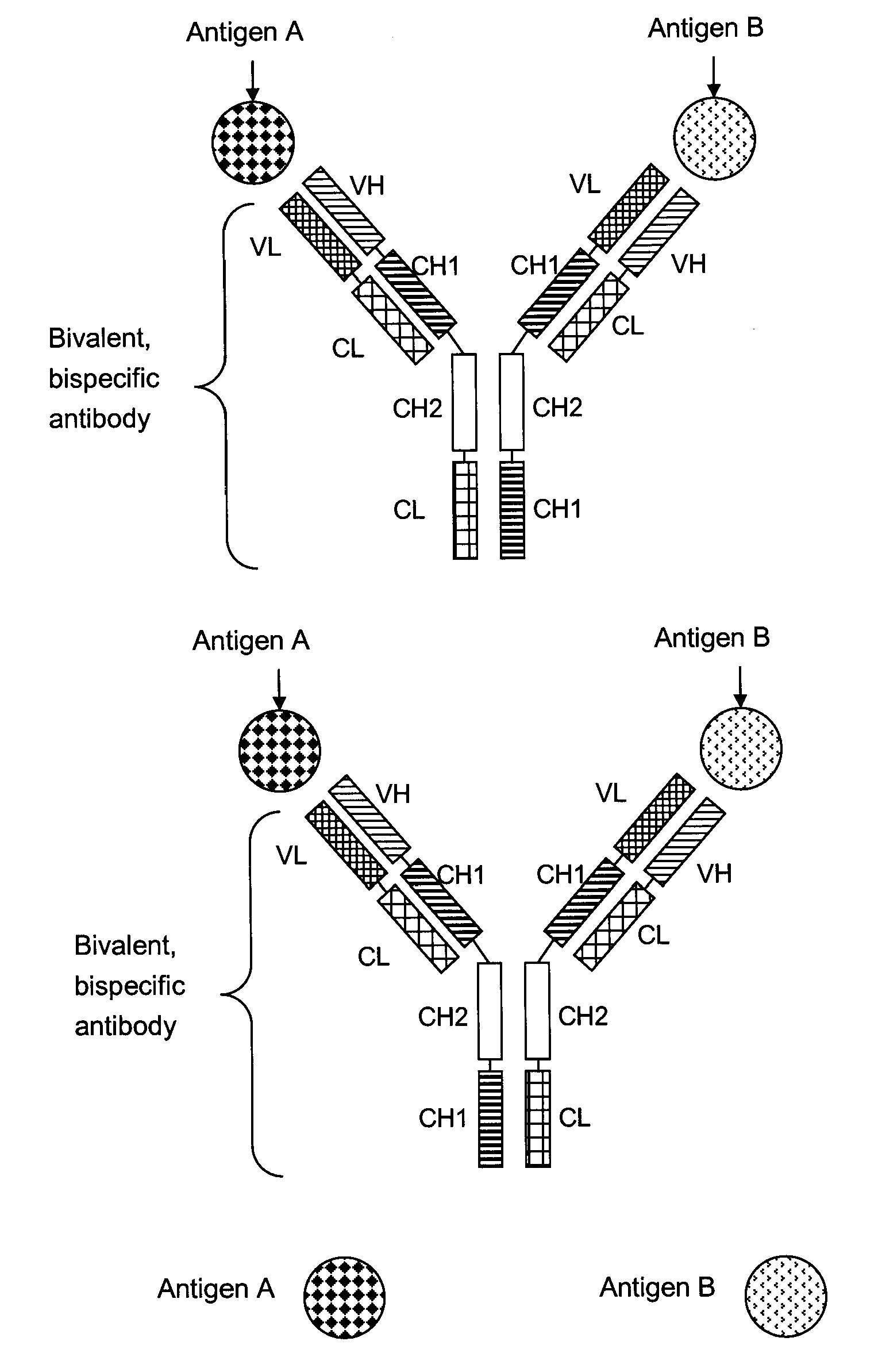
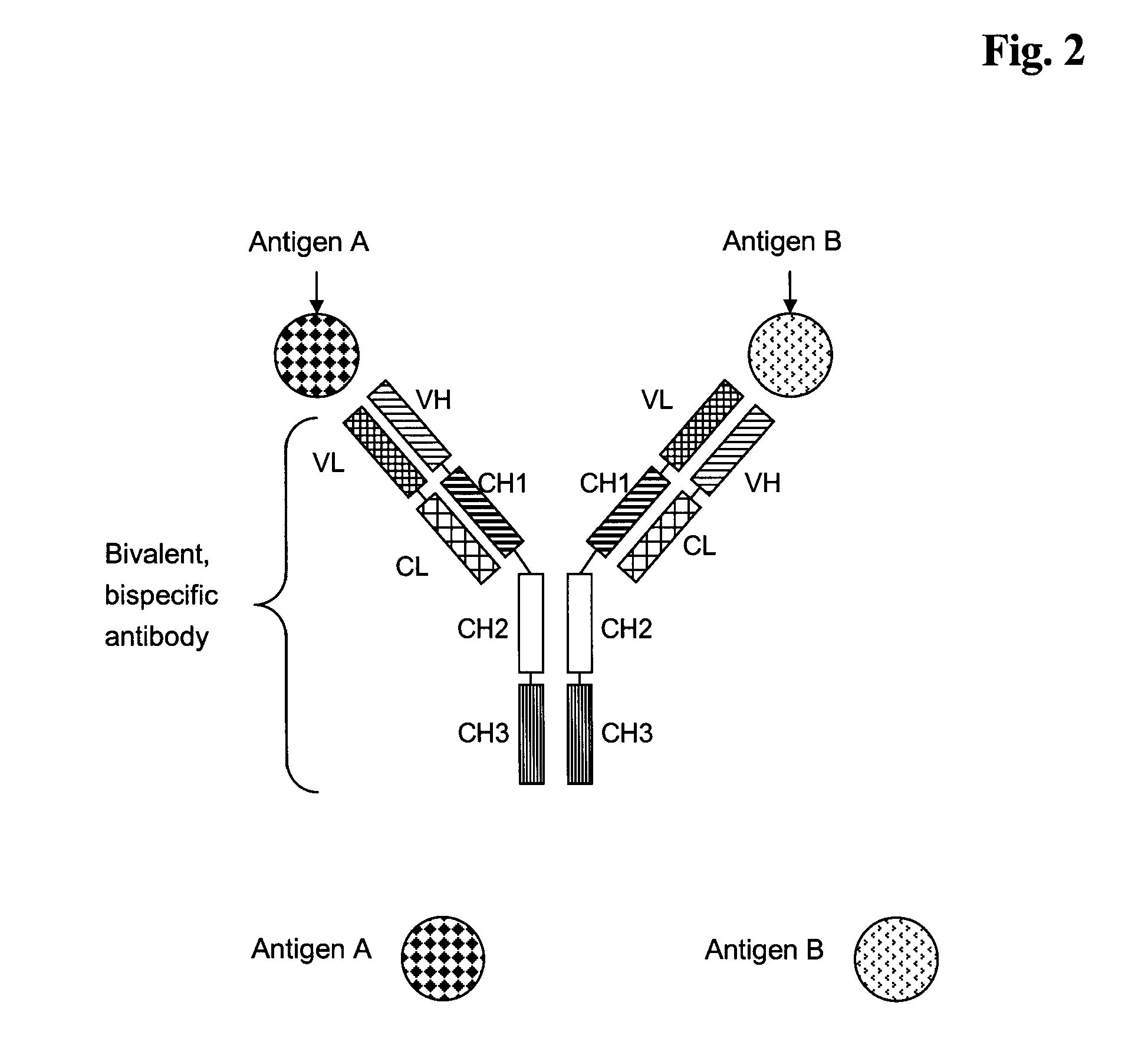
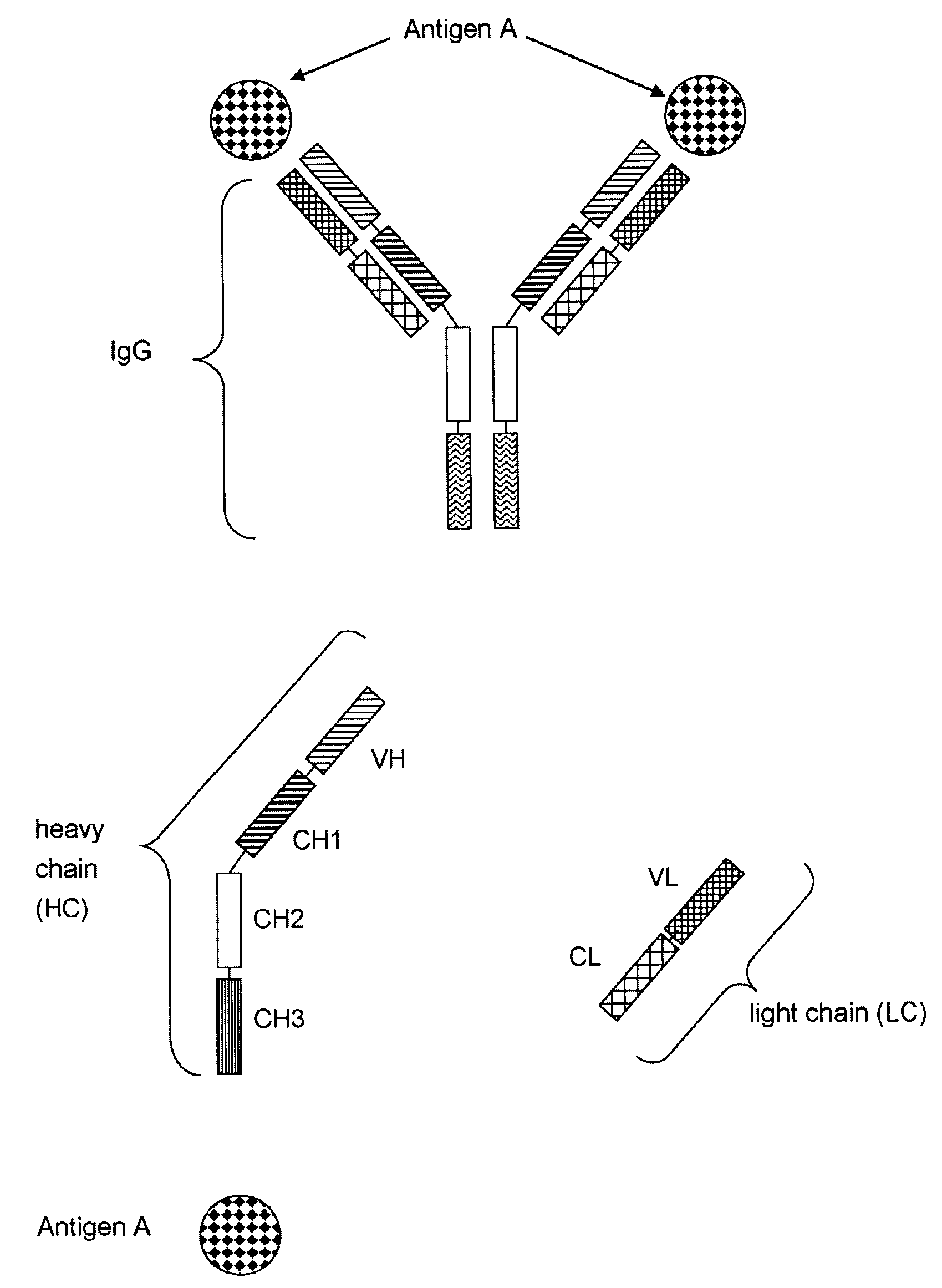
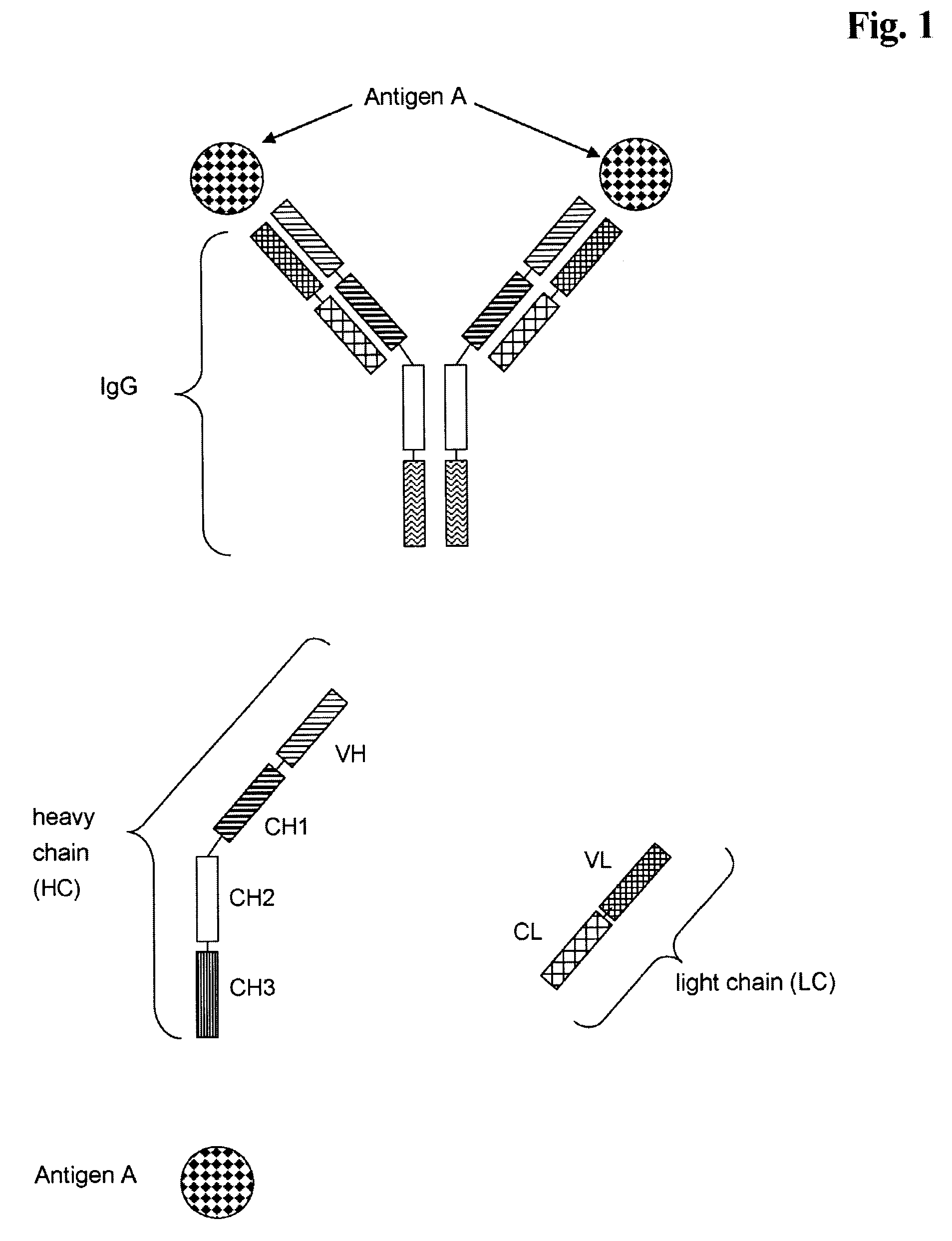
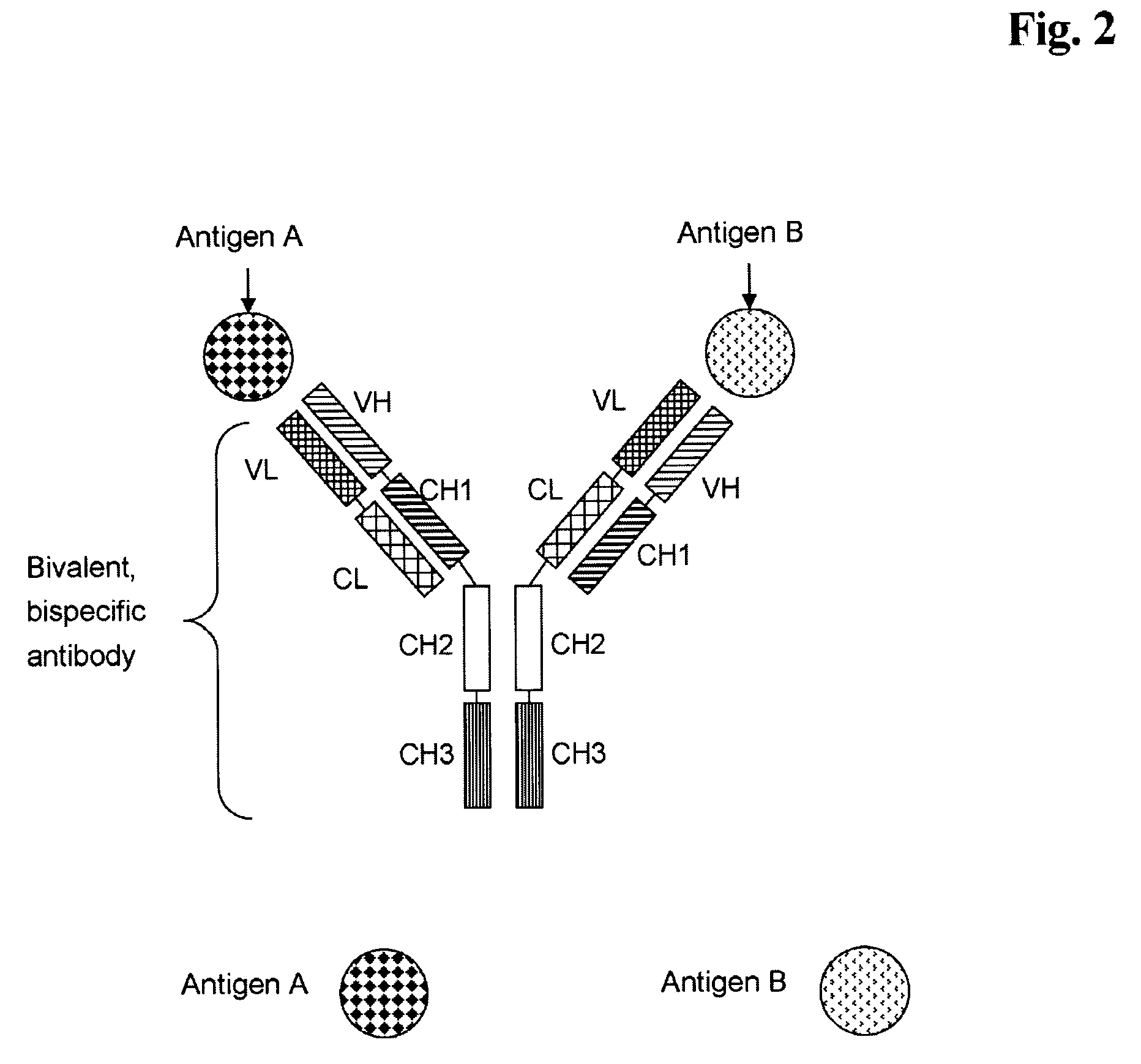
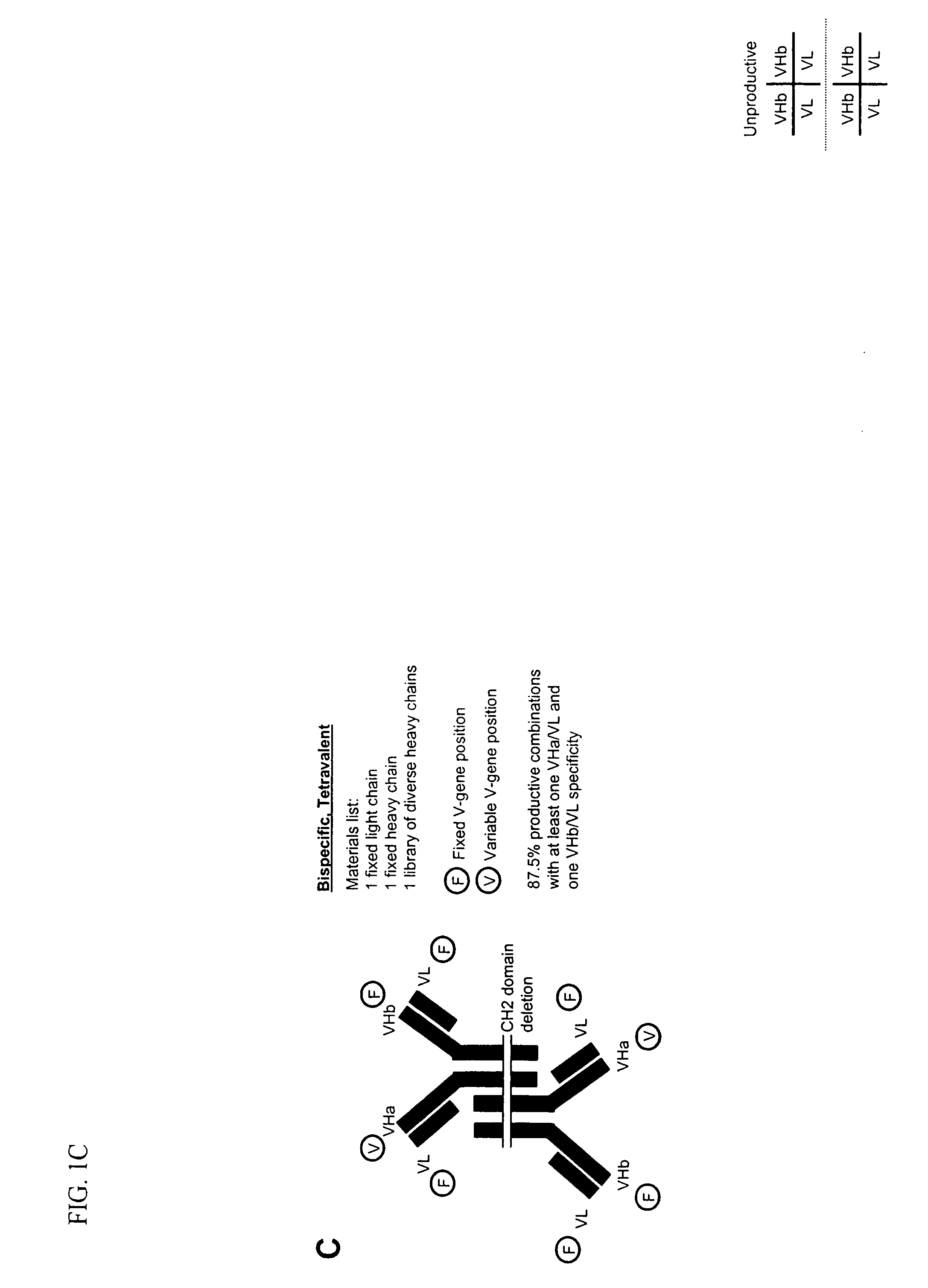
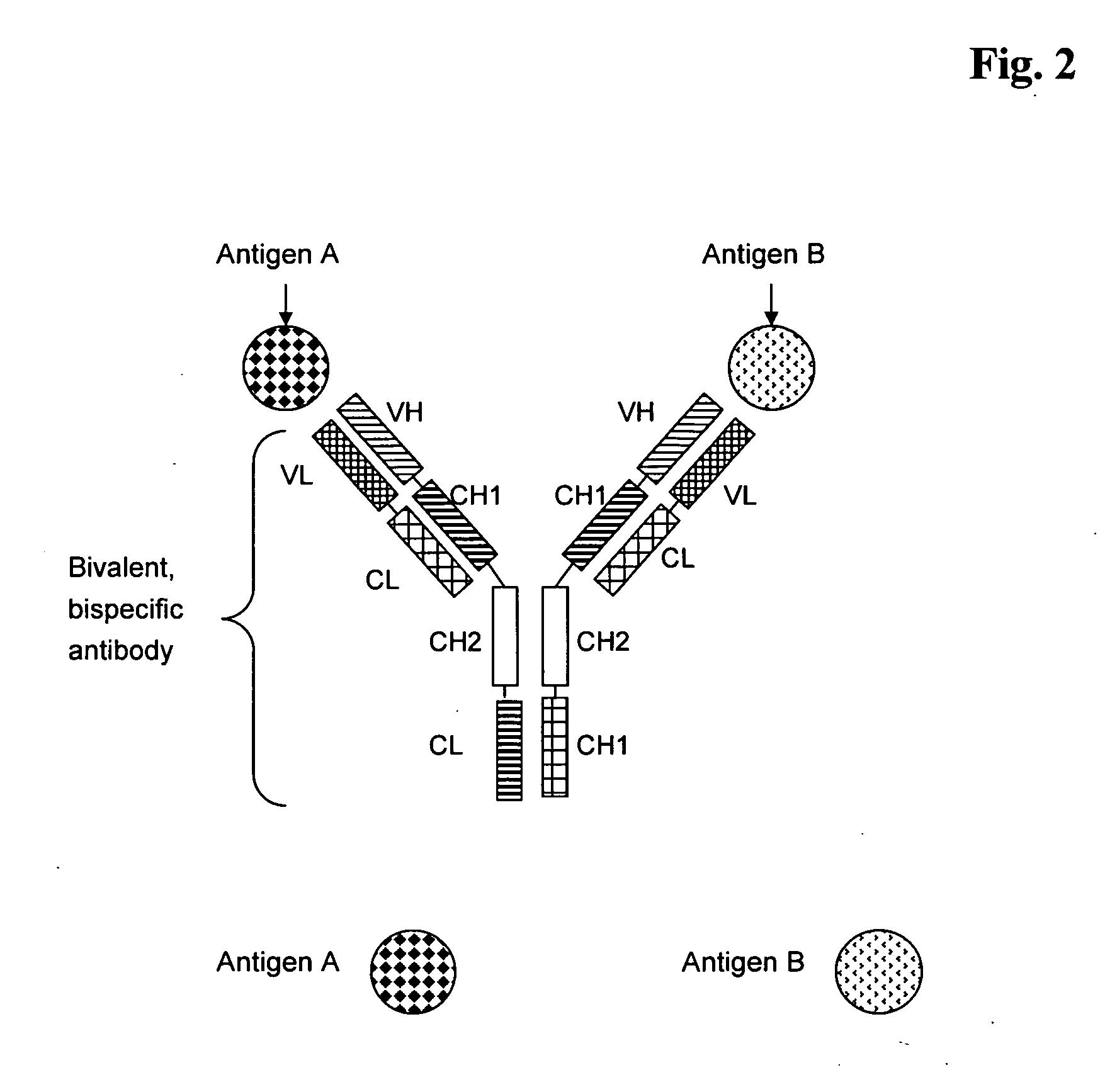
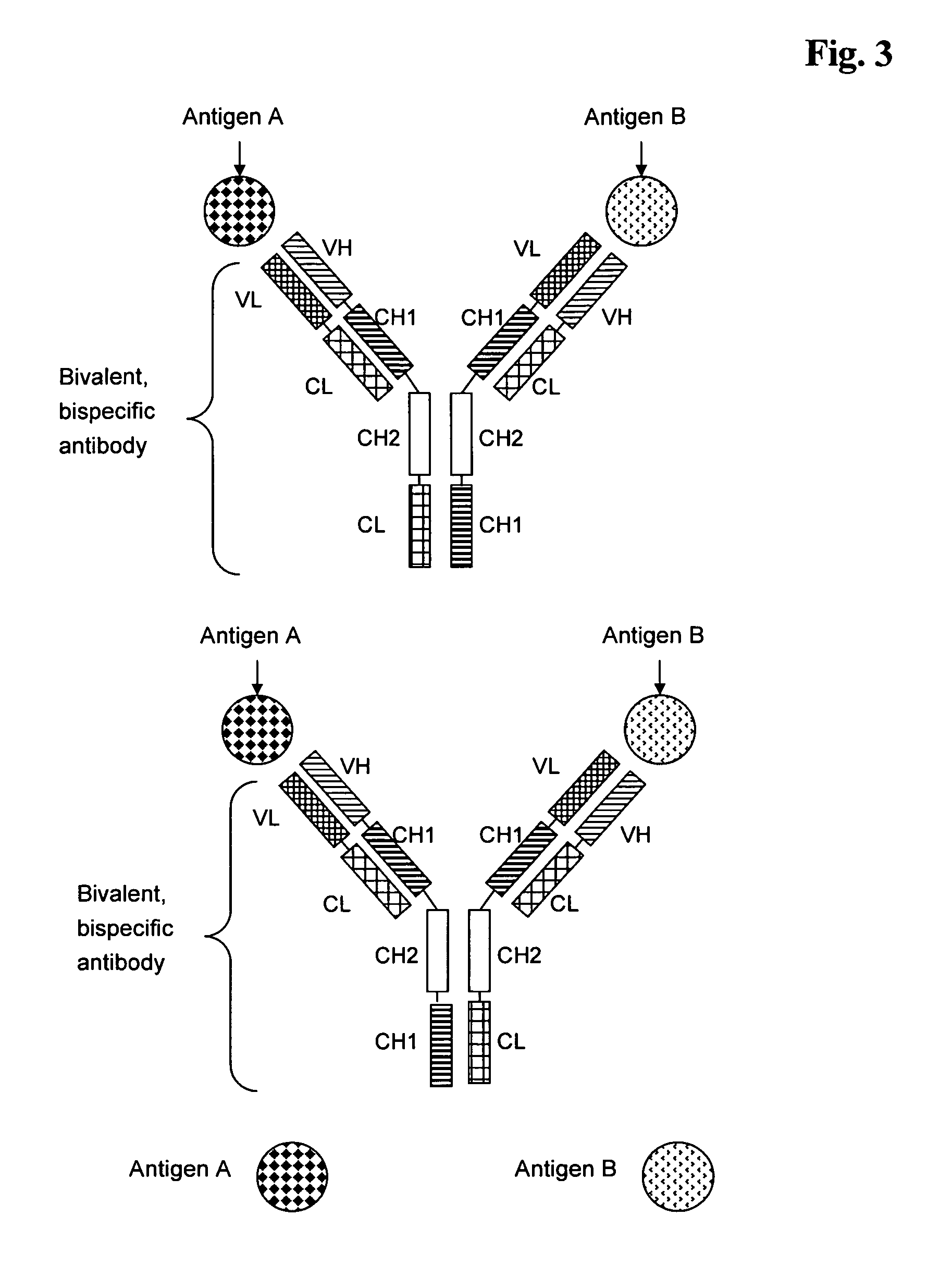
Bivalent, bispecific antibodies
ActiveUS20090162360A1Increase heterodimerisationIncrease productionAnimal cellsHybrid immunoglobulinsBispecific antibodySpecific antibody
Owner:F HOFFMANN LA ROCHE & CO AG
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Methods for the treatment of lymphoma by administration of a B cell-specific antibody are described. The invention encompasses providing to a patient both unlabeled antibodies and antibodies labeled with a radioisotope. A principal advantage of the method is that tumor responses can be obtained in a radiometric dose range that does not require hematopoietic stem cell replacement as an adjunct therapy also described is a composition useful in the treatment of lymphoma.
Owner:RGT UNIV OF MICHIGAN +2
Bispecific antibody devoid of Fc region and method of treatment using same
InactiveUS20060263367A1Efficient productionAvoids undesirable effector functionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsBispecific antibodyAntigen binding
Bispecific antibody derivatives are disclosed which are comprised of a first region which binds to a first antigen and a second region which binds to a second antigen different from the first antigen. The first and second regions of the bispecific antibody are each stabilized by an additional internal disulfide bridge, and connected by a flexible polypeptide linker. The bispecific antibody is devoid of an Fc portion and is encoded as a single chain-sequence.
Owner:FRIEDRICH ALEXANDER UNIV ERLANGEN NURNBERG
Bivalent, bispecific antibodies
InactiveUS20090162359A1Increase productionImprove purification effectAnimal cellsHybrid immunoglobulinsBispecific antibodySpecific antibody
Owner:F HOFFMANN LA ROCHE & CO AG
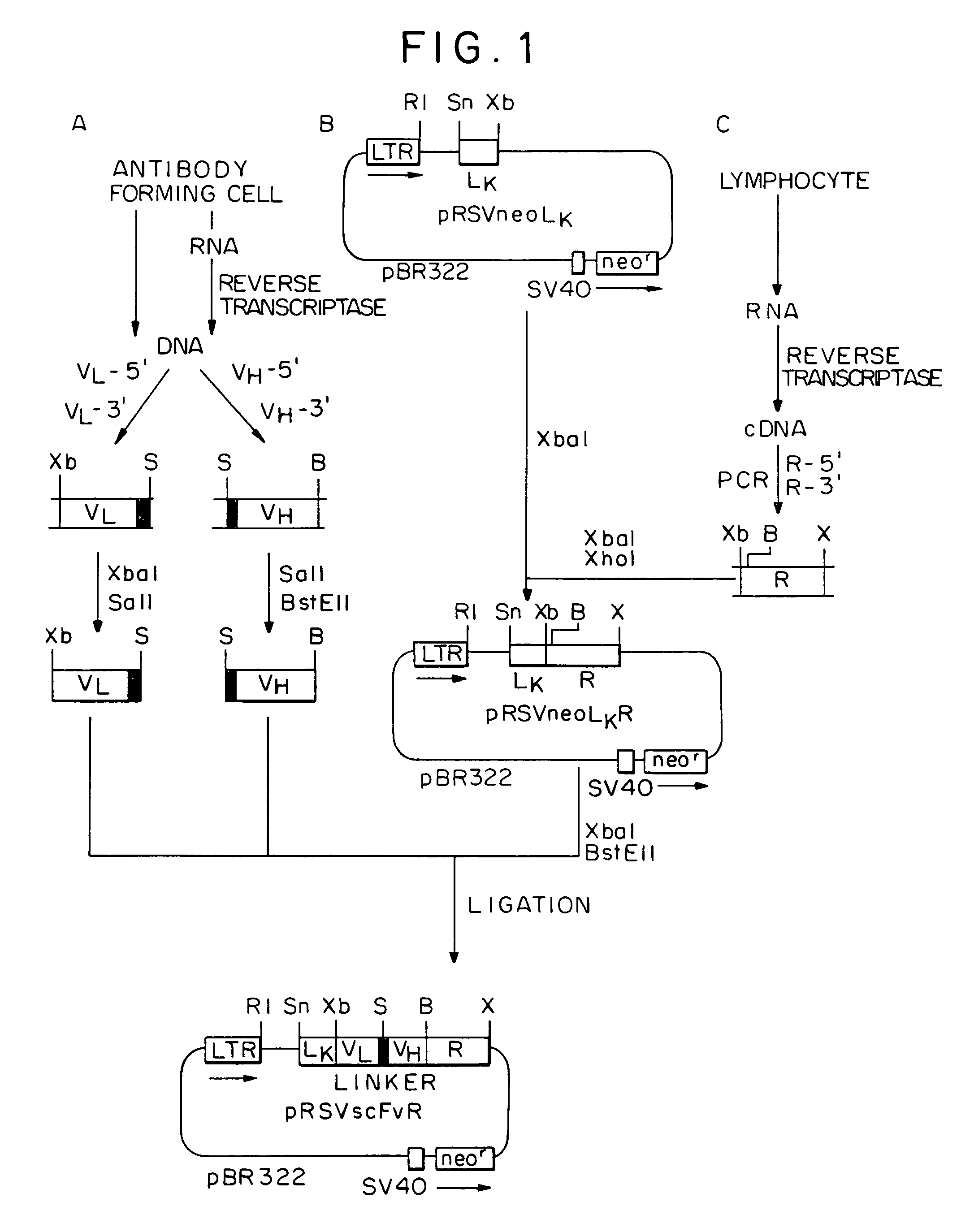
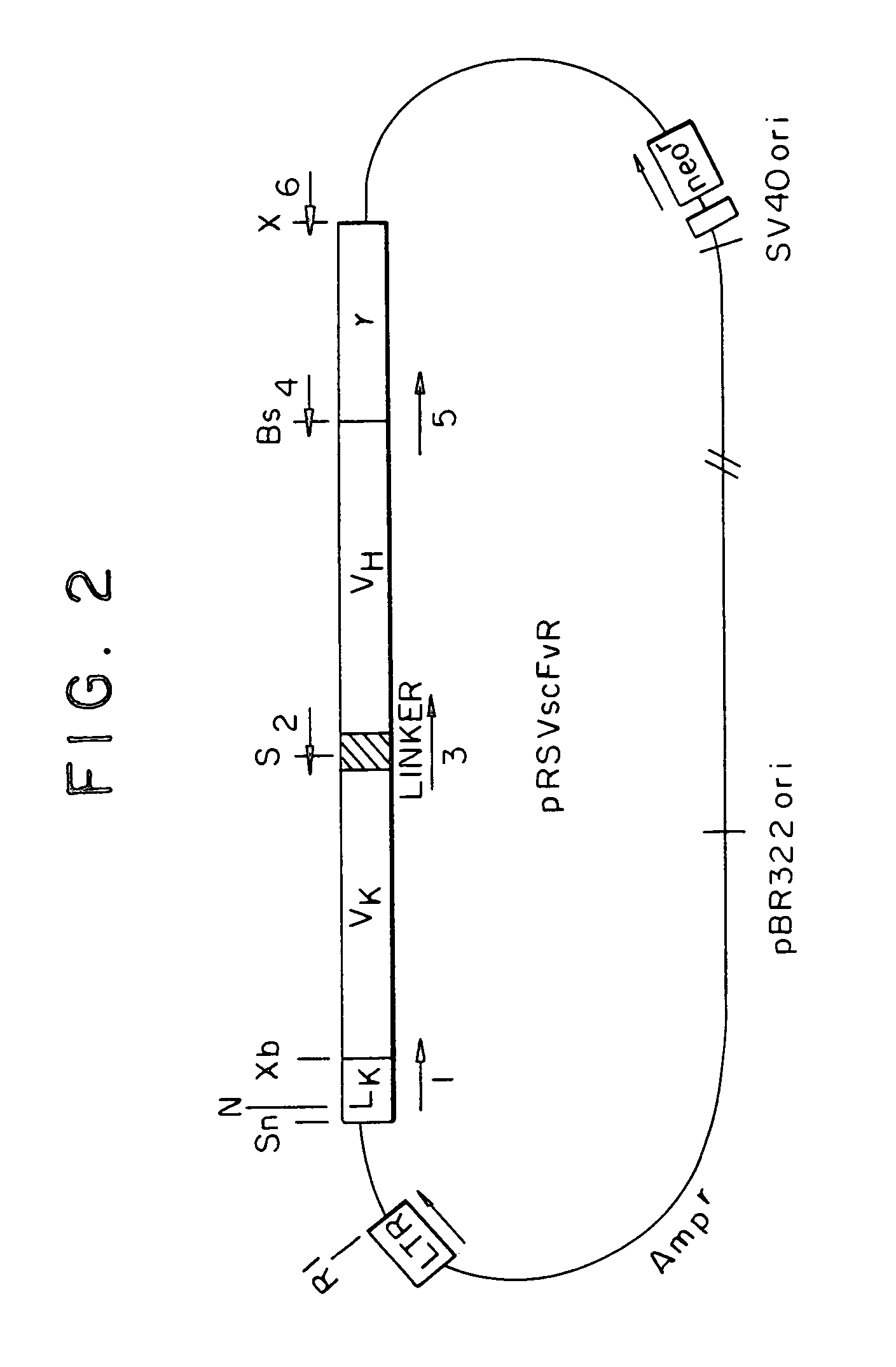
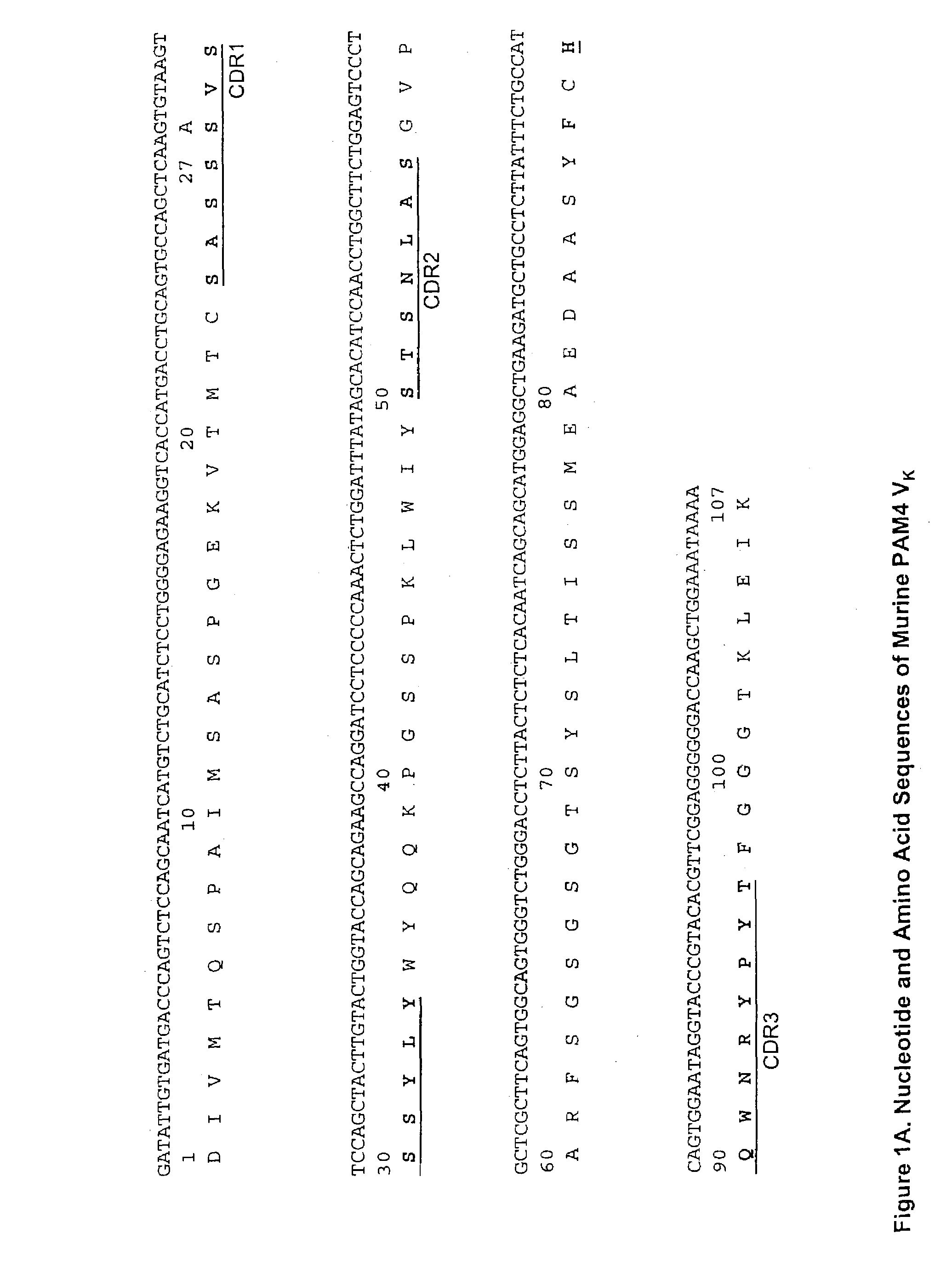
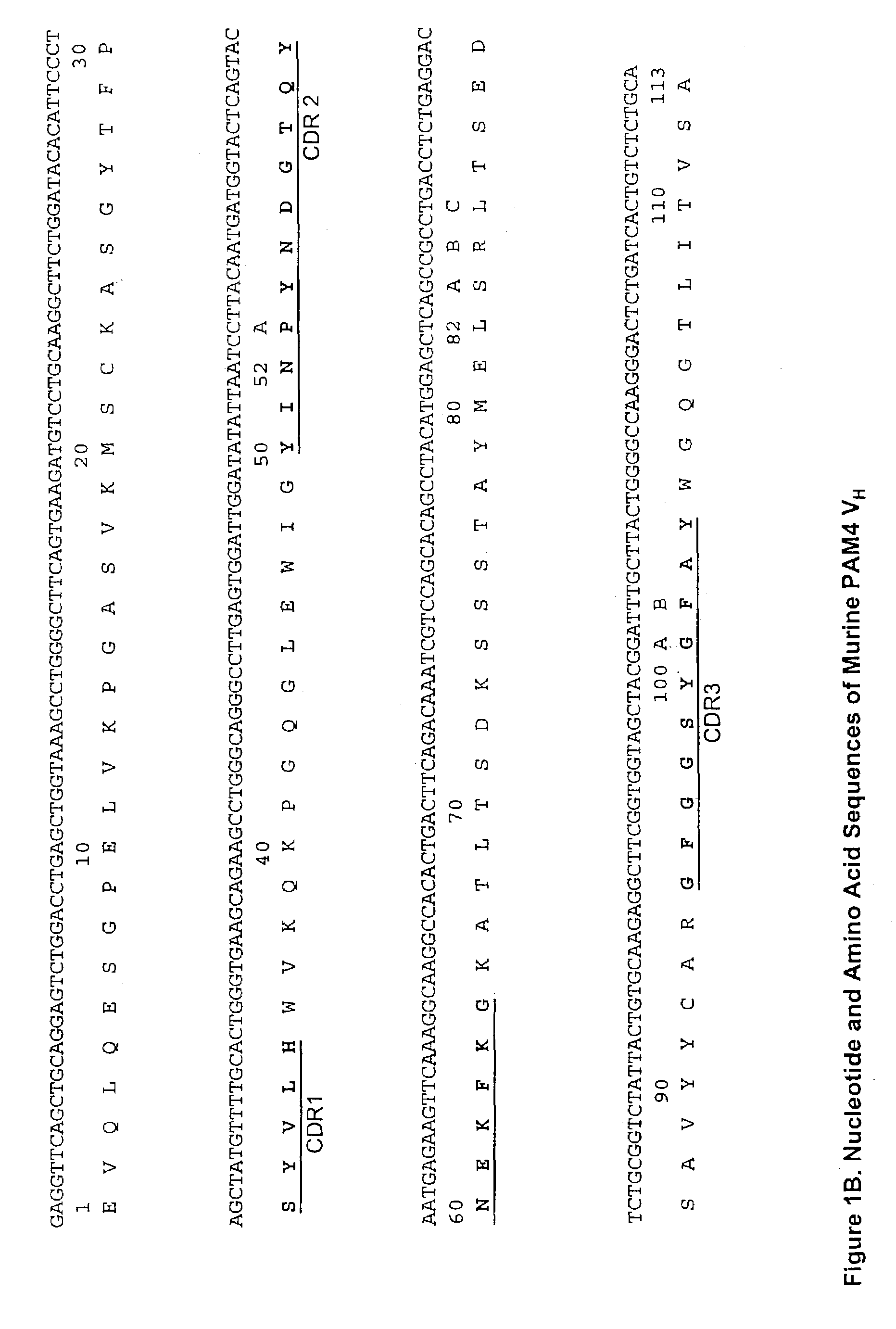
Monoclonal antibody hPAM4
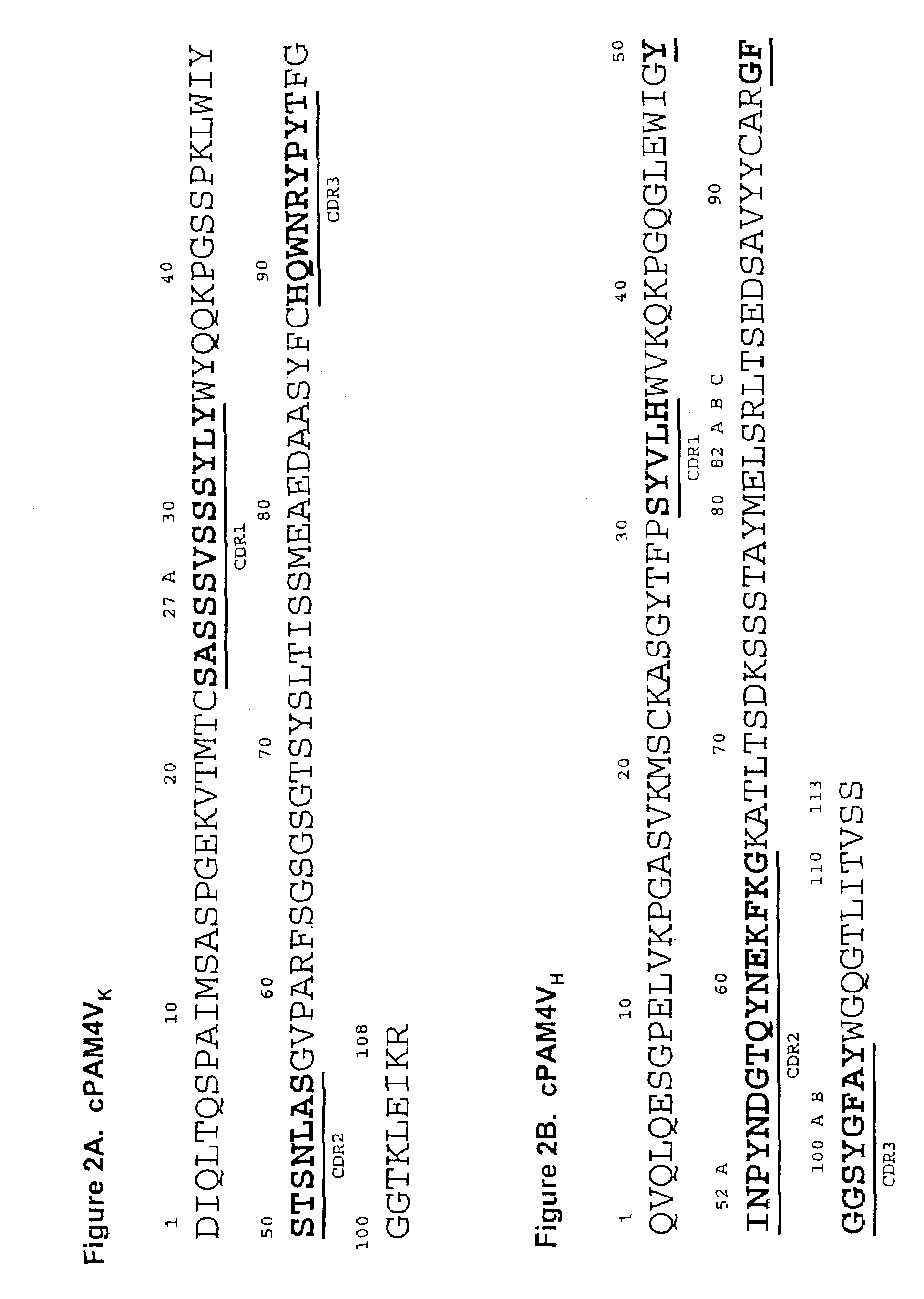
This invention relates to monovalent and multivalent, monospecific antibodies and to multivalent, multispecific antibodies. One embodiment of these antibodies has one or more identical binding sites where each binding site binds with a target antigen or an epitope on a target antigen. Another embodiment of these antibodies has two or more binding sites where these binding sites have affinity towards different epitopes on a target antigen or different target antigens, or have affinity towards a target antigen and a hapten. The present invention further relates to recombinant vectors useful for the expression of these functional antibodies in a host. More specifically, the present invention relates to the tumor-associated antibody designated PAM4. The invention further relates to humanized and human PAM4 antibodies, and the use of such antibodies in diagnosis and therapy.
Owner:IMMUNOMEDICS INC
Bivalent, bispecific antibodies
ActiveUS20090232811A1Increase productionImprove purification effectAnimal cellsAntibody mimetics/scaffoldsBispecific antibodySpecific antibody
Owner:F HOFFMANN LA ROCHE & CO AG
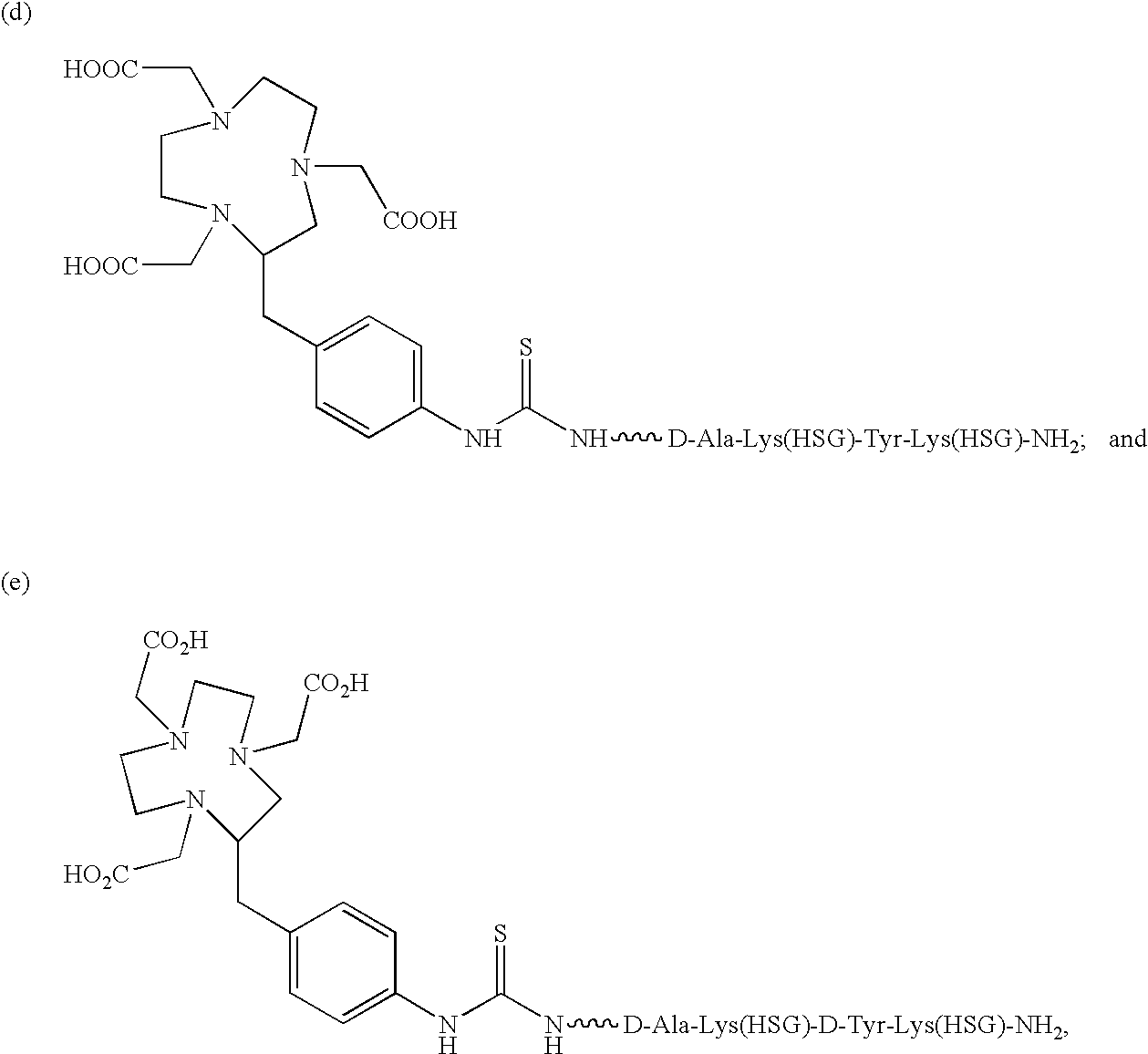
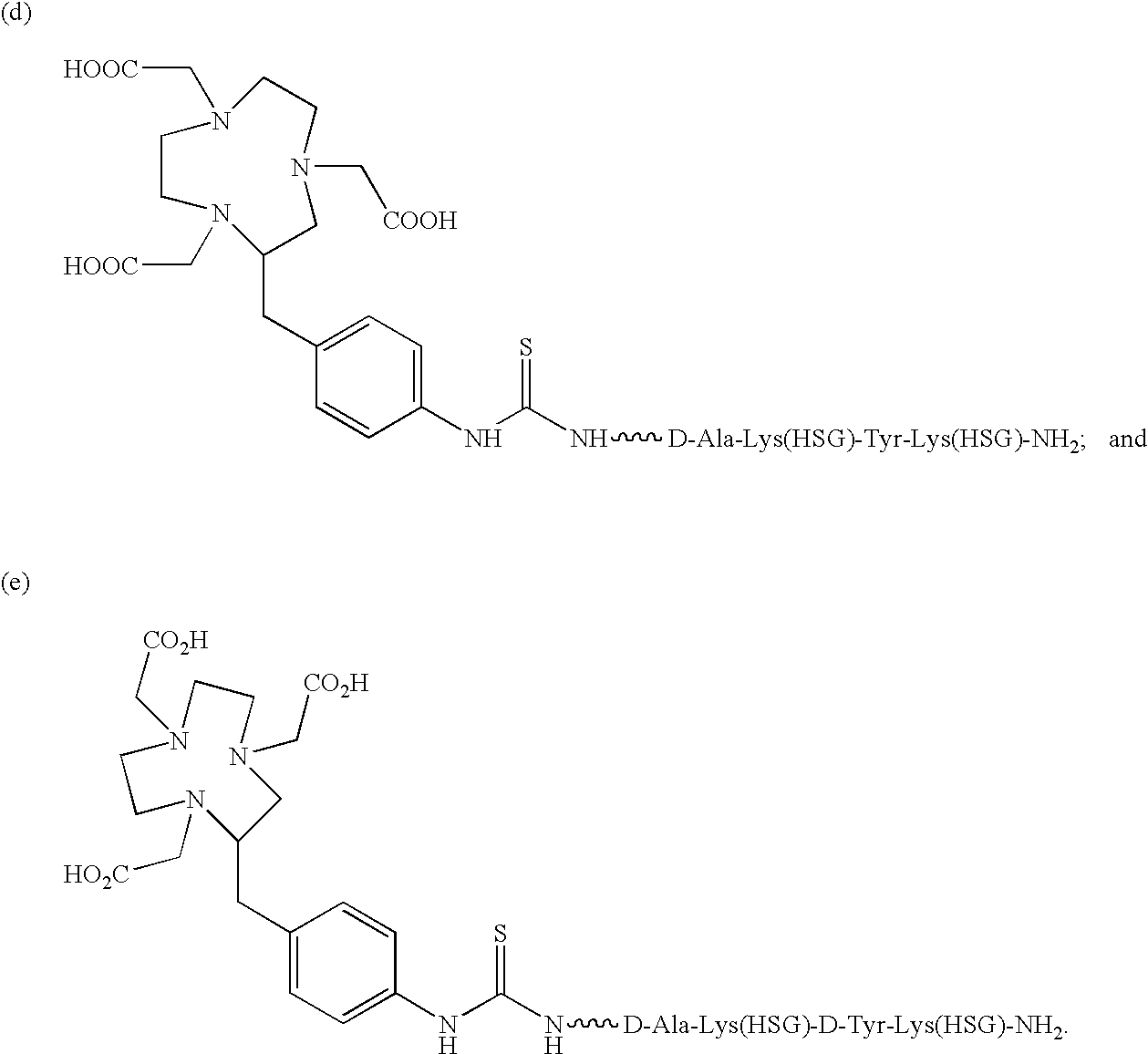
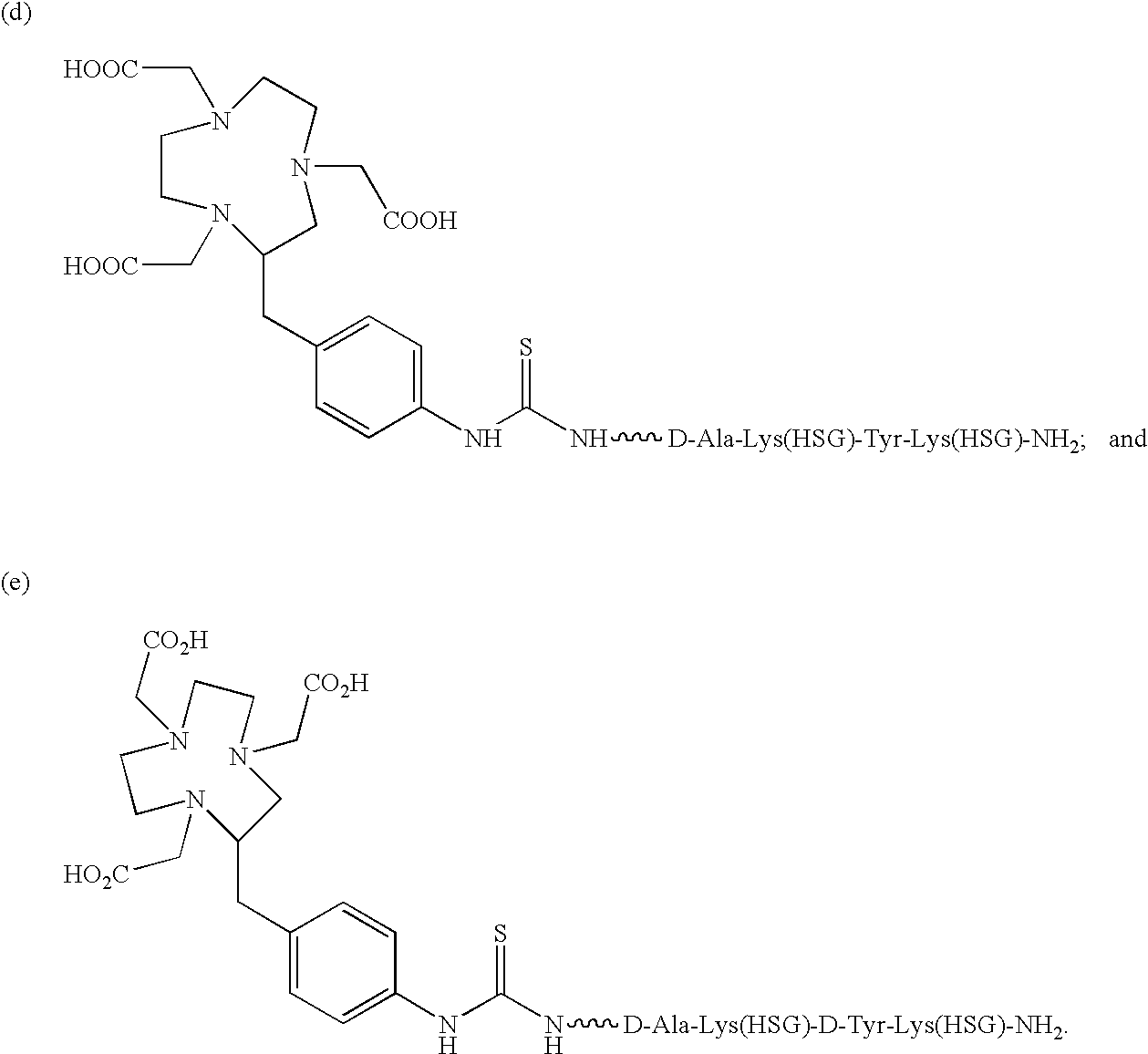
Production and use of novel peptide-based agents for use with bi-specific antibodies
The present invention relates to a bi-specific antibody or antibody fragment having at least one arm that is reactive against a targeted tissue and at least one other arm that is reactive against a linker moiety. The linker moiety encompasses a hapten to which antibodies have been prepared. The antigenic linker is conjugated to one or more therapeutic or diagnostic agents or enzymes. The invention provides constructs and methods for producing the bispecific antibodies or antibody fragments, as well as methods for using them.
Owner:IMMUNOMEDICS INC
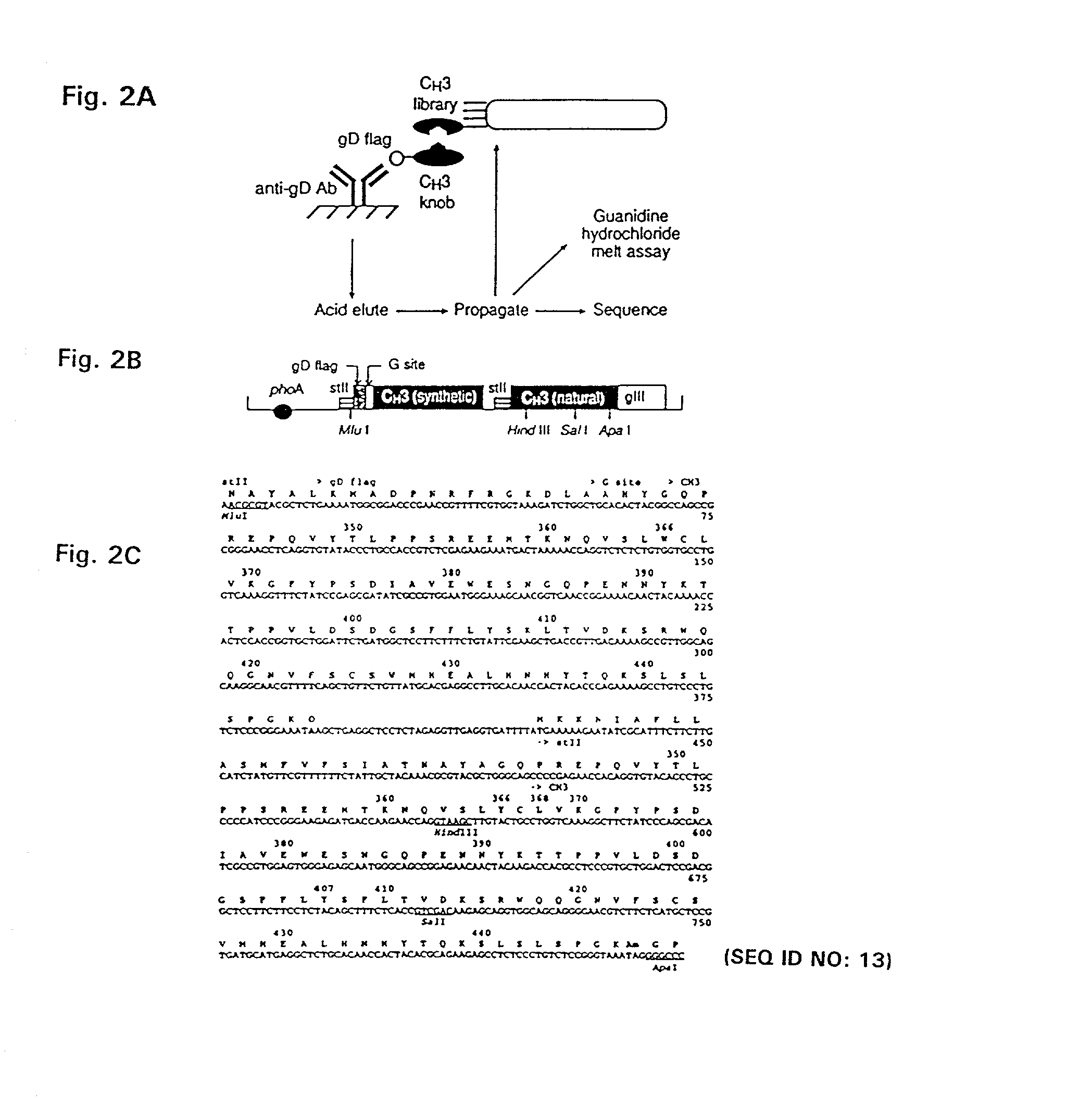
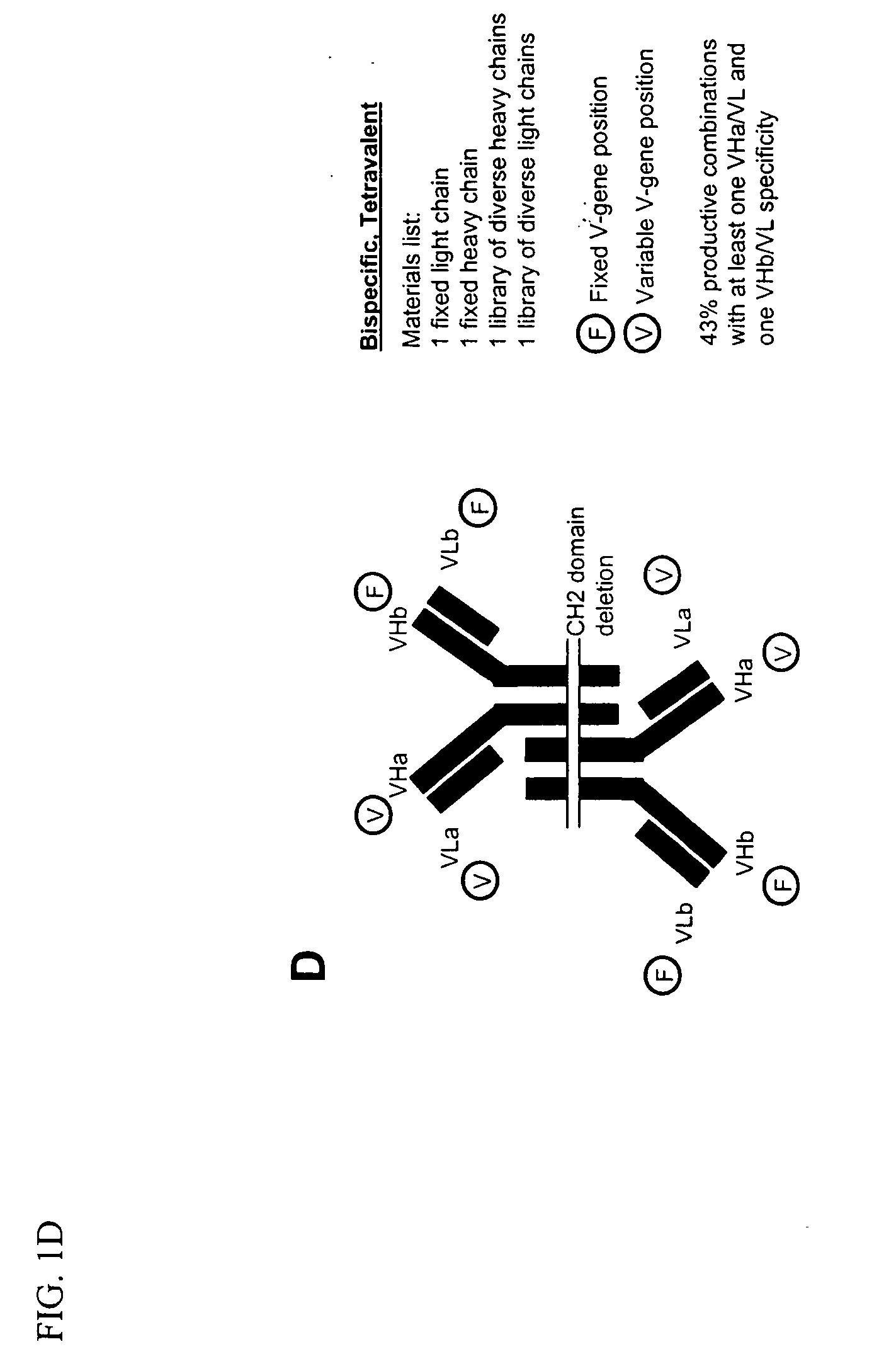
Methods for producing and identifying multispecific antibodies
The present invention relates to a high efficiency method of expressing multispecific antibodies in eukaryotic cells. The invention is further drawn to a method of producing immunoglobulin heavy and light chain libraries, particularly using the trimolecular recombination method, for expression in eukaryotic cells. The invention further provides methods of selecting and screening for multispecific antibodies, and antigen-binding fragments thereof. The invention also provides kits for producing, screening and selecting multispecific antibodies. Finally, the invention provides multispecific antibodies, and antigen-binding fragments thereof, produced by the methods provided herein.
Owner:VACCINEX
Fcgamma riib specific antibodies and methods of use thereof
InactiveUS20050215767A1Enhance immune responseImprove responseSenses disorderAntipyreticTherapeutic antibodyAntiendomysial antibodies
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Fcgamma-RIIB-specific antibodies and methods of use thereof
InactiveUS20050260213A1Good curative effectAvoid managementHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen Binding FragmentTherapeutic effect
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Bivalent, bispecific antibodies
InactiveUS20090175851A1Increase productionImprove purification effectAntibacterial agentsAntibody mimetics/scaffoldsBispecific antibodySpecific antibody
Owner:F HOFFMANN LA ROCHE INC
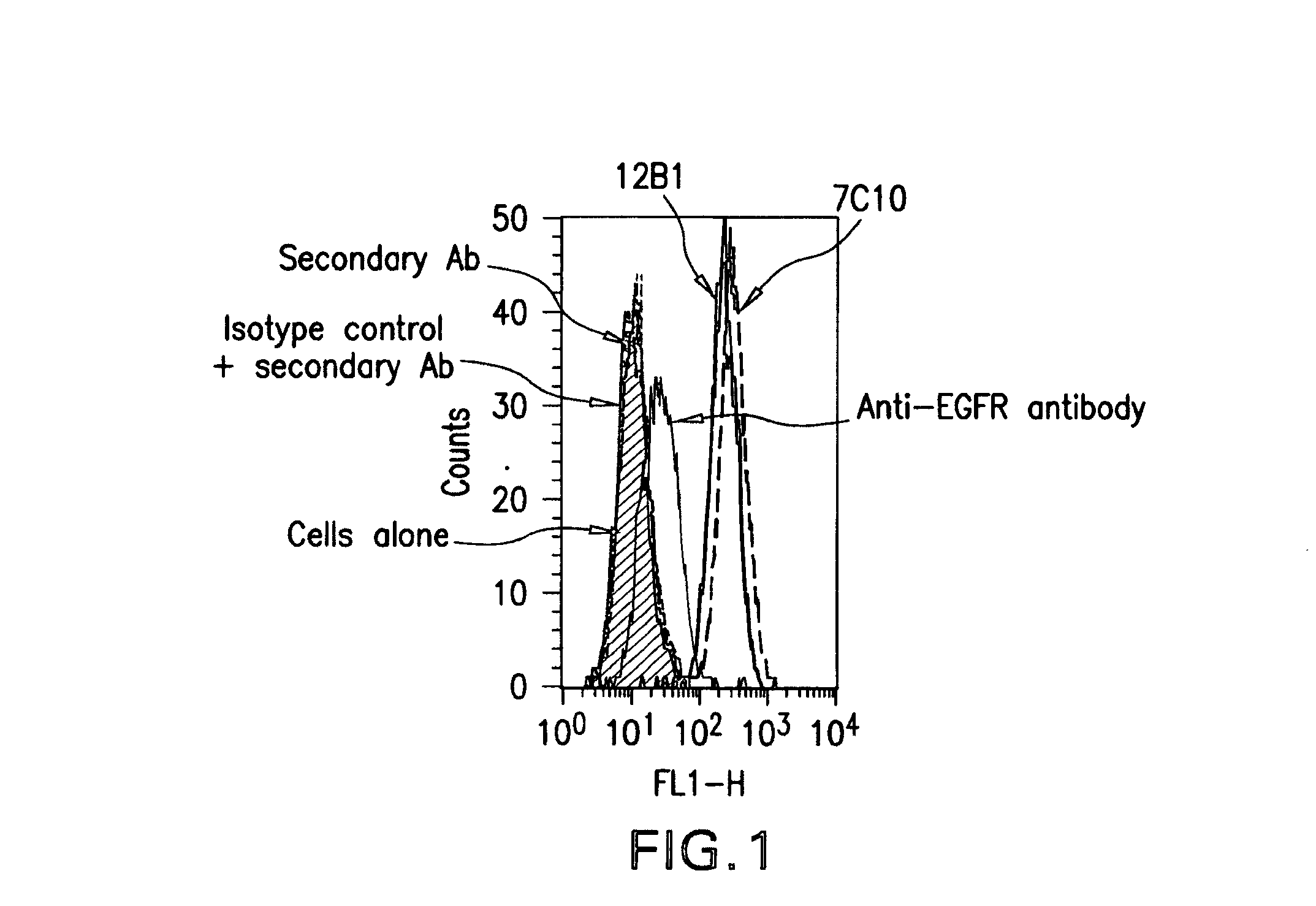
Igf-1r specific antibodies useful in the detection and diagnosis of cellular proliferative disorders
InactiveUS20130084243A1High affinityUseful in detectionAnimal cellsIn-vivo radioactive preparationsDiseaseSingle-Chain Antibodies
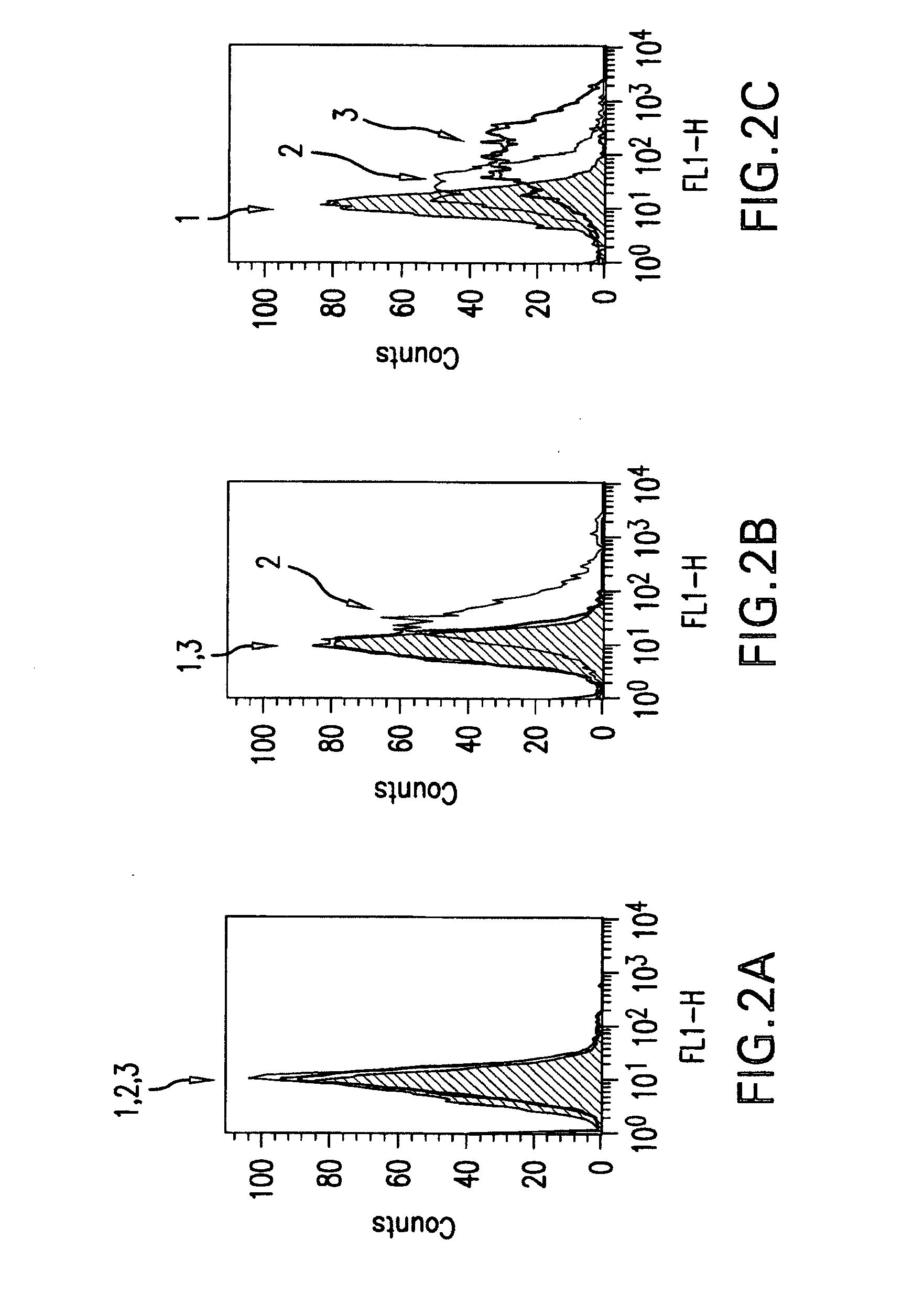
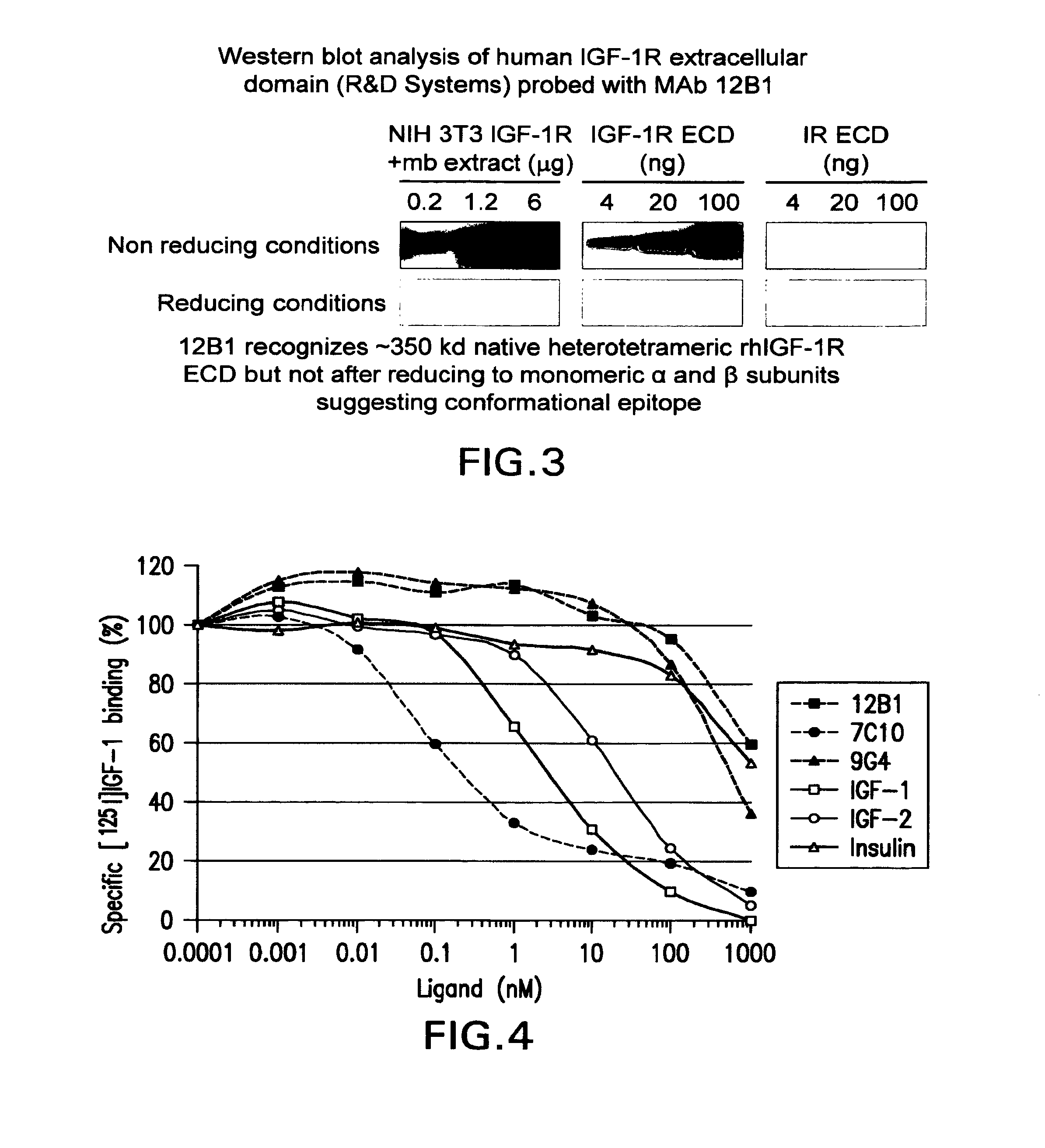
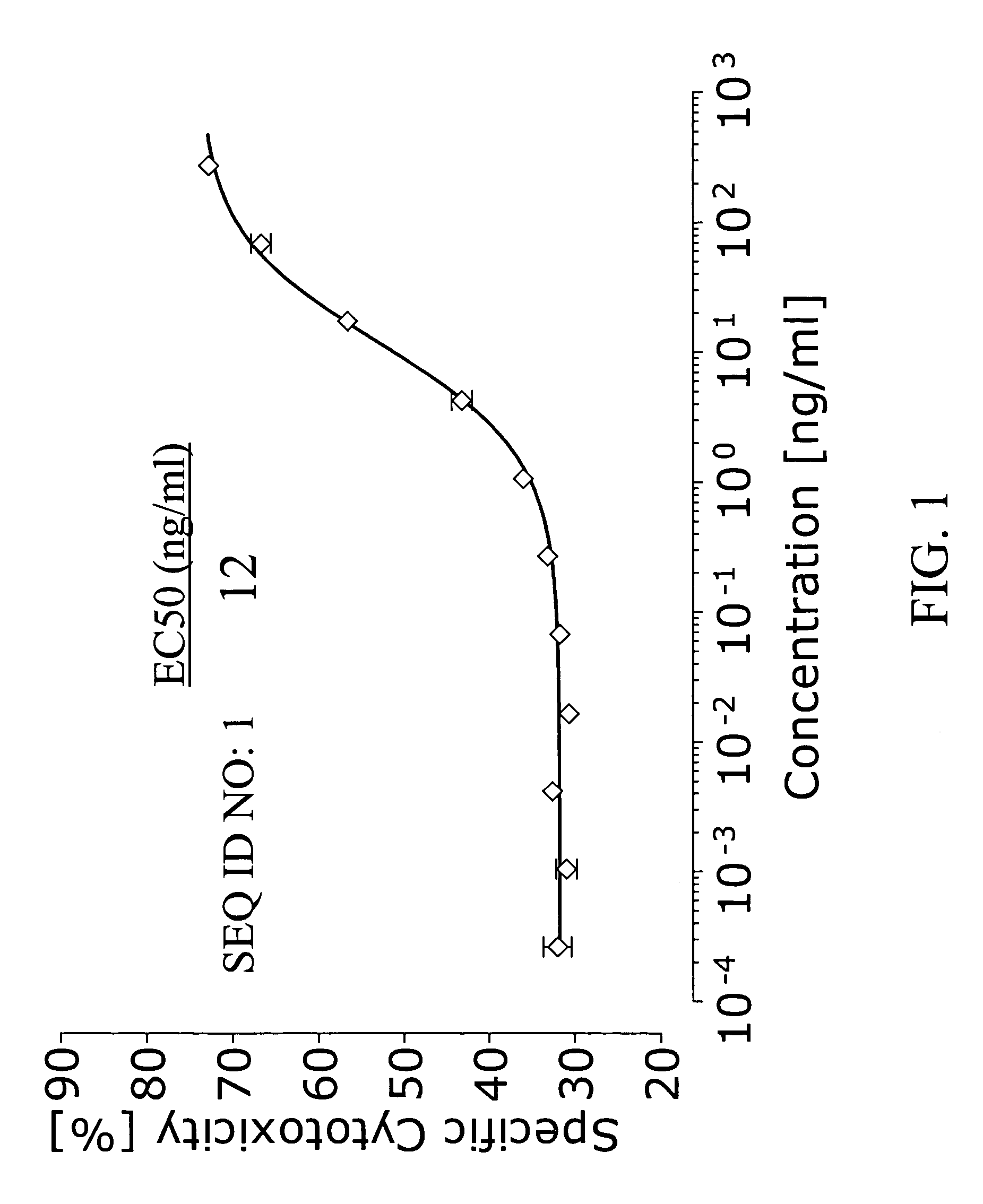
The present invention relates to mammalian antibodies, designated 12B1 and antigen-binding portions thereof that specifically bind to insulin-like growth factor I receptor (IGF-IR), preferably human IGF-IR. Also included are chimeric, bispecific, derivatized, single chain antibodies derived from the antibodies disclosed herein. Nucleic acid molecules encoding the mammalian antibodies as well as methods of use thereof are also disclosed. Also included are pharmaceutical compositions comprising these antibodies and methods of using the antibodies and compositions thereof for treatment and diagnosis of pathological hyperproliferative oncogenic disorders associated with expression of IGf-1R.
Owner:GOETSCH LILIANE +4
Bispecific antibodies
ActiveUS7235641B2Improve productivityImprove efficiencyAntipyreticAntibody mimetics/scaffoldsBispecific antibodyImmune effector cell
Owner:AMGEN RES (MUNICH) GMBH
Bispecific Antibody Point Mutations for Enhancing Rate of Clearance
A mutant bispecific antibody that includes (a) a human hinge constant region from IgG having one or more amino acid mutations in the CH2 domain, (b) two scFvs; and (c) two Fvs has been constructed. This type of antibody displays enhanced clearance, which has been found to be particularly useful in the context of pre-targeting methods.
Owner:IMMUNOMEDICS INC
Chimeric receptor genes and cells transformed therewith
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:UNITED STATES OF AMERICA +1
Method for making multispecific antibodies having heteromultimeric and common components
InactiveUS7951917B1Increased formationIncrease productionHybrid immunoglobulinsAntibody ingredientsSpecific immunityBispecific antibody
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method provides a multispecific antibody having a common light chain associated with each heteromeric polypeptide having an antibody binding domain. Additionally the method further involves introducing into the multispecific antibody a specific and complementary interaction at the interface of a first polypeptide and the interface of a second polypeptide, so as to promote heteromultimer formation and hinder homomultimer formation; and / or a free thiol-containing residue at the interface of a first polypeptide and a corresponding free thiol-containing residue in the interface of a second polypeptide, such that a non-naturally occurring disulfide bond is formed between the first and second polypeptide. The method allows for the enhanced formation of the desired heteromultimer relative to undesired heteromultimers and homomultimers.
Owner:GENENTECH INC
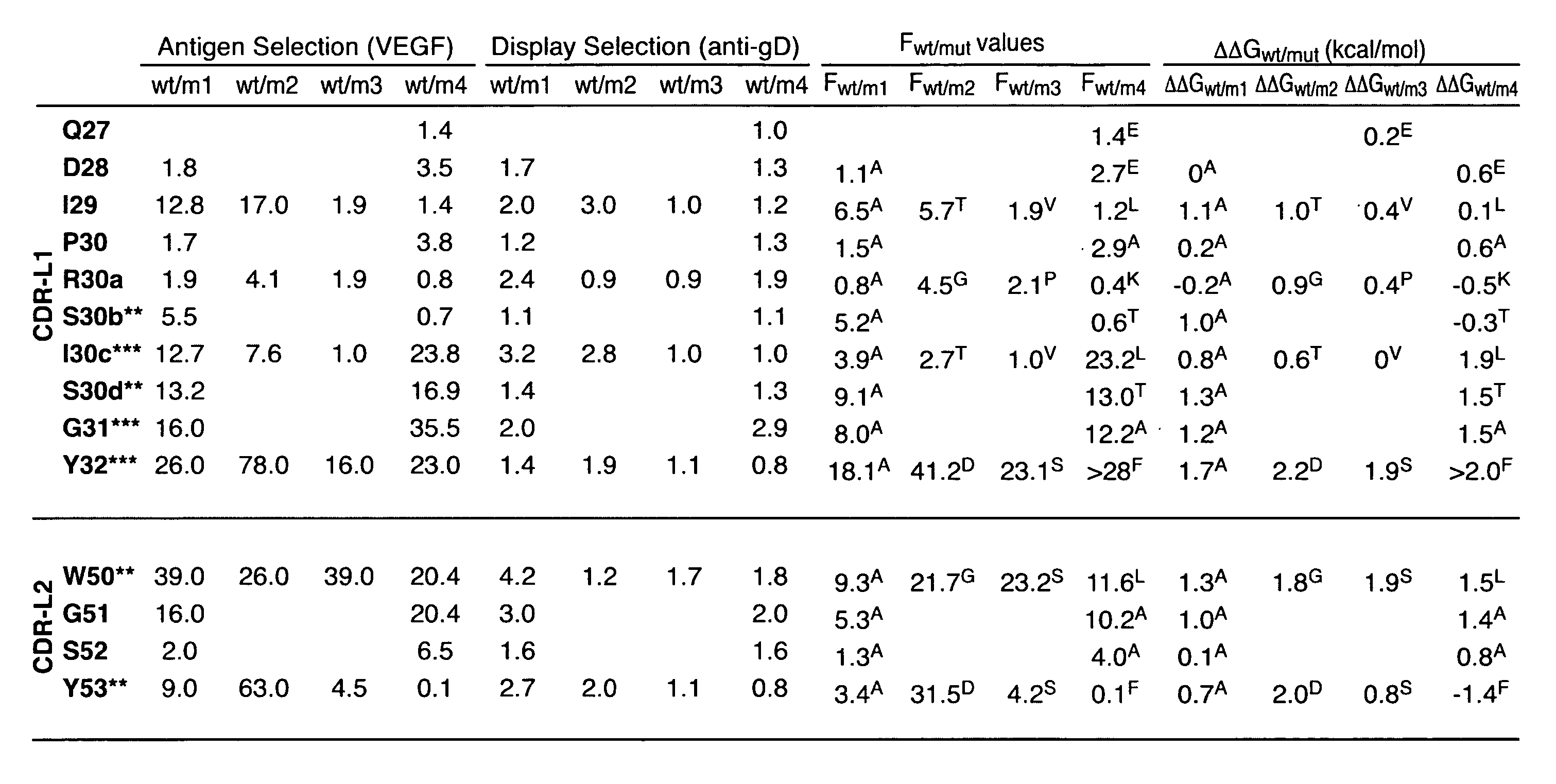
Method of Modifying Isoelectric Point of Antibody Via Amino Acid Substitution in CDR
ActiveUS20110076275A1Enhanced antigen-neutralizing activityImprove retentionSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsComplementarity determining regionAmino acid substitution
The present inventors provide methods for modifying the isoelectric point of an antibody while retaining its antigen-binding activity, comprising modifying the charge of at least one exposable amino acid residue on the surface of the complementarity determining region (CDR). The present invention also provides methods for purifying multispecific antibodies, comprising modifying isoelectric point, and methods for improving the plasma pharmacokinetics of antibodies, comprising modifying isoelectric point. The present invention further provides antibodies with a modified isoelectric point, pharmaceutical compositions comprising the antibodies as an active ingredient, and methods for producing the antibodies and compositions.
Owner:CHUGAI PHARMA CO LTD
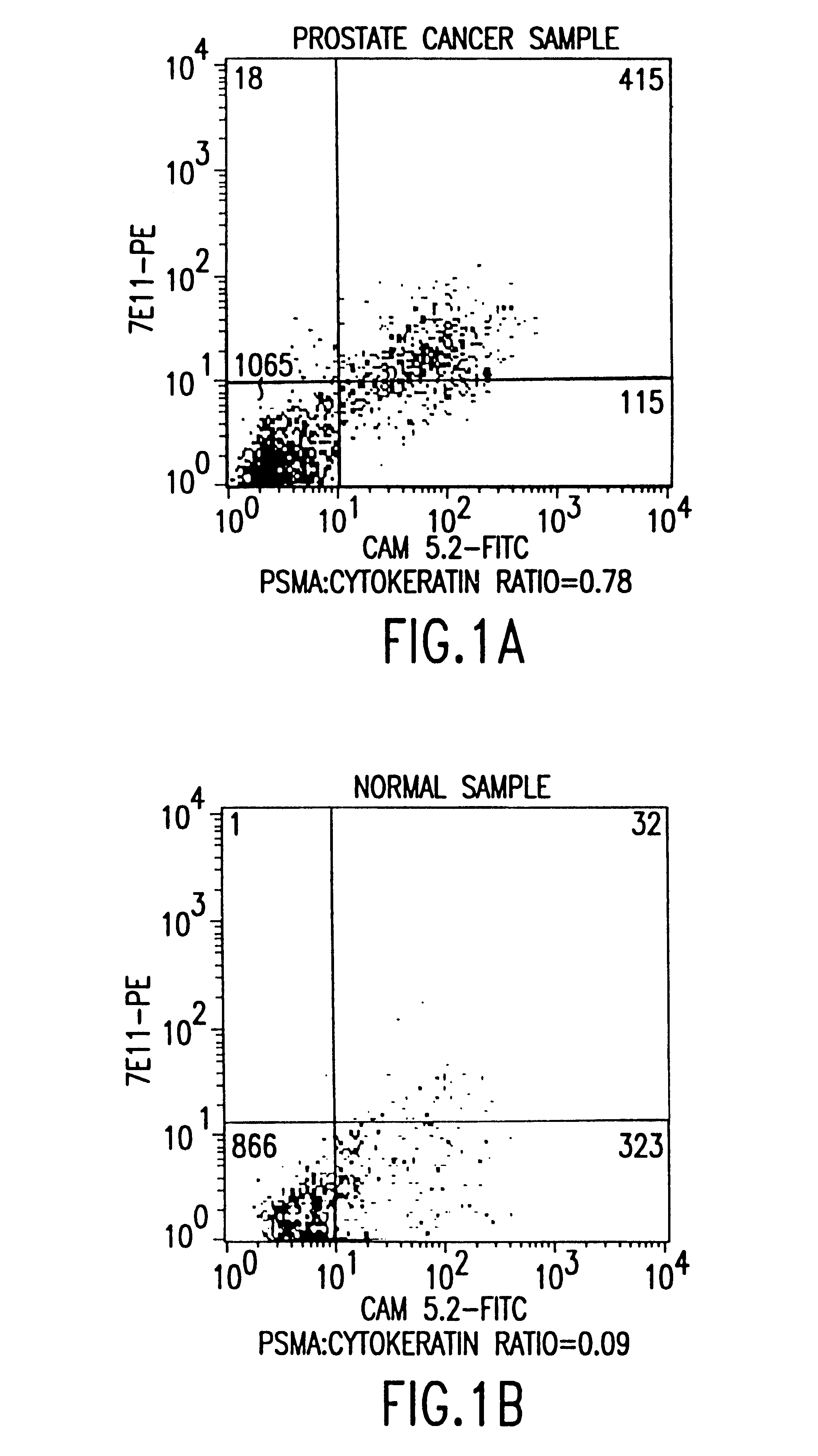
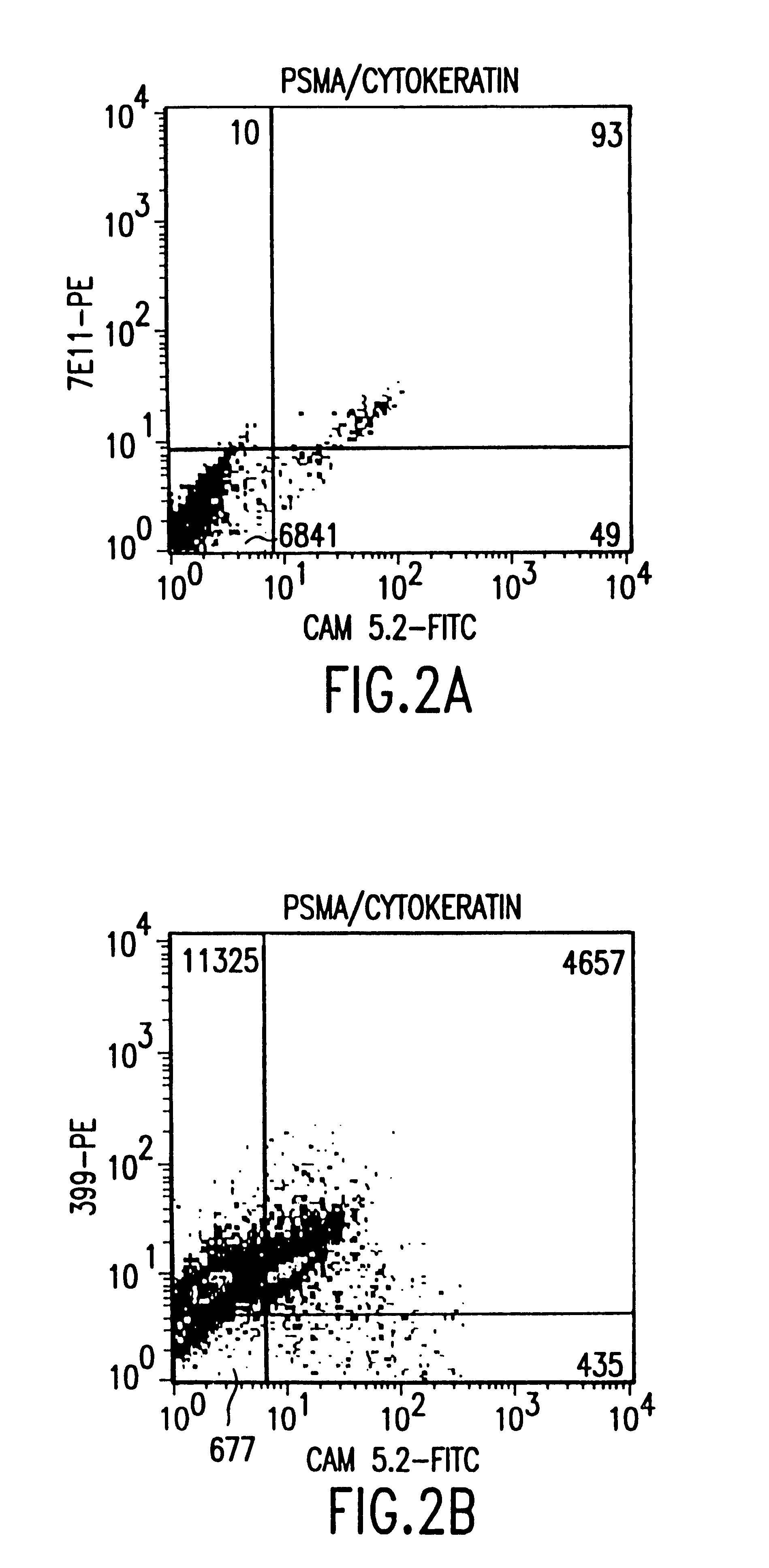
Non-invasive method to detect prostate cancer
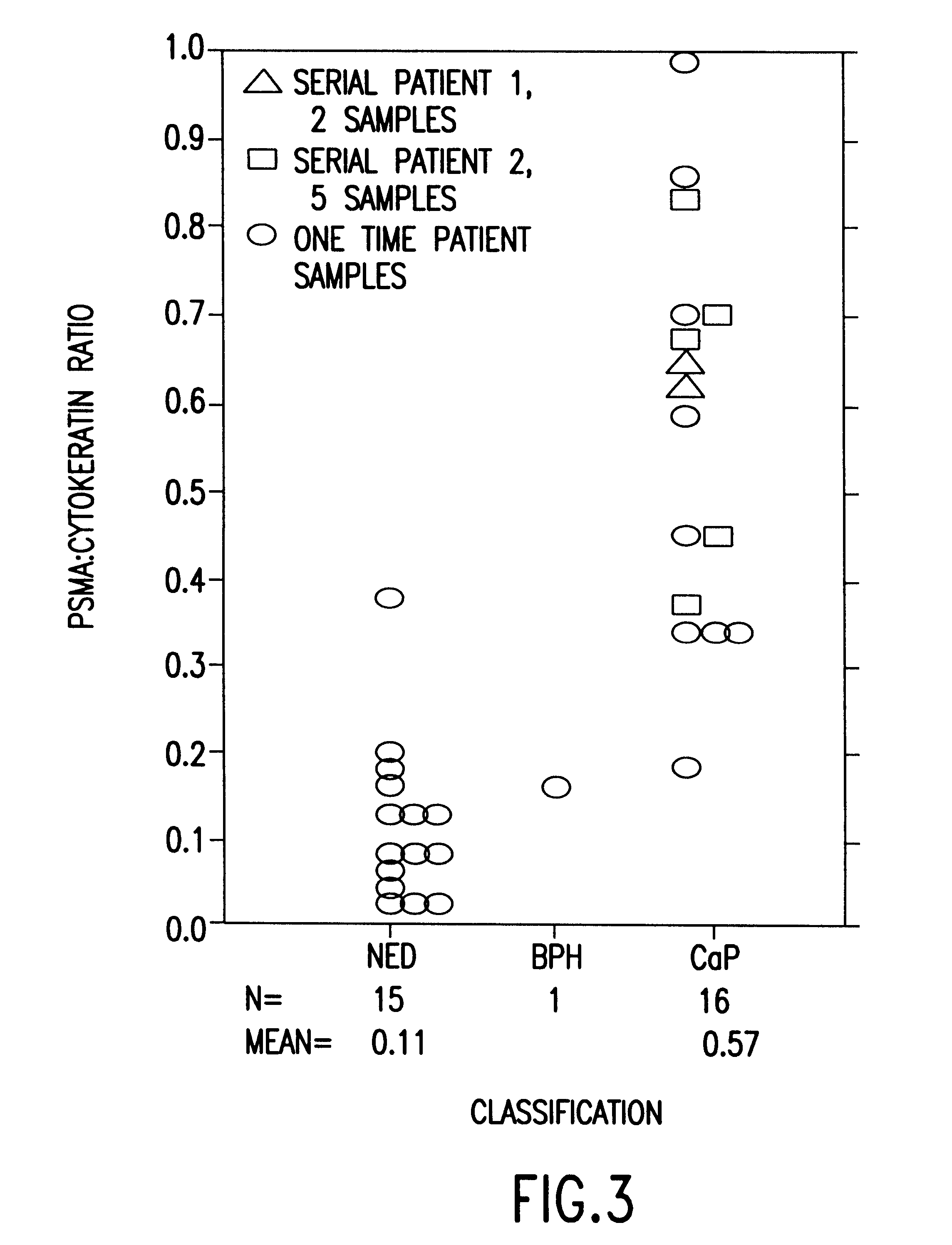
InactiveUS6383759B1Microbiological testing/measurementImmunoglobulinsAbnormal tissue growthNon invasive
The present invention is directed to methods of detecting prostate cancer in a sample of a body fluid with prostate cell marker-specific and epithelial cell marker-specific antibodies as well as to kits comprising such antibodies for use in the detection of prostate cancer. The present invention is also directed to methods of detecting prostate cancer in a sample of a body fluid with prostate cell marker-specific and tumor associated marker-specific antibodies as well as to kits comprising such antibodies for use in the detection of prostate cancer.
Owner:GERALD P MURPHY CANCER FOUND
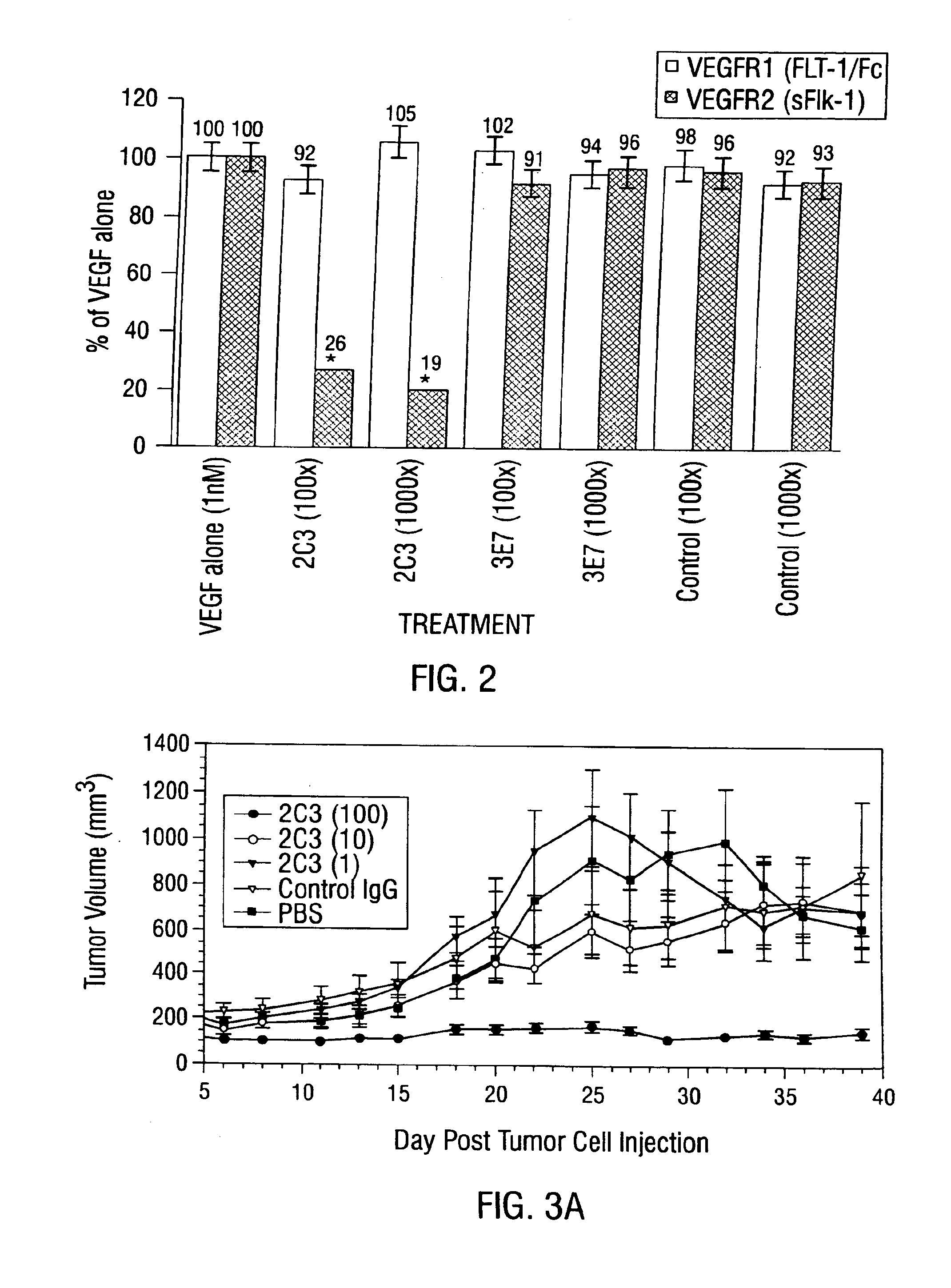
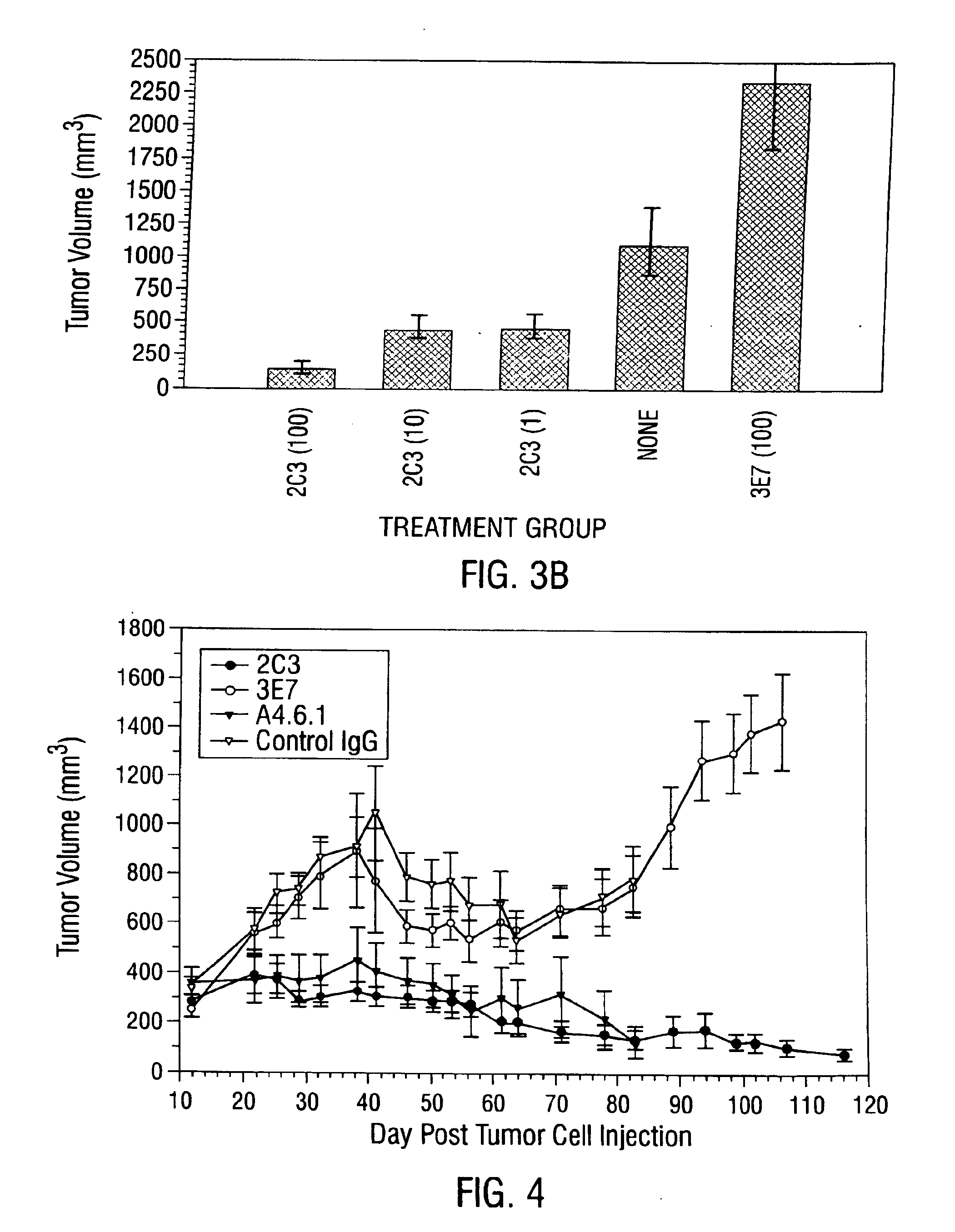
Antibody methods for selectively inhibiting VEGF
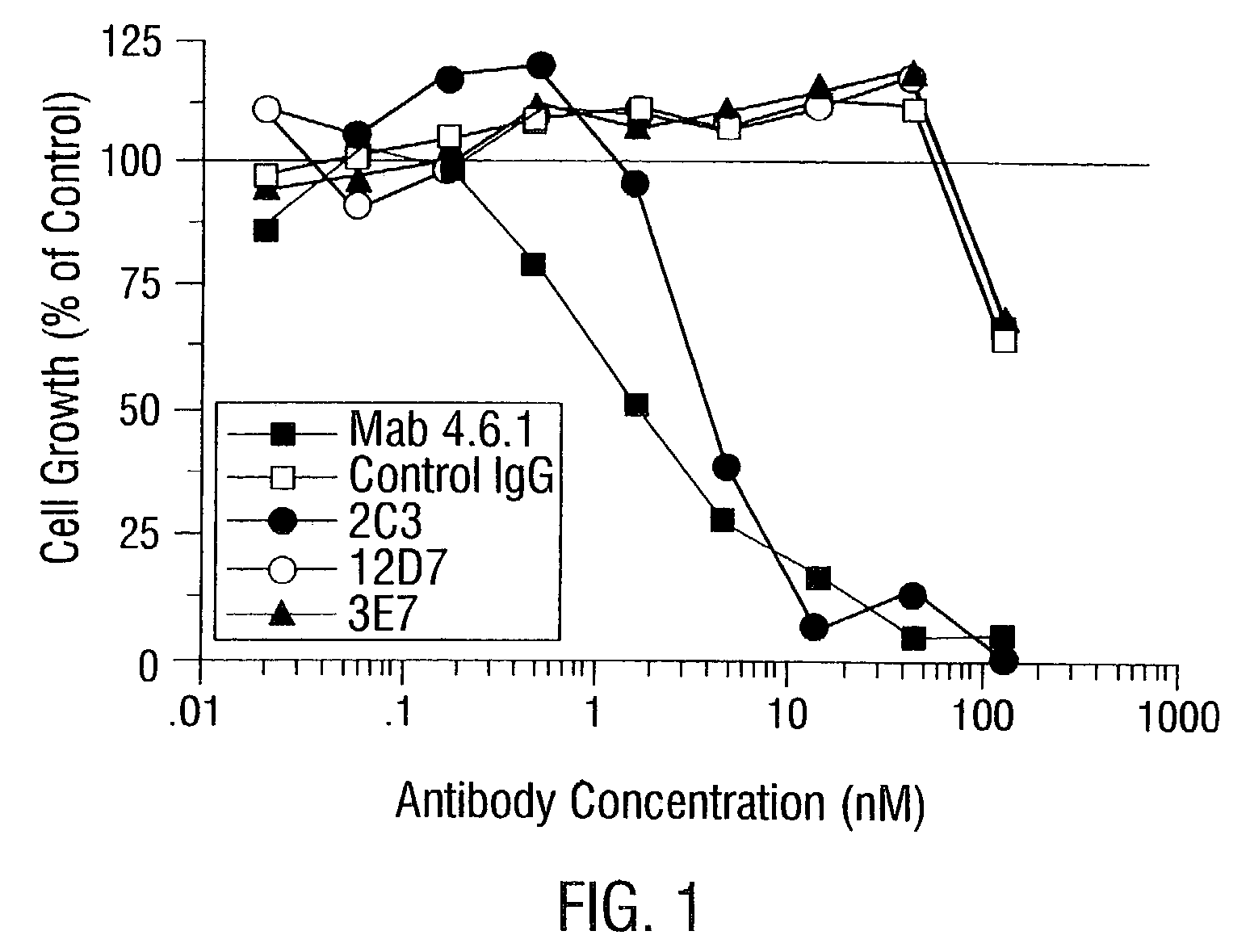
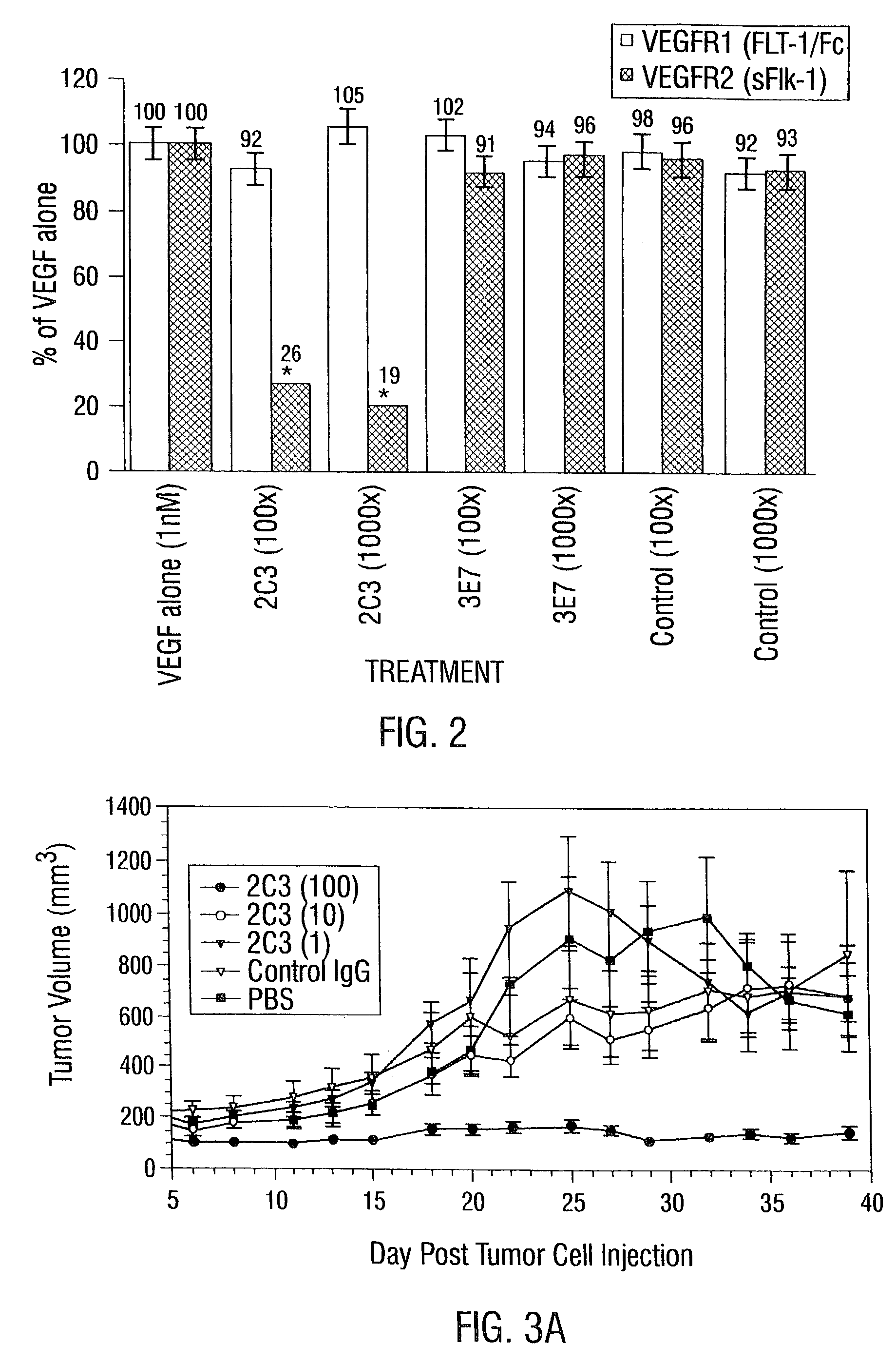
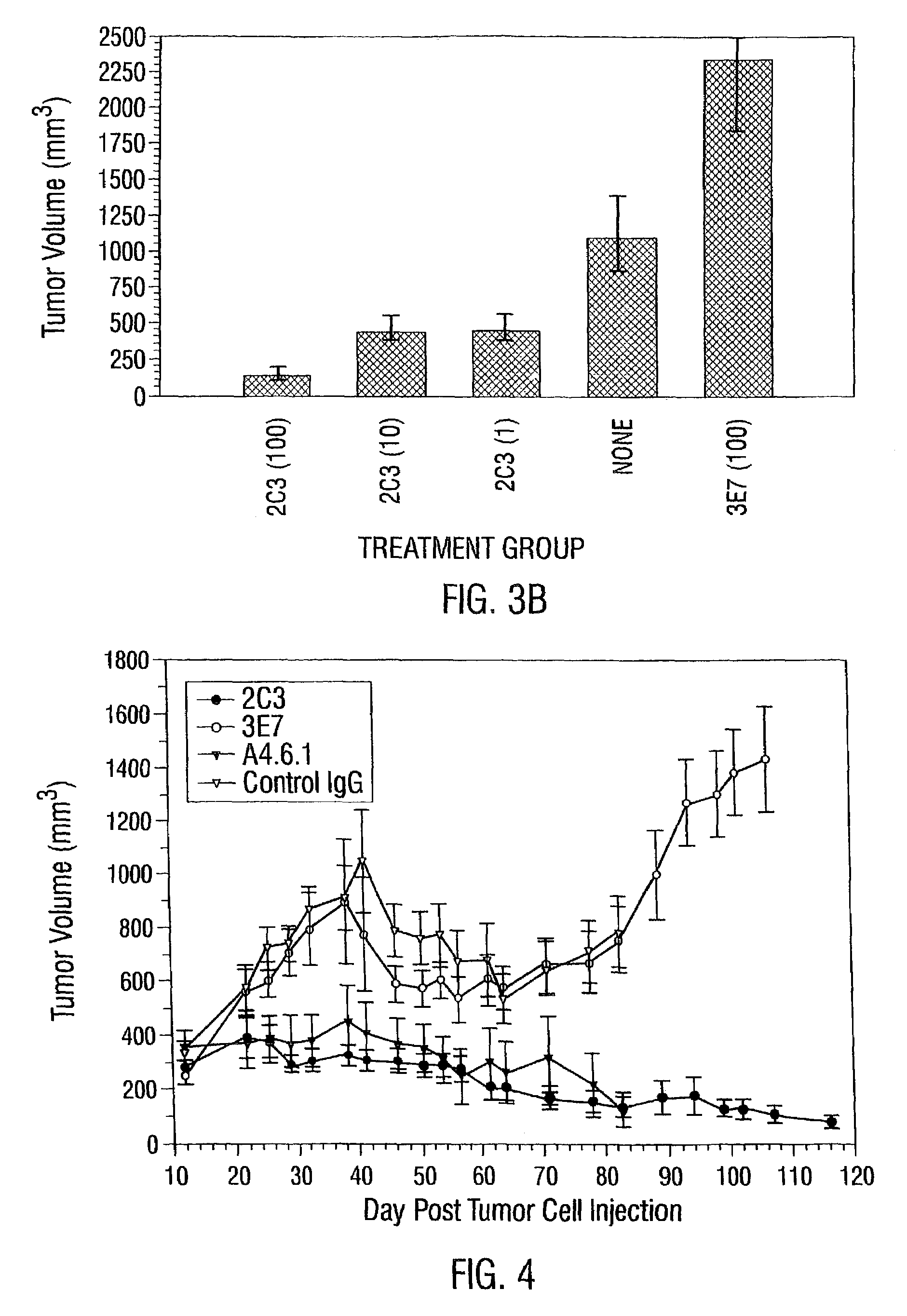
InactiveUS7056509B2Inhibit bindingAntibacterial agentsSenses disorderDiseaseAngiogenesis growth factor
Disclosed are antibodies that specifically inhibit VEGF binding to only one (VEGFR2) of the two VEGF receptors. The antibodies effectively inhibit angiogenesis and induce tumor regression, and yet have improved safety due to their specificity. The present invention thus provides new antibody-based compositions, methods and combined protocols for treating cancer and other angiogenic diseases. Advantageous immunoconjugate and prodrug compositions and methods using the new VEGF-specific antibodies are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Coleopteran-toxic polypeptide compositions and insect-resistant transgenic plants
InactiveUS6555655B1Easy to storeInhibit microbial growthBiocideBacteriaDelta endotoxinPolynucleotide
Disclosed are novel insecticidal polypeptides, and compositions comprising these polypeptides, peptide fragments thereof, and antibodies specific therefor. Also disclosed are vectors, transformed host cells, and transgenic plants that contain nucleic acid segments that encode the disclosed delta-endotoxin polypeptides. Also disclosed are methods of identifying related polypeptides and polynucleotides, methods of making and using transgenic cells comprising these polynucleotide sequences, as well as methods for controlling an insect population, such as Colorado potato beetle, southern corn rootworm and western corn rootworm, and for conferring to a plant resistance to a target insect species.
Owner:MONSANTO TECH LLC
Antibody kits for selectively inhibiting VEGF
InactiveUS6887468B1Reduce control antibody bindingReduce the binding forceAntibacterial agentsSenses disorderDiseaseAngiogenesis growth factor
Disclosed are antibodies that specifically inhibit VEGF binding to only one (VEGFR2) of the two VEGF receptors. The antibodies effectively inhibit angiogenesis and induce tumor regression, and yet have improved safety due to their specificity. The present invention thus provides new antibody-based compositions, methods and combined protocols for treating cancer and other angiogenic diseases. Advantageous immunoconjugate and prodrug compositions and methods using the new VEGF-specific antibodies are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Bivalent, bispecific antibodies
InactiveUS8227577B2Raise the ratioIncrease productionAntibacterial agentsAntibody mimetics/scaffoldsAntiendomysial antibodiesBispecific antibody
Owner:F HOFFMANN LA ROCHE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com