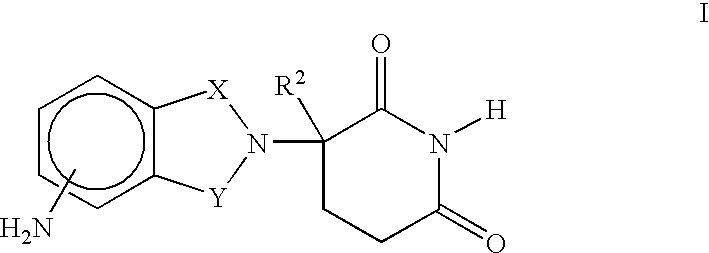
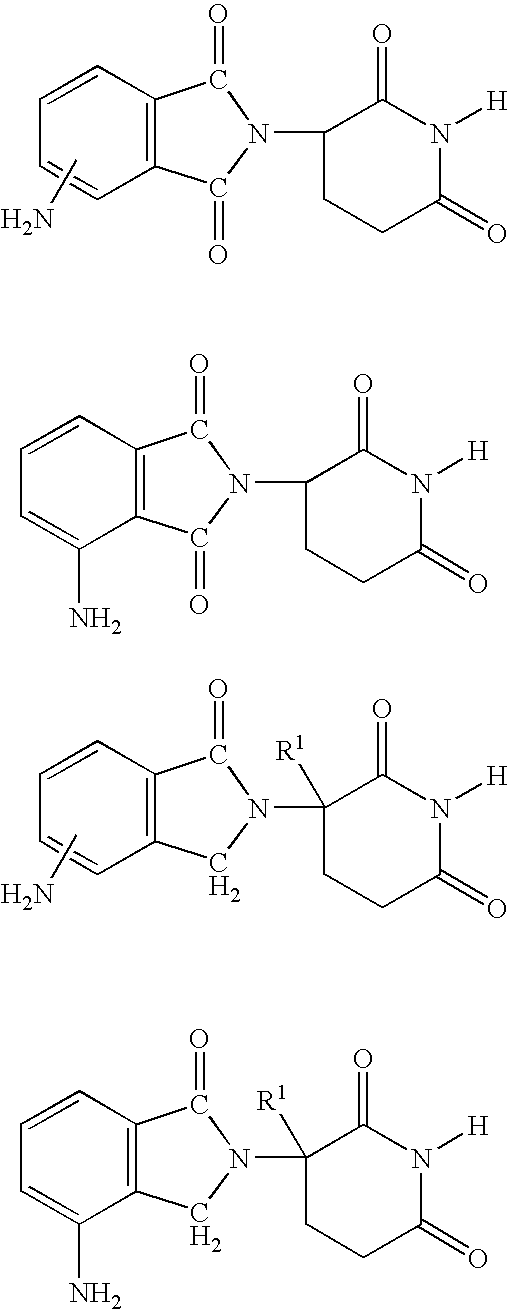
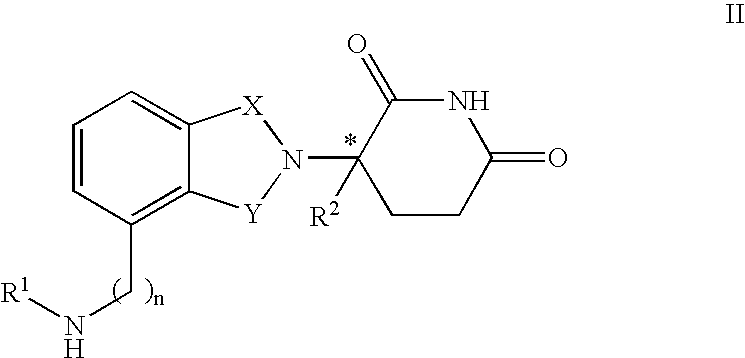
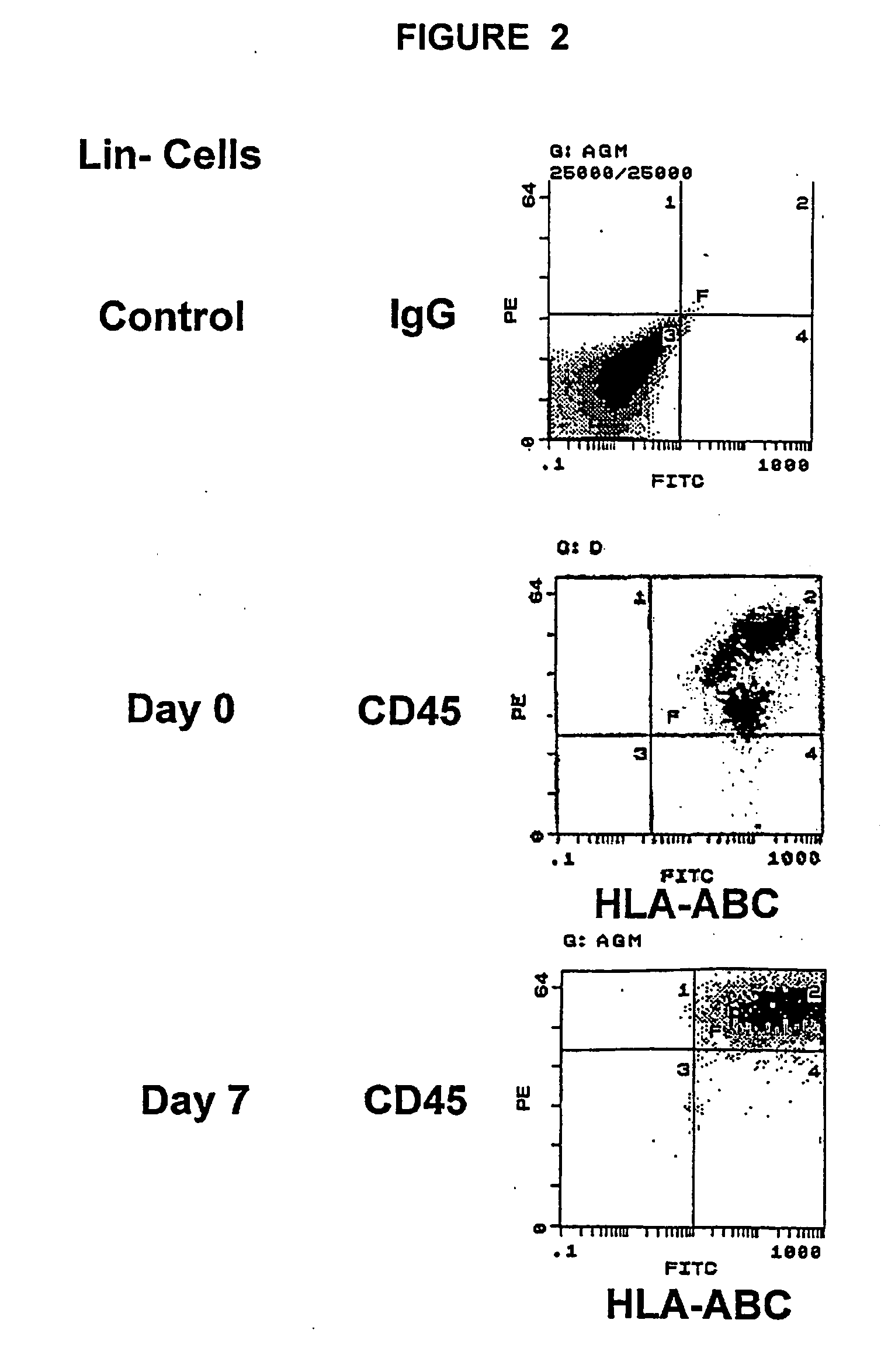
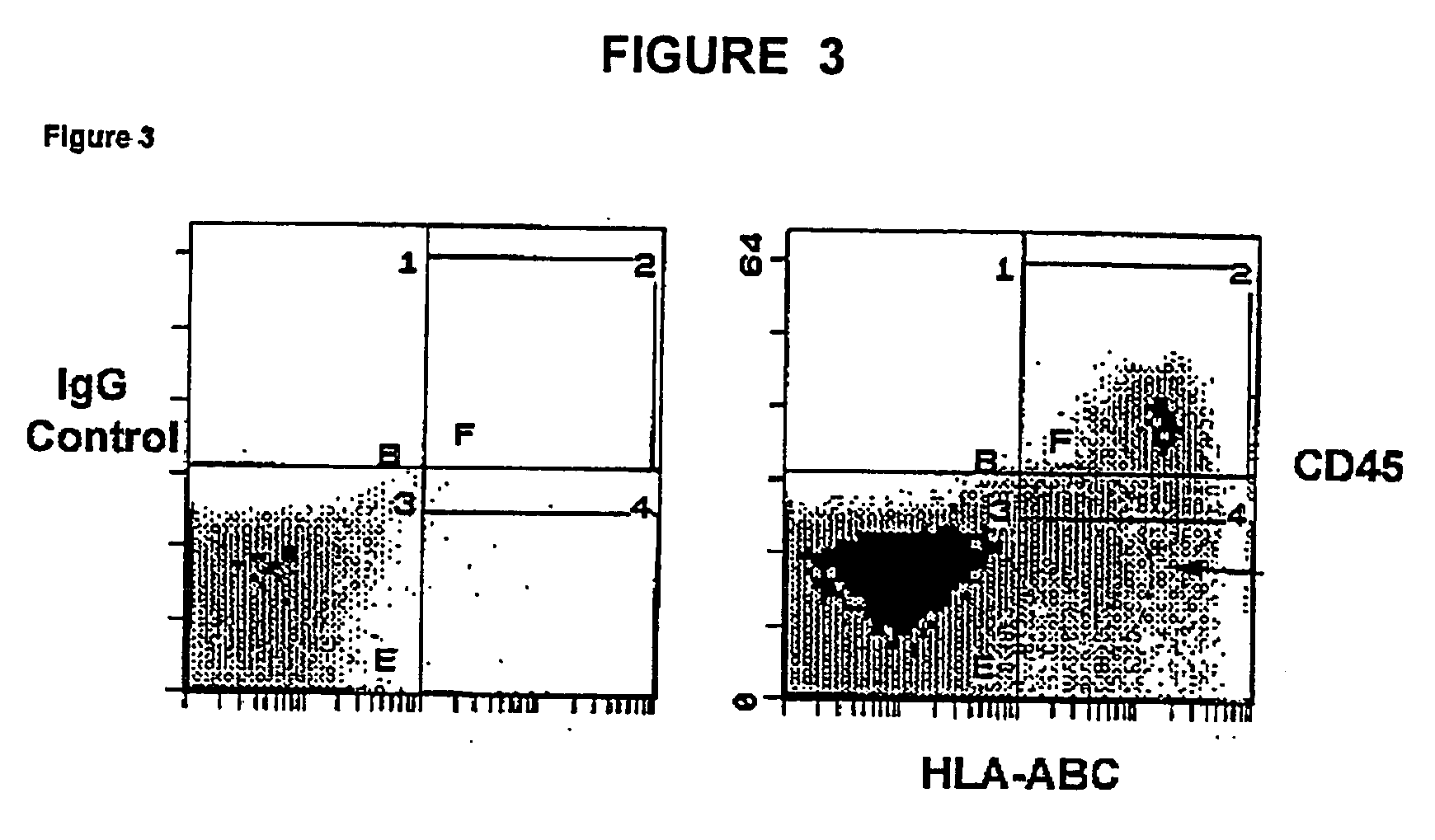
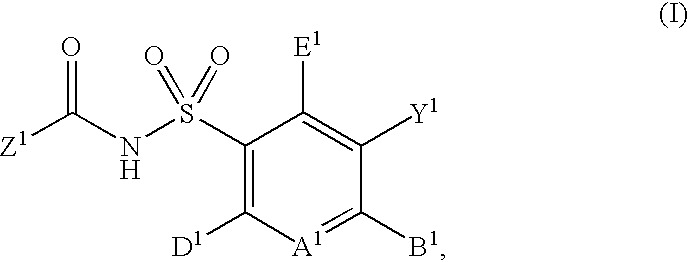
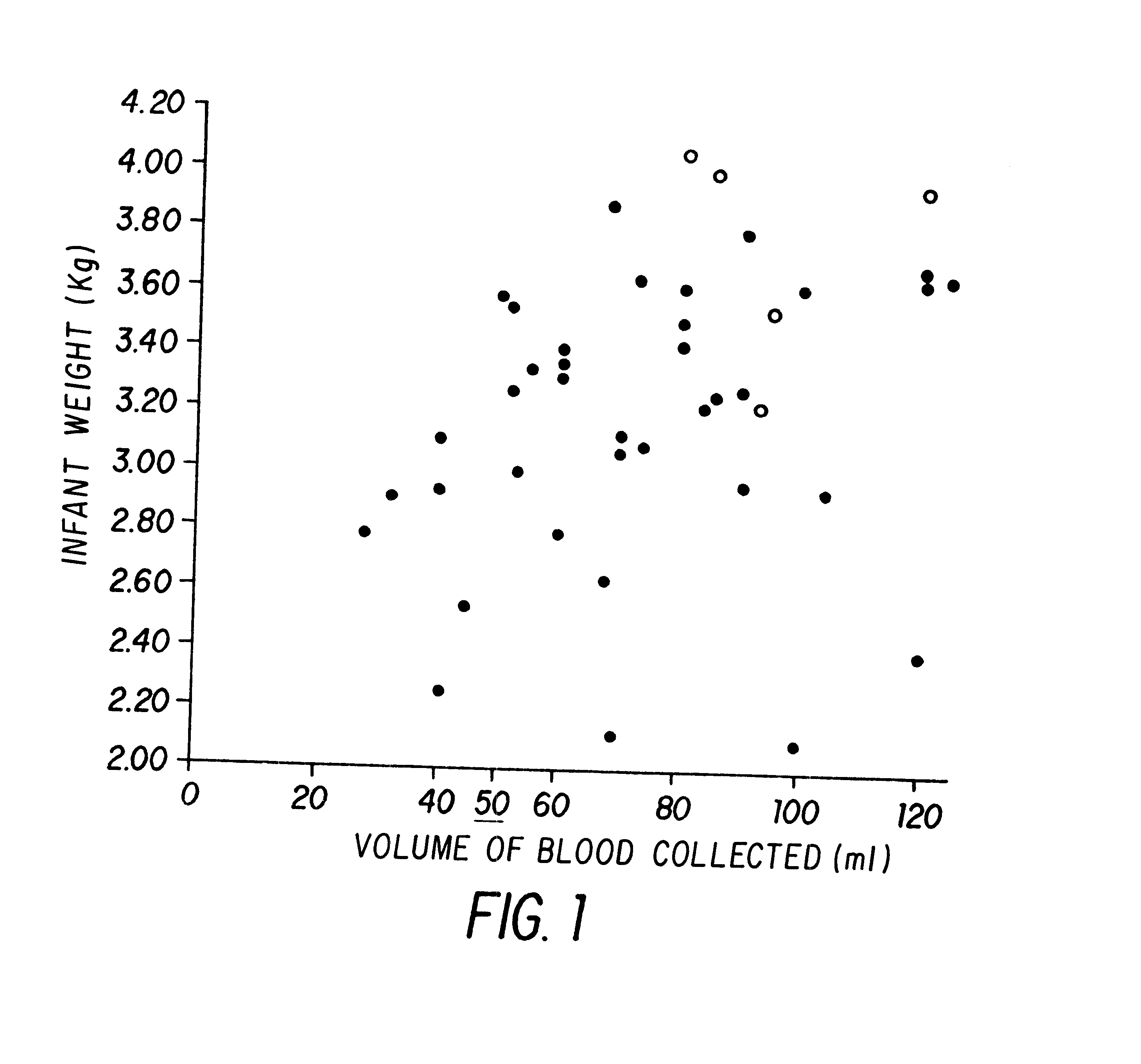
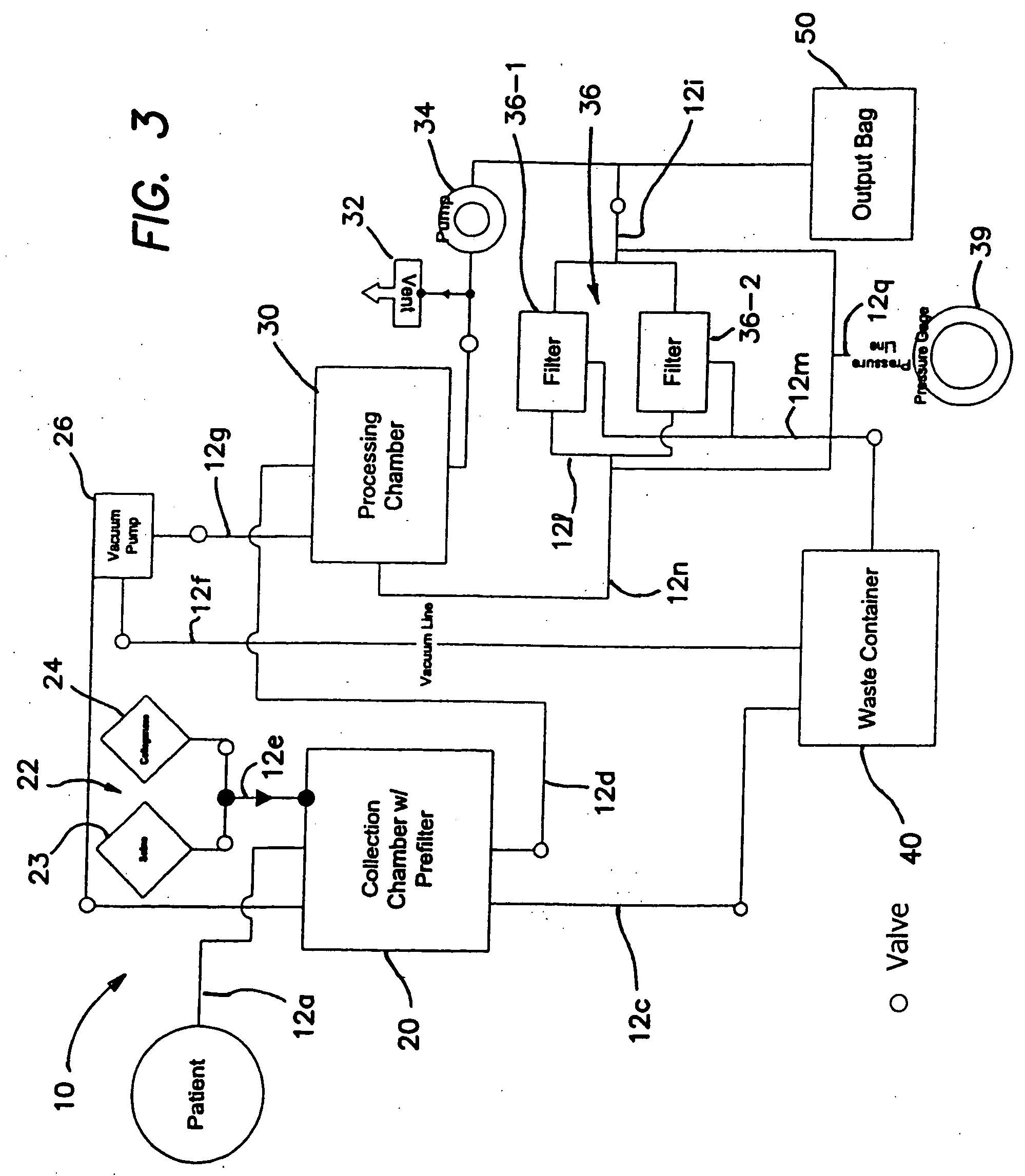
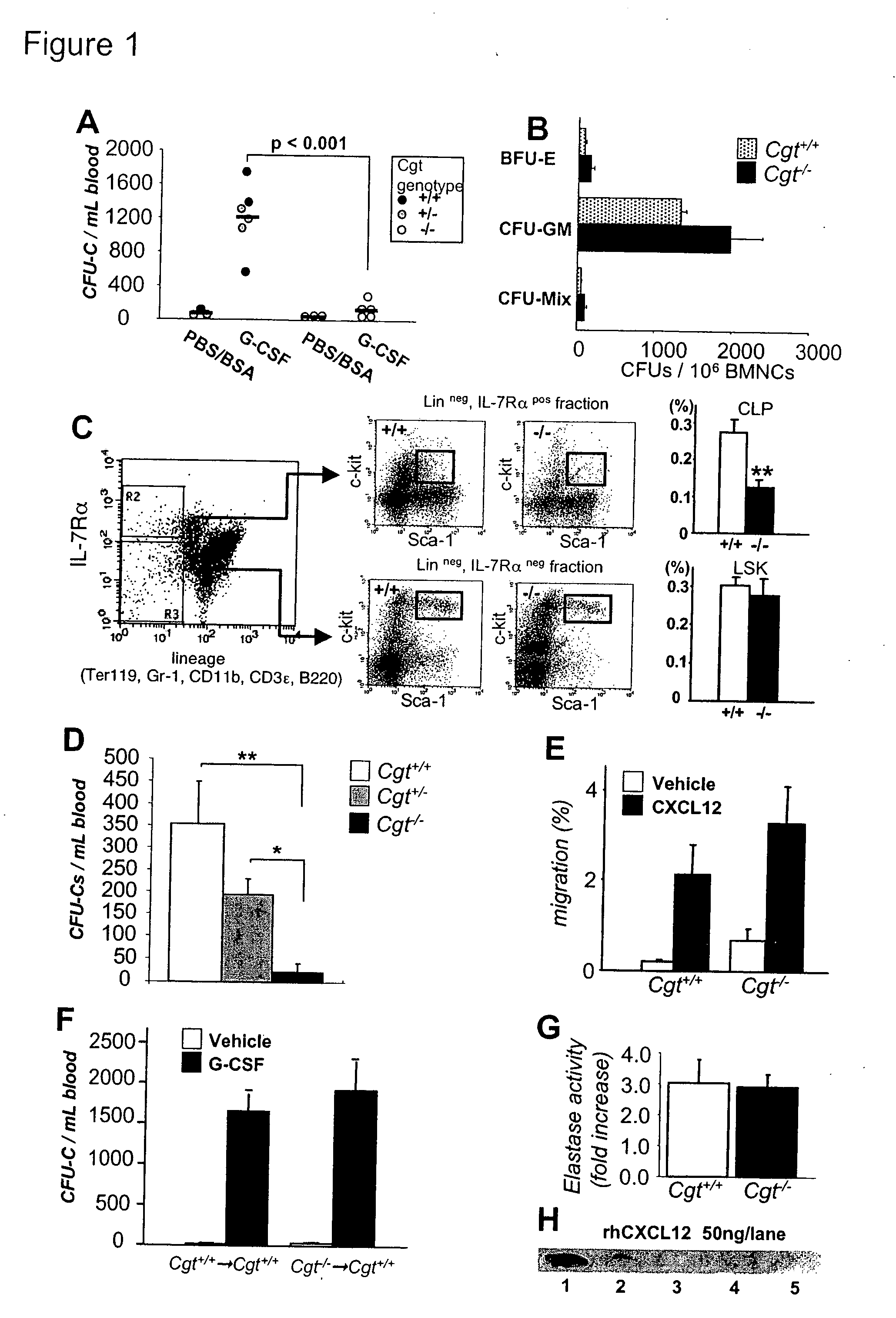
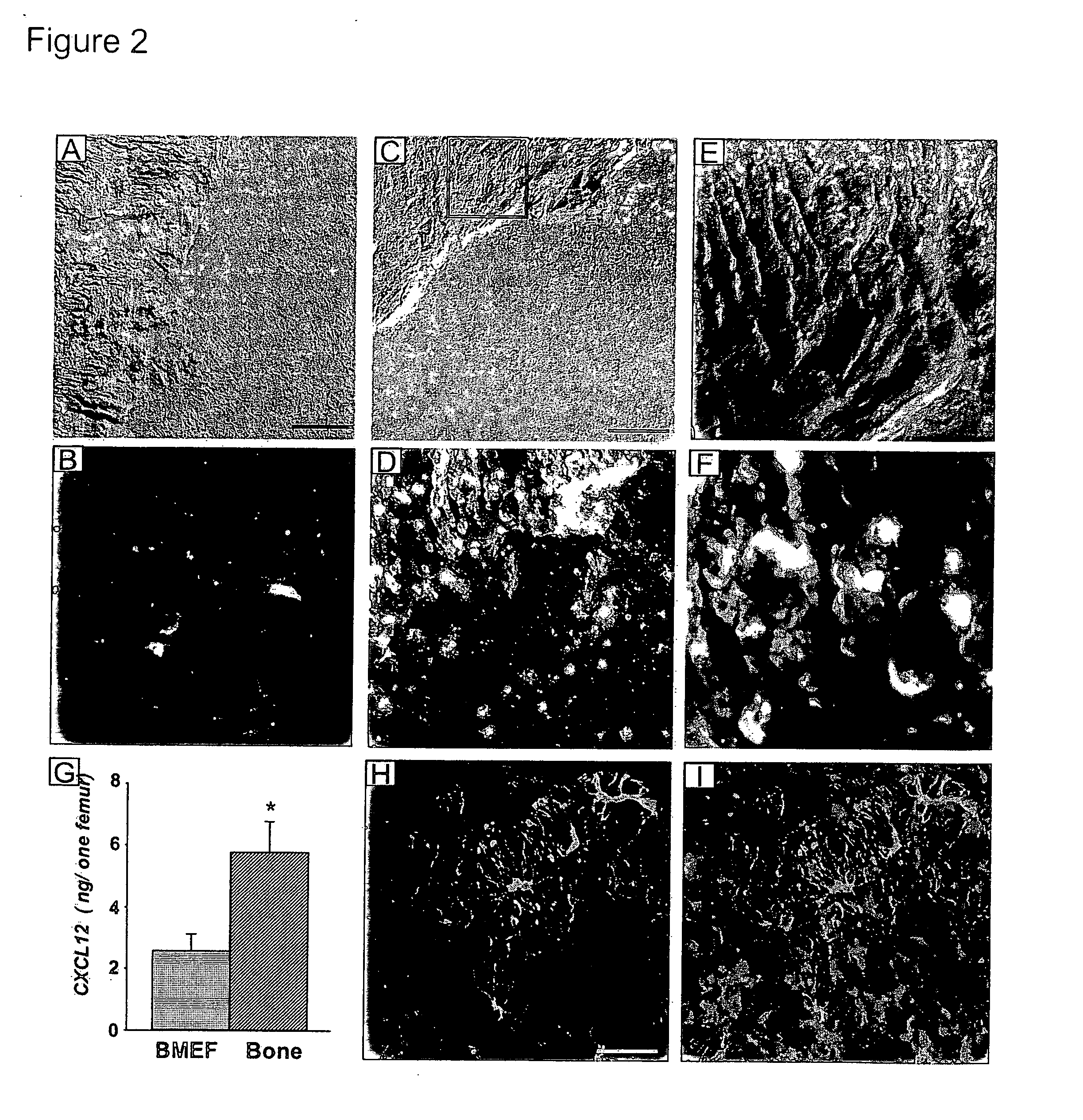
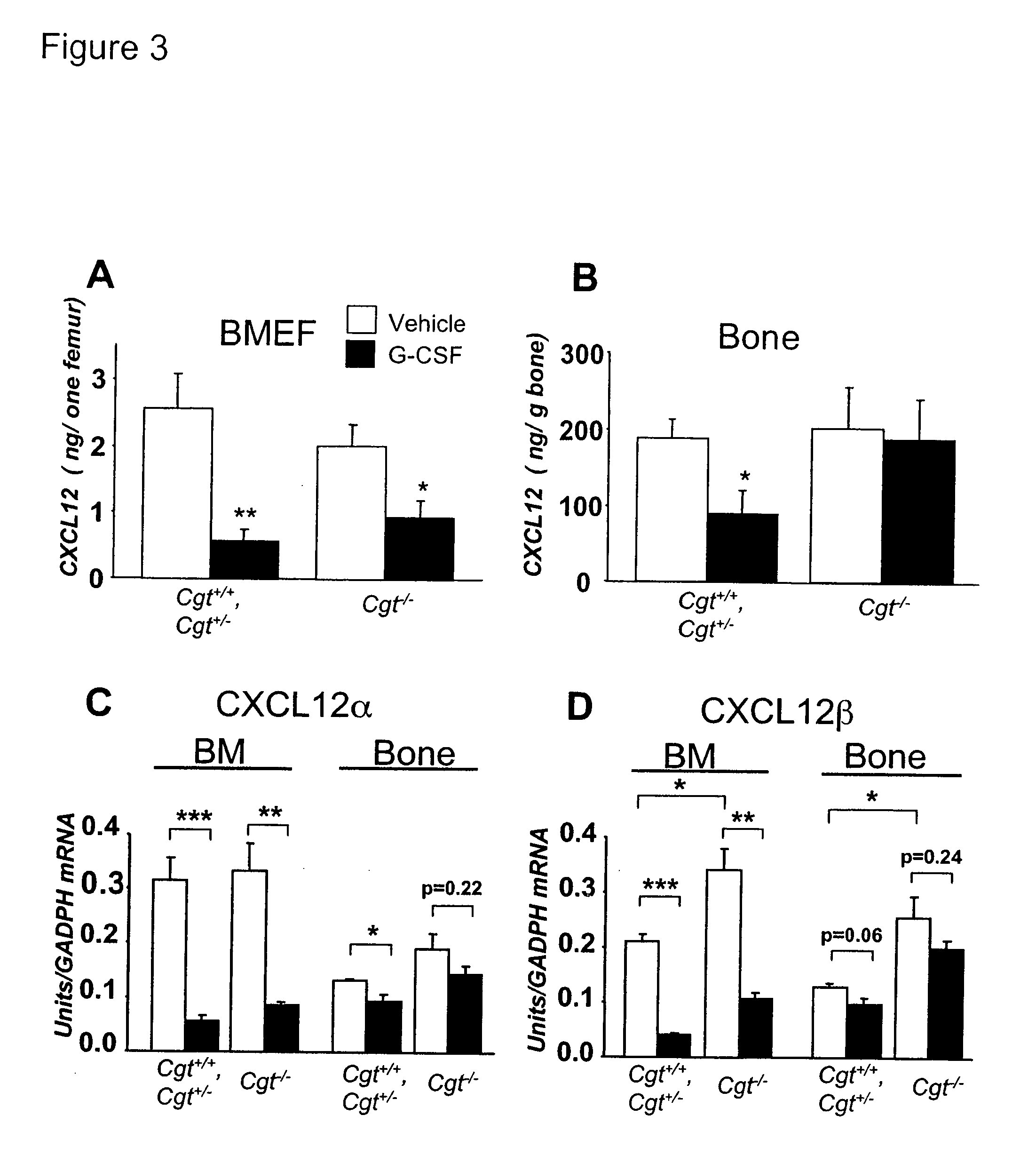
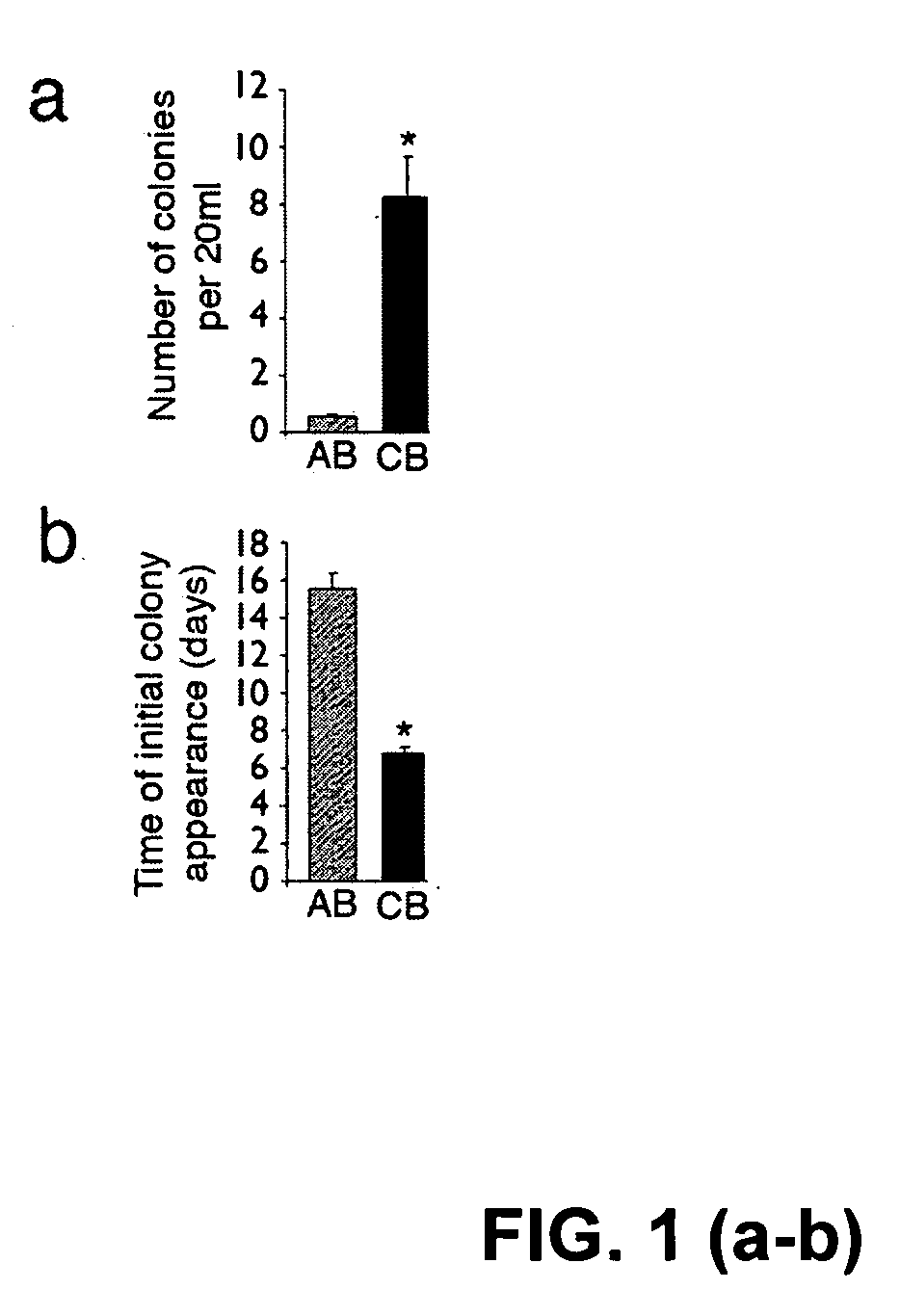
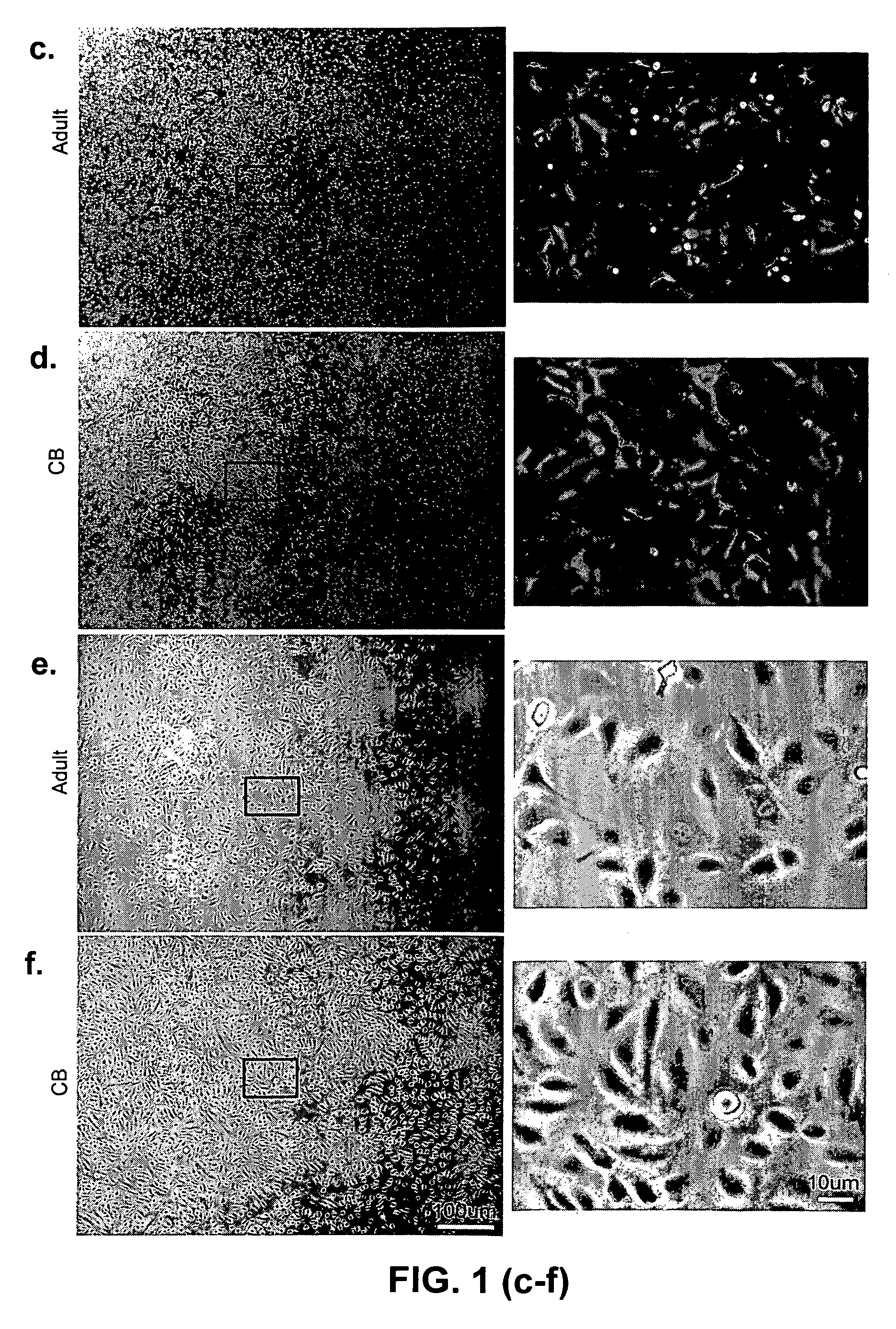
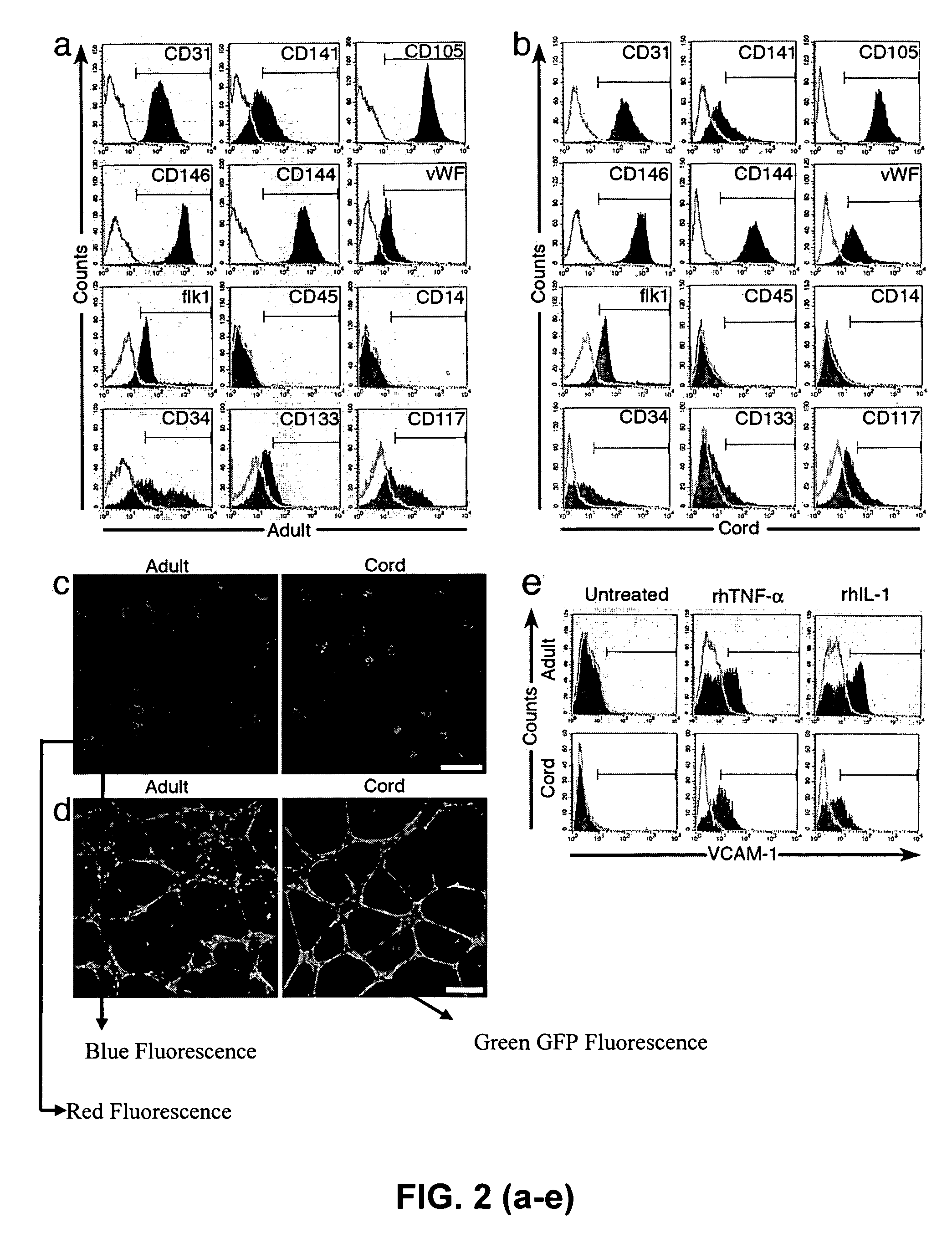
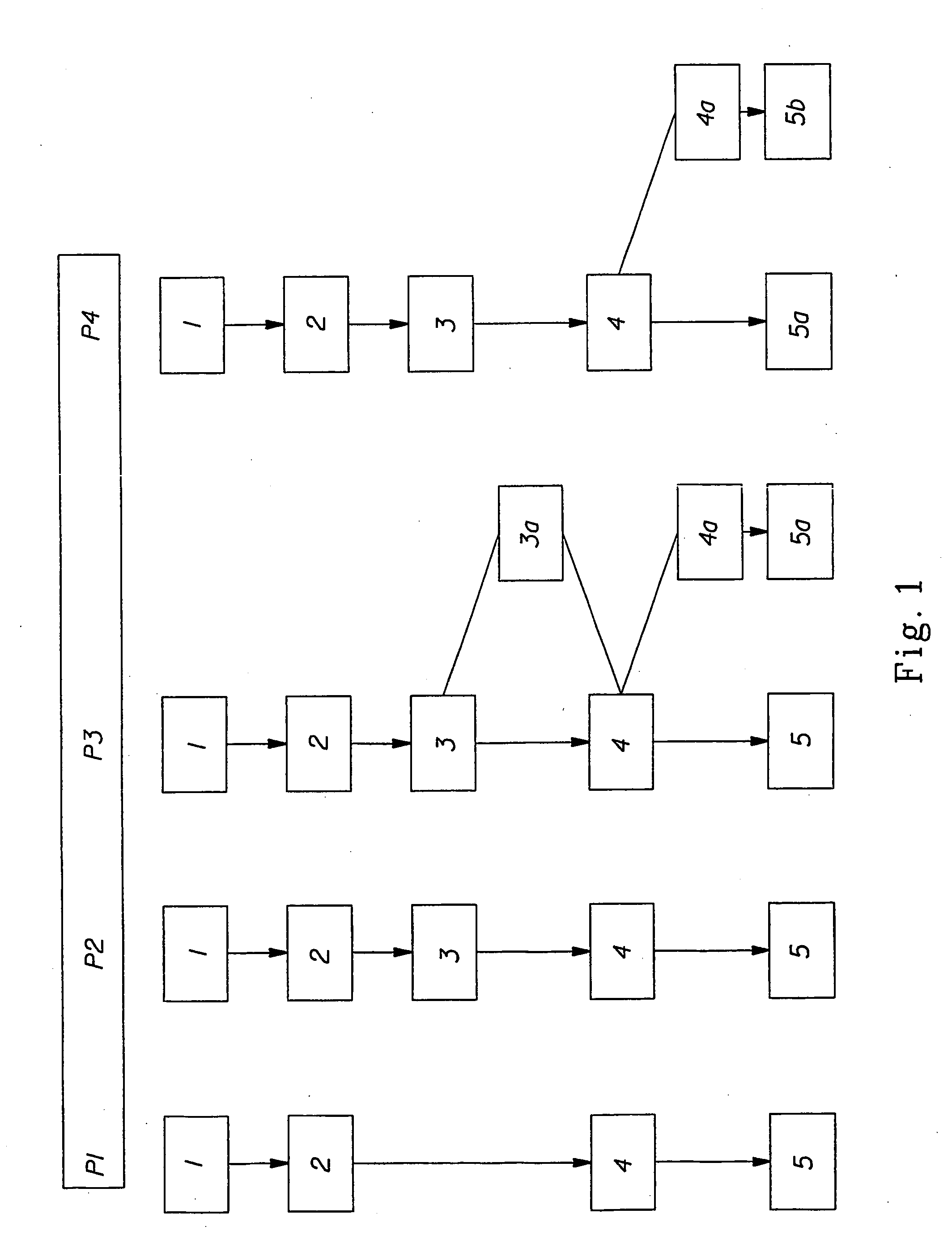
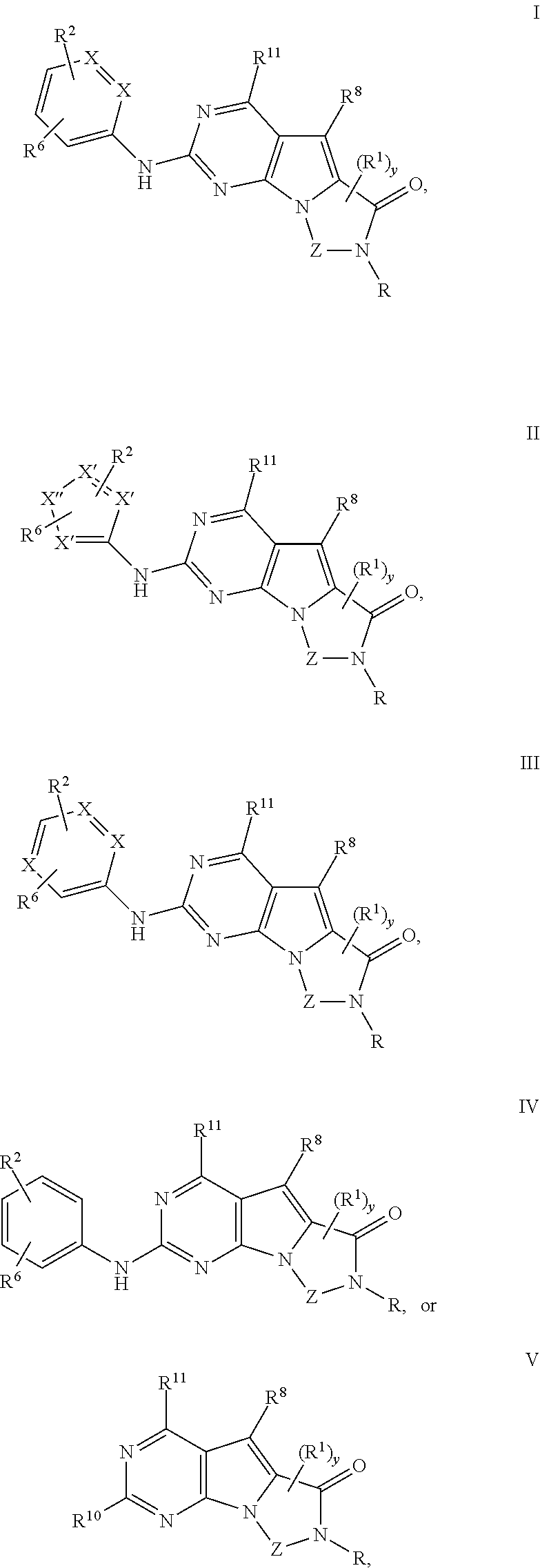
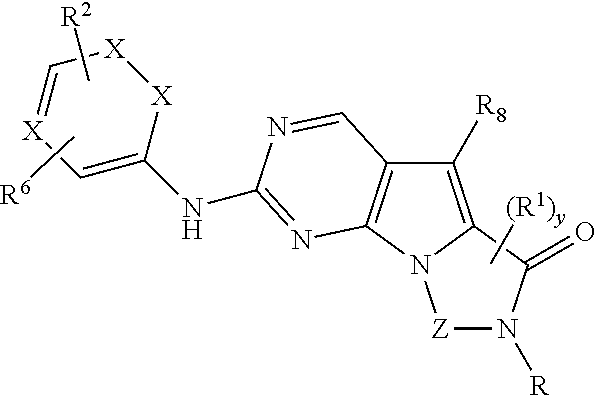
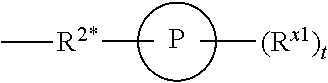Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1378 results about "Hematopoietic stem cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. This process occurs in the red bone marrow, in the core of most bones. In embryonic development, the red bone marrow is derived from the layer of the embryo called the mesoderm.
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of myeloproliferative diseases
InactiveUS20040087546A1Reduce adverse effectsImprove toleranceBiocideNervous disorderActive agentMyeloproliferative disease
Methods of treating, preventing and / or managing a myeloproliferative disease are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent, and / or the transplantation of blood or cells. Particular second active agents are capable of suppressing the overproduction of hematopoietic stem cells or ameliorating one or more of the symptoms of a myeloproliferative disease. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Owner:GLAXO SMITHKLINE LLC
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Methods for the treatment of lymphoma by administration of a B cell-specific antibody are described. The invention encompasses providing to a patient both unlabeled antibodies and antibodies labeled with a radioisotope. A principal advantage of the method is that tumor responses can be obtained in a radiometric dose range that does not require hematopoietic stem cell replacement as an adjunct therapy also described is a composition useful in the treatment of lymphoma.
Owner:RGT UNIV OF MICHIGAN +2
Embryonic-like stem cells derived from post-partum mammalian placenta, and uses and methods of treatment using said cells
Owner:CELULARITY INC
Cellular compositions and methods of making and using them
InactiveUS20050074435A1Increase the number ofEasy to collectPeptide/protein ingredientsDrug screeningProgenitorHematopoietic cell
The invention relates to cellular compositions comprising hematopoietic cells with the potential or increased potential to form non-hematopoietic cells; methods for producing such cellular compositions; methods for differeentitation of cells of cellular compositions of the invention into cells that exhibit morphological, physiological, functional, and / or immunological features of non-hematopoietic cells; and uses of the cellular compositions. The invention also relates to a method for the expansion of hemtopoietic stem and progenitor cells.
Owner:MOUNT SINAI HOSPITAL
Method of preventing or treating organ, hematopoietic stem cell or bone marrow transplant rejection
InactiveUS20080182845A1Inhibitory activityOrganic chemistryImmunological disordersBone marrow transplantationSurgery
Methods of preventing or treating organ, hematopoietic stem cell or bone marrow transplant rejection are disclosed.
Owner:ABBOTT LAB INC
Isolation and preservation of fetal and neonatal hematopoietic stem and progenitor cells of the blood
The present invention relates to hematopoietic stem and progenitor cells of neonatal or fetal blood that are cryopreserved, and the therapeutic uses of such stem and progenitor cells upon thawing. In particular, the present invention relates to the therapeutic use of fetal or neonatal stem cells for hematopoietic (or immune) reconstitution. Hematopoietic reconstitution with the cells of the invention can be valuable in the treatment or prevention of various diseases and disorders such as anemias, malignancies, autoimmune disorders, and various immune dysfunctions and deficiencies. In another embodiment, fetal or neonatal hematopoietic stem and progenitor cells which contain a heterologous gene sequence can be used for hematopoietic reconstitution in gene therapy. In a preferred embodiment of the invention, neonatal or fetal blood cells that have been cryopreserved and thawed can be used for autologous (self) reconstitution.
Owner:PHARMASTEM THERAPEUTICS
Method of producing undifferentiated hemopoietic stem cells using a stationary phase plug-flow bioreactor
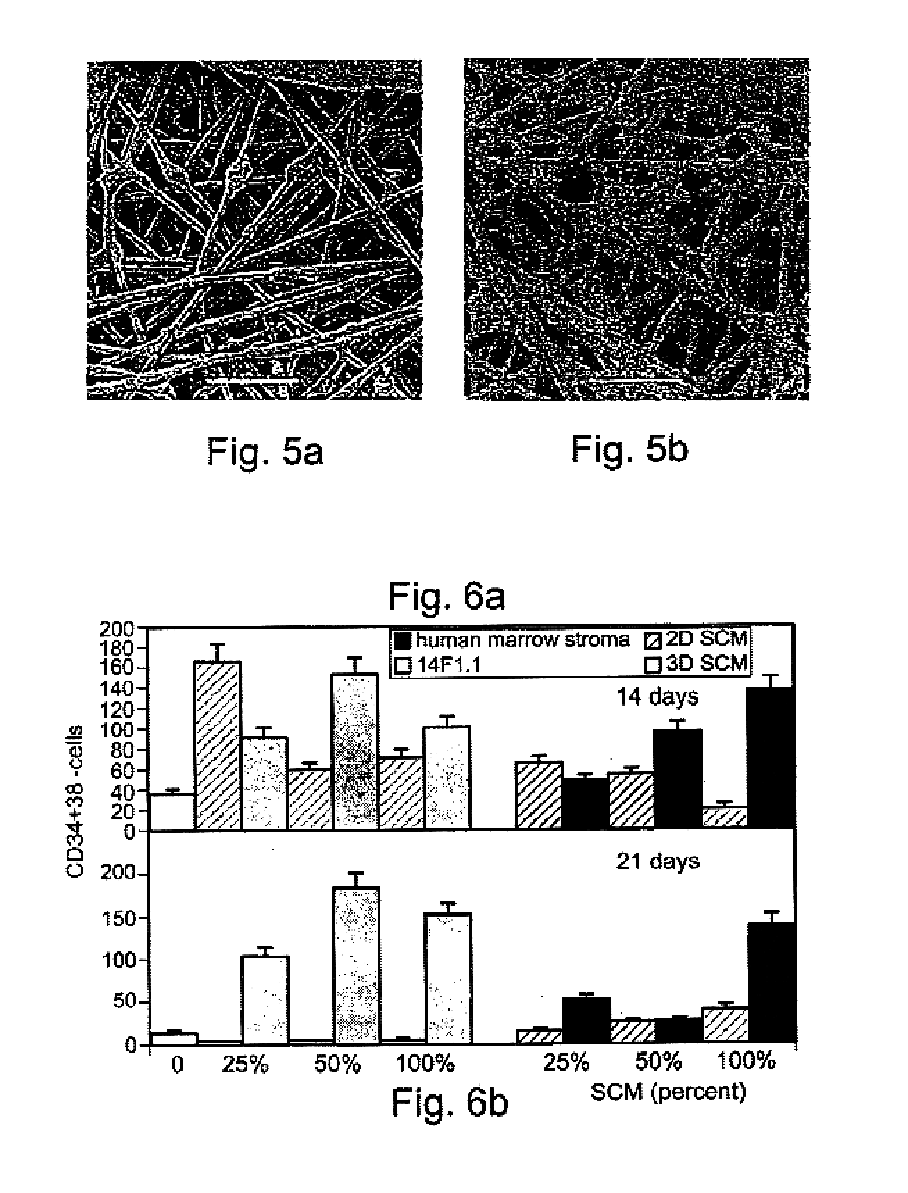
A method of expanding / maintaining undifferentiated hemopoietic stem cells or progenitor cells by obtaining undifferentiated hemopoietic stem cells or progenitor cells; and either seeding the undifferentiated hemopoietic stem cells or progenitor cells into a stationary phase plug-flow bioreactor in which a three-dimensional stromal cell culture has been pre-established on a substrate in the form of a sheet, the substrate including a non-woven fibrous matrix forming a physiologically acceptable three-dimensional network of fibers, thereby expanding / maintaining undifferentiated hemopoietic stem cells or progenitor cells, or culturing the undifferentiated hemopoietic stem cells or progenitor cells in conditioned medium obtained from such a reactor.
Owner:PLURISTEAM LTD +1
Methods for diagnosing and evaluating treatment of blood disorders
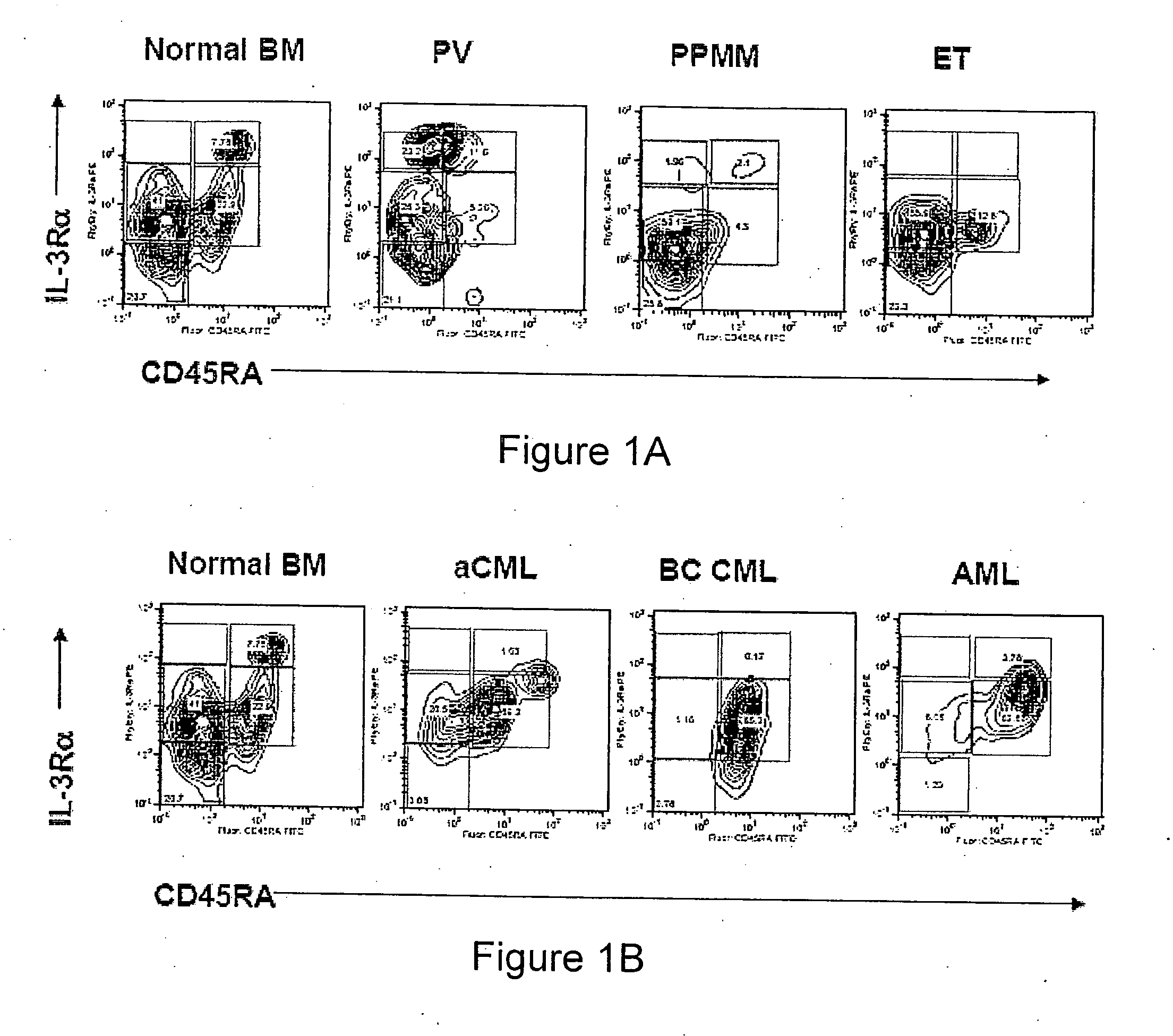
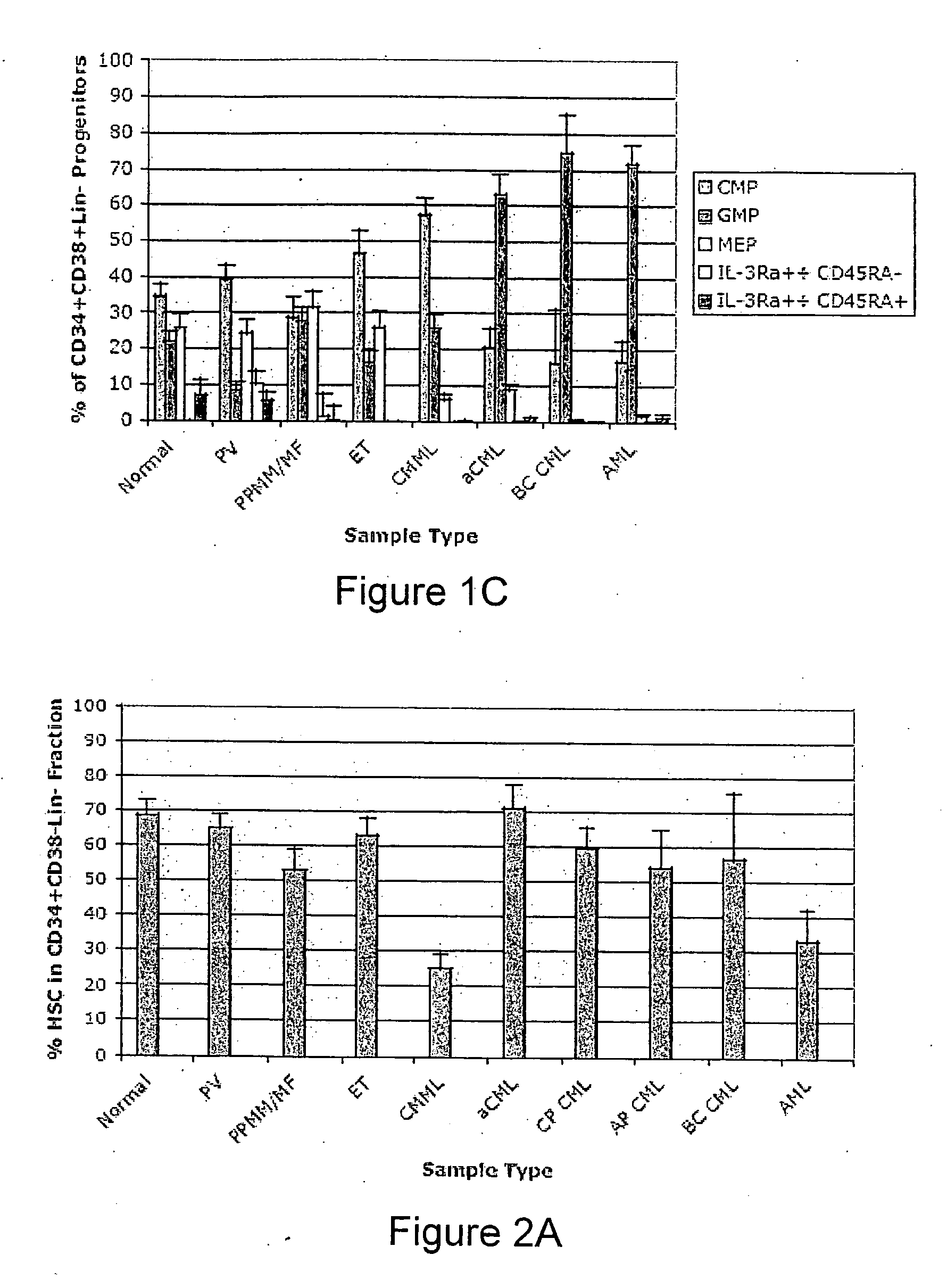
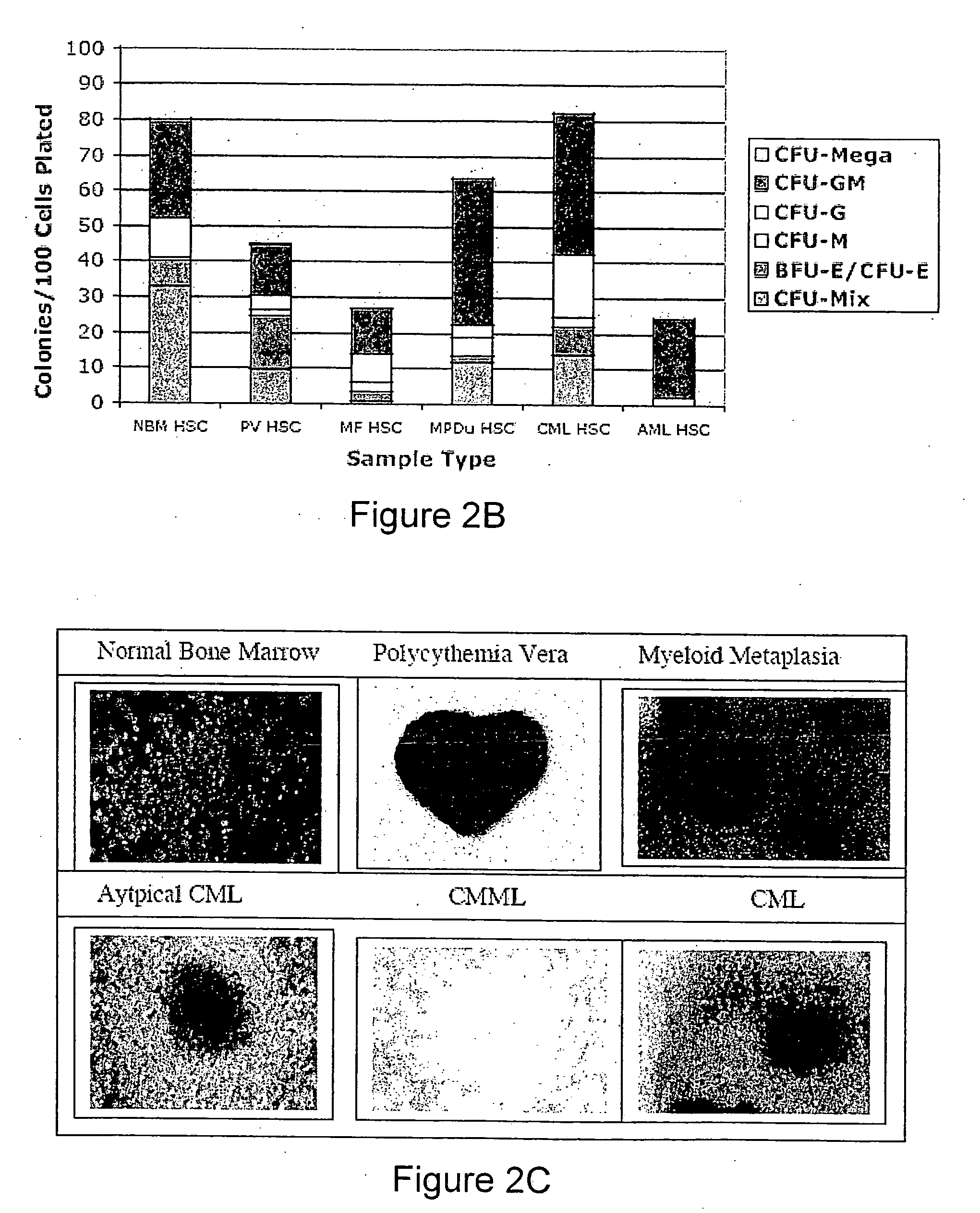
Methods, systems and kits are provided for the clinical staging of blood disorders including myelodysplastic syndrome, myeloproliferative diseases and leukemias by differential analysis of hematologic samples for the distribution of one or more hematopoietic stem or progenitor cell subsets. Additional functional, genetic, gene expression, proteomic or other molecular analyses of the hematopoietic stem and progenitor cells from the patients can also be employed in the staging methods of the invention.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method and apparatus for sorting biological cells with a MEMS device
InactiveUS6838056B2Bioreactor/fermenter combinationsBiological substance pretreatmentsBiological cellLaser light
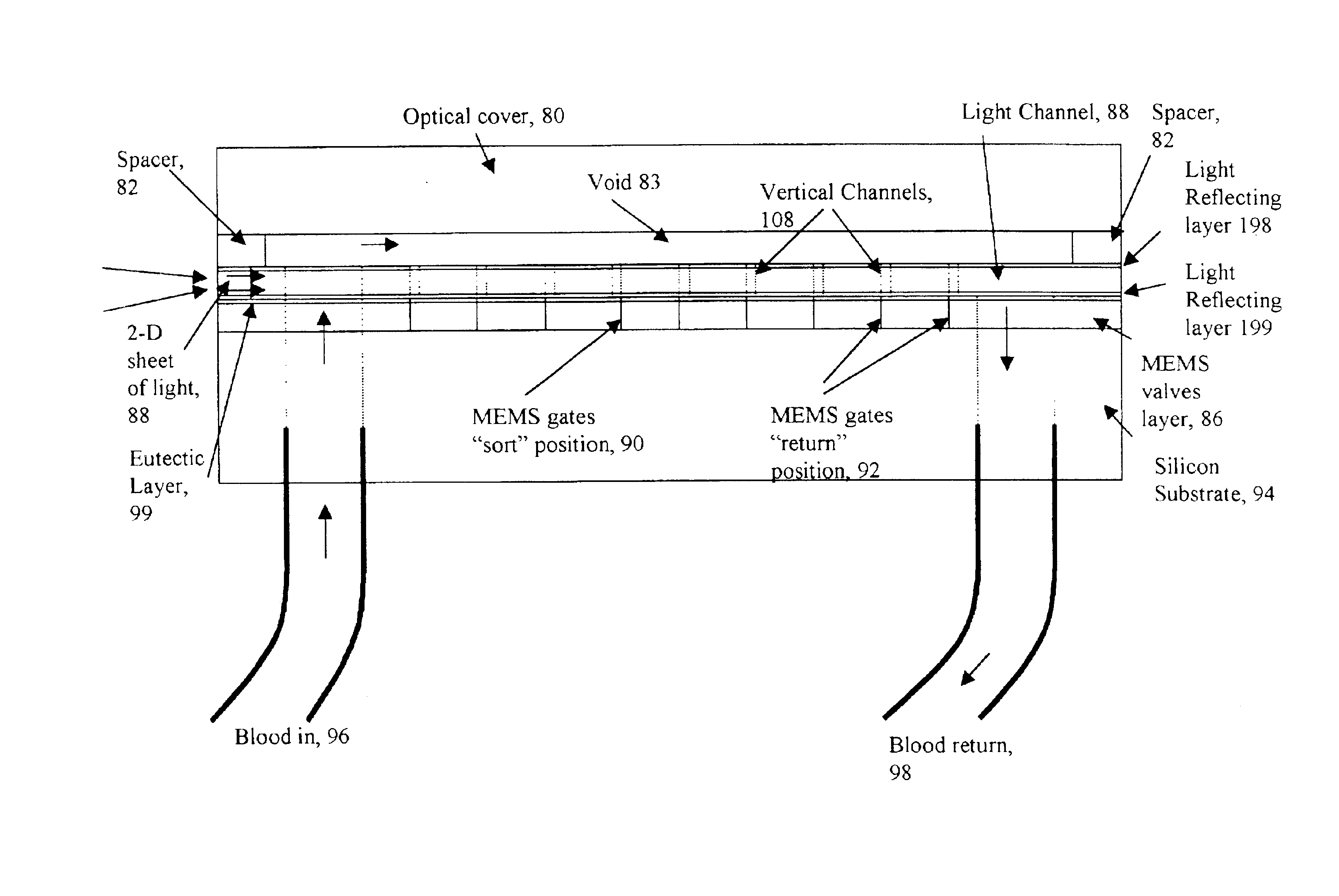
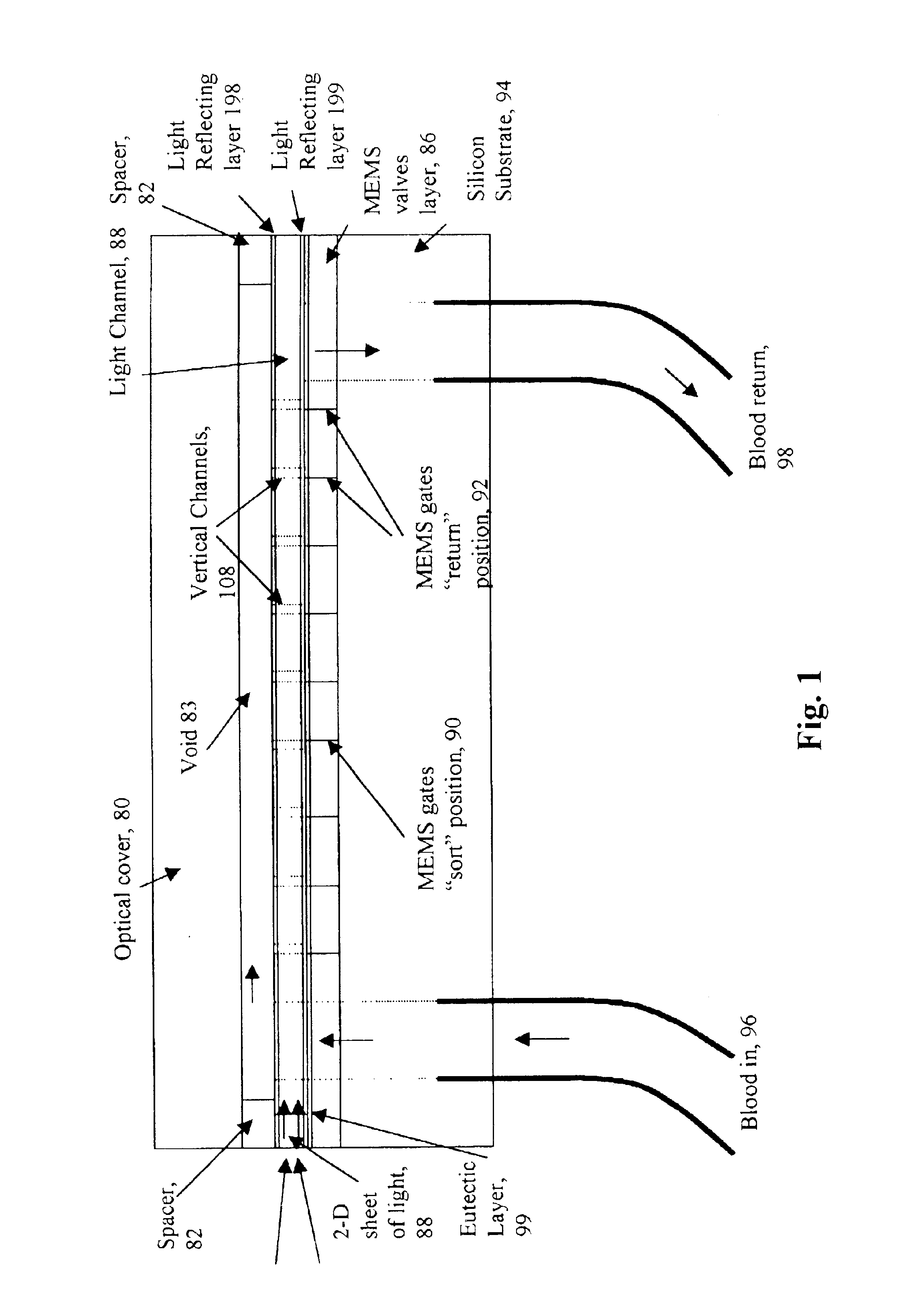
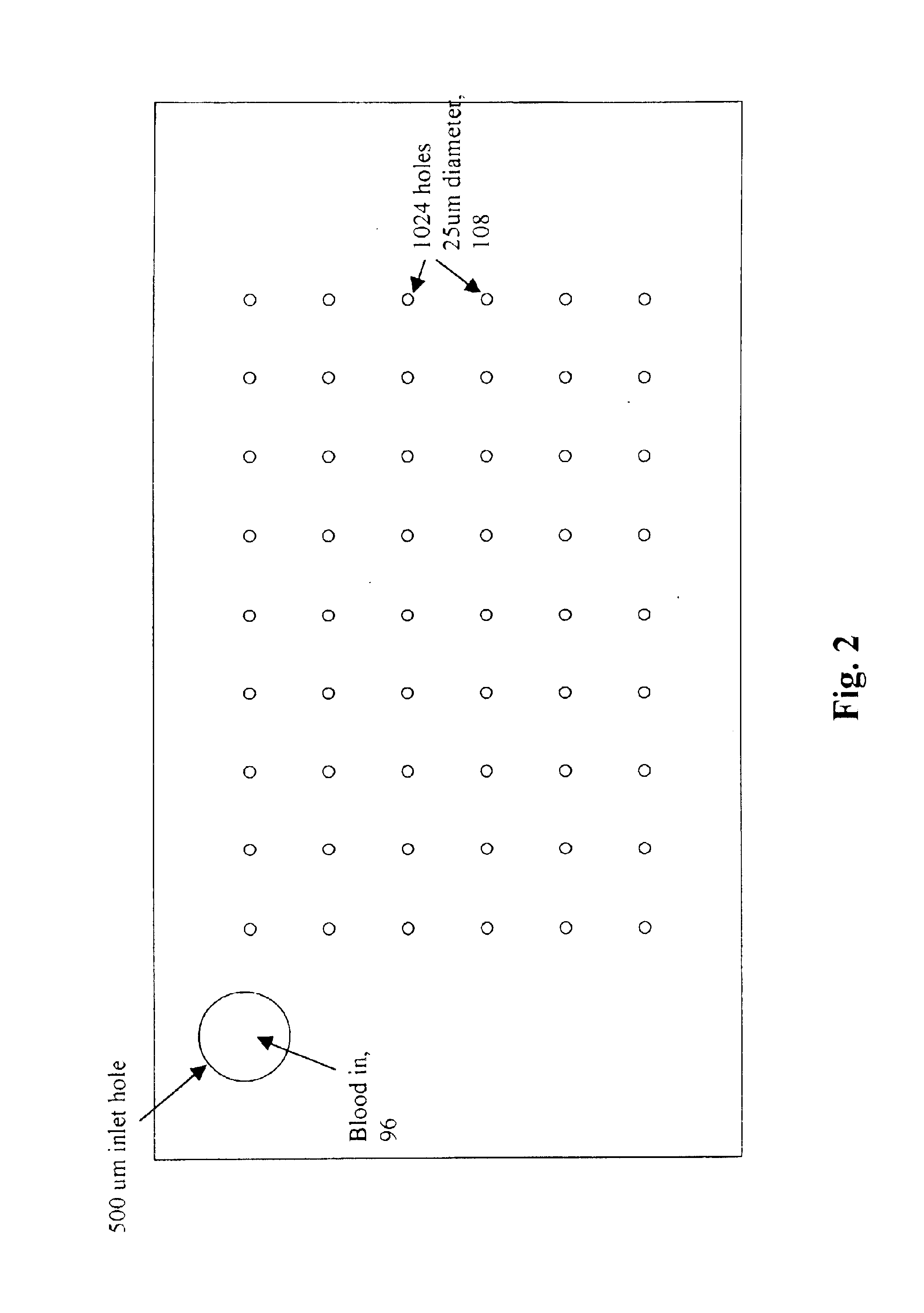
A micromechanical actuator for sorting hematopoietic stem cells for use in cancer therapies. The actuator operates by diverting cells into one of a number of possible pathways fabricated in the fabrication substrate of the micromechanical actuator, when fluorescence is detected emanating from the cells. The fluorescence results from irradiating the cells with laser light, which excites a fluorescent tag attached to the cell. The micromechanical actuator thereby sorts the cells individually, with an operation rate of 3.3 kHz, however with the massively parallel 1024-fold device described herein, a throughput of 3.3 million events / second is achievable.
Owner:OWL BIOMEDICAL
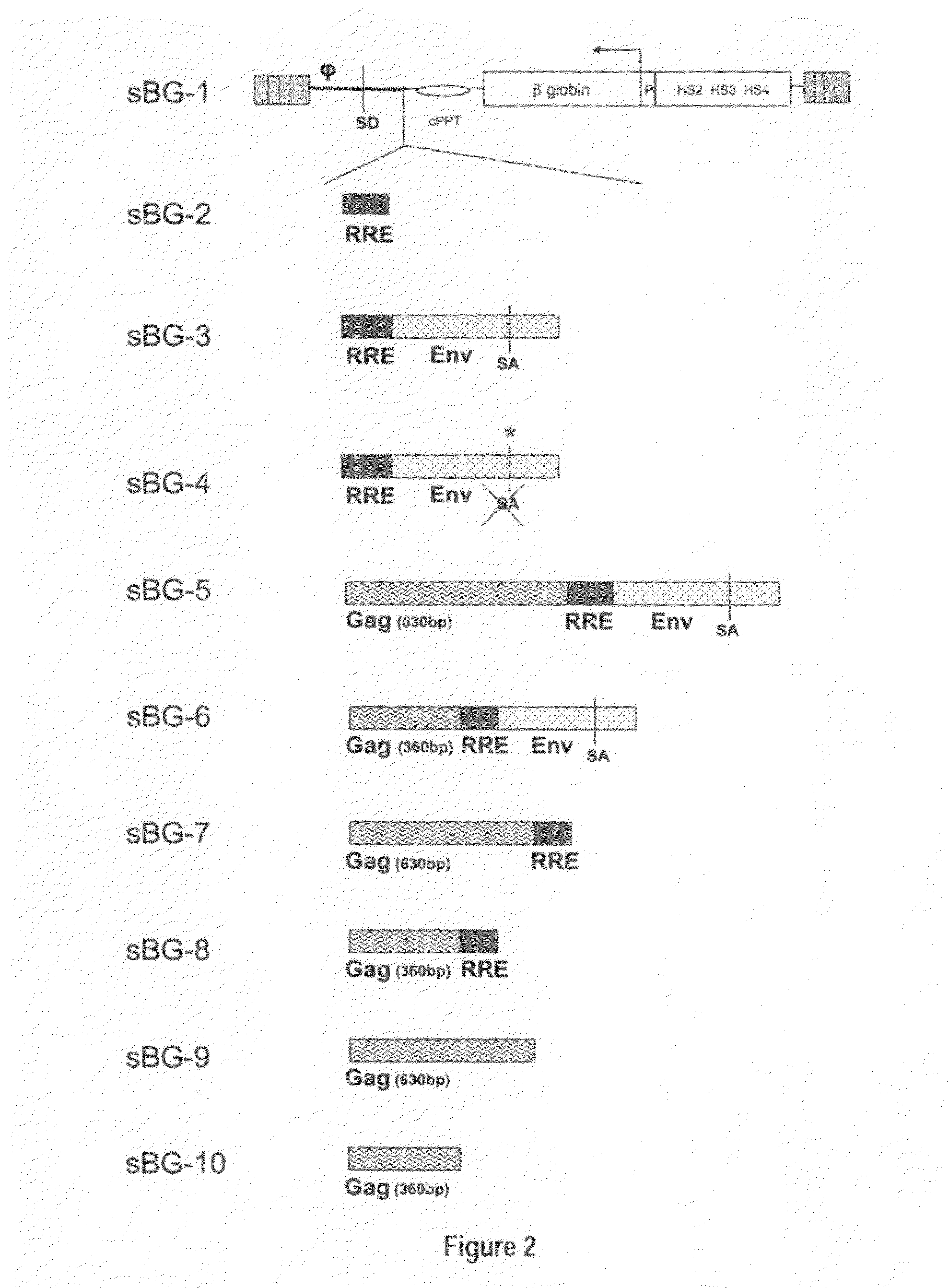
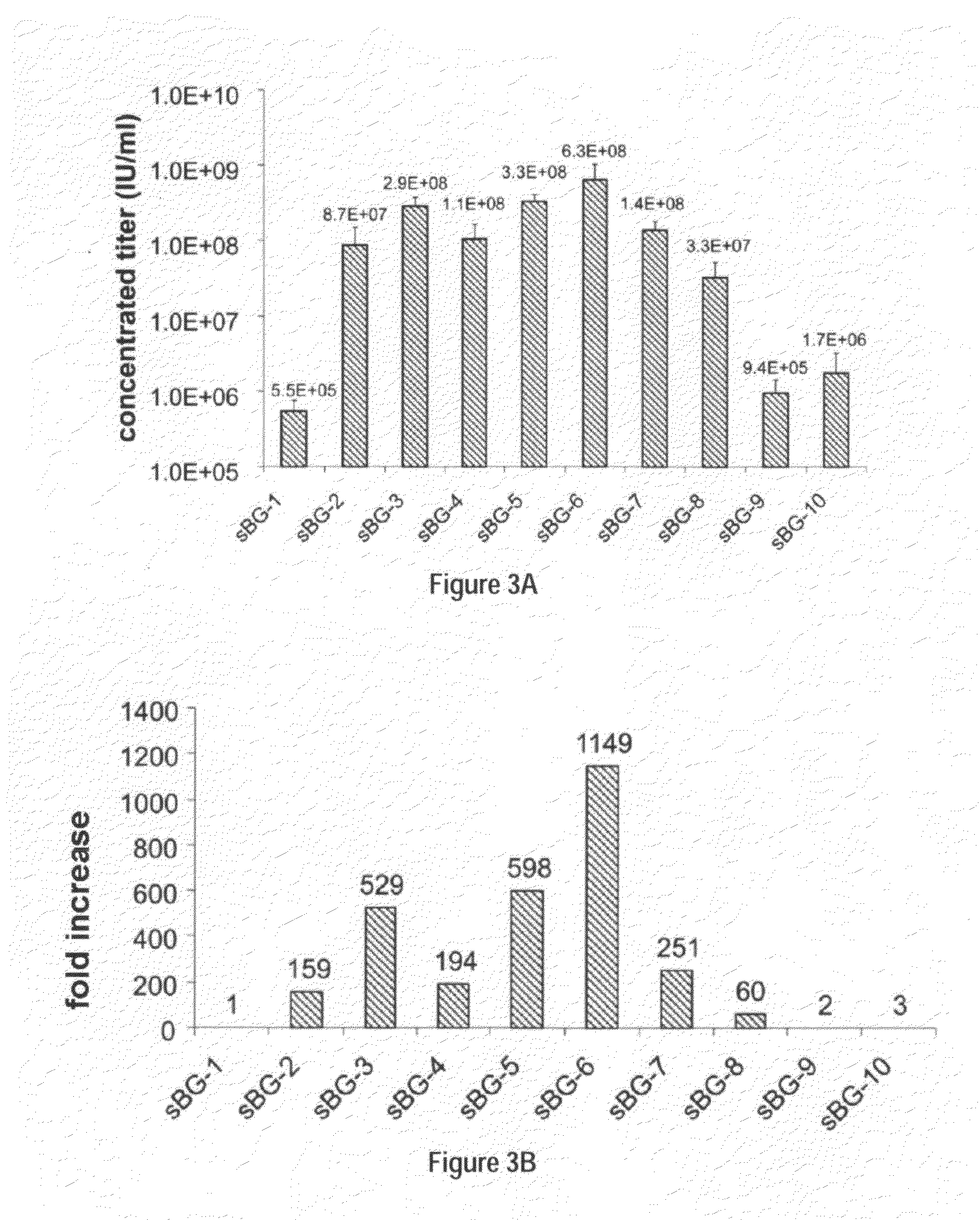
Optimization of determinants for successful genetic correction of diseases, mediated by hematopoietic stem cells
InactiveUS20110294114A1Improve stabilityImprove securityVectorsSugar derivativesNervous systemSickle cell anemia
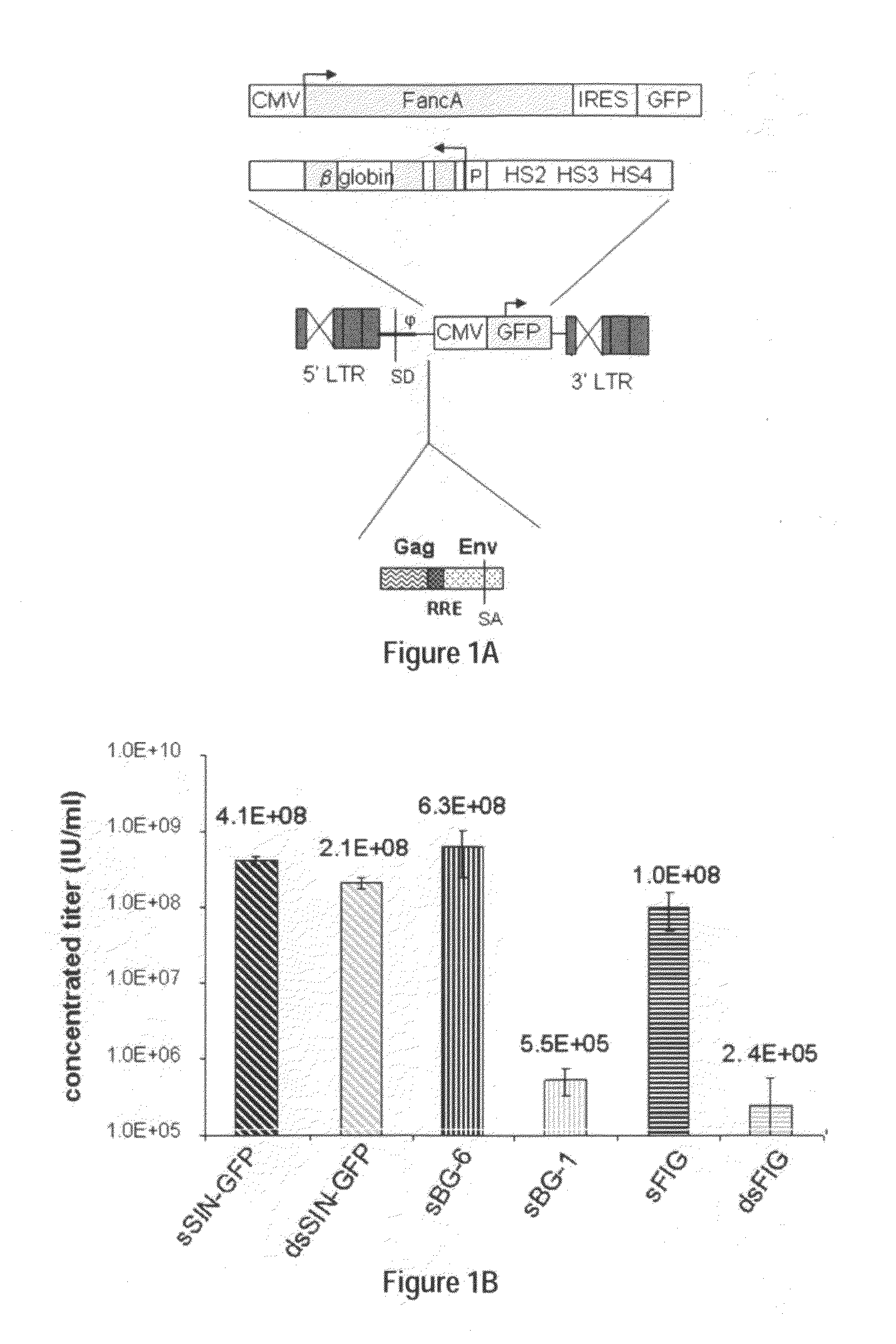
Methods and compositions disclosed herein generally relates to methods of determining minimum hematopoietic stem cell (HSC) chimerism and gene dosage for correction of a hematopoietic disease; in particular, in in vivo models. The invention also relates to modified lentiviral expression vectors for increase a viral titer and various methods for increasing such titers as well as expression vectors capable of enhancing such titers. The invention also relates to CHS4 chromatin insulator-derived functional insulator sequences. The invention further relates to methods for genetic correction of diseases or reducing symptoms thereof, such as sickle cell anemia, a lysosomal storage disease. The invention further relates to a method of improving and / or correcting one or more central nervous system (CNS) abnormalities caused by one or more lysosomal storage disease. The invention further relates to methods of improving titer in transfection-based bioreactor culture production or transfection-based production systems using eukaryotic cells.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
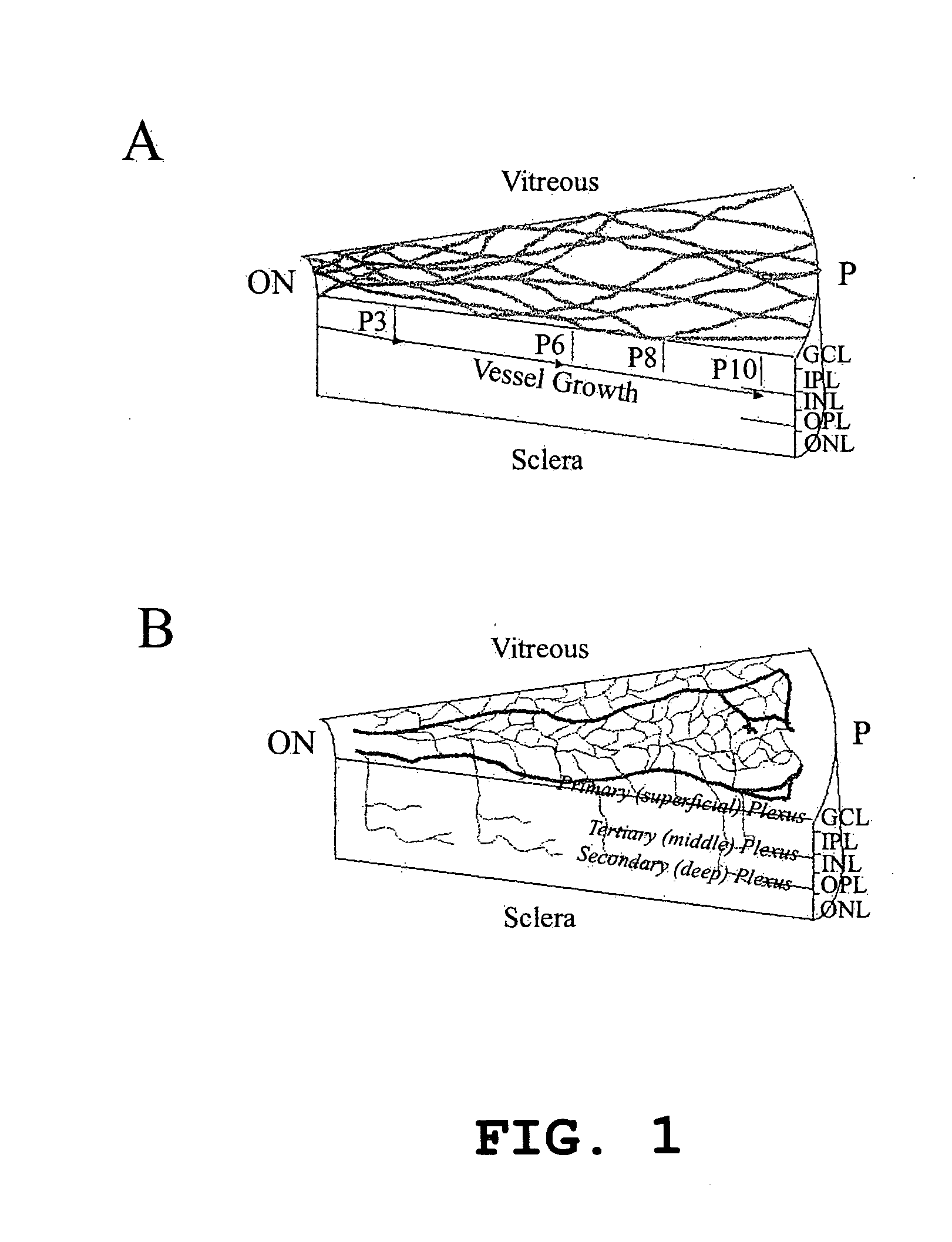
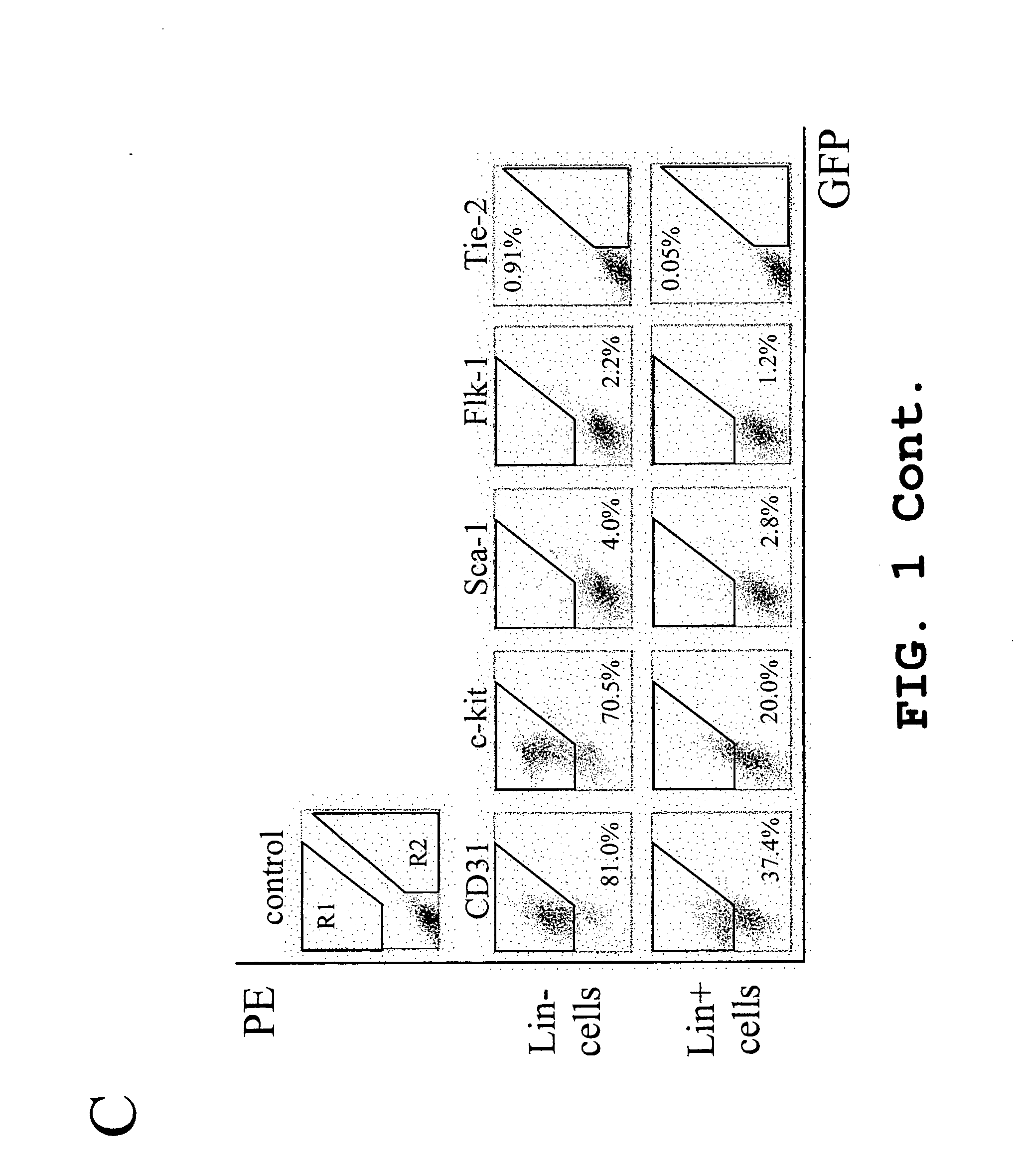
Hematopoietic stem cells and methods of treatment of neovascular eye diseases therewith
InactiveUS20050063961A1Stably incorporated into neovasculature of the eyePromote repairBiocideSenses disorderDiseaseProgenitor
Isolated, mammalian, adult bone marrow-derived, lineage negative hematopoietic stem cell populations (Lin− HSCs) contain endothelial progenitor cells (EPCs) capable of rescuing retinal blood vessels and neuronal networks in the eye. Preferably at least about 20% of the cells in the isolated Lin− HSCs express the cell surface antigen CD31. The isolated Lin− HSC populations are useful for treatment of ocular vascular diseases. In a preferred embodiment, the Lin− HSCs are isolated by extracting bone marrow from an adult mammal; separating a plurality of monocytes from the bone marrow; labeling the monocytes with biotin-conjugated lineage panel antibodies to one or more lineage surface antigens; removing of monocytes that are positive for the lineage surface antigens from the plurality of monocytes, and recovering a Lin− HSC population containing EPCs. Isolated Lin− HSCs that have been transfected with therapeutically useful genes are also provided, and are useful for delivering genes to the eye for cell-based gene therapy. Methods of preparing isolated stem cell populations of the invention, and methods of treating ocular diseases and injury are also described.
Owner:THE SCRIPPS RES INST
Method of judging leukemia, pre-leukemia or aleukemic malignant blood disease and diagnostic therefor
InactiveUS7479371B2Biological material analysisImmunoglobulins against growth factorsGackstroemiaPreleukemia
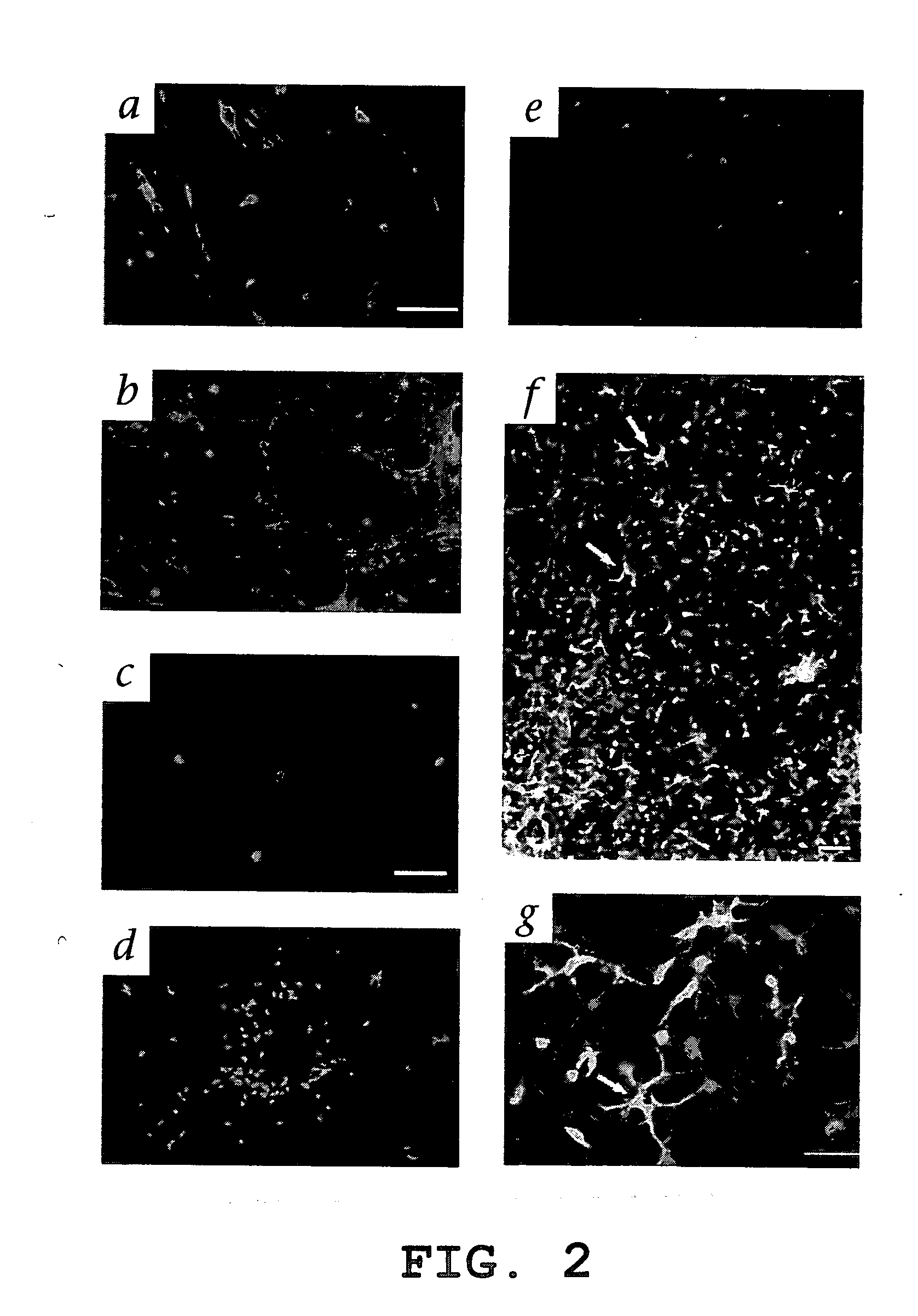
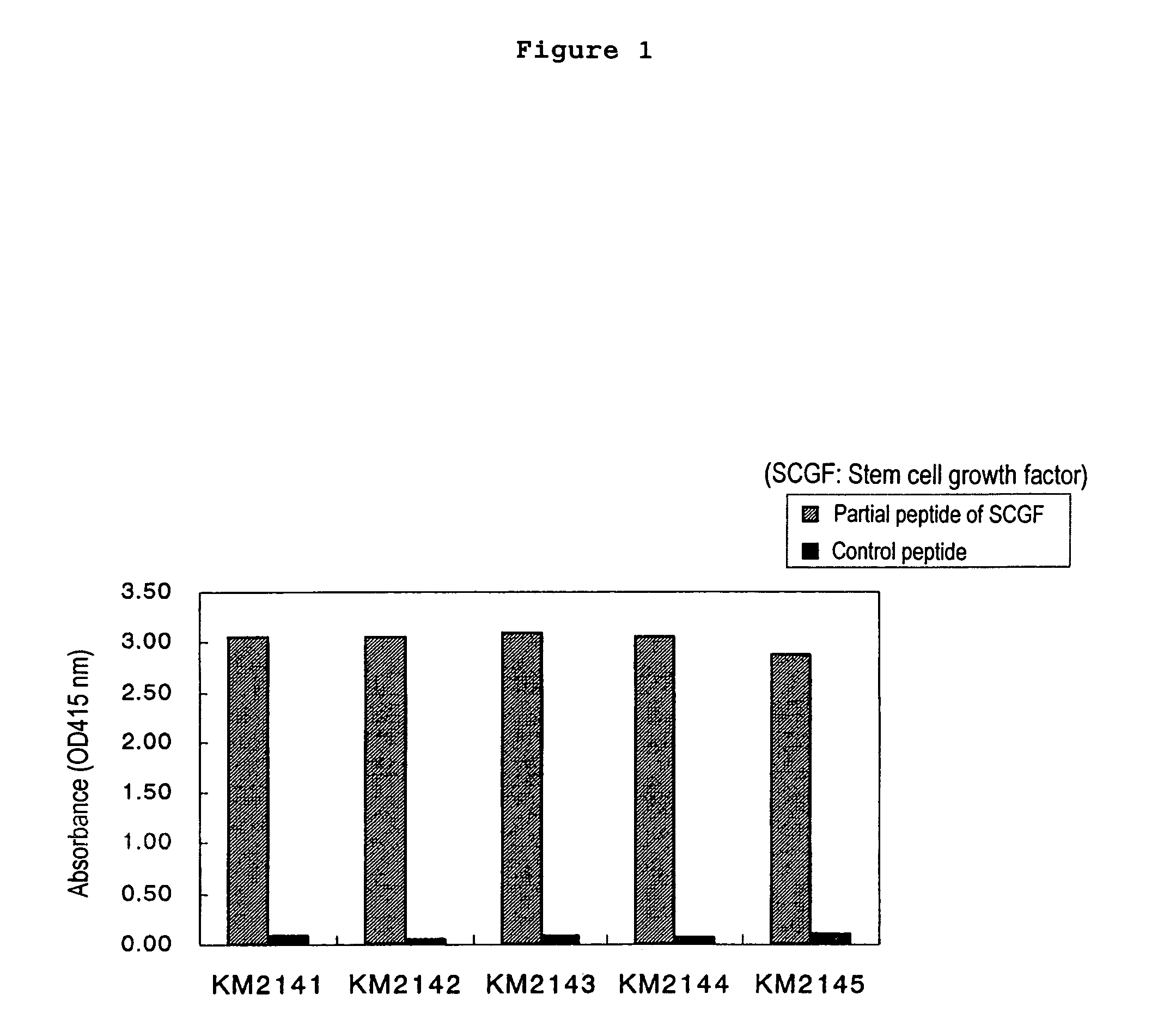
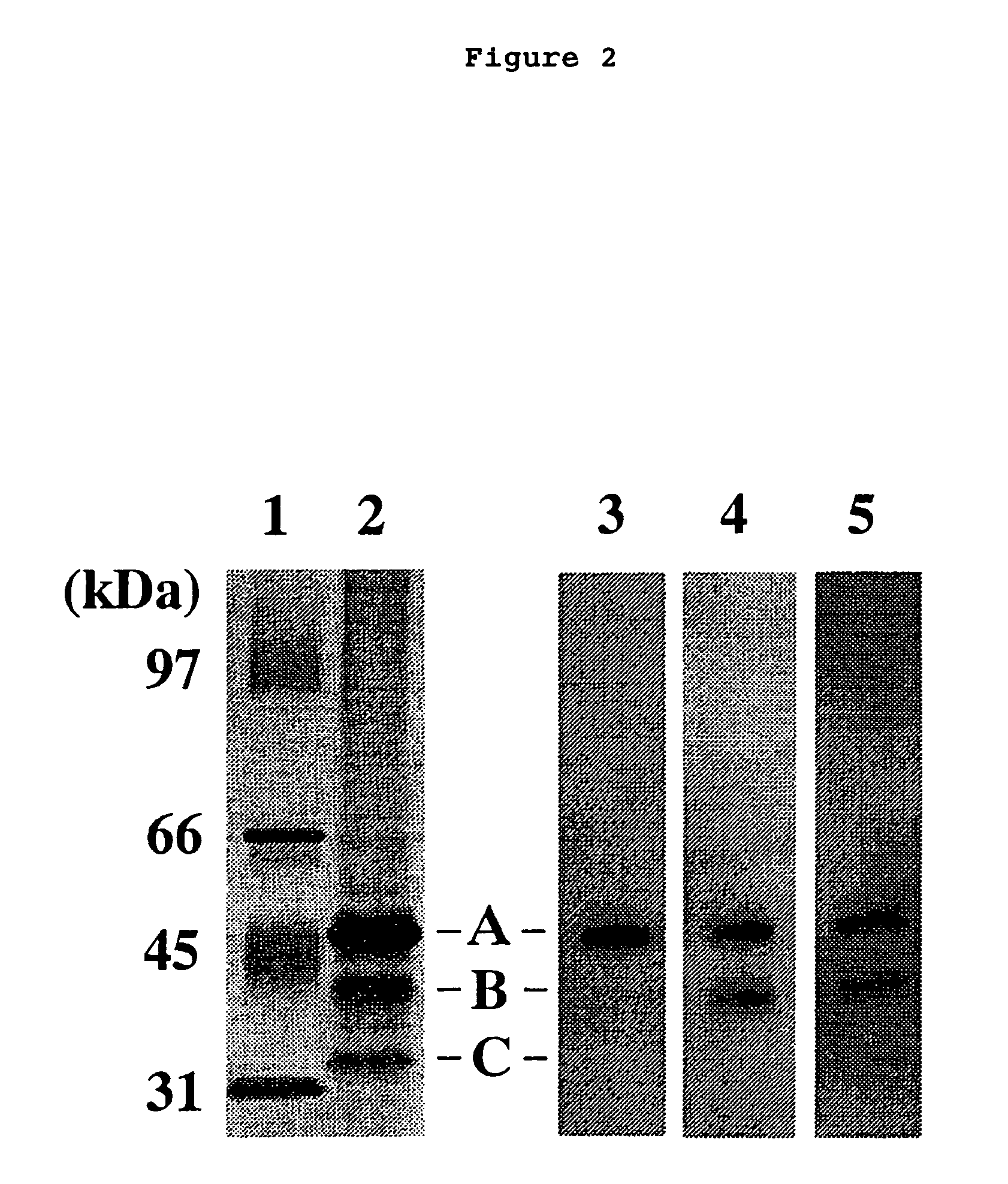
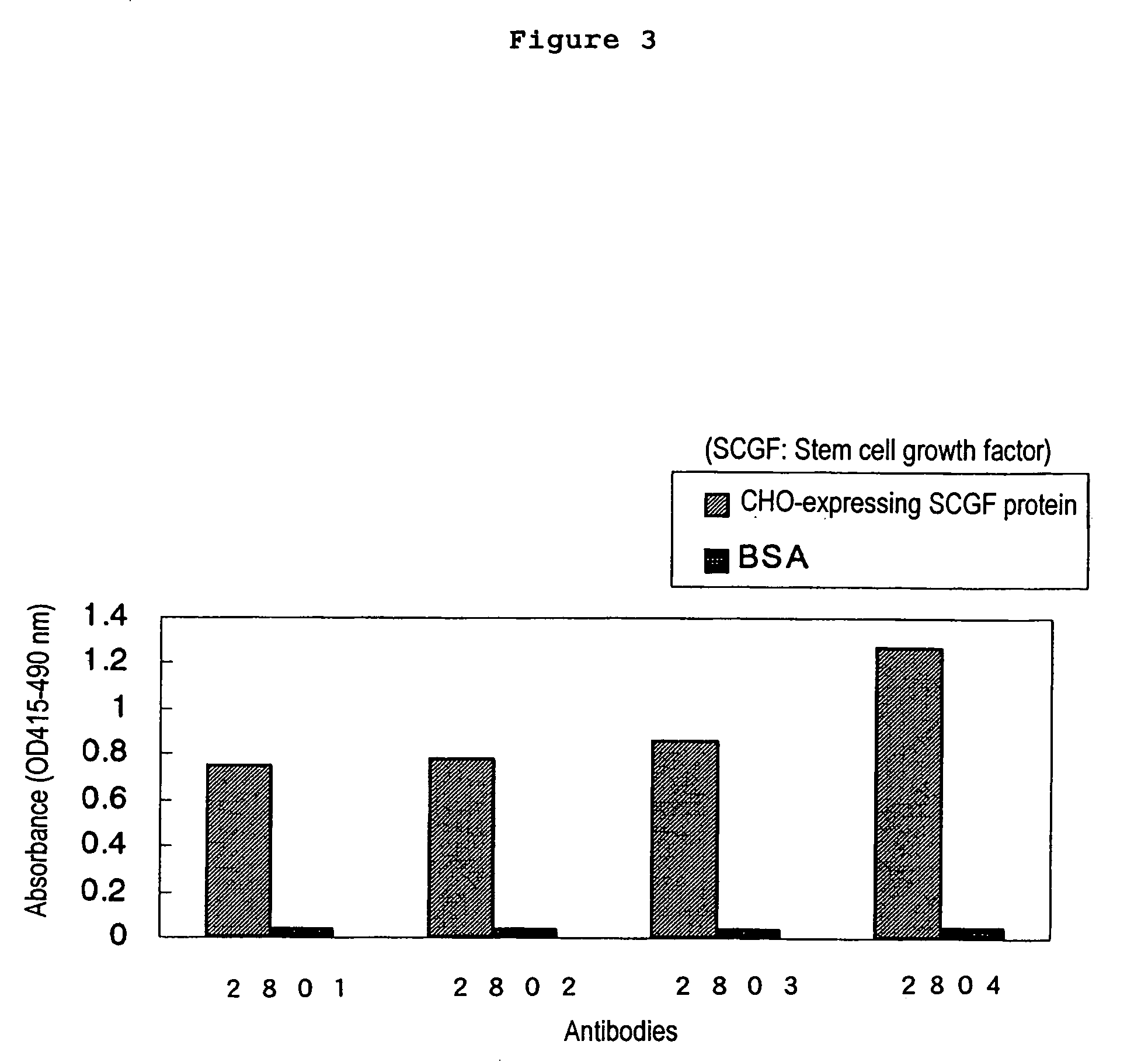
The present invention relates to a method for diagnosing leukemia, pre-leukemia or aleukemic malignant blood diseases, a method of discriminating leukemia from pre-leukemia or aleukemic malignant blood diseases, a method of discriminating aplastic anemia from myelodysplastic syndrome, a method of diagnosing delayed engraftment of the hematopoietic stem cells after transplantation of the hematopoietic stem cells, and a method of diagnosing the graft versus host disease, each of said methods comprising quantifying stem cell growth factor (SCGF). The present invention also makes it possible to provide an agent for diagnosing leukemia, pre-leukemia or aleukemic malignant blood diseases and an agent for diagnosing delayed engraftment of the hematopoietic stem cells after transplantation of the hematopoietic stem cells or an agent for diagnosing graft versus host disease (GVHD), each containing as an active ingredient an antibody reacting with stem cell growth factor (SCGF).
Owner:TOKAI UNIV +2
Isolation and preservation of fetal and neonatal hematopoietic stem and progenitor cells of the blood
The present invention relates to hematopoietic stem and progenitor cells of neonatal or fetal blood that are cryopreserved, and the therapeutic uses of such stem and progenitor cells upon thawing. In particular, the present invention relates to the therapeutic use of fetal or neonatal stem cells for hematopoietic (or immune) reconstitution. Hematopoietic reconstitution with the cells of the invention can be valuable in the treatment or prevention of various diseases and disorders such as anemias, malignancies, autoimmune disorders, and various immune dysfunctions and deficiencies. In another embodiment, fetal or neonatal hematopoietic stem and progenitor cells which contain a heterologous gene sequence can be used for hematopoietic reconstitution in gene therapy. In a preferred embodiment of the invention, neonatal or fetal blood cells that have been cryopreserved and thawed can be used for autologous (self) reconstitution.
Owner:PHARMASTEM THERAPEUTICS
Drug delivery device
InactiveUS20050187607A1Reduce inflammationReduce proliferationStentsBlood vesselsGrowth retardantProgenitor
A medical device for drug delivery is provided which includes a stent structure and a biologically active structure attached to the stent structure. The biologically active structure is comprised of a plurality of layers, including a first layer having a first biologically active compound, such as an anti-proliferative, cytostatic or cytotoxic drug, for delivery at a first target site, such as the vascular tissue; a second layer having a second biologically active compound, such as a growth factor, for delivery into the arterial lumen and capable of promoting engraftment and differentiation of hematopoietic stem cells and / or endothelial progenitor cells at a second site of myocardial injury; and a third, middle layer which is substantially or selectively impermeable to both the first and second biologically active compounds.
Owner:AKHTAR ADIL JAMAL +1
Ligand/lytic peptide compositions and methods of use
InactiveUS6635740B1Prevents sexual maturationInhibition of maturationHormone peptidesPeptide/protein ingredientsLytic peptideAbnormal tissue growth
Amphipathic lytic peptides are ideally suited to use in a ligand / cytotoxin combination to specifically inhibit cells that are driven by or are dependent upon a specific ligand interaction; for example, to induce sterility or long-term contraception, or to attack tumor cells, or to selectively lyse virally-infected cells, or to attack lymphocytes responsible for autoimmune diseases. The peptides act directly on cell membranes, and need not be internalized. Administering a combination of gonadotropin-releasing hormone (GnRH) (or a GnRH agonist) and a membrane-active lytic peptide produces long-term contraception or sterilization in animals in vivo. Administering in vivo a combination of a ligand and a membrane-active lytic peptide kills cells with a receptor for the ligand. The compounds are relatively small, and are not antigenic. Lysis of gonadotropes has been observed to be very rapid (on the order of ten minutes.) Lysis of tumor cells is rapid. The two components-the ligand and the lytic peptide-may optionally be administered as a fusion peptide, or they may be administered separately, with the ligand administered slightly before the lytic peptide, to activate cells with receptors for the ligand, and thereby make those cells susceptible to lysis by the lytic peptide. The compounds may be used in gene therapy to treat malignant or non-malignant tumors, and other diseases caused by clones or populations of "normal" host cells bearing specific receptors (such as lymphocytes), because genes encoding a lytic peptide or encoding a lytic peptide / peptide hormone fusion may readily be inserted into hematopoietic stem cells or myeloid precursor cells.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
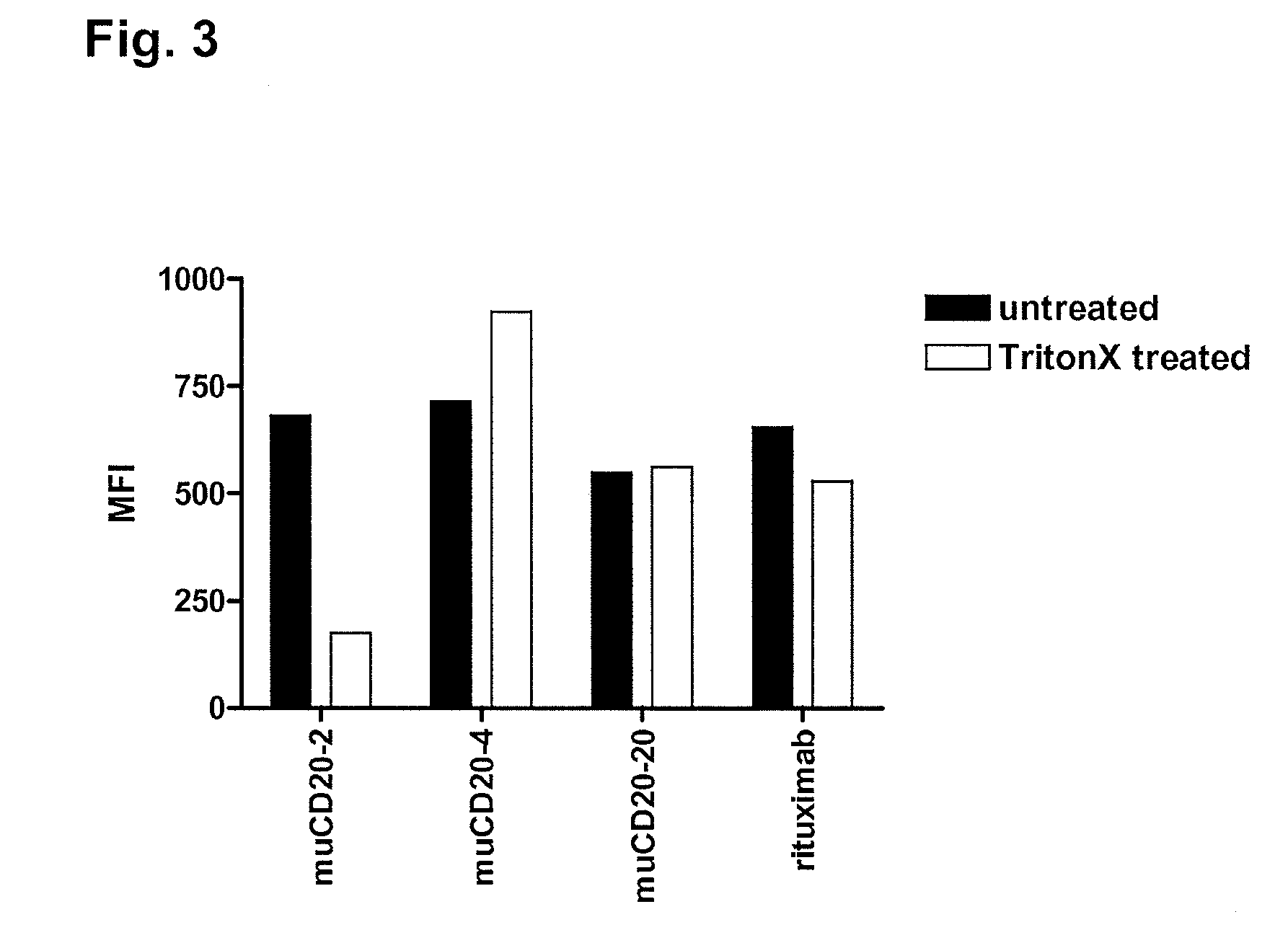
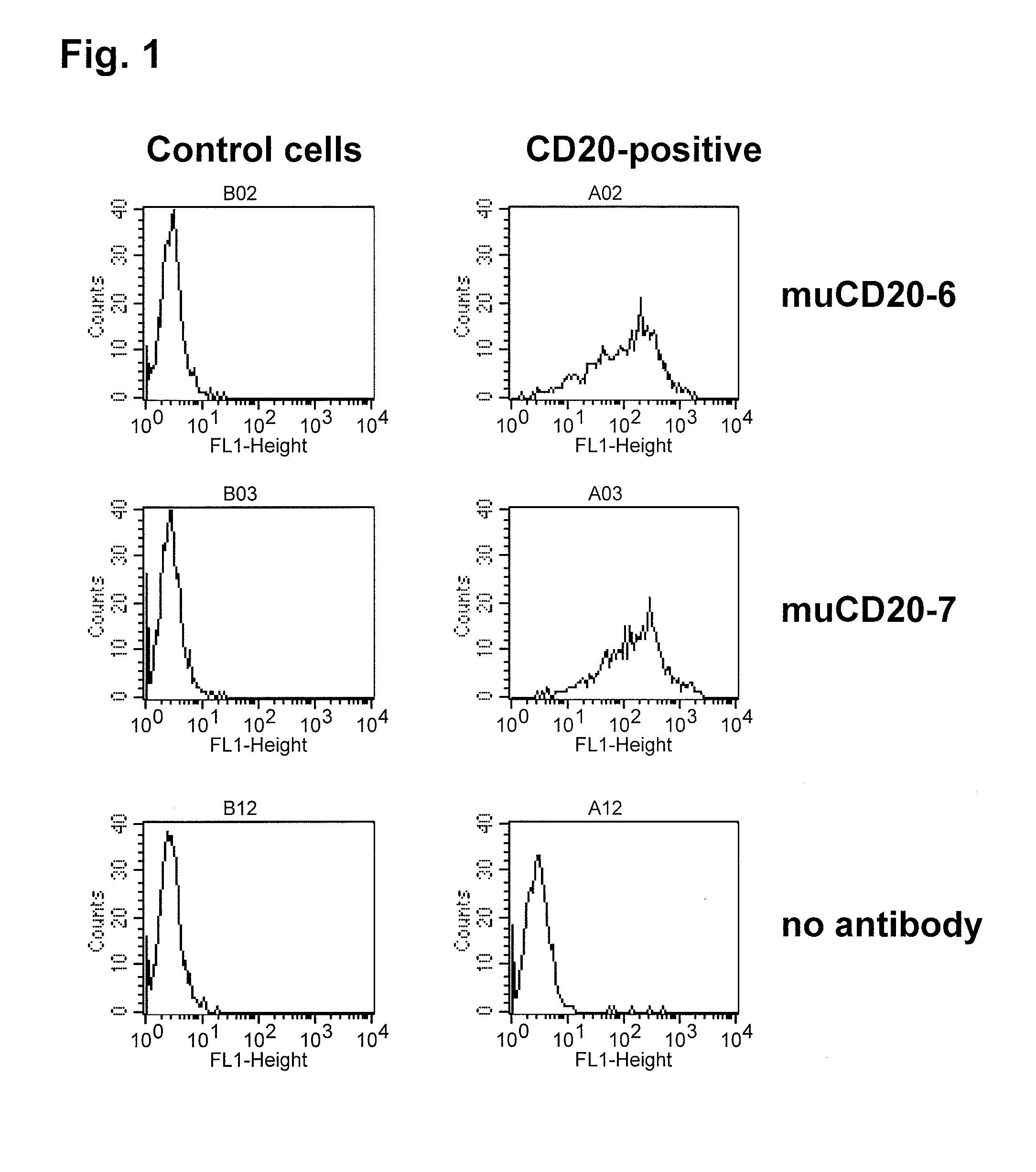
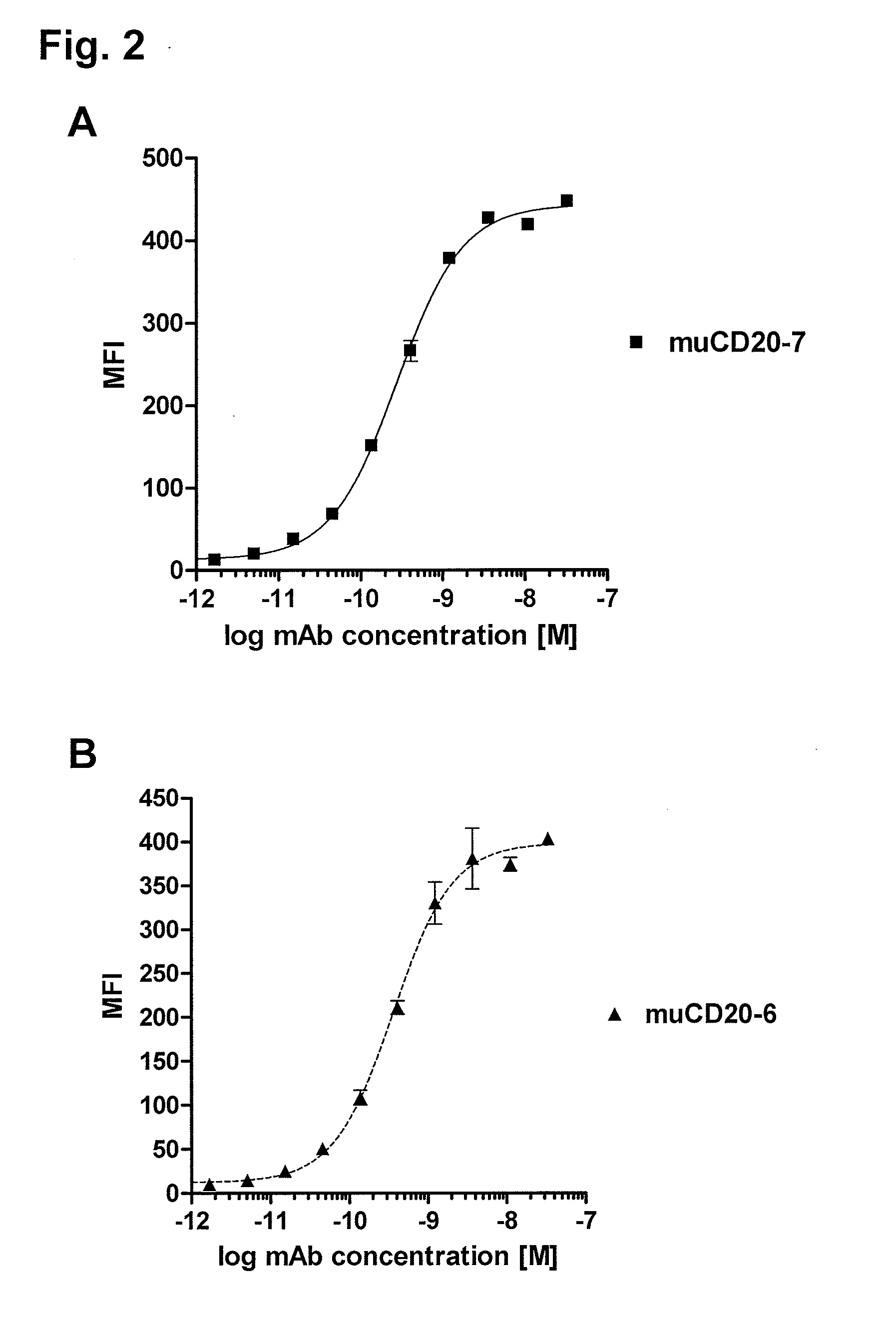
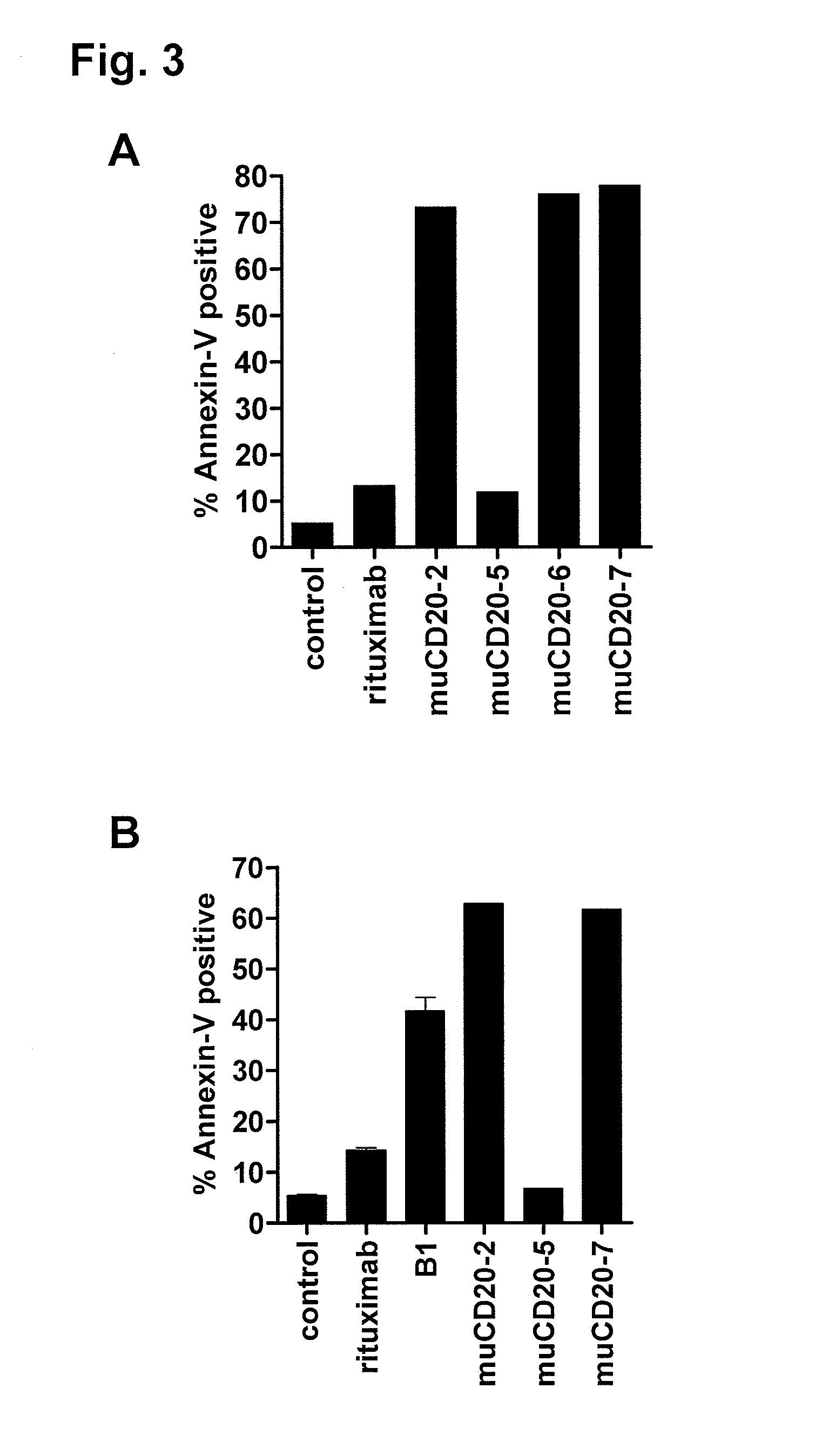
Cd20 antibodies and uses thereof
CD20 is a transmembrane protein of the tetra-spanin family expressed on the surface of B-cells and has been found on B-cells from peripheral blood as well as lymphoid tissues. CD20 expression persists from the early pre-B cell stage until the plasma cell differentiation stage. Conversely, it is not found on hematopoietic stem cells, pro-B cells, differentiated plasma cells or non-lymphoid tissues. In addition to expression in normal B-cells, CD20 is expressed in B-cell derived malignancies such as non-Hodgkin's lymphoma (NHL) and B-cell chronic lymphocytic leukemia (CLL). CD20 expressing cells are known to play a role in other diseases and disorders, including inflammation. The present invention includes anti-CD20 antibodies, forms and fragments, having superior physical and functional properties; immunoconjugates, compositions, diagnostic reagents, methods for inhibiting growth, therapeutic methods, improved antibodies and cell lines; and polynucleotides, vectors and genetic constructs encoding same.
Owner:IMMUNOGEN INC
Cd20 antibodies and uses thereof
InactiveUS20110195021A1Improve stabilityImproved ADCC functionSenses disorderAntipyreticDiseaseMalignancy
CD20 is a transmembrane protein of the tetra-spanin family expressed on the surface of B-cells and has been found on B-cells from peripheral blood as well as lymphoid tissues. CD20 expression persists from the early pre-B cell stage until the plasma cell differentiation stage. Conversely, it is not found on hematopoietic stem cells, pro-B cells, differentiated plasma cells or non-lymphoid tissues. In addition to expression in normal B-cells, CD20 is expressed in B-cell derived malignancies such as non-Hodgkin's lymphoma (NHL) and B-cell chronic lymphocytic leukemia (CLL). CD20 expressing cells are known to play a role in other diseases and disorders, including inflammation. The present invention includes anti-CD20 antibodies, forms and fragments, having superior physical and functional properties; immunoconjugates, compositions, diagnostic reagents, methods for inhibiting growth, therapeutic methods, improved antibodies and cell lines; and polynucleotides, vectors and genetic constructs encoding same.
Owner:IMMUNOGEN INC
Methods for manipulating phagocytosis mediated by CD47
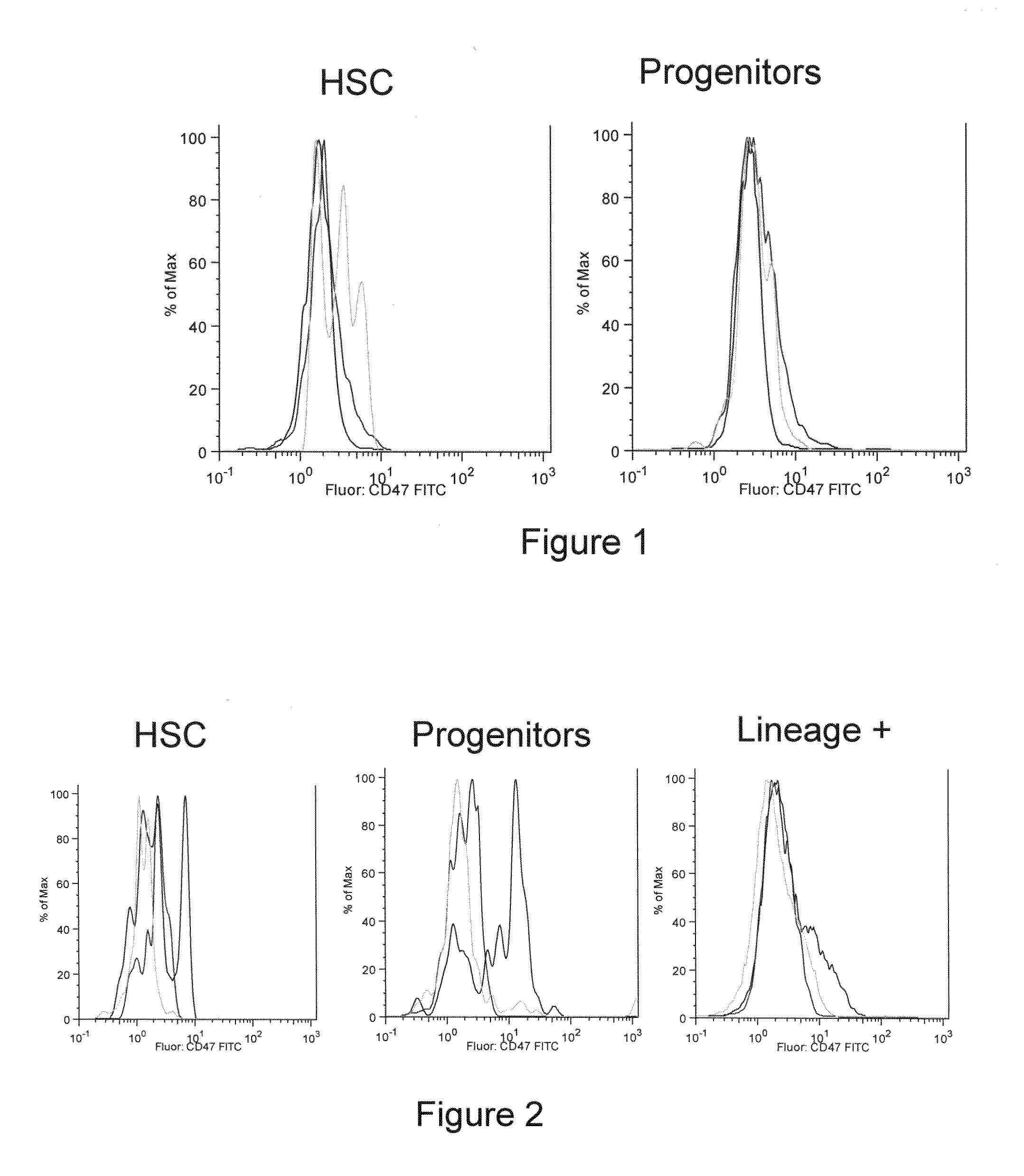
InactiveUS20090191202A1Enhance phagocytosisPeptide/protein ingredientsMammal material medical ingredientsHematopoietic cellSolid tumor
Methods are provided to manipulate phagocytosis of cells, including hematopoietic cells, e.g. circulating hematopoietic cells, bone marrow cells, etc.; and solid tumor cells. In some embodiments of the invention the circulating cells are hematopoietic stem cells, or hematopoietic progenitor cells, particularly in a transplantation context, where protection from phagocytosis is desirable. In other embodiments the circulating cells are leukemia cells, particularly acute myeloid leukemia (AML), where increased phagocytosis is desirable.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
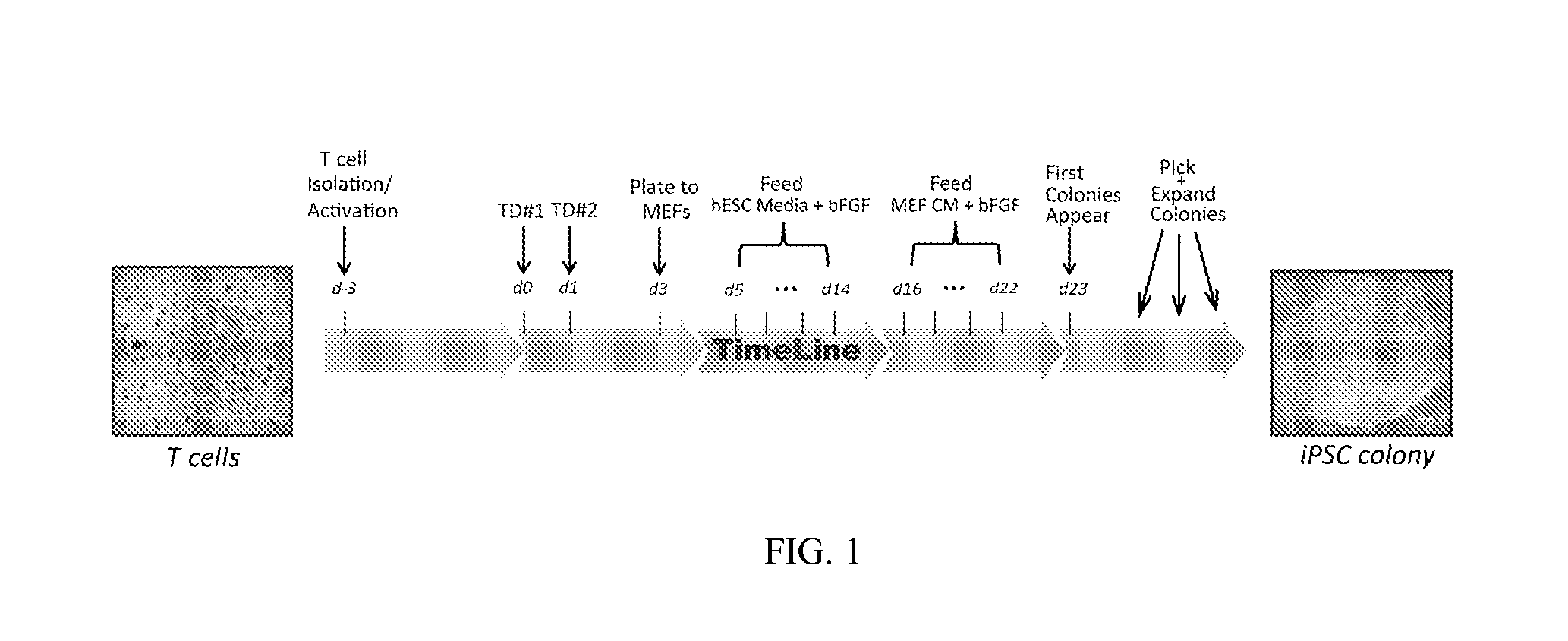
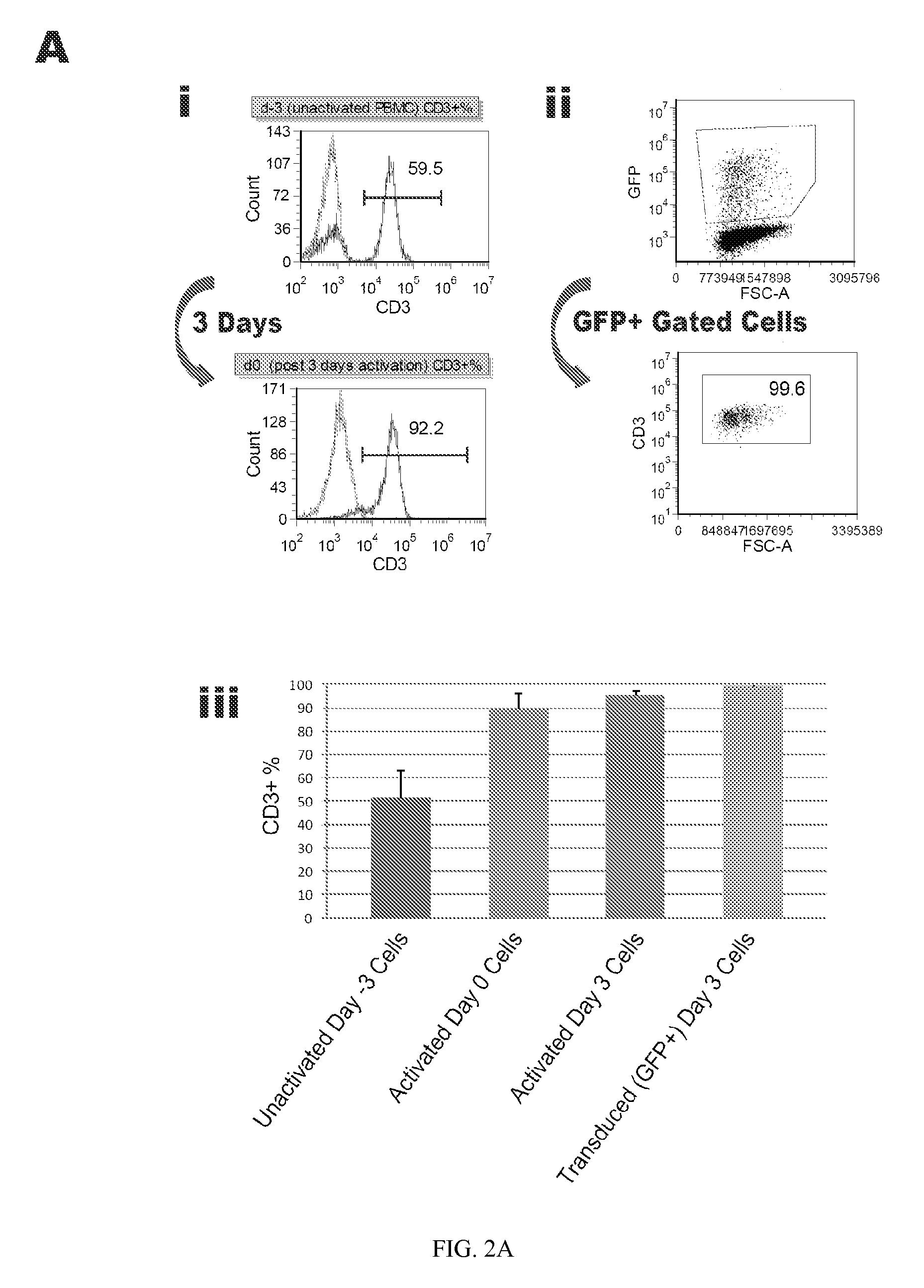
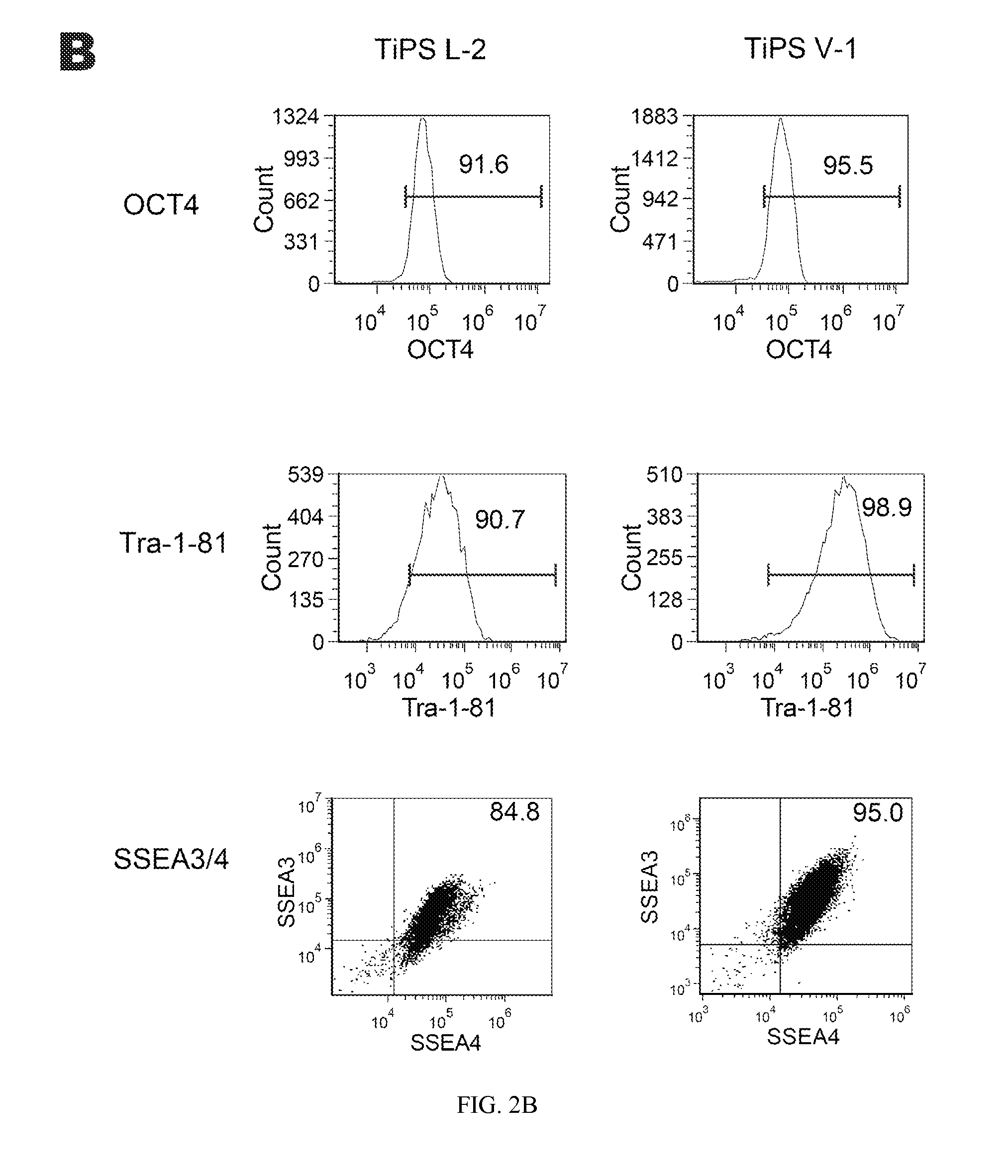
Reprogramming T cells and hematopoietic cells
ActiveUS8741648B2Increase capacityGenetically modified cellsArtificial cell constructsProgenitorHematopoietic cell
Methods and compositions relating to the production of induced pluripotent stem cells (iPS cells) are disclosed. For example, induced pluripotent stem cells may be generated from CD34+ hematopoietic cells, such as human CD34+ blood progenitor cells, or T cells. Various iPS cell lines are also provided. In certain embodiments, the invention provides novel induced pluripotent stem cells with a genome comprising genetic rearrangement of T cell receptors.
Owner:FUJIFILM CELLULAR DYNAMICS INC
Compositions and methods for the stimulation or enhancement of bone formation and the self-renewal of cells
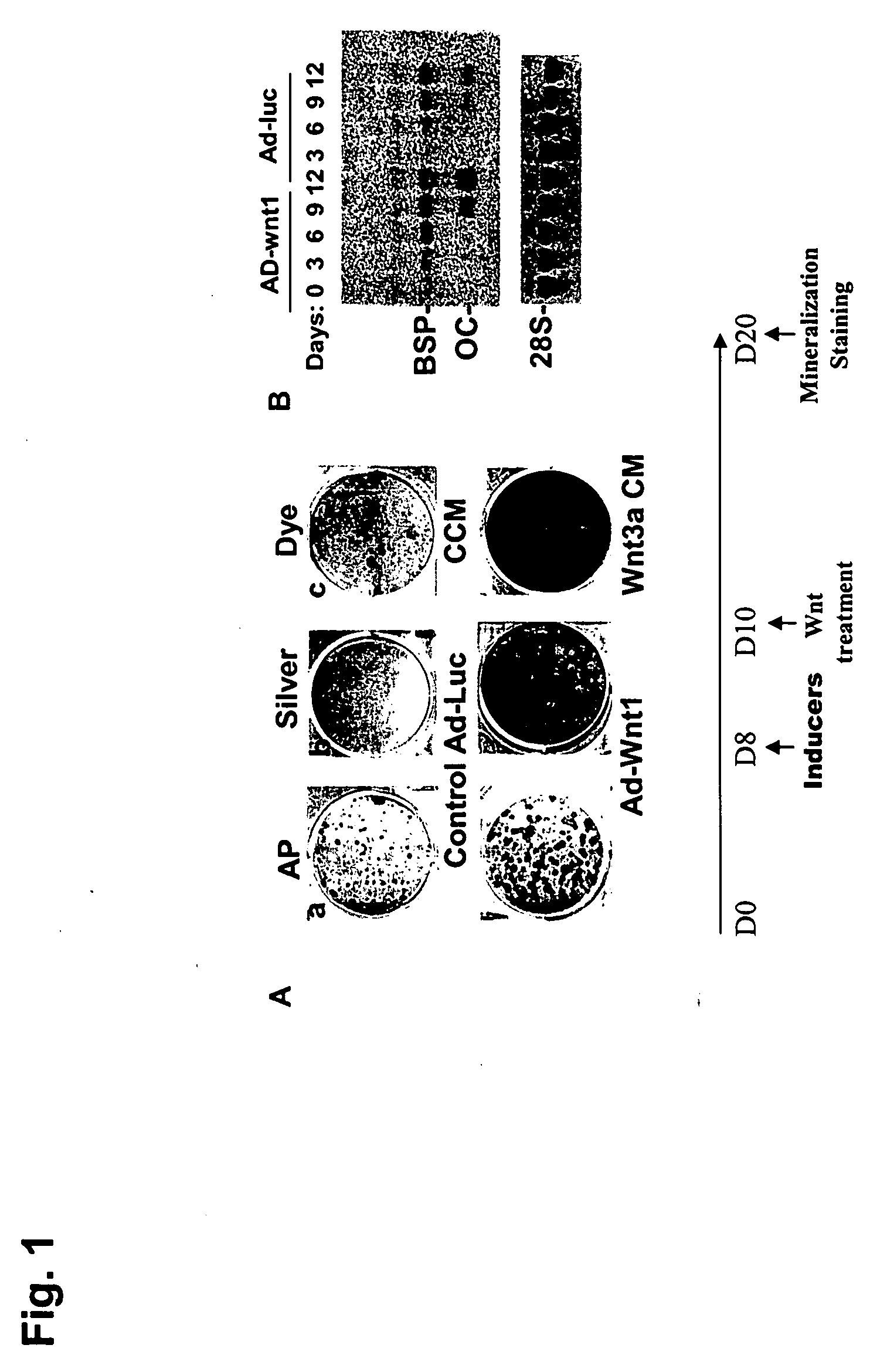
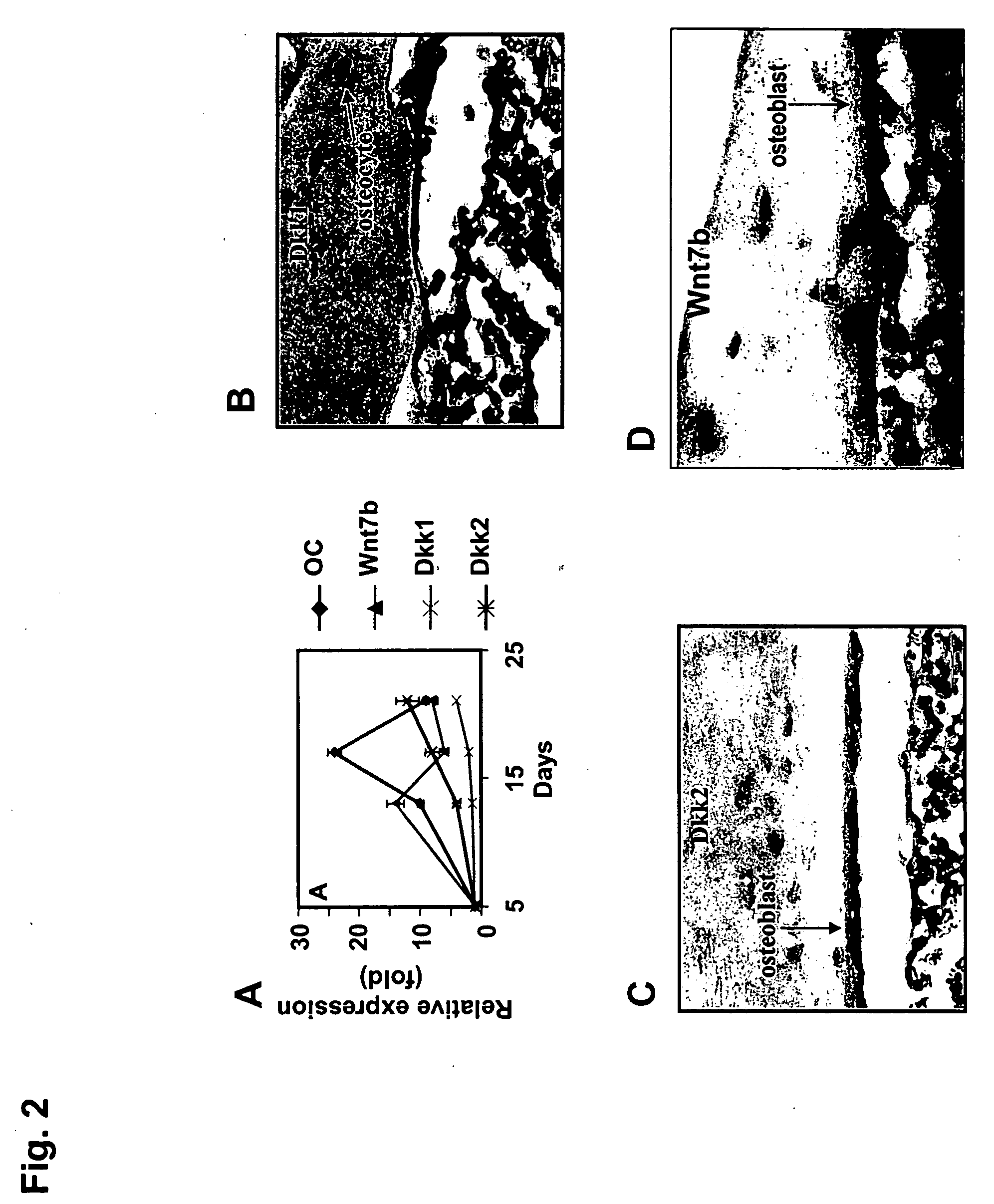
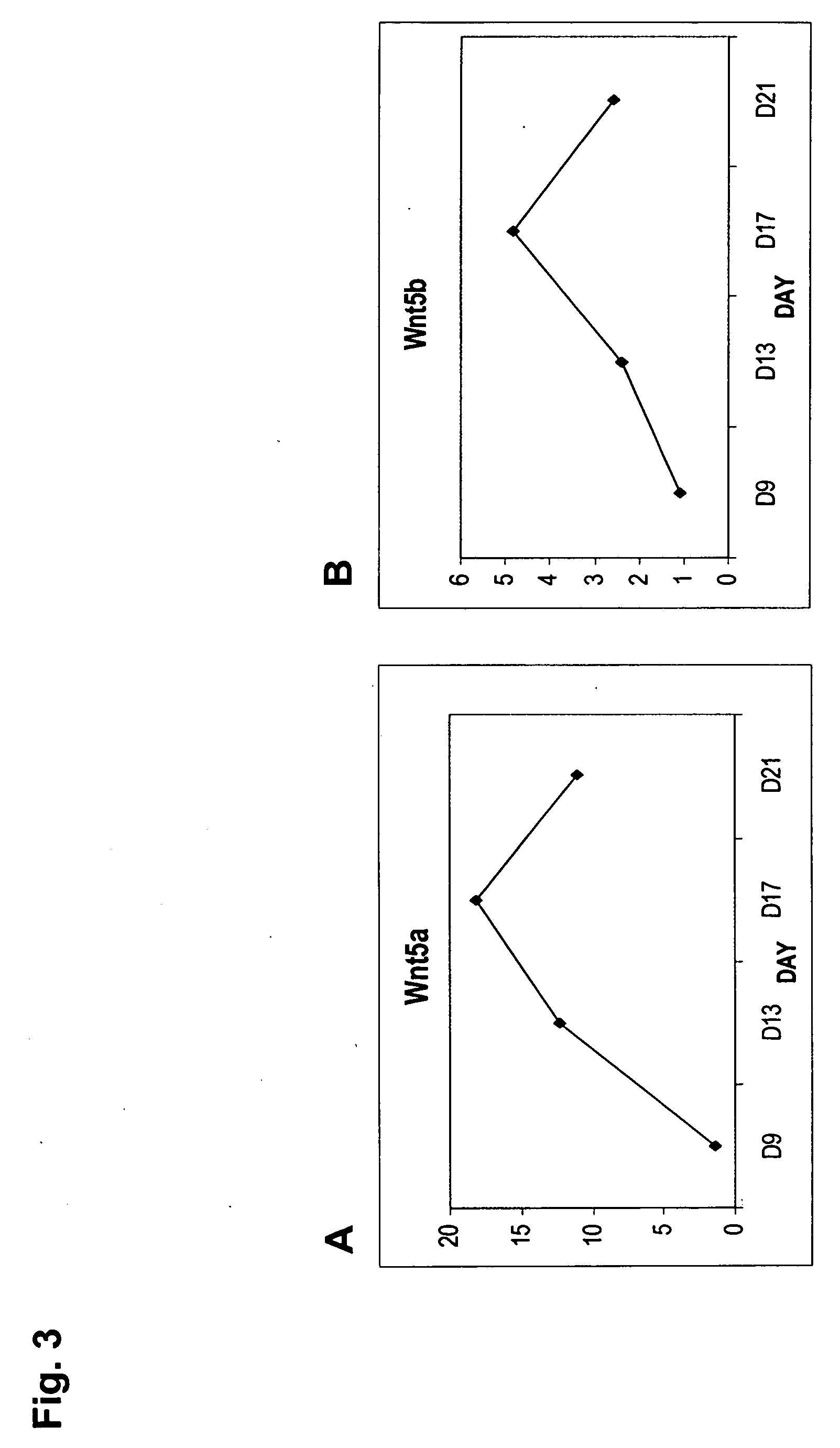
ActiveUS20050261181A1Improve biological activityPromote mineralizationBiocidePeptide/protein ingredientsOsteoblastWnt inhibitor
Compositions and methods for the treatment of bone diseases, bone fractures, bone injuries and other bone abnormalities involving the use of Dkk protein, a Wnt antagonist, a Wnt inhibitor, or any other related protein for the stimulation or enhancement of mineralization and for stimulating the renewal of cells. One Dkk protein, Dickkopf-2 (Dkk-2), acts to stimulate bone formation independently of Wnt proteins which may be inhibited and / or antagonized by Dkk-2. Dkk-2 displayed enhanced specific targeting ability and enhanced biological activity in stimulating or enhancing mineralization. Dkk-2 also played a role in the differentiation and self-renewal of hematopoietic stem cells and mesenchymal stem cells, particularly in osteoblastogenesis and osteoclastogenesis.
Owner:ENZO BIOCHEM
Antigen specific T cell therapy
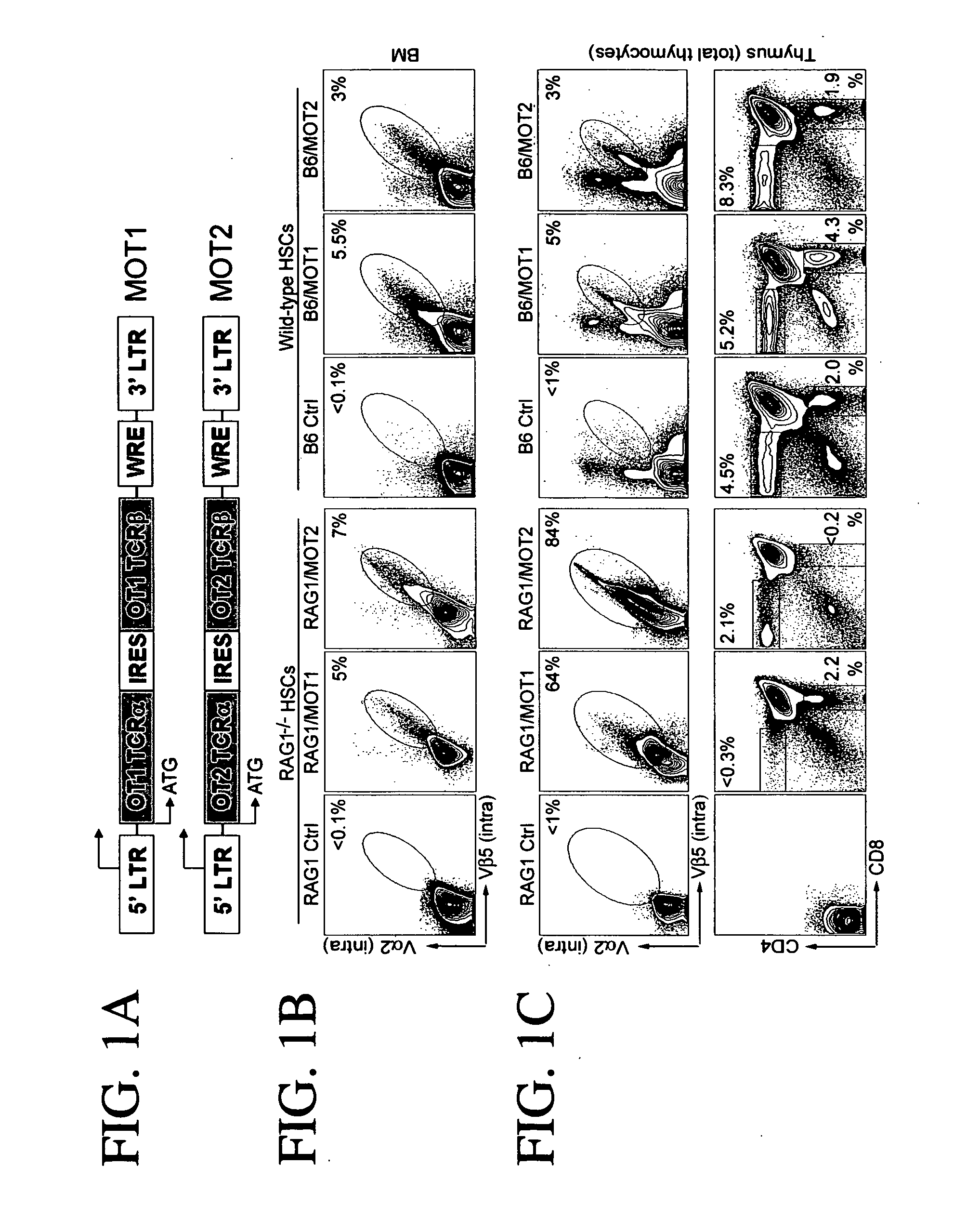
Provided are methods for generating immune cells of the desired type and specificity in a host. The methods may be used to treat a disease or disorder, such as a tumor in a patient. Target cells, preferably hematopoietic stem cells such as primary bone marrow cells are transfected with a polynucleotide encoding a T cell receptor with the desired specificity. The transfected cells are then transferred to the host where they develop into mature, functional immune cells. The source of the T cell receptor can determine the stem cell's fate. Thus transfecting stem cells with TCRs from cytotoxic cells will lead to the generation of cytotoxic T cells in the host, while TCRs from helper cells will produce helper cells. Both arms of T cell immunity can be generated simultaneously in a host. Additionally, the immune response to the desired antigen can be further stimulated by immunizing the host with the antigen.
Owner:CALIFORNIA INST OF TECH
Methods of using regenerative cells in the treatment of inherited and acquired disorders of the bone, bone marrow, liver, and other tissues
InactiveUS20050048036A1Promote differentiationBiocideMammal material medical ingredientsAdipose tissueDisease cause
Stem cells, e.g. HSC and MSC-like stem cells, present in adipose tissue are used to treat diseases and disorders that would benefit from a hematopoietic stem cell transplant. Methods of treating patients include processing adipose tissue to deliver a concentrated amount of stem cells obtained from the adipose tissue to a patient. The methods may be practiced in a closed system so that the stem cells are not exposed to an external environment prior to being administered to a patient. Accordingly, in a preferred method, cells present in adipose tissue are placed directly into a recipient along with such additives necessary to promote, engender or support a therapeutic renal benefit.
Owner:LOREM VASCULAR PTE LTD
Methods and compositions for modulating the mobilization of stem cells
InactiveUS20070190023A1Enhance mobilizationPrevent egressBiocidePeptide/protein ingredientsDiseaseCXCR4
Methods and compositions for modulating the mobilization of stem cells, particularly for promoting or increasing the mobilization of hematopoietic stem cells from the bone marrow to the peripheral blood are disclosed. In particular, the invention relates to the use of adrenergic agonists that act in concert with a mobilization compound or agent. The mobilization agent(s) may act to decrease the expression or function of the chemokine, CXCL12, or may act to block or antagonize CXCR4. The invention also relates to methods of using these compounds or agents for enhancing the mobilization of hematopoietic stem cells when harvesting of the stem cells is necessary for the treatment of diseases, disabilities or conditions whereby transplantation of such cells would be beneficial in ameliorating the symptoms associated with such diseases, disabilities or conditions. Methods of screening for novel agents and pharmaceutical compositions comprising these agents are also disclosed.
Owner:MT SINAI SCHOOL OF MEDICINE
Isolation, expansion and use of clonogenic endothelial progenitor cells
InactiveUS20050266556A1Increase in HSCHigh activityBiological material analysisMammal material medical ingredientsCord blood stem cellFeeder Layer
A hierarchy of endothelial colony forming cells (EPCs) was identified from mammalian cord blood, umbilical vein and aorta. A newly isolated cell named high proliferative potential—endothelial colony forming cell (HPP-ECFC) was isolated and characterized. Single cell assays were developed that test the proliferative and clonogenic potential of endothelial cells derived from cord blood, or from HUVECs and HAECs. EPCs were found to reside in vessel walls. Use of a feeder layer of cells derived from high proliferative potential-endothelial colony forming cells (HPP-ECPCS) from human umbilical cord blood, stimulates growth and survival of repopulating hematopoietic stem and progenitor cells. Stimulation of growth and survival was determined by increased numbers of progenitor cells in in vitro cultures and increased levels of human cell engraftment in the NOD / SCID immunodeficient mouse transplant system.
Owner:INDIANA UNIV RES & TECH CORP
Cellular compositions which facilitate engraftment of hematopoietic stem cells while minimizing the risk of gvhd
The present invention provides for cellular compositions which facilitate engraftment of hematopoietic stem cells from a syngeneic, allogeneic or xenogeneic donor. The cellular compositions of the invention facilitate engraftment while minimizing the risk of graft versus host disease in the graft recipient. According to a preferred embodiment of the invention, a cell composition is provided, which cell composition comprises hematopoietic stem cells, such as CD34+ cells and / or facilitating cells, in combination with αβ TCR+ T cells. The invention also relates to methods of using the cellular compositions of the invention to induce donor specific tolerance in a recipient, thus allowing the transplantation of donor organs, cells and tissues. Also disclosed are methods of treating leukemia and cancer as well as infectious diseases caused by viruses.
Owner:JEWISH HOSPITAL HEALTHCARE SERVICES
Methods for diagnosing and evaluating treatment of blood disorders
Methods, systems and kits are provided for the clinical staging of blood disorders including myelodysplastic syndrome, myeloproliferative diseases and leukemias by differential analysis of hematologic samples for the distribution of one or more hematopoietic stem or progenitor cell subsets. Additional functional, genetic, gene expression, proteomic or other molecular analyses of the hematopoietic stem and progenitor cells from the patients can also be employed in the staging methods of the invention.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Therapeutic compositions containing glutathione analogs
InactiveUS7029695B2Improve bioavailabilityPromote absorptionTripeptide ingredientsImmunoglobulinsLipid formationHematopoietic cell
Pharmaceutical compositions and methods of using them. Lipid formulations of a glutathione analog and methods of manufacturing them. Their use to stimulate hematopoiesis, protect hematopoietic cells from damage caused by radiation or chemotherapy, or potentiate the stimulatory action of one or a combination of cytokines on colony formation by hematopoietic progenitor cells, protect a subject from a destructive effect of a chemotherapeutic agent or irradiation, or to potentiate the effect of a chemotherapeutic agent.
Owner:TELIK INC
HSPC-Sparing Treatments for RB-Positive Abnormal Cellular Proliferation
ActiveUS20140271466A1MinimizingDeleterious effectBiocideOrganic chemistryProgenitorTreatment choices
This invention is in the area of improved compounds for and methods of treating selected RB-positive cancers and other Rb-positive abnormal cellular proliferative disorders while minimizing the deleterious effects on healthy cells, for example healthy Hematopoietic Stem Cells and Progenitor Cells (HSPCs), associated with current treatment modalities. In one aspect, improved treatment of select RB-positive cancers is disclosed using specific compounds disclosed herein. In certain embodiments, the compounds described herein act as highly selective and, in certain embodiments, short, transiently-acting cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitors when administered to subjects.
Owner:G1 THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com