Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
234 results about "Cord blood" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
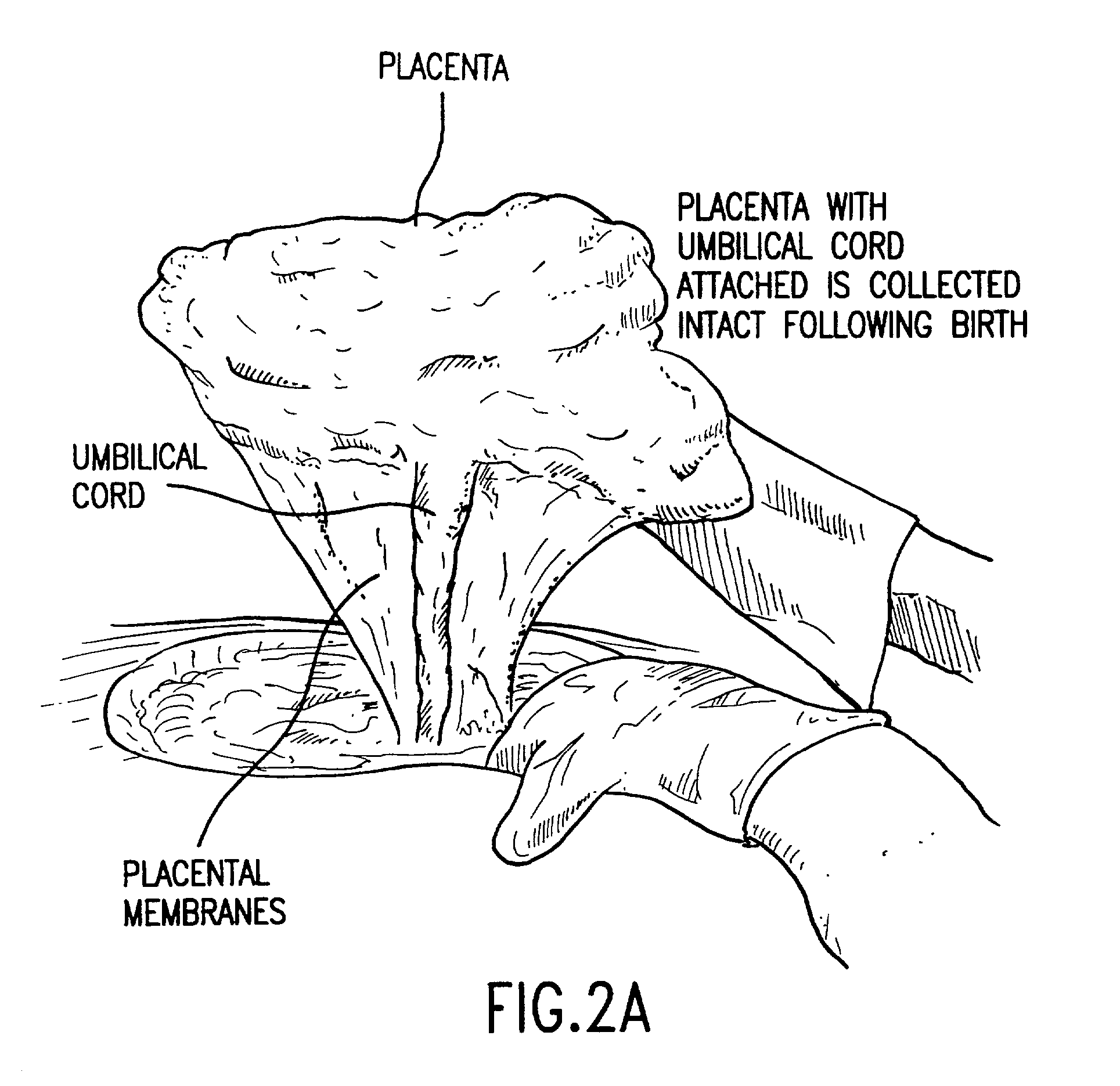
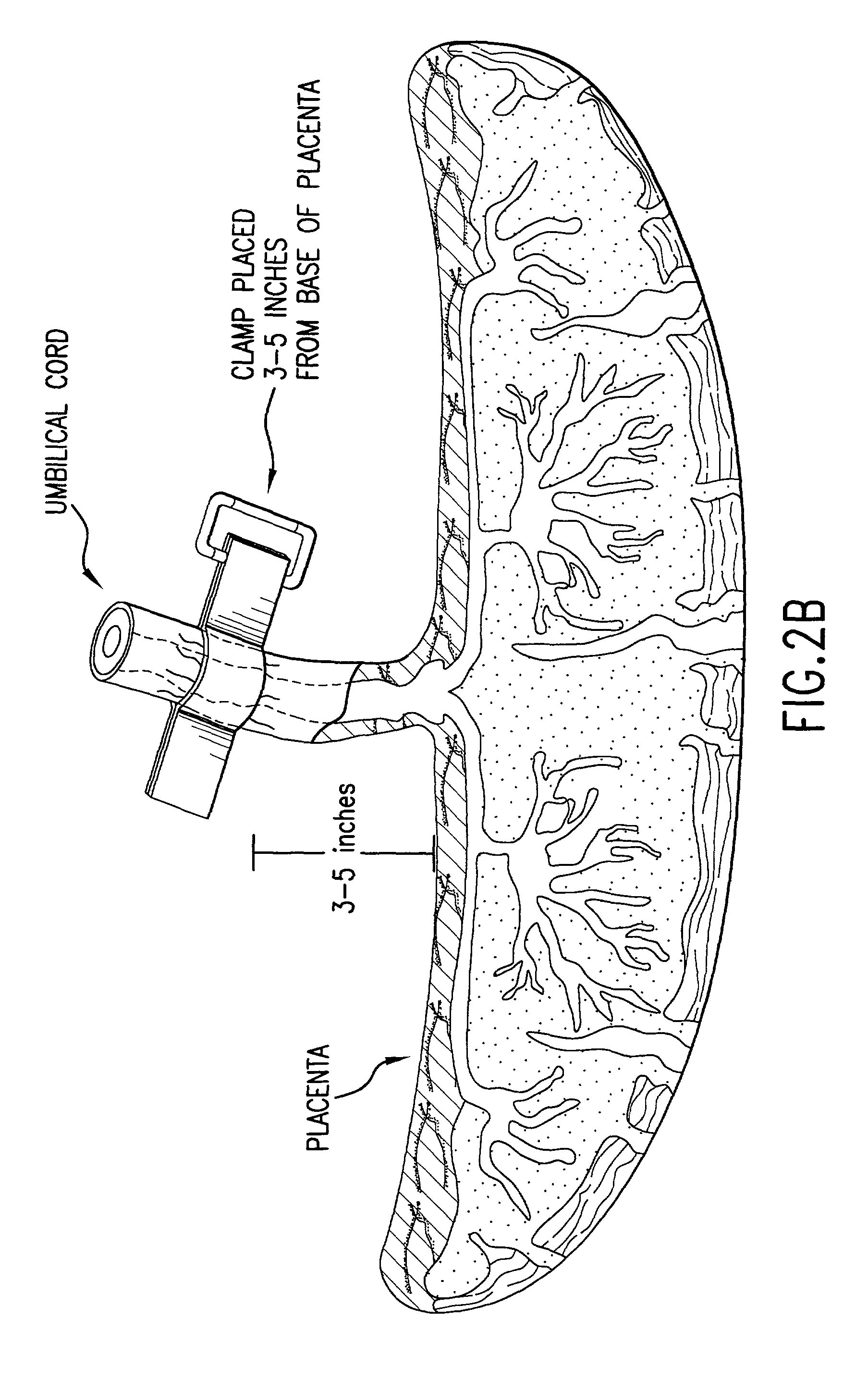
Cord blood (umbilical cord blood) is blood that remains in the placenta and in the attached umbilical cord after childbirth. Cord blood is collected because it contains stem cells, which can be used to treat hematopoietic and genetic disorders.
Post-partum mammalian placenta, its use and placental stem cells therefrom
InactiveUS20030032179A1Enhance exsanguinationEnhance sterile conditionSenses disorderAntipyreticAnticoagulant AgentEmbryo
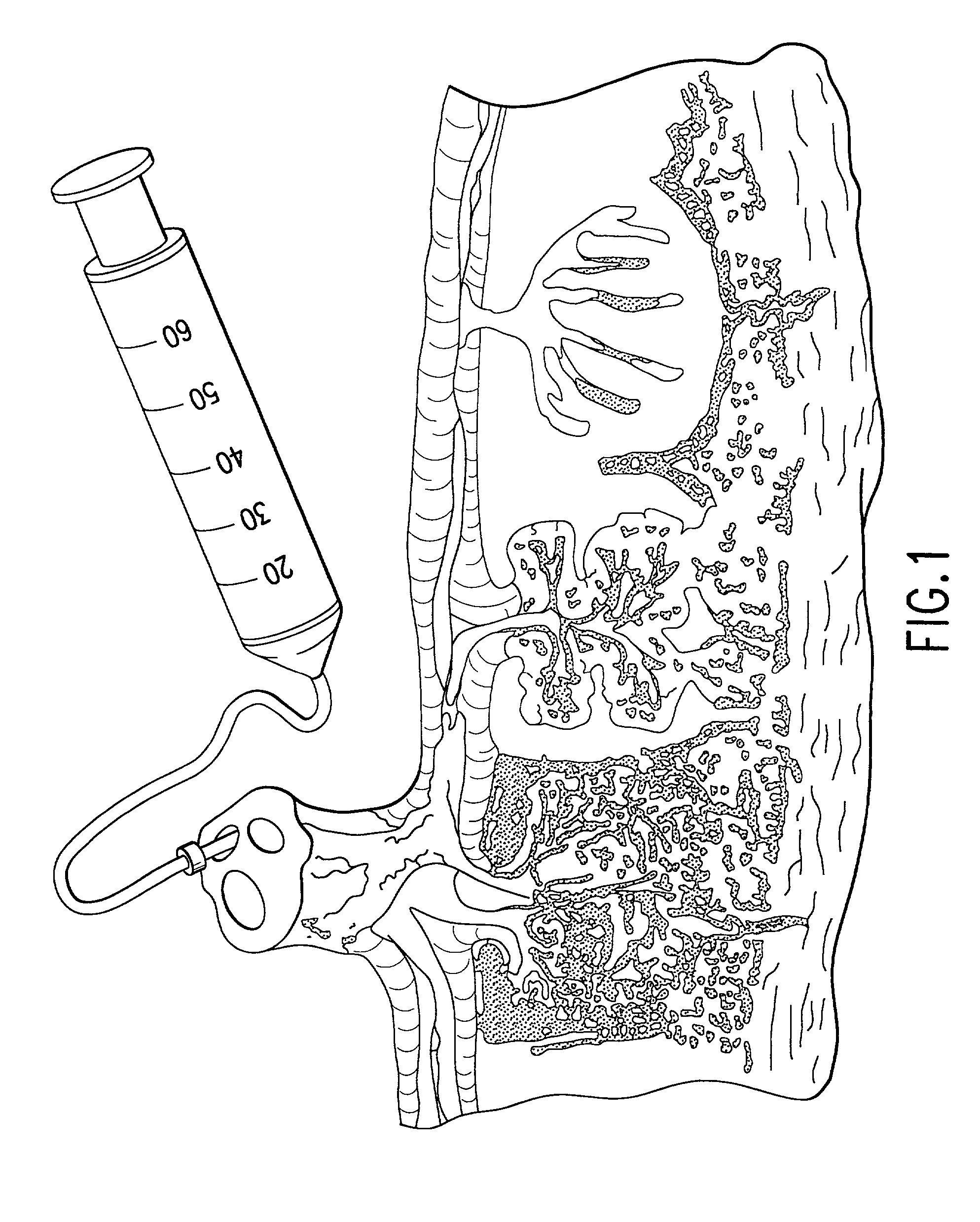
The present invention provides a method of extracting and recovering embryonic-like stem cells, including, but not limited to pluripotent or multipotent stem cells, from an exsanguinated human placenta. A placenta is treated to remove residual umbilical cord blood by perfusing an exsanguinated placenta, preferably with an anticoagulant solution, to flush out residual cells. The residual cells and perfusion liquid from the exsanguinated placenta are collected, and the embryonic-like stem cells are separated from the residual cells and perfusion liquid. The invention also provides a method of utilizing the isolated and perfused placenta as a bioreactor in which to propagate endogenous cells, including, but not limited to, embryonic-like stem cells. The invention also provides methods for propagation of exogenous cells in a placental bioreactor and collecting the propagated exogenous cells and bioactive molecules therefrom.
Owner:CELULARITY INC
Agonist antibody to human thrombopoietin receptor
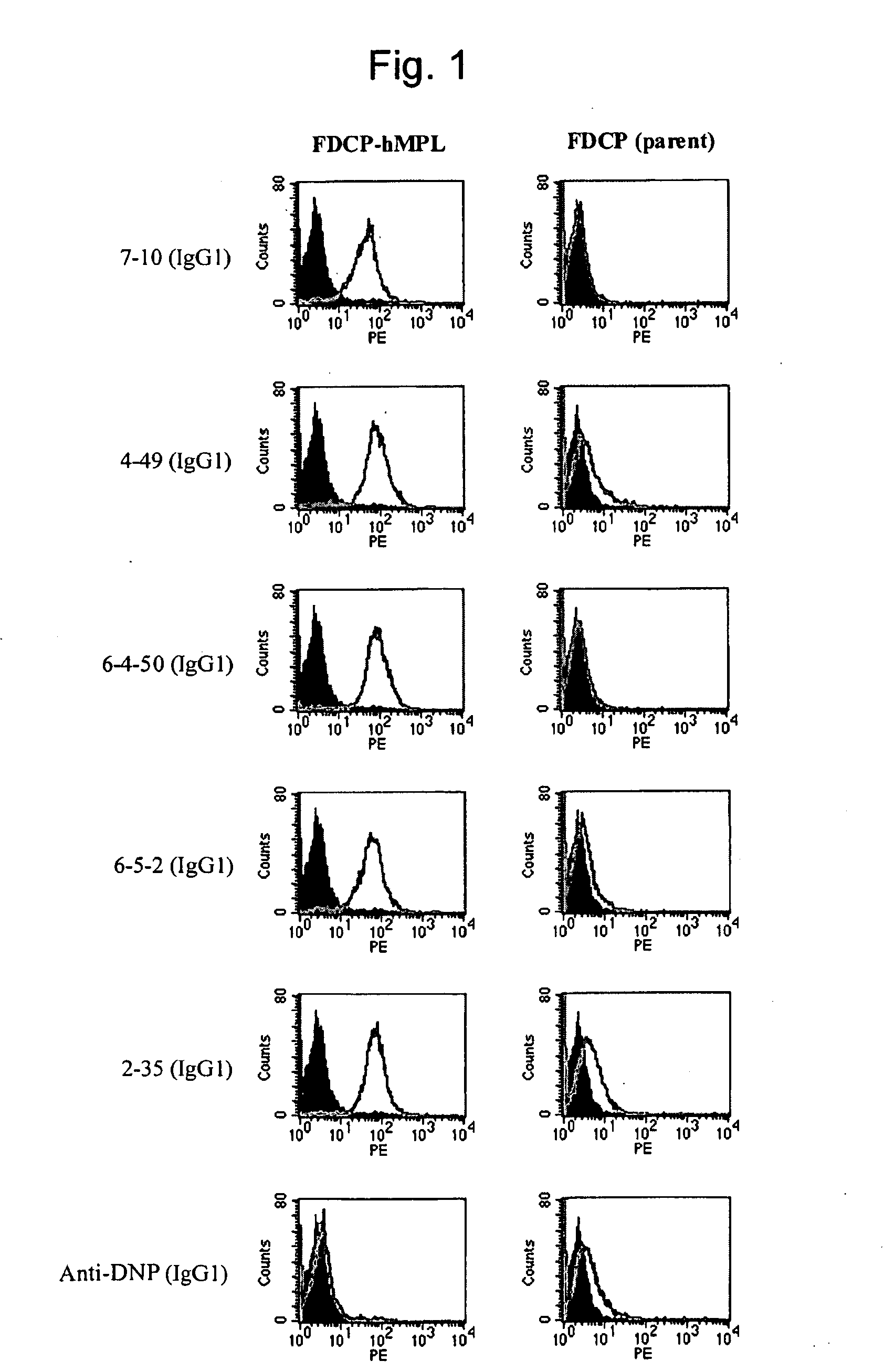
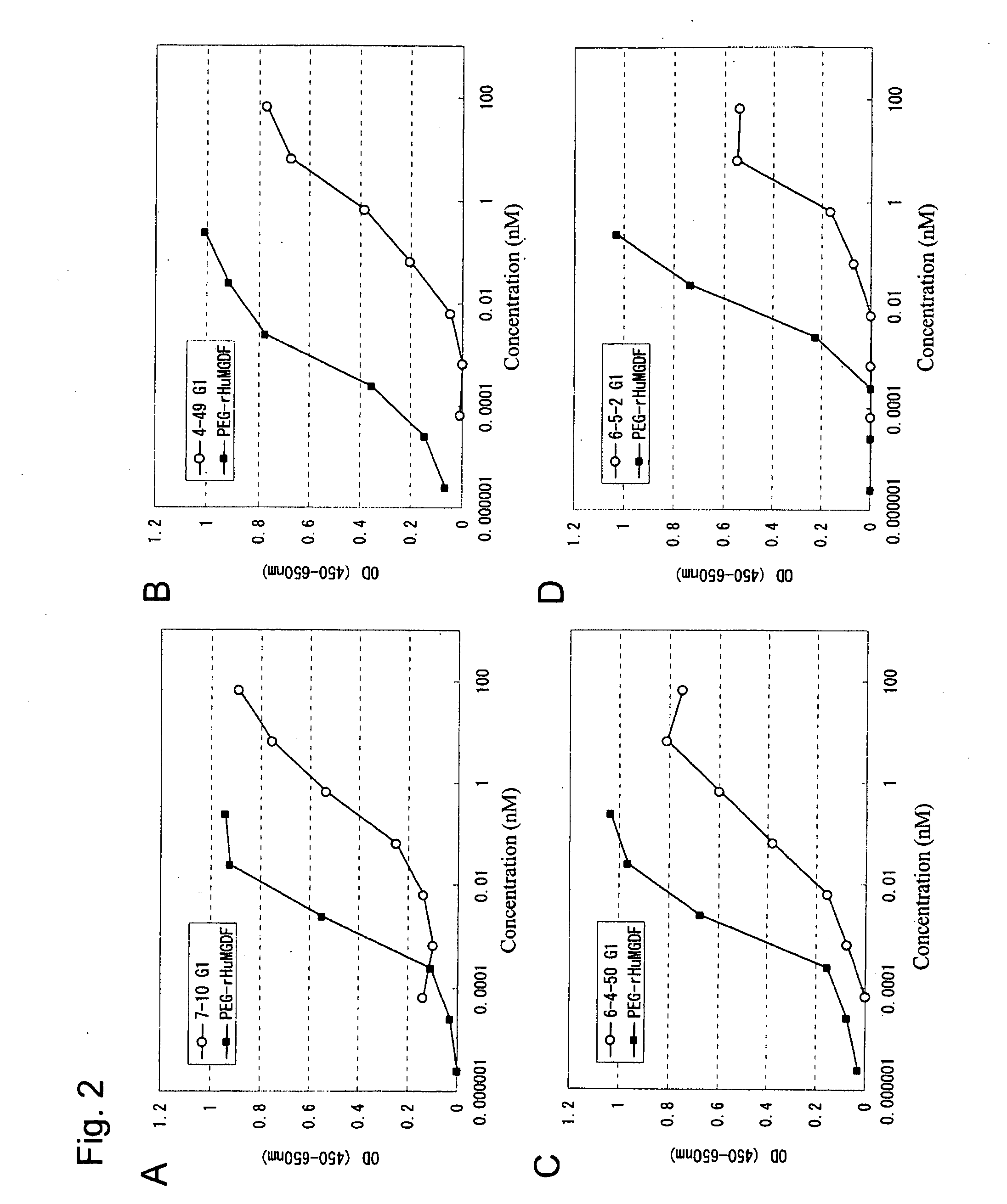
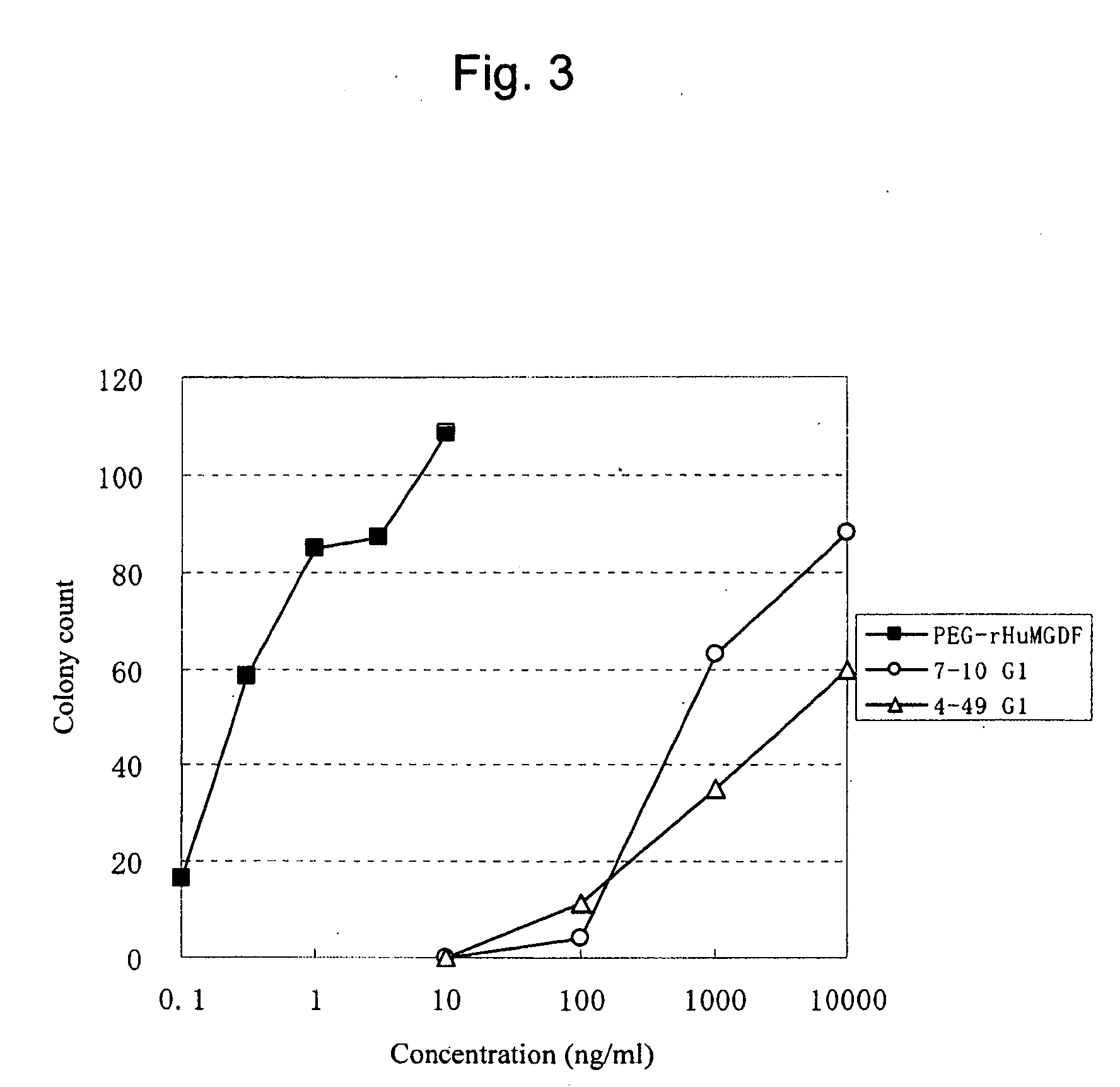
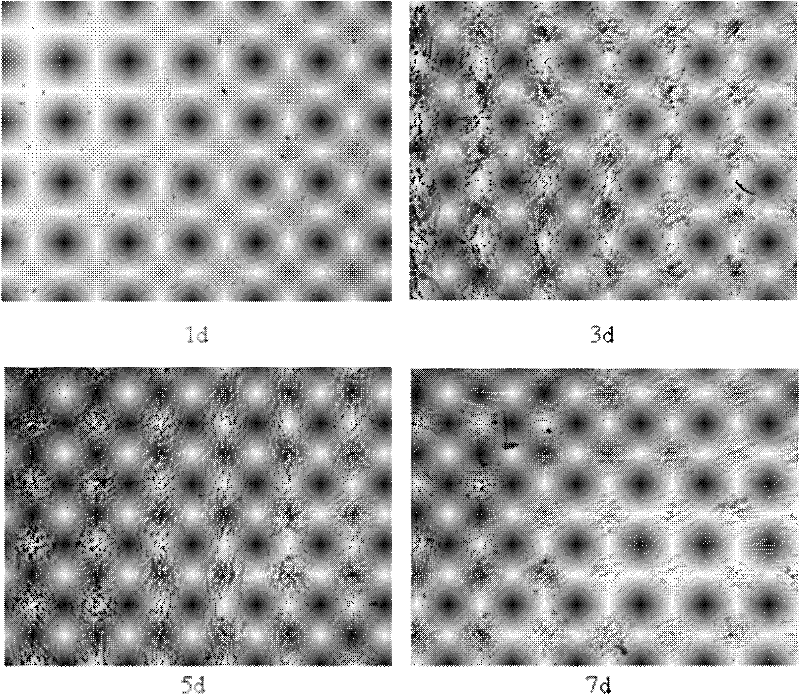
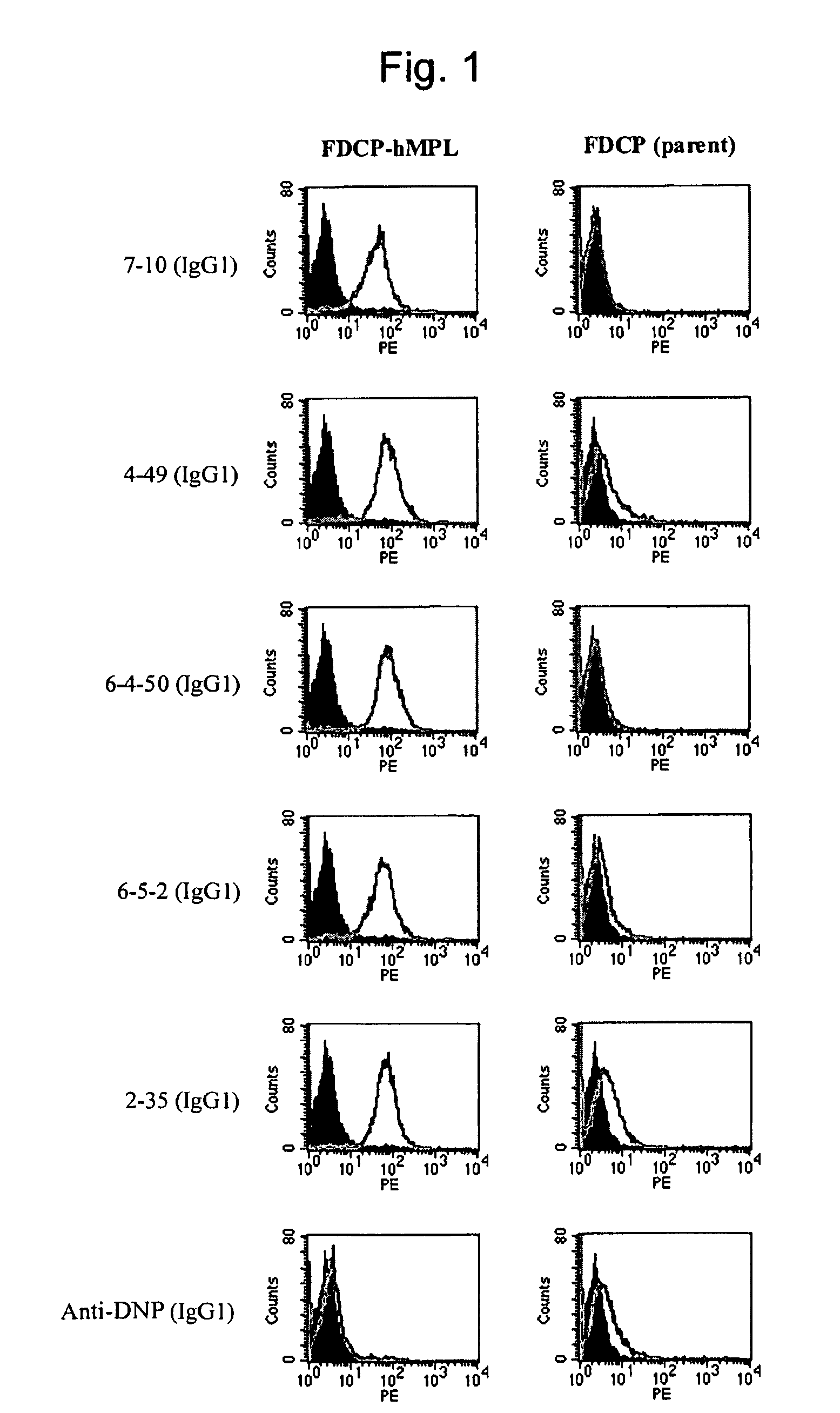
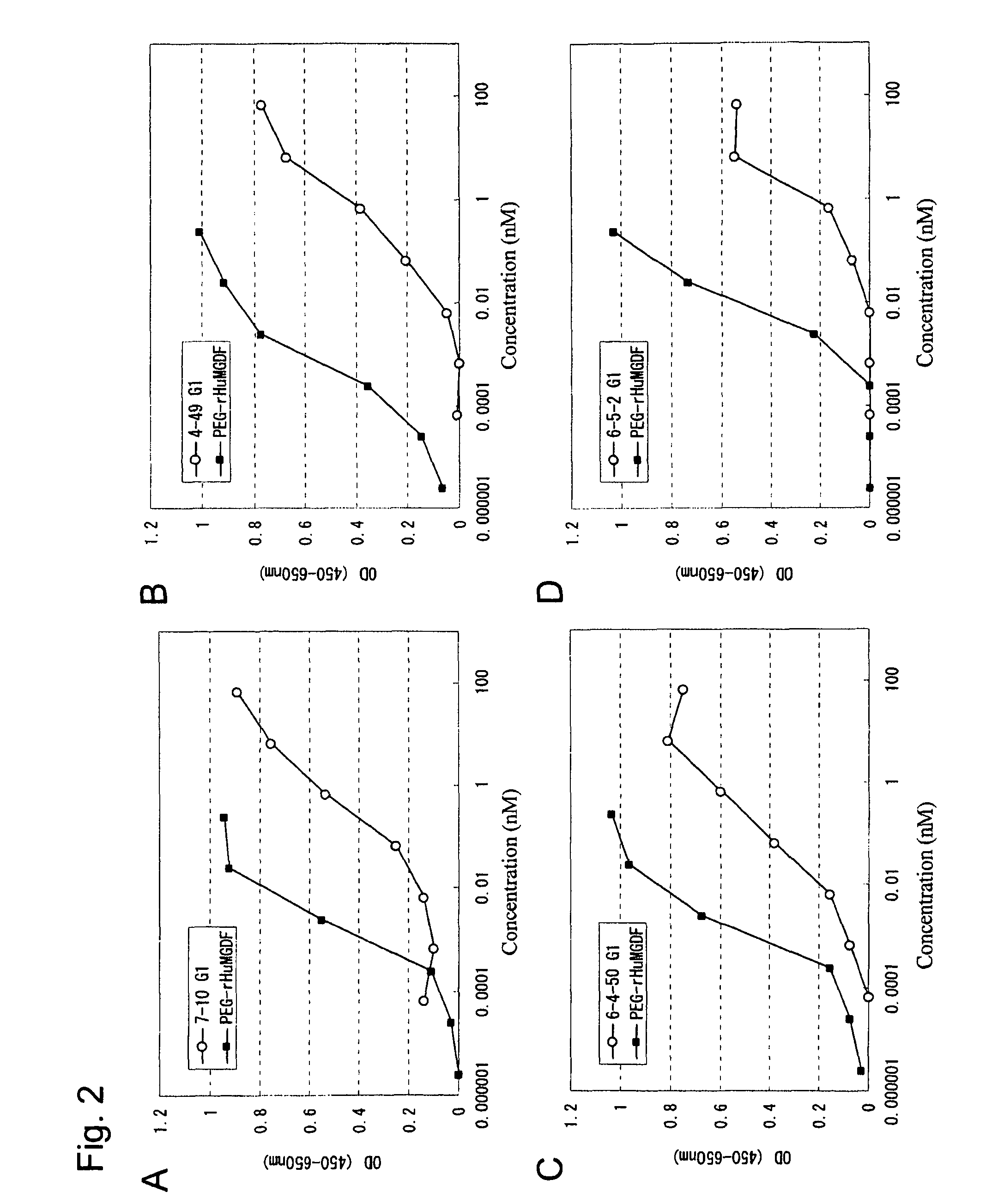
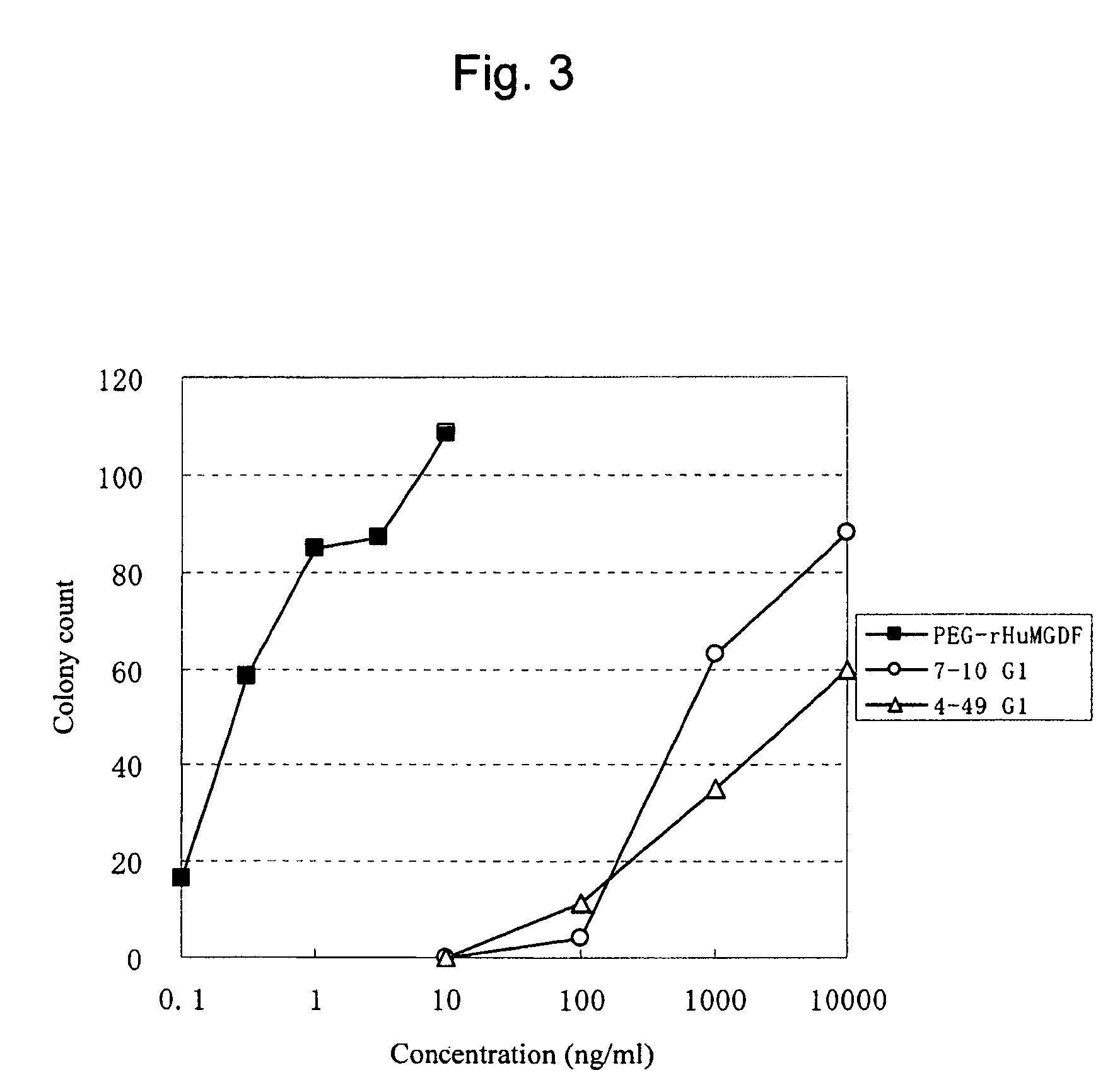
InactiveUS20100004429A1High activityLow antigenicityThrombopoietinHybrid immunoglobulinsHuman plateletUmbilical cord
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Additive solution for blood preservation
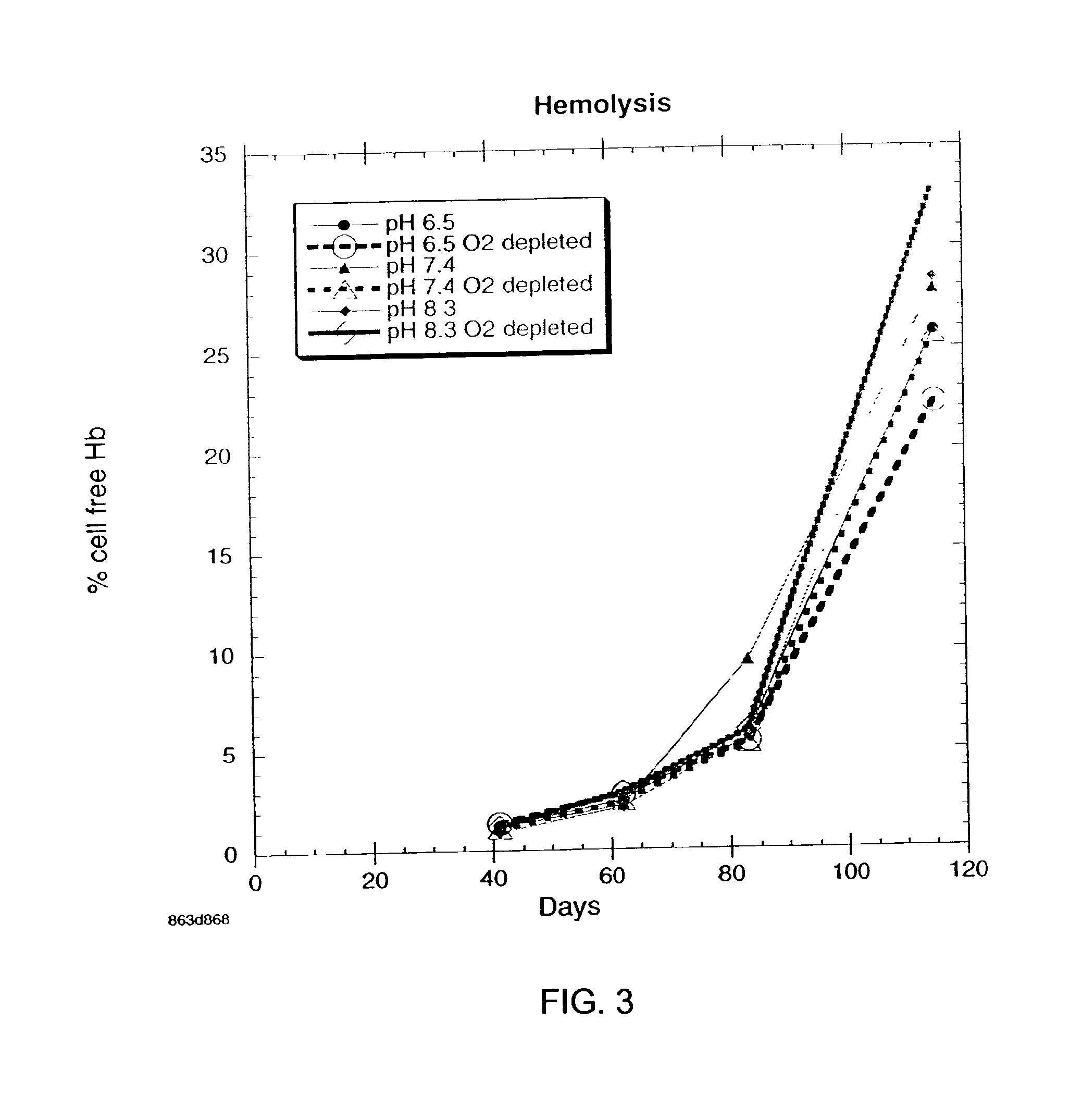
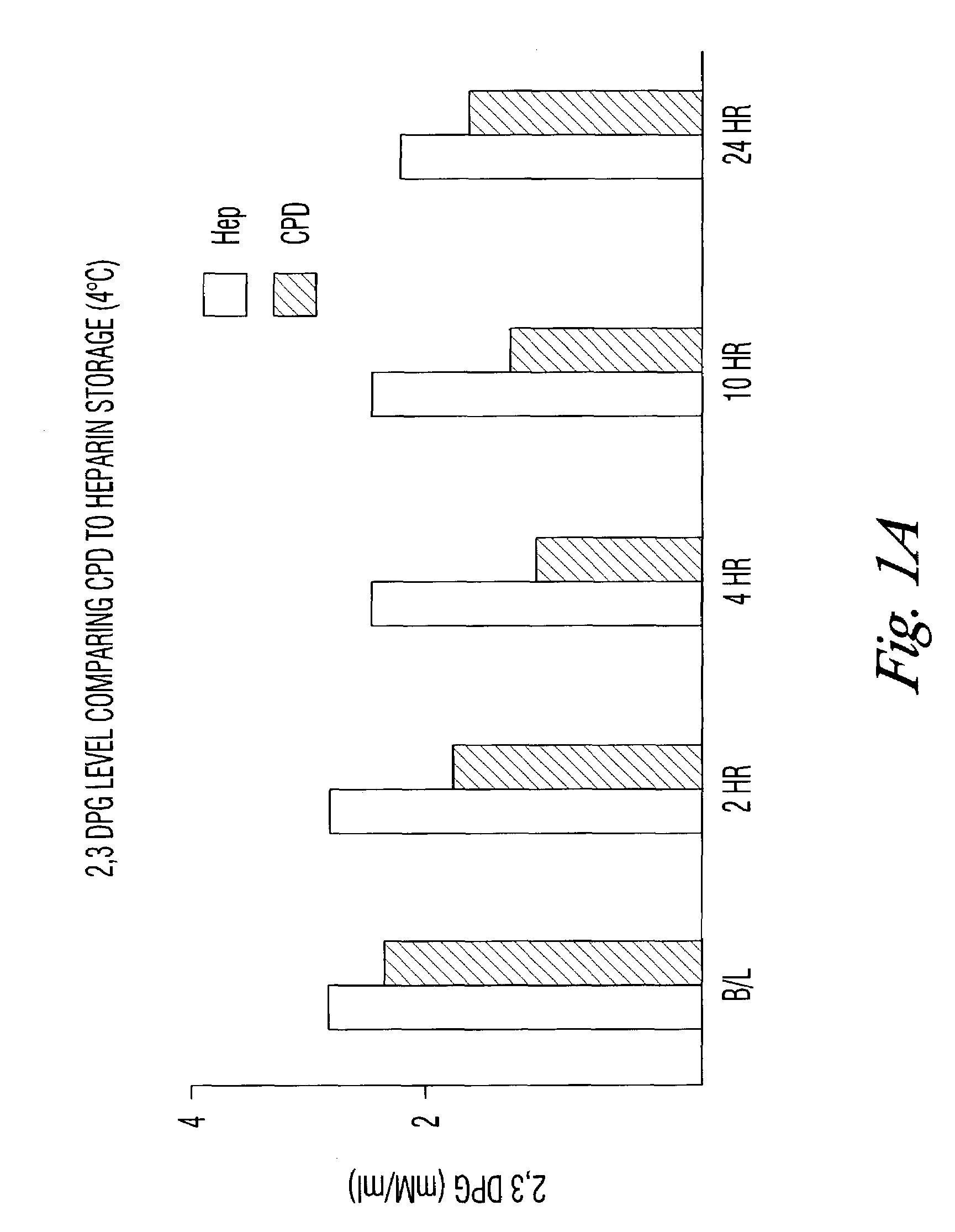
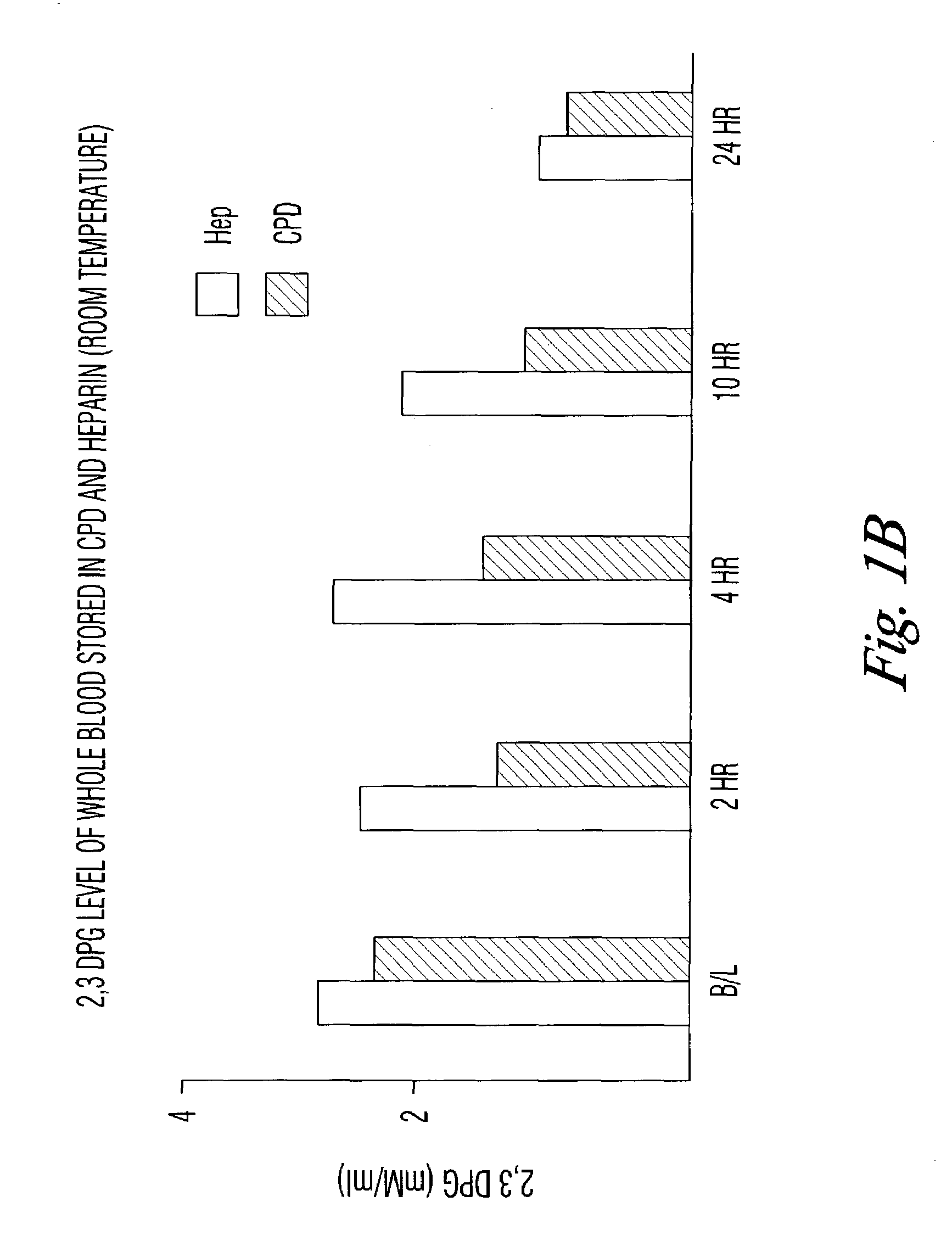
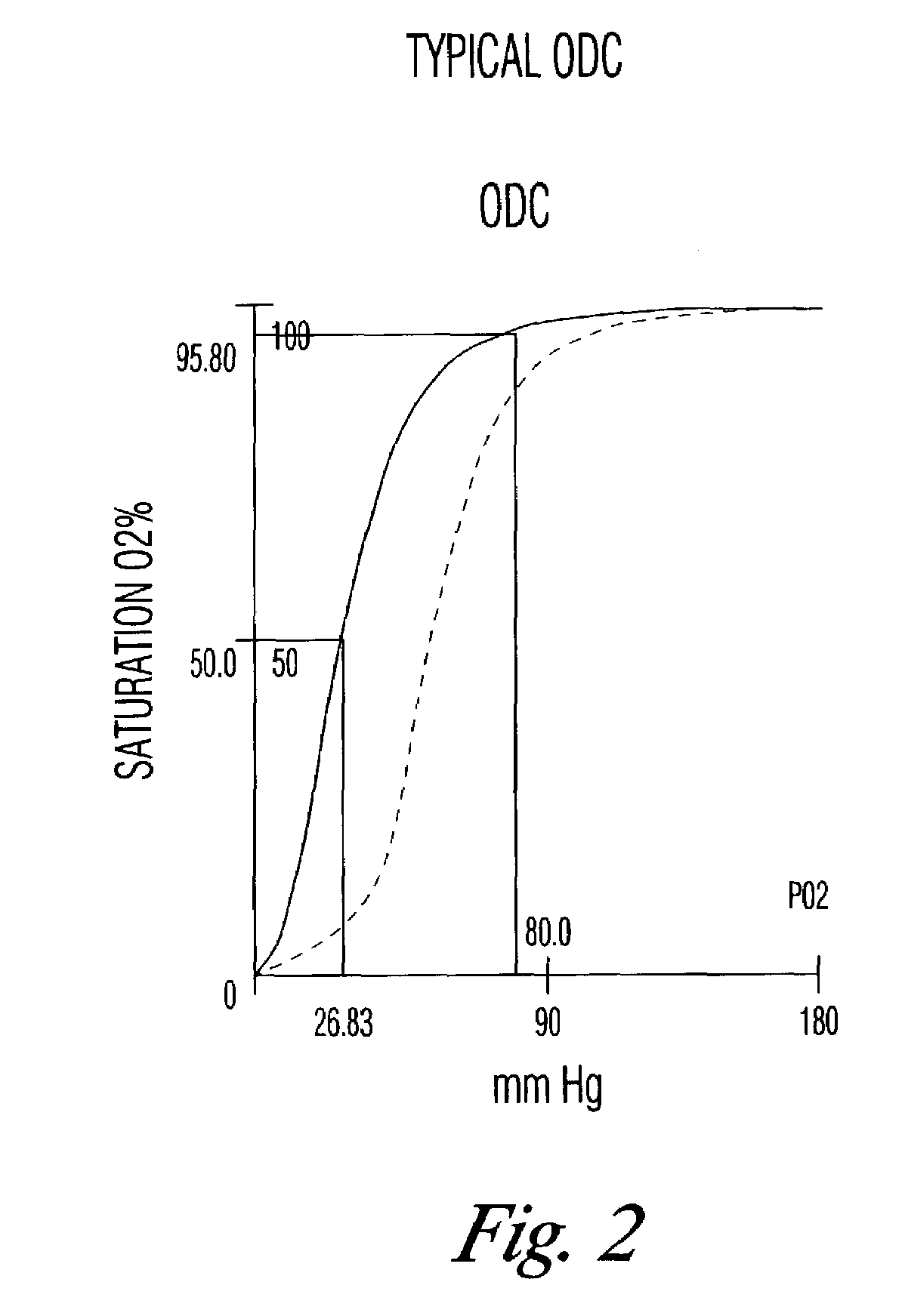
There is provided compositions and methods for the storage of red blood cells. The compositions are additive solutions comprising adenine, dextrose, mannitol, NaH2PO4, and optionally NaCl and / or NH4Cl. Composition are preferably used with oxygen-depletion refrigerated storage of red blood cells and may optionally be employed with nutrient supplements extending the useful shelf life of stored blood.
Owner:TRUSTEES OF BOSTON UNIV +1
Method for separating exosome from human umbilical cord mesenchymal stem cell source and reagent used by method
The invention relates to a method for separating exosome from a human umbilical cord mesenchymal stem cell source and a reagent used by the method. The method comprises the following steps: (1) cultivating umbilical cord mesenchymal stem cells; and (2) extracting umbilical cord blood MSC exosome, wherein the umbilical cord mesenchymal stem cells are cultivated to P3 generation; and extraction forthe umbilical cord blood MSC exosome comprises the following steps: pre-treating exosome suspension; and extracting exosome. The reagent used by the method realtes to a solution which has a special formula and is used as a buffer pad. The method has the excellent technical effects as described in the specification.
Owner:FIVE DIMENSION BY INCOSC HEALTH MANAGEMENT JIANGSU
Stem Cell Populations and Methods of Use
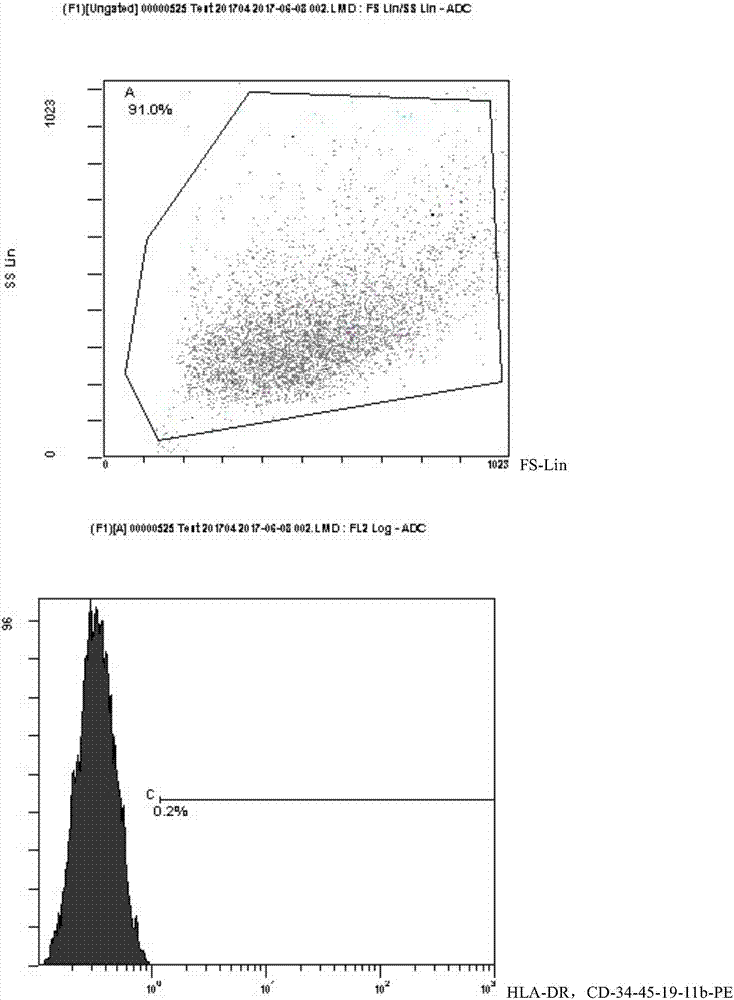
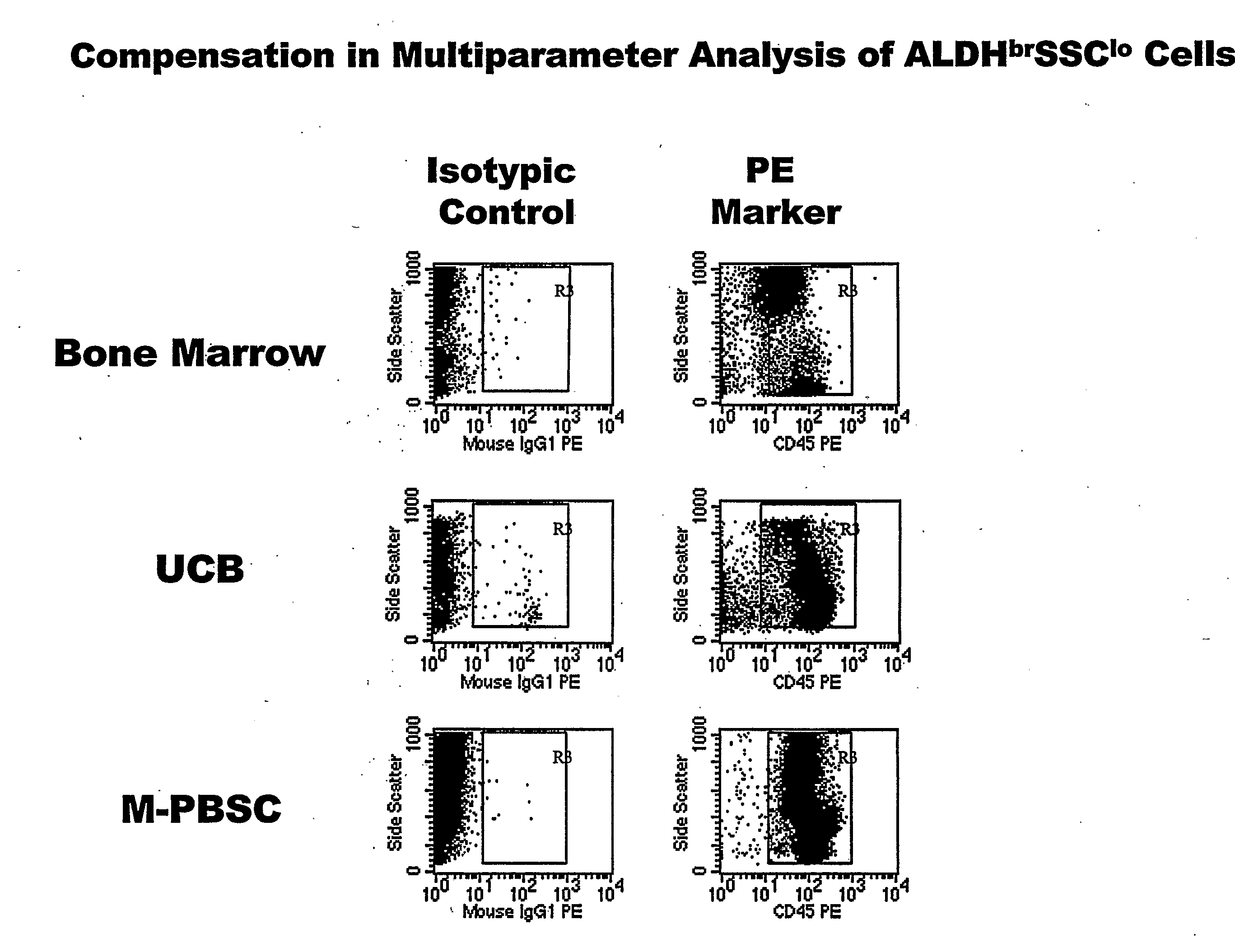
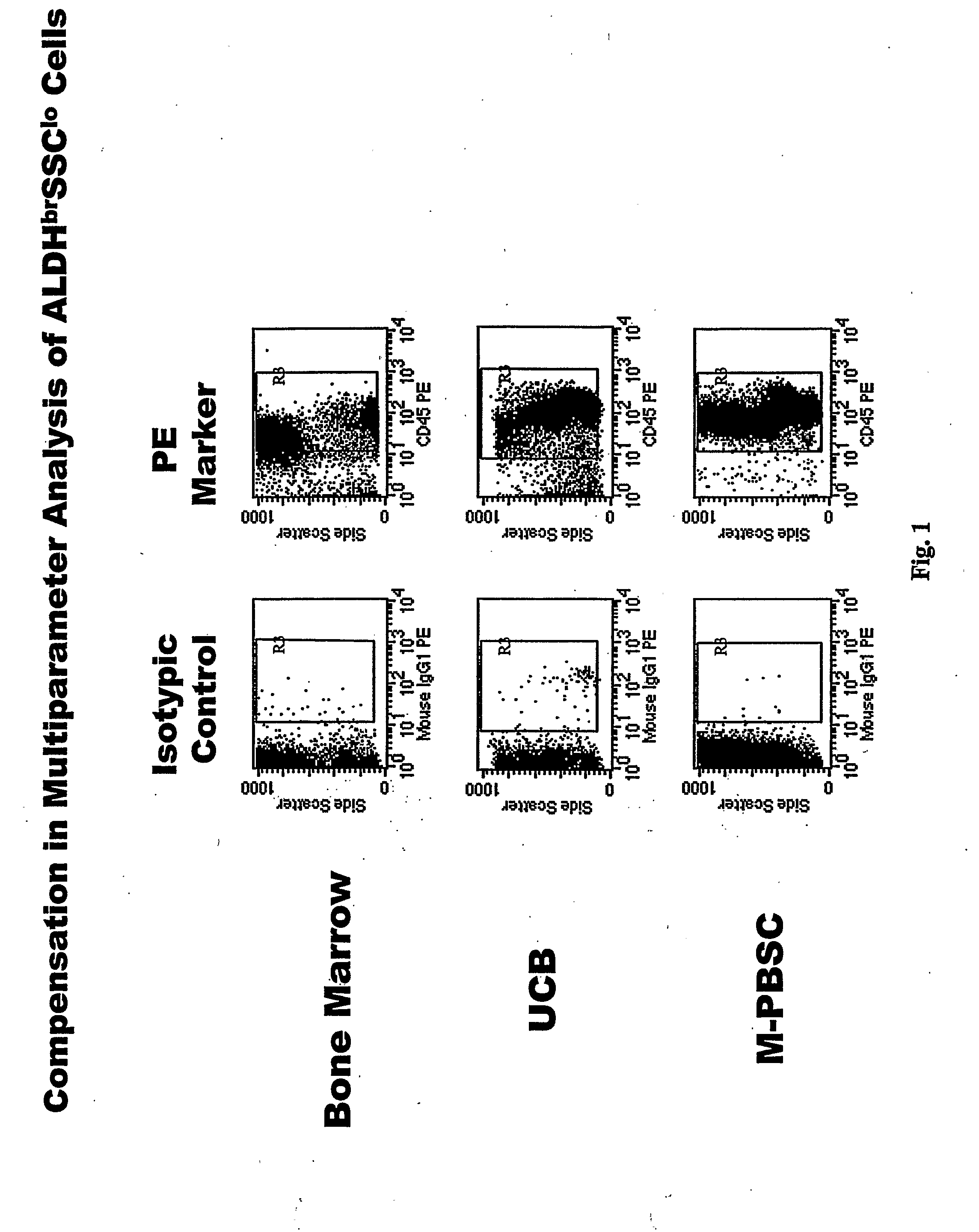
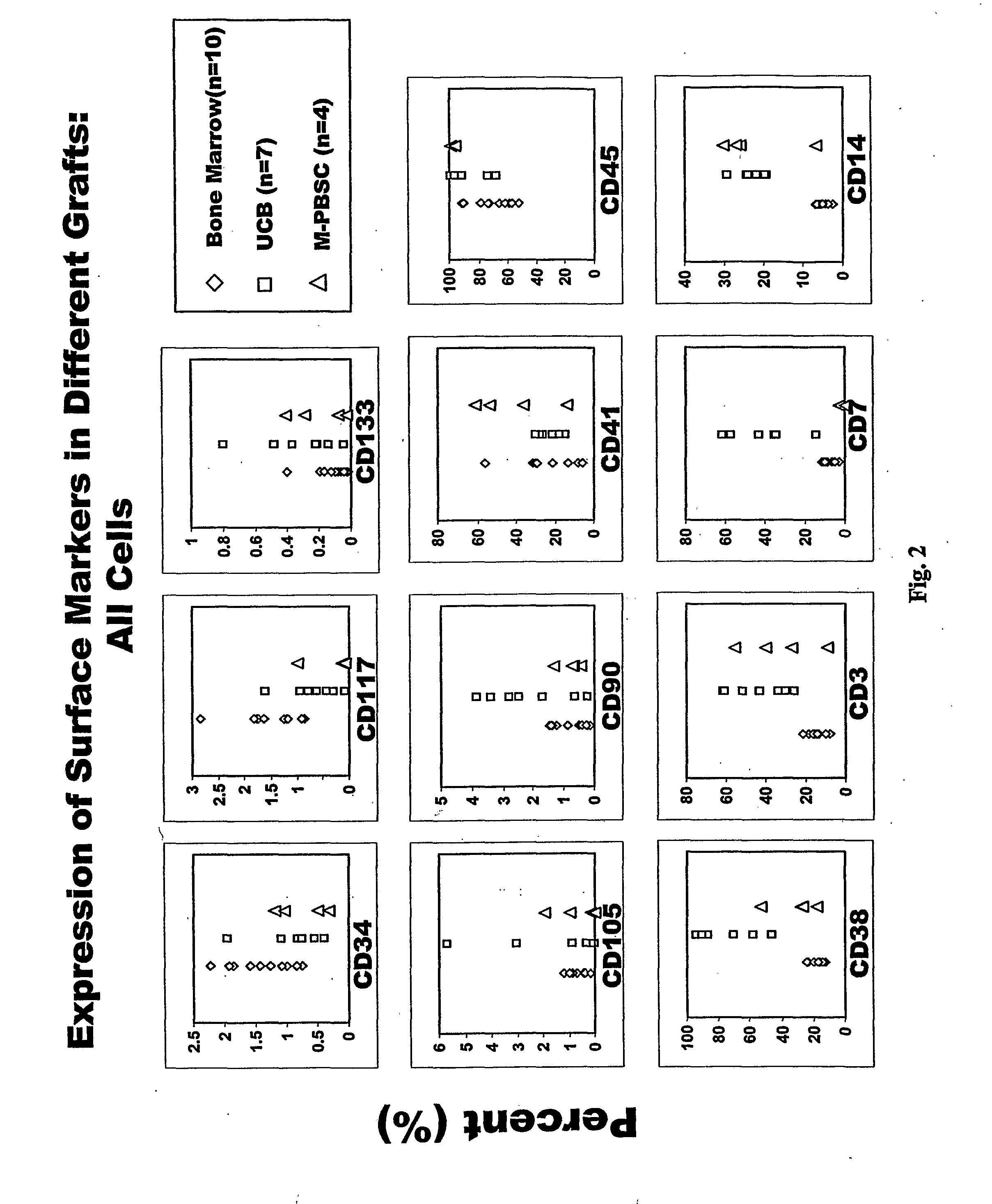
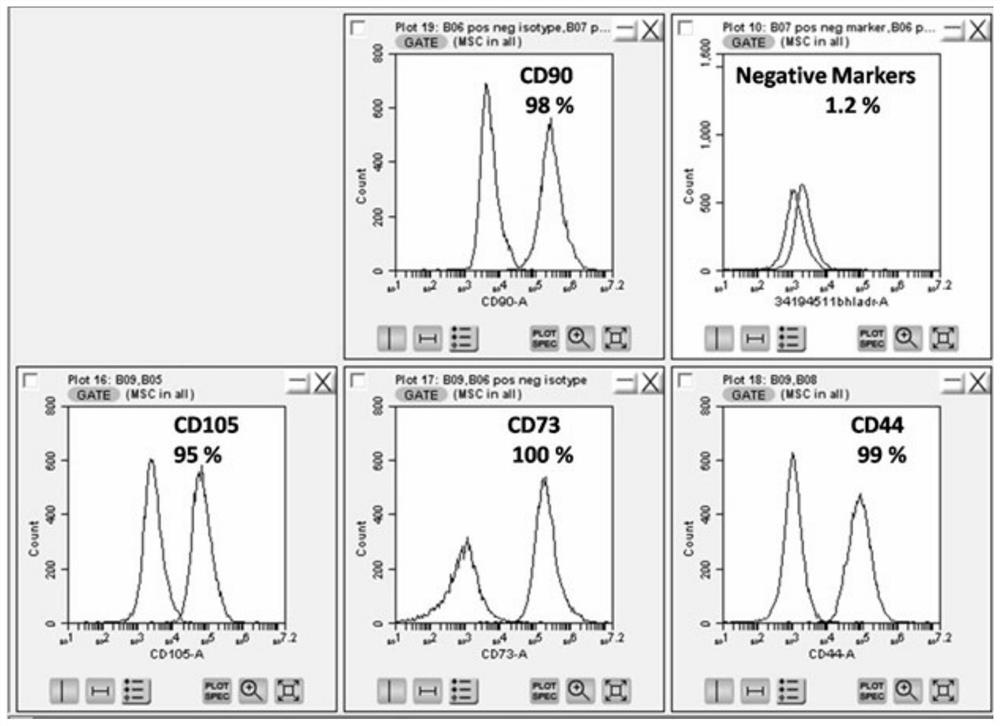
Populations of stem cells and methods for their isolation and use are provided. These stem cell populations comprise aldehyde dehydrogenase positive (ALDHbr) cells isolated from bone marrow, and ALDHbr CD105+ cells derived from any stem cell source. These populations may also comprise cells expressing such surface markers as CD34, CD38, CD41, CD45, CD105, CD133, CD135, CD117, and HLA-DR, and / or are substantially free from such cell surface markers as CD3, CD7, CD 10, CD 13, CD 14, C1319, CD33, CD35, CD56, CD 127, CD 138, and glycophorin A. The population may also comprise cells expressing CD90. The stem cell populations of the invention are isolated from a stem cell source such as bone marrow, peripheral blood, umbilical cord blood, and fetal liver. Methods of the invention comprise isolating and purifying stem cell populations from stem cell sources, and methods of using these cells to reconstitute, repair, and regenerate tissues.
Owner:ALDAGEN
Composition for treatment of articular cartilage damage
InactiveUS7459307B2Good effectEasy to operateBiocideSkeletal disorderCellular componentArticular pain
Disclosed herein is a composition for the treatment of articular cartilage damage or loss or defect. The composition for the treatment of articular cartilage injury of the present invention includes (i) cellular components separated, proliferated, and / or differentiated from the umbilical cord blood, (ii) a culture medium; and (iii) a biocompatible polymer. The composition has very superior ability of proliferation and differentiation and easier to be collected and acquired.
Owner:MEDIPOST +1
Complete medium and human amnion-derived mesenchymal stem cell culture method
The invention discloses a complete medium and a human amnion-derived mesenchymal stem cell (hAMSCs) culture method. The complete medium is prepared by adding 3 to 10 percent of autologous umbilical cord blood serum into low-sugar Dulbecco minimum essential medium solution according to a volume ratio. The culture method comprises: (1) separation; (2) primary culture; and (3) subculture. The methodusing the complete medium in the hAMSCs culture has the advantages that: the risk of using fetal calf serum is avoided; although the need of adding L-glutamine, non-essential amino acid, 2-mercapitoethanol, pyruvic acid and the like is obviated, the high proliferation properties and phenotypic characteristics of the hAMSCs and expression of multilineage differentiation marker genes sand proteins of some stem cells can still be retained; and in subculture, the wall adherence fastness of the hAMSCs is much lower than that in fetal bovine serum (FBS) culture, the digestion time is reduced obviously, and the damage of trypsinization to cells and loss of cells are reduced.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Agonist antibody to human thrombopoietin receptor
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
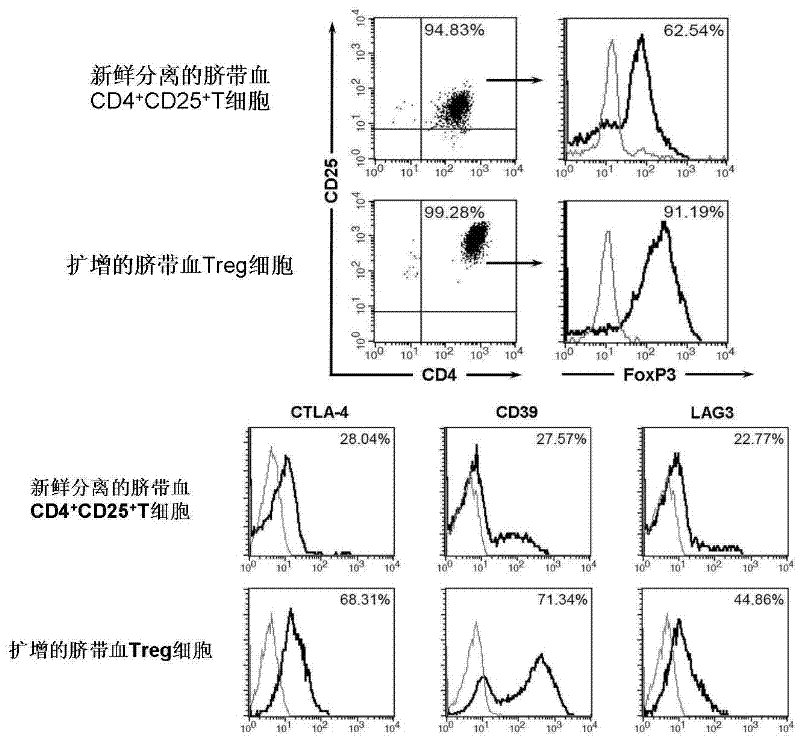
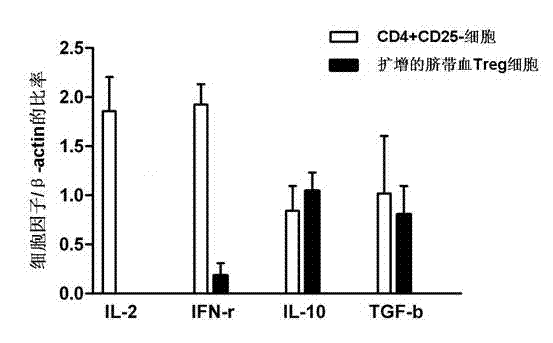
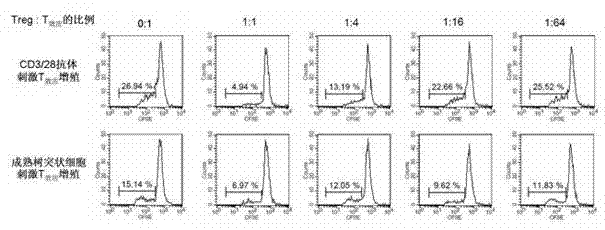
In vitro amplification and low-temperature storage method for regulatory T cells of umbilical cord blood
ActiveCN102517253AMeet needsDead animal preservationMammal material medical ingredientsImmunologic disordersRegulatory T cell
The invention relates to an in vitro amplification and low-temperature storage method for regulatory T cells of umbilical cord blood. The method comprises the following steps of: separating a CD4+CD25+T cell group rich in the regulatory T cells from the umbilical cord blood; amplifying the enriched CD4+CD25+T cell group to obtain high-purity regulatory T cells; and storing the amplified regulatory T cells in liquid nitrogen at a low temperature to serve as third-party unrelated donor regulatory T cells for treating clinical diseases. The method has the advantages that: a large quantity of regulatory T cells can be obtained from the umbilical cord blood and stored for a long term in the liquid nitrogen, can meet the requirements of clinic for regulatory T cell products, and are used for treating graft versus host disease, organ transplantation rejection and autoimmune disease.
Owner:SHANGHAI BLOOD CENT
Composition for treating a disease caused by neuronal insult comprising a human umbilical cord blood-derived mesenchymal stem cell as an active ingredient
InactiveUS20080131405A1Improve abilitiesResilienceBiocideNervous disorderHuntingtons choreaRisk stroke
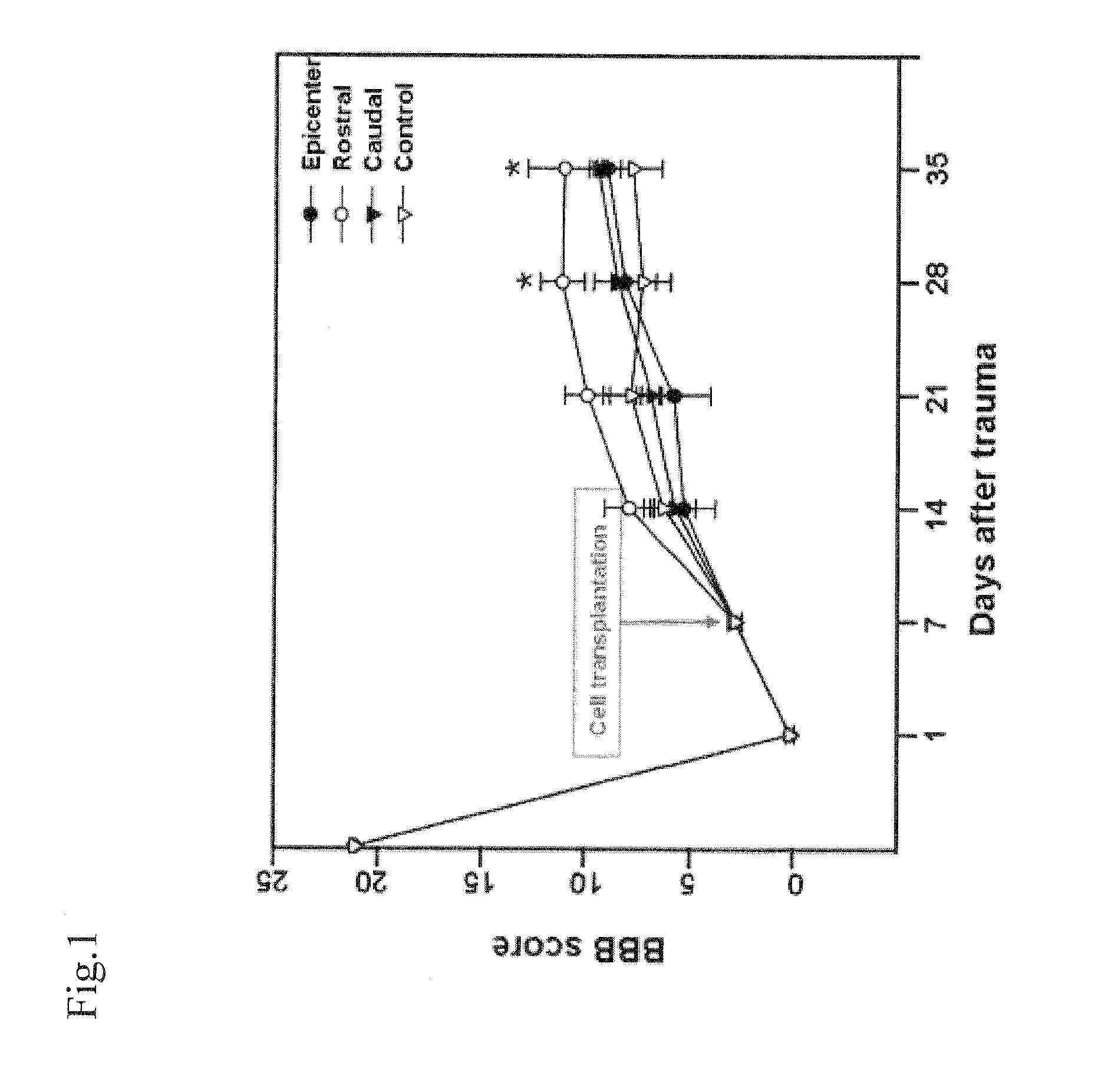
Provided is a composition for treating nerve damage-related diseases. The composition includes a human umbilical cord blood-derived mesenchymal stem cell as an active ingredient. The mesenchymal stem cell isolated and incubated from the human umbilical cord blood migrates to an injured area to be differentiated into a nerve cell or a neuroglial cell at the time of in vivo transplantation. Thus, the mesenchymal stem cell and a composition including the same can be effectively used in cell replacement therapy and gene therapy for treating diseases caused by nerve damage including a stroke, Parkinson's disease, Alzheimer's disease, Pick's disease, Huntington's disease, amyotrophic lateral sclerosis, traumatic central nervous system disease and a spinal cord injury.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOP FOUND
Medical kit and using method thereof
InactiveUS20100034783A1Easy to useReduce decreaseBiocidePower operated devicesBlood collectionOsteoblast
An aseptic / sterile medical kits are comprising a cartilage regeneration kit, a bone regeneration kit or an umbilical cord blood storage kit in a configuration that each process performs according to functionally-specialized kit sets for each step, via division of overall processes into corresponding steps for isolation, culture, collection and storage of cells, and implantation of desired cells into target sites of the body. The cartilage is regenerated by cartilage tissue collection; chondrocyte isolation; chondrocyte medium change and subculture; preparation of chondrocyte therapy product; media for isolation / culture / preparation / cryopreservation of cells; and media for isolation / culture / cryopreservation of cells, using the cartilage regeneration kit. The bone is regenerated by bone marrow collection; osteoblast isolation; osteoblast medium change and subculture; and preparation of osteoblast therapy product, using the bone regeneration kit. Additionally, the umbilical cord blood is stored by umbilical cord blood collection; hematopoietic stem cell isolation; and cryopreservation of hematopoietic stem cells, using the umbilical cord blood storage kit.
Owner:SEWON CELLONTECH CO LTD
Method for in vitro mass amplification of human mature high-activity dendritic cells and application thereof
ActiveCN108546679AHigh activityImprove proliferative abilityVirus peptidesAntiviralsGene ModificationDendritic cell proliferation
Owner:CELARTICS BIOPHARMA CO LTD
Method for preparing PRP (platelet-rich plasma) of umbilical cord blood
A method for preparing PRP (platelet-rich plasma) of umbilical cord blood includes steps: subjecting fresh umbilical cord blood to low-speed centrifuging, removing a lower cell layer, and subjecting upper platelet containing plasma to ultrahigh-speed centrifuging until the liquid is separated into two layers including an upper plasma layer and a lower layer of precipitates which are platelets; removing the upper plasma layer, leaving a small quantity of plasma in an original centrifugal tube, well mixing, vibrating, counting the platelets, and well mixing according to a proportion that 1ml of plasma contains 1.2+ / -0.125 billion of platelets approximately. The platelet-rich plasma is effective in treatment of cartilage injuries, pain alleviation and function restoration and especially remarkable in treatment of senile osteoarthritis.
Owner:孔五一
Preparation method for dendritic cell of umbilical cord blood source and dendritic cell vaccine
ActiveCN102676455ABlood/immune system cellsAntibody medical ingredientsHematopoietic cellCell culture media
The invention discloses a preparation method for the dendritic cell (DC) of an umbilical cord blood source and a dendritic cell (DC) vaccine, which relates to a preparation method for the dendritic cell. According to the method, various cell factors are adopted to induce DC obtained by umbilical cord blood separation, and then the DC is stimulated by a tumor specific antigen so as to improve the specific antigen presentation capability of the DC; and a stem cell growth factor and Flt3-L are added into a cell culture medium so as to effectively accelerate a hematopoietic cell in the umbilical cord blood to induce and proliferate to an immune cell. The DC vaccine prepared with the method has the specific antigen presentation capability, can be combined with a CIK (cytokine induced killer) cell to mutually treat the malignant tumor when being used as a tumor immunotherapy product, and is used as an important adjuvant therapy after operations and chemoradiotherapy. Recurrence and metastasis after the operations can be effectively prevented, and toxic and side effects caused by the chemoradiotherapy on patients are lowered so as to improve the treatment effect.
Owner:北京和泽普瑞生物科技有限公司 +1
Freezing storage protective solution for human umbilical Wharton's jelly tissue block
InactiveCN101971799AGuaranteed osmotic balanceLow toxicityDead animal preservationSucroseCell membrane
The invention discloses freezing storage protective solution for a human umbilical Wharton's jelly tissue block. 1 liter of the freezing storage protective solution comprises 0.8 to 1.2 mol of glycol, 0.4 to 0.6 mol of dimethyl sulfoxide, 0.1 to 0.3 mol of cane sugar, 100 to 200 milliliters of cord blood autoserum and the balance of culture solution DMEM / F12. In the freezing storage protective solution, the cord blood autoserum and the culture solution DMEM / F12 serve as base solution to provide nutrient for the tissue block, so osmotic equilibrium inside and outside cells is guaranteed, the cell environment in the tissue block is guaranteed to be similar to the in vivo environment, and the freezing storage effect is good; the low-concentration glycol and dimethyl sulfoxide serve as a permeability freezing protective agent, so the toxicity of the freezing storage protective solution to the cells is reduced; and the cane sugar serves as a non-permeability freezing protective agent, so the viscosity of the solution is increased and the osmotic pressure inside and outside cell membranes is relieved when the cells are frozen and stored. The human umbilical Wharton's jelly tissue block can be directly frozen and stored by the freezing storage protective solution, so the freezing storage effect is good, the cell damage is little, and high-quality human umbilical mesenchymal stem cells can be provided for clinic.
Owner:江苏省北科生物科技有限公司
Lumbar booster therapy belt
InactiveCN101268982AAchieve therapeuticAchieve the purpose of preventionElectrotherapyChiropractic devicesAir compressorAirbag
The invention relates to a medical device, in particular to a lumbar booster physiotherapy cord which can cure and ease diseases such as human sciatic nerve, lumbar muscle strain, and lumbar disc protrusion, etc. The cord body is bonded with the four sides of an airbag cavity in the thermal sealing way. Lumbar acupoint magnetic blocks are vertically arranged in the two sides of the central lumbar of the airbag pasted on the body. A traditional Chinese drug bag is crossly arranged on the two sides of the central lumbar of the airbag pasted on the body. The output pipe of an airbag switch is connected with the cord body. The input end of the airbag switch is connected with a gas space through a rubber hose. The gas space is provided with an adjustable pressure maintaining valve and a release valve and a micro-air compressor which are communicated with the gas space. The lumbar booster physiotherapy cord is provided with the airbag cavity which can play the roles of support and massage. The medication point and the magnetic therapy point provided by the lumbar booster physiotherapy cord can play the function of nourishing the kidney and activating the blood flow in order to cure a plurality of lumbar diseases quickly. The lumbar booster physiotherapy cord is suitable for the cure and the ease of the human lumbar diseases.
Owner:刘恒志
Ex-vivo expansion culture medium of umbilical cord blood hematopoietic stem cells and application thereof
ActiveCN105112374AMaintain self-renewal abilityHigh proliferation rateBlood/immune system cellsReceptorUmbilical cord
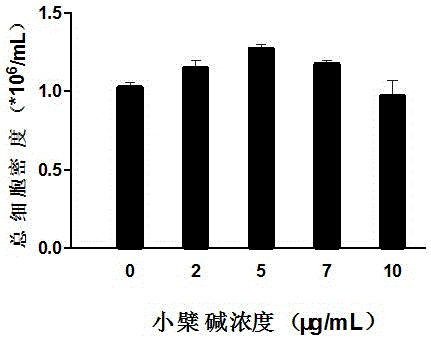
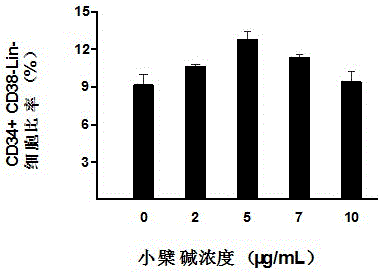
The invention belongs to the technical field of stem cells, and relates to an ex-vivo expansion culture medium of umbilical cord blood hematopoietic stem cells and an application thereof. The ex-vivo expansion culture medium comprises an IMEM basal culture medium, serum, thrombopoietin, stem cell factors, FMS-liketyrosinc kinase 3 ligand, interleukin-1, interleukin-6, stem cell growth factors and berberine. The ex-vivo expansion culture medium of the umbilical cord blood hematopoietic stem cells has the advantages of being high in hematopoietic stem cell proliferation rate, capable of significantly improving the implantation capacity of the hematopoietic stem cells transplanted into a receptor and the reconstruction capacity of a hematopoietic system and capable of well maintaining the properties of the hematopoietic stem cells and is an ideal ex-vivo expansion culture medium of the umbilical cord blood hematopoietic stem cells.
Owner:广东美赛尔细胞生物科技有限公司
Rejuvenation of stored blood
D-Ribose, a buffer and an anticoagulant are added to whole blood or packed red cells to extend function in storage beyond 42 days. Methods are disclosed to rejuvenate suboptimally functional red cells. The methods are comprised of incubation of the cells at 37° C. for 10 to 60 minutes in the presence of D-ribose.
Owner:VIACELL
Exosome preparation prepared from umbilical cord mesenchymal stem cells, and preparation method of exosome preparation
PendingCN111647554AHigh purityNo reduction in in vitro biological activityPowder deliveryPancreatic cellsEngineeringUmbilical cord
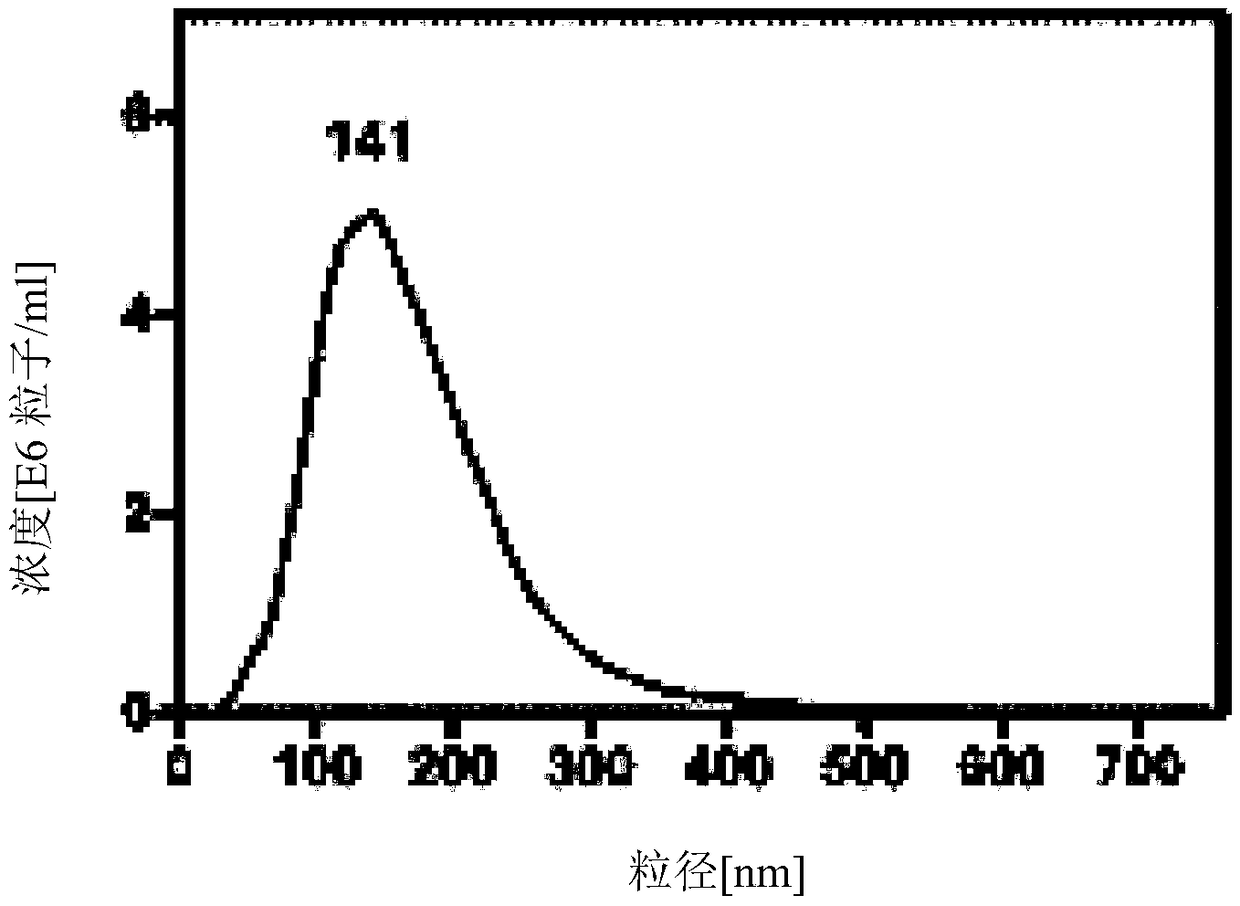
The invention relates to an exosome preparation prepared from umbilical cord blood bone marrow mesenchymal stem cells, and a preparation method of the exosome preparation. The exosome is obtained froma cell culture containing at least 10<4> cells. When the cell culture reaches fusion, washing is performed in PBS, a KO-DMEM culture medium is added and the cell culture is put back into an incubatorcontaining carbon dioxide for culture, the cell culture is cultured for 2-20 days, and the exosome is prepared through the ultracentrifugation technology, size exclusion filtration, chemical precipitation, discontinuous density gradient, immunoaffinity, ultrafiltration and / or high performance liquid chromatography, and preferably through PEG precipitation. The exosome prepared by the method is stable, high in purity, and comprehensive in positive markers, and can be used for activating proliferation and regeneration, promoting wound healing, and other medical clinical uses.
Owner:广州达康基因技术有限公司
Method for preparing tumor-specific DC vaccine by applying CD34+ cells of umbilical cord blood
ActiveCN103405759ABreak immune toleranceBlood/immune system cellsAntibody medical ingredientsAntigenDc vaccine
The invention discloses a method for preparing a tumor-specific DC vaccine by applying CD34+ cells in umbilical cord blood. The method comprises (1) a step of preparing autologous tumor-related holoantigen; (2) a step of obtaining the umbilical cord blood; (3) a step of obtaining mononuclear cells derived from the umbilical cord blood; (4) a step of purifying CD34+ cells in the mononuclear cells derived from the umbilical cord blood; (5) performing induction culture for a precursor DC; (6) a step of performing amplification and culture of an immature DC; and (7) a step of preparing the DC vaccine.
Owner:玥特农生物科技河北有限责任公司
Method for cryopreservation of DC cells and CIK seed cells in blood, prepared cells and application
InactiveCN105831106AImprove recycling efficiencyMeet needsCulture processDead animal preservationCryopreservationBlood sampling
The invention discloses a method for freezing DC cells and CIK seed cells in blood, and the prepared cells and their application. The mononuclear cells in peripheral blood or umbilical cord blood are frozen in autologous serum after appropriate treatment, and the cell The cryoprotectant makes the survival rate of the cells reach 80%. The resuscitated cells can be used to prepare DC-cells and CIK cells at the same time, and finally prepare DC-CIK cell preparations that can meet the needs of clinical applications. In this way, the requirement of multiple DC-CIK clinical treatments can be realized in a single blood collection, thus providing support for the large-scale development of immune cell therapy.
Owner:TIANJIN PURUI SAIER BIOLOGICAL TECH CO LTD
Kit for treating human bone marrow, umbilical cord blood, and peripheral blood cells, and cell treatment method
InactiveCN102965339AAvoid interferenceAvoid the risk of rejectionBlood/immune system cellsArtificially induced pluripotent cellsHuman bodySeparation technology
The invention discloses a kit for treating human bone marrow, umbilical cord blood, and peripheral blood cells, and provides a kit for treating human bone marrow, umbilical cord blood, and peripheral blood cells and a cell treatment method which are strong in operationality, high in clinical safety, and convenient for clinical popularization. The kit comprises four reagents: No. A liquid is a diluent; No. B liquid is density fluid; No. C liquid is washing liquid; No. D liquid is erythrocyte-removing liquid. The invention fundamentally solves the problems of high cost, low cell activity, undefined human body influence for markers entering human body, pain for patients due to mobilization agent injection, cumbersome and inapplicable operations, and the like for current cell separation technology.
Owner:WUHAN HAMILTON BIOTECH
Use of umbilical cord blood derived exosomes for tissue repair
The subject invention provides a method for promoting wound healing in a patient in need thereof comprising contacting a wound of the patient with exosomes secreted by umbilical cord blood mononuclearcells (UCBMNCs) so as to thereby promote wound healing in the patient. The subject application also provides a method for promoting wound healing in a patient in need thereof comprising contacting awound of the patient with a composition comprising one or more miRNA and a pharmaceutically acceptable carrier so as to thereby promote wound healing in the patient.
Owner:ステムラボエスエイ +1
Preparation method for DC-CIK cells originated from umbilical cord blood mononuclear cells and preparation
InactiveCN105112371AImprove production efficiencyShorten the timeMammal material medical ingredientsBlood/immune system cellsCulture cellUmbilical cord
The invention provides a DC-CIK co-culture cell preparation method. According to the invention, mononuclear cells separated from umbilical cord blood are separately differentiated into DC cells and CIK cells, and then the DC cells and the CIK cells are co-cultured; and mature DC cells are obtained through two-stage differentiation of the mononuclear cells separated from umbilical cord blood in virtue of a plurality of factors. The DC-CIK co-culture cell preparation method provided by the invention shortens time needed for obtainment of the mature DC cells by at least two folds and improves DC-CIK cell co-culture efficiency.
Owner:中国干细胞集团上海生物科技有限公司 +10
Natural killer cell differentiated from human pluripotent stem cells as well as preparation method and application thereof
PendingCN112608895AEfficient and stable differentiationExtended processing timeCulture processMammal material medical ingredientsPluripotential stem cellNatural Killer Cell Inhibitory Receptors
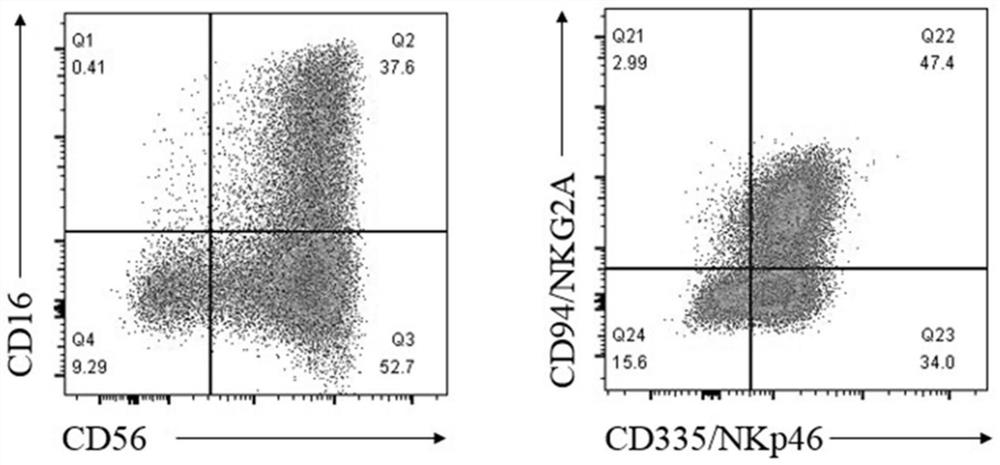
The invention relates to the field of stem cell biology, and discloses a preparation method of natural killer cells differentiated from human pluripotent stem cells. The preparation method comprises the steps of S1, forming an embryoid body; S2, performing directional permanent hematopoiesis differentiation on the embryoid body to obtain hematopoietic precursor cells; and S3, differentiating the hematopoietic precursor cells to obtain natural killer cells. The invention also discloses application of the natural killer cell prepared by the method as a pharmaceutical composition for preventing and / or treating tumors by cells. According to the method, efficient and stable differentiation of the NK cells is achieved, EB adherent differentiation is achieved in the induced differentiation period, TGFB inhibitor treatment is prolonged, 10% or above of CD34+CD45+permanent hematopoietic HPCs can be obtained through rapid induction. The method has the advantages of being efficient, stable, low in cost and suitable for large-scale cell preparation production; and the method is easy and convenient to operate, the NK cells obtained through differentiation have typical NK cell surface marking molecules, the anti-tumor function of the NK cells is superior to that of the NK cells derived from umbilical cord blood, and great scientific research and clinical application potentials are achieved.
Owner:深圳市济因生物科技有限公司
Multipotent adult stem cells having an ability of oct4 expression derived from umbilical cord blood and method for preparing the same
InactiveUS20090305413A1Negative immunological responseBlood/immune system cellsCell culture active agentsGerm layerDisease
The present invention relates to multipotent adult stem cells expressing Oct4, derived from umbilical cord blood (UCB) and also these cell are expressing CD29, CD31, CD44, simultaneously, a method for preparing the same, and more specifically to multipotent adult stem cells which are obtained by culturing umbilical cord blood-derived monocytes in a medium containing bFGF (basic fibroblast growth factor) and human serum or plasma. In addition, multipotent adult stem cells expressing Oct-4 from UCB are morphologically spindle or round shaped cells Although the stem cells according to the present invention are adult stem cells, they are multipotent and capable of differentiating into ectodermal-, messodermal-, endodermal-originated tissue or cells including osteogenic cells or nerve cells etc, thus they can be effectively used in the treatment of intractable diseases and incurable diseases.
Owner:SEOUL NAT UNIV R&DB FOUND
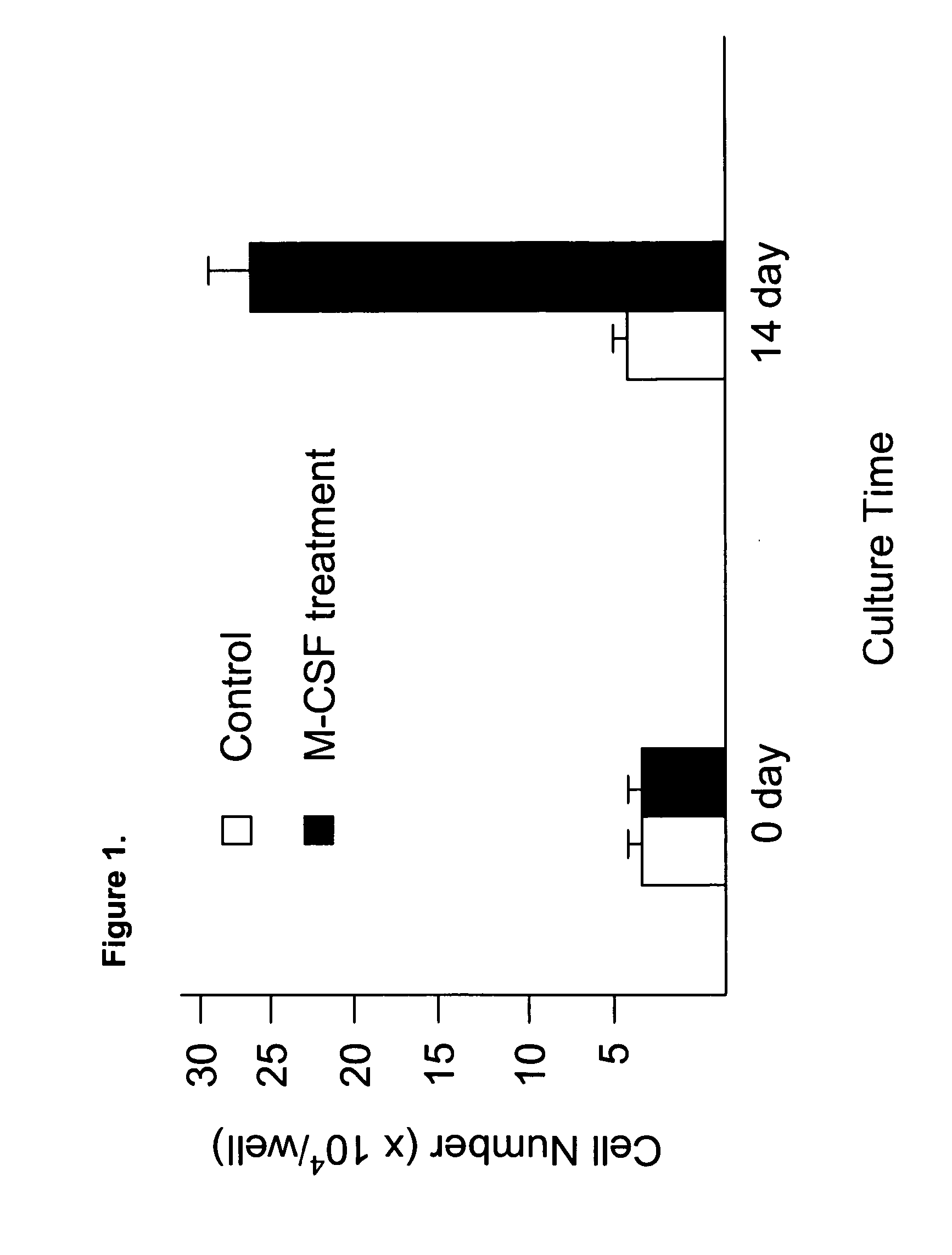
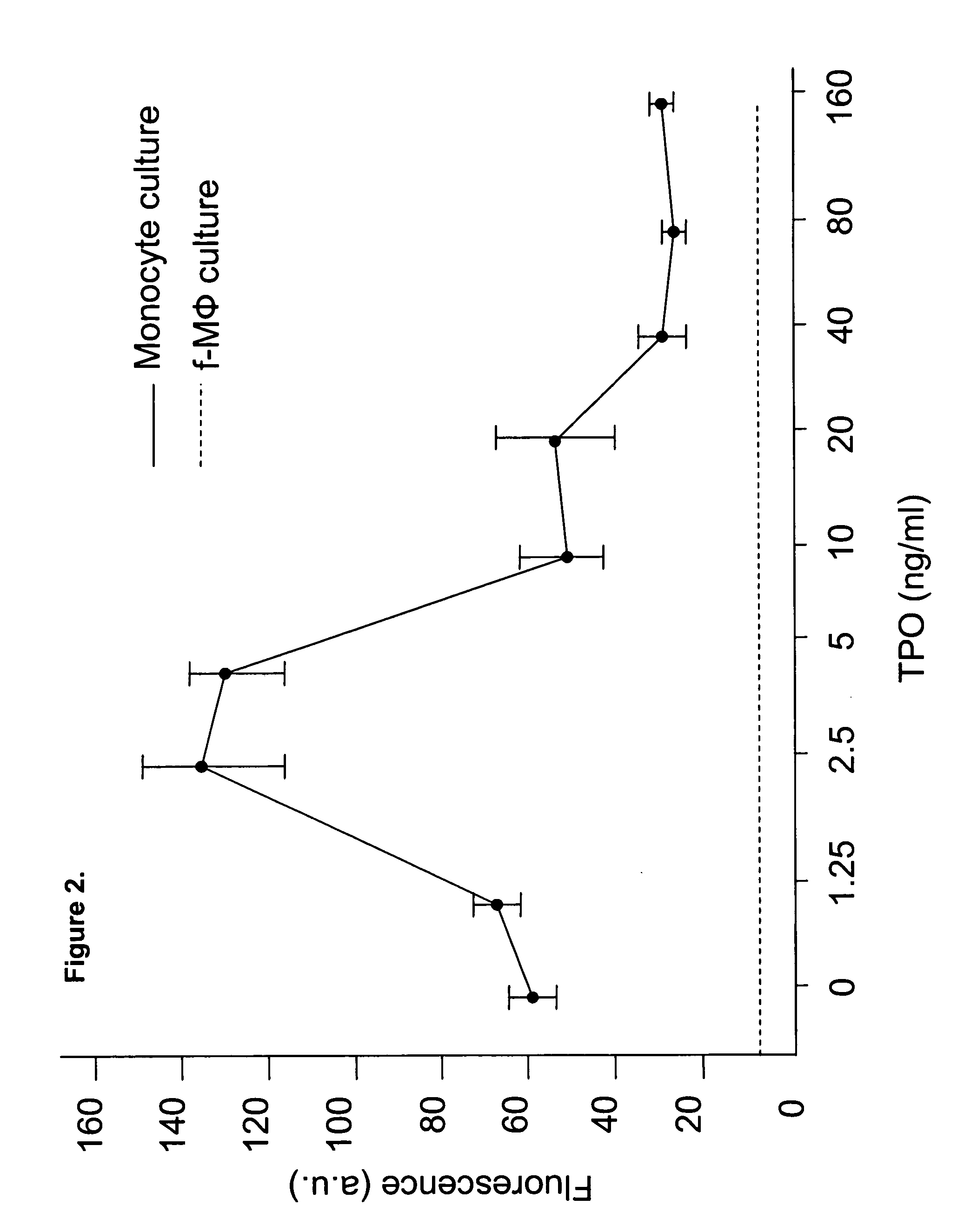
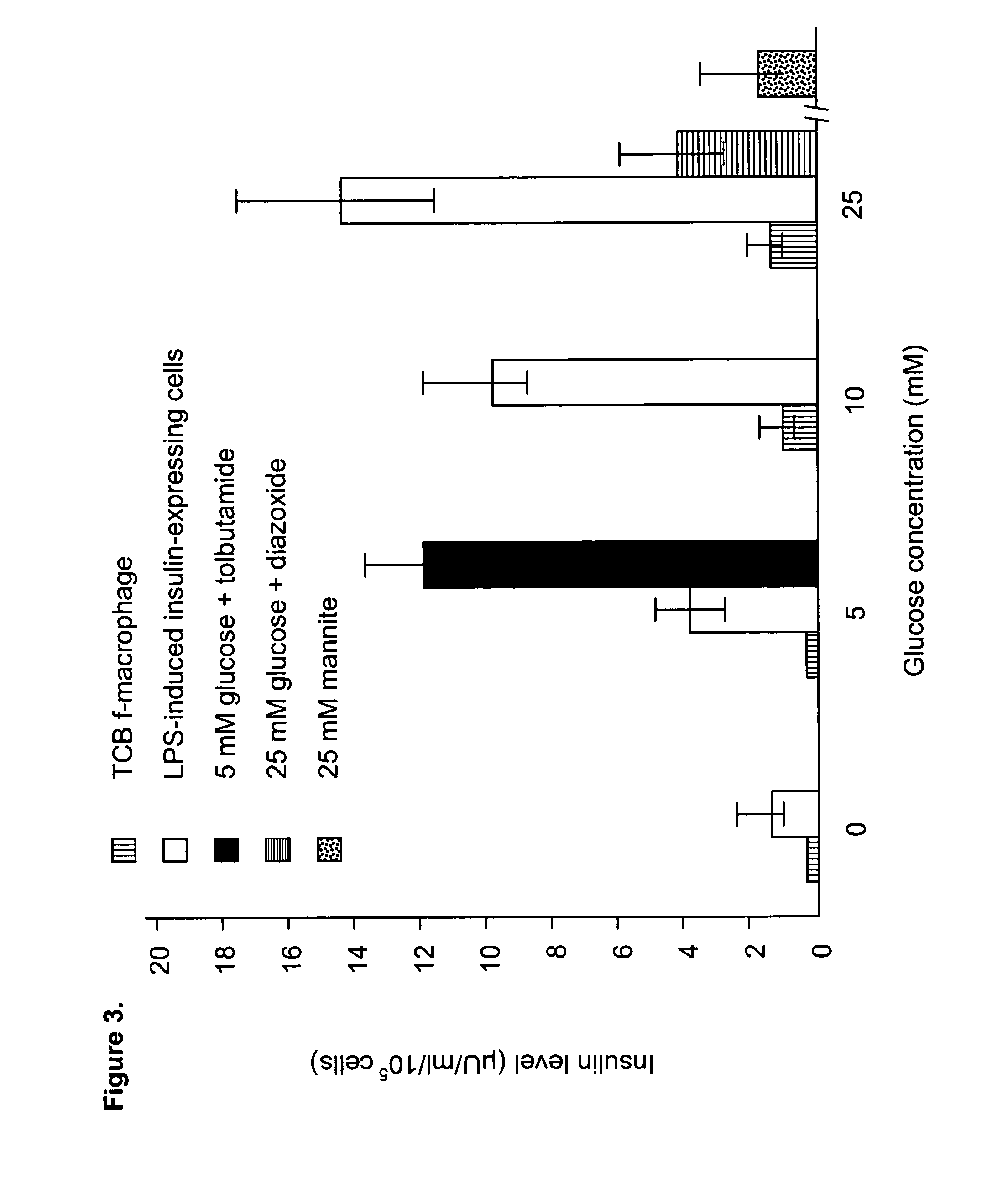
Human umbilical cord blood-derived pluripotent fibroblast-like-macrophages
InactiveUS20070059824A1Great likelihoodExpand numberPancreatic cellsCulture processPopulationPeripheral
The present invention relates to a purified population of fibroblast-like macrophage (f-macrophage, f-MΦ) and methods using the same. The f-MΦ can be expanded in vitro and differentiated into several lineages, including insulin-expressing cells, endothelial cells, and neuronal cells. The f-MΦ described herein have been generated from human umbilical cord blood (CB f-MΦ) and have characteristics similar to f-MΦ derived from peripheral blood. Thrombopoietin (TPO), at low dosage, significantly stimulates the proliferation of CB f-MΦ, wherein the TPO-expanded CB f-MΦ retain their pluripotent differentiation potential.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Method for Expanding Postembryonic Stem and Progenitor Cells from Umbilical Cord Blood and Immunotherapeutic Agent
Method for obtaining and expanding postembryonic hematopoietic stem cells from umbilical cord blood while avoiding unwanted differentiation. Initial cells from umbilical cord blood are proliferated and multiplied ex vivo in a stroma-free medium and in the presence of a regio-modified glycan or glycosaminoglycan. The regio-modified glycan or glycosaminoglycan, e.g. a heparin derivative, is N-desulfated, and N-reacetylated or N-reacylated, in essence, on C2 atoms. The heparin derivative advantageously comprises less than 5 percent of C3-O-sulfate, at least 60 percent C2-O-sulfate, and it is preferably added in a quantity of 15 to 50 mg / L to the medium in order to stop an unwanted differentiation. The stem cells generated in this manner can differentiate, after expansion, into myeloma cells and lymphatic cells, and they can be used as an immunotherapeutic agent against many diseases.
Owner:GLYCOSTEM
Preparation method for monkshood polysaccharide-induced nature killer T (NKT) cell proliferation and application thereof
The invention provides a culture method for monkshood polysaccharide-induced nature killer T cell (Nature Killer T cell, NKT) proliferation. The method comprises the characteristics as follows: human peripheral blood, marrow or a mononuclear cell with an umbilical cord blood source are continuously cultivated by traditional Chinese medicine monkshood polysaccharide and recombinant human interleukin 2, a lot of nature kill T cells are obtained, NK<1.1+> and CD<8+> are expressed, interferon gamma is secreted, the ratio in lymphocyte at the 14th day is more than 16.15%, the order of magnitude can be up to over 5*10<9>, and the nature kill T cell have effective anti-tumor and anti-virus functions. A clinical application of the NKT cell obtained by the method is also provided by the invention.
Owner:李金珍
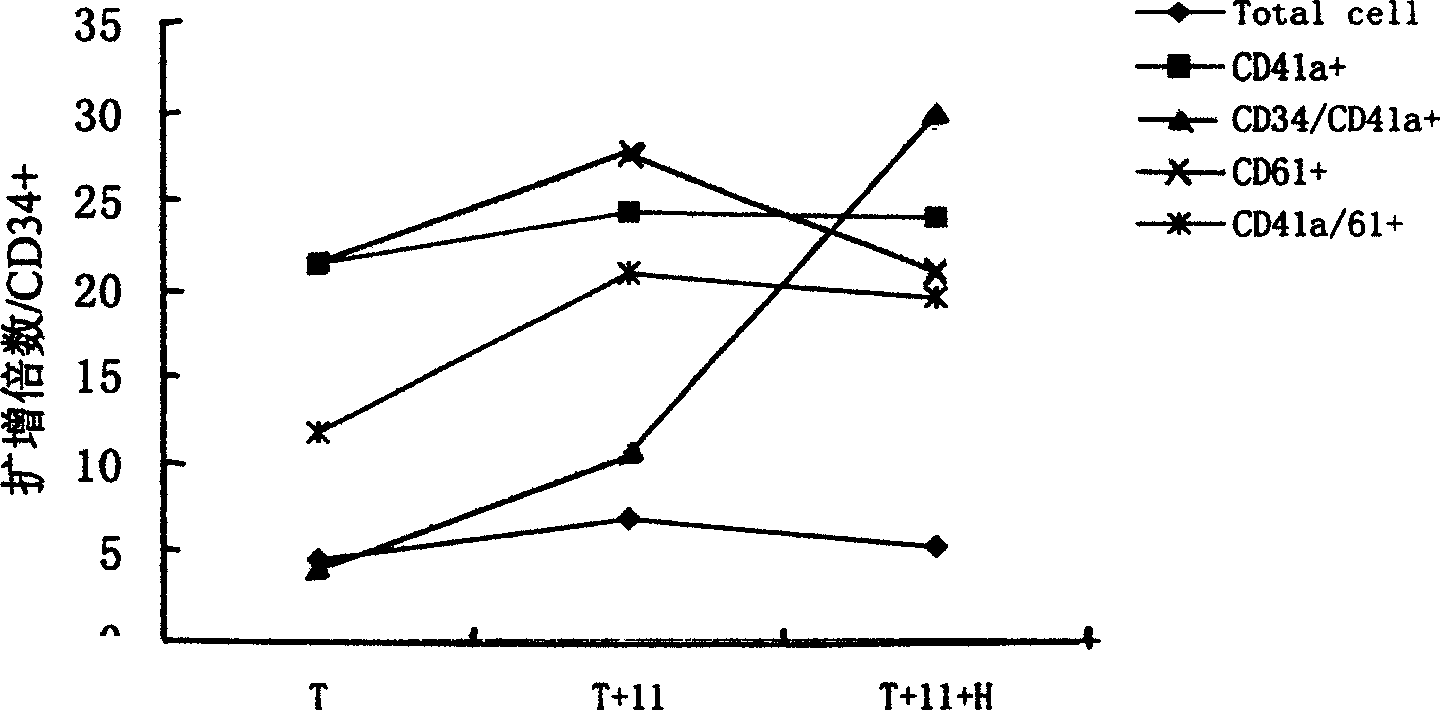
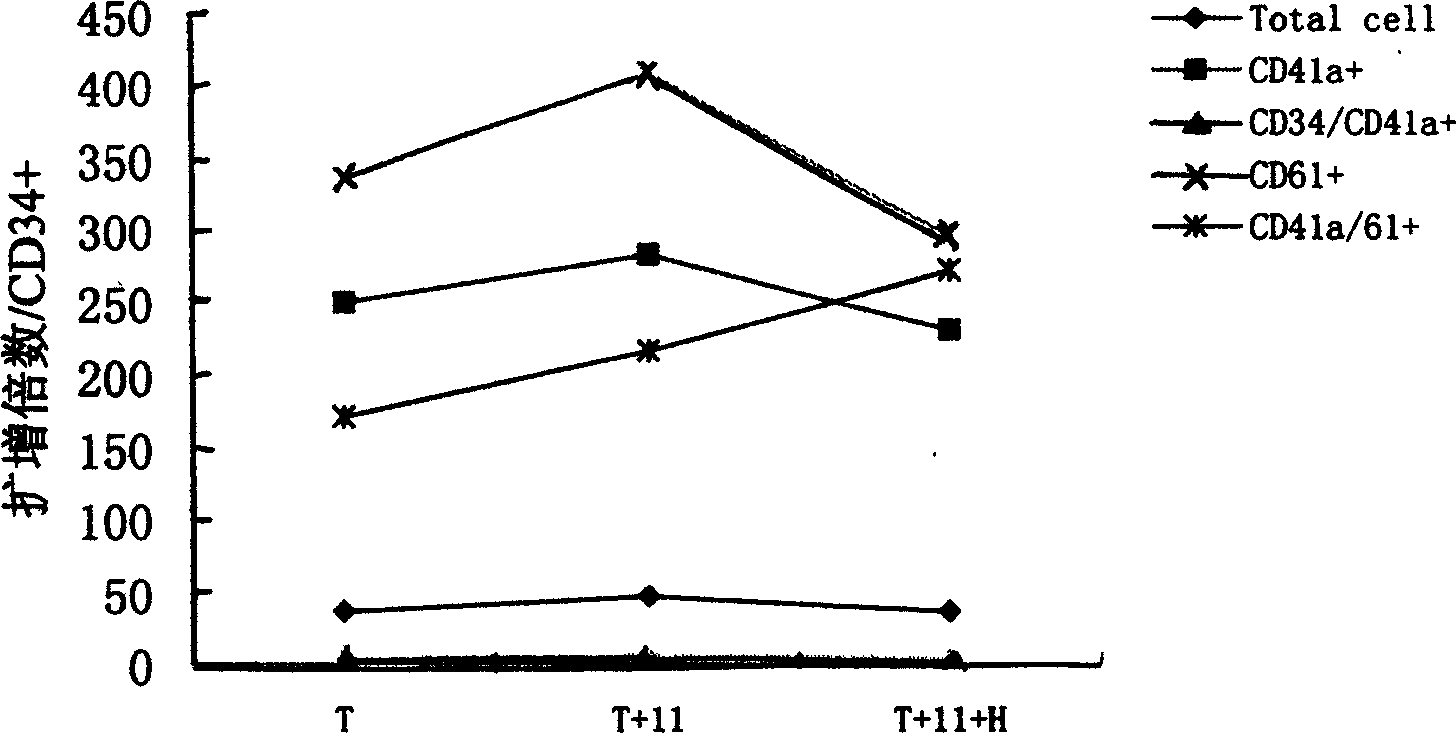
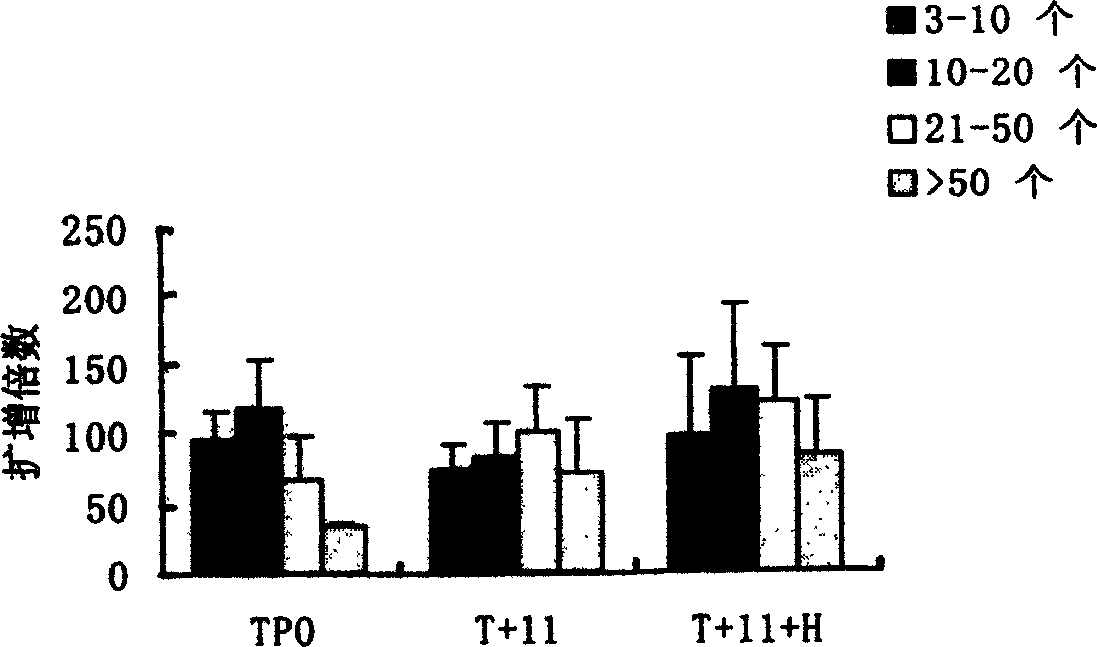
Method for preparing megakaryocytic preparation by amplifying macronucleus ancestral cell and mature megacaryocyte and use
The present invention relates to mainly one system of efficient culturing and directive inducing stem cell differentiation for culturing and proliferating megakaryocyte. The system has umbilical cord blood and marrow stem cell as seed cell, and the serum-free culture medium comprising TPO, IL-11 and heparin as the main component for megakaryocyte proliferation. The megakaryocyte preparation prepared based on the present invention contains great amount of macronucleus ancestral cells and mature megakaryocyte as well as CD34+ cells, and has the functions of treating thrombocytopenia and improving the blood-forming function after stem cell transplantation. The present invention provides novel efficient cell treating preparation for various thrombocytopenia and hemocyitopenia.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com