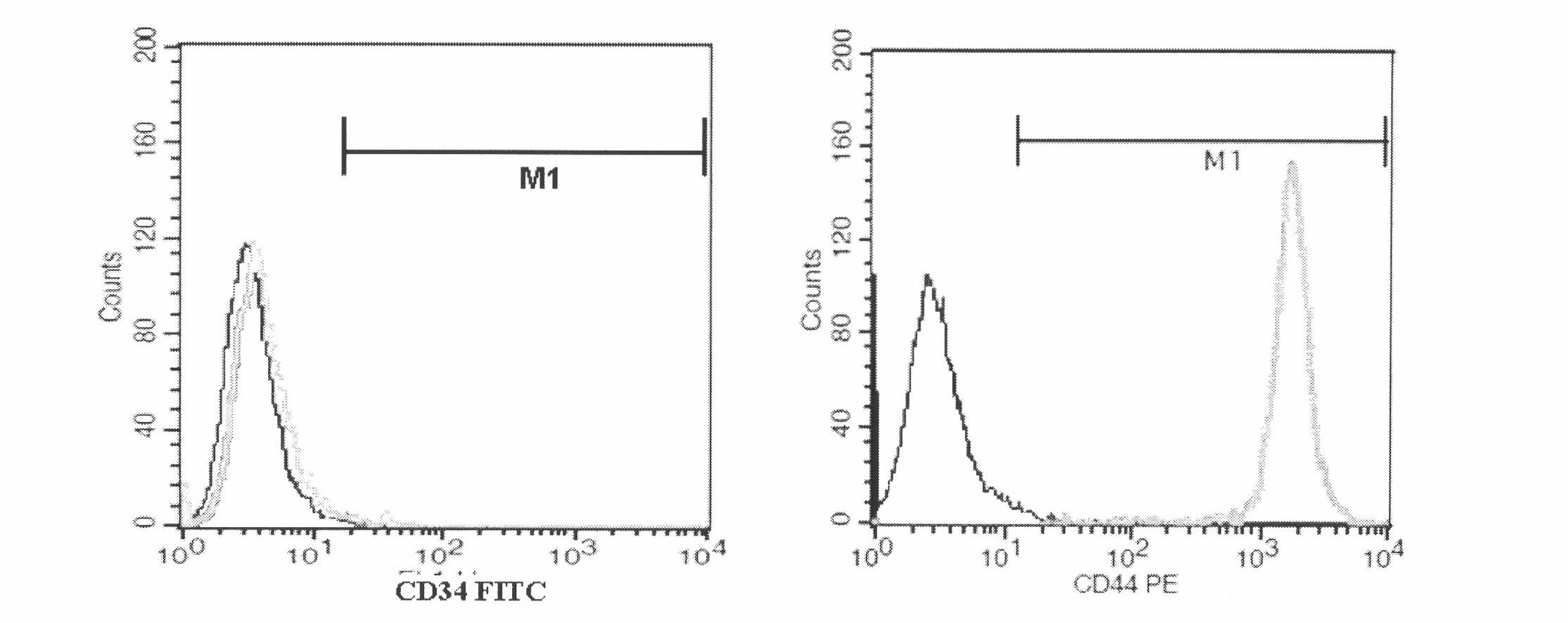
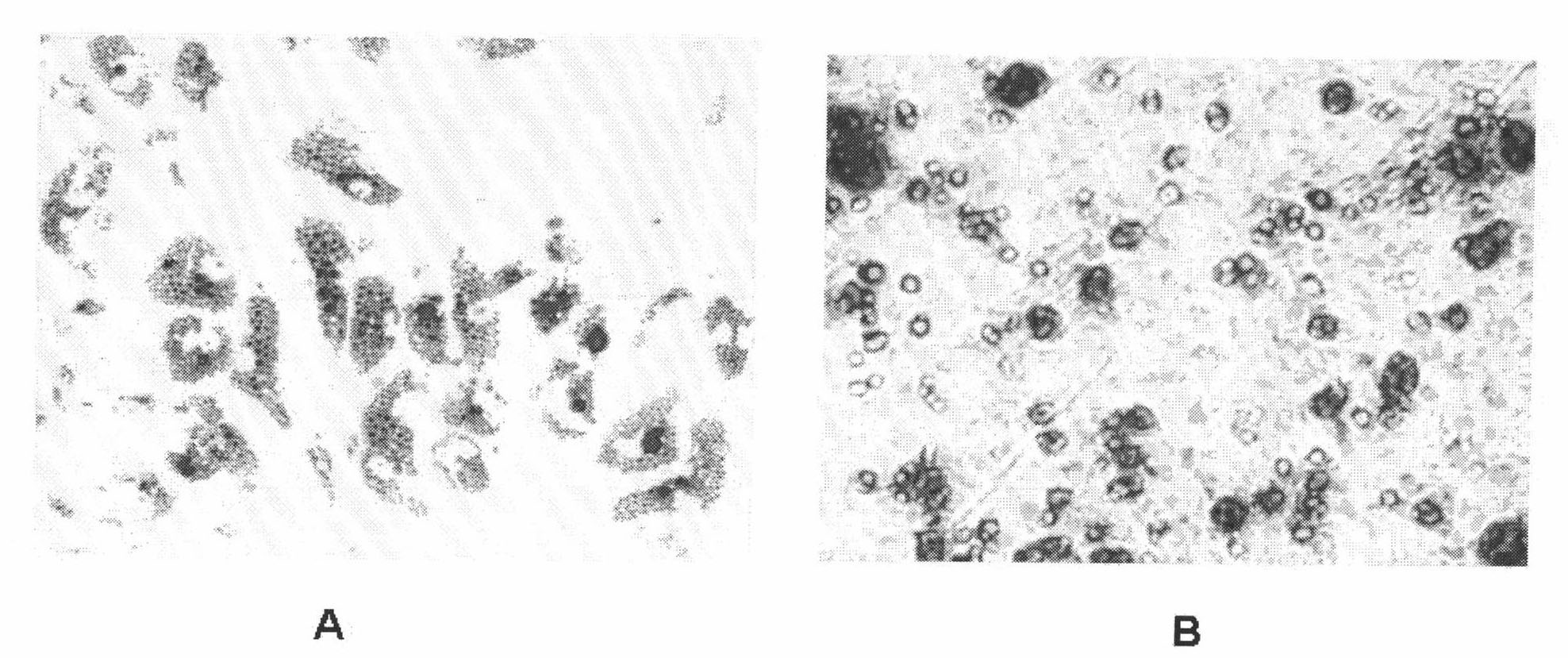
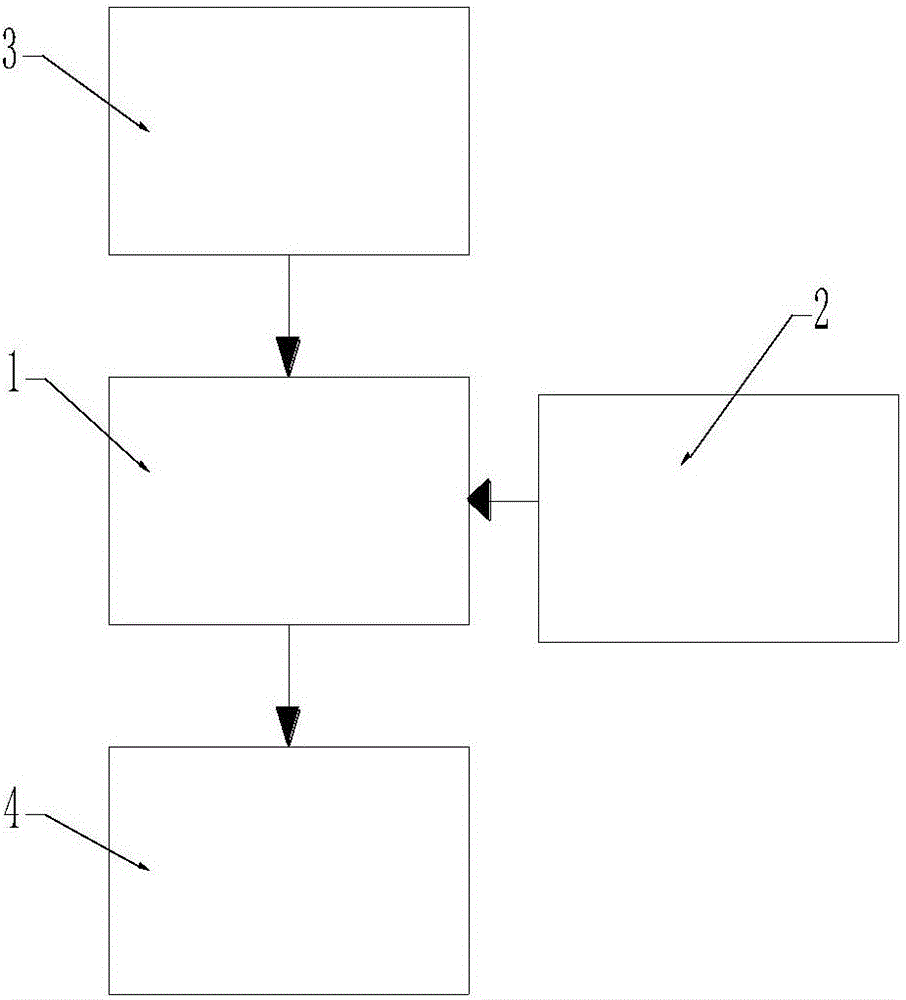
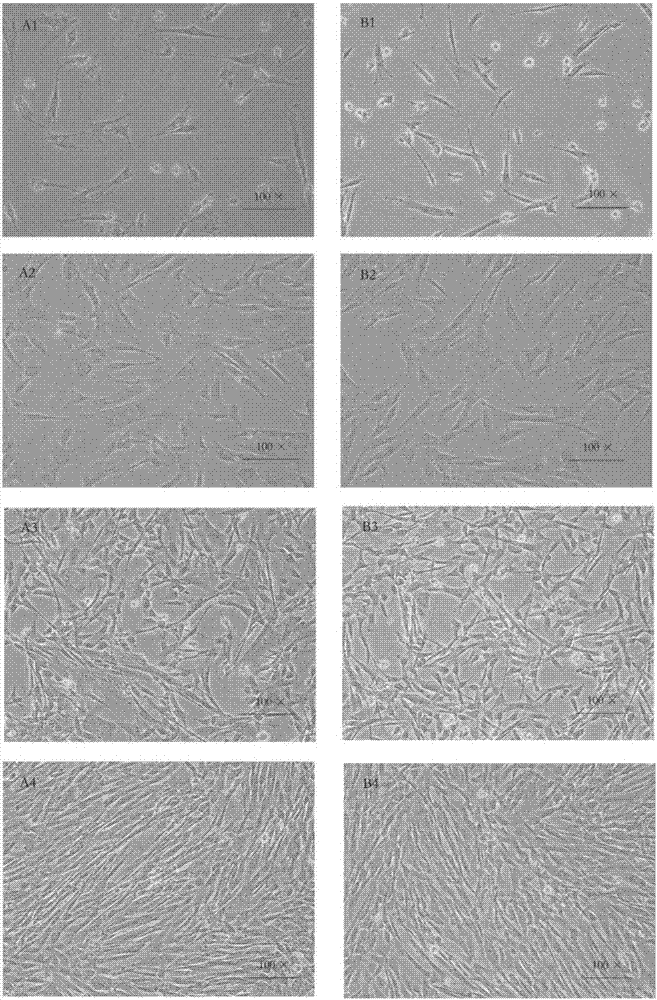
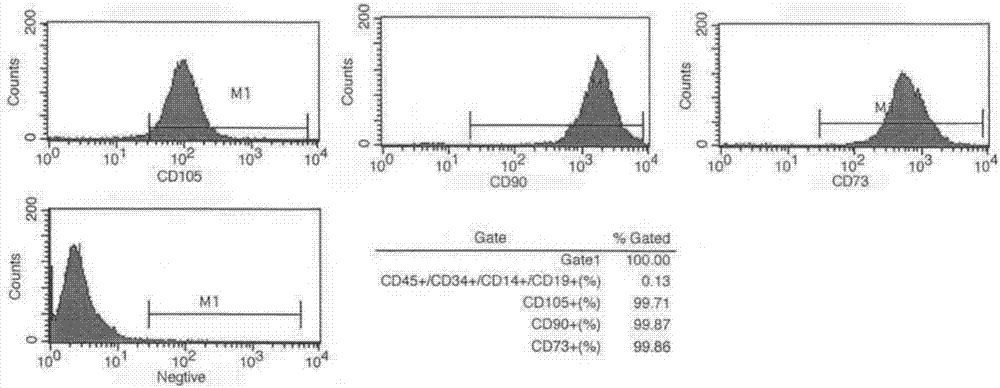
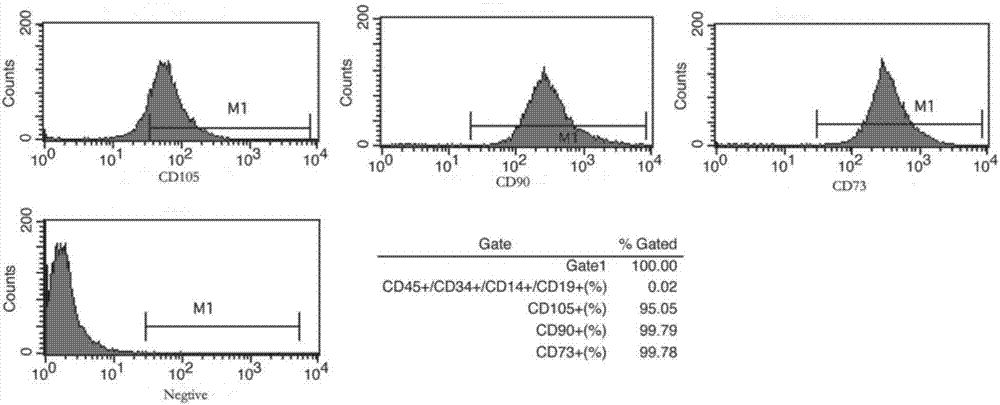
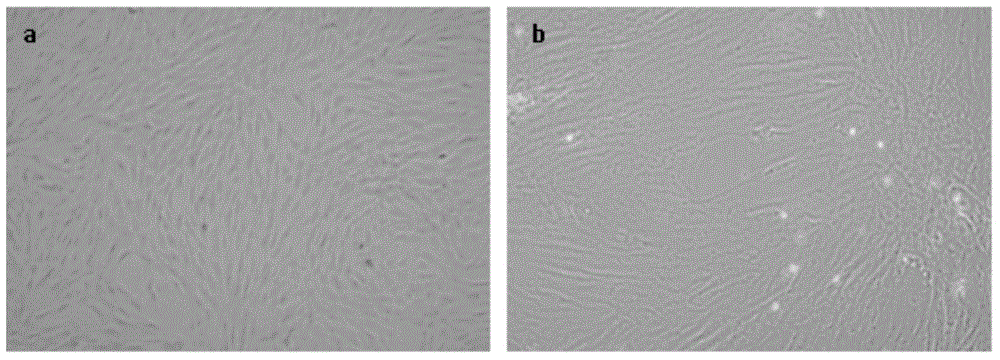
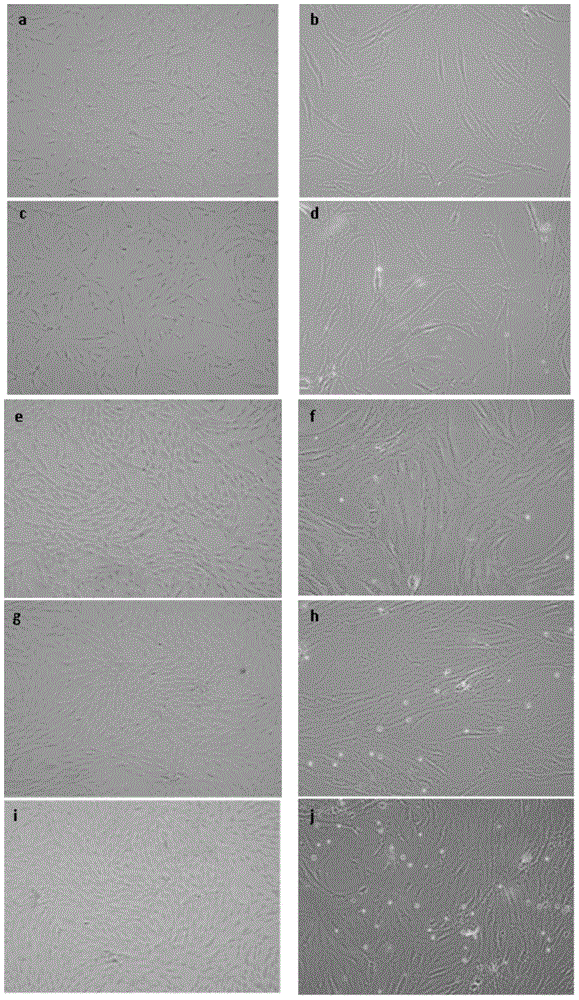
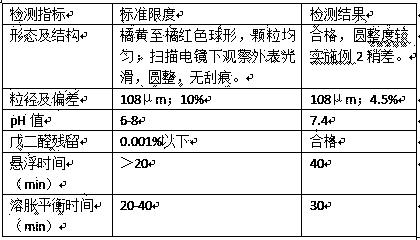
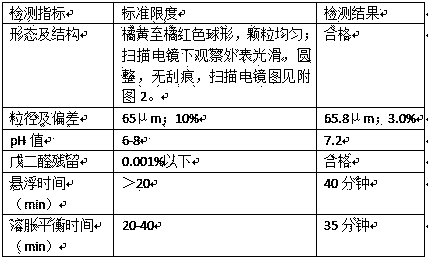
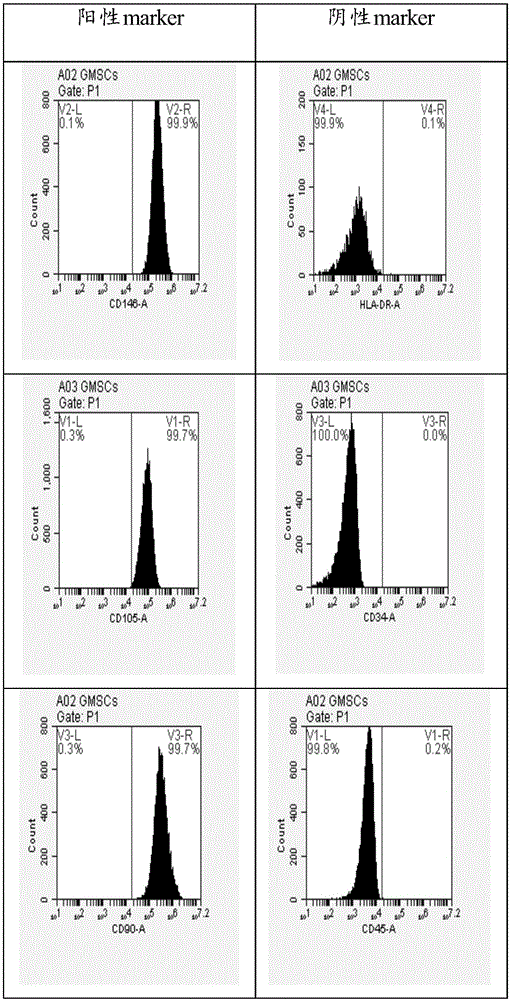
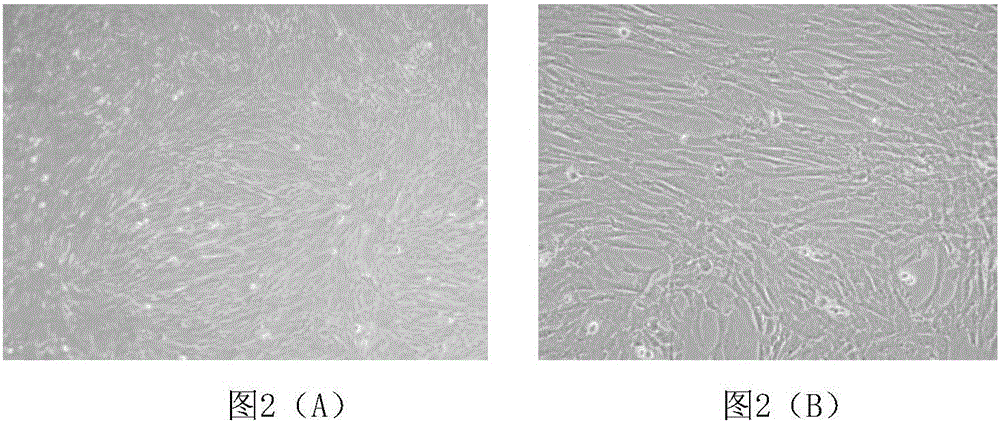
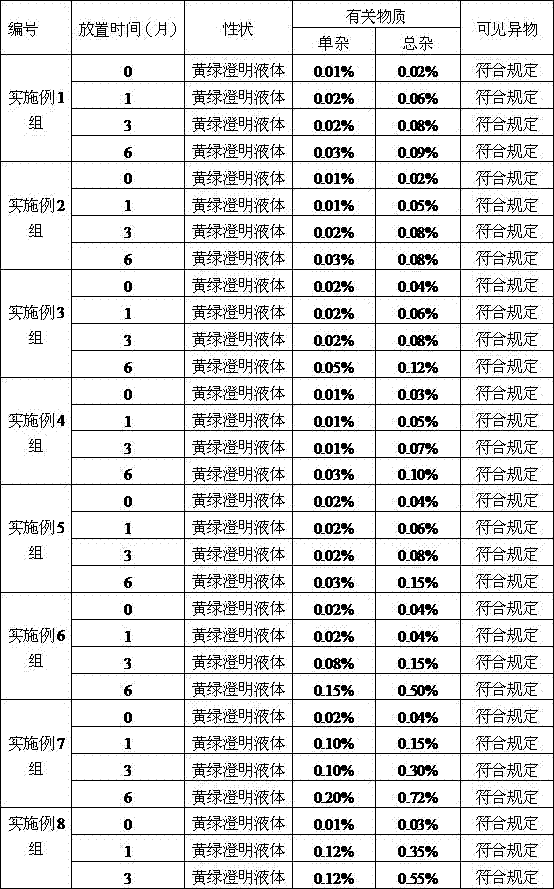
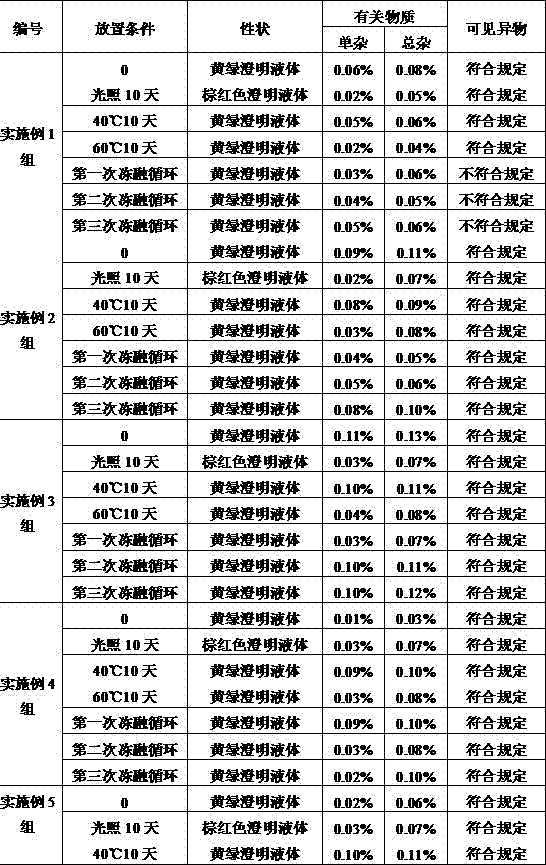
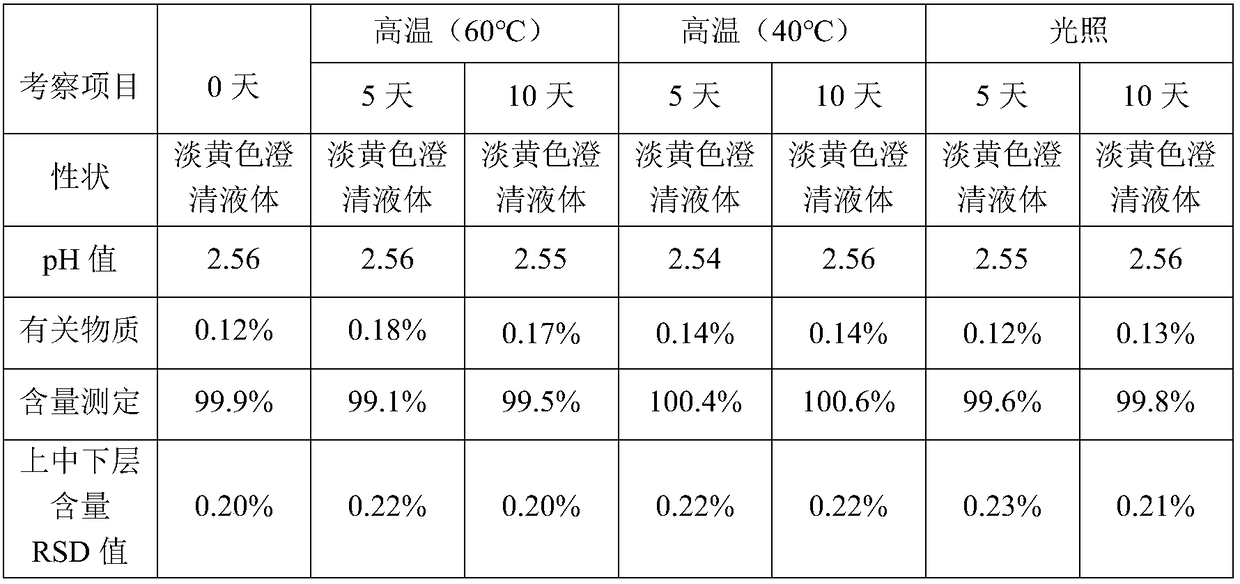
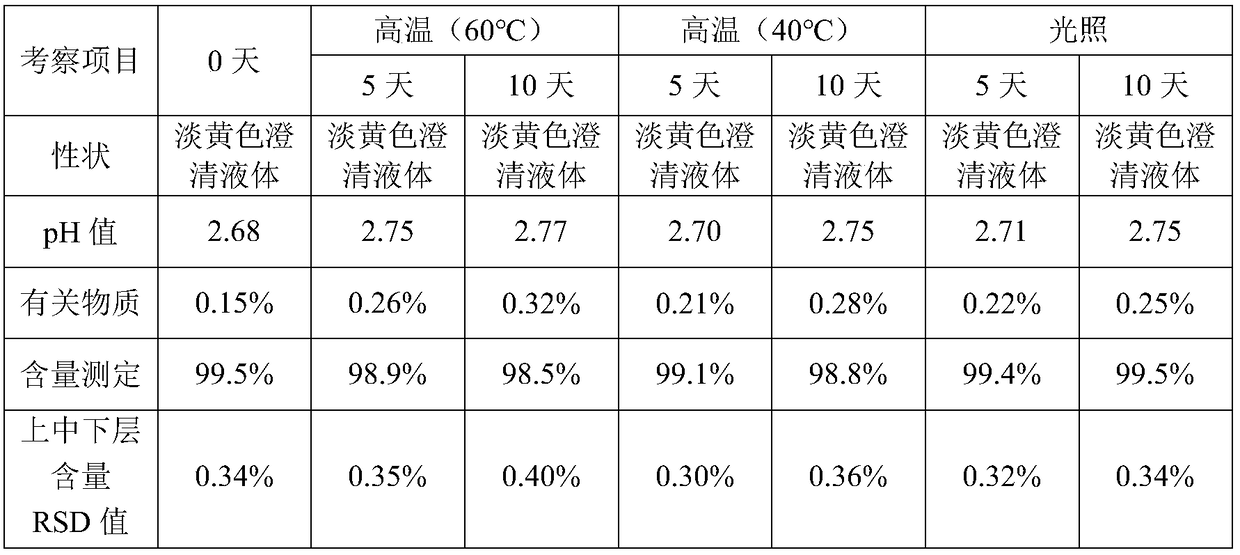
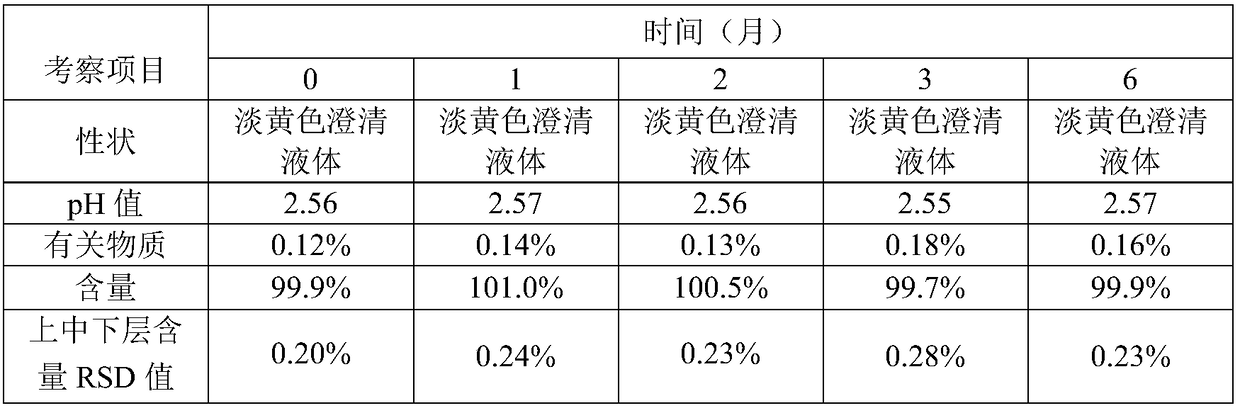
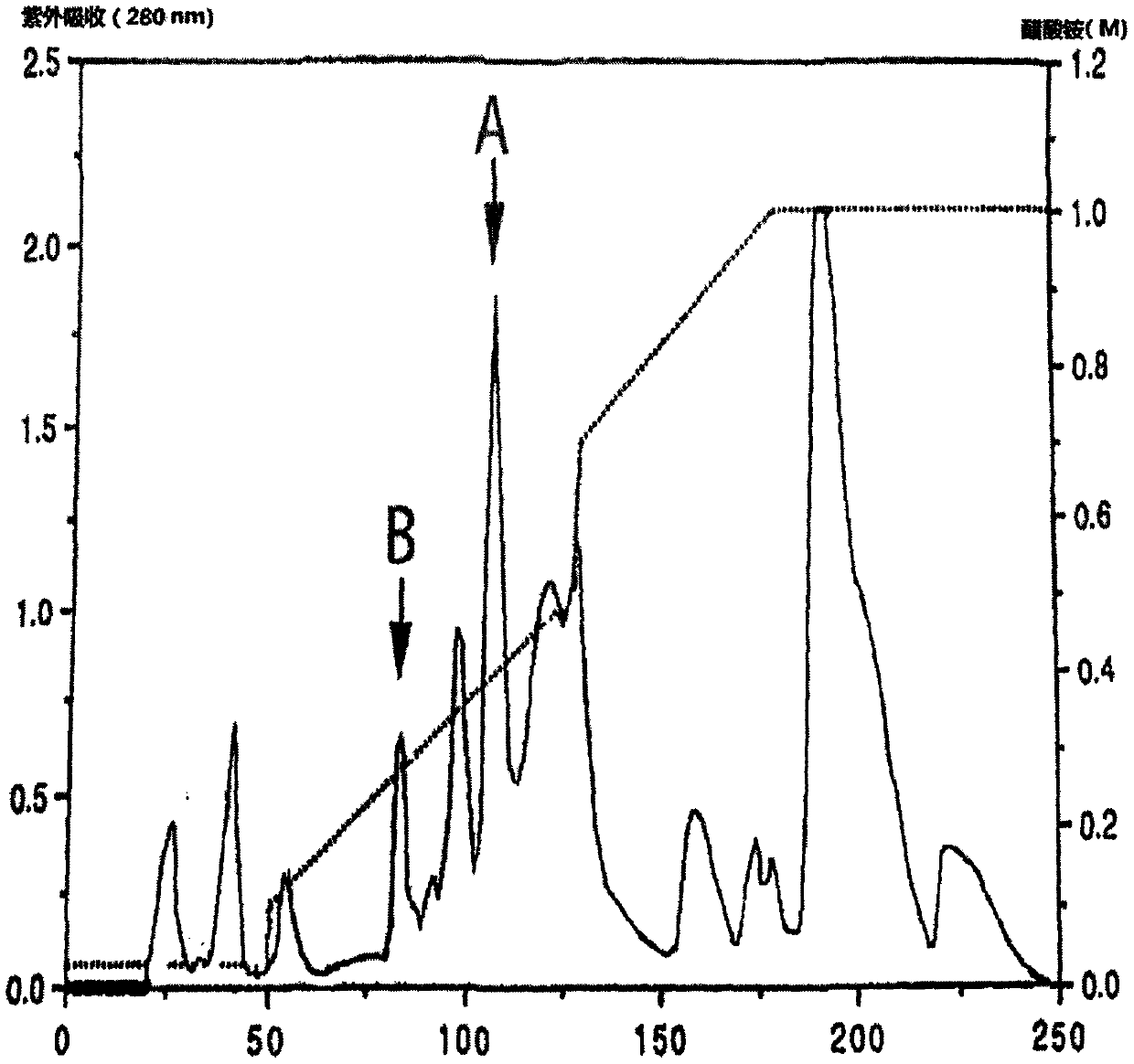
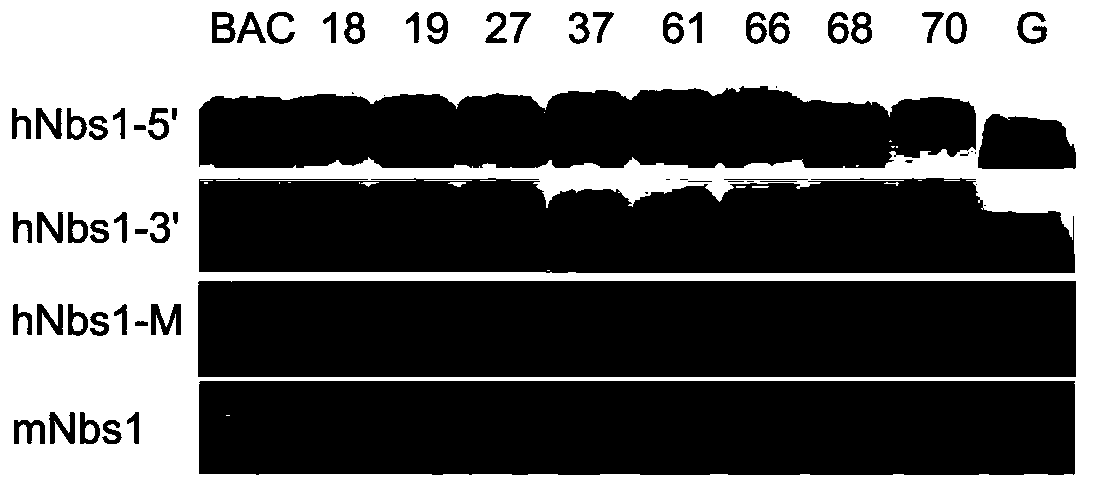Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
167 results about "Clinical safety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clinical Safety. Patient-care is a uniquely hazardous occupation. Clinical personal confront potential exposure to infectious diseases, chemical, physical, and radiological hazards. The safety of clinical personnel is of paramount importance to EH&S, which manages and responds to all issues and concerns that involves chemical, biological,...
Vascular interventional surgery robot and device
ActiveCN110200700AImprove securityPlay a monitoring roleMedical devicesCatheterEngineeringAxial force
The invention provides a vascular interventional surgery robot and a vascular interventional device. The suffered axial force and torque of a guide wire can be truly measured by a guide wire rotationand force feedback integrated module of the robot, and a hardware basis is laid for developing a human-computer interaction function. The robot is additionally provided with a detachable outer shell to meet the needs of surgical disinfection and ensure clinical safety and hygiene requirements. At the same time, the vascular interventional device is additionally provided with a four-degree-of-freedom mechanical arm structure on the basis of the vascular interventional robot, the height, position and stable support of the guide wire propulsion module are adjusted according to operation requirements, and wheels are arranged below a mechanical arm to facilitate the overall transportation and movement of the vascular interventional surgery robot. In addition, in the vascular interventional surgery robot and device, the size of the propulsion mechanism module is reduced as much as possible, the production materials are saved and the occupied space is saved.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Process for preparing cross-linked sodium hyaluronate microspheres capable of being adopted as emboliaztion agent by adopting sodium hyaluronate as raw material
The present invention provides a process for preparing cross-linked sodium hyaluronate microspheres capable of being adopted as an emboliaztion agent by adopting sodium hyaluronate as a raw material. The process comprises the following steps: preparing a sodium hyaluronate alkaline solution gel with a concentration of 10-30% g / ml; adding the sodium hyaluronate alkaline solution gel to an emulsifier-containing oil phase, and carrying out high speed emulsification through a shearing machine, wherein an emulsification speed is 500-2000 rpm, and a time is 10-20 min; adding a certain amount of a cross-linking agent, stirring for 4-6 h at a room temperature, carrying out a cross-linking reaction, and standing overnight after completing the reaction, wherein a mass percentage of the cross-linking agent in the oil phase is 0.2-2%; and adopting a water-soluble organic solvent to wash to remove the oil phase remained on the surface of the microspheres, and finally drying to obtain the cross-linked sodium hyaluronate microspheres. According to the present invention, the preparation process is simple; and the size of the obtained microspheres is suitable for routine blood vessel emboliaztion, and the obtained microspheres have characteristics of controllable particle size, good microsphere shape, easy screening, elasticity, expandibility, no toxic-side effect on human body, good biocompatibility, good biodegradability, and ensured clinical safety.
Owner:HANGZHOU SINGCLEAN MEDICAL PROD
Enhanced safety medication administration system
InactiveUS20050021368A1Data processing applicationsDrug and medicationsSafe handlingMedication administration
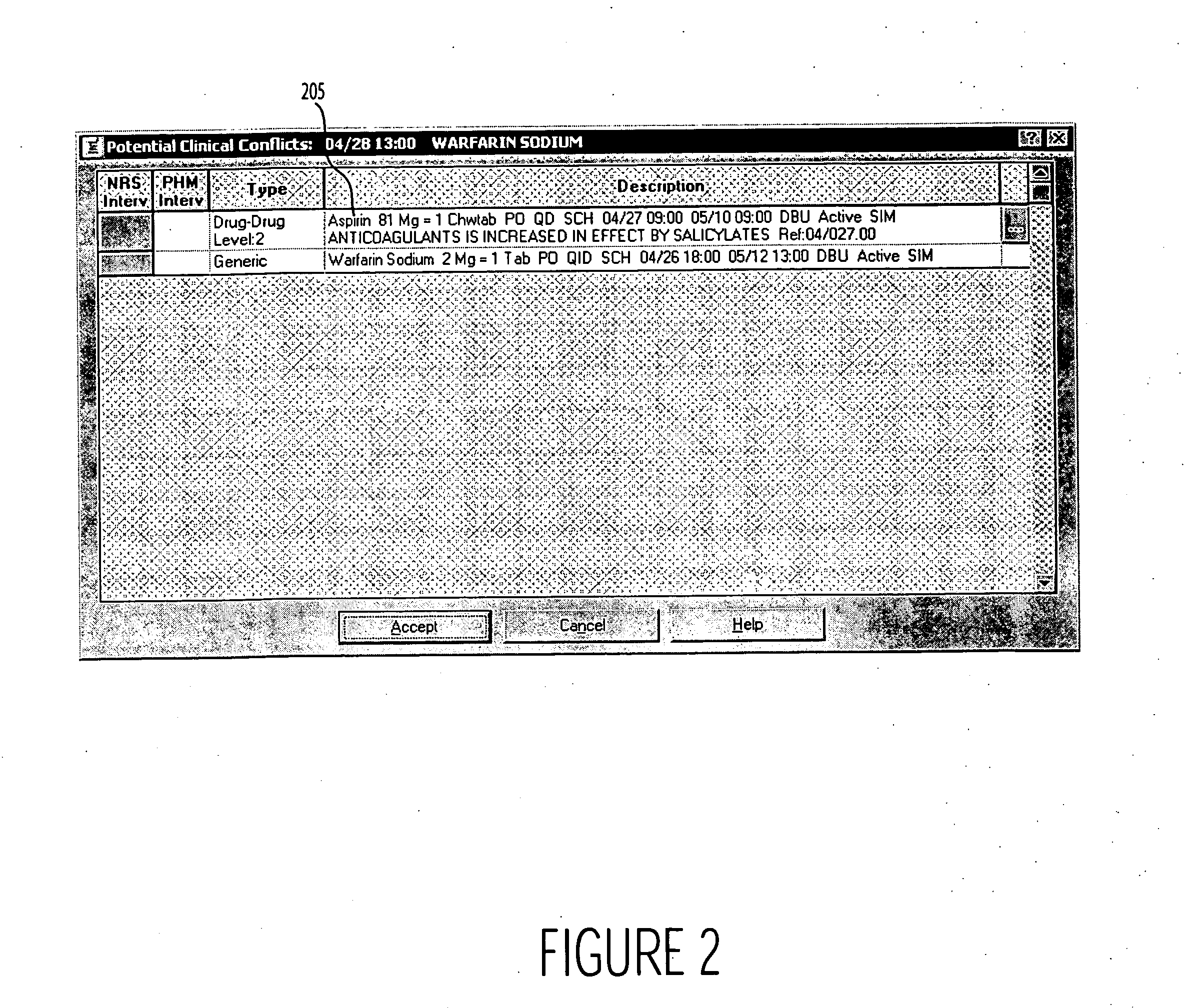
A system provides dual independent medication administration clinical safety checks comprising a check upon medication ordering and a check substantially at the time of medication administration. A system checks safety of medication administration to a patient using an input processor for receiving, an identifier identifying a particular medication and an identifier identifying a particular patient. A first safety processor uses the received identifiers in checking for a clinical condition indicative of potentially impaired safety in administering the particular medication to the particular patient. The checking occurs substantially at the time of administering the particular medication to the particular patient. A display generator initiates generation of data representing at least one display image including a message alerting a user to the safety impairment. A second safety processor checks for a clinical condition indicative of potentially impaired safety in administering the particular medication to the particular patient using received information associated with an order prescribing the particular medication is to be administered to the particular patient.
Owner:SIEMENS MEDICAL SOLUTIONS HEALTH SERVICES CORPORAT
Drug model explorer
InactiveUS20050079511A1Enhanced interactionUniform and consistent evaluationMedical simulationLocal control/monitoringProbit modelInformation type
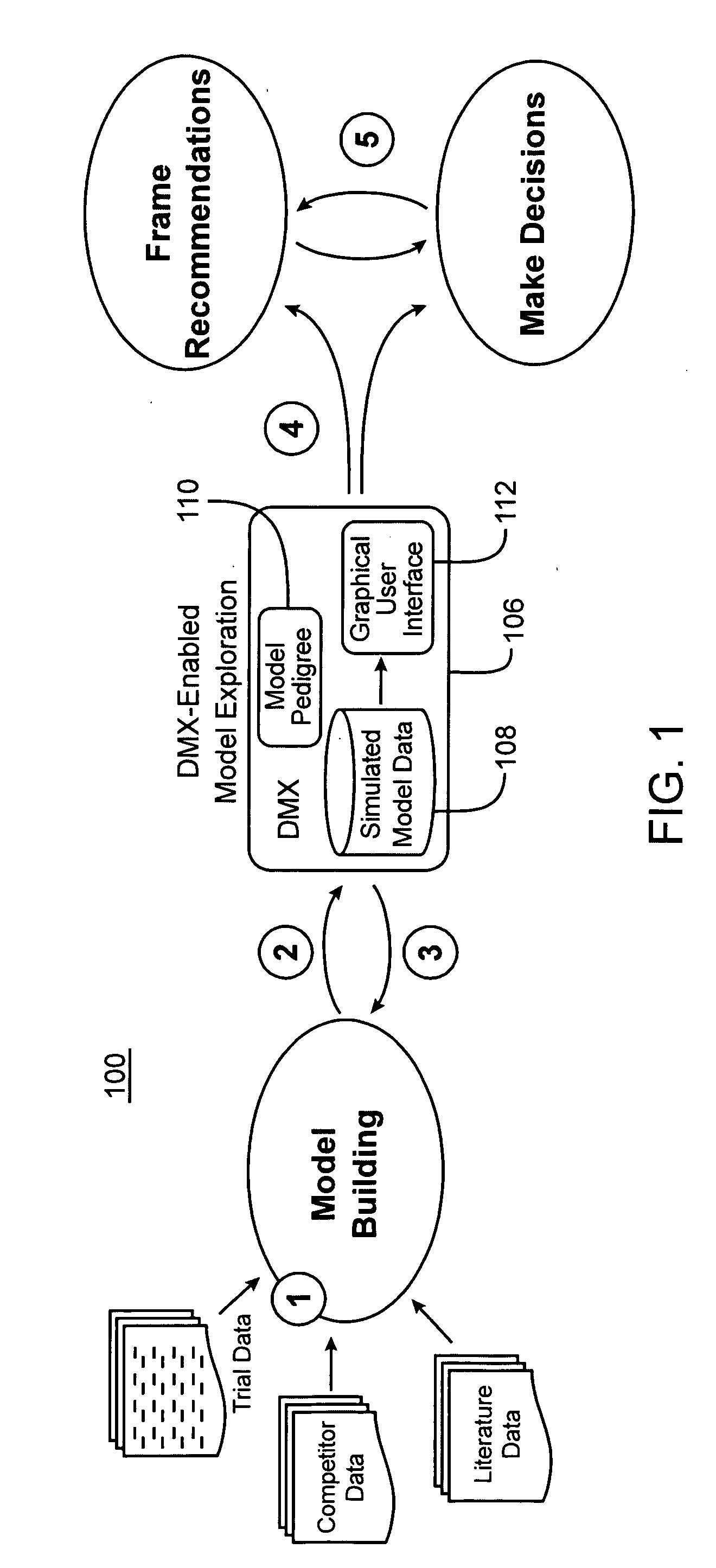
Computer systems and methods facilitate exploring results of drug candidate modeling. In one embodiment, the software is configured to receive raw data simulated by a probabilistic model of clinical safety, tolerability, and efficacy of a drug candidate. Index information is extracted from the raw data and then referenced to generate a metadata file, the structure of the metadata file explicitly reflecting a hierarchical structure of the model. The metadata file is in turn used to convert the raw data into a binary file, the metadata file explicitly identifying locations within the binary file, of treatment scenario information types and output performance information types. The metadata file is also referenced to generate an interface configured to receive inputs from a non-expert audience, and in turn present relevant subsets of the binary file in a limited number of plot and tabular formats. By standardizing presentation and manipulation of data from different models, software and methods in accordance with the present invention facilitate meaningful interaction between a non-expert audience, and the complex abstract mathematical models predicting drug behavior. The heightened audience-model interaction afforded by the present invention in turn promotes uniform and consistent evaluation of modeled data in the process of drug development.
Owner:TRIPOS
Mesenchymal stem cell low-temperature preserving fluid and preparation method thereof
InactiveCN106922648AHigh activityProlong the duration of activityDead animal preservationSodium acetateSide effect
The invention discloses mesenchymal stem cell low-temperature preserving fluid and a preparation method thereof. The mesenchymal stem cell low-temperature preserving fluid is prepared from human albumin injection and compound electrolyte injection according to a volume ratio of (1:100) to (1:5). Per 25ml of human albumin injection contains 5g of human albumin; per 1000 mL of the compound electrolyte injection contains 5.26g of sodium chloride, 5.02g of sodium gluconate, 3.68g of sodium acetate, 0.37g of potassium chloride and 0.30g of magnesium chloride. The invention also discloses an injection prepared from the low-temperature preserving fluid and a preparation method thereof. The use of freeze storage protection agents having the toxic and side effects and causing injury on cells is avoided; the freeze storage and unfreezing steps are avoided; the clinic safety, simplicity and convenience are greatly improved; meanwhile, the activity maintenance time of stem cells is prolonged; the condition that the cell activity can still meet the clinic use requirements after the 24h transportation. The mesenchymal stem cell low-temperature preserving fluid has the advantages that the safety is high; the transportation is convenient; the cell activity is high; the cost is low.
Owner:浙江新生泉细胞科技有限公司
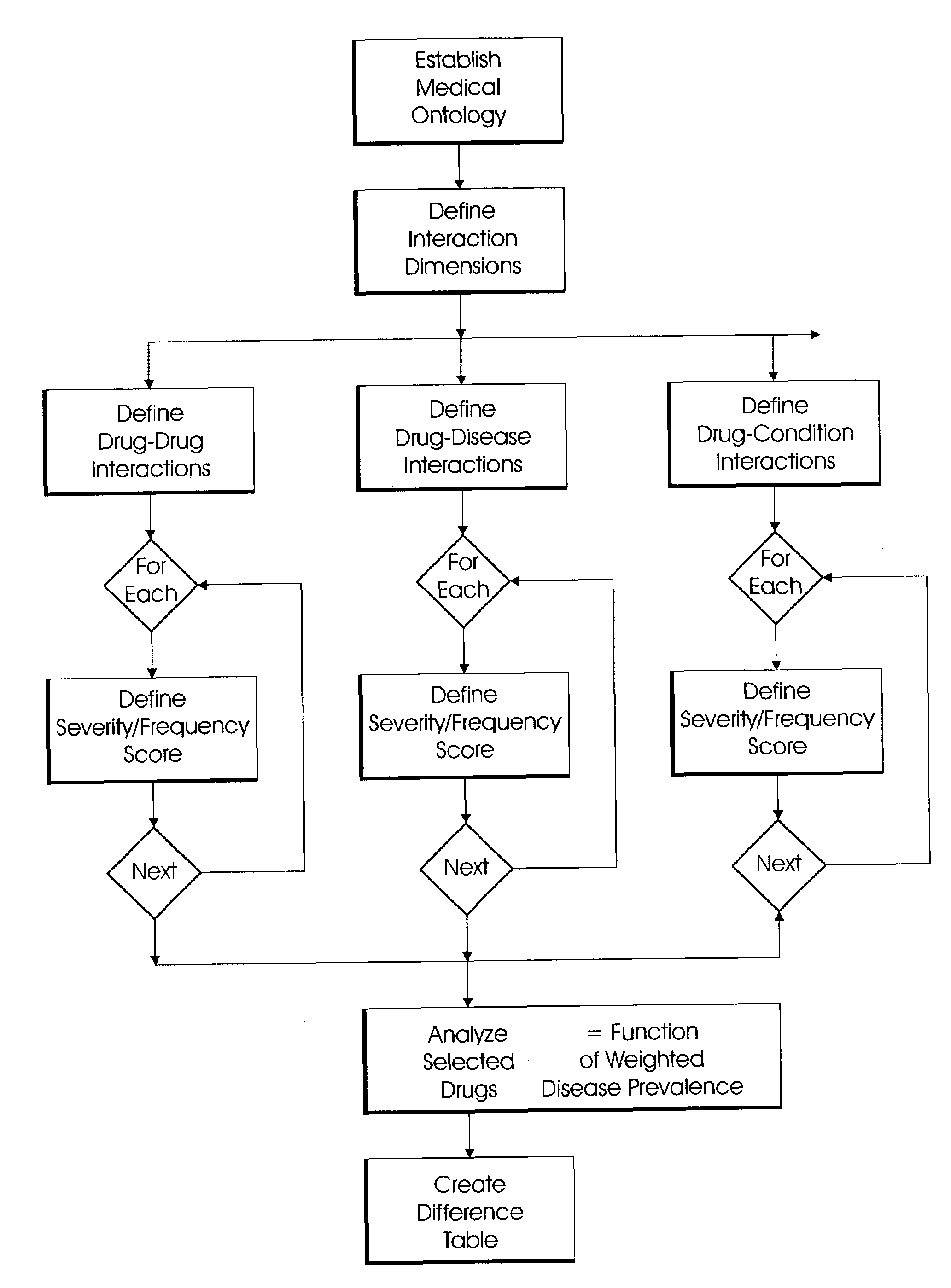
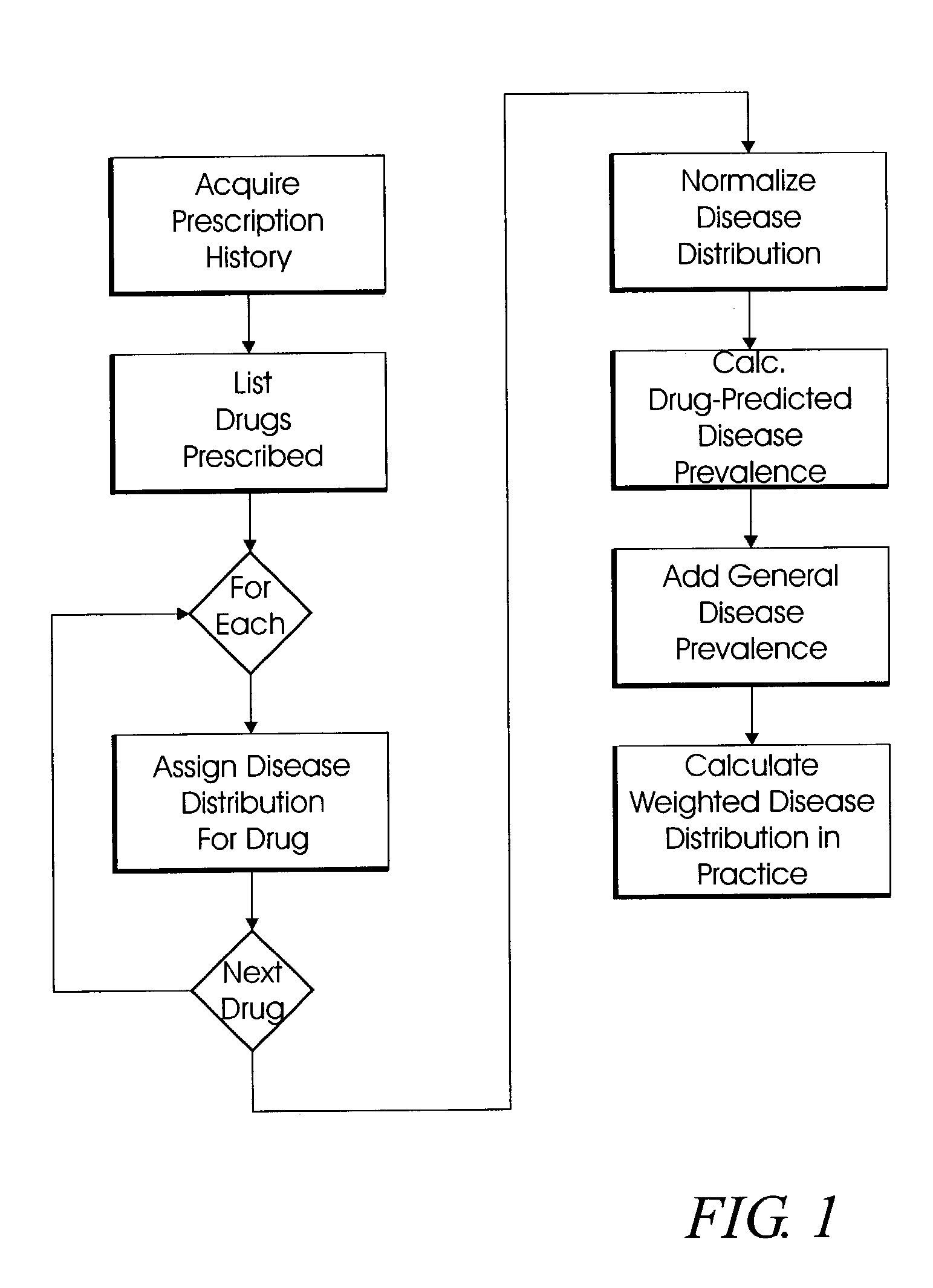
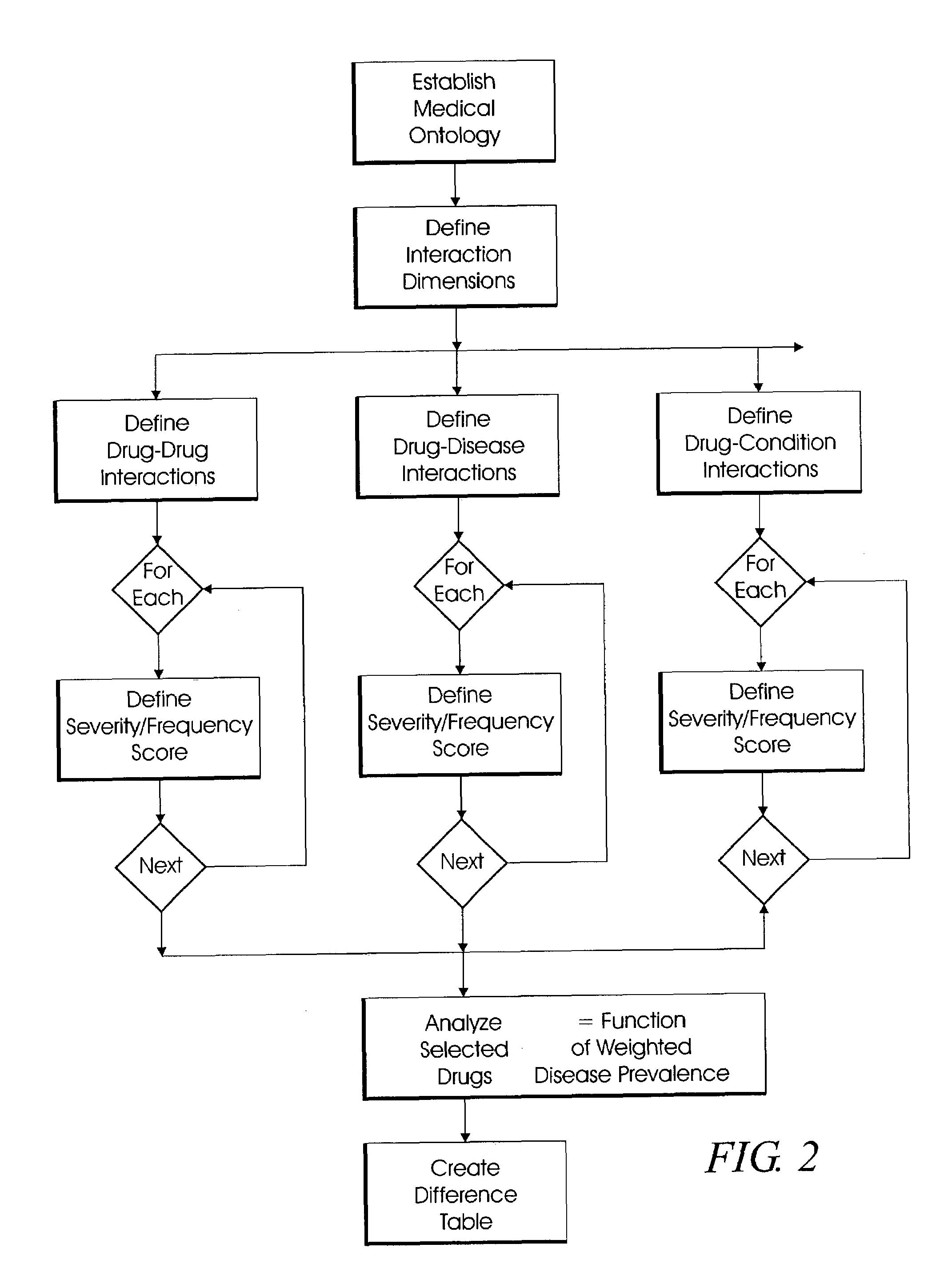
System and method for multi-dimensional physician-specific data mining for pharmaceutical sales and marketing
ActiveUS7698157B2Facilitate electronicFacilitate algorithmic computationDigital data processing detailsDrug and medicationsDiseaseSide effect
The present invention relates to a system and method for electronic and algorithmic data mining of an individual physician's prescribing history to determine the approximate distribution of diseases within their practice population for optimizing pharmaceutical sales and marketing. Rapid and large-scale determination of specific clinical safety and efficacy attributes of a marketed drug which are most pertinent and relevant to a given physician, when compared to a competitor's drug, are defined and tabularized. Major clinical characteristics taken into account include a drug's safety, efficacy, cost, dosing convenience, formulary insurance coverage, side effect profiles, and FDA approval for the intended use. A symbolic representation of knowledge is employed in which the marketed drug and each competitor's drug are compared algorithmically against each other with a scoring system that is based upon machine analysis of each major clinical characteristic. The score is further refined according to the number and severity of safety interactions which are relevant to the comparison, and also based upon predicted prevalence of such interactions within a specific physician's practice.
Owner:HUMANA INC
Method for controlling medicament release rate of orally disintegrating tablet
InactiveCN102716097ASimple processIncrease productivityPharmaceutical non-active ingredientsPill deliveryOrally disintegrating tabletWater insoluble
The invention relates to a preparation method of an orally disintegrating tablet. The orally disintegrating tablet contains active ingredients, a water-insoluble film-forming material accounting for 0.1%-4% of the total weight of the tablet and other pharmaceutically acceptable additives, and is prepared through a wet granulation process. The orally disintegrating tablet prepared according to the method disclosed by the invention can control the release rate of the active ingredients and is favorable for ensuring the bioequiavailability, clinical safety and efficacy of a product. Furthermore, the method disclosed by the invention has the advantages of simple process flow and easiness in realization of industrial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Method for extracting mesenchymal stem cells from trace human fatty tissues and massively culturing
InactiveCN101974486ARelieve painOvercoming barriers to access to adipose tissueSkeletal/connective tissue cellsAnimal productBiology
The invention belongs to the field of biomedicine, and in particular discloses a method for extracting mesenchymal stem cells from trace human fatty tissues and massively culturing. The method for extracting mesenchymal stem cells from trace human fatty tissues comprises the steps of: locally narcotizing in small area by injecting lidocaine, extracting trace fatty tissues of a patient by using a micro draining needle; then soaking with a phosphate buffer, centrifugally washing and obtaining fatty tissues in a sterile state; and digesting with collagenase, centrifuging with gradient and filtering and then culturing in an in-vitro culturing system. The invention effectively reduces the pain of the patient, eliminates the barrier that the thin patient can not obtain the fatty tissues, avoids using animal products in the whole cell culturing system, especially serum-free products, and meets the demands on future clinical safety application.
Owner:王泰华
Patient self-control sedative target control infusion system based on anesthesia depth monitoring
InactiveCN106730110AAvoid pressing requestsDeeper sedationInfusion devicesEvaluation of blood vesselsTarget controlSedation
The invention discloses a patient self-control sedative target control infusion system based on the anesthesia depth monitoring. The infusion system comprises the four modules of a central processor control module, a BIS sedation depth and vital sign monitoring feedback module, an output request module, and an output execution module. According to the infusion system, the target controlled infusion is used as the mode of the self-controlled dosing for a patient, the target controlled infusion is a therapeutic scheme calculated based on the group drug generation-medicine efficacy power theory by a computer, and blood plasma / effect compartment medicine concentration is used as the target control objective, the target controlled infusion can be rapidly achieved or modulated, the stability and a certain degree of safety of the sedative level can be guaranteed, and the BIS sedation depth monitoring system is used to monitor the sedation depth of the patient, when combined with the vital sign indicator, the clinical safety is increased to a greater extent.
Owner:谢言虎
Method for preparing gelatin microballoon embolization agent
InactiveCN103006573AGood biocompatibilityPromote degradationGranular deliveryMacromolecular non-active ingredientsCross-linkSide effect
The invention relates to a method for preparing a gelatin microballoon embolization agent, which comprises the following steps of dissolving gelatin or mixture of gelatin and medicine at 30-70 DEG C, filtering to obtain gelatin solution with 15%-60% of solid content, adding gelatin solution into oil phase liquid paraffin with mass ratio of 1%-2% of stabilizer Span 80, wherein the volume ratio of water and oil is 1 / 1-1 / 10, stirring for 15 minutes by 200-800r / min, adding cross-linking agent aldehyde compound with mass percent of 2%-50% at low temperature of 0-10 DEG C, solidifying for 1-2 hours, washing or drying the cross-linking agent to obtain the microballoon embolization agent after freeze drying or dehydration. The method has the beneficial effects that the gelatin microballoon embolization agent has certain elasticity and expansibility, the surface is smooth, the sphere is obvious, the size and the shape of the sphere is uniform, the grain size is controllable, clinical embolization effect can be improved, and side effect can be lowered, the clinical controllability is strong, antitumor drug can be added into the microballoon in manufacture process, so that double therapeutical effect of medicine treatment and embolization can be realized. The gelatin has good biocompatibility and biodegradablity, and clinical safety can be guaranteed.
Owner:杭州艾力康医药科技有限公司
Serum-free cryoprotectant, and application thereof in cryopreservation of mesenchymal stem cells
ActiveCN107494517AImprove survival rateIncreased clinical allergen riskDead animal preservationUmbilical cordResuscitation
A serum-free cryoprotectant includes: 8-15 v / v% of DMSO, 85-92 v / v% of a DMEM basic culture medium, and a nutritional additive. The nutritional additive includes: fibroblast growth factors, insulin, growth hormone, transferrin, bone morphogenetic protein 4, glutamine, sodium pyruvate, beta-mercaptoethanol, human epidermal growth factor, sodium selenite, and various amino acids and vitamins. The cryoprotectant is free of animal sourced serum and avoids pollution and risk of allergen, and has better clinical safety. The serum-free cryoprotectant is suitable for cryopreservation of human placenta sourced, umbilical cord sourced and cord blood sourced mesenchymal stem cells; compared with common serum cryoprotectants, perinatal mesenchymal stem cells preserved in the cryoprotectant have high cell survival rate after resuscitation, have excellent adherence growth status and maintain biological characters well.
Owner:章毅 +7
Human adipose tissue-derived stromal cell frozen stock solution
InactiveCN104472474AGood stem cell propertiesAvoid pollutionDead animal preservationMotilityStromal cell
The invention relates to the field of stem cells, discloses a frozen stock solution of stem cells, and in particular discloses a human adipose tissue-derived stromal cell frozen stock solution which consists of human plasma and dimaethyl sulfoxide. Compared with an ordinary cell frozen stock solution, the human adipose tissue-derived stromal cell frozen stock solution disclosed by the invention is relatively high in clinical security as no non-human source serum or protein is provided and the risk that contamination or allergen is introduced is avoided. The experience shows that compared with the ordinary cell frozen stock solution, the human adipose tissue-derived stromal cell frozen stock solution disclosed by the invention is relatively high in motility rate of cells in frozen stock, good stem cell characteristics are maintained, and human fat stem cells can be preserved and applied for a long time.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
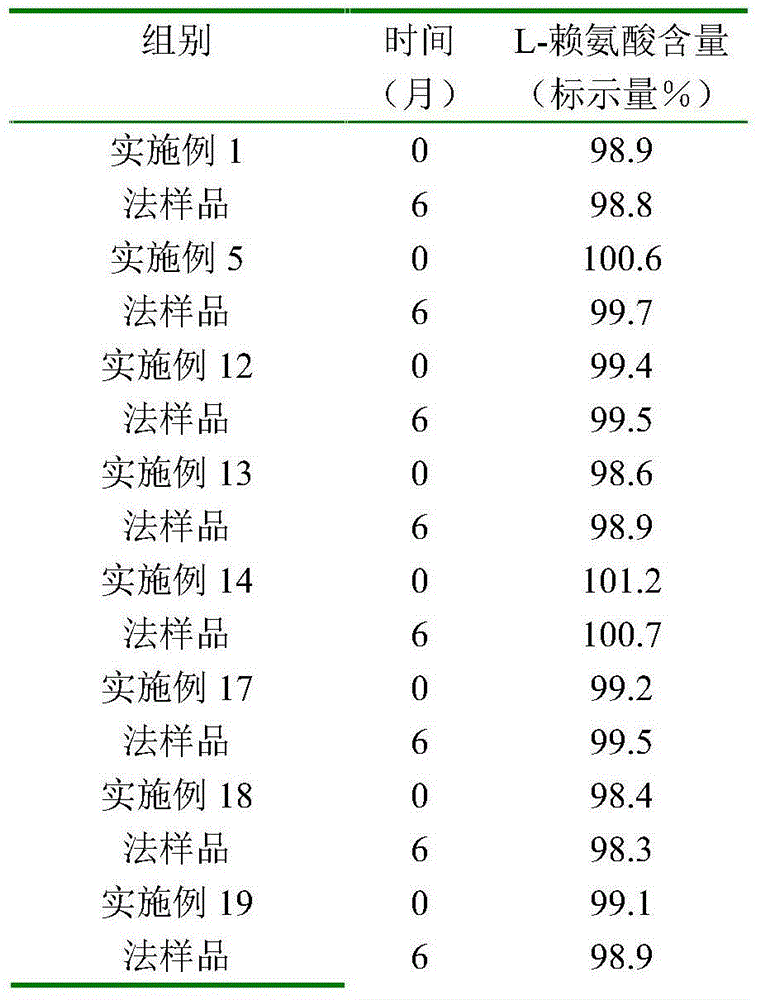
Lysine-vitamin pharmaceutical composition containing chiral isocompounds and use thereof
InactiveCN104688763APromote absorptionIncrease profitNervous disorderHydroxy compound active ingredientsOxygenBrain traumas
The invention provides a pharmaceutical composition of lysine and vitamin containing chiral isocompounds, the pharmaceutical composition is mainly used for preventing or treating growth retardation of mammals and human, malnutrition, inappetence, cerebral ischemia, brain trauma, anemia, hair growth, hair loss, fatty liver, hepatitis, atherosclerosis, hyperlipidaemia or oxiunt radical scavenger and the like, the composition can better adapt to patients with metabolic acidosis and hyperchloremia, and the pharmaceutical composition can adapt to new applicable people on clinic use or can have better pertinence or better clinical safety.
Owner:刘力
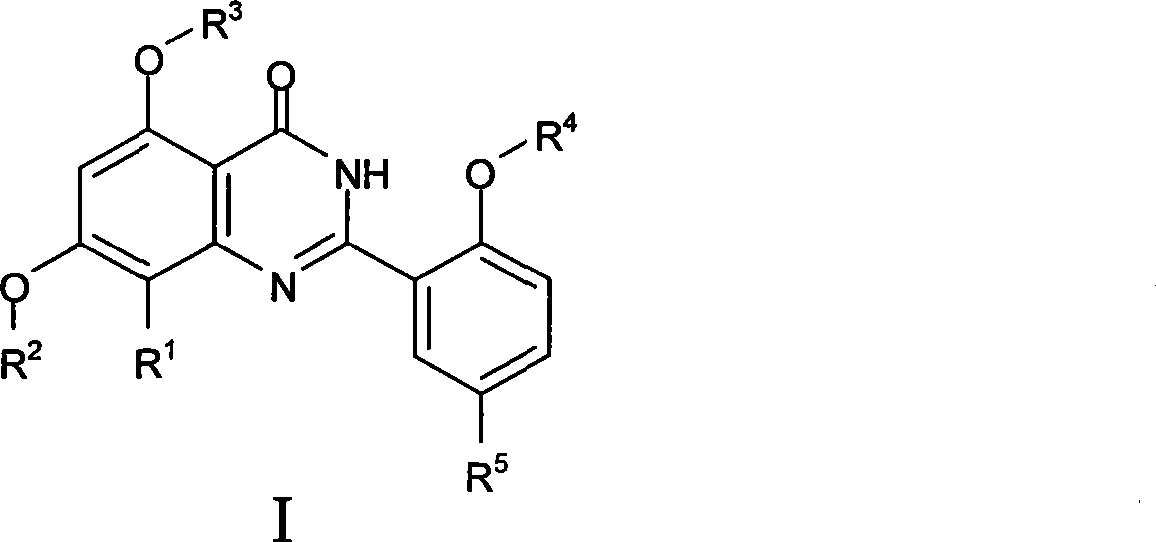
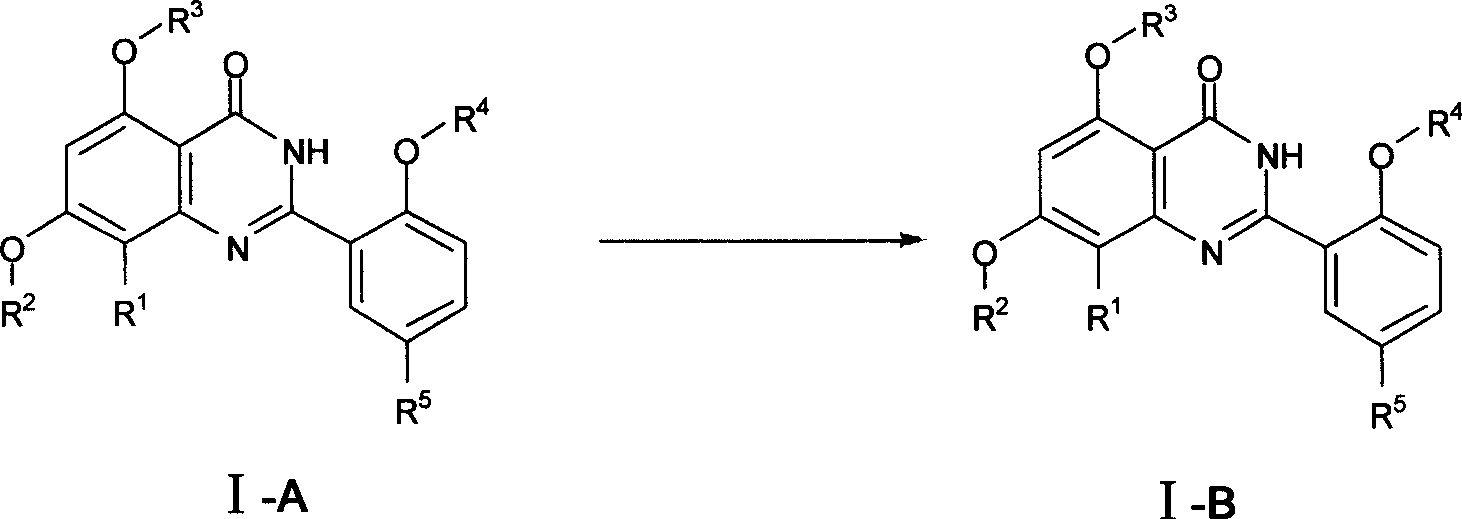
Quinazoline ketone derivant, preparation method and application thereof
InactiveCN101429166AStrong inhibitory activityHigh selectivityOrganic active ingredientsSenses disorderPde5 inhibitionKetone
The invention relates to the technical field of medicine, in particular to a quinazolinone derivative, a preparation method and application thereof. The invention discloses quinazolinone compounds as structural general formula (I) or pharmaceutically acceptable salt thereof. Most of the compounds have stronger PDE5 inhibition activity than that of Sildenafil, and have higher selectivity than thatof PDE 6 distributed on a retina. Therefore, the compounds are expected to show better clinical safety and effectiveness, and have wide clinical application prospect.
Owner:TOPHARMAN SHANGHAI +1
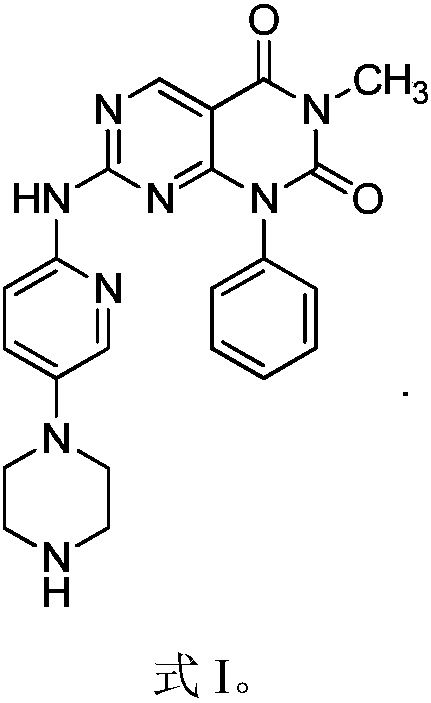
Pharmaceutical composition prepared by combining paclitaxel with CDKS kinase inhibitor for use
InactiveCN107929276AAdvantages and Notable ImprovementsSignificant progressOrganic active ingredientsAntineoplastic agentsHigh dosesBULK ACTIVE INGREDIENT
The present invention provides a combined pharmaceutical composition of paclitaxel and CDKS kinase inhibitors, comprising active ingredients and pharmaceutically acceptable excipients, characterized in that: the active ingredients are composed of paclitaxel and CDK4 / 6 kinase represented by formula I Inhibitors or pharmaceutically acceptable salts thereof, the mass ratio of paclitaxel and CDK4 / 6 kinase inhibitors or pharmaceutically acceptable salts thereof in the active ingredient is (2‑8):1. The pharmaceutical composition has good anticancer curative effect and low toxic and side effects; due to the sensitivity of CDK4 / 6 kinase inhibitors to paclitaxel, the combination of the two produces a synergistic effect, thereby reducing the clinical dosage of capecitabine and reducing the large dosage Toxic and side effects produced by using capecitabine can improve the safety index of clinical treatment and have good clinical application prospects.
Owner:南京众慧网络科技有限公司
Serum-free culture medium suitable for immune cell large-scale culture
ActiveCN105039253AHigh clinical safetyProliferation effect is goodBlood/immune system cellsSodium bicarbonateHuman albumin
The invention discloses a serum-free culture medium suitable for immune cell large-scale culture, and belongs to the field of cell biology and medical immunology. The serum-free culture medium takes an RPMI1640 culture medium as a basis, additive components are further contained and include HEPES, phenol red sodium salt, L-glutamate and sodium bicarbonate. Insulin, transferrin, selenium, human albumin and interleukin-2 can be further added. Components contained in the culture medium are chemical defined components, no animal serum components are contained, and the serum-free culture medium is suitable for large-scale culture of immunity cells and is high in clinical safety and good in proliferation effect.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Comprehensive burn treatment bed
The invention discloses a comprehensive burn treatment bed which is novel in structure, convenient and safe to use and capable of conducting automatic operation, reducing treatment time, improving treatment effects and reducing working intensity and is driven by a machine. The bed comprises a bed body, a lifting mechanism, a rotating mechanism, an electric device and an immersion bath. A body turning bed and a body turning frame are arranged and matched in use, a patient lies flatly when the bed is turned upwards, transposition is conducted when the bed is turned downwards, irradiation treatment is conducted on the patient on the body turning frame, and pains of the patient caused by the fact that the patient is bound and hung for back irradiation are removed. An automatic location fixed separation moving device is arranged to replace manual transportation of the body turning frame, an infrared electric heater case all-dimensional radiation effect is achieved in use, the immersion bath is added clinically, and ventilation suspension is conducted on the patient on time to prevent pressure sores. A worm reducer and a bedside guardrail are provided to ensure clinical safety of the patient. The treatment bed is clinically applied to moderate and severe burn patients.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
Aluminum hydroxide gel-polysaccharide composite immunologic adjuvant and preparation method and application thereof
ActiveCN102526724AAntigen releaseRelease stabilityDigestive systemAntiviralsImmune effectsImmunocompetence
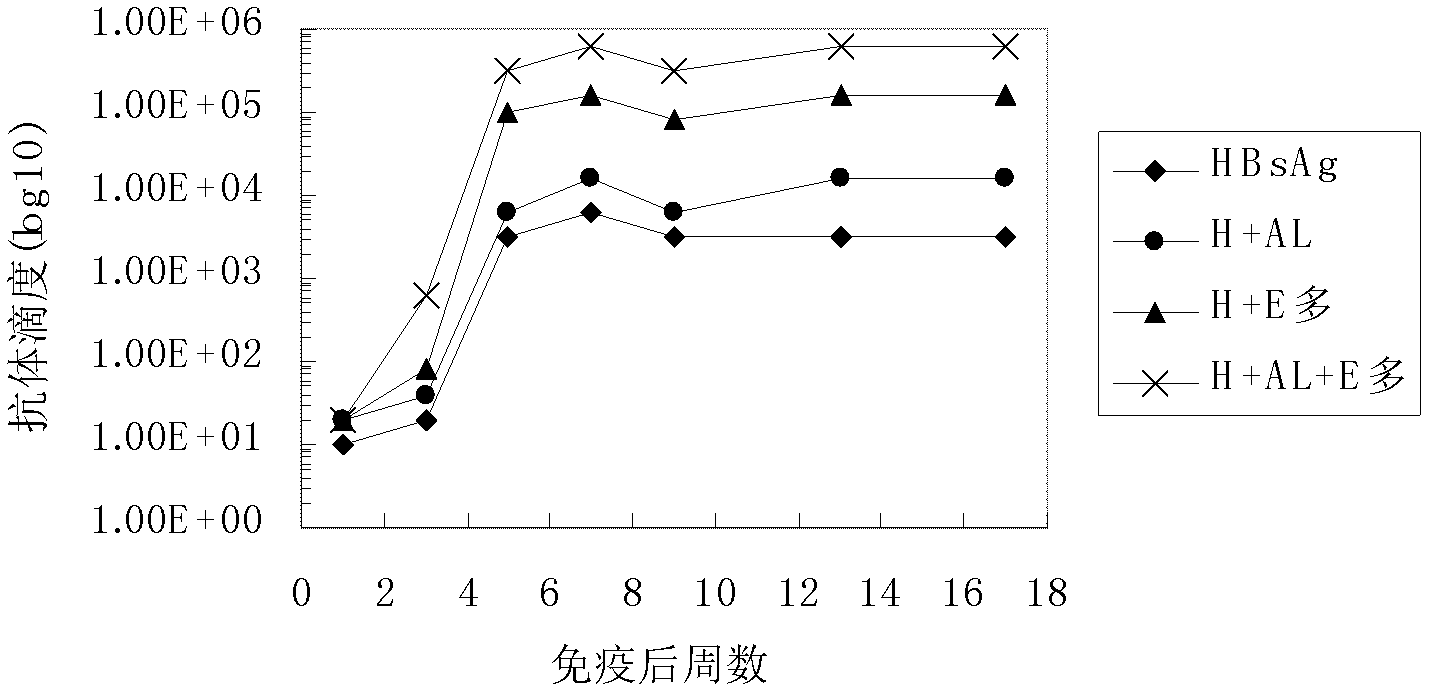
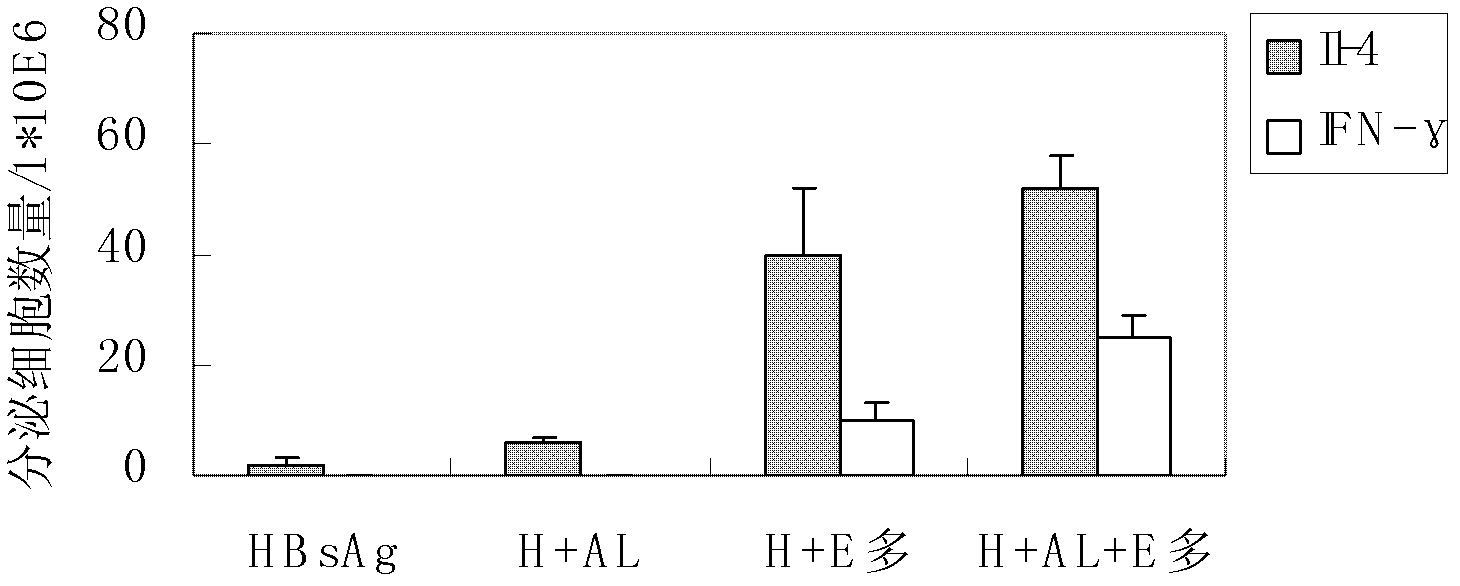
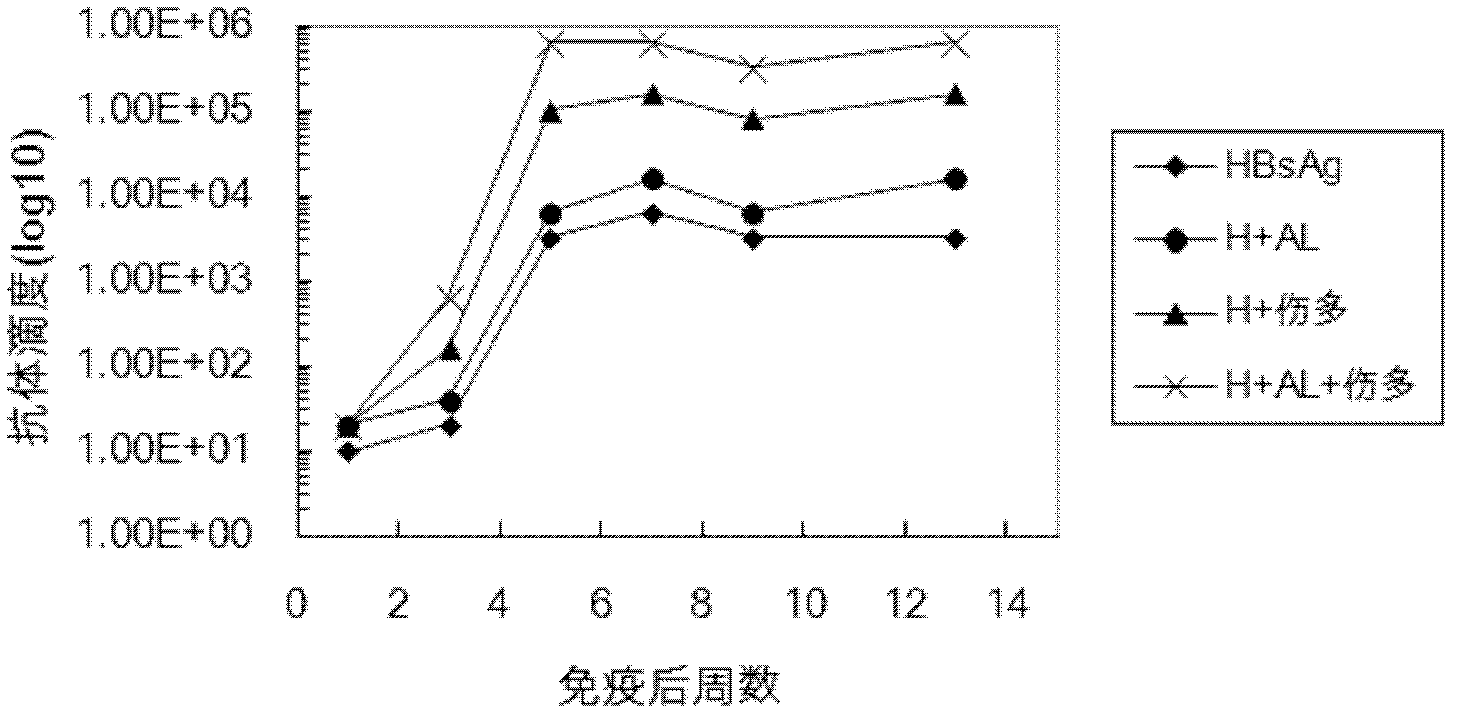
The invention belongs to the field of biomedicine, and particularly relates to an aluminum hydroxide gel-polysaccharide composite immunologic adjuvant and a preparation method and application thereof. The invention aims to provide a new immunologic adjuvant with good performance. The technical scheme is to provide a composite immunologic adjuvant. The composite immunologic adjuvant mainly contains aluminum hydroxide gel and polysaccharide serving as bacteria source. The aluminum hydroxide gel-polysaccharide composite immunologic adjuvant provided by the invention has the advantages of strong immunocompetence, high clinical safety and the like, and is an excellent composite immunologic adjuvant aiming at various antigens. The hepatitis vaccine and tumor vaccine prepared by taking the aluminum hydroxide gel-polysaccharide composite provided by the invention as adjuvant has stronger immune effect and anti-cancer effect, and thus new selection is provided for the development and application of vaccine.
Owner:WEST VAC BIOPHARMA CO LTD
Chitosan microspheres and preparation method thereof
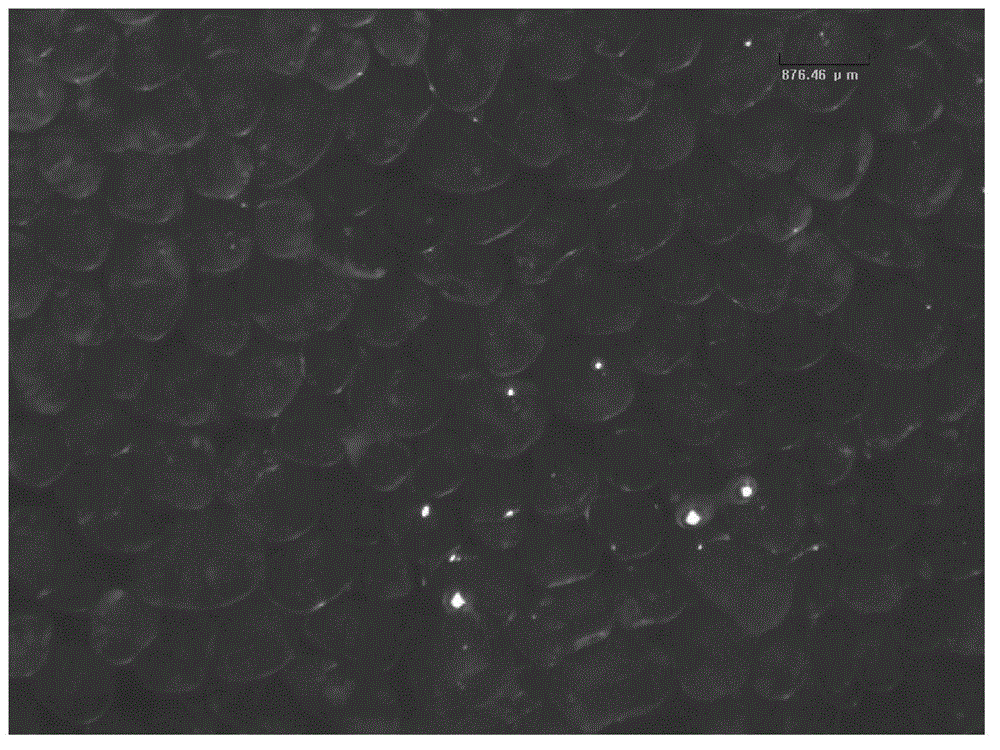
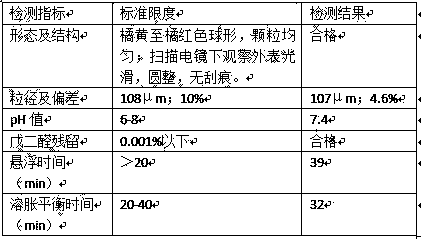
The invention relates to chitosan microspheres. The microspheres are solid spheres which have smooth surface and uniform granularity and are formed by crosslinking of chitosan and a crosslinking agent, wherein the grain diameter of each microsphere is 50-1200 mu m, preferably 50-600mu m, and further preferably 150-300mu m; and the grain diameter difference is not more than 20 percent, and preferably not more than 10 percent. The microspheres provided by the invention have the characteristics of small grain diameters and high uniformity, and are capable of improving the safety effectiveness of embolism in interventional therapy, reinforcing the clinical operability, reducing the clinical safety accident rate and reducing the production cost.
Owner:SHIJIAZHUANG YISHENGTANG MEDICAL SUPPLIES
Cell cryoprotectant and cryopreservation method
The invention relates to the field of cells, in particular to a cell cryoprotectant and a cryopreservation method. The cell cryoprotectant is prepared from DMSO, human albumin and a serum-free medium. The cryprotectant does not contain animal serum, the risks of introducing contamination and allergens are avoided, and the higher clinical safety is achieved compared with a conventional cell cryoprotectant. Meanwhile, the GMSCs cryoprotectant can well keep the activity of cryopreserved cells.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
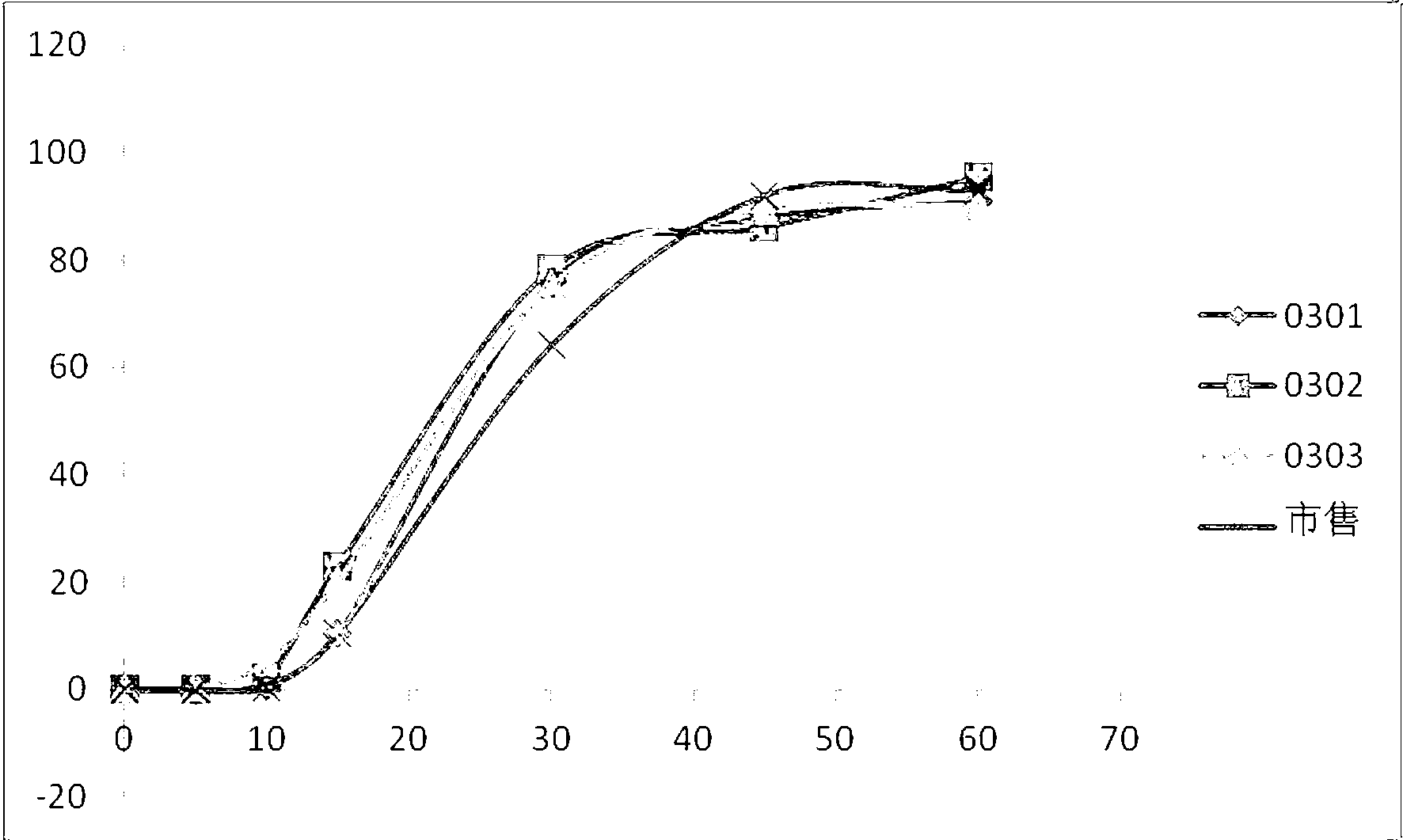
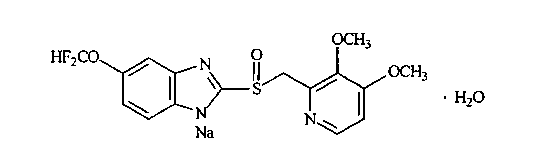
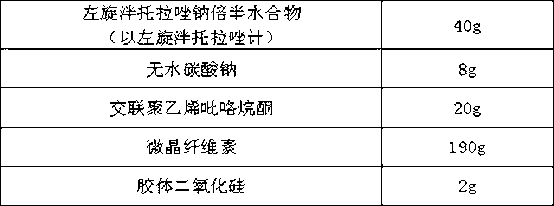
Levo-pantoprazole salt aquo-complex enteric-coated tablet and preparation method thereof
InactiveCN103211778AOne-sided bright and tidyAccurate measurementOrganic active ingredientsDigestive systemMedicineIsolation layer
The invention discloses a levo-pantoprazole salt aquo-complex enteric-coated tablet which comprises a tablet core, an isolation layer and an enteric-coated layer. The tablet is characterized in that the tablet core comprises a levo-pantoprazole salt aquo-complex, a shaping agent, a pH conditioning agent, a disintegrating agent and a lubricant; the levo-pantoprazole salt aquo-complex is 15-25% of the total weight of the tablet core by the weight of levo-pantoprazole; the shaping agent is 60-75% of the total weight of the tablet core; the disintegrating agent is 5-10% of the total weight of the tablet core; the pH conditioning agent is 3-7% of the total weight of the tablet core; the lubricant is 0.5-1.5% of the total weight of the tablet core; and the percentage is of percentage by weight. The enteric-coated tablet of the levo-pantoprazole or the salt of the levo-pantoprazole is convenient to carry over, transport and take; the enteric-coated tablet is high in process operability and is applicable to industrial production; and compared with a racemate preparation, the enteric-coated tablet reduces the dosage and avoids adverse effects of dextroisomer, thereby being better in clinical security.
Owner:沈阳双鼎制药有限公司
Kit for treating human bone marrow, umbilical cord blood, and peripheral blood cells, and cell treatment method
InactiveCN102965339AAvoid interferenceAvoid the risk of rejectionBlood/immune system cellsArtificially induced pluripotent cellsHuman bodySeparation technology
The invention discloses a kit for treating human bone marrow, umbilical cord blood, and peripheral blood cells, and provides a kit for treating human bone marrow, umbilical cord blood, and peripheral blood cells and a cell treatment method which are strong in operationality, high in clinical safety, and convenient for clinical popularization. The kit comprises four reagents: No. A liquid is a diluent; No. B liquid is density fluid; No. C liquid is washing liquid; No. D liquid is erythrocyte-removing liquid. The invention fundamentally solves the problems of high cost, low cell activity, undefined human body influence for markers entering human body, pain for patients due to mobilization agent injection, cumbersome and inapplicable operations, and the like for current cell separation technology.
Owner:WUHAN HAMILTON BIOTECH
Construction method and application of humanized mouse model
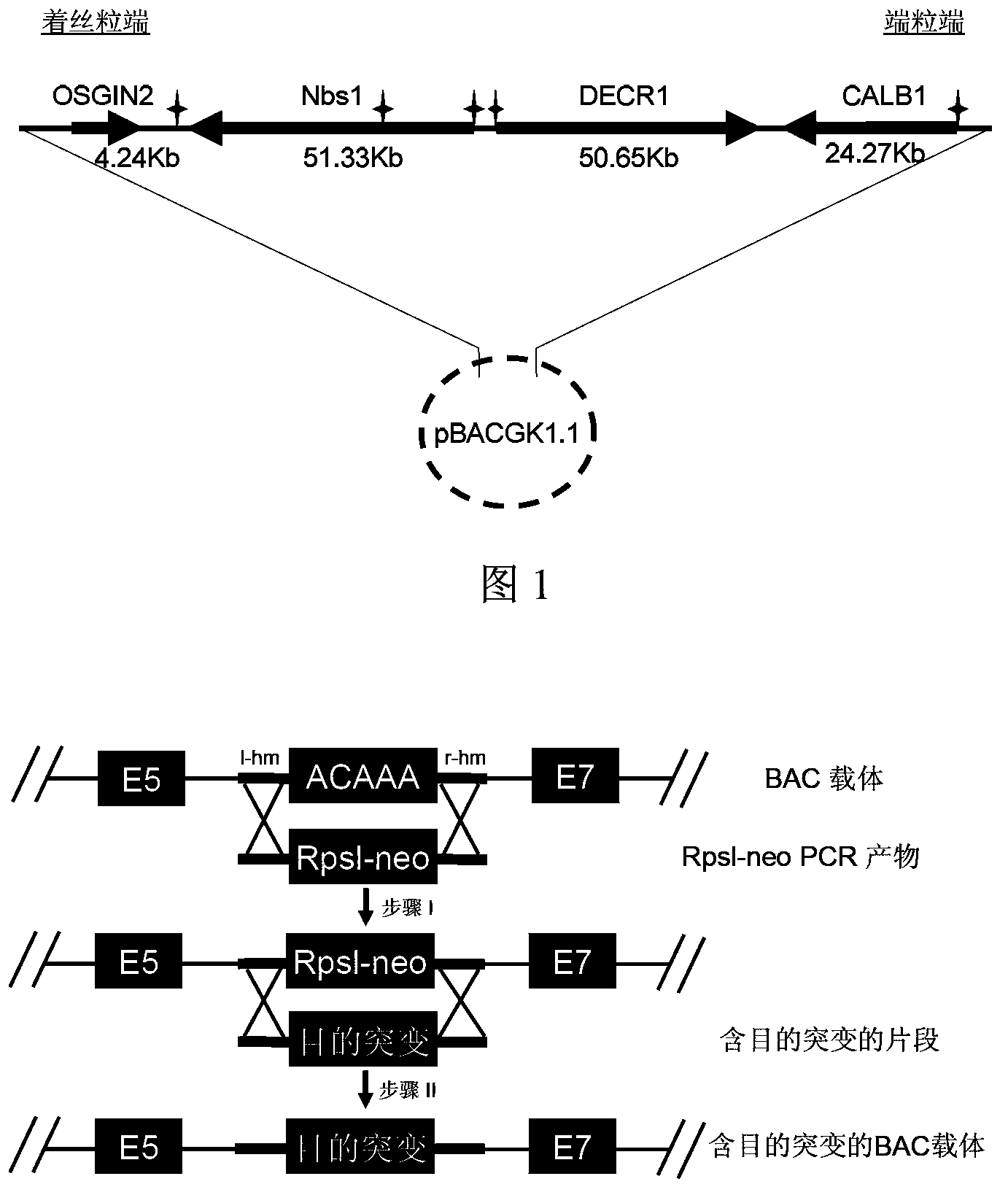
InactiveCN104046644AMicrobiological testing/measurementVector-based foreign material introductionDiseaseDevelopmental anomaly
The invention relates to a construction method and application of a humanized mouse model, in particular to a construction method and application of a transgenic mouse model of a human Nbs1<c.657del5> gene. The method includes: constructing a 5bp deletion mutation-containing BAC carrier in the human Nbs1 gene, conducting pronuclei microinjection, and performing screening to obtain 3 stable transgenic lines for high expression and low expression of the human Nbs1 gene. The transgenic mouse involved in the invention has the phenotypes of delayed puberty, uniform shortening of body length and bone dysplasia at certain proportion in one of the lines, and a new mouse model is established for Nijmegen breakage syndrome diseases. At the same time, as the Nbs1 gene function impairment is closely related to cancers, the transgenic model can be applied to short-term carcinogenic tests in drug pre-clinical safety evaluation, thus providing a potential substitution model for traditional biennium carcinogenic tests and also providing an effective tool for research of carcinogenesis mechanisms.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Remote controlled modicine reliesing electronic capsule capable of partly degradation
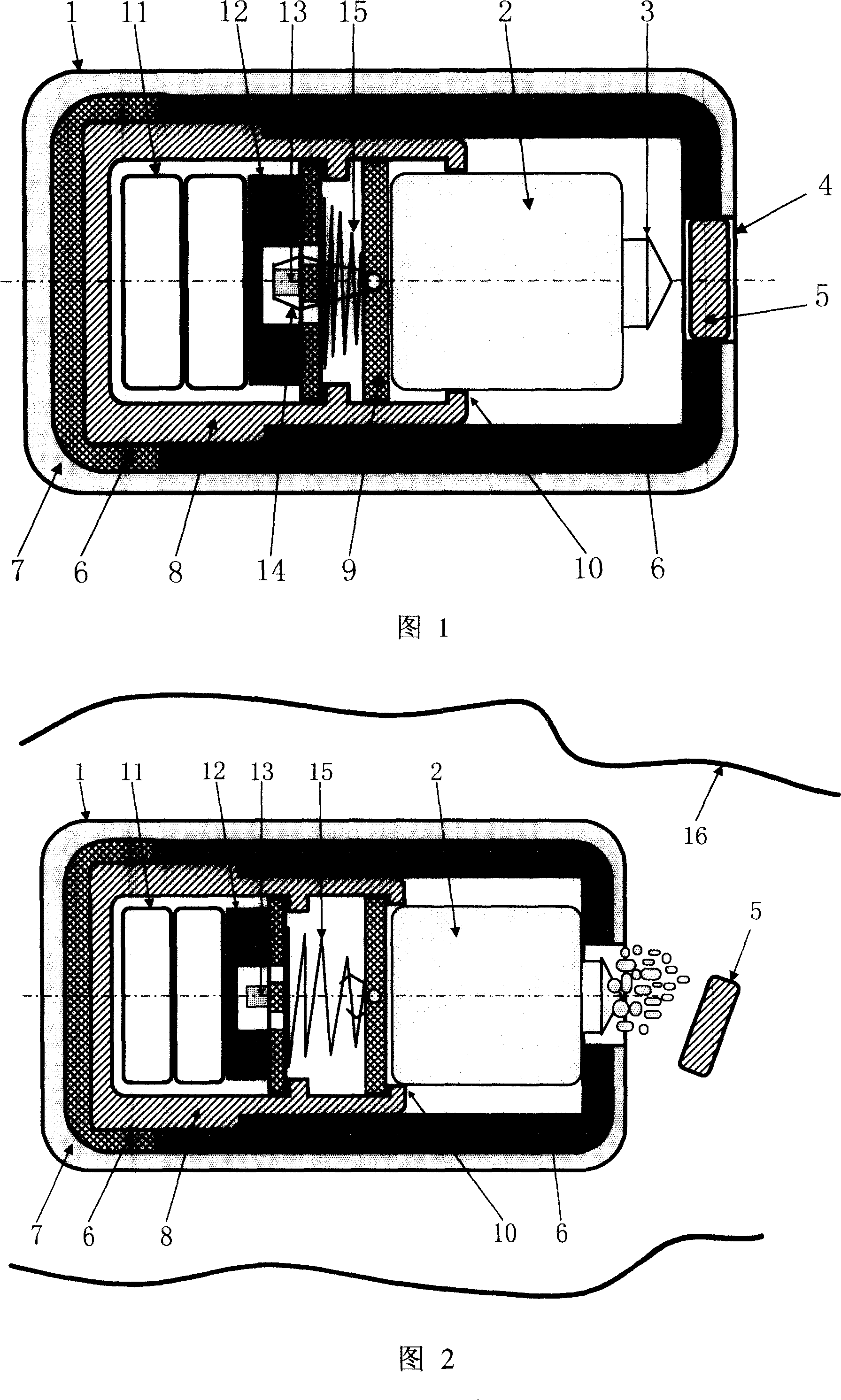
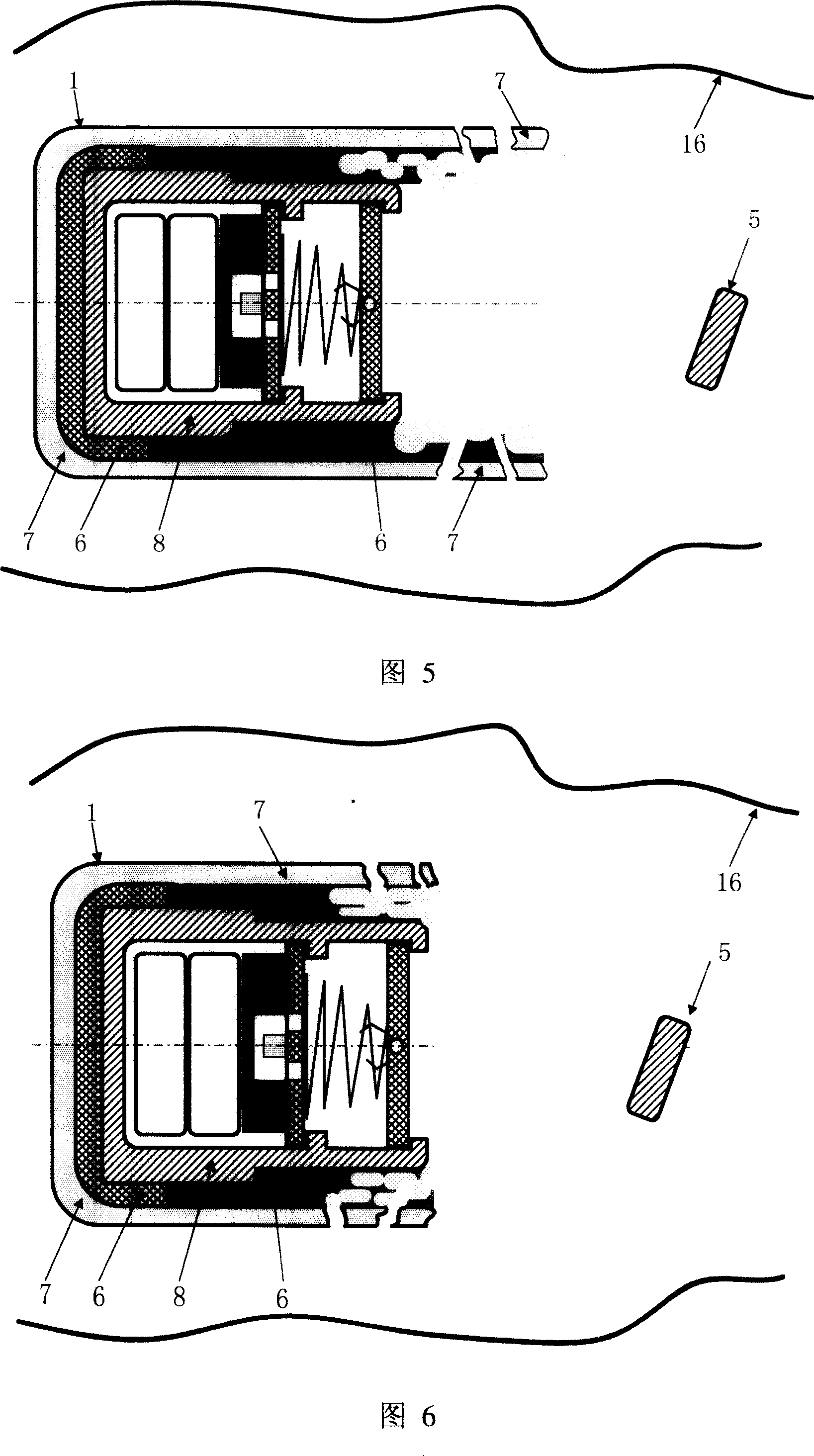
InactiveCN100998906AHigh clinical safetySimple structureMedical devicesDiagnostic recording/measuringRemote controlDrug release
A remotely controlled slow-releasing electronic capsule able to be partially degradated for releasing medicine, food or making substance in digestive tract is composed of casing, remotely controlled driver, slow-releasing channel of medicine and a medicine tablet core with a conic or cylindrical boss at its one end. Under the drive of said driver, said boss on medicine tablet can move forward to open the medicine releasing channel for slowly releasing the medicine. Said casing consists of the scaffold able to be degradated and a coated layer.
Owner:CHONGQING UNIV
Preparation method and application of cross-linked hyaluronic acid gel microspheres
ActiveCN108478875AUniform crosslinkingThe particle distribution is round and uniformPharmaceutical delivery mechanismProsthesisCross-linkTissue repair
The invention discloses a preparation method and application of cross-linked hyaluronic acid gel microspheres. The preparation method comprises the steps that a hyaluronic acid alkaline solution withthe concentration being 5-30% g / ml is prepared, inorganic dispersants are added into organic phase cyclohexane under stirring, full stirring is conducted, and thus the inorganic dispersants are uniformly dispersed. By adopting the technical scheme, the preparation method is simple, products are controllable in particle size, intact in form, low in impurity content, high in purity and highly transparent, and meanwhile, the degradation period can also be controlled within the range of 30-300 days. In the preparation method and the application of the cross-linked hyaluronic acid gel microspheres,the cross-linked hyaluronic acid gel microspheres prepared through the preparation method can be used for producing medical or prevention products and used for injection, tissue repair and tissue strength in animals and especially in humans; and because of the extremely-low impurity content, the products have good biocompatibility and degradability, and clinical safety is ensured.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY +1
Parodontium stem cell cryoprotectant and cryopreservation method thereof
InactiveCN109601527ALow costAvoid introducingDead animal preservationPeriodontal ligament stem cellsCellular viability
The invention relates to the technical field of stem cell cryopreservation, in particular to a parodontium stem cell cryoprotectant and a cryopreservation method thereof. The parodontium stem cell cryoprotectant is prepared from, 500-2000 mg / L of trehalose, 5-20vt% of acetamide, 5-15vt% of DMSO and the balance a basal culture medium. Compared with a conventional cell cryoprotectant, the parodontium stem cell cryoprotectant and the cryopreservation method thereof have the advantages that the cryoprotectant has a less damage effect on cells during cell cryopreservation, the cell viability is higher, the cryopreservation effect of the cryoprotectant is obviously superior to that of the conventional cell cryoprotectant; on one hand, the cost of the cell cryopreservation can be reduced to a certain degree, on the other hand, the introduction of heterologous matter can be avoided, and the clinical safety is higher.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Drug model explorer
InactiveUS20060161354A1Facilitate exploring resultEnhanced interactionMedical simulationLocal control/monitoringProbit modelInformation type
Owner:TRIPOS
Ornidazole injection and preparation method thereof
InactiveCN107496351AThe finished product has less impuritiesImprove stabilityAntibacterial agentsPharmaceutical delivery mechanismAlcoholPolyethylene glycol
The invention discloses an ornidazole injection and a preparation method thereof. Mixed liquid of absolute ethyl alcohol and polyethylene glycol-15-hydroxystearate serves as a solvent of the ornidazole injection, wherein the polyethylene glycol-15-hydroxystearate accounts for 6-14% in terms of the mass percentage of the injection. The use of a propylene glycol solvent can be prevented, and the preparation (the injection) is good in stability and good in clinical safety. The invention also discloses a preparation method of the injection; and the preparation process (the preparation method) is simple and easy to implement.
Owner:SHANDONG HUBBLE KISEN BIOLOGICAL TECH CO LTD
Loratadine syrup and preparation method thereof
InactiveCN109498569AUniform contentStable contentOrganic active ingredientsDispersion deliveryGlycerolSolvent
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a loratadine syrup and a preparation method thereof. The loratadine syrup, in terms of 1000ml,comprises the following components: 0.9-1.1g of loratadine, 650-750g of a sweetener, 80-120ml of propylene glycol, 40-60ml of glycerol, 0.9-1.1g of ethylenediaminetetraacetic acid disodium salt, 15-25g of an acidity regulator, 2-4g of a preservative, and water added until the total amount is 1000ml. The loratadine syrup prepared by the method provided by the invention can meet a requirement of uniform dispersion of bulk drugs; the effective components of the loratadine syrup can be uniformly dispersed in the syrup, the contents of the upper layer, the middle layer and the lower layer of the syrup are uniform, and the quality is uniform; and the loratadine syrup produced by the preparation method provided by the invention does not contain an ethanol solvent, has no ethanol residue, and hasgood clinical safety. The loratadine syrup prepared by the method provided by the invention has stable quality and a shelf life of more than 36 months.
Owner:HUBEI KANGYUAN PHARMA
Application of group of cobra neurotoxin molecules having high affinity with nicotinic acetylcholine receptors for rapid acting on pain easing
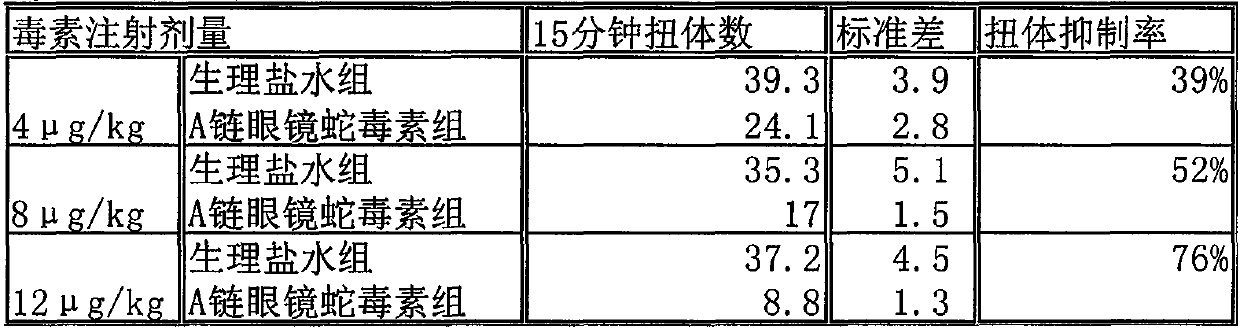
The cobra neurotoxin is capable of binding to nicotinic acetylcholine receptors after nerve synapses to block neuronal caudal ion flow to realize analgesic effect, however, the product on the market takes 2 hours to be effective and the curative effect is unstable, which cannot meet clinical requirement. The neurotoxins need to pass a blood cerebral barrier in the brain to play an analgesic effect, and the product on the market has no clear components, including various molecular weight proteins, so the speed for permeating the blood cerebral barrier is slow; and at the same time, affinity with the nicotinic acetylcholine receptors is also inconsistent. The application screens a group of neurotoxin molecular monomers having high affinity with the nicotinic acetylcholine receptor, can quickly pass the blood cerebral barrier, and has analgesic effect and stable therapeutic effect in 30 minutes. The neurotoxin molecular monomer overcomes the defects that a mixture cannot specify which neurotoxin is present, the protein primary structure is not existed, the quality cannot be precisely controlled; according to the invention, the quality is controlled, clinical safety and effectiveness are better guaranteed.
Owner:祁展楷
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com