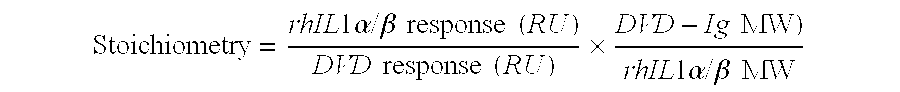Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
14085results about "Macromolecular non-active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
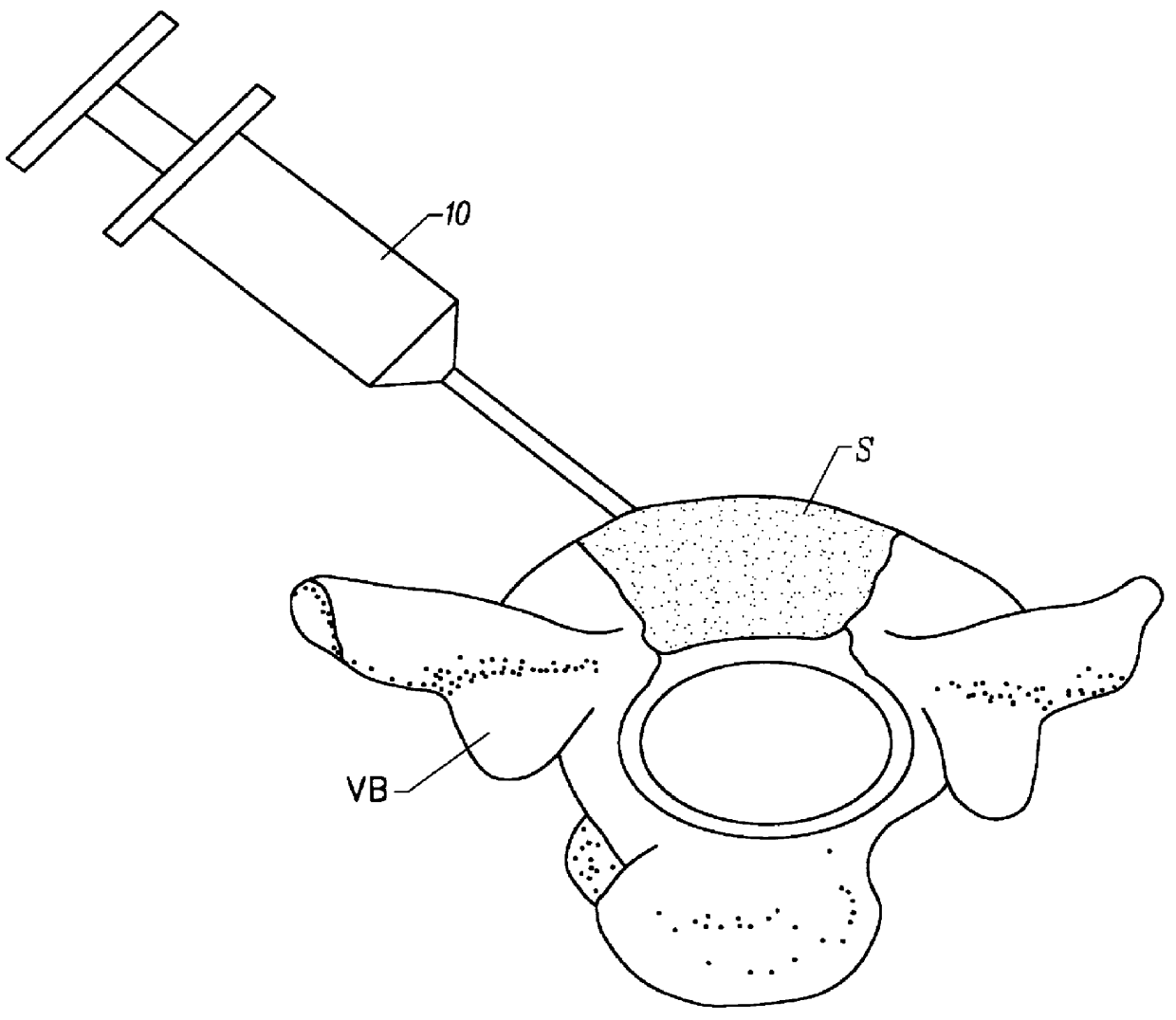
Drug administration method
InactiveUS7427607B2Reduce weightPrevention of the adhesion of an organBiocidePowder deliveryBiopolymerDrug administration
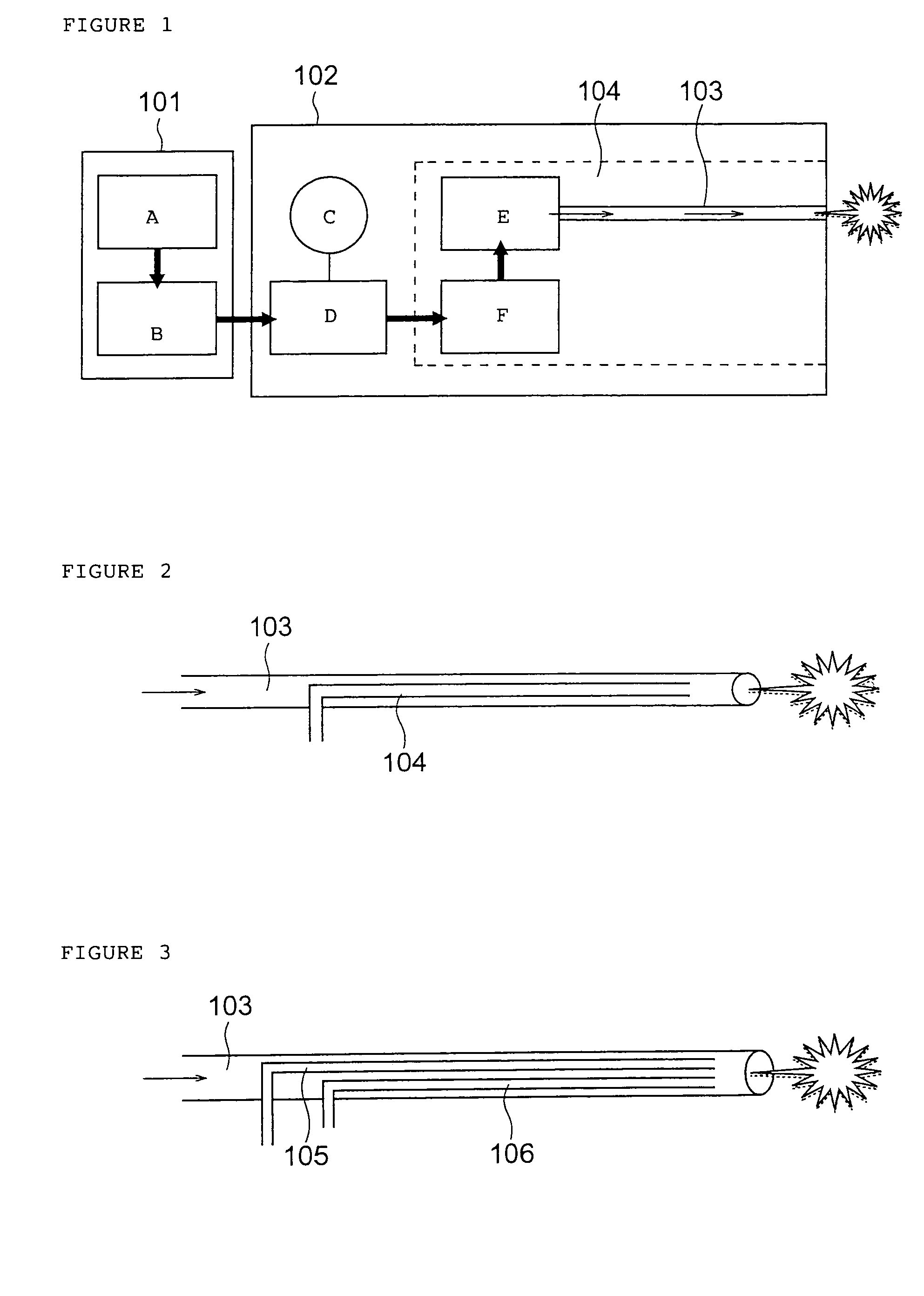
A method of administering a drug whereby a fine drug powder can be accurately administered to a target site (in particular, a target site in the body cavity) via fluidization and spraying with a gas by using a micro tube. Concerning the administration mode, in particular, the drug alone or a biopolymer is administered or the biopolymer is employed as a carrier in the above method. More specifically speaking, a method of administering a fine drug powder which comprises finely milling one or more types fine particles of the drug and / or the biopolymer, blending them each other, fluidizing the blend with a gas, then transporting the fluidized matter in a micro tube by the gas stream and spraying the fine drug powder from the tip of the micro tube toward the target site. Further, an administration method which comprises concentrically providing a capillary tube in the micro tube, supplying an aqueous solution of the drug and / or the biopolymer from the capillary tube into the gas stream and then mixing it with other fine particles of the drug and / or the biopolymer under transportation by the gas.
Owner:NEXT21 KK
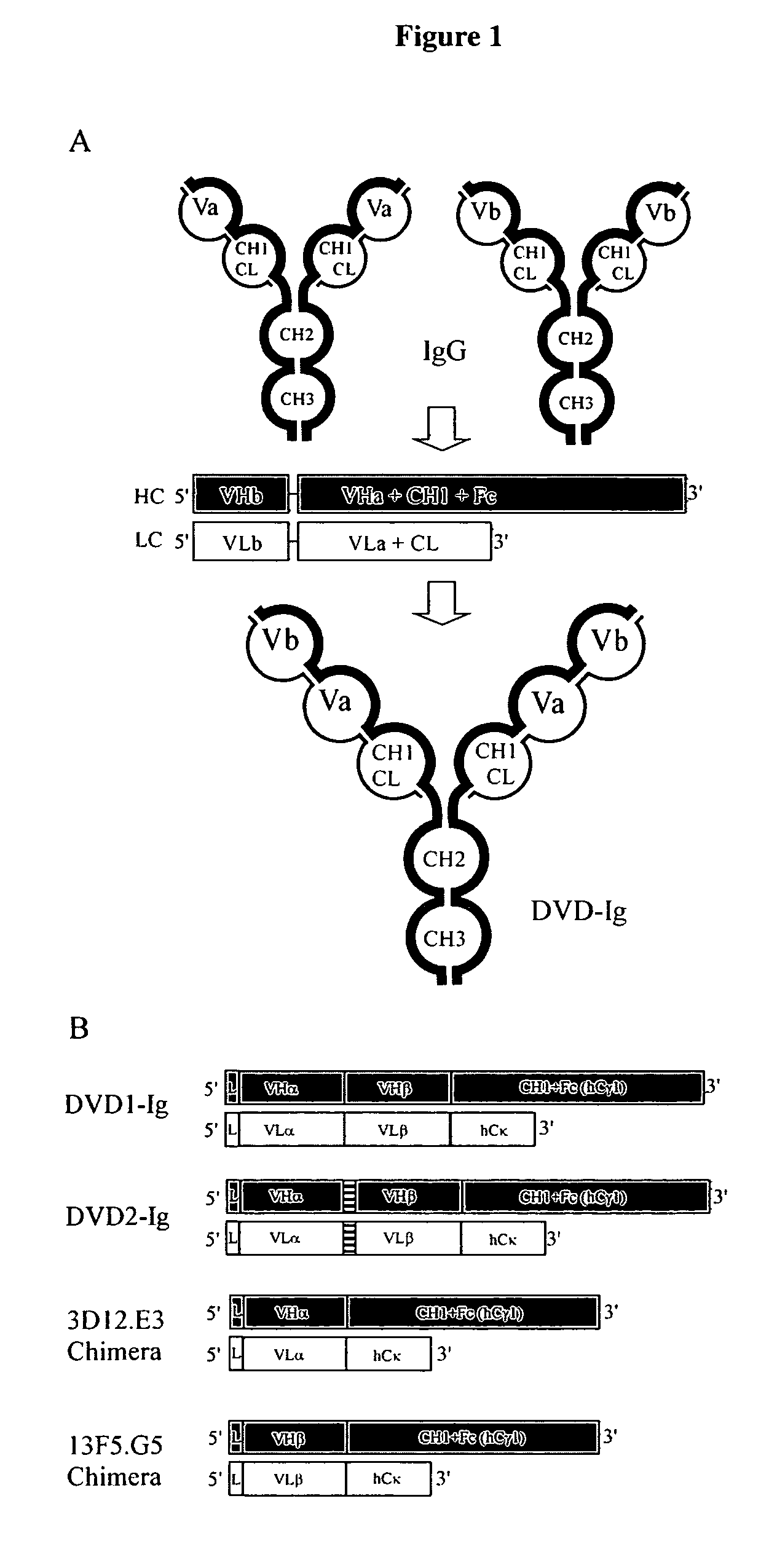
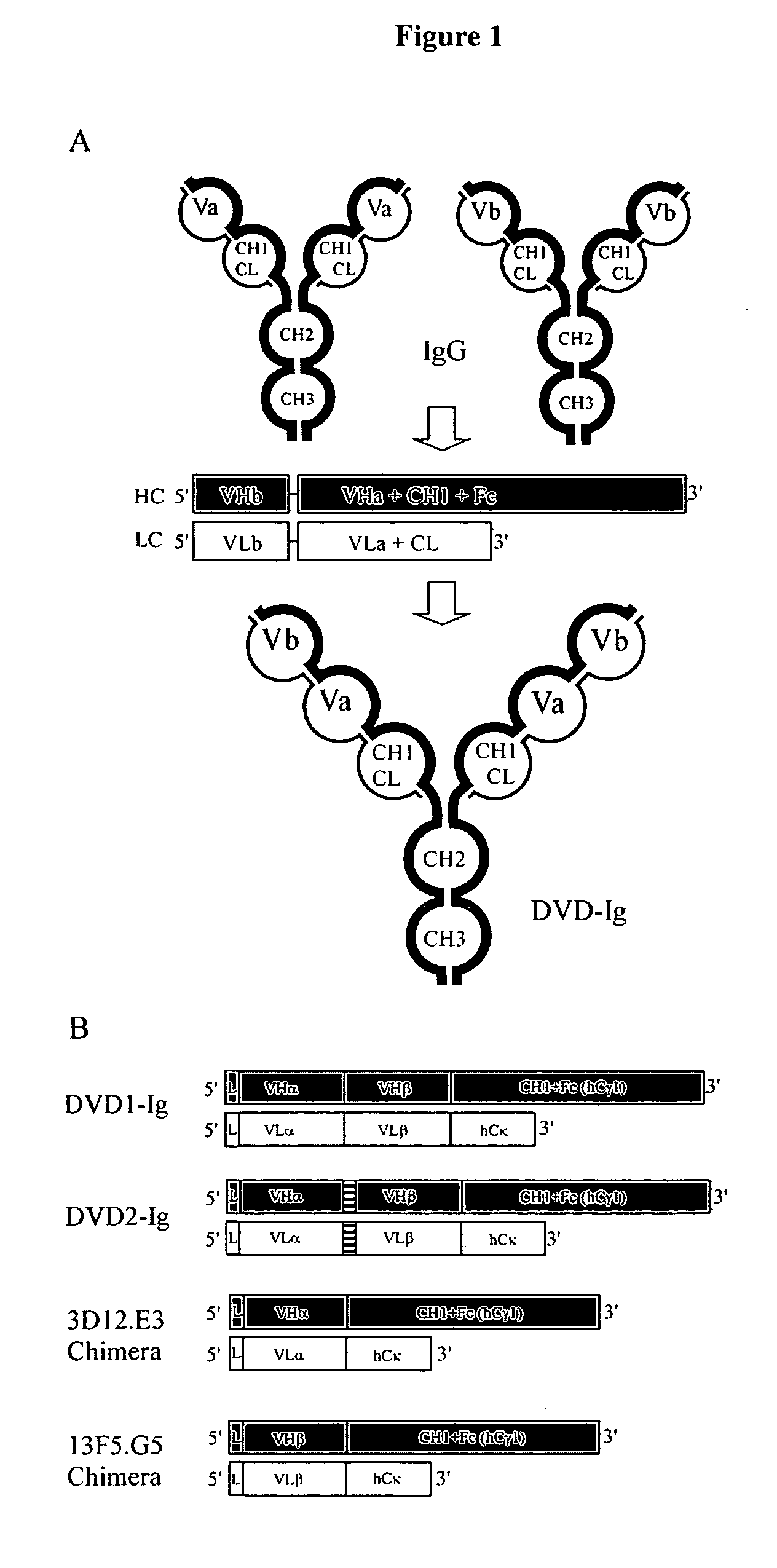
Dual variable domain immunoglobulin and uses thereof
Owner:ABBVIE INC
Dual variable domain immunoglobulin and uses thereof
Owner:ABBVIE INC
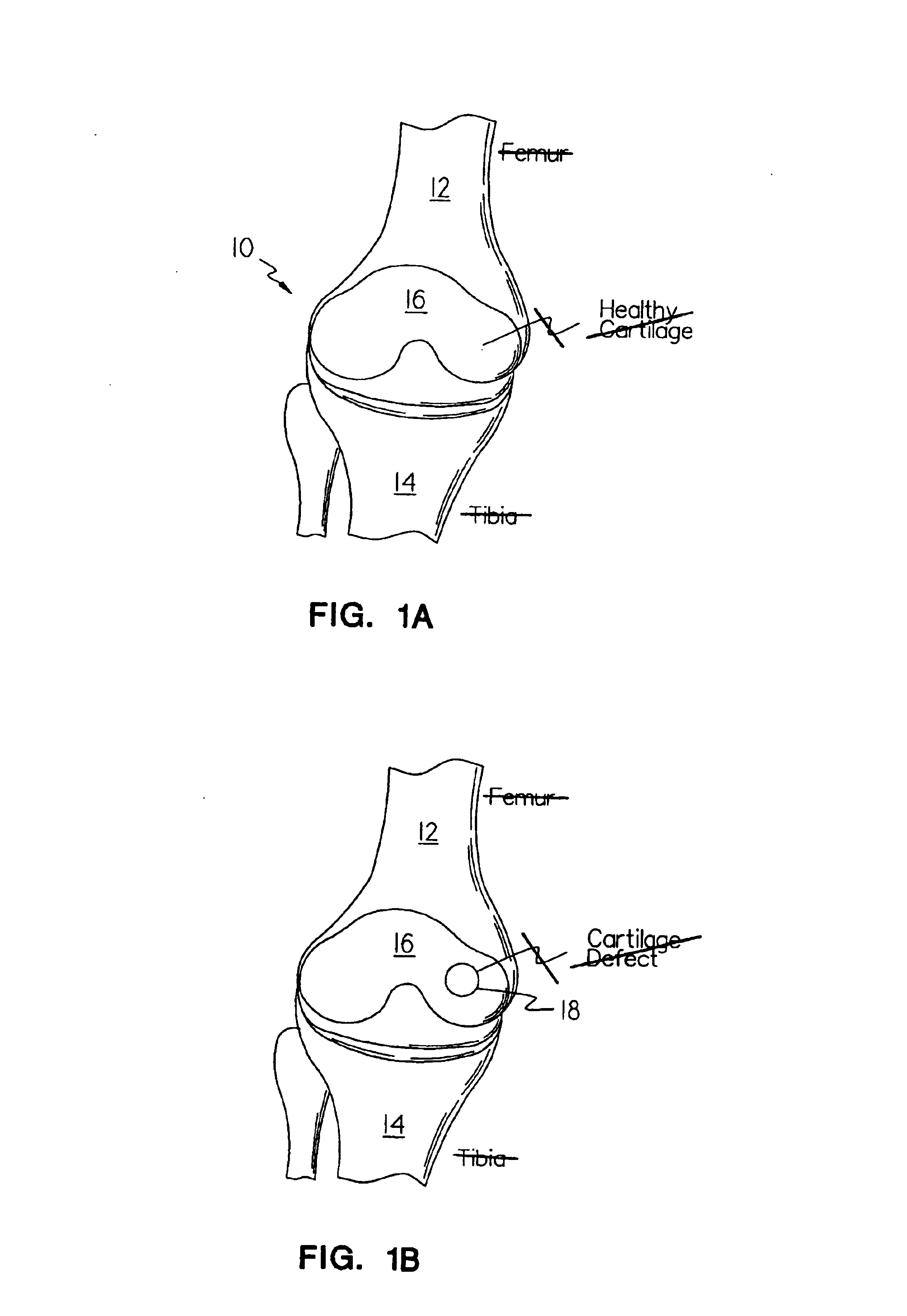
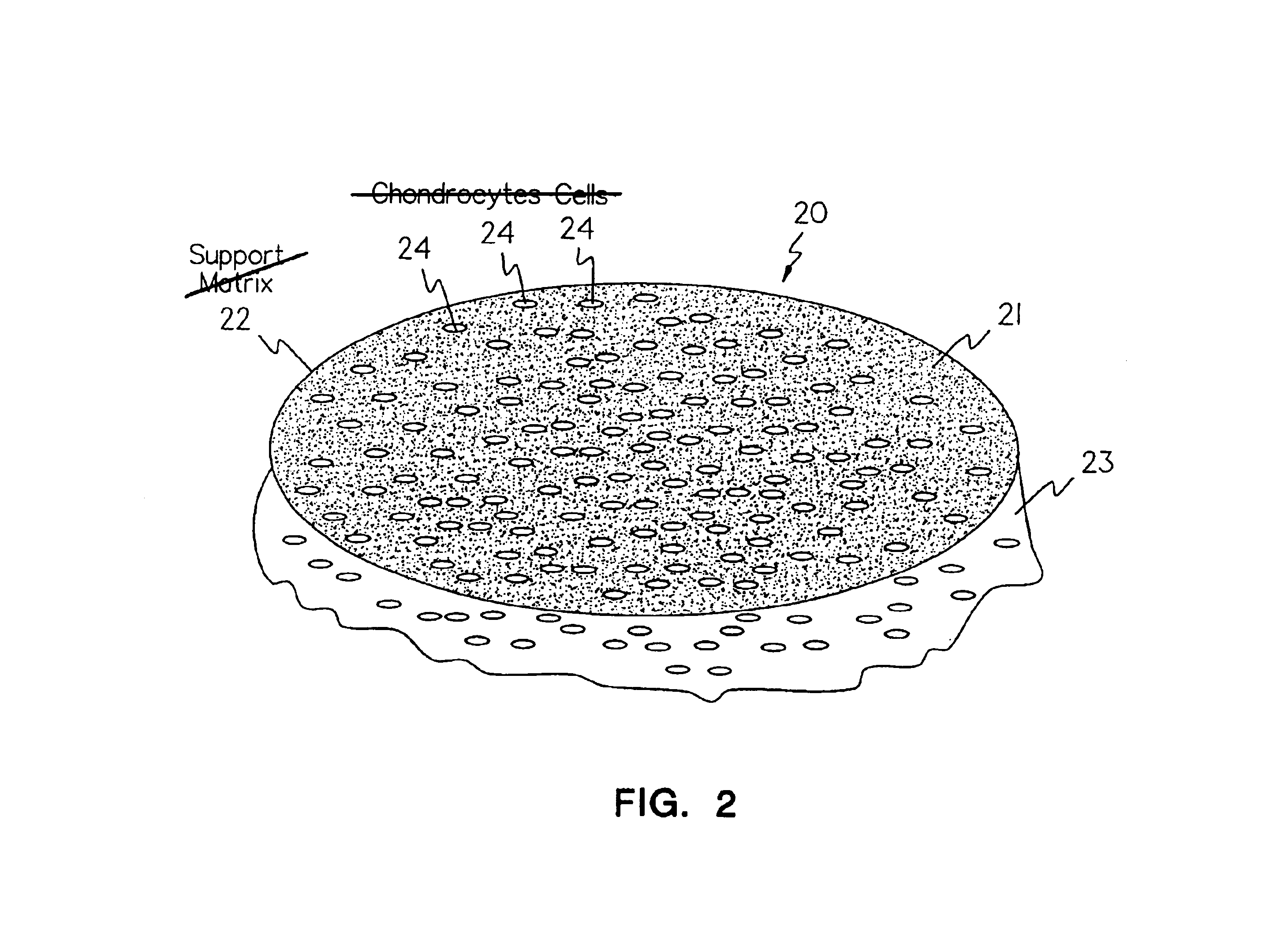
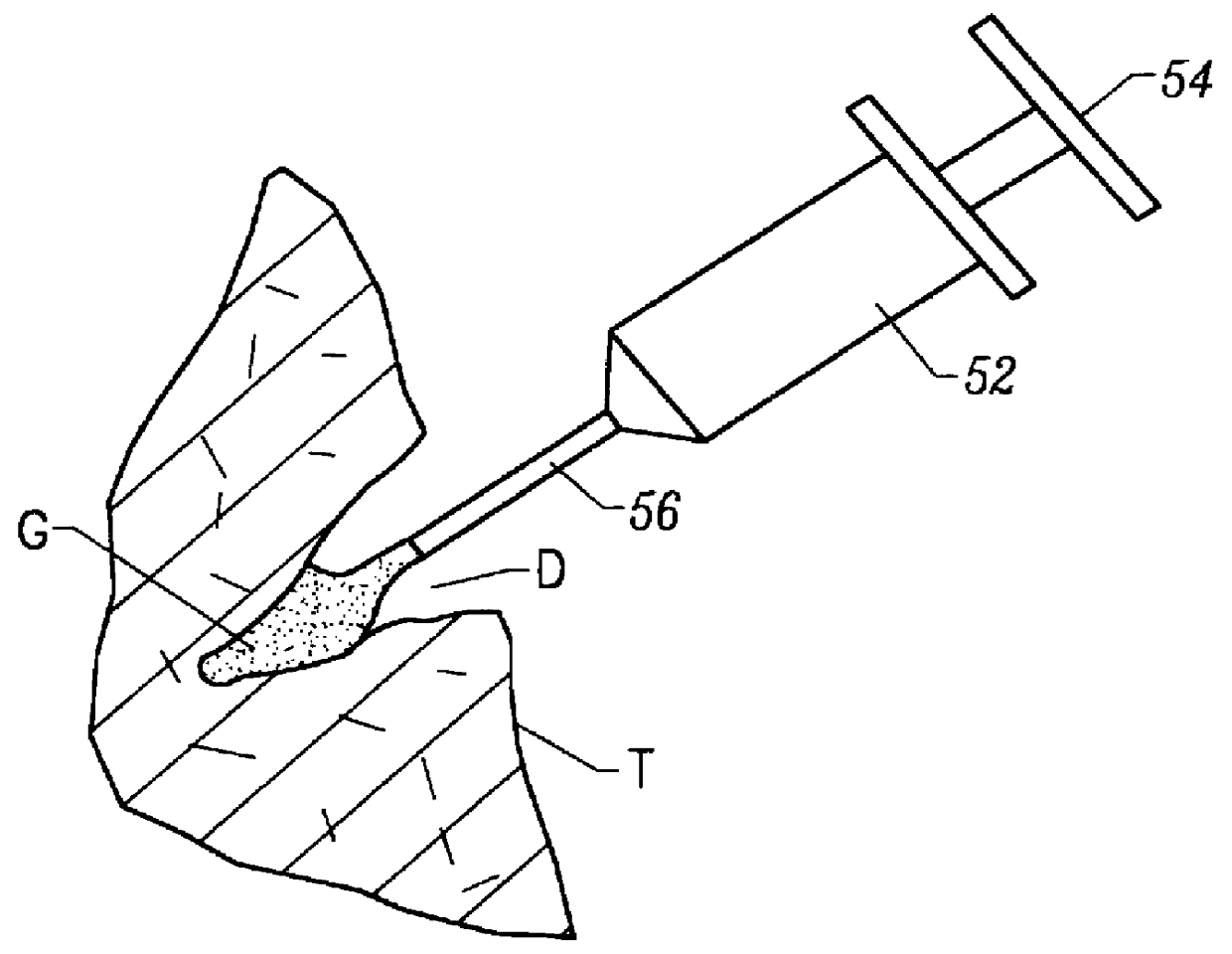
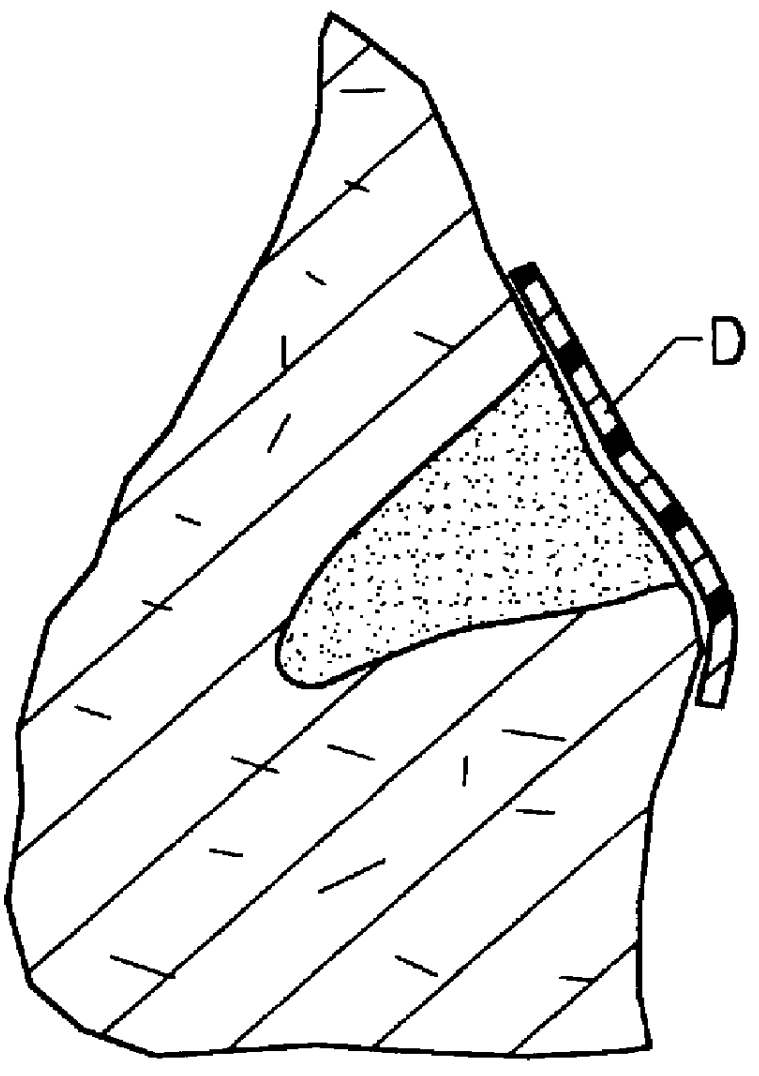
Methods, instruments and materials for chondrocyte cell transplantation
InactiveUS6866668B2Effective treatmentSuture equipmentsSurgical adhesivesSupport matrixTreated animal
A method for the effective treatment of articulating joint surface cartilage in an animal by the transplantation of an implantable article including chondrocyte cells retained to an absorbable support matrix. An instrument for placing and manipulating the implantable article at the site of implantation, and a retention device for securing the implantable article to the site of implantation. An implantable article for cartilage repair in an animal, the implantable article including chondrocyte cells retained on an absorbable support matrix, and a method of making same. An article comprising an absorbable flexible support matrix for living cells grown and adhered thereto.
Owner:VERIGEN TRANSPLANTATION SERVICE INT
Biocompatible crosslinked polymers
InactiveUS7009034B2Improve performanceImprove visibilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryWound dressingPost operative
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
Fragmented polymeric compositions and methods for their use
InactiveUS6063061AImprove liquidityEasy to controlSurgical adhesivesSurgical drugsCross-linkBreast implant
Molecular cross-linked gels comprise a variety of biologic and non-biologic polymers, such as proteins, polysaccharides, and synthetic polymers. Such molecular gels may be applied to target sites in a patient's body by extruding the gel through an orifice at the target site. Alternatively, the gels may be mechanically disrupted and used in implantable articles, such as breast implants. When used in vivo, the compositions are useful for inhibiting post-surgical spinal and other tissue adhesions, for filling tissue divots, tissue tracts, body cavities, surgical defects, and the like.
Owner:BAXTER INT INC +1
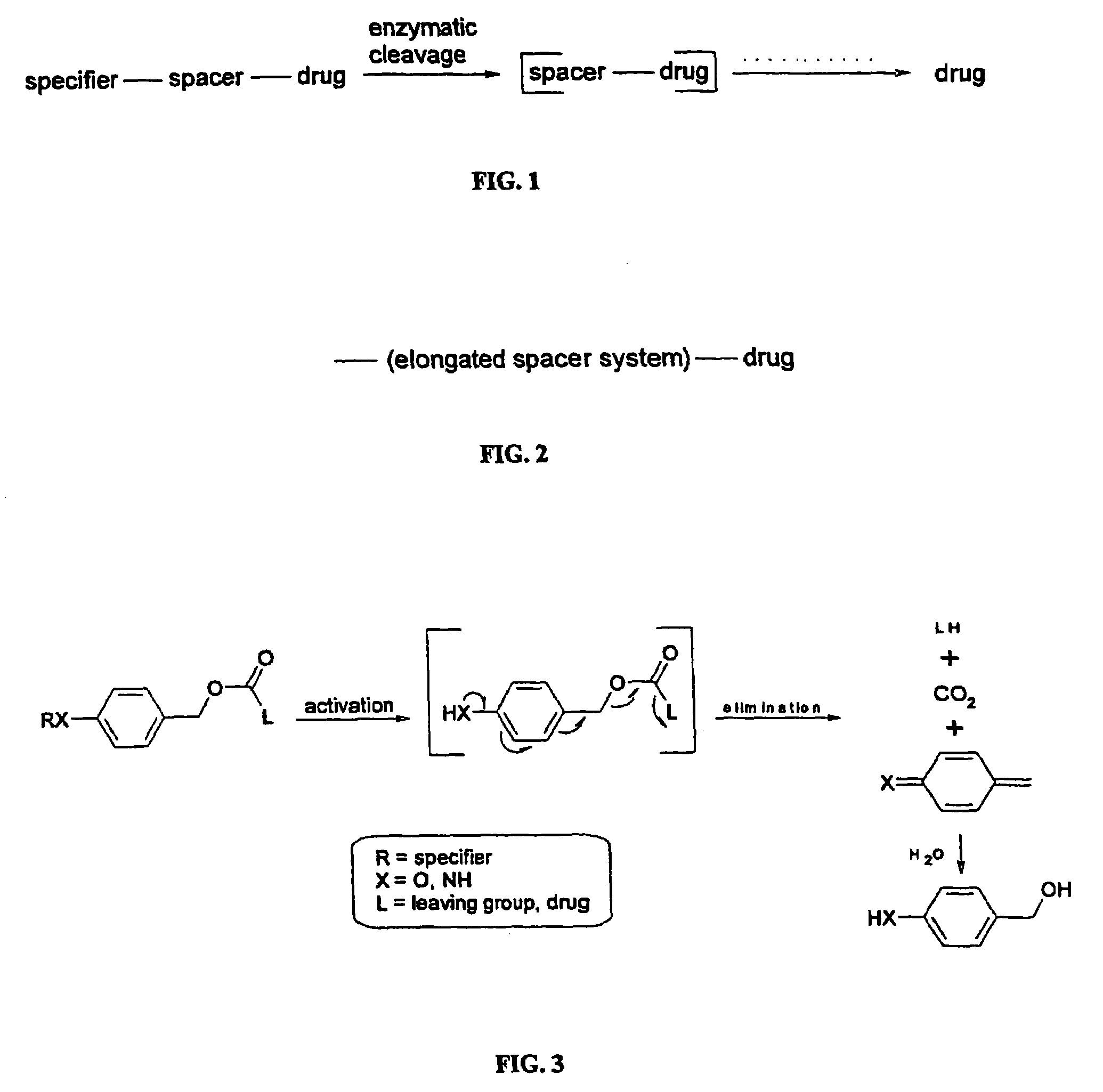
Elongated and multiple spacers in activatible prodrugs
InactiveUS7223837B2Improved kineticsFacilitate enzymatic cleavageAntibacterial agentsOrganic active ingredientsTumor cellsChemistry
This invention is directed to prodrugs that can be activated at the preferred site of action in order to selectively deliver the corresponding therapeutic parent drugs to target cells or to the target site. This invention will therefore primarily but not exclusively relate to tumor cells as target cells. More specifically the prodrugs are compounds of the formula V—(W)k—(X)l—A—Z, wherein: V is a specifier; (W)k—(X)l—A is an elongated self-elimination spacer system; W and X are each a 1,(4+2n) electronic cascade spacer, being the same or different; A is either a spacer group of formula (Y)m wherein: Y is a 1,(4+2n) electronic cascade spacer, or a group of formula U being a cyclization elimination spacer; Z is a therapeutic drug; k, l and m are integers from 0 (included) to 5 (included); n is an integer of 0 (included) to 10 (included), with the provisos that: —when A is (Y)m: k+l+m≧1, and if k+l+m=1; —when A is U: k+l≧1.
Owner:BYONDIS BV
Lipid compositions
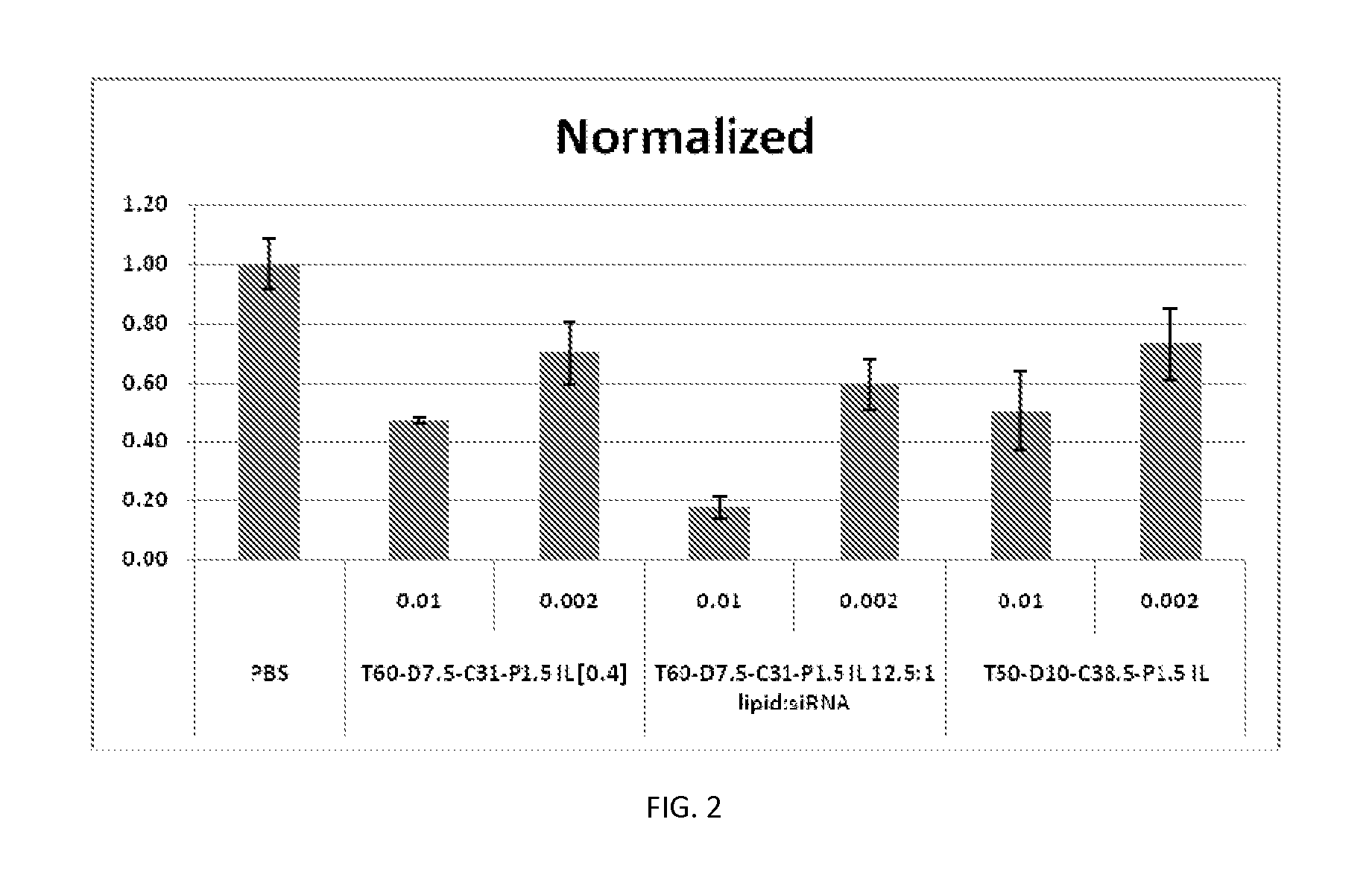
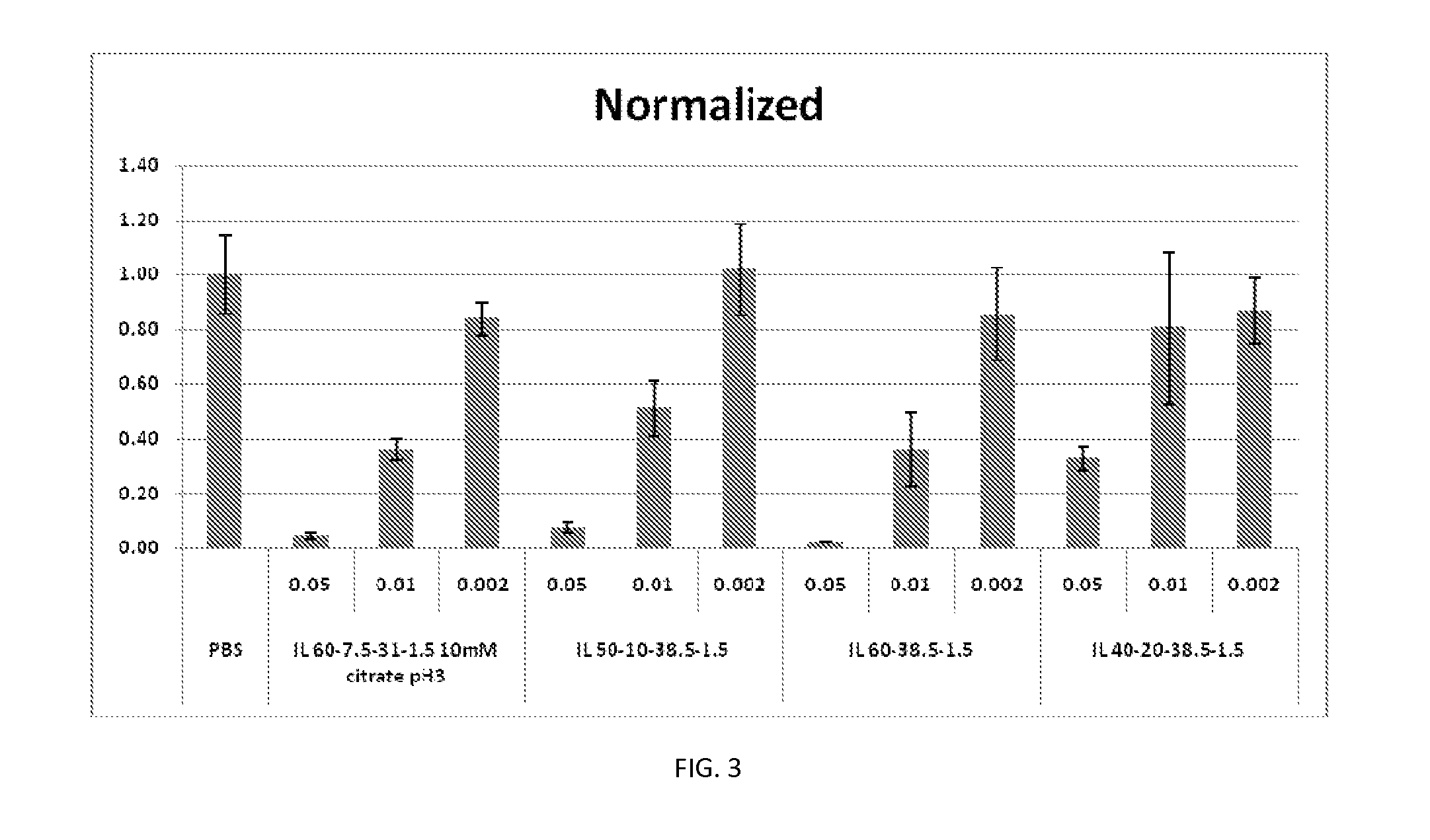
Disclosed herein are lipid compositions comprising a cationic lipid of formula (I), a neutral lipid, a sterol and a PEG or PEG-modified lipid, wherein formula (I) is (F). Also disclosed are methods of producing the cationic lipid of formula (I).
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Intervertebral disc treatment devices and methods
InactiveUS7318840B2Promote tissue growthFunction increasePeptide/protein ingredientsBone implantFibrous bodyIntervertebral disk
Intervertebral disc treatment devices and methods are provided. An intervertebral disc treatment device includes a fibrous body sized for introduction into a disc cavity of a damaged disc wherein the body incorporates an effective amount of a tissue growth factor. Intervertebral disc treatment apparatuses are also described that include such a disc treatment device in combination with a delivery apparatus for retaining and selectively releasing the device into the disc cavity. Methods for treatment include providing a disc treatment device as described above and inserting the device into an opening in an annulus fibrous and into the disc cavity. The methods further include stimulating tissue growth within the disc cavity of the intervertebral disc.
Owner:SDGI HLDG
Protein formulations and methods of making same
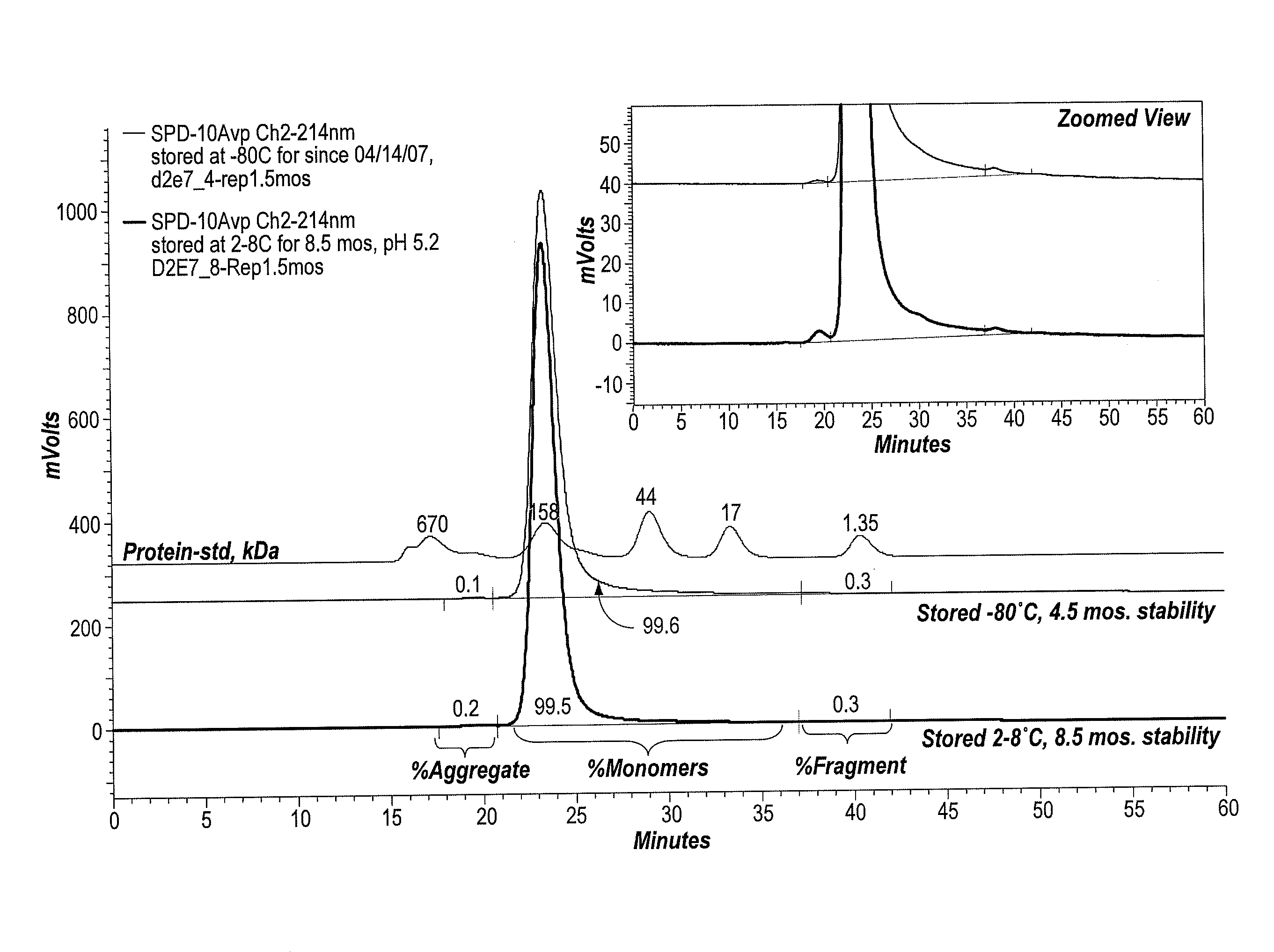
ActiveUS20090291062A1Improve consistencyAccurate concentrationPeptide/protein ingredientsAntipyreticOsmolar ConcentrationExcipient
The invention provides an aqueous formulation comprising water and a protein, and methods of making the same. The aqueous formulation of the invention may be a high protein formulation and / or may have low levels of conductivity resulting from the low levels of ionic excipients. Also included in the invention are formulations comprising water and proteins having low osmolality.
Owner:ABBVIE BIOTECHNOLOGY LTD
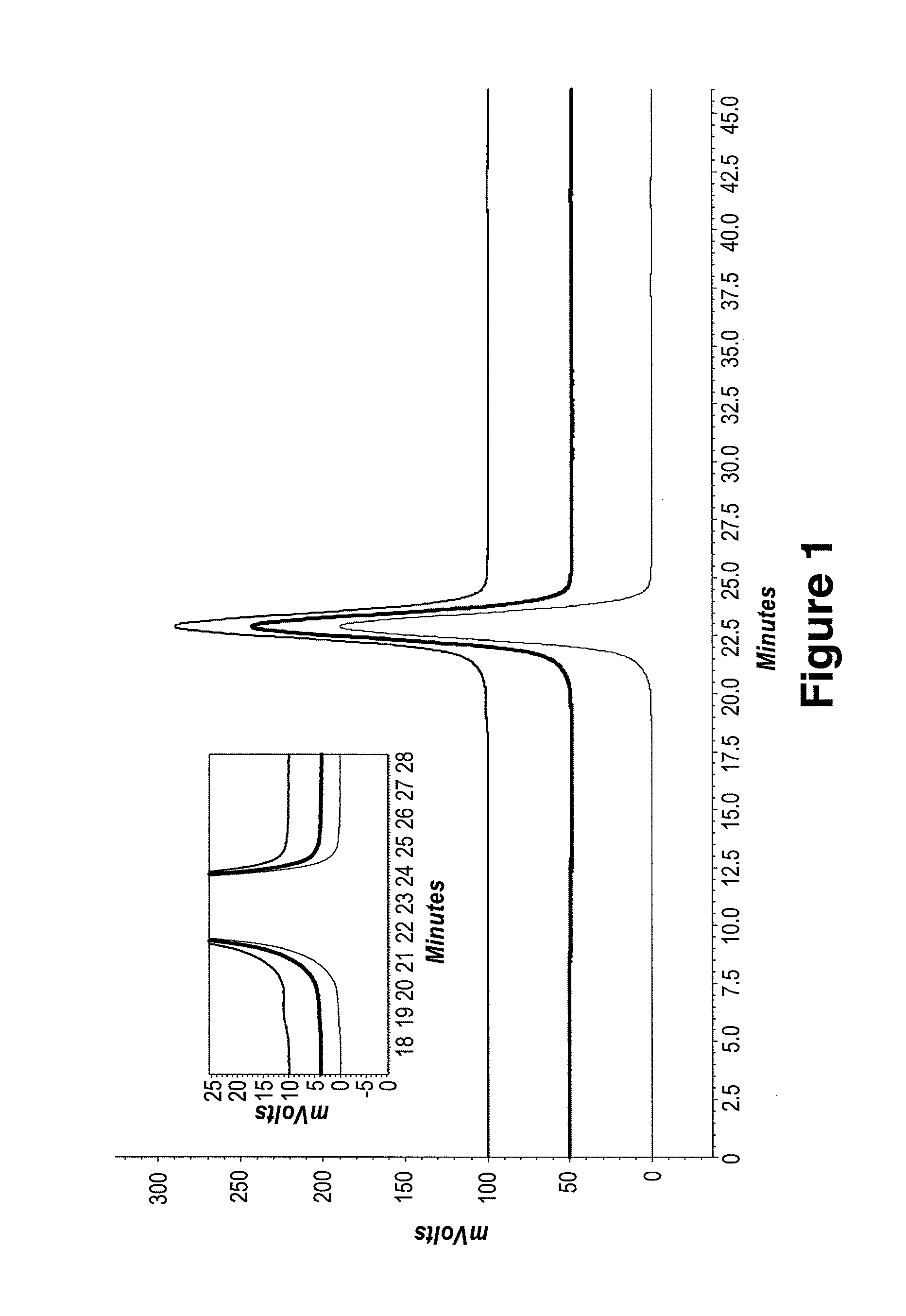
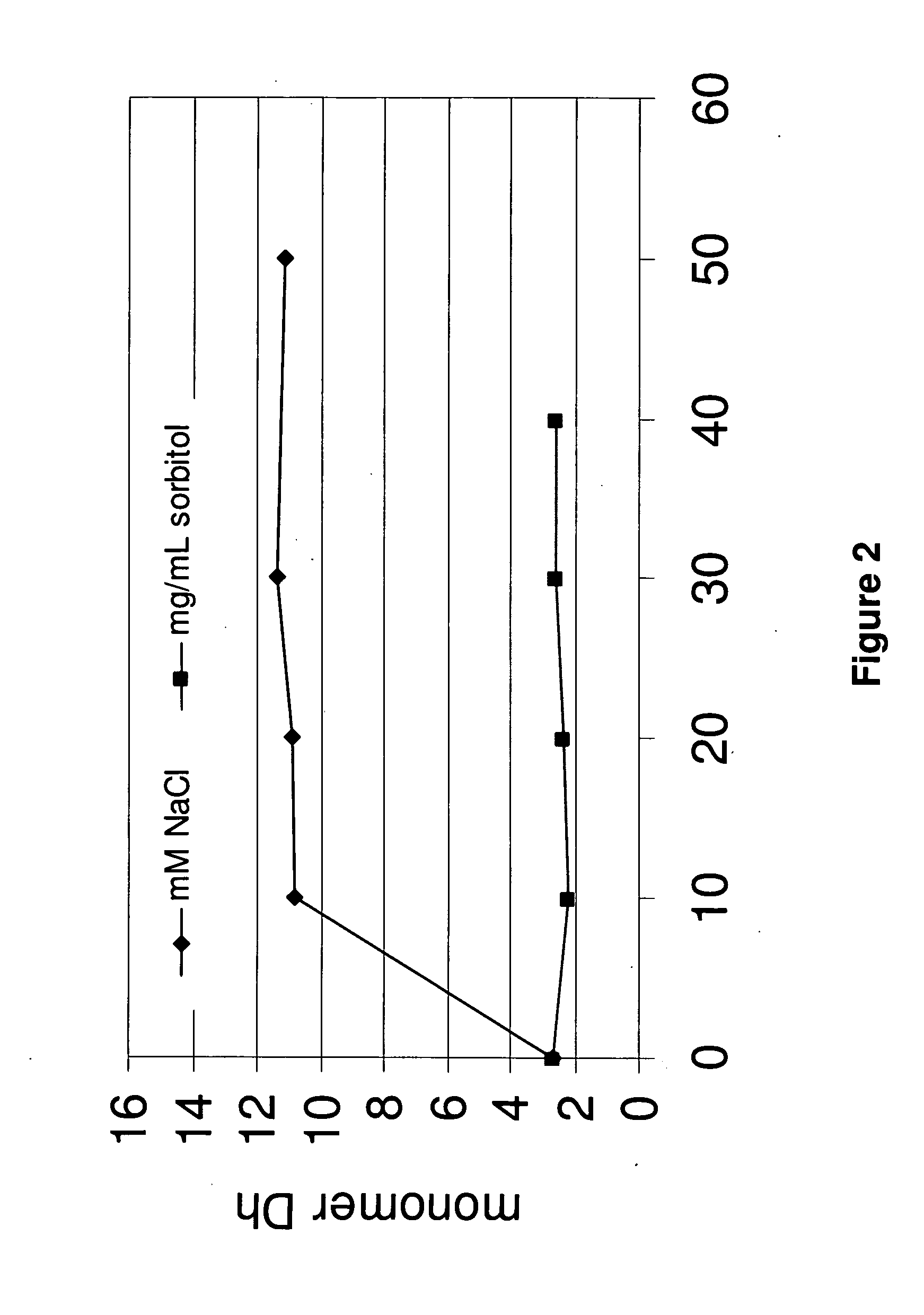
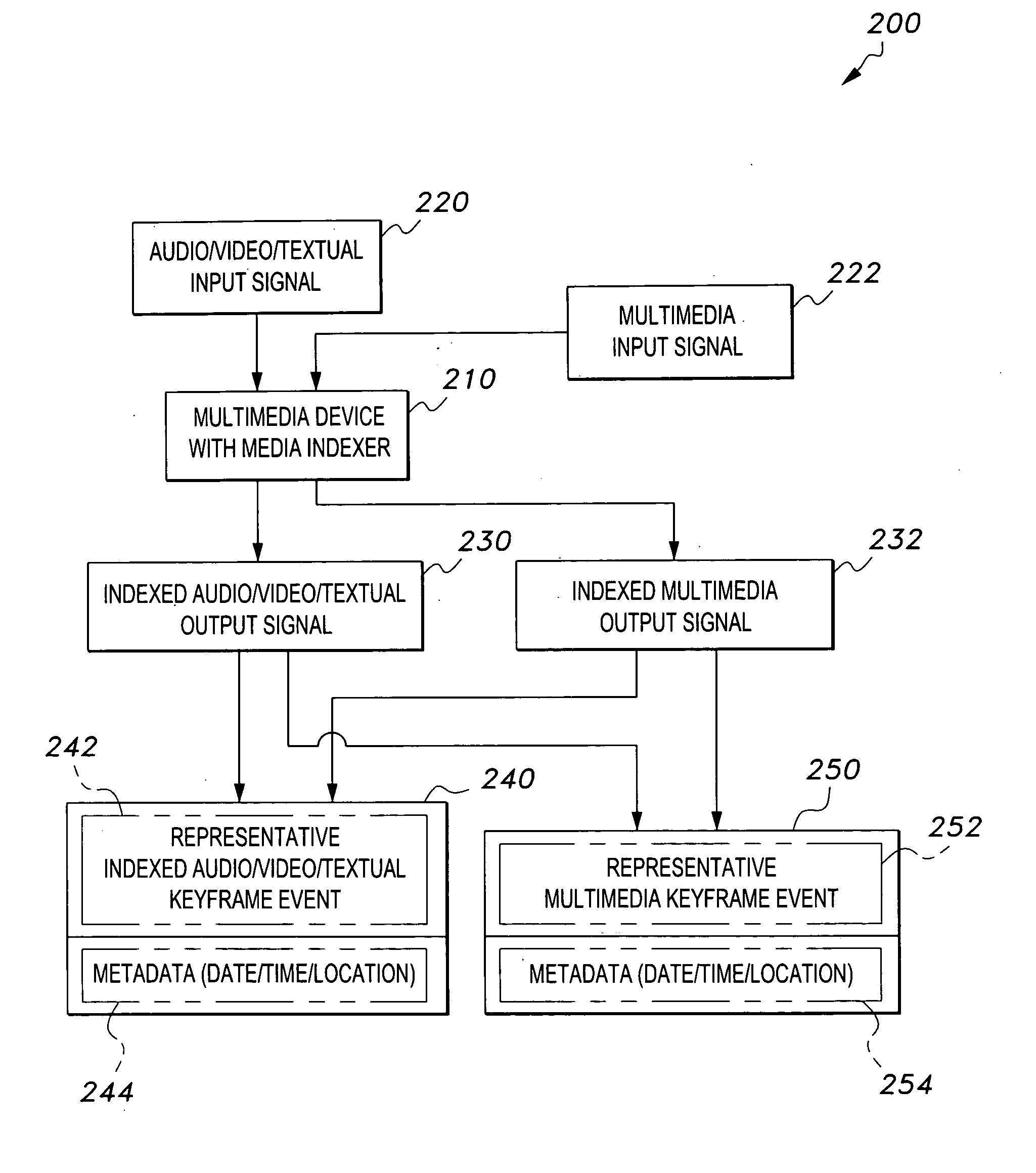
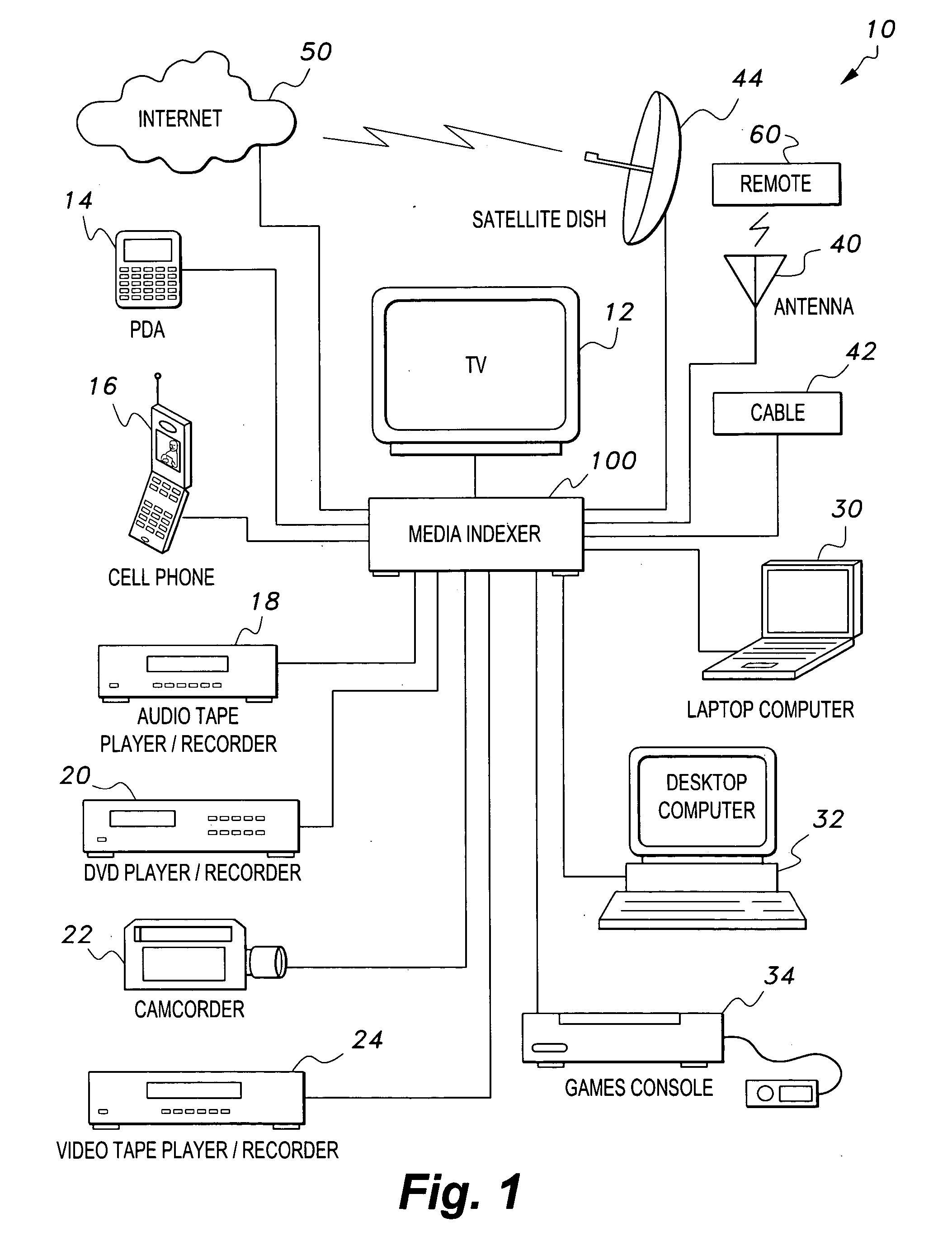
Media indexer
InactiveUS20050033758A1Inexpensive and dependable and fully effectiveTelevision system detailsPeptide/protein ingredientsData operationsFile sharing
A media indexer method, a media indexer, and / or a media indexer computer useable medium. The media indexer includes a central processor and a memory carrying thereon media indexer software which carries out steps including receiving a media signal, identifying keyframes of the media signal, establishing metadata for each identified keyframe, tagging each identified keyframe with metadata established for the associated keyframe, and outputting the media signal in a form unchanged from the received media signal, and / or a form including identified keyframes of the received media signal, each identified keyframe including a representative media keyframe event with metadata associated with the corresponding media keyframe event. The media indexer can generate parallel index signals that are synchronized to the time rate of the received media signal, and can input and output data using standard compatible file formats for file sharing and data manipulations with other compatible files and software.
Owner:BAXTER BRENT A
Compositions and methods of delivery of pharmacological agents
InactiveUS20050004002A1Reducing one or more side effectsInhibiting oxidation in the pharmaceutical compositionAntibacterial agentsOrganic active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serum albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
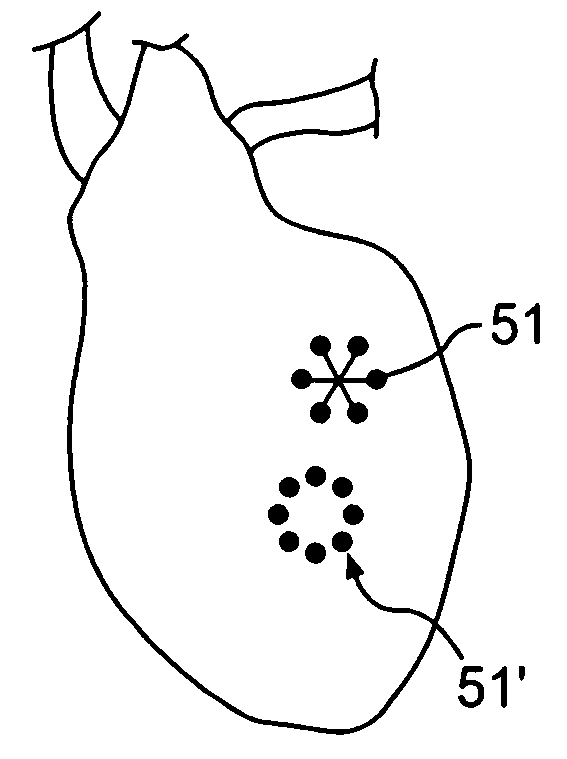
Prevention of myocardial infarction induced ventricular expansion and remodeling
ActiveUS20050080402A1Prevent further deteriorationInhibit swellingSuture equipmentsPowder deliveryCardiac muscleTherapeutic treatment
A method for direct therapeutic treatment of myocardial tissue in a localized region of a heart having a pathological condition. The method includes identifying a target region of the myocardium and applying material directly and substantially only to at least a portion of the myocardial tissue of the target region. The material applied results in a physically modification the mechanical properties, including stiffness, of said tissue. Various devices and modes of practicing the method are disclosed for stiffening, restraining and constraining myocardial tissue for the treatment of conditions including myocardial infarction or mitral valve regurgitation.
Owner:MYOMEND
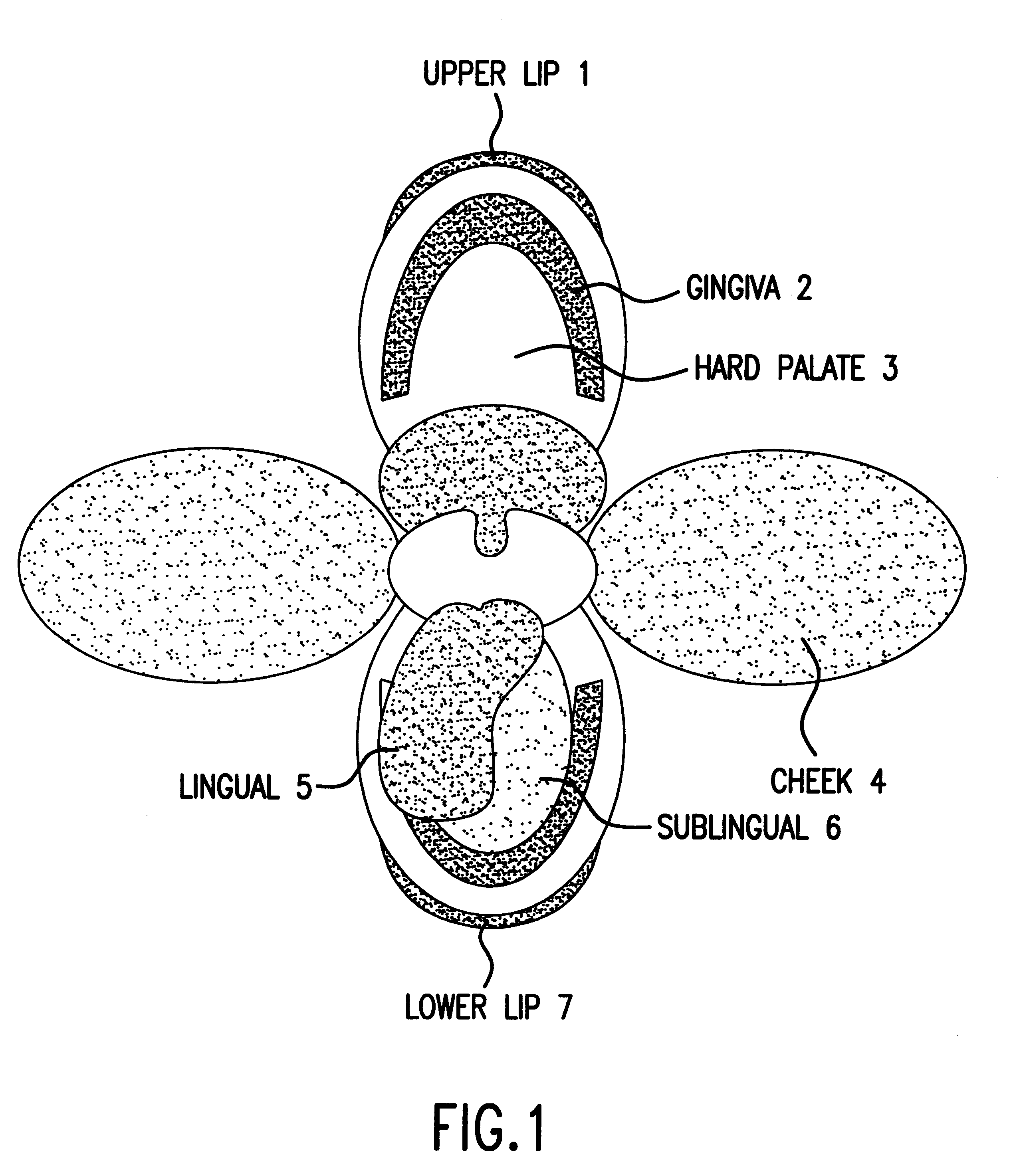
Compositions and methods for mucosal delivery
Mucosal surface-coat-forming film dosage units containing a water-soluble hydrocolloid, an effective dose of an active agent and a mucosal adhesion enhancer; wherein the active agent is encapsulated within a polymer which is chemically or physically distinct from the hydocolloid; wherein the mucosal adhesion enhancer is a starch graft copolymer; wherein the film exhibits a dry tack value of less than 3.5 g, a wet tack of greater than 35 g, a gelation temperature that is greater than 70° C. for a 2% polymer solution, a dry film thickness of less than 20 mil, a water content of 0.5 to 10%, a tensile strength greater than 1500 psi, a modulus in the range of 35,000 to 300,000 psi, a % elongation of less than 20%, a tear probagation resistance of 0.001 to 1 N, and a dissolution time in the range of 1 to 600 seconds upon application to an oral mucosal surface.
Owner:THALLIUM HLDG CO LLC
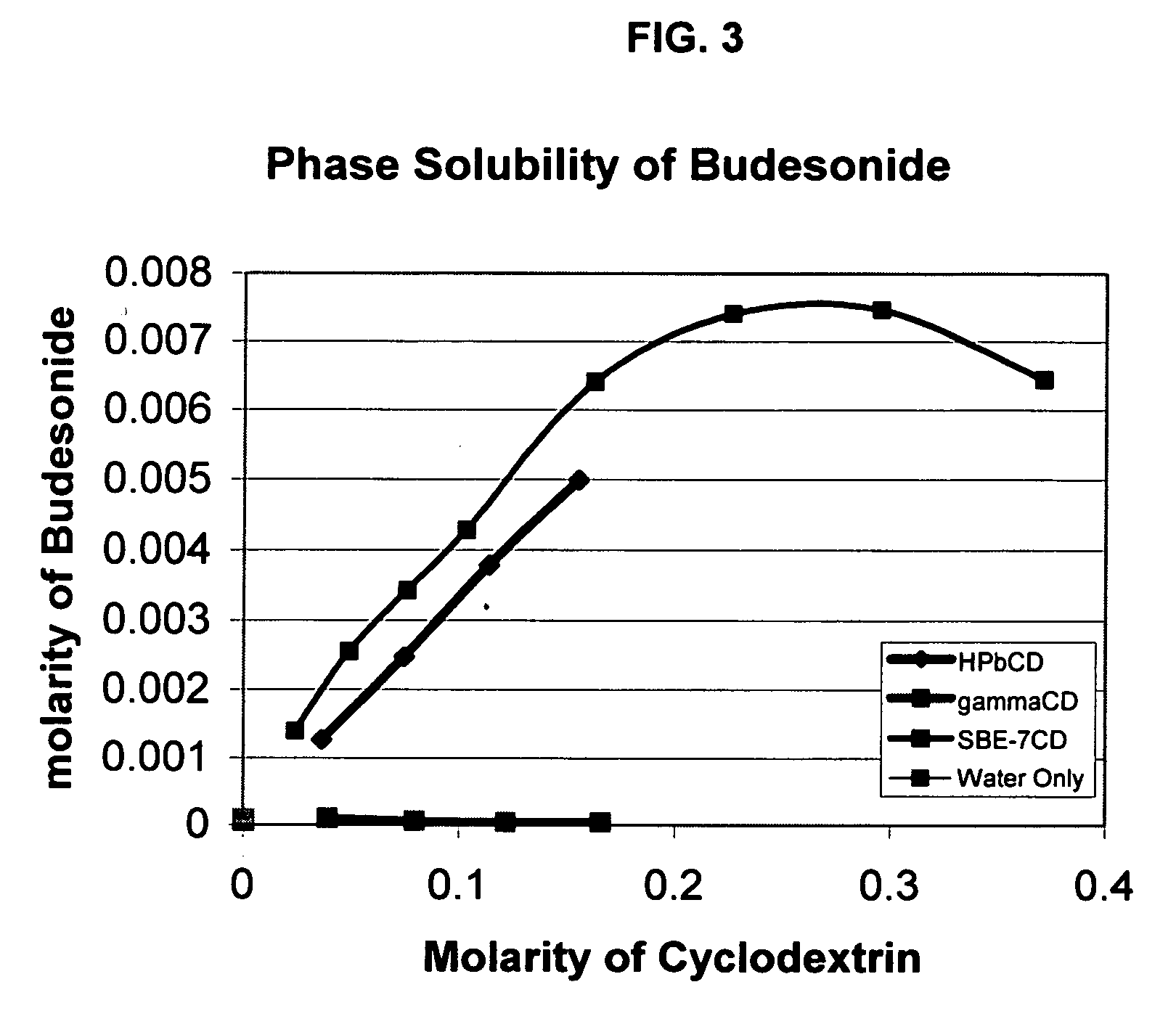
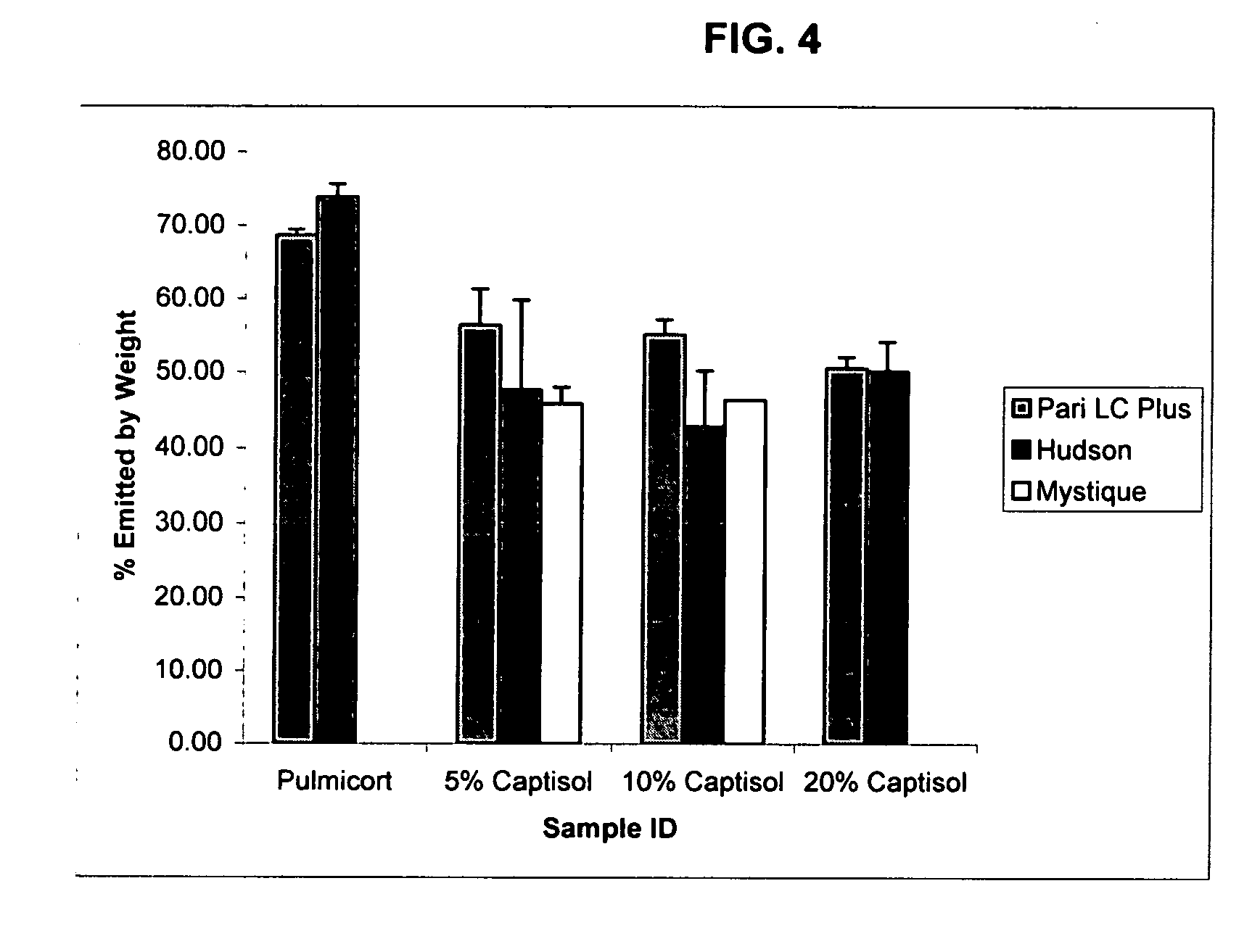
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Poly(amino acid) targeting moieties
The present invention generally relates to polymers and macromolecules, in particular, to polymers useful in particles such as nanoparticles. One aspect of the invention is directed to a method of developing nanoparticles with desired properties. In one set of embodiments, the method includes producing libraries of nanoparticles having highly controlled properties, which can be formed by mixing together two or more macromolecules in different ratios. One or more of the macromolecules may be a polymeric conjugate of a moiety to a biocompatible polymer. In some cases, the nanoparticle may contain a drug. Other aspects of the invention are directed to methods using nanoparticle libraries.
Owner:THE BRIGHAM & WOMENS HOSPITAL INC +1
Albumin fusion proteins
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disordrs or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
Combinations and modes of administration of therapeutic agents and combination therapy
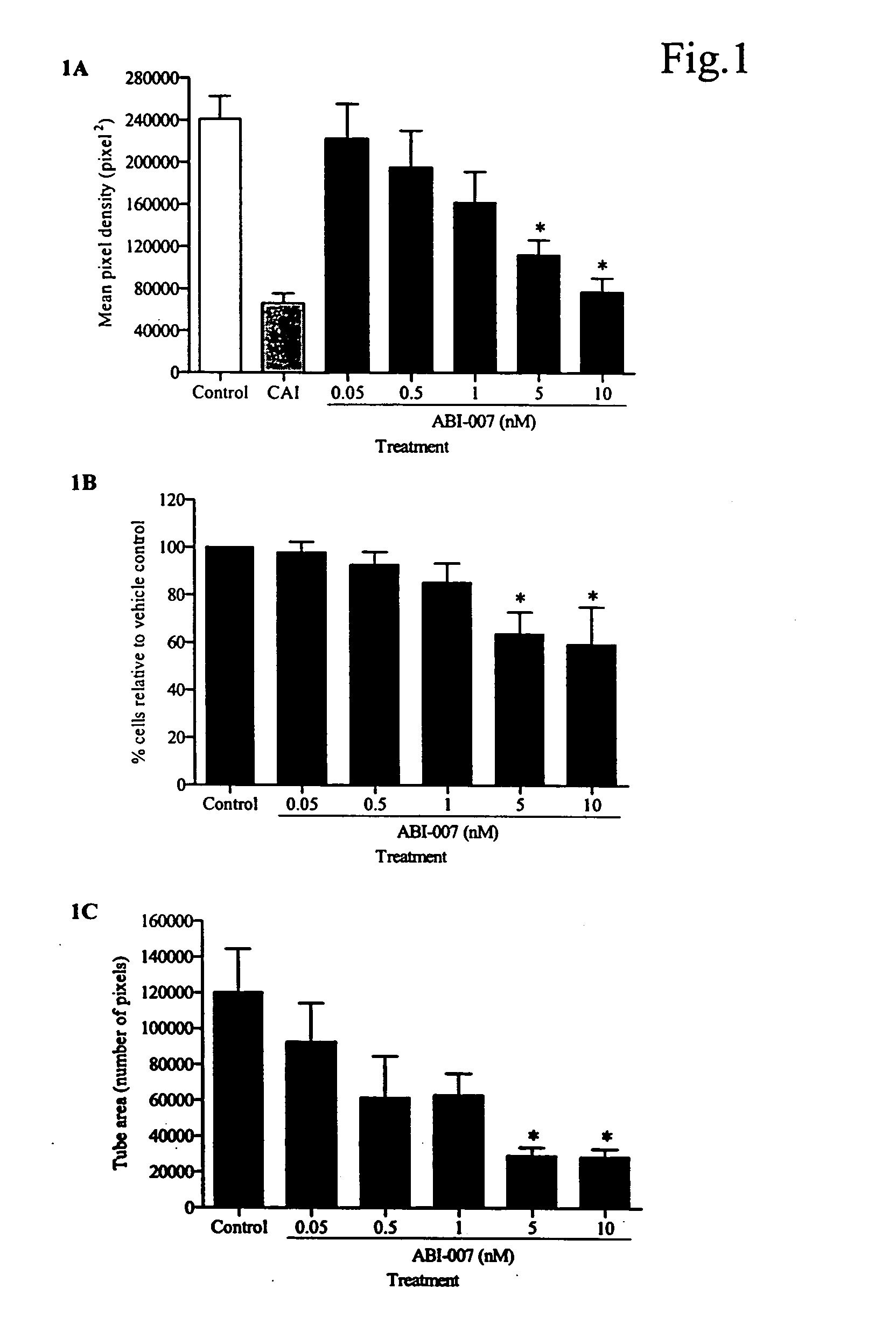
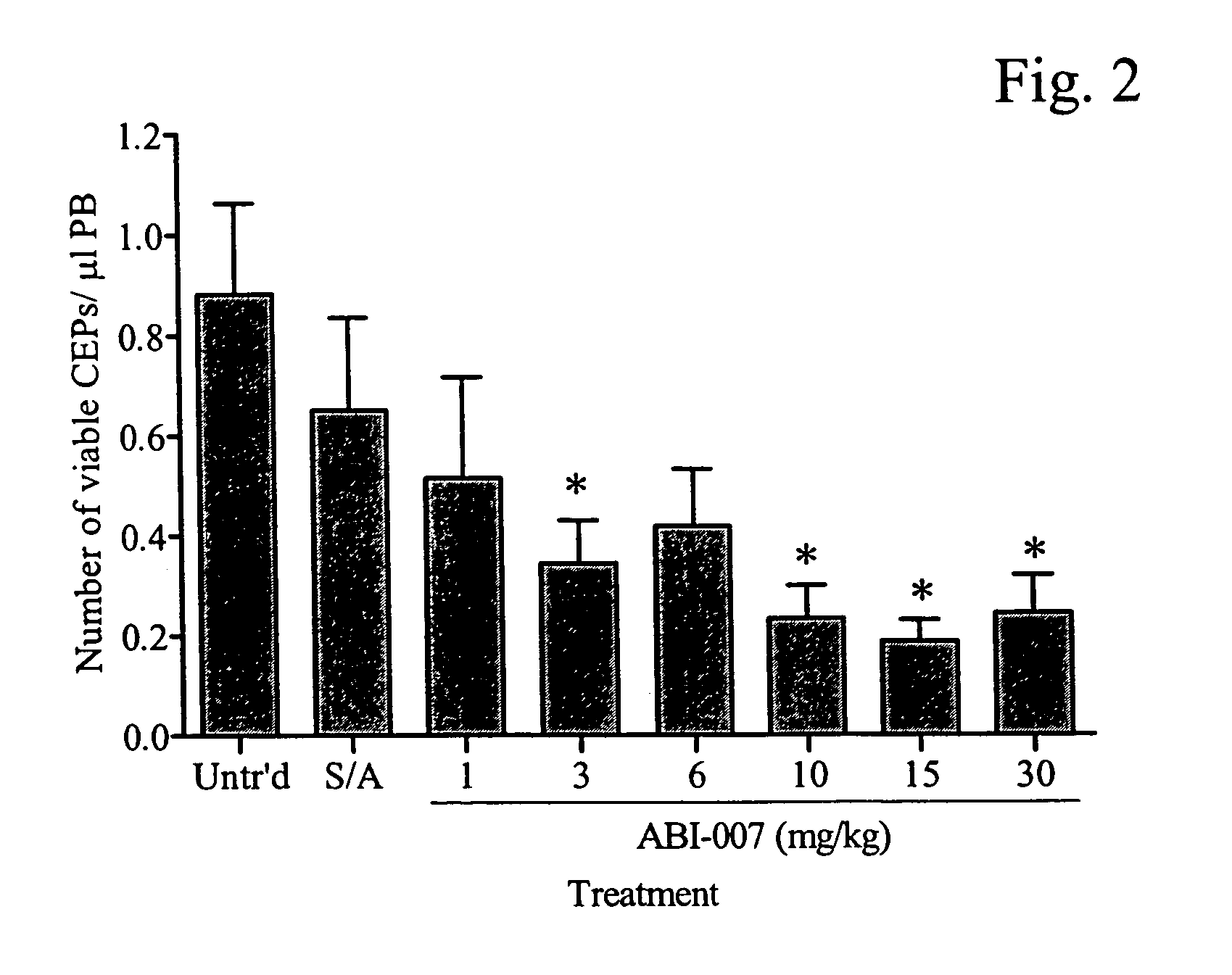
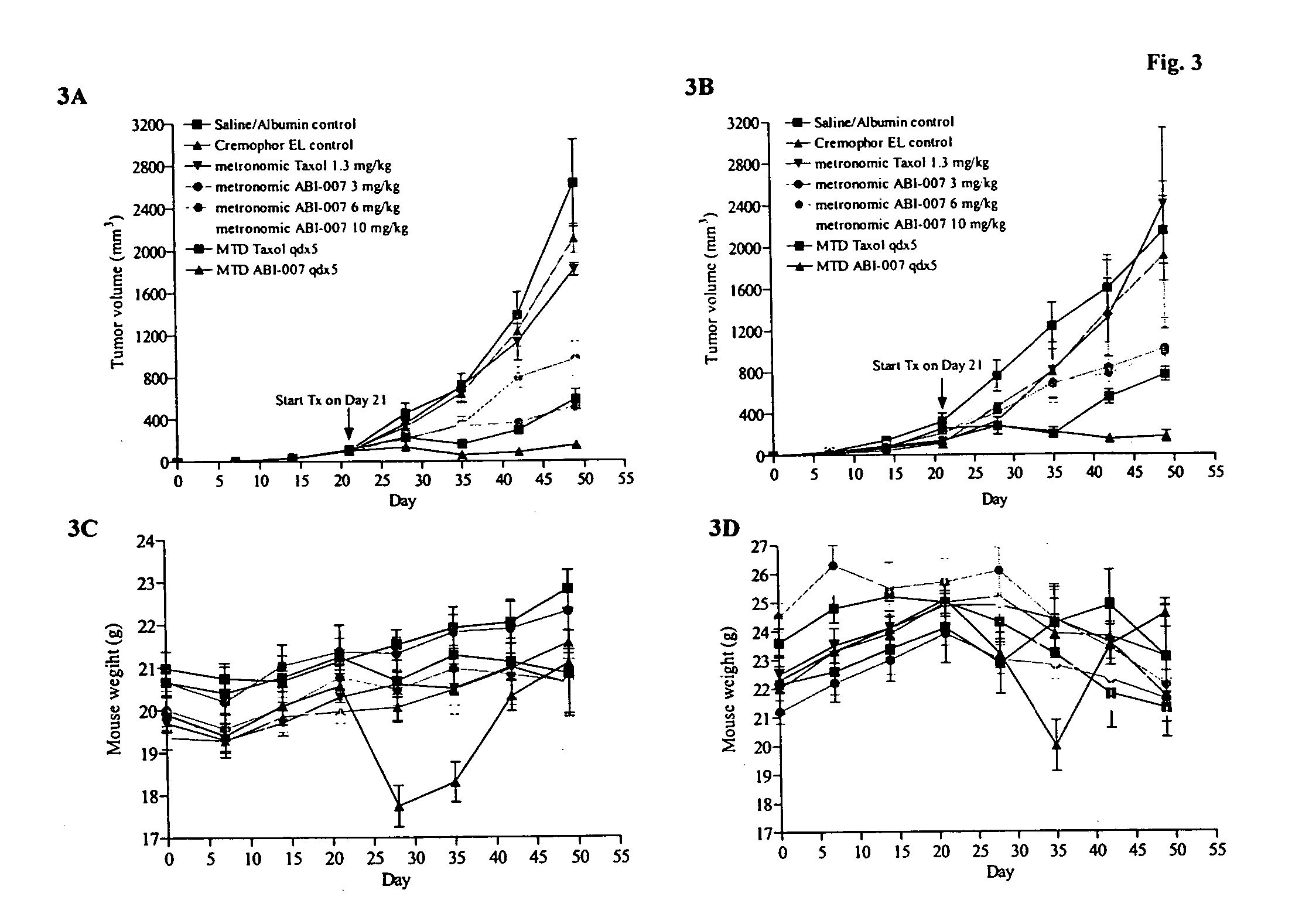
The present invention provides combination therapy methods of treating proliferative diseases (such as cancer) comprising a first therapy comprising administering to an individual an effective amount of a taxane in a nanoparticle composition, and a second therapy which may include, for example, radiation, surgery, administration of chemotherapeutic agents, or combinations thereof. Also provided are methods of administering to an individual a drug taxane in a nanoparticle composition based on a metronomic dosing regime.
Owner:ABRAXIS BIOSCI LLC
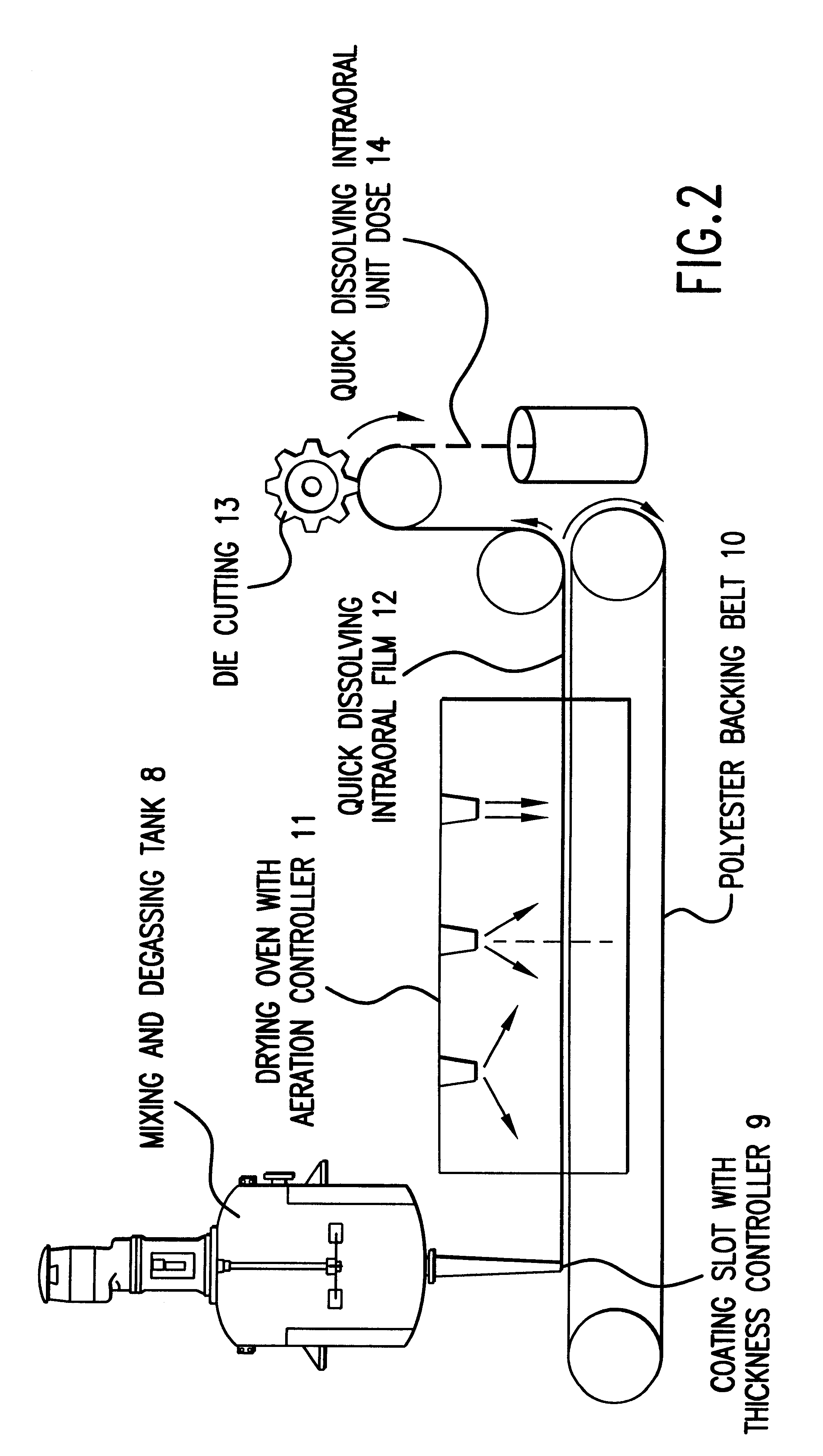
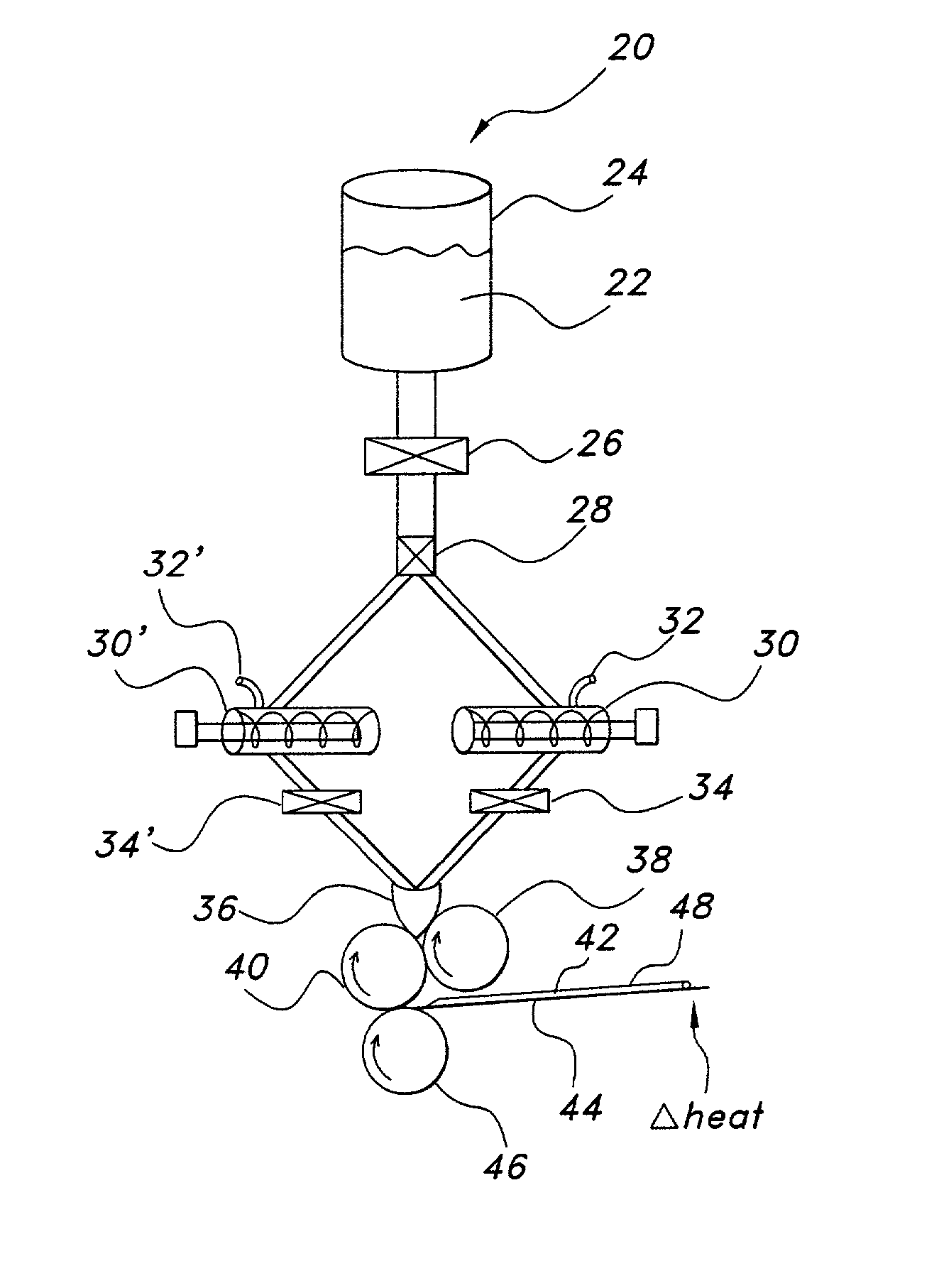
Thin film with non-self-aggregating uniform heterogeneity and drug delivery systems made therefrom
The invention relates to the film products and methods of their preparation that demonstrate a non-self-aggregating uniform heterogeneity. Desirably the films disintegrate in water and may be formed by a controlled drying process, or other process that maintains the required uniformity of the film.
Owner:AQUESTIVE THERAPEUTICS INC
Compositions and methods for topical application and transdermal delivery of botulinum toxins
ActiveUS20050196414A1Reduce hypersecretionReduce sweatingCosmetic preparationsSenses disorderEpitheliumMedicine
A composition for topical application of a botulinum toxin (including botulinum toxin derivatives) comprises a botulinum toxin and a carrier comprising a polymeric backbone comprising a long-chain polypeptide or nonpeptidyl polymer having attached positively charged branching or “efficiency” groups. The invention also relates to methods for reducing muscle paralysis and other conditions that may be treated with a botulinum toxin, particularly paralysis of subcutaneous, and most particularly, facial, muscles, by topically applying an effective amount of the botulinum toxin and carrier, in conjunction, to the subject's skin or epithelium. Kits for administration are also described.
Owner:REVANCE THERAPEUTICS INC
Lipid formulation
The invention features an improved lipid formulation comprising a cationic lipid of formula (A), a neutral lipid, a sterol and a PEG or PEG-modified lipid, where R1 and R2 are independently alkyl, alkenyl or alkynyl, each can be optionally substituted, and R3 and R4 are independently lower alkyl or R3 and R4 can be taken together to form an optionally substituted heterocyclic ring. In one embodiment, R1 and R2 are independently selected from oleoyl, pamitoyl, steroyl, linoleyl and R3 and R4 are methyl. Also disclosed are targeting lipids, and specific lipid formulations comprising such targeting lipids.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Antigen delivery system and method of production
The present invention concerns polymer particle vaccine delivery systems in which a water insoluble protein antigen, e.g. a lipidated HpaA protein, is incorporated with particles comprising a polymer matrix. The present invention also concerns a method for incorporating such a water insoluble protein antigen with a polymer matrix in order to produce a polymer particle vaccine delivery system. In addition, the invention also provides a vaccine composition comprising the polymer particle delivery system. The vaccine can be used to treat and / or reduce the risk of for example Helicobacter infection.
Owner:ASTRAZENECA AB
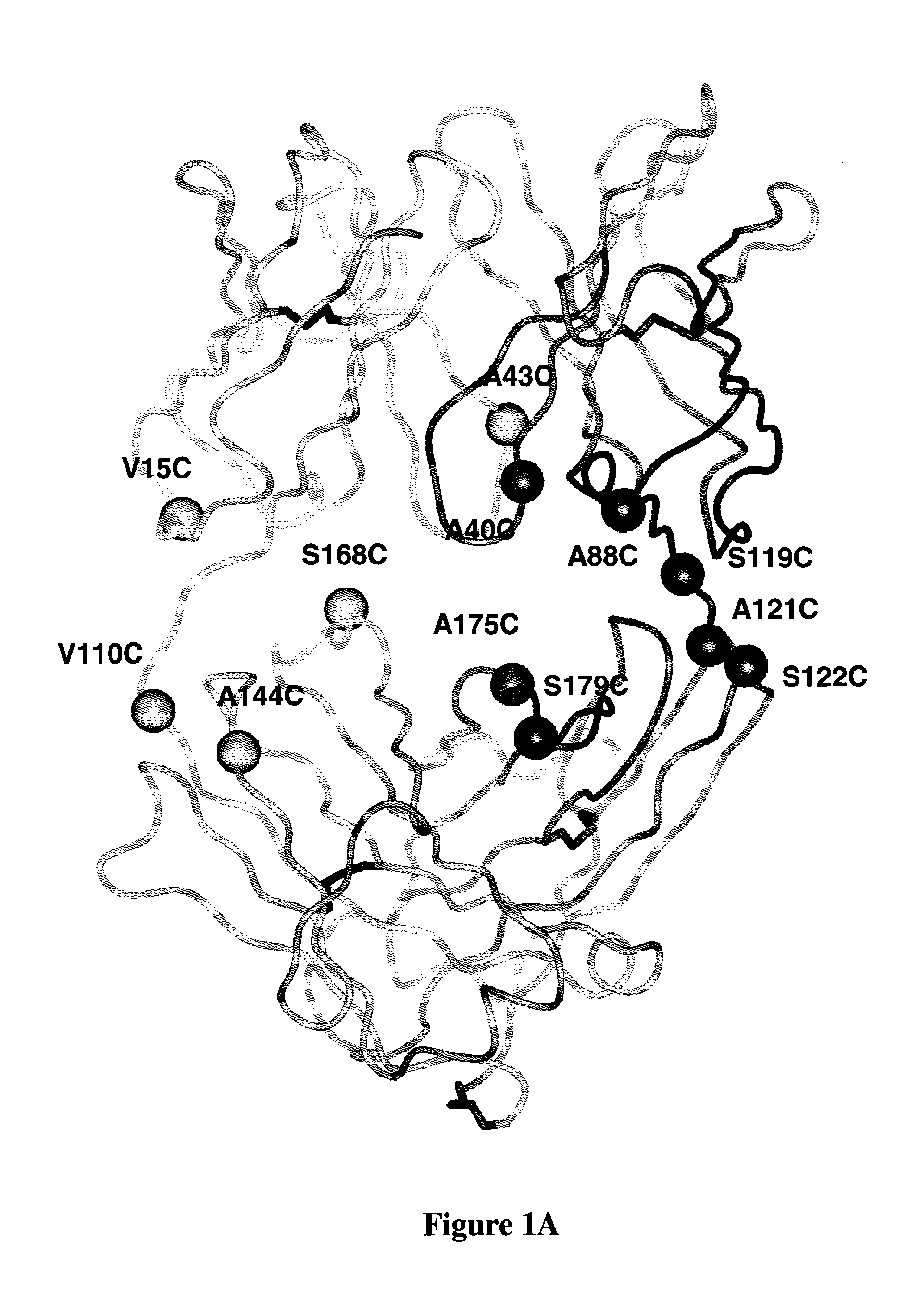
Zirconium-radiolabeled, cysteine engineered antibody conjugates
InactiveUS20100111856A1Peptide/protein ingredientsGenetic material ingredientsAntibody conjugateAntibody fragments
Antibodies are engineered by replacing one or more amino acids of a parent antibody with non cross-linked, highly reactive cysteine amino acids. Antibody fragments may also be engineered with one or more cysteine amino acids to form cysteine engineered antibody fragments (ThioFab). Methods of design, preparation, screening, and selection of the cysteine engineered antibodies are provided. Cysteine engineered antibodies (Ab) are conjugated with one or more zirconium complex (Z) labels through a linker (L) to form cysteine engineered zirconium-labeled antibody conjugates having Formula I:Ab-(L-Z)p Iwhere p is 1 to 4. Imaging methods and diagnostic uses for zirconium-radiolabeled, cysteine engineered antibody conjugate compositions are disclosed.
Owner:F HOFFMANN LA ROCHE & CO AG
Pharmaceutical compositions for buccal and pulmonary application
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and at least three different micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal region of the mouth.
Owner:GENEREX PHARMA INC +1
Compositions and methods for improved skin care
InactiveUS20070077292A1Safe and effective amount of collagenPrevent adverse side effectsOrganic active ingredientsCosmetic preparationsMedicineInstability
Compositions and methods for administering collagen to a human subject have been developed. The collagen-containing lipid vesicles of the invention provide a delivery system for human collagen which eliminates problems associated with chemical and physical instability of the collagen as well as immune responses to non-human collagen.
Owner:PINSKY MARK A
Methods and devices for detection and therapy of atheromatous plaque
InactiveUS20030082105A1Easy to detectHigh sensitivityUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsVulnerable plaqueFluorescence
The present invention relates to devices for detection and therapy of active atheromatous plaque and / or thin-capped fibro-atheroma ("vulnerable plaque"), using selectively targeted fluorescent, radiolabeled, or fluorescent and radiolabeled compositions. The present invention further relates to methods and devices for detection and theraphy of active atheromatous plaques and / or vulnerable plaques, using selectively targeted beta-emitting compositions, optionally comprising fluorescent compositions.
Owner:THE GENERAL HOSPITAL CORP
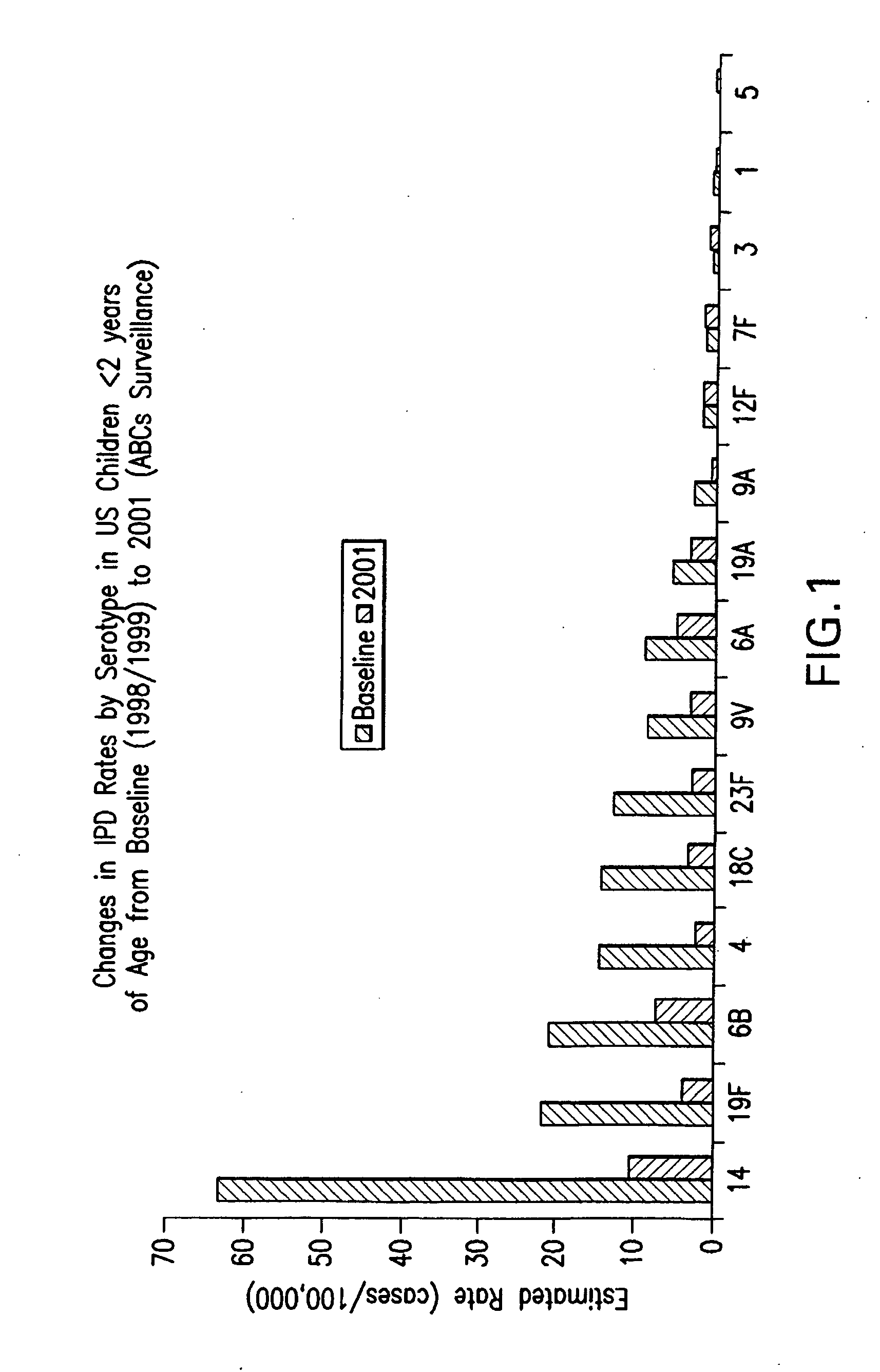
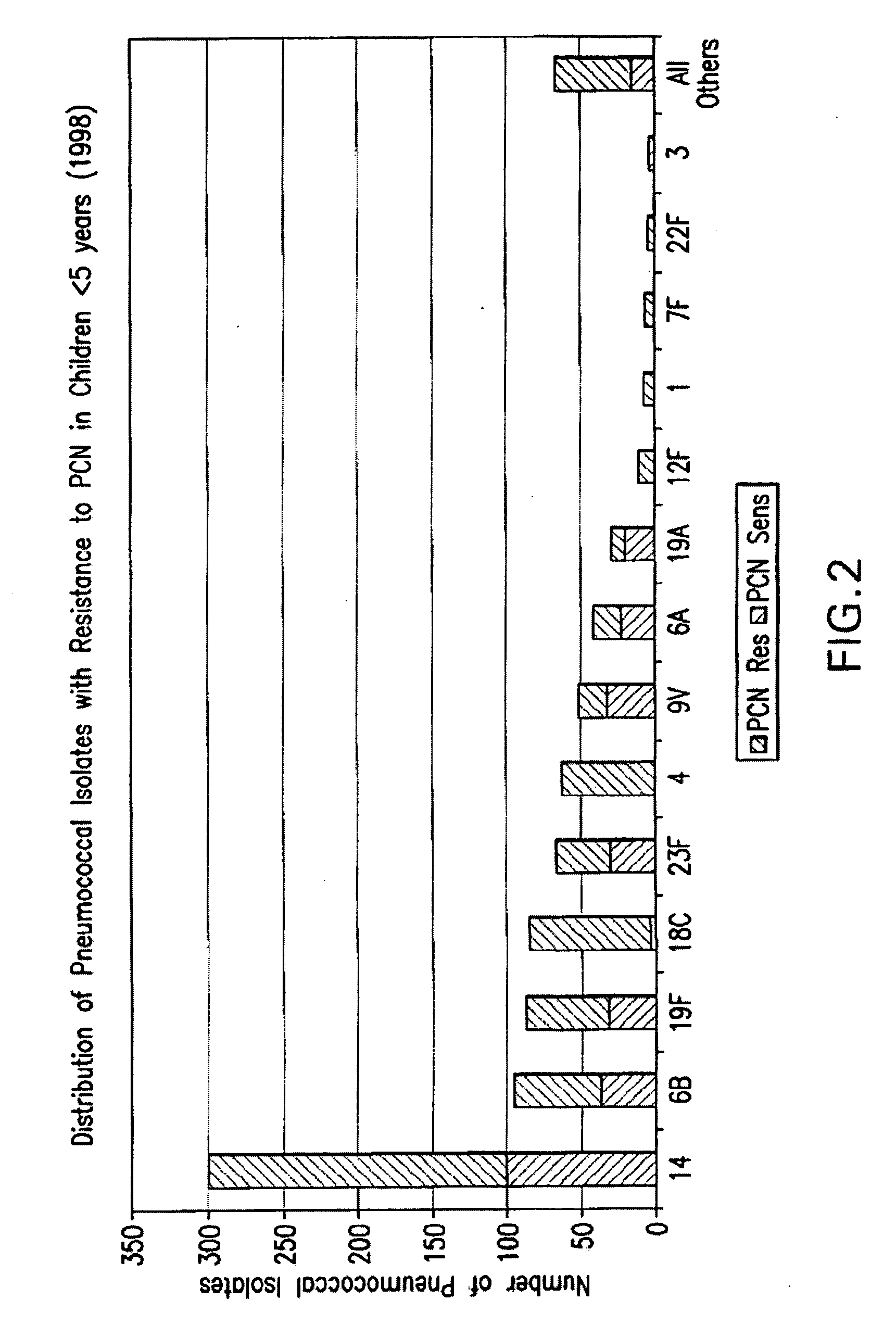
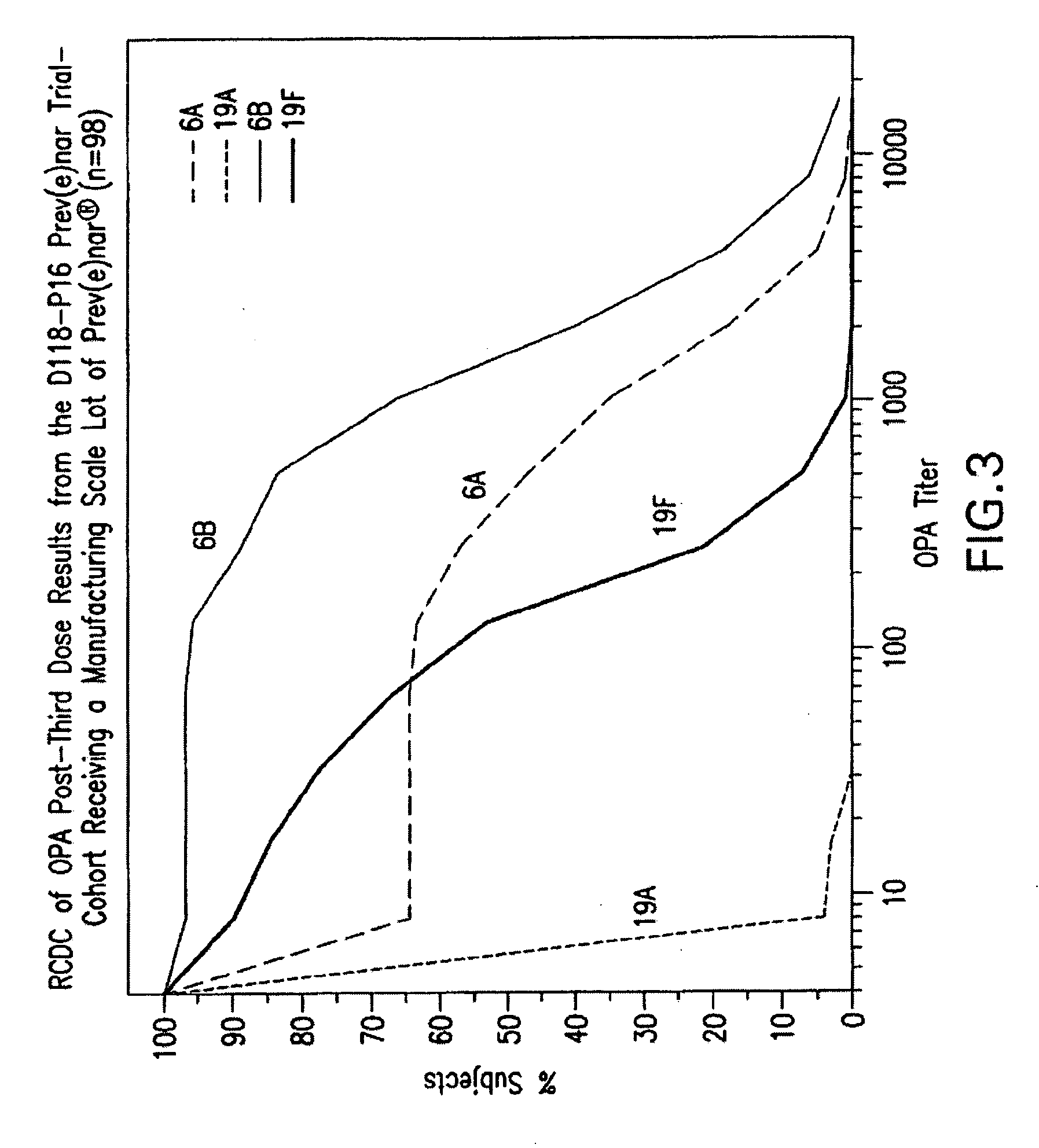
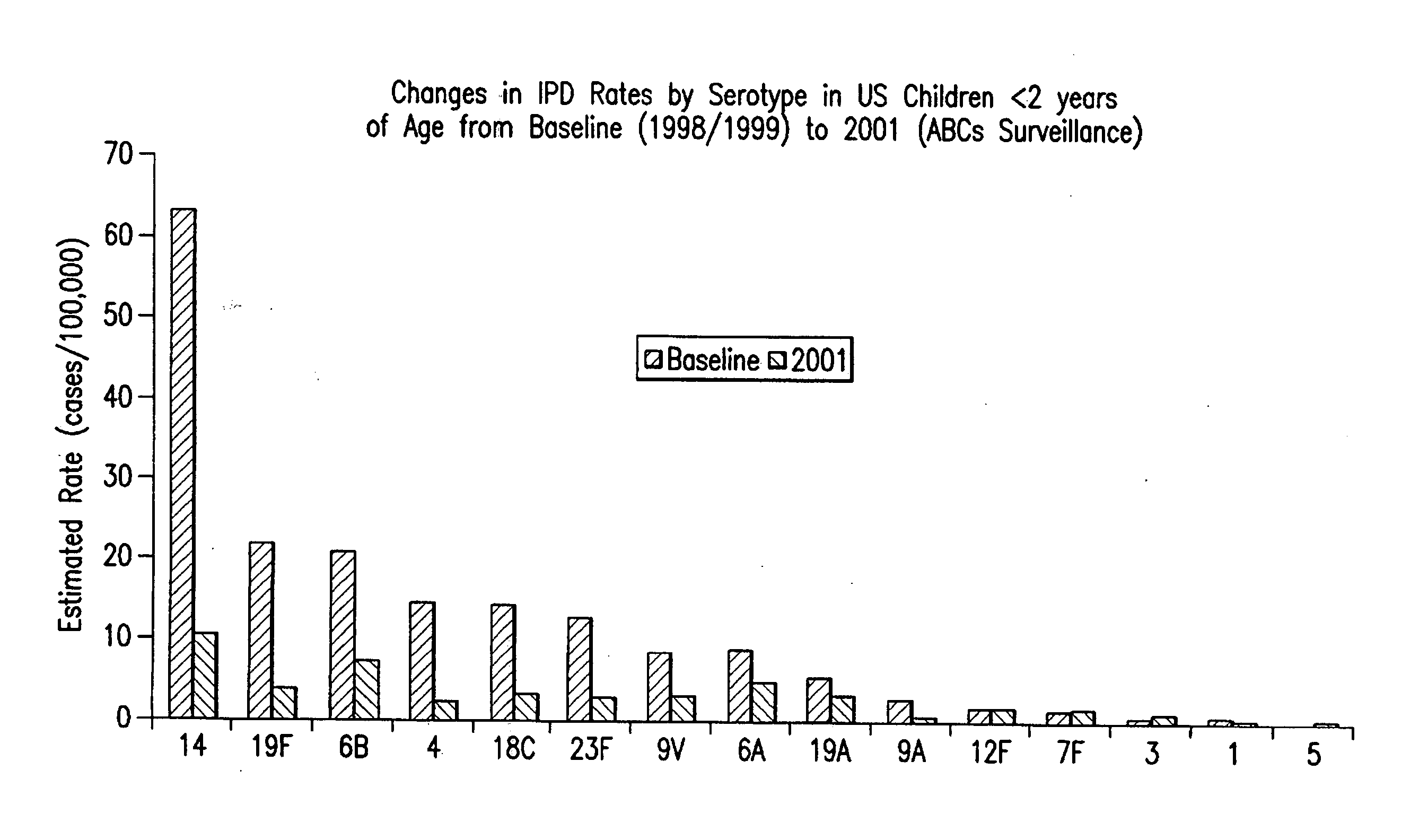
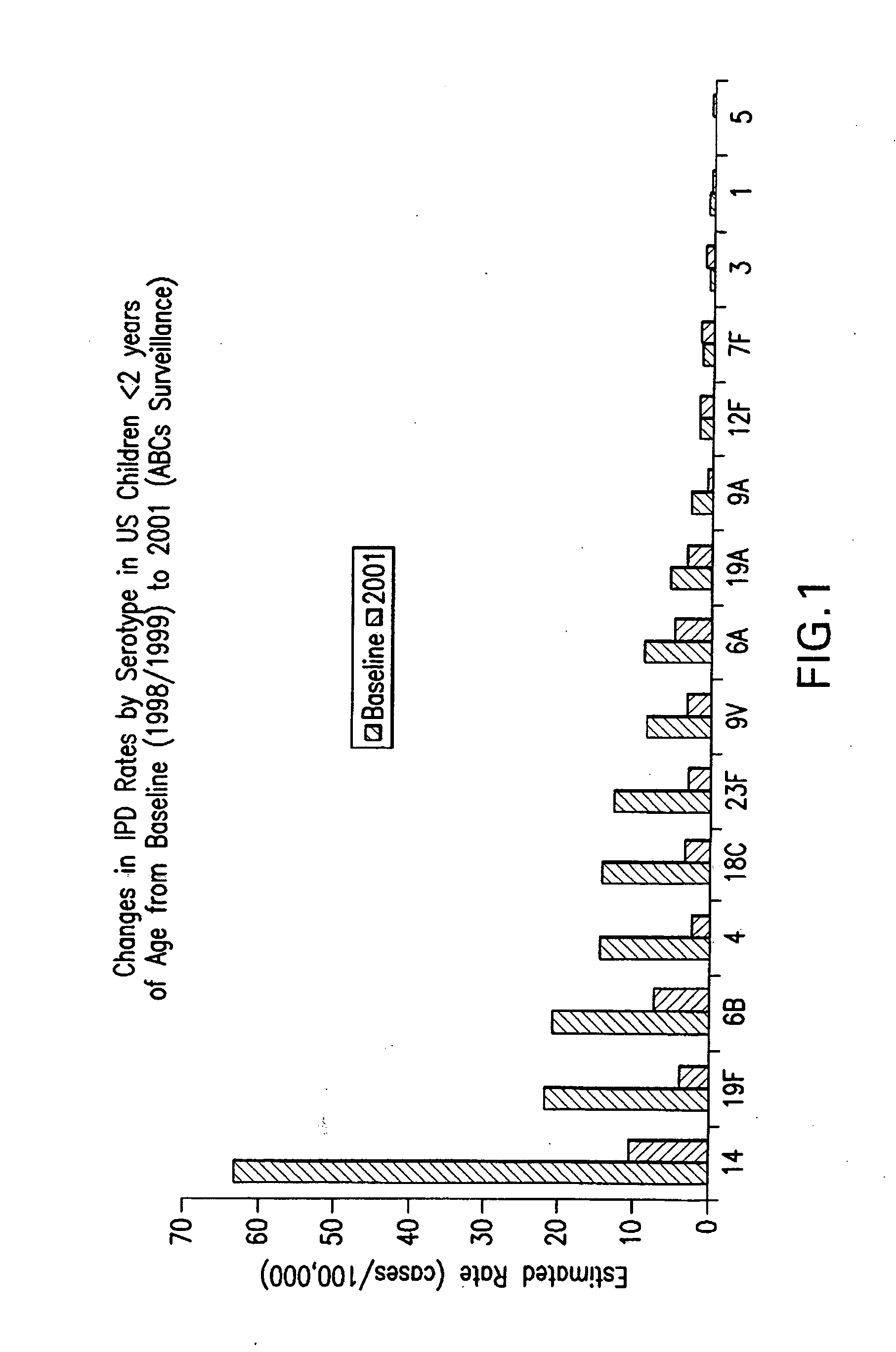
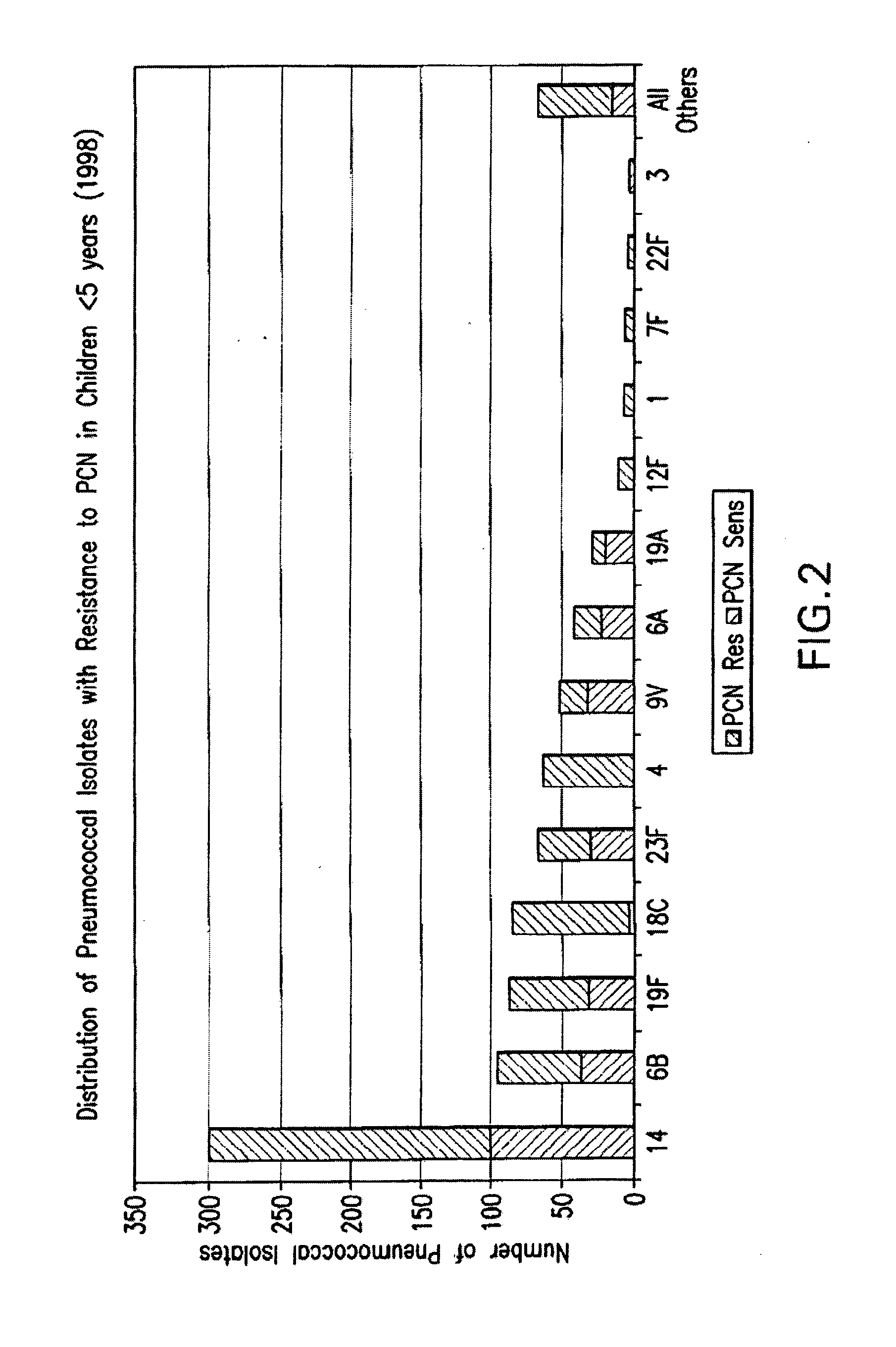
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 1 polysaccharide covalently linked to a carrier protein, the method including partial de-O-acetylation of the polysaccharide by mild hydrolysis in an alkaline pH buffer.
Owner:WYETH LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 3 polysaccharide covalently linked to a carrier protein, the method including periodic acid oxidation of the polysaccharide in the presence of bivalent cations.
Owner:WYETH LLC
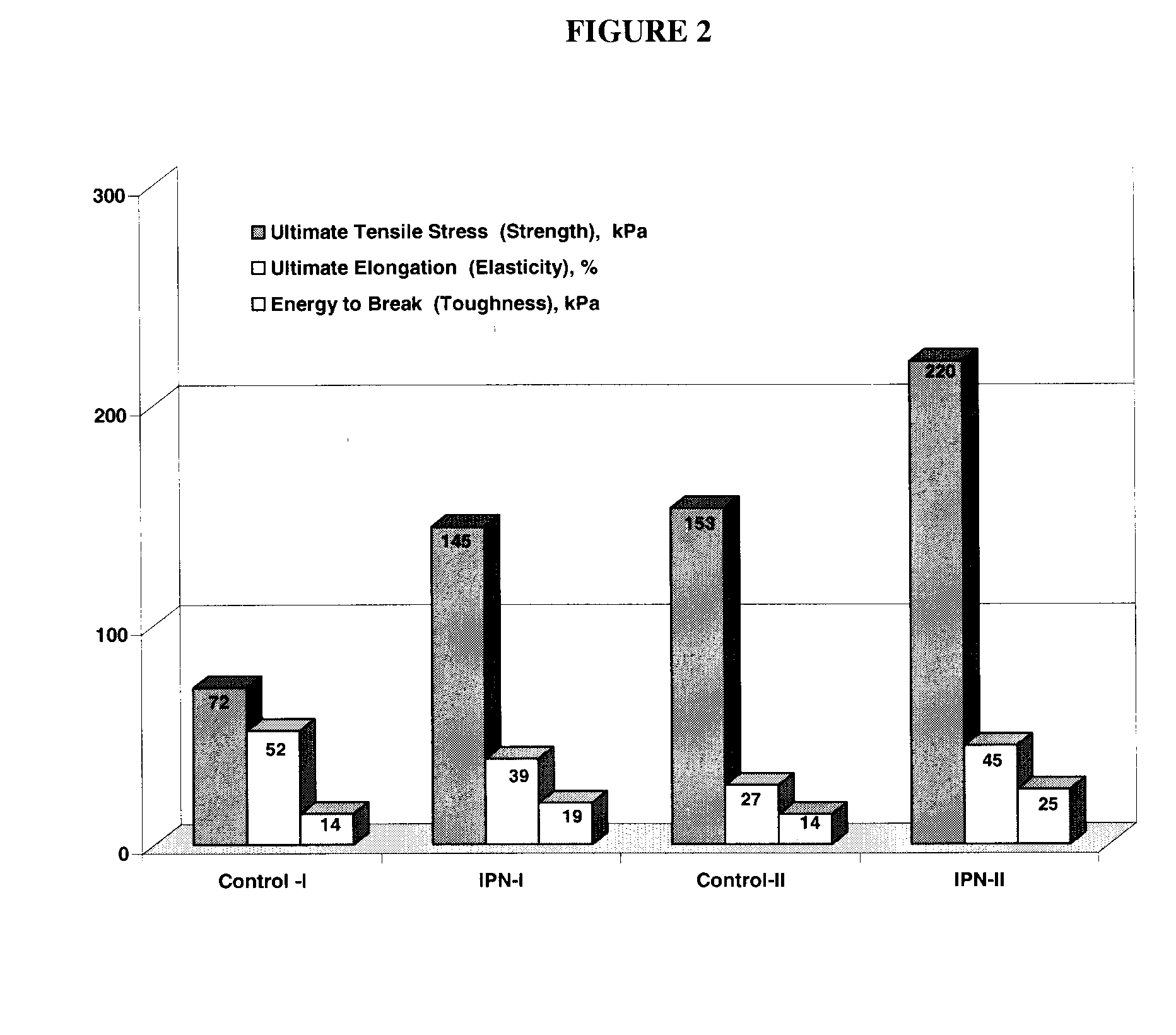
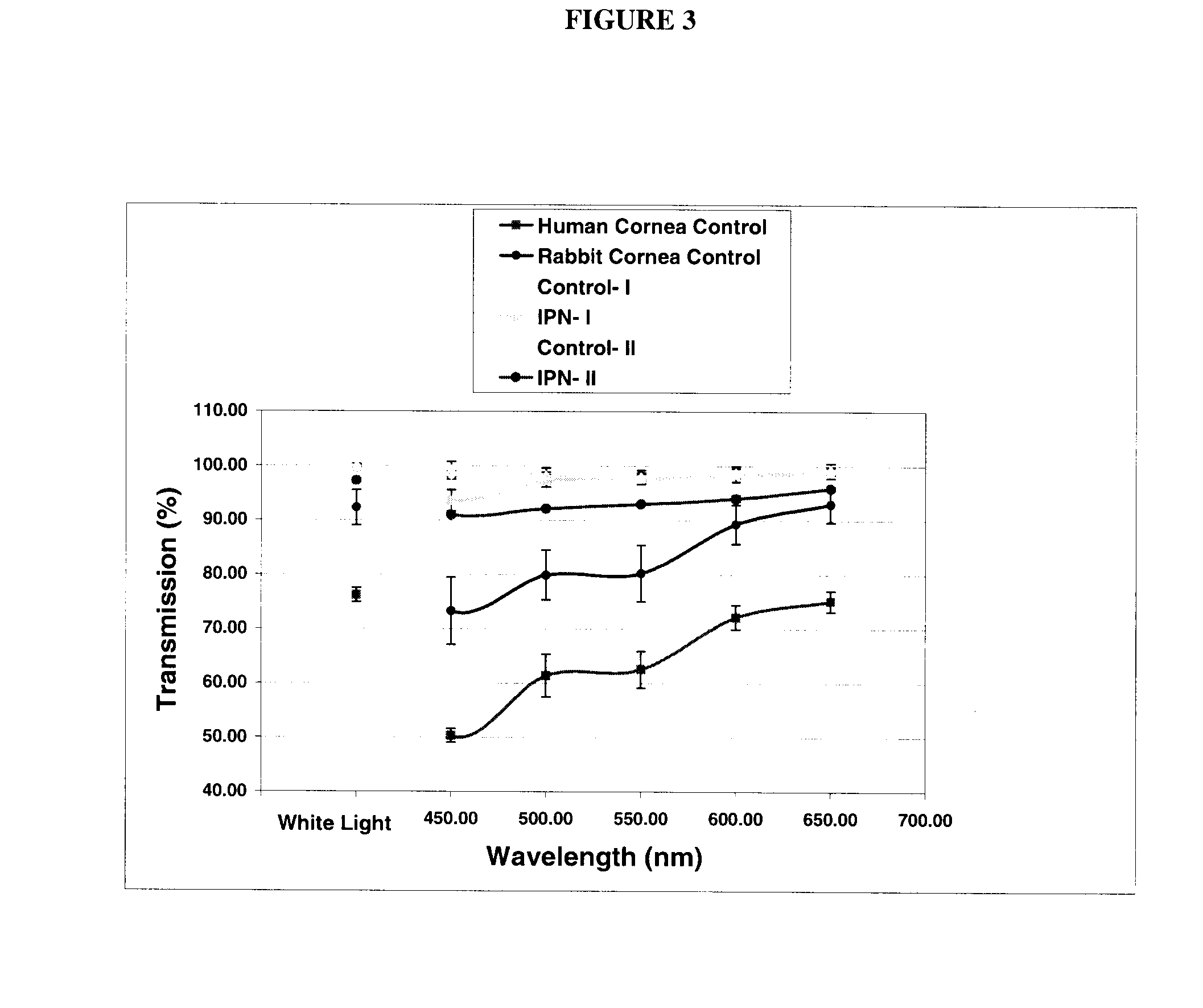
Interpenetrating Networks, and Related Methods and Compositions
The present invention provides interpenetrating polymeric networks (IPNs), and related methods and compositions. The hydrogel material of this invention comprises an interpenetrating network of two or more polymer networks, wherein at least one of the polymer networks is based on a biopolymer. Also provided is a method of producing the hydrogel material comprising, combining a first polymeric network with a second polymeric network, wherein the first polymeric network or the second polymeric network is based on a biopolymer. The present application also discloses devices manufactured from the IPN hydrogel material and uses thereof.
Owner:OTTAWA HOSPITAL RES INST +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com