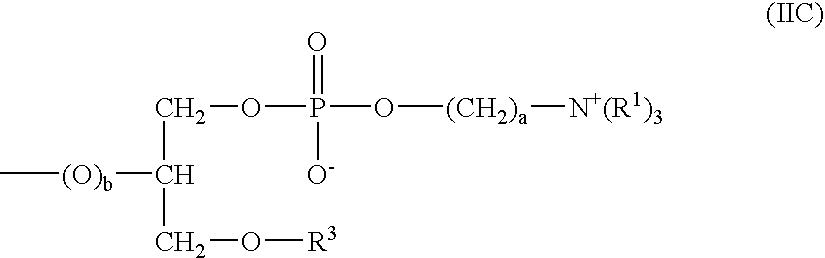
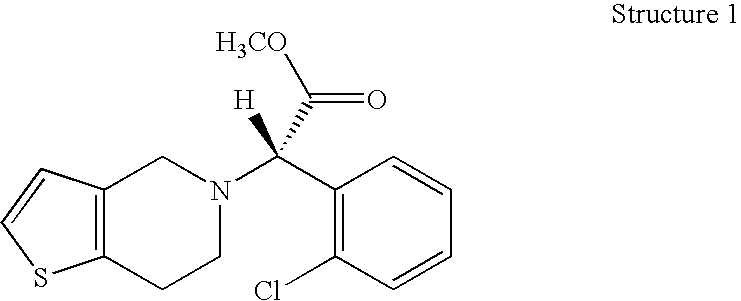
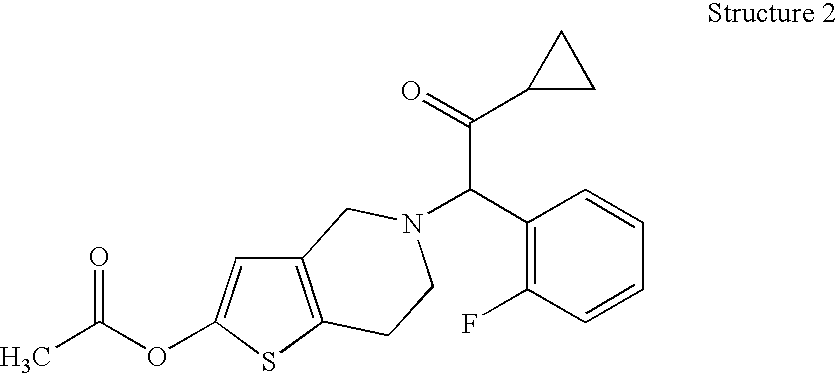
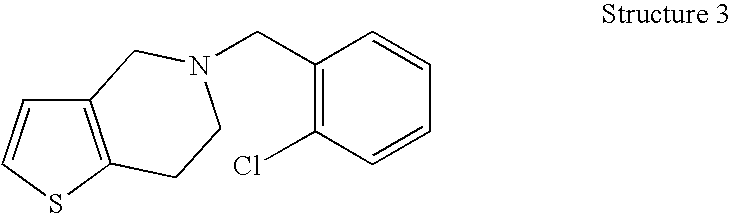
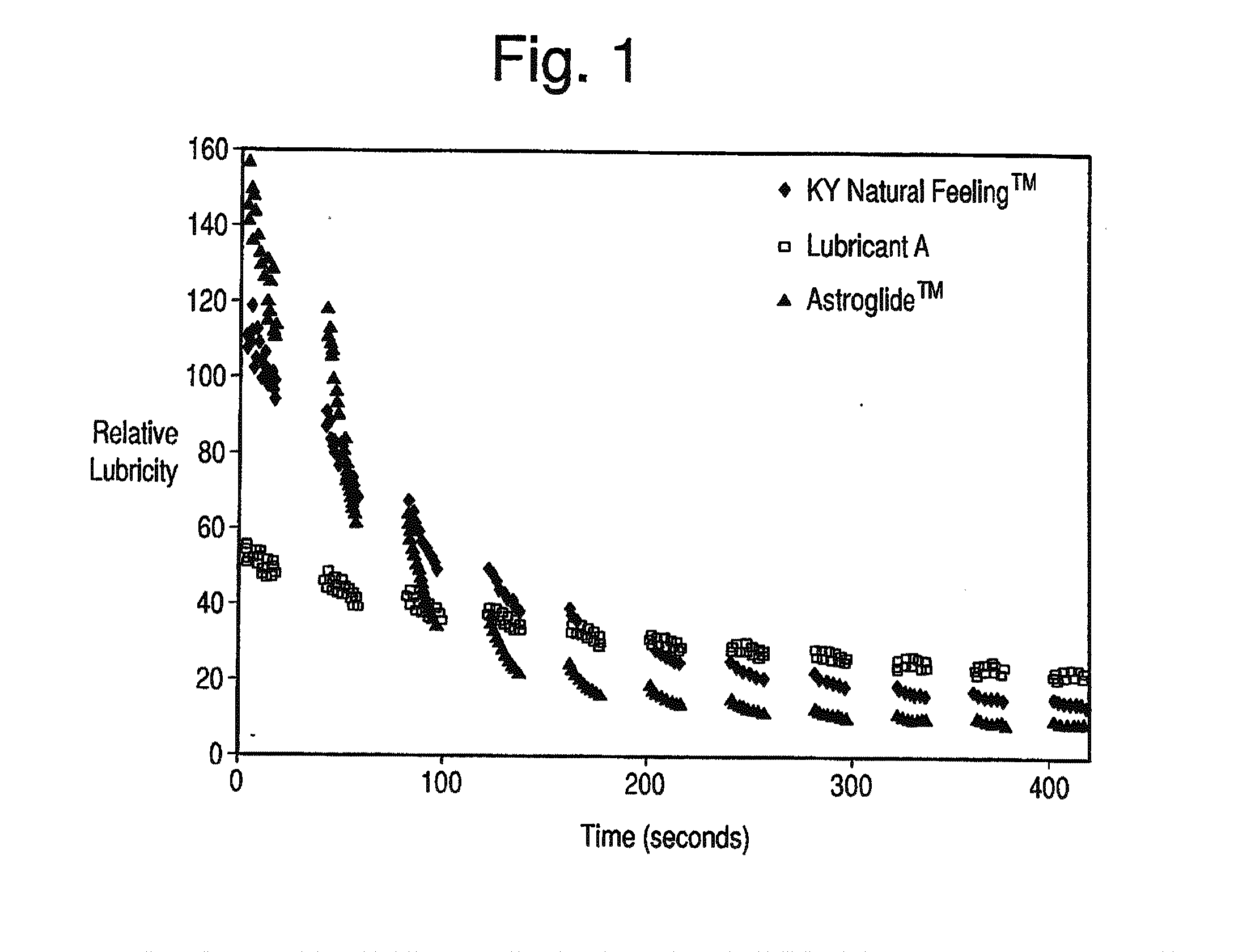
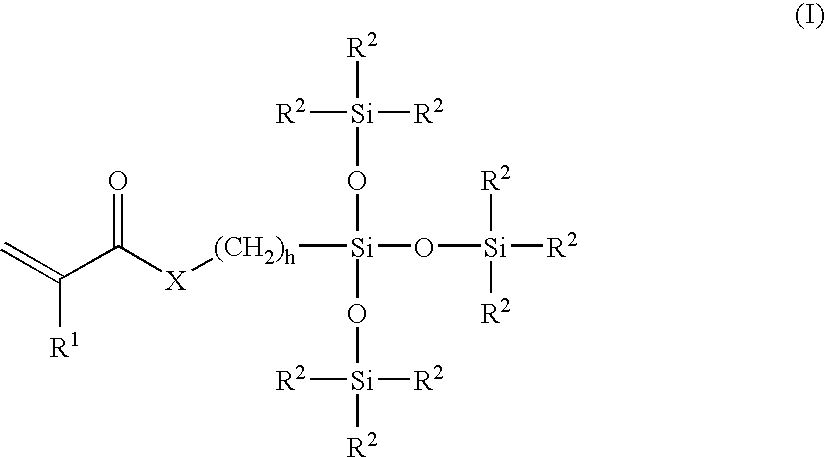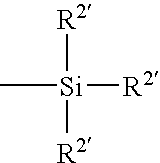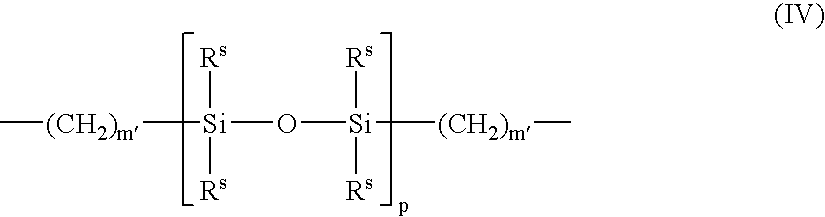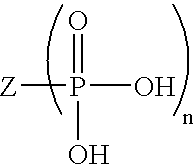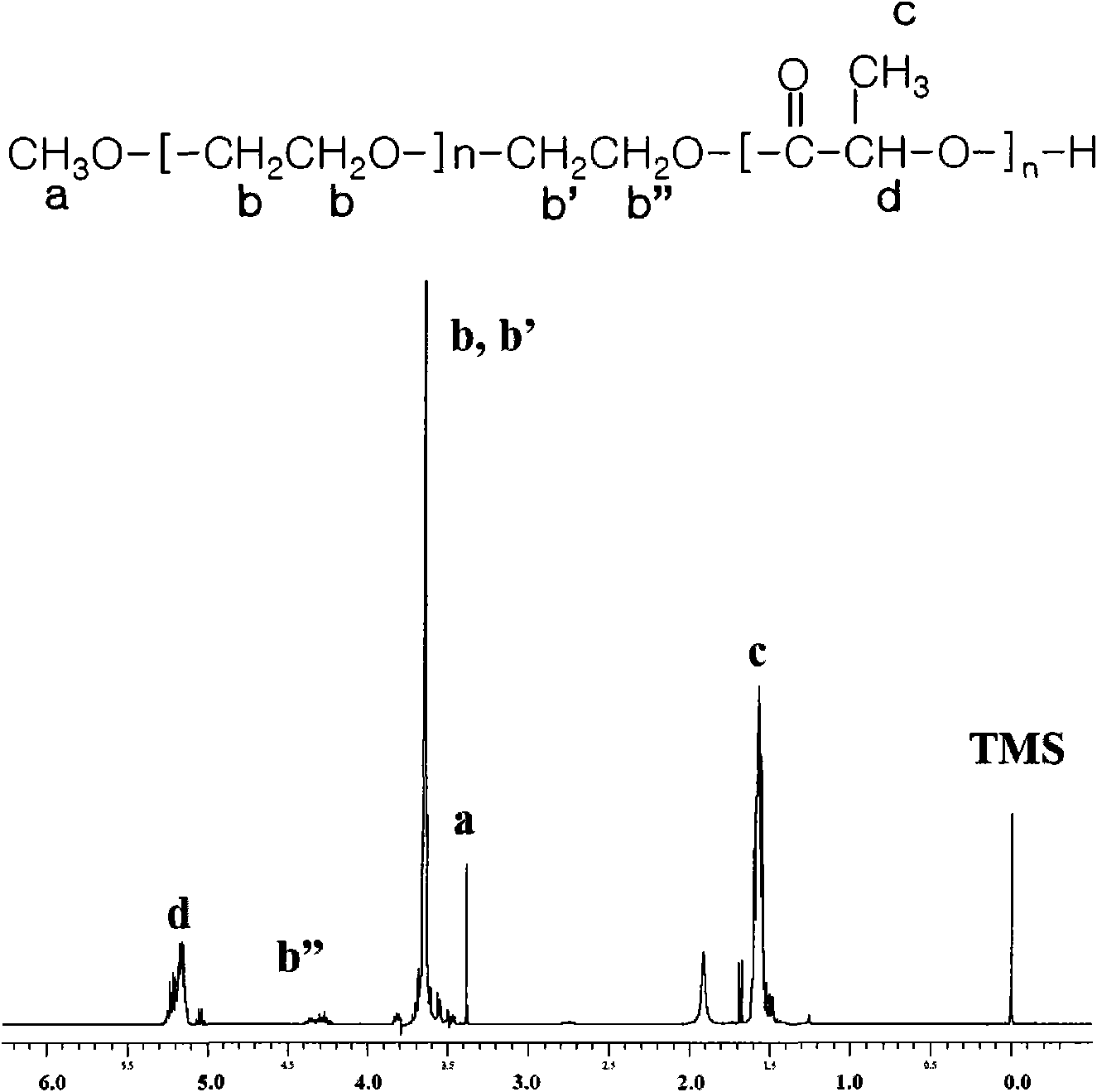Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
82 results about "Osmolar Concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The concentration of osmotically active particles in solution expressed in terms of osmoles of solute per liter of solution. Osmolality is expressed in terms of osmoles of solute per kilogram of solvent.
Protein formulations and methods of making same
ActiveUS20090291062A1Improve consistencyAccurate concentrationPeptide/protein ingredientsAntipyreticOsmolar ConcentrationExcipient
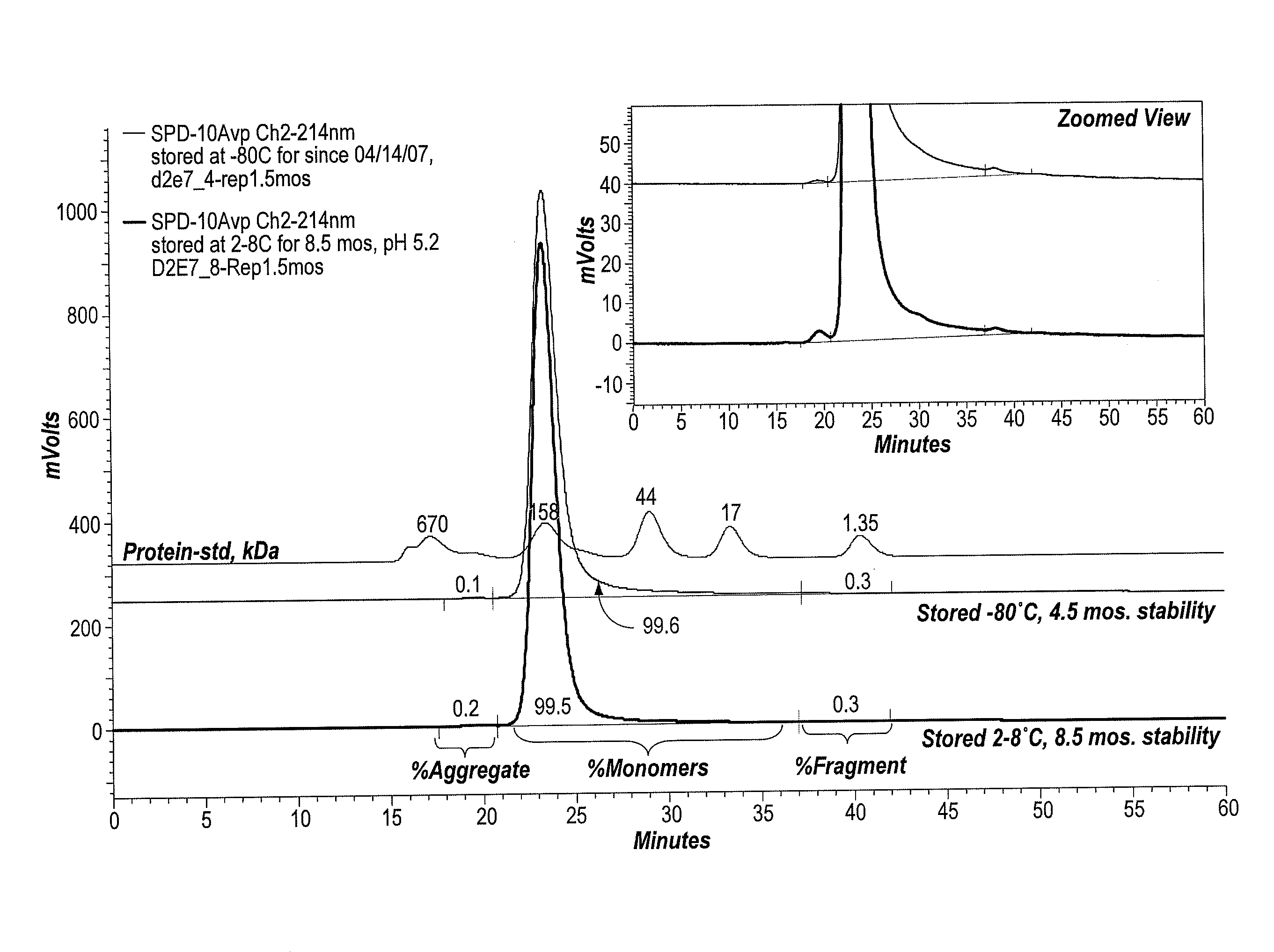
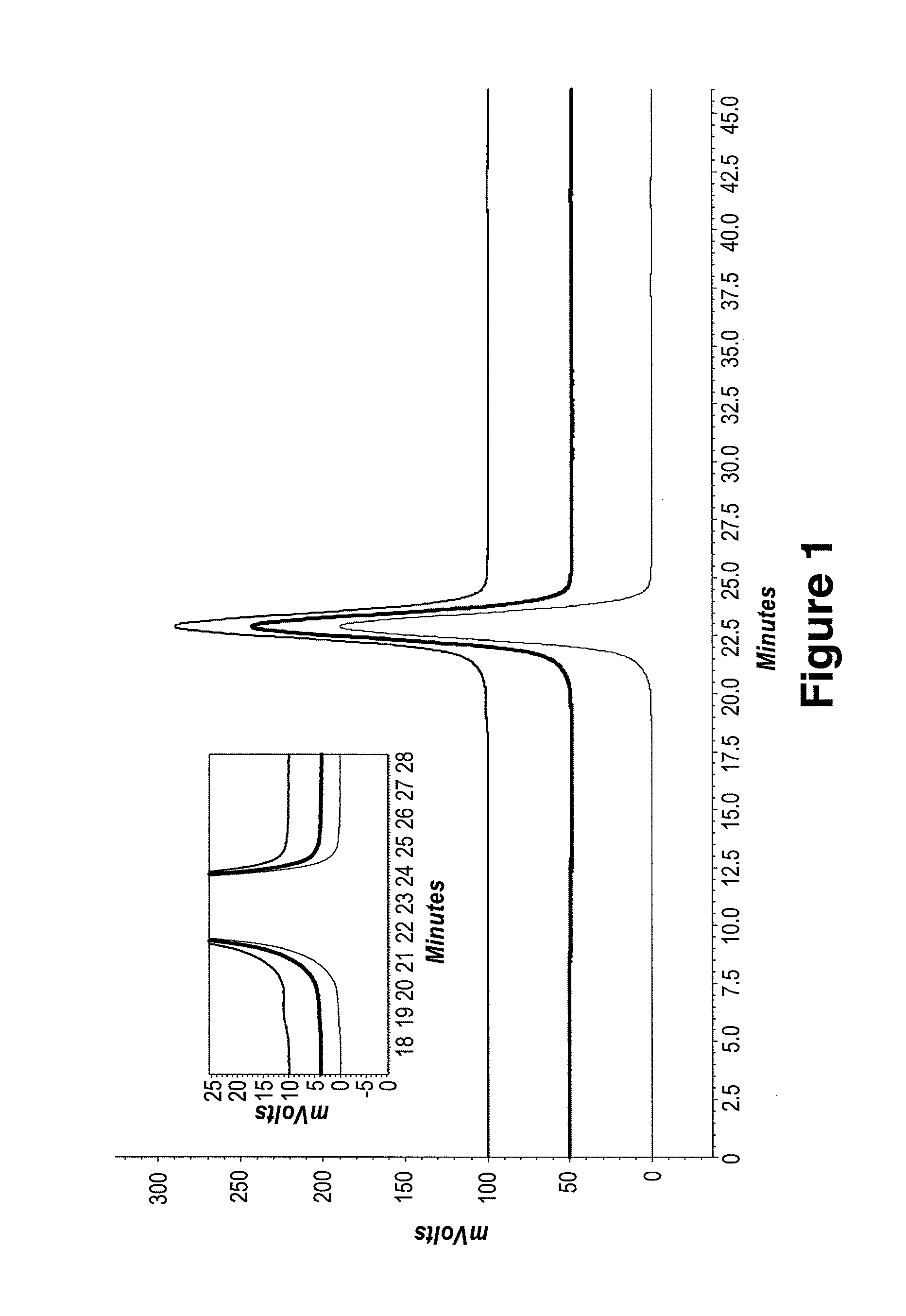
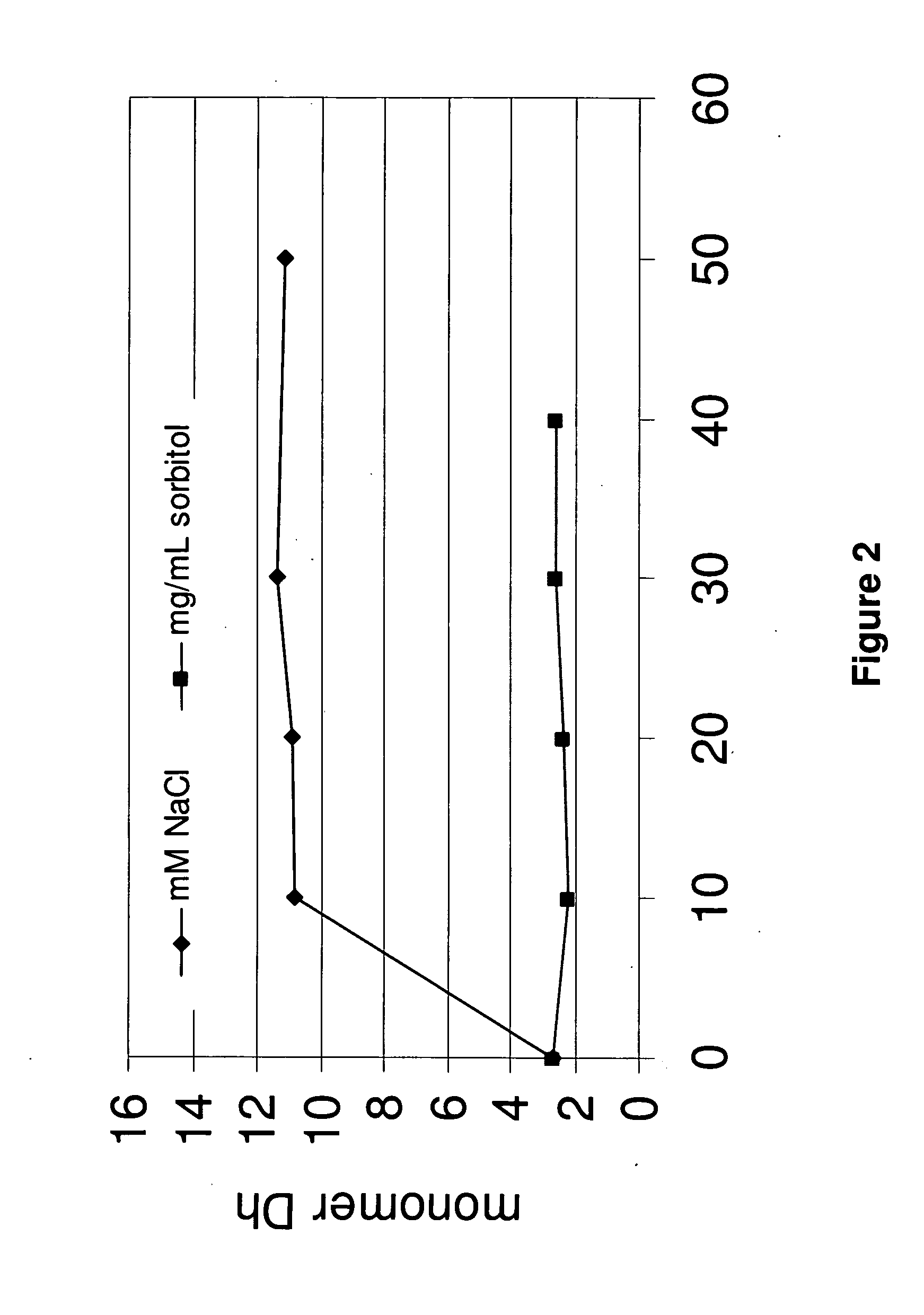
The invention provides an aqueous formulation comprising water and a protein, and methods of making the same. The aqueous formulation of the invention may be a high protein formulation and / or may have low levels of conductivity resulting from the low levels of ionic excipients. Also included in the invention are formulations comprising water and proteins having low osmolality.
Owner:ABBVIE BIOTECHNOLOGY LTD
Ophthalmic Solutions
InactiveUS20080314767A1Avoid pollutionLens cleaning compositionsPackage sterilisationOsmolar ConcentrationNon ionic
Disclosed are ophthalmic solutions such as packaging solutions for storing ophthalmic devices and lens care solutions for cleaning, disinfecting, rinsing and / or storing ophthalmic devices. The ophthalmic solutions contain at least a polymerization product obtained from a monomeric mixture comprising (a) a monomer bearing a center of permanent positive charge and (b) a non-ionic ethylenically unsaturated monomer, wherein the solution has an osmolality of at least about 200 mOsm / kg, and a pH of about 4 to about 9.
Owner:BAUSCH & LOMB INC
Packaging Solutions
ActiveUS20090100801A1Avoid pollutionComfortable to wearPackage sterilisationPackaging corrosive chemicalsPhosphorylcholineOsmolar Concentration
A packaging system for the storage of an ionic, hydrogel contact lens employs an aqueous packaging solution including a phosphorylcholine polymer. Preferably, the solution has an osmolality of at least about 200 mOsm / kg, a pH of about 6 to about 8 and is heat sterilized.
Owner:BAUSCH & LOMB INC
Human tumor necrosis factor-immunoglobulin(TNFR1-IgG1) chimera composition
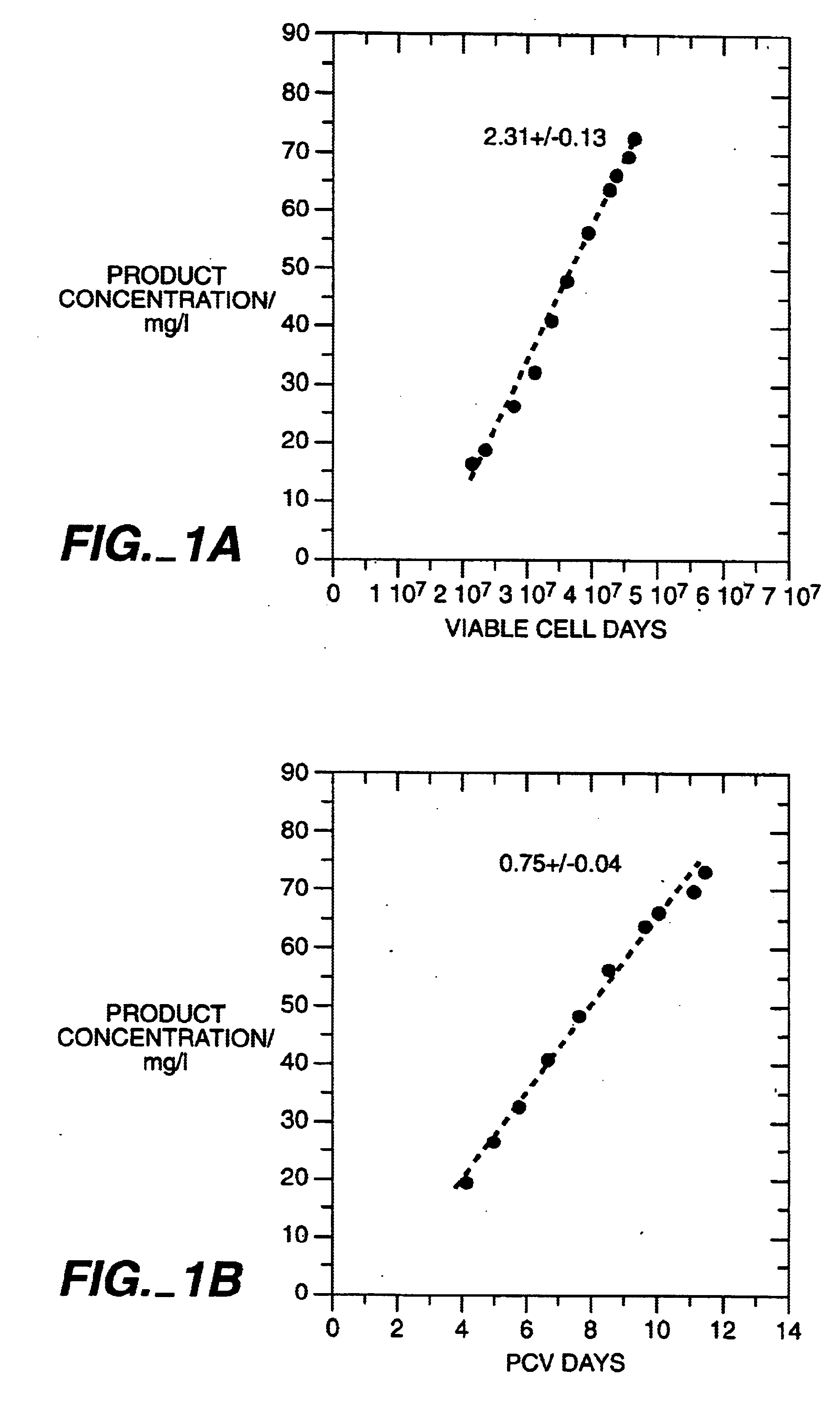
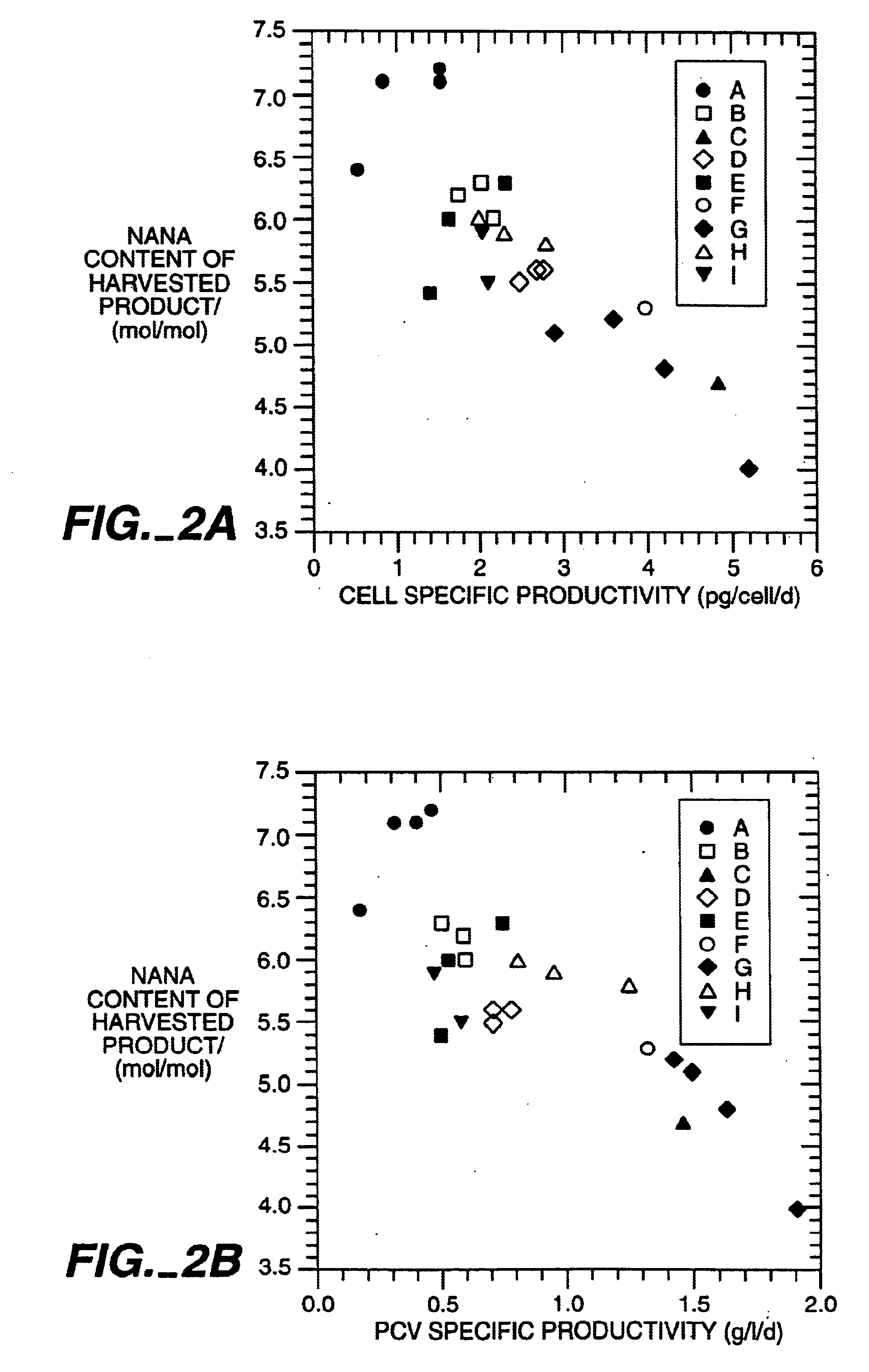
The present invention relates to novel process for the preparation of glycoproteins by mammalian cell culture wherein the sialic acid content of the glycoprotein produced is controlled over a broad range of values by manipulating the cell culture environment. The invention provides for processes in which the sialic acid content of the glycoprotein is modified by changes in cell culture parameters which affect cell specific productivity. Preferred embodiments of the invention include cell culture processes in the osmolality of the cell culture is controlled as well as the concentration of a transcription enhancer during the production phase of the cell culture. The invention further provides for novel preparations of soluble type 1 tumor necrosis factor immunoglobulin G1 and their uses in the treatment of inflammatory or immune related disorders.
Owner:GENENTECH INC
Novel strategy to reduce lactic acid production and control ph in animal cell culture
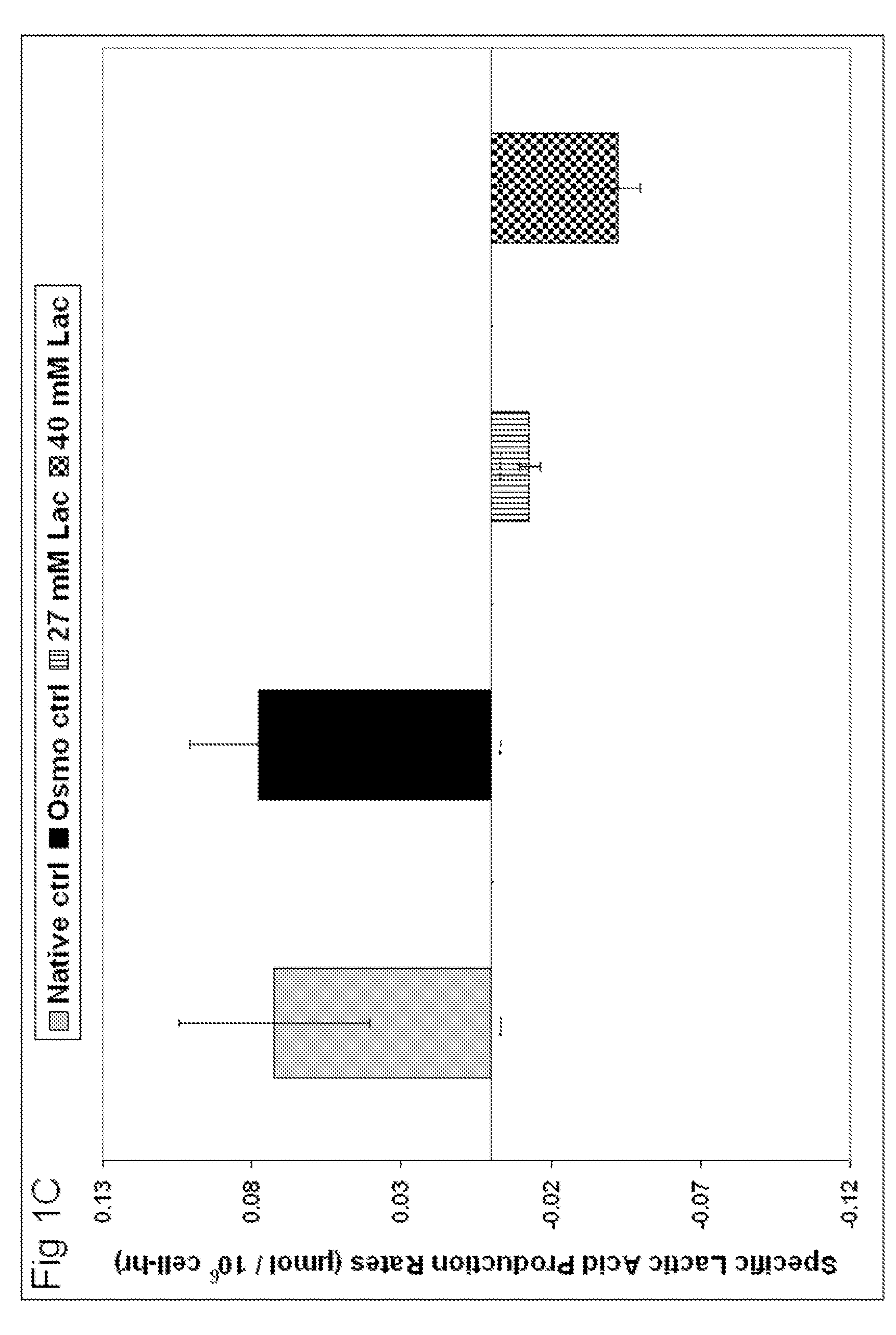
ActiveUS20110104734A1Reduce lactic acid productionReduces specific lactic acid production rateAnimal cellsMicrobiological testing/measurementOsmolar ConcentrationAntibody
The present disclosure provides a method for culturing cells in exogenous lactic acid. Certain aspects of the present disclosure include the production of recombinant proteins, such as antibodies and fragments thereof. Certain aspects of the present disclosure also relate to methods of controlling lactic acid production, pH stability and osmolality in cell culture.
Owner:CROUGHAN MATTHEW S
Ophthalmic composition containing a polyol-acid copolymer
An ophthalmic composition comprising a copolymer and one or more cationic antimicrobial components. The copolymer comprises monomeric units of one or more polymerizable alcohols or polymerizable polyols, and monomeric units of one or more polymerizable carboxylic acids. Also, the composition has an osmolality from 200 mOsmol / kg to 400 mOsmol / kg.
Owner:BAUSCH & LOMB INC
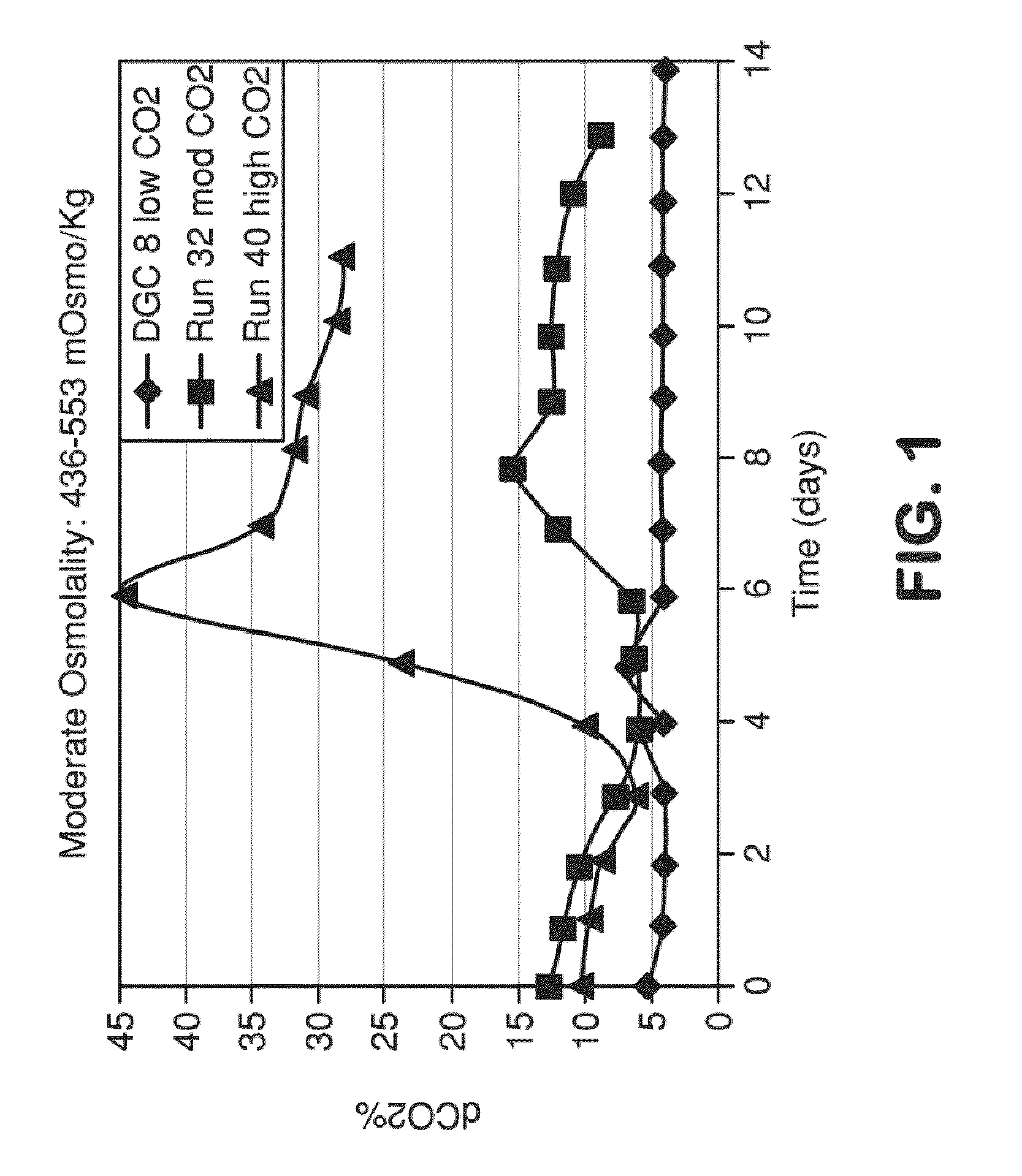
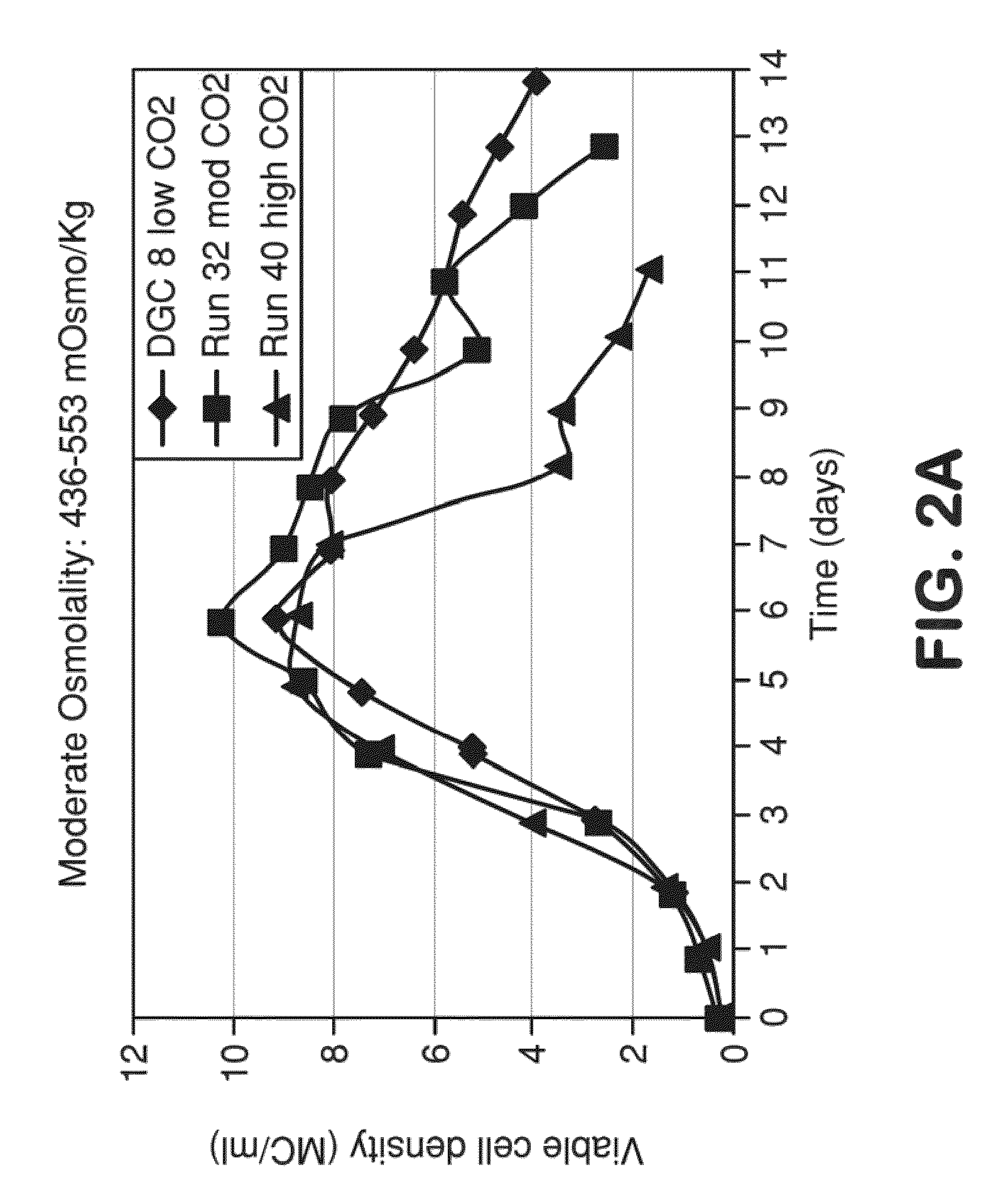
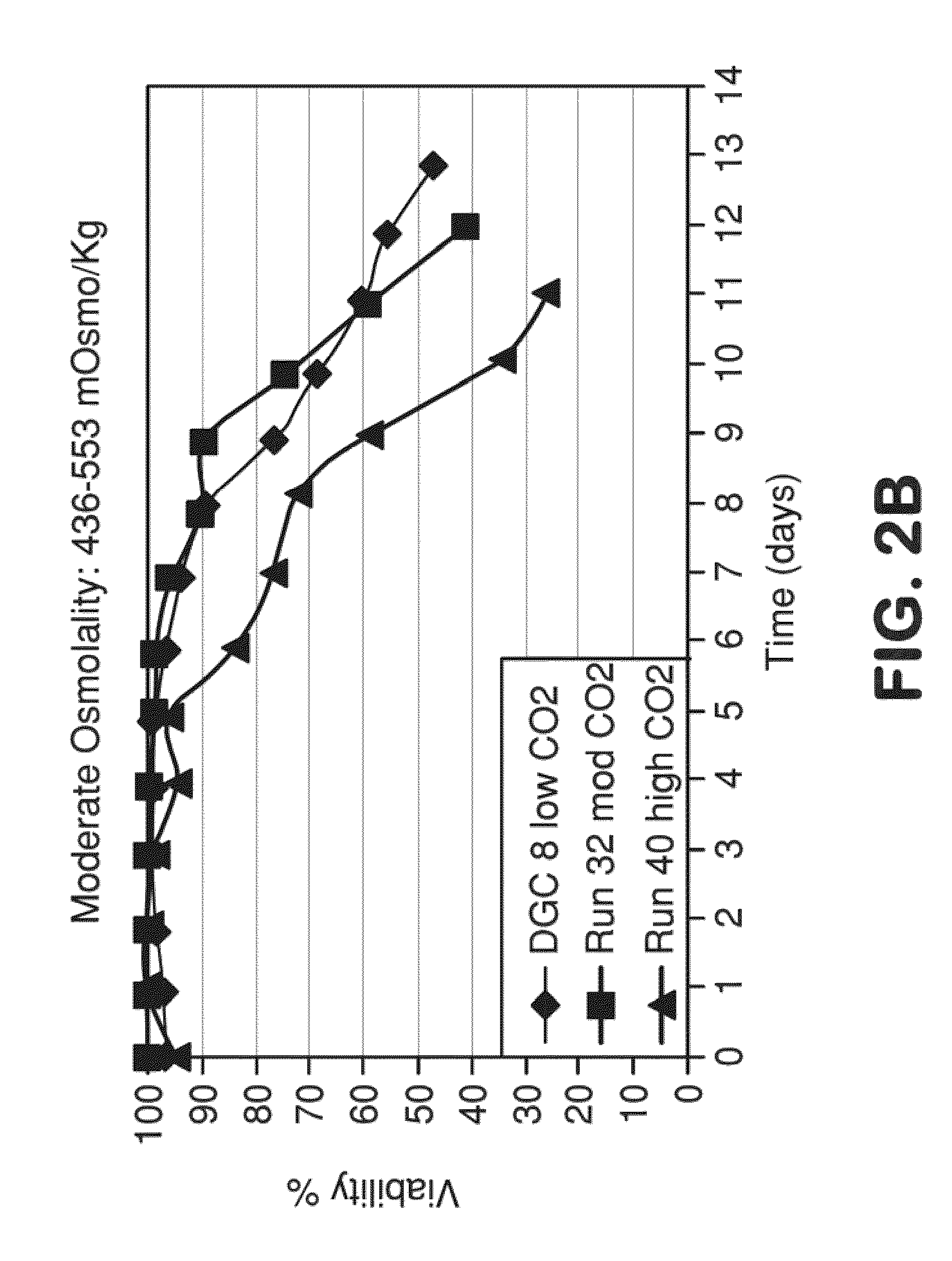
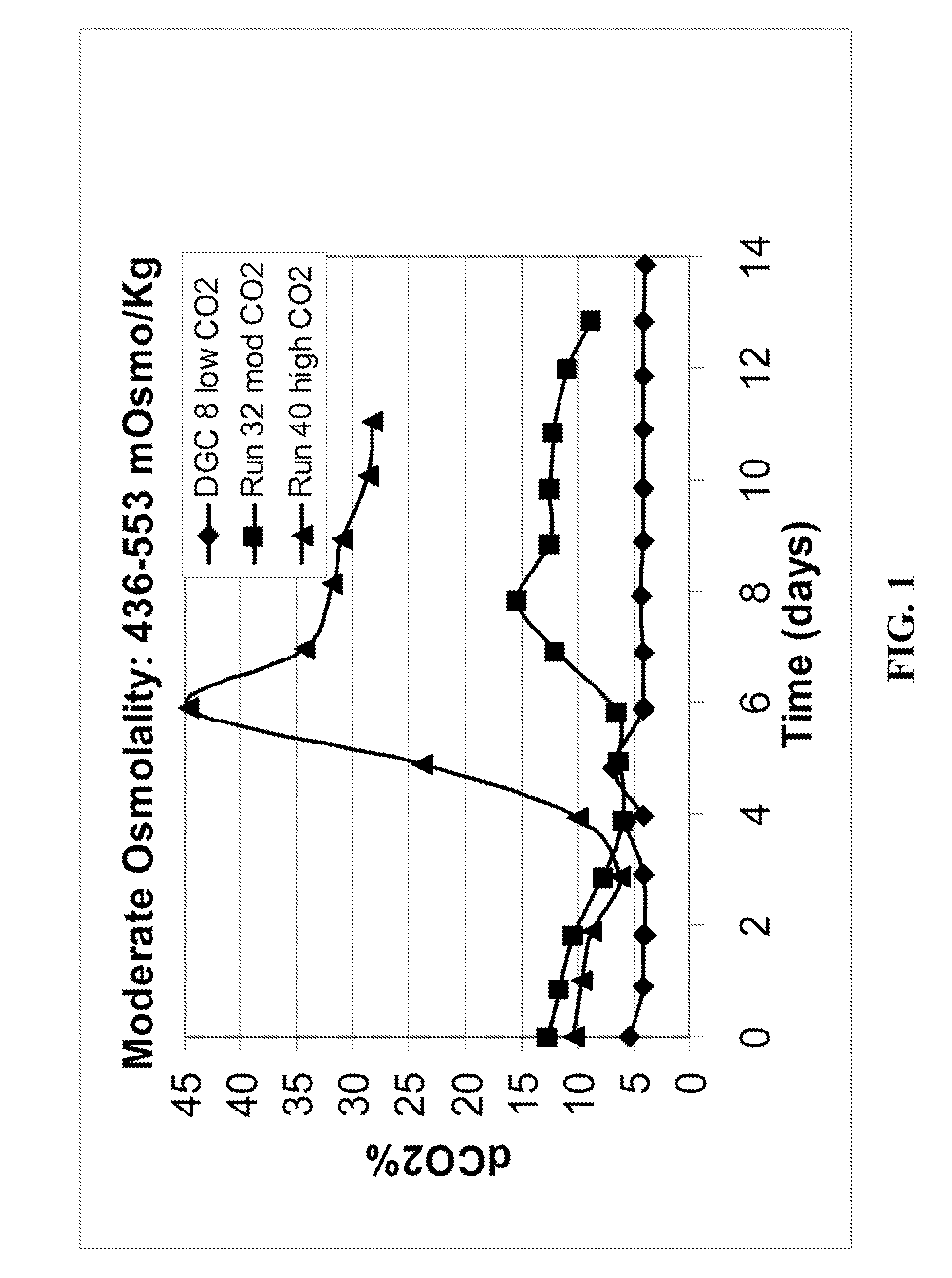
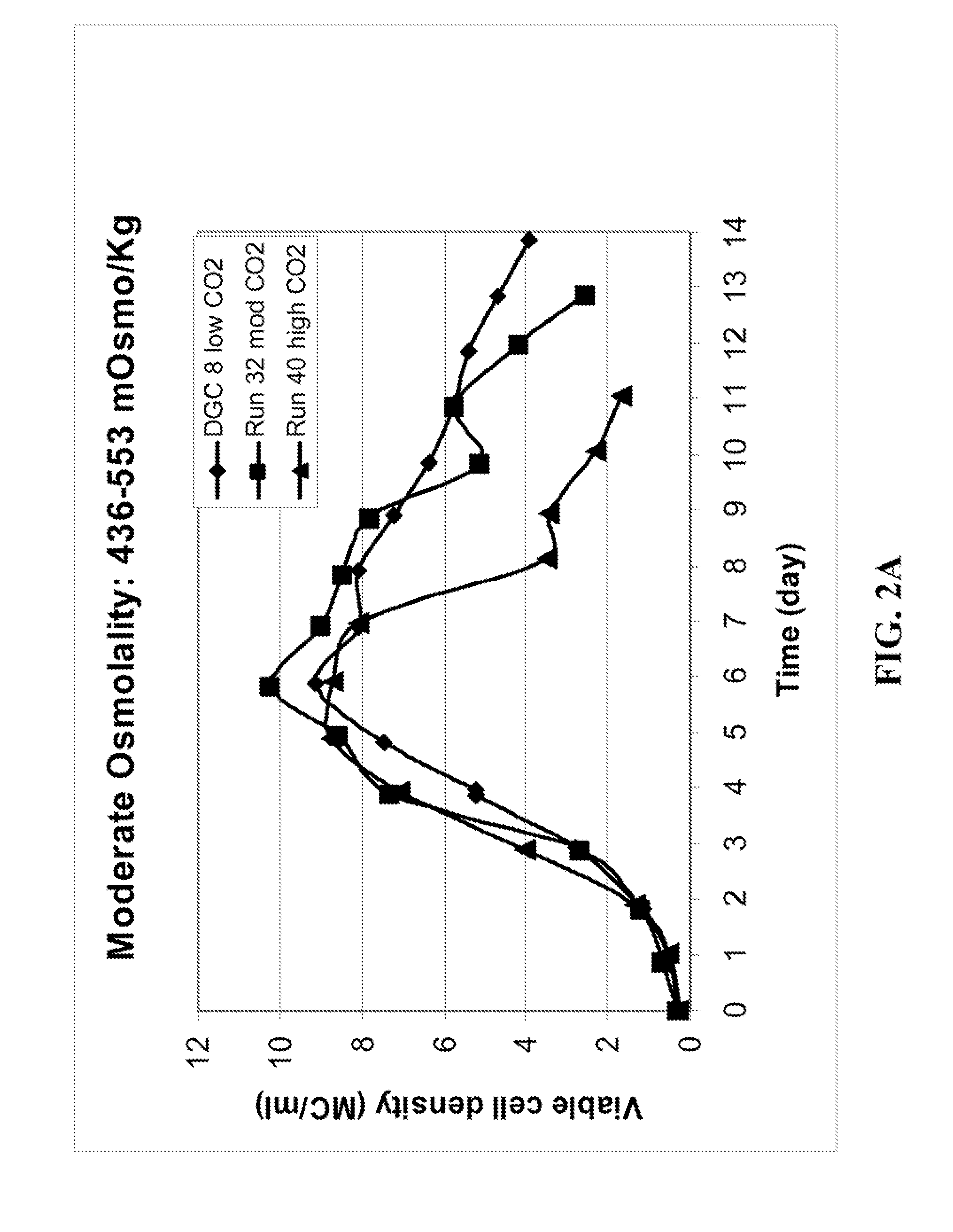
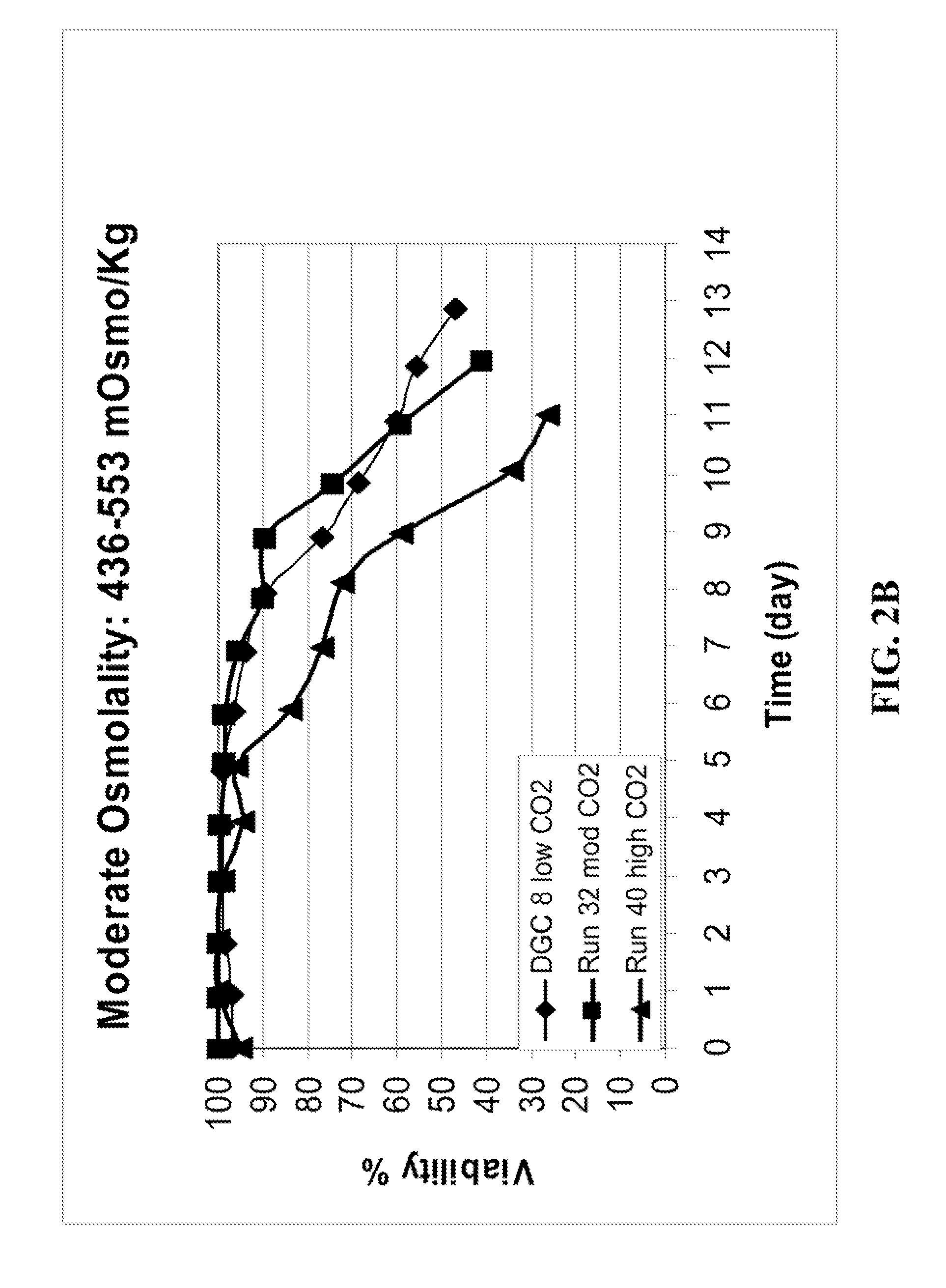
METHOD FOR CONTROLLING pH, OSMOLALITY AND DISSOLVED CARBON DIOXIDE LEVELS IN A MAMMALIAN CELL CULTURE PROCESS TO ENHANCE CELL VIABILITY AND BIOLOGIC PRODUCT YIELD
ActiveUS20100184147A1Facilitate communicationImprove production yieldBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyPh control
Methods for controlling the level of dissolved carbon dioxide and limiting osmolality in a mammalian cell culture process to enhance cell growth, viability and density, and increase biologic product concentration and yield are provided. Such control of the level of dissolved carbon dioxide and pH as well as the resulting ability to limit osmolality in a mammalian cell culture process is achieved by adopting alternative pH control strategies and CO2 stripping techniques during a mammalian cell culture process. Such pH control techniques and carbon dioxide stripping occur without foam and with little or no damage to the mammalian cells.
Owner:PRAXAIR TECH INC
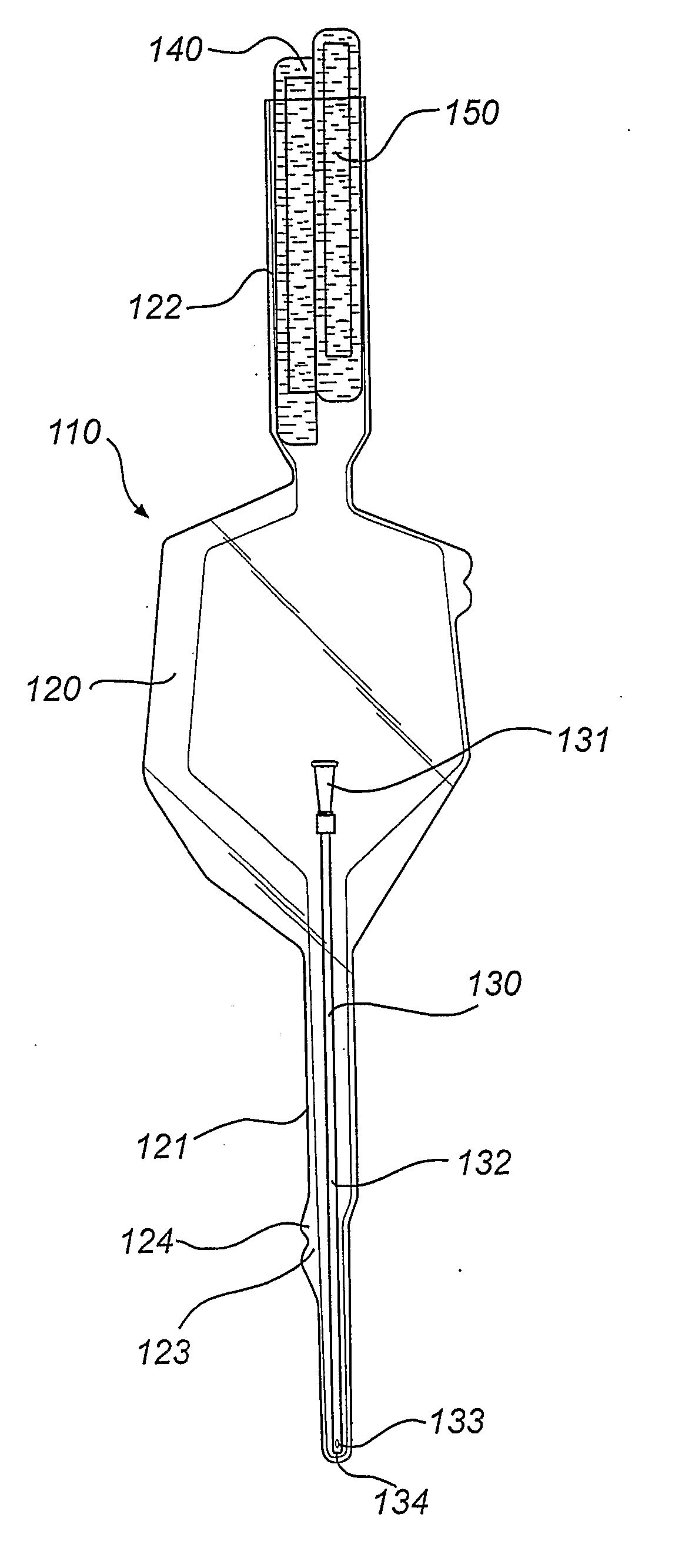
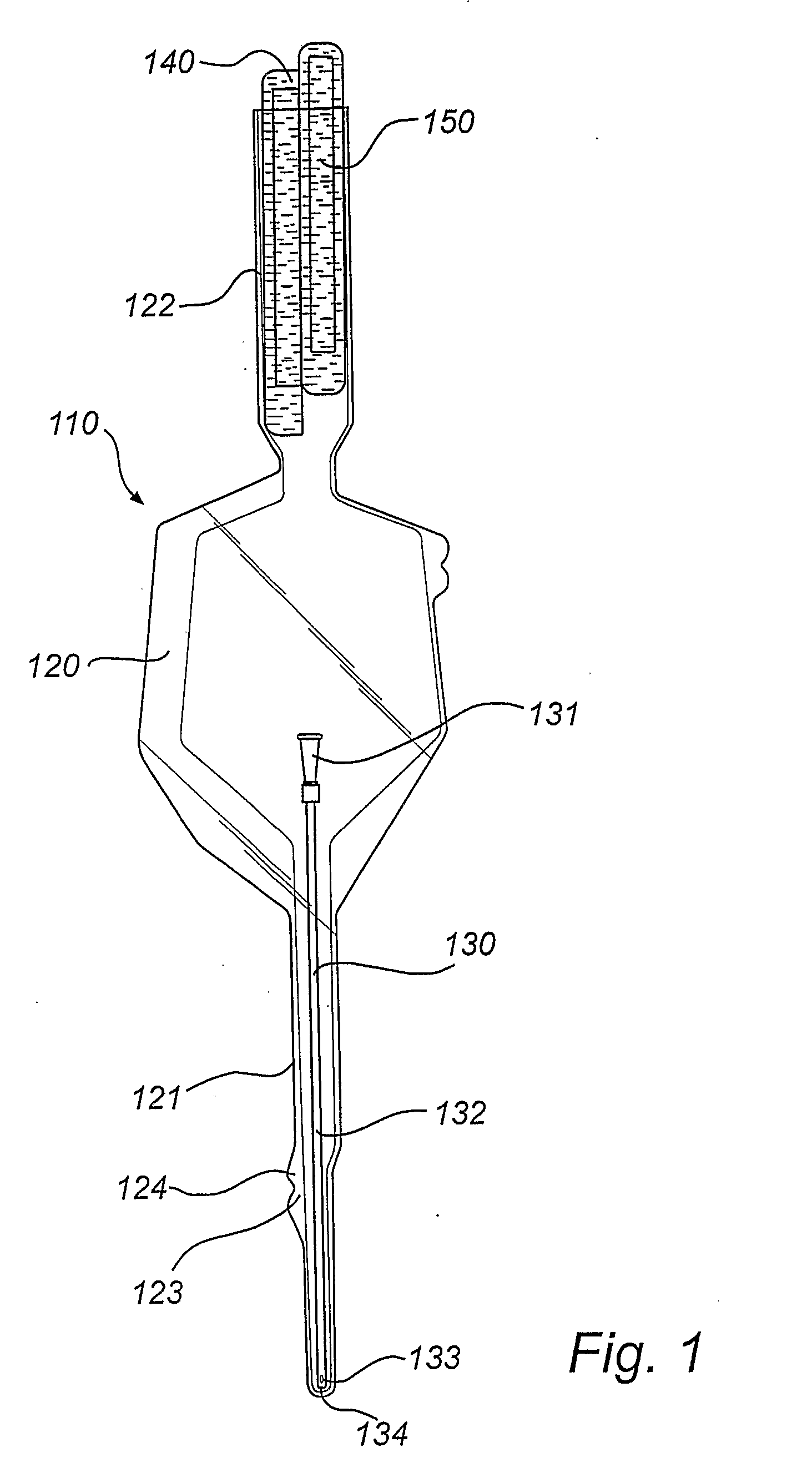
Catheter assembly with osmolality-increasing fluid
ActiveUS20070149929A1Improve propertiesReduce forceTransportation and packagingWound drainsHigh concentrationHydrophilic coating
A catheter assembly is disclosed comprising: a hydrophilic catheter; a wetting fluid for wetting of the catheter; and a receptacle enclosing at least the insertable part of the catheter and the wetting fluid. Further, the wetting fluid is a solution incorporating at least one osmolality-increasing compound, and the total concentration of the osmolality-increasing compound(s) is very high, preferably exceeding 600 mOsm / dm3. The wetting fluid could either be arranged in contact with the hydrophilic surface layer of the catheter in the receptacle, for preservation of the hydrophilic surface layer in a wetted state during accommodation in said receptacle and provision of a ready-to-use catheter assembly, or be arranged to keep the wetting fluid separated from the hydrophilic surface layer of the catheter during storage, but to be brought into contact with said hydrophilic surface layer upon activation before an intended use of the catheter. A similar method and wetting fluid is disclosed as well. The provision of the osmolality-increasing compound in the wetting fluid provides several advantages per se, such as a improved properties of the hydrophilic coating, a more predictable and controllable wetting process, a more expedient and cost efficient production, etc. Further, the use of this very high concentration of osmolality-increasing compound in the wetting fluid has proven remarkably efficient.
Owner:ASTRA TECH SE
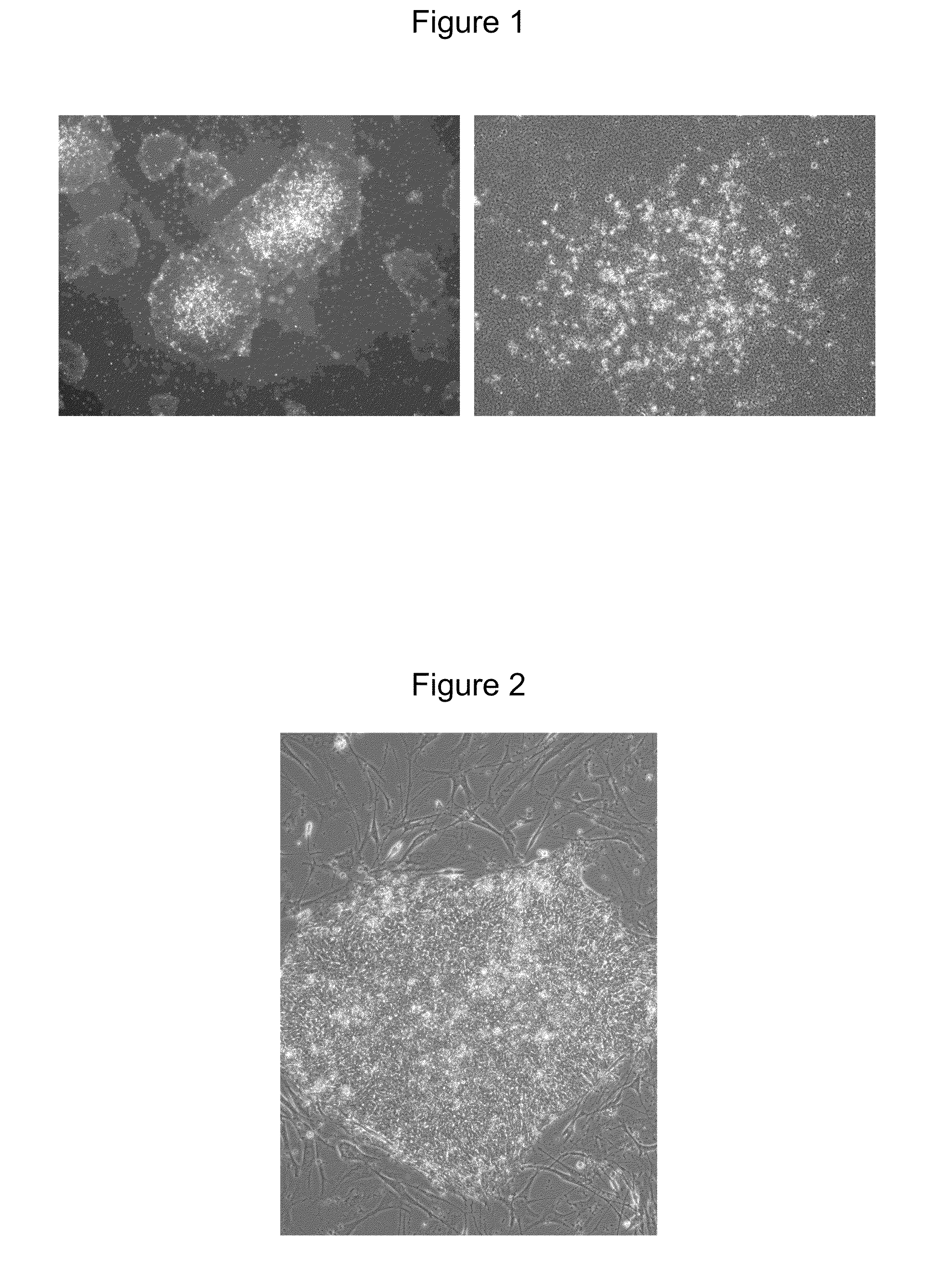
Method of Differentiating Stem Cells
ActiveUS20110086379A1Cell dissociation methodsMicrobiological testing/measurementCell lineageProgenitor
The present disclosure provides methods of generating germ layers from stem cells comprising culturing the stem cells in a culture medium having an osmolality less than 340 mOsm / kg. The present disclosure also includes a method to generate different cell lineages from the germ layers as well as to detect them by immunological methods. The present disclosure further provides methods for the generation, isolation, cultivation and propagation of committed progenitor cells and for the production of differentiated cells from the three germ layers. The present disclosure also provides culture media for use in inducing the three germ layers.
Owner:STEMCELL TECHNOLOGIES
Computerized System for Blood Chemistry Monitoring
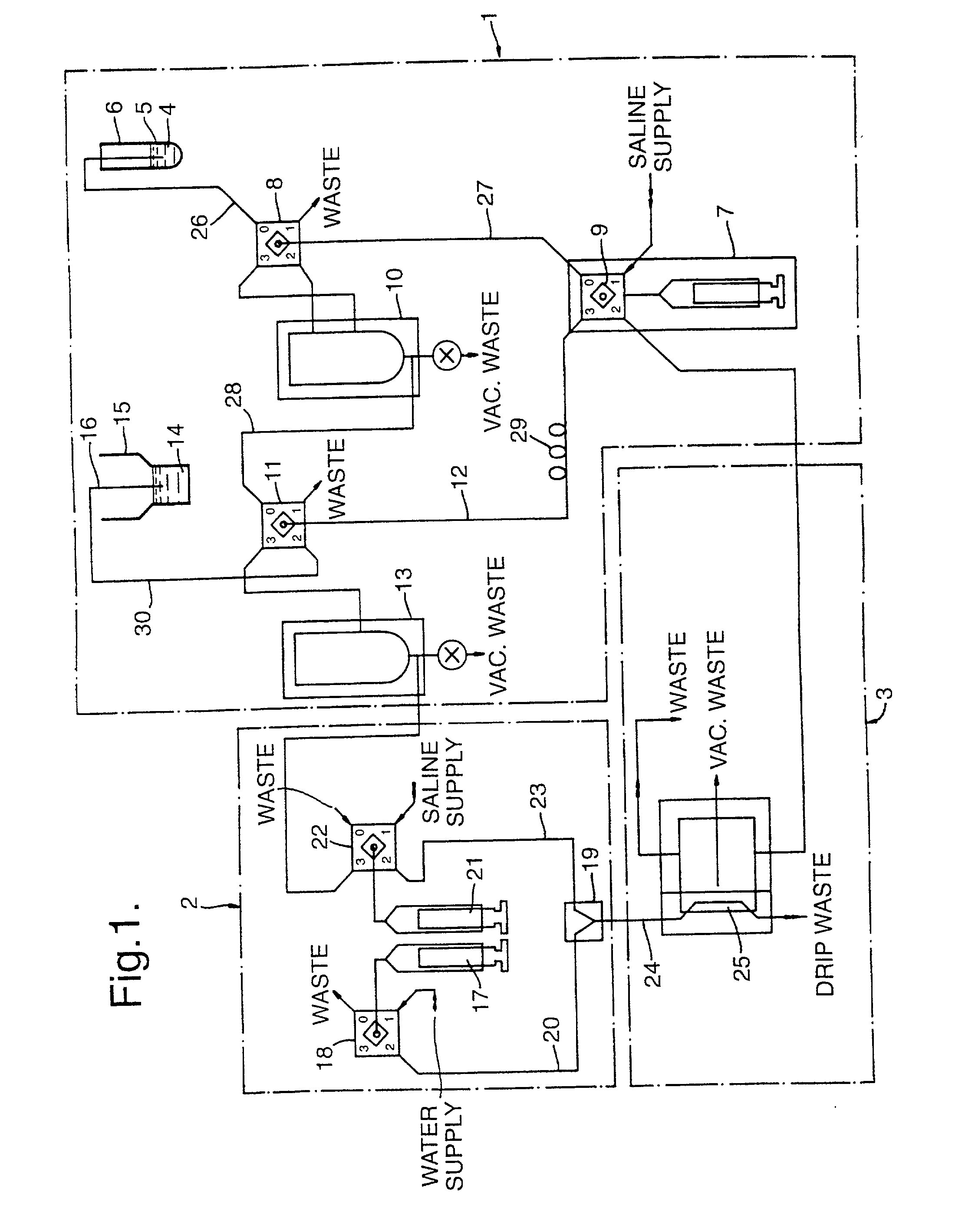
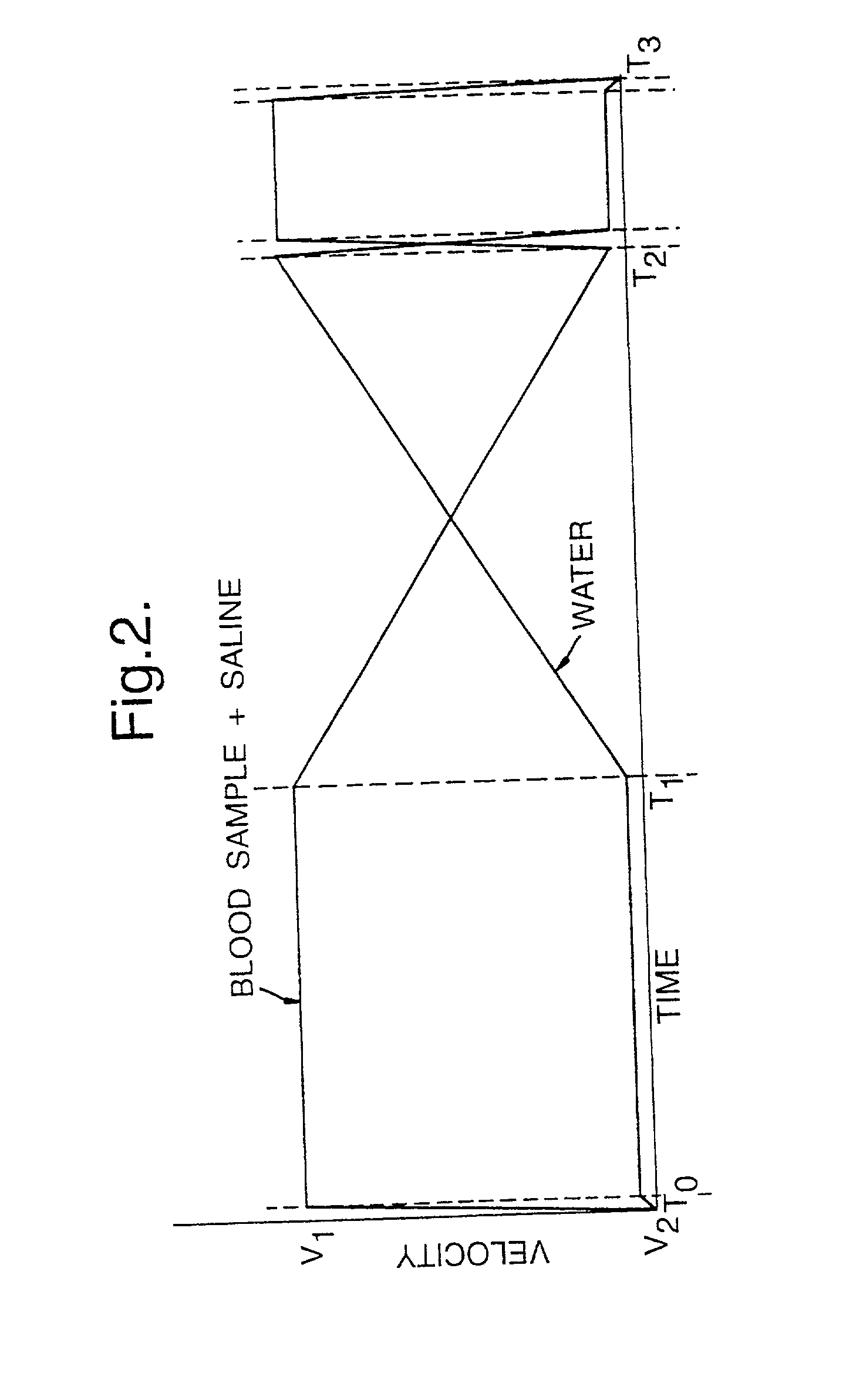
ActiveUS20100217238A1Stabilize blood sugar levelsPharmaceutical delivery mechanismMedical devicesDiseaseOsmolar Concentration
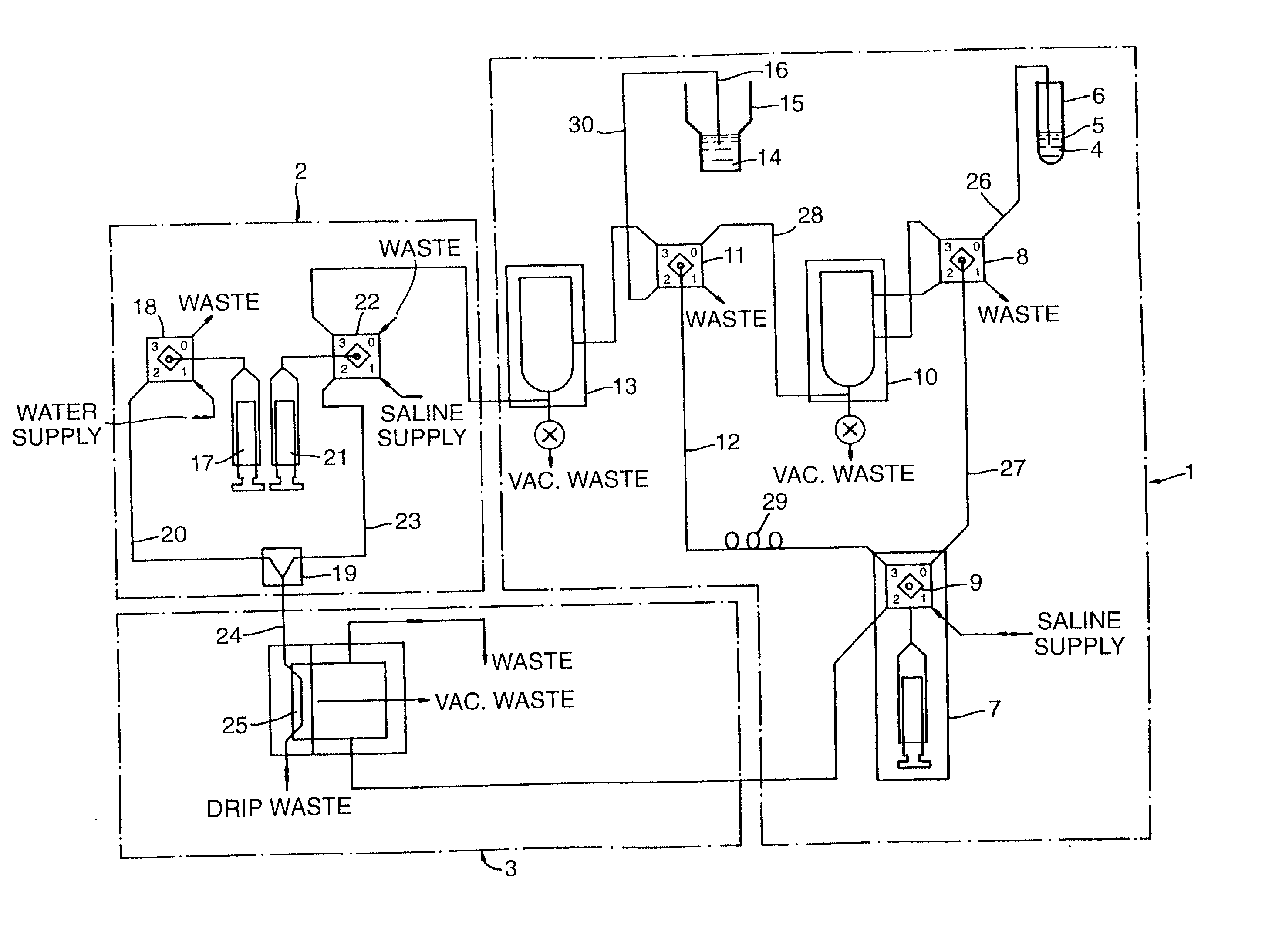
An apparatus and computerized method of intravenously monitoring a patient's blood chemistry transmits real time measurements to an electronically controlled closed loop system that auto-regulates blood osmolality and glucose level with medications infused through a catheter designed for such purpose. The closed loop system utilizes a glucose algorithm and an osmolality algorithm implemented in hardware and software to control the flow of dextrose, insulin and hypertonic saline to a patient in an effort to achieve better patient outcomes in instances of trauma, surgery and medical illnesses.
Owner:IDEAL MEDICAL TECH
Isotonic juice drink for children
An isotonic drink that is especially designed for children between the ages of 6 and 12 years. The drink contains water, carbohydrates including fruit juice, a calcium source and vitamin C. The drink has an osmolality of about 300 to about 380 mOsm / kg and a pH of between about 3 and about 4.5. The drink improves hydration compliance when consumed by children.
Owner:NESTEC SA
METHOD FOR CONTROLLING pH, OSMOLALITY AND DISSOLVED CARBON DIOXIDE LEVELS IN A MAMMALIAN CELL CULTURE PROCESS TO ENHANCE CELL VIABILITY AND BIOLOGIC PRODUCT YIELD
InactiveUS20100035342A1Increase concentrationHigh densityVertebrate cellsEnzymology/microbiology apparatusBiotechnologyPh control
Methods for controlling the level of dissolved carbon dioxide and limiting osmolality in a mammalian cell culture process to enhance cell growth, viability and density, and increase biologic product concentration and yield are provided. Such control of the level of dissolved carbon dioxide and pH as well as the resulting ability to limit osmolality in a mammalian cell culture process is achieved by adopting alternative pH control strategies and CO2 stripping techniques during a mammalian cell culture process. Such pH control techniques and carbon dioxide stripping occur with little or no damage to the mammalian cells.
Owner:PRAXAIR TECH INC
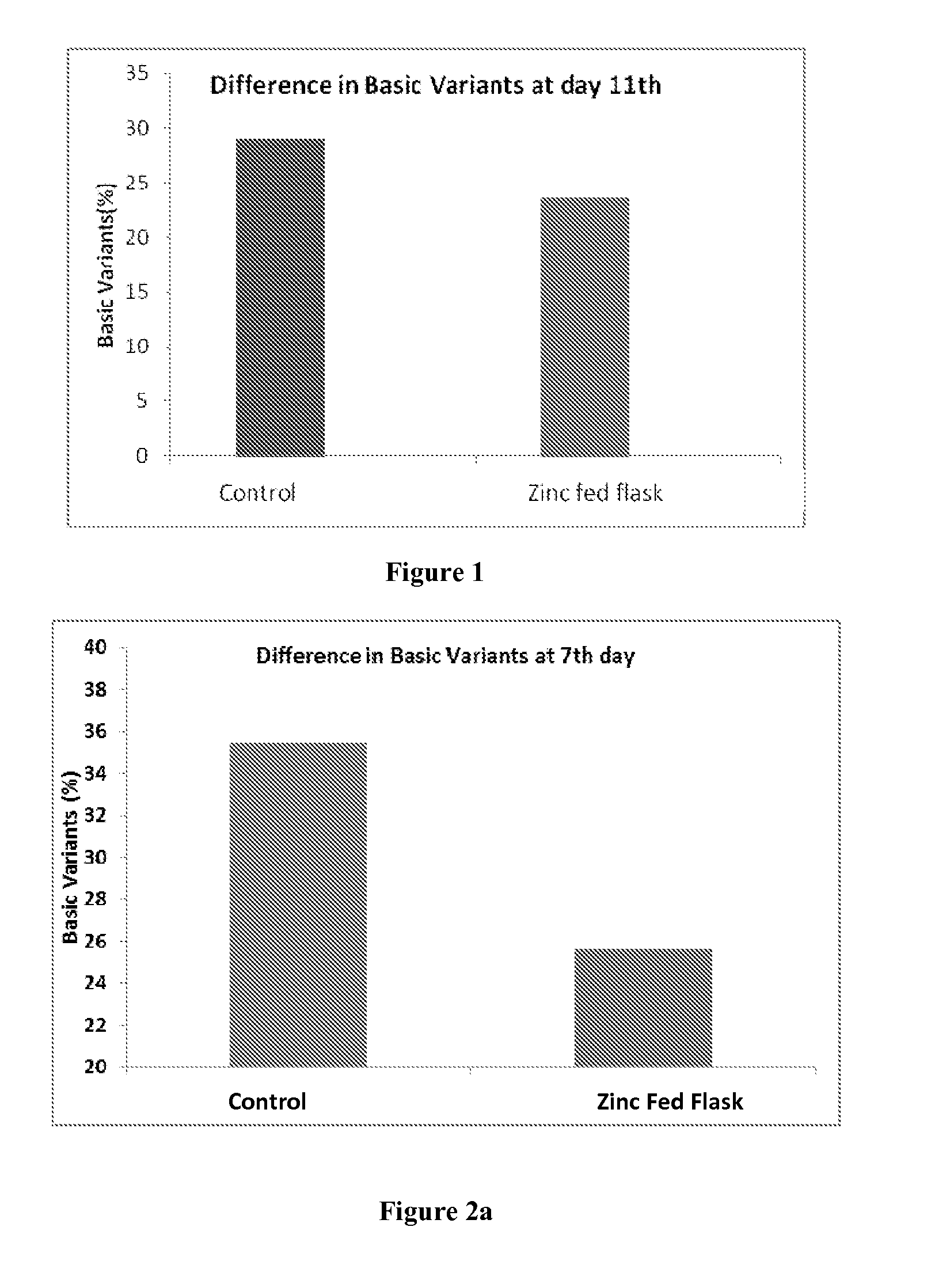
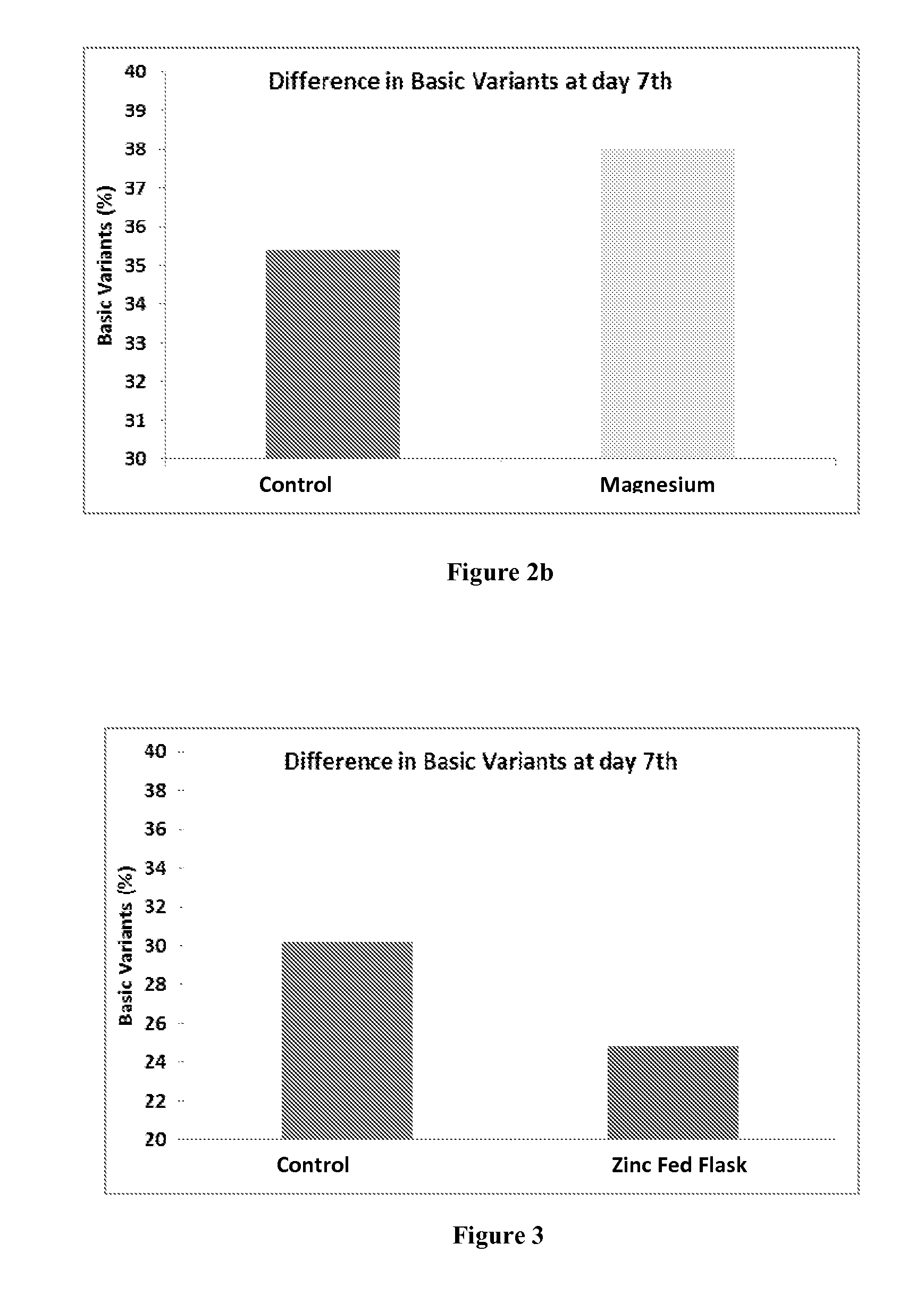
Method for Reducing Heterogeneity of Antibodies and a Process of Producing the Antibodies Thereof
ActiveUS20140199729A1Low heterogeneityAntibody ingredientsImmunoglobulinsCulture cellCell culture media
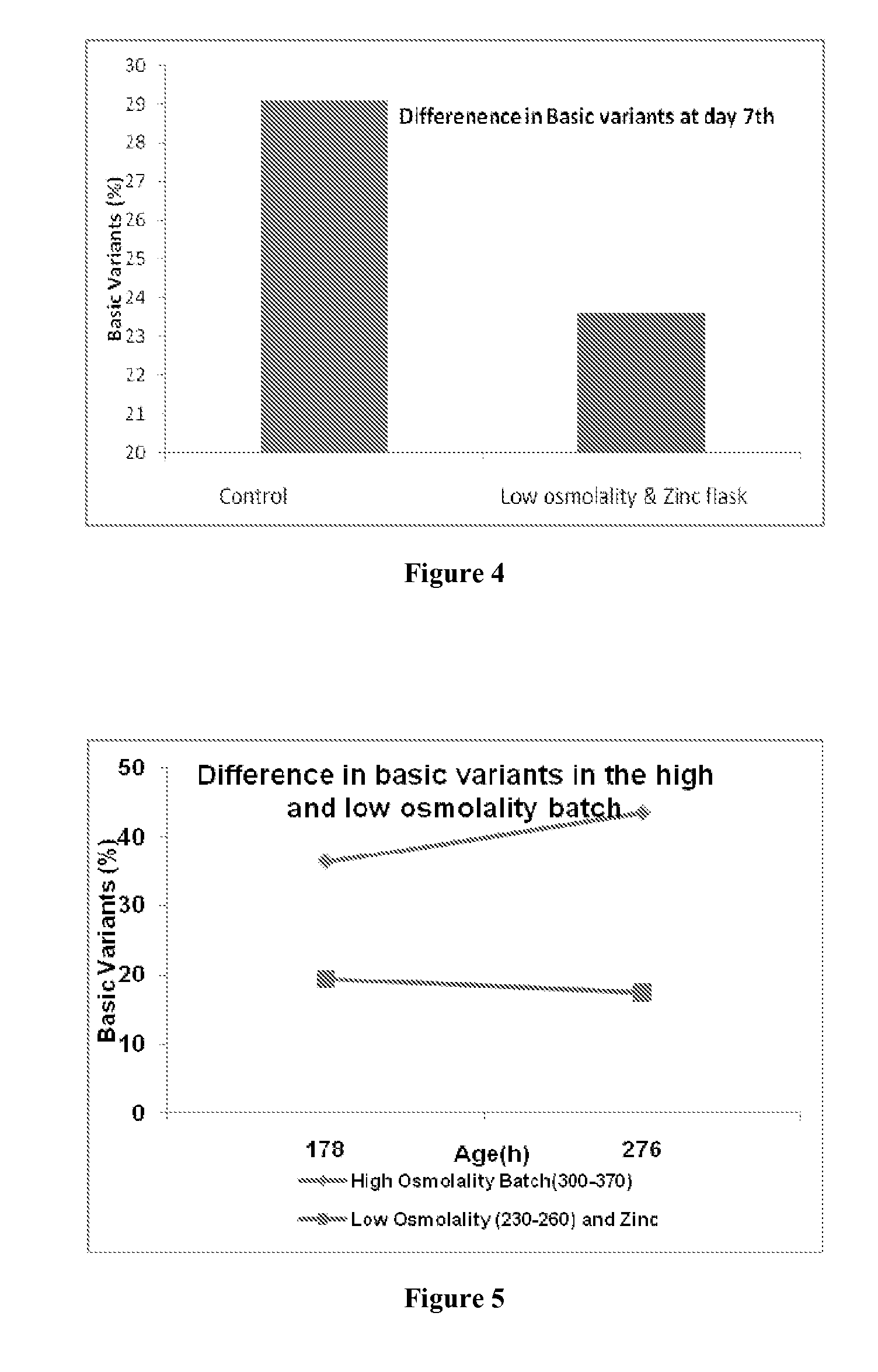
The present disclosure relates to a method of reducing heterogeneity in antibodies during culturing, wherein the heterogeneity is due to proportion of charge variant of the antibody. The disclosure also comprises a process of growing cells in a cell culture system that results in antibodies with the reduced heterogeneity. In one embodiment antibody heterogeneity is reduced by the addition of divalent transitional metal ions such as zinc (Zn2+) to the cell culture medium. In another embodiment antibody heterogeneity is reduced by decreasing the osmolality of the cell culture medium.
Owner:BIOCON BIOLOGICS INDIA LTD
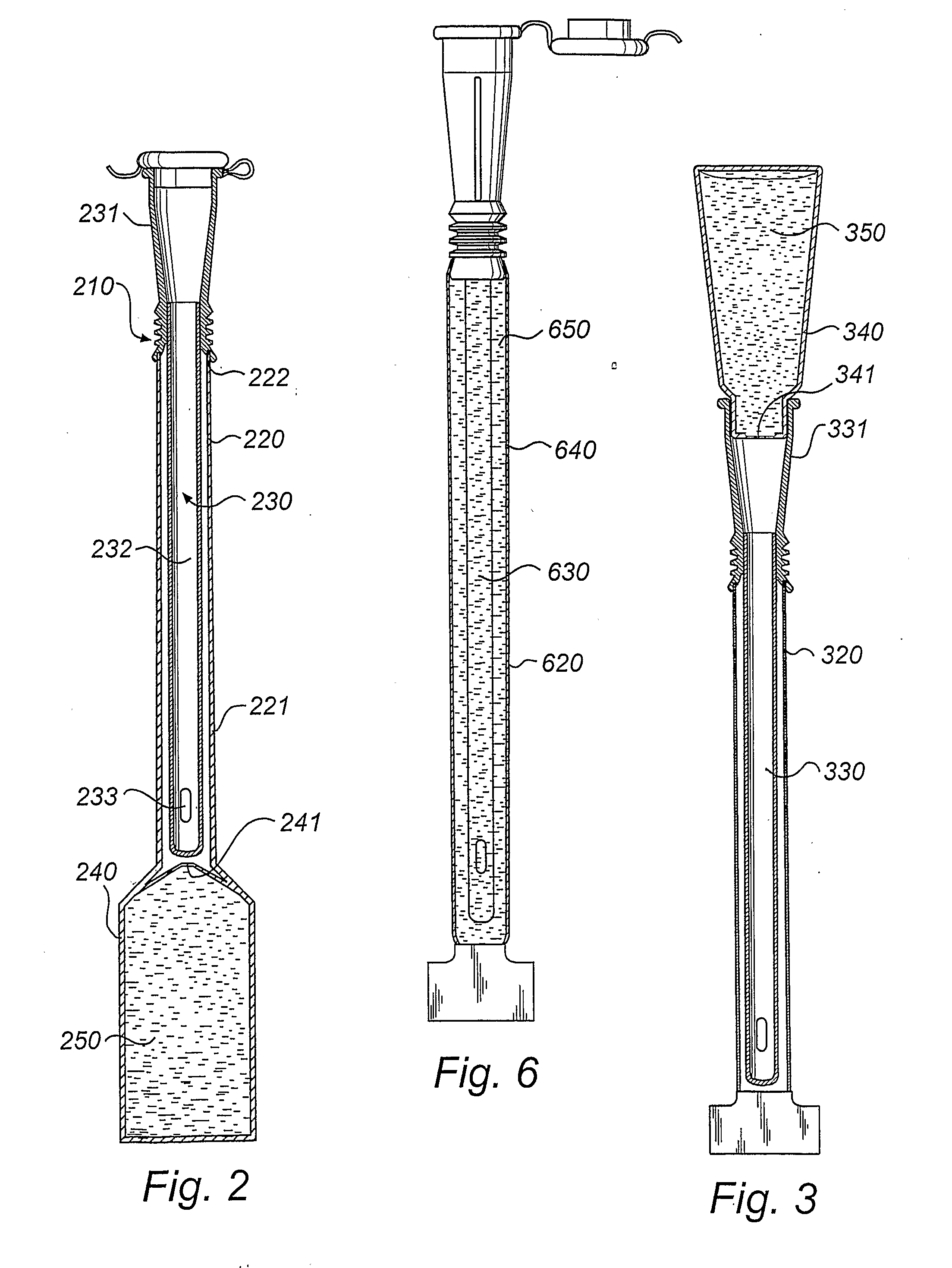
An assembly for the preparation of a medical device having a coating comprising hydrogen peroxide
ActiveCN1753702AInhibit the development of infectionReduced stabilitySurgeryCatheterGuide wiresPolyvinylpyrrolidone
The present invention provides an assembly for the preparation of a medical device having a porous coating comprising hydrogen peroxide. Particularly interesting medical devices are catheters (such as urinary catheters), endoscopes, laryngoscopes, tubes for feeding, tubes for drainage, guide wires, condoms, urisheaths, barrier coatings e.g. for gloves, stents and other implants, extra corporeal blood conduits, membranes e.g. for dialysis, blood filters, devices for circulatory assistance, dressings for wound care, and ostomy bags. The coating is in particular a hydrophilic coating formed from cross-linked polyvinylpyrrolidone. In one embodiment, the assembly holds a dry catheter element in one compartment of a package and an aqueous hydrogen peroxide solution in another compartment. The solution may also comprise stabilizers, e.g. chelators, and osmolality increasing agents. The catheter for insertion in the urethra is useful for the treatment, alleviation or prophylaxis of microbial infections such as urinary tract infections (UTI).
Owner:COLOPLAST AS
Formulations of Tetrahydropyridine Antiplatelet Agents for Parenteral or Oral Administration
A pharmaceutical composition for the oral or parenteral administration of a compound of Formula (I) comprising an oil in water emulsion, wherein the oil phase comprises the free base of or a pharmaceutically acceptable salt thereof of a compound of Formula (I), and one or more surfactants which are soluble in the oil phase and / or the aqueous phase. The emulsion optionally contains one or more excipients that are soluble in the oil phase and / or the aqueous phase, such as pH modifying agents such as buffers, osmolality / tonicity modifying agents, emulsifying agents, water-soluble polymers, and preservatives. The compound of Formula (I) can be formulated as a solid material and stored until needed. Kits for forming the emulsion are provided. Prior to administration, the solid material can be reconstituted in an aqueous medium to form the emulsion.
Owner:ACUSPHERE INC
Cell culture process
The invention provides a method for producing a recombinant polypeptide of interest which method comprises: (a) providing a host cell which comprises a nucleotide sequence which encodes the recombinant polypeptide of interest and which directs expression of the recombinant polypeptide of interest in the host cell; (b) providing a serum-free culture medium which comprises (i) water, a plant-derived peptone, an osmolatity regulator, a buffer, an energy source, at least one amino acid, a lipid source or precursor, a source of iron, non-ferrous metal ions and optionally one or more vitamins and cofactors; and (ii) does not contain any full-length polypeptides; and (c) culturing the host cell in the culture medium under conditions that allow for expression of the recombinant polypeptide of interest.
Owner:SANDOZ AG
Aqueous lubricant composition
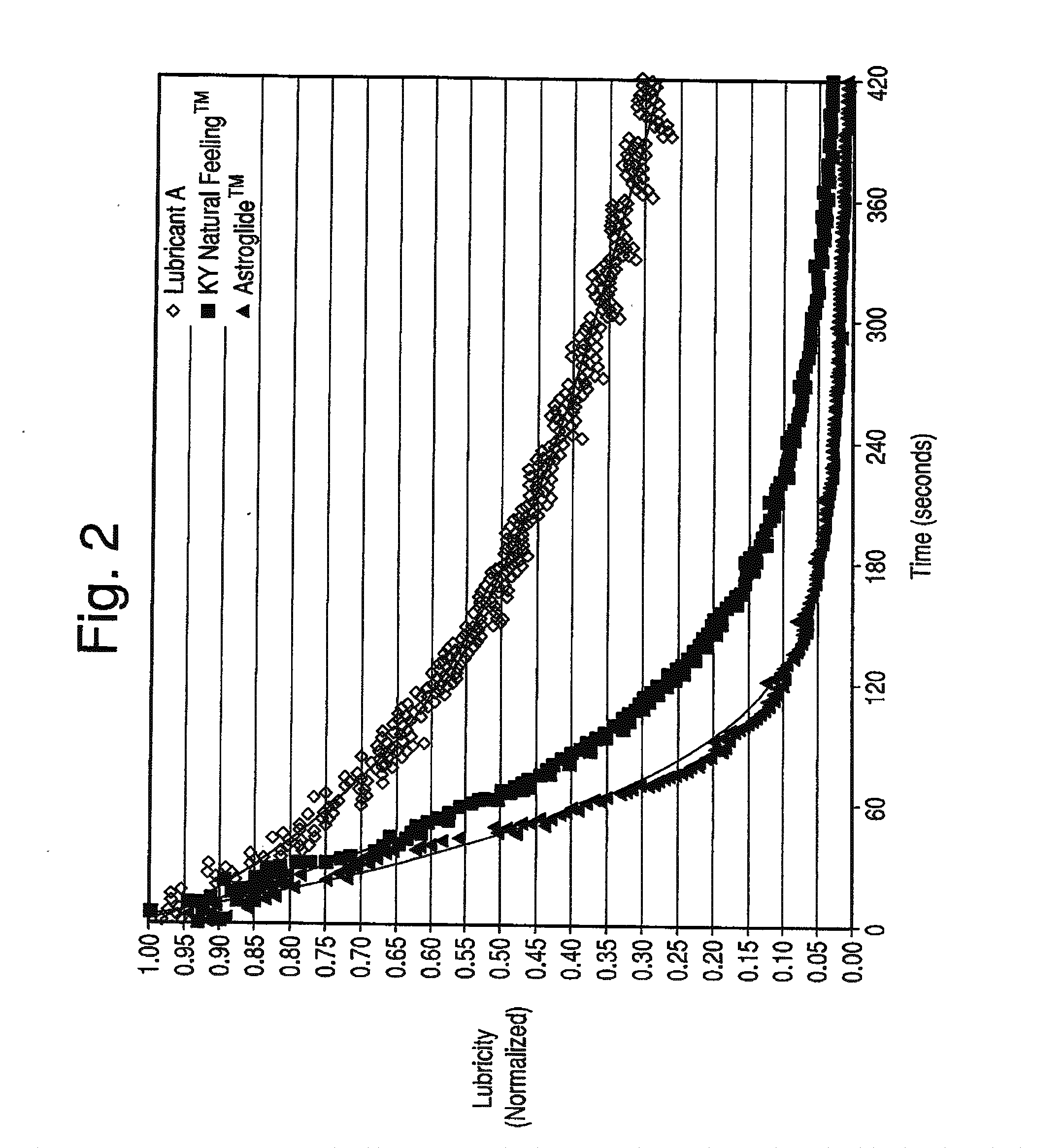
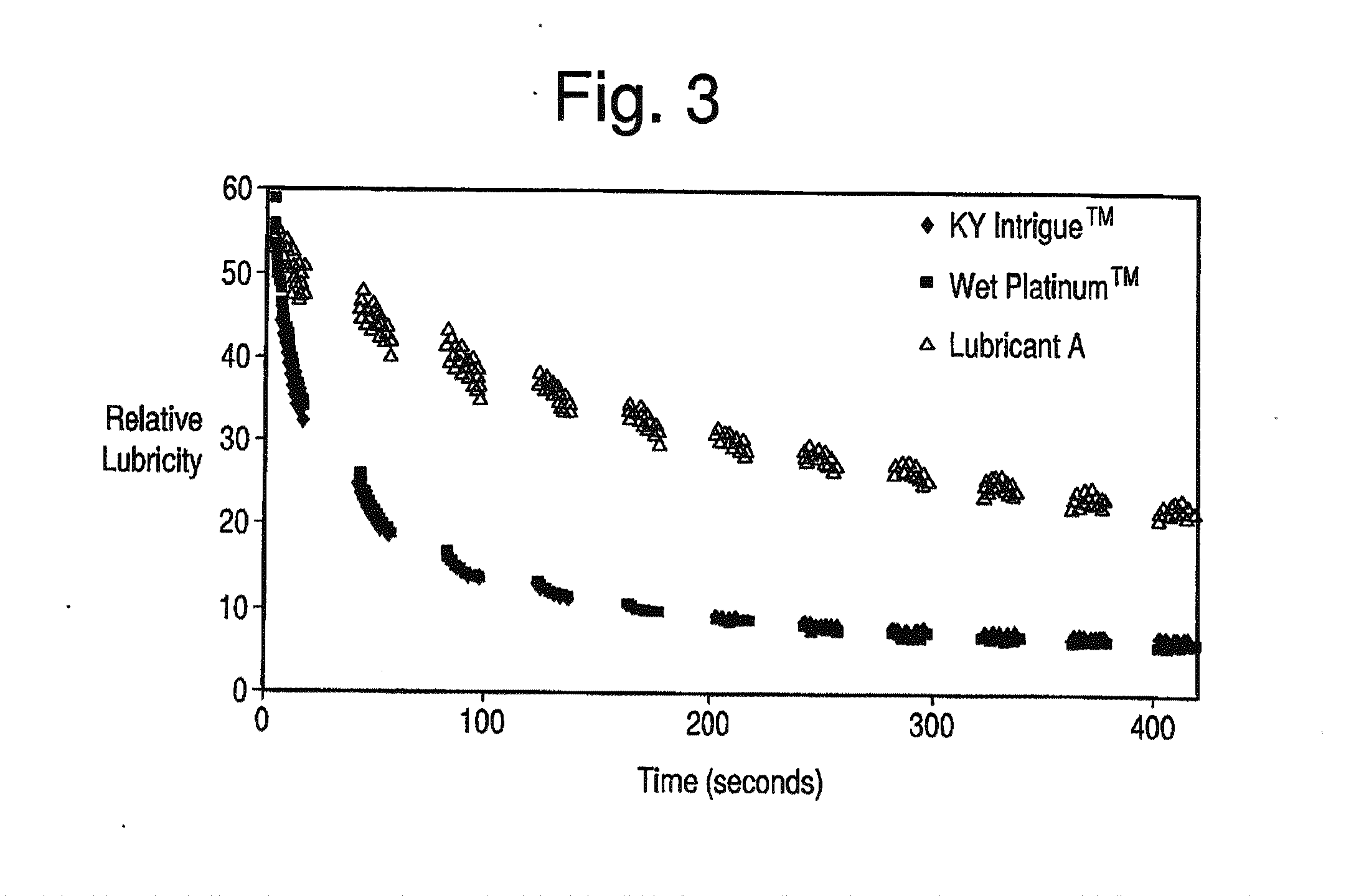
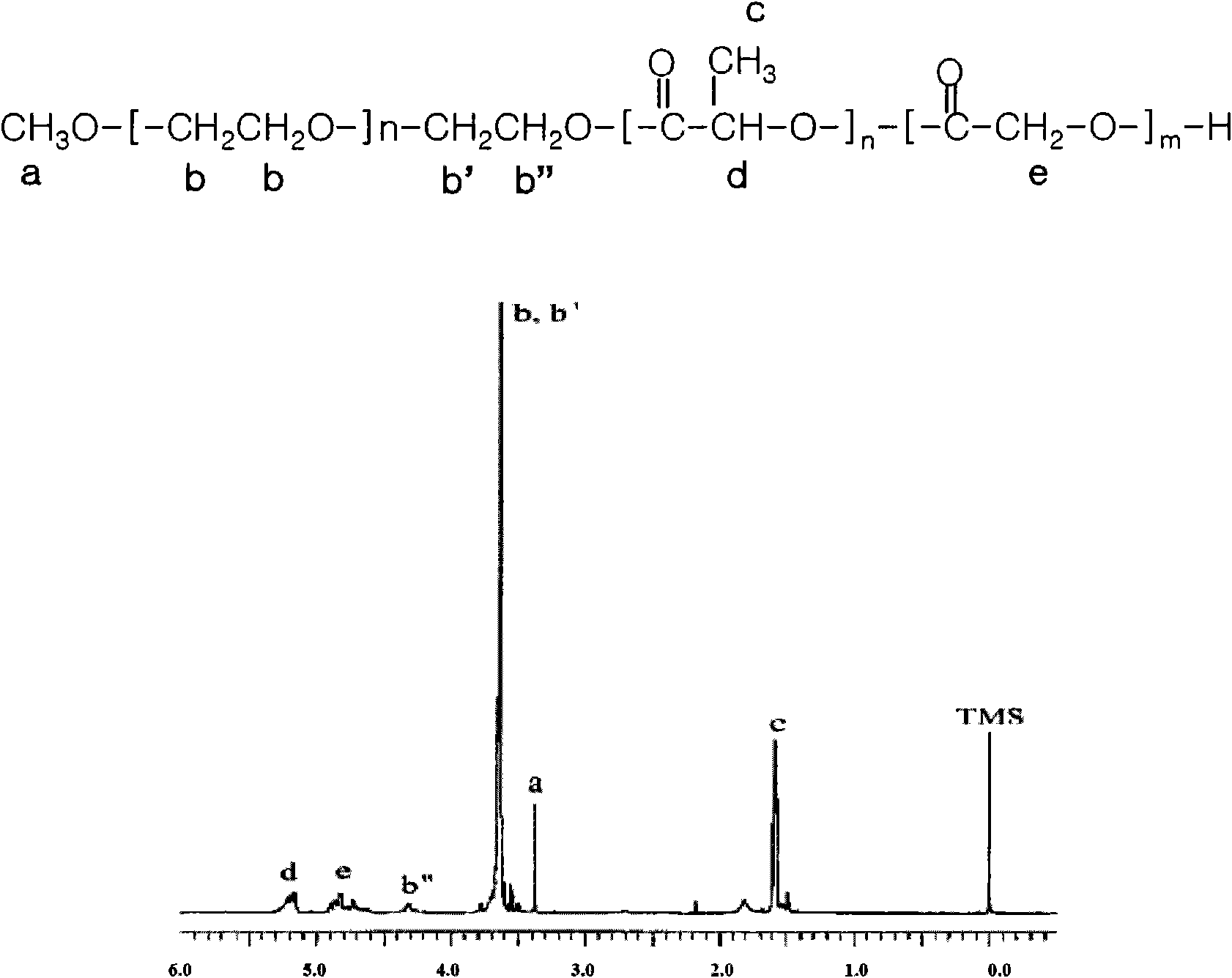
The present invention is directed towards an aqueous lubricant composition comprising hyaluronic acid, organic acid, salts and water, and has a pH of between 5.5 and 7.0 and an osmolality between about 150 and 1,500 mOsm / kg. The lubricant surprisingly has long-lasting lubricity and low osmolality as compared to conventional lubricants on the market.
Owner:CHURCH & DWIGHT CO INC
Amphiphilic block copolymer micelle composition containing taxane and manufacturing process of the same
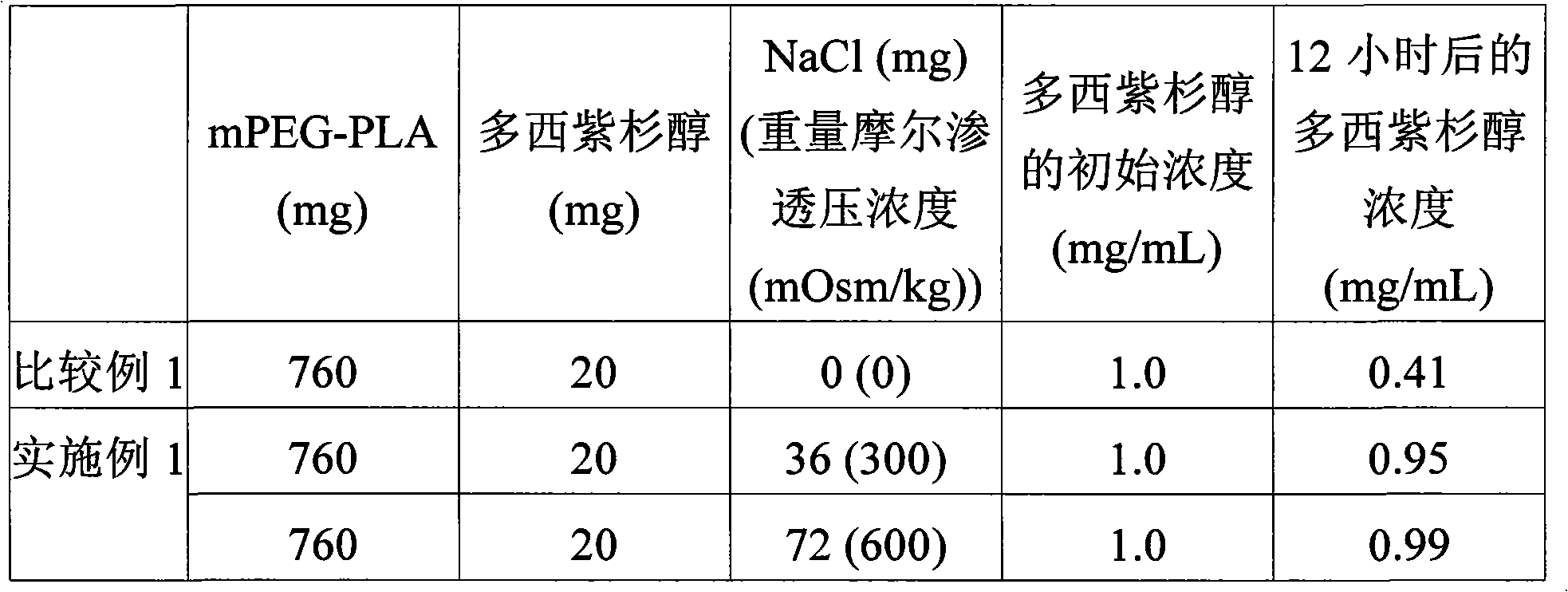
ActiveCN101910274AImprove stabilityQuick releaseOrganic active ingredientsEmulsion deliveryOsmolar ConcentrationCopolymer
A taxane-containing amphiphilic block copolymer micelle composition, including taxane, an amphiphilic block copolymer containing a hydrophilic block and a hydrophobic block, and an osmolality adjusting agent, is discribed. Also described are a method for preparing the same composition. The composition has excellent stability so that it can prevent rapid release of a drug and can improve a desired pharmacological effect. Additionally, the method enables highly efficient preparation of the composition.
Owner:SAMYANG HLDG CORP
Isotonic juice drink for children
InactiveUS20020132034A1Animal feeding stuffFood ingredient as taste affecting agentFruit juiceVitamin C
An isotonic drink that is especially designed for children between the ages of 6 and 12 years. The drink contains water, carbohydrates including fruit juice, a calcium source and vitamin C. The drink has an osmolality of about 300 to about 380 mOsm / kg and a pH of between about 3 and about 4.5. The drink improves hydration compliance when consumed by children.
Owner:NESTEC SA
Targeted Lung Delivery of Citrulline and/or Another Nitric Oxide Precursor and a Method for Treatment of Pulmonary Deficiency of Nitric Oxide in Cystic Fibrosis and Other Pulmonary Diseases
A method of treatment of cystic fibrosis and other pulmonary diseases identified by nitric oxide deficiency, comprising administration of a nebulized solution of citrulline and / or another nitric oxide precursor as an inhalable aerosol or an inhalable dry powder for targeted delivery into conducting and central airways. Citrulline or another nitric oxide precursor is formulated as a composition having predetermined limited volume, salinity, pH and osmolality. The composition is nebulized into an aerosol having a mass median aerodynamic diameter (MMAD) between 2 μm and 6 μm.
Owner:ACTIVAERO
Methods for staining cells for identification and sorting
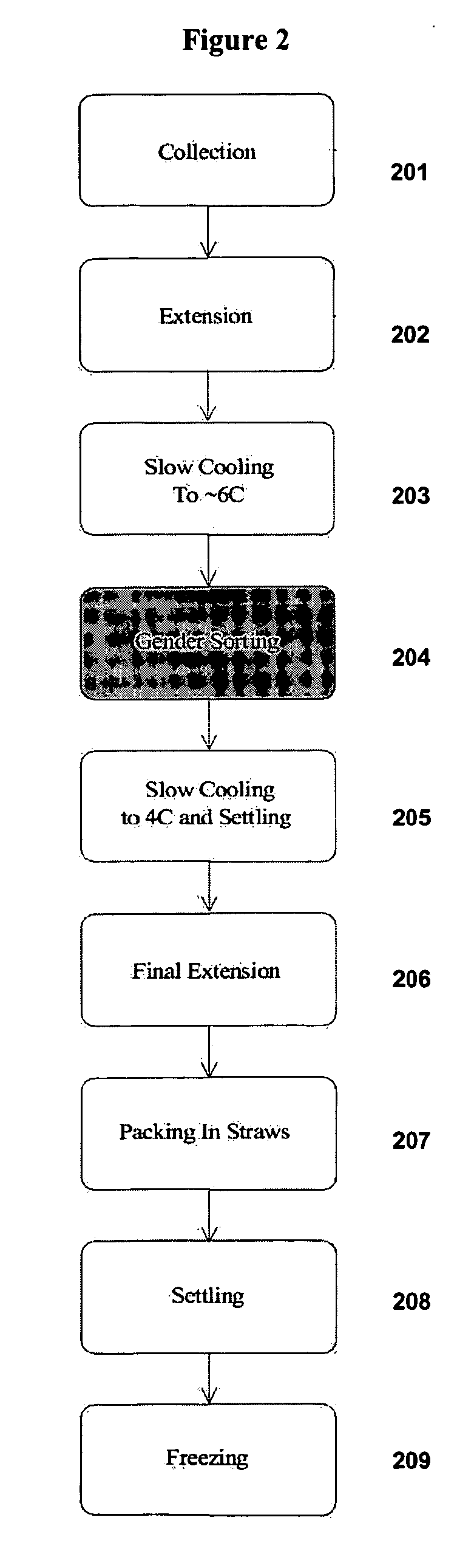
InactiveUS20060172315A1Convenient introductionImprove sperm motilityAnimal reproductionMicrobiological testing/measurementOsmolar ConcentrationCell staining
The present invention provides novel methods of cell staining, such as bovine sperm, using electroporation or osmolality treatments at viability-enhancing temperatures. Furthermore, methods of highly efficient cell sorting that are especially suitable in sorting bovine sperm using novel cell staining procedures are also provided.
Owner:ARRYX INC
Method for testing a cell sample
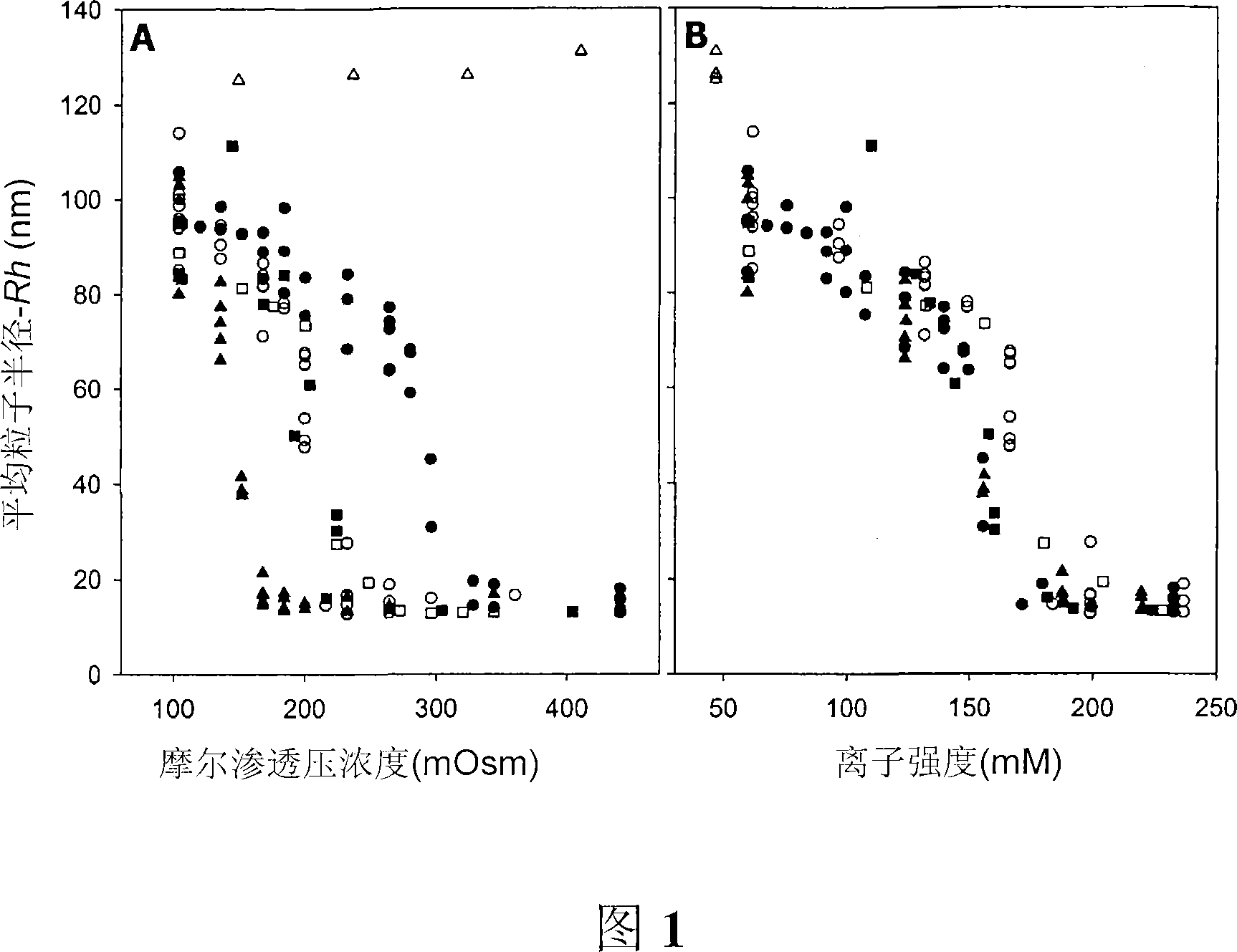
InactiveUS20020035879A1Increase the number ofImprove accuracyFlow propertiesWithdrawing sample devicesOsmolar ConcentrationCell parameter
In this invention, a cell parameter, for example cell volume, is determined by subjecting one or more aliquots of a sample cell suspension to one or more alterations of at least one parameter of the cell environment to identify a point at which the cells achieve a particular shape to obtain a sample specific shape compensation factor. Preferably, the environmental parameter change is a reduction in osmolality.
Owner:SHINE THOMAS ADAM +1
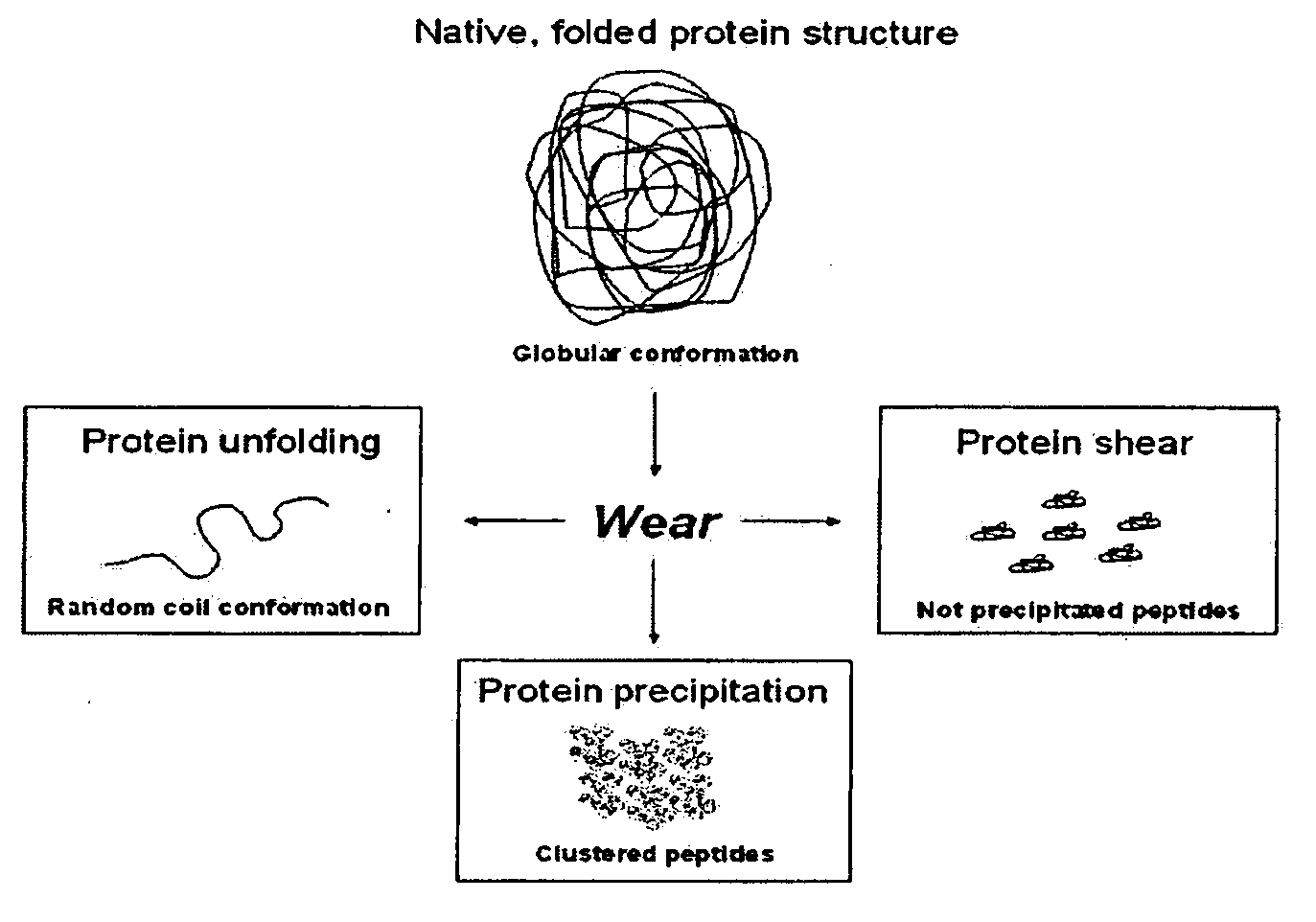
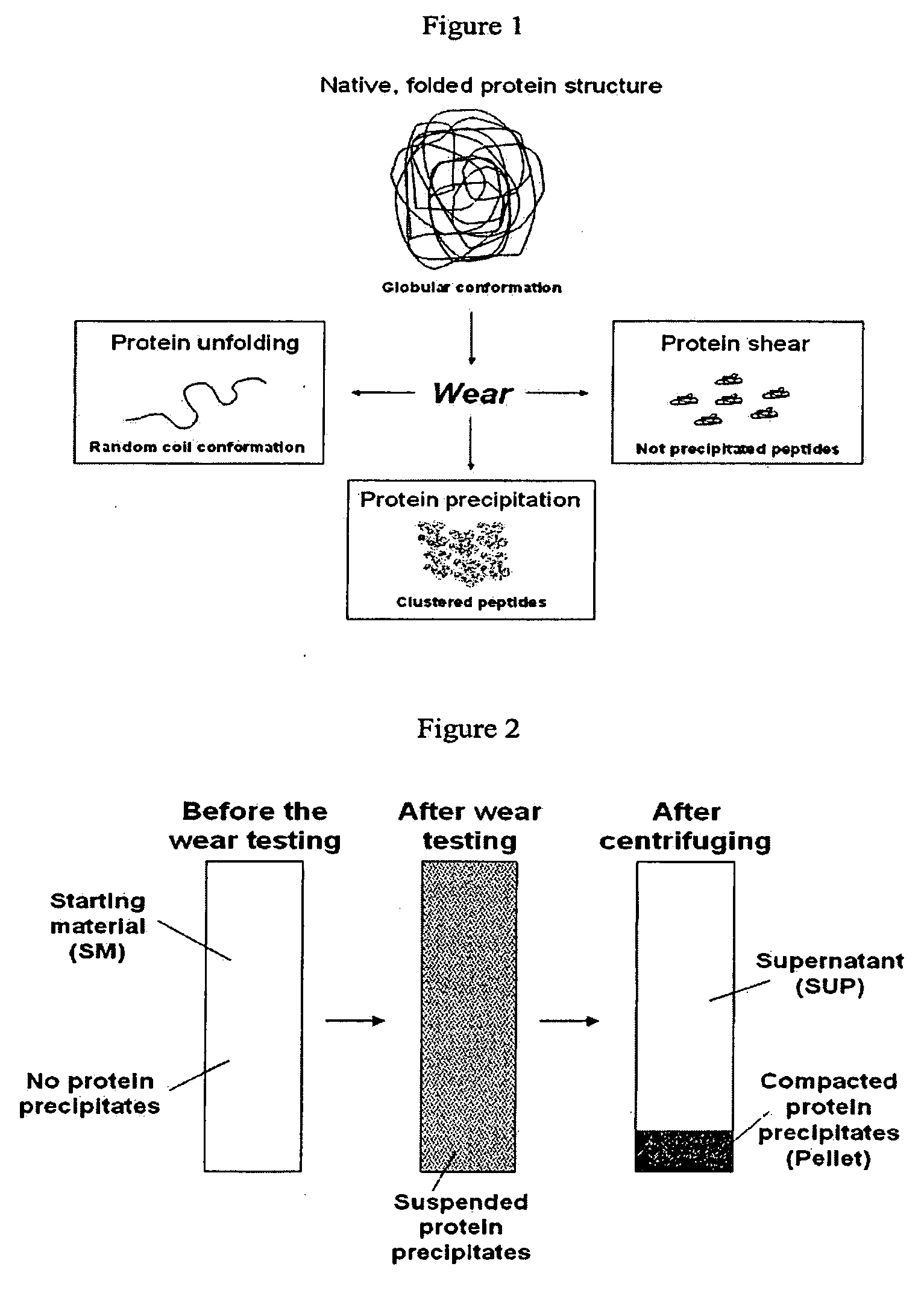
Lubricant for wear testing of joint replacements and associated materials
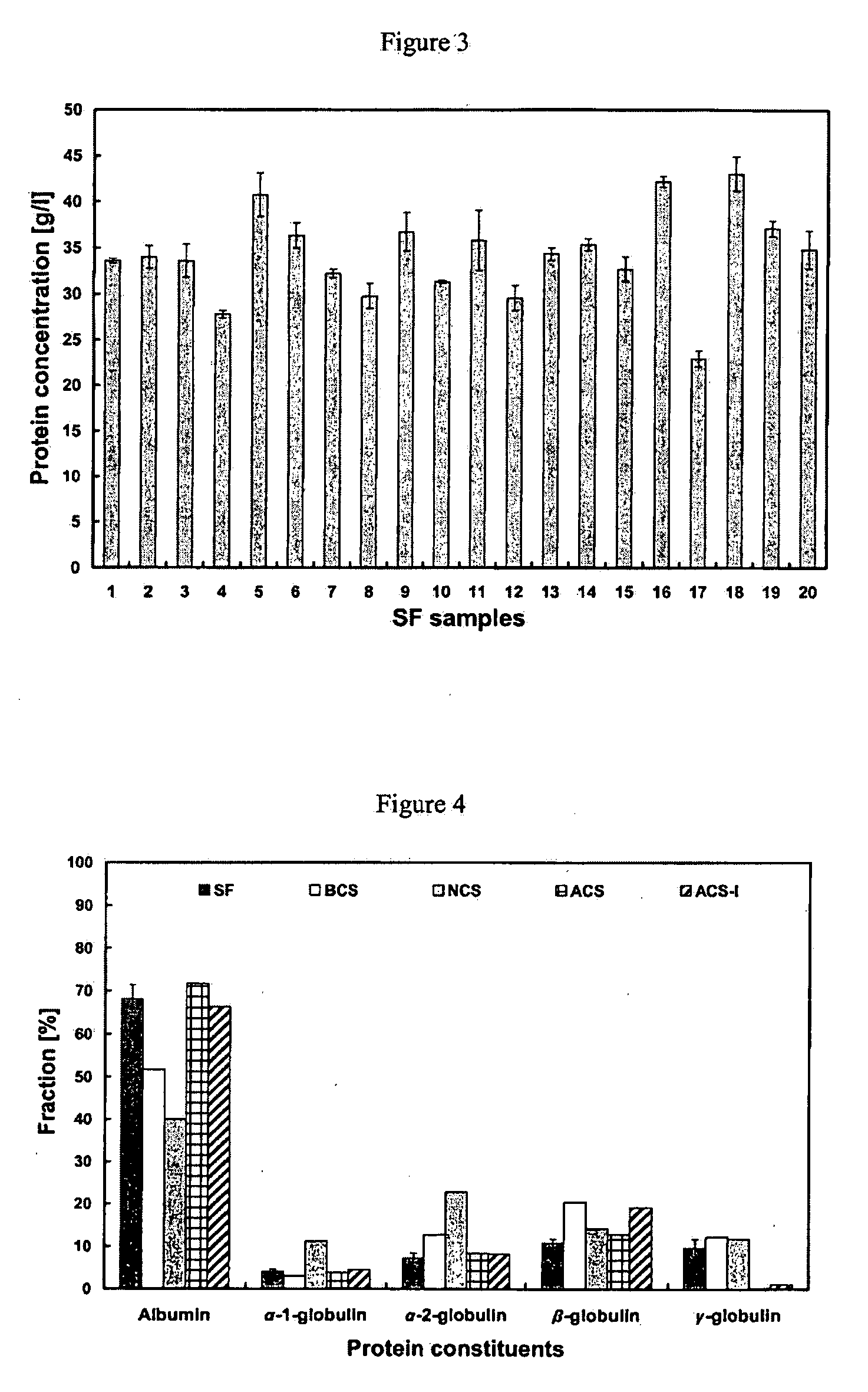
The present invention relates to an artificial synovial fluid composition that mimics the tribological properties of human, osteoarthritic synovial fluid (SF) for the main purpose of in vitro wear testing. There is described an artificial synovial fluid composition comprising: a) a mammalian serum having i) a total protein concentration of from about 10 to about 45 g / L; ii) an albumin fraction of from about 55% to about 80% w / w %; iii) an α-1 globulin fraction of from about 3% to about 6% w / w %; iv) an α-2 globulin fraction of from about 5% to about 10% w / w %; v) a β-globulin fraction of from about 3% to about 20% w / w %; vi) a γ-globulin fraction of from about 5% to about 20% w / w %; vii) a calcium (Ca) concentration of from about 0.1 mmol / L to about 3 mmol / L; viii) a magnesium (Mg) concentration of from about 0.05 mmol / L to about 0.8 mmol / L; ix) an inorganic phosphate (P) concentration of from about 0.1 mmol / L to about 1.5 mmol / L; x) an iron (Fe) concentration of from about 0.001 mmol / L to about 0.1 mmol / L; xi) a peptide concentration at a 2,000 Da molecular weight cut-off of from about 0.005 g / L to about 10 g / L; xii) a maximal transition midpoint temperature, Tm-cp-max, of from about 330 K to about 350 K at a concentration molality of from about 0.005 to about 0.15 mmol / L measured at a scan rate of from about 20 K / hour to about 100 K / hour; xiii) a total enthalpy change, ΔH, of from about 650 kJ mol−1 to about 1200 kJ mol−1 at a set concentration molality of 0.05 mmol / l measured at a scan rate of 60 K / hour; xiv) a total entropy change, ΔS, of from about 1.5 kJ mol−1 K−1 to about 4 kJ mol−1 K−1 at a concentration molality of from about 0.005 mmol / l to about 0.15 mmol / l and a scan rate from about 20 K / hour to 100 K / hour; wherein the ratio of albumin to total globulin (α-1 globulin+α-2 globulin+β-globulin+γ-globulin fractions) in solution is from about 1.5 to about 3; b) a buffer for maintaining a solution osmolality of from about 200 mmol / kg to about 400 mmol / kg; c) hyaluronic acid (HA) at a concentration of from about 0.1 g / L to about 6 g / L; d) hyaluronic acid (HA) with a molecular weight of from 1 MDa to 4 MDa; and e) a pH level of about 7 to about 8.
Owner:BRANDT JAN M +1
Method for enriching target cells from biology specimen and method for removing leucocyte
ActiveCN101469310ALow costImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsWhite blood cellOsmolar Concentration
The invention relates to a method for enriching target cells from biological samples and a method for removing white blood cells from the biological samples. The invention practically provides the method for enriching the target cells from the biological samples, which comprises: using a mode based on mol osmotic pressure concentration to remove red blood cells, and using immune affinity chromatography to remove the white blood cells. The method not only has low cost but also highly efficiently removes the white blood cells and the red blood cells, so as to enrich the target cells and be easy for subsequent detection. The invention also provides the method for removing the white blood cells from the biological samples, which comprises: adopting Affi-Gel 10 coupled by CD45 antibodies to remove the white blood cells.
Owner:杭州岳晨医药科技有限公司
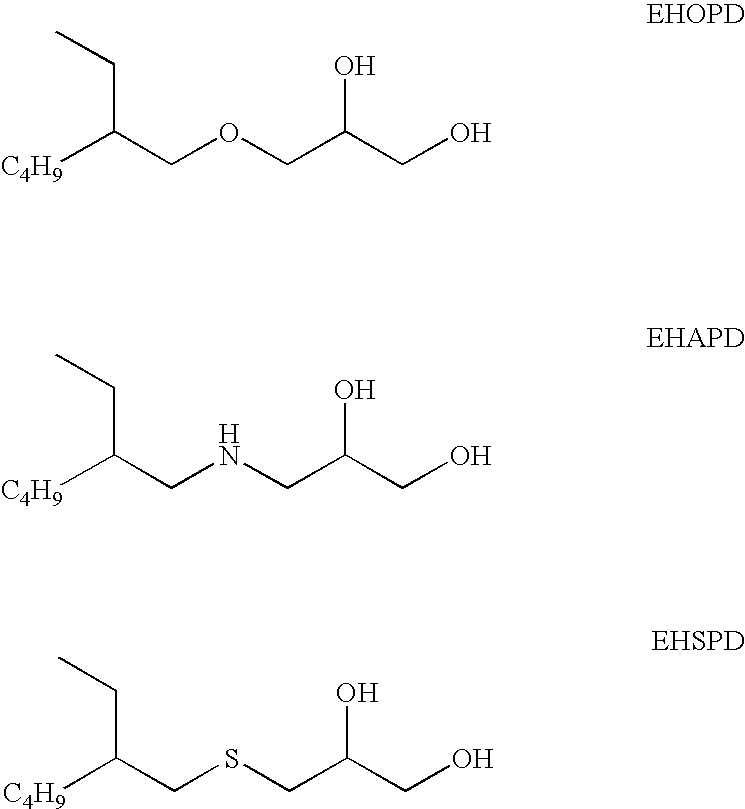
Ophthalmic compositions comprising a branched, glycerol monoalkyl compound and a fatty acid monoester
InactiveUS7670997B2Cosmetic preparationsOrganic detergent compounding agentsOsmolar ConcentrationGlycerol
An aqueous ophthalmic composition comprising a branched, glycerol monoalkyl compound and a fatty acid monoester. The fatty acid monoester comprises an aliphatic fatty acid portion having six to fourteen carbon atoms and an aliphatic hydroxyl portion. The composition will also have an osmolality in a range from 200 mOsmol / kg to 400 mOsmol / kg. The invention is also directed to a method of inhibiting the formation of foam in an aqueous ophthalmic composition that includes a surfactant as well as to a method of enhancing the biocidal efficacy of an aqueous ophthalmic composition containing a fatty acid monoester.
Owner:BAUSCH & LOMB INC
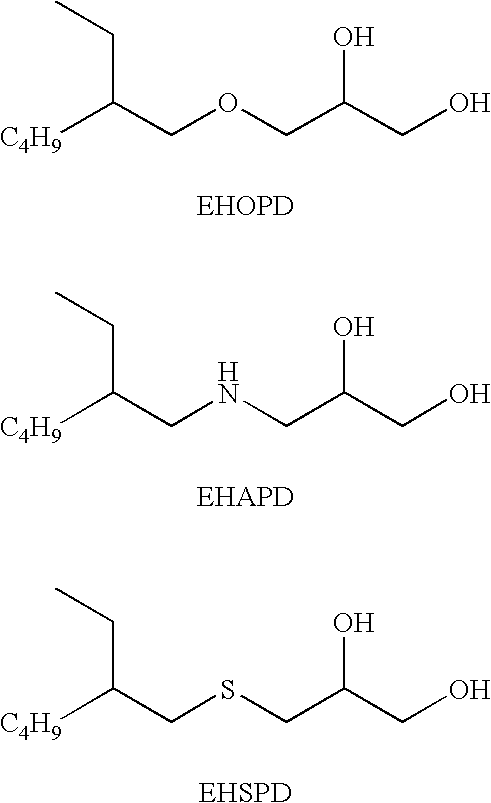
Ophthalmic Compositions Comprising A Branched, Glycerol Compound
InactiveUS20070287752A1Improve the bactericidal effectReduce total usageBiocideSenses disorderOsmolar ConcentrationGlycerol
An aqueous ophthalmic composition comprising a branched glycerol compound selected from the group consisting of a branched, glycerol monoalkyl ether, a branched, glycerol monoalkyl amine, a branched, glycerol monoalkyl sulfide, or any mixture thereof, present in a total amount of from 0.05 ppm to 1,000 ppm; a cationic antimicrobial component, and having an osmolality in a range from 200 mOsmol / kg to 400 mOsmol / kg. The presence of the branched glycerol compound enhances the biocidal efficacy of the aqueous ophthalmic composition containing one or more cationic antimicrobial components.
Owner:BAUSCH & LOMB INC
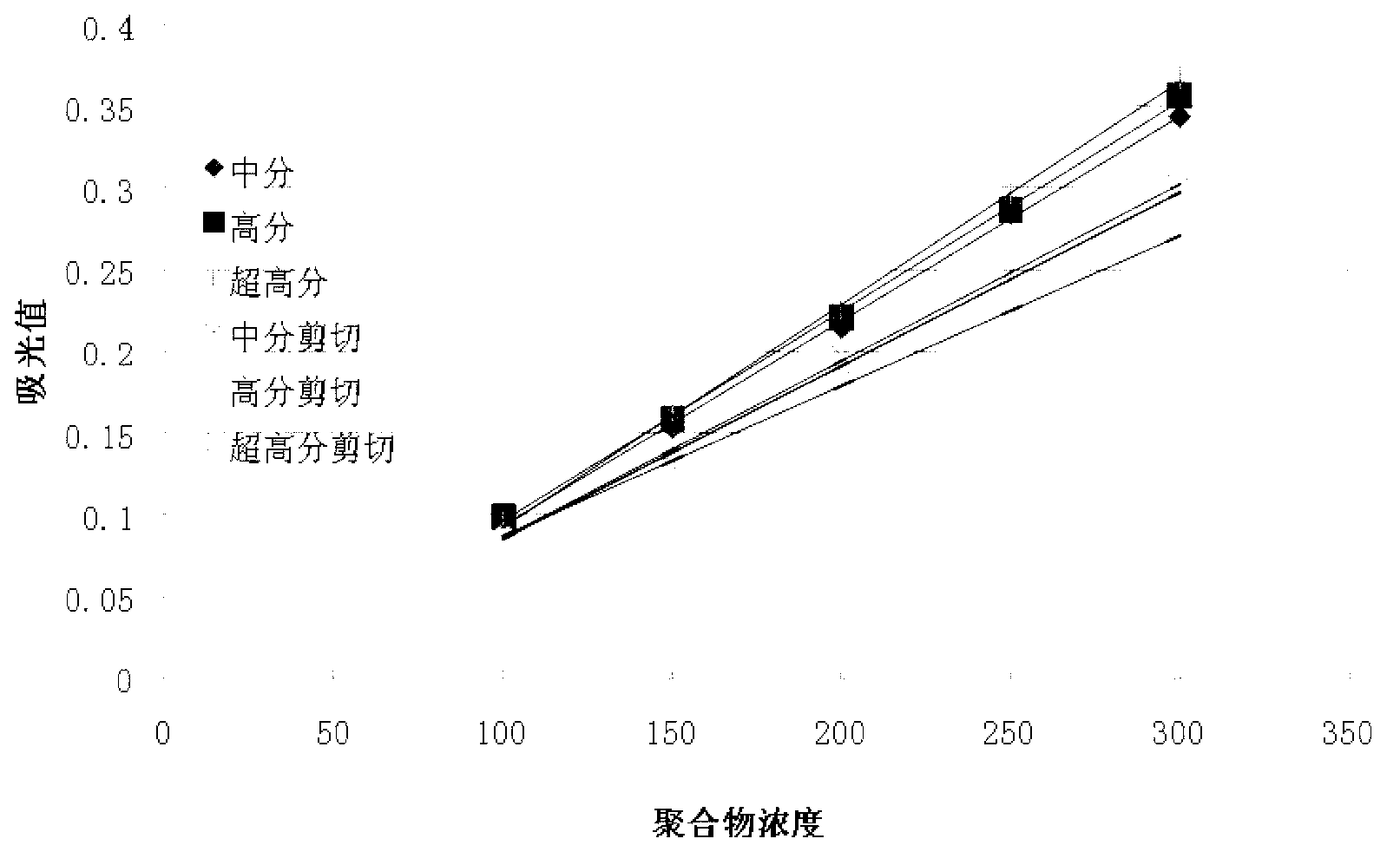
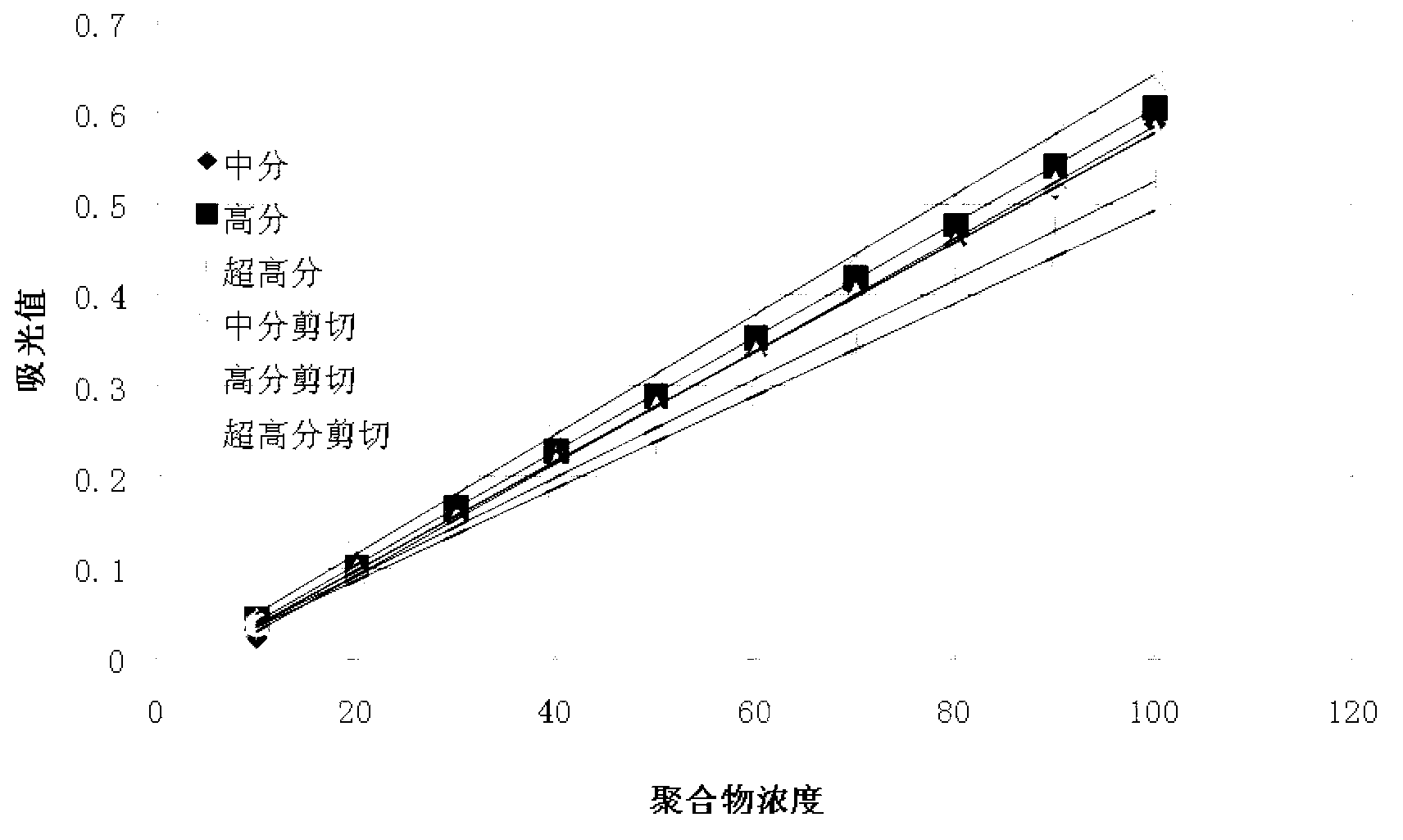
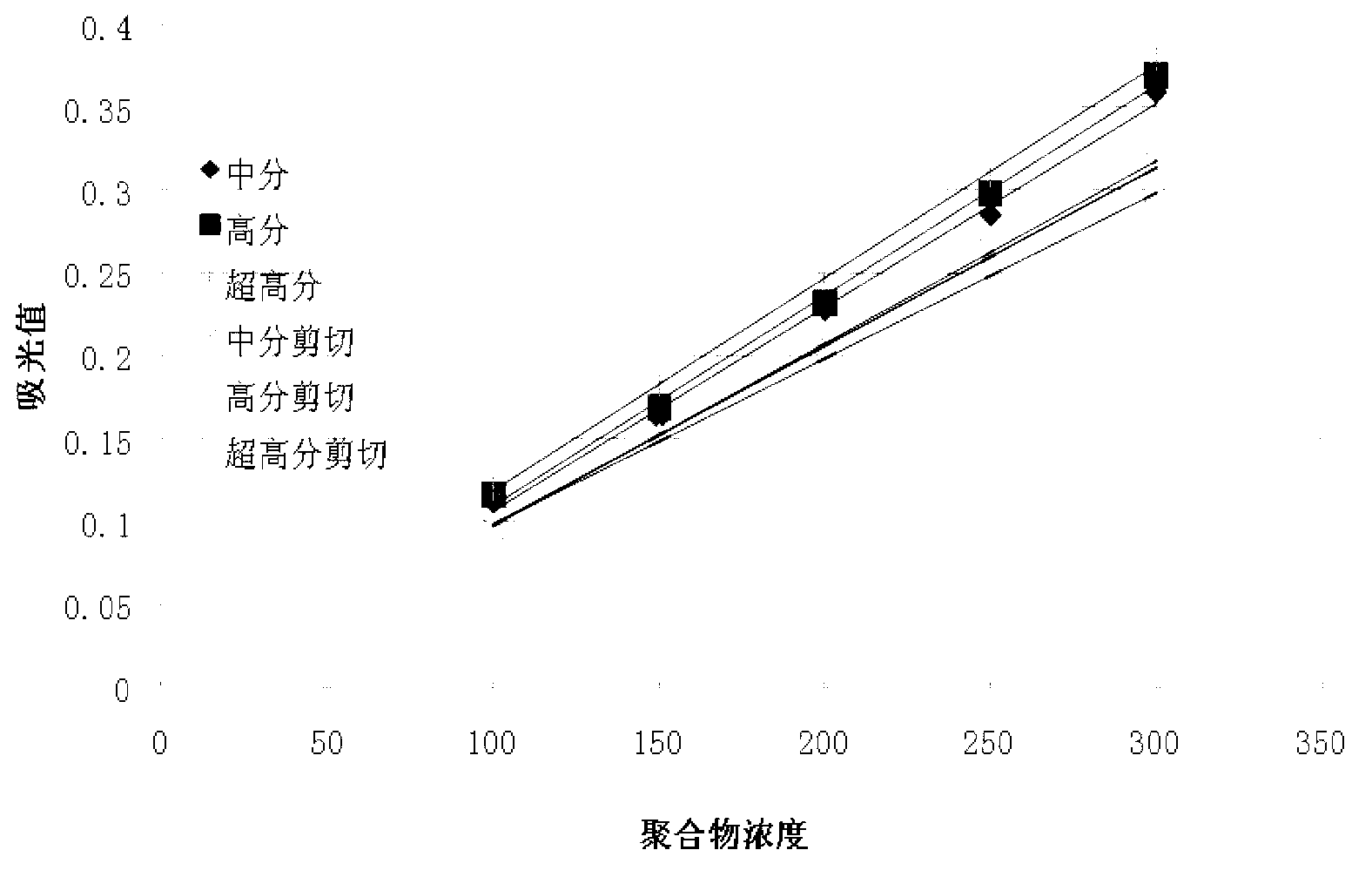
Detection method of polyacrylamide concentration in tertiary oil recovery liquid
ActiveCN103226104AImprove concentration detection accuracyEliminate errorsMaterial analysis by observing effect on chemical indicatorTurbidityOsmolar Concentration
The invention provides a detection method of polyacrylamide concentration in a tertiary oil recovery liquid. The method comprises: drawing a standard curve, preparing standard polyacrylamide solutions of different concentrations, shearing for at least 30 seconds under rotate speeds of 11000-22000 r / min, determining light absorption values of the solutions, drawing a standard curve, detecting to-be-detected samples, determining light absorption values of the to-be-detected samples, and determining the concentration of the polyacrylamide of the samples referring to the drawed standard curve, wherein a nephelometry or a starch-cadmium iodide method is used to determine the light absorption values of the solutions. According to the invention, the method can eliminate relatively large errors of polymer concentrations of the recovery liquid determined by the traditional nephelometry and the starch-cadmium iodide methods, thereby increasing a detection precision of the polymer concentrations of the recovery liquid.
Owner:PETROCHINA CO LTD +1
Chitosan stenting paste
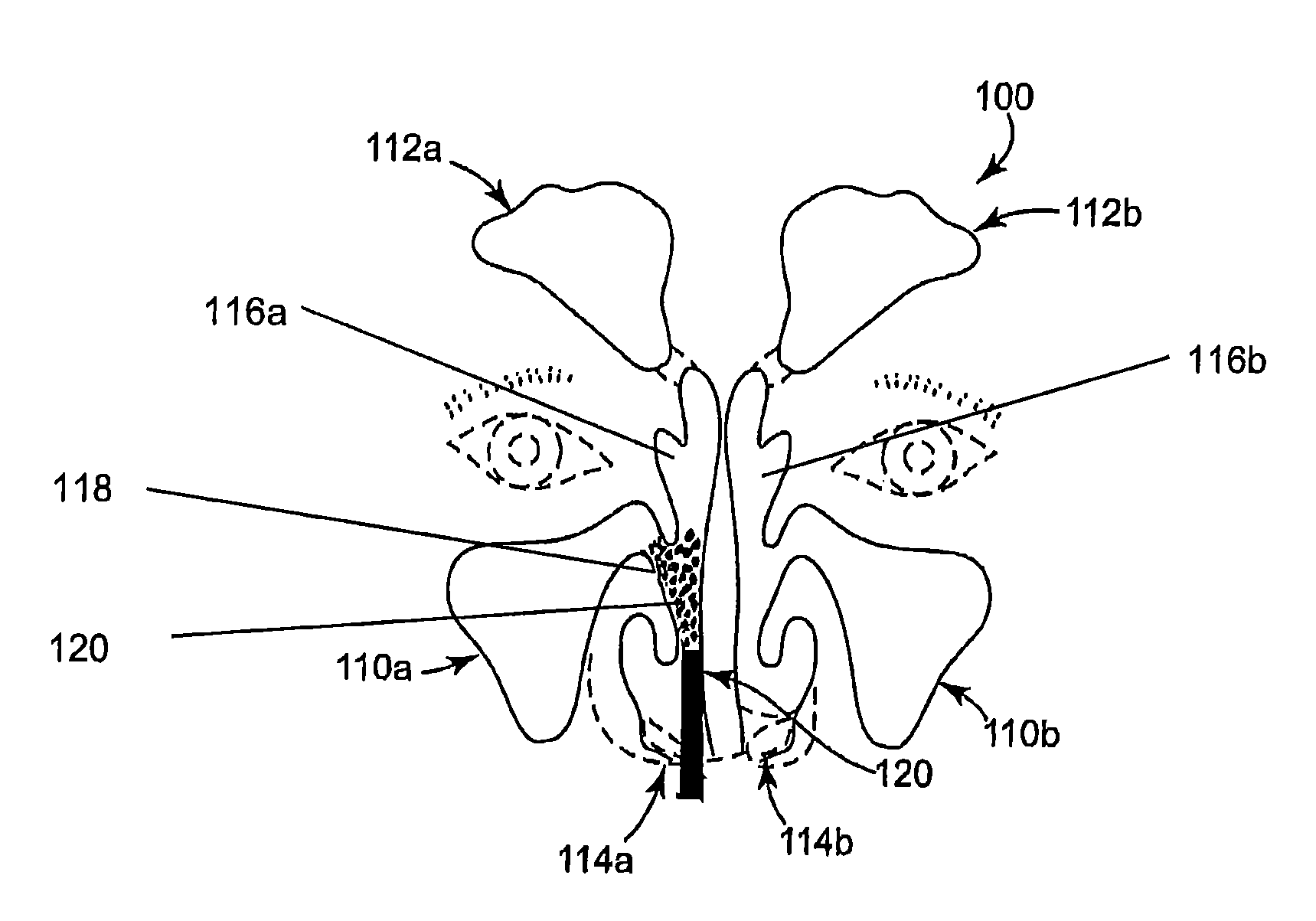
Nasal or sinus site may be treated with a ready-to-use paste having a high concentration of a water-soluble chitosan, an osmolality reducing agent in a phosphate-containing solution. The paste forms at room temperature, has a pH of at least 4 and the osmolality reducing agent does not crosslink with the water-soluble chitosan. The paste provides stenting and adheres to the nasal or sinus site and has a residence time of at least 1 day.
Owner:MEDTRONIC XOMED INC
Compositions and methods to prevent AAV vector aggregation
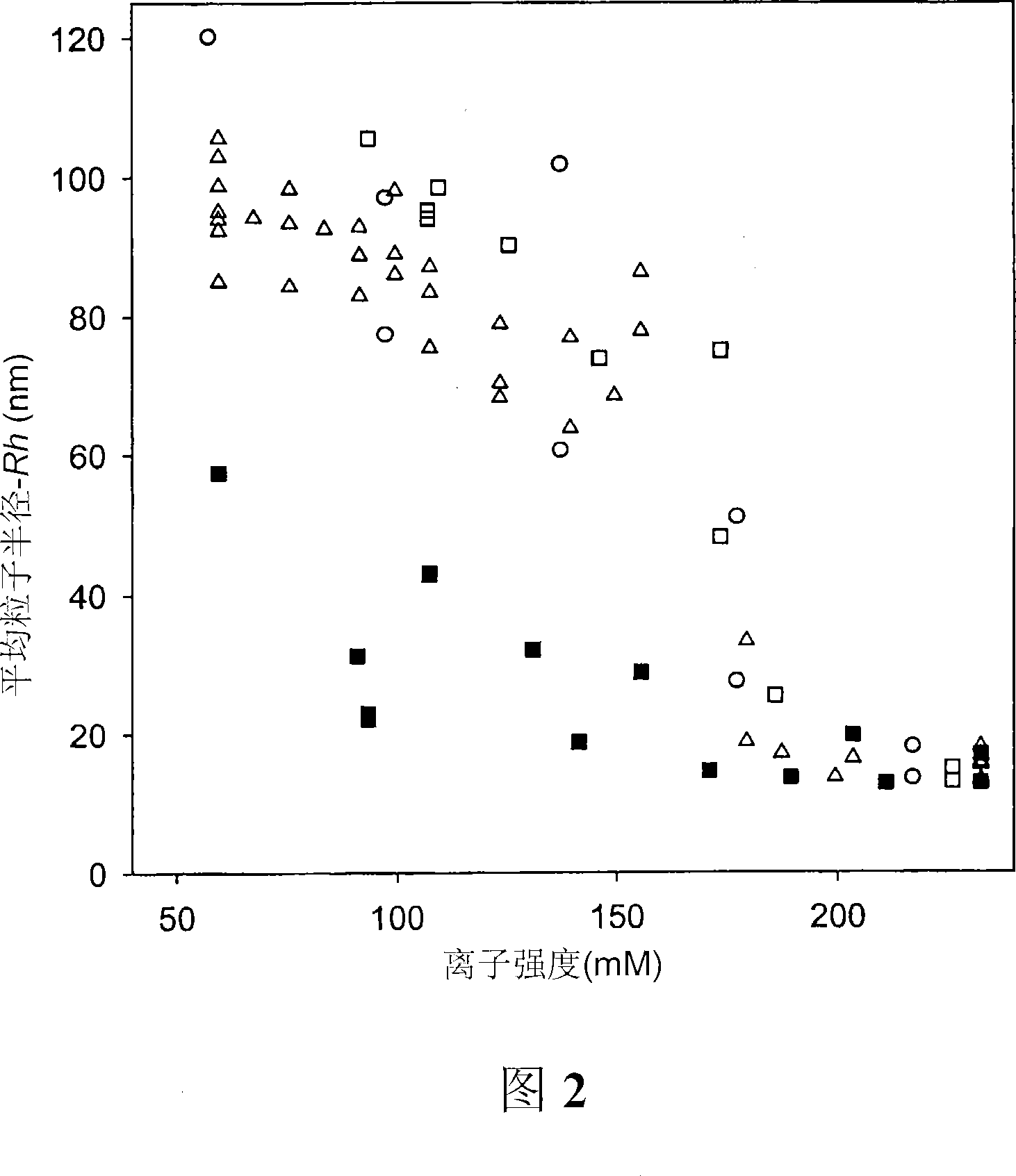
Compositions and methods are provided for preparation of concentrated stock solutions of AAV virions without aggregation. Formulations for AAV preparation and storage are high ionic strength solutions (e.g. mu~500mM) that are nonetheless isotonic with the intended target tissue. This combination of high ionic strength and modest osmolarity is achieved using salts of high valency, such as sodium citrate. AAV stock solutions up to 6.4x10<13> vg / mL are possible using the formulations of the invention, with no aggregation being observed even after ten freeze-thaw cycles. The surfactant Pluronic(R) F68 may be added at 0.001% to prevent losses of virions to surfaces during handling. Virion preparations can also be treated with nucleases to eliminate small nucleic acid strands on virions surfaces that exacerbate aggregation.
Owner:GENZYME CORP
Mammalian cell culture process
The present invention relates to novel process for the preparation of glycoproteins by mammalian cell culture wherein the sialic acid content of the glycoprotein produced is controlled over a broad range of values by manipulating the cell culture environment. The invention provides for processes in which the sialic acid content of the glycoprotein is modified by changes in cell culture parameters which affect cell specific productivity. Preferred embodiments of the invention include cell culture processes in the osmolality of the cell culture is controlled as well as the concentration of a transcription enhancer during the production phase of the cell culture. The invention further provides for novel preparations of soluble type 1 tumor necrosis factor immunoglobulin G1 and their uses in the treatment of inflammatory or immune related disorders.
Owner:ETCHEVERRY TINA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com