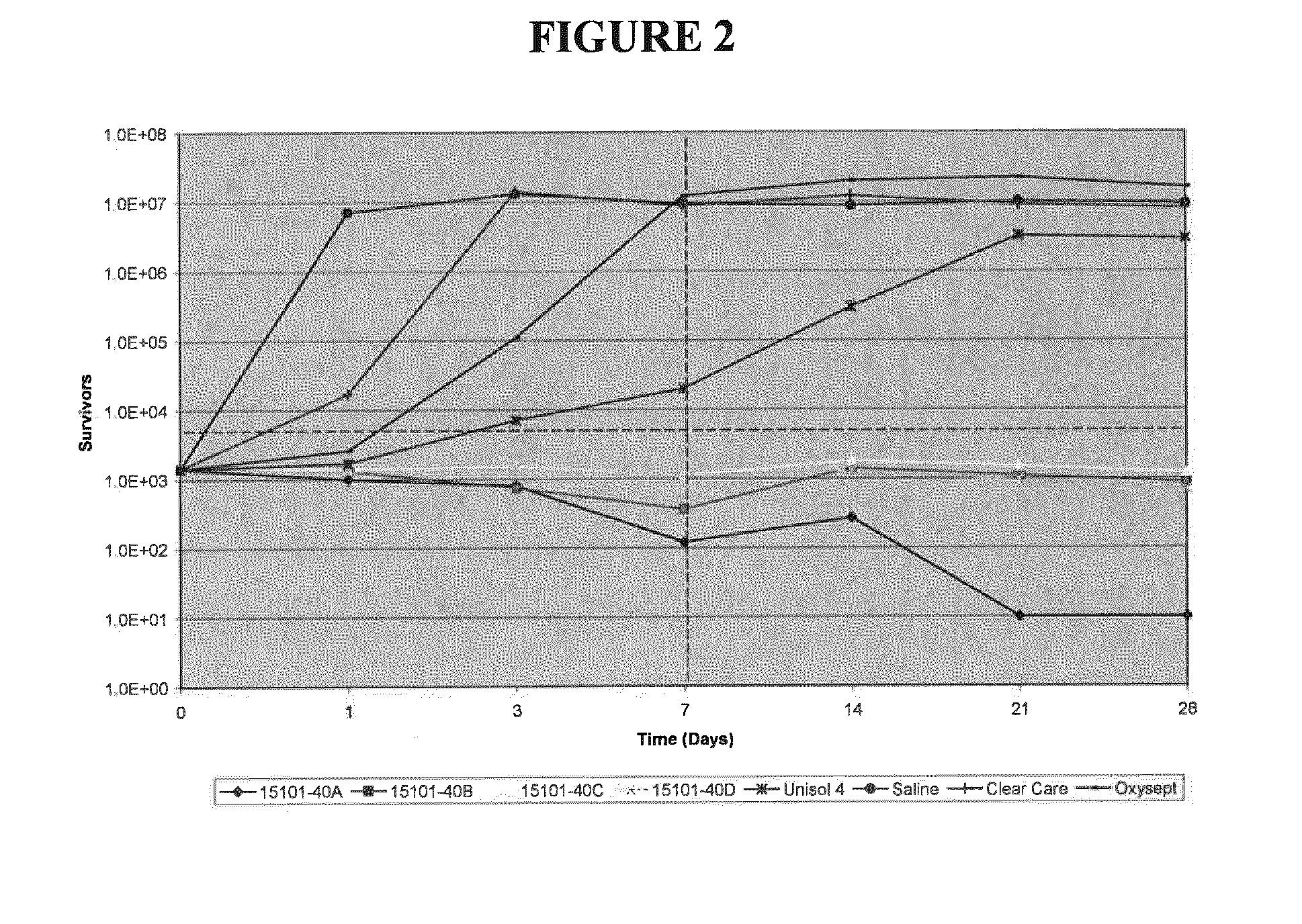
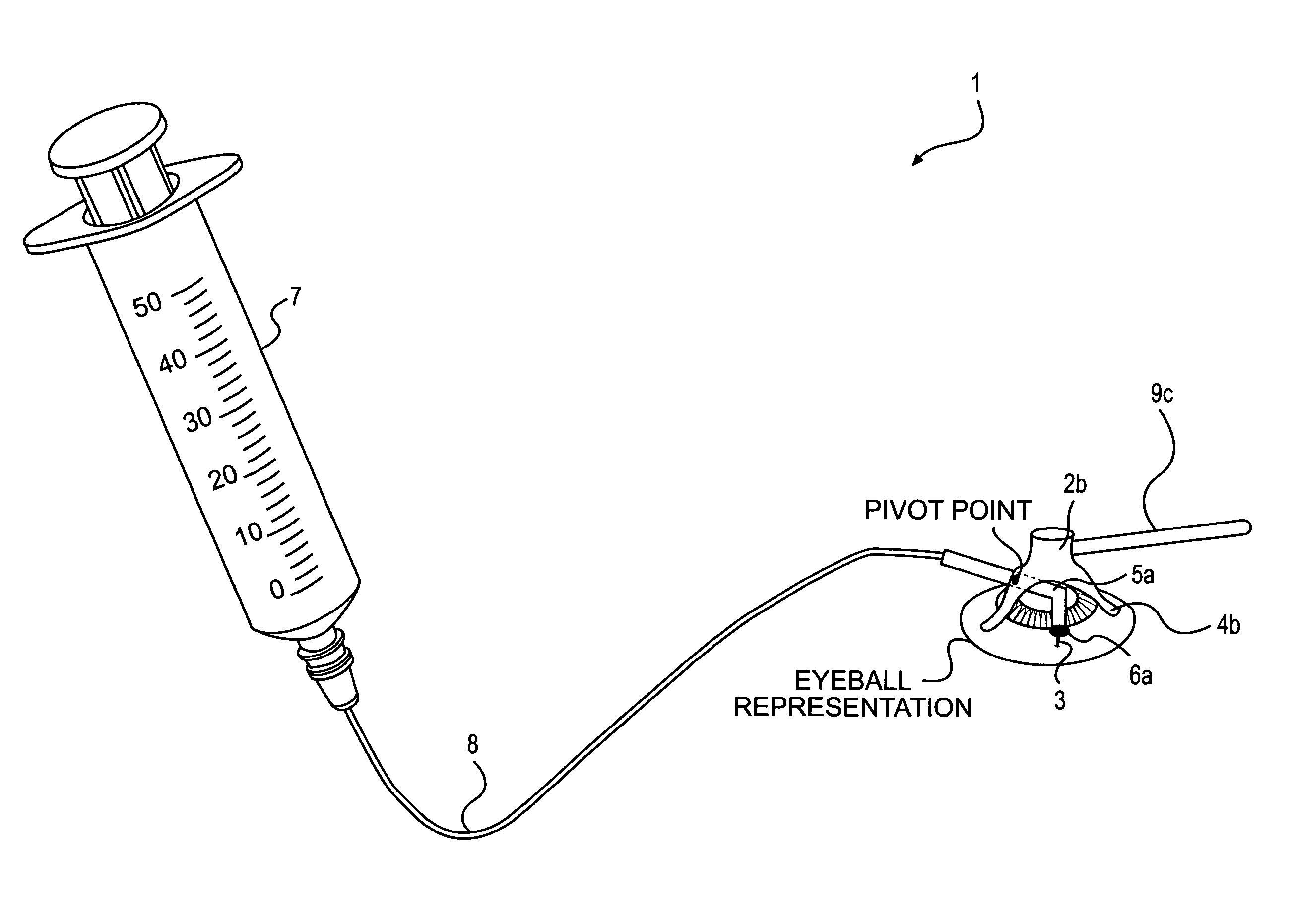
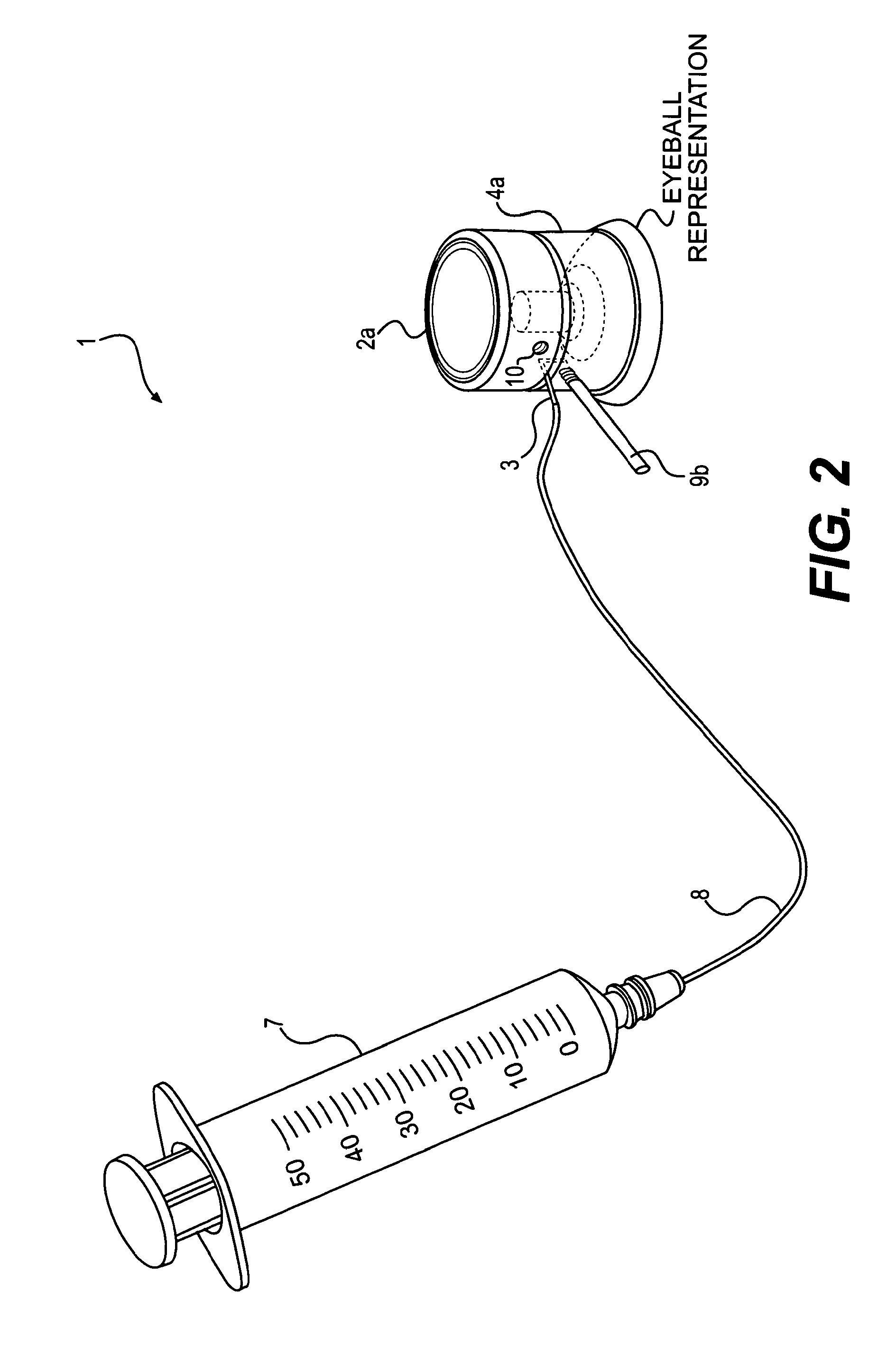
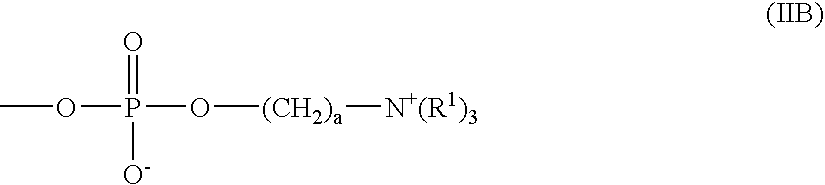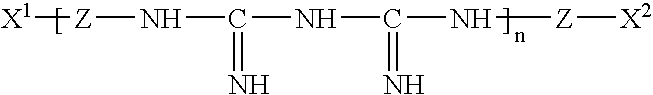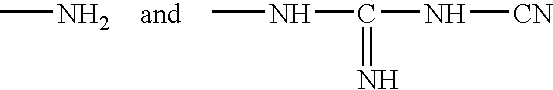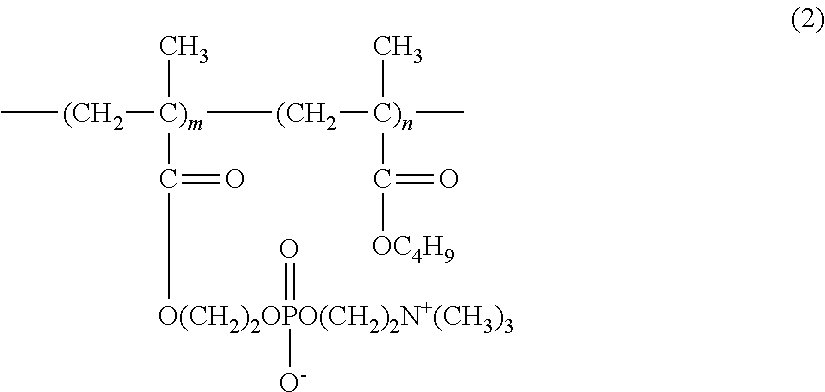Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Ophthalmic solutions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tobramycin Ophthalmic Solution USP, 0.3% is a topical antibiotic indicated in the treatment of external infections of the eye and its adnexa caused by susceptible bacteria.
Ophthalmic Solutions
InactiveUS20080314767A1Avoid pollutionLens cleaning compositionsPackage sterilisationOsmolar ConcentrationNon ionic
Disclosed are ophthalmic solutions such as packaging solutions for storing ophthalmic devices and lens care solutions for cleaning, disinfecting, rinsing and / or storing ophthalmic devices. The ophthalmic solutions contain at least a polymerization product obtained from a monomeric mixture comprising (a) a monomer bearing a center of permanent positive charge and (b) a non-ionic ethylenically unsaturated monomer, wherein the solution has an osmolality of at least about 200 mOsm / kg, and a pH of about 4 to about 9.
Owner:BAUSCH & LOMB INC
Sustained release delivery of one or more agents
InactiveUS20100209477A1Improve permeabilityReduce the amount requiredBiocideOrganic active ingredientsCross-linkExcipient
The lacrimal implant delivery systems and methods described herein provide for controlled release of a therapeutic agent for the treatment of disease, including the treatment of glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agents. Treatment of disease, including glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agent in conjunction with penetration enhancer, such as benzalkonium chloride, and / or artificial tears is also provided. Also provided are implants containing a drug core emplacable in a punctum adjacent to an eye of a patient for controlled release of a therapeutic agent such as latanoprost for the treatment of glaucoma, the drug core containing a polymer such as cross-linked silicone, a therapeutic agent, and an excipient, wherein the excipient can increase the rate of release of the agent from the drug core, or can increase the drug loading in the core without loss of desirable homogeneity of the agent within the core, or can improve retention of the agent in the eye or in tear fluid, or can increase corneal penetration of the agent into the eye.
Owner:MATI THERAPEUTICS
Ophthalmic and contact lens solutions containing forms of vitamin b
InactiveUS20070104744A1Discomfort to userDegree of reductionOrganic detergent compounding agentsLens cleaning compositionsOphthalmic solutionsBiology
The present invention relates to improved ophthalmic solutions that employ select B vitamins; pyridoxine and its salts; and thiamine and its salts in order to more effectively preserve solutions and to reduce the degree to which cationic preservatives will deposit on contact lenses. Ophthalmic solutions are here understood to include contact lens treatment solutions, such as cleaners, soaking solutions, conditioning solutions and lens storage solutions, as well as wetting solutions and in-eye solutions for treatment of eye conditions.
Owner:FXS VENTURES LLC
Stabilized ophthalmic solution for the treatment of glaucoma and lowering intraocular pressure
InactiveUS20060073172A1Solution stableStable ophthalmicBiocideInorganic non-active ingredientsCompound (substance)Serotonergic Neuron
The present invention provides stable ophthalmic solutions comprising a compound with serotonergic 5-HT2 receptor activity and at least one stabilizer, together with methods of using such solutions to treat glaucoma and to lower intraocular pressure.
Owner:SCHNEIDER L WAYNE +5
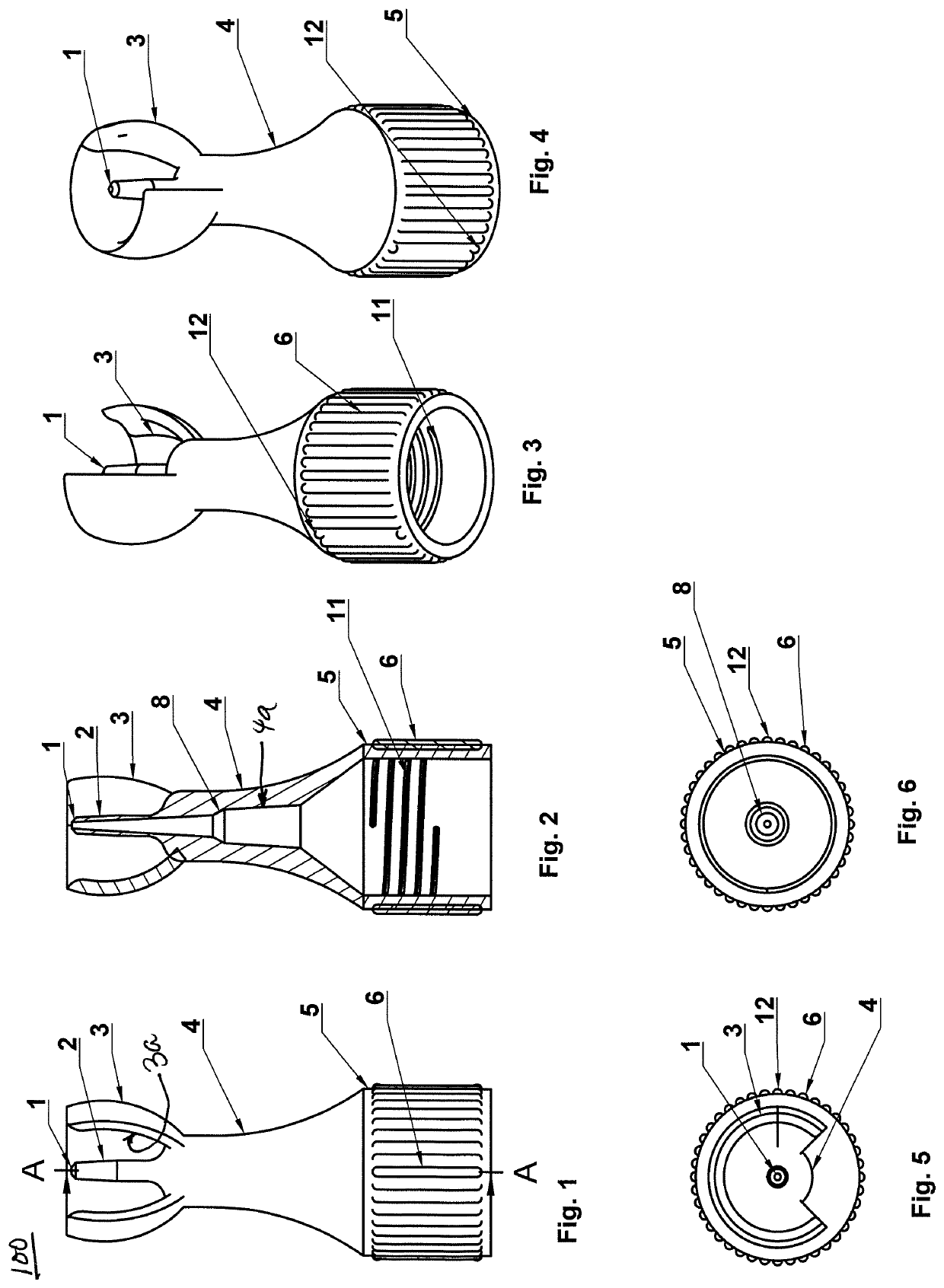
Ophthalmic solution dispensing device
InactiveUS20090207373A1Auxillary optical partsNon-optical adjunctsOphthalmic solutionsBiomedical engineering
A device for dispensing ophthalmic solutions to a user's eyes comprising a container for holding ophthalmic solutions, transfer tubing attached to the container for dispensing and applying said solution and a clip attached to the container for attaching said applicator to eyeglasses. The transfer tubing is the clip by being formed into the shape of a clip for attaching said eye solution applicator to eyeglasses.
Owner:STINSON STEVE
Ophthalmic Drug Dispensing Tip
InactiveUS20080039807A1Increase in sizeSave both the patient and the healthcare system moneyMedical applicatorsEye treatmentOphthalmic drugBottle
A dispensing tip apparatus for an eye drop dispenser to administer topical ophthalmic solutions is described. The apparatus integrates an ophthalmic solution-dispensing tip with an optical gauging assembly. The tip provides continuous visual feedback about it orientation and relationship to the eye. The dispensing tip when attached to any standard topical ophthalmic solution dispensing bottle or reservoir enables the user to view a target, visually align the dispenser tip, and administer an eye drop with precision. There is also a visual feedback by which the dispenser tip is prevented from gaining too close proximity and contacting the eye, thus preventing contamination of the medication and its dispenser. The visual feedback can also contain textual or graphic information that serves as a promotional advertisement.
Owner:PINE JERROLD SCOTT
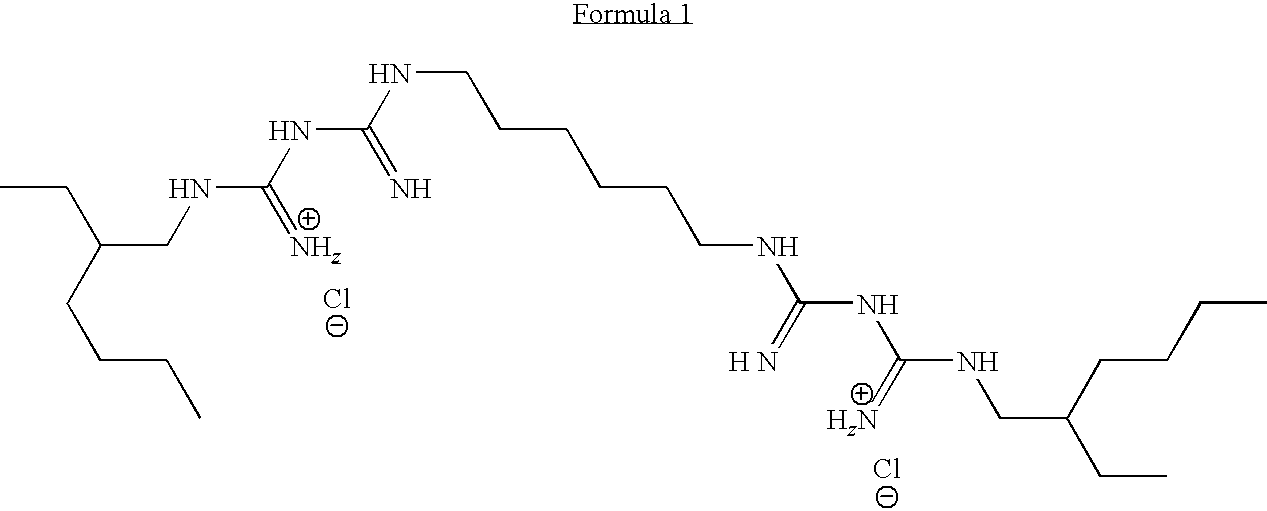
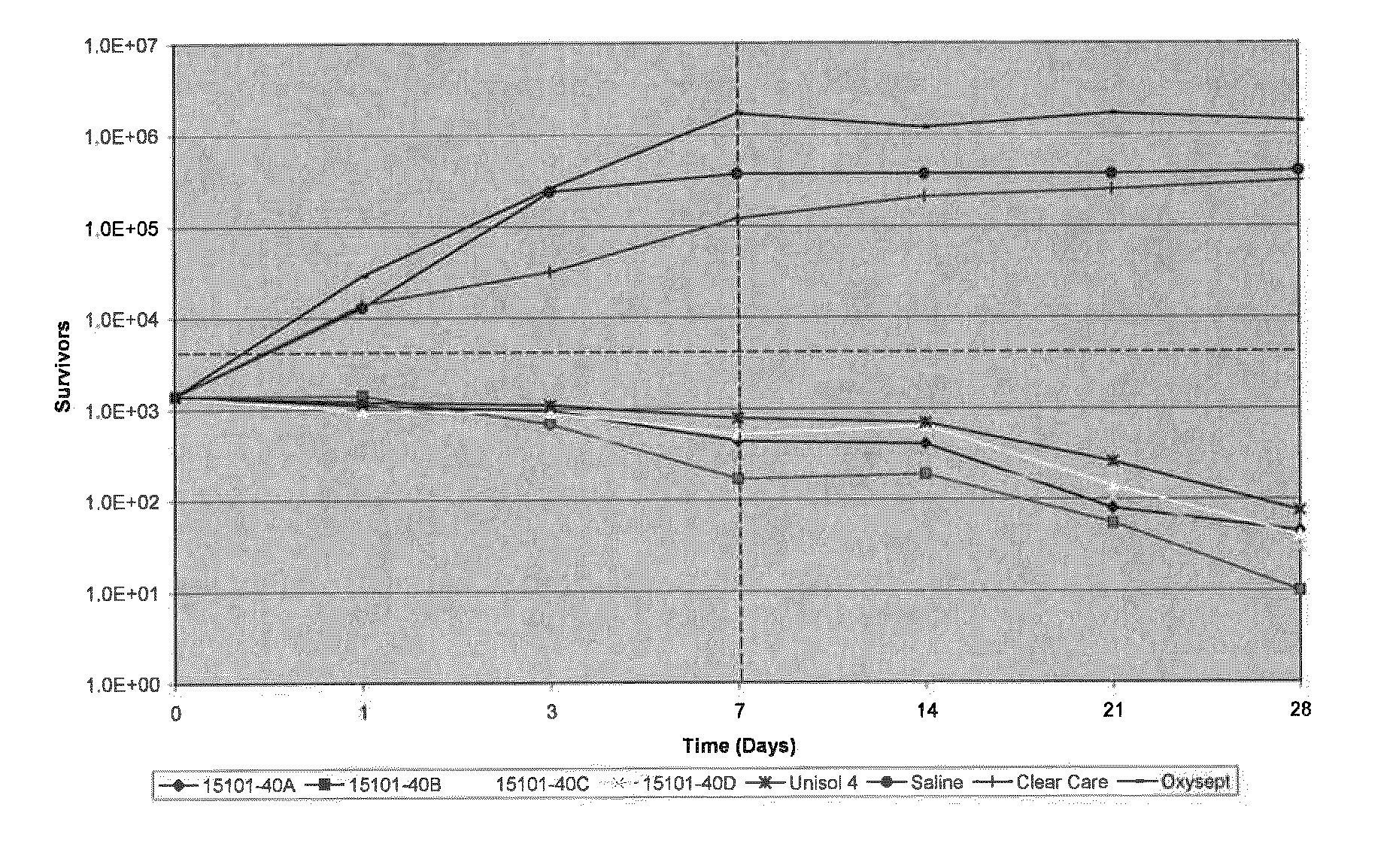
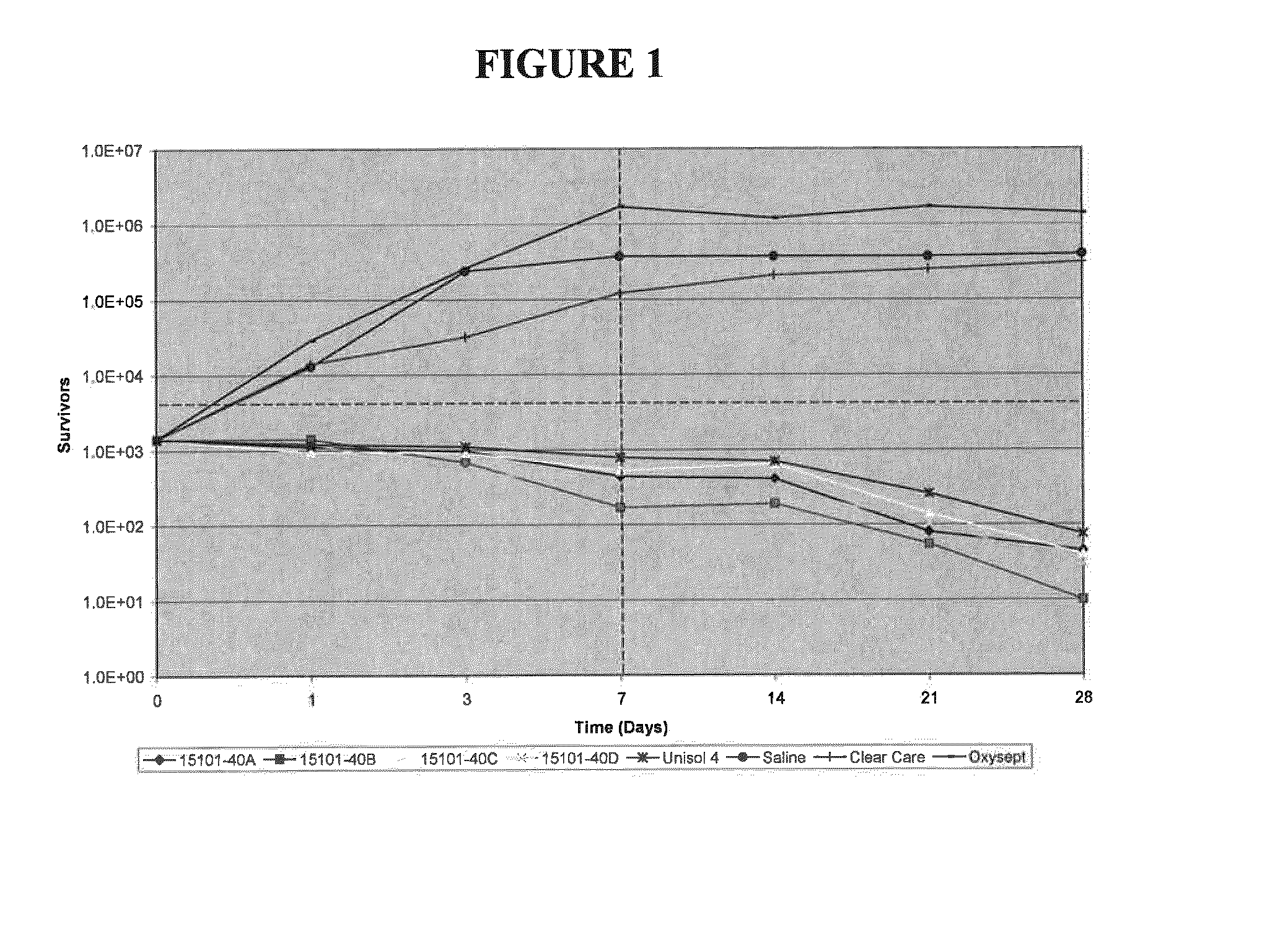
Complex of Polymeric Quaternary Ammonium and Anionic Polymers as a New Antimicrobial Agent for Ophthalmic Compositions
Disclosed herein are ophthalmic solutions and methods of using the solutions which comprise a complex of at least one cationic component and an anionic polymer, wherein the complex can be used as an antimicrobial agent. The solutions described have complexes which can be dissolved in aqueous ophthalmic solutions due to both lower charge load and lower total polymer concentrations than commonly taught. The complexes display different antimicrobial profiles than the non-complexed antimicrobial agents.
Owner:ABBOTT MEDICAL OPTICS INC
Mucomimetic compositions and uses therefore
The present invention relates to mucomimetic and ophthalmic solutions comprising a cationic multimeric antimicrobial agent such as polyaminopropyl biguanide and a magnesium, calcium or magnesium / calcium complex of an anionic polymer such as hyaluronate, alginate, carboxymethyl cellulose, chondroitin sulfate or mixtures thereof. In specific embodiments, the solutions include additional components such as a surfactant, preferably polysorbate 20, a viscosity-modifying agent, preferably hydroxypropylmethyl cellulose, carboxymethyl cellulose or hydroxyethyl cellulose, a tonicity agent and a buffer. The solutions are biocompatible with and are highly comfortable when administered to mucous membranes, including those of the eye, as well as are effective disinfectants.
Owner:SCHAEFER ROLF
Ophthalmic and contact lens solutions containing forms of vitamin b
InactiveUS20060148665A1Degree of reductionEffectively preserve solutionsCosmetic preparationsOrganic detergent compounding agentsDiseasePreservative
The present invention relates to improved ophthalmic solutions that employ select B vitamins; pyridoxine and its salts; and thiamine and its salts in order to more effectively preserve solutions and to reduce the degree to which cationic preservatives will deposit on contact lenses. Ophthalmic solutions are here understood to include contact lens treatment solutions, such as cleaners, soaking solutions, conditioning solutions and lens storage solutions, as well as wetting solutions and in-eye solutions for treatment of eye conditions.
Owner:FXS VENTURES LLC
L-histidine in ophthalmic solutions
InactiveUS20070110782A1Avoid lens disclorationOrganic active ingredientsBiocidePreservativeFungal microorganisms
The invention relates to an aqueous ophthalmic solution comprising 0.00001 to about 10.0 percent by weight L-histidine, 0.0001 to 3.0 percent by weight hydrogen peroxide, and optionally 0.1 to 500 parts per million of a preservative that provides superior preservative efficacy especially as against fungal microbes. These solutions may be employed in various ways including cleaning contact lenses, rinsing lenses while in the eye, storing lenses and in delivering active pharmaceutical agents to the eye.
Owner:FXS VENTURES LLC
Ophthalmic solutions with improved disinfection profiles
InactiveUS20110151017A1Desirable profileReduce concentrationAntibacterial agentsBiocideARS solutionAntimicrobial compound
The present invention relates to ophthalmic solutions with antimicrobial activity. The solutions have an antimicrobial compound such as hydrogen peroxide and a boron compound. In one embodiment, the solutions contain a boron compound such as sodium borate that provides antimicrobial activity in addition to that of hydrogen peroxide, particularly during periods following disinfection and neutralization of such solutions. This additional activity reduces the likelihood of microbial growth in contact lens disinfection applications that neutralize hydrogen peroxide.
Owner:ALCON RES LTD
L-histidine in ophthalmic solutions
The invention relates to an aqueous ophthalmic solution comprising 0.01 to about 1.0 percent by weight L-histidine; 0.01 to 0.0001 percent by weight hydrogen peroxide; 0.1 to 500 parts per million of a cationic polymeric preservative that provides superior preservative efficacy especially as against fungal microbes. These solutions may be employed in various ways including cleaning contact lenses, rinsing lenses while in the eye, storing lenses and in delivering active pharmaceutical agents to the eye.
Owner:FXS VENTURES LLC
Ophthalmic solutions, including contact lens care and eye drops comprising carnosine, preferably in combination with dexpanthenol and/or hyaluronic acid
InactiveUS20110230424A1Prevent of even repair damagePrevent and reduce stainingOrganic active ingredientsSenses disorderDexpanthenolOphthalmic solutions
The current invention relates to a contact lens care solution comprising a compound suitable for treating the eye, or the contact lens, use of such compound in such contact lens care solutions, and methods for introducing such compound in the eye of a person wearing contact lenses. With the current invention damage to the eye, in particular the cornea, in particular damage to the eye, in particular the cornea, of a person wearing a contact lens, or as a consequence of wearing a contact lens is prevented or reduced. / esp.
Owner:WAGENAAR LOUIS JOHAN
Ophthalmic solutions for glaucoma and conjunctivitis treatment
This invention generally relates to an ophthalmic solution comprising cannabinoids for the treatment of glaucoma. Also disclosed is an ophthalmic solution comprising cannabinoids for symptomatic relief of conjunctival inflammation. Cannabinoids are selected to achieve the specific purpose of the respective ophthalmic solution.
Owner:APIRX PHARMA USA LLC
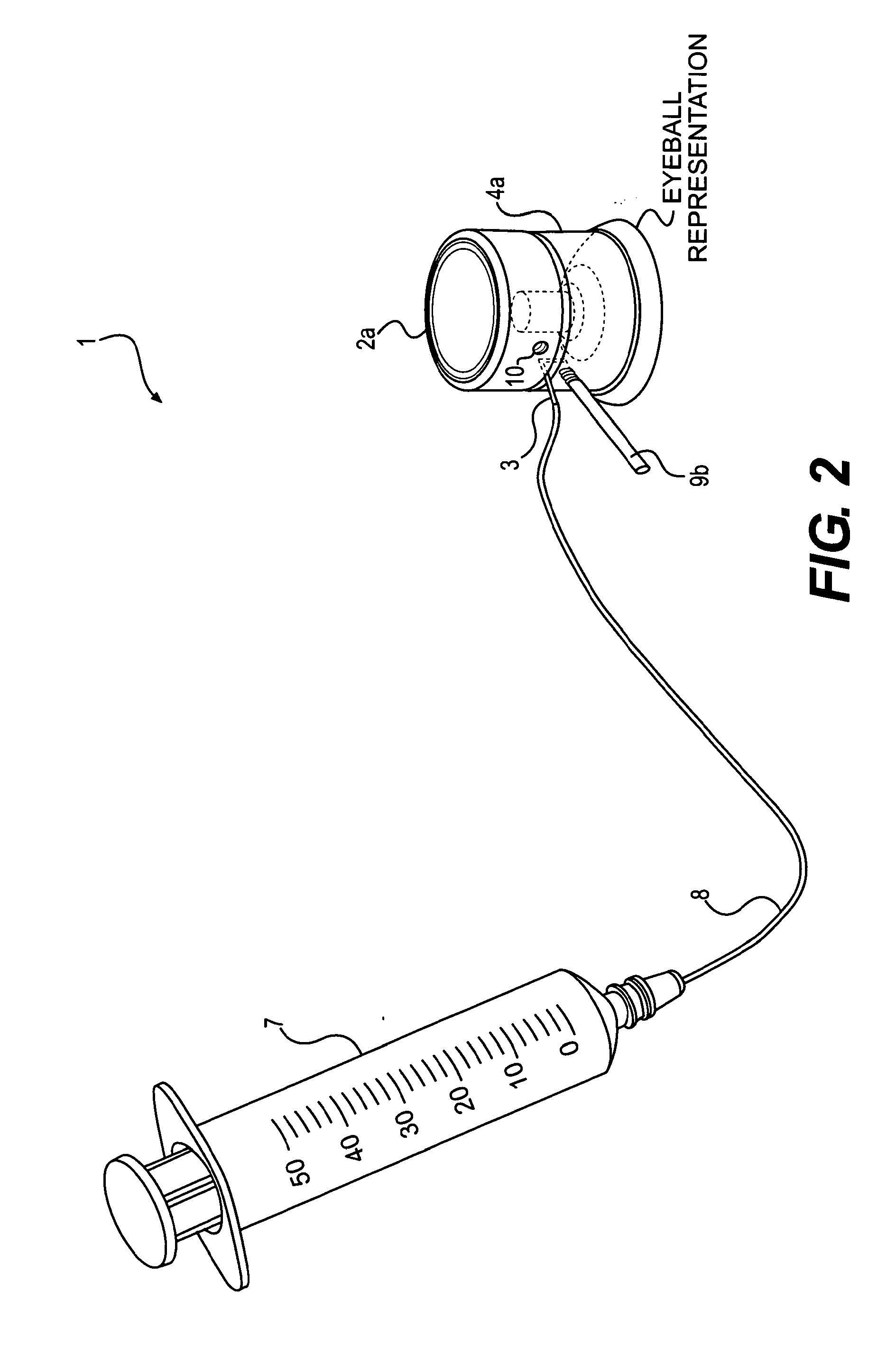
Device and method for the controlled delivery of ophthalmic solutions
ActiveUS9399102B2Peptide/protein ingredientsPharmaceutical delivery mechanismOphthalmic solutionsControlled delivery
Owner:EUCLID SYST CORP
Ophthalmic solutions
InactiveCN101730645ACationic surface-active compoundsOrganic detergent compounding agentsNon ionicOphthalmic solutions
Owner:BAUSCH & LOMB INC
Ophthalmic solution dispensing device
InactiveUS7784936B2Auxillary optical partsNon-optical adjunctsOphthalmic solutionsBiomedical engineering
A device for dispensing ophthalmic solutions to a user's eyes comprising a container for holding ophthalmic solutions, transfer tubing attached to the container for dispensing and applying said solution and a clip attached to the container for attaching said applicator to eyeglasses. The transfer tubing is the clip by being formed into the shape of a clip for attaching said eye solution applicator to eyeglasses.
Owner:STINSON STEVE
Lubricious ophthalmic solutions
There are provided lubricious ophthalmic solutions having a pH of between about 7.0 and about 8.0, consisting essentially of an aqueous solution of from about 0.5–4% by weight of a monographed polyol, borate calculated as the borate equivalent of 20–100% by weight of boric acid relative to said polyol, from about 0.1 to about 1.00% by weight of monographed polysorbate, a monographed preservative and a buffer. Suitably the polyol is polyvinyl alcohol In addition to polyvinyl alcohol (hereinafter PVA) the solution may include other monographed polymers as well as monographed pharmacologically active substances.
Owner:MACKLES LEONARD
Ophthalmic solution containing diquafosol, method for producing same, and method for preventing formation of insoluble precipitate
InactiveCN103282039AStable physical and chemical propertiesReduce filter effectOrganic active ingredientsSenses disorderFiltrationOphthalmic solutions
An ophthalmic solution containing diquafosol to which a chelating agent is added, wherein the formation of an insoluble precipitate during storage and a lowering in filtration performance in a production process (a filtration / sterilization process), said insoluble precipitate formation and said lowering in filtration performance being observed in conventional ophthalmic solutions containing diquafosol, are inhibited. It is also confirmed that the ophthalmic solution containing diquafosol to which a chelating agent is added shows an improved ability to retain the effect during storage. Therefore, the present invention provides an ophthalmic solution containing diquafosol, said ophthalmic solution having stable physicochemical properties in the course of production, distribution and storage by a patient, in particular, enabling efficient filtration / sterilization in the production process, and, moreover, having an excellent ability to retain the effect during storage. Also, the present invention provides a method for preventing the formation of an insoluble precipitate from an aqueous ophthalmic solution containing diquafosol or a salt thereof, said method comprising adding a chelating agent to the aqueous ophthalmic solution.
Owner:SANTEN PHARMA CO LTD
Ophthalmic solutions displaying improved efficacy
The present invention relates to ophthalmic compositions comprising a pH between about 6 and about 8 and about 50 to about 1000 ppm hydrogen peroxide, about 100 ppm to about 2000 ppm of at least one chlorite compound and about 20 to 100 ppm of at least one saturated, polymeric quaternium salt. The ophthalmic compositions of the present invention display improved antifungal efficacy against fusarium solani.
Owner:JOHNSON & JOHNSON VISION CARE INC
Ophthalmic drop dispensing tip assembly
ActiveUS20100211027A1Save both the patient and the healthcare system moneyConvenient and accurateBathing devicesMedical applicatorsEngineeringBottle
A dispensing tip apparatus for an eye drop dispenser to administer topical ophthalmic solutions is described. The apparatus integrates an ophthalmic solution-dispensing tip with an optical gauging assembly. The tip provides continuous visual feedback about it orientation and relationship to the eye. The dispensing tip when attached to any standard topical ophthalmic solution dispensing bottle or reservoir enables the user to view a target, visually align the dispenser tip, and administer an eye drop with precision. There is also a visual feedback by which the dispenser tip is prevented from gaining too close proximity and contacting the eye, thus preventing contamination of the medication and its dispenser. The visual feedback can also contain textual or graphic information that serves as a promotional advertisement. The is assembly can be attached to the neck of an eye drop bottle or attached to the tip of an eye drop bottle.
Owner:PINE JERROLD
Device and method for the controlled delivery of ophthalmic solutions
ActiveUS20130144260A1Peptide/protein ingredientsMedical devicesOphthalmic solutionsControlled delivery
Owner:EUCLID SYST CORP
Eyedrop of Chinese traditional medicine for treating ophthalmic diseases, and preparation method
InactiveCN101049359AGood effectLow costSenses disorderHydroxy compound active ingredientsDiseaseXerophthalmia
A Chinese medicine in the form of eyedrops for treating ophthalmopathy, such as cataract, presbyopia, muscae volitantes, xerophthalmia, etc, is prepared from 10 Chinese-medicinal materials including coptis root, ledebouriella root, ash bark, alum, etc through sequentially decocting, filtering, distilling, cooling and sterilizing.
Owner:程艳蕊
Method and composition for eliminating ocular hypoxic acidosis
InactiveUS20060209253A1Difficult to absorbImprove stabilityHeavy metal active ingredientsOrganic detergent compounding agentsRubidiumOphthalmic solutions
The methods and compositions of the present invention generally relate to ophthalmic solutions. Specifically, the methods and compositions relate to at least the following: ophthalmic solutions that are usable as eye drops for contact lens wearers, eye rinsing solutions, and ophthalmic solutions that are usable as contact lens wetting agents. In a wetting solution aspect, the present invention provides a contact lens wetting solution that includes: a cesium salt, a rubidium salt or a combination thereof; and, water, wherein the wetting solution has a viscosity of 1 to 3 cps.
Owner:GILES BRIAN C
Phosphate buffered ophthalmic solutions displaying improved efficacy
The present invention relates to ophthalmic compositions comprising a pH between about 6 and about 8 and about 50 to about 1000 ppm hydrogen peroxide and at least one phosphate buffer. The ophthalmic compositions of the present invention display improved antifungal efficacy against fungi, including candidas albicans and fusarium solani.
Owner:JOHNSON & JOHNSON VISION CARE INC
Ophthalmic contact lens solutions containing forms of vitamin B
InactiveUS9308264B2Degree of reductionEffectively preserve solutionsBiocideOrganic detergent compounding agentsPreservativeB Vitamin Family
The present invention relates to improved ophthalmic solutions that employ select B vitamins; pyridoxine and its salts; and thiamine and its salts in order to more effectively preserve solutions and to reduce the degree to which cationic preservatives will deposit on contact lenses. Ophthalmic solutions are here understood to include contact lens treatment solutions, such as cleaners, soaking solutions, conditioning solutions and lens storage solutions, as well as wetting solutions and in-eye solutions for treatment of eye conditions.
Owner:FXS VENTURES LLC
Stabilized ophthalmic solutions
The present invention relates to stabilized ophthalmic solutions comprising at least one peroxide. In one embodiment, the present invention relates to an ophthalmic solution comprising a pH between about 6 and about 8 and about 50 to about 1000 ppm hydrogen peroxide and about 0.005 wt % (50 ppm) to about 0.05 wt % (500 ppm) at least one salt of diethylenetriamine pentaacetic acid selected from the group consisting of calcium salts of diethylenetriamine pentaacetic acid, zinc salts of diethylenetriamine pentaacetic acid and mixed calcium / zinc salts of diethylenetriamine pentaacetic acid.The present invention further relates to a method for preserving ophthalmic solution comprising at least one peroxide.
Owner:JOHNSON & JOHNSON VISION CARE INC
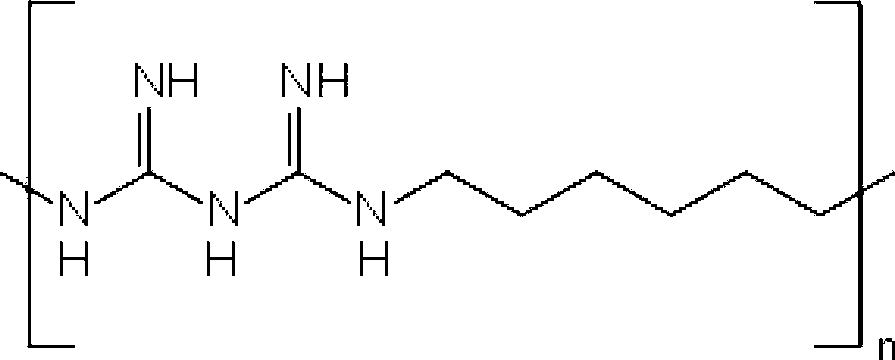
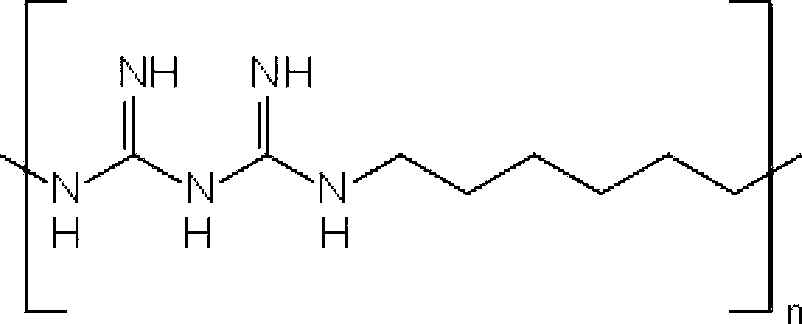
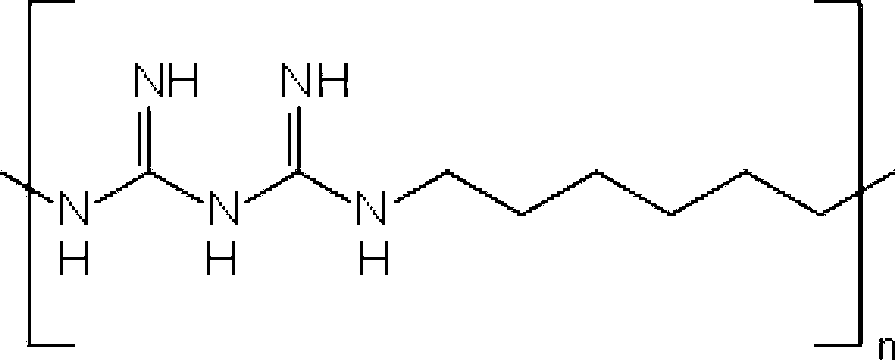
Combinations of preservatives for ophthalmic compositions
The present invention provides a preservative composition for protecting ophthalmic solutions from microbial attack comprising a combination of benzalkonium ion and polyhexamethylene biguanide (PHMB) wherein the combined concentrations of benzalkonium ion and polyhexamethylene biguanide (PHMB) in said composition is sufficient to provide protection against microbial attack when said composition is added to an ophtalmic solution as compared to said ophthalmic soulution having the same concentration of benzalkonium ion and polyhexamethylene biguanide (PHMB), alone.
Owner:ALLERGAN INC
Eye drop dispensing apparatus
An ophthalmic solution discharging apparatus is described. The ophthalmic solution discharging apparatus comprises: a base portion comprising a cylindrical base for receiving and removably engaging a dropper of an ophthalmic solution container; a head portion comprising a micro nozzle portion through which ophthalmic solution is discharged, a shield portion that cylindrically surrounds the micro nozzle portion, the shield portion comprising a wall containing an opening for viewing a portion of the micro nozzle portion during discharge; a neck portion that connects the base portion and the head portion; and an internal cavity portion defined by the base, head and neck portions and comprising a (1) container engagement portion for receiving and removably engaging the ophthalmic solution container, and (2) a funnel portion for transporting and discharging ophthalmic solution through the micro nozzle portion at a lesser volume than would otherwise be discharged by the dropper of the ophthalmic solution container.
Owner:HOSSAIN SYED SHADMAN +1
Ophthalmic solution
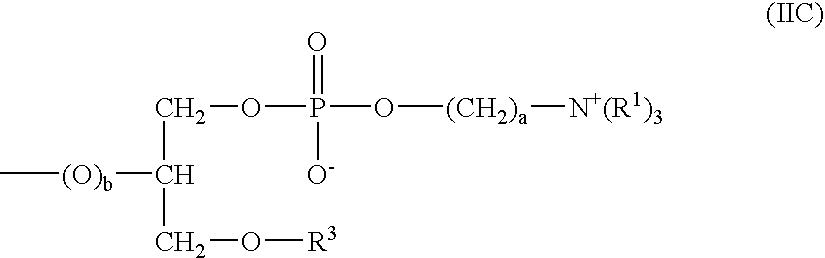
ActiveUS20160199526A1Improve securityEasy to useOrganic active ingredientsBiocidePhosphorylcholineEthyl group
Provided is an ophthalmic solution of which preservative component, hexamethylene biguanide, contained therein is inhibited from being adsorbed on soft contact lenses and eye droppers. The ophthalmic solution contains, at a specific ratio, (A) hydroxypropyl methylcellulose having 28.0 to 30.0 mass % methoxy groups and 7.0 to 12.0 mass % hydroxypropoxy groups, both with respect to the total amount of the hydroxypropyl methylcellulose, at a methoxy group / hydroxypropoxy group ratio of 2.5 to 4.0 by mass, wherein a 2 mass % aqueous solution of the hydroxypropyl methylcellulose at 20° C. has a viscosity of 50 to 4000 mPa·s; (B) sodium chloride or potassium chloride; (C) hexamethylene biguanide or a salt thereof; (D) 2-methacryloyloxyethyl phosphorylcholine / butyl methacrylate copolymer; and (E) water.
Owner:NOF CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com