Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
118 results about "Lacrimal punctum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
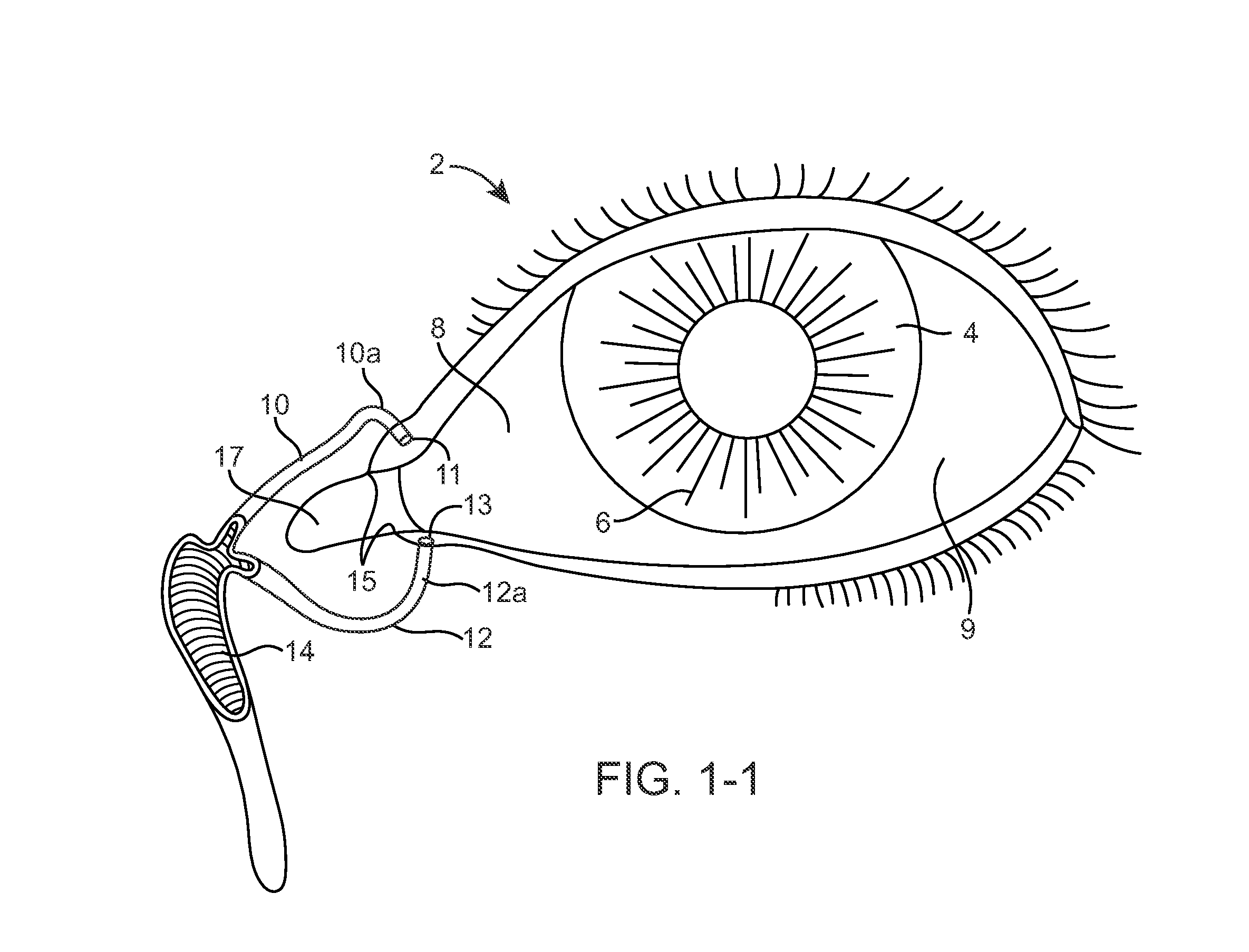
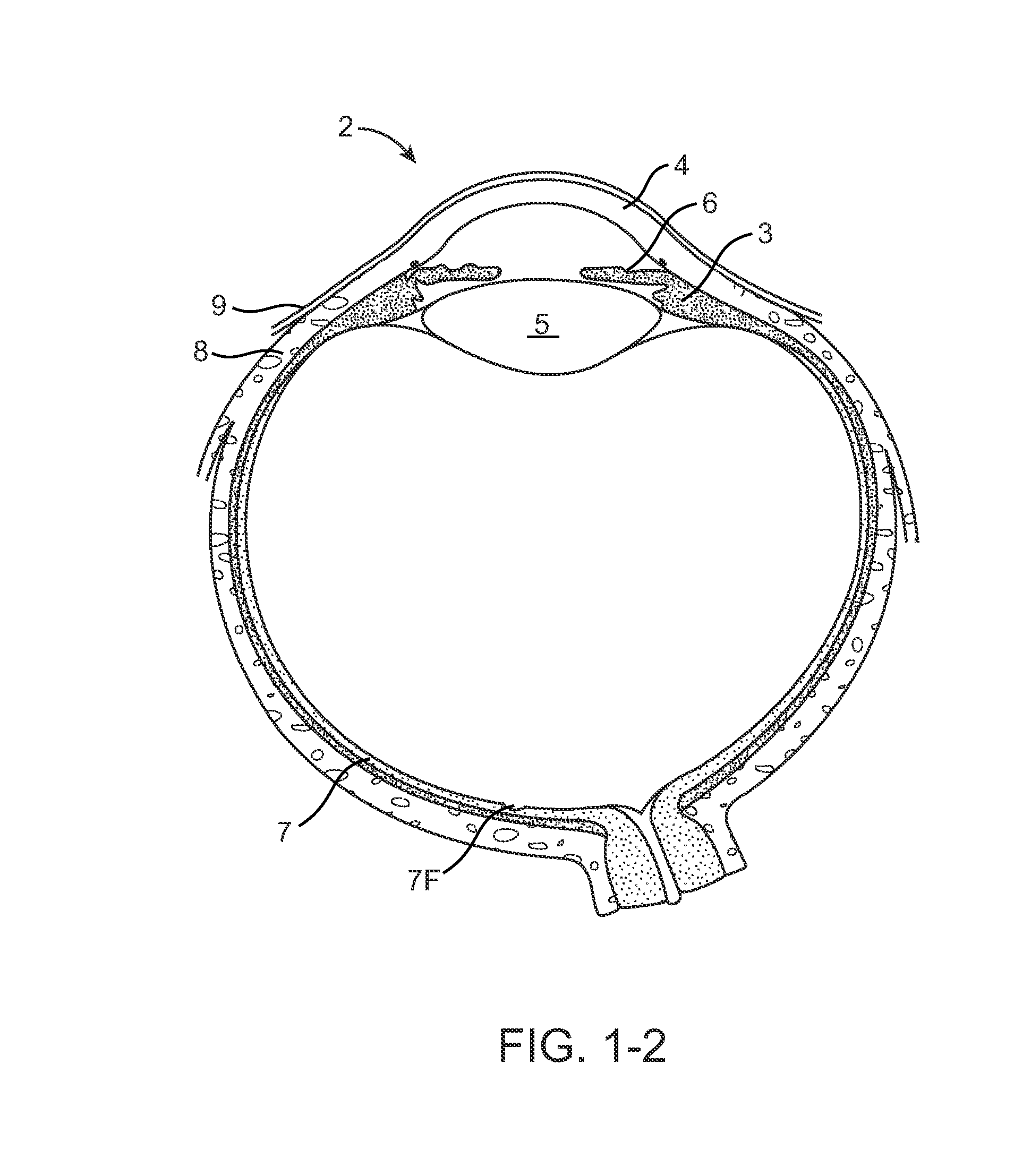
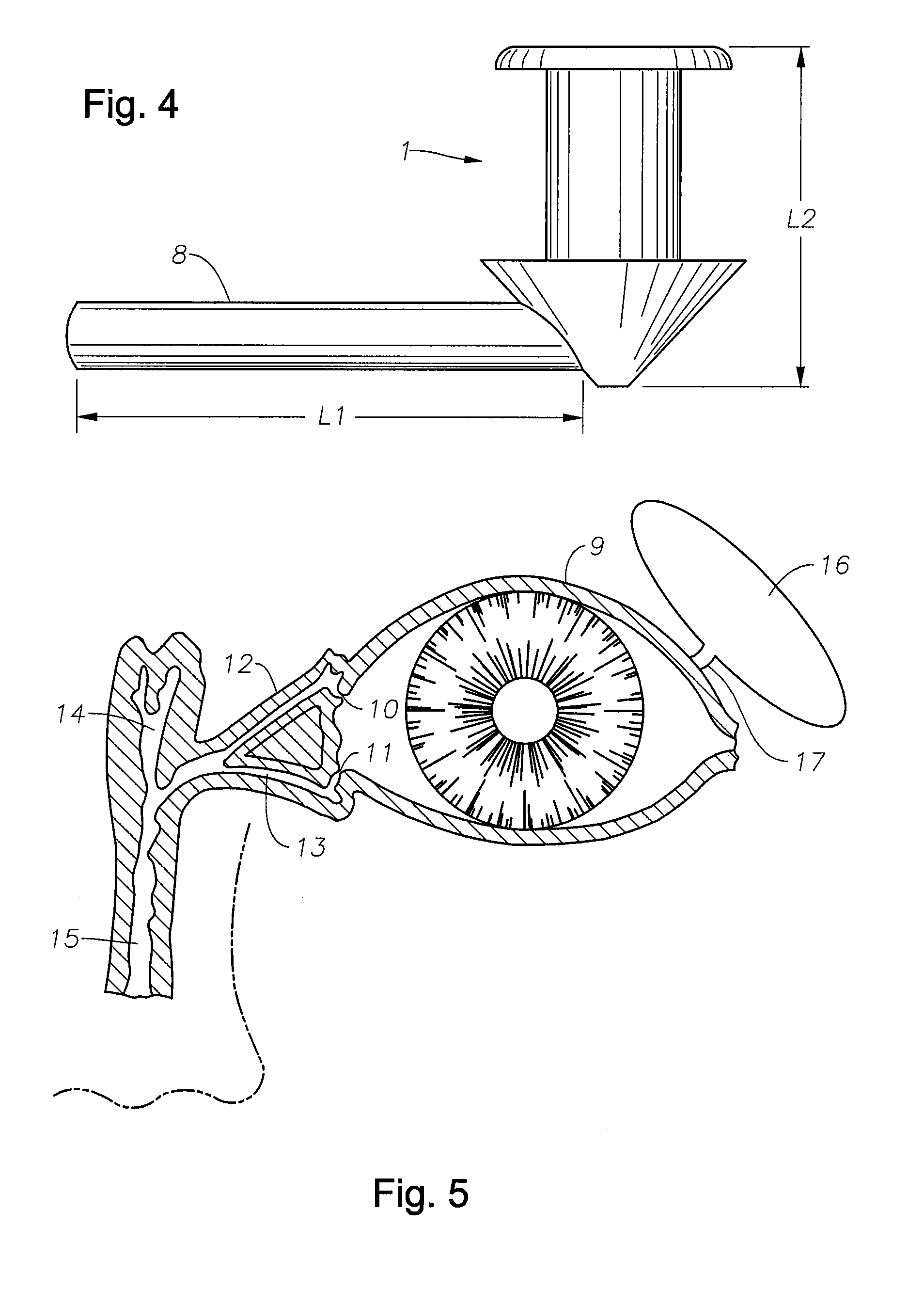
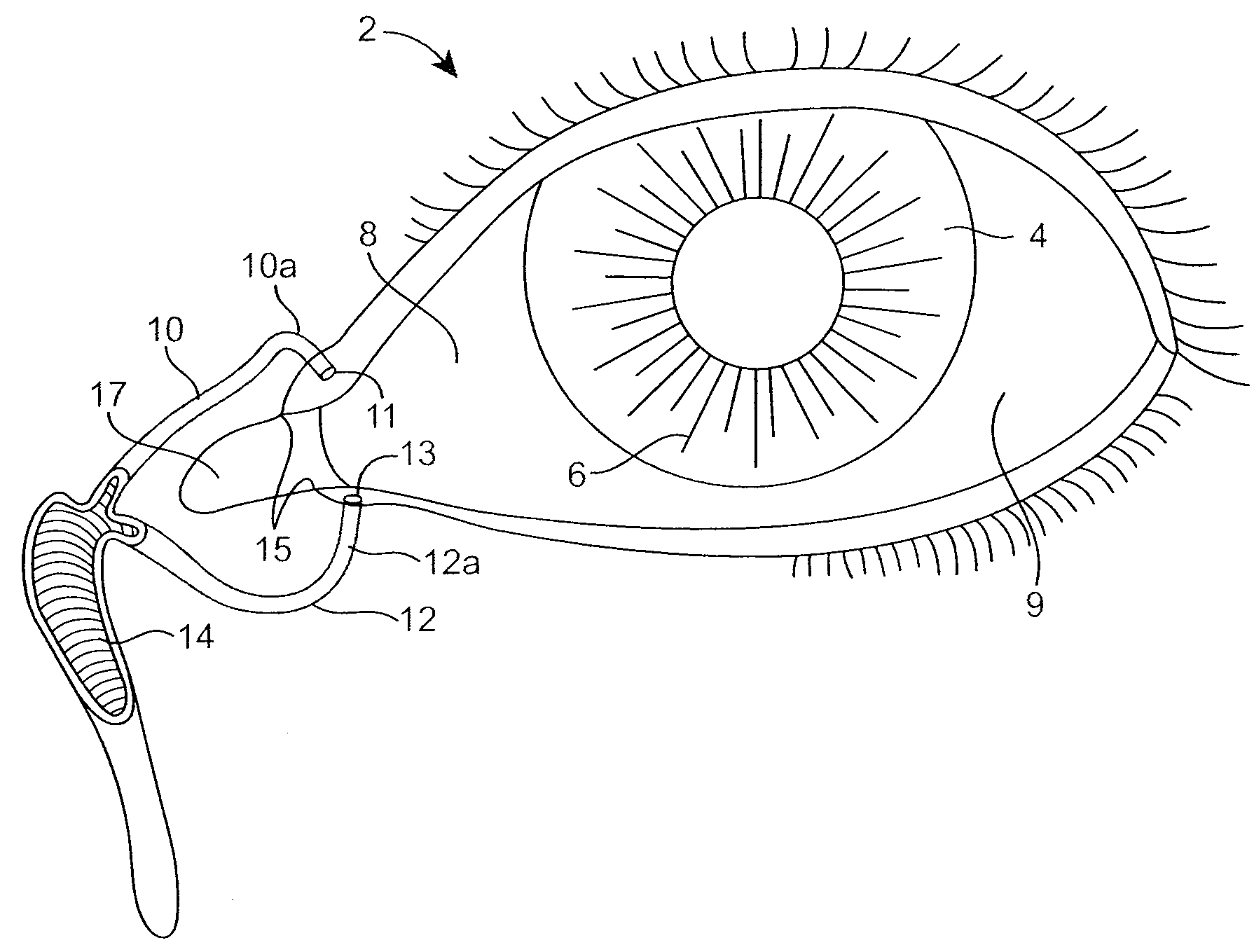
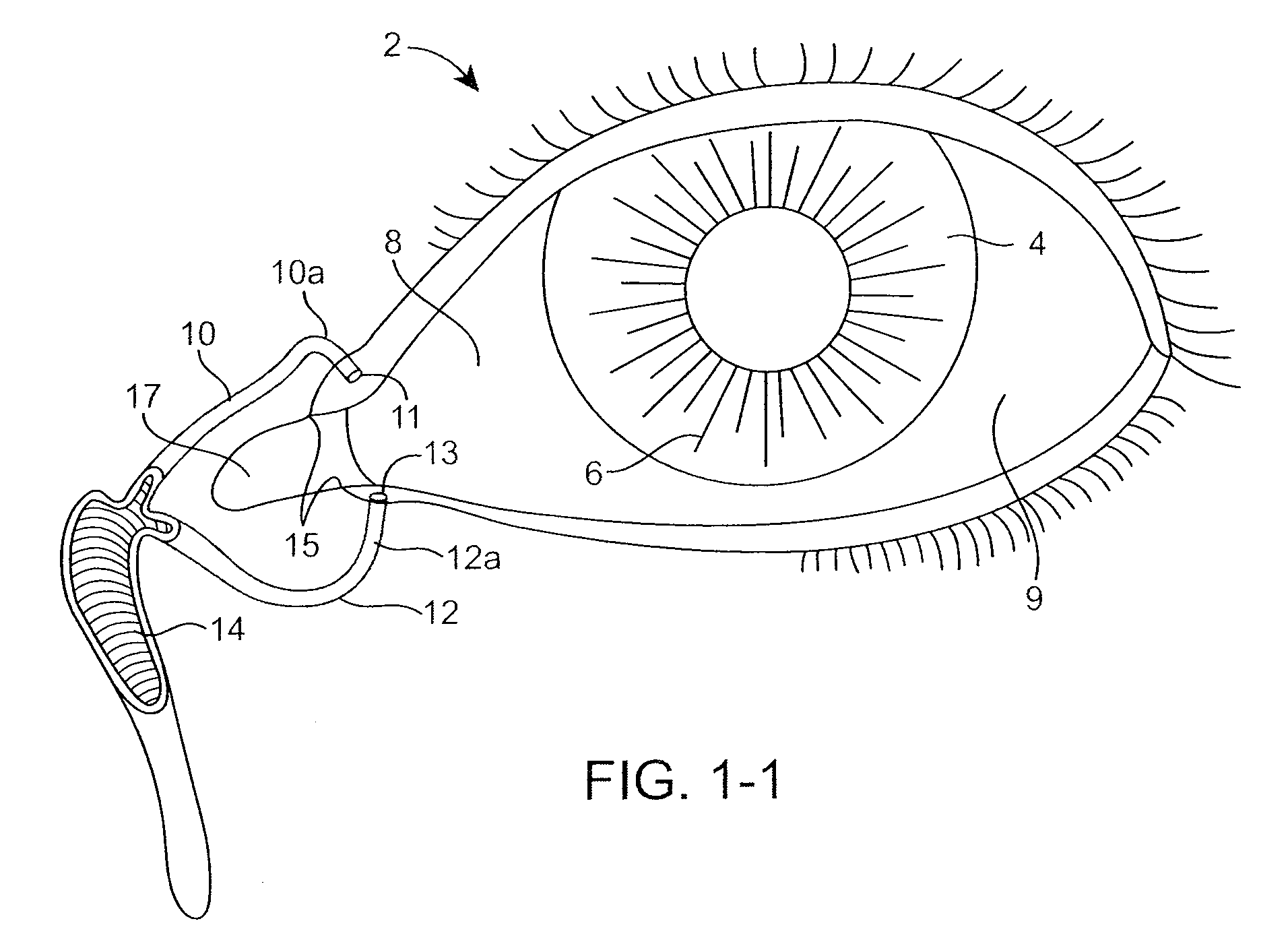
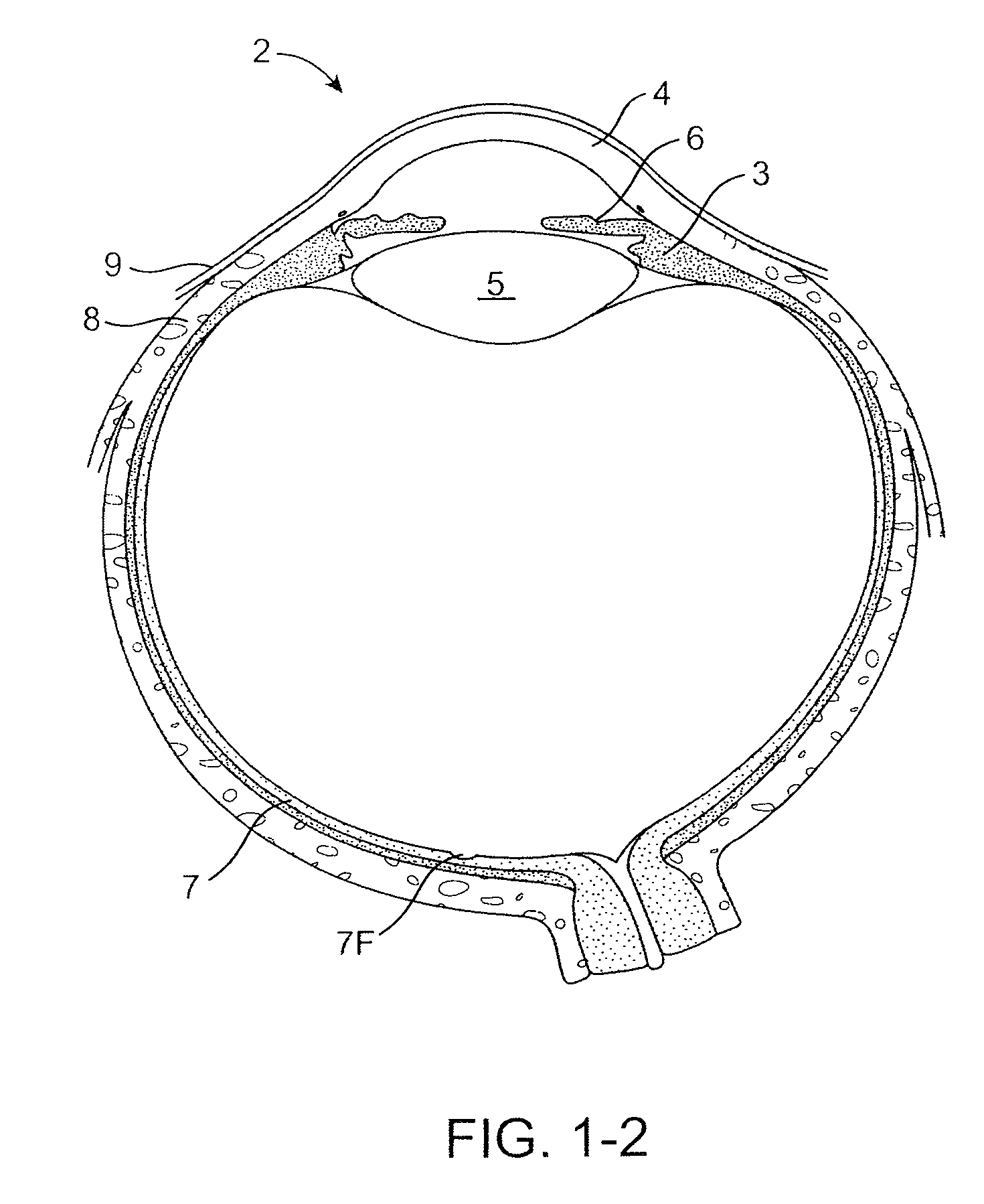
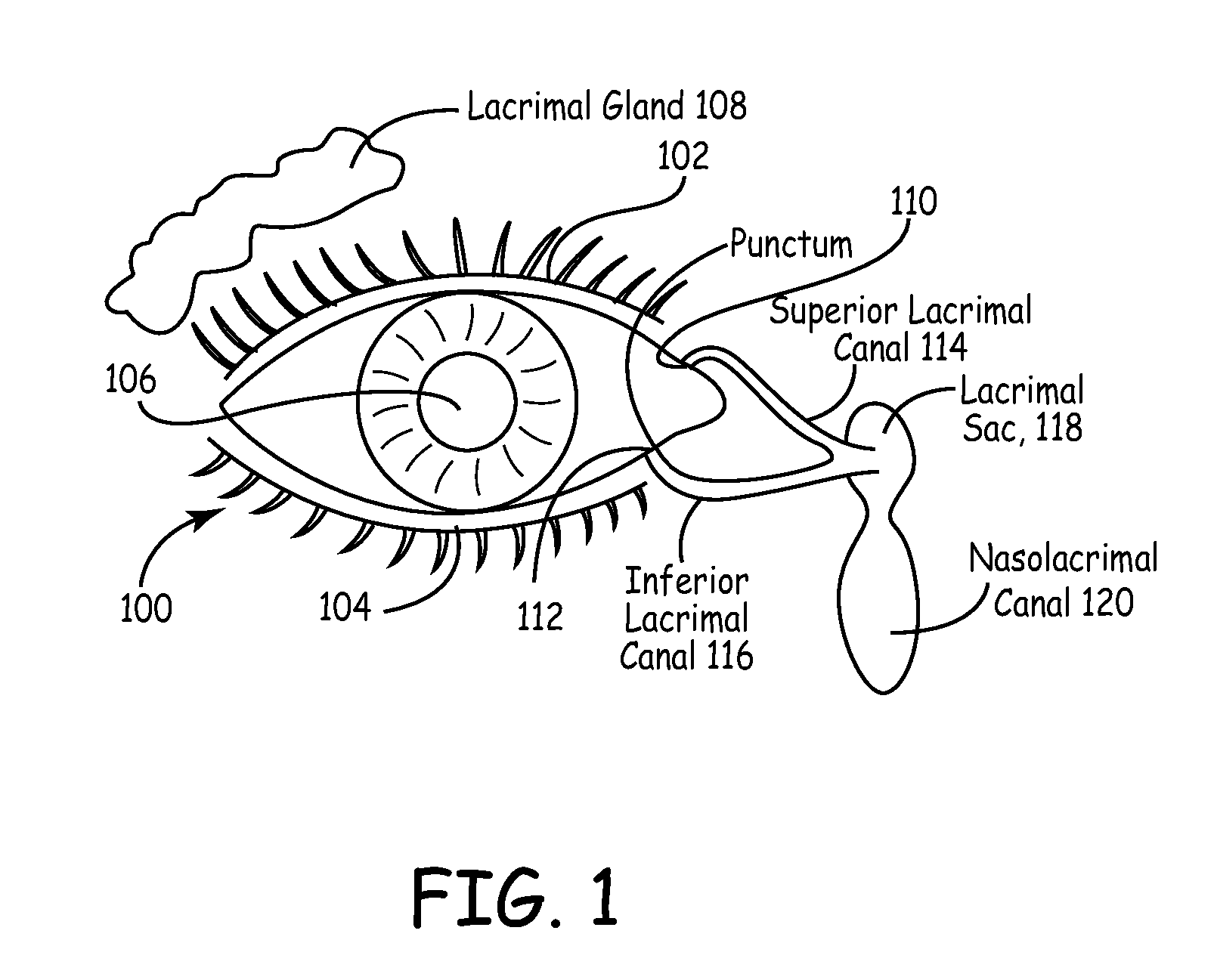
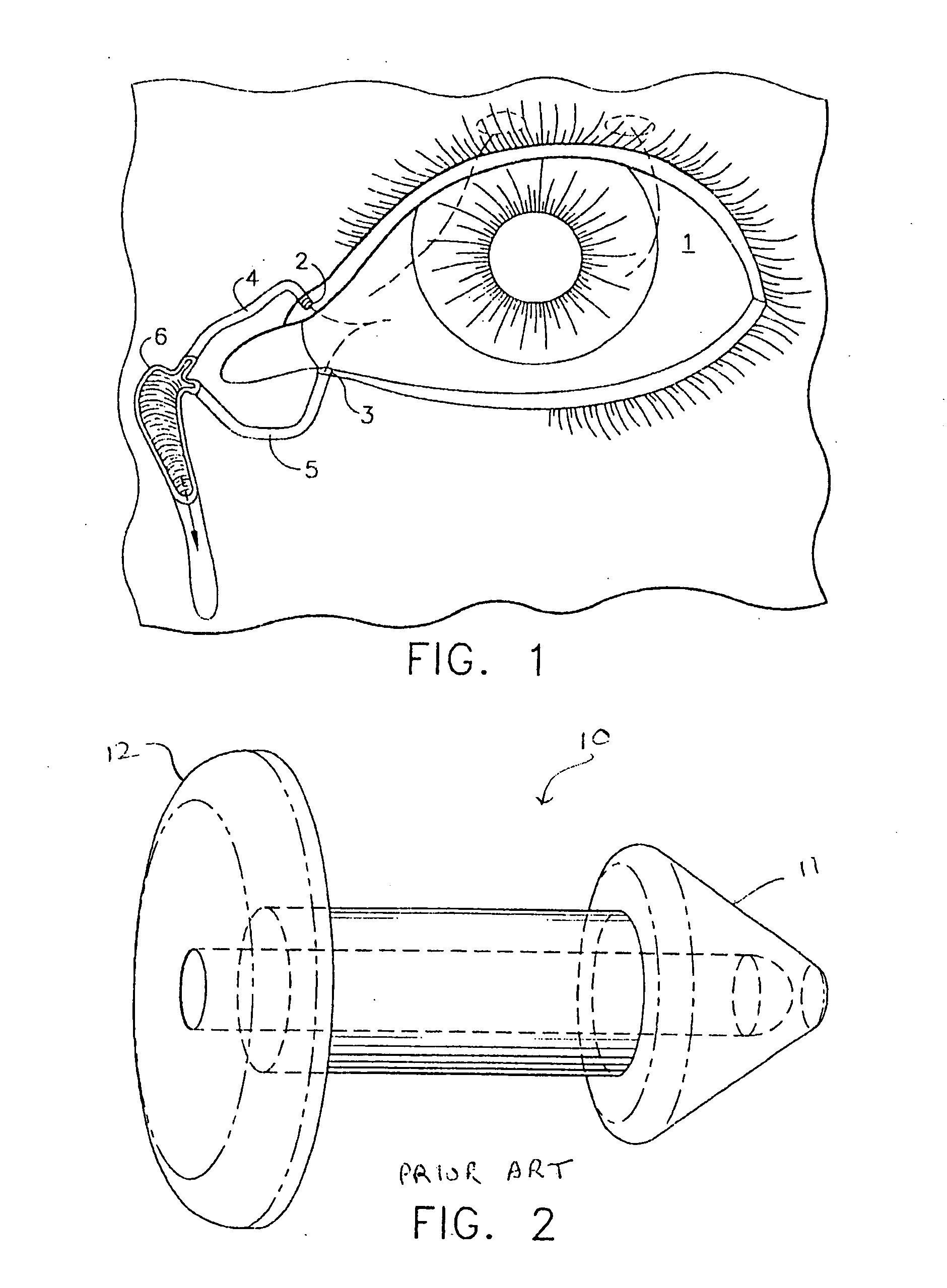
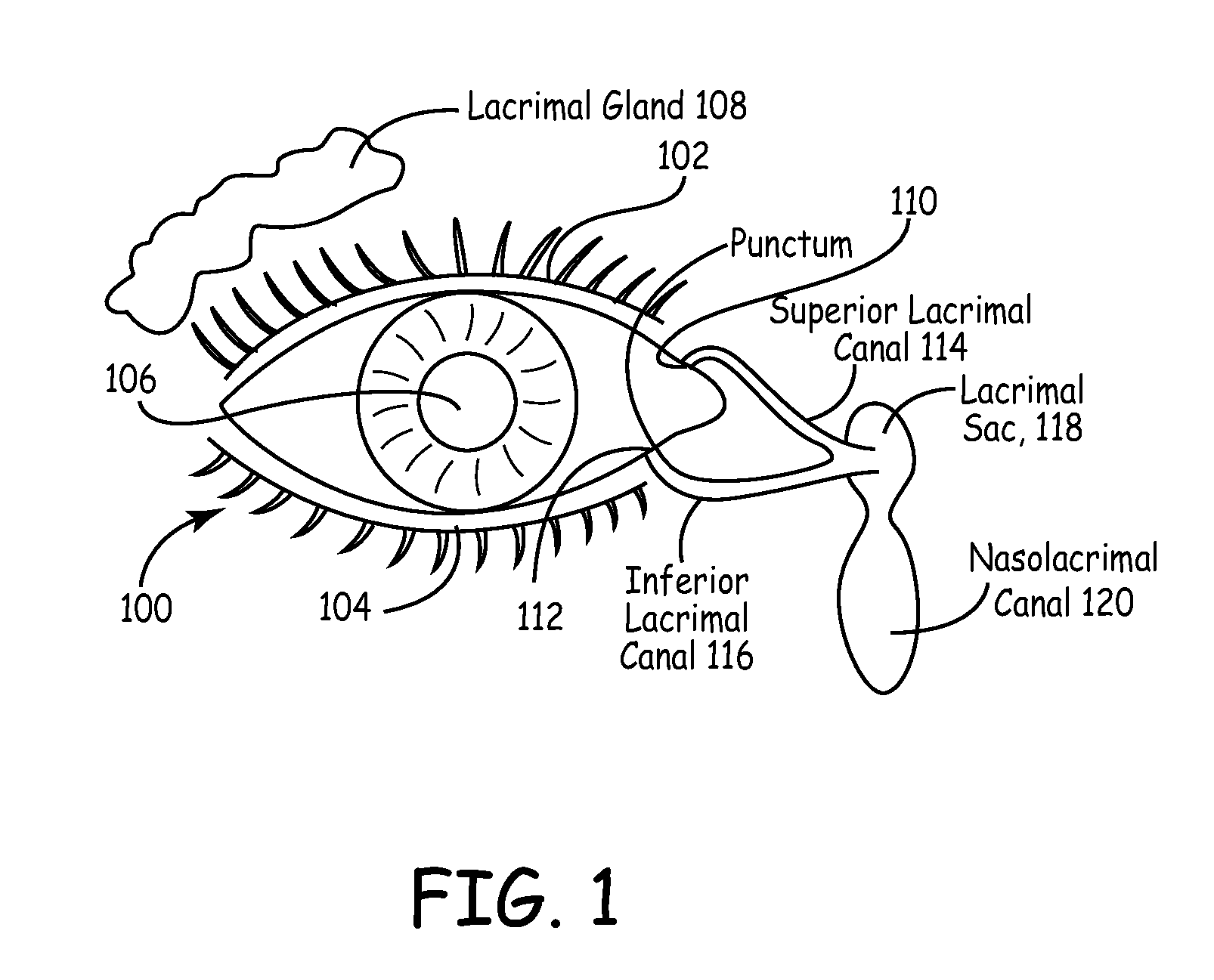
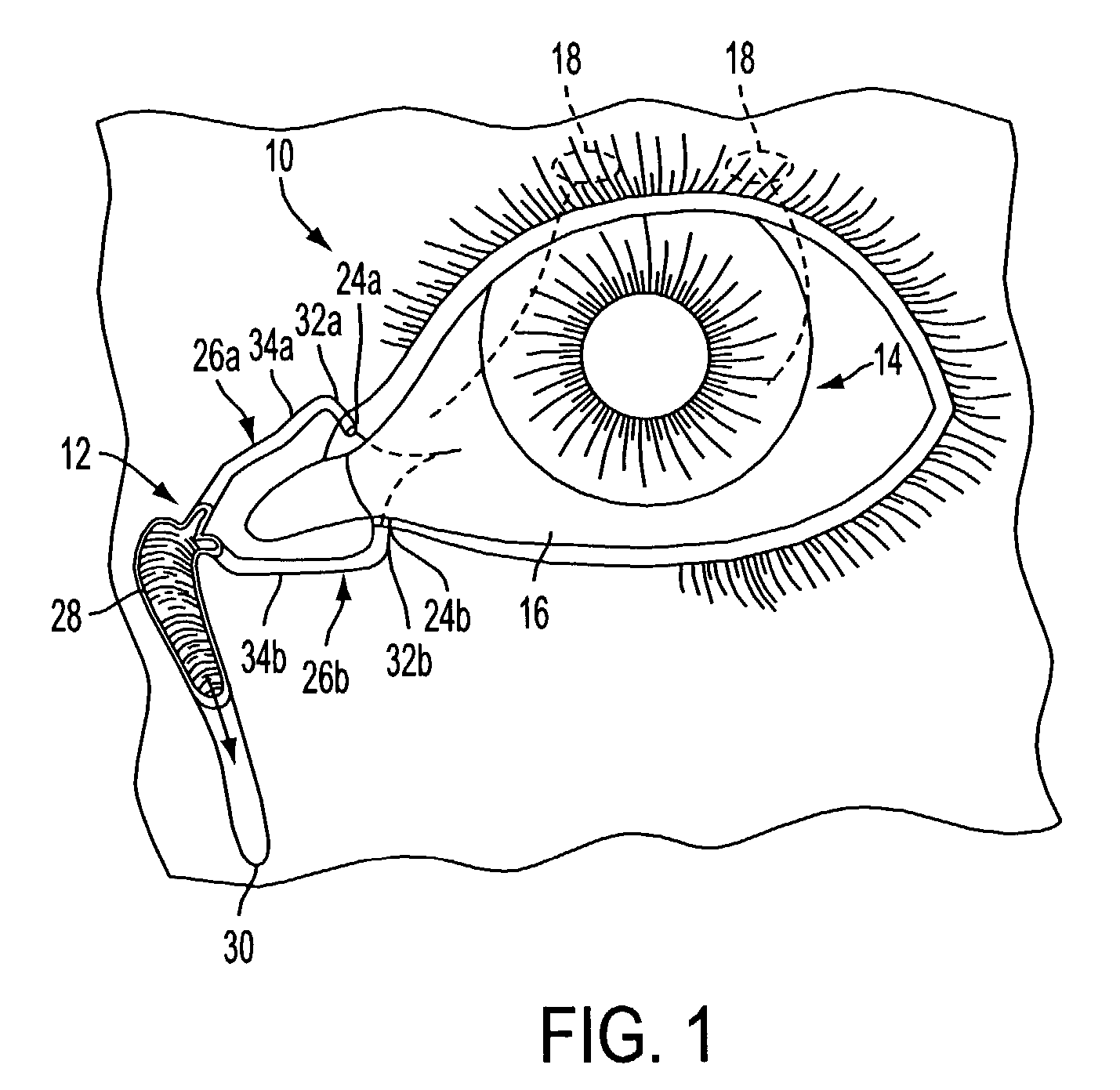
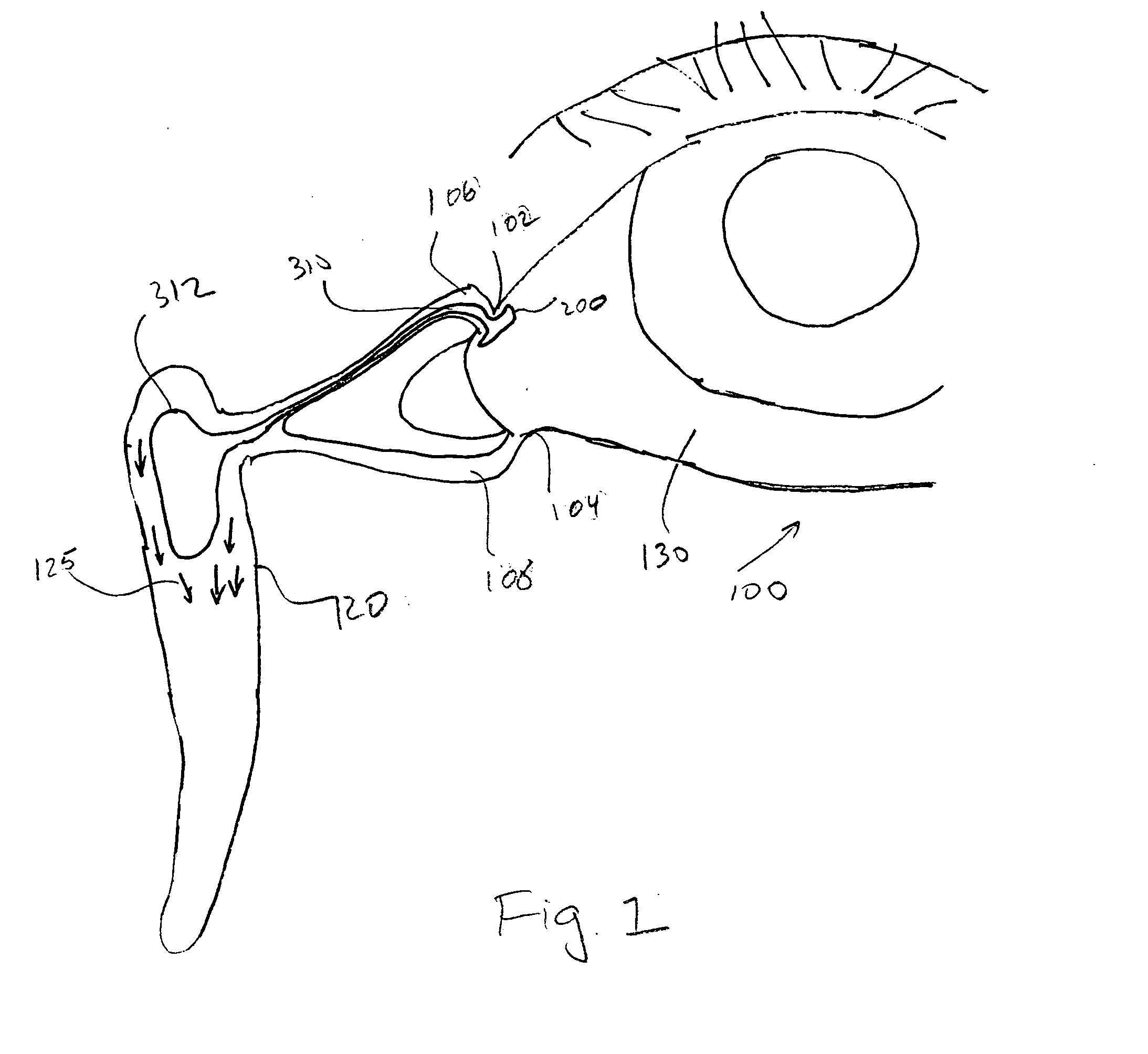
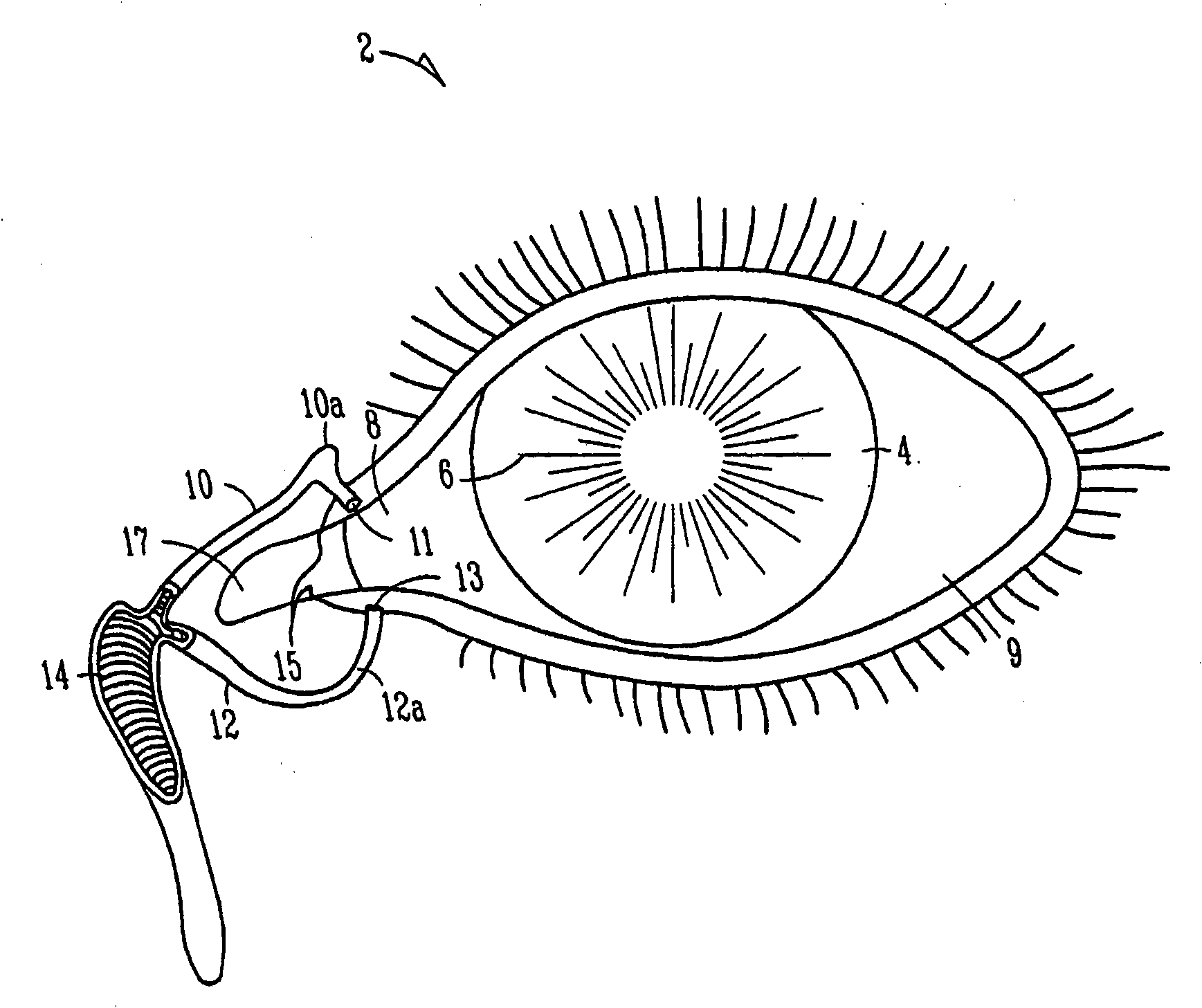
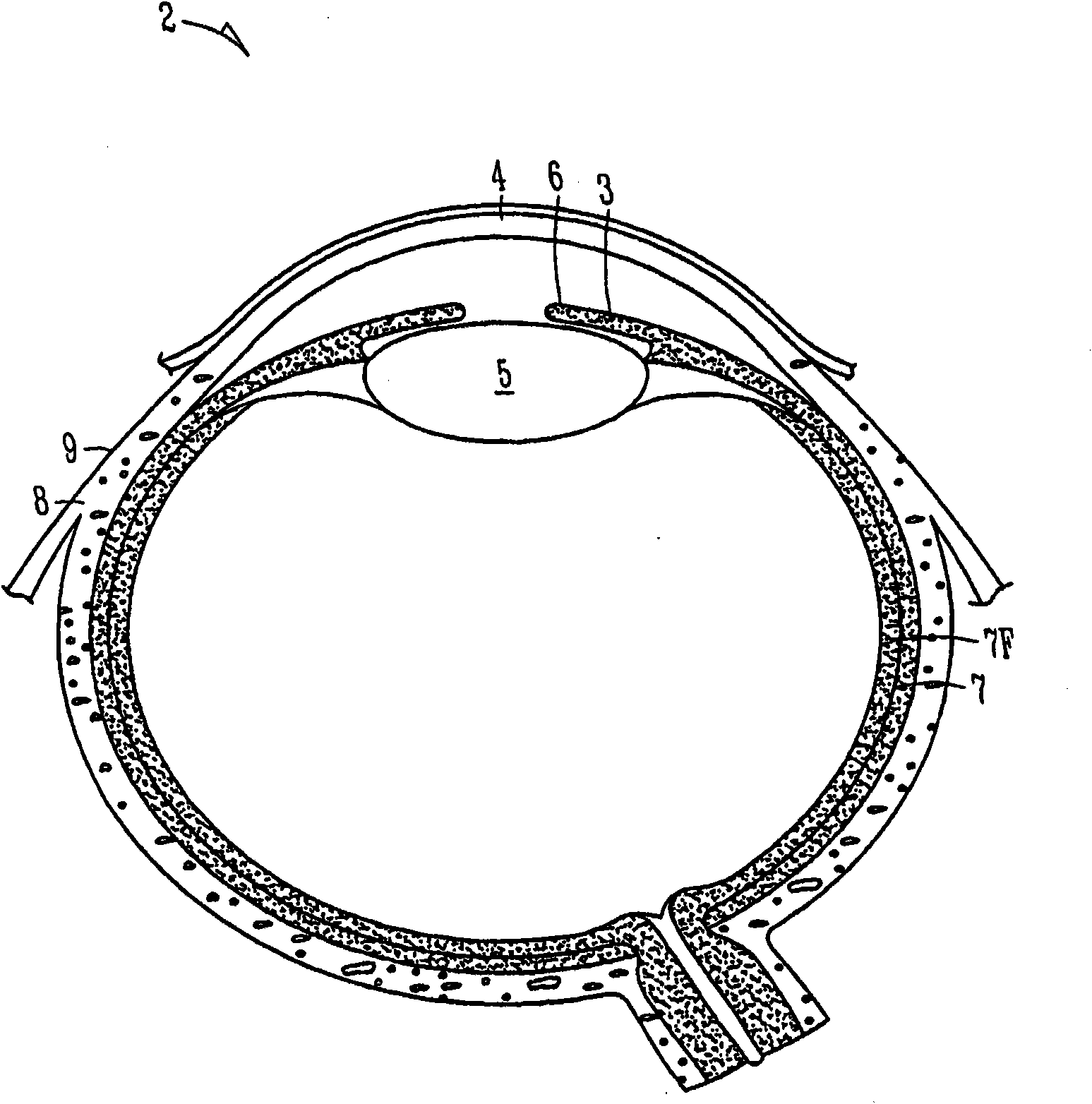
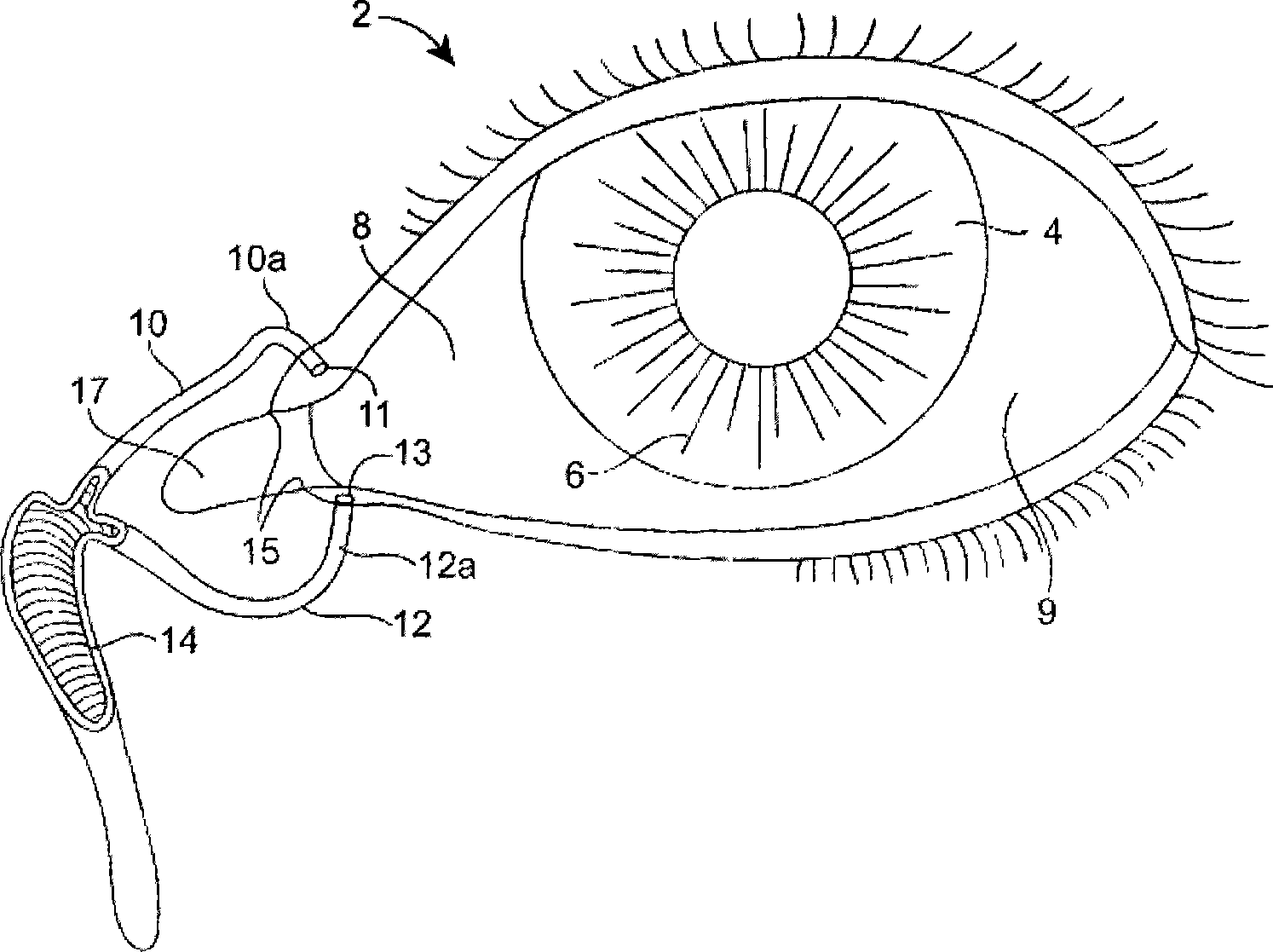
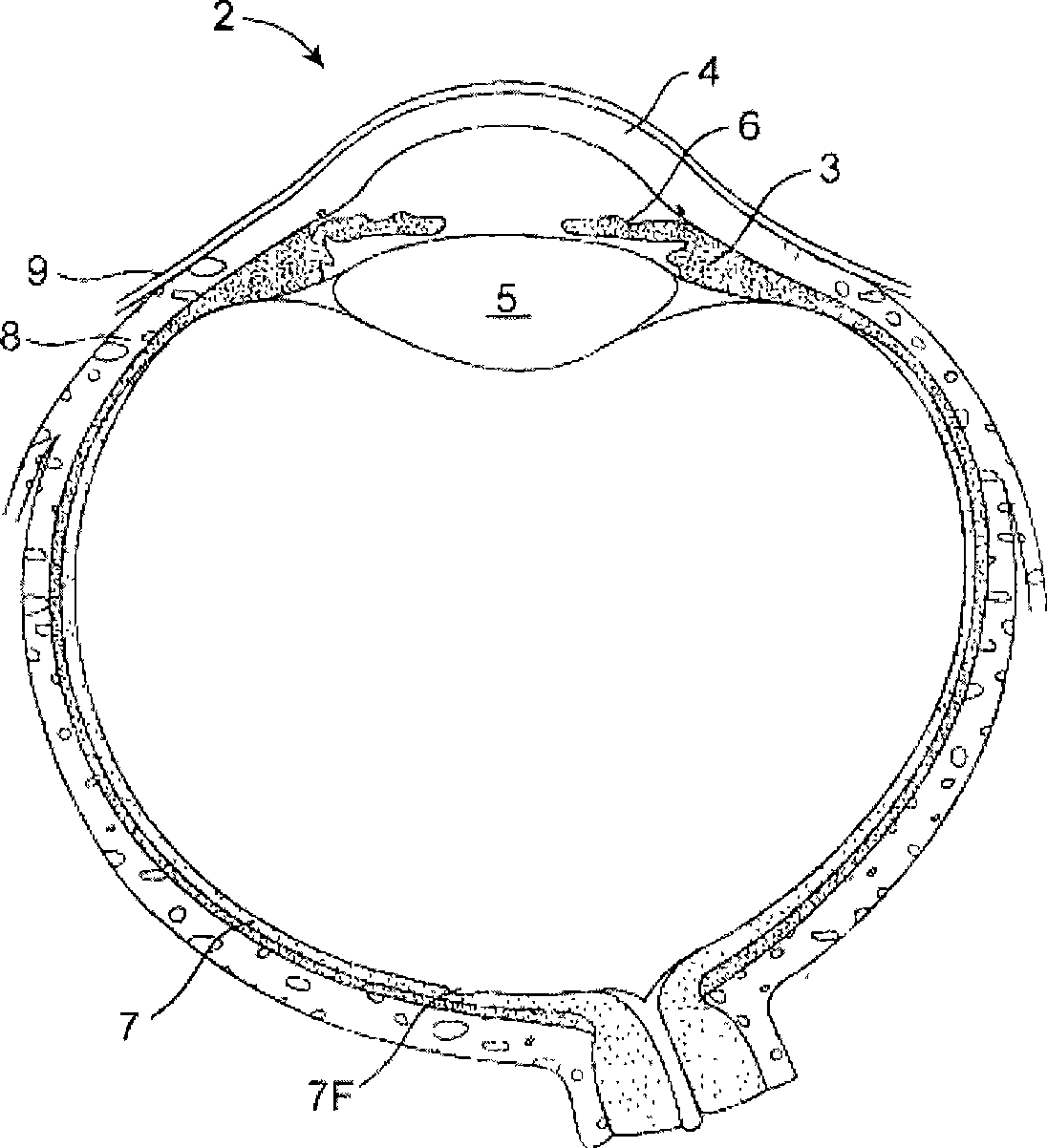
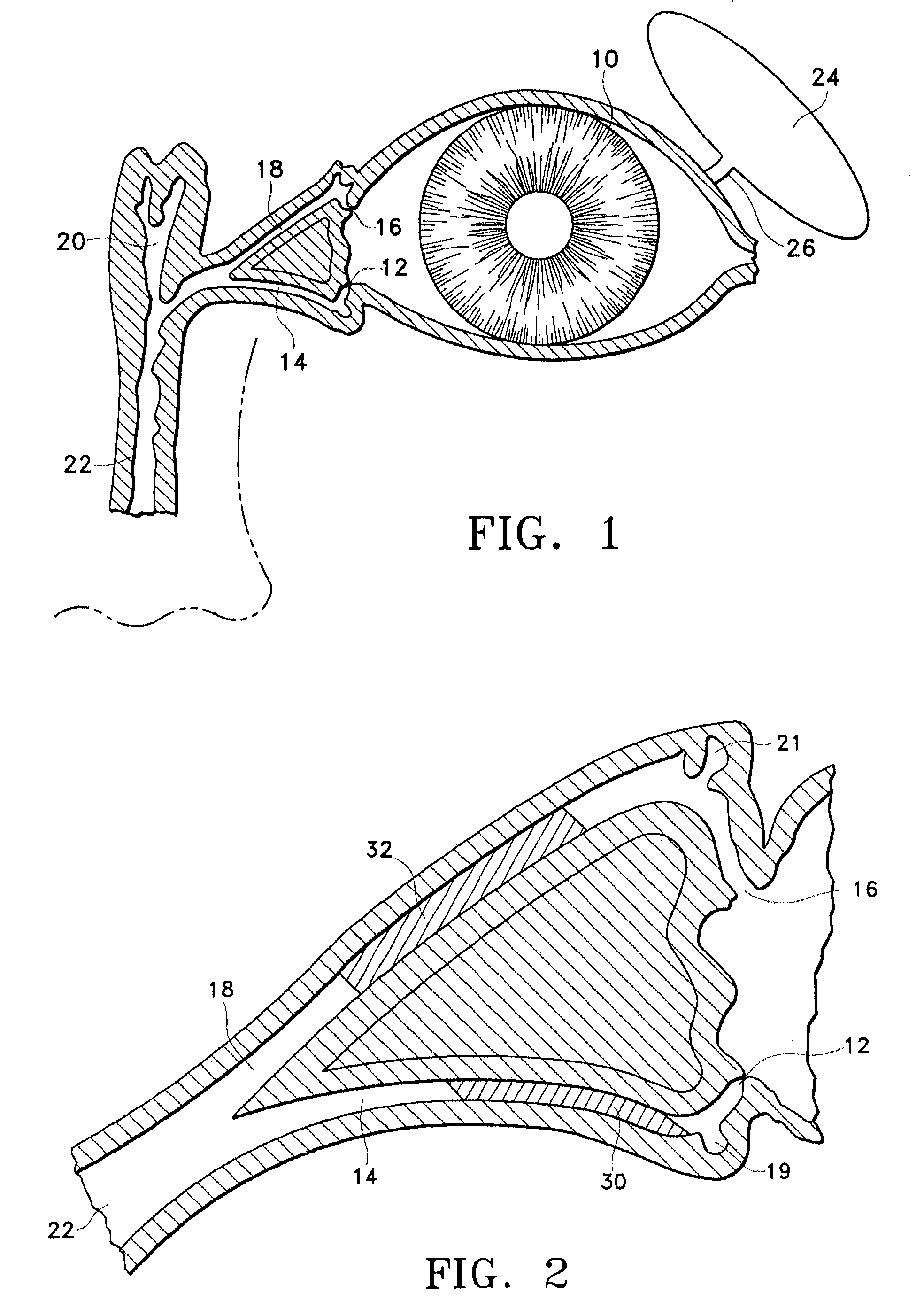
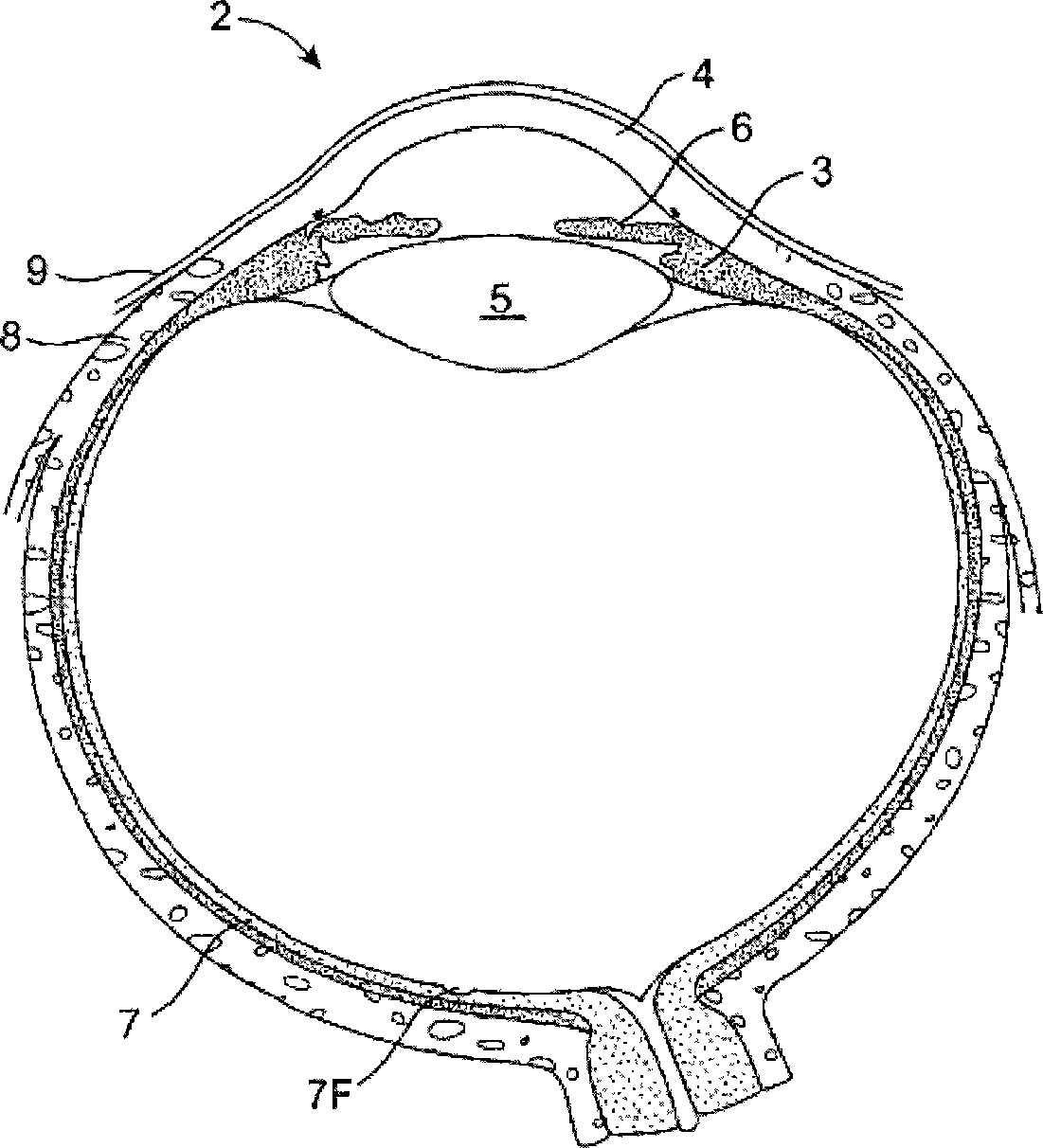
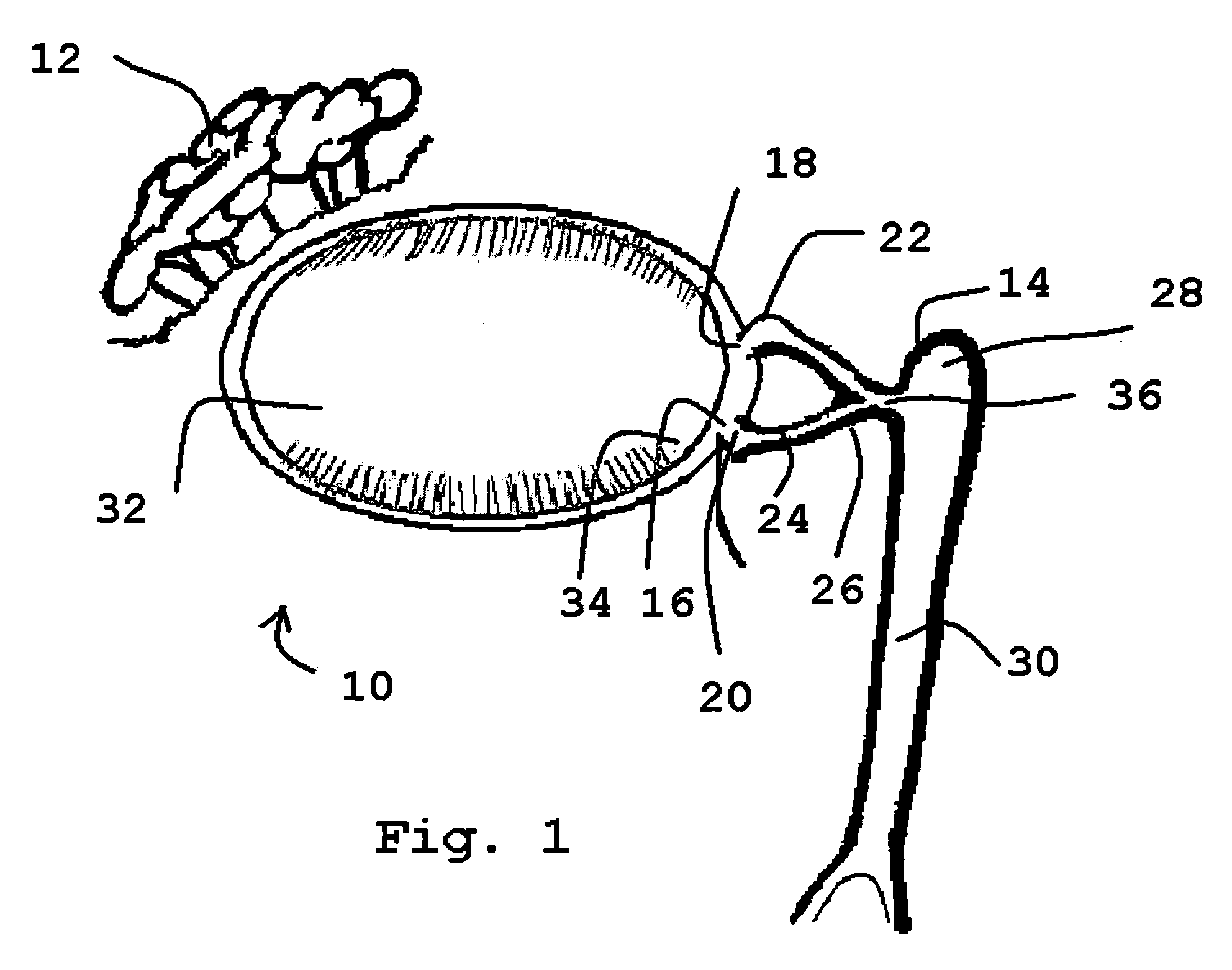
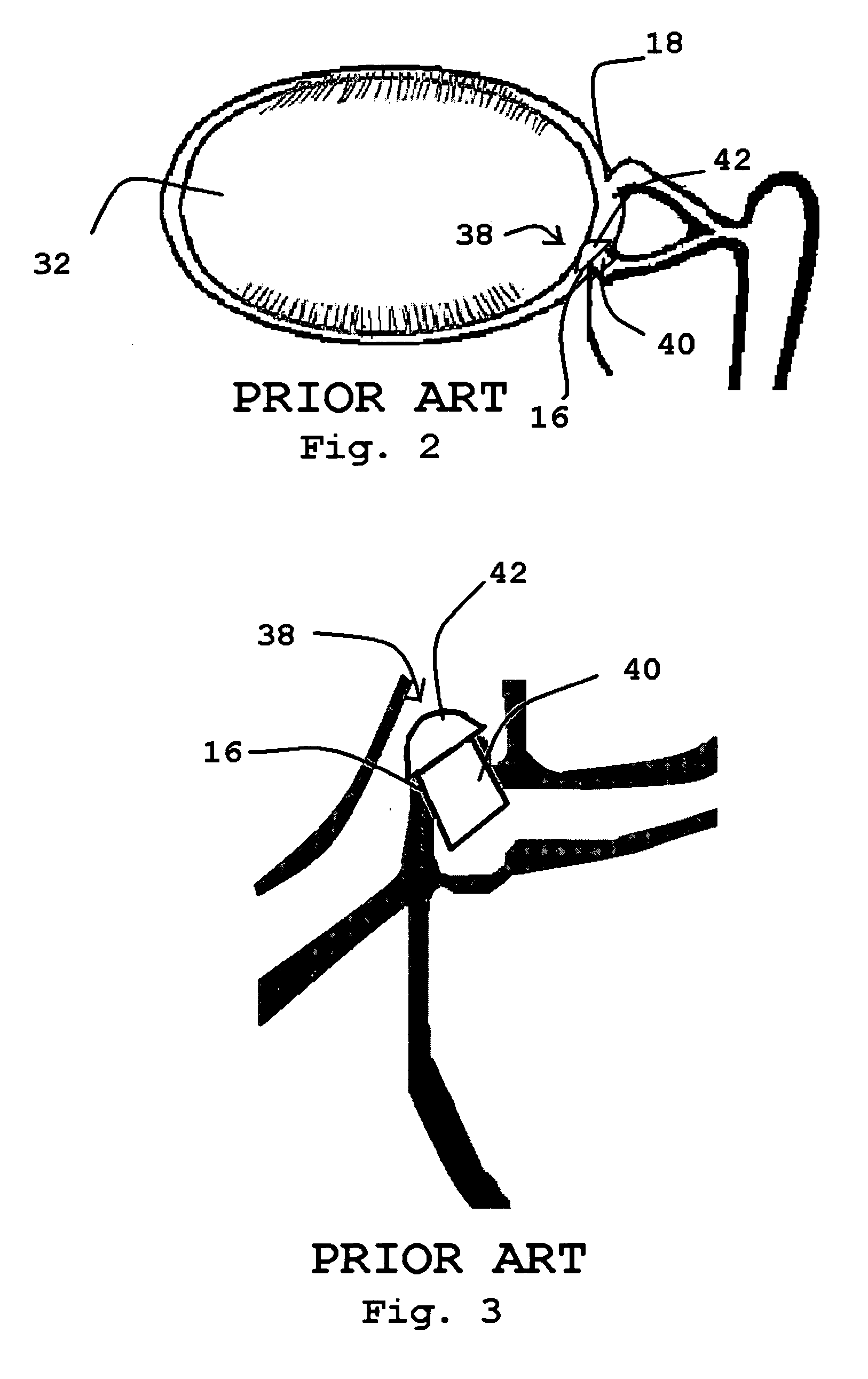
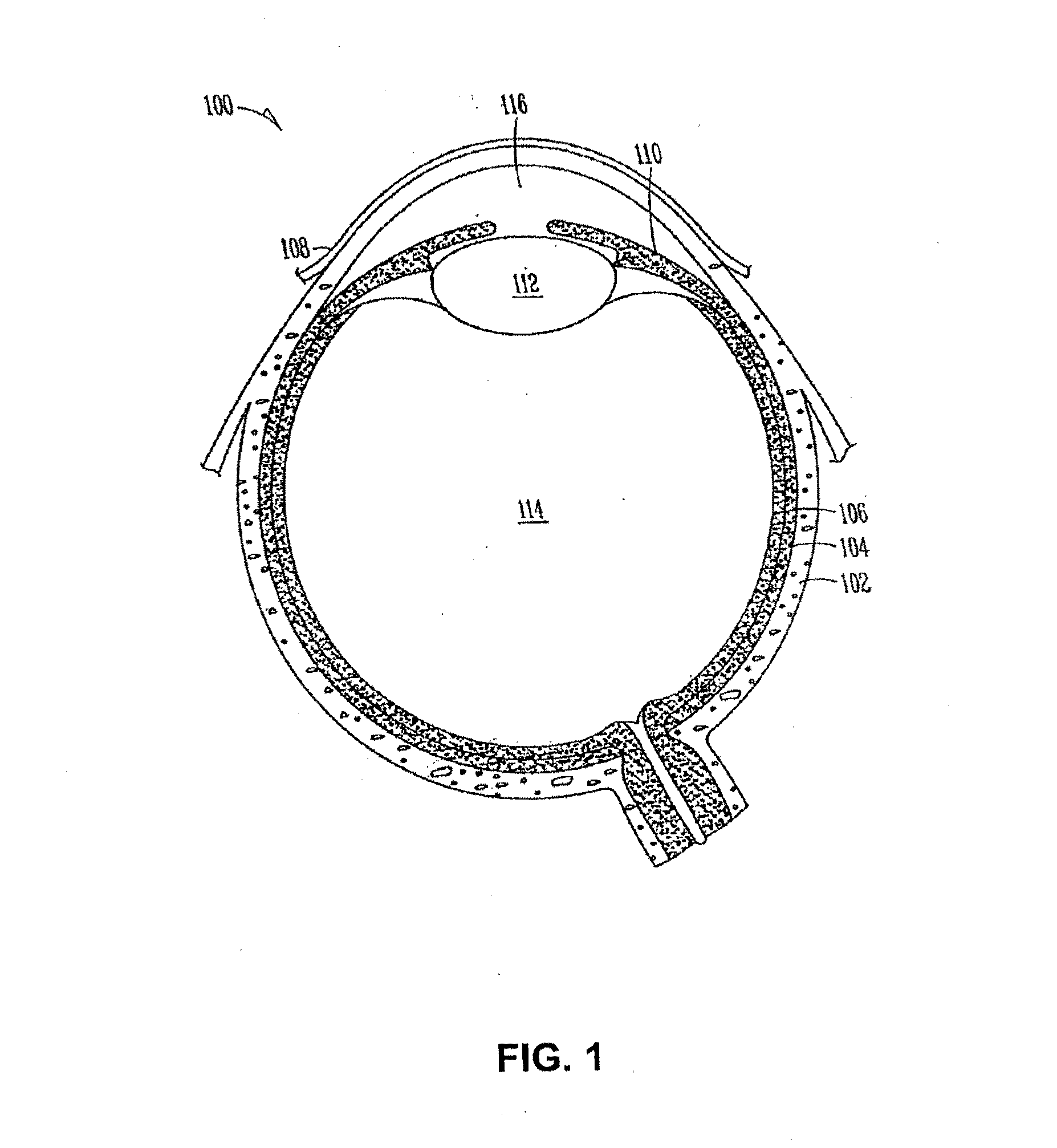
The lacrimal punctum (plural puncta) or lacrimal point, is a minute opening on the summits of the lacrimal papillae, seen on the margins of the eyelids at the lateral extremity of the lacrimal lake. There are two lacrimal puncta in the medial (inside) portion of each eyelid.
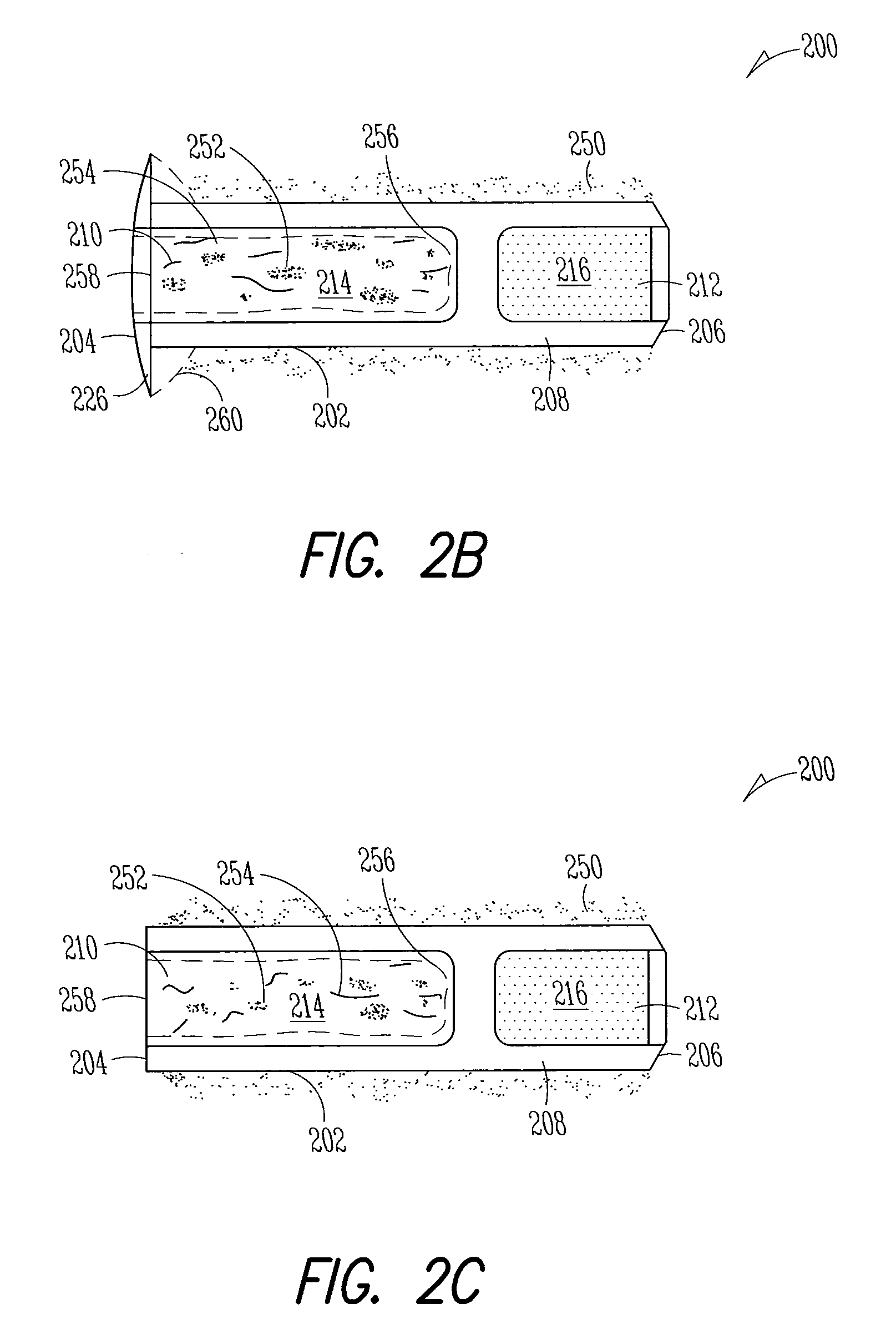
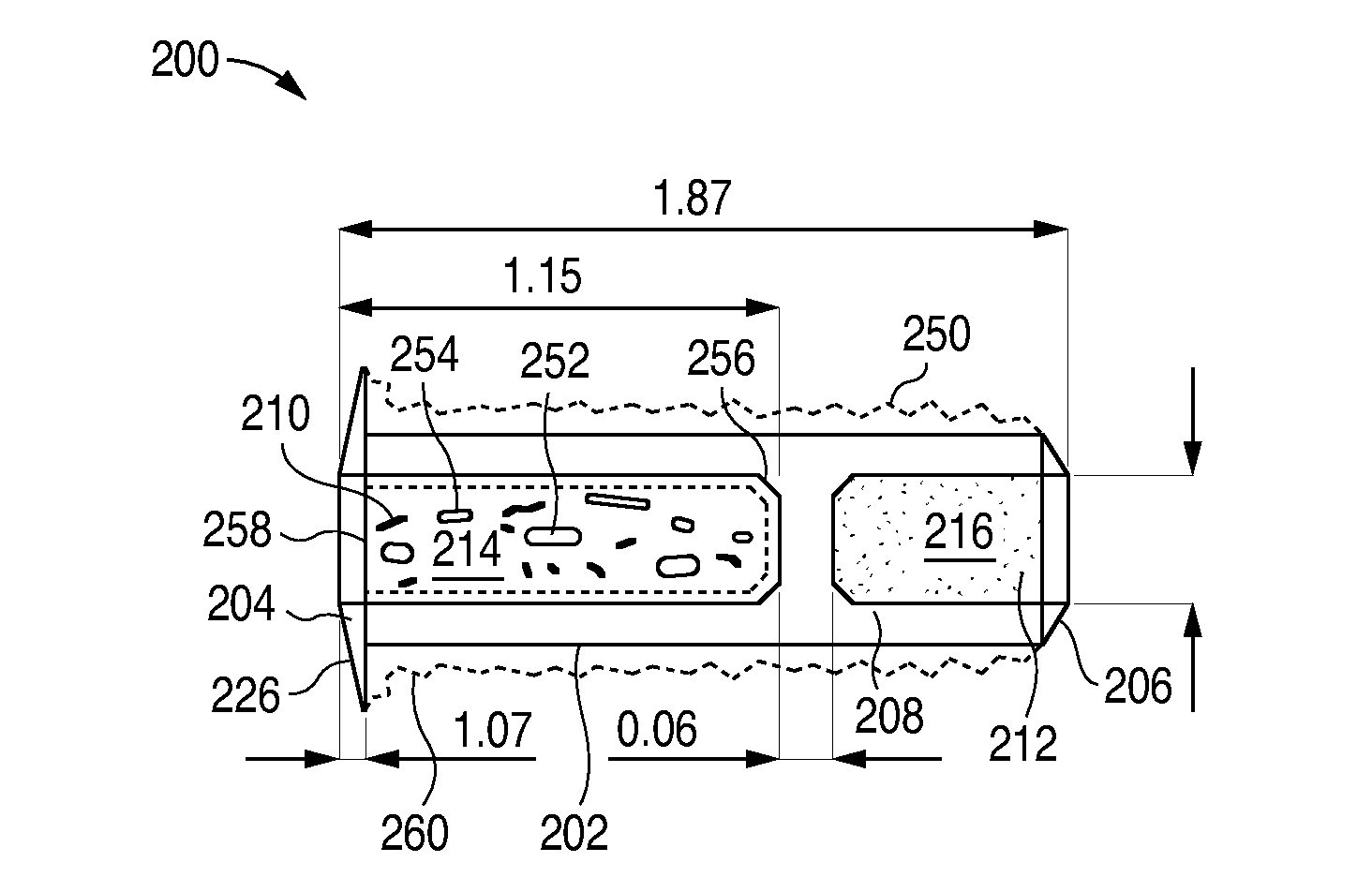
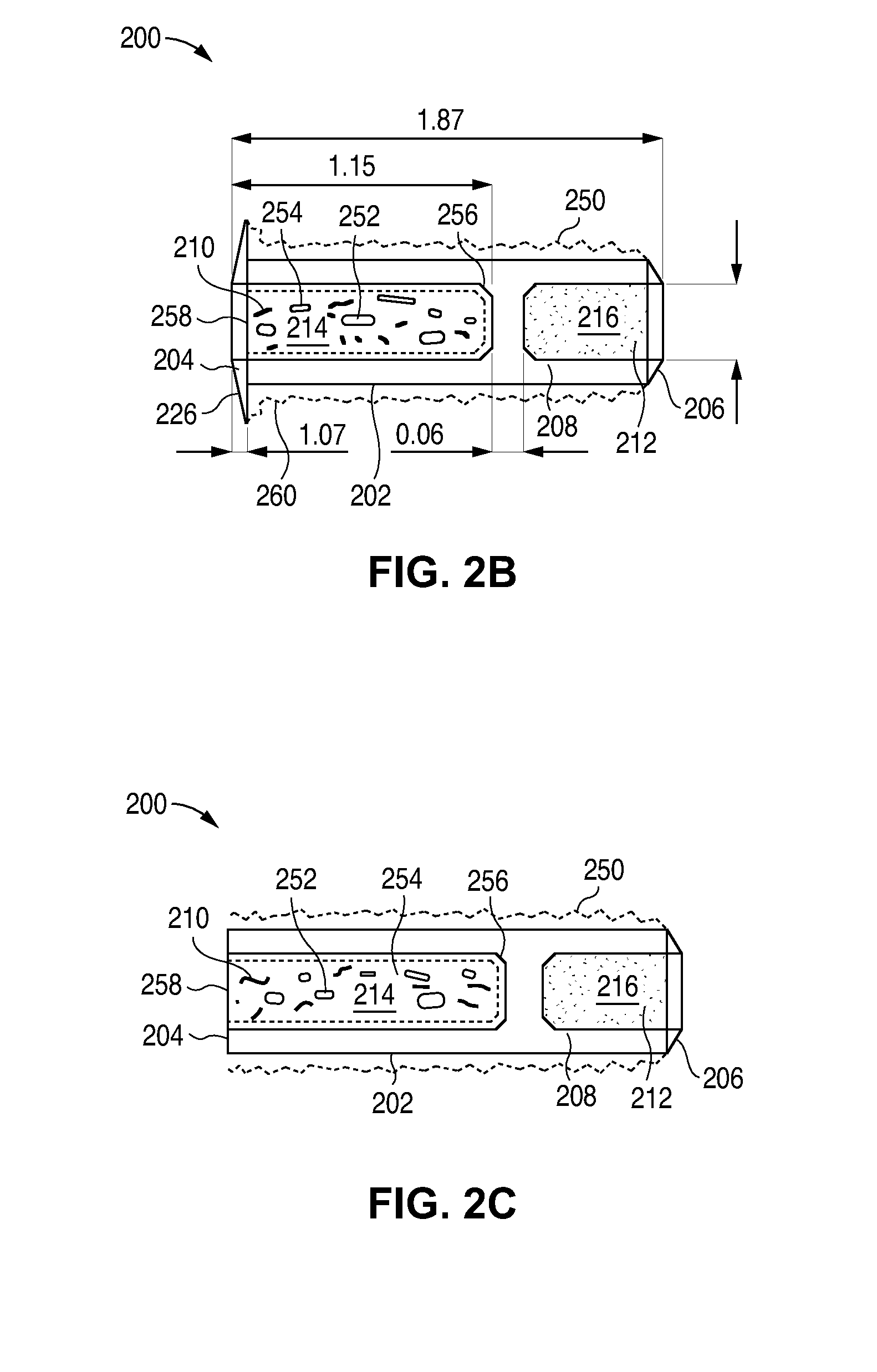
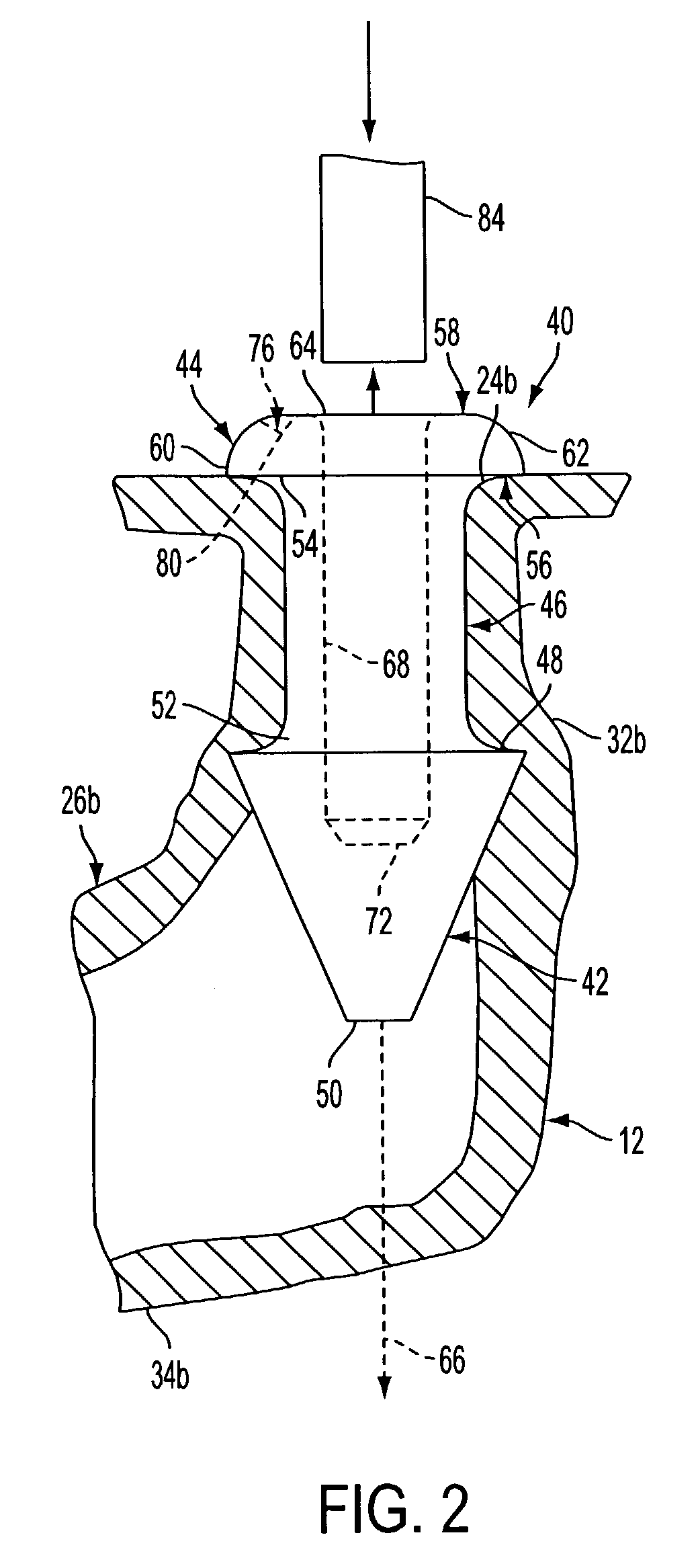
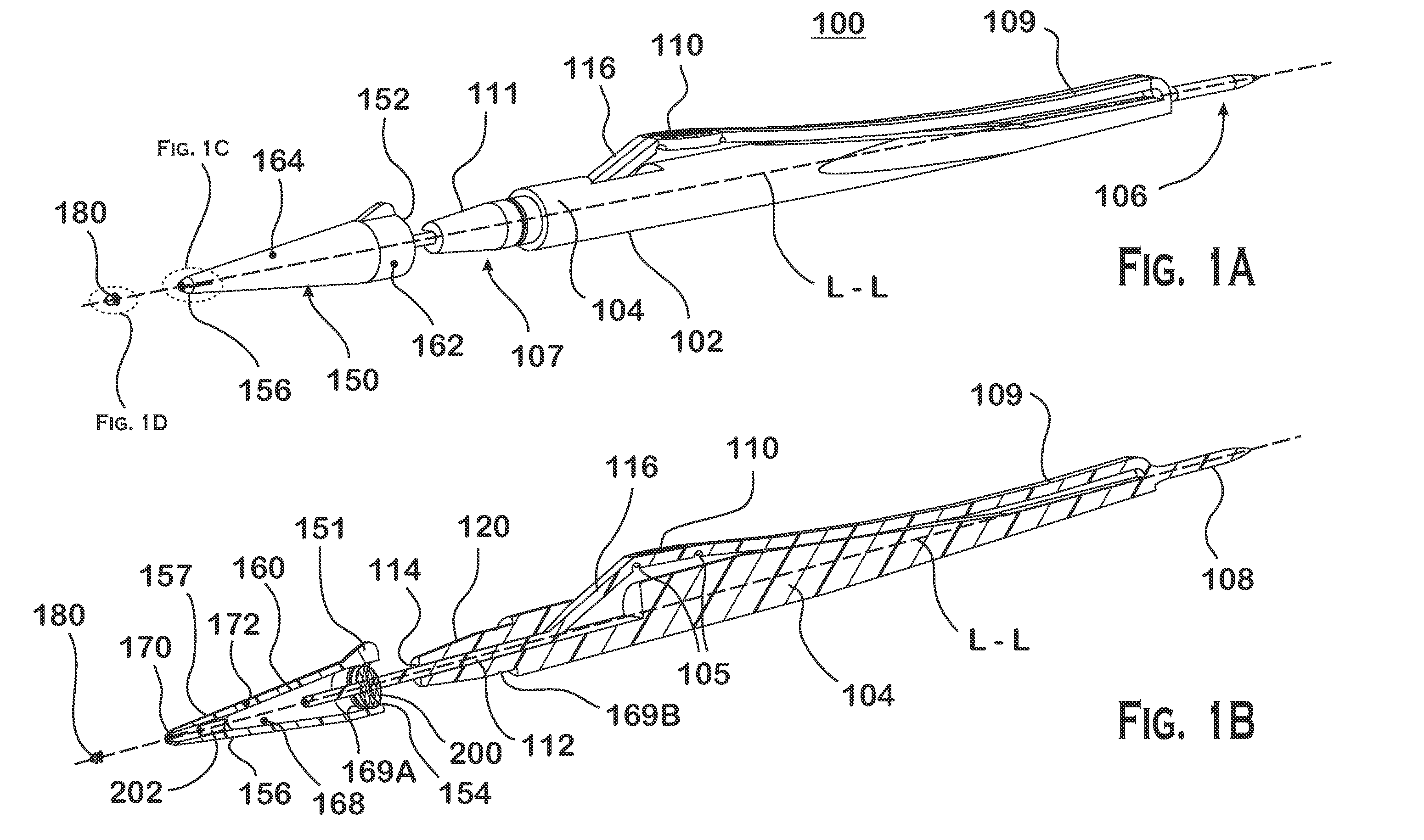
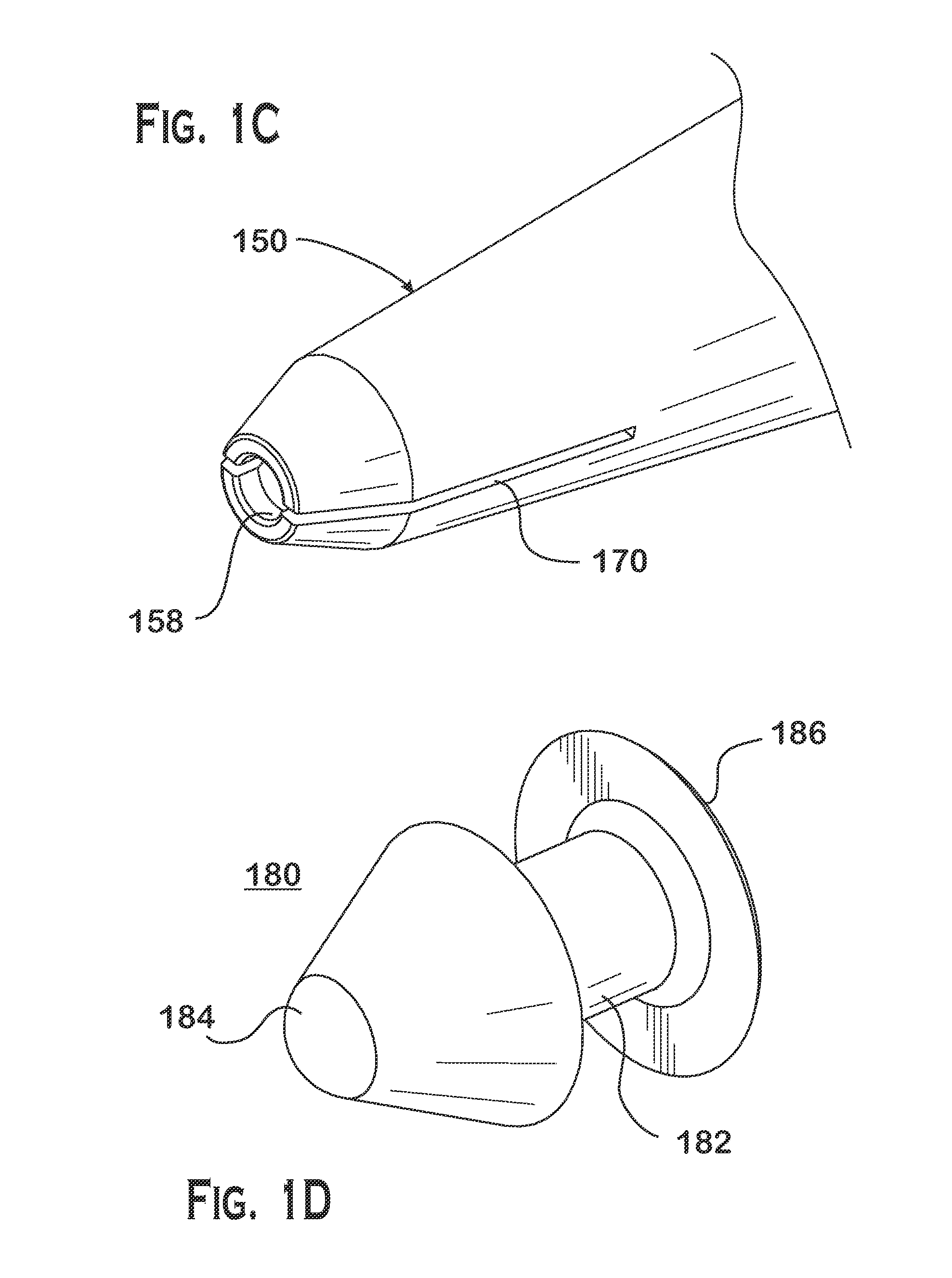
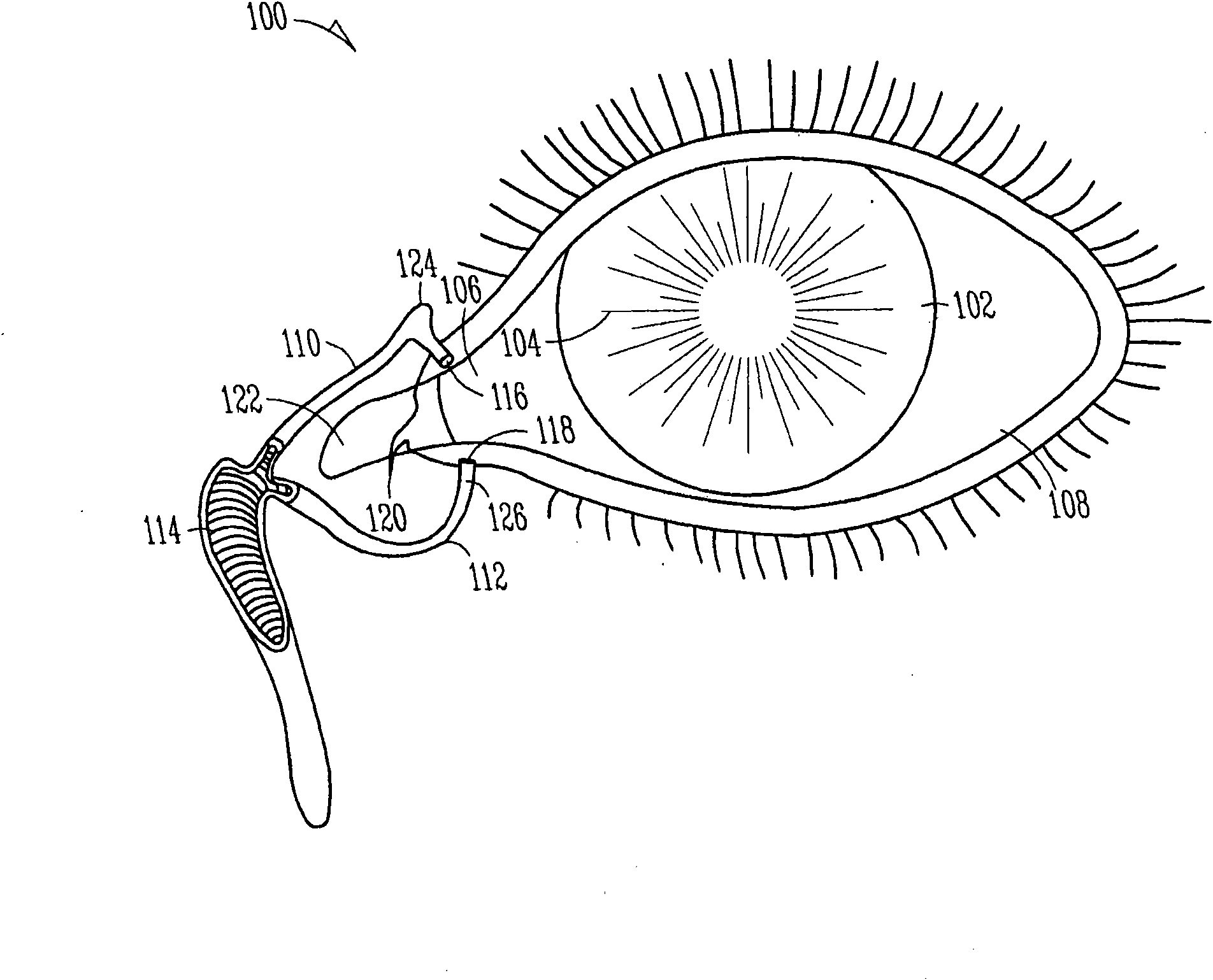
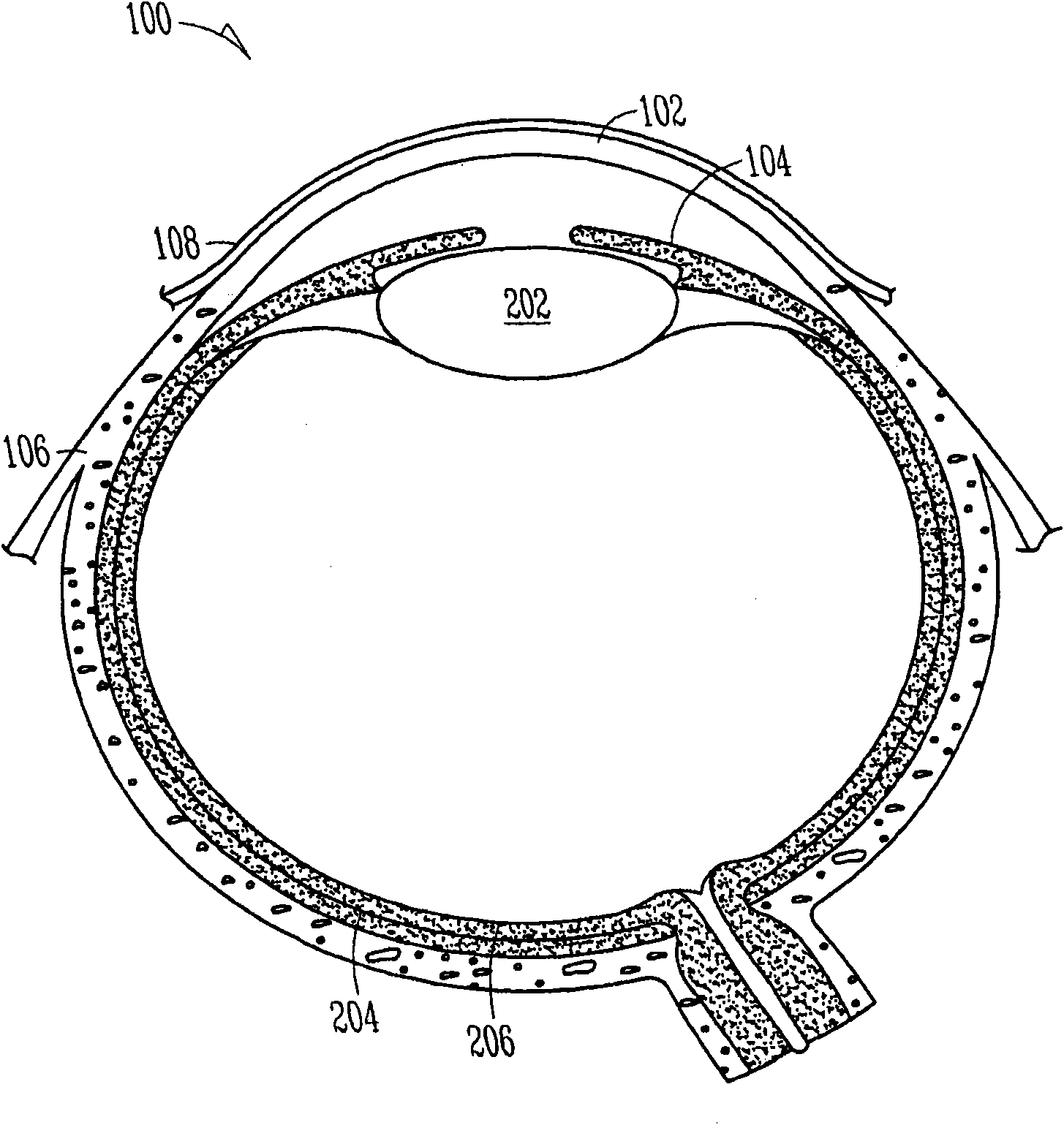
Nasolacrimal Drainage System Implants for Drug Therapy
ActiveUS20070243230A1Reduce deliveryAvoid flowAntibacterial agentsSenses disorderShape-memory alloyImplanted device
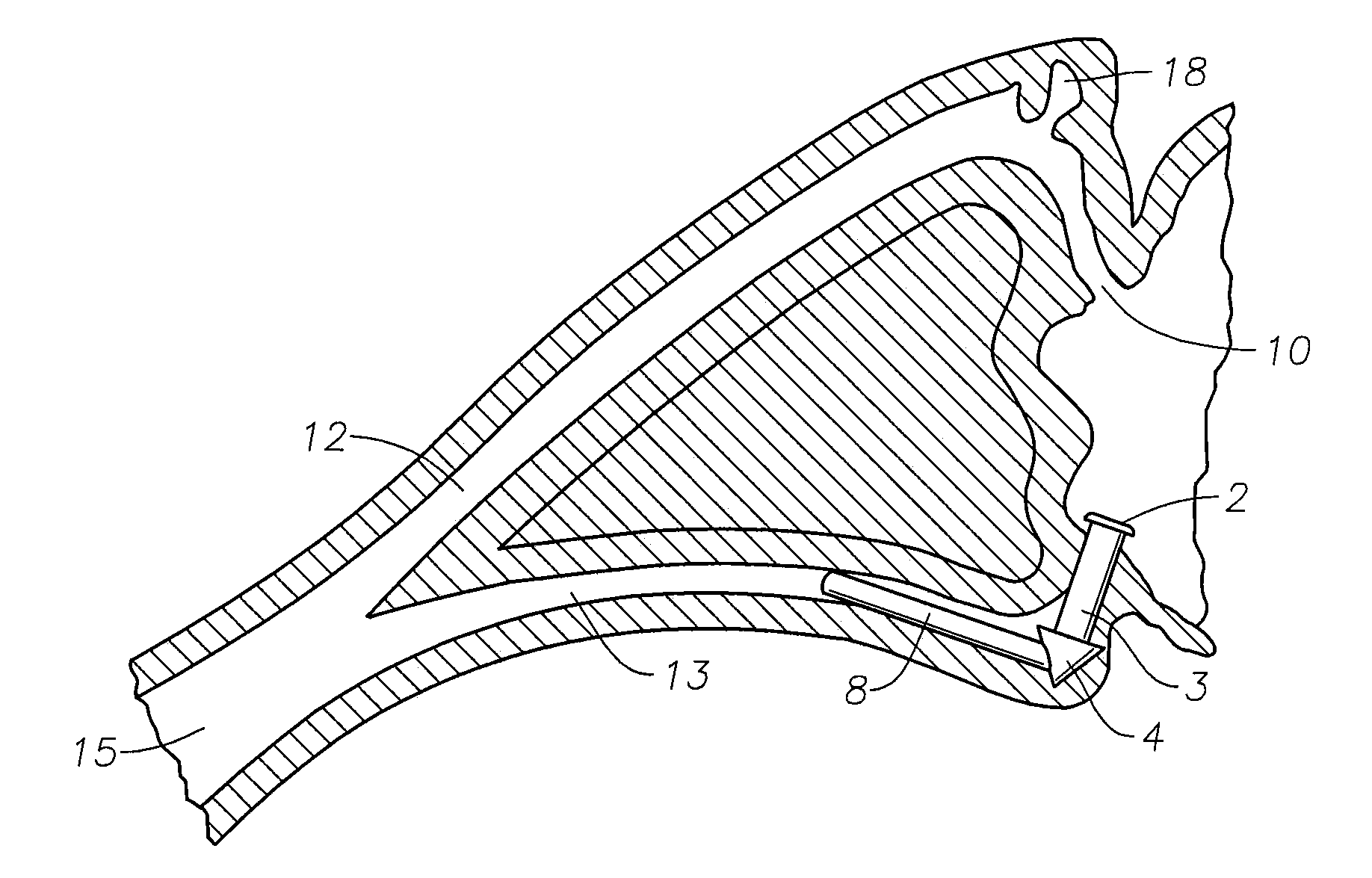
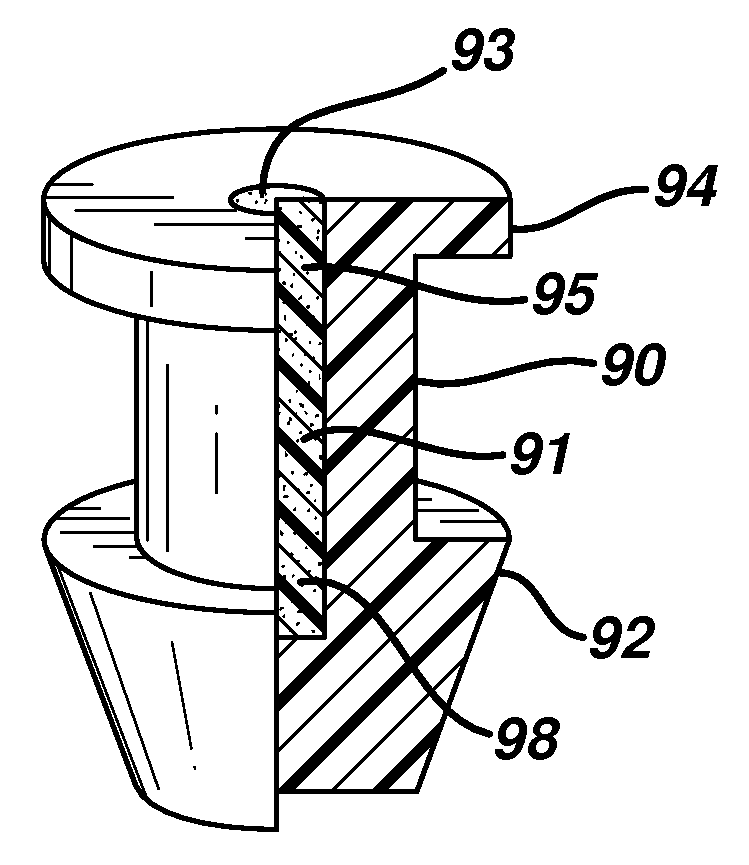
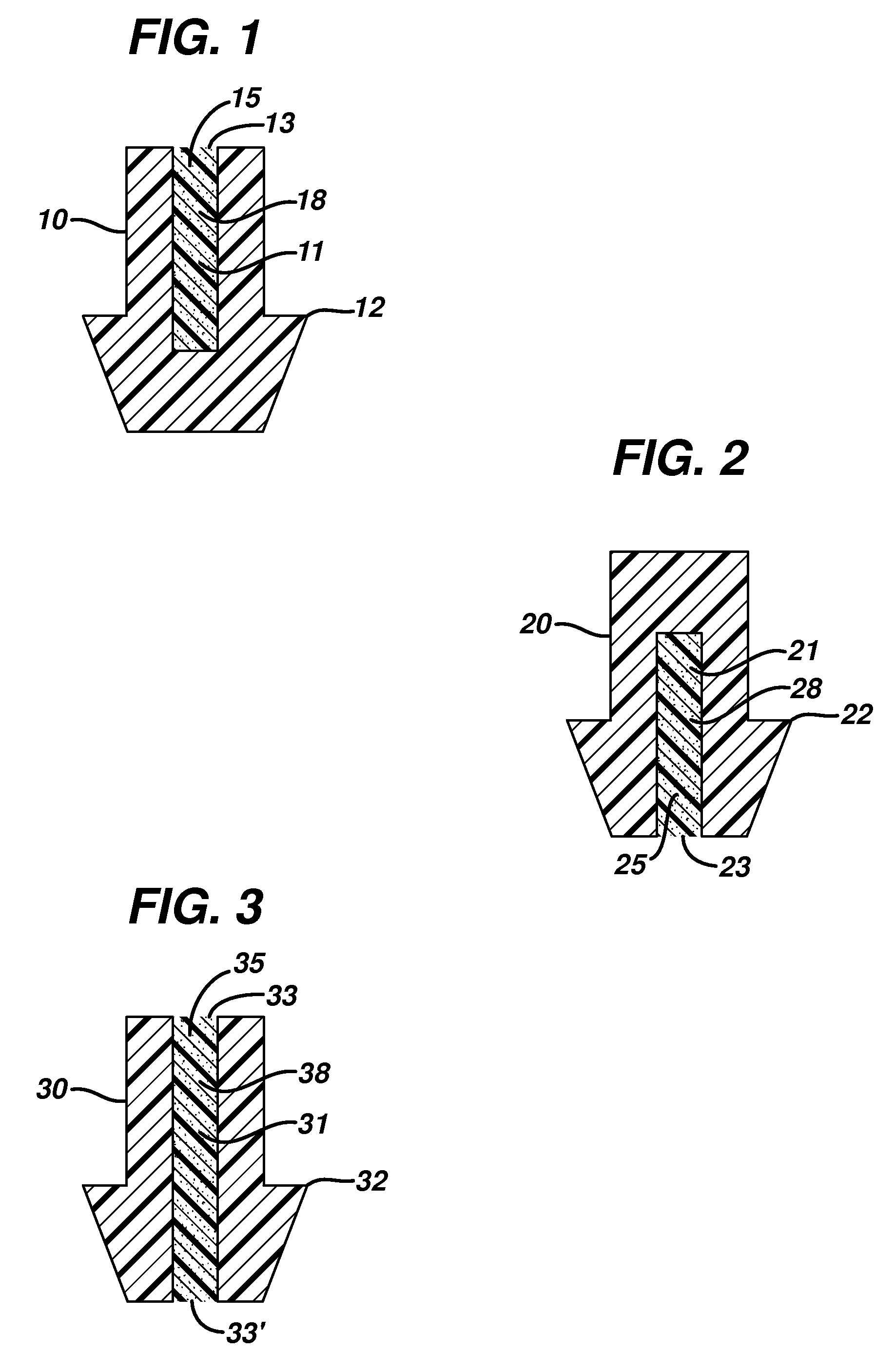
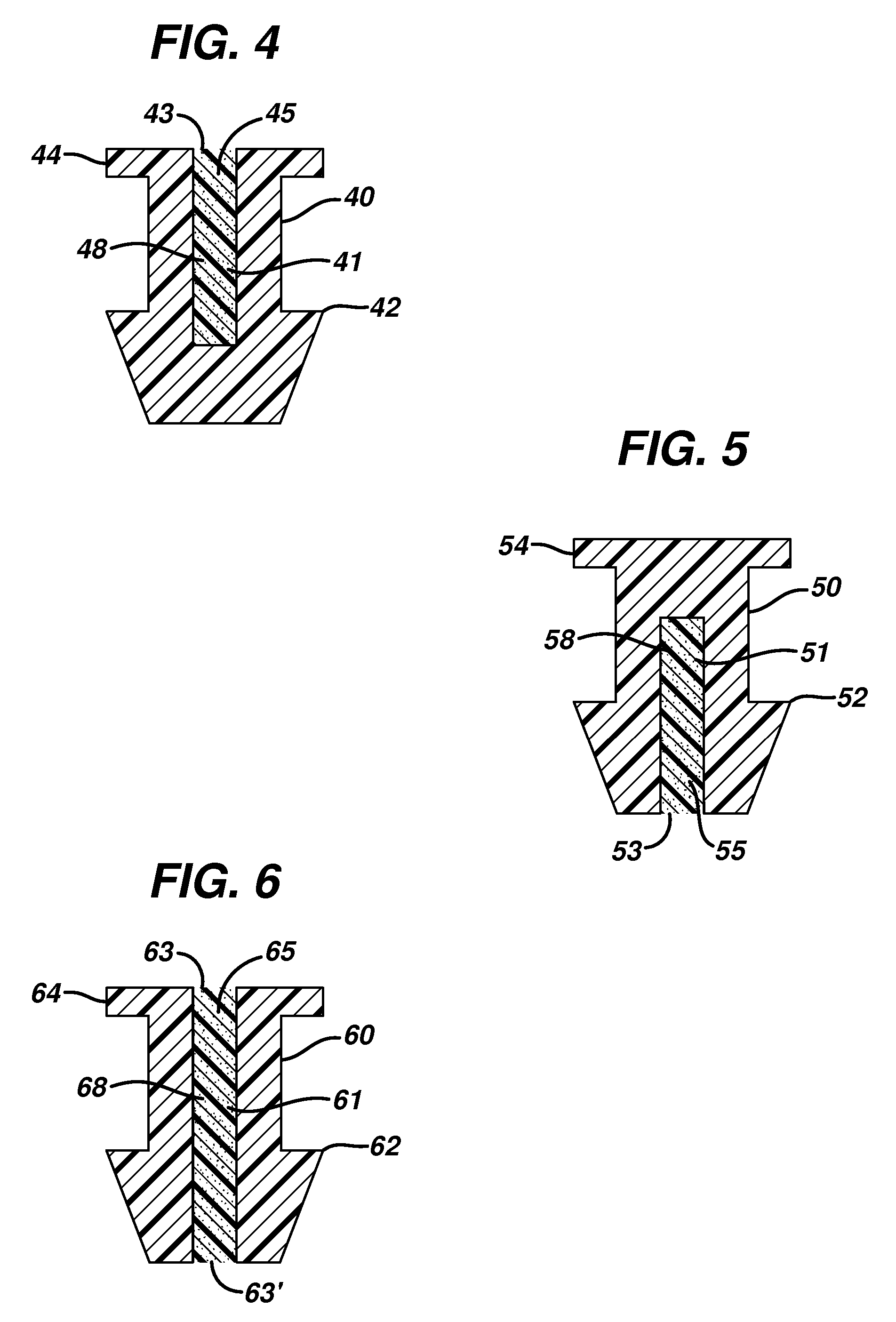
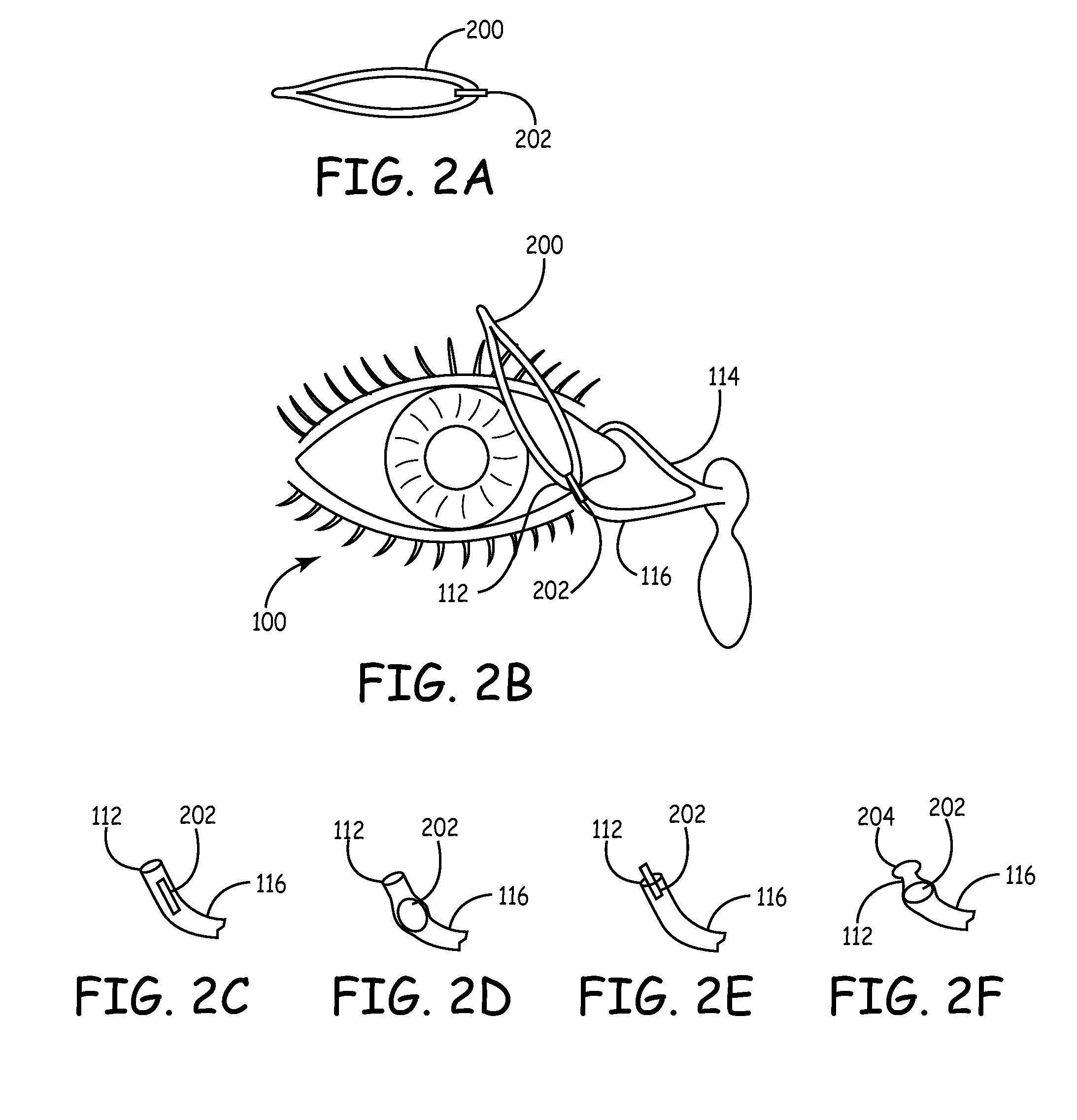
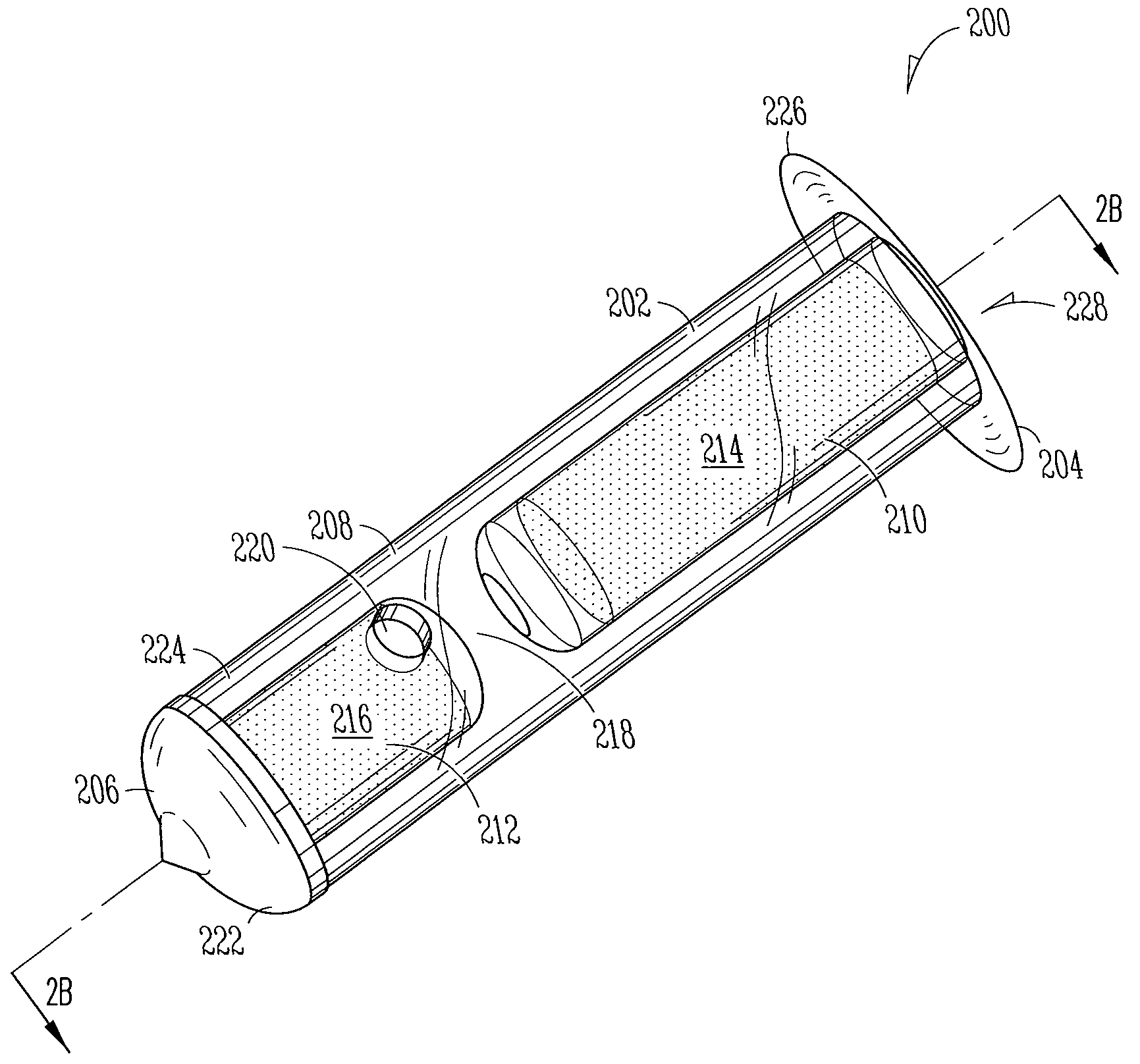
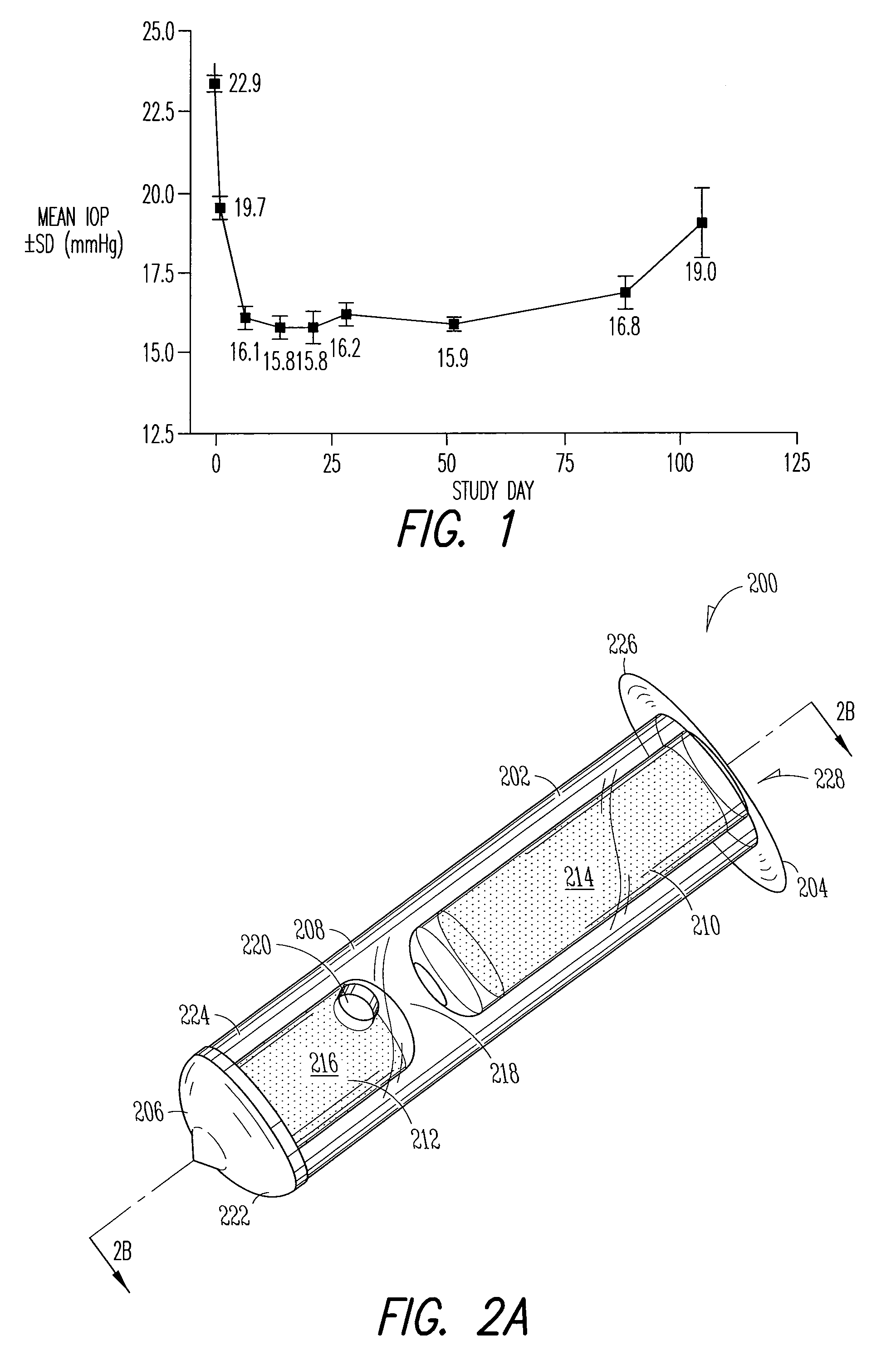
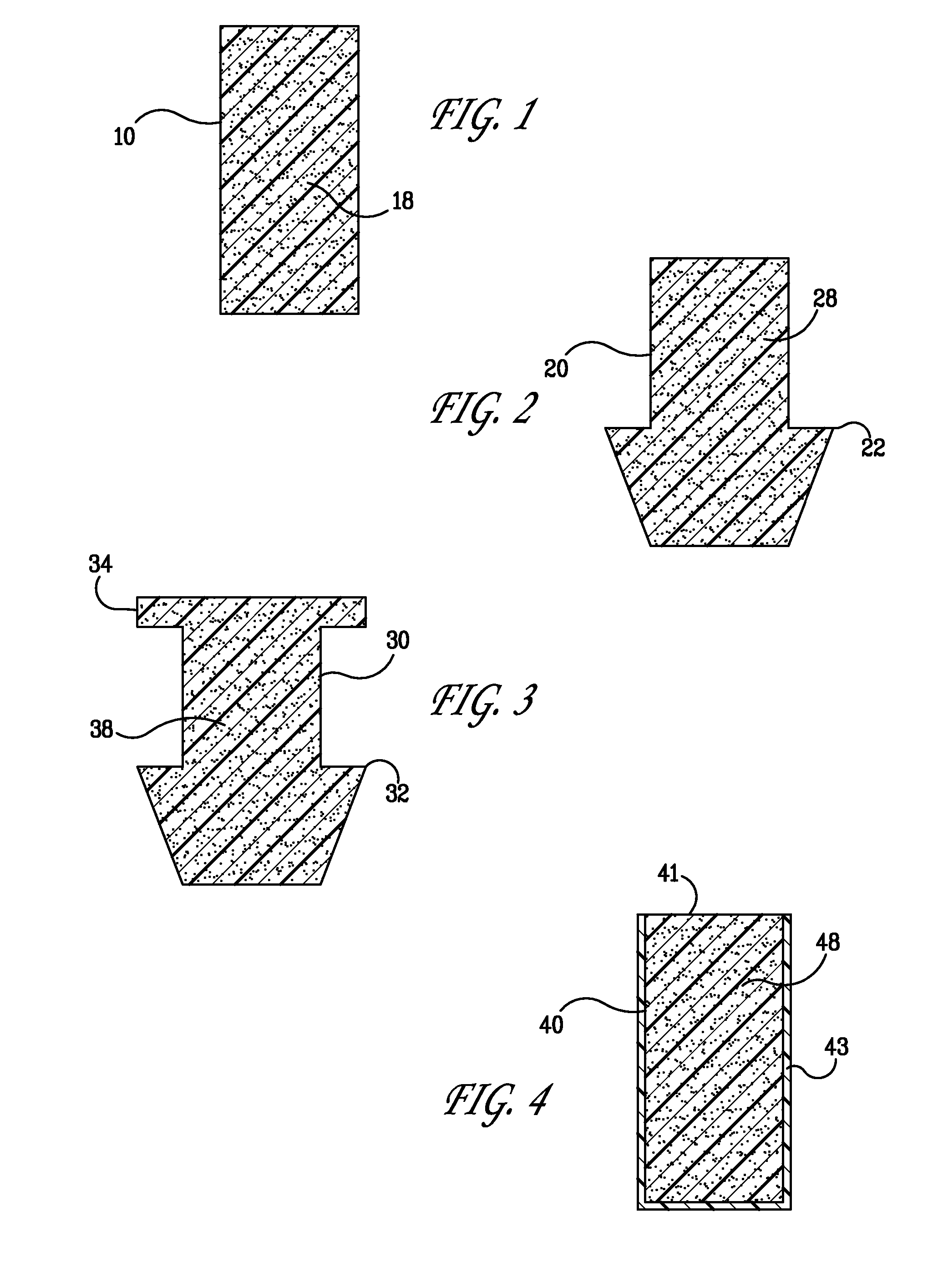
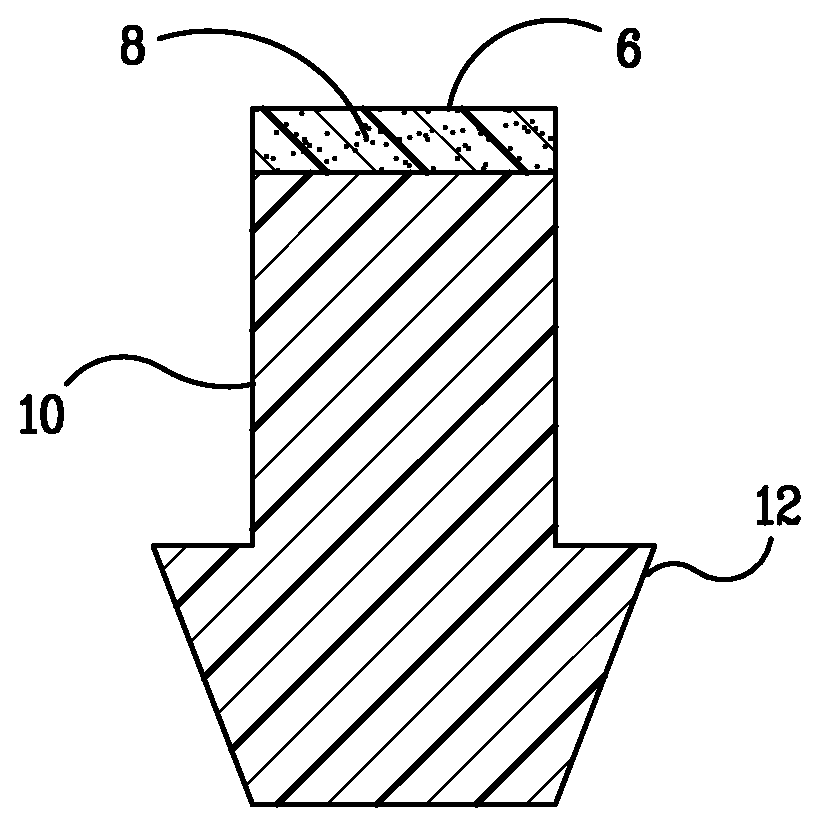
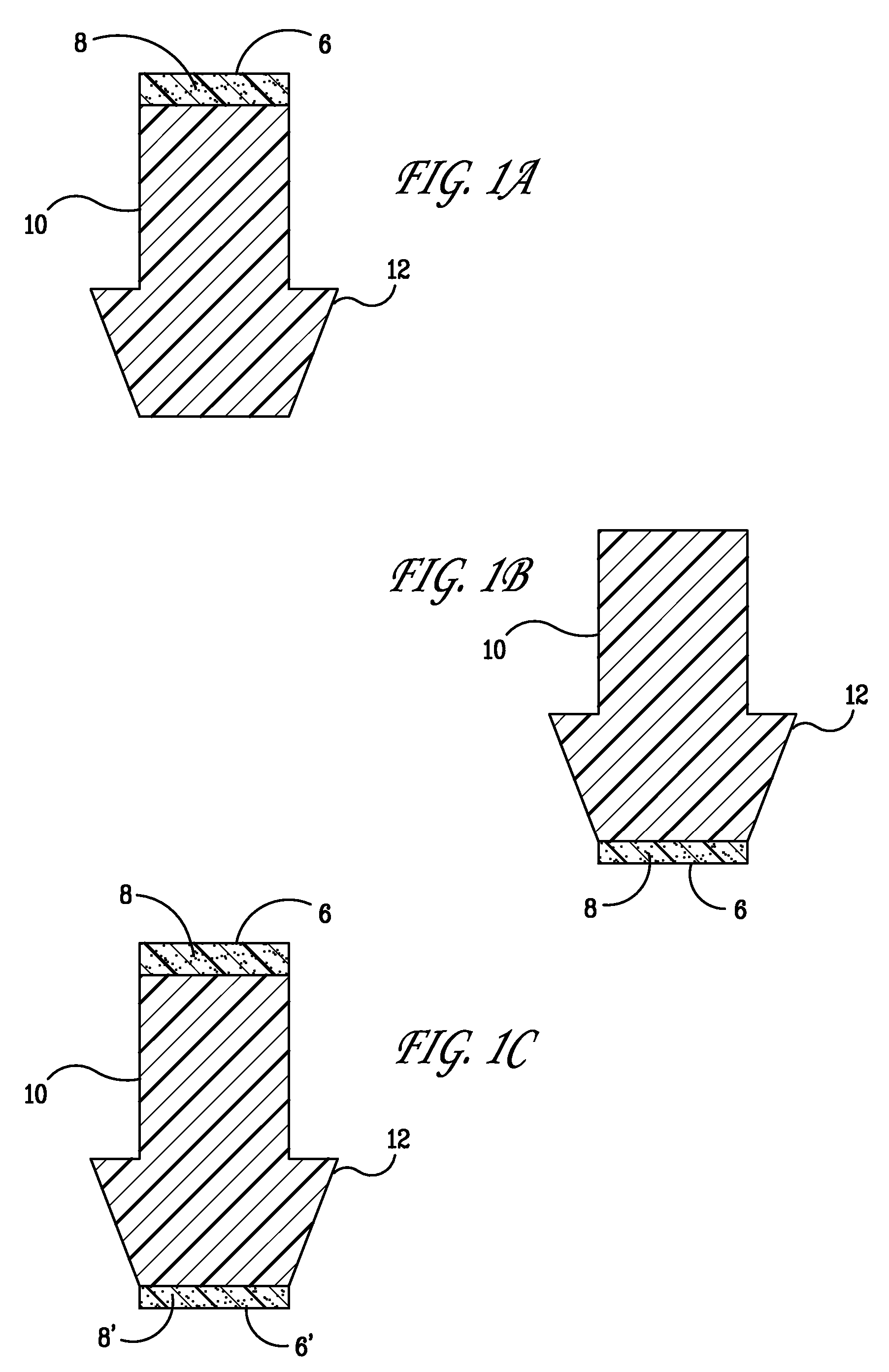
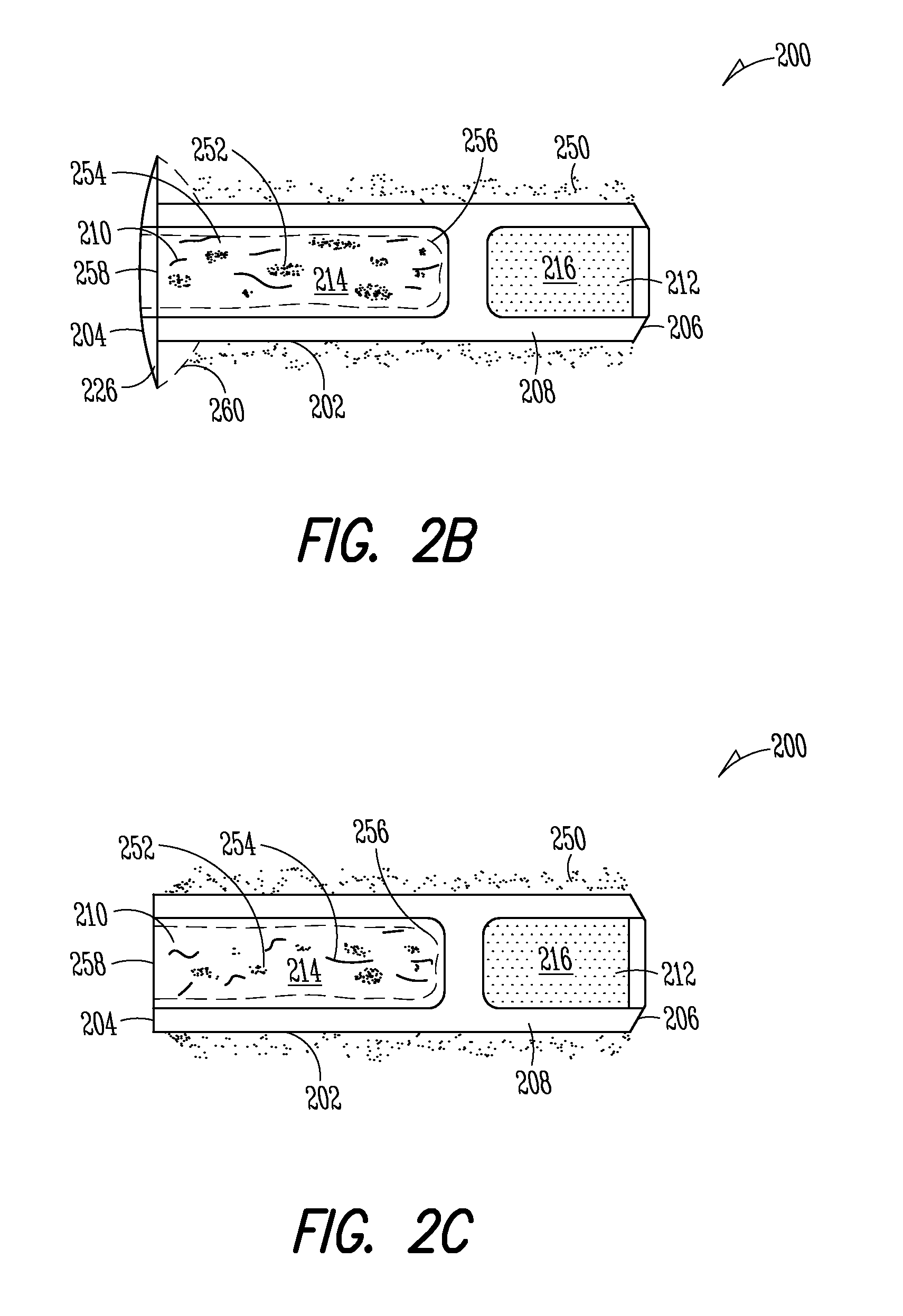
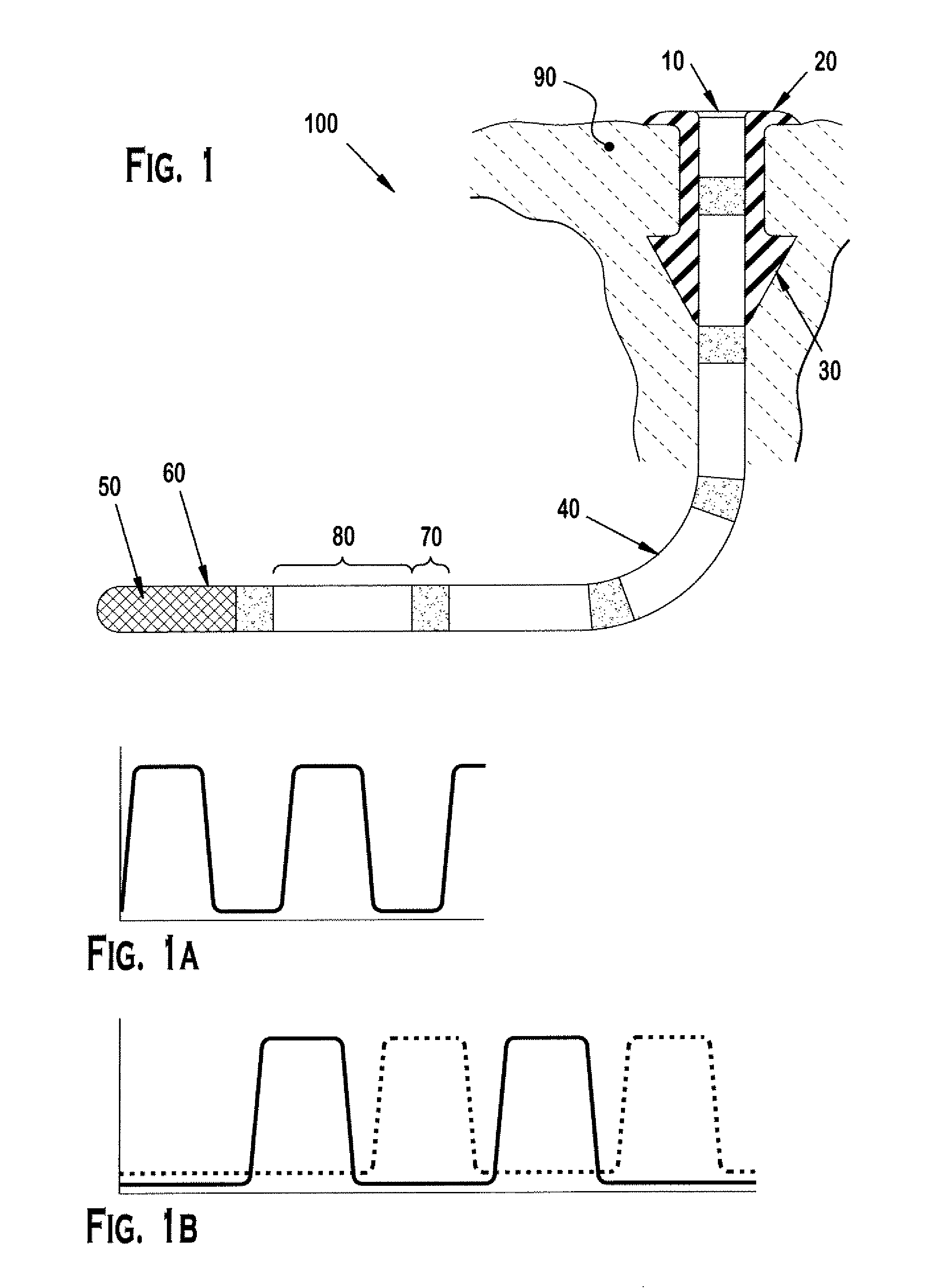
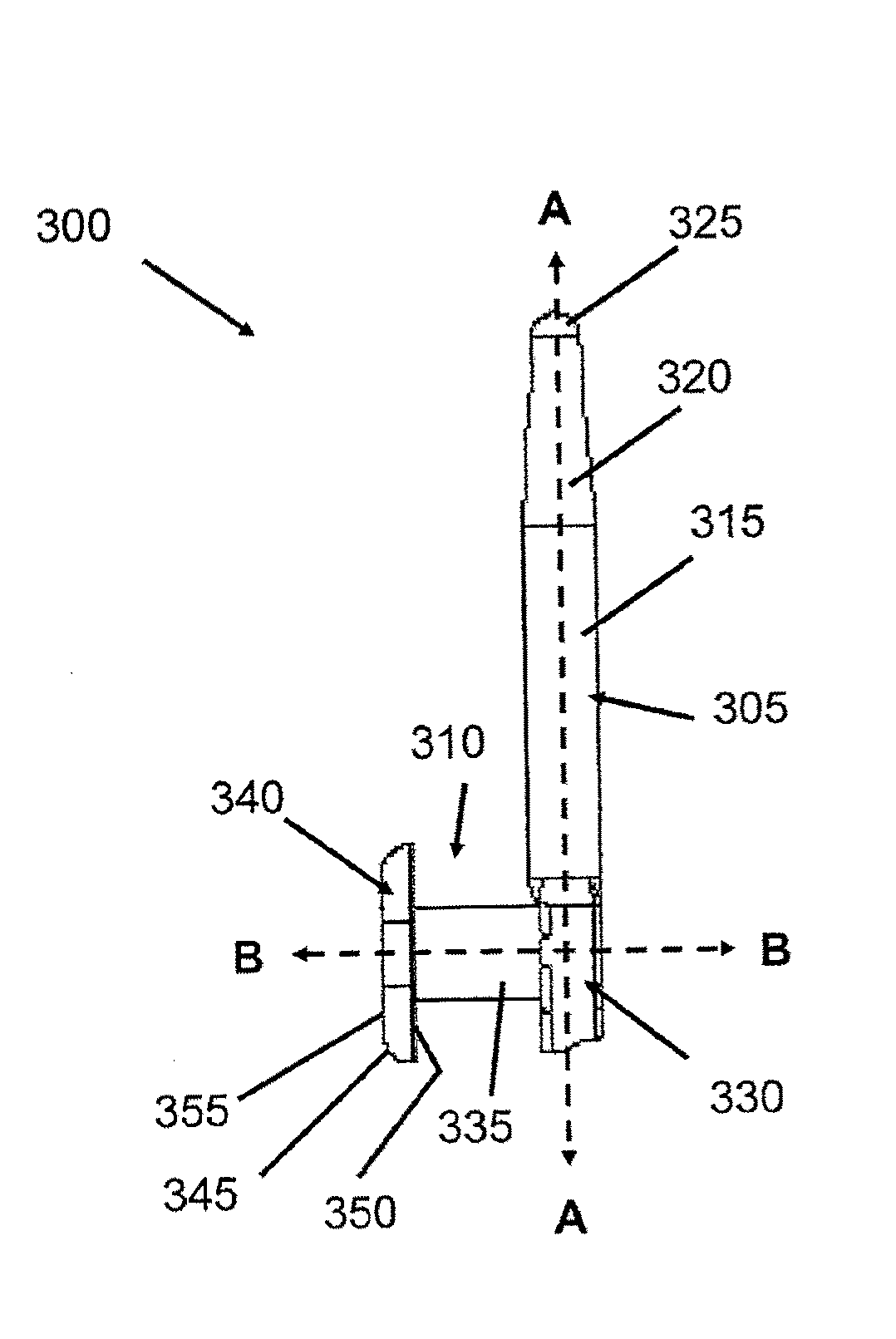
Implant devices, systems and methods for insertion into a punctum of a patient optionally comprises a drug core and a sheath body disposed over the drug core. The drug core includes a therapeutic agent deliverable into the eye, and the sheath defines at least one exposed surface of the drug core. The exposed surface(s) of the drug core may contact a tear or tear film fluid and release the therapeutic agent at therapeutic levels over a sustained period when the implant is implanted for use. The implant may include a retention element to retain the drug core and sheath body near the punctum, optionally comprising a shape memory alloy that can resiliently expand. An occlusive element may be attached to the retention element to at least partially occlude tear flow through the canalicular lumen.
Owner:MATI THERAPEUTICS
Drug Delivery Methods, Structures, and Compositions for Nasolacrimal System
ActiveUS20070269487A1Avoid expulsionInhibition releaseAntibacterial agentsPowder deliveryEffective treatmentBiomedical engineering
Owner:MATI THERAPEUTICS
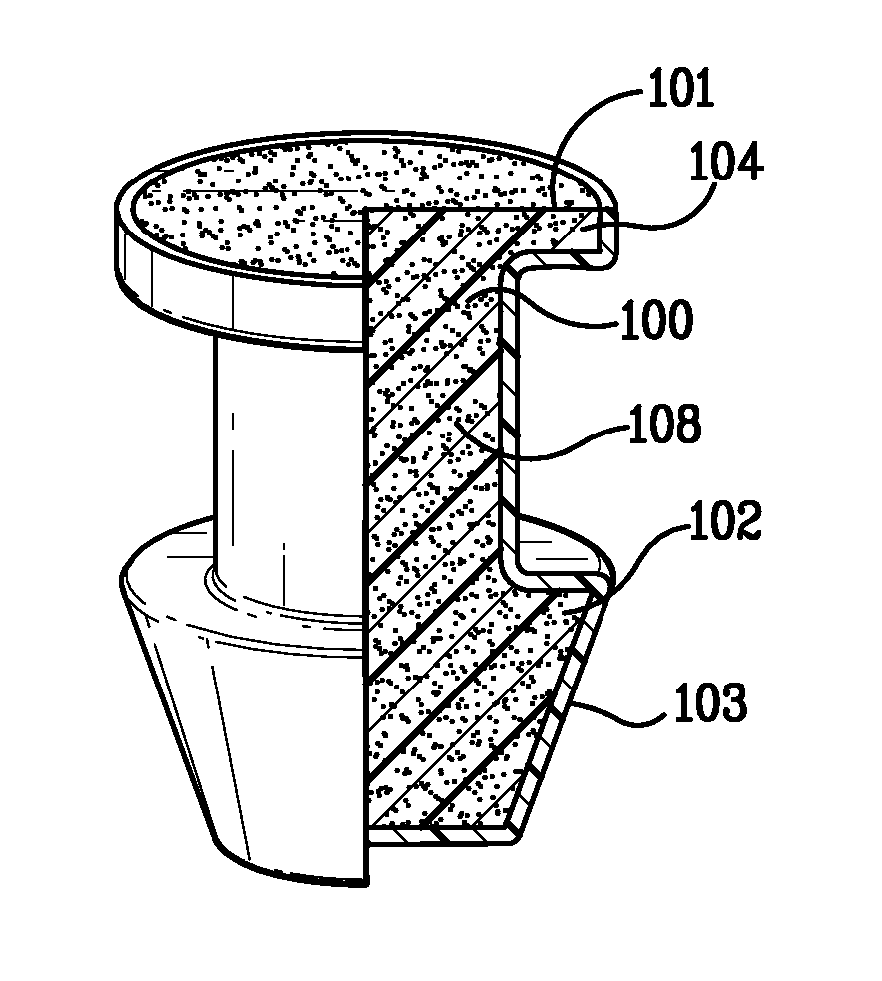
Punctal Plugs and Methods of Delivering Therapeutic Agents
ActiveUS20080181930A1High retention rateIncrease stiffnessBiocideSenses disorderCollagen Punctal PlugsParylene coating
The present invention concerns implantable ocular devices for the sustained release of medication to the eye, and methods for manufacturing and using such devices. In one embodiment, the present invention provides a device comprising: (a) a body comprising a matrix of a prostaglandin and a silicone; (b) a parylene coating on the outer surface of the body; and (c) one or more pores extending from the outer surface of the parylene coating to the outer surface of the body.
Owner:NOVARTIS AG
Drug Delivery Methods, Structures, and Compositions for Nasolacrimal System
InactiveUS20090092654A1Avoid expulsionInhibition releaseAntibacterial agentsBiocideEffective treatmentBioabsorbable polymer
An implant for insertion into a punctum of a patient comprises a body. The body has a distal end, a proximal end, and an axis therebetween. The distal end of the body is insertable distally through the punctum into the canalicular lumen. The body comprises a therapeutic agent included within an agent matrix drug core. Exposure of the agent matrix to the tear fluid effects an effective therapeutic agent release into the tear fluid over a sustained period. The body has a sheath disposed over the agent matrix to inhibit release of the agent away from the proximal end. The body also has an outer surface configured to engage luminal wall tissues so as to inhibit expulsion when disposed therein. In specific embodiments, the agent matrix comprises a non-bioabsorbable polymer, for example silicone in a non-homogenous mixture with the agent.
Owner:MATI THERAPEUTICS
Punctal plugs for the delivery of active agents
The invention provides punctal plugs for the delivery of active agent to one or both of the tear fluid of the eye and to the nasolacrimal duct. The plugs of the invention have a body, a reservoir contained within the body, and optionally a collarette. The reservoir has at least one opening and contains a polymeric material and at least one active agent.
Owner:JOHNSON & JOHNSON VISION CARE INC
Drug delivery through hydrogel plugs
ActiveUS20100209478A1Improve securityIncrease chanceAntibacterial agentsBiocideProsthesisWater content
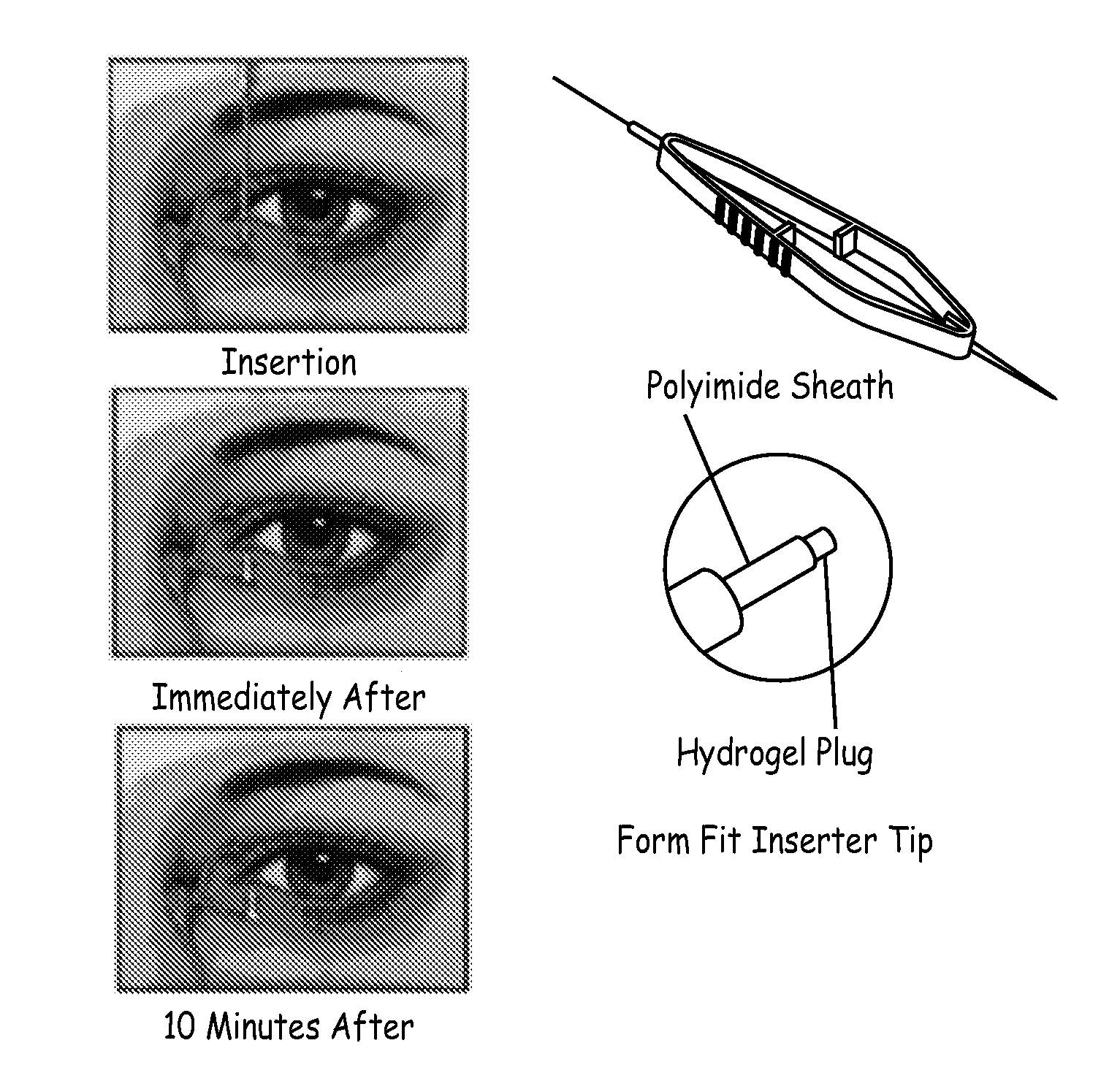
An embodiment is a medical prosthesis for blocking or reducing tear flow through a punctum or canaliculus of a human eye and delivering a drug to the eye that comprises a dehydrated covalently crosslinked synthetic hydrophilic polymer hydrogel with dimensions to pass through a puncta lacrimali, with the dehydrated hydrogel absorbing physiological water to swell to at least 1 mm in cross-sectional width and conformably fit a canaliculus, with the hydrogel comprising a therapeutic agent dispersed through the hydrogel for release to an eye, with the hydrogel having a water content of at least about 50% by weight or volume when allowed to fully hydrate in vitro in physiological saline.
Owner:INCEPT LLC
Sustained release delivery of active agents to treat glaucoma and ocular hypertension
ActiveUS20090280158A1Lower eye pressureReduction in patient noncomplianceBiocideSenses disorderLatanoprostActive agent
The methods described herein provide treatment of glaucoma, ocular hypertension, and elevated intraocular pressure with latanoprost or other therapeutic agent(s). Implant devices for insertion into a punctum of a patient provide sustained release of latanoprost or other therapeutic agent(s) that is maintained for 7, 14, 21, 30, 45, 60, or 90 days or more, thus avoiding patient noncompliance and reducing or lowering adverse events associated with eye drop administration of latanoprost or other therapeutic agent(s) and other therapeutic agent(s).
Owner:MATI THERAPEUTICS
Ocular occluder and method of insertion
InactiveUS20060074370A1Shorten the timeSmall inventoryEye surgeryIntravenous devicesCylindromaOcular occluder
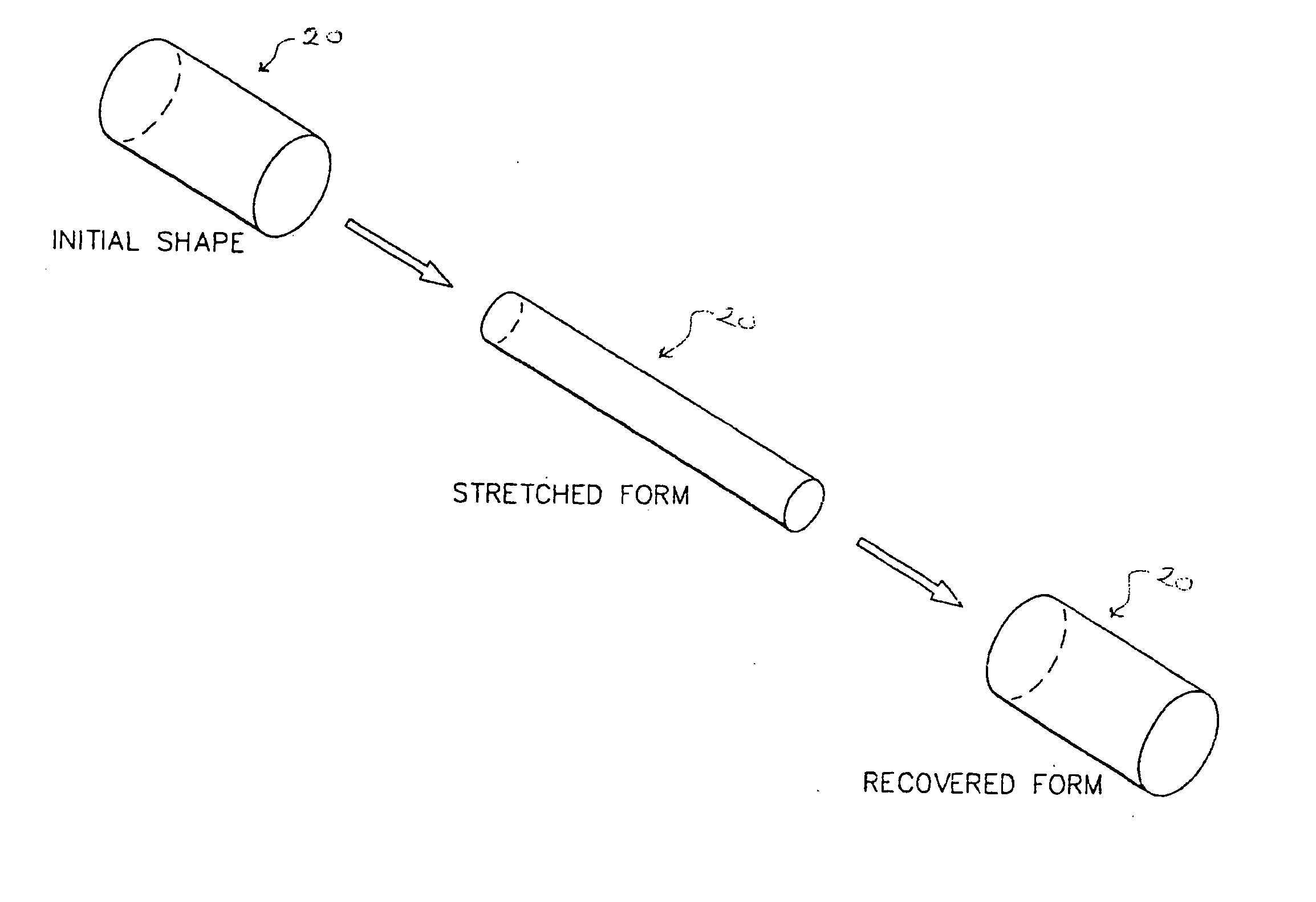
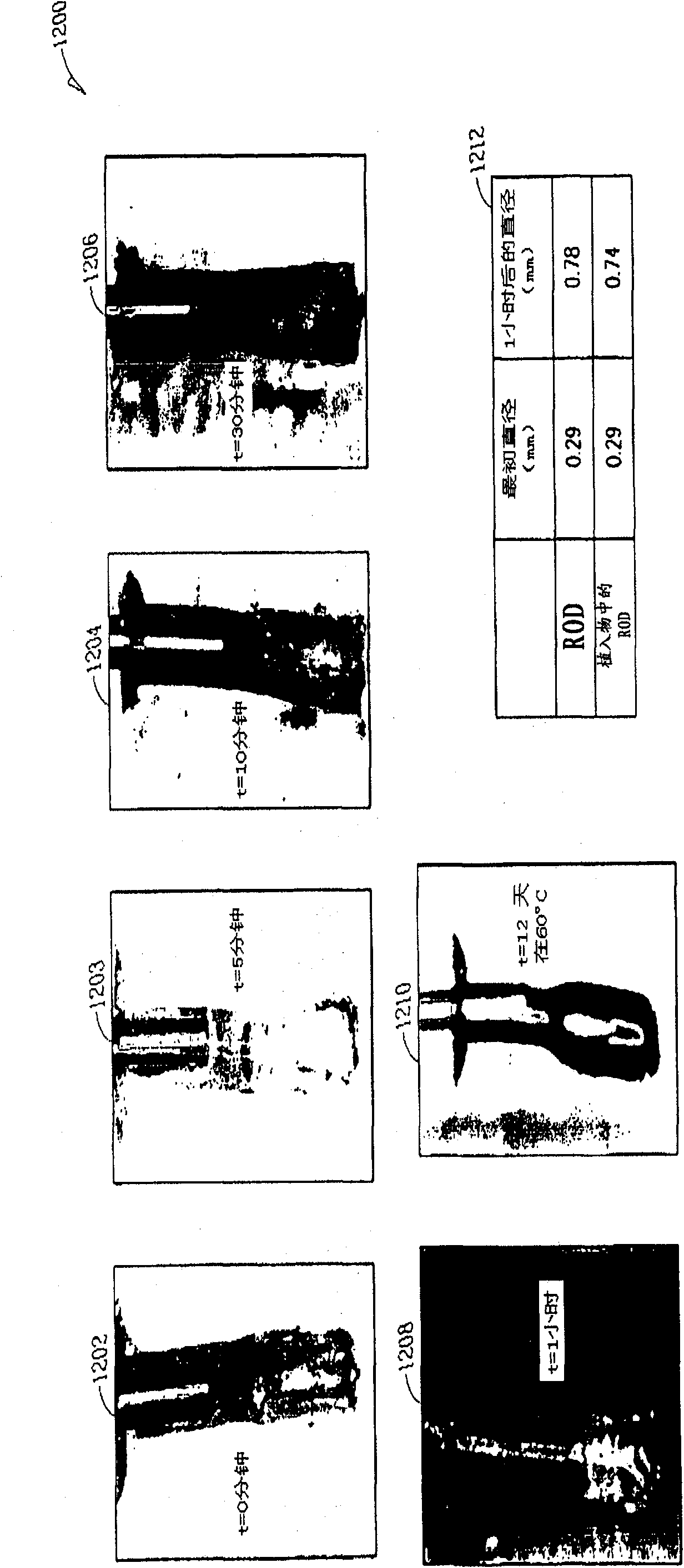
An ocular plug design and method of insertion is described for the treatment of dry eye. This ocular plug is generally a narrow rod-like cylinder of appropriate diameter, which may be tapered at one end, for insertion into an ocular channel, such as the punctum or the canaliculus. The plug is prepared from a hydrophilic polymeric material which forms a hydrogel upon absorption of water, but is rigid in its nonhydrated form. The plug is hydrated, formed into a length and diameter which is appropriate for insertion into an ocular channel (i.e., it is elongated), and dried so as to become frozen in its elongated state prior to insertion into the ocular channel. Once inserted into the ocular channel, the plug absorbs water, thereby becoming a hydrogel which is soft and pliable, and it expands to adapt to the size and shape of the patient's punctum or canaliculus. Once the plug expands to the size of a particular ocular channel, the plug is met with resistance from the surrounding tissue. At that point, expansion of the plug ceases and the plug can effectively block tear drainage through the ocular channel.
Owner:MEDENNIUM
Sustained release delivery of one or more agents
InactiveUS20100209477A1Improve permeabilityReduce the amount requiredBiocideOrganic active ingredientsCross-linkExcipient
The lacrimal implant delivery systems and methods described herein provide for controlled release of a therapeutic agent for the treatment of disease, including the treatment of glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agents. Treatment of disease, including glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agent in conjunction with penetration enhancer, such as benzalkonium chloride, and / or artificial tears is also provided. Also provided are implants containing a drug core emplacable in a punctum adjacent to an eye of a patient for controlled release of a therapeutic agent such as latanoprost for the treatment of glaucoma, the drug core containing a polymer such as cross-linked silicone, a therapeutic agent, and an excipient, wherein the excipient can increase the rate of release of the agent from the drug core, or can increase the drug loading in the core without loss of desirable homogeneity of the agent within the core, or can improve retention of the agent in the eye or in tear fluid, or can increase corneal penetration of the agent into the eye.
Owner:MATI THERAPEUTICS
Punctal plugs for the delivery of active agents
The invention provides punctal plugs for the delivery of active agents. The plugs have a body throughout which at least one active agent is dispersed or that is coated with a polymeric material containing at least one active agent.
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs for the delivery of active agents
The invention provides punctal plugs for the delivery of active agent to one or both of the tear fluid of the eye and to the nasolacrimal duct that comprise a body, at least one cap, and optionally a collarette.
Owner:JOHNSON & JOHNSON VISION CARE INC
Drug delivery through hydrogel plugs
An embodiment is a medical prosthesis for blocking or reducing tear flow through a punctum or canaliculus of a human eye and delivering a drug to the eye that comprises a dehydrated covalently crosslinked synthetic hydrophilic polymer hydrogel with dimensions to pass through a puncta lacrimali, with the dehydrated hydrogel absorbing physiological water to swell to at least 1 mm in cross-sectional width and conformably fit a canaliculus, with the hydrogel comprising a therapeutic agent dispersed through the hydrogel for release to an eye, with the hydrogel having a water content of at least about 50% by weight or volume when allowed to fully hydrate in vitro in physiological saline.
Owner:INCEPT LLC
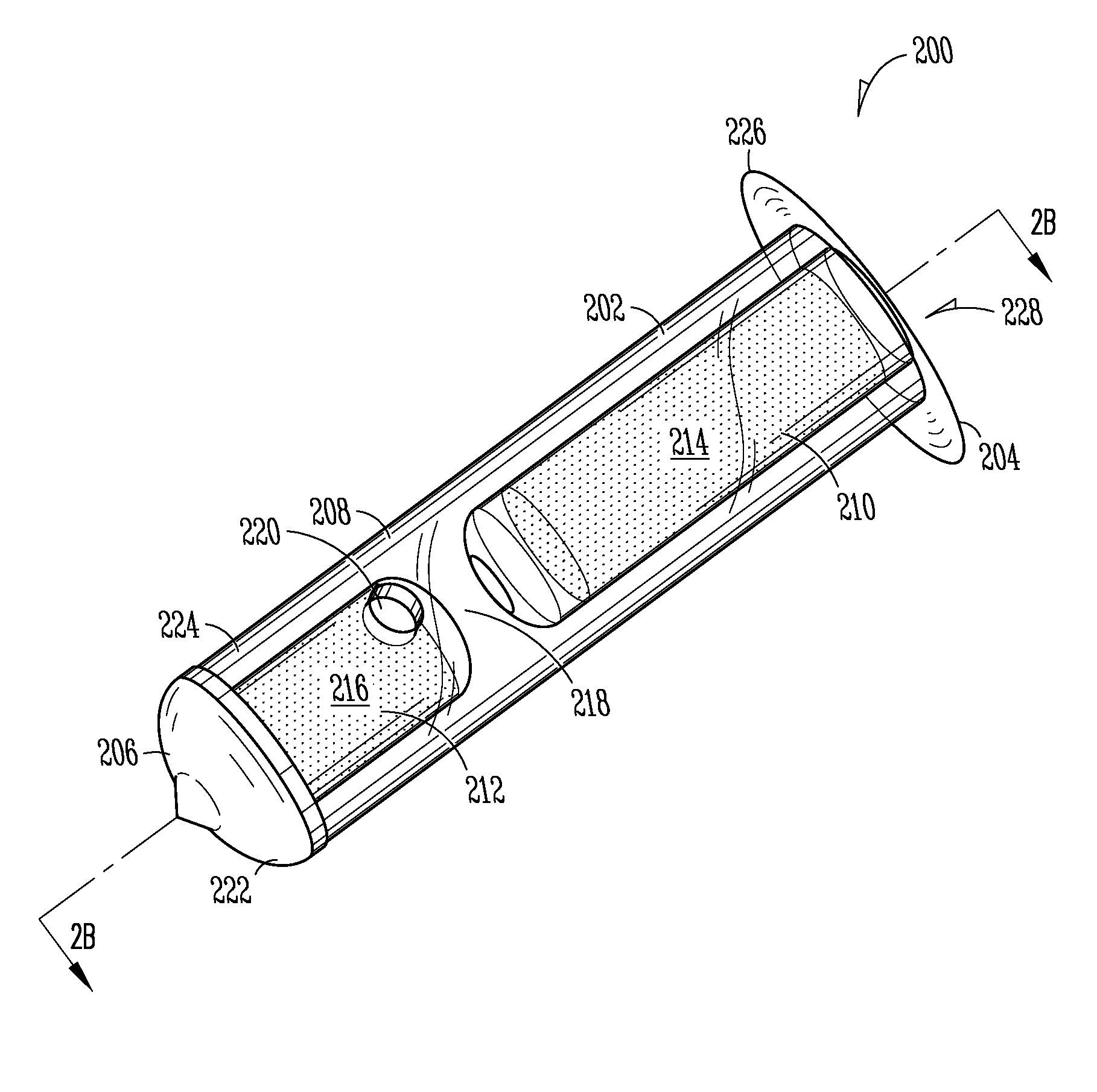
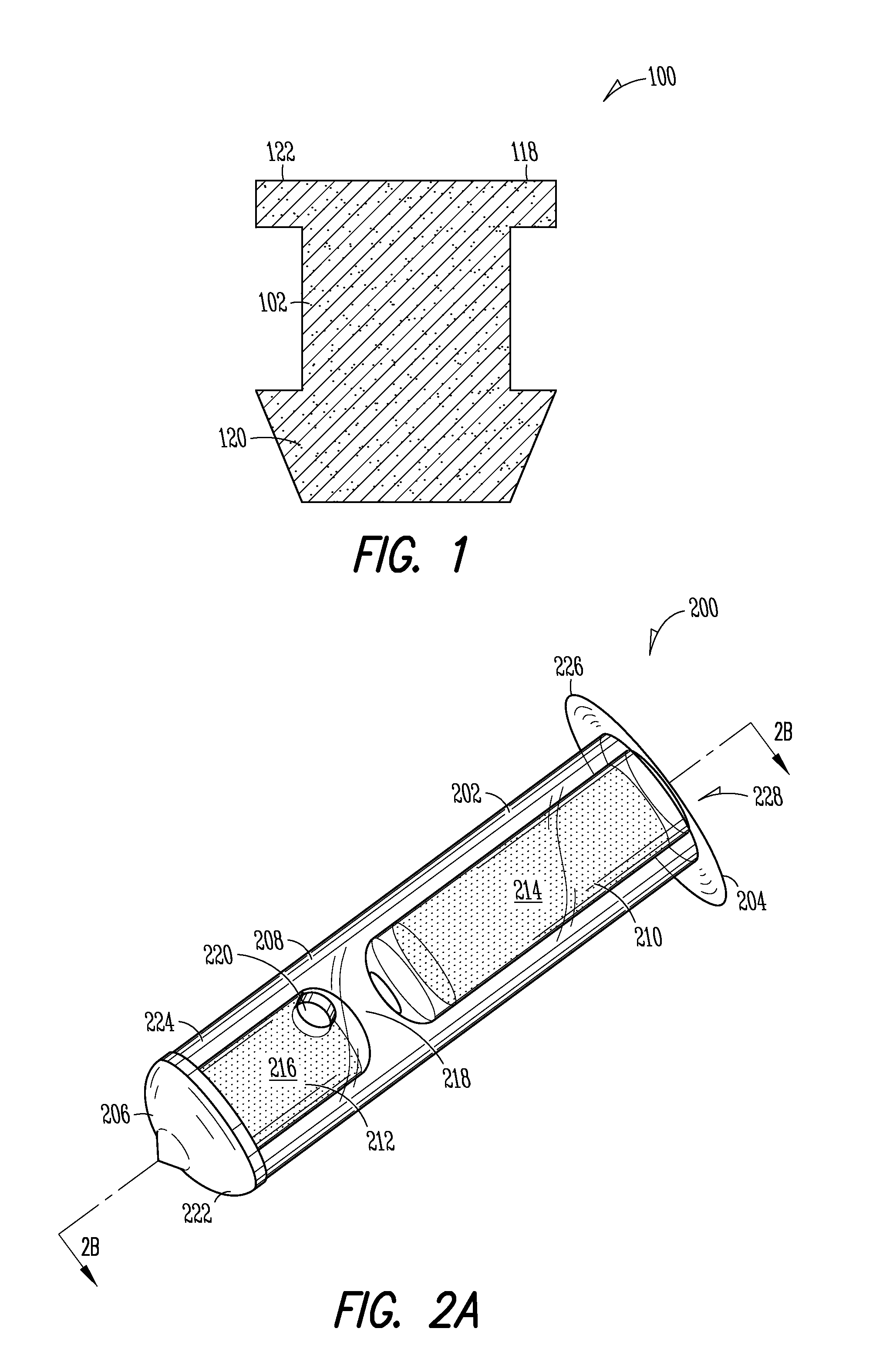
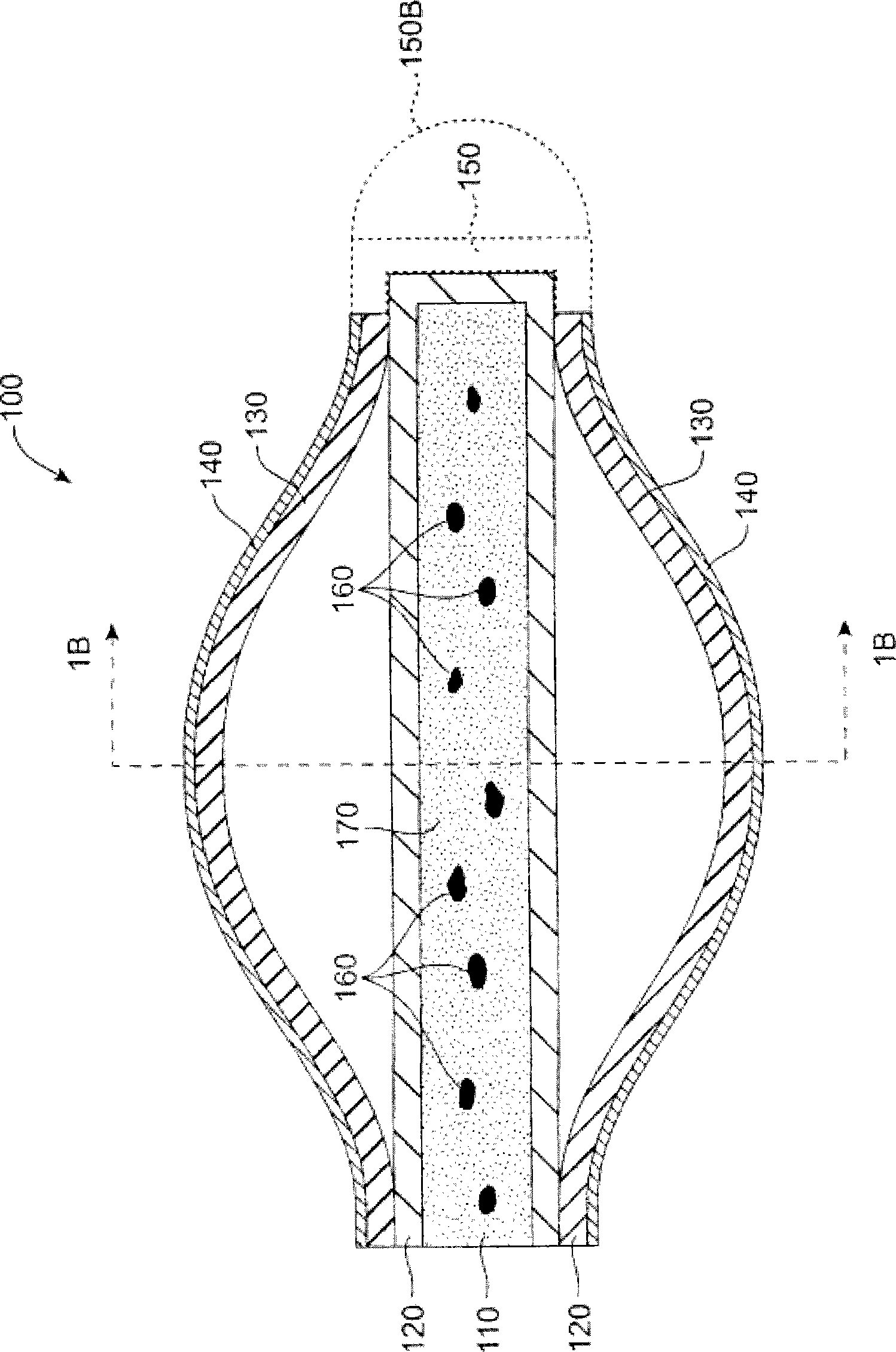
More easily visualized punctum plug configurations
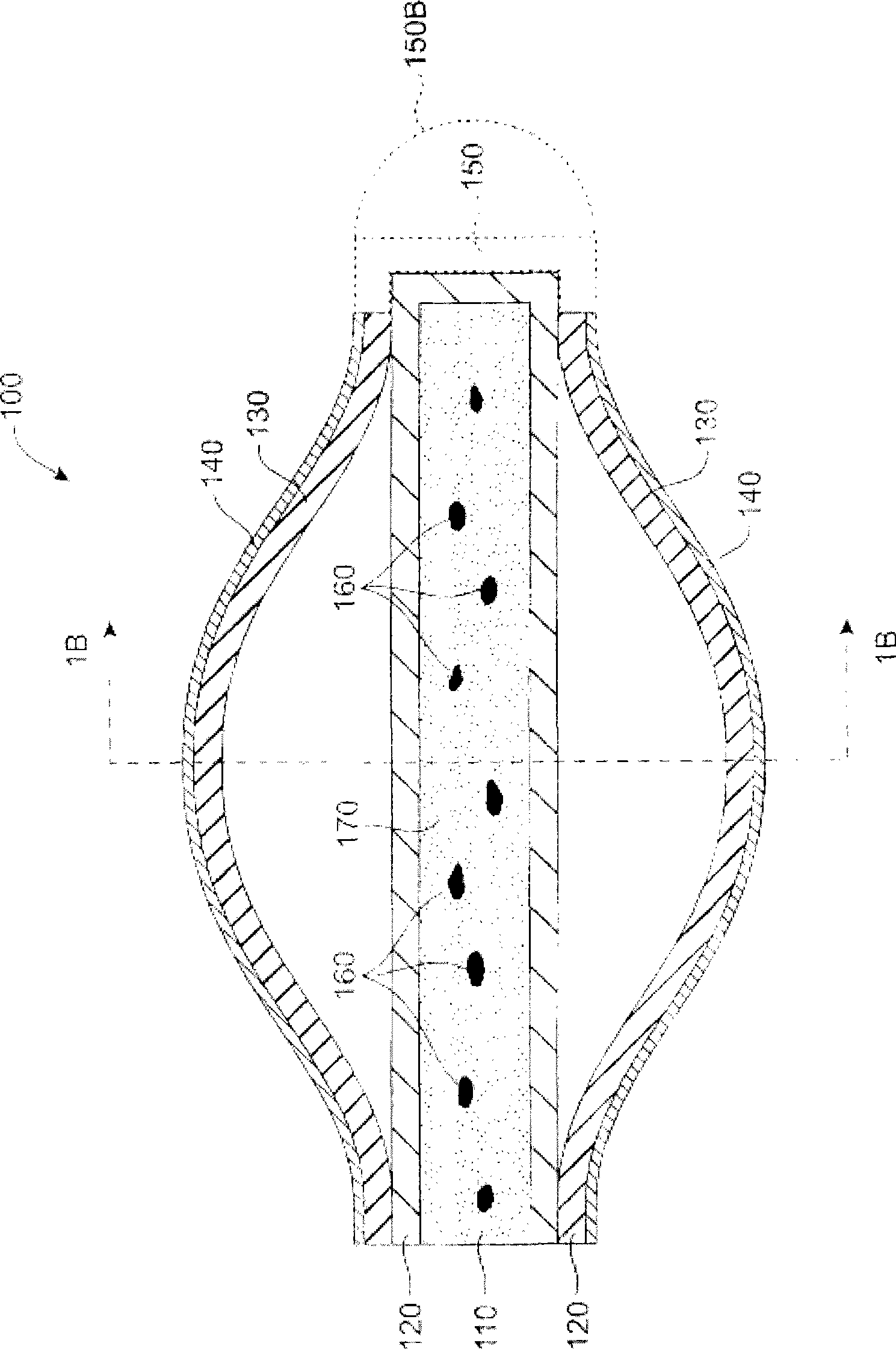
An improved punctum plug is more easily visualized when positioned within a punctual canal of a recipient. The body of the plug features an outwardly exposed surface when properly positioned, and a substance causing at least the outwardly exposed surface to contrast with surrounding tissue, such that the use of the substance causes the plug to be more easily visualized than if the substance were not present. The substance, which may be disposed on the outwardly exposed surface or within the body of the plug, may include a saturated coloration, or may be phosphorescent, fluorescent or otherwise operative to reflect or re-radiate light to assist in visualization. For example, the substance may include an organic or inorganic phosphor or fluorescent material, reflective beads, quantum dots, a dye or pigment. Such reflection or re-radiation may occur at the same or different wavelength(s) compared to the illumination wavelength(s), whether or not either or both are within the visible part of the spectrum. If outside the visible region, a detector may be employed according to the invention for detecting the radiated light. A system for determining whether or not a punctum plug is positioned within the punctal canal of a person's eye is also enclosed, including at least one optical element permitting the eye to view itself, to be viewed by the other eye, or by a second person.
Owner:DIGGER MONTANA
Punctum plugs having fluid collecting recesses and methods of punctal occlusion
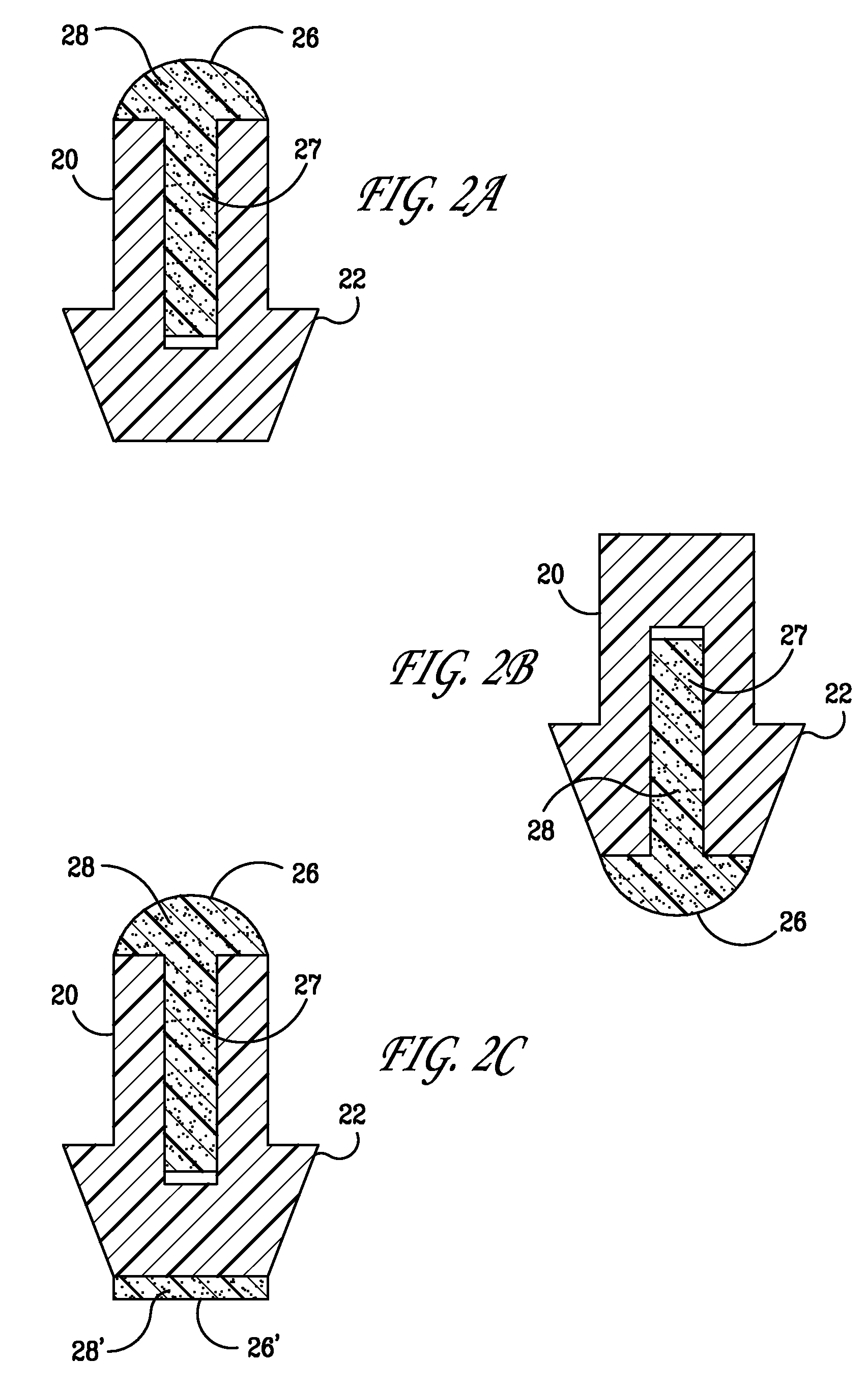
ActiveUS6994684B2Safely implantedSafe removalEar treatmentEye surgeryEngineeringBiomedical engineering
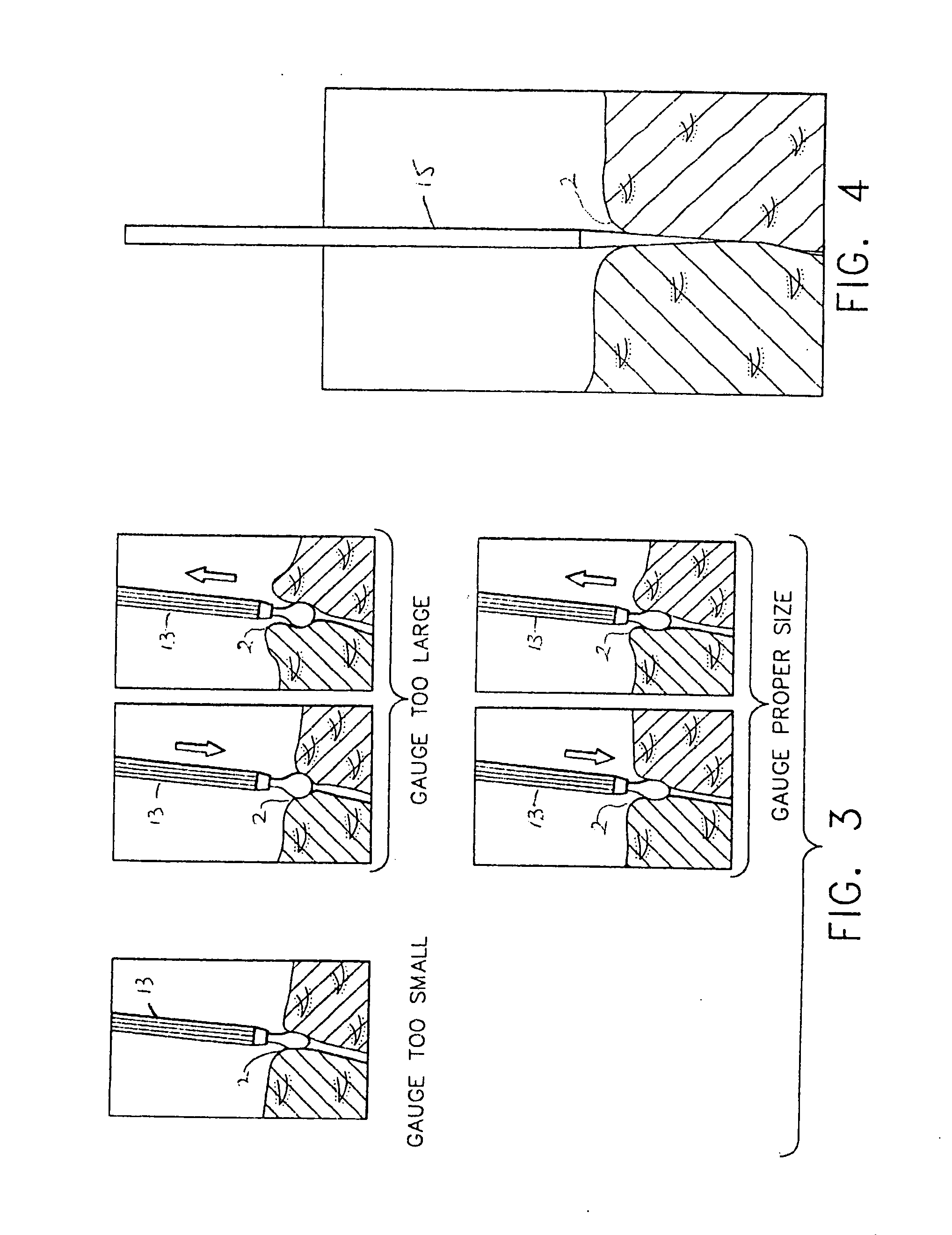
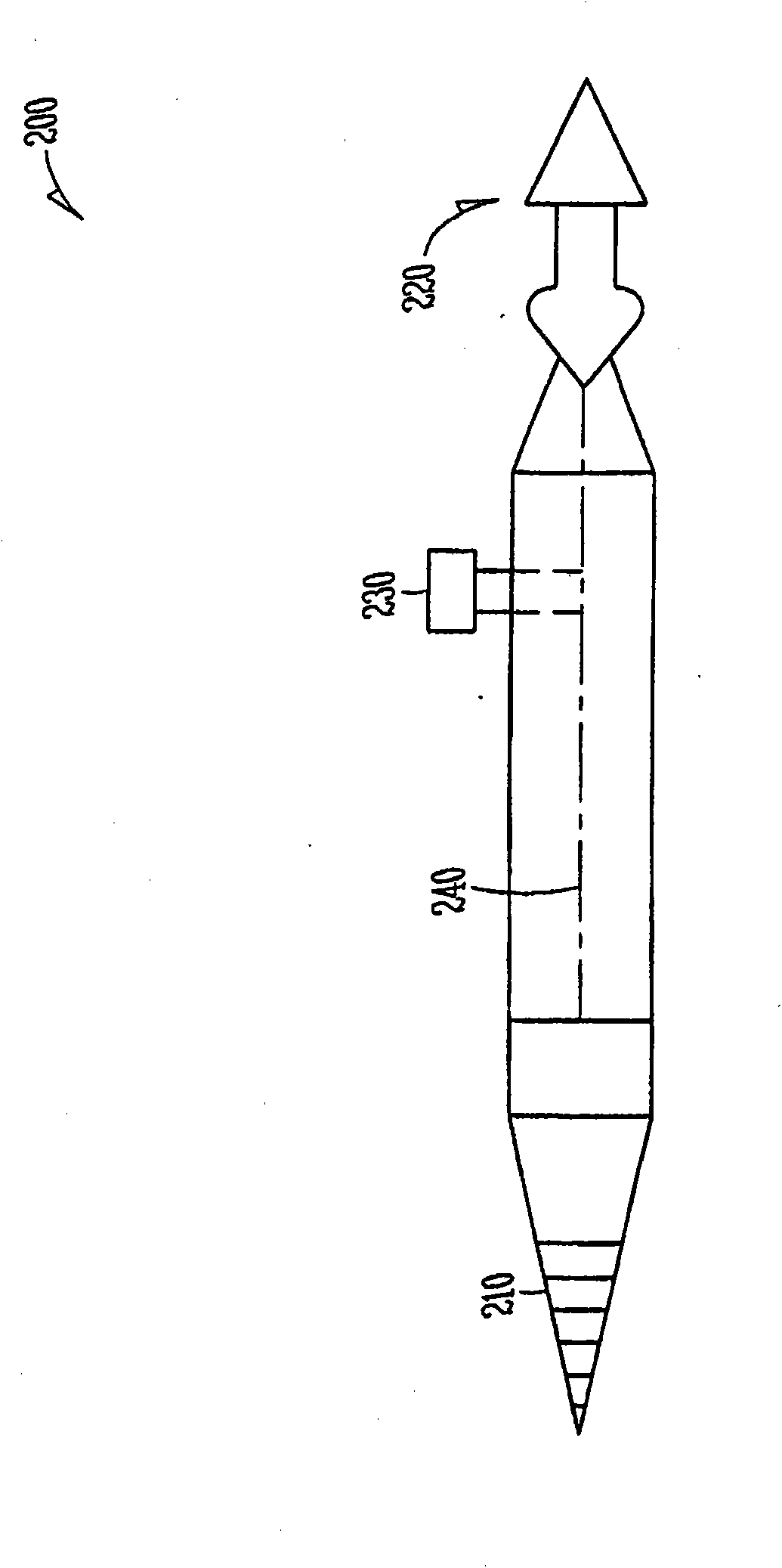
A punctum plug includes a distal tip, a proximal cap and a body connecting the tip to the cap. The cap has a proximal surface which remains exposed in the eye upon implantation of the punctum plug. A passage for an insertion tool extends distally in the punctum plug from an opening along the proximal surface. One or more recesses in the proximal surface collect tear fluid by surface tension, and each recess is laterally offset in its entirety from the opening and does not overlap or align with the opening. The number of recesses is indicative of the size of the punctum plug. A method of punctal occlusion involves collecting tear fluid in the one or more recesses and adhering the tear fluid in the one or more recesses by surface tension.
Owner:MISTEN VISION CORP
Combination treatment of glaucoma
InactiveUS20090318549A1Lower eye pressureOrganic active ingredientsBiocideLatanoprostSustained Release Formulations
The methods described herein provide reduction of intraocular pressure by administering a sustained release formulation including latanoprost and a pharmaceutically acceptable vehicle and administering an eye drop adjunctive composition to the eye of a patient. The sustained release formulation can release latanoprost continuously for at least 90 days from a punctum plug delivery system. The eye drop adjunctive composition can also include latanoprost.
Owner:MATI THERAPEUTICS
Sustained release delivery of active agents to treat glaucoma and ocular hypertension
InactiveUS20130053794A1Improved patient comfortImproved implant retentionOrganic active ingredientsSenses disorderActive agentOcular hypertension
A method of decreasing intraocular pressure (IOP) in an eye of a patient in need thereof includes implanting a first lacrimal implant through a firsts punctum and into a first lacrimal canaliculus of the eye of the patient. The method may further comprise implanting a second lacrimal implant through a second punctum and into a second lacrimal canaliculus of the eye of the patient, and releasing, on a sustained basis a therapeutically effective amount of an intraocular pressure-reducing therapeutic agent.
Owner:MATI THERAPEUTICS
Dextran-based polymer tissue adhesive for medical use
A tissue adhesive formed by reacting an aminodextran containing primary amine groups with an oxidized dextran containing aldehyde groups is described. The dextran-based polymer tissue adhesive is particularly useful in medical applications where low swell and slow degradation are needed, for example sealing the dura, ophthalmic procedures, tissue repair, antiadhesive applications, drug delivery, and as a plug to seal a fistula or the punctum.
Owner:ACTAMAX SURGICAL MATERIALS
Punctal plug containing drug formulation
ActiveUS20120059338A1Easy insertion stabilityEasy long-term stabilityEye surgeryMedical devicesControlled releaseEyelid
Disclosed are lacrimal inserts and their method of use for delivery of medication to the eye. The plug includes a body portion sized to pass through a lacrimal punctum and be positioned within a lacrimal canaliculus of the eyelid. The plug may contain a core, or reservoir, at least partially within the body portion comprising a therapeutic agent that is configured to controlled release into the eye and is configured for release medication via a designated port, valve, or orifice in the insert housing and inhibits diffusion of medication via the housing itself.
Owner:JOHNSON & JOHNSON VISION CARE INC
Ophthalmic insert
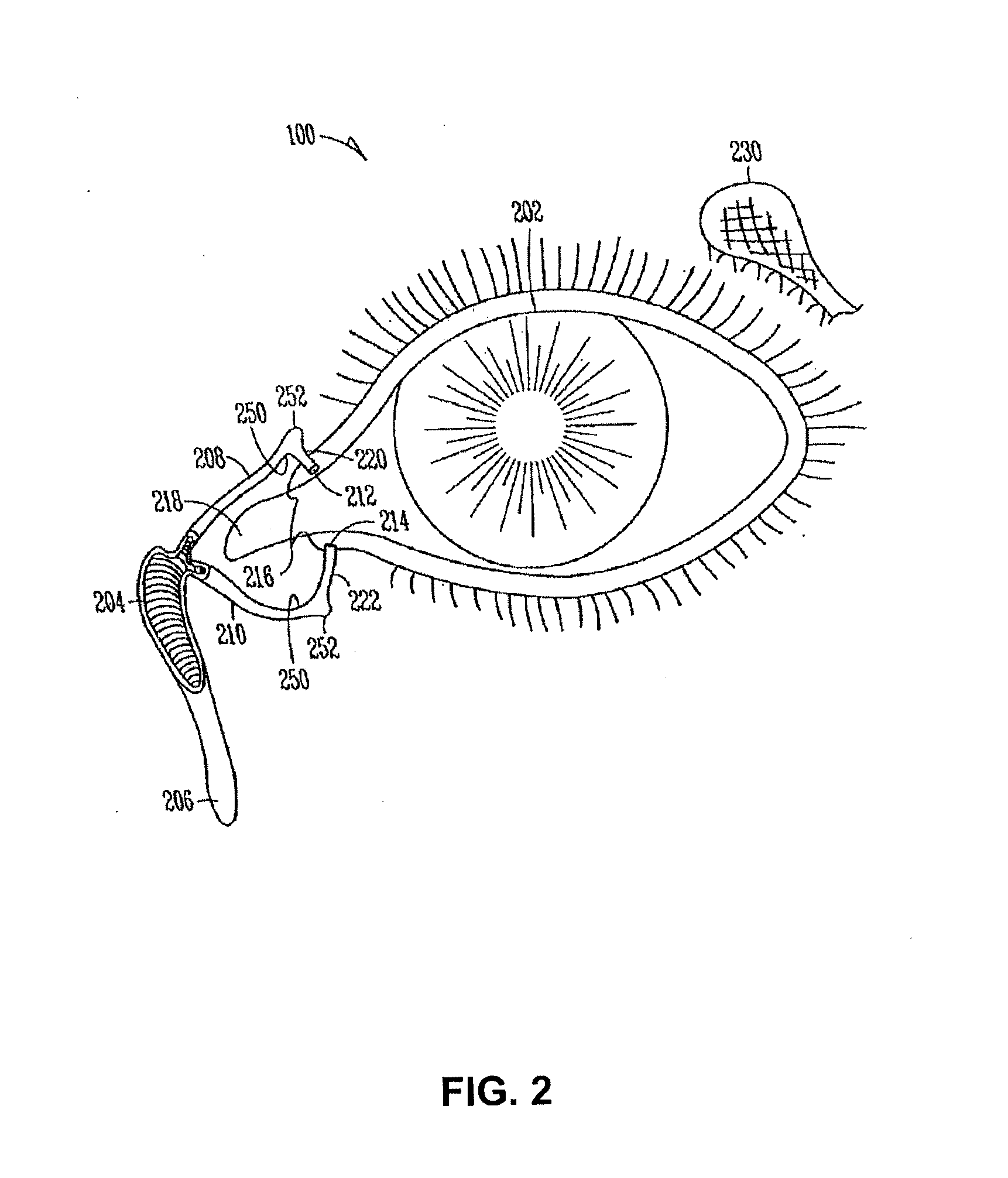
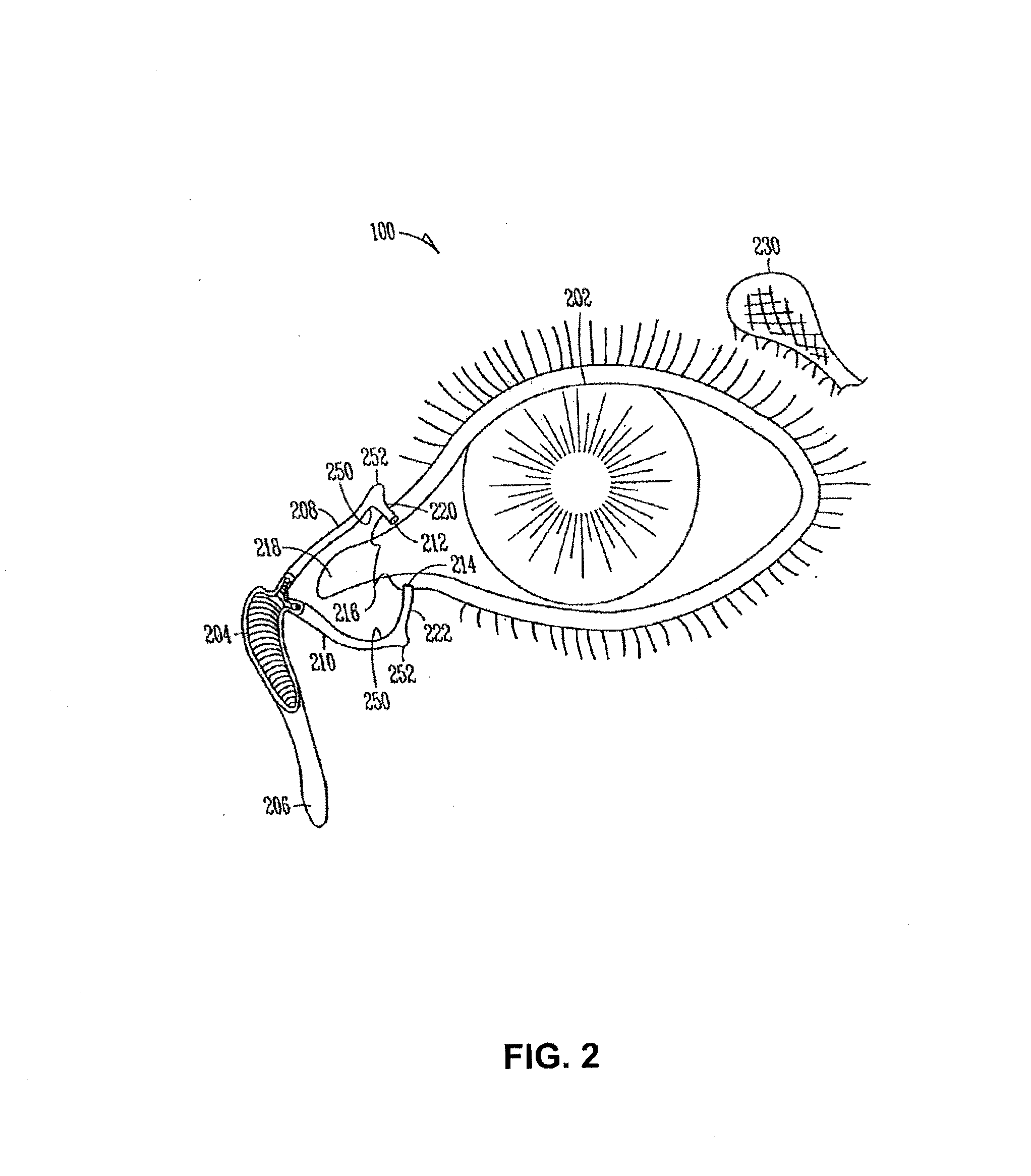
A medical device for delivering medication to an eye includes a stent having a proximal end and a distal end, the proximal end includes a collarette configured to be secured against a punctum of the nasal lacrimal system, and the distal end includes an expandable pouch for storing the medication to be delivered to the eye. The stent has a length substantially equivalent to a length of a canaliculus that is joined to the punctum such that, when implanted, the expandable pouch of the medical device is disposed in the nasal lacrimal sac of the patient and the medication is thereafter released through an opening in the collarette. A mechanical pumping mechanism in the expandable pouch and / or a membrane covering the opening in the collarette may be provided to better control release of the fluid. The stent may also include anchoring pegs disposed on an exterior surface thereof that, when the device is implanted, contact an interior surface of the canaliculus. An inserter tool is also described.
Owner:FREILICH DAVID
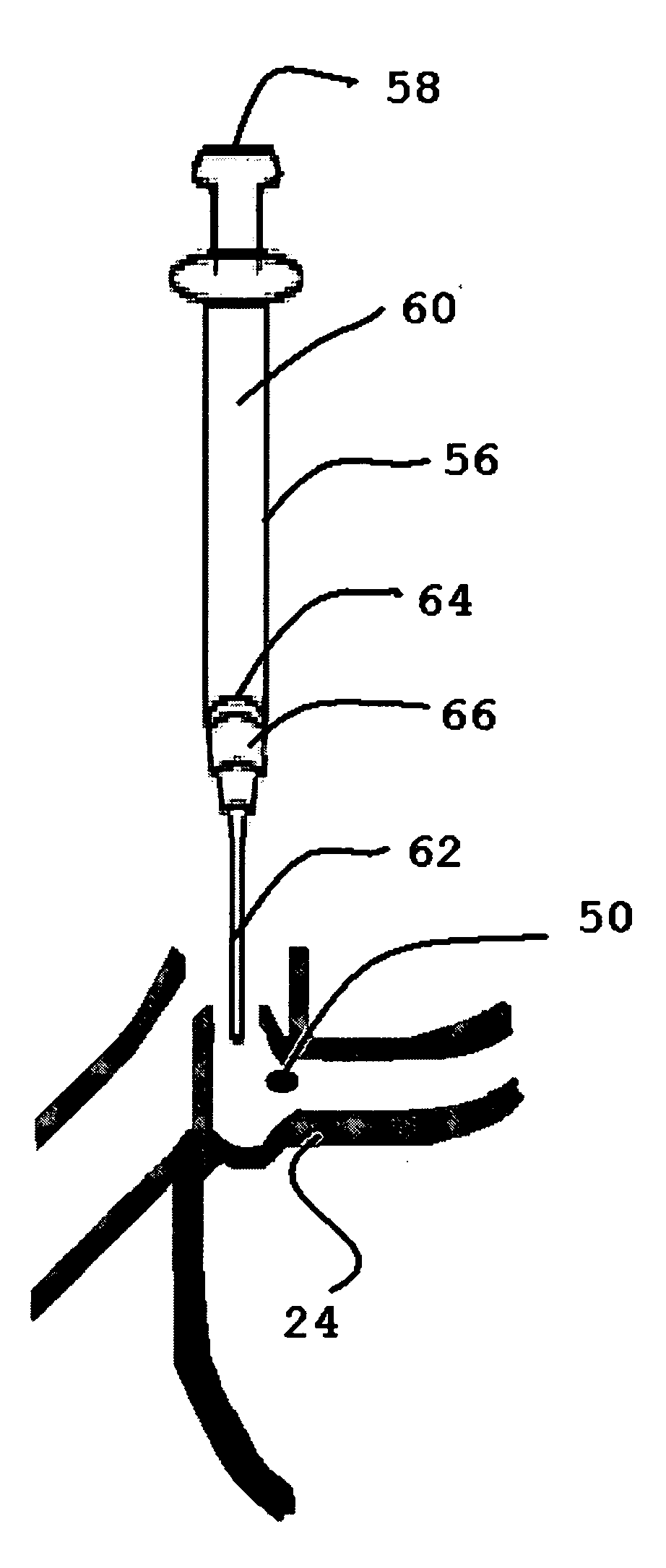
Implantation instruments, system, and kit for punctal implants
Described and illustrated are various insertion instruments, cap, plug, method and kit. In one aspect, a cap for a punctal insertion instrument is shown and described. In a further aspect, a punctum plug insertion system that includes the instrument and the cap is provided. In yet a further aspect, a method of releasing a punctum plug from a plug holder is provided. Additionally, a kit that contains the plug insertion instrument, cap, plugs, and instructions for use is provided.
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal Plugs
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs for controlled release of therapeutic agents
Disclosed are lacrimal inserts and their method of use for delivery of medication to the eye. The plug includes a body portion sized to pass through a lacrimal punctum and be positioned within a lacrimal canaliculus of the eyelid. The plug may contain a core, or reservoir, at least partially within the body portion comprising a therapeutic agent that is configured for controlled release into the eye by means of an osmotic engine.
Owner:JOHNSON & JOHNSON VISION CARE INC
Insertion and extraction tools for lacrimal implants
Owner:QLT INC
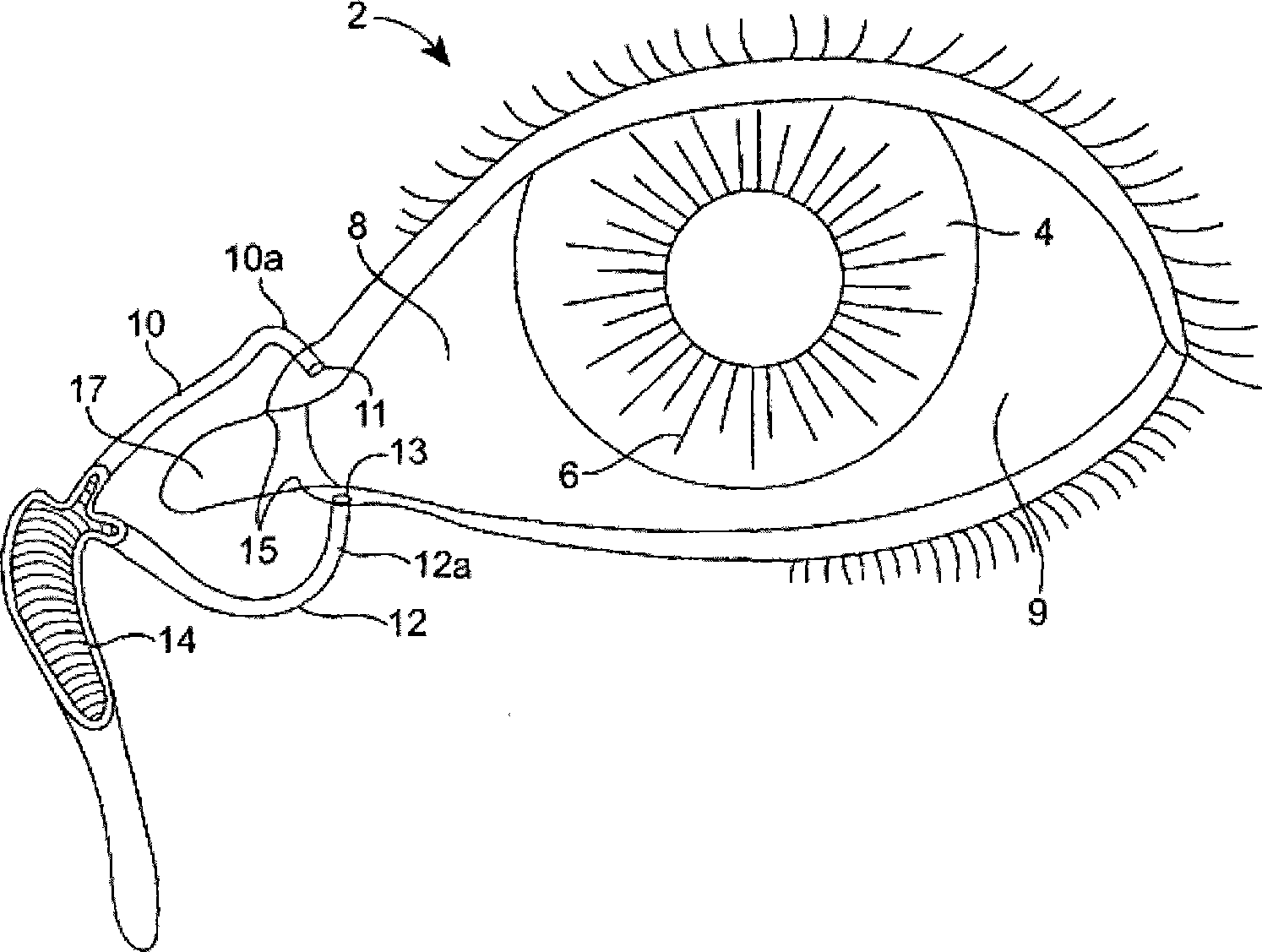
Nasolacrimal drainage system implants for drug therapy
ActiveCN101505681AReduce deliveryOrganic active ingredientsEye surgeryImplanted deviceShape-memory alloy
Implant devices, systems and methods for insertion into a punctum of a patient optionally comprises a drug core and a sheath body disposed over the drug core. The drug core includes a therapeutic agent deliverable into the eye, and the sheath defines at least one exposed surface of the drug core. The exposed surface(s) of the drug core may contact a tear or tear film fluid and release the therapeutic agent at therapeutic levels over a sustained period when the implant is implanted for use. The implant may include a retention element to retain the drug core and sheath body near the punctum, optionally comprising a shape memory alloy that can resiliently expand. An occlusive element may be attached to the retention element to at least partially occlude tear flow through the canalicular lumen.
Owner:MATI THERAPEUTICS
Punctal plug comprising a water-insoluble polymeric matrix
InactiveUS20080114076A1Senses disorderPharmaceutical delivery mechanismCollagen Punctal PlugsPolyester
Disclosed is a pharmaceutical composition comprising a water insoluble polymer matrix that comprises a bioerodable polyester polymer or a fatty acid based polyester polymer, or a mixture of both polymers, wherein the polymer matrix has a melting point of less than 60° C., and wherein the composition is liquid or paste at room temperature and is formulated to occlude a punctual channel in a subject and conforms to the shape of the canalicular or punctal channel.
Owner:ALCON RES LTD
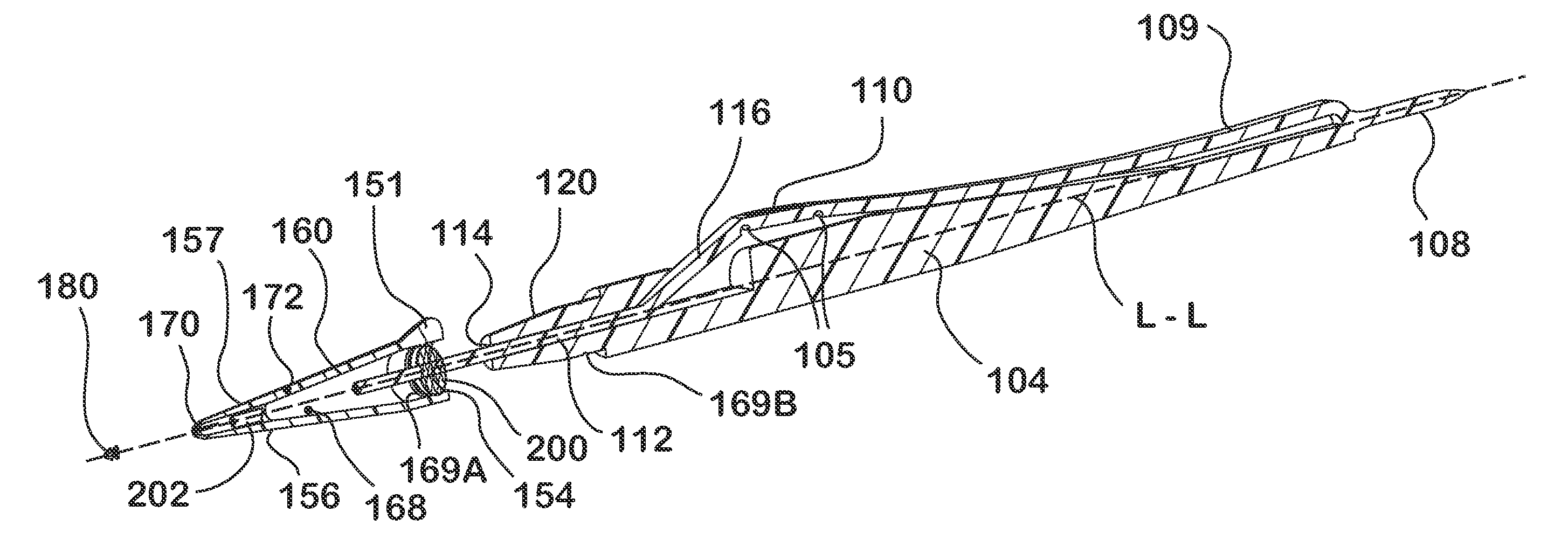
Lacrimal implants and related methods
Lacrimal implants and related methods providing secure retention within the lacrimal punctum of an eye are described. The lacrimal implants can comprise a implant body configured for at least partial insertion through the lacrimal punctum and into a lacrimal canaliculus. The implant body can include a deformable retention structure that can be configured to substantially encapsulate an expandable retention element. In some examples, the expandable retention element can include a fluid absorbing material, which can be exposed to fluid such as via a fluid permeable retainer or a fluid permeable aperture. As the fluid absorbing material retains fluid (i.e., upon acceptance of fluid into the retention structure), its size increases and its shape can change to urge one or more portions of the retention structure outward, such as against a wall of the lacrimal canaliculus, thereby securely retaining the lacrimal implant within the punctum.
Owner:MATI THERAPEUTICS
Drug delivery methods, structures, and compositions for nasolacrimal system
An implant which is provided by the invention for being inserted into the lacrimal punctum of patient comprises a main body. The main body comprises a distant end, a near end and a shaft between the distant end and the near end. The distant end of main body can be inserted into the inner cavity of lacrimal duct to the distant end through the lacrimal punctum. The main body comprises a therapeuticagent included in the flux core of pharmaceutical base. The exposing of pharmaceutical base to the tear causes that the effective therapeutic agent is released into the tear in duration. The main body is provided with a sheath which is configured on the pharmaceutical base for inhibiting the releasing of agent from the near end. The body is also provided with an outer surface which is combined with the tissue of inner cavity wall that restricts the discharging of tissue of inner cavity wall. In a specific execution mode, the pharmaceutical base comprises a non-biological absorptive polymer, such as the silicone in the non-homogeneous phase compound comprising the agent.
Owner:MATI THERAPEUTICS
Lacrimal filler
ActiveUS20110066138A1Avoid erosionPrevent outflowSurgical adhesivesEye surgeryDilatorBiological materials
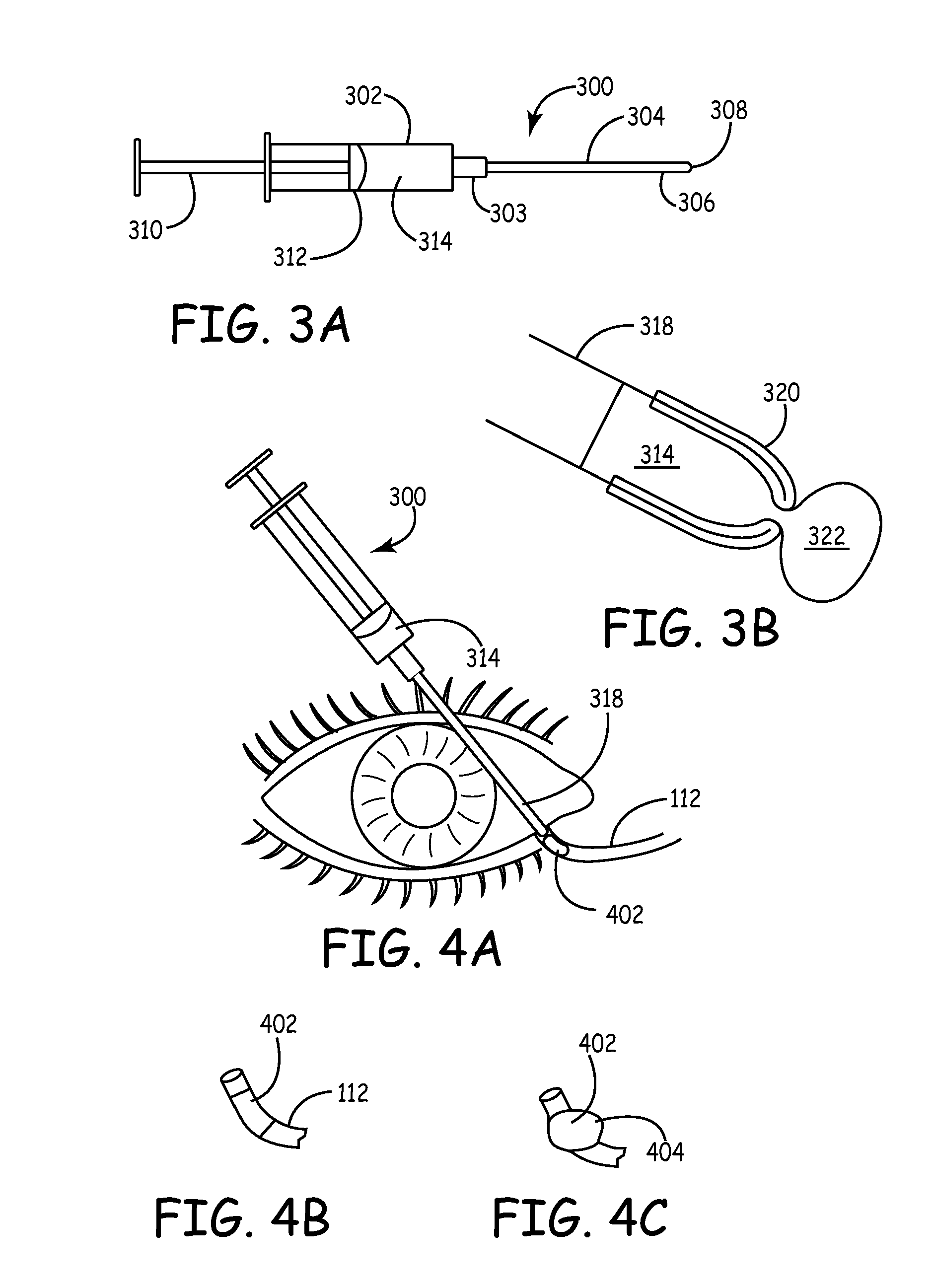
A biomaterial mass is inserted through a punctum into a canaliculus in the lacrimal outflow system with a syringe. The mass has an outer cross section that is less than the inner cross section of the canaliculus. The mass absorbs liquid to swell to form a lacrimal filler or sealing mechanism. The lacrimal filler has an external cross section that conforms to the internal cross section of the canaliculus and a soft outer surface relative to the surrounding tissues to prevent erosion of the canaliculus lining. The lacrimal filler forms an occlusion that prevents the outflow of liquid through the lacrimal outflow system to retain tears within the eye to maintain eye lubrication and wetness. The mass and syringe are provided in kit with a rubber stopper and, optionally, a punctal dilator, an injecting catheter, and an enzyme for dissolving or degrading the lacrimal filler at a later time.
Owner:FEZZA FAMILY PROPERTIES
Drug delivery system and methods of treating open angle glaucoma and ocular hypertension
ActiveUS20140025022A1Improved patient comfortImproved implant retentionOrganic active ingredientsEye surgeryOpen angle glaucomaOcular hypertension
A method of decreasing intraocular pressure (IOP) in an eye of a patient in need thereof includes implanting a first lacrimal implant through an upper punctum and into an upper lacrimal canaliculus of the eye of the patient. The method may further comprise implanting a second lacrimal implant through a lower punctum and into a lower lacrimal canaliculus of the eye of the patient, and releasing, on a sustained basis a therapeutically effective amount of an intraocular pressure-reducing therapeutic agent.
Owner:MATI THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com