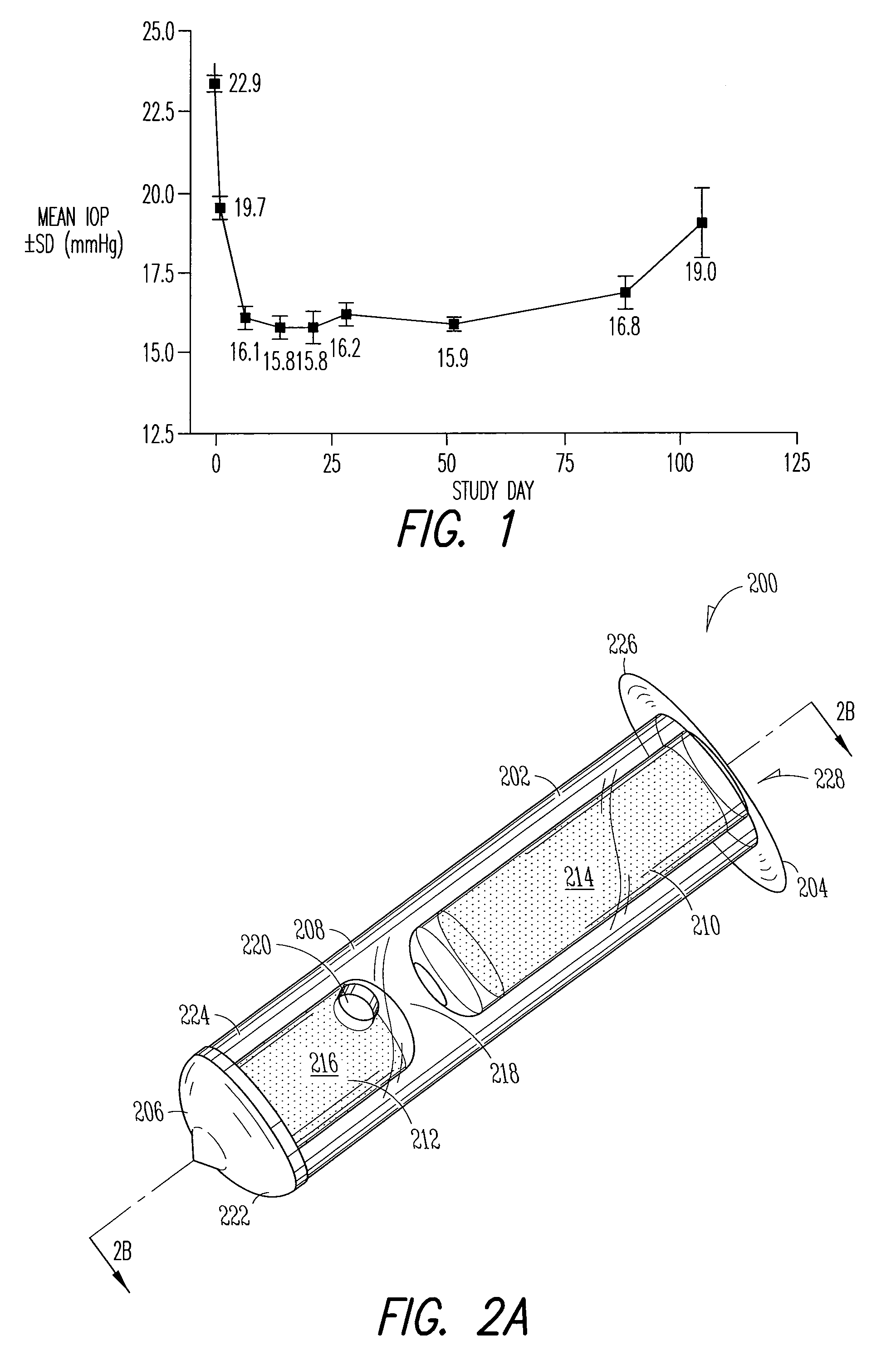
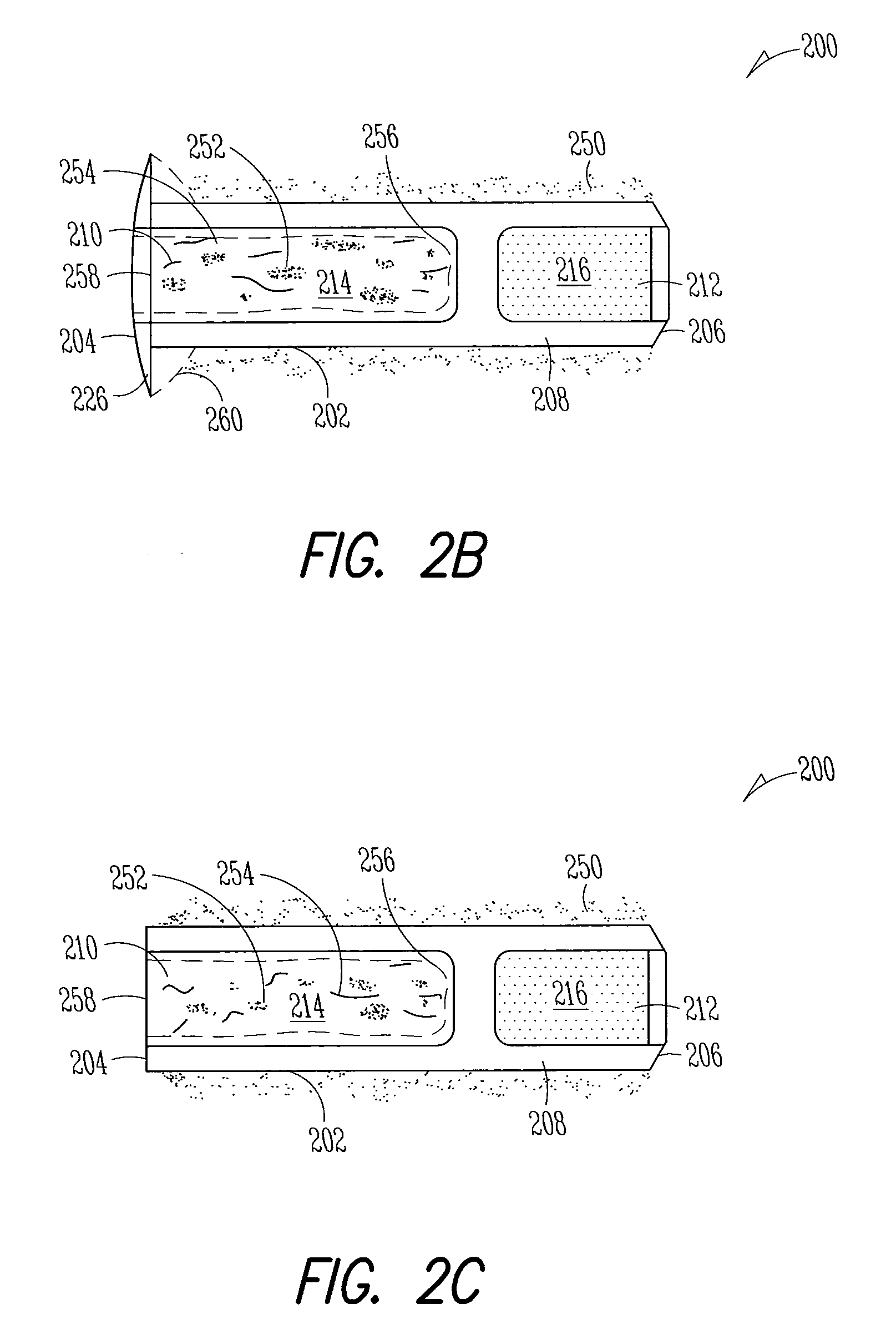
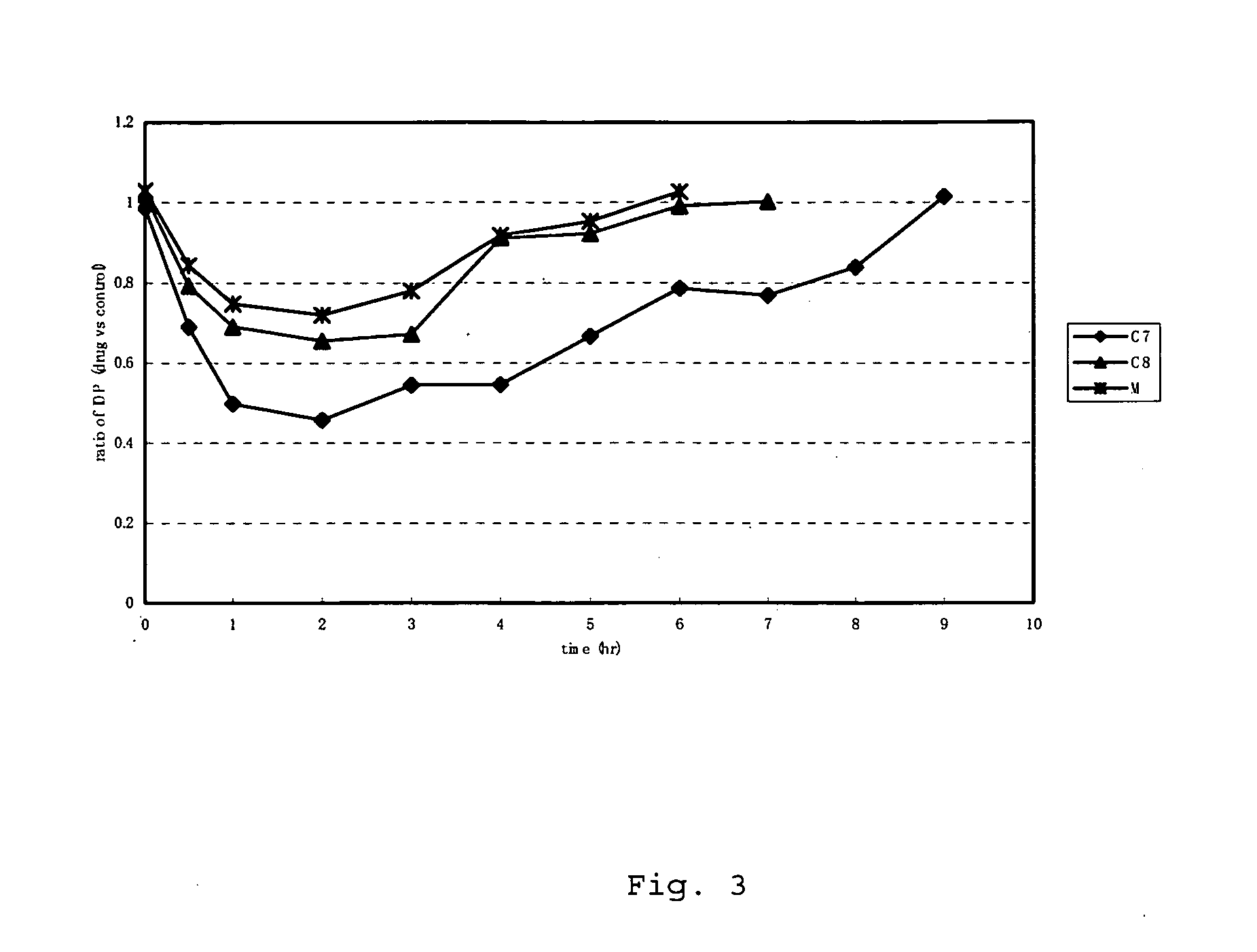
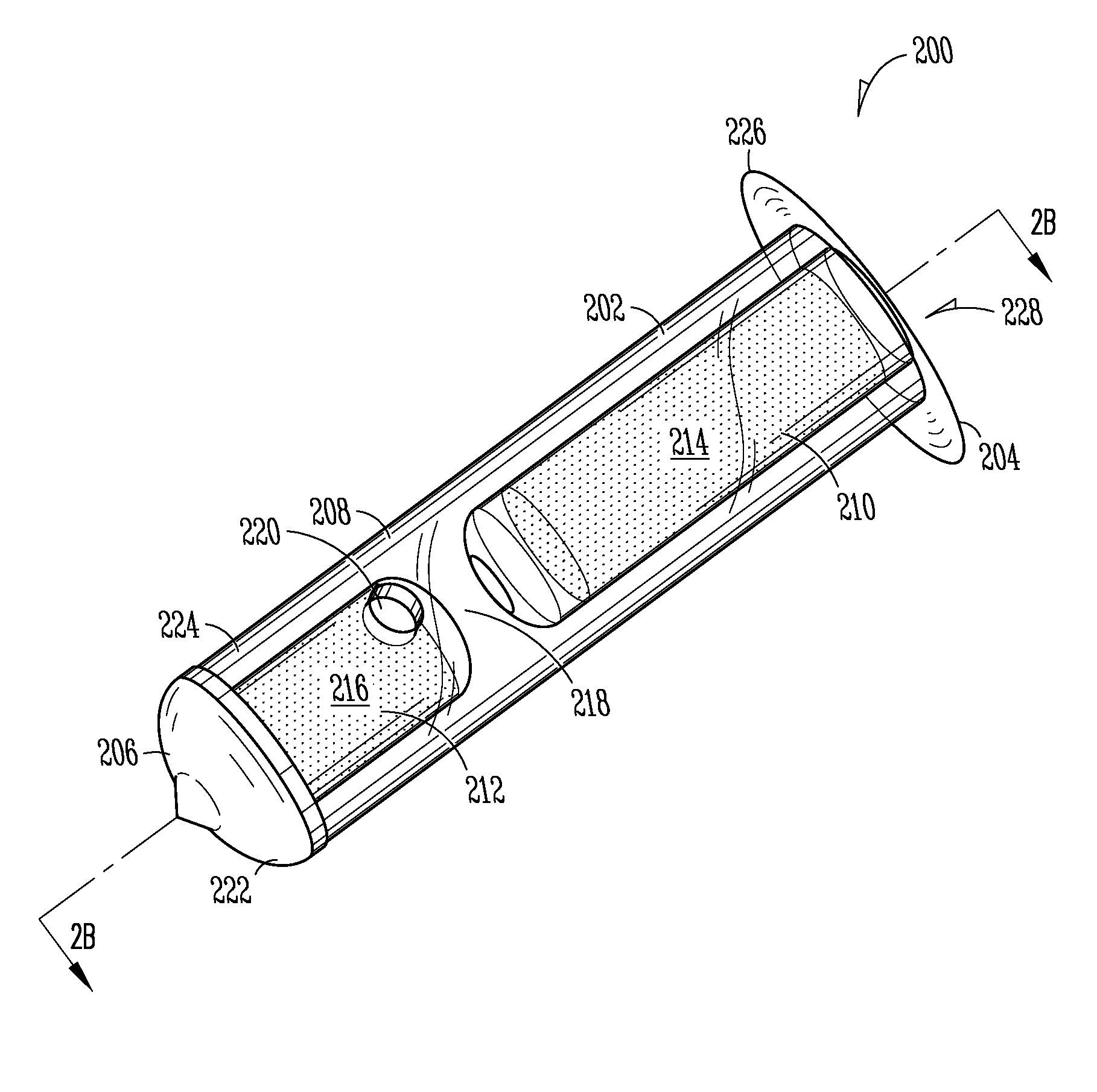
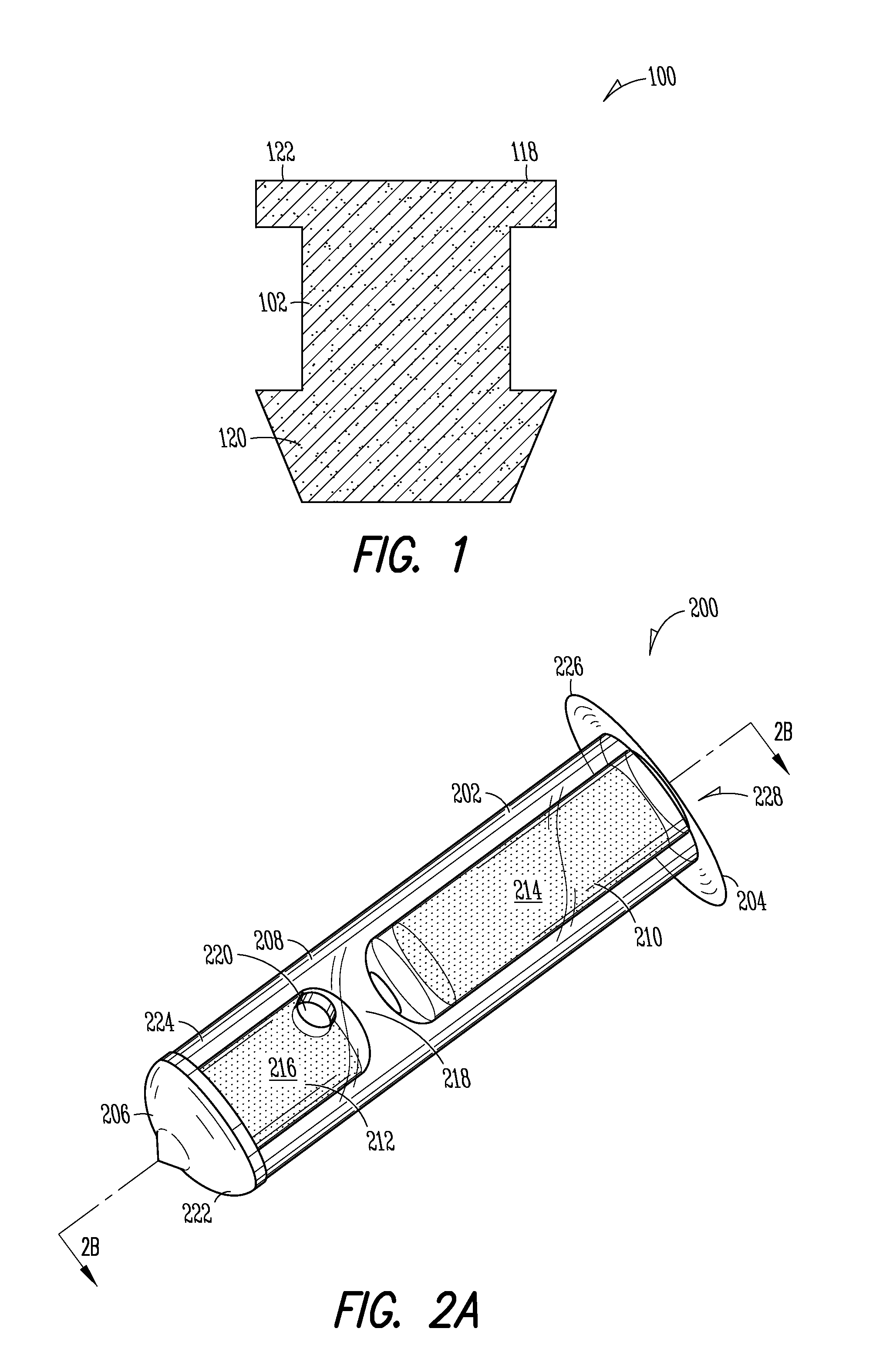
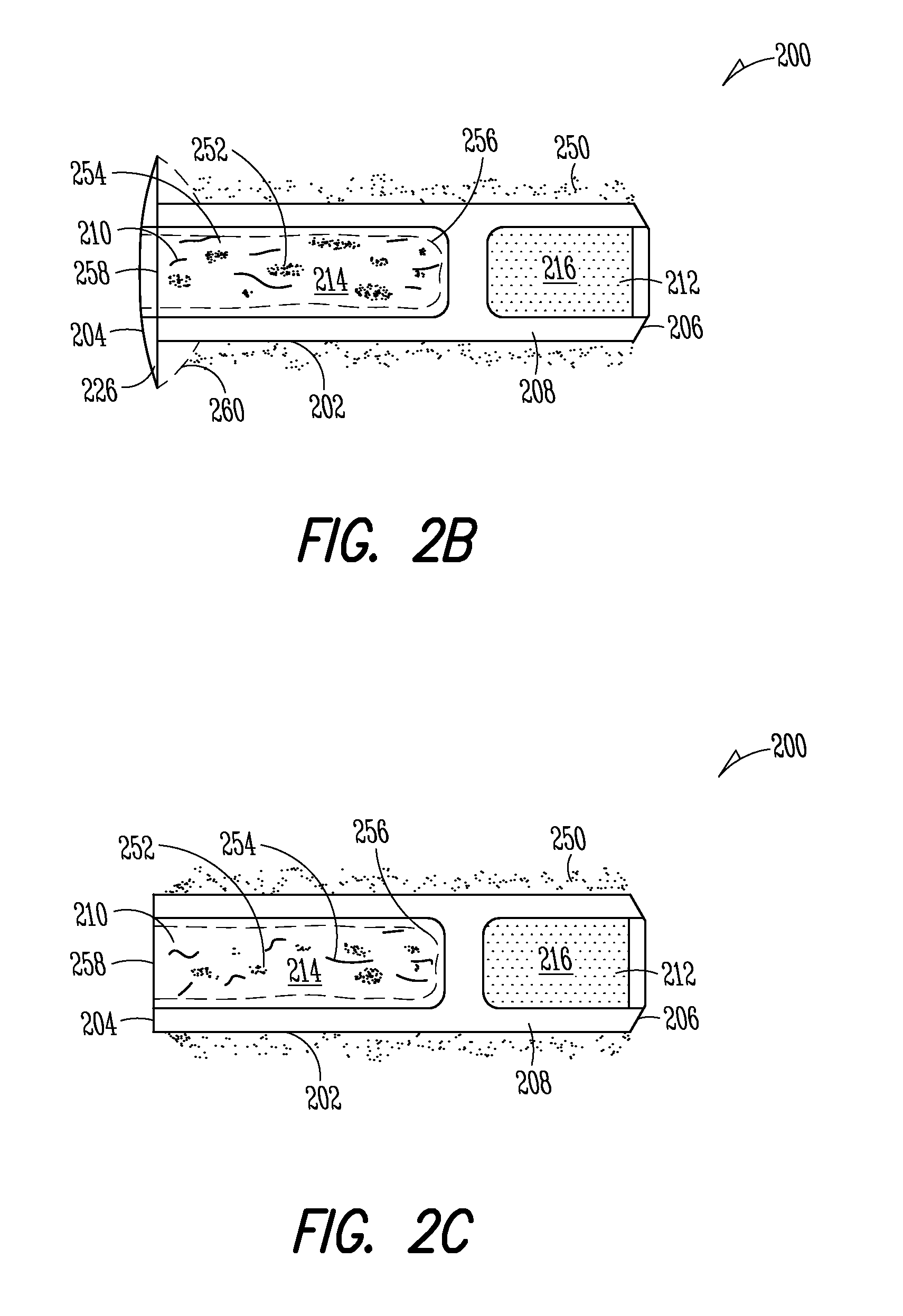
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1029 results about "Eye drop" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Eye drops are saline-containing drops used as an ocular route to administer. Depending on the condition being treated, they may contain steroids, antihistamines, sympathomimetics, beta receptor blockers, parasympathomimetics, parasympatholytics, prostaglandins, nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, antifungal, or topical anesthetics. Eye drops sometimes do not have medications in them and are only lubricating and tear-replacing solutions.
Hydrogels used to deliver medicaments to the eye for the treatment of posterior segment diseases
This invention provides a polymeric drug delivery system including a hydrogel containing one or more drugs for the treatment of a posterior segment disease. Exemplary drugs are anti-angiogenesis compounds for the treatment of macular degeneration. Allowing passive transference of this drug from a dilute solution into the hydrogel produces the delivery system. The hydrogel, when placed in contact with the eye, delivers the drug. The delivery of the drug is sustained over an extended period of time, which is of particular utility in the eye, which is periodically flushed with tears. This sustained delivery accelerates the treatment process while avoiding potential damaging effects of localized delivery of high concentrations of compounds, e.g., from eye drops.
Owner:DIRECTCONTACT
Methods and articles for the delivery of medicaments to the eye for the treatment of posterior segment diseases
InactiveUS20050255144A1Pharmaceutical delivery mechanismEye treatmentHigh concentrationDelivery system
This invention provides articles and methods for drug delivery including a hydrogel containing one or more drugs for the treatment of a posterior segment disease and / or dry eye conditions. Exemplary drugs are anti-angiogenesis compounds for the treatment of macular degeneration. Allowing passive transference of this drug from a dilute solution into the hydrogel produces the delivery system. The hydrogel, when placed in contact with the eye, delivers the drug. The delivery of the drug is sustained over an extended period of time, which is of particular utility in the eye, which is periodically flushed with tears. This sustained delivery accelerates the treatment process while avoiding potential damaging effects of localized delivery of high concentrations of compounds, e.g., from eye drops.
Owner:DIRECTCONTACT
Cyclodextrin nanotechnology for ophthalmic drug delivery
The invention provides an ophthalmic composition which is an aqueous suspension comprising drug, cyclodextrin and water, the composition having an aqueous phase of from about 0.1% (w / v) to about 90% (w / v) of the drug in solution, as dissolved free drug and as dissolved drug / cyclodextrin complex(es), and a solid phase of from about 10% (w / v) to about 99.9% (w / v) of the drug as solid drug / cyclodextrin particles, suspended in the aqueous phase; the size of the solid particles being from about 10 nm to about 1 mm, the drug / cyclodextrin particles being capable of dissolving in aqueous tear fluid within 24 hours of application to the eye surface. The aqueous eye suspension can be in the form of eye drops, eye gel or eye mist. Further, the invention provides a method for treating a condition of the posterior segment and / or anterior segment of the eye comprising applying to the eye surface, in an amount which delivers to said segment or segments a therapeutically effective amount of a drug suitable for treating said condition, an ophthalmic composition which is as defined above. Nasal compositions and methods and ophthalmic and nasal compositions in powder form are also provided.
Owner:OCULIS EHF
Sustained release delivery of active agents to treat glaucoma and ocular hypertension
ActiveUS20090280158A1Lower eye pressureReduction in patient noncomplianceBiocideSenses disorderLatanoprostActive agent
The methods described herein provide treatment of glaucoma, ocular hypertension, and elevated intraocular pressure with latanoprost or other therapeutic agent(s). Implant devices for insertion into a punctum of a patient provide sustained release of latanoprost or other therapeutic agent(s) that is maintained for 7, 14, 21, 30, 45, 60, or 90 days or more, thus avoiding patient noncompliance and reducing or lowering adverse events associated with eye drop administration of latanoprost or other therapeutic agent(s) and other therapeutic agent(s).
Owner:MATI THERAPEUTICS
Agent for treating eye diseases
An agent for treating various kinds of diseases which contains at least one selected from the group consisting of sexual steroid hormone such as estrogen or its metabolites, its derivative, structural analogues thereof, estrogen acting substance or SERM non-feminizing estrogen (non-hormonal estrogen), and an activator of sirtuin and has a form of eye drops or eye washes, oral preparation, etc. An agent for treating eye diseases which has excellent treatment effects, reduced in side action can be provided.
Owner:ADVANCED MEDICINE RES INST
Drug delivery to the anterior and posterior segments of the eye using eye drops
InactiveUS20110104155A1Detection is simple and fastBiocideSenses disorderAdrenergic DrugsAdrenergic Agent
A method and means for delivery of drugs to the chorio-retina and the optic nerve head which comprises contacting the surface of the eye with an effective amount of drug for treatment of chorio-retina and optic nerve head and a physiologically acceptable adrenergic agent for enhancing delivery of the drug to these tissues in an ophtalmologically acceptable carrier, said adrenergic agent being selected from the group consisting of alpha adrenergic agonist agents, derivatives of the alpha adrenergic agonist agents, beta-blocking agents, derivatives of the beta-blocking agents and mixtures thereof.
Owner:RAOUF REKIK
Therapeutic agent for treating glaucoma
InactiveUS20080064681A1Lower eye pressureBiocideAnimal repellantsIntraocular pressureTherapeutic effect
The present invention provides compounds useful as therapeutic agent for treating glaucoma and methods for treating glaucoma. That is, a therapeutic agent for treating glaucoma containing, as an active ingredient, a compound represented by formula (1) below is synthesized, and the therapeutic agent is administered in the form of eye drops to a glaucoma patient. Thus, the intraocular pressure is reduced.In the above formula, ring A represents a 5- to 11-membered cyclic amino group, which may have a substituted group, and X represents a halogen.
Owner:D WESTERN THERAPEUTICS INST
Combination treatment of glaucoma
InactiveUS20090318549A1Lower eye pressureOrganic active ingredientsBiocideLatanoprostSustained Release Formulations
The methods described herein provide reduction of intraocular pressure by administering a sustained release formulation including latanoprost and a pharmaceutically acceptable vehicle and administering an eye drop adjunctive composition to the eye of a patient. The sustained release formulation can release latanoprost continuously for at least 90 days from a punctum plug delivery system. The eye drop adjunctive composition can also include latanoprost.
Owner:MATI THERAPEUTICS
Method for treating visual impairment through the prophylactic administration of a Morinda citrifolia-based naturaceutical
InactiveUS20030134002A1Promote resultsInhibit and prevent and reverse macular degenerationBiocideUnknown materialsDiabetic retinopathyRetinitis pigmentosa
Implementation of the present invention takes place in association with the utilization of one or more processed products produced from the Indian Mulberry plant, scientifically known as Morinda citrifolia L., to treat one or more eye disorders that affect vision, such as glaucoma, diabetic retinopathy, retinitis pigmentosa, cataracts, age-related macular degeneration, night blindness, color blindness, and other related conditions. The processed Morinda citrifolia products from the Indian Mulberry plant may be in the form of a dietary supplement, eye drops, or in another suitable form.
Owner:TAHITIAN NONI INT INC
Stabilized ocular solutions
InactiveUS20050065091A1Improve the ability to solveAntioxidant inhibitionBiocideSenses disorderVolume replacementAntioxidant
Ocular solutions containing an antioxidant provide beneficial properties, for example, the antioxidant scavenges free radicals in the solution which may cause the solution to deteriorate. However, antioxidants are themselves extremely susceptible to oxidation. A stabilizing agent for the antioxidant retards or prevents the antioxidant from undesirable reactions and thus enhances its ability to stabilize the ocular solution. This in turn enhances the physiological properties of the ocular solution, which may be a topical solution such as eye drops, or a surgical ocular irrigation or volume replacement solution.
Owner:MINU
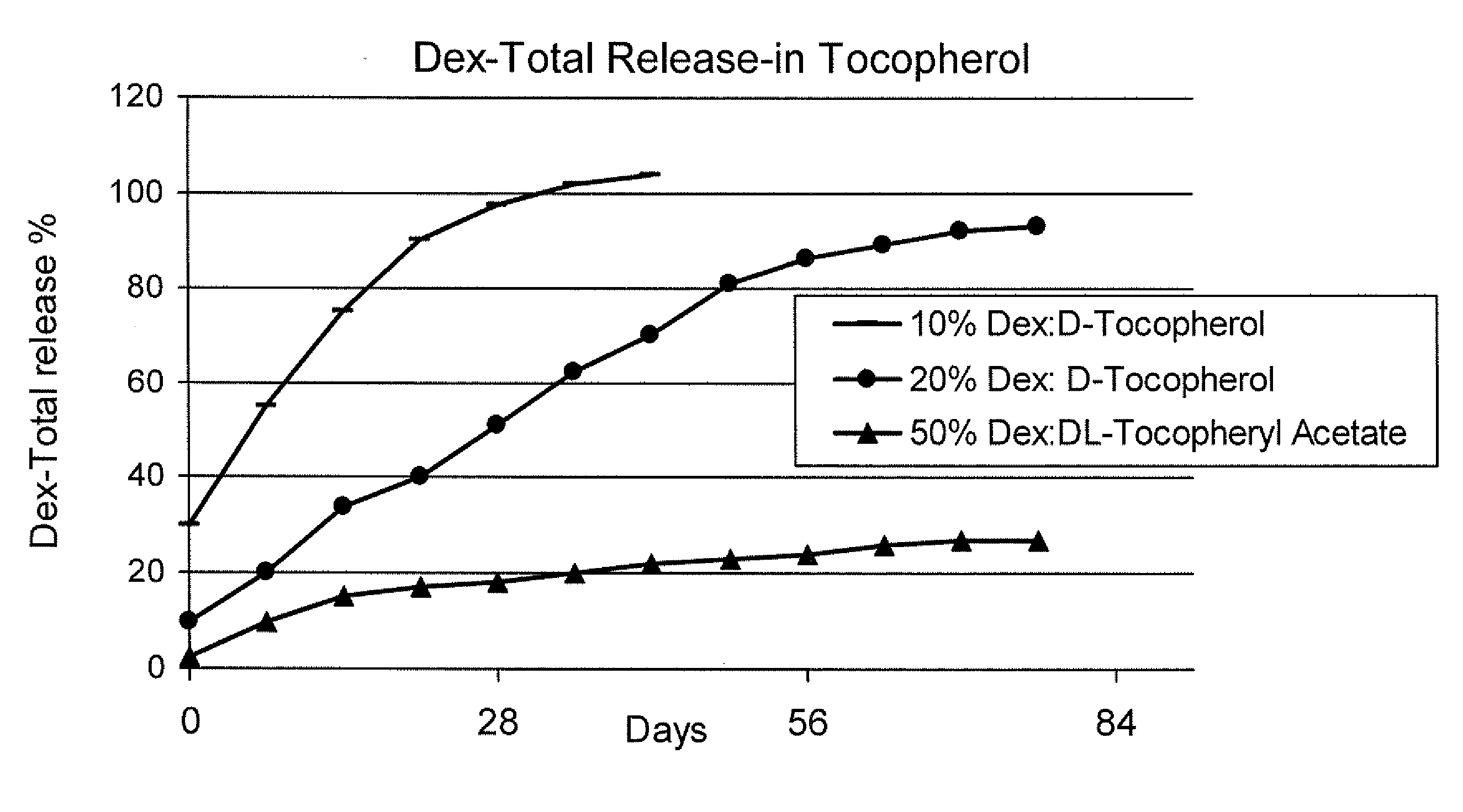
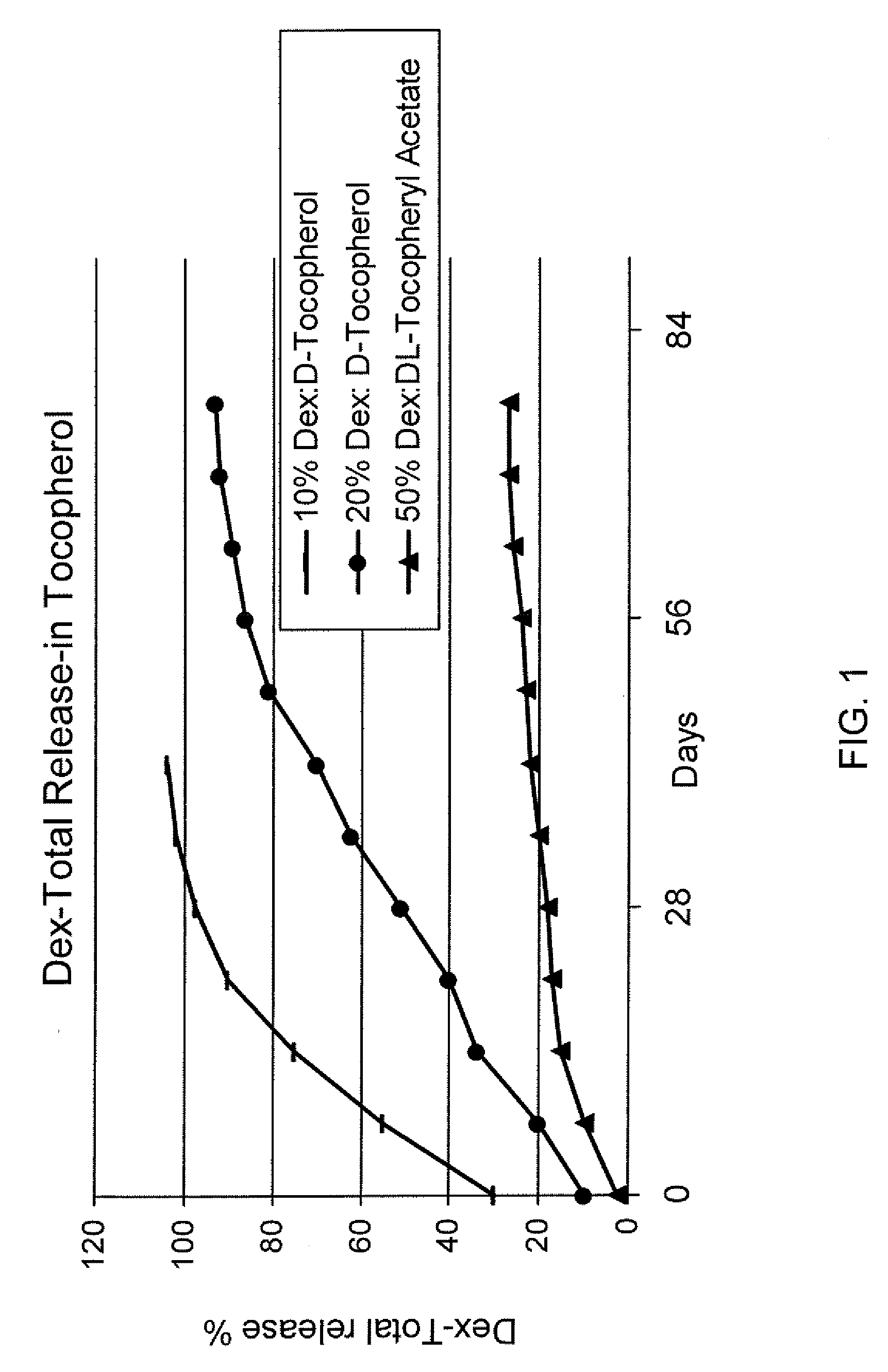
Sustained release eye drop formulations
ActiveUS20090136445A1Economical and practical and efficientEasy to produceAntibacterial agentsBiocideSolubilityIrritation
This invention provides for biocompatible, biodegradable eye drop pharmaceutical formulations useful for the treatment of ocular indications. In particular, tocopherols and their esters of low water solubility, notably α-tocopheryl acetate, are exceptional vehicles for biocompatible, nonirritating topical eye drop formulations that provide sustained release of active agents.
Owner:RAMSCOR
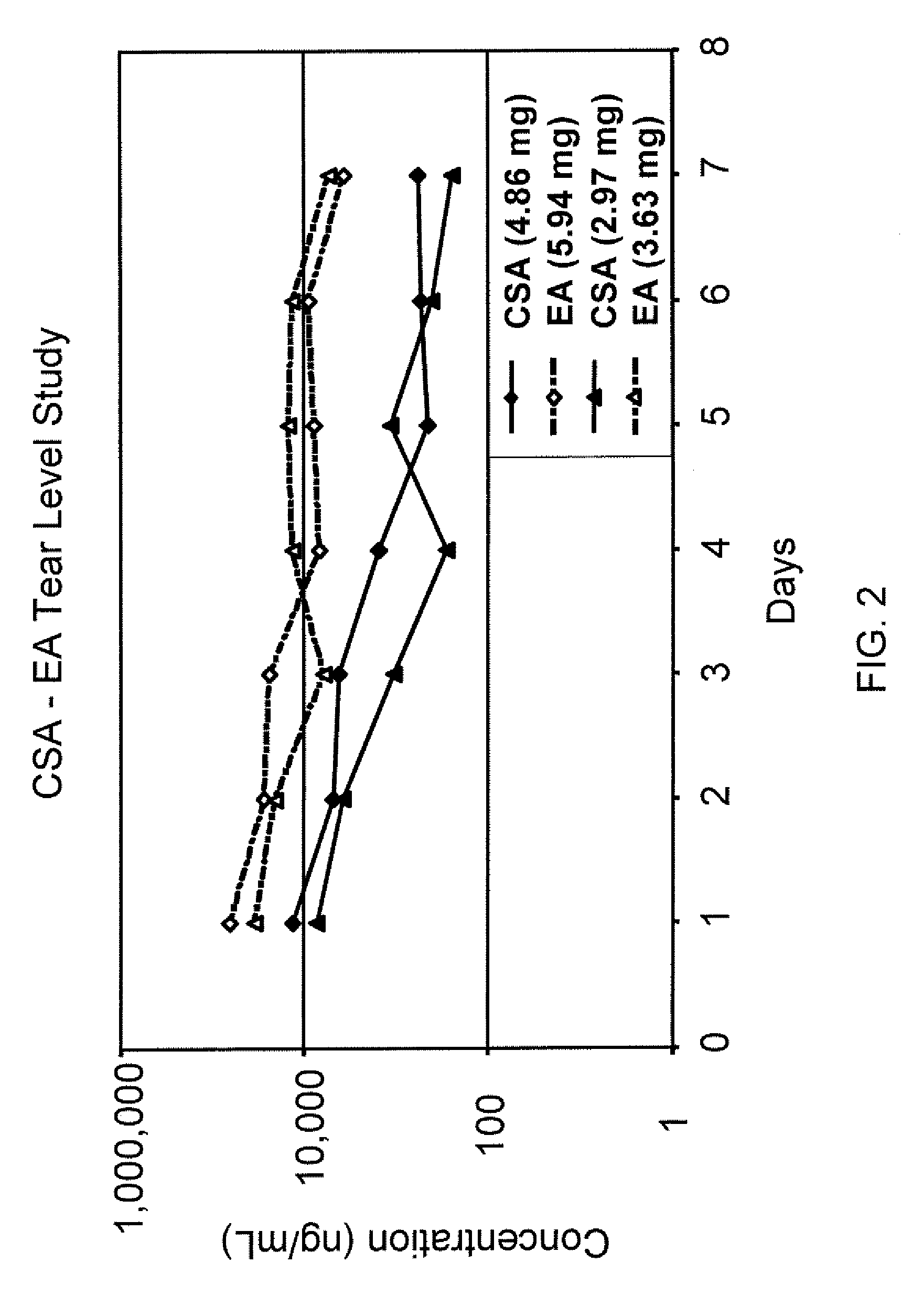
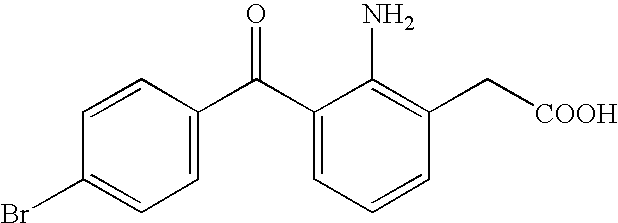
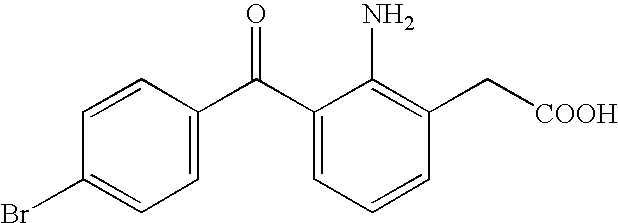
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
ActiveUS20050239895A1No irritationInhibit deteriorationBiocideOrganic active ingredientsScleritisPhenylacetic acid
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
Contact lens and eye drop rewetter compositions and methods
Stable ophthalmic formulations comprising hyaluronic acid (sodium hyaluronate) as the primary active demulcent ingredient, stabilized oxy-chloro complex (available commercially as OcuPure(tm) from Advanced Medical Optics, Purite® from Allergan, and Purogene from Biocide) for preservative efficacy, balanced salts mimicking the tear film, and sodium borate as a buffer are disclosed. In one embodiment, preferred stable formulations may be used in the human eye with or without contact lenses. In another embodiment preferred formulations may also be used as a storage and conditioning solution for contact lenses following disinfection.
Owner:ADVANCED MEDICAL OPTICS
Aqueous suspension preparations with excellent redispersibility
InactiveUS6274634B1Reduce surface tensionSurface tensionBiocideSolution deliveryOral medicationLotion
The aqueous suspension can be prepared by incorporating, in an aqueous suspension of a hardly soluble drug, a water-soluble polymer within the concentration range from the concentration at which the surface tension of the aqueous suspension of the drug begins to decrease up to the concentration at which the reduction in surface tension ceases. The resulting aqueous suspension shows ready redispersibility and will not undergo aggregation of dispersed particles or caking. Because of its good redispersibility, the suspension is useful as a parenteral preparation, eye drops, nasal drops, a preparation for oral administration, a lotion or the like.
Owner:SENJU PHARMA CO LTD
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
Mangiferin-berberine composition
InactiveCN101066275AHighlight substantive featuresSignificant progressOrganic active ingredientsSenses disorderSolubilityBerberine
The present invention relates to one kind of mangiferin-berberine composition with relatively high water solubility and its preparation process and medicinal application. The mangiferin-berberine composition has water solubility up to 25 mg / ml. Animal test shows that the mangiferin-berberine composition has functions of inhibiting bacteria, lowering blood sugar, diminishing inflammation, protecting liver, etc. The mangiferin-berberine composition may be used in preparing eye drops, medicine for women's vaginal diseases, medicine for lowering blood sugar and medicine for treating hepatitis.
Owner:广西中医学院
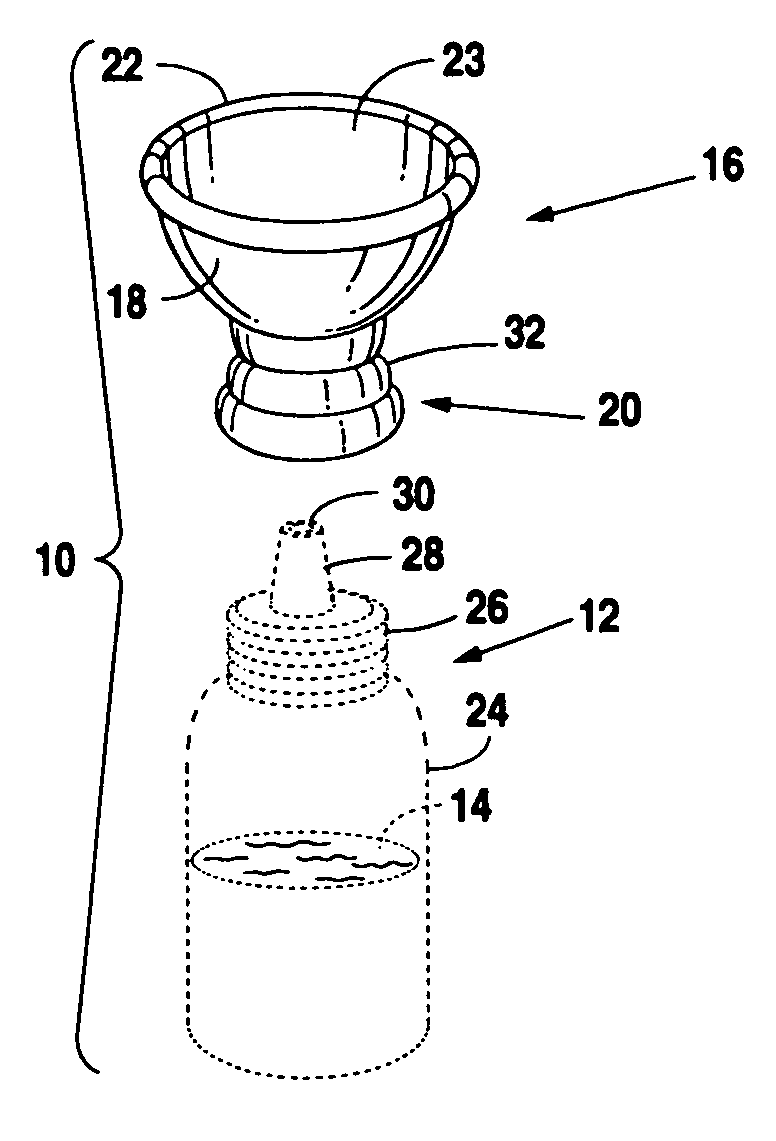
Eye drop applicator
InactiveUS20060129113A1Avoid pollutionEasy to manufactureMedical applicatorsEye treatmentEngineeringSpherical shaped
A novel applicator for engagement to an eye drop vial. The eye drop vial would be squeezable and contain a liquid therein that is compatible with the eye. The adapter includes a neck having a multiplicity of inner diameters adapted to a multiplicity of eye drop vials and a cup. The cup is semi-spherical shaped and has a rim that is collapsible between a closed position, which would seal off the vial, and an open position which would allow application of liquid from the vial to the eye of a user.
Owner:MERRICK JAMES
Methods and articles for the delivery of medicaments to the eye for the treatment of posterior segment diseases
This invention provides articles and methods for drug delivery including a hydrogel containing one or more drugs for the treatment of a posterior segment disease and / or dry eye conditions. Exemplary drugs are anti-angiogenesis compounds for the treatment of macular degeneration. Allowing passive transference of this drug from a dilute solution into the hydrogel produces the delivery system. The hydrogel, when placed in contact with the eye, delivers the drug. The delivery of the drug is sustained over an extended period of time, which is of particular utility in the eye, which is periodically flushed with tears. This sustained delivery accelerates the treatment process while avoiding potential damaging effects of localized delivery of high concentrations of compounds, e.g., from eye drops.
Owner:DIRECTCONTACT
Voriconazole eye drops and preparation method thereof
InactiveCN101849905AImprove comfortGood for antifungal effectOrganic active ingredientsSenses disorderMedicineBromine
The invention discloses voriconazole eye drops. Every 1000 milliliters of eye drops contain 3-50g of voriconazole powder, 20-500g of hydroxypropyl-beta-cyclodextrin, 0.1-0.3g of bacteriostat, 0.5-2.5g of sodium hyaluronate and the balance of water for injection, wherein the bacteriostat is benzalkonium chloride, benzalkonium bromine or ethylparaben. The invention also discloses a preparation method of the voriconazole eye drops. The invention greatly increases the solubility of voriconazole in water to 0.3-5%, so that the medicine can exist in water solution in the form of molecules, thereby providing convenience for exerting the antifungal action of the medicine. Meanwhile, when being applied to eyes, the invention provides convenience for exerting the antifungal action of the medicine through cornea. The preparation method can be carried out at room temperature without affecting the medicine stability, and has the advantages of simple and easy realization and low cost.
Owner:河南省眼科研究所
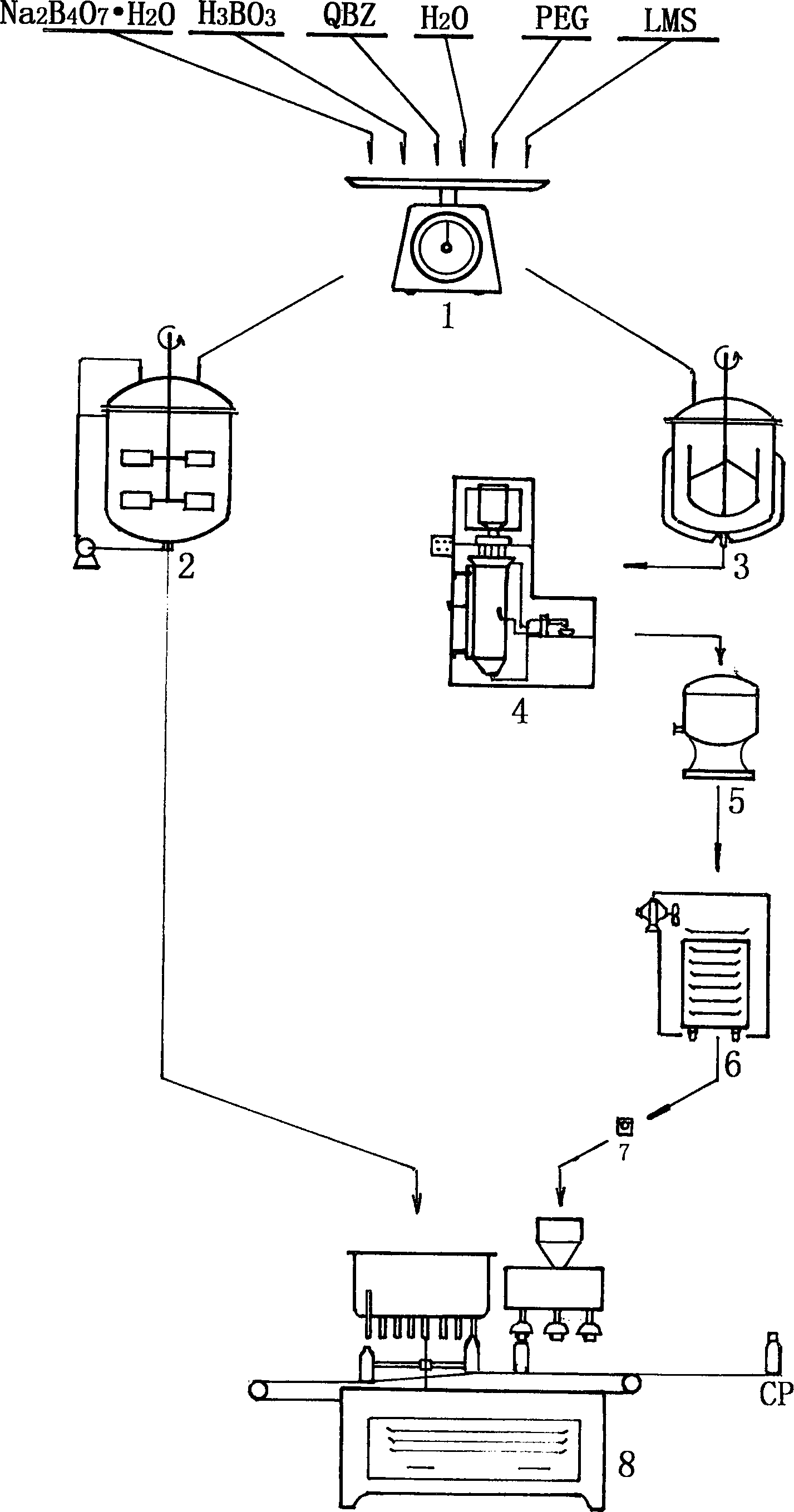
Drop pill type eye drip fluid of chloramphenicol, and preparation method
InactiveCN1872038AEnsure stable qualityImprove stabilityOrganic active ingredientsSenses disorderDiseasePolyethylene glycol
A dripping pill of Chloromycetin eye drops for treating trachnoma, keratitis, conjunctivitis, eyelid inflammation, etc is proportionally prepared from polyethanediol as matrix and Chloromycetin. It is applied in conjunction with the buffer liquid prepared from borax, boric acid, hydroxyphenyl ethylether and the water for injection. Its preparing process is also disclosed.
Owner:济宁光明制药有限公司
Transparent eye drops containing latanoprost
InactiveUS20060069162A1White turbidity can be preventedAvoid turbidityBiocideSenses disorderPreservativeMedicine
An object of the present invention is to provide better formulations of a latanoprost ophthalmic solution. The present invention provides a clear ophthalmic solution comprising latanoprost as an active ingredient and benzalkonium chloride as a preservative wherein white turbidity due to a change of formulation is prevented by at least one means selected from the following 1) to 3); 1) adding a surfactant, 2) using benzalkonium chloride represented by the formula of [C6H5CH2N(CH3)2R]Cl (wherein R is alkyl having 12 carbon atoms) as the preservative and 3) adding a nonionic tonicity agent as a tonicity agent.
Owner:SANTEN PHARMA CO LTD
Container for eye drops
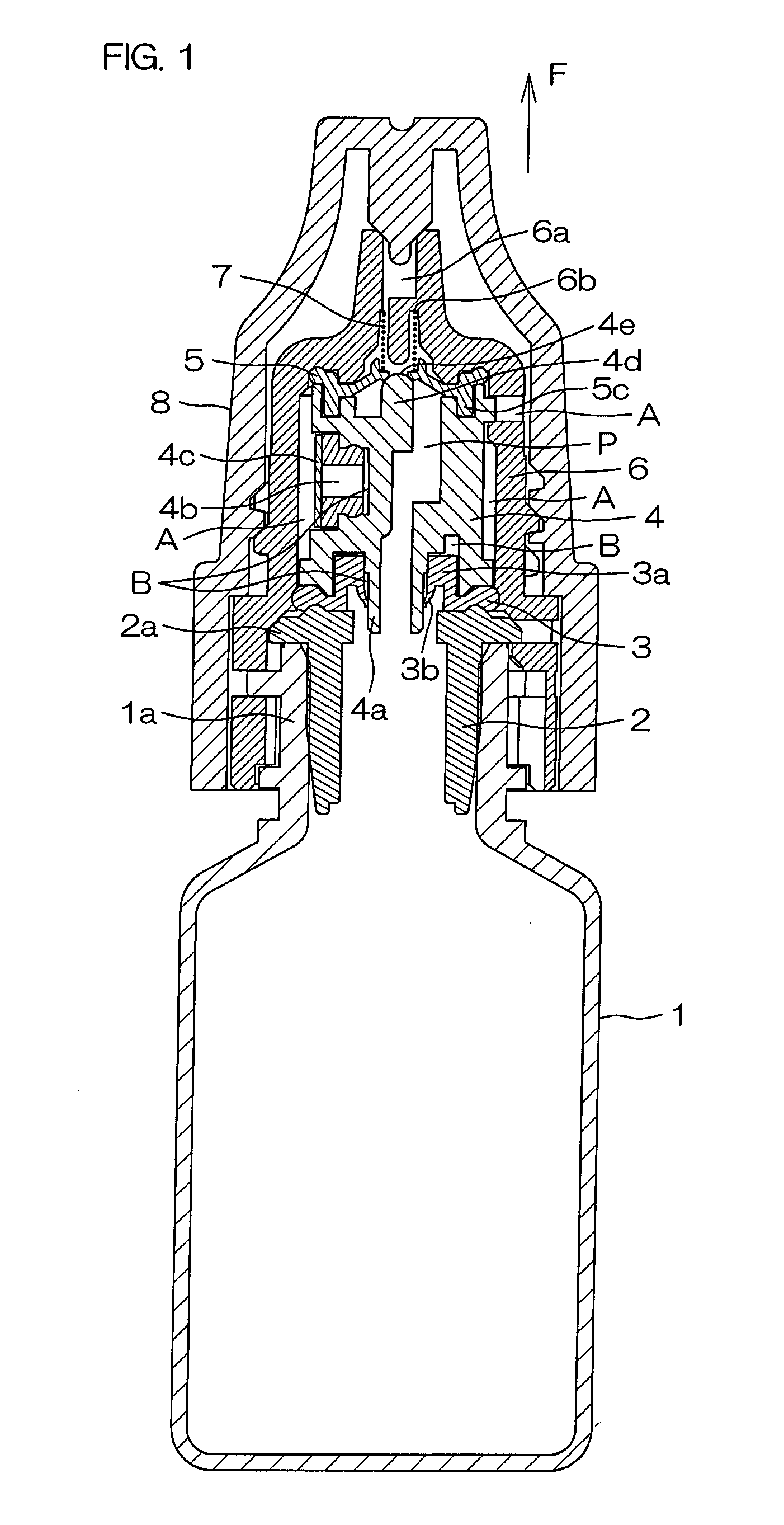
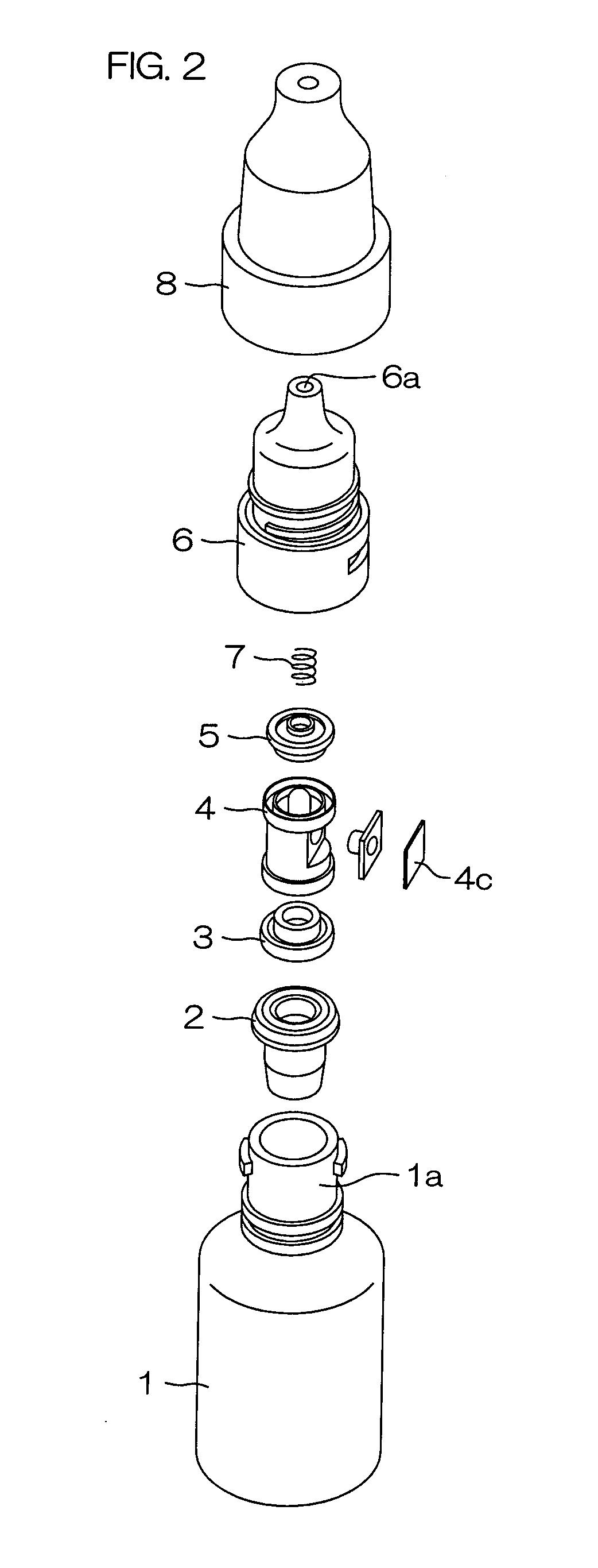
InactiveUS20120067926A1Improve breathabilityPrevent wettingClosuresDispensing apparatusEngineeringMechanical engineering
Provided are a flexible and restorable container main body (1) for housing a medicinal solution; a stopper member (4d; 14d) fixed in the inside of a medicinal solution passage (P) communicated with the container main body (1) for discharging the medicinal solution when the container main body (1) is pressed and deformed; a pressure valve (5) arranged in the medicinal solution passage (P) at a downstream side in the medicinal solution discharge direction further than the stopper member (4d; 14d) being formed with an opening having a circular cross section which contacts the tip portion (4e; 14e) of the stopper member (4d; 14d); and a biasing member (7; 7a) at a downstream side for biasing the pressure valve (5) in a direction opposite to the medicinal solution discharge direction by abutting against a peripheral portion (5b) of the opening of the pressure valve (5).
Owner:OTSUKA PHARM CO LTD
Contact lens and eye drop rewetter compositions and methods
Stable ophthalmic formulations comprising hyaluronic acid (sodium hyaluronate) as the primary active demulcent ingredient, stabilized oxy-chloro complex (available commercially as OcuPure™ from Advanced Medical Optics, Purite® from Allergan, and Purogene from Biocide) for preservative efficacy, balanced salts mimicking the tear film, and sodium borate as a buffer are disclosed. In one embodiment, preferred stable formulations may be used in the human eye with or without contact lenses. In another embodiment preferred formulations may also be used as a storage and conditioning solution for contact lenses following disinfection.
Owner:ADVANCED MEDICAL OPTICS
Use of a VEGF Antagonist in Treating Retinopathy of Prematurity
InactiveUS20160159893A1Stopping abnormal blood vessel growthExtended half-lifeLaser surgerySenses disorderDiseaseRetina
The present invention relates to the use of a VEGF antagonist in the treatment of retinal neovascular disorders in infants. In particular, the invention provides a method for treating an infant having retinopathy of prematurity (ROP), wherein said method comprises administering to the eye of an infant a VEGF antagonist that either does not enter or is rapidly cleared from the systemic circulation. The term “infant” is typically used to refer to young children from birth up to the age of 12 months. The VEGF antagonist may be administered intravitreally, e.g. through injection, or topically, e.g. in form of eye drops.
Owner:NOVARTIS AG
Liquid eye drop composition
A composition that is used as an eye treatment contains reduced glutathione, vitamin A and vitamin E, as well as one or more of zinc sulfate, boric acid and potassium as buffering agents. The composition also may contain a lubricant and a preservative. The composition is a sterile isotonic solution. The composition is used in a method of treating eyes for the alleviation of irritations and / or dryness, as well as for the prevention and treatment of cataracts.
Owner:BRASWELL A GLENN +2
Use of VEGF antagonist in treating chorioretinal neovascular and permeability disorders in paediatric patients
InactiveUS20160168240A1Minimize handlingExtended maintenance periodLaser surgerySenses disorderDiseasePaediatric patients
The present invention relates to the use of a VEGF antagonist in the treatment of chorioretinal neovascular or permeability disorders in children. In particular, the invention provides a VEGF antagonist for use in a method for treating a child having CNV or ME, wherein said method comprises administering to the eye of a child a VEGF antagonist that either does not enter or is rapidly cleared from the systemic circulation. The VEGF antagonist may be administered intravitreally, e.g. through injection, or topically, e.g. in form of eye drops. The invention further provides the use of a VEGF antagonist in the manufacture of a medicament for treating a child having a chorioretinal neovascular or permeability disorder.
Owner:NOVARTIS AG
Ophthalmic composition
InactiveUS20100239518A1Relieve pressureImproving eye drynessSenses disorderHydroxy compound active ingredientsCelluloseEye dryness
The present invention provides an ophthalmic composition which stabilizes the tear film during wearing contact lens, prevents eye dryness, imparts a favorable sensation in using, is highly convenient with no risk of misuse and shows a high efficiency in the course from manufacturing to sales. More specifically, the present invention provides a wetting solution—eye drops for contact lenses comprising (A) one or more member (s) selected from the group consisting of a cellulose-based polymer, a vinyl-based polymer, polyethylene glycol and dextran; and (B) a terpenoid.
Owner:ROHTO PHARM CO LTD
Eye-drops prepns. contg. tetrandrine and its application for preparing medicine therewith
InactiveCN1785192AImprove stabilityGood curative effectOrganic active ingredientsSenses disorderDiseaseIsoquinoline
An eye medicine containing tetrandrine for treating ophthalmitis, allergic ophthalmopathy, glaucoma, catarart, etc is prepared from tetrandrine (0.001-2 Wt%), other medicines (0-5), and excipient (93-99.999).
Owner:胡世兴 +1
Compounding use of sodium hyaluronate for eye preparation
InactiveCN1488404AImprove stabilityImprove water holding capacitySenses disorderPharmaceutical non-active ingredientsGlycerolWater soluble
The invention refers to the compound applied technique of two components of adjunct medical material-sodium hyaluronate and glycerin, in ophthalmic preparation. Its main technique: the compound base material is added to the ophthalmic preparation to make into stable water-soluble transparent eye drops; sodium hyaluronate is 0.05%-0.5% (g / ml) and glycerin 0.1%-2.5% (g / ml), and the mixed proportion: the former : the latter=1:1-1:4, and the optimal project: the former 0.05%-0.25%, the latter 0.1%-0.5%, and the mixed proportion: 1:2-1:4, and they can be added in multiple eye drops.
Owner:刘继东
Eye Drops for the Treatment of Dry Eye
InactiveUS20070265353A1Eliminate the effects ofSuppression problemBiocideSenses disorderSqualaneCompound (substance)
Eye drops for the treatment of dry eye is disclosed. The eye drops is a solution-type, emulsion-type or two separate phase-type eye drops containing squalane and is, among others, an oil-in-water-type emulsion further containing a water-soluble macromolecular compound such as polyvinylpyrrolidone. Also disclosed are use of squalane for the production of eye drops for the treatment of dry eye, and a method for the treatment of dry eye.
Owner:SENJU PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com