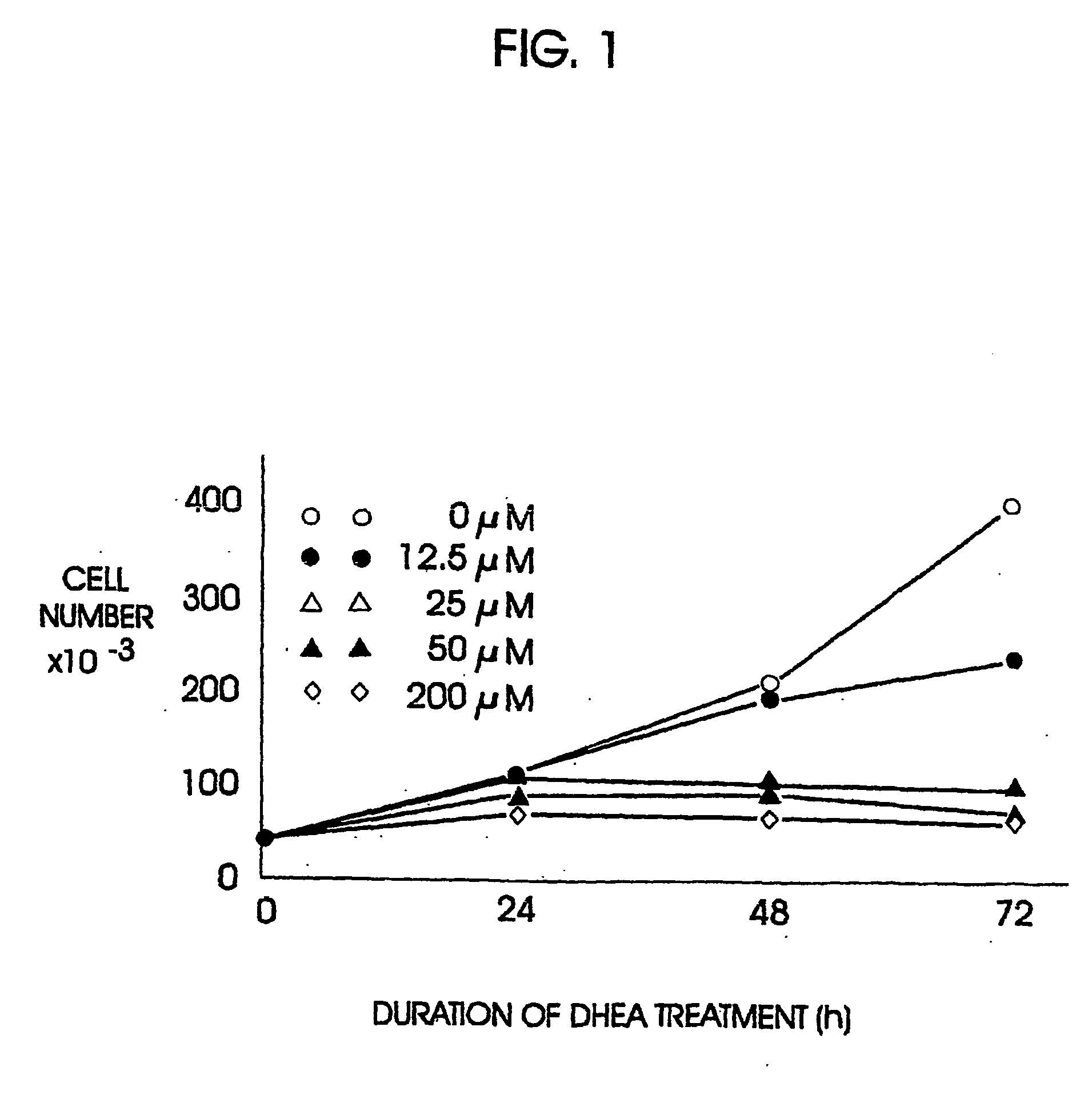
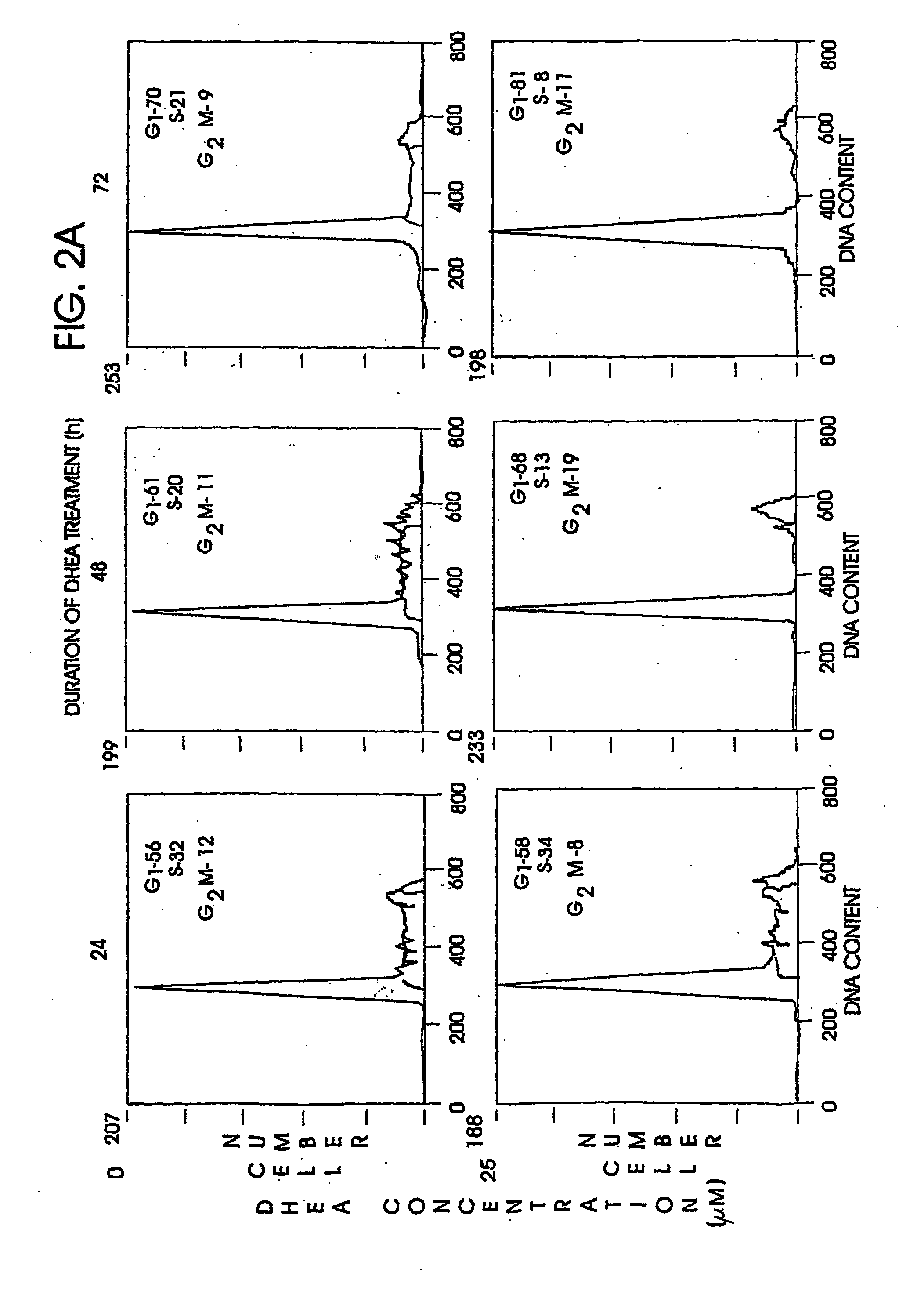
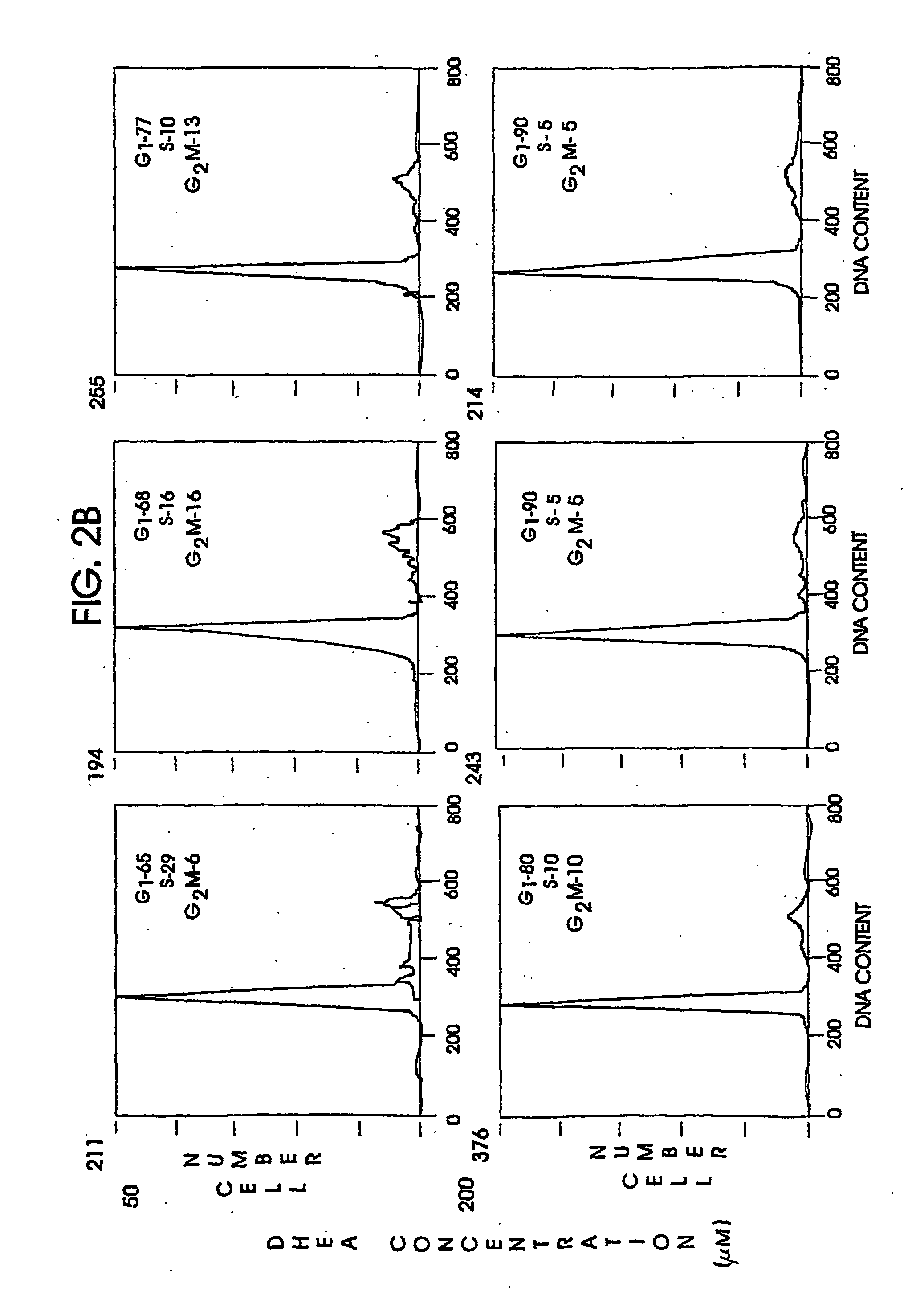
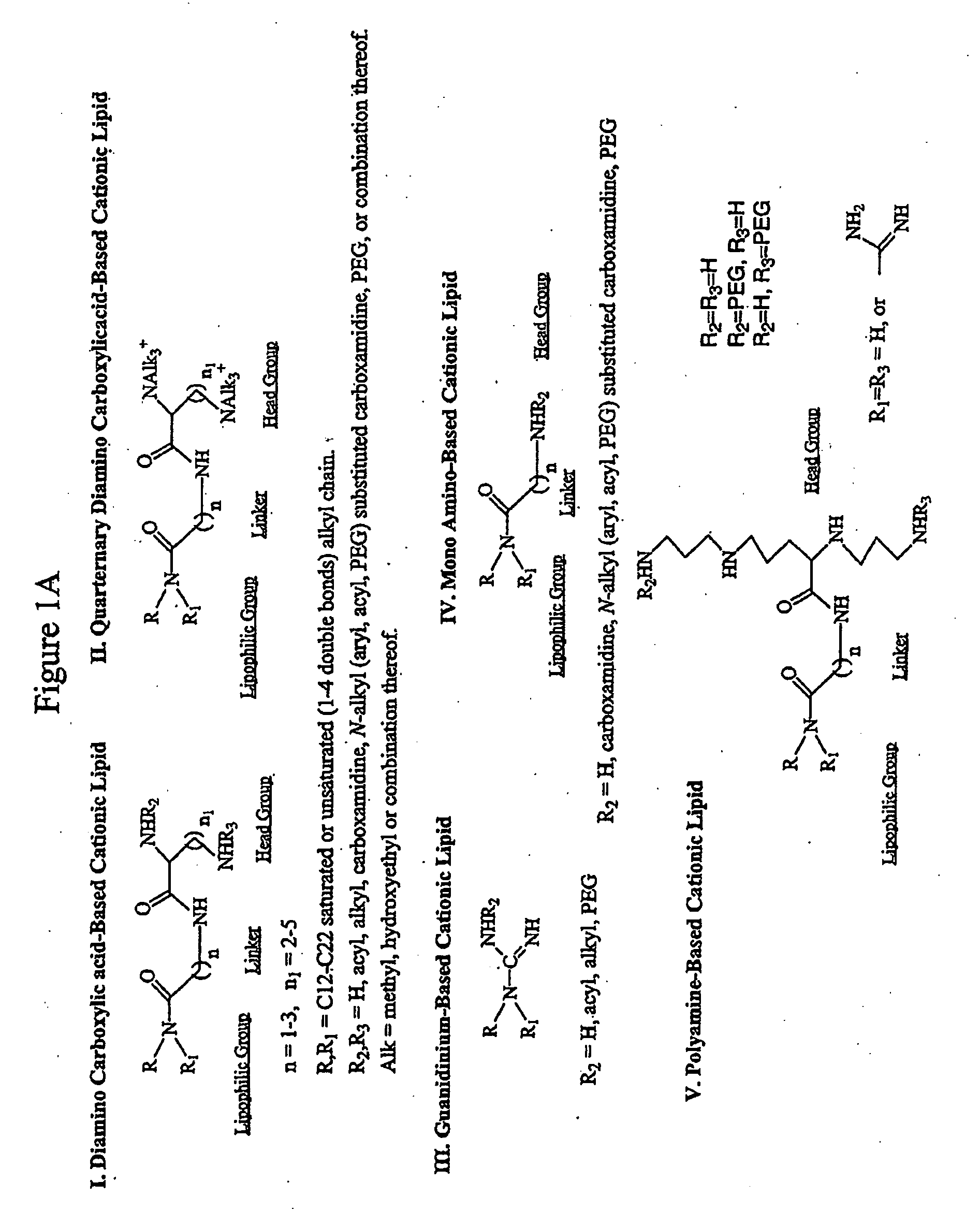
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1694 results about "Steroid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes which alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.
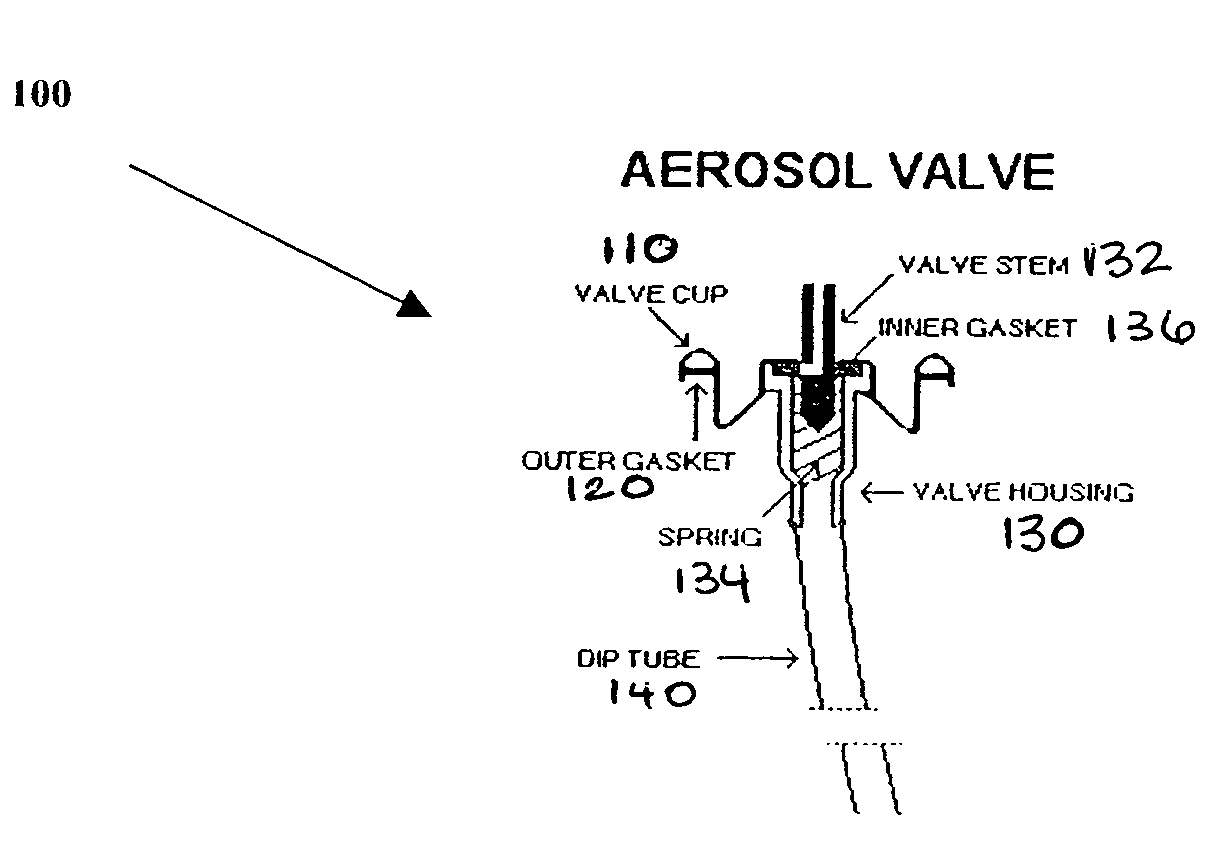
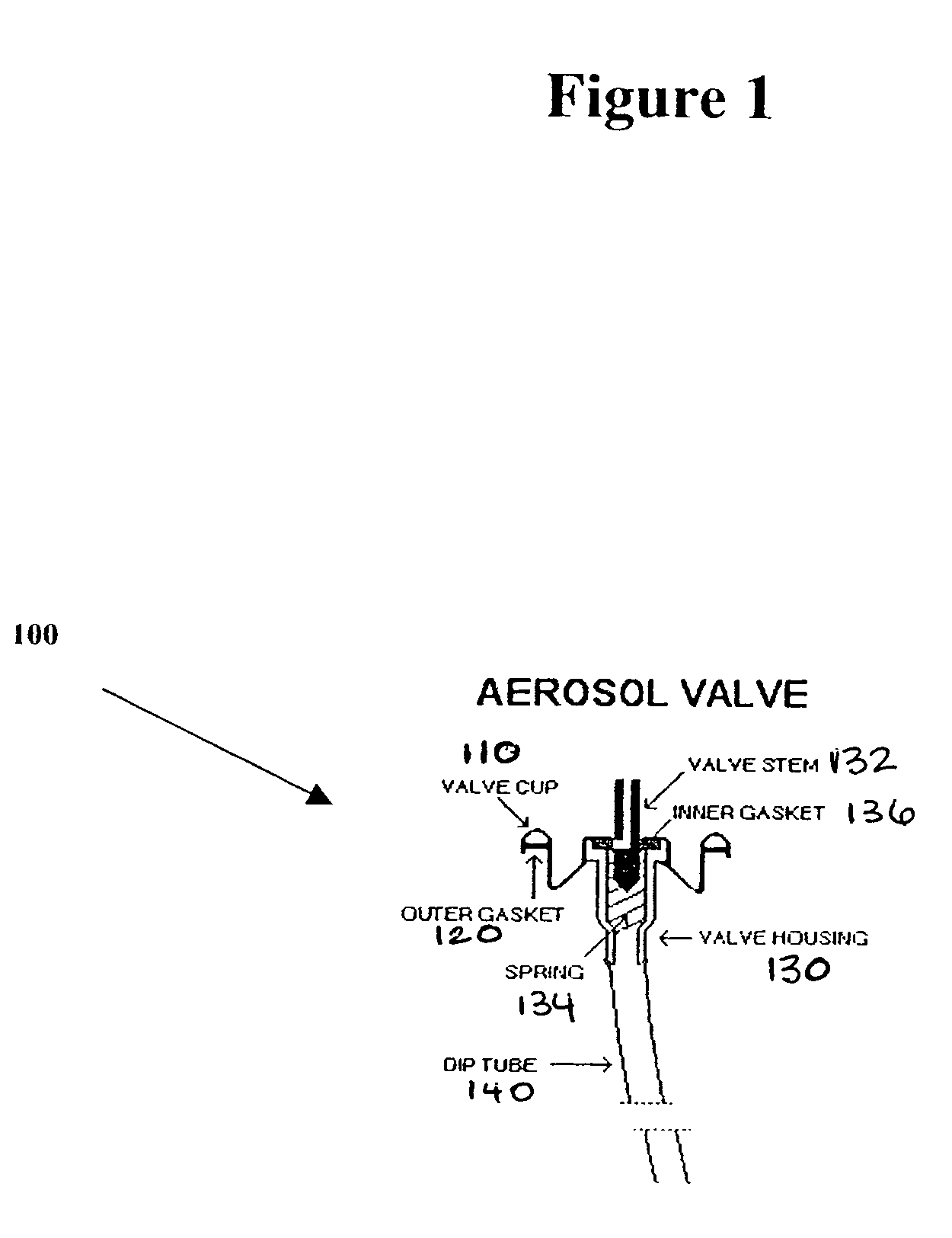
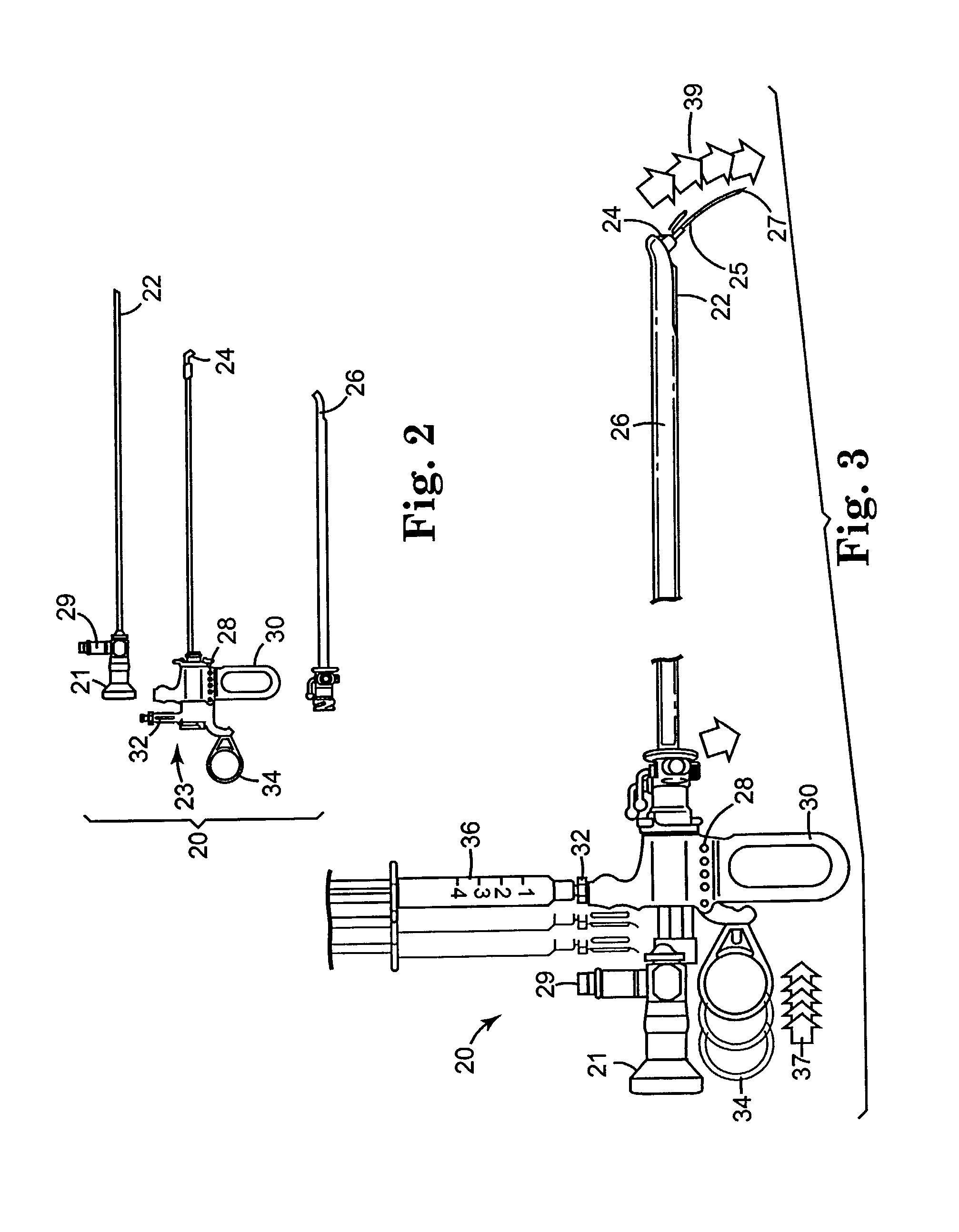
Steroid kit and foamable composition and uses thereof
InactiveUS20060018937A1Preventing and alleviatingCosmetic preparationsSenses disorderActive agentFilm-forming agent
A composition and therapeutic kit including an aerosol packaging assembly including a container accommodating a pressurized product and an outlet capable of releasing a foamable composition, including a steroid as a foam. The pressurized product includes a foamable composition including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam; wherein the pressurized product comprises a foamable composition including: i. a steroid; ii. at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, a polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight; iii. a surface-active agent; iv. about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent; v. water; and vi. liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition. The composition further may include a therapeutically active foam adjuvant, selected from the group consisting of a fatty alcohol, a fatty acid, a hydroxyl fatty acid; and mixtures thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
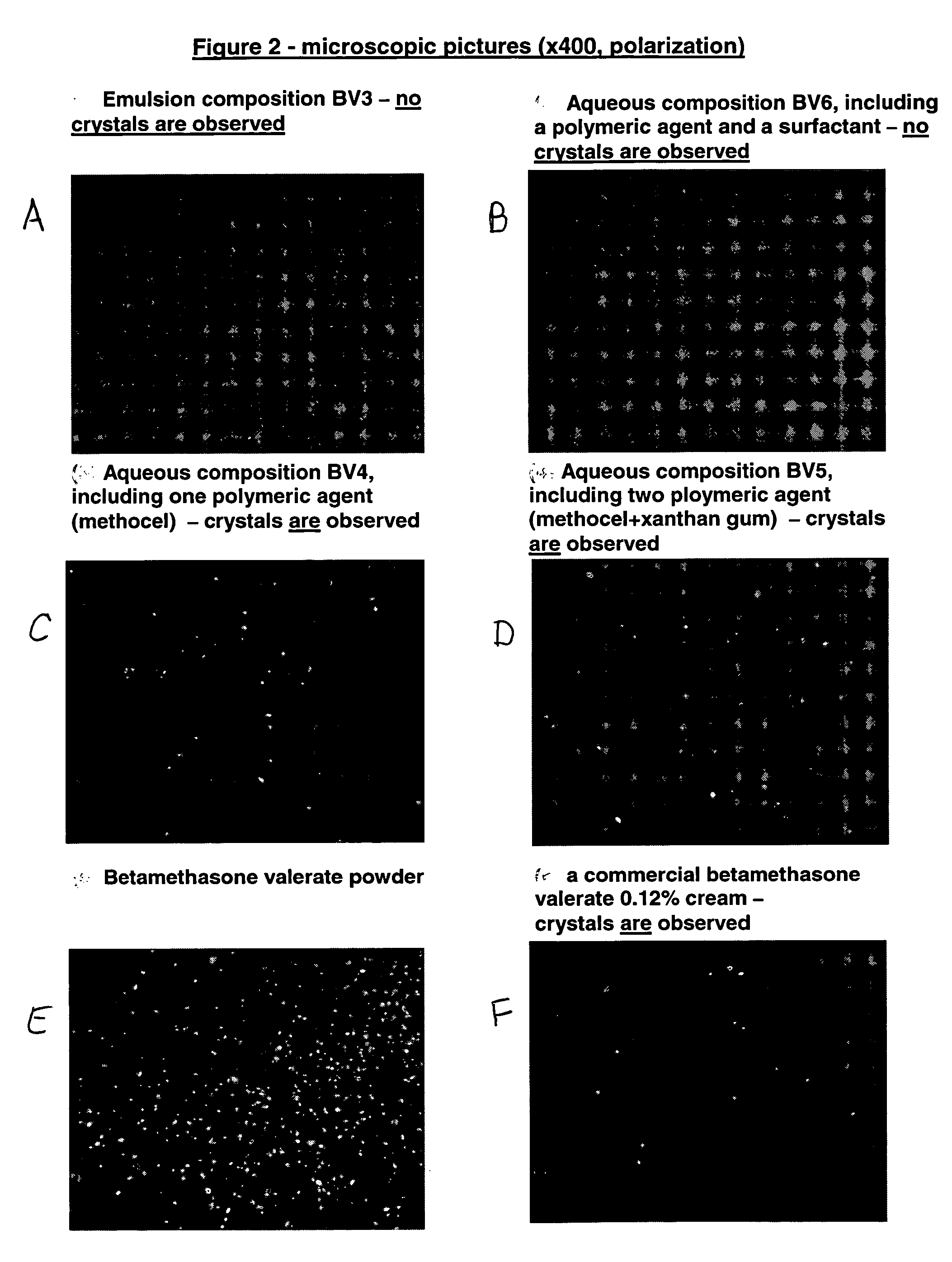
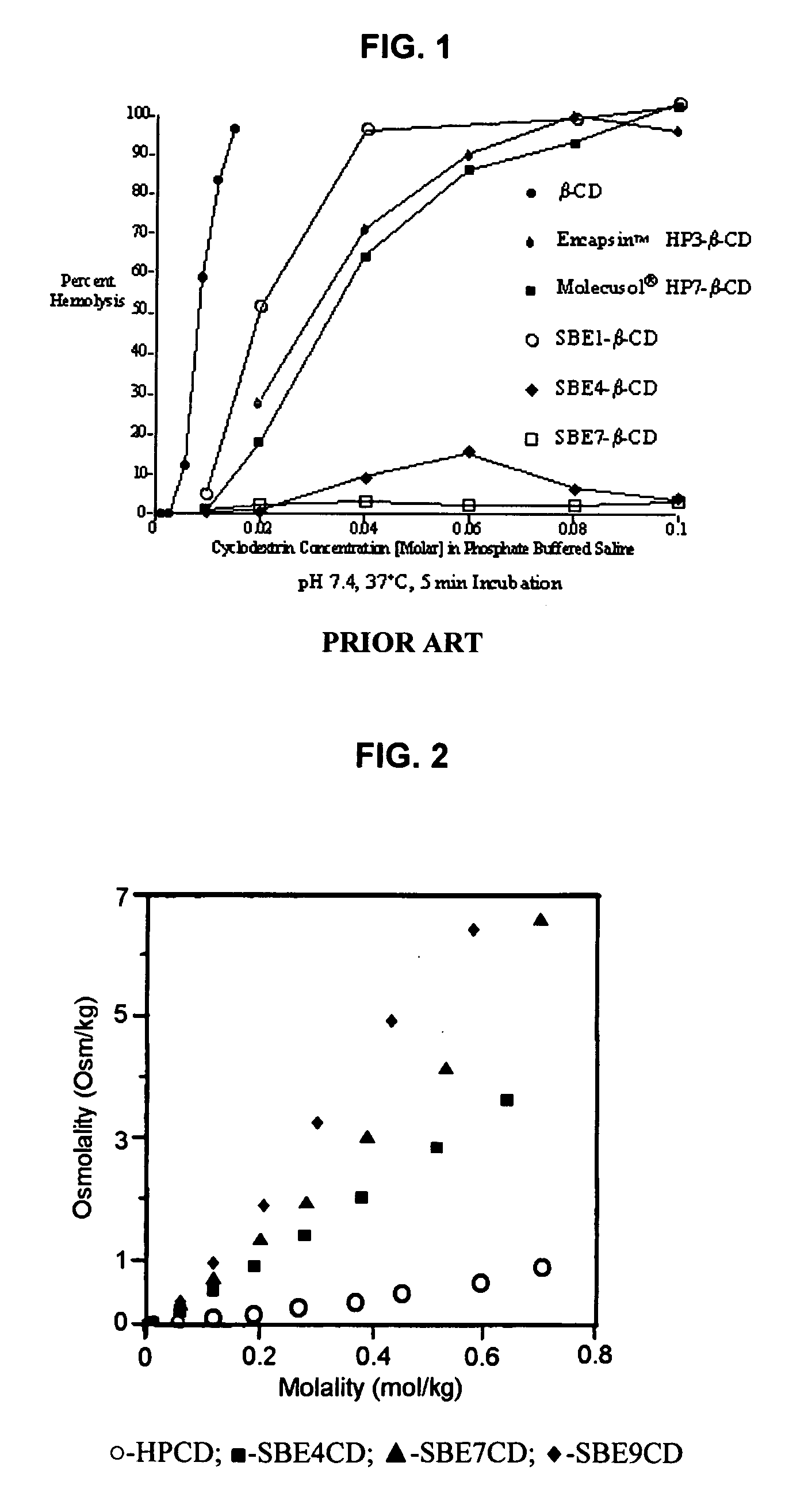
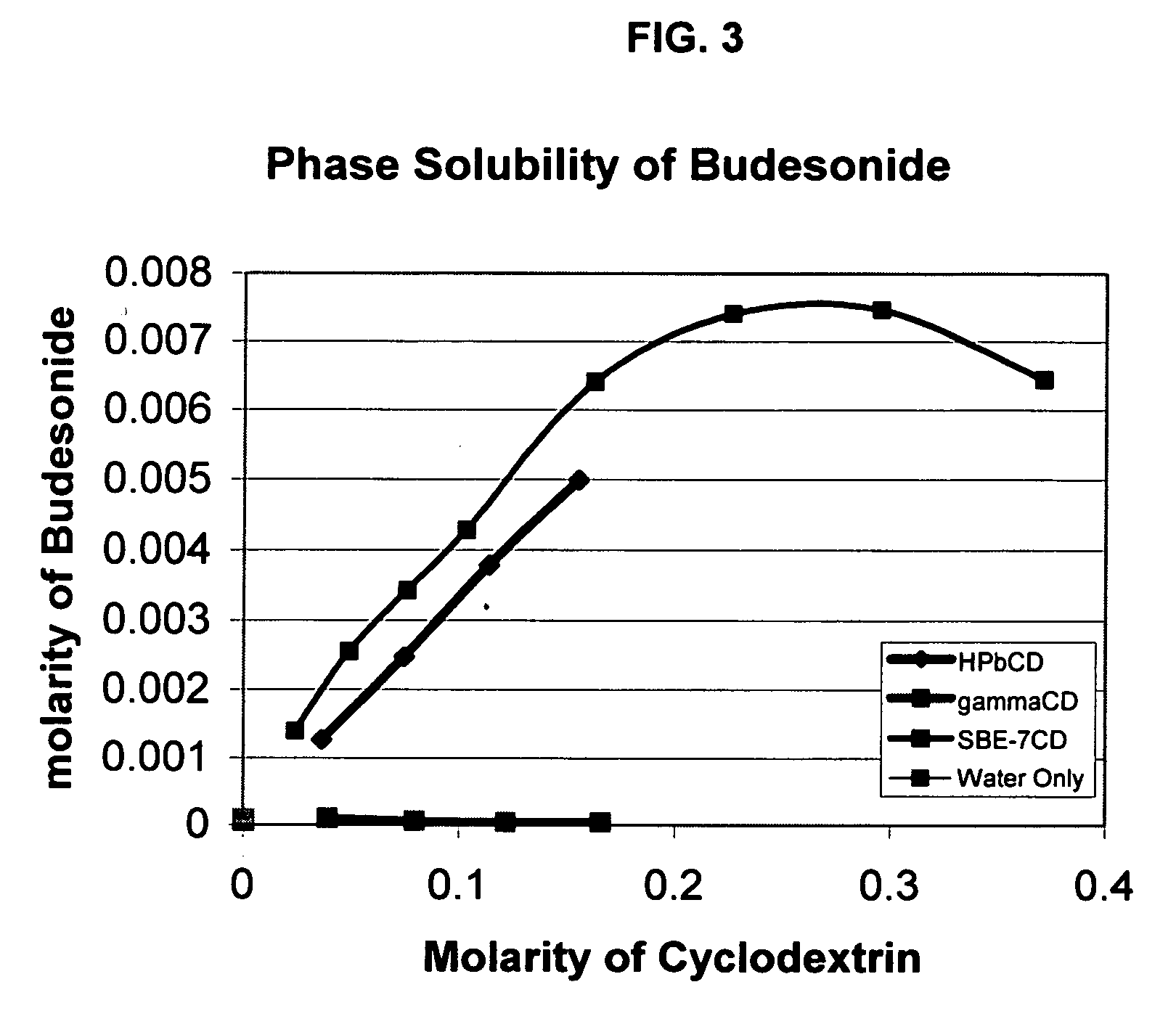
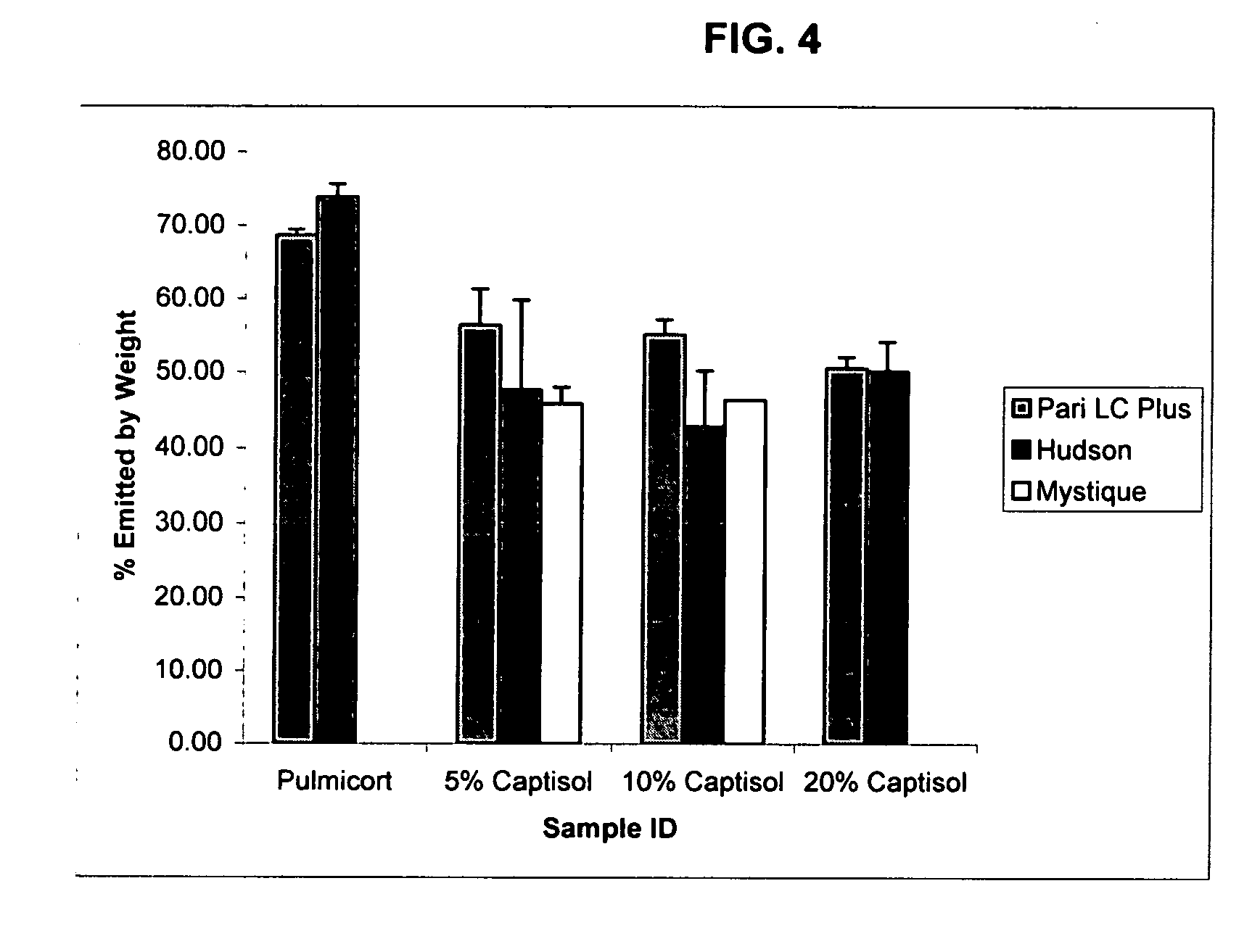
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Regimen for treating prostate tissue and surgical kit for use in the regimen
InactiveUS7015253B2Decreasing prostate sizeSmall sizeBiocideHydroxy compound active ingredientsSteroidal antiandrogenRegimen
The present invention provides treatment regimens for treating diseased prostate tissue, including the steps of chemically ablating prostate tissue and coadministering an antiandrogen. In some embodiments, prostate tissue is chemically ablated by injection of ethanol, or an injectable gel comprising ethanol, into prostate tissue. Steroidal and non-steroidal antiandrogens are suitable antiandrogens. One suitable non-steroidal antiandrogen is bicalutamide. The treatment regimen is suitable for treatment of prostate tissue diseases including benign prostatic hyperplasia and prostatic carcinoma. The invention further provides a treatment regimen for treating benign prostatic hyperplasia, including the steps of damaging prostate tissue and coadministering an antiandrogen. Also provided by the present invention is a kit for treating a human male, including a means for necrosing prostate tissue, an antiandrogen drug, and a means for administering the antiandrogen drug. A kit including a first surgical device for delivering a chemoablation fluid to prostate tissue transurethrally, an antiandrogen drug such as bicalutamide, and a second surgical device for administering the antiandrogen drug, is further provided.
Owner:BOSTON SCI SCIMED INC
Topologically segregated, encoded solid phase libraries comprising linkers having an enzymatically susceptible bond
The invention relates to libraries of synthetic test compound attached to separate phase synthesis supports. In particular, the invention relates to libraries of synthetic test compound attached to separate phase synthesis supports that also contain coding molecules that encode the structure of the synthetic test compound. The molecules may be polymers or multiple nonpolymeric molecules. Each of the solid phase synthesis support beads contains a single type of synthetic test compound. The synthetic test compound can have backbone structures with linkages such as amide, urea, carbamate (i.e., urethane), ester, amino, sulfide, disulfide, or carbon-carbon, such as alkane and alkene, or any combination thereof. Examples of subunits suited for the different linkage chemistries are provided. The synthetic test compound can also be molecular scaffolds, such as derivatives of monocyclic of bicyclic carbohydrates, steroids, sugars, heterocyclic structures, polyaromatic structures, or other structures capable of acting as a scaffolding. Examples of suitable molecular scaffolds are provided. The invention also relates to methods of synthesizing such libraries and the use of such libraries to identify and characterize molecules of interest from among the library of synthetic test compound.
Owner:AVENTIS PHARMA INC
Compositions, formulations and kit with anti-sense oligonucleotide and anti-inflammatory steroid and/or obiquinone for treatment of respiratory and lung disesase
InactiveUS20070021360A1Decreased airwayOrganic active ingredientsBiocideDiseaseAntiendomysial antibodies
A pharmaceutical composition and formulations comprise preventative, prophylactic or therapeutic amounts of an oligo(s) anti-sense to a specific gene(s) or its corresponding mRNA(s), and a glucocorticoid and / or non-glucocorticoid steroid or a ubiquinone or their salts. The agents, composition and formulations are used for treatment of ailments associated with impaired respiration, bronchoconstriction, lung allergy(ies) or inflammation, and abnormal levels of adenosine, adenosine receptors, sensitivity to adenosine, lung surfactant and ubiquinone, such as pulmonary fibrosis, vasoconstriction, inflammation, allergies, allergic rhinitis, asthma, impeded respiration, lung pain, cystic fibrosis, bronchoconstriction, COPD, RDS, ARDS, cancer, and others. The present treatment is effectively administered by itself for conditions without known therapies, as a substitute for therapies exhibiting undesirable side effects, or in combination with other treatments, e.g. before, during and after other respiratory system therapies, radiation, chemotherapy, antibody therapy and surgery, among others. Each of the agents of this invention may be administered directly into the respiratory system so that they gain direct access to the lungs, or by other effective routes of administration. A kit comprises a delivery device, the agents and instructions for its use.
Owner:EPIGENESIS PHARMA LLC
Novel Compositions for the Delivery of Negatively Charged Molecules
InactiveUS20100036115A1Facilitated DiffusionReduce deliverySugar derivativesDipeptide ingredientsMembrane permeabilityAntisense nucleic acid
This invention features permeability enhancer molecules, and methods, to increase membrane permeability of various molecules, such as nucleic acids, polynucleotides, oligonucleotides, enzymatic nucleic acid molecules, antisense nucleic acid molecules, 2-5A antisense chimeras, triplex forming oligonucleotides, decoy RNAs, dsRNAs, siRNAs, aptamers, or antisense nucleic acids containing nucleic acid cleaving chemical groups, peptides, polypeptides, proteins, carbohydrates, steroids, metals and small molecules, thereby facilitating cellular uptake of such molecules.
Owner:SIRNA THERAPEUTICS INC
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
Nanoparticulate corticosteroid and antihistamine formulations
InactiveUS20060216353A1Less liver toxicityUseful in prophylaxis and chronic treatment of asthmaBiocidePowder deliveryPediatric patientMicroparticle
Compositions comprising a nanoparticulate corticosteroid and an antihistamine are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining an antihistamine with a nanoparticulate corticosteroid in a single formulation results in improved efficacy.
Owner:ALKERMES PHARMA IRELAND LTD
Vitamin D3 mimics
The present invention relates to non-secosteroidal compounds which activate and modulate the vitamin D receptor (VDR). Because compounds of the present invention display many of the beneficial properties of 1,25(OH)2D3, but with reduced calcium mobilization effects, they may be used advantageously to treat and prevent conditions that show vitamin D sensitivity. Such disease states typically show abnormal calcium regulatory, abnormal immune responsive, hyperproliferative, and / or neurodegenerative characteristics.
Owner:LIGAND PHARMA INC
Nitric oxide donor composition and method for treatment of anal disorders
A pharmaceutical composition contains a nitric oxide donor and advantageously an optional corticosteroid and / or topical anesthetic. The composition is useful in a method for treating anal disorders such as anal fissure, anal ulcer, hemorrhoidal disease, levator spasm, and so forth, by topical application to or proximate the affected area.
Owner:STRAKAN INT S A R L
Steroids as agonists for FXR
InactiveUS7138390B2Organic active ingredientsIn-vivo radioactive preparationsCombinatorial chemistryAgonist
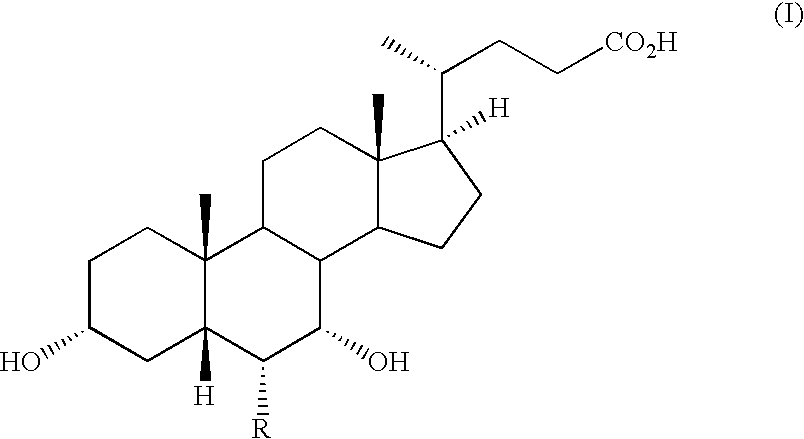
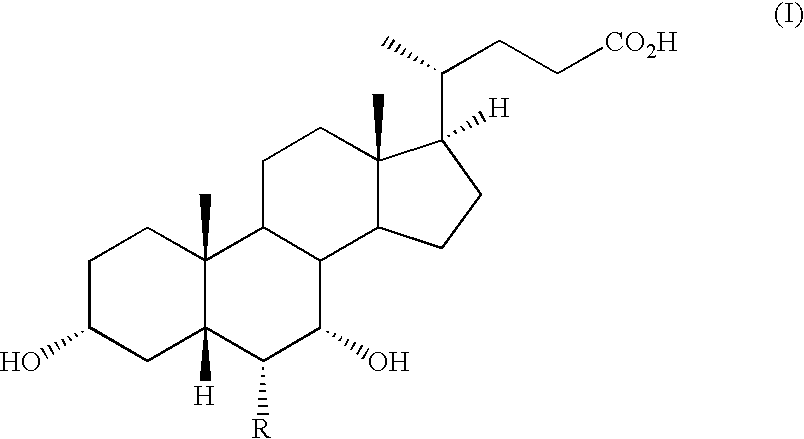
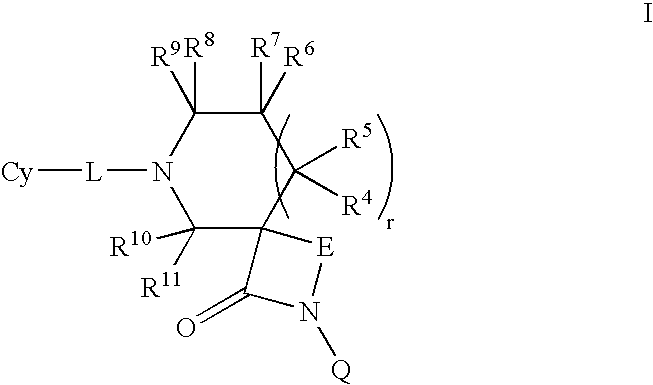
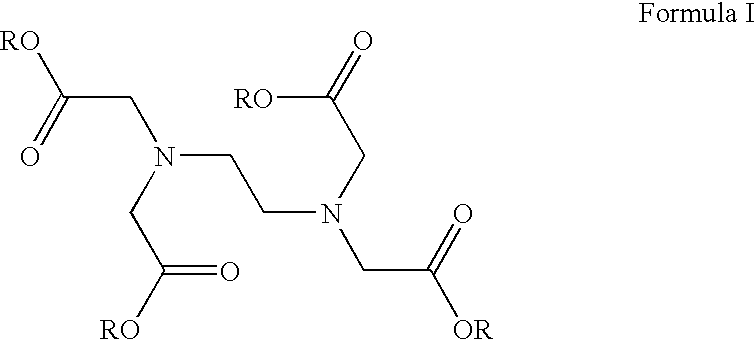
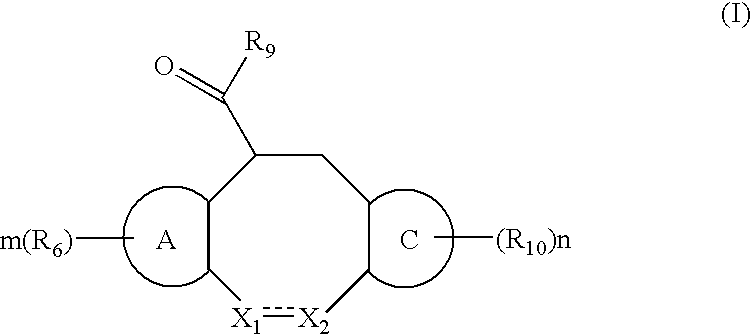
The invention relates to compounds of formula (I):wherein R is ethyl and pharmaceutically acceptable salts, solvates or amino acid conjugates thereof. The compounds of formula (I) are useful as FXR agonists.
Owner:INTERCEPT PHARMA INC
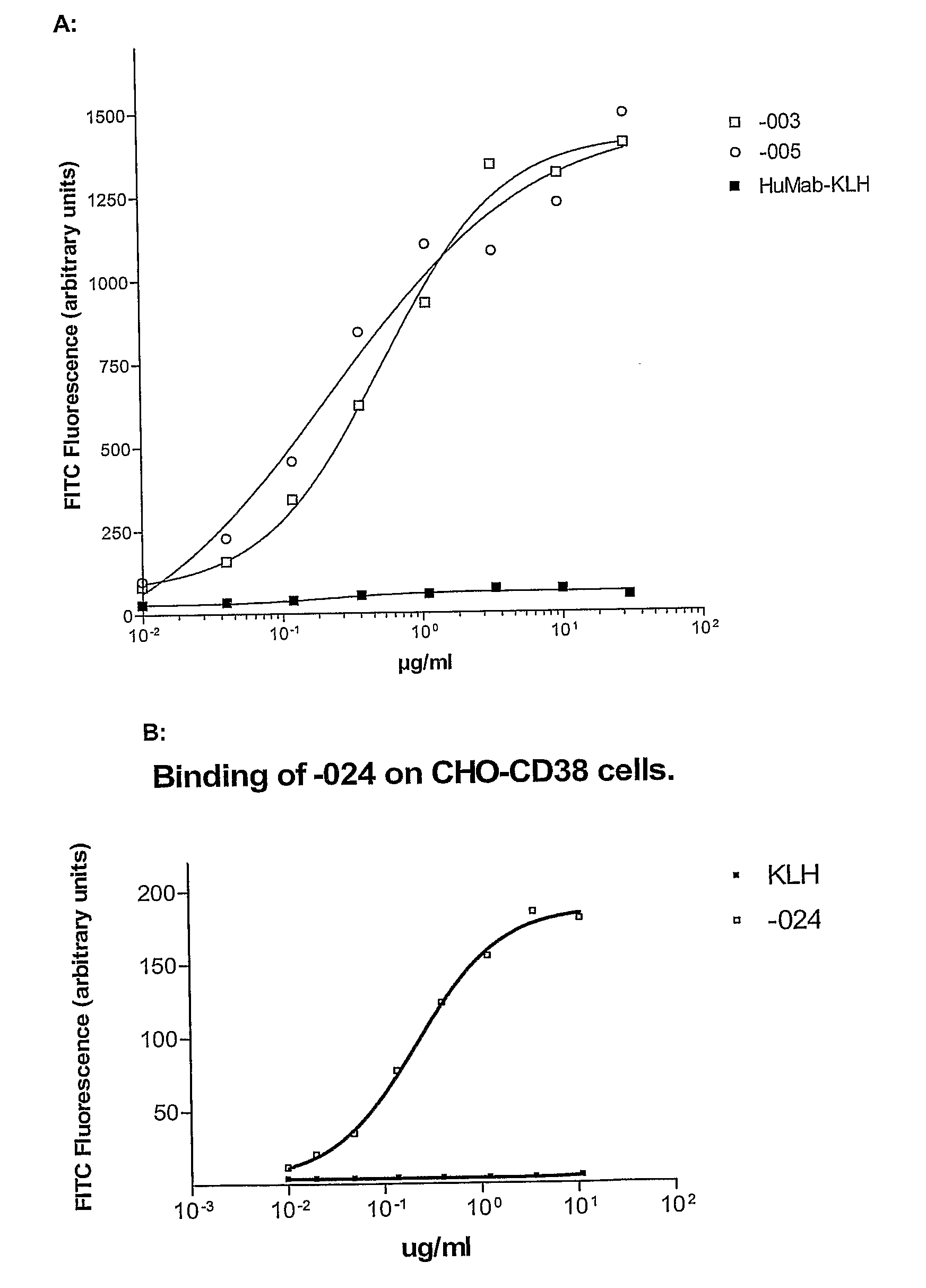
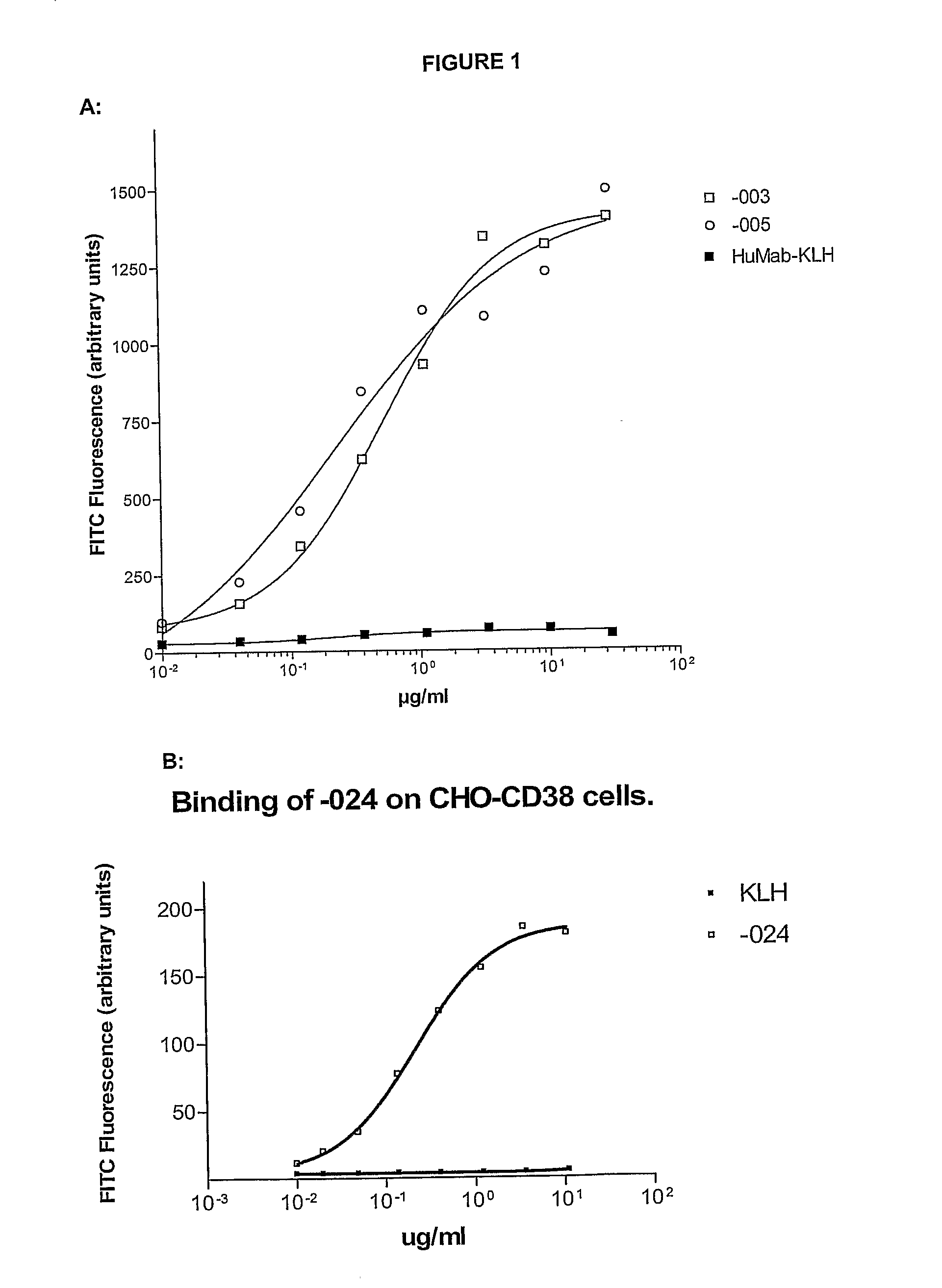
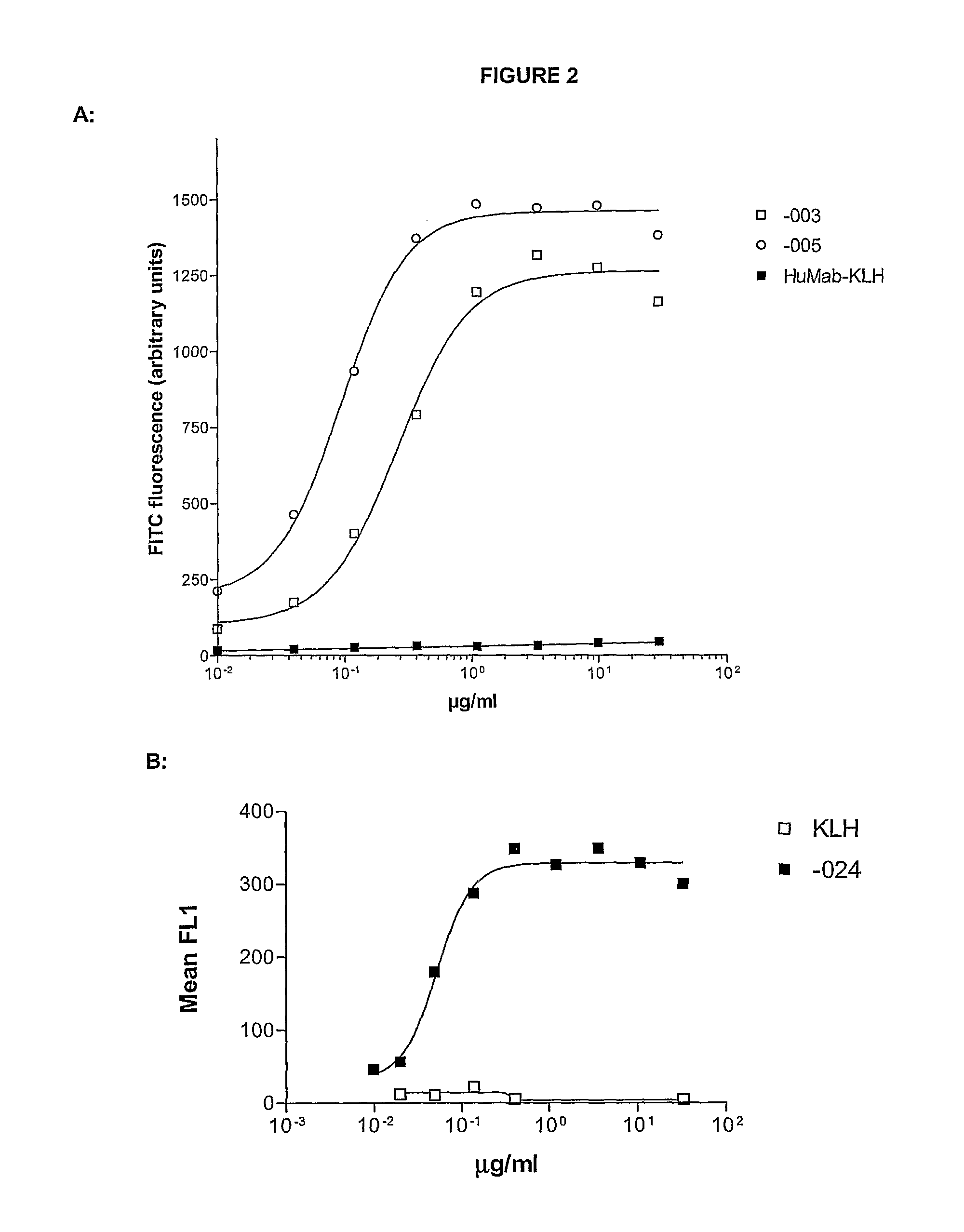
Combination treatment of cd38-expressing tumors
ActiveUS20100092489A1Good curative effectImprove survivalBoron compound active ingredientsSkeletal disorderAntiendomysial antibodiesOncology
The invention relates to novel method for the treatment of cancer using a combination therapy comprising an antibody that binds CD38, a corticosteroid and a non- corticosteroid chemotherapeutic agent.
Owner:GENMAB AS
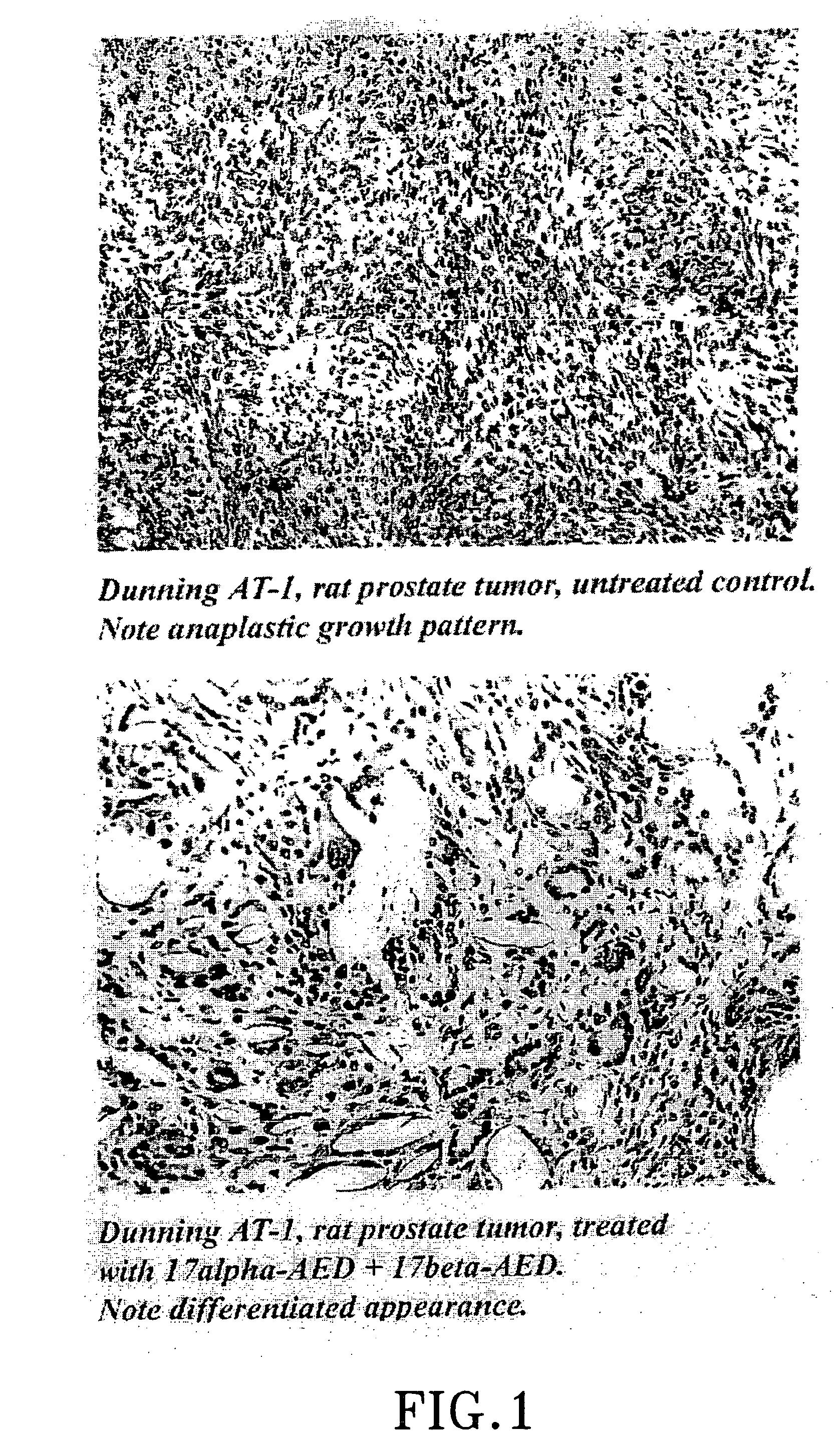
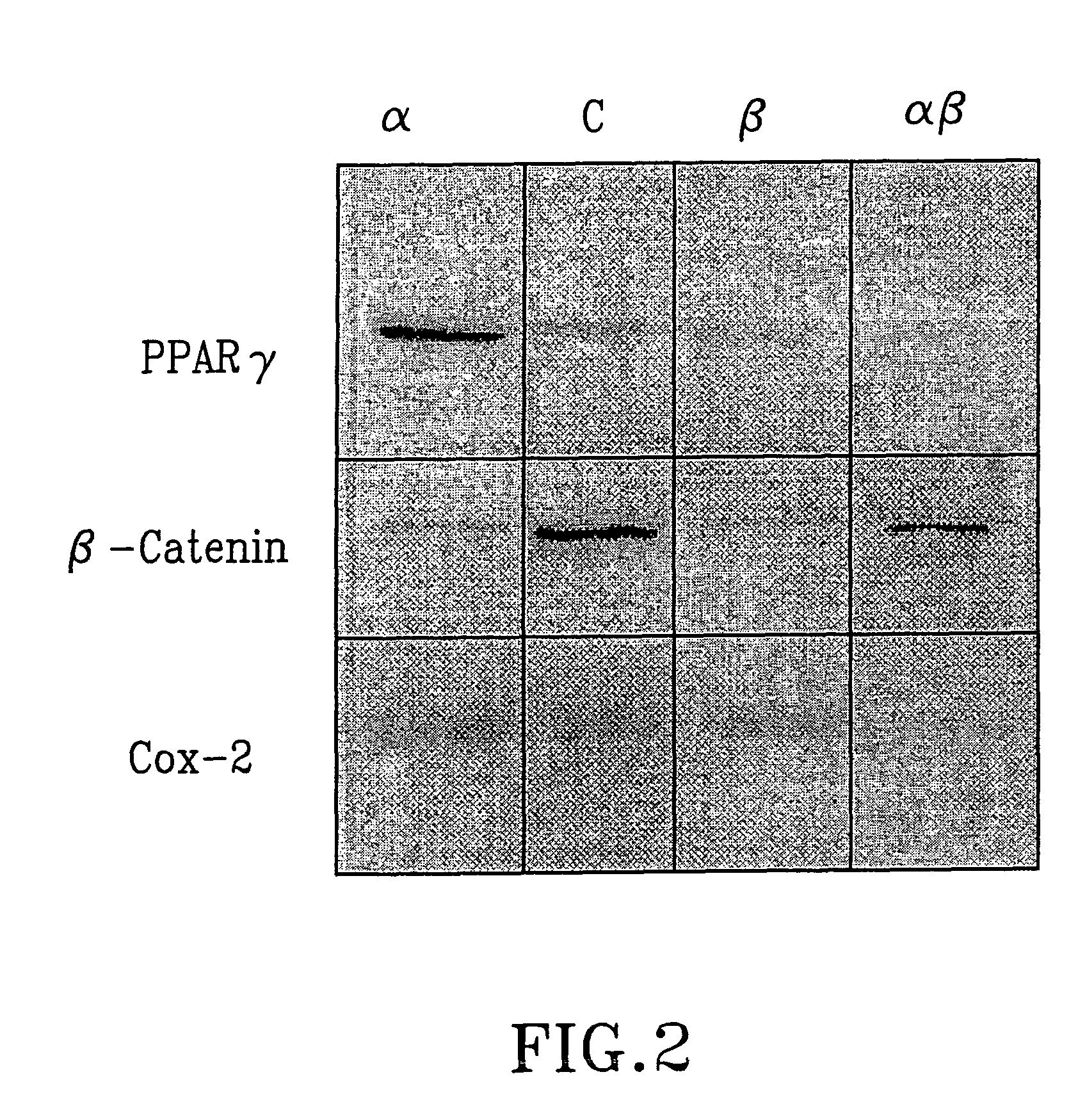
Treatment of tumours
InactiveUS20050192262A1Good curative effectSquelching unwanted PPARγ-activityOrganic active ingredientsSteroidsDiseaseAndrostane
The present invention refers to steroid derivatives for use as medicaments. More specifically, the invention also relates to the use of a steroid derivative of 5-androstene-, 5-pregnenolone or corresponding saturated derivatives (androstane- or pregnane-) in the manufacture of a medicament for the treatment of a benign and / or malignant tumour, which medicament is capable of interrupting disturbances in Wnt-signaling, such as cell-cycle arrest in G1-phase, and / or providing an angiostatic effect. Examples of such steroid derivatives are -5-androstene-17-ol, androstane-17-ol-pregnane-17-ol or pregnane-17-ol derivatives. In a further aspect, the invention relates to a method of producing a medicament for the treatment of a benign and / or malignant tumour and / or an inflammatory condition comprising the steps of contacting 5-androstane-3β,17-diol or androstane-3β-diol, an enzyme and a sulfotransferase to provide 5-androstene-17-ol-3β-sulfate or corresponding andros tane derivative (17-AEDS or 17-AADS); and mixing the 17-AEDS or 17-AADS so produced with a suitable carrier; whereby a medicament which is capable of acting as a ligand to peroxisome proliferators-activated receptor-(PPAR) is produced.
Owner:HAGSTROM TOMAS
Inhibitors of the 11-beta-hydroxysteroid dehydrogenase type 1 enzyme
ActiveUS20060149070A1Improve throughputLimit deliveryOrganic active ingredientsOrganic chemistryInsulin dependentEnzyme inhibitor
The present invention relates to compounds that are inhibitors of the 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme. The present invention further relates to the use of inhibitors of 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme for the treatment of non-insulin dependent type 2 diabetes, insulin resistance, obesity, lipid disorders, metabolic syndrome and other diseases and conditions that are mediated by excessive glucocorticoid action.
Owner:ABBVIE INC
Corticosteroid-containing pharmaceutical composition
InactiveUS7078058B2Improve permeabilityEasy to handleOrganic active ingredientsBiocideScalp psoriasisDisease
A foamable pharmaceutical composition comprising a corticosteroid, a quick-break foaming agent, a propellant and a buffering agent, sufficient to buffer the composition to within the range of pH 3.0 to 6.0 is disclosed. The quick-break foaming agent typically comprises an aliphatic alcohol, water, a fatty alcohol and a surface active agent. Due to the nature of the compositions of the invention, they are especially well-suited for use in the treatment of various skin diseases, and in particular, in the treatment of scalp psoriasis.
Owner:STIEFEL WEST COAST
Methods and compositions for use in treatment of patients with autoantibody positive disease
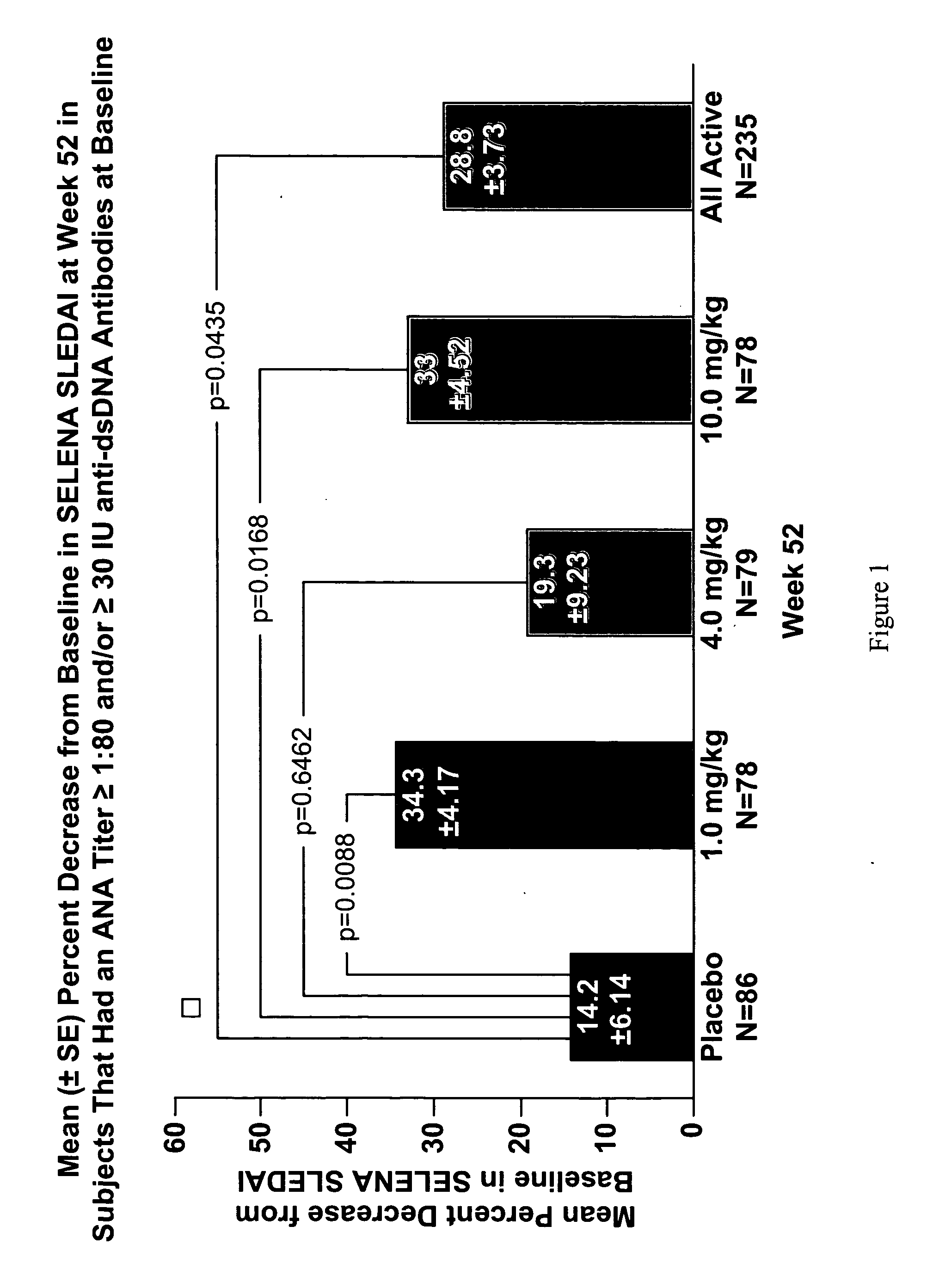
InactiveUS20070086979A1Reduce frequencyReduce in quantityPeptide/protein ingredientsAntipyreticAnti-dsDNA antibodiesSystemic lupus erythematosus
The present invention relates to methods and compositions for use in treatment of patients with autoantibody positive disease. In a specific embodiment, the present invention relates to a method of treating a patient that has an ANA titer of 1:80 or greater and / or greater than or equal to 30 IU / ml of anti-dsDNA antibodies in his / her blood plasma or serum comprising administering a therapeutically effective amount of an immunomodulatory agent, such as an antagonist of Neutrokine-alpha. Additionally provided is a method of reducing the frequency and / or quantity of corticosteroid administration to patients. In preferred embodiments, the patient has systemic lupus erythematosus. Methods for determining if a lupus patient is responding to medical treatment are also provided.
Owner:HUMAN GENOME SCI INC
Methods of neuroprotection using neuroprotective steroids and a vitamin d
InactiveUS20110306579A1Inhibiting neurodegenerationPromote physical recoveryBiocideOrganic active ingredientsNervous systemProgesterones
Described herein are compositions and methods for treating or preventing nervous system injury. In particular, the methods and compositions relate to the use of at least one neuroprotective steroid, such as progesterone, and vitamin D.
Owner:EMORY UNIVERSITY
Novel photoacid generator, resist composition, and patterning process
ActiveUS20090274978A1Addressing Insufficient ControlInhibits the formation of defectsPhotosensitive materialsPhoto-taking processesResistHigh energy
Photoacid generators generate sulfonic acids of formula (1a) or (1b) upon exposure to high-energy radiation.R1—COOCH2CF2SO3−H+ (1a)R1—O—COOCH2CF2SO3−H+ (1b)R1 is a monovalent C20-C50 hydrocarbon group of steroid structure which may contain a heteroatom. The bulky steroid structure ensures adequate control of acid diffusion. The photoacid generators are compatible with resins and suited for use in chemically amplified resist compositions.
Owner:SHIN ETSU CHEM IND CO LTD
Lactam compounds and their use as pharmaceuticals
InactiveUS20060116382A1Avoid conversionInhibit productionBiocideSenses disorderDiseaseMineralocorticoid receptor
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1, antagonists of the mineralocorticoid receptor (MR), and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1 and / or diseases associated with aldosterone excess.
Owner:INCYTE
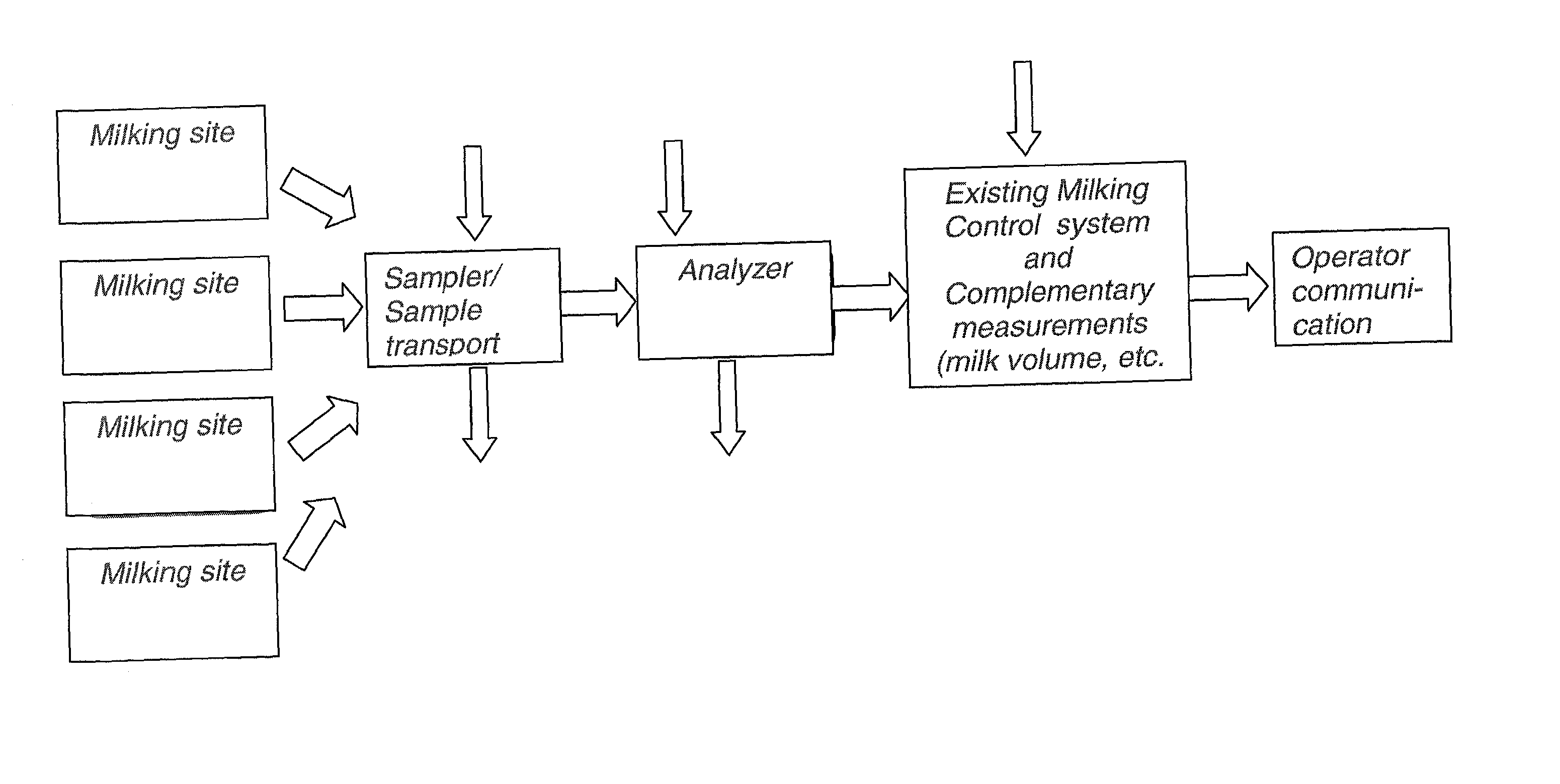
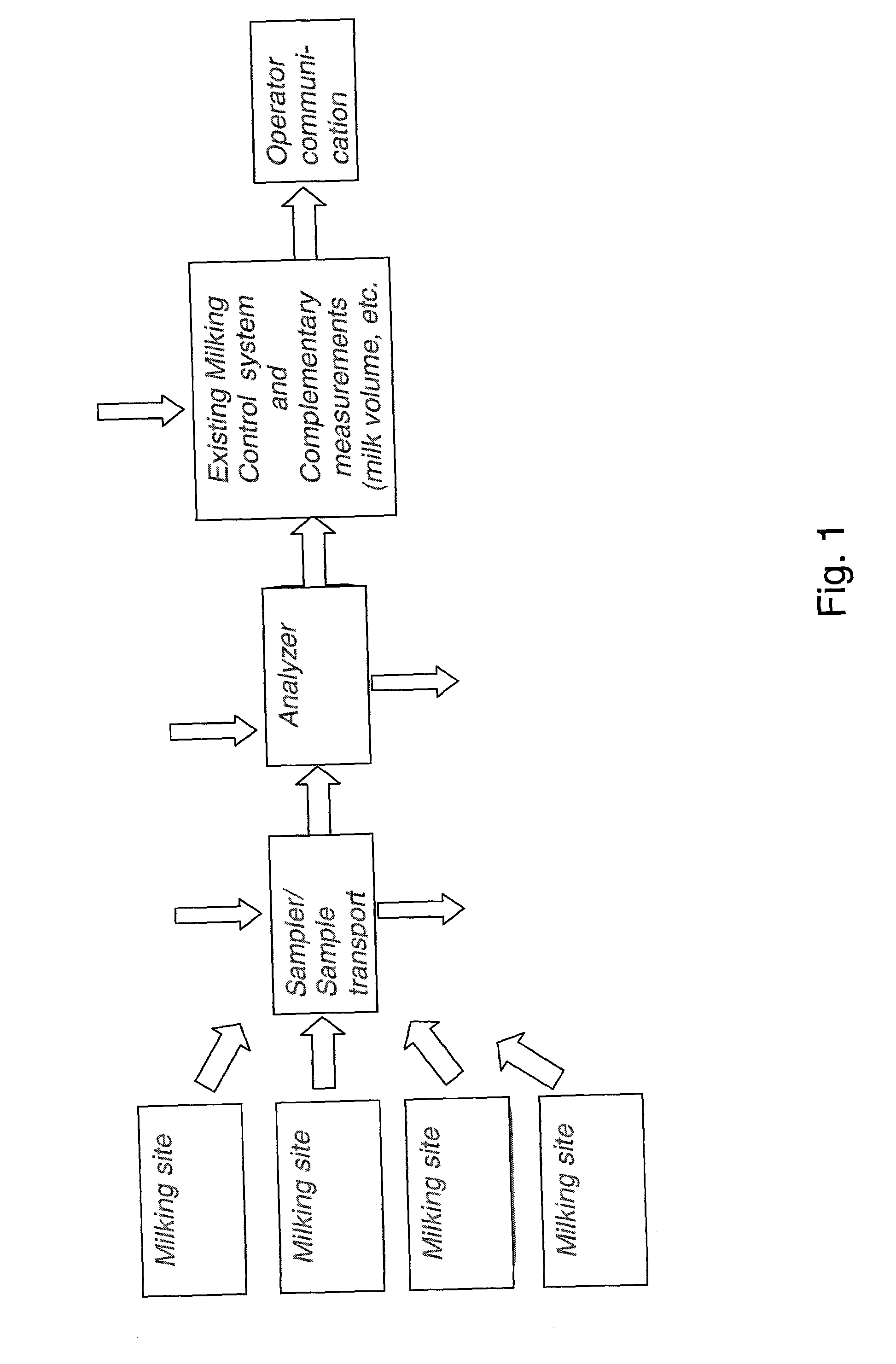
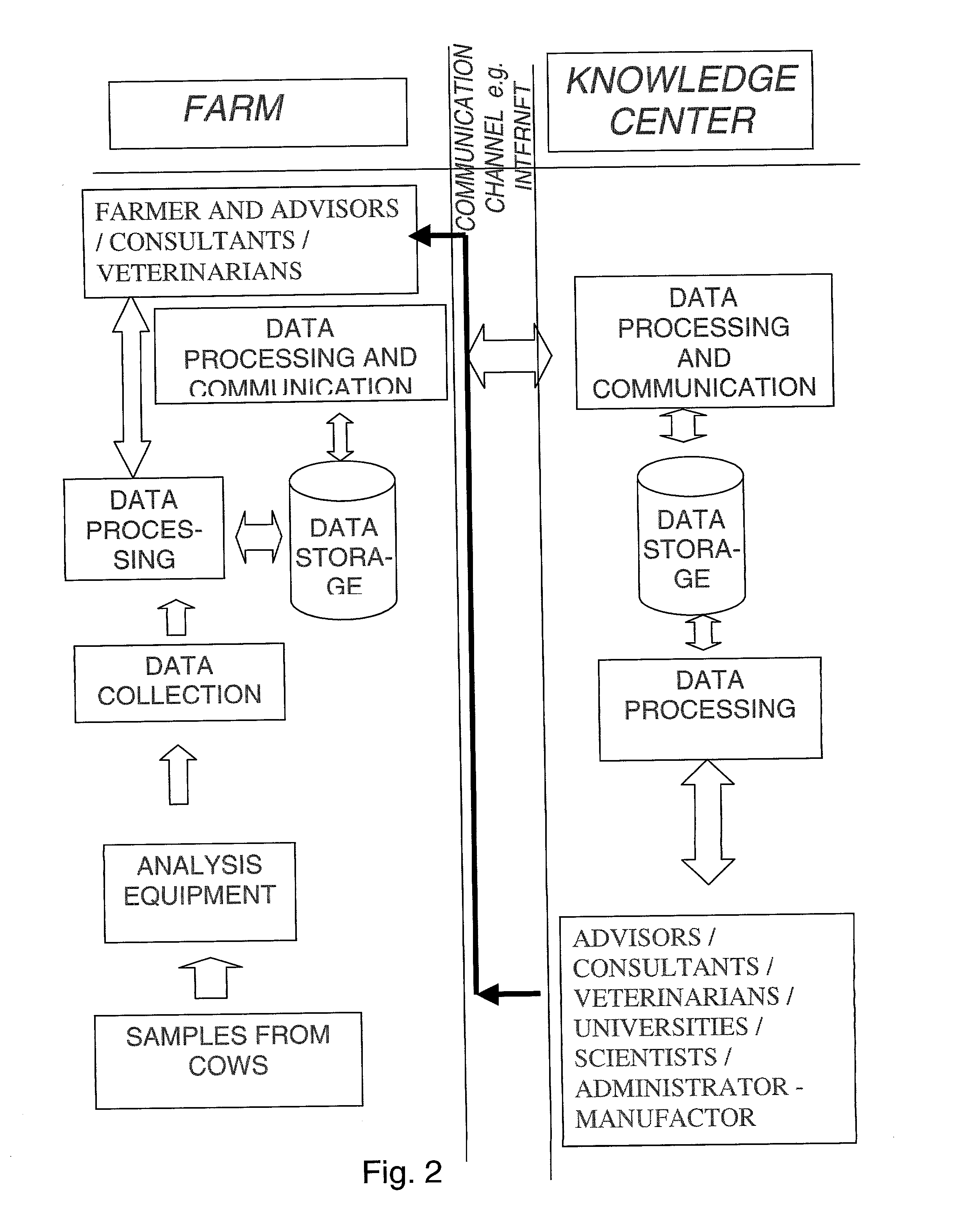
System for optimising the production performance of a milk producing animal herd
InactiveUS20020124803A1Excels in productivityImprove productivitySamplingCathetersReproductive cycleSteroid Compound
A system for optimizing the production performance of a milk producing animal herd is provided. The system comprises milk sampling means, analytical means comprising separate means for analyzing compounds or parameters that in the presence of compounds indicative of the physiological or nutritional condition of the herd member, generates detectable signals, and means for directing a part of the milk sample to each separate analyzing means which is controlled by data for the physiological and nutritional state of a herd member such that the directing means is only activated at pre-selected points in time or at pre-selected time intervals in the production and or lactation cycles. Specific compounds are compounds indicative of mastitis, including beta-N-acetylhexosaminidase (NAGase) E.C. 3.2.1.52 and lactate dehydrogenase (LDH), protein balance, including milk urea nitrogen (MUN) and total protein, ketosis, including acetolactate, beta-hydroxybutyrate, acetone and lipids, fat and state in reproduction cycle, including a steroid or peptide hormone such as progesterone. Furthermore, the system comprises signal detection means for recording and processing the signals, means for data storage and data output means. Additionally there are provided methods for optimizing the production performance of a milk producing animal herd and an apparatus herefor.
Owner:LATTEC
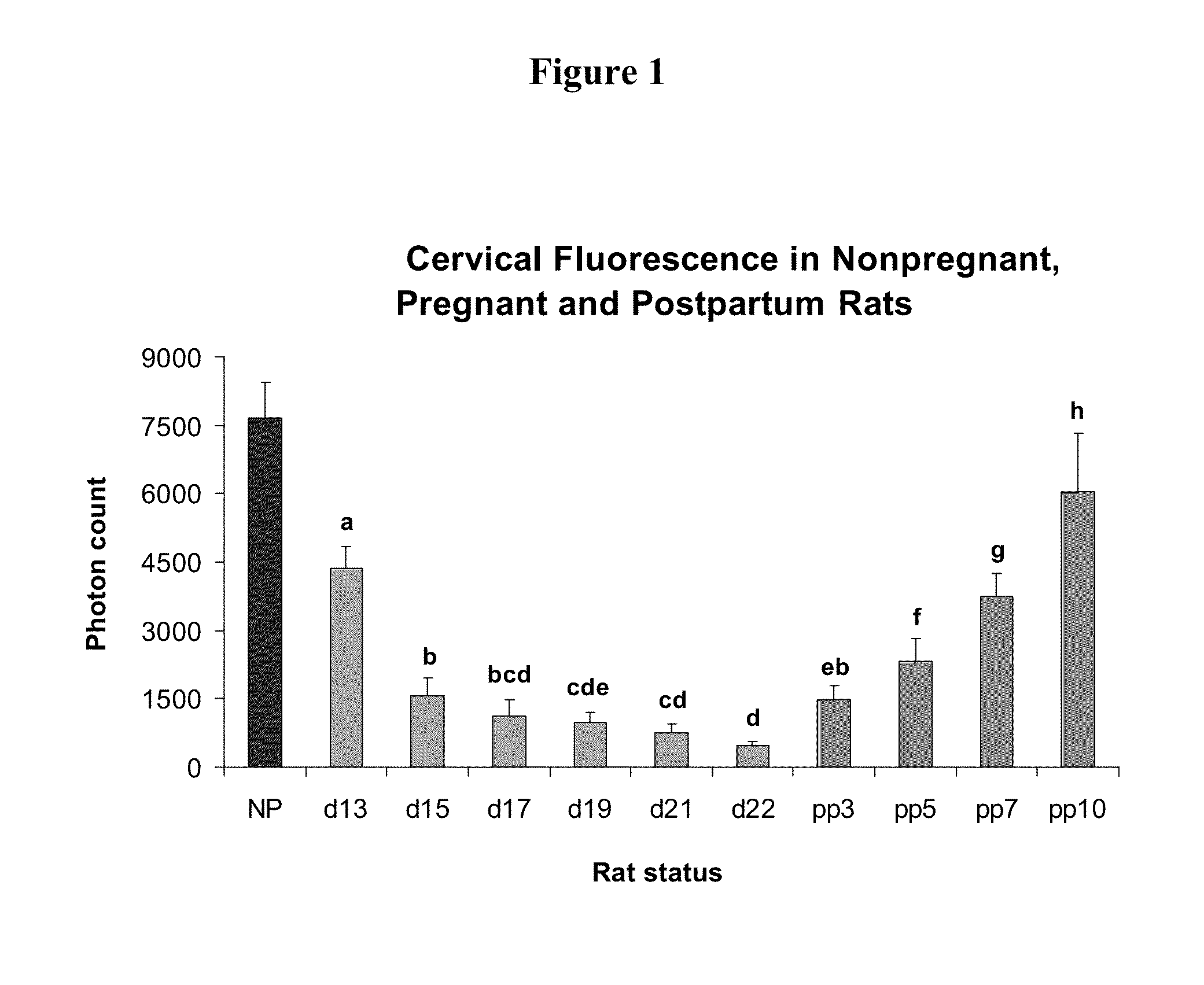
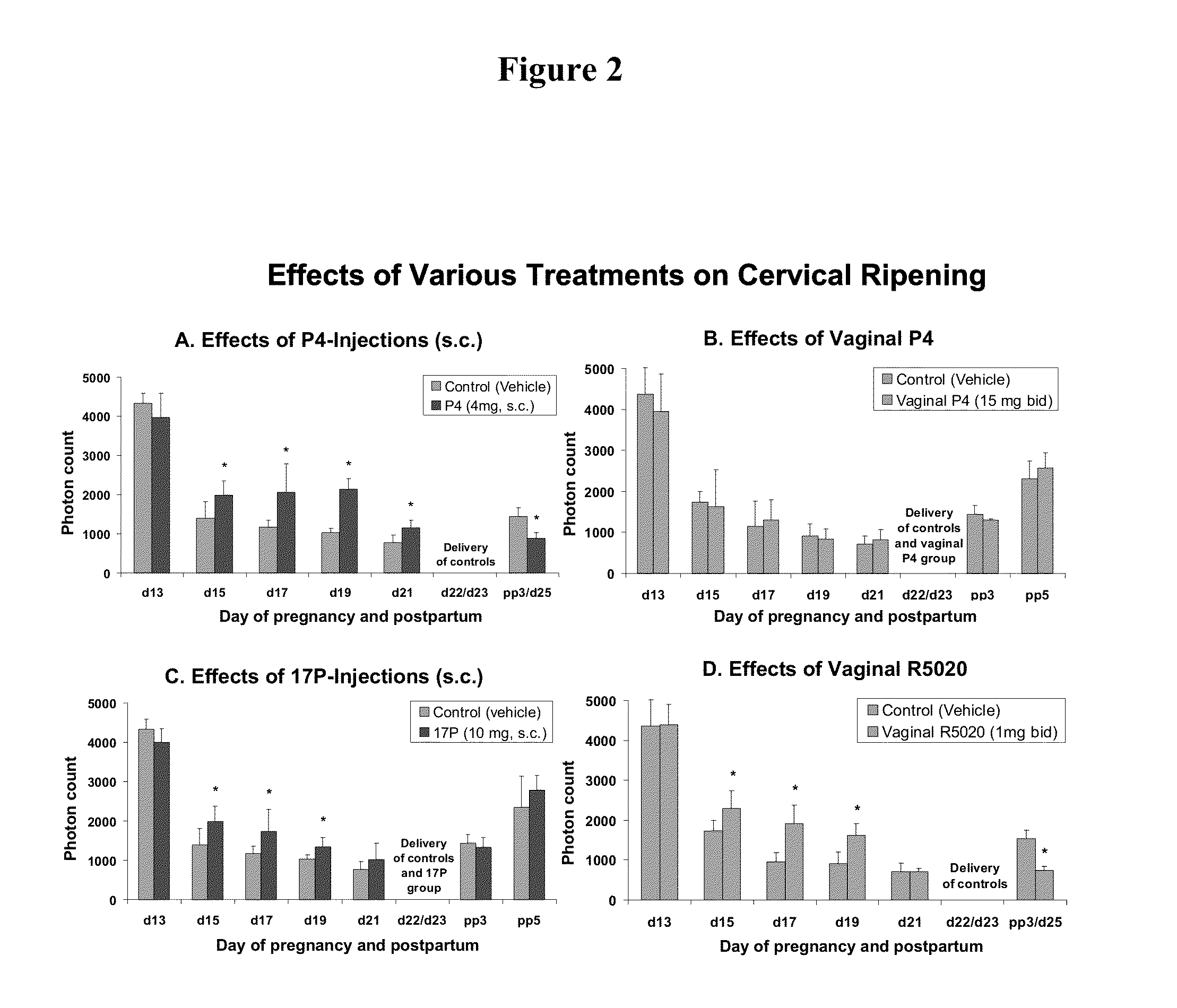
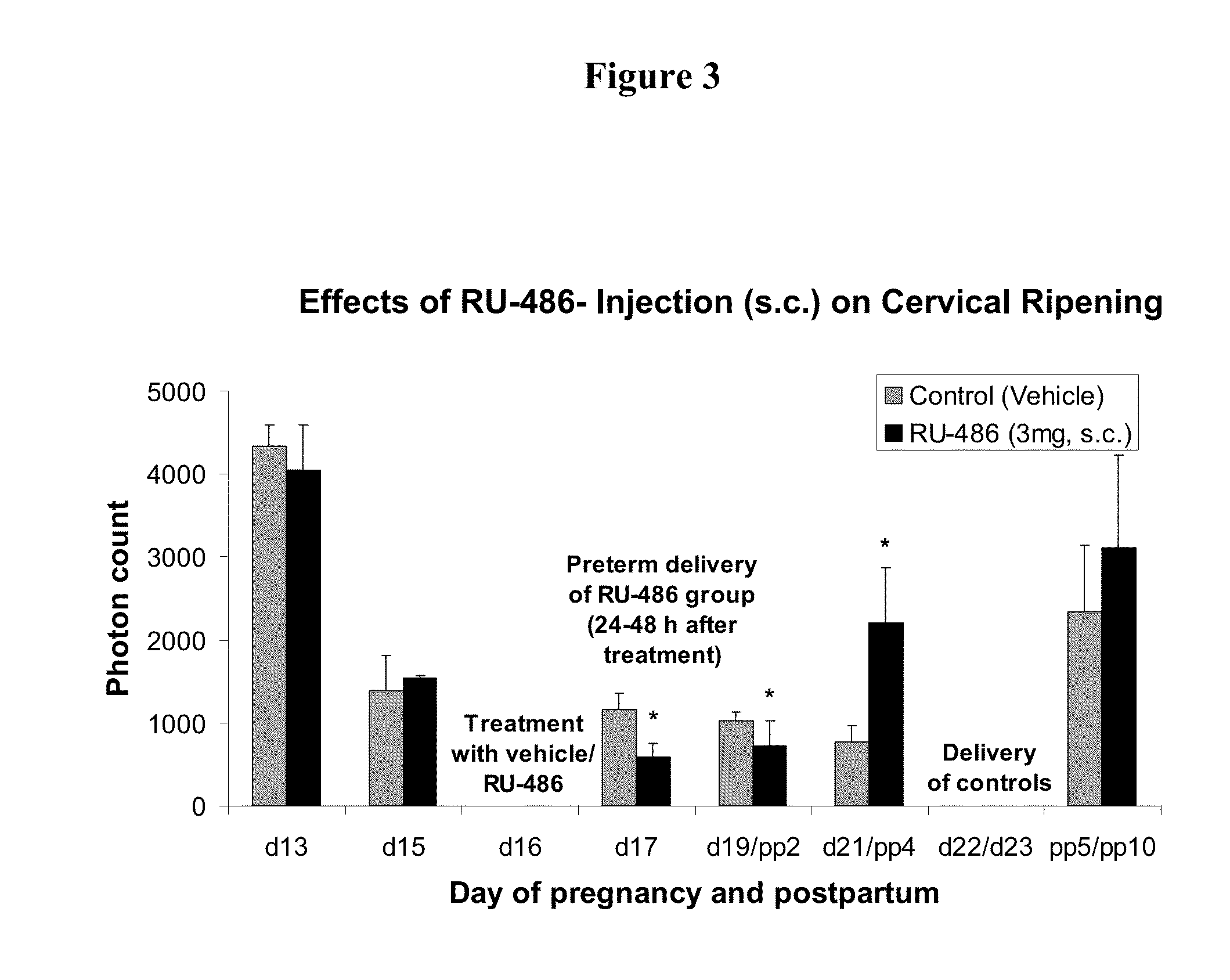
Methods for inhibiting preterm labor and uterine contractility disorders and preventing cervical ripening
InactiveUS20130023505A1Inhibit uterine contractilityInhibit cervical ripeningOrganic active ingredientsPharmaceutical delivery mechanismMyometrial contractilityGynecology
The invention relates to methods and pharmaceutical compositions for inhibiting or preventing preterm birth, inhibiting or delaying cervical ripening, inhibiting myometrial contractility and treating or inhibiting uterine contractility disorders. The methods comprise administering an effective amount of a composition comprising steroid hormones such as soluble progesterone.
Owner:DIGNITY HEALTH
Combination products
Owner:SIGMOID PHARM LIMITED
Hormone receptor functional dimers and methods of their use
InactiveUS7057015B1Enhance possibility of producingIncrease flexibilityFusion with DNA-binding domainSugar derivativesADAMTS ProteinsProtein Unit
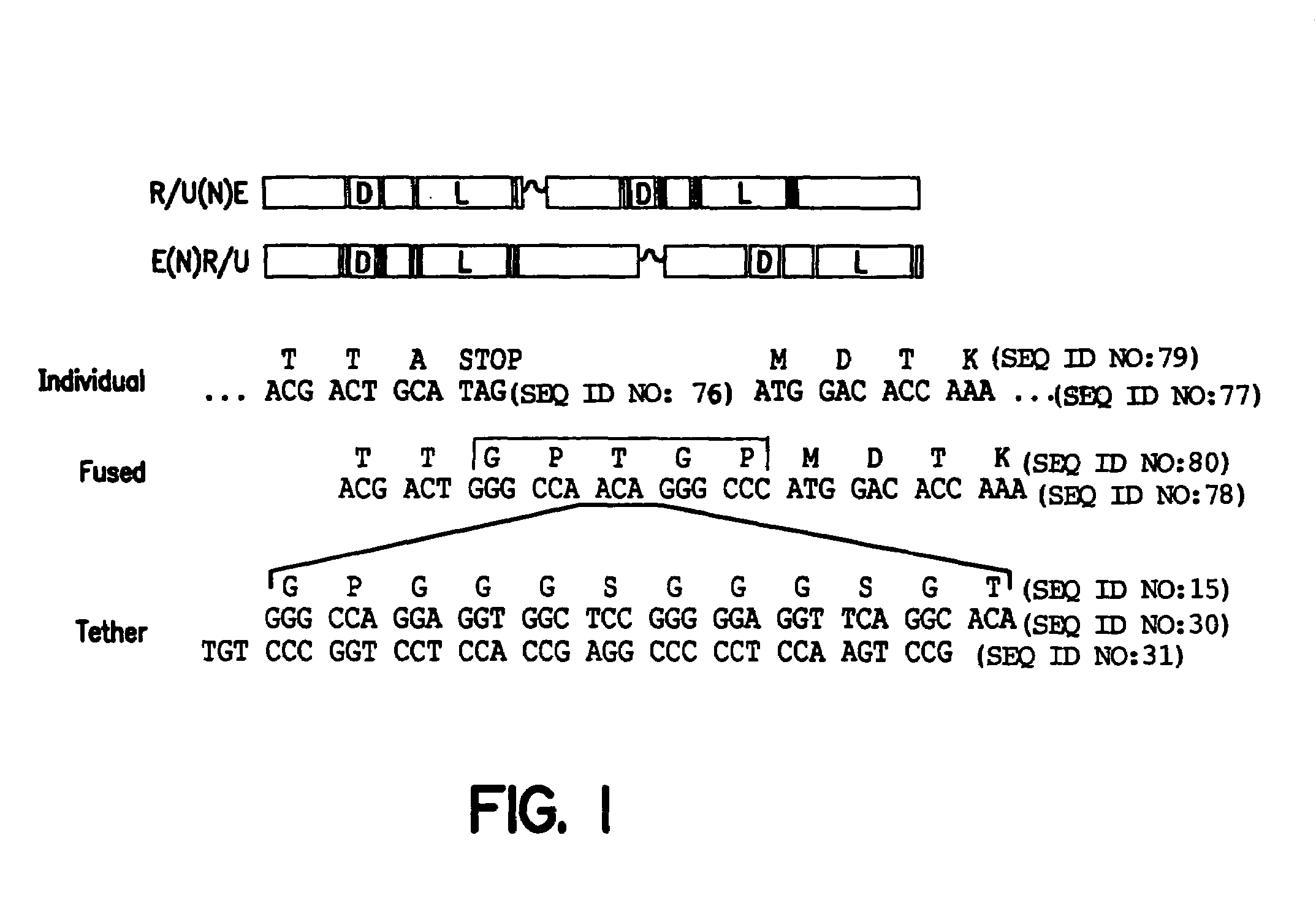
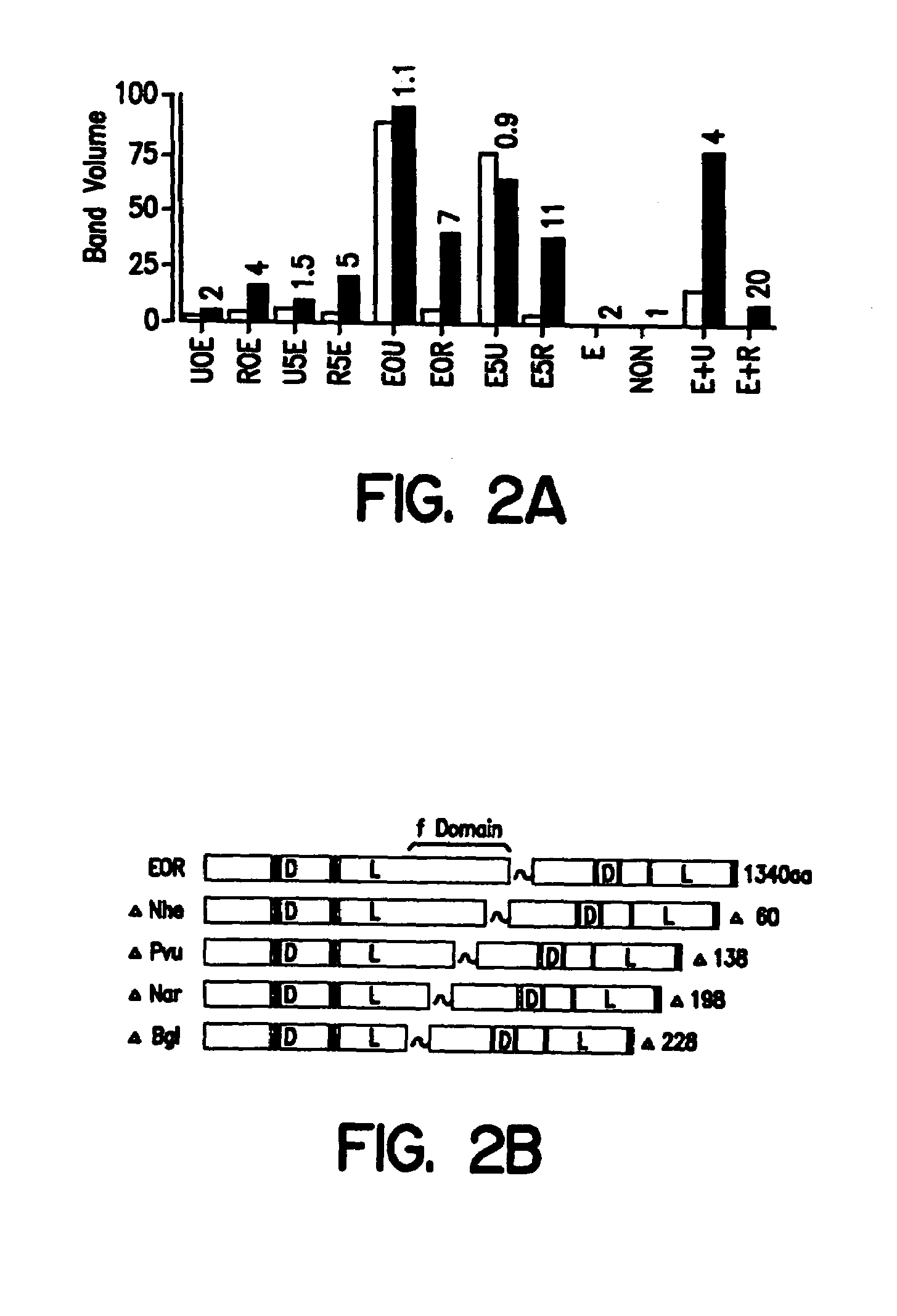
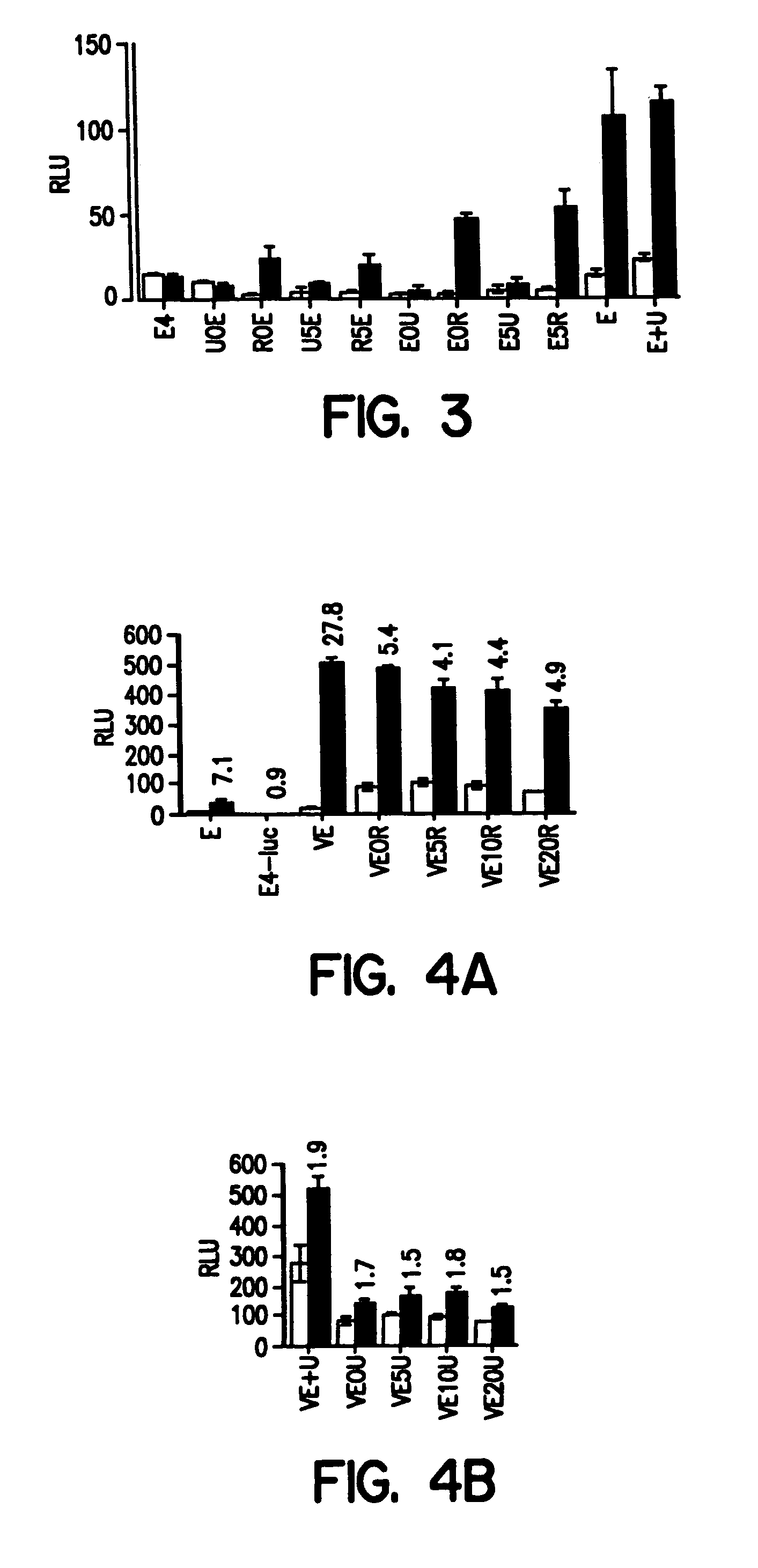
The invention provides chimeric proteins having at least two functional protein units, each containing the dimerization domain of a member of the steroid / thyroid hormone nuclear receptor superfamily. The chimeric proteins can fold under crystallization conditions to form functional entities. The functional entities optionally contain a novel flexible peptide linker of variable lengths between at least two of the protein units. In a preferred embodiment, the linker is designed to be increased in increments of 12 amino acids each to aid in preparation of variant chimeric proteins. The DNA binding characteristics of the invention functional entities differ from those of wild-type complexes formed between “monomeric” receptors and their binding partners. Some functional entities, e.g. dimers expressed as fusion proteins, transactivate responsive promoters in a manner similar to wild-type complexes, while others do not promote transactivation and function instead essentially as constitutive repressors. The invention further provides nucleotide sequences encoding the invention chimeric proteins, cells containing such nucleotide sequences, and methods for using the invention chimeric proteins to modulate expression of one or more exogenous genes in a subject organism. In addition, isolated protein crystals suitable for x-ray diffraction analysis and methods for obtaining putative ligands for the invention chimeric proteins are provided.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Corticosteroid compositions
ActiveUS20090123550A1Good dispersionEasily resuspendedBiocideOrganic active ingredientsDiseaseInflammation
Provided herein are methods for treating, preventing or alleviating the symptoms of and inflammation associated with inflammatory diseases and conditions of the gastrointestinal tract, for example, those involving the esophagus. Also provided herein are pharmaceutical compositions useful for the methods of the present invention.
Owner:VIROPHARMA HLDG
Topical composition
ActiveUS20080234239A1Improve solubilityImprove permeabilityOrganic active ingredientsBiocideChemical compositionDermatology
A composition suitable for topical application comprising a continuous phase and at least one discontinuous phase, said composition comprising at least one polyaphron dispersion, at least one vitamin D or vitamin D analogue and at least one corticosteroid.
Owner:MC2 THERAPEUTICS LTD
Phase change inks containing gelator additives
Disclosed is a phase change ink composition comprising an ink vehicle, a colorant, and a nonpolymeric organic gelator selected from the group consisting of anthracene-based compounds, steroid compounds, partially fluorinated high molecular weight alkanes, high molecular weight alkanes with exactly one hetero atom, chiral tartrate compounds, chiral butenolide-based compounds, bis-urea compounds, guanines, barbiturates, oxamide compounds, ureidopyrimidone compounds, and mixtures thereof, said organic gelator being present in the ink in an amount of no more than about 20 percent by weight of the ink, said ink having a melting point at or below which the ink is a solid, said ink having a gel point at or above which the ink is a liquid, and said ink exhibiting a gel state between the melting point and the gel point, said ink exhibiting reversible transitions between the solid state and the gel state upon heating and cooling, said ink exhibiting reversible transitions between the gel state and the liquid state upon heating and cooling, said melting point being greater than about 35° C., said gel point being greater than said melting point. Also disclosed are imaging processes employing phase change inks containing gelator additives.
Owner:MONTREAL UNIV OF
Infusion apparatus with modulated flow control
InactiveUS20050033232A1Easy to useEasy constructionFlexible member pumpsMedical devicesStored energyConstant force
A compact fluid dispenser for use in controllably dispensing fluid medicaments, such as antibiotics, oncolytics, hormones, steroids, blood clotting agents, analgesics, and like medicinal agents from prefilled containers at a uniform rate. The dispenser uniquely includes a stored energy source that is provided in the form of a substantially constant-force, compressible-expandable wave spring that provides the force necessary to continuously and uniformly expel fluid from the device reservoir. The device further includes a fluid flow control assembly that precisely controls the flow of medicament solution to the patient. Additionally, the device includes a novel modulating assembly for controllably modulating the force exerted by the wave spring tending to expel the fluid from the device reservoir.
Owner:BIOQUIDDITY
Implantable sensor with biocompatible coating for controlling or inhibiting tissue growth
ActiveUS20060008500A1Inhibit growthPrevent formation of tissueMaterial nanotechnologyElectrotherapySteroid CompoundAdhesion process
All or a portion of a surface of an implantable sensor is covered with a biocompatible coating formed at least partially of a biomaterial matrix having properties that promote a substantially even growth of tissue cells over the surface of the coating. Additional materials, such as growth factors, agents that recruit endogenous stem cells, and cell adhesion motif arginine, glycine, aspartic acid may be included in the coating. Autologous cells may be added to the coating prior to implantation. The sensor surface may also be textured, by etching or abrading, in order to promote even tissue growth. Alternatively, the sensor surface may be covered with a coating having properties that inhibit the growth of tissue. These coatings may include a biomaterial, a biomaterial matrix having a drug, such as a sirolimus or a steroid, an active component, or a self assembled monolayer.
Owner:CARDIAC PACEMAKERS INC
Fused heterotricyclic compounds as inhibitors of 17beta-hydroxysteroid dehydrogenase 3
Owner:BRISTOL MYERS SQUIBB CO
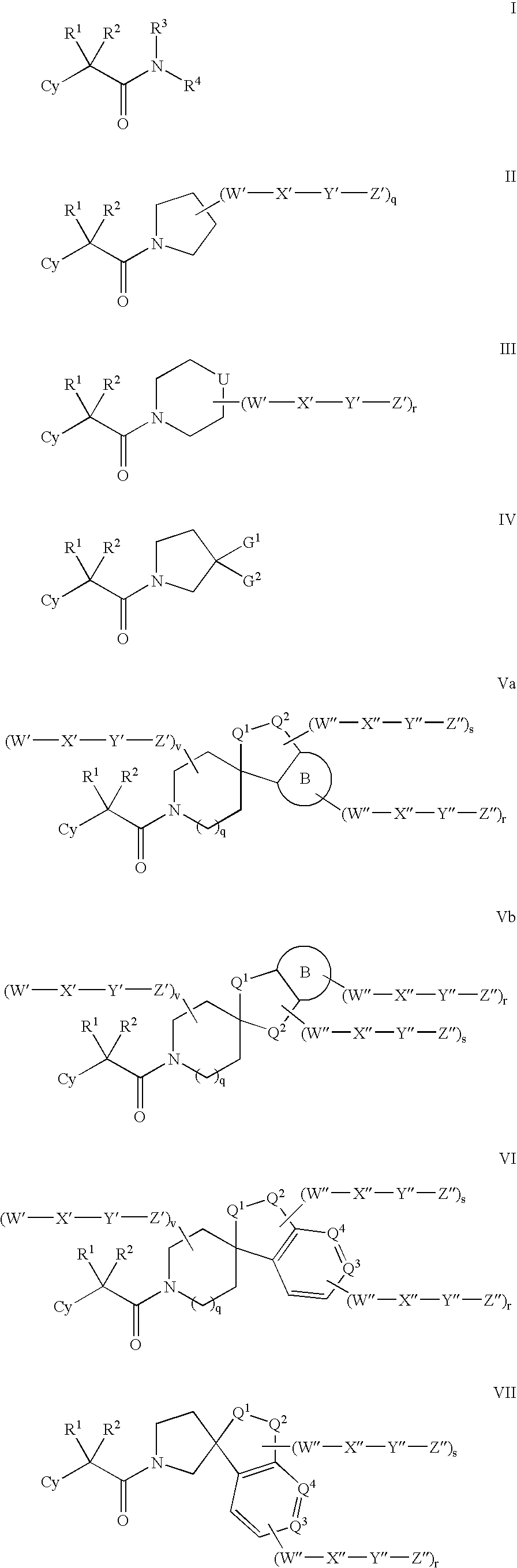
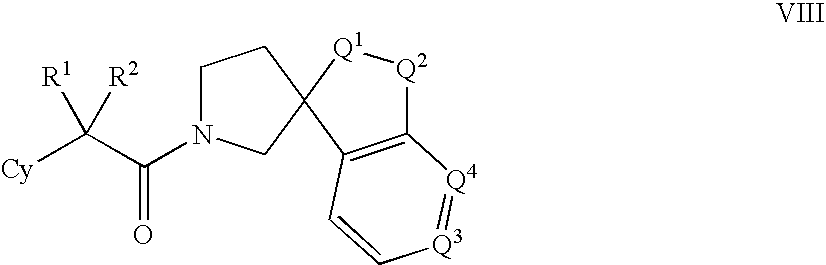
Amido compounds and their use as pharmaceuticals
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1, antagonists of the mineralocorticoid receptor (MR), and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1 and / or diseases associated with aldosterone excess.
Owner:INCYTE HLDG CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com