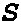Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
80 results about "Allergic conjunctivitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Allergic conjunctivitis is inflammation of the conjunctiva (the membrane covering the white part of the eye) due to allergy. Although allergens differ among patients, the most common cause is hay fever. Symptoms consist of redness (mainly due to vasodilation of the peripheral small blood vessels), edema (swelling) of the conjunctiva, itching, and increased lacrimation (production of tears). If this is combined with rhinitis, the condition is termed allergic rhinoconjunctivitis.
Nanoparticulate corticosteroid and antihistamine formulations
InactiveUS20060216353A1Less liver toxicityUseful in prophylaxis and chronic treatment of asthmaBiocidePowder deliveryPediatric patientMicroparticle
Compositions comprising a nanoparticulate corticosteroid and an antihistamine are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining an antihistamine with a nanoparticulate corticosteroid in a single formulation results in improved efficacy.
Owner:ALKERMES PHARMA IRELAND LTD
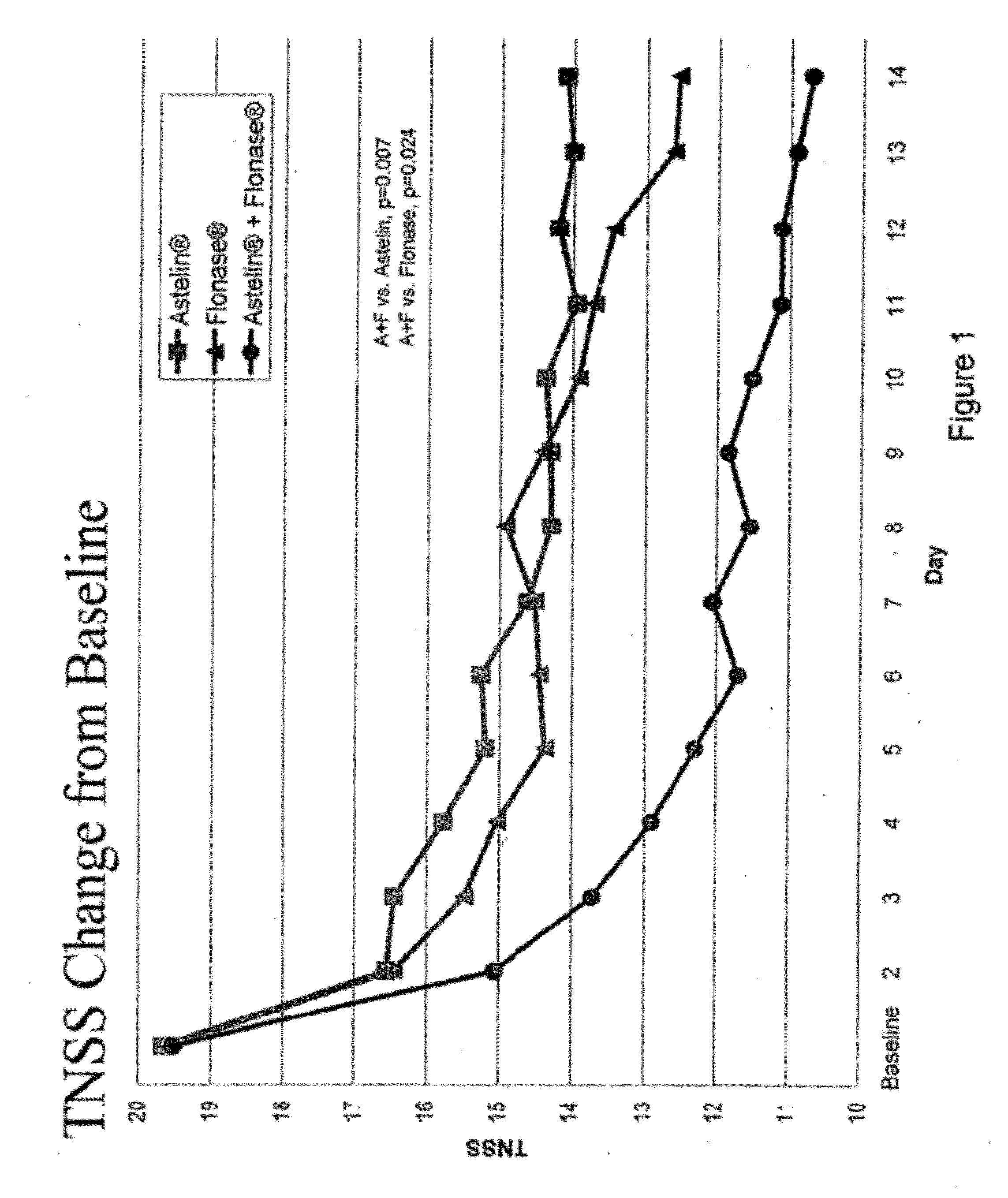
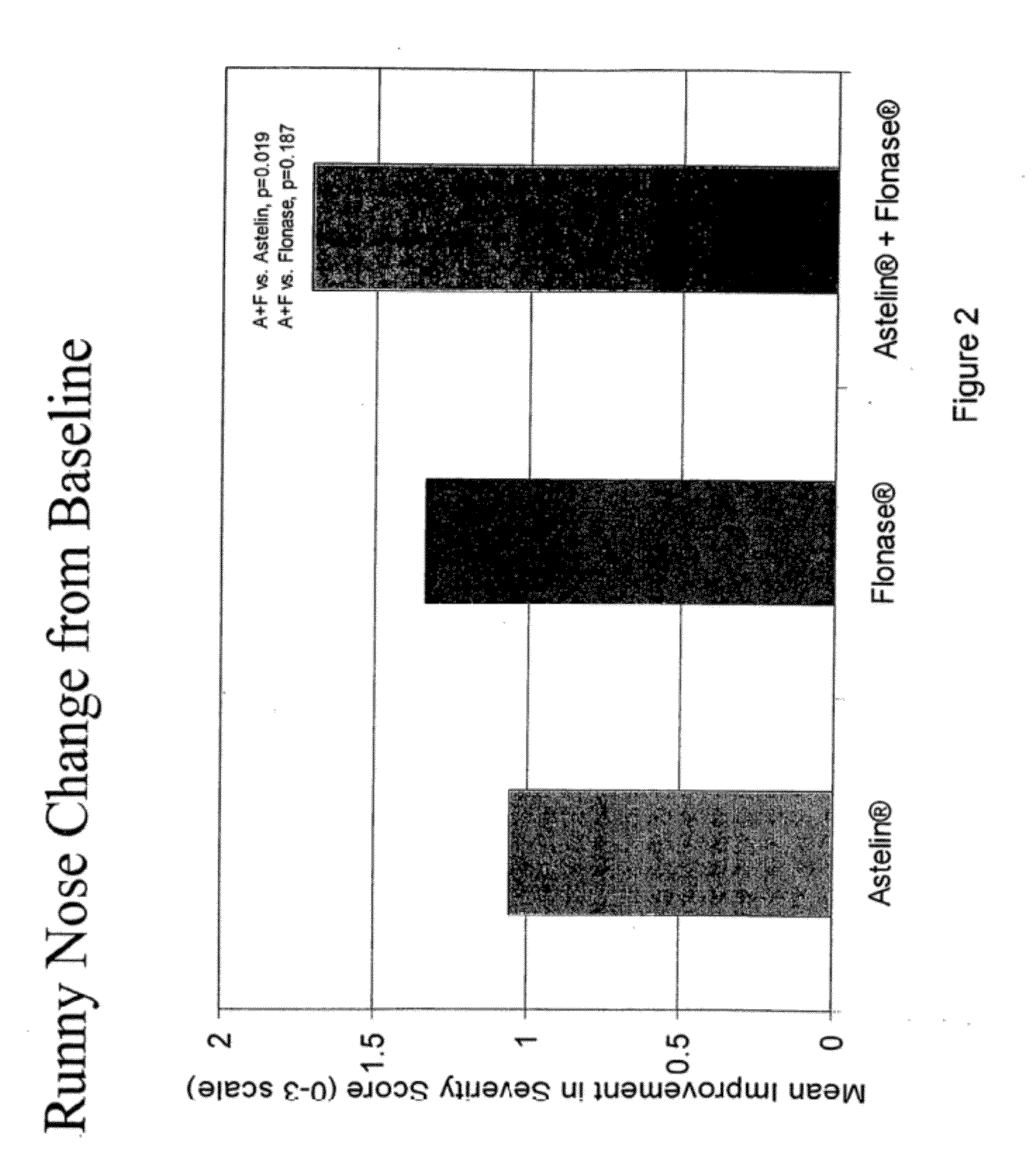
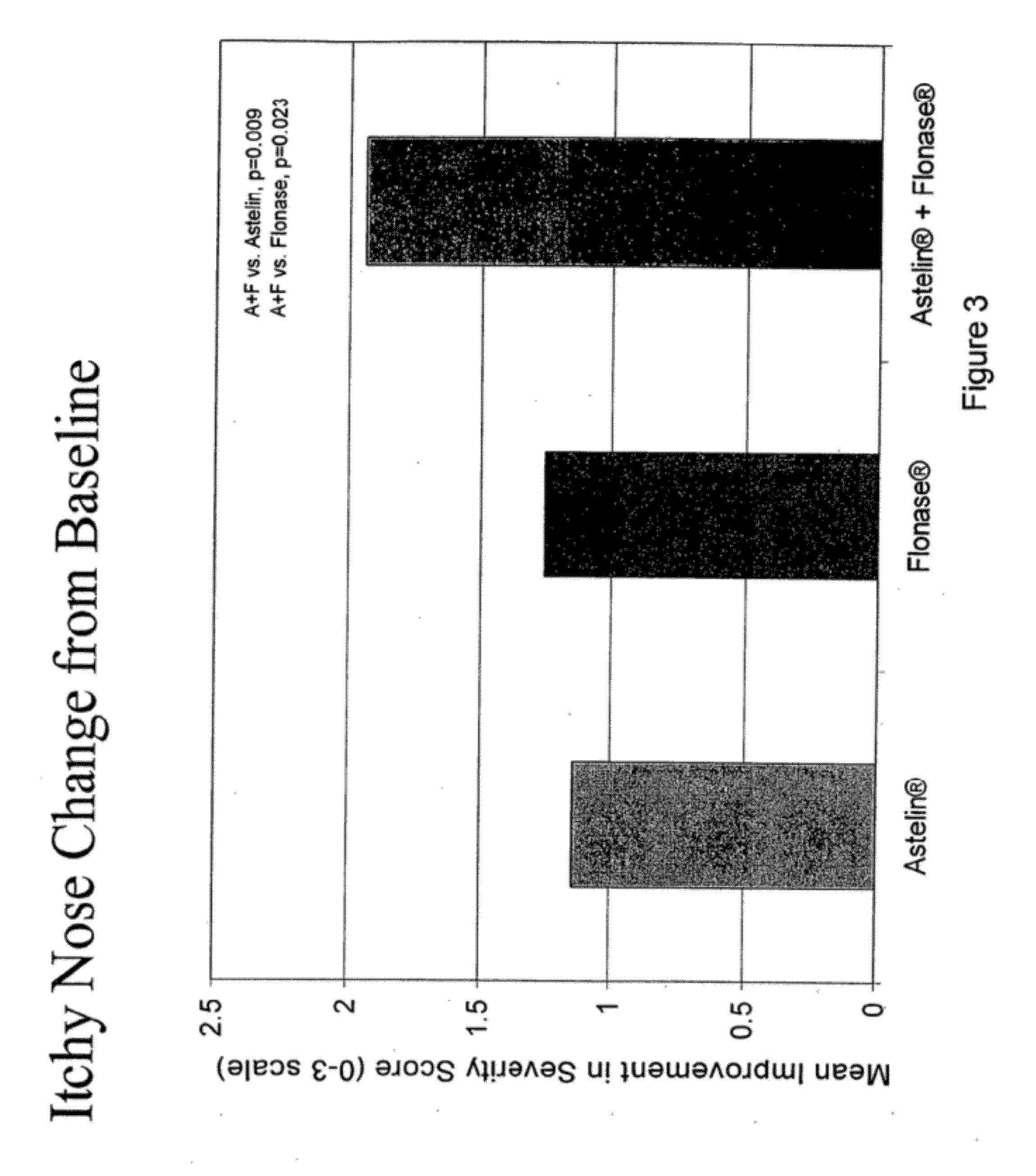
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Method of treating mast cell activation-induced diseases with a proteoglycan
InactiveUS6689748B1Decrease in urinaryBiocidePeptide/protein ingredientsInflammatory Bowel DiseasesInterstitial cystitis
The invention provides a method for preventing and treating the harmful biological effects of biochemicals secreted from activated mast cells in the organism of warm blooded animals and more especially human beings, said effects being associated with allergy (including but not limited to allergic conjunctivitis, allergic rhinitis, allergic otitis, asthma, allergic uticaria, food allergy and atopic dermatitis), hyperproliferative diseases such as leukemia and systemic mastocytosis, interstitial cystitis, inflammatory bowel disease, irritable bowel syndrome, osteoporosis and scleroderma. The method consists in administering to said animals and especially to human beings an effective amount of a proteoglycan such as chondroitin sulfate with mast cell secretion inhibitory activity, alone or in combination with one or more synergistic adjuvants such those belonging to the class of flavonoids or compounds with histamine-1 receptor antagonist activity.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
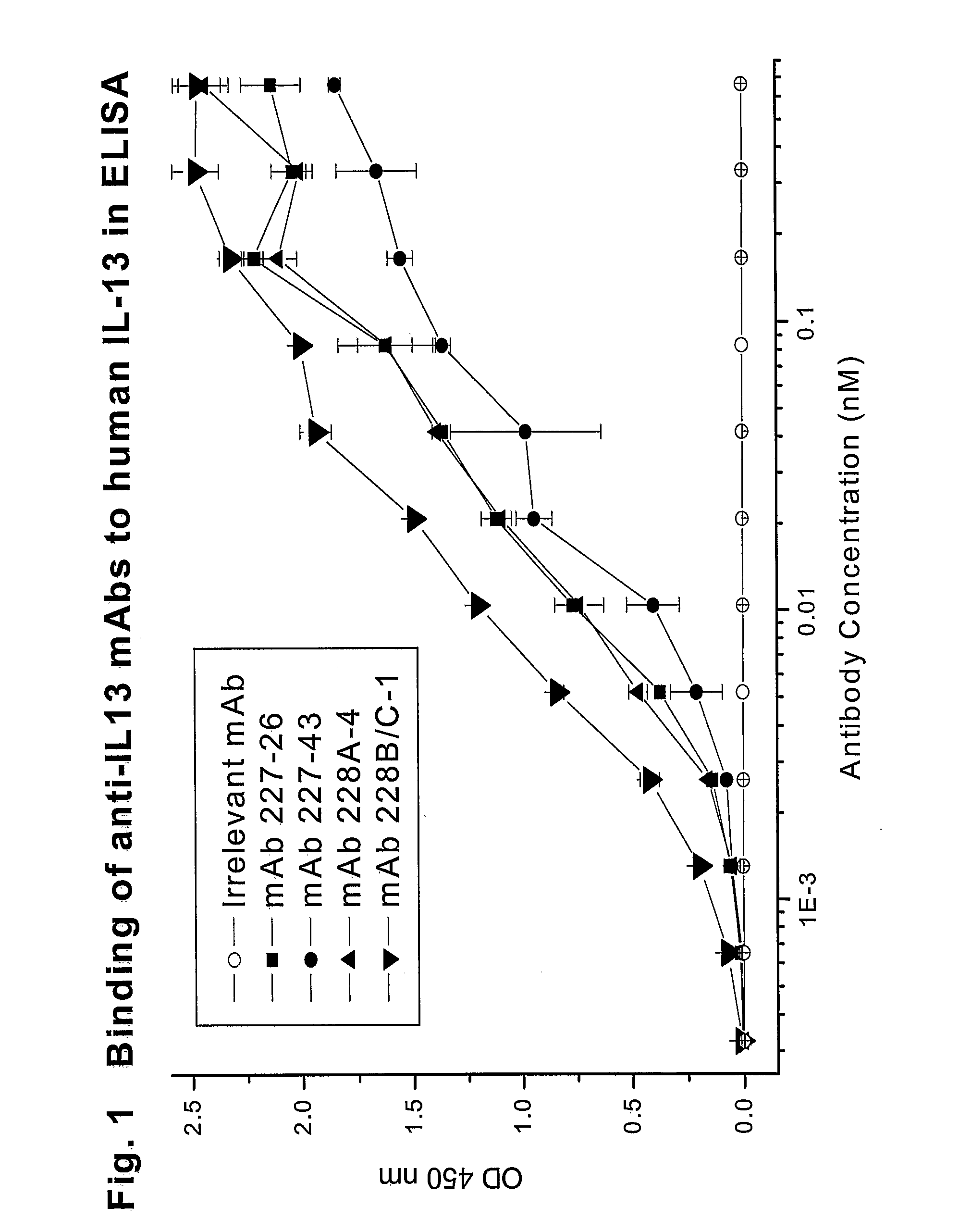
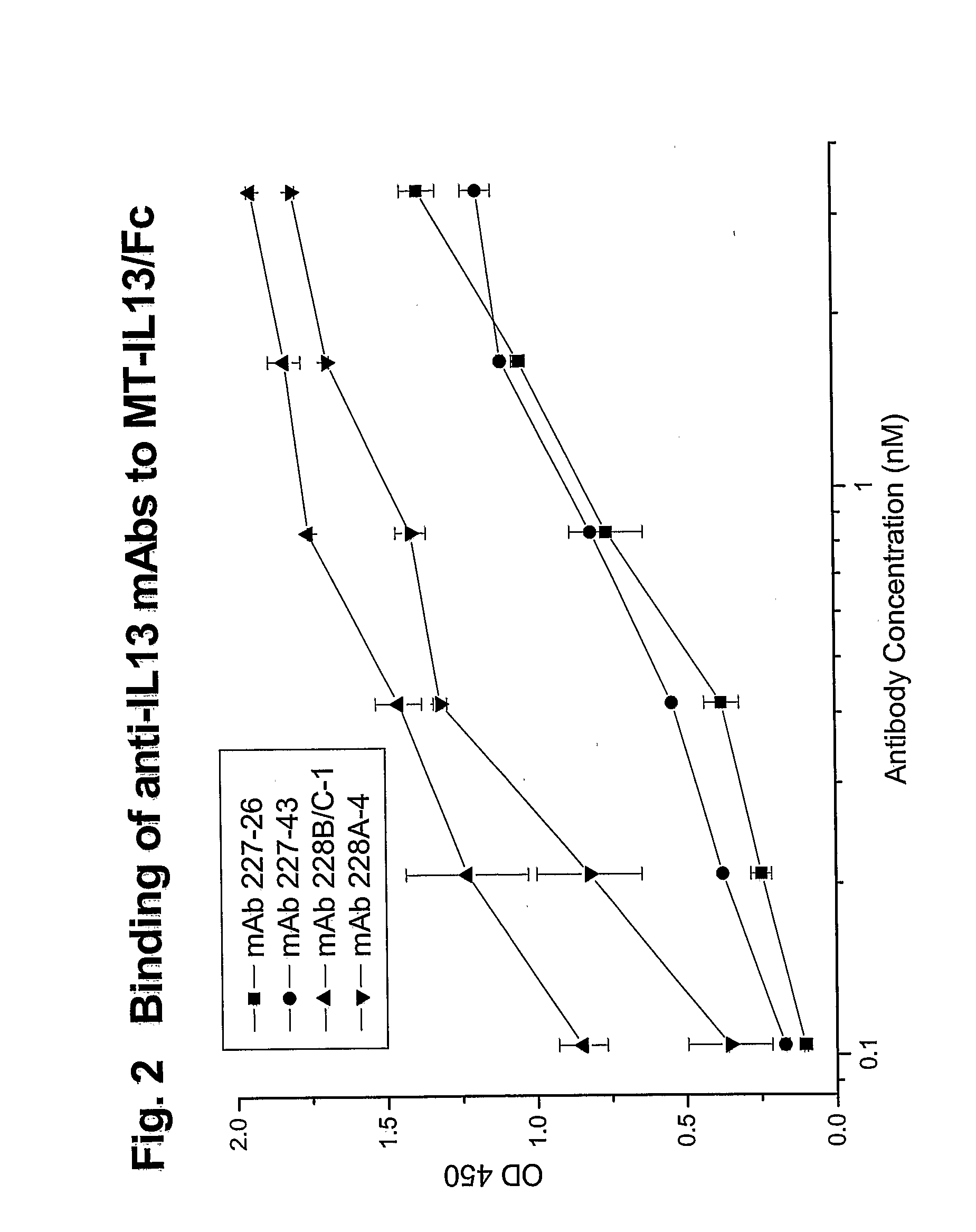
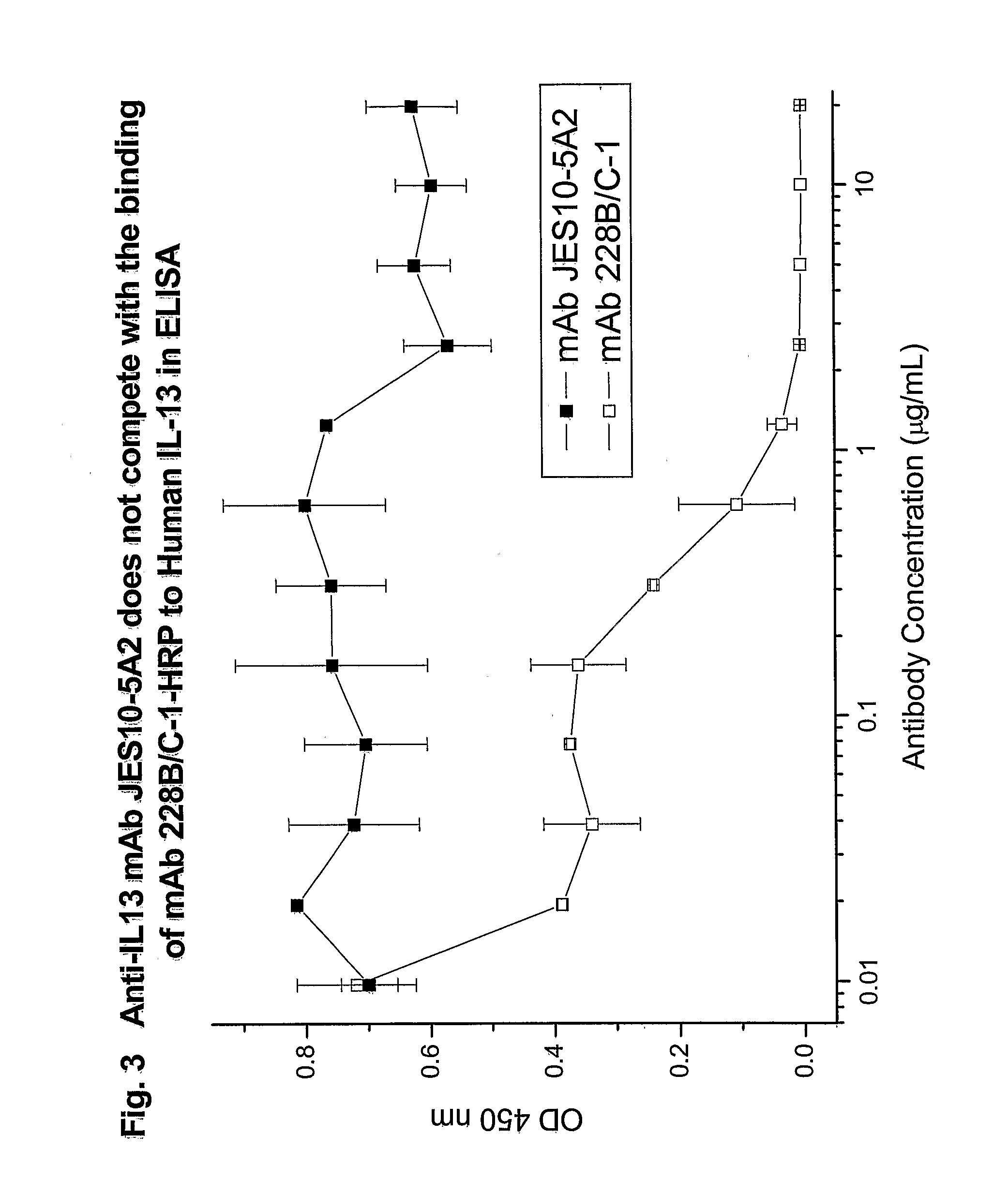
Novel anti-IL13 antibodies and uses thereof
ActiveUS20090214523A1Inhibiting antibody productionRelieve symptomsSenses disorderAntipyreticUveitisNonallergic rhinitis
The present invention relates to anti-IL13 antibodies that bind specifically and with high affinity to both glycosylated and non-glycosylated human IL13, does not bind mouse IL13, and neutralize human IL13 activity at an approximate molar ratio of 1:2 (MAb:IL13). The invention also relates to the use of these antibodies in the treatment of IL13-mediated diseases, such as allergic disease, including asthma, allergic asthma, non-allergic (intrinsic) asthma, allergic rhinitis, atopic dermatitis, allergic conjunctivitis, eczema, urticaria, food allergies, chronic obstructive pulmonary disease, ulcerative colitis, RSV infection, uveitis, scleroderma, and osteoporosis.
Owner:GENENTECH INC
Nanoparticulate leukotriene receptor antagonist/corticosteroid formulations
InactiveUS20070065374A1Useful in prophylaxis and chronic treatment of asthmaGood curative effectPowder deliveryBiocidePediatric patientPatient compliance
Nanoparticulate compositions comprising a corticosteroid and a leukotriene receptor antagonist are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining a leukotriene receptor antagonist with a corticosteroid in a particle size ranges of less than 2000 nm in a single formulation results in improved efficacy. In addition, patient compliance is enhanced since only one dosage form is needed. Furthermore, local administration of the leukotriene receptor antagonist results in less liver toxicity since the liver will be exposed to lower amounts of drug than happens following oral administration. The drug compositions according to the invention can be formulated into inhalation, nasal, or ocular formulations.
Owner:ELAN PHRMA INT LTD
Method for treating ophthalmic diseases using rho kinase inhibitor compounds
This invention is directed to methods of preventing or treating ocular diseases with inflammation, excessive cell proliferation, remodeling, neurite retraction, corneal neurodegeneration, excessive vaso-permeability and edema. Particularly, this invention relates to methods treating ocular diseases such as allergic conjunctivitis, corneal hyposensitivity, neurotrophic keratopathy, dry eye disease, proliferative vitreal retinopathy, macular edema, macular degeneration, and blepharitis, using novel Rho kinase inhibitor compounds. The method comprises identifying a subject in need of the treatment, and administering to the subject an effective amount of a novel Rho kinase inhibitor compound to treat the disease.
Owner:INSPIRE PHARMA
Use of an Inhibitor of TNFa Plus an Antihistamine to Treat Allergic Rhinitis and Allergic Conjunctivitis
InactiveUS20080254029A1Immediate and long-term reliefOvercomes drawbackBiocideSenses disorderNonallergic rhinitisAllergic conjunctivitis
Disclosed are methods of treating allergic conjunctivitis and allergic rhinitis in a subject that involve topically administering to the subject a composition comprising a pharmaceutically effective amount of an H1 antagonist and an anti-TNFα compound.
Owner:ALCON RES LTD
Ophthalmic formulations of fluticasone and methods of use
InactiveUS20110105450A1Relieve signRelieve symptomsSenses disorderInorganic non-active ingredientsOphthalmologyAllergic conjunctivitis
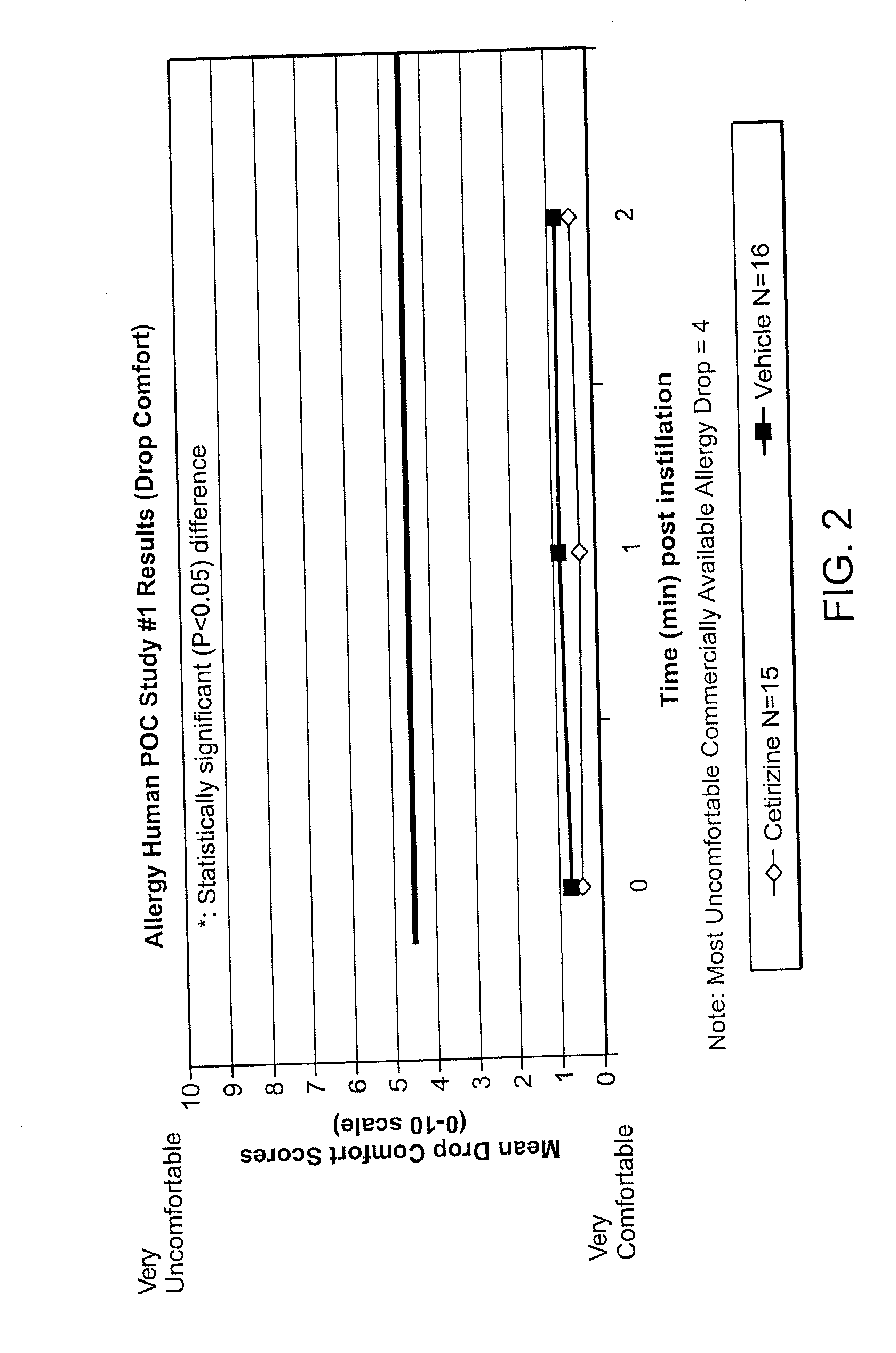
The present invention provides ophthalmic formulations of fluticasone that provide a comfortable formulation when instilled in the eye and is effective in the treatment of allergic conjunctivitis and / or allergic conjunctivitis. The invention further provides methods of treating allergic conjunctivitis and / or allergic conjunctivitis in a subject in need of such treatment by topical application of the fluticasone formulations of the invention directly to the eye.
Owner:ACIEX THERAPEUTICS INC
Method for treating ophthalmic diseases using kinase inhibitor compounds in prodrug forms
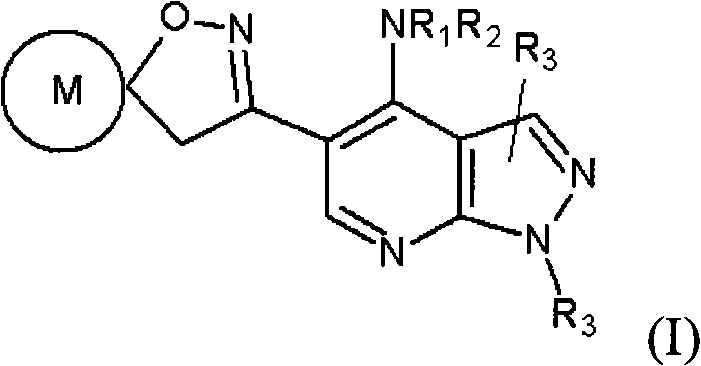
This invention is directed to prodrugs of rho kinase (ROCK) inhibitors. These prodrugs are in general the ester or the amide derivatives of the parent compounds. These prodrugs are often weak inhibitors of ROCK, but their parent compounds have good activities. Upon instillation into the eyes, the ester or the amide group of these prodrugs is rapidly hydrolyzed into alcohol, amine, or acid, and the prodrugs are converted into the active base compounds. The prodrugs of ROCK inhibitors provide several advantages such as delivery of higher concentrations of the active species into the target site and reduction of ocular discomfort. The invention is also directed to a method of treating ophthalmic diseases such as glaucoma, allergic conjunctivitis, macular edema, macular degeneration, and blepharitis, by administering an effective amount of a ROCK prodrug compound of Formula I to the eyes of the patient in need of.
Owner:MERCK SHARP & DOHME CORP
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
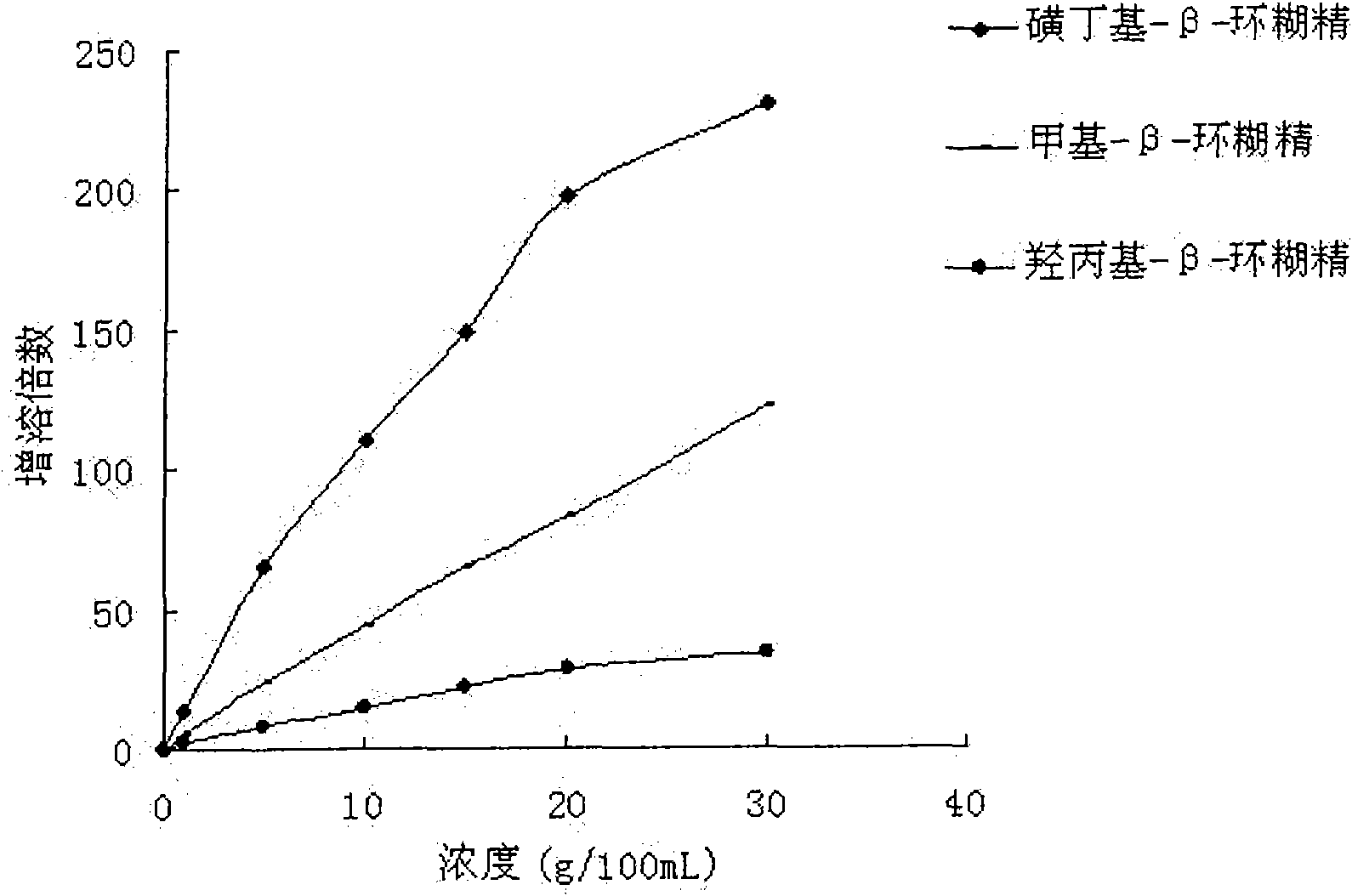
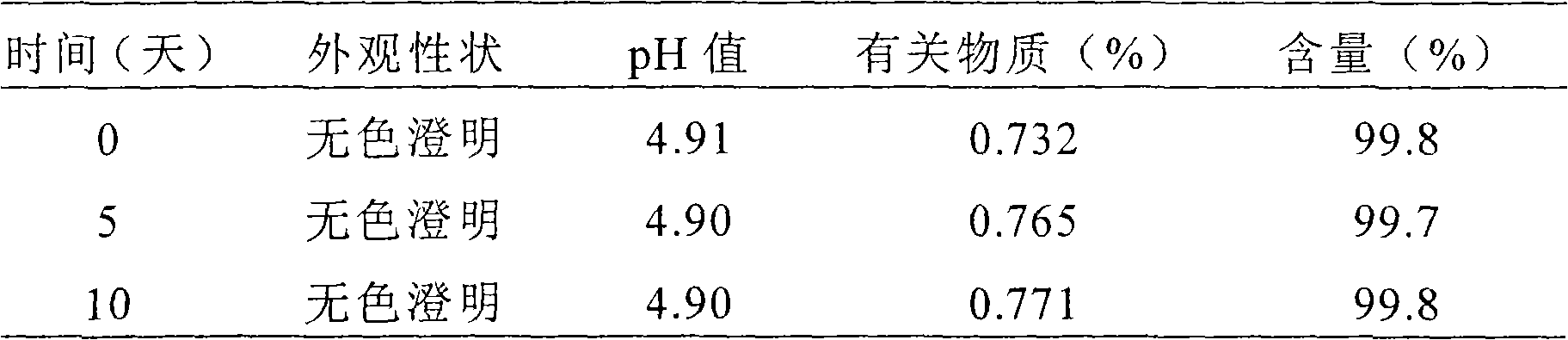
Liquid preparation for dosing eyes and method for making the same
The invention relates to a liquid preparation for dosing eyes, which comprises 0.1 to 25 g / 100 mL of rupatadine and 0.5 to 30 g / 100 mL of cyclodextrin compound. The invention also relates to a methodfor making the liquid preparation. The liquid preparation of the invention has the characteristics of rapid absorption, convenient use, obvious curative effect and stability, and has excellent curative effect for treating conjunctivitis, in particular for treating allergic conjunctivitis.
Owner:广州达信生物技术有限公司
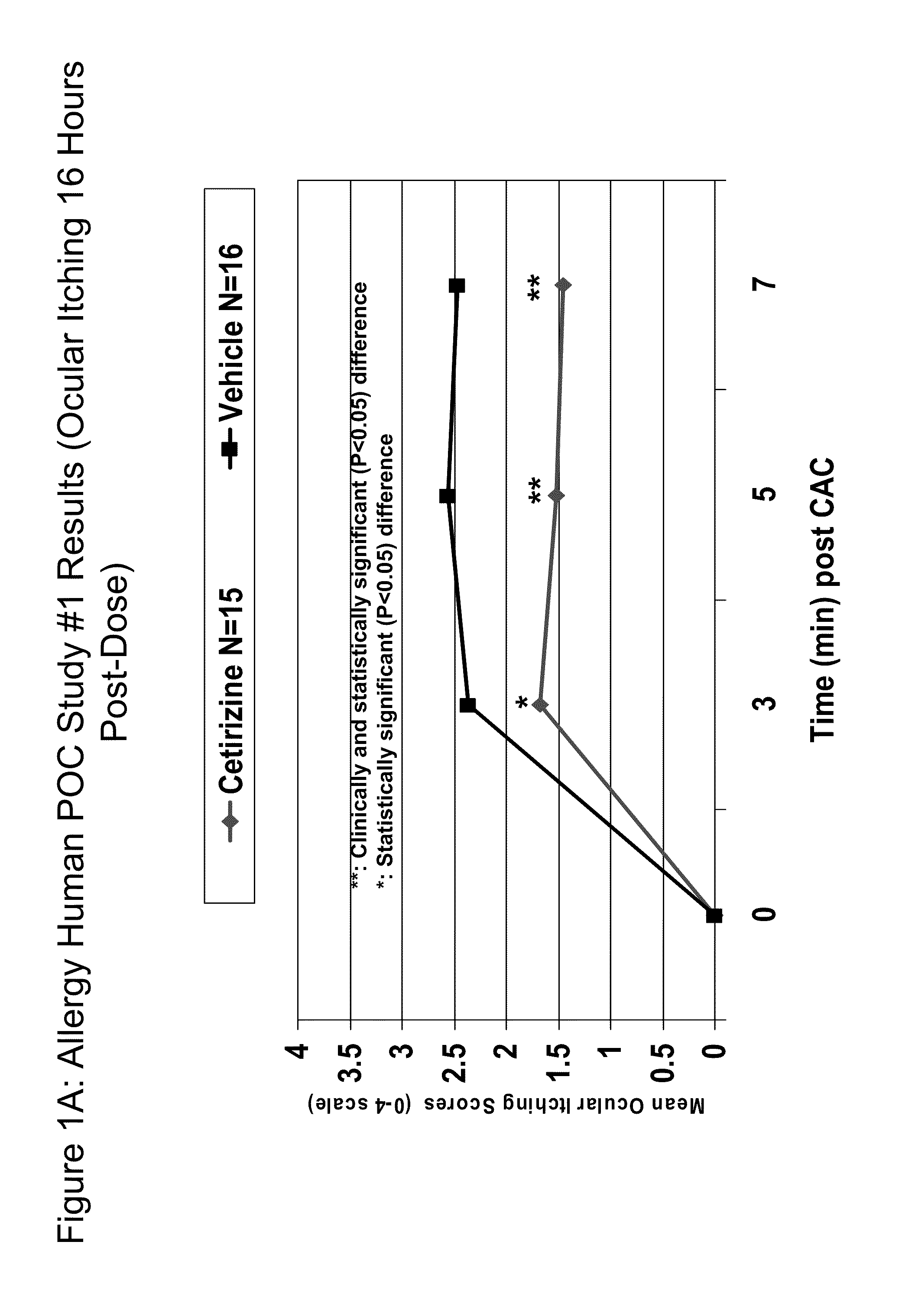
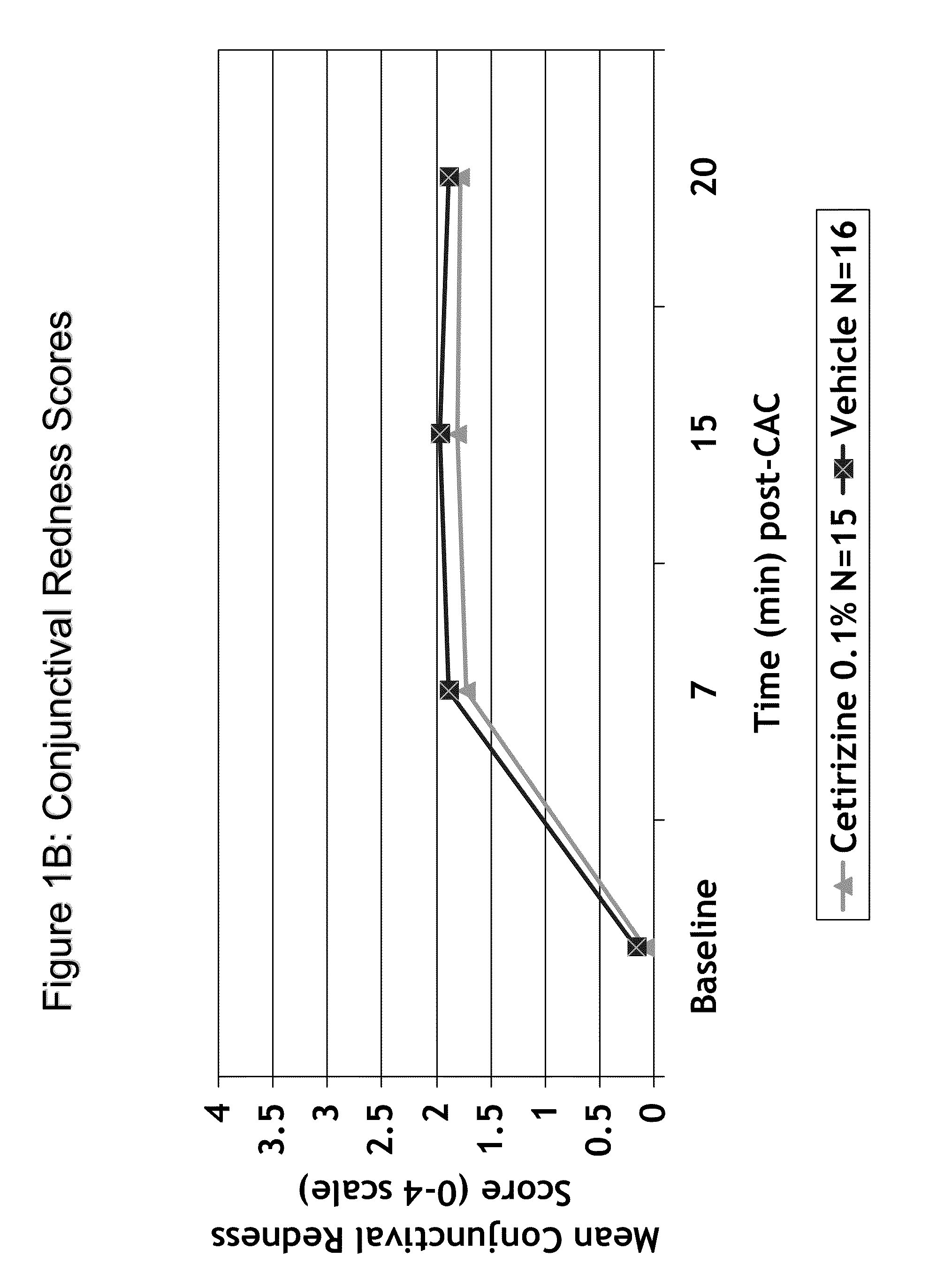
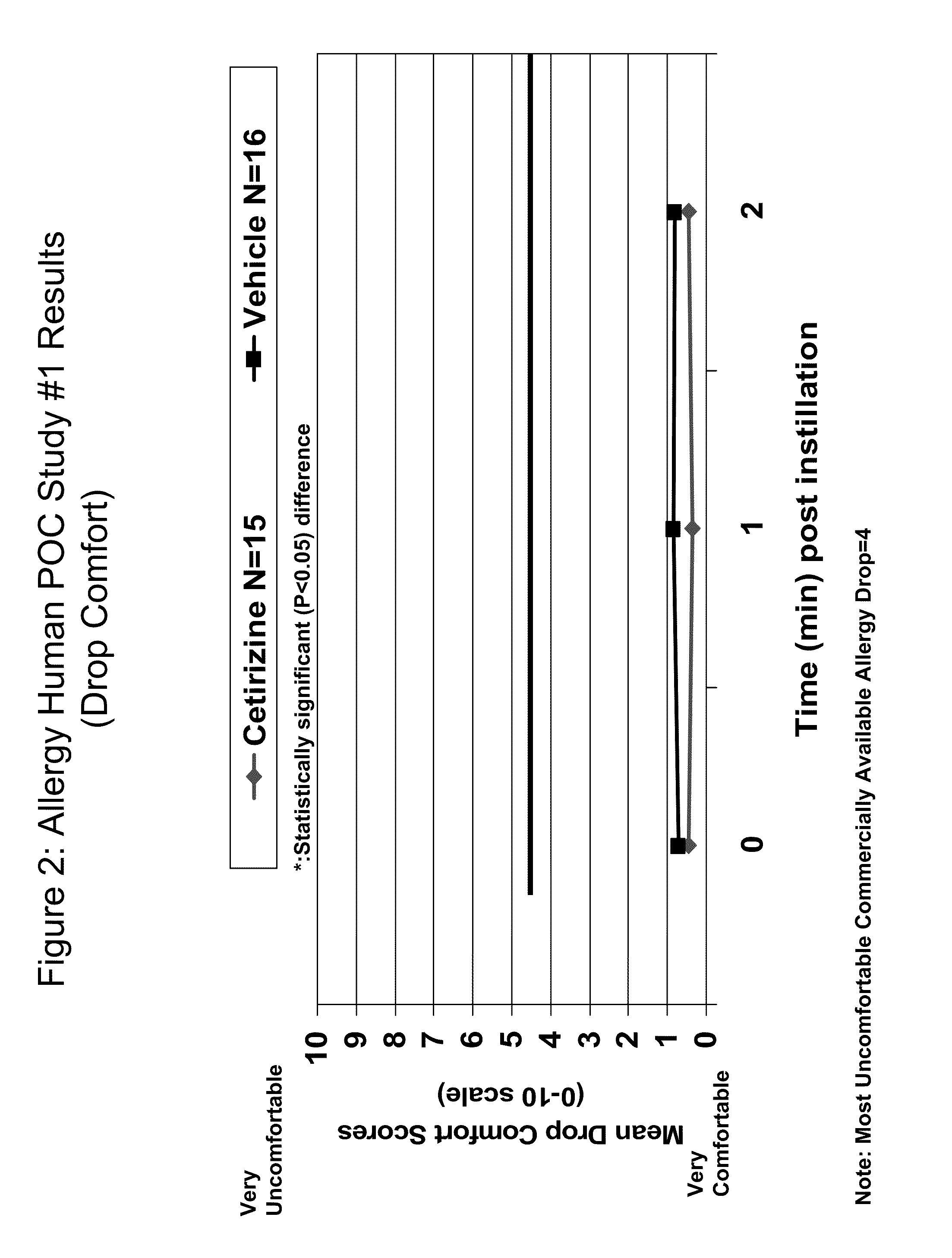
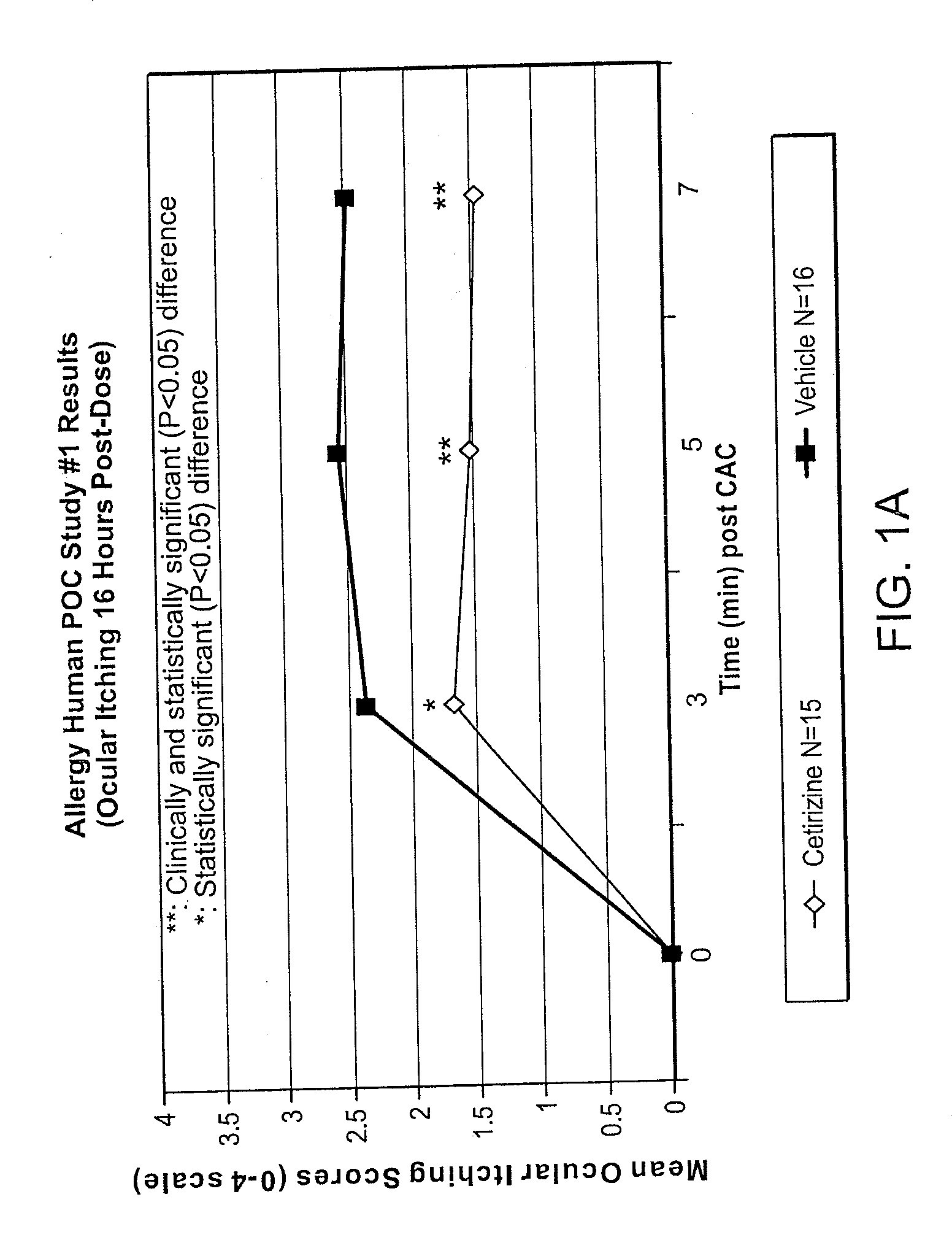
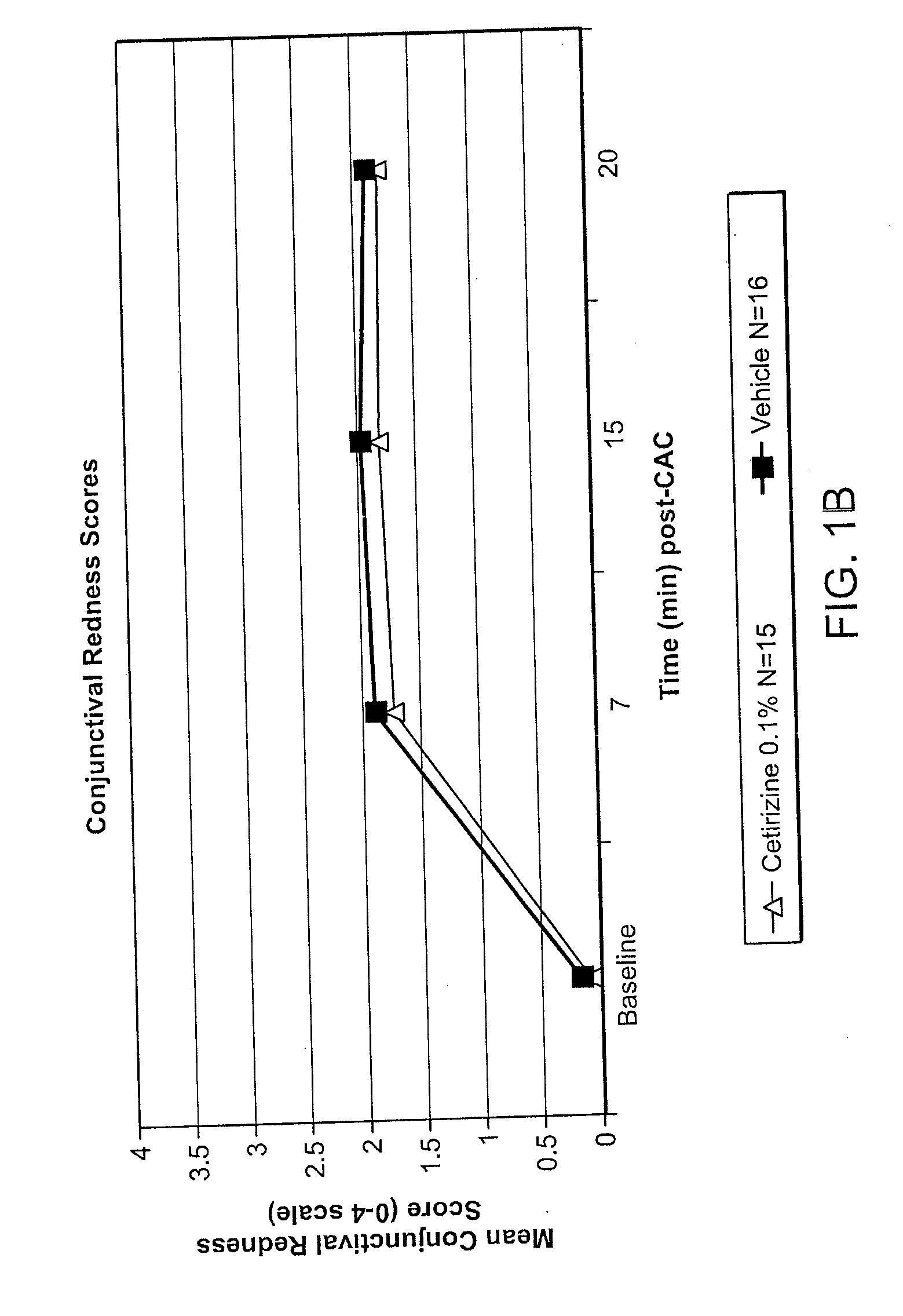
Ophthalmic Formulations of Cetirizine and Methods of Use
ActiveUS20100240625A1Relieve symptomsEffectively masks itchingBiocideOrganic active ingredientsAllergic conjunctivitisCetirizine
The present invention provides stable topical formulations of cetirizine that provide a comfortable formulation when instilled in the eye and is effective in the treatment of allergic conjunctivitis and / or allergic conjunctivitis. The invention further provides methods of treating allergic conjunctivitis and / or allergic rhinoconjunctivitis in a subject in need of such treatment by topical application of the cetirizine formulations of the invention directly to the eye.
Owner:NICOX OPHTHALMICS
Nanoparticulate corticosteroid and antihistamine formulations methods of making, and methods of administering thereof
InactiveUS8003127B2Useful in prophylaxis and chronic treatment of asthmaGood curative effectBiocidePowder deliveryPediatric patientMicroparticle
Compositions comprising a nanoparticulate corticosteroid and an antihistamine are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining an antihistamine with a nanoparticulate corticosteroid in a single formulation results in improved efficacy.
Owner:ALKERMES PHARMA IRELAND LTD
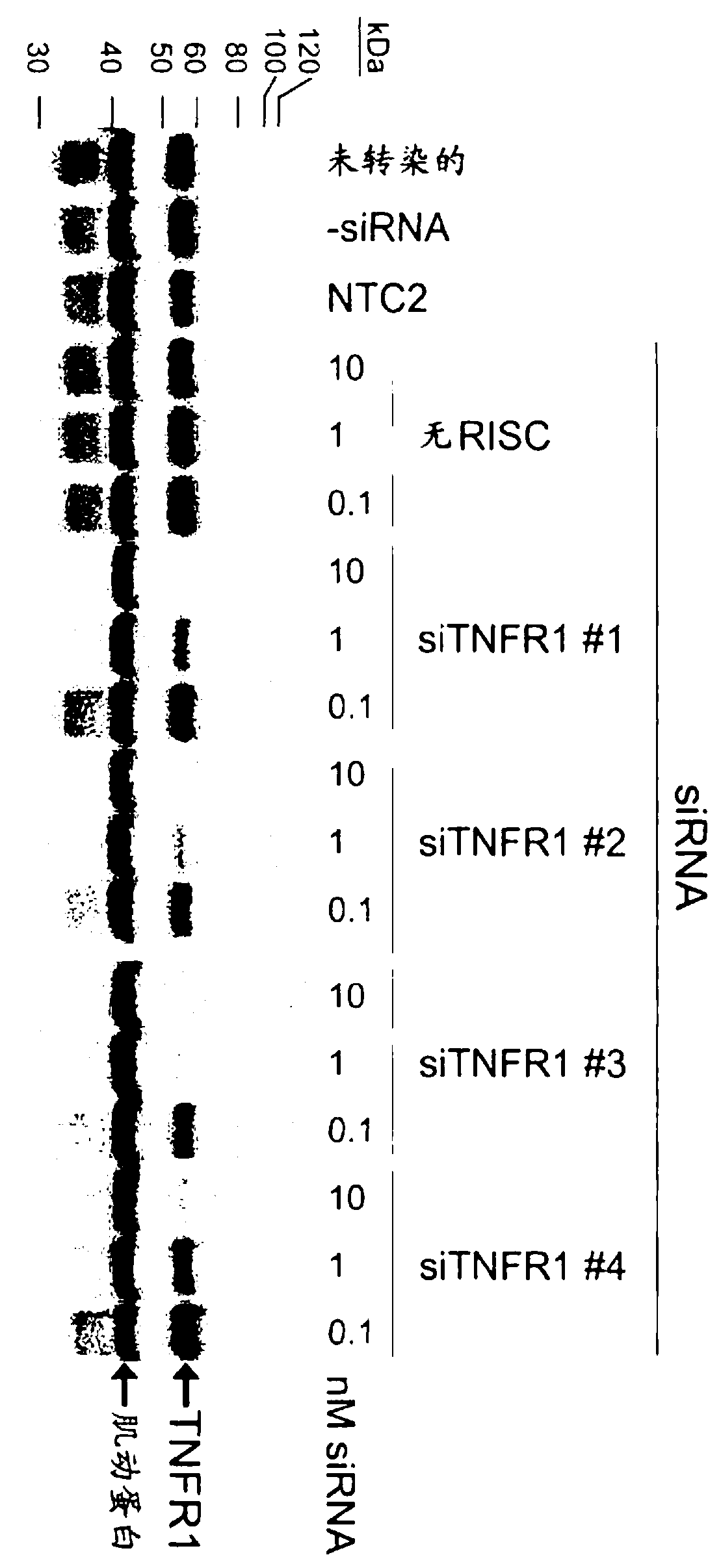
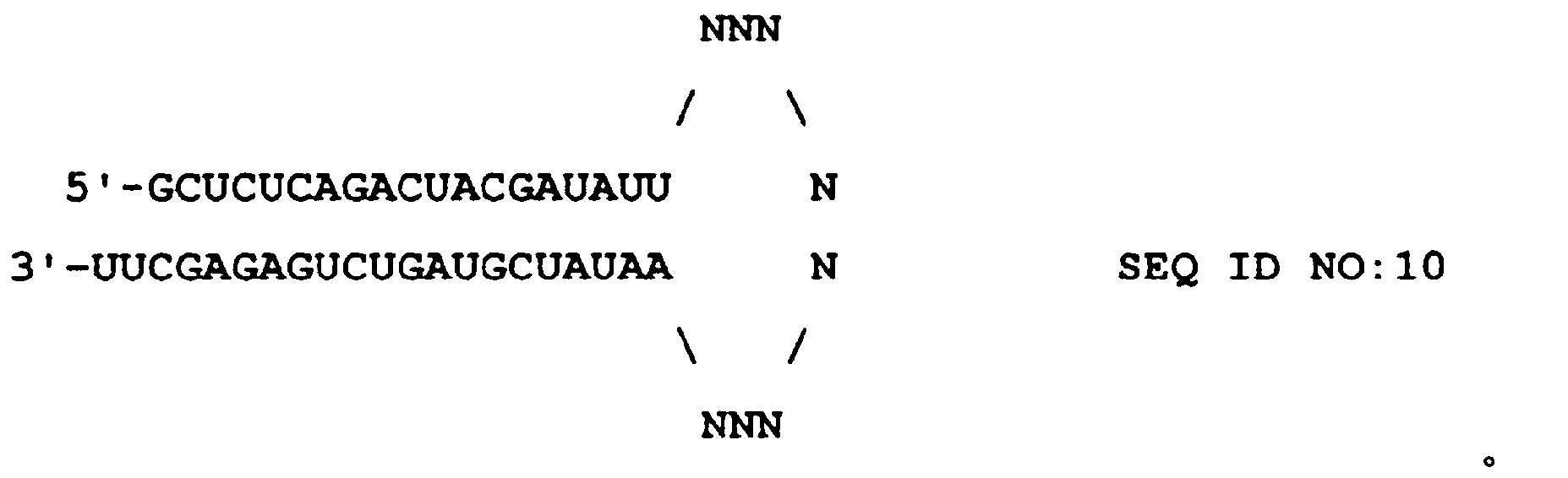
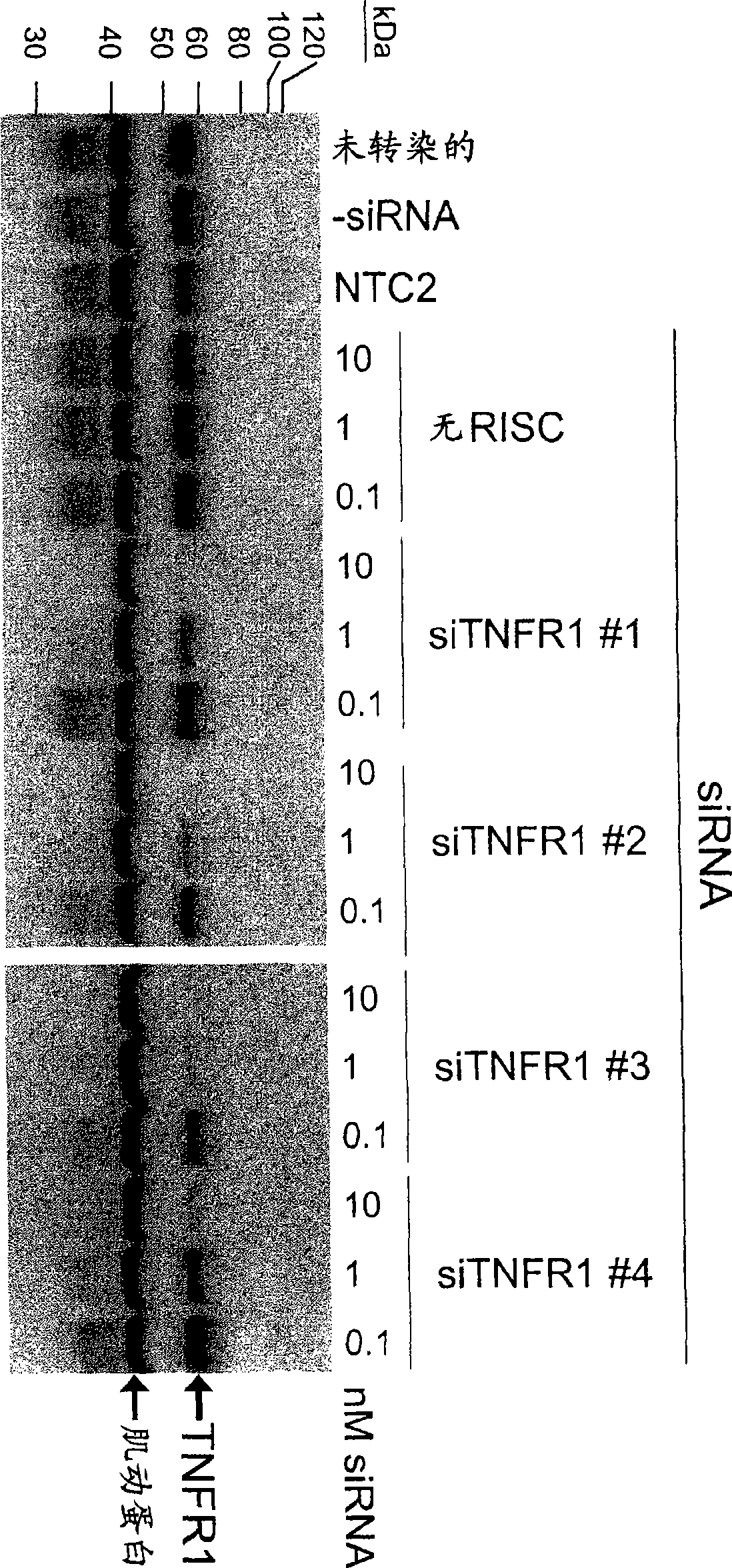
RNAi-mediated inhibition of tumor necrosis factor alpha-related conditions
RNA interference is provided for inhibition of tumor necrosis factor alpha (TNFalpha) by silencing TNFalpha cell surface receptor TNF receptor-1 (TNFR1) mRNA expression, or by silencing TNFalpha converting enzyme (TACE / ADAM17) mRNA expression. Silencing such TNFalpha targets, in particular, is useful for treating patients having a TNFalpha-related condition or at risk of developing a TNFalpha-related condition such as the ocular conditions dry eye, allergic conjunctivitis, or ocular inflammation, or such as dermatitis, rhinitis, or asthma, for example.
Owner:ARROWHEAD RES CORP
Ophthalmic Formulations Of Cetirizine And Methods Of Use
ActiveUS20110257136A1Alleviate and reduce and systemic exposureStable, comfortable, efficacious and safeBiocideOrganic active ingredientsAllergic conjunctivitisCetirizine
The present invention provides stable topical formulations of cetirizine that provide a comfortable formulation when instilled in the eye and is effective in the treatment of allergic conjunctivitis and / or allergic conjunctivitis. The invention further provides methods of treating allergic conjunctivitis rhinitis, and / or allergic rhinoconjunctivitis in a subject in need of such treatment by topical application of the cetirizine formulations of the invention directly to the eye.
Owner:NICOX OPHTHALMICS
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders.
Owner:MEDA PHARMA INC
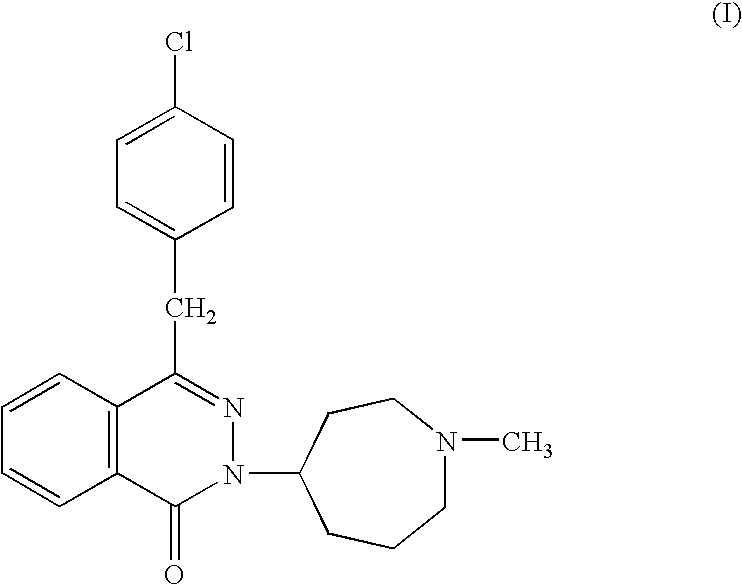
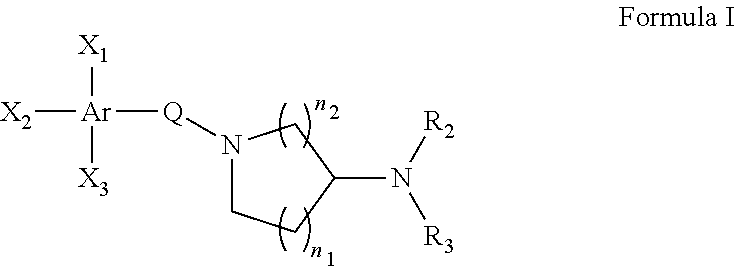
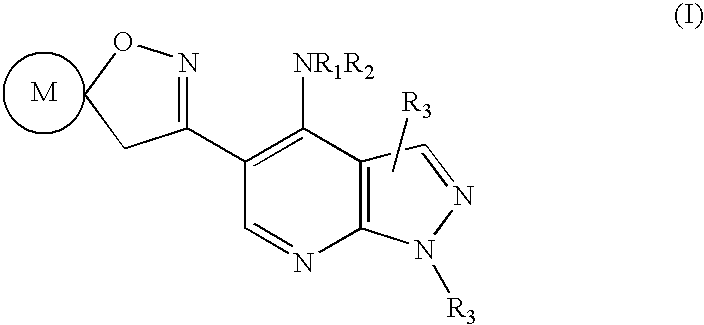
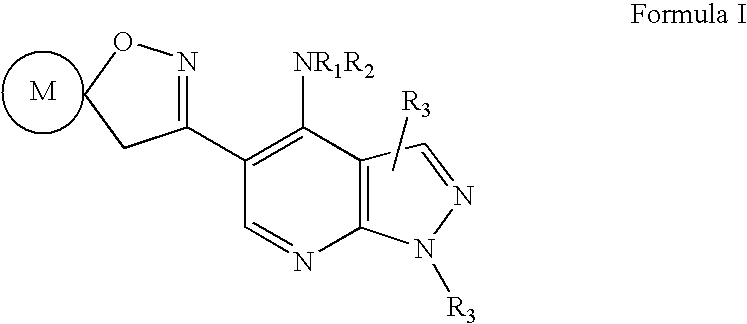
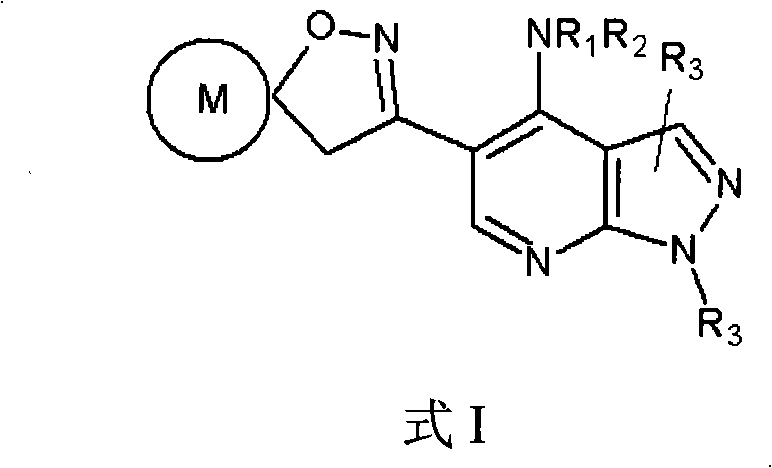
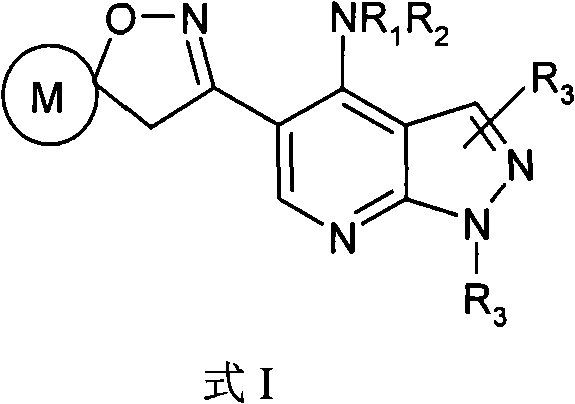
Pyrazolo (3, 4-b) pyridine derivatives as phosphodiesterase inhibitors
The present invention relates to phosphodiesterase (PDE) type 4, phosphodiesterase (PDE) type 7 and dual PDE type 4 / PDE type 7 inhibitors. Compounds disclosed herein having the structure of Formula 1: can be useful in the treatment, prevention, inhibition or suppression of CNS diseases, for example, multiple sclerosis; various pathological conditions such as diseases affecting the immune system, including AIDS, rejection of transplant, auto-immune disorders such as T-cell related diseases, for example, rheumatoid arthritis; inflammatory diseases such as respiratory inflammation diseases including chronic obstructive pulmonary disease (COPD), asthma, bronchitis, allergic rhinitis, adult respiratory distress syndrome (ARDS) and other inflammatory diseases including but not limited to psoriasis, shock, atopic dermatitis, eosinophilic granuloma, allergic conjunctivitis, osteoarthritis; gastrointestinal inflammation diseases such as Crohn's disease, colitis, pancreatitis as well as different types of cancers including leukaemia; especially in humans. Processes for the preparation of disclosed compounds, pharmaceutical compositions containing the disclosed compounds and their use as PDE type 4, PDE type 7 and dual PDE type 4 / PDE type 7 inhibitors are provided.
Owner:SUN PHARMA INDS
Pharmaceutical composition comprising glutarimide derivatives and use thereof in the treatment of eosinophilic diseases
ActiveUS20160279114A1Suppress eosinophiliaShorten the counting processSenses disorderOrganic chemistryEsophagitisEosinophilic syndrome
The present invention relates to novel biologically active glutarimide derivatives of general formula (I) or a pharmaceutically acceptable salt thereof, their use as a therapeutic agent for the treatment of eosinophilic diseases, preferably of allergic nature, in particular bronchial asthma, allergic rhinitis, polypous rhinosinusopathies, eosinophilic colitis, eosinophilic syndrome, allergic conjunctivitis, atopic dermatitis, Churg-Strauss syndrome, anaphylactic shock, Quincke's edema, eosinophilic vasculitis, eosinophylic esophagitis, eosinophilic gastroenteritis, or fibroses. The invention also relates to pharmaceutical compositions comprising glutarimide derivatives of general formula (I):
Owner:OBSCHESTVO S OGRANICHENNOI OTVETABTVENNOSTIYU PHARMENTERPRISES
Pharmaceutical composition
InactiveUS20060110449A1Reduce adverse effectsImproved degradation profileBiocideSenses disorderDiseaseAnticholinergic Drugs
Owner:SCHERING CORP
Desloratadine oral dispersible film
InactiveCN102940617ADisintegrates quicklyQuick effectOrganic active ingredientsImmunological disordersAllergic dermatitisIrritation
The invention relates to a desloratadine oral dispersible film which comprises desloratadine, biologically-acceptable water-soluble polymers, plasticizers, purified water and the like, can be dispersed or dissolved quickly in the oral cavity, and is used for treating allergic rhinitis, allergic rhinitis and asthma syndrome, allergic nasal conjunctivitis, allergic dermatitis, allergic asthma and the like. The desloratadine oral dispersible film is free of irritation, convenient to take and carry, fast in effect, good in taste and particularly suitable for being used by the olds and children.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Treatment of allergic diseases with recombinant antibodies
Recombinant antibodies (including binding fragments thereof) that bind IgE are described, along with compositions and methods of using the same in the treatment of subjects in need thereof, including subjects afflicted with atopic dermatitis, allergic rhinitis, allergic conjunctivitis, urticaria, gastro-intestinal inflammation, or oral-pharyngeal inflammation. In some embodiments, the antibody is a single chain variable fragment (scFv) or a disulfide linked variable fragment (sdFv). In some embodiments, the subject is a dog, cat, or horse.
Owner:NORTH CAROLINA STATE UNIV
Chinese herbal preparation used for curing allergic conjunctivitis
InactiveCN104383275ALow recurrence rateGuaranteed curative effectSenses disorderAnthropod material medical ingredientsDiseaseHerbal preparations
The invention provides Chinese herbal preparation used for curing the allergic conjunctivitis. The Chinese herbal preparation comprises the active ingredients of ephedra, broom cypress fruit, radix sileris, notopterygium root, honeysuckle, densefruit pittany root-bark, hairyvein agrimonia herb and bud, scouring rush, orthostachyus, angelica dahurica, vine of multiflower knotweed, cnidium fruit, polygonum aviculare, euphorbia hirta, paniculate swallowwort root, honeycomb, hypericum sampsonii hance, loranthus parasiticus, talinum crassifolium, centipeda minima, fragrant solomonseal rhizome, liparis nervosa, lindernia ruellioides and liquorice. The Chinese herbal preparation can reduce rate of relapse, can act on ocular surface diseases in relatively long time, maintains curative effect, and reduces side effect.
Owner:NINGBO UNIV
Traditional Chinese medical composition for treating allergic conjunctivitis
InactiveCN104096141AEffective cureShort course of treatmentSenses disorderAnthropod material medical ingredientsRegimenSide effect
The invention discloses a traditional Chinese medical composition for treating allergic conjunctivitis, and belongs to the field of traditional Chinese medicine. The traditional Chinese medical composition is prepared from the following drug substances in parts by weight: 8-12 parts of ash bark, 8-12 parts of concha haliotidis, 8-10 parts of natural indigo, 10-15 parts of wild chrysanthemum flowers, 8-10 parts of plantain seeds, 8-12 parts of dandelion, 6-10 parts of rhizoma anemarrhenae, 6-10 parts of broom cypress fruits, 5-8 parts of honeycombs, 5-8 parts of cicada ecdysis, 5-8 parts of Asiatic moonseed rhizome, 5-8 parts of fragrant solomonseal rhizome, 6-10 parts of selfheal, 5-8 parts of poria cocos and 5-8 parts of bunge corydalis herb. The traditional Chinese medical composition adopts natural traditional Chinese medicine as raw materials, is short in treatment course, definitive in therapeutic effect and free of toxic and side effects, can effectively cure allergic conjunctivitis, and prevents relapse of allergic conjunctivitis, and the total effective rate reaches up to 98%.
Owner:马玲
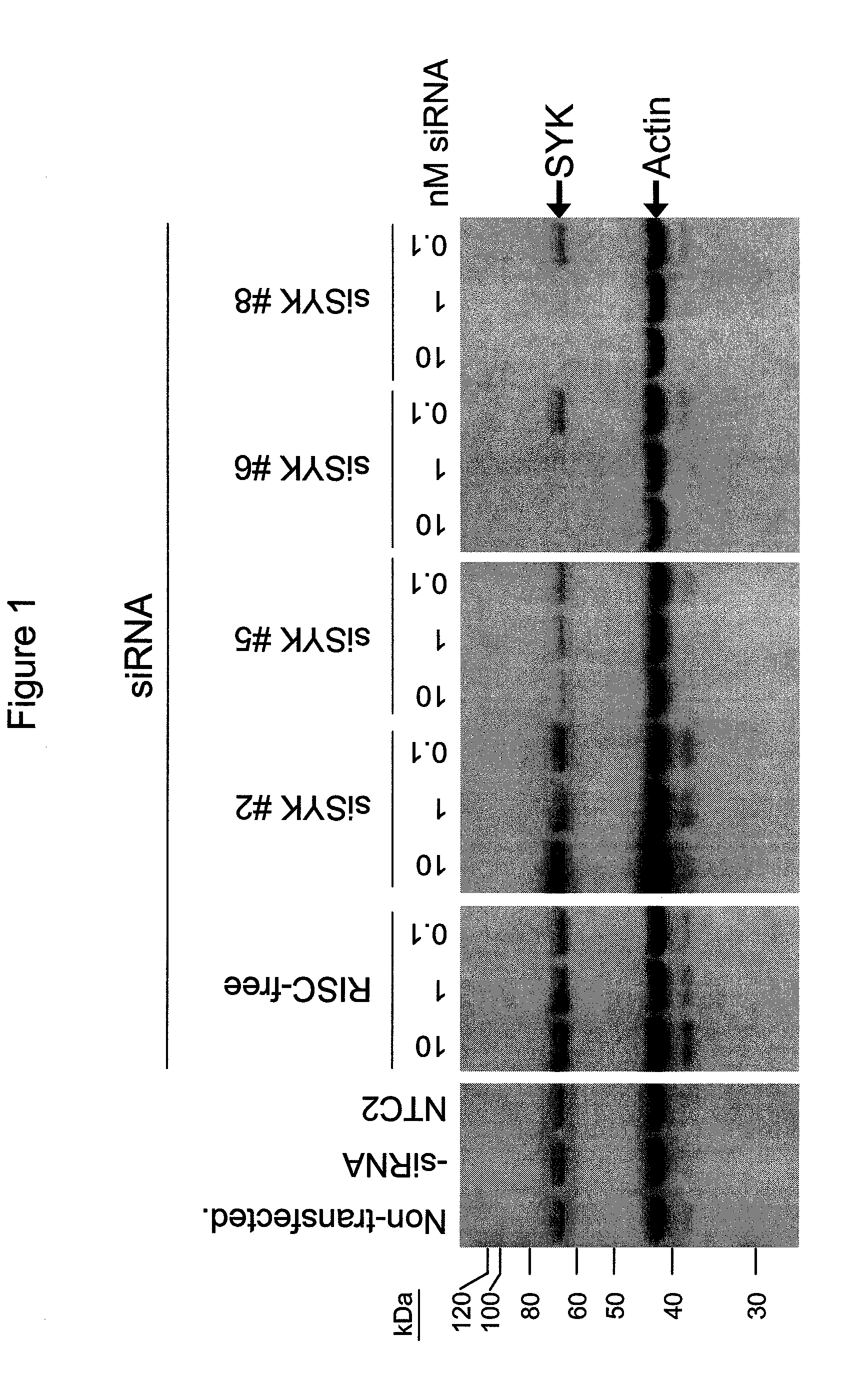
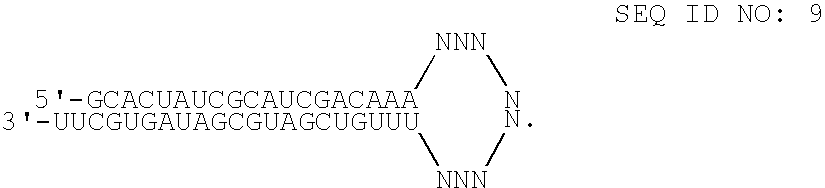
RNAi-mediated inhibition of spleen tyrosine kinase-related inflammatory conditions
Owner:ARROWHEAD RES CORP
Pyrazolo (3, 4-b) pyridine derivatives as phosphodiesterase inhibitors
InactiveCN101801376AOrganic active ingredientsSenses disorderRESPIRATORY DISTRESS SYNDROME ADULTT cell
The present invention relates to phosphodiesterase (PDE) type 4, phosphodiesterase (PDE) type 7 and dual PDE type 4 / PDE type 7 inhibitors. Compounds disclosed herein having the structure of Formura 1: can be useful in the treatment, prevention, inhibition or suppression of CNS diseases, for example, multiple sclerosis; various pathological conditions such as diseases affecting the immune system, including AIDS, rejection of transplant, auto-immune disorders such as T-cell related diseases, for example, rheumatoid arthritis; inflammatory diseases such as respiratory inflammation diseases including chronic obstructive pulmonary disease (COPD), asthma, bronchitis, allergic rhinitis, adult respiratory distress syndrome (ARDS) and other inflammatory diseases including but not limited to psoriasis, shock, atopic dermatitis, eosinophilic granuloma, allergic conjunctivitis, osteoarthritis; gastrointestinal inflammation diseases such as Crohn's disease, colitis, pancreatitis as well as different types of cancers including leukaemia; especially in humans. Processes for the preparation of disclosed compounds, pharmaceutical compositions containing the disclosed compounds and their use as PDE type 4, PDE type 7 and dual PDE type 4 / PDE type 7 inhibitors are provided.
Owner:RANBAXY LAB LTD
Ophthalmic compositions and methods of use
ActiveUS20180333414A1Small amountEliminate side effectsOrganic active ingredientsSenses disorderAntigenHerpes zoster keratitis
The present invention provides a composition comprising two or more of the following pharmaceutically active compounds: (i) an alpha 2 adrenergic agonist; (ii) a corticosteroid; (iii) a lymphocyte function-associated antigen antagonist; (iv) a non-steroidal anti-inflammatory drug (NSAID); (v) a sodium channel blocker; and (vi) an antibiotic, provided at least one of the pharmaceutically active compound is selected from the group consisting of (i) alpha 2 adrenergic agonist and (ii) corticosteroid. The present invention also provides a method for using such composition to treat an eye disorder such as a dry eye syndrome; ocular graft-versus-host-disease; ocular rosacea; allergic conjunctivitis; autoimmune ocular surface disease; thygeson's superficial punctuate keratopathy; herpes zoster keratitis; Stevens-Johnson syndrome; keratitis; conjunctivitis; blepharitis; blepharochalasis; conjunctivochalasis; blepharoconjunctivitis; blepharokeratoconjunctivitis; post-operative inflammation or pain from ocular surgery; scleritis; episcleritis; anterior uveitis; iritis; cyclitis; ocular surface vascular disorder; ulcerative keratitis; photokeratitis; dacryocystitis; eyelid disorder; congenital alacrima; xerophthalmia; dacryoadenitis; vernal keratoconjunctivitis; pinguecula; and / or ocular surface disorder induced by chemical burns, thermal burns, or physical insult to the ocular surface.
Owner:OCUGEN INC
RNAi-mediated inhibition of tumor necrosis factor alpha-related conditions
The present invention provides a RNA interferencefor inhibition of tumor necrosis factor a (TNFa) by silencing TNFa cell surface receptor TNF receptor-1 (TNFR1) mRNA expression, or by silencing TNFa converting enzyme (TACE / ADAM17) mRNA expression. Silencing such TNFa targets, in particular, is useful for treating patients having a TNFa-related condition or at risk of developing a TNFa-related condition such as the ocular conditions dry eye, allergic conjunctivitis, or ocular inflammation, or such as dermatitis, rhinitis, or asthma, for example.
Owner:ARROWHEAD RES CORP
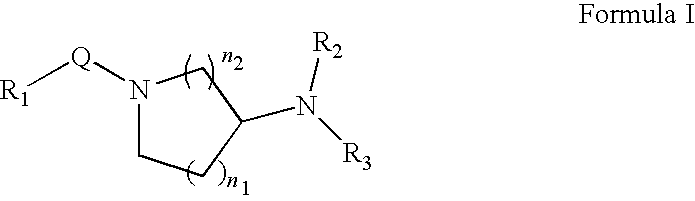
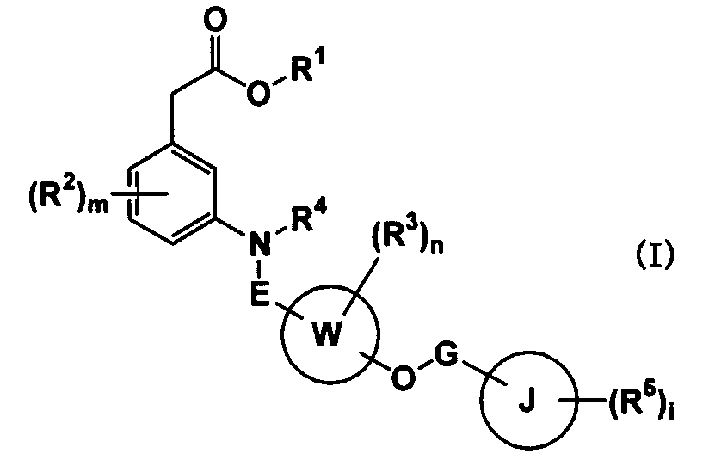
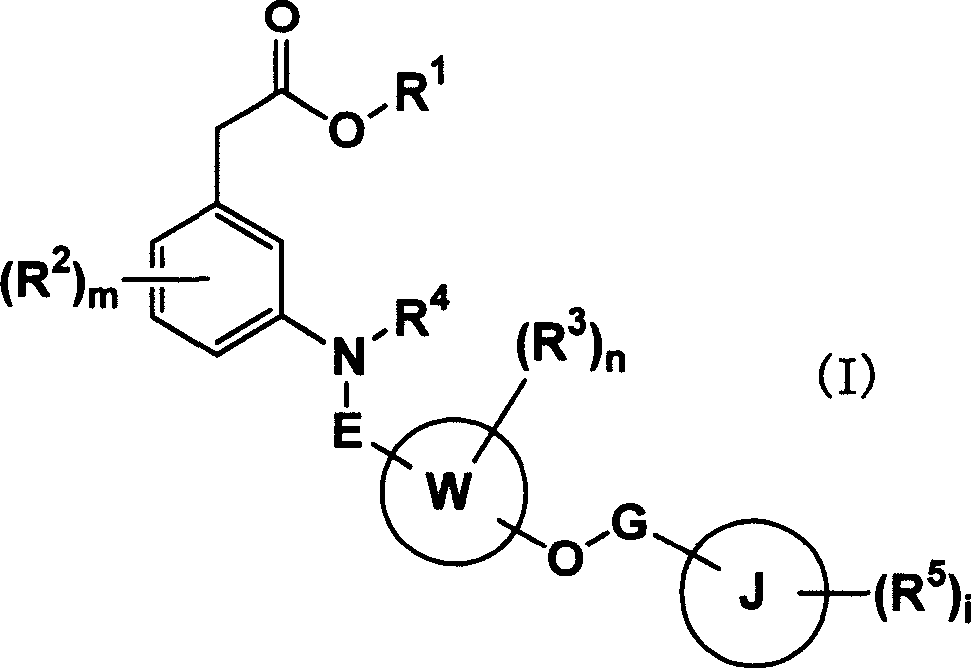
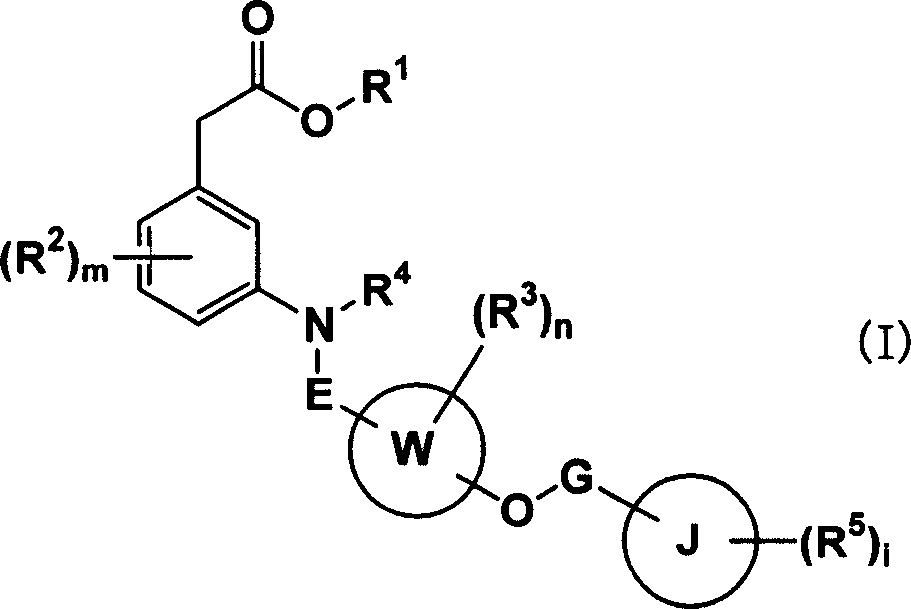
Carboxylic acid compounds and drugs containing the compounds as the active ingredient
A carboxylic acid compound represented by formula (I): (meanings of the symbols in the formula are as mentioned in the specification) and a pharmaceutical agent comprising the compound. <>Since the compound represented by formula (I) binds to a DP receptor and shows antagonistic activity for the DP receptor, it is useful for prevention and / or treatment of diseases such as allergic disease (such as allergic rhinitis, allergic conjunctivitis, atopic dermatitis, bronchial asthma and food allergy), systemic mastocytosis, disorders accompanied by systemic mast cell activation, anaphylaxis shock, bronchoconstriction, urticaria, eczema, diseases accompanied by itch (such as atopic dermatitis and urticaria), diseases (such as cataract, retinal detachment, inflammation, infection and sleeping disorders) which is generated secondarily as a result of behavior accompanied by itch (such as scratching and beating), inflammation, chronic obstructive pulmonary diseases, ischemic reperfusion injury, cerebrovascular accident, chronic rheumatoid arthritis, pleurisy, ulcerative colitis and the like. Carboxylic acid compounds represented by the following general formula (I) (wherein each symbol is as defined in the description) and drugs containing these compounds: (I) Because of binding to DP receptor and antagonizing the same, the compounds represented by the general formula (I) are useful in preventing and / or treating allergic diseases (allergic nephritis, allergic conjunctivitis, atopic dermatitis, bronchial asthma, food allergy, etc.), systemic mast cell disease, systemic mast cell activation failure, anaphylactic shock, respiratory tract contraction, urticaria, eczema, diseases associated with itch (atopic dermatitis, urticaria, etc.), diseases (cataract, retinal detachment, inflammation, infection, sleep disorder, etc.) secondarily caused by behaviors associating itch (scratching, beating.
Owner:ONO PHARMA CO LTD
Eye drop containing olopatadine hydrochloride and preparation method thereof
InactiveCN110013498AImprove stabilityGuaranteed stabilityOrganic active ingredientsSenses disorderTreatment effectSide effect
The present invention belongs to the technical field of medicines and particularly relates to an eye drop containing olopatadine hydrochloride and a preparation method thereof. The provided eye drop containing the olopatadine hydrochloride mainly consists of the olopatadine hydrochloride, a cassia seed extract, pharmaceutically acceptable eye drop accessory materials and water for injection. Every100 mL of the eye drop contains 0.095-0.110 g of the olopatadine hydrochloride, and a weight ratio of the olopatadine hydrochloride to the cassia seed extract is 1:(1-3). The provided eye drop containing the olopatadine hydrochloride has advantages of remarkable therapeutic effects, high in stability, small toxic and side effects, etc., and is a relatively ideal eye drop for treating allergic conjunctivitis.
Owner:合肥华威药业有限公司
Benzothiophenecarboxamide derivatives and PGD2 antagonists comprising them
The present invention provides a compound, a pharmaceutically acceptable salt thereof, or a hydrate thereof having PGD2-antagonistic activities, inhibitory activities against infiltration of eosinophils, and being useful as a drug for treating diseases, such as systemic mastocytosis and disorder of systemic mast cell activation, as well as tracheal contraction, asthma, allergic rhinitis, allergic conjunctivitis, urticaria, ischemic reperfusion injury, inflammation and atopic dermatitis, which is shown by the following formula (I).
Owner:SHIONOGI & CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com