Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
381 results about "Ocular surface" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
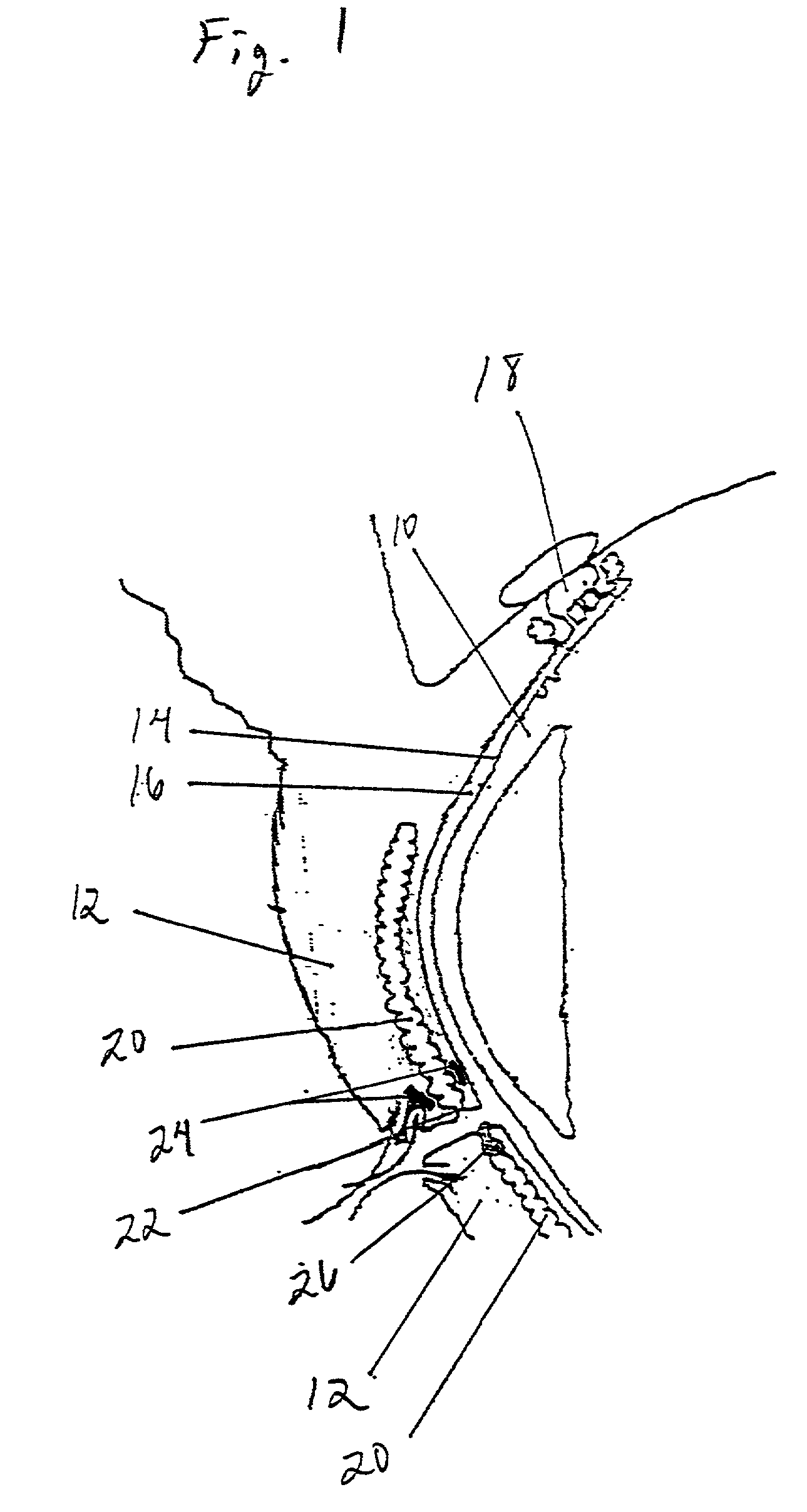
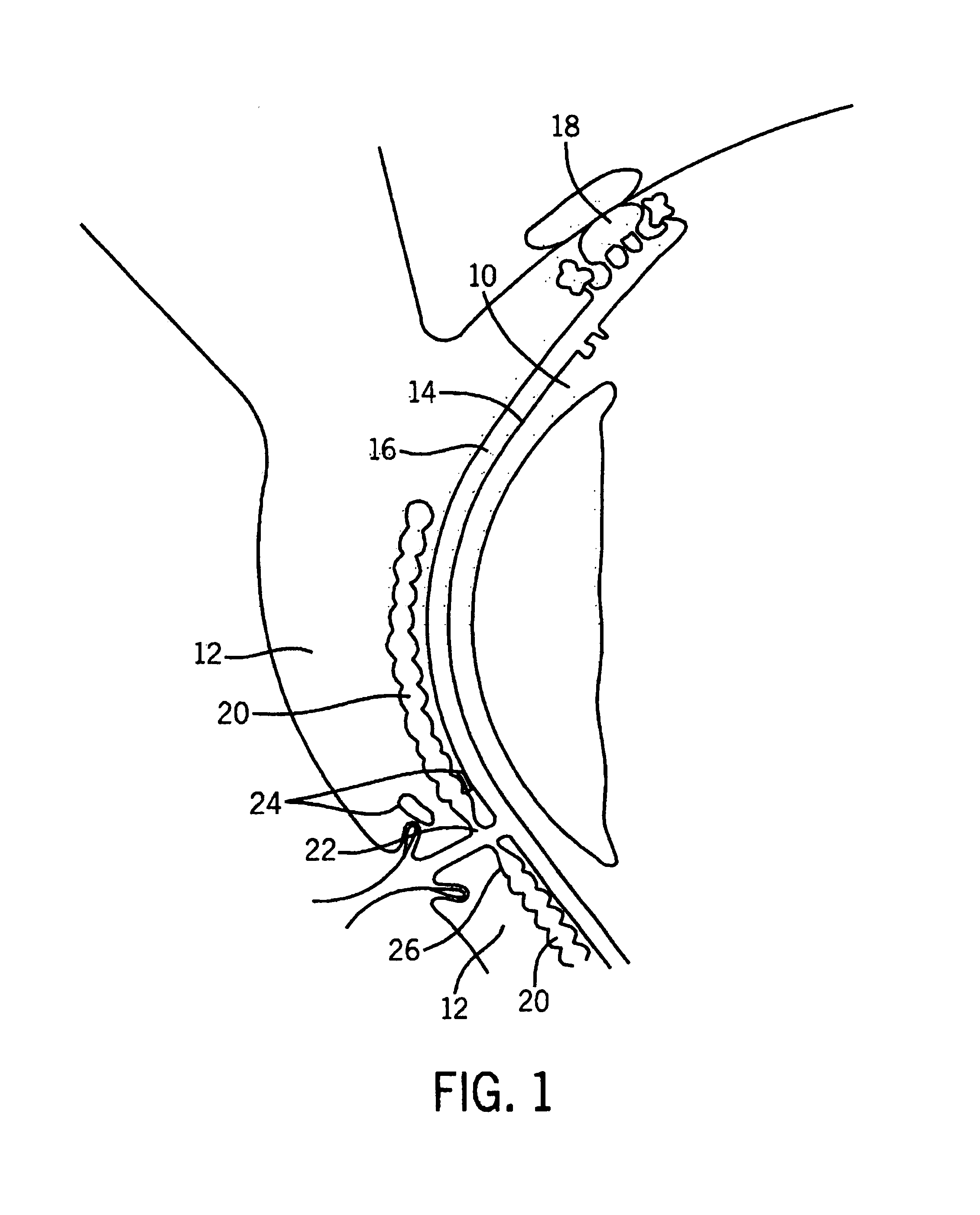
Method and apparatus for preventing and treating eyelid problems
A method and apparatus are provided by which eyelid diseases may be treated and eyelid hygiene may be performed. In particular, the method and apparatus stimulate eyelid muscles and facial muscles, allowing stimulation of glandular eyelid components that allow optimization of the tear film and the ocular surface. In this manner, the symptoms associated with ocular irritation or with eyelid disorders may be treated by maintaining proper tear film composition.
Owner:SEEFIT
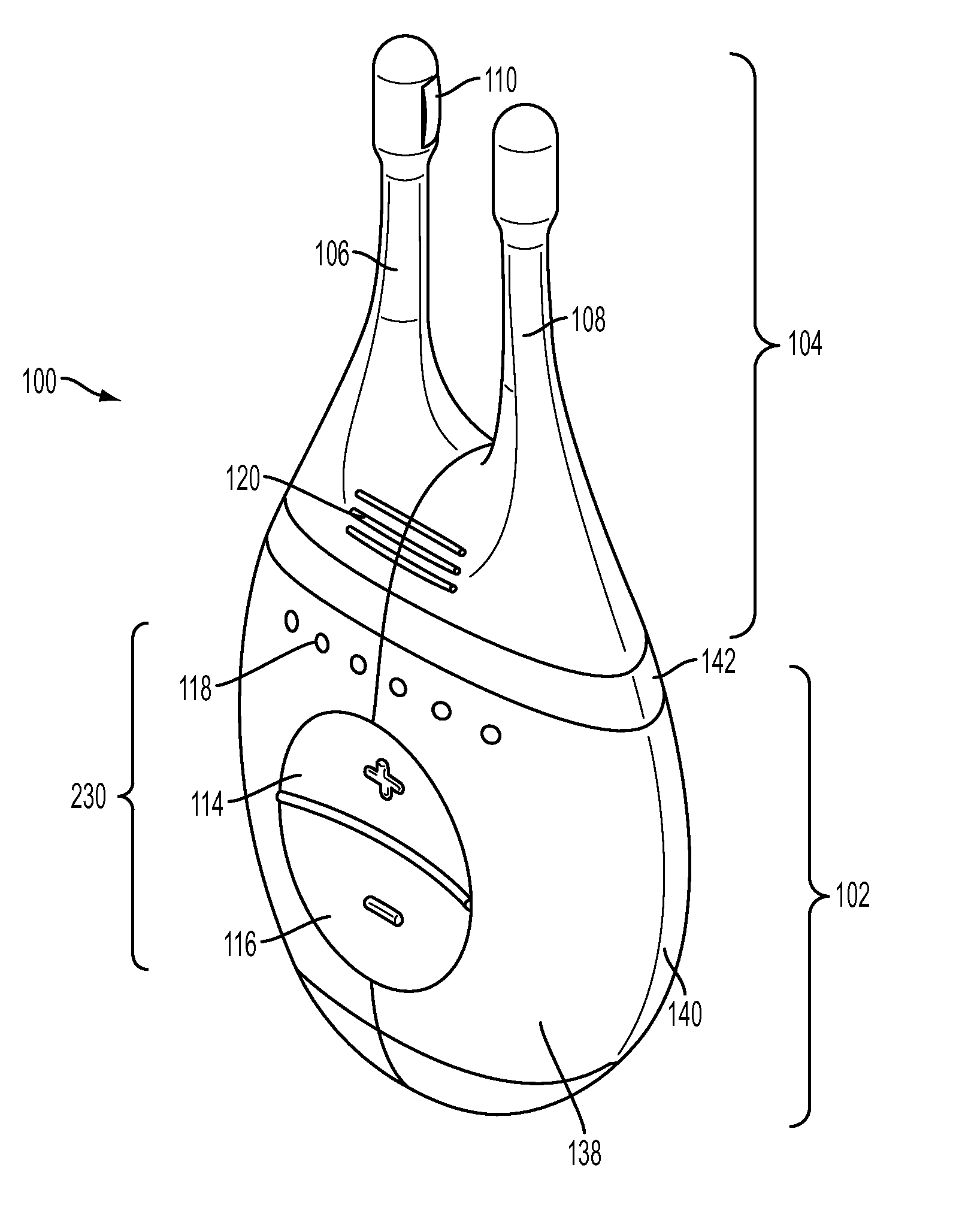
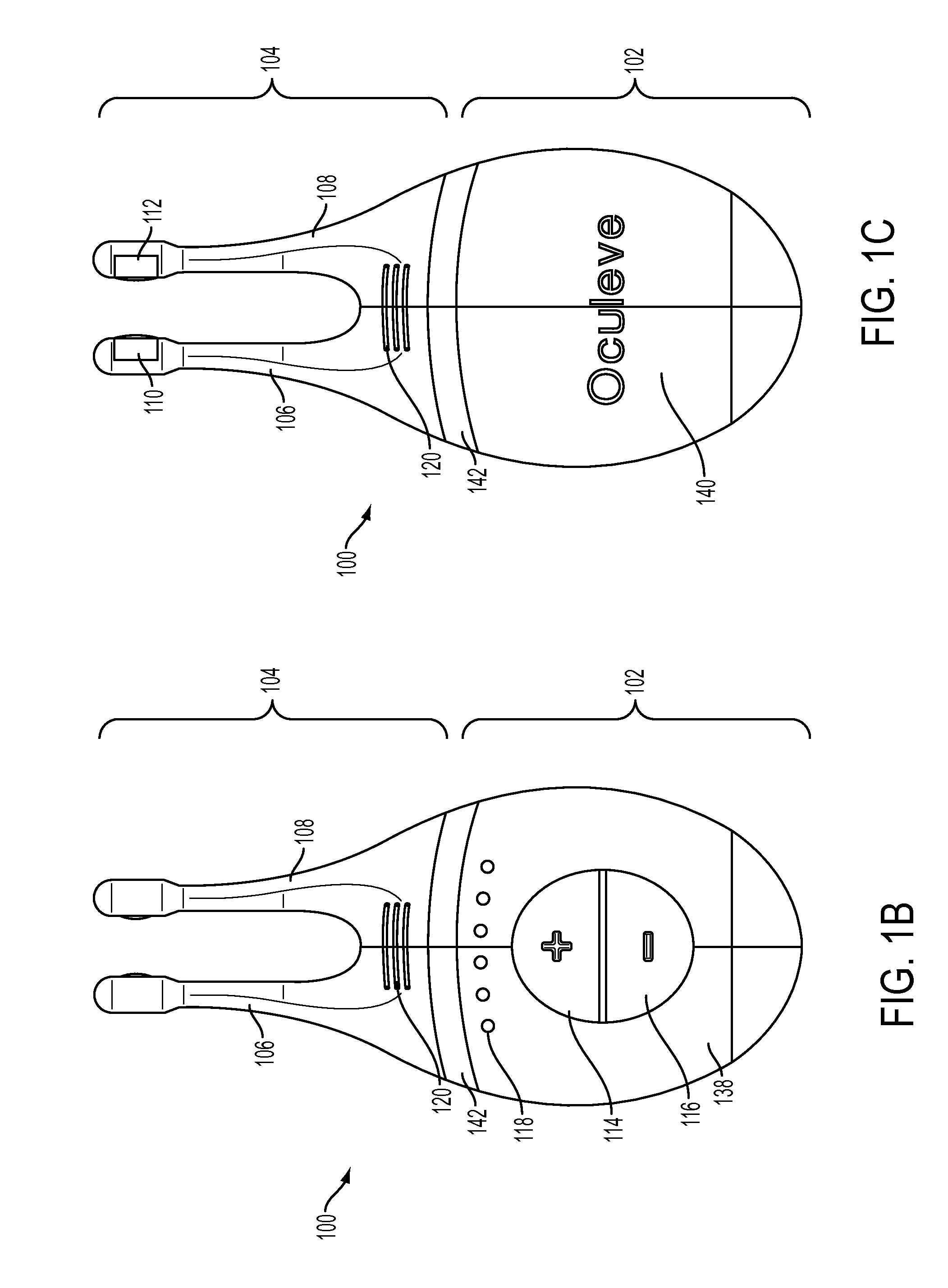
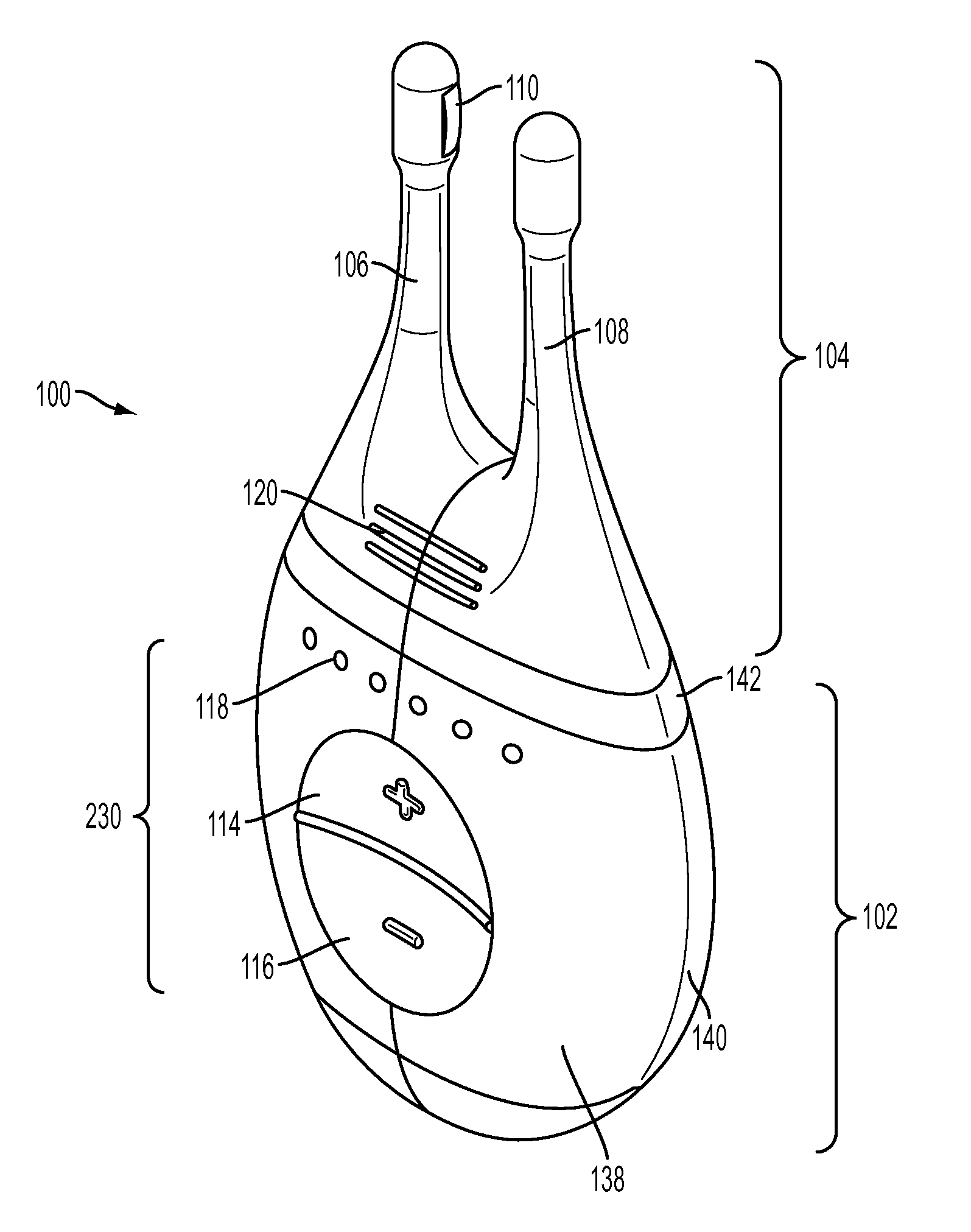
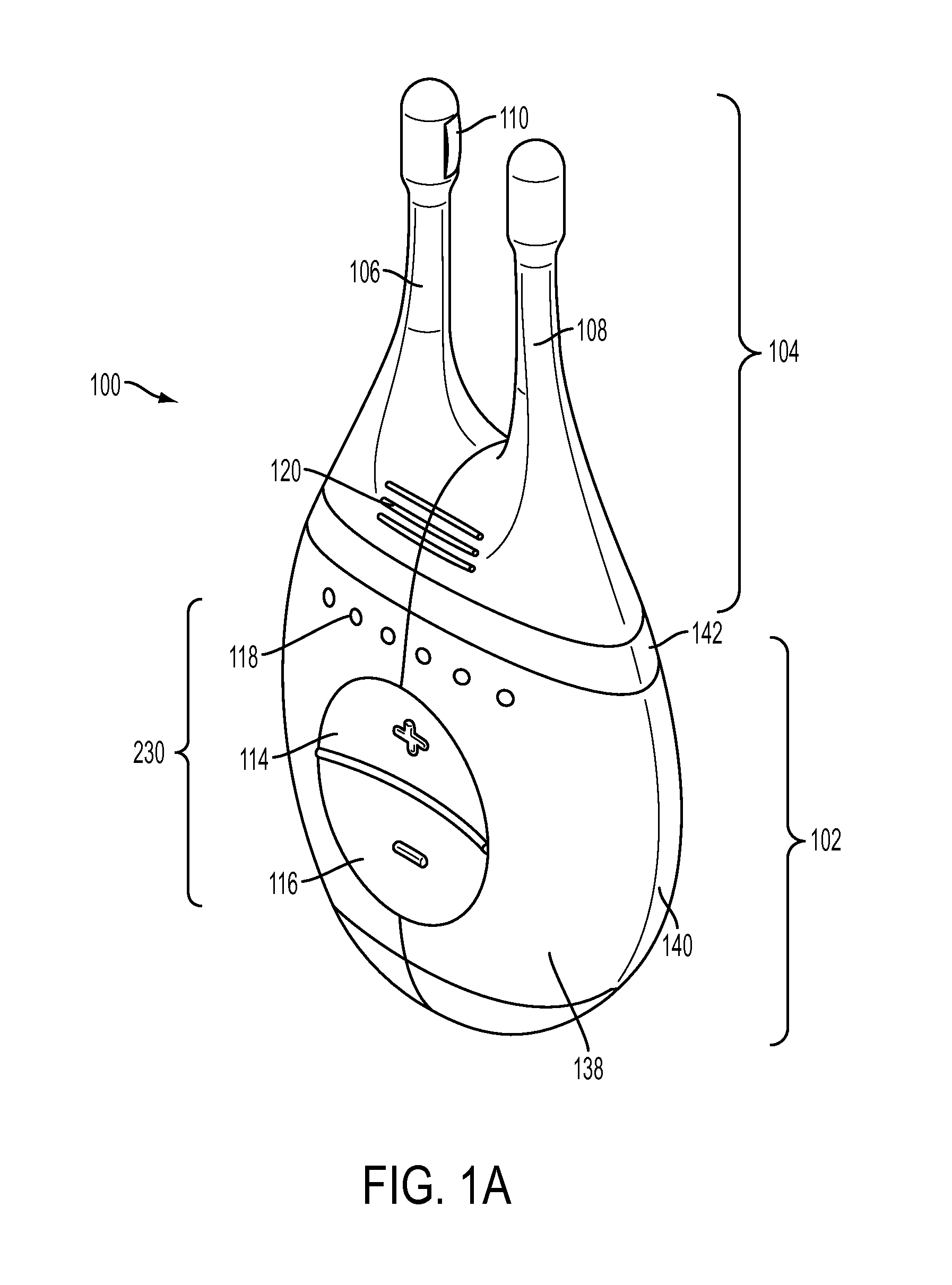
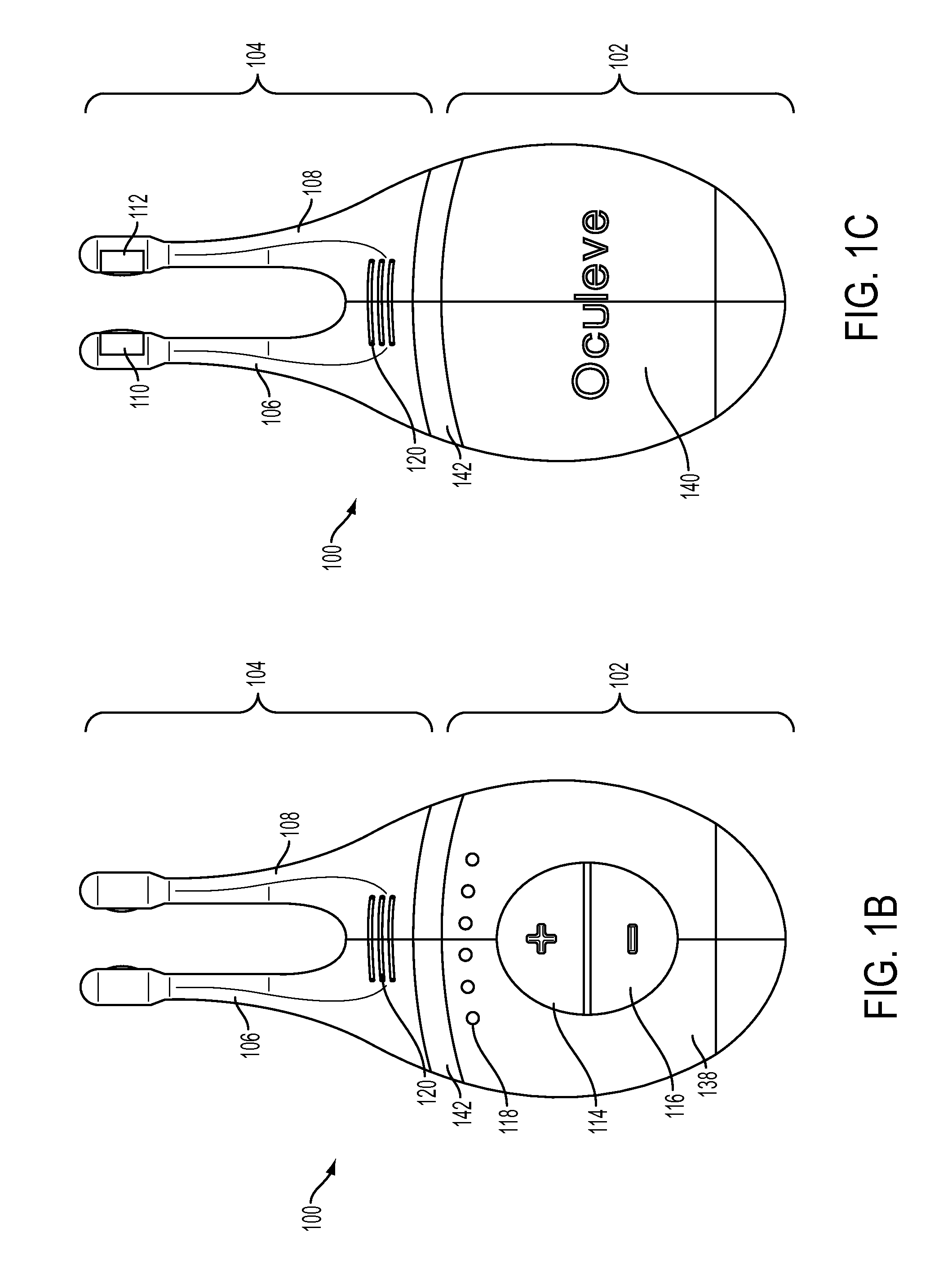
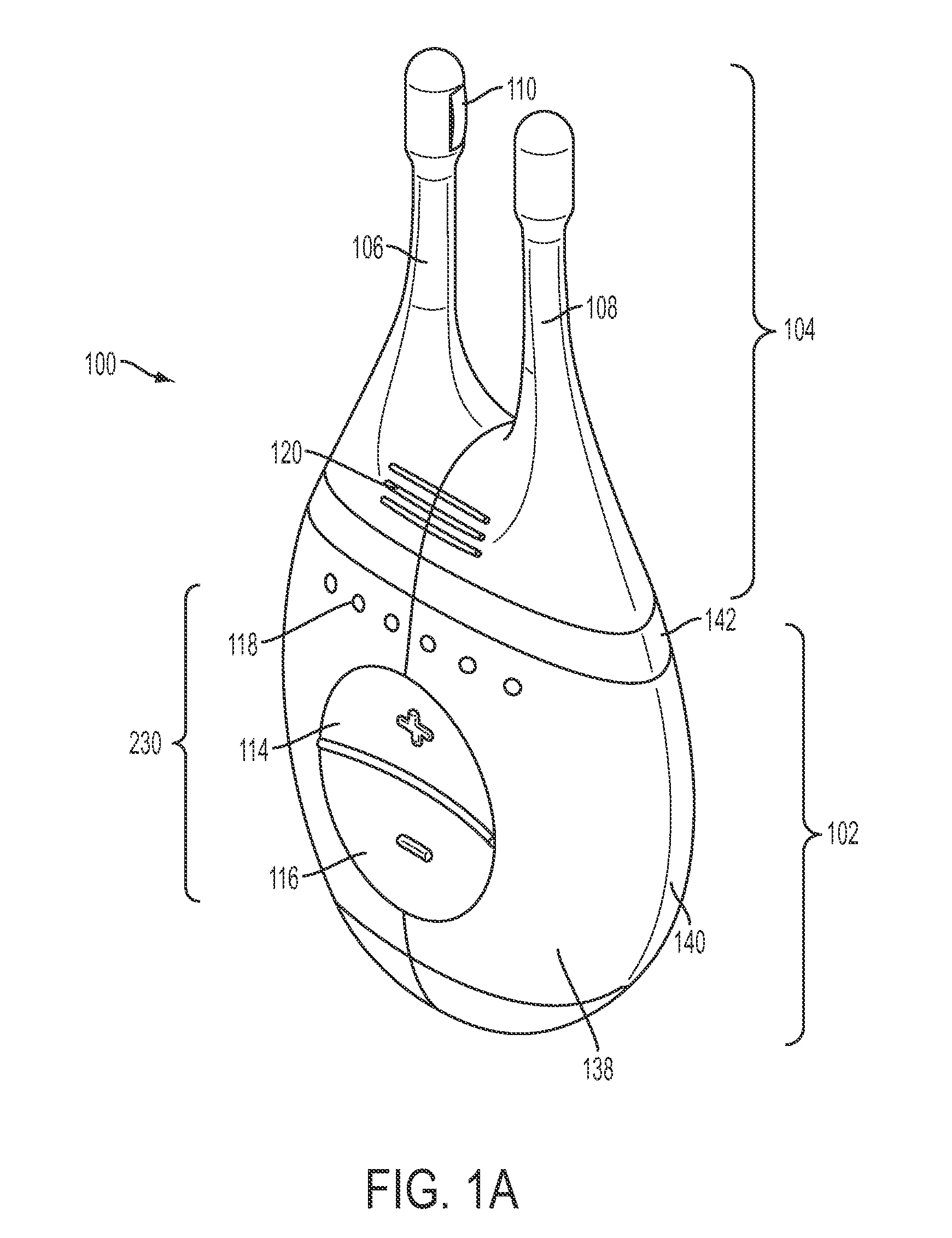
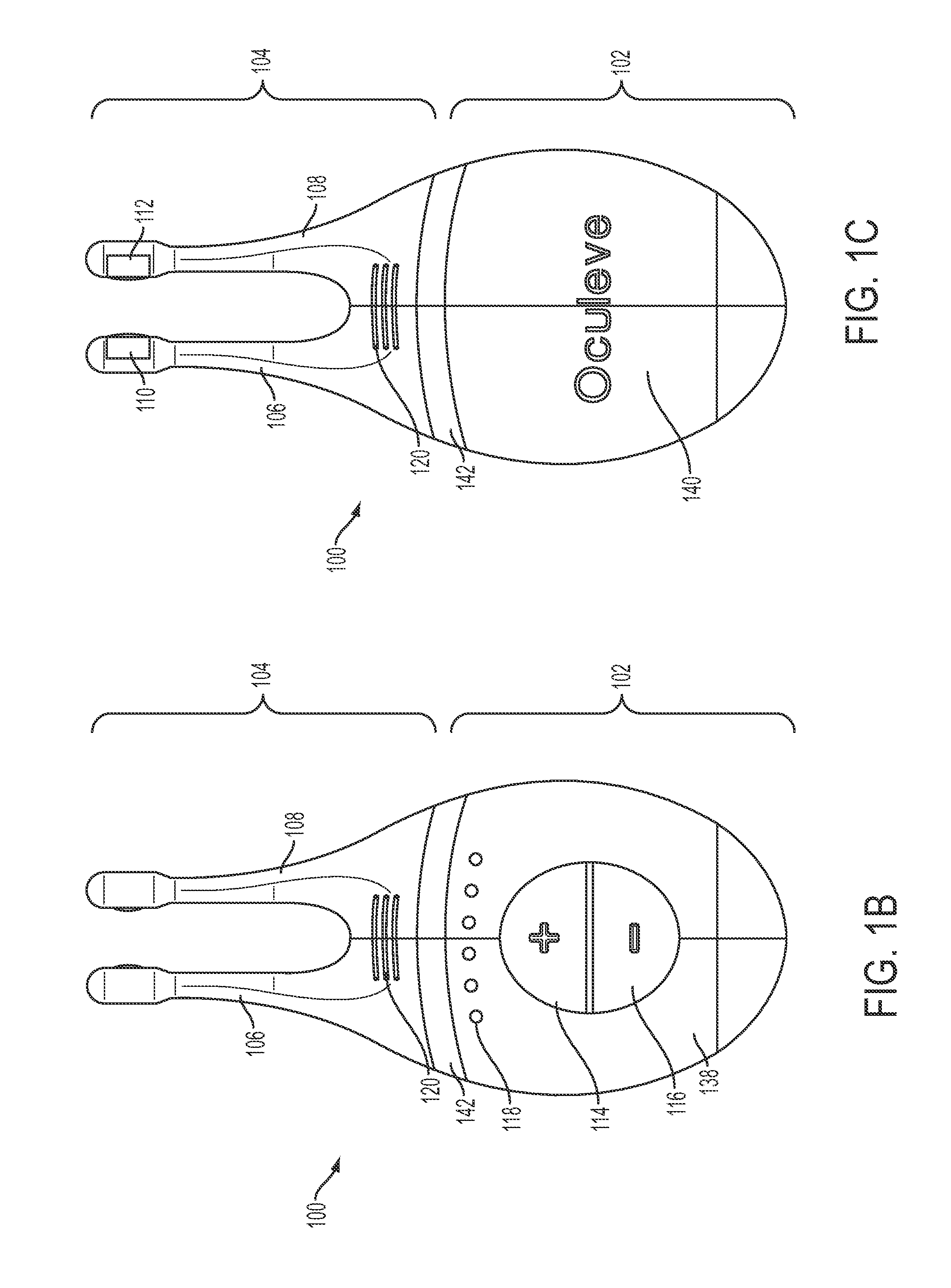
Nasal stimulation devices and methods
ActiveUS20140316310A1Promotes eye healthLowering indexUltrasound therapyBatteries circuit arrangementsNoseHand held devices
Described here are devices, systems, and methods for treating one or more conditions (such as dry eye) or improving ocular health by providing stimulation to nasal or sinus tissue. Generally, the devices may be handheld or implantable. In some variations, the handheld devices may have a stimulator body and a stimulator probe having one or more nasal insertion prongs. When the devices and systems are used to treat dry eye, nasal or sinus tissue may be stimulated to increase tear production, reduce the symptoms of dry eye, and / or improve ocular surface health.
Owner:OCULEVE
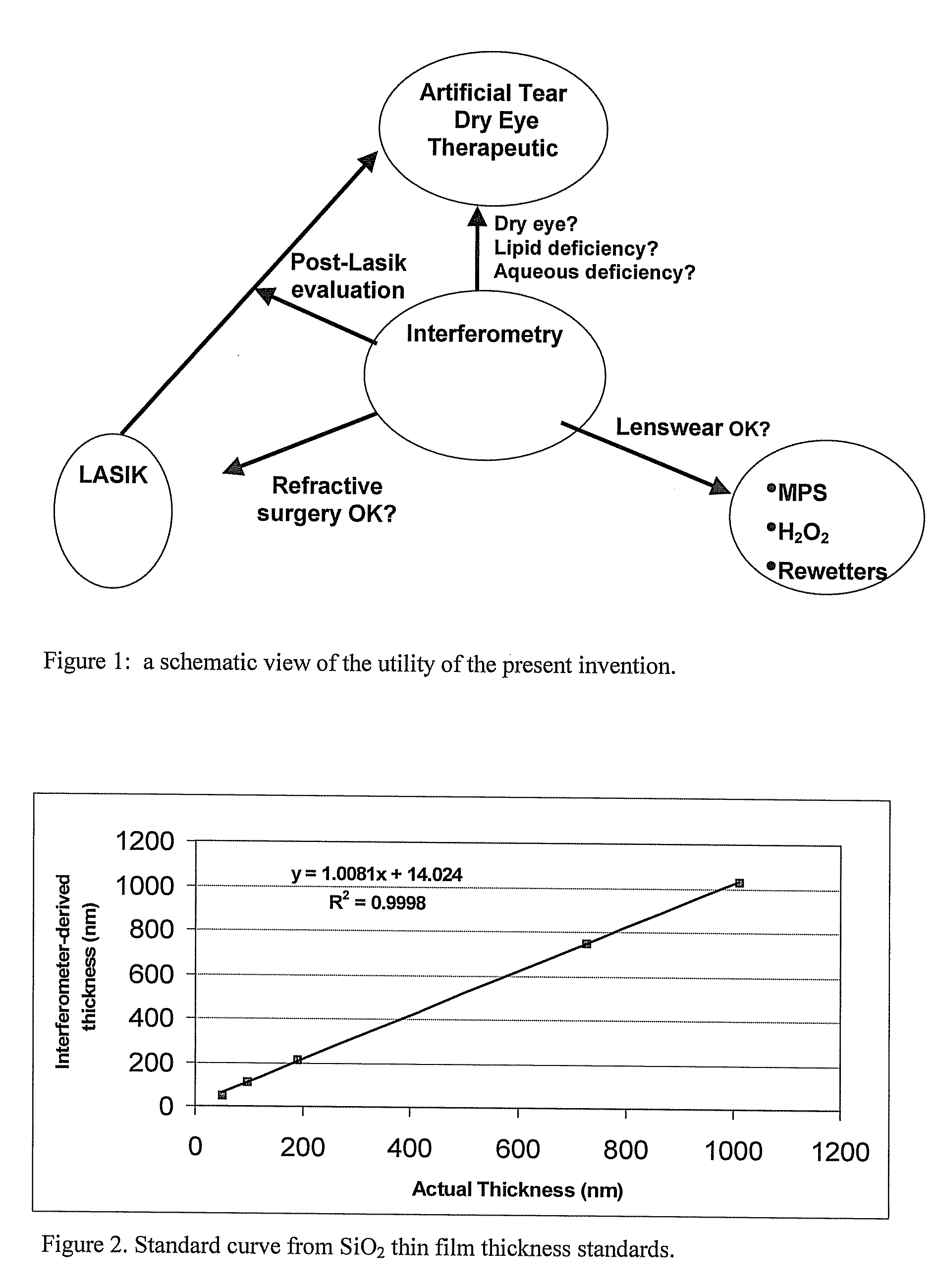
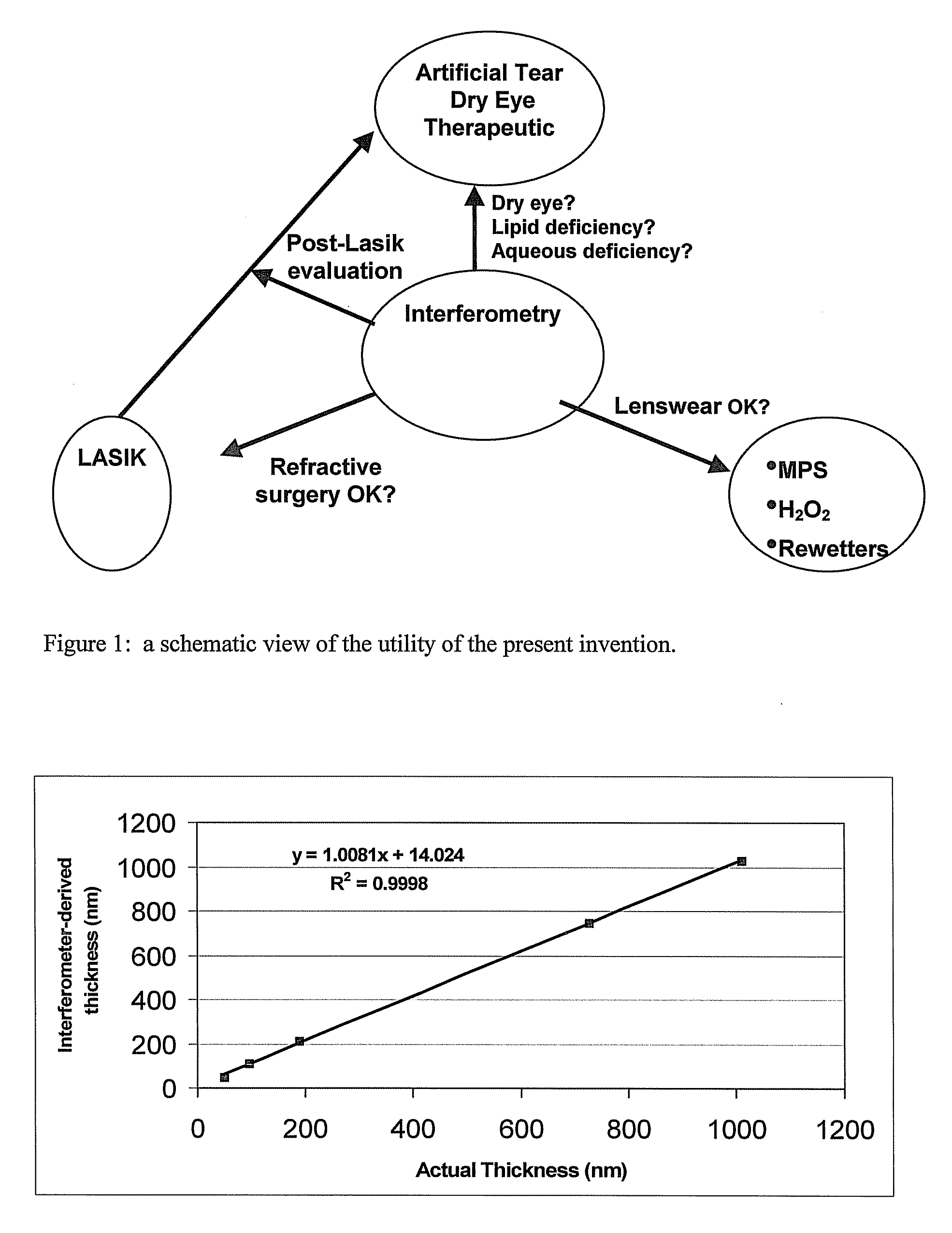
Methods and devices for measuring tear film and diagnosing tear disorders
Methods and devices measure eye blinks and tear film lipid and aqueous layer thickness before and following ophthalmic formula application onto the ocular surface, especially wherein the ophthalmic formula is an artificial tear. The methods and devices are suitable for dry eye diagnosis. The methods and devices are suitable for use to evaluate ophthalmic formula effects on the tear film and to use such information to diagnose ophthalmic formula treatment of ocular disease conditions such as dry eye in the absence of contact lens wear or post-surgical eye drop treatment and diagnosis. The methods and devices are also suitable for use in the optimization of ophthalmic drug dosage forms and sustained drug release.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Methods and devices for measuring tear film and diagnosing tear disorders
Methods and devices measure eye blinks and tear film lipid and aqueous layer thickness before and following ophthalmic formula application onto the ocular surface, especially wherein the ophthalmic formula is an artificial tear. The methods and devices are suitable for dry eye diagnosis. The methods and devices are suitable for use to evaluate ophthalmic formula effects on the tear film and to use such information to diagnose ophthalmic formula treatment of ocular disease conditions such as dry eye in the absence of contact lens wear or post-surgical eye drop treatment and diagnosis. The methods and devices are also suitable for use in the optimization of ophthalmic drug dosage forms and sustained drug release.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Intraocular delivery compositions and methods
The present invention relates to intraocular drug delivery for treating ocular diseases. Particularly, the invention relates to particles useful for the delivery of certain pharmacologically active agents to treat ocular diseases. The particles contain calcium phosphate core particles, particularly nanoparticles, as delivery agents and adjuvants. The invention also relates to methods of making such particles and to methods of treating ocular disease by delivery of a therapeutic drug to an ocular surface using the particles of this invention. The invention further relates to methods of regulating ocular pressure using certain formulations according to the present invention.
Owner:BIOSANTE PHARMA
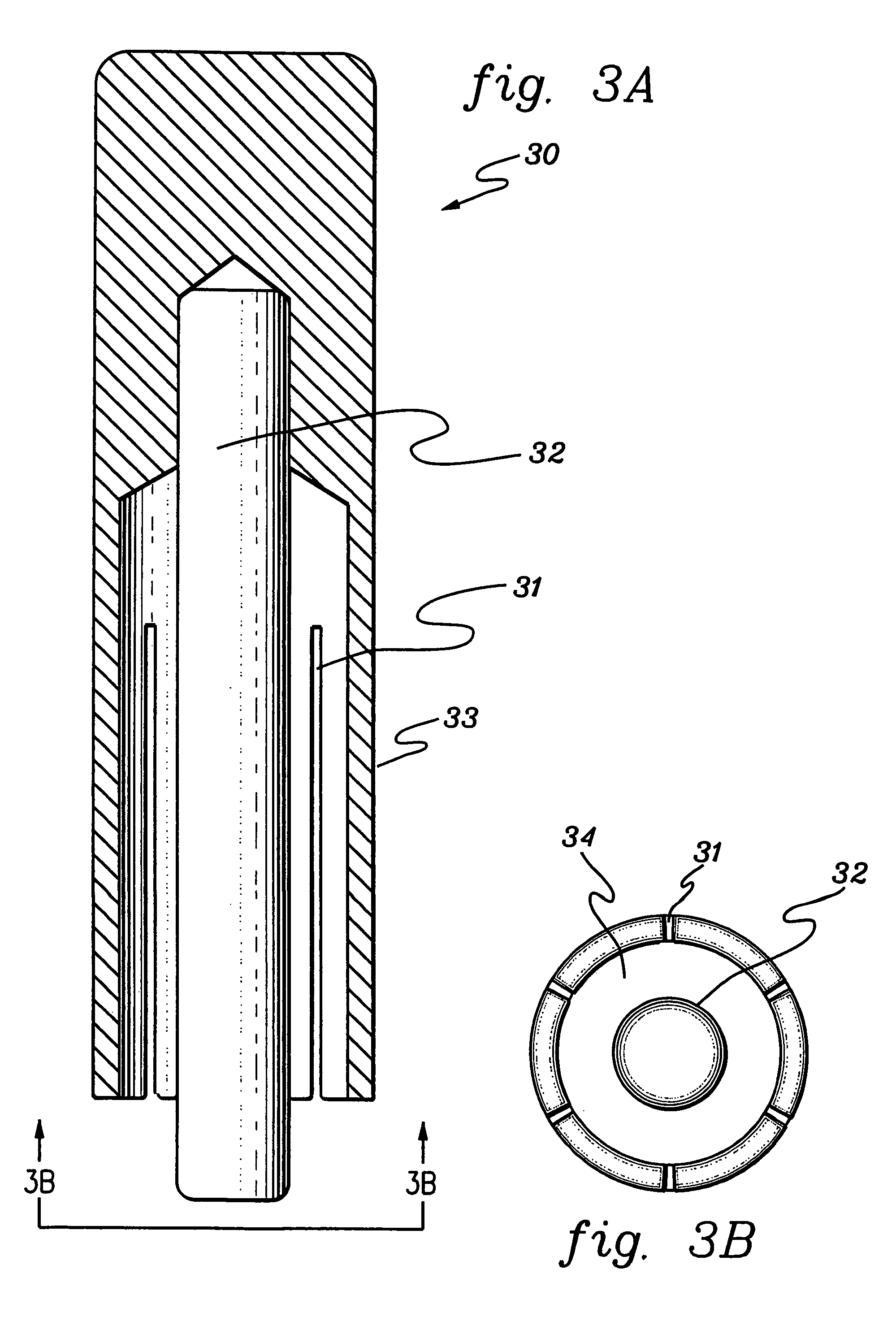
Amniotic membrane covering for a tissue surface and devices facilitating fastening of membranes
ActiveUS7494802B2Reduce inflammationPromote wound healingBioreactor/fermenter combinationsBiological substance pretreatmentsBandage contact lensDelivery vehicle
The present invention relates to a biopolymer covering for a tissue surface including, for example, a dressing, a bandage, a drape such as a bandage contact lens, a composition or covering to protect tissue, a covering to prevent adhesions, to exclude bacteria, to inhibit bacterial activity, or to promote healing or growth of tissue. An example of such a composition is an amniotic membrane covering for an ocular surface. Use of a covering for a tissue surface according to the invention eliminates the need for suturing. The invention also includes devices facilitating the fastening of a membrane to a support, culture inserts, compositions, methods, and kits for making and using coverings for a tissue surface and culture inserts. Compositions according to the invention may include cells grown on a membrane or attached to a membrane, and such compositions may be used as scaffolds for tissue engineering or tissue grafts. A method of preparing and using an amniotic membrane covering for a tissue surface as a controlled release drug delivery vehicle is also disclosed.
Owner:TISSUETECH INC
Lubricant for the ocular surface
InactiveUS20050202097A1Easy to spreadProcess stabilityBiocideSenses disorderEye/ear dropsBlurred vision
A formulation has been developed for treatment of the symptoms of dry eye which incorporates the natural product jojoba wax, or components thereof, to enhance the spreading of the artificial tear and eyedrop as well as stabilize the eyedrop. The improved performance of the jojoba wax supplemented tear relieves irritation and discomfort as well as sharpens the blurred vision.
Owner:MELBJ HLDG
Nasal stimulation devices and methods
ActiveUS20140316485A1Promotes eye healthLowering indexUltrasound therapyBatteries circuit arrangementsNoseHand held devices
Described here are devices, systems, and methods for treating one or more conditions (such as dry eye) or improving ocular health by providing stimulation to nasal or sinus tissue. Generally, the devices may be handheld or implantable. In some variations, the handheld devices may have a stimulator body and a stimulator probe having one or more nasal insertion prongs. When the devices and systems are used to treat dry eye, nasal or sinus tissue may be stimulated to increase tear production, reduce the symptoms of dry eye, and / or improve ocular surface health.
Owner:OCULEVE
Ophthalmic compositions and methods for treating eyes
ActiveUS20060106104A1Harm reductionEasy and cost-effective to manufactureBiocideSenses disorderOcular surfaceAdverse effect
Ophthalmic compositions including compatible solute components and / or polyanionic components are useful in treating eyes, for example, to relieve dry eye syndrome, to protect the eyes against hypertonic insult and / or the adverse effects of cationic species on the ocular surfaces of eyes and / or to facilitate recovery from eye surgery.
Owner:ALLERGAN INC
Use of connective tissue mast cell stabilizers to facilitate ocular surface re-epithelization and wound repair
InactiveUS20080139531A1Inhibition releaseOvercomes drawbackBiocideOrganic chemistryConjunctival woundDihydropyridine
Disclosed are methods of treating a wound in a subject that involve administering to the subject a pharmaceutically effective amount of a composition that includes one or more human connective tissue mast cell stabilizers, wherein administration of the composition results in treatment of the wound. In particular embodiments, the wound is an ophthalmic or dermal wound, such as a corneal epithelial defect, a conjunctival wound, or dermal abrasion. Administration, for example, may be by topical application of the composition to the ocular surface or skin. Exemplary mast cell stabilizers include olopatadine, variants of olopatadine, alcaftidine, derivatives of alcaftidine, dihydropyridines, and spleen tyrosine kinase inhibitors.
Owner:ALCON RES LTD
Nasal stimulation devices and methods
ActiveUS20140371812A1Promotes eye healthDecreased corneal staining or conjunctival stainingUltrasound therapyBatteries circuit arrangementsNoseHand held devices
Described here are devices, systems, and methods for treating one or more conditions (such as dry eye) or improving ocular health by providing stimulation to nasal or sinus tissue. Generally, the devices may be handheld or implantable. In some variations, the handheld devices may have a stimulator body and a stimulator probe having one or more nasal insertion prongs. When the devices and systems are used to treat dry eye, nasal or sinus tissue may be stimulated to increase tear production, reduce the symptoms of dry eye, and / or improve ocular surface health.
Owner:OCULEVE
Lubricant for the ocular surface
InactiveUS20120128763A1Easy to spreadStabilize the tear filmOrganic active ingredientsSenses disorderNatural productIrritation
Owner:MELBJ HLDG
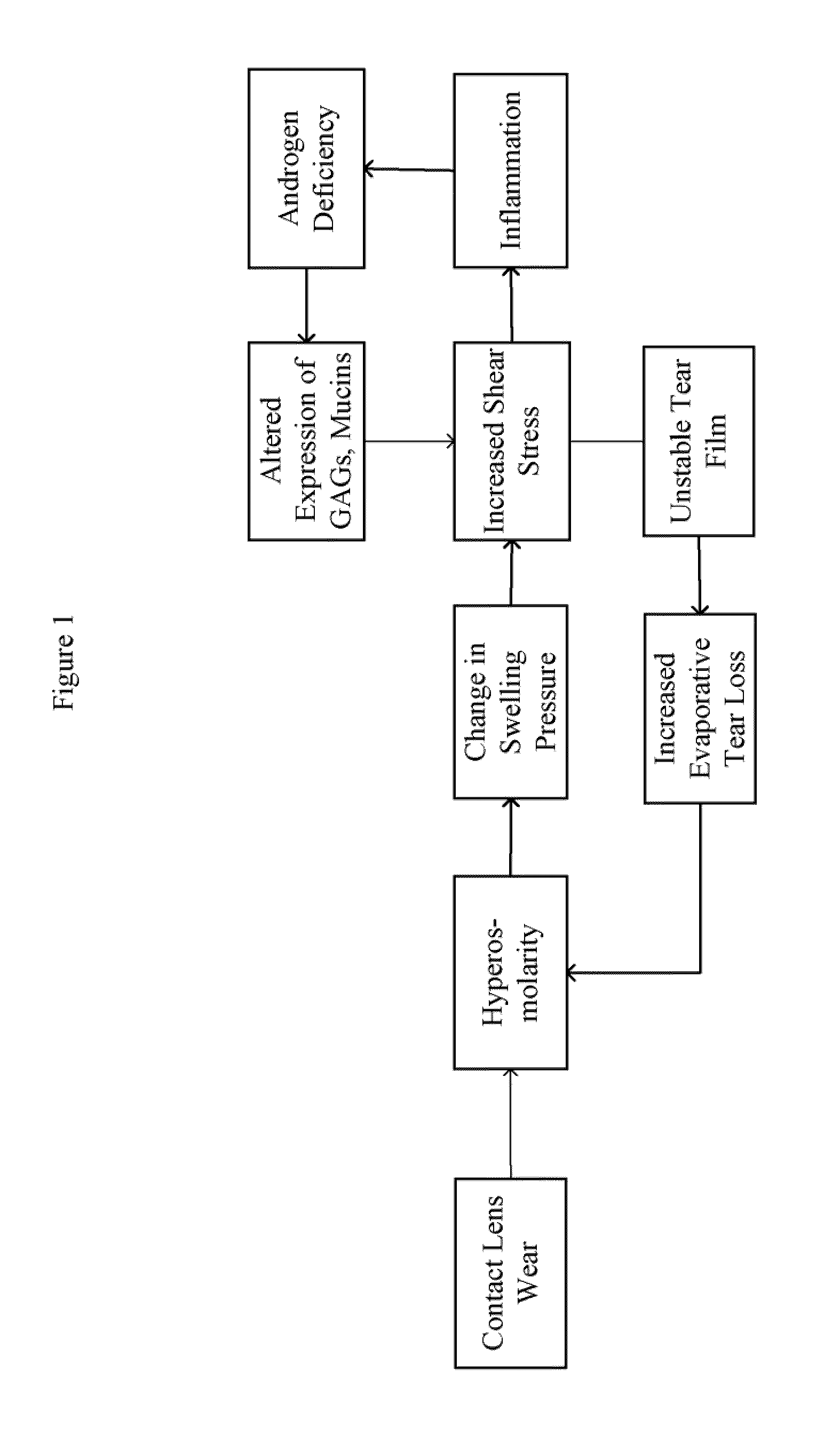
Treatment for dry eye
The present invention comprises a composition and method treating eye diseases using a composition having a therapeutically effective amount of a progestagen and a pharmaceutically acceptable carrier, wherein the composition is applied to the palpebral part of the eye and / or ocular surface.
Owner:RHODES PHARMA LP
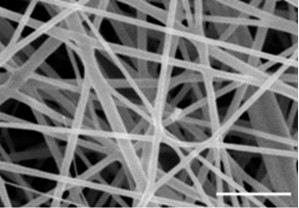
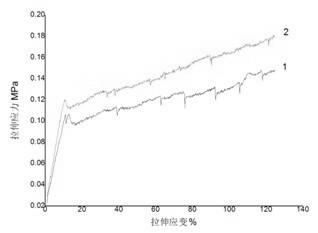
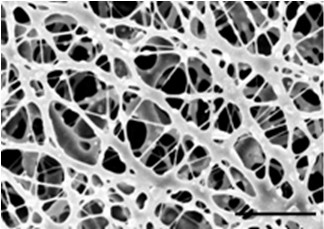
Medical bionic transparent film implanting material, and preparation method and application of material
InactiveCN102580166ALarge specific surface areaPorous and vapor permeableSurgeryAbsorbent padsMembrana tectoriaElectrospinning
The invention discloses a medical bionic transparent film implanting material which is a transparent porous material generated by electrostatic spinning and crosslinking. The thickness of the film ranges from 0.400 mu m to 800 mu m, the transparency of the film ranges from 75.0% to 99.9%, and the film comprises the following components in percentage by weight: 75.00%-95.00% of collagen, 1.50%-15.00% of polysaccharides, 0.01%-3.00% of therapeutic substances, and the balance being water. The film is prepared by an electrostatic spinning method. According to the invention, the technology is simple, the whole process is free of pollution, the transparency of the film is high, and the implanting material has a function of slow releasing on the loaded therapeutic substances, can remarkably improve a human tissue damage repair effect, and can be applied to ocular surface damage membrana tectoria, skin wound dressing, bone tooth defect regeneration guide films and other viscera repair separation films.
Owner:ZHEJIANG UNIV
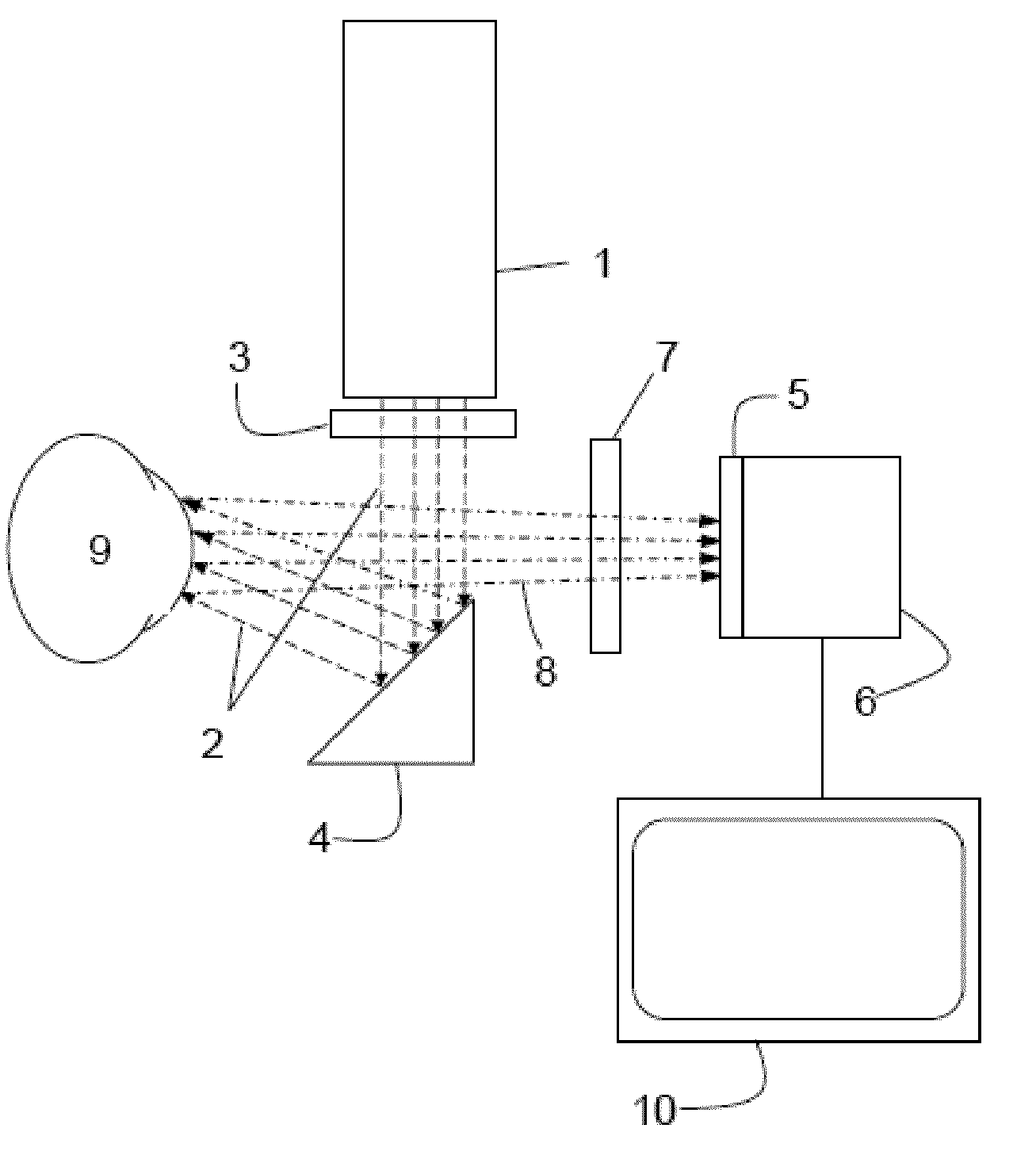
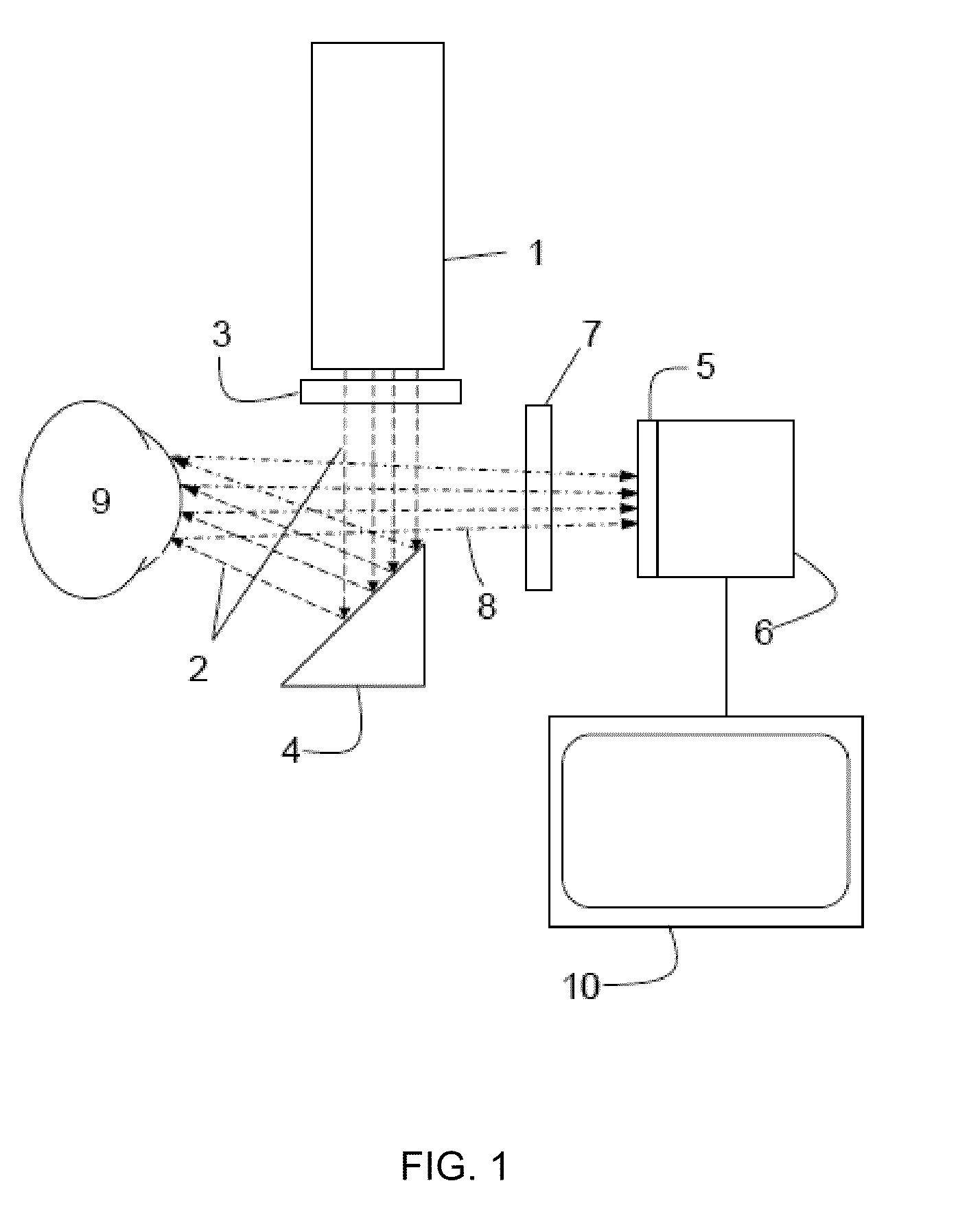
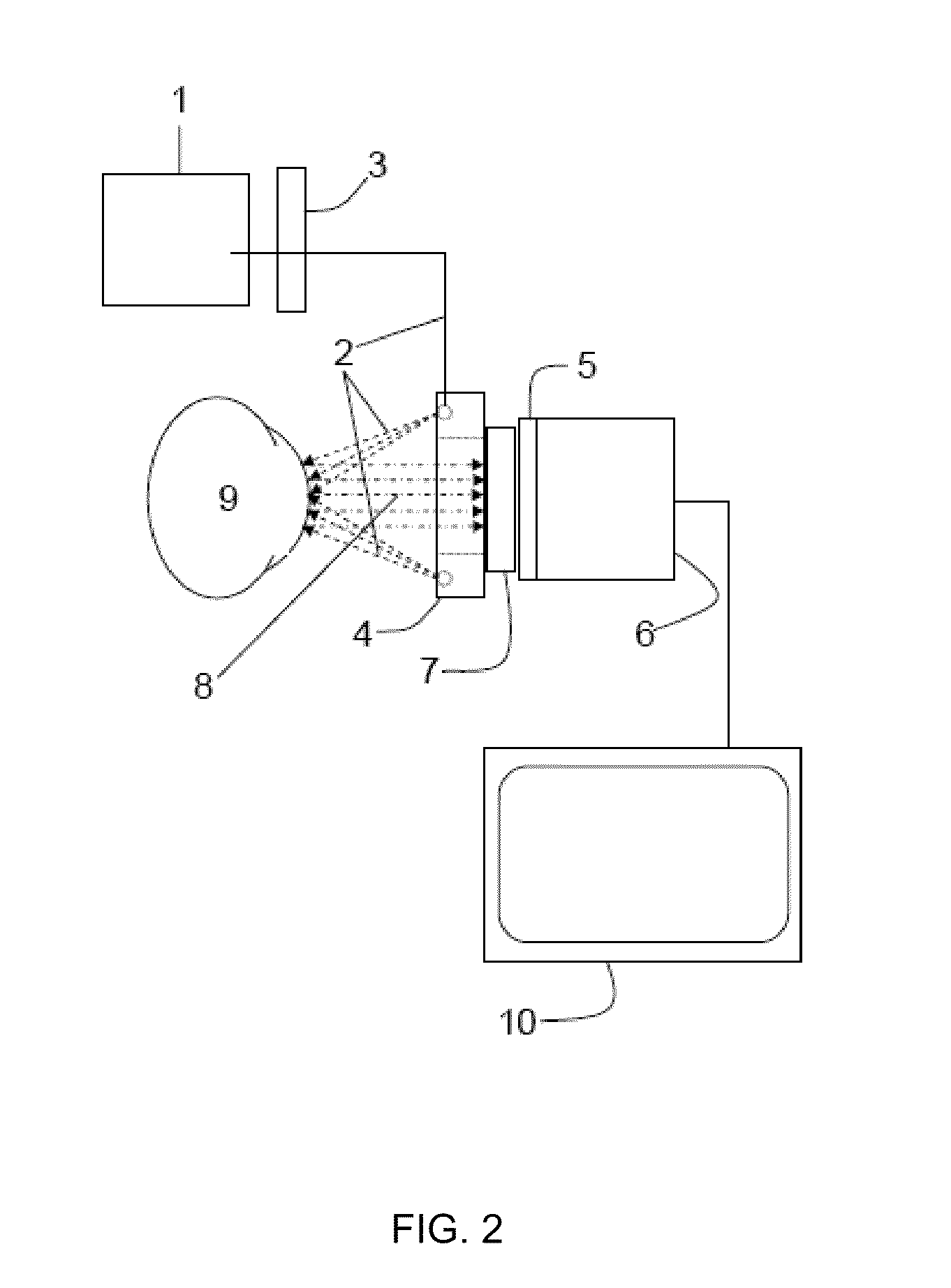
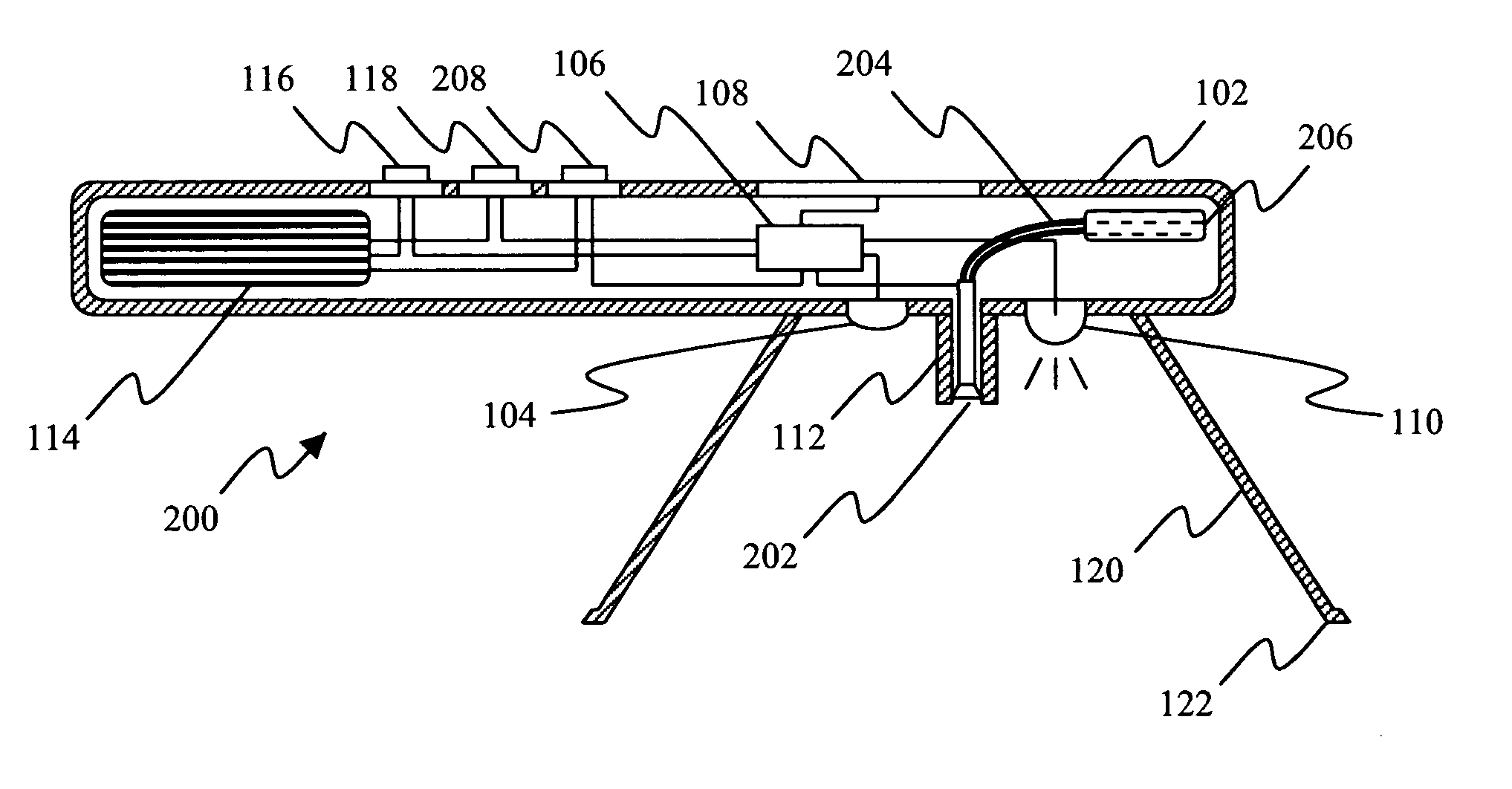
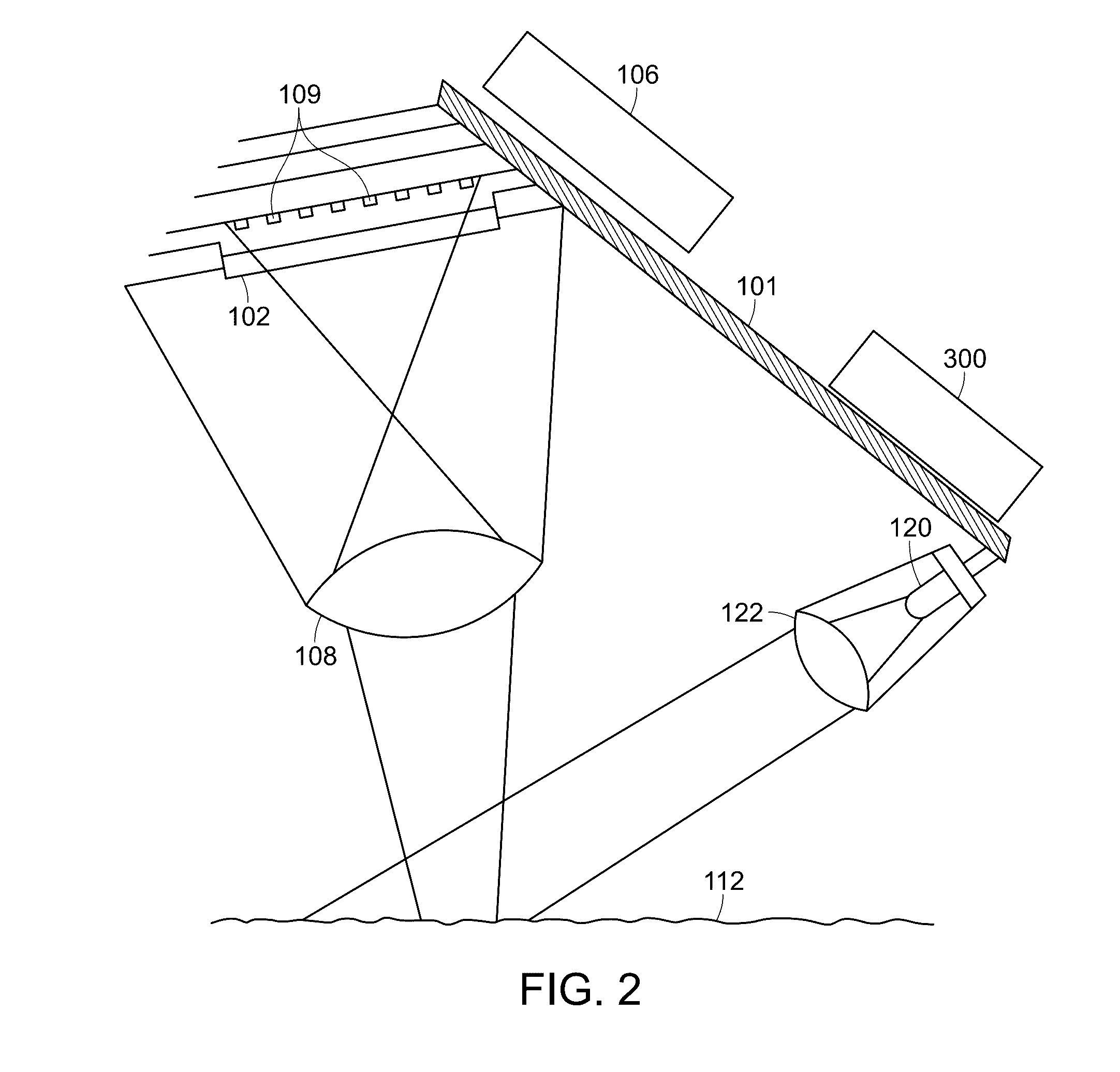
Method and apparatus for ocular surface imaging
The invention provides apparatuses and methods for detecting corneal surface defects. The methods and / or an apparatus of the invention can be used to detect corneal surface diseases, such as dry eye.
Owner:ALCON INC
Eye state sensor
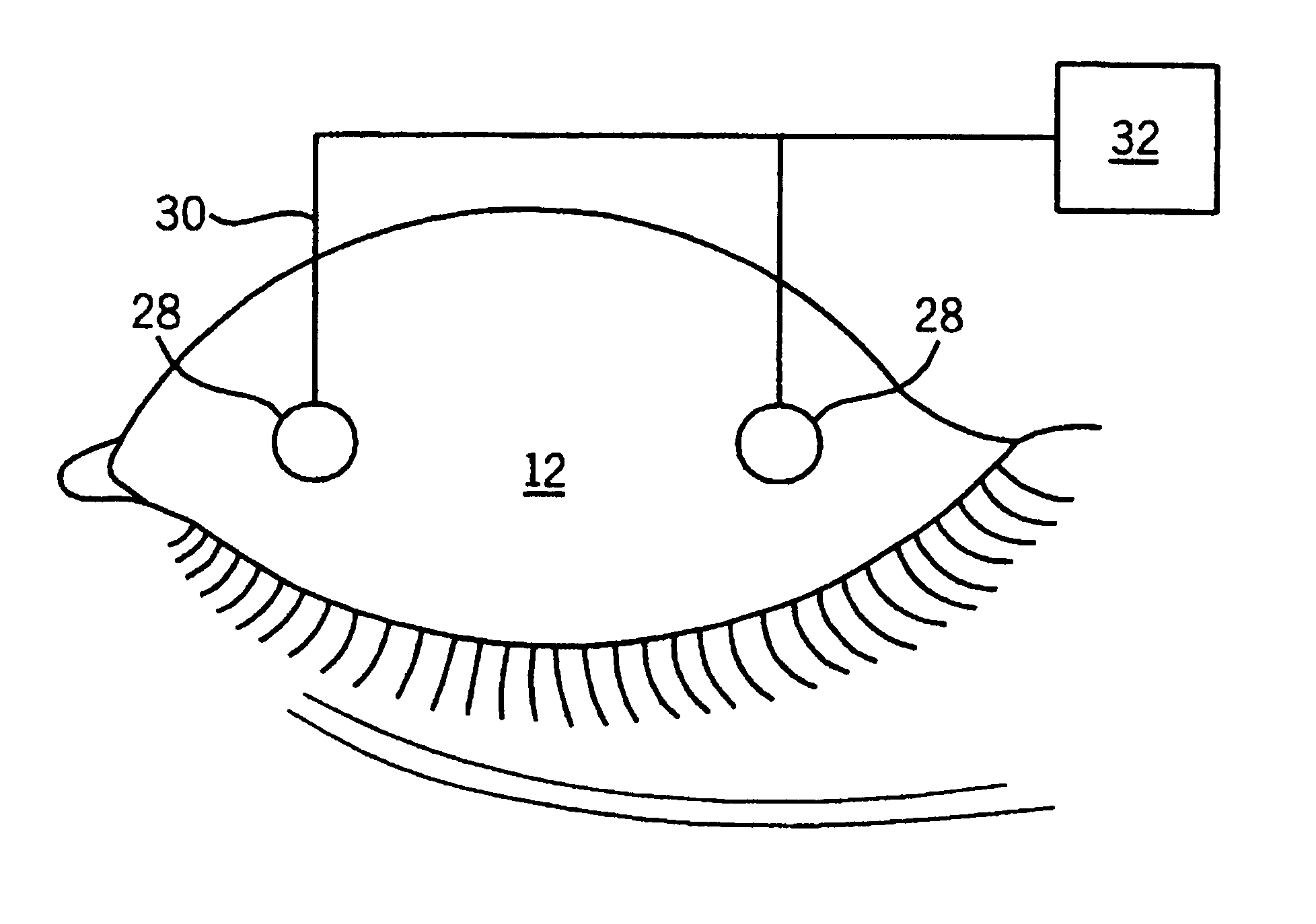
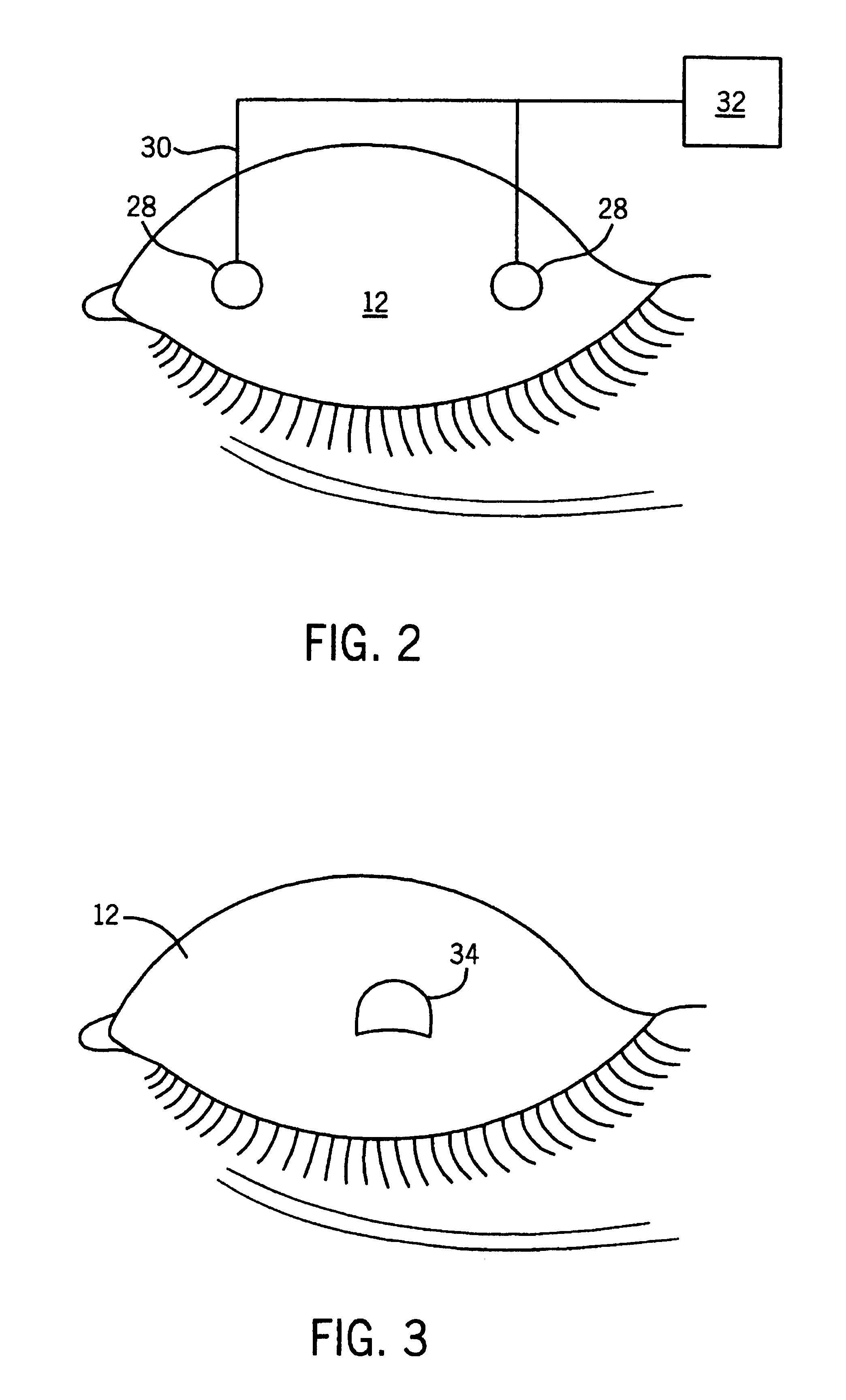
A method for sensing the state of an eye of a subject comprises measuring light reflected from an ocular surface and comparing the measured light to a reference. A method for treating an eye of a subject comprises sensing the state of the eye as described above, and controlling whether a substance is delivered to the eye whereby the substance is so delivered only when the eye is sensed to be open. A device for sensing the state of an eye comprises a light source that directs light to an ocular surface of a subject, and a sensor for measuring light reflected from the ocular surface. An apparatus for treating an eye of a subject comprises a device for sensing the state of an eye as described above, an applicator for delivering a substance to the eye, and a control system that permits delivery of the substance when the sensing device detects that the eye is open but prevents delivery of the substance when the sensing device detects that the eye is closed.
Owner:PHARMACIA CORP
Conjunctival tissue system
InactiveUS20070218039A1Improve clinical successShorten recovery timeBiocideCulture processConjunctivaBiology
The present disclosure describes a tissue system with conjunctival cells, including conjunctival stem cells. The conjunctival tissue system is derived from isolated tissue comprising conjunctival cells, and is suitable for restoring ocular surface impairments, particularly those that result from damaged or diseased conjunctiva. The tissue system is generated using a simple single medium culture scheme, and a support material, such as human amniotic membrane. The conjunctival tissue system generate is suitable for transplantation to treat the ocular surface of an eye of a subject that is damaged or diseased.
Owner:RELIANCE LIFE SCI PVT
Ophthalmic Device, and Method of Use Thereof, for Increasing Ocular Boundary Lubrication
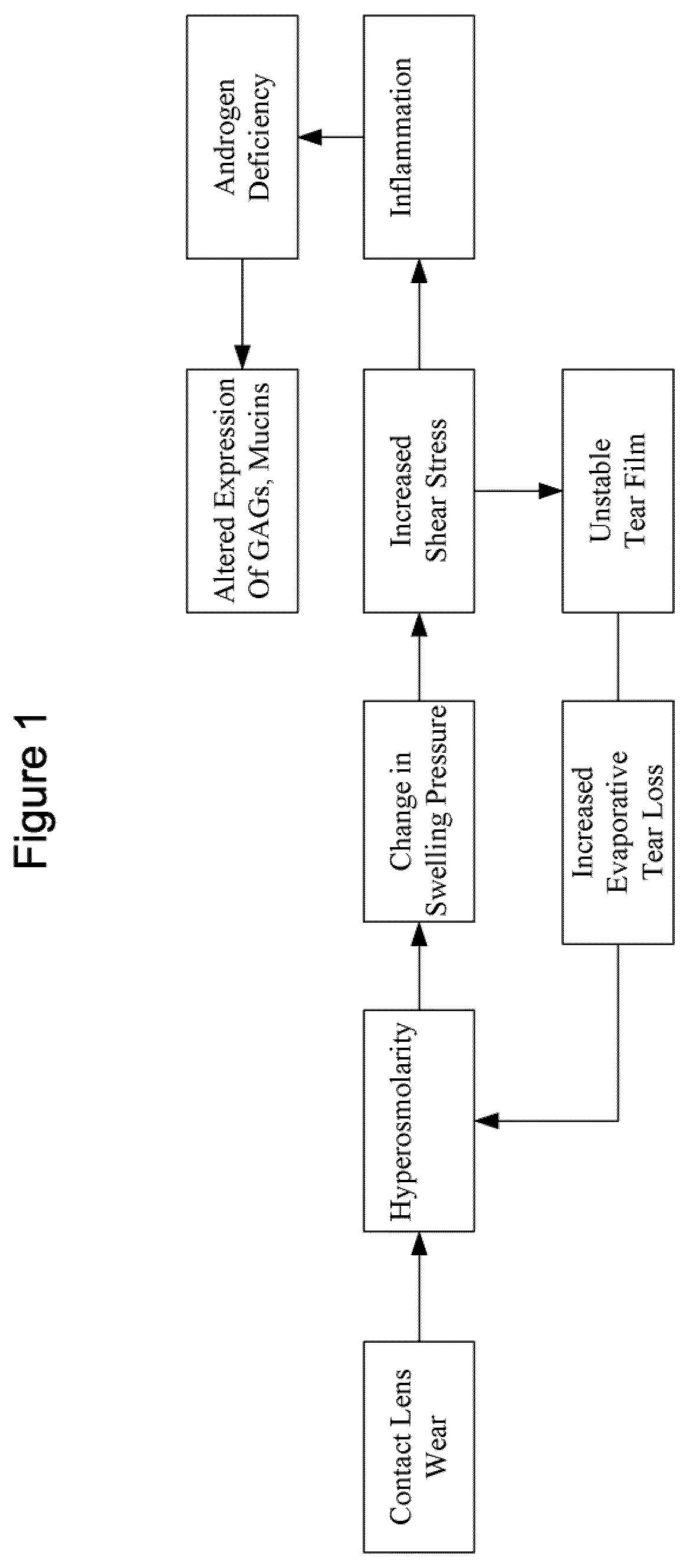
ActiveUS20110142908A1Reduce lubricationPromote hyperosmolarityOrganic active ingredientsSenses disorderLipid formationShear stress
The present invention provides an ophthalmic device, and method of use thereof, for an individual wearing an ophthalmic lens to increase ocular surface boundary lubrication. The invention device comprises an ophthalmic lens and a sacrificial mechanism disposed on the ophthalmic lens, wherein the sacrificial mechanism comprises a plurality of surface bound receptors, such as PRG4, hyaluronic acid, and DNA aptamers, that reversibly bound to a lubricating composition comprising a gel forming agent, a surfactant, or a combination thereof, effectively inhibiting or preventing protein and lipid adsorption on the surface of the lens, and mitigate shear stress and reduce the friction between the lens and the ocular surface of the individual in need.
Owner:RGT UNIV OF CALIFORNIA +1
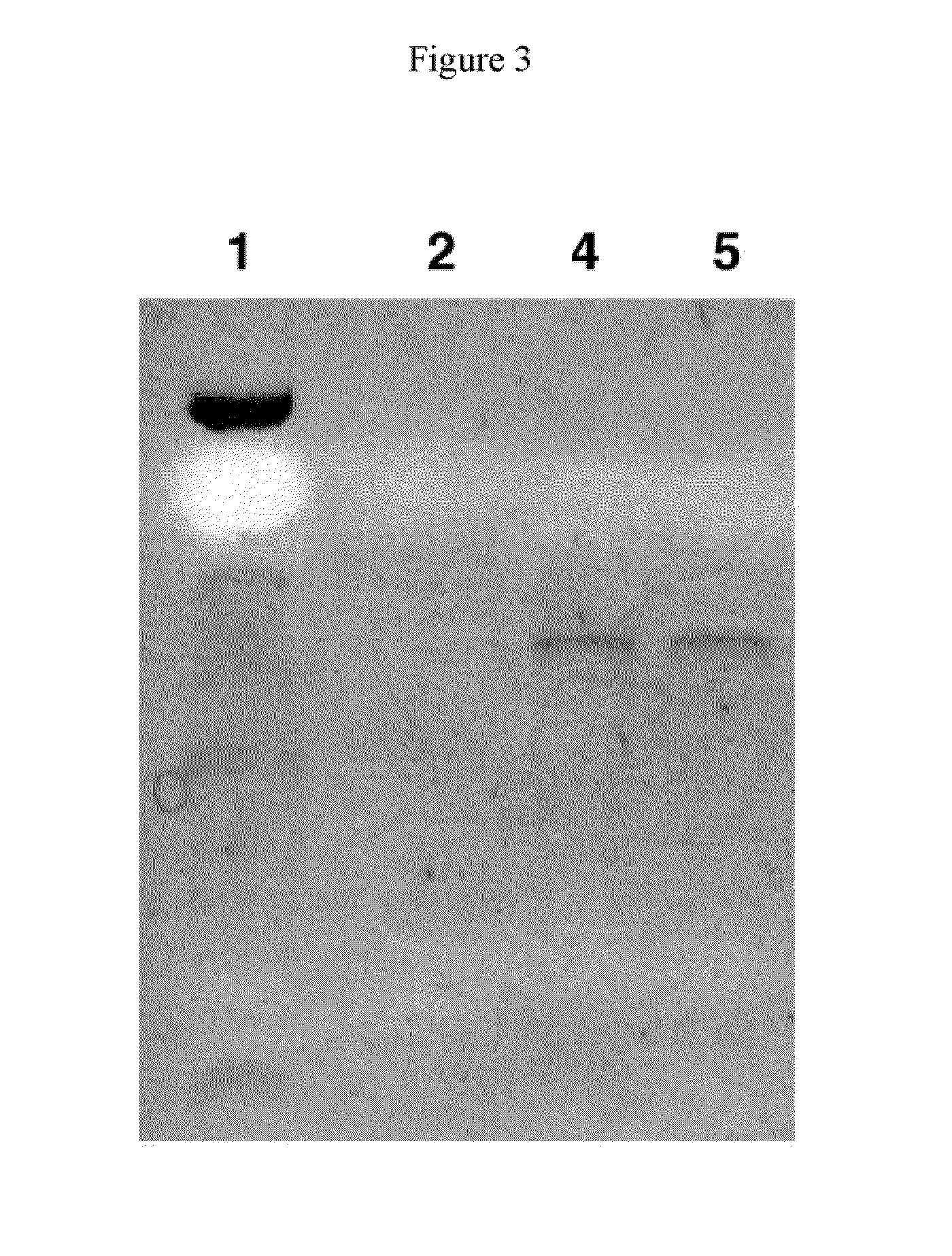
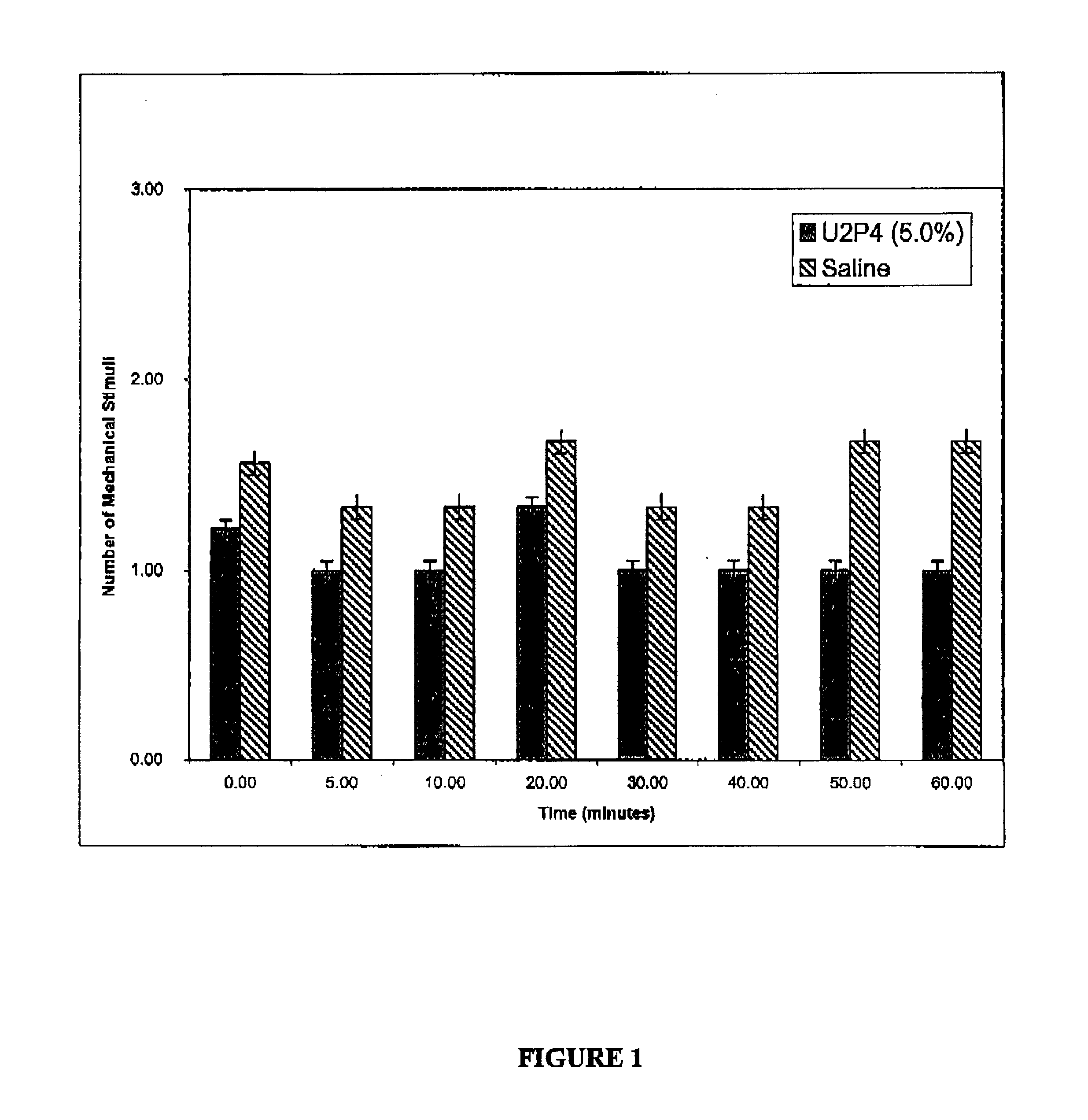
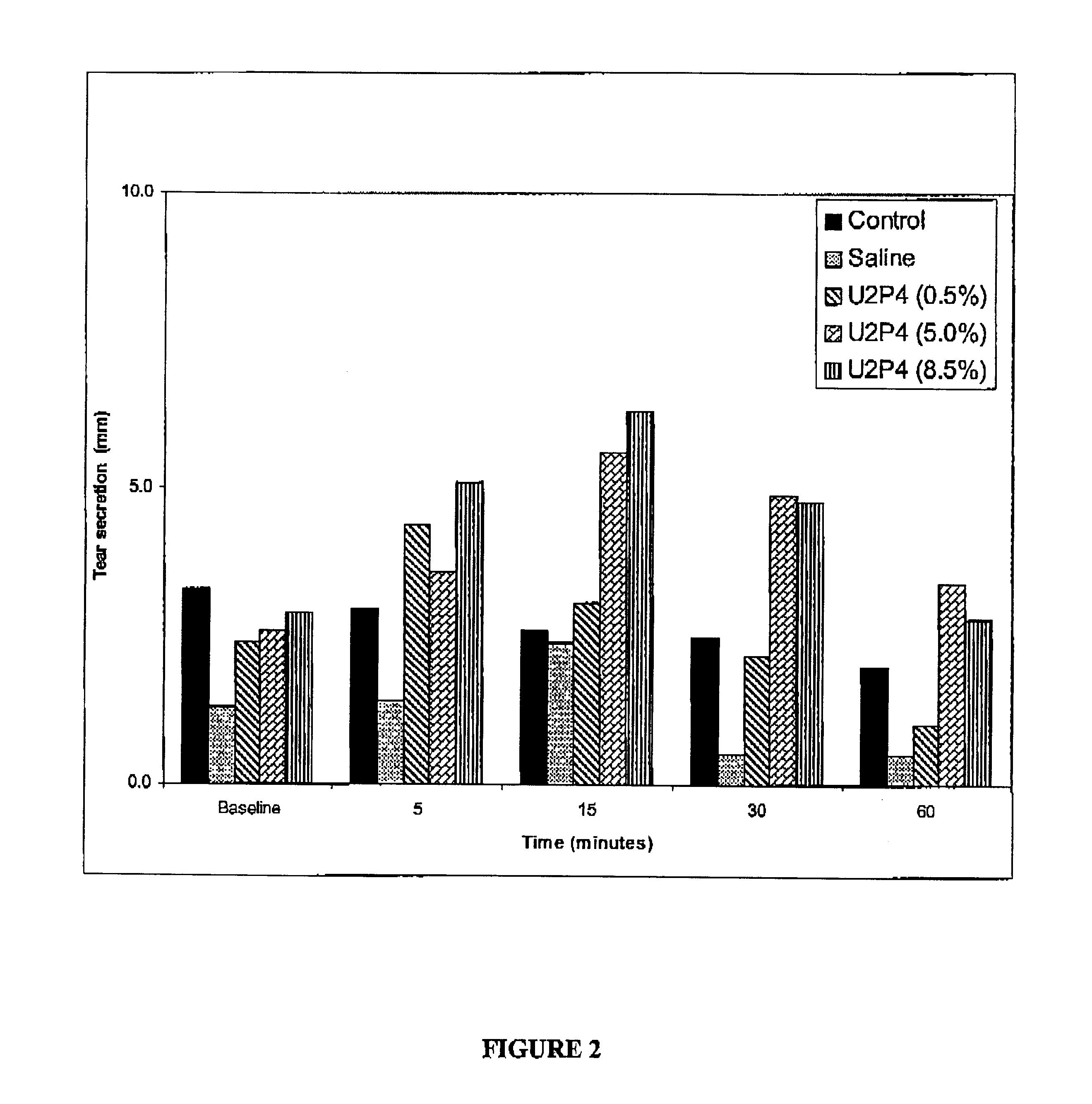
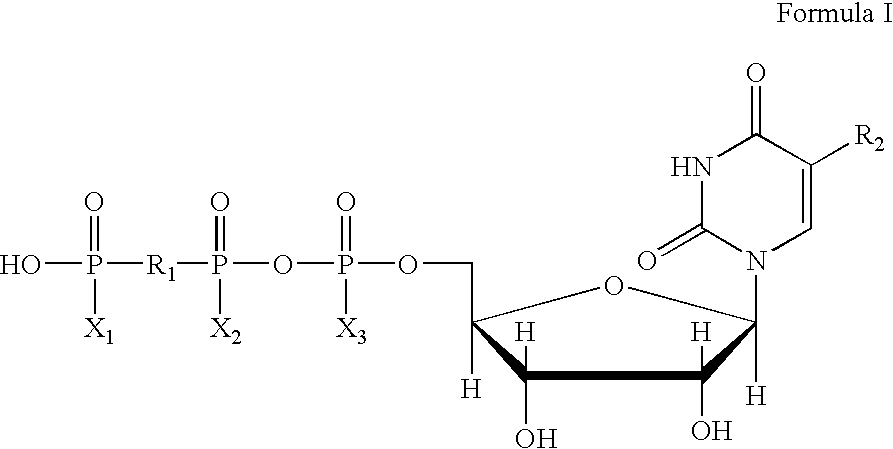
Method of treating dry eye disease with purinergic receptor agonists
A method and preparation for the stimulation of tear secretion in a subject in need of such treatment is disclosed. The method comprises administering to the ocular surfaces of the subject a purinergic receptor agonist such as uridine 5′-triphosphate [UTP], dinucleotides, cytidine 5′-triphosphate [CTP], adenosine 5′-triphosphate [ATP], or their therapeutically useful analogs and derivatives, in an amount effective to stimulate tear fluid secretion and enhance drainage of the lacrimal system. Pharmaceutical formulations and methods of making the same are also disclosed. Methods of administering the same would include: topical administration via a liquid, gel, cream, or as part of a contact lens or selective release membrane; or systemic administration via nasal drops or spray, inhalation by nebulizer or other device, oral form (liquid or pill), injectable, intra-operative instillation or suppository form.
Owner:MERCK SHARP & DOHME CORP
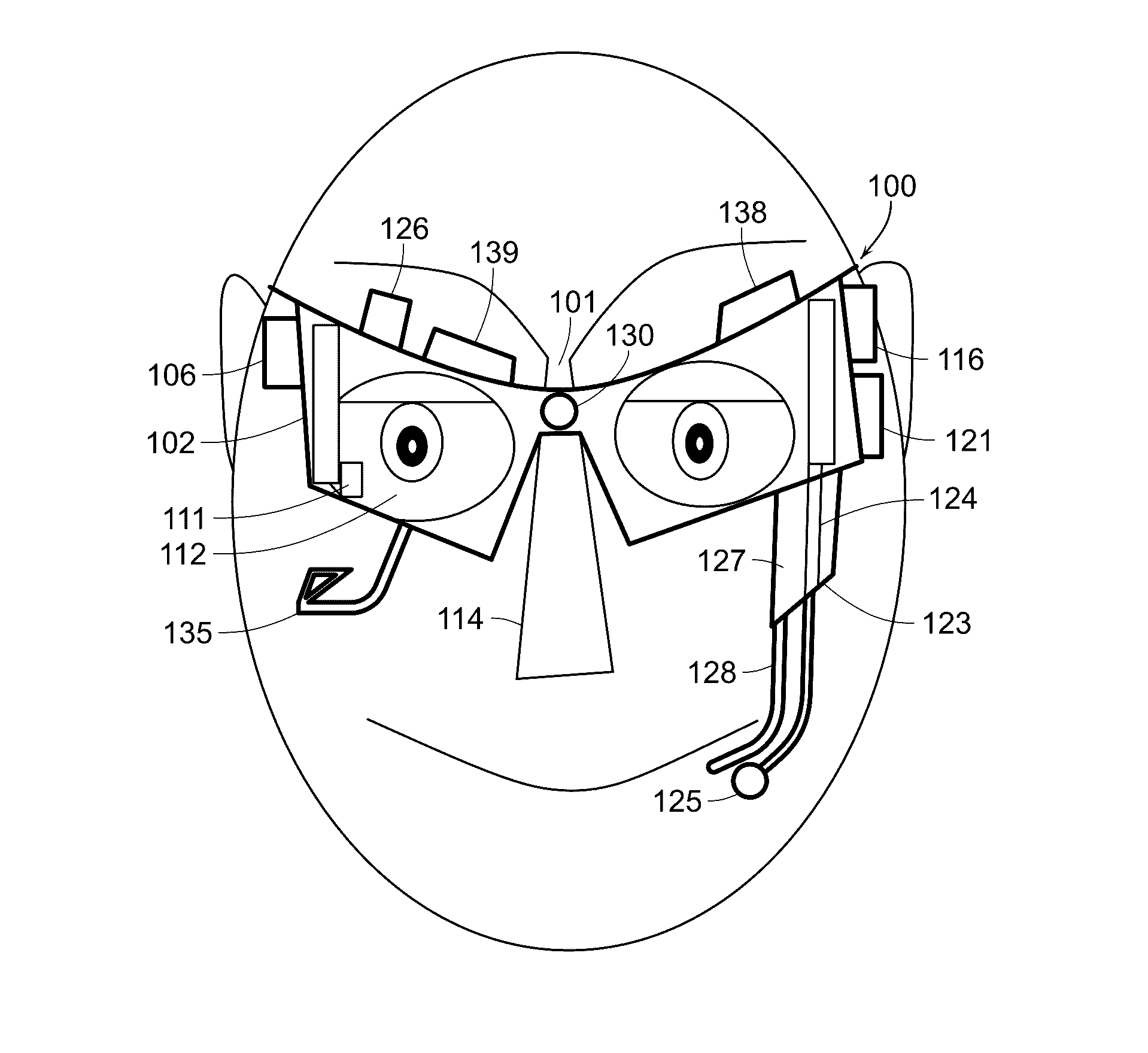
Eye and head tracking device
ActiveUS20160342206A1Improve accuracyInput/output for user-computer interactionImage enhancementSensor arrayHead movements
An apparatus and method of use for tracking eye and head movement comprising (1) at least one optoelectronic array sensor or optical flow sensor formed of a plurality of optoelectronic sensor cells; (2) a body configured to support the optoelectronic array sensor with focusing means along with a source of light with collimating means in front of and in proximity to an eye of a user; (3) an optical focusing means to focus an image of the ocular surface of the user on the optoelectronic array sensor; (4) a focusing lens with a source of light; (5) a means to detect blinking; (6) a driver configured to receive signals from the sensor array to generate coordinate signals corresponding to changes in the position of the ocular surface relative to the sensor array; and (7) a means to detect user's additional input and gestures.
Owner:SHAZLY TAREK A +1
Treatment for dry eye using testosterone and progestagen
InactiveUS20100016264A1Minimizes and avoids systemic treatmentAvoid disadvantagesOrganic active ingredientsBiocideOcular surfaceProgestin
The present invention comprises a composition and methods for treating eye conditions using a composition having a therapeutically effective amount of a progestagen, or a therapeutically effective amount of a progestagen with a testosterone; and pharmaceutically acceptable carrier, wherein the composition is applied to the palpebral part of the eye and / or ocular surface.
Owner:CONNOR CHARLES G +1
Compositions and methods for surface treatment in medical and surgical procedures
The present invention comprises compositions and methods for contemporaneously anesthetizing and antiseptically treating or pretreating anatomic surfaces for invasive surgical or treatment procedures. In particular, compositions and methods according to the present invention are used to treat ocular surfaces for intravitreal injections and other ophthalmic procedures. Compositions of the present invention comprise antiseptic agents and anesthetic agents in an aqueous gel or semi-gel formulation base to provide for enhanced methods of treating anatomic surfaces such as the eye prior to surgical or other invasive procedures.
Owner:LADD BYRON S
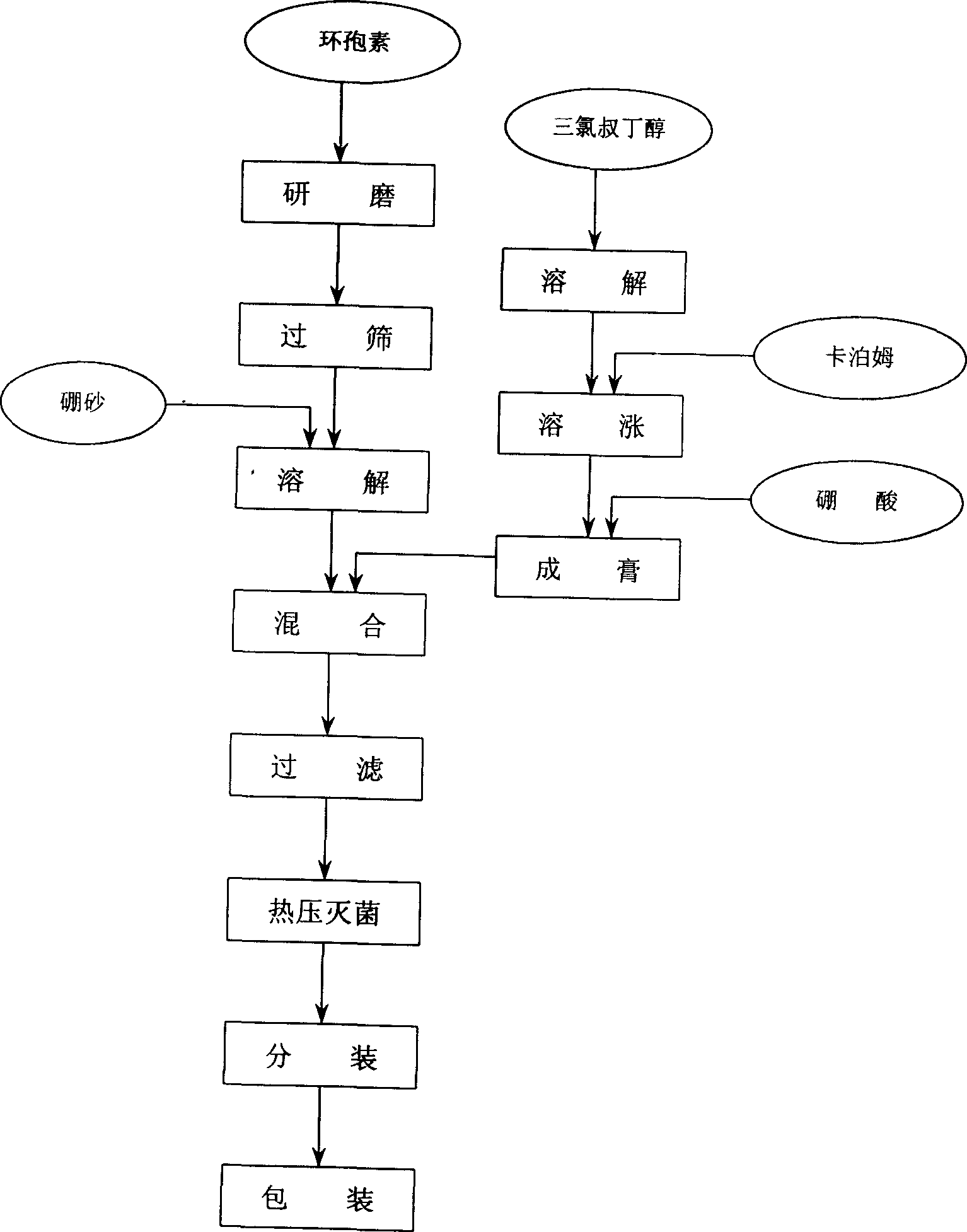
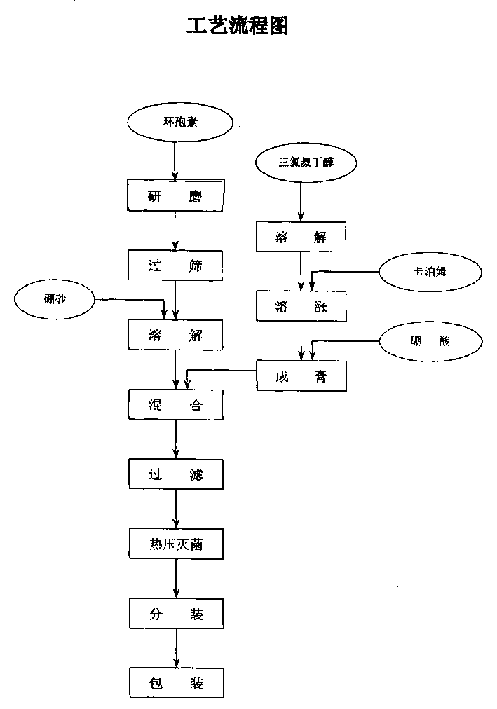
Eye cyclosporin gel
InactiveCN1456350ANot easy to diluteGood water solubilitySenses disorderPharmaceutical delivery mechanismCyclosporinsWhole body
A cyclosporin eye gel for treating the rejection reaction of corneal transplantation, keratoconjunctival xerosis, catarrhal conjunctivitis, and chemical burn of eye is prepared from cyclosporin, boric acid, borax, trichloro-tert-butanol, etc. Its advantages are long stay time in eye and high curative effect.
Owner:刘继东
Therapeutic Modulation of Ocular Surface Lubrication
ActiveUS20110070222A1Protect conjunctivaProtect corneaOrganic active ingredientsSenses disorderBoundary lubricationOcular surface
Provided herein are ophthalmically acceptable pharmaceutical compositions comprising a PRG4 inducing compound in combination with PRG4 (including a lubricant fragments, homologs, or isoforms thereof), and methods of using the same. The PRG4 inducing compound in the pharmaceutical composition of the present invention upregulates PRG4 expression and localization in the ocular surface for efficient surface boundary lubrication. In some instances, pharmaceutical compositions described herein are utilized for treating ophthalmic conditions, e.g., ocular boundary deficiency and symptoms associated therewith.
Owner:RGT UNIV OF CALIFORNIA +1
Systems and methods for mapping the ocular surface
ActiveUS20150131055A1Fine surfaceEasy to measureEye diagnosticsOptical partsAnterior surfaceKeratoconus
Examples of methods and apparatus for an accurate measurement of the anterior surface of the eye including the corneal and scleral regions are disclosed. The measurements provide a three-dimensional map of the surface which can be used for a variety of ophthalmic and optometric applications from astigmatism and keratoconus diagnostics to scleral lens fitting.
Owner:PRECISION OCULAR METROLOGY LLC
Drug composition comprising albumin as active ingredient
InactiveUS6043213AIncrease heightFine surfaceSenses disorderPeptide/protein ingredientsAdditive ingredientConjunctival lesion
PCT No. PCT / JP97 / 01329 Sec. 371 Date Dec. 19, 1997 Sec. 102(e) Date Dec. 19, 1997 PCT Filed Apr. 17, 1997 PCT Pub. No. WO97 / 39769 PCT Pub. Date Oct. 30, 1997The present invention provides a pharmaceutical composition for treatment of corneal and conjunctival lesion, and dry eye comprising albumin as an active ingredient. The pharmaceutical composition is also useful for increase of eye surface epithelium mucin secretion. The present invention further provides a method for treatment of corneal and conjunctival lesion, and dry eye, which comprises administering, to a subject in need of such treatment, albumin in an amount effective. In addition, the present invention provides a use of albumin for manufacture of a pharmaceutical composition of the present invention.
Owner:R TECH UENO
Silk compositions and methods of using same
The present invention provides for silk-derived compositions for treating a wide variety of ocular conditions. The composition is produced by processing the silk cocoon into a water-based solution (i.e., a dissolved silk), which is then cast into a film. The film may be transparent to visible light, and curved in shape for easy application to the ocular surface. The silk film may either self-adhere or be sutured to cover the wound. The degradation time of the film may range from 1 minute to 24 hours, or from 2 hours to 20 hours. The present compositions can help regenerate damaged corneal tissue, thus promoting healing.
Owner:CORNELL UNIVERSITY
Passive intraocular drug delivery devices and associated methods
The present invention provides methods and devices for the passive delivery of active agents into the eye of a subject. In one aspect, for example, a device for passively delivering an active agent into an eye of a subject may include a housing configured to conform to at least a portion of an eye surface, and a first reservoir component located within the housing and configured to be fluidically coupled to a first ocular surface, where the first reservoir contains an ionized active agent. The device may further include a second reservoir component located within the housing and configured to be fluidically coupled to a second ocular surface located adjacent to the first ocular surface, where the second reservoir component contains a depot forming agent having a charge that is opposite in polarity as compared to the ionized active agent. The ionized active agent and the depot forming agent are configured to form a precipitate when contacted together at room to body temperature and at a pH of between about 4 to about 8 in an aqueous medium. Additionally, the device does not include an electrode.
Owner:ACIONT
Dry eye treatment
This invention relates to an emulsion composition for the formation of an artificial tear film over the ocular surface of the eye capable of providing mechanical lubrication for the ocular surface while reducing evaporation of fluid therefrom. The emulsion is desirably in the form of a meta stable emulsion and is characterized by the use of a surfactant comprising a combination of a primary and secondary surfactant where the primary surfactant permits formation of the emulsion and the secondary surfactant permits autoclaving of the surfactant. The invention also includes a method for the formation of such an emulsion.
Owner:OCULAR RES OF BOSTON INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com