Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1594 results about "Nebulizer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
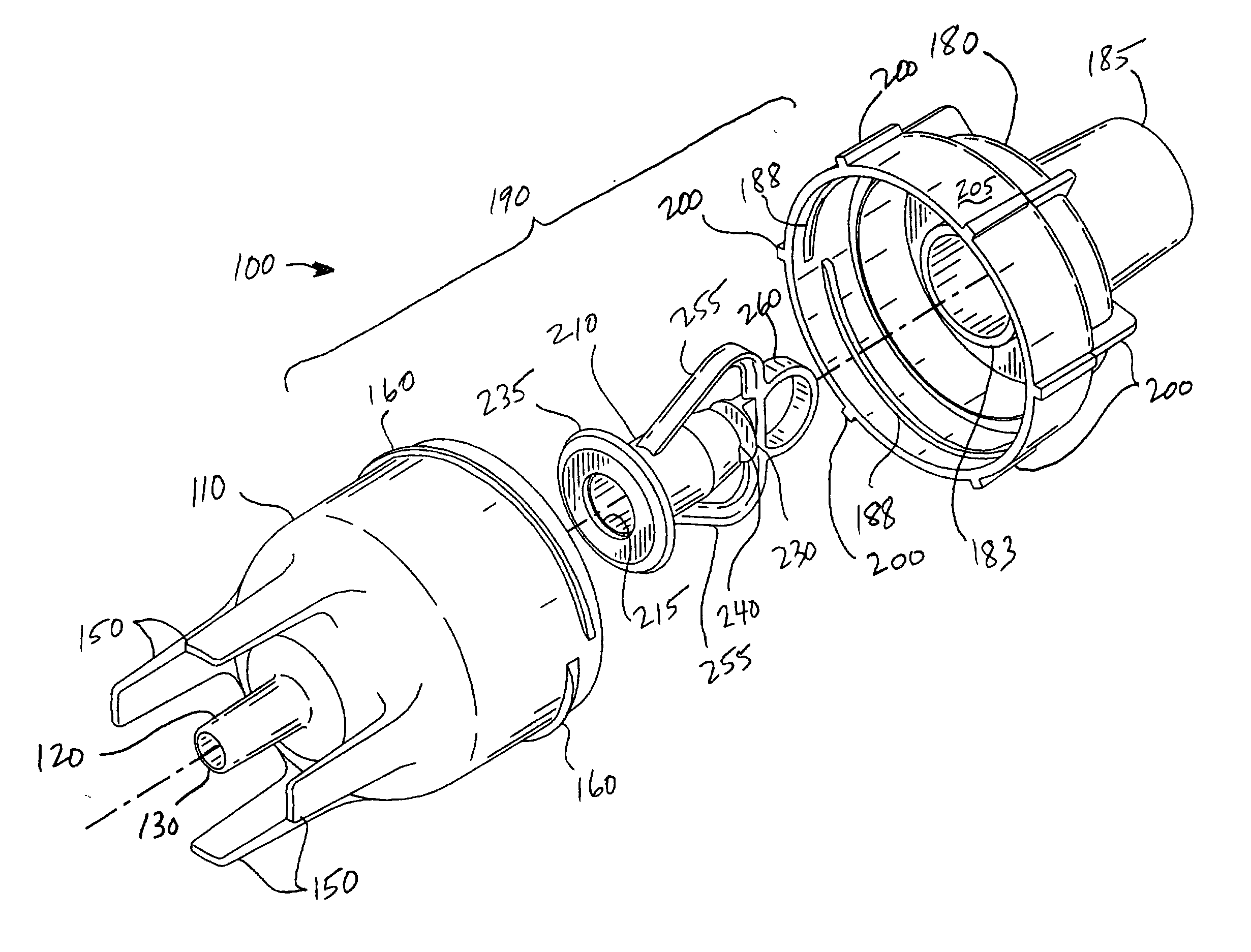
In medicine, a nebulizer or nebuliser (see spelling differences) is a drug delivery device used to administer medication in the form of a mist inhaled into the lungs. Nebulizers are commonly used for the treatment of asthma, cystic fibrosis, COPD and other respiratory diseases or disorders.
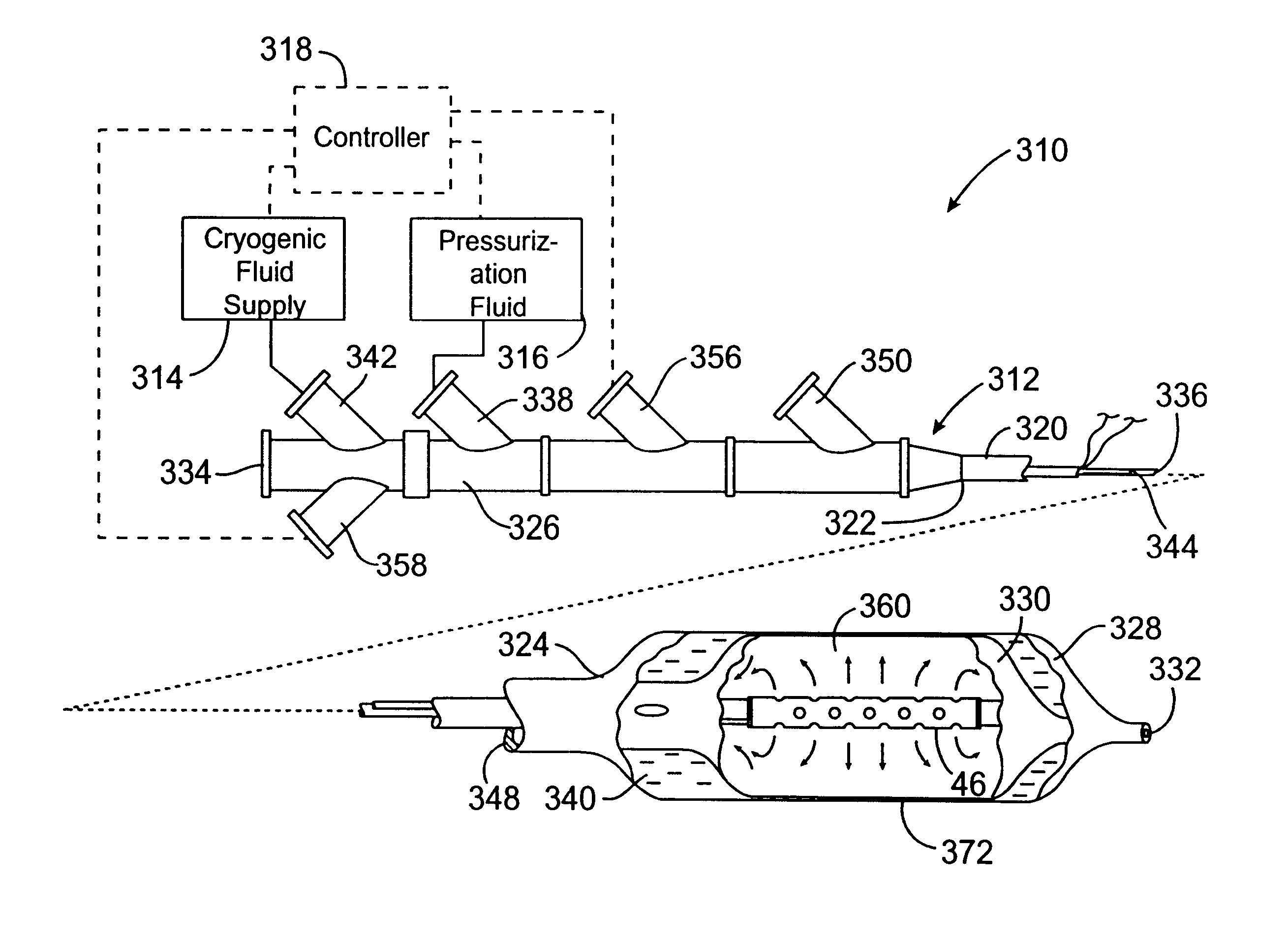
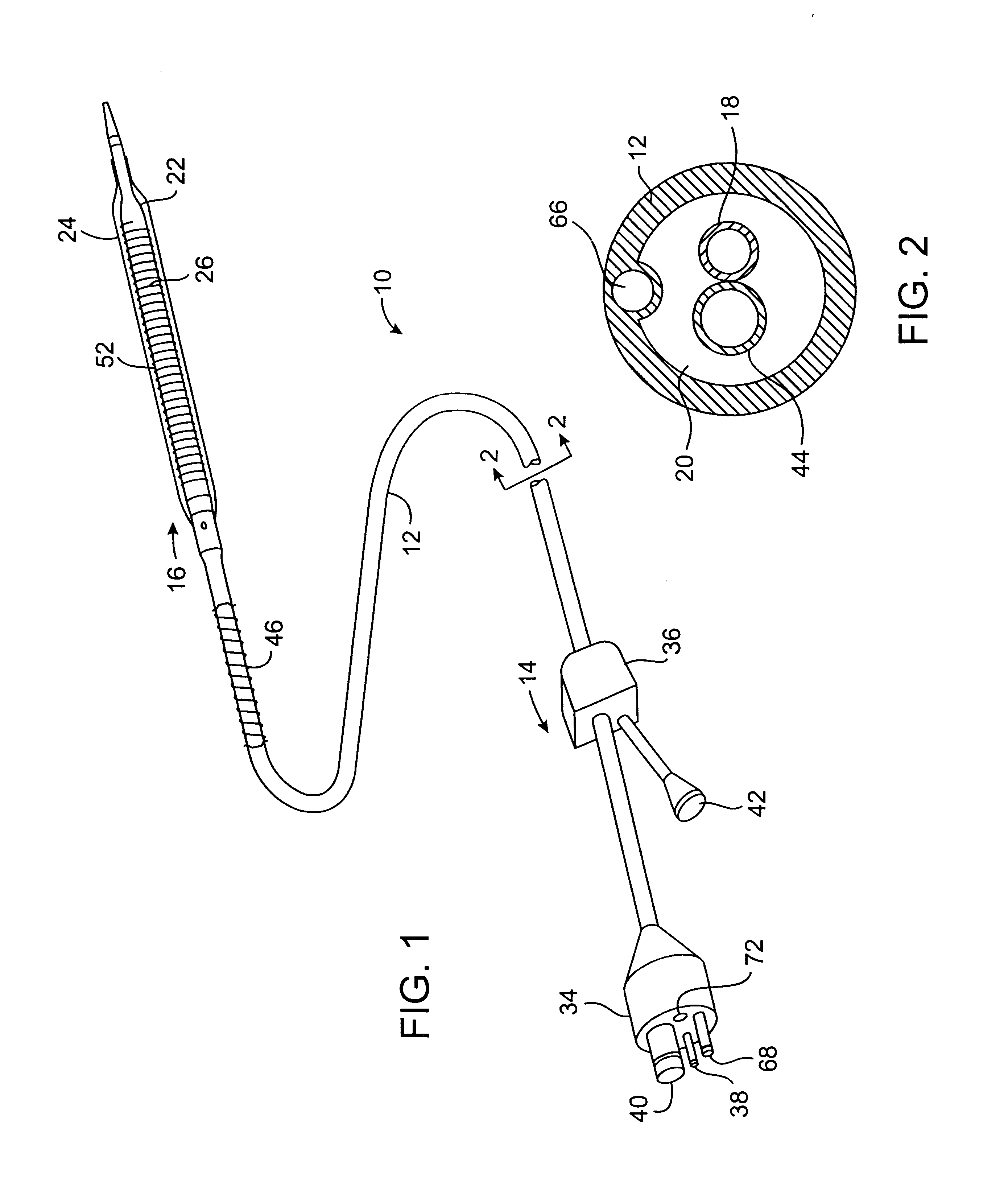
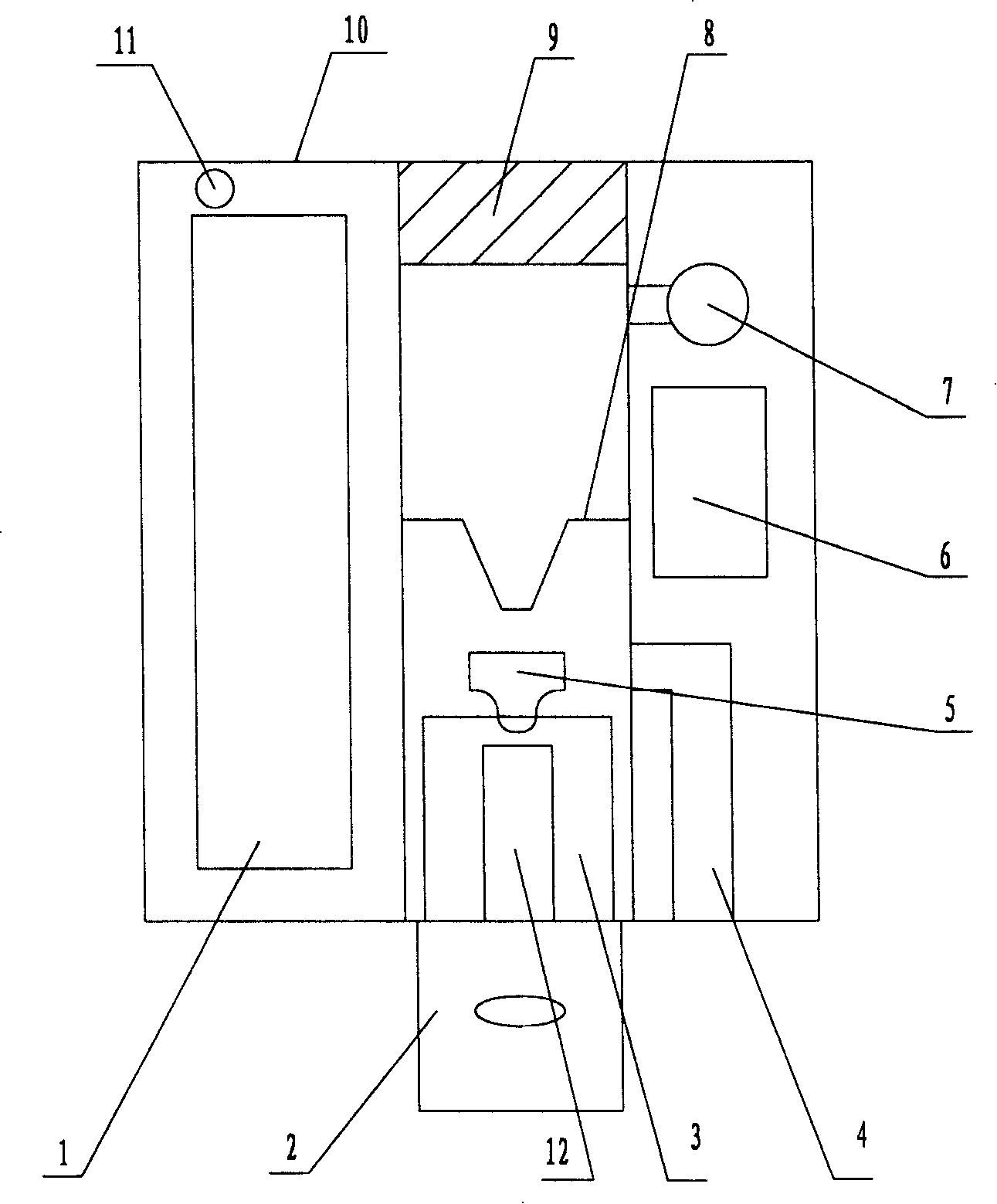
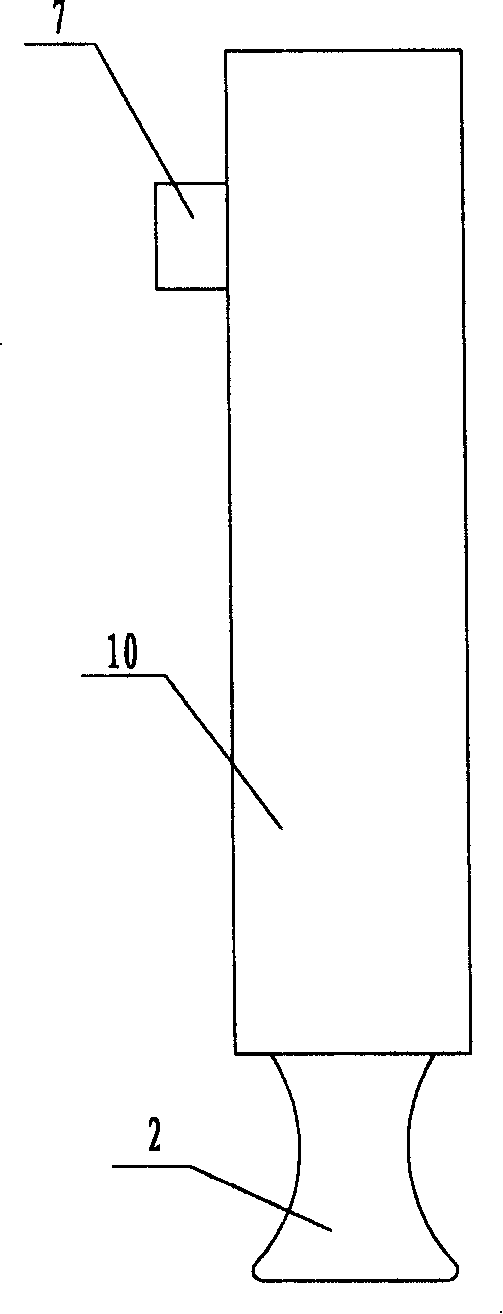
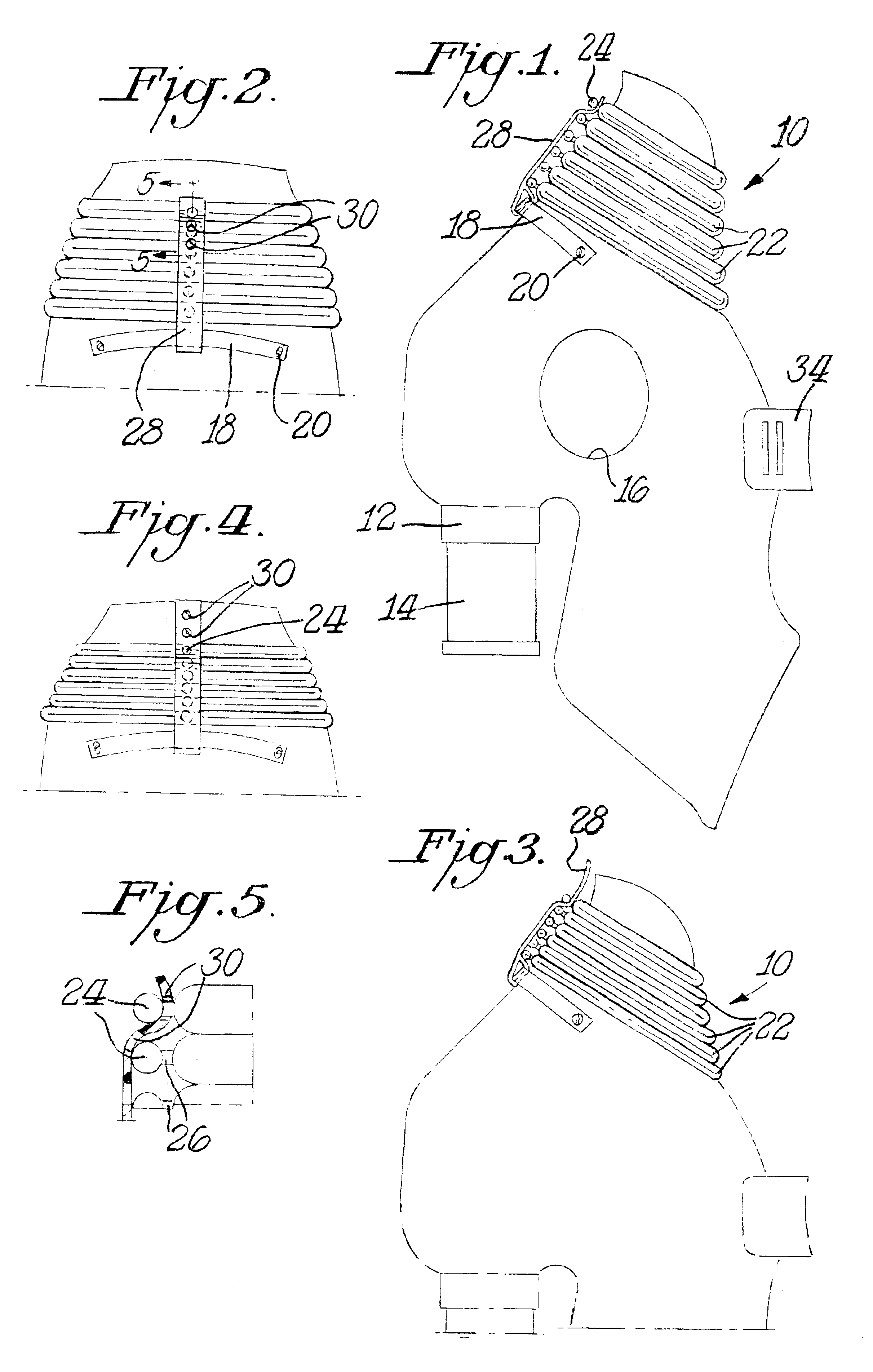
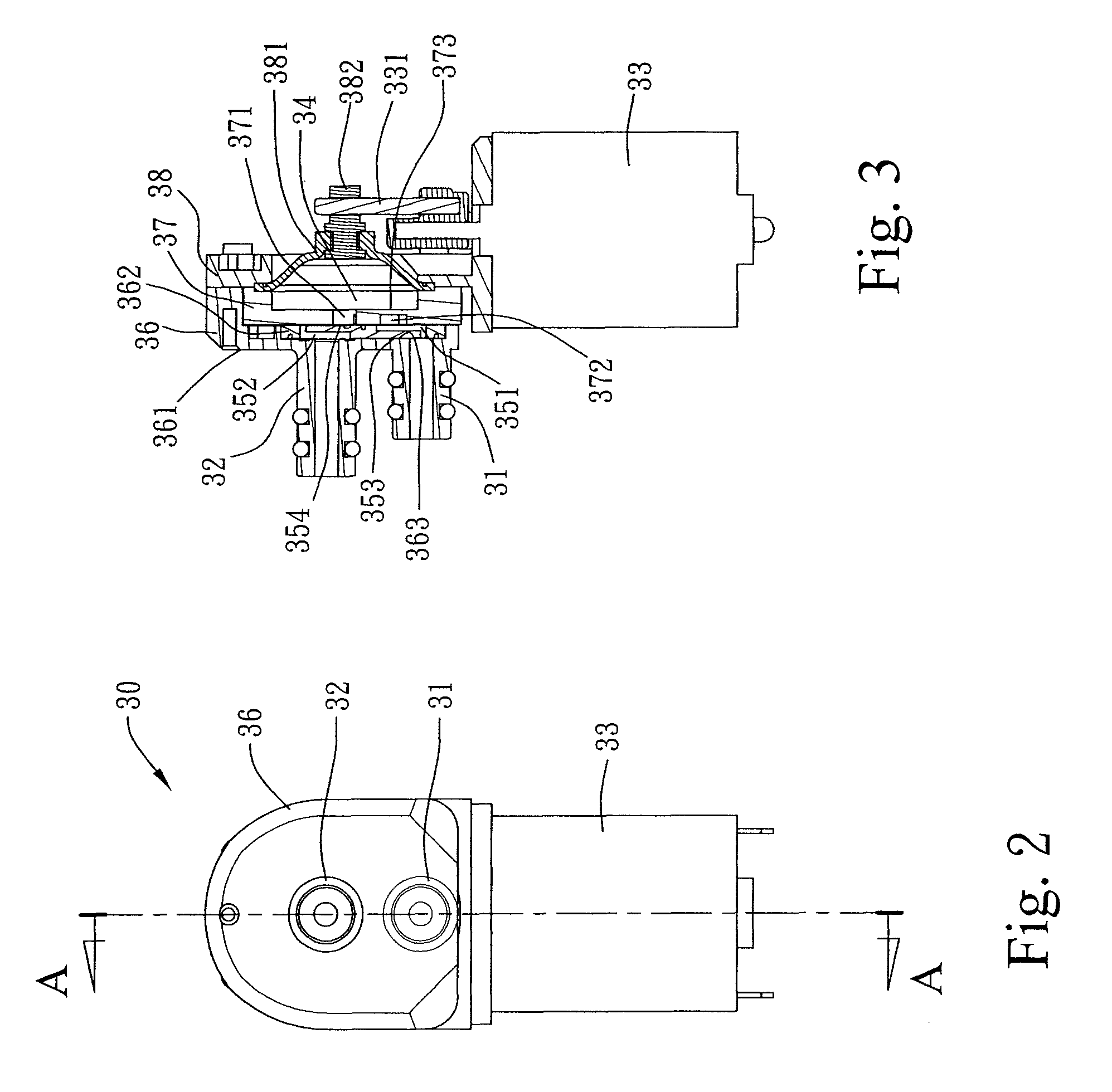
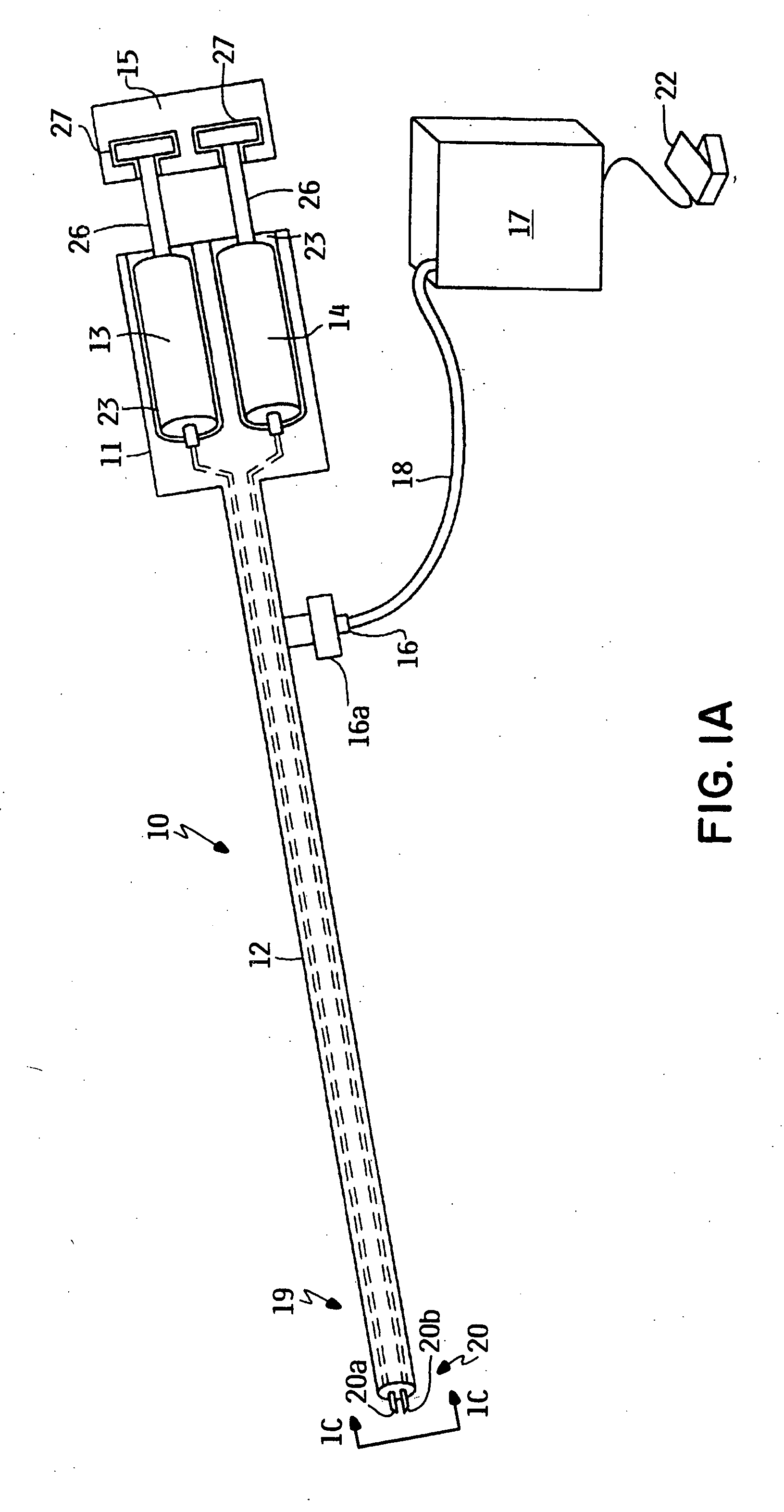
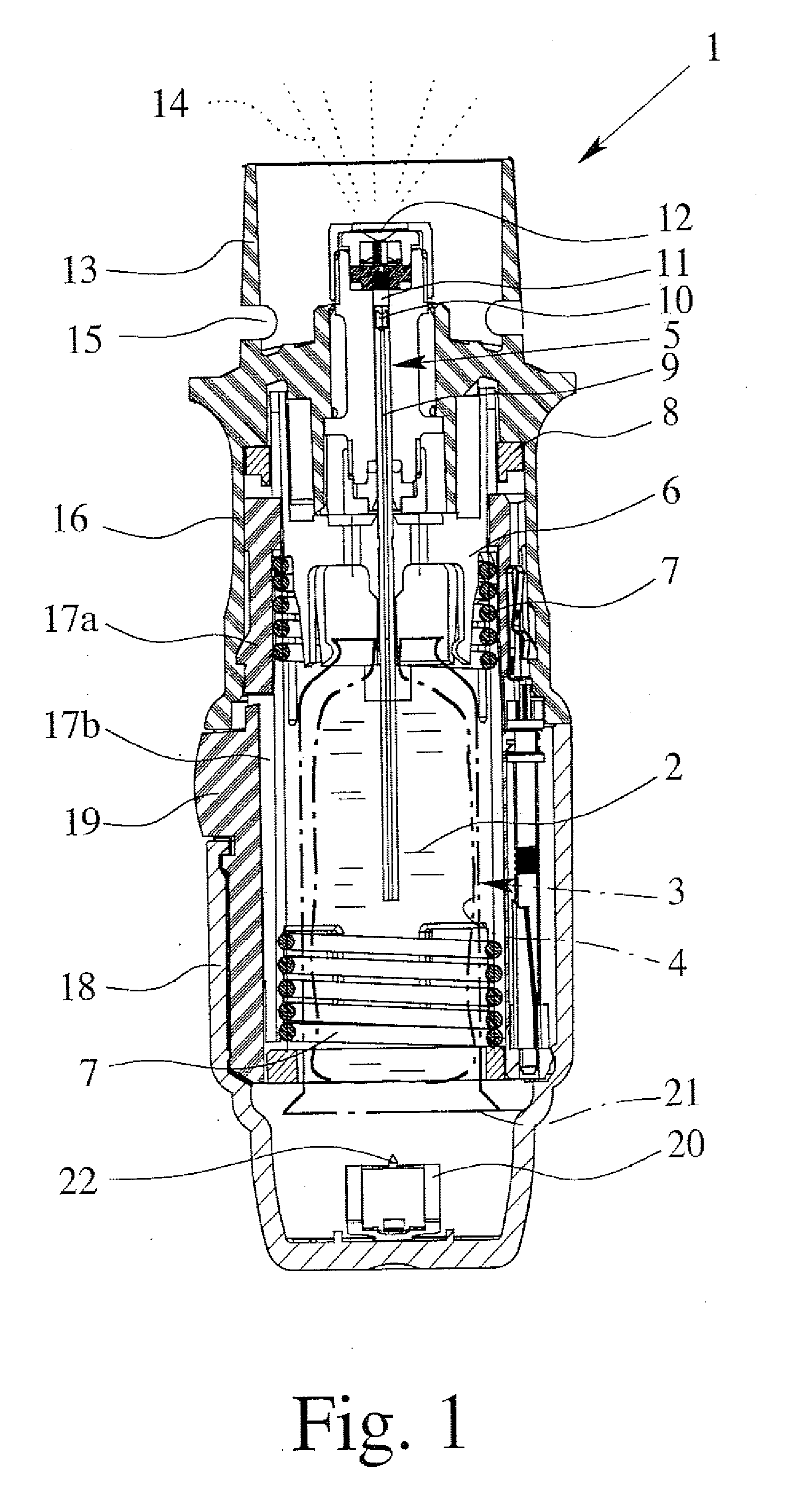
Safety cryotherapy catheter
InactiveUS6514245B1Keep openReduce and/or eliminate any associated hyperplasiaDomestic cooling apparatusLighting and heating apparatusNebulizerTarget tissue
Improved devices, systems, and methods for inhibiting hyperplasia in blood vessels provide controlled and safe cryotherapy treatment of a target portion within a body lumen of a patient. Efficacy of endoluminal cryogenic cooling can be enhanced by limiting cooling of target tissues using a thermal barrier disposed between a dual balloon cryotherapy catheter. Containment of both balloons can be monitored by applying a vacuum within a space between the first and second balloons, and by coupling the vacuum space to a fluid shutoff so as to inhibit flow of cryogenic fluid in response to a change in the vacuum space. Controlled cooling of the vessel can be improved by use of a nebulizer in fluid communication with a cryogenic liquid supply lumen and a gas supply lumen.
Owner:BOSTON SCI SCIMED INC
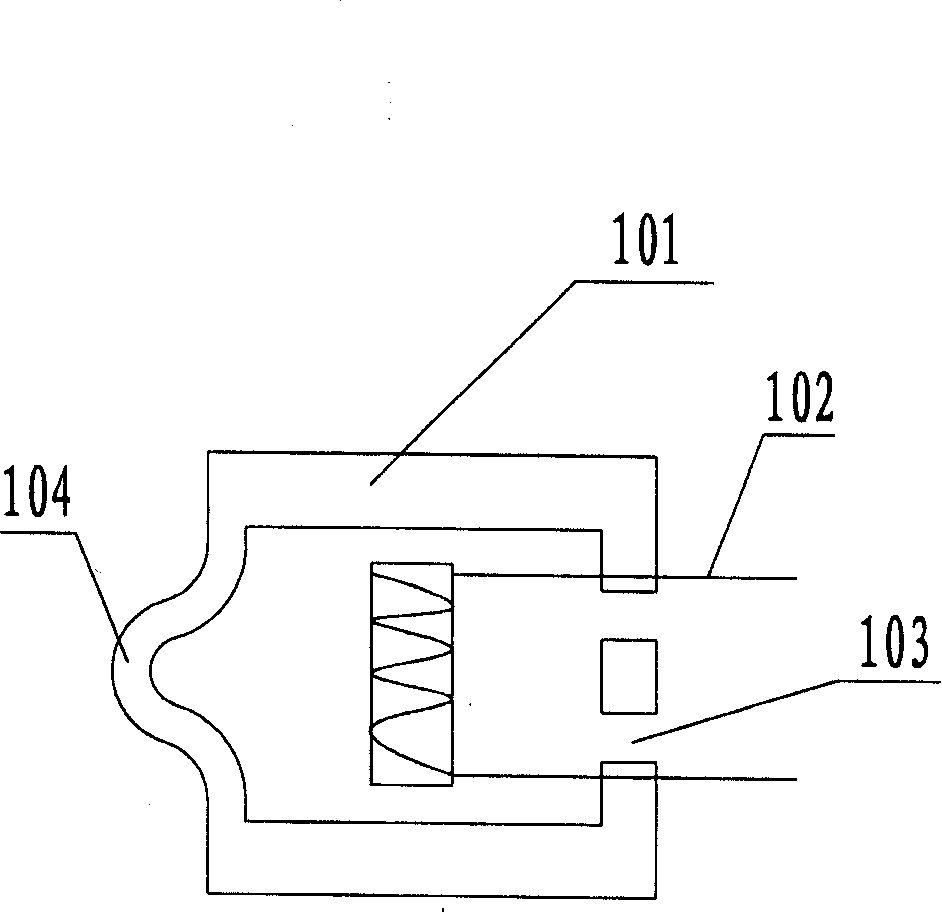
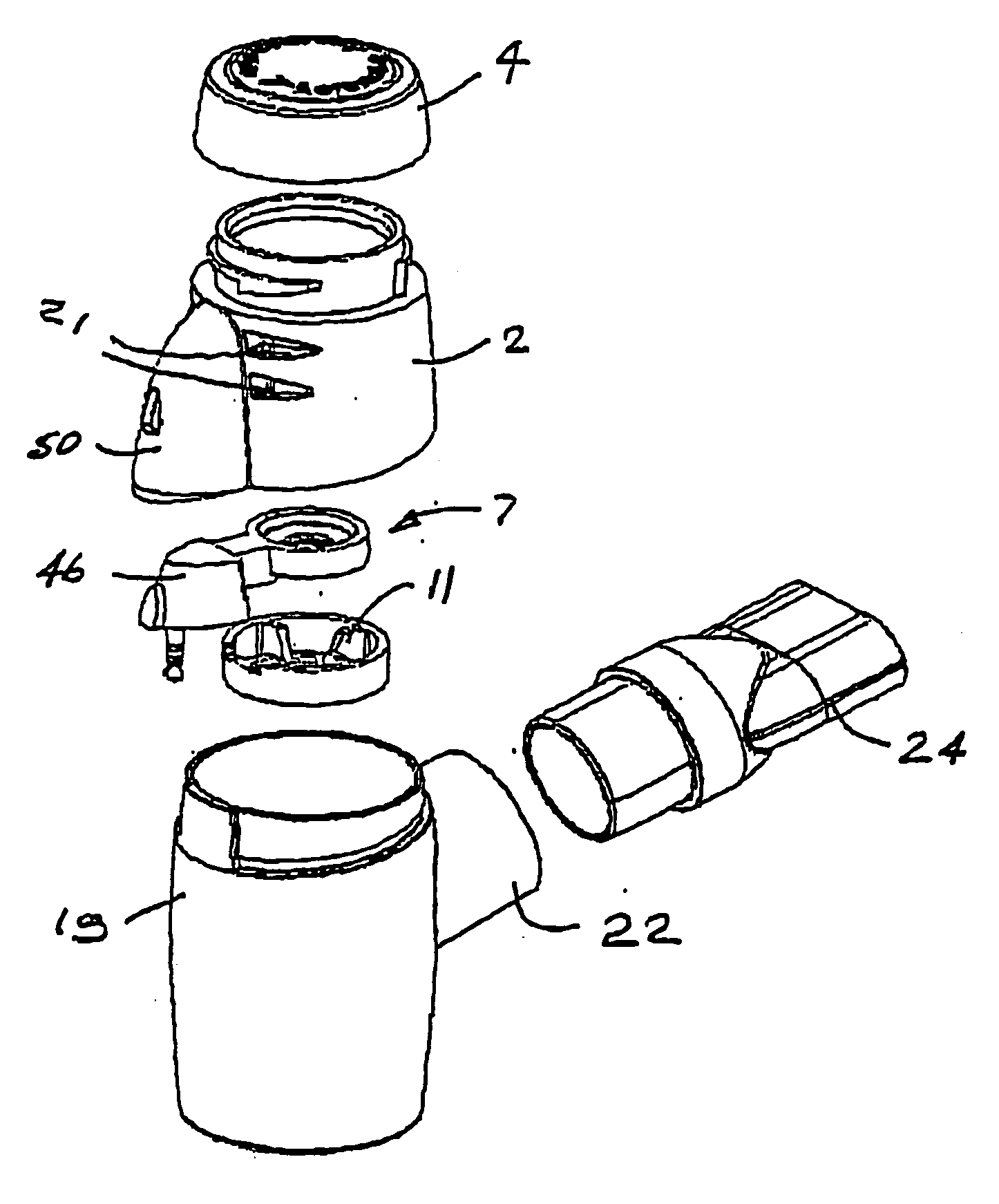
Inhalation nebulizer
InactiveUS6962151B1Prevent escapePrevent inflowMovable spraying apparatusSpray nozzlesNebulizerInhalation
An inhalation nebulizer (1) includes an aerosol generator (2) that has a diaphragm (22) vibrated by a vibration generator (23). The inhalation nebulizer (1) includes a liquid storage container (21) that is in fluid contact with the diaphragm (22). A liquid contained in the storage container (21) is atomized into a mixing chamber (3) through openings in the diaphragm and can subsequently be inhaled by a patient.
Owner:PARI PHARMA GMBH
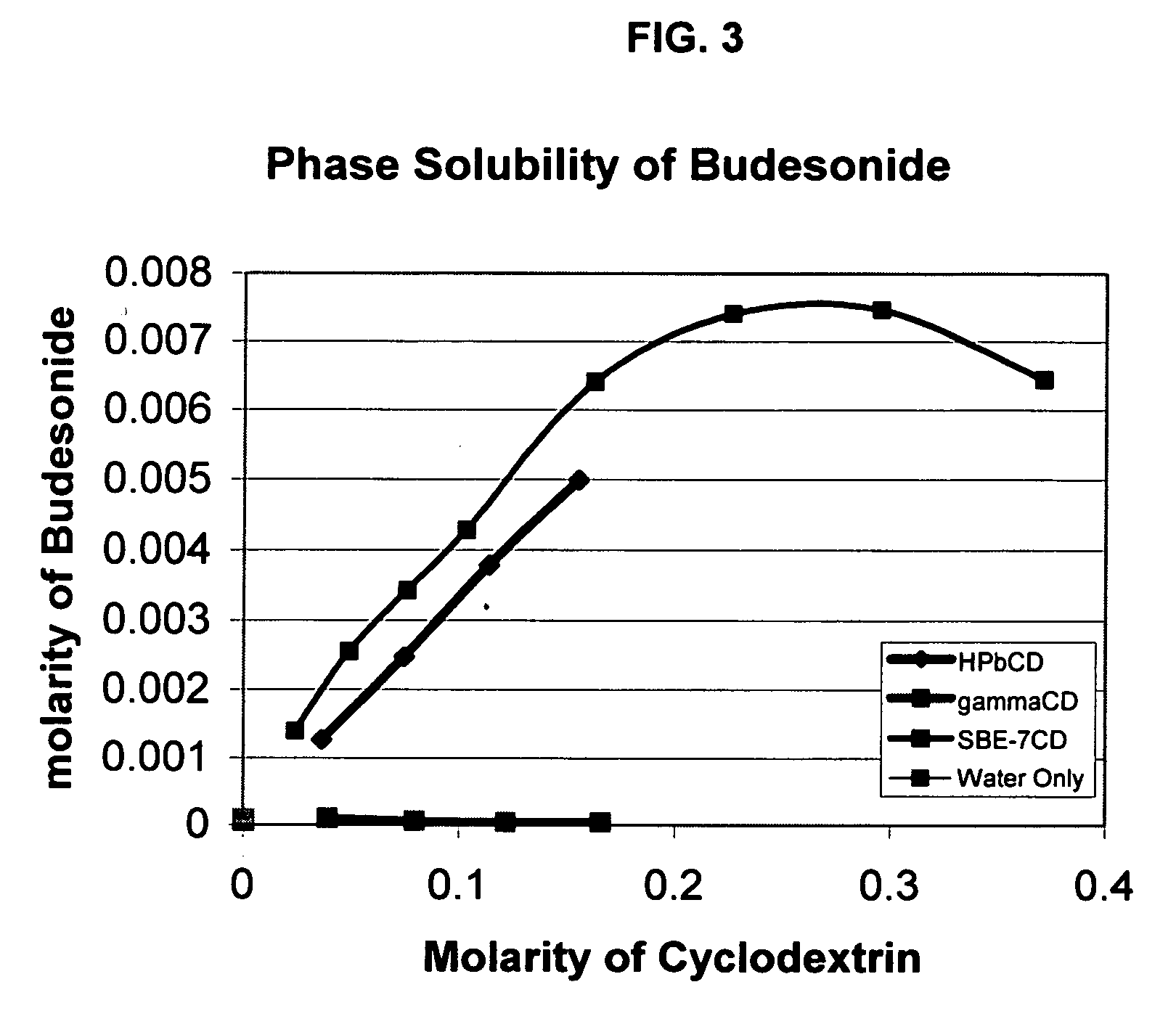
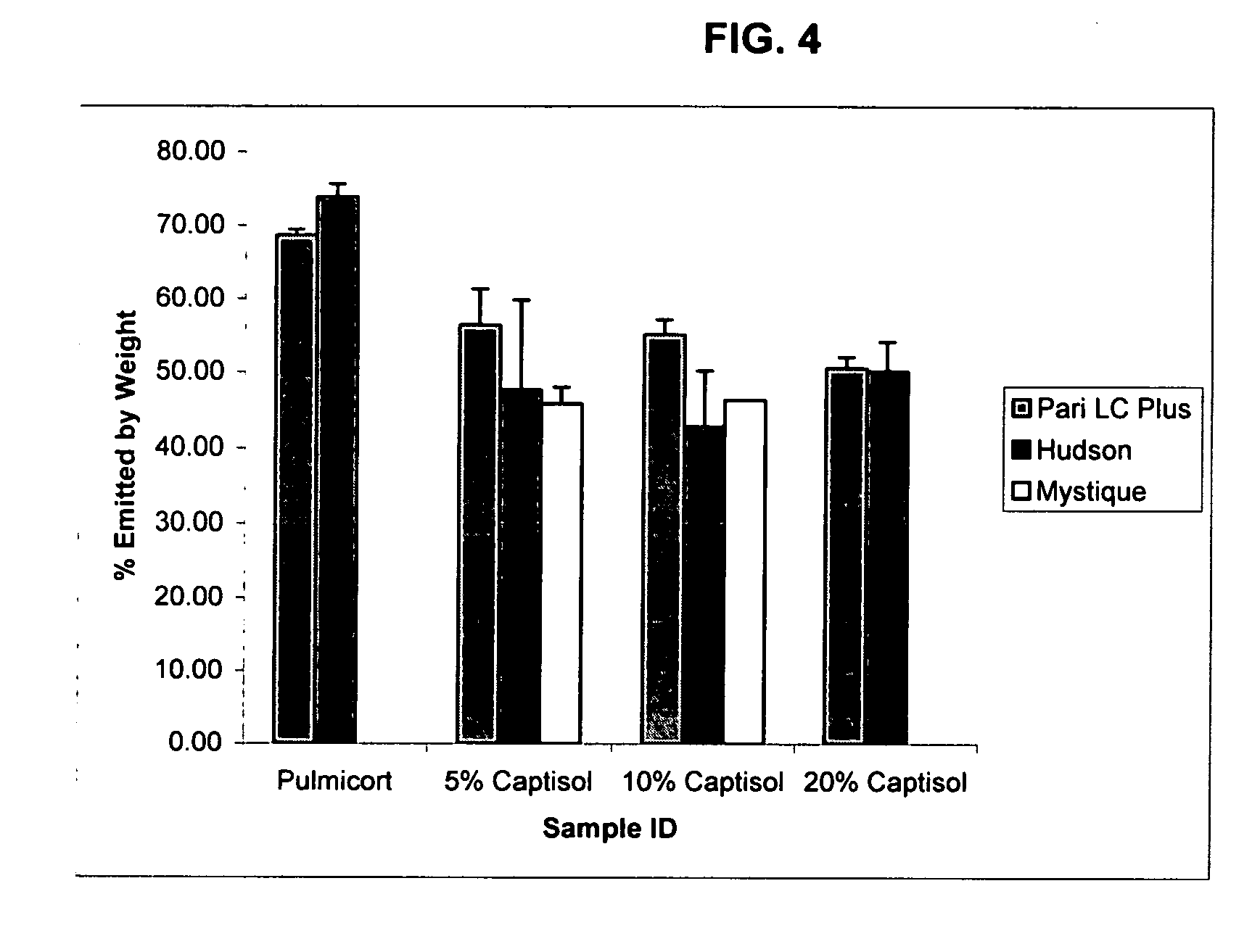
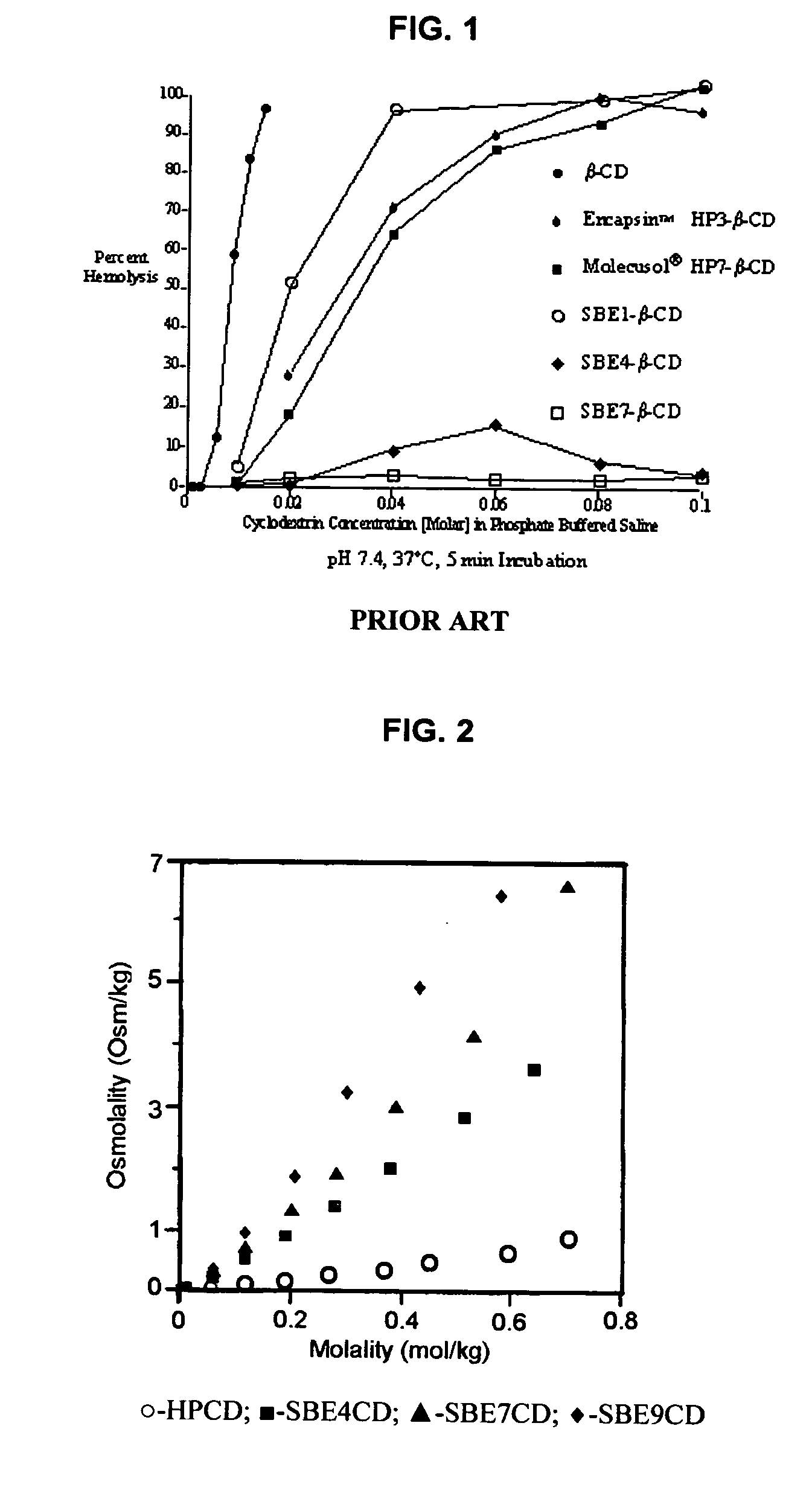
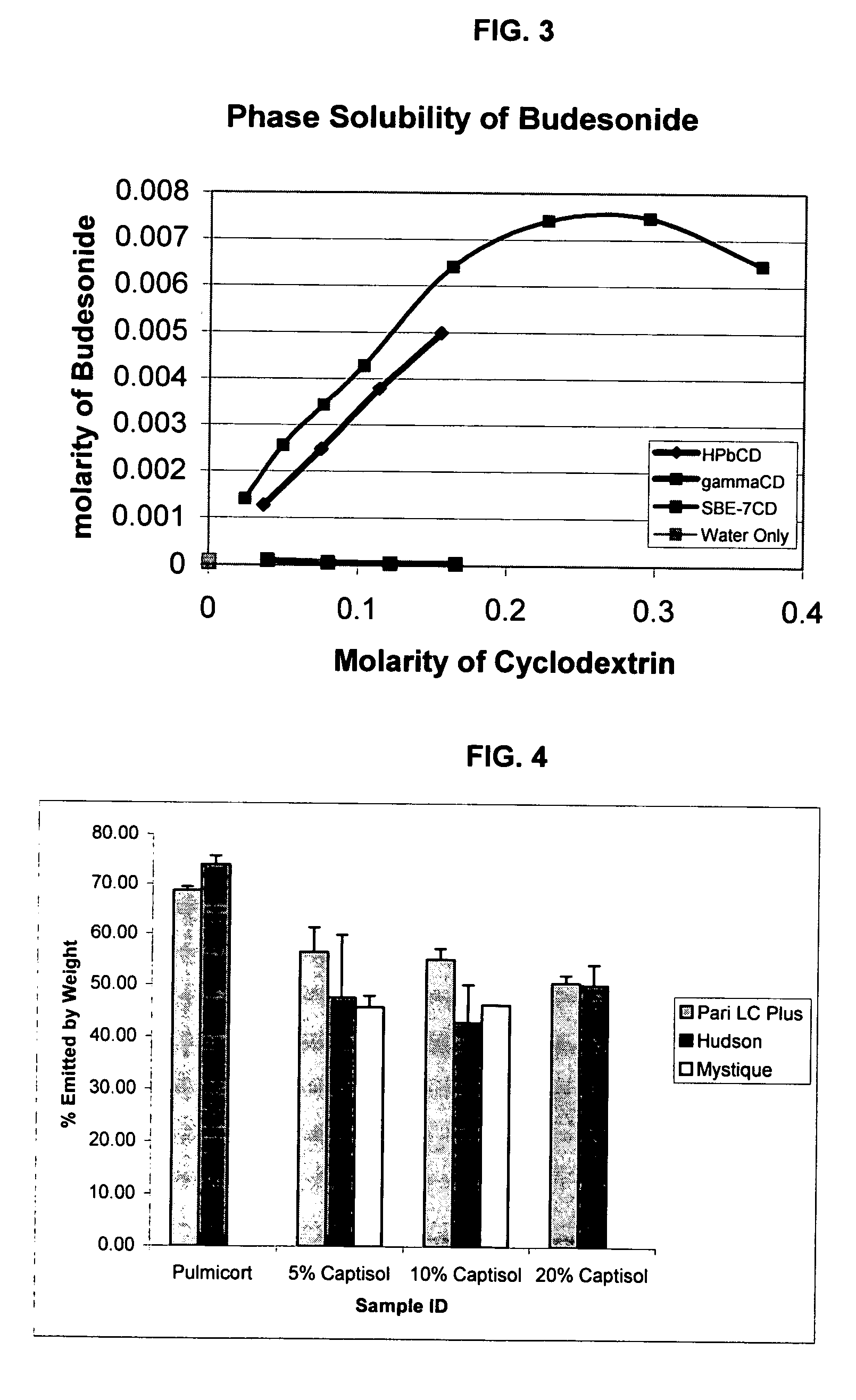
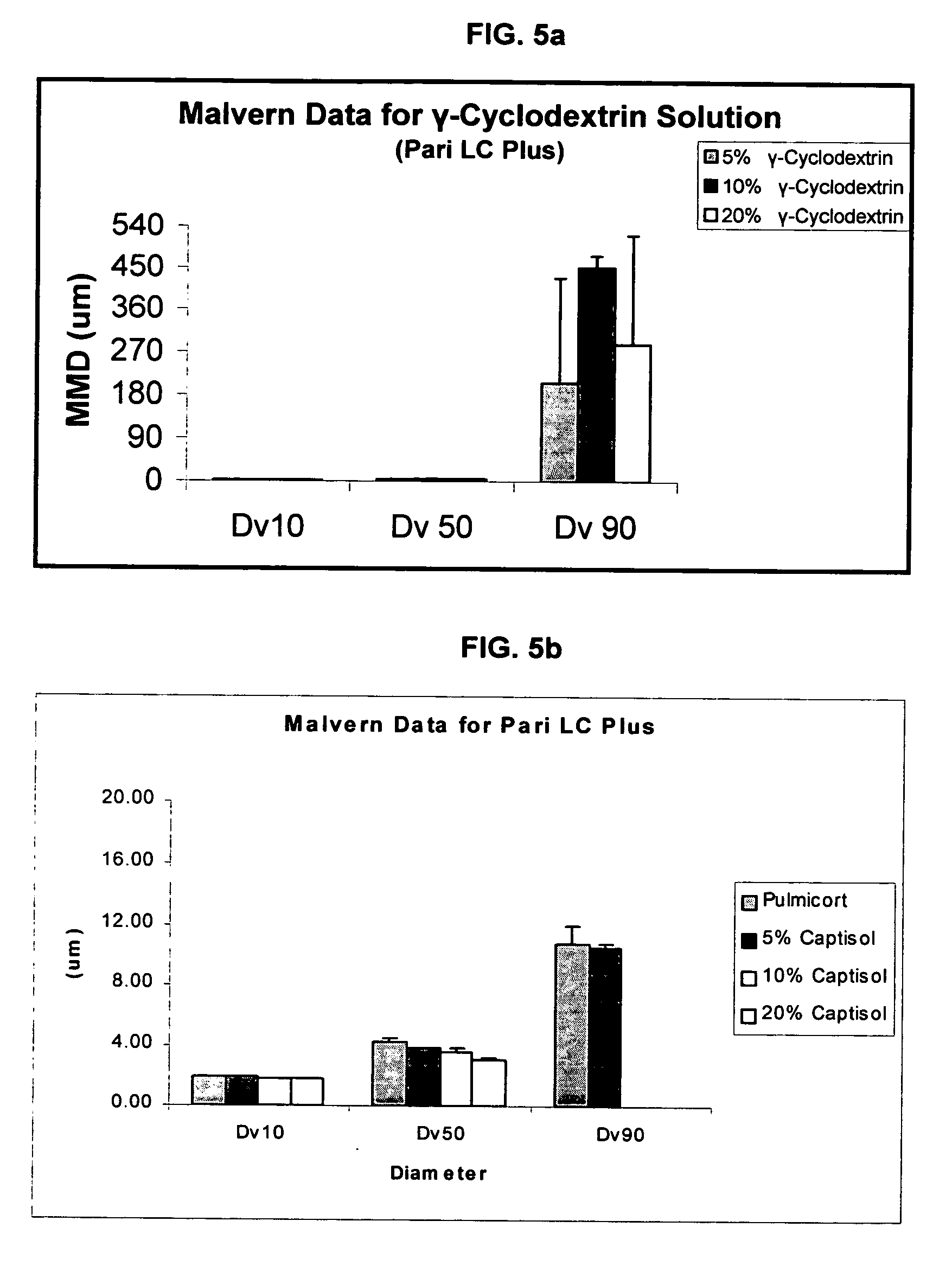
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Atomizer for an aerosol delivery device and related input, aerosol production assembly, cartridge, and method
The present disclosure relates to atomizers for an aerosol delivery device such as a smoking article. The atomizer may include a liquid transport element and a wire extending along at least a portion of a longitudinal length thereof. The wire may define contact portions configured to engage heater terminals and a heating portion configured to produce heat. The heating portion may include a variable coil spacing. In other atomizers, the wire may extend at least partially through the liquid transport element proximate the contact portions. Related inputs, cartridges, aerosol production assemblies, and methods of forming atomizers are also provided.
Owner:RAI STRATEGIC HLDG INC
Device for feeding drug into pulmones
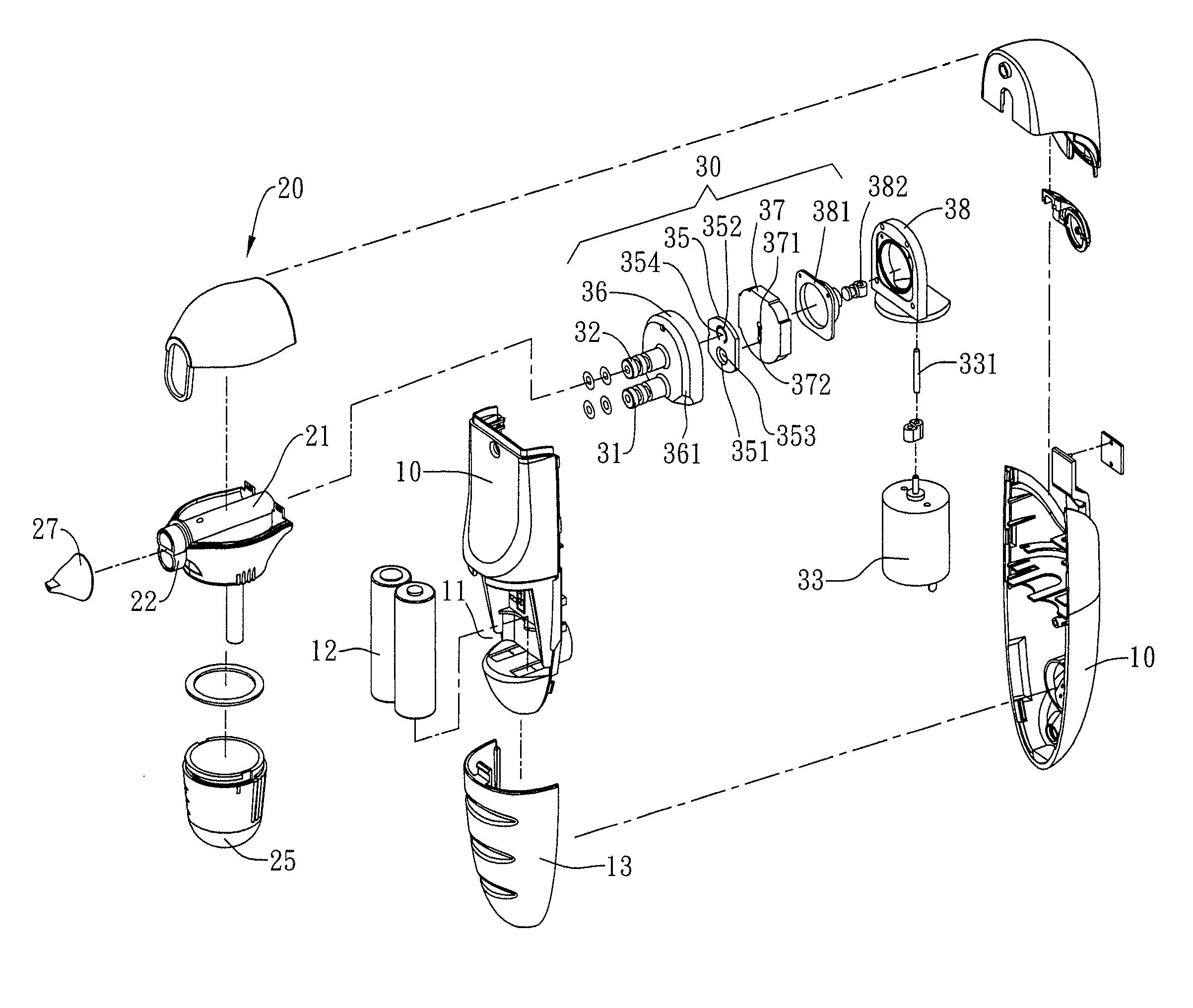
InactiveCN101176805AUniform atomizationAtomized particles are smallMedical atomisersInhalatorsNebulizerMedicine
The invention relates to a medical instrument used for drug absorption of pulmonary alveoli, in particular to an intrapulmonary drug administration device atomizing drugs dissolved in liquid into fine particles reaching the pulmonary alveoli, which comprises a casing and a suction nozzle arranged at one side of the casing. The invention is characterized in that a battery, a liquid supplying bottle and an atomizer are arranged in the casing, wherein, one end of the liquid supplying bottle is communicated with the suction nozzle, while the other end is connected with the atomizer, an air inlet is arranged on the casing above the atomizer, and an air passage is arranged on the outer surface of the liquid supplying bottle; an action switch is arranged on the casing. The invention has the advantages that the drug can be atomized into uniform particles, the particles are small, and the average diameter can reach about 1 micron, which is the best technical parameter for intrapulmonary absorption; a quantitative device arranged in the invention can control the drug administration amount; the invention can be used for drug administration of bronchioles and pulmonary alveoli.
Owner:DAFUBAO INT
Respiratory mask
InactiveUS6851428B2Adjustable sizeEasy to adjustBreathing masksRespiratory masksNebulizerFolded form
A respiratory mask is reduced in size to better fit patients with smaller faces by either (a) folding one or more accordian folds formed in an upper portion of the mask, or (b) tearing away a part of the upper portion of the mask. The respiratory mask for adult patients can thus be adjusted to fit smaller adults and larger children. The respiratory mask for children can thus be adjusted to fit smaller children. A ball joint swivel connector is provided to connect the gas inlet of the masks to the hose connection for respiratory or nebulizer treating gases or aerosols.
Owner:DENNIS CARNELL K
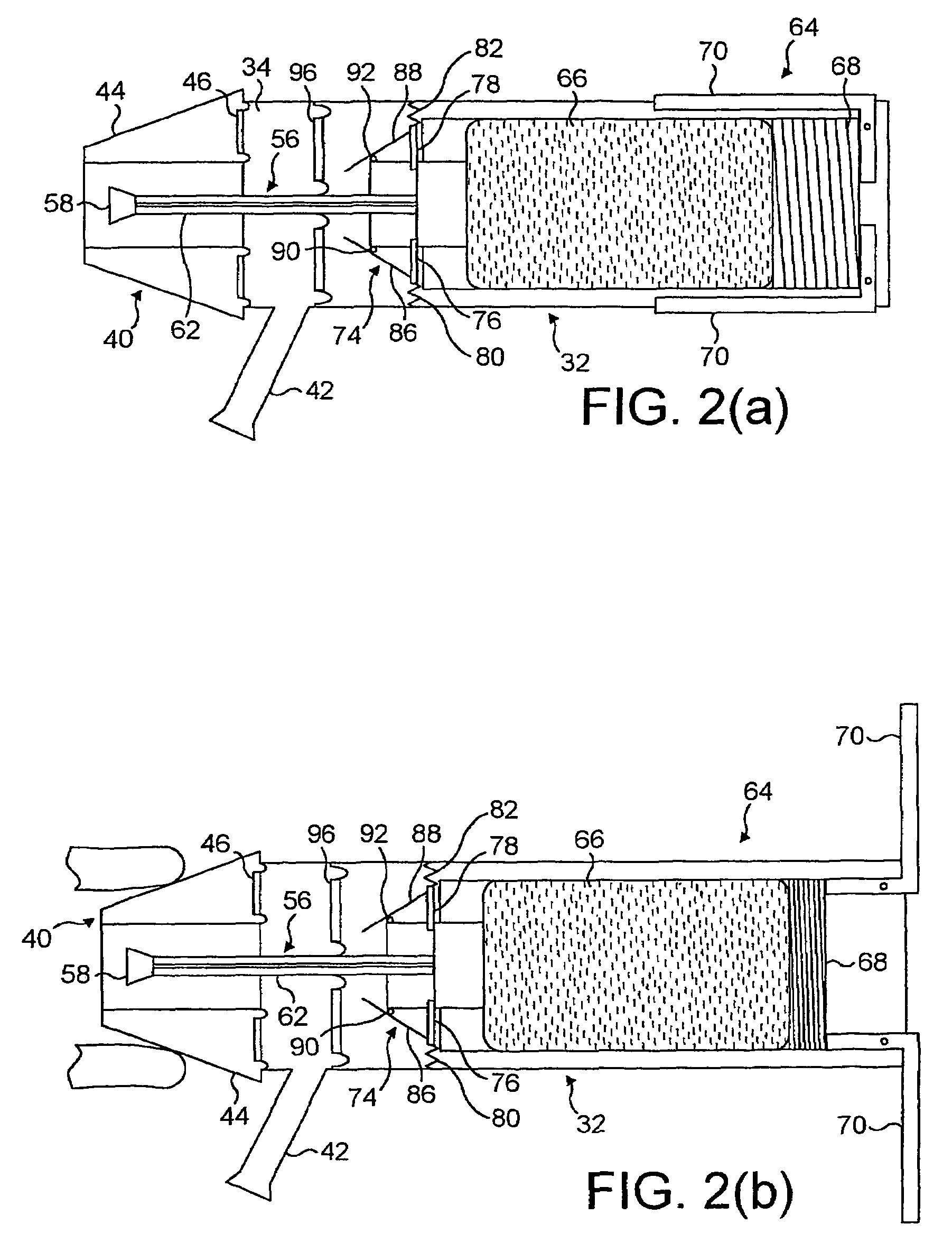
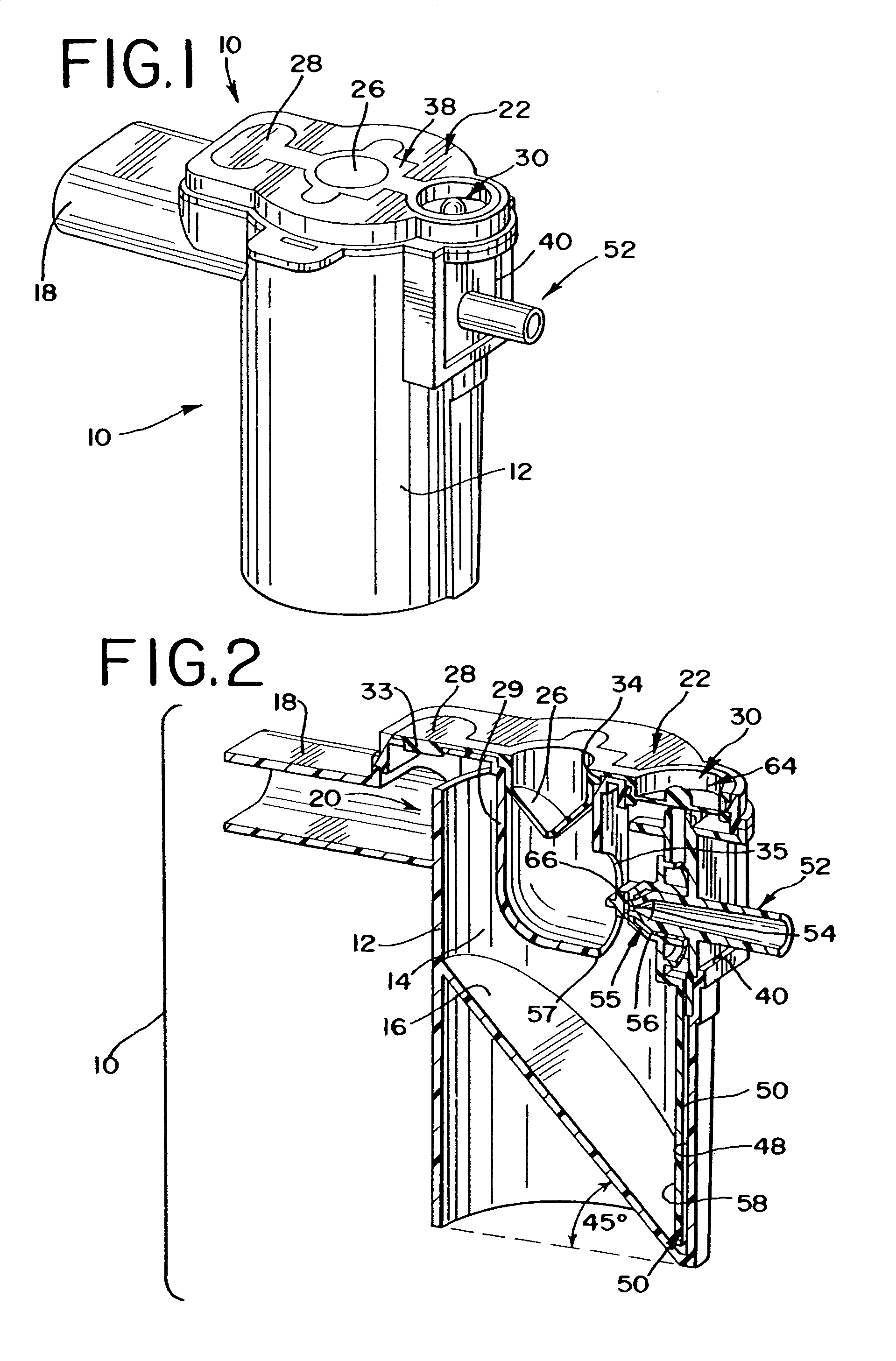
Nebuliser for the production of aerosolized medication
A nebuliser to deliver a medicament that includes a housing having a reservoir for the medicament, an aerosol generator that can be supplied the medicament from the reservoir, where the generator aerosolizes at least a portion of the medicament into an aerosol, a gas venting inlet to permit a gas to enter the nebuliser and form a mixture with the aerosol, and a passage through which the mixture of the aerosol and the gas is delivered to an outlet port of the nebuliser.
Owner:NOVARTIS AG
Aerosolization device
InactiveUS20110108025A1Reduce needImprove security levelTracheal tubesRespiratory masksNebulizerAerosol deposition
An aerosol transfer device (10) for medical aerosol generators comprises a body (12), fluidically coupled to a nebulizer (14) and to a patient interface (16). An ambient air intake (20) is formed into a lower body (12C). The body is shaped and configured to optimize mixing of ambient air from the ambient air intake and the aerosol generated by the nebulizer, resulting in the formation of an aerosol plume having optimum characteristics for delivery of the aerosol to the patient's pulmonary system, such as the central or deep lung regions. The shape and dimensions of the body are further designed to minimize aerosol deposition, thus improving delivery efficiency.
Owner:NEKTAR THERAPEUTICS INC
High efficiency medical nebulizer
A pneumatic nebulizer that produces a high volume of aerosols for inhalent delivery of medications and other constituencies. High pressure gas formed into a gas jet is passed through a thin choked region of fluid that is entrailed and impinged upon an aerosol amplifier which creates a spray whose aerosol components are directed up through vents to an aerosol outlet for delivery. Larger-sized liquid particles are caused to pool up in a region surrounding the aerosol amplifer and then drip down back into the liquid medication reservoir.
Owner:MERCURY ENTERPRISES
Aerosol delivery device including a wirelessly-heated atomizer and related method
The present disclosure relates to an aerosol delivery device configured to wirelessly heat an atomizer. The aerosol delivery device may include a control body and a cartridge. The control body may include an induction transmitter. The cartridge may include an induction receiver and an aerosol precursor composition. When electrical current is directed to the induction transmitter, the induction receiver may be inductively heated. The heat produced by the induction receiver may form an aerosol from the aerosol precursor composition at the substrate. Related methods are also provided.
Owner:RAI STRATEGIC HLDG INC
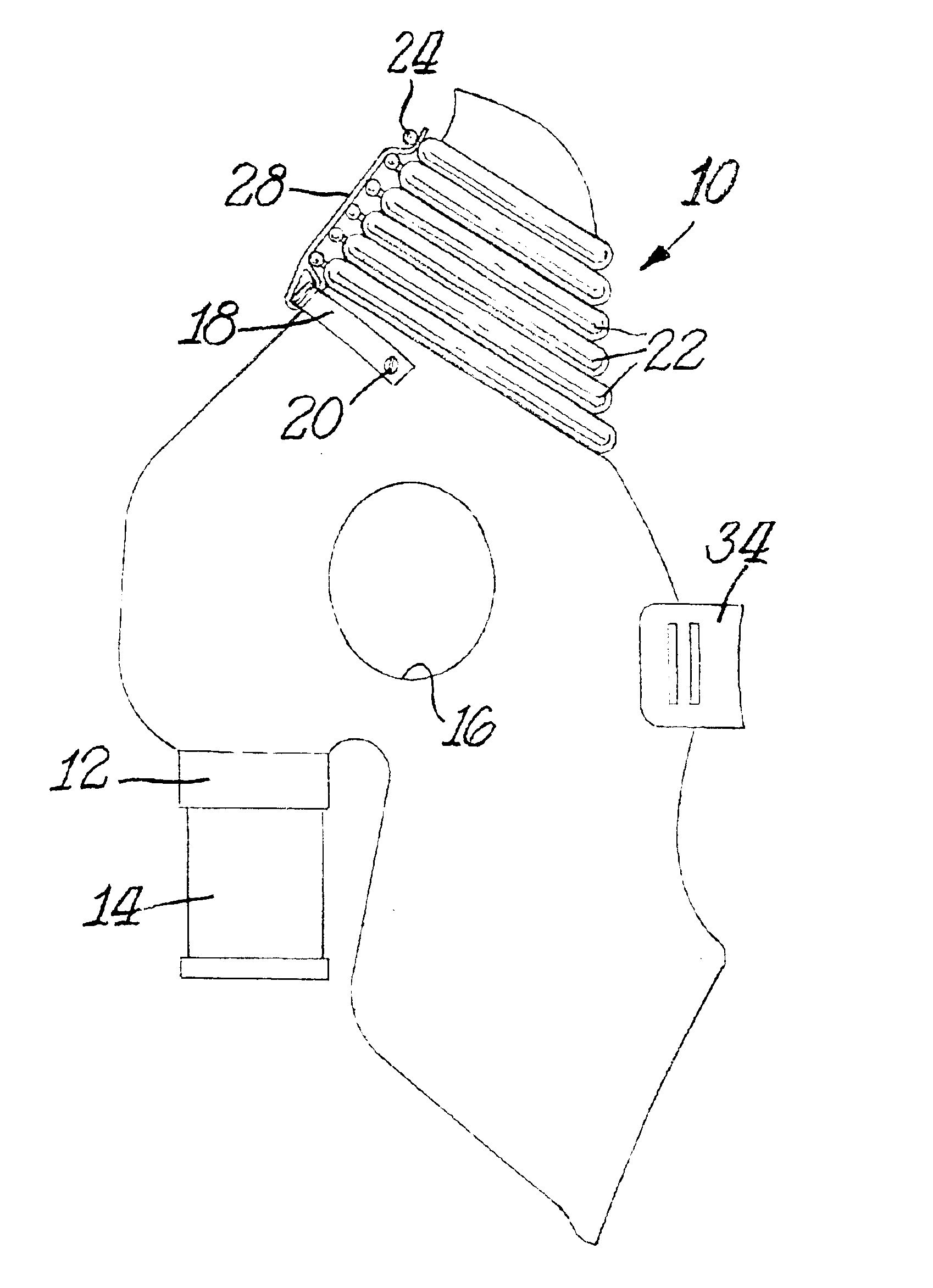
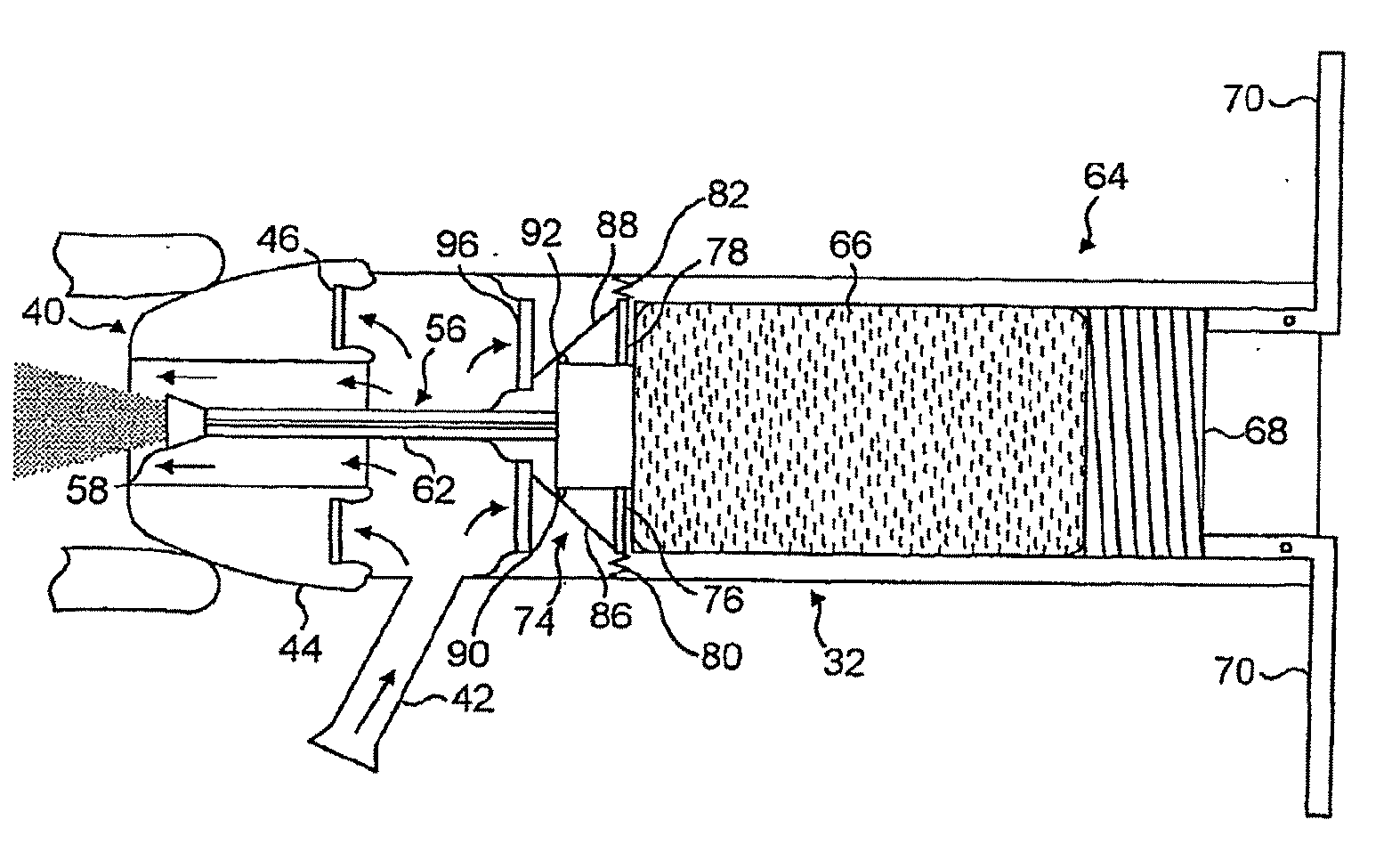
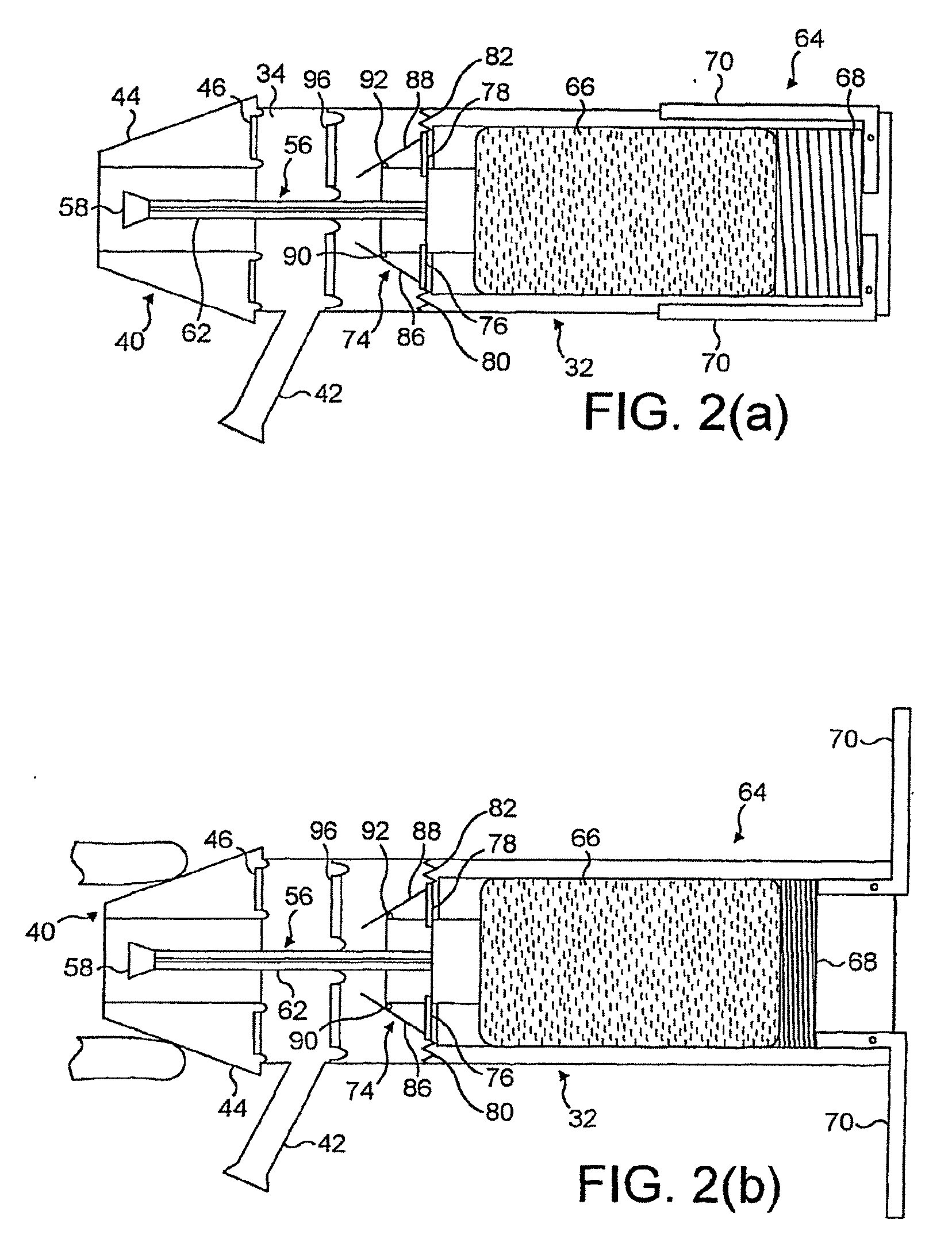
Nasal delivery devices
InactiveUS7347201B2Small particle sizeAvoid inhalationRespiratorsLiquid surface applicatorsNostrilNasal cavity
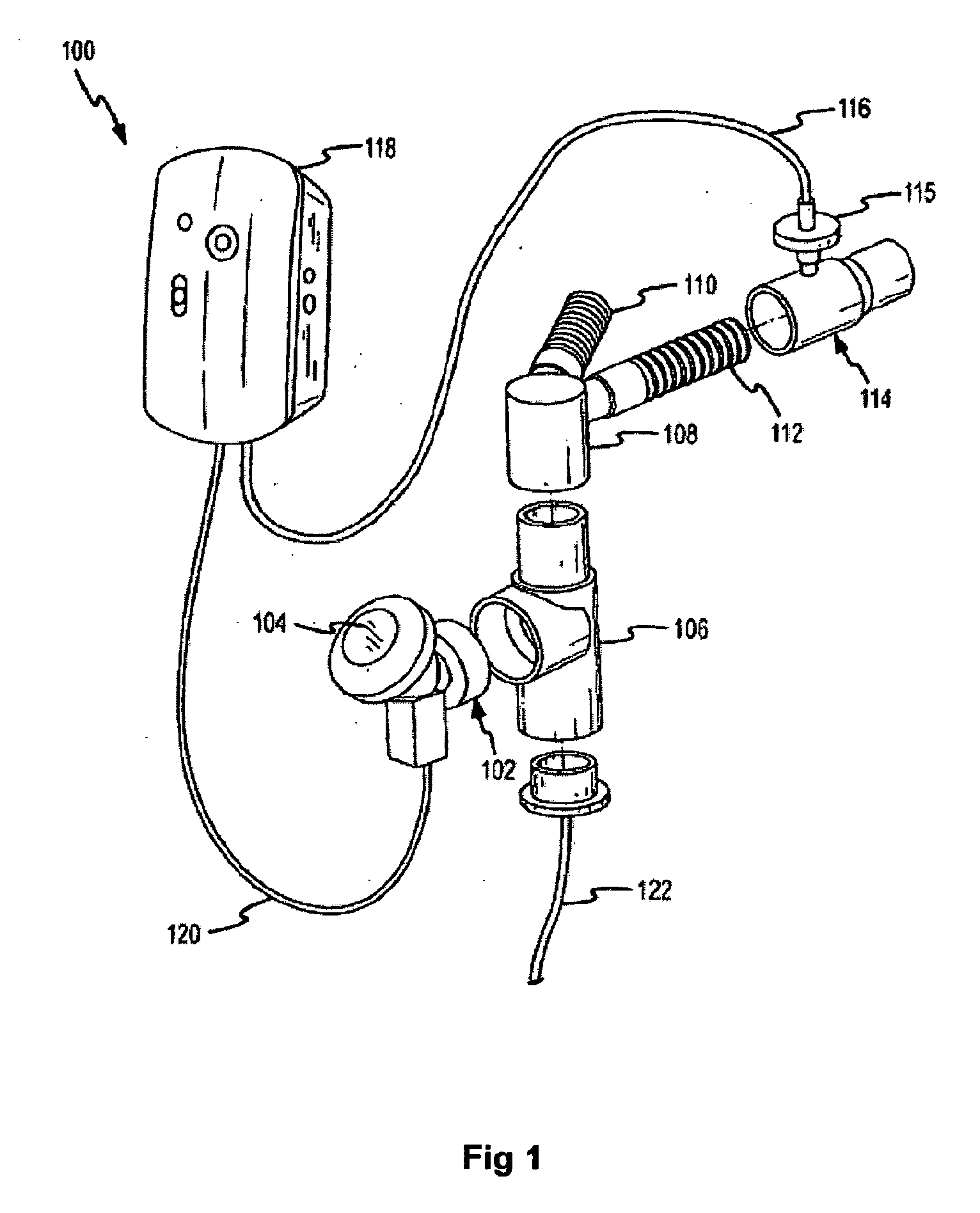
An exhalation breath-actuated nasal delivery device for and a method of delivering a substance to a nasal cavity of a subject, the delivery device comprising: a nosepiece (40) for fitting to a nostril of a subject; a mouthpiece (42) through which the subject in use exhales; and delivery unit (64), as one of a mechanical delivery pump (66) or a nebulizer (115), for delivering a substance to the nosepiece (40); and an actuation mechanism (74) for actuating the delivery unit in response to oral exhalation through the mouthpiece, and preferably when at least one or both of the pressure at or the flow rate through the nosepiece exceeds a predetermined threshold.
Owner:OPTINOSE INC
Engineered particles and methods of use
InactiveUS7306787B2Reduce deliveryLess attractivePowder deliveryOrganic active ingredientsNebulizerActive agent
Engineered particles are provided may be used for the delivery of a bioactive agent to the respiratory tract of a patient. The particles may be used in the form of dry powders or in the form of stabilized dispersions comprising a nonaqueous continuous phase. In particularly preferred embodiments the particles may be used in conjunction with an inhalation device such as a dry powder inhaler, metered dose inhaler or a nebulizer.
Owner:NOVARTIS AG
Combined nasal spray and aspirator device
ActiveUS7862536B2Present invention is physically more favorable for usersGood for healthEar treatmentCannulasNasal cavityNebulizer
Owner:AVITA CORP
Spray for fluent materials
ActiveUS20060189944A1Easy to measureWell mixedSurgeryIntravenous devicesSprayerBiocompatible coating
Certain embodiments relate to a sprayer or other medical apparatus for applying a biocompatible coating in situ. Such an apparatus may have a first conduit connected to a first exit opening and a second conduit connected to a second exit opening to deliver a first composition through the first conduit and a second composition through the second conduit to mix the first composition and the second composition outside both the first conduit and the second conduit. The first composition may be, e.g., a precursor to a material formed after the mixing of the first composition and the second composition. The first exit opening and the second exit opening may be approximately adjacent to each other and define an angle that is less than about 140 degrees.
Owner:CONFLUENT SURGICAL
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
Multipurpose therapeutic face mask
A therapeutic face mask comprises a face-engaging portion and a single connector having a mask-engaging end and a single treatment-receiving end which has a single attachment mounting for detachably sealingly receiving a treatment attachment, such as an oxygen reservoir bag or a nebulizer. A one-way inhalation valve in the connector permits fluid flow from the treatment-receiving end to the mask-engaging end during inhalation and inhibits fluid flow in the other direction. The mask also includes a valve-governed exhalation port and an anti-asphyxia valve assembly configured to permit fluid flow from ambient to the face-engaging portion during inhalation only when inspiratory effort during inhalation exceeds fluid flow to the treatment-receiving end of the connector. Also provided is an oxygen reservoir bag having a neck shaped for removable coupling to a mating connector of a therapeutic face mask. An oxygen reservoir bag may have a metered-dose inhaler port defined in its neck.
Owner:FLYNN SR STEPHEN DONALD
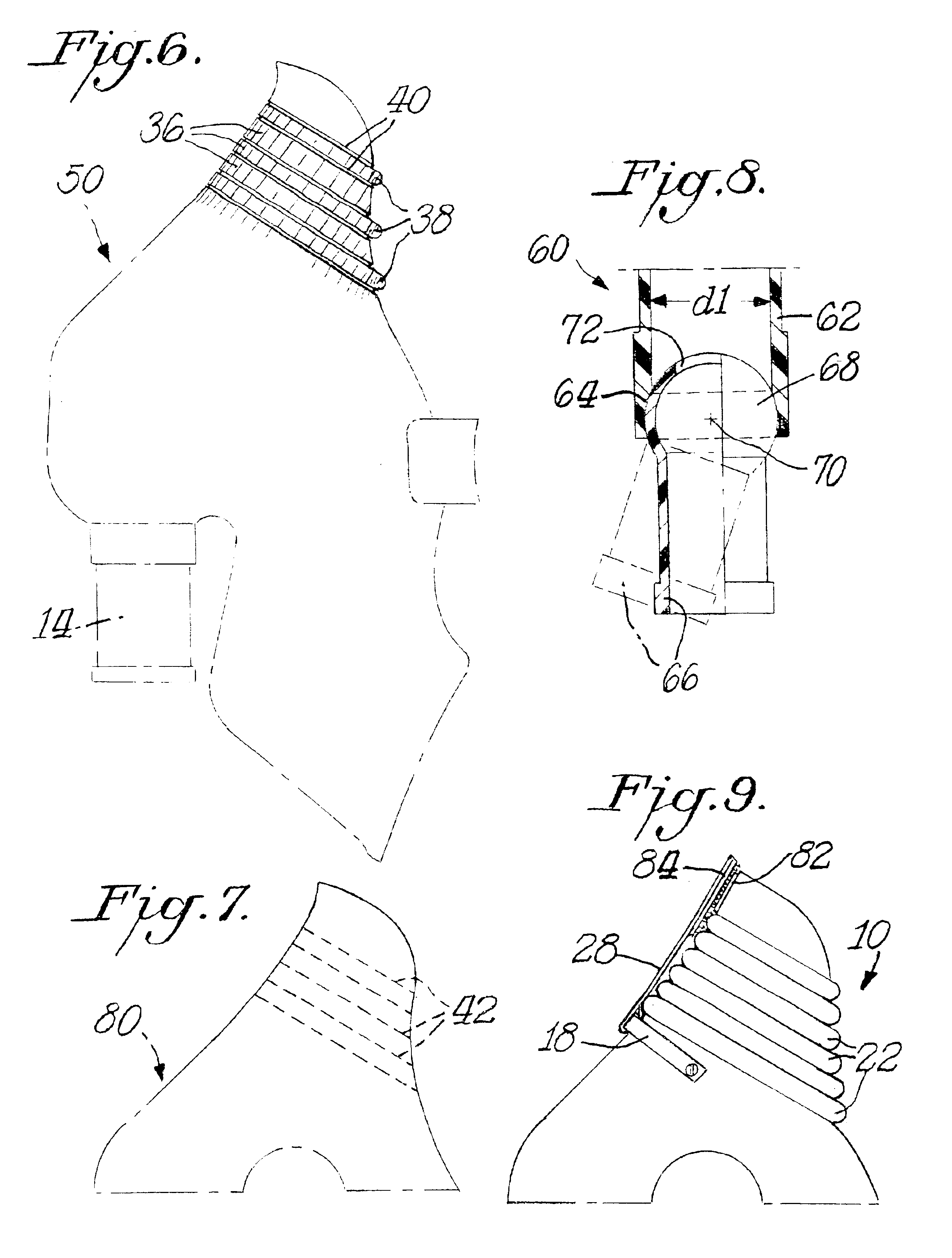
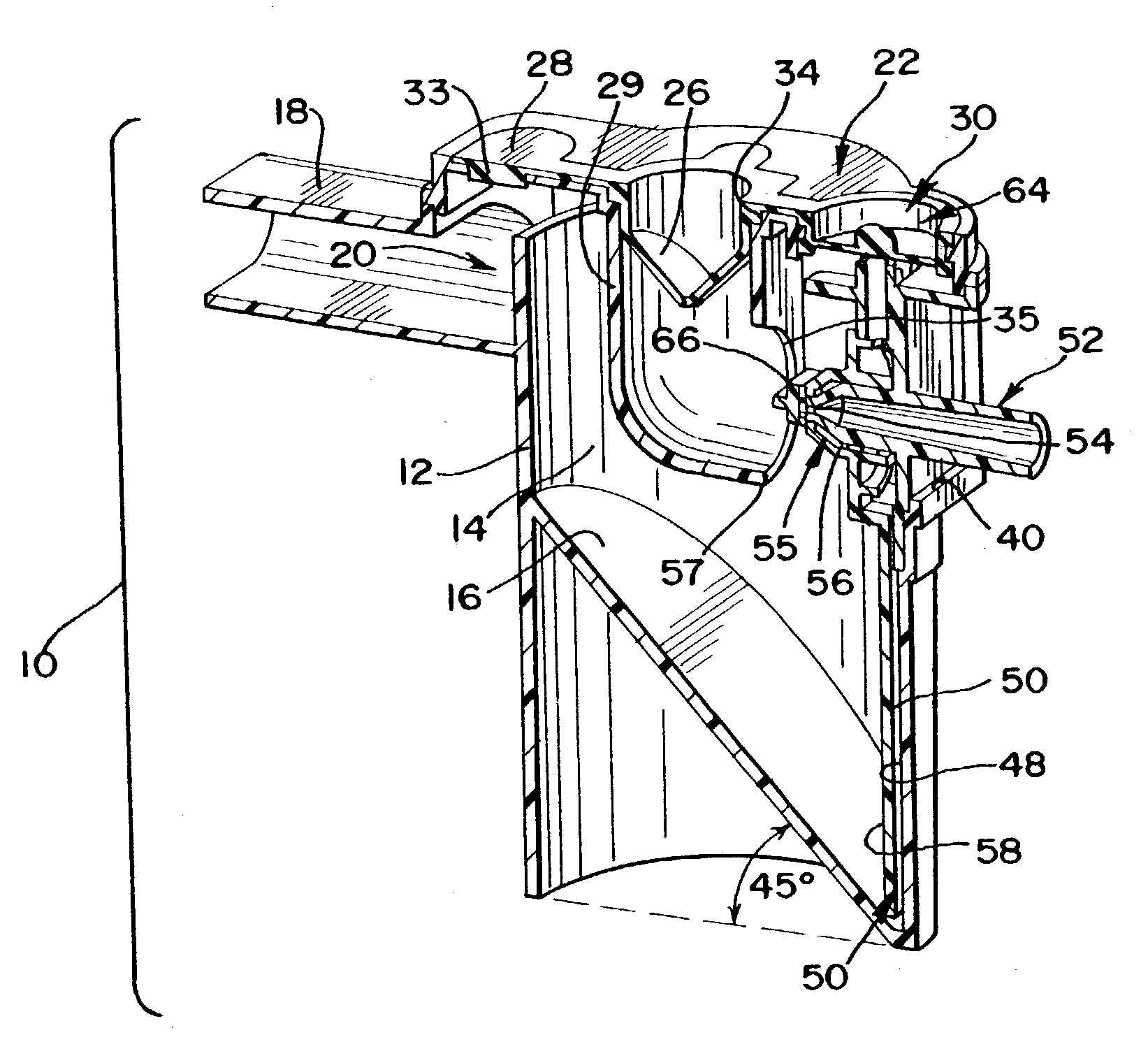
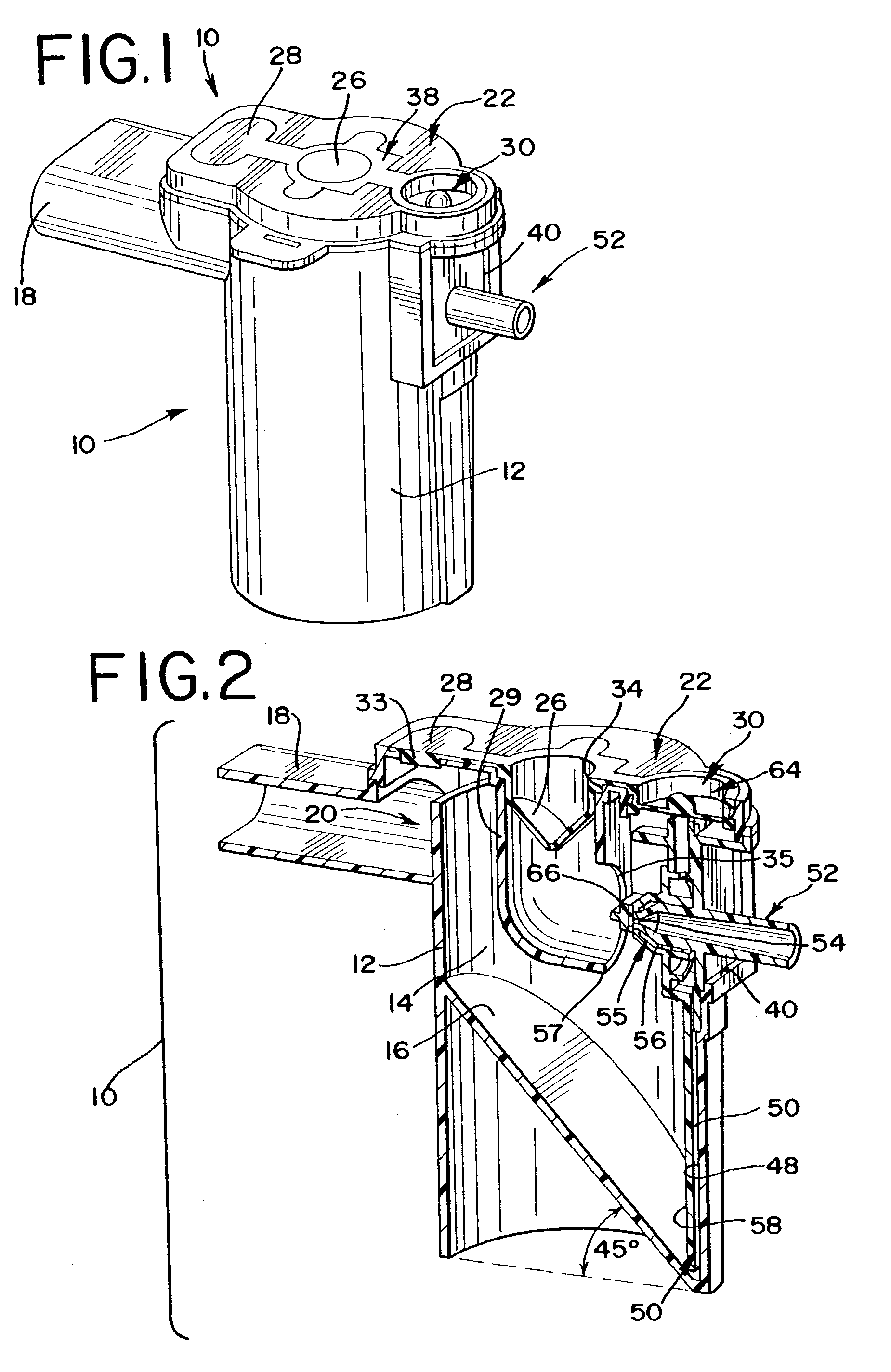
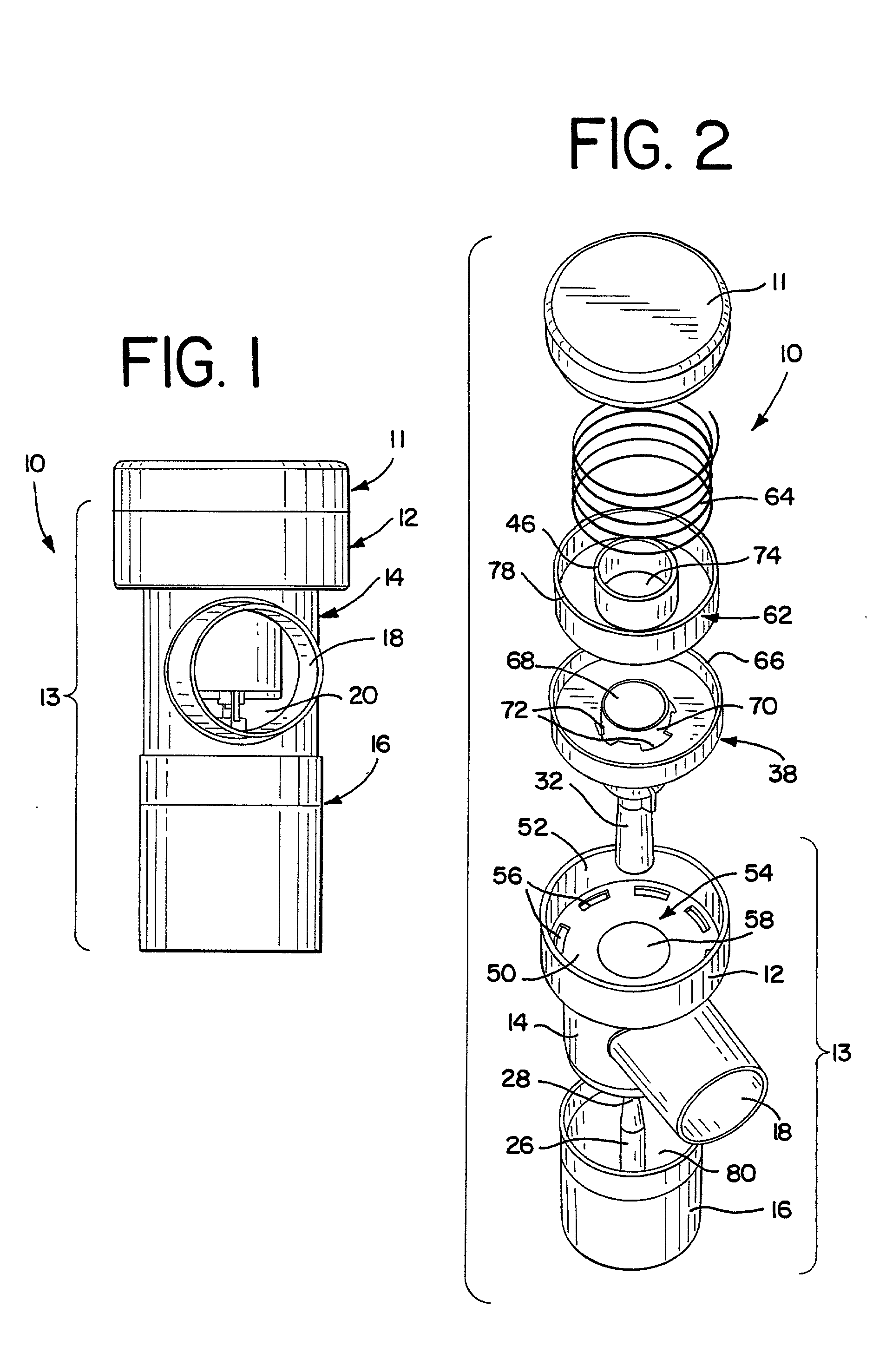
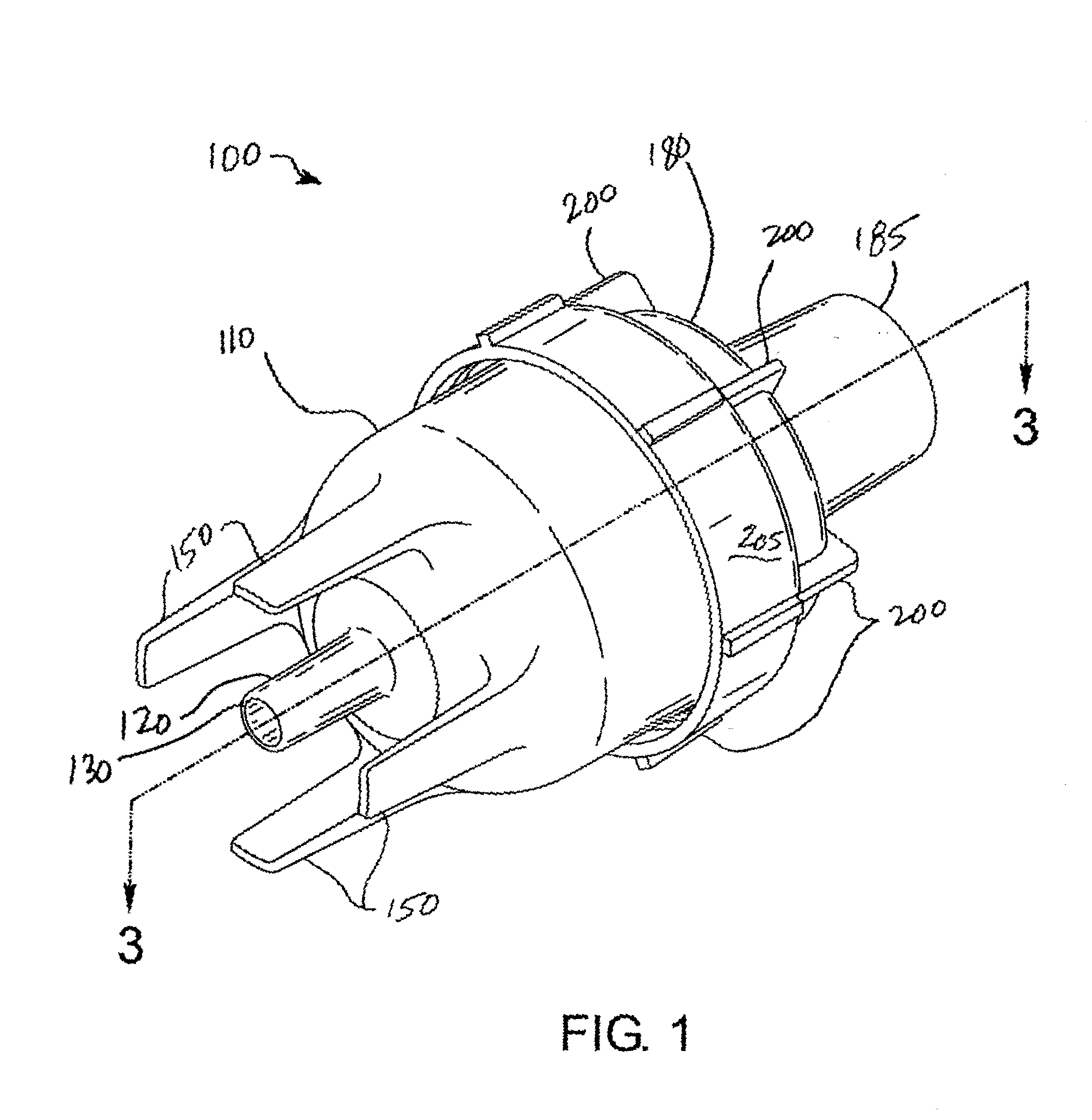
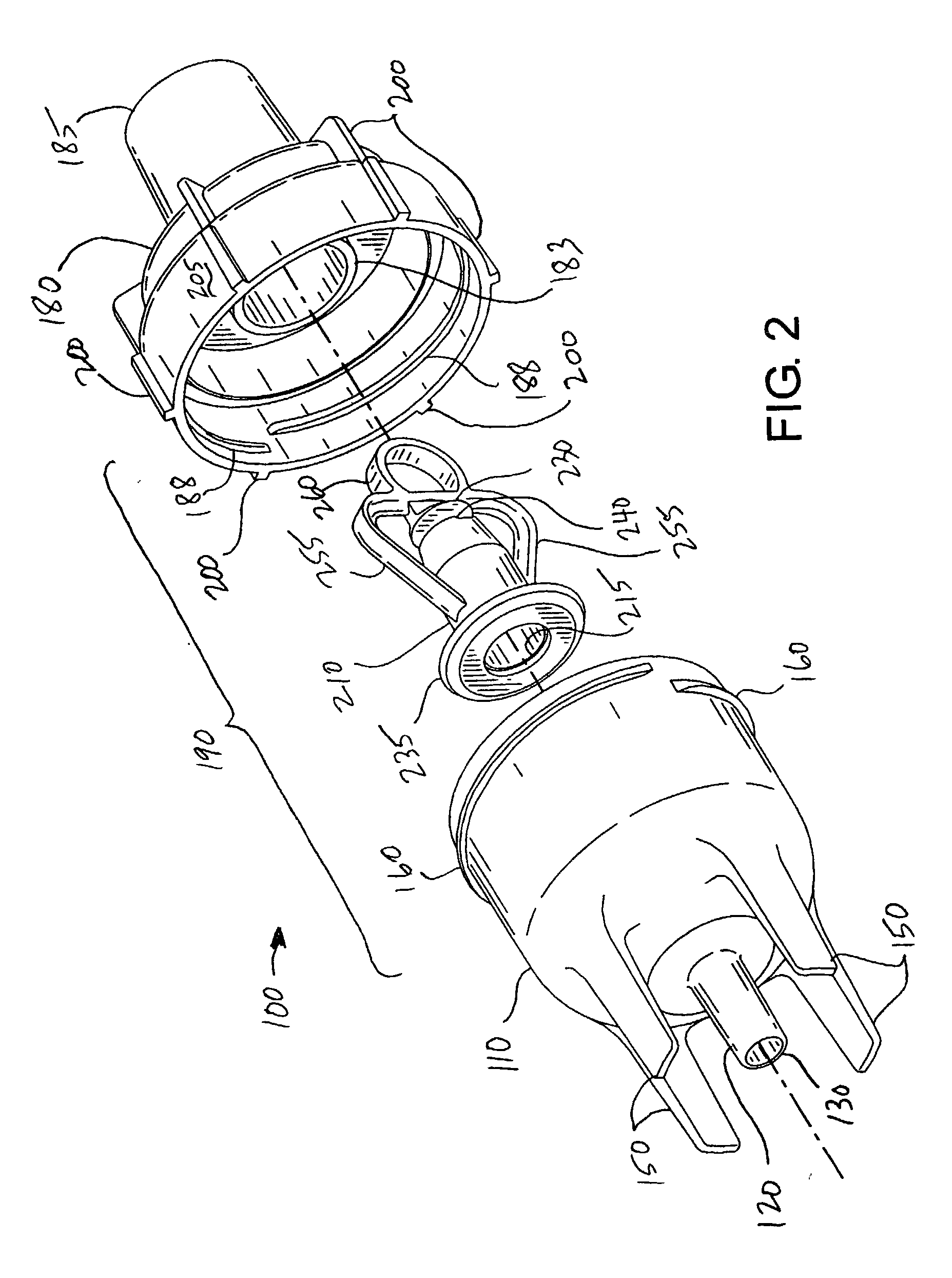
Nebulizer apparatus and method
A nebulizer for efficiently and reliably delivering aerosolized fluid to an inhaling patient is disclosed. The nebulizer includes a fixed diverter and a movable fluid orifice or fluid pathway connected with an actuator for responding to an inhalation or a manual actuation and beginning the nebulization process. Also provided is a method of providing nebulization including the steps of moving a fluid orifice or fluid pathway connected to an actuator so that the fluid orifice or fluid pathway reaches a nebulizing position during inhalation.
Owner:TRUDELL MEDICAL INT INC
Nebulizer apparatus and method
InactiveUS6994083B2Reduce effortContinuous nebulizationSpray nozzlesMedical atomisersNebulizerInhalation
A nebulizer for efficiently and reliably delivering aerosolized fluid to an inhaling patient is disclosed. The nebulizer includes a fluid channel air inlet and fluid channel air inlet valve responsive to either a manual force external of the nebulizer, or a patient's breathing, to begin the nebulization process. Also provided is a method of providing nebulization including the steps of moving a fluid channel air inlet valve against a fluid channel air inlet so that a negative pressure may build up over the fluid in the fluid channel to draw fluid from the fluid reservoir and begin nebulization during inhalation.
Owner:TRUDELL MEDICAL INT INC
Atomizer, atomizing assembly and inhaler
An atomizer for an inhaler includes a housing, a gasket, a liquid absorbing sheet, a wick, and an atomizing element. The housing includes a first housing and a second housing, the first housing and the second housing form a liquid reservoir therebetween for storing liquid. The gasket is sleeved on the first housing, the gasket defines a liquid conducting hole in communication with the liquid reservoir. The liquid absorbing sheet is sleeved on the first housing in contact with the gasket, the liquid absorbing sheet is configured to absorb the liquid in the liquid reservoir via the liquid conducting hole. The wick is in contact with the liquid absorbing sheet and configured to draw the liquid from the liquid absorbing sheet. The atomizing element is fixed to the wick and configured to atomize the liquid in the wick.
Owner:SHENZHEN SMOORE TECH LTD
Nasal Delivery Devices
InactiveUS20080223363A1Increase surface areaIncrease blood flowRespiratorsMedical devicesNostrilNasal cavity
Owner:OPTINOSE AS
Nebulizer apparatus and method
InactiveUS20030136399A1Alleviate inhalation effortContinuous nebulizationSpray nozzlesMedical atomisersNebulizerInhalation
A nebulizer for efficiently and reliably delivering aerosolized fluid to an inhaling patient is disclosed. The nebulizer includes a fluid channel air inlet and fluid channel air inlet valve responsive to either a manual force external of the nebulizer, or a patient's breathing, to begin the nebulization process. Also provided is a method of providing nebulization including the steps of moving a fluid channel air inlet valve against a fluid channel air inlet so that a negative pressure may build up over the fluid in the fluid channel to draw fluid from the fluid reservoir and begin nebulization during inhalation.
Owner:TRUDELL MEDICAL INT INC
Pressure atomizer nozzle
InactiveUS6045058ASlow disintegrationReduce friction lossBurnersSpray nozzlesNebulizerAtomizer nozzle
The invention relates to a two-stage pressure atomizer nozzle with a nozzle body (30) having a mixing chamber (39) which is connected to an outside space via a nozzle outlet bore (33), and with a first feed duct (42) with a feed bore (41) for a liquid (37) to be atomized, through which feed bore said liquid (37) can be fed, free of swirling and under pressure, at least one further feed duct (36) for a portion of the liquid (37) to be atomized or for a second liquid (37') to be atomized opening into the chamber (39), through which feed duct said liquid (37, 37') can be fed under pressure and with swirling. The feed bore (41) of the first feed duct (42) lies on one axis (34) with the nozzle outlet bore (33). It is defined in that the outlet-side diameter (da) of the nozzle outlet bore (33) is at most as large as the diameter (dz) of the feed bore (41) and the length (L) of the nozzle outlet bore (33) is at least twice to at most ten times the outlet-side diameter (da) of the nozzle outlet bore (33).
Owner:ANSALDO ENERGIA SWITZERLAND AG
Nebulizer apparatus and method
A nebulizer for efficiently and reliably delivering aerosolized fluid to an inhaling patient is disclosed. The nebulizer includes a fixed diverter and a movable fluid orifice or fluid pathway connected with an actuator for responding to an inhalation or a manual actuation and beginning the nebulization process. Also provided is a method of providing nebulization including the steps of moving a fluid orifice or fluid pathway connected to an actuator so that the fluid orifice or fluid pathway reaches a nebulizing position during inhalation.
Owner:TRUDELL MEDICAL INT INC
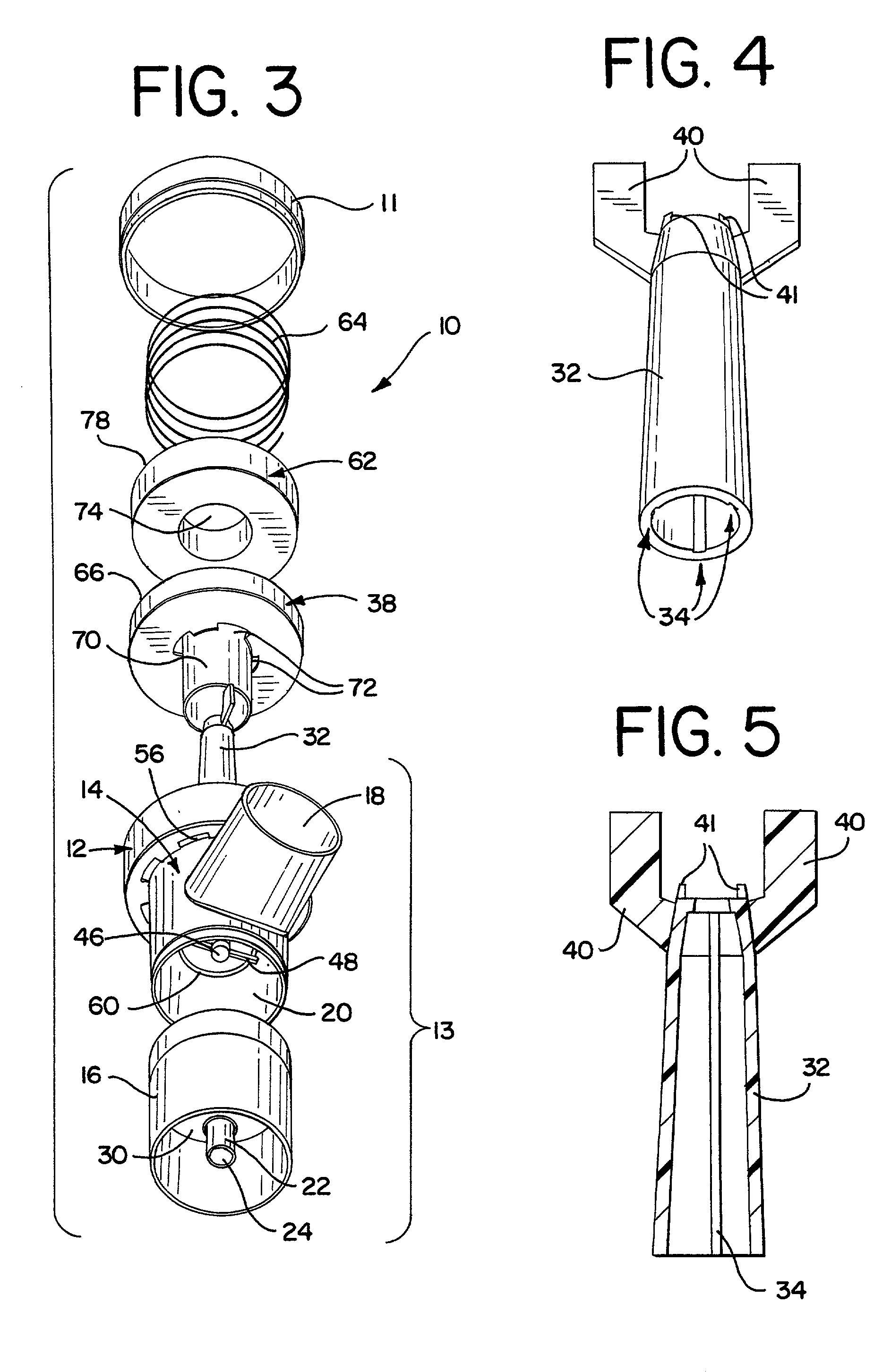
Pharmaceutical delivery system for oral inhalation through nebulization consisting of inert substrate impregnated with substance (S) to be solubilized or suspended prior to use
InactiveUS20040045546A1Good chemical stabilitySolvent evaporates quicklyDispersion deliverySolution deliveryBULK ACTIVE INGREDIENTDelivery system
A pharmaceutical delivery system for oral inhalation is disclosed through nebulization consisting of an inert supporting material impregnated with or deposited with pharmaceutically active ingredient which must be solubilized or suspended in a pharmaceutical solvent to form a solution or suspension prior to administration. Each pharmaceutical delivery unit dosage form comprises one or more therapeutically effective and safe amounts of pharmaceutically active ingredient uniformly impregnated in or deposited on a supporting material which is a natural or synthetic polymer, woven or non-woven fabrics, inert paper, inorganic materials such as foil and combination thereof in a single or multi-layer lamination in a form of a sheet or strip or film or membrane or sponge-like or cup or well. The dosage form of this invention is to be administered to a patient through oral or nasal inhalation using a nebulizer after reconstitution with a reconstituting solvent.
Owner:COLLEGIUM PHARMA INC
Atomizer
An atomizer is disclosed. The atomizer of the present invention comprises a main unit, a pipe, and a vaporizer. The vaporizer of the present invention comprises a pneumatic switch which is triggered by the airflow from the main unit. There is no conducting wire between the main unit and the vaporizer. The length of the pipe is determined according to the requirement of the user. In one embodiment of the present invention, the atomizer is remote controllable.
Owner:TSAI WEI LI
Low leakage liquid atomization device
InactiveUS6843430B2Minimize migrationImprove device performanceMovable spraying apparatusSpray nozzlesElectricityNebulizer
A battery operated atomizer device comprising, in a housing (22), a liquid reservoir (30) from which a capillary type liquid delivery system (38) extends to contact a piezoelectric actuator an atomization plate assembly (34), the assembly (34) being supported by means of wire-like elements (36) in cantilever fashion over the liquid delivery system, the liquid delivery system comprising an outer tubular member (52) and a solid rod (56) which have facing surfaces configured to define between them, longitudinal capillary liquid passages.
Owner:SC JOHNSON & SON INC
Treatment of pulmonary disorders with aerosolized medicaments such as vancomycin
InactiveUS20100282247A1Reduce in quantityReduce the amount requiredAntibacterial agentsAntimycoticsDiseaseGlycopeptide
A method of administering an aerosolized anti-infective, such as a glycopeptide, to the respiratory system of a patient. A ratio of an amount of the glycopeptide, such as vancomycin, delivered to the pulmonary system of the patient in a 24 hour period to a minimum inhibitory amount for the target organ for the same period is about 2 or more. A system to introduce aerosolized medicament to a patient may include a humidifier coupled to an inspiratory limb of a ventilator circuit wye, where the humidifier supplies heated and humidified air to the patient, and an endotracheal tube having a proximal end coupled to a distal end of the ventilator circuit wye. The system may also include a nebulizer coupled to the endotracheal tube, where the nebulizer generates the aerosolized medicament.
Owner:NEKTAR THERAPEUTICS INC
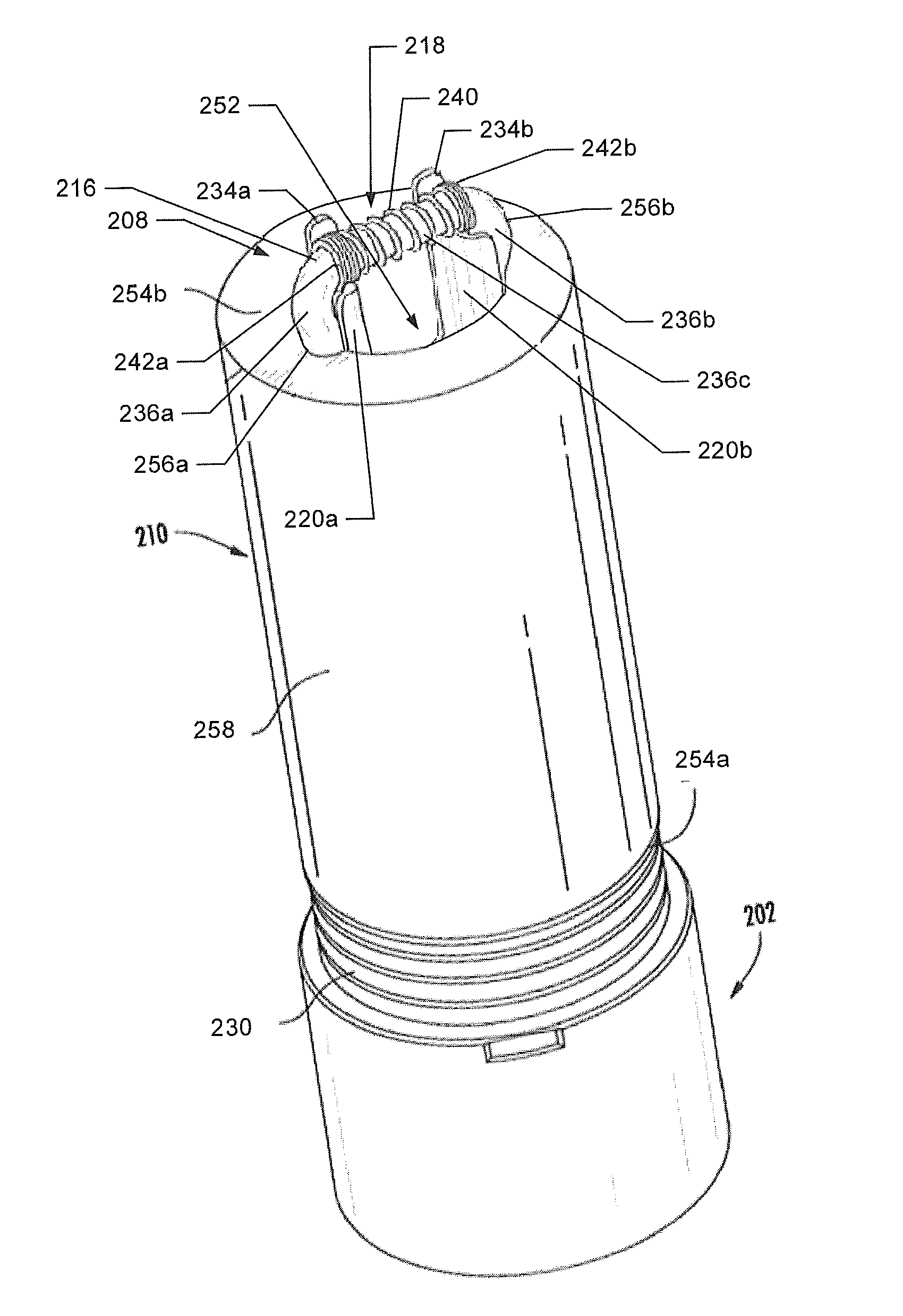
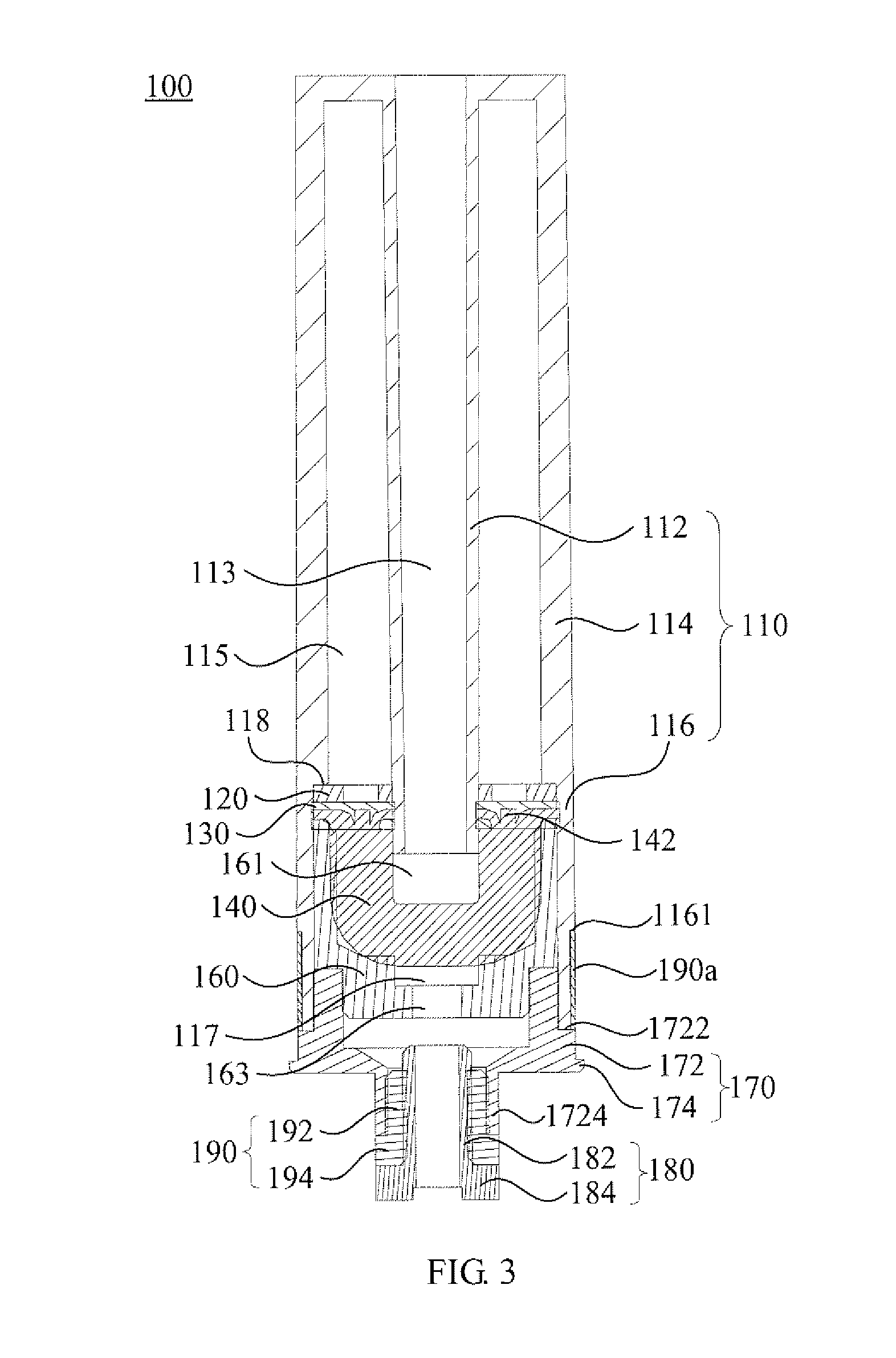
Small volume nebulizer
ActiveUS20040031485A1Minimize energy lossMinimize decelerationRespiratorsSpray nozzlesNebulizerProximate
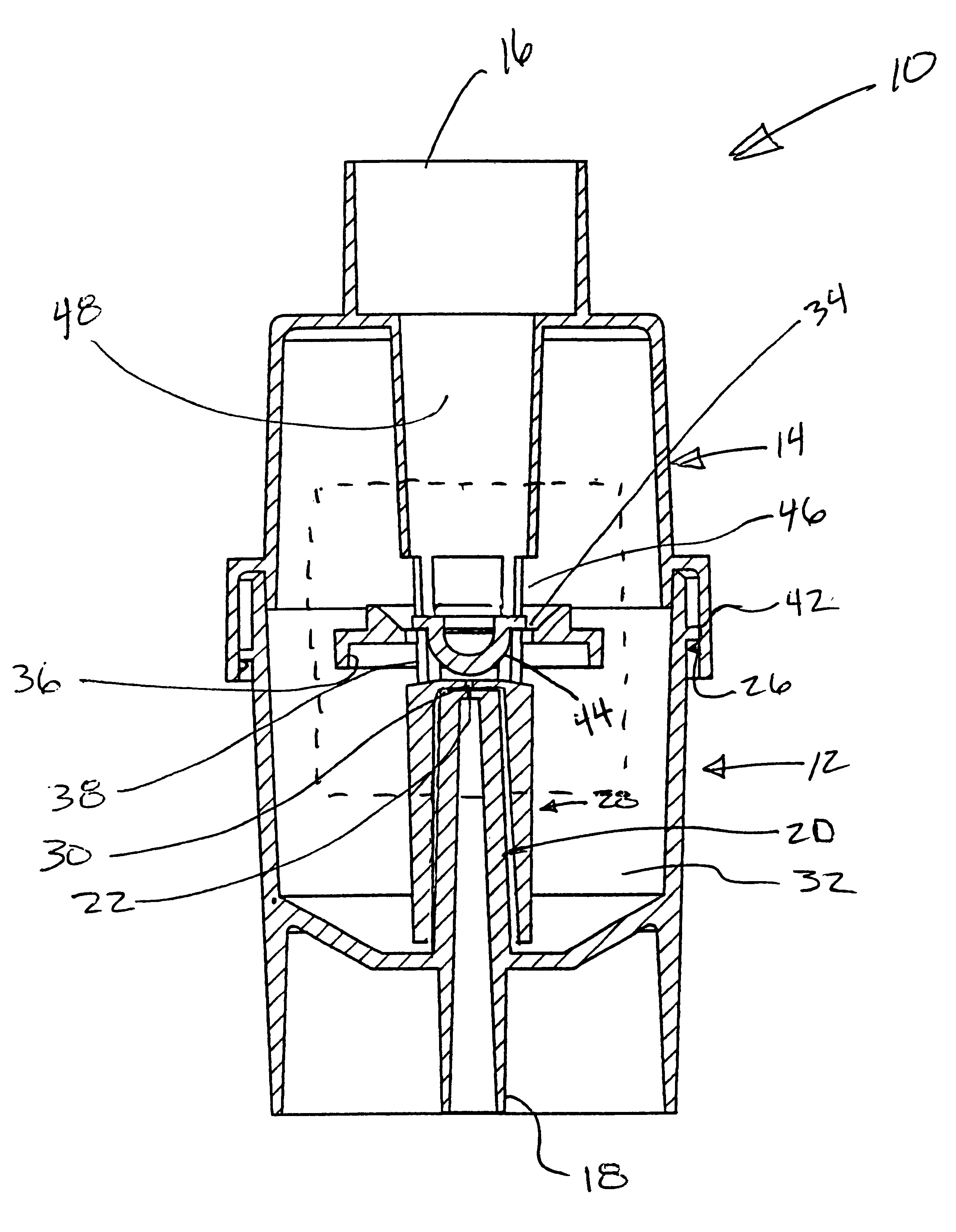
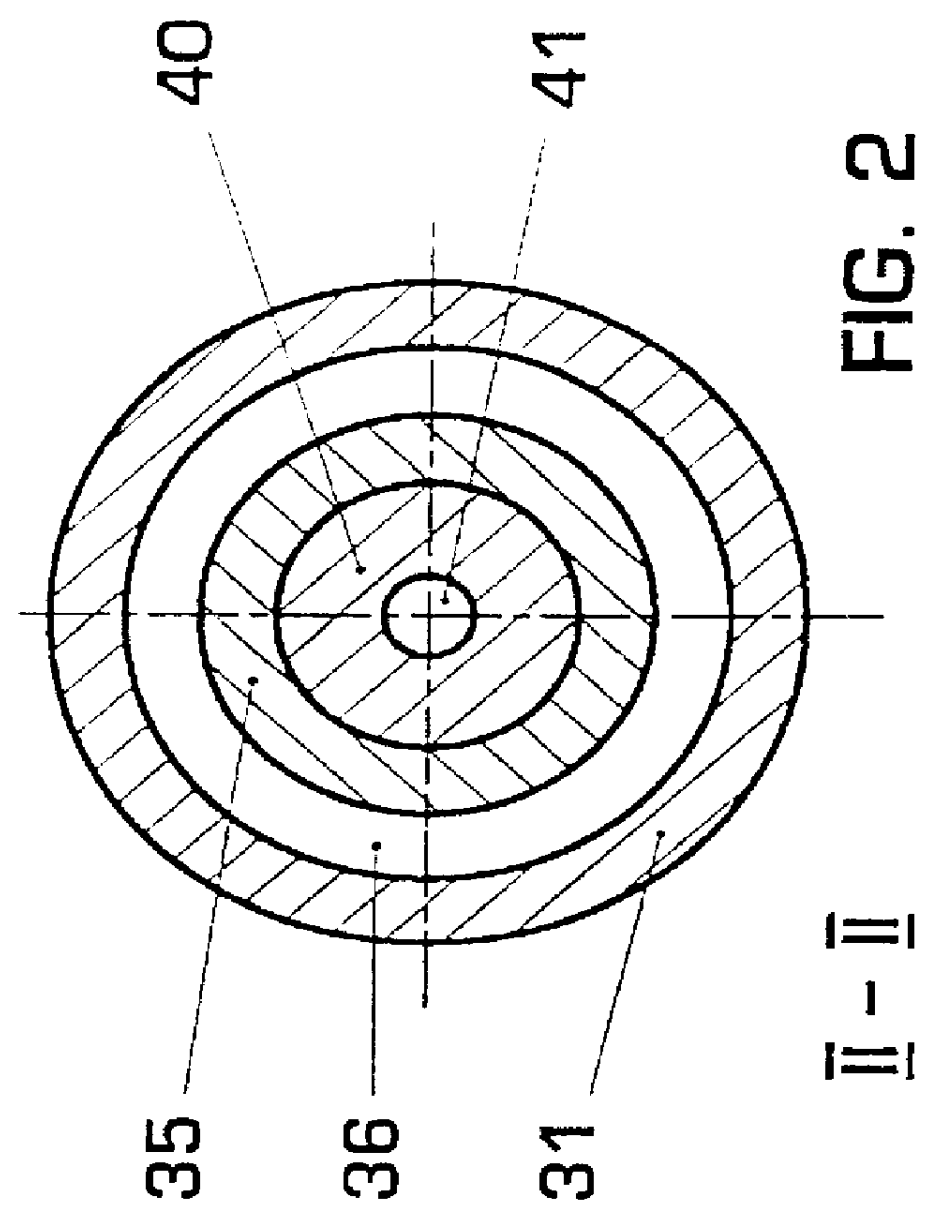
An atomizing nebulizer for dispensing a substance or medicament is described. The nebulizer is formed with a reservoir base releasably secured to an effluent vent cap that together capture a diffuser and integral dispersing baffle that are further formed with an uptake lumen or channel terminating with a nozzle jet. The diffuser dispersing baffle is positioned relative to the jet nozzle to optimize atomization of any of a number of such substances so as to maximize disbursement of the substance. The reservoir base also incorporates a pressurized fluid-accelerating inlet tube terminated with a metering orifice that cooperates with the nozzle jet when the inlet tube is received within the diffuser uptake lumen or channel. When so received, the nozzle jet axially registers proximate and superior to the orifice to establish a vacuum space that is in fluid communication with a capillary interstice established between the walls of the exterior of the inlet tube and the confronting interior surface of the diffuser lumen or channel. When a pressurized fluid is communicated through the lumen, the orifice, and into the vacuum space towards the nozzle jet, a vacuum develops in the vacuum space that, in combination with the capillary action of the interstice, draws the fluid proximate to the orifice and disperses it into droplets that are then entrained into a fluid stream to be further atomized upon impact with the baffle and then dispensed.
Owner:SUNMED GRP HLDG LLC
Atomizer
ActiveUS20070062518A1Easy to operateImprove the safety of useJet injection syringesMedical devicesNebulizerEngineering
An atomizer for a fluid, in particular, a fluid for medical aerosol therapy. In order to allow simplified operation and improved safety in use, the atomizer has a counter device for counting the number of operations of the atomizer and the number of containers inserted. The atomizer is locked against further operation and / or against removal of the current container or insertion of a new container, if a certain number of operations of the atomizer and / or a certain number of containers inserted has / have been reached or exceeded.
Owner:BOEHRINGER INGELHEIM INT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com