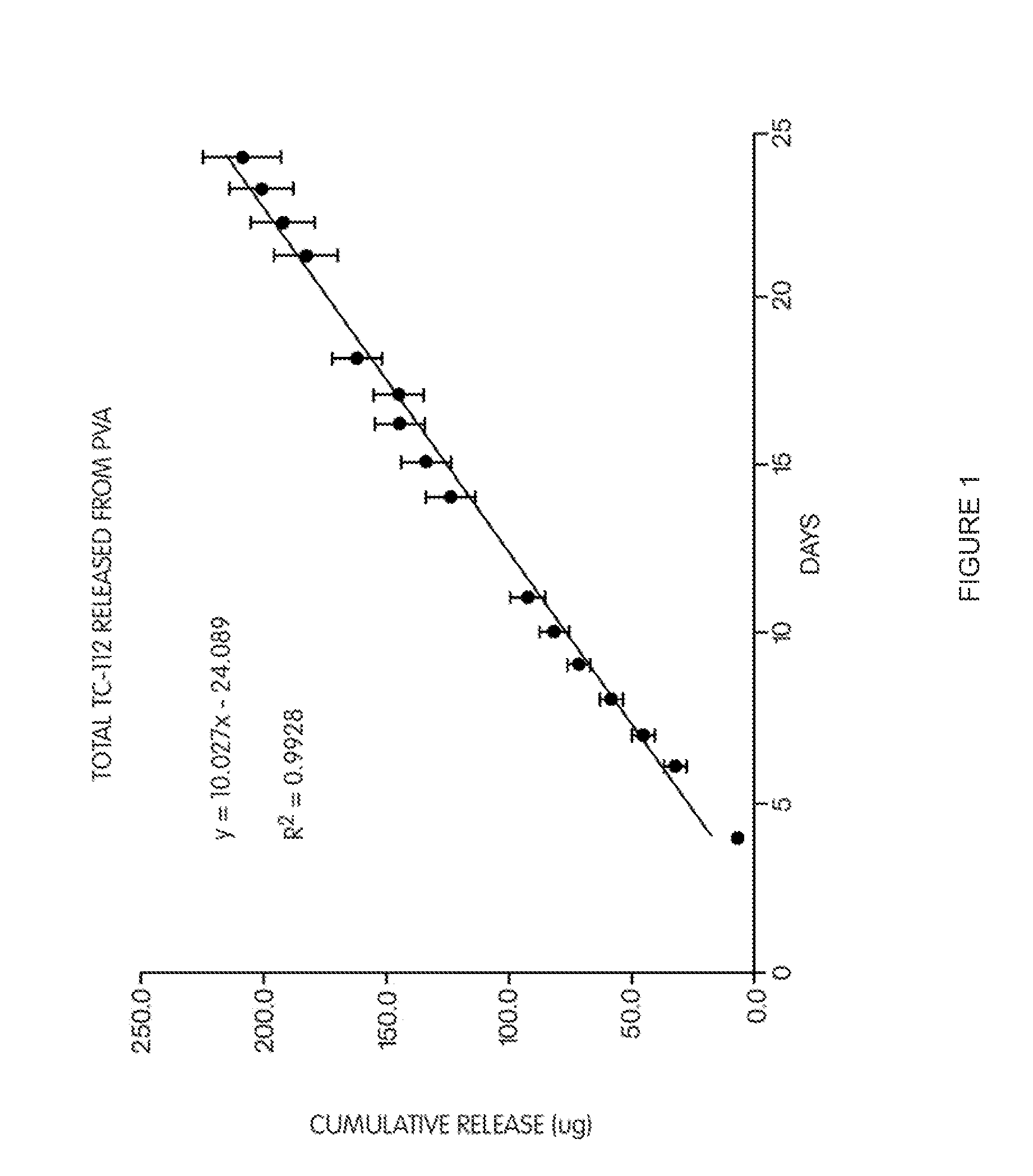
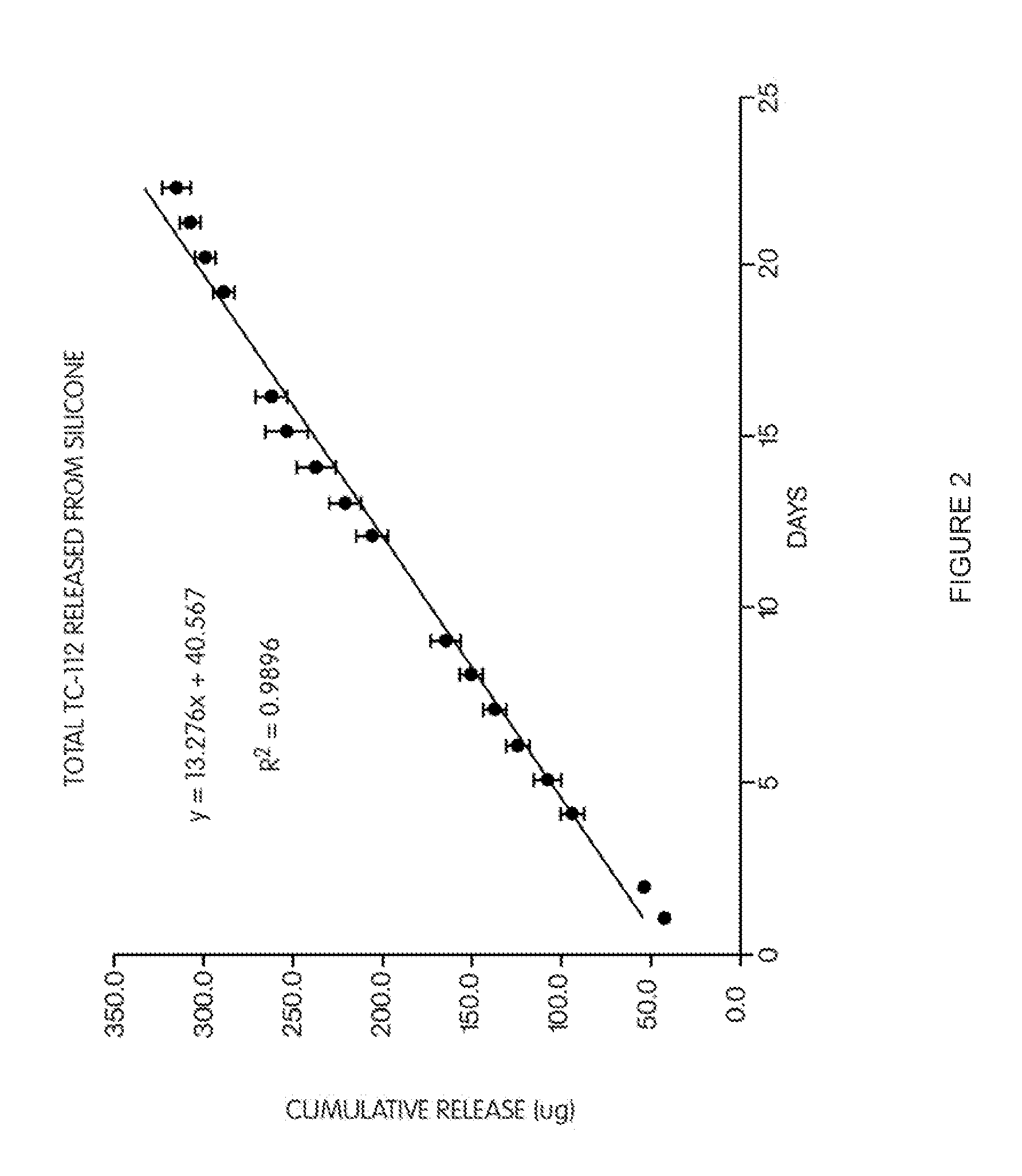
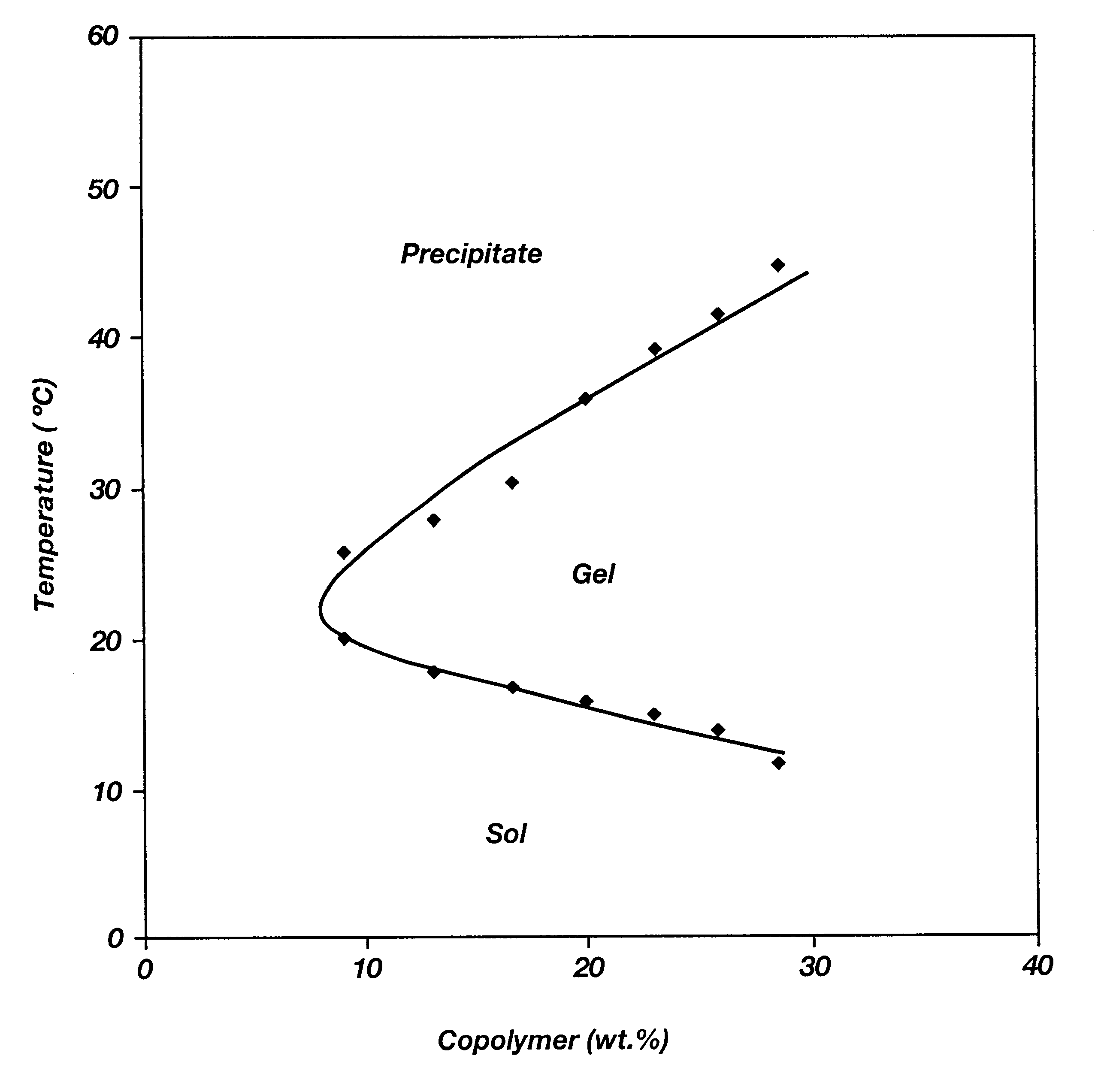
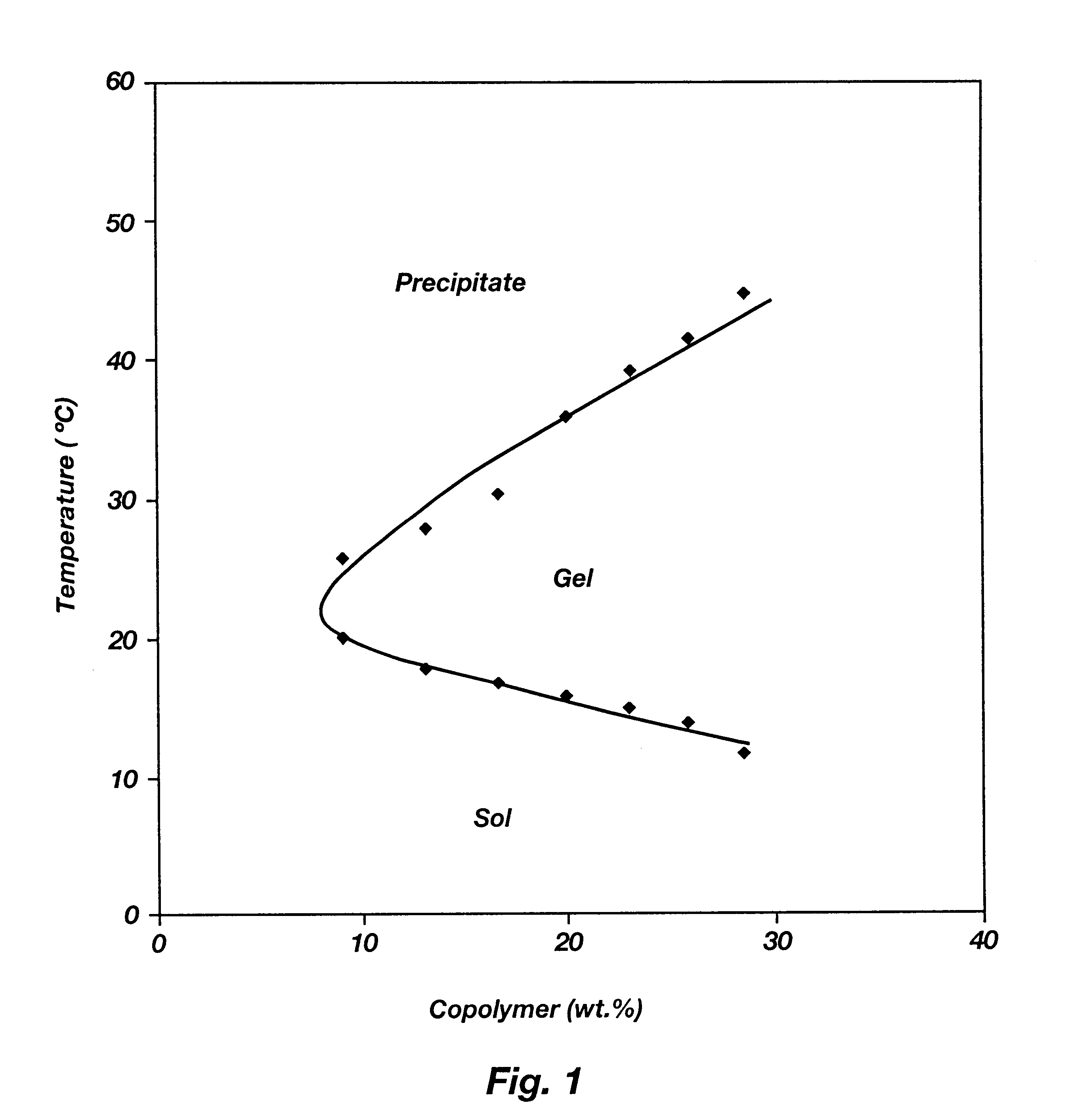
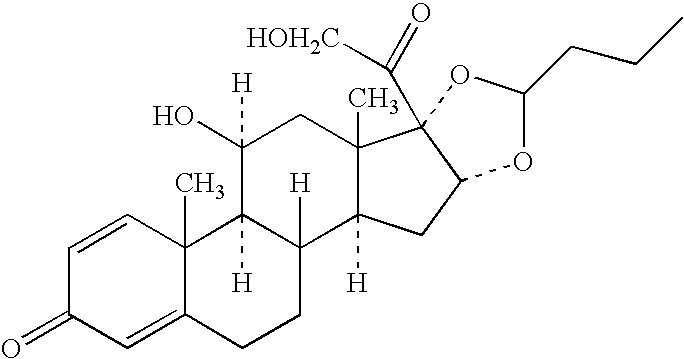
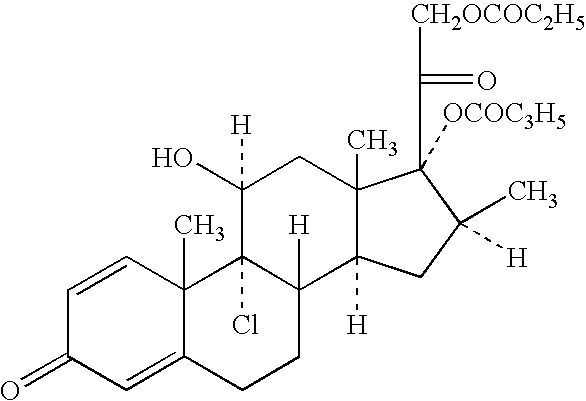
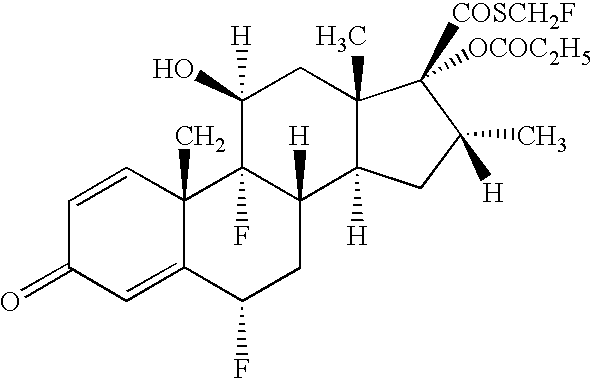
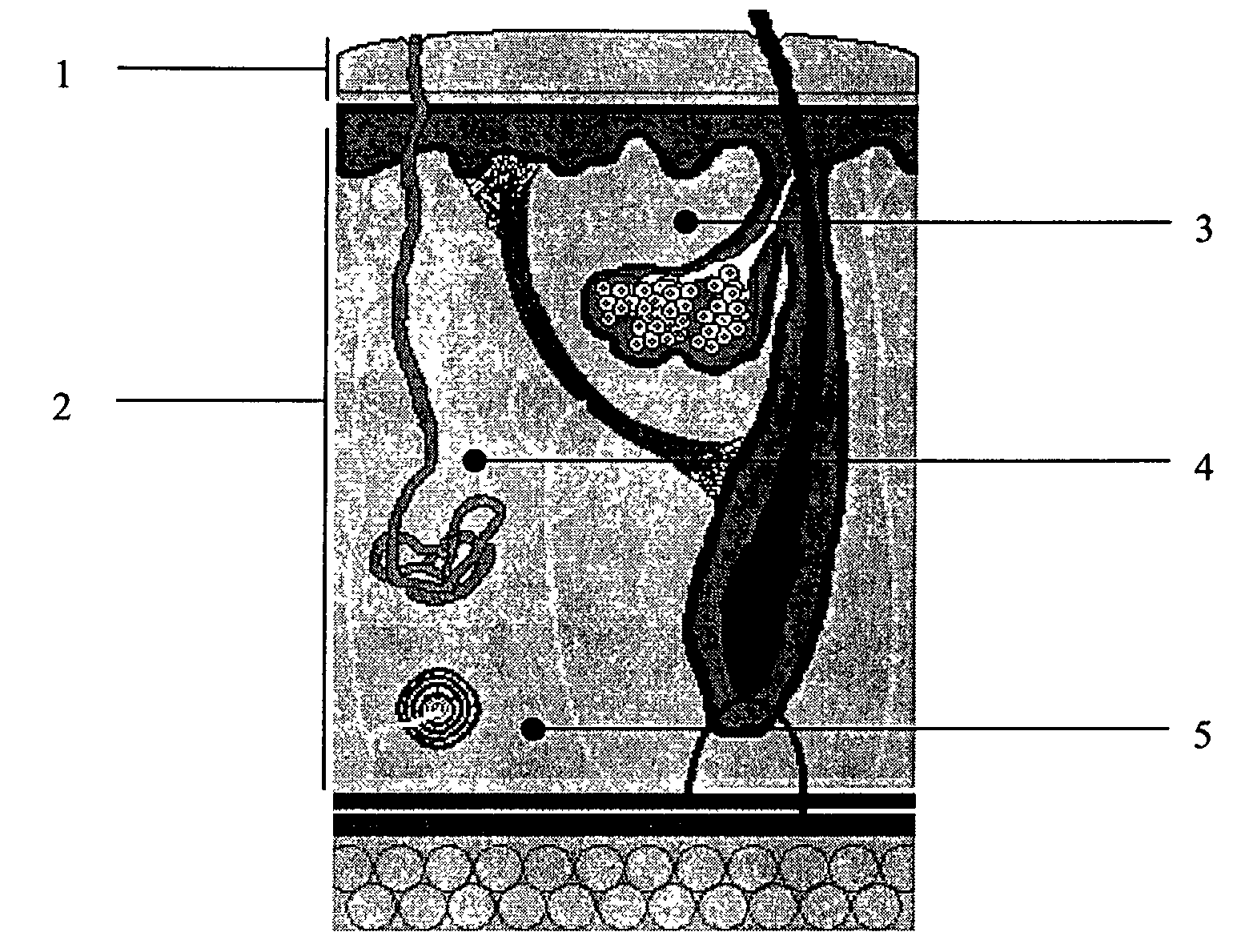
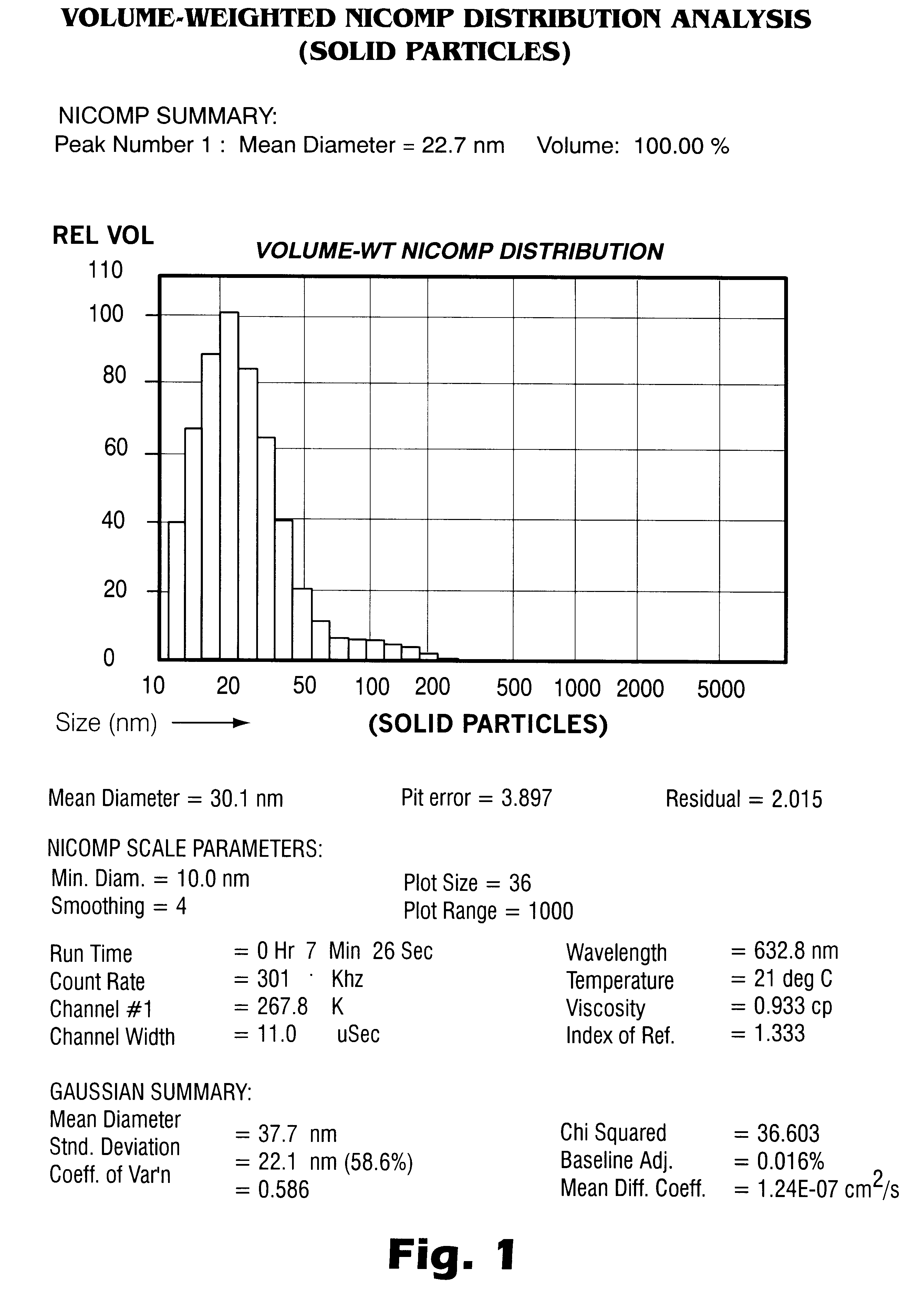
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
26468results about "Solution delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug administration method
InactiveUS7427607B2Reduce weightPrevention of the adhesion of an organBiocidePowder deliveryBiopolymerDrug administration
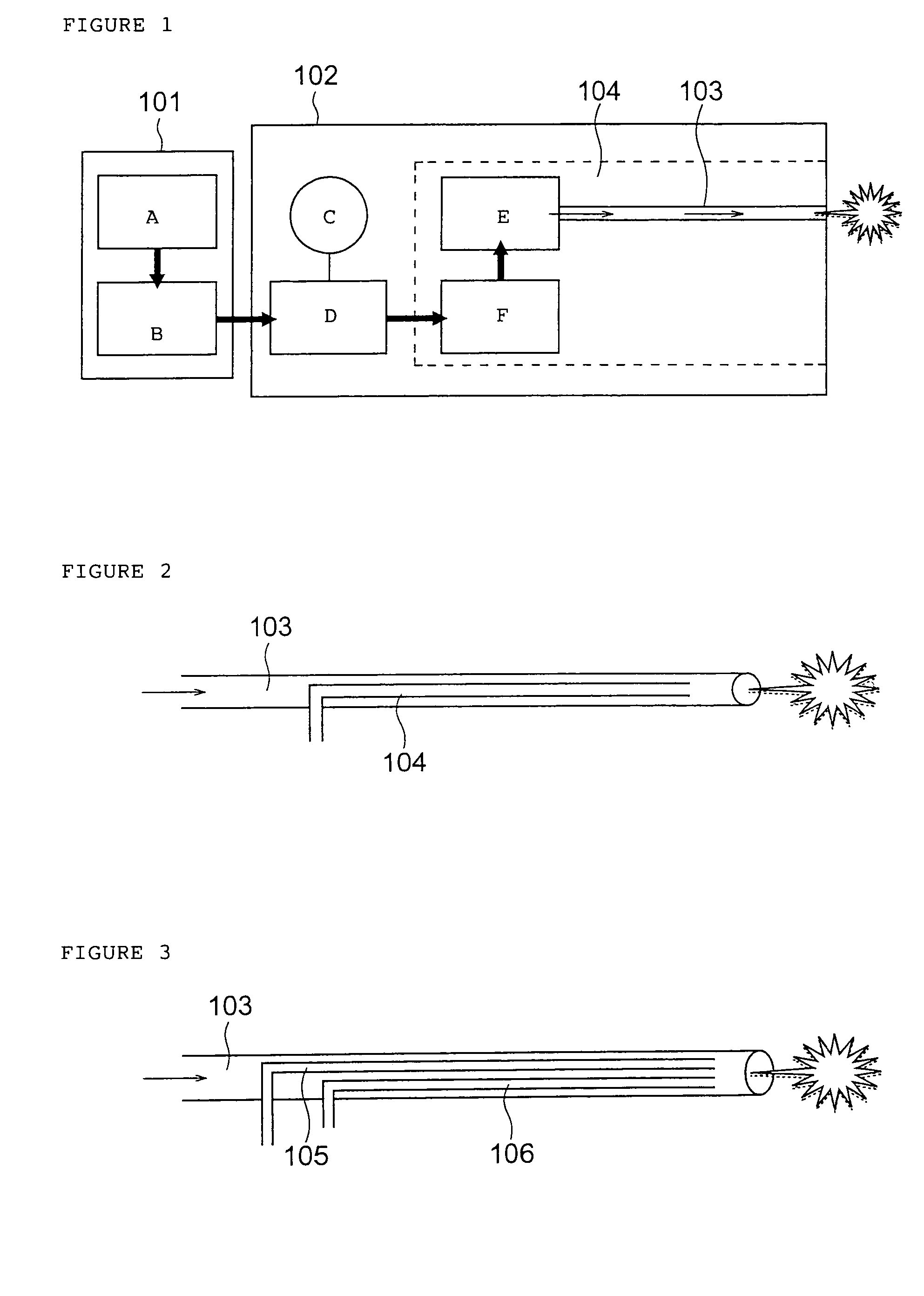
A method of administering a drug whereby a fine drug powder can be accurately administered to a target site (in particular, a target site in the body cavity) via fluidization and spraying with a gas by using a micro tube. Concerning the administration mode, in particular, the drug alone or a biopolymer is administered or the biopolymer is employed as a carrier in the above method. More specifically speaking, a method of administering a fine drug powder which comprises finely milling one or more types fine particles of the drug and / or the biopolymer, blending them each other, fluidizing the blend with a gas, then transporting the fluidized matter in a micro tube by the gas stream and spraying the fine drug powder from the tip of the micro tube toward the target site. Further, an administration method which comprises concentrically providing a capillary tube in the micro tube, supplying an aqueous solution of the drug and / or the biopolymer from the capillary tube into the gas stream and then mixing it with other fine particles of the drug and / or the biopolymer under transportation by the gas.
Owner:NEXT21 KK
Polymer-based, sustained release drug delivery system
InactiveUS20120016467A1Reduce interactionSuture equipmentsAntibacterial agentsRate limitingSolubility
Disclosed is a sustained release system that includes a polymer and a prodrug having a solubility less than about 1 mg / ml dispersed in the polymer. Advantageously, the polymer is permeable to the prodrug and may be non-release rate limiting with respect to the rate of release of the prodrug from the polymer. This permits improved drug delivery within a body in the vicinity of a surgery via sustained release rate kinetics over a prolonged period of time, while not requiring complicated manufacturing processes.
Owner:PSIVIDA INC
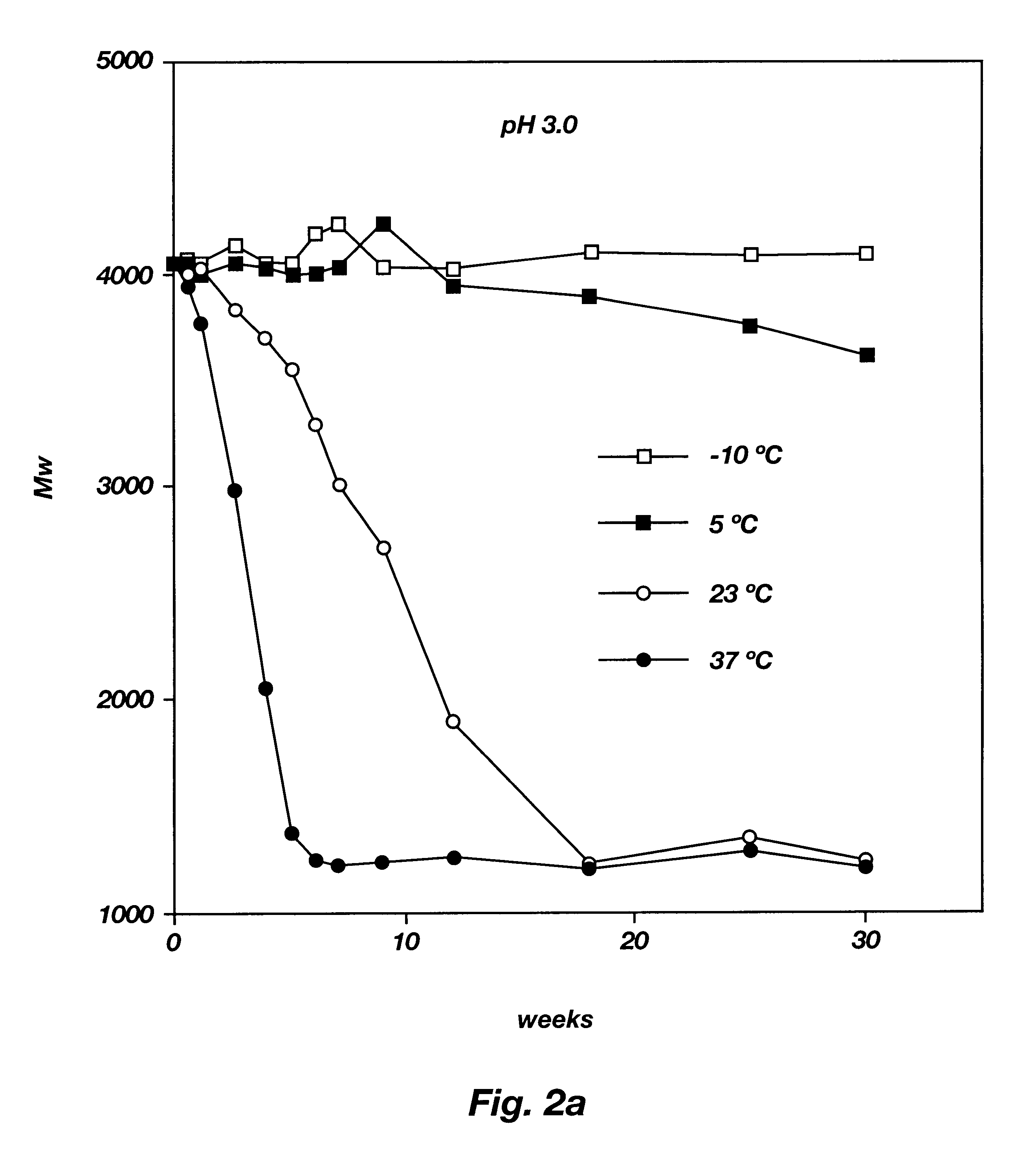
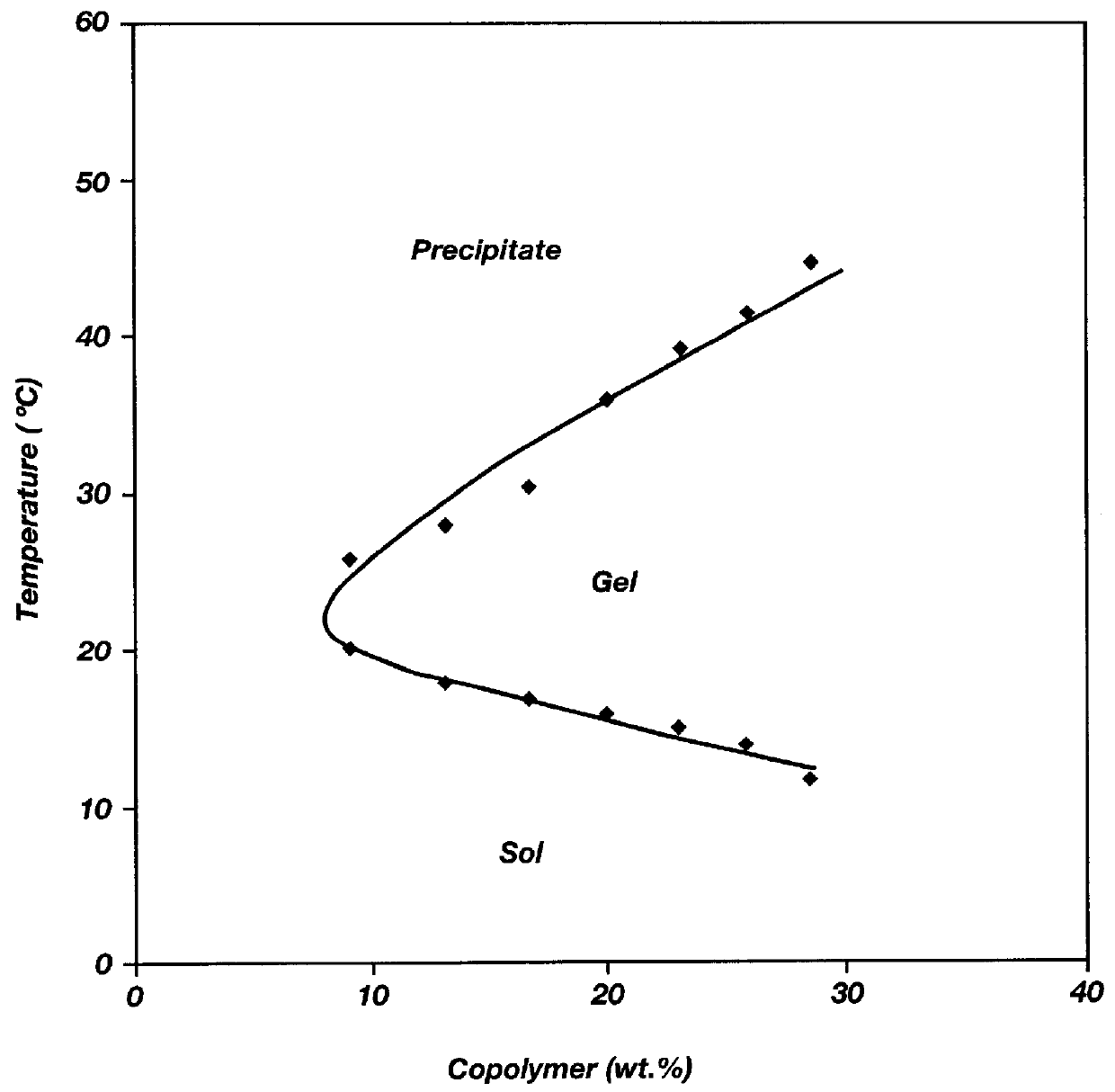
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Methods and compositions useful for administration of chemotherapeutic agents
InactiveUS6096331AReduce morbidityLow toxicityPowder deliveryEchographic/ultrasound-imaging preparationsActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
Bioactive agent delivering system comprised of microparticles within a biodegradable to improve release profiles
InactiveUS6589549B2Improve stabilityPowder deliveryPeptide/protein ingredientsActive agentEngineering
A composition and method for releasing a bio-active agent or a drug within a biological environment in a controlled manner is disclosed. The composition is a dual phase polymeric agent-delivery composition comprising a continuous biocompatible gel phase, a discontinuous particulate phase comprising defined microparticles and an agent to be delivered. A microparticle containing a bio-active agent is releasably entrained within a biocompatible polymeric gel matrix. The bioactive agent release may be contained in the microparticle phase alone or in both the microparticles and the gel matrix. The release of the agent is prolonged over a period of time, and the delivery may be modulated and / or controlled. In addition, a second agent may be loaded in some of the microparticles and / or the gel matrix.
Owner:BTG INT LTD
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
Pharmaceutical and cosmetic carrier or composition for topical application
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
N-acetyl aldosamines, n-acetylamino acids and related n-acetyl compounds and their topical use
Compositions comprising N-acetyl-aldosamines, N-acetylamino acids, and related N-acetyl compounds are useful to alleviate or improve various cosmetic conditions and dermatological disorders, including changes or damage to skin, nail and hair associated with intrinsic aging and / or extrinsic aging, as well as changes or damage caused by extrinsic factors. N-acetyl-aldosamines, N-acetylamino acids, and related N-acetyl composition may further comprise a cosmetic, pharmaceutical or other topical agent to enhance or create synergetic effects.
Owner:TRISTRATA TECH
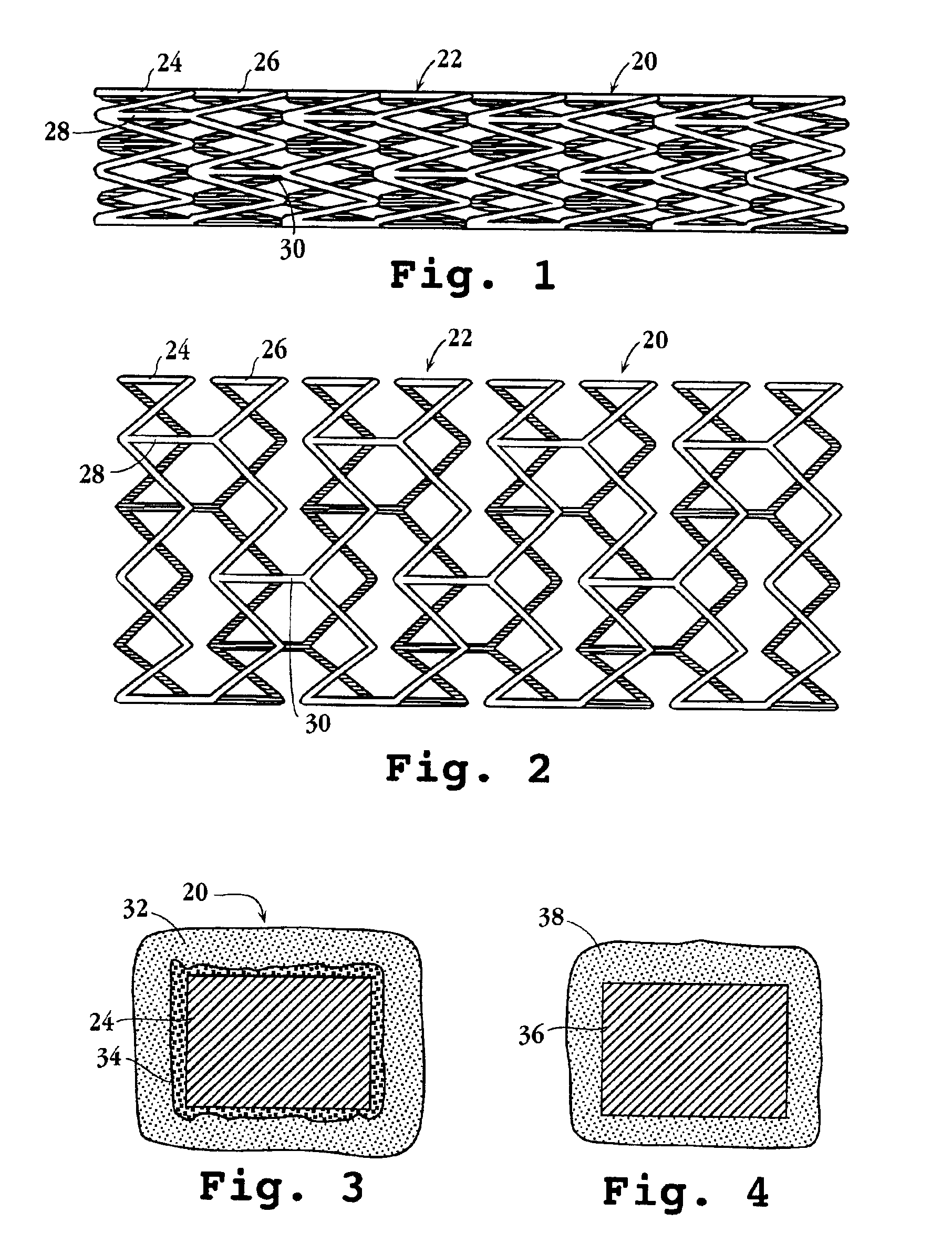
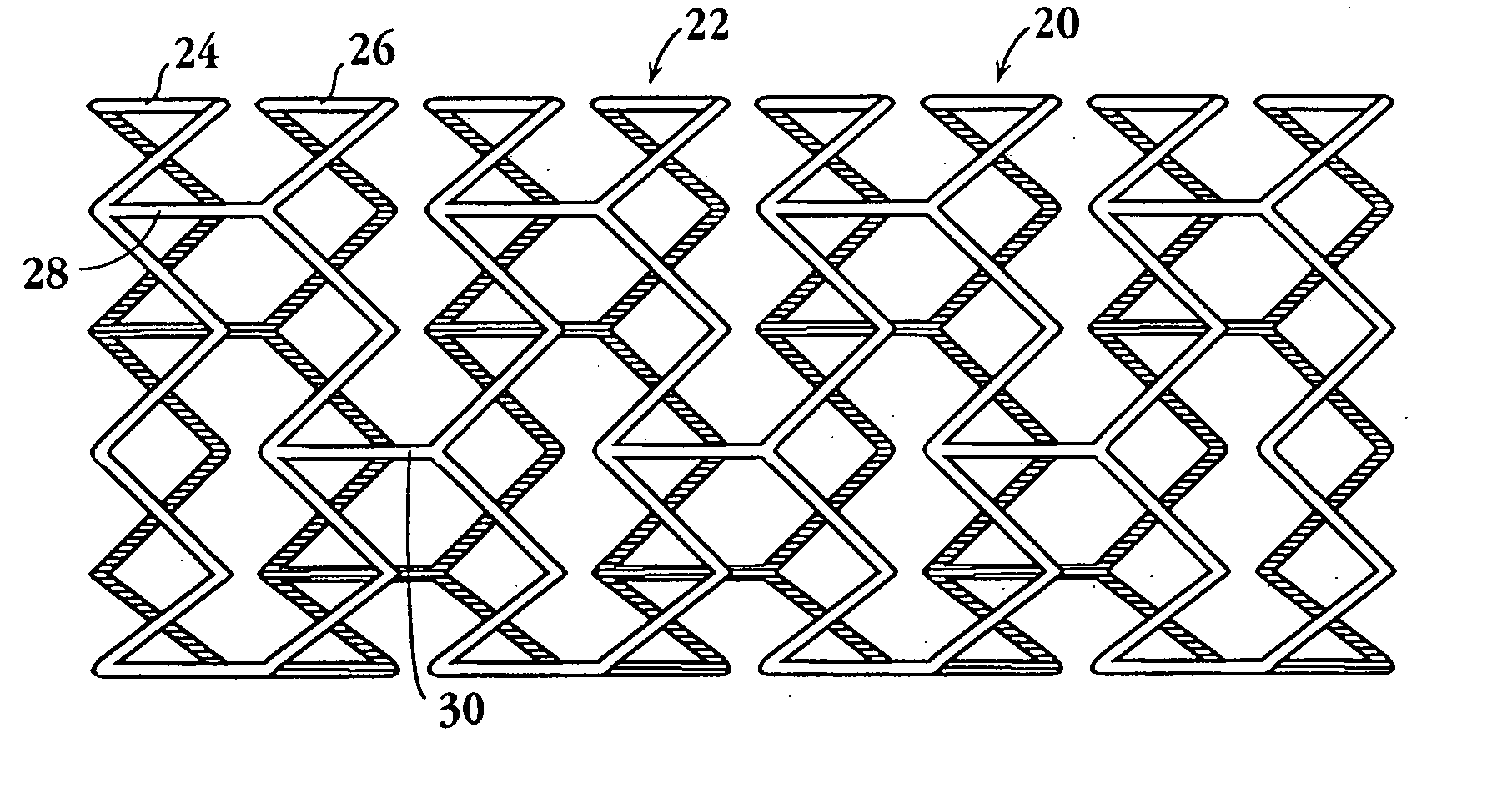
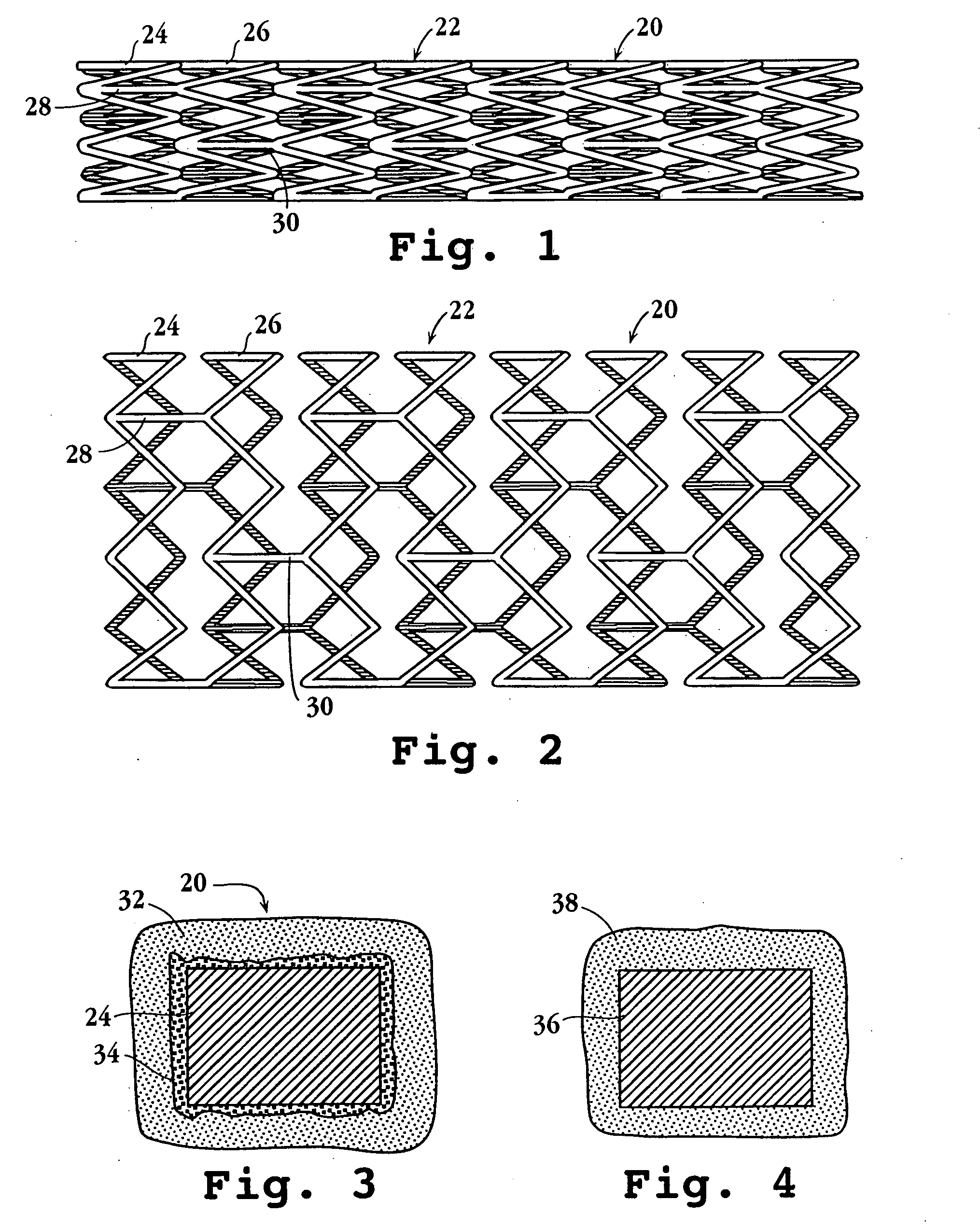
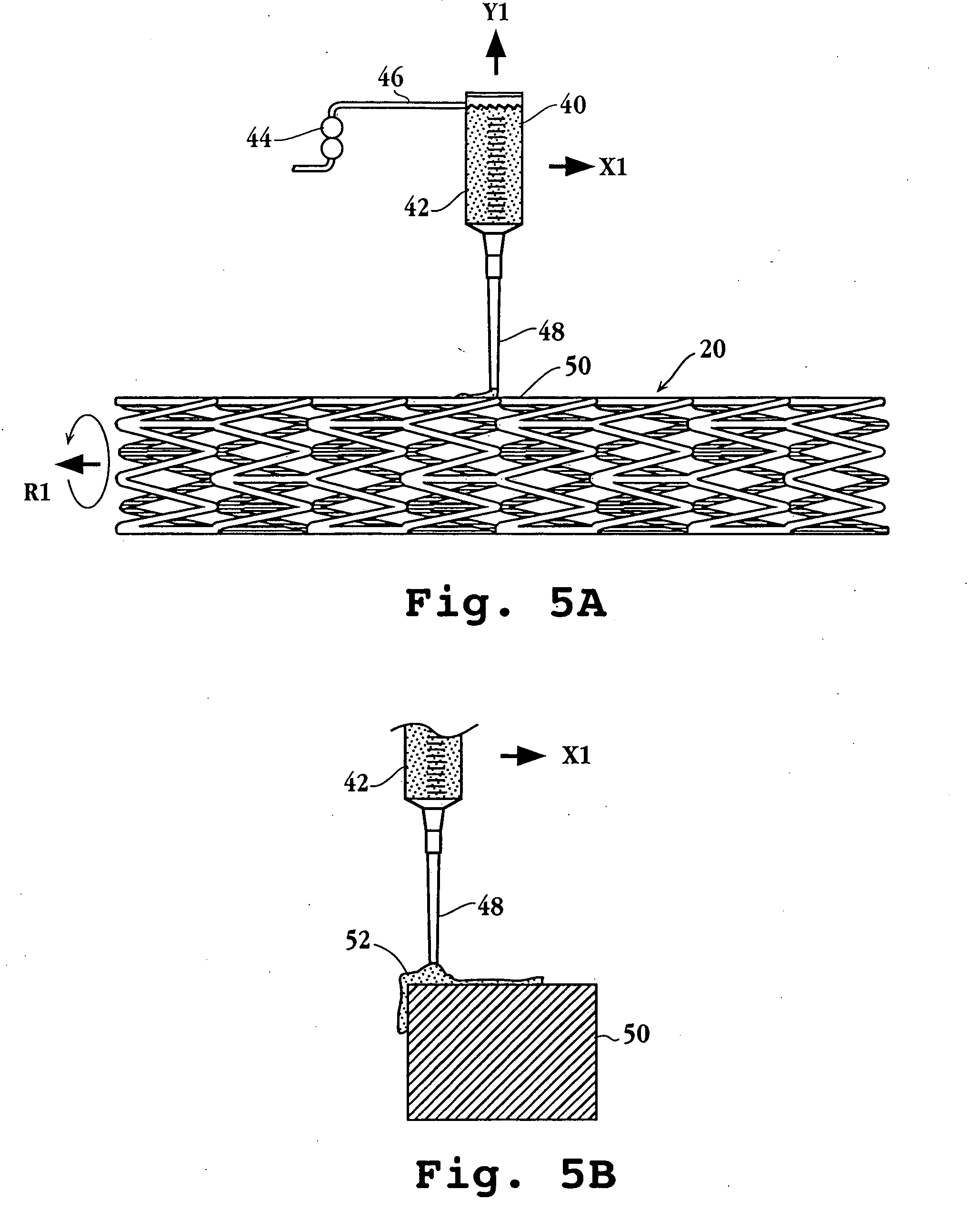
Drug-delivery endovascular stent and method for treating restenosis
InactiveUS6939376B2Efficient releaseOrganic active ingredientsOrganic chemistryRestenosisPoly dl lactide
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Compositions and methods for treating a posterior segment of an eye
InactiveUS20050101582A1Improve abilitiesIncrease viscosity of compositionAntibacterial agentsOrganic active ingredientsShear ratePharmacology
Compositions, and methods of using such compositions, useful for injection into the posterior segments of human or animal eyes are provided. Such compositions include corticosteroid component-containing particles present in a therapeutically effective amount, a viscosity inducing component, and an aqueous carrier component. The compositions have viscosities of at least about 10 cps or about 100 cps at a shear rate of 0.1 / second. In a preferred embodiment, the viscosity is in the range of from about 140,000 cps to about 300,000 cps. The compositions advantageously suspend the particles for prolonged periods of time.
Owner:ALLERGAN INC
Compositions and methods of delivery of pharmacological agents
InactiveUS20050004002A1Reducing one or more side effectsInhibiting oxidation in the pharmaceutical compositionAntibacterial agentsOrganic active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serum albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
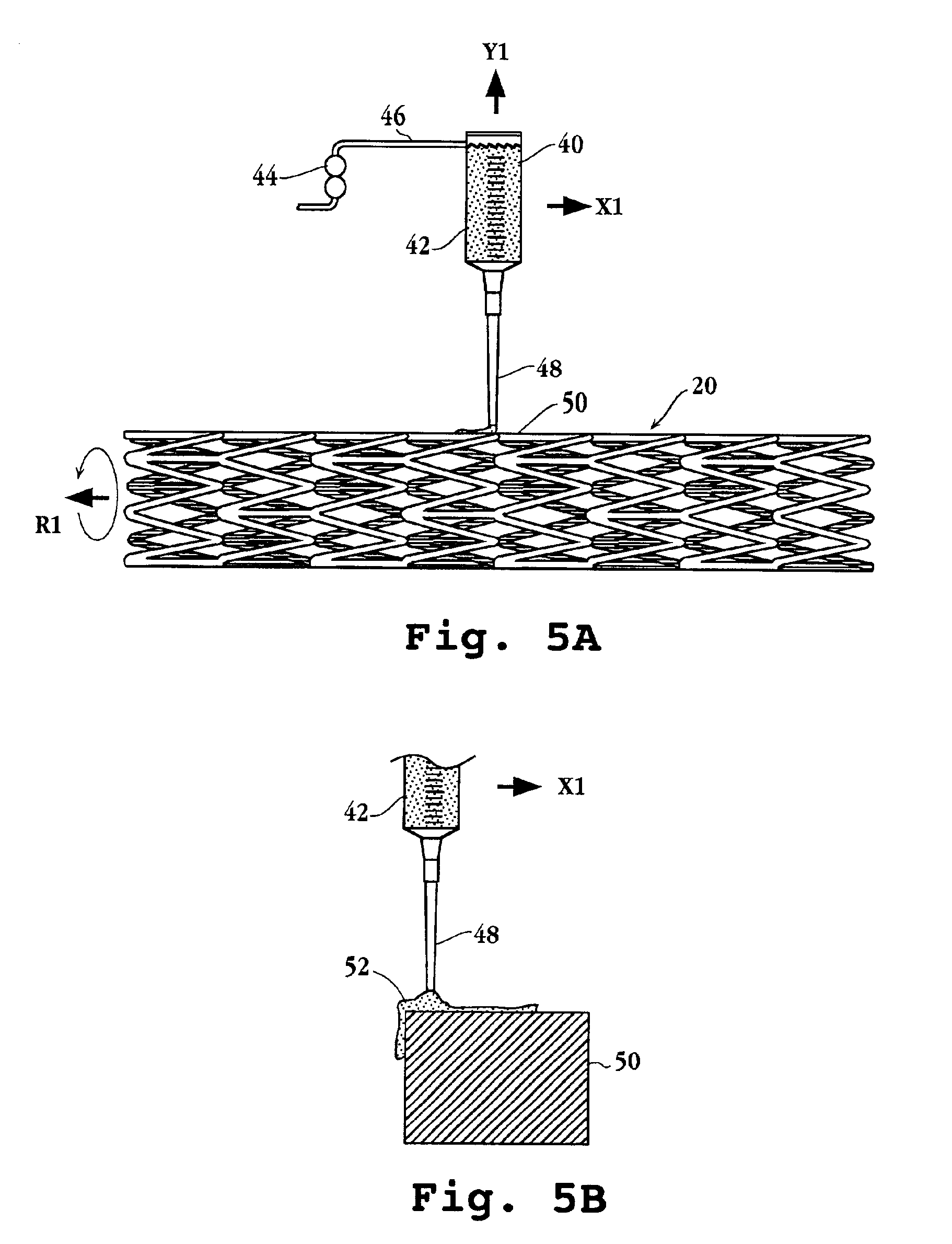
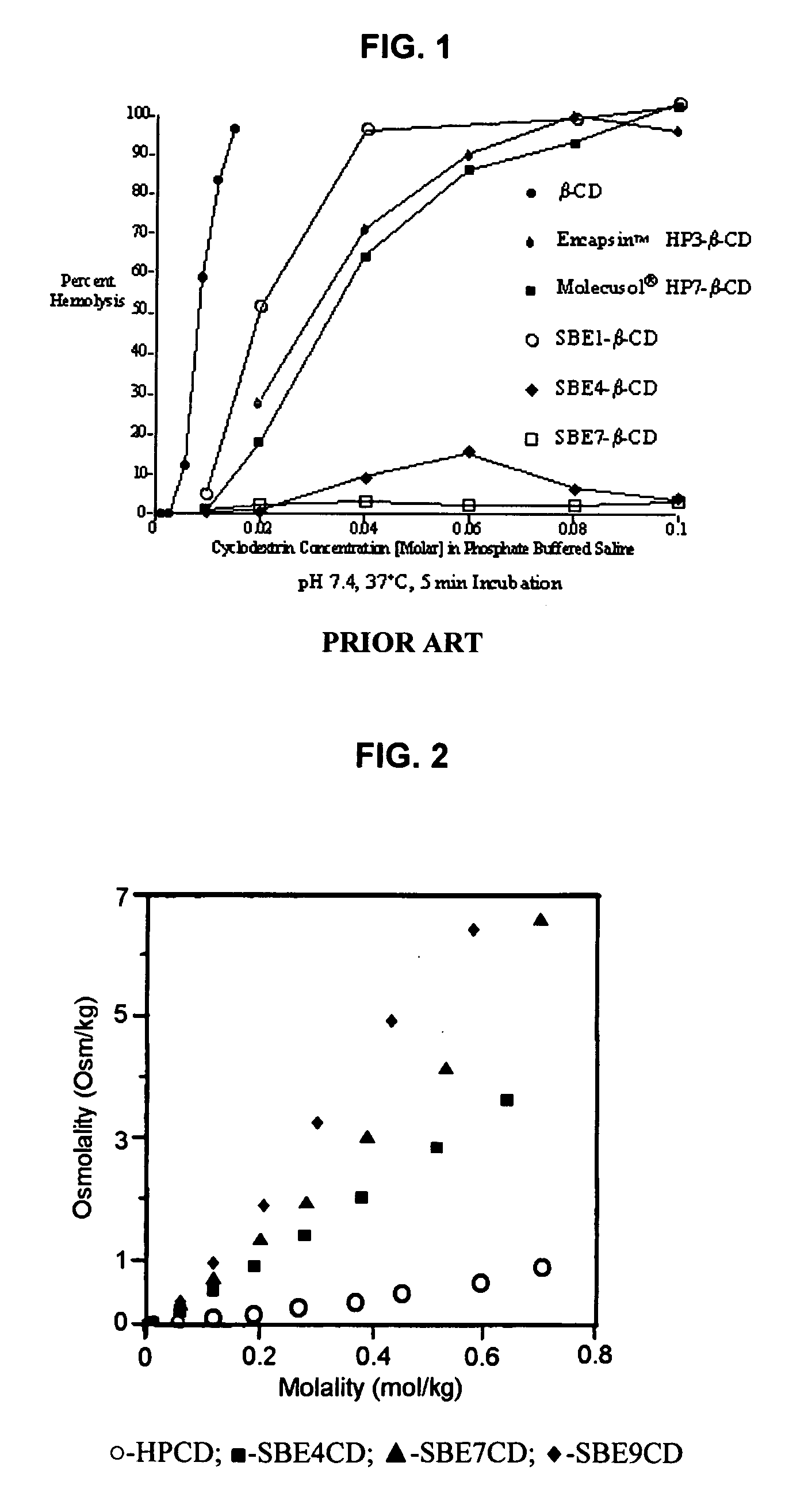
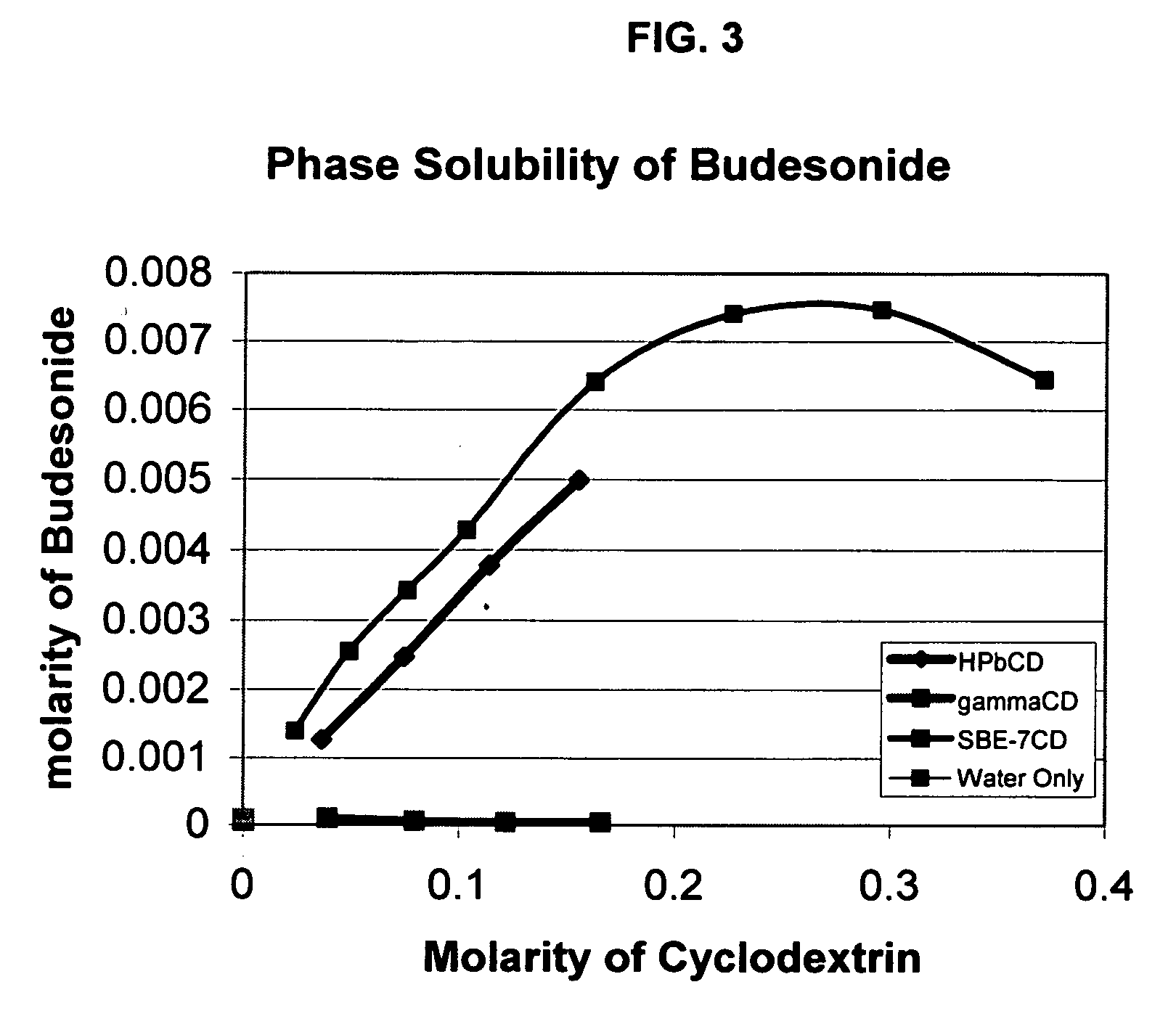
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Poly(vinyl alcohol) hydrogel
InactiveUS6231605B1Exemption stepsLow biocompatibilityOrganic active ingredientsBone implantBearing surfaceSacroiliac joint
The present invention relate to a poly(vinyl alcohol) hydrogel construct having a wide range of mechanical strengths for use as a human tissue replacement. The hydrogel construct may include a tissue scaffolding, a low bearing surface within a joint, or any other structure which is suitable for supporting the growth of tissue.
Owner:GEORGIA TECH RES CORP
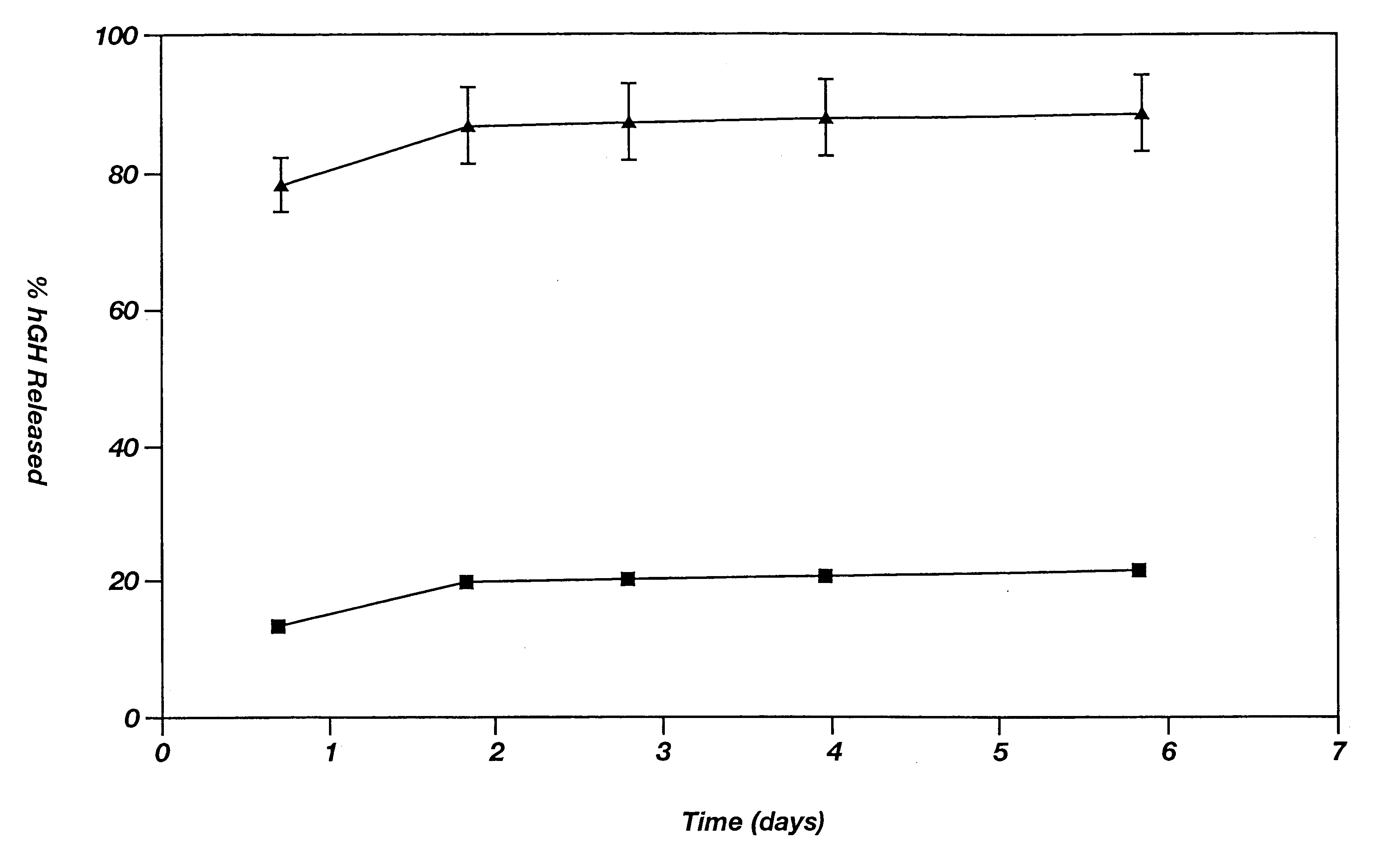
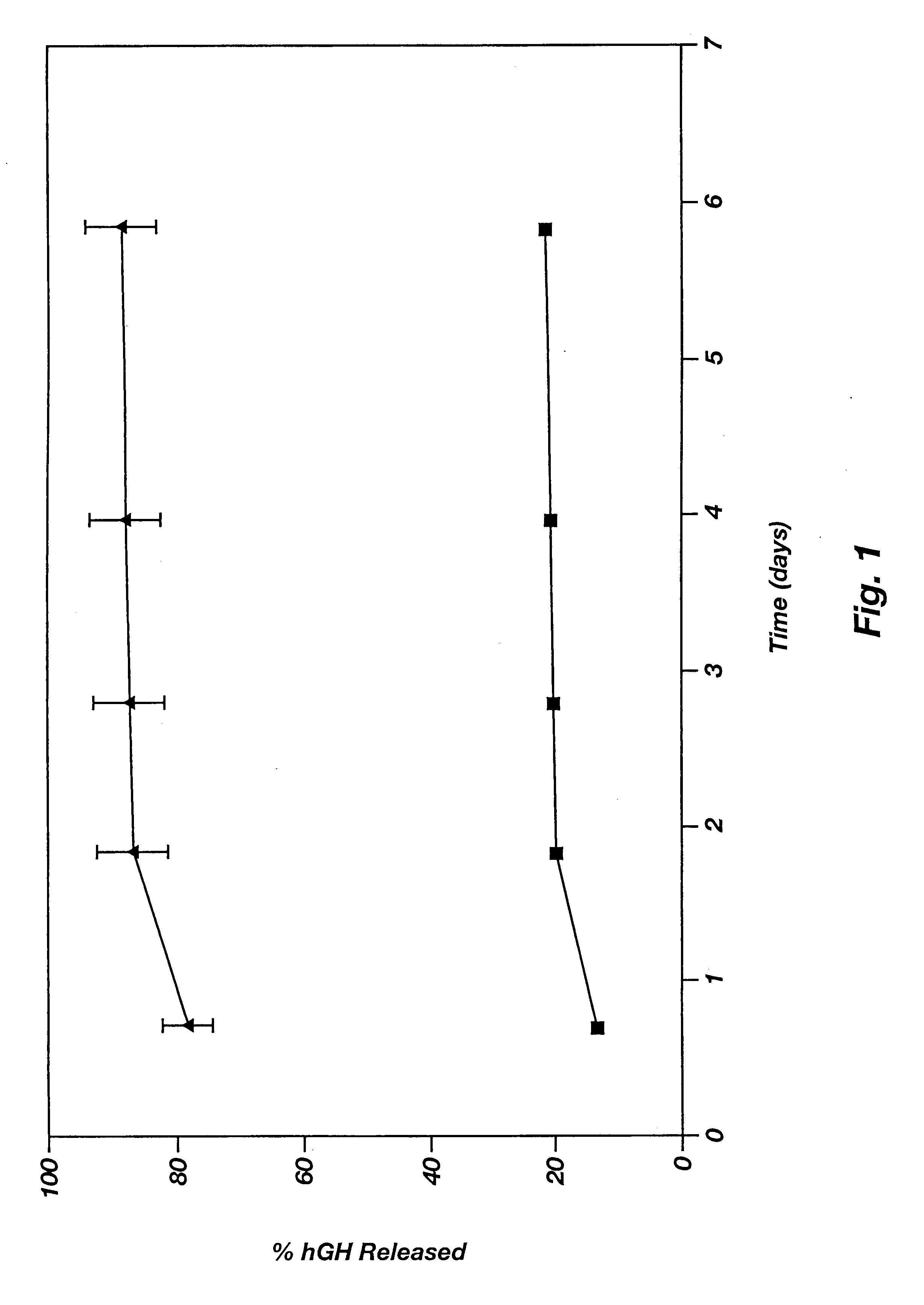
Dosage form exhibiting rapid disperse properties, methods of use and process for the manufacture of same
InactiveUS6471992B1Disperse fastHigh hardnessPowder deliveryAdditive manufacturing apparatusThree dimensional shapeBULK ACTIVE INGREDIENT
A rapidly dispersing dosage form is described, which releases its active ingredients within a period of less than about ninety seconds. These dosage forms exhibit a three-dimensional shape that is retained for adequate storage but is readily dispersed in the presence of excess moisture. Also disclosed are methods of administration of a medicament and a process for the preparation of rapidly dispersing dosage forms.
Owner:MASSACHUSETTS INST OF TECH +2
Solid hydrogel coupling for ultrasound imaging and therapy
InactiveUS7070565B2Lower the volumeInhibition of polymerizationUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyUltrasound imagingUltrasonic sensor
Owner:UNIV OF WASHINGTON
Biodegradable injectable implants and related methods of manufacture and use
InactiveUS20030093157A1Broaden applicationSolution deliveryPharmaceutical non-active ingredientsGlycolic acidImplant
This invention is directed to the field of medical implants, and more specifically to biodegradable injectable implants and their methods of manufacture and use. The injectable implants disclosed herein comprise glycolic acid and bio-compatible / bio-absorbable polymeric particles containing a polymer of lactic acid. The particles are small enough to be injected through a needle but large enough to avoid engulfment by macrophages. The injectables of this invention may be in a pre-activated solid form or an activated form (e.g., injectable suspension or emulsion).
Owner:MEDGRAFT MICROTECH
Drug-delivery endovascular stent and method of forming the same
ActiveUS20050038505A1Prevent restenosisEfficient releaseOrganic active ingredientsOrganic chemistryPolymer substrateInsertion stent
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-d / -lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
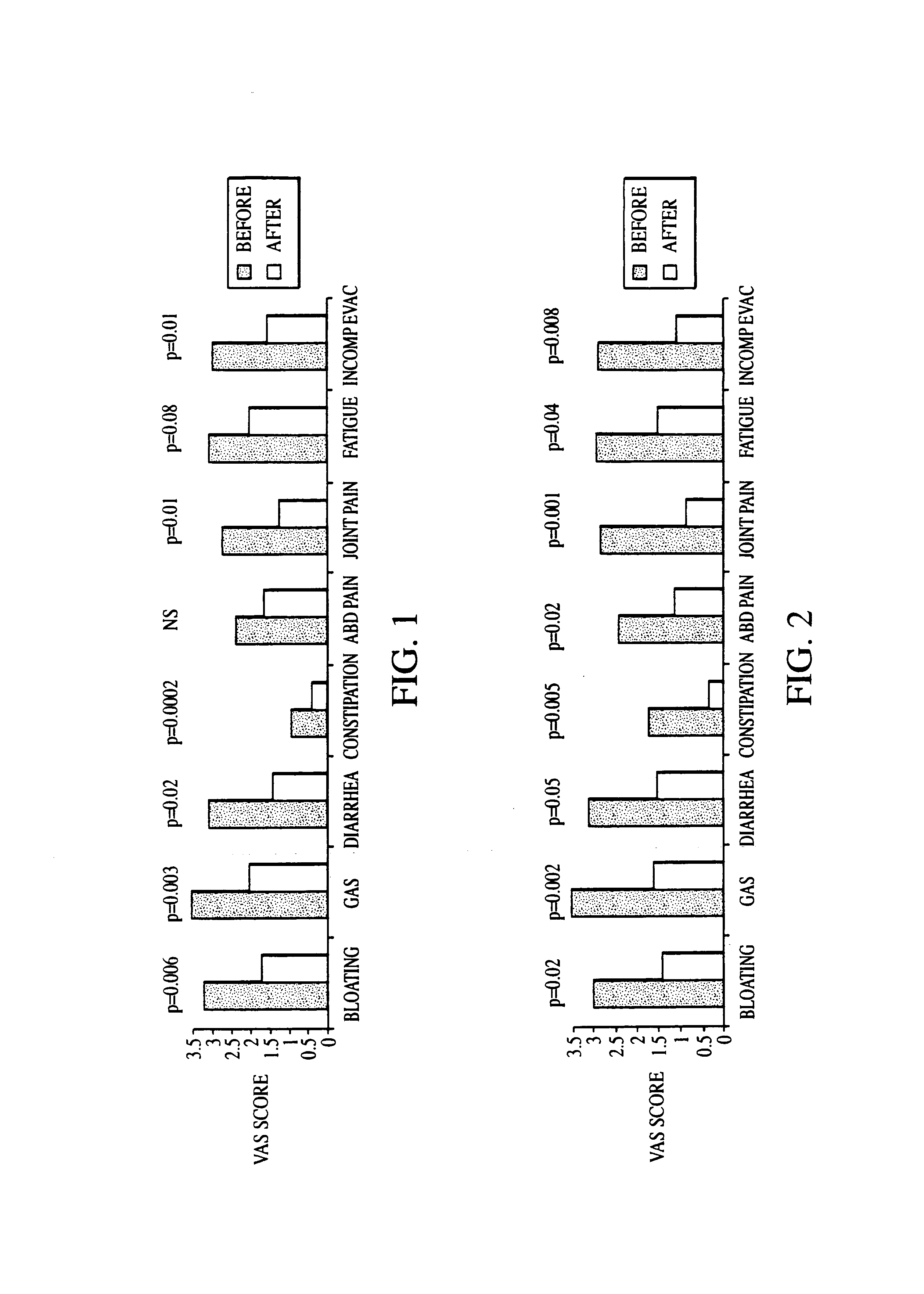
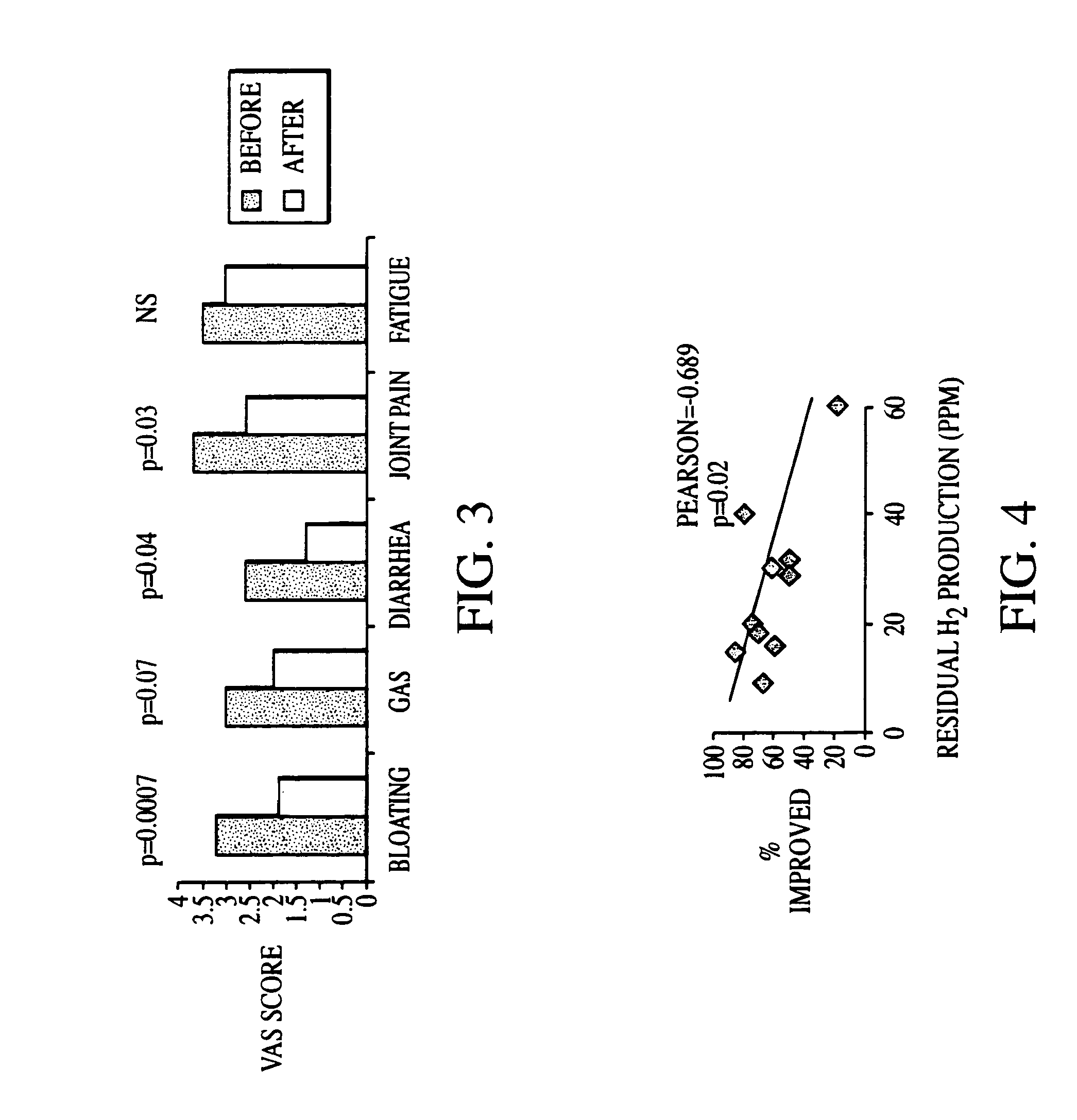
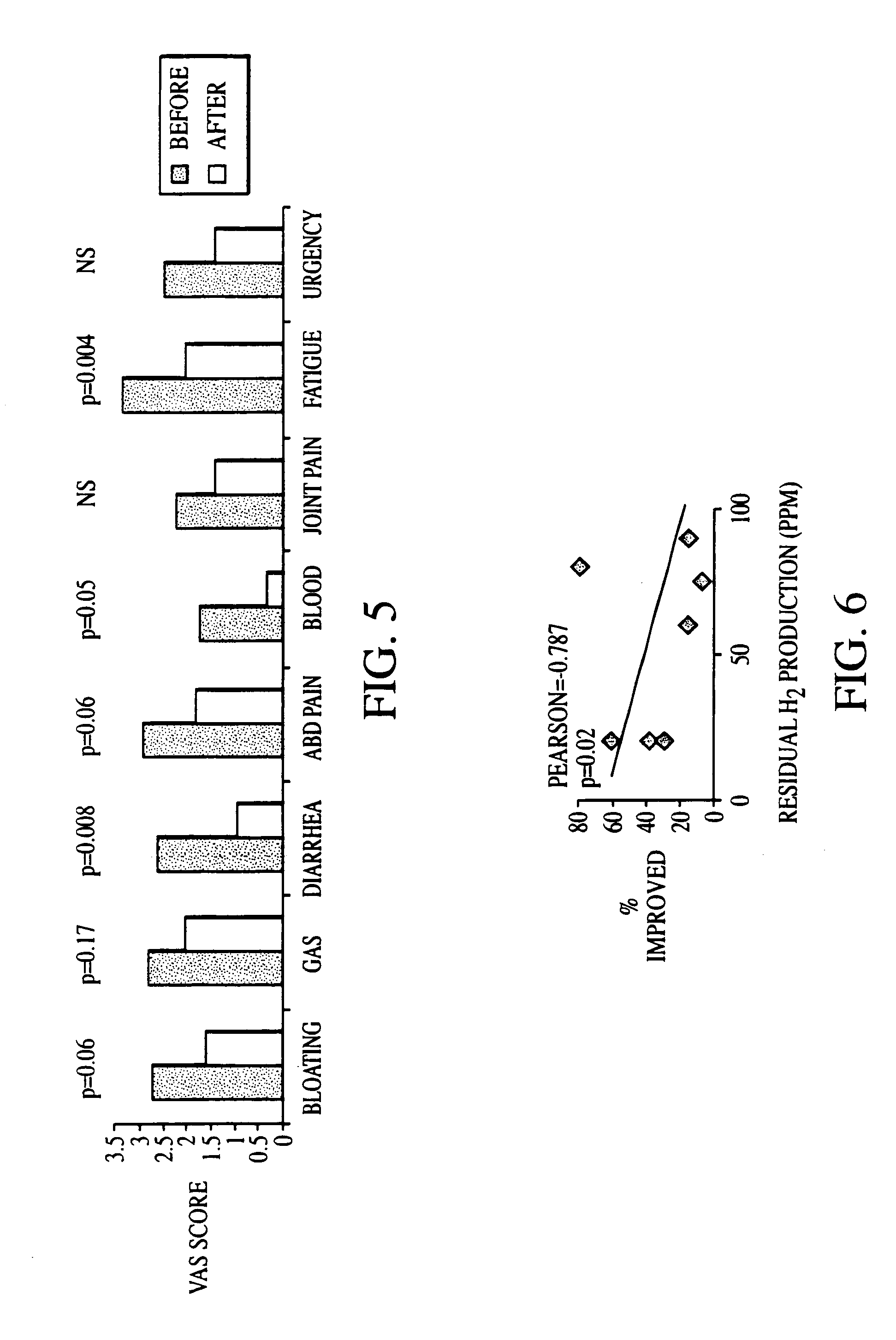
Methods of diagnosing and treating small intestinal bacterial overgrowth (SIBO) and SIBO-related conditions
InactiveUS7048906B2Prevent further growthReduced magnitudeAntibacterial agentsCompounds screening/testingImmunologic disordersPhysiology
Disclosed is a method of treating small intestinal bacterial overgrowth (SIBO) or a SIBO-caused condition in a human subject. SIBO-caused conditions include irritable bowel syndrome, fibromyalgia, chronic pelvic pain syndrome, chronic fatigue syndrome, depression, impaired mentation, impaired memory, halitosis, tinnitus, sugar craving, autism, attention deficit / hyperactivity disorder, drug sensitivity, an autoimmune disease, and Crohn's disease. Also disclosed are a method of screening for the abnormally likely presence of SIBO in a human subject and a method of detecting SIBO in a human subject. A method of determining the relative severity of SIBO or a SIBO-caused condition in a human subject, in whom small intestinal bacterial overgrowth (SIBO) has been detected, is also disclosed.
Owner:CEDARS SINAI MEDICAL CENT
Pharmaceutical compositions for buccal and pulmonary application
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and at least three different micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal region of the mouth.
Owner:GENEREX PHARMA INC +1
Sterilized nanoparticulate glucocorticosteroid formulations
InactiveUS20070178051A1Readily heat sterilizedImprove the heating effectOrganic active ingredientsPowder deliveryPediatric patientMicroparticle
The invention is directed sterile to compositions of glucocorticosteroids useful in the prophylaxis and chronic treatment of asthma and other allergic and inflammatory conditions in adults and pediatric patients.
Owner:ELAN PHRMA INT LTD
Biodegradable injectable implants containing glycolic acid
InactiveUS7314636B2Non-migratoryEasy to moveSolution deliveryPharmaceutical non-active ingredientsEmulsionGlycolic acid
Owner:MEDGRAFT MICROTECH
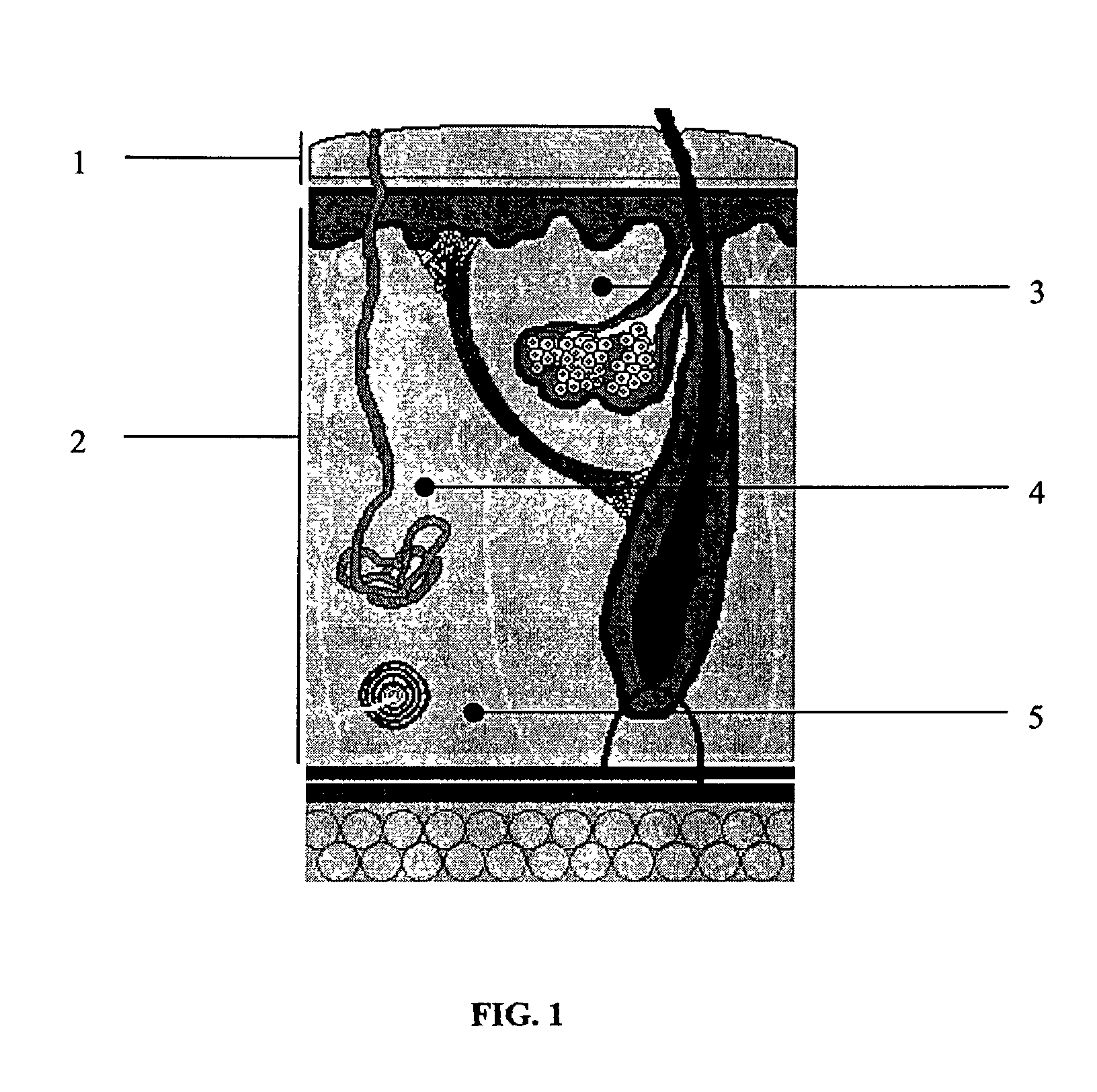
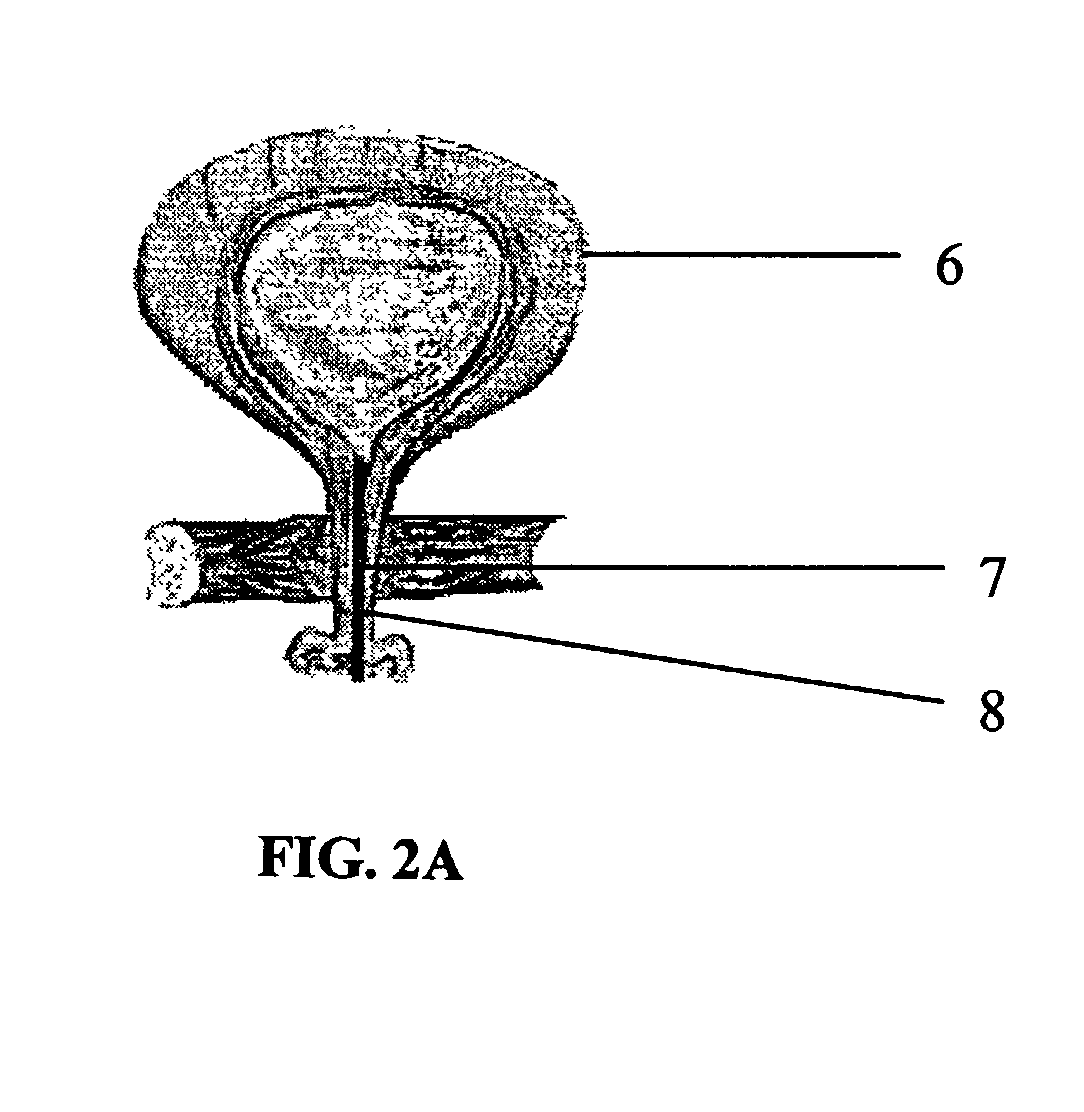
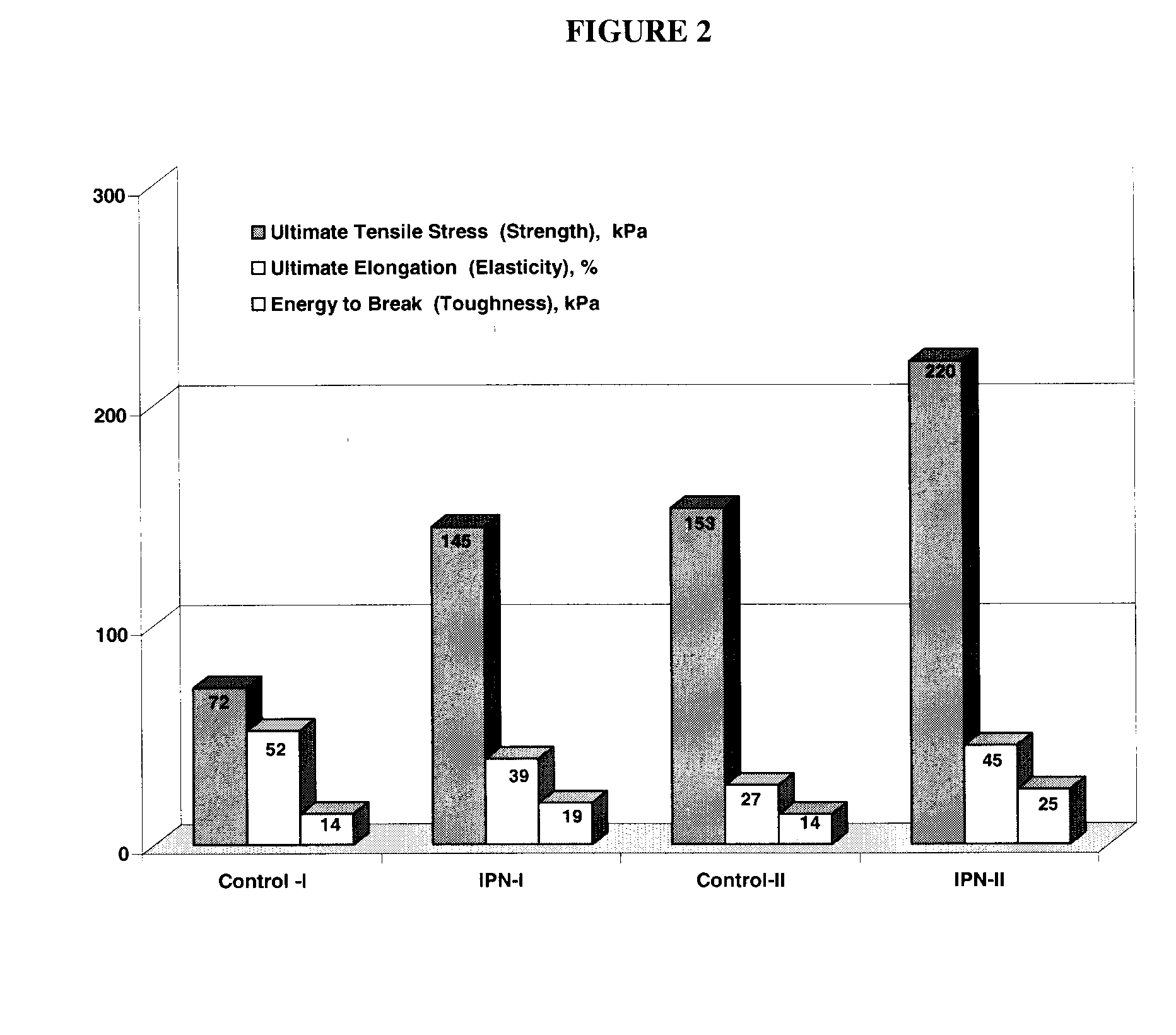
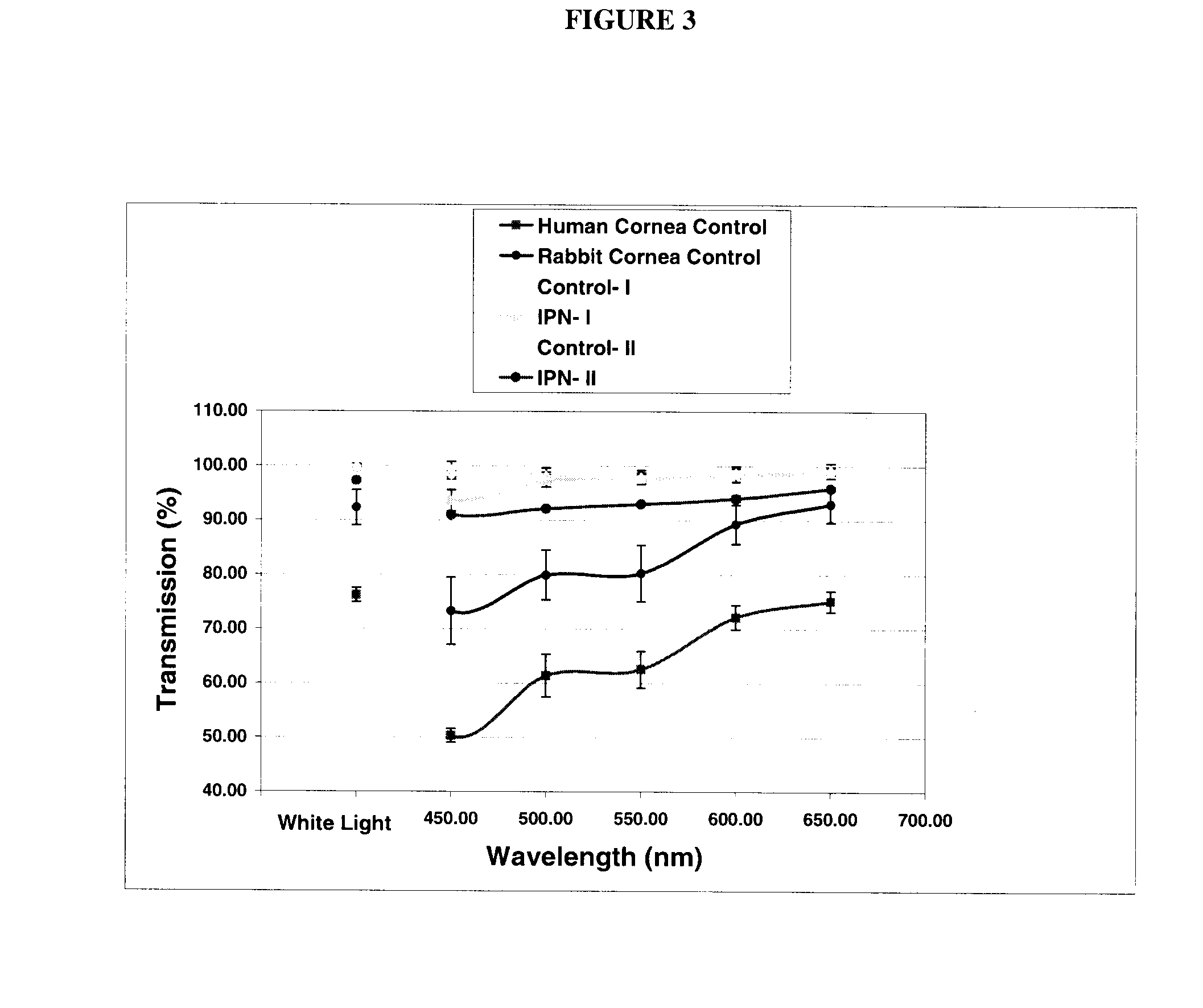
Interpenetrating Networks, and Related Methods and Compositions
The present invention provides interpenetrating polymeric networks (IPNs), and related methods and compositions. The hydrogel material of this invention comprises an interpenetrating network of two or more polymer networks, wherein at least one of the polymer networks is based on a biopolymer. Also provided is a method of producing the hydrogel material comprising, combining a first polymeric network with a second polymeric network, wherein the first polymeric network or the second polymeric network is based on a biopolymer. The present application also discloses devices manufactured from the IPN hydrogel material and uses thereof.
Owner:OTTAWA HOSPITAL RES INST +1
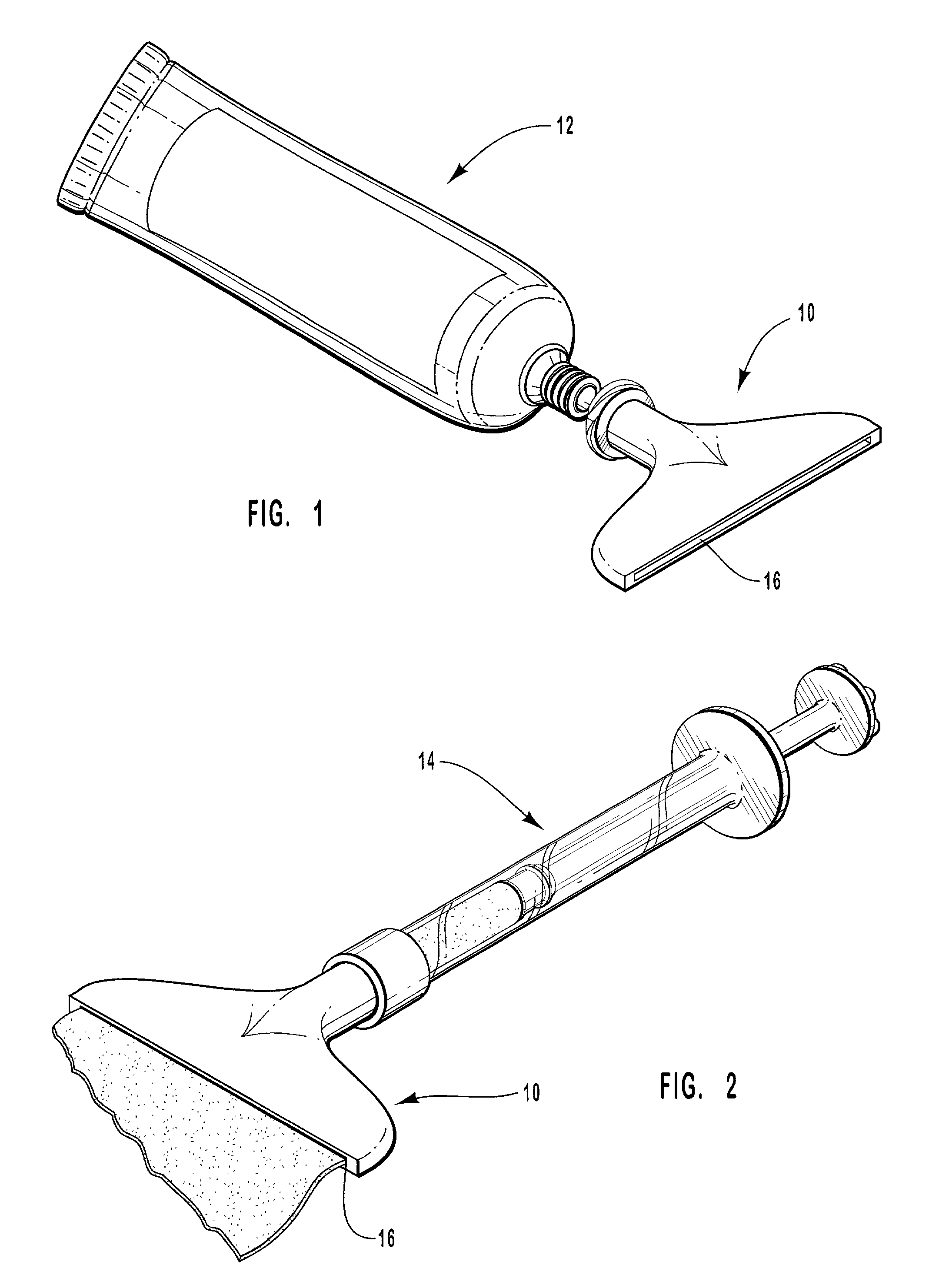
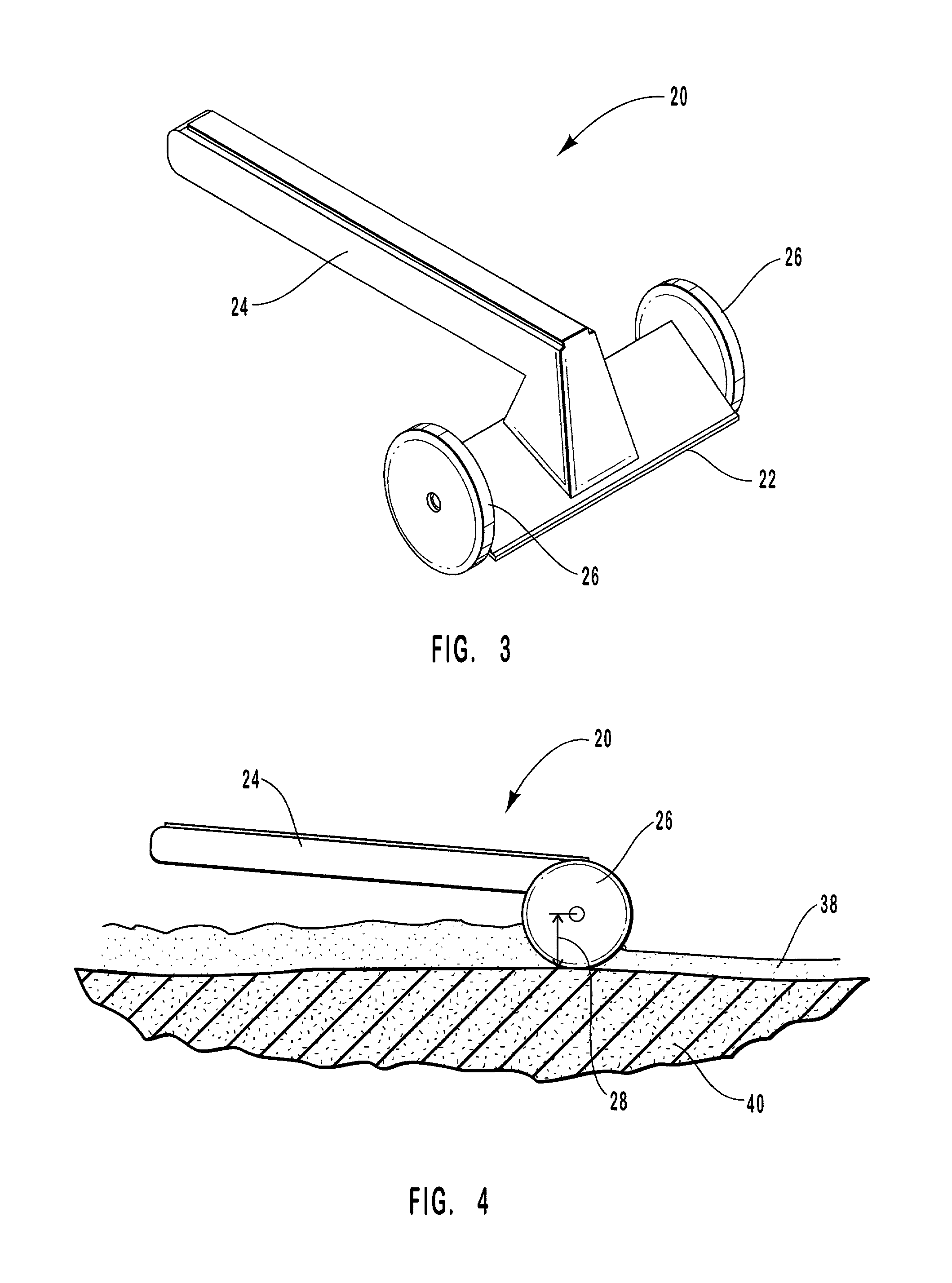
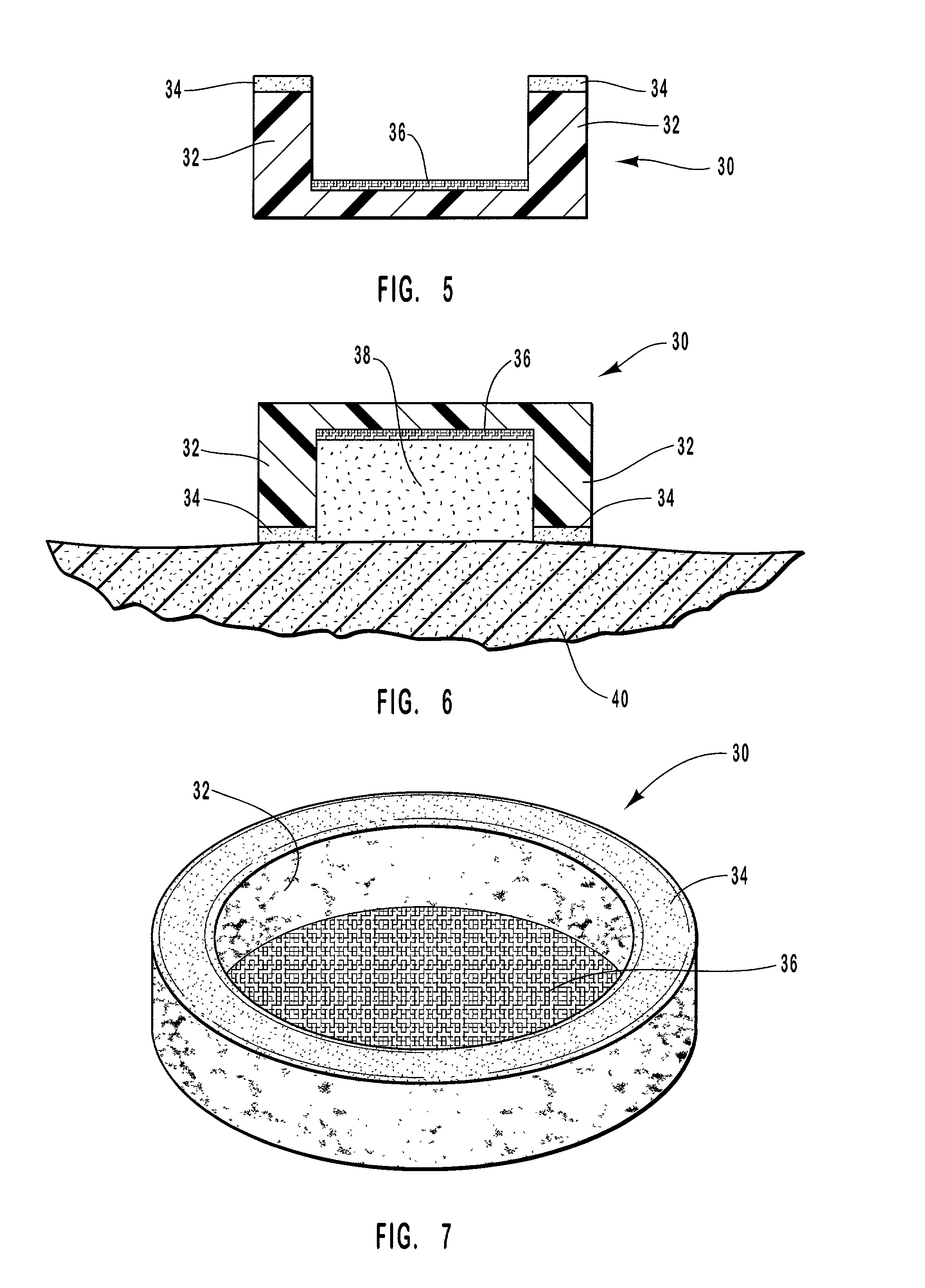
Methods and apparatus for drug delivery involving phase changing formulations
InactiveUS20020004063A1Cleanly peeled from the skinMaintenance of such surfaceSalicyclic acid active ingredientsAnaestheticsPharmaceutical formulationSoft solids
This invention relates to an apparatus and method of drug delivery on a human body surface. The formulation comprises a drug, a conversion agent capable of converting the formulation from a less solid phase to a coherent, soft, solid phase, and a vehicle medium or carrier for the drug and conversion agent. The drug formulation is applied to this human body surface in its less than solid phase and is subsequently converted to a soft solid phase while the drug is being delivered through the human body surface. After delivery of the drug is complete, the soft solid formulation can be removed or peeled from the body surface as a coherent solid formulation. The drug formulation provides control over drug delivery rates and allows the formulation to be removed without leaving a messy, residual formulation on the body surface.
Owner:CRESCITA THERAPEUTICS INC
Processes to generate submicron particles of water-insoluble compounds
InactiveUS6177103B1Rapid of surface stabilizedRapid attainmentOrganic active ingredientsPowder deliveryWater insolubleChemical compound
Submicron particles of water-insoluble compounds, particularly drugs, are prepared by simultaneously stabilizing microparticulate suspensions of same with surface modifier molecules by rapid expansion into an aqueous medium from a compressed solution of the compound and surface modifiers in a liquefied gas and optionally homogenizing the aqueous suspension thus formed with a high pressure homogenizer.
Owner:JAGOTEC AG +1
Oral care compositions containing combinations of anti-bacterial and host-response modulating agents
InactiveUS20070053849A1Potent anti-inflammatory activityPromote progressAntibacterial agentsCosmetic preparationsWhole bodyOral bacterial infection
The present invention encompasses topical oral care compositions comprising the combination of an anti-bacterial agent with an anti-inflammatory agent in an orally acceptable carrier for effective treatment and prevention of bacteria-mediated diseases and conditions in the oral cavity and for modulating host reaction to bacterial pathogens present in the oral cavity and to the toxins, endotoxins, inflammatory cytokines and mediators released by or prompted by these pathogens. The present invention also encompasses methods of use of these compositions comprising topical application to the oral cavity. The benefits of the present compositions and methods extend beyond treating and preventing oral bacterial infections in the oral cavity to promoting whole body or systemic health.
Owner:THE PROCTER & GAMBLE COMPANY
Nano-Chinese medicinal biological product and its preparation
A nano-class biologic product of Chinese-medicinal material for higher biological utilization rate and target nature, lower toxic by-effect and improved curative effect is prepared through preparing the nanoparticles of Chinese-medicinal materials, and preparing nano-class (50-80 nm) biologic product.
Owner:陈永丽
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
Methods and systems for operating an aerosol generator
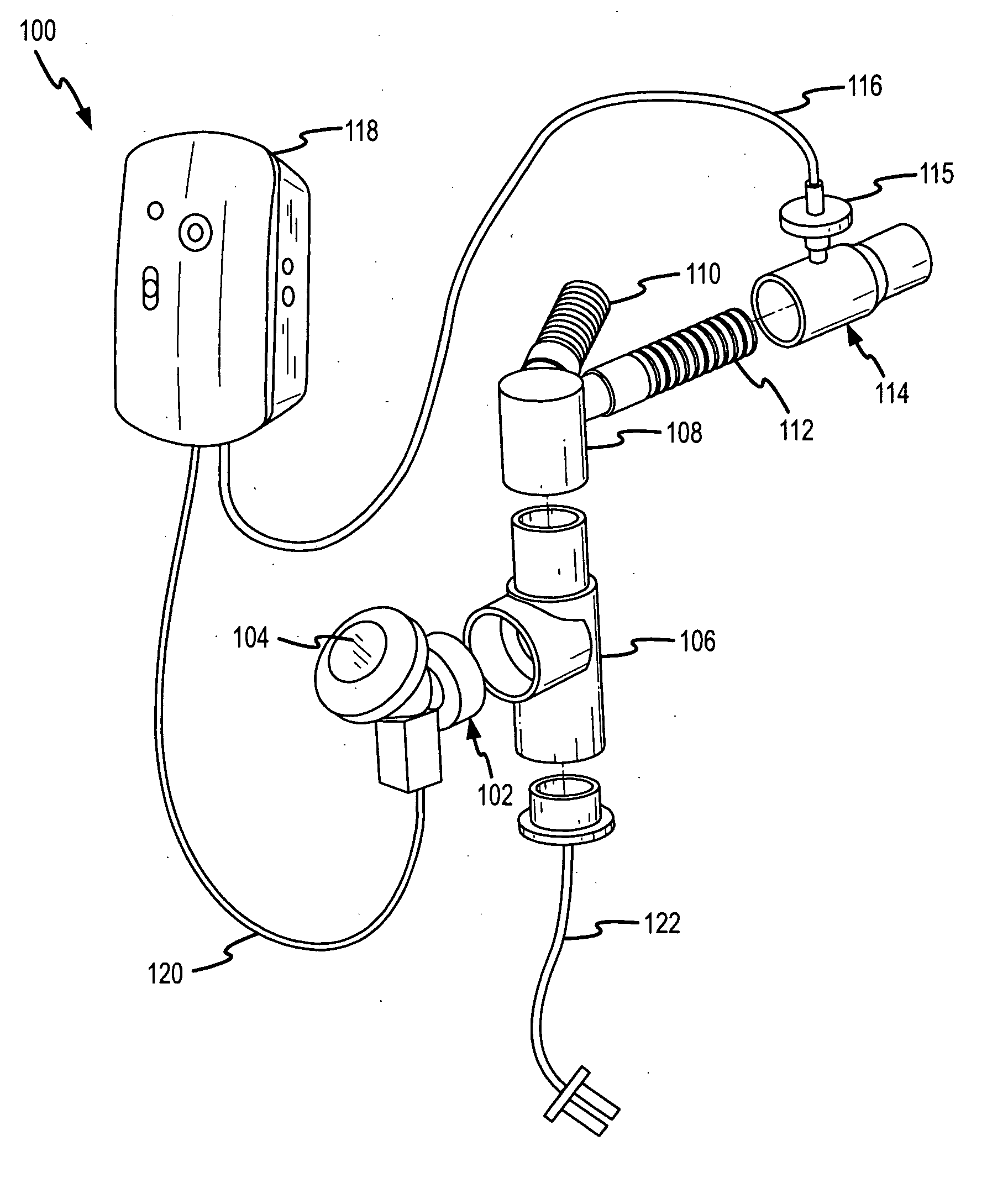
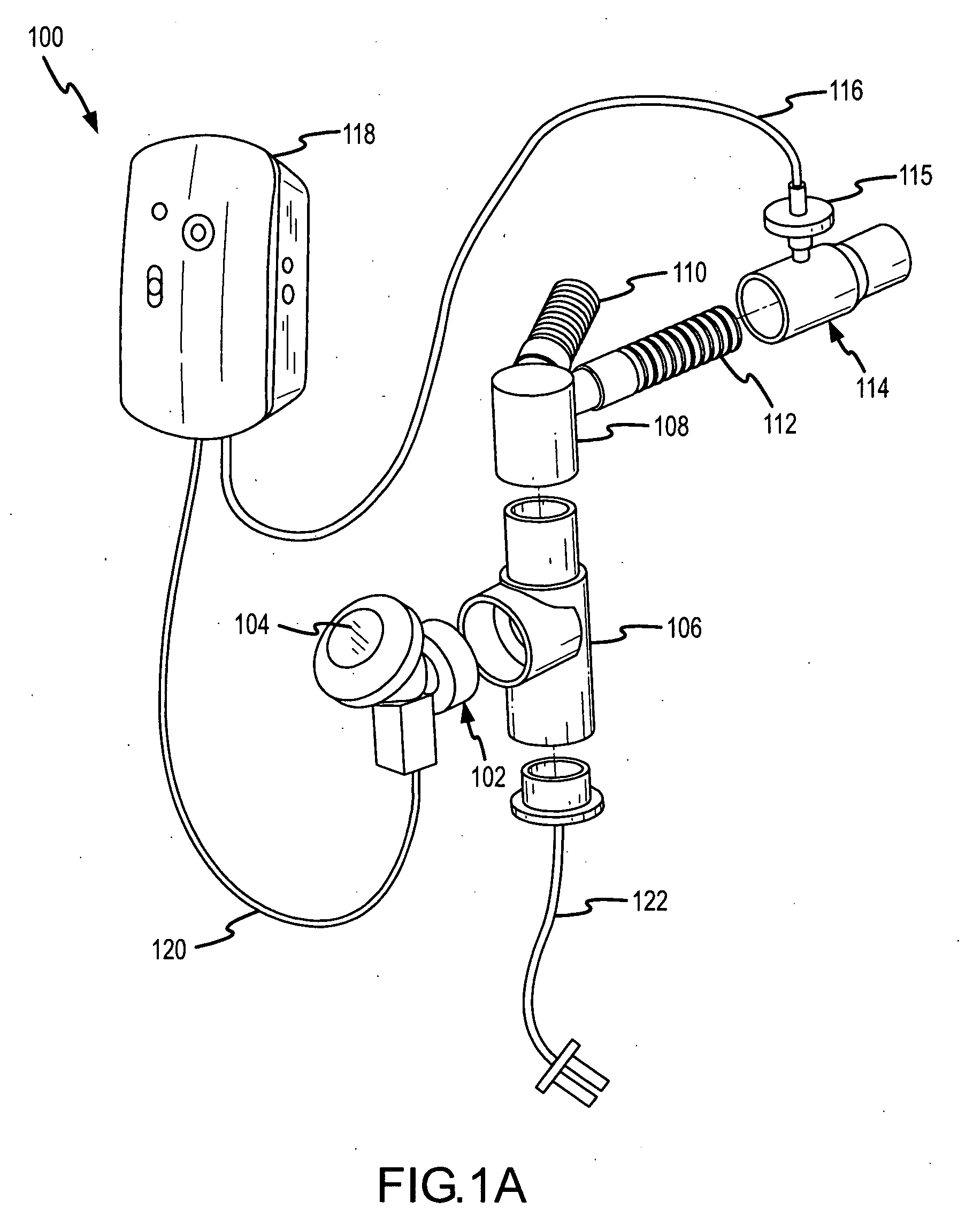
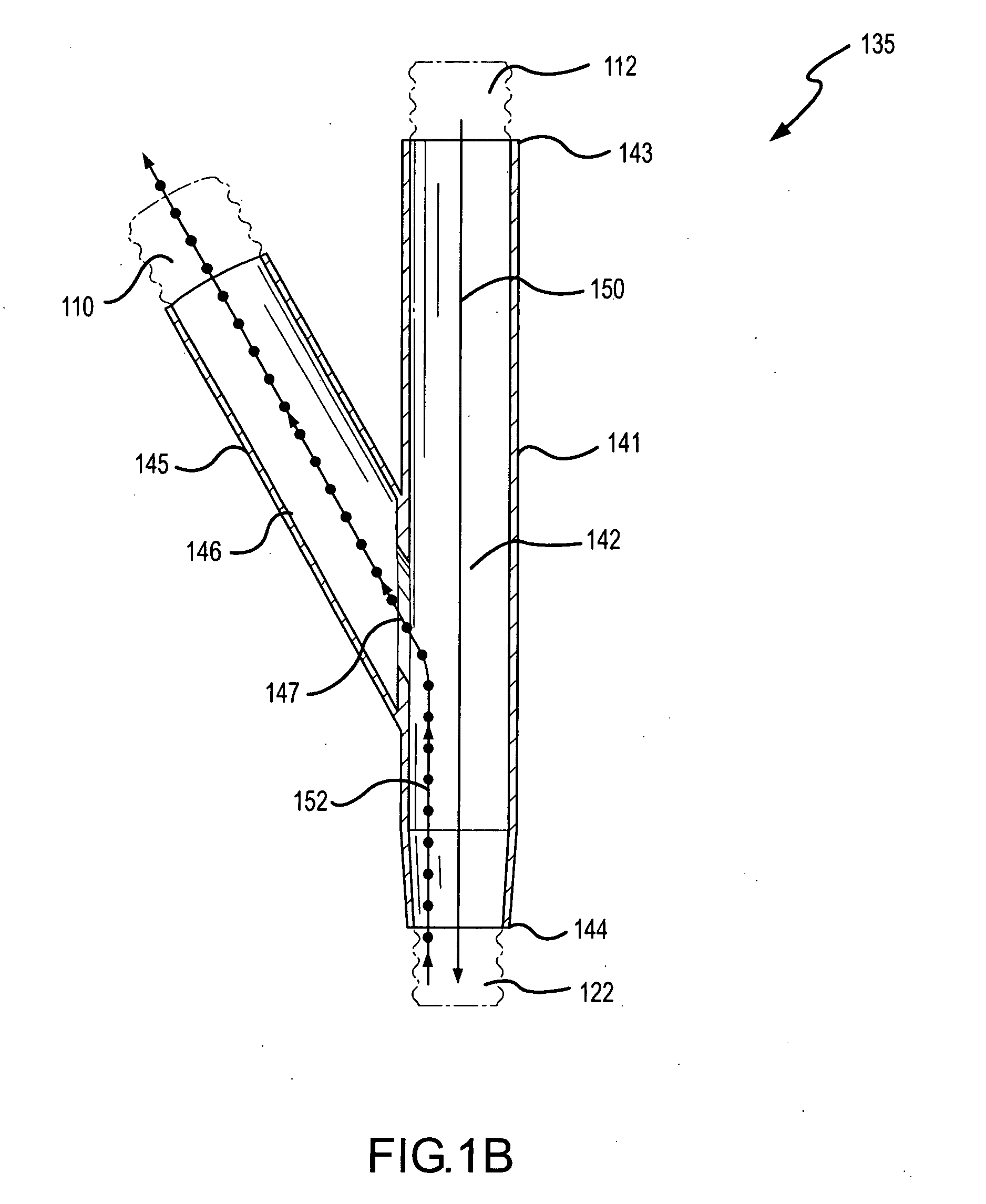
InactiveUS20050217666A1Improve security levelImprove delivery efficiencyAntibacterial agentsOrganic active ingredientsDiseaseAmikacin
A method of treating a patient with a pulmonary disease, where the method includes delivering a dose of aerosolized medicament intermittently to a ventilator circuit coupled to the respiratory system of the patient. Also, a method of treating a patient with a pulmonary disease, where the method includes taking the patient off a ventilator, and administering to the patient, a nebulized aerosol comprising from about 100 μg to about 500 mg of a medicament. Additionally, an aerosolized medicament for the treatment of a pulmonary disease, where the medicament includes amikacin mixed with an aqueous solution having an adjusted pH from about 5.5 to about 6.3. The pH is adjusted by adding hydrochloric acid and sodium hydroxide to the aqueous solution.
Owner:NOVARTIS AG
Topical nitric oxide donor compositions
InactiveUS6287601B1Reduced and failing organ functionPoor appetitePowder deliveryBiocideLipid formationEquine Species
Compositions and methods of the topical treatment of equine laminitis are disclosed. In particular, combinations of a fast acting nitric oxide (NO) donor, a sustained acting NO donor and an NSAID mixed in a lipid-based carrier are described. The application of such combinations to the affected areas, e.g., the hoofs and surrounding tissues, of an equine afflicted with laminitis provides relief from the debilitating effects of this painful, often life-threatening condition.
Owner:STREHKEHN INT LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com