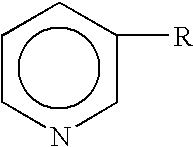
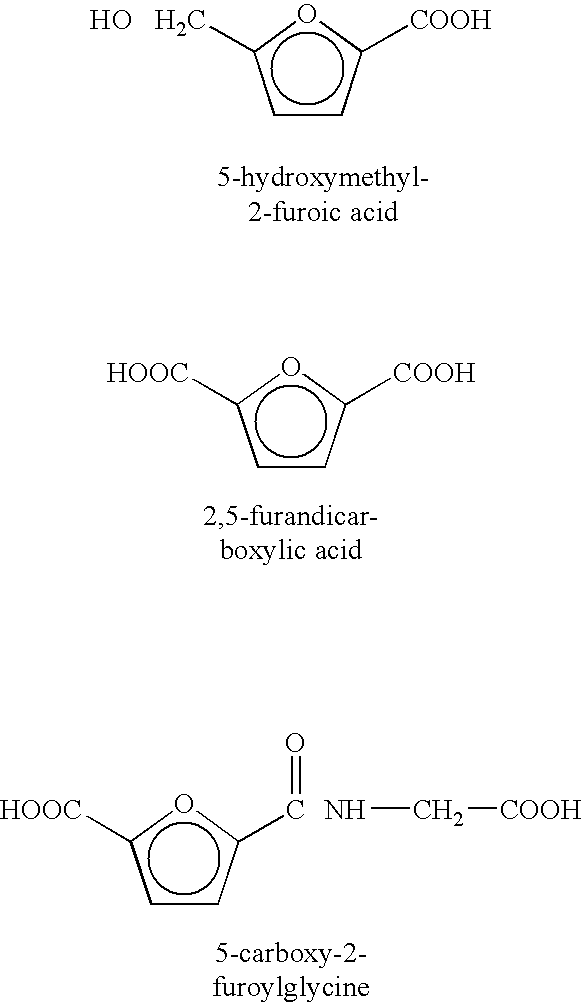
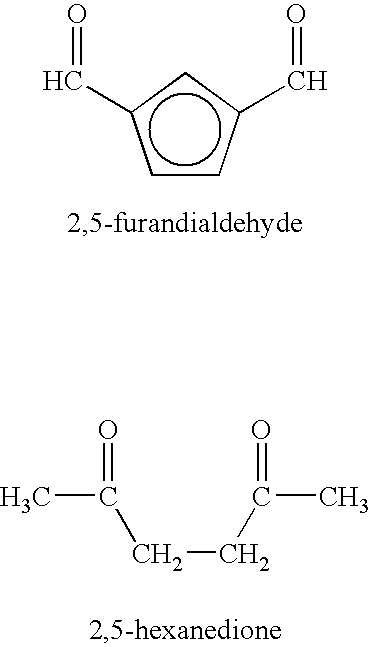
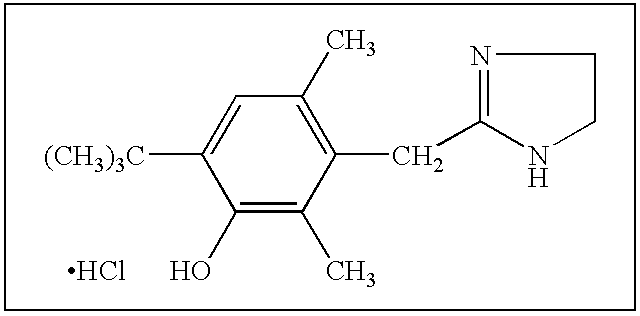
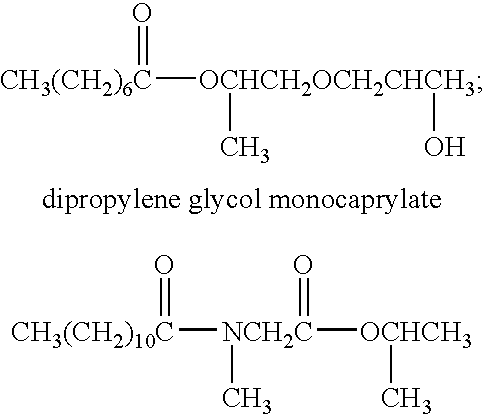Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2506results about "Salicyclic acid active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
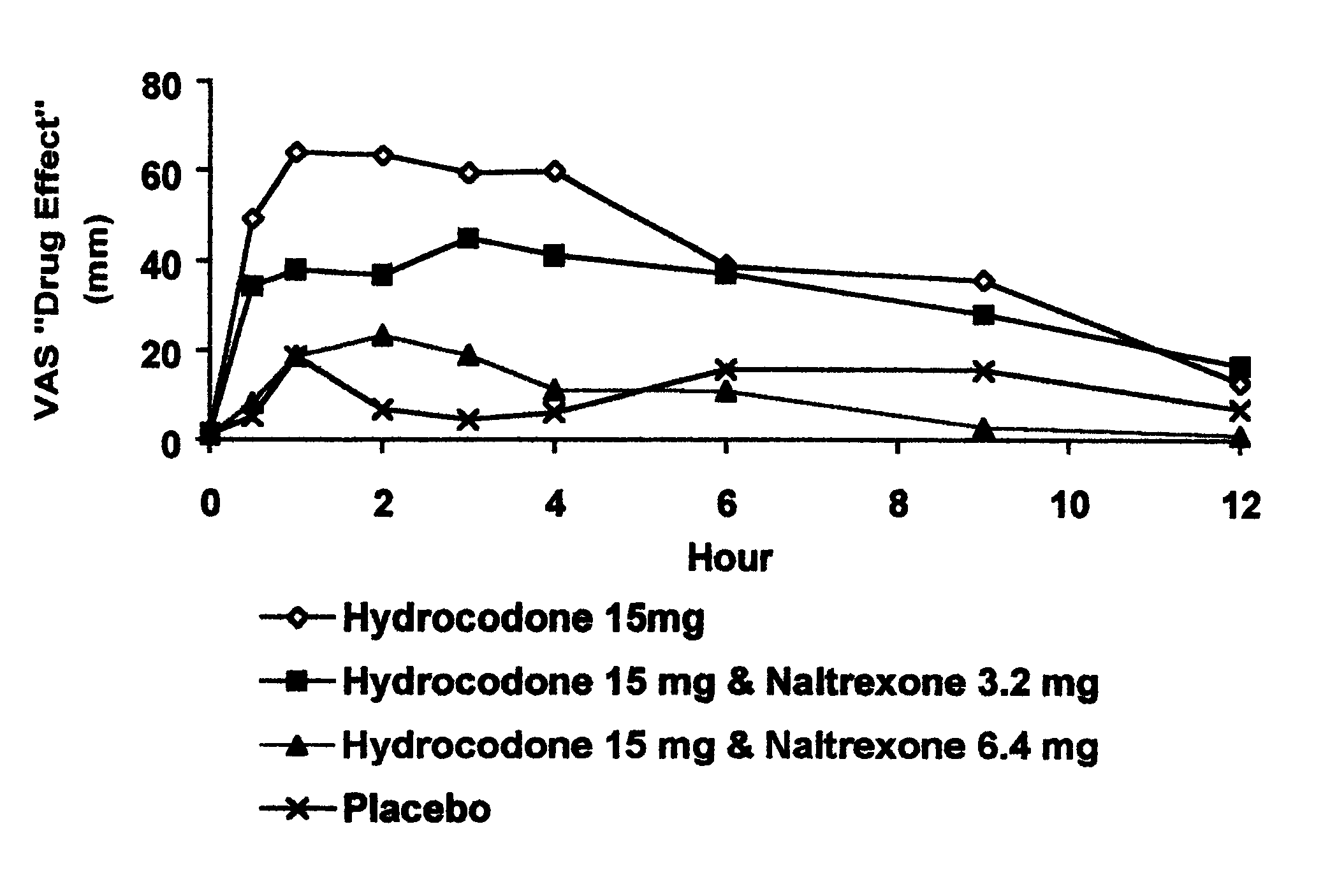
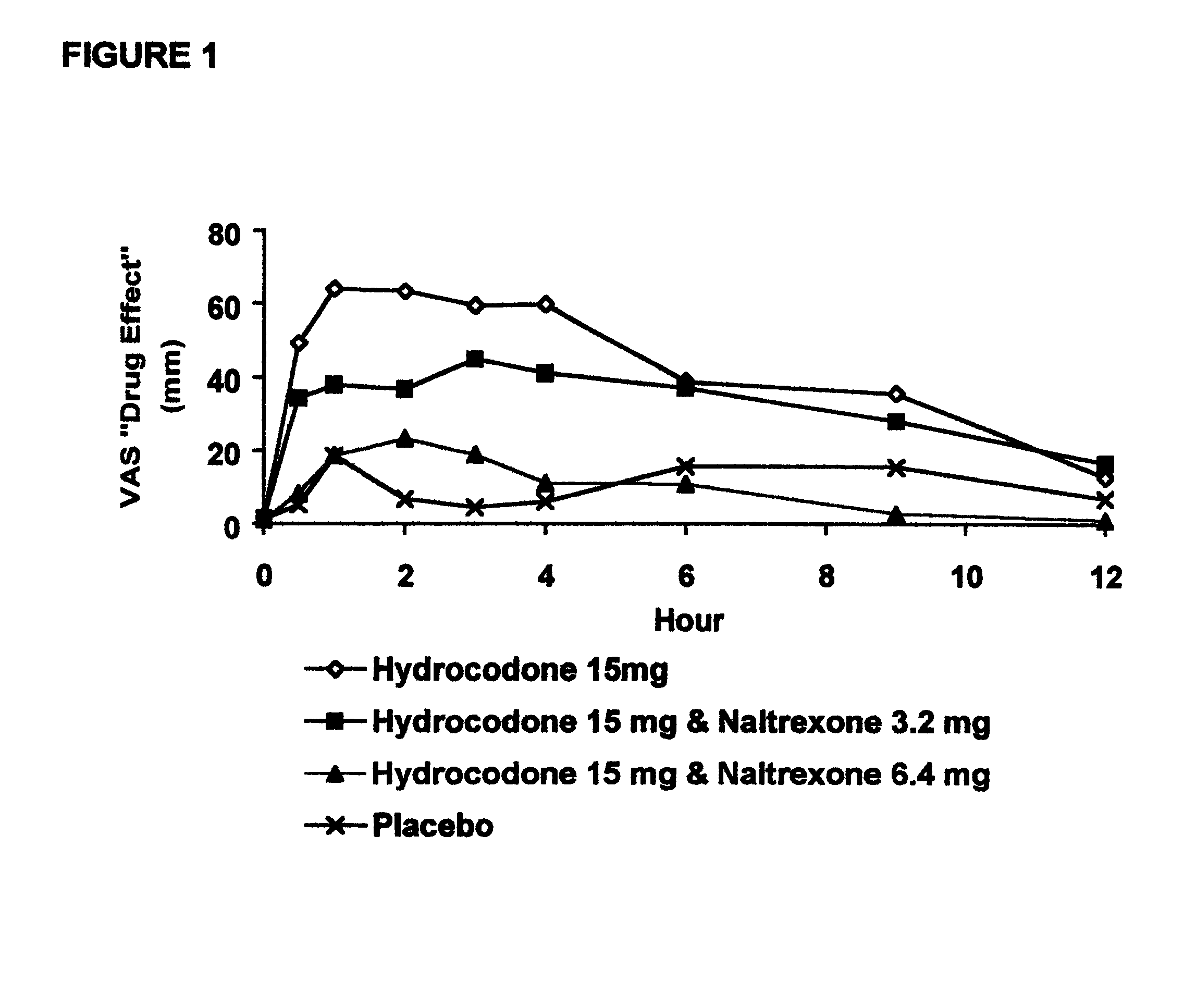
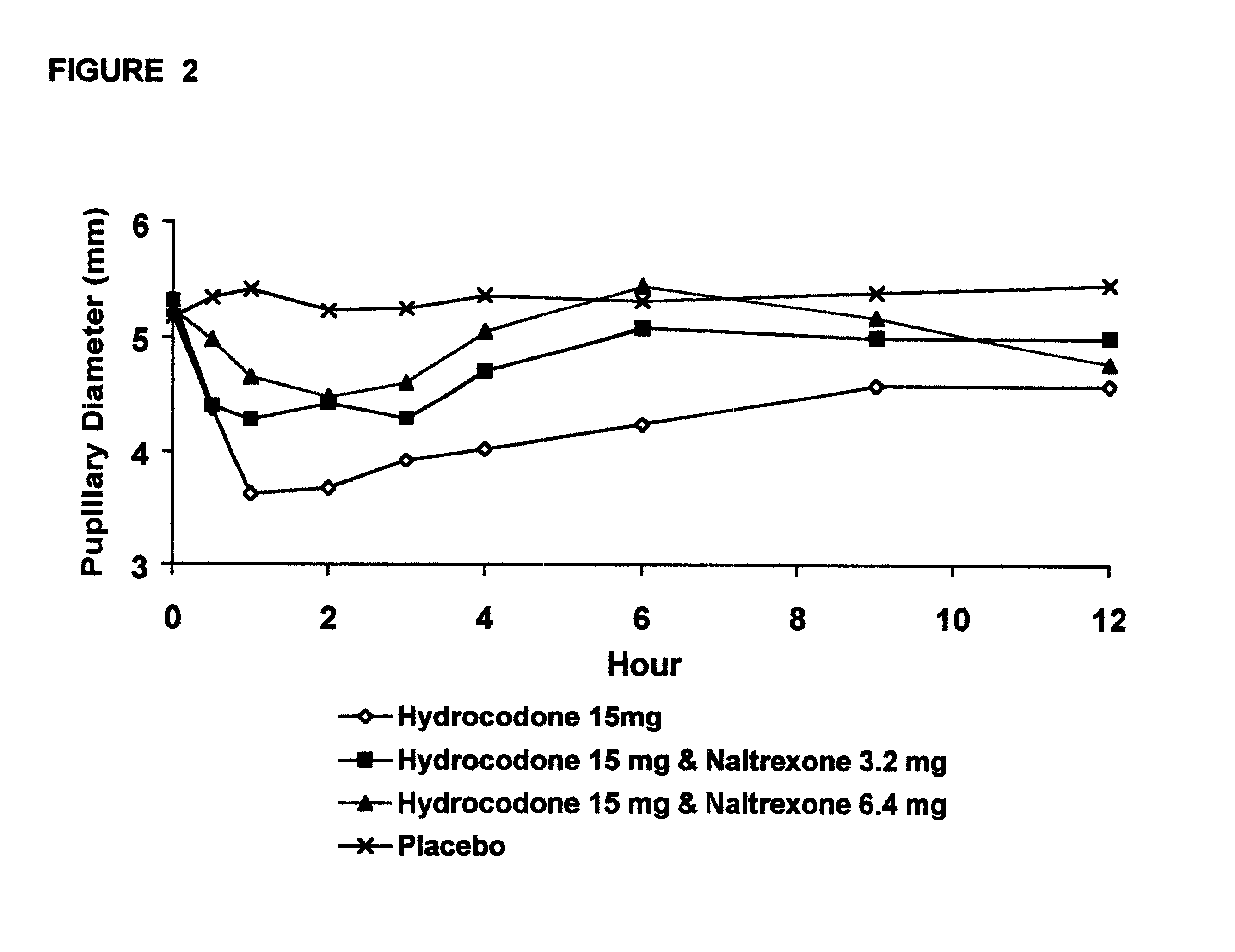
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
N-acetyl aldosamines, n-acetylamino acids and related n-acetyl compounds and their topical use
Compositions comprising N-acetyl-aldosamines, N-acetylamino acids, and related N-acetyl compounds are useful to alleviate or improve various cosmetic conditions and dermatological disorders, including changes or damage to skin, nail and hair associated with intrinsic aging and / or extrinsic aging, as well as changes or damage caused by extrinsic factors. N-acetyl-aldosamines, N-acetylamino acids, and related N-acetyl composition may further comprise a cosmetic, pharmaceutical or other topical agent to enhance or create synergetic effects.
Owner:TRISTRATA TECH
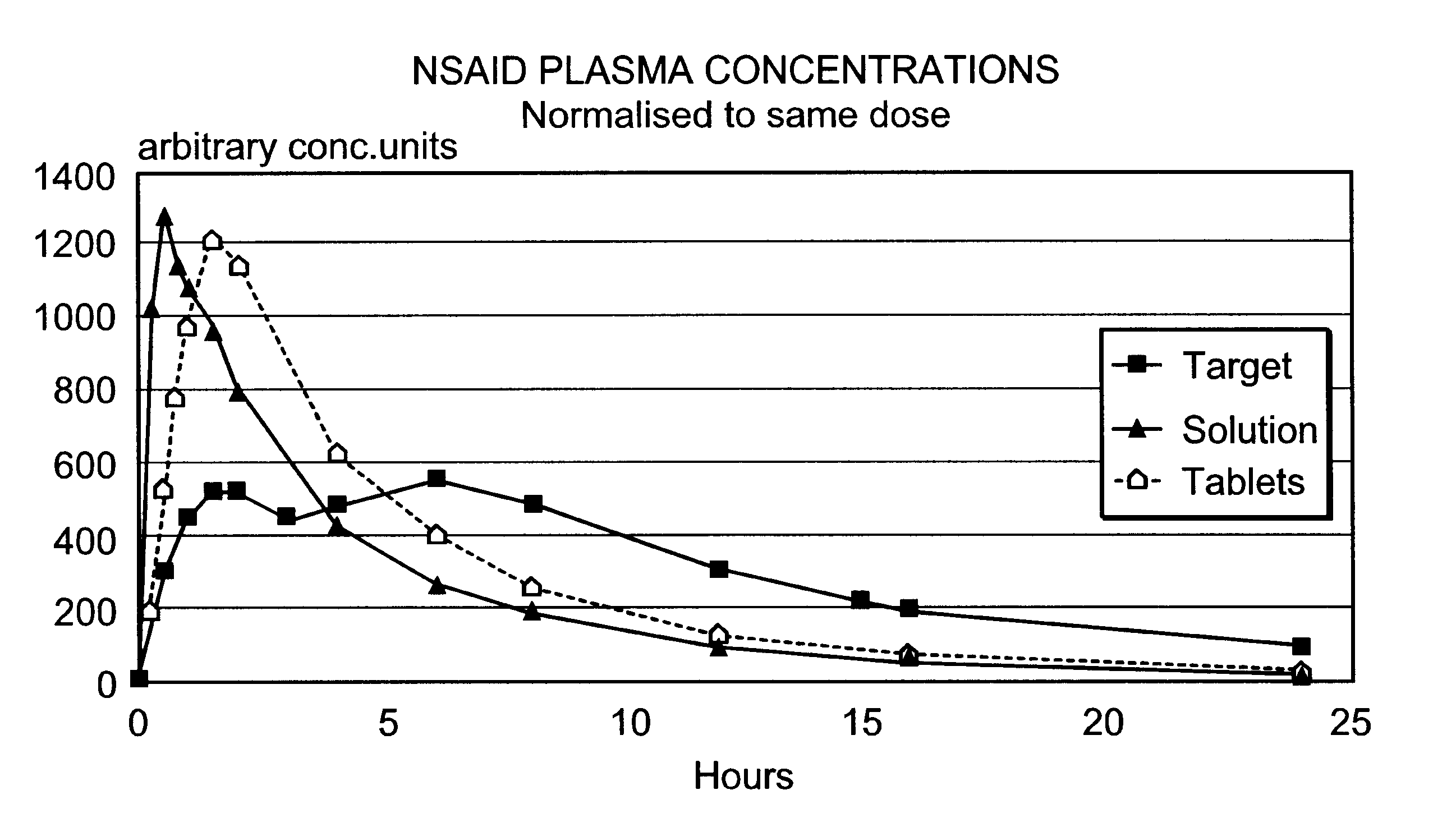
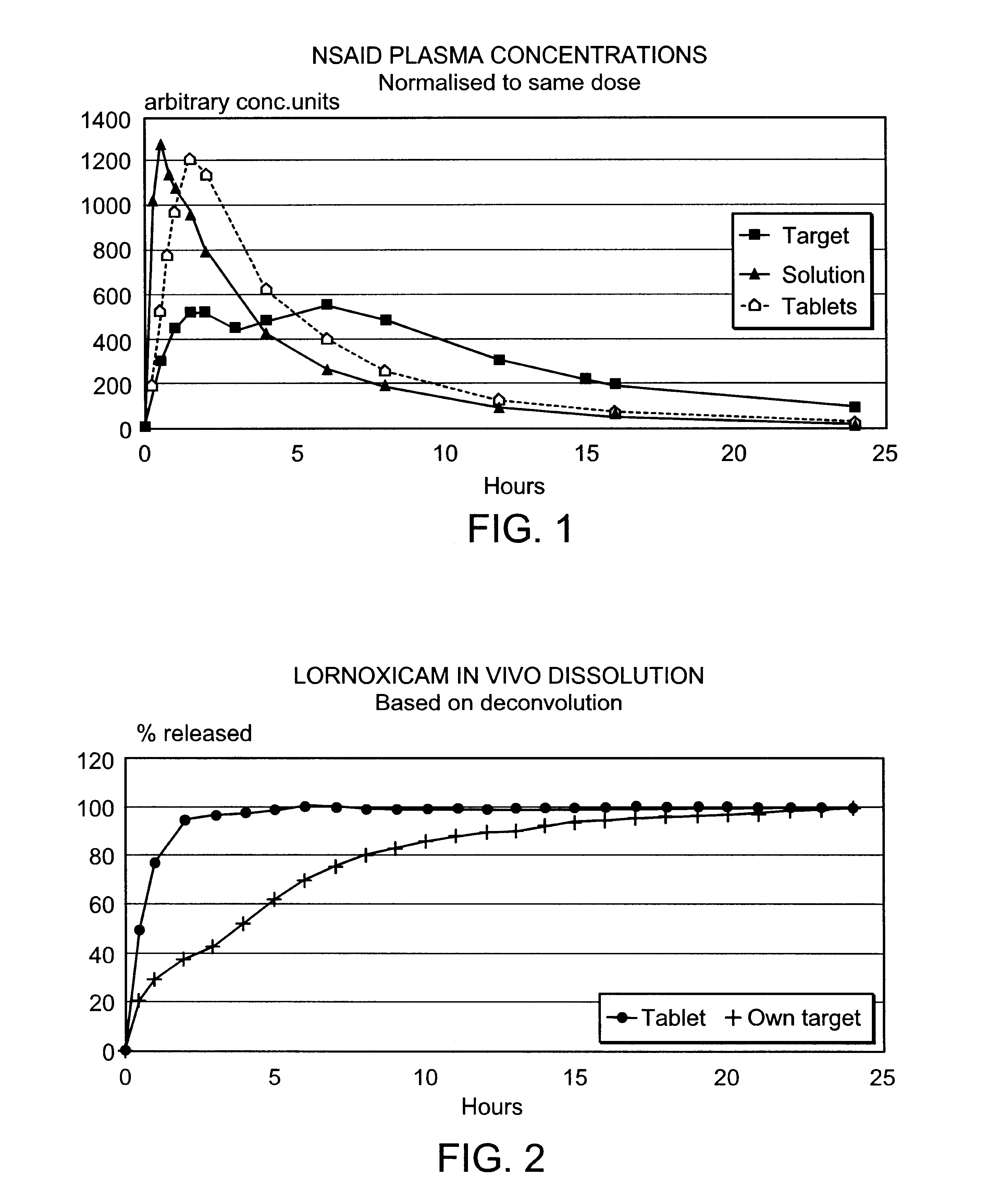
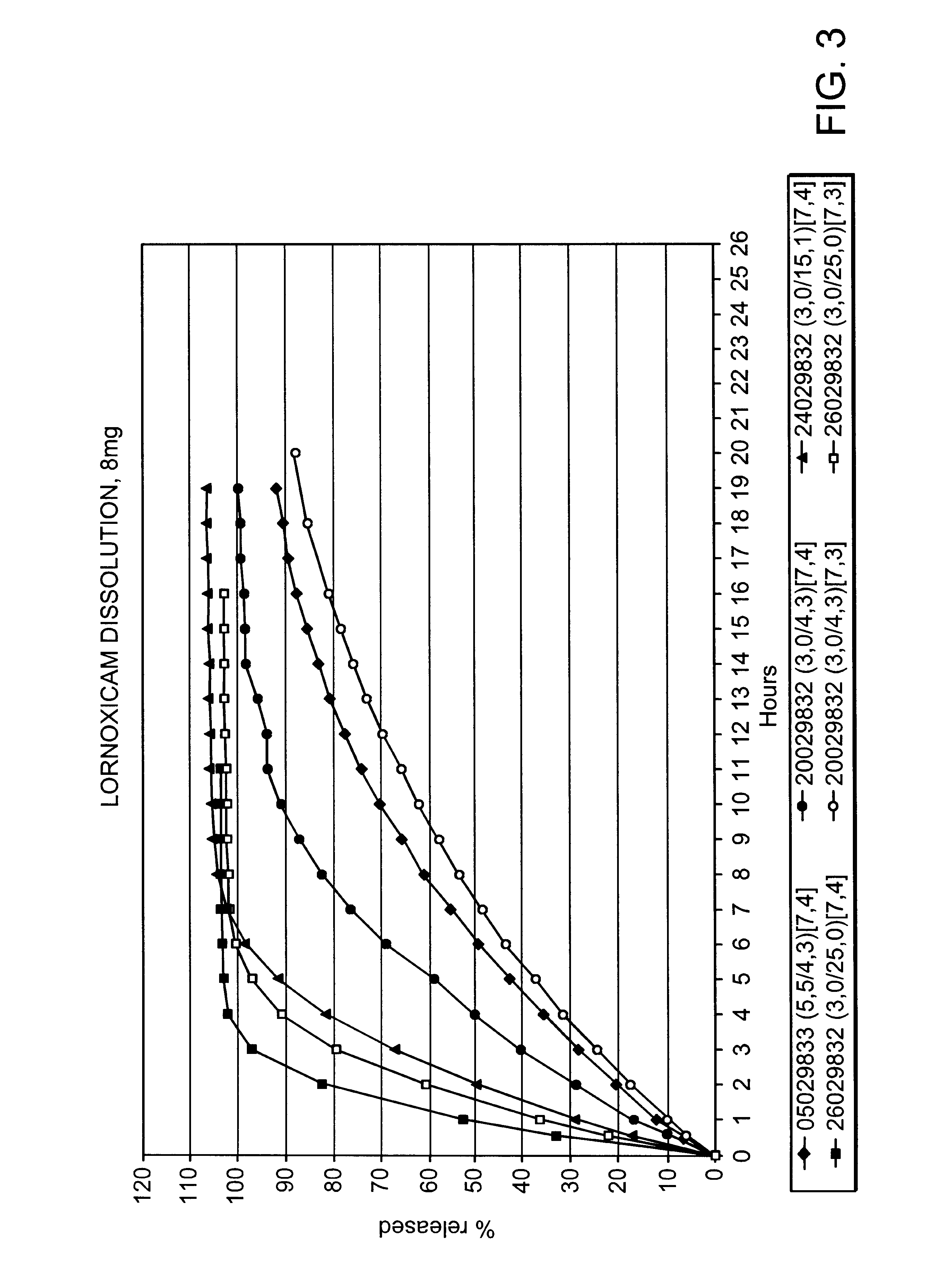
Modified release multiple-units compositions of non-steroid anti-inflammatory drug substances (NSAIDs)
InactiveUS6599529B1Keep low levelQuick releasePowder deliveryNervous disorderNon steroid anti inflammatory drugTherapeutic effect
An oral pharmaceutical modified release multiple-units composition for the administration of a therapeutically and / or prophylactically effective amount of a non-steroid anti-inflammatory drug substance to obtain both a relatively fast onset of the therapeutic effect and the maintenance of a therapeutically active plasma concentration for a relatively long period of time is disclosed.
Owner:TAKEDA PHARMA AS +1
Method of treating depression using a TNF-alpha antibody
InactiveUS20070041905A1Inhibiting TNFα activityImprove depressionSalicyclic acid active ingredientsBiocideAntiendomysial antibodiesAntigen binding
The invention describes methods of treating depression comprising administering a TNFα antibody, such as a human TNFα antibody. The invention also provides a method for treating depression comprising inhibiting TNFα activity in a subject suffering from depression by systemically administering to the subject a human anti-TNFα antibody, or an antigen-binding portion thereof, such that depression is treated. Also described is a method for the treatment or alleviation of depression or other affective disorders comprising administering an amount of an anti-inflammatory agent effective to treat or alleviate depression or other affective disorder to a subject in need thereof, wherein said anti-inflammatory agent down-regulates peripheral cytokine levels to thereby treat or alleviate depression or other affective disorder.
Owner:HOFFMAN REBECCA S +3
Fast dissolving orally consumable films
InactiveUS6923981B2Dissolve fastGood curative effectAntibacterial agentsCosmetic preparationsPolymer sciencePullulan
Physiologically acceptable films, including edible films, are disclosed. The films include a water soluble film-forming polymer such as pullulan. Edible films are disclosed that include pullulan and antimicrobially effective amounts of the essential oils thymol, methyl salicylate, eucalyptol and menthol. The edible films are effective at killing the plaque-producing germs that cause dental plaque, gingivitis and bad breath. The film can also contain pharmaceutically active agents. Methods for producing the films are also disclosed.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Use of NSAIDs for prevention and treatment of cellular abnormalities of the lung or bronchial pathway
InactiveUS20030004142A1High concentrationMinimal exposureBiocideSalicyclic acid active ingredientsSurgeryNon steroidal anti inflammatory
Owner:PRIOR CHRISTOPHER P +2
Topically Bioavailable Acne and Rosacea Treatment Compositions
InactiveUS20040156873A1Reduce stimulationSynergistic superior anti-acneBiocideCosmetic preparationsAdditive ingredientIrritation
The present invention relates to acne and rosacea compositions by a six-prong synergistic combination treatment strategy that includes (1) control of excess sebum production, (2) control of undesirable bacteria or mites, (3) control of inflammation, (4) enhanced desquamation of follicular infundibulum cells, (5) reduction of irritation from anti-acne or rosacea compositions themselves, and (6) enhancement of the topical bioavailability of anti-acne and rosacea compositions. This is achieved by a synergistic combination of commonly utilized topical anti-acne and rosacea ingredients with a topical bioavailability enhancement composition, which results in enhanced anti-acne and rosacea action from such ingredients. Moreover, additional inclusion of an anti-inflammatory composition, and also a vascular micro-circulation enhancement composition, further results in synergistic superior anti-acne and rosacea benefits from such compositions. The present invention discloses additional surprising synergistic combinations for the control of acne and rosacea that are suitable for a variety of delivery systems and packaging forms.
Owner:GUPTA SHYAM K
Methods and apparatus for drug delivery involving phase changing formulations
InactiveUS20020004063A1Cleanly peeled from the skinMaintenance of such surfaceSalicyclic acid active ingredientsAnaestheticsPharmaceutical formulationSoft solids
This invention relates to an apparatus and method of drug delivery on a human body surface. The formulation comprises a drug, a conversion agent capable of converting the formulation from a less solid phase to a coherent, soft, solid phase, and a vehicle medium or carrier for the drug and conversion agent. The drug formulation is applied to this human body surface in its less than solid phase and is subsequently converted to a soft solid phase while the drug is being delivered through the human body surface. After delivery of the drug is complete, the soft solid formulation can be removed or peeled from the body surface as a coherent solid formulation. The drug formulation provides control over drug delivery rates and allows the formulation to be removed without leaving a messy, residual formulation on the body surface.
Owner:CRESCITA THERAPEUTICS INC
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of skin diseases or disorders
Methods of treating, preventing, correcting and / or managing skin diseases or disorders characterized by overgrowths of the epidermis, keratoses, scleroderma, cutaneous vasculitis, acne or wrinkles are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent. Specific second active ingredients are capable of affecting or inhibiting cell growth or proliferation, removing or improving acne scars, or reducing or correcting wrinkle lines. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
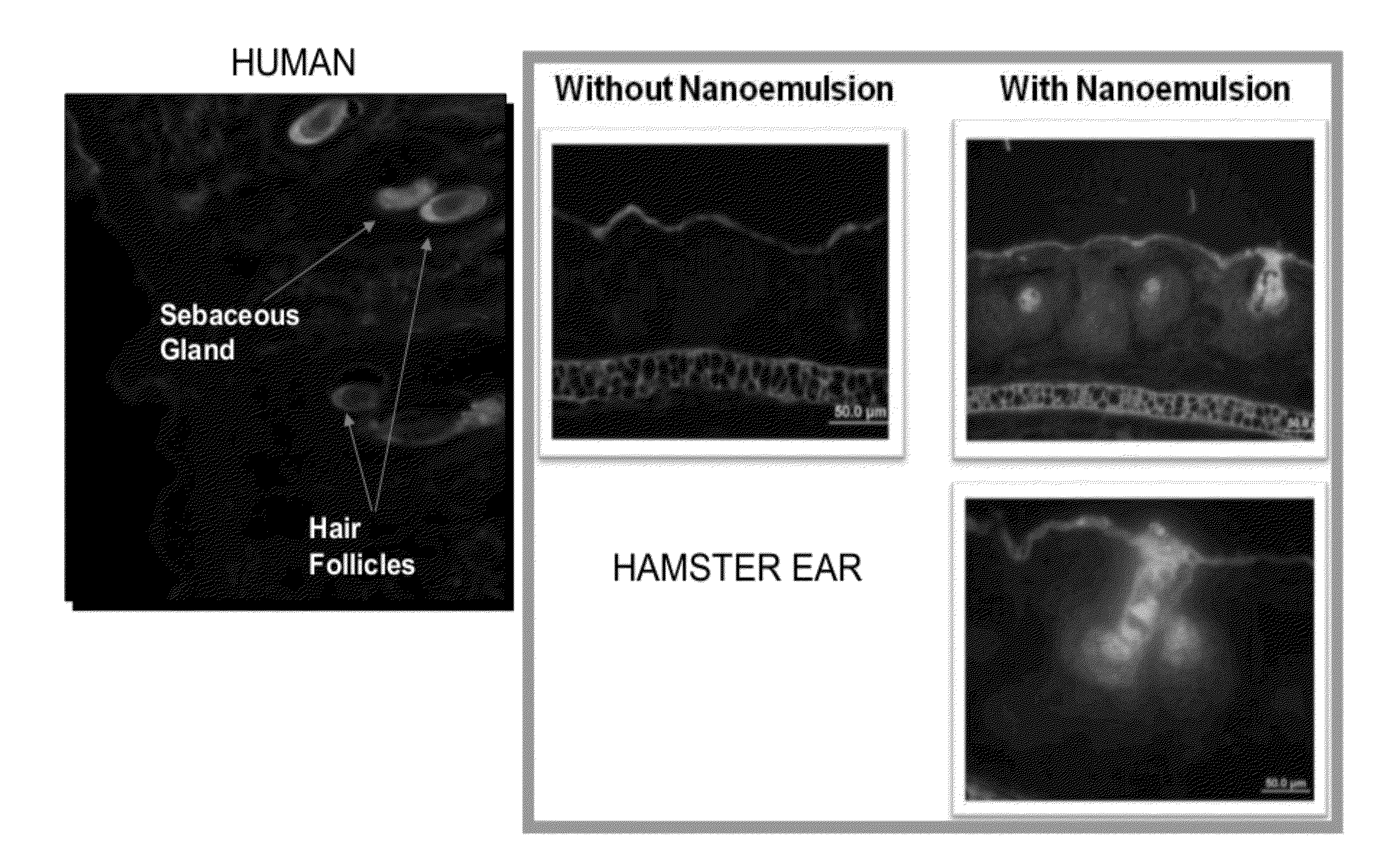
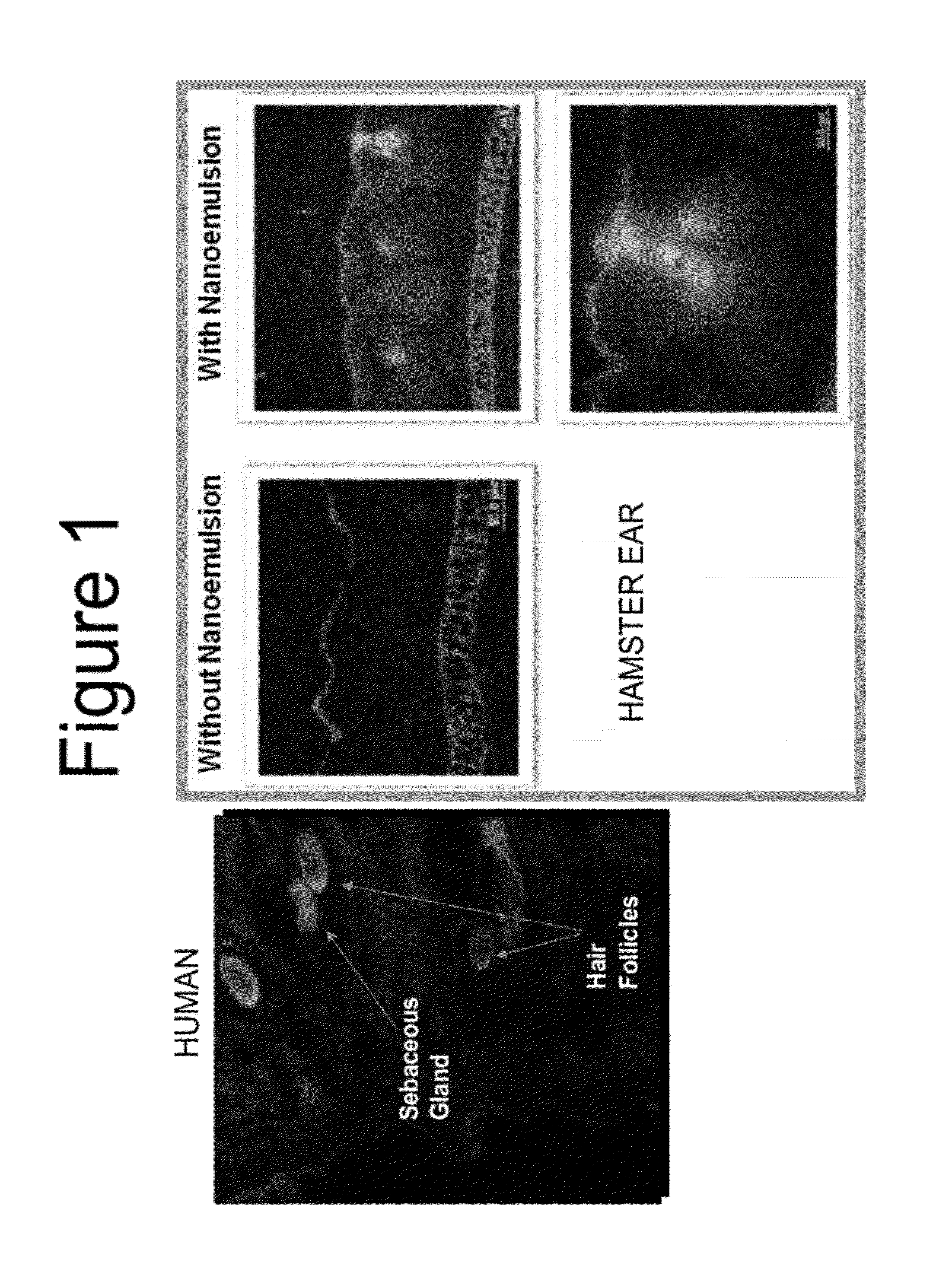
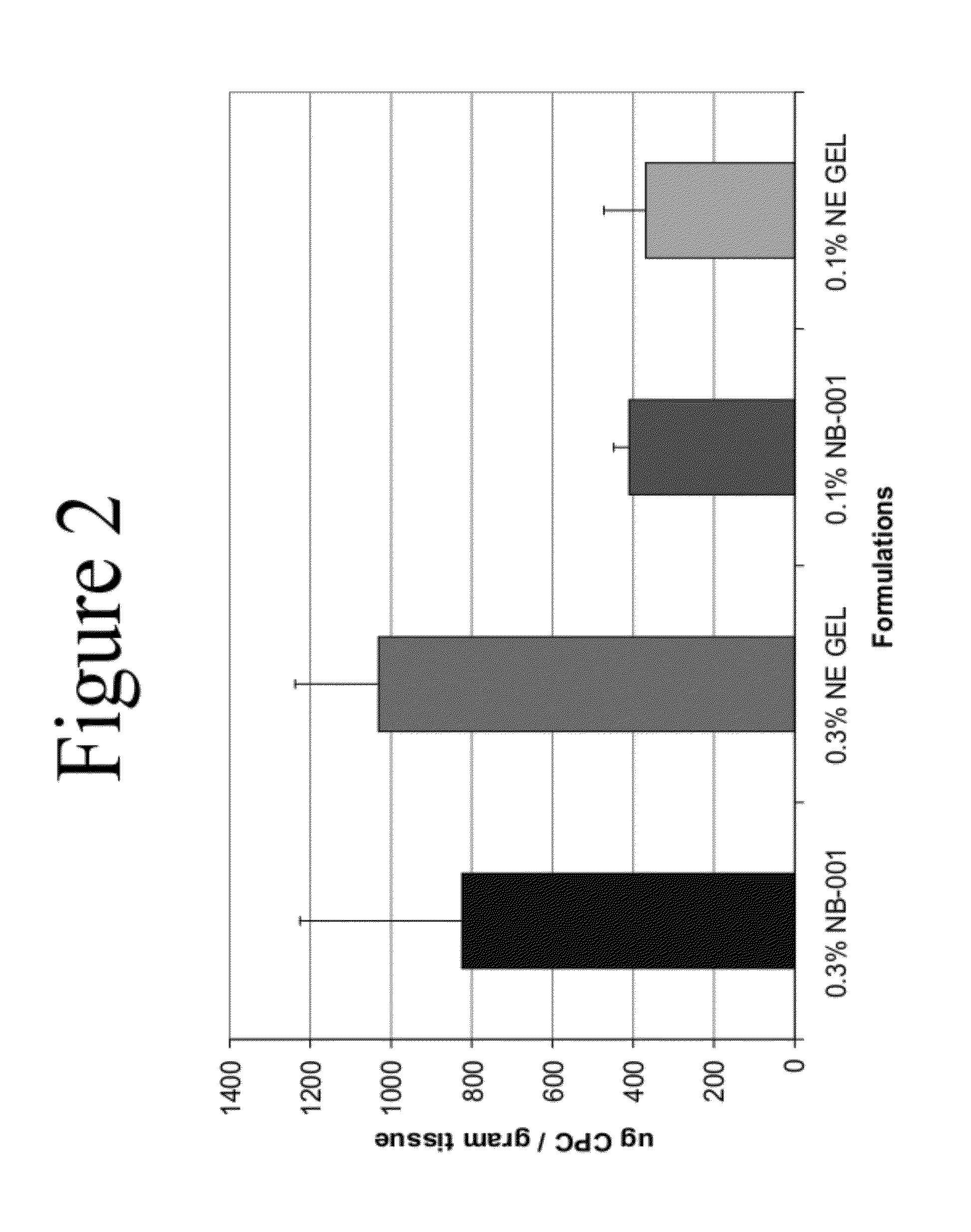
Anti-aging and wrinkle treatment methods using nanoemulsion compositions
InactiveUS20120064136A1Effective treatmentEffective preventionBiocideCosmetic preparationsWrinkle skinMedicine
The present invention relates to methods for treating, preventing, minimizing, and / or diminishing signs of aging in the skin comprising administering to the subject in need thereof a nanoemulsion composition.
Owner:NANOBIO CORP
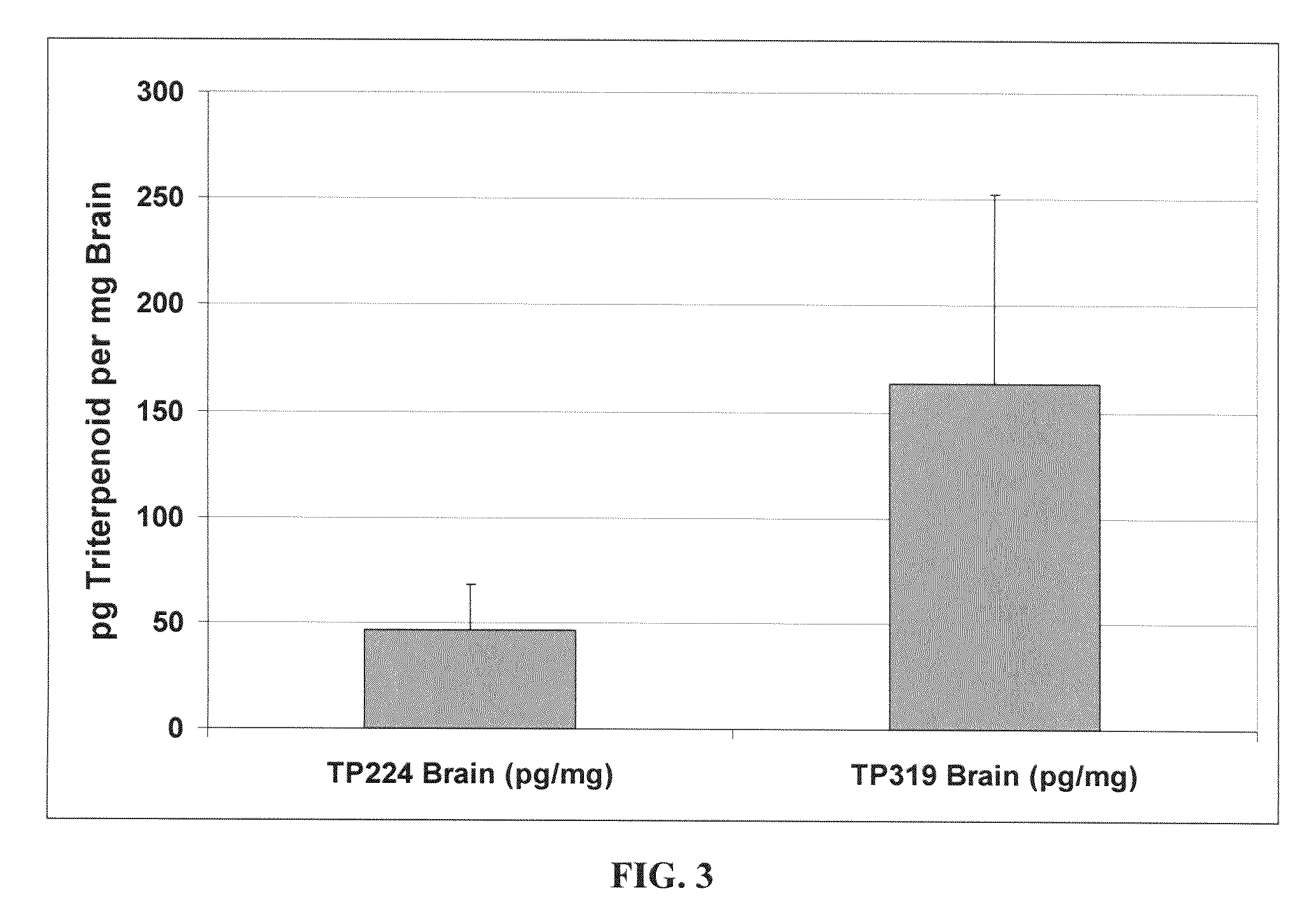
Novel synthetic triterpenoids and methods of use in the treatment and prevention of multiple scleroris
InactiveUS20090060873A1Improving glomerular filtration rateImproving creatinine clearanceBiocideSalicyclic acid active ingredientsDiseaseBipolar mood disorder
The present invention overcomes limitations of the prior art by providing new compounds and methods for the treatment of conditions, such as neurodegenerative diseases (e.g., multiple sclerosis), psychiatric disorders (e.g., psychosis, bipolar disorder, depression, neuropathic pain), conditions involving CNS-mediated chronic pain, spinal cord injuries, and other diseases or injuries.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE +1
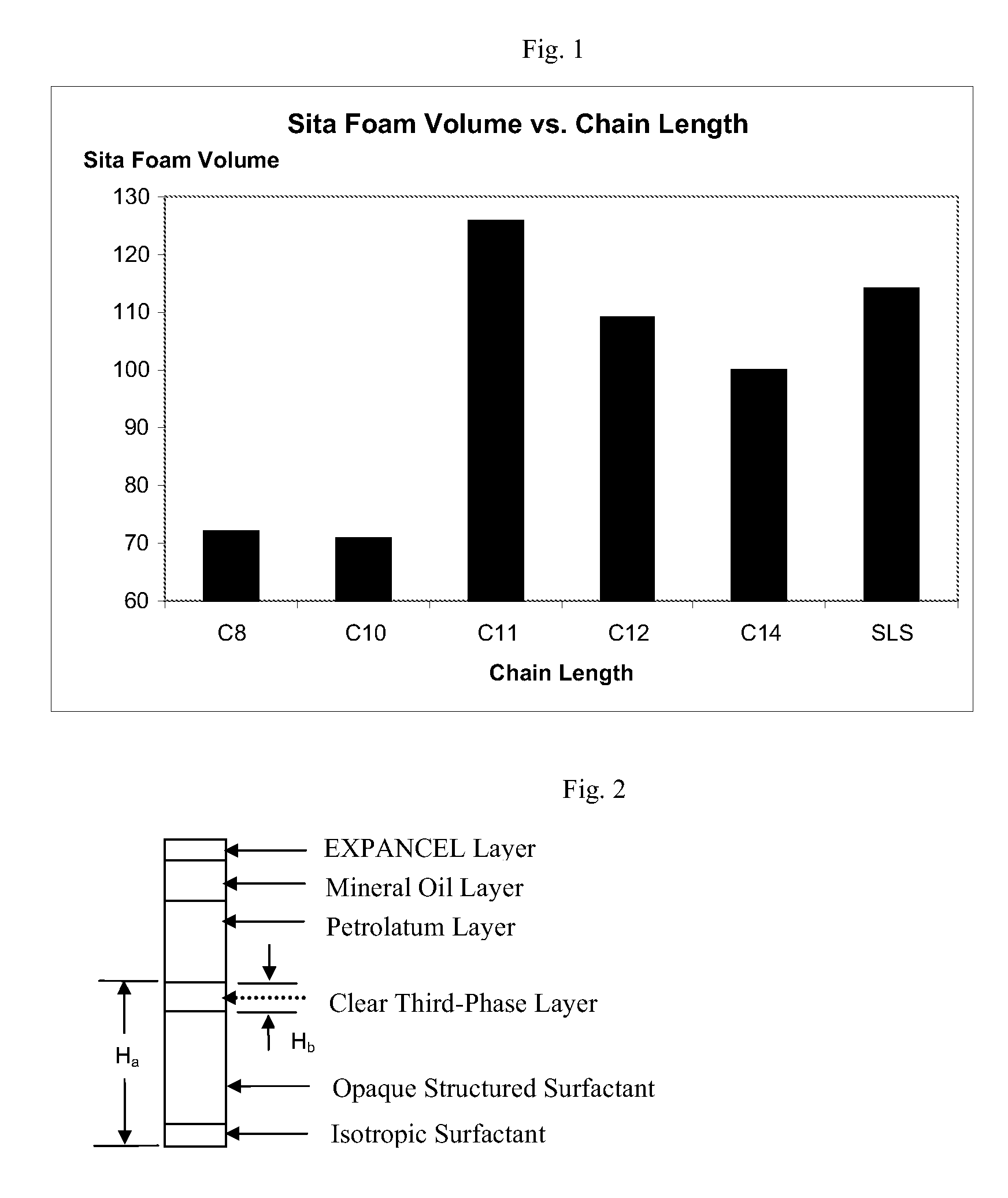
Personal Care Compositions Comprising Undecyl Sulfates
A personal care composition utilizing undecyl sulfates and / or ethoxylated undecyl sulfates (undeceth sulfates).
Owner:THE PROCTER & GAMBLE COMPANY
Topical nitric oxide donor compositions
InactiveUS6287601B1Reduced and failing organ functionPoor appetitePowder deliveryBiocideLipid formationEquine Species
Compositions and methods of the topical treatment of equine laminitis are disclosed. In particular, combinations of a fast acting nitric oxide (NO) donor, a sustained acting NO donor and an NSAID mixed in a lipid-based carrier are described. The application of such combinations to the affected areas, e.g., the hoofs and surrounding tissues, of an equine afflicted with laminitis provides relief from the debilitating effects of this painful, often life-threatening condition.
Owner:STREHKEHN INT LTD
Method for the treatment or prevention of dermatological disorders with a cyclooxygenase-2 inhibitor alone and in combination with a dermatological treatment agent and compositions therewith
A method for preventing or treating dermatological disorders and dermatological disorder-related complications in a subject involves a monotherapy with a Cox-2 inhibitor or a combination therapy with a Cox-2 inhibitor and a dermatological treatment agent. Also described are therapeutic compositions comprising a Cox-2 inhibitor and a dermatological treatment agent. Pharmaceutical compositions and kits for implementing the present method are also described.
Owner:PHARMACIA CORP
Antimicrobial Compositions and Methods for Locking Catheters
Antimicrobial compositions for use in locking catheters and other devices are provided. In some embodiments, the composition includes at least one alcohol, at least one biocidal agent which is not an alcohol, and one or more poloxamers; in other embodiments, the composition comprises at least one poloxamer and at least one alcohol. The composition can provide long-lasting antimicrobial activity. Methods of using the composition are also provided.
Owner:BECTON DICKINSON & CO
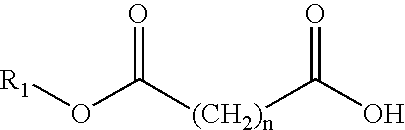
Compositions comprising lipophilic active compounds and method for their preparation
ActiveUS20090098200A1Quick releaseImprove bioavailabilityPowder deliveryBiocideHydrophilic polymersCompound (substance)
Compositions are provided comprising a lipophilic active compound, e.g., a human or veterinary drug or a nutraceutical, interwoven with a polymeric matrix formed by two or more polymers, wherein one of the polymers is an amphiphilic polymer and the other polymer is either an amphiphilic polymer with a different hydrophobic-hydrophilic balance or a hydrophilic polymer, and the active lipophilic compound has modified physicochemical properties. The composition forms colloidal nanodispersion upon contact with aqueous media.
Owner:SOLUBEST
Regulation of Mammalian Keratinous Tissue Using Skin and/or Hair Care Actives
Personal care compositions containing an active selected from the group consisting of phlorogine, phlorgine BG, deoxyArbutin, sucrose dilaurate, bakuchiol, pyrenoine, millet, arlatone dioic acid, cinnamic acid, ferulic acid, achromaxyl, methyl nicotinamide, oil soluble licorice extract, folic acid, undecylenic acid, zinc undecylenate, L-tryptophan, thiamine HCl, hexylresorcinol, lipidami red vine, dragosine, methyl gentisate, inositol, symdiol 68, laminaine, their salts, their derivatives, their precursors, and / or combinations thereof. Methods for regulating the condition of mammalian keratinous tissue by topically applying the personal care compositions are also provided.
Owner:THE PROCTER & GAMBLE COMPANY
Method of treating neurological diseases and etiologically related symptomology using carbonyl trapping agents in combination with medicaments
This invention defines a novel method for treatment of several neurological diseases and pathophysiologically related symptomology, said diseases including peripheral neuropathies, secondary symptomology of diabetes, Alzheimer's disease, Parkinson's disease, alcoholic polyneuropathy and age-onset symptomology, as well as analogous veterinary disease states. An opportunity exists for pharmacological intervention in some neurological diseases by use of water soluble, small molecular weight primary amine agents and chemical derivatives thereof. Examples of such primary pharmacological agents include 4-aminobenzoic acid and derivatives thereof. The present invention also includes: (1) oral use of optional non-absorbable polyamine polymeric co-agents such as chitosan, (2) oral use of optional known antioxidant co-agents and nutritional factors related thereto, and (3) use of the primary agents and co-agents noted above in optional combination with medicaments recognized as effective for treatment of the diseases addressed herein or symptoms thereof.
Owner:SECANT PHARMA
Multifunctional and biologically active matrices from multicomponent polymeric solutions
The present invention relates to a biologically active functionalized electrospun matrix to permit immobilization and long-term delivery of biologically active agents. In particular the invention relates to a functionalized polymer matrix comprising a matrix polymer, a compatibilizing polymer and a biomolecule or other small functioning molecule. In certain aspects the electrospun polymer fibers comprise at least one biologically active molecule functionalized with low molecular weight heparin. Examples of active molecules that may be used with the multicomponent polymer of the invention include, for example, a drug, a biopolymer, for example a growth factor, a protein, a peptide, a nucleotide, a polysaccharide, a biological macromolecule or the like. The invention is further directed to the formation of functionalized crosslinked matrices, such as hydrogels, that include at least one functionalized compatibilizing polymer capable of assembly.
Owner:UNIVERSITY OF DELAWARE
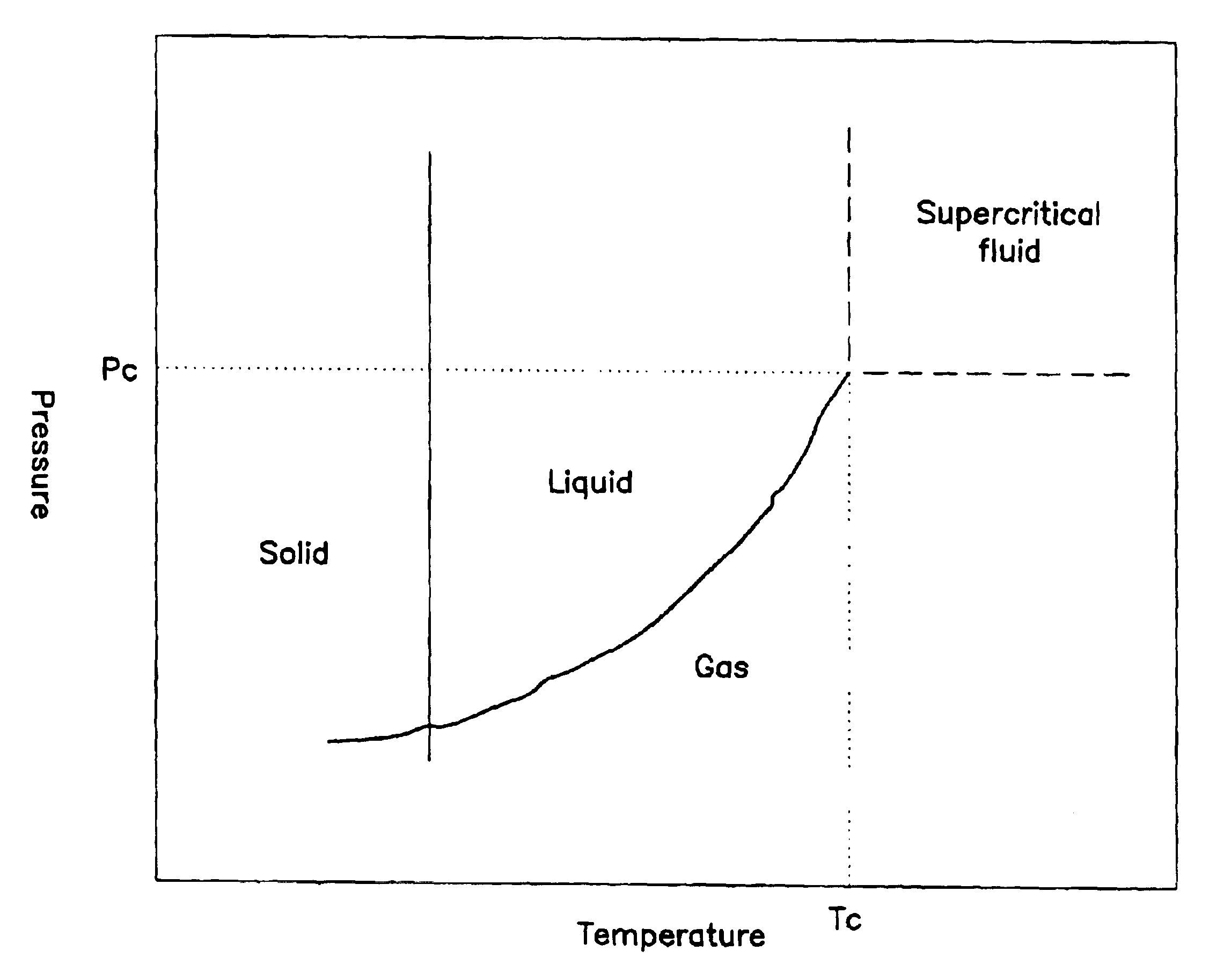
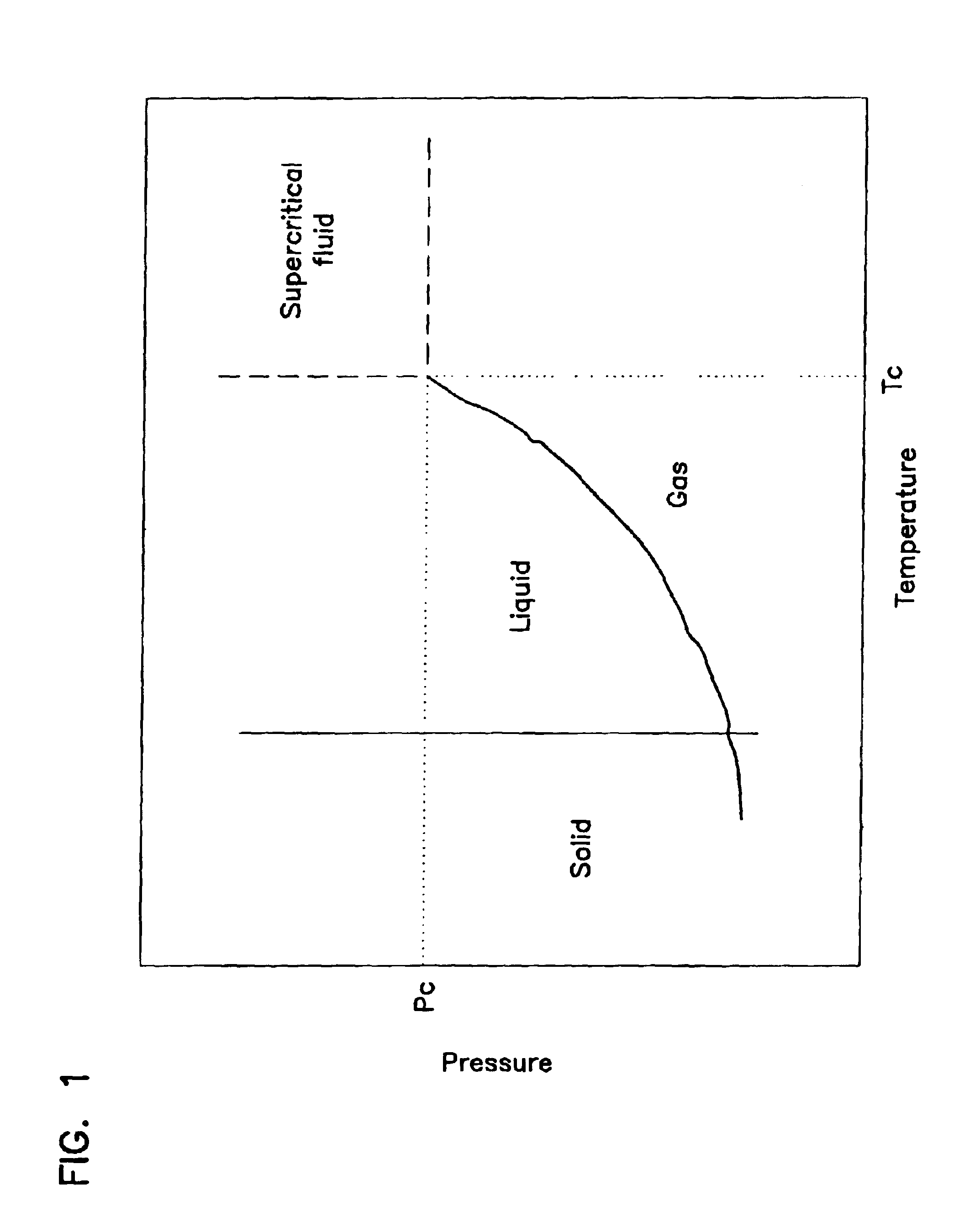
Critical fluid antimicrobial compositions and their use and generation
The present invention relates to antimicrobial compositions including a critical, near critical, or supercritical (densified) fluid and an antimicrobial agent, to methods of forming these compositions, and to methods employing these compositions. An antimicrobial agent can be generated in the presence of a densified fluid, for example, by reacting an oxidizing agent with a precursor to the antimicrobial agent.
Owner:ECOLAB USA INC
Alcohol-containing antimicrobial compositions having improved efficacy
InactiveUS20070281999A1Improve efficacyReduce skin irritationSalicyclic acid active ingredientsBiocideOrganic acidAlcohol
Antimicrobial compositions having a rapid antiviral and antibacterial effectiveness, and a persistent antiviral effectiveness, are disclosed. The antimicrobial compositions contain (a) a disinfecting alcohol, (b) a blend containing a C12 to C22 alcohol and an ethoxylated C12 to C22 alcohol, such as a cetearyl alcohol and cetereth-20 blend, a cetearyl alcohol, steareth-20, and steareth-10 blend, or a mixture thereof, (c) an optional organic acid, and (c) water.
Owner:DIAL CORPORATION
Methods and apparatus for drug delivery involving phase changing formulations
InactiveUS6528086B2Cleanly peeled from the skinMaintenance of such surfaceBiocideAdhesive dressingsHuman bodyPharmaceutical formulation
This invention relates to an apparatus and method of drug delivery on a human body surface. The formulation comprises a drug, a conversion agent capable of converting the formulation from a less solid phase to a coherent, soft, solid phase, and a vehicle medium or carrier for the drug and conversion agent. The drug formulation is applied to this human body surface in its less than solid phase and is subsequently converted to a soft solid phase while the drug is being delivered through the human body surface. After delivery of the drug is complete, the soft solid formulation can be removed or peeled from the body surface as a coherent solid formulation. The drug formulation provides control over drug delivery rates and allows the formulation to be removed without leaving a messy, residual formulation on the body surface.
Owner:CRESCITA THERAPEUTICS INC
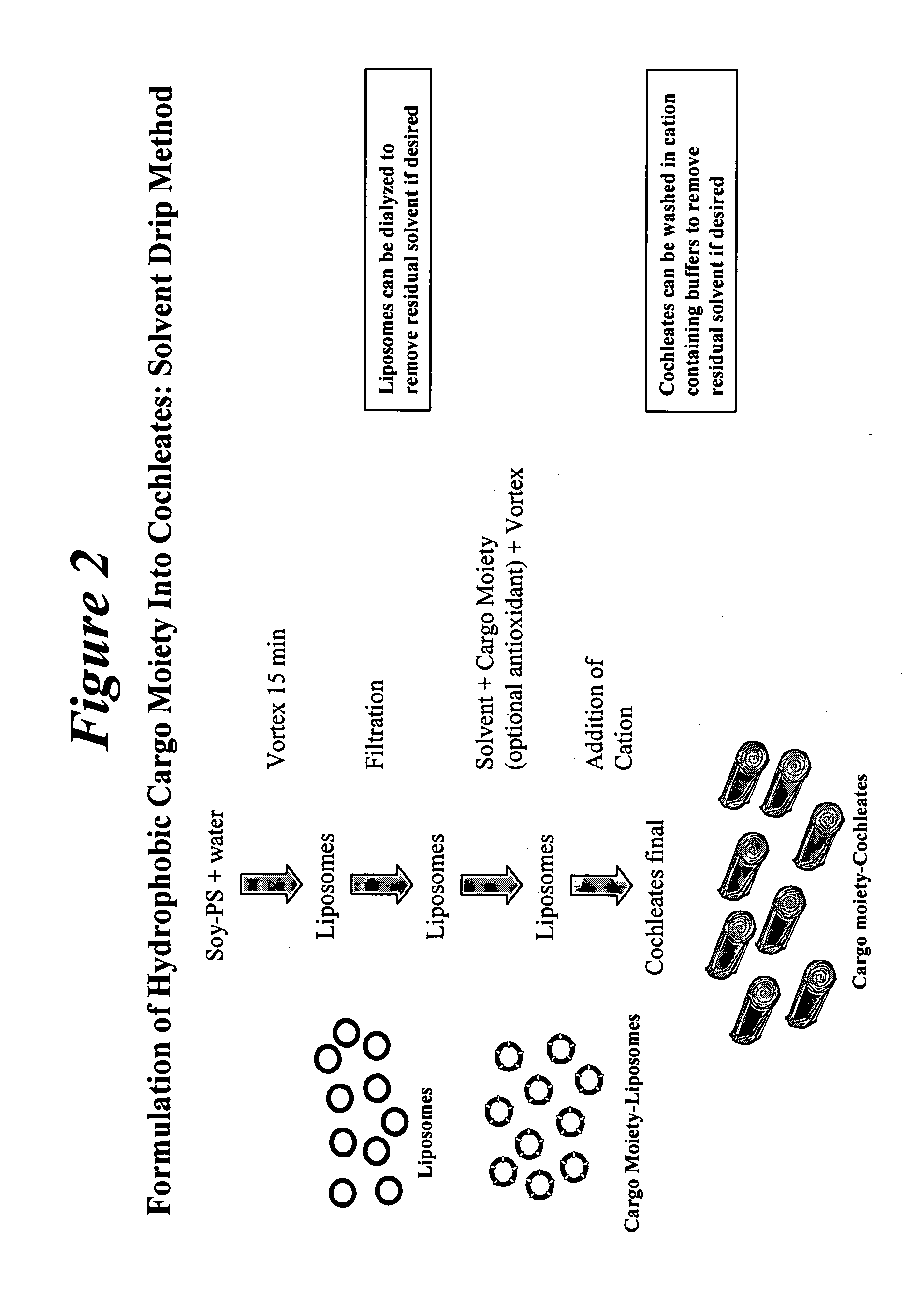
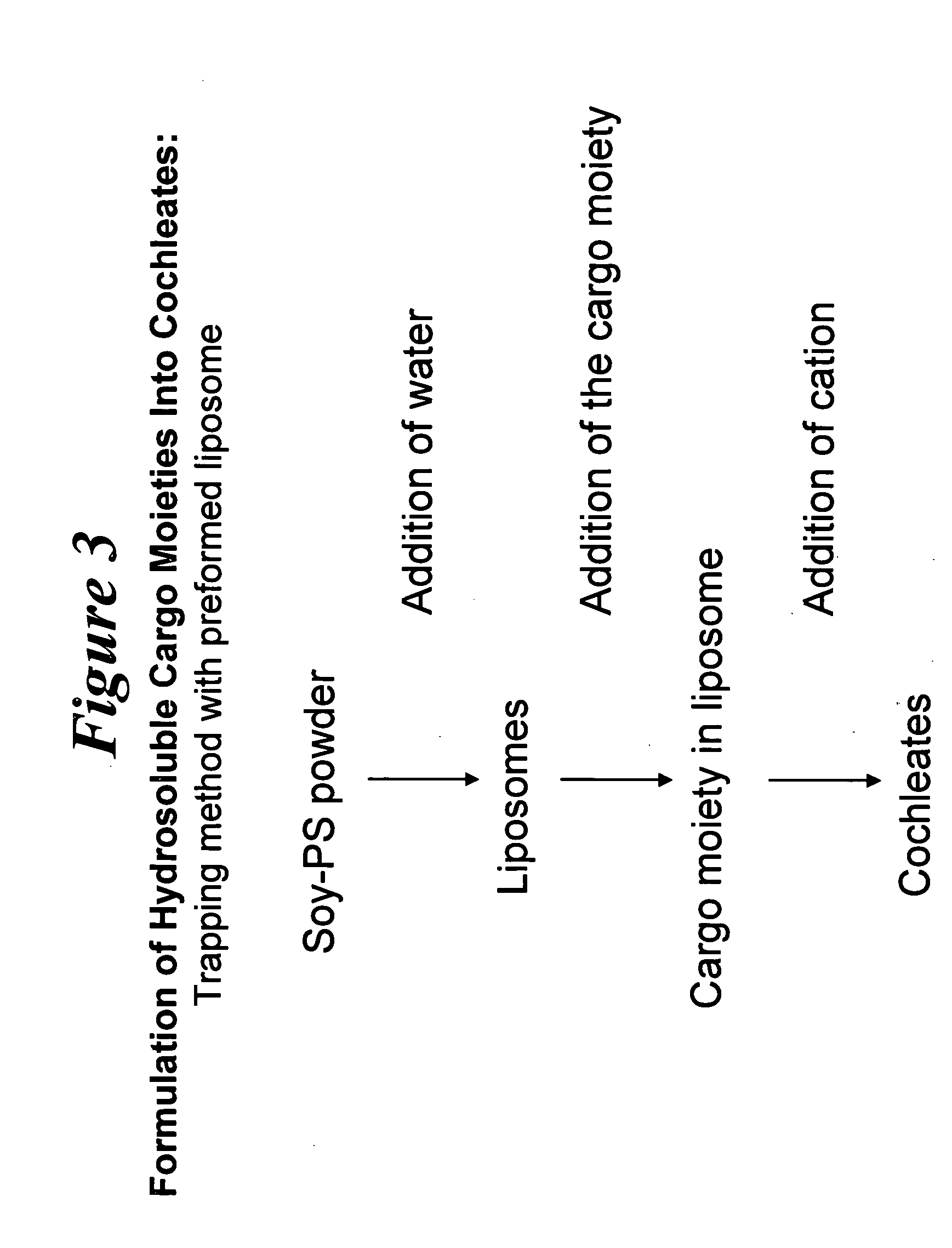
Novel encochleation methods, cochleates and methods of use
InactiveUS20050013854A1Efficiently and easily scaledQuick and efficientSalicyclic acid active ingredientsMicroencapsulation basedLipid formationDivalent metal
Disclosed are novel methods for making cochleates and cochleate compositions that include introducing a cargo moiety to a liposome in the presence of a solvent. Also disclosed are cochleates and cochleate compositions that include an aggregation inhibitor, and optionally, a cargo moiety. Additionally, anhydrous cochleates that include a protonized cargo moiety, a divalent metal cation and a negatively charge lipid are disclosed. Methods of using the cochleate compositions of the invention, including methods of administration, are also disclosed.
Owner:BIODELIVERY SCI +1
Methods for treating gastrointestinal disorders
InactiveUS20110097401A1Suitable for treatmentSalicyclic acid active ingredientsBiocideGastrointestinal disorderGastrointestinal tract
Provided herein are compositions and formulations suitable for the treatment of gastrointestinal disorders. Also provided are methods for treating, preventing, or alleviating disorders of the gastrointestinal tract, for example, those involving the esophagus.
Owner:MERITAGE PHARMA INC
Method and composition to reduce cancer incidence
InactiveUS6090414AReduce distractionsInhibition formationBiocideTetrapeptide ingredientsHydroxybenzoate EthersS oxidation
The five component composition consisting essentially of: (1) Water soluble antioxidant vitamin C or ascorbic acid, or any of its forms or derivatives, or mixtures thereof. (2) Oil soluble antioxidant vitamin E or Alpha-tocophorol, or any of its forms or derivatives, or mixtures thereof. (3) The element selenium, or a chemical (or composition) containing it, or mixtures thereof. The most preferred chemical containing selenium is dimethyl selenide and mixtures thereof. The words "dimethyl selenide" here and hereinafter mean dimethyl selenide and / or it's oxidation products, including dimethyl selenoxide. (4) A sulfur amino acid, in any form, or a sulfur peptide, or a sulfur protein, or any of their derivatives, or mixtures thereof. The mixture of methionine and cysteine, which contains as impurities some seleno-methionine and some selenocysteine, is preferred,-the tripeptide glutathione containing cysteine is also preferred. (5) Another antioxidant, other than vitamin C and other than vitamin E, which is synthetic or natural and water soluble or oil soluble, or a mixture of such antioxidants, or a combination of such forms thereof. The mixtures of butylated hydroxyanisole and ethoxyquin is preferred.
Owner:LIFE SCI LAB
Method and therapeutic/cosmetic topical compositions for the treatment of rosacea and skin erythema using a1-adrenoceptor agonists
ActiveUS20050165079A1ConstrictMinimize rednessSalicyclic acid active ingredientsBiocideAdrenergicHyper reactivity
The present invention is directed to the treatment of skin erythema as exhibited in rosacea and other conditions characterized by increased erythema (redness) of the skin. These conditions exhibit dilation of blood vessels due to a cutaneous vascular hyper-reactivity. In particular, the present invention is directed to a novel composition and method for the treatment of skin erythema using α1-adrenergic receptor (α1-adrenoceptor) agonists incorporated into cosmetic, pharmacological or dermatological compositions for topical application to the skin.
Owner:ALLERGAN INC
Sustained Release Dosage Forms For Delivery of Agents to an Oral Cavity of a User
Aspects of the invention include a sustained release dosage form that can be administered to an oral cavity, e.g., the mouth. In certain embodiments, the sustained release dosage form is formulated as a lozenge or gum that may be administered to an oral cavity of a user for the purpose of dissolving over a prolonged period of time and thereby delivering an essential oil component therein. In certain embodiments, the sustained release dosage form includes a beneficial agent and, therefore, not only provides for the prolonged delivery of an essential oil component to an oral cavity, but also provides for the sustained release of a beneficial agent thereto. In certain embodiments, the sustained release dosage form includes a biocompatible, water-insoluble polymer, e.g., ethylcellulose and an essential oil component, which are combined in such a manner so as to produce a dosage form that substantially dissolves over a prolonged period of time when positioned within an aqueous environment, such as an oral cavity of a user. In certain embodiments, the sustained release dosage form may include an additional water soluble agent, such as gum arabic, which may be included so as to further provide the dosage form with a desired dissolution characteristic. In certain embodiments, the dosage form may also include a beneficial agent to be delivered to the mouth. Methods of formulating such dosage forms and administering them to an oral cavity for the treatment of an adverse condition are also provided herein.
Owner:BENNES
Transdermal and topical administration of drugs using basic permeation enhancers
InactiveUS20050074487A1Improve throughputEffective amountCosmetic preparationsBiocideActive agentIrritation
Methods are provided for enhancing the permeability of skin or mucosal tissue to topical or transdermal application of pharmacologically or cosmeceutically active agents. The methods entail the use of a base in order to increase the flux of the active agent through a body surface while minimizing the likelihood of skin damage, irritation or sensitization. The permeation enhancer can be an inorganic or organic base. Compositions and transdermal systems are also described.
Owner:DERMATRENDS INC
Topical administration of basic antifungal compositions to treat fungal infections of the nails
Methods and topical pharmaceutical formulations are provided for the treatment of nail fungus (onychomycosis). The invention involves a pharmacologically active antifungal agent, plus a pharmaceutically acceptable base in a formulation having a pH of 7.5 to about 13.0, preferably about 8.0 to 11.5, and most preferably about 8.5 to 10.5. These basic formulations permeate the nail and are effective in treating fungal infections of the nail and surrounding tissues.
Owner:DERMATRENDS INC
Dosage form containing promethazine and another drug
A pharmaceutical dosage form which comprises promethazine and / or a pharmaceutically acceptable salt thereof and at least one second drug. The dosage form provides a plasma concentration within the therapeutic range of the at least one second drug over a period which is coextensive with a substantial part of the period over which the dosage form provides a plasma concentration within the therapeutic range of promethazine or salt thereof. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com