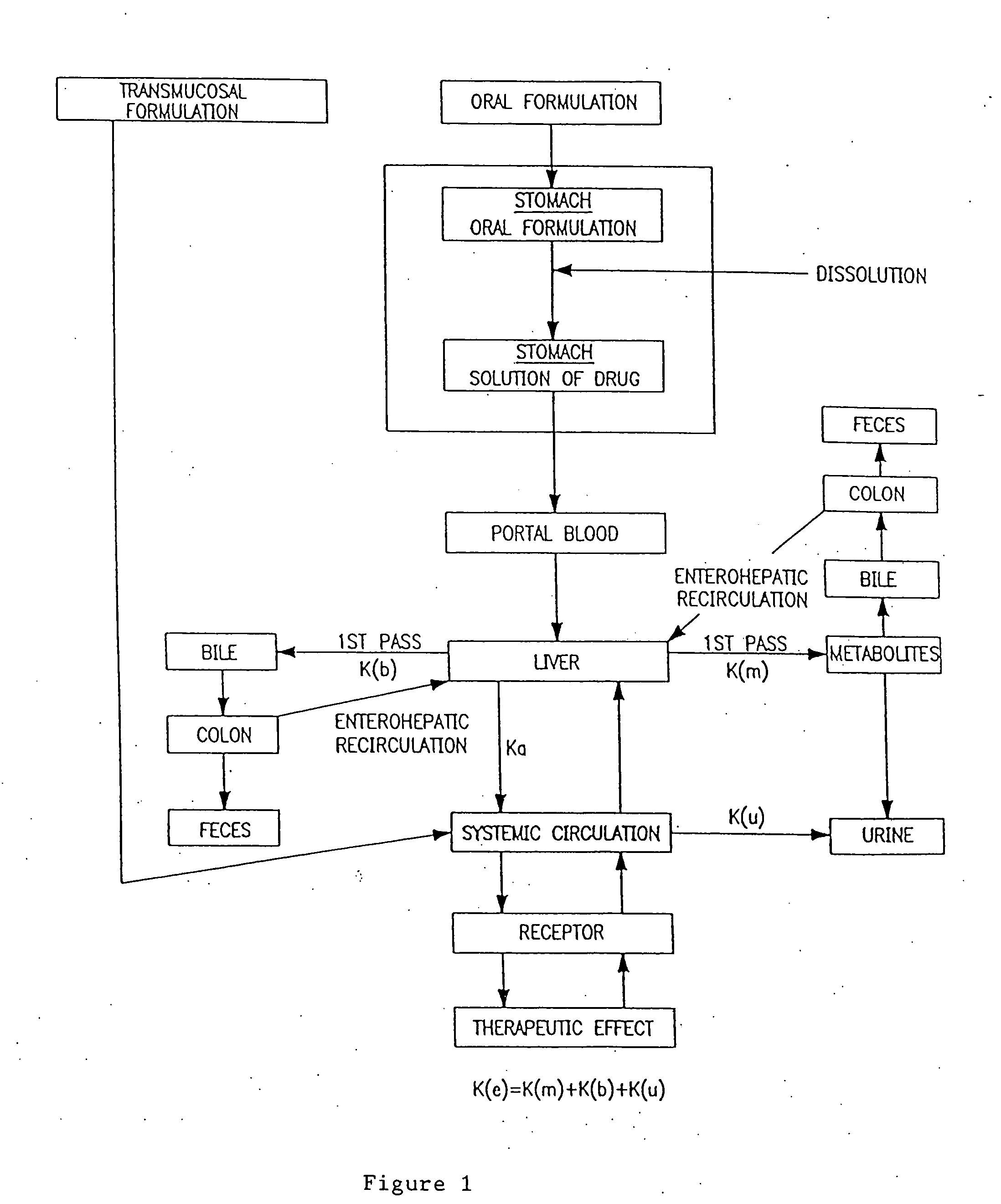
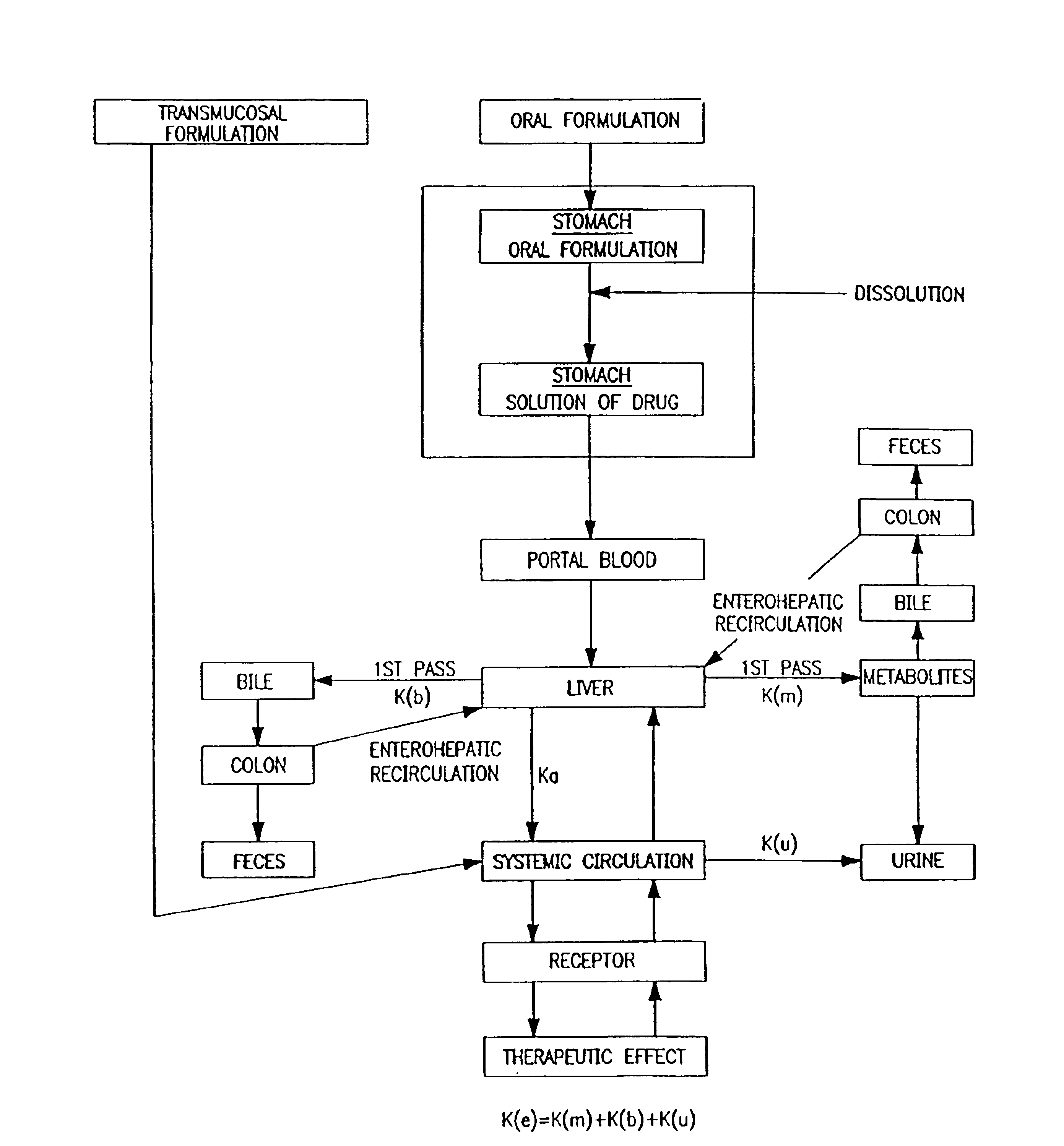
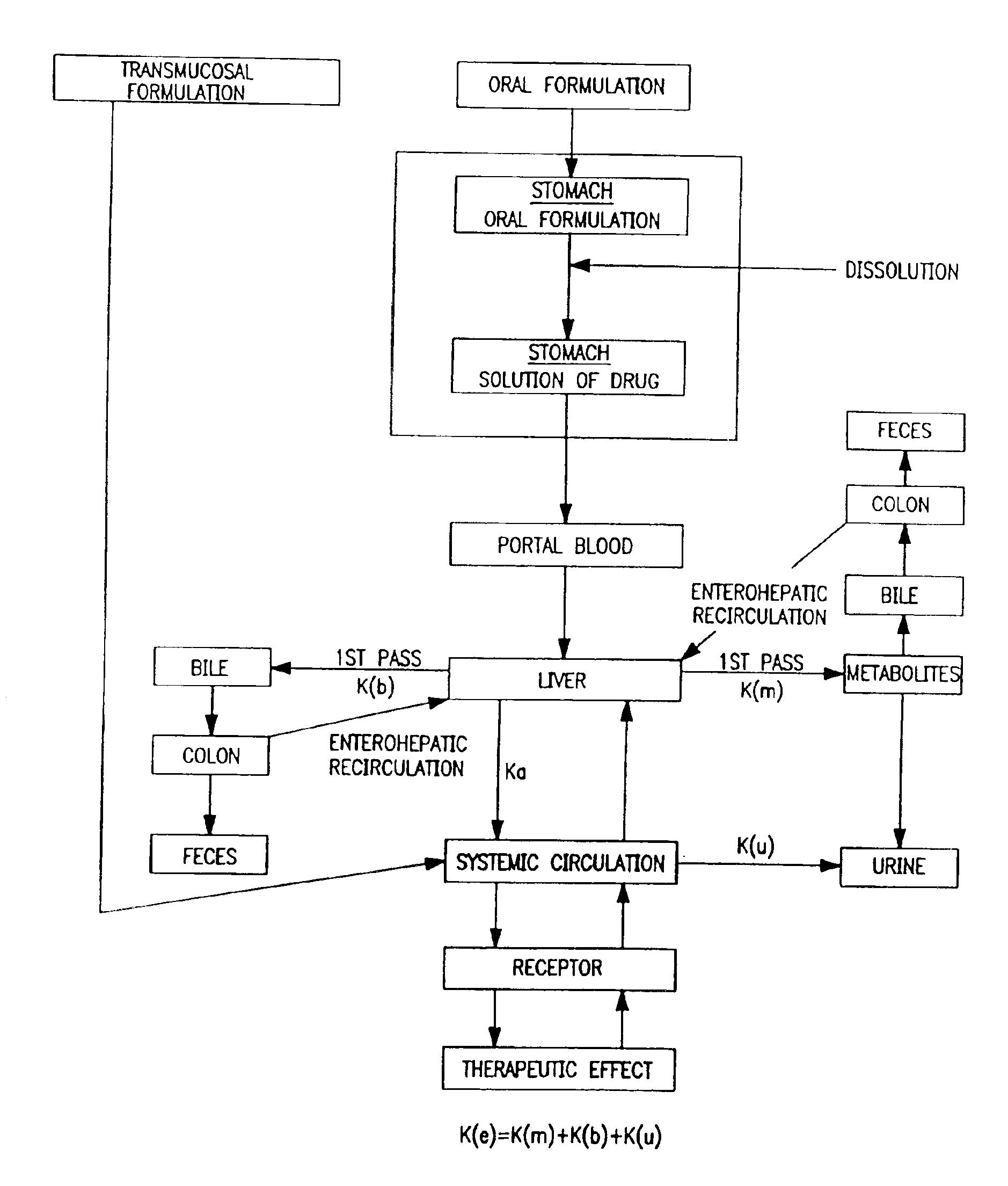
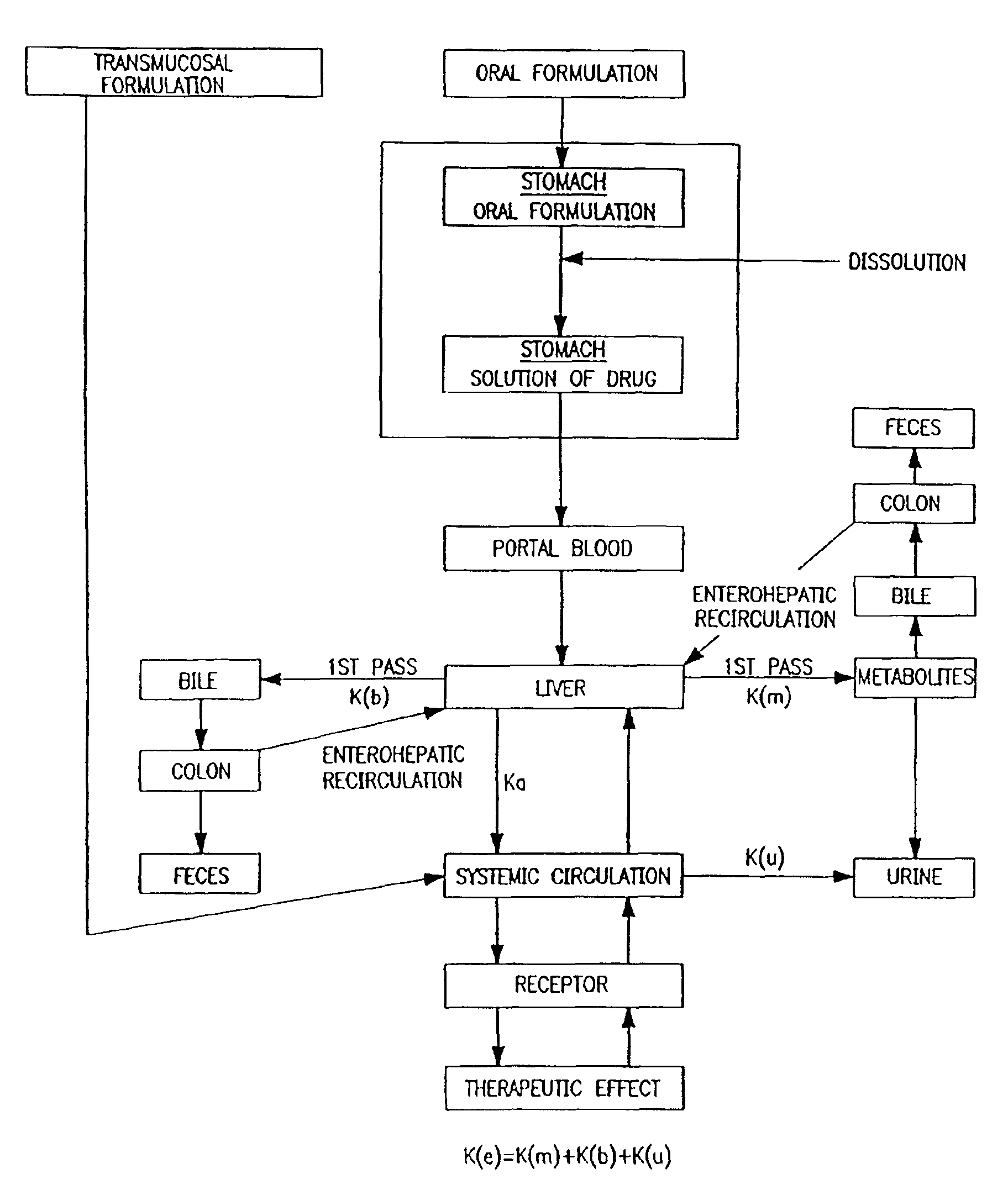
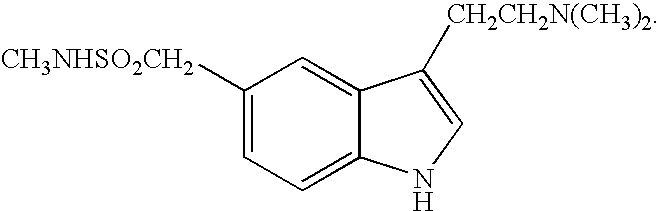
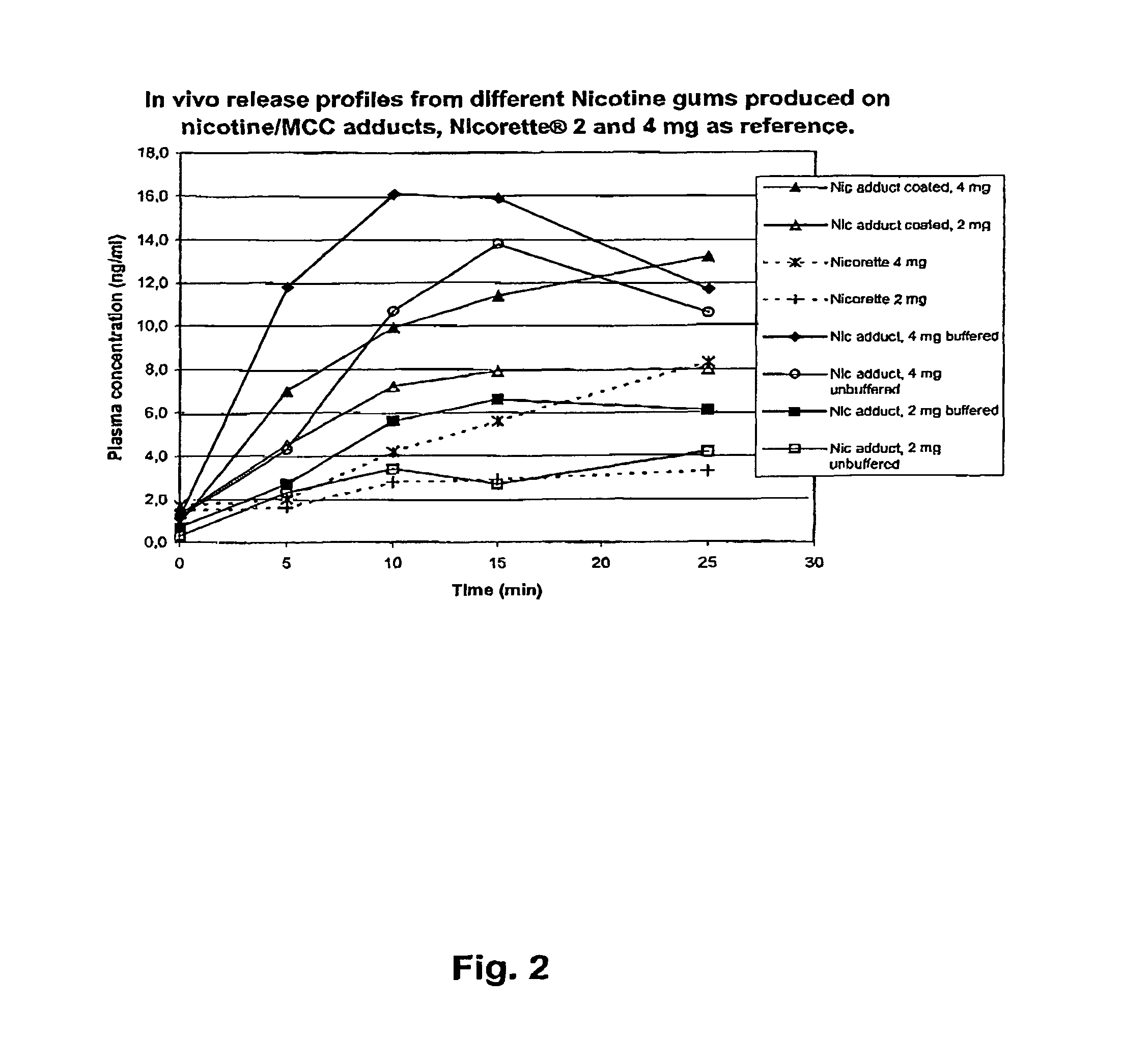
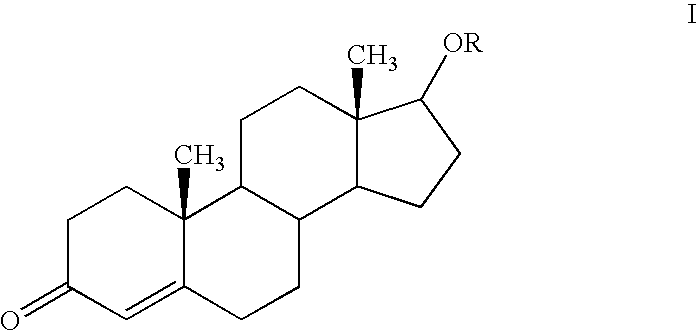
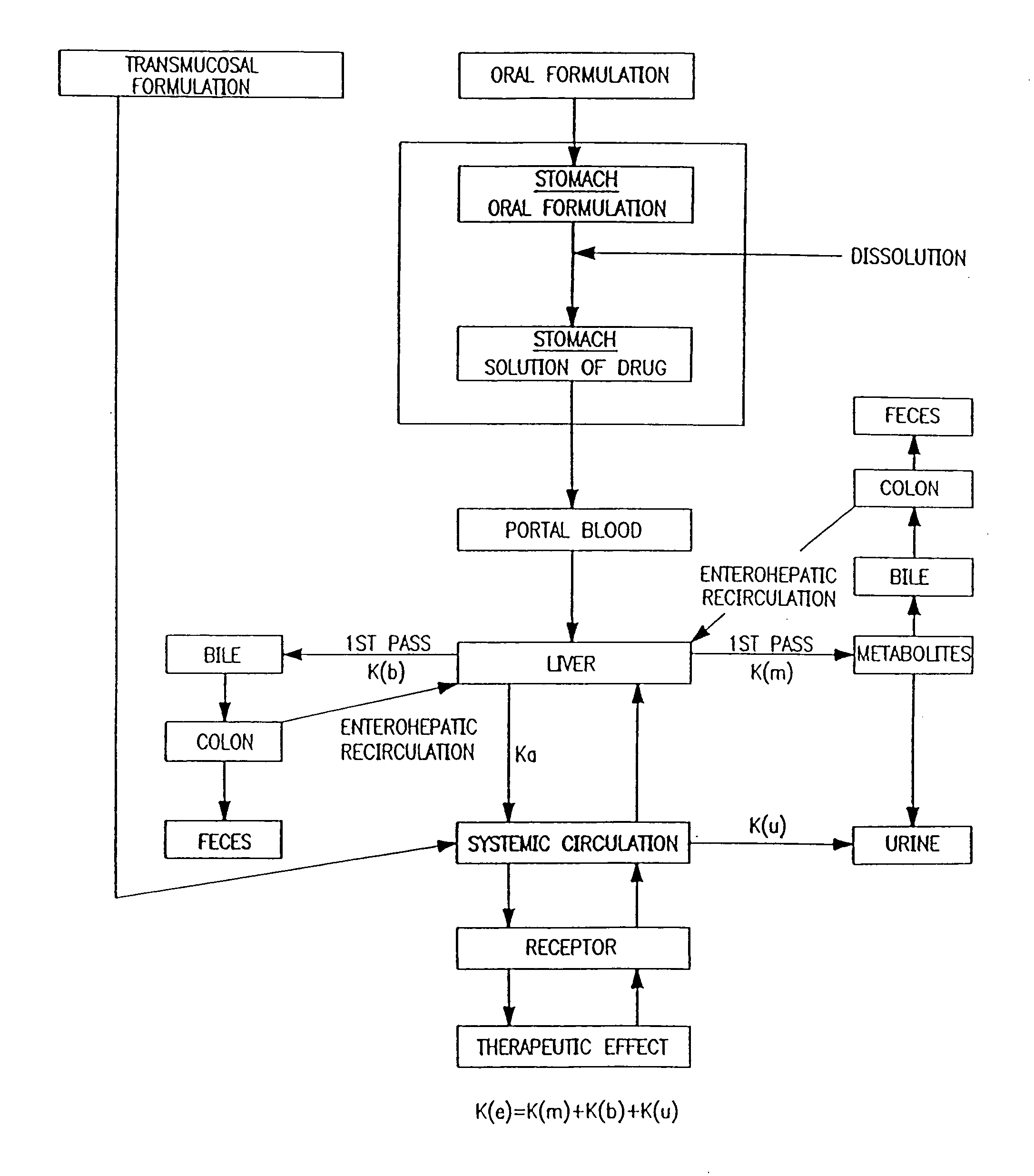
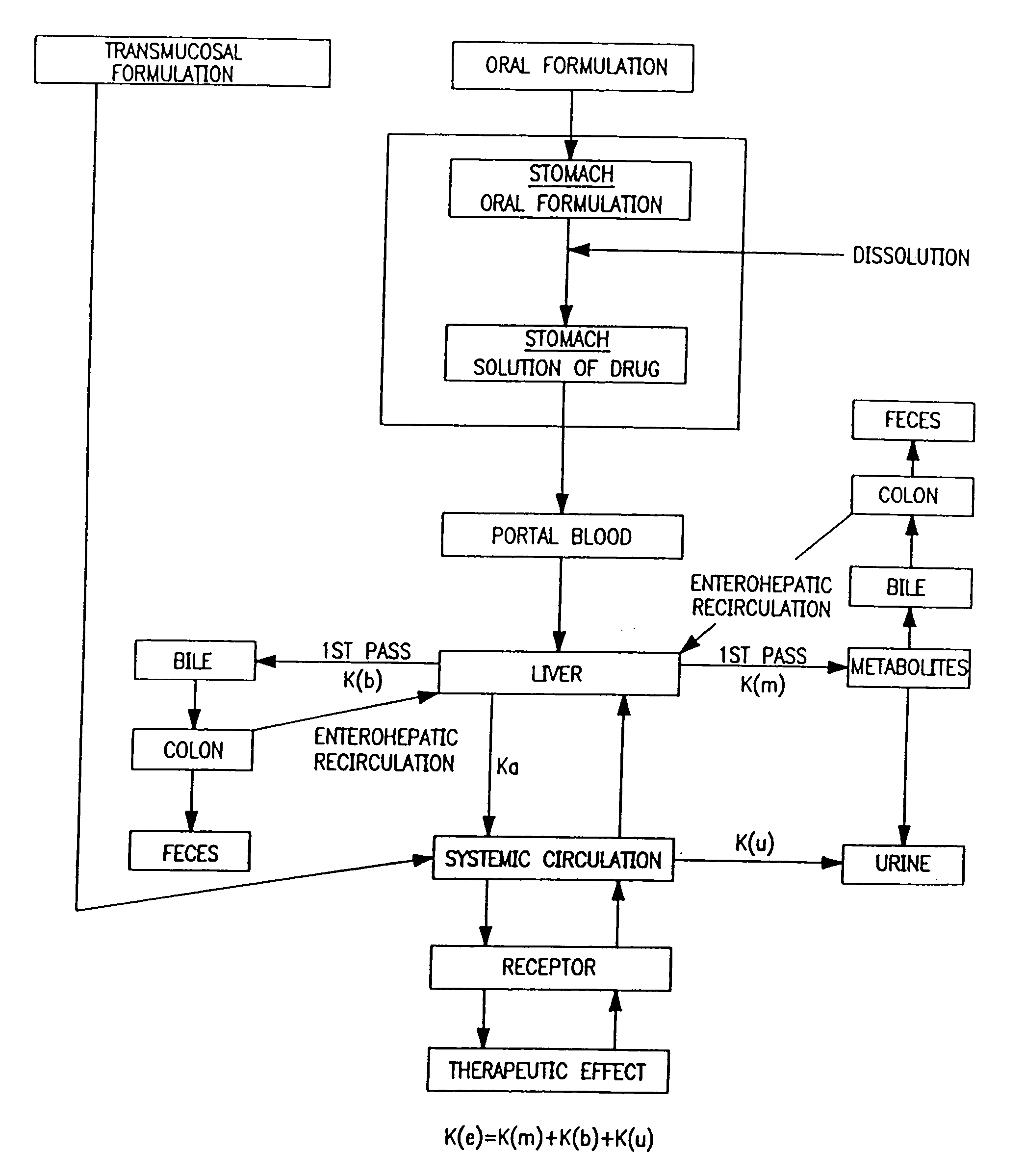
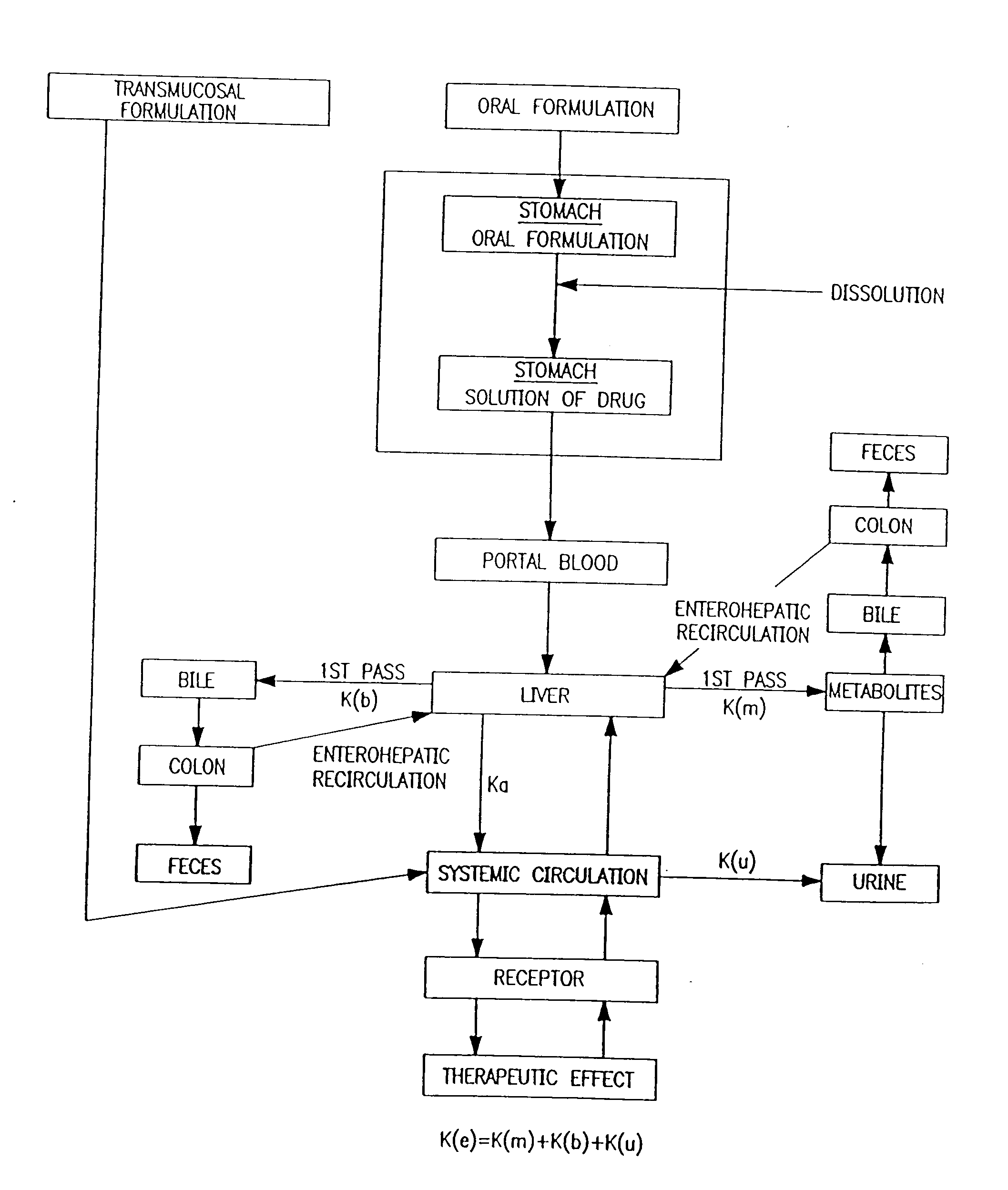
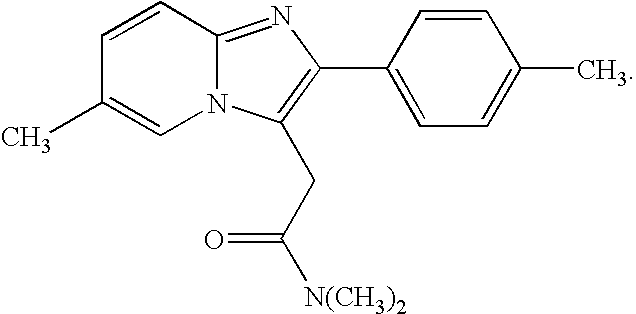
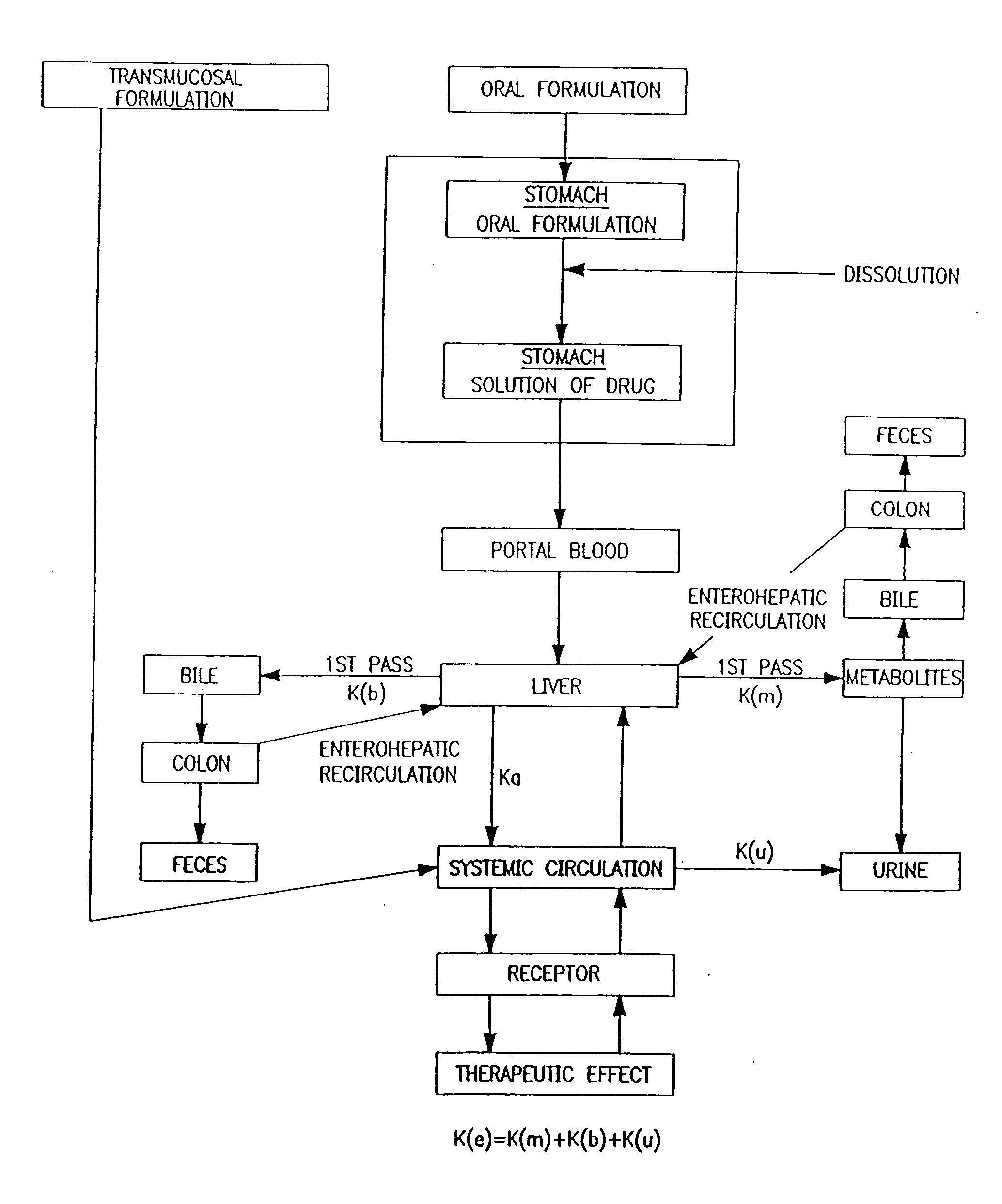
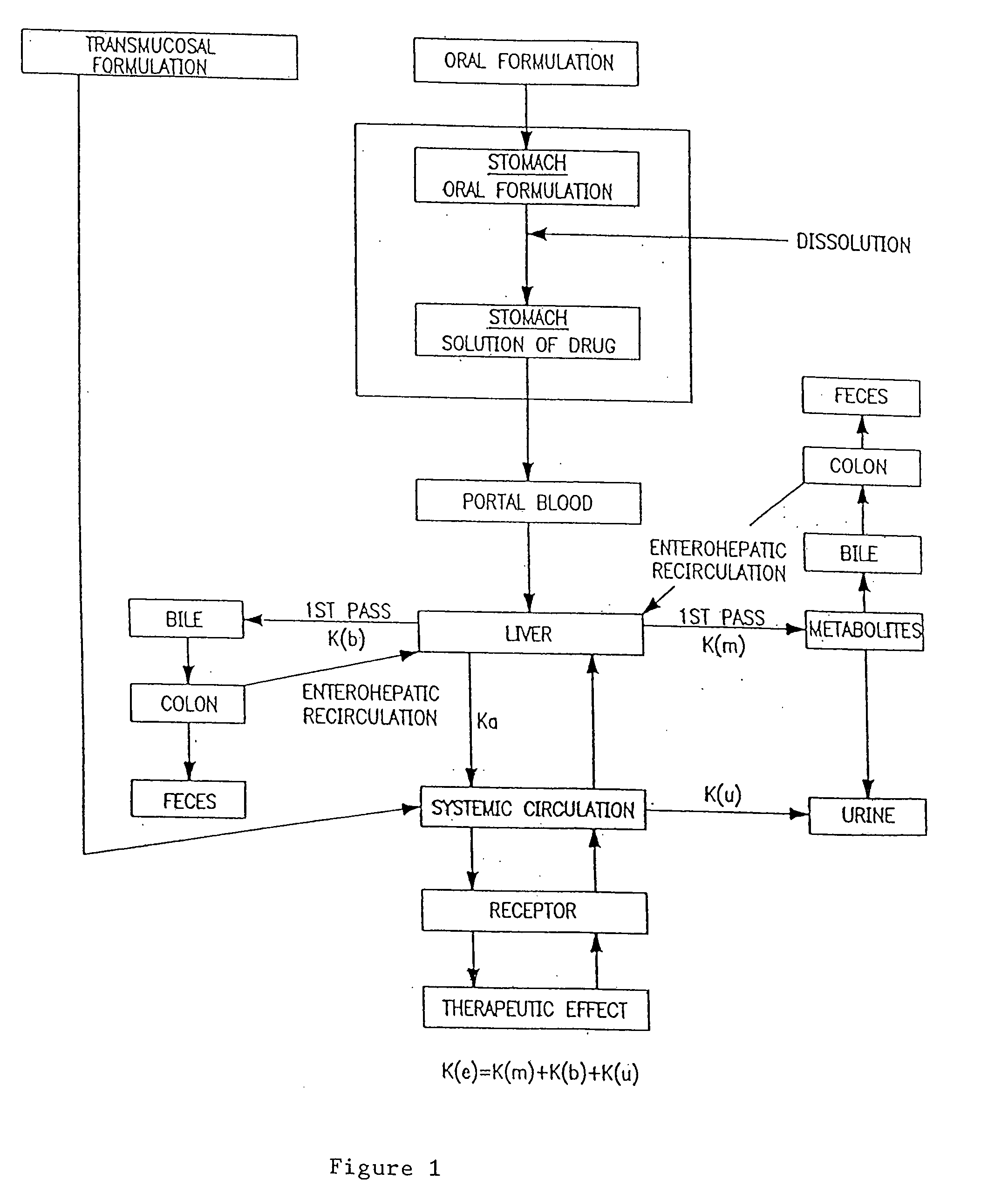
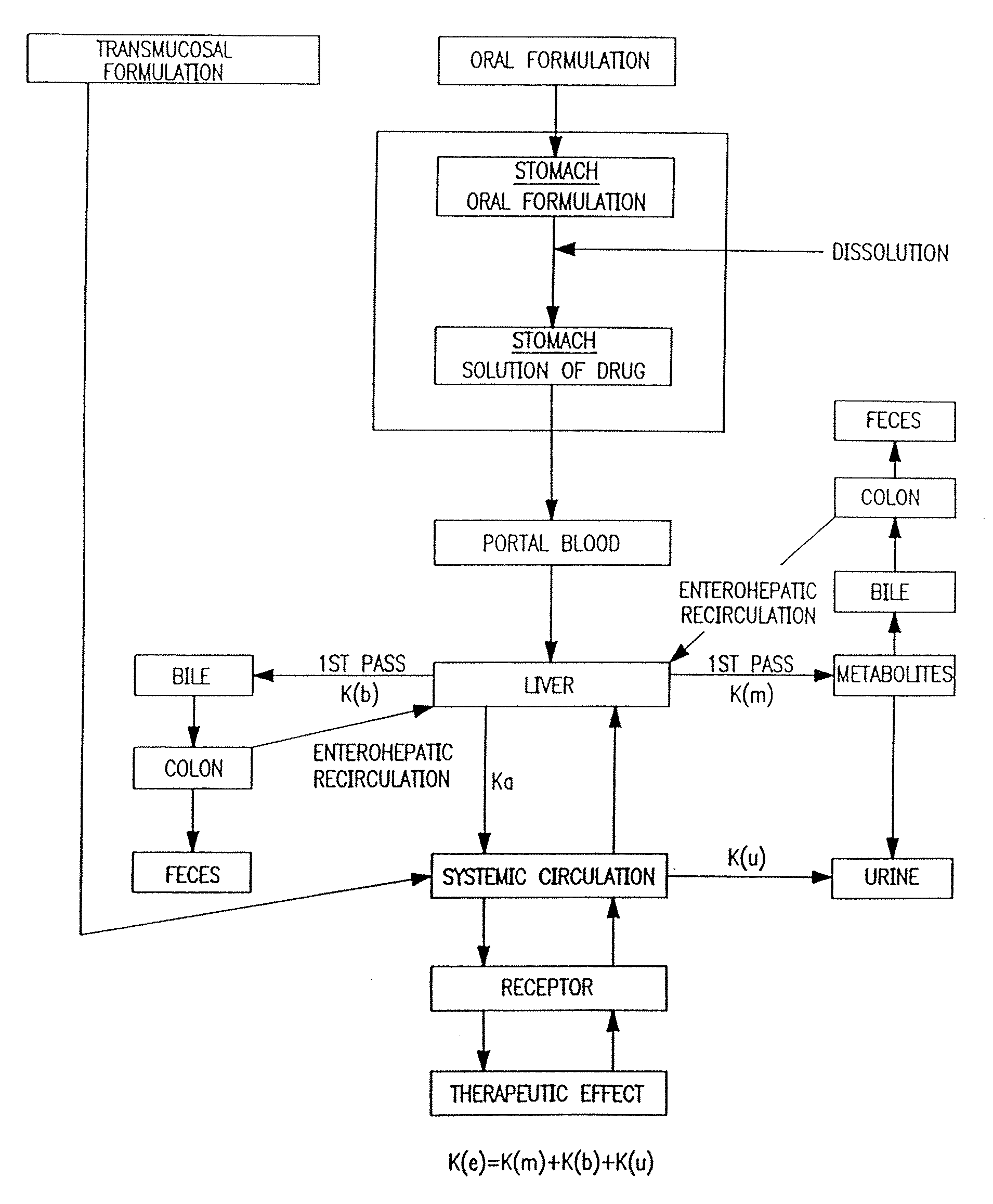
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
191 results about "Fast onset" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
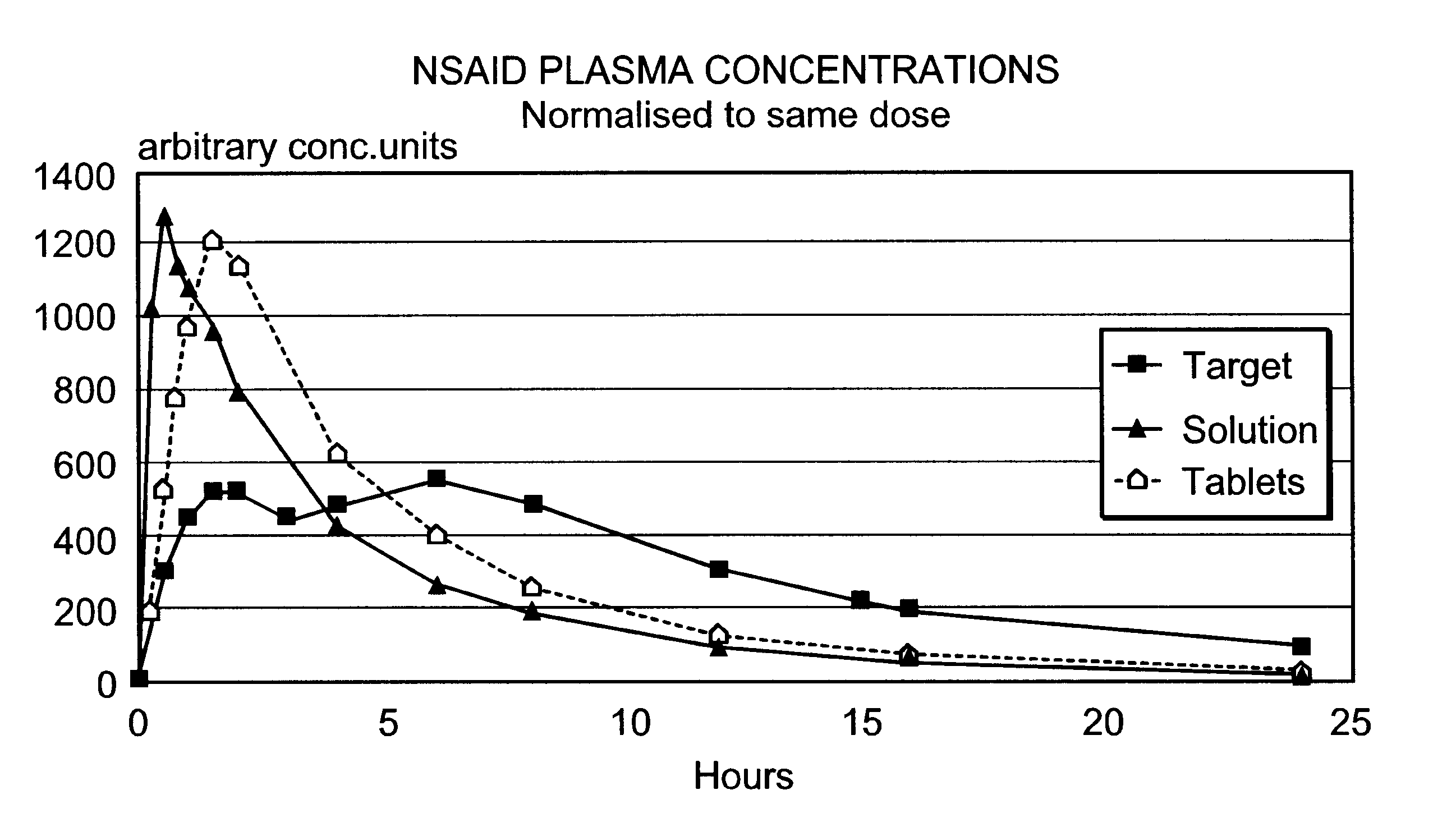
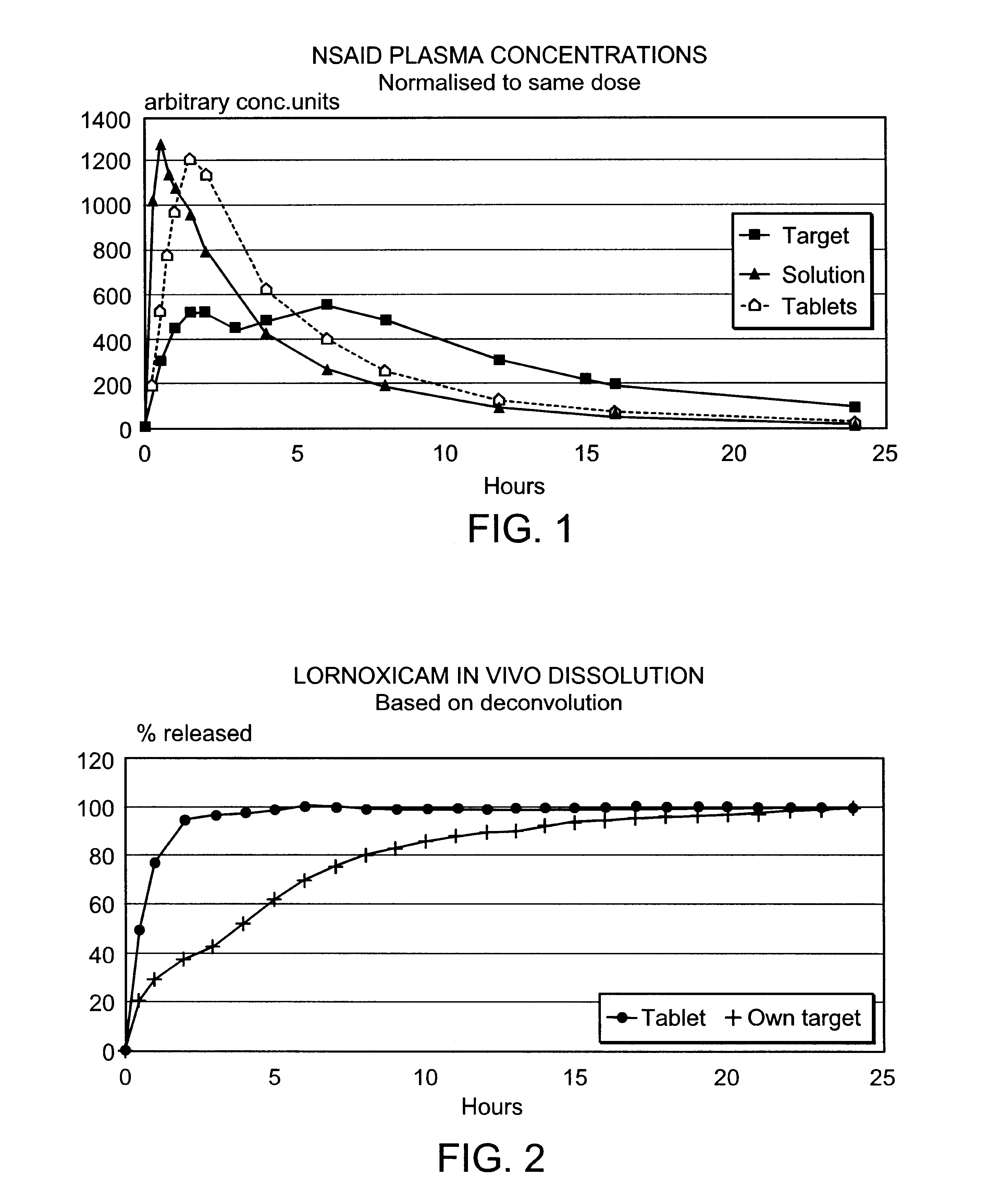
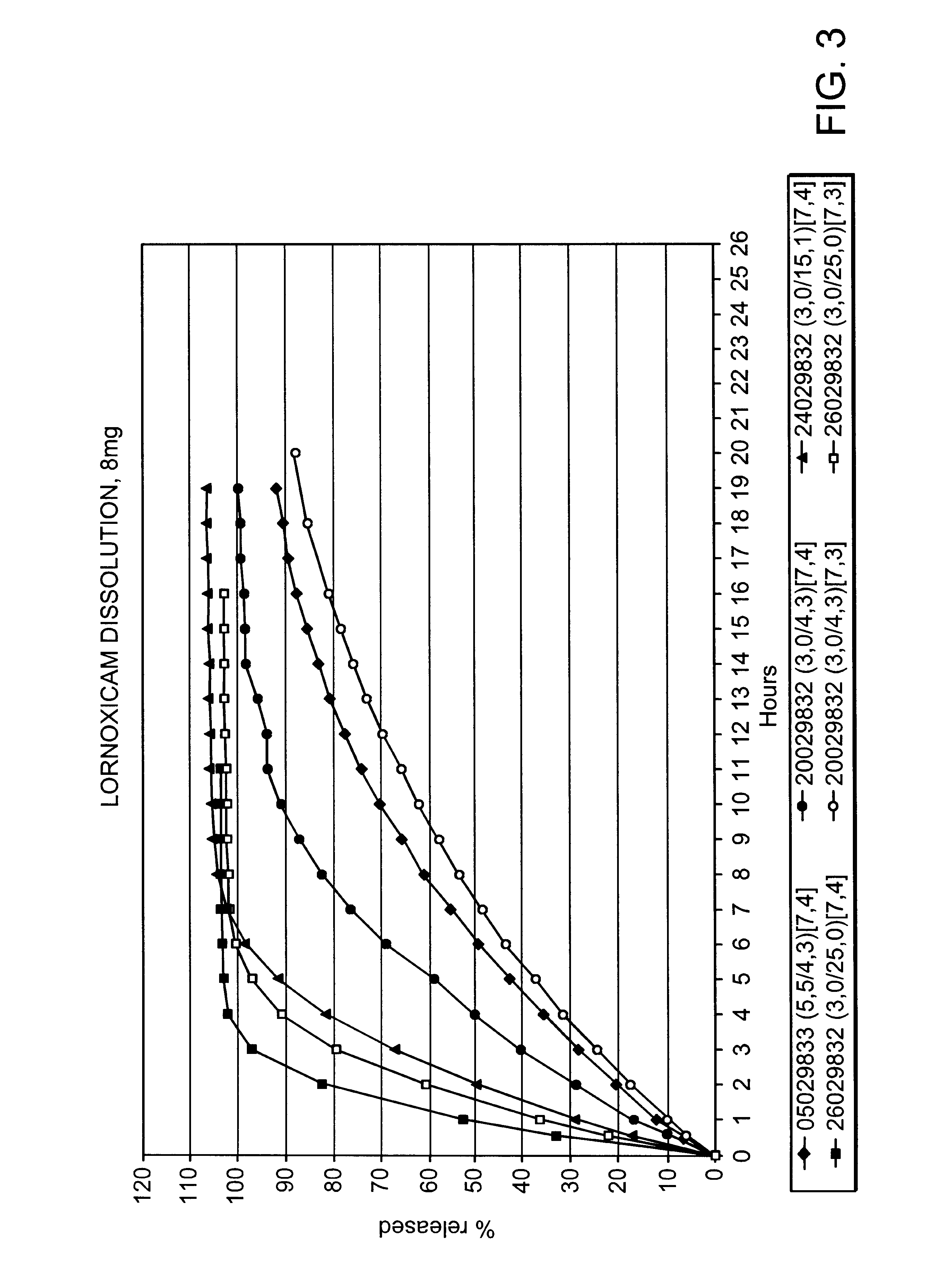
Modified release multiple-units compositions of non-steroid anti-inflammatory drug substances (NSAIDs)
InactiveUS6599529B1Keep low levelQuick releasePowder deliveryNervous disorderNon steroid anti inflammatory drugTherapeutic effect
An oral pharmaceutical modified release multiple-units composition for the administration of a therapeutically and / or prophylactically effective amount of a non-steroid anti-inflammatory drug substance to obtain both a relatively fast onset of the therapeutic effect and the maintenance of a therapeutically active plasma concentration for a relatively long period of time is disclosed.
Owner:TAKEDA PHARMA AS +1
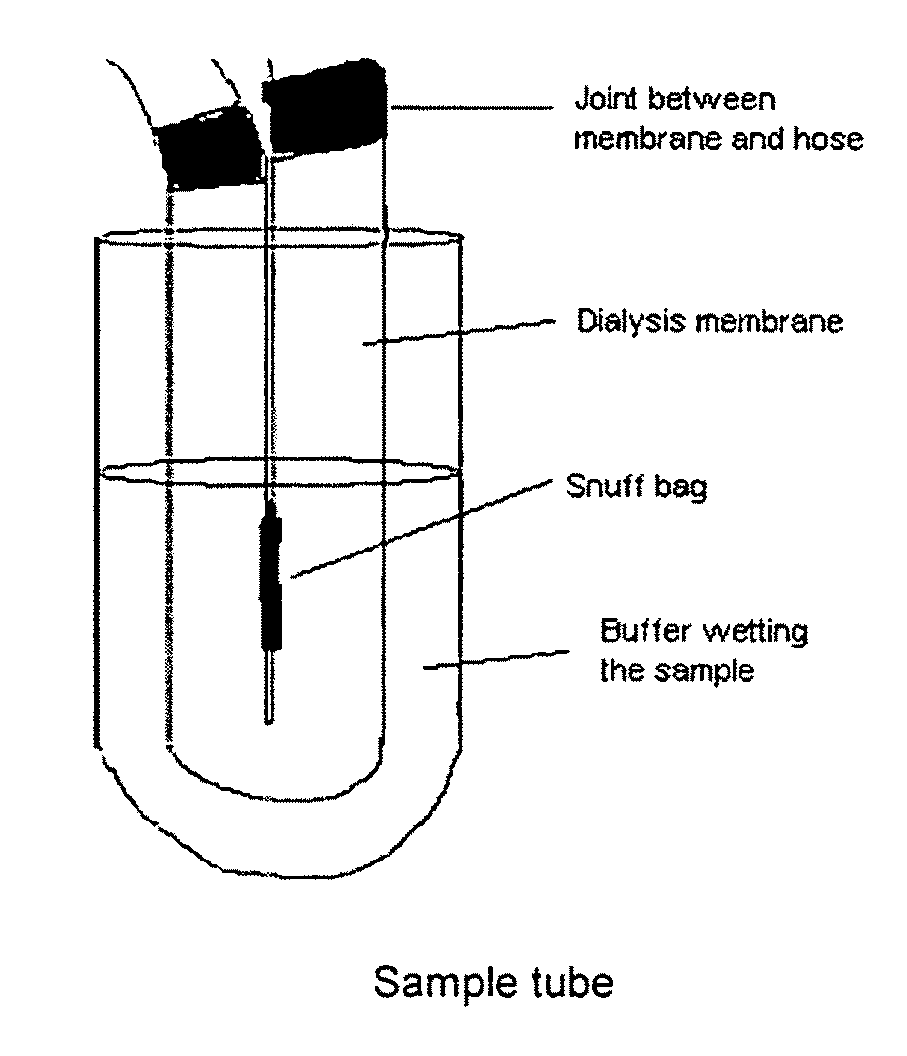
Snuff Composition
ActiveUS20090293895A1Quick effectRapid onsetPowder deliveryOrganic active ingredientsCellulosePharmacology
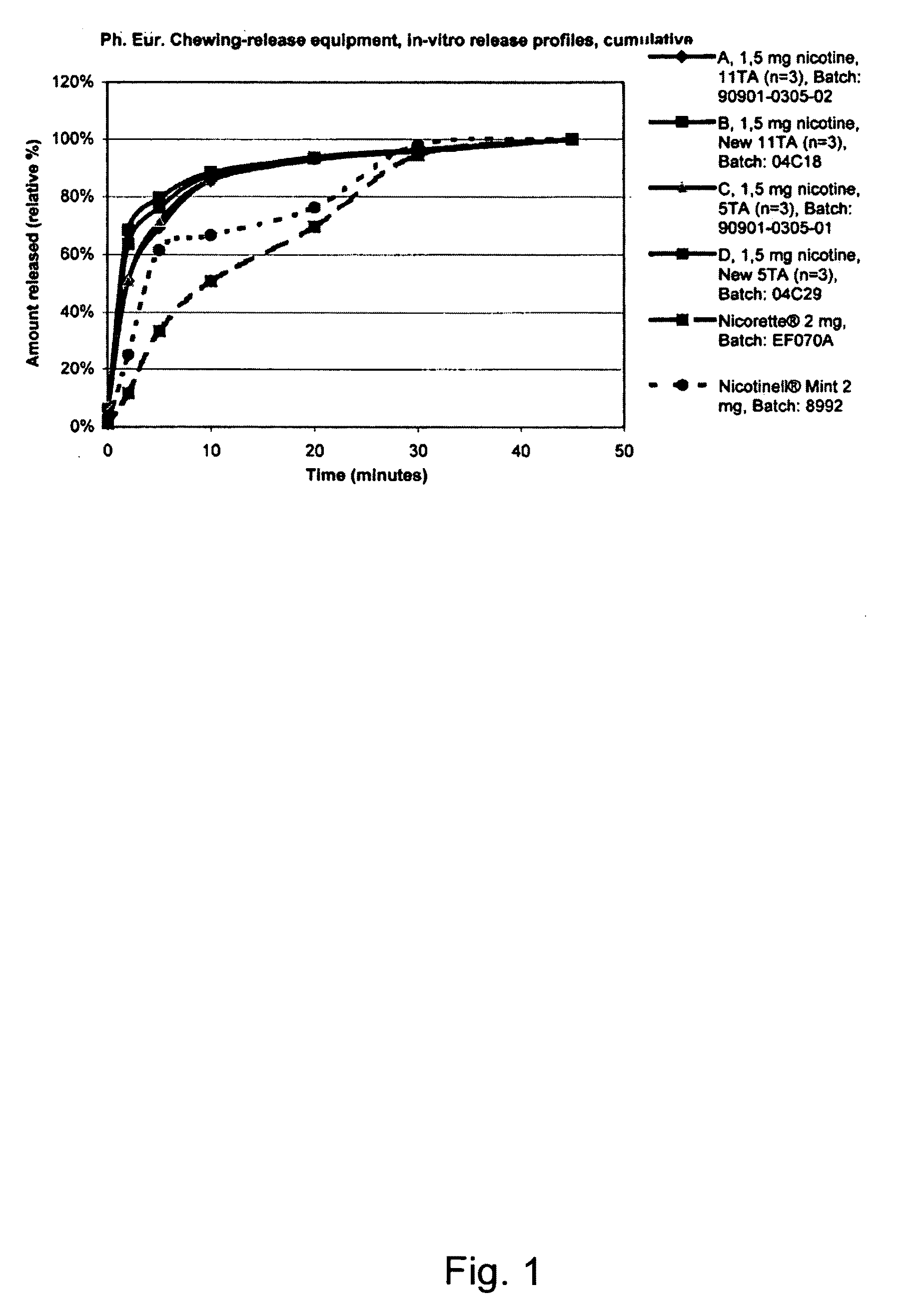
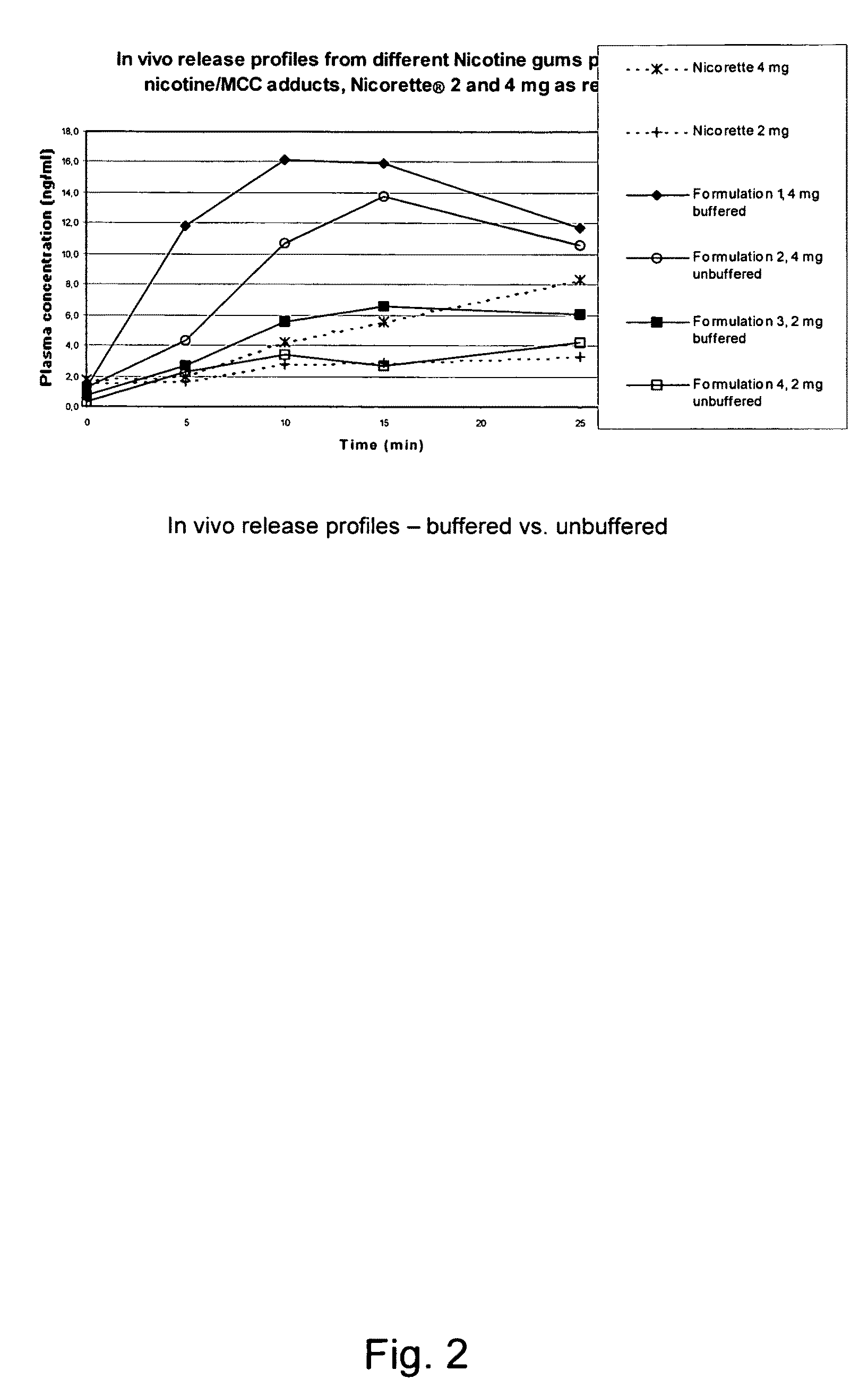
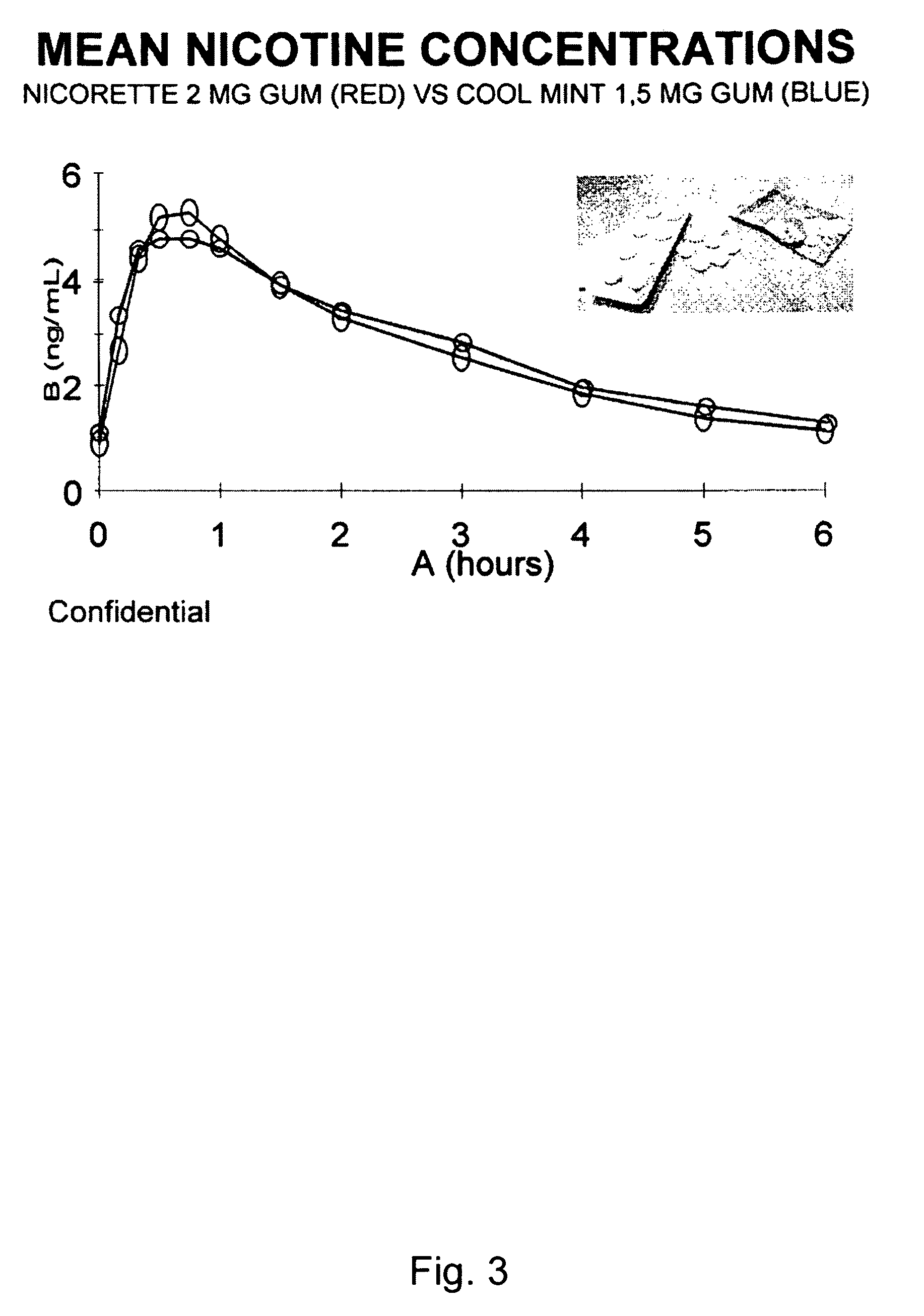
Use of a nicotine-cellulose combination for the preparation of a snuff composition for achievement of a fast onset of action of nicotine after application of the snuff composition to the oral cavity of a subject, wherein the composition has a high release rate so that when subjected to an in vitro dissolution test about 45% or more of the total content of nicotine is released within 30 minutes. Moreover, the invention relates to an improved snuff composition for application to the oral cavity.
Owner:MODORAL BRANDS INC
Modified release multiple-units dosage composition for release of opioid compounds
InactiveUS6159501AQuick releaseShort timePowder deliveryOrganic active ingredientsFast releaseAnalgesics effects
PCT No. PCT / DK97 / 00101 Sec. 371 Date Jun. 22, 1998 Sec. 102(e) Date Jun. 22, 1998 PCT Filed Mar. 7, 1997 PCT Pub. No. WO97 / 32573 PCT Pub. Date Sep. 12, 1997An oral pharmaceutical modified release multiple-units composition for the administration of an analgesically effective amount of an opoid. The composition comprises at least two fractions wherein individual units containing an opoid are coated with a sustained release coating. A first fraction is adapted to relatively fast release while a second fraction is adapted to a delayed release. Such compositions make possible to obtain both a relatively fast onset of the analgesic effect and the maintenance of analgesically active plasma concentration for a relatively long period of time. The invention further relates to a process for the preparation of a composition according to the invention.
Owner:TAKEDA PHARMA AS
Buccal, polar and non-polar sprays containing propofol
InactiveUS20050002867A1Fast absorptionRapid onsetBiocideHydroxy compound active ingredientsAerosol spraySolvent
Buccal aerosol sprays using polar and / or non-polar solvents have now been developed which provide propofol for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: propofol, a polar solvent and an optional flavoring agent; formulation II: propofol, a polar solvent, a propellant, and an optionally flavoring agent; formulation III: propofol, a non-polar solvent, and an optional flavoring agent; formulation IV: propofol, a non-polar solvent, a propellant, and an optional flavoring agent; formulation V: propofol, a mixture of a polar solvent and a non-polar solvent, and an optional flavoring agent; and formulation VI: propofol, a mixture of a polar solvent and a non-polar solvent, a propellant, and an optional flavoring agent.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray or capsule containing drugs for treating disorders of the central nervous system
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:SUDA
Buccal, polar and non-polar spray or capsule
InactiveUS6998110B2Rapid onsetFast absorptionBatteries circuit arrangementsAerosol deliverySolventPharmacology
Owner:SUDA
Buccal, polar and non-polar spray or capsule containing drugs for treating pain
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:SUDA
Chewing Gum Compositions Providing Rapid Release of Nicotine
PendingUS20100061940A1Quick releaseIncrease concentrationOrganic active ingredientsNervous disorderCelluloseGum base
Use of a nicotine-cellulose combination and a gum base for the preparation of a chewing gum composition for achieving a fast onset of nicotine effect after initiation of chewing the chewing gum composition by a subject. The chewing gum composition is preferably prepared by direct compression and it does not disintegrate during chewing. The invention also relates to chewing gum compositions comprising nicotine, which compositions provide a rapid release of nicotine.
Owner:NICONOVUM AB
Buccal, polar and non-polar spray or capsule containing cardiovascular or renal drugs
InactiveUS20050025713A1Fast absorptionRapid onsetElcosanoid active ingredientsAerosol deliverySolventBioactive compound
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
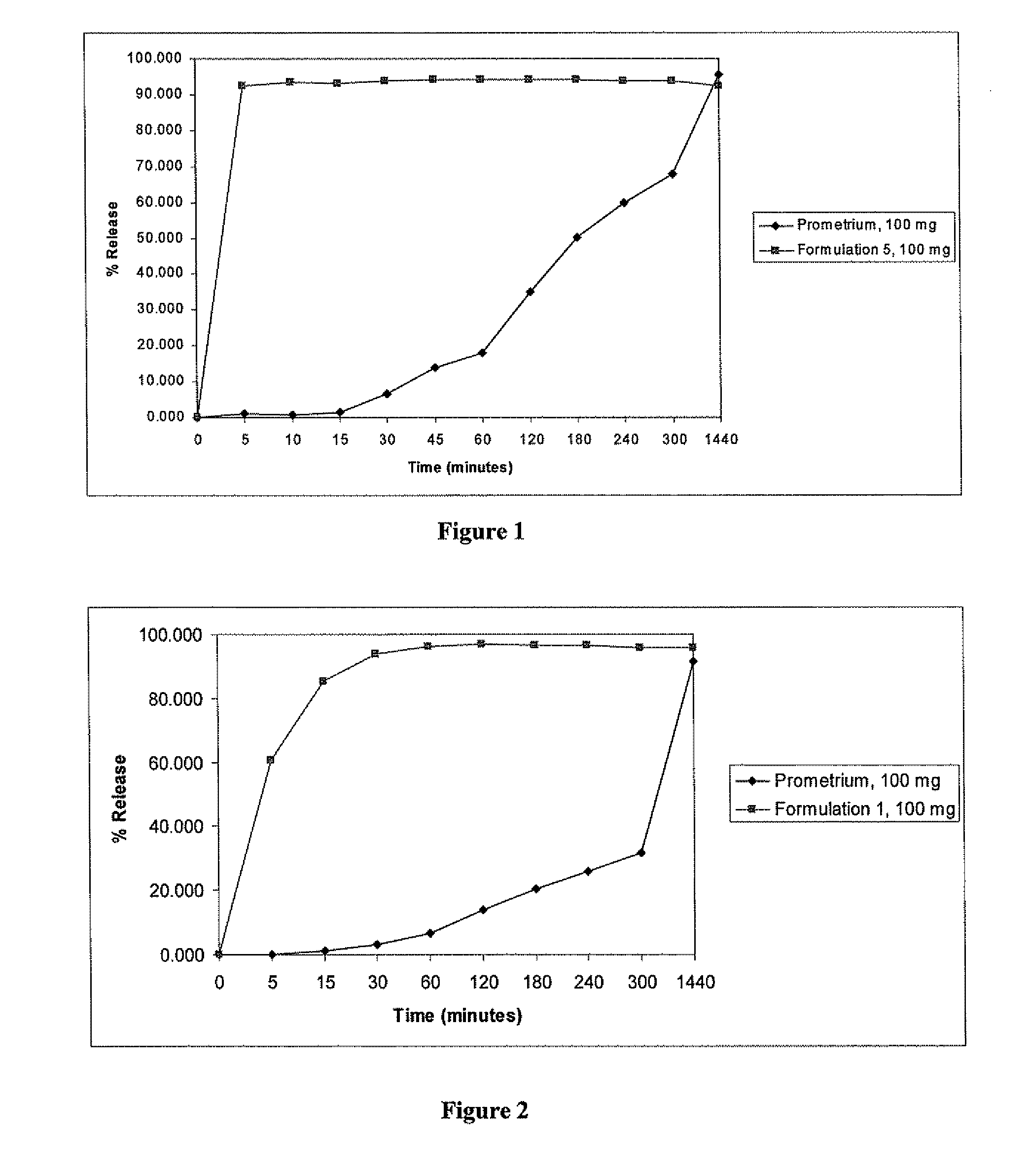
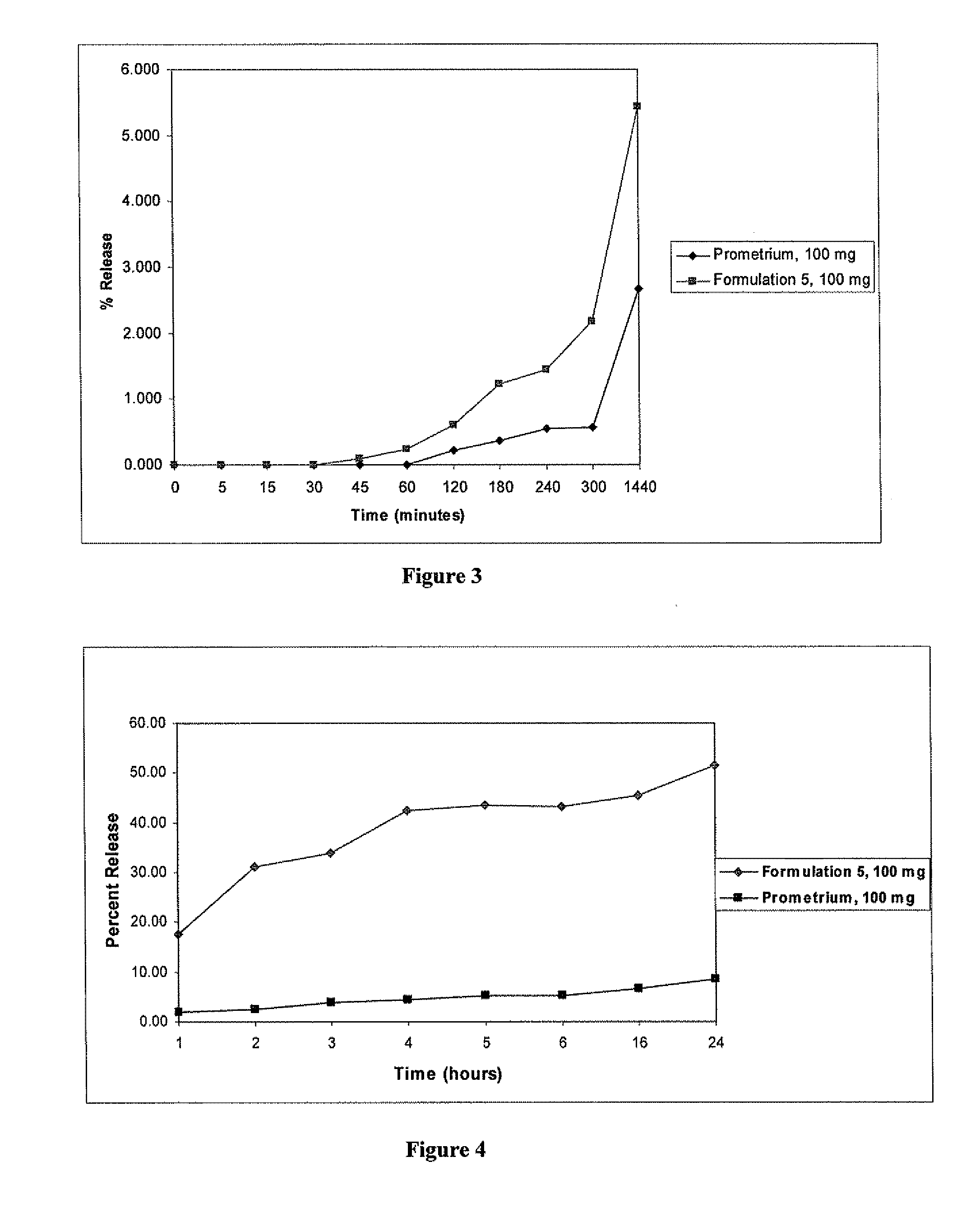
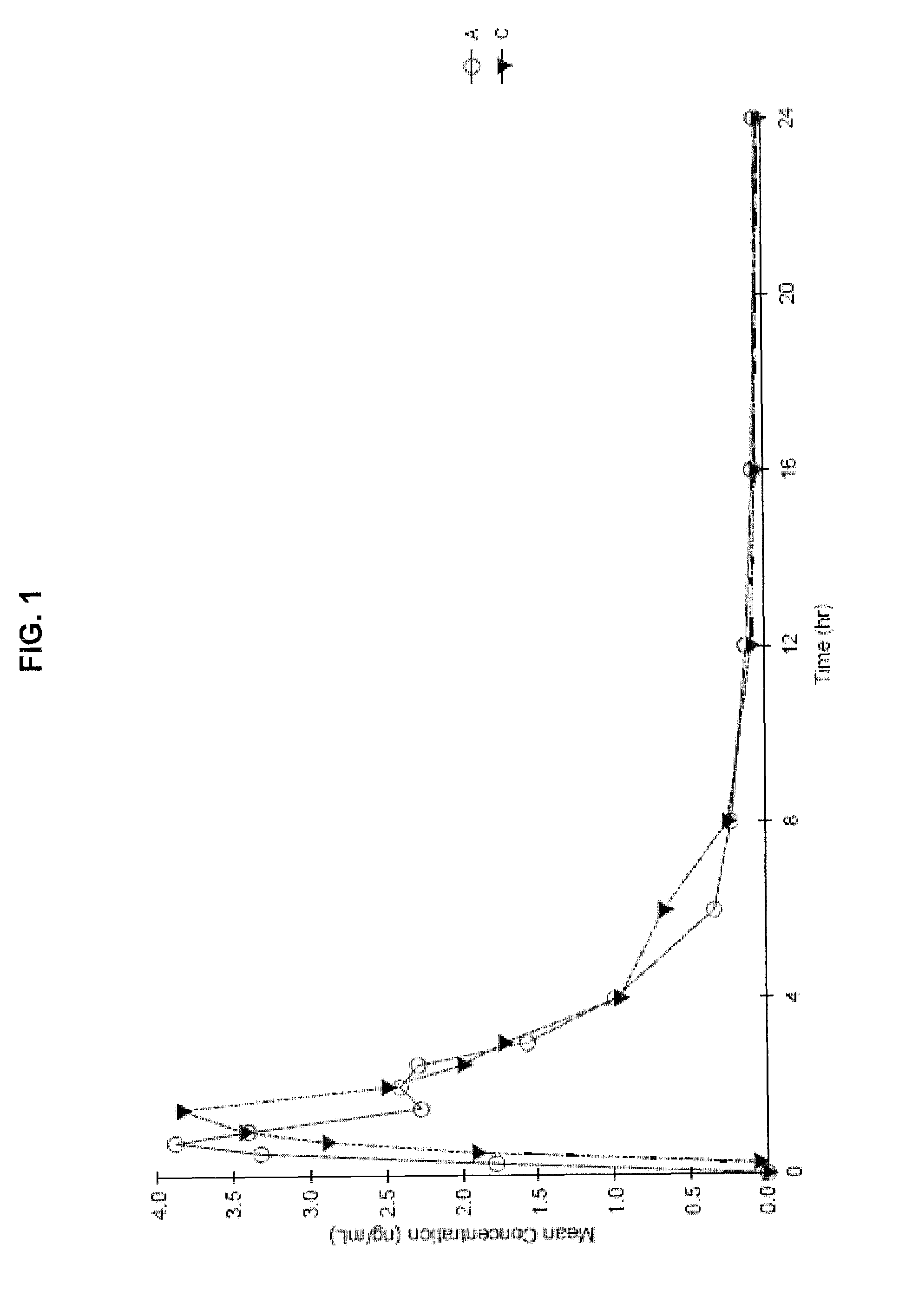
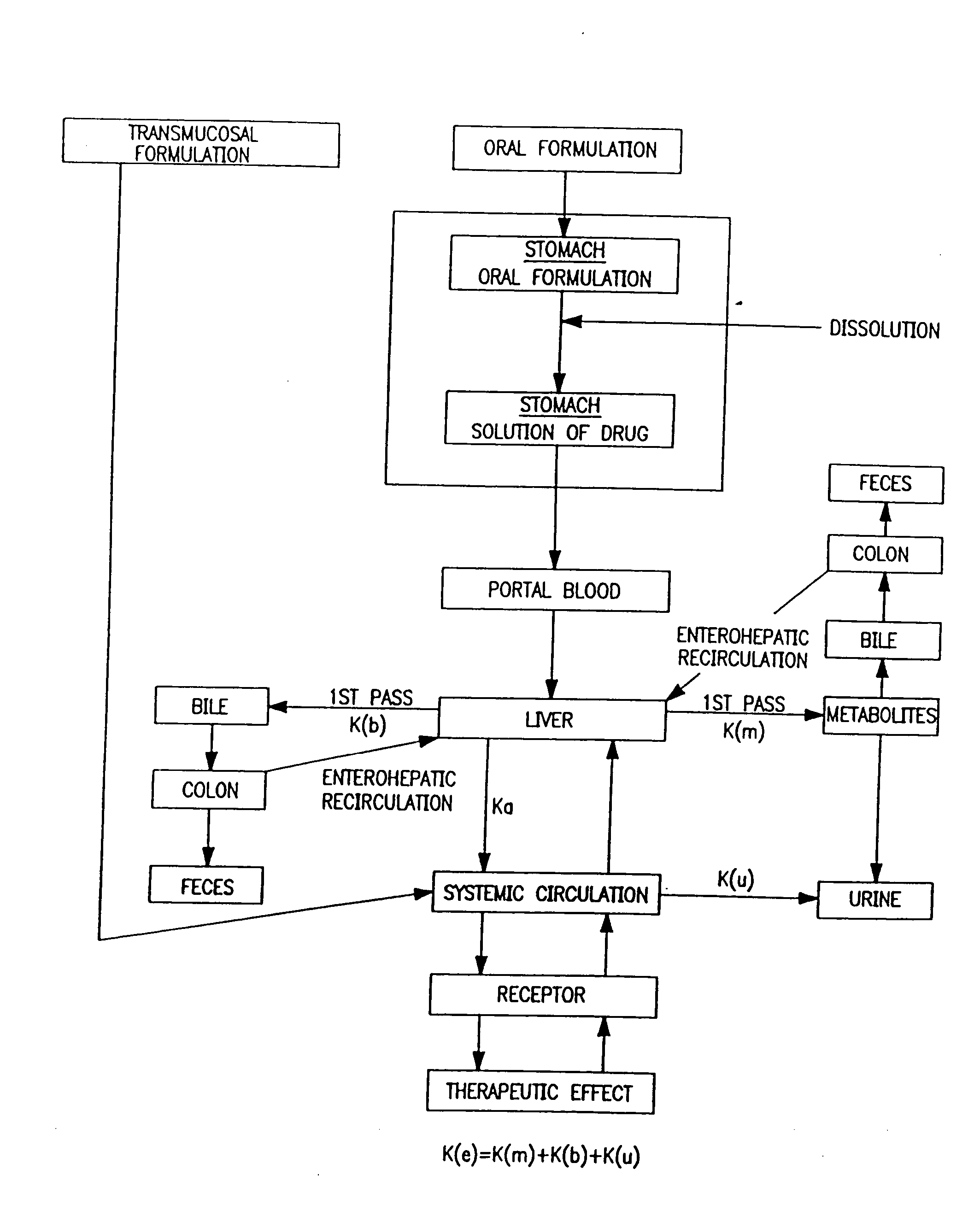
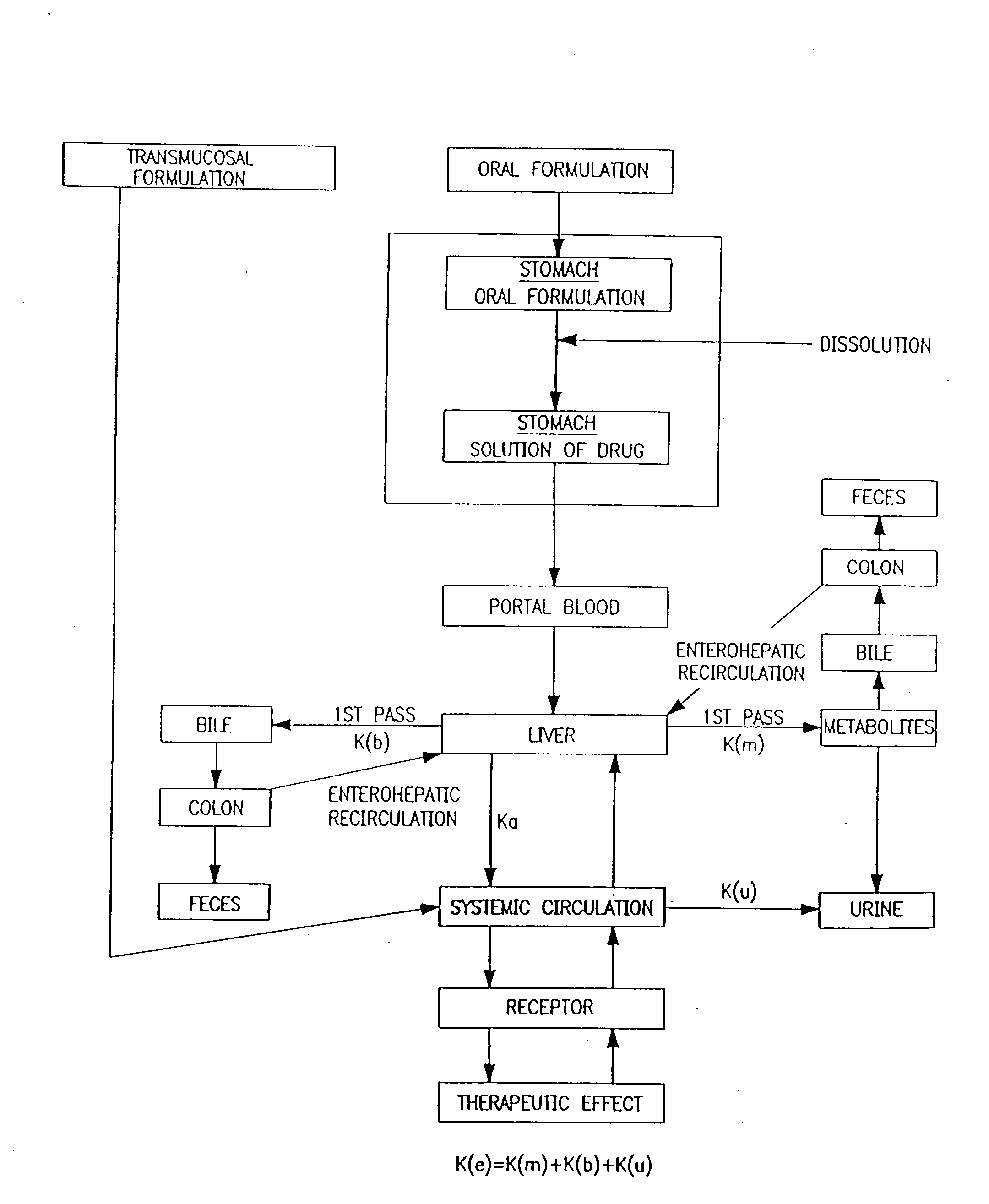
Progesterone Solutions for Increased Bioavailability
ActiveUS20100255085A1Improve bioavailabilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsDissolutionProgesterones
Fill materials for hydrophobic drugs, such as progesterone, and methods of making and using thereof are described herein. The fill material contains the hydrophobic drug dissolved in one or more fatty acids. The concentration of the hydrophobic drug is typically from about 7% to about 50% by weight of the fill material. The concentration of the one or more fatty acids is from about 60% to about 95% by weight of the carrier. The formulation also contains an organic acid and one or both of one or more pharmaceutically acceptable alcohols and one or more pharmaceutically acceptable mono-, di-, or triesters of medium or long chain fatty acids. The fill material can be encapsulated in a hard or soft capsule. The formulations described herein have a higher dissolution rate and faster onset of dissolution compared to micronized progesterone suspended in an oil and thus should have increased bioavailability in vivo.
Owner:PATHEON SOFTGELS INC
Liquid cannabinoid formulations
Oral cannabinoid formulations, including an aqueous-based oral dronabinol solution, that are stable at room or refrigerated temperatures and may possess improved in vivo absorption profiles with faster onset and lower inter-subject variability.
Owner:BENUVIA OPERATIONS LLC
Buccal, polar and non-polar spray containing sumatriptan
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide sumatriptan for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, sumatriptan, and optional flavoring agent; formulation II: aqueous polar solvent, sumatriptan, optionally flavoring agent, and propellant; formulation III: non-polar solvent, sumatriptan, and optional flavoring agent; formulation IV: non-polar solvent, sumatriptan, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, sumatriptan, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, sumatriptan, optional flavoring agent, and propellant.
Owner:DUGGER HARRY A III +1
Physically and chemically stable nicotine-containing particulate material
ActiveUS8741348B2Fast absorptionQuick releasePowder deliveryOrganic active ingredientsMedicineNicotine
A nicotine-containing particulate material for release of nicotine, the material comprising a combination of nicotine or a pharmaceutically acceptable salt, complex or solvate thereof and a microcrystalline cellulose. The particulate material is stable upon storage and releases nicotine relatively fast. The particulate material can be used in the manufacture of nicotine containing pharmaceutical composition, wherein the release of nicotine can be designed to be relatively fast so as to obtain a fast onset of action.
Owner:MODORAL BRANDS INC
Oral cannabinoid formulations
ActiveUS20140100269A1Improve stabilityEffective amountBiocideDispersion deliveryIn vivo absorptionSubject variability
Oral cannabinoid formulations, including an aqueous-based oral dronabinol solution, that are stable at room or refrigerated temperatures and may possess improved in vivo absorption profiles with faster onset and lower inter-subject variability.
Owner:BENUVIA OPERATIONS LLC
Buccal, polar and non-polar spray containing testosterone
InactiveUS20050180923A1Fast absorptionRapid onsetAerosol deliveryOrganic non-active ingredientsSolventPharmacology
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide testosterone for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, testosterone or a pharmaceutically acceptable ester thereof, and optional flavoring agent; formulation II: aqueous polar solvent, testosterone or a pharmaceutically acceptable ester thereof, optionally flavoring agent, and propellant; formulation III: non-polar solvent, testosterone or a pharmaceutically acceptable ester thereof, and optional flavoring agent; and formulation IV: non-polar solvent, testosterone or a pharmaceutically acceptable ester thereof, optional flavoring agent, and propellant; formulation V: a mixture of a polar and a non-polar solvent, testosterone or a pharmaceutically acceptable ester thereof, and optional flavoring agent; formulation VI: a mixture of a polar and a non-polar solvent, testosterone or a pharmaceutically acceptable ester thereof, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray or capsule containing drugs for treating disorders of the central nervous system
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:DUGGER HARRY A III
Buccal, polar and non-polar spray or capsule containing drugs for treating disorders of the gastrointestinal tract or urinary tract
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
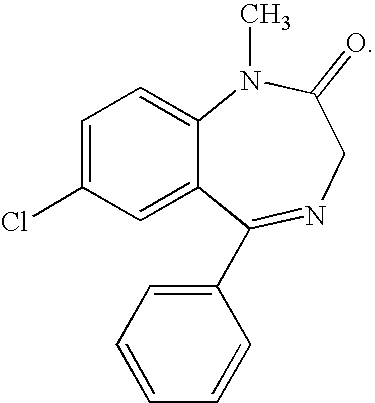
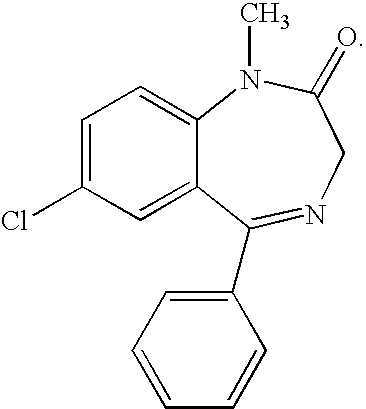
Buccal, polar and non-polar spray containing diazepam
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide diazepam for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, diazepam, and optional flavoring agent; formulation II: aqueous polar solvent, diazepam, optionally flavoring agent, and propellant; formulation III: non-polar solvent, diazepam, and optional flavoring agent; and formulation IV: non-polar solvent, diazepam, optional flavoring agent, and propellant; formulation V: a mixture of a polar and a non-polar solvent, diazepam, and optional flavoring agent; formulation VI: a mixture of a polar and a non-polar solvent, diazepam, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
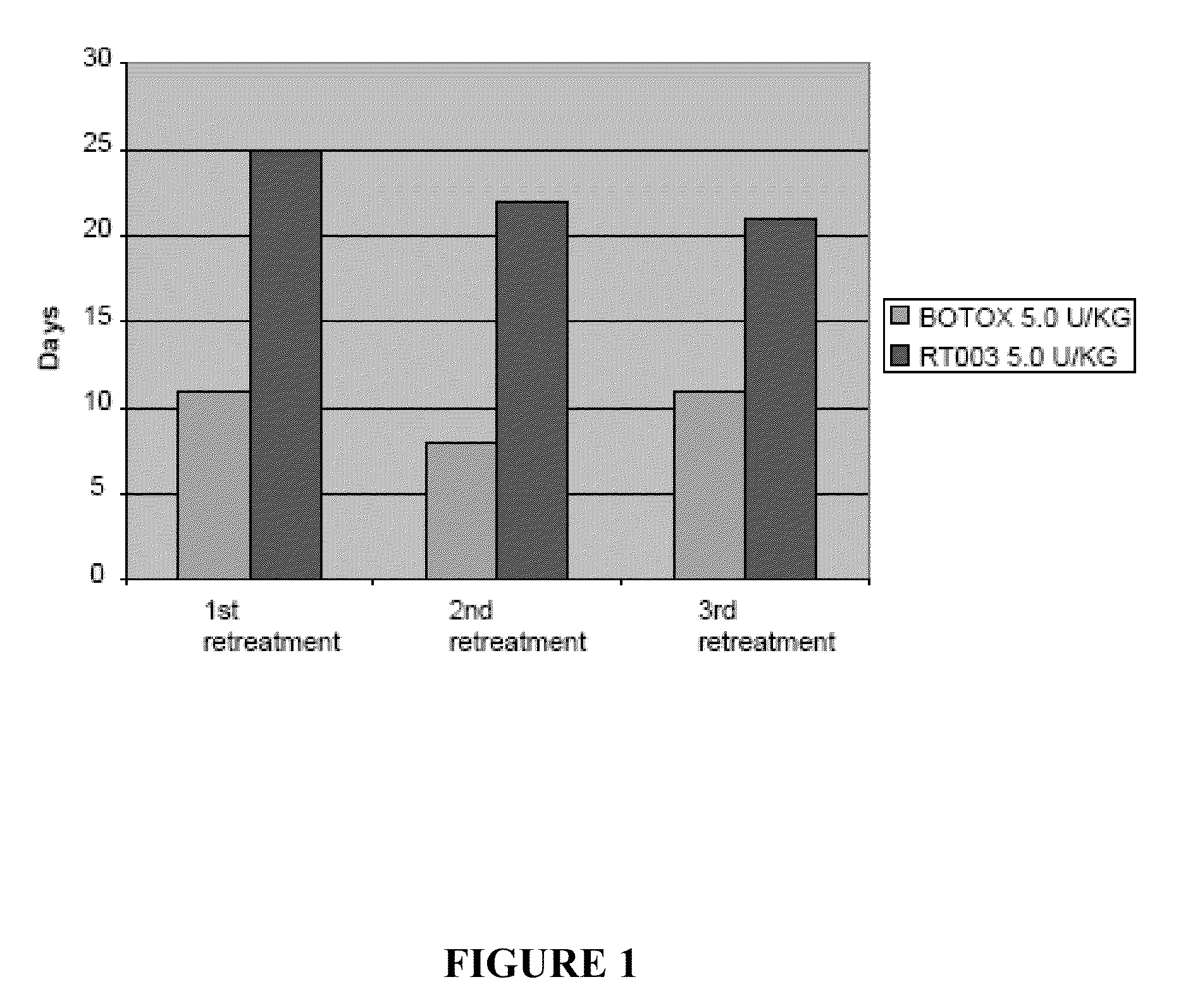
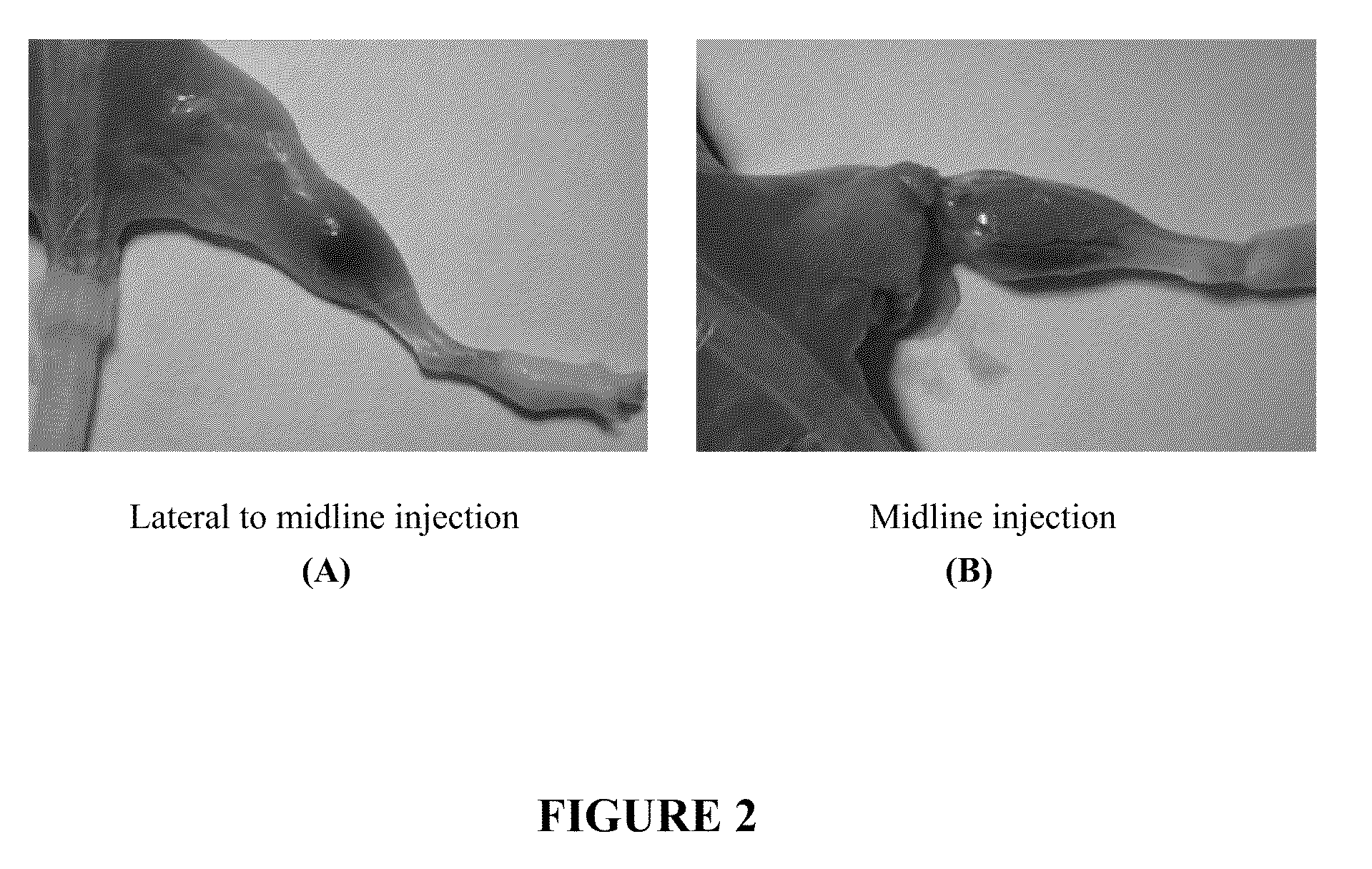
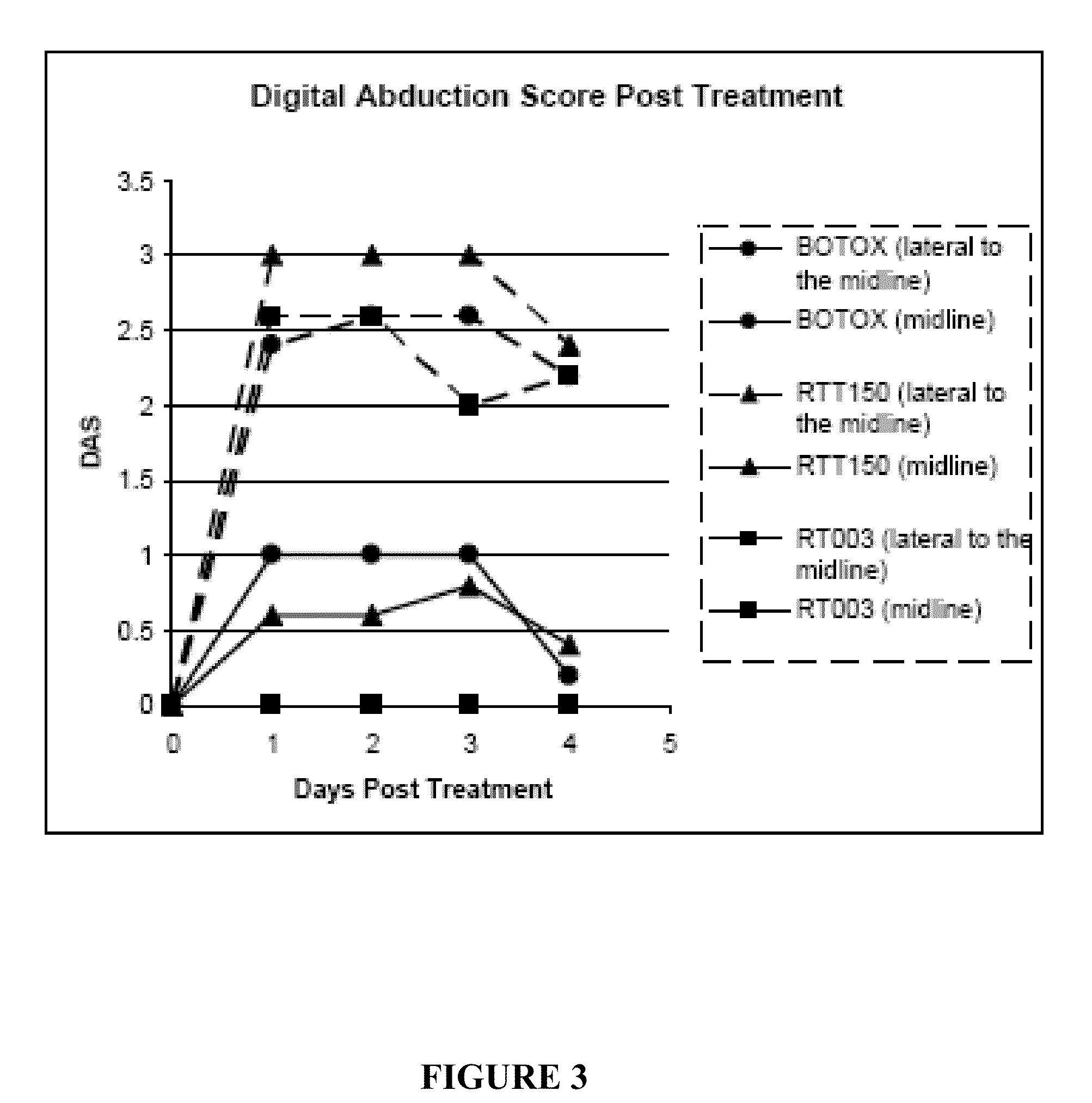
Injectable Botulinum Toxin Formulations
InactiveUS20100168023A1Low antigenicityExtended durationCosmetic preparationsSenses disorderClinical efficacyBotulinum toxin type
This invention provides novel injectable compositions comprising botulinum toxin that may be administered to a subject for various therapeutic, aesthetic and / or cosmetic purposes. The injectable compositions contemplated by the invention exhibit one or more advantages over conventional botulinum toxin formulations, including reduced antigenicity, a reduced tendency to undergo unwanted localized diffusion following injection, increased duration of clinical efficacy or enhanced potency relative, faster onset of clinical efficacy, and / or improved stability.
Owner:REVANCE THERAPEUTICS INC
Buccal, polar and non-polar spray or capsule containing drugs for treating endocrine disorders
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray or capsule containing drugs for treating an infectious disease or cancer
InactiveUS20050142069A1Fast absorptionRapid onsetGenetic material ingredientsAerosol deliverySolventBioactive compound
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray containing zolpidem
InactiveUS20060216240A1Rapid onsetFast absorptionOrganic active ingredientsNervous disorderSolventFast onset
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide zolpidem for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, zolpidem, and optional flavoring agent; formulation II: aqueous polar solvent, zolpidem, optionally flavoring agent, and propellant; formulation III: non-polar solvent, zolpidem, and optional flavoring agent; formulation IV: non-polar solvent, zolpidem, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, zolpidem, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, zolpidem, optional flavoring agent, and propellant.
Owner:MAGNA PHARMA INC
Buccal, polar and non-polar spray containing zolpidem
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide zolpidem for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, zolpidem, and optional flavoring agent; formulation II: aqueous polar solvent, zolpidem, optionally flavoring agent, and propellant; formulation III: non-polar solvent, zolpidem, and optional flavoring agent; formulation IV: non-polar solvent, zolpidem, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, zolpidem, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, zolpidem, optional flavoring agent, and propellant.
Owner:MAGNA PHARMA INC
Buccal, polar and non-polar sprays containing propofol
InactiveUS20060222597A1Rapid onsetFast absorptionBiocideHydroxy compound active ingredientsAerosol spraySolvent
Buccal aerosol sprays using polar and / or non-polar solvents have now been developed which provide propofol for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: propofol, a polar solvent and an optional flavoring agent; formulation II: propofol, a polar solvent, a propellant, and an optionally flavoring agent; formulation III: propofol, a non-polar solvent, and an optional flavoring agent; formulation IV: propofol, a non-polar solvent, a propellant, and an optional flavoring agent; formulation V: propofol, a mixture of a polar solvent and a non-polar solvent, and an optional flavoring agent; and formulation VI: propofol, a mixture of a polar solvent and a non-polar solvent, a propellant, and an optional flavoring agent.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray or capsule containing drugs for treating pain
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:DUGGER III HARRY A
Buccal, polar and non-polar spray containing testosterone
InactiveUS20060210484A1Rapid onsetFast absorptionAerosol deliveryOrganic non-active ingredientsSolventPharmacology
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide testosterone for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, testosterone or a pharmaceutically acceptable ester thereof, and optional flavoring agent; formulation II: aqueous polar solvent, testosterone or a pharmaceutically acceptable ester thereof, optionally flavoring agent, and propellant; formulation III: non-polar solvent, testosterone or a pharmaceutically acceptable ester thereof, and optional flavoring agent; and formulation IV: non-polar solvent, testosterone or a pharmaceutically acceptable ester thereof, optional flavoring agent, and propellant; formulation V: a mixture of a polar and a non-polar solvent, testosterone or a pharmaceutically acceptable ester thereof, and optional flavoring agent; formulation VI: a mixture of a polar and a non-polar solvent, testosterone or a pharmaceutically acceptable ester thereof, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray containing atropine
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide atropine for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, atropine, and optional taste mask and / or flavoring agent; formulation II: aqueous polar solvent, atropine, optionally flavoring agent, and propellant; formulation III: non-polar solvent, atropine, and optional flavoring agent; and formulation IV: non-polar solvent, atropine, optional flavoring agent, and propellant; formulation V: a mixture of a polar and a non-polar solvent, atropine, and optional flavoring agent; formulation VI: a mixture of a polar and a non-polar solvent, atropine, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray containing alprazolam
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide alprazolam for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, alprazolam, and optional flavoring agent; formulation II: aqueous polar solvent, alprazolam, optionally flavoring agent, and propellant; formulation III: non-polar solvent, alprazolam, and optional flavoring agent; formulation IV: non-polar solvent, alprazolam, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, alprazolam, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, alprazolam, optional flavoring agent, and propellant.
Owner:DUGGER HARRY A III +1
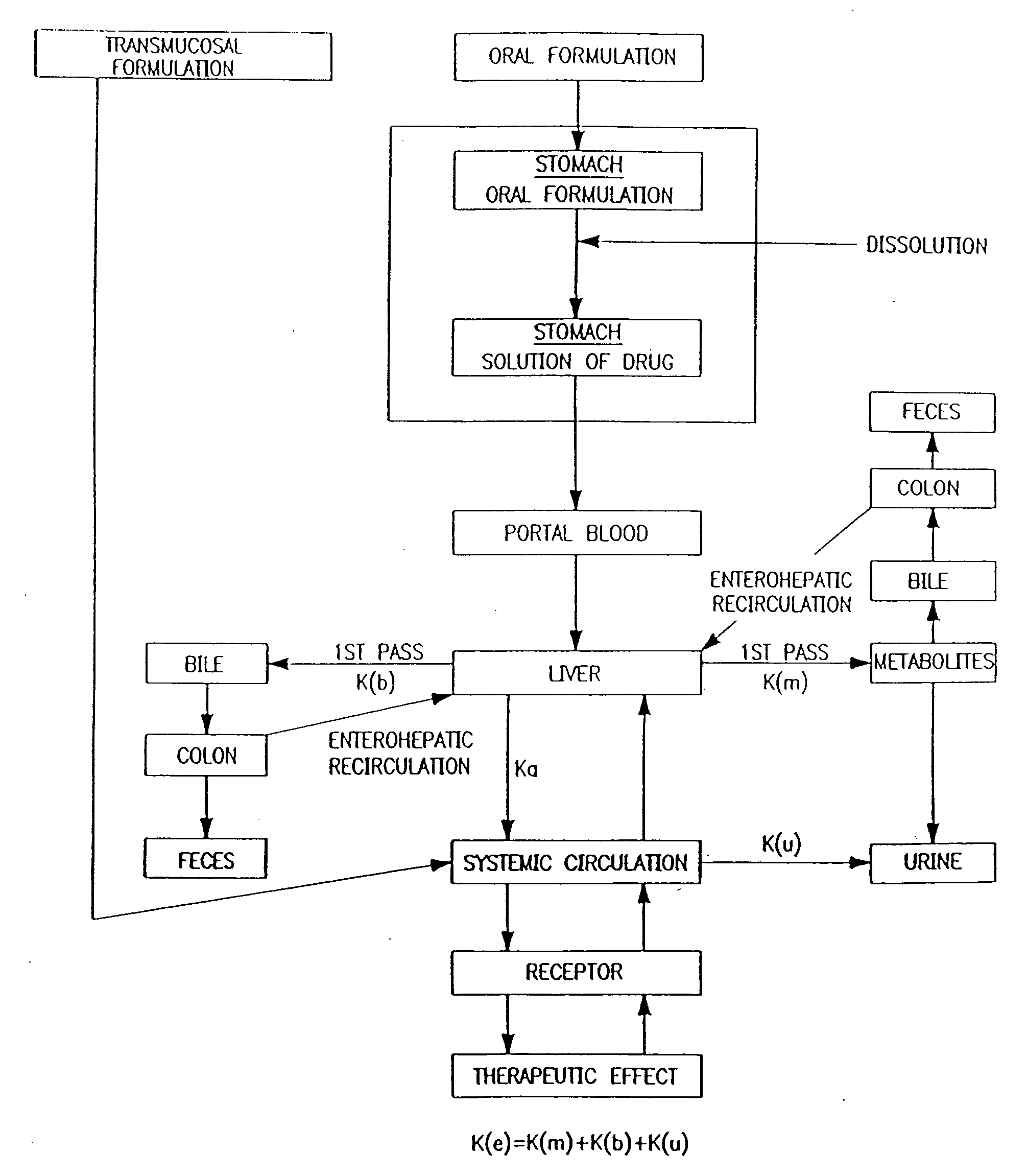
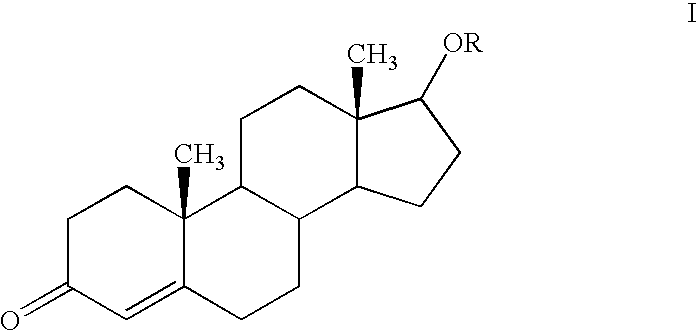
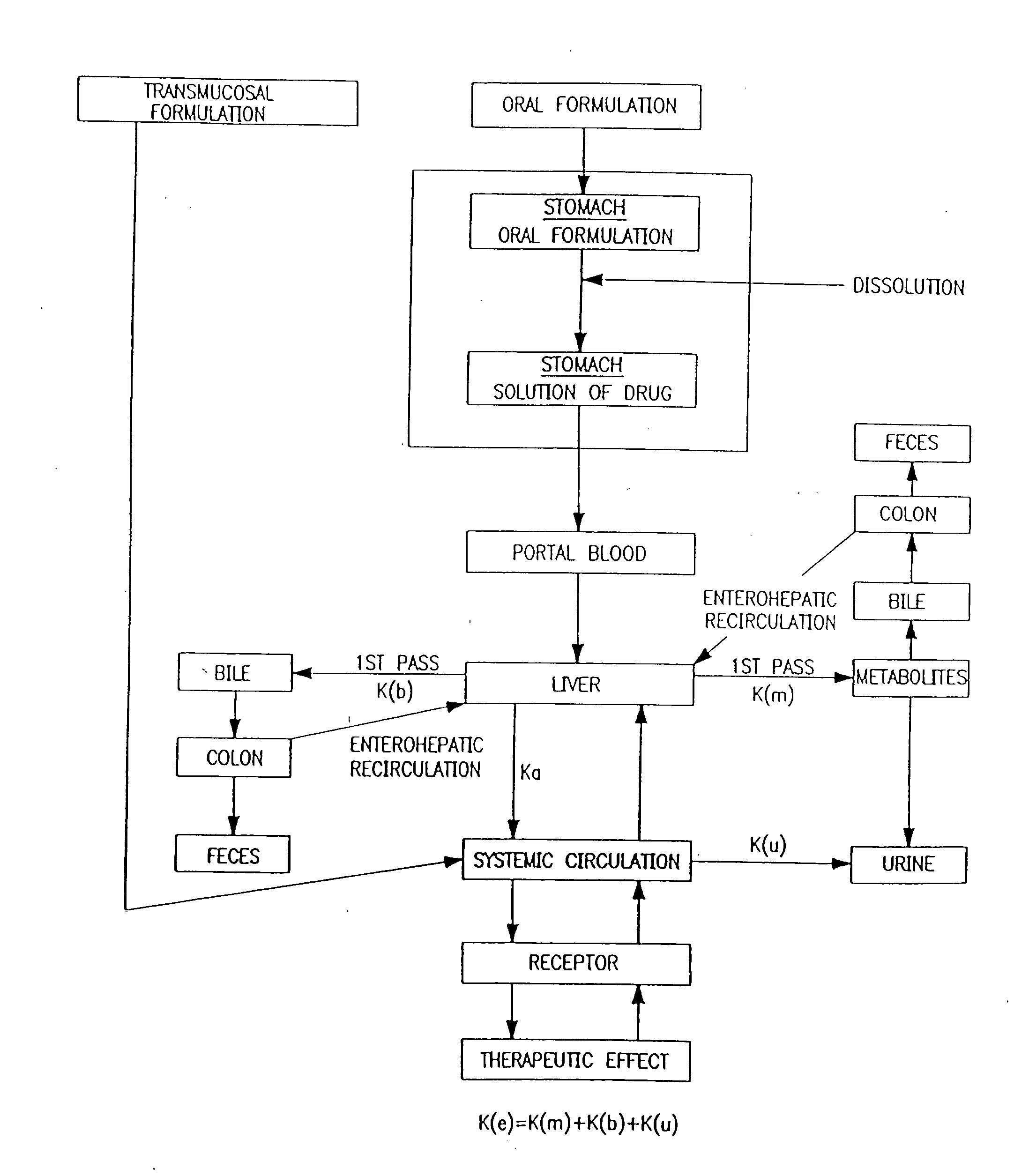
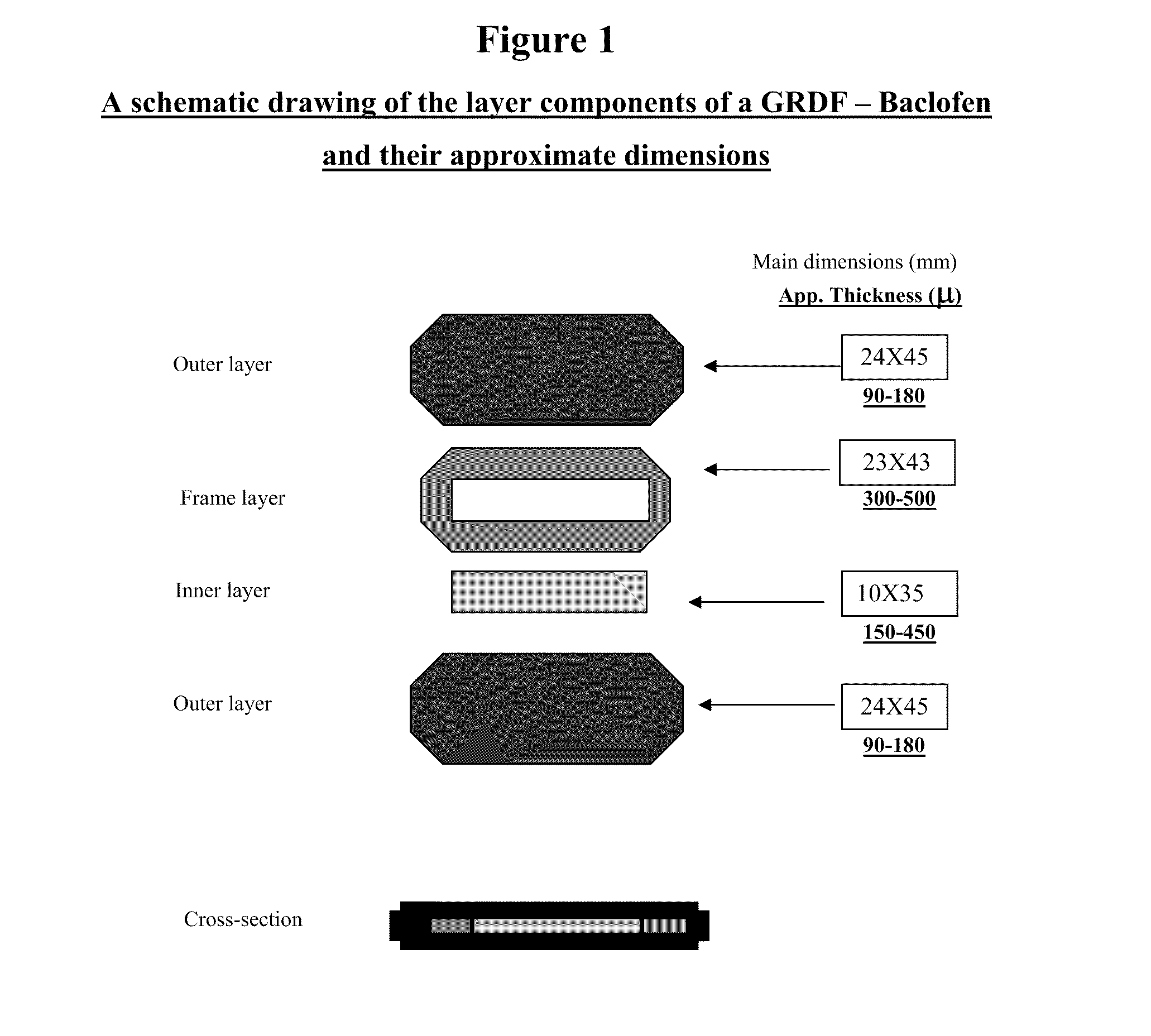
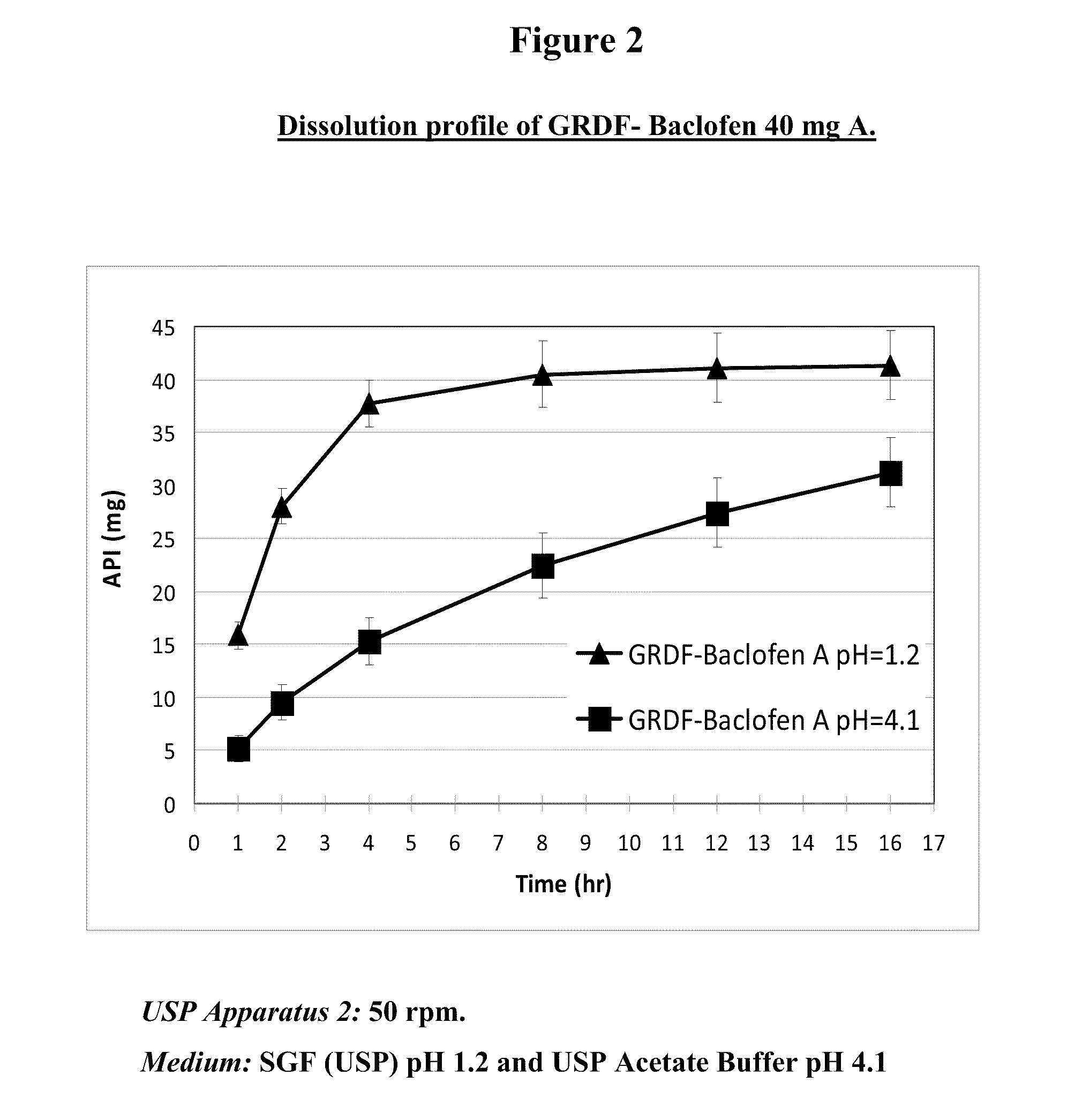
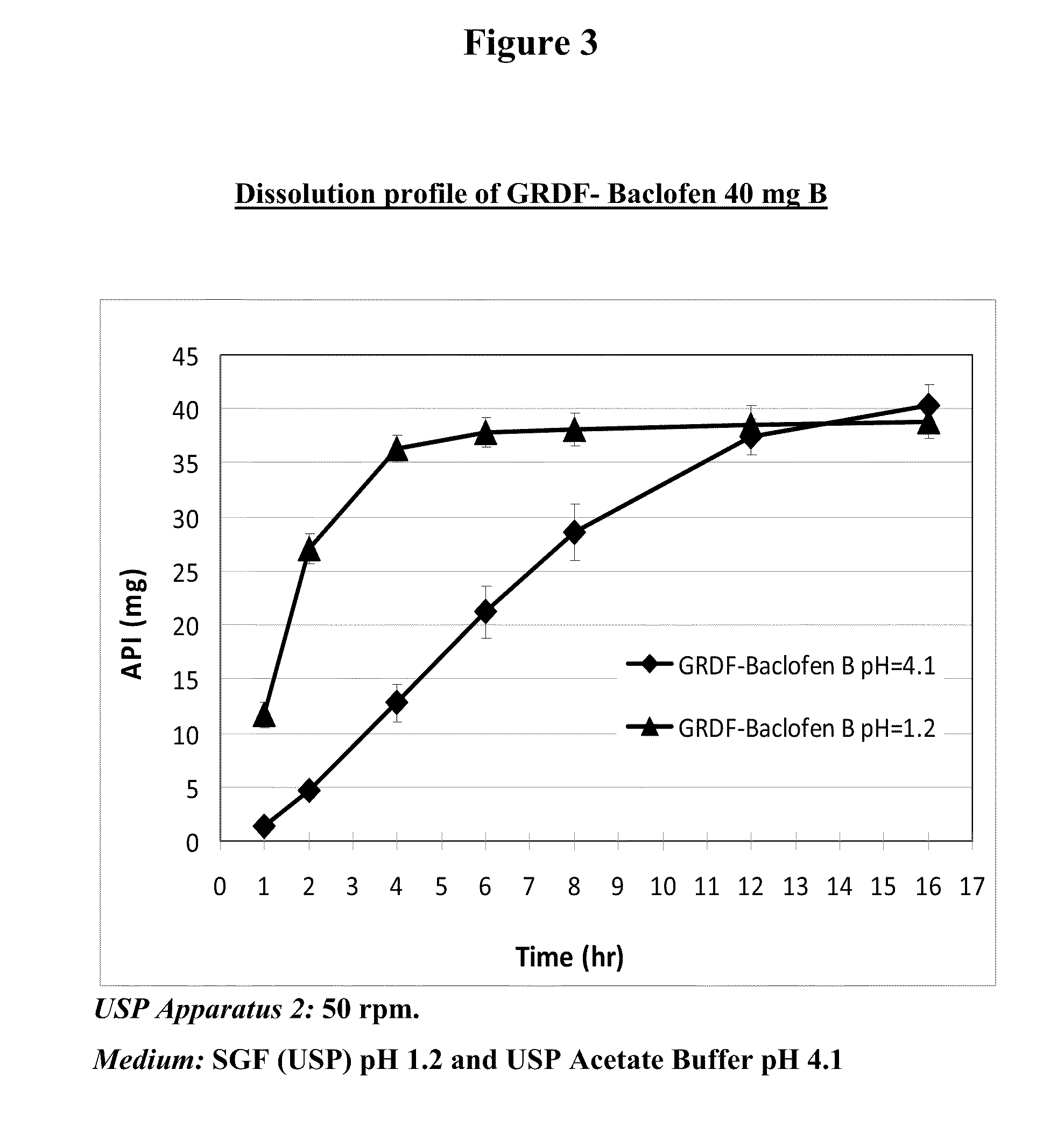
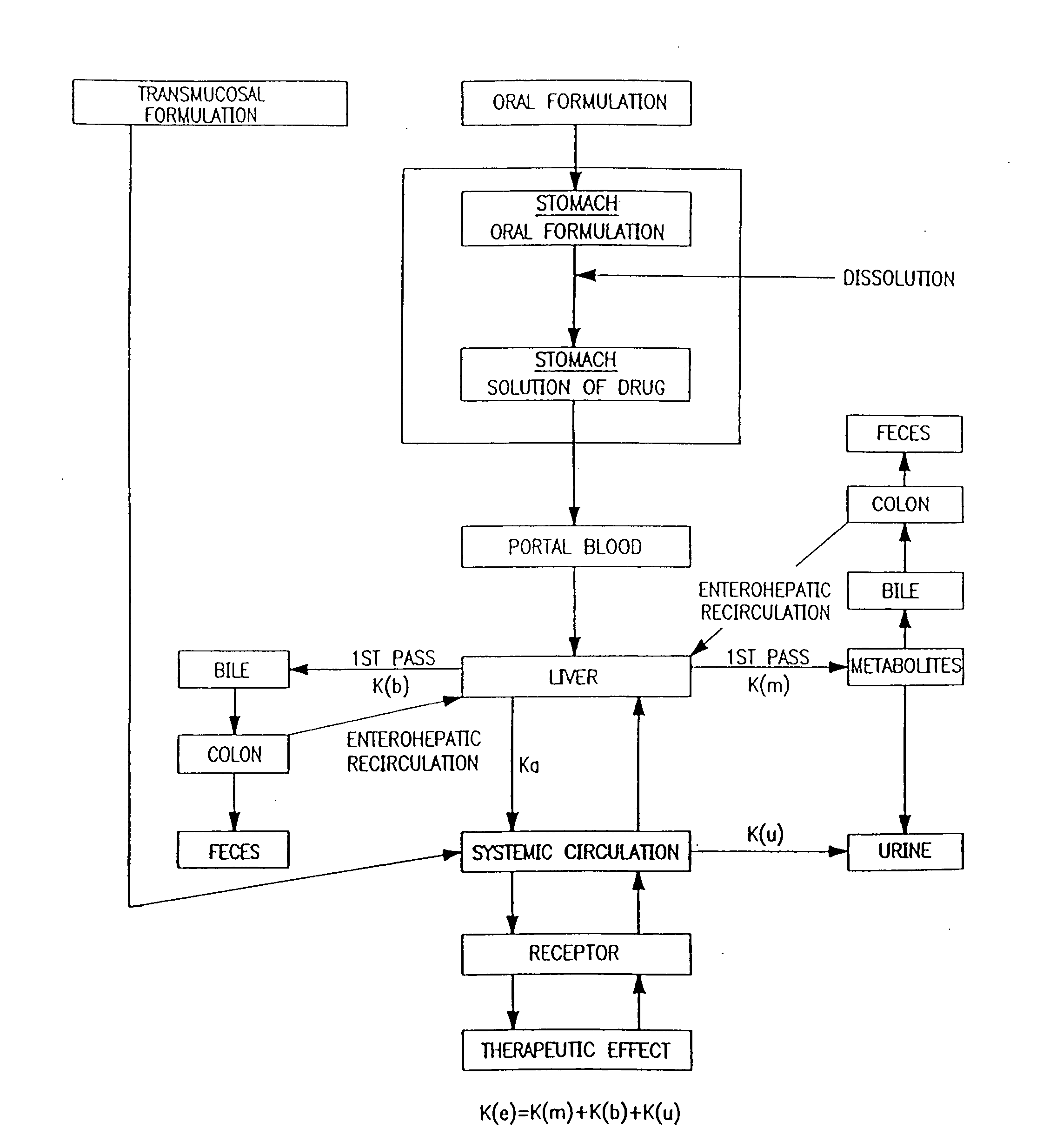
Baclofen and r-baclofen gastroretentive drug delivery systems
InactiveUS20110091542A1Reduce concentrationStrong therapeutic activityOrganic active ingredientsBiocideControlled releaseSide effect
A biodegradable, multi-layered controlled release gastroretentive baclofen or R-baclofen dosage form which is optionally divided into a first dosage of baclofen or R-baclofen for immediate release and a second dosage of baclofen or R-baclofen for controlled release in the stomach and gastrointestinal tract of a patient, folded into a capsule which disintegrates upon contact with gastric juice and the dosage form unfolds rapidly upon contact with gastric juice. The biodegradable, multi-layered gastroretentive dosage forms of the invention provide fast onset of baclofen or R-baclofen activity with prolonged absorption and minimal undesirable side effects.
Owner:INTEC PHARMA
Buccal, polar and non-polar spray containing diazepam
InactiveUS20060216241A1Rapid onsetFast absorptionOrganic active ingredientsNervous disorderDiazepamSolvent
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide diazepam for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, diazepam, and optional flavoring agent; formulation II: aqueous polar solvent, diazepam, optionally flavoring agent, and propellant; formulation III: non-polar solvent, diazepam, and optional flavoring agent; and formulation IV: non-polar solvent, diazepam, optional flavoring agent, and propellant; formulation V: a mixture of a polar and a non-polar solvent, diazepam, and optional flavoring agent; formulation VI: a mixture of a polar and a non-polar solvent, diazepam, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com