Injectable Botulinum Toxin Formulations
a technology of botulinum toxin and injection composition, which is applied in the direction of drug compositions, peptide/protein ingredients, immunological disorders, etc., can solve the problems of improper sterilization and sealing of food containers, unwanted paralysis in surrounding areas of the body, and dry skin, so as to reduce the tendency to undergo diffusion, reduce the effect of antigenicity and increase the duration of clinical efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Duration Of Local Muscle Paralysis In A Murine Model
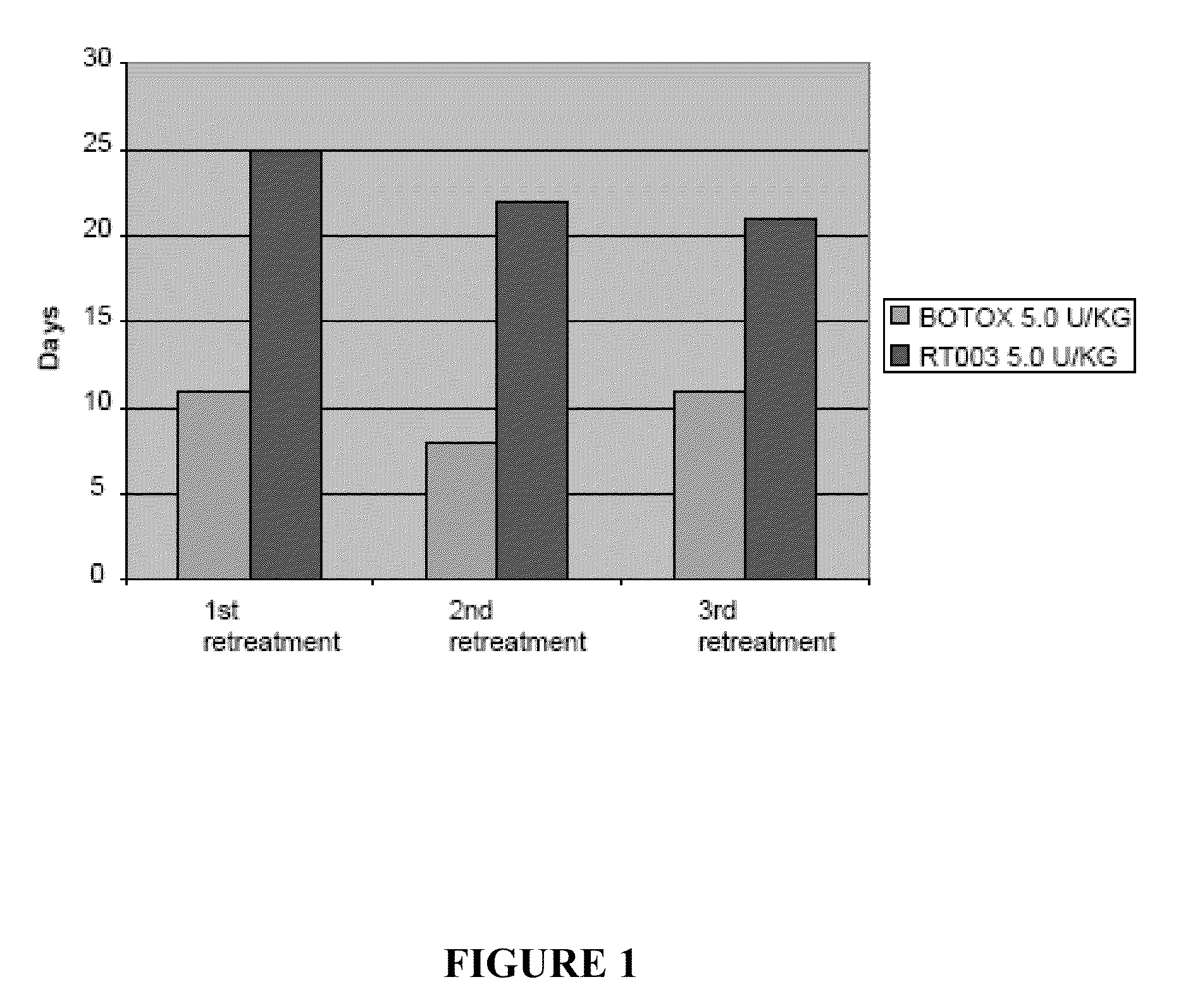
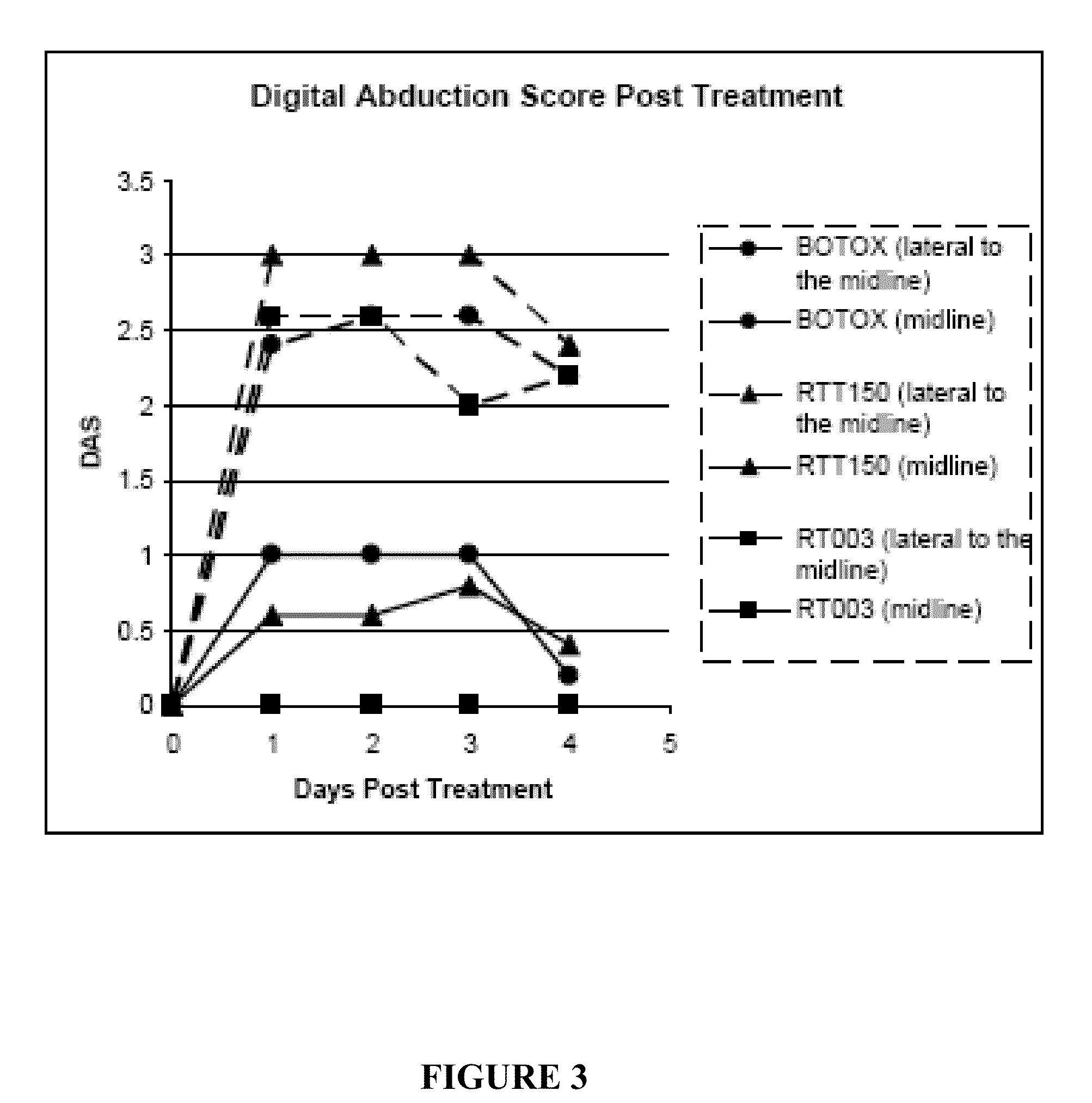
[0056]This example compares the duration of local muscle paralysis in mice injected with either RT003 or BOTOX®. RT003 is an exemplary injectable formulation according to the invention that contains type A botulinum toxin (purified to remove all endogenous non-toxin proteins) and positively charged carrier with the sequence RKKRRQRRRG-(K)15-GRKKRRQRRR. BOTOX® also contains type A botulinum toxin, but exogenous albumin is added to stabilize the type A botulinum toxin molecule.
[0057]The muscle paralysis was measured using digit abduction score (DAS) assay as reported by Aoki, K. R. in “A comparison of the safety margins of botulinum neurotoxin serotypes A, B, and F in mice”, Toxicon 2001; 39(12):1815-1820. In the DAS assay, a mouse is briefly suspended by its tail to cause a characteristic startle response in which the mouse extends its hind limbs and abducts its hind digits. The extent to which the mouse is able to exhibit this star...
example 2
Injectable Botulinum Toxin Formulations With an Improved Safety Profile
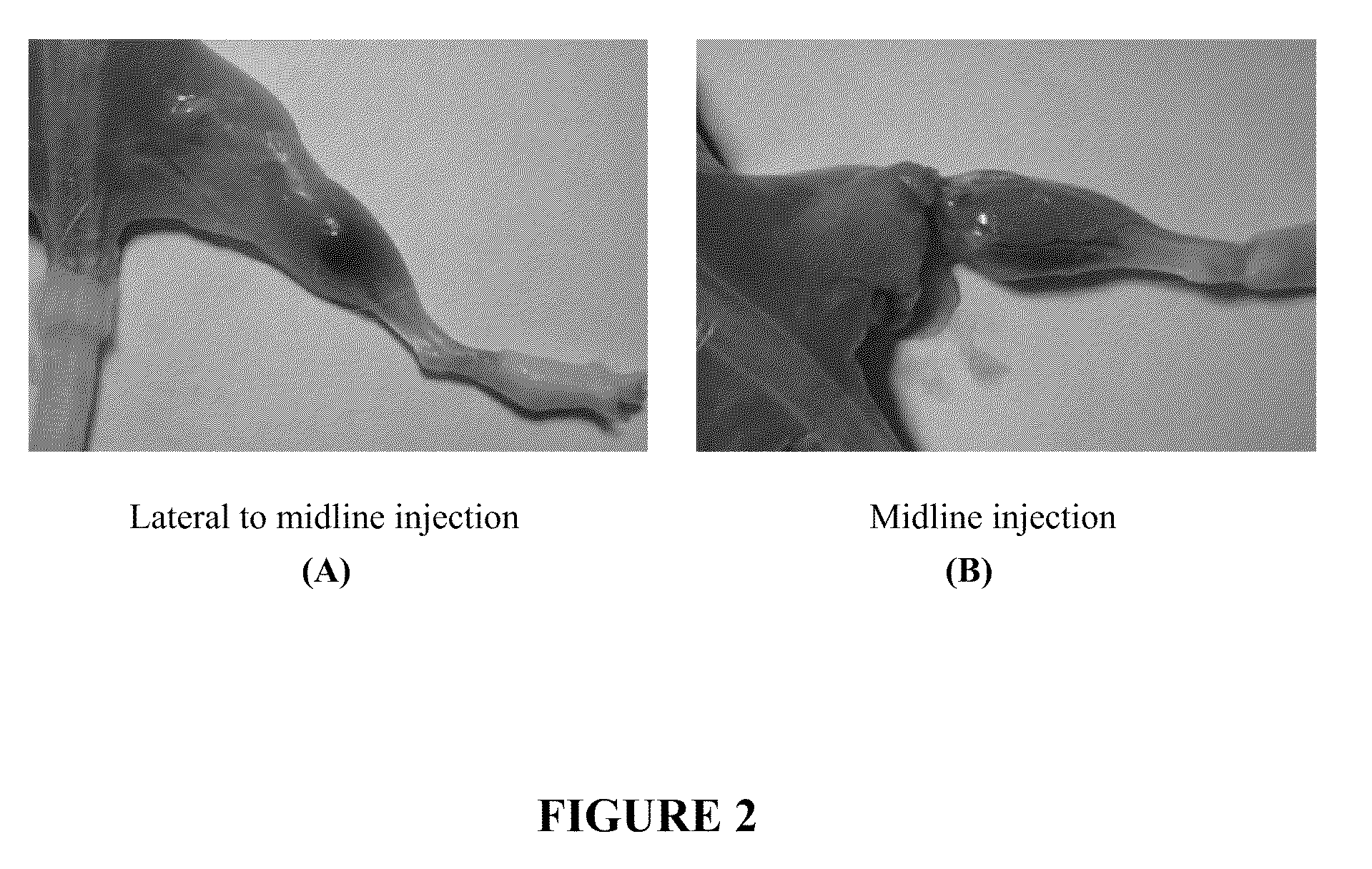
[0060]Over the last few decades, botulinum toxin has found use as a therapeutic agent for treating a variety of conditions, including wrinkles, hyperhidrosis, and muscle spasms. However, as botulinum toxin is the most potent naturally occurring toxin known to humans, improper administration of the toxin can be extremely dangerous. For instance, accidental systemic delivery of botulinum toxin can lead to paralysis, difficulty breathing, and even death. Moreover, even if botulinum toxin were properly delivered to a localized region of the body as a part of a therapeutic treatment, the toxin has a natural tendency to diffuse over time, thereby increasing the risk of unwanted paralysis in other parts of the body. For example, when botulinum toxin is injected around the eyes to treat wrinkles, it may diffuse to the muscles that control the movement of the eyelids. If this happens, the eyelid muscles may become partial...
example 3
Injectable Botulinum Toxin Formulations with Reduced Tendency to Generate Antibodies
[0072]When botulinum toxin is periodically injected into a patient to treat an unwanted condition such as wrinkles, it is often observed that efficacy of the botulinum toxin decreases with successive injections, even though the duration of the effects of the botulinum toxin may remain the same. This phenomenon is believed to be the result of the formation of antibodies to the botulinum toxin by the immune system of the patient. From a treatment perspective, the formation of antibodies to botulinum toxin by the patient is undesirable, because increasingly larger doses of botulinum toxin are then required to achieve the same effect, which presents serious issues related to both safety and cost.
[0073]In certain embodiments, this invention provides injectable botulinum toxin formulations that have a decreased tendency to induce antibody formation, as compared to currently available commercial injectable ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dynamic viscosity | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com