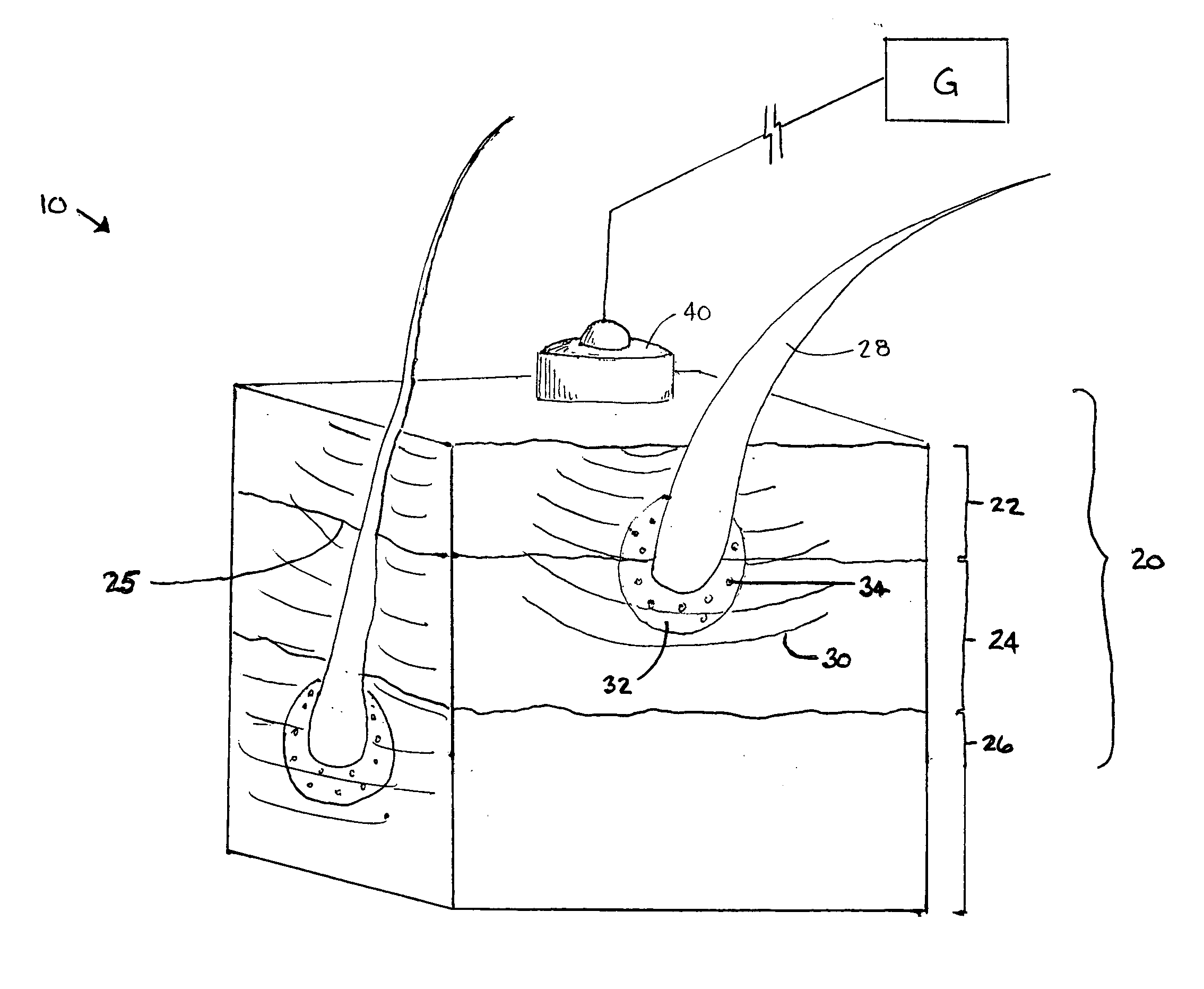
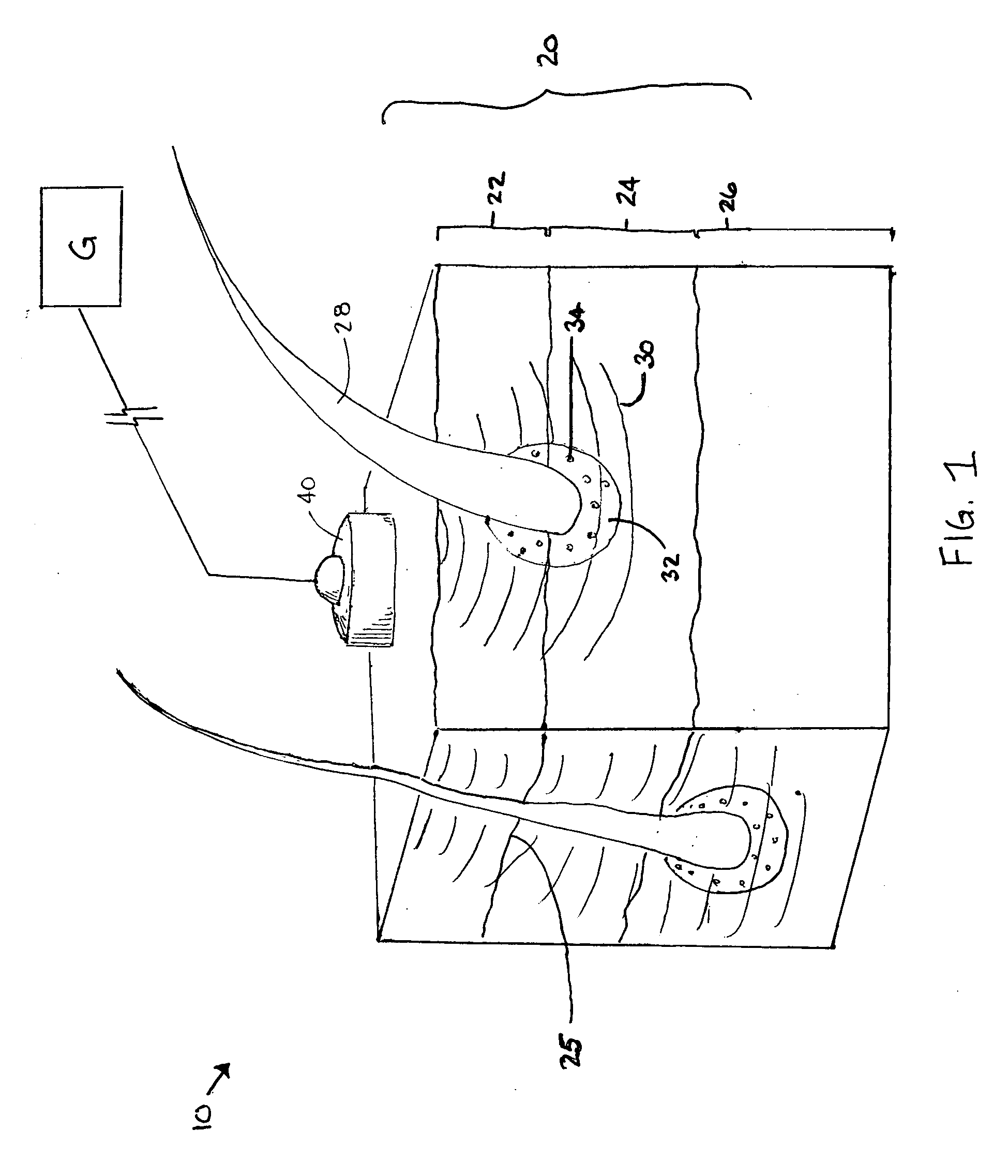
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2152 results about "Dermis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
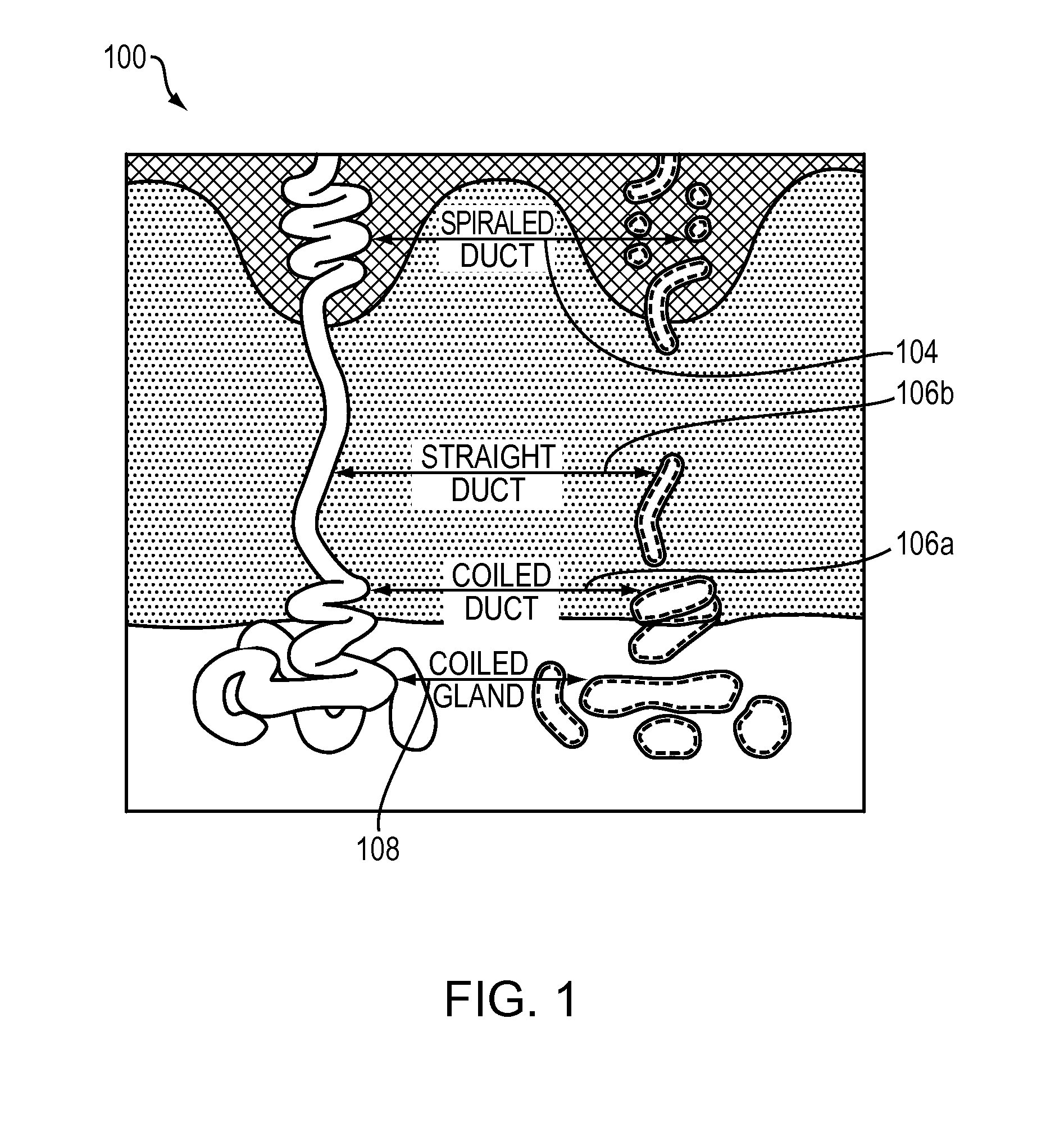
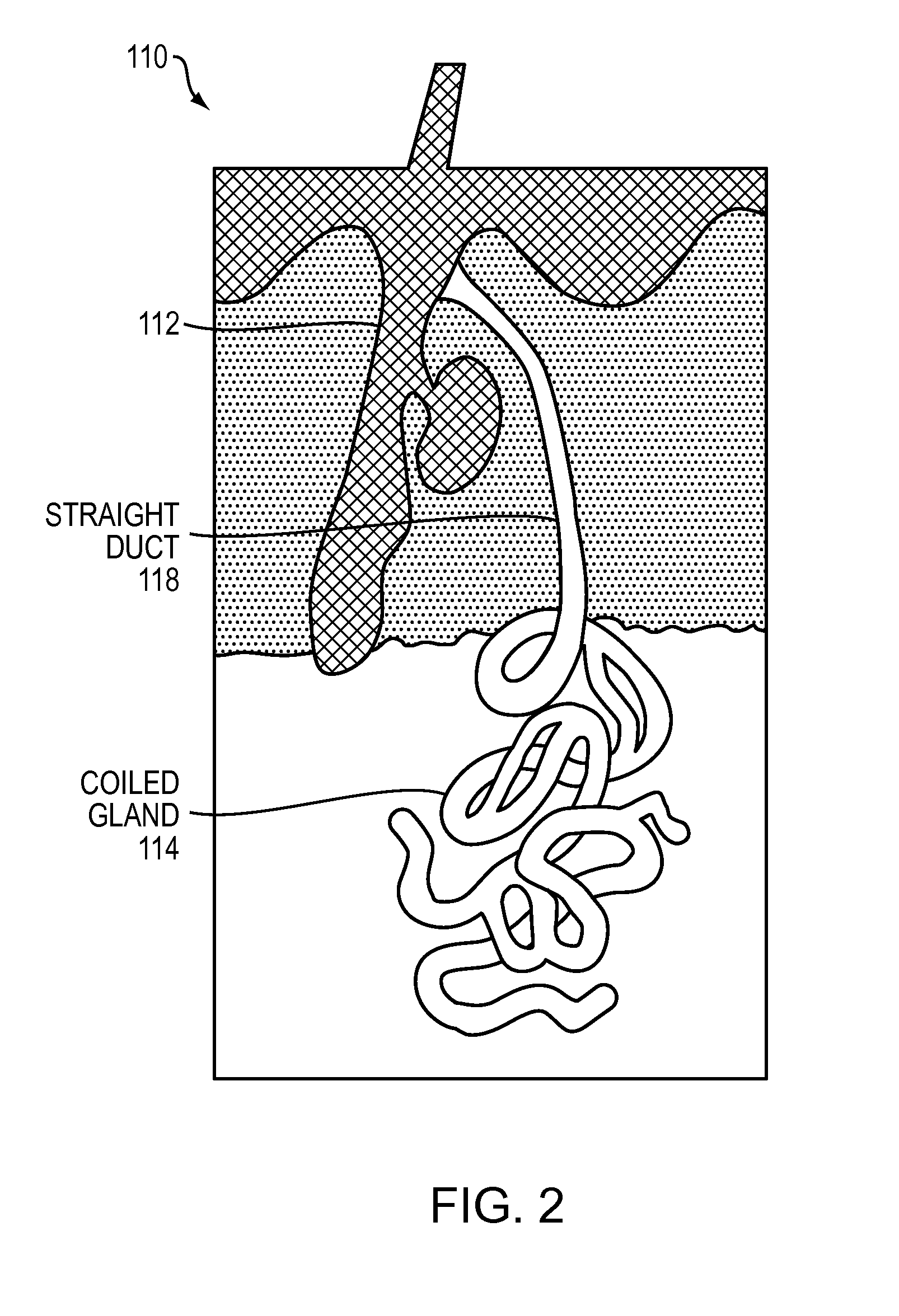
The dermis or corium is a layer of skin between the epidermis (with which it makes up the cutis) and subcutaneous tissues, that primarily consists of dense irregular connective tissue and cushions the body from stress and strain. It is divided into two layers, the superficial area adjacent to the epidermis called the papillary region and a deep thicker area known as the reticular dermis. The dermis is tightly connected to the epidermis through a basement membrane. Structural components of the dermis are collagen, elastic fibers, and extrafibrillar matrix. It also contains mechanoreceptors that provide the sense of touch and thermoreceptors that provide the sense of heat. In addition, hair follicles, sweat glands, sebaceous glands (oil glands), apocrine glands, lymphatic vessels, nerves and blood vessels are present in the dermis. Those blood vessels provide nourishment and waste removal for both dermal and epidermal cells.
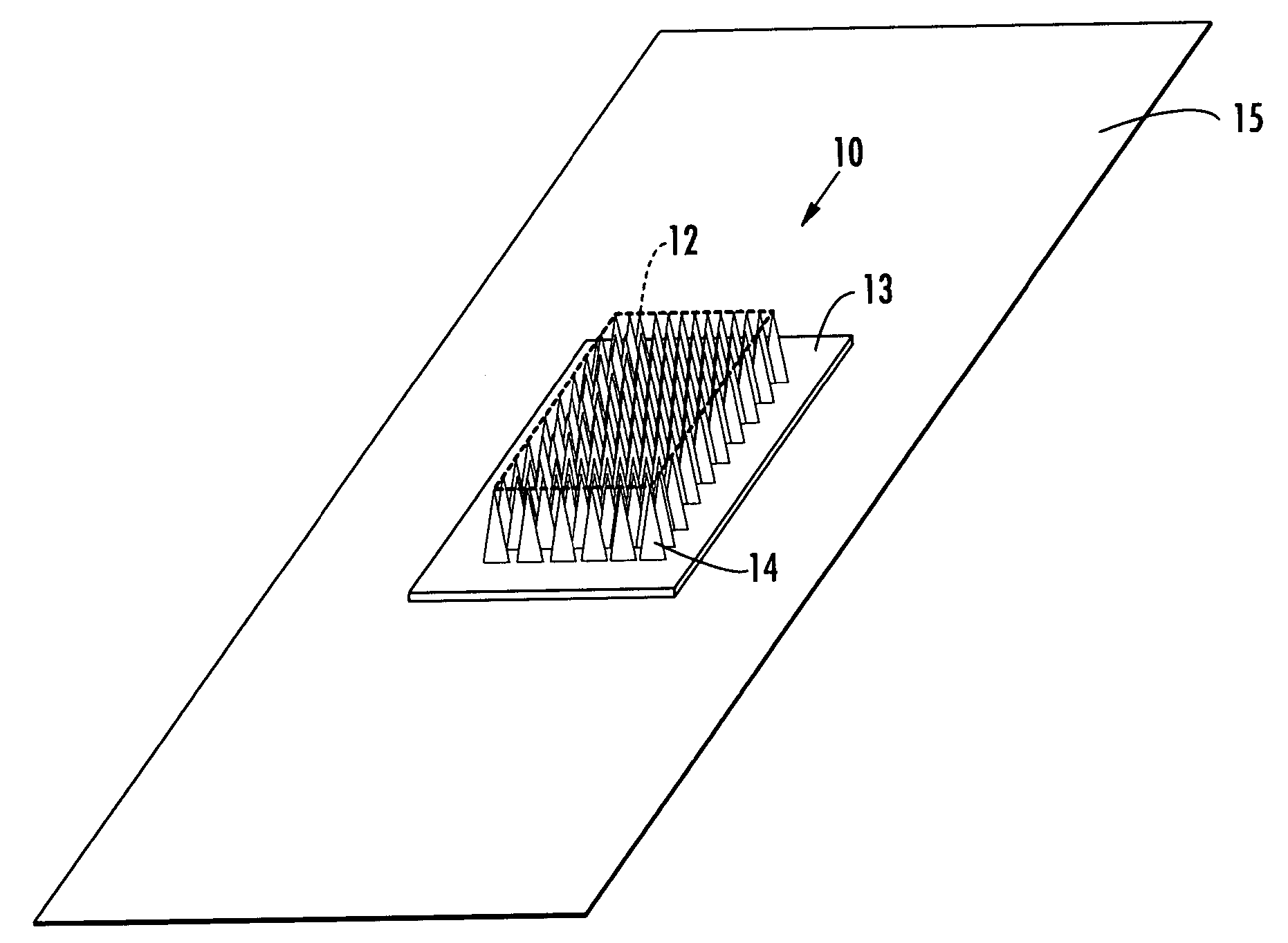
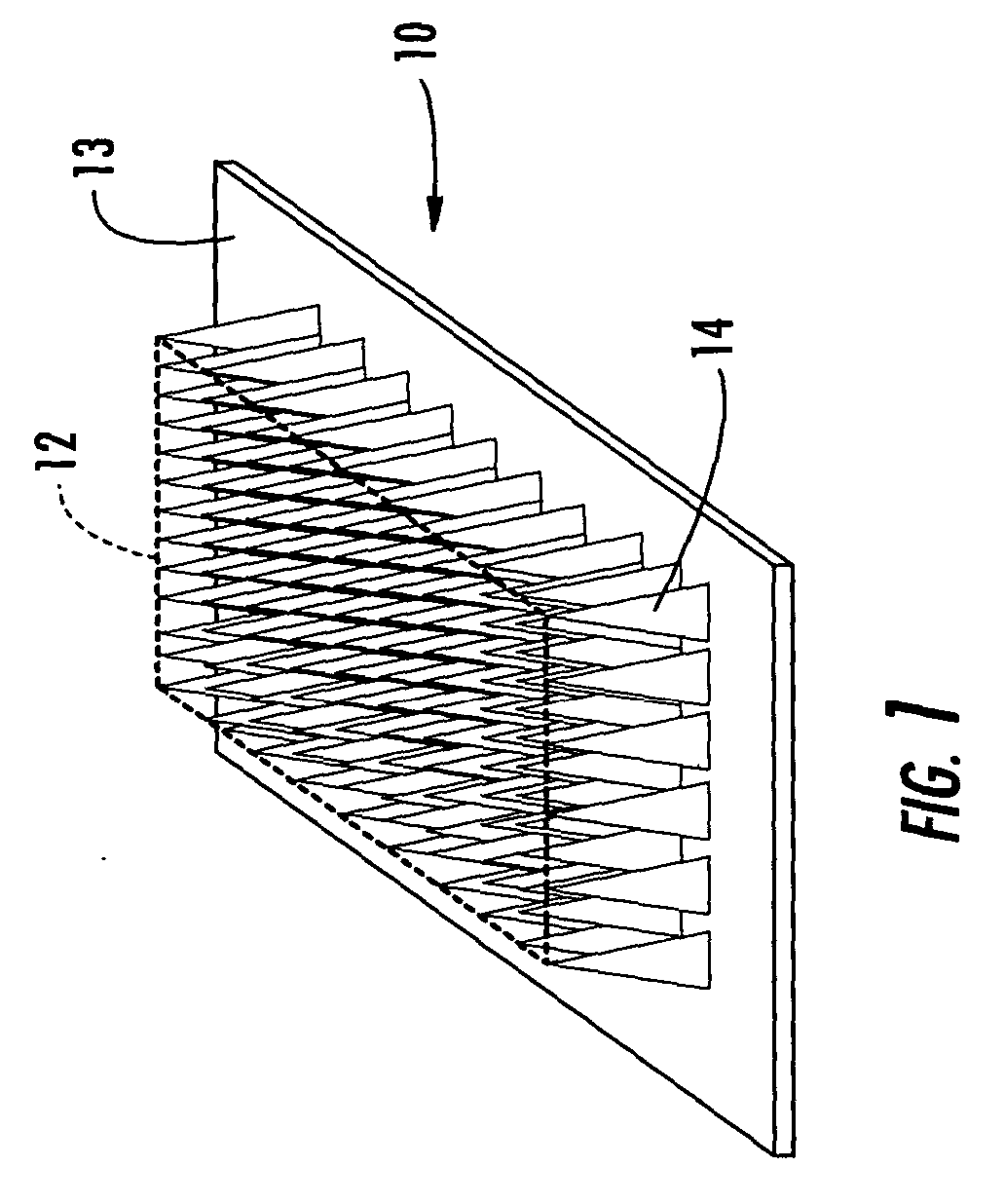
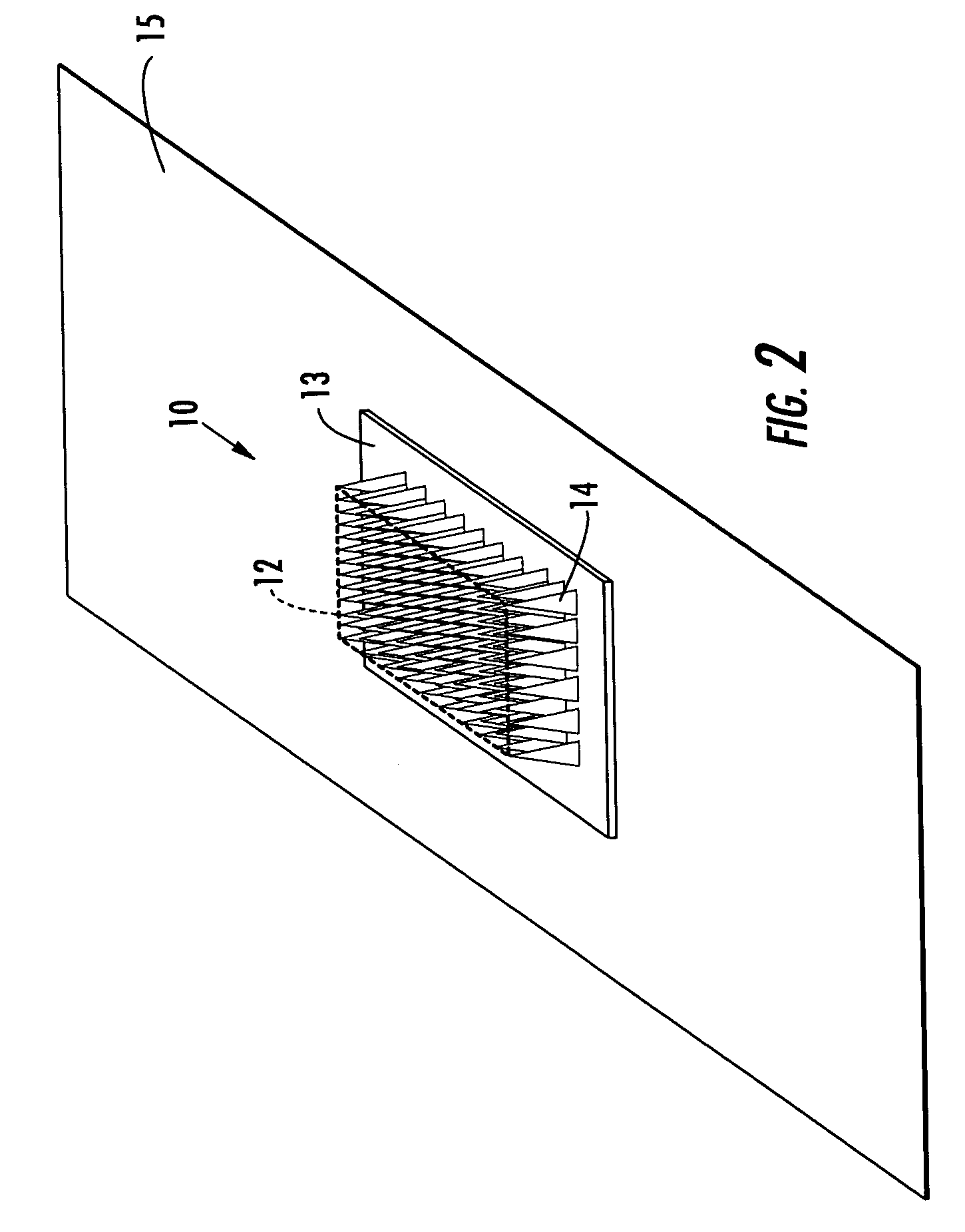
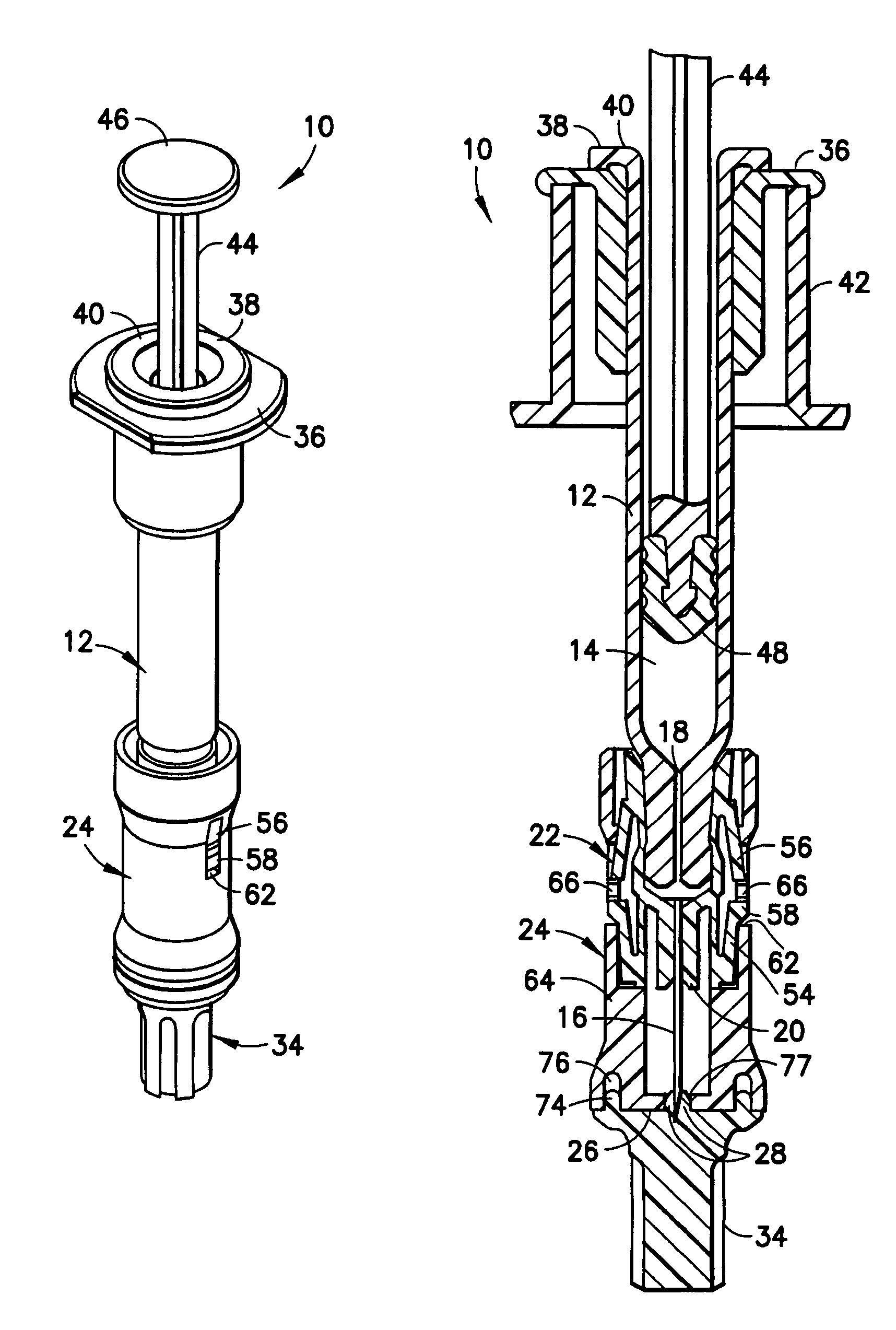
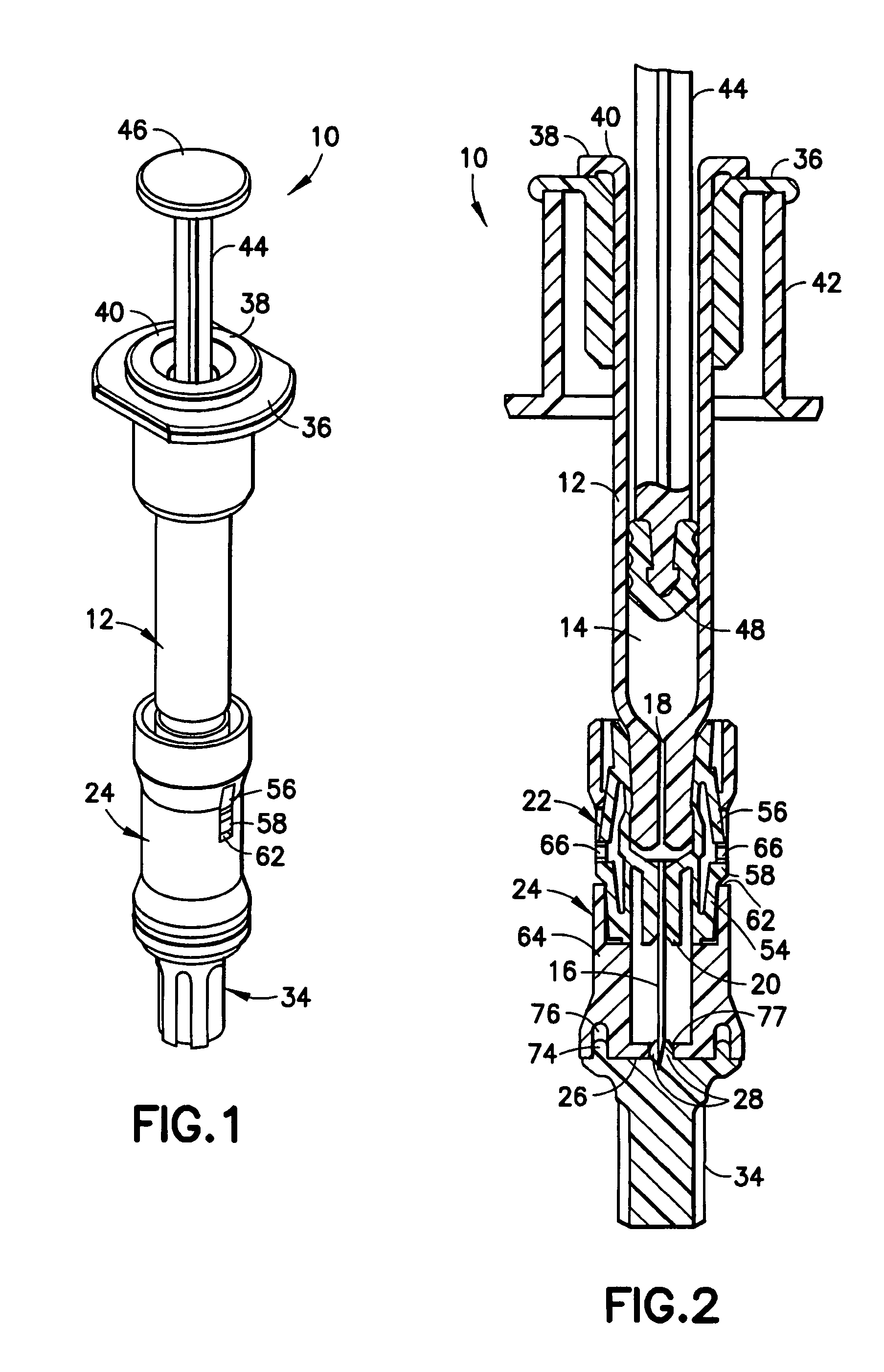
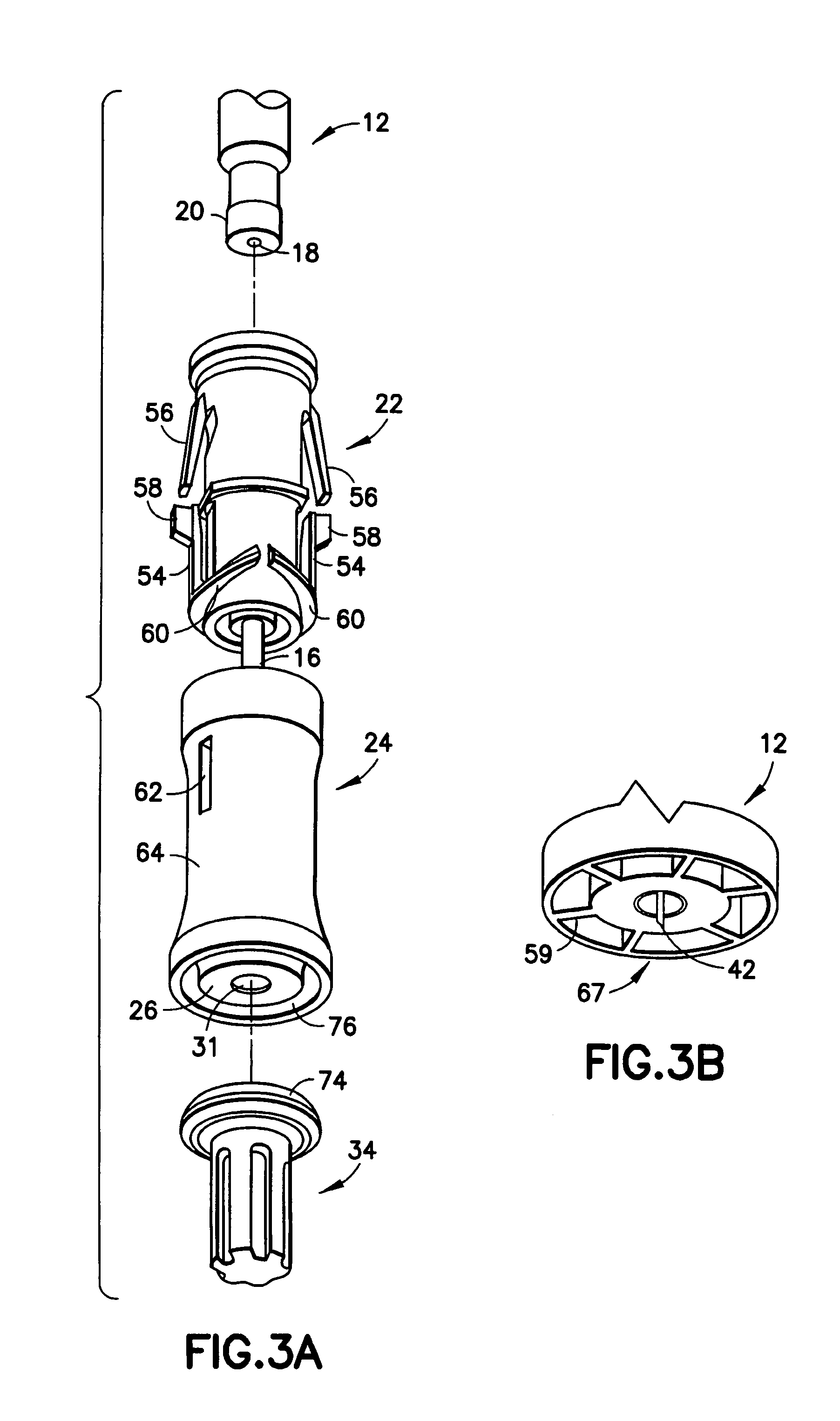
Interfaced medical implant assembly
ActiveUS8425600B2Reduce and eliminate capsular contracturePromote formationMammary implantsTissue regenerationMedicineBreast prostheses
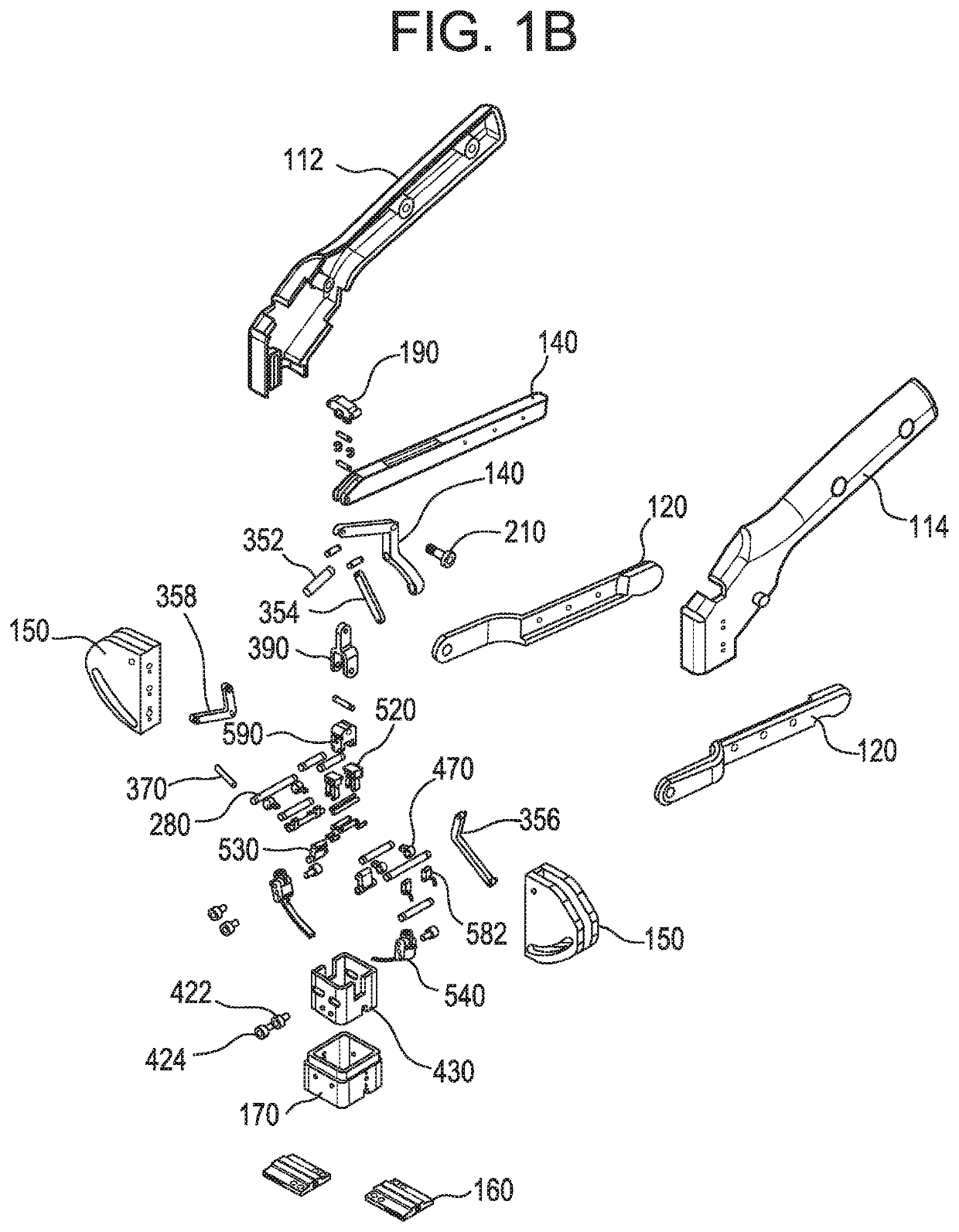
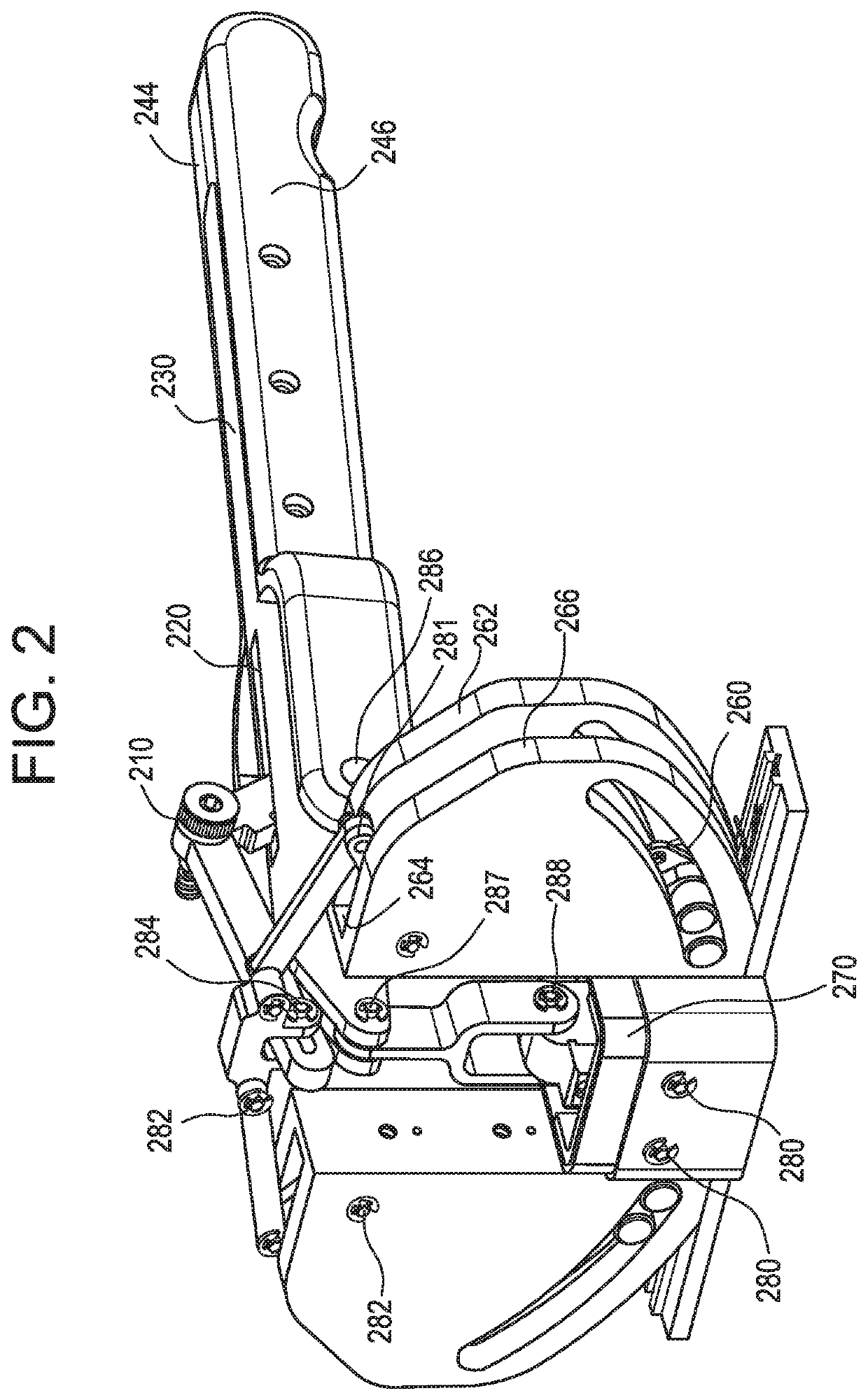
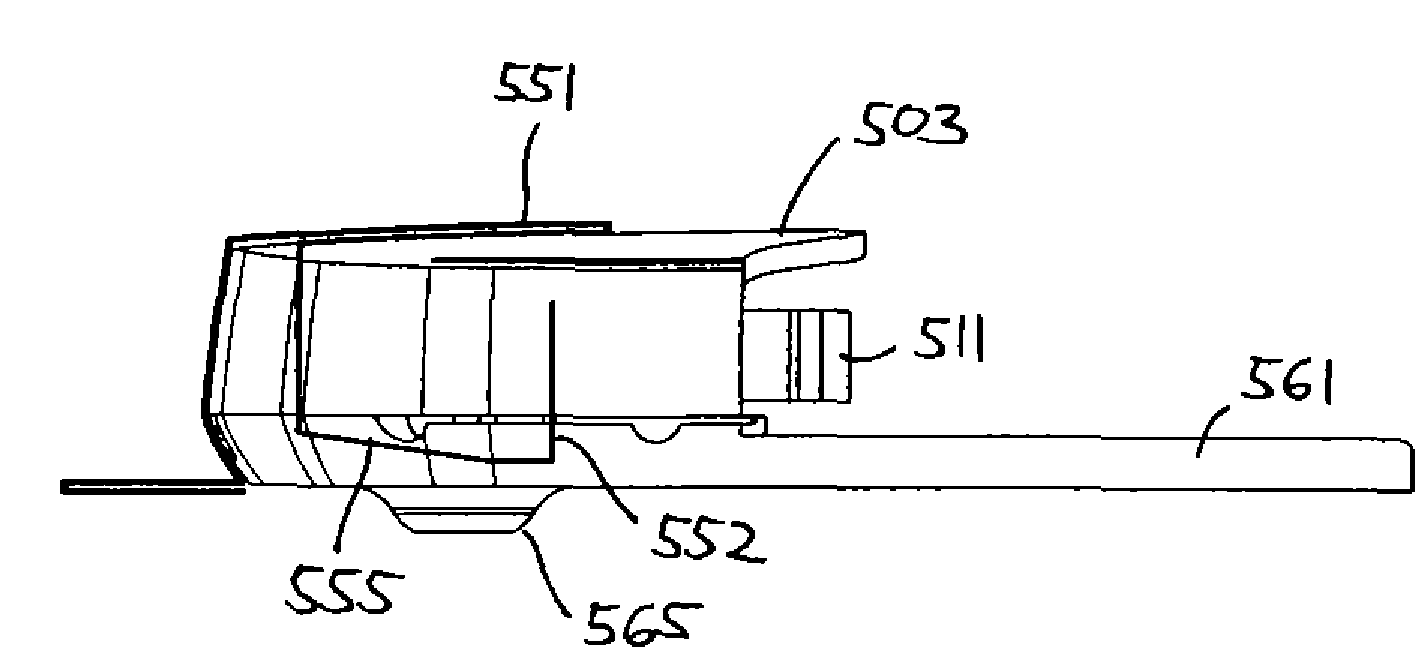
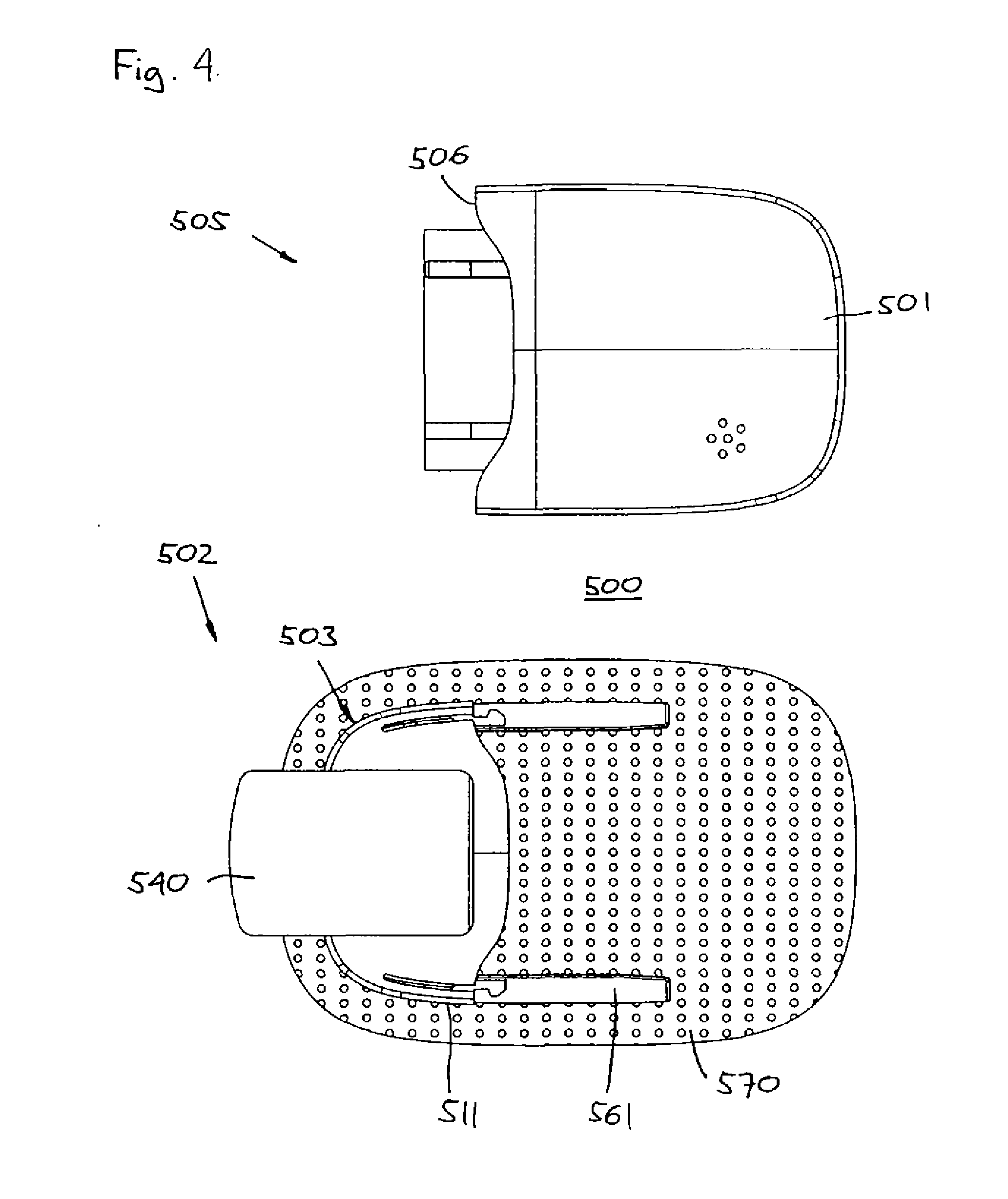
A medical implant assembly and method having a medical implant, e.g. a breast prostheses, attached to a biological interface. The biological interface is comprised of a dermal material with capsular contracture inhibiting properties so that once the medical assembly is inserted into the host, the biological interface, which is intimately coupled to the implant, prevents / reduces capsular contracture formation around the implant. The biological interface comprises a plurality of apertures along its periphery, and attaches to the medical implant by receiving a plurality of attachment flaps or appendages located on the exterior surface of the medical implant within or through the apertures. The attachment of the biological interface is such that the assembly remains intact even where the attachment flaps loosen upon expansion of the implant after insertion into a host, as where the implant is therein injected to a desired dimension.
Owner:MAXWELL G PATRICK
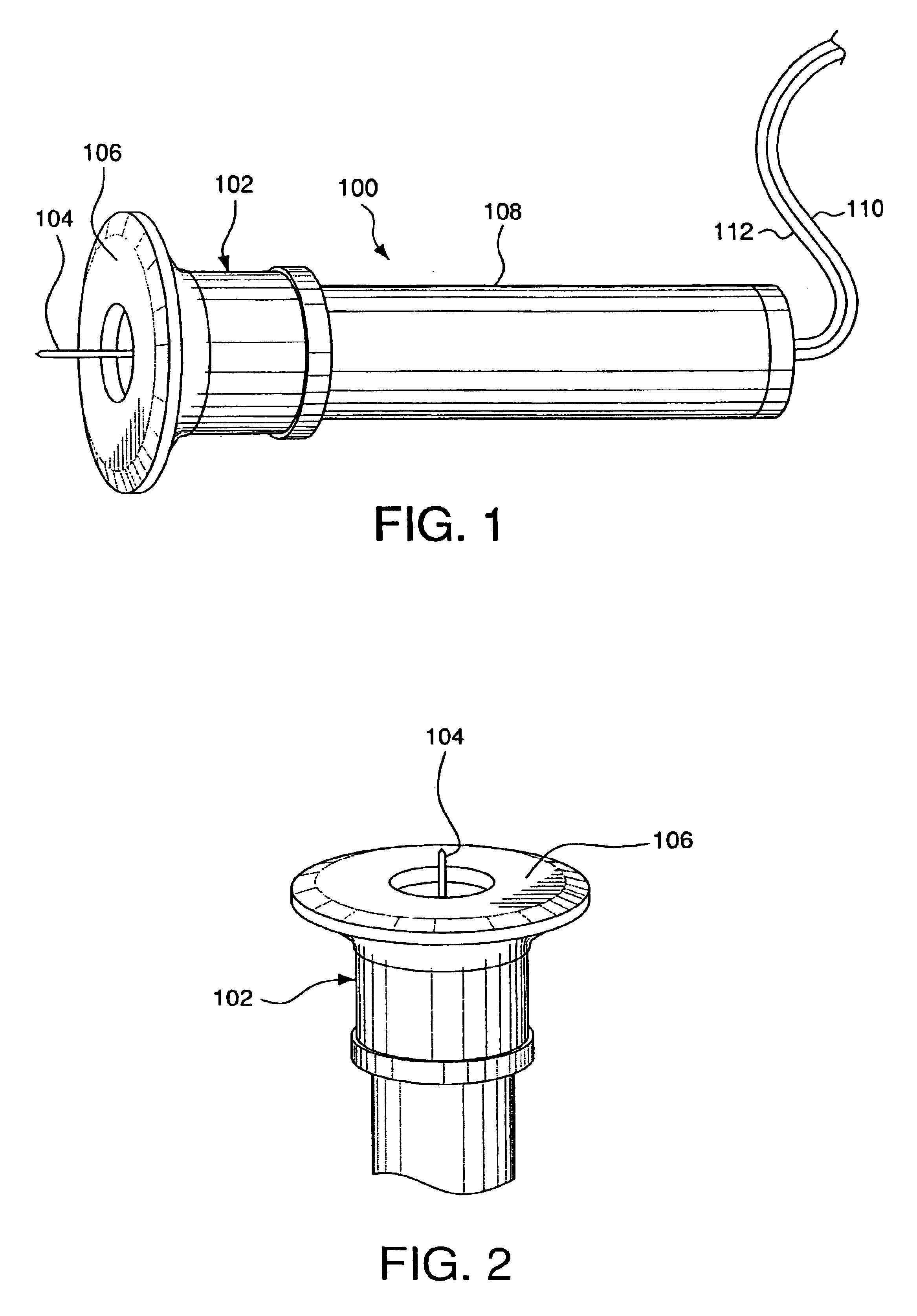
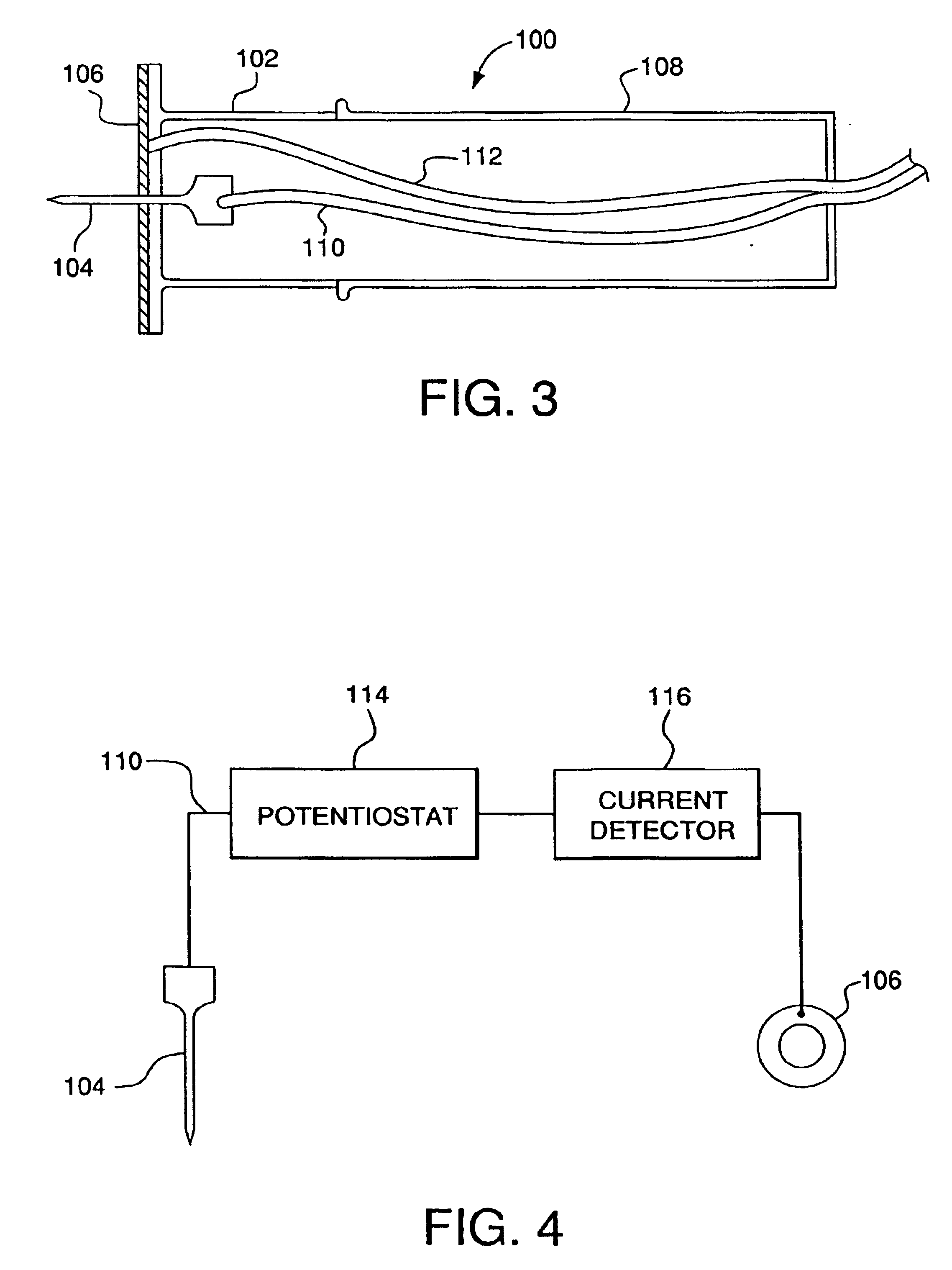
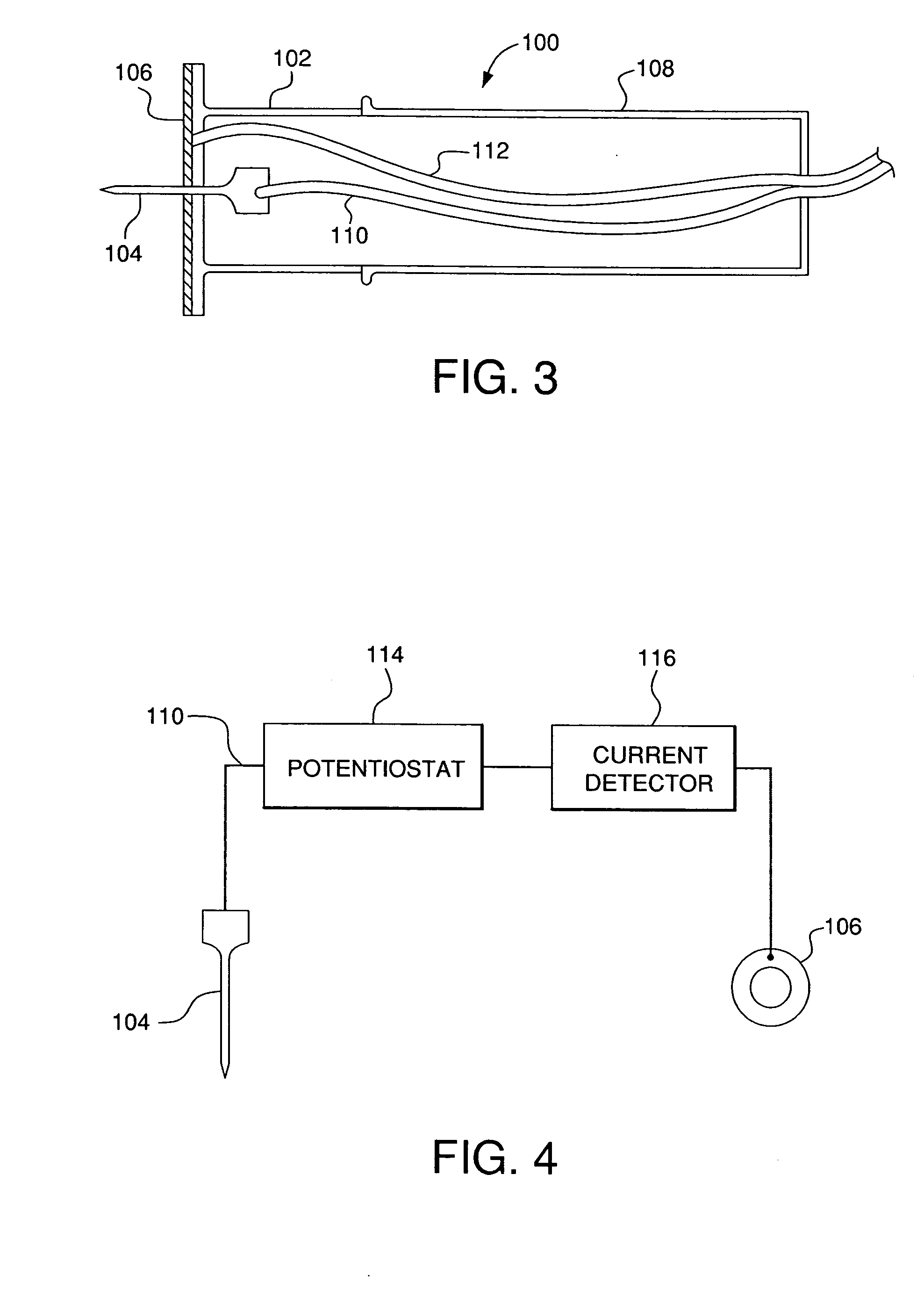
Minimally-invasive system and method for monitoring analyte levels
InactiveUS6952604B2Improve responseImprove signal and performanceCatheterSensorsAnalyteStratum corneum
A minimally-invasive analyte detecting device and method for using the same. The system and method employ a device having an active electrode optionally coated with a substance, and a counter-electrode that is configured at least partially surround the active electrode. The configuration of the auxiliary electrode and active electrode improves the current flow through the device and increases the sensitivity of the device. When the device is placed against the patient's skin, the active electrode is adapted to enter through the stratum corneum of a patient to a depth less than a depth in the dermis at which nerve endings reside. An electric potential is applied to the active electrode and the analyte level is determined based on the amount of current or charge flowing through the device.
Owner:BECTON DICKINSON & CO
Biopsy and sutureless device
InactiveUS8540646B2Speed up the processEliminate needVaccination/ovulation diagnosticsExcision instrumentsTissue sampleSurgical department
A dermal punch device for automatically extracting a sample of tissue of a predetermined size and shape from a body comprising a retractable cutter and a sutureless biopsy closure mechanism that includes a wound closure fastener member adapted to be disposed over a biopsy region after the performance of the biopsy, wherein wound closure fastener member is applied without the need of several instruments to seal the wound. The wound closure fastener member is dispensed by a sutureless biopsy closure dispenser located at the same distal end of the biopsy punch device surrounding the biopsy punch cutter assembly avoiding the need of separates instruments, reducing the wound closing steps and surgical procedure time.
Owner:MENDEZ COLL JOSE ARTURO
Methods and apparatus for skin treatment
InactiveUS6920883B2Thermal damage is minimizedMinimizes and avoids damageDiagnosticsSurgical instruments for heatingDermisFace lifting
Methods and apparatus for electrosurgically treating human skin. The skin may be treated by applying thermal energy to the dermis to shrink the skin following liposuction, or to induce collagen deposition at the site of a wrinkle for wrinkle reduction or removal. In another embodiment, a method involves electrosurgically removing or modifying tissue in the head or neck to provide a face-lift or a neck-lift. In one embodiment, the working end of an electrosurgical instrument is positioned in at least close proximity to the dermis by approaching the dermis from the underside (reverse side) of the skin.
Owner:ARTHROCARE
Method and apparatus for vacuum-assisted light-based treatments of the skin
InactiveUS20060259102A1Efficacy and utilityUltrasound therapyPneumatic massageVacuum assistedThree vessels
A method and apparatus are disclosed for enhancing the absorption of light in targeted skin structures and for the inhibition of pain transmission during light based treatments of the skin. After applying a vacuum to a vacuum chamber placed on a skin target and modulating the applied vacuum, the concentration of blood and / or blood vessels is increased within a predetermined depth below the skin surface of the skin target. Optical energy associated with light directed in a direction substantially normal to a skin surface adjoining the skin target is absorbed within the predetermined depth. The apparatus is suitable for treating vascular lesions with a reduced treatment energy density level than that of the prior art and for evacuating condensed vapors produced during the cooling of skin. The vacuum chamber may also have a skin flattening transmitting element which inhibits pain transmission upon applying a sufficiently high vacuum.
Owner:CANDELA CORP
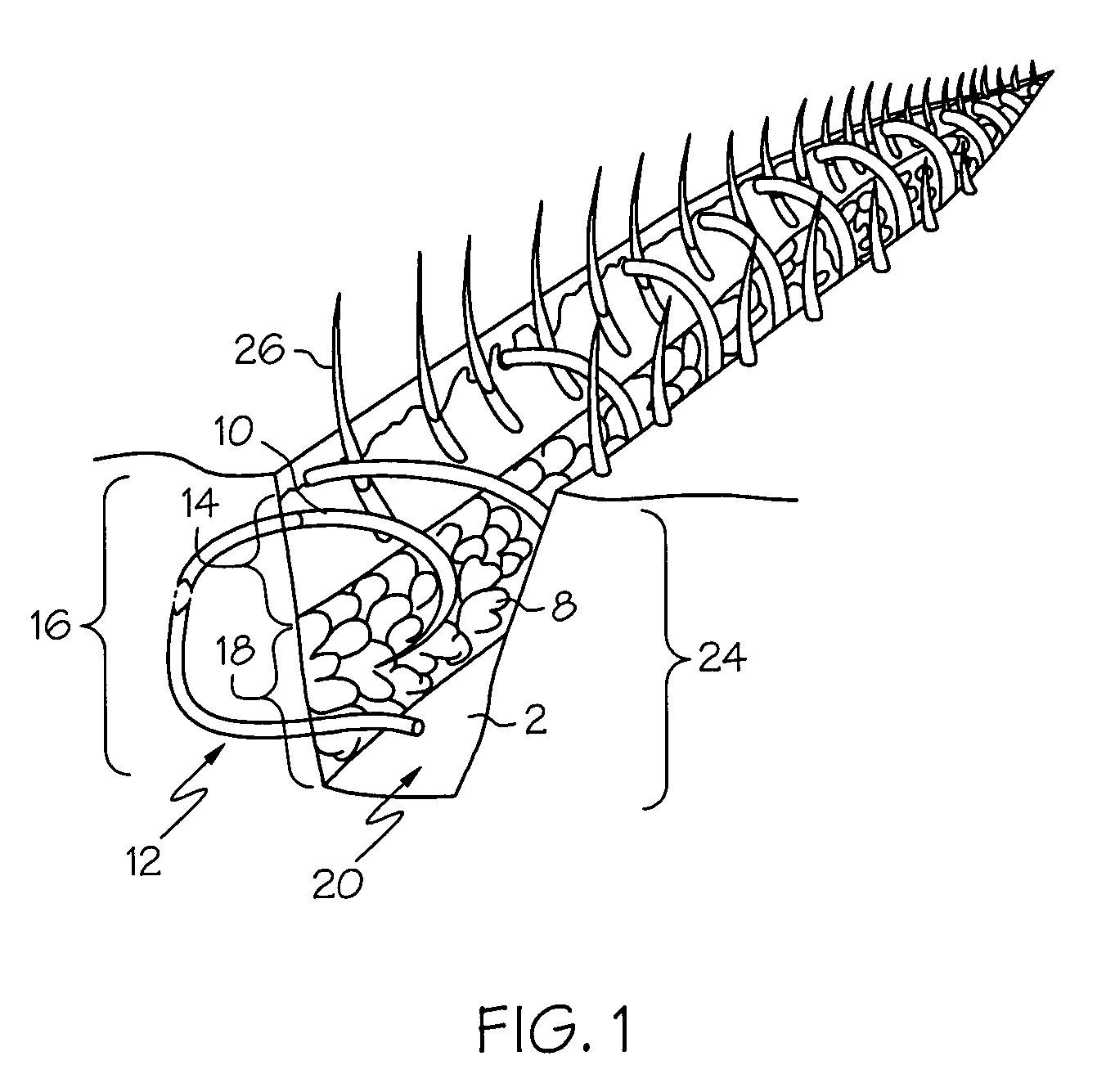
Intra dermal tissue fixation device
A device is disclosed for the securement of dermal tissues. The device provides approximation and eversion of the tissue as well as the placement of a fixation element that bridges a wound. The fixation element may be produced in several fixed or dynamic configurations that may or may not alter the ability to engage tissue in response to stresses placed upon the fixation member post-deployment. The delivery device inserts the fastener percutaneously through the epidermis into the sub-dermal position without entering the wound margins with any part of the approximation portion of the delivery device.
Owner:ETHICON INC
Minimally-invasive system and method for monitoring analyte levels
A minimally-invasive analyte detecting device and method for using the same. The system and method employ a device having an active electrode optionally coated with a substance, and a counter-electrode that is configured at least partially surround the active electrode. The configuration of the auxiliary electrode and active electrode improves the current flow through the device and increases the sensitivity of the device. When the device is placed against the patient's skin, the active electrode is adapted to enter through the stratum corneum of a patient to a depth less than a depth in the dermis at which nerve endings reside. An electric potential is applied to the active electrode and the analyte level is determined based on the amount of current or charge flowing through the device.
Owner:BECTON DICKINSON & CO
Solid solution perforator for drug delivery and other applications
InactiveUS6945952B2Fast biodegradationBarrier property can be diminished and controlledSurgeryMicroneedlesDrug reservoirDrugs solution
A solid drug solution perforator (SSP) system and an associated drug reservoir are provided for delivering therapeutic, prophylactic and / or cosmetic compounds, for nutrient delivery and for drug targeting. For drug delivery, the SSP system includes an active drug ingredient and a matrix of perforator material that biodegrades or dissolves quickly upon contact with a patient's body. The SSP system provides a skin barrier perforator and a controller for prompt initiation and cut-off drug delivery. In a preferred method of transdermal drug delivery, an SSP system containing a selected drug penetrates into an epidermis or dermis, and the drug is promptly released from the (dissolving) SSP system perforator. An additional drug is optionally delivered from a patch reservoir through skin pores created by insertion of the perforator. Formulation and fabrication procedures for the SSP and associated reservoir are also provided. An SSP system can be fabricated with variety of shapes and dimensions.
Owner:THERAJECT INC.
Applicator for applying functional substances into human skin
An applicator for applying functional substances, such as cosmetic powder, food color marking, India ink effect marks, or drugs into human skin, having a base, a plurality of microneedles fixed to and projecting from the base a distance only sufficient to penetrate into the stratum corneum or dermis, with the microneedles being of a material that is capable of disintegration and dispersion into the stratum corneum or dermis, such as maltose. The needles contain the functional substance for delivery into the stratum corneum or dermis. The microneedles are of a length approximately 0.5 to 500 μm when used to apply a functional substance to the stratum corneum, or are of a length of approximately 500 to 5,000 μm when used to apply a functional substance to the dermis.
Owner:TOBINAGA YOSHIKAZU +1
Soft and calcified tissue implants
InactiveUS20050101957A1Minimize movementReduce degradationInternal osteosythesisSurgical adhesivesLigament structurePlastic surgery
Disclosed herein is processed dermis graft for use in orthopedic surgical procedures. Specifically exemplified herein is a processed dermis graft comprising one or more bone blocks having a groove cut into the surface thereof, wherein said groove is sufficient to accommodate a fixation screw. Also disclosed is a method of processing dermis that results in a dermis derived implant suitable to replace a tendon or ligament in a recipient in need thereof. Other compositions and applications of a dermis derived implant, and methods of manufacture and use, are disclosed.
Owner:RTI BIOLOGICS INC
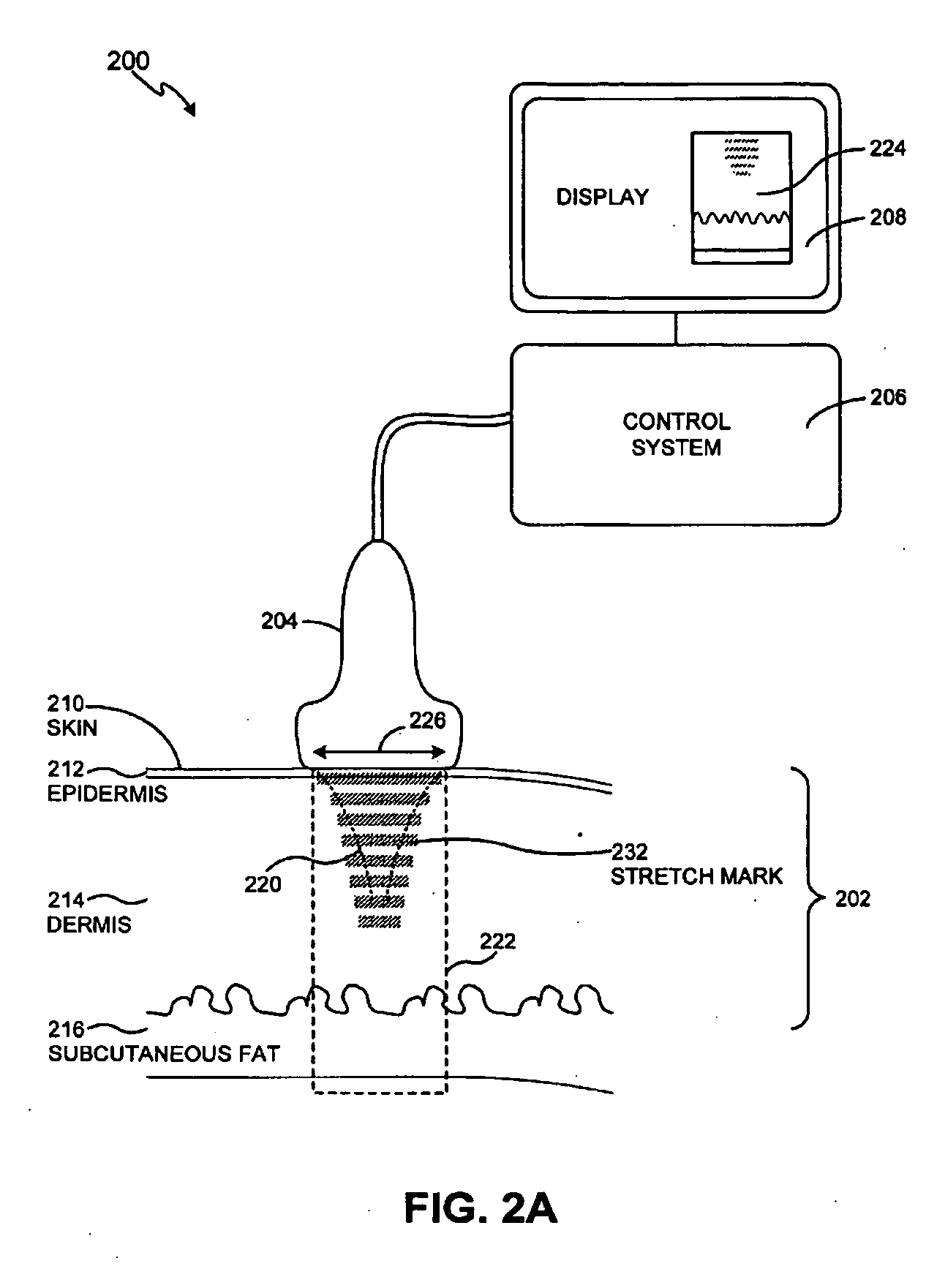
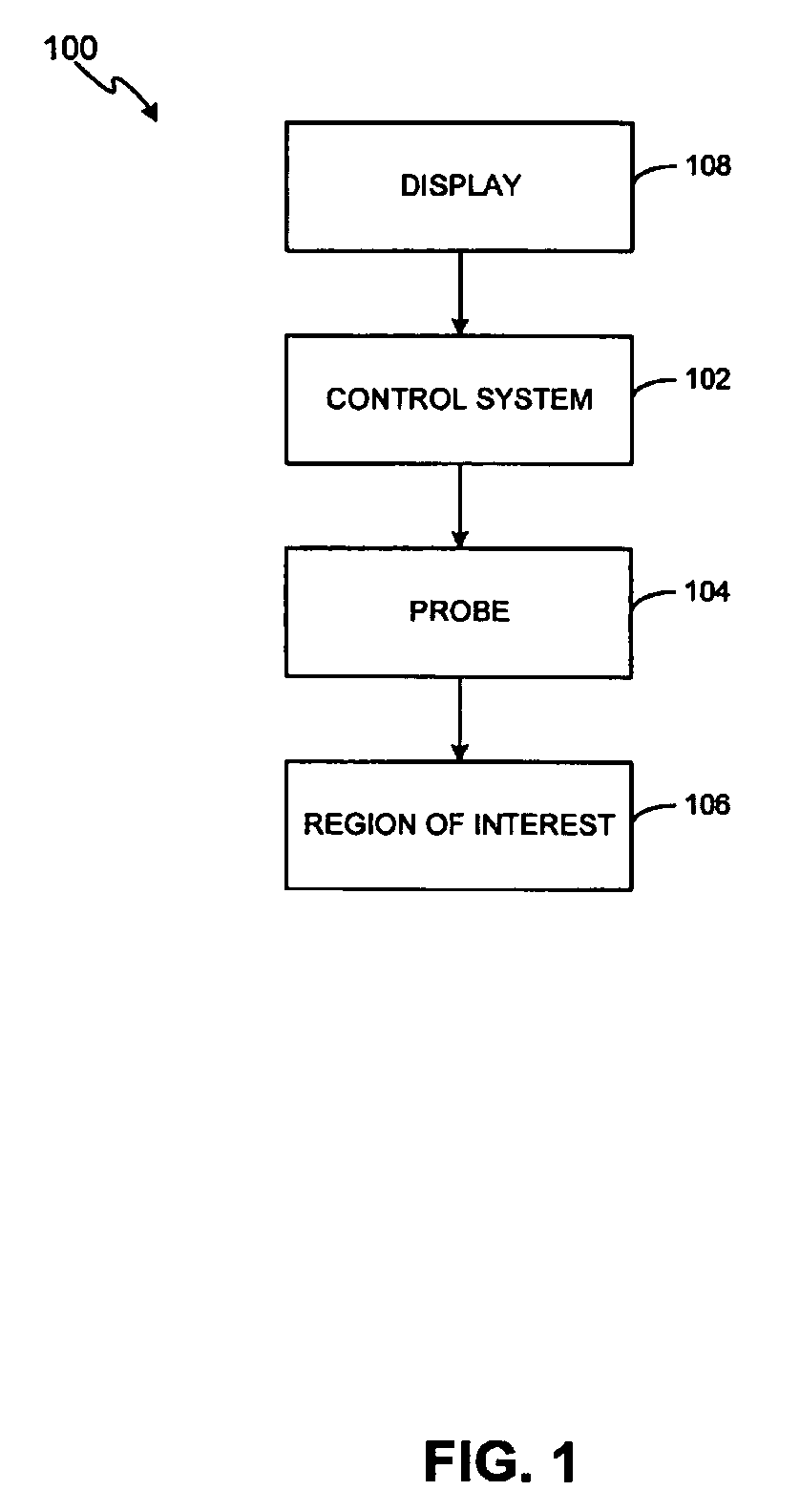
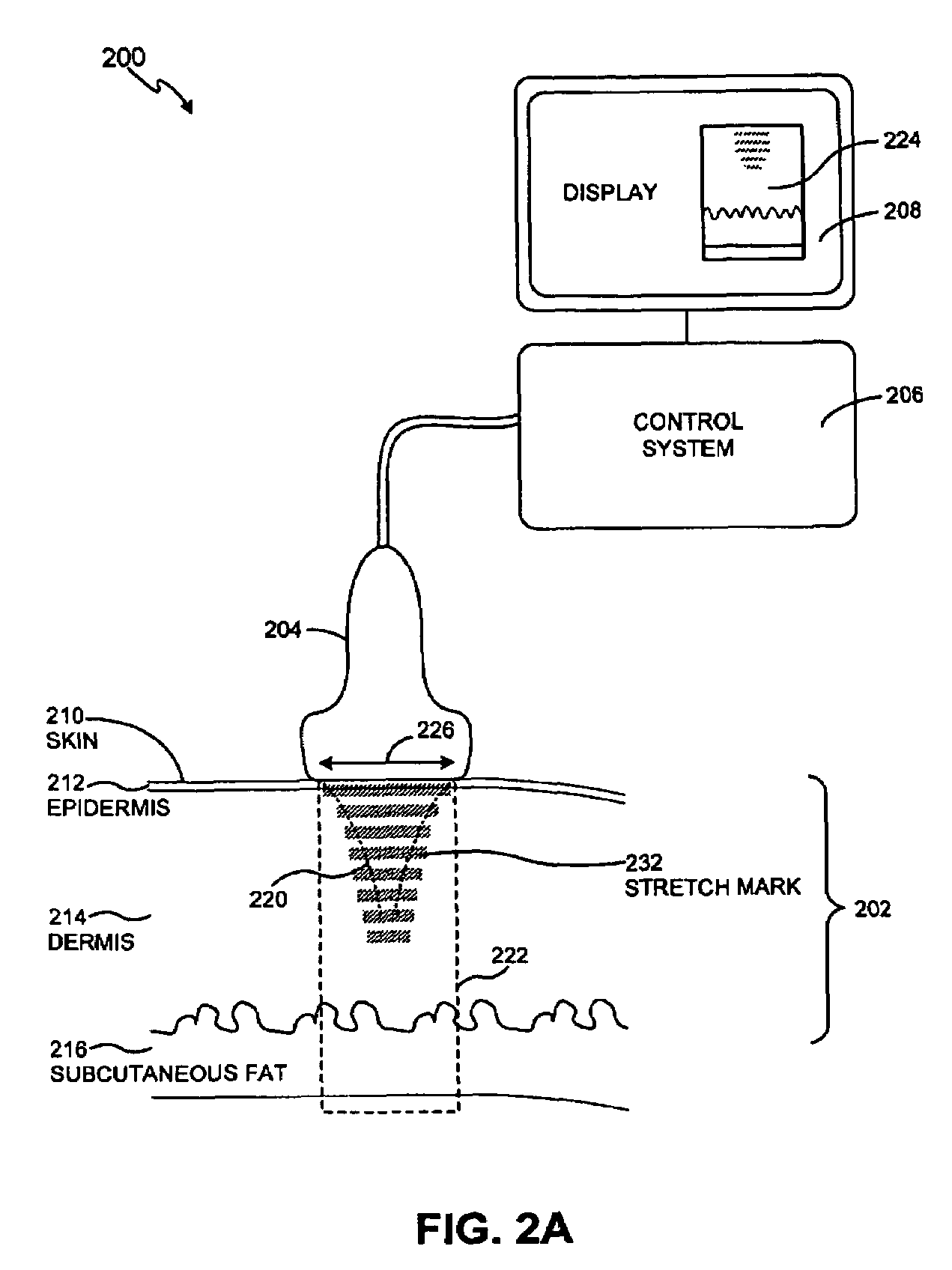
Method and system for treating stretch marks
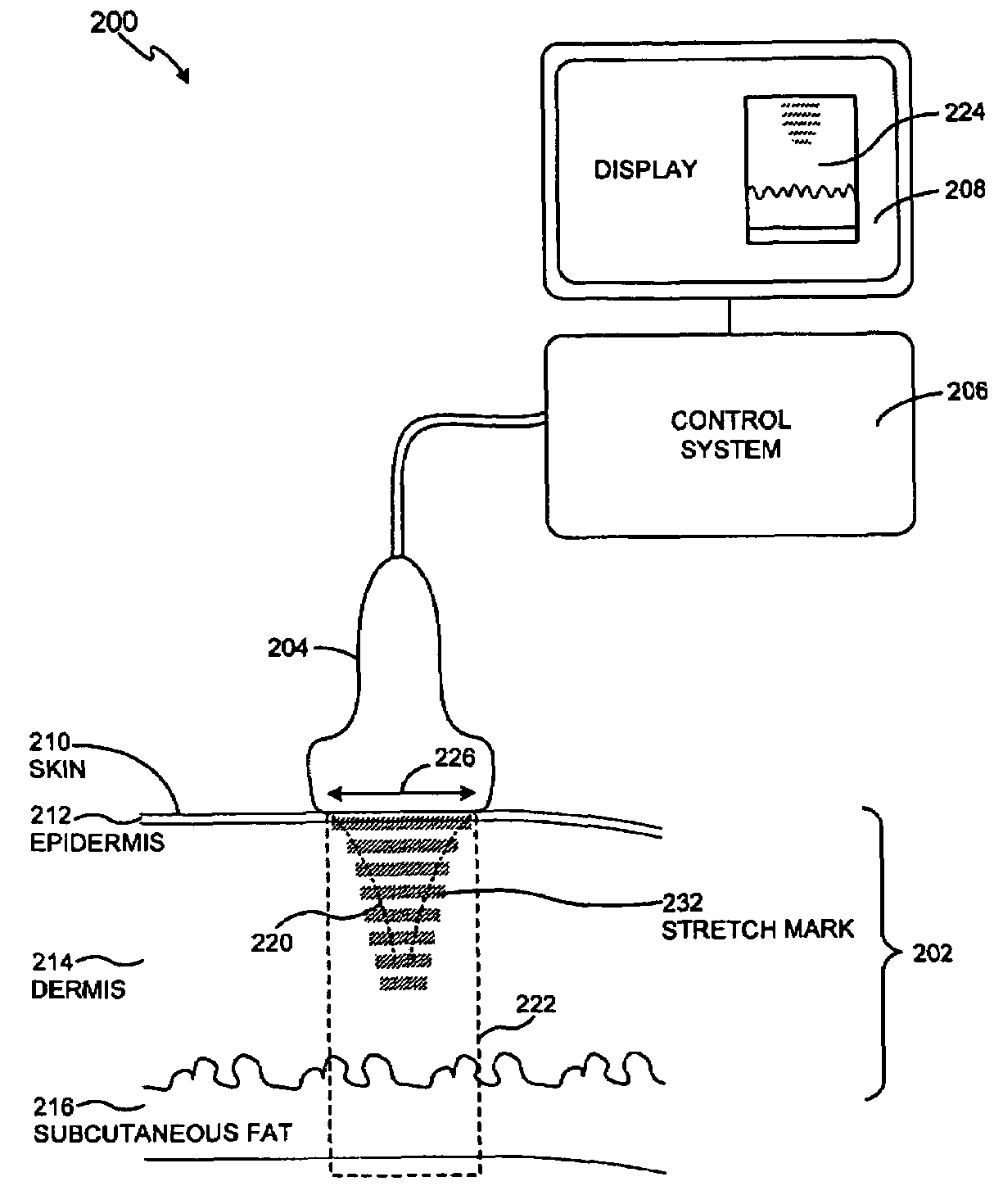
Methods and systems for treating stretch marks through deep tissue tightening with ultrasound are provided. An exemplary method and system comprise a therapeutic ultrasound system configured for providing ultrasound treatment to a shallow tissue region, such as a region comprising an epidermis, a dermis and a deep dermis. In accordance with various exemplary embodiments, a therapeutic ultrasound system can be configured to achieve depth from 0 mm to 1 cm with a conformal selective deposition of ultrasound energy without damaging an intervening tissue in the range of frequencies from 2 to 50 MHz. In addition, a therapeutic ultrasound can also be configured in combination with ultrasound imaging or imaging / monitoring capabilities, either separately configured with imaging, therapy and monitoring systems or any level of integration thereof.
Owner:GUIDED THERAPY SYSTEMS LLC
Intra Dermal Tissue Fixation Device
A device is disclosed for the securement of dermal tissues. The device provides approximation and eversion of the tissue as well as the placement of a fixation element that bridges a wound. The fixation element may be produced in several fixed or dynamic configurations that may or may not alter the ability to engage tissue in response to stresses placed upon the fixation member post-deployment. The delivery device inserts the fastener percutaneously through the epidermis into the sub-dermal position without entering the wound margins with any part of the approximation portion of the delivery device.
Owner:ETHICON INC
Transcutaneous Device Assembly
InactiveUS20090054866A1Convenient and cost-effective mannerSafe and reliable treatment of medicalAutomatic syringesMedical devicesDistal portionMedical device
A medical device is provided comprising a cannula having a distal portion adapted to be arranged through the skin of the subject, and an outer needle arranged coaxially with and being axially moveable relative to the cannula, the needle comprising a pointed distal end, wherein the device is adapted for advancing the cannula with the distal end of the needle projecting there from through the dermis of the subject, and subsequently retract the needle.
Owner:NOVO NORDISK AS
Method and system for treating stretch marks
Methods and systems for treating stretch marks through deep tissue tightening with ultrasound are provided. An exemplary method and system comprise a therapeutic ultrasound system configured for providing ultrasound treatment to a shallow tissue region, such as a region comprising an epidermis, a dermis and a deep dermis. In accordance with various exemplary embodiments, a therapeutic ultrasound system can be configured to achieve depth from 0 mm to 1 cm with a conformal selective deposition of ultrasound energy without damaging an intervening tissue in the range of frequencies from 2 to 50 MHz. In addition, a therapeutic ultrasound can also be configured in combination with ultrasound imaging or imaging / monitoring capabilities, either separately configured with imaging, therapy and monitoring systems or any level of integration thereof.
Owner:GUIDED THERAPY SYSTEMS LLC
Systems and methods for intradermal collagen stimulation
InactiveUS20020128641A1Minimizing and suppressing wound healing phaseReduce removalDiagnosticsSurgical needlesWound healingWrinkle skin
The present invention provides systems, apparatus and methods for selective applying energy to a patient's dermis tissue to generate the growth of new collagen in this tissue, while minimizing the effect on the outer epidermis layer, thereby minimizing or suppressing the wound healing phase of the procedure. In one aspect of the invention, a method includes positioning a first electrode adjacent to, or in contact with, a region on or within a patient's skin, and applying a sufficient high frequency voltage between the first electrode and a second electrode to create a heat injury to a target tissue within the patient's dermis layer without ablating the epidermis layer overlying the target tissue. Typically, the voltage applied to the first and second electrodes is sufficient to induce heating of the dermis layer to about 60.degree.-80.degree. C., preferably about 65.degree.-75.degree. C. This induced heating causes the patient's body to undergo a wound healing response in the slightly inflamed tissue of the dermis. The wound healing process involves the generation of neo-collagen in the dermis layer, which fills in the wrinkle in the patient's skin.
Owner:ARTHROCARE
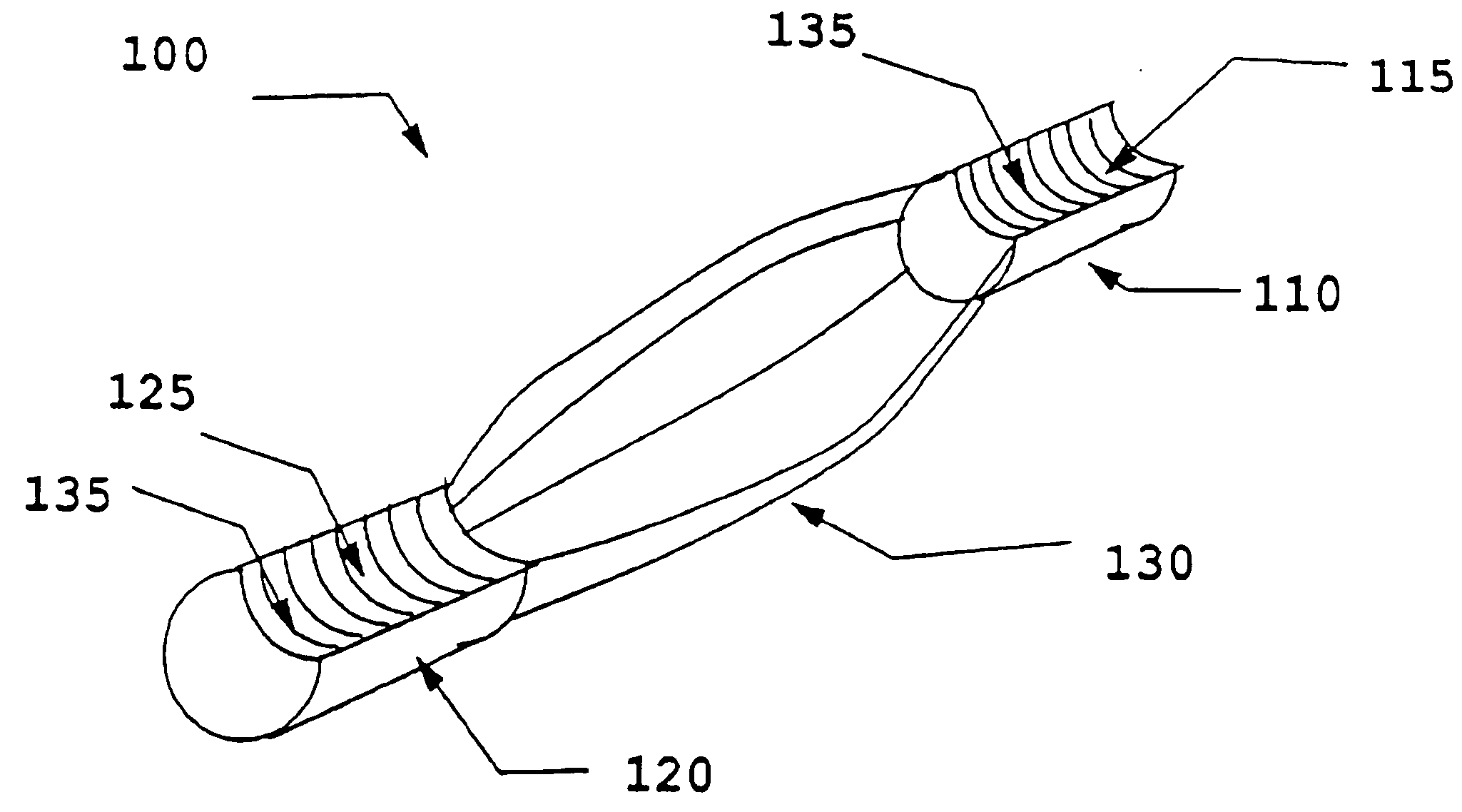
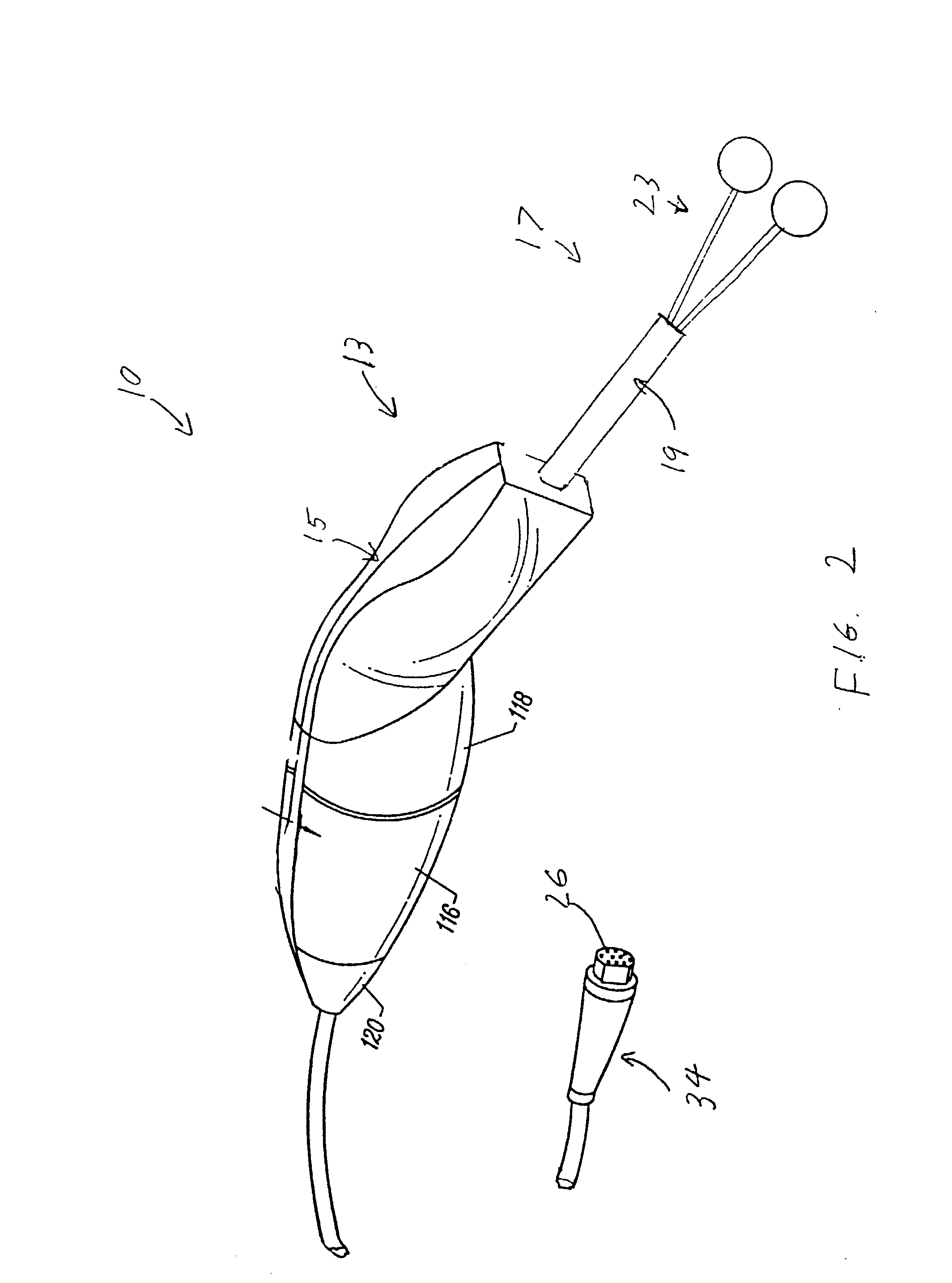
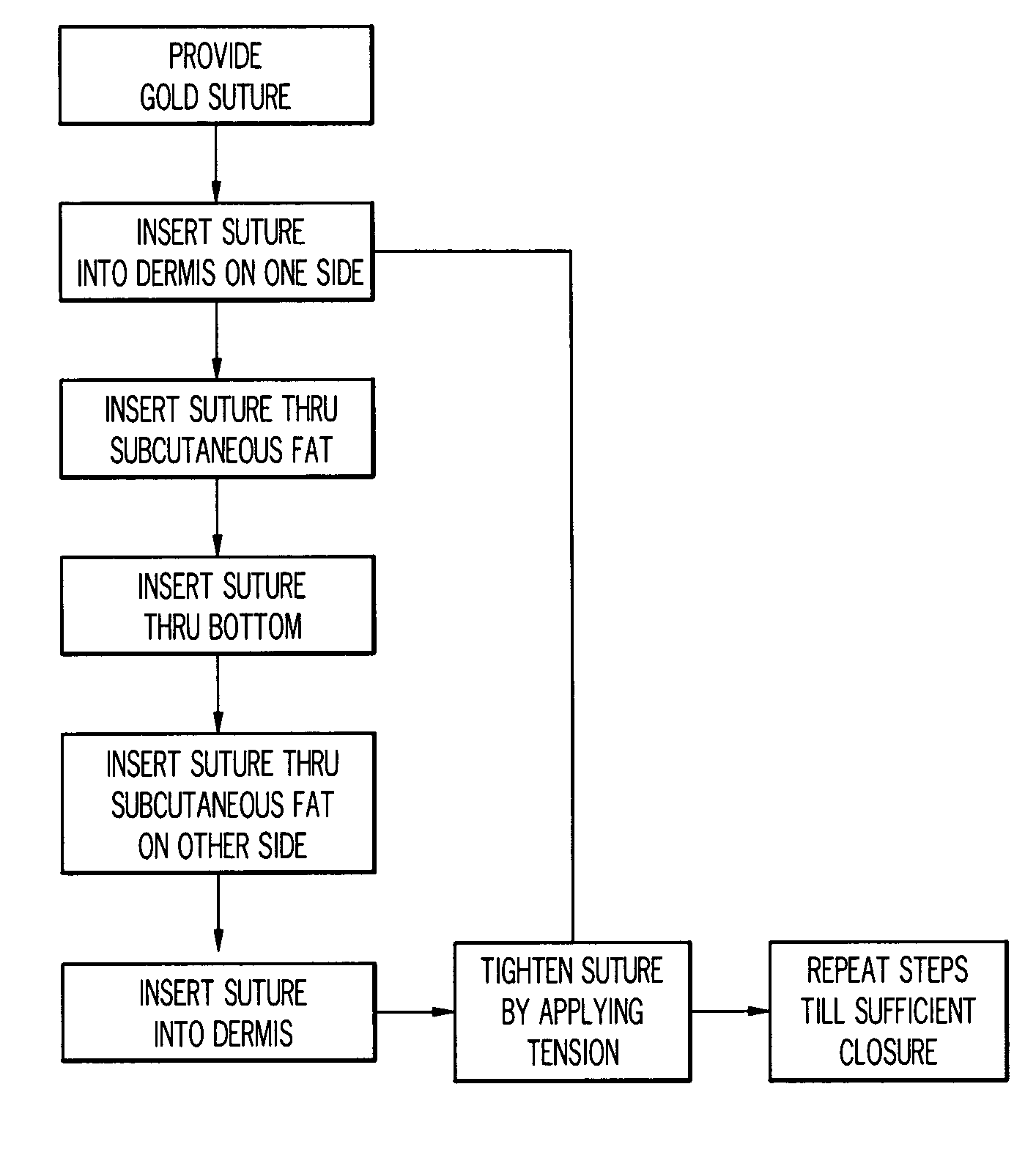
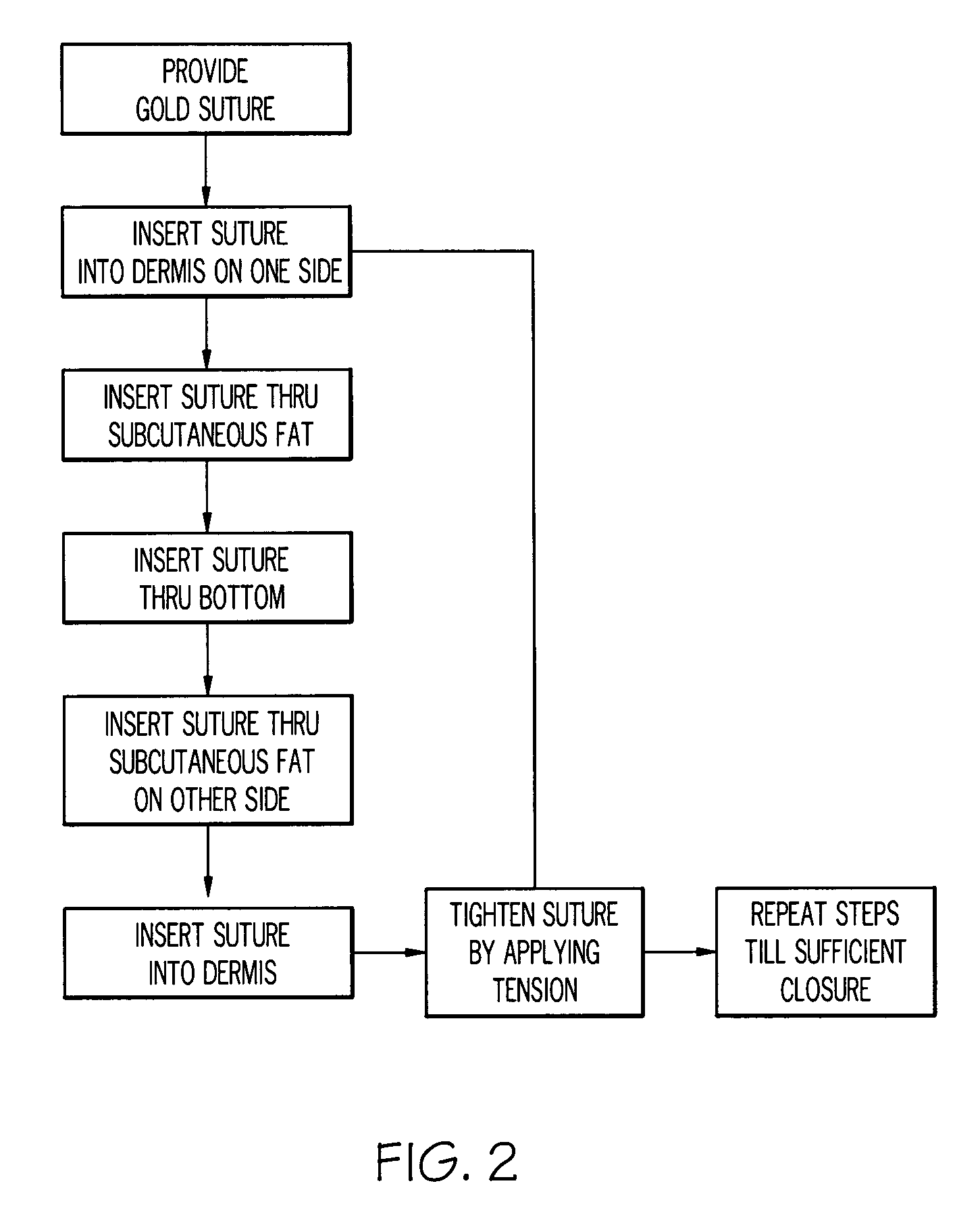
Gold suture and method of use in wound closure
A method of wound closure comprising providing a gold suture and closing the laceration with the gold suture. The laceration is closed by: inserting the suture into the dermis and through the subcutaneous fat layer on one side of the laceration; inserting the suture at the base of the laceration; inserting the suture through the subcutaneous fat layer and into the dermis on the other side of the laceration to form a loop under the skin; repeating the steps in another insertion 3 to 5 mm from the loop in a continuous or interrupted manner to close the laceration; and tightening the suture line by applying tension to the loops to bring one side of the laceration into contact with the other side of the laceration.
Owner:GOLD THREAD
Prefillable intradermal delivery device
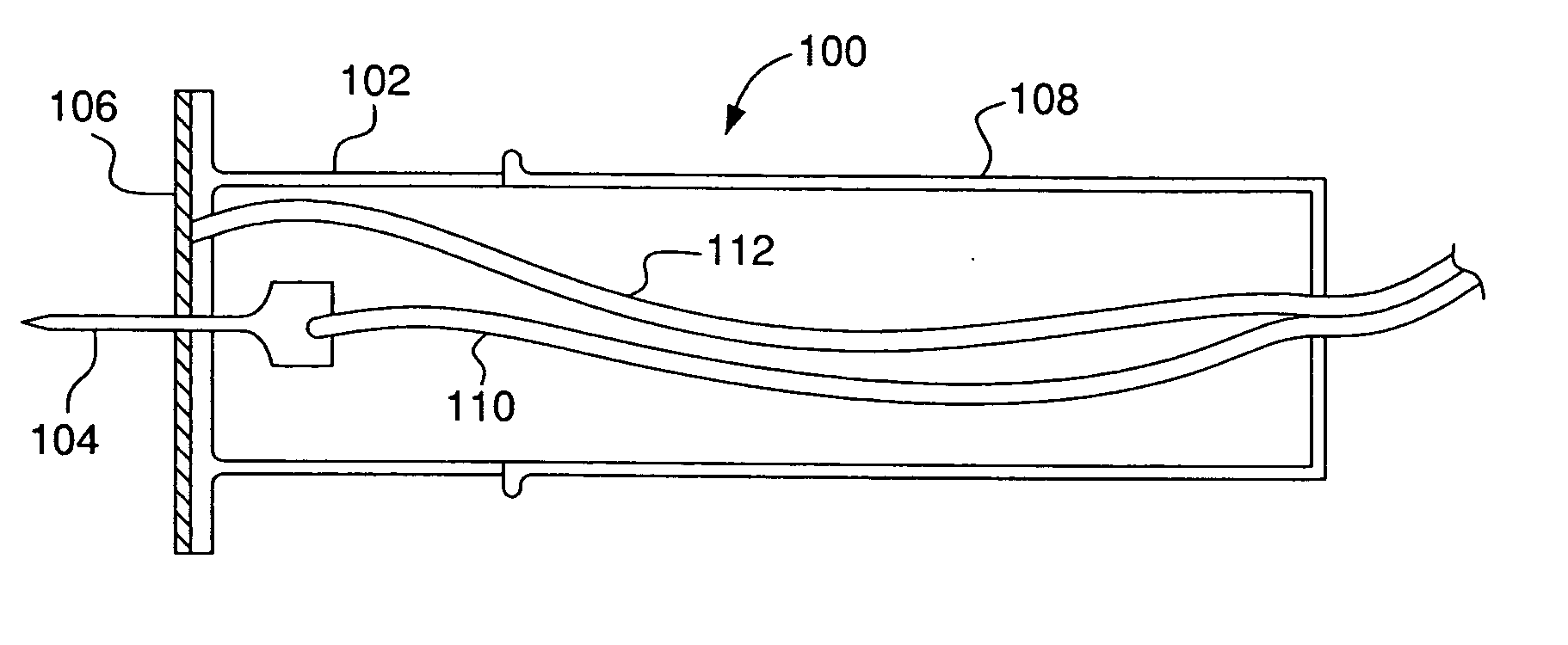
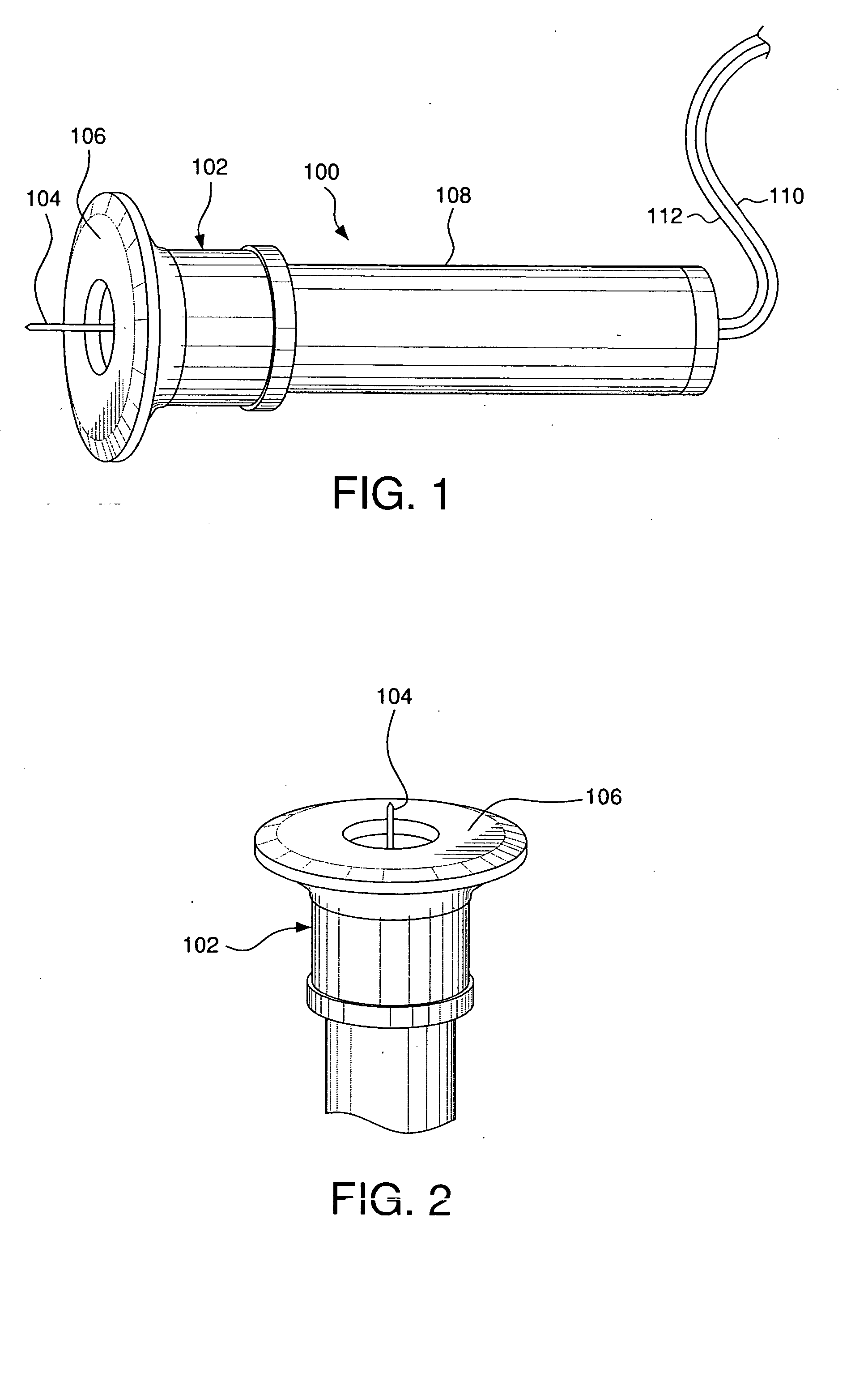
An intradermal delivery device for use in intradermally injecting substances into the skin of an animal includes a needle cannula supported by a hub portion that is attachable to a prefillable container. A limiter portion surrounds the needle cannula and extends away from the hub portion toward a forward tip of the needle cannula. The limiter portion includes a skin engaging surface extending in a plane generally perpendicular to an axis of the needle cannula. The skin engaging surface is received against skin of an animal to administer an intradermal injection. The forward tip extends beyond the skin engaging surface a distance that enables penetration of the needle cannula into the dermis layer of the skin of the animal enabling injection of the substance into the dermis layer of the animal. The device includes enclosure means that is moveable for concealing the needle cannula after the injection has been administered.
Owner:BECTON DICKINSON & CO
Soft and calcified tissue implants
InactiveUS6893462B2Minimize movementReduce degradationSurgical adhesivesBone implantLigament structurePlastic surgery
Disclosed herein is processed dermis graft for use in orthopedic surgical procedures. Specifically exemplified herein is a processed dermis graft comprising one or more bone blocks having a groove cut into the surface thereof, wherein said groove is sufficient to accommodate a fixation screw. Also disclosed is a method of processing dermis that results in a dermis derived implant suitable to replace a tendon or ligament in a recipient in need thereof. Other compositions and applications of a dermis derived implant, and methods of manufacture and use, are disclosed.
Owner:RTI BIOLOGICS INC
Method and system for treating cellulite
ActiveUS8133180B2Good lookingUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyThermal injuryUltrasound imaging
Owner:GUIDED THERAPY SYSTEMS LLC
Fluidic Tissue Augmentation Compositions and Methods
InactiveUS20070212385A1Quality improvementExtending and improving qualityBiocideCosmetic preparationsBiologyTissue augmentation
Owner:KYTHERA BIOPHARMLS INC
Packaged medical device with a deployable dermal tissue penetration member
A packaged medical device includes upper and lower flexible sheets, a lance body and a test strip. The lance body includes upper and lower surfaces, an opening that extends between the upper and lower surfaces and a dermal tissue penetration member that projects into the lance body opening. The test strip has an opening therethrough and is attached to the lance body lower surface such that the dermal tissue penetration member is operatively aligned with the test strip opening. The upper flexible sheet is attached to the lance body upper surface and covers the lance body opening, while the lower flexible sheet is detachably attached to the test strip and covers the test strip opening. The upper flexible sheet, lance body and test strip are configured such that, when the lower flexible sheet has been detached to uncover the test strip opening, the upper flexible sheet, lance body and test strip can be bent to deploy the dermal tissue penetration member from the lance body opening. A kit includes the packaged medical device described above and a deployment device for detaching the lower flexible sheet and bending the upper flexible sheet, lance body and test strip to deploy the dermal tissue penetration member. A method for deploying a dermal tissue penetration member of a packed medical device includes providing the packaged medical device described above, detaching the lower flexible sheet to uncover the test strip opening and bending the upper flexible sheet, lance body and test strip to deploy the dermal tissue penetration member.
Owner:LIFESCAN IP HLDG LLC
Interfaced Medical Implant Assembly
ActiveUS20090125107A1Reduce and eliminate capsular contracturePromote formationMammary implantsTissue regenerationBreast prosthesesAppendage
A medical implant assembly and method having a medical implant, e.g. a breast prostheses, attached to a biological interface. The biological interface is comprised of a dermal material with capsular contracture inhibiting properties so that once the medical assembly is inserted into the host, the biological interface, which is intimately coupled to the implant, prevents / reduces capsular contracture formation around the implant. The biological interface comprises a plurality of apertures along its periphery, and attaches to the medical implant by receiving a plurality of attachment flaps or appendages located on the exterior surface of the medical implant within or through the apertures. The attachment of the biological interface is such that the assembly remains intact even where the attachment flaps loosen upon expansion of the implant after insertion into a host, as where the implant is therein injected to a desired dimension.
Owner:MAXWELL G PATRICK
Packaged medical device with a deployable dermal tissue penetration member
A packaged medical device includes upper and lower flexible sheets, a lance body and a test strip. The lance body includes upper and lower surfaces, an opening that extends between the upper and lower surfaces and a dermal tissue penetration member that projects into the lance body opening. The test strip has an opening therethrough and is attached to the lance body lower surface such that the dermal tissue penetration member is operatively aligned with the test strip opening. The upper flexible sheet is attached to the lance body upper surface and covers the lance body opening, while the lower flexible sheet is detachably attached to the test strip and covers the test strip opening. The upper flexible sheet, lance body and test strip are configured such that, when the lower flexible sheet has been detached to uncover the test strip opening, the upper flexible sheet, lance body and test strip can be bent to deploy the dermal tissue penetration member from the lance body opening. A kit includes the packaged medical device described above and a deployment device for detaching the lower flexible sheet and bending the upper flexible sheet, lance body and test strip to deploy the dermal tissue penetration member. A method for deploying a dermal tissue penetration member of a packed medical device includes providing the packaged medical device described above, detaching the lower flexible sheet to uncover the test strip opening and bending the upper flexible sheet, lance body and test strip to deploy the dermal tissue penetration member.
Owner:LIFESCAN IP HLDG LLC
Method and apparatus for selective treatment of biological tissue using ultrasound energy
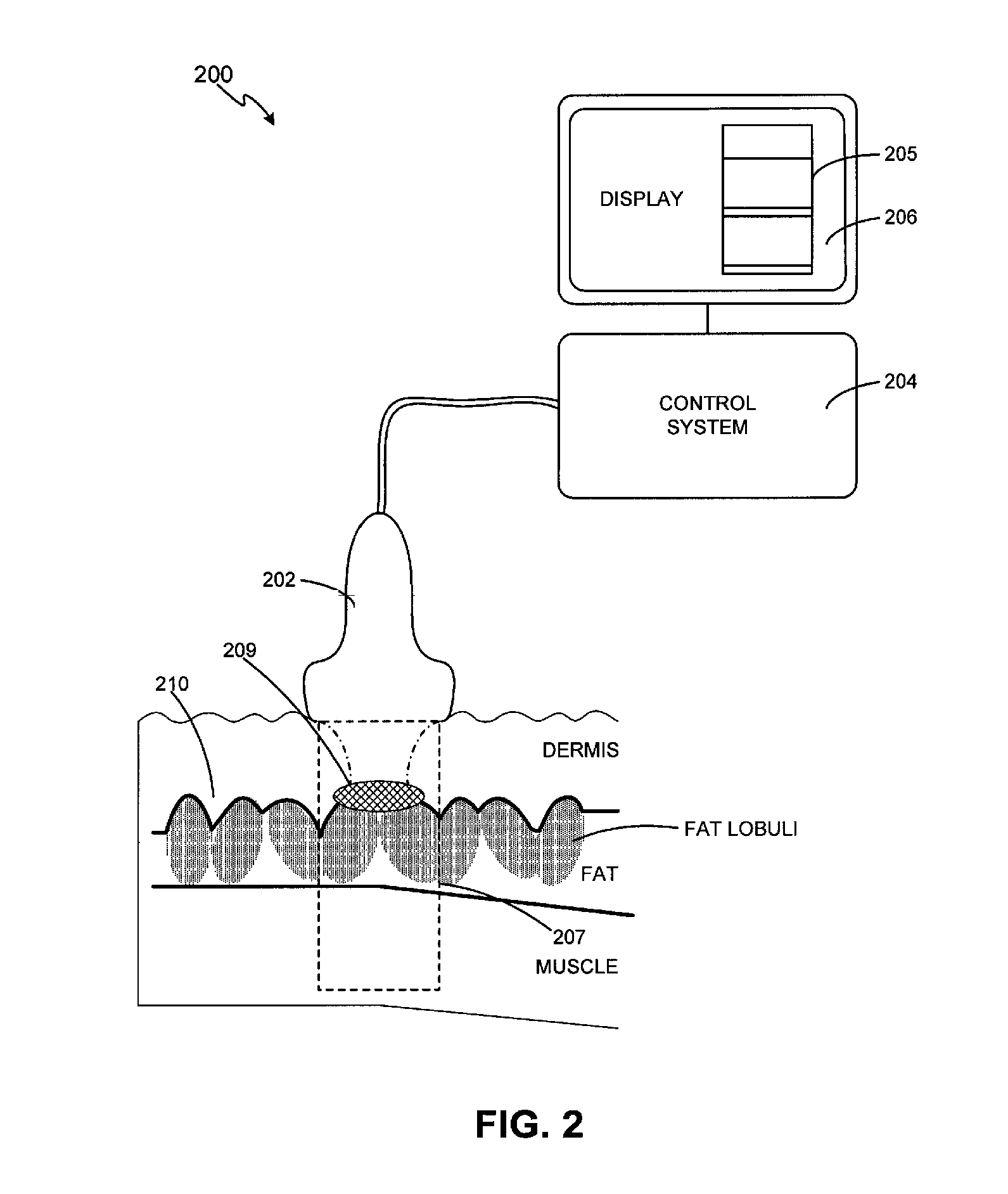
A method and apparatus are provided for dermatological treatment by focusing ultrasound energy in a volume of tissue below the dermis to obtain selective heating and thermal damage of certain portions of the volume while sparing other portions of the treatment volume from thermal damage. Selective heating of fibrous septae can be achieved while relatively sparing surrounding fatty tissue, which can lead to some shrinkage of the fibrous septae and reduction in the appearance of wrinkles. The matrix of hair follicles can also be selectively heated to provide relatively safe temporary or permanent hair removal. The superficial musculoaponeurotic system can also be selectively heated to obtain a tightening of the overlying skin.
Owner:THE GENERAL HOSPITAL CORP
Compositions of polyacids and polyethers and methods for their use as dermal fillers
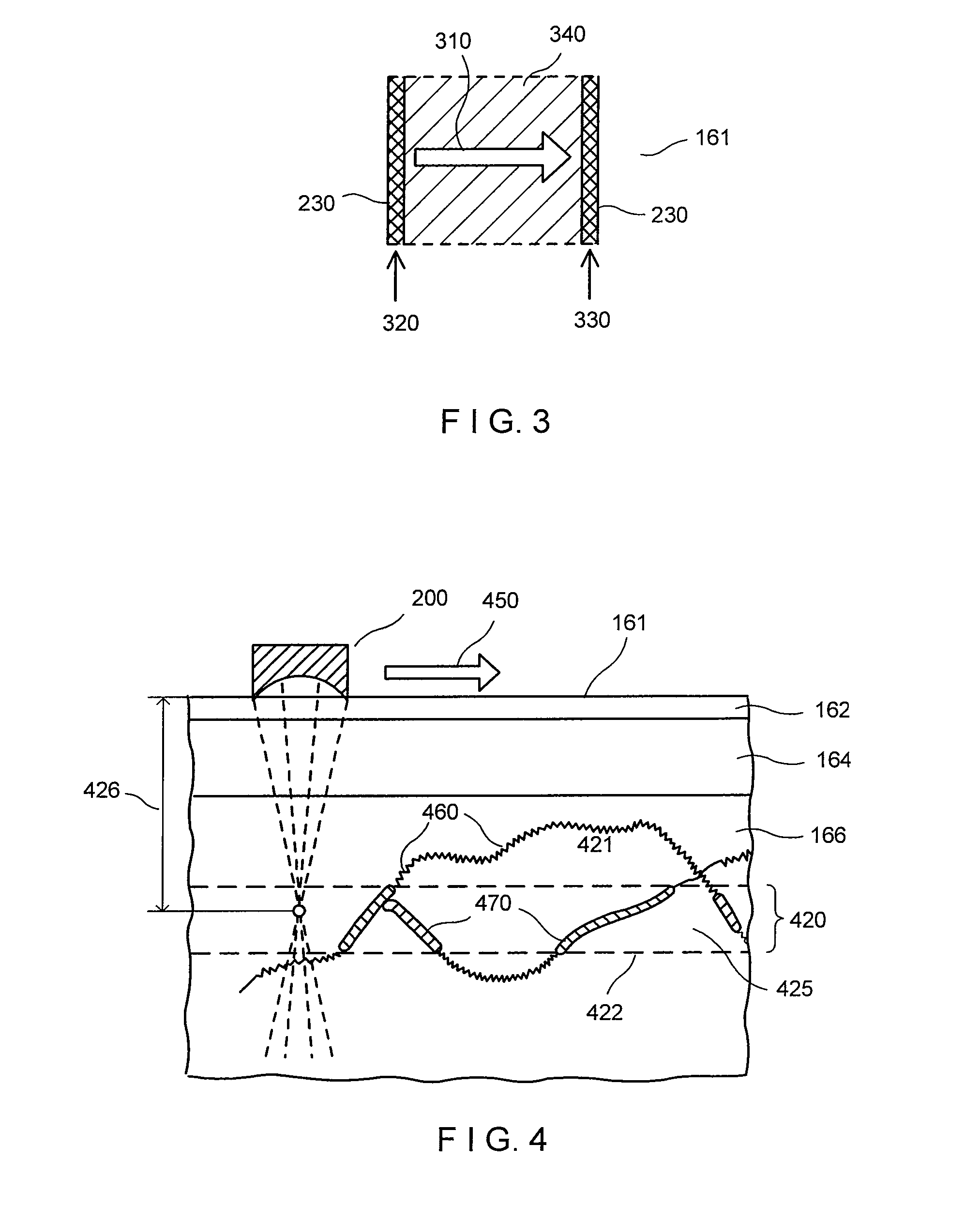
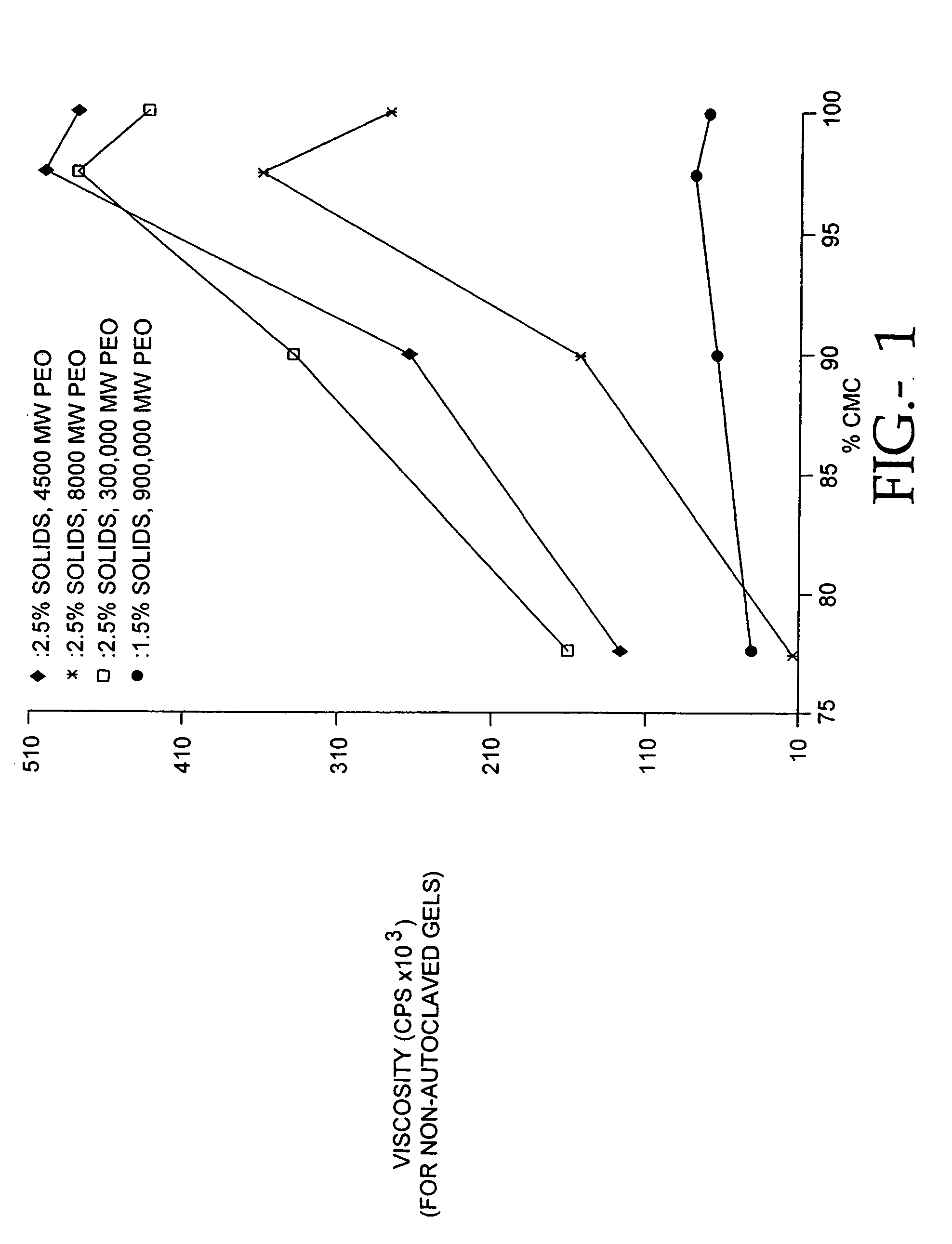
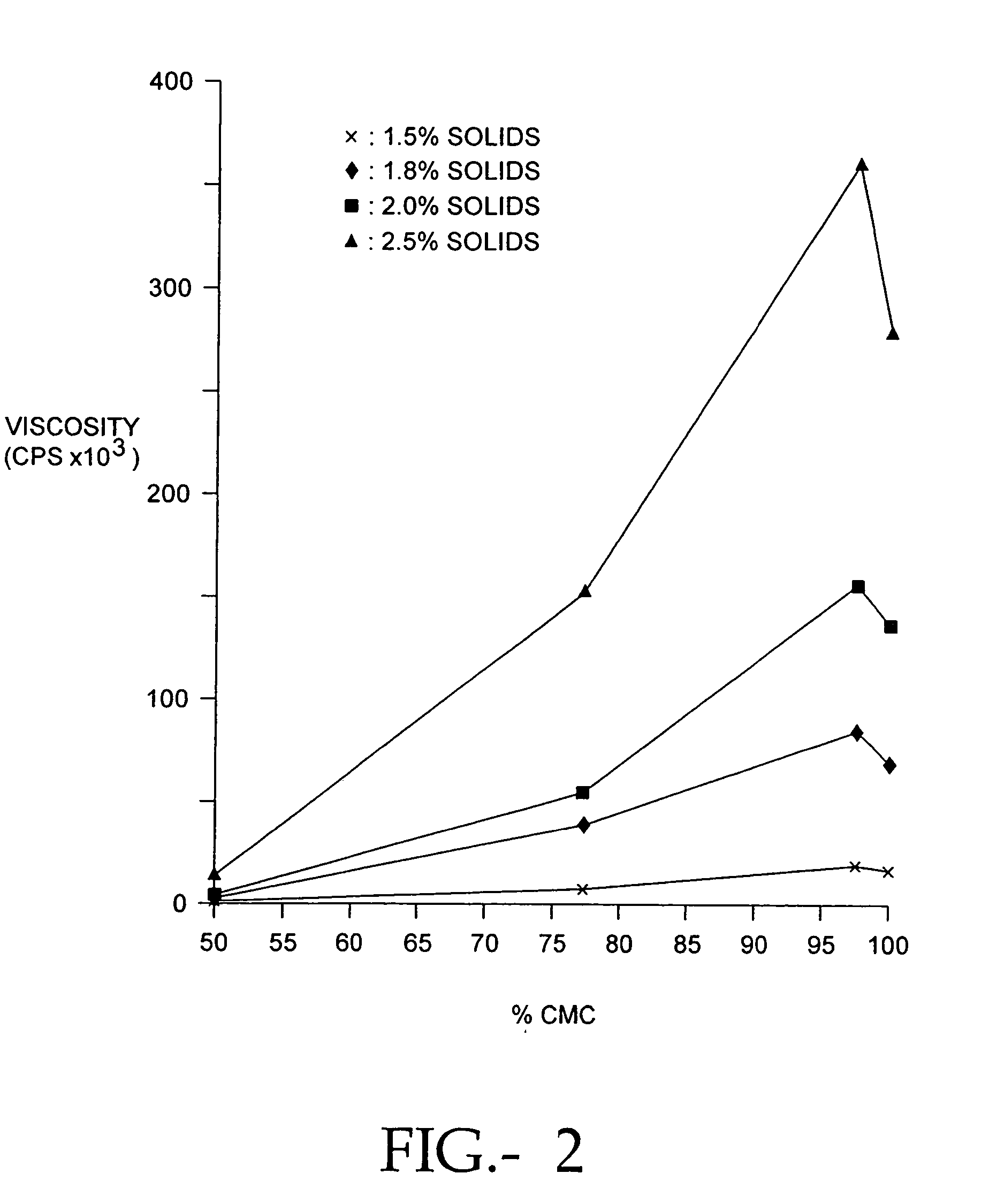
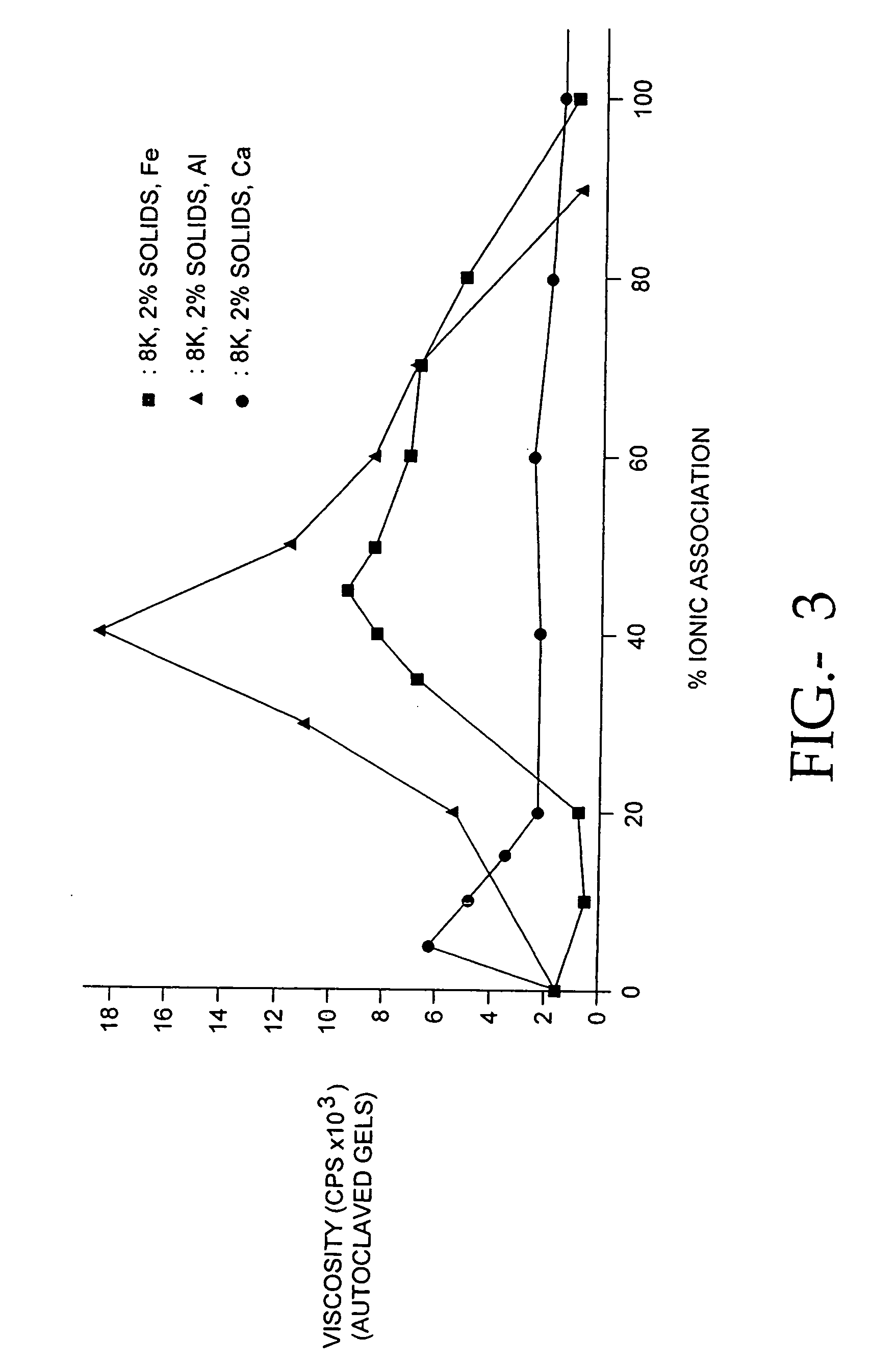
The present invention relates to improved methods for filling the skin for cosmetic or medical purposes. Compositions comprising carboxymethyl cellulose (CMC), polyethylene oxide (PEO) and calcium ions can be made and have physical properties that depend on the amounts and types of CMC, PEO, and calcium ions to form ioniclaly cross-linked gels. Compositions can be formed into microspheres, coascervates, gels, or membranes. Gels, microspheres and coascervates can be injected directly into a site for dermal filling. Membranes can be surgically introduced, where they swell to form hydrated gels. After introduction, the dermal filler persists for a period of time and then can disintegrate and be removed from the body.
Owner:FZIOMED
Ultrasound assisted transdermal vaccine delivery method and system
InactiveUS20050112135A1Adequate buffering capacityHigh viscosityViral antigen ingredientsSurgeryVaccine deliveryBiocompatible coating
An apparatus and method for transdermally delivering a vaccine comprising a delivery system having (i) a microprojection member (or system) that includes a plurality of microprojections (or array thereof) that are adapted to pierce through the stratum corneum into the underlying epidermis layer, or epidermis and dermis layers and (ii) an ultrasonic device. In one embodiment, the vaccine is contained in a biocompatible coating that is applied to the microprojection member. In a further embodiment, the delivery system includes a gel pack having a vaccine-containing hydrogel formulation that is disposed on the microprojection member after application to the skin of a patient. In an alternative embodiment, the vaccine is contained in both the coating and the hydrogel formulation.
Owner:ALZA CORP
Treatment of sweat glands
InactiveUS20110190745A1Less side effectsLow costSurgical instruments using microwavesRadiation therapyThermal injuryCuticle
A treatment of sweat glands in a target region of skin includes generating electromagnetic radiation having a wavelength of about 1,064 nm to about 1,800 nm. To decrease sweat production in a plurality of sweat glands, the electromagnetic radiation is delivered to a dermal interface defined by a dermal region and a subcutaneous fat region in the target region of skin to cause thermal injury to at least one of the dermal region, the subcutaneous fat region or the dermal interface. An epidermal region of the skin can be cooled at least one of before, during or after delivering the electromagnetic radiation to the dermal interface in the target region of skin.
Owner:CANDELA CORP
Methods and devices for intradermal injection
ActiveUS20100137831A1Minimal trainingMinimizes skillInfusion syringesMedical devicesBiomedical engineeringSkin surfaces
Devices and methods for intradermal (ID) administration of diagnostic and therapeutic agents, vaccines and other compounds into the dermal layer of the skin are disclosed. The devices and the methods simplify the ID injection process and increase the consistency of the placement of the needle tip in the dermal space close to the skin surface allowing for a shallow cannula placement in the dermis. Furthermore, the devices and methods broaden the number of sites suitable for ID injection and make the ID injection possible with limited training.
Owner:SID TECH LLC +1
Aesthetic thermal sculpting of skin
Owner:APSARA MEDICAL
Ultrasound-based treatment methods for therapeutic treatment of skin and subcutaneous tissues
The disclosure provides a method and apparatus for noninvasive and minimally-invasive treatment of skin and subcutaneous tissues with ultrasound with or without nano- or microparticles. The treatment includes, but is not limited to, hair removal, skin rejuvenation (wrinkle removal), scar removal, treatment of spider veins and varicose veins, removal of birthmarks, acne treatment, wound treatment, abnormal pigmentation and stretch mark removal, abnormal tissues in skin and subcutaneous layers, and tattoo removal. Skin and subcutaneous tissues which can be treated with the methods described include, but are not limited to, the dermis, epidermis, subcutaneous fat, connective tissue, muscle tissue, blood vessels, scar tissues, tendons, and cartilage tissues, and abnormal tissues in skin and subcutaneous layers. The disclosure is especially applicable to hair removal and skin rejuvenation.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com