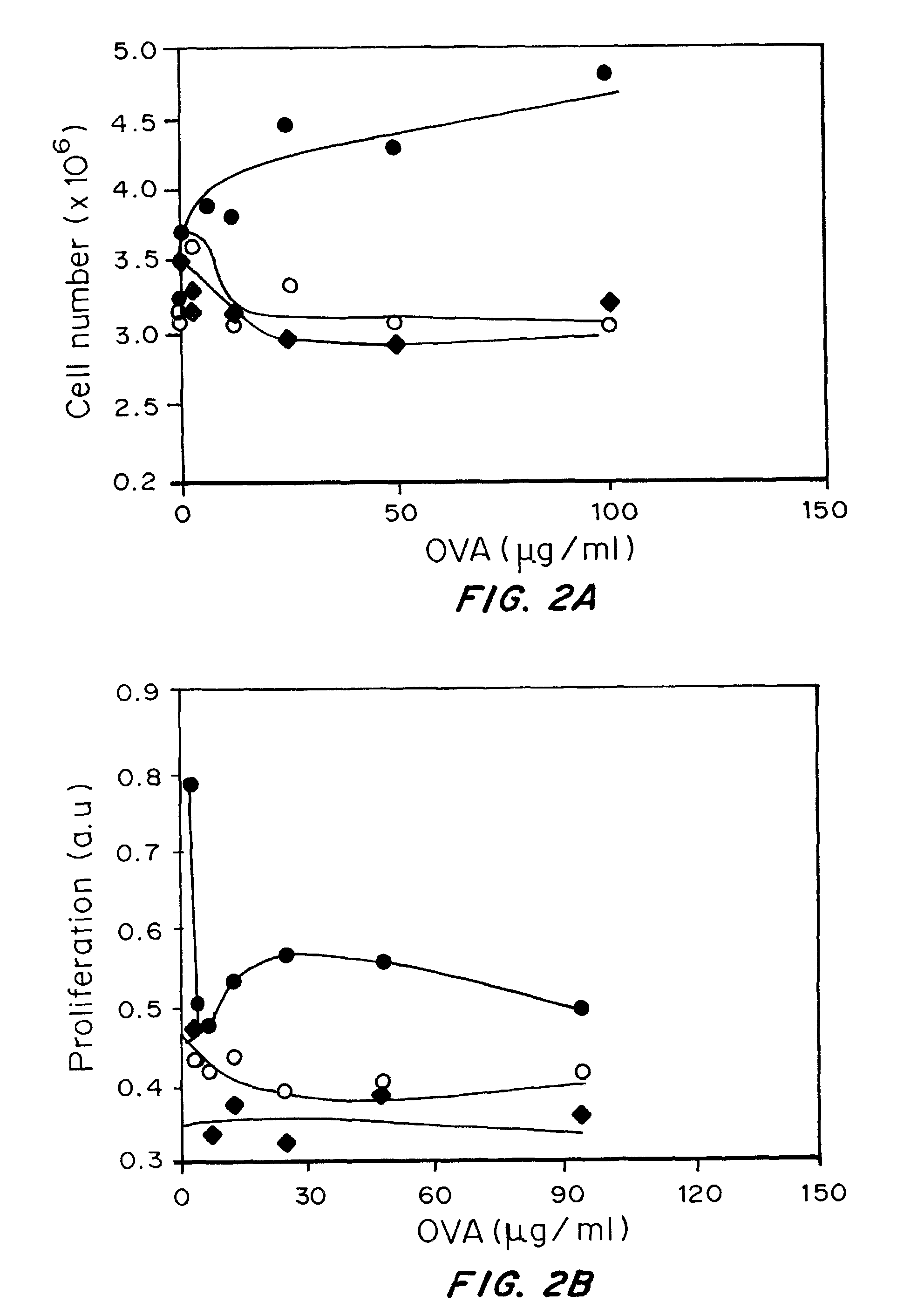
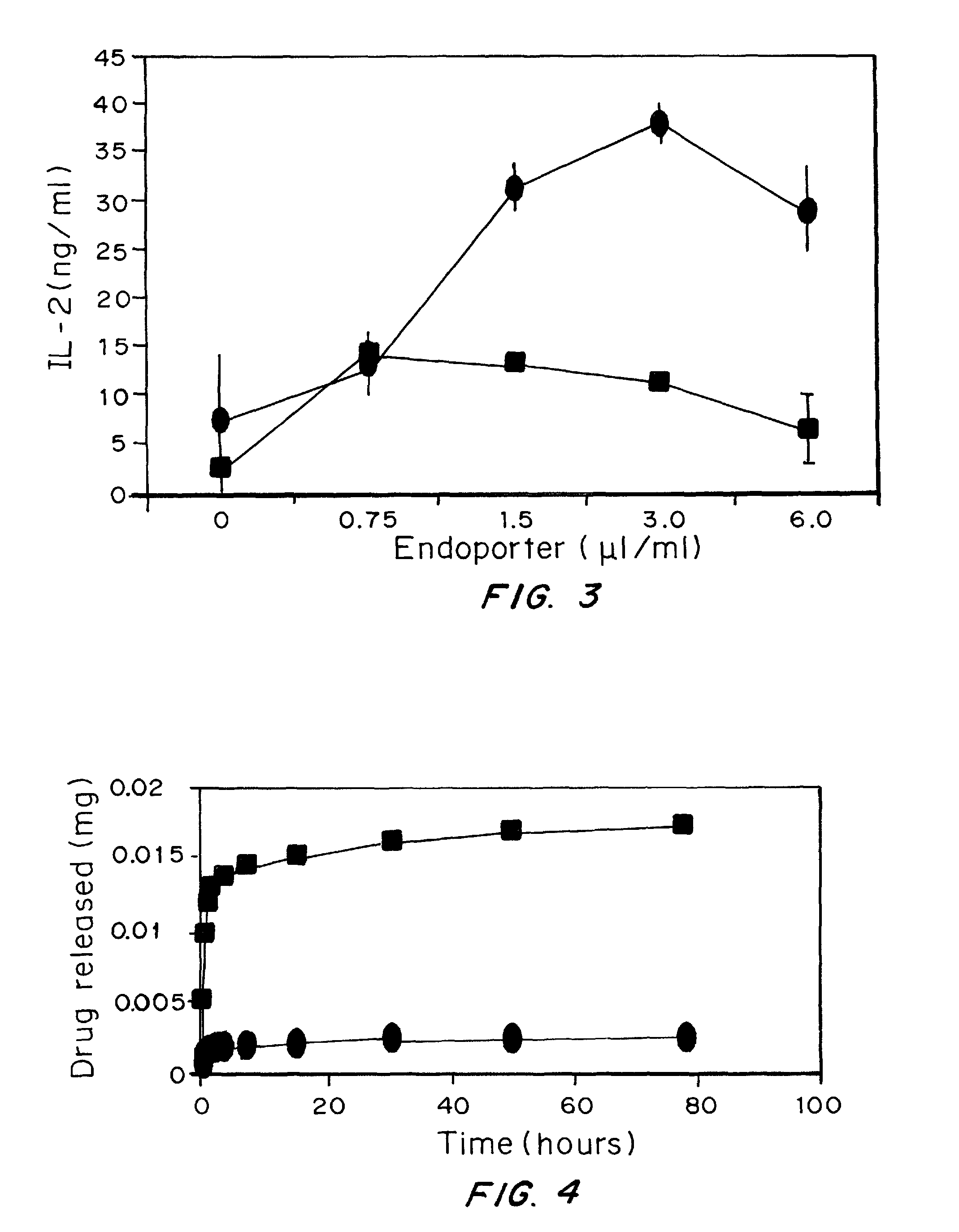
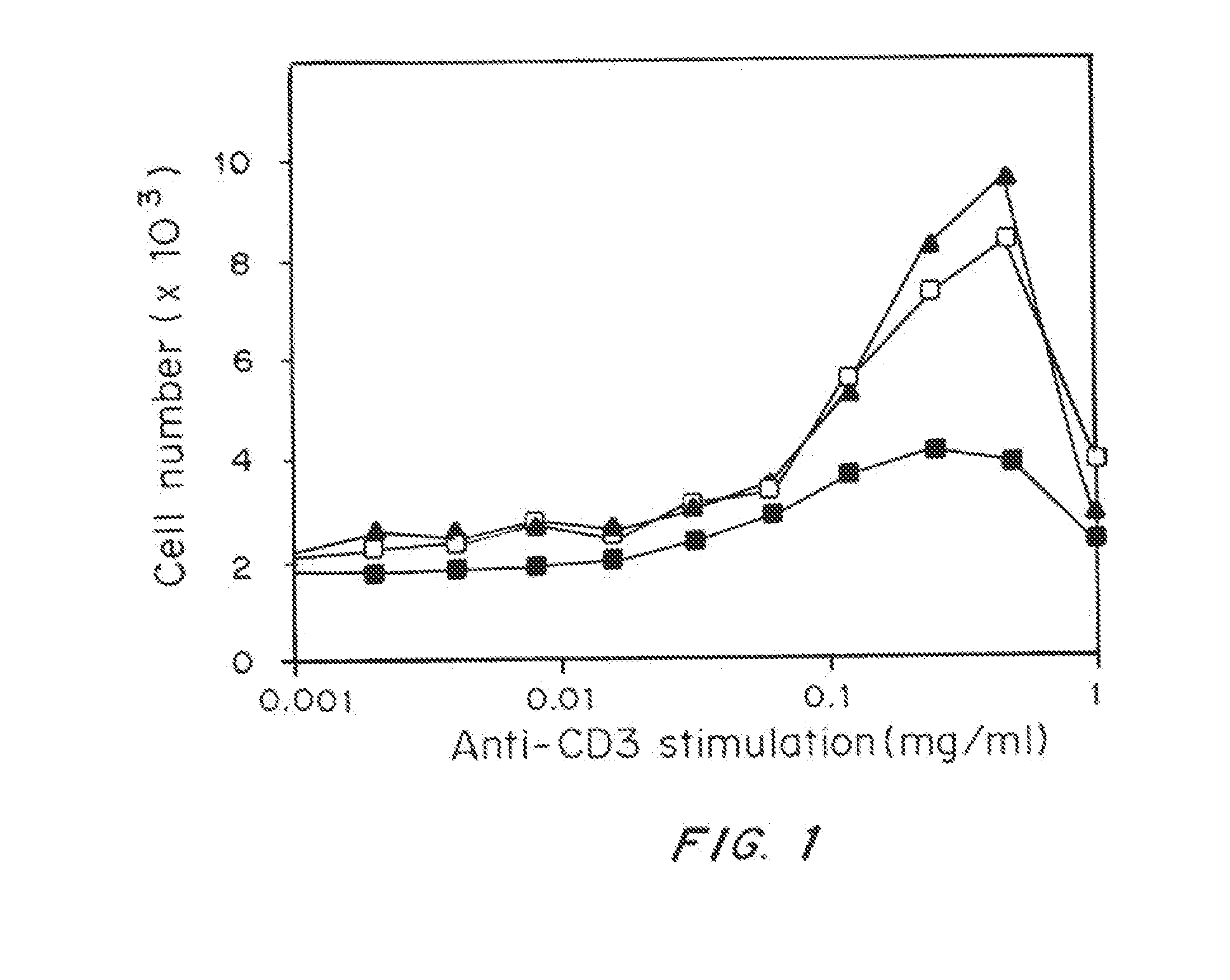
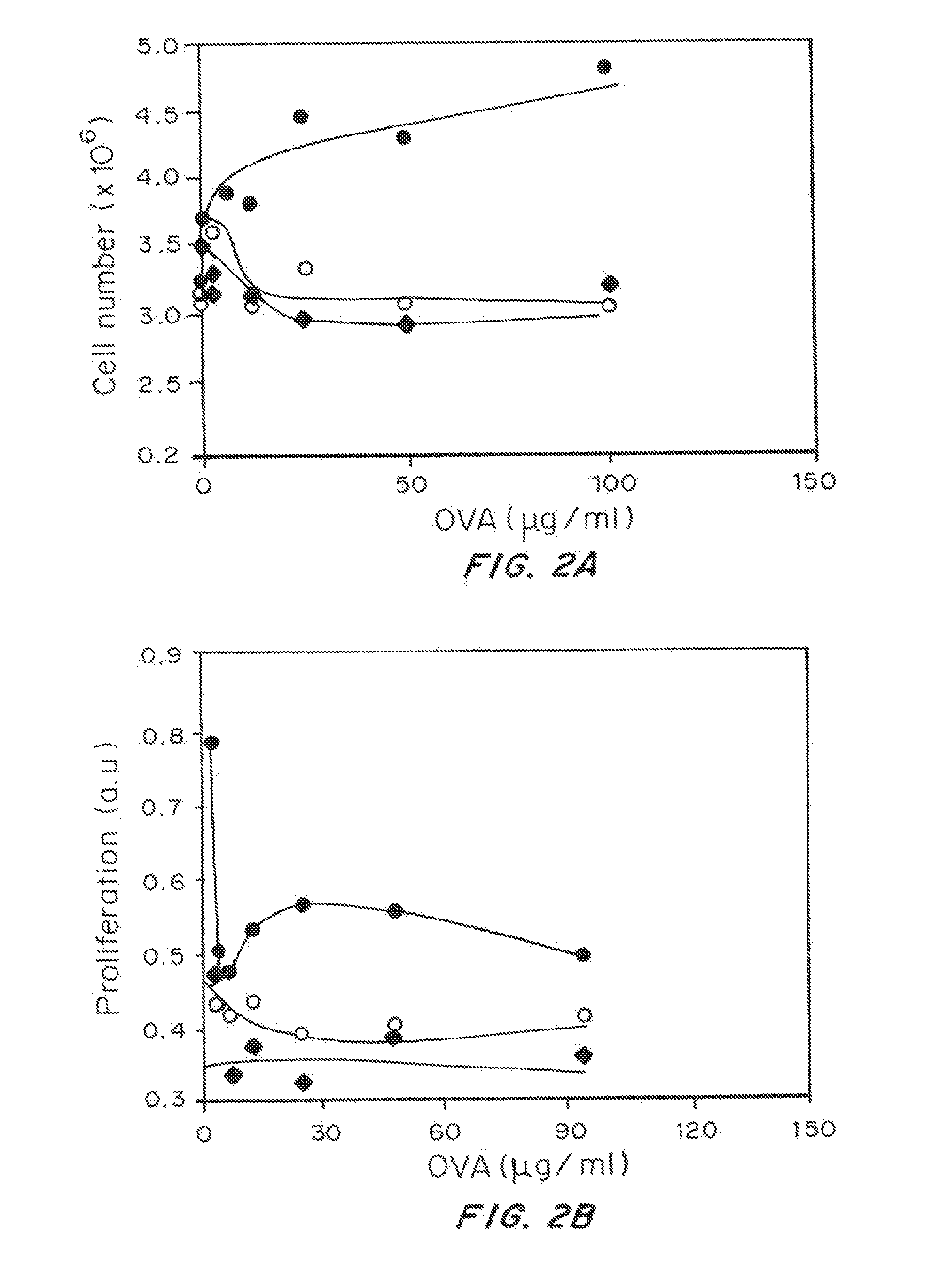
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
120 results about "Vaccine delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antigen delivery system and method of production
The present invention concerns polymer particle vaccine delivery systems in which a water insoluble protein antigen, e.g. a lipidated HpaA protein, is incorporated with particles comprising a polymer matrix. The present invention also concerns a method for incorporating such a water insoluble protein antigen with a polymer matrix in order to produce a polymer particle vaccine delivery system. In addition, the invention also provides a vaccine composition comprising the polymer particle delivery system. The vaccine can be used to treat and / or reduce the risk of for example Helicobacter infection.
Owner:ASTRAZENECA AB
Ultrasound assisted transdermal vaccine delivery method and system
InactiveUS20050112135A1Adequate buffering capacityHigh viscosityViral antigen ingredientsSurgeryVaccine deliveryBiocompatible coating
An apparatus and method for transdermally delivering a vaccine comprising a delivery system having (i) a microprojection member (or system) that includes a plurality of microprojections (or array thereof) that are adapted to pierce through the stratum corneum into the underlying epidermis layer, or epidermis and dermis layers and (ii) an ultrasonic device. In one embodiment, the vaccine is contained in a biocompatible coating that is applied to the microprojection member. In a further embodiment, the delivery system includes a gel pack having a vaccine-containing hydrogel formulation that is disposed on the microprojection member after application to the skin of a patient. In an alternative embodiment, the vaccine is contained in both the coating and the hydrogel formulation.
Owner:ALZA CORP
Vaccine delivery compositions and methods of use
InactiveUS20080160089A1Easy to produceImprove efficiencySsRNA viruses negative-sensePowder deliveryPolyesterMHC class I
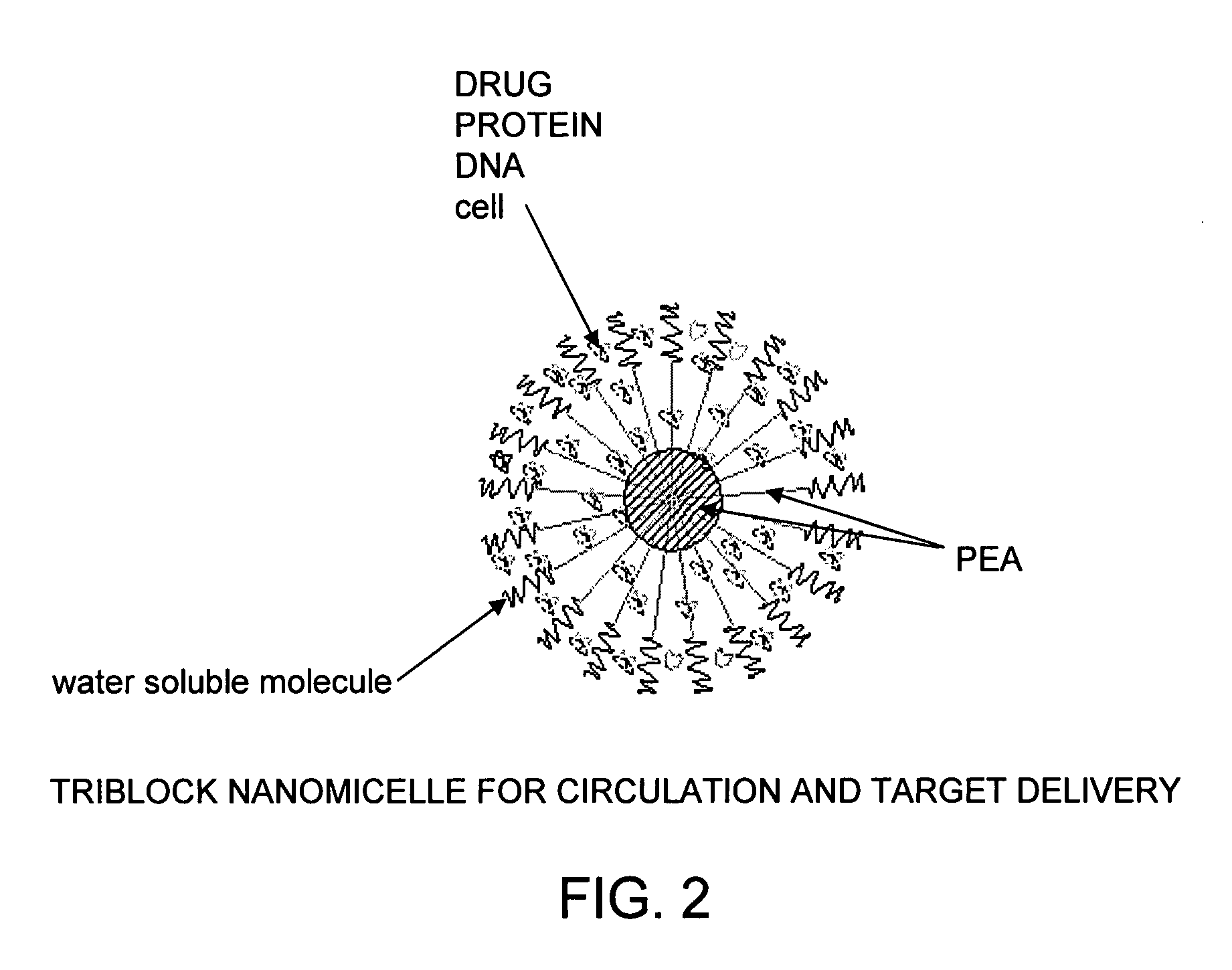
The present invention provides synthetic vaccines against a variety of pathogenic organisms and tumor cells in humans and other mammals based on biodegradable polymers containing polyester amide (PEA), polyester urethane (PEUR), and polyester urea (PEU) and immunostimulatory adjuvants. The vaccines can be formulated as a liquid dispersion of polymer particles or molecules in which are dispersed an immunostimulatory adjuvant, such as a TLR agonist, and whole protein or peptidic antigens containing MHC class I or class II epitopes derived from organism or tumor cell proteins. Methods of inducing an immune response via intracellular mechanisms to the pathogenic organism or tumor cells specific for the antigen in the invention compositions are also included.
Owner:MEDIVAS LLC
System and method for transdermal vaccine delivery
InactiveUS20050123565A1Ease of conditionsBacterial antigen ingredientsElectrotherapyVaccine deliveryBiocompatible coating
A system and method for transdermally delivering a vaccine to a patient including an iontophoresis delivery device having a donor electrode, a counter electrode, and electric circuitry for supplying iontophoresis energy to the electrodes, and a non-electroactive microprojection member having a plurality of stratum corneum-piercing microprojections extending therefrom. The vaccine can be contained in a hydrogel formulation in an agent reservoir disposed proximate the donor electrode, in a biocompatible coating that is disposed on the microprojections or in both.
Owner:ALZA CORP
Intradermal delivery of biological agents
InactiveUS20090012494A1Significant comprehensive benefitsMinimal expertiseBiocidePeptide/protein ingredientsActive agentWhole body
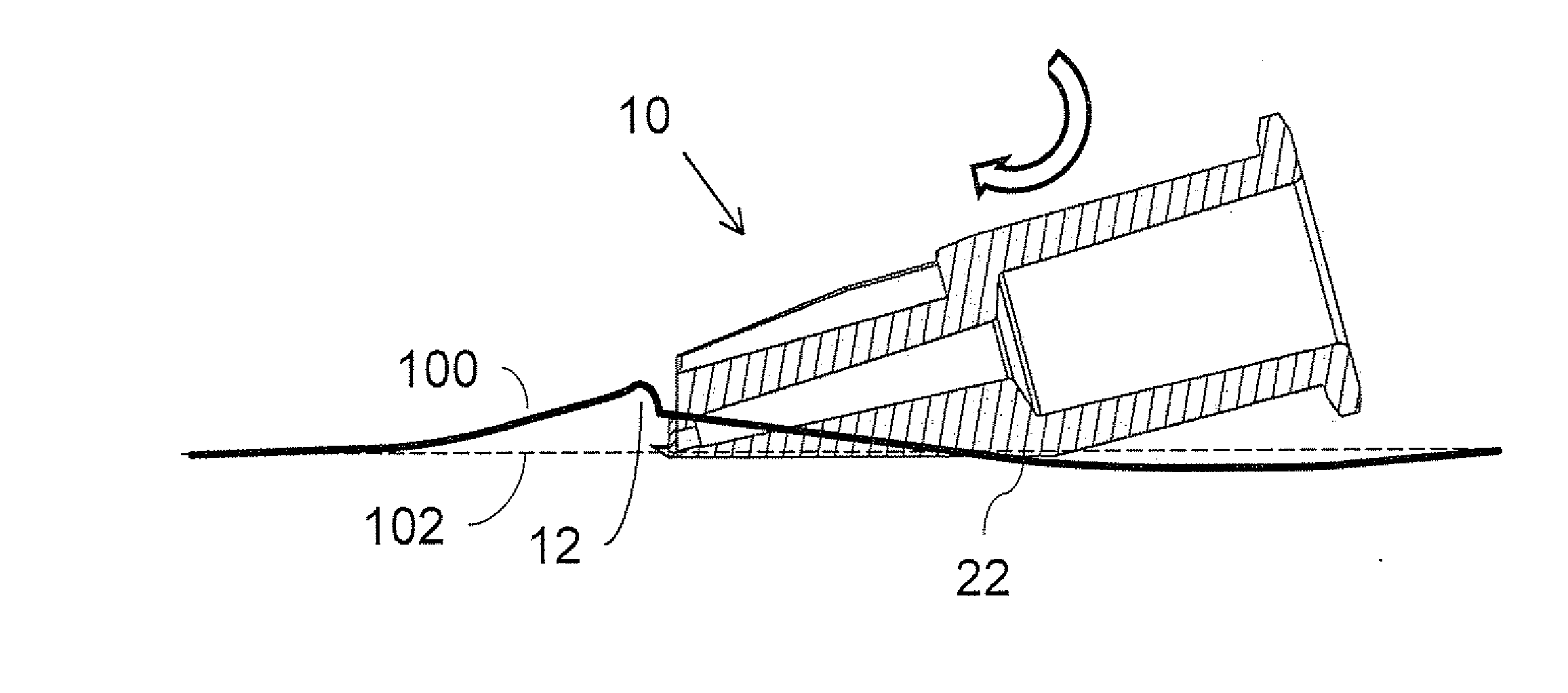
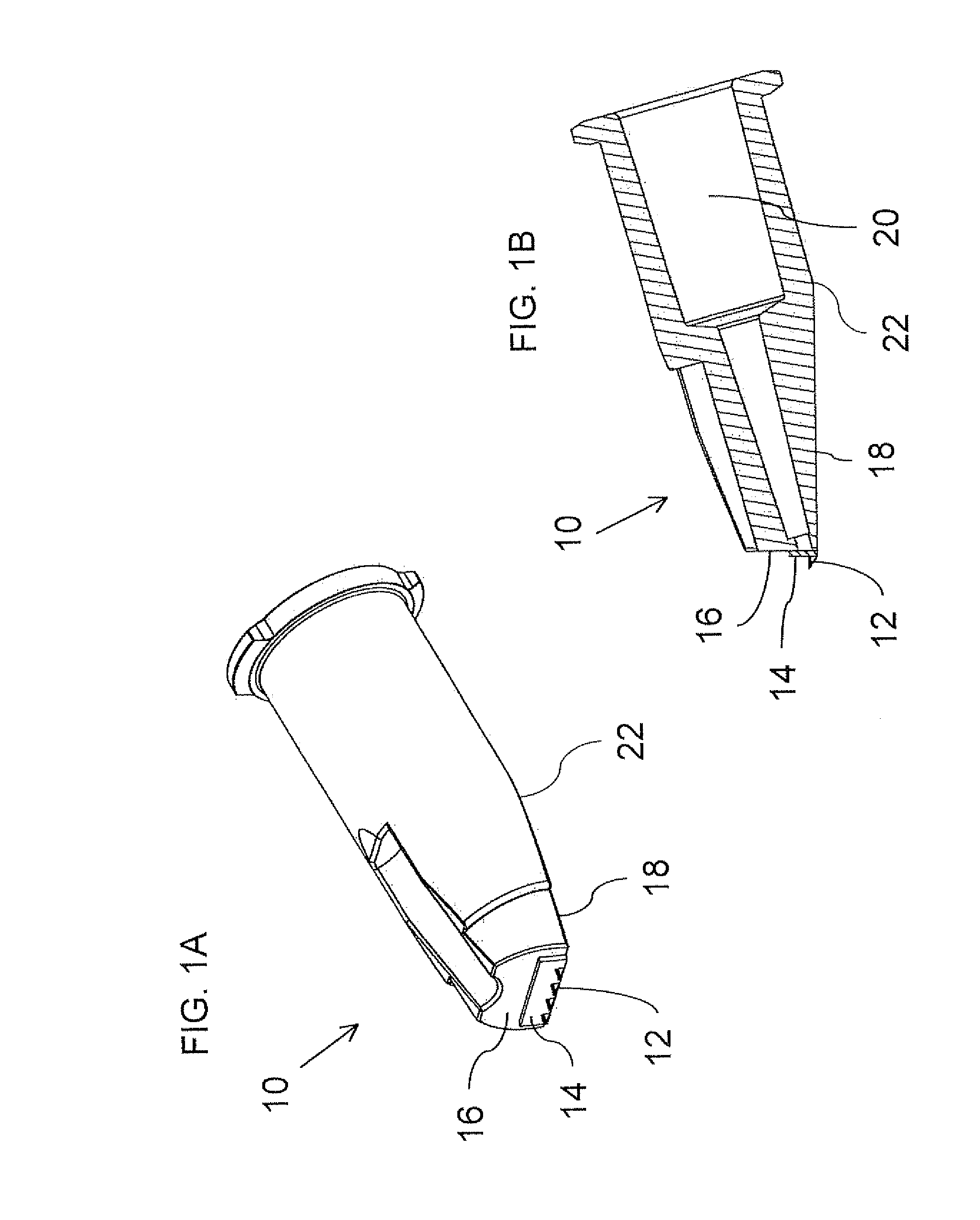
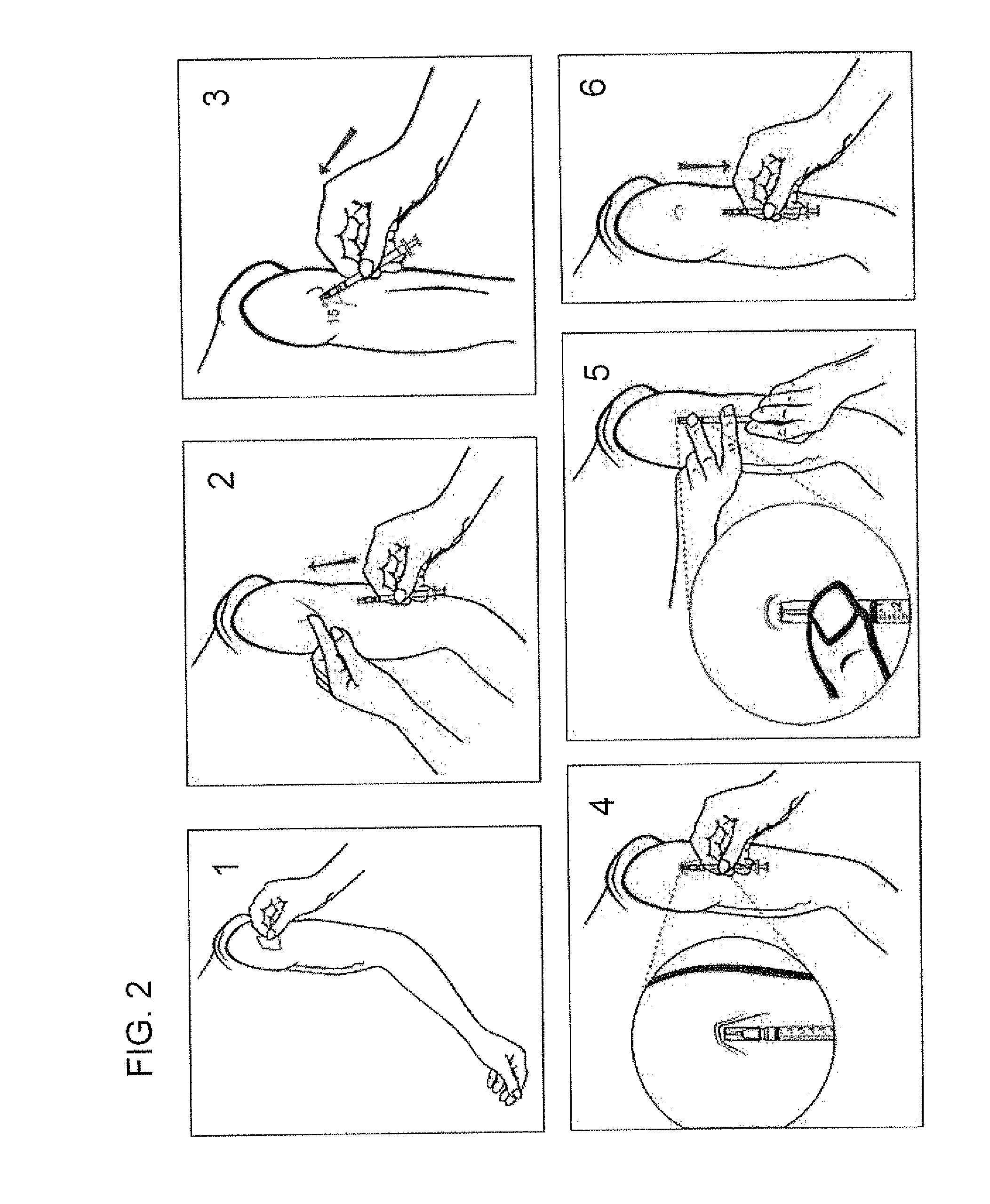
The present invention relates to methods for intradermally delivering one or more biologically active agents such as vaccines and therapeutic agents into the dermis layer of the skin of a subject to obtain systemic delivery or an immune response using a microneedle drug delivery device containing the agent to be delivered. The methods employ a microneedle device with a row of hollow microneedles. The microneedles penetrate the skin of the subject and assume an anchored state in which the microneedles are anchored in the skin and project laterally from the device. A pivotal motion is then performed with the device so that the skin in which the microneedles are engaged is lifted above the initial plane of the surface of the skin while the biologically active agent is delivered. The methods of the invention elicit increased humoral and / or cellular response as compared to conventional vaccine delivery routes, facilitating dose sparing.
Owner:NANOPASS TECH LTD
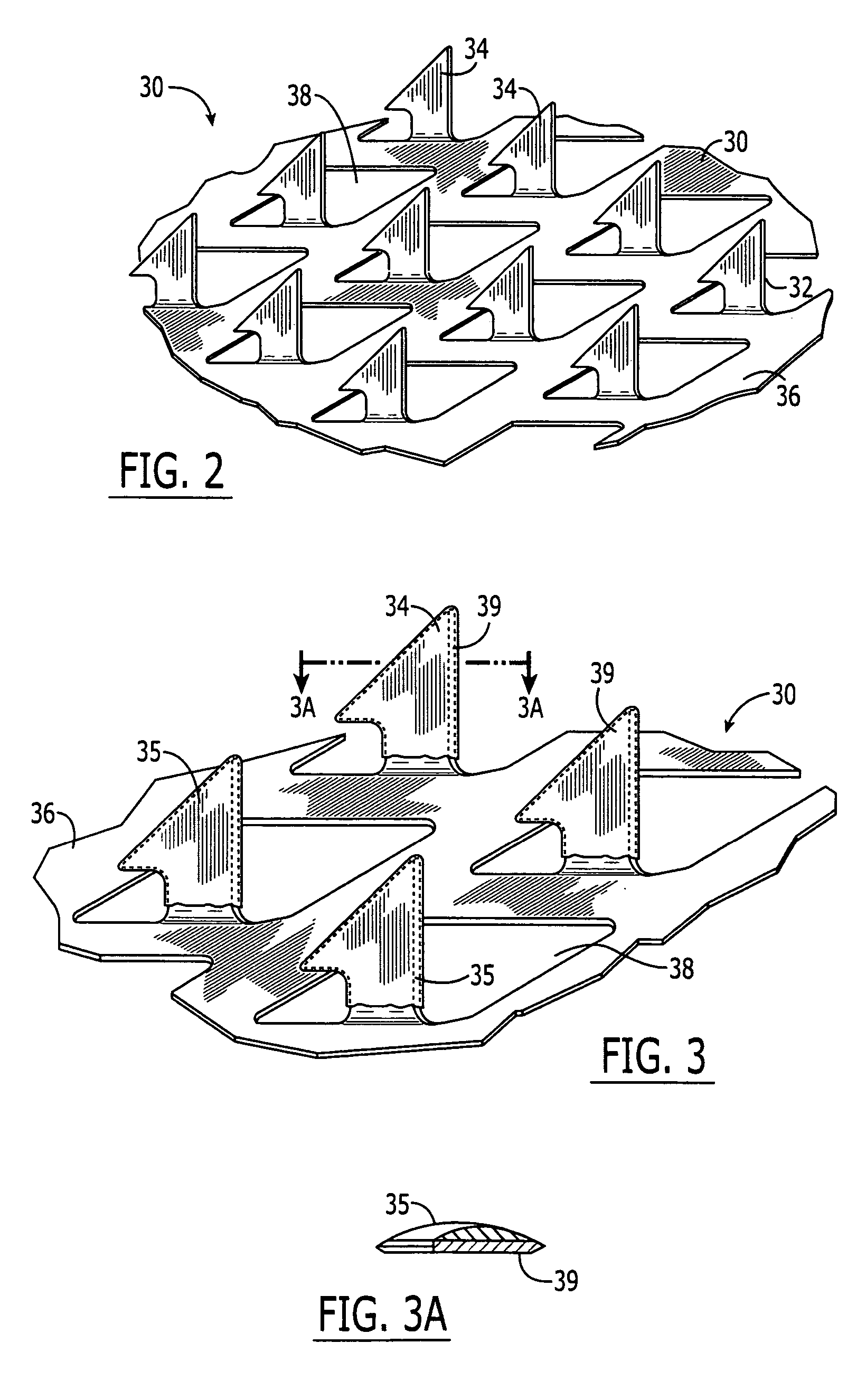
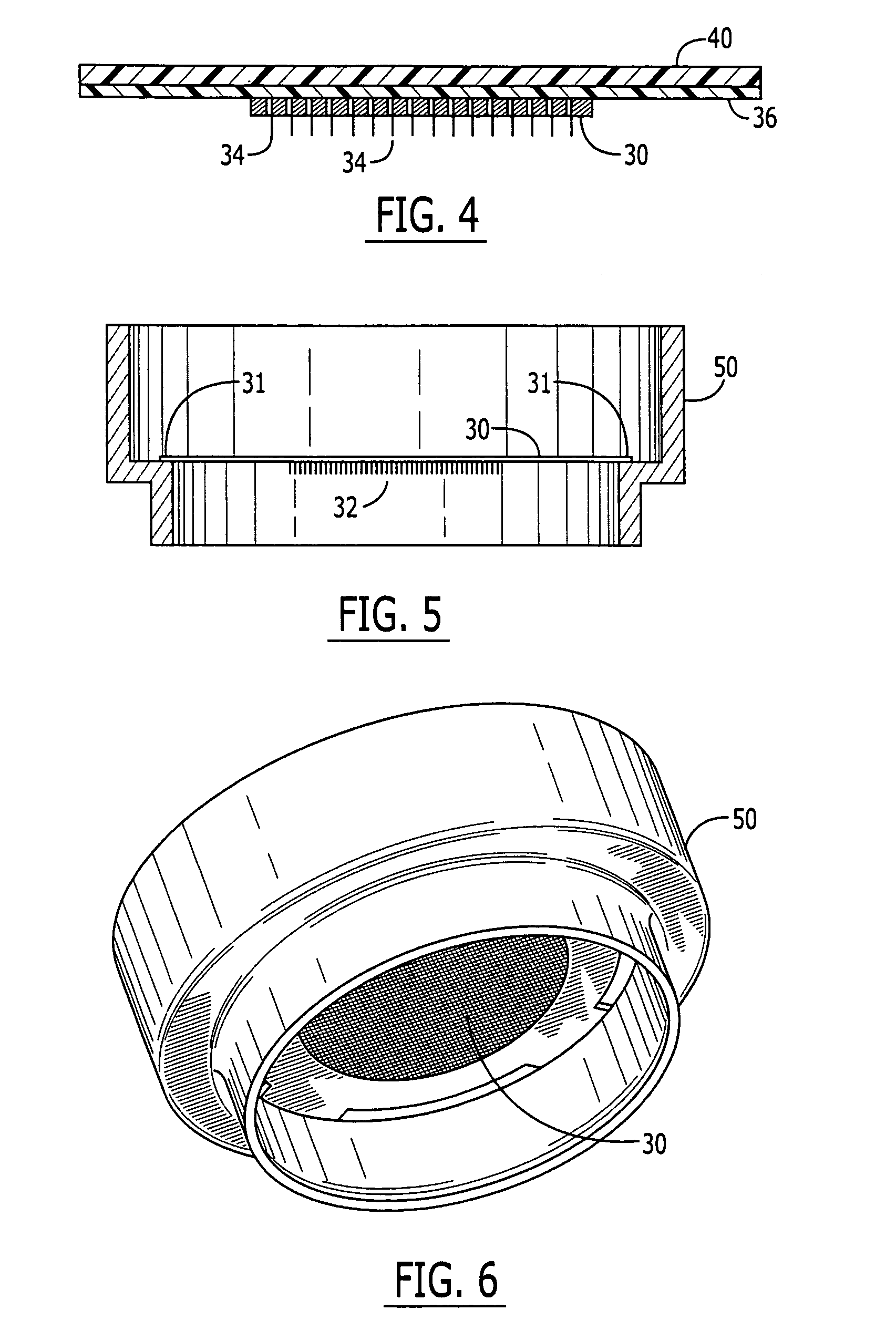
Vaccine Delivery via Microneedle Arrays
ActiveUS20090155330A1Effective meanAntibacterial agentsBacterial antigen ingredientsVaccine deliveryDisease
A microprojection array is provided, comprising an approximately planar base and a plurality of microprojections, wherein the array comprises a vaccine and a polymeric material. The array may have multiple layers. The vaccine may be placed in only one layer. In another embodiment of the invention, a method of preventing a disease is provided, comprising insertion into the skin of a patient an array of microprojections comprising a layer which comprises a vaccine for that disease and a polymer.
Owner:CORIUM PHARMA SOLUTIONS INC
Emulsion and micellar formulations for the delivery of biologically active substances to cells
InactiveUS6120794AChange concentrationReduce deliveryPeptide/protein ingredientsGenetic material ingredientsLipid formationVaccine delivery
New emulsion and micelle formulations are described as are complexes of these formulations with biologically active substances. The novel formulations are different from cationic lipid vectors such as cationic liposomes in that the complexes formed between biologically active substances and the emulsion and micellar formulations of this invention are physically stable and their transfection activity is resistant to the presence of serum. These novel formulations are disclosed to be useful in areas such as gene therapy or vaccine delivery.
Owner:UNIVERSITY OF PITTSBURGH
Antigen delivery compositions and methods of use
ActiveUS20060008472A1Prevent formation of newEnhance cellular immunityNanotechBacterial antigen ingredientsVaccine deliveryAntigen delivery
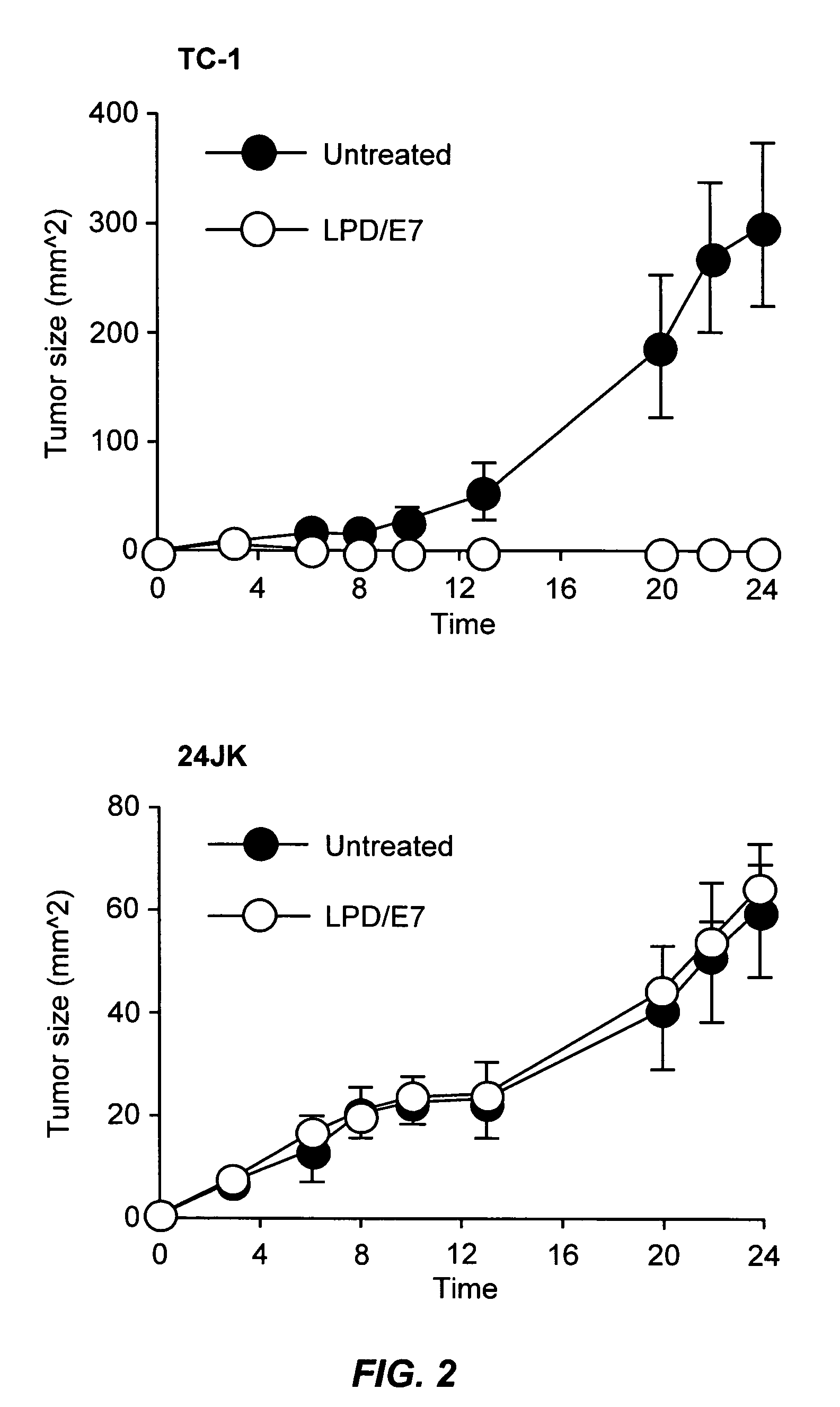
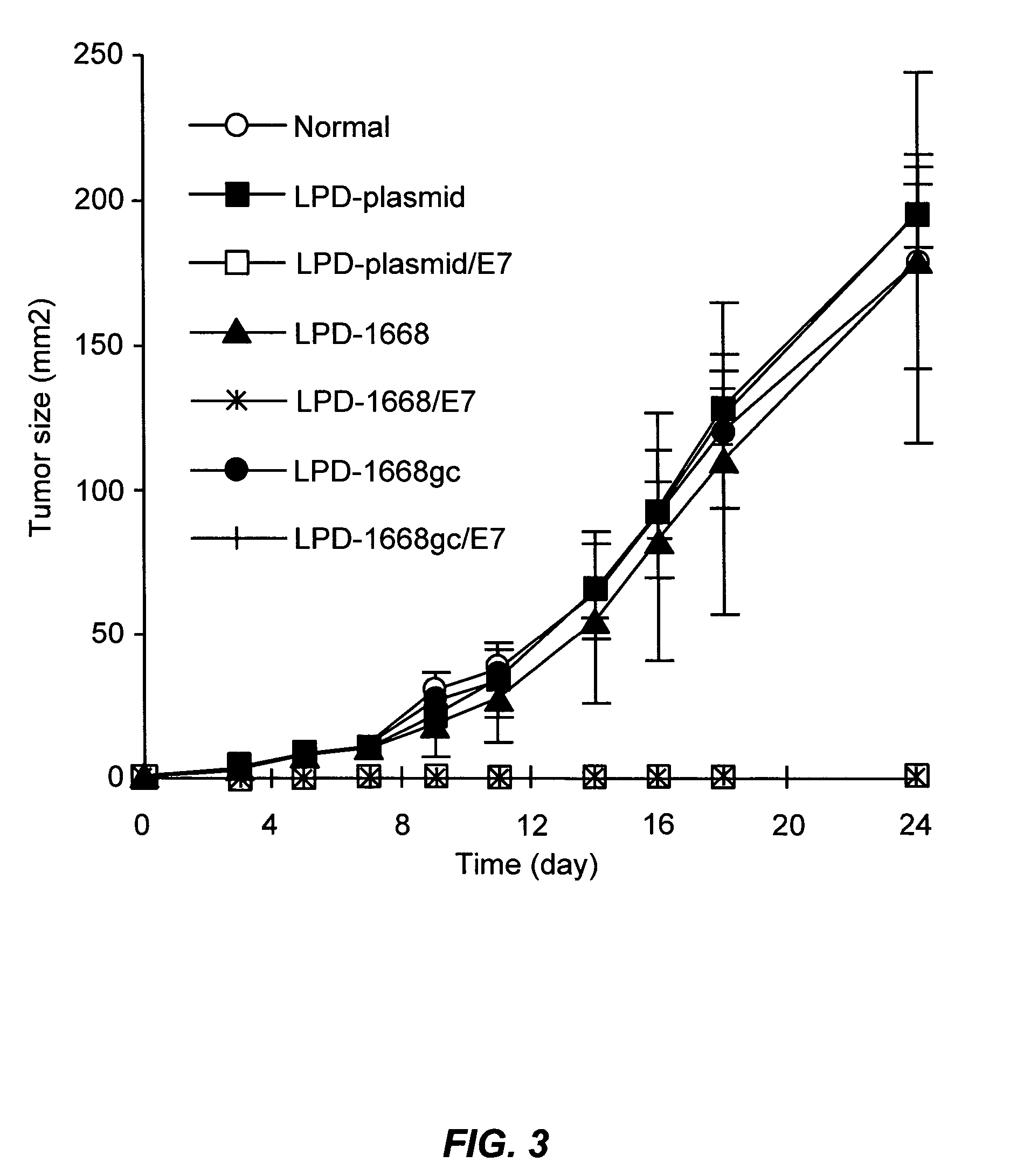
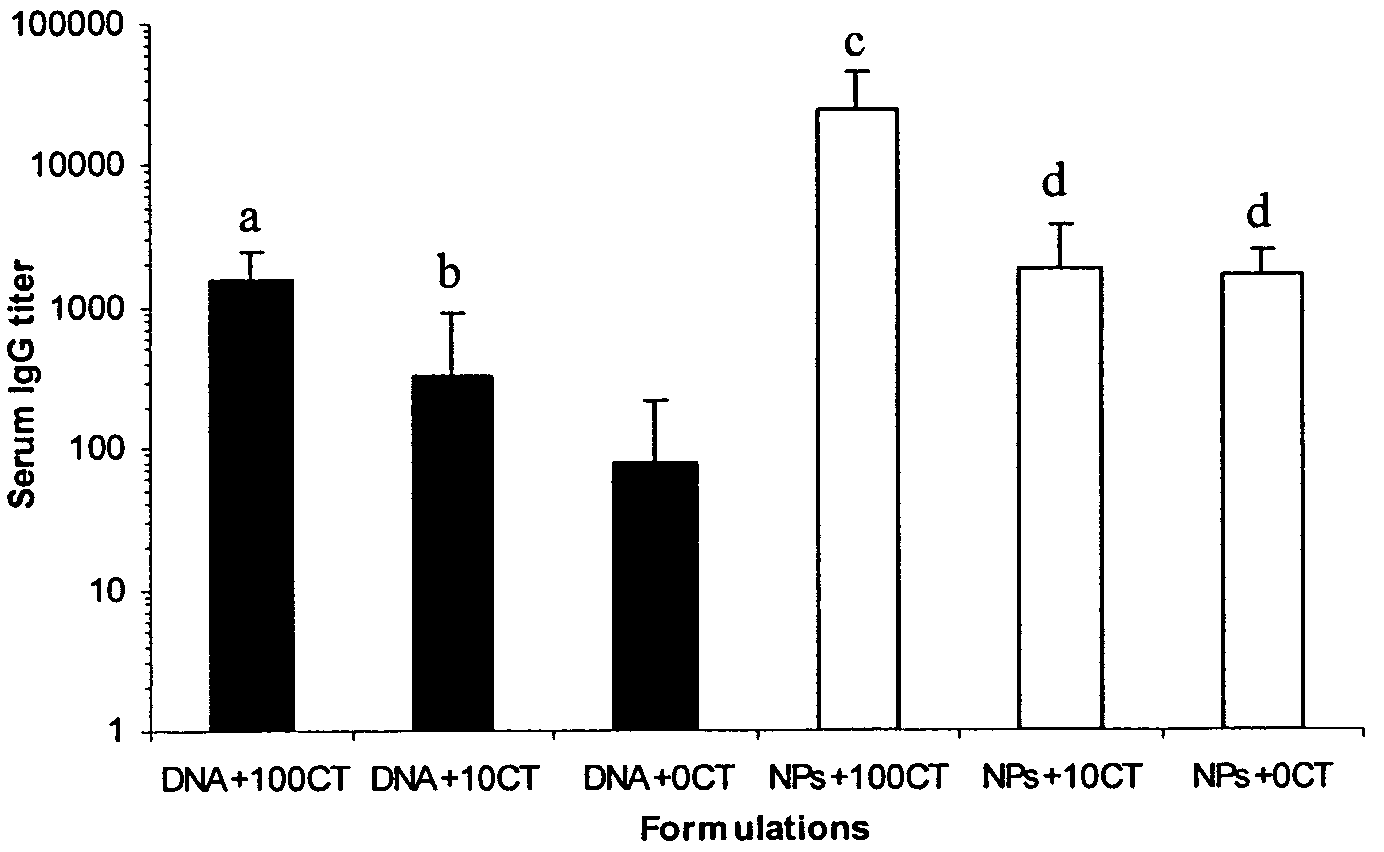
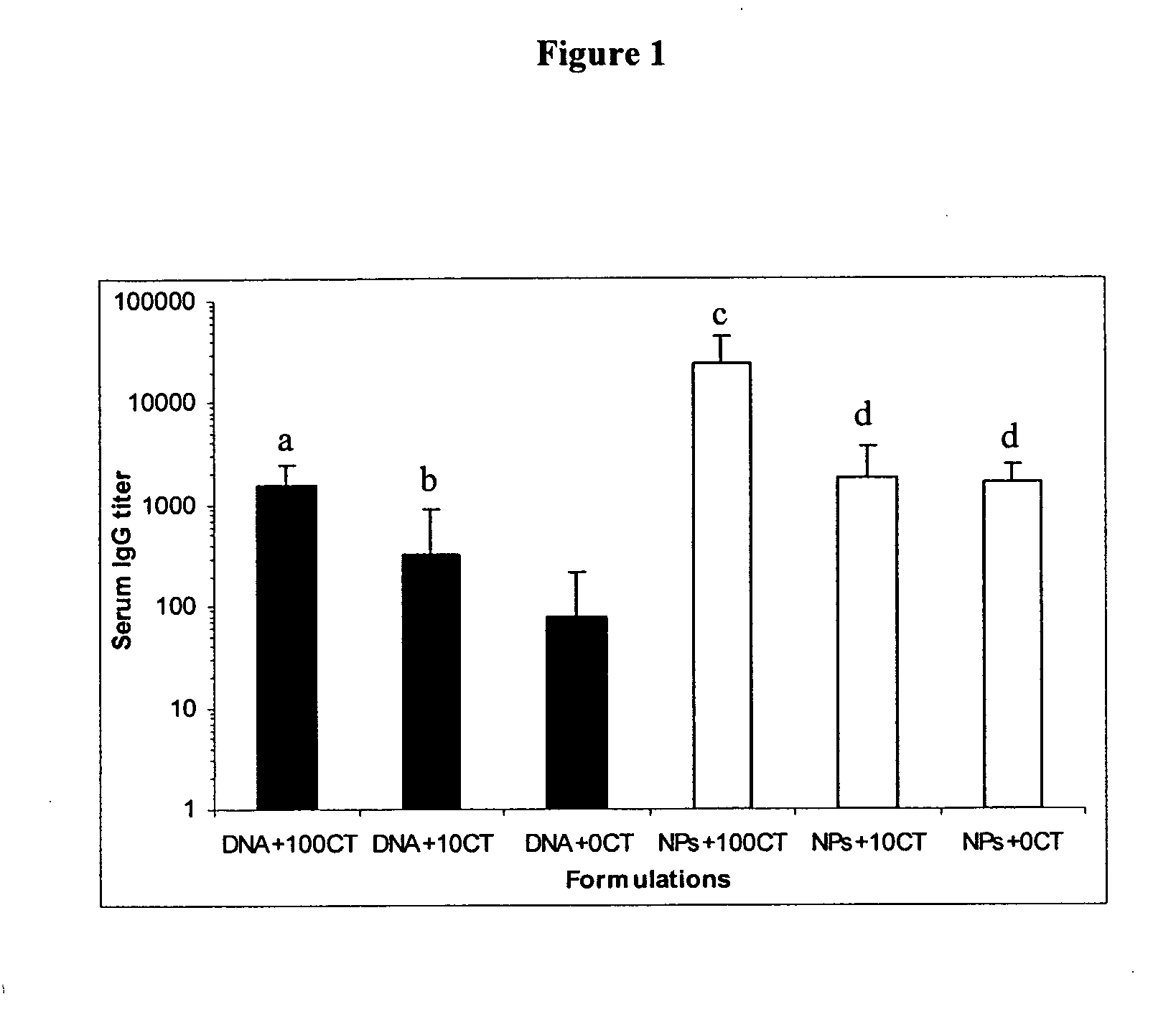
The present invention provides antigen delivery compositions and methods of using same to prevent or to treat cancers and other infectious diseases. More particularly, the present invention provides a lipid-protamine-DNA (LPD) vaccine delivery system and methods of using such LPD vaccine delivery system for the prevention and treatment of cancer and other infectious diseases.
Owner:PDS BIOTECH
Nanoparticle-based vaccine delivery system containing adjuvant
InactiveUS20080124350A1Genetic material ingredientsSnake antigen ingredientsVaccine deliveryAdjuvant
A vaccine delivery system comprising adjuvant and nanoparticles comprising an immunogenic agent is provided. A method of immunizing an animal comprising administering a nanoparticle-based vaccine delivery system is also provided.
Owner:UNIV OF KENTUCKY RES FOUND
Recombinant vaccines comprising immunogenic attenuated bacteria having RpoS positive phenotype
InactiveUS7083794B2Improve balanceImproving immunogenicityAntibacterial agentsBiocideSalmonella entericaSalmonella serotype typhi
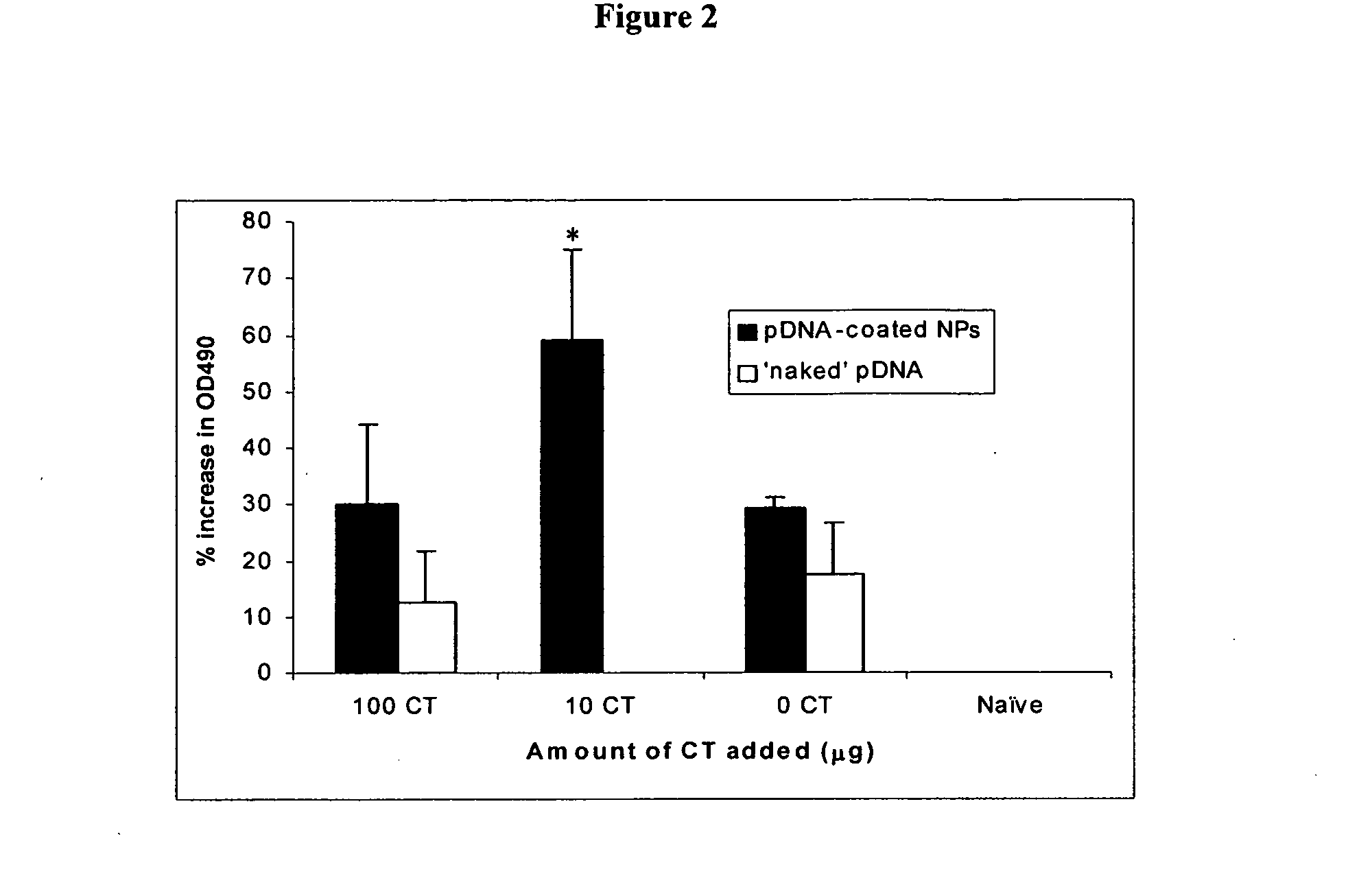
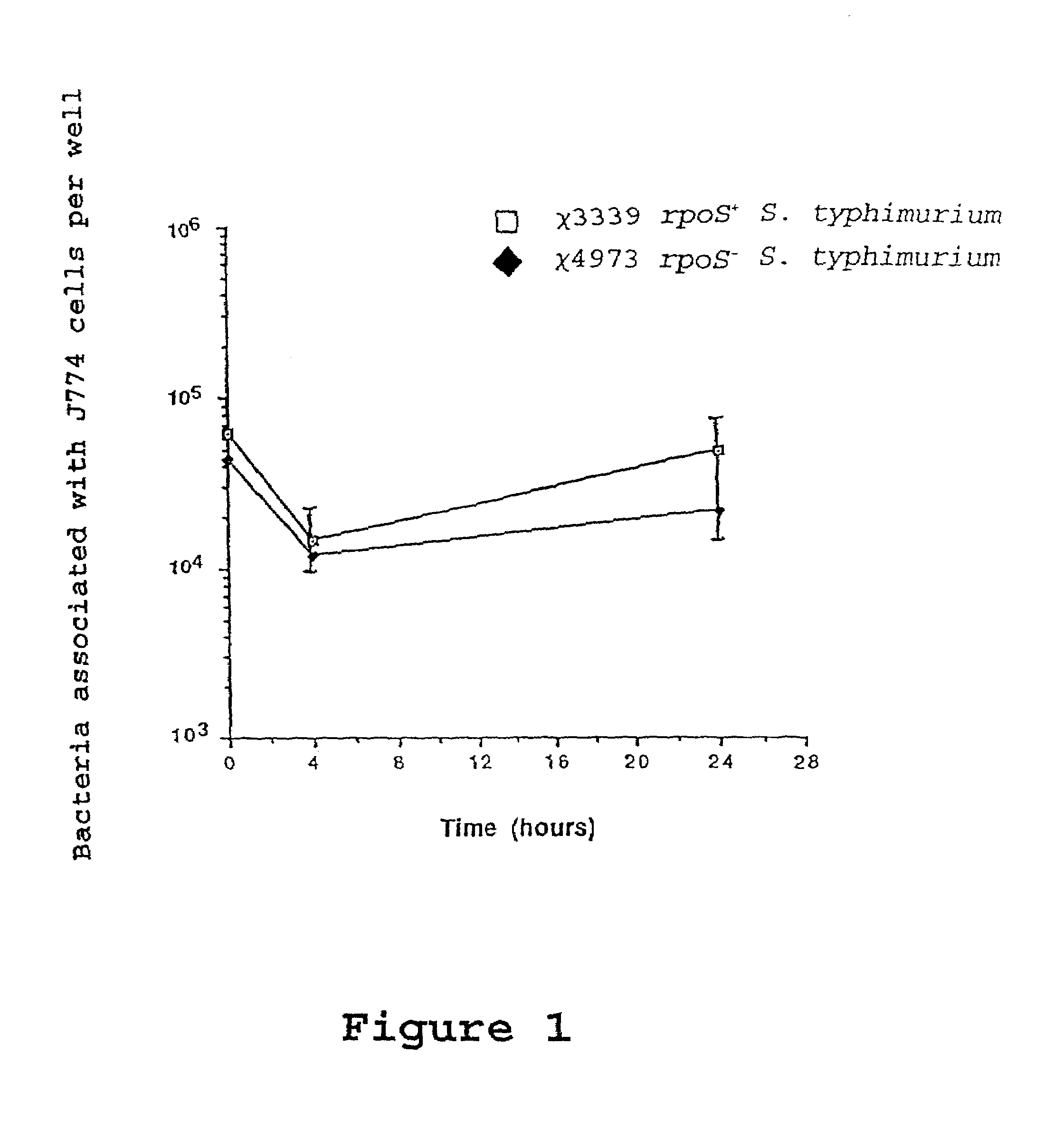
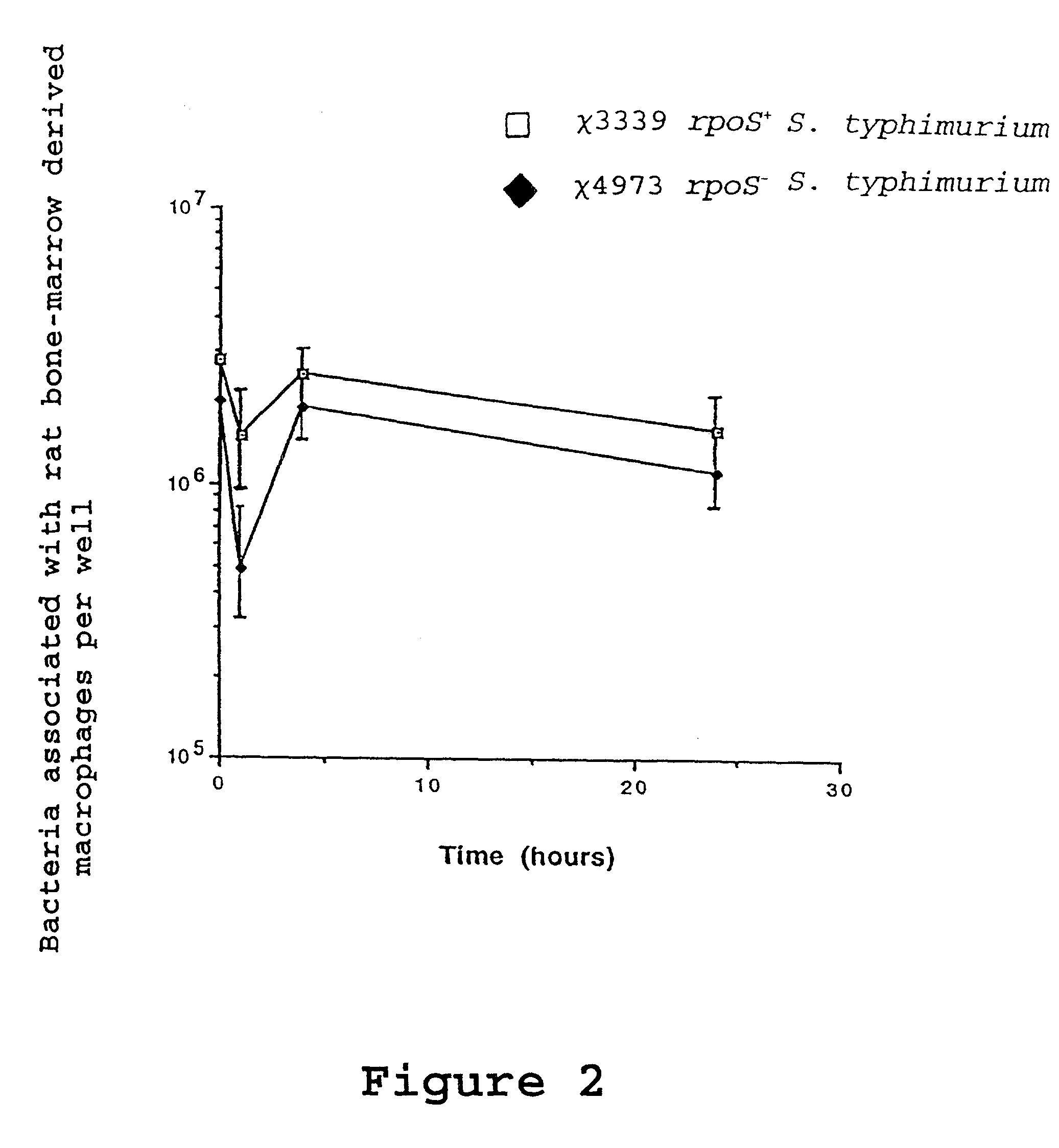
Attenuated immunogenic bacteria having an RpoS+ phenotype, in particular, Salmonella enterica serotype Typhi having an RpoS+ phenotype and methods therefor are disclosed. The Salmonella have in addition to an RpoS+ phenotype, an inactivating mutation in one or more genes which render the microbe attenuated, and a recombinant gene capable of expressing a desired protein. The Salmonella are attenuated and have high immunogenicity so that they can be used in vaccines and as delivery vehicles for genes and gene products. Also disclosed are methods for preparing the vaccine delivery vehicles.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Modular nanoparticles for adaptable vaccines
ActiveUS20100104503A1Rapid productionWell representedPowder deliveryBacterial antigen ingredientsVaccine deliveryAdjuvant
Modular nanoparticle vaccine compositions and methods of making and using the same have been developed. Modular nanoparticle vaccine compositions comprise an antigen encapsulated in a polymeric particle and adaptor elements which modularly couple functional elements to the particle. The modular design of these vaccine compositions, which involves flexible addition and subtraction of antigen, adjuvant, immune potentiators, molecular recognition and transport mediation elements, as well as intracellular uptake mediators, allows for exquisite control over variables that are important in optimizing an effective vaccine delivery system.
Owner:YALE UNIV
Use of polymeric nanoparticles for vaccine delivery
InactiveUS20080044484A1Enhance antigen presentationImprove efficiencyBiocidePowder deliveryCancer cellT lymphocyte
The invention relates generally to the treatment and prevention of human cancer and viral diseases. More specifically, this invention relates to development of a new generation of vaccines that rely on eliciting cellular immune responses, specifically induction of cytotoxic T lymphocytes (CTL), against cancer cells and virus-infected cells via administration of a polymeric nanoparticle containing a vaccine comprising a fusion peptide or a modified peptide. Such a fusion peptide is composed of an insertion signal sequence and a peptide derived from a tumor antigen or a viral antigen, which improves antigen presentation and induces CTL with higher efficiency against cancer cells and virus-infected cells. An exemplary peptide utilized in the invention is Mart-1:27-35 peptide.
Owner:RGT UNIV OF CALIFORNIA
Nanoparticle-Based vaccine delivery system containing adjuvant
A vaccine delivery system comprising adjuvant and nanoparticles comprising an immunogenic agent is provided. A method of immunizing an animal comprising administering a nanoparticle-based vaccine delivery system is also provided.
Owner:UNIV OF KENTUCKY RES FOUND
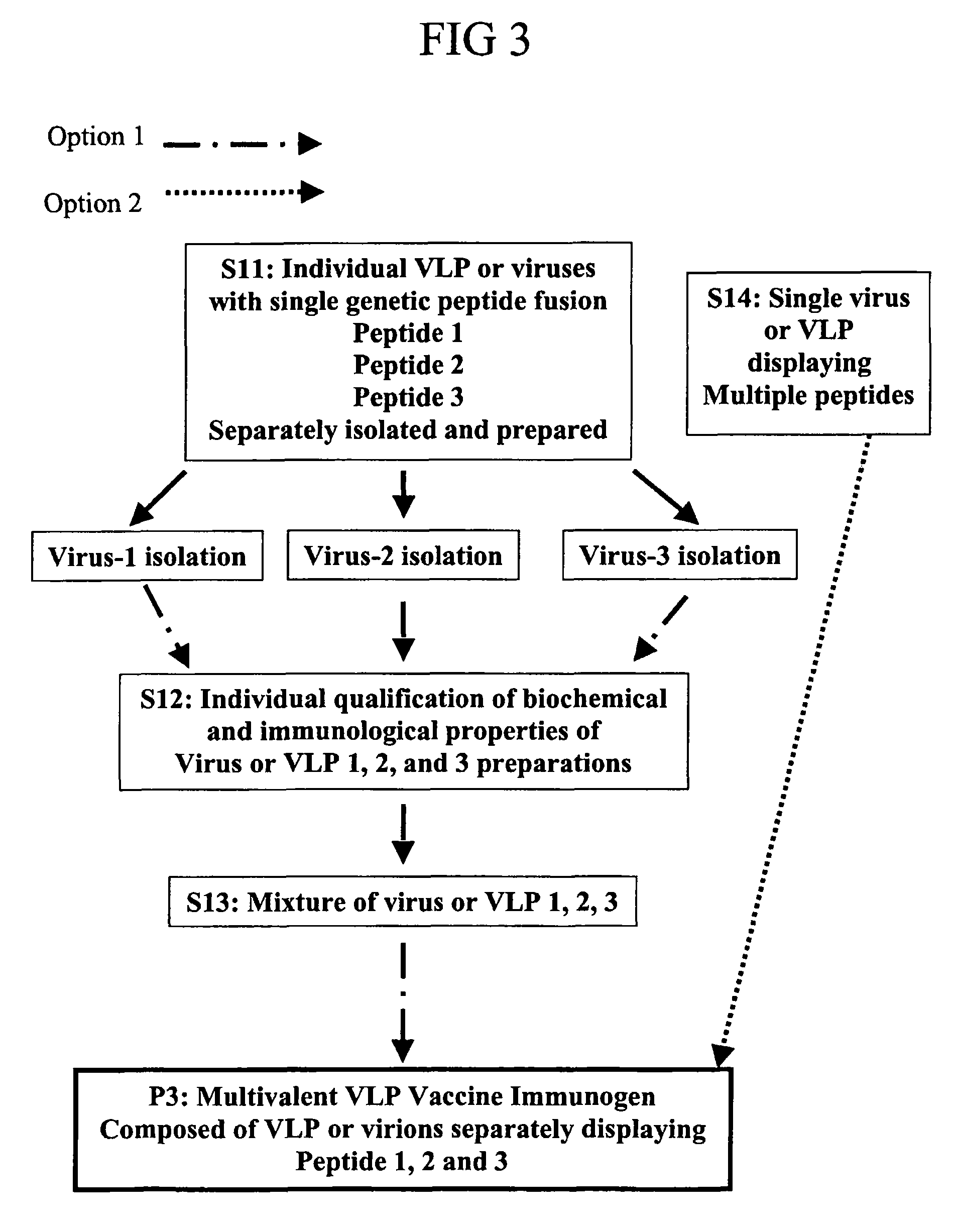
Flexible vaccine assembly and vaccine delivery platform
ActiveUS7939318B2Modulate host immune responseEfficient identificationVirusesAntibody mimetics/scaffoldsVaccine deliveryVirus-like particle
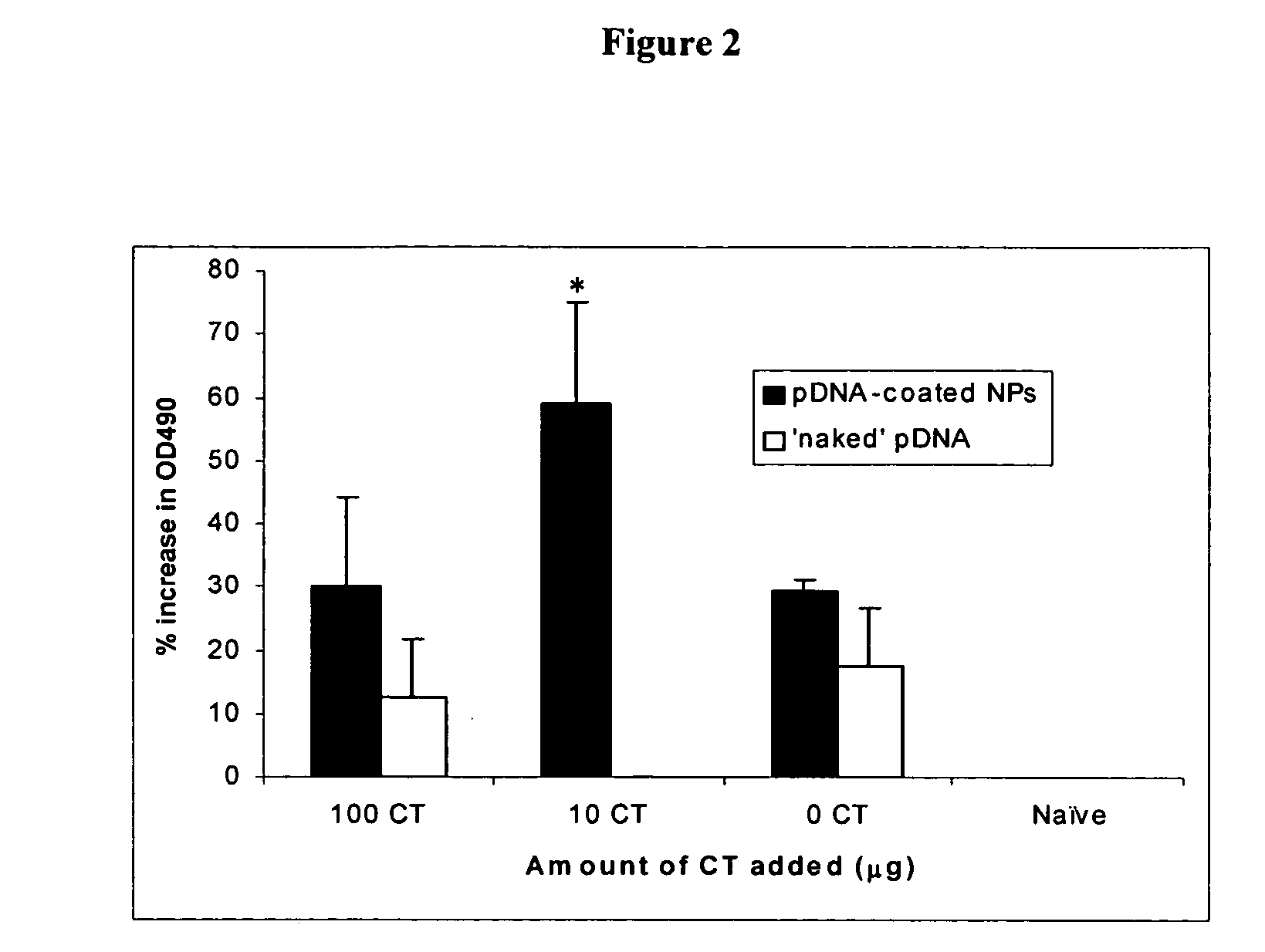
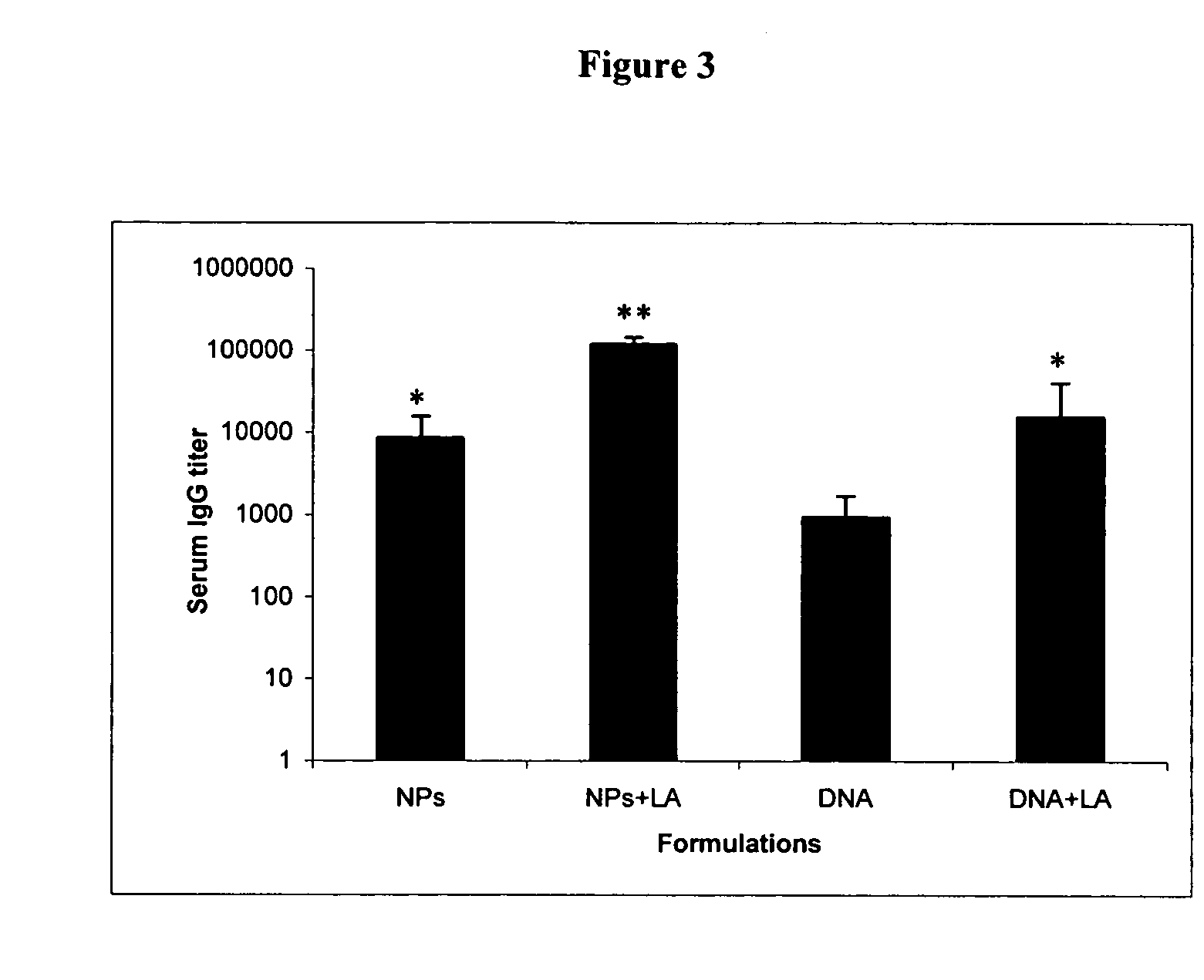
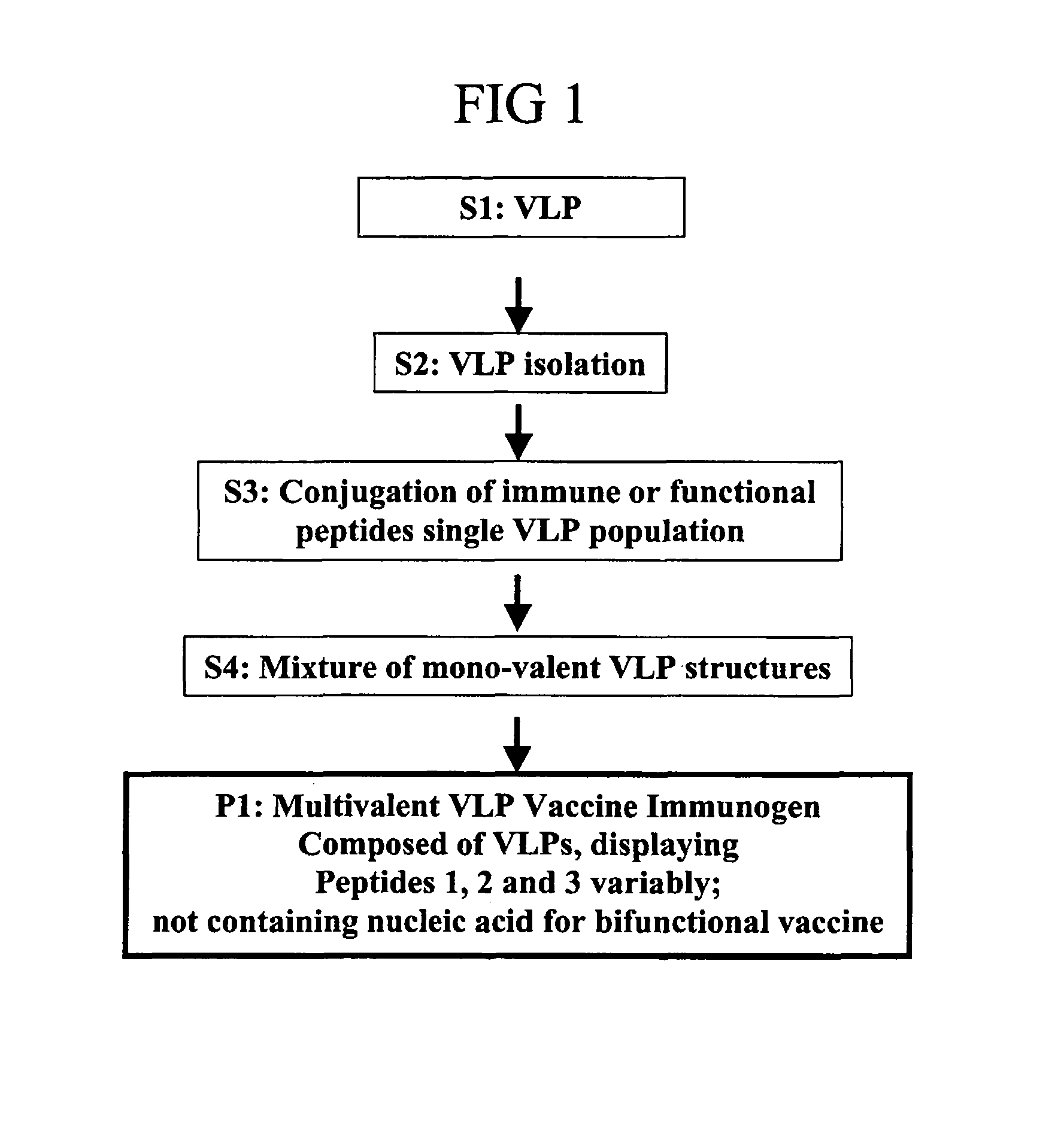
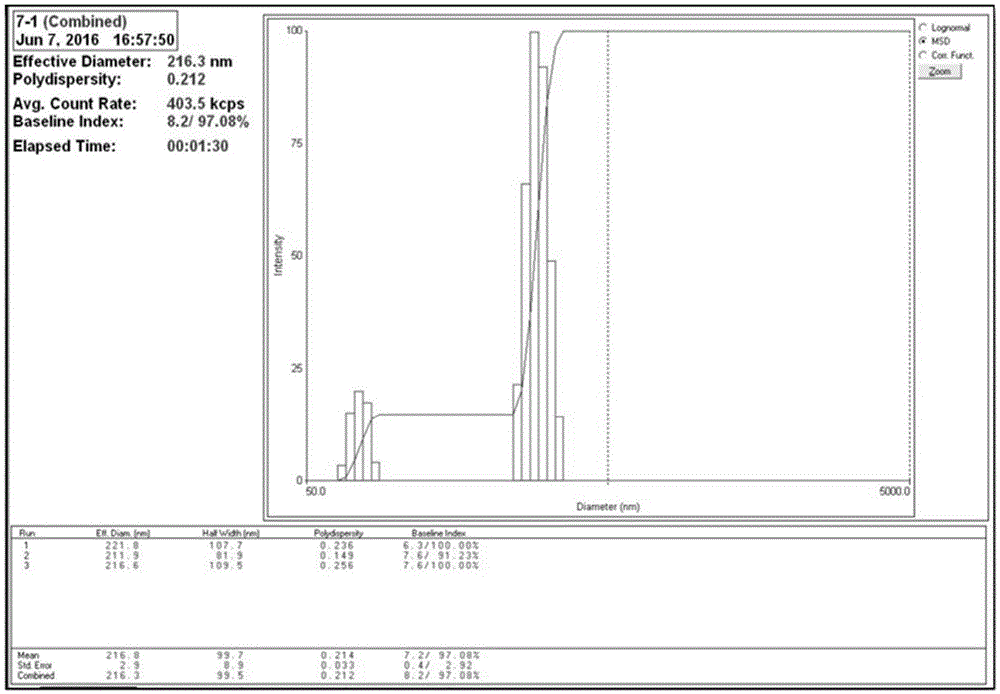
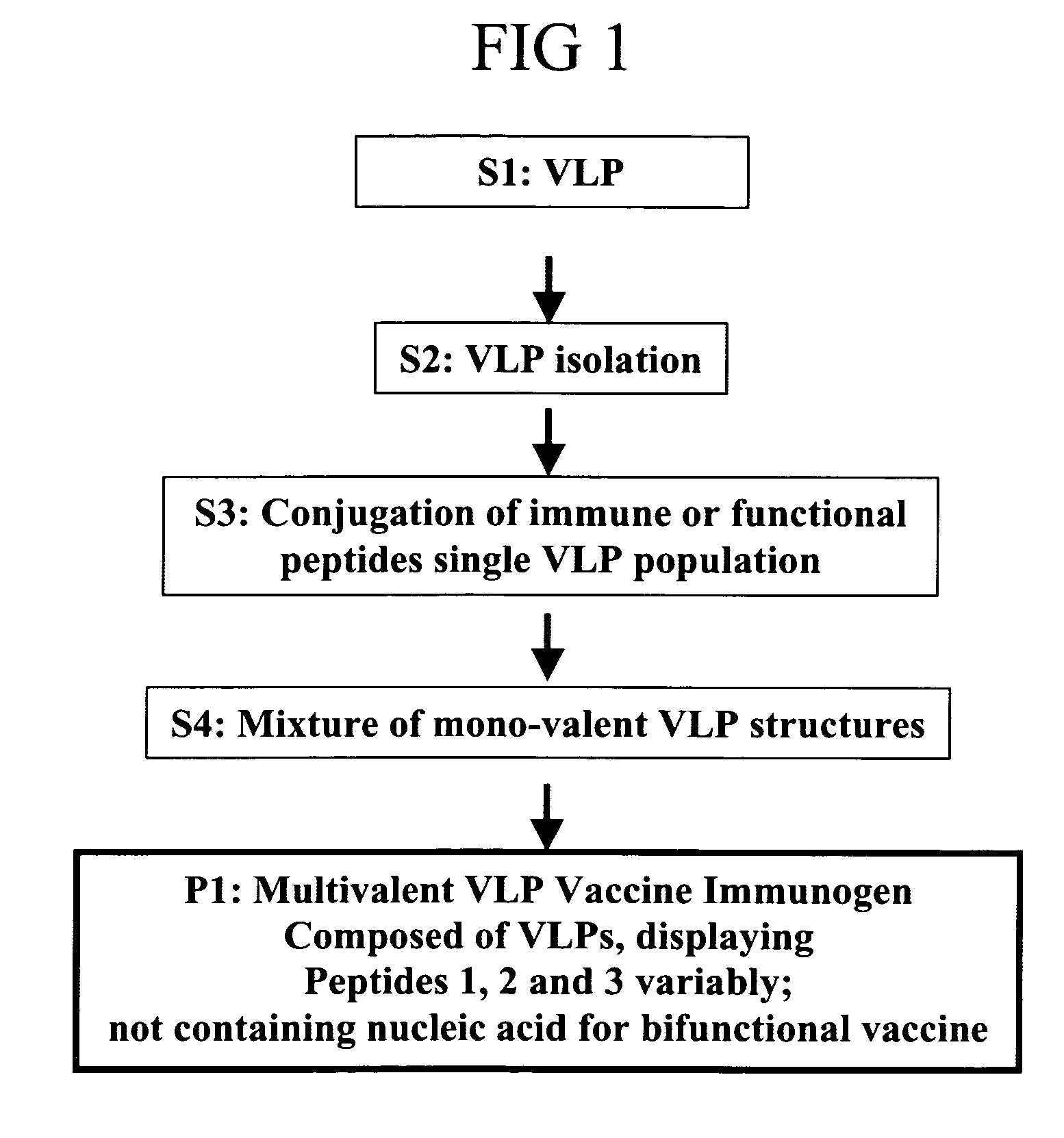
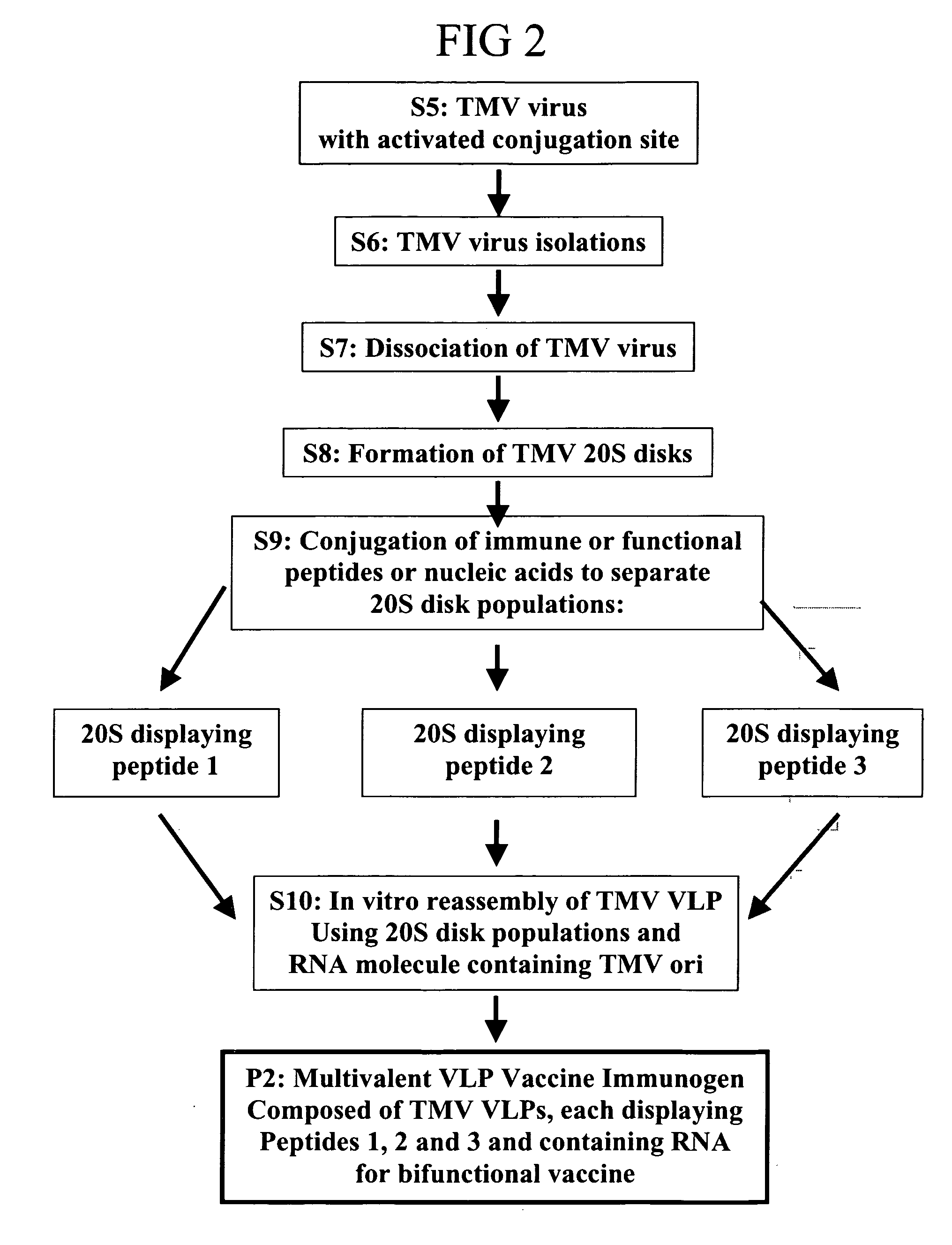
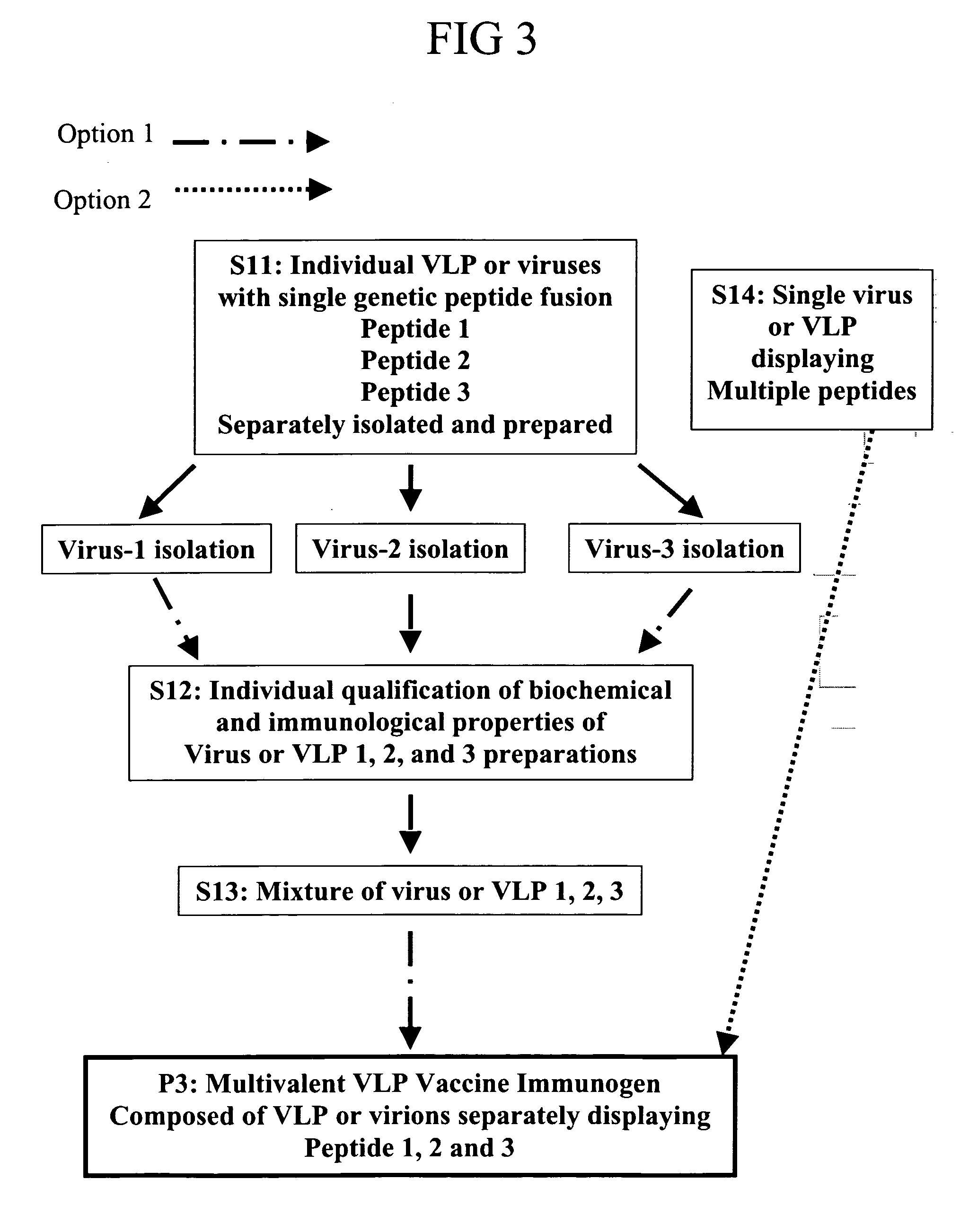
Herein-described are various methods for making a vaccine that are made of re-assembled virus like particles (VLP). First, the VLPs are disassembled into encapsidation intermediate populations. Each encapsidation intermediate population undergoes, for instance, chemical conjugation of unique peptide or nucleic moieties to form separate populations. Thereafter, a predetermined amount of each of the several (one or more) different encapsidation intermediates from the different populations is mixed and joined, forming intact VLPs, surrounding a nucleic acid core, that are composed of different encapsidation intermediate such that the reassembled VLP displays more than one peptide or nucleic acid. The nucleic acid can function either as a scaffold alone or can be engineered for the expression of an immunomodulatory protein in a eukaryotic cell.
Owner:KBIO HLDG LTD
Vaccine delivery compositions and methods of use
InactiveUS20060188469A1Easy to produceSsRNA viruses negative-senseViral antigen ingredientsPolyesterOrganism
The present invention provides synthetic vaccine delivery compositions based on polyester amide (PEA), polyester urethane (PEUR), and polyester urea (PEU) polymers for stimulating an immune response to a variety of pathogenic organisms and tumor cells in humans and other mammals. The vaccine delivery compositions are formulated as a liquid dispersion of polymer particles or molecules including class I or class II antigen peptides derived from organism or tumor cell proteins, which are taken up by antigen presenting cells of the mammal to induce an immune response in the mammal. Methods of inducing an immune response to the pathogenic organism or tumor cells in the invention compositions are also included.
Owner:MEDIVAS LLC
Intradermal delivery of vacccines and therapeutic agents
InactiveUS20060018877A1Improve responseEfficacious improved responsivenessSsRNA viruses negative-senseBiocideVaccine deliveryIntramuscular route
The present invention relates to methods and devices for administration of vaccines and therapeutic agents into the intradermal layer of the skin. The methods of the present invention elicit increased humoral and / or cellular response as compared to conventional vaccine delivery methods, e.g., intramuscular route. Furthermore, the methods of the present invention facilitate induction of an immune response by an amount of vaccine which is otherwise insufficient for inducing an immune response when delivered via conventional vaccine routes, e.g., intramuscular route.
Owner:BECTON DICKINSON & CO
Modular nanoparticles for adaptable vaccines
ActiveUS8889117B2Rapid productionWell representedPowder deliveryBacterial antigen ingredientsVaccine deliveryAdjuvant
Modular nanoparticle vaccine compositions and methods of making and using the same have been developed. Modular nanoparticle vaccine compositions comprise an antigen encapsulated in a polymeric particle and adaptor elements which modularly couple functional elements to the particle. The modular design of these vaccine compositions, which involves flexible addition and subtraction of antigen, adjuvant, immune potentiators, molecular recognition and transport mediation elements, as well as intracellular uptake mediators, allows for exquisite control over variables that are important in optimizing an effective vaccine delivery system.
Owner:YALE UNIV
Modular nanodevices for smart adaptable vaccines
ActiveUS20150125384A1Promote absorptionGood curative effectSsRNA viruses negative-sensePowder deliveryVaccine deliveryAdjuvant
Modular nanoparticle vaccine compositions and methods of making and using the same have been developed. Modular nanoparticle vaccine compositions comprise an antigen encapsulated in a polymeric particle and adaptor elements which modularly couple functional elements to the particle. The modular design of these vaccine compositions, which involves flexible addition and subtraction of antigen, adjuvant, immune potentiators, molecular recognition and transport mediation elements, as well as intracellular uptake mediators, allows for exquisite control over variables that are important in optimizing an effective vaccine delivery system.
Owner:YALE UNIV
Vaccine delivery via microneedle arrays
A microprojection array is provided, comprising an approximately planar base and a plurality of microprojections, wherein the array comprises a vaccine and a polymeric material. The array may have multiple layers. The vaccine may be placed in only one layer. In another embodiment of the invention, a method of preventing a disease is provided, comprising insertion into the skin of a patient an array of microprojections comprising a layer which comprises a vaccine for that disease and a polymer.
Owner:CORIUM PHARMA SOLUTIONS INC
Method of vaccine delivery via microneedle arrays
A microprojection array is provided, comprising an approximately planar base and a plurality of microprojections, wherein the array comprises a vaccine and a polymeric material. The array may have multiple layers. The vaccine may be placed in only one layer. In another embodiment of the invention, a method of preventing a disease is provided, comprising insertion into the skin of a patient an array of microprojections comprising a layer which comprises a vaccine for that disease and a polymer.
Owner:CORIUM PHARMA SOLUTIONS INC
Subunit vaccine delivery platform for robust humoral and cellular immune responses
ActiveUS9808517B2Suppress immunityModulates T-Cell Cycling and ExpansionSsRNA viruses negative-senseBacterial antigen ingredientsVaccine deliveryVesicle/vacuole
Owner:CORNELL UNIVERSITY
Coiled-coil lipopeptide helical bundles and synthetic virus-like particles
ActiveUS20100015173A1Efficient inductionVirus peptidesSaccharide peptide ingredientsChemical synthesisCoiled coil
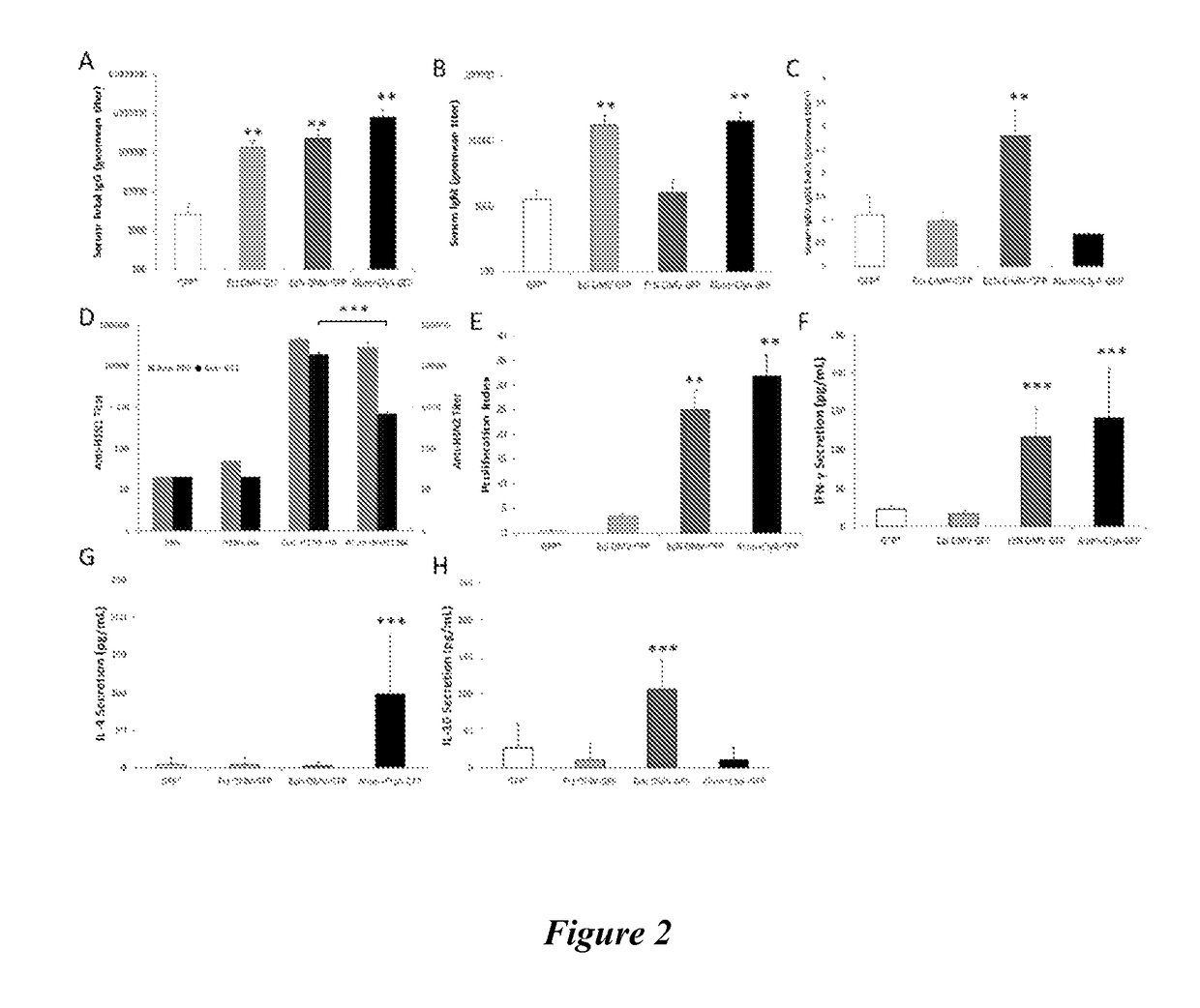
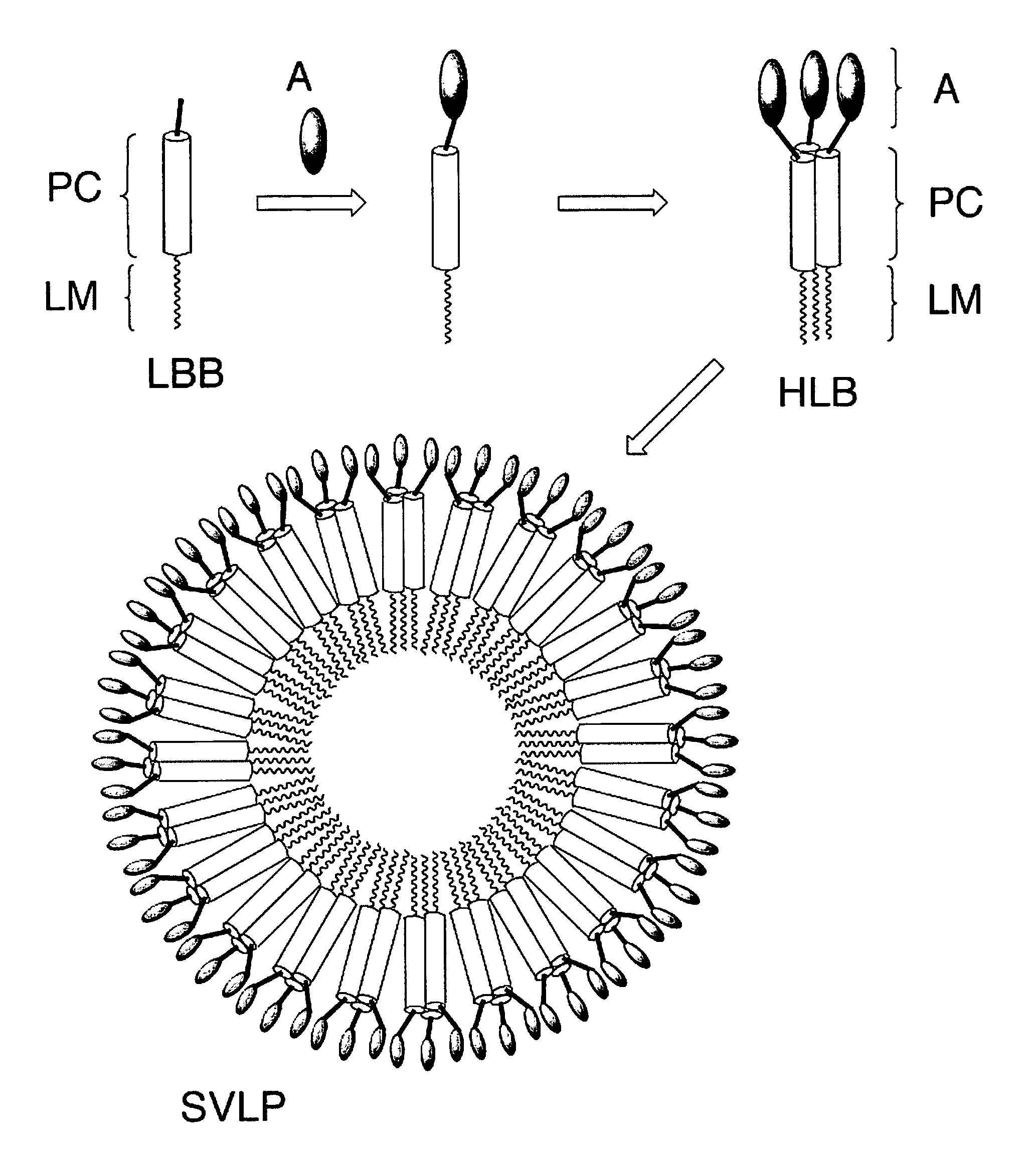
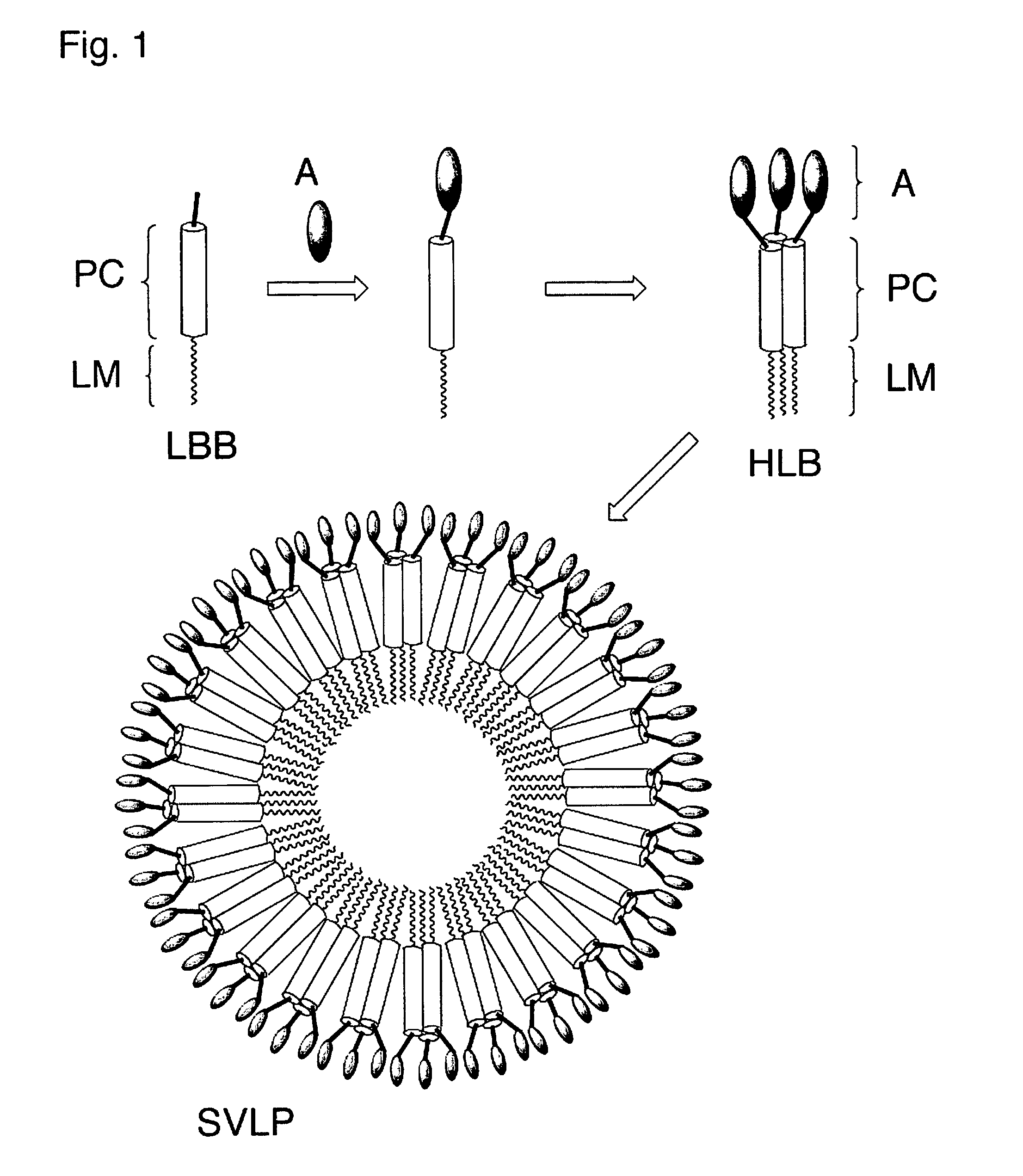
The invention relates to lipopeptide building blocks consisting of a peptide chain comprising a coiled-coil domain, linked covalently to a lipid moiety comprising long alkyl or alkenyl chains, and optionally linked to an antigen; and to helical lipopeptide bundles and synthetic virus-like particles formed by aggregation. The nanometer size and shape of these bundles and particles, their stability under aqueous physiological conditions, their chemical composition, the possibility to incorporate B- and T-cell epitopes, and their production by chemical synthesis, make them highly suitable as vaccine delivery vehicles.
Owner:UNIV ZURICH
Electroporation leading-in apparatus for medicament
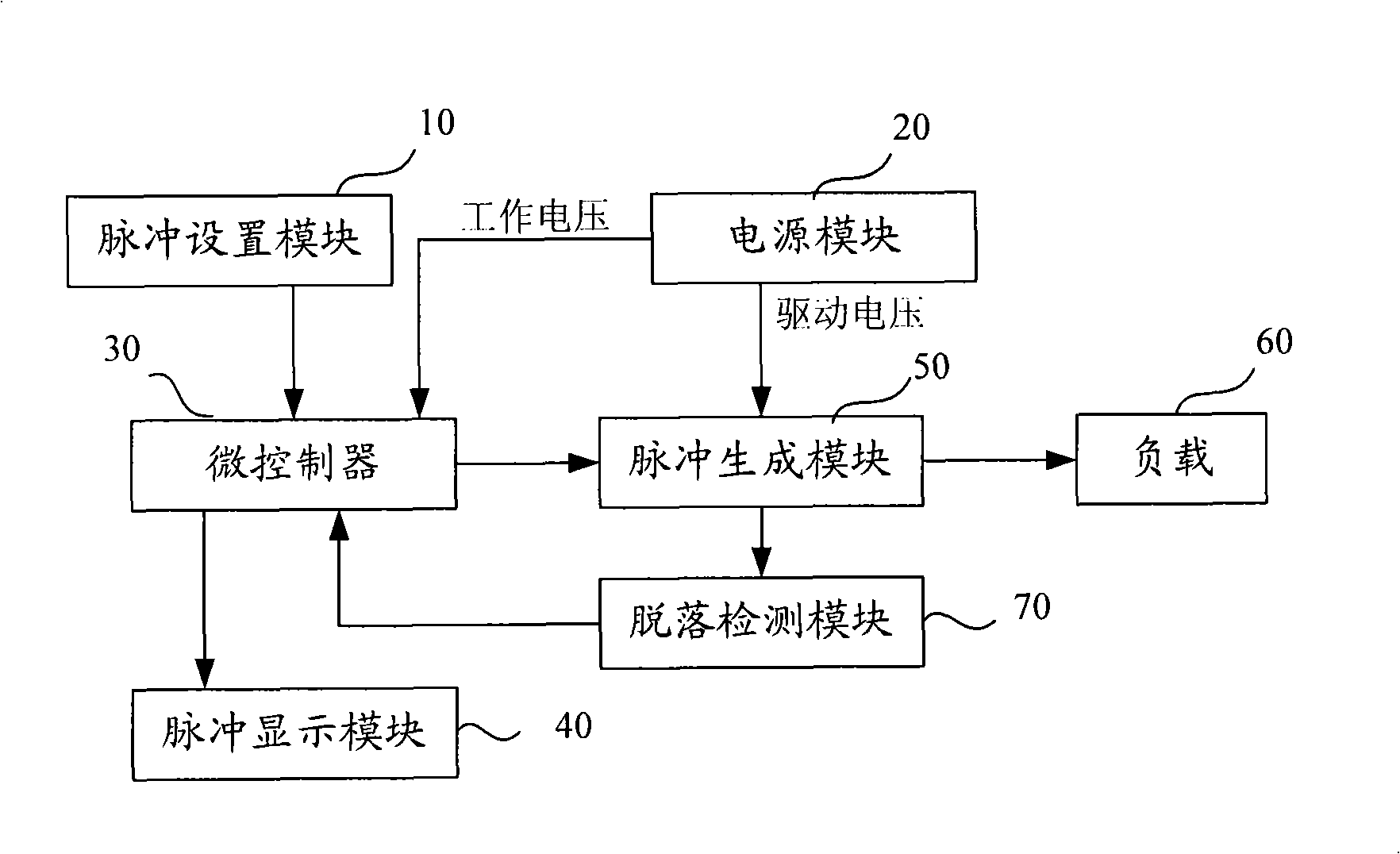
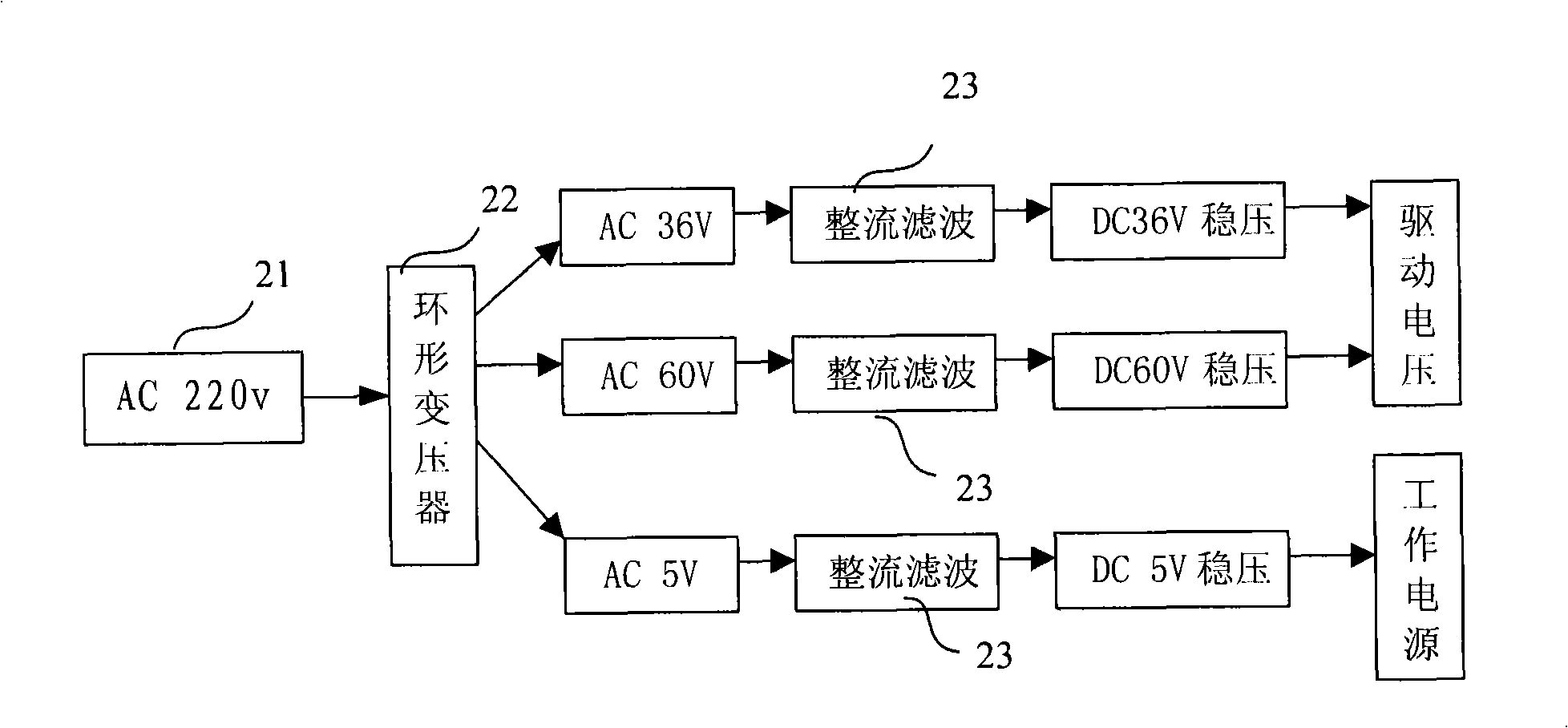
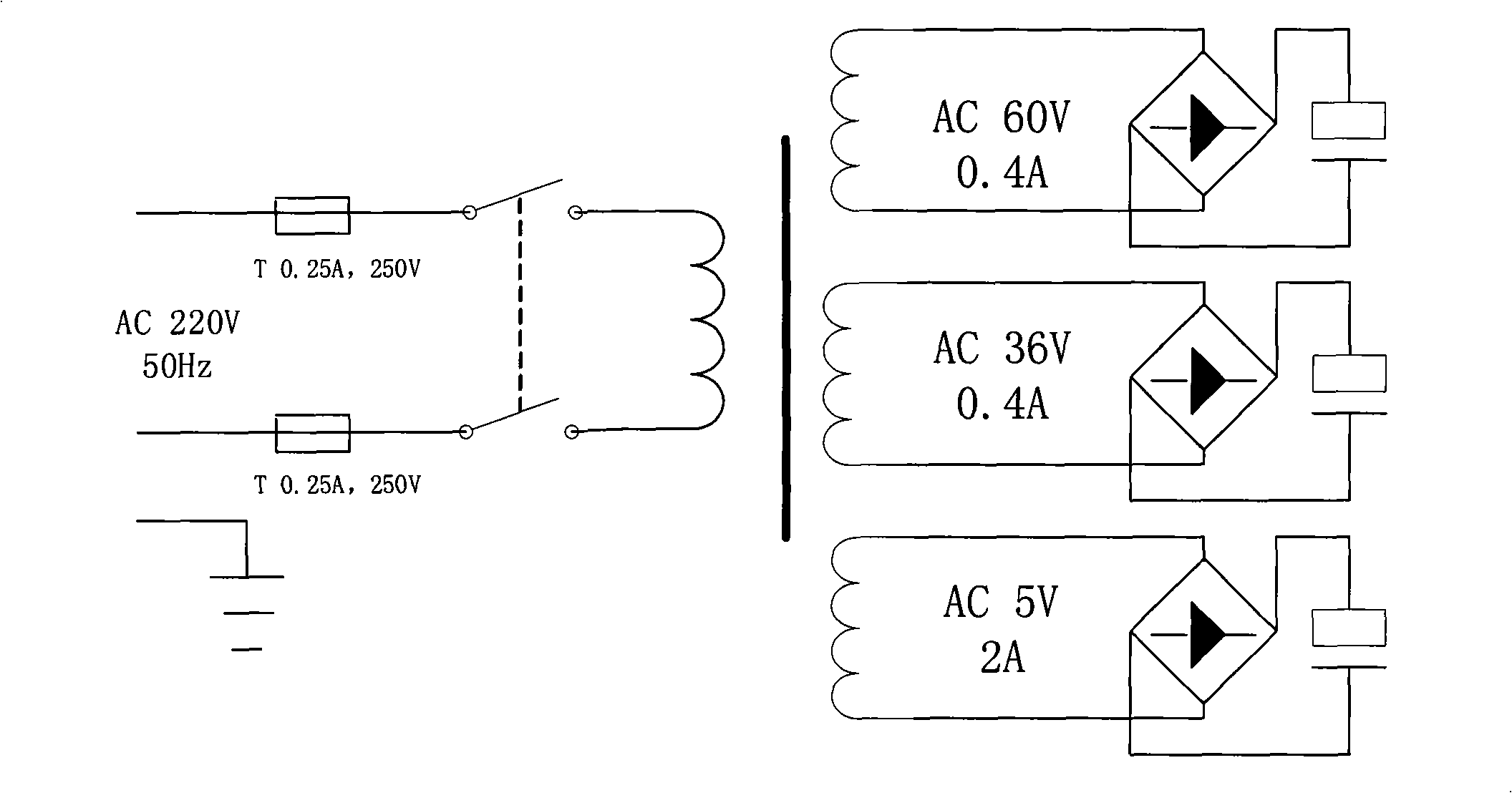
InactiveCN101342390AEasy accessIncrease profitMedical devicesIntravenous devicesMicrocontrollerVaccine delivery
The present invention discloses a medicine deliverer adopting electroperation, which improves the delivering effect of vaccinal medicines, decreases the dosage of medicines and reduces the untoward effect of medicines. In the technical scheme, the medicine deliverer adopting electroperation, which can deliver medicine into a target tissue by means of low-frequency electrical pulses, comprises a pulse-setting module, a power supply module, a microcontroller, a pulse-generating module and a load; wherein, the pulse-setting module receives an electrical pulse parameter inputted by a user; the power supply module generates a direct-current signal; the microcontroller receives the electrical pulse parameter of the pulse-setting module and outputs a control signal according to the electrical pulse parameter; the pulse-generating module receives the control signal outputted by the microcontroller and processes the direct-current signal supplied by the power supply module into an electrical pulse signal, which is then outputted; and the load receives the electrical pulse signal outputted by the pulse-generating module. The medicine deliverer adopting electroperation is applied to the field of medicinal vaccine delivery.
Owner:SHANGHAI TERESA BIO TECH
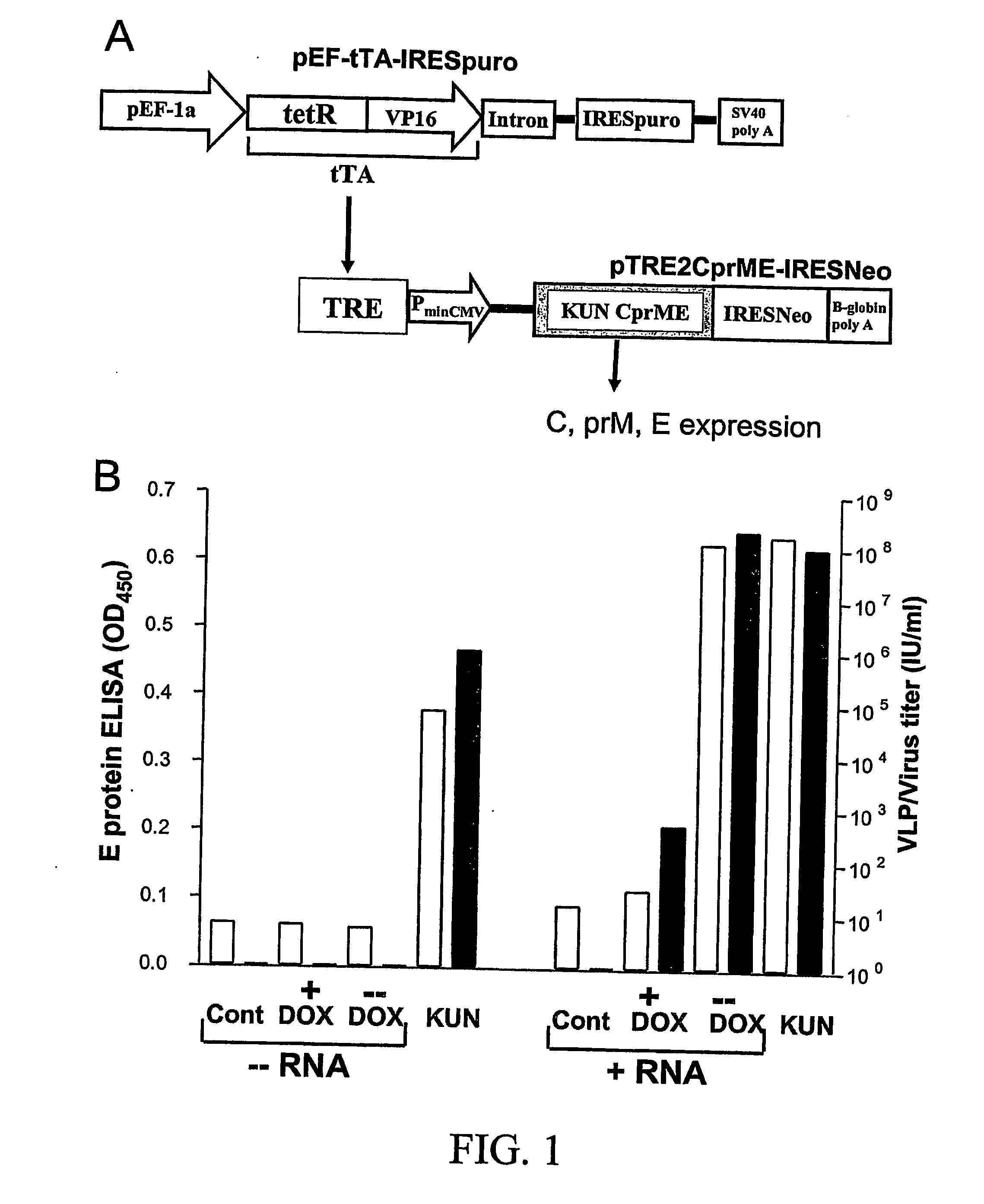
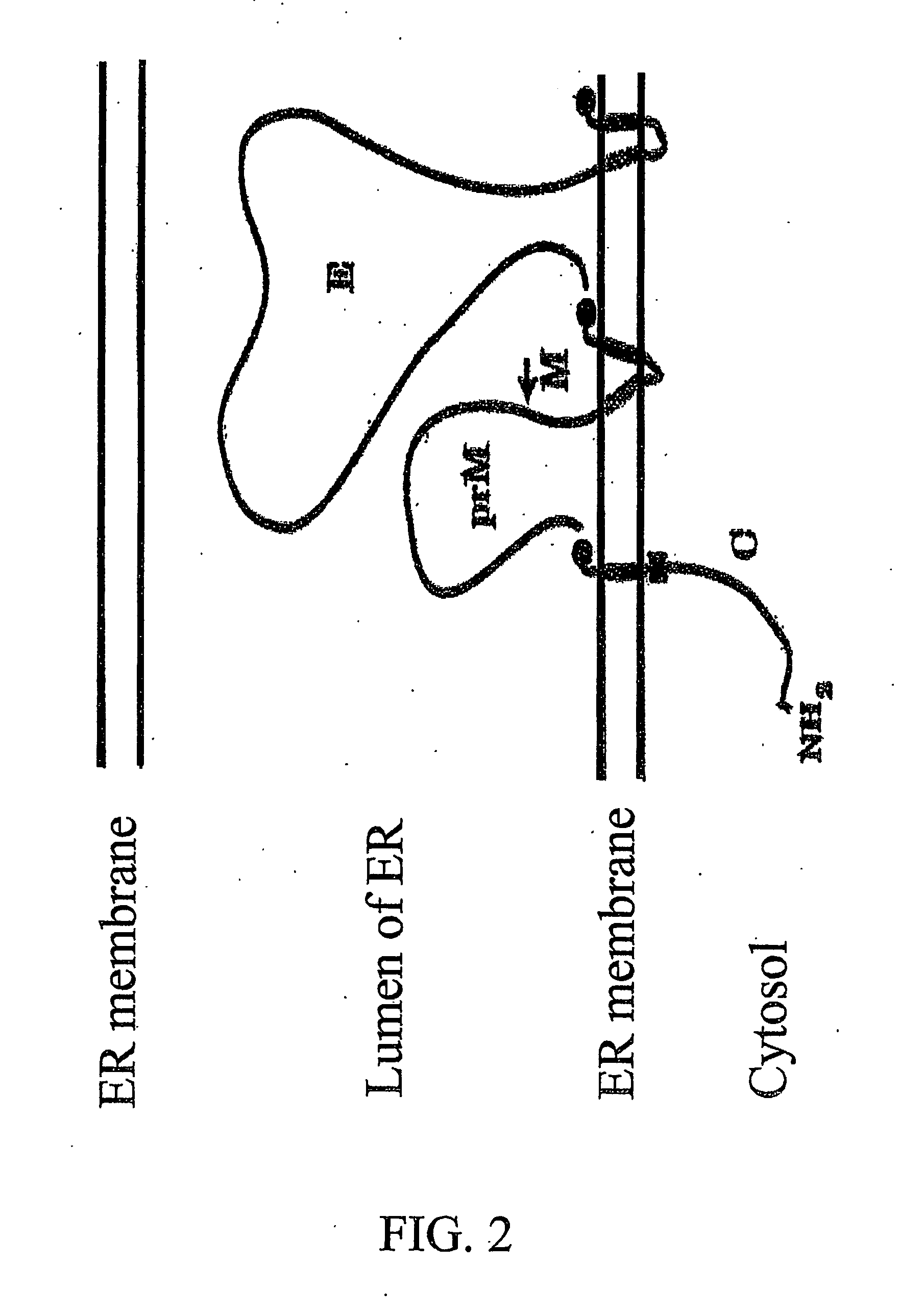
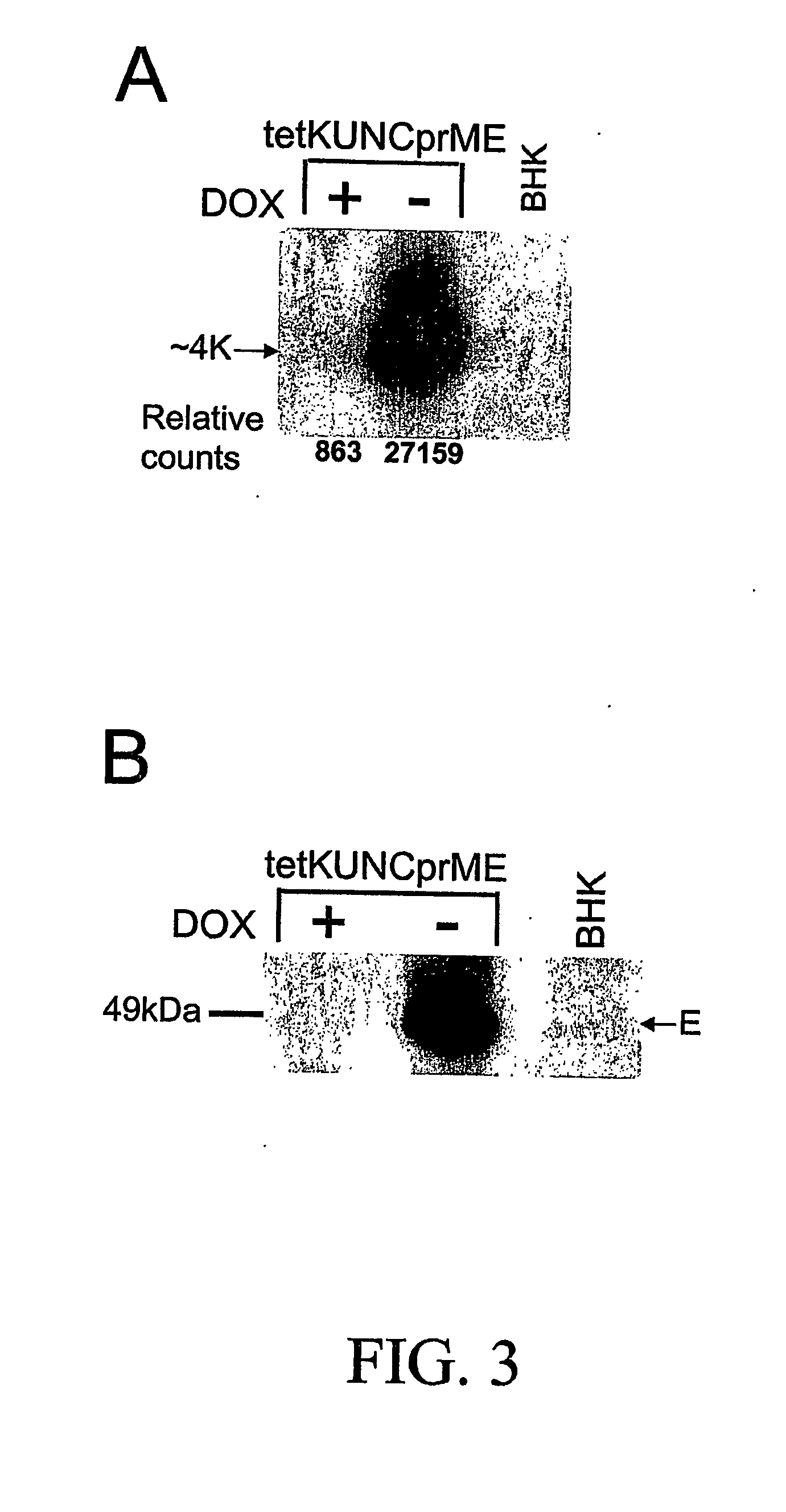
Flavivirus vaccine delivery system
A tetracycline regulatable flaviviral packaging system is provided that facilitates expression of flaviviral structural proteins necessary for flaviviral RNA replicon packaging and virus like particle production in animal cells. This regulatable packaging system is compatible with Kunjin, Dengue and West Nile virus and other flaviviral replicon-based expression systems and produces unexpectedly high titres of virus-like particles. A particular application of this packaging system is the production of virus-like particles that package RNA comprising a flaviviral replicon and encoding a heterologous protein or peptide for expression in animal cells. Even more particularly, the packaging system is capable of delivering immunogens that induce a protective CD8 T cell-mediated immune response.
Owner:REPLIKUN BIOTECH
Subunit vaccine delivery platform for robust humoral and cellular immune responses
ActiveUS20150056246A1Suppress local immunityEnhancing natural adjuvanting mechanismSsRNA viruses negative-senseBacterial antigen ingredientsVaccine deliveryVesicle/vacuole
The present invention relates to a probiotic cell transformed with a construct suitable to overexpress and display on the surface of the probiotic cell a fusion protein comprising at least a portion of a transport protein coupled to at least a portion of one or more antigenic proteins or peptides. Probiotic-derived vesicles displaying this fusion protein as well as methods of inducing an immune response using the probiotic cells or vesicles are also disclosed.
Owner:CORNELL UNIVERSITY
Combined dna/protein vaccine compositions
InactiveUS20050129712A1Bacterial antigen ingredientsViral antigen ingredientsVaccine deliveryAdjuvant
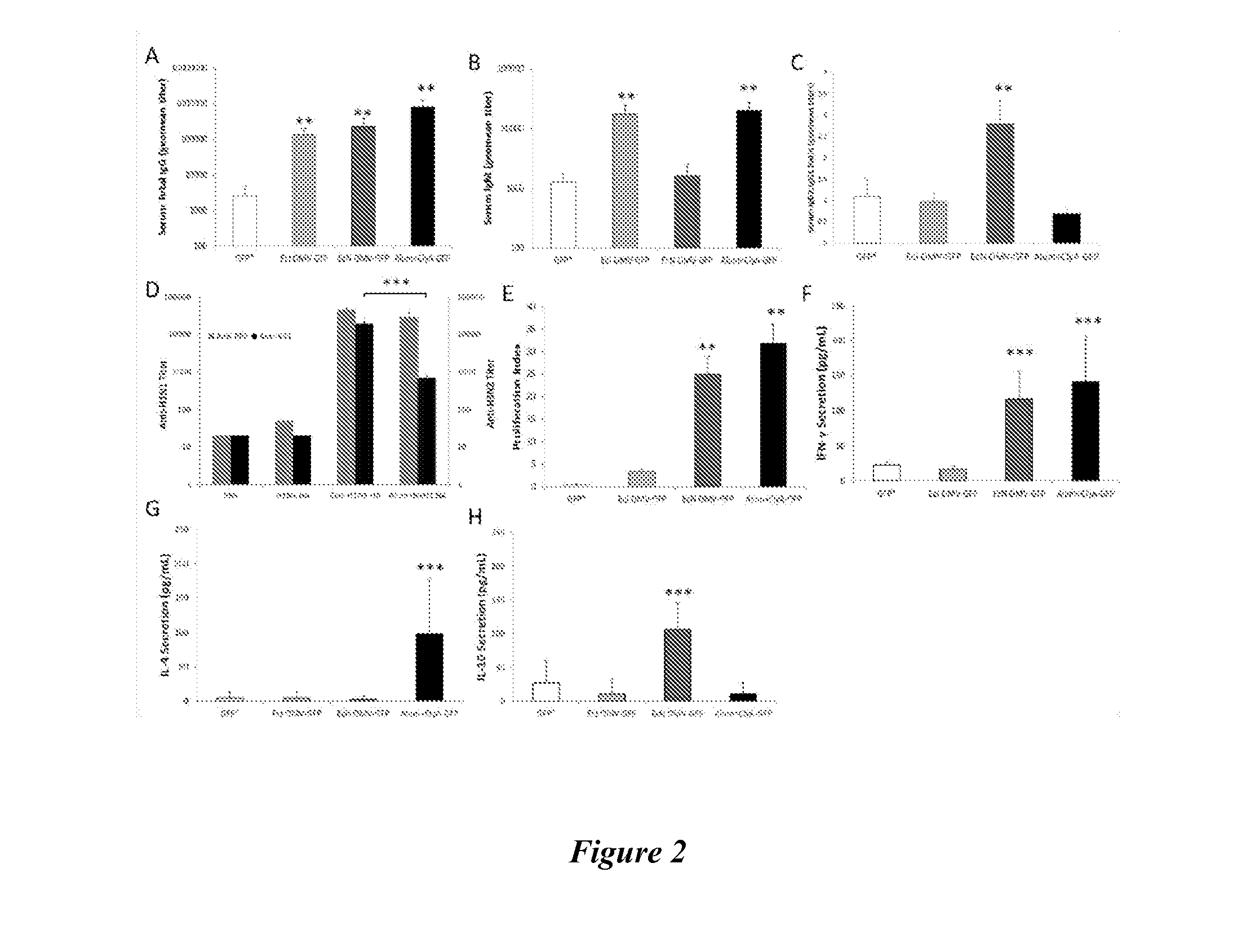
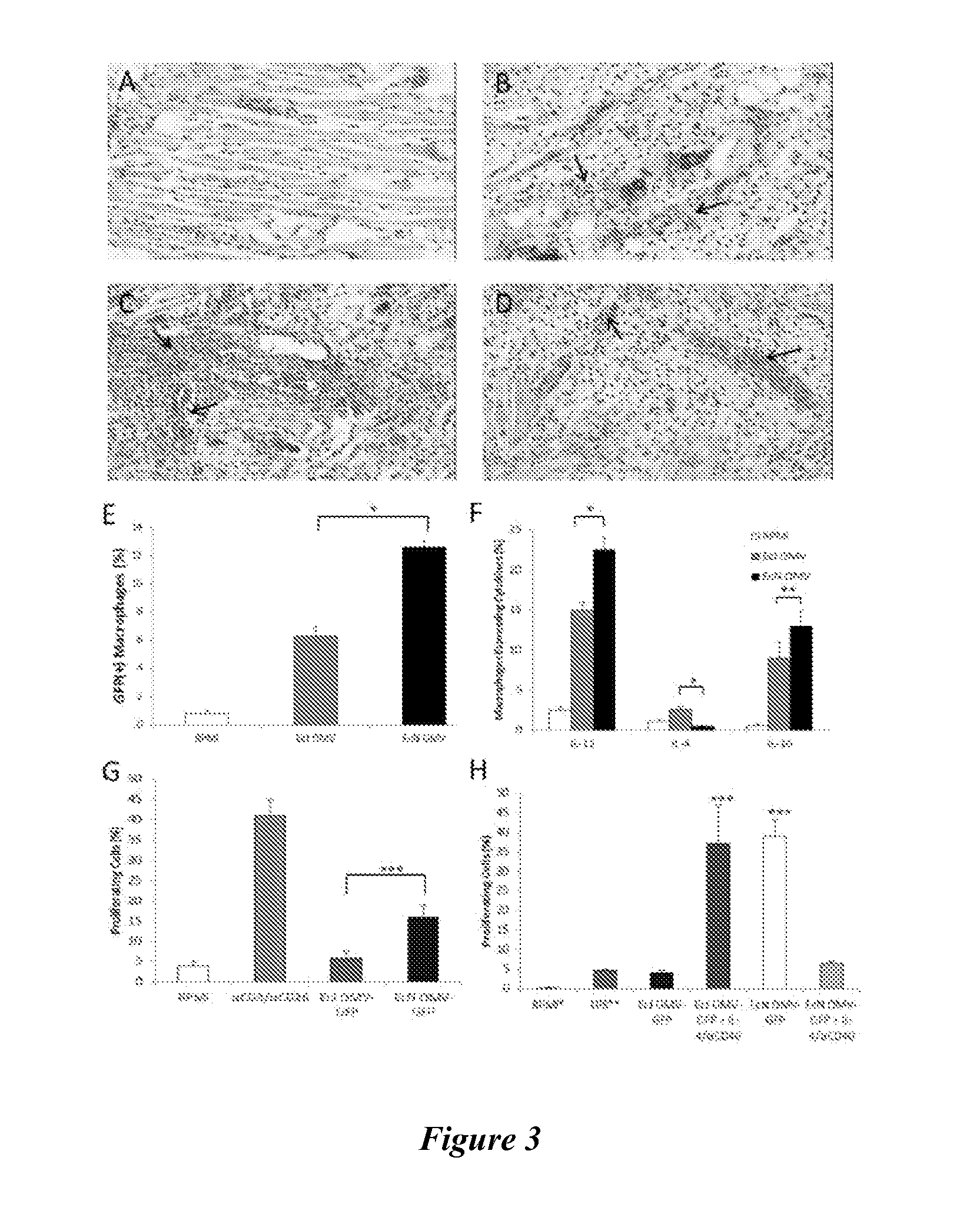
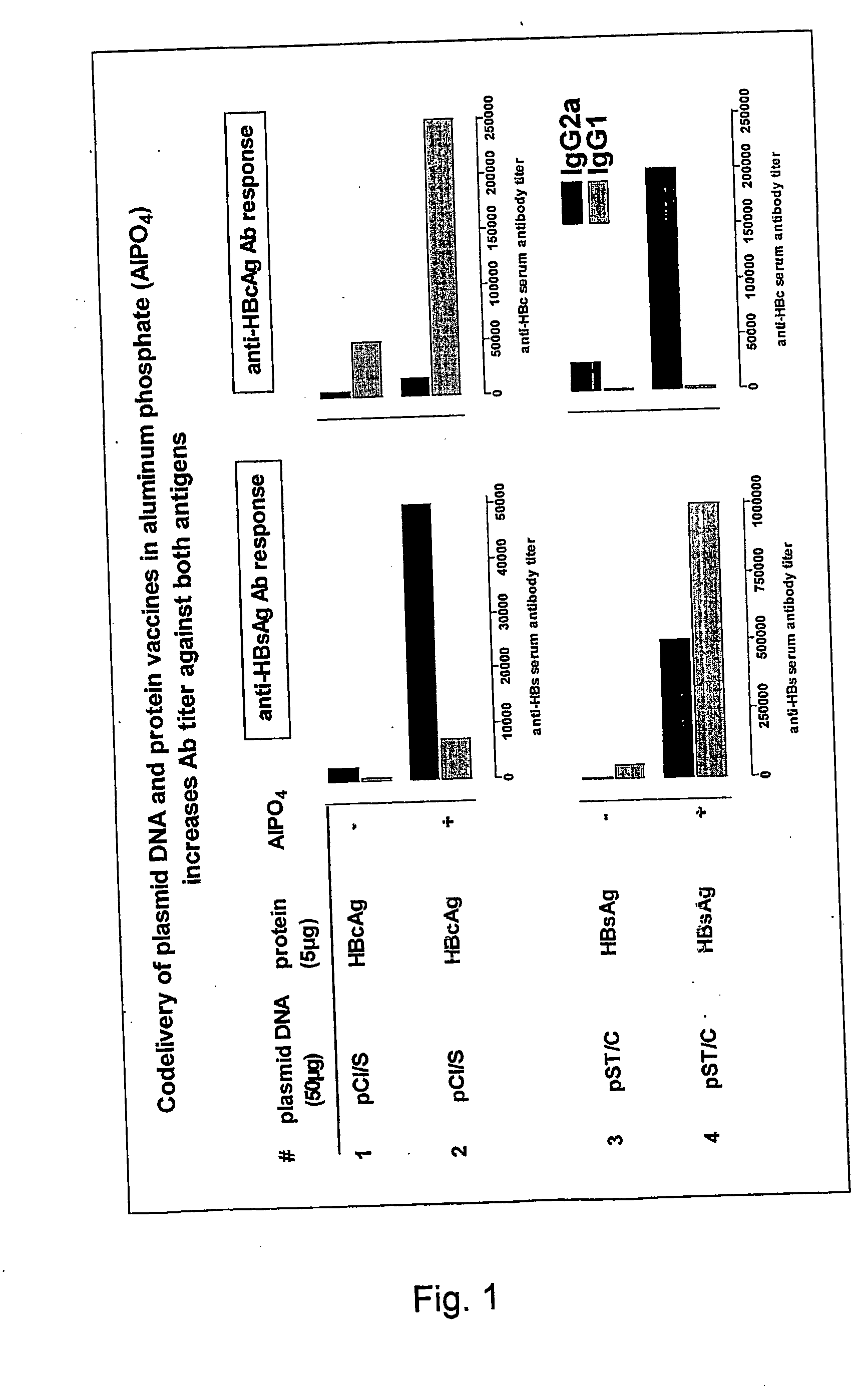
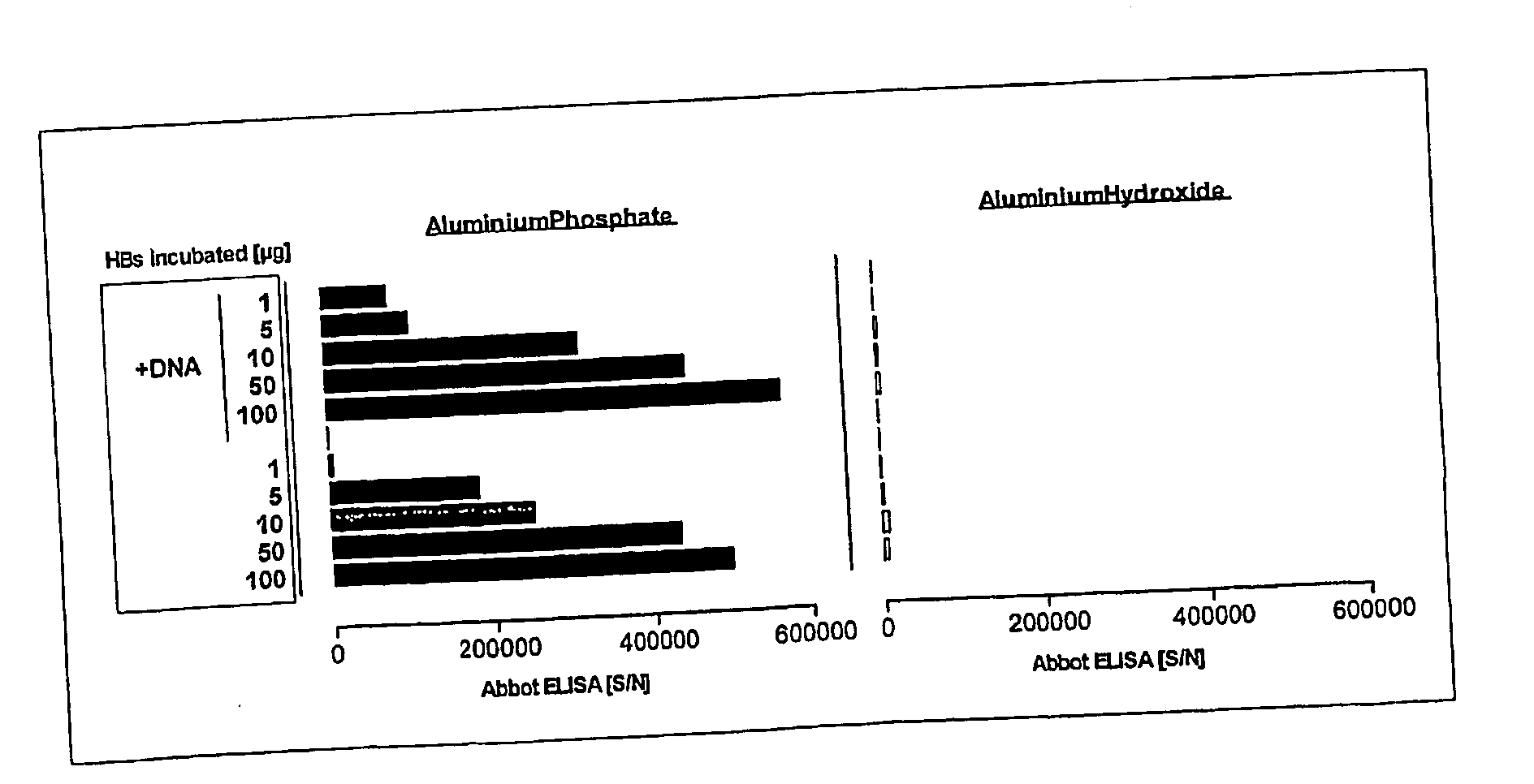
The present invention relates to a combined DNA / Protein antigen vaccine composition which includes nucleic acid molecules and a mineral-based, negatively charged adjuvant, so as to enhance the immunogenicity of the DNA vaccine efficiently, enhancing not only plasmid DNA vaccines but also providing a novel strategy for immunogenic, multivalent combined protein / DNA vaccine delivery. Preferred adjuvants are aluminum or calcium salts, in particular aluminum phosphate.
Owner:BRENNTAG BIOSECTOR
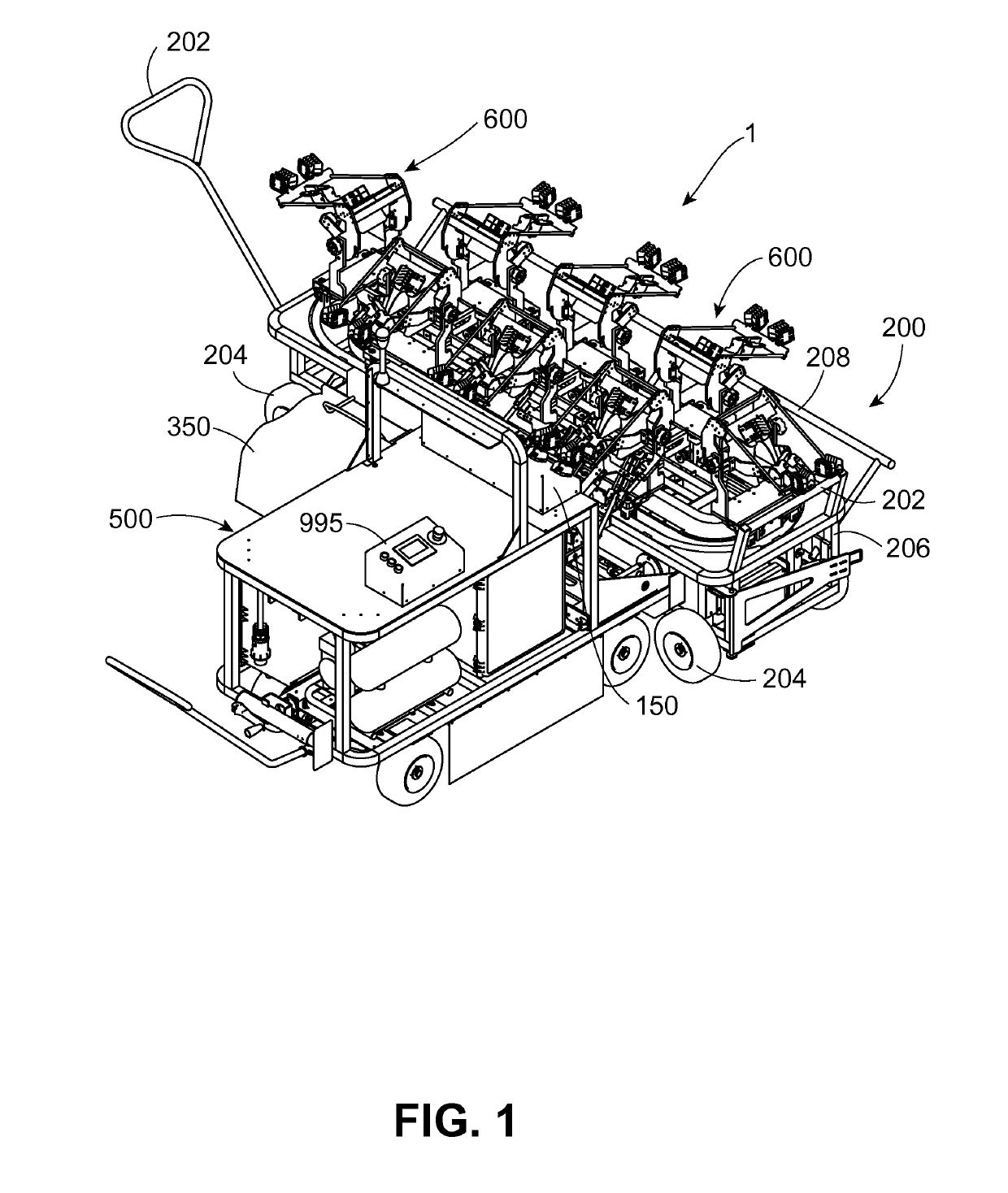
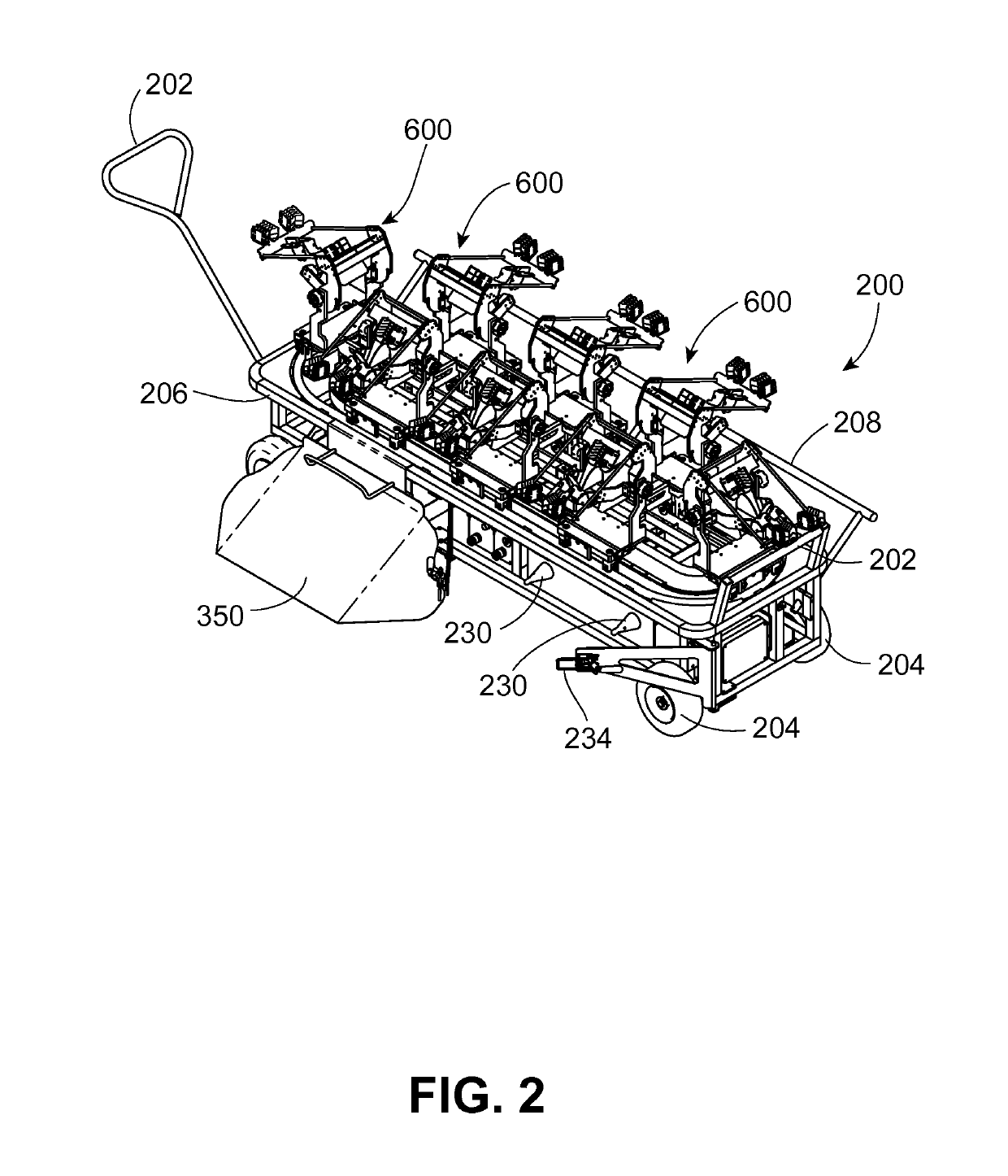
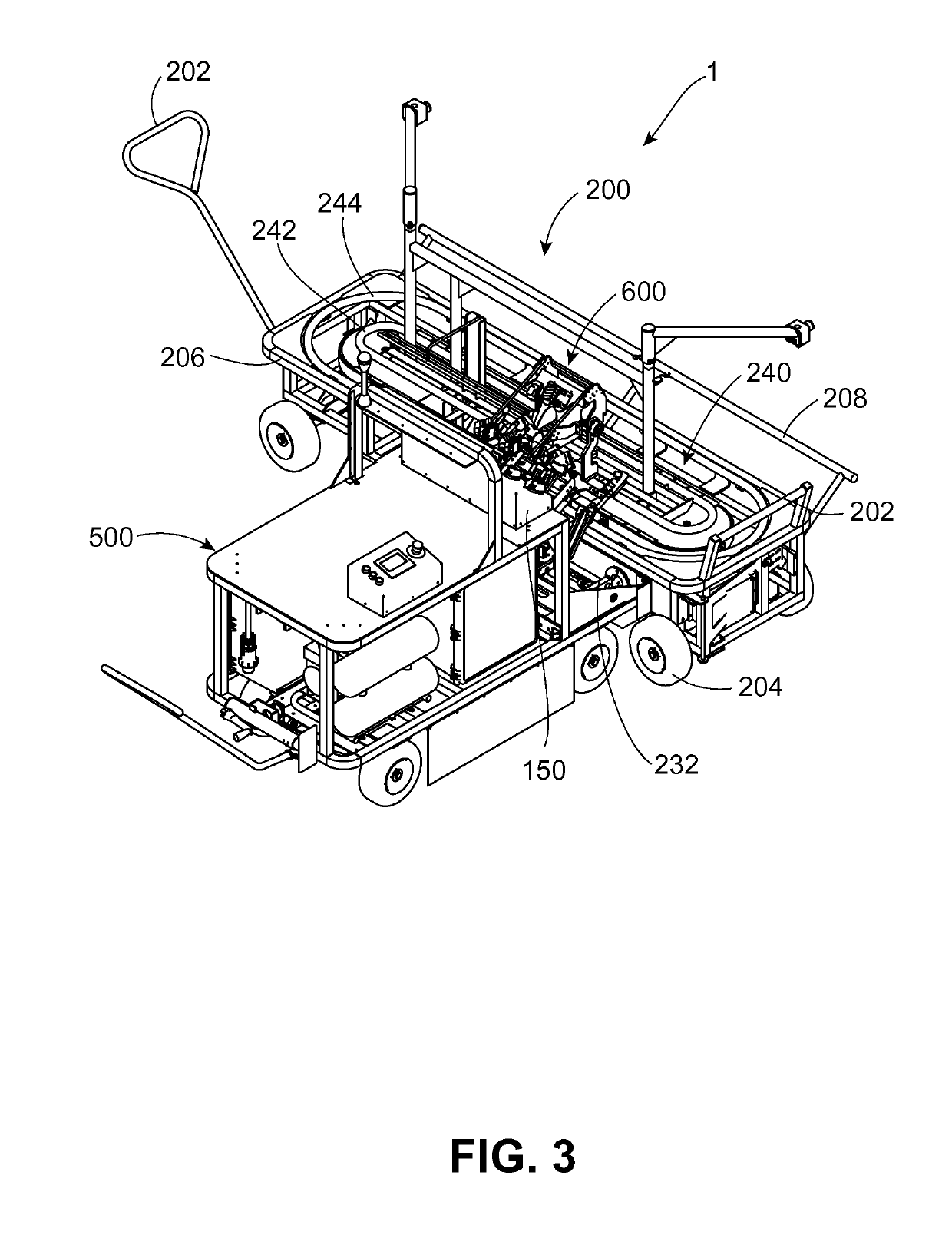
Vaccination system for delivering vaccine to avian pullets, and associated methods, devices, and assemblies
A vaccination system for delivering a vaccine substance to avian pullets is provided. Such a system includes a vaccine delivery assembly configured to perform a vaccine delivery procedure for delivering a vaccine substance to the avian pullets. A plurality of positioning devices is provided, with each positioning device receiving an avian pullet for presentation to the vaccine delivery assembly. Each positioning device is transported to individually mate with the vaccine delivery assembly to deliver the vaccine substance during the vaccine delivery procedure. Associated methods, devices, and assemblies are also provided.
Owner:ZOETIS SERVICE LLC
Oral vaccine delivery system and application thereof
InactiveCN106215181AGood biocompatibilityNo side effectsAntibacterial agentsBacterial antigen ingredientsDiseaseAdjuvant
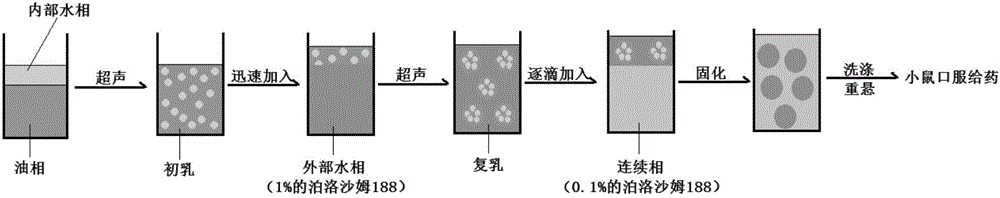
The invention discloses an oral vaccine delivery system. W1 of W1 / O / W2 type nanoparticles is a vaccine medicine phase, an organic phase O is selected from PLGA and HP55, an external water phase W2 and a continuous water phase are selected from poloxamer 188, and a vaccine medicine is a double epitope-double adjuvant helicobacter pylori vaccine. The method adopts a polymer material having good biological safety and good compatibility to prepare the nanoparticles, a preparation process and a formula are optimized, the oral vaccine delivery system is non-toxic, free of any side effects, easy to manufacture and low in cost. In the field of biological medicines, the nanoparticles can be used for oral taking of a helicobacter pylori subunit vaccine, accordingly helicobacter pylori infection related diseases are prevented and treated, and huge economic benefits and social benefits are brought.
Owner:CHINA PHARM UNIV
Flexible vaccine assembly and vaccine delivery platform
ActiveUS20060188991A1Modulate host immune responseFacilitate efficient immune cell recognitionVirusesAntibody mimetics/scaffoldsVaccine deliveryVirus-like particle
Herein-described are various methods for making a vaccine that are made of re-assembled virus like particles (VLP). First, the VLPs are disassembled into encapsidation intermediate populations. Each encapsidation intermediate population undergoes, for instance, chemical conjugation of unique peptide or nucleic moieties to form separate populations. Thereafter, a predetermined amount of each of the several (one or more) different encapsidation intermediates from the different populations is mixed and joined, forming intact VLPs, surrounding a nucleic acid core, that are composed of different encapsidation intermediate such that the reassembled VLP displays more than one peptide or nucleic acid. The nucleic acid can function either as a scaffold alone or can be engineered for the expression of an immunomodulatory protein in a eukaryotic cell.
Owner:KBIO HLDG LTD
Targeted nano-vaccine preparation based on metal-polyphenol network structure and product of targeted nano-vaccine preparation
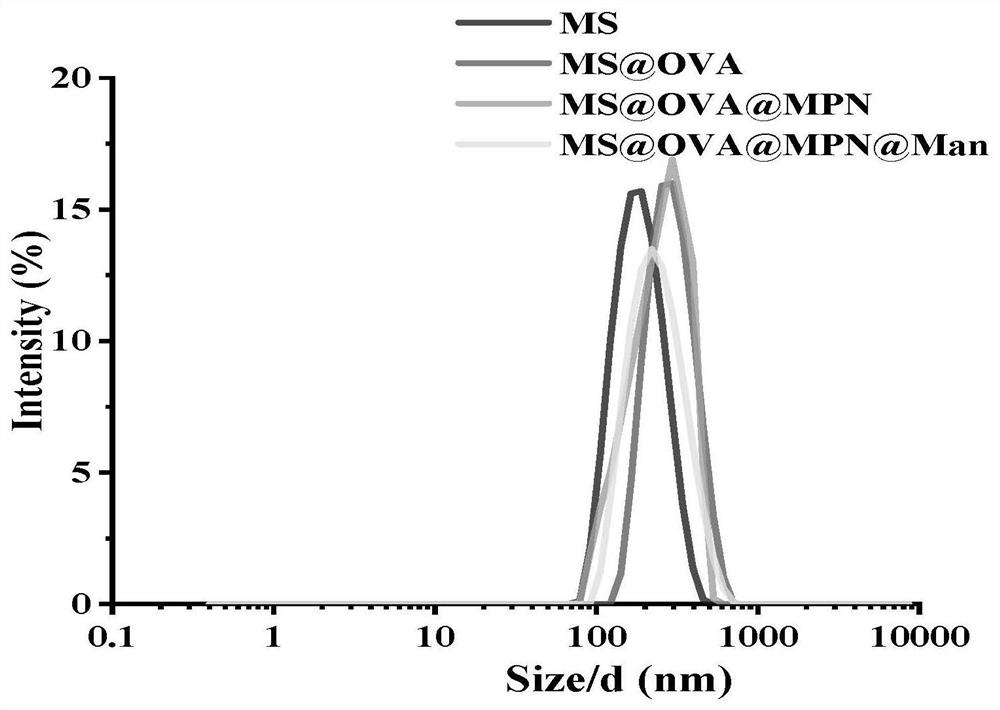
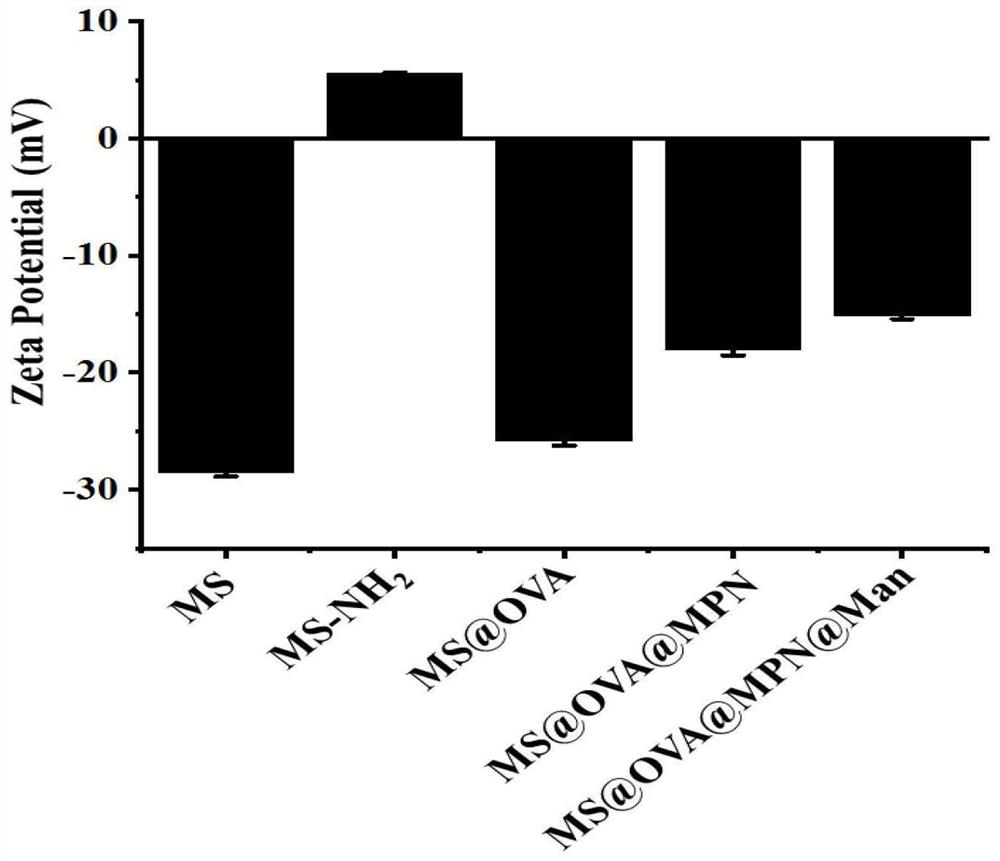
InactiveCN112245407APrevent leakageAdd escape functionCancer antigen ingredientsPharmaceutical non-active ingredientsLysosomeMesoporous silica
The invention discloses a preparation method of a targeted nano vaccine based on a metal-polyphenol network structure. The preparation method comprises the following steps that mesoporous silica nanoparticles loaded with ovalbumin OVA are prepared, mannose-modified tannic acid molecules are synthesized, and the surfaces of the mesoporous silica nanoparticles loaded with the ovalbumin OVA are coated with metal-polyphenol network coatings, so that the mesoporous silica nanoparticles loaded with the OVA and with the surfaces coated with the metal-polyphenol network coatings are obtained, therefore the targeted nano-vaccine based on the metal-polyphenol network structures is obtained, and the targeted nano-vaccine is named as MS@OVA@MPN@Man. According to the preparation method, the surfaces are coated with the metal-polyphenol network coatings, so that leakage of OVA is prevented, and the lysosome escape function of the nanoparticles is increased; and meanwhile, mannose is modified on thesurface to target a mannose receptor on the surface of an immune cell, so that the uptake capacity of the cell to the mannose receptor is enhanced to improve the vaccine delivery efficiency, and the problems of targeting of the nano vaccine and lysosome escape efficiency are solved.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com