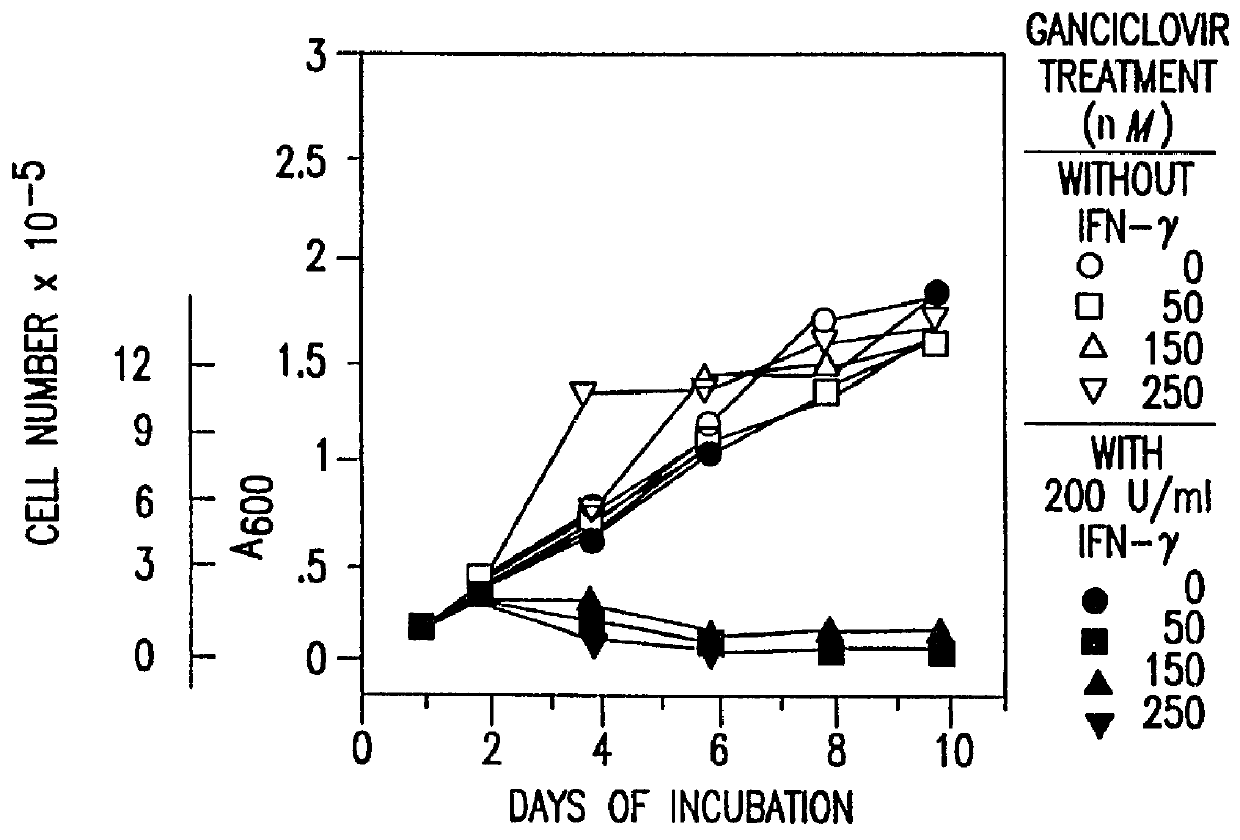
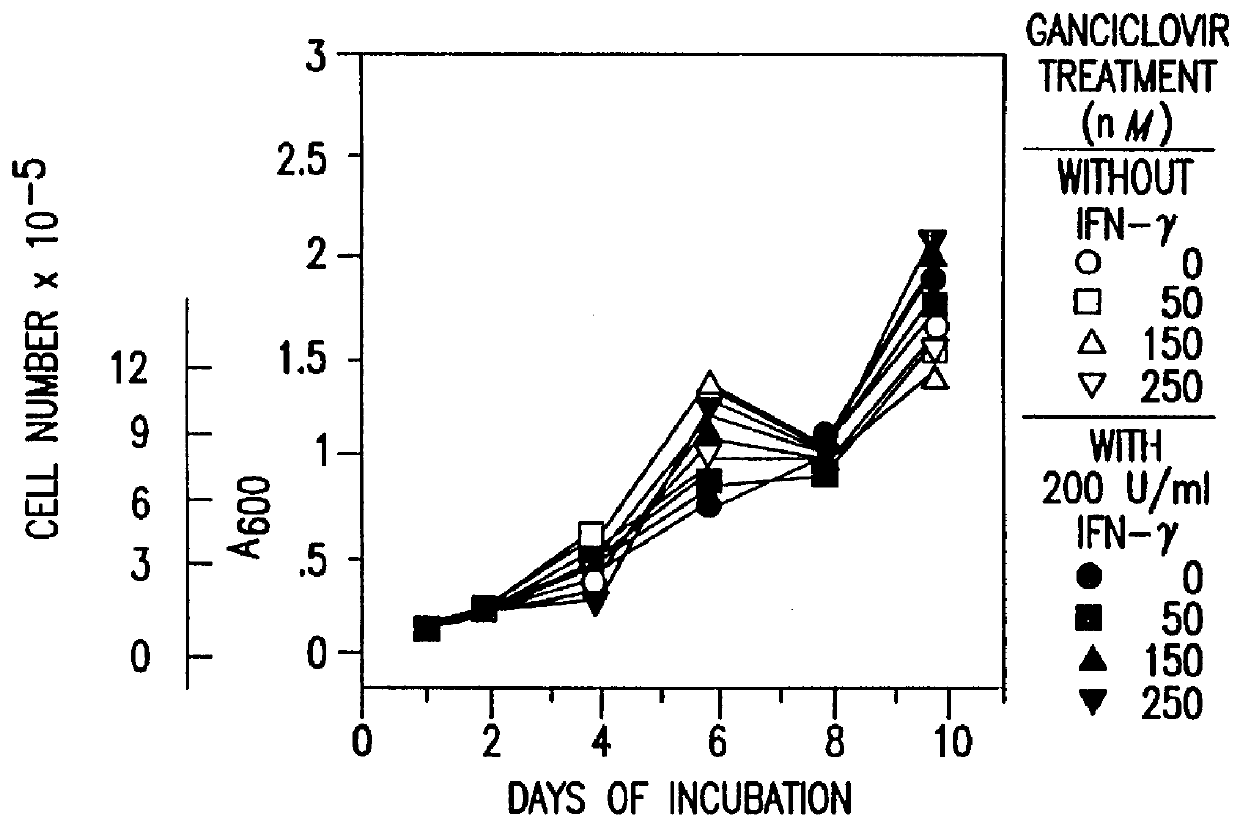
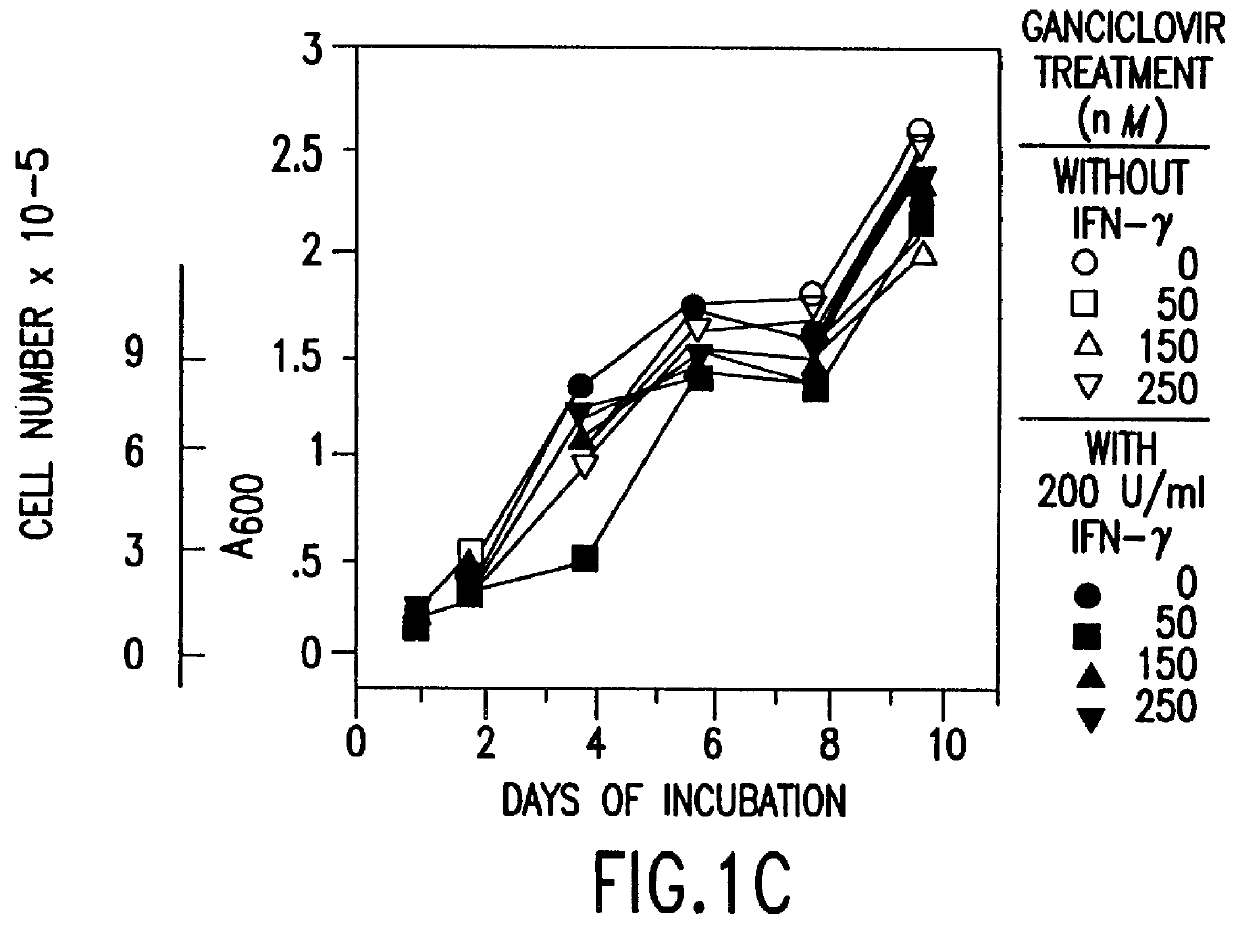
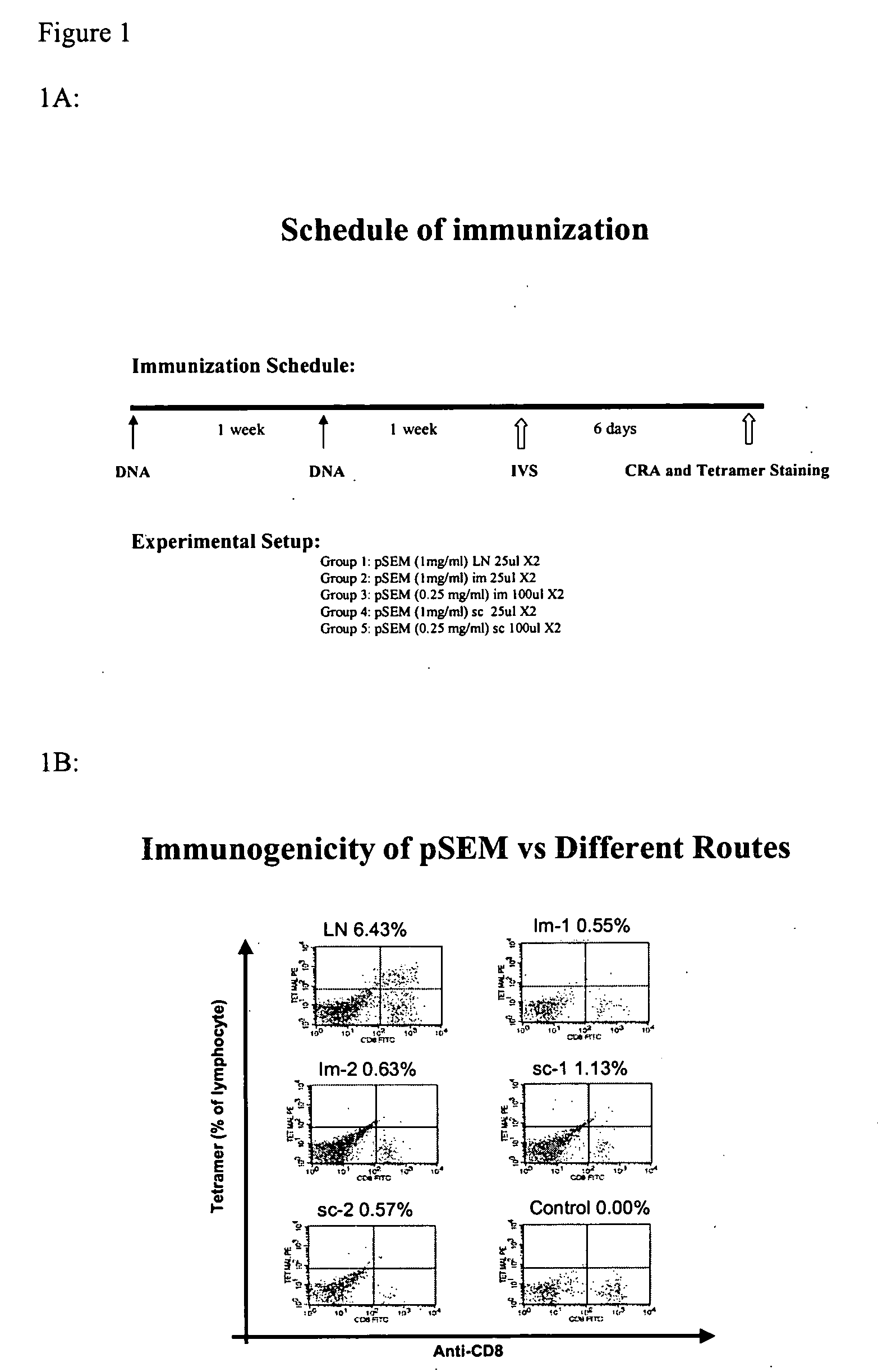
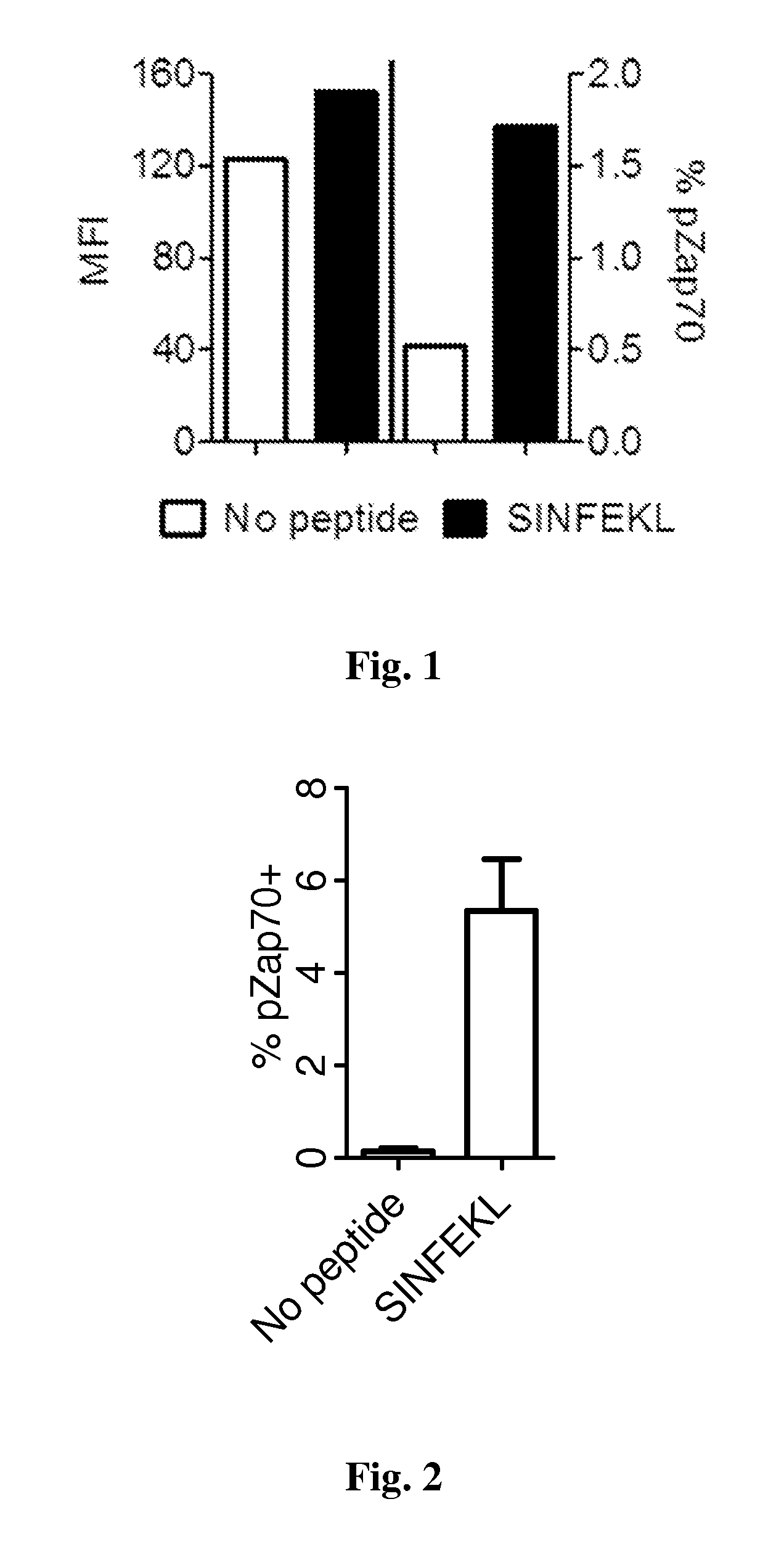
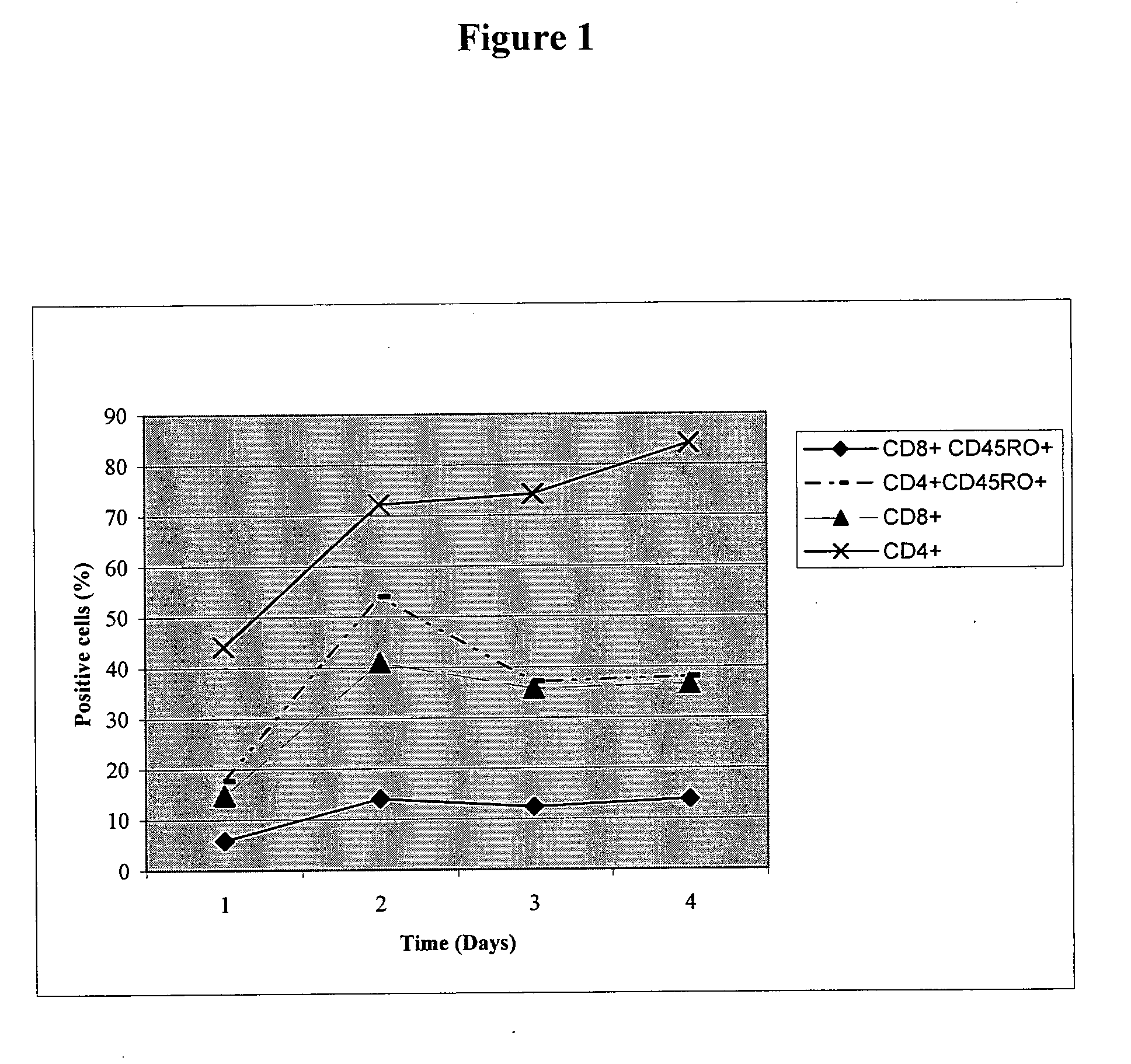
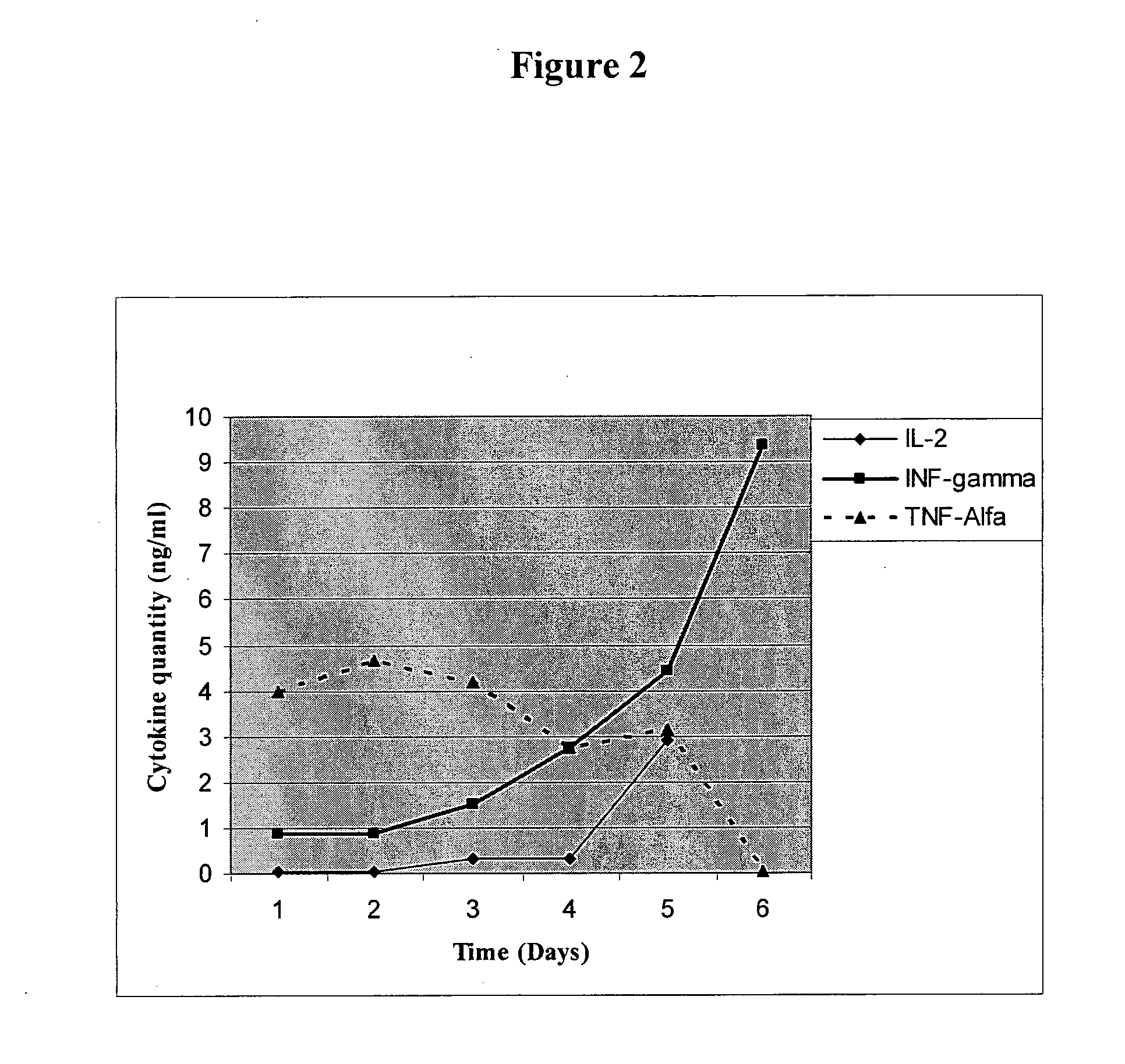
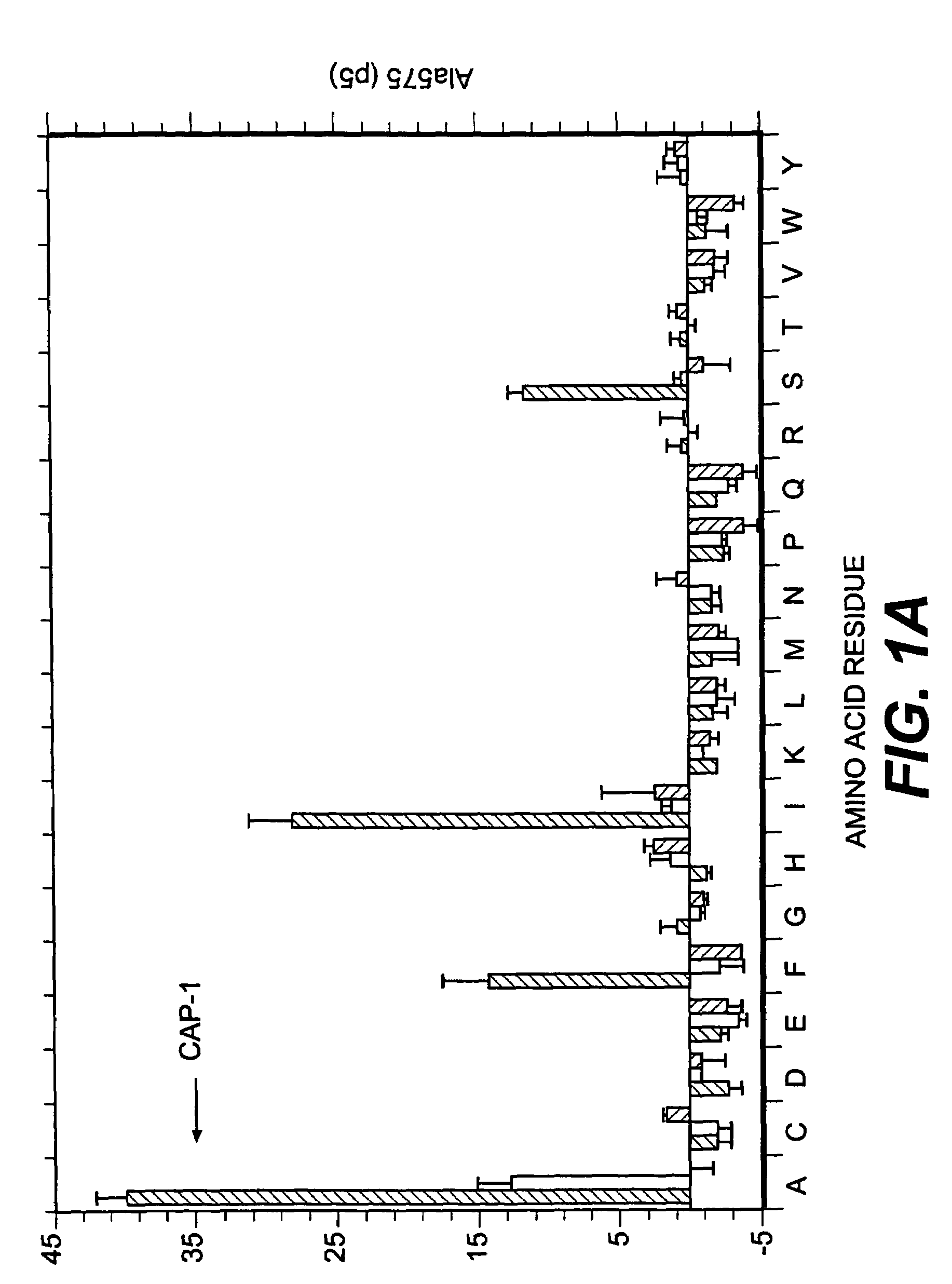
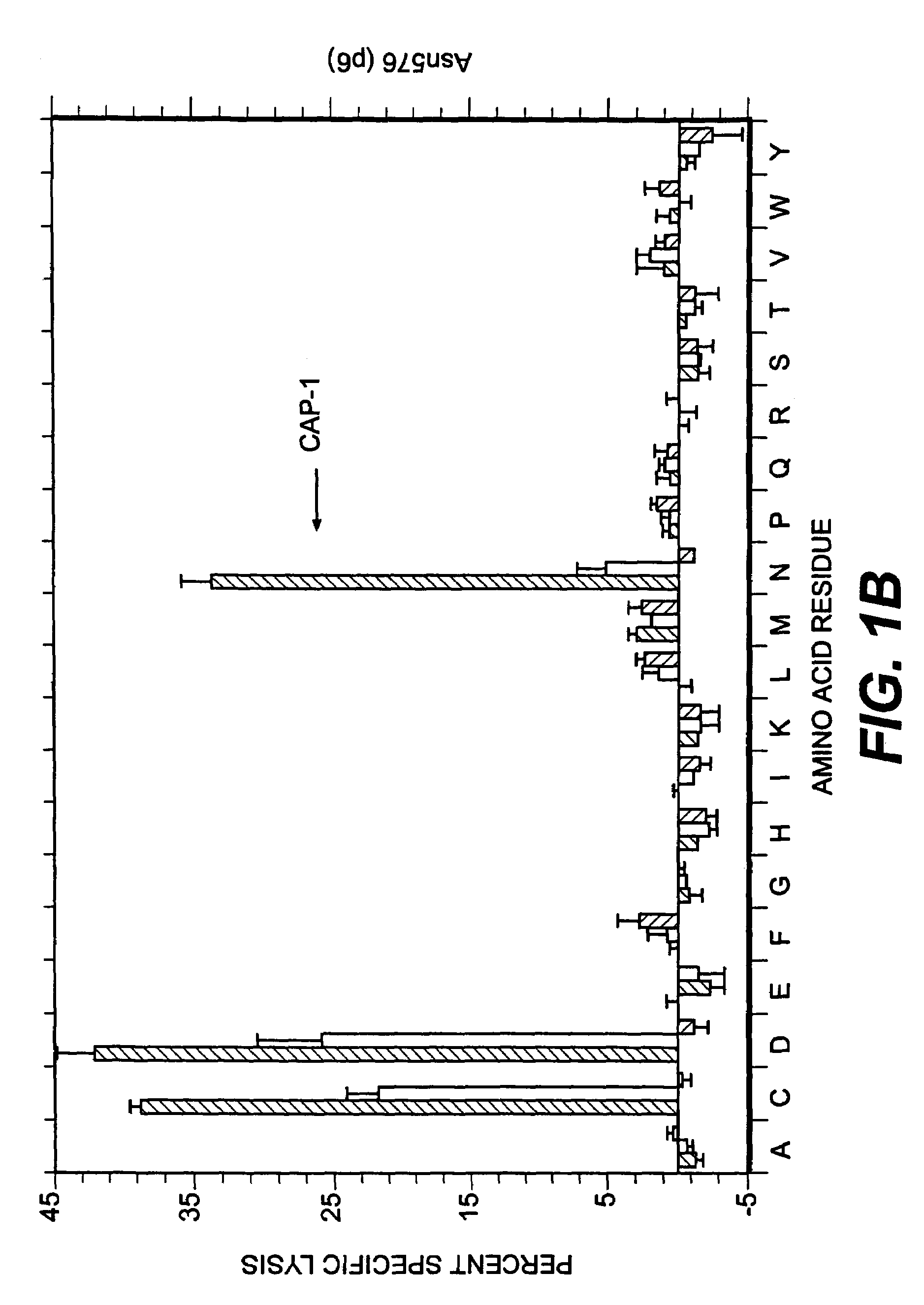
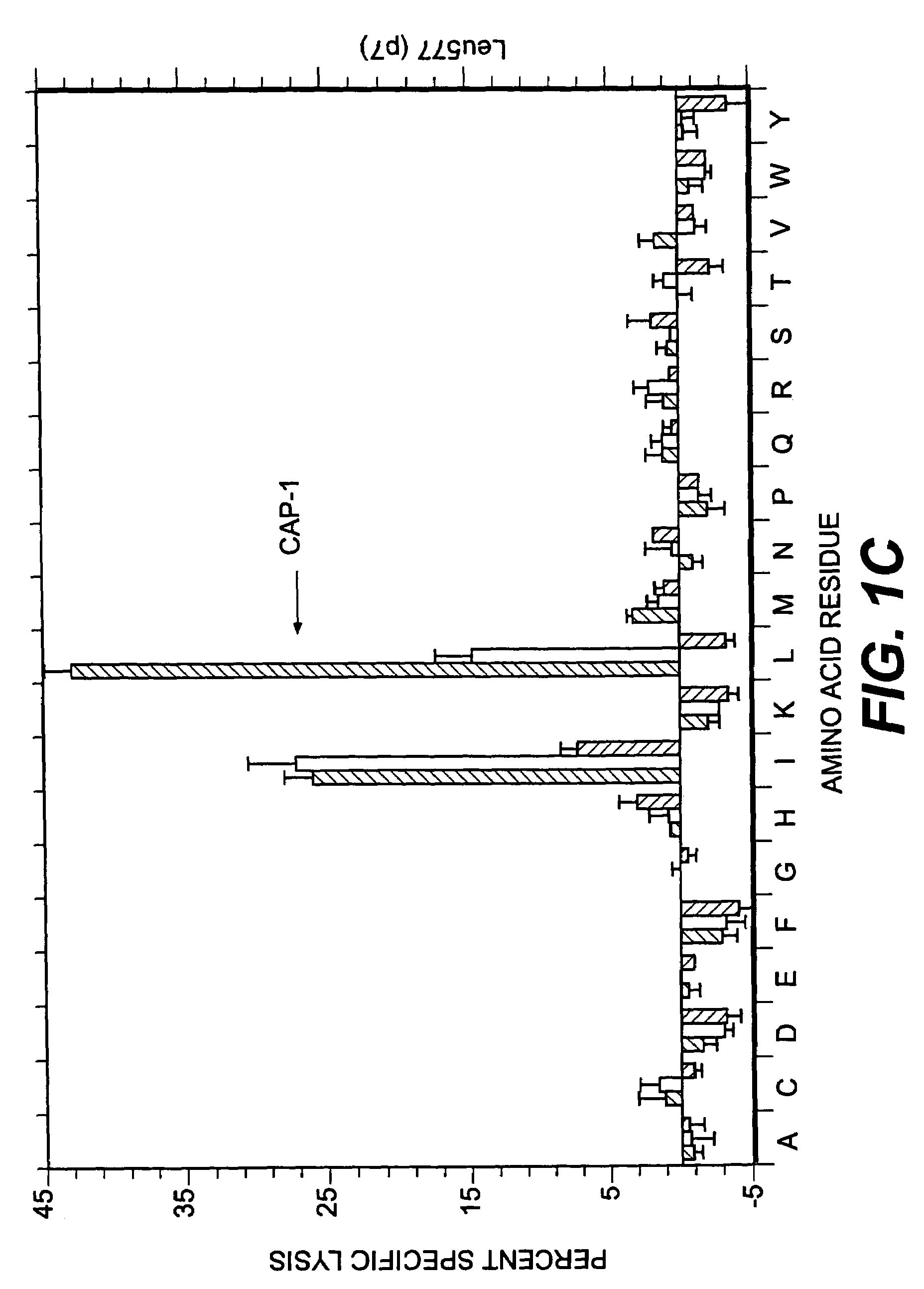
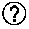Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
197 results about "MHC class I" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
MHC class I molecules are one of two primary classes of major histocompatibility complex (MHC) molecules (the other being MHC class II) and are found on the cell surface of all nucleated cells in the bodies of jawed vertebrates. They also occur on platelets, but not on red blood cells. Their function is to display peptide fragments of proteins from within the cell to cytotoxic T cells; this will trigger an immediate response from the immune system against a particular non-self antigen displayed with the help of an MHC class I protein. Because MHC class I molecules present peptides derived from cytosolic proteins, the pathway of MHC class I presentation is often called cytosolic or endogenous pathway.
Regulation of gene expression
InactiveUS6022863APrevention of fetal rejectionSugar derivativesGenetic material ingredientsMHC class IDisease
The present invention relates to utrons, RNA molecules which contain promoter regulatory motif(s) and DNA analogs thereof and DNA molecules that can be transcribed to produce the foregoing. In particular, the invention provides gene promoter suppressing nucleic acids which suppress transcription from a promoter of interest. In a preferred embodiment, the invention provides the TSU gene, nucleotide sequences of the TSU gene and RNA, as well as fragments, homologs and derivatives thereof. Methods of isolating TSU genes are also provided. Therapeutic and diagnostic methods and pharmaceutical compositions are also provided. In particular, the invention relates to methods for cell replacement therapy, gene therapy or organ transplantation wherein TSU nucleic acids suppress MHC class I and II gene expression, thus preventing immuno-rejection of non-autologous cells or organs. The invention also provides methods for treatment of diseases or disorders by suppression of MHC class I, MHC class II, ICAM-1, B7-1, B7-2, and / or Fc gamma R expression by provision of TSU function.
Owner:YALE UNIV
Methods of elicit, enhance and sustain immune responses against MHC class I-restricted epitopes, for prophylactic or therapeutic purposes
InactiveUS20050079152A1Improve responseEfficient amplificationAntibacterial agentsBiocideEpitopeMHC class I
Embodiments relate to methods and compositions for eliciting, enhancing, and sustaining immune responses, preferably against MHC class I-restricted epitopes. The methods and compositions can be used for prophylactic or therapeutic purposes.
Owner:MANNKIND CORP
Vaccine delivery compositions and methods of use
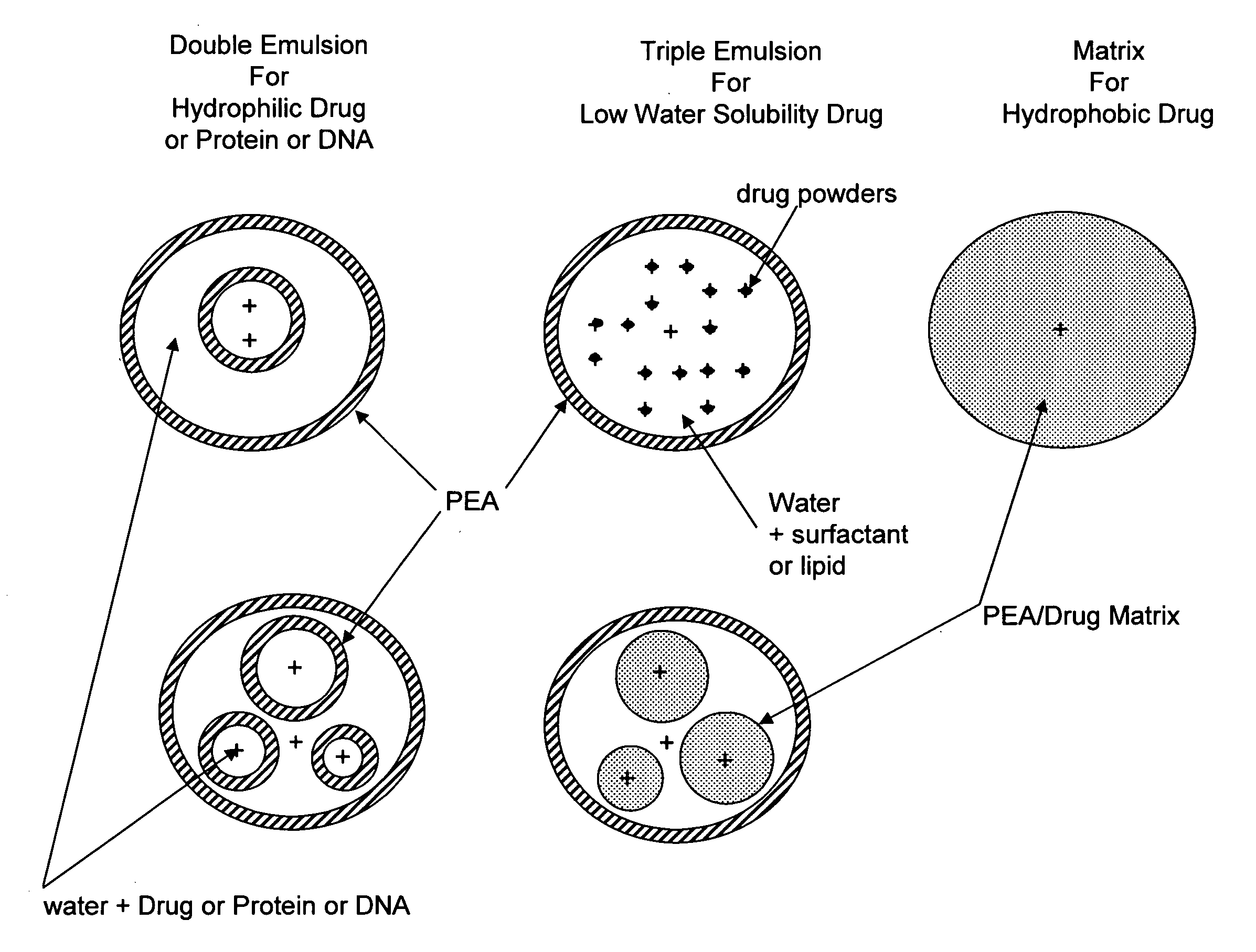
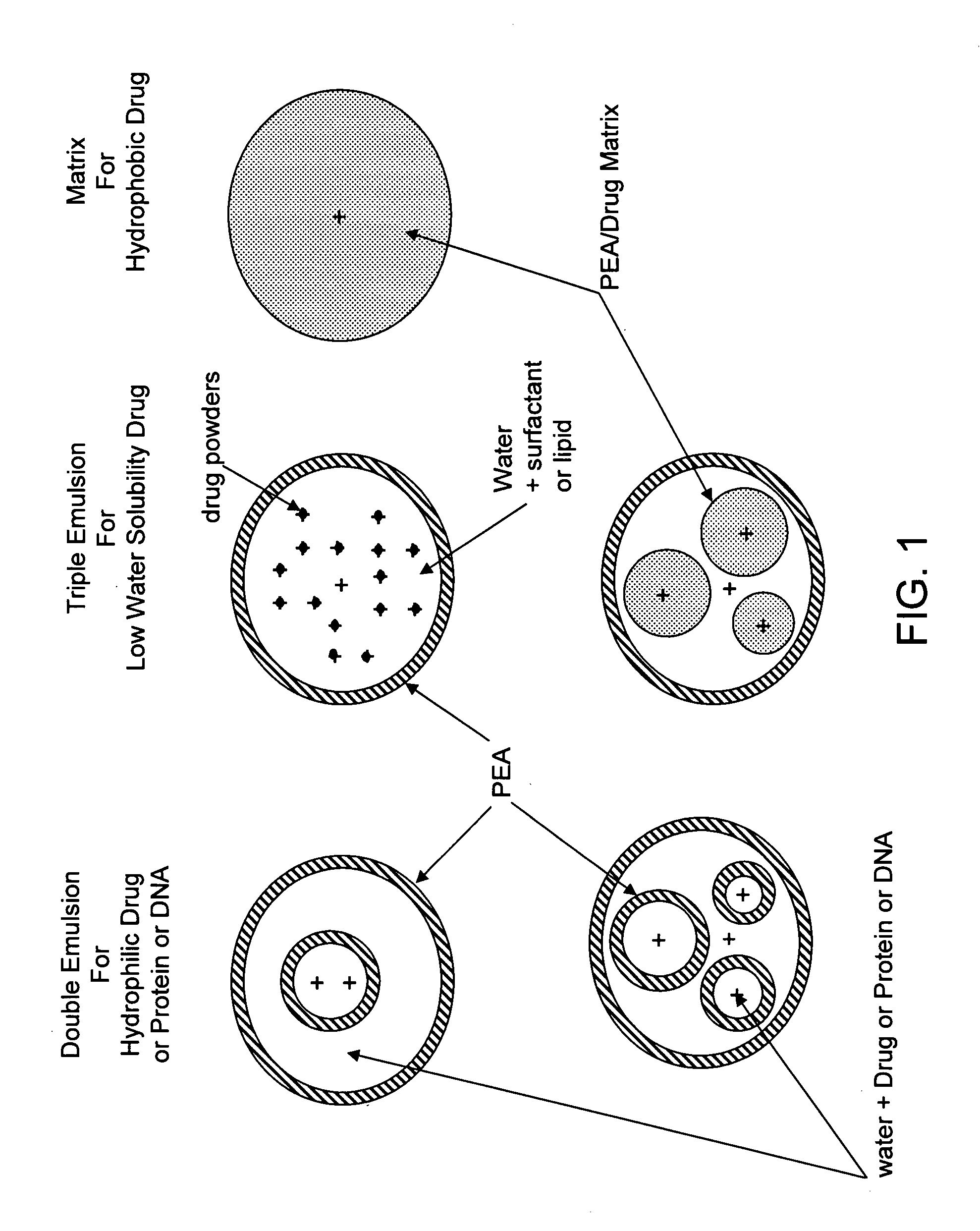
InactiveUS20080160089A1Easy to produceImprove efficiencySsRNA viruses negative-sensePowder deliveryPolyesterMHC class I
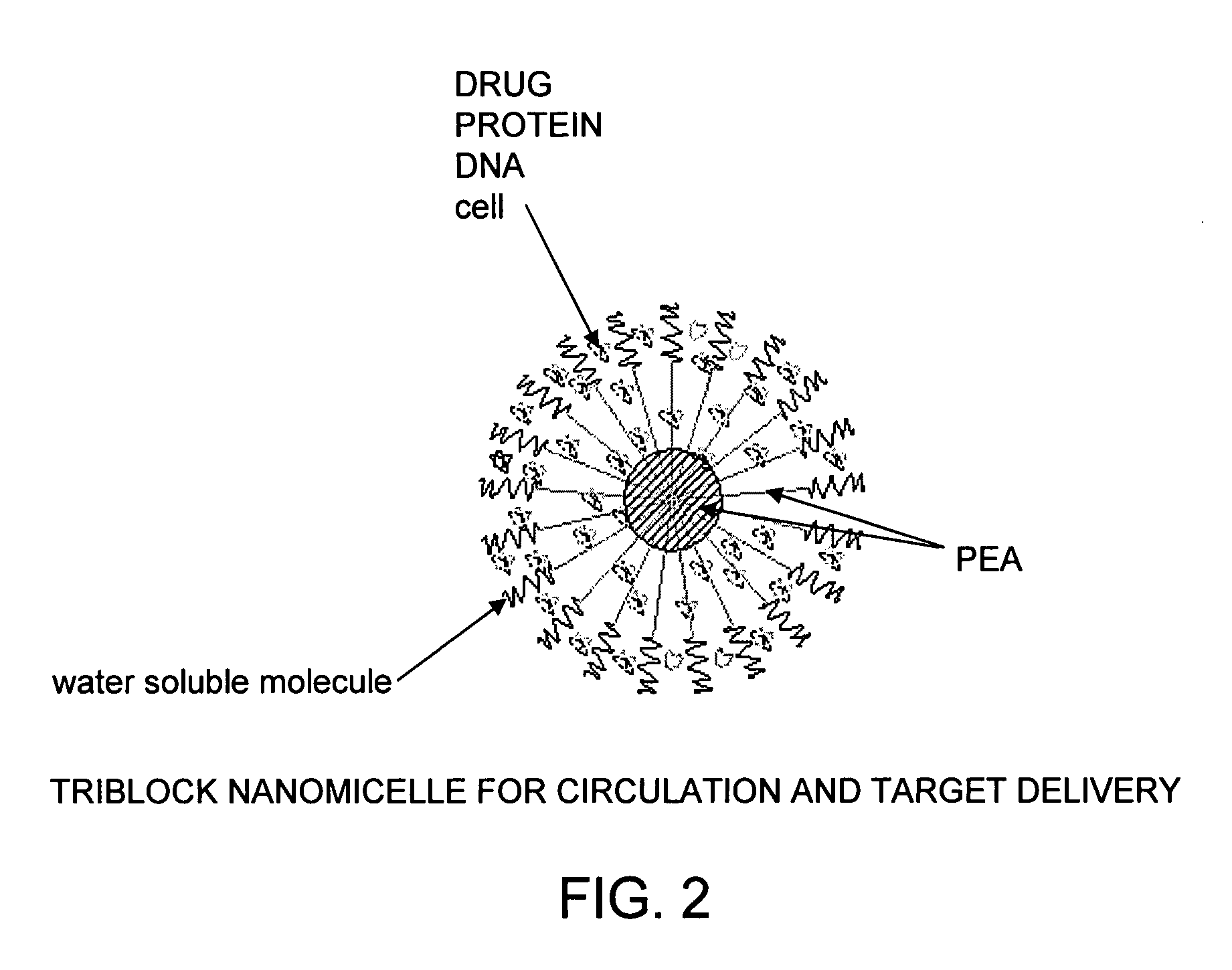
The present invention provides synthetic vaccines against a variety of pathogenic organisms and tumor cells in humans and other mammals based on biodegradable polymers containing polyester amide (PEA), polyester urethane (PEUR), and polyester urea (PEU) and immunostimulatory adjuvants. The vaccines can be formulated as a liquid dispersion of polymer particles or molecules in which are dispersed an immunostimulatory adjuvant, such as a TLR agonist, and whole protein or peptidic antigens containing MHC class I or class II epitopes derived from organism or tumor cell proteins. Methods of inducing an immune response via intracellular mechanisms to the pathogenic organism or tumor cells specific for the antigen in the invention compositions are also included.
Owner:MEDIVAS LLC
Peptide composition
InactiveUS8343500B2Simplify the development processReduce capacityNervous disorderPeptide/protein ingredientsDiseaseMHC class I
Owner:APITOPE TECH BRISTOL
Purification of antigen-specific T cells
InactiveUS20060134125A1Snake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMHC class IAbnormal tissue growth
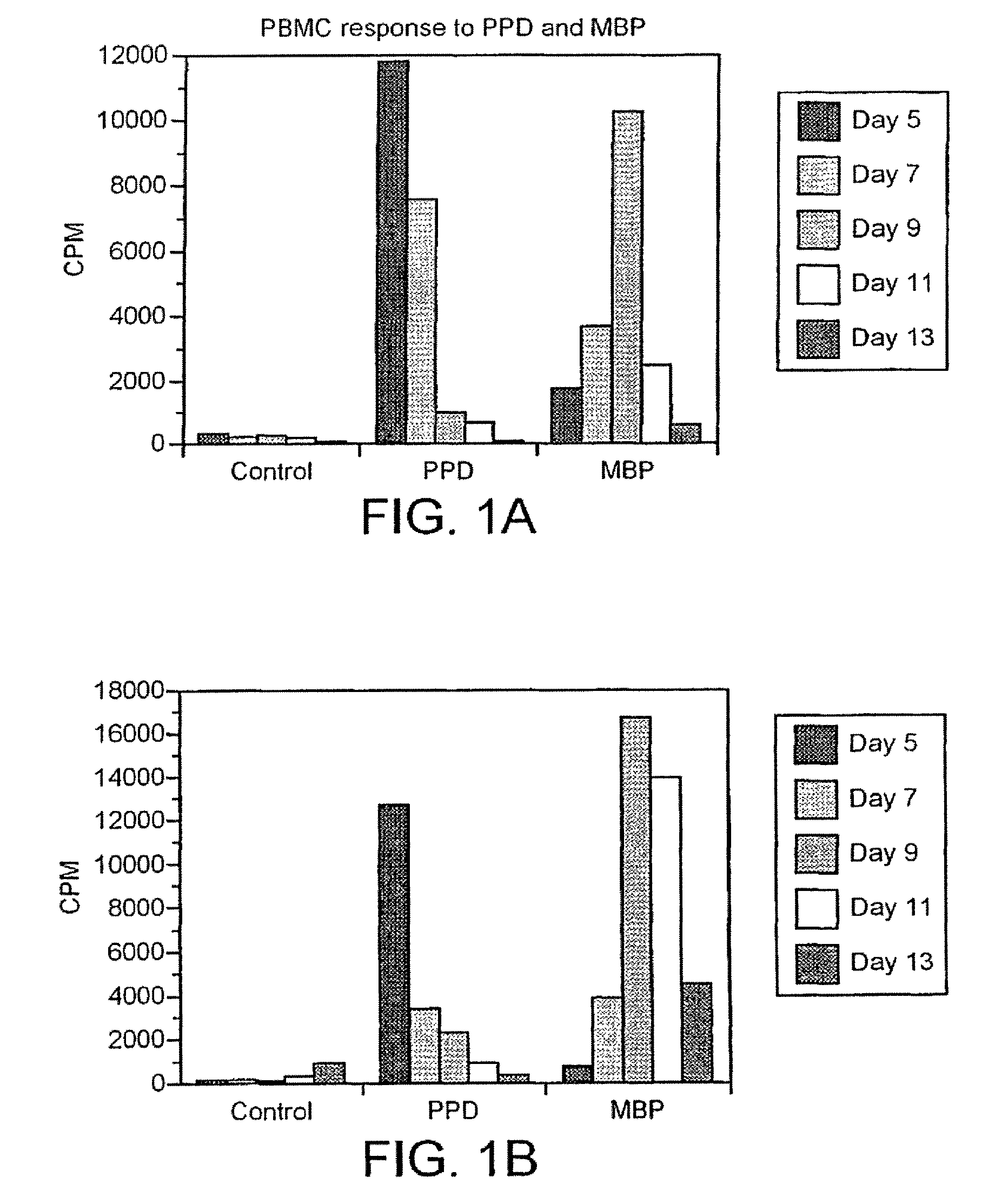
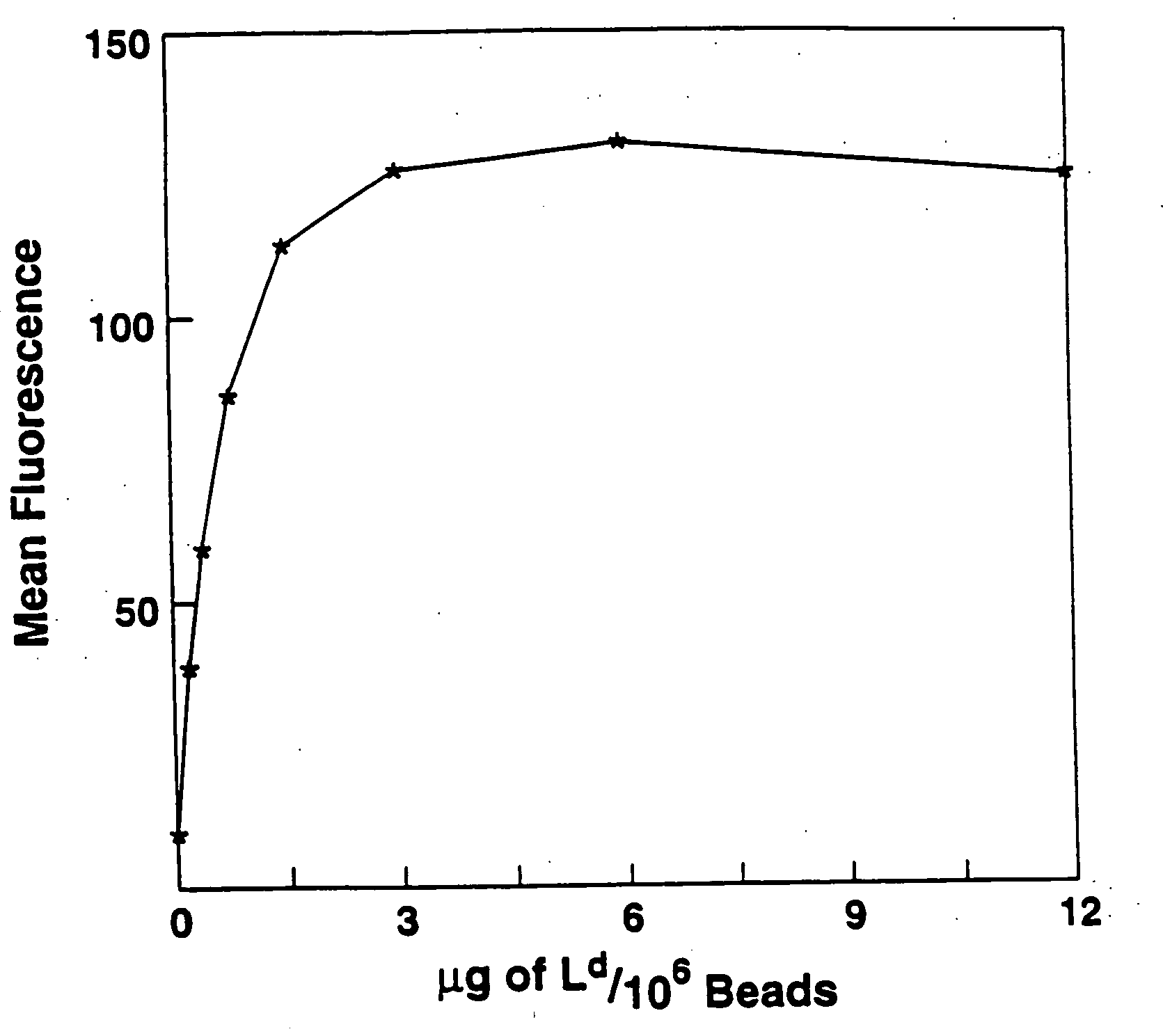
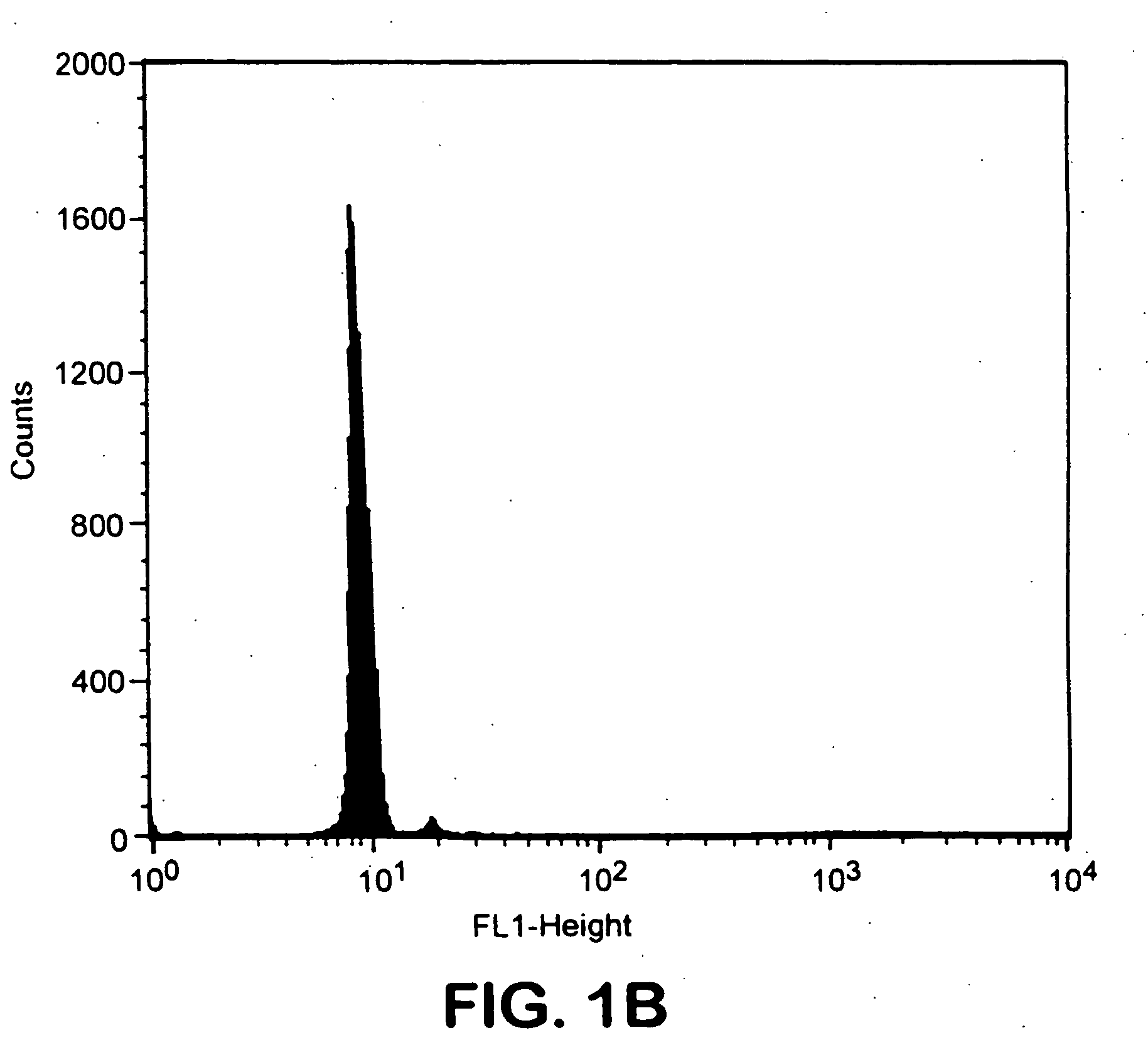
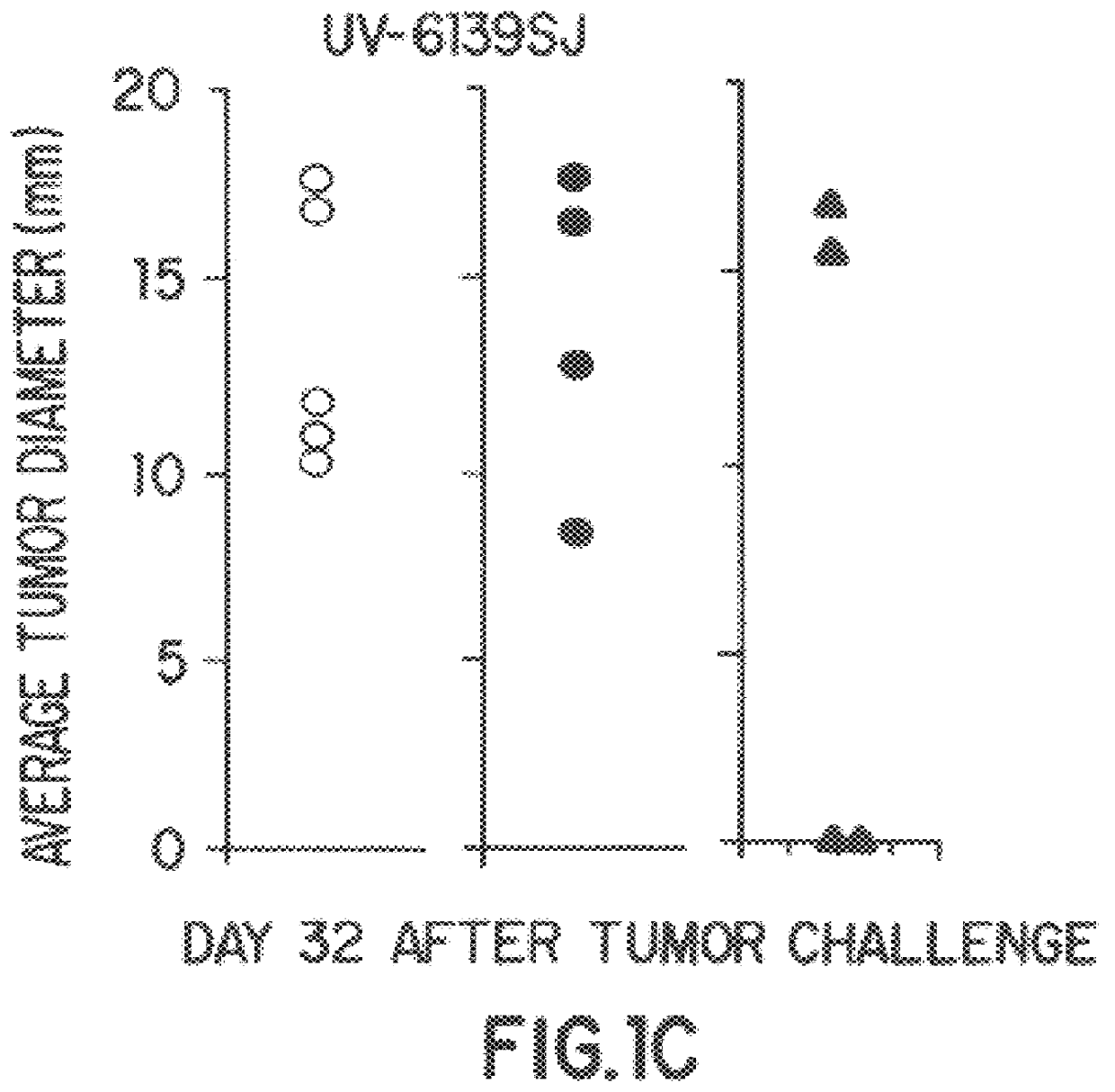
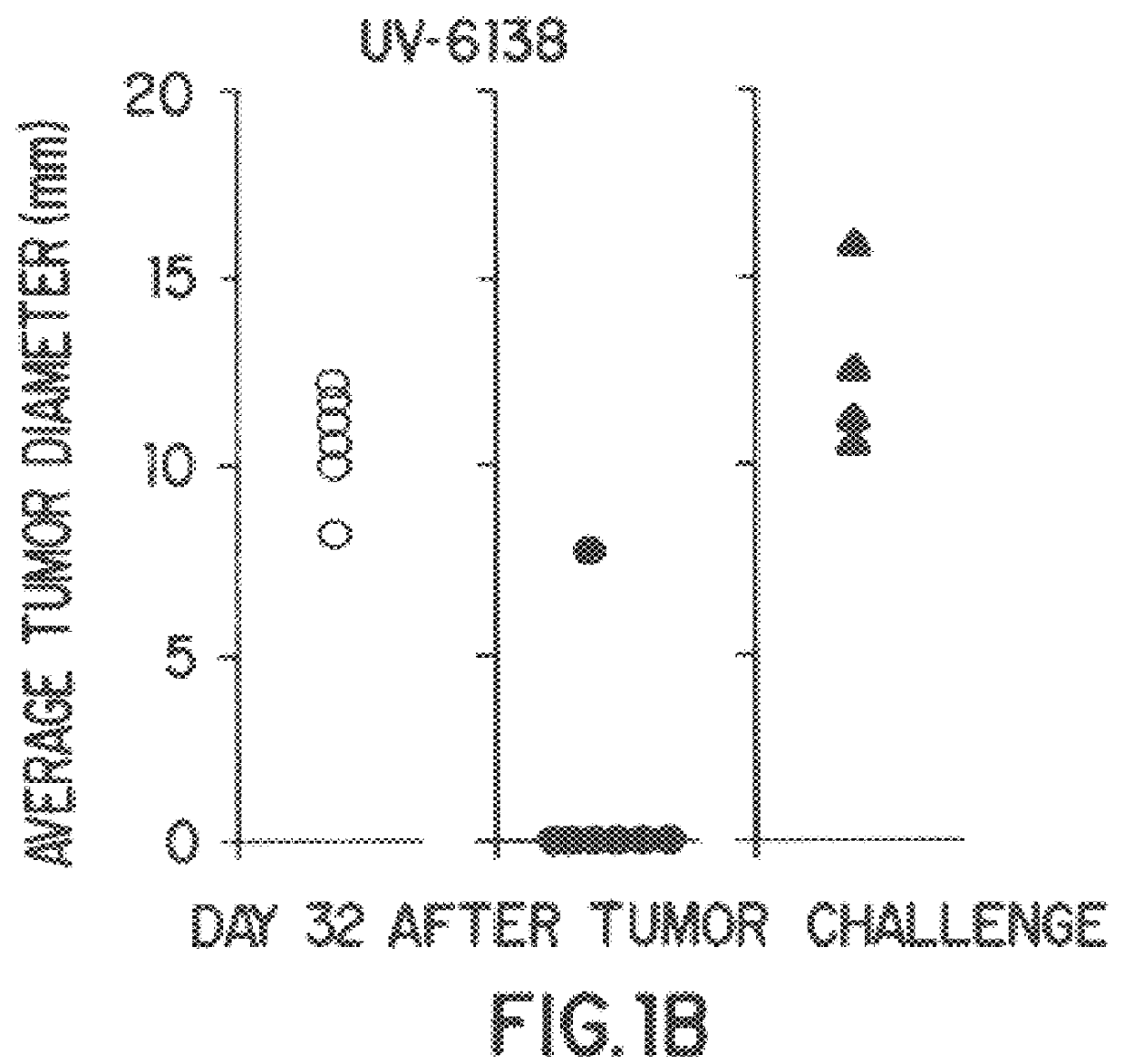
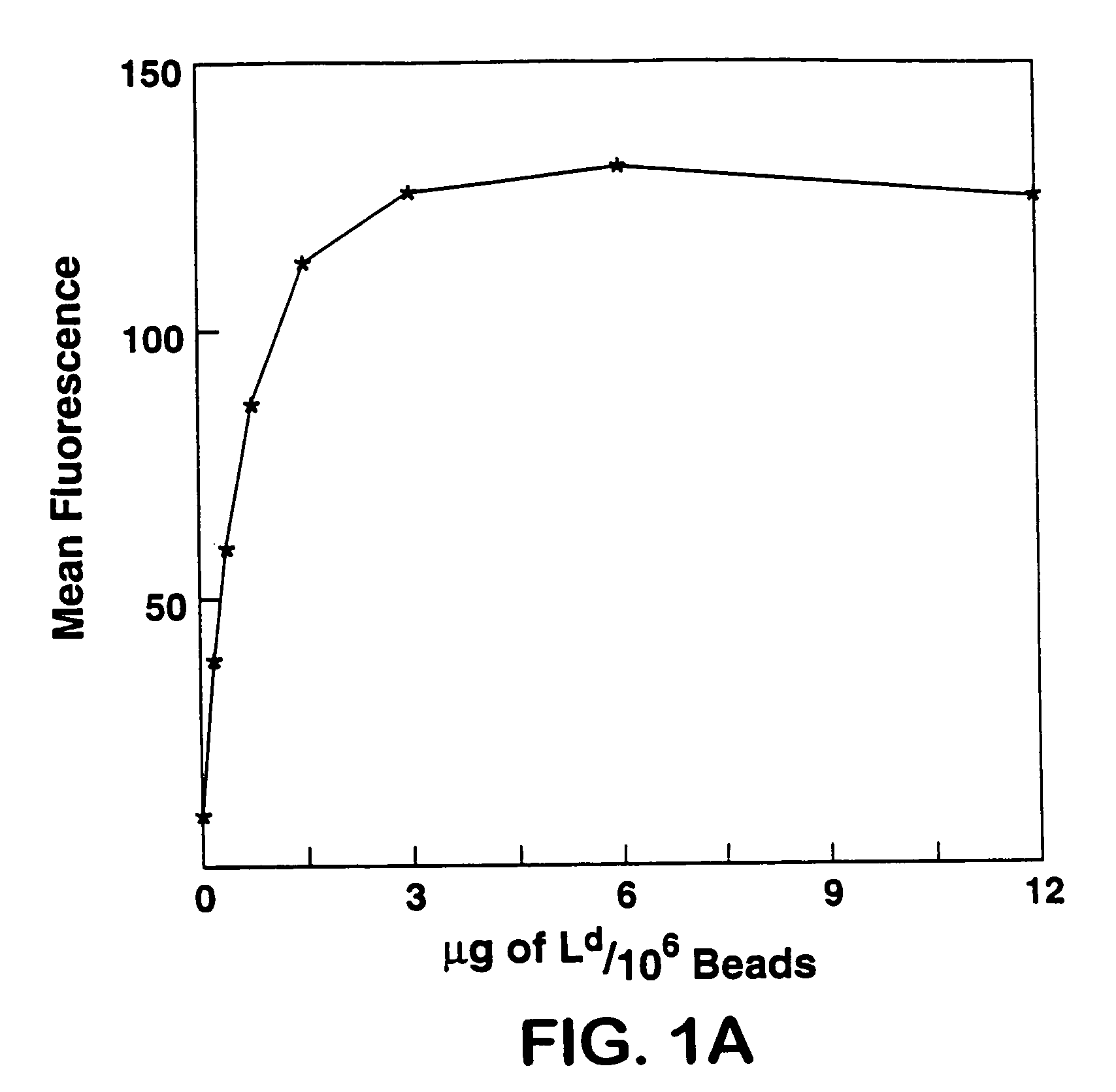
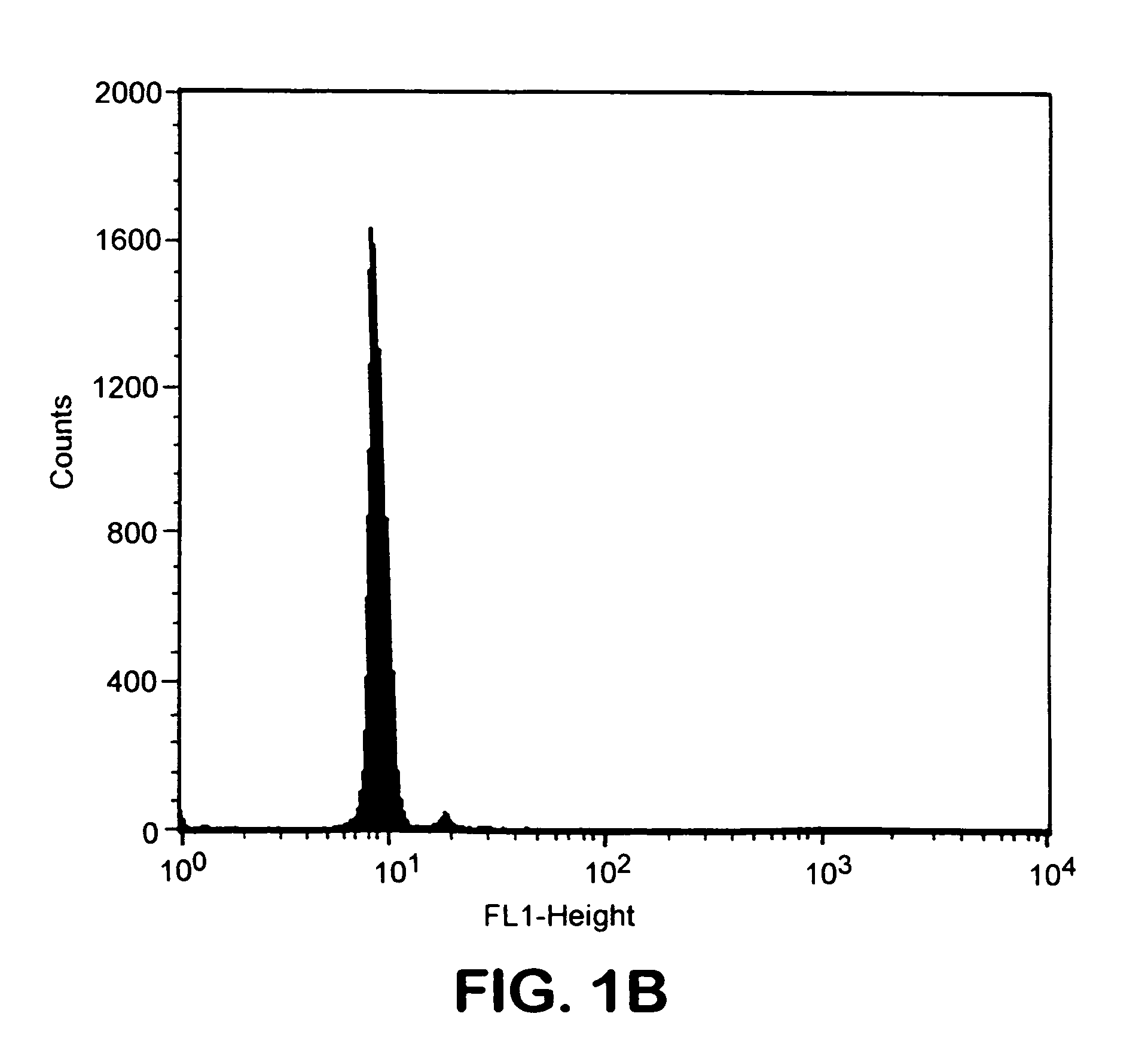
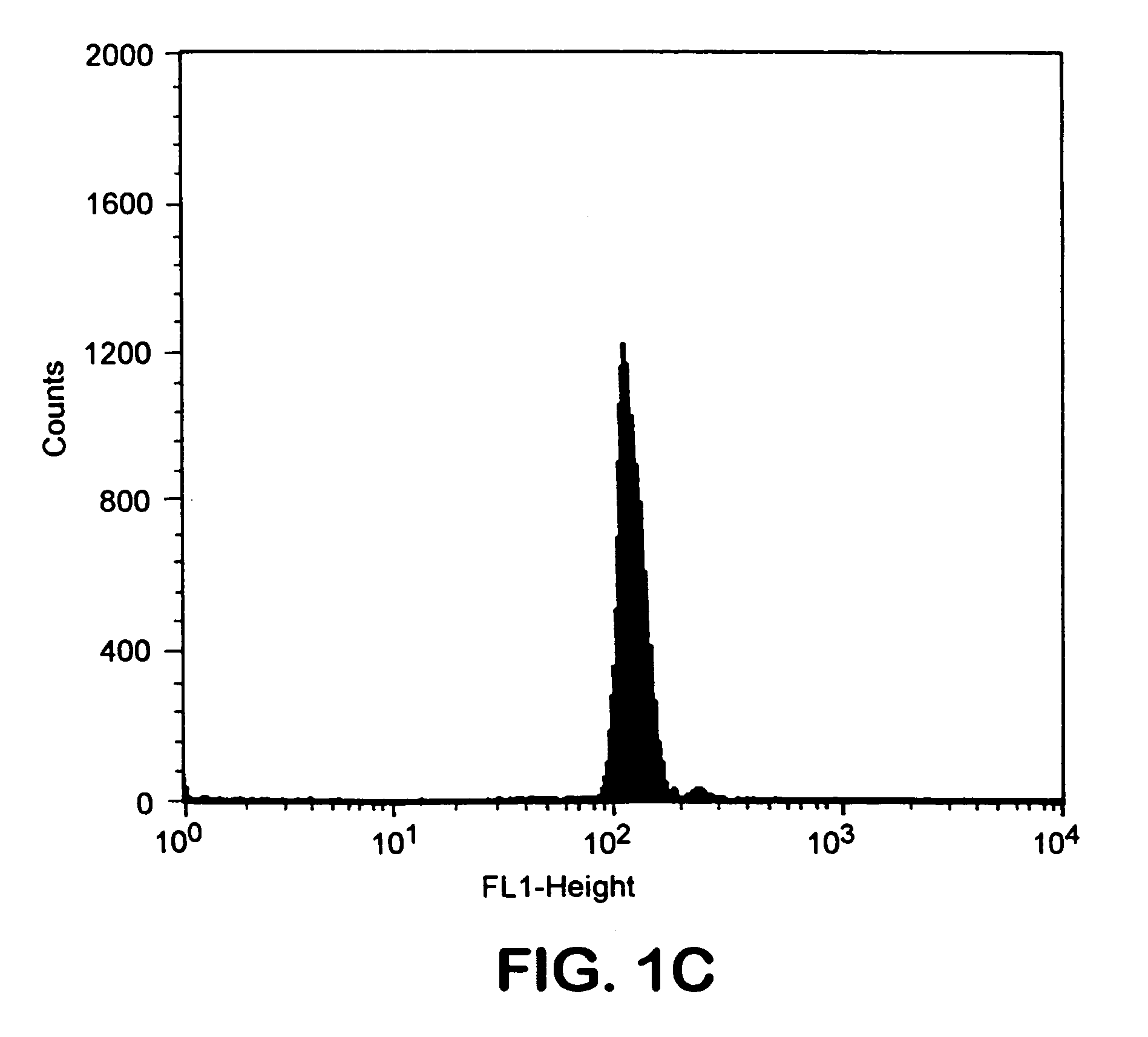
A new method to capture, purify and expand antigen-specific T lymphocytes has been developed using magnetic beads coated with recombinant MHC class I molecules. This method was optimized using homogenous populations of naive T cells purified from mice transgenic for the 2C T cell receptor (TCR). These T cells were captured on beads coated with MHC class I molecules and the relevant antigenic peptides. MHC and peptide specificity was confirmed by the usage of irrelevant MHC peptide combinations. An enrichment of 800 to 1600 fold was measured, using 2C T cells mixed with irrelevant T cells, starting from a 2C T cell frequency of 1 / 3000. The same approach was used to purify antigen-specific CD8+ T cells from total CD8+ T cells from naive mice. The recovered cells could be expanded and specifically kill target cells in vitro; they had a significant effect in vivo as well. We expect this procedure to be suitable to purify and expand in vitro tumor- and virus-specific killer T cells for use in cell therapy.
Owner:ORTHO PHARMA
Compositions and methods using complexes of heat shock protein 90 and antigenic molecules for the treatment and prevention of neoplastic diseases
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. The effective amounts of the complex are in the range of 10-600 micrograms for complexes comprising hsp7o, 50-1000 micrograms for hsp9o, and 10-600 micrograms for gp96. The invention also provides a method for measuring tumor rejection in viva in an individual, preferably a human, comprising measuring the generation by the individual of MHC Class I-restricted CD8+ cytotoxic T lymphocytes specific to the tumor. Methods of purifying hsp7o-peptide complexes are also provided.
Owner:FORDHAM UNIVERSITY
Tolerogenic synthetic nanocarriers for antigen-specific deletion of t effector cells
Disclosed are synthetic nanocarrier methods, and related compositions, comprising administering immunosuppressants and MHC Class I-restricted and / or MHC Class II-restricted epitopes that can generate tolerogenic immune responses (e.g., antigen-specific T effector cell deletion).
Owner:SELECTA BIOSCI
Fusion proteins, uses thereof and processes for producing same
InactiveUS20080014208A1Efficient expressionAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsMHC class IAmino acid
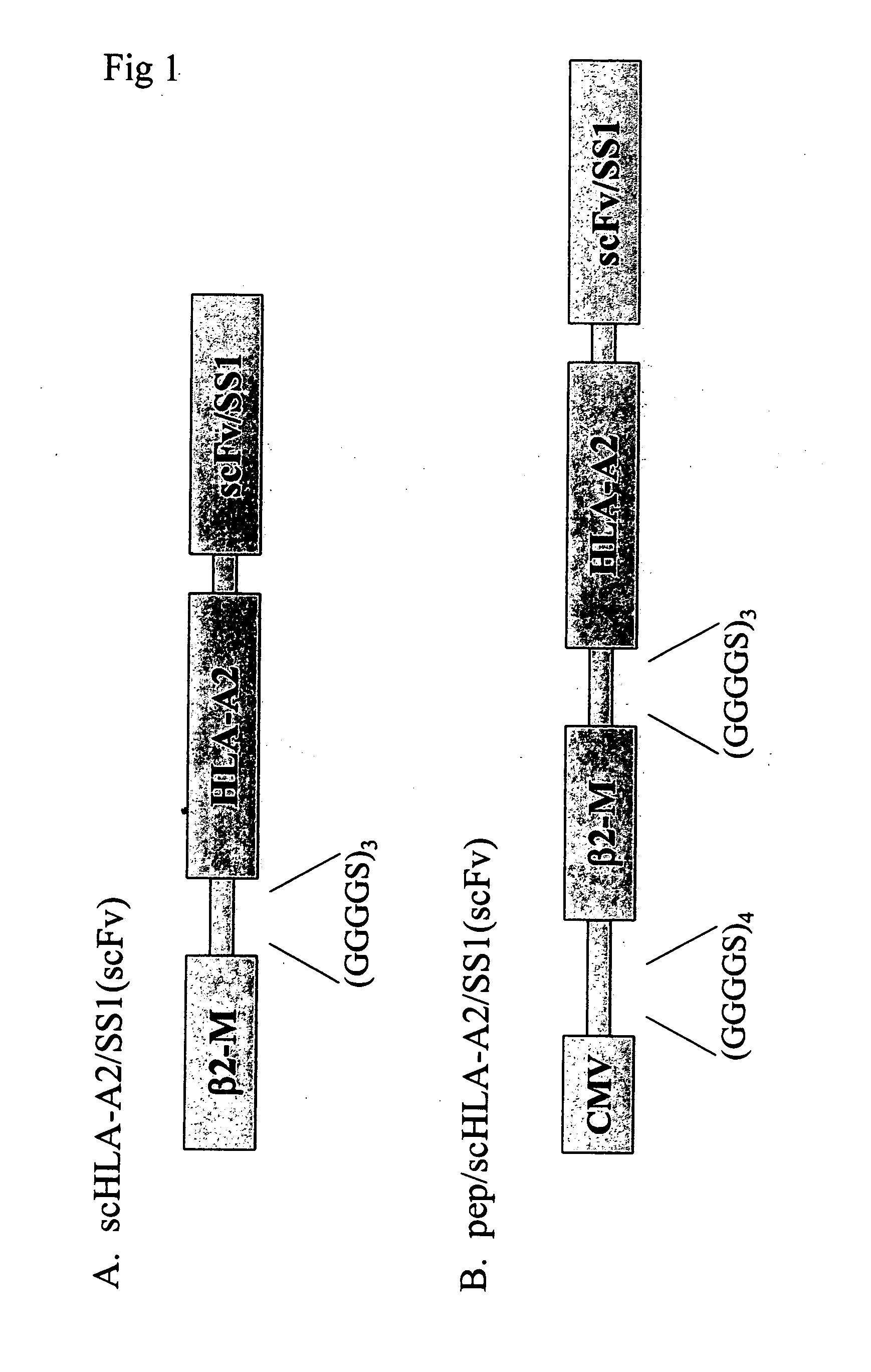
This invention provides fusion proteins comprising consecutive amino acids which beginning at the amino terminus of the protein correspond to consecutive amino acids present in (i) a cytomegalovirus human MHC-restricted peptide, (ii) a first peptide linker, (iii) a human β-2 microglobulin, (iv) a second peptide linker, (v) a HLA-A2 chain of a human MHC class I molecule, (vi) a third peptide linker, (vii) a variable region from a heavy chain of a scFv fragment of an antibody, and (viii) a variable region from a light chain of such scFv fragment, wherein the consecutive amino acids which correspond to (vii) and (viii) are bound together directly by a peptide bond or by consecutive amino acids which correspond to a fourth peptide linker, wherein the antibody from which the scFv fragment is derived specifically binds to mesothelin. This invention provides nucleic acid constructs encoding same, processes for producing same, compositions, and uses thereof.
Owner:TECHNION RES & DEV FOUND LTD
Methods to elicit, enhance and sustain immune responses against MHC class I-restricted epitopes, for prophylactic or therapeutic purposes
Embodiments relate to methods and compositions for eliciting, enhancing, and sustaining immune responses, preferably multivalent responses, preferably against MHC class I-restricted epitopes. The methods and compositions can be used for prophylactic or therapeutic purposes.
Owner:MANNKIND CORP
Engineered antibody-stress protein fusions
ActiveUS7749501B2Inexpensively fused to a stress proteinHigh affinityAntipyreticAntibody mimetics/scaffoldsEscherichia coliMHC class I
Provided are fusion polypeptides comprising an engineered antibody and a stress protein that bind to antigens with high affinity, are highly immunogenic, exhibit MHC class I priming and are able to be produced in non-mammalian cells, such as E. coli.
Owner:THE GENERAL HOSPITAL CORP
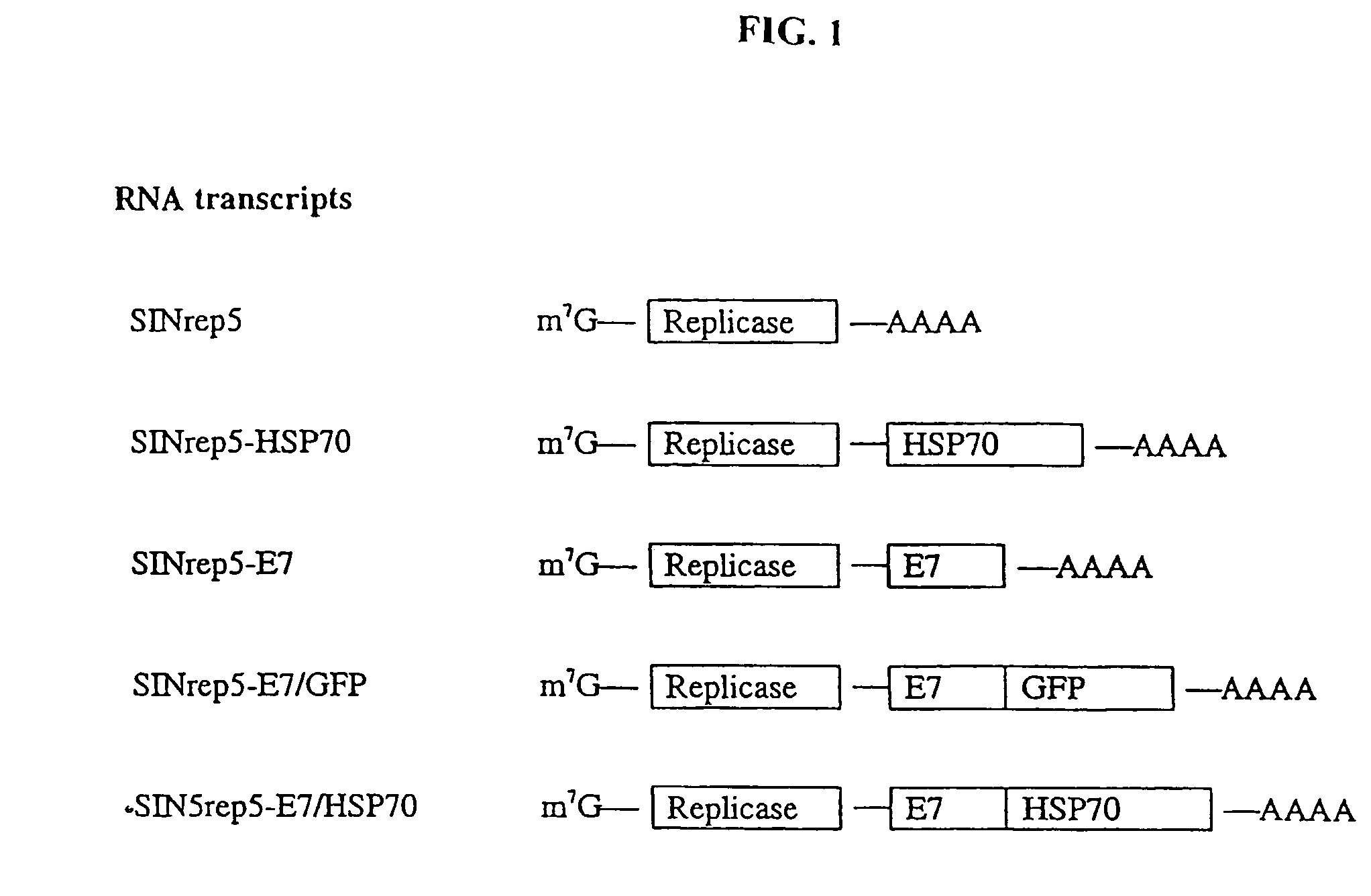
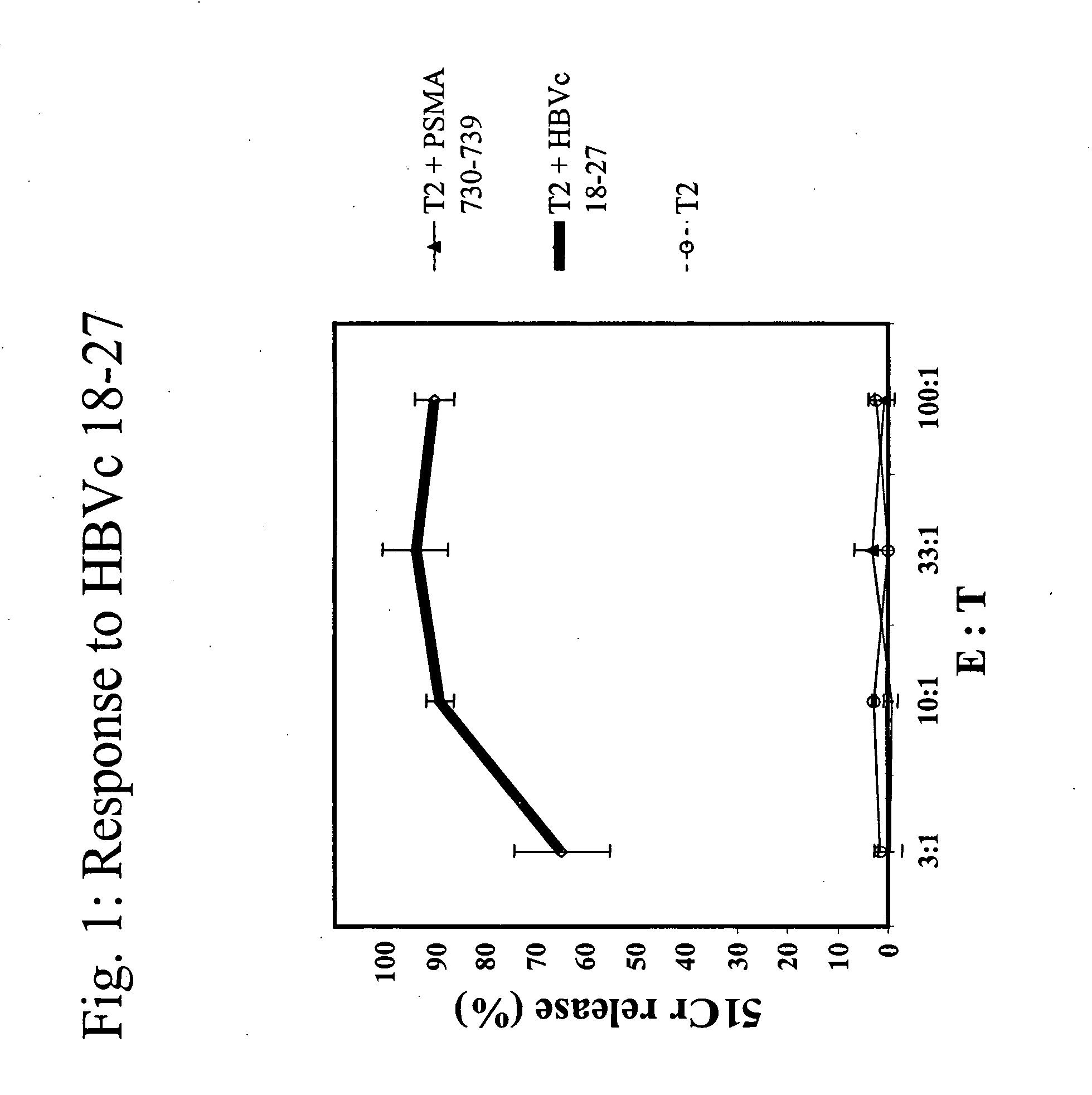
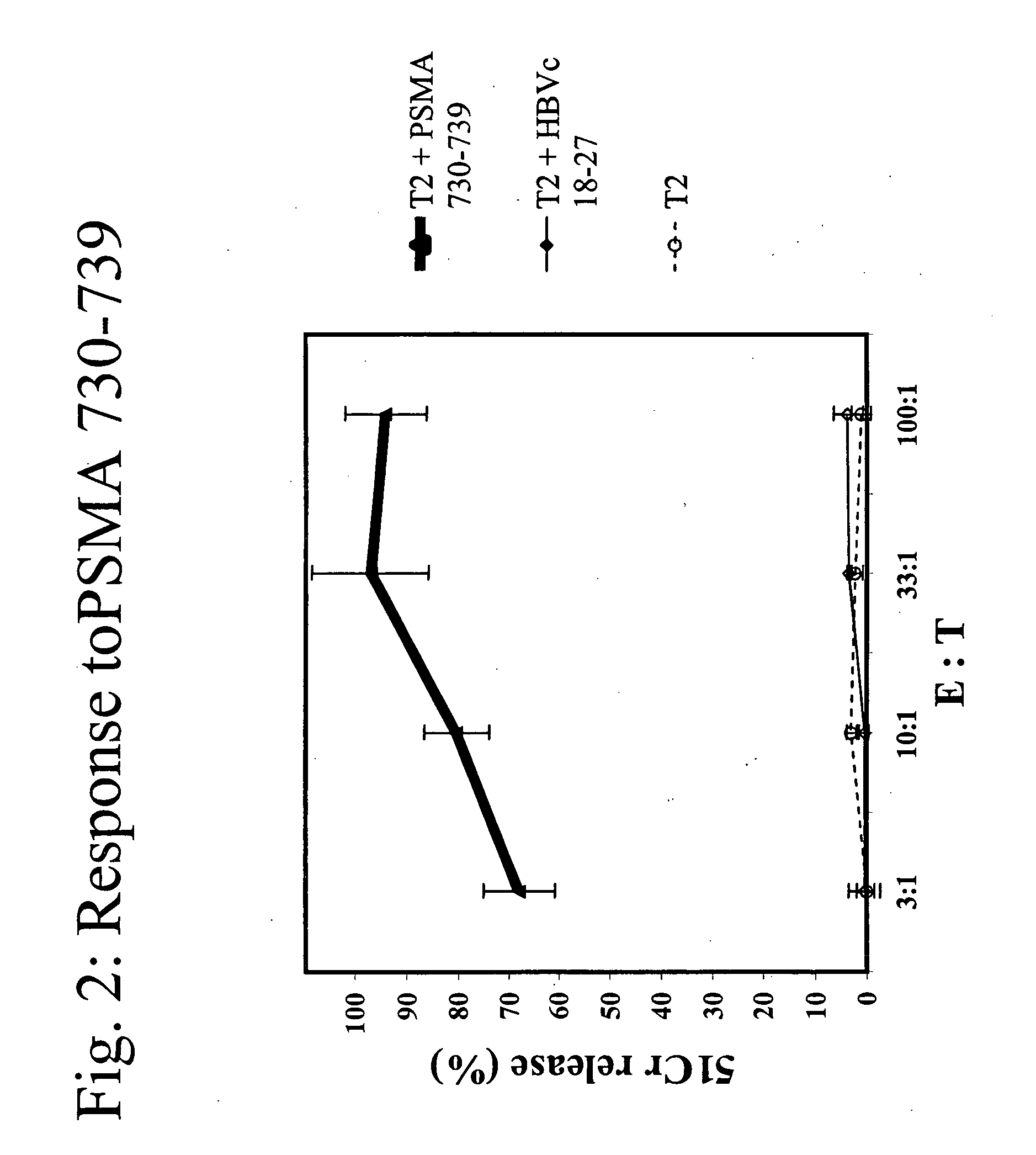
Superior molecular vaccine based on self-replicating RNA, suicidal DNA or naked DNA vector, that links antigen with polypeptide that promotes antigen presentation
InactiveUS7557200B2Efficiently presentedImprove effectivenessSsRNA viruses positive-senseAntibody mimetics/scaffoldsDiseaseMHC class I
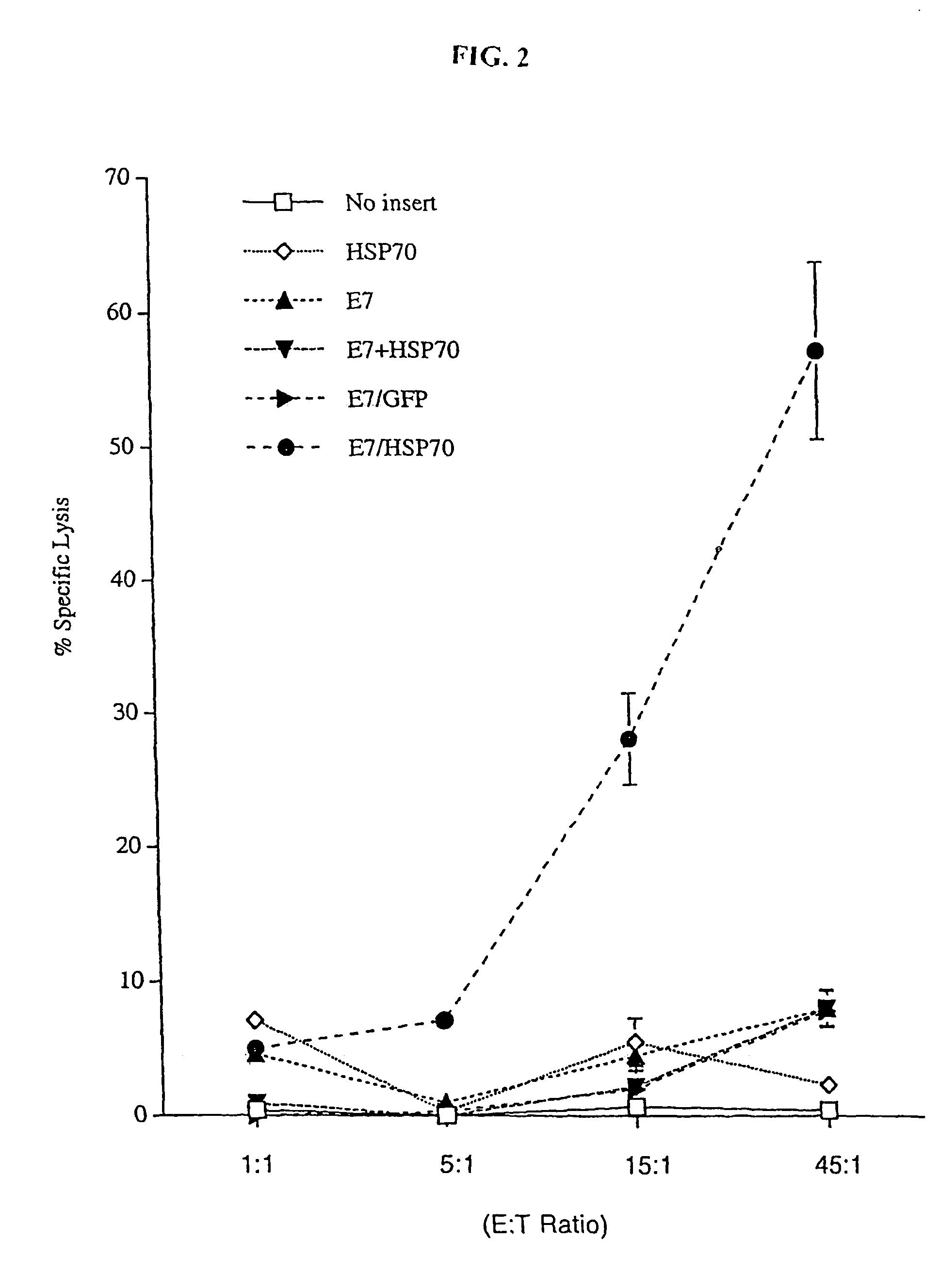
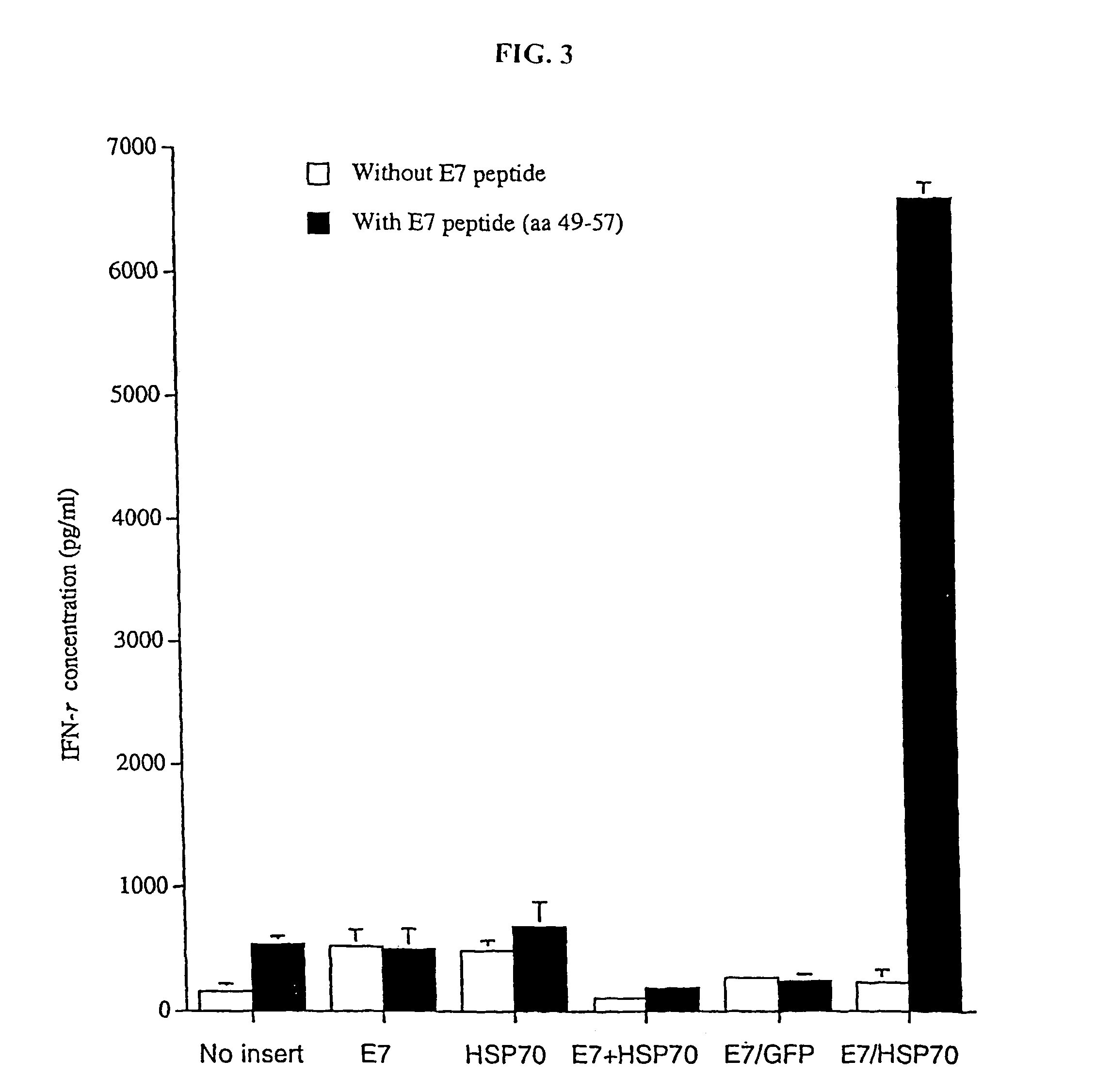
Improved molecular vaccines comprise nucleic acid vectors that encode a fusion polypeptide that includes polypeptide or peptide physically linked to an antigen. The linked polypeptide is one that (a) promotes processing of the expressed fusion polypeptide via the MHC class I pathway and / or (b) promotes development or activity of antigen presenting cells, primarily dendritic cells. These vaccines employ one of several types of nucleic acid vectors, each with its own relative advantages: naked DNA plasmids, self-replicating RNA replicons and suicidal DNA-based on viral RNA replicons. Administration of such a vaccine results in enhance immune responses, primarily those mediated by CD8+ cytotoxic T lymphocytes, directed against the immunizing antigen part of the fusion polypeptide. Such vaccines are useful against tumor antigens, viral antigens and antigens of other pathogenic microorganisms and can be used in the prevention or treatment of diseases that include cancer and infections.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Beta-2 microglobulin-deficient cells
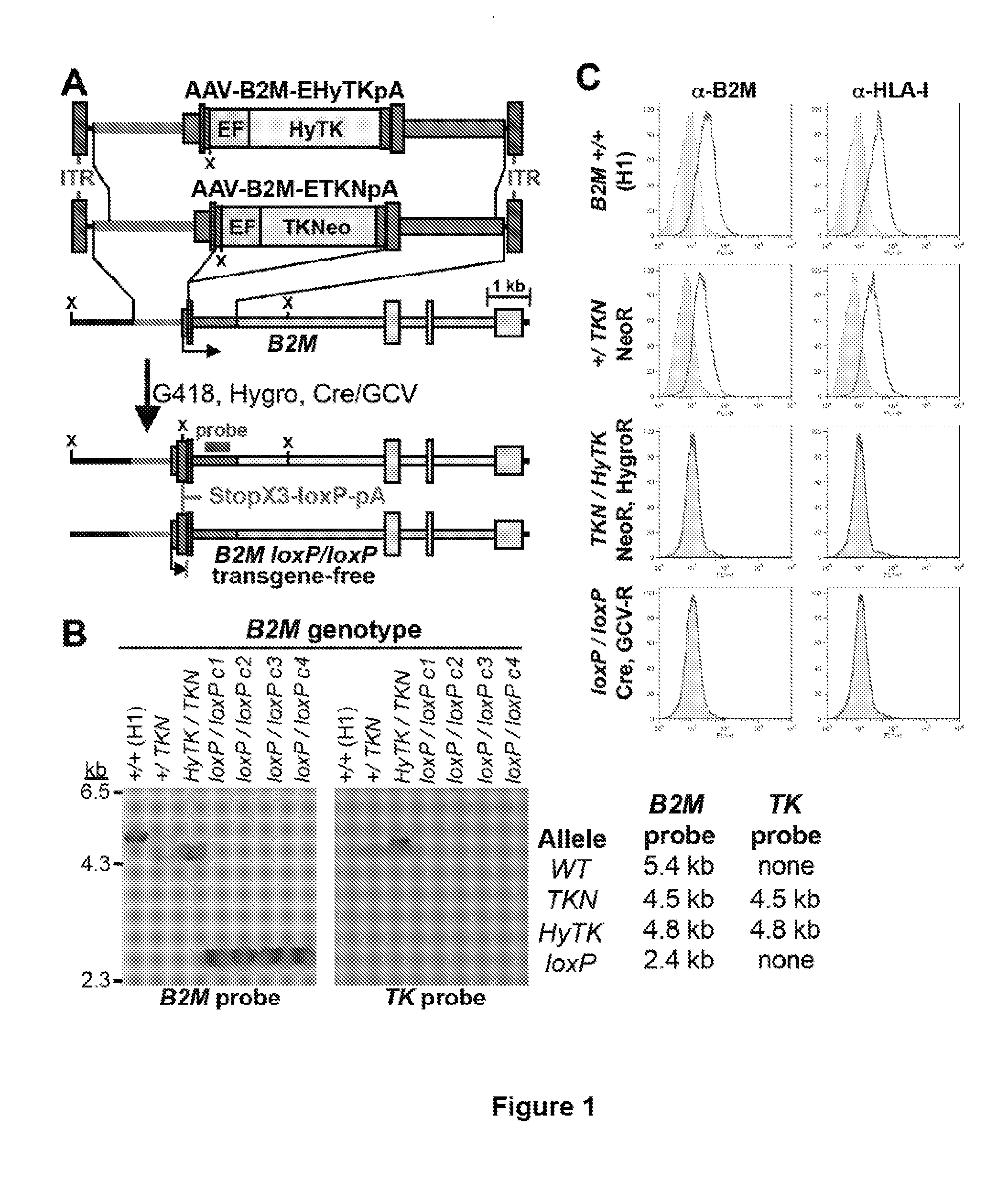
The invention provides isolated primate cells preferably human cells that comprise a genetically engineered disruption in a beta-2 microglobulin (B2M) gene, which results in deficiency in MHC class I expression and function. Also provided are the method of using the cells for transplantation and treating a disease condition.
Owner:UNIV OF WASHINGTON CENT FOR COMMERICIALIZATION
Monomeric recombinant MHC molecules useful for manipulation of antigen-specific T cells
InactiveUS20050142142A1Powerful and epitope-specific effects on T-cell activationEasy to producePeptide/protein ingredientsImmunoglobulinsMHC class IAutoimmune condition
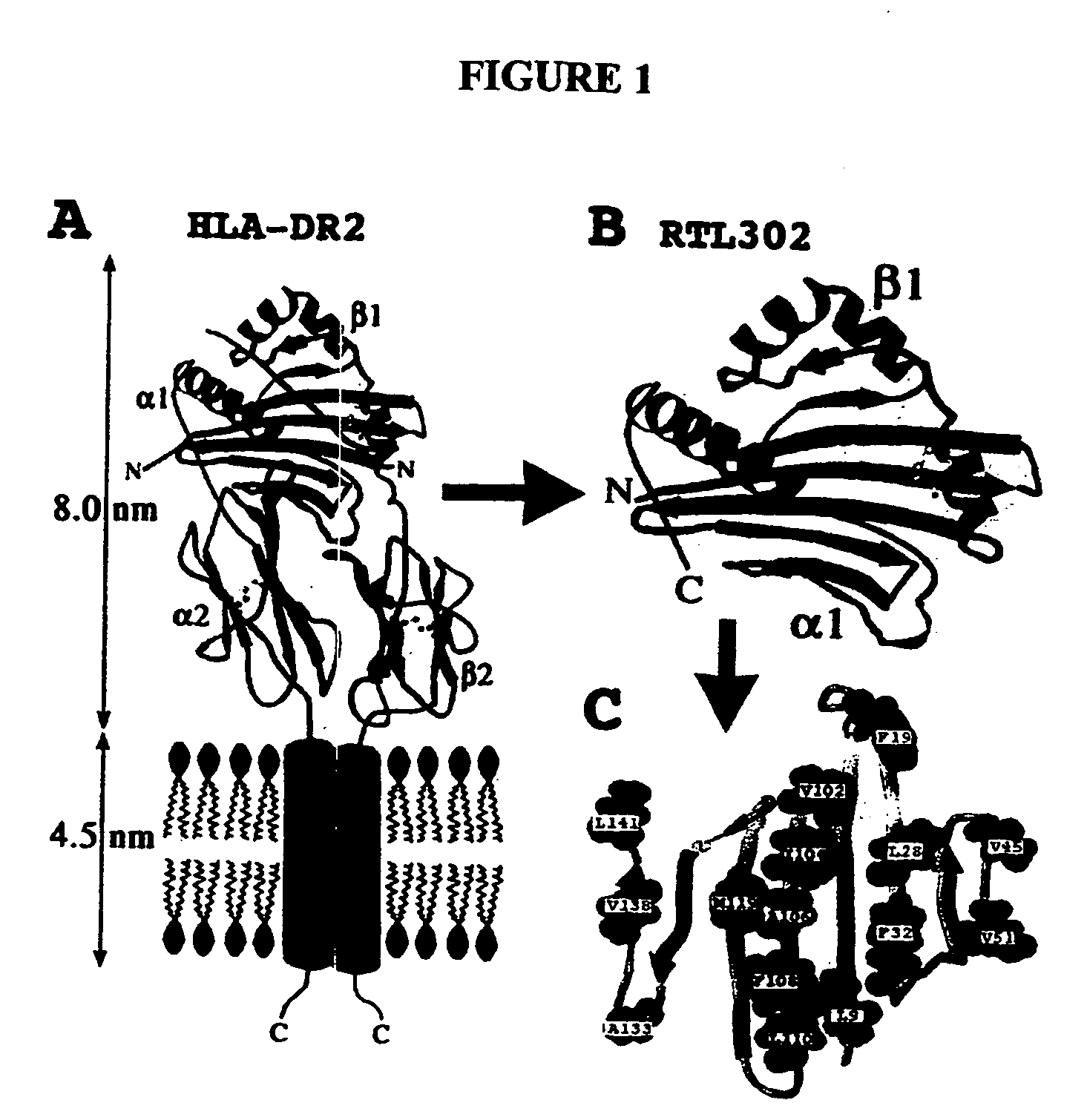
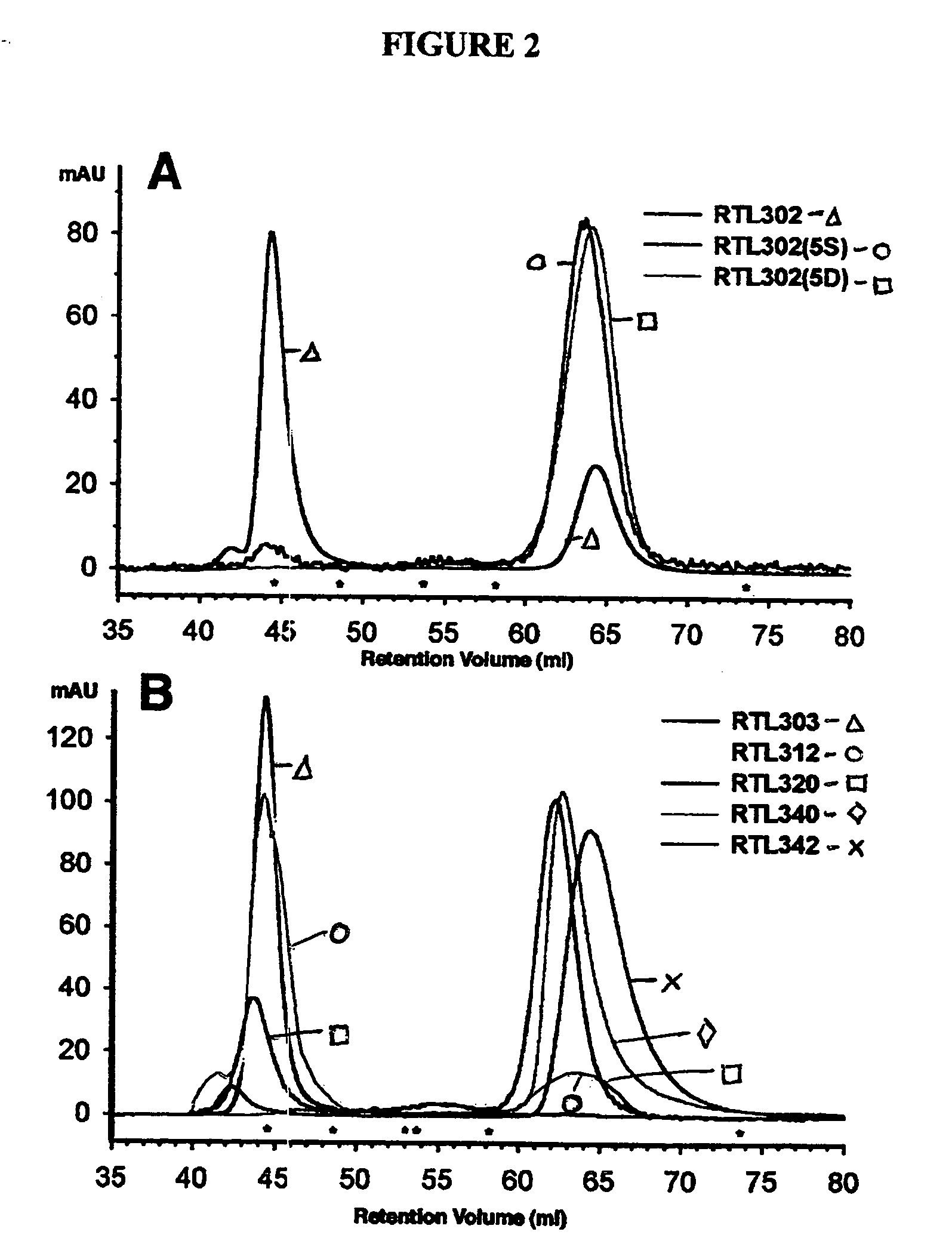
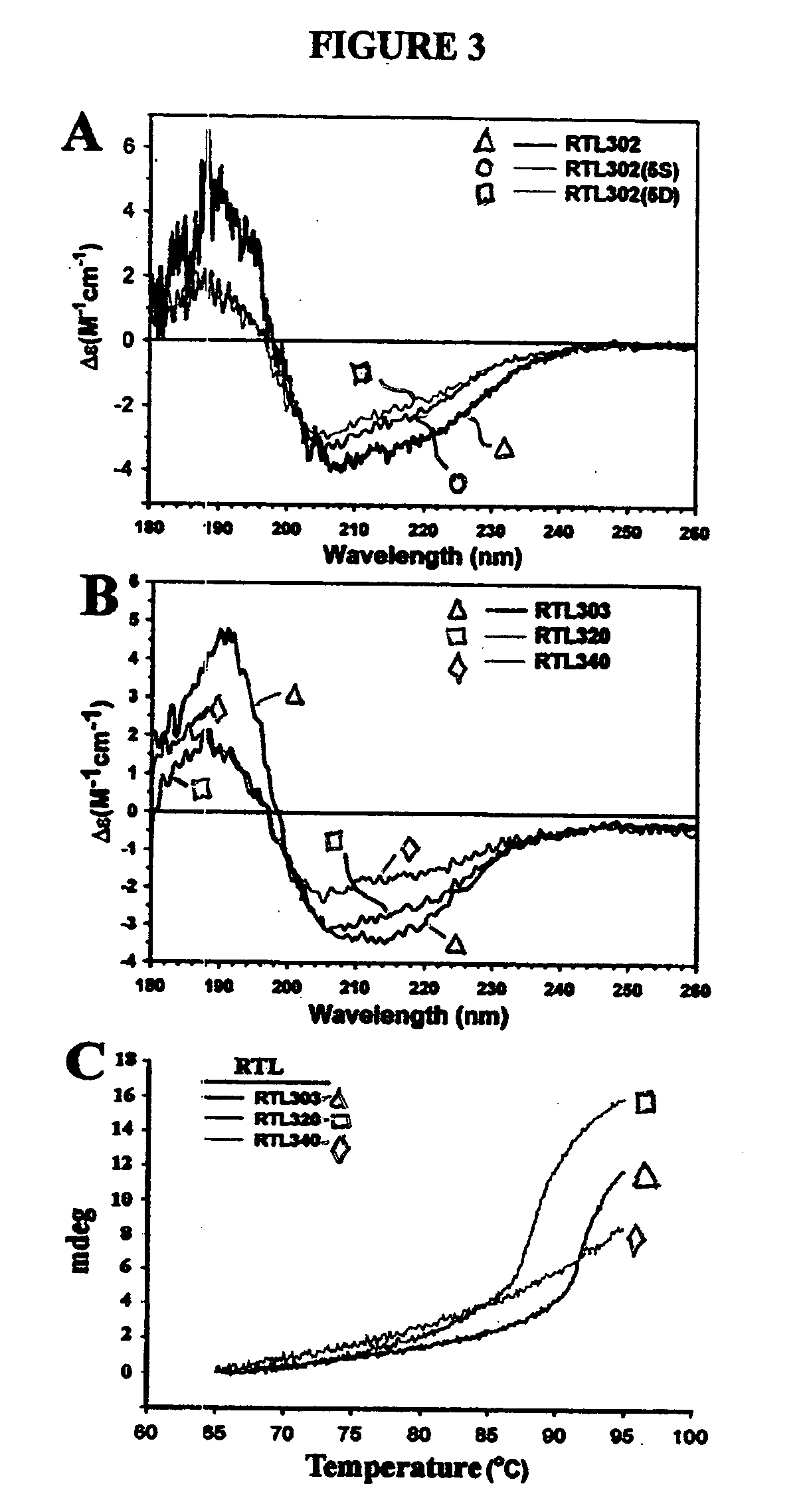
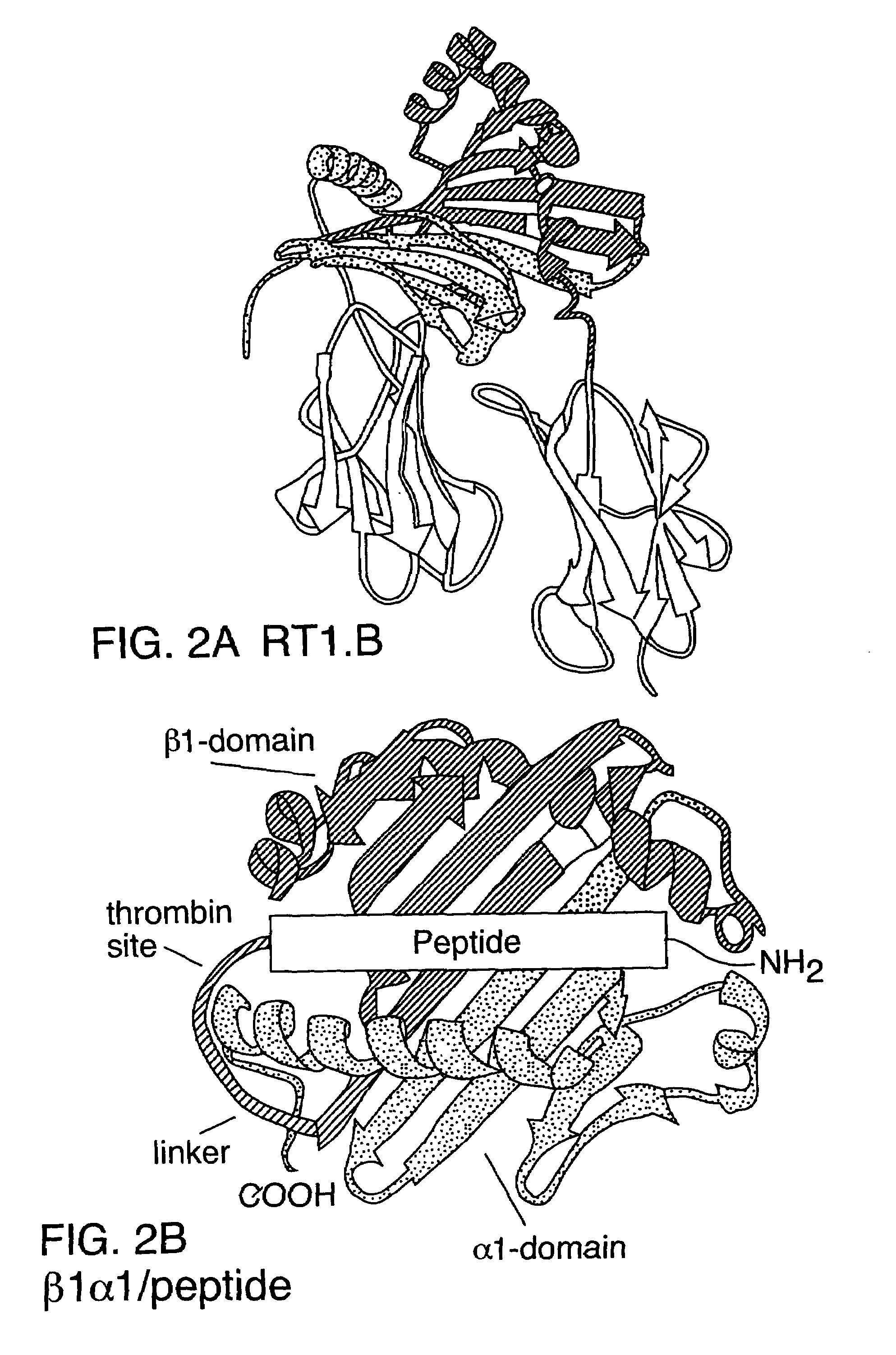
The present invention provides, in particular embodiments, for modified recombinant T cell receptor (TCR) ligands (RTLs) comprising a MHC class I or MHC class II component. The modified RTLs have redesigned surface features that preclude or reduce aggregation, wherein the modified molecules retain the ability to bind Ag-peptides, target antigen-specific T cells, inhibit T cell proliferation in an Ag-specific manner and have utility to treat, inter alia, autoimmune disease and other conditions mediated by antigen-specific T cells in vivo.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Disulfide trap MHC class I molecules and uses therefor
ActiveUS8992937B2Extended cell surface half-lifeLow efficiencyBiocideAntibody mimetics/scaffoldsAntigenMHC class I
Owner:WASHINGTON UNIV IN SAINT LOUIS
Compositions and methods related to induced tolerogenic dendritic cells externally loaded with mhc class i-restricted epitopes
Disclosed are induced tolerogenic dendritic cells (itDCs) produced by combining itDCs with externally loadable MHC Class I-restricted epitopes, as well as related compositions and methods.
Owner:SELECTA BIOSCI
Antigen specific multi epitope vaccines
ActiveUS20100074925A1Strong and comprehensive responseEffective immune responseTumor rejection antigen precursorsSugar derivativesMHC class IProtein target
The present invention relates to cancer vaccines composed of the signal peptide domain of tumor associated antigens or proteins. The peptide vaccines of the invention are characterized by having multiple MHC class I and class II epitopes which are highly abundant in the population. Therefore, these vaccines are likely to induce a strong, comprehensive immune response against the target proteins in the majority of the vaccinated population, and thereby induce an immune reaction against tumors expressing such target proteins. Specifically, the invention relates to peptide vaccines composed of the signal peptide domain of Mucin (MUC1), BAGE-1 or ARMET, and their use for the treatment of cancers which express Mucin (MUC1), BAGE-1 or ARMET.
Owner:VAXIL BIOTHERAPEUTICS
Recombinant MHC molecules useful for manipulation of antigen-specific T-cells
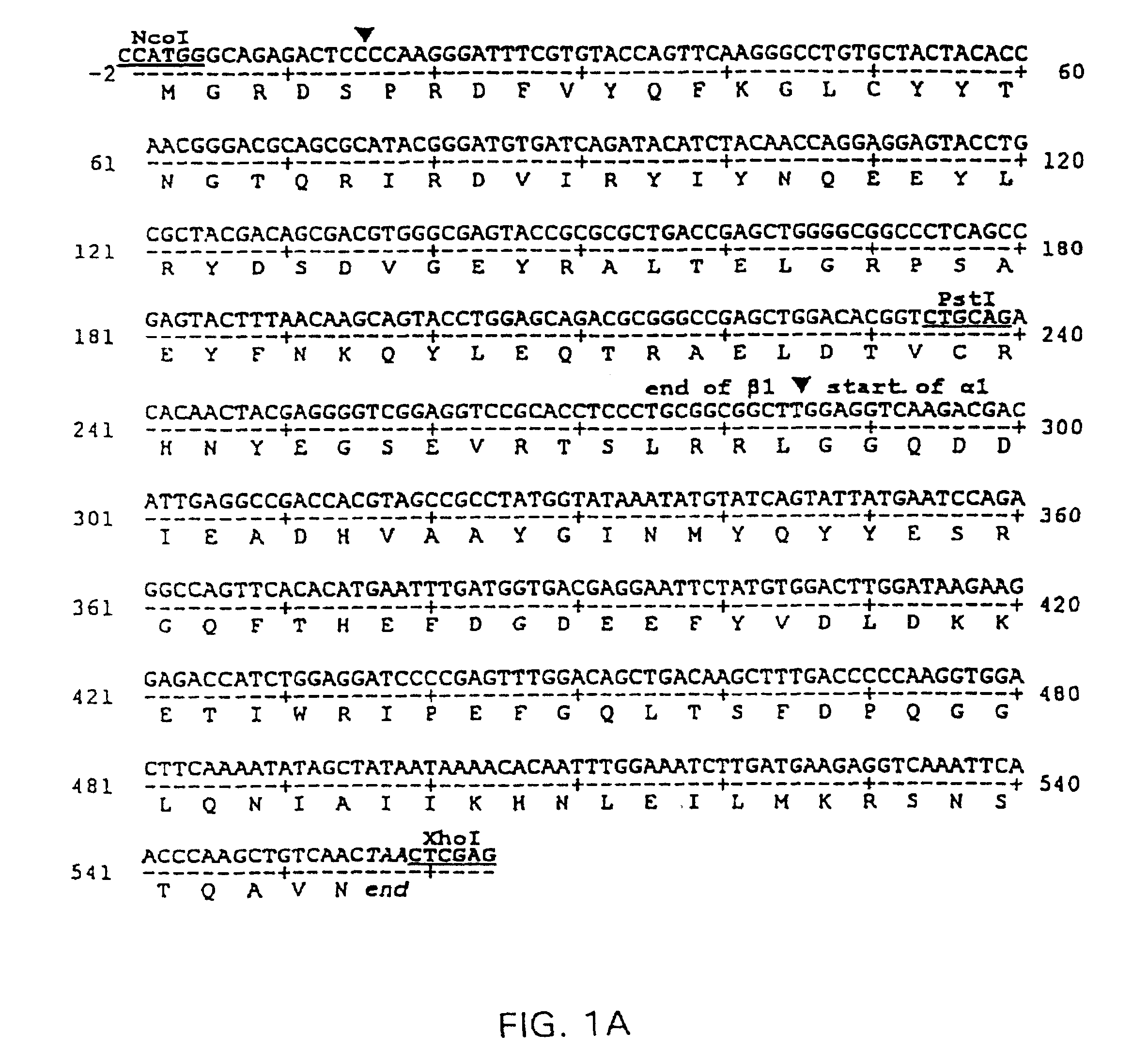
Two-domain MHC polypeptides useful for manipulation of antigen-specific T-cells are disclosed. These polypeptides include MHC class II-based molecules that comprise covalently linked β1 and α1 domains, and MHC class I-based molecules that comprise covalently linked α1 and α2 domains. These polypeptides may also include covalently linked antigenic determinants, toxic moieties, and / or detectable labels. The disclosed polypeptides can be used to target antigen-specific T-cells, and are useful, among other things, to detect and purify antigen-specific T-cells, to induce or activate T-cells, and to treat conditions mediated by antigen-specific T-cells.
Owner:OREGON HEALTH & SCI UNIV
Methods to bypass CD4+
InactiveUS20080124352A1Enhance antigen-specific immune responseSustained responseViral antigen ingredientsSnake antigen ingredientsMHC class IVaccination
Embodiments of the invention disclosed herein relate to methods and compositions for bypassing the involvement of CD4+ cells when generating antibody and MHC class I-restricted immune responses, controlling the nature and magnitude of the response, and promoting effective immunologic intervention in viral pathogenesis. More specifically, embodiments relate to immunogenic compositions for vaccination particularly therapeutic vaccination, against HIV and other microbial pathogens that impact functioning of the immune system, their nature, and the order, timing, and route of administration by which they are effectively used.
Owner:MANNKIND CORP
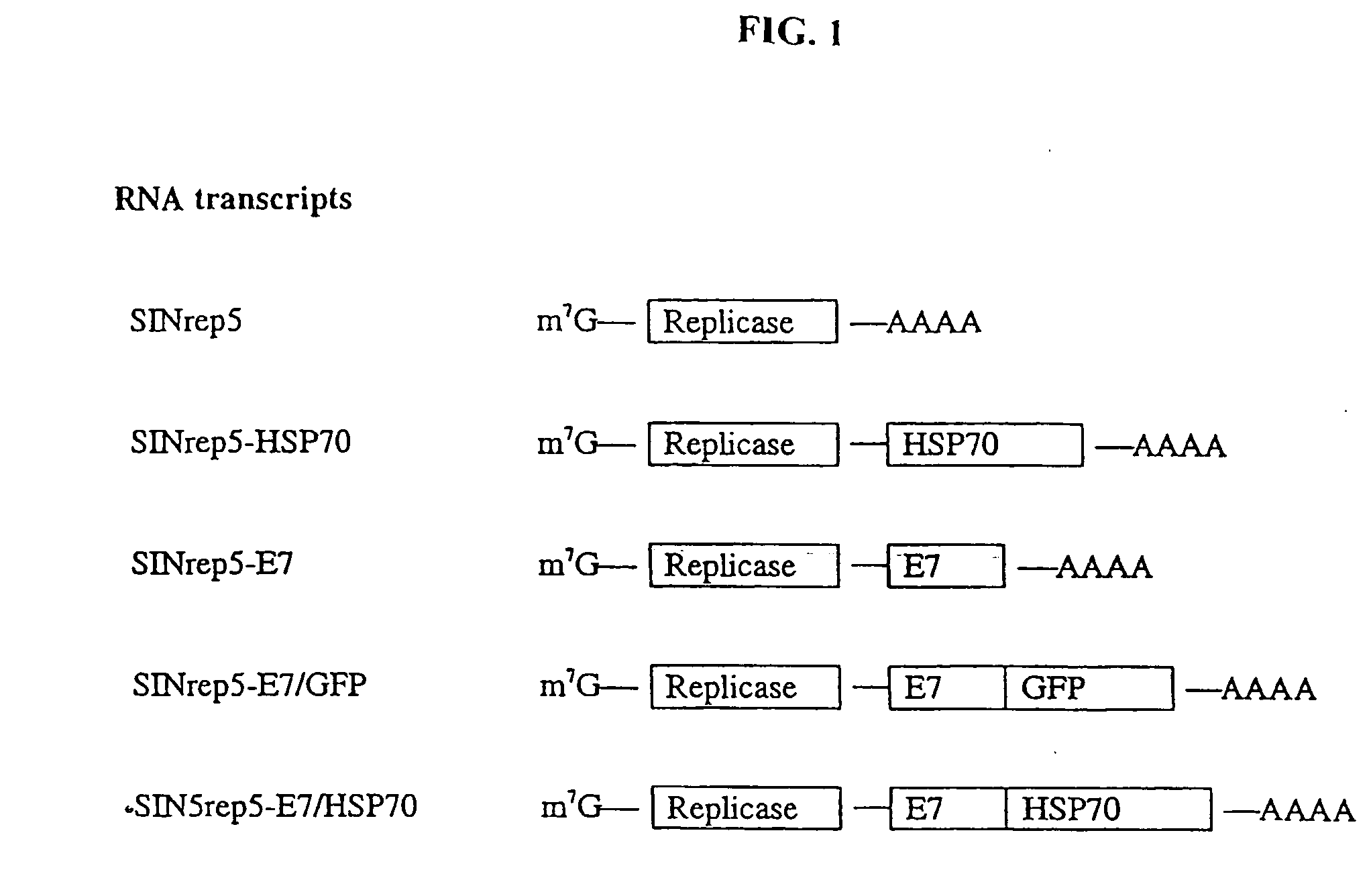
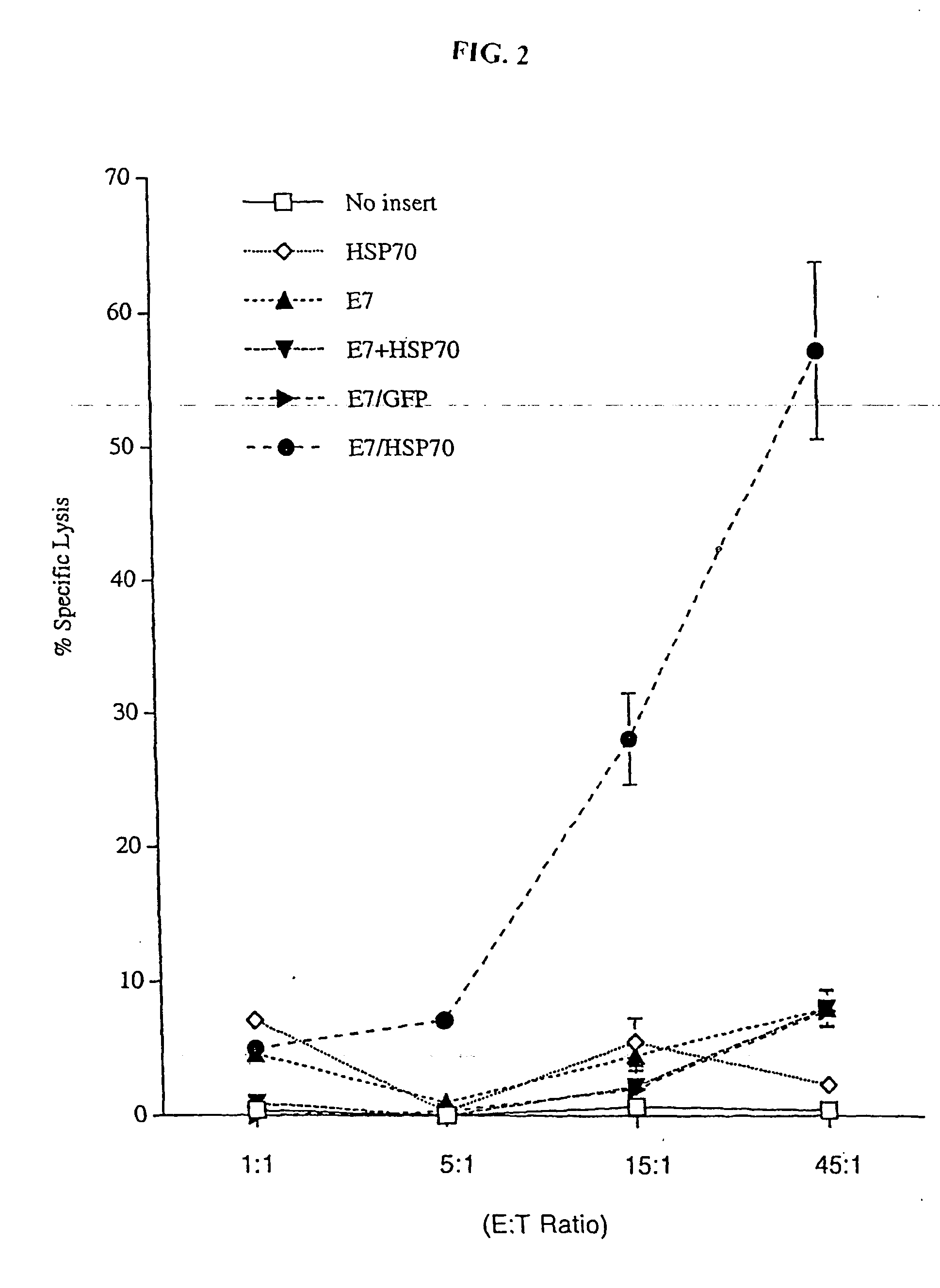
Superior molecular vaccine based on self-replicating rna, suicidal dna or naked dna vector, that links antigen with polypeptide that promotes antigen presentation
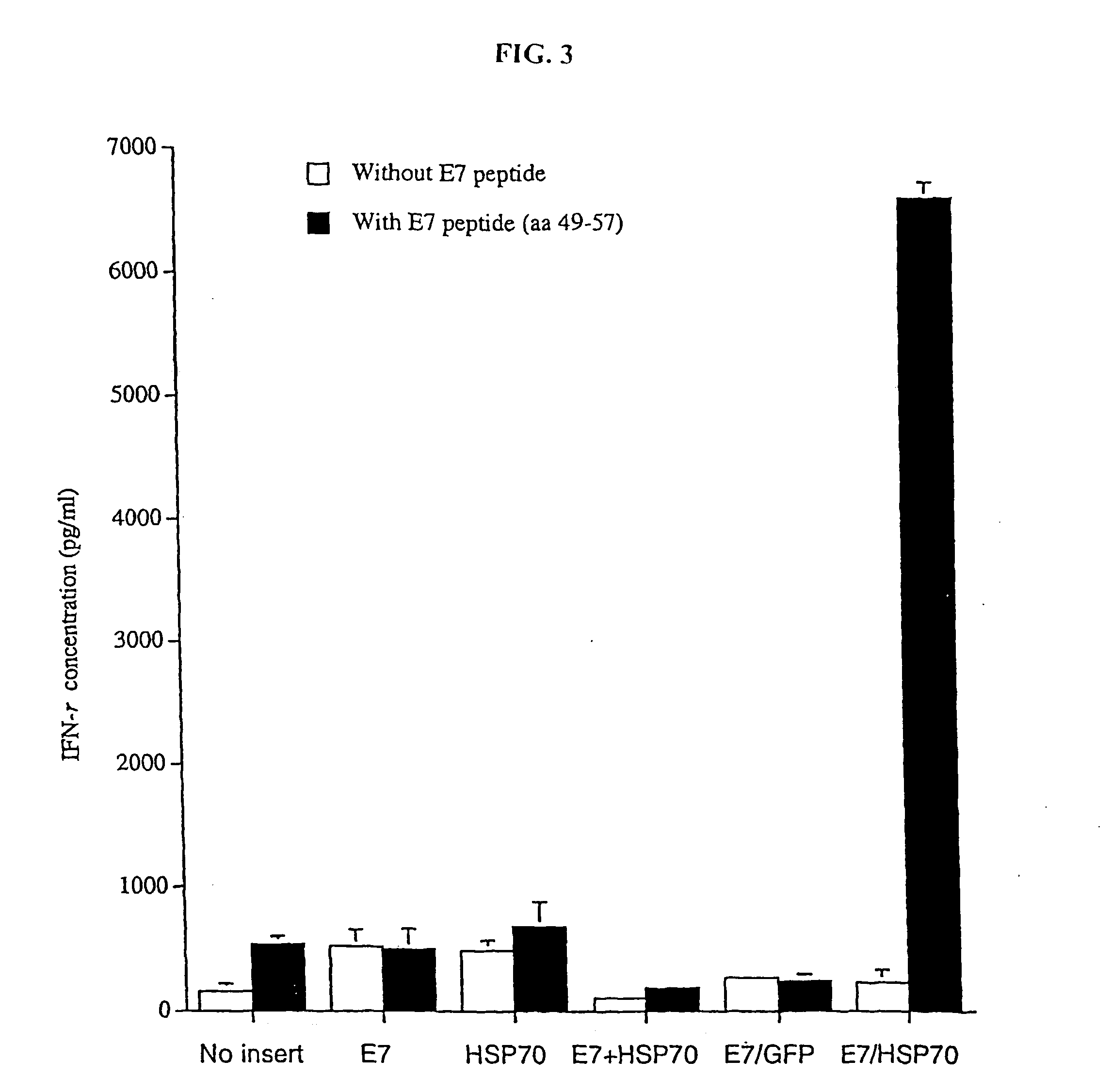
InactiveUS20050277605A1Great E7-specific T cell-mediated immunityEfficiently presentedSsRNA viruses positive-senseAntibody mimetics/scaffoldsMHC class IDisease
Improved molecular vaccines comprise nucleic acid vectors that encode a fusion polypeptide that includes polypeptide or peptide physically linked to an antigen. The linked polypeptide is one that (a) promotes processing of the expressed fusion polypeptide via the MHC class I pathway and / or (b) promotes development or activity of antigen presenting cells, primarily dendritic cells. These vaccines employ one of several types of nucleic acid vectors, each with its own relative advantages: naked DNA plasmids, self-replicating RNA replicons and suicidal DNA-based on viral RNA replicons. Administration of such a vaccine results in enhance immune responses, primarily those mediated by CD8+ cytotoxic T lymphocytes, directed against the immunizing antigen part of the fusion polypeptide. Such vaccines are useful against tumor antigens, viral antigens and antigens of other pathogenic microorganisms and can be used in the prevention or treatment of diseases that include cancer and infections.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compositions and methods using complexes of heat shock protein gp96 and antigenic molecules for the treatment and prevention of infectious diseases
InactiveUS6143299AElicit immune responseOrganic active ingredientsPeptide/protein ingredientsMHC class IAntigenic valence
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. The effective amounts of the complex are in the range of 10-600 micrograms for complexes comprising hsp70, 50-1000 micrograms for hsp90, and 10-600 micrograms for gp96. The invention also provides a method for measuring tumor rejection in vivo in an individual, preferably a human, comprising measuring the generation by the individual of MHC Class I-restricted CD8+ cytotoxic T lymphocytes specific to the tumor. Methods of purifying hsp70-peptide complexes are also provided.
Owner:FORDHAM UNIVERSITY
Mhc class i epitope delivering polypeptides and cell-targeted molecules for direct cell killing and immune stimulation via mhc class i presentation and methods regarding the same
InactiveUS20160347798A1Increase probabilityReduce probabilityAntibacterial agentsHydrolasesMHC class IHeterologous
The present invention is directed to T-cell epitope delivering polypeptides which deliver one or more CD8+ T-cell epitopes to the MHC class I presentation pathway of a cell, including toxin-derived polypeptides which comprise embedded T-cell epitopes and are de-immunized. The present invention provides cell-targeted, CD8+ T-cell epitope delivering molecules for the targeted delivery of cytotoxicity to certain cells, e.g., infected or malignant cells, for the targeted killing of specific cell types, and the treatment of a variety of diseases, disorders, and conditions, including cancers, immune disorders, and microbial infections. The present invention also provides methods of generating polypeptides capable of delivering one or more heterologous T-cell epitopes to the MHC class I presentation pathway, including polypeptides which are 1) B-cell and / or CD4+ T-cell de-immunized, 2) comprise embedded T-cell epitopes, and / or 3) comprises toxin effectors which retain toxin functions.
Owner:MOLECULAR TEMPLATES
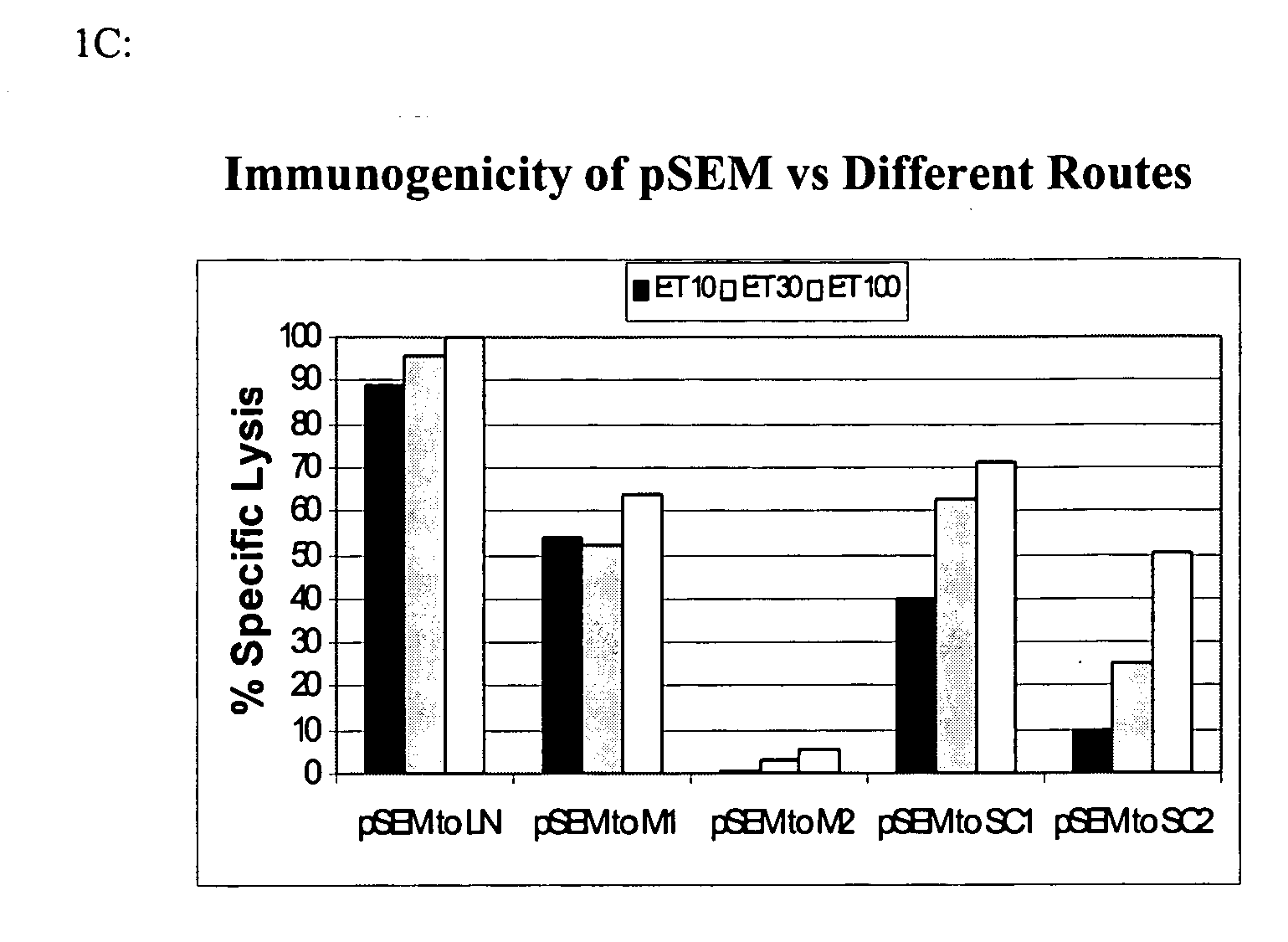
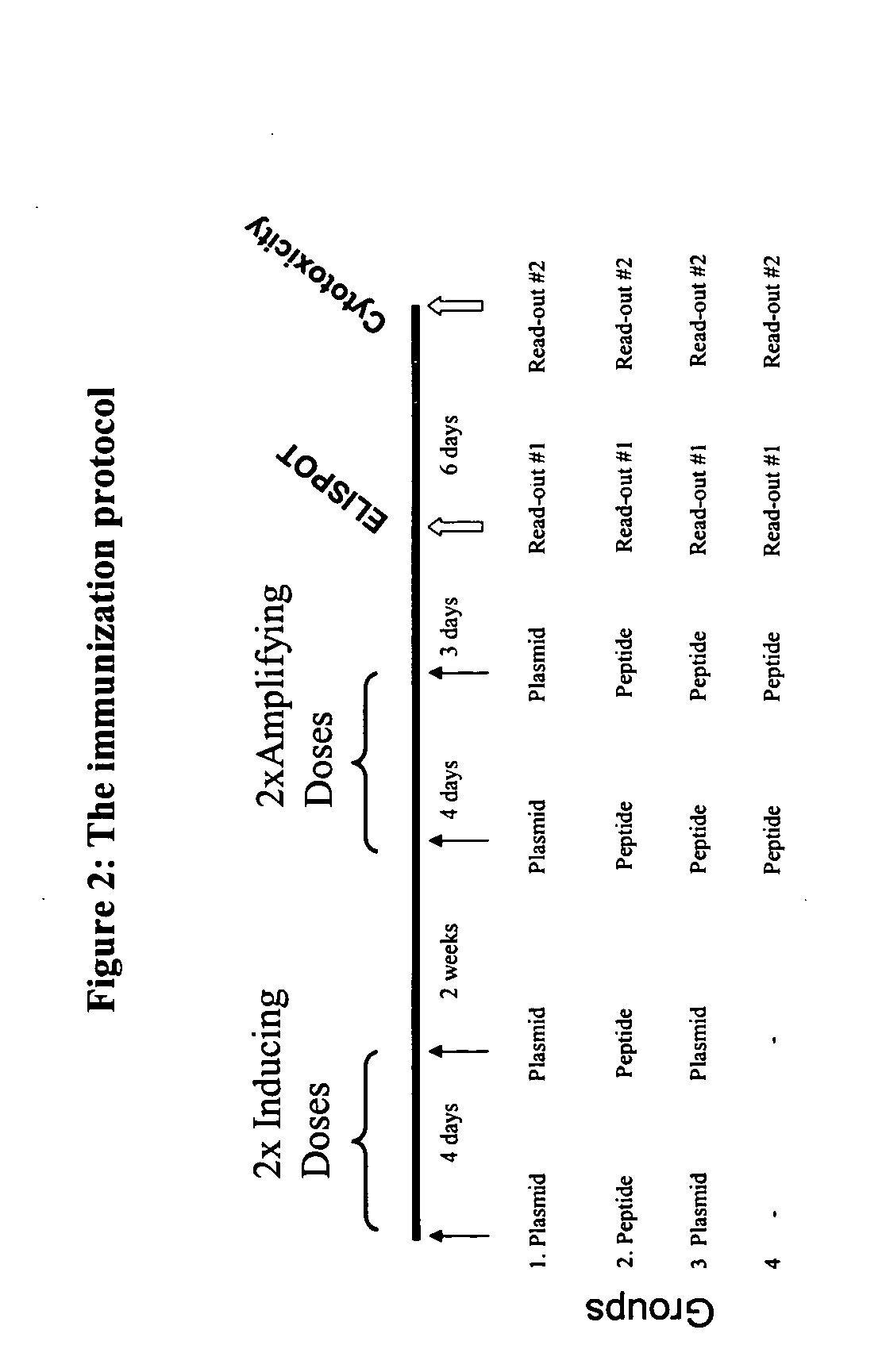
Multivalent entrain-and-amplify immunotherapeutics for carcinoma
InactiveUS20070003563A1Tumor rejection antigen precursorsPeptide/protein ingredientsMHC class IEpitope
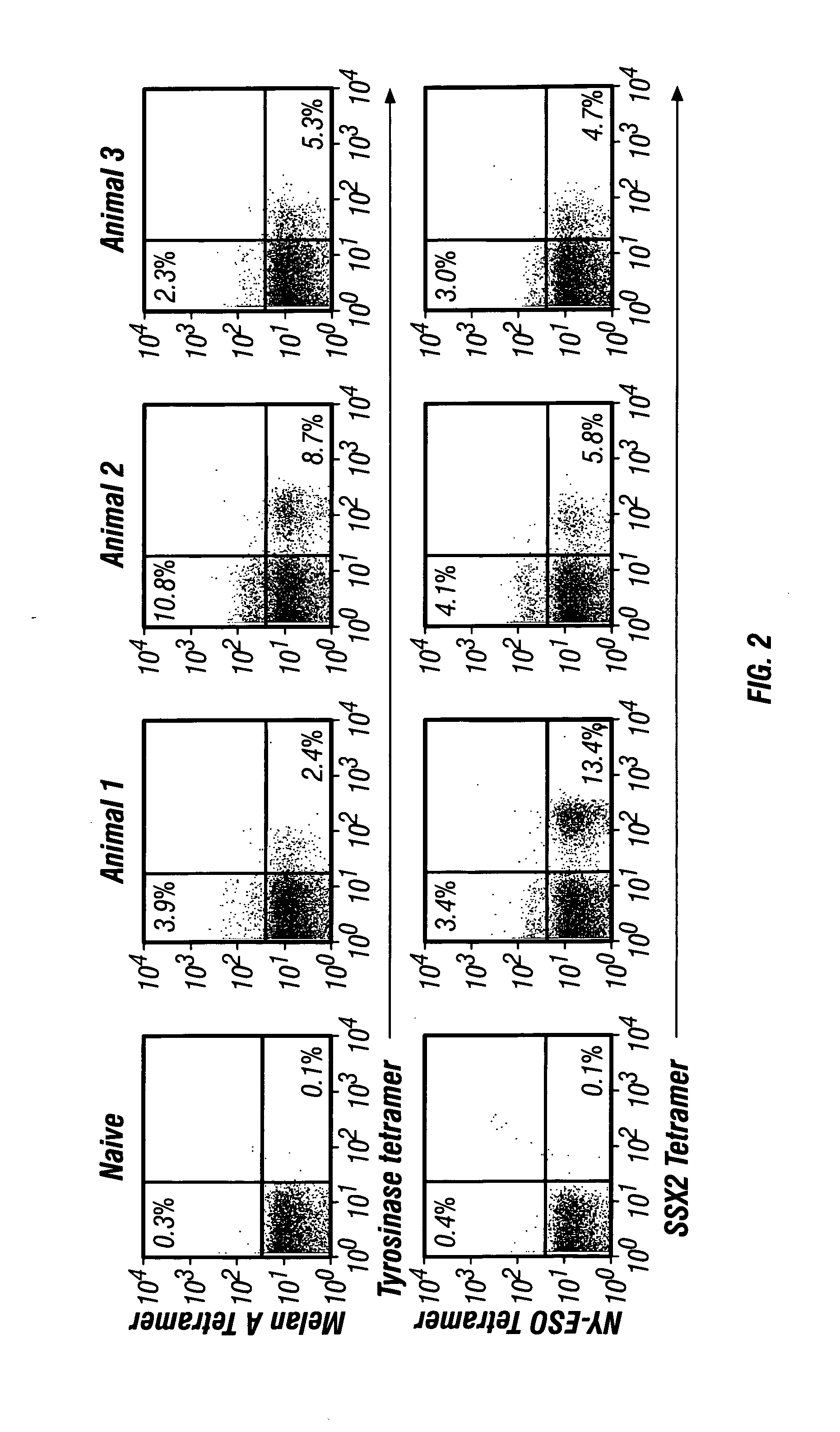
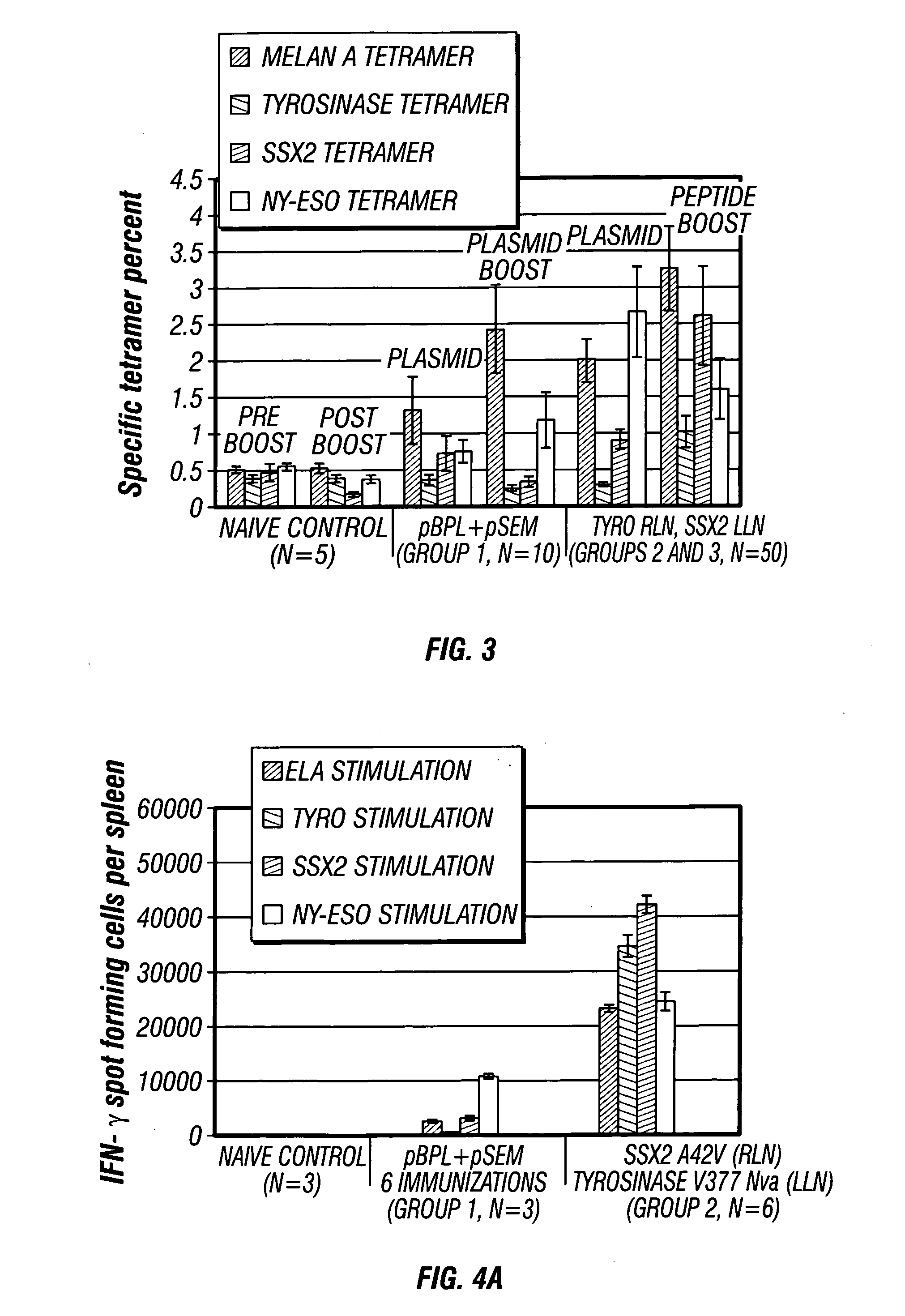
The present invention provides a method of treating a cell proliferative disease such as cancer by providing to a subject in need thereof an immunogenic composition comprising plasmid and peptide(s) or analogues thereof. In embodiments of the present invention there is provided methods and compositions for inducing, entraining, and / or amplifying the immune response to MHC class-I restricted epitopes of carcinoma antigens to generate an effective anti-cancer immune response.
Owner:MANNKIND CORP
Survivin-derived peptides and use thereof
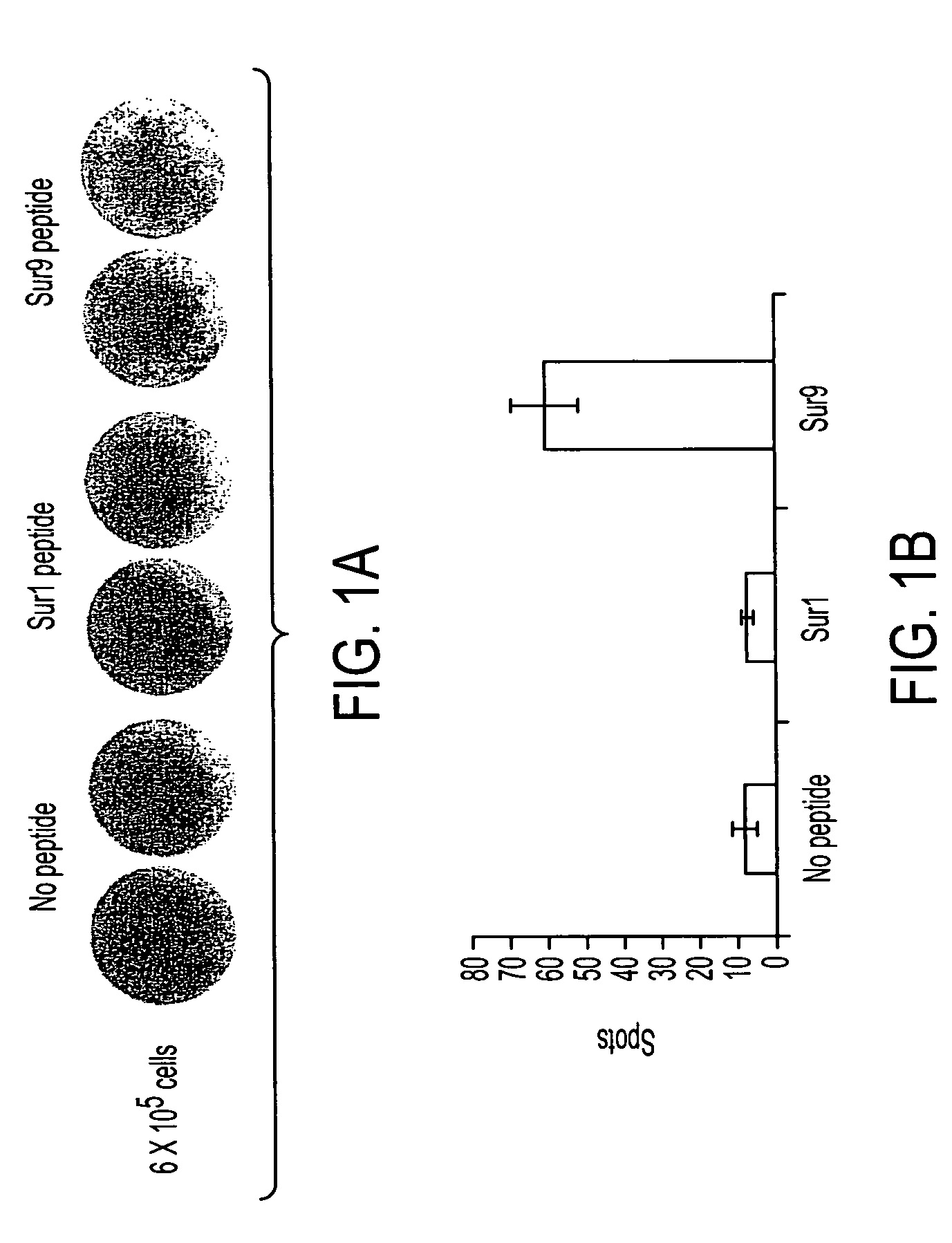
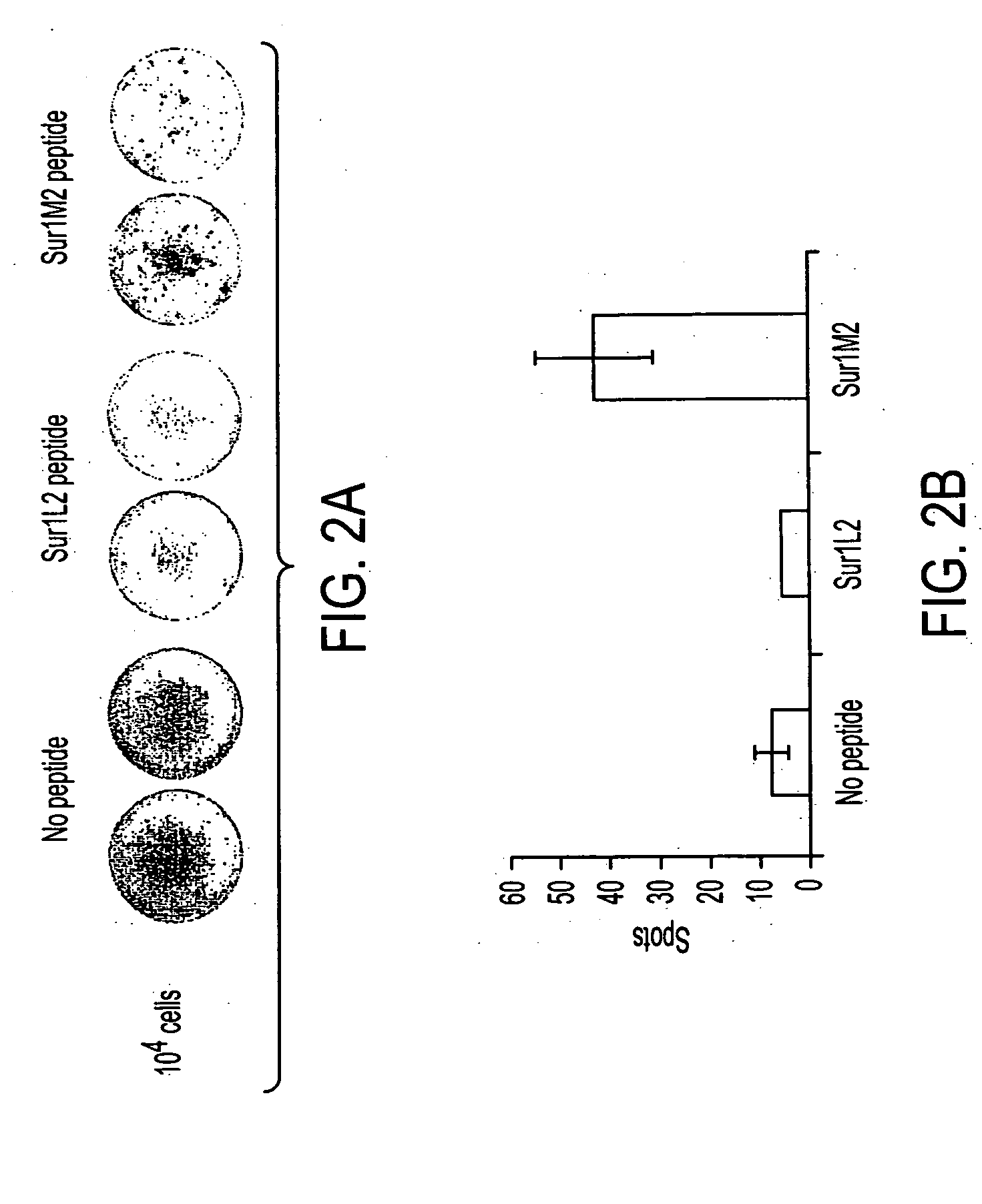
InactiveUS20040210035A1Easy to filterWeak affinityPeptide/protein ingredientsDepsipeptidesMHC class IWilms' tumor
MHC Class I-restricted peptides derived from the tumor associated antigen, survivin, which peptides are capable of binding to Class I HLA molecules at a high affinity, capable of eliciting INF-gamma-producing cells in a PBL population of a cancer patient and capable of in situ detection of cytotoxic T cells in a tumor tissue, therapeutic and diagnostic composition comprising the peptide and uses hereof.
Owner:SURVAC
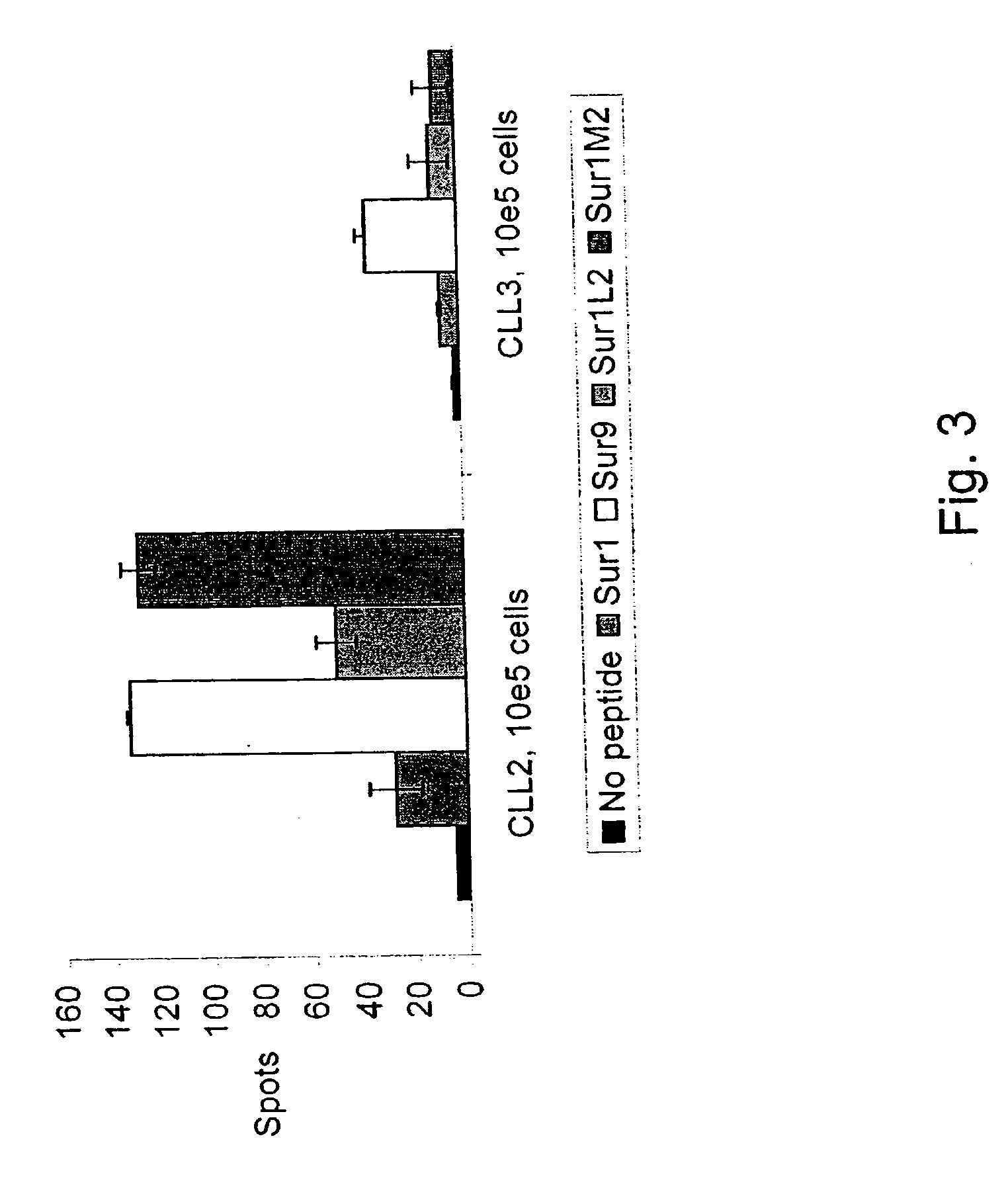
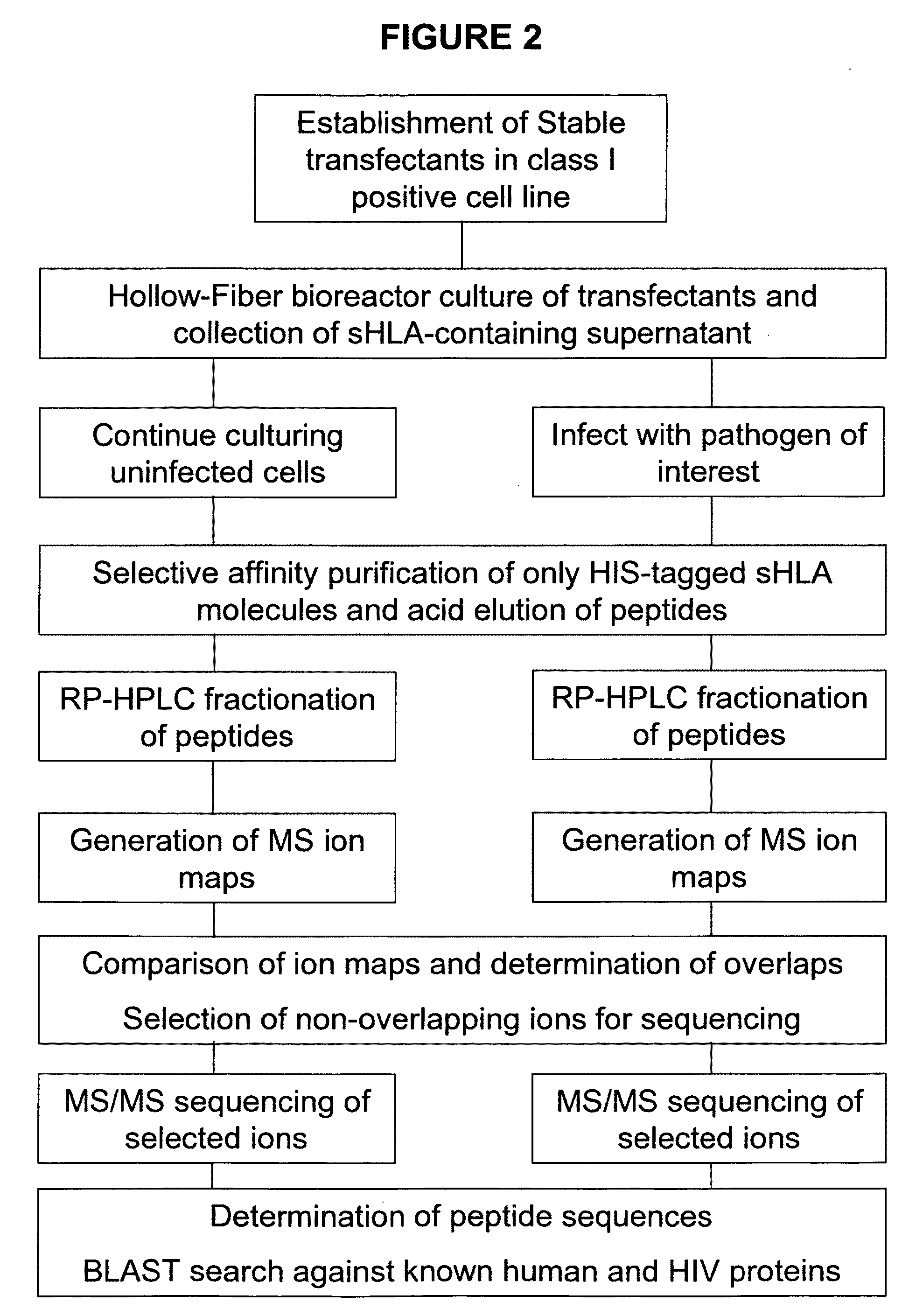
Comparative ligand mapping from MHC class I positive cells
The present invention relates generally to a methodology for the isolation, purification and identification of peptide ligands presented by MHC positive cells. In particular, the methodology of the present invention relates to the isolation, purification and identification of these peptide ligands from soluble class I and class II MHC molecules which may be from uninfected, infected, or tumorigenic cells. The methodology of the present invention broadly allows for these peptide ligands and their cognate source proteins thereof to be identified and used as markers for infected versus uninfected cells and / or tumorigenic versus nontumorigenic cells, with said identification being useful for marking or targeting a cell for therapeutic treatment or priming the immune response against infected / tumorigenic cells.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
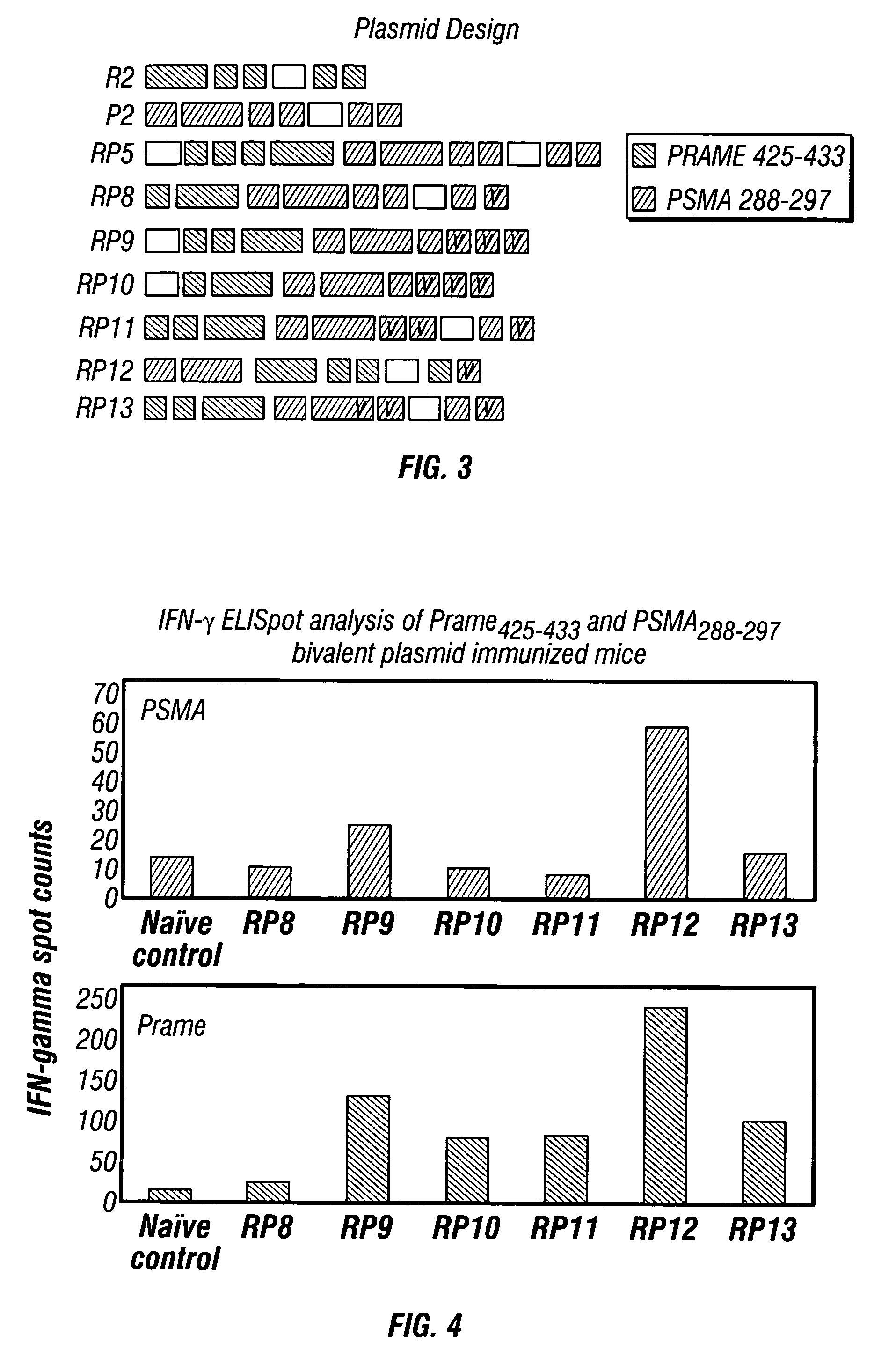
Methods and compositions to elicit multivalent immune responses against dominant and subdominant epitopes, expressed on cancer cells and tumor stroma
InactiveUS20070004662A1Effective controlBeneficial implicationTumor rejection antigen precursorsGenetic material ingredientsMHC class IEpitope
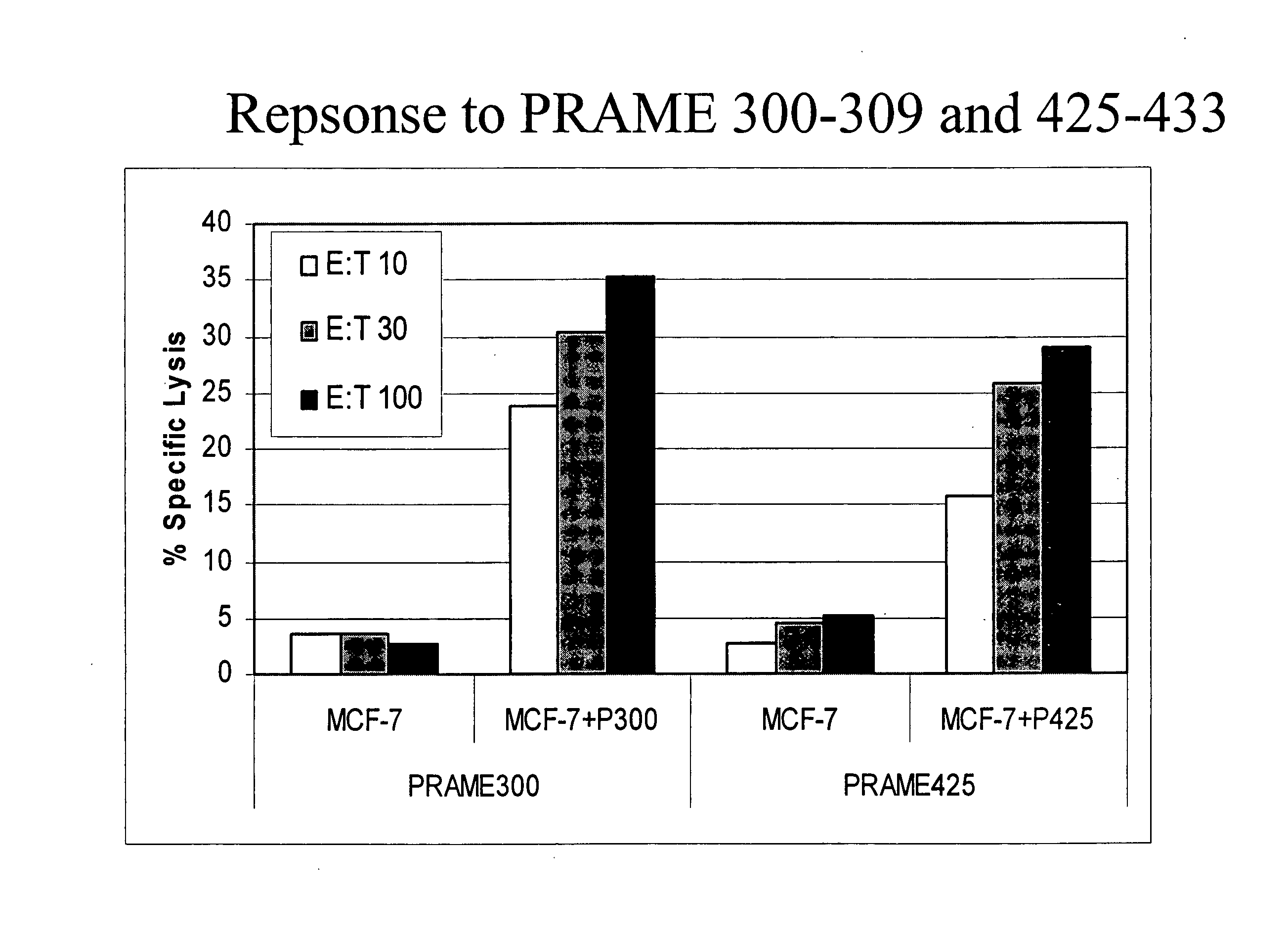
The present invention provides a method of treating cancer by providing to a subject in need thereof an immunogenic composition comprising a nucleic acid construct encoding a polypeptide comprising CTL epitopes PSMA288-297 and PRAME425-433, or a cross-reactive analogue. In embodiments of the present invention there is provided methods and compositions for inducing, entraining, and / or amplifying the immune response to MHC class-I restricted epitopes of carcinoma antigens to generate an effective anti-cancer immune response.
Owner:MANNKIND CORP
Purification of antigen-specific T cells
InactiveUS7125964B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMHC class IMagnetic bead
Owner:ORTHO MCNEIL PHARM INC
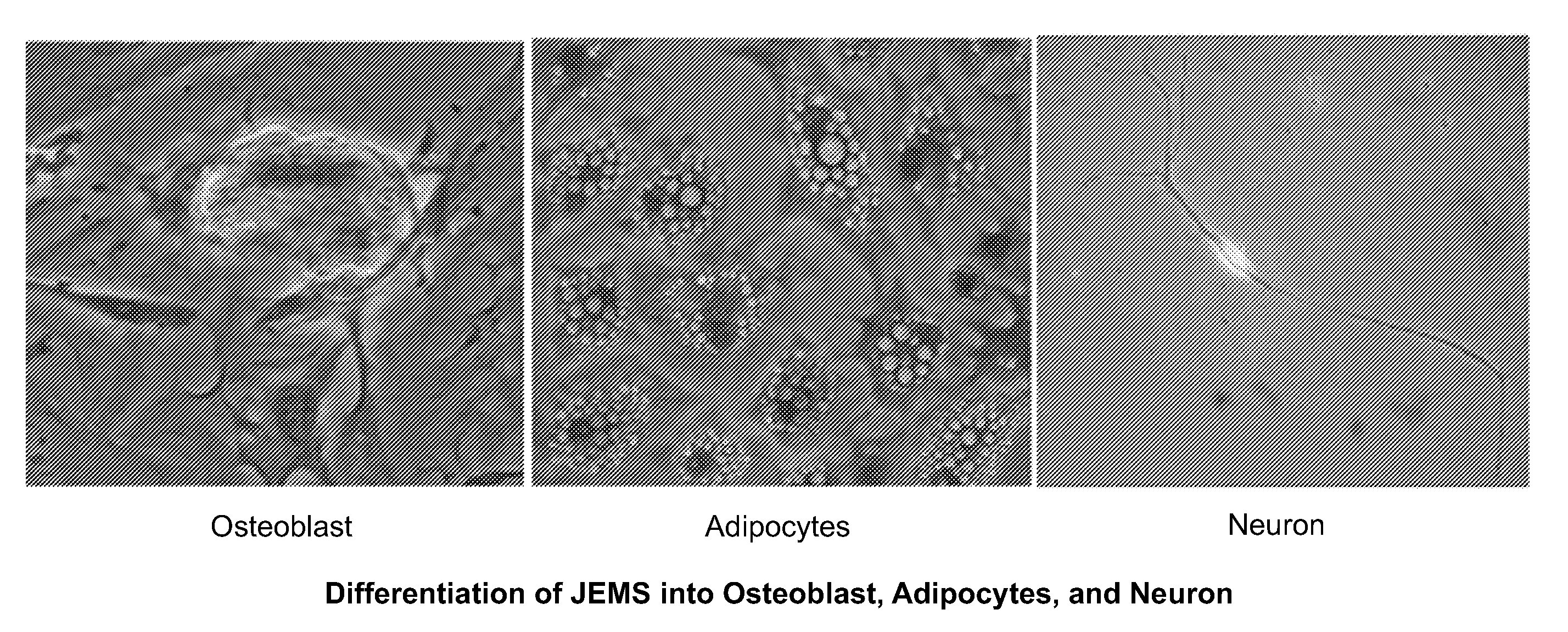
Multipotent stem cells and uses thereof
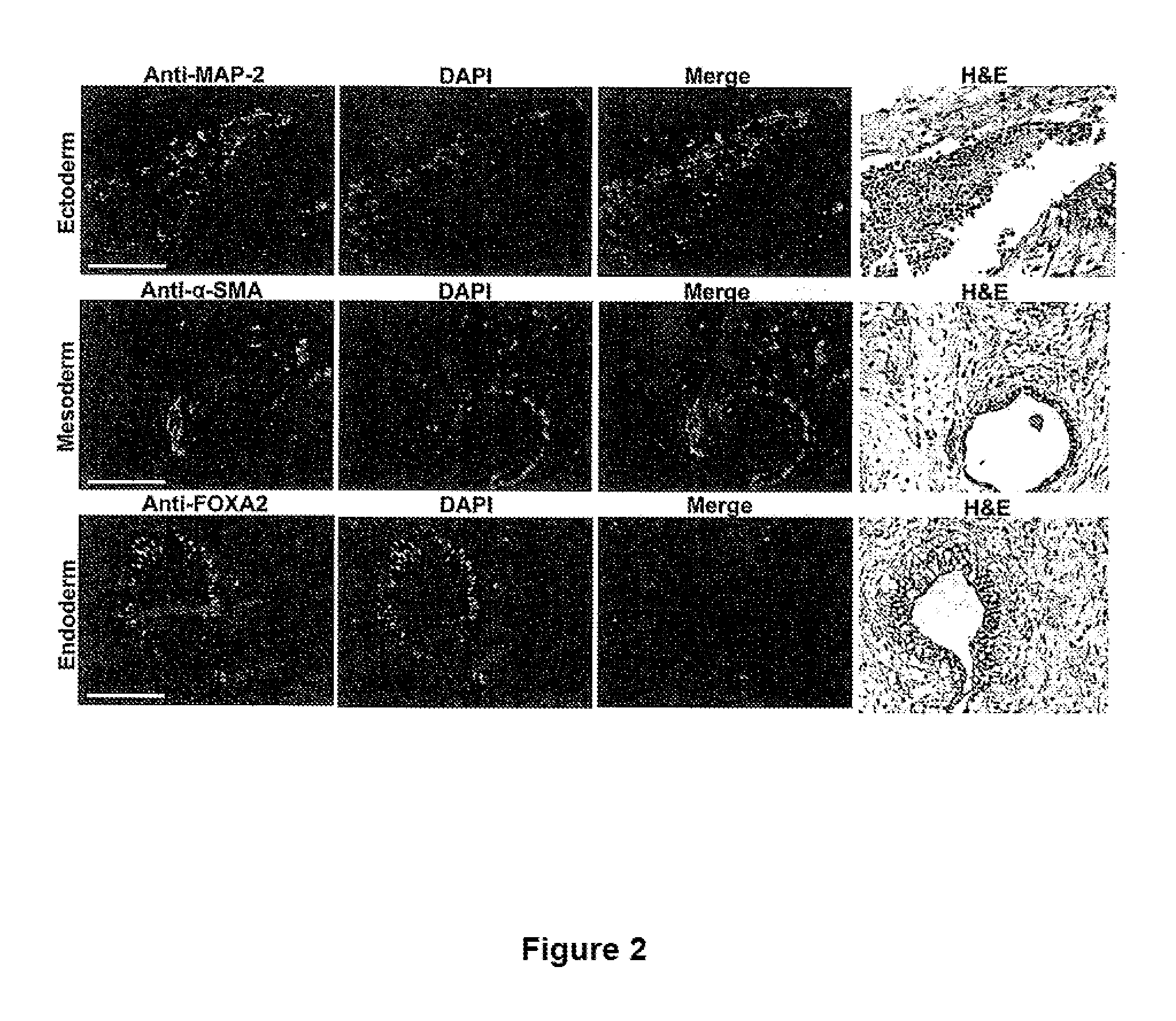
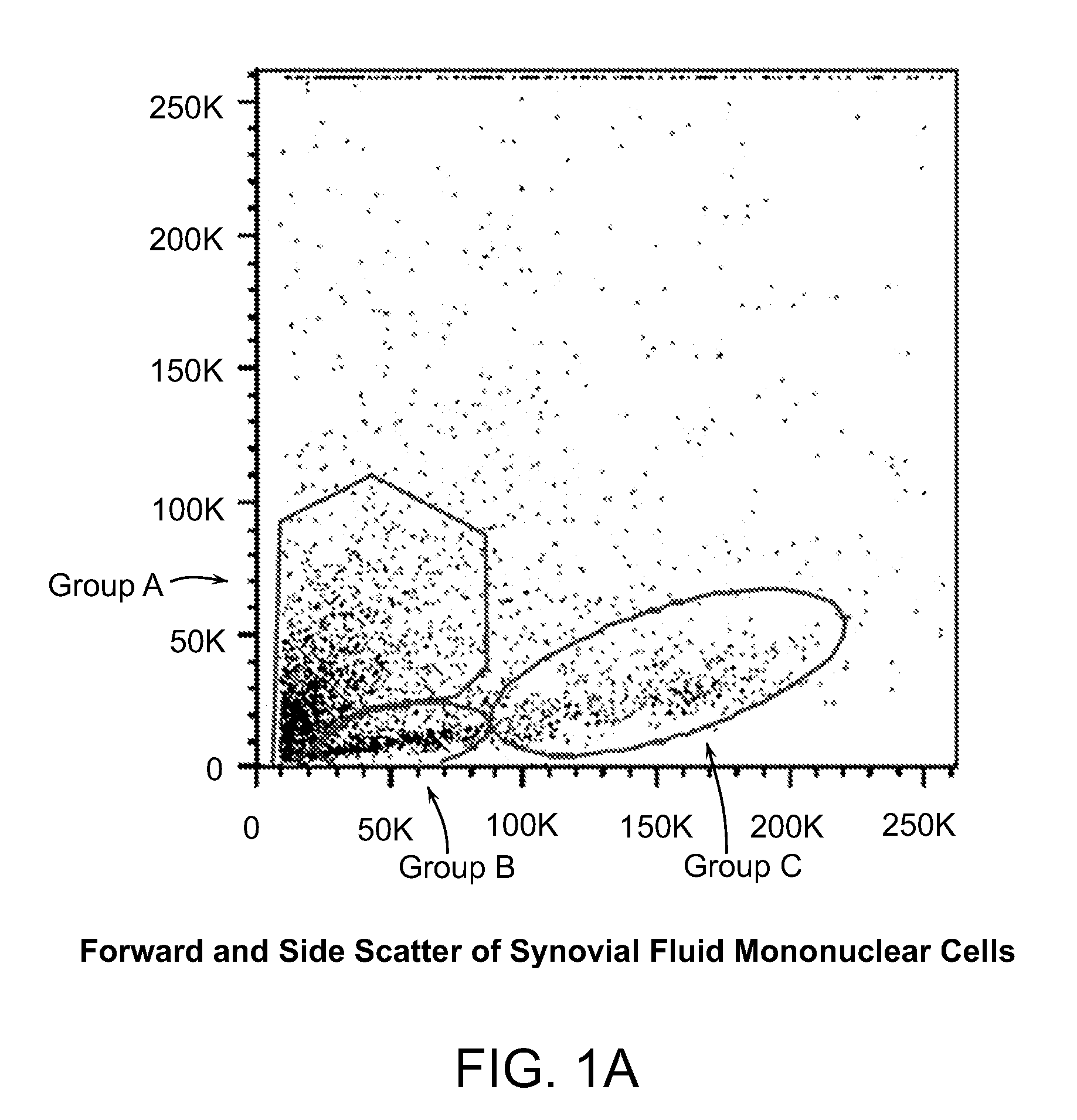
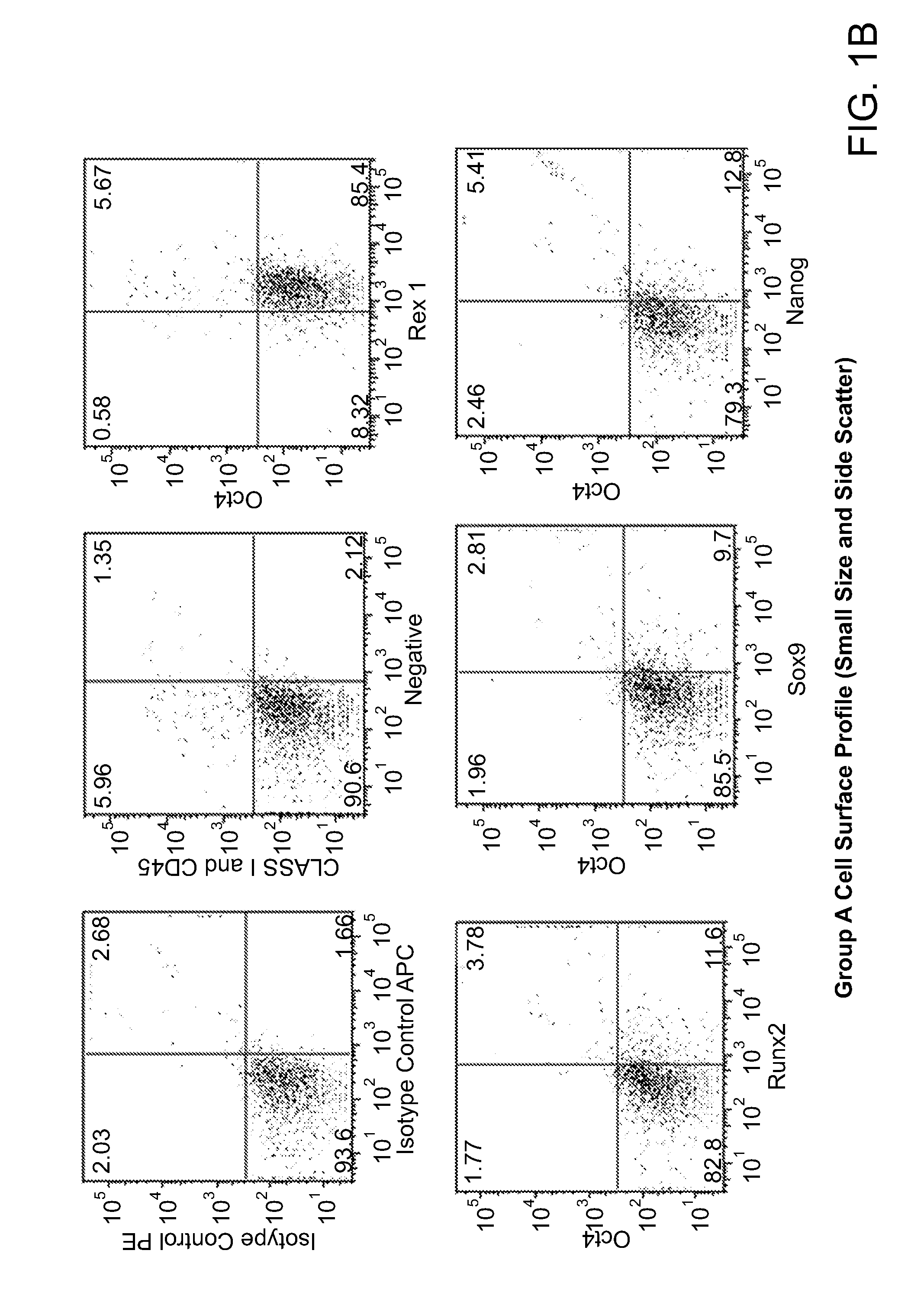
ActiveUS20110070205A1Easily attainable embryonic-likePromote wound healingBiocideNervous disorderGerm layerMHC class I
The invention provides a quiescent stem cell having the capacity to differentiate into ectoderm, mesoderm and endoderm, and which does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105 CD90, CD66A, CD66E, CXCR4, CD133 or an SSEA. The invention further provides a proliferative stem cell, which expresses genes including Oct-4, Nanog, Sox2, GDF3, P16INK4, BMI, Notch, HDAC4, TERT, Rex-1, TWIST, KLF-4 and Stella but does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105, CD90, CD66A, CD66E, CXCR4, CD133 or an SSEA. The cells of the invention can be isolated from adult mammals, have embryonic cell characteristics, and can form embryoid bodies. Methods for obtaining the stem cells, as well as methods of treating diseases and differentiated the stem cells, are also provided.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Hat acetylation promoters and uses of compositions thereof in promoting immunogenicity
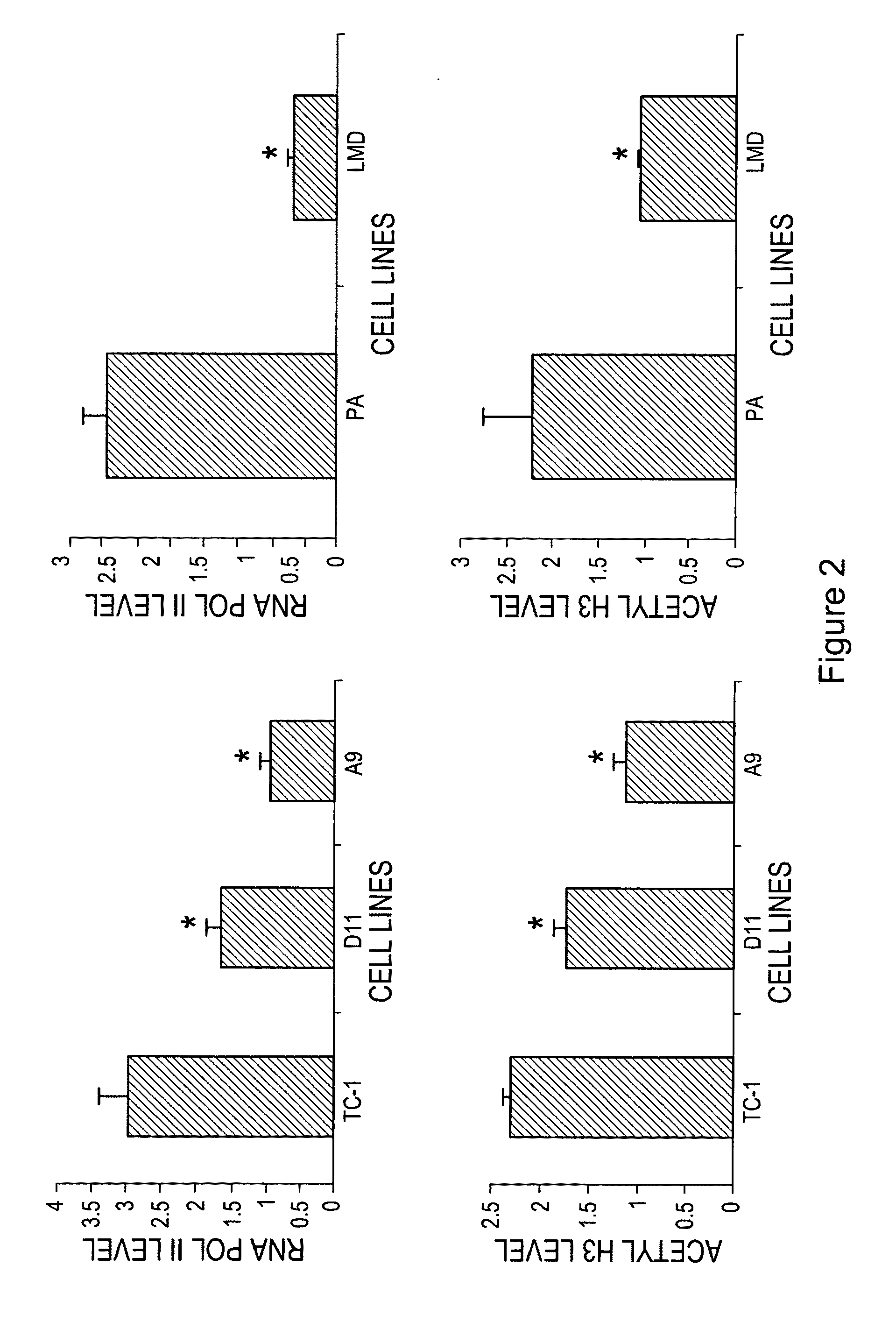
InactiveUS20100166781A1Improving immunogenicityPromoting surfaceAntibacterial agentsBiocideMHC class IFactor ii
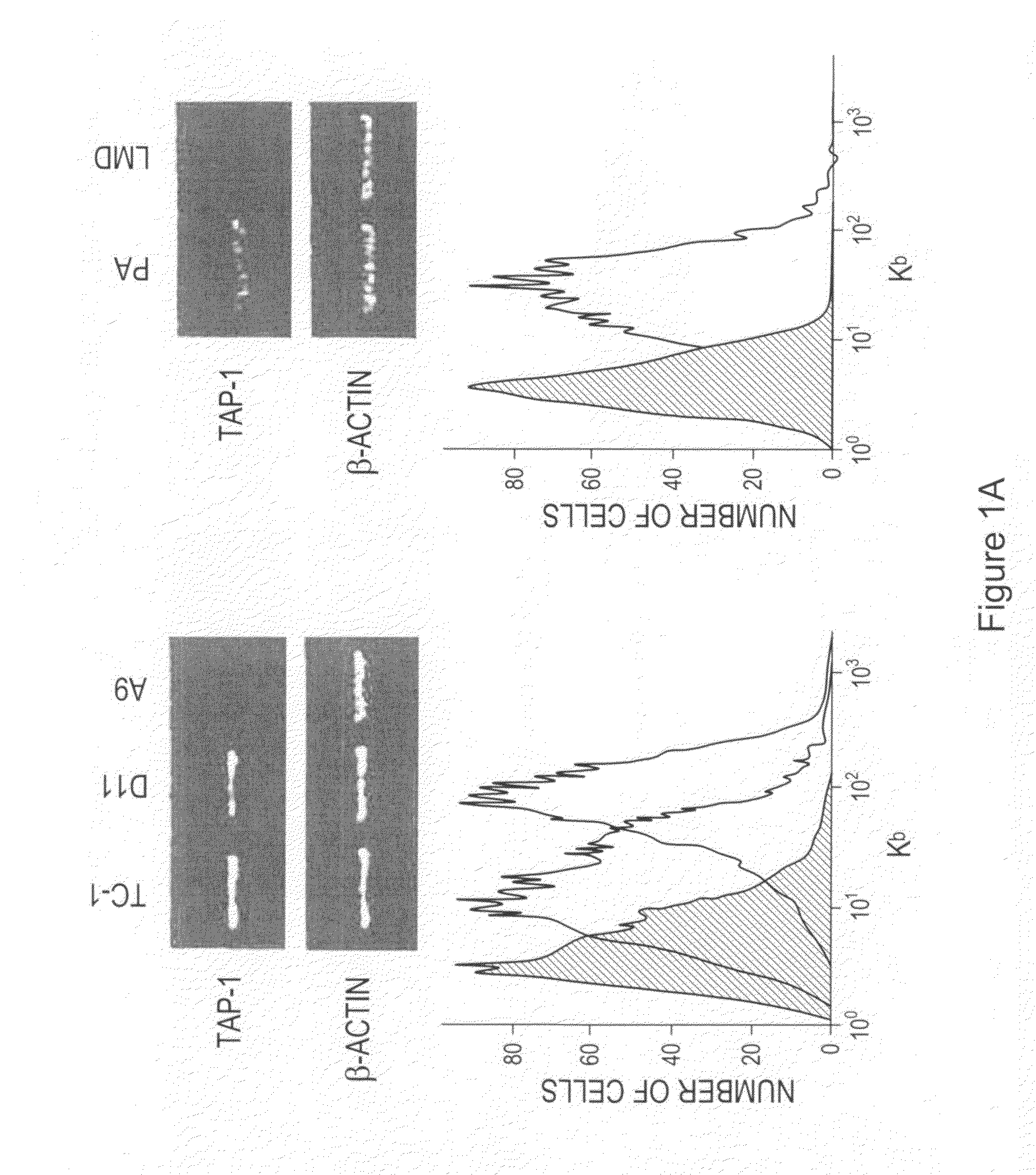
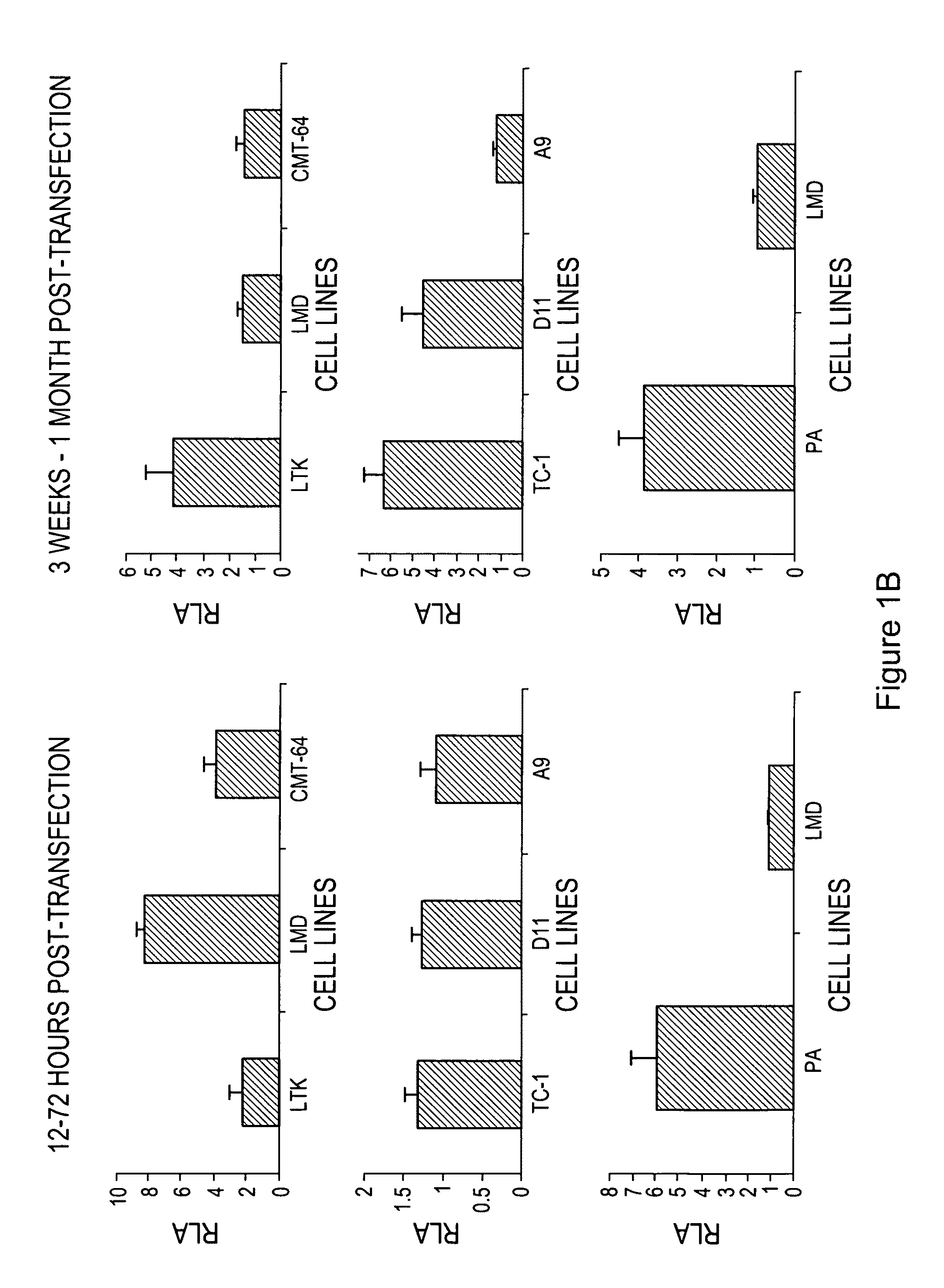
The invention provides processes and compositions for enhancing the immunogenicity of TAP-1 expression-deficient cells by increasing the presentation of MHC Class I surface molecules for detection by cytotoxic T-lymphocyte cells through increased TAP-1 expression, which comprises administering to the TAP-1 expression-deficient cells a TAP-1 expression increasing amount of a bio-acceptable substance that promotes transcription of TAP-1 gene in the cells to cause enhanced MHC Class I surface expression of the cells. The bio-acceptable substance may be a histone H3 deacetylase inhibitor, such as trichostatin A, a transcriptional co-activator having intrinsic histone acetyl transferase activity or a histone acetyl transferase comprising at least one member of the CBP / p300 protein family. The process and compositions increase the immunogenicity of the target cells to enhance their destruction by cytotoxic lymphocytes.
Owner:JEFFERIES WILFRED
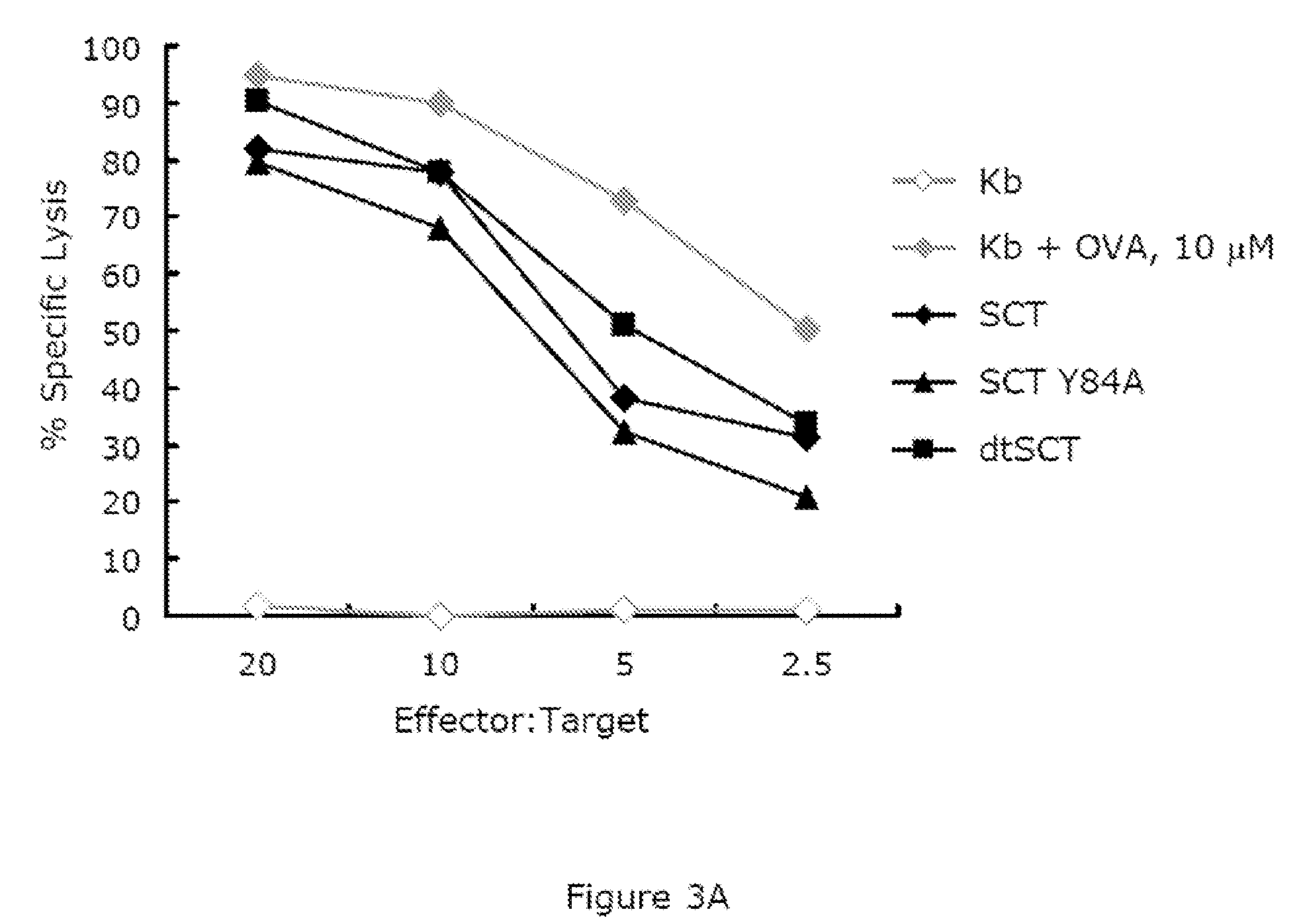
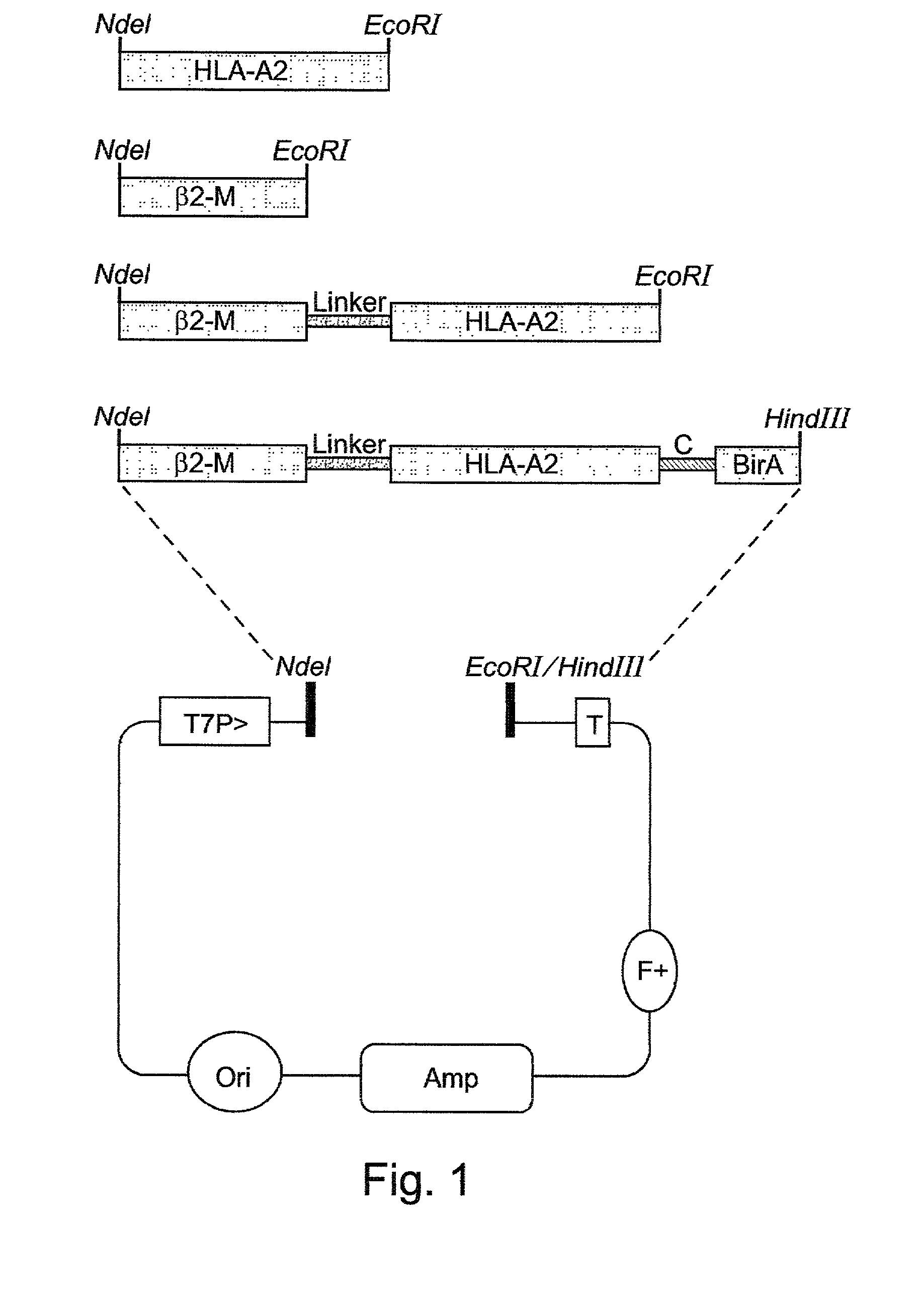
Single chain class I major histo-compatibility complexes
A recombinant polypeptide and nucleic acid constructs capable of expressing the recombinant polypeptide are provided. The recombinant polypeptide comprises a chimeric polypeptide including an antigenic peptide being capable of binding a human MHC class I, a functional human beta-2 microglobulin and a functional human MHC class I heavy chain.
Owner:TECHNION RES & DEV FOUND LTD
Agonist peptides of carcinoembryonic antigen (CEA) and nucleic acid sequences encoding the agonist peptides
InactiveUS7723096B2Reduce eliminate CEA-specific T-cellReduce or eliminate CEA-specific T-cell activation and killingBacteriaPeptide/protein ingredientsMHC class ICarcinoembryonic antigen
A nucleic acid molecule encoding an amino acid sequence consisting of an agonist of a MHC class I binding native sequence of CEA having an amino acid substitution and enhanced immunogenicity compared to the native sequence is described. A vector comprising the nucleic acid molecule, host cell comprising the vector and a kit comprising the encoded agonist peptide are also described.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com