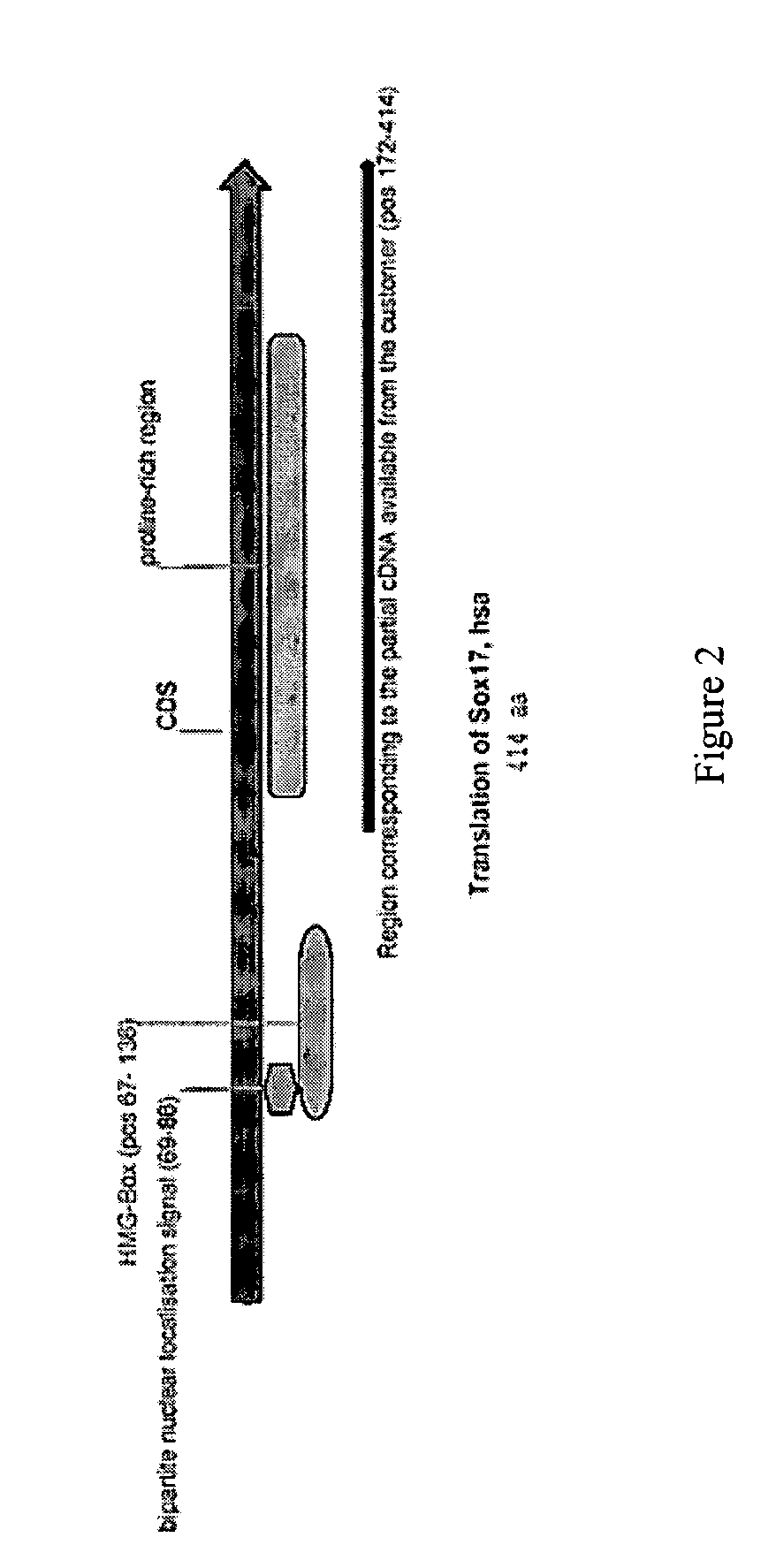
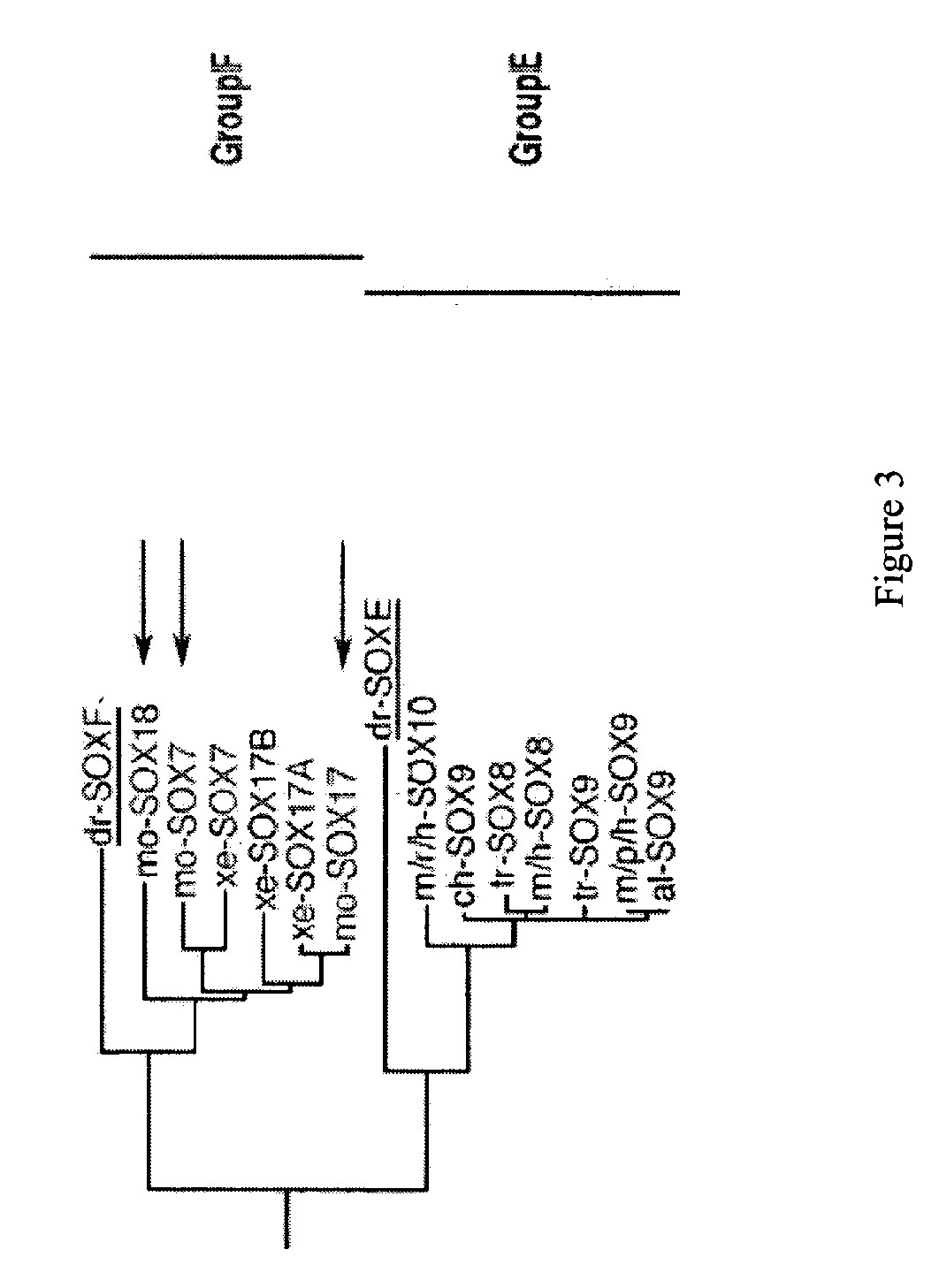
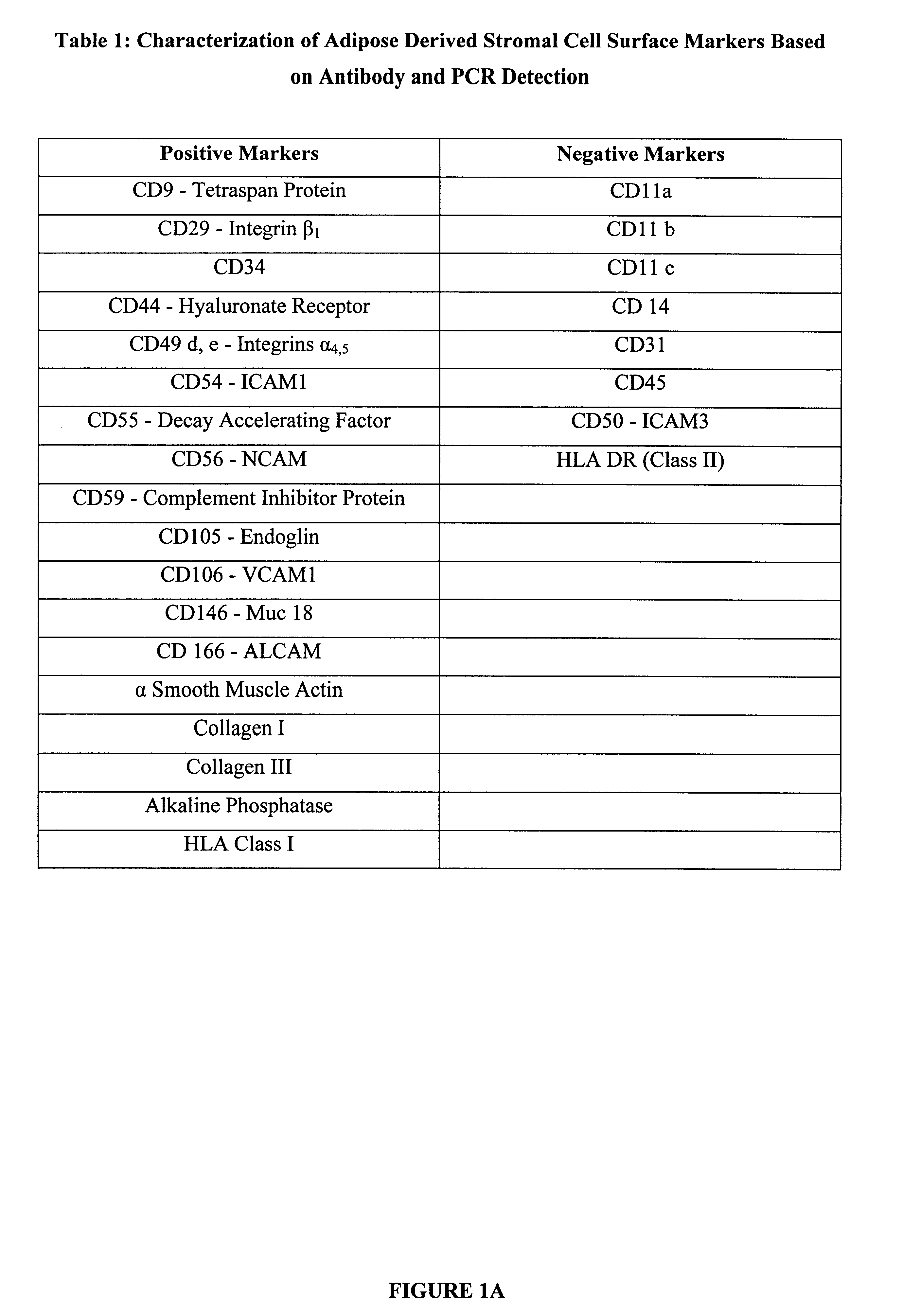
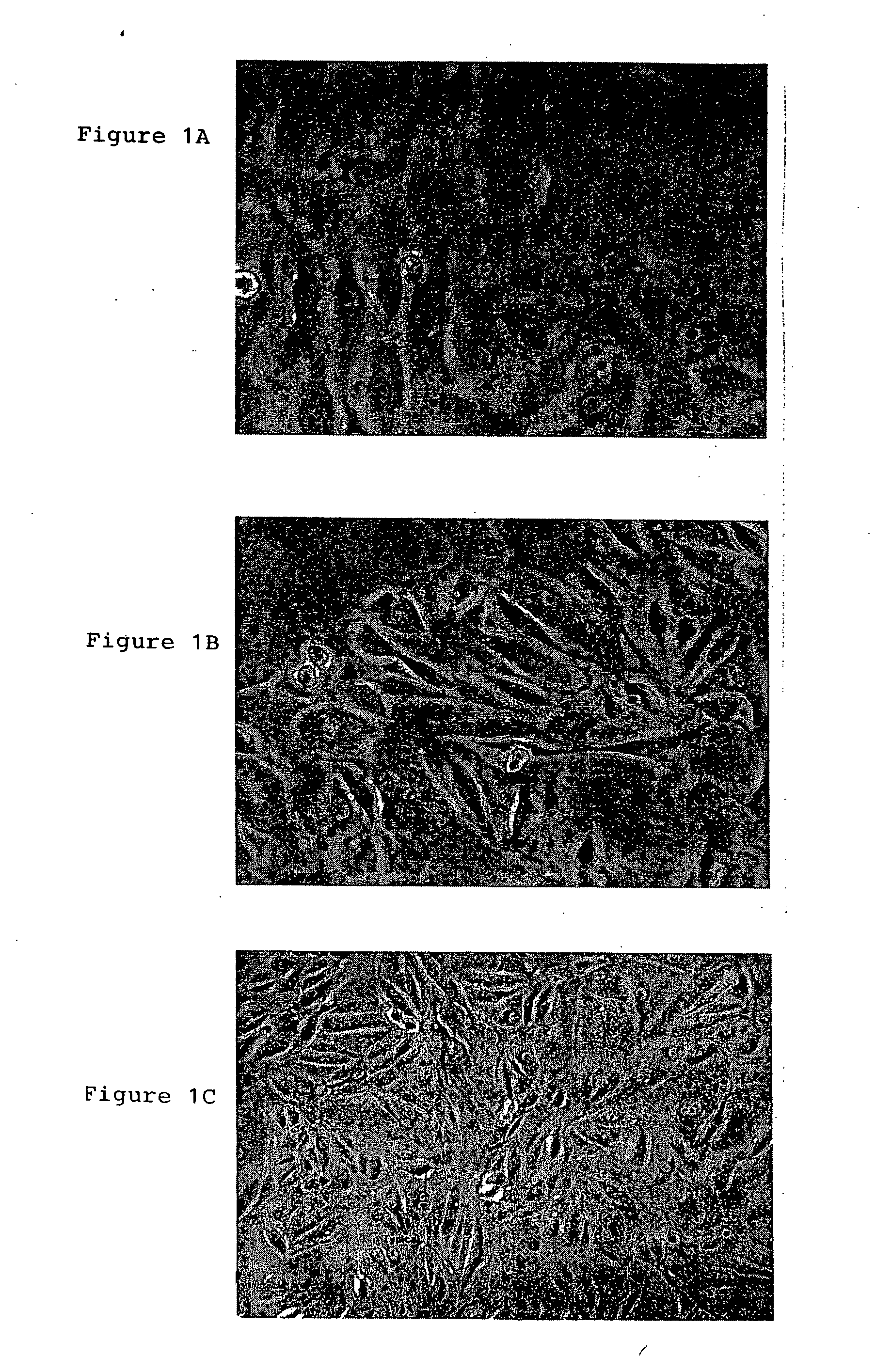
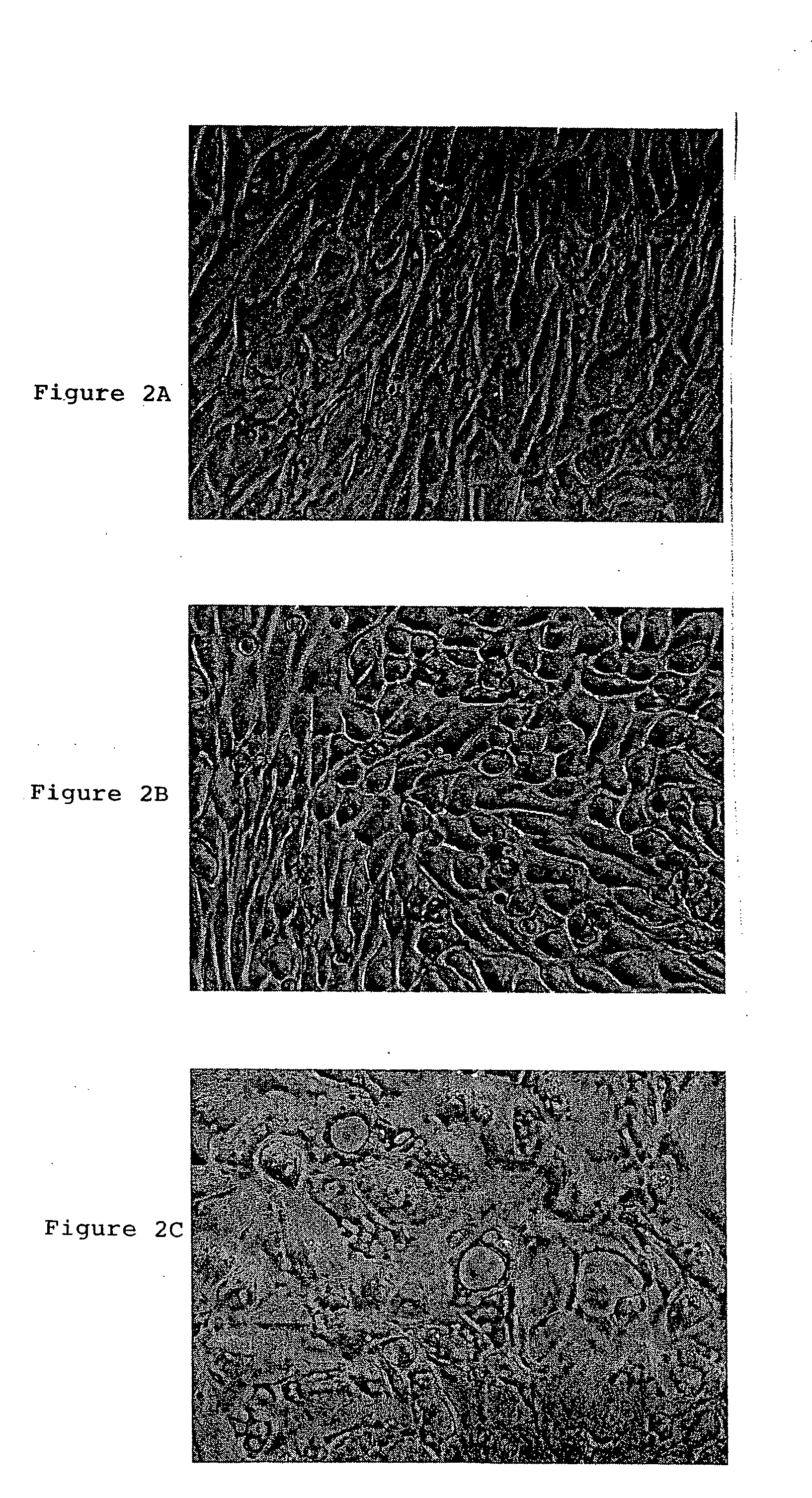
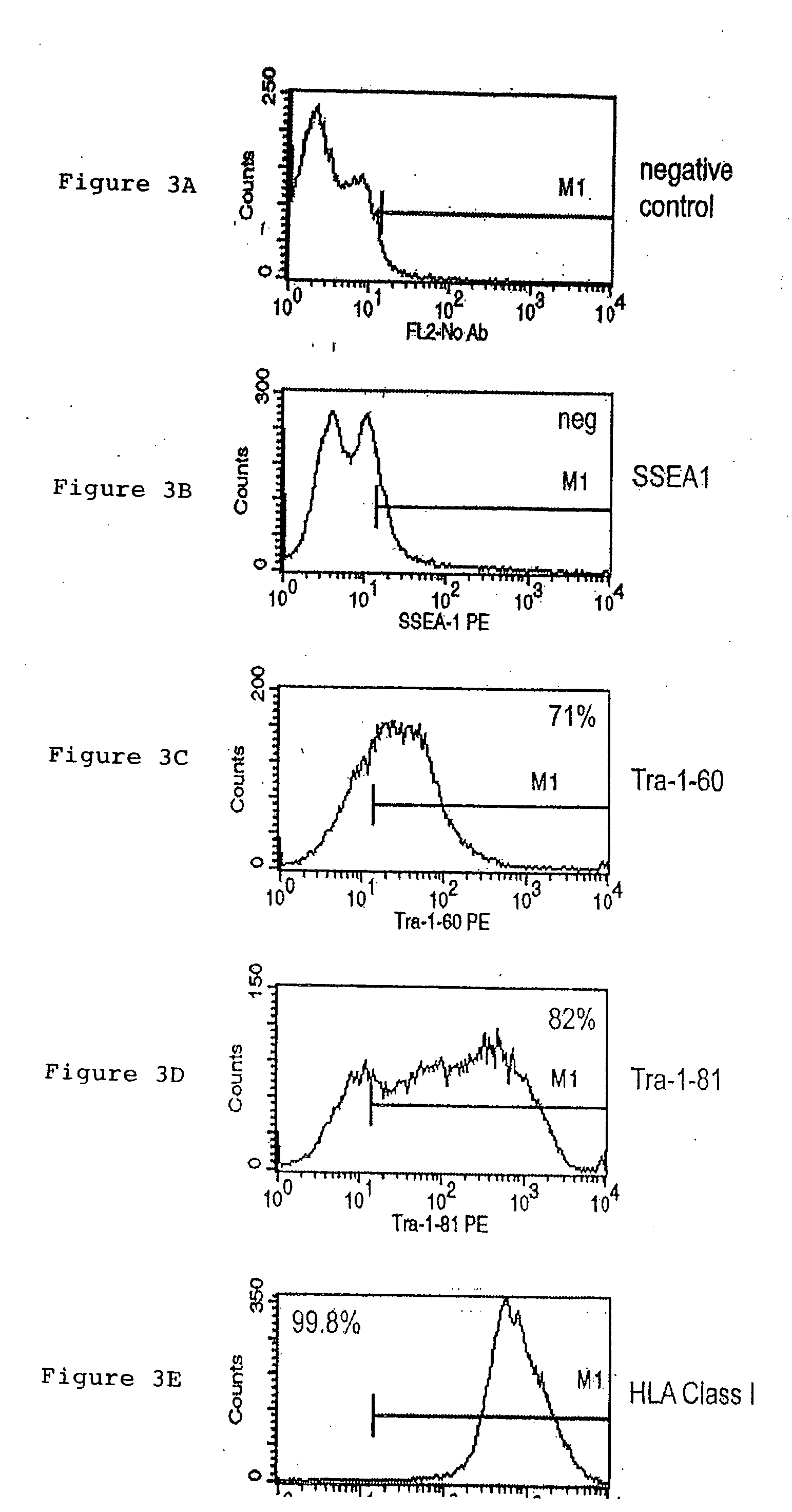
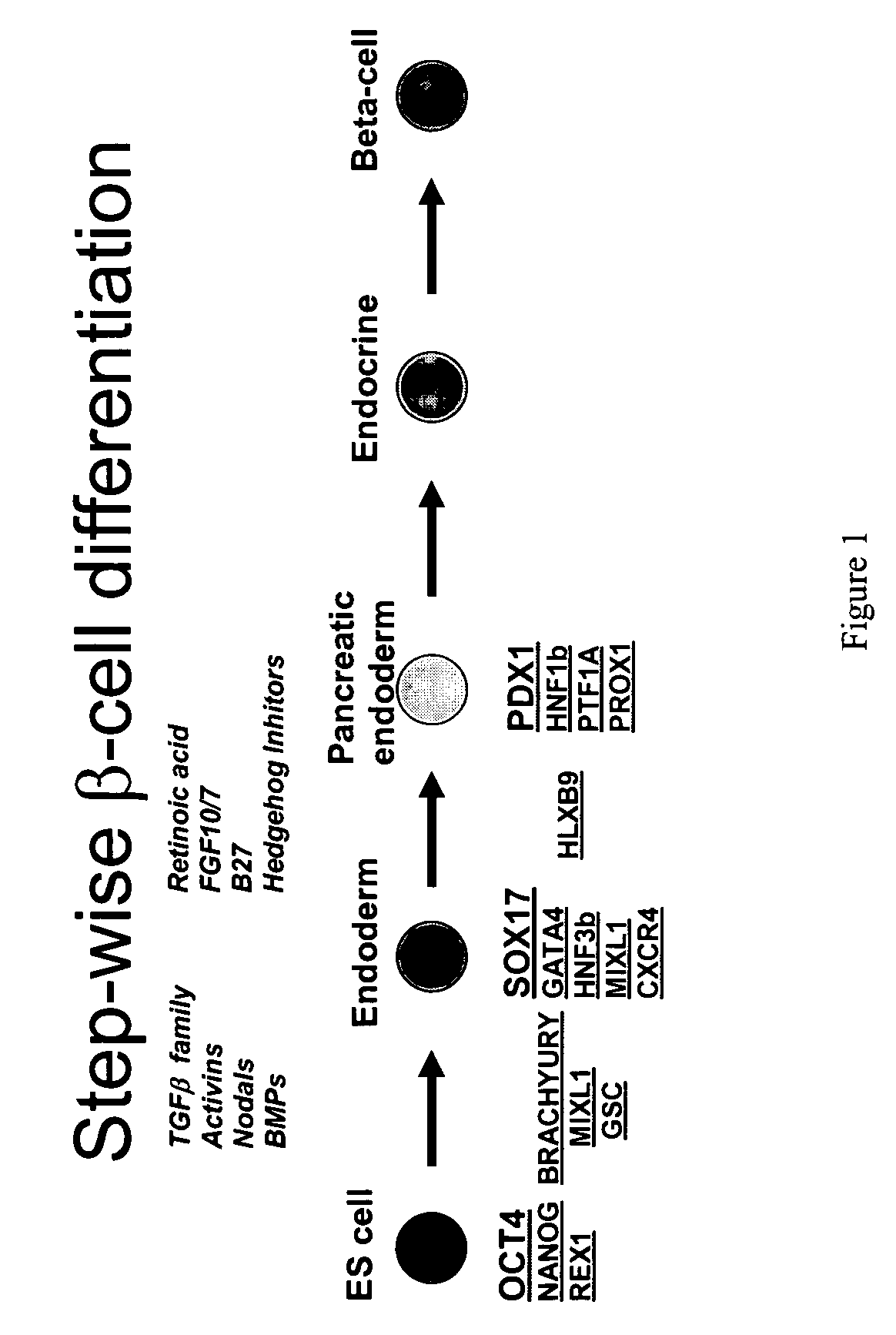
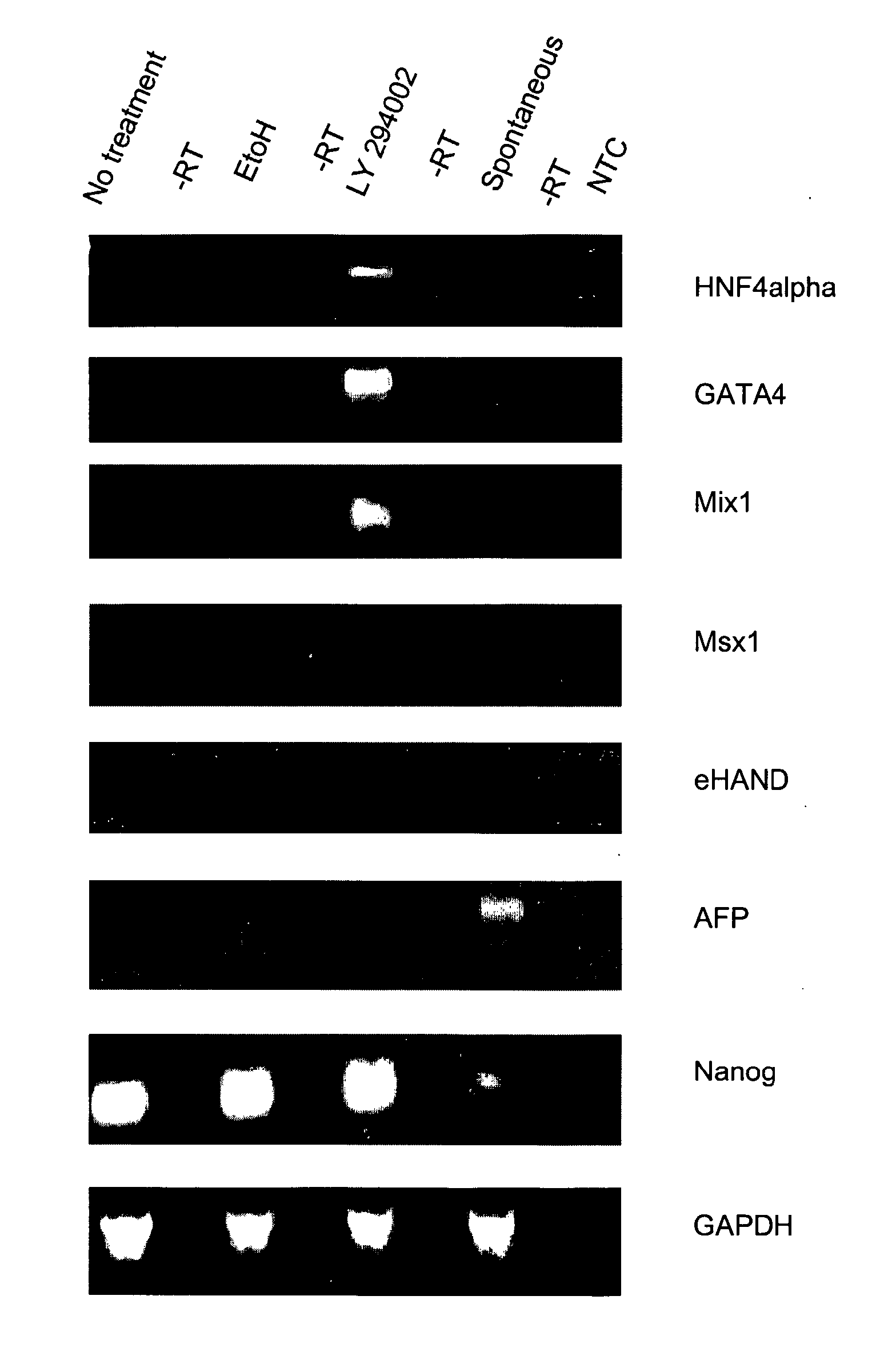
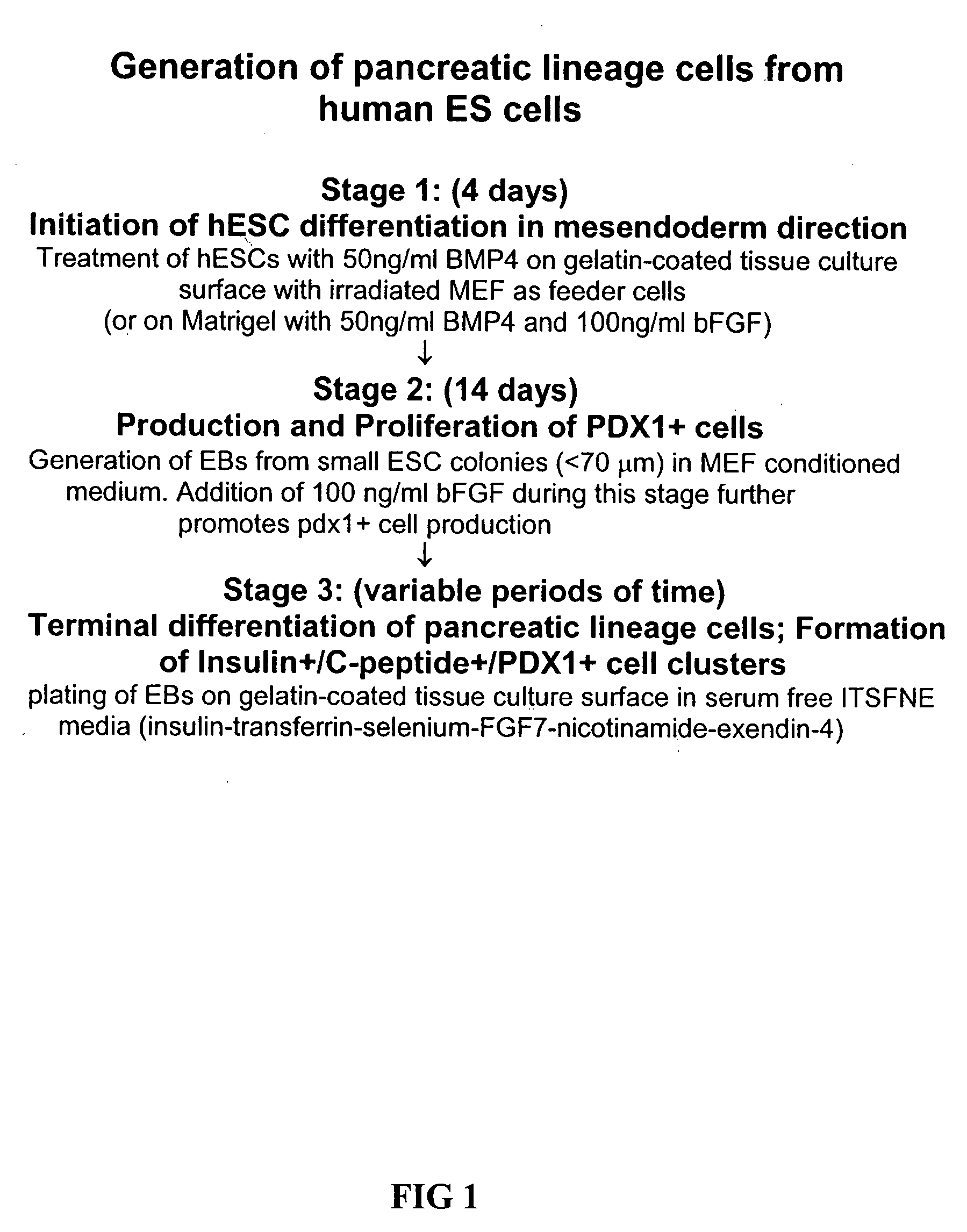
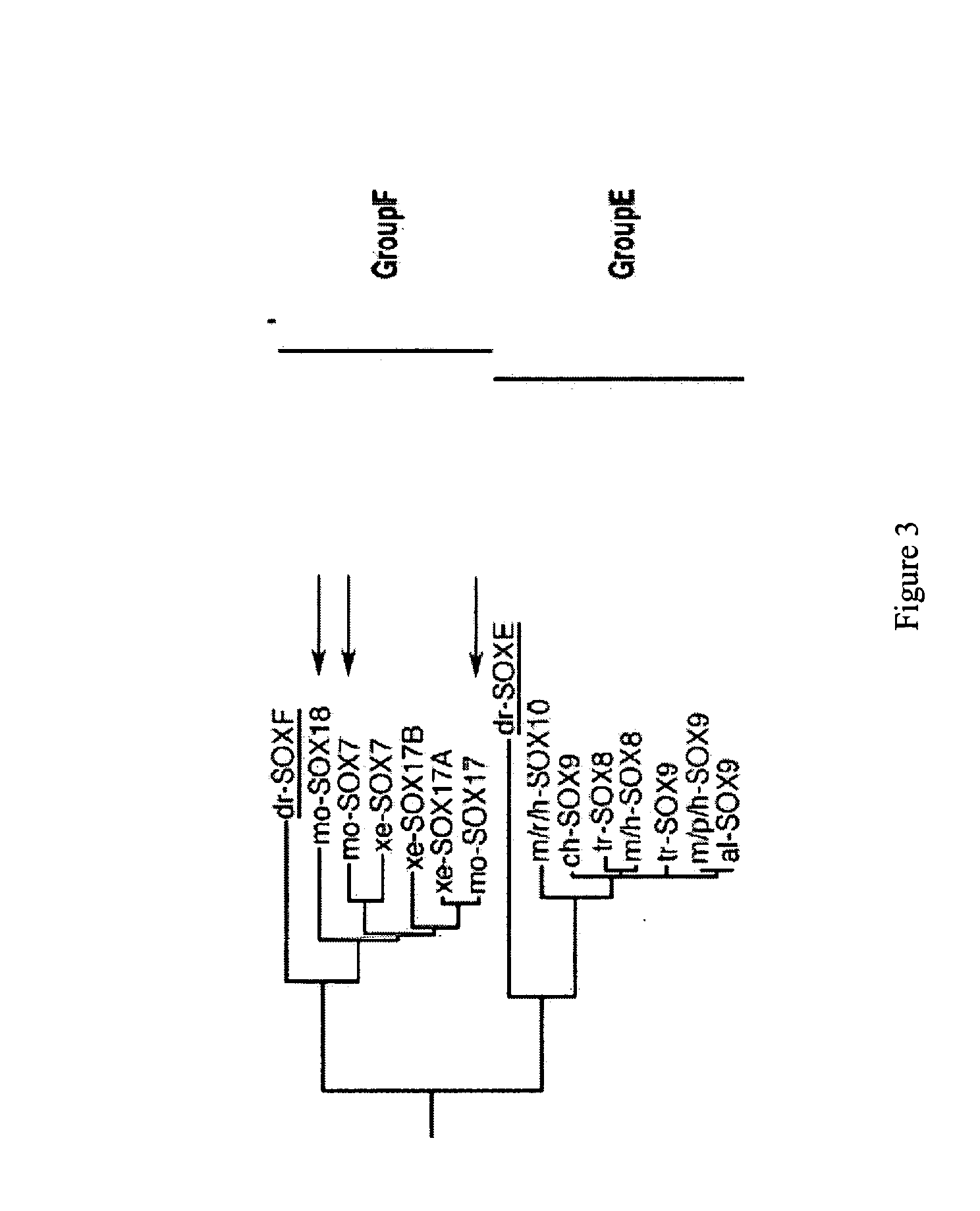
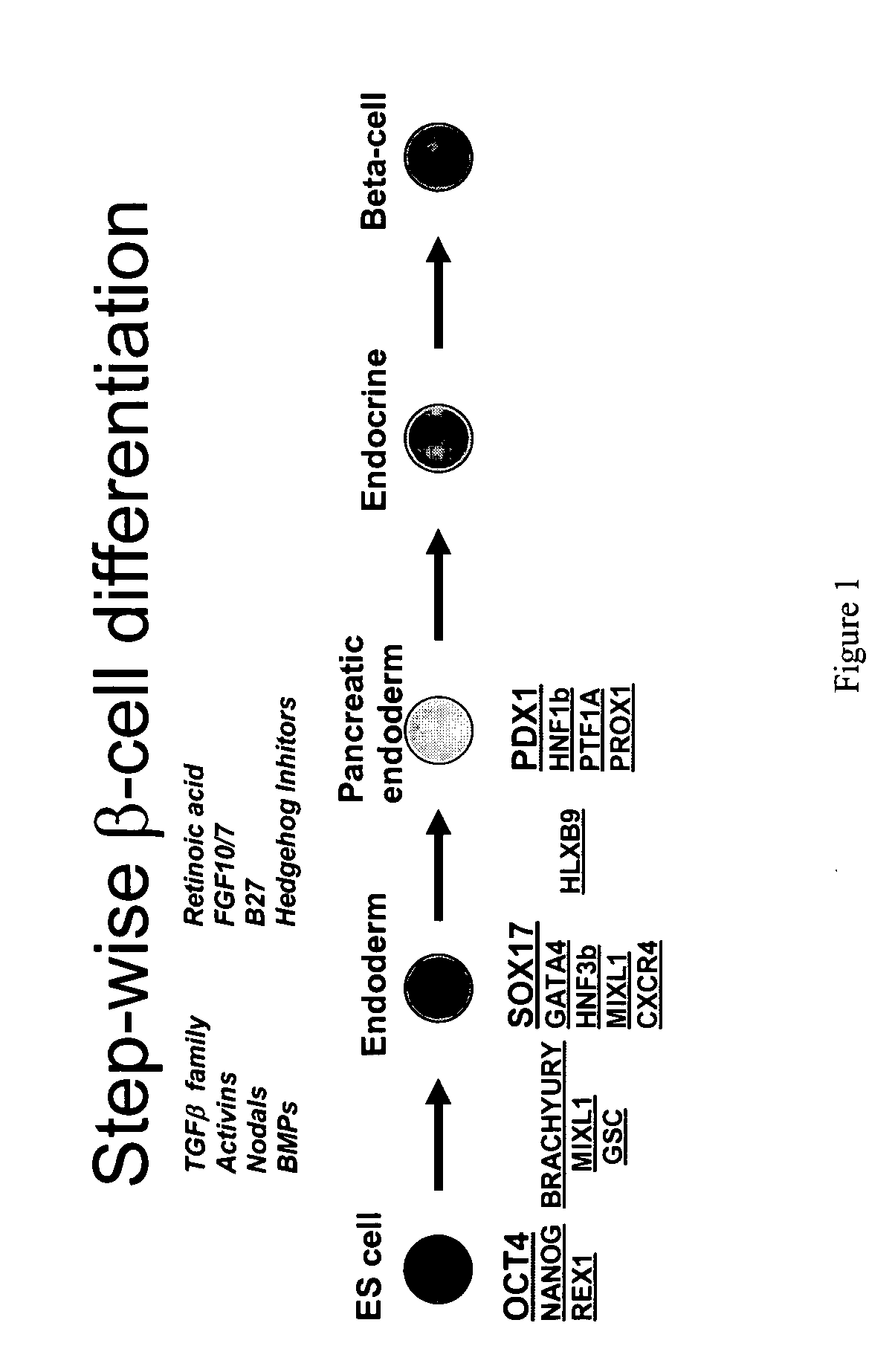
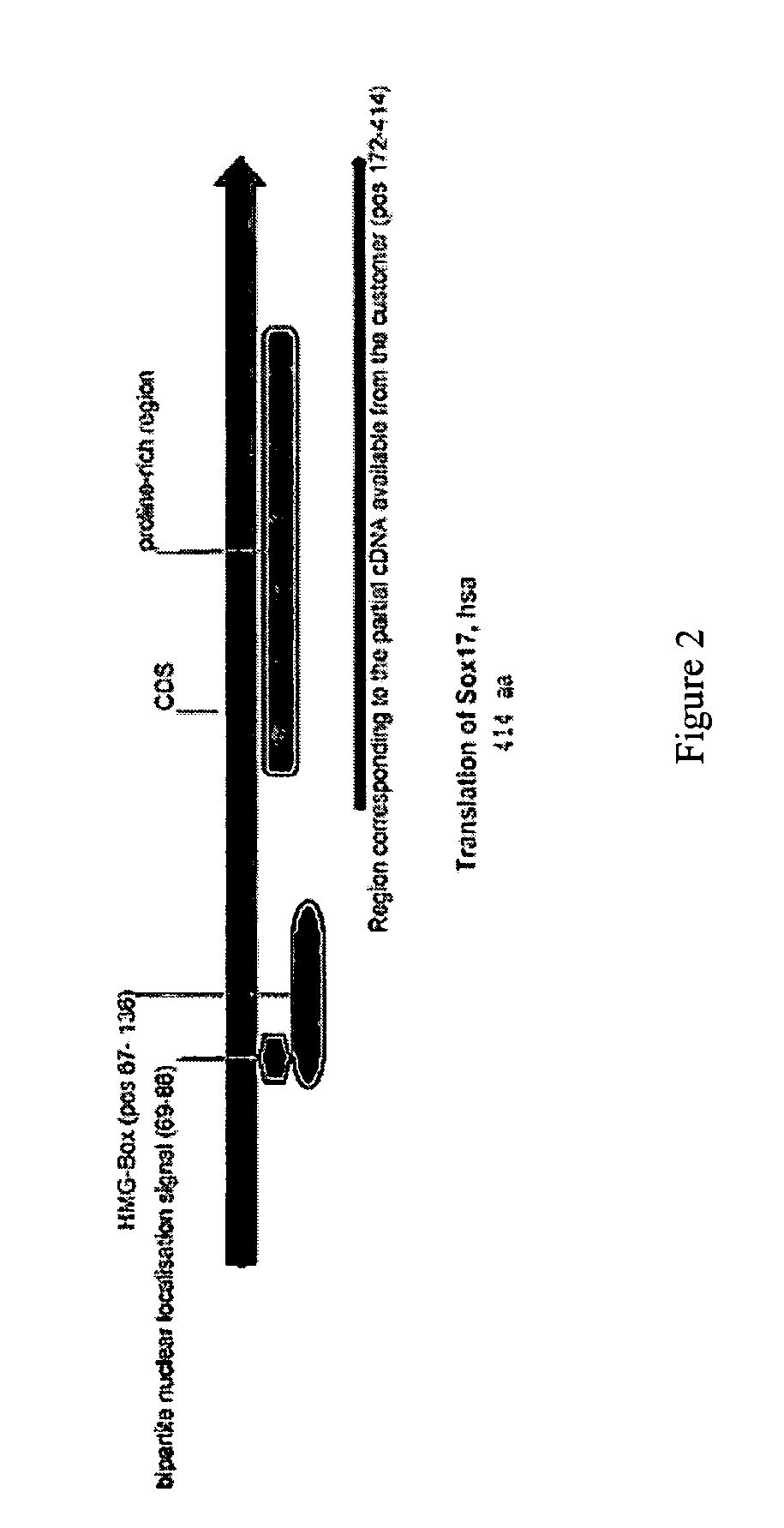
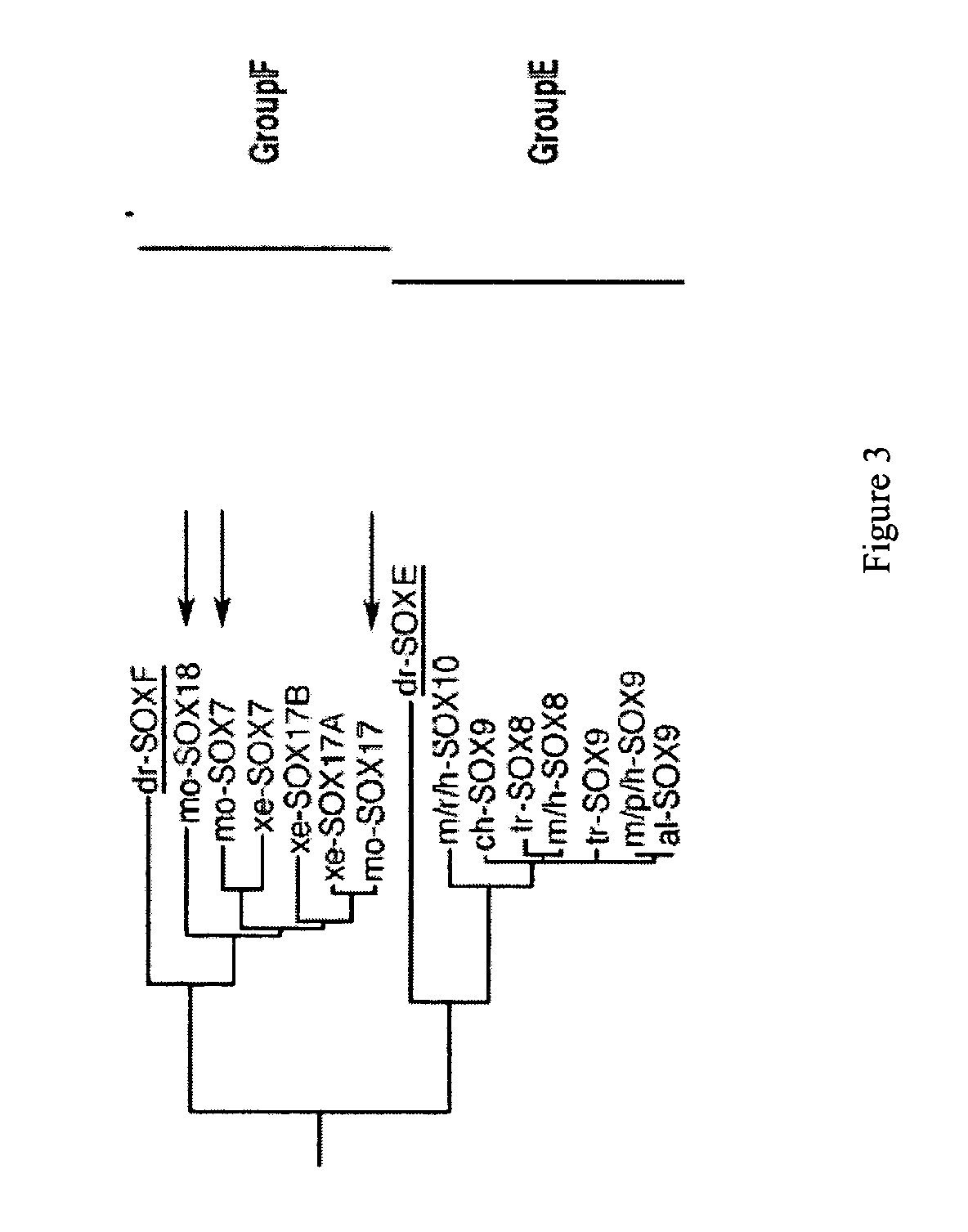
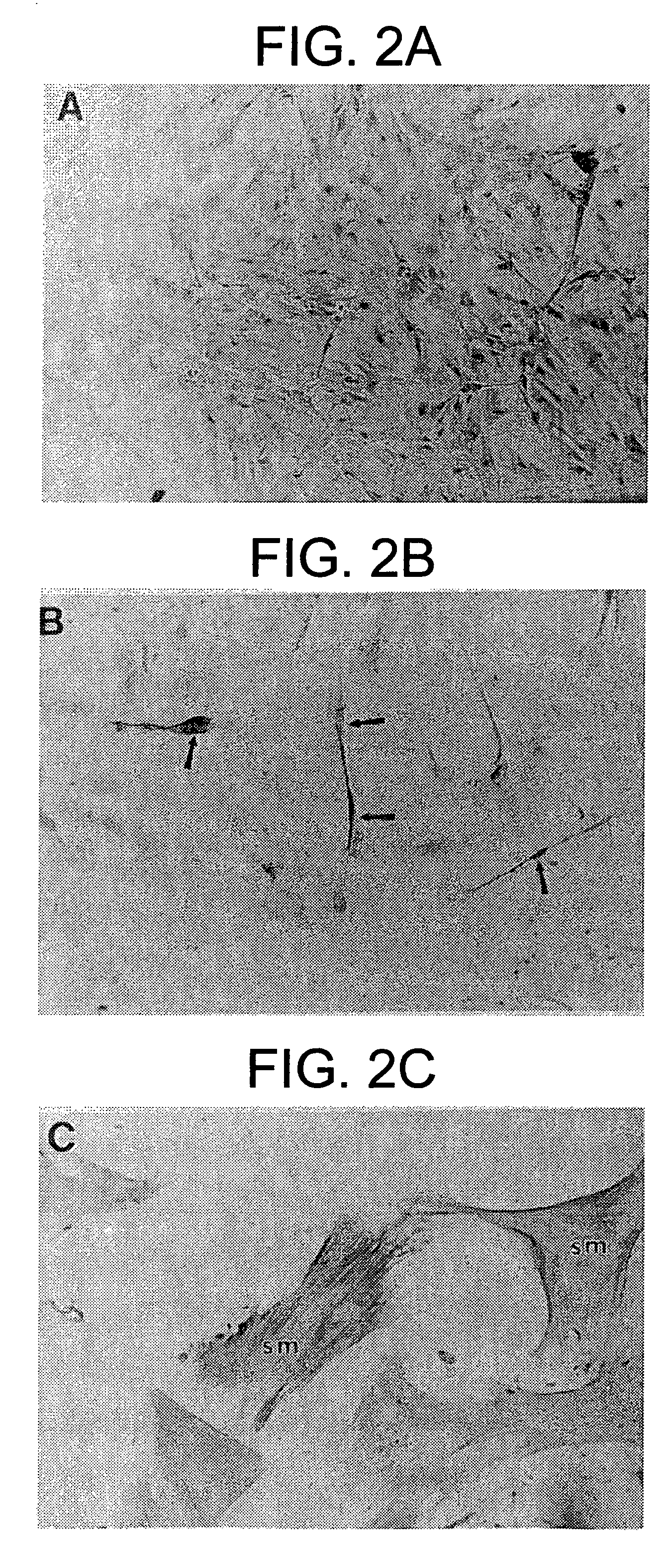
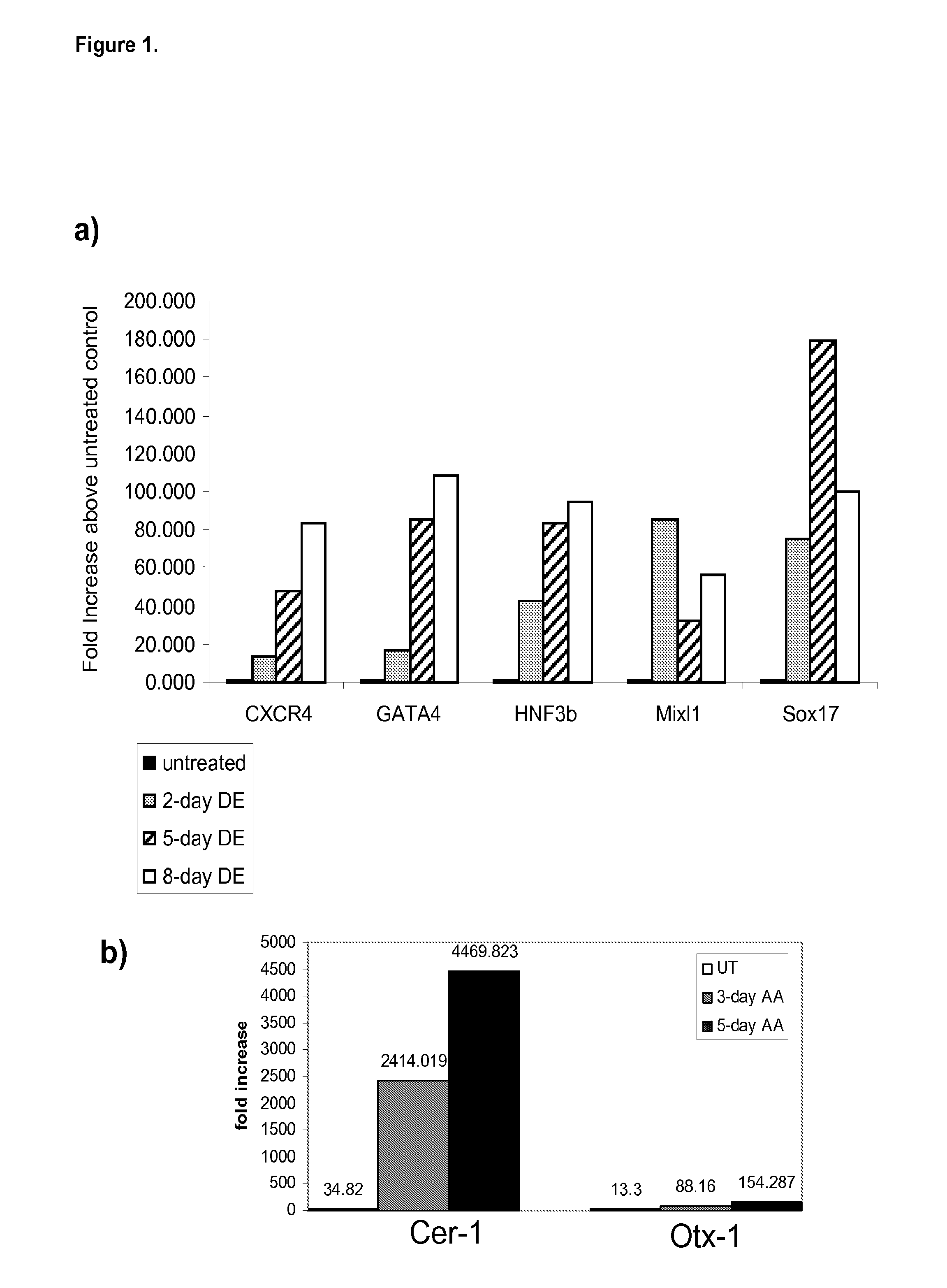
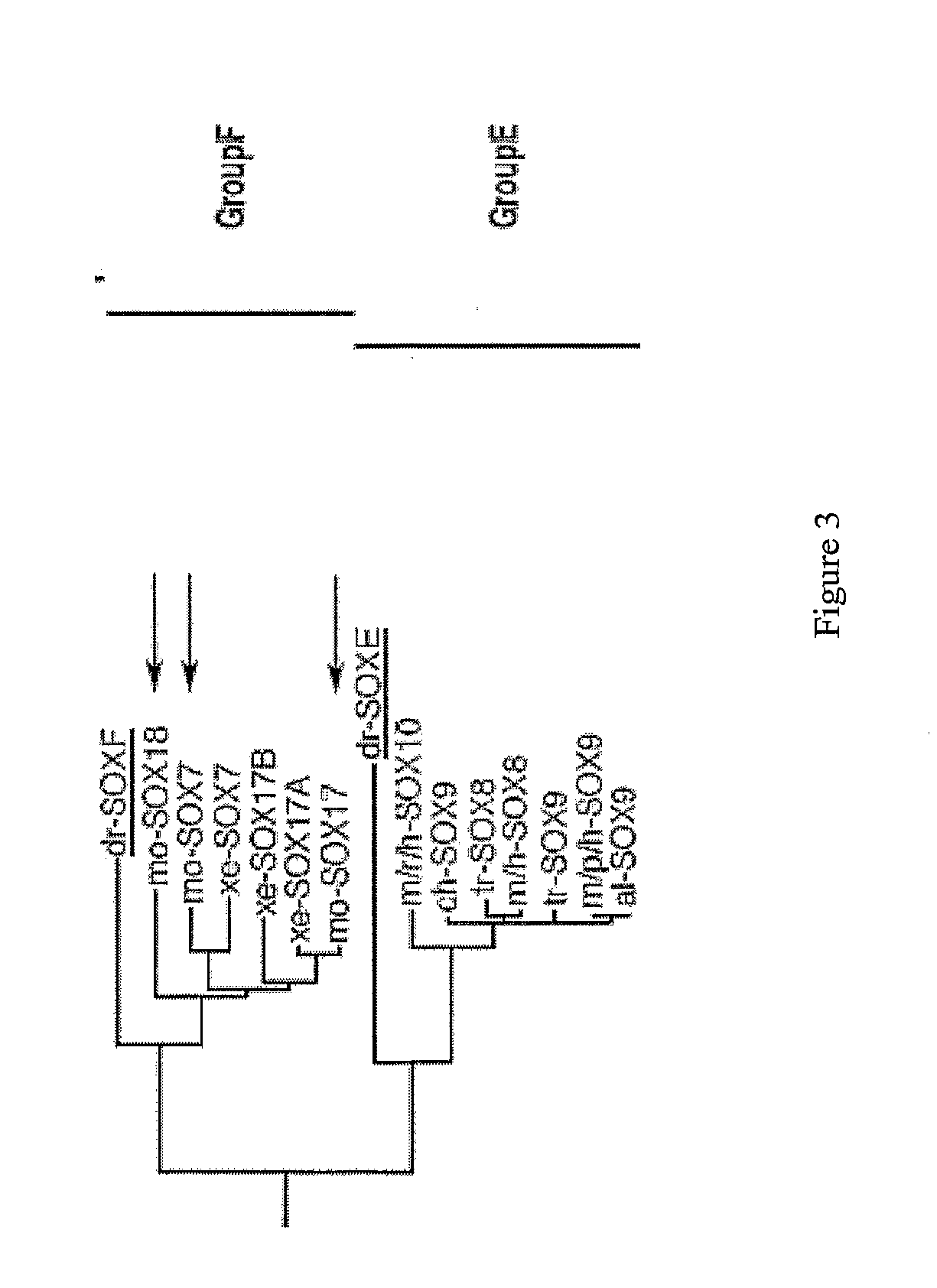
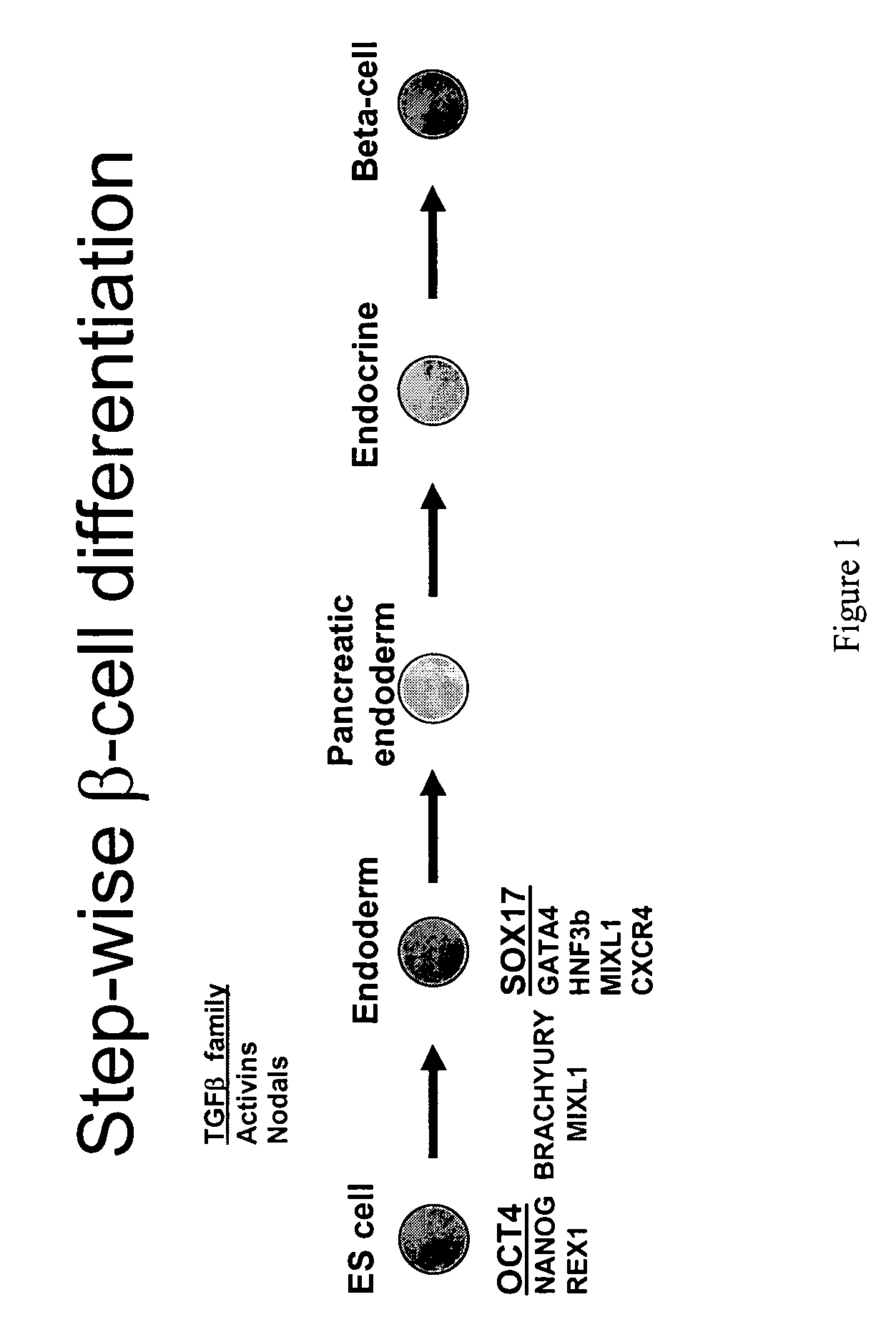
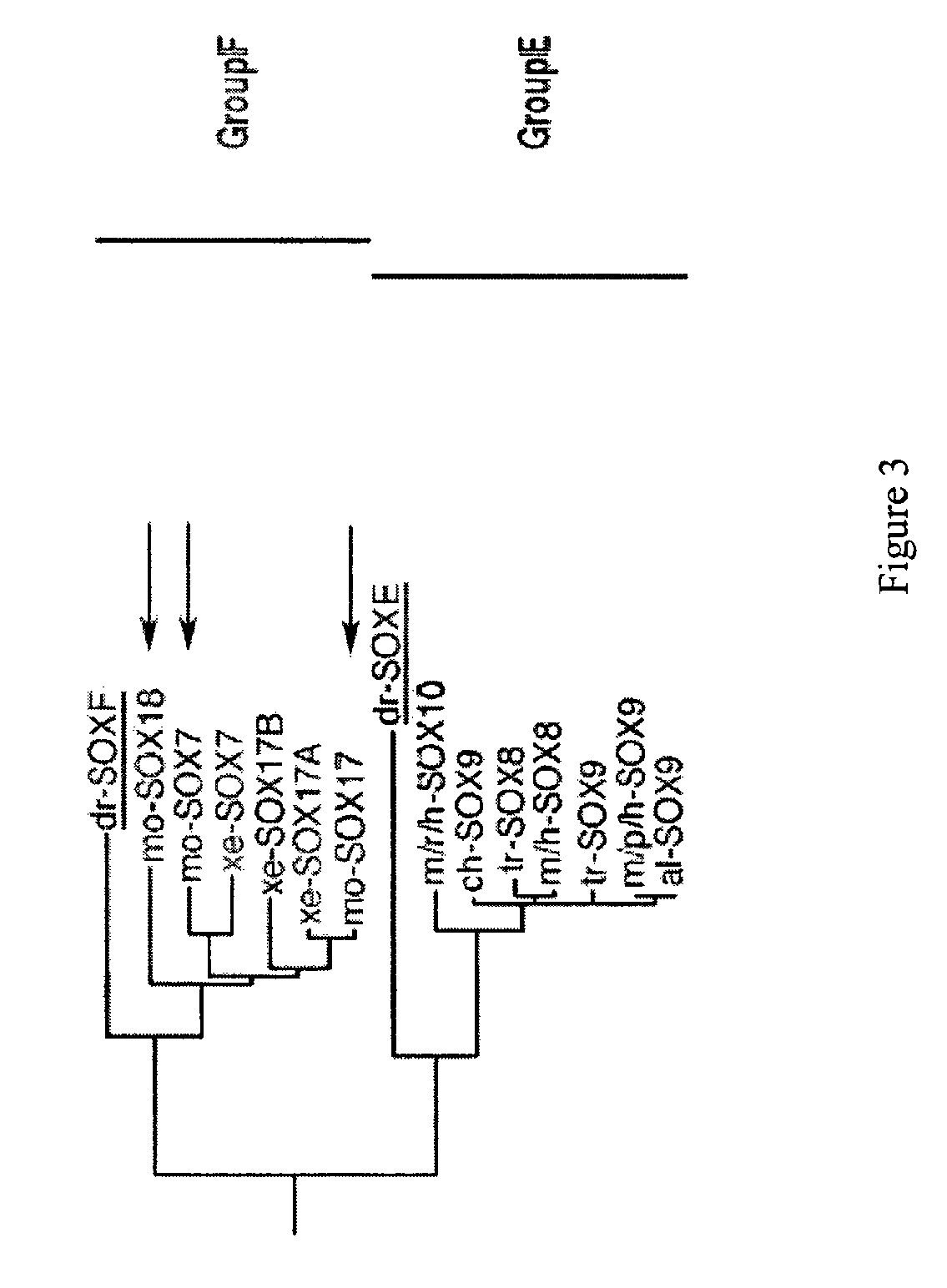
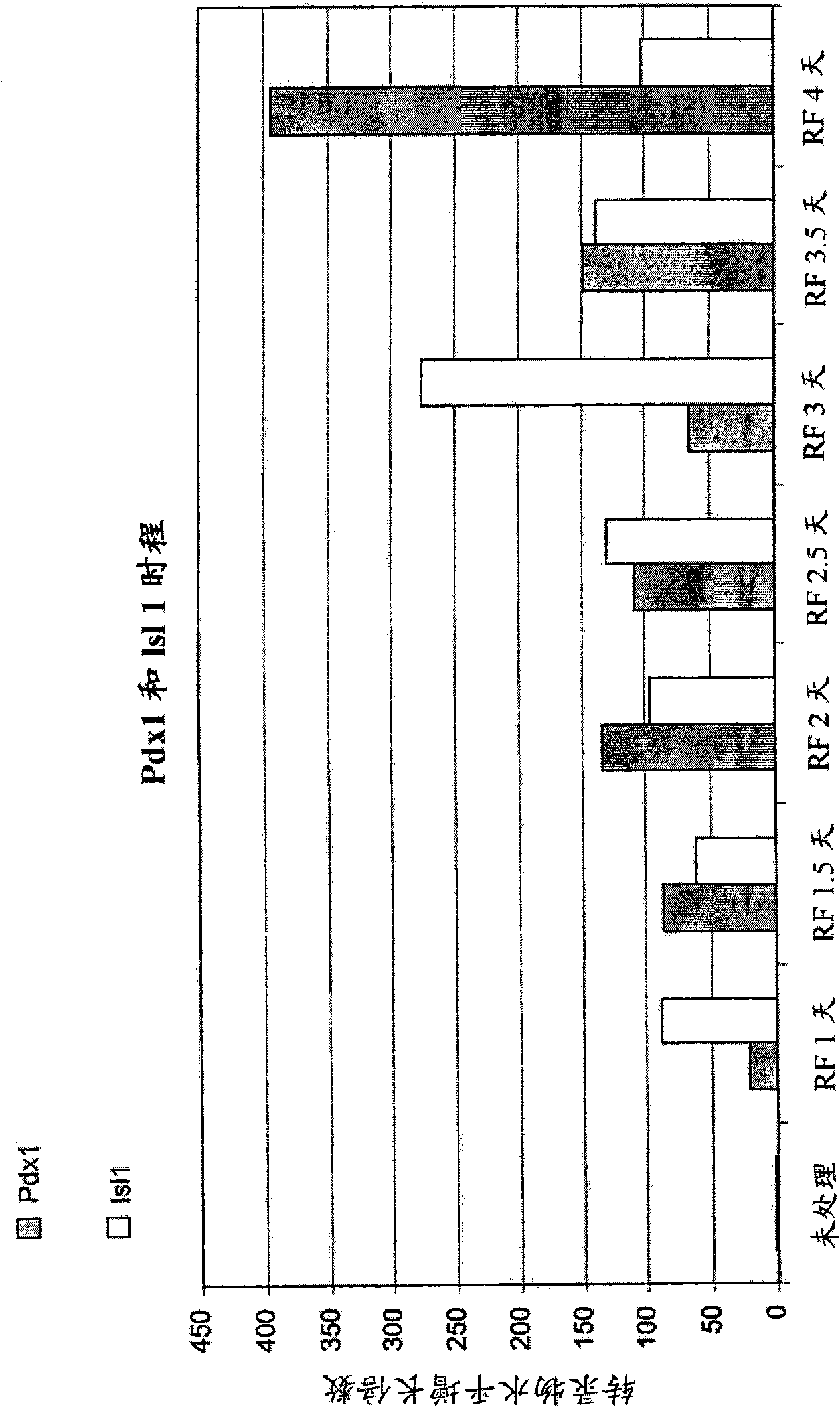
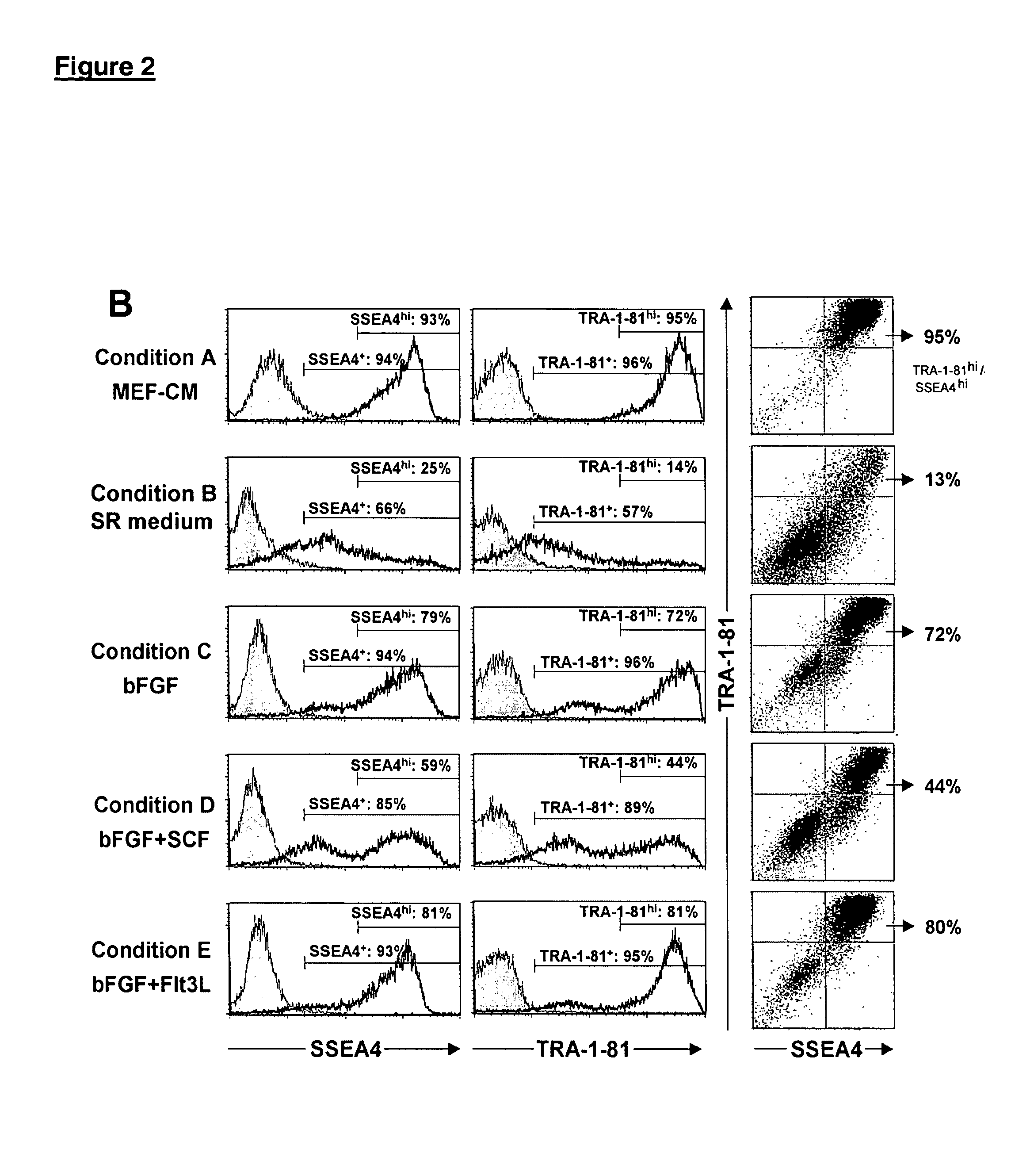
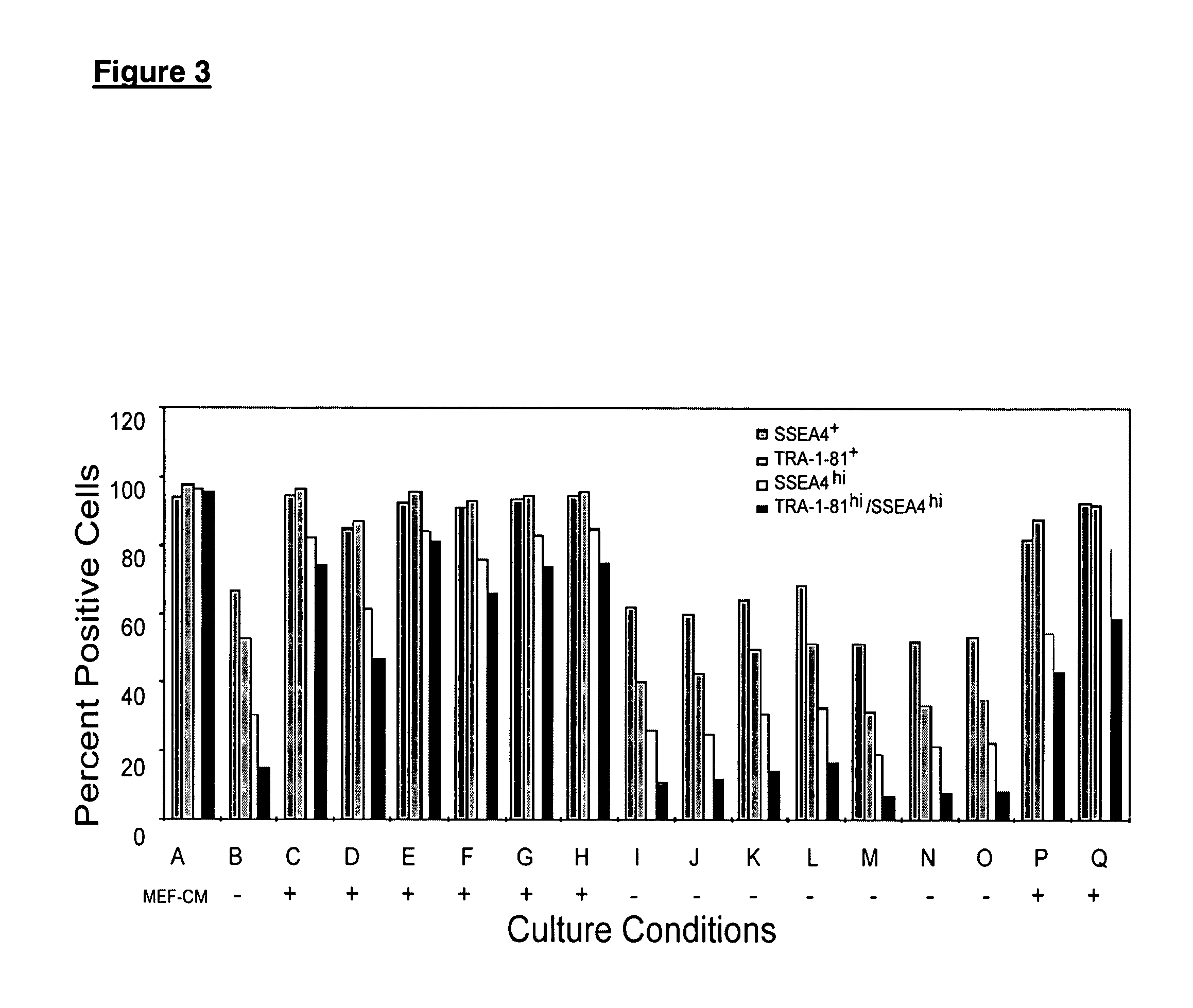
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
485 results about "Germ layer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
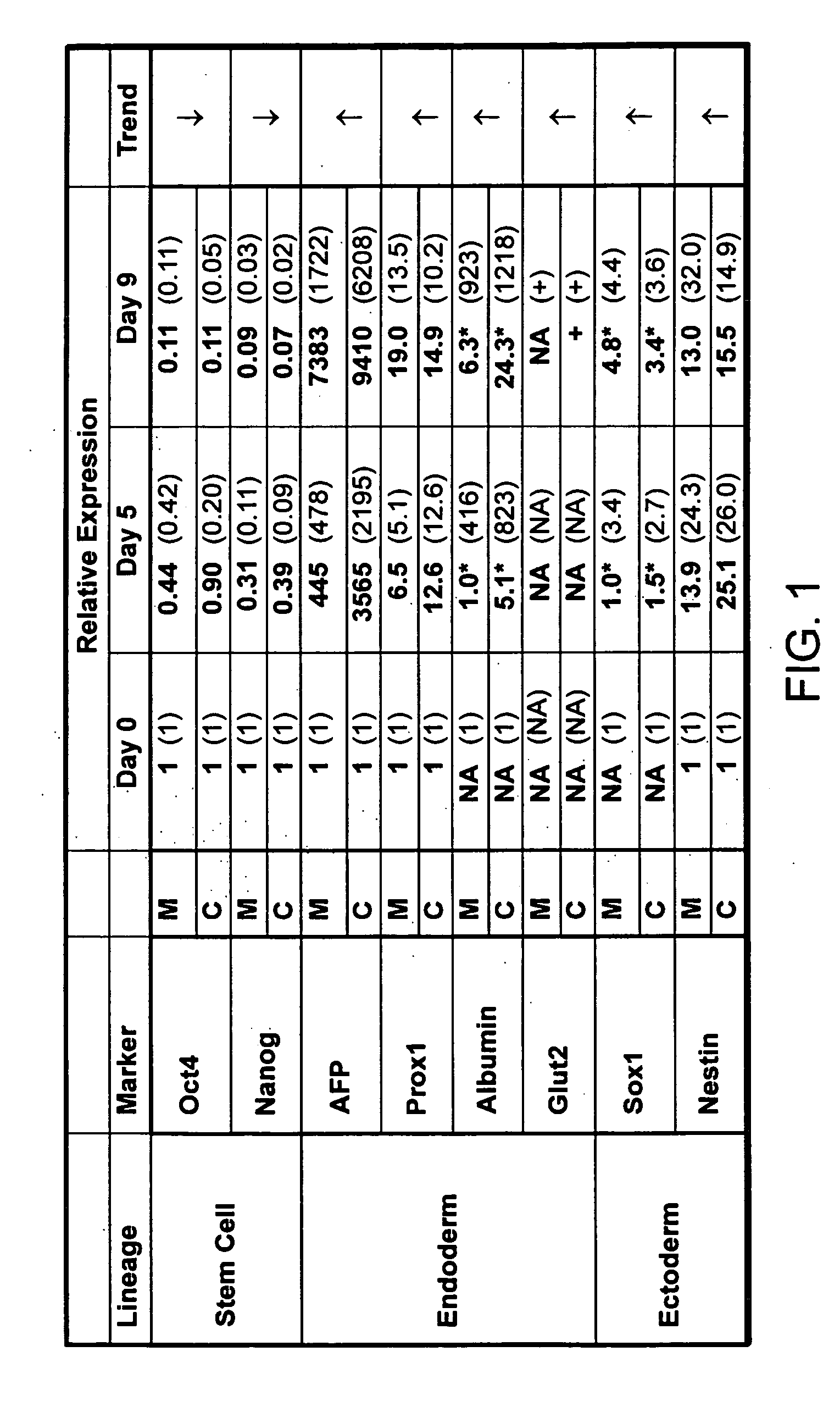
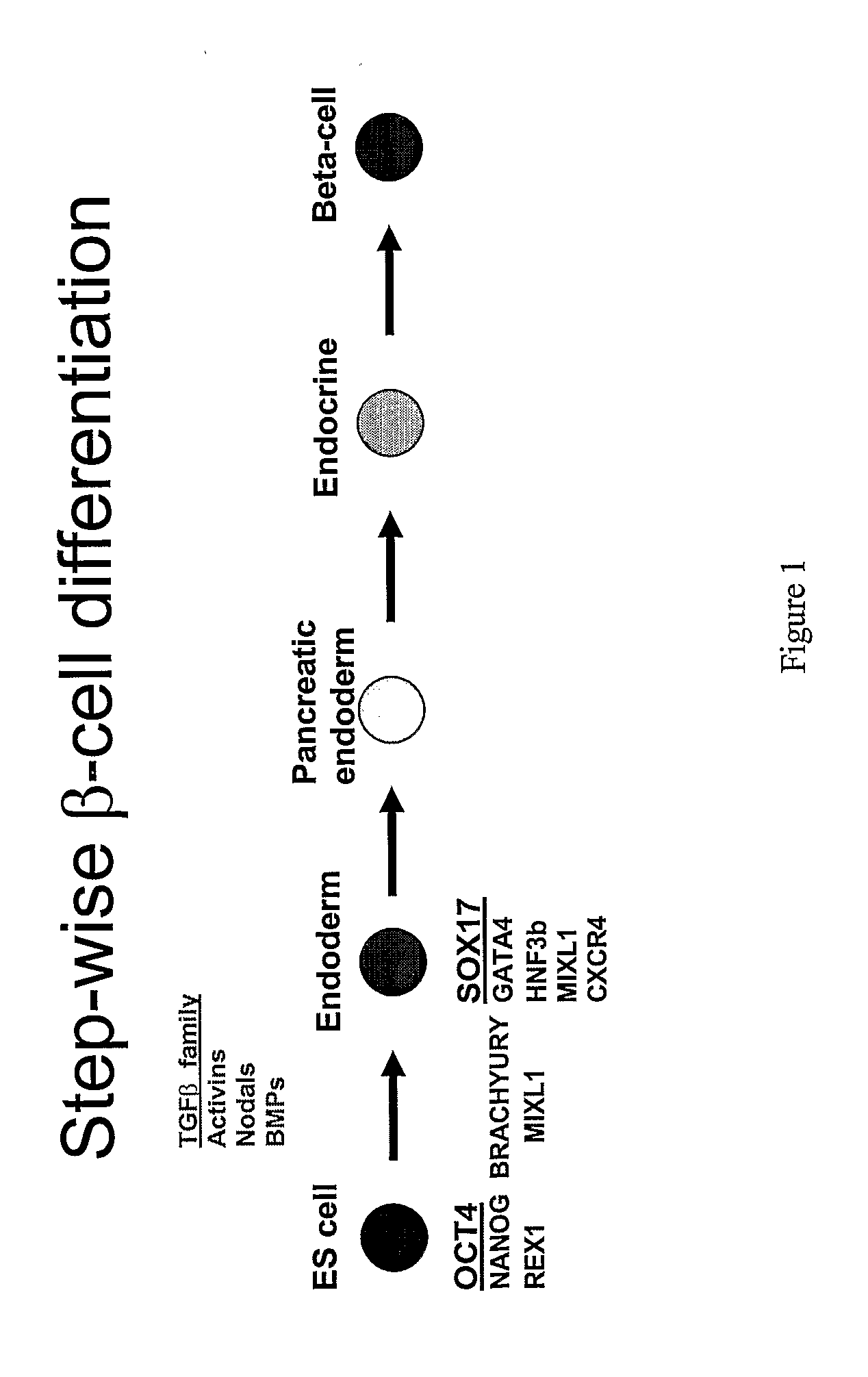
A germ layer is a primary layer of cells that forms during embryonic development. The three germ layers in vertebrates are particularly pronounced; however, all eumetazoans (animals more complex than the sponge) produce two or three primary germ layers. Some animals, like cnidarians, produce two germ layers (the ectoderm and endoderm) making them diploblastic. Other animals such as chordates produce a third layer (the mesoderm) between these two layers, making them triploblastic. Germ layers eventually give rise to all of an animal’s tissues and organs through the process of organogenesis.
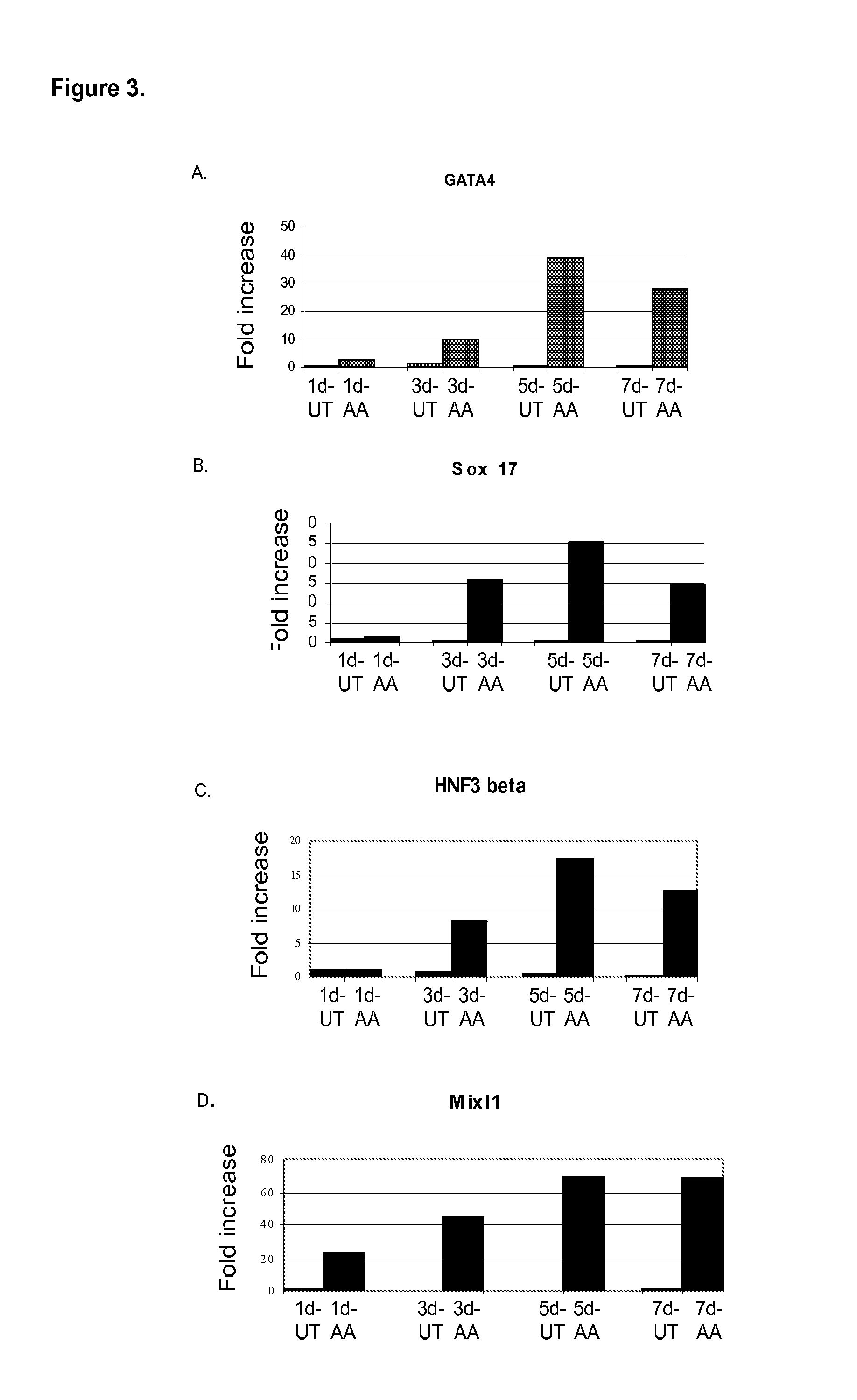
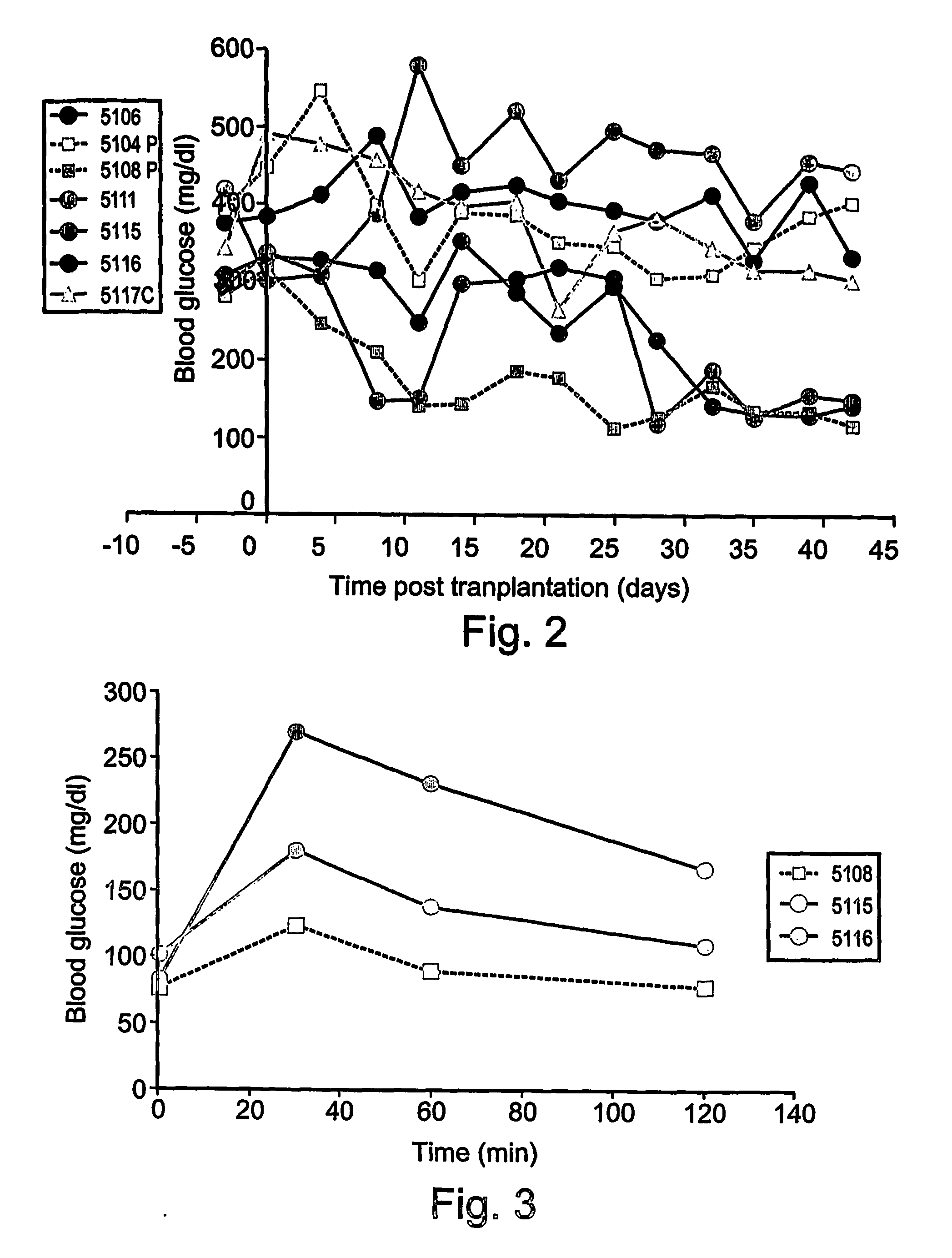
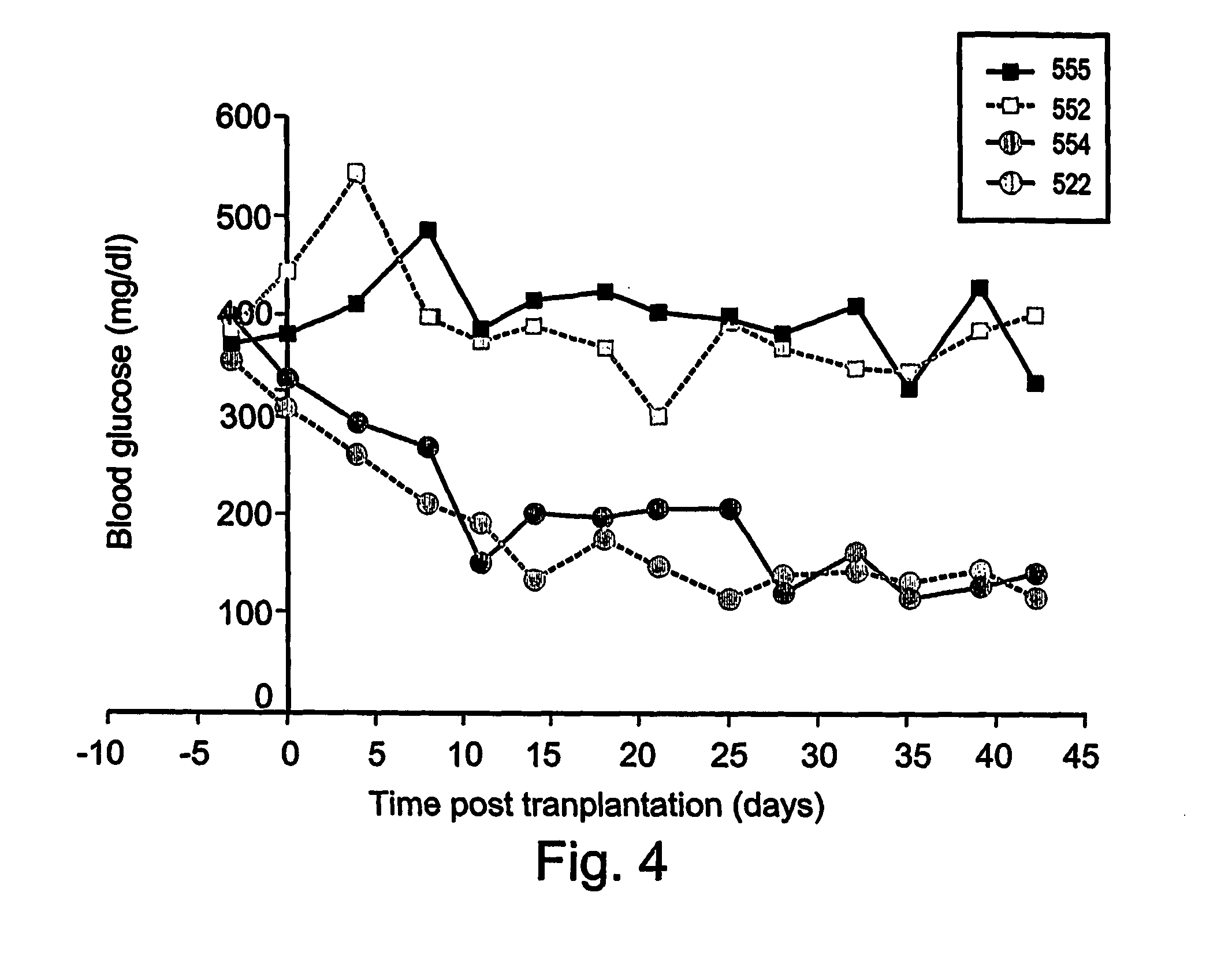
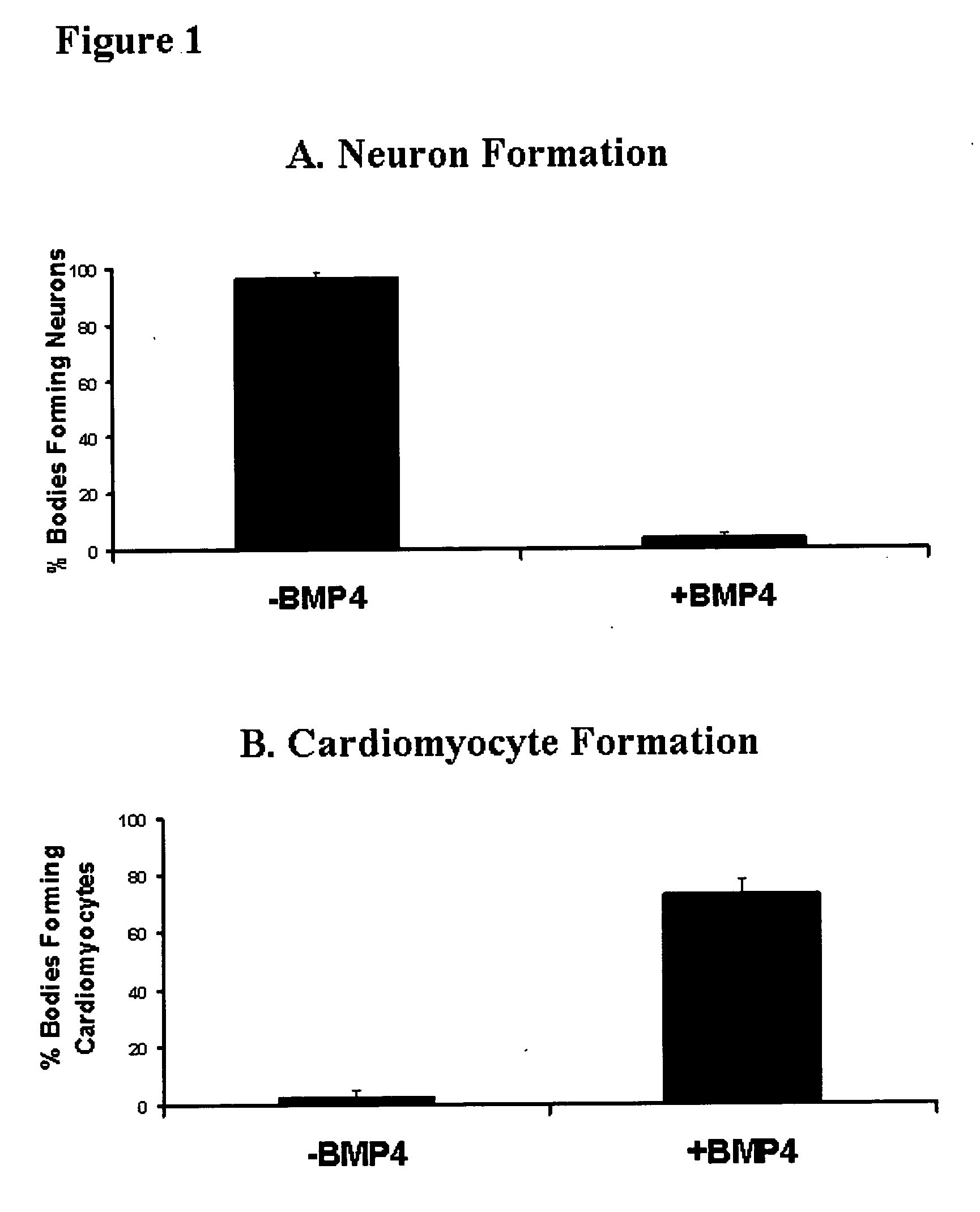
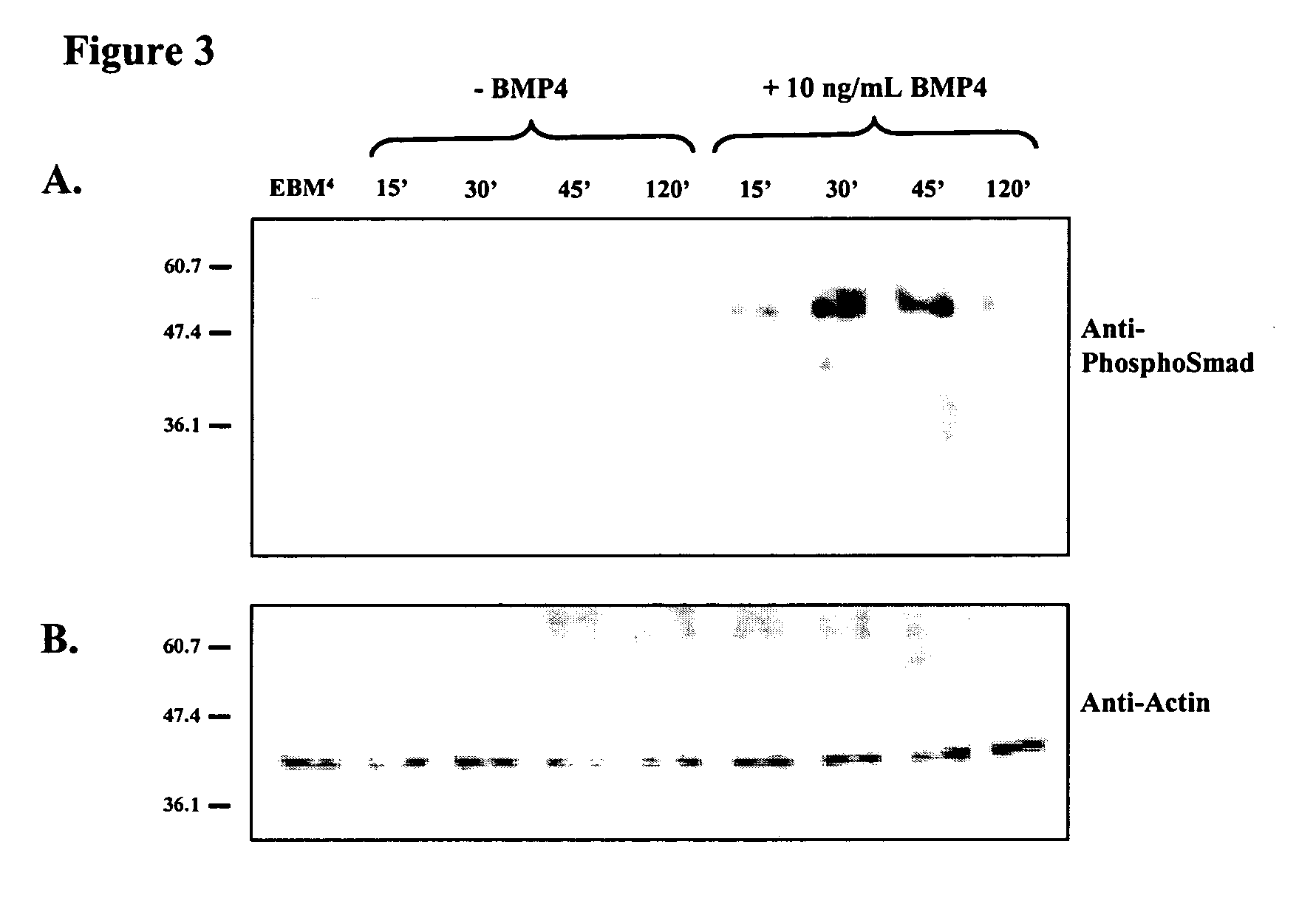
Differentiation of human embryonic stem cells
ActiveUS20070254359A1Inhibits Notch signalingPancreatic cellsArtificial cell constructsGerm layerPluripotential stem cell
The present invention provides methods to promote the differentiation of pluripotent stem cells. In particular, the present invention provides an improved method for the formation of pancreatic endoderm, pancreatic hormone expressing cells and pancreatic hormone secreting cells. The present invention also provides methods to promote the differentiation of pluripotent stem cells without the use of a feeder cell layer.
Owner:LIFESCAN INC
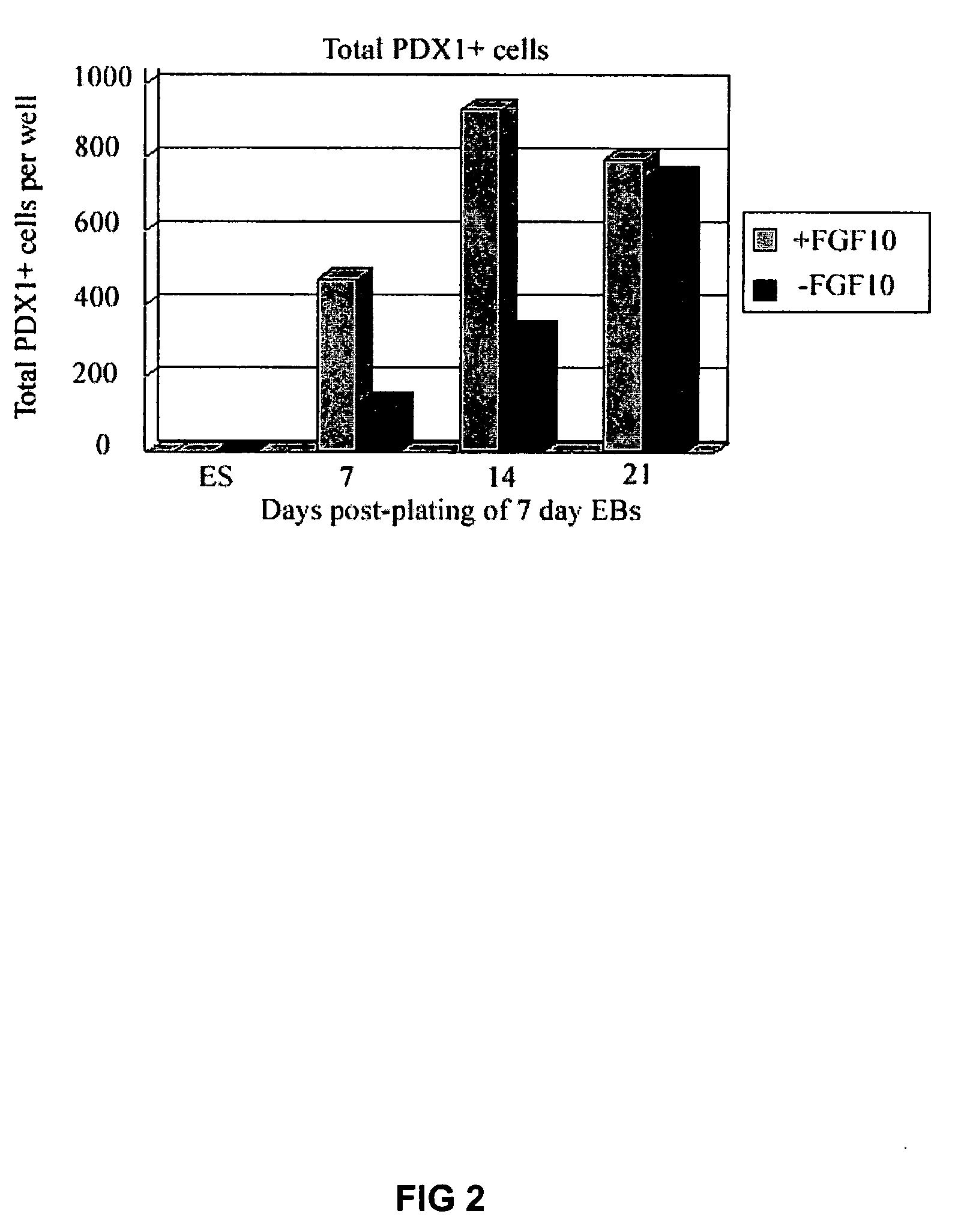
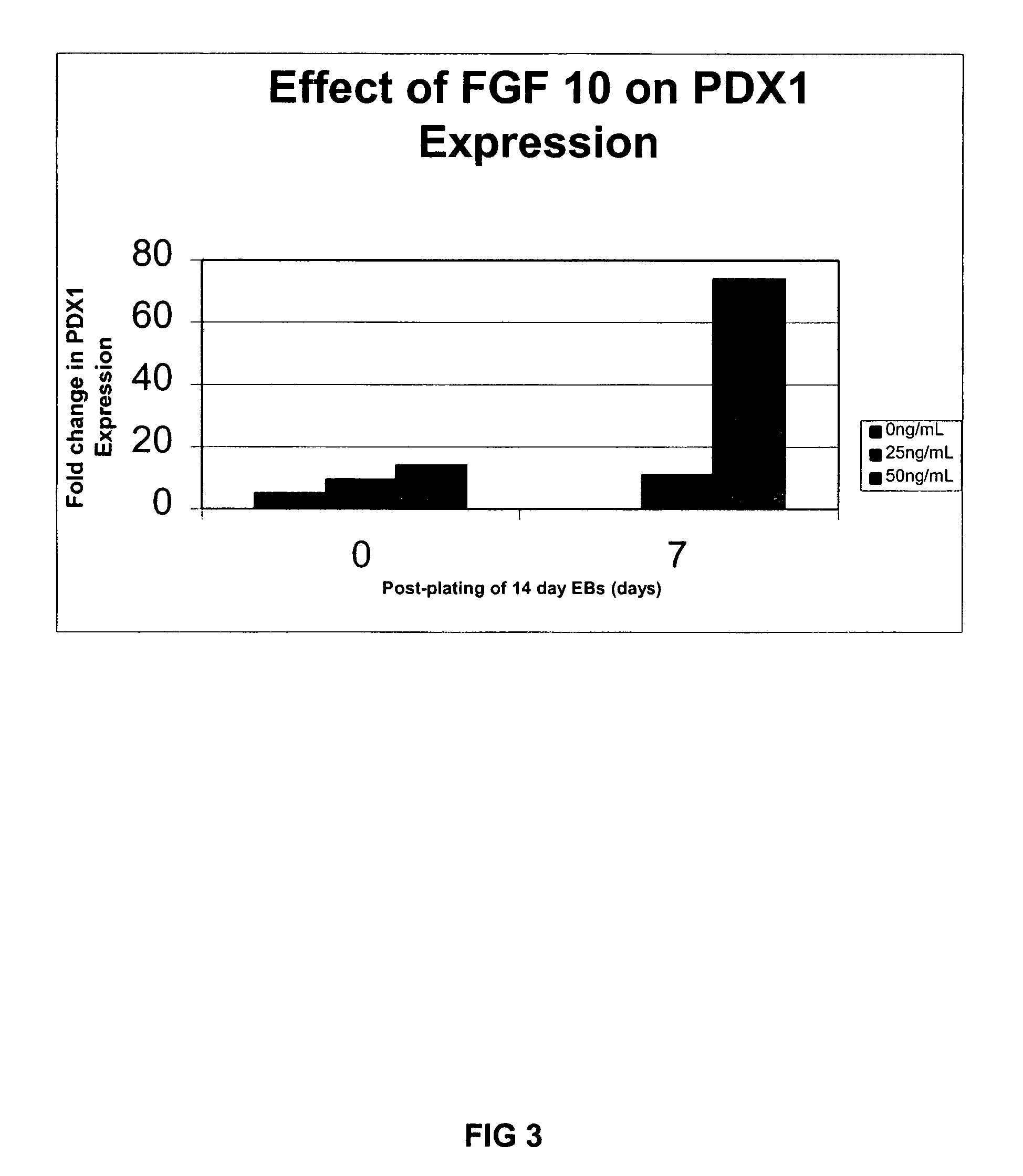
PDX1 expressing endoderm
InactiveUS20050266554A1Increase differentiationIncrease productionGastrointestinal cellsDiagnosticsGerm layerCell type
Disclosed herein are cell cultures comprising PDX1-positive endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified PDX1-positive endoderm cells as well as methods for enriching, isolating and purifying PDX1-positive endoderm cells from other cell types. Methods of identifying differentiation factors capable of promoting the differentiation of endoderm cells, such as PDX1-positive foregut endoderm cells and PDX1-negative definitive endoderm cells, are also disclosed.
Owner:CYTHERA
Multiple mesodermal lineage differentiation potentials for adipose tissue-derived stromal cells and uses thereof
InactiveUS6555374B1Enhance and inhibit differentiationIncrease differentiationMicrobiological testing/measurementMammal material medical ingredientsGerm layerSmooth muscle
The invention relates to methods and compositions for the differentiation of stromal cells from adipose tissue into hematopoietic supporting stromal cells and myocytes of both the skeletal and smooth muscle type. The cells produced by the methods are useful in providing a source of fully differentiated and functional cells for research, transplantation and development of tissue engineering products for the treatment of human diseases and traumatic tissue injury repair.
Owner:COGNATE BIOSERVICES
Multipotent amniotic fetal stem cells
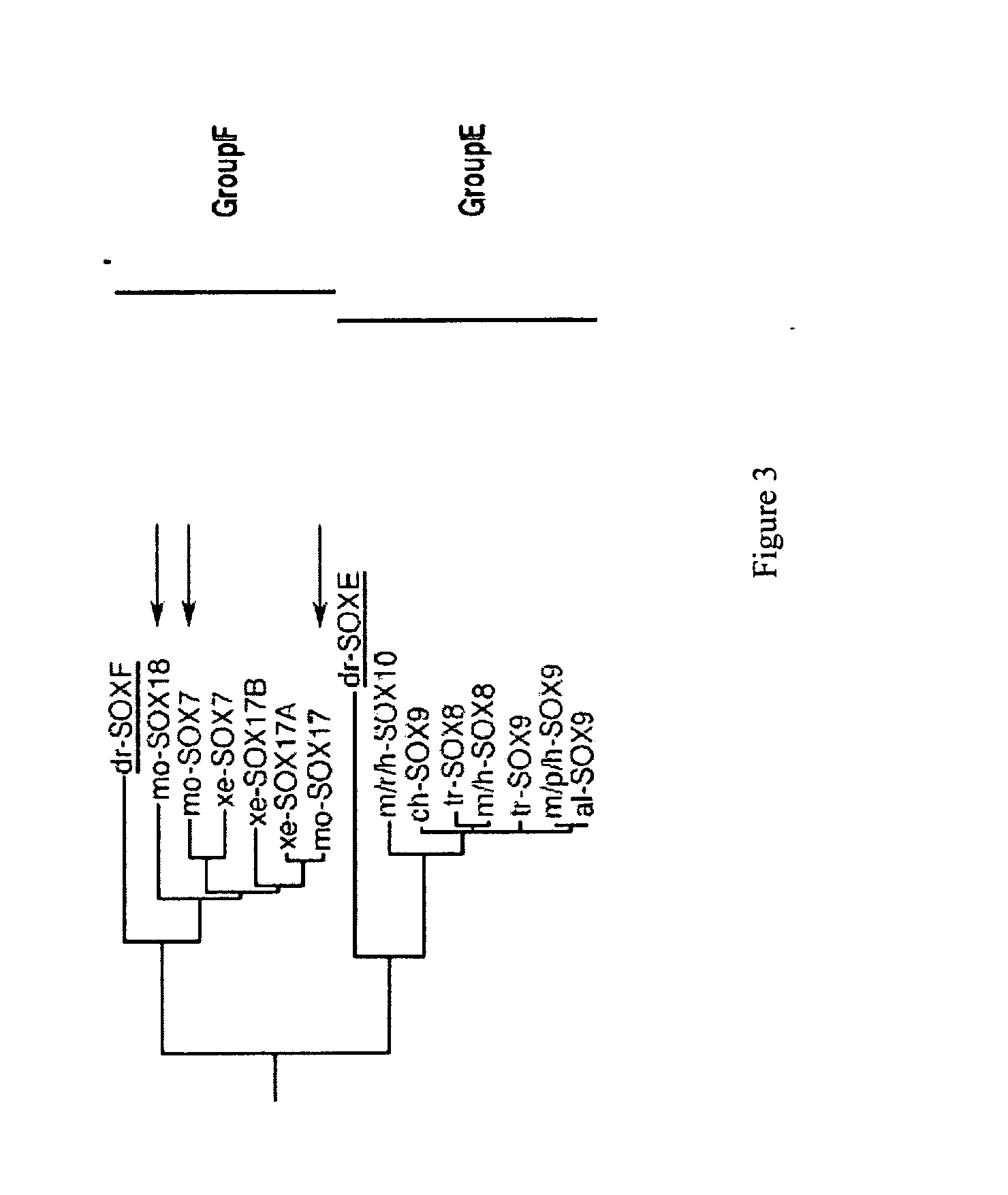
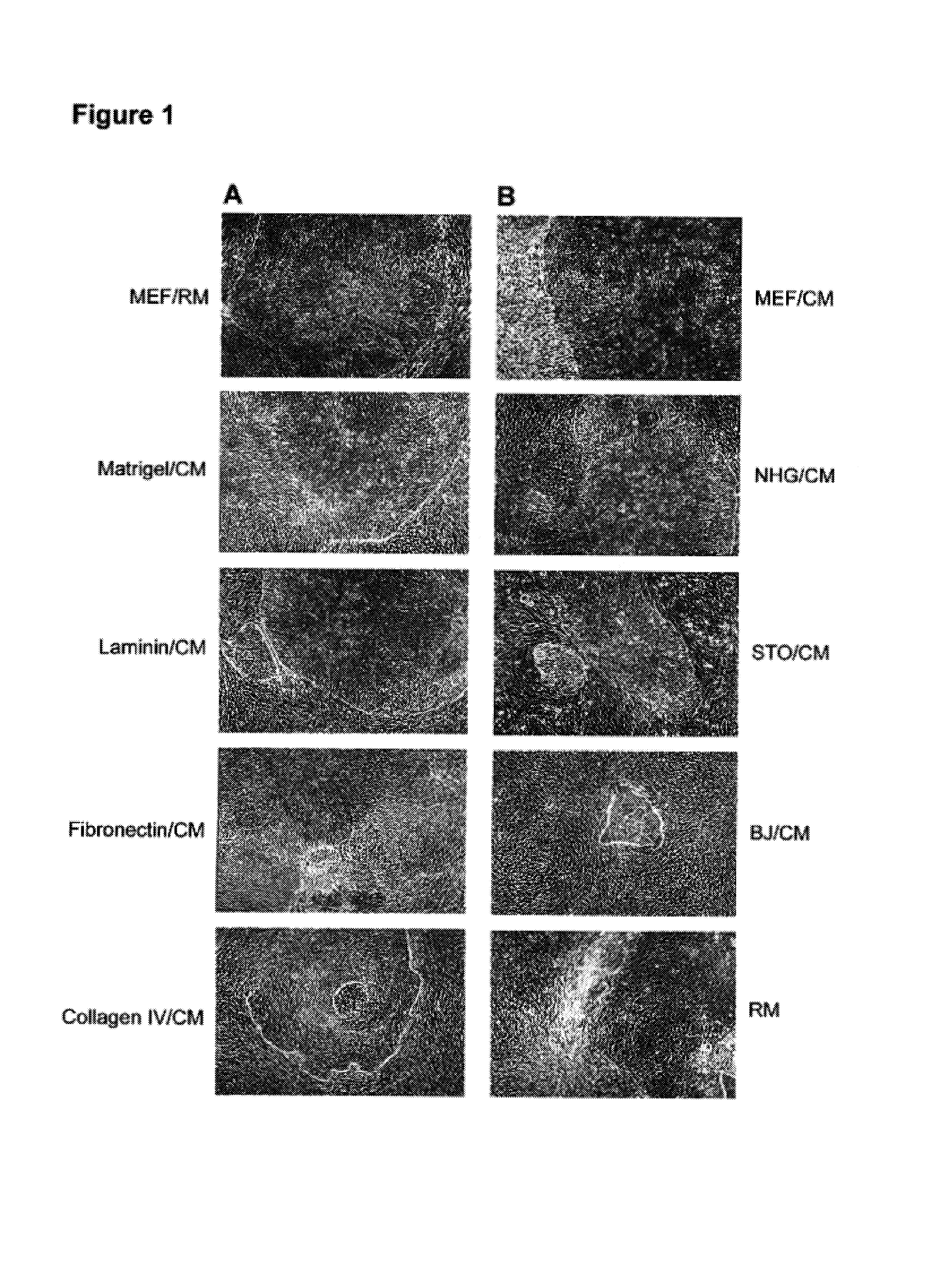
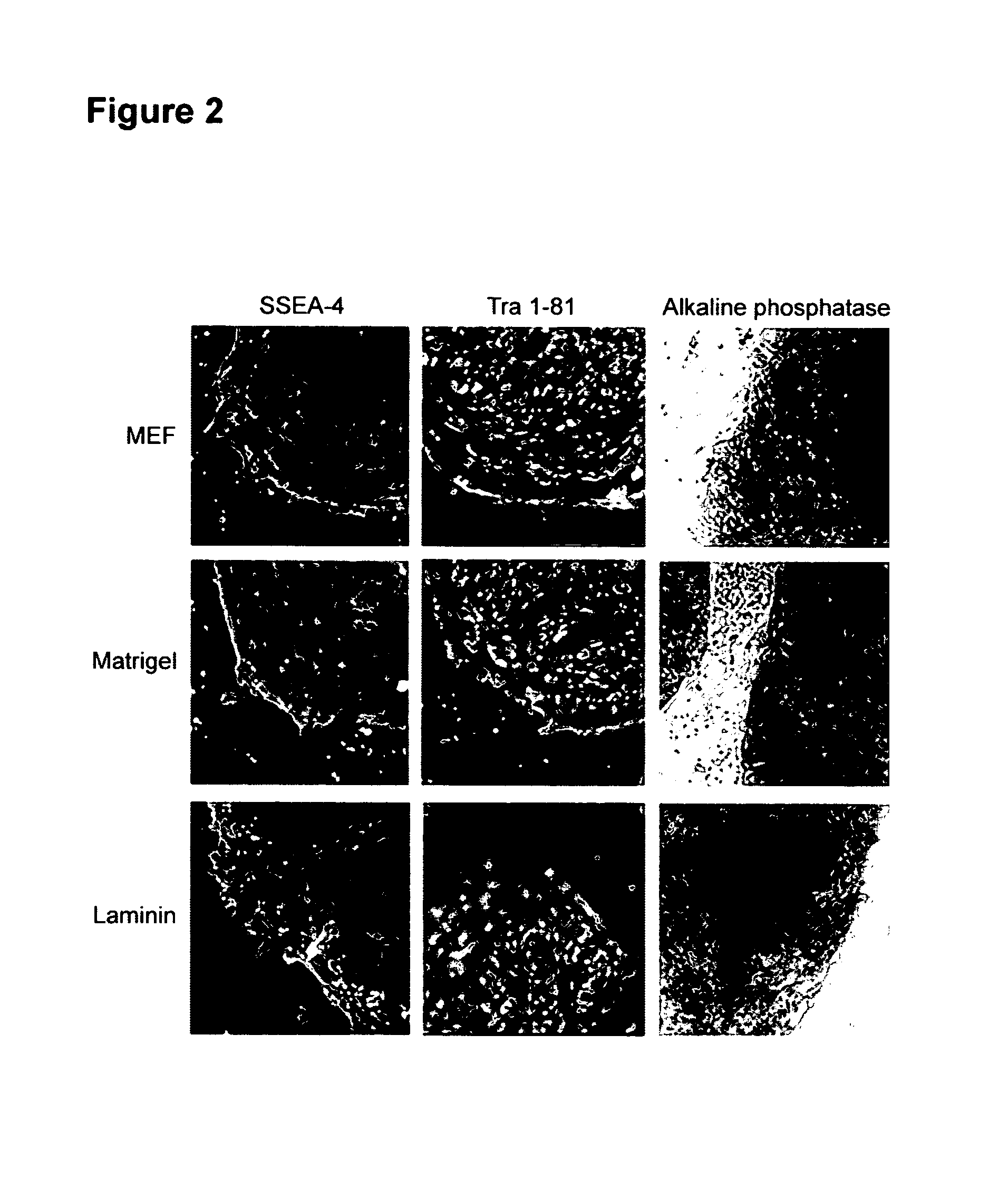
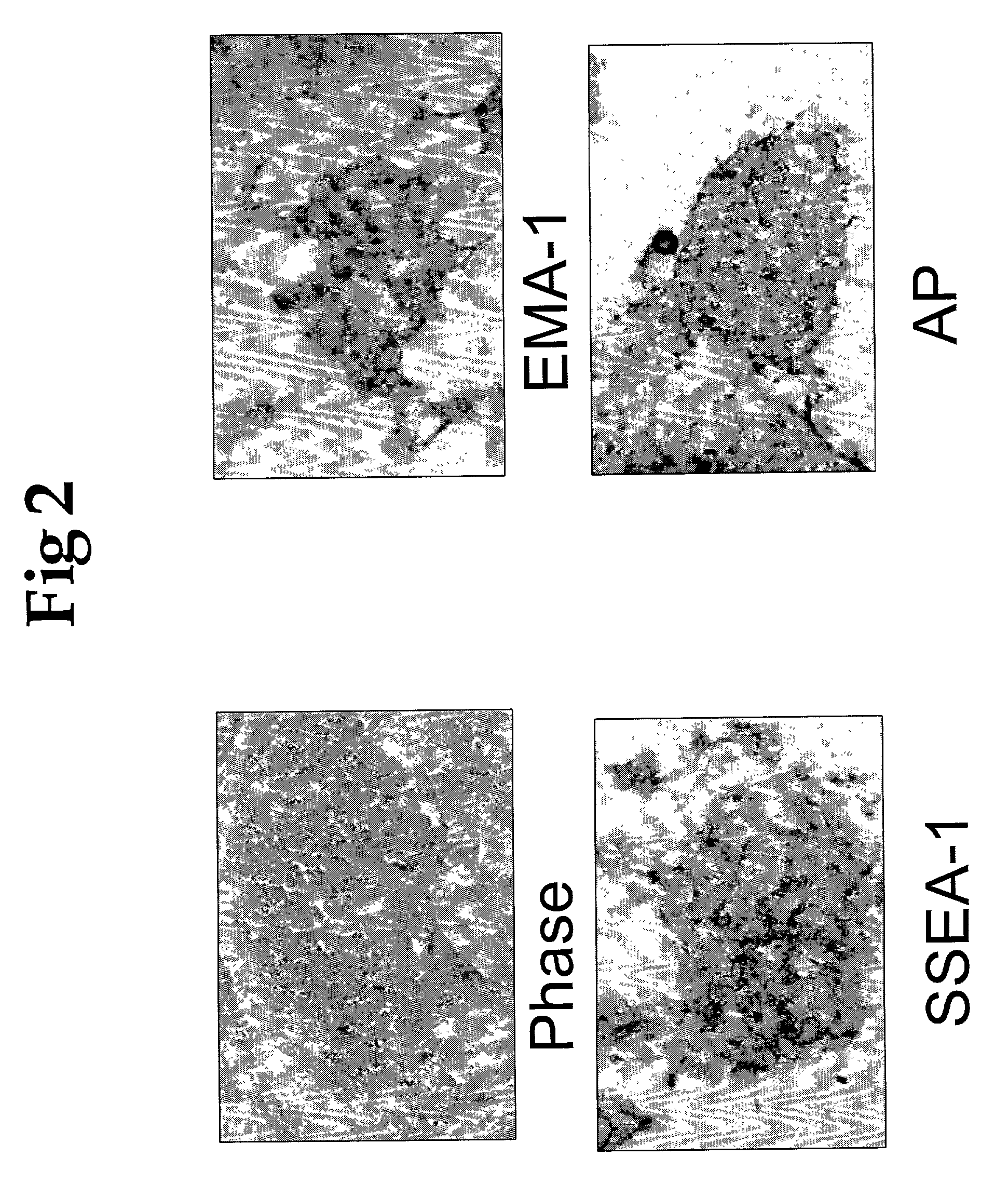
A source of multipotent amniotic fluid / fetal stem cells (MAFSCs) is disclosed. MAFSC are of fetal origin and have a normal diploid karyotype. These cells are characterized by the following cell surface markers: SSEA3, SSEA4, Tra-1-60, Tra-1-81, Tra-2-54, HLA class I, CD13, CD44, CD49b, CD105 and are distinguished by the absence of the antigen markers CD34, CD45, and HLA Class II, but are distinguished from mouse embryonic stem cells in that these cells do not express the cell surface marker SSEA1. MAFSC express the stem cell transcription factor Oct-4. MAFSC cells can be propagated for an indefinite period of time in continuous culture in an undifferentiated state. The MAFSCs have the ability to differentiate in culture in a regulated manner, into three or more subphenotypes. Cells can then be differentiated into endodermal, mesodermal and ectodermal derived tissues in vitro and in vivo. A method for isolating, identifying, expanding and differentiating MAFSCs is disclosed.
Owner:RGT UNIV OF CALIFORNIA
Methods for identifying factors for differentiating definitive endoderm
Disclosed herein are methods of identifying one or more differentiation factors that are useful for differentiating cells in a cell population comprising definitive endoderm cells into cells which are capable of forming tissues and / or organs that are derived from the gut tube.
Owner:VIACYTE INC
Pluripotent stem cells derived without the use of embryos or fetal tissue
InactiveUS20030113910A1New breed animal cellsArtificial cell constructsPluripotential stem cellGerm layer
Owner:STEMA
Compositions And Methods For Self-Renewal And Differentiation In Human Embryonic Stem Cells
ActiveUS20070281355A1Efficient productionEffectively lead to differentiationPeptide/protein ingredientsMetabolism disorderGerm layerFeeder Layer
The present invention provides compositions and methods for the production of differentiated mammalian cells. More particularly, the present invention provides cellular differentiation methods employing culturing the cells on a feeder layer or under feeder-free conditions in cell culture and further contacting the cells with an inhibitor of the PI3-kinase pathway for the generation of differentiated mammalian cells from pluripotent mammalian stem cells. Preferably, the differentiated cell is selected from the group consisting of a mesendodermal cell, a mesodermal cell, and an endodermal cell.
Owner:UNIV OF GEORGIA RES FOUND INC +1
Method of differentiating stem cells into cells of the endoderm and pancreatic lineage
InactiveUS20070259423A1Promote differentiationMass productionPancreatic cellsCulture processPluripotential stem cellGerm layer
Methods are described to more efficiently produce cells of the endoderm and pancreatic lineage from mammalian pluripotent stem cells. These methods provide a simple, reproducible culture protocol using defined media components to enable consistent, large-scale production of pancreatic cell types for research or therapeutic uses.
Owner:WISCONSIN ALUMNI RES FOUND
PDX1-expressing dorsal and ventral foregut endoderm
Disclosed herein are cell cultures comprising dorsal and / or ventral PDX1-positive foregut endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified dorsal and / or ventral PDX1-positive foregut endoderm cells as well as methods for enriching, isolating and purifying dorsal and / or ventral PDX1-positive foregut endoderm cells from other cell types. Methods of identifying differentiation factors capable of promoting the differentiation of dorsal and / or ventral PDX1-positive foregut endoderm cells, are also disclosed.
Owner:CYTHERA
Medium for growing human embryonic stem cells
InactiveUS7297539B2Rapid productionExpanding primate pluripotent stem (pPS) cellsHepatocytesGastrointestinal cellsGerm layerFiber
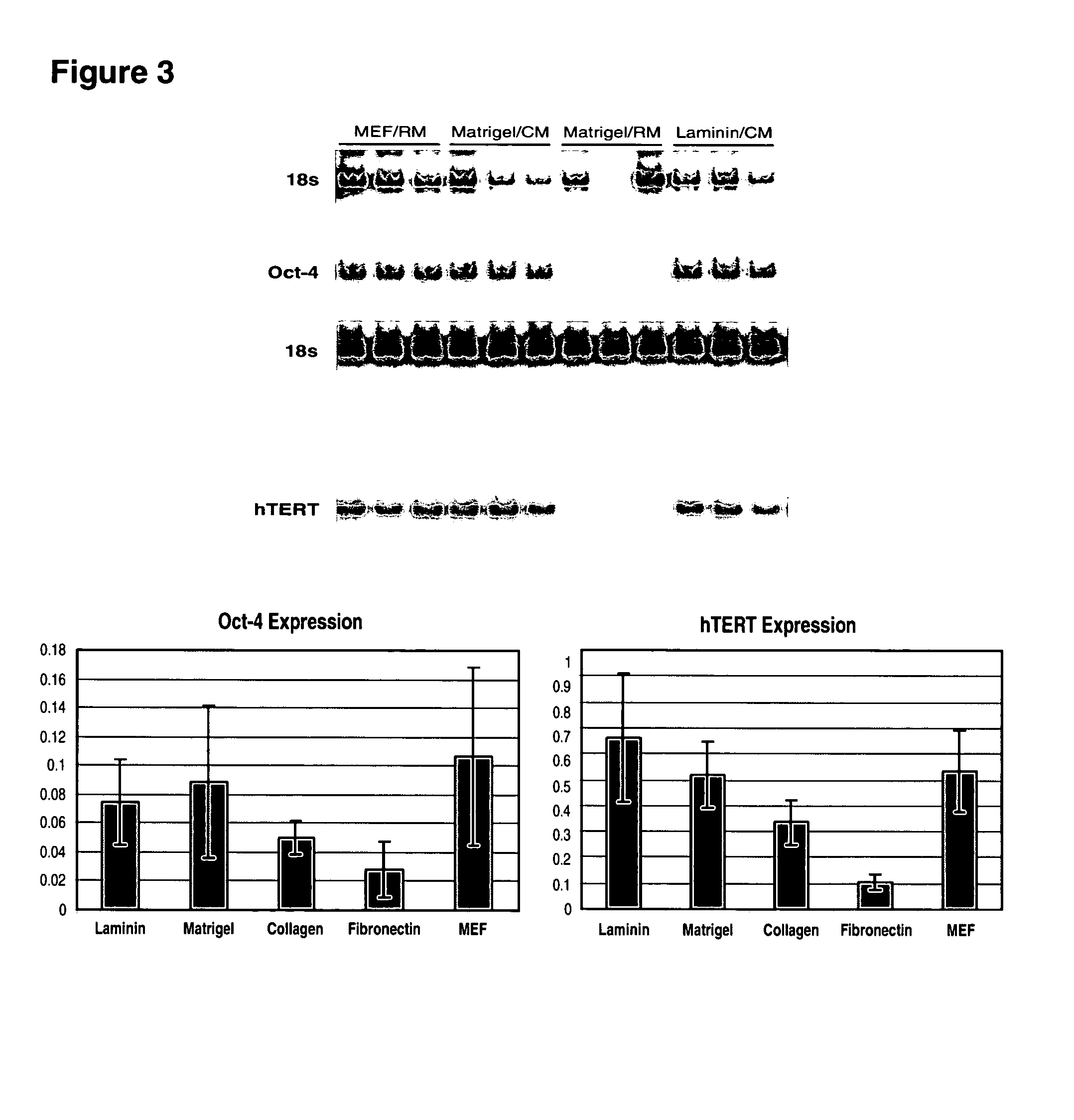
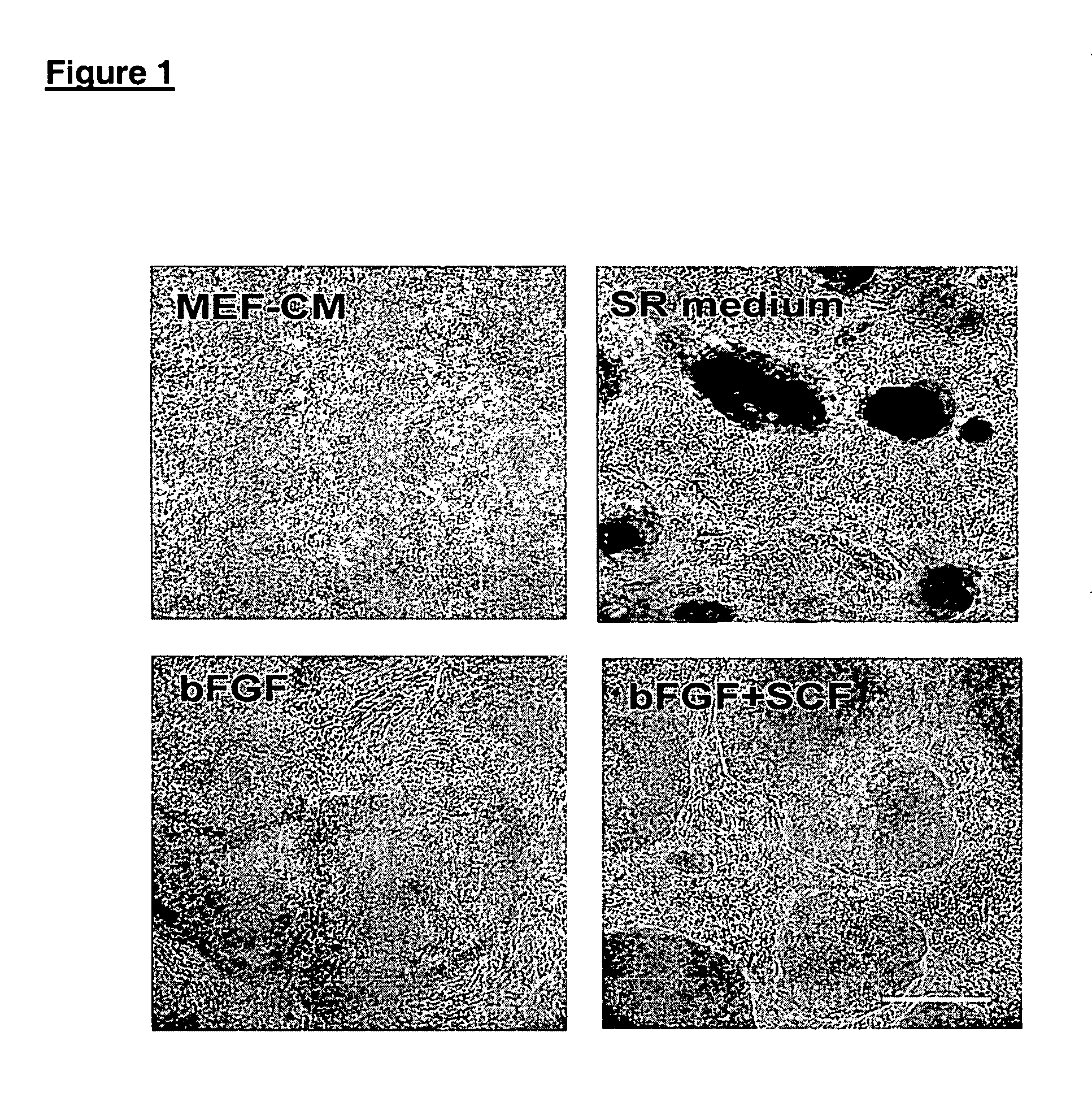
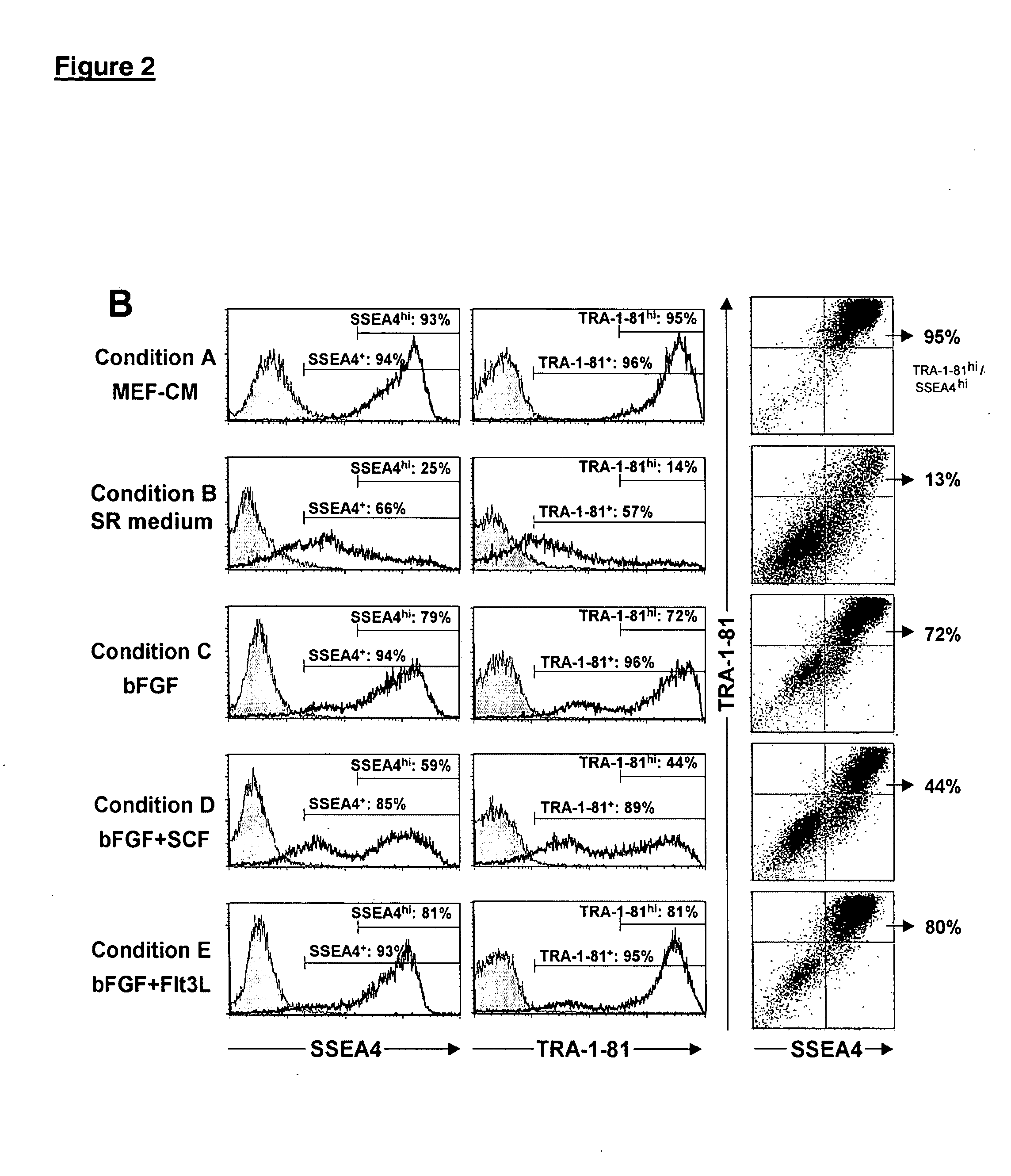
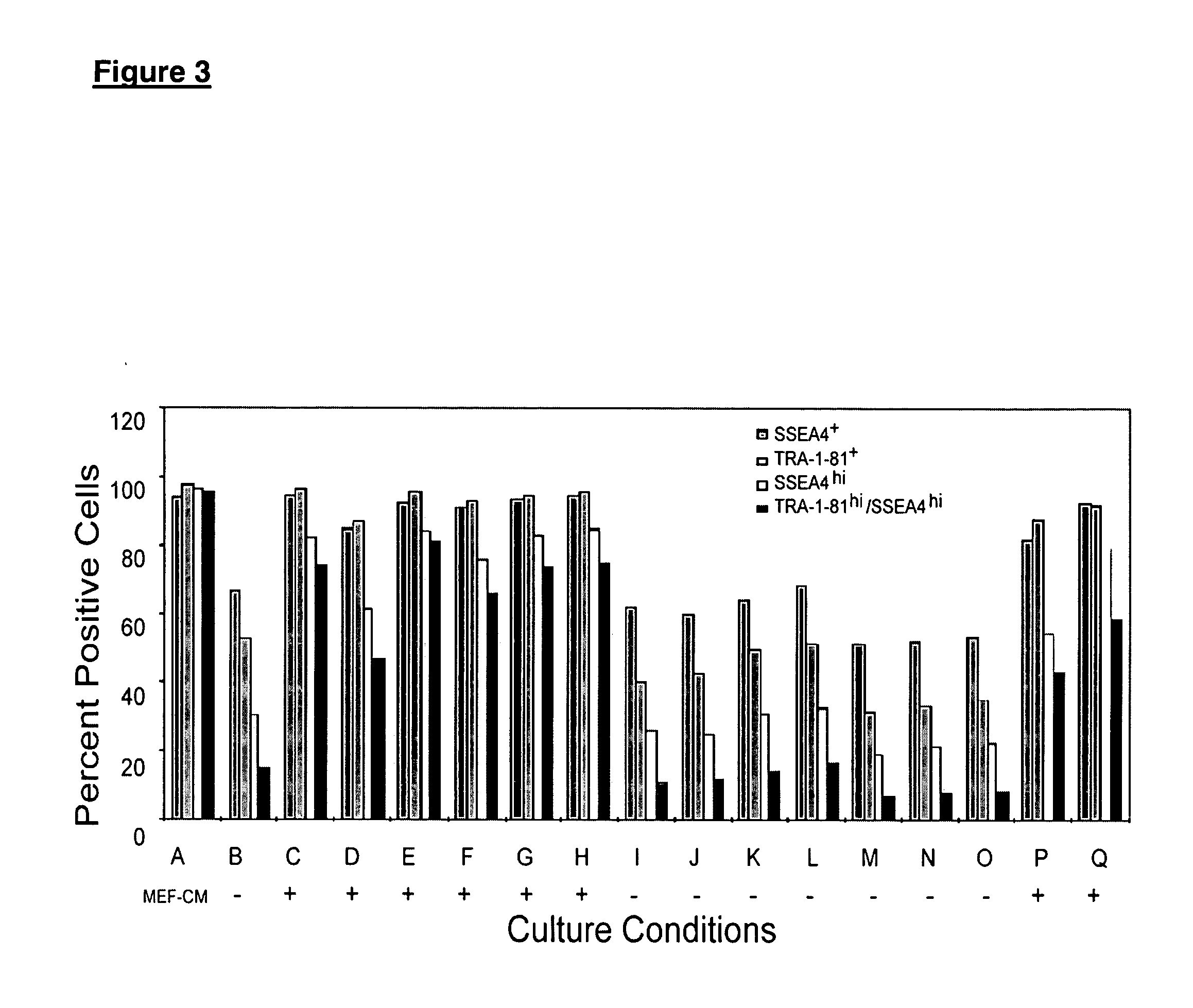
This disclosure provides an improved system for culturing human pluripotent stem cells. Traditionally, pluripotent stem cells are cultured on a layer of feeder cells (such as mouse embryonic fibroblasts) to prevent them from differentiating. In the system described here, the role of feeder cells is replaced by components added to the culture environment that support rapid proliferation without differentiation. Effective features are a suitable support structure for the cells, and an effective medium that can be added fresh to the culture without being preconditioned by another cell type. Culturing human embryonic stem cells in fresh medium according to this invention causes the cells to expand surprisingly rapidly, while retaining the ability to differentiate into cells representing all three embryonic germ layers. This new culture system allows for bulk proliferation of pPS cells for commercial production of important products for use in drug screening and human therapy.
Owner:ASTERIAS BIOTHERAPEUTICS INC
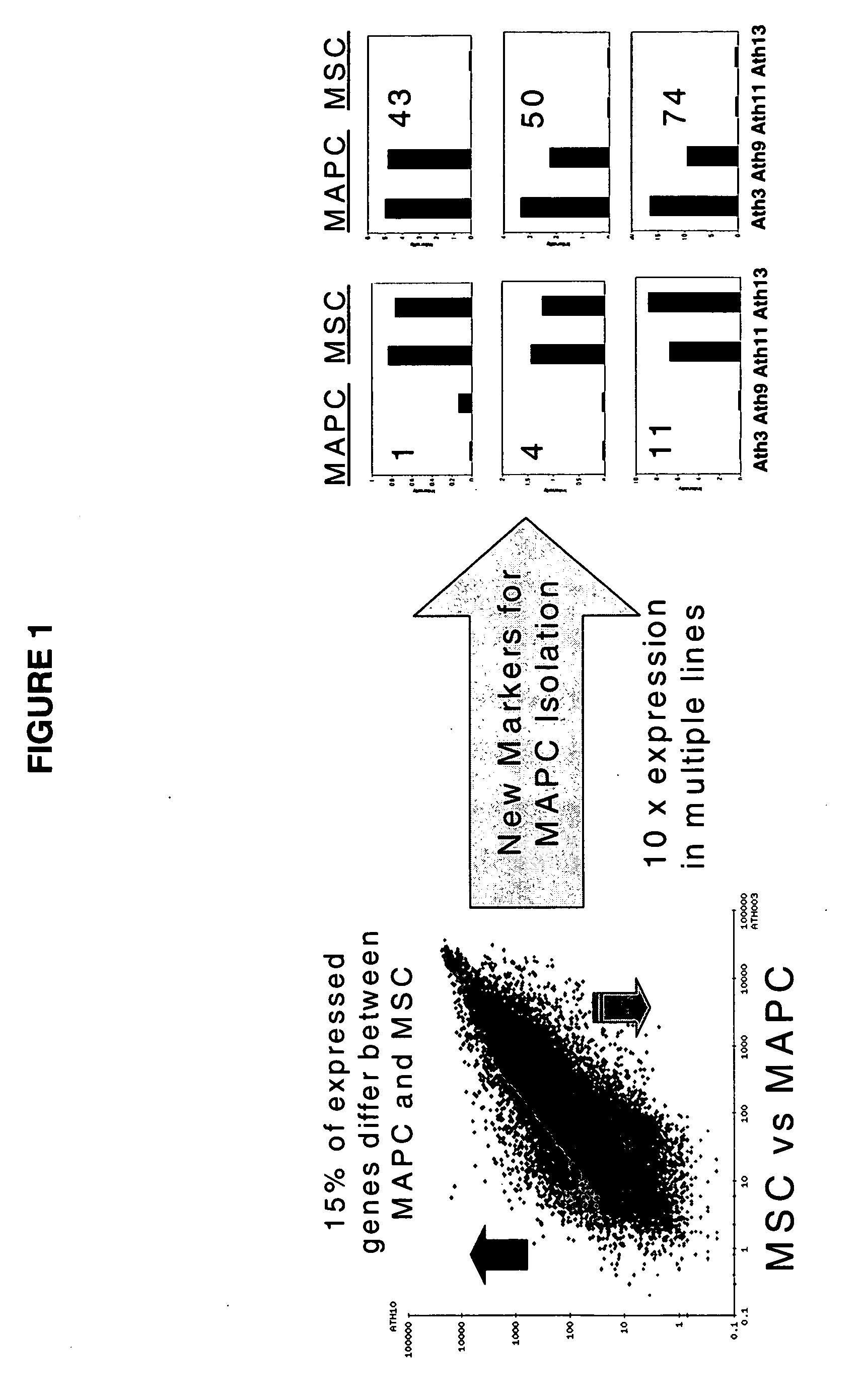
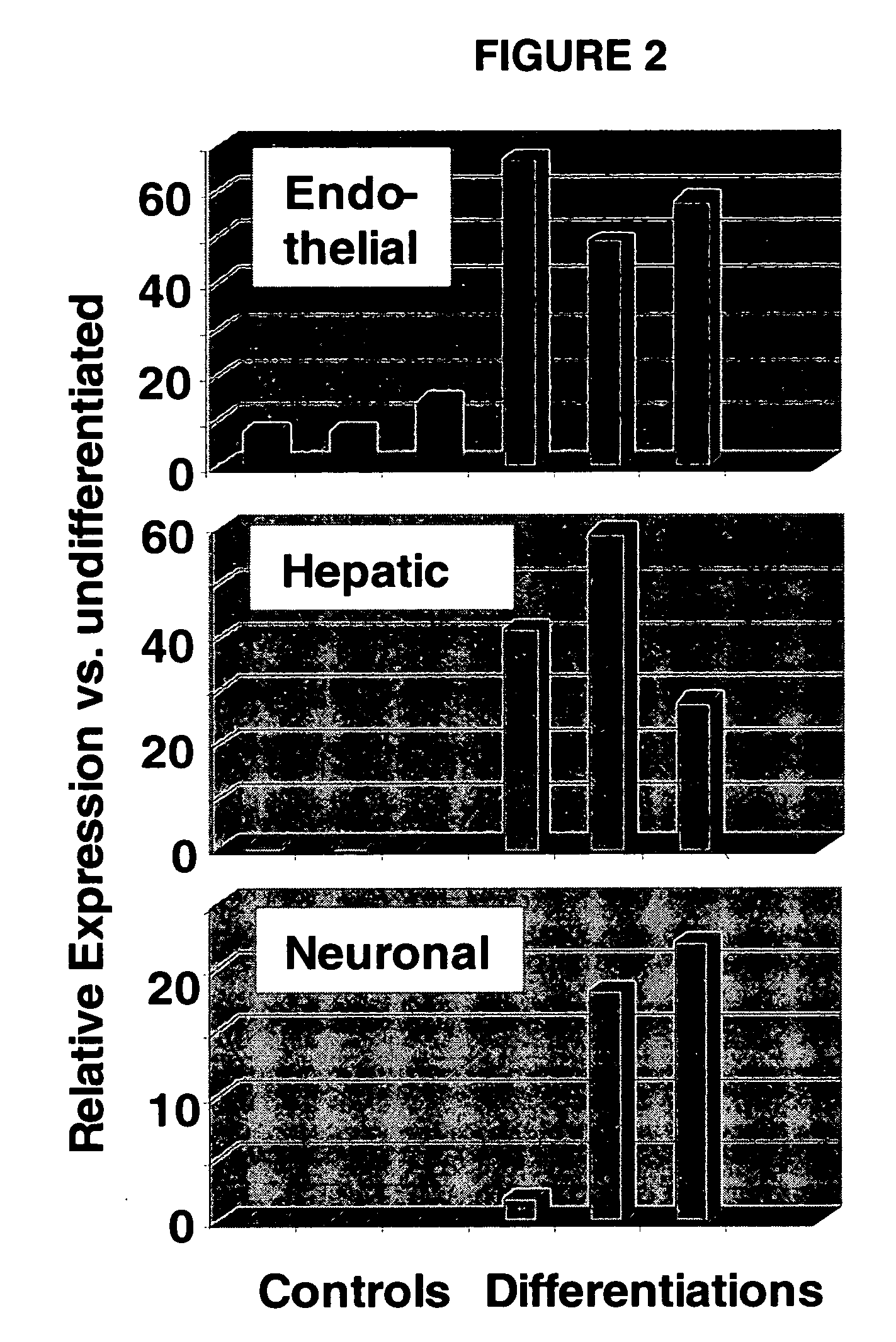
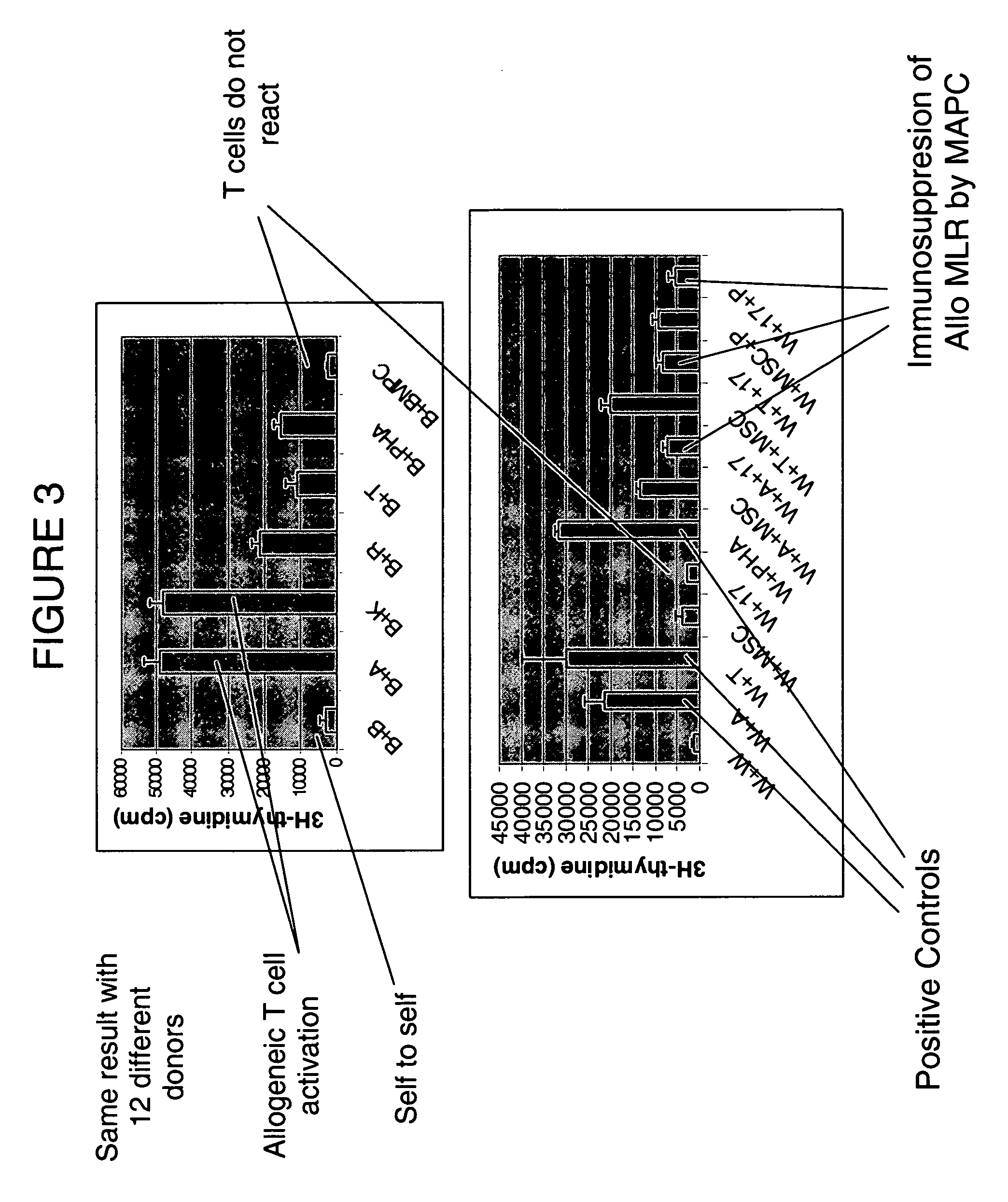
Immunomodulatory properties of multipotent adult progenitor cells and uses thereof
Isolated cells are described that are not embryonic stem cells, not embryonic germ cells, and not germ cells. The cells can differentiate into at least one cell type of each of at least two of the endodermal, ectodermal, and mesodermal lineages. The cells do not provoke a harmful immune response. The cells can modulate immune responses. As an example, the cells can suppress an immune response in a host engendered by allogeneic cells, tissues, and organs. Methods are described for using the cells, by themselves or adjunctively, to treat subjects. For instance, the cells can be used adjunctively for immunosuppression in transplant therapy. Methods for obtaining the cells and compositions for using them also are described.
Owner:ABT HOLDING COMPANY +1
Directed differentiation of embryonic stem cells and uses thereof
The present invention provides methods for the directed differentiation of embryonic stem cells along the endodermal lineage, especially the pancreatic lineage.
Owner:ES CELL INT
Definitive endoderm
ActiveUS20050158853A1Enough timeGastrointestinal cellsMicrobiological testing/measurementGerm layerCell type
Disclosed herein are cell cultures comprising definitive endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified definitive endoderm cells as well as methods for enriching, isolating and purifying definitive endoderm cells from other cell types.
Owner:VIACYTE INC
Methods for identifying factors for differentiating definitive endoderm
Disclosed herein are methods of identifying one or more differentiation factors that are useful for differentiating cells in a cell population comprising definitive endoderm cells into cells which are capable of forming tissues and / or organs that are derived from the gut tube.
Owner:VIACYTE INC
Methods for increasing definitive endoderm differentiation of pluripotent human embryonic stem cells with PI-3 kinase inhibitors
ActiveUS8187878B2Efficient productionEffectively lead to differentiationPeptide/protein ingredientsMetabolism disorderGerm layerFeeder Layer
Owner:UNIV OF GEORGIA RES FOUND INC +1
Pluripotent embryonic-like stem cells, compositions, methods and uses thereof
InactiveUS20050255588A1Avoid developmentInhibit progressBone marrow stroma cellsGenetic material ingredientsGerm layerIn vivo
The present invention relates to pluripotent stem cells, particularly to pluripotent embryonic-like stem cells. The invention further relates to methods of purifying pluripotent embryonic-like stem cells and to compositions, cultures and clones thereof. The present invention also relates to a method of transplanting the pluripotent stem cells of the present invention in a mammalian host, such as human, comprising introducing the stem cells, into the host. The invention further relates to methods of in vivo administration of a protein or gene of interest comprising transfecting a pluripotent stem cell with a construct comprising DNA which encodes a protein of interest and then introducing the stem cell into the host where the protein or gene of interest is expressed. The present also relates to methods of producing mesodermal, endodermal or ectodermal lineage-committed cells by culturing or transplantation of the pluripotent stem cells of the present invention.
Owner:ABT HOLDING COMPANY
Differentiation of Human Embryonic Stem Cells
The present invention provides methods to promote the differentiation of pluripotent stem cells. In particular, the present invention provides an improved method for the formation of pancreatic endoderm, pancreatic hormone expressing cells and pancreatic hormone secreting cells. The present invention also provides methods to promote the differentiation of pluripotent stem cells without the use of a feeder cell layer.
Owner:LIFESCAN INC
Ex vivo progenitor and stem cell expansion for use in the treatment of disease of endodermally-derived organs
Methods of ex-vivo expansion of endodermally-derived and non-endodermally-derived progenitor and stem cells, expanded populations of renewable progenitor and stem cells and to their uses in therapeutic applications such as the production of endocrine hormones and the prevention and treatment of liver and pancreatic disease.
Owner:GAMIDA CELL
Medium for growing human embryonic stem cells
InactiveUS20050037492A1Rapid productionCost-effectiveDrug screeningNervous system cellsGerm layerLipid formation
This disclosure provides an improved system for culturing human pluripotent stem cells. Traditionally, pluripotent stem cells are cultured on a layer of mouse embryonic fibroblast feeder cells to prevent them from differentiating. In the system described here, the role of feeder cells is replaced by defined components added to the culture environment that support rapid proliferation without differentiation. The medium contains an isotonic buffer, a blend of essential nutrients such as protein and lipids, and an effective growth factor or combination of factors that promote proliferation while inhibiting differentiation. Culturing human embryonic stem cells in fresh medium on an extracellular matrix according to this invention causes the cells to expand surprisingly rapidly, while retaining the ability to differentiate into cells representing all three embryonic germ layers. This new culture system allows for bulk proliferation of pPS cells for commercial production of important products for use in drug screening and human therapy.
Owner:ASTERIAS BIOTHERAPEUTICS INC
Chimeric bird from embryonic stem cells
Sustained cultures of avian embryonic stem cells are provided. Injection of avian embryonic stem cells into recipient embryos yields chimeras with a significant contribution from the embryonic stem cell phenotype. Transgene encoding exogenous proteins are stably integrated in the embryonic stem cells and are present in the somatic tissue of the resulting chimeras. The transgenes may encode exogenous proteins expressed in endodermal, ectodermal, mesodermal, or extra embryonic tissue. Breeding the resulting chimera yields transgenic birds whose genome is comprised of exogenous DNA.
Owner:SYNAGEVA BIOPHARMA CORP
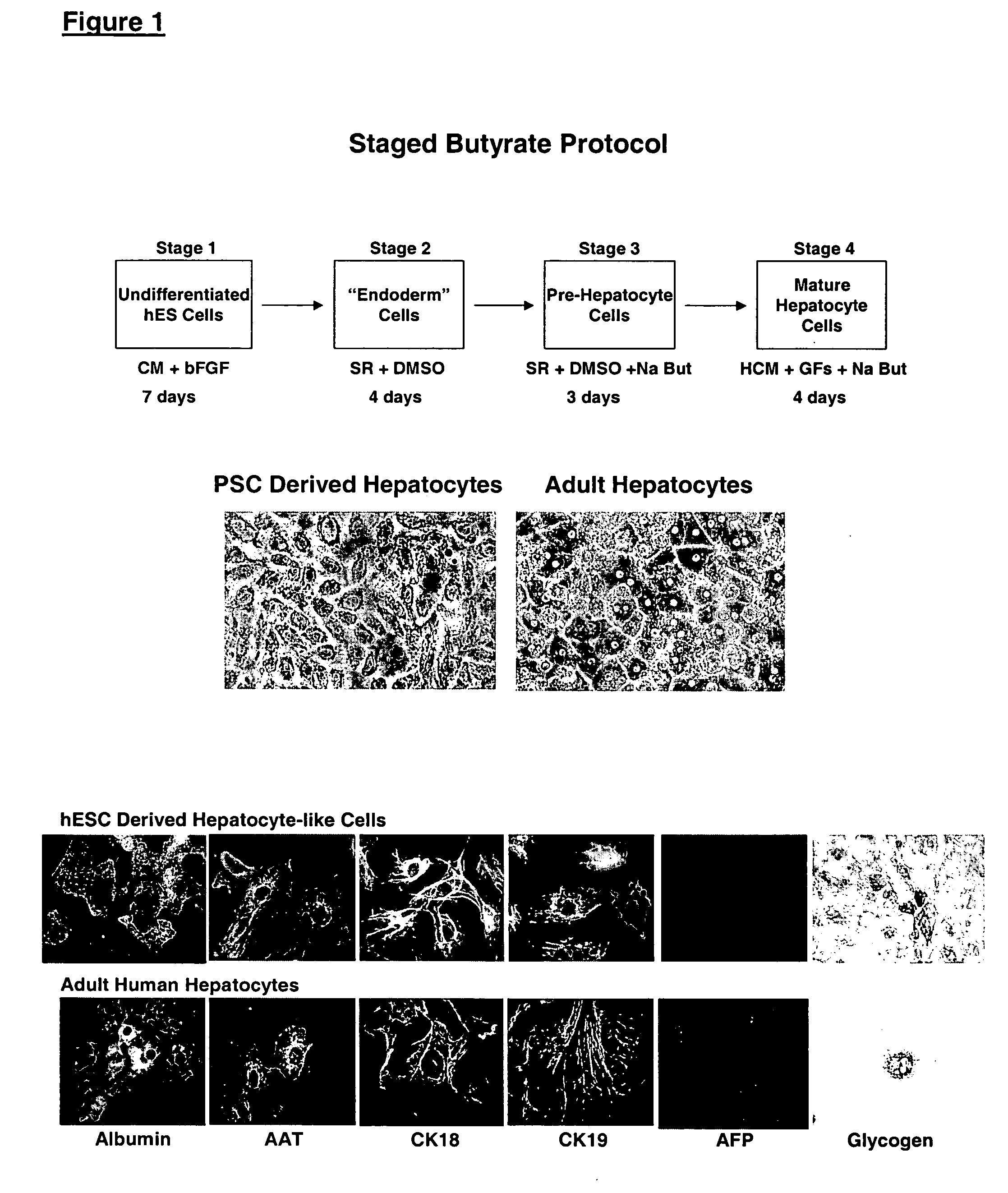
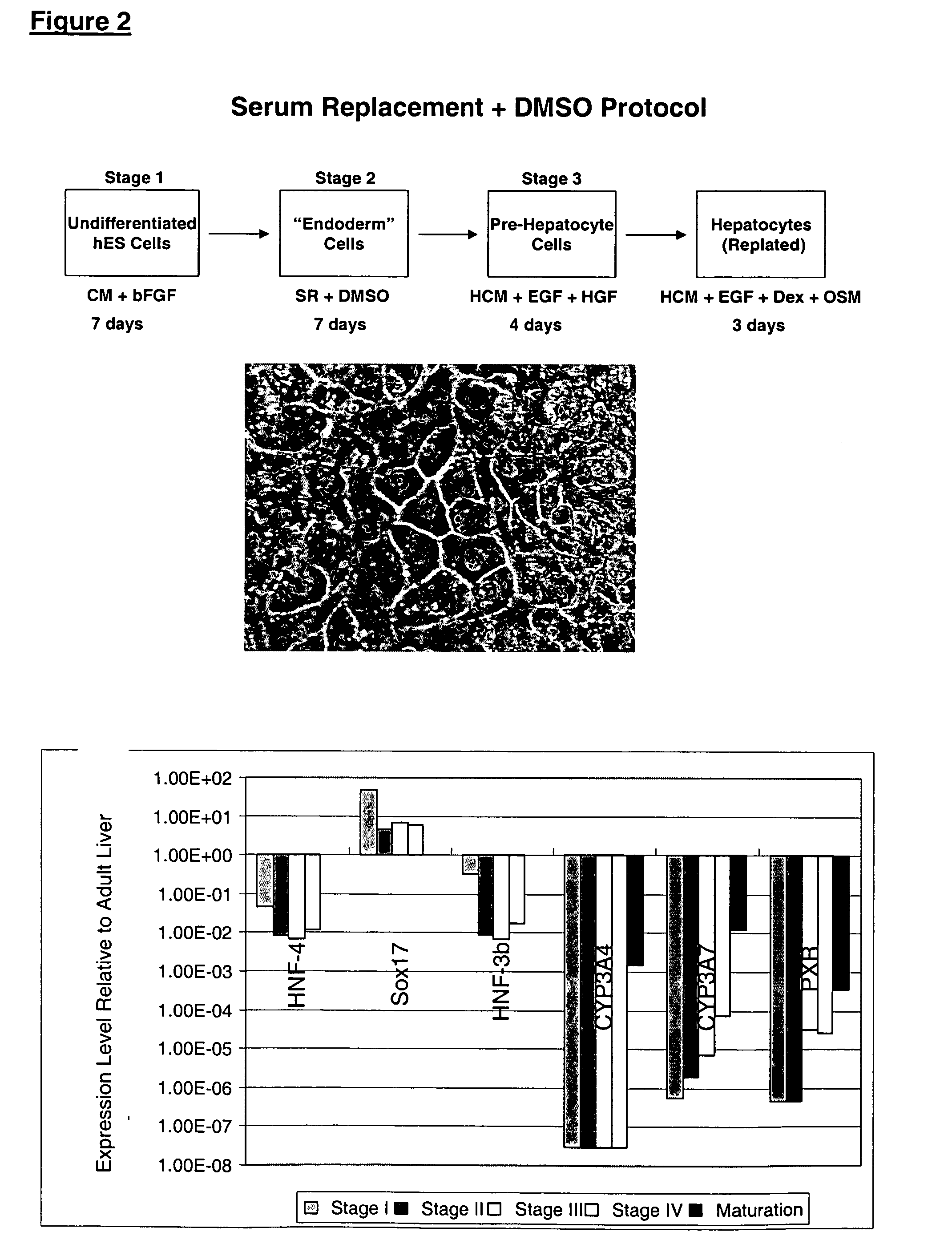
Protocols for making hepatocytes from embryonic stem cells
InactiveUS20050037493A1Promote cell differentiationCulture processDrug screeningGerm layerPluripotential stem cell
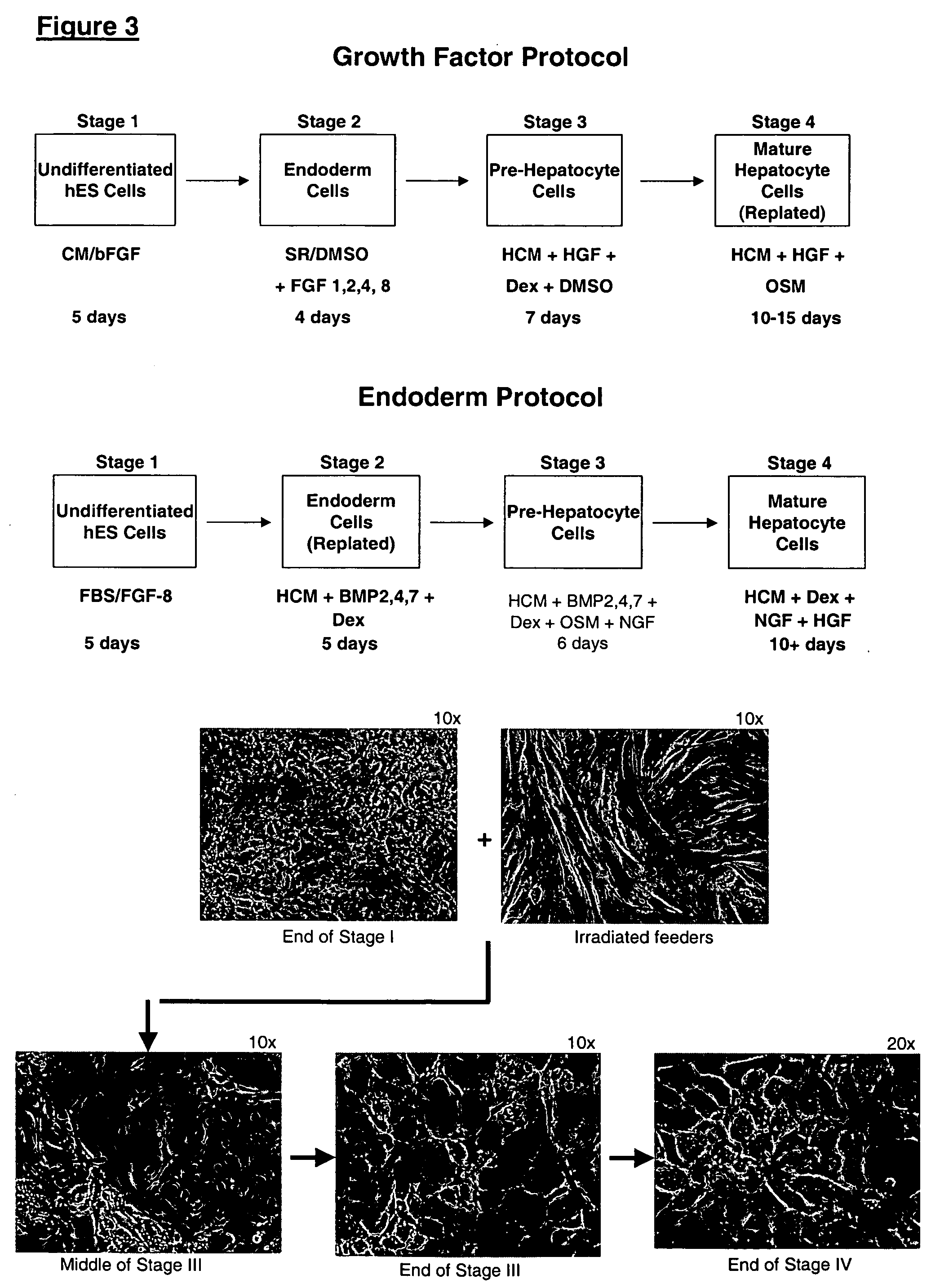
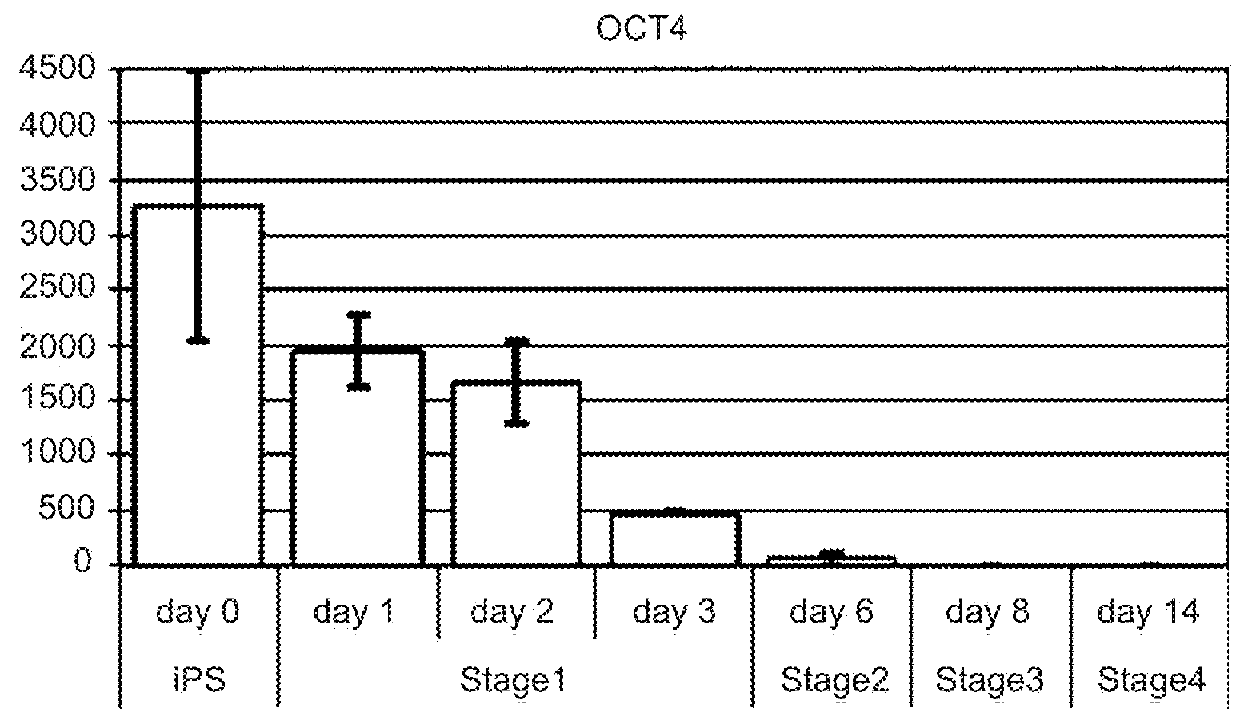
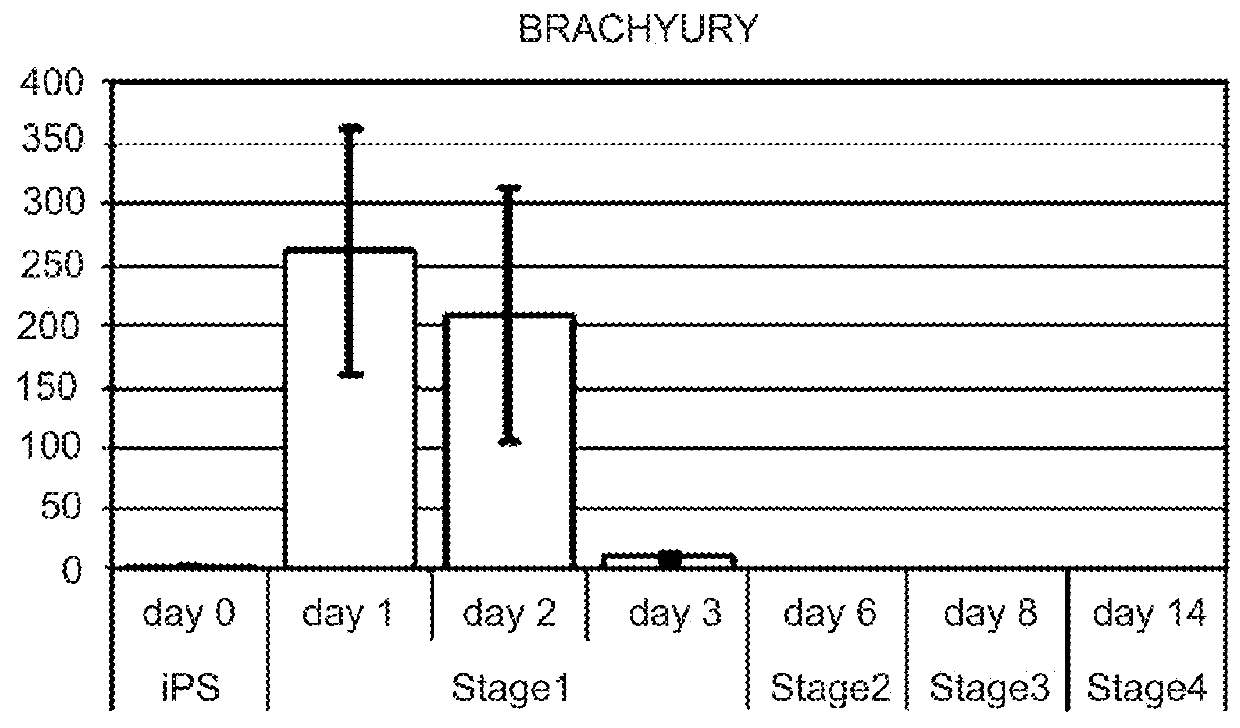
This disclosure provides a newly developed strategy and particular options for differentiating pluripotent stem cells into cells of the hepatocyte lineage. Many of the protocols are based on a strategy in which the cells are first differentiated into early germ layer cells, then into hepatocyte precursors, and then into mature cells. The cells obtained have morphological features and phenotypic markers characteristic of human adult hepatocytes. They also show evidence of cytochrome p450 enzyme activity, validating their utility for commercial applications such as drug screening, or use in the manufacture of medicaments and medical devices for clinical therapy.
Owner:ASTERIAS BIOTHERAPEUTICS INC
In vitro differentiation of pluripotent stem cells to pancreatic endoderm cells (PEC) and endocrine cells
A human immature endocrine cell population and methods for making an immature endocrine cell population are provided. Specifically, immature beta cells and methods for production of immature beta cells are described. Immature beta cells co-express INS and NKX6.1 and are uni-potent and thereby develop into mature beta cells when implanted in vivo. The mature beta cells in vivo are capable of producing insulin in response to glucose stimulation.
Owner:VIACYTE INC
Mesoporous silica nanoparticle-mediated delivery of DNA into arabidopsis root
InactiveUS20130185823A1MicroorganismsOther foreign material introduction processesGerm layerGene delivery
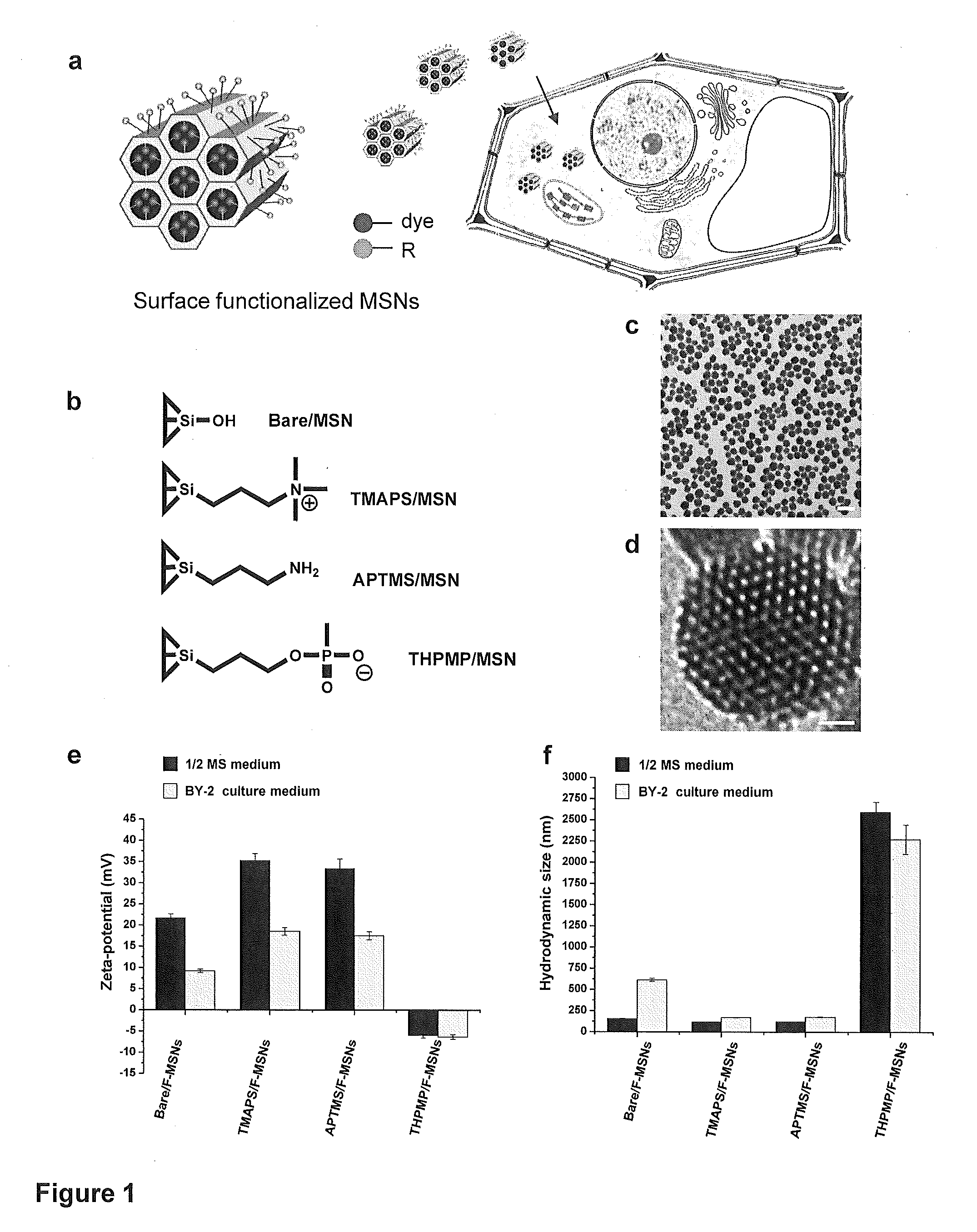
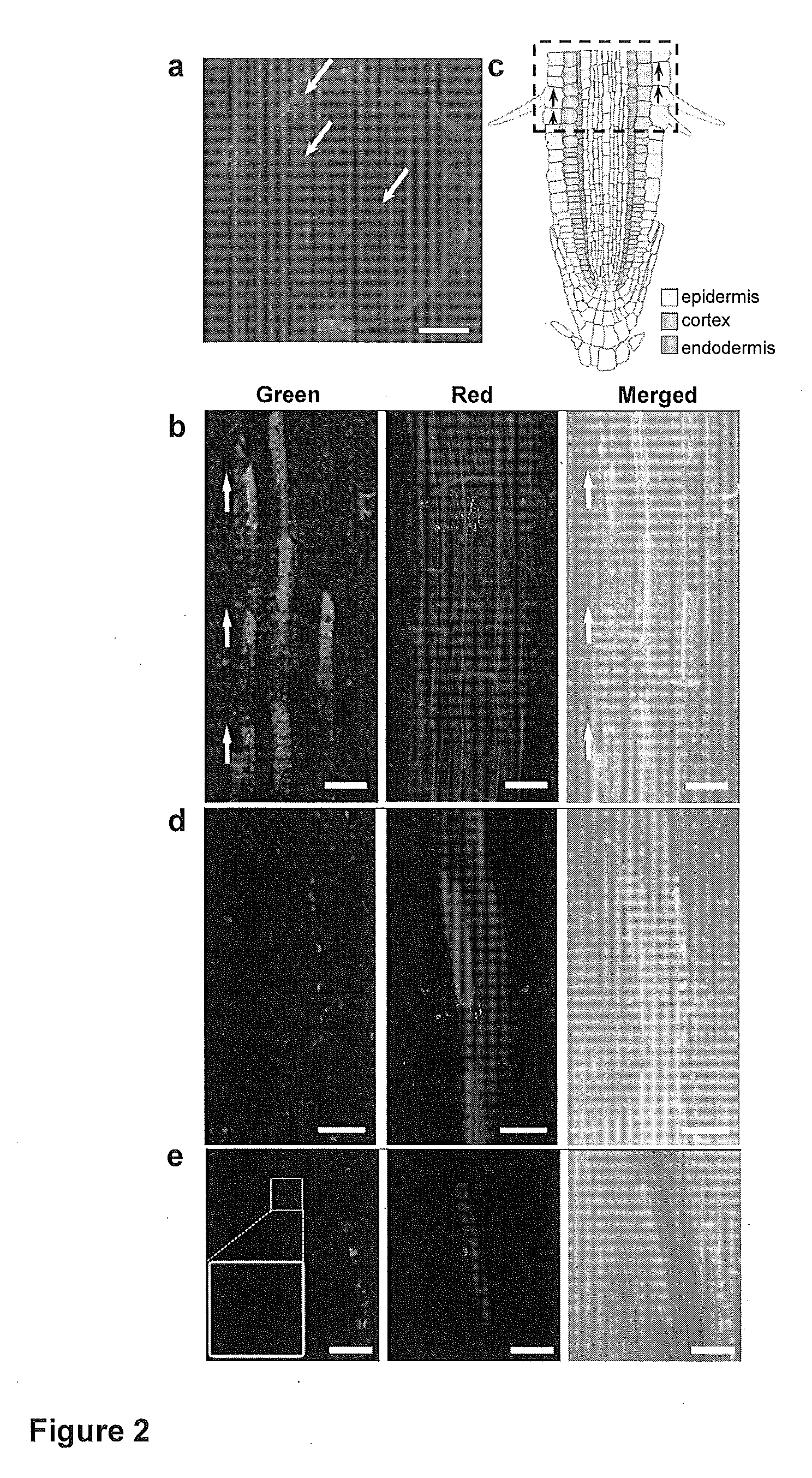
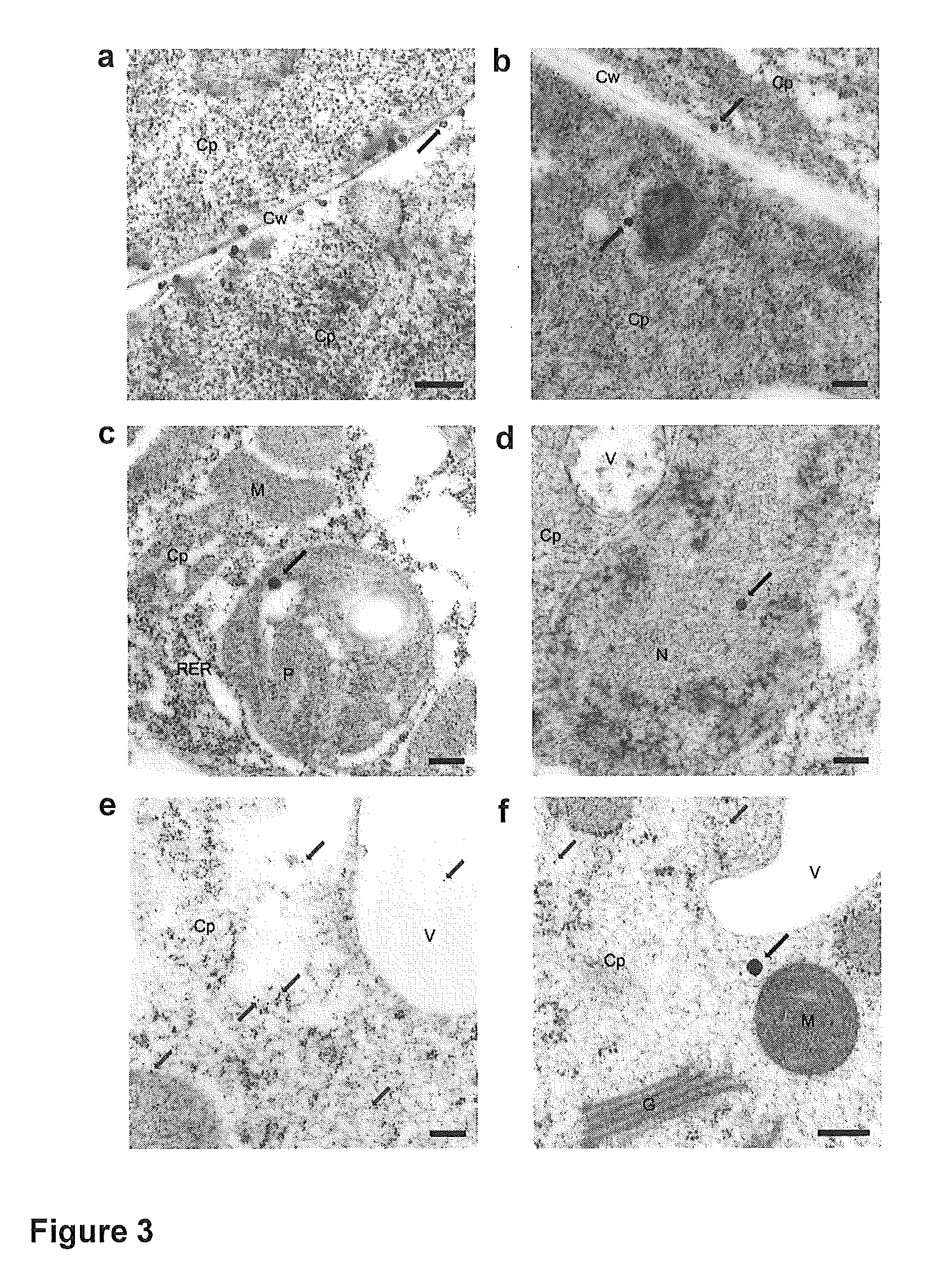
Transient gene expression is a powerful tool for plant genomics studies. Recently, the use of nanomaterials has drawn great interest. Delivery with mesoporous silica nanoparticles (MSNs) has many advantages. We used surface-functionalized MSNs to deliver and express foreign DNA in Arabidopsis thaliana root cells without the aid of particle bombardment. Gene expression was detected in the epidermis layer and in the more inner cortex and endodermis root tissues. This method is superior to the conventional gene-gun method to deliver DNA, which delivers the gene to the epidermis layer only. Less DNA is needed for the MSN method. Our system is the first use of nanoparticles to deliver DNA to plants with good efficiency and without external aids. MSNs, with multifunctionality and the capability of cargo delivery to plant cells as we demonstrated, provide a versatile system for biomolecule delivery, organelle targeting, and even agriculture, such as improved nutrient uptake.
Owner:ACAD SINIC +1
Definitive endoderm
Disclosed herein are cell cultures comprising definitive endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified definitive endoderm cells as well as methods for enriching, isolating and purifying definitive endoderm cells from other cell types.
Owner:VIACYTE INC
Expansion of definitive endoderm cells
Disclosed herein are cell cultures comprising expanded definitive endoderm cells as well as methods for expanding definitive endoderm cells in culture.
Owner:VIACYTE INC
Method for the preparation of cells of mesodermal lineage
The present invention relates generally to the generation of cells of mesodermal lineage. More particularly, the present invention contemplates a method for the preparation of differentiated or partially differentiated mesodermal cells and their use in tissue repair, regeneration and / or augmentation therapy. The identification and generation of the mesodermal cells further provides a source of transcriptome or proteome data to assess the expression profile of genes associated with the maintenance of mesodermal cells as well as their differentiation, proliferation, expansion and / or renewal potential.
Owner:ADELAIDE RES & INNOVATION PTY LTD
Isolation of inner cell mass for the establishment of human embryonic stem cell (hESC) lines
InactiveUS7294508B2Easy to useAvoid possibilityMammal material medical ingredientsDead animal preservationGerm layerHuman embryonic stem cell line
A method for isolating an inner cell mass comprising the steps of immobilizing a blastocyst stage embryo having a zona pellucida, trophectoderm, and inner cell mass, creating an aperture in the blastocyst stage embryo by laser ablation, and removing the inner cell mass from the blastocyst stage embryo through the aperture. The aperture is through the zona pellucida and the trophectoderm. The laser ablation is acheived using a non-contact diode laser. The inner cell mass removed from the blastocyst stage embryo is used to establish human Embryonic Stem Cell lines.
Owner:RELIANCE LIFE SCI PVT
Pancreatic and liver endoderm cells and tissue by differentiation of definitive endoderm cells obtained from human embryonic stems
The invention relates to methods that allow for the efficient differentiation to form pancreatic endoderm cells from pluripotent stem cells such as human embryonic stem cells and definitive endoderm cells. The invention is directly applicable to the ultimate generation of pancreatic beta cells that could be used as part of a therapy to treat or even cure diabetes. Additionally, the present invention may be used to generate liver endoderm cells from human embryonic stem cells and definite endoderm cells as well. This invention relates to a method for generating definitive endoderm and pancreatic endoderm cells from stem cells, preferably human embryonic stem cells using defined media in the absence of feeder cells. A simply two step procedure to provide pancreatic endoderm cells from embryonic stem cells represents further embodiments of the present invention.
Owner:UNIV OF GEORGIA RES FOUND INC
Medium for growing human embryonic stem cells
InactiveUS7455983B2Cost-effectiveRapid productionMicroorganismsDrug screeningGerm layerLipid formation
This disclosure provides an improved system for culturing human pluripotent stem cells. Traditionally, pluripotent stem cells are cultured on a layer of mouse embryonic fibroblast feeder cells to prevent them from differentiating. In the system described here, the role of feeder cells is replaced by defined components added to the culture environment that support rapid proliferation without differentiation. The medium contains an isotonic buffer, a blend of essential nutrients such as protein and lipids, and an effective growth factor or combination of factors that promote proliferation while inhibiting differentiation. Culturing human embryonic stem cells in fresh medium on an extracellular matrix according to this invention causes the cells to expand surprisingly rapidly, while retaining the ability to differentiate into cells representing all three embryonic germ layers. This new culture system allows for bulk proliferation of pPS cells for commercial production of important products for use in drug screening and human therapy.
Owner:ASTERIAS BIOTHERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com