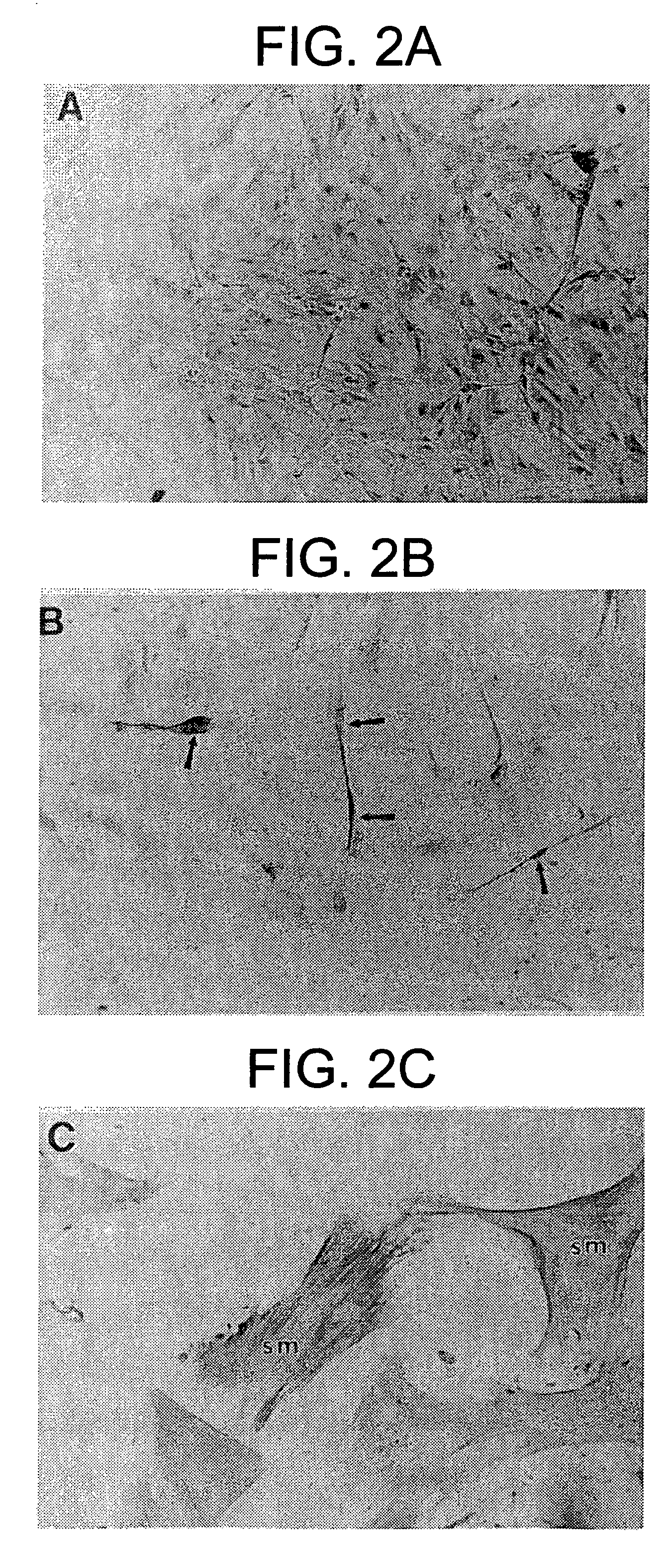
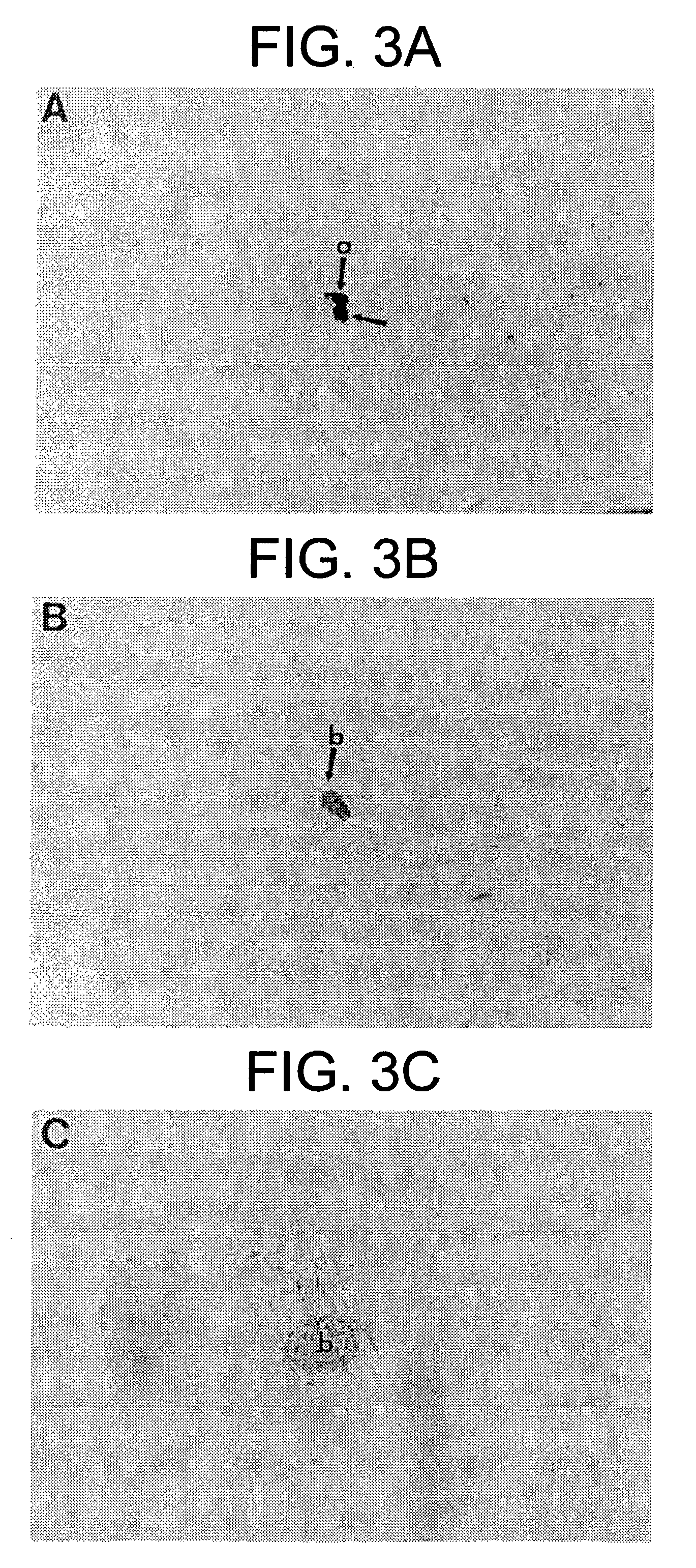
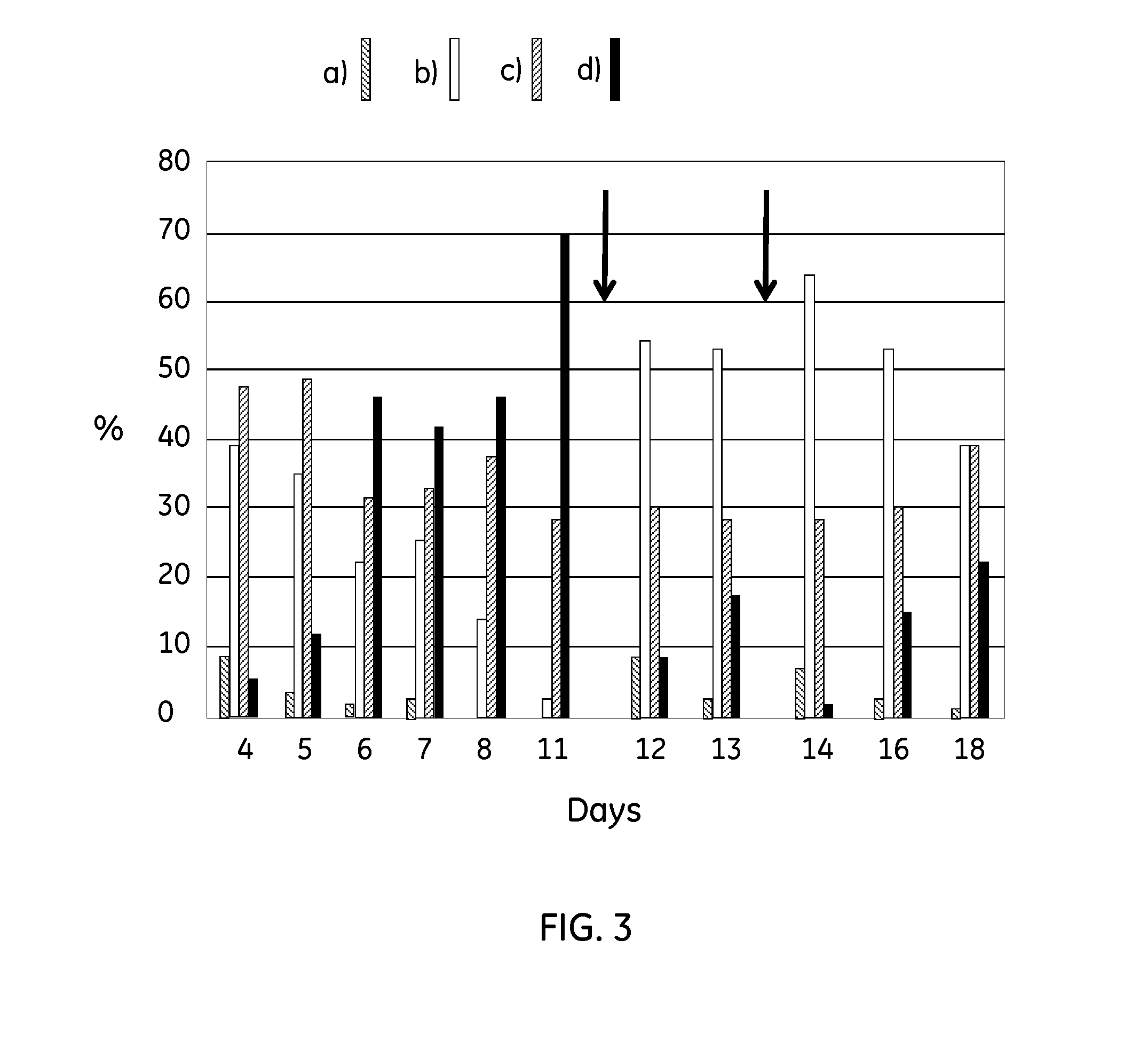
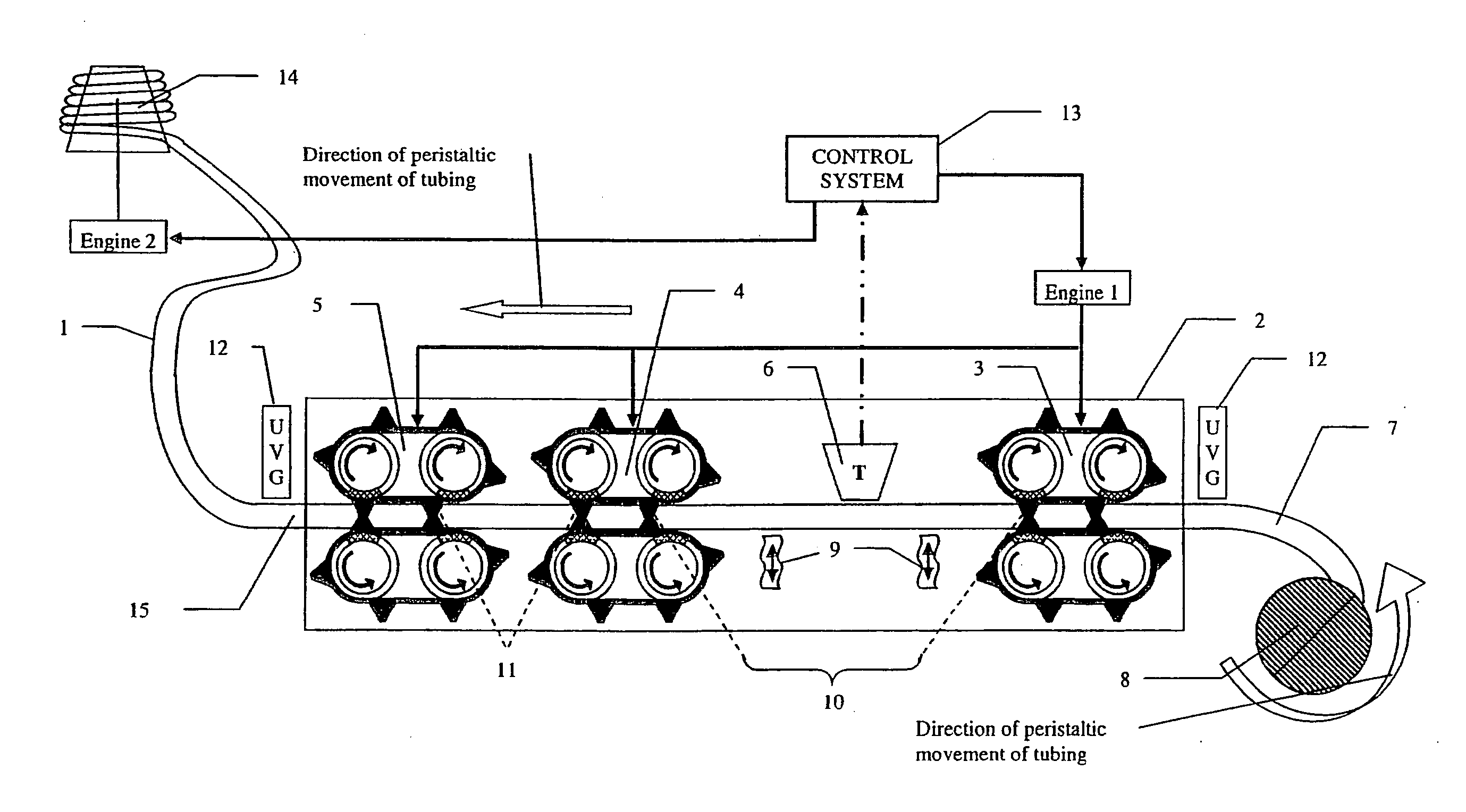
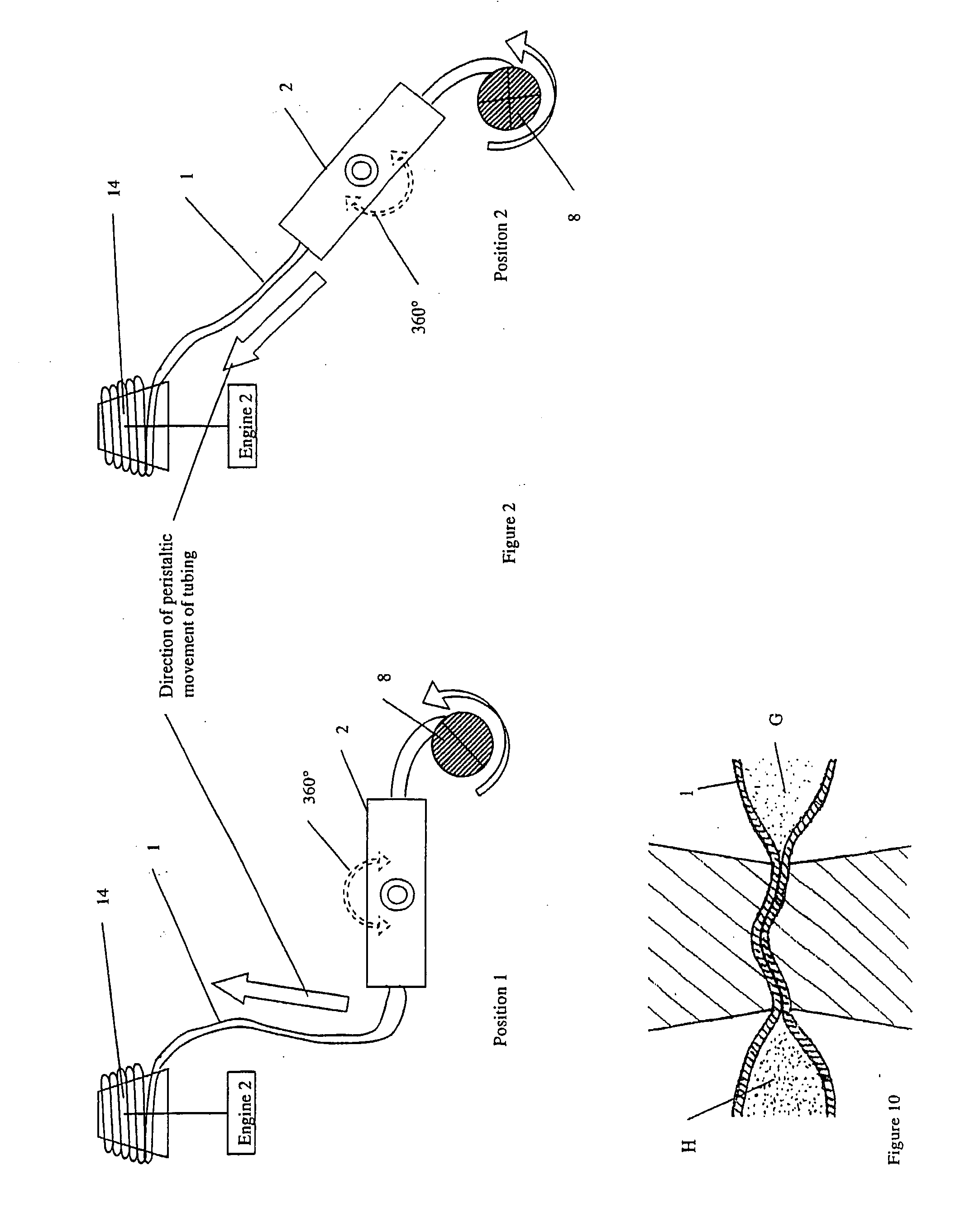
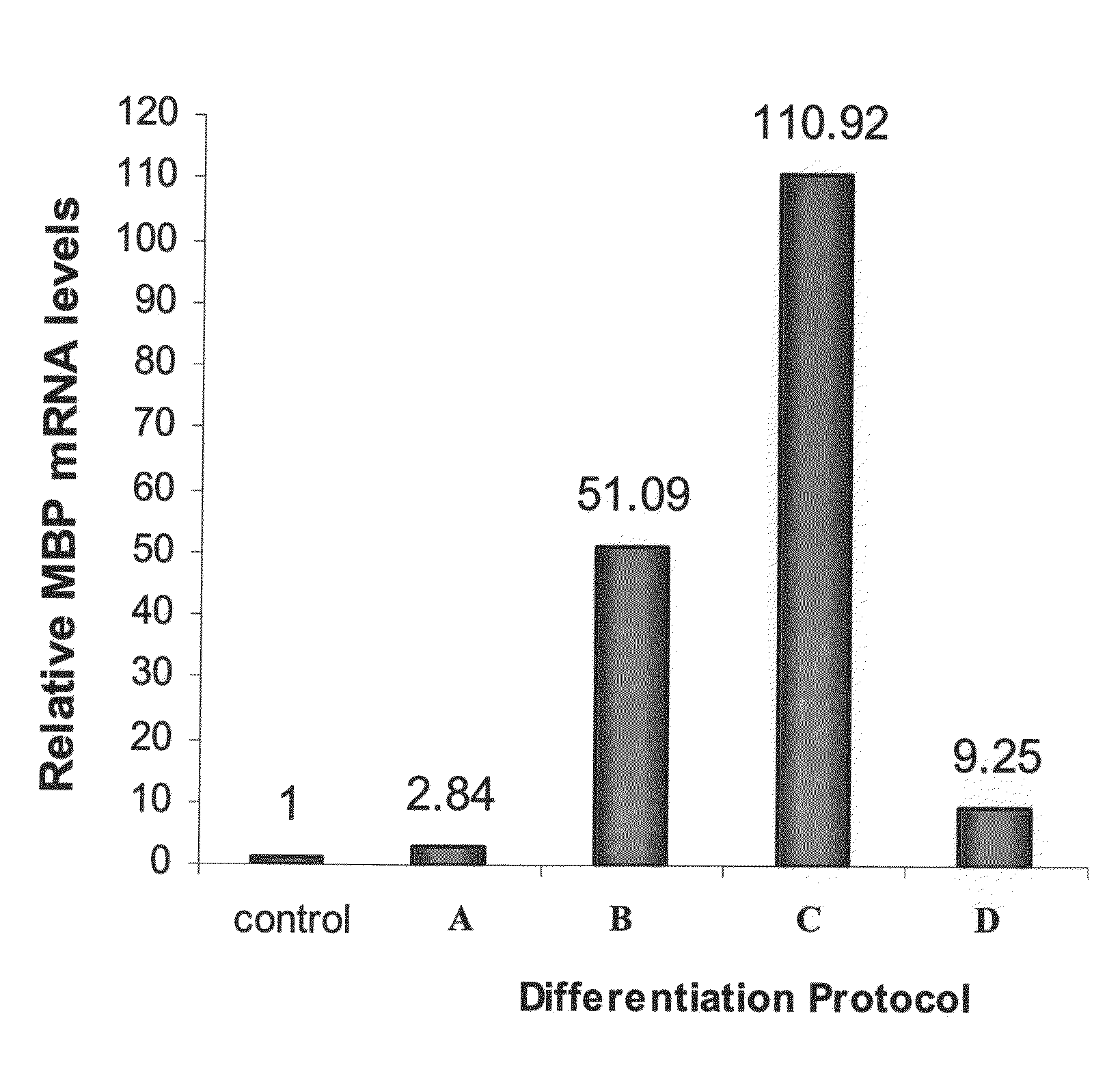
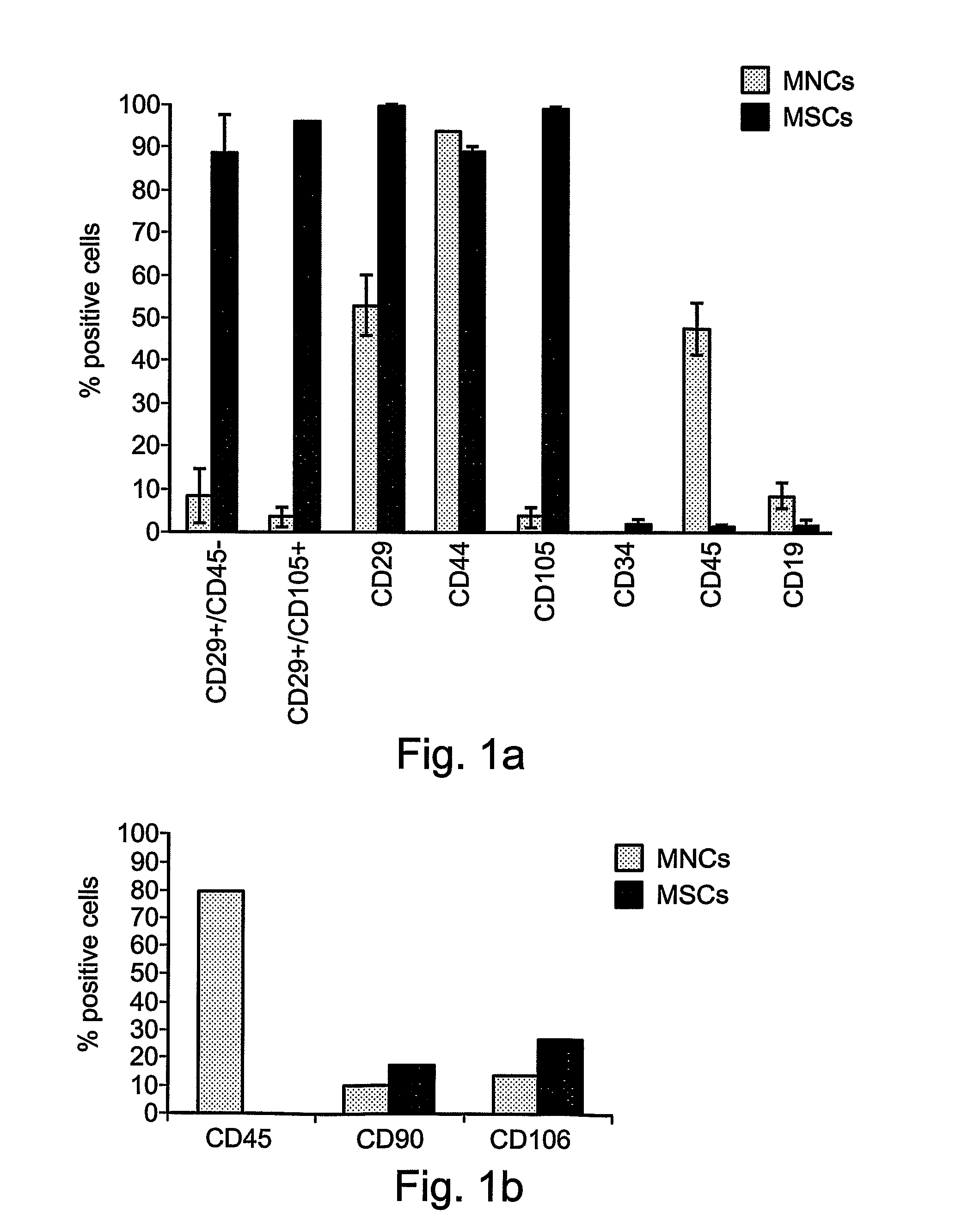
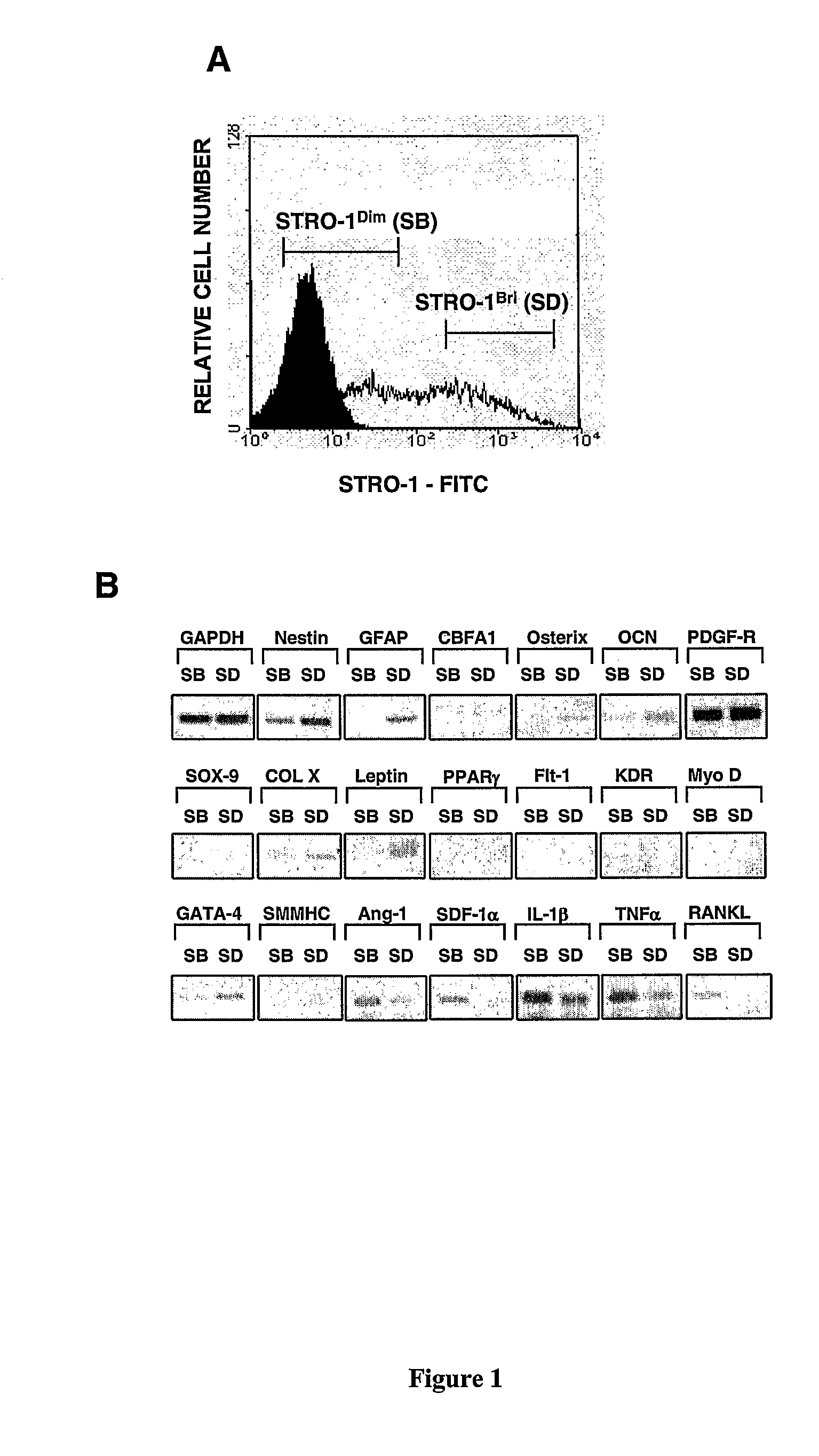
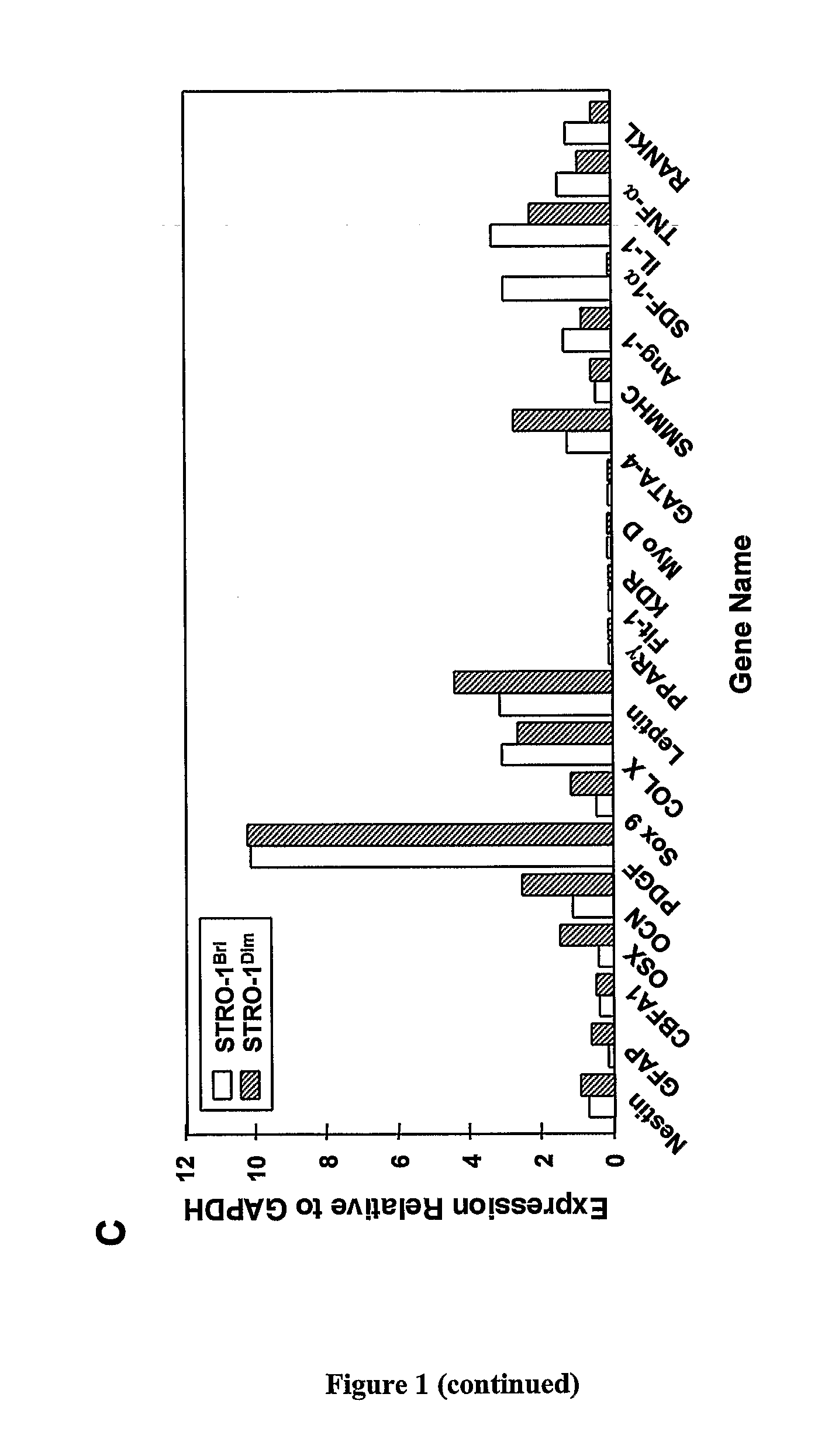
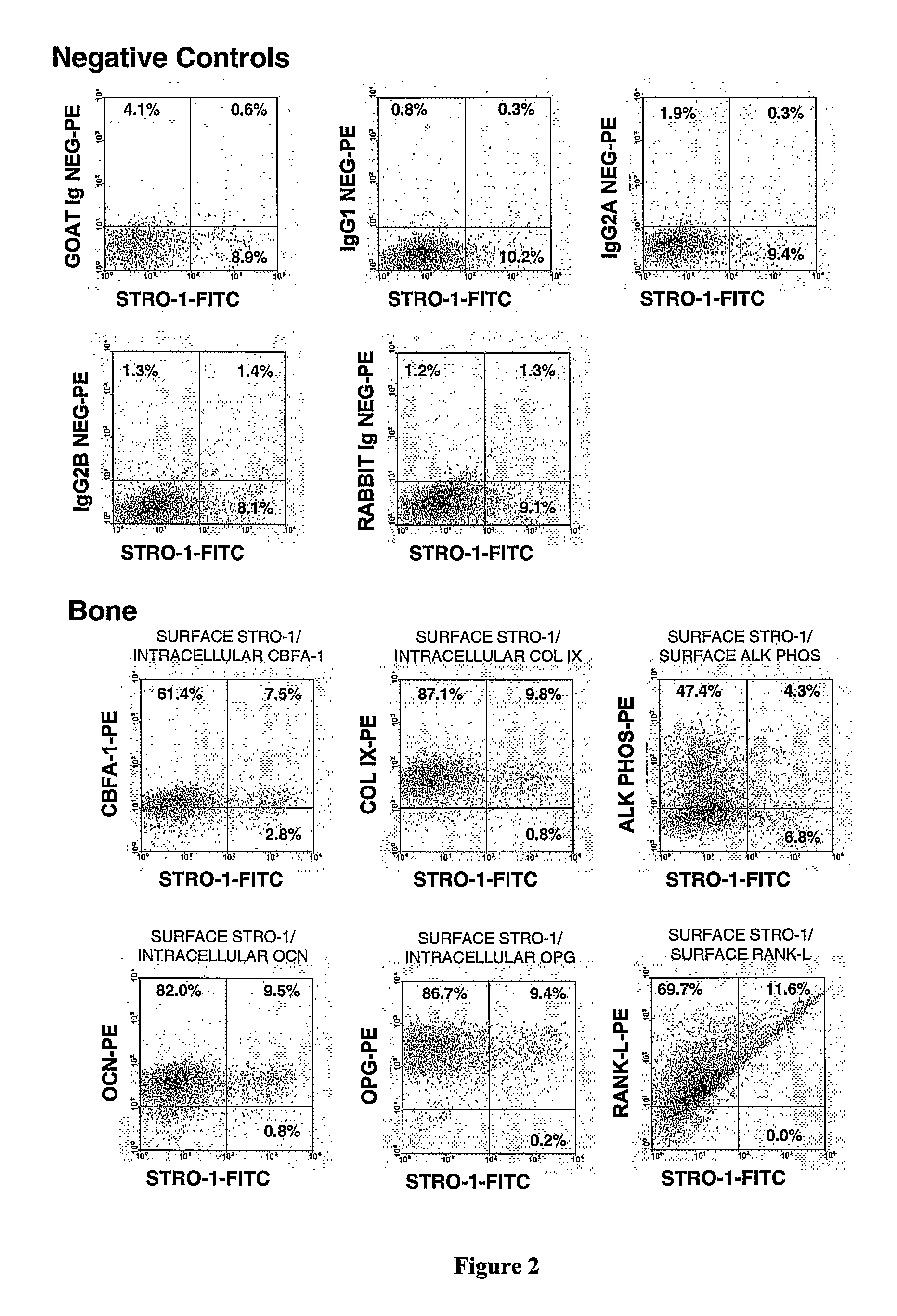
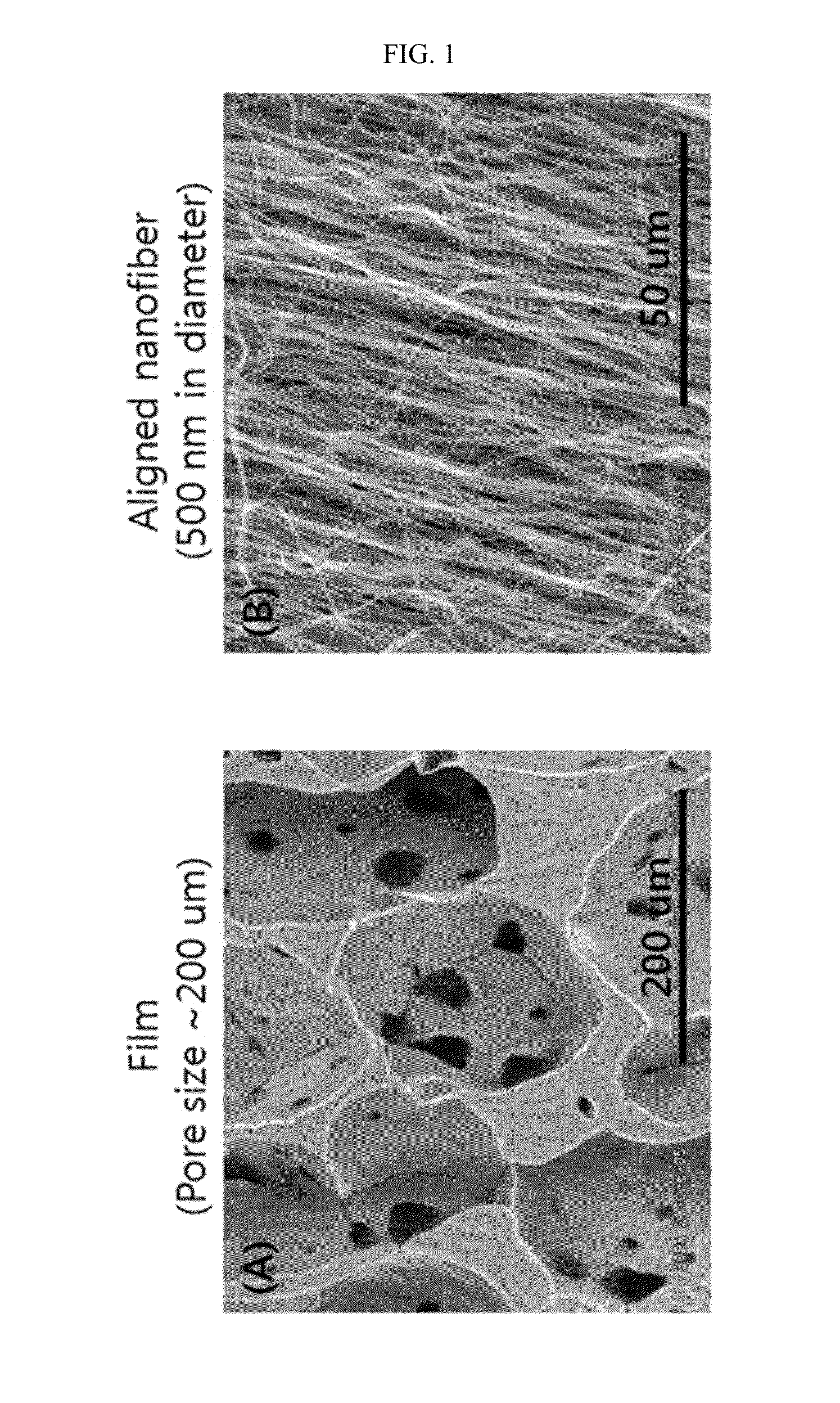
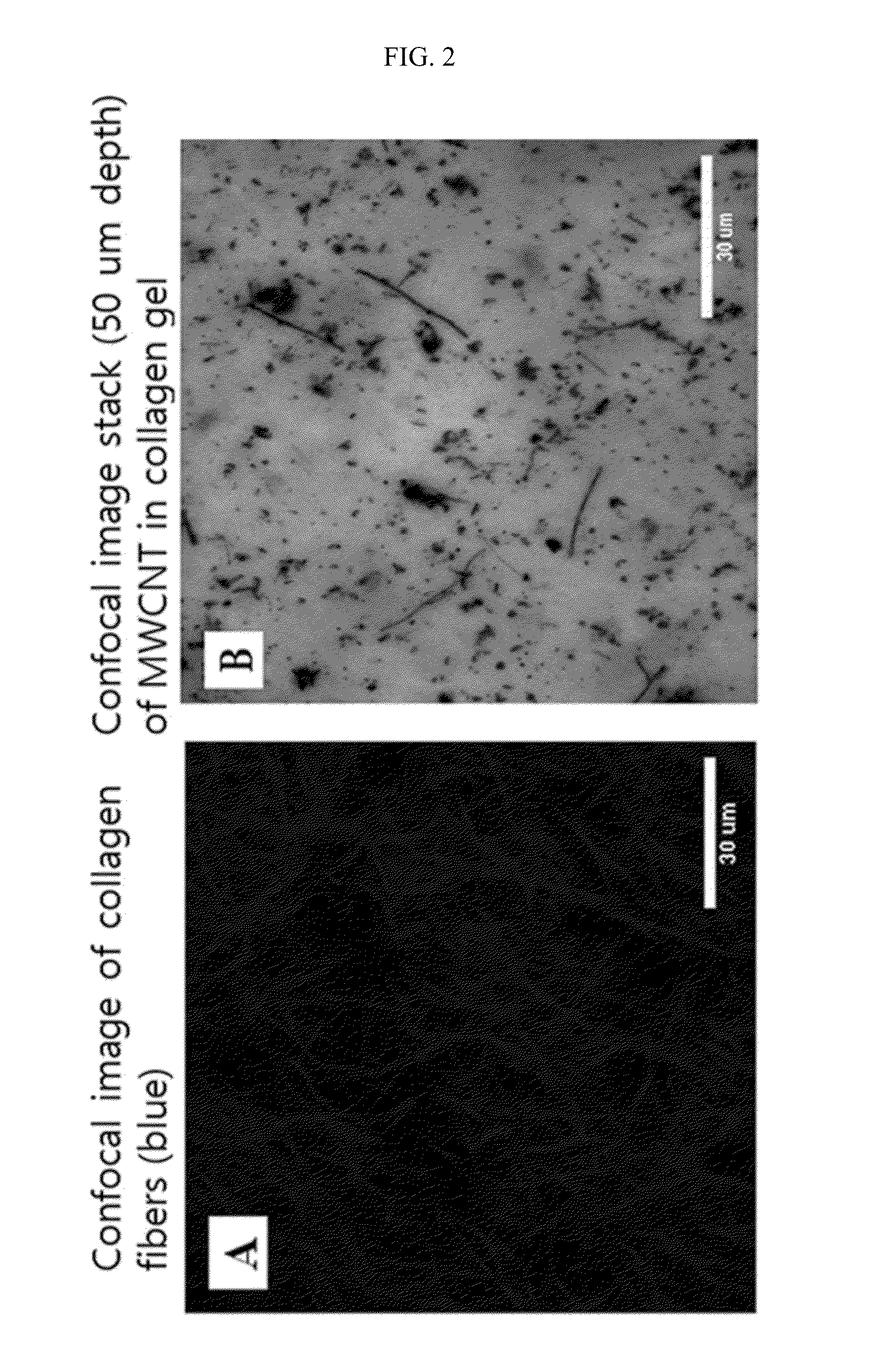
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56results about "Bone marrow stroma cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Regeneration and augmentation of bone using mesenchymal stem cells
InactiveUS6863900B2Reduce functionReduce the numberBiocideBioreactor/fermenter combinationsMedicineBiopolymer
Disclosed are compositions and methods for augmenting bone formation by administering isolated human mesenchymal stem cells (hMSCs) with a ceramic material or matrix or by administering hMSCs; fresh, whole marrow; or combinations thereof in a resorbable biopolymer which supports their differentiation into the osteogenic lineage. Contemplated is the delivery of (i) isolated, culture-expanded, human mesenchymal stem cells; (ii) freshly aspirated bone marrow; or (iii) their combination in a carrier material or matrix.
Owner:MESOBLAST INT
Pluripotent embryonic-like stem cells, compositions, methods and uses thereof
InactiveUS20050255588A1Avoid developmentInhibit progressBone marrow stroma cellsGenetic material ingredientsGerm layerIn vivo
The present invention relates to pluripotent stem cells, particularly to pluripotent embryonic-like stem cells. The invention further relates to methods of purifying pluripotent embryonic-like stem cells and to compositions, cultures and clones thereof. The present invention also relates to a method of transplanting the pluripotent stem cells of the present invention in a mammalian host, such as human, comprising introducing the stem cells, into the host. The invention further relates to methods of in vivo administration of a protein or gene of interest comprising transfecting a pluripotent stem cell with a construct comprising DNA which encodes a protein of interest and then introducing the stem cell into the host where the protein or gene of interest is expressed. The present also relates to methods of producing mesodermal, endodermal or ectodermal lineage-committed cells by culturing or transplantation of the pluripotent stem cells of the present invention.
Owner:ABT HOLDING COMPANY
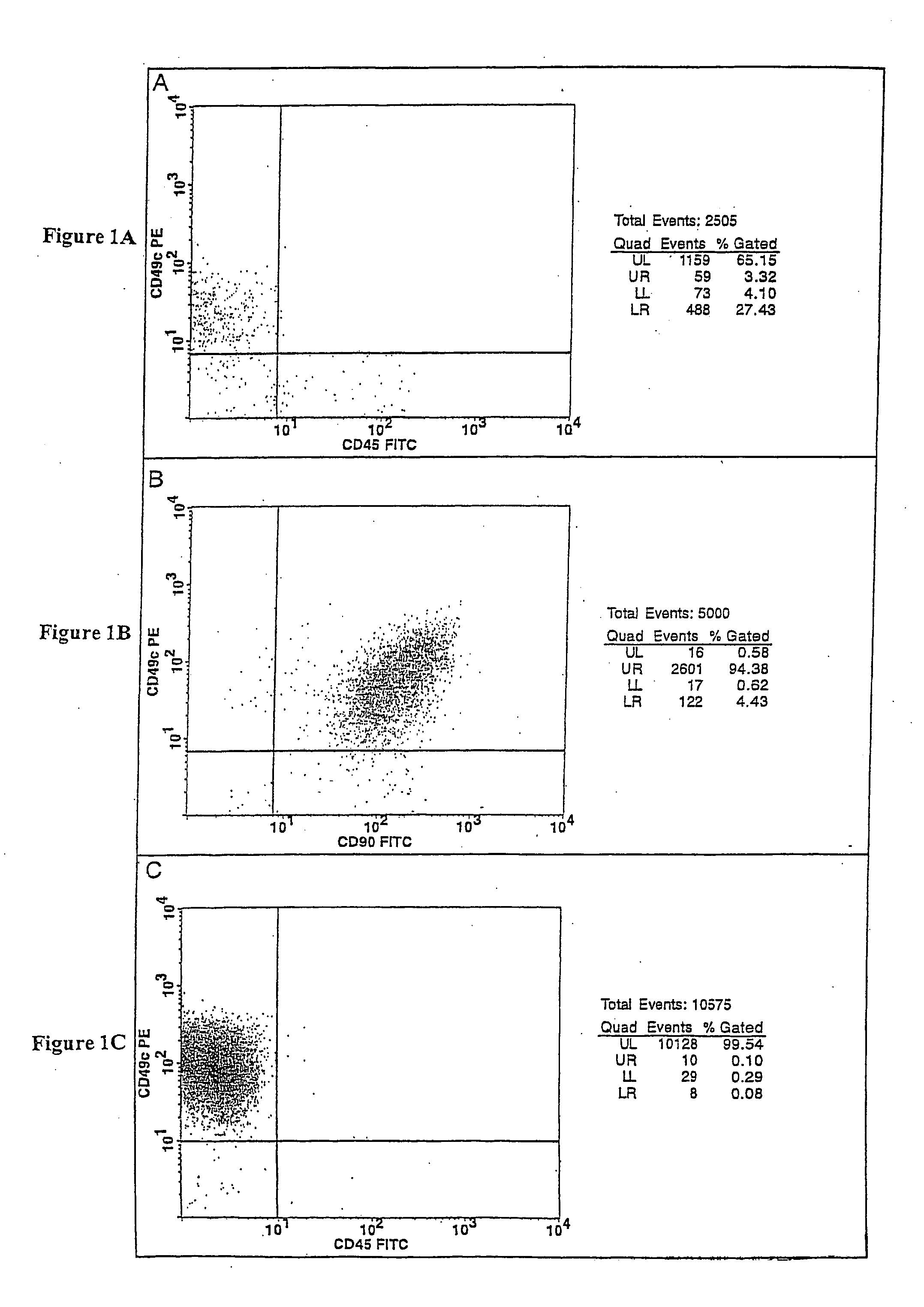
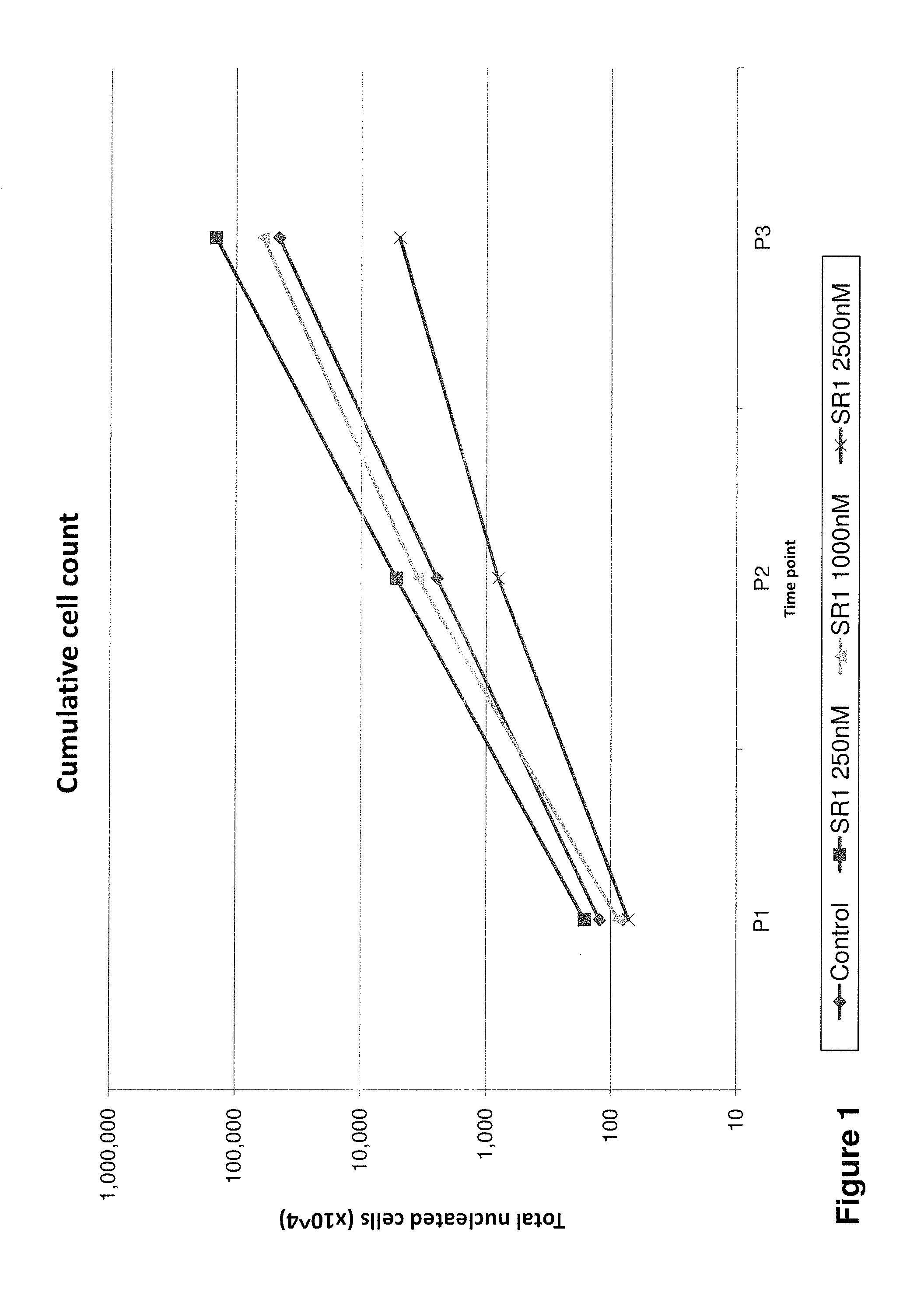
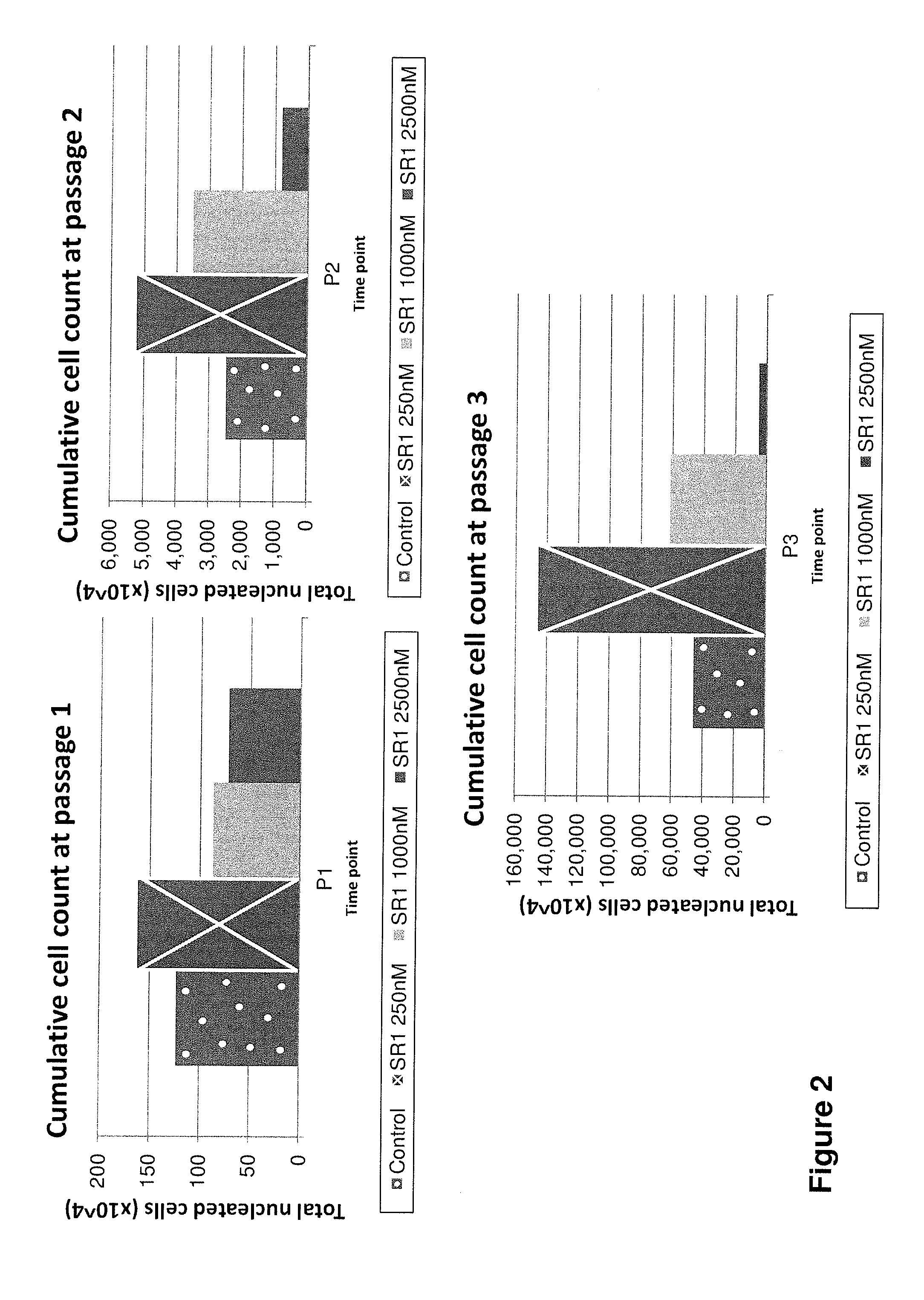
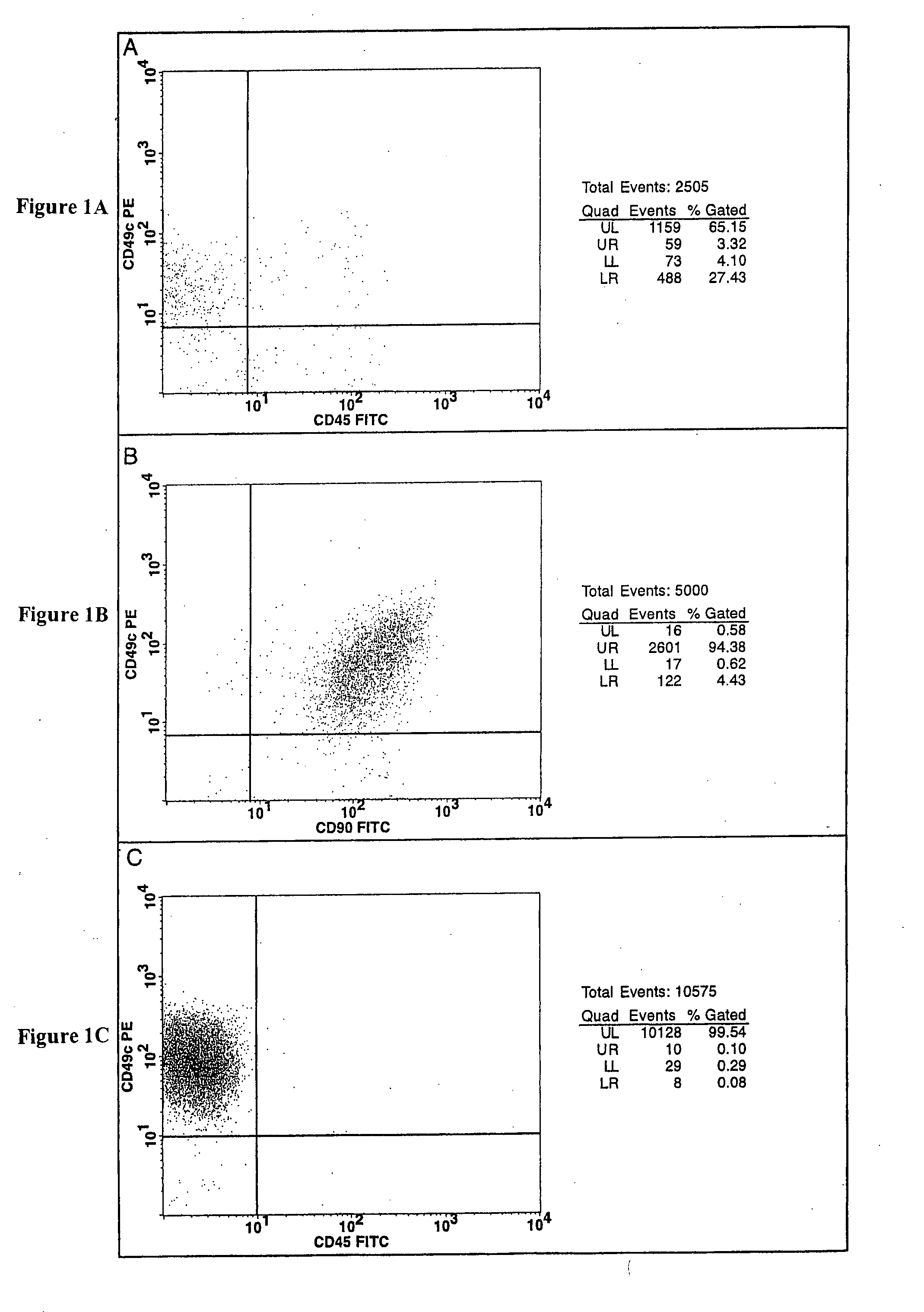
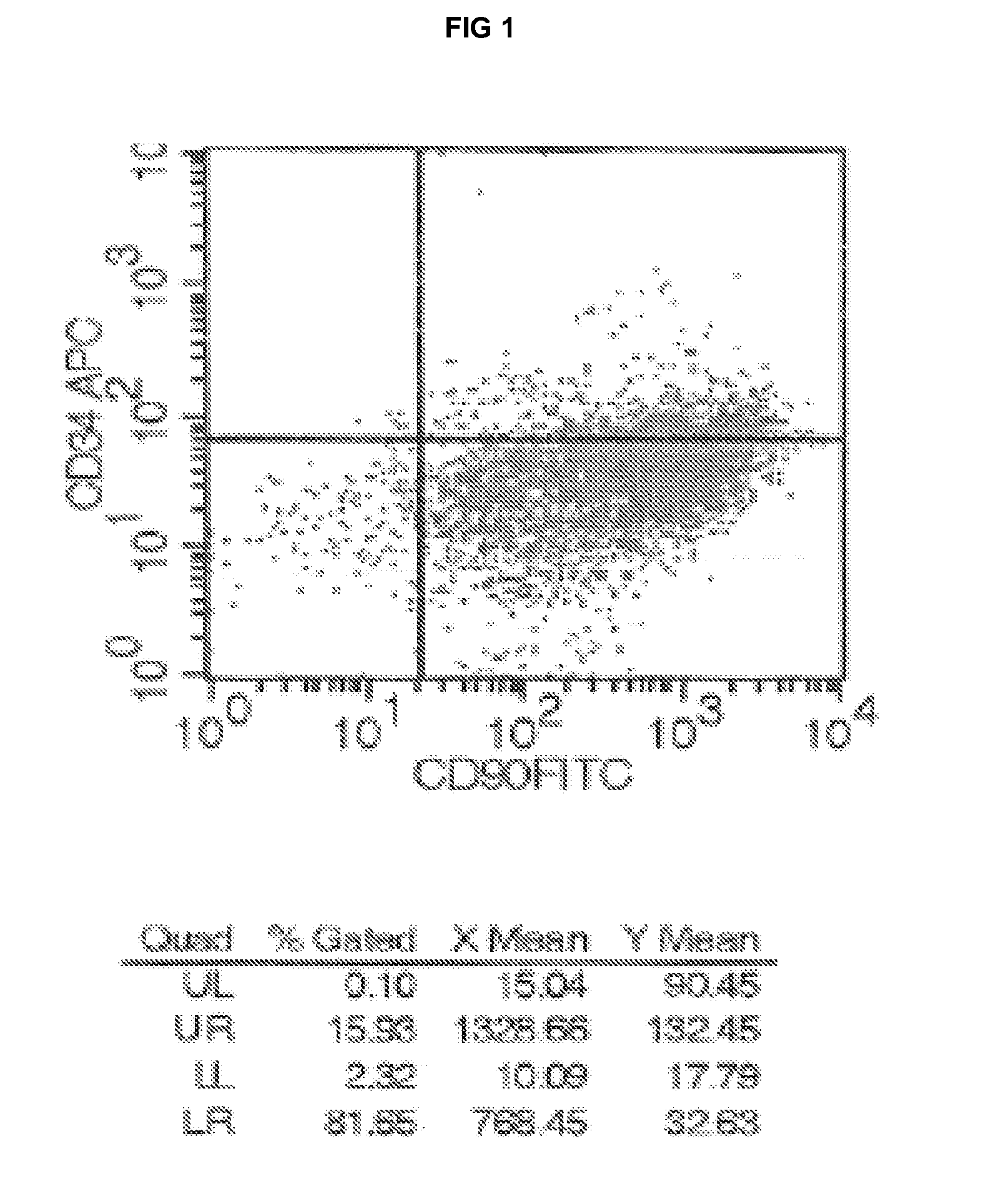
Cell populations which co-express CD49c and CD90
ActiveUS20050233452A1Promote repairIncreasing of life life expectancyBone marrow stroma cellsNervous disorderTelomeraseProgenitor
Substantially homogenous cells populations which co-express CD49c, CD90 and telomerase are made. In one embodiment, humans suffering from a degenerative, traumatic, acute injury, cardiac or neurological condition are treated with the substantially homogenous cells populations which co-express CD49c, CD90 and telomerase. In another embodiment, committed progenitor cells are made are made by selecting from a cultured source of a cell population which co-express CD49c and CD90 and modifying the cell population. The committed progenitor cells can be employed to treat a human suffering from a degenerative, traumatic, acute injury, cardiac or neurological condition and formulate pharmaceutical compositions.
Owner:GARNET BIOTHERAPEUTICS
Mesenchymal stem cells and related therapies
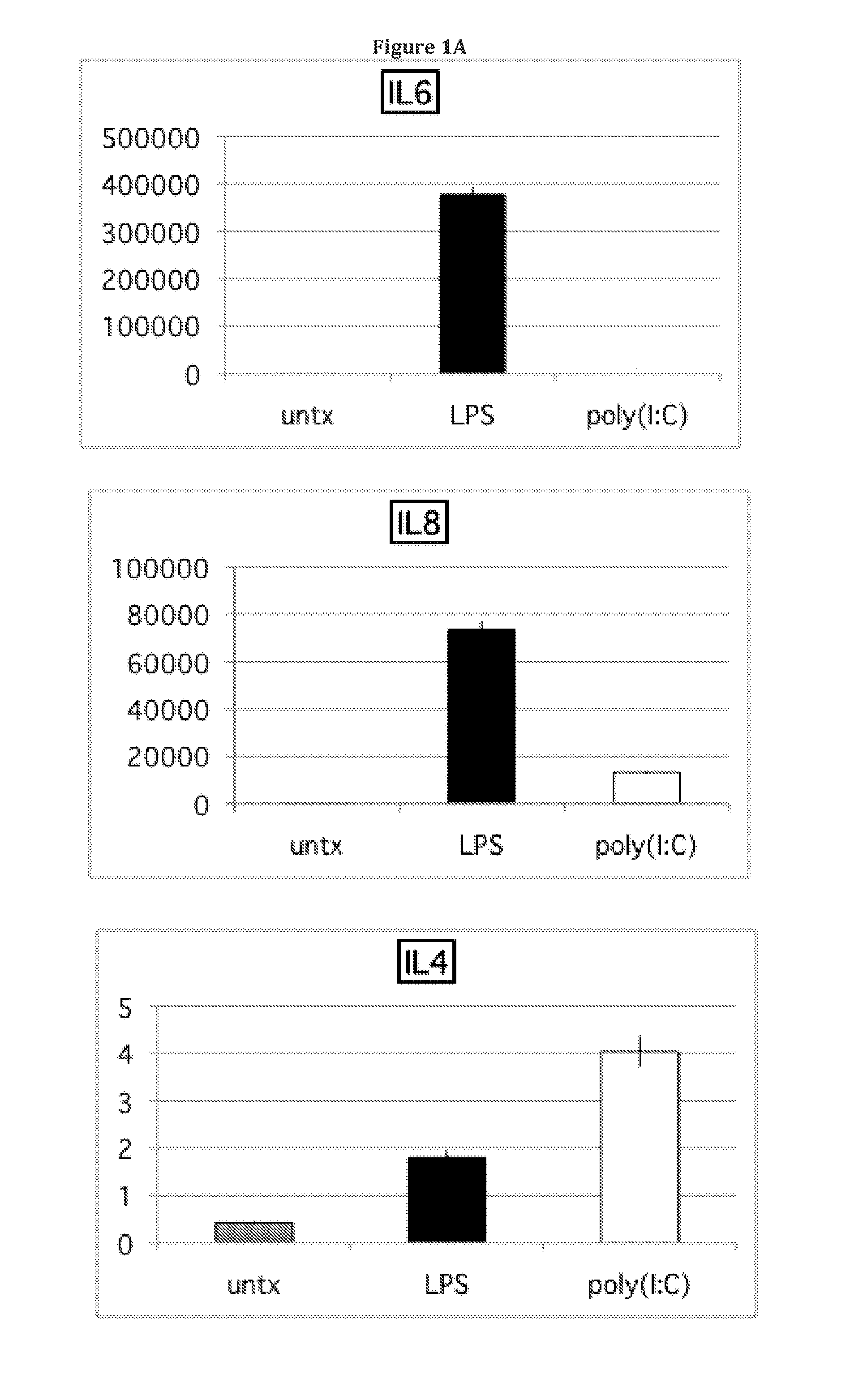
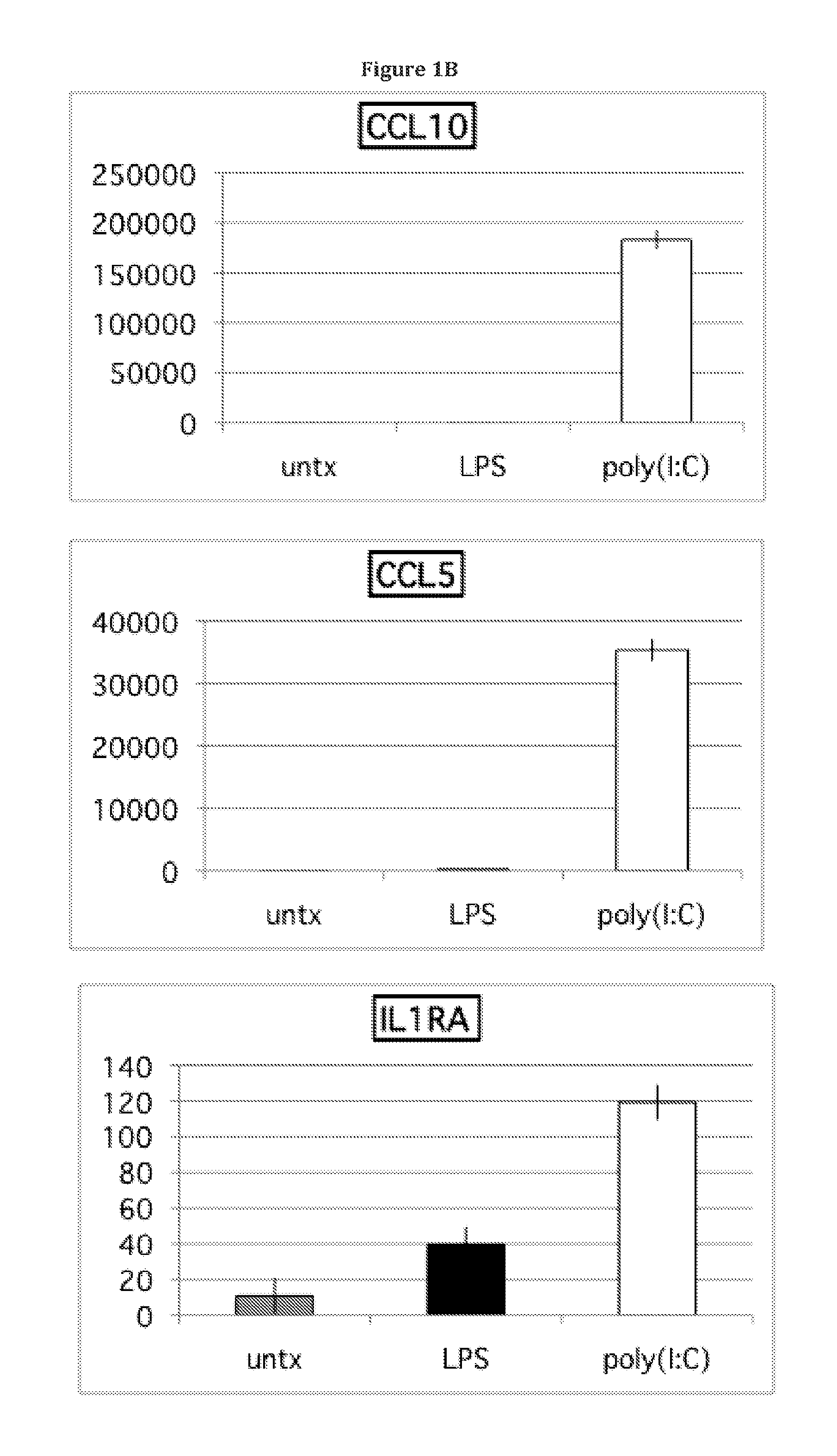
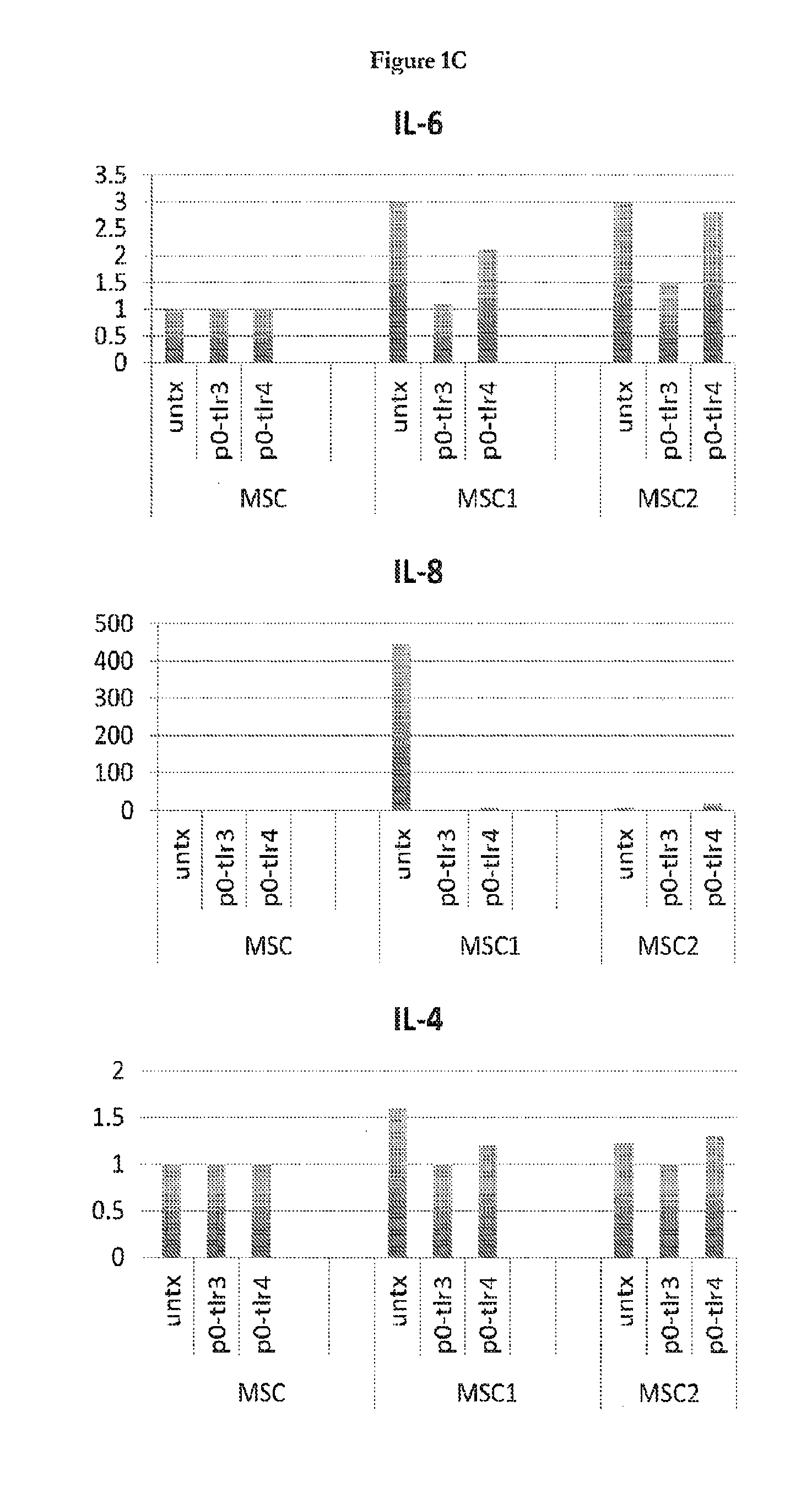
InactiveUS20140017787A1Elevated secretion of IL4Reduce secretionBone marrow stroma cellsSkeletal/connective tissue cellsMesenchymal stem cellCancer research
Mesenchymal stem cells that selectively promote or suppress inflammation are provided, as well as methods of producing and using the same.
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND
Pluripotent embryonic-like stem cells, compositions, methods and uses thereof
The present invention relates to pluripotent stem cells, particularly to pluripotent embryonic-like stem cells. The invention further relates to methods of purifying pluripotent embryonic-like stem cells and to compositions, cultures and clones thereof. The present invention also relates to a method of transplanting the pluripotent stem cells of the present invention in a mammalian host, such as human, comprising introducing the stem cells, into the host. The invention further relates to methods of in vivo administration of a protein or gene of interest comprising transfecting a pluripotent stem cell with a construct comprising DNA which encodes a protein of interest and then introducing the stem cell into the host where the protein or gene of interest is expressed. The present also relates to methods of producing mesodermal, endodermal or ectodermal lineage-committed cells by culturing or transplantation of the pluripotent stem cells of the present invention.
Owner:ABT HOLDING COMPANY
Multipotent Adult Stem Cells And Uses of Multipotent Adult Stem Cells To Treat Inflammation
InactiveUS20100172885A1Effective treatmentIncrease the number ofBiocideBone marrow stroma cellsDiseaseAutoimmune responses
Disclosed are cell preparations comprising multipotent adult stem cells and methods for using multipotent adult stem cells to treat autoimmune diseases, treat allergic responses, treat cancer, treat inflammatory diseases, treat fibrotic disorders, reduce inflammation and / or fibrosis, promote would healing, repair epithelial damage, and / or promote angiogenesis.
Owner:MESOBLAST INT
Defined cell culturing surfaces and methods of use
ActiveUS20100021998A1Excellent Adhesive PropertiesAdvantageously avoidedBone marrow stroma cellsLiquid surface applicatorsCoated surfaceCell-Extracellular Matrix
In one aspect, there is provided a cell culturing substrate including: a cell culture surface having a film attached thereto, wherein the film includes one or more plasma polymerized monomers; and a coating on the film-coated surface, the coating deposited from a coating solution comprising one or more extracellular matrix proteins and an aqueous solvent, where the total extracellular matrix protein concentration in the coating solution is about 1 ng / mL to about 1 mg / mL.
Owner:CORNING INC
Perfusion Device and Method
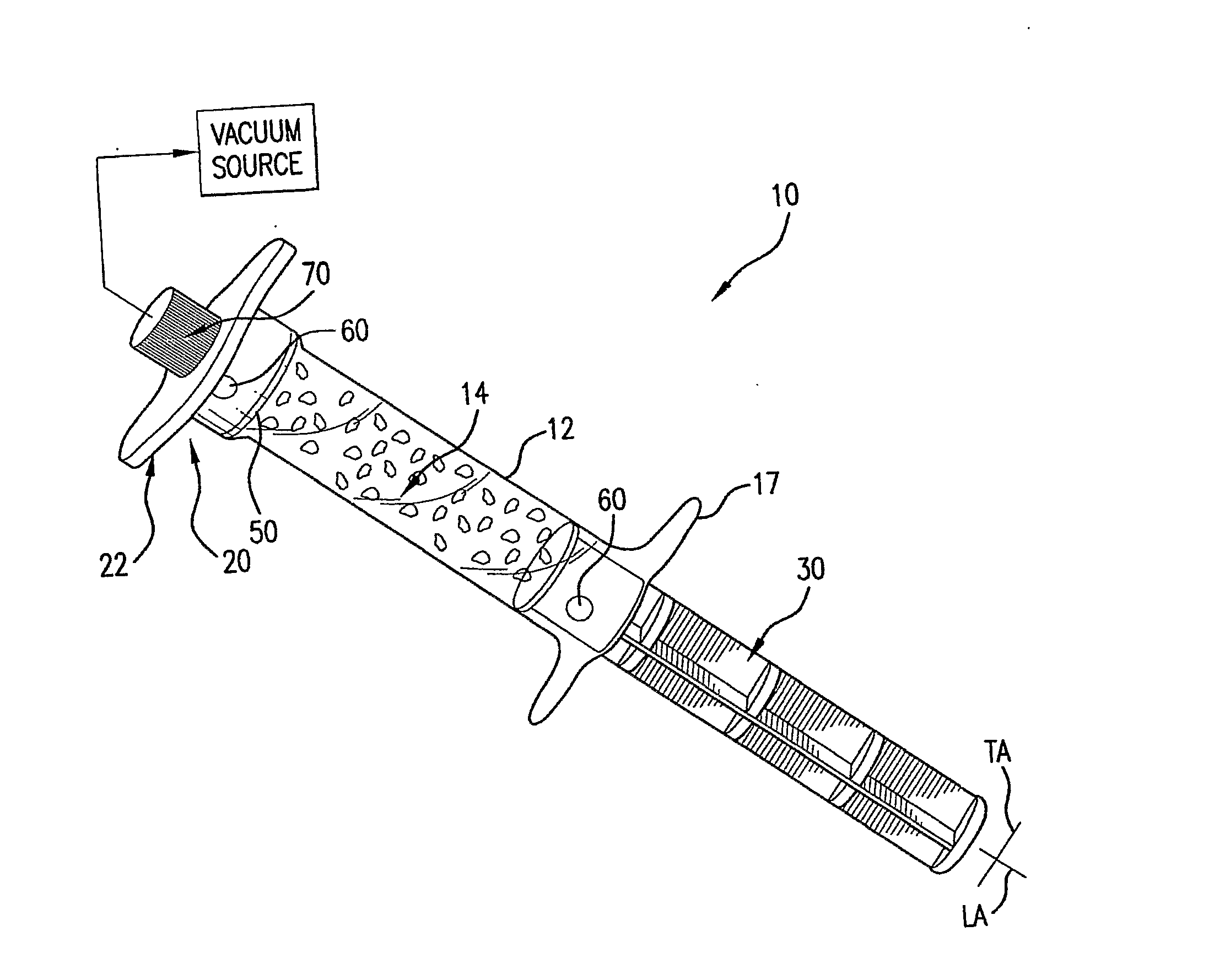
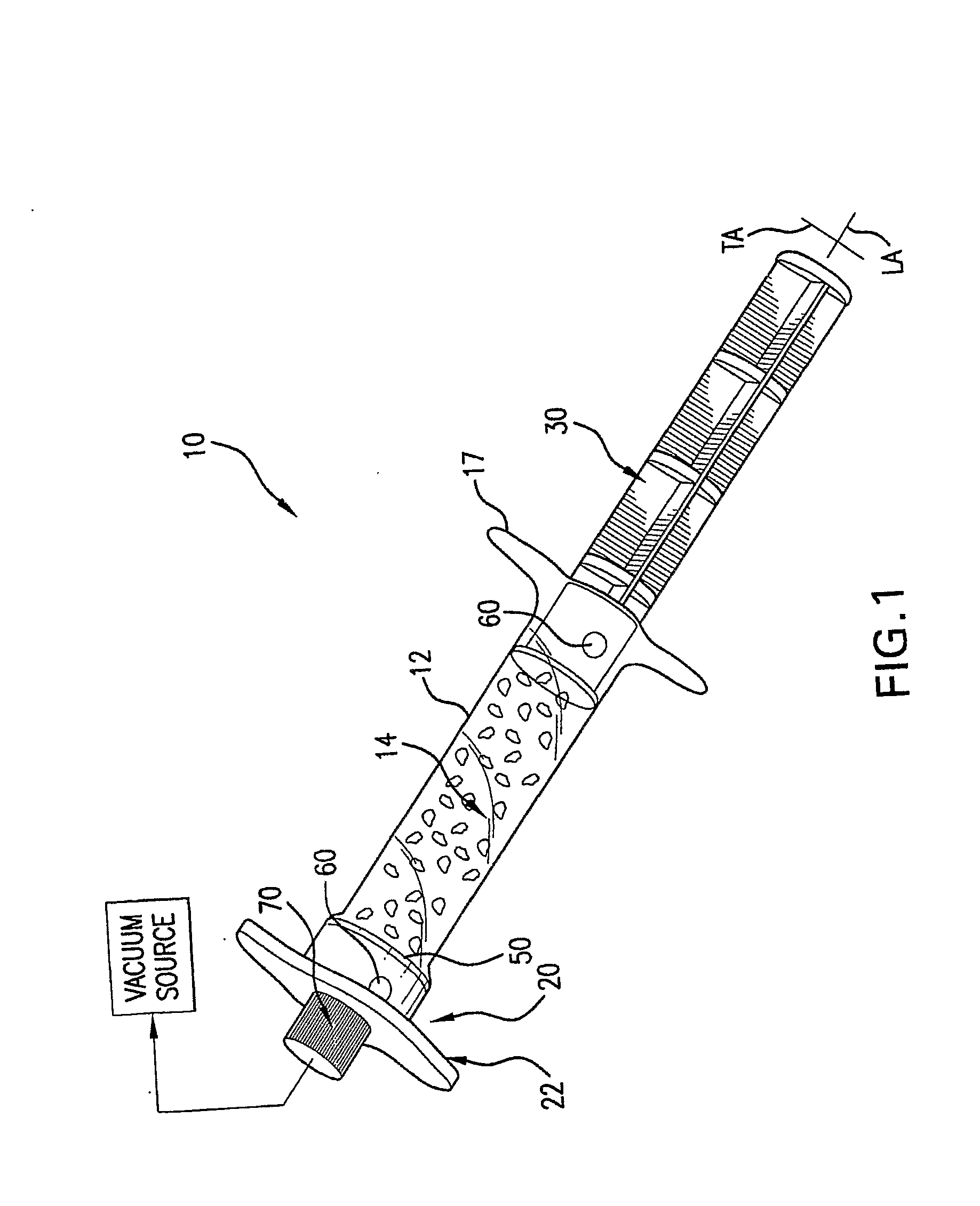
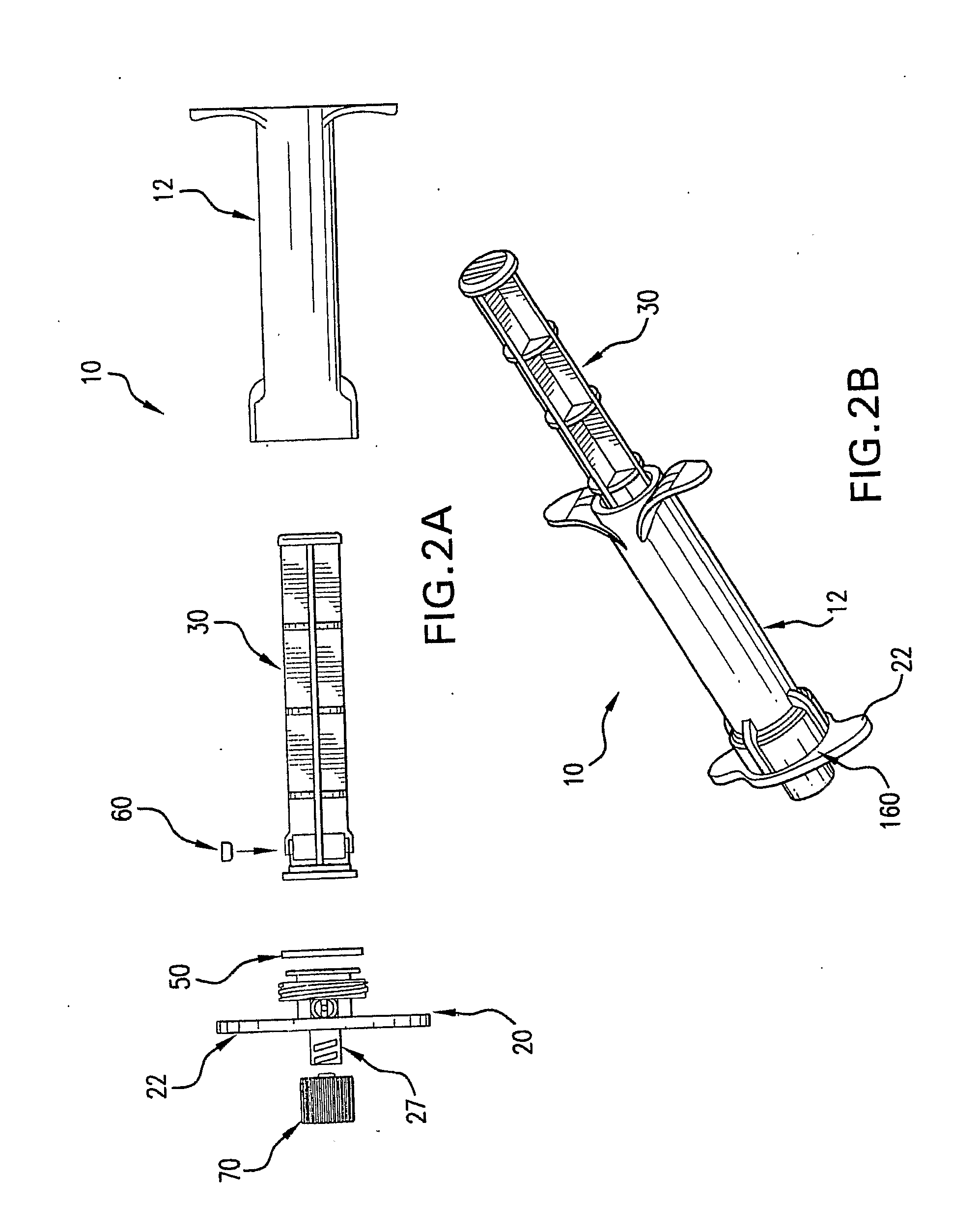
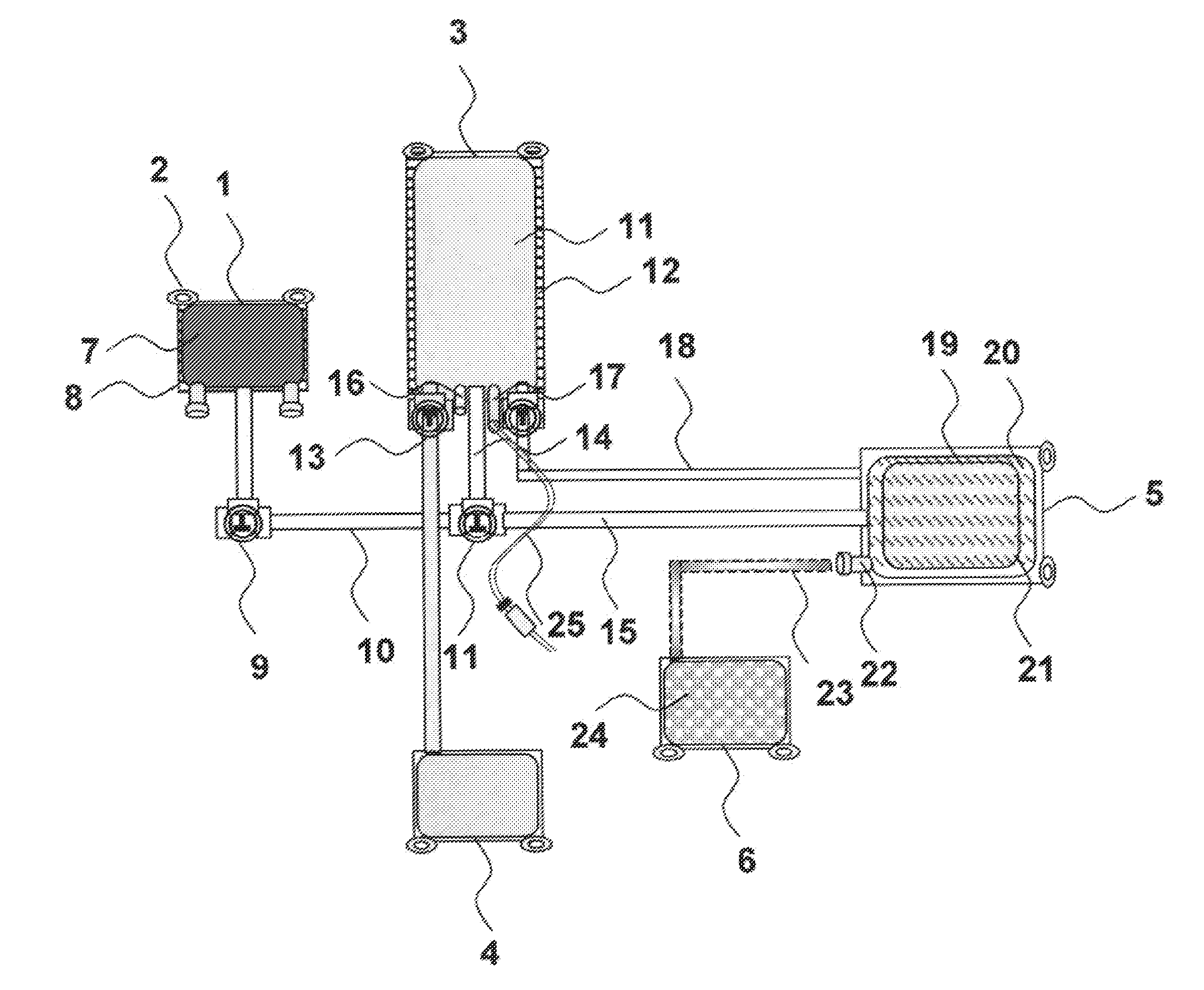
ActiveUS20080214998A1Great perfusionShorten the timeBone marrow stroma cellsDiagnosticsBiological materialsPerfusion
A perfusion device and method is provided. In one embodiment, the device includes a container having a first internal chamber configured to hold the material; a port for introducing the liquid into the chamber; a vent for releasing gas and liquid from the chamber; and a means for sealing the vent to allow a vacuum to be drawn on the first chamber The material may be biomaterial, such as a bone graft material in any form. In one embodiment, the container is a syringe that defines the internal material chamber and includes an end cap and a plunger The vent may be formed by a venting passageway in plunger and / or cap in some embodiments. In one embodiment, the vacuum may be created by a medical syringe coupled to the container, and which in some embodiments may also be used to deliver the liquid into the container The liquid may be bone marrow aspirate in some embodiments.
Owner:SYNTHES GMBH
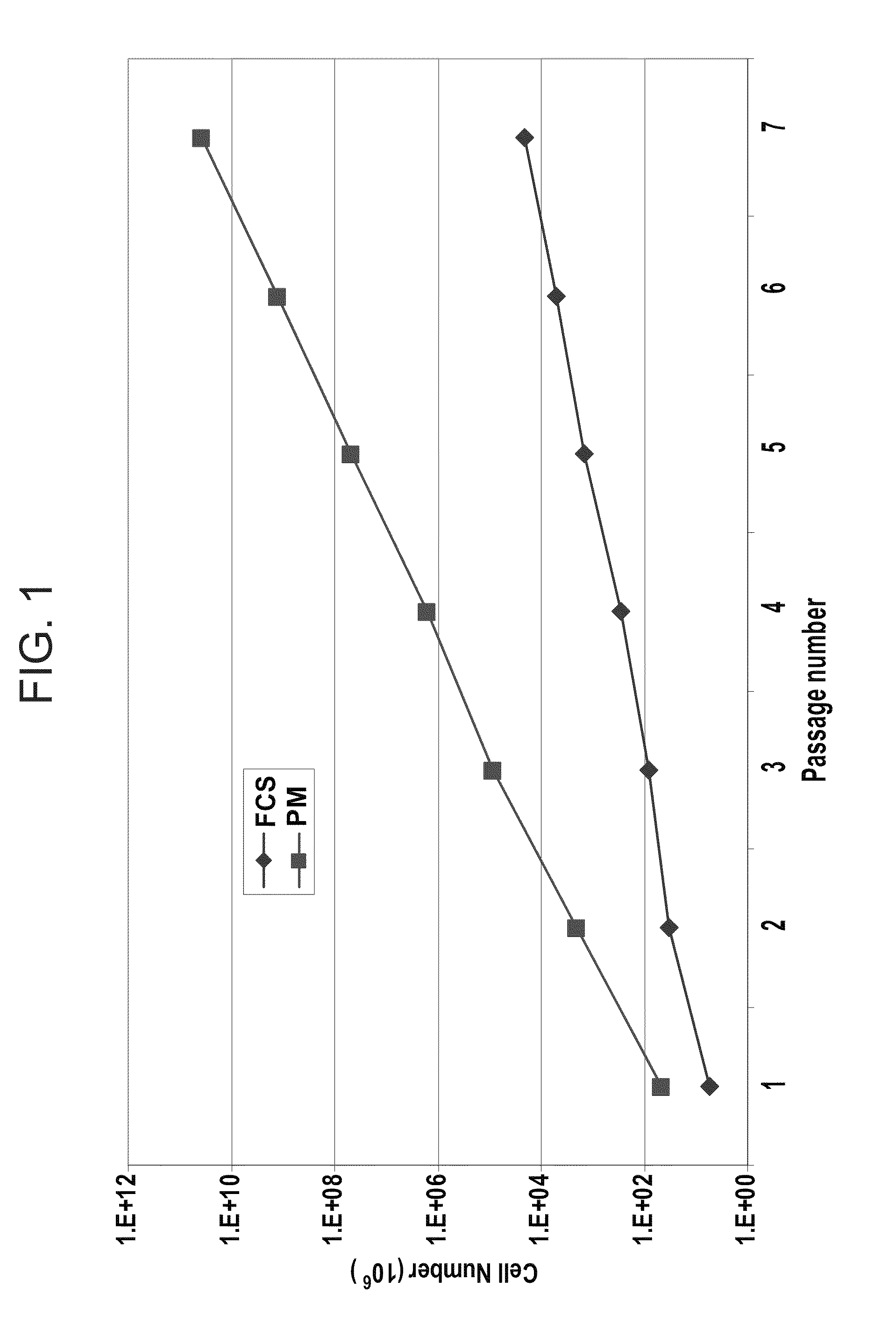
Method for cell expansion
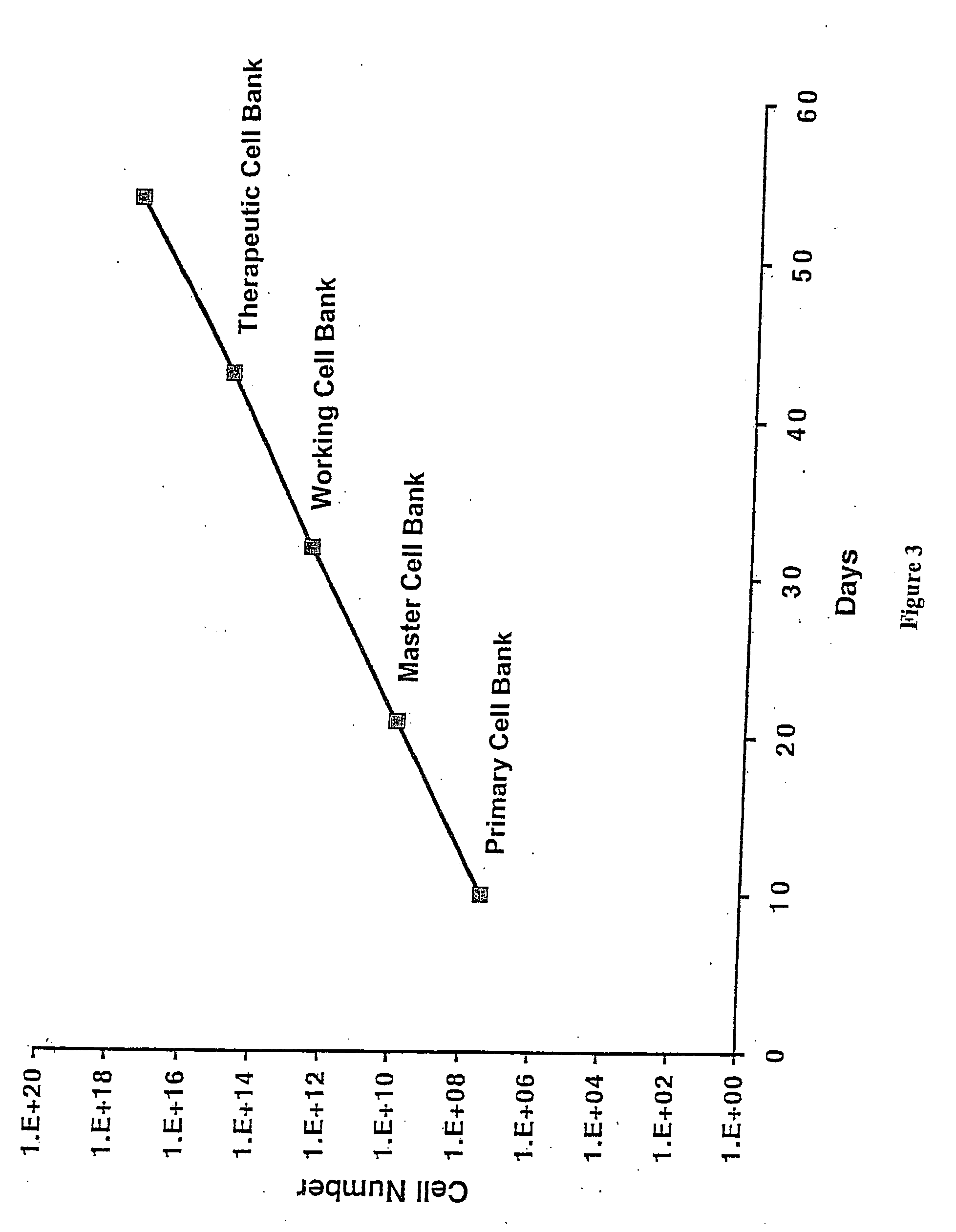
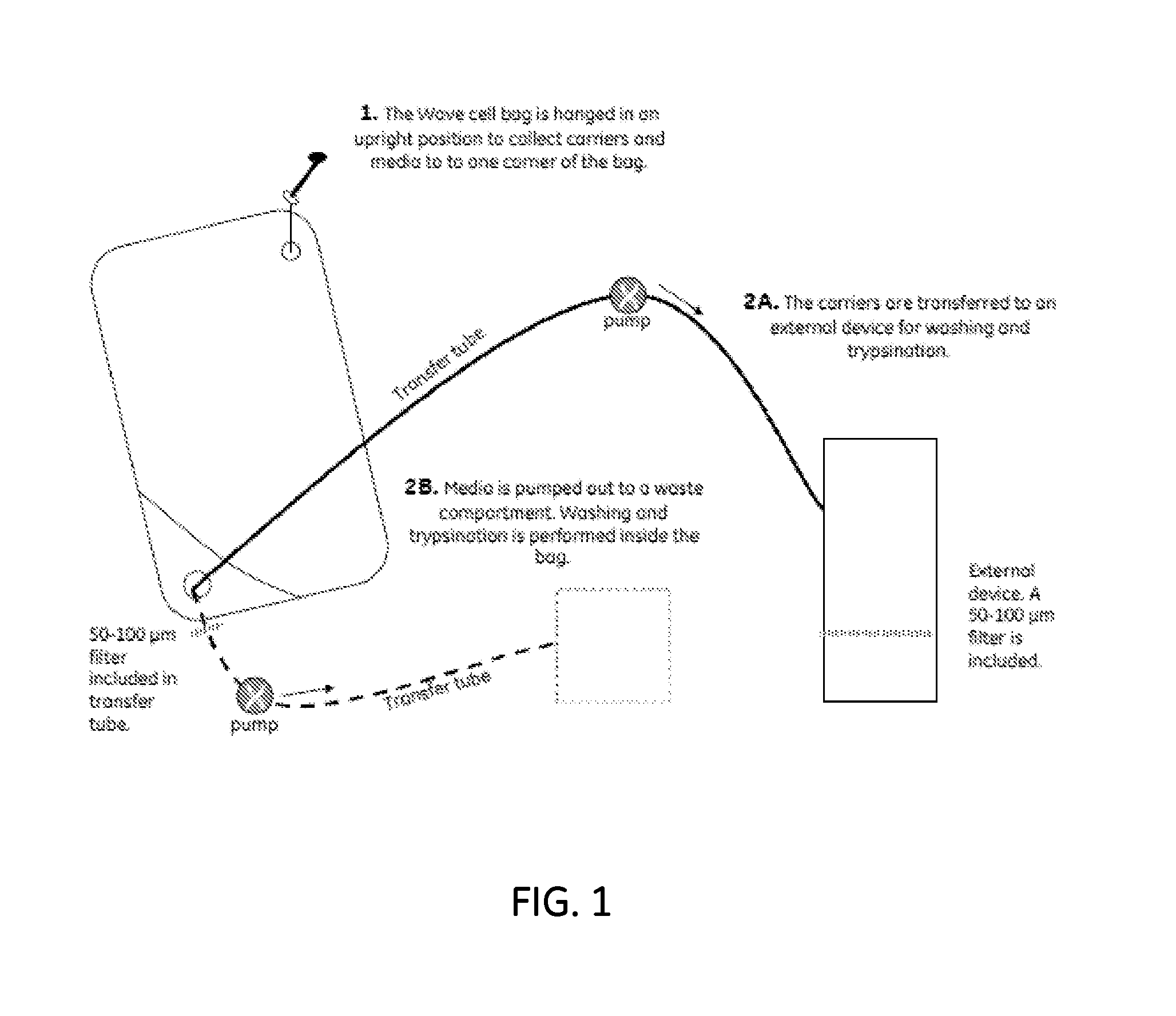
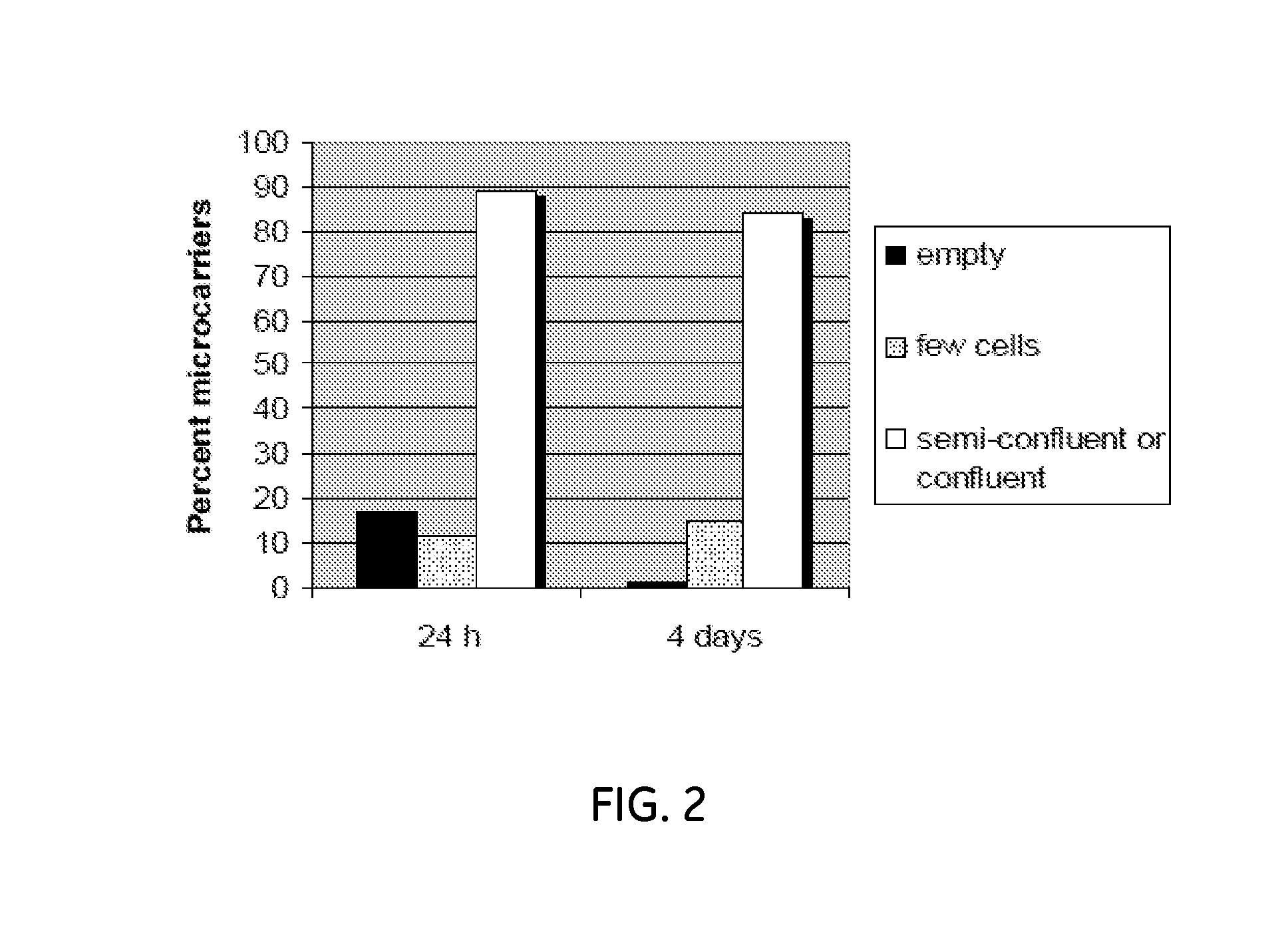
ActiveUS20130236970A1Increase surface areaBone marrow stroma cellsBiomass after-treatmentCell adhesionCell culture media
The present invention relates to a method for cell expansion. More closely, it relates to a method for expansion of cells, such as mesenchymal stem cells, on microcarriers in a plastic bag bioreactor. The invention enables expansion to therapeutic amounts of stem cells. The method comprises the following steps: a) addition of cells in cell culture medium and microcarriers to a plastic bag container; b) allowing the cells to adhere to the microcarriers while the container is kept substantially still; c) addition of further cell culture medium once the cells have adhered; d) culturing the cells under gentle and constant agitation; e) increase the surface area for continued culturing; and f) final harvesting of cells by an active detachment and separation step.
Owner:CYTIVA SWEDEN AB
Continuous culture apparatus with mobile vessel, allowing selection of fitter cell variants and producing a culture in a continuous manner
InactiveUS20070037276A1Improve securityBone marrow stroma cellsBioreactor/fermenter combinationsZoologyStem cell
Owner:DE CRECY EUDES FRANCOIS MARIE
Isolated Oligodendrocyte-Like Cells and Populations Comprising Same for the Treatment of CNS Diseases
Isolated human cells and populations thereof are provided comprising at least one oligodendrocyte phenotype and at least one mesenchymal stem cell phenotype, wherein the mesenchymal stem cell phenotype is not an oligodendrocyte phenotype. Methods of generating and using same are also provided.
Owner:RAMOT AT TEL AVIV UNIV LTD
Composition and method for culturing potentially regenerative cells and functional tissue-organs in vitro
Compositions and methods are provided for culturing in vitro potentially regenerative cells (PRCs) from which functional tissue-organs are regenerated. In one aspect of the invention, a tissue culture medium is provided which comprises at least 50% of water and a sterol compound that is dissolved in a fatty acid-containing oil at a concentration at least 0.1% by weight based on the weight of the oil and added to the water. The culture medium can be used to culture PRCs that are isolated from the body of a mammal to generate functional tissue-organs in vitro with substantially the same physiological structure and function as the corresponding ones existing in vivo and in situ. The cultured PRCs, tissues, and tissue-organs can serve as valuable models for scientific investigation in life sciences, nutraceutical discovery, drug screening, pharmacokinetic studies, medical devices and tissue / organ transplantation.
Owner:RONGXIANG XU
Skeletal muscle regeneration using mesenchymal system cells
ActiveUS20130259843A1Enhancing functional recovery and structural regenerationRelieve painBone marrow stroma cellsBiocideMedicineMesenchymal stem cell
The present invention relates to a therapeutic substance and / or medicament and methods relating to the use of said substance and / or medicament for skeletal muscle regeneration using mesenchymal stem cells (MSCs) which can be applied directly or shortly after muscle damage or injury.
Owner:PLURI BIOTECH LTD
Cardiac muscle regeneration using mesenchymal stem cells
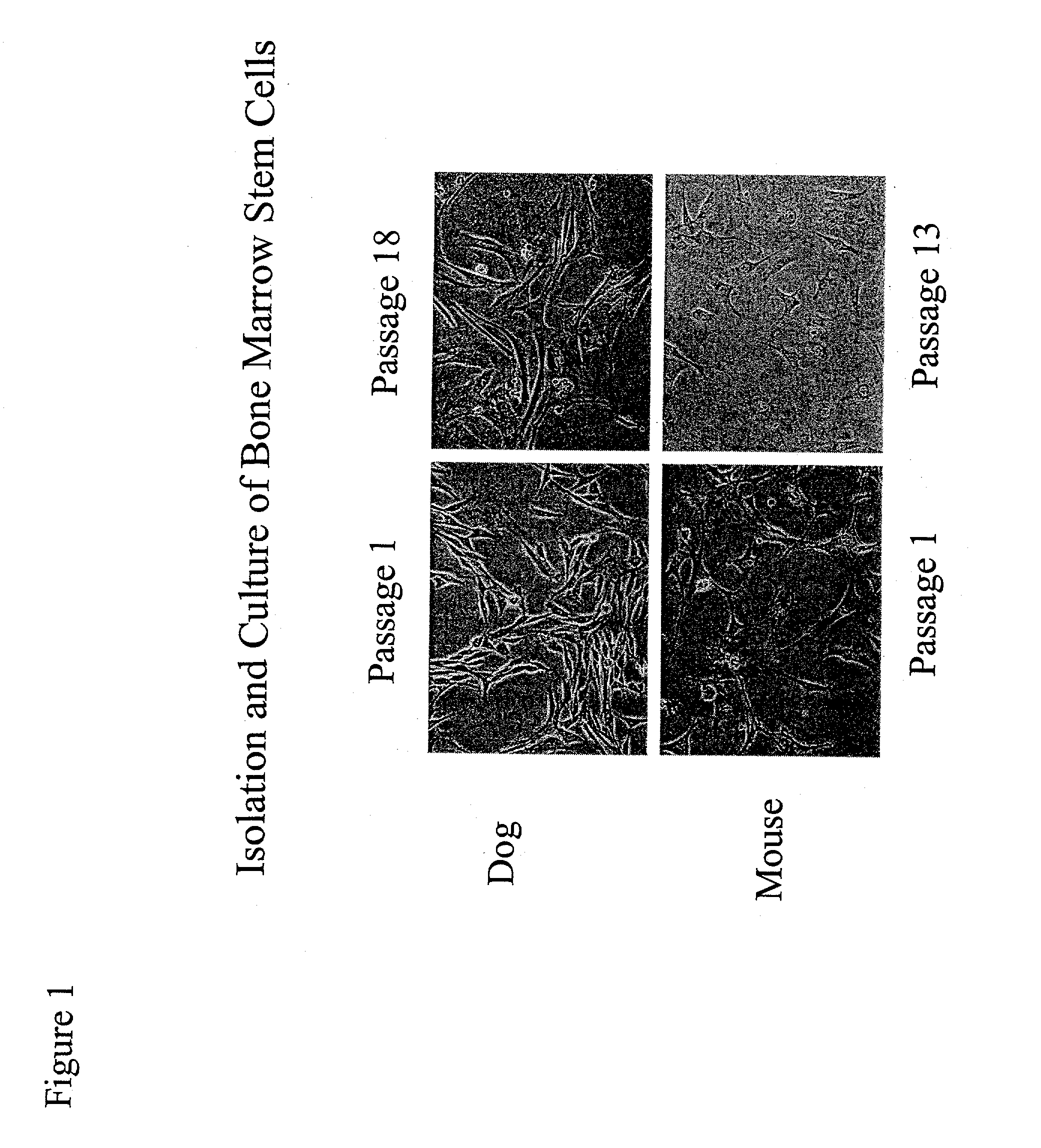
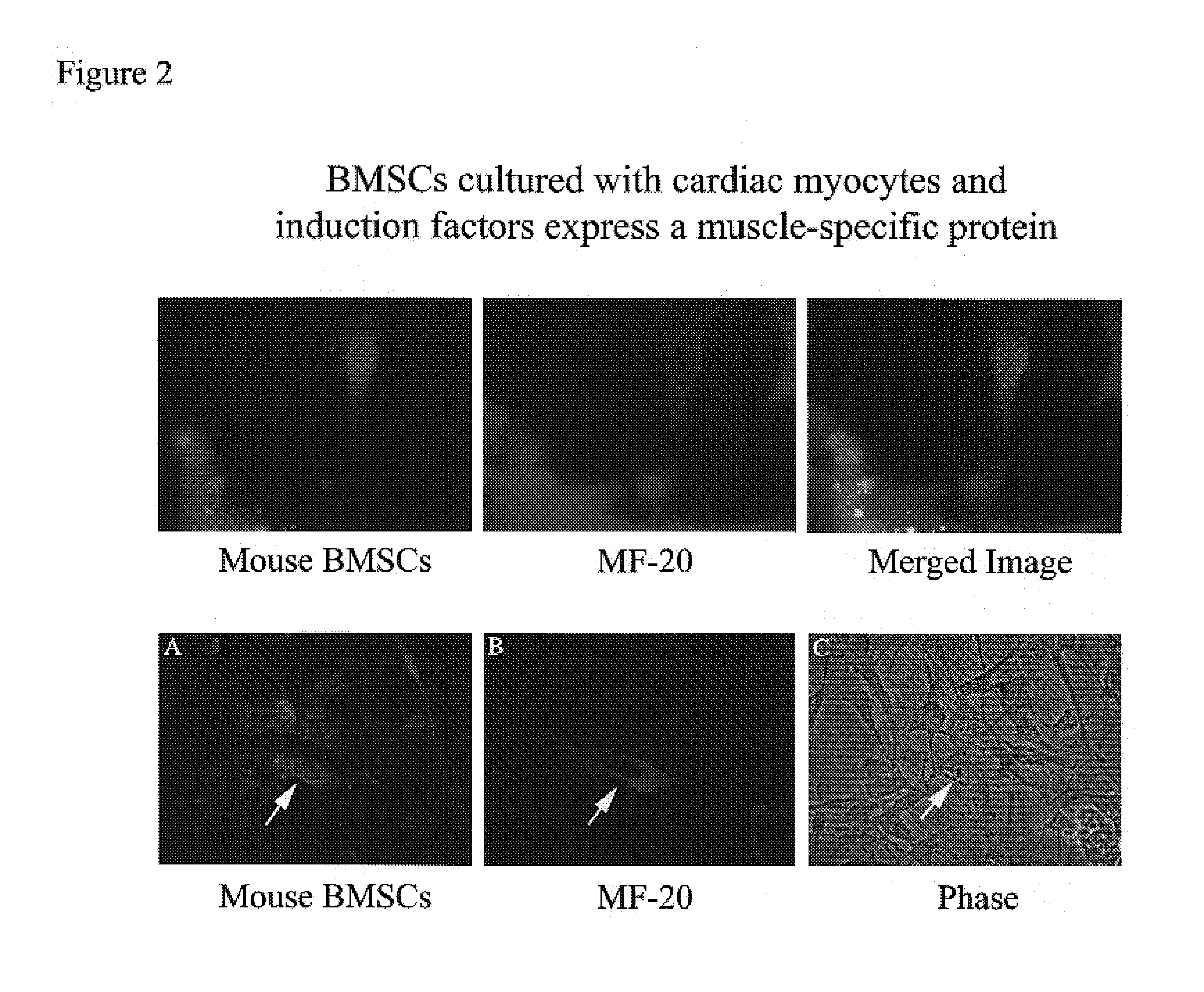
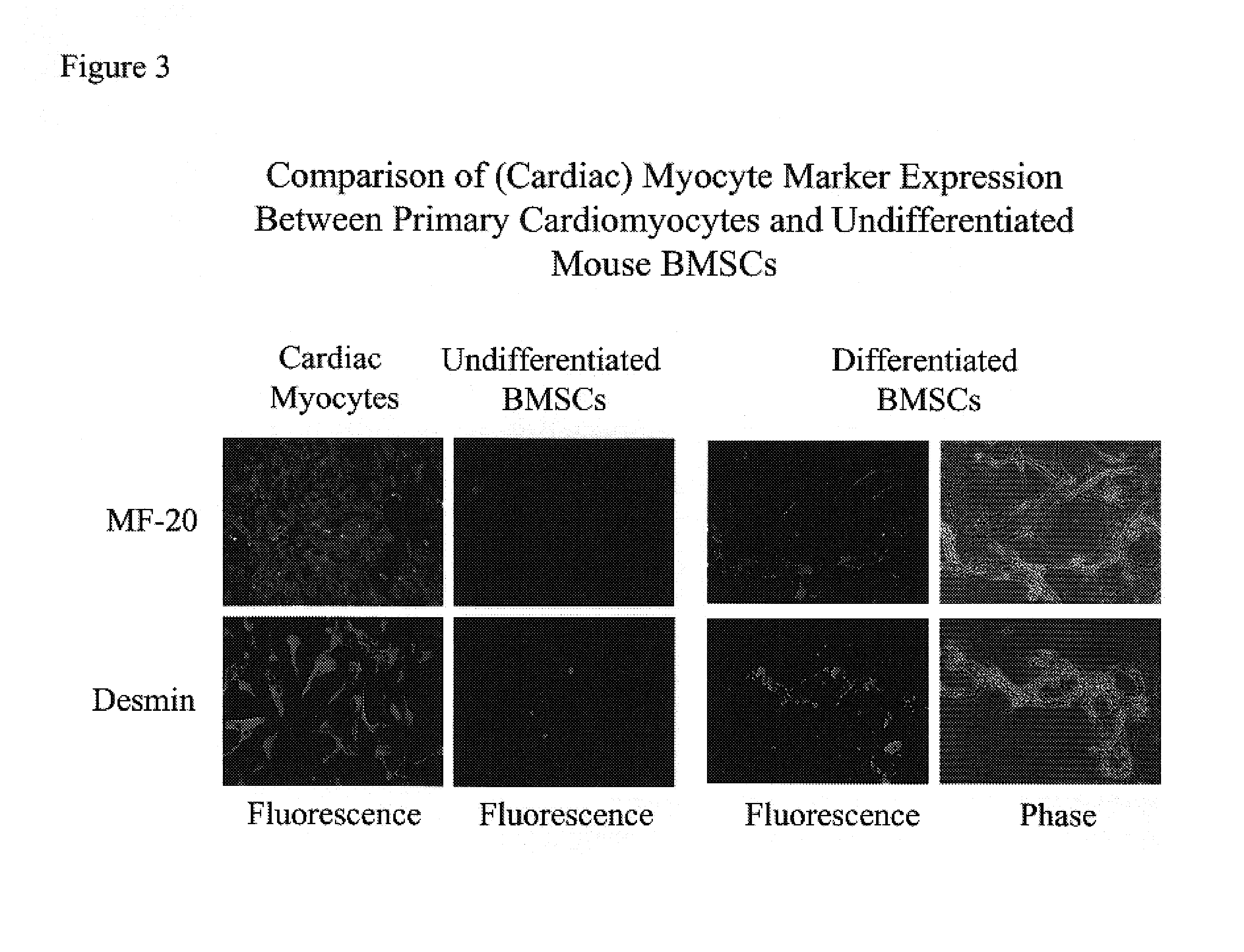
InactiveUS20070003530A1Shorten the timeEasy to convertBone marrow stroma cellsBiocideCardiac muscleMesenchymal stem cell
Disclosed is a method for producing cardiomyocytes in vivo by administering to the heart of an individual a cardiomyocyte producing amount of mesenchymal stem cells. These cells can be administered as a liquid injectible or as a preparation of cells in a matrix which is or becomes solid or semi-solid. The cells can be genetically modified to enhance myocardial differentiation and integration. Also disclosed is a method for replacing cells ex vivo in a heart valve for implantation.
Owner:MESOBLAST INT
Thermoresponsive cell culture supports
ActiveUS20140193911A1Increased cell-cell interactionEliminate riskBone marrow stroma cellsSkeletal/connective tissue cellsPolymerAdhesion promoters
The present invention relates to a cell culture support comprising a substrate and a thermoresponsive polymeric blend layer, wherein the polymeric blend layer comprises at least one thermoresponsive polymer and at least one network forming adhesion promoter. The present invention further relates a method of making a cell culture complex comprising: providing a substrate; blending at least one thermoresponsive polymer and at least one network forming adhesion promoter to provide a polymeric blend; applying a thin film of said polymeric blend to the substrate to provide a polymeric blend layer on the substrate; curing the polymeric blend layer on the substrate to provide a cell culture support; and depositing cells onto said cell culture support, wherein the cells may optionally further comprise medium, to provide a cell culture complex.
Owner:THE UNIVERSITY OF AKRON
Closed system separation of adherent bone marrow stem cells for regenerative medicine applications
ActiveUS20130101561A1Elicit neuronal and astrocytic differentiationMinimally invasiveBiocideBone marrow stroma cellsCell adhesionBone Marrow Stem Cell
A method for isolating and processing bone marrow derived stem cells, including the steps of: (a) collecting a biological sample containing adherent bone marrow stem cells in a receptacle with interior walls coated with a cell-adherent substrate; (b) incubating the bone marrow cells on the adherent substrate so that a layer of adherent bone marrow stem cells adheres to the substrate; (c) washing any non-adherent cells from the substrate; and (d) collecting the bone marrow stem cell layer. Isolation kits and use of bone marrow cells harvested for cell therapies are also described.
Owner:RUTGERS THE STATE UNIV
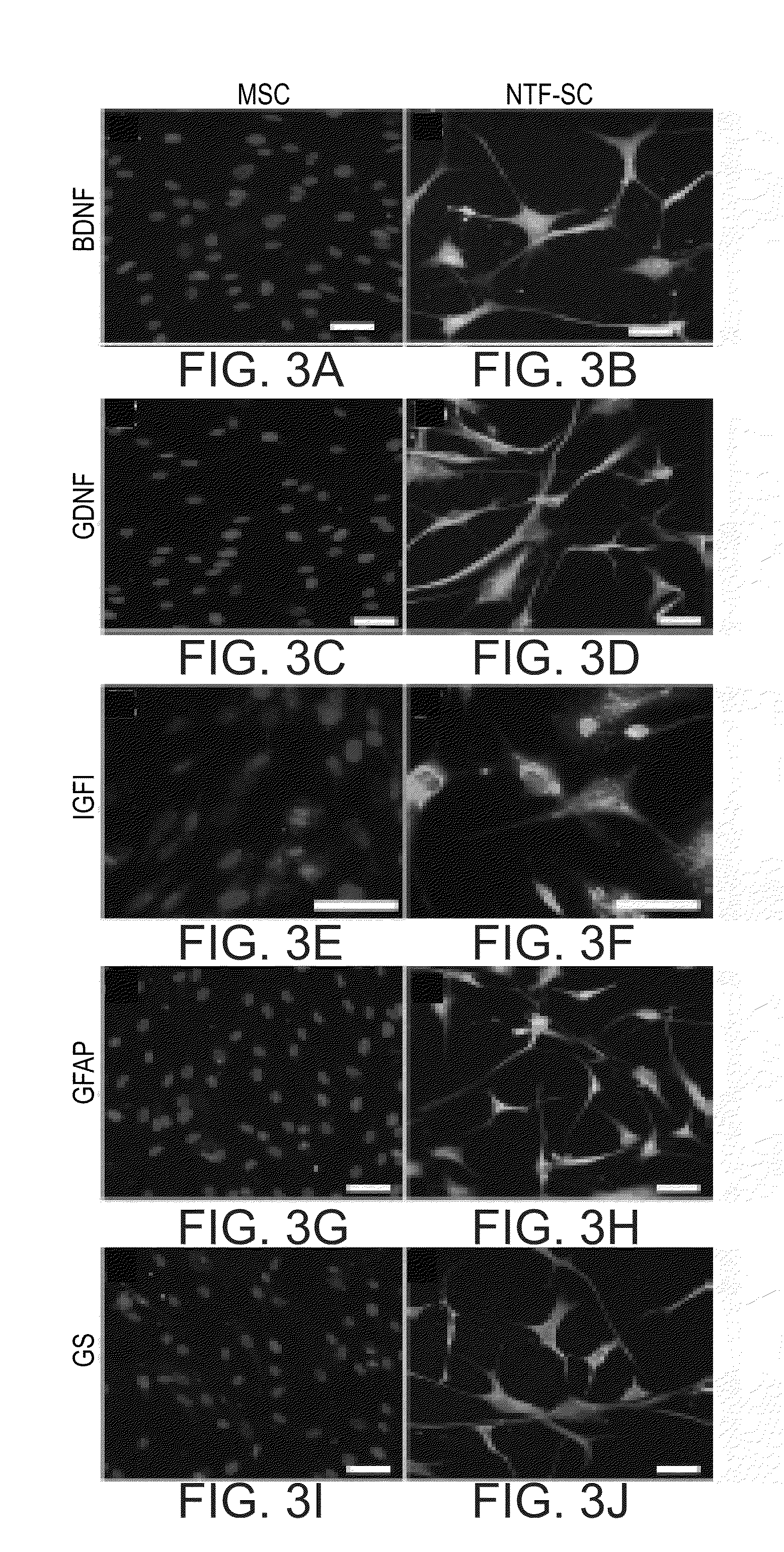
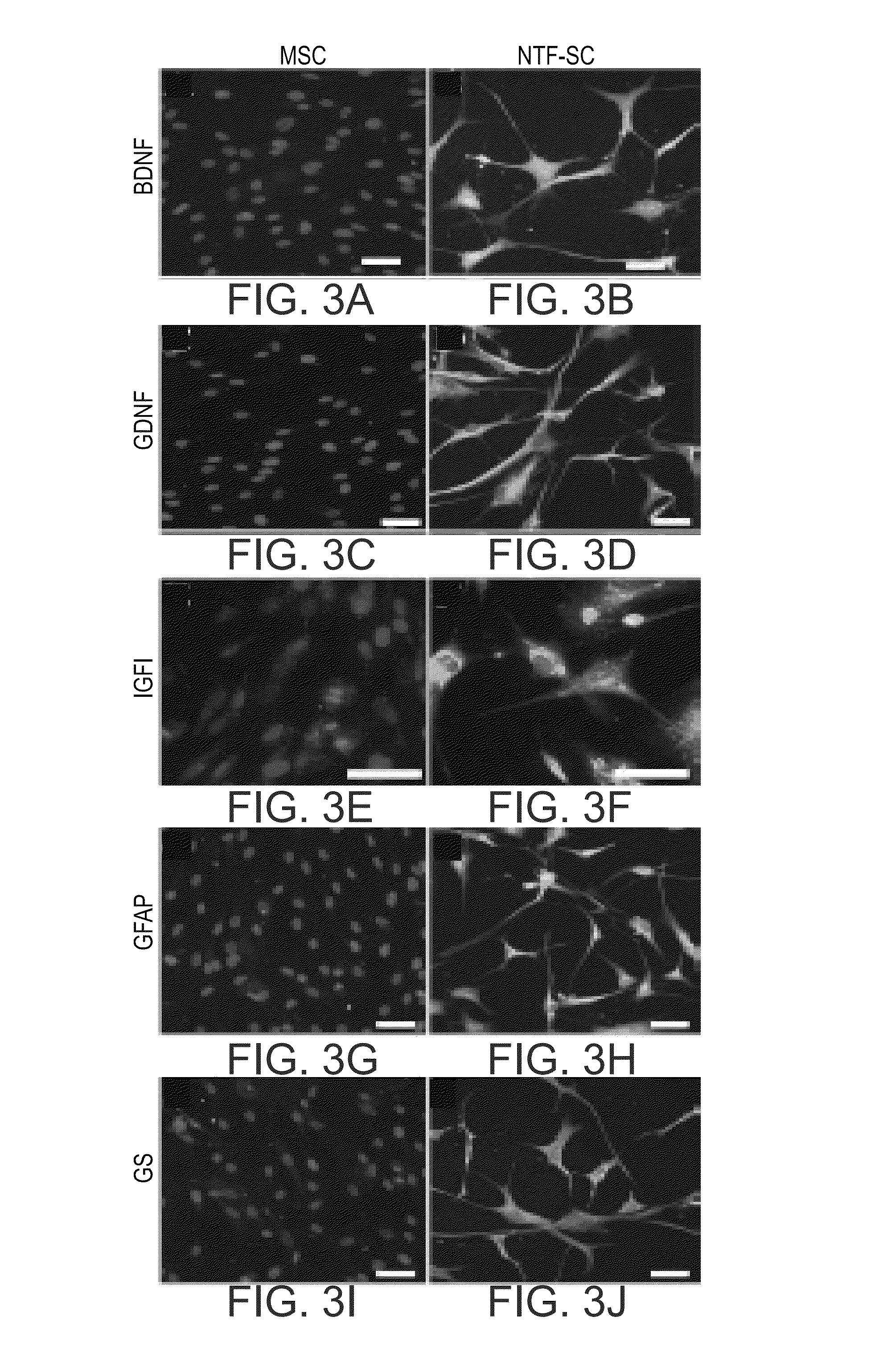
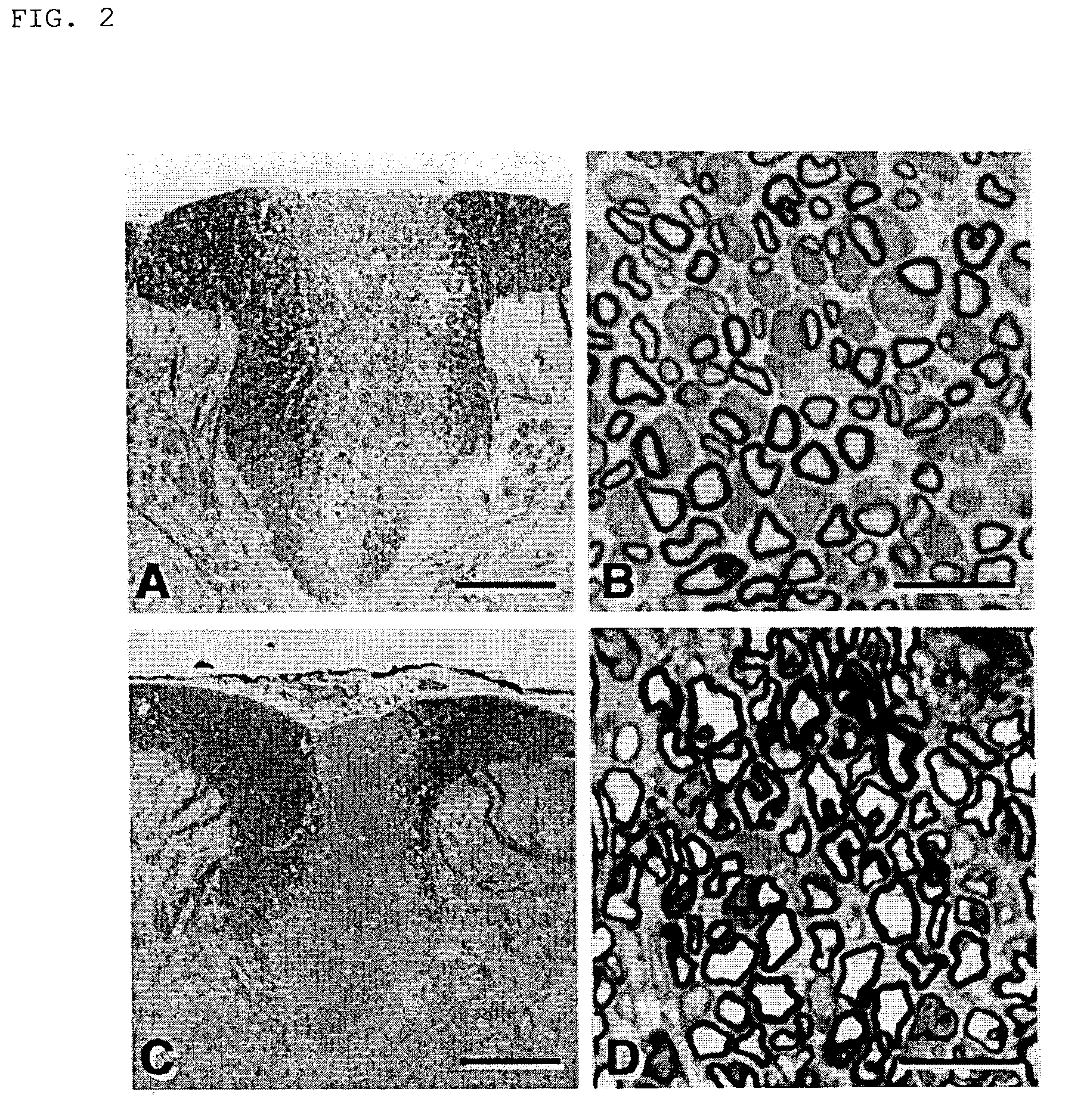
Mesenchymal stem cells for the treatment of CNS diseases
ActiveUS20120009673A1Bone marrow stroma cellsNervous disorderCns diseaseBrain-derived neurotrophic factor
An isolated human cell is disclosed comprising at least one mesenchymal stem cell phenotype and secreting brain-derived neurotrophic factor (BDNF), wherein a basal secretion of the BDNF is at least five times greater than a basal secretion of the BDNF in a mesenchymal stem cell. Methods of generating same and uses of same are also disclosed.
Owner:BRAINSTORM CELL THERAPEUTICS +1
Methods of Culturing and Expanding Mesenchymal Stem Cells and Isolated Cell Populations Generated Thereby
A method of culturing mesenchymal stem cells (MSCs) is provided. The method comprising culturing a population of the MSCs in a medium comprising an aryl hydrocarbon receptor antagonist, thereby culturing MSCs.
Owner:GAMIDA CELL
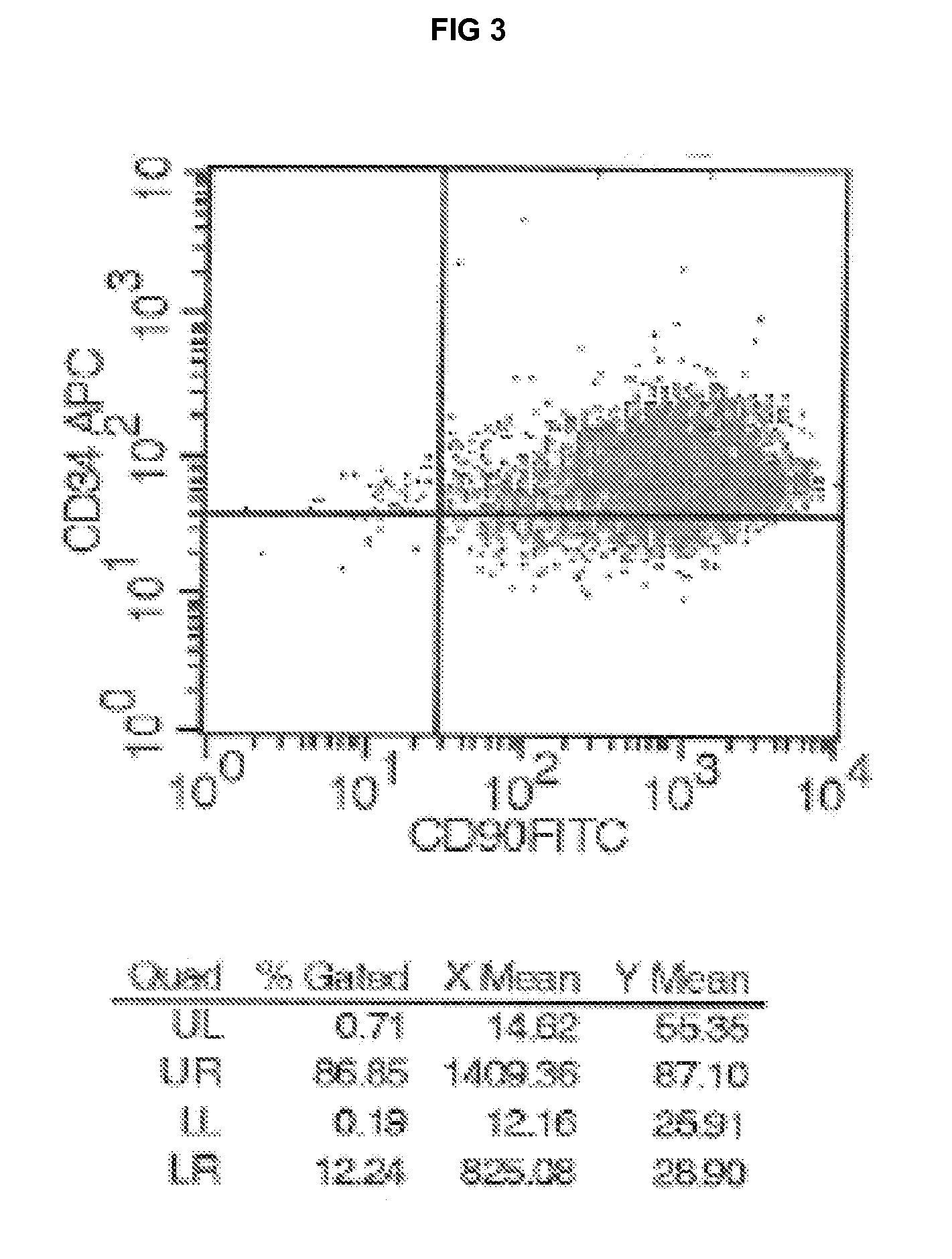
Cell populations which co-express CD49c and CD90
InactiveUS20070231309A1Promote repairIncreasing quality of life and life expectancyBiocideBone marrow stroma cellsTelomeraseProgenitor
Substantially homogenous cells populations which co-express CD49c, CD90 and telomerase are made. In one embodiment, humans suffering from a degenerative, traumatic, acute injury, cardiac or neurological condition are treated with the substantially homogenous cells populations which co-express CD49c, CD90 and telomerase. In another embodiment, committed progenitor cells are made are made by selecting from a cultured source of a cell population which co-express CD49c and CD90 and modifying the cell population. The committed progenitor cells can be employed to treat a human suffering from a degenerative, traumatic, acute injury cardiac or neurological condition and formulate pharmaceutical compositions.
Owner:GARNET BIOTHERAPEUTICS
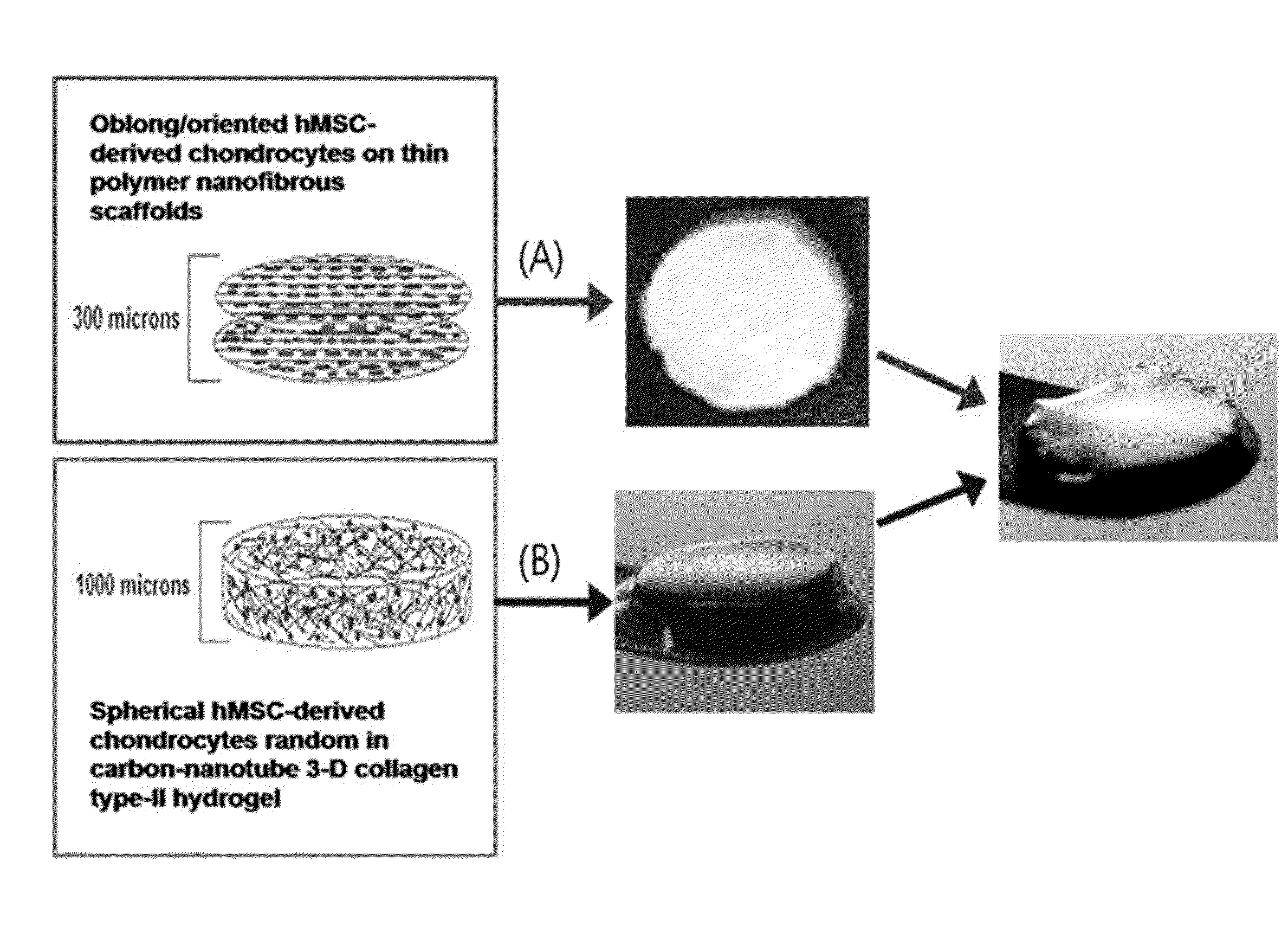
Scaffold for articular cartilage regeneration and method for manufacturing same
InactiveUS20130084636A1Improve mechanical propertiesEnhance cell viabilityBone marrow stroma cellsNanostructure manufactureDiseaseCell adhesion
Disclosed are scaffolds for regeneration of articular cartilage which are applicable to both the superficial zone and the middle zone of articular cartilage, and a method for manufacturing the same. The scaffolds have sufficient mechanical properties to support the implantation and regeneration of chondrocytes, and allow cells to show high cell viability with a high content of sulfated glycosaminoglycans (GAGs). In addition, being applicable to both the superficial zone and the middle zone of articular cartilage, the scaffolds facilitate cell adhesion and provide biomimetic surface environments that are effective for growing and differentiating stem cells. Therefore, the scaffolds are helpful in regenerating damaged articular cartilage, thus finding applications in stem cell therapy for articular cartilage damage and disease. Also, the application of the scaffolds can be extended to prostheses of the ear and the nose in plastic surgery.
Owner:TE BIOS
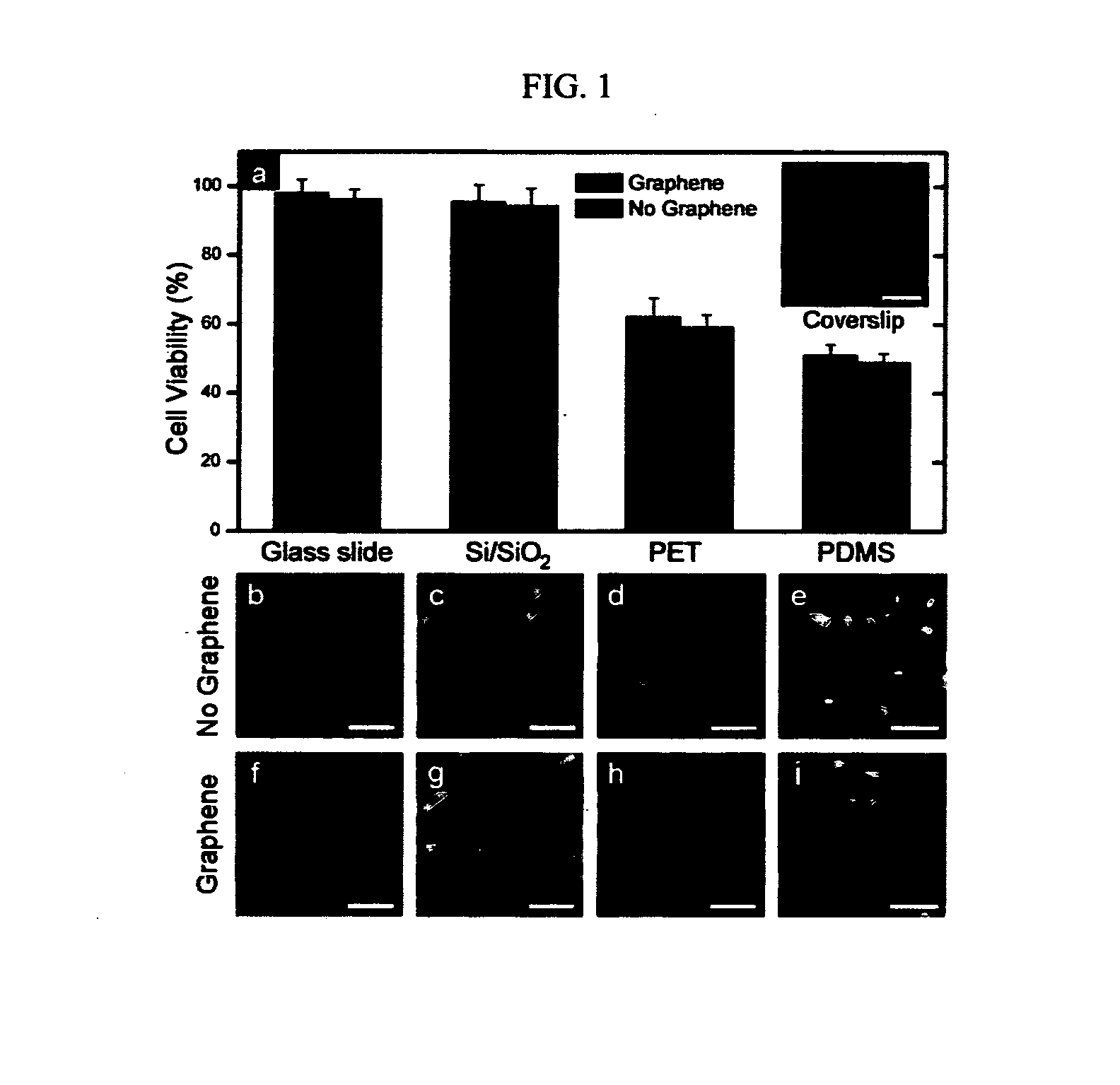
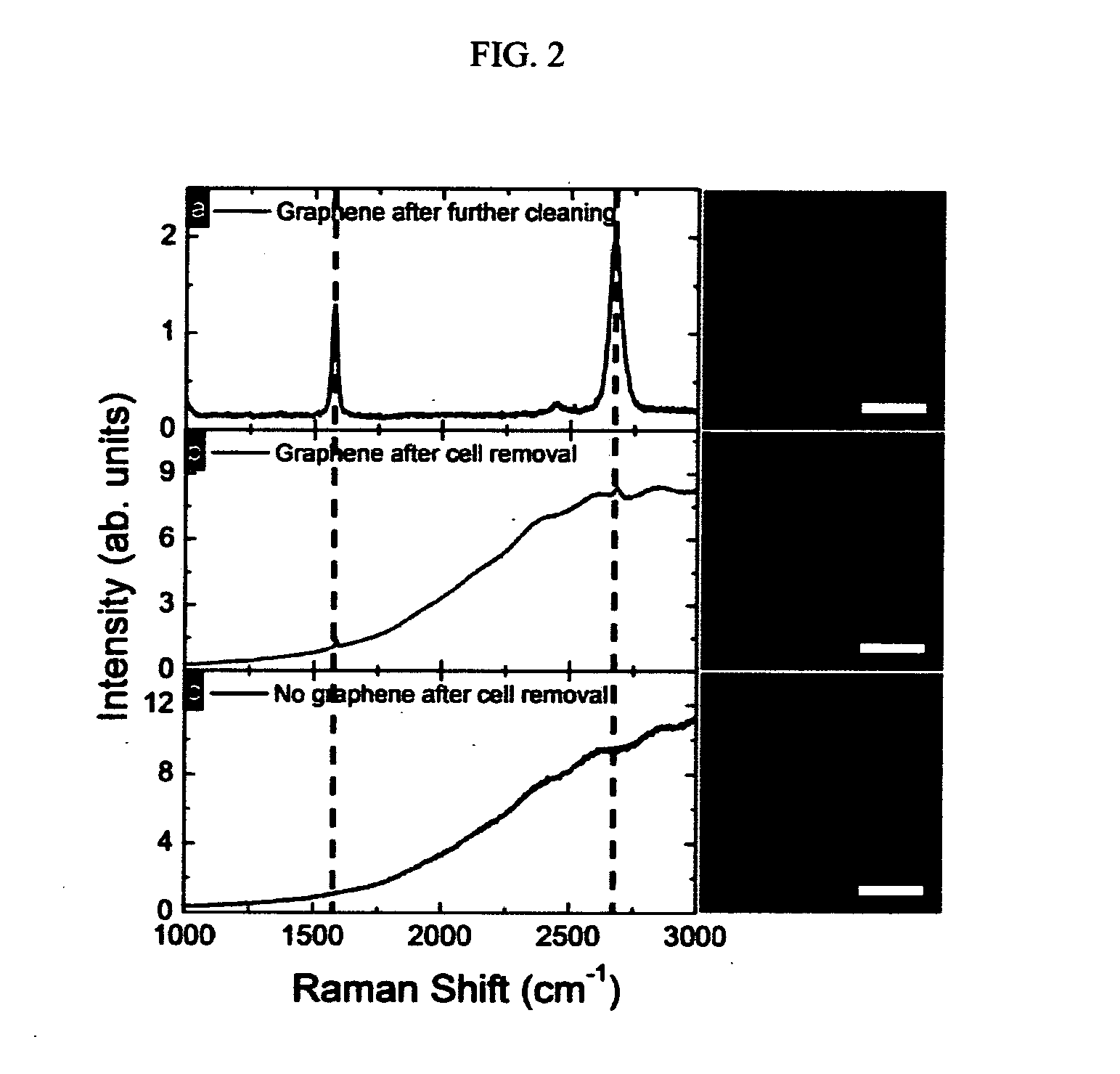
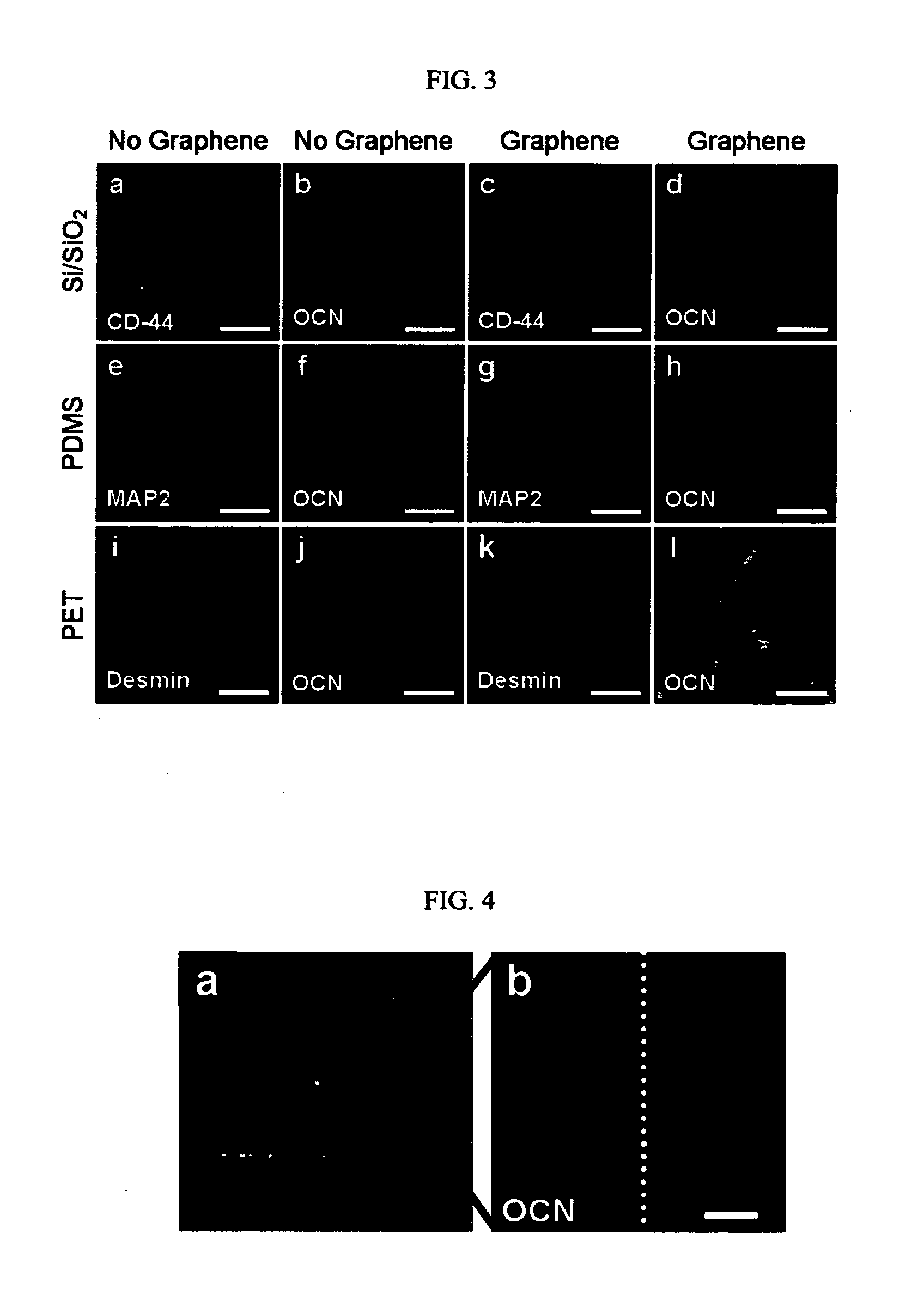
Method For Controlling And Accelerating Differentiation Of Stem Cells Using Graphene Substrates
ActiveUS20130095083A1Low costNot hamper proliferationBone marrow stroma cellsBiocideBone tissueCell growth
The invention relates to methods for directing differentiation of stem cells comprising graphene. In additional embodiments, the invention relates to methods for repairing and improving bone tissue functions comprising accelerating differentiation in stem cell growth by exposing stem cells to graphene and transplanting the graphene with the exposed stem cells in the tissue at the site of repair.
Owner:NAT UNIV OF SINGAPORE
Tissue culture medium for culturing potentially regenerative cells and functional tissue-organs in vitro
Compositions and methods are provided for culturing in vitro potentially regenerative cells (PRCs) from which functional tissue-organs are regenerated. In one aspect of the invention, a tissue culture medium is provided which comprises at least 50% of water and a sterol compound that is dissolved in a fatty acid-containing oil at a concentration at least 0.1% by weight based on the weight of the oil and added to the water. The culture medium can be used to culture PRCs that are isolated from the body of a mammal to generate functional tissue-organs in vitro with substantially the same physiological structure and function as the corresponding ones existing in vivo and in situ. The cultured PRCs, tissues, and tissue-organs can serve as valuable models for scientific investigation in life sciences, nutraceutical discovery, drug screening, pharmacokinetic studies, medical devices and tissue / organ transplantation.
Owner:XU RONGXIANG
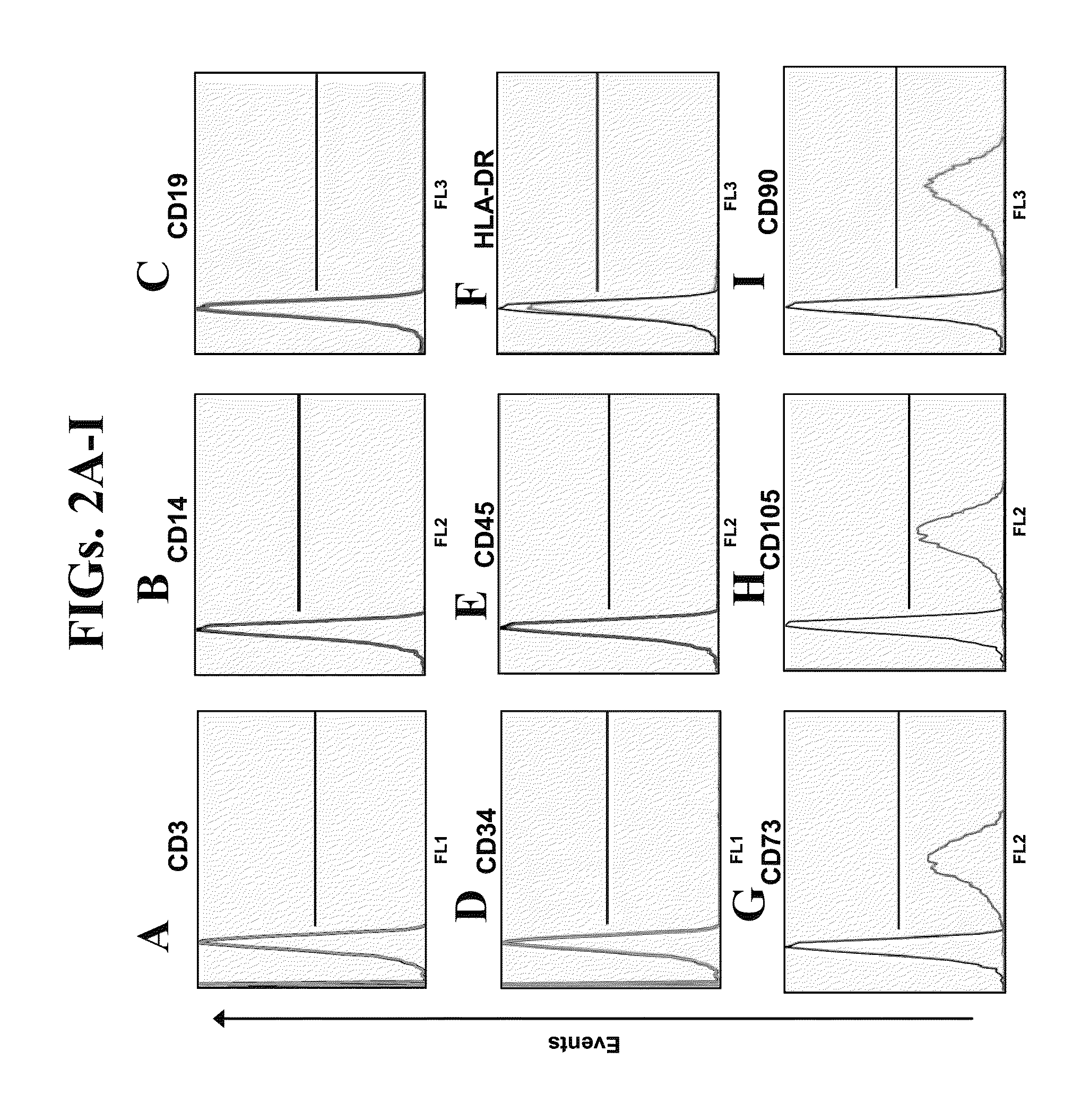
Novel Mesenchymal Stem Cells and Bone-Forming Cells
ActiveUS20110262404A1Improve featuresStimulate differentiationBone marrow stroma cellsBiocideMedicineMesenchymal stem cell
The invention relates to a new type of mesenchymal stem cells (MSC) which co-express at least one mesenchymal marker, preferably at least CD105 and CD34. Also provided are bone-forming cells having an analogous phenotype. The invention also provides the cells and cell populations, as well as further products comprising such and uses thereof in bone therapy.
Owner:BONE THRAPEUTICS SA
Novel Carbohydrate Profile Compositions From Human Cells and Methods for Analysis and Modification Thereof
Owner:GLYKOS FINLAND
Mesenchymal stem cells for the treatment of CNS diseases
ActiveUS8663987B2Bone marrow stroma cellsNervous disorderCns diseaseBrain-derived neurotrophic factor
An isolated human cell is disclosed comprising at least one mesenchymal stem cell phenotype and secreting brain-derived neurotrophic factor (BDNF), wherein a basal secretion of the BDNF is at least five times greater than a basal secretion of the BDNF in a mesenchymal stem cell. Methods of generating same and uses of same are also disclosed.
Owner:BRAINSTORM CELL THERAPEUTICS LTD +1
Method for remyelinating a demyelinized lesion due to injury in the brain or spinal cord
InactiveUS7098027B2Prepared safely and readilyBeneficial in medical industryBone marrow stroma cellsBiocideMedicineBone marrow cell
Demyelinated axons were remyelinated in the demyelinated rat model by collecting bone marrow cells from mouse bone marrow and transplanting the mononuclear cell fraction separated from these bone marrow cells.
Owner:MITSUI SUMITOMO INSURANCE CARE NETWORK CO LTD RECEIVES 5 +2
Mesenchymal Stem Cells and Uses Therefor
InactiveUS20110027238A1Limit neurodegenerationReduce inflammationBone marrow stroma cellsBiocideAnaphylaxisAutoimmune disease
Methods of treating autoimmune diseases, allergic responses, cancer, or inflammatory diseases in an animal, promoting would healing, repairing epithelial damage and promoting angiogenesis in an organ or tissue of an animal by administering to the animal mesenchymal stem cells in an effective amount.
Owner:MESOBLAST INT
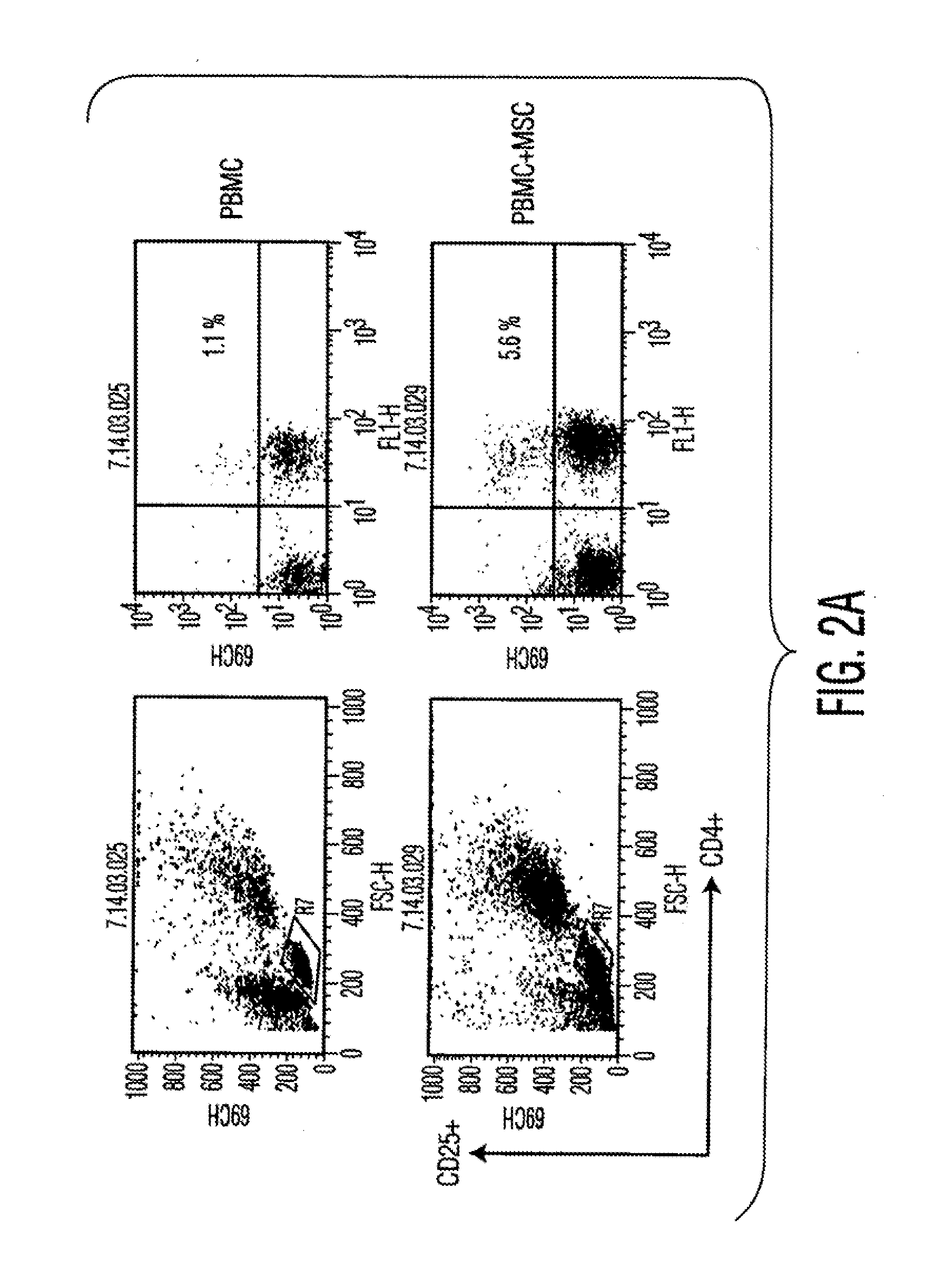
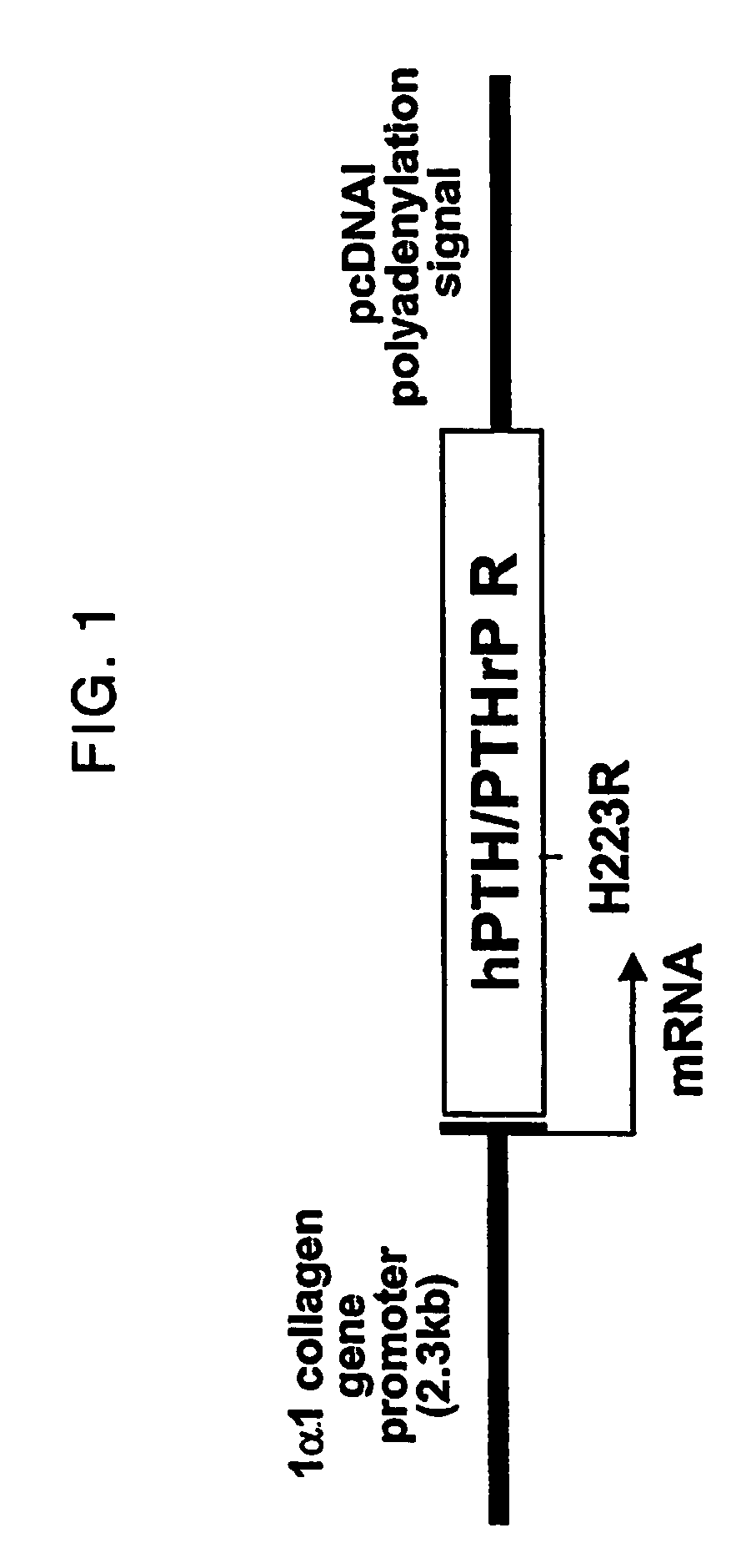
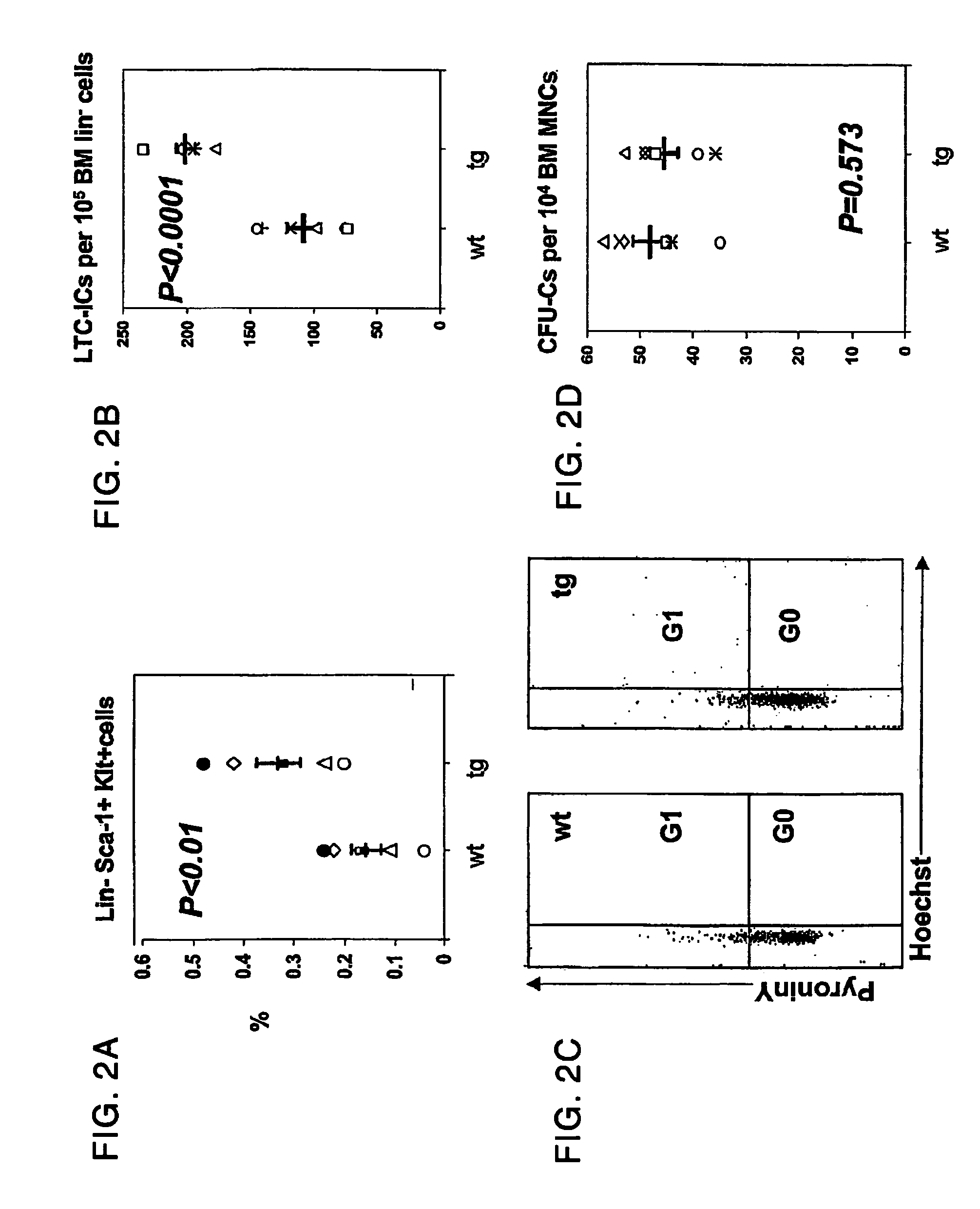
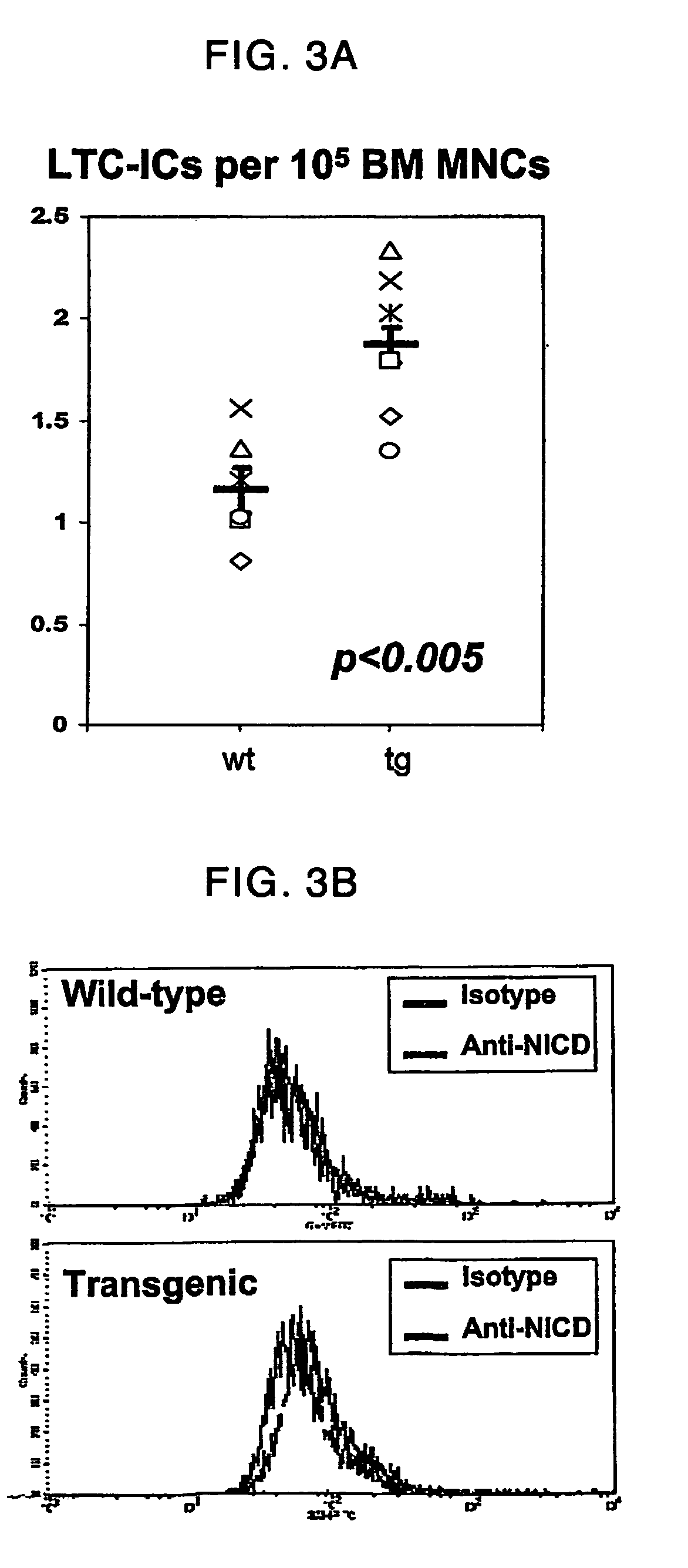
Parathyroid hormone receptor activation and stem and progenitor cell expansion
The invention relates to methods for manipulating hematopoietic stem or progenitor cells, mesenchymal stem cells, epithelial stem cells, neural stem cells and related products through activation of the PTH / PTHrP receptor in neighboring cells.
Owner:THE GENERAL HOSPITAL CORP
Encapsulated cell indicator system
The invention features an encapsulated cell indicator system that includes (a) indicator cells having a signal-responsive element operably linked to a reporter gene; (b) encapsulating material; and (c) a permeable membrane. In this encapsulated cell indicator system, the indicator cells are encapsulated in the encapsulated material and the encapsulated material and the indicator cells are surrounded by the permeable membrane.
Owner:CARDIO3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com