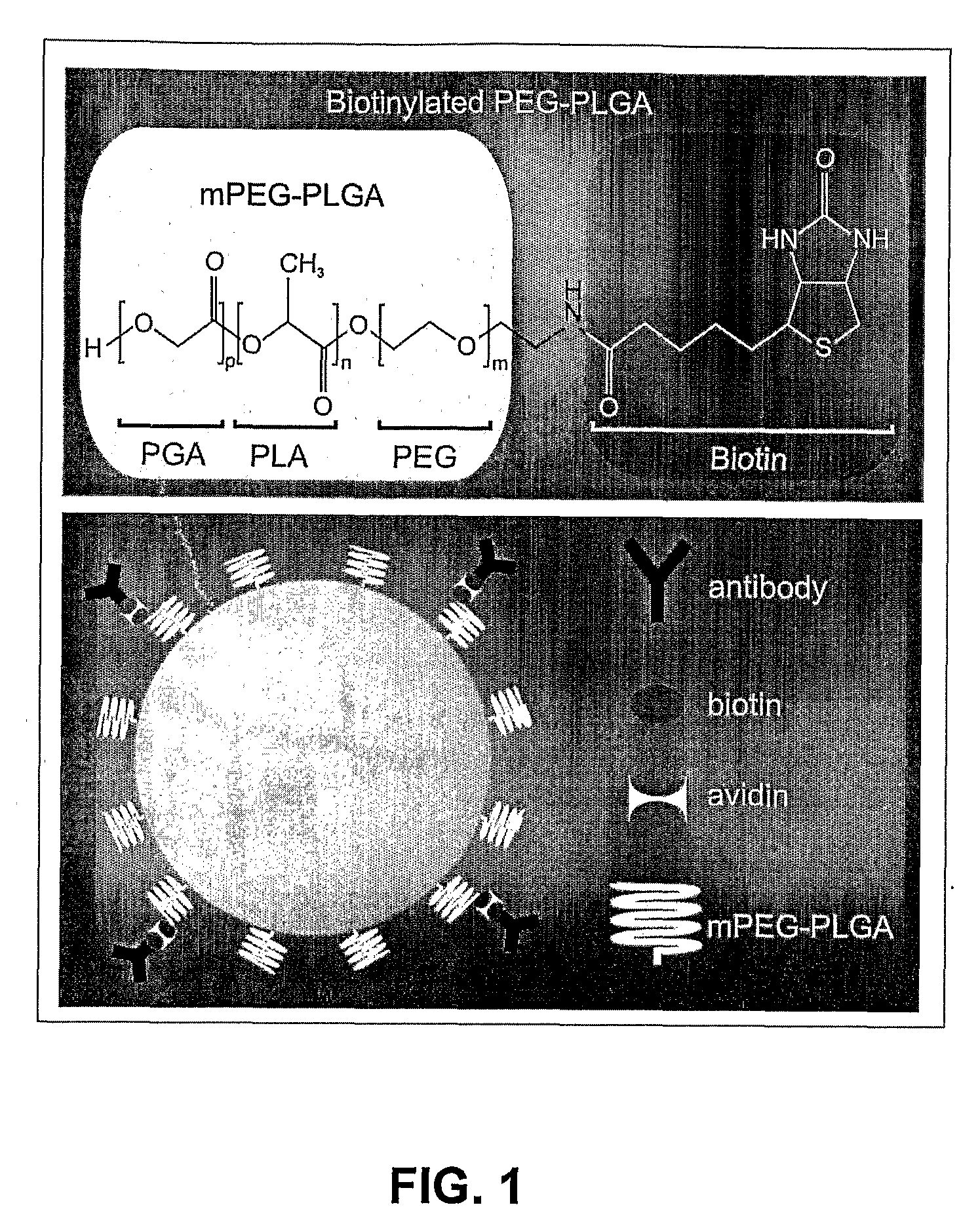
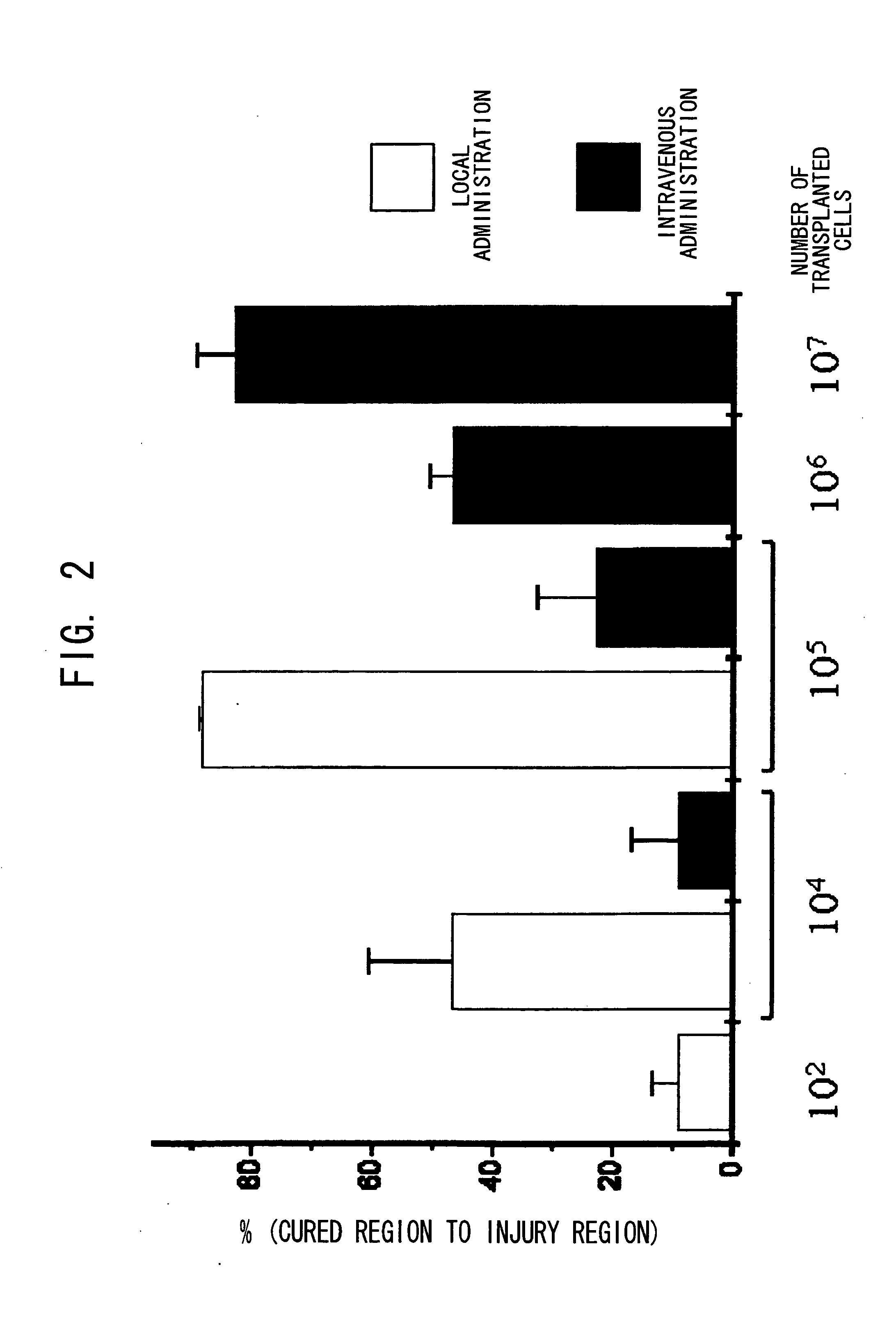
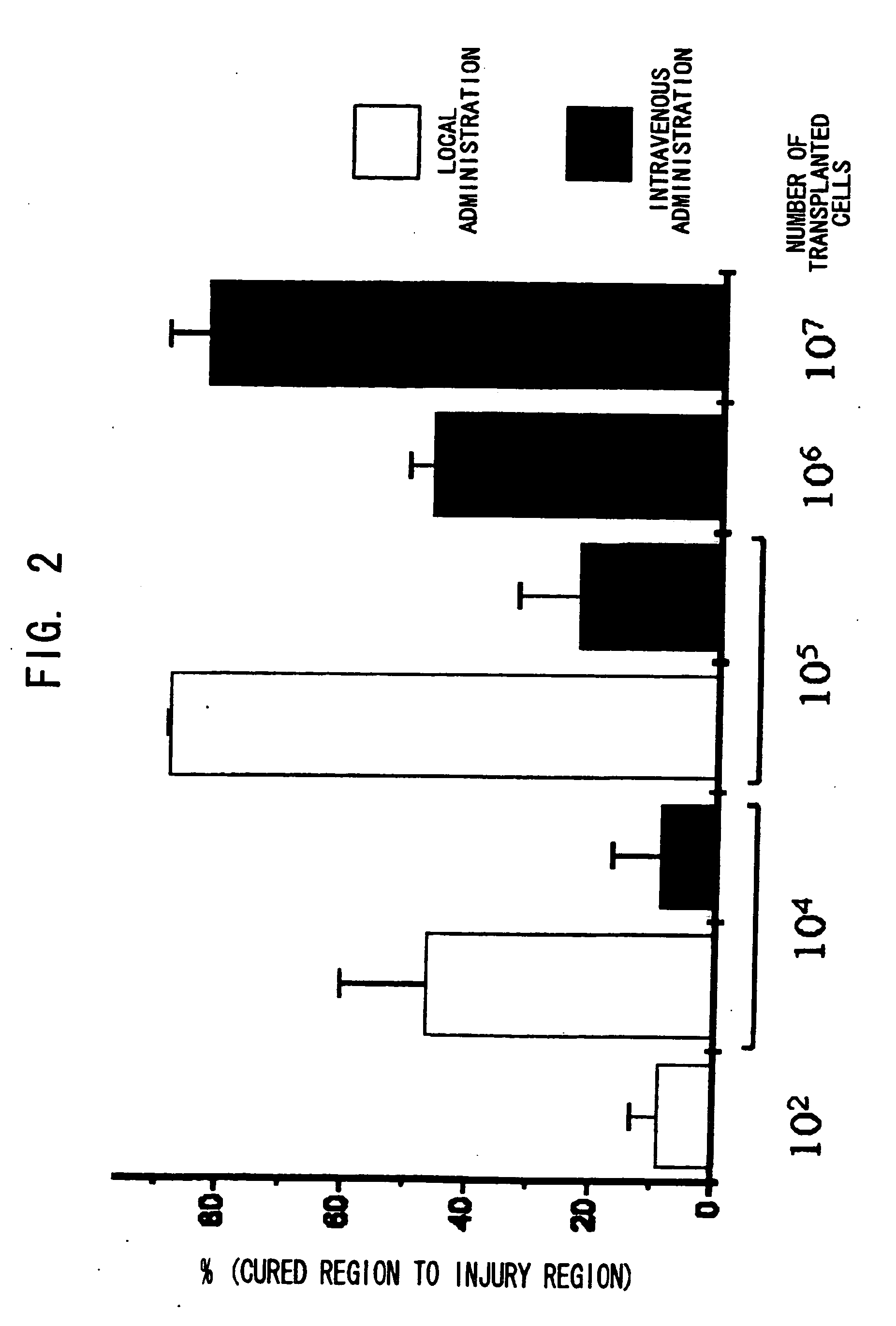
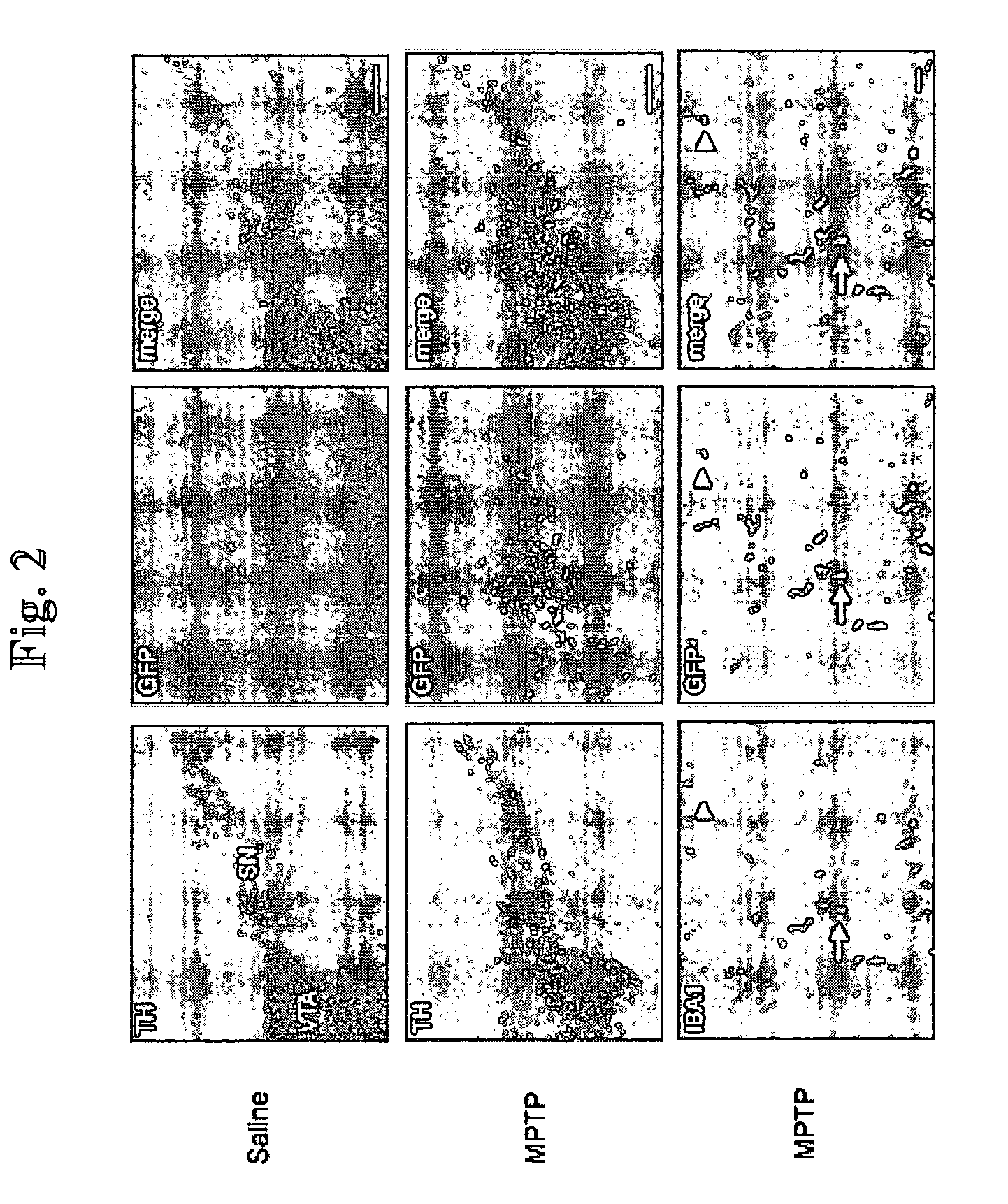
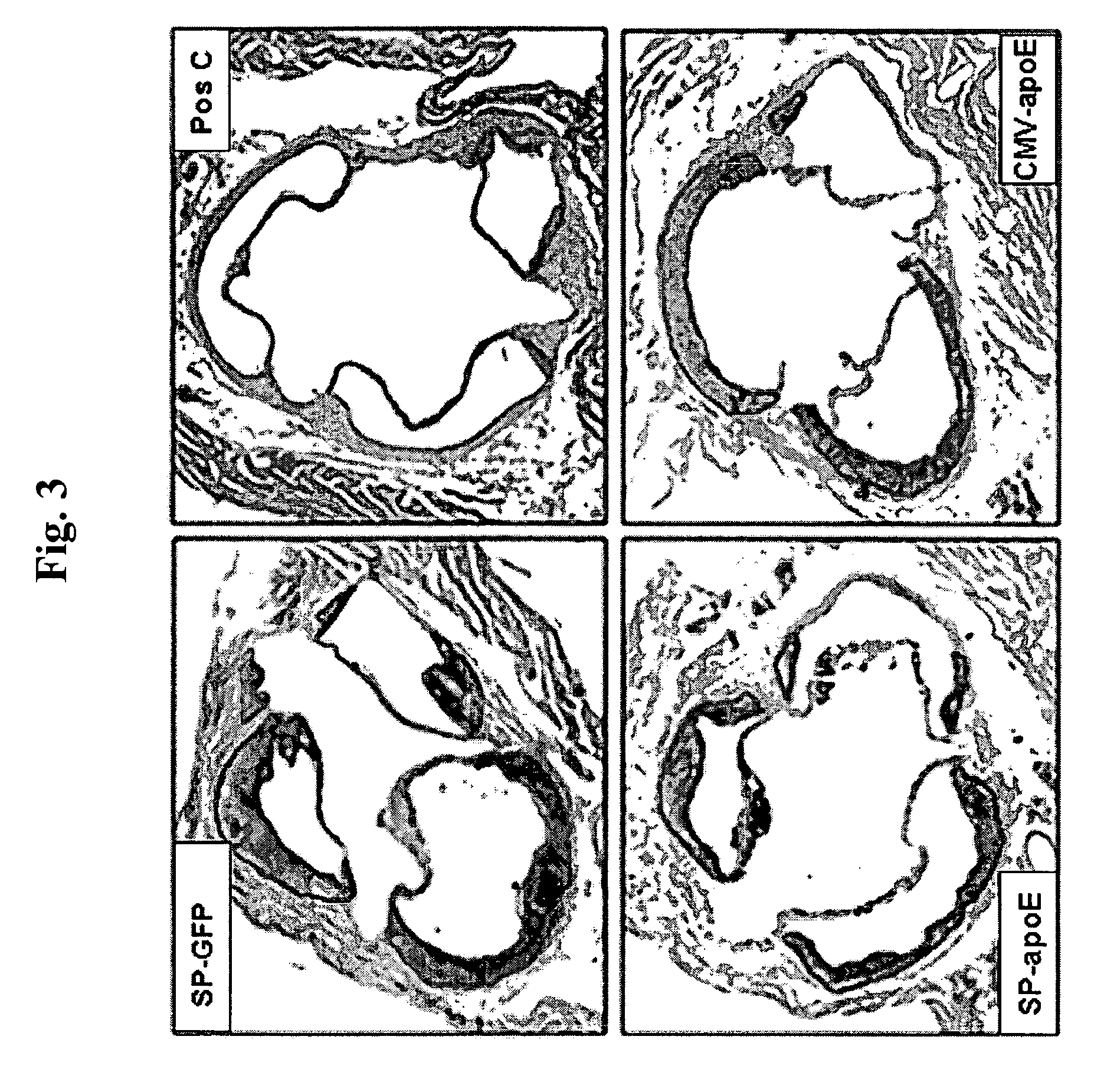
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
164 results about "Bone Marrow Stem Cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Primitive blood cells residing in the bone marrow, derived from embryonic mesenchyme, and capable of differentiating into any of the blood cell line progenitor cells (erythroblasts, young granulocytic series cells, megakaryocytes, etc.)
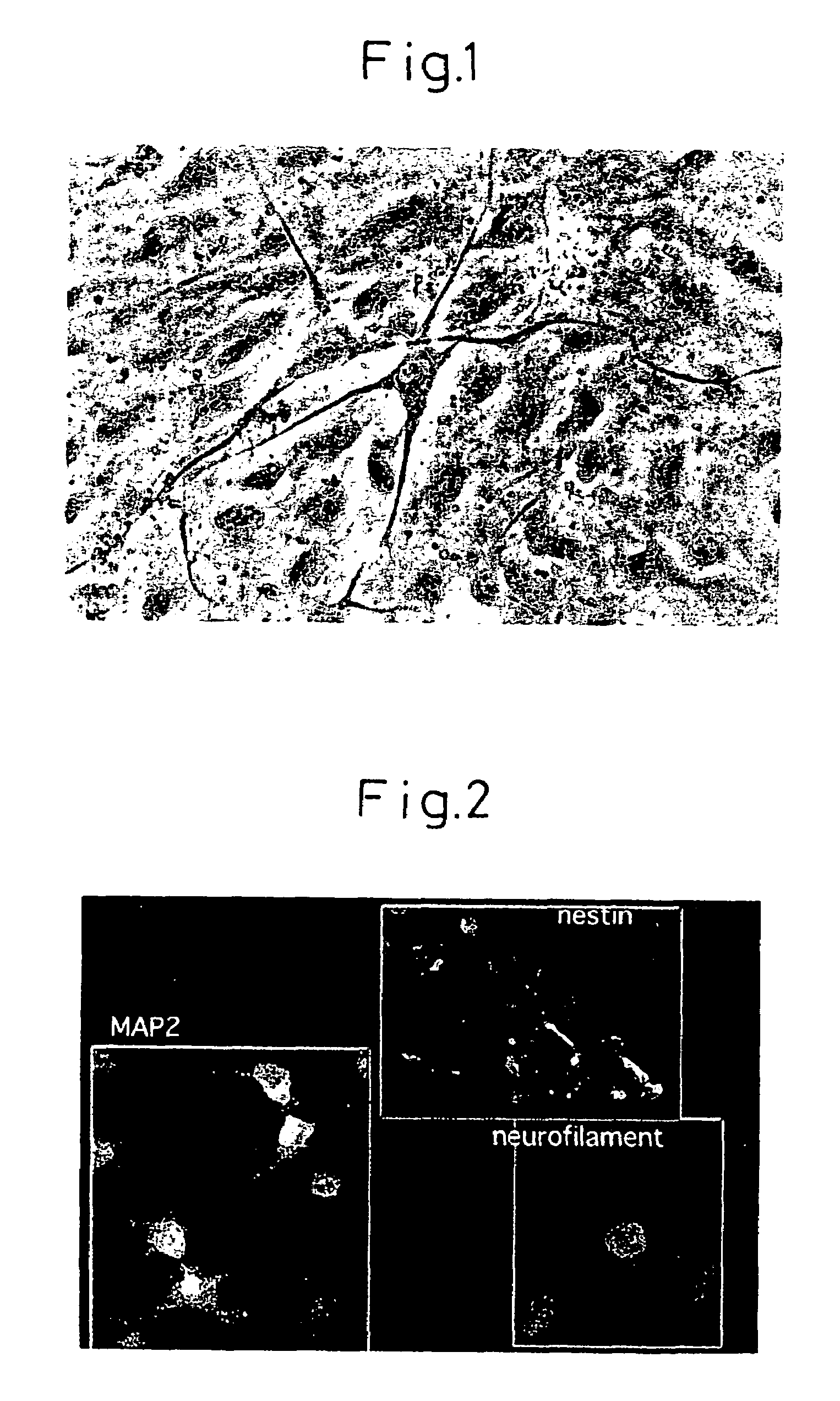
Differentiation of bone marrow stromal cells to neural cells or skeletal muscle cells by introduction of notch gene
There is provided a method of inducing differentiation of bone marrow stromal cells to neural cells or skeletal muscle cells by introduction of a Notch gene. Specifically, the invention provides a method of inducing differentiation of bone marrow stromal cells to neural cells or skeletal muscle cells in vitro, which method comprises introducing a Notch gene and / or a Notch signaling related gene into the cells, wherein the finally obtained differentiated cells are the result of cell division of the bone marrow stromal cells into which the Notch gene and / or Notch signaling related gene have been introduced. The invention also provides a method of inducing further differentiation of the differentiation-induced neural cells to dopaminergic neurons or acetylcholinergic neurons. The invention yet further provides a treatment method for neurodegenerative and skeletal muscle degenerative diseases which employs neural precursor cells, neural cells or skeletal muscle cells produced by the method of the invention.
Owner:SANBIO
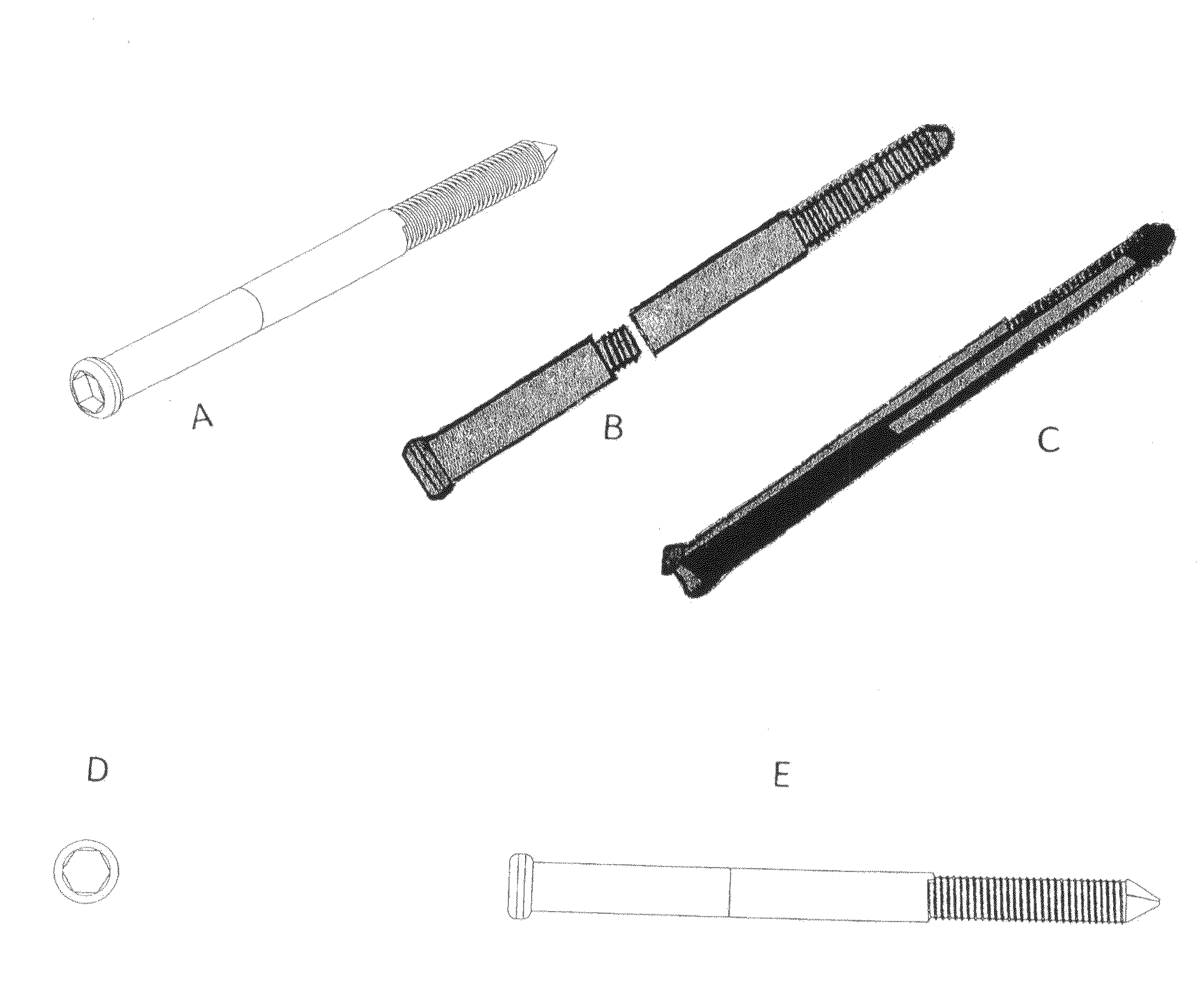
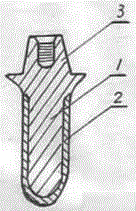
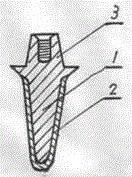
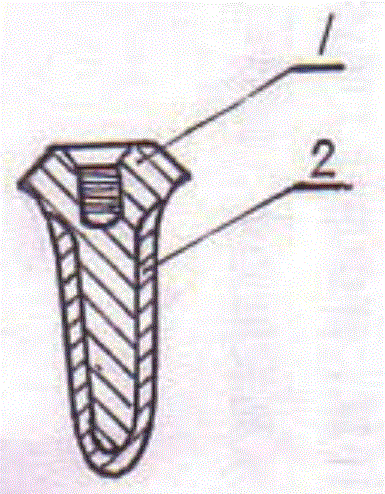
Poly-porous hollow screw for target delivery of growth factors and stem cells:the design and potential clinical application
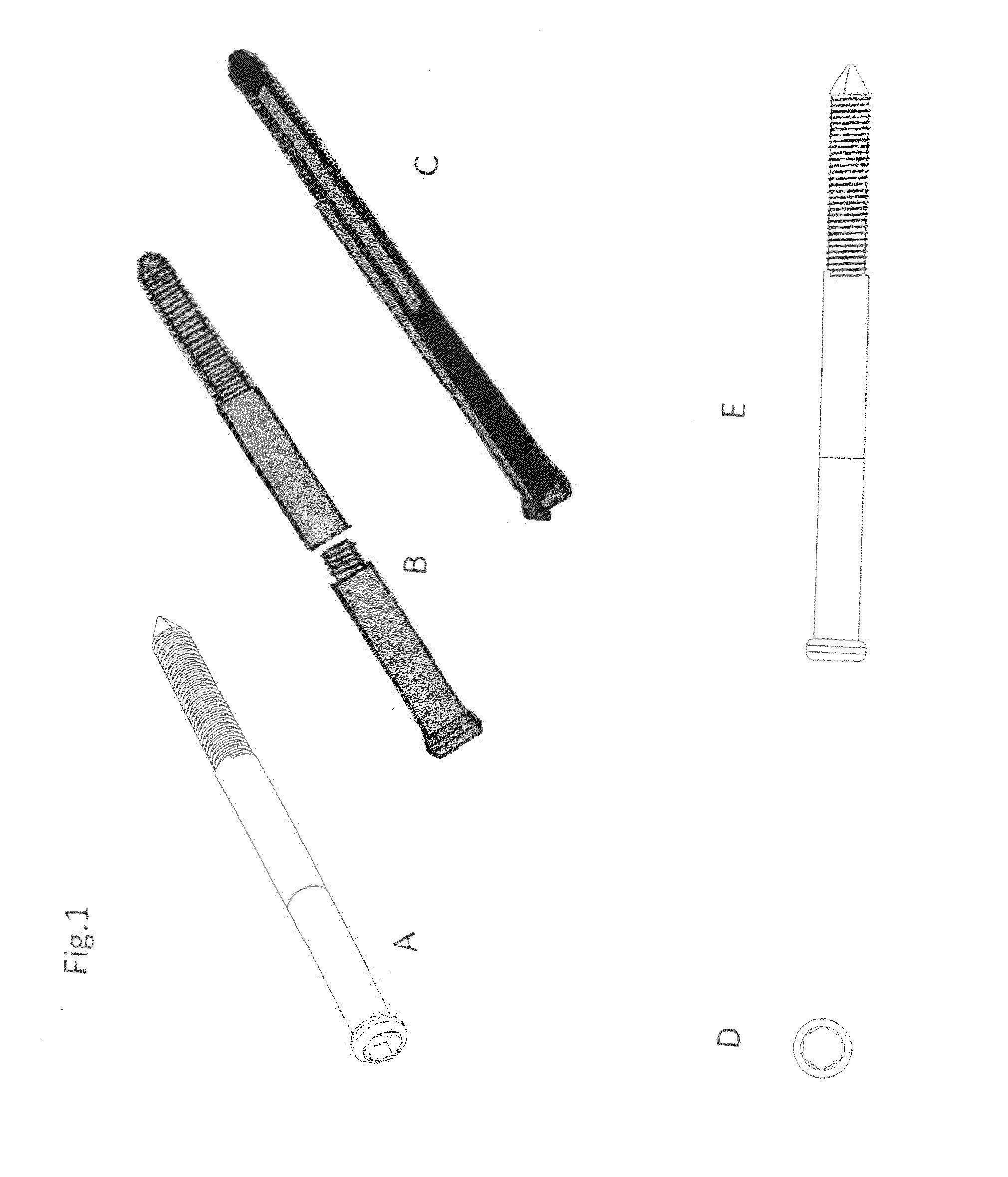
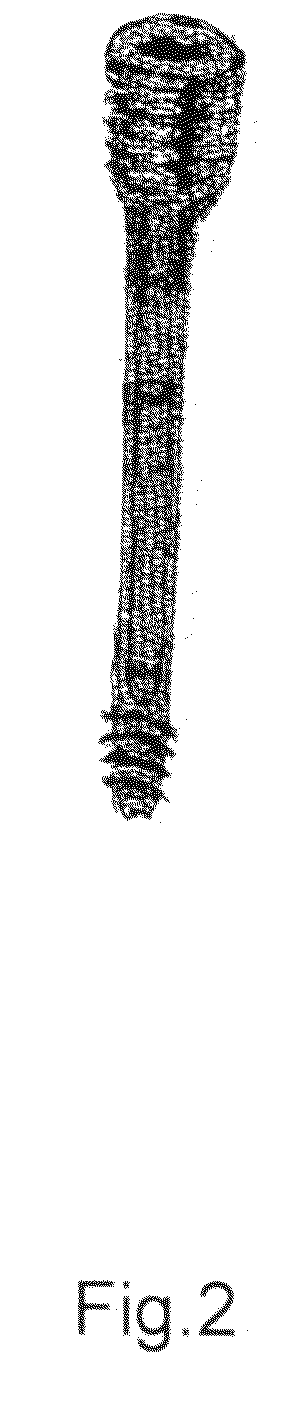
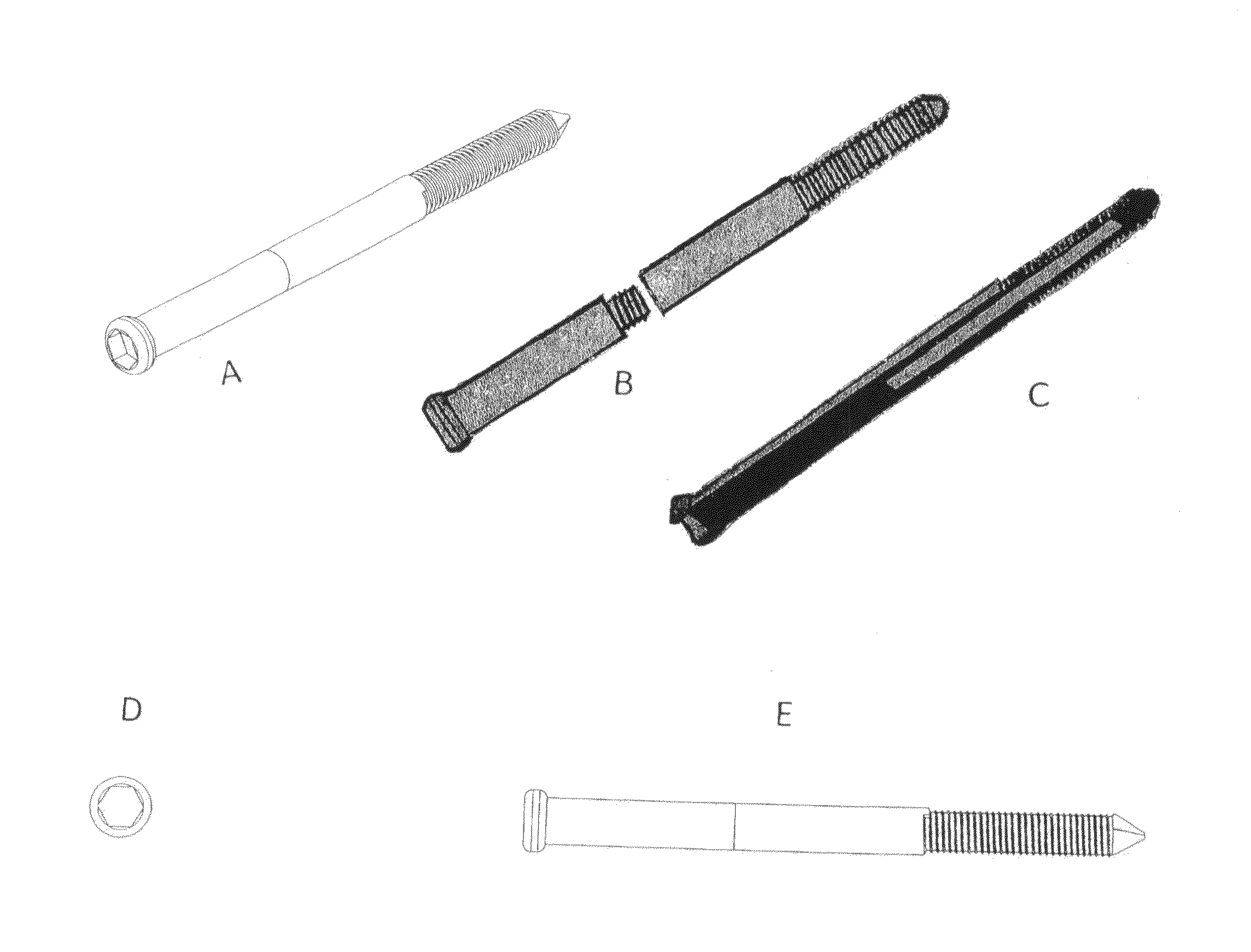
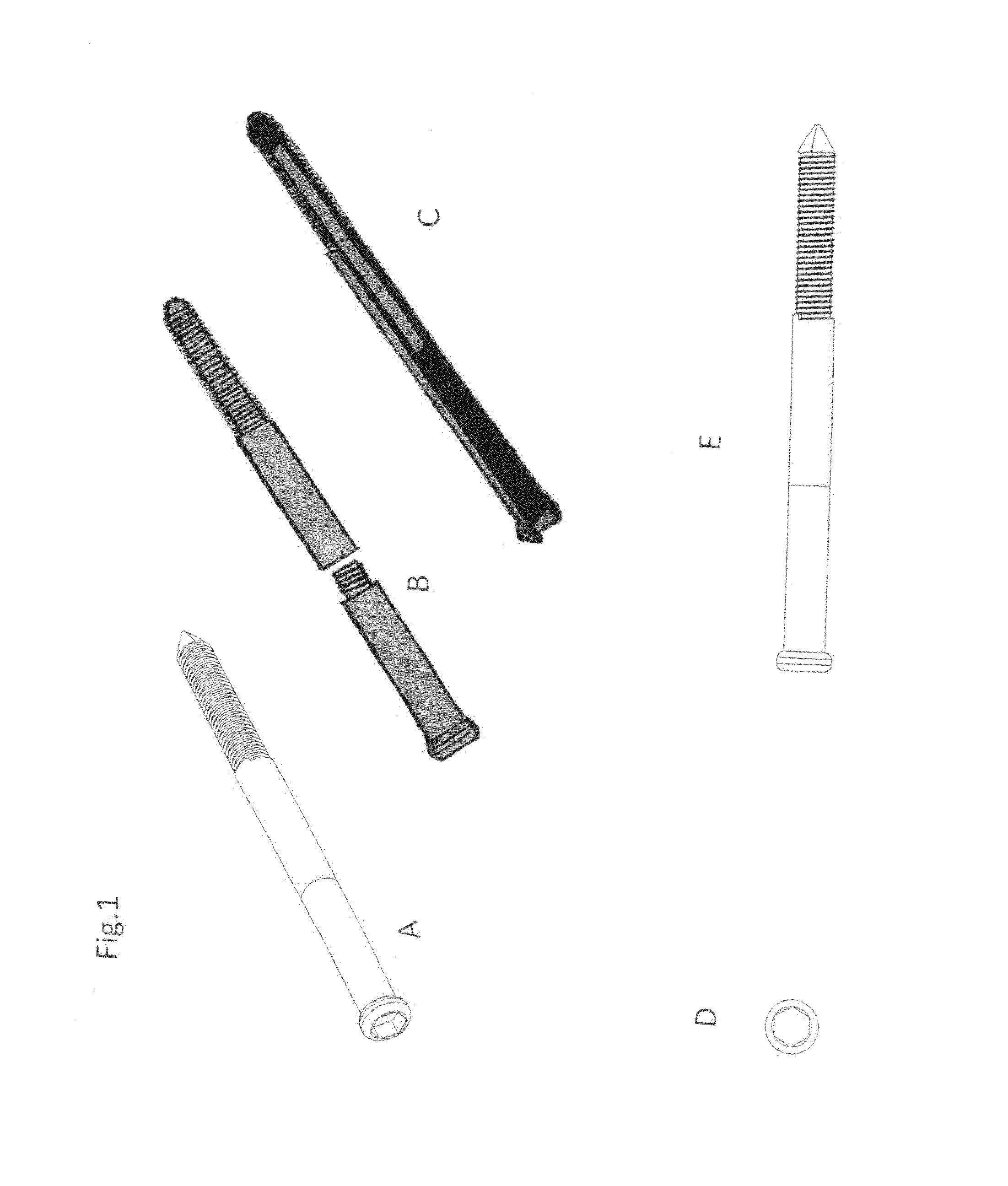
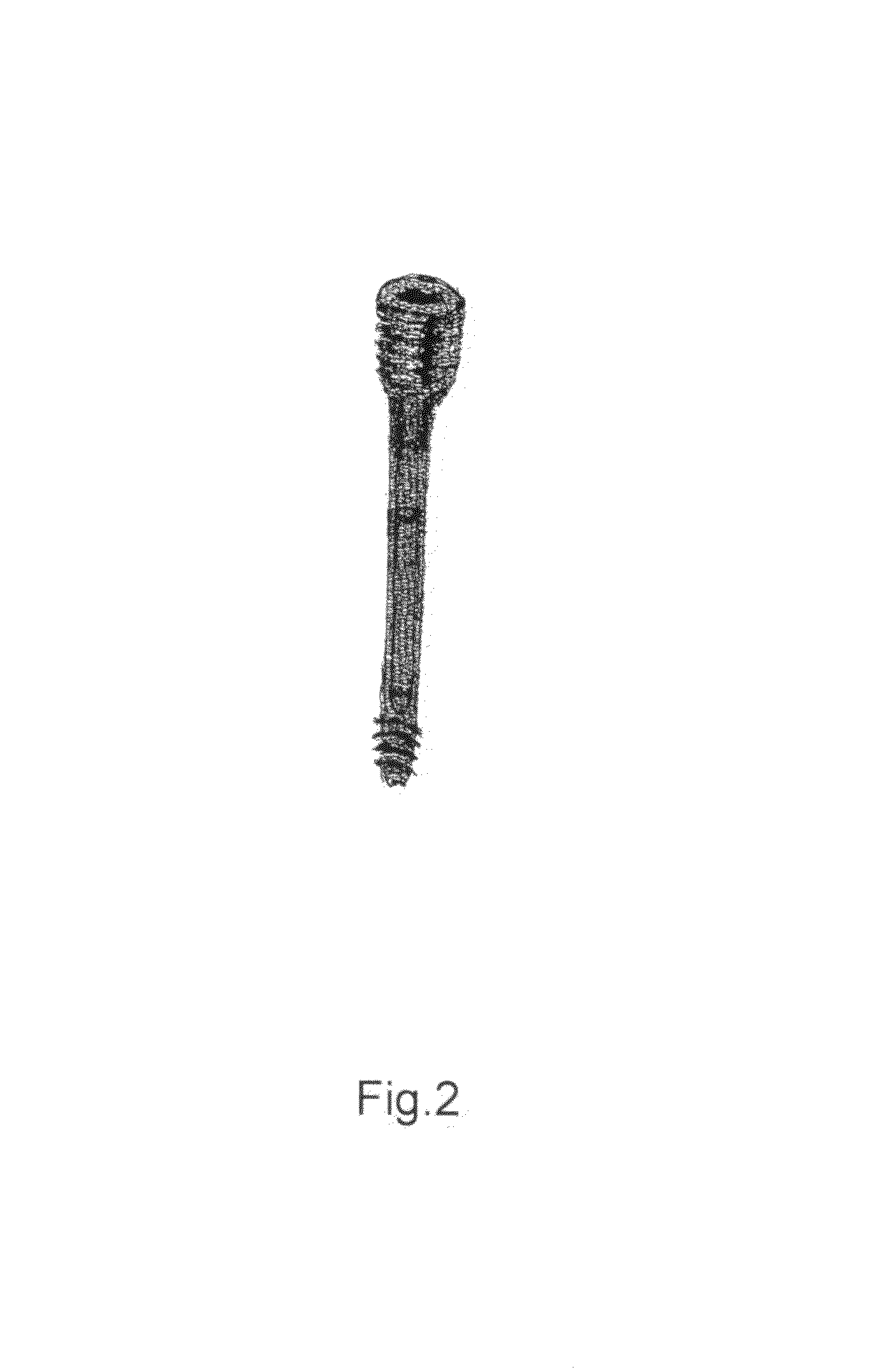
Present invention depicts a poly-porous (micropore) hollow screws as diffusion chamber filled with core matrix for targeted delivery of growth factors and bone marrow stem cells. The screws comprise at least two parts: the distal part of the screw consists of the tip of the screw made of poly porous material and hollow inside proximally. It has threaded navel attached to the threaded nipple of the distal part of the proximal screw which has the screw head and is made of the solid material of the same kind. The screw head had hexagonal recess targeted for screw driver insertion. Assembly of screw created a chamber in the middle of the screw. The chamber is filled with core matrix consisting of gelatin nano-particles pre-impregnated with BMPs (BMP2 / BMP7 for bone or BMP12 for tendon, ligament) and fibrin sealants or Chitosan dispersed with bone marrow stem cells and / or other growth factors. Bioactive protein core material is prepared during the surgery and filled the chamber of the screw by the surgeon. Fibrin sealants or Chitosan will polymerize to form a gel to hold the growth factors and stem cell in place. The screw can be used as the lag screw or other function to provide mechanical fixation in variety of condition. Once the screw implanted in the human body, the fibrin sealant or Chitosin / gelatin nano-particles are gradually degraded and slowly release growth factors and stem cells via micropores of screw to facilitate the bone healing and regeneration. The gelatin nanoparticles and fibril sealant / or Chitosan matrix also serve as the scaffold and platform for bone in-growth to the screw or alternatively, the stem cell inside of screw can regenerate new bone, providing the biological fixation. At the mean time as the bone regenerate and / or in growth, mechanical strength of the screw increased.
Owner:WU YANGGUAN
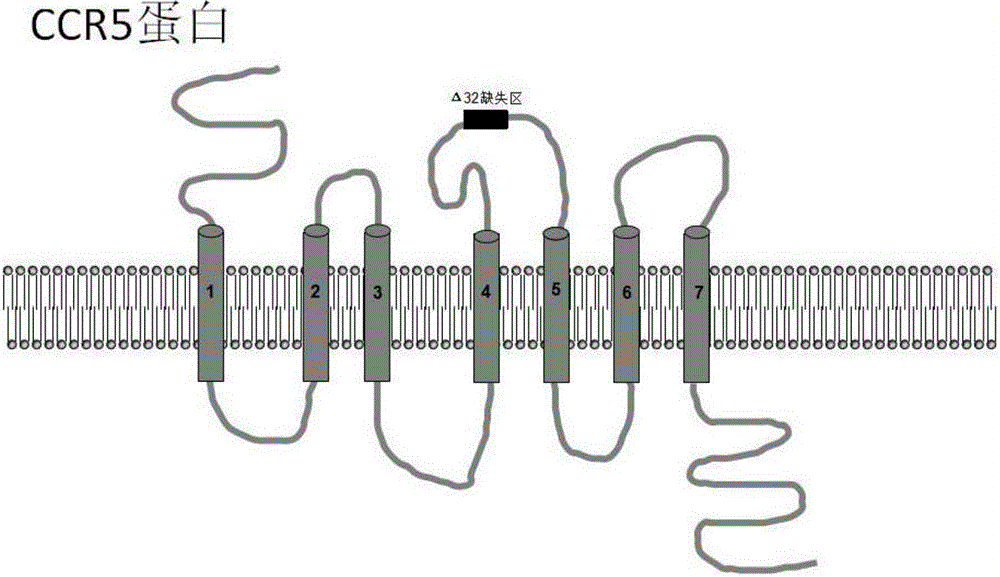
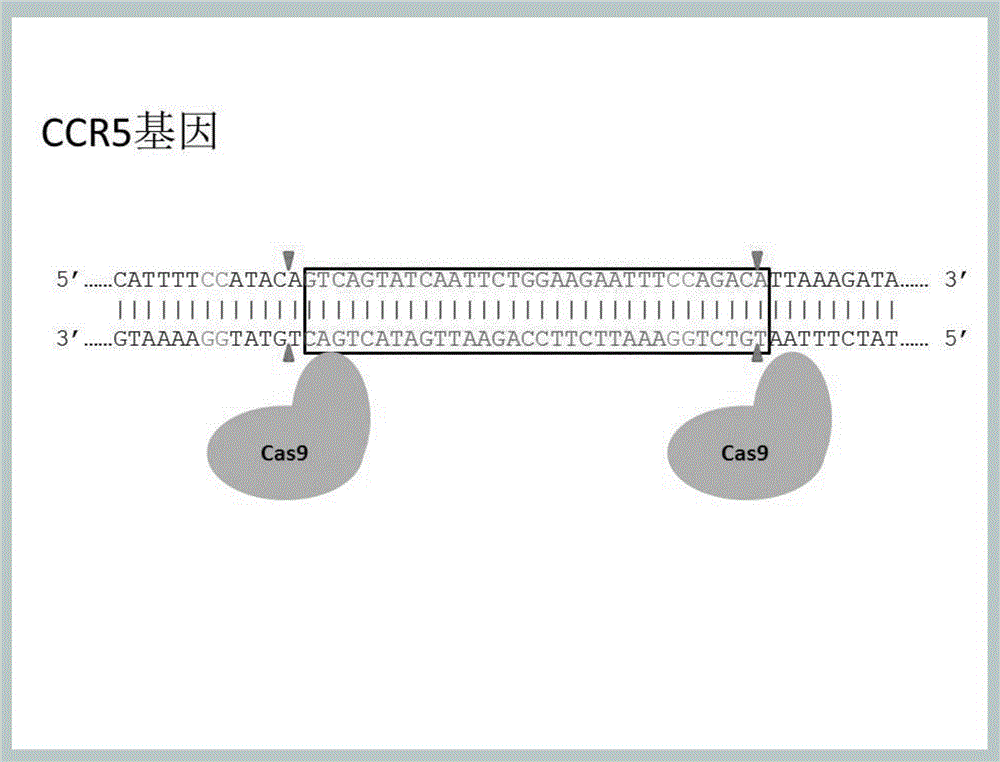
Method for inducing CCR5-delta32 deletion with genome editing technology CRISPR-Cas9
The invention relates to a method for successfully inducing cell chemokine receptor CCR5 genes to be mutated into CCR5-delta32 deletion-type genes with a new genome editing technology CRISPR-Cas9. CCR5 is an important receptor for human immunodeficiency viruses (HIV) to invade personal host cells. CCR5-delta32 deletion means deletion of 32 basic groups occurs in a CCR5 coding region, so that the sequence after the 185th amino acid is changed, and early termination occurs. CCR5-delta32 biallelic-gene homozygous deletion has natural resistance to HIV infection, and can not be infected by HIV. By means of the method, a slow virus packaging system and the CRISPR technology are used at the same time; as the slow virus infecting host range is wide, the method can be applied to cells such as bone marrow stem cells and CD4T cells, and the CCR5-delta32 deletion-type genes hopefully become medicine for treating acquired immune deficiency syndrome or other diseases.
Owner:NANKAI UNIV
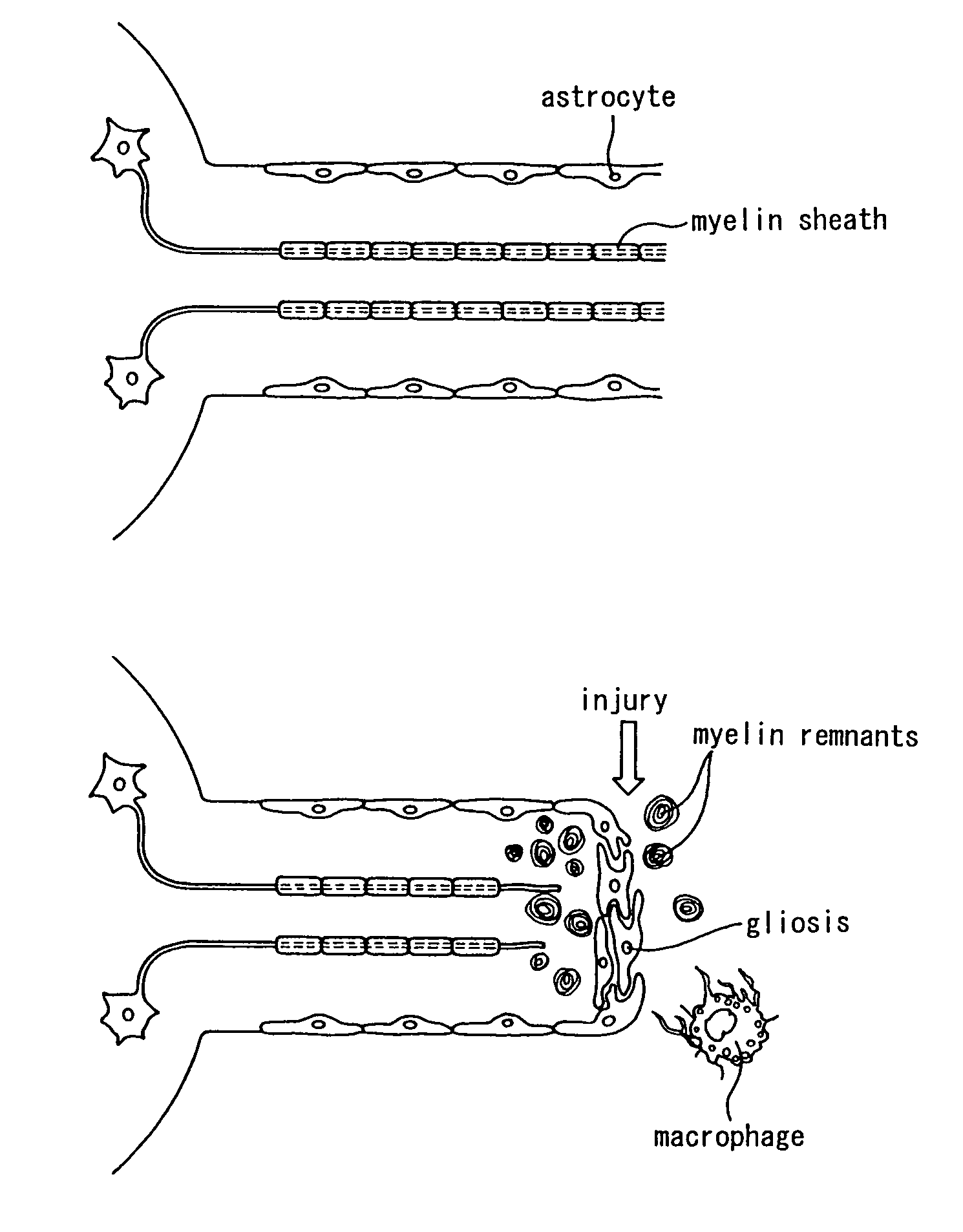
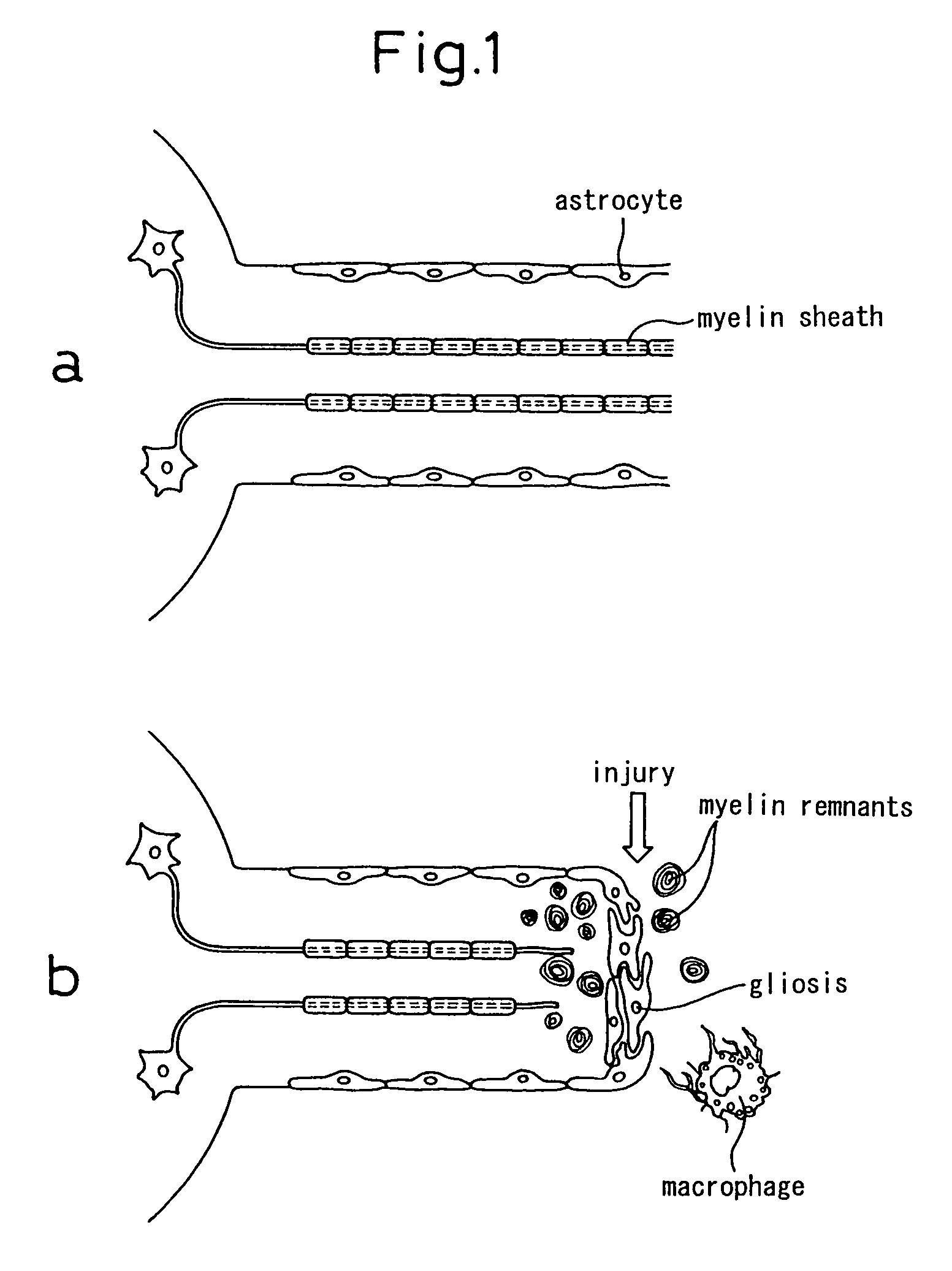
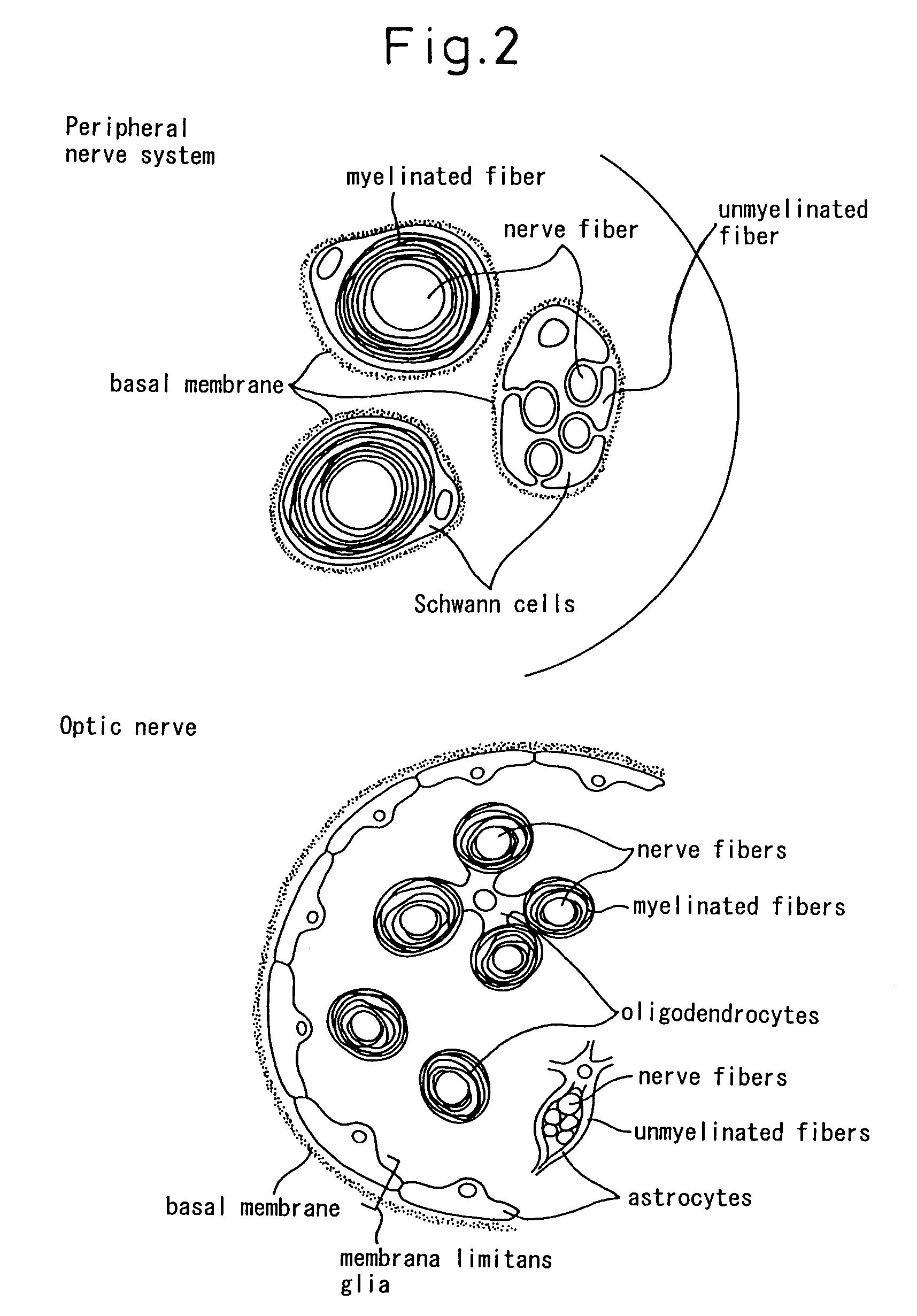
Schwann cells originating in myeloid interstitial cells
There is provided a method of inducing bone marrow stromal cells to differentiate into bone marrow stromal cell-derived Schwann cells in vitro, comprising the steps of: collecting bone marrow stromal cells from bone marrow and culturing the cells in a standard essential culture medium supplemented with a serum; adding a reducing agent to the culture medium and further culturing the cells; adding a differentiation inducing agent to the culture medium and further culturing the cells; and adding a cyclic AMP-augmenting agent or a cyclic AMP analogue and / or a glial cell differentiation and survival stimulating factor to the culture medium, and further culturing the cells to obtain the bone marrow stromal cell-derived Schwann cells. There are also provided bone marrow stromal cell-derived Schwann cells obtained thereby and a pharmaceutical composition for neural regeneration that comprises them.
Owner:SANBIO
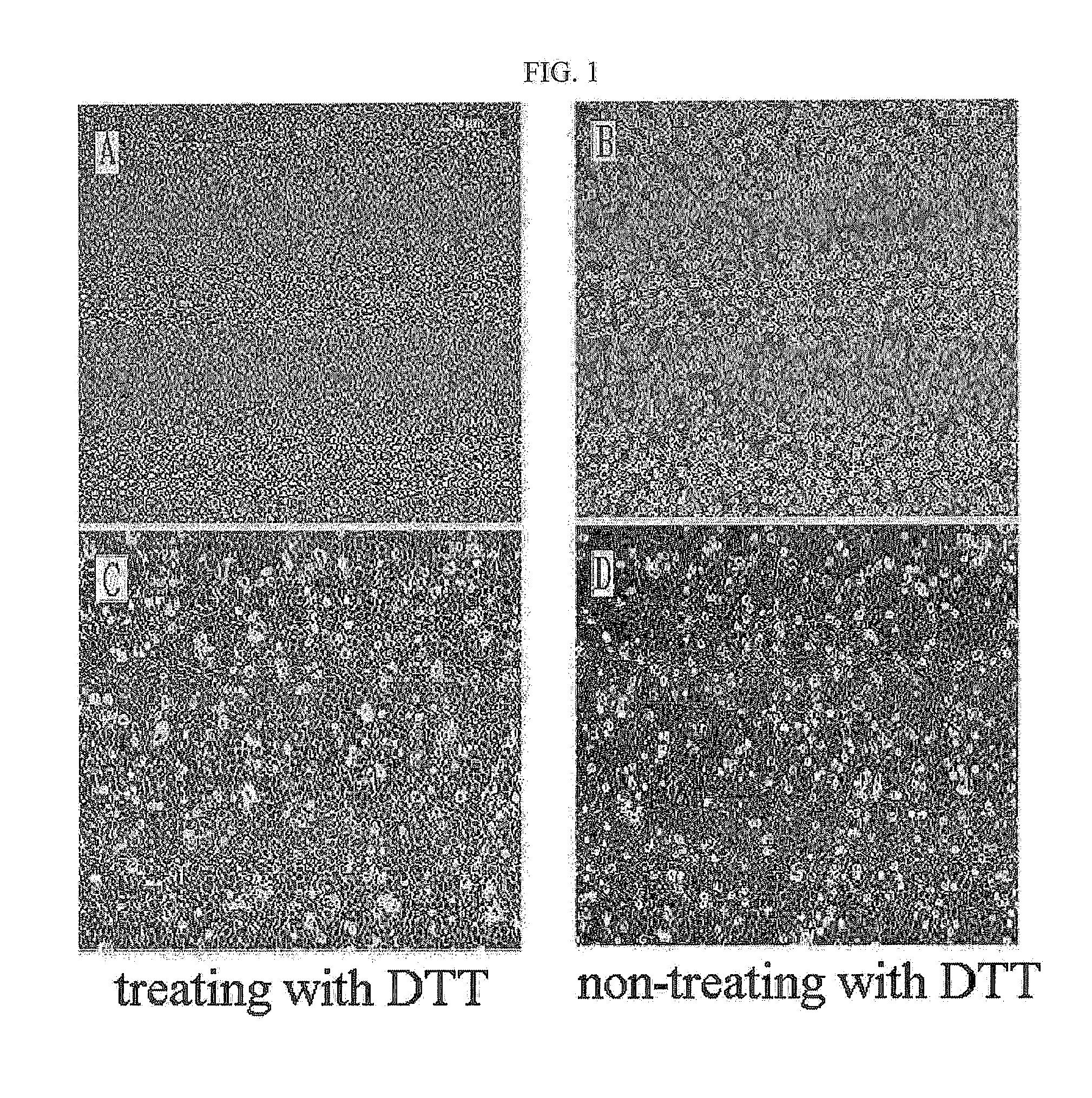
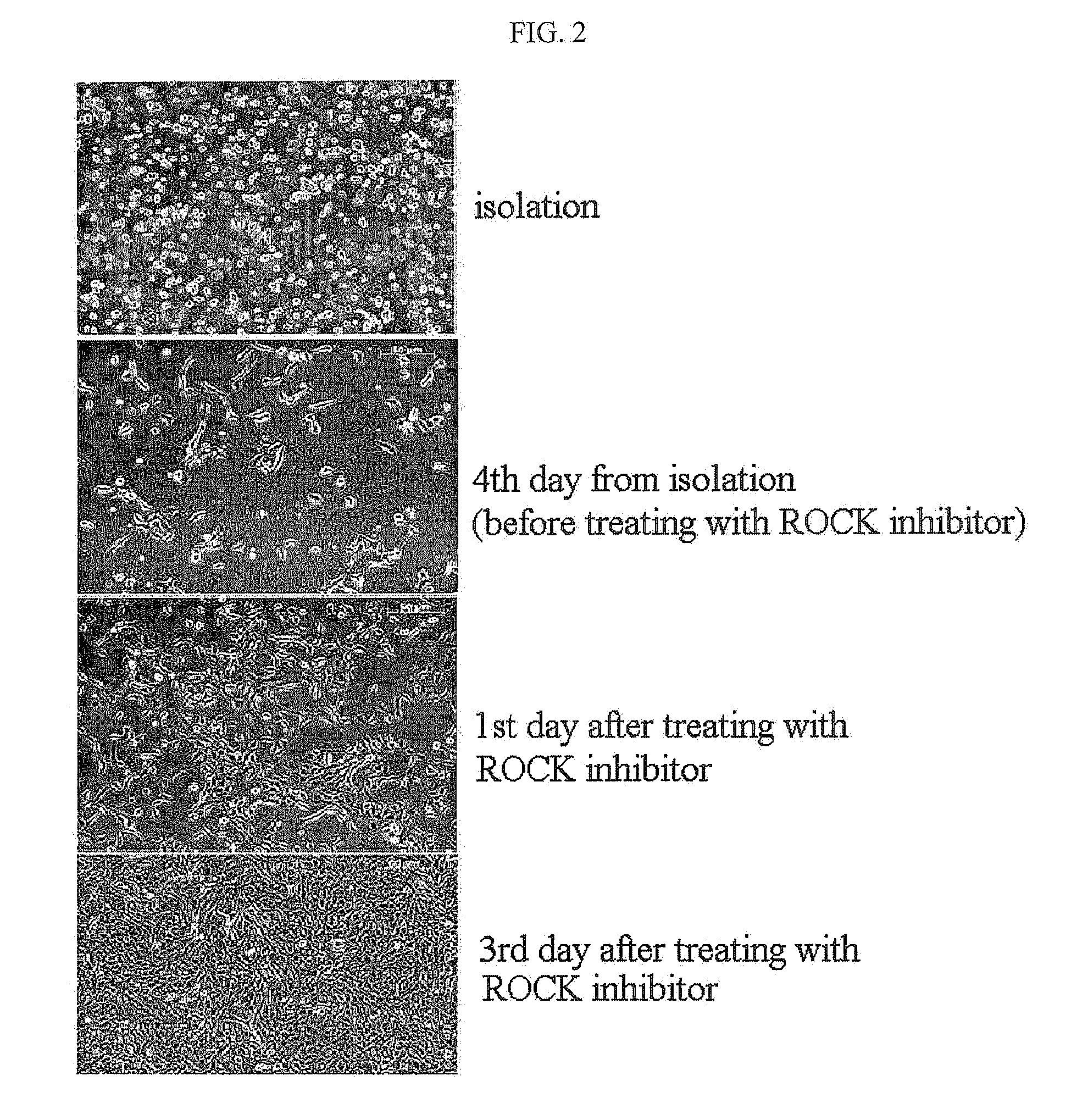
Method for isolating and culturing adult stem cells derived from human amniotic epithelium
The present invention relates to a method for isolating and culturing adult stem cells derived from human amniotic membrane in high yield, and more particularly to a method for obtaining a large amount of adult stem cells, the method comprising obtaining amniotic epithelial cells from human amniotic tissue in high yield by treatment with dithiothreitol (DTT) and a low concentration of trypsin and culturing the amniotic epithelial cells in a medium containing a Rho-associated kinase inhibitor. The human amniotic epithelial cell-derived stem cells are easily extracted compared to existing therapeutic stem cells such as umbilical cord blood stem cells and bone marrow stem cells, the yield and proliferation thereof are significantly increased by DTT treatment, the addition of the ROCK inhibitor or the replacement of medium. Thus, the method can be used to efficiently prepare adult stem cells.
Owner:RNL BIO
Bone cartilage repair material and preparation method of scaffold for tissue engineering
InactiveCN109364302AEasy to prepareRealize the loadAdditive manufacturing apparatusTissue regenerationOsteoblastSolvent
The invention discloses a bone cartilage repair material and a preparation method of a scaffold for tissue engineering. The material comprises a subchondral bone layer, an interface layer and a cartilage layer arranged in sequence, wherein the interface layer is positioned between the subchondral bone layer and the cartilage layer; the material contains the following components in parts by weight:10-40 parts of oil-soluble high polymer materials, 10-50 parts of biological ceramic powder, 50-100 parts of oily solvents, 0.1-1 part of a water-soluble bio-active material, 2-30 parts of water, more than 0 and less than 1 part of an emulsifier, 10-30 parts of a hydrogel material, 0-10 parts of seed cells and 50-100 parts of a culture medium. The preparation method comprises the following steps:preparing printing ink according to the ratio of the materials, and transferring into 3D printing equipment for printing molding. The bone cartilage scaffold having a personalized appearance can be prepared, load and controlled release of different growth factors or peptides in different zones of the scaffold can be realized, and directional differentiation of bone marrow stem cells at differentparts to osteoblast and chondrocyte can be promoted.
Owner:王翀
Compositions and Method for Brain Specific Targeted Delivery of Therapeutic Agents
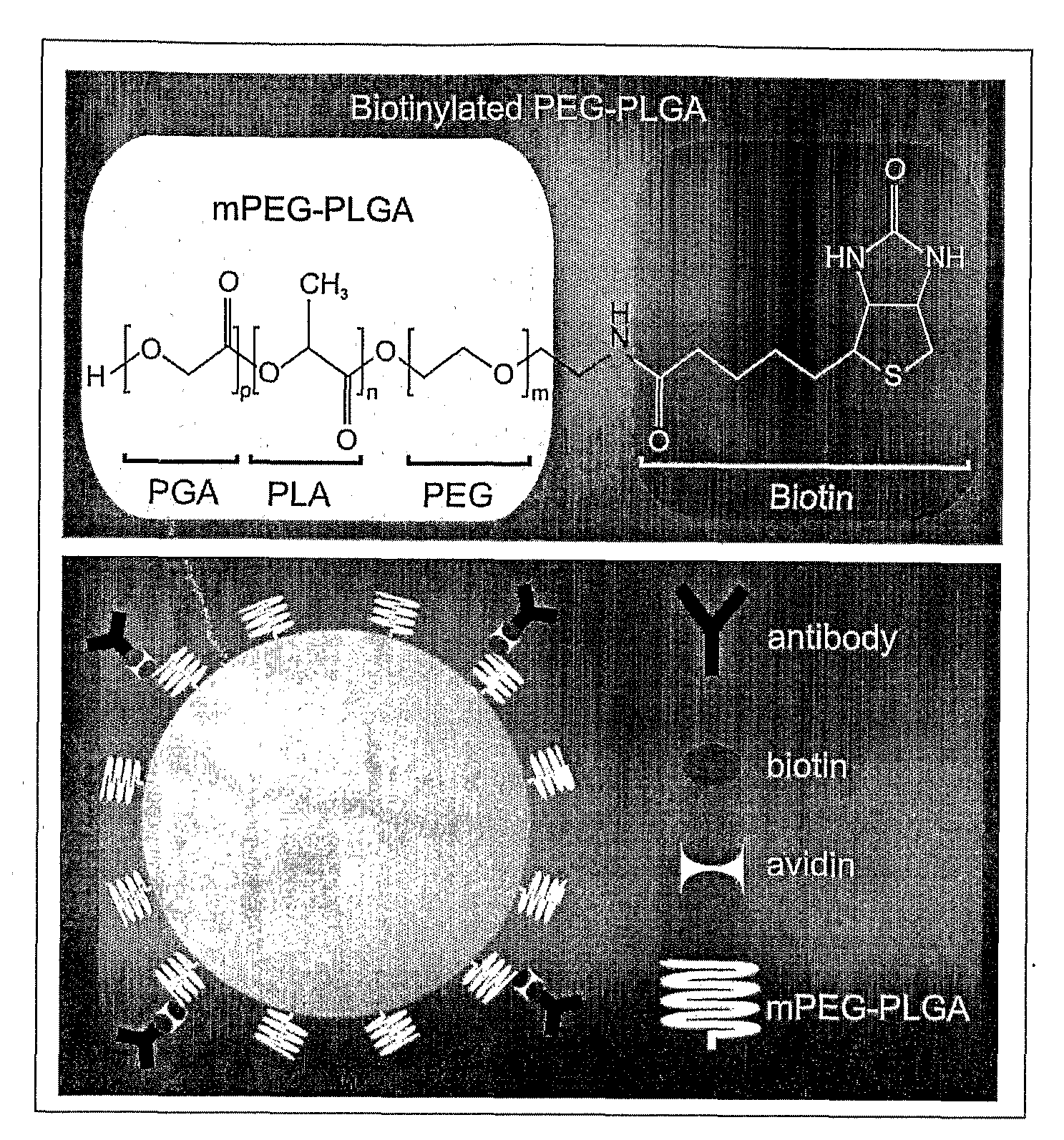
Disclosed are methods and compositions for delivering a therapeutic agent to target organs or tissues, such as brain. The methods and compositions use bone marrow stem cells, monocytes, macrophages or microglial cells to deliver the therapeutic agent associated with nanoparticles to the target organ or tissue.
Owner:UNIVERSITY OF CHICAGO
Method for osteogenic differentiation of bone marrow stem cells (BMSC) and uses thereof
ActiveUS20090081169A1Reduce chanceLess of contaminationBiocideAntipyreticBone Marrow Stem CellOsteocyte
Methods for obtaining osteoprogenitors, osteoblasts or osteoblast phenotype cells, as well as cell populations including such cells, from human bone marrow stem cells in vitro or ex vivo are disclosed. Bone marrow stem cells are contacted with human serum or plasma and a growth factor or a biologically active variant or derivative thereof. In addition, osteoprogenitor, osteoblast or osteoblast phenotype cell types and cell populations are provided. The cell populations may include additional cell types, such as endothelial cells or progenitors. The osteoprogenitors, osteoblasts or osteoblast phenotype cells may be used in therapy, particularly bone therapy.
Owner:UNIV LIBRE DE BRUXELIES
Internally administered therapeutic agents for diseases in central and peripheral nervous system comprising mesenchymal cells as an active ingredient
InactiveUS20070178591A1Effective treatmentReduce riskVectorsPeptide/protein ingredientsNervous systemCord blood stem cell
Intravenous administration of bone marrow cells collected from rat bone marrow to a rat cerebral infarction model was found to be effective in treating cerebral infarction. Human and murine bone marrow stem cells showed similar effects. Mesenchymal cells such as bone marrow cells, cord blood cells, or peripheral blood cells can be used as agents for in vivo administration against cranial nerve diseases.
Owner:NC MEDICAL RES +2
Human Bone Stem Cells From Amniotic Mesenchymal Cell Layer
A bone stem cell which may be supplied stably and which is free from the problem about the compatibility in transplantation is disclosed. The bone stem cell according to the present invention is separated from human amniotic mesenchymal cell layer. The bone stem cell may be used for osteogenesis in a bone defect or the like.
Owner:SAKURAGAWA NORIO +1
Internally administered therapeutic agents for cranial nerve diseases comprising mesenchymal cells as an active ingredient
InactiveUS20060210544A1Easily and safely obtainedInhibit ischemic damageBiocideGenetic material ingredientsDiseaseCord blood stem cell
Intravenous administration of bone marrow cells collected from rat bone marrow or peripheral blood to a rat cerebral infarction model was found to be effective in treating cerebral infarction. Human and murine bone marrow stem cells showed similar effects. Mesenchymal cells such as bone marrow cells, cord blood cells, or peripheral blood cells can be used as agents for in vivo administration against cranial nerve diseases.
Owner:NC MEDICAL RES
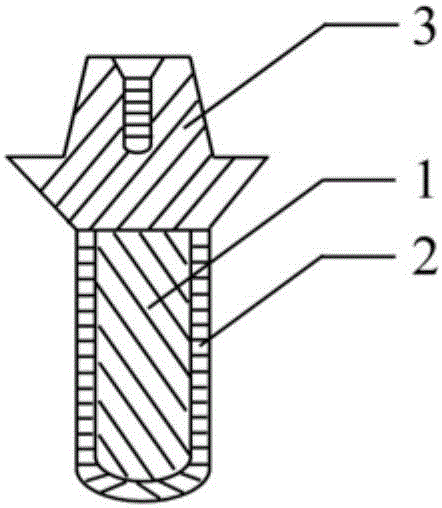
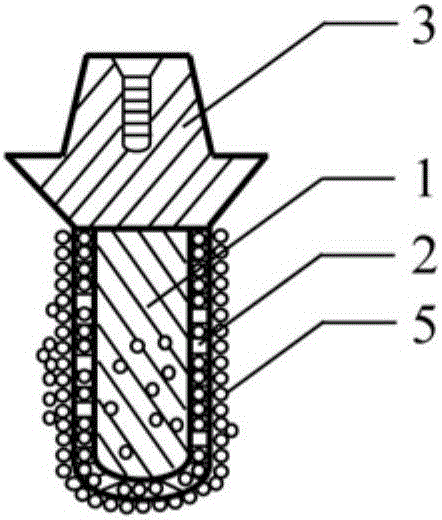
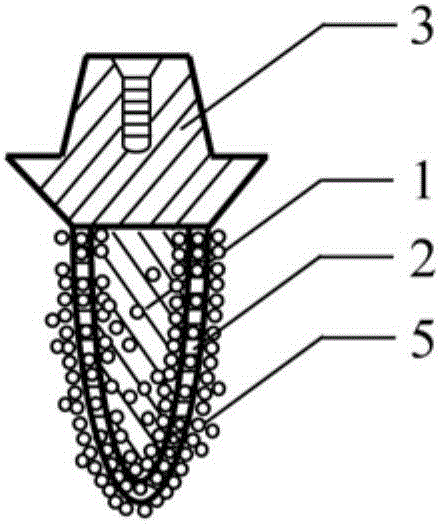
Polyporous hollow bone screw
Present invention depicts a poly-porous (micropore) hollow screws as diffusion chamber filled with core matrix for targeted delivery of growth factors and bone marrow stem cells. The screws comprise at least two parts: the distal part of the screw consists of the tip of the screw made of poly porous material and hollow inside proximally. It has threaded navel attached to the threaded nipple of the distal part of the proximal screw which has the screw head and is made of the solid material of the same kind. The screw head had hexagonal recess targeted for screw driver insertion. Assembly of screw created a chamber in the middle of the screw. The chamber is filled with core matrix consisting of gelatin nano-particles pre-impregnated with BMPs (BMP2 / BMP7 for bone or BMP12 for tendon, ligament) and fibrin sealants or Chitosan dispersed with bone marrow stem cells and / or other growth factors. Bioactive protein core material is prepared during the surgery and filled the chamber of the screw by the surgeon. Fibrin sealants or Chitosan will polymerize to form a gel to hold the growth factors and stem cell in place. The screw can be used as the lag screw or other function to provide mechanical fixation in variety of condition. Once the screw implanted in the human body, the fibrin sealant or Chitosin / gelatin nano-particles are gradually degraded and slowly release growth factors and stem cells via micropores of screw to facilitate the bone healing and regeneration. The gelatin nanoparticles and fibril sealant / or Chitosan matrix also serve as the scaffold and platform for bone in-growth to the screw or alternatively, the stem cell inside of screw can regenerate new bone, providing the biological fixation. At the mean time as the bone regenerate and / or in growth, mechanical strength of the screw increased.
Owner:WU YANGGUAN
Closed system separation of adherent bone marrow stem cells for regenerative medicine applications
ActiveUS20130101561A1Elicit neuronal and astrocytic differentiationMinimally invasiveBiocideBone marrow stroma cellsCell adhesionBone Marrow Stem Cell
A method for isolating and processing bone marrow derived stem cells, including the steps of: (a) collecting a biological sample containing adherent bone marrow stem cells in a receptacle with interior walls coated with a cell-adherent substrate; (b) incubating the bone marrow cells on the adherent substrate so that a layer of adherent bone marrow stem cells adheres to the substrate; (c) washing any non-adherent cells from the substrate; and (d) collecting the bone marrow stem cell layer. Isolation kits and use of bone marrow cells harvested for cell therapies are also described.
Owner:RUTGERS THE STATE UNIV
Bone marrow stem cell protection solution and preparation method thereof
InactiveCN107668024AEffective protectionExpand the scope of clinical applicationDead animal preservationCell activityInosamycins
The present invention provides a bone marrow stem cell protection solution, which is mainly prepared by dissolving a hypoxic protection agent, heparin sodium and an aminoglycoside antibiotic in a DMEM / F12 culture medium aqueous solution, wherein per mL of the DMEM / F12 culture medium aqueous solution can respectively dissolve 5-10 mg of the hypoxic protection agent, 0.01-0.05 U of the heparin sodium and 50-200 U of the aminoglycoside antibiotic, and the concentration of the DMEM / F12 culture medium aqueous solution is 30-40 mg / ml. According to the present invention, the bone marrow stem cell protection solution has advantages of stable performance, safety and no toxicity, can effectively protect bone marrow stem cells, can make bone marrow stem cells be preserved for a long time and can maintain the cell activity in vitro, can maintain the original multidirectional differentiation ability, and can expand the clinical application of bone marrow stem cells, wherein the bone marrow stem cells can be transported to most domestic cities and neighboring countries by using the existing transportation tools.
Owner:CENTURY BIOSTRENGTH BEIJING PTY LTD
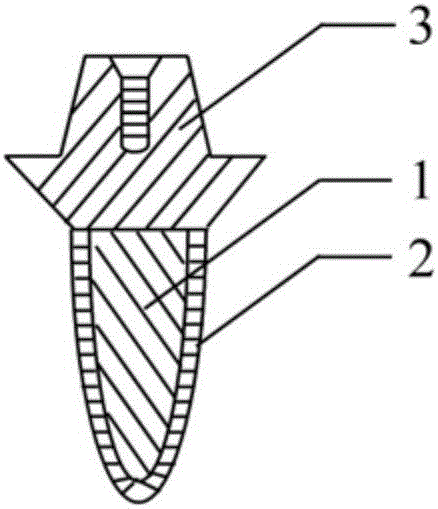
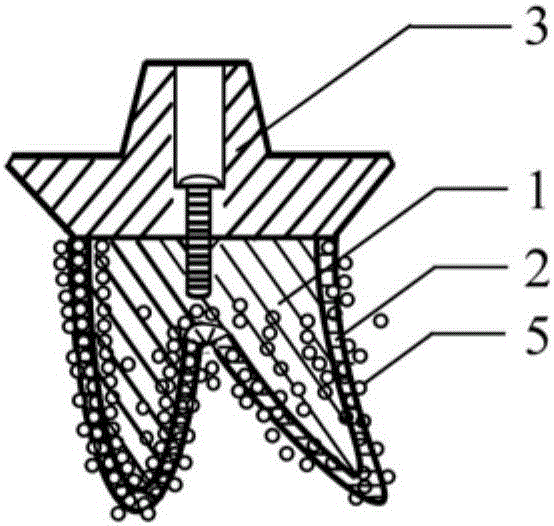
Individualized biomimetic dental implant and manufacture method thereof
InactiveCN106037965ALow elastic modulusImprove biomechanical compatibilityDental implants3d printCell adhesion
The invention relates to an individualized biomimetic dental implant and a manufacture method thereof. The invention is characterized in that the dental implant comprises an implant body for imitating the original tooth root tissue structure of a patient's missing tooth and also comprises a biomimetic artificial alveolar bone structure outer-layer for imitating a patient's alveolar bone tissue structure. The biomimetic artificial alveolar bone structure outer-layer and the external surface of the implant body are combined as a whole. The biomimetic tooth root implant body's outer layer is a 3D printed biomimetic artificial alveolar bone structure outer-layer which is combined with the implant body to form a whole structure, and is similar to the tissue structures of missing tooth's original tooth root and alveolar bone. Thus, the invention provides an ideal implementation scheme of a biomimetic artificial tooth root structure. What is the most important is that the invention provides a structure which is beneficial to bone marrow stem cell and osteogenesis-related cell adhesion, proliferation and mineralization and gives play to final osteogenic function and has good bioactivity. Therefore, a microenvironment for promoting osteogenesis is created, osteocyte generation is induced, and early rapid and firm synosteosis is realized.
Owner:STOMATOLOGICAL HOSPITAL TIANJIN MEDICAL UNIV
Culture methods of bone marrow stromal cells and mesenchymal stem cells, and manufacture method of graft cells for central nerve system diseases therapy
ActiveUS20120115224A1Improve survival rateGood treatment effectBioreactor/fermenter combinationsNervous disorderDiseaseBone Marrow Stromal Cell
Owner:YUGE LOUIS +1
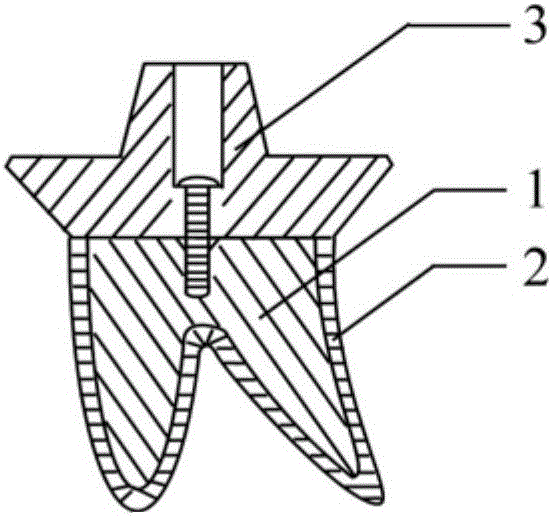
Medical dental implant with surface biomimetic function and manufacturing method thereof
The invention relates to a medical dental implant with the surface biomimetic function and a manufacturing method thereof. The medical dental implant is characterized in that the medical dental implant comprises an implant body and further comprises an outer layer of a bionic artificial bone structure, the outer layer is integrally combined with the outer surface of the implant body and capable of simulating the alveolar cavity bone tissue structure around the tooth root portion of a missing tooth of a patient. According to the medical dental implant and the manufacturing method thereof, the outer layer of the bionic bone structure, which is formed in a 3D printing mode and of a massive structure, serves as the outer layer of the tooth implant body, and therefore an ideal embodiment used for simulating the artificial bone surface structure is provided. More importantly, a good activity surface layer structure foundation beneficial to bone marrow stem cell and bone-formation related cell adhesion, proliferation and growth and capable of playing the final bone-formation function is provided, the surface of the foundation is loaded with chemical components such as calcium and phosphate and bioactive molecules such as collagens, hence, a bone-formation promotion microenvironment is created, bone cell generation is induced, and fast and firm bone combination can be achieved at the early stage.
Owner:李莺
Medicament for treating tumor and hemorrhoid and method for producing the same
InactiveCN101485669ANo painNo sequelaeHeavy metal active ingredientsHydroxy compound active ingredientsBone Marrow Stem CellTreatment period
The invention relates to a medicine for treating tumor and hemorrhoid and a manufacturing method thereof. The medicine aims at the disadvantages that: the prior treatment method for removing the hemorrhoid and the tumor can only kill or remove partial tumor cells, has large pain and high recurrence rate during the treatment period, has the phenomena of lassitude, hypodynamia, nausea, vomiting and hemogram reduction during the radiotherapy period, causes low immunity, damages self-immunologic function of a human body, inhibits functions of bone marrow stem cells, is easy to produce drug dependence, and is easy for recurrence. The invention prepares tannic acid, atropine, gallic acid, quinin hydrochloride, ferrous sulfate, caffeine, pure sulfuric acid, urethane, carbolic acid, sterile water and the like, into medicines for local parts of pathological changes, does not hurt normal tissues, has no pain, does not need to treat for a long time, and has the advantages of taking effect by injecting at one time, no recurrence, wide raw materials, low cost, wide clinical application, and small side effect.
Owner:秦克骏
Method for treating chronic nerve tissue injury using a cell therapy strategy
ActiveUS20120276068A1Improved motor coordinationReduction of targeted neurodegenerative conditionBiocideNervous disorderInjury SiteTraumatic injury
A method for treating a degenerative or traumatic injury to a nerve tissue or the brain by administering at or near the injury site a composition containing adherent bone marrow stem cells suspended in a pharmaceutically acceptable liquid in an amount effective to elicit axonal regeneration or re-myelination at the site of injury.
Owner:RUTGERS THE STATE UNIV
Methods and compositions for bone marrow stem cell-derived macrophage delivery of genes for gene therapy
The present invention provides an isolated nucleic acid comprising a promoter operably linked to a nucleic acid encoding a peptide or protein and / or an RNA (e.g., antisense or ribozyme), wherein the promoter comprises elements that can include, but are not limited to, a) a myeloid specific promoter element comprising a core sequence GAGGAA; b) a myeloid specific promoter element comprising a core sequence AAGGAGAAG; c) a myeloid specific promoter element comprising a core sequence TTTCCAAA; d) a myeloid specific promoter element comprising a core sequence TGTGGTTGC; e) a myeloid specific promoter element comprising a core sequence TGAGTCA; f) a myeloid associated promoter element comprising a core sequence CCGCCC; and g) any combination of (a), (b), (c), (d), (e) and / or (f), any combination of multiples of (a), (b), (c), (d), (e) and / or (f), in any order and / or in any orientation (forward or reverse).
Owner:BOARD OF RGT UNIV OF TEXAS THE
Method for inducing self-body stem cell differentiation to be nerve stem cell with liquor cerebrospinalis
The invention relates to a method of the induction and differentiation of the neural stem cells and the neural cells from bone marrow stromal cells which are cultured by autologous bone marrow, autologous serum and autologous cerebrospinal fluid. The method pertains to the methods for differentiating the bone marrow stem cells into the neural stem cells. A conventional low-sugar DMEM / F 12 culture medium is adopted, the autologous serum replaces the original fetal bovine serum, the autologous cerebrospinal fluid replaces the original additive and a stimulating factor, a small amount of autologous bone marrow is used for culturing more cells with the number that can meet the clinical needs. The method has the advantages of low cost and easy material selection, avoiding zoonosis and foreign protein exclusive reaction, and so on. The method of inducing the bone marrow stromal cells into the neural stem cells by using cerebrospinal fluid can effectively solve the shortcomings of the traditional methods.
Owner:万美蓉 +1
Individual biomimetic dental implant and making method thereof
ActiveCN106333753AEarly rapid and strong osseointegrationFast and strong osseointegrationDental implantsCell adhesionBone structure
The invention relates to an individual biomimetic dental implant and a making method thereof, and the individual biomimetic dental implant includes an implant body imitating an original teeth root tissue structure of a missing tooth of a patient, a bionic artificial alveolar bone structure outer layer which is bonded with the outer surface of the implant body into one body, and biological protein molecule-bone morphogenetic protein (BMP) which is attached to the bionic artificial alveolar bone structure outer layer. The implant body and the bionic artificial alveolar bone structure outer layer are overall 3D-printed, and are similar to the original teeth root tissue structure and the alveolar bone tissue structure of the missing tooth of the patient, and the BMP-modified biological protein molecule promoting rapid bone formation is added, so that an ideal bionic artificial teeth root tissue structure is provided. The most important is to provide a good biological activity structure which is conducive to bone marrow stem cell and bone formation-related cell adhesion, proliferation and mineralization to finally play the osteogenic function, a bone formation promotion microenvironment is created, bone cell formation is induced, and early rapid and strong bone combination can be realized.
Owner:STOMATOLOGICAL HOSPITAL TIANJIN MEDICAL UNIV
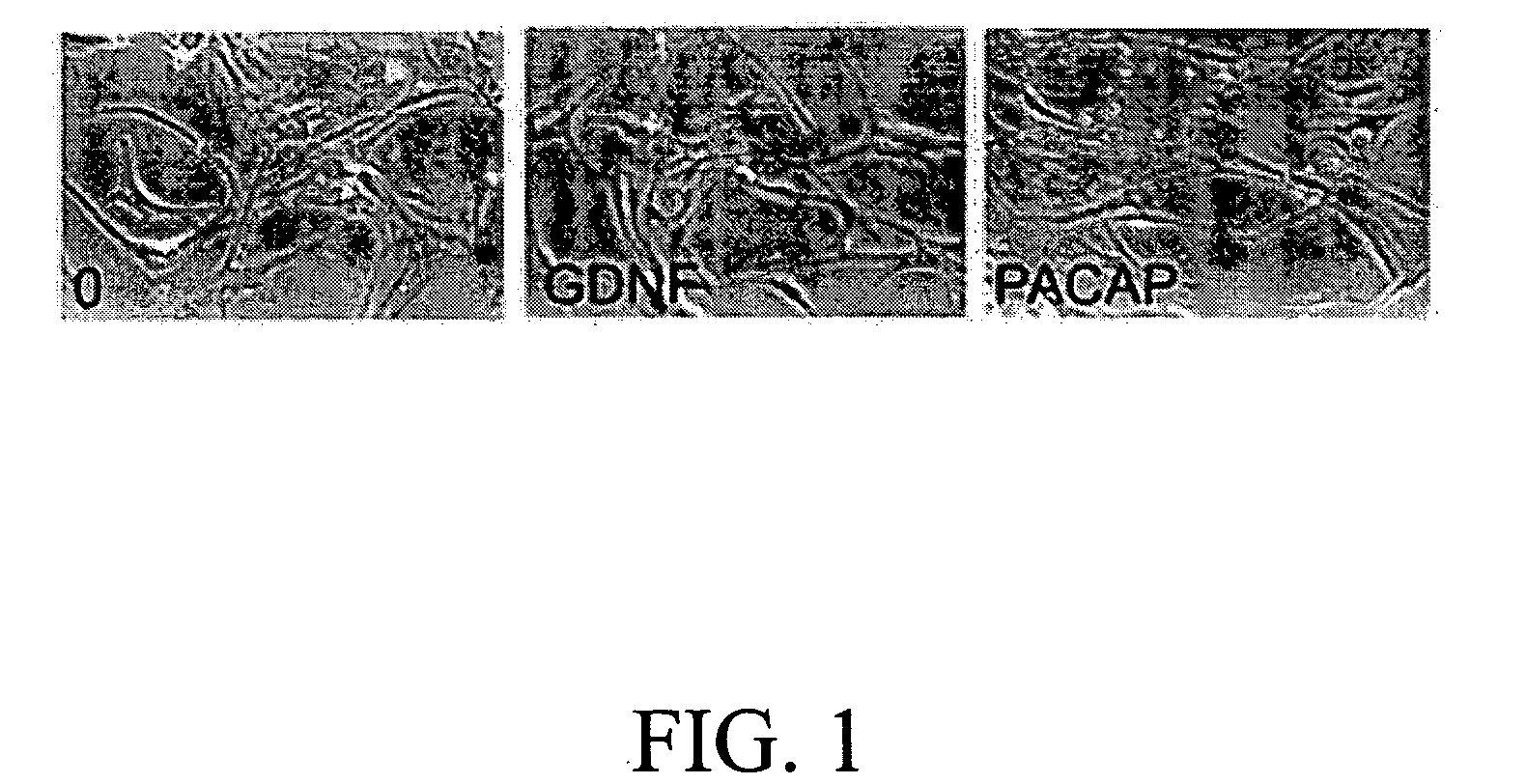
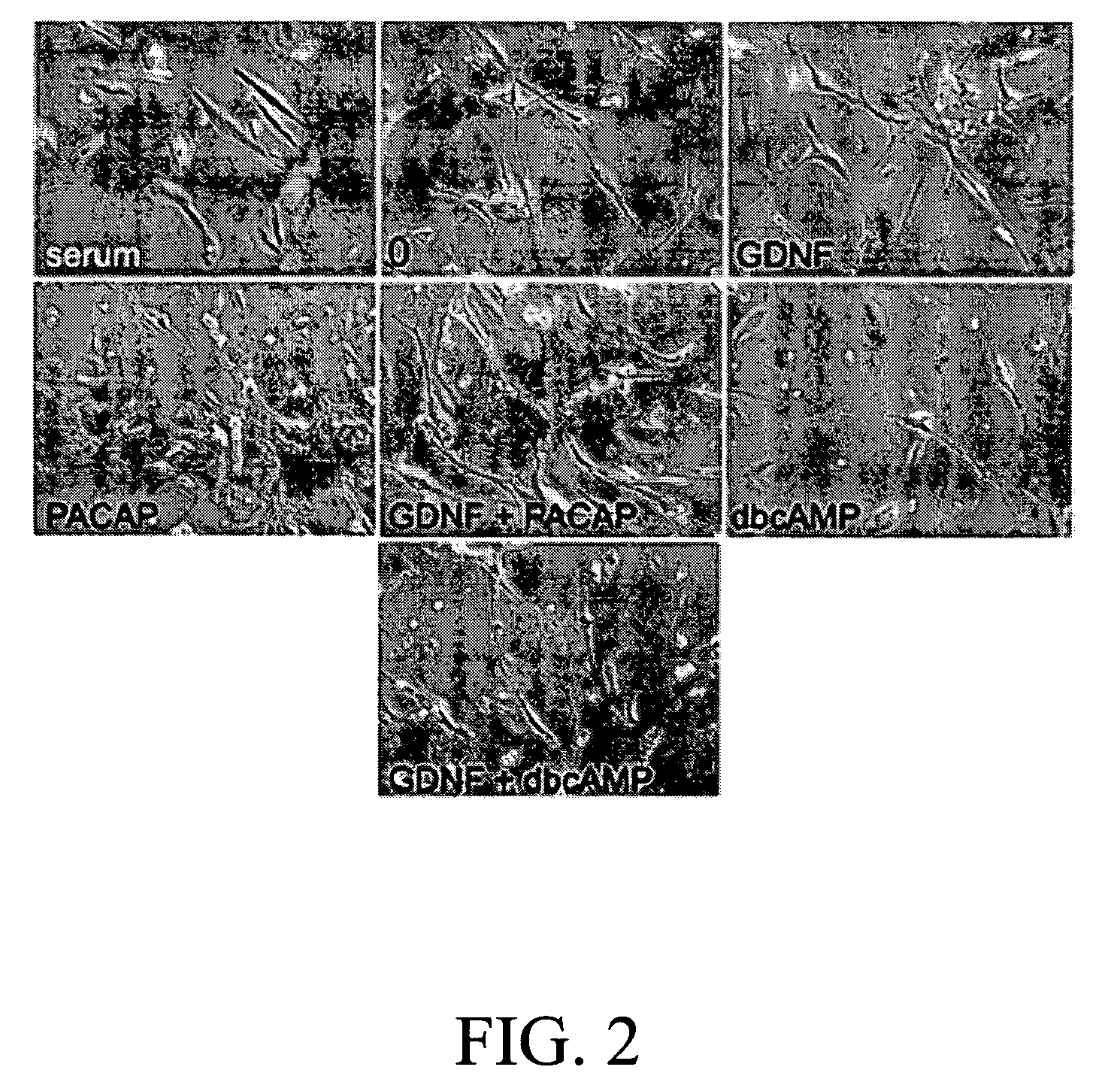
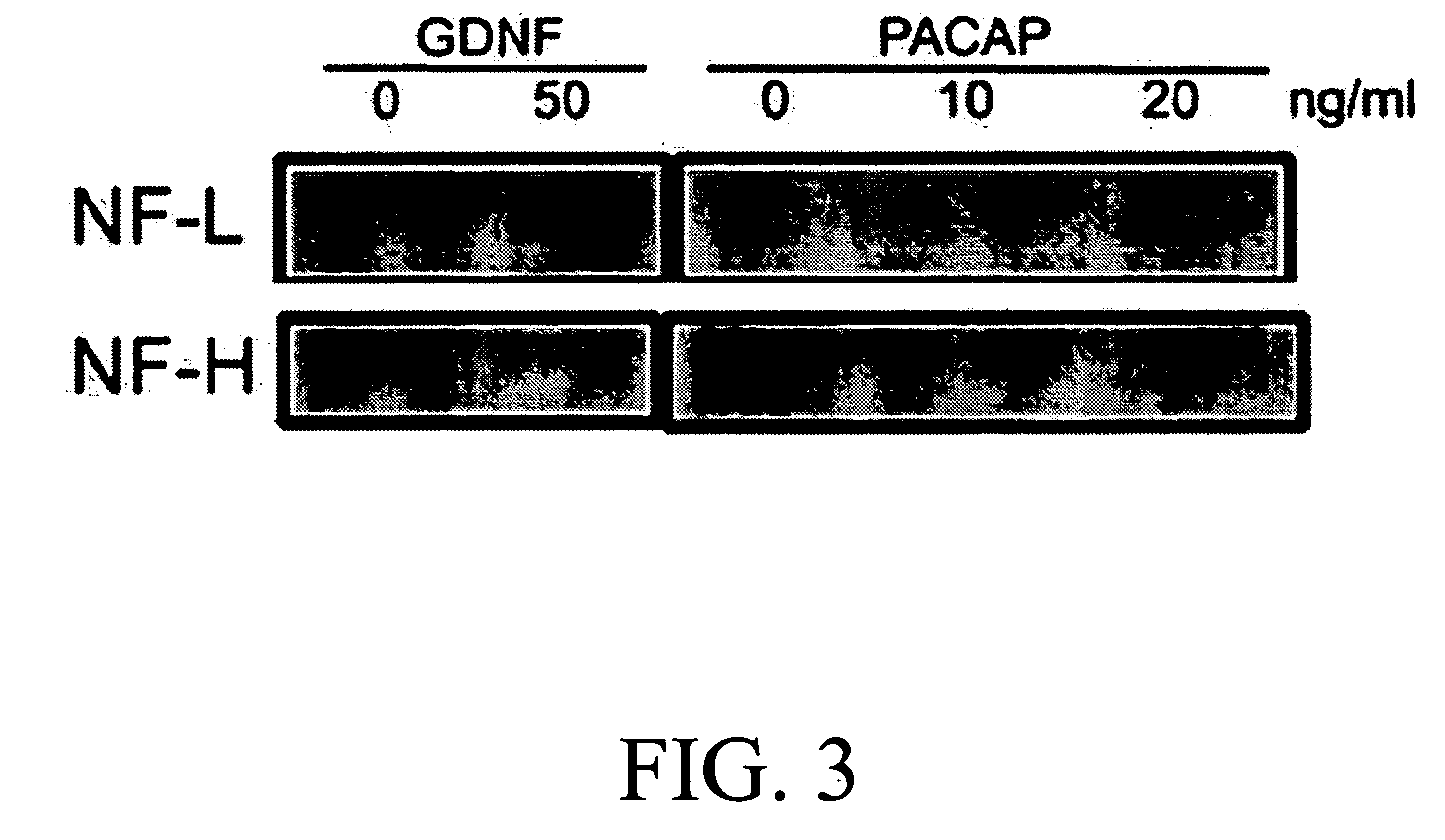
Method for inducing neural differentiation
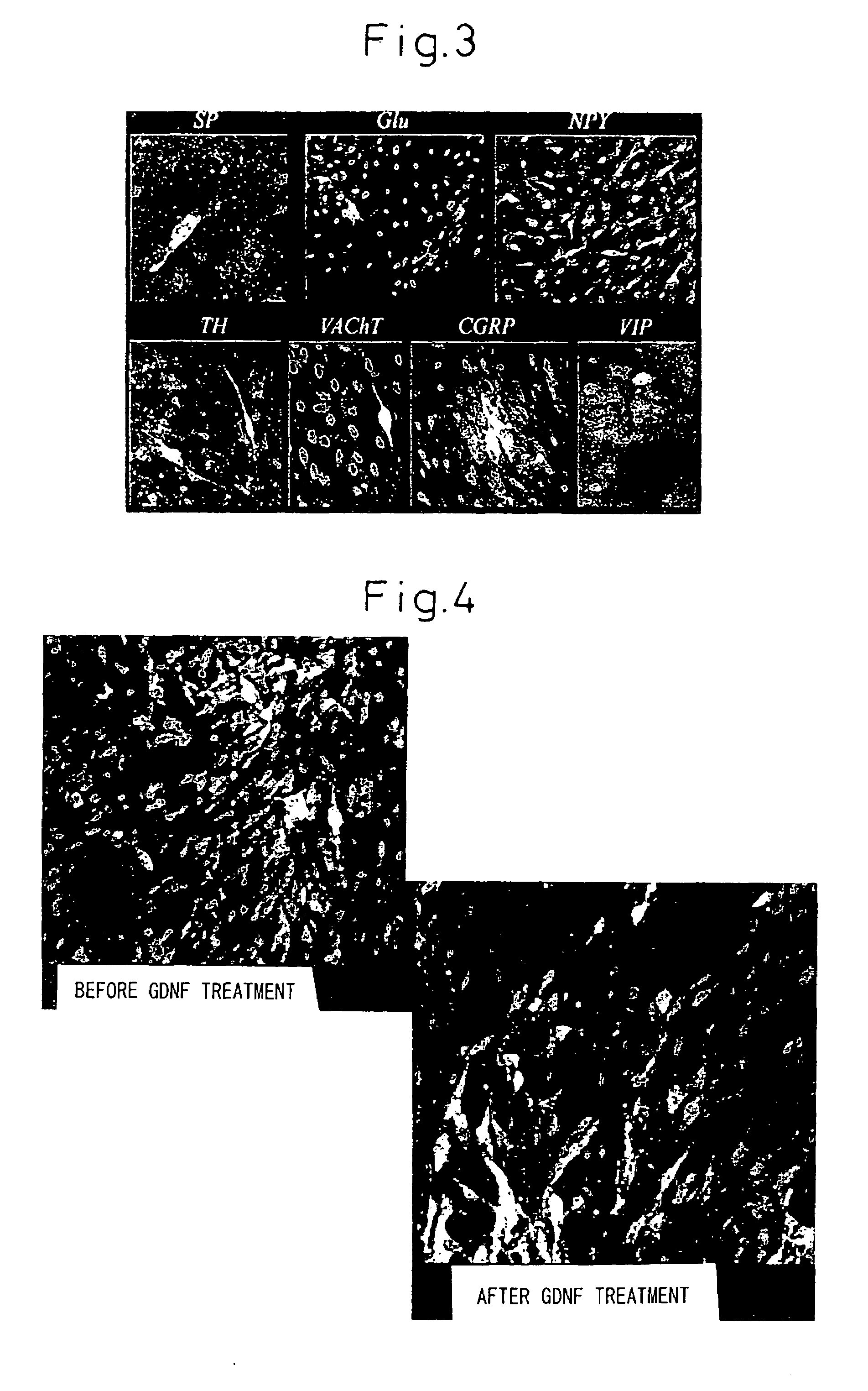
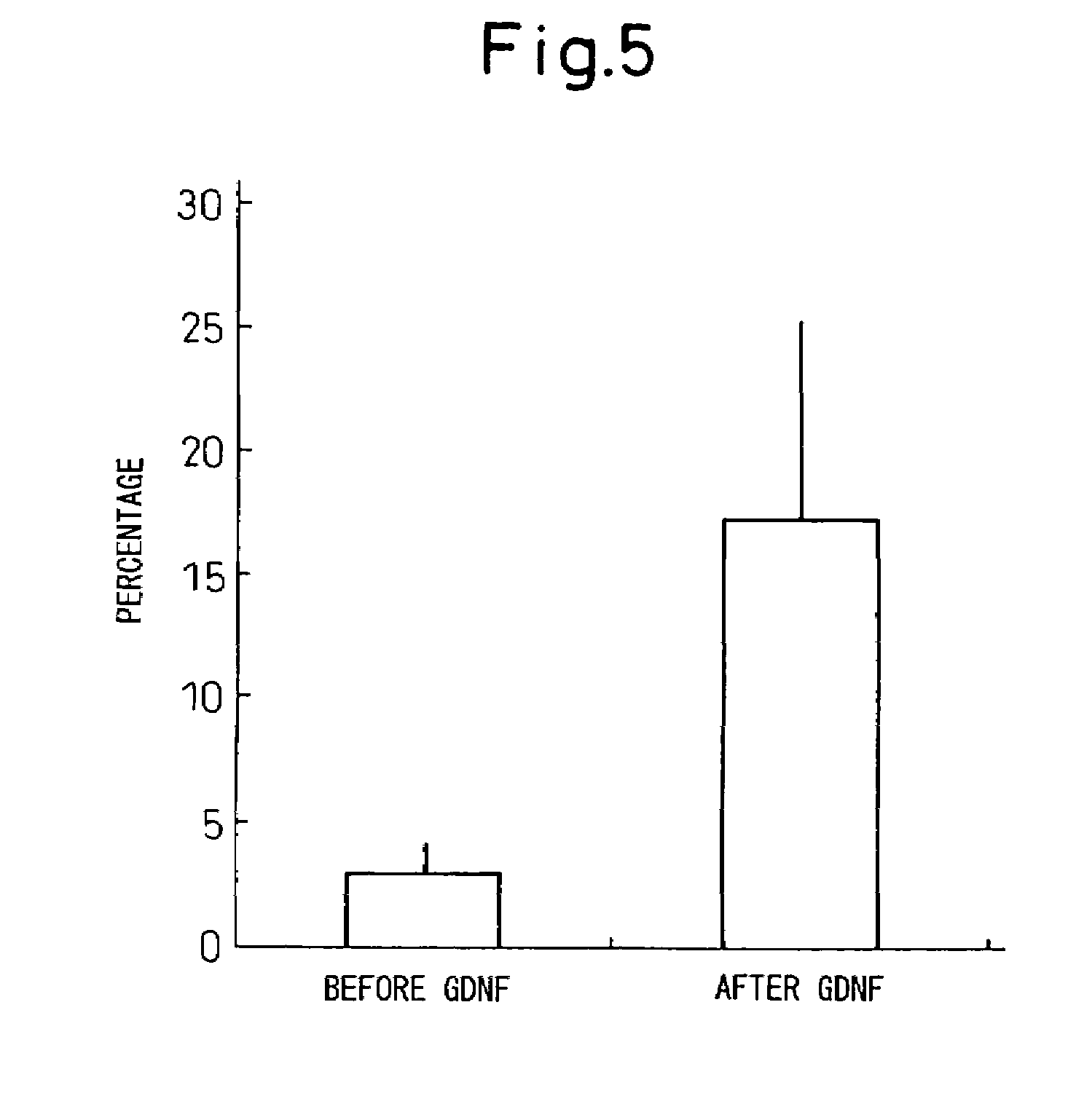
InactiveUS20050287665A1Safe and effective in changeFix bugsOrganic active ingredientsNervous disorderGlial cell line-derived neurotrophic factorBone Marrow Stem Cell
The present invention provides a method for inducing neural differentiation comprising treating a bone marrow stem cell with a neurotrophic factor and / or dibutyryl cAMP (dbcAMP), wherein the neurotrophic factor comprises glial cell line-derived neurotrophic factor (GDNF) or pituitary adenylate cyclase-activating polypeptide (PACAP).
Owner:HENRICH CHENG
Common stonecrop herb and use of salidroside in stem cell committed differentiation to hepatocyte lineage
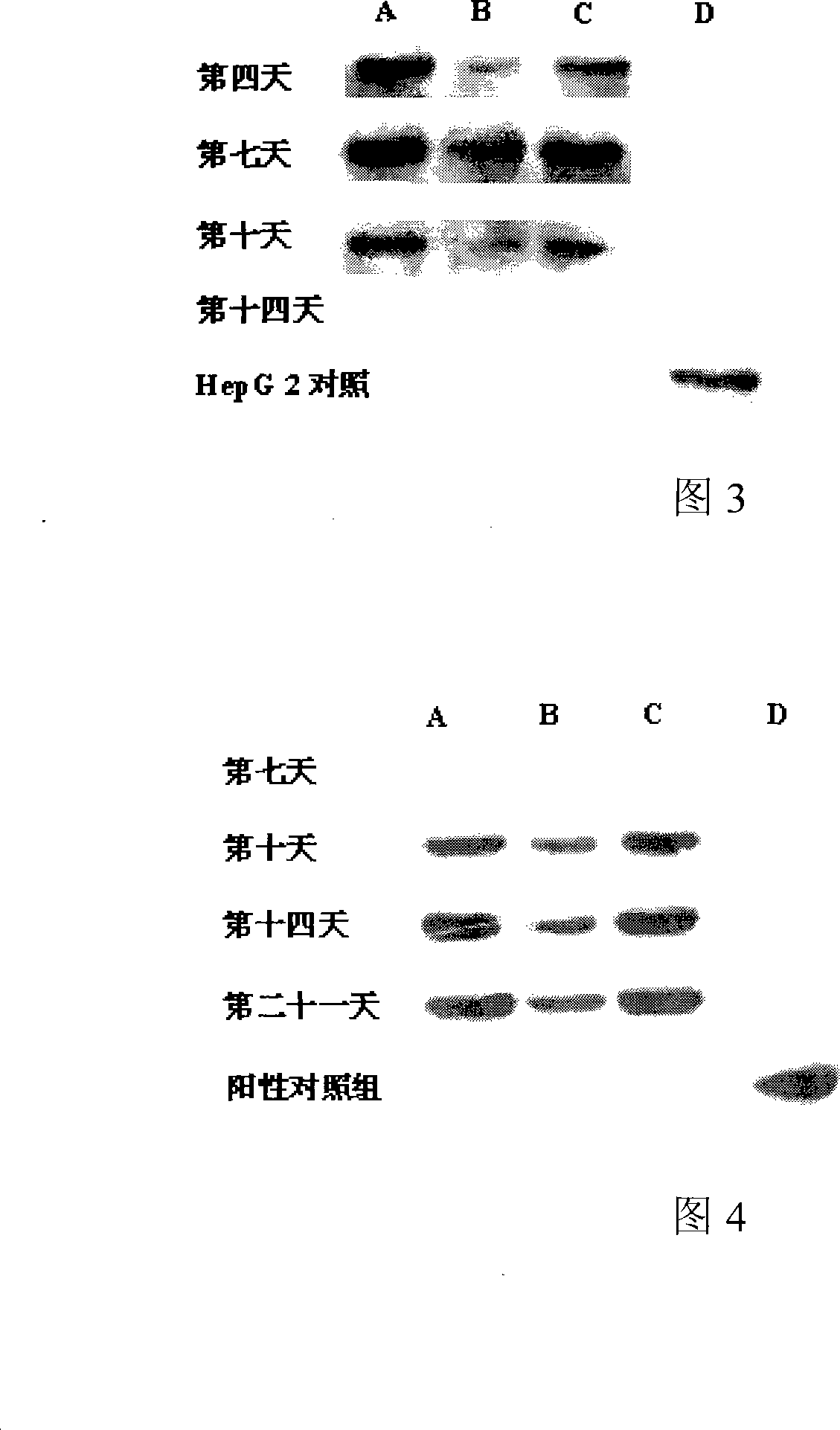
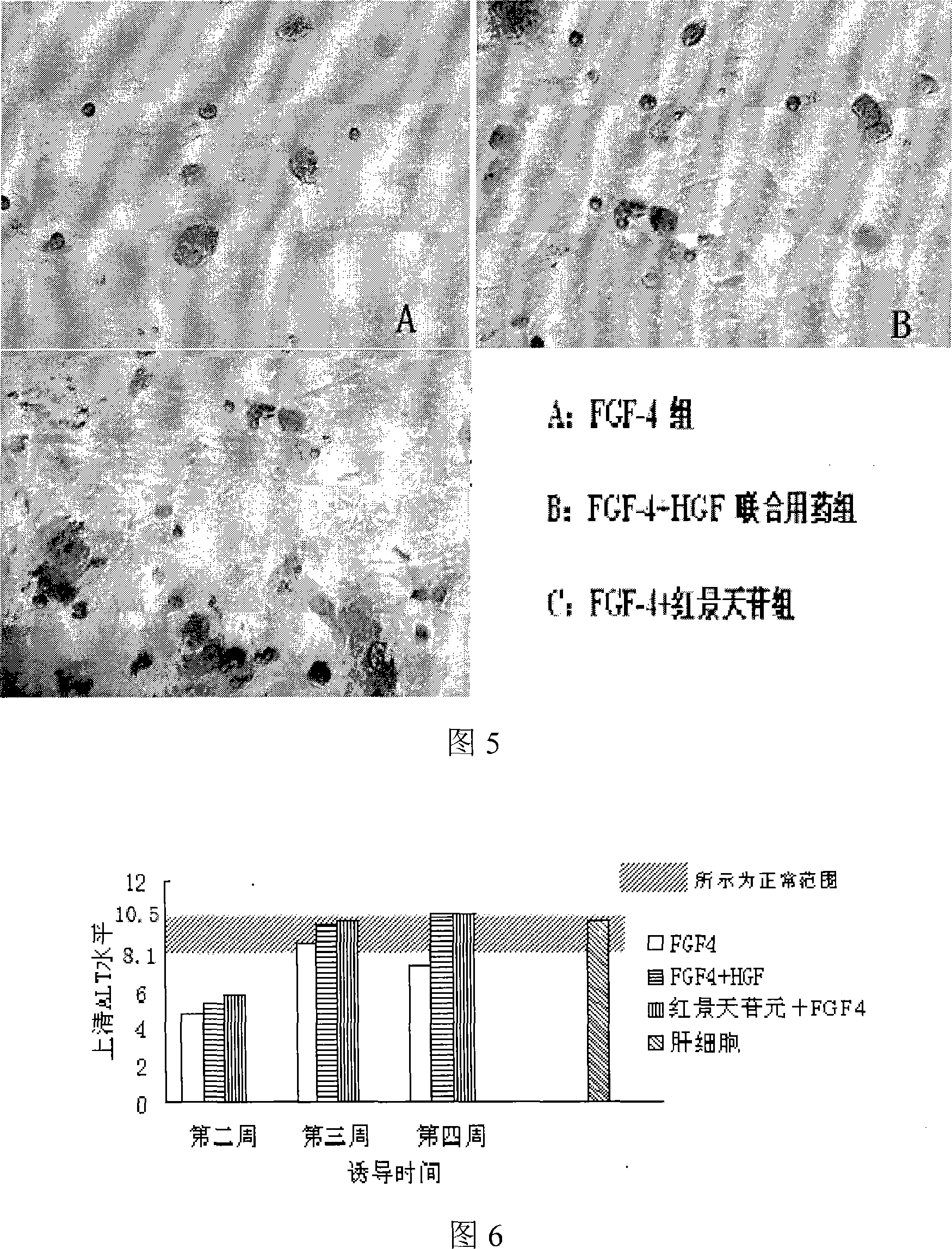
ActiveCN101225374AConducive to in-depth researchDigestive systemArtificial cell constructsSalidrosideRHODIOLA ROSEA ROOT
The invention relates to a rhodiola rosea and a rhodioside application for inducing stem cells to directionally differentiate into hepatocytes, in particular to a compound inducer of the rhodioside and cell growth factor FGF-4 used for inducing mesenchymal stem cells to directionally differentiate into hepatocytes. The rhodiola rosea develops the new application of rhodiola, which induces mesenchymal stem cells and other stem cells to directionally differentiate into hepatocytes in vitro, and provides the effect of the rhodiola rosea and the rhodioside on inducing the adult stem cells to directionally differentiate into hepatocytes, which is beneficial to further research of the in vivo stem cells transplantation for treating acute or chronic hepatic injury and middle-advanced liver disease field, and provides the basic for new drug development.
Owner:北京瑞草堂三圣(汤阴)药业有限公司
Methods and compositions for enhancing stem cell mobilization
ActiveUS20130108587A1Increasing stem cell mobilizationBiocideOrganic active ingredientsDosing regimenMicroorganism
A method of using fucoidan to enhance stem cell mobilization in a subject, including hematopoietic stem cells (HSCs) and bone marrow stem cells (BMSCs) is provided. In the method, a blended composition of fruits, mushrooms, microorganisms, maternal fluids, and extracts thereof are used to promote trafficking of stem cells, resulting in migration of the stem cells to specific sties of maintenance an and repair within tissues and / or organs. The method also involves the use of fucoidan obtained from particular algae species to support release and circulation of HSCs, as demonstrated by significantly increase circulation of HSCs in the peripheral blood. Increased circulation of HSCs and / or BMSCs and migration towards sites of maintenance and the natural regeneration mechanisms in the body. Further provided is a dosing regimen for the administration of fucoidan and a method of enhancing release and circulation of stem cells.
Owner:STEMTECH INT
Compounds for stimulating stem cell proliferation including spirulina
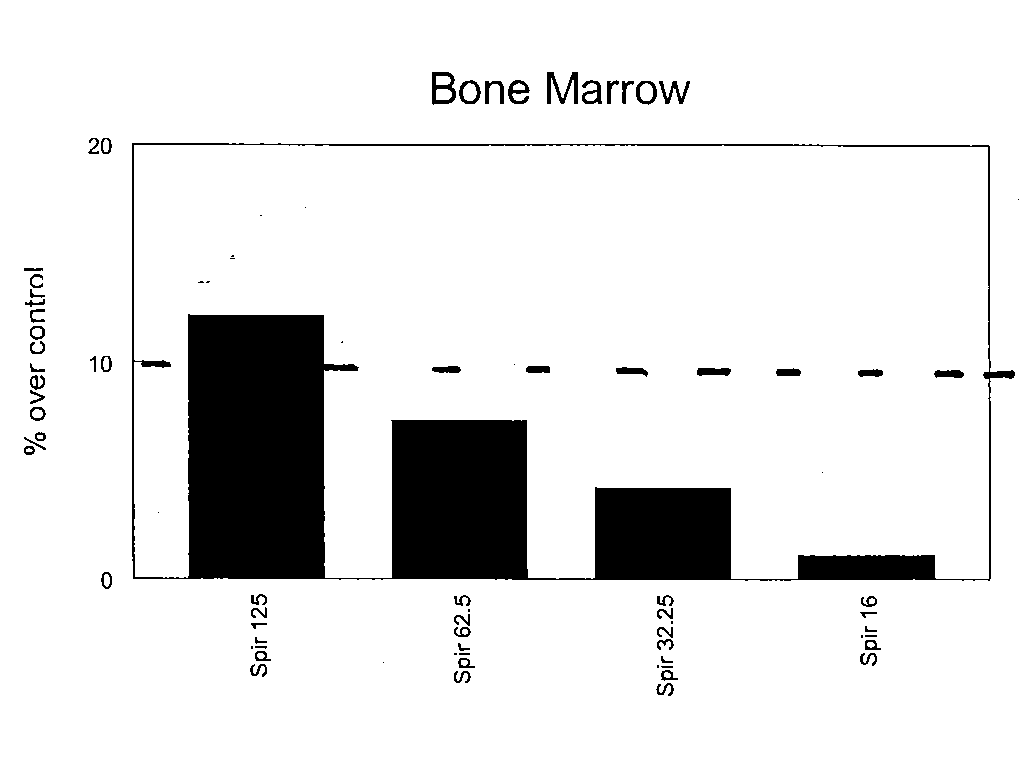
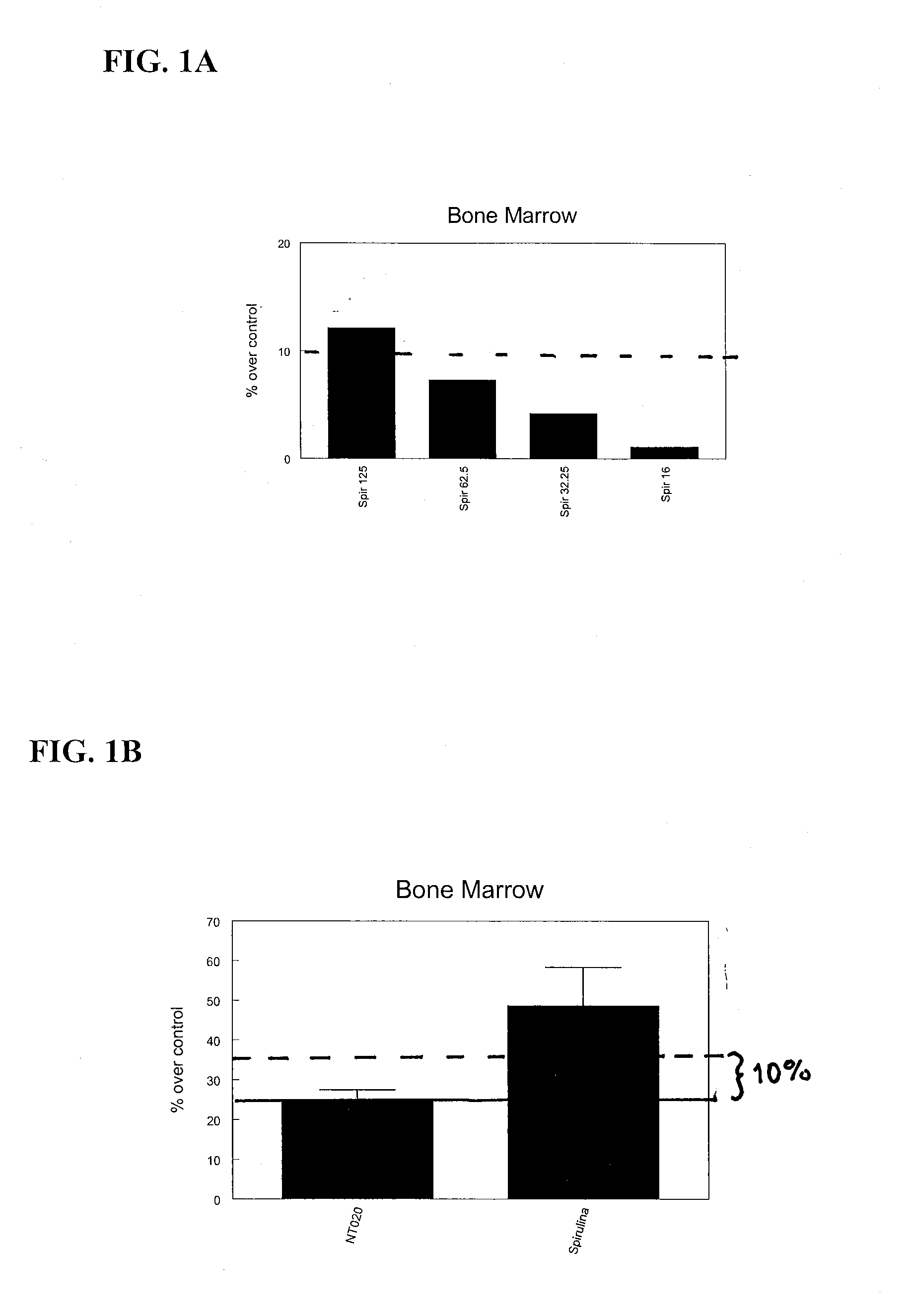
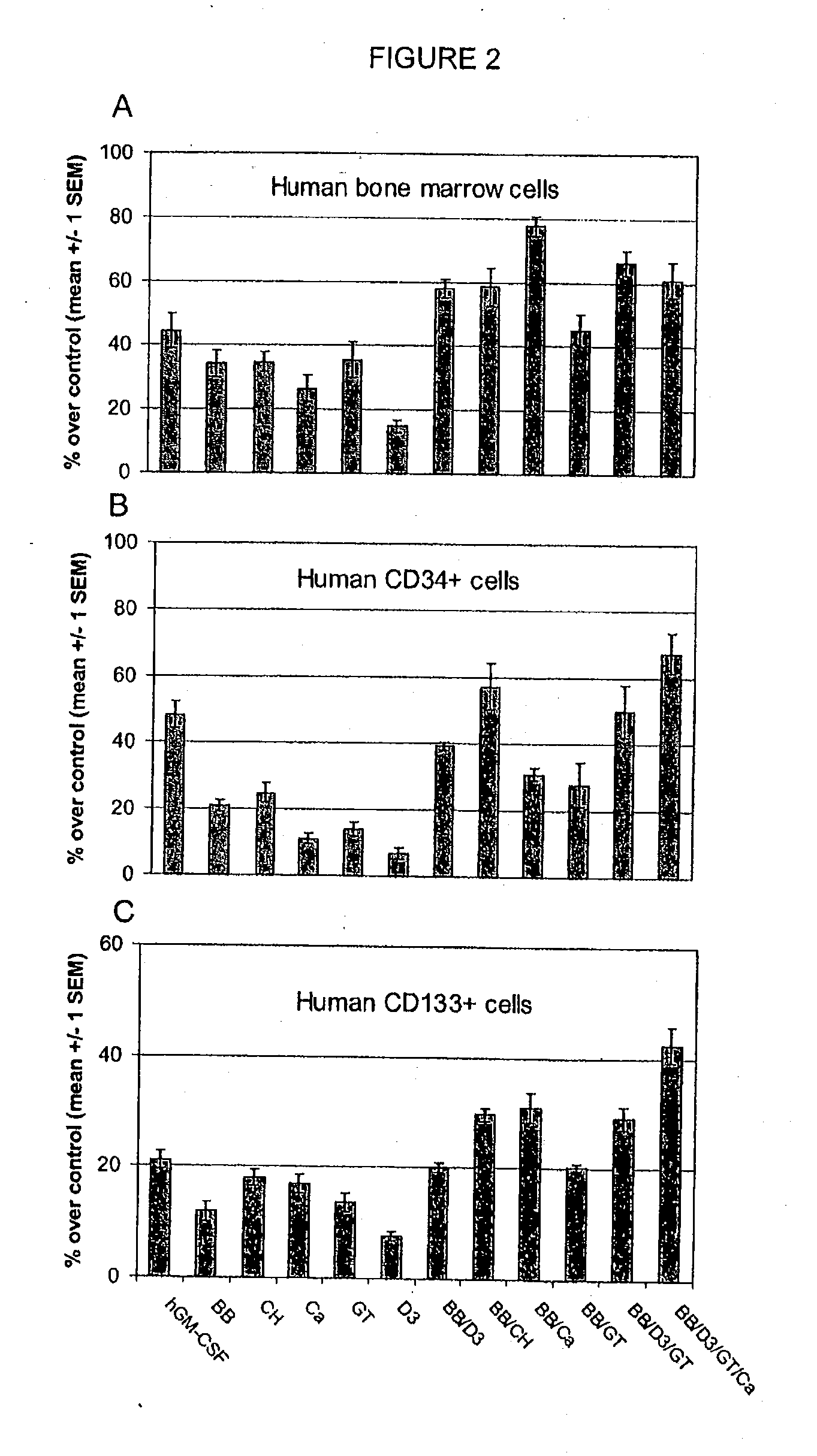
InactiveUS20080085330A1Increasing stem cell proliferationIncrease measurable indicator of proliferationOrganic active ingredientsBiocideDiseaseDamages tissue
A method and composition for stimulating the proliferation and differentiation of stem cells is used to self-repair injury in mammals. A supplement is administered having an effective dose of substances selected from blueberry, carnosine, catechin, green tea extract, VitaBlue, Vitamin D3, Spirulina, AFA or effective combinations and derivatives of these. For example, a supplement comprising wild blueberry, green tea extract, carnosine, vitamin D3, and a Spirulina and / or an AFA-Omega (EtOH) exhibited a synergistic and unexpected association with substantially increased proliferation of bone marrow stem cells and CD34+. A therapeutic amount of a substance that is associated with a substantial increase in stem cells may be used to prevent and repair damages tissues of the brain, due to stroke, the heart, due to coronary artery disease or a heart attack.
Owner:UNIV OF SOUTH FLORIDA
Novel multipotent stem cells and use thereof
InactiveUS20070253937A1Reduce severityPrevent and treat and reduce severityBiocideArtificial cell constructsSmooth muscleCell marker
Disclosed is an isolated bone marrow stem cell (BMSC) having undetectable or low levels of selected cell markers including those typical of endothelial, neuronal and smooth muscle cells. Also disclosed are grafts and pharmaceutical products that include such cells. Methods of making the BMSCs are also provided. The invention has a wide spectrum of useful applications including use in the prevention and treatment of cardiovascular disease.
Owner:STEWARD RES & SPECIALTY PROJECTS
Transgenic therapeutic stem cells and methods for their use and manufacture
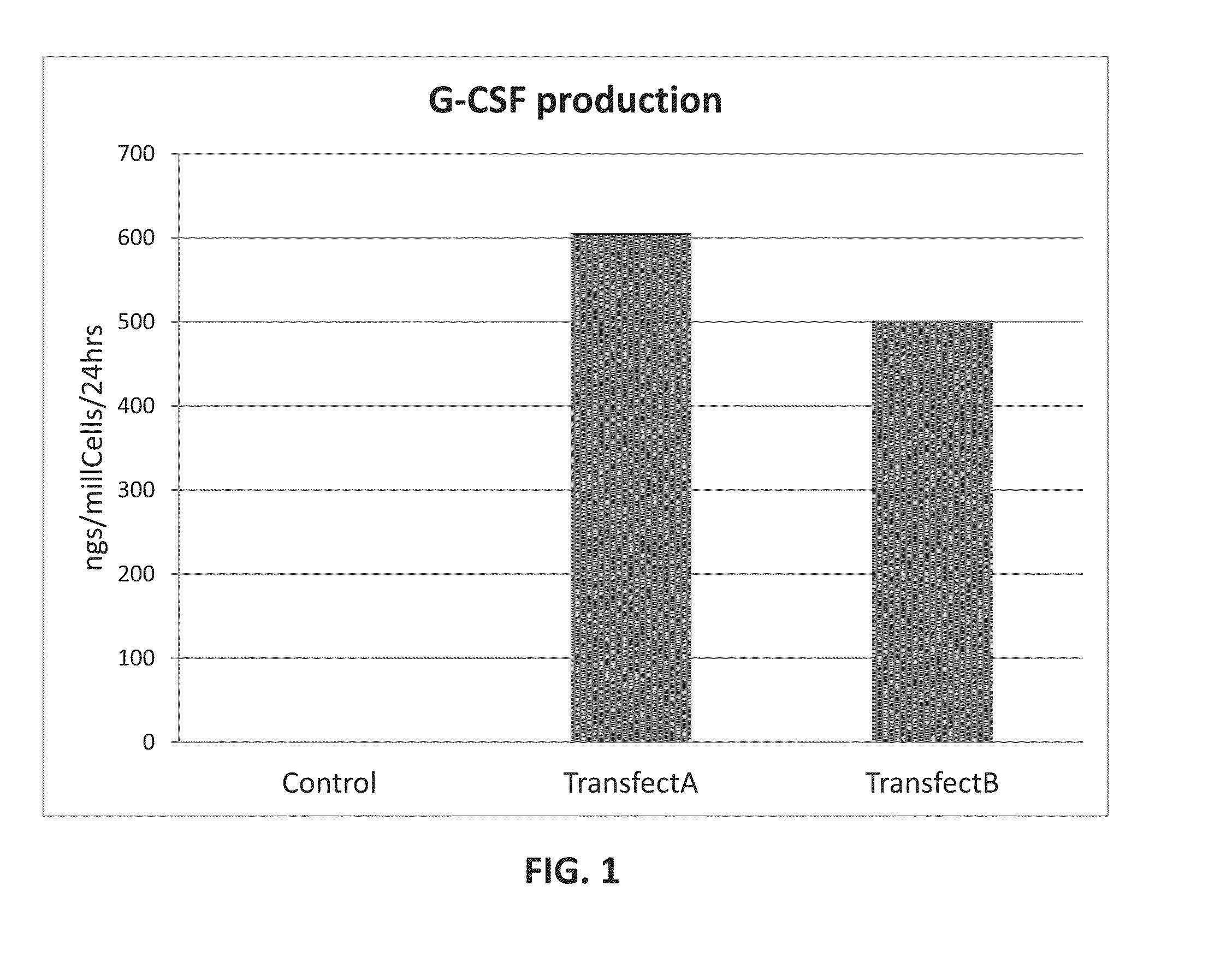
The invention relates to transgenic stem cells for therapeutic use. Stem cells according to the invention comprise at least one therapeutic trans gene that is expressed when transplanted to a subject. Methods of use and manufacturing the transgenic stem cells of the invention are also contemplated, including the use of transiently transgenic G-CSF bone marrow stem cells for treating the penumbra of ischemic tissues such as central nervous system tissues.
Owner:STEMEDICA CELL TECH
Myeloid-derived suppressor cells generated in vitro
InactiveUS20120225038A1BiocideArtificial cell constructsMyeloid-derived Suppressor CellBone Marrow Stem Cell
A population of myeloid-derived suppressor cells and the culture procedure to obtain these in vitro starting with bone marrow cells of mice, other animals and human beings, in the presence of specific cytokine combinations used to determine concentrations, is provided.
Owner:IST ONCOLOGICO VENETO
Tissue engineered bone tissue
InactiveCN1410037AGood biocompatibilityGood bone conductionBone implantSurgeryBone tissueBone defect
A histo-engineered bone tissue is composed of osteocortex frame as externally layer and central loose bone inoculated by bone marrow stem cells. It can be moulding-shape according to the actual operation scope predefined by CT film and CT reconstitution data of deficit part.
Owner:谭强 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com