Novel multipotent stem cells and use thereof
a multi-potent stem cell, stem cell technology, applied in the field of bone marrow stem cells, can solve the problems of unclear whether such immature cells have much clinical potential, no medication or procedure used clinically has shown the efficacy of replacing myocardial scars with functioning contractile tissue, and it is not certain that isolation and expansion of highly undifferentiated stem cells from a single cell level will be successful, so as to prevent, treat or reduce the severity of a cardiovascular (hear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Culture and Characteristics of hBMSC
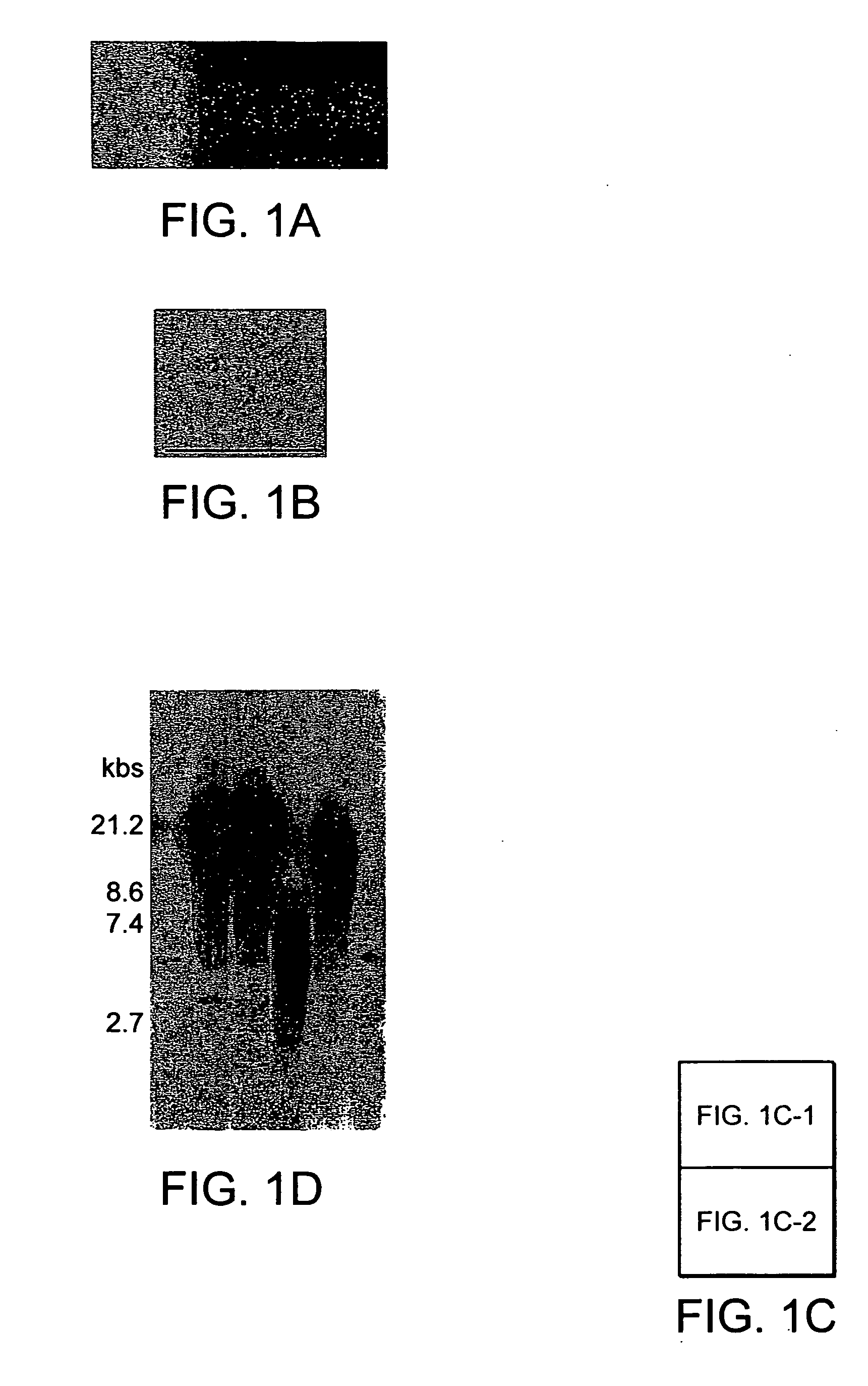
[0087] The clonality, surface epitopes, euploidy and proliferation of hBMSCs was evaluated as follows. Fresh unprocessed human BMs from young male donors were purchased. Three different marrow specimens from three different donors for SC cultures were used. After serial culture of total marrow cells in plastic dishes in Dulbecco's modified eagle's medium (DMEM) with low (1 g) glucose containing 17% of fetal bovine serum (FBS), cells were labeled with red fluorescent dye, DiI. After limiting dilution (1-2 cells per well in 96 well plate) wells containing a single cell visualized by fluorescent microscopy were selected. Of wells containing a single cell (FIG. 1a), 6±4% (range 2-13%) demonstrated survival and proliferation of cells. When cells were grown to 40-50% confluence, cells from each well (one clone) were reseeded into one well of 6-well plates and thereafter serially reseeded in 25 cm2 tissue culture flask (T25), T75 and T175 at a density o...
example 2
Plasticity of hBMSC: In Vitro Differentiation
[0089] The in vitro differentiation potential of hBMSC was tested by adopting and modifying previously published culture conditions for adult and embryonic stem cells, which primarily requires lineage-specific cytokines.
[0090] To induce differentiation into ECs, hBMSC were replated at 5×104 cells / cm2 in either 0.1% gelatin or fibronectin coated glass chamber slides in DMEM or EBM-2 (Clonetics) with 2% FBS, 10−8 dexamethasone and 10 ng / ml VEGF. Five days after culture, hBMSC formed vascular tube-like structures (FIG. 2a, left upper panel). Fourteen days after culture, hBMSC exhibited EC specific phenotypes such as von Willebrand factor (vWF), Flk1, VE-Cadherin, CD31, human-specific EC marker Ulex europaeus lectin type 1 (UAE-1) (FIG. 2a). At 14 days, 63±8% (VE-Cadherin) to 85±12% (UAE-1) of hBMSC demonstrated EC phenotypes by immunocytochemistry. RT-PCR of EC specific genes, VE-Cadherin, CD34, Flk-1, Tie2 and CD31 also confirmed the diff...
example 3
Cardiomyogenic Differentiation of BMSC after Co-Culture with Neonatal Rat Cardiomyocyte (NRCM)
[0096] To induce cardiomyogenic differentiation, hBMSC were co-cultured with neonatal rat cardiomyocytes (NRCM). NRCM were plated at 1×105 cells / cm2 and cultured in DMEM (low glucose) containing 10% fetal calf serum (FCS). On day 3, hBMSC labeled with DiI were added to the cultured NRCM at a 1:4 ratio [Condorelli, 2001] and cultured up to 2 wks. After fixation and staining with antibodies against cardiac specific markers, immunofluorescent images were obtained. DiI-labeled hBMSC (FIG. 4b, f, j) exhibit red fluorescence from the DiI label and cells positive for CMC specific proteins, such as cardiac troponin I(cTnI), atrial natriuretic peptide(ANP), α-myosin heavy chain (α-MHC) appear green (FIG. 4c, g, k). The merged images illustrate a fraction of DiI-labeled hBMSC which also stained positive for the CMC specific proteins indicating that a subpopulation of hBMSC were displaying features o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com