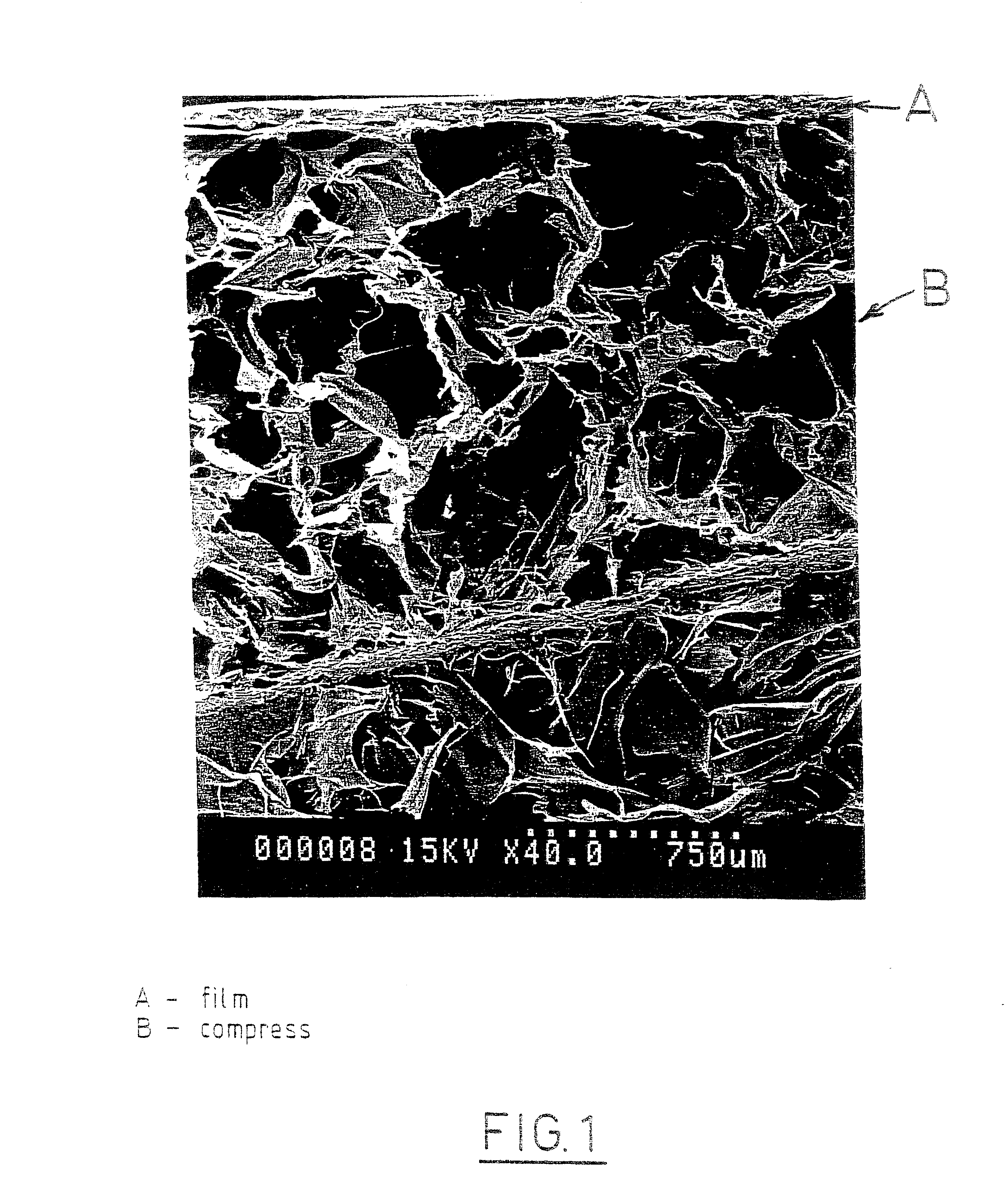
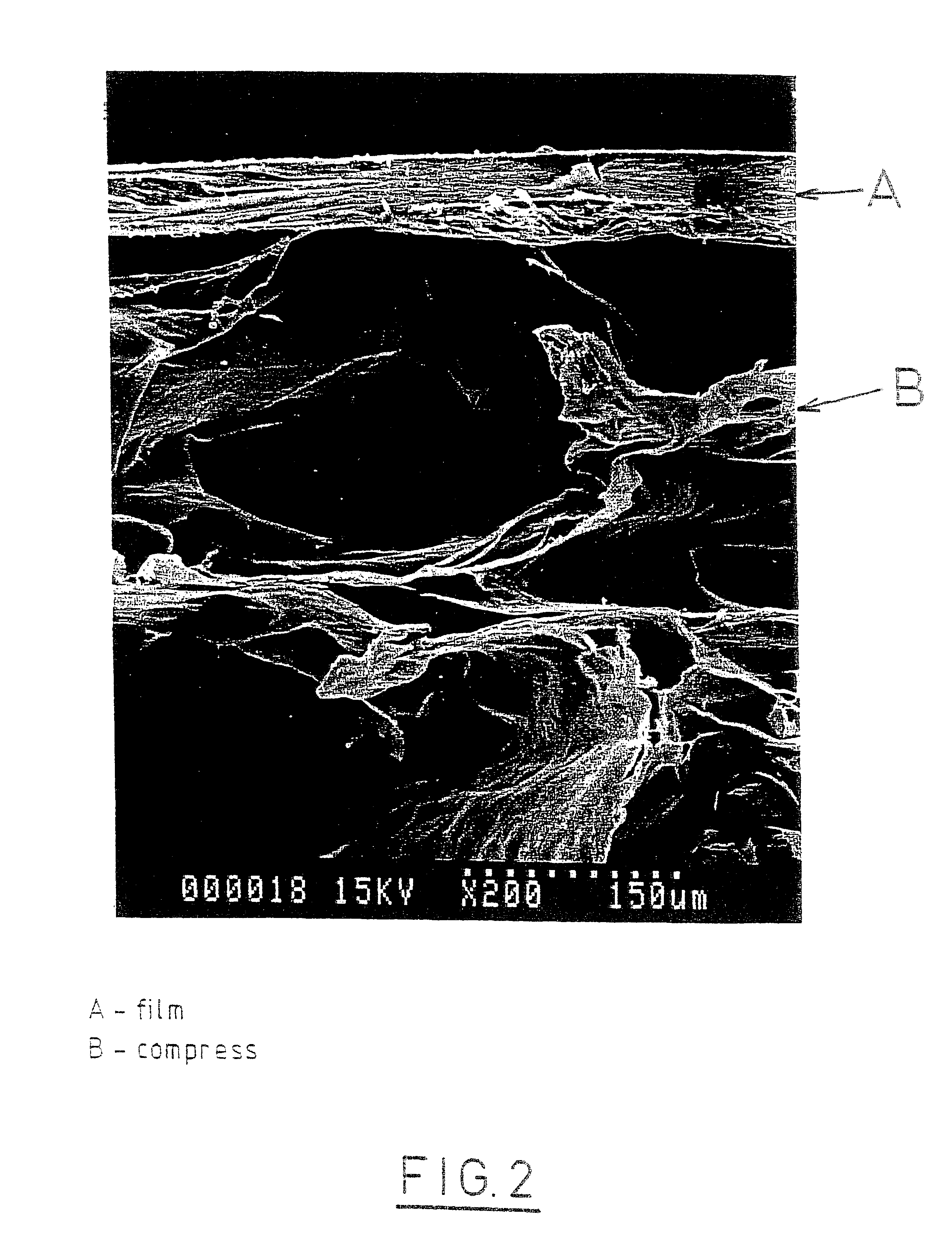
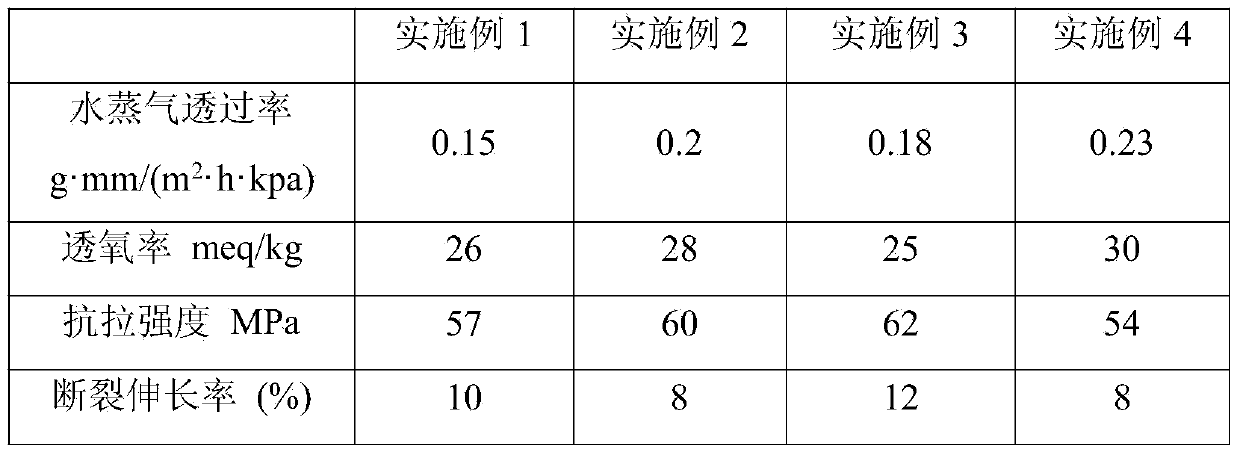
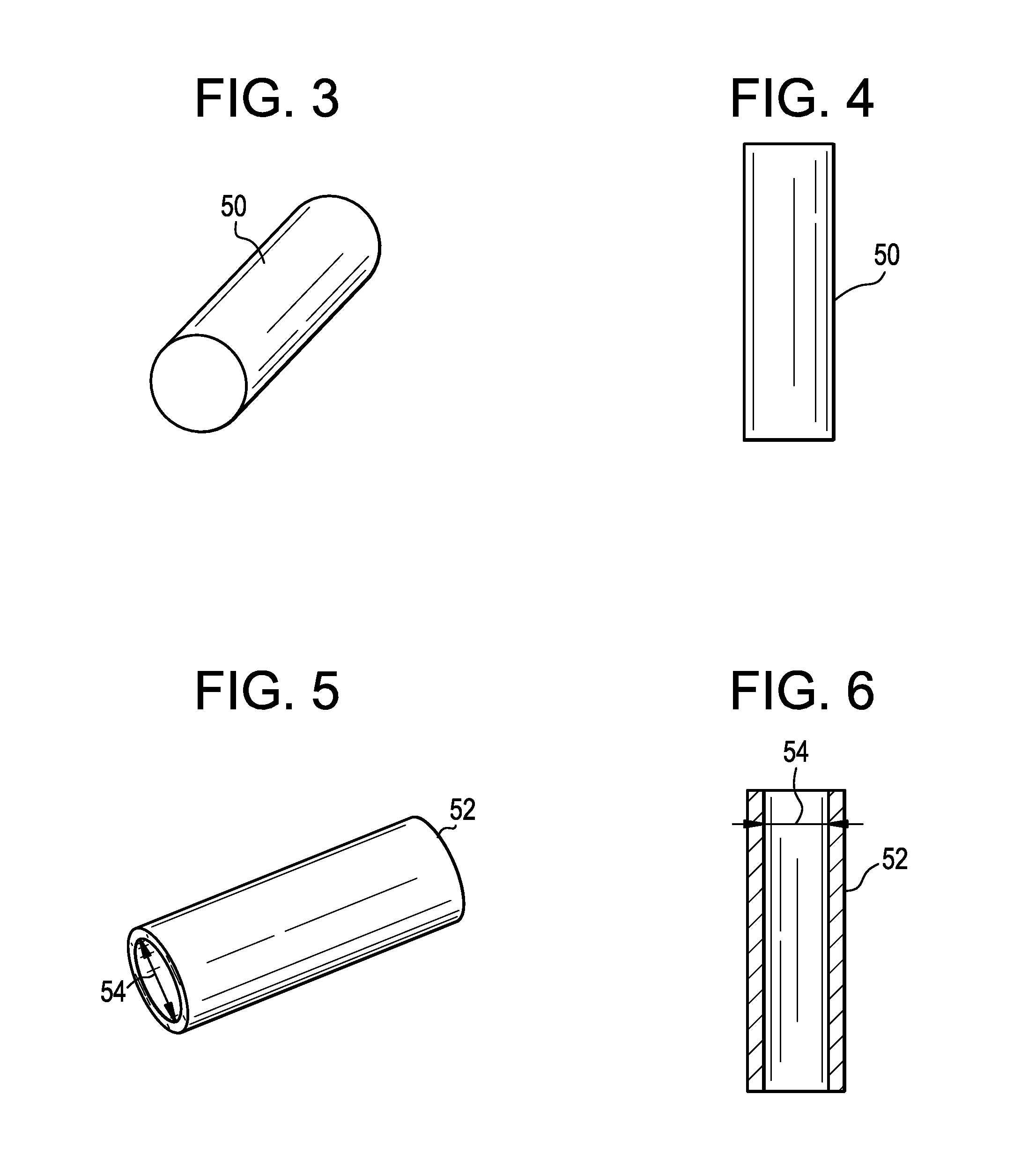
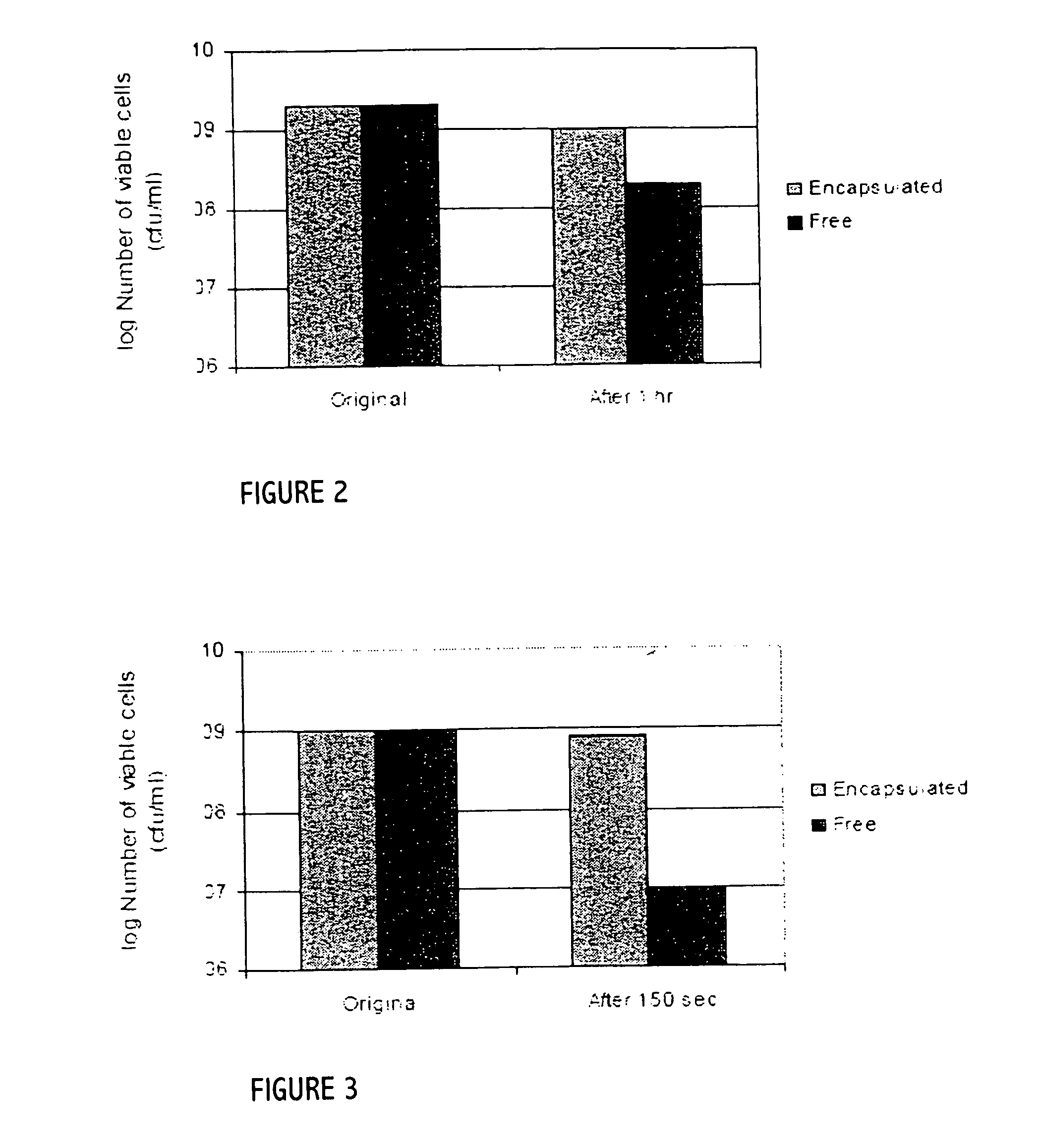
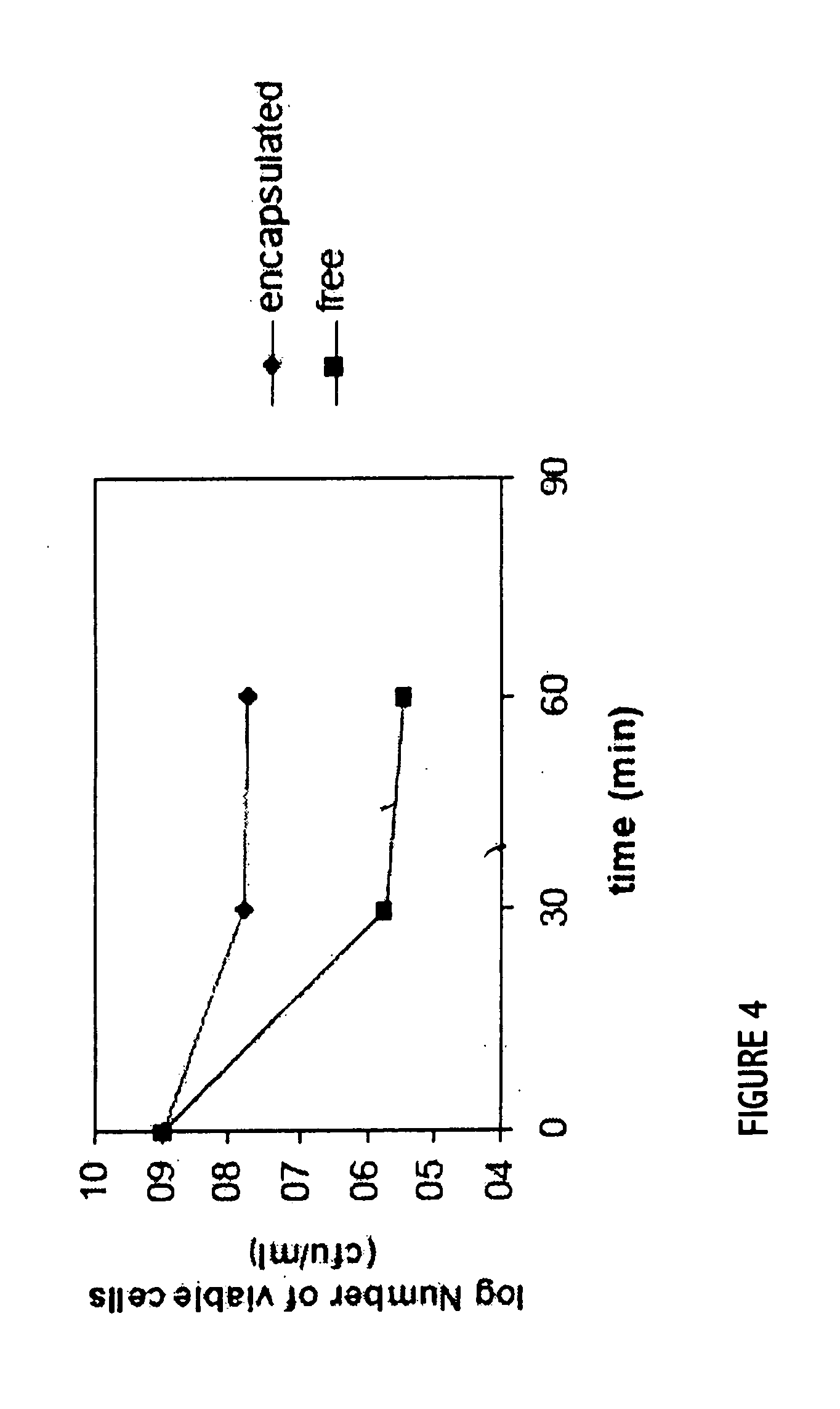
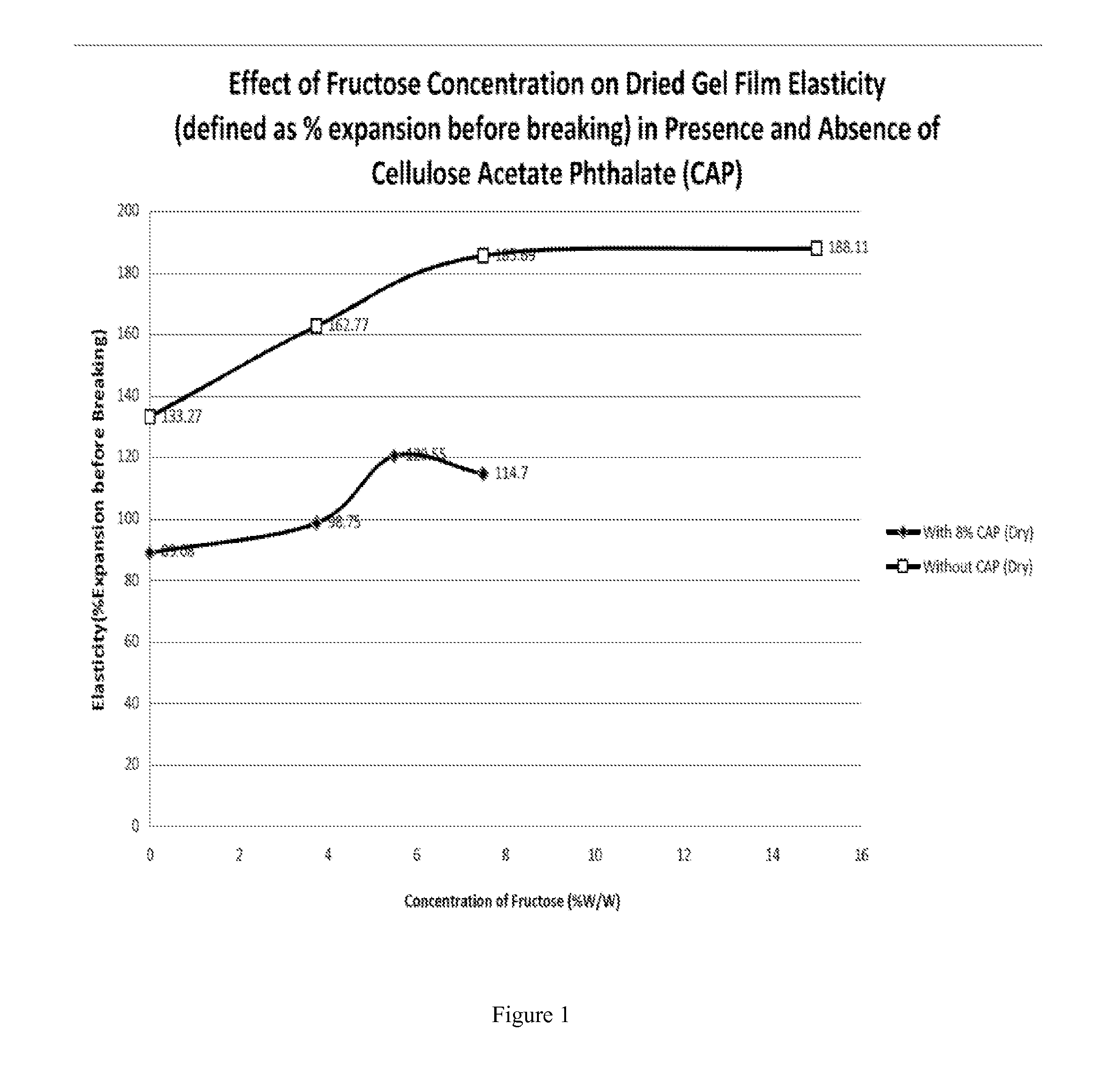
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
12873 results about "Gelatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gelatin or gelatine (from Latin: gelatus meaning "stiff" or "frozen") is a translucent, colorless, flavorless food ingredient, derived from collagen taken from animal body parts. Brittle when dry and gummy when moist, it is also called hydrolyzed collagen, collagen hydrolysate, gelatine hydrolysate, hydrolyzed gelatine, and collagen peptides. It is commonly used as a gelling agent in food, medications, drug and vitamin capsules, photographic films and papers, and cosmetics.
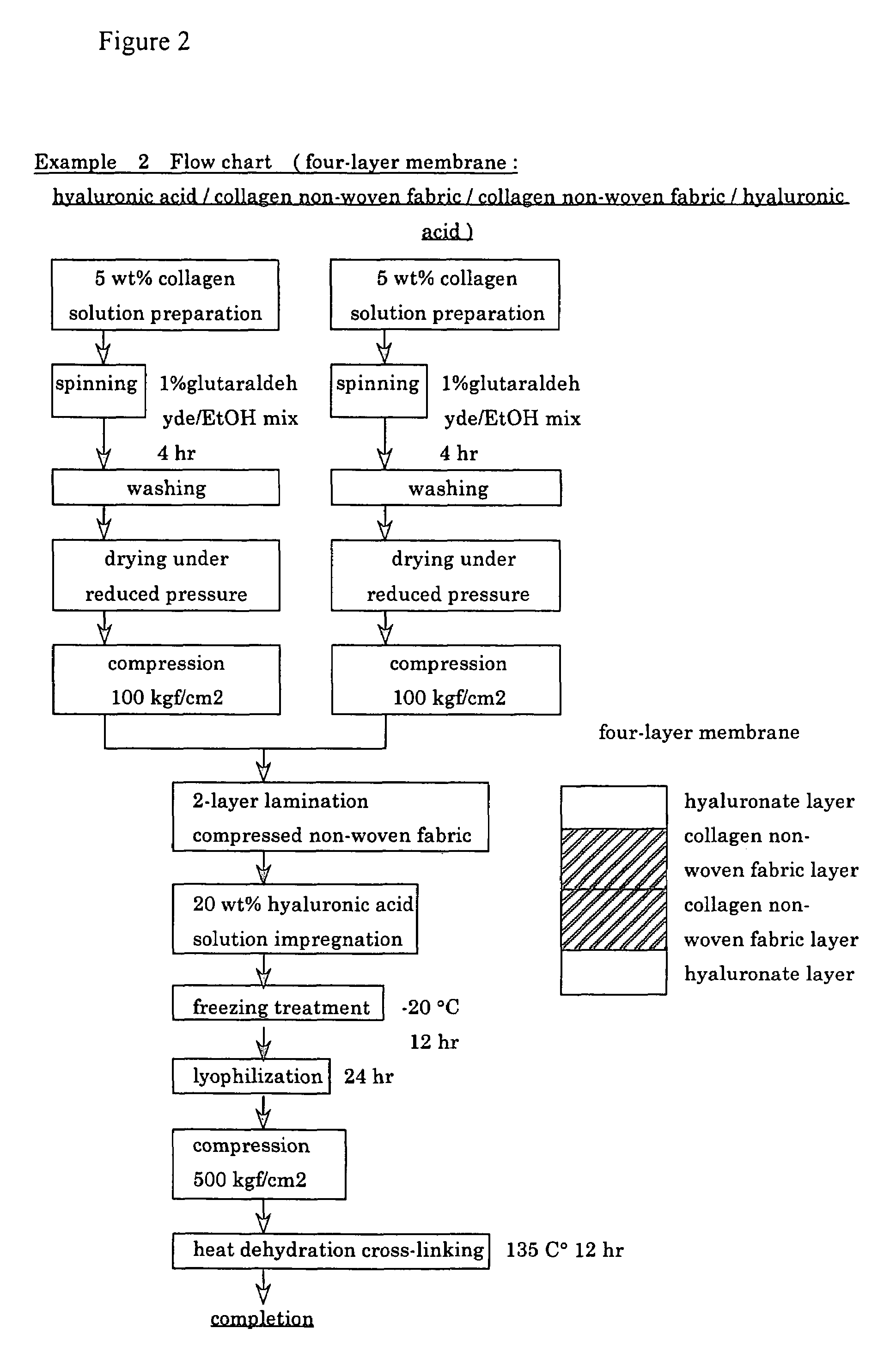
Method for preparing two-layer bicomposite collagen material for preventing post-operative adhesions
InactiveUS6596304B1Improve propertiesAvoid stickingPeptide/protein ingredientsSurgerySurgical operationPost operative
A bicomposite material based on collagen is prepared which has two closely bound layers and is biocompatible, non-toxic, hemostatic and biodegradable in less than a month, and can be used in surgery to achieve hemostasis and prevent post-surgical adhesion. To prepare the material, a solution of collagen or gelatin, which may contain glycerine and a hydrophilic additive such as polyethylene glycol or a polysaccharide, is poured onto an inert support to form a layer 30 .mu.m to less than 100 .mu.m thick. Then a polymeric porous fibrous layer is applied during gelling of the collagen or gelatin, and the resultant material is dried. The polymeric porous fibrous layer may be made of collagen or a polysaccharide, and have a density of not more than 75 mg / cm.sup.2, a pore size from 30 .mu.m to 300 .mu.m and a thickness of 0.2 cm to 1.5 cm.
Owner:IMEDEX BIOMATERIAUX CHAPONOST
Encapsulated unsaturated fatty acid substance and method for producing the same
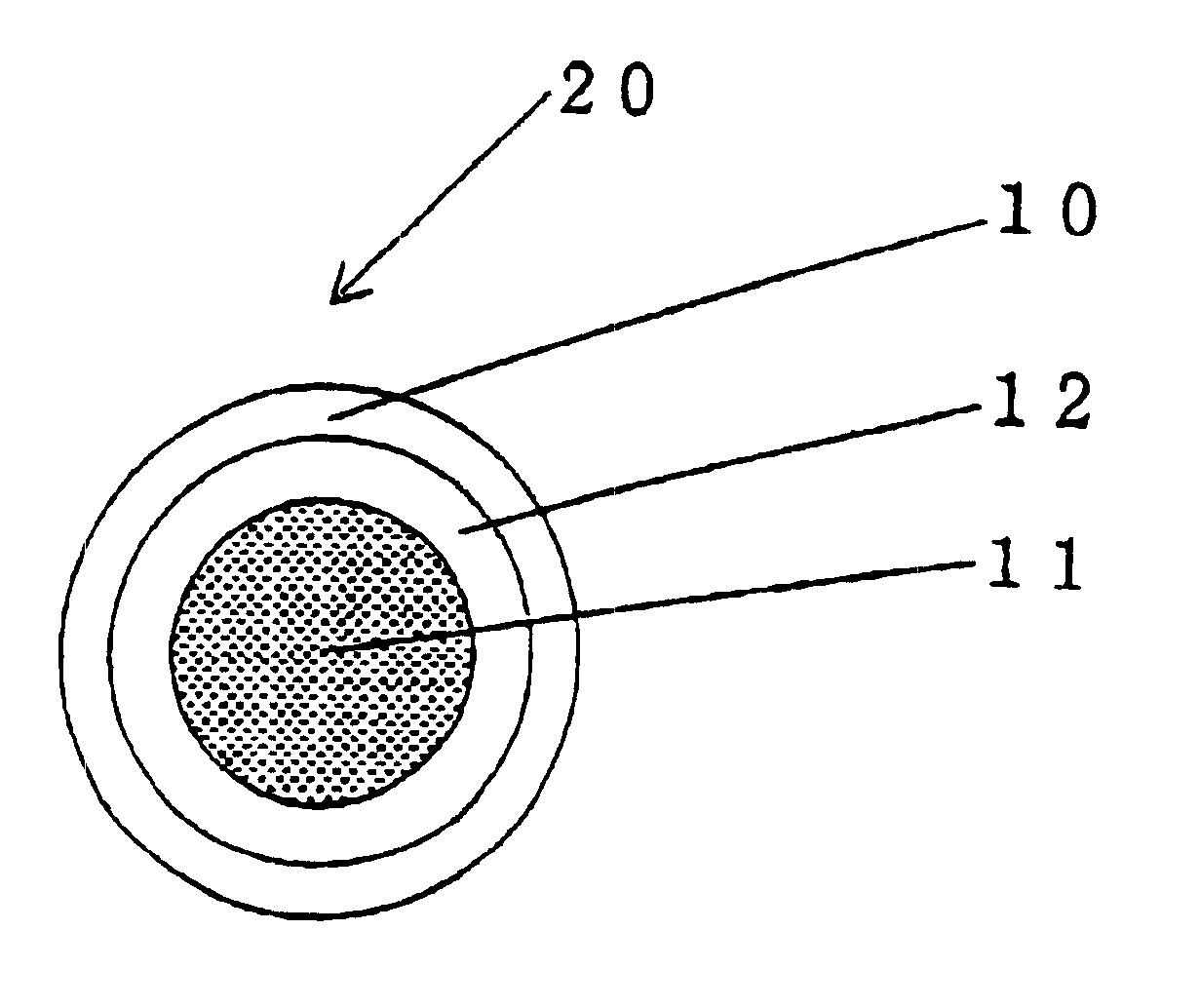
InactiveUS6531150B1Improve product qualityInhibit oxygen-permeabilityPowder deliveryGranular deliveryWater solubleGelatin
The present invention relates to an encapsulated unsaturated fatty acid substance in a form of a three-layered capsule, comprising an unsaturated fatty acid or a derivative thereof (11) as a content and a coating layer (10) mainly containing gelatin, encapsulating the content (11), wherein a water-soluble gel layer (12) containing an acid or an acid salt thereof is present between the coating layer (10) and the content (11). The encapsulated unsaturated fatty acid substance of the present invention is characterized by that it has neither insolubility nor deterioration with time, and that it is enteric.
Owner:MORISHITA JINTAN CO LTD
Methods for glucagon suppression using modified exendins
InactiveUS7153825B2Saccharide peptide ingredientsVasoactive intestinal peptideDiseaseBiological half-life
We claim a method of lowering plasma glucagon in a subject in need thereof comprising administering to the subject a composition comprising a modified exendin or modified exendin analog, wherein said modification comprises one or more molecule linked to an exendin or the exendin analog wherein said molecule is selected from the group consisiting of polyethylene glycol, gelatin and / or albumin. The modified exendin or the modified exendin analog has activity of suppressing glucagon secretion and / or lowering glucagon levels in the subject and possesses increased biological half-life compared to unmodified exendin or unmodified exendin analog. The method is useful in treating hyperglucagonemia and other disorders that would be benefited by lowering plasma glucagon or suppressing glucagon secretion.
Owner:AMYLIN PHARMA INC
Biodegradable polymer coils for intraluminal implants
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
Allograft bone composition having a gelatin binder
InactiveUS7045141B2Low tensile strengthIncrease delayImpression capsSurgical adhesivesCross-linkSolid structure
The invention is directed toward an osteoimplant for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing composition of demineralized allograft bone material mixed with an aqueous phosphate buffered gelatin which when lyophilized to remove water from the composition cross links the gelatin to form a solid structure.
Owner:MUSCULOSKELETAL TRANSPLANT
Seamless capsule
InactiveUS20100119598A1Improve stabilityIncrease contentBiocideAnimal repellantsPolymer sciencePlasticizer
The present invention aims to provide a seamless capsule free of an interfacial tension modifier and a gelling agent. The present invention provides a shell formed by a shell composition containing gelatin and a plasticizer, but free of an interfacial tension modifier and a gelling agent, and a seamless capsule comprising a capsule content free of an interfacial tension modifier and a gelling agent.
Owner:TAKEDA PHARMA CO LTD
Diet for dietotherapy and health preservation
ActiveCN102423073AReduce adverse reactionsReduce dosageConfectionerySweetmeatsPumpkin seedDry weight
The invention relates to a diet for dietotherapy and health preservation which is prepared by medicine-food materials, and belongs to the field of nutrition and health preservation; the basic formula comprises the following raw materials on a dry weight basis: 30-60 parts of black sesame, 4-6 parts of poria cocos, 2-8 parts of hawthorn, 2-8 parts of medlar, 2-5 parts of coix seeds, 1-5 parts of spine date seeds, 2-5 parts of lotus seeds, 2-5 parts of Chinese yam, 1-5 parts of lily, 5-10 parts of jujube, 1-5 parts of donkey-hide gelatin, 1-4 parts of roses, 2-8 parts of carrots, 2-5 parts of walnut kernels, 2-5 parts of pumpkin seeds, 2-5 parts of black soybeans, 2-5 parts of black fungus, 1-3 parts of honeysuckles, 1-3 parts of sea-tangle, and 1-3 parts of mulberry. The invention can alsobe combined with other materials to form a secondary formula by using the basic formula as a main component. The formula of the invention is not excessively cold or hot, has a moderate character and taste, is convenient for using, has a less using amount, can be eaten frequently for a long term, has a lot of efficacy and effect and definite effect, and the efficacy is confirmed to reach the expectation and to be satisfied through long-term eating by a lot of people.
Owner:李超建
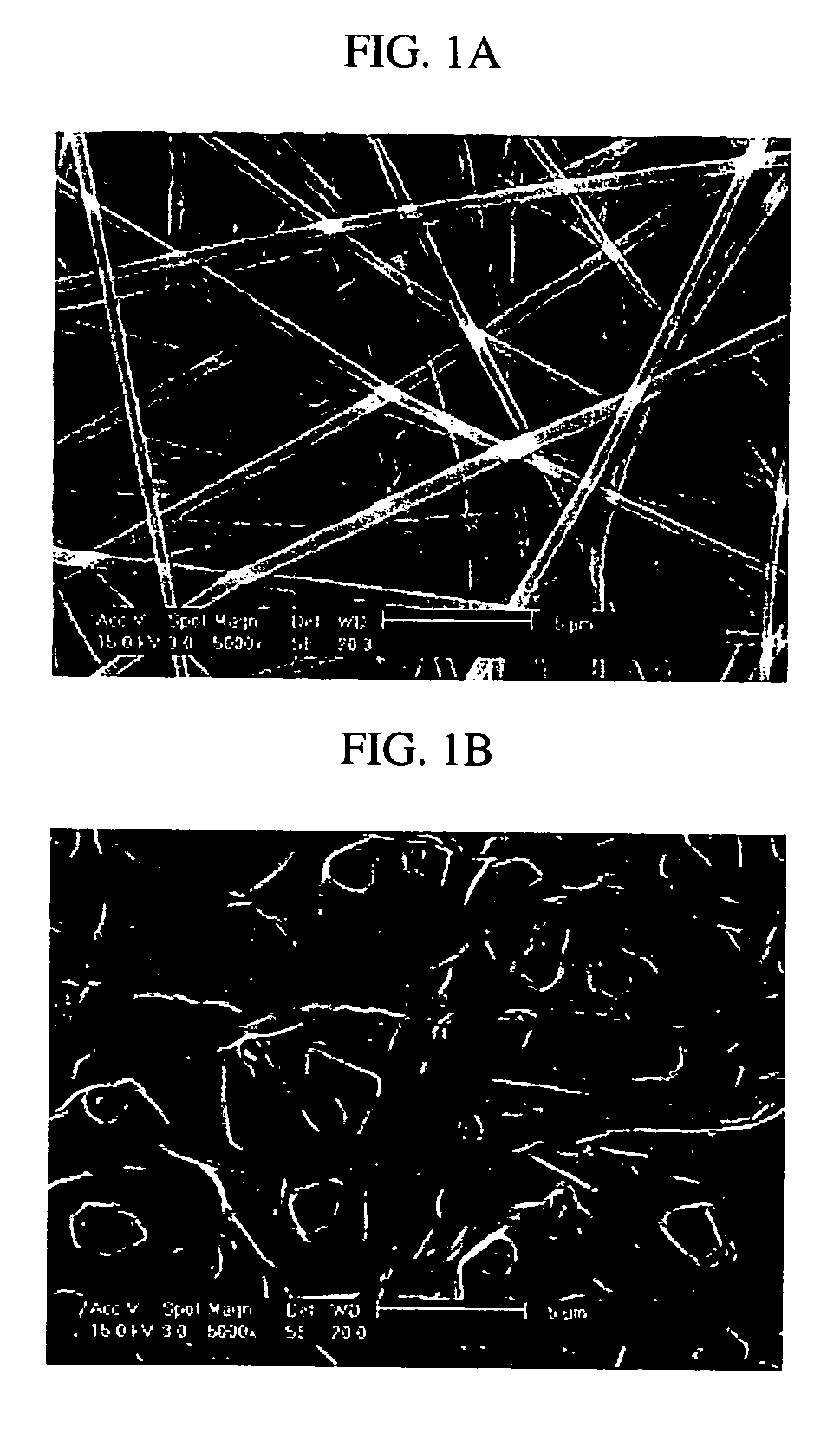
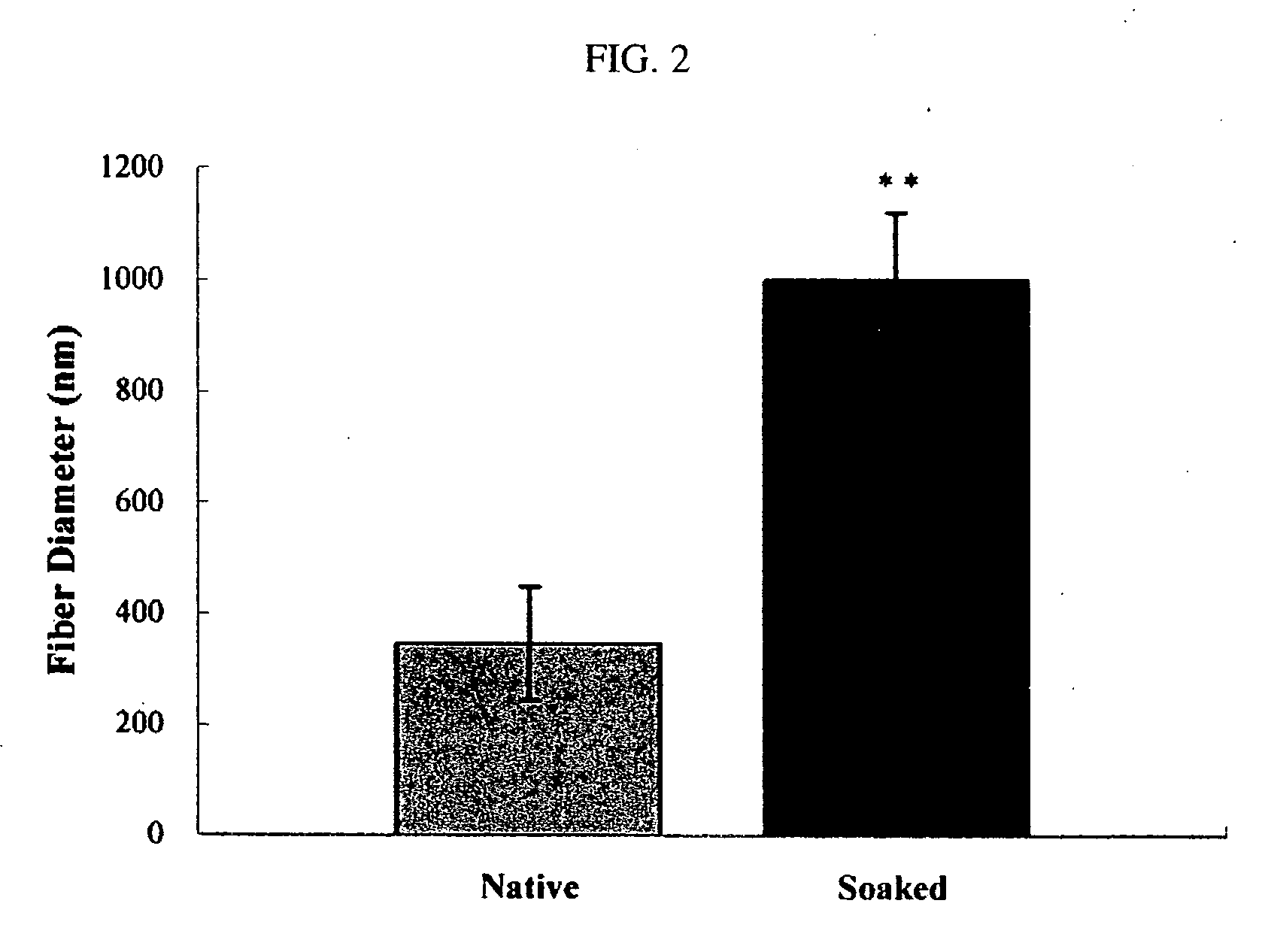
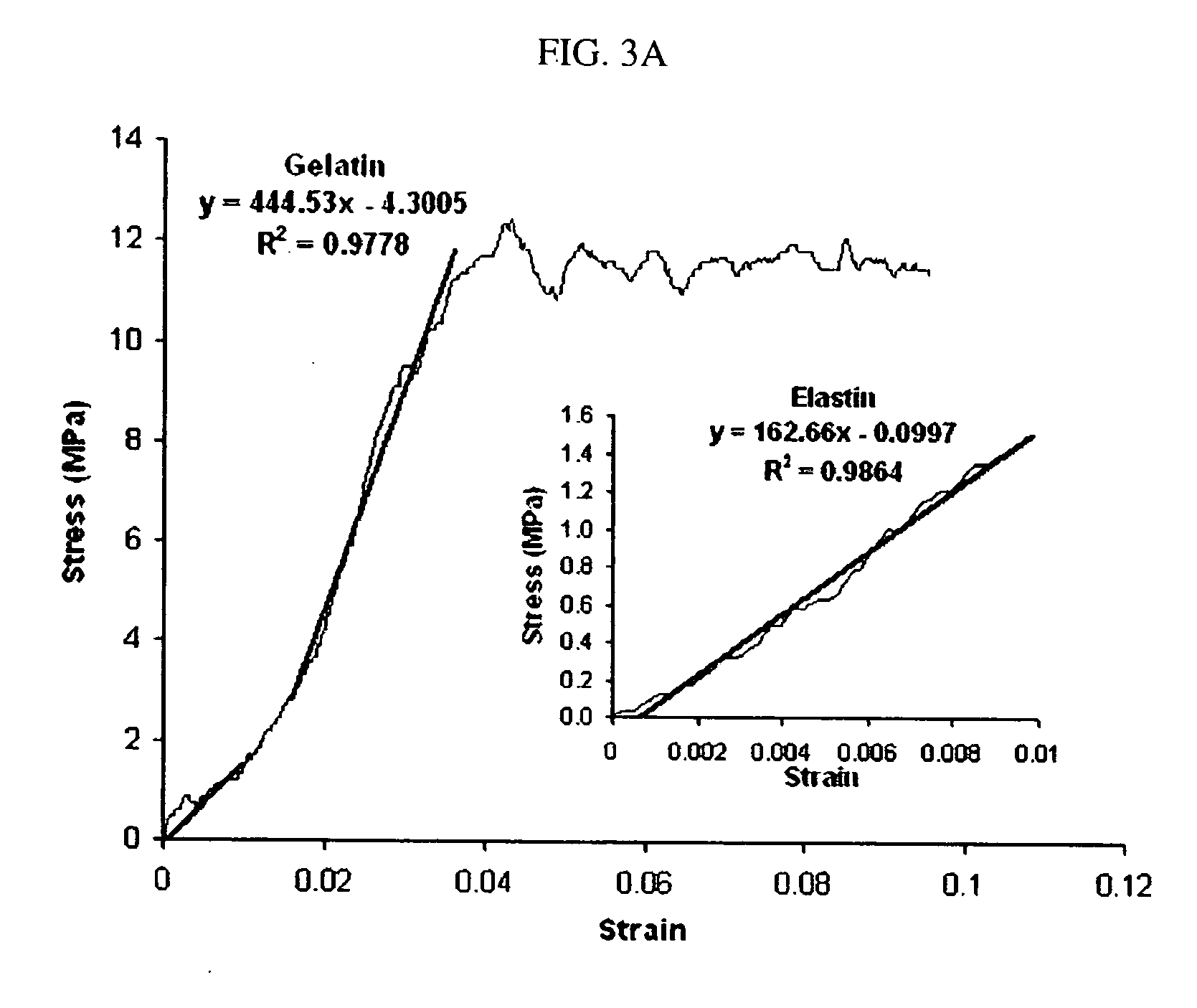
Electrospun blends of natural and synthetic polymer fibers as tissue engineering scaffolds
InactiveUS20060263417A1Facilitate cell penetrationFacilitate proliferationBiocidePeptide/protein ingredientsFiberPolymer science
Non-woven fibrous scaffolds made by electrospinning from the synthetic biodegradable polymer such as, for example, poly(lactic-co-glycolic acid) (PLGA) and natural proteins, such as, for example, gelatin (denatured collagen) and elastin and a method of making thereof.
Owner:DREXEL UNIV
Artificial neural canal
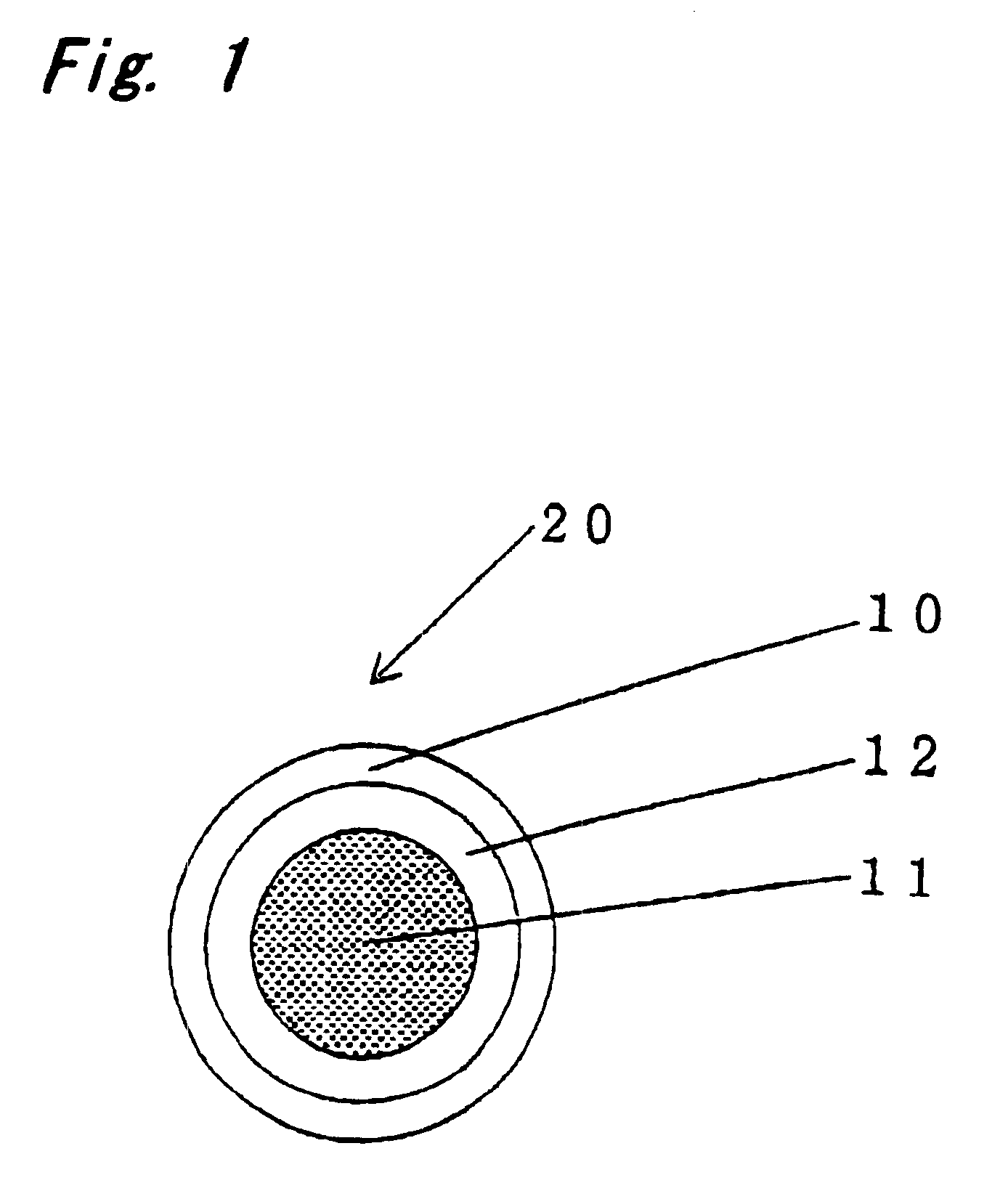
InactiveUS6090117AStay in shapePrevent intrusionTubular organ implantsTissue regenerationMedicineBlood capillary
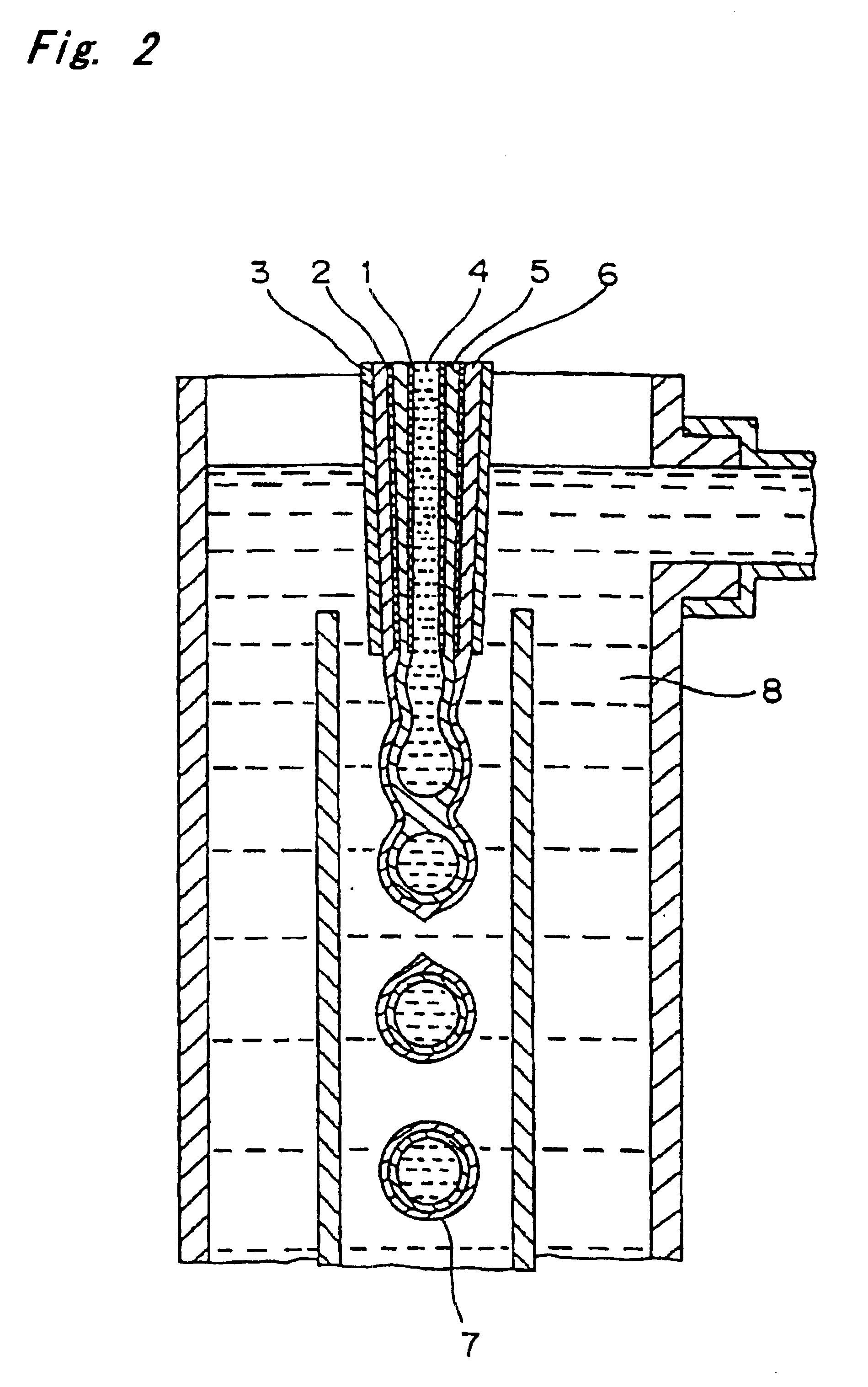
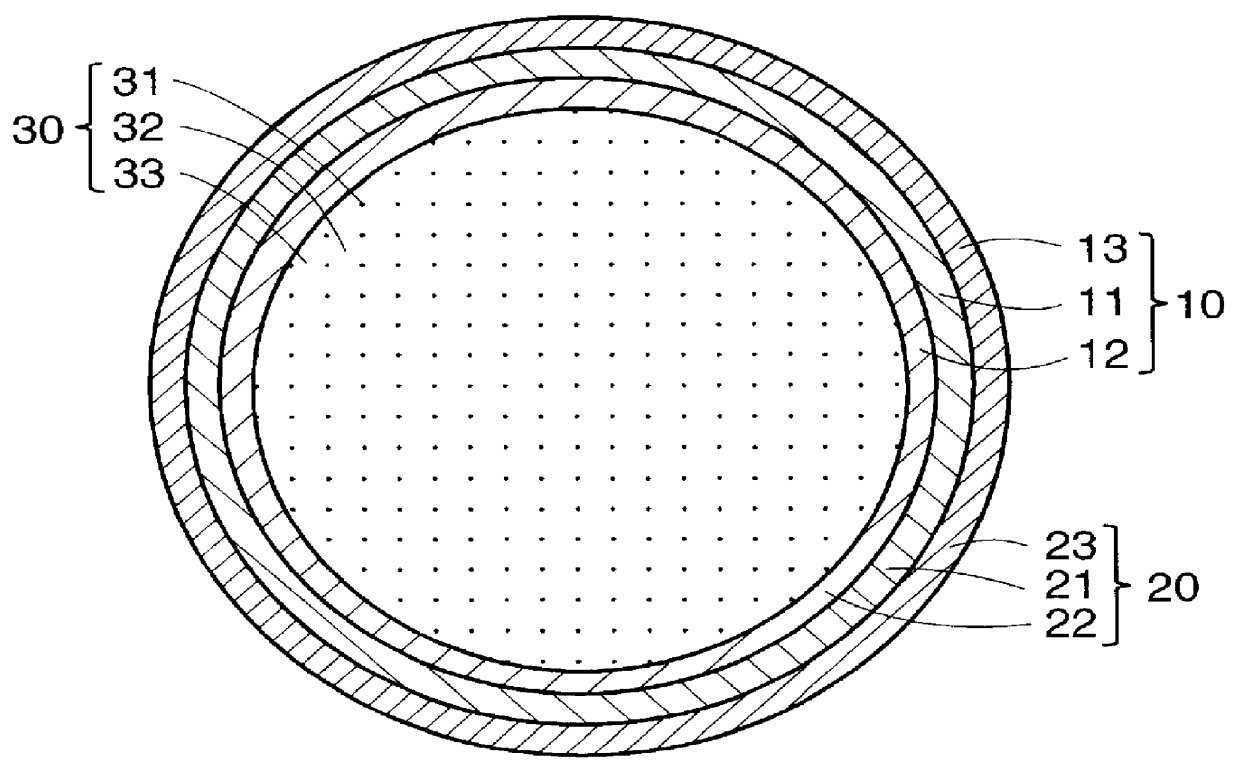
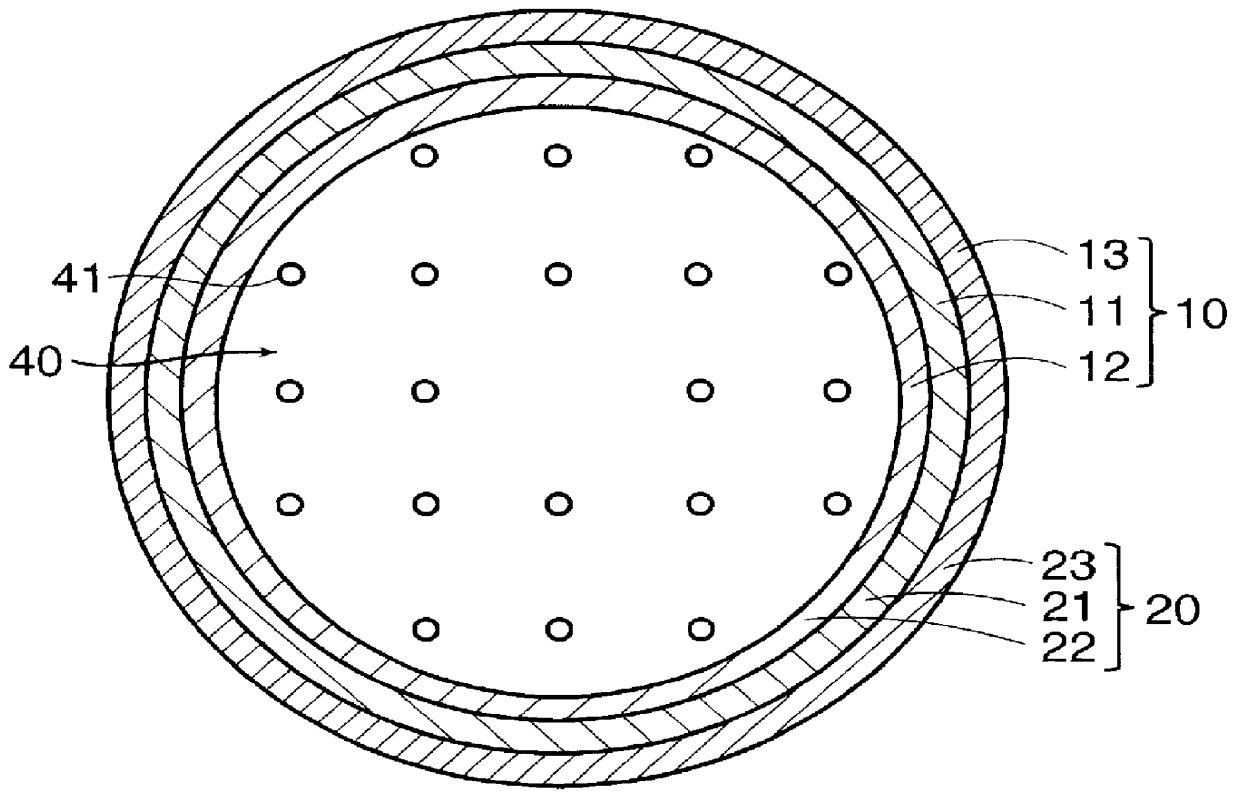
PCT No. PCT / JP97 / 04203 Sec. 371 Date May 20, 1999 Sec. 102(e) Date May 20, 1999 PCT Filed Nov. 19, 1997 PCT Pub. No. WO98 / 22155 PCT Pub. Date May 28, 1998The present invention offers an artificial tube for nerve which can remain in the body until the nerve regenerates while does not remain as a foreign body in the body following nerve regeneration, and which induces axons regenerated from severed nerve stumps, can promote infiltration of blood capillaries from the body and regeneration of nerve tissue. The present invention comprises a tube 10 or 20 having coating layers 12, 13 or 22, 23 composed of gelatin or collagen on the inner and outer surfaces of a tube 11 or 21 composed of a material being biodegradable and absorbable in vivo, and a collagen body 30 or 40 having cavities 32, 33 or 41 which pass through said tube so as to be substantially parallel to the axis of said tube; wherein, said cavities are filled with a matrix gel.
Owner:TAPIC INT
Suturable adhesion-preventing membrane
InactiveUS6977231B1High strengthGood biocompatibilitySuture equipmentsSynthetic resin layered productsCross-linkDecomposition
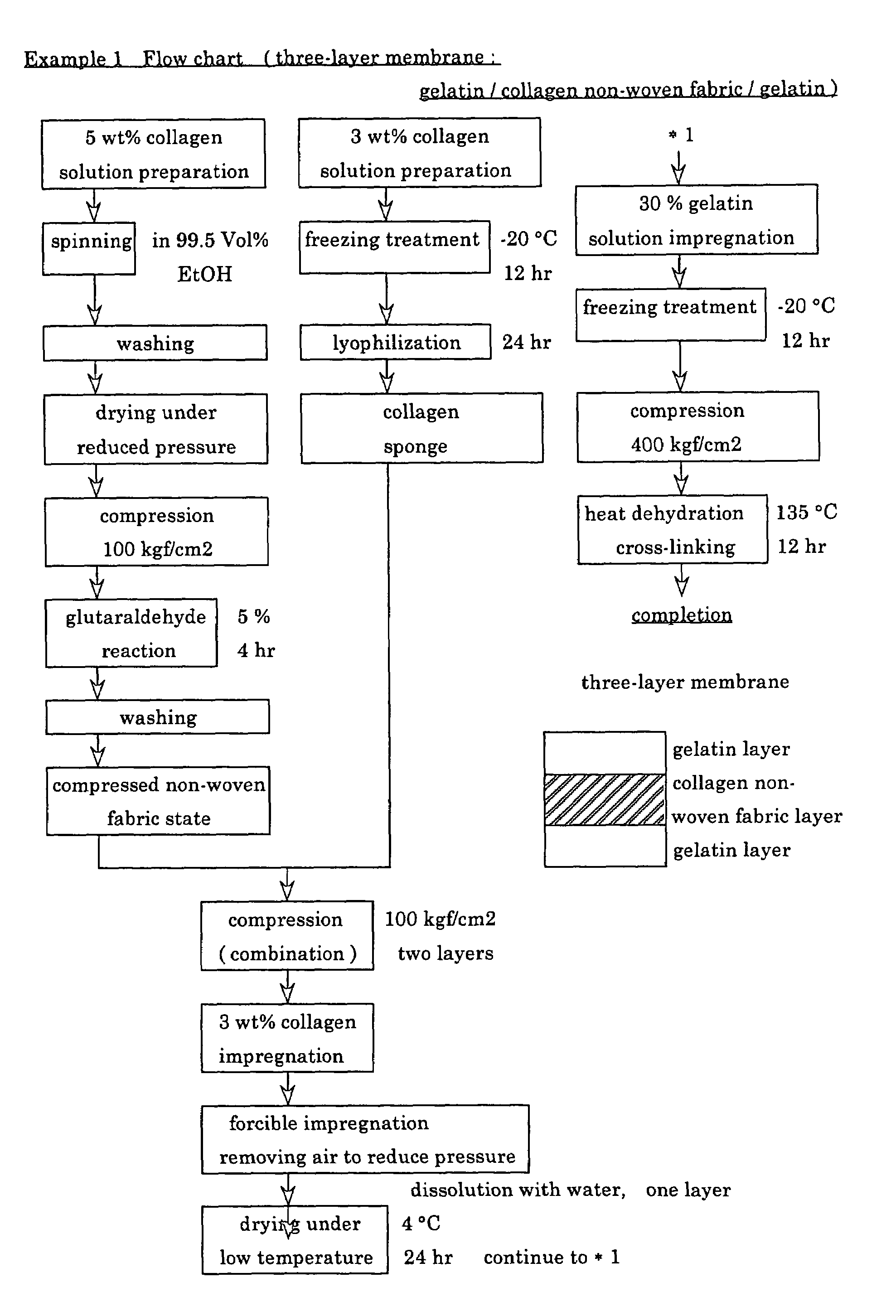
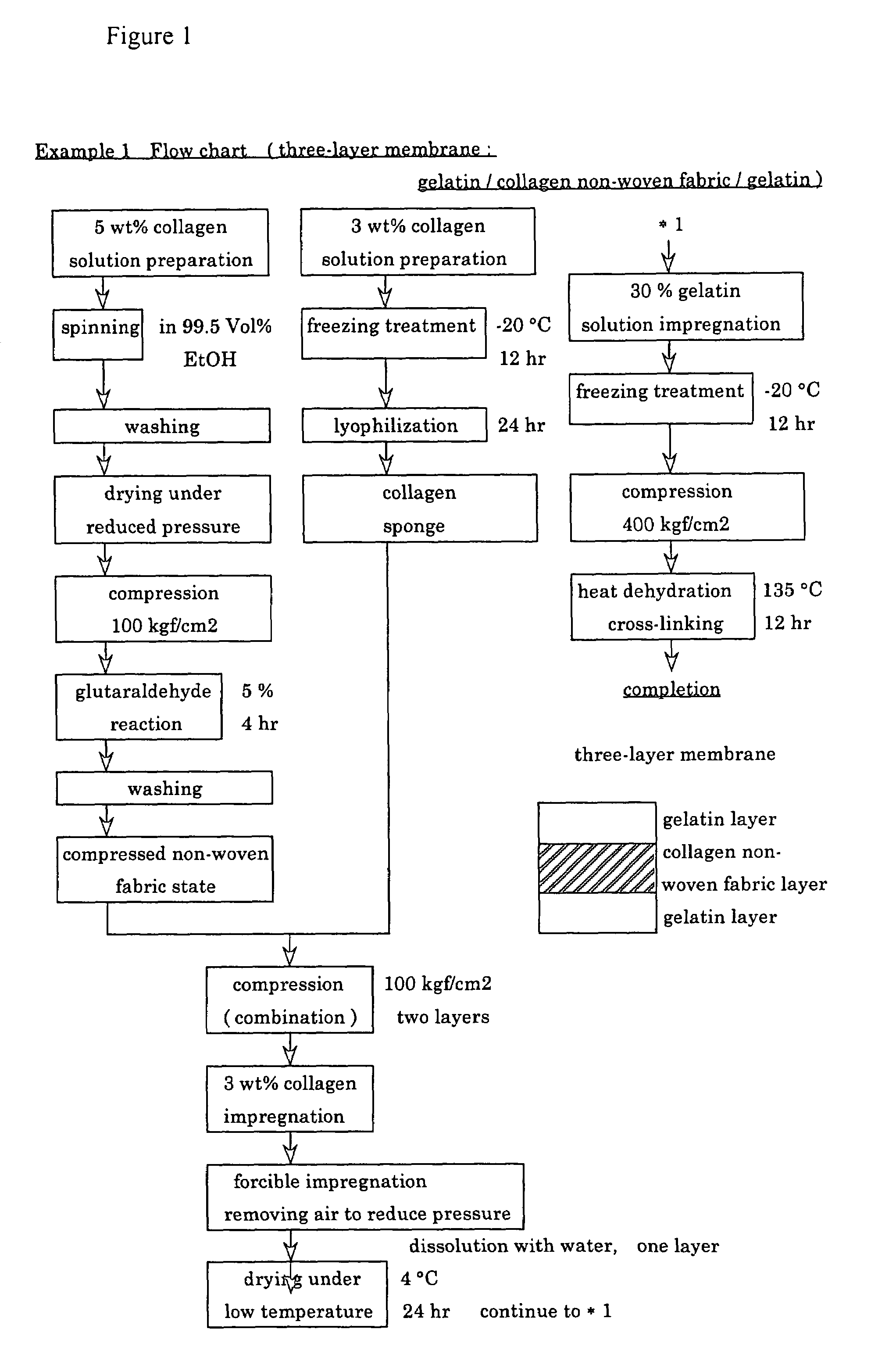
A suturable adhesion-preventing membrane has high suture strength, good biocompatibility, decomposition and absorption in a living body, sufficient adhesion-preventing effect, and desirable guided tissue regeneration. The membrane is composed of at least one non-woven fabric layer made of collagen fibers, or a laminated membranous substance consisting of at least one non-woven fabric layer made of collagen fibers and at least one sponge layer made of collagen, and a coating layer of gelatin or hyaluronic acid on the surface or surfaces of the above membrane. Preferably, the membrane comprises one to six compressed cross-linked collagen non-woven fabric layers wherein a layer has a fibers having a fiber diameter of 0.05 mm to 1.0 mm, a bulk density of 5.0×10−4 to 5 g / cm3 and a thickness of 0.1 mm to 50 mm, and a coating layer containing gelatin or hyaluronic acid and having a thickness of 0.05 mm to 20 mm, wherein the coating layer covers one or both sides or a part or whole of the surface of the membrane.
Owner:NIPRO CORP
Nutrition noodles containing Chinese medicine components and preparation method thereof
The invention provides nutrition noodles containing Chinese medicine components and a preparation method thereof; the nutrition noodles are prepared from the raw materials which comprise flour and the following Chinese medicine components, wherein every 5kg of flour is mixed with the Chinese medicine components by weight: 4 to 10g of ginseng, 3 to 6g of radix codonopsitis, 3 to 6g of astragalus, 4 to 8g of polygonatum, 2 to 5g of atractylode, 10 to 20g of yam, 3 to 6g of rehmannia, 4 to 8g of polygonum, 3 to 8g of angelica, 4 to 10g of donkey-hide gelatin, 5 to 10g of medlar, 4 to 6g of root of herbaceous peony, 4 to 8g of radix, 3 to 6g of asparagine, 4 to 8g of polygonatum, 4 to 8g of root of straight ladybell, 10 to 15g of lily, 4 to 8g of ligustrum lucidum, 5 to 8g of psoralen, 4 to 8g of cistanche, 3 to 8g of morinda, 4 to 7g of dodder, 5 to 10g of cornus, 3 to 7g of schisandra, 3 to 7g of poria, 5 to 8g of coix seed, 3 to 6g of clove, 4 to 8g of nutmeg, 3 to 6g of fructus tsaoko, 4 to 8g of rhizoma gastrodiae, 4 to 8g of kidney tonifying nut, 3 to 6g of fructus momordicae, 10 to 20g of lotus seeds and 3 to 7g of notoginseng. The nutrition noodles have various health care effects.
Owner:杨占良
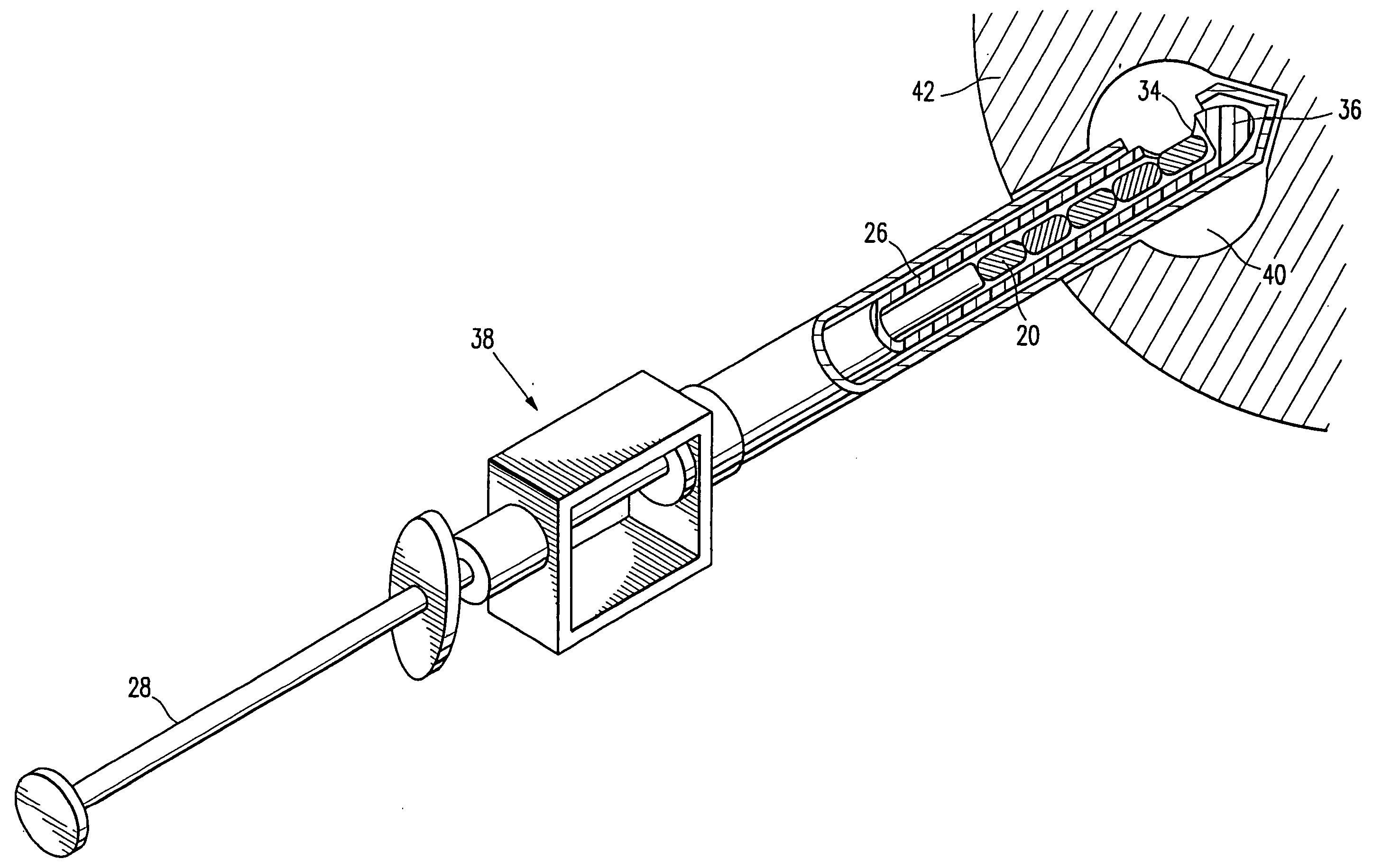
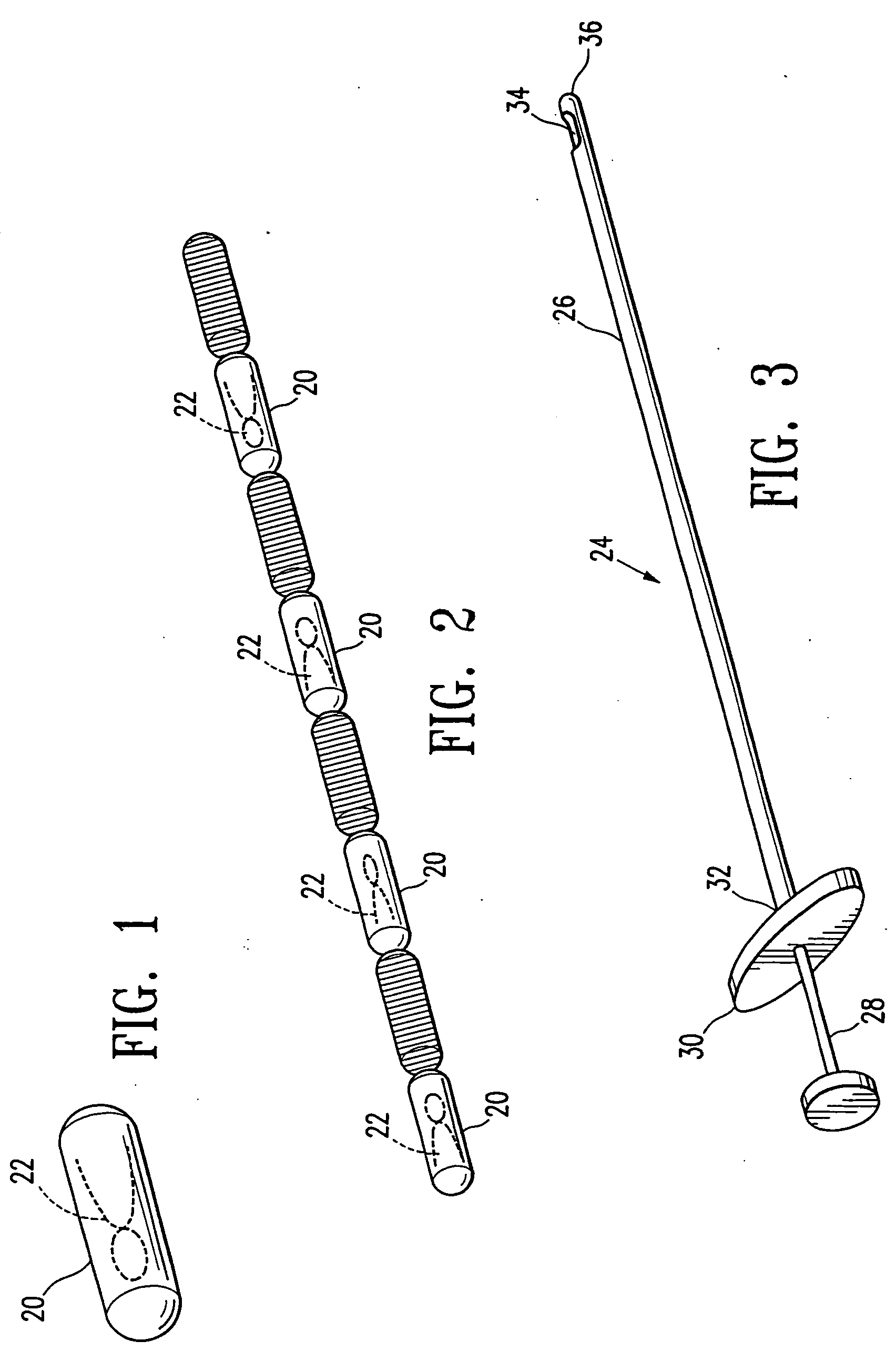
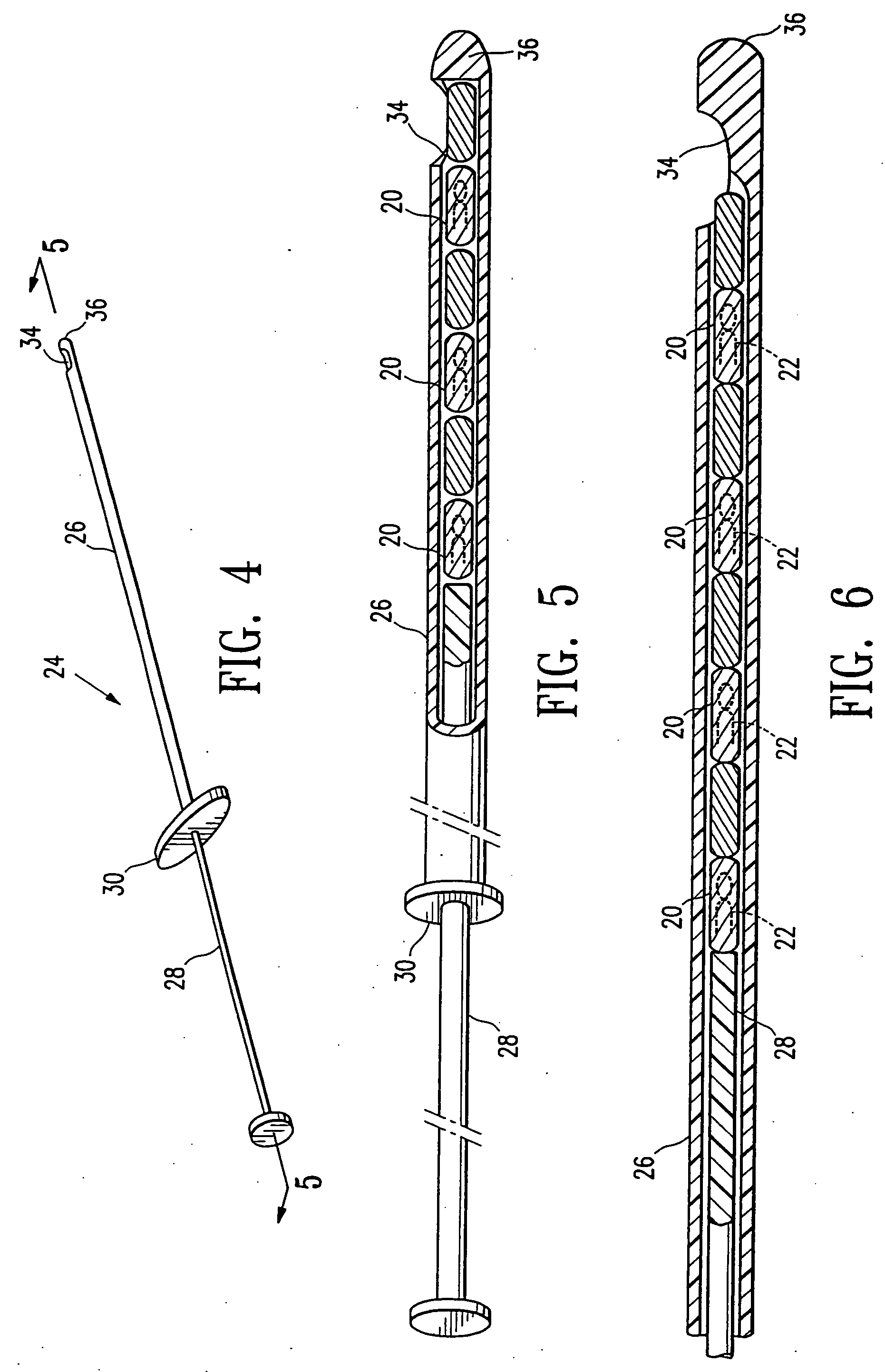
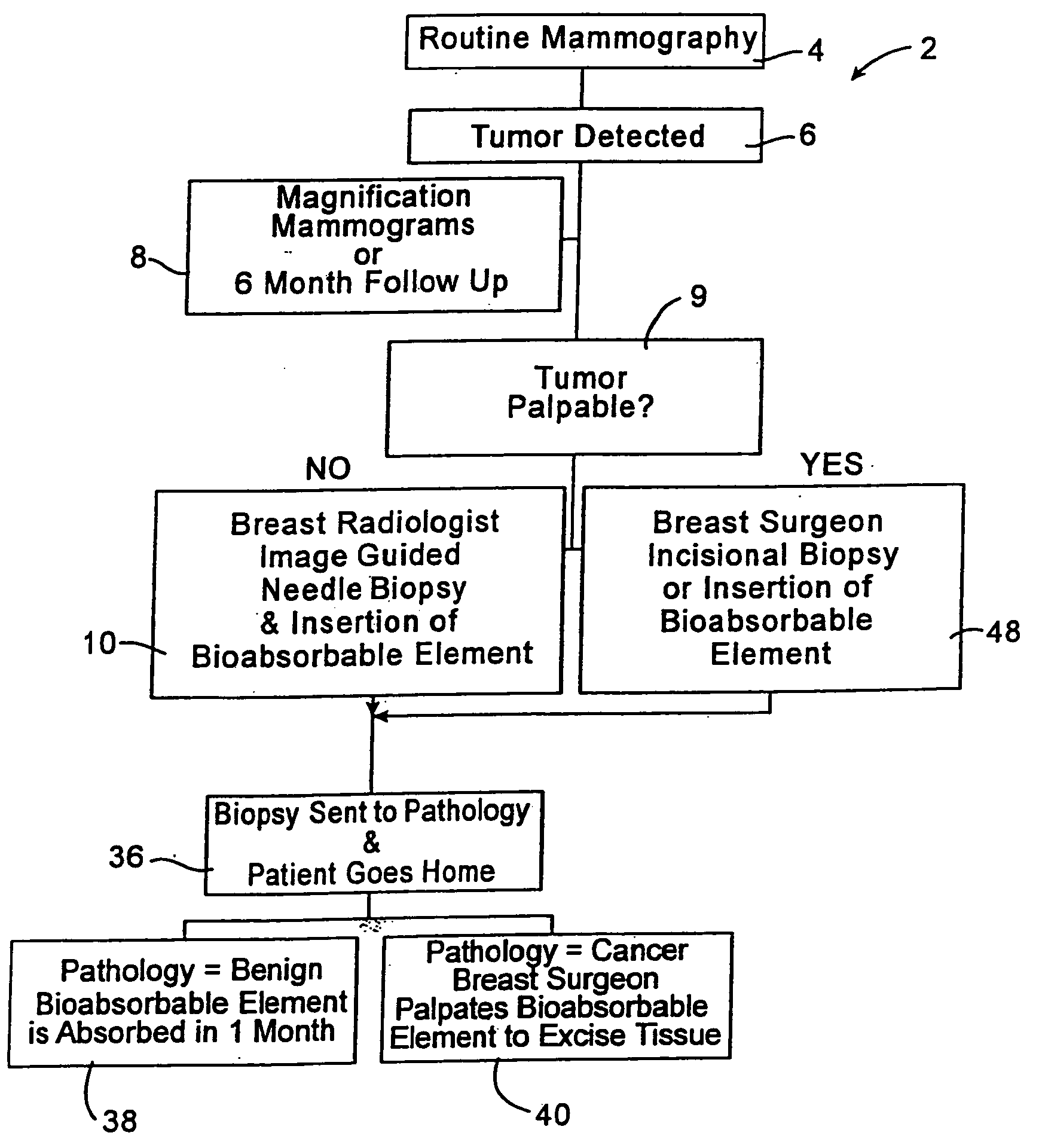
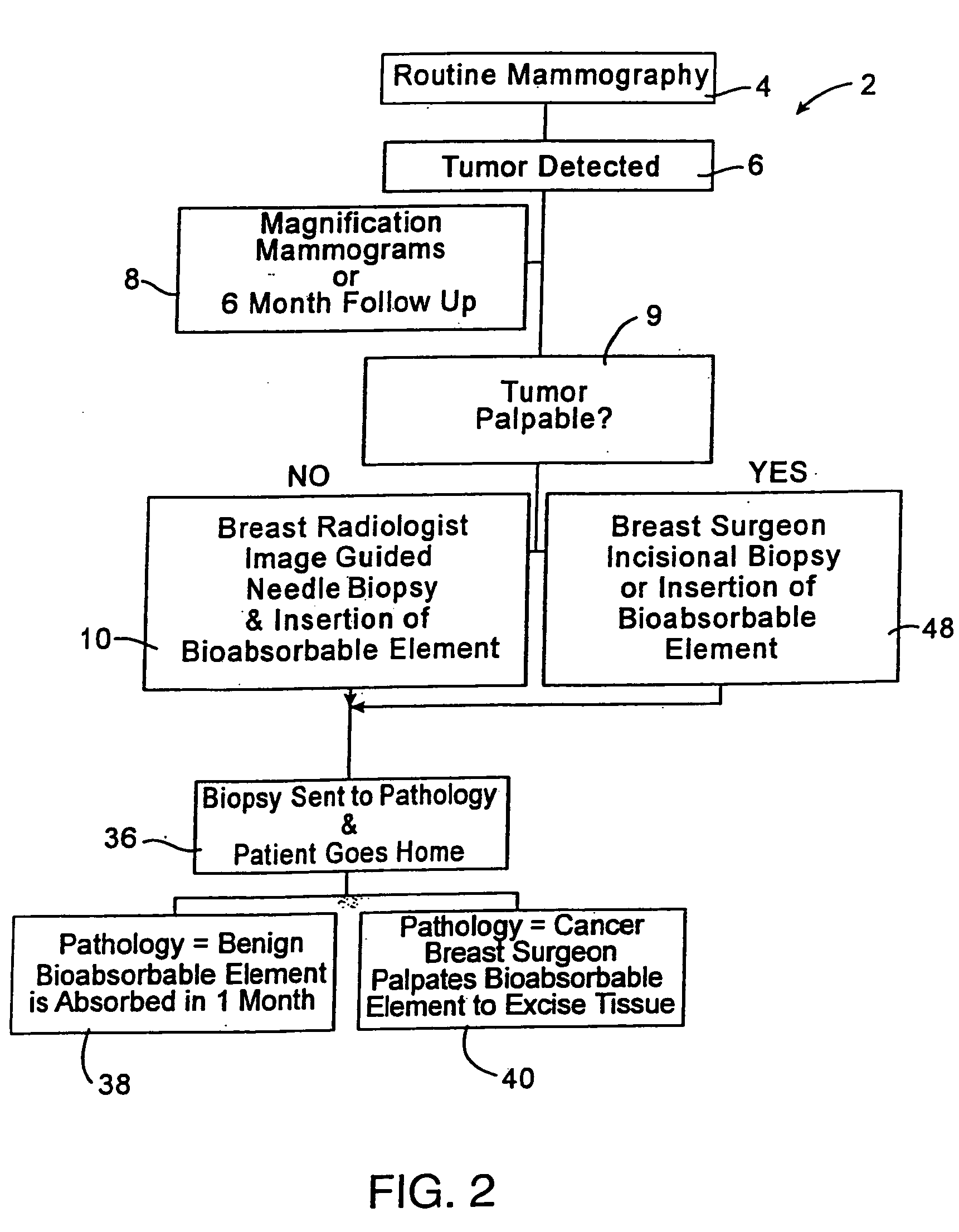
Imageable biopsy site marker
InactiveUS20060122503A1Accurately excise and remove a quantityMark accuratelyLuminescence/biological staining preparationOrgan movement/changes detectionRadiologyPiston
A biopsy site marker having at least one small marker body or pellet of bioresorbable material such as gelatin, collagen, polylactic acid, polyglycolic acid which has a radiopaque object, preferably with a non-biological configuration. The at least one bioresorbable body or pellet with a radiopaque object is deposited into the biopsy site, by an delivery device that includes an elongated tubular body with a piston slidable within the tubular body. One end of the tube is placed into the biopsy site. At least one but preferably several marker bodies or pellets are deposited sequentially into the biopsy site through the tube. At least the bioresorbable materials of the detectable markers remain present in sufficient quantity to permit detection and location of the biopsy site at a first time point (e.g., 2 weeks) after introduction but clear from the biopsy site or otherwise do not interfere with imaging of tissues adjacent the biopsy site at a second time point (e.g., 5-7 months) after introduction.
Owner:SENORX
Microcapsules having improved printing and efficiency
InactiveUS6544926B1Efficient use ofSharpness of image printing qualitiesPretreated surfacesGlass/slag layered productsChromogenicColloid
Dual shell microcapsule aggregate particles and copy materials coated therewith, such aggregate particles having inner shells surrounding chromogenic nucleus material, and outer shells encompassing multiple such inner shells to form aggregate particles thereof. The inner shells are derived from polar pre-polymer compositions. The outer shells are derived from complex colloids such as gelatin and gelatin derivatives. The outer shell material causes agglomeration of the inner shells into aggregate particles, thus increasing the sizes of the particles without increasing the sizes of the respective inner-shell microcapsules which contain the chromogenic material.
Owner:ENCAPSYS LLC
Edible biological preservative film and preparation method thereof
ActiveCN104194354AImprove mechanical propertiesImprove barrier propertiesFlexible coversWrappersAntioxidantPlasticizer
The invention discloses an edible biological preservative film and a preparation method thereof. The edible biological preservative film comprises the following components in parts by weight: 30-60 parts of a film former, 1-10 parts of a natural antioxidant, 1-10 parts of a natural bacterial inhibitor, 1-4 parts of a plasticizer and 0.5-4 parts of an emulsifier. The preparation method comprises the following steps: mixing the components in proportion to form preservative film liquid, and preparing the preservative film by adopting a tape-casting process. According to the preservative film, excellent film forming performances of materials such as marine polysaccharides, chitosan, gelatin and the like are utilized, the natural antioxidant and the natural bacterial inhibitor are selected and used, and the modification process is carried out through the plasticizer, the emulsifier and the like, so that the prepared preservative film is good in mechanical property, delays the spoilage of an aquatic product and can effectively prolong the shelf life of the aquatic product.
Owner:MARINE BIOLOGY INST OF SHANDONG PROVINCE
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
Green tonic lucid ganoderma (three immortals) cream for preventing sub-health and preparation method thereof
InactiveCN101856430AGood Prescription CombinationThe primary and secondary collocations are orderly and appropriateAerosol deliveryDigestive systemDiseaseMedicine
The invention discloses green tonic lucid ganoderma (three immortals) cream for preventing sub-health and a preparation method thereof. Lucid ganoderma is used as a main raw material. The preparation method comprises the following steps of: scientifically blending the lucid ganoderma and other Chinese medicinal materials, soaking, decocting and concentrating the materials, then properly adding nutrient or dietary supplements into the concentrate, and finally adding refined honey (or honey) or colloid medicament such as previously melted donkey-hide gelatin and the like serving as a cream collecting agent into the mixture to form the green tonic lucid ganoderma (three immortals) cream. The green tonic lucid ganoderma (three immortals) cream has the effects of nourishing and building the body, prolonging the life, preventing and treating diseases, effectively preventing sub-health and the like, and has unique health promotion significance for promoting the three aspects of 'essence, energy, spirit' of the human body in particular.
Owner:周艳
Fibrin glue without fibrinogen and biosealant compositions and methods
InactiveUS6168788B1Stop blood flowPrevents unwantedSurgical adhesivesPeptide/protein ingredientsFibrin glueClot formation
The invention is a fibrin glue that avoids the use of fibrinogen and thus eliminates the need for premixing and premature clot formation. The fibrin glue of the invention comprises thrombin, thromboplastin and calcium and may have clotting Factors, VII, IX and X, and the like. The invention also comprises a biosealant for use with the fibrin glue without fibrinogen or for use alone. The biosealant is a two component mixture of gelatin / resorcinol and glyoxal / glutaraldehyde / 4-(p-maleimidophenyl) butyric acid. The two components are mixed on use.
Owner:WORTHAM LEON
Ethanol-free gel formulation cartridge for e-vaping device
A cartridge for an e-vaping device includes an ethanol-free gel formulation. The ethanol-free gel formulation includes a vapor former, water, and a biopolymer. The biopolymer may be included in an amount ranging from about 0.01% by weight based on the weight of the ethanol-free gel formulation to about 2.0% by weight based on the weight of the ethanol-free gel formulation. The biopolymer may be one or more of agar, kappa carrageenan, gelatin, sodium alginate, gellan gum, pectin, and combinations thereof. The cartridge also includes a heater configured to heat liquid from the gel formulation to a temperature sufficient to release a liquid / semi-liquid component from the gel, which component thereupon forms a vapor.
Owner:AKRIA CLIENT SERVICES LLC
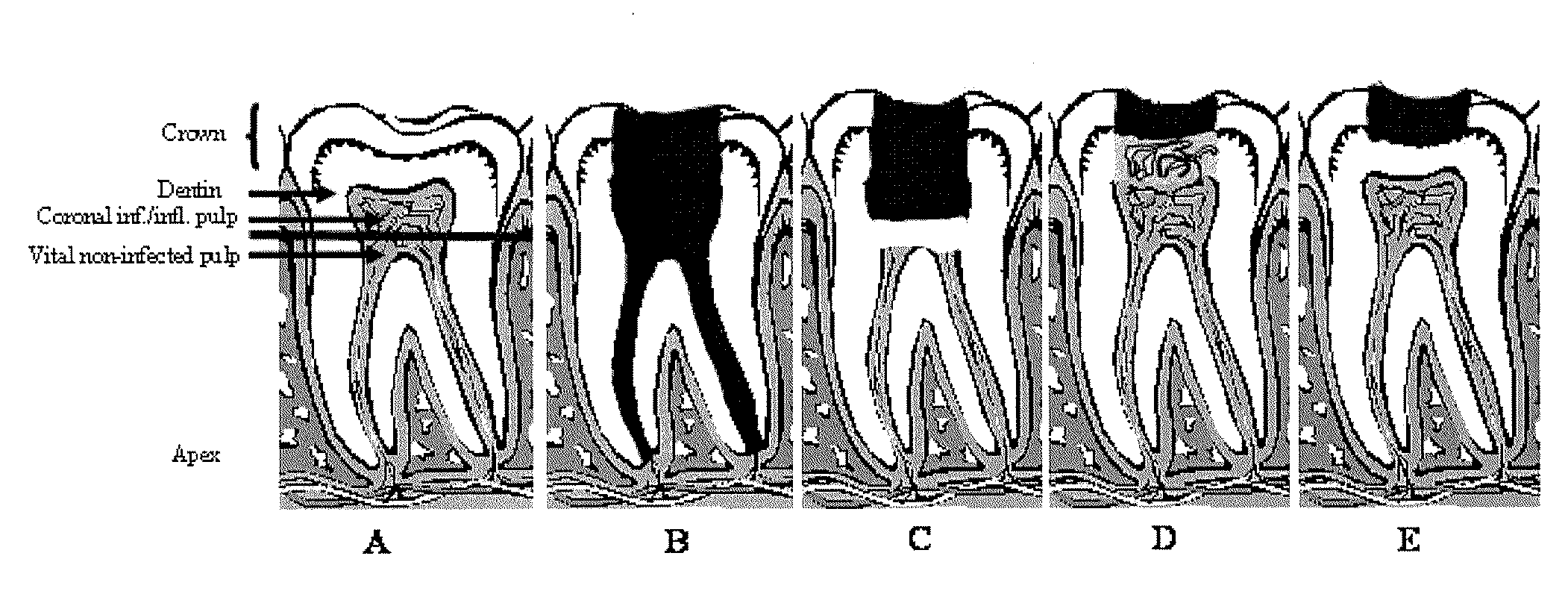
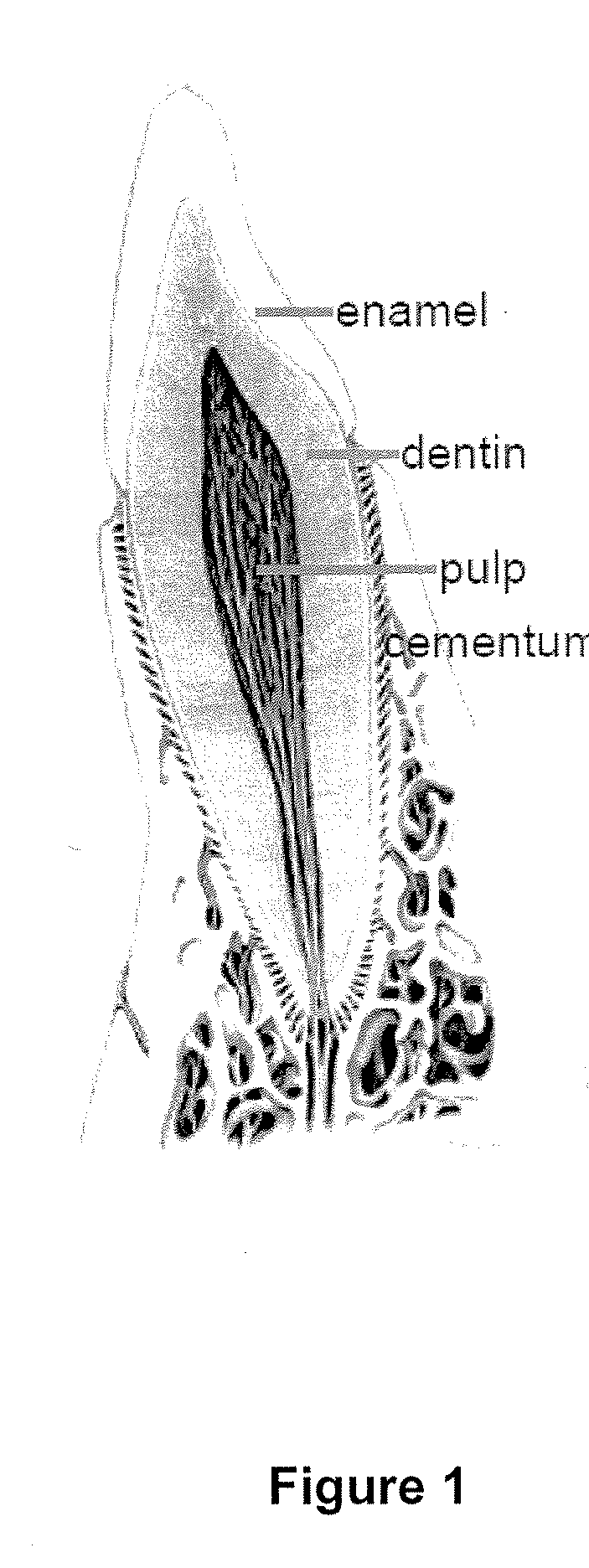
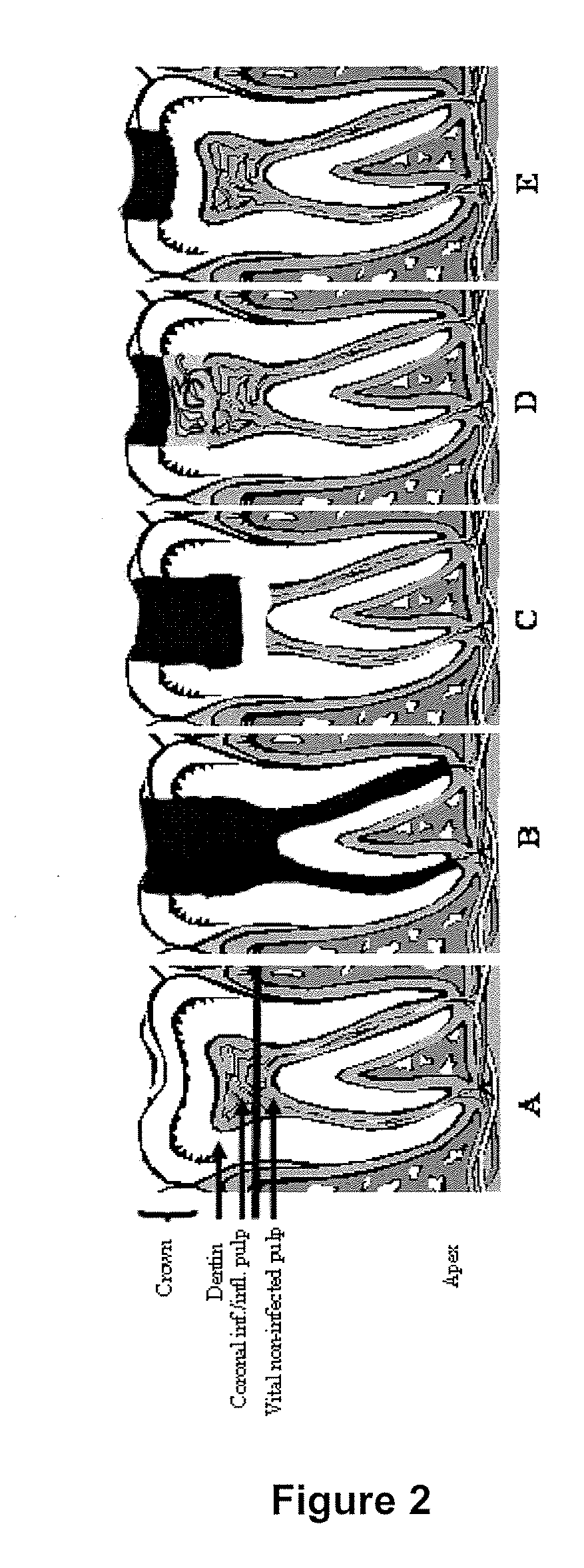
Compositions and methods for treating pulp inflammations caused by infection or trauma
The present disclosed subject matter relates to methods and compositions for restoring a diseased or damaged tooth such that infection is inhibited or eliminated and pulp regeneration is facilitated. The disclosed subject matter also includes a composition comprising a physiologically acceptable matrix seeded with pulp cells. The matrix can be capable of being injected into the pulp chamber of a tooth. In some embodiments, the matrix of a composition includes a hydrogel (e.g., collagen, chitosan, alginate, MATRIGEL™, gelatin, JELL-O®, fibrin), a mesh (e.g., polylactide-coglycolide (PLGA) mesh, polylactide (PLA) mesh, or polyglycolide (PGA) mesh, a cross-linked fiber mesh, a nanofiber mesh, a mesh fabric, biodegradable polymer mesh), a microsphere (biodegradable polymer microsphere, a hydrogel microsphere), or a combination of any of the foregoing. In yet other embodiments, the matrix includes a nanofiber, an artificial three-dimensional scaffold material, or a synthetic three-dimensional scaffold material.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Biopsy localization method and device
Owner:ARTEMIS MEDICAL
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20050065214A1Stimulate bone healing processLower potentialBiocidePowder deliveryBarium saltTG - Triglyceride
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
Intra-serum and intra-gel for modeling human skin tissue
InactiveUS6475800B1Diagnostics using spectroscopyScattering properties measurementsConfocalCrosslinking reagent
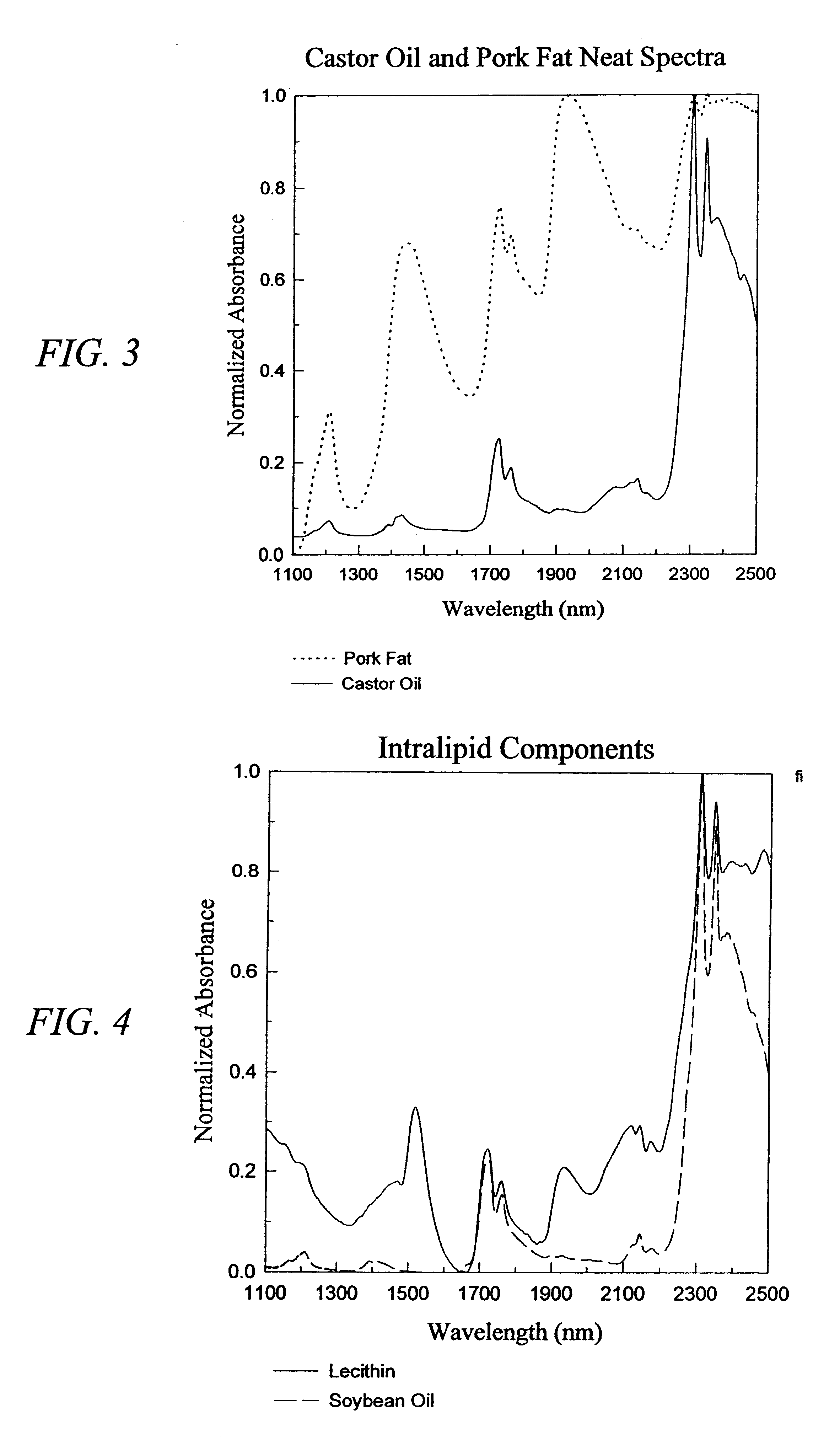
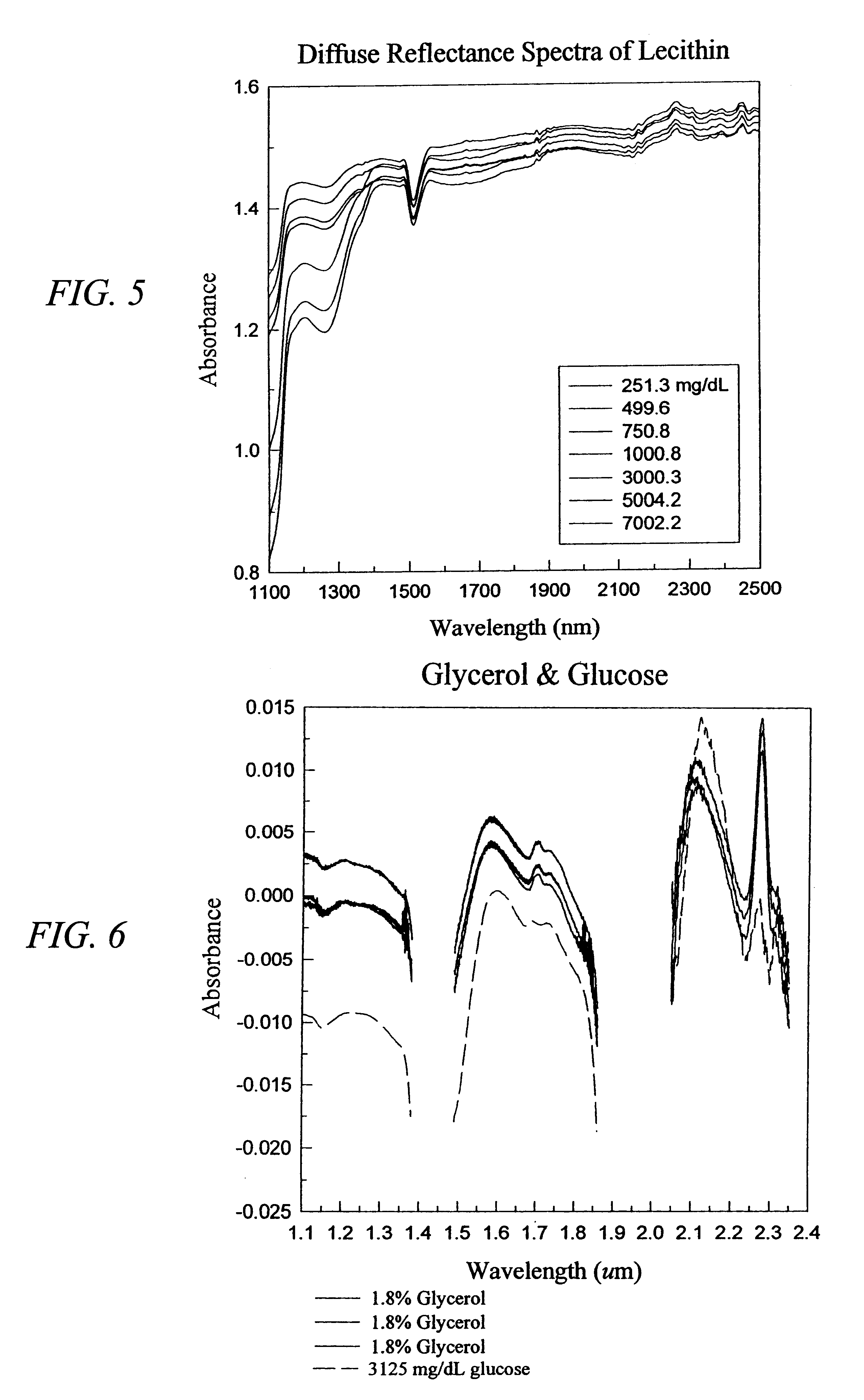
The invention provides a class of samples that model the human body. This family of samples is based upon emulsions of oil in water with lecithin acting as the emulsifier. These solutions that have varying particle sizes may be spiked with basis set components (albumin, urea and glucose) to simulate skin tissues further. The family of samples is such that other organic compounds such as collagen, elastin, globulin and bilirubin may be added, as can salts such as Na+, K+ and Cl-. Layers of varying thickness with known index of refraction and particle size distributions may be generated using simple crosslinking reagents, such as collagen (gelatin). The resulting samples are flexible in each analyte's concentration and match the skin layers of the body in terms of the samples reduced scattering and absorption coefficients, mums and muma. This family of samples is provided for use in the medical field where lasers and spectroscopy based analyzers are used in treatment of the body. In particular, knowledge may be gained on net analyte signal, photon depth of penetration, photon radial diffusion, photon interaction between tissue layers, photon density (all as a function of frequency) and on instrument parameter specifications such as resolution and required dynamic range (A / D bits required). In particular, applications to delineate such parameters have been developed for the application of noninvasive glucose determination in the near-IR region from 700 to 2500 nm with an emphasis on the region 1000 to 2500 nm (10,000 to 4,000 cm-1).
Owner:GLT ACQUISITION
Cross-linked gelatin composition comprising a wetting agent
Disclosed is a biocompatible, hemostatic, cross-linked gelatin composition comprising a biocompatible material comprising cross-linked gelatin and a sufficient amount of wetting agent to permit uniform wetting of the sponge in the presence of an aqueous solution.
Owner:BOSTON SCI SCIMED INC +11
Chondroitinase, process for preparing the same, and pharmaceutical composition comprising the same
InactiveUS6184023B1Avoid stickingInhibit productionBacteriaHydrolasesChondroitinase ABCConcentration gradient
A crystallizable, purified chondroitinase ABC having a molecular weight of about 100,000 dalton by the measurement of the SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and the measurement by the gel permeation chromatography method, having alanine as the N-terminal amino acid and proline as the C-terminal amino acid. A process for the purification of the crystallizable purified chondroitinase ABC comprising removing nucleic acid from an surfactant solution extract obtained from cells of chondroitinase ABC-producing microorganisms and chromatographically treating by concentration gradient elution using a weak cation exchange resin or a strong cation exchange resin. A composition comprising a chondroitinase and serum albumin, gelatin, or a nonionic surfactant.
Owner:SEIKAGAKU KOGYO CO LTD
Layered bio-adhesive compositions and uses thereof
The invention generally provides compositions and methods for promoting and enhancing wound closure and healing. Specifically, the invention provides a biologic composition which comprises a support layer which serves as transport scaffold, for example made of gelatin, which is coated or impregnated with a bio-adhesive molecule such as rose bengal or glyceraldehyde. The composition can also comprise an artificial or biological matrix, optionally processed (i.e. cleaned and coated with extracellular matrix proteins) to enhance cell attachment and survival. The composition can further comprise a monolayer of epithelial, endothelial cells or mesenchymal cells. The invention provides methods for using the compositions for treating wounds due to disease, trauma or surgery. Specific methods for treating ocular wounds are provided.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Probiotic products for pet applications
An exemplary embodiment providing one or more improvements includes feeding pets with probiotic microbes encapsulated in a mixture of xanthan gum and chitosan, or in gelatin, specifically Pediococcus acidilactici and Saccharomyces boulardii. Such encapsulation protects the viability of the probiotic microbes against unfavorable temperatures. Such feeding has the benefit of reducing odors, and improving digestion in pets which have these problems.
Owner:IMAGILIN TECH LLC
Acid-resistant soft gel compositions
The present disclosure describes a delivery device for administration of nutraceuticals or pharmaceuticals, which device contains a soft gel shell comprising a gelatin-based water soluble film forming polymer, an acid insoluble polymer, and at least one reducing sugar and water, including processes, gel mixtures used for device production, and coatings containing such gel mixtures.
Owner:HASSAN EMADELDIN M
Blumea oil microcapsule textile composite finishing agent and use thereof
InactiveCN101591859AStir wellSmall particle sizeFibre treatmentMicroballoon preparationCross-linkChemical Linkage
The invention discloses a blumea oil microcapsule textile composite finishing agent and use thereof. Gelatin or acacia of natural polymers is used the main ingredient of a wall material, the blumea oil serving as a Chinese medicinal material is used as a core material, and complex coacervation is adopted to prepare an blumea oil microcapsule aqueous emulsion; a shrink-resistance crosslinker and a catalyst are added to be combined with the blumea oil microcapsule aqueous emulsion into a multifunctional blumea oil microcapsule textile composite finishing agent; researches on the use of blumea oil microcapsules in textile finishing are carried out; and a novel cross linked graft method is used to perform the microcapsule finishing of textiles to form chemical bonds between the microcapsules and the textiles though the shrink-resistance crosslinker and free formaldehyde molecules in 2D resin further participate in a cross-linking and curing reaction as a microcapsule curing agent, so the textiles achieve a long lasting antibacterial and health-care function, a shrink-resistance function and low formaldehyde release content performance.
Owner:SOUTH CHINA AGRI UNIV
Resorbable bone graft materials
Ceramic materials operable to repair a defect in bone of a human or animal subject comprising a porous ceramic scaffold having a bioresorbable coating, and a carrier comprising denatured demineralized bone. The ceramic may contain a material selected from the group consisting of hydroxyapatite, tricalcium phosphate, calcium phosphates, calcium carbonates, calcium sulfates, and combinations thereof. The compositions may also contain a bone material selected from the group consisting of: bone powder, bone chips, bone shavings, and combinations thereof. The bioresorbable coating may be, for example, demineralized bone matrix, gelatin, collagen, hyaluronic acid, chitosan, polyglycolic acid, polylactic acid, polypropylenefumarate, polyethylene glycol, or mixtures thereof.
Owner:BIOMET MFG CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com