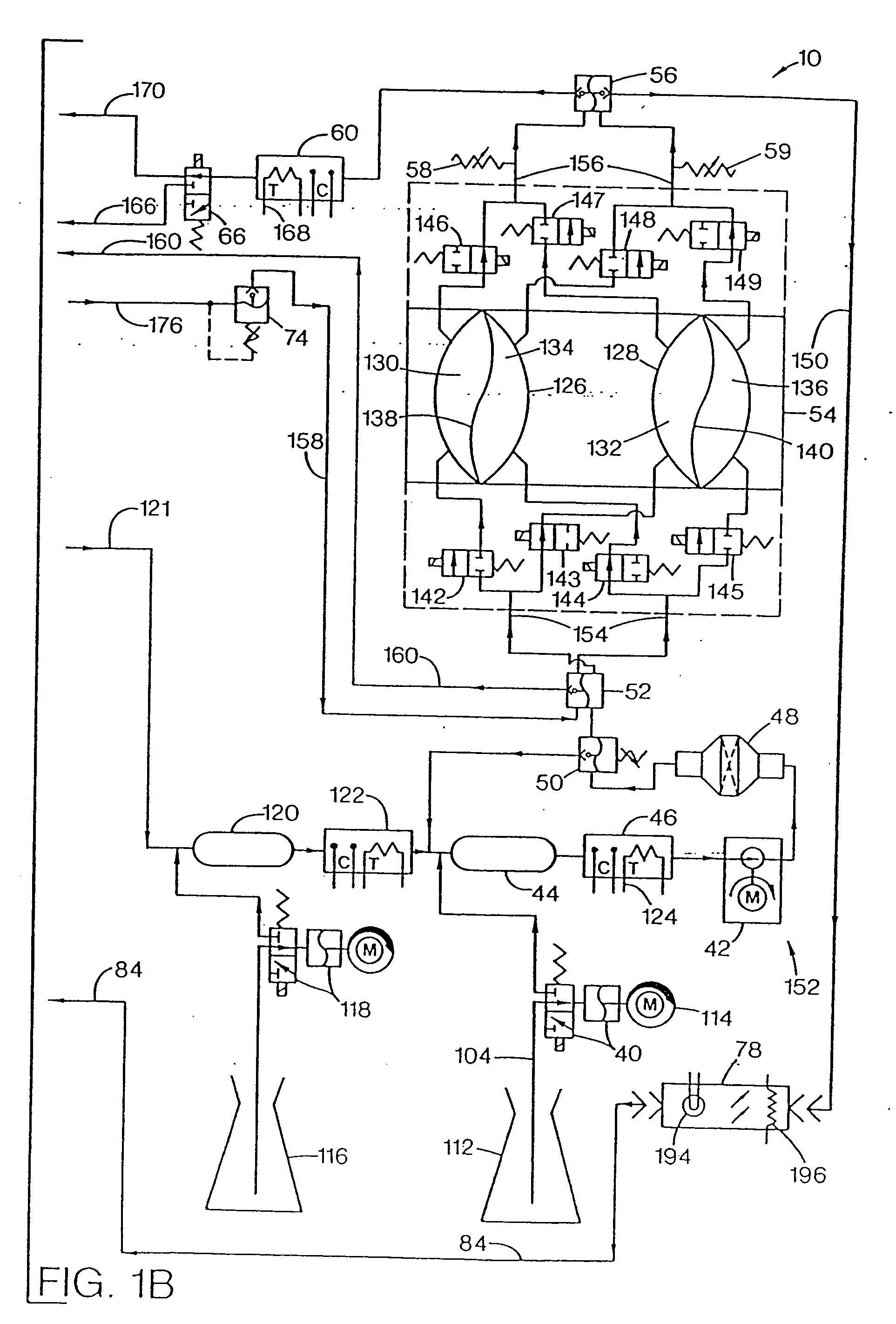
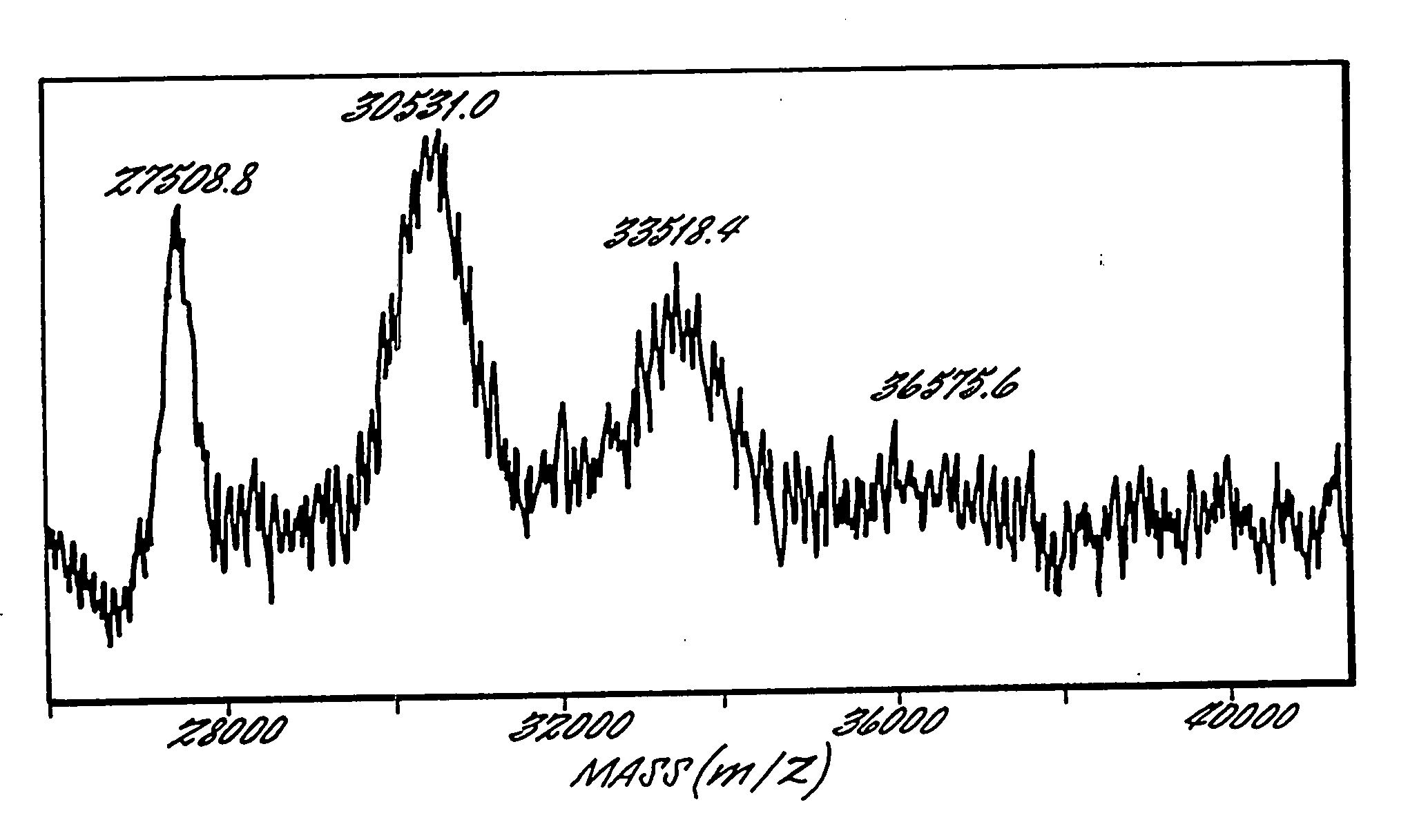
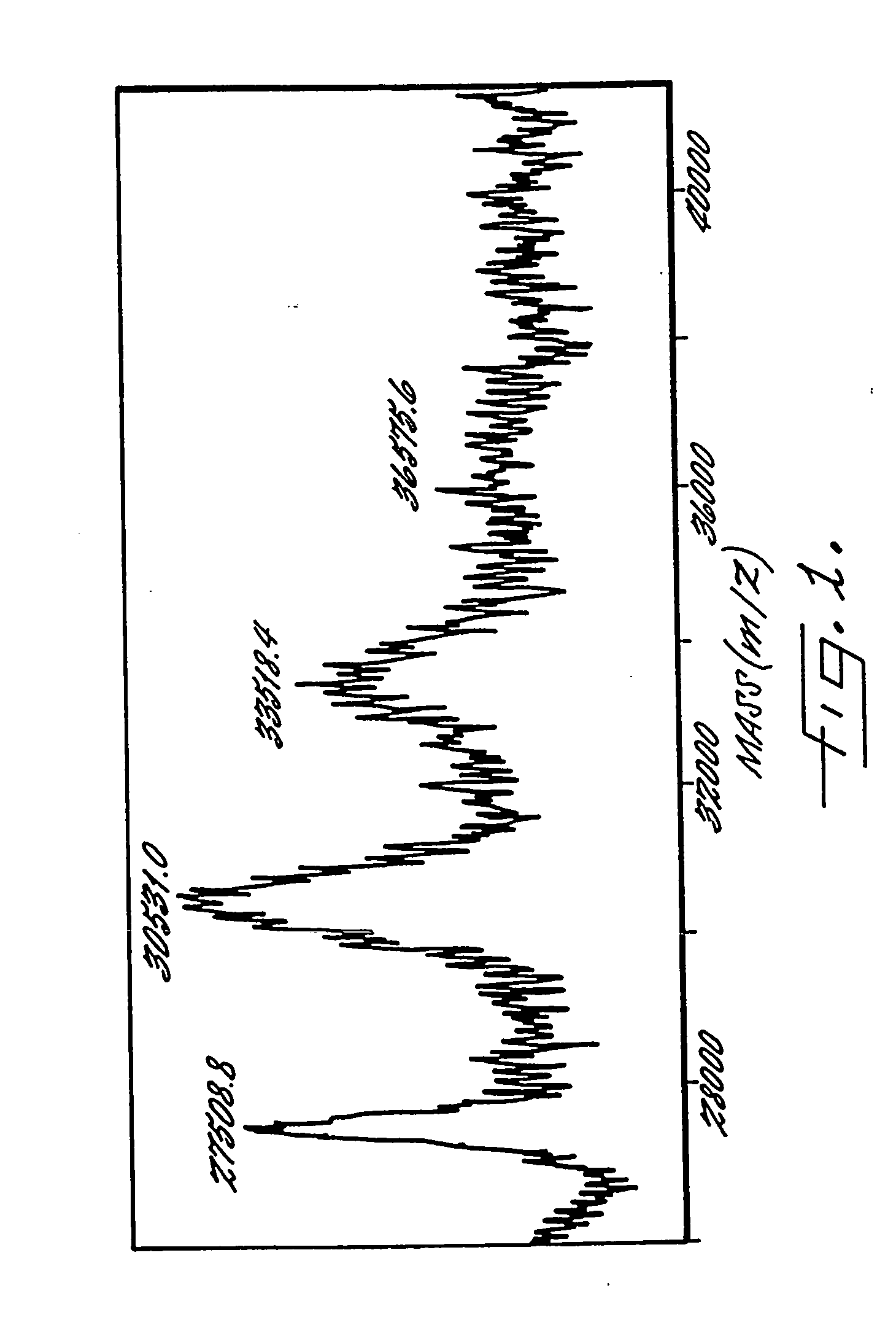
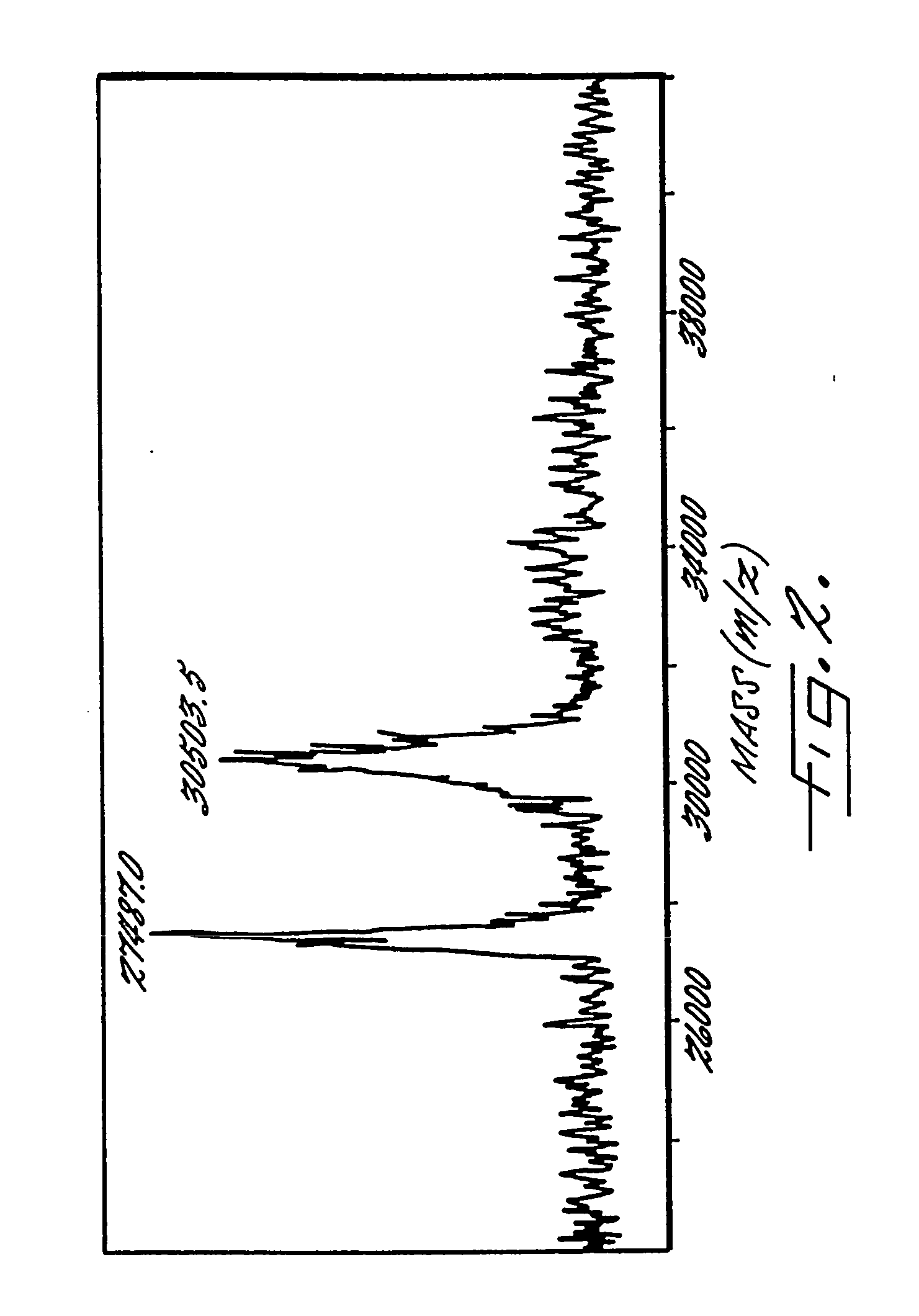
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
13135 results about "Kidney" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The kidneys are two bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about 11 centimetres (4.3 in) in length. They receive blood from the paired renal arteries; blood exits into the paired renal veins. Each kidney is attached to a ureter, a tube that carries excreted urine to the bladder.
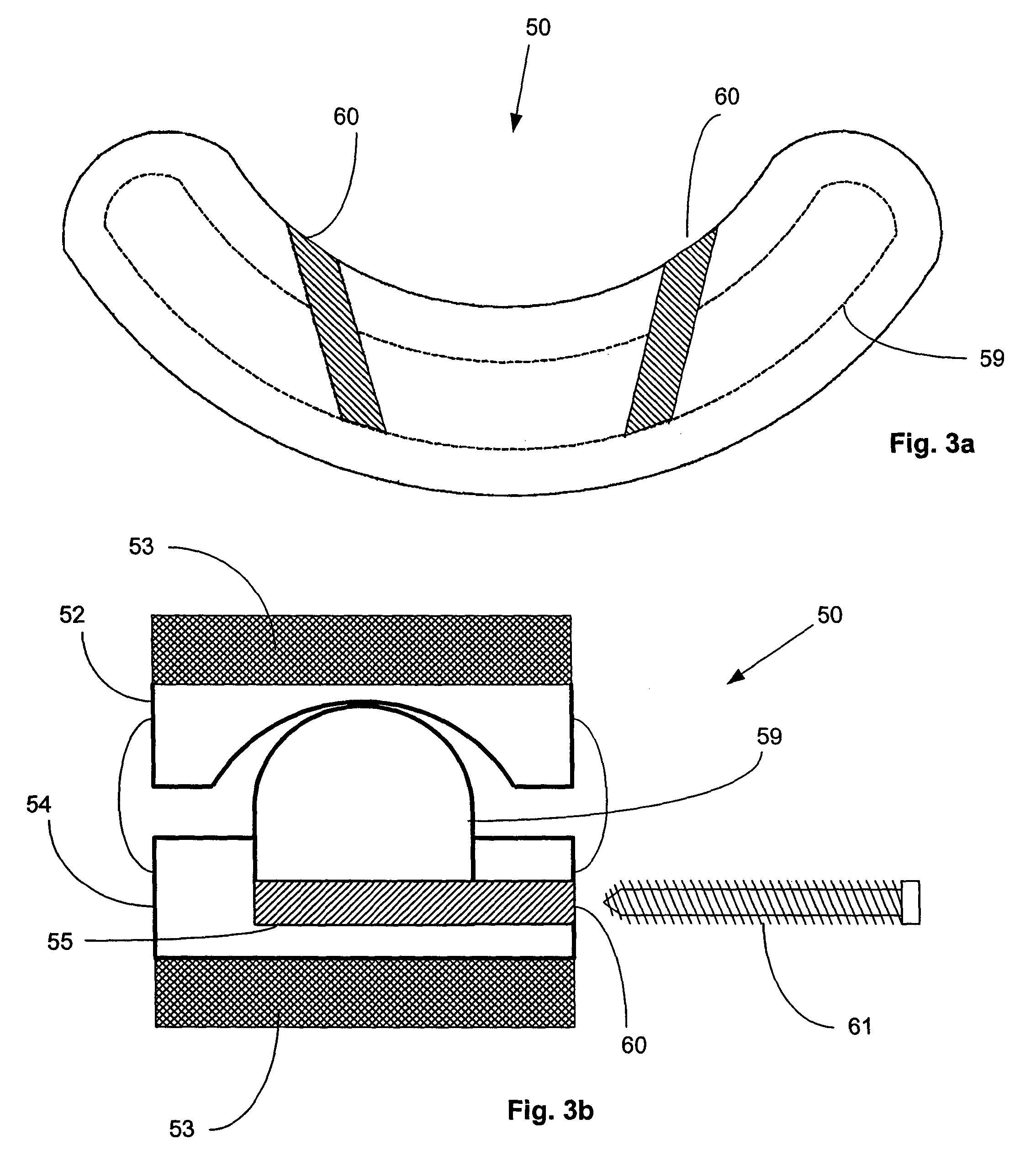
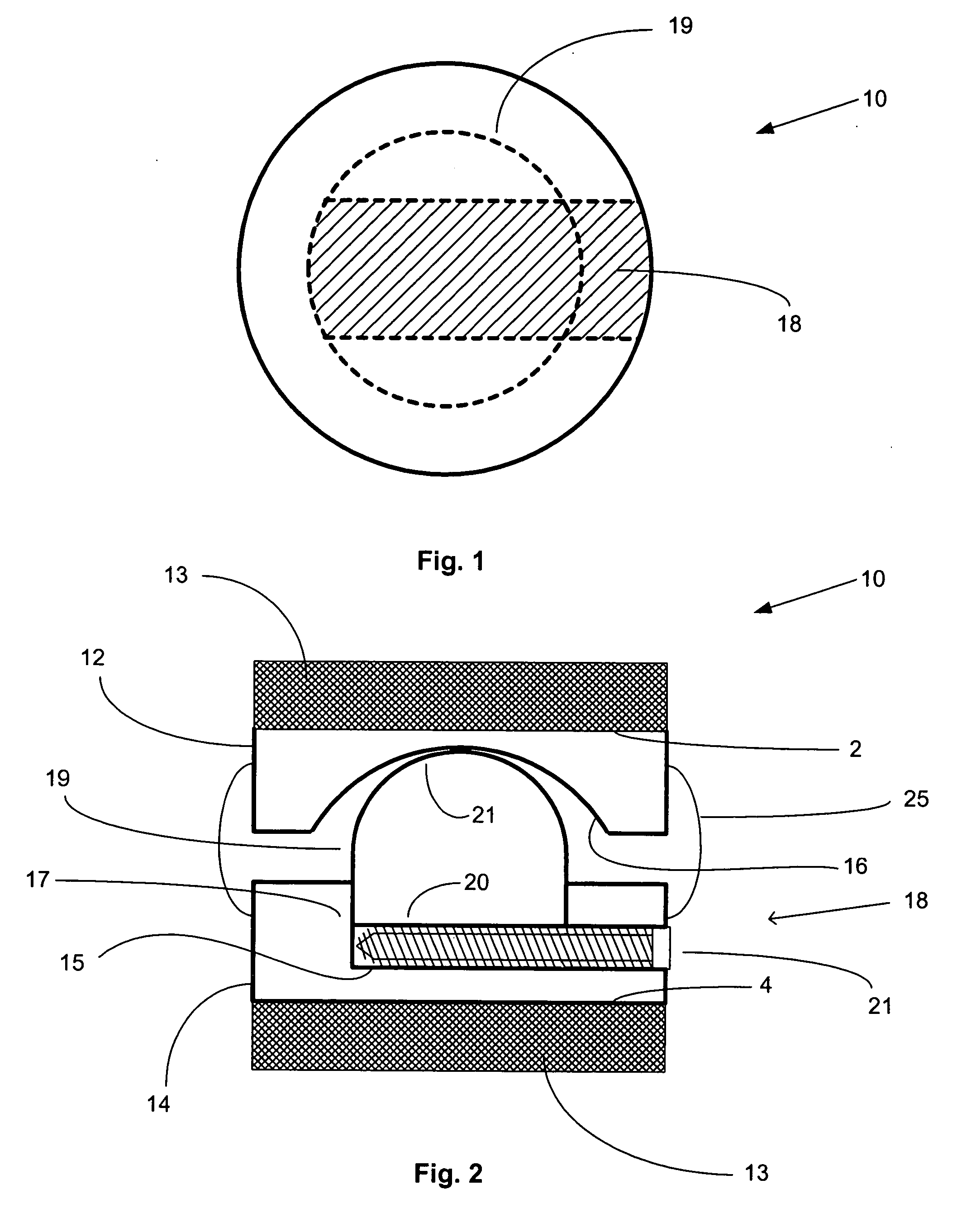
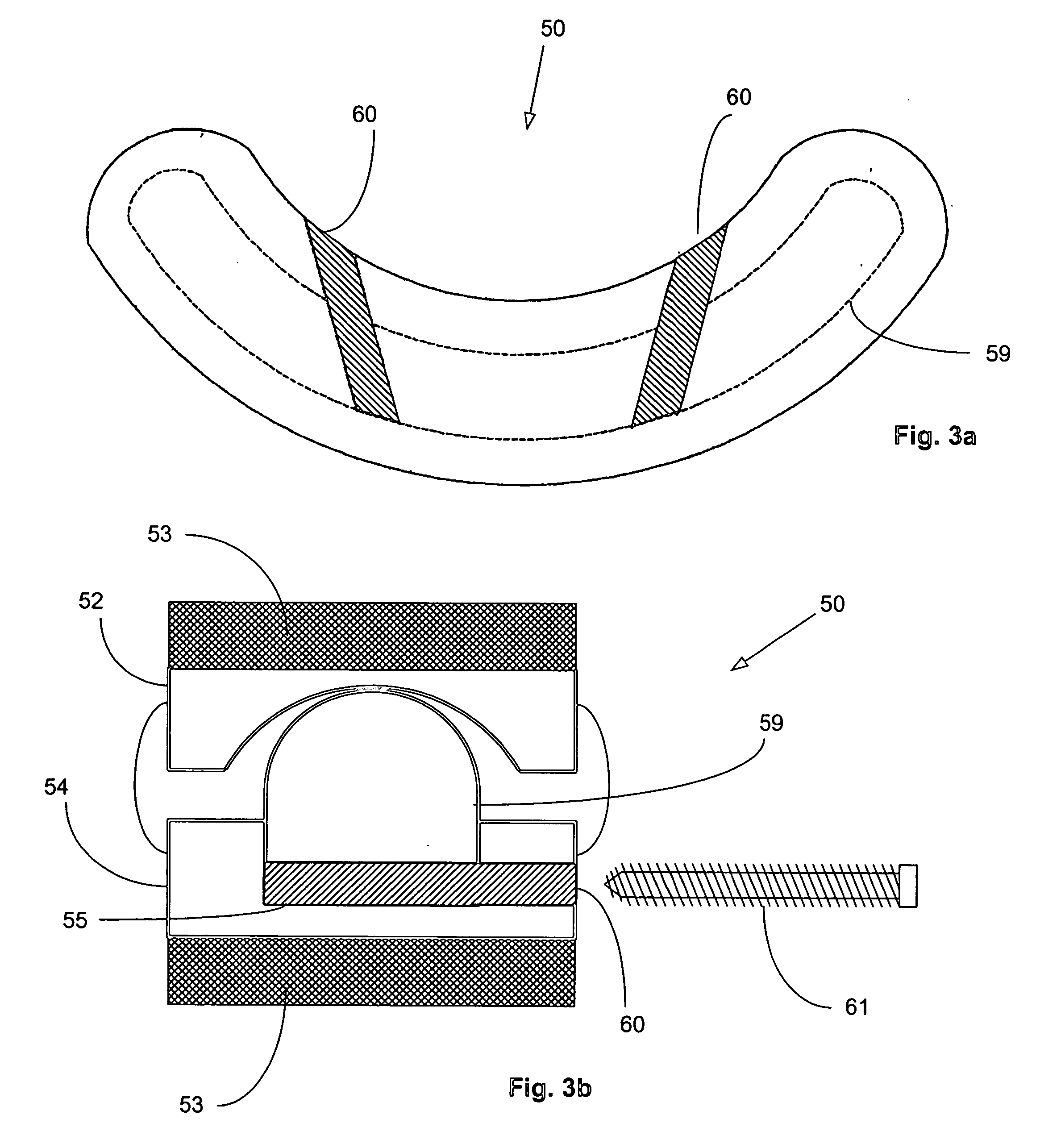
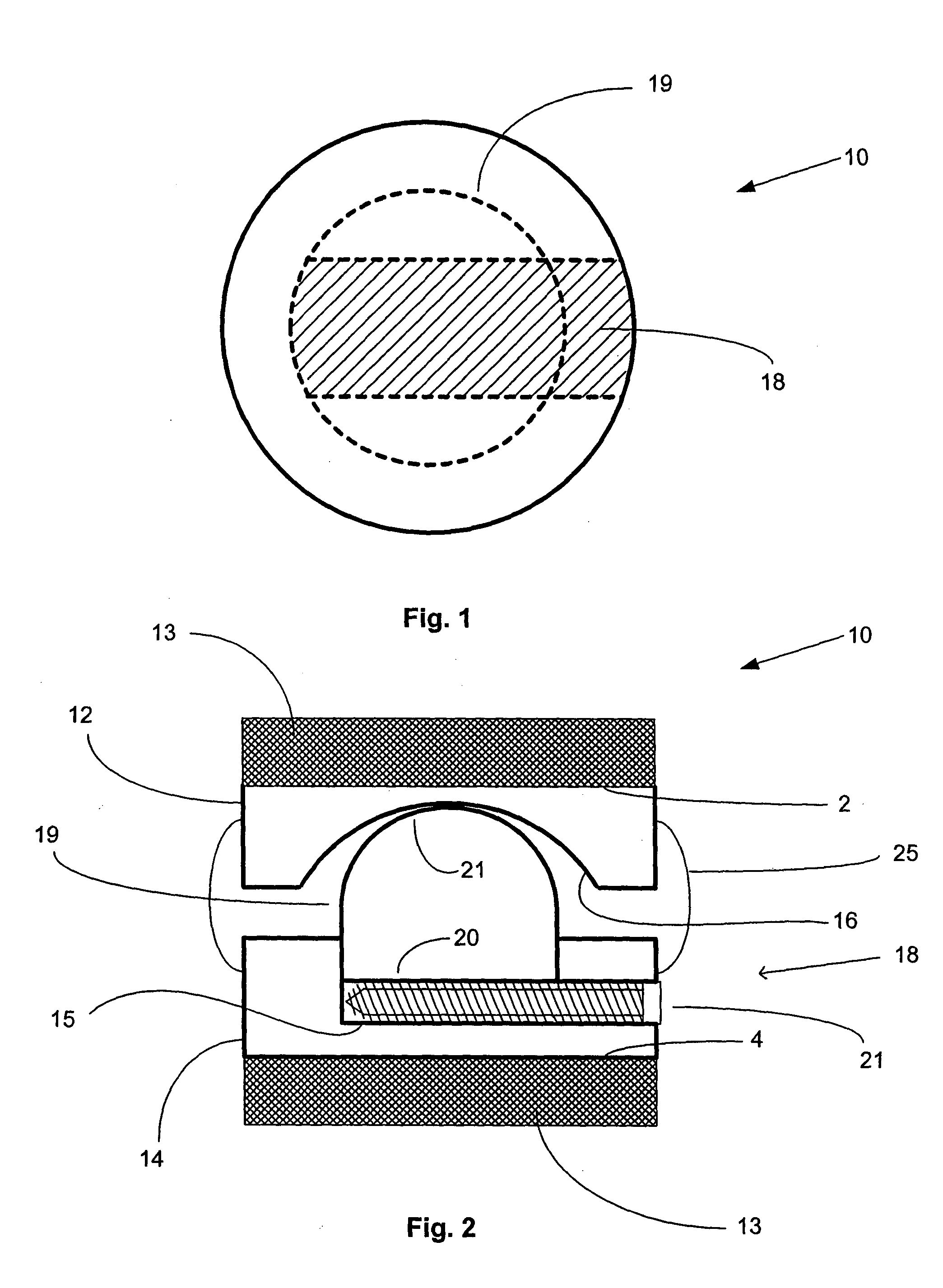
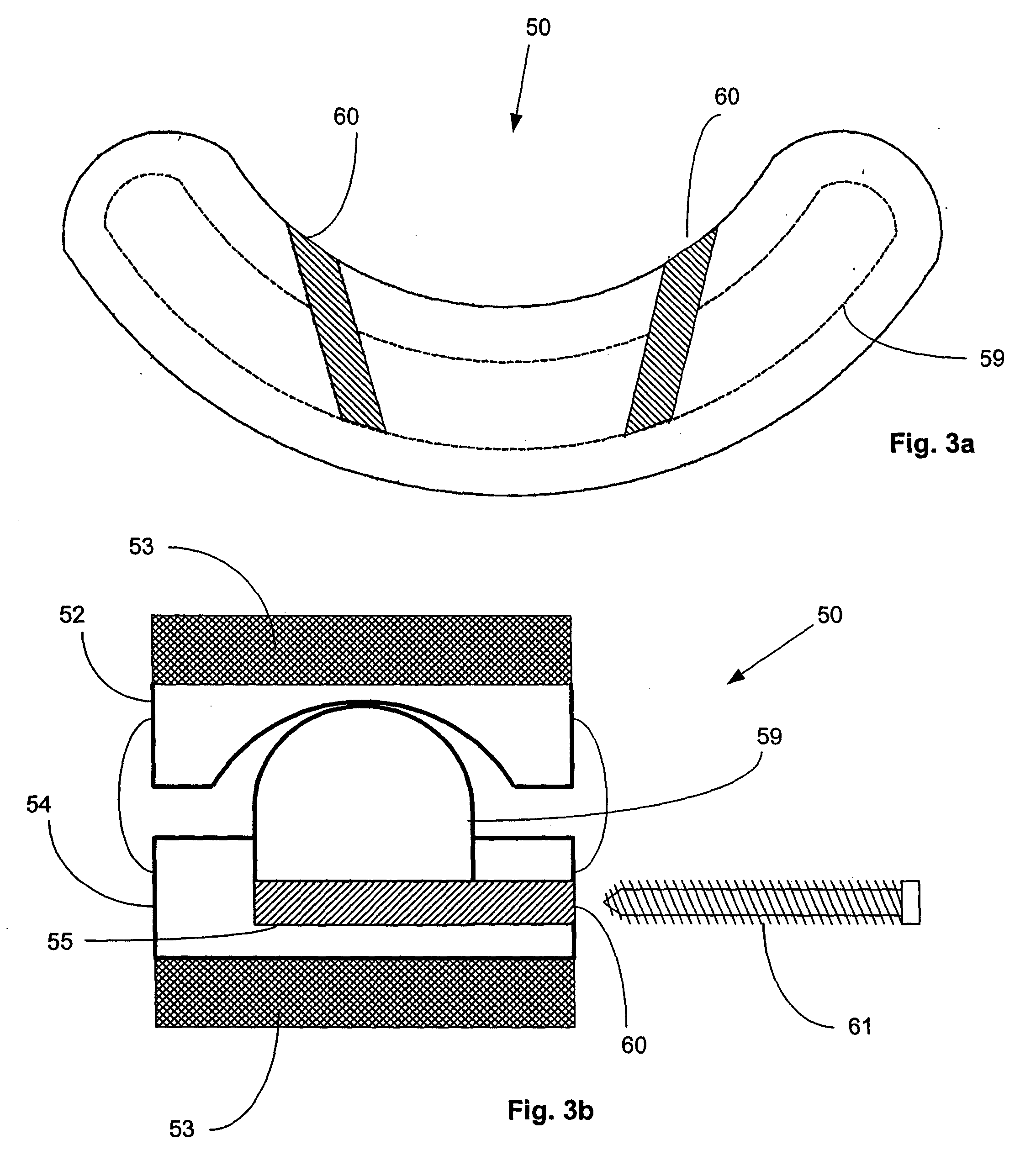
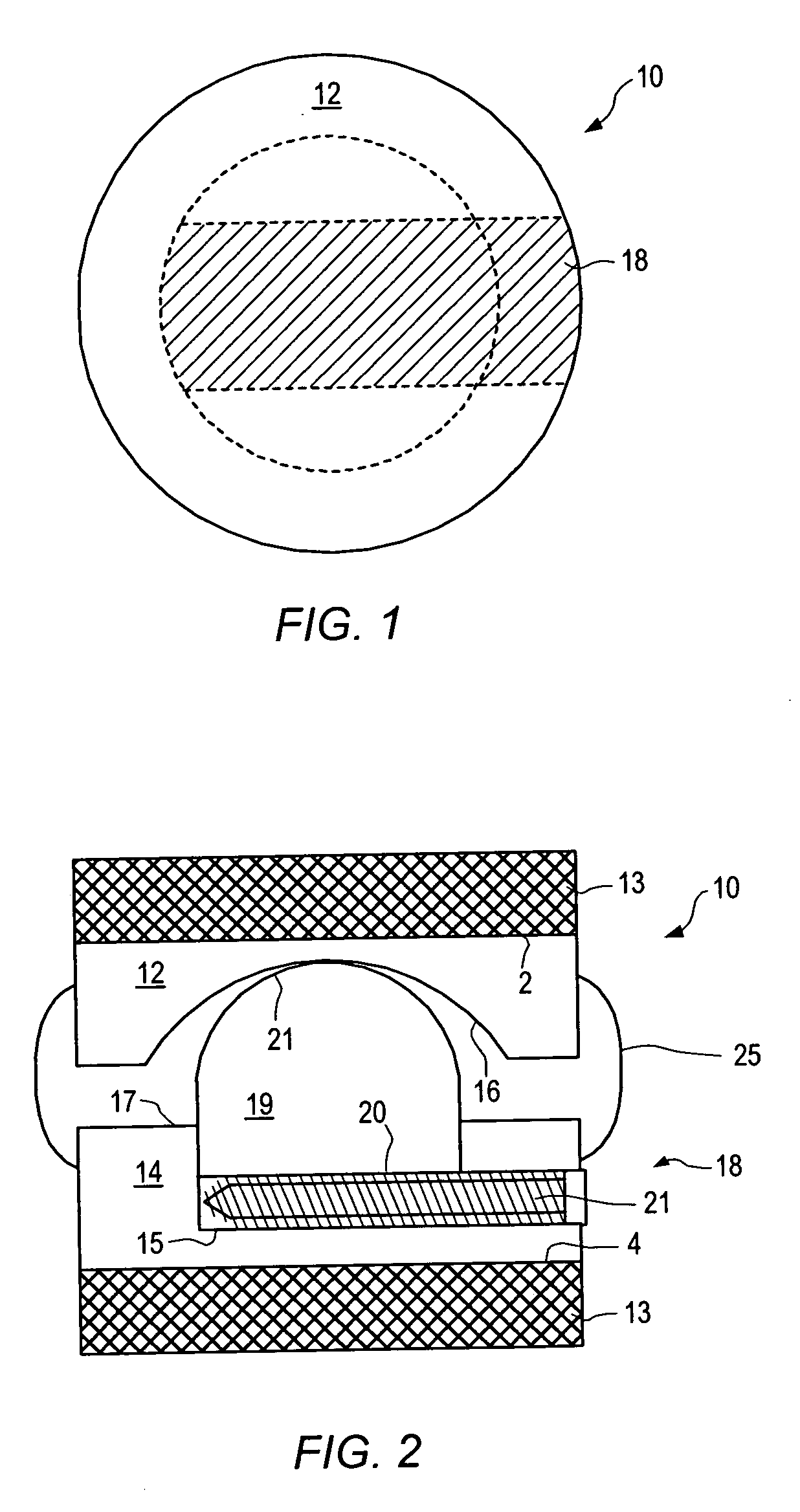
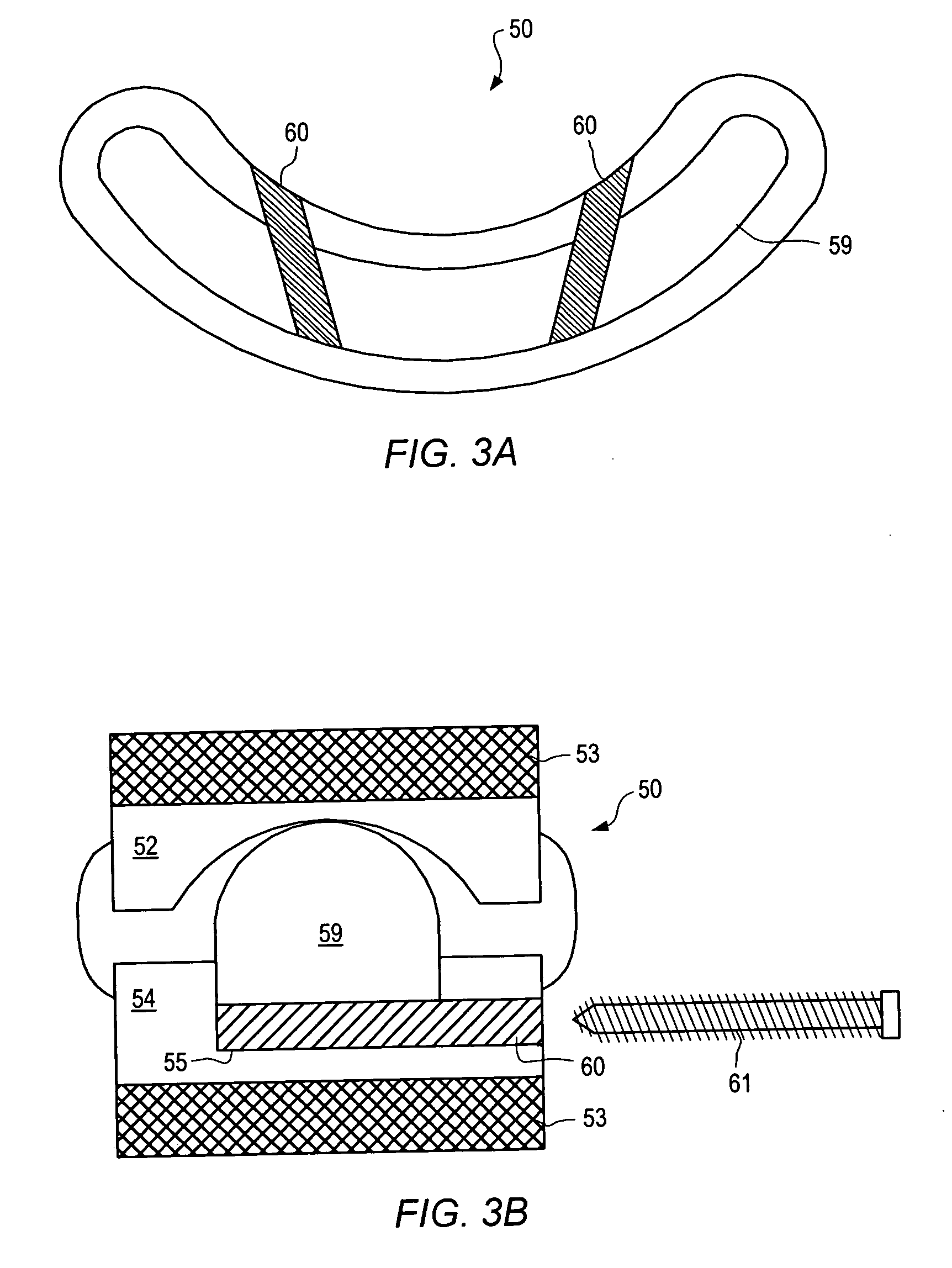
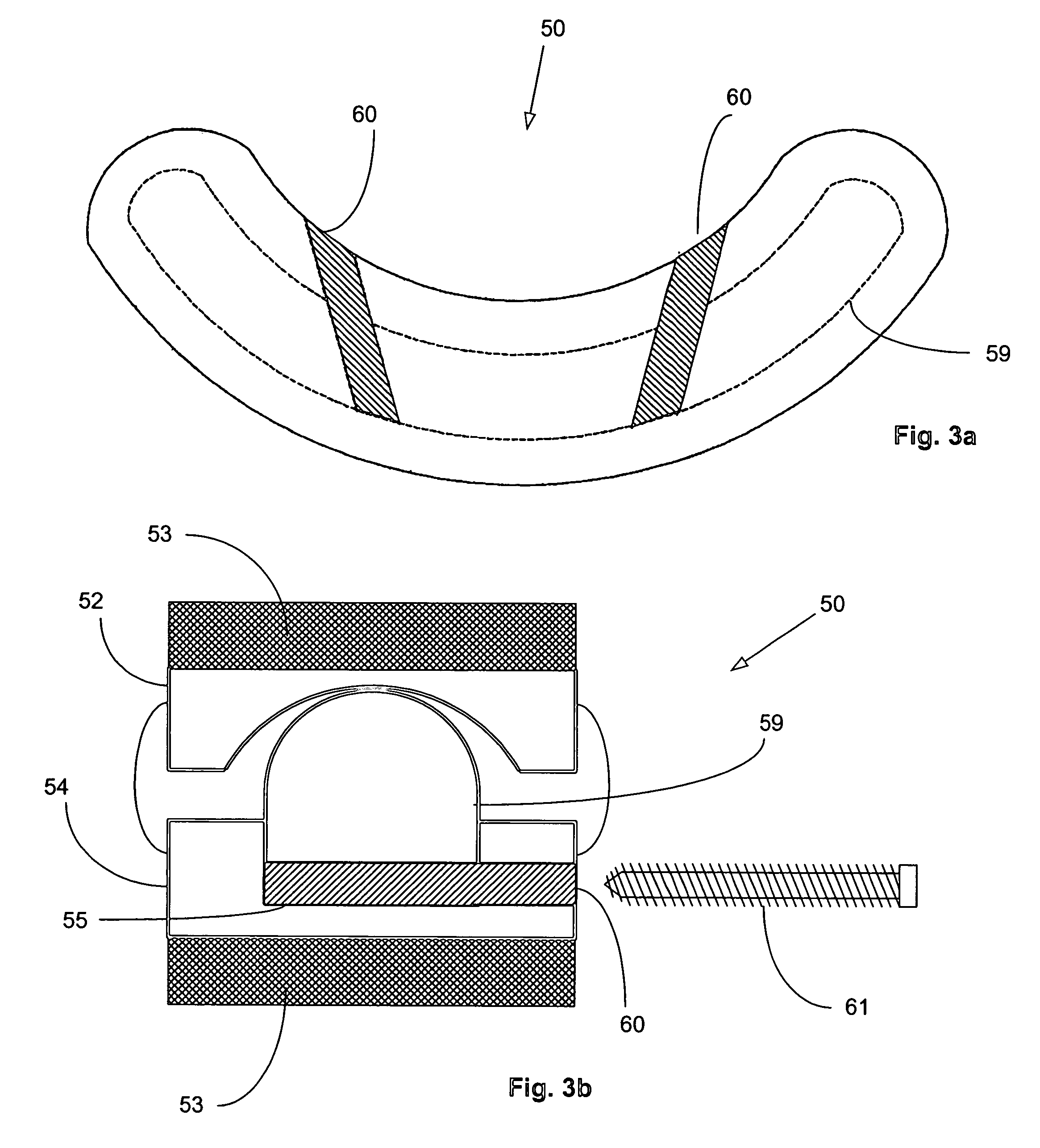
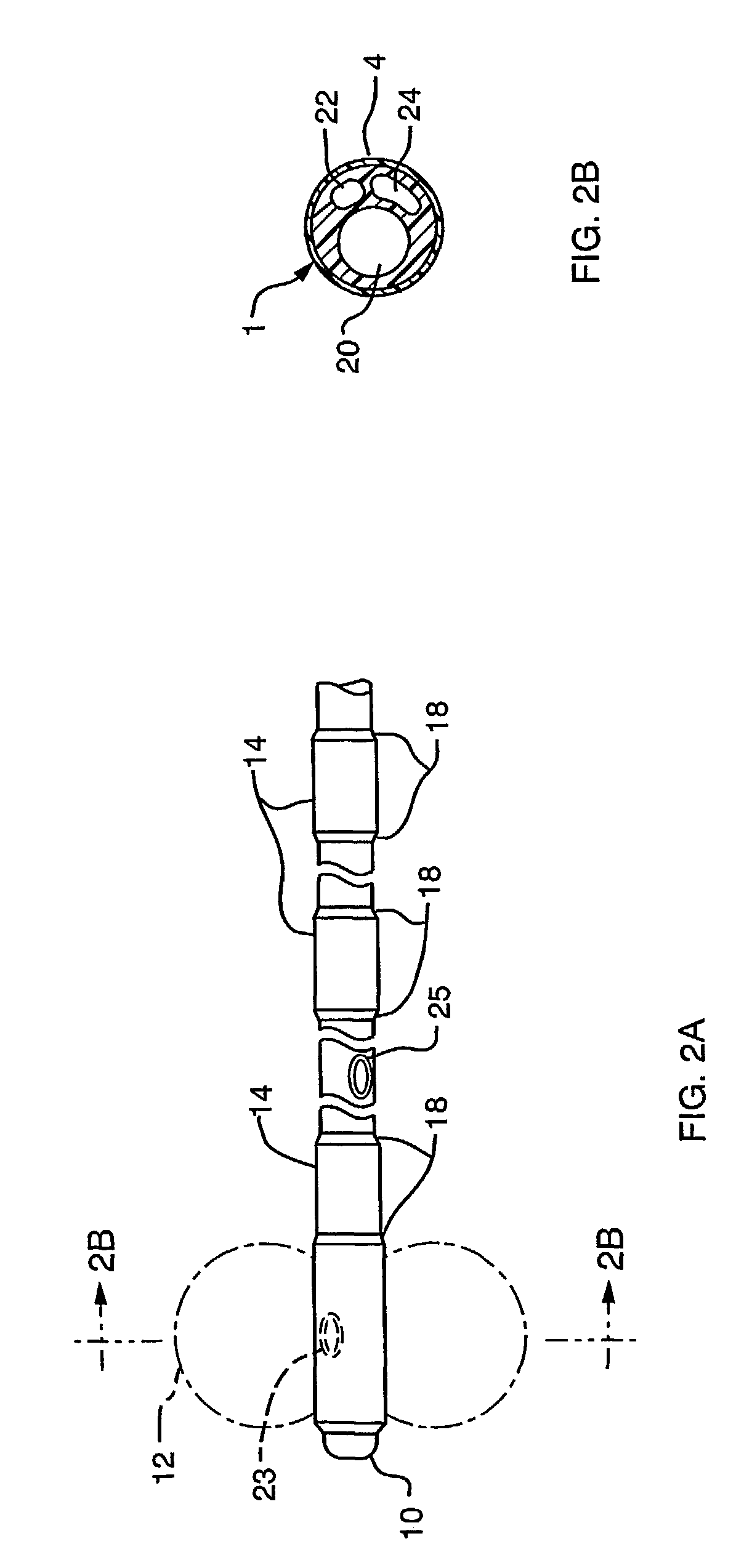
Surgical staple and staple pocket for forming kidney-shaped staple
ActiveUS9561030B2Improve sealingFacilitating the healing of the surrounding tissueStaplesNailsSurgical stapleEngineering
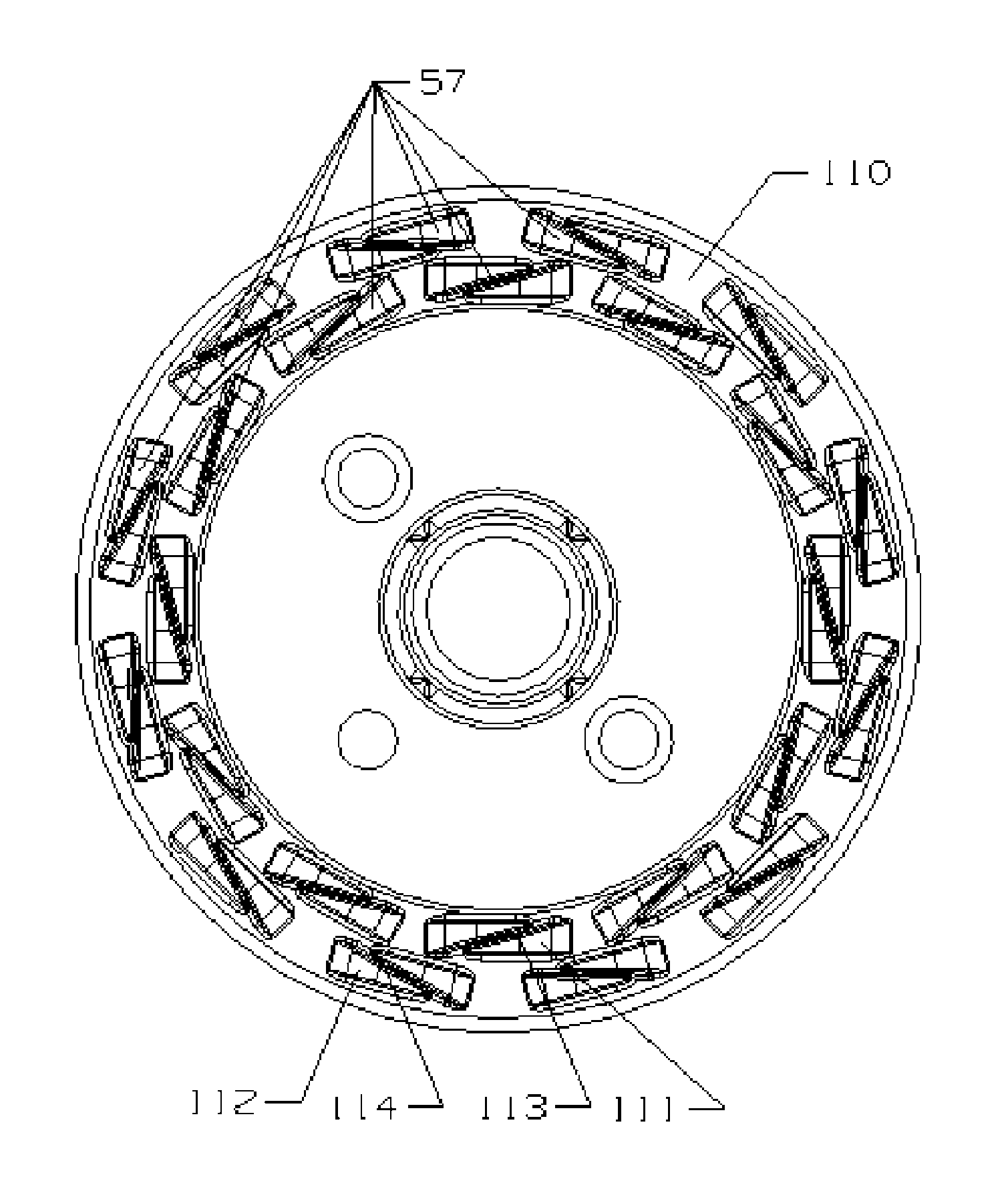
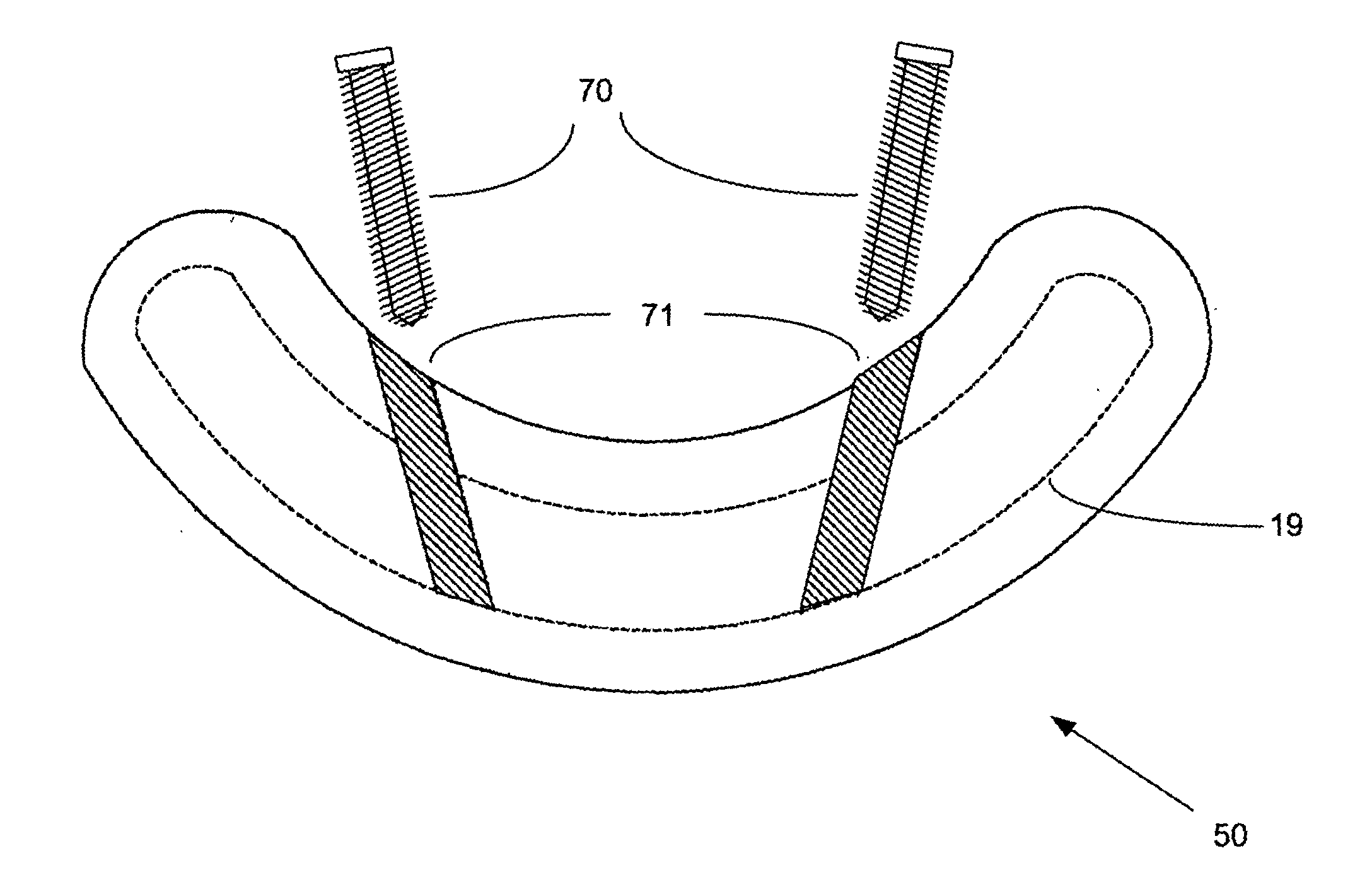
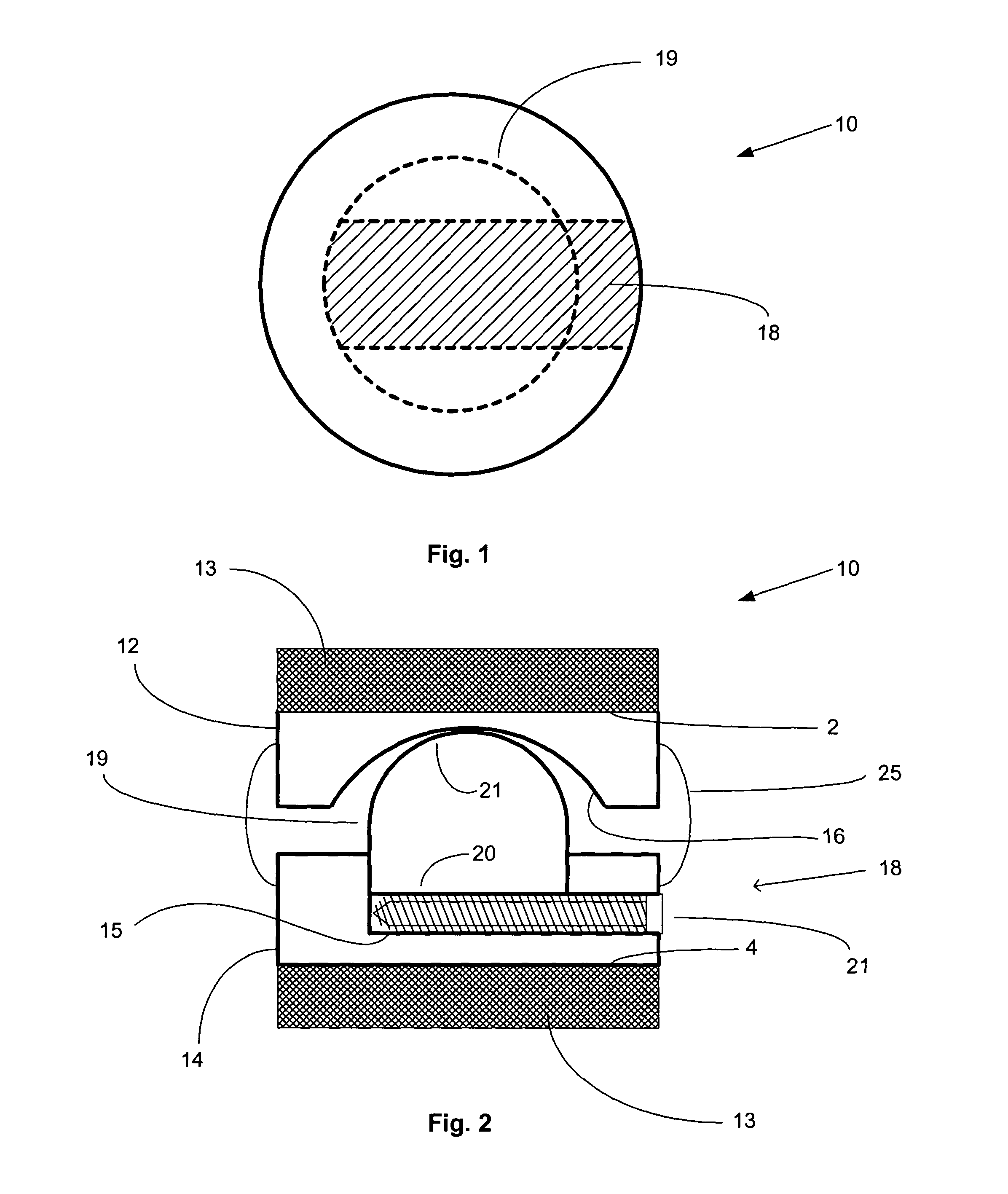
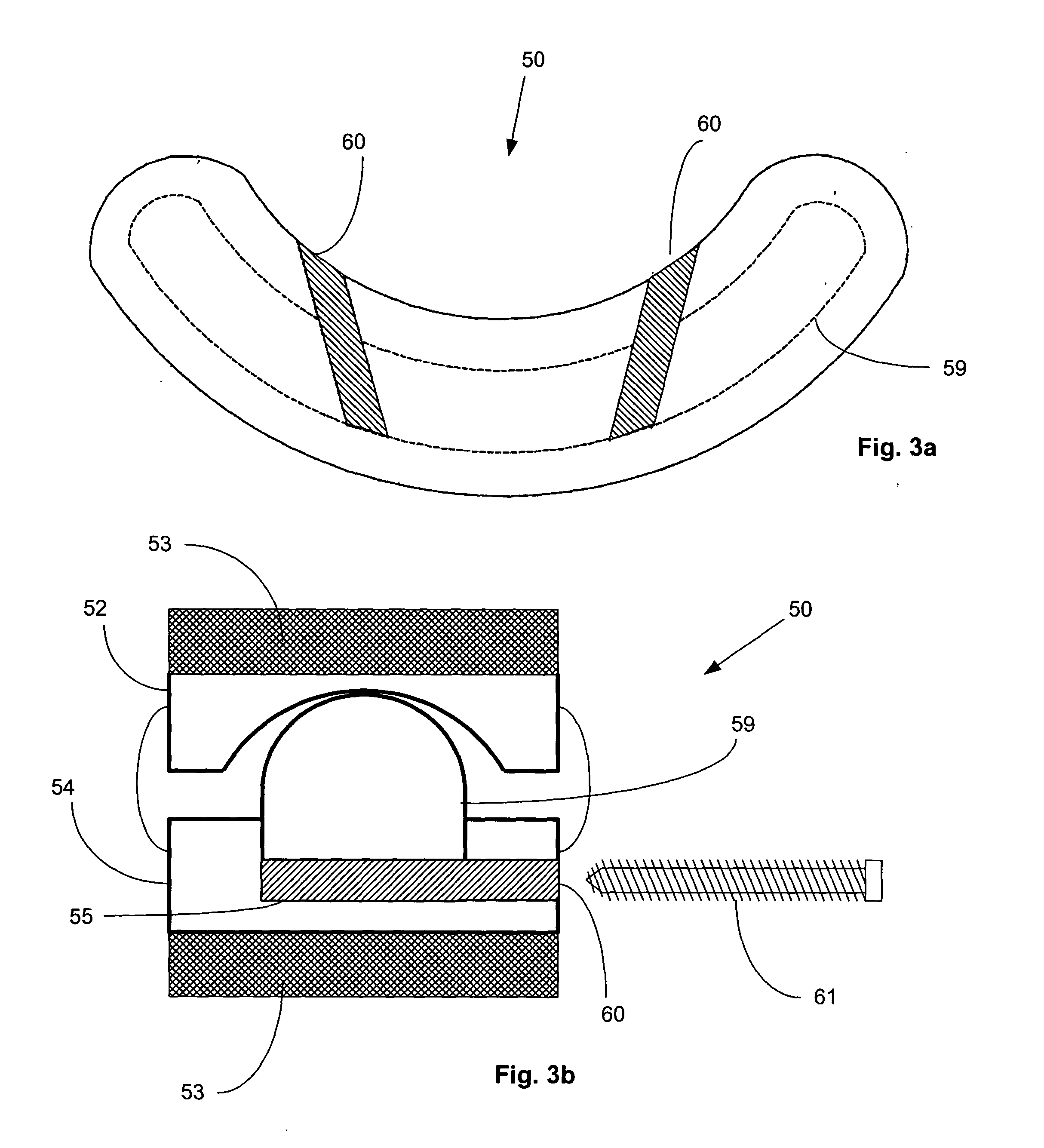
The present invention disclosed a surgical staple (50) and staple pocket (57) for forming kidney-shaped staple therefor, with the staple pocket (57) being recessed inward on the tissue contacting surface of the staple anvil (58), and with M-shaped staple (50) being used, wherein the degree of projection of the middle portion of the back span (51) of the M-shaped staple (50) arranged at the inner circle or arranged at a proximal side of a cutter slot (121) is larger than that of the back span (51) of the M-shaped staple (50) arranged at the outer circle or arranged at a distal side of the cutter slot (121), so that when the staple (50) of the same height are adapted to staple tissues to be stapled of different thickness, staple driver (68) bends the M-shaped staple (50) on the staple pocket (57) to form kidney-shaped staple (50) so as to achieve the operational effect of being able to seal the stapled tissue, to stop bleeding and to heal the tissue therearound.
Owner:CHANGZHOU KANGDI MEDICAL STAPLER
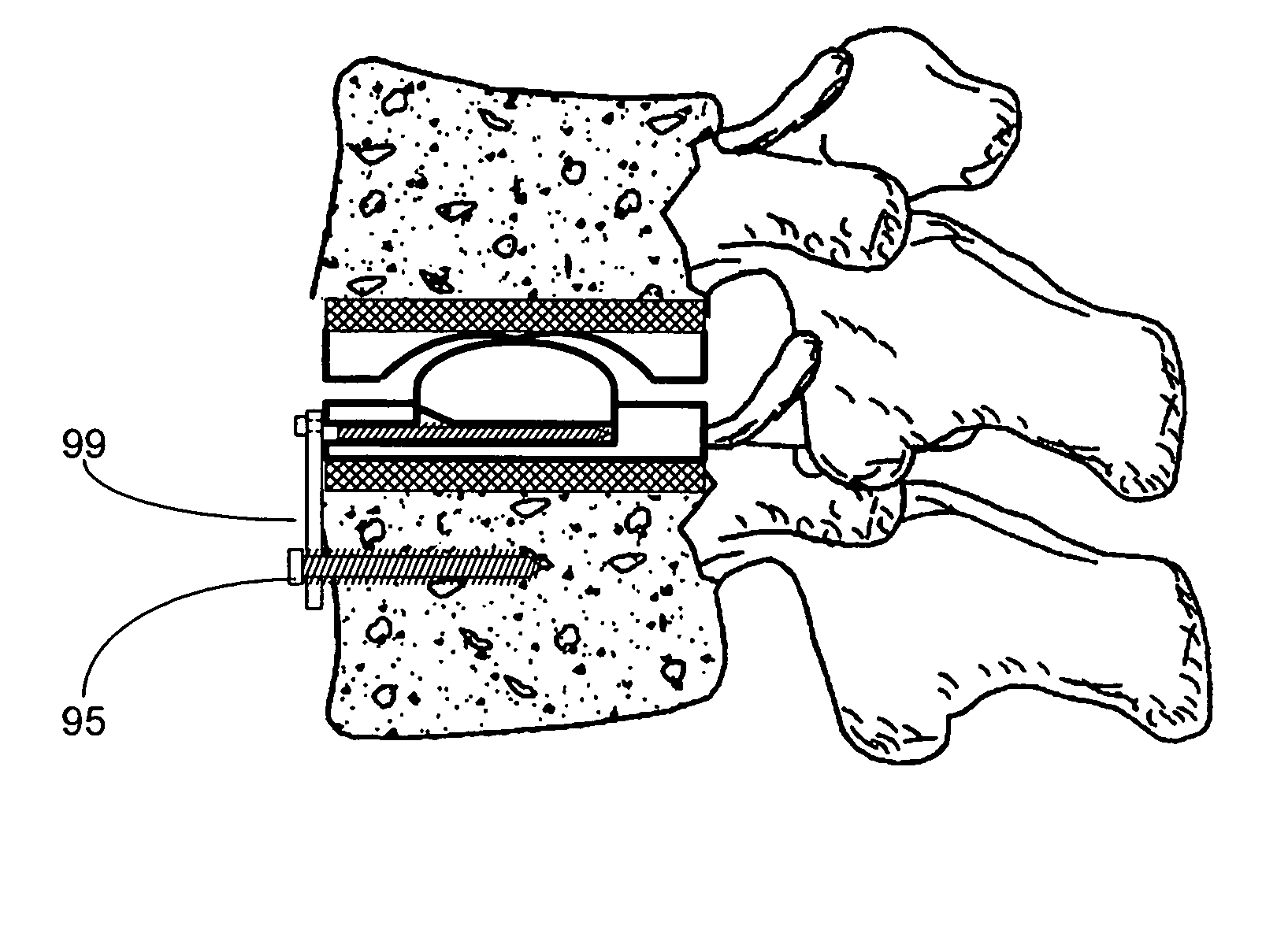
Artificial functional spinal unit assemblies
InactiveUS7316714B2Promote growthPromoting bony end growthInternal osteosythesisJoint implantsSurgical operationSurgical approach
An artificial functional spinal unit is provided comprising, generally, an expandable artificial intervertebral implant that can be placed via a posterior surgical approach and used in conjunction with one or more artificial facet joints to provide an anatomically correct range of motion. Expandable artificial intervertebral implants in both lordotic and non-lordotic designs are disclosed, as well as lordotic and non-lordotic expandable cages for both PLIF (posterior lumber interbody fusion) and TLIF (transforaminal lumbar interbody fusion) procedures. The expandable implants may have various shapes, such as round, square, rectangular, banana-shaped, kidney-shaped, or other similar shapes. By virtue of their posteriorly implanted approach, the disclosed artificial FSU's allow for posterior decompression of the neural elements, reconstruction of all or part of the natural functional spinal unit, restoration and maintenance of lordosis, maintenance of motion, and restoration and maintenance of disc space height.
Owner:FLEXUSPINE INC
Artificial spinal unit assemblies
ActiveUS20050033432A1Prohibit some movementPrevent movementInternal osteosythesisJoint implantsSpinal columnSurgical approach
An artificial functional spinal unit is provided comprising, generally, an expandable artificial intervertebral implant that can be placed via a posterior surgical approach and used in conjunction with one or more artificial facet joints to provide an anatomically correct range of motion. Expandable artificial intervertebral implants in both lordotic and non-lordotic designs are disclosed, as well as lordotic and non-lordotic expandable cages for both PLIF (posterior lumber interbody fusion) and TLIF (transforaminal lumbar interbody fusion) procedures. The expandable implants may have various shapes, such as round, square, rectangular, banana-shaped, kidney-shaped, or other similar shapes. By virtue of their posteriorly implanted approach, the disclosed artificial FSU's allow for posterior decompression of the neural elements, reconstruction of all or part of the natural functional spinal unit, restoration and maintenance of lordosis, maintenance of motion, and restoration and maintenance of disc space height.
Owner:FLEXUSPINE
Artificial functional spinal unit assemblies
ActiveUS20050033439A1Promote growthPromoting bony end growthInternal osteosythesisJoint implantsSurgical approachFunctional spinal unit
An artificial functional spinal unit is provided comprising, generally, an expandable artificial intervertebral implant that can be placed via a posterior surgical approach and used in conjunction with one or more artificial facet joints to provide an anatomically correct range of motion. Expandable artificial intervertebral implants in both lordotic and non-lordotic designs are disclosed, as well as lordotic and non-lordotic expandable cages for both PLIF (posterior lumber interbody fusion) and TLIF (transforaminal lumbar interbody fusion) procedures. The expandable implants may have various shapes, such as round, square, rectangular, banana-shaped, kidney-shaped, or other similar shapes. By virtue of their posteriorly implanted approach, the disclosed artificial FSU's allow for posterior decompression of the neural elements, reconstruction of all or part of the natural functional spinal unit, restoration and maintenance of lordosis, maintenance of motion, and restoration and maintenance of disc space height.
Owner:FLEXUSPINE INC
Artificial functional spinal unit assemblies
InactiveUS20050033431A1Promote growthPromoting bony end growthInternal osteosythesisJoint implantsSurgical approachFunctional spinal unit
An artificial functional spinal unit is provided comprising, generally, an expandable artificial intervertebral implant that can be placed via a posterior surgical approach and used in conjunction with one or more artificial facet joints to provide an anatomically correct range of motion. Expandable artificial intervertebral implants in both lordotic and non-lordotic designs are disclosed, as well as lordotic and non-lordotic expandable cages for both PLIF (posterior lumber interbody fusion) and TLIF (transforaminal lumbar interbody fusion) procedures. The expandable implants may have various shapes, such as round, square, rectangular, banana-shaped, kidney-shaped, or other similar shapes. By virtue of their posteriorly implanted approach, the disclosed artificial FSU's allow for posterior decompression of the neural elements, reconstruction of all or part of the natural functional spinal unit, restoration and maintenance of lordosis, maintenance of motion, and restoration and maintenance of disc space height.
Owner:FLEXUSPINE INC
Artificial functional spinal unit assemblies
InactiveUS20060195192A1Promote growthPromoting bony end growthInternal osteosythesisJoint implantsSurgical approachFunctional spinal unit
An artificial functional spinal unit is provided comprising, generally, an expandable artificial intervertebral implant that can be placed via a posterior surgical approach and used in conjunction with one or more artificial facet joints to provide an anatomically correct range of motion. Expandable artificial intervertebral implants in both lordotic and non-lordotic designs are disclosed, as well as lordotic and non-lordotic expandable cages for both PLIF (posterior lumber interbody fusion) and TLIF (transforaminal lumbar interbody fusion) procedures. The expandable implants may have various shapes, such as round, square, rectangular, banana-shaped, kidney-shaped, or other similar shapes. By virtue of their posteriorly implanted approach, the disclosed artificial FSU's allow for posterior decompression of the neural elements, reconstruction of all or part of the natural functional spinal unit, restoration and maintenance of lordosis, maintenance of motion, and restoration and maintenance of disc space height.
Owner:FLEXUSPINE INC
Artificial spinal unit assemblies
ActiveUS7909869B2Promote growthPromoting bony end growthInternal osteosythesisJoint implantsSpinal columnSurgical operation
An artificial functional spinal unit is provided comprising, generally, an expandable artificial intervertebral implant that can be placed via a posterior surgical approach and used in conjunction with one or more artificial facet joints to provide an anatomically correct range of motion. Expandable artificial intervertebral implants in both lordotic and non-lordotic designs are disclosed, as well as lordotic and non-lordotic expandable cages for both PLIF (posterior lumber interbody fusion) and TLIF (transforaminal lumbar interbody fusion) procedures. The expandable implants may have various shapes, such as round, square, rectangular, banana-shaped, kidney-shaped, or other similar shapes. By virtue of their posteriorly implanted approach, the disclosed artificial FSU's allow for posterior decompression of the neural elements, reconstruction of all or part of the natural functional spinal unit, restoration and maintenance of lordosis, maintenance of motion, and restoration and maintenance of disc space height.
Owner:FLEXUSPINE INC
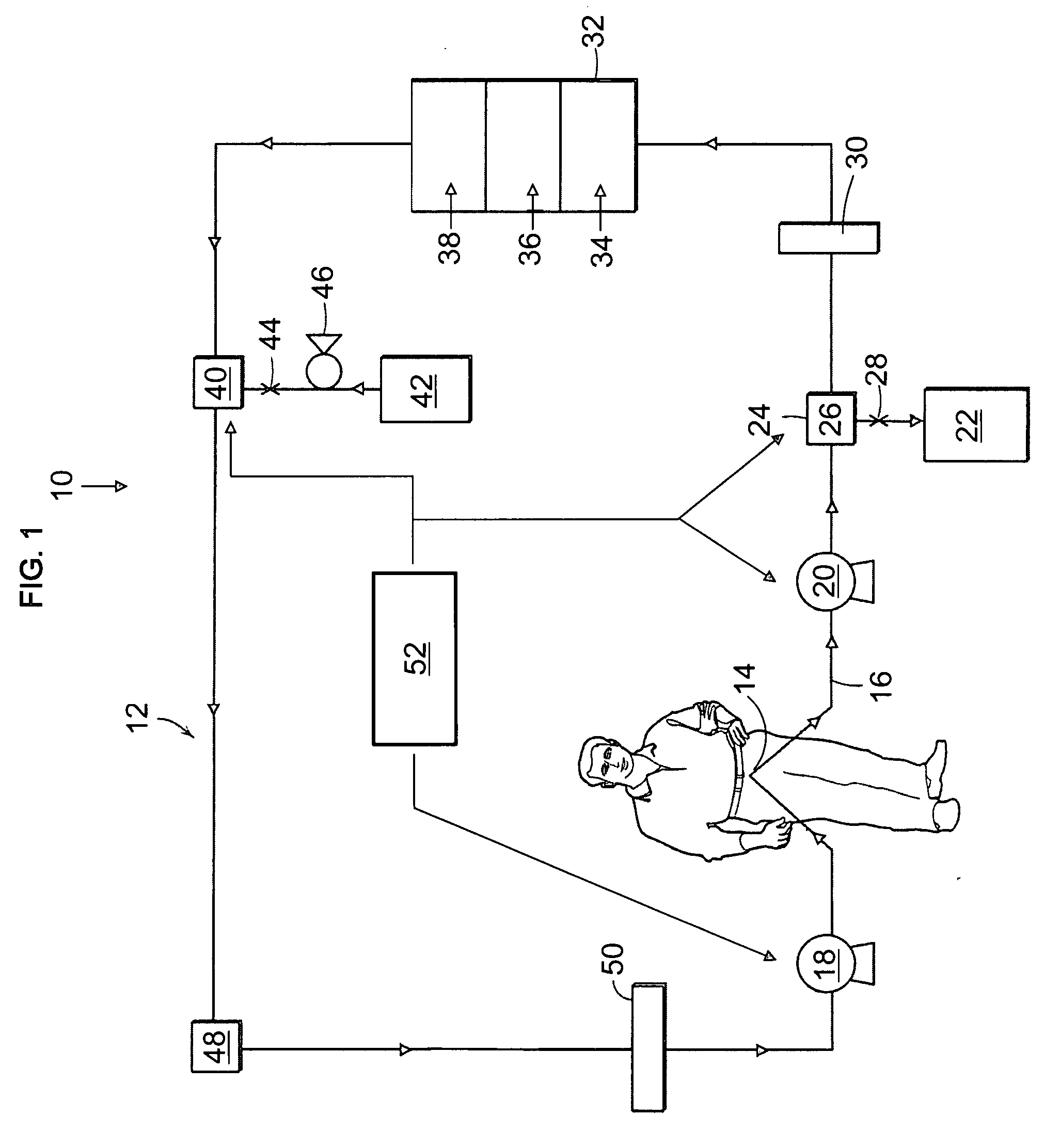
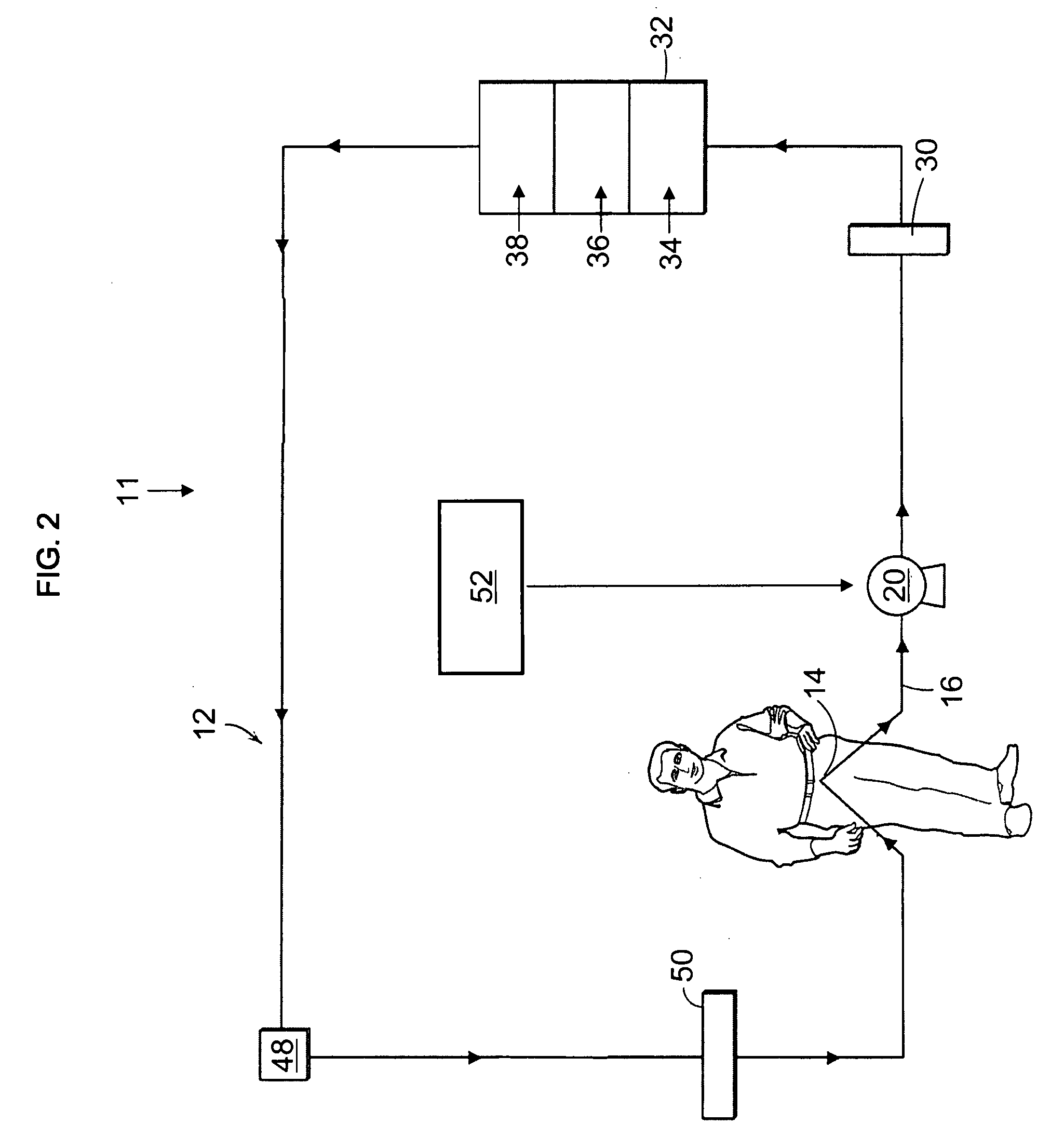
Cardiac rhythm management device and sensor-suite for the optimal control of ultrafiltration and renal replacement therapies
ActiveUS20070175827A1ElectrotherapyMechanical/radiation/invasive therapiesUltrafiltrationOptimal control
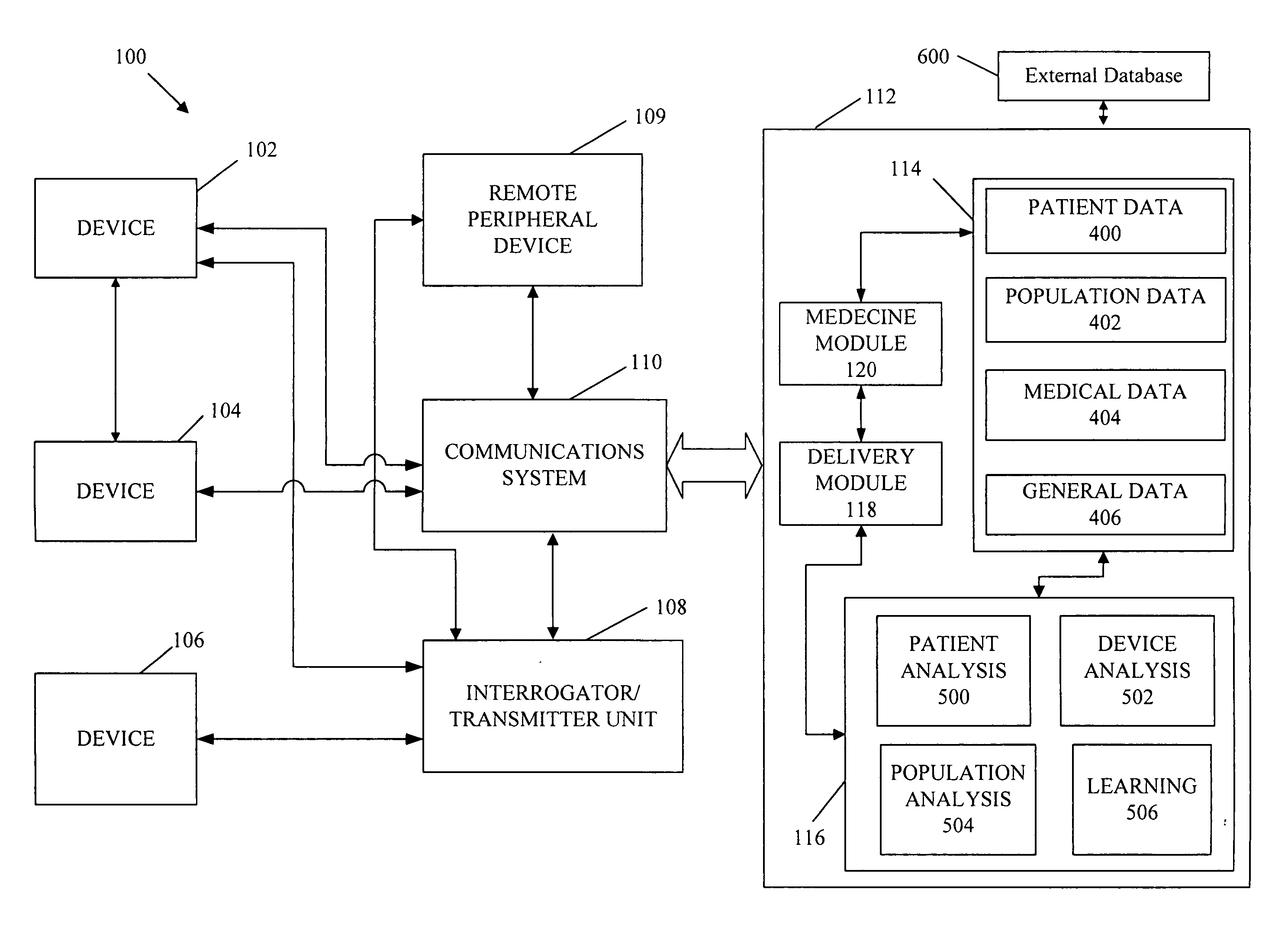
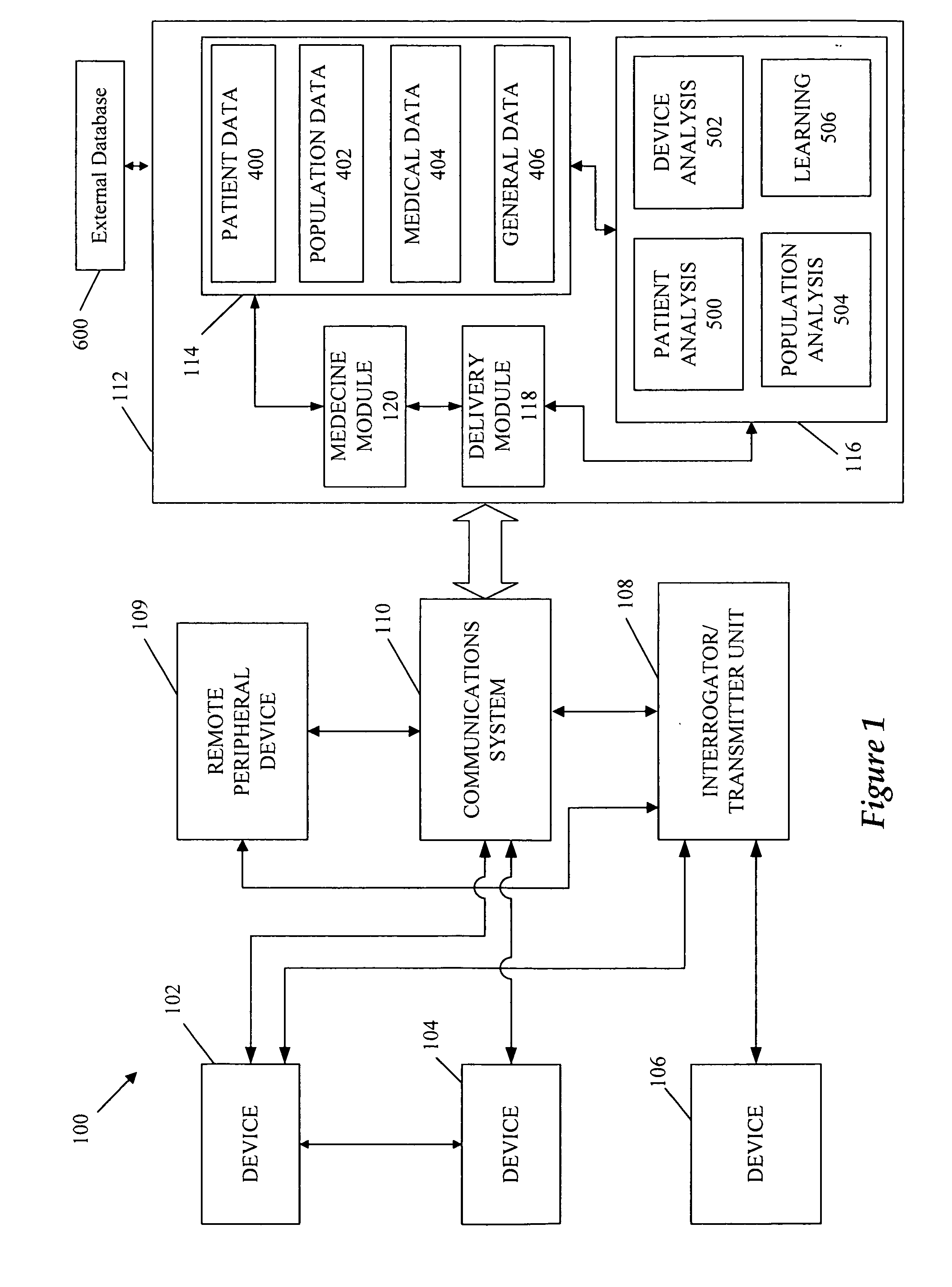
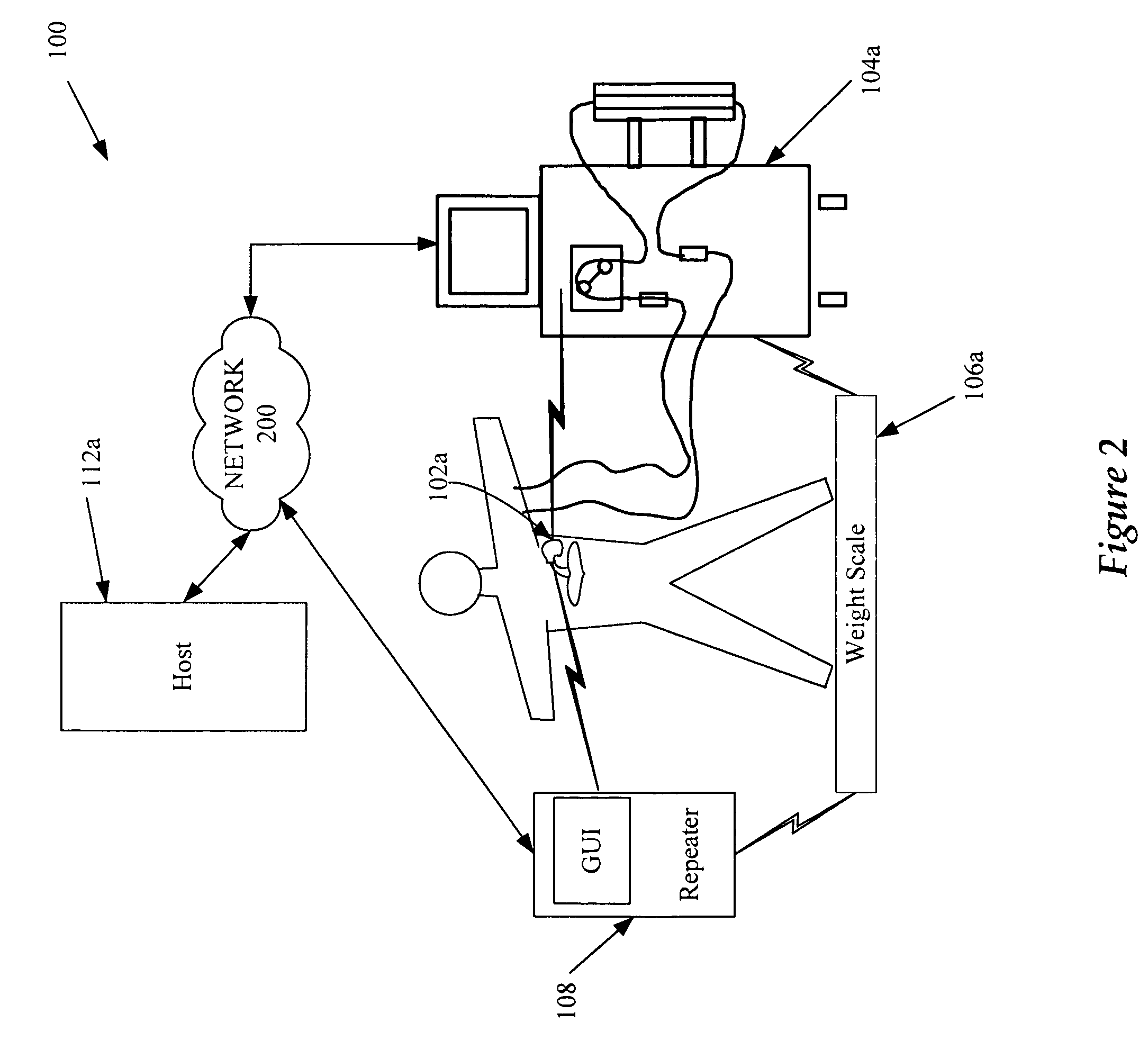
A cardiorenal patient monitoring system comprising, either implanted or non-implanted device(s), remote peripheral device(s), computer network(s), host, and communication means between the device(s), computer network(s), and host. The preferred embodiment shows an advanced patient monitoring system for using an implanted cardiac device and a dialysis machine in renal therapy. In addition, the method of advanced patient monitoring is in conjunction with the advanced patient monitoring system is disclosed.
Owner:CARDIAC PACEMAKERS INC
Methods and compositions for treating a renal disease condition in a subject
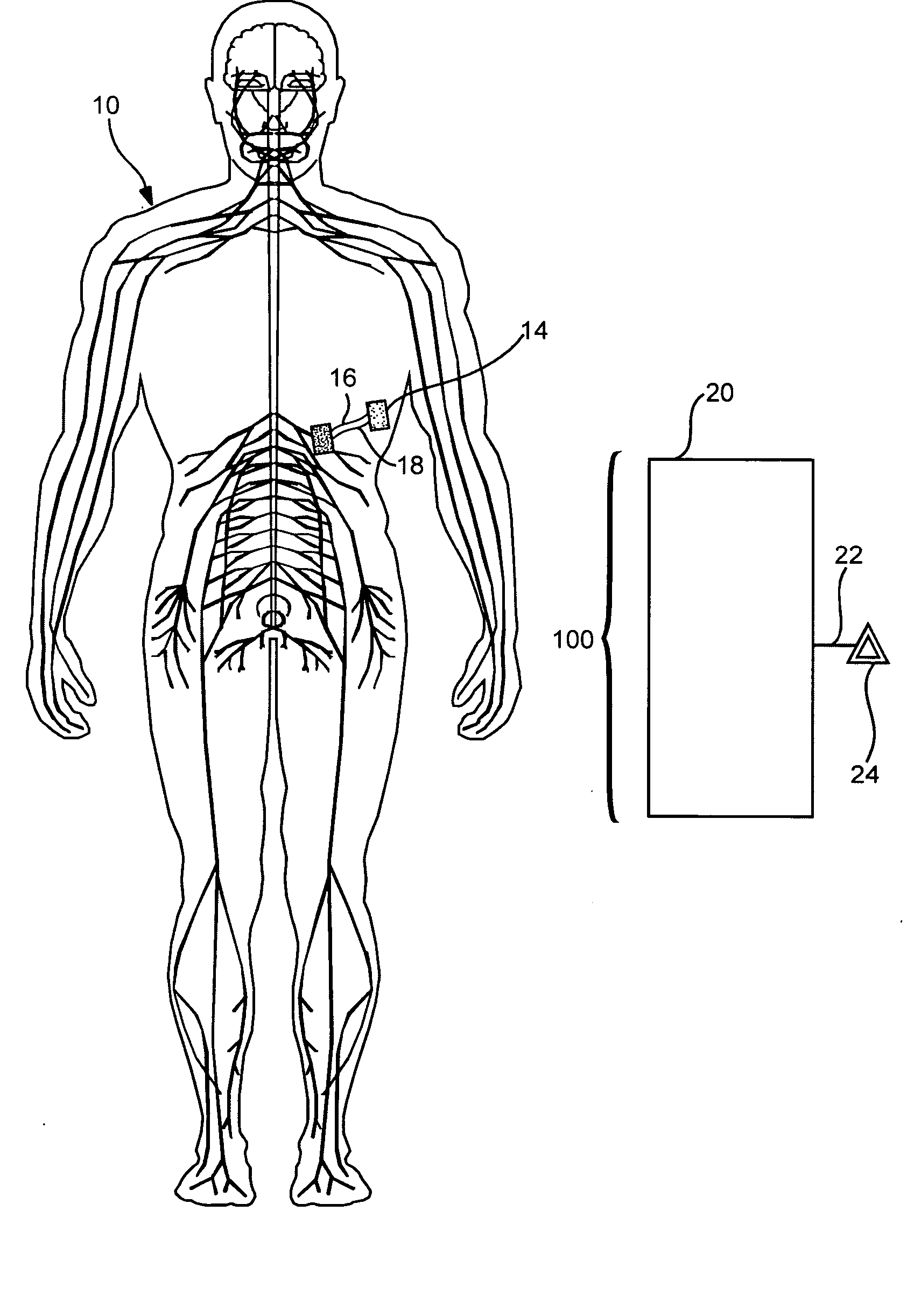
Methods for treating a renal associated disease condition in a subject are provided. The subject methods are characterized by modulating at least one portion of the subject's autonomic nervous system in a manner effective to treat a renal condition in the subject. Specifically, the methods may include modulating, e.g., increasing, a parasympathetic / sympathetic activity ratio in the subject. Also provided are compositions, kits and systems for practicing the subject methods.
Owner:PALO ALTO INVESTORS
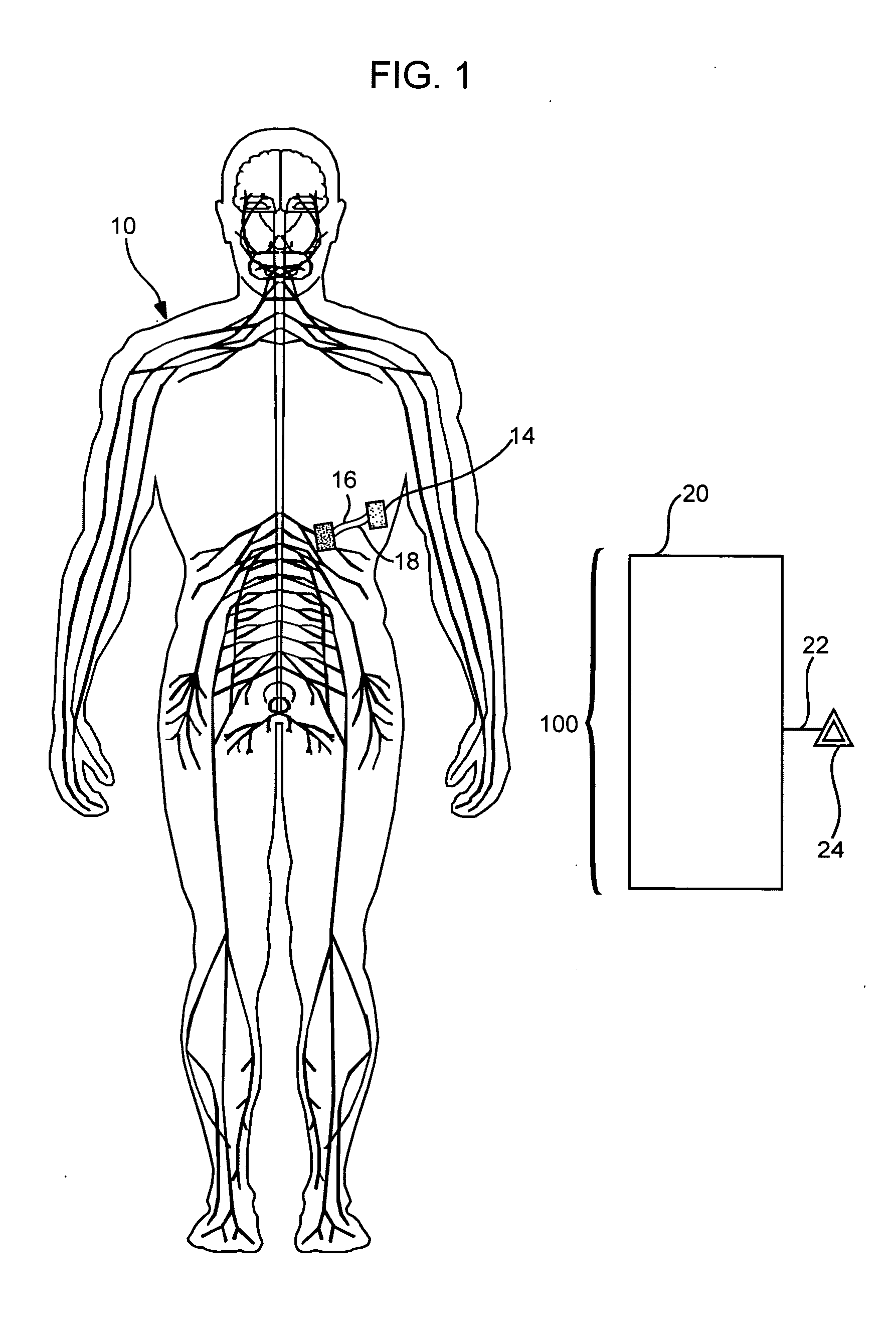
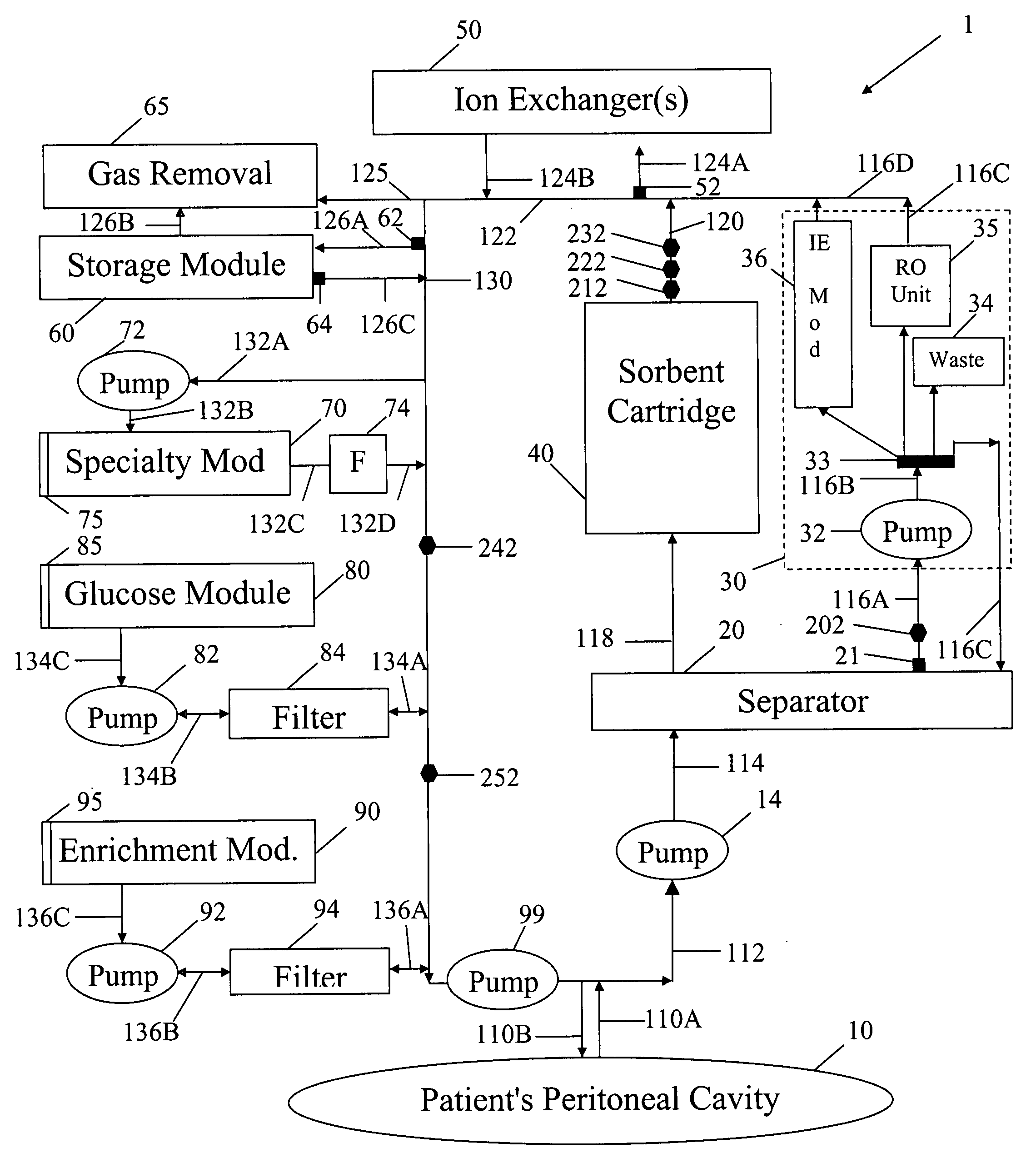
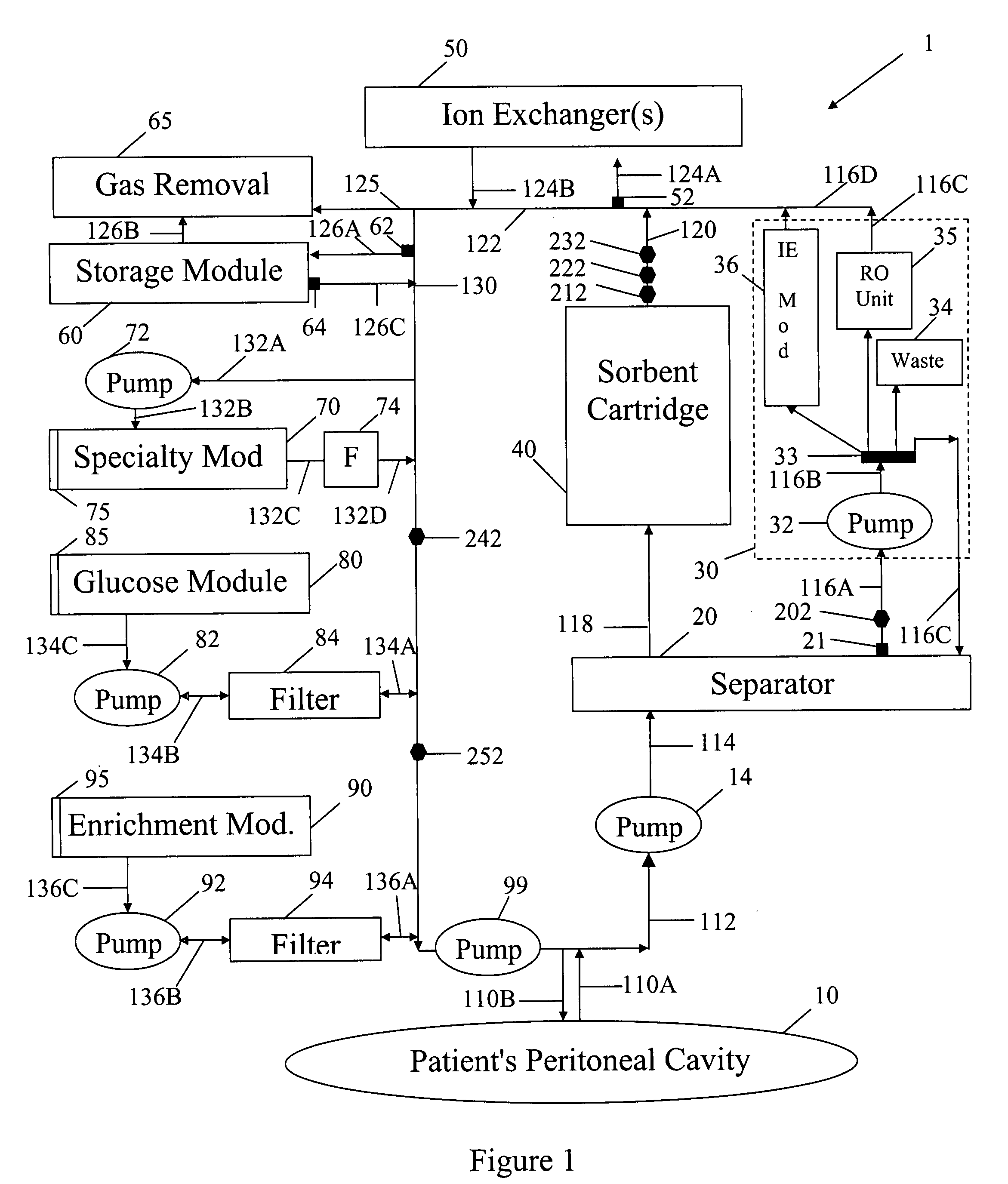
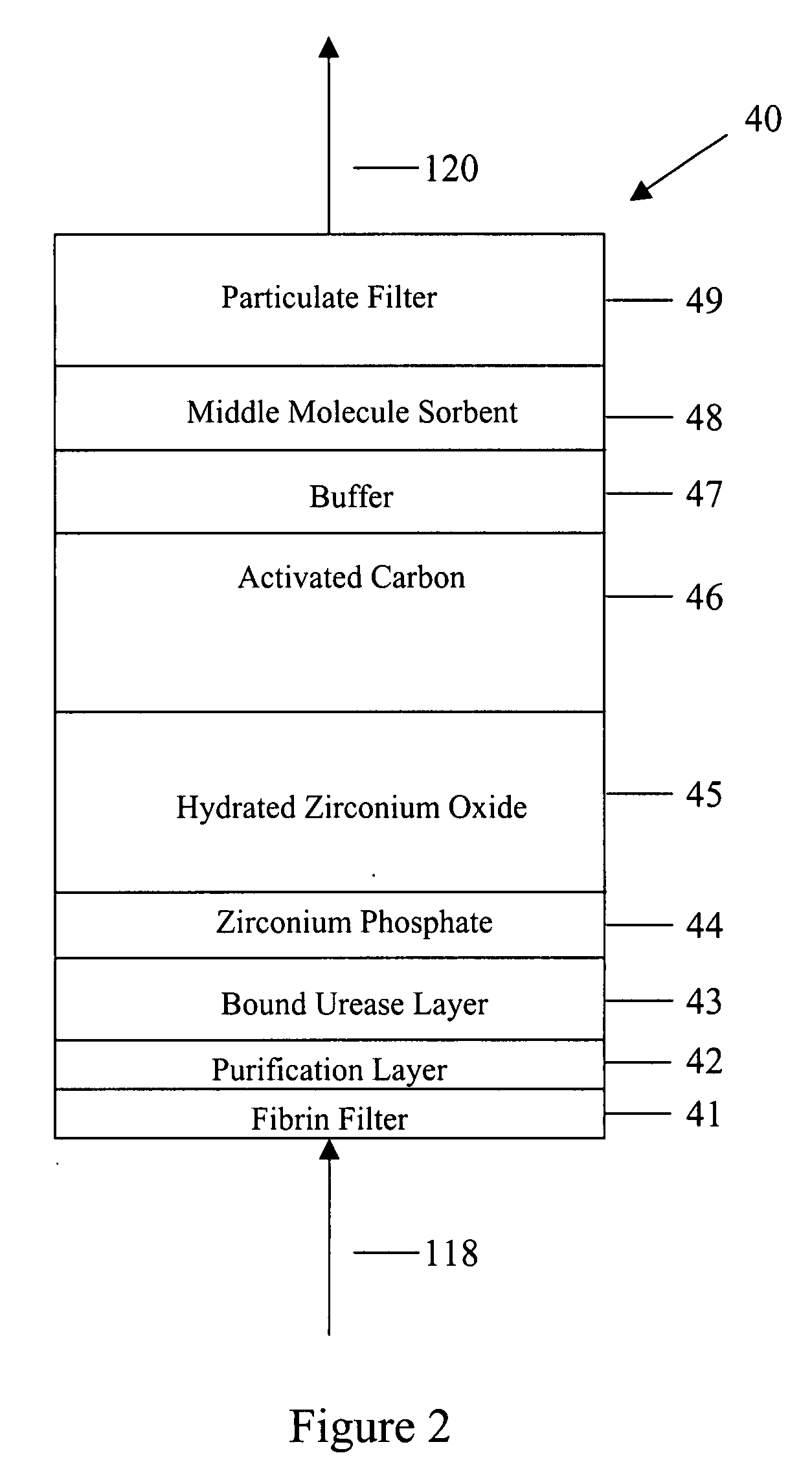
Peritoneal dialysis methods and apparatus
ActiveUS20070179431A1Increase percentageImprove wear resistancePeritoneal dialysisProtein compositionPeritoneal fluid
A peritoneal-based (“bloodless”) artificial kidney processes peritoneal fluid without need for additional fluids (“waterless”). Fluid is separated into a protein-rich stream and a protein-free stream. The protein-rich stream is regenerated using a sorbent assembly, and its protein composition can be modified by removal of selected protein(s) (“dialysate-pheresis”). It is then reconstituted with additives and returned into the peritoneal cavity, thereby reducing protein-loss and providing oncotic-pressure for ultrafiltration. The protein-free stream is used to produce free water, and an alkaline or acid fluid for optimization of the composition of the regenerated stream. The unused protein-free stream can be used to “reverse flush” the separator to maintain its patency and the excess discarded for fluid-balance regulation. Compared to prior art, immobilization of urease allows more protein rich fluid to be regenerated and re-circulated into the peritoneal cavity for toxin removal and allows practicable development of portable and wearable artificial kidneys.
Owner:RGT UNIV OF CALIFORNIA +1
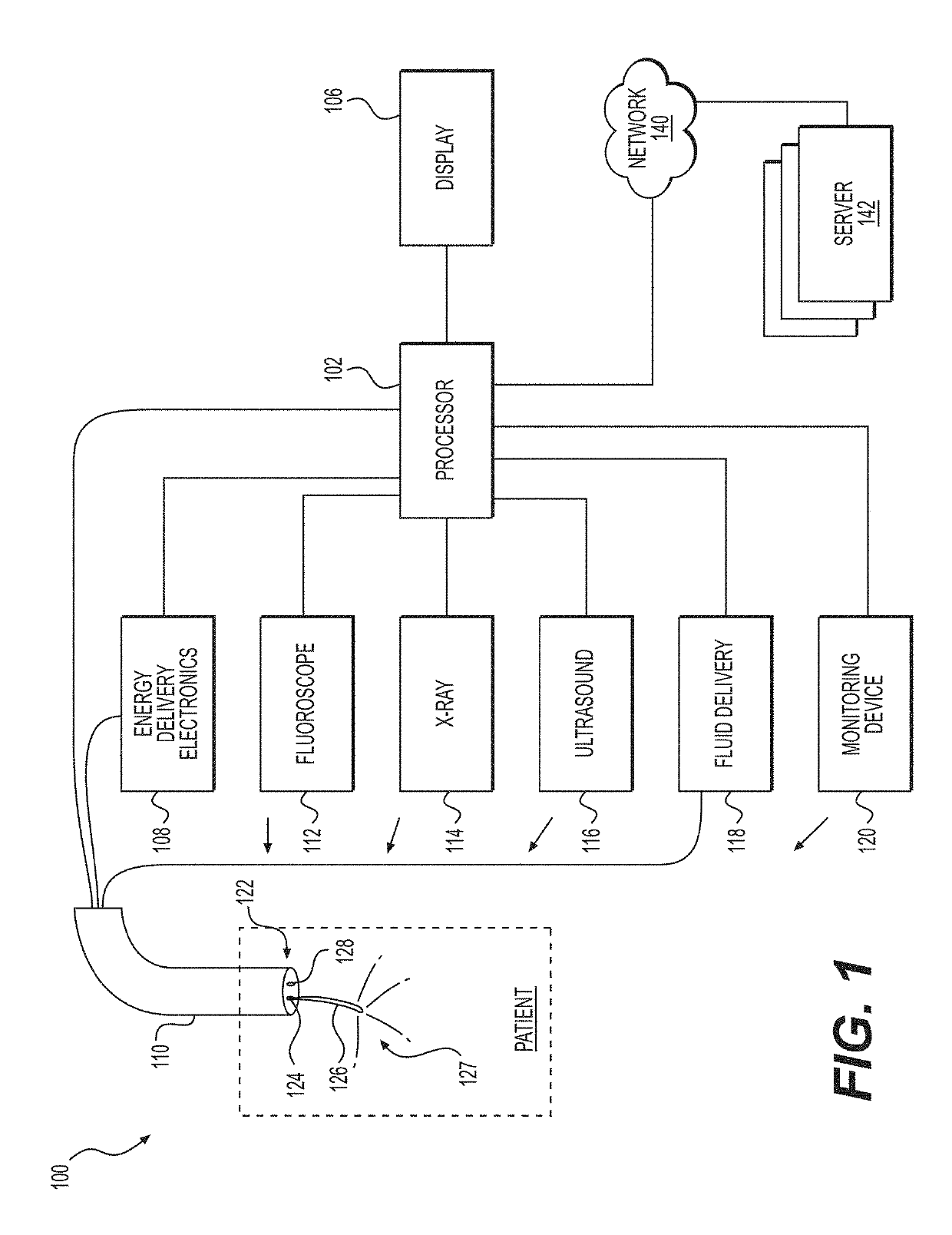
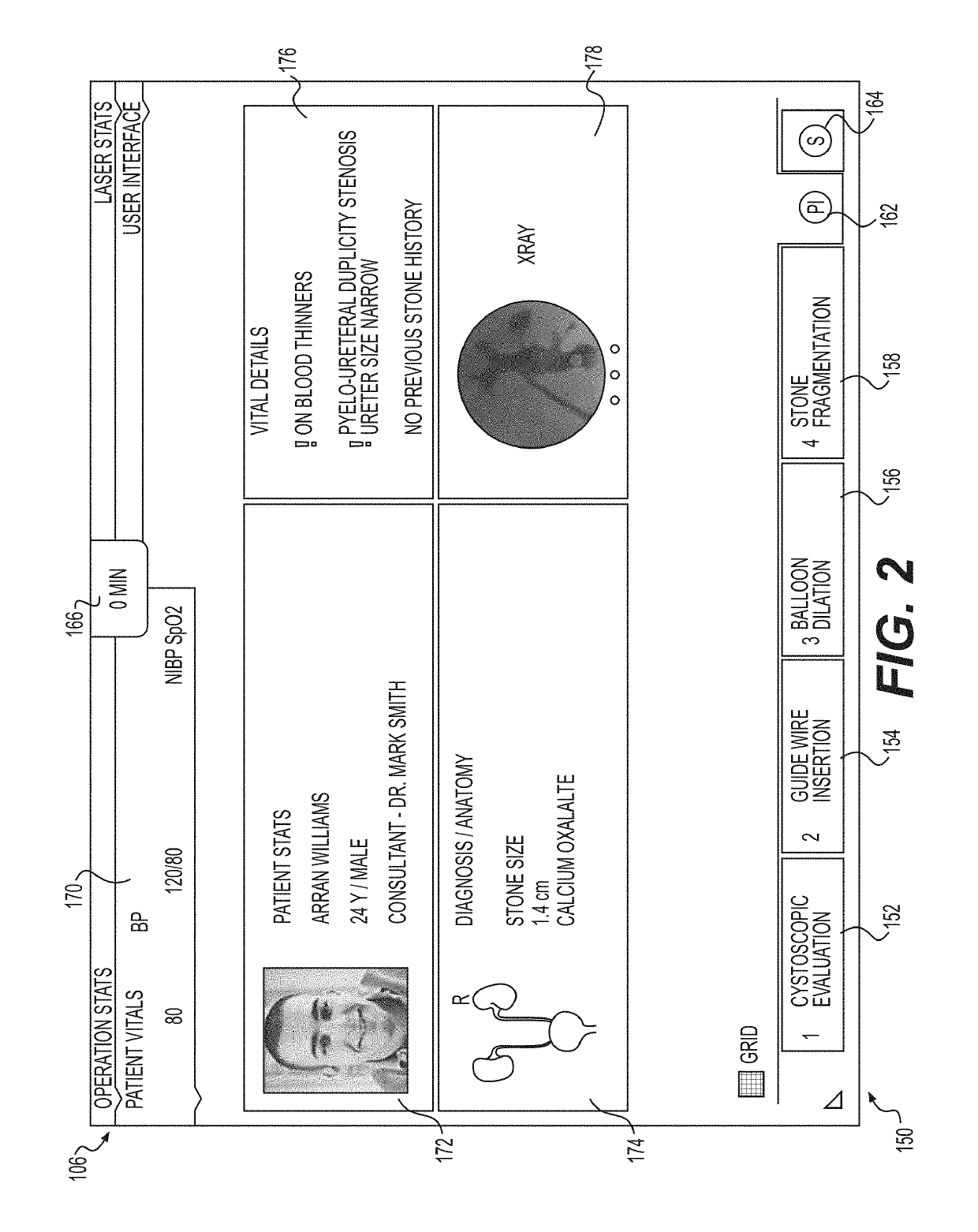
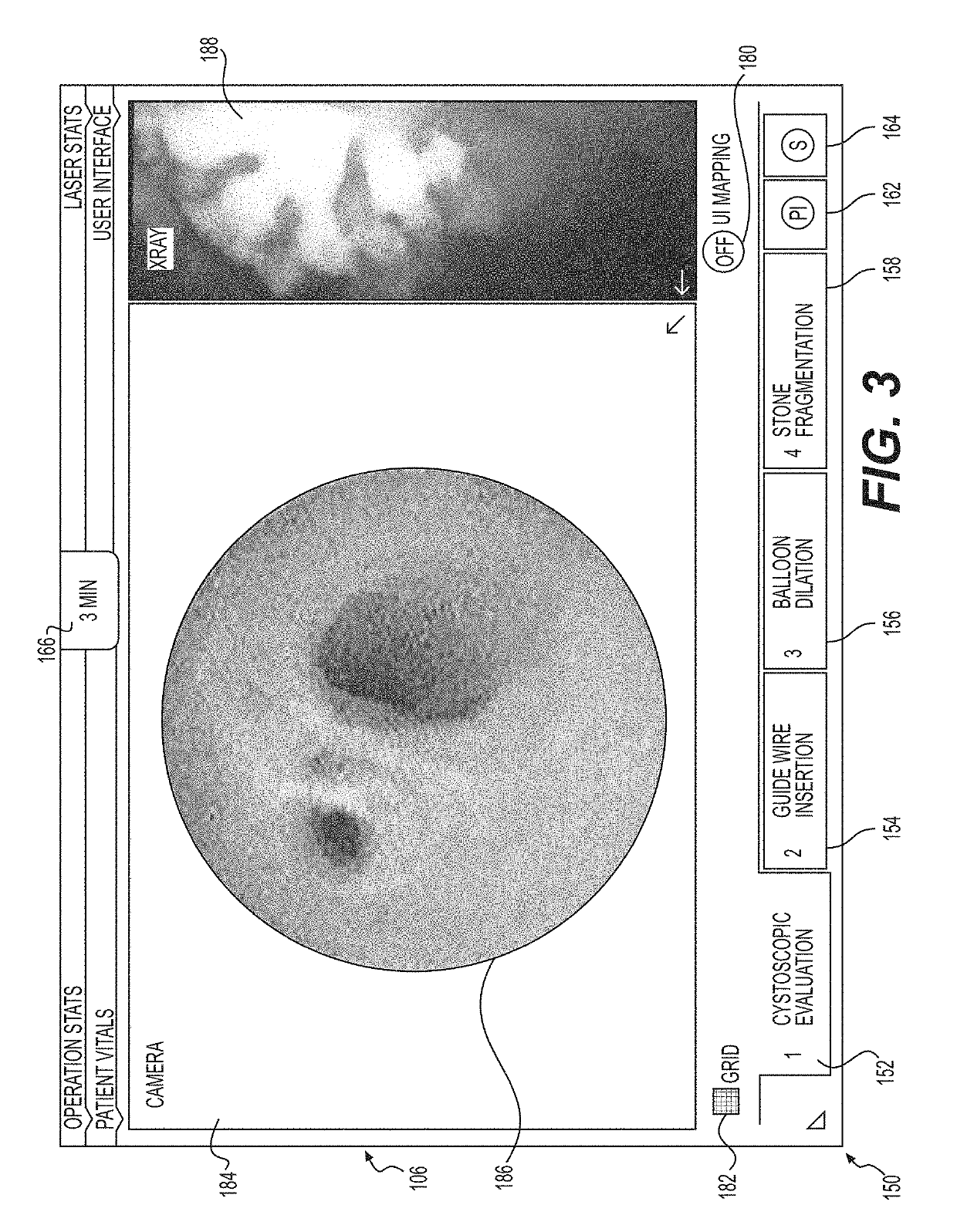
Medical user interfaces and related methods of use
A medical system for use in a lithotripsy procedure may include a processor configured to receive input from a first imaging device, wherein the first imaging device may be configured to send image data representative of an image captured in a lumen of a kidney, bladder, or ureter to the processor. The processor may be configured to display the image on a display device coupled to the processor, and analyze the image to sense the presence of an object within the image. If an object was sensed within the image, the processor may analyze the image to estimate a size of the object, and display the estimate on the display device.
Owner:BOSTON SCI MEDICAL DEVICE LTD
Reduction of porcine circovirus-2 viral load with inactivated PCV-2
InactiveUS6517843B1Improving immunogenicityImprove performanceBiocideGenetic material ingredientsDiseaseStaining
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6th and 8th and / or the 10th and 13th weeks of gestation, can prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:QUEENS UNIV OF BELFAST +4
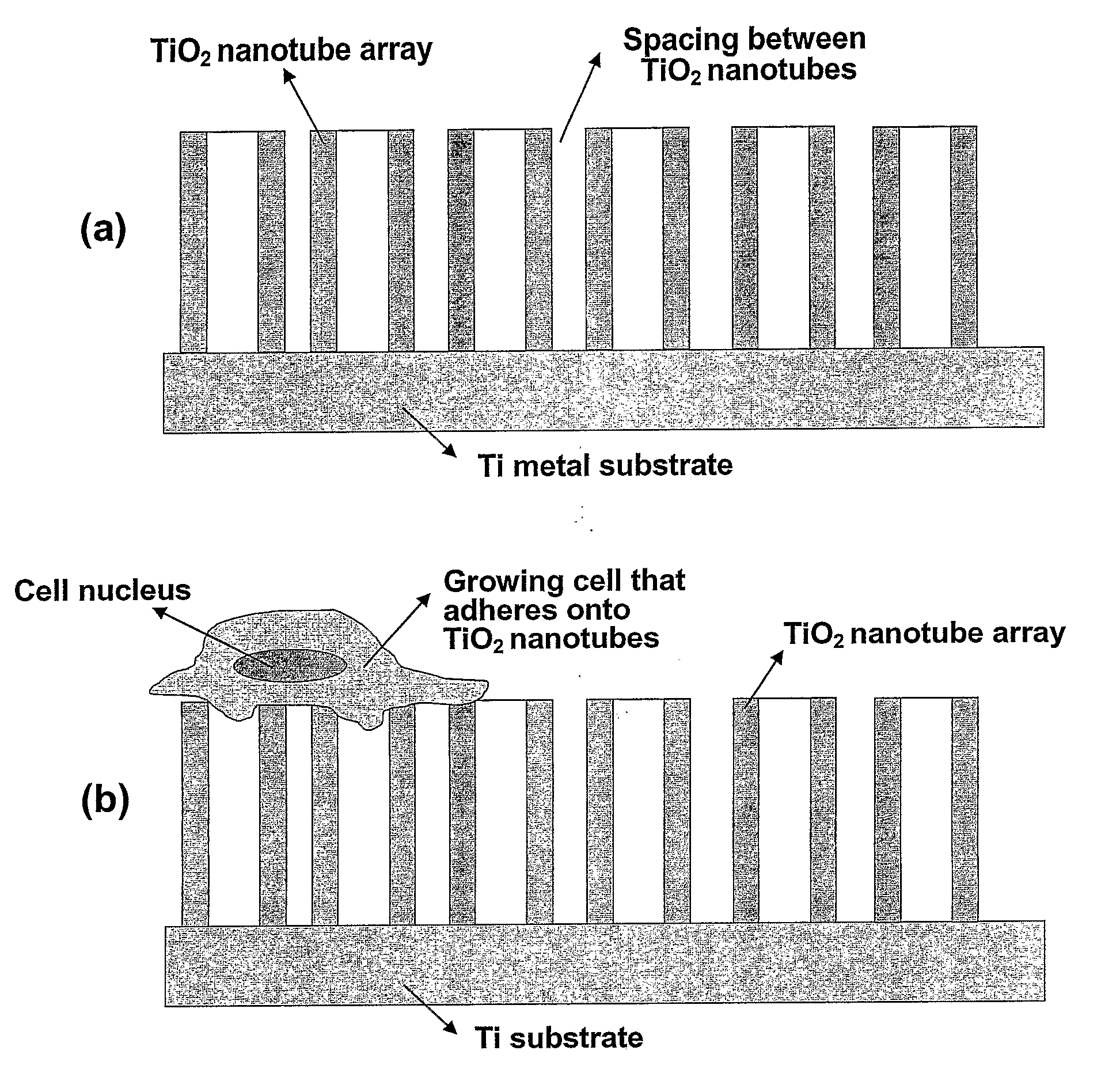
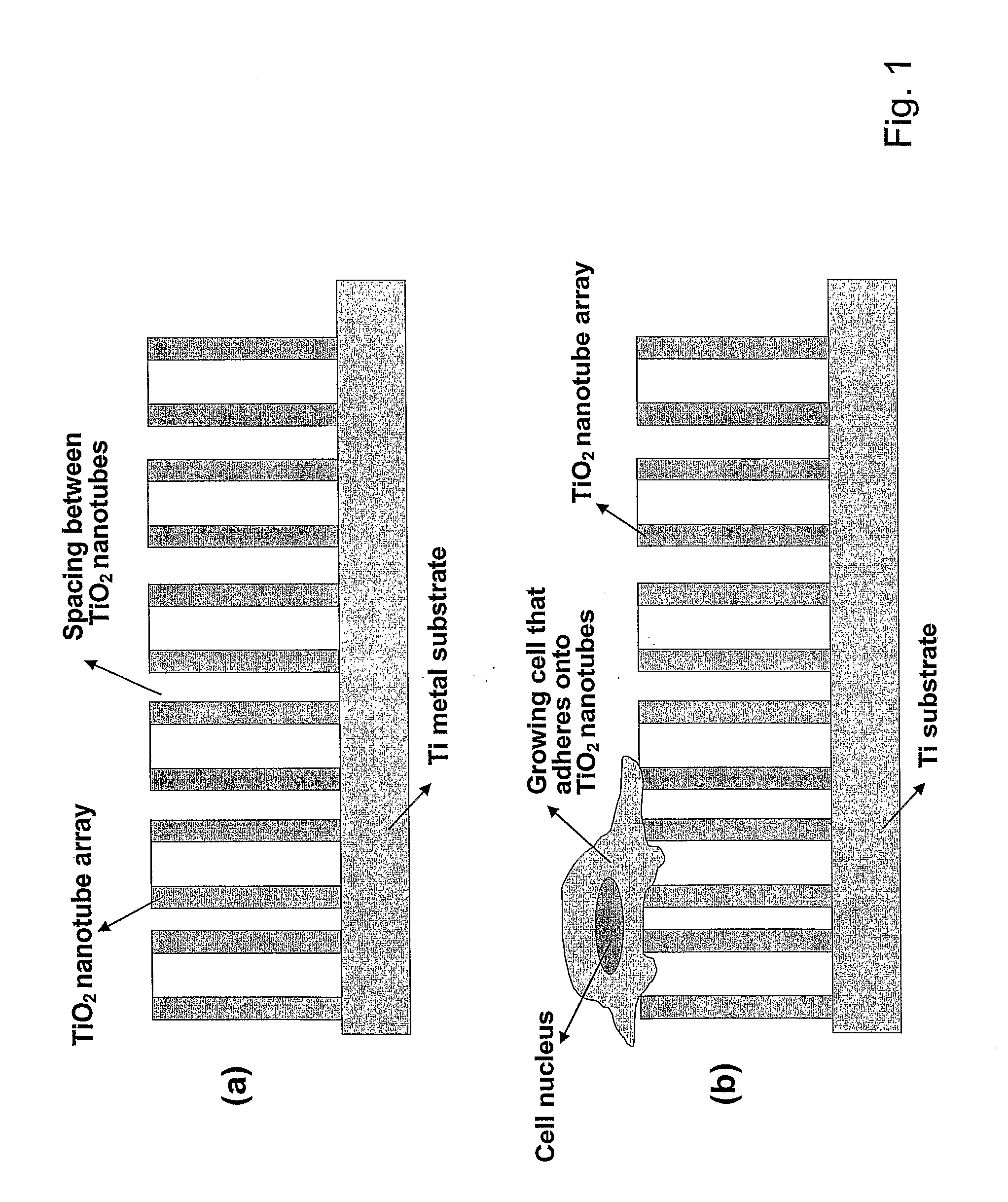
Compositions comprising nanostructures for cell, tissue and artificial organ growth, and methods for making and using same
ActiveUS20090220561A1Improve bone formationIncreased durabilityBioreactor/fermenter combinationsElectrolysis componentsIn vivoNanostructure
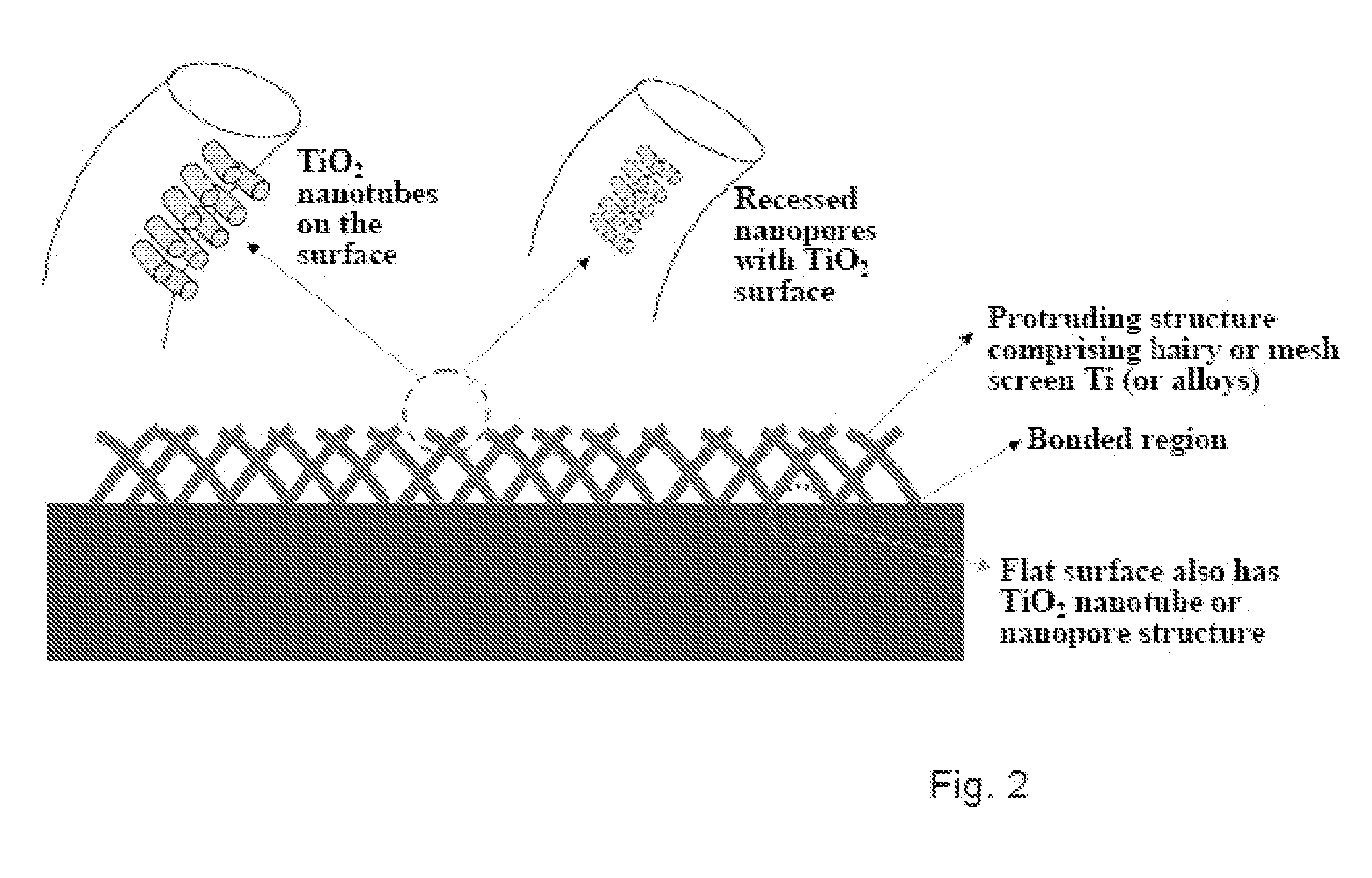
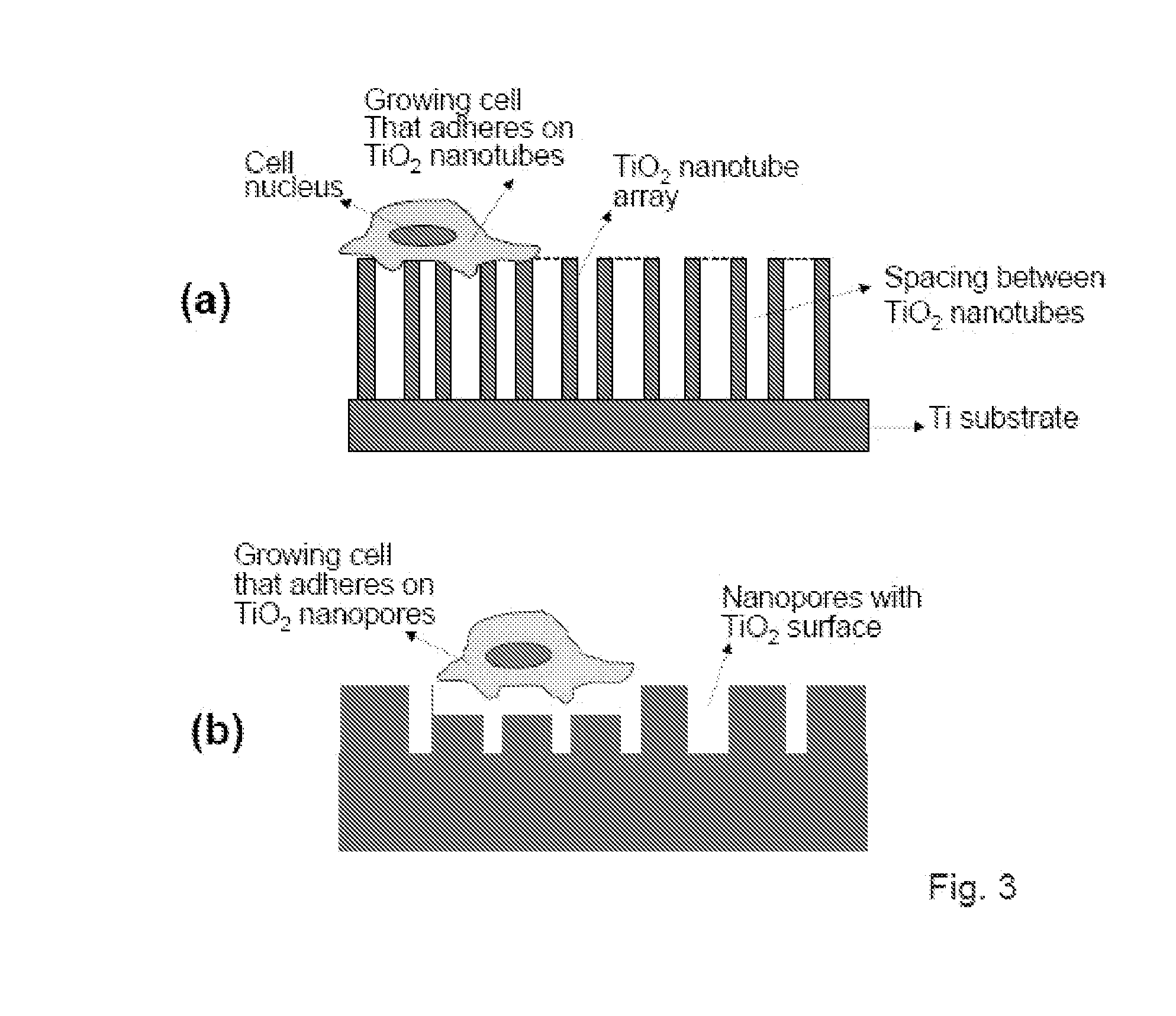
The invention provides articles of manufacture comprising biocompatible nanostructures comprising nanotubes and nanopores for, e.g., organ, tissue and / or cell growth, e.g., for bone, kidney or liver growth, and uses thereof, e.g., for in vitro testing, in vivo implants, including their use in making and using artificial organs, and related therapeutics. The invention provides lock-in nanostructures comprising a plurality of nanopores or nanotubes, wherein the nanopore or nanotube entrance has a smaller diameter or size than the rest (the interior) of the nanopore or nanotube. The invention also provides dual structured biomaterial comprising micro- or macro-pores and nanopores. The invention provides biomaterials having a surface comprising a plurality of enlarged diameter nanopores and / or nanotubes.
Owner:RGT UNIV OF CALIFORNIA
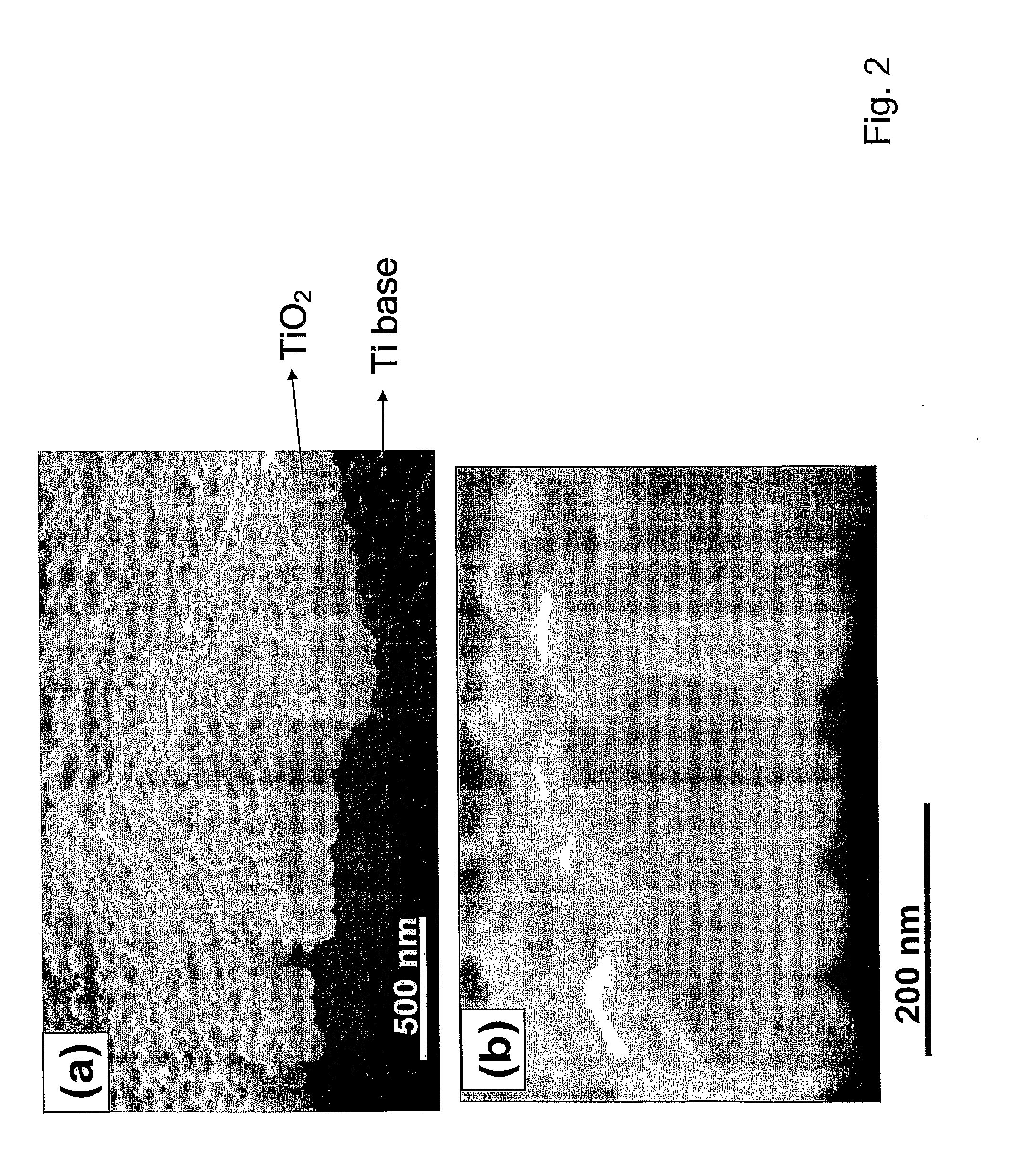
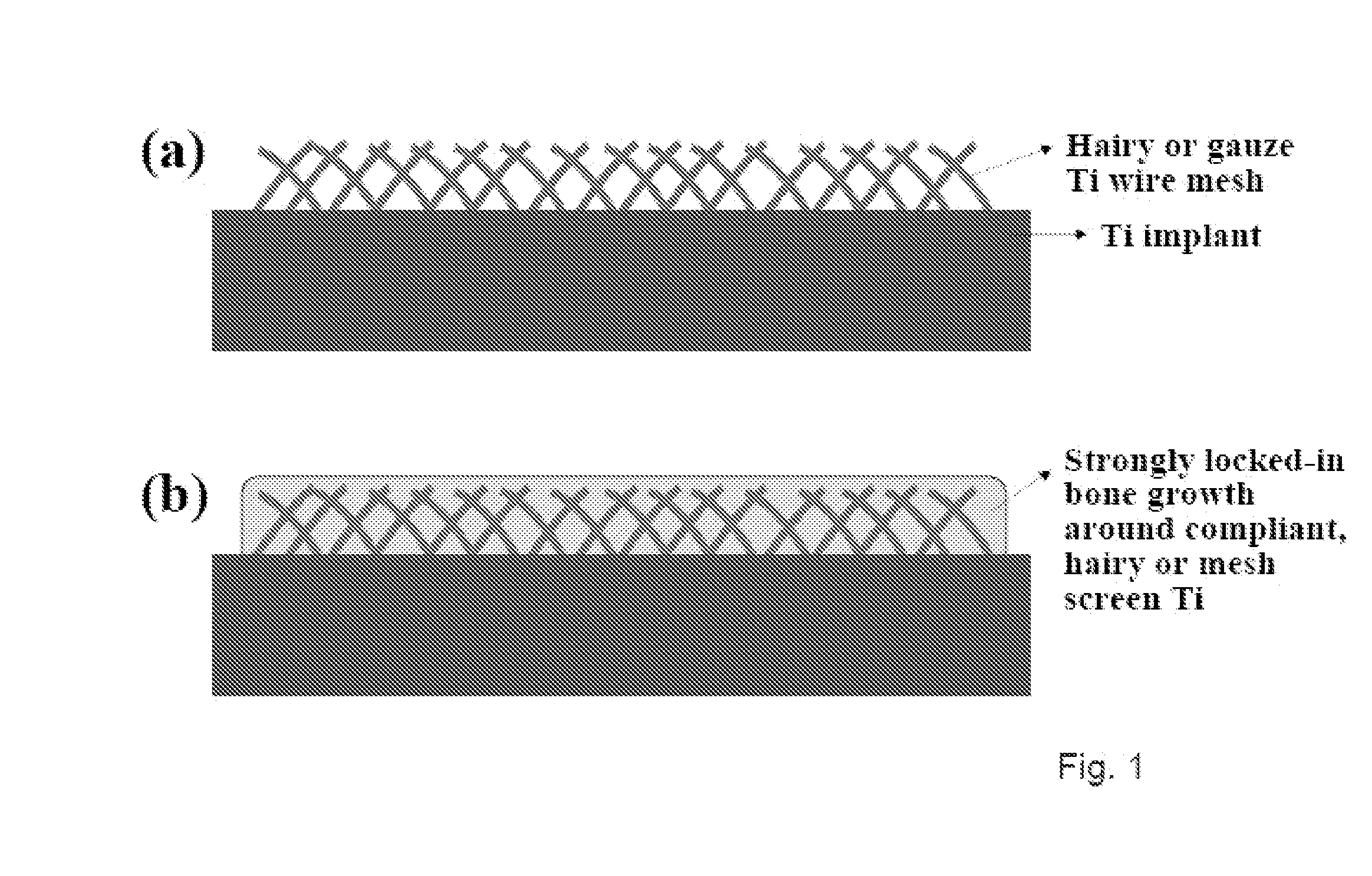
Articles comprising large-surface-area bio-compatible materials and methods for making and using them
ActiveUS20100303722A1Improve cell adhesionAccelerated cell growth characteristicImmobilised enzymesBioreactor/fermenter combinationsCell culture mediaBone growth
The present invention provides articles of manufacture comprising biocompatible nanostructures comprising significantly increased surface area for, e.g., organ, tissue and / or cell growth, e.g., for bone, tooth, kidney or liver growth, and uses thereof, e.g., for in vitro testing of drugs, chemicals or toxins, or as in vivo implants, including their use in making and using artificial tissues and organs, and related, diagnostic, screening, research and development and therapeutic uses, e.g., as drug delivery devices. The present invention provides biocompatible nanostructures with significantly increased surface area, such as with nanotube and nanopore array on the surface of metallic, ceramic, or polymer materials for enhanced cell and bone growth, for in vitro and in vivo testing, cleansing reaction, implants and therapeutics. The present invention provides optically transparent or translucent cell-culturing substrates. The present invention provides biocompatible and cell-growth-enhancing culture substrates comprising elastically compliant protruding nanostructure substrates coated with Ti, TiO2 or related metal and metal oxide films.
Owner:RGT UNIV OF CALIFORNIA
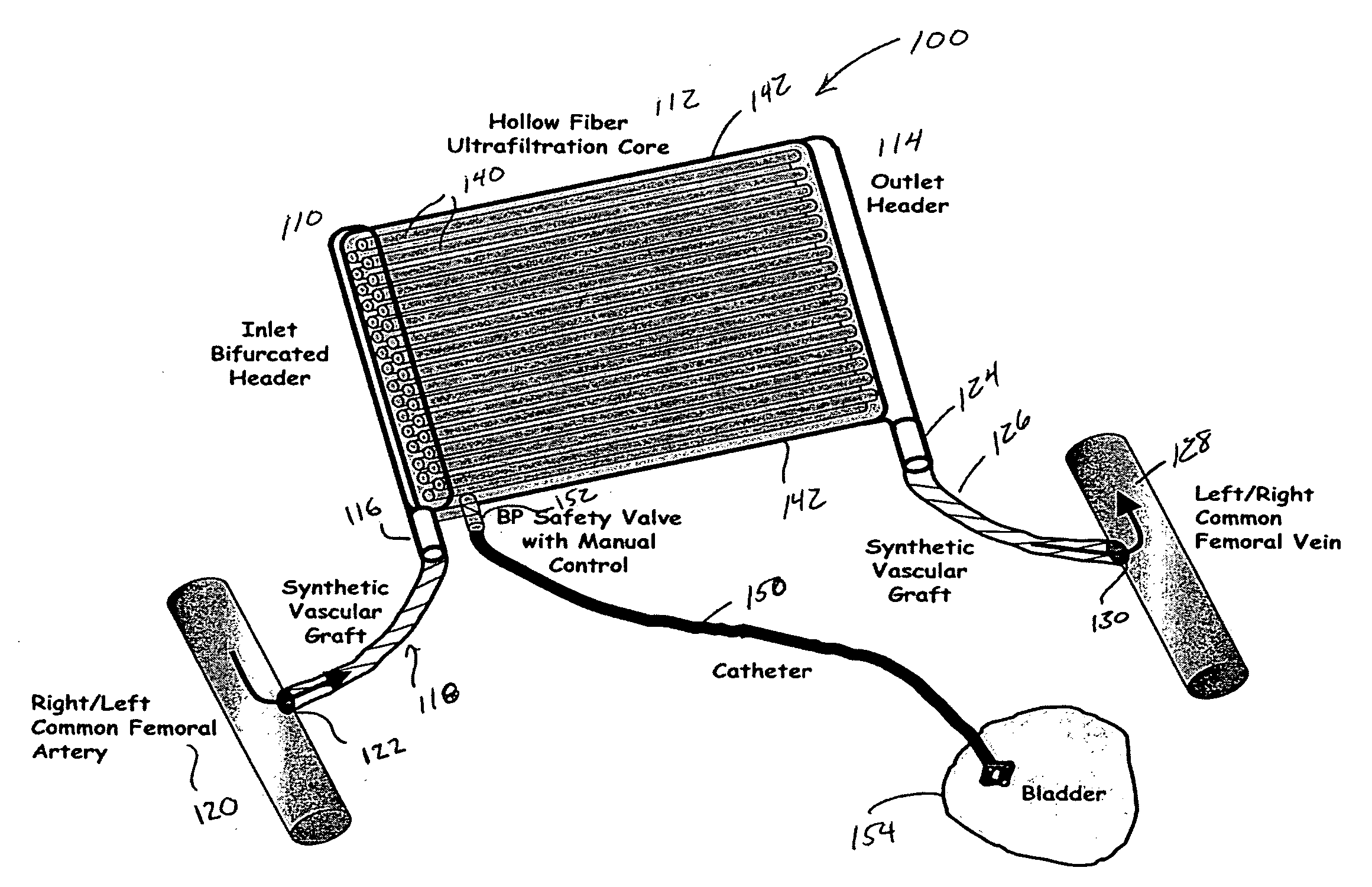
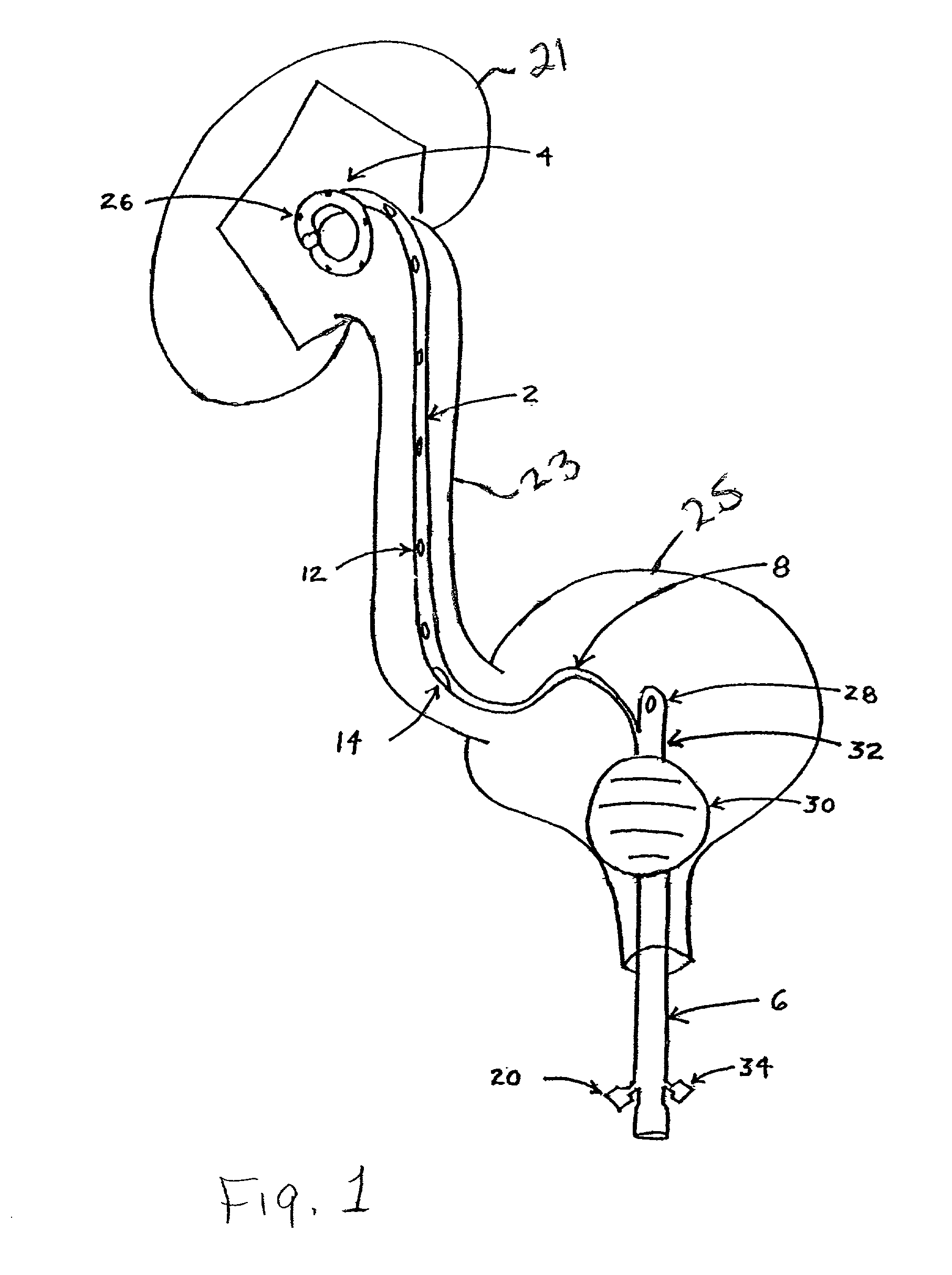
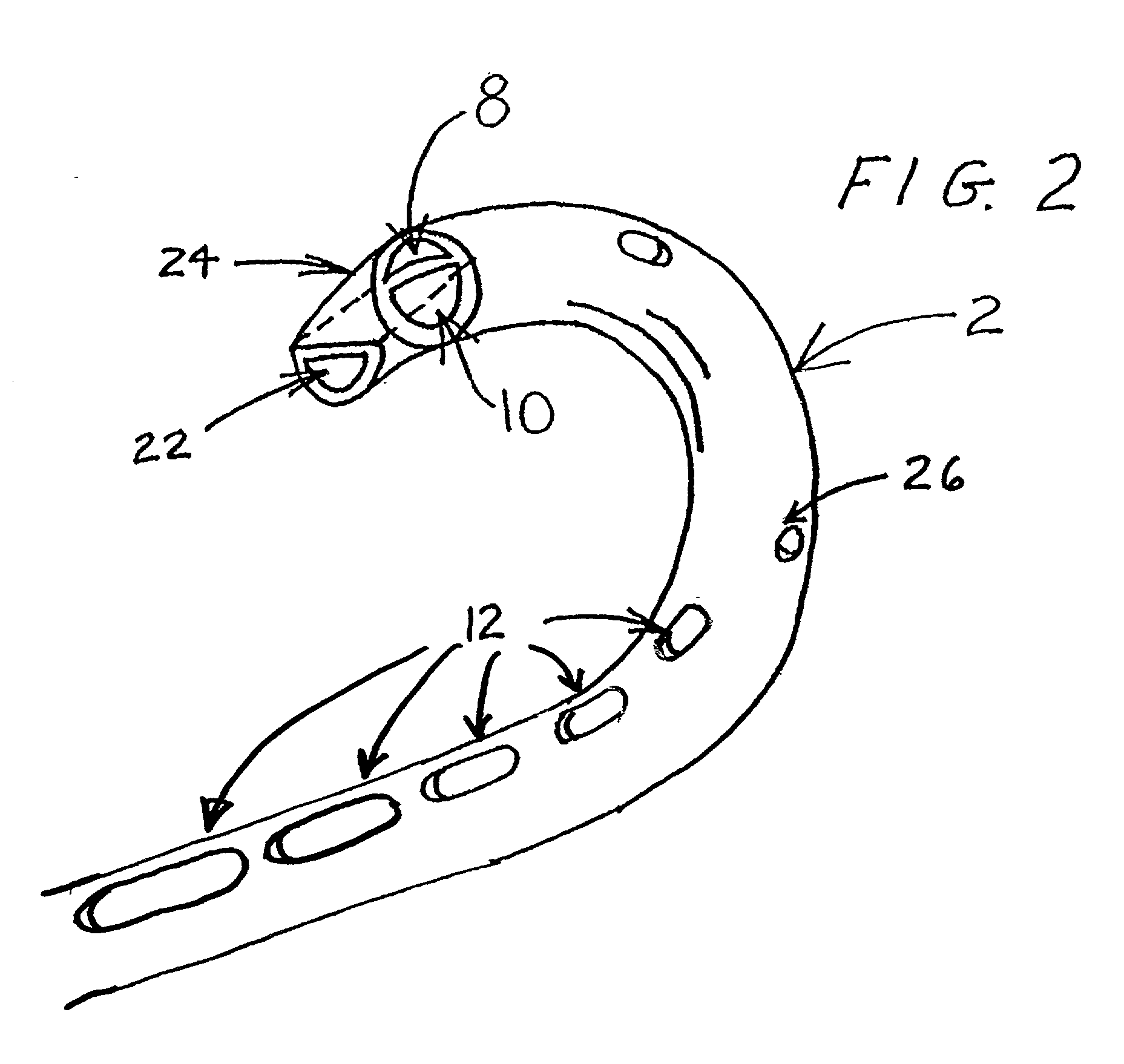
Method and apparatus for kidney dialysis
InactiveUS20050242034A1Mechanical/radiation/invasive therapiesOther blood circulation devicesUltrafiltrationDischarge measurements
Owner:BAXTER INT INC
Soluble, degradable poly(ethylene glycol) derivatives for conrollable release of bound molecules into solution
InactiveUS20050171328A1Reduce rateEnhance solubility and circulation lifetimePharmaceutical non-active ingredientsSynthetic polymeric active ingredientsSolubilityIncreased sizes
PEG and related polymer derivatives having weak, hydrolytically unstable linkages near the reactive end of the polymer are provided for conjugation to drugs, including proteins, enzymes, small molecules, and others. These derivatives provide a sufficient circulation period for a drug-PEG conjugate, followed by hydrolytic breakdown of the conjugate and release of the bound molecule. In some cases, drugs that demonstrate reduced activity when permanently coupled to PEG maintain a therapeutically suitable activity when coupled to a degradable PEG in accordance with the invention. The PEG derivatives of the invention can be used to impart improved water solubility, increased size, a slower rate of kidney clearance, and reduced immunogenicity to a conjugate formed by attachment thereto. Controlled hydrolytic release of the bound molecule into an aqueous environment can then enhance the drug's delivery profile by providing a delivery system which employs such polymers and utilizes the teachings provided herein.
Owner:NEKTAR THERAPEUTICS INC
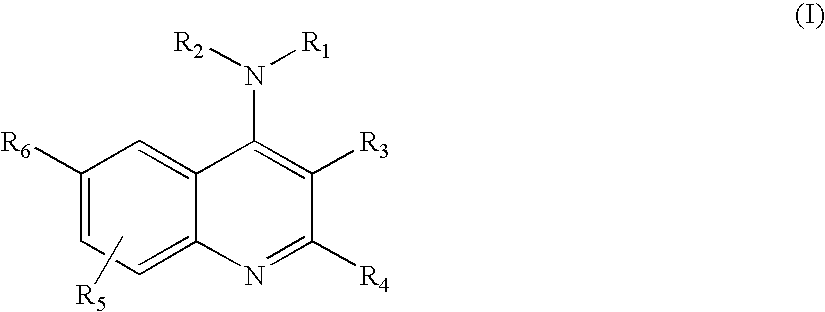
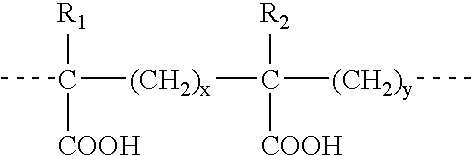
4-Aminoquinoline compounds
The present invention is concerned with compounds of the general Formula I: and pharmaceutically acceptable salts thereof, which are useful as melanin concentrating hormone receptor antagonists, particularly MCH-1R antagonists. As such, compounds of the present invention are useful for the treatment or prevention of obesity or eating disorders associated with excessive food intake and complications thereof, osteoarthritis, certain cancers, AIDS wasting, cachexia, frailty (particularly in elderly), mental disorders stress, cognitive disorders, sexual function, reproductive function, kidney function, locomotor disorders, attention deficit disorder (ADD), substance abuse disorders and dyskinesias, Huntington's disease, epilepsy, memory function, and spinal muscular atrophy. Compounds of formula I may therefore be used in the treatment of these conditions, and in the manufacture of a medicament useful in treating these conditions. Pharmaceutical formulations comprising one of the compounds of formula (I) as an active ingredient are disclosed, as are processes for preparing these compounds.
Owner:DEVITA ROBERT J +5
Compositions and methods for the treatment of cancer
InactiveUS20020128228A1Reducing and avoiding adverse effectImprove toleranceBiocideAnimal repellantsIntestinal structureCancer prevention
This invention relates to compositions comprising temozolomide and thalidomide which can be used in the treatment or prevention of cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof. A particular composition comprises temozolomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof, and thalidomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof. The invention also relates to methods of treating or preventing cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof, which comprise the administration of temozolomide and thalidomide and another anti-cancer drug to a patient in need of such treatment or prevention. The invention further relates to methods of reducing or avoiding adverse side effects associated with the administration of cancer chemotherapy or radiation therapy which comprise the administration of temozolomide and thalidomide to a patient in need of such reduction or avoidance.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
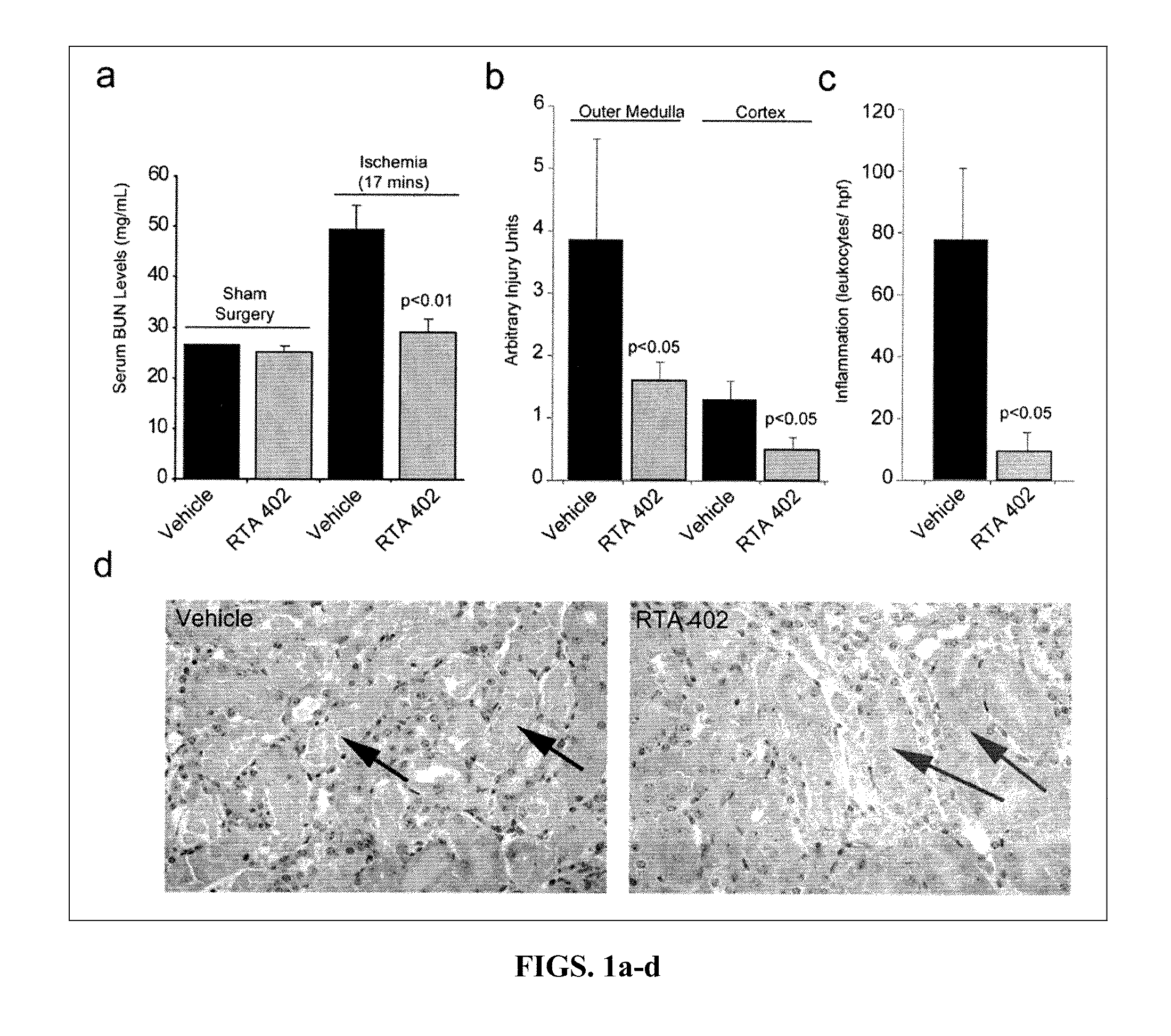
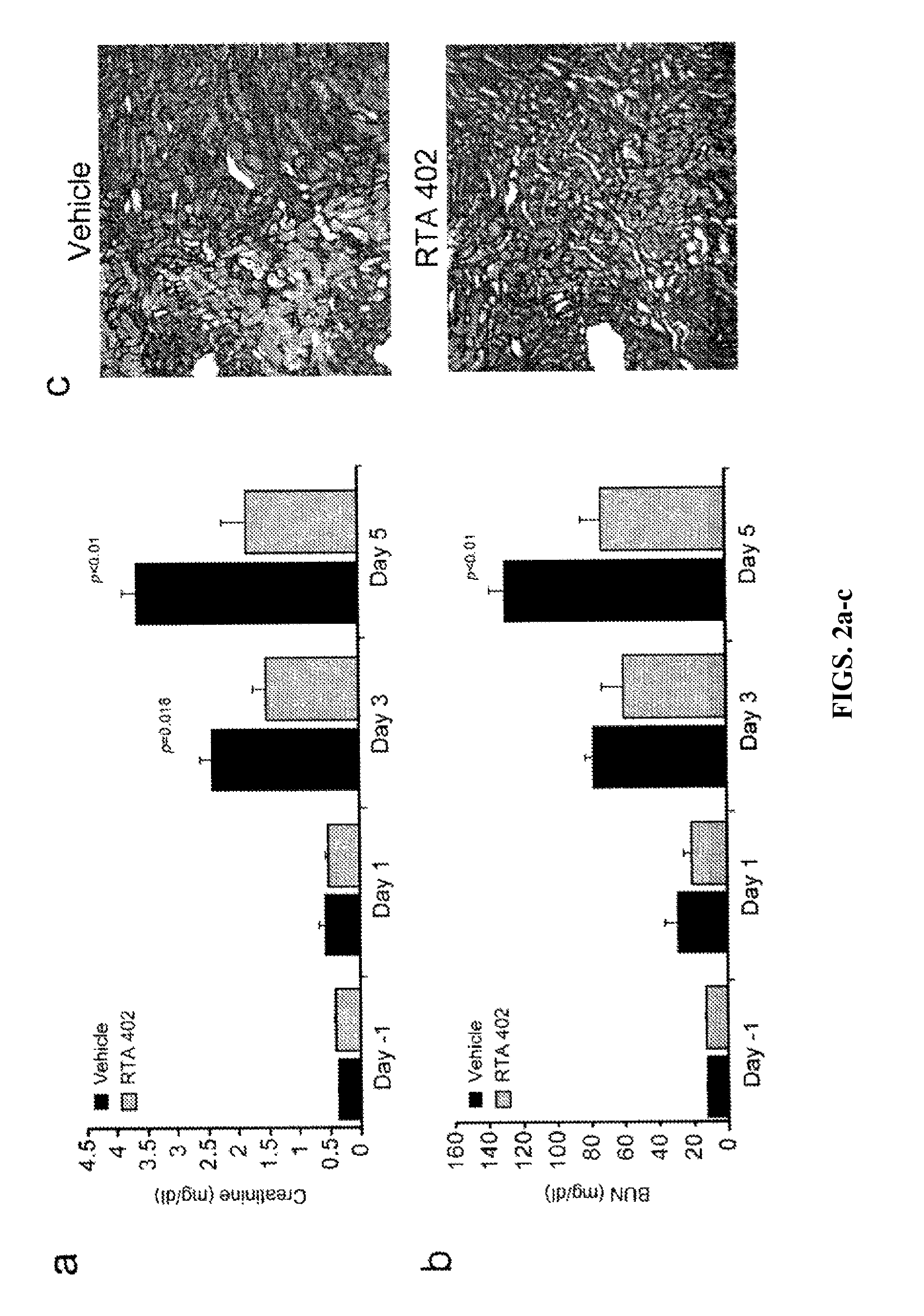
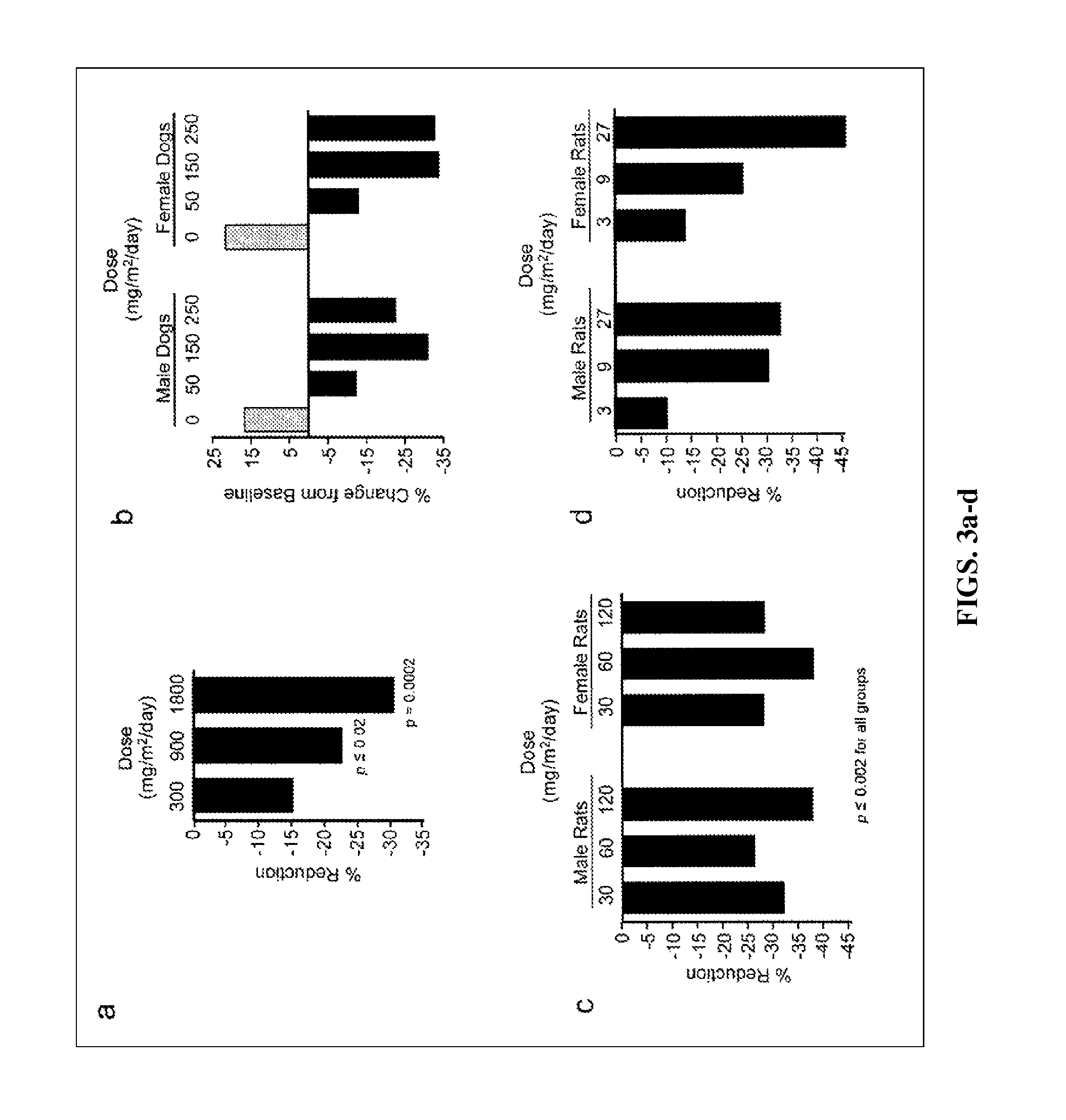
Synthetic triterpenoids and methods of use in the treatment of disease
ActiveUS8129429B2Improve clearanceIncrease ratingsBiocideSenses disorderFatty liverEndothelial dysfunction
The present invention concerns methods for treating and preventing renal / kidney disease, insulin resistance / diabetes, fatty liver disease, and / or endothelial dysfunction / cardiovascular disease using synthetic triterpenoids, optionally in combination with a second treatment or prophylaxis.
Owner:REATA PHARM HLDG LLC +1
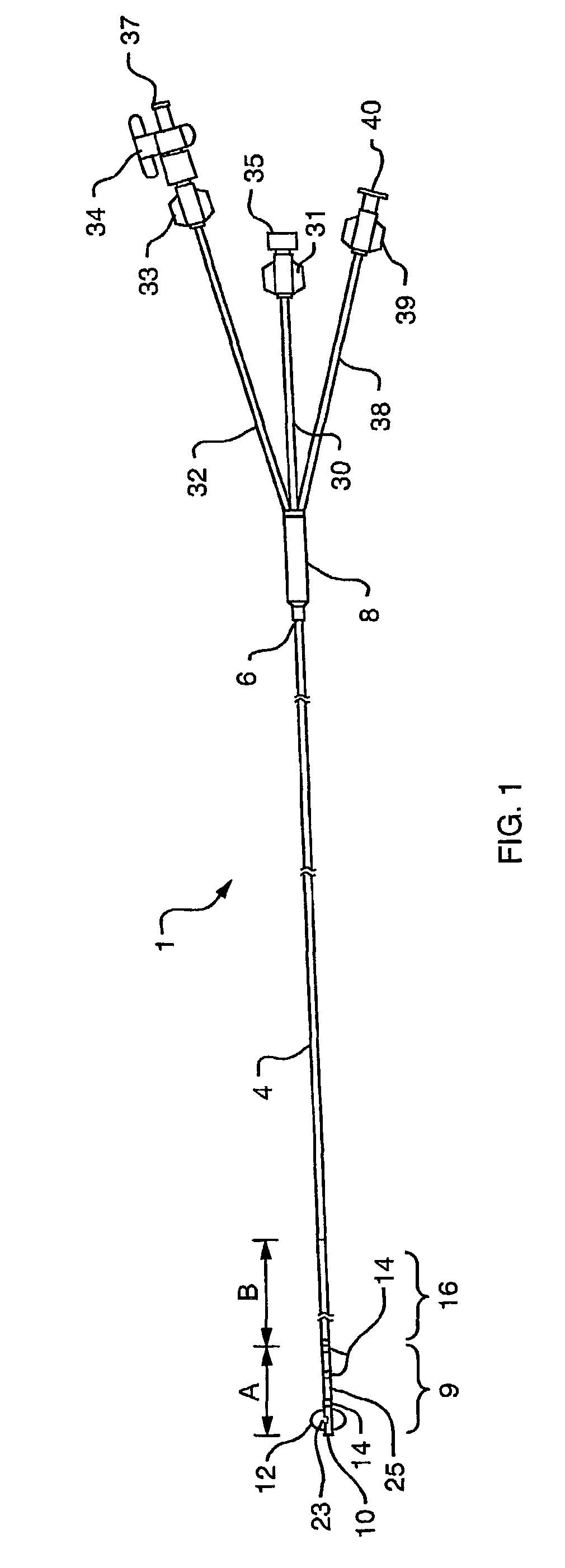
Triple lumen stone balloon catheter and method
InactiveUS7481800B2Acceptable inflationAcceptable deflation rateStentsBalloon catheterBalloon catheterKidney
A triple lumen stone balloon catheter (1) having a tapered distal end (9). A lumen (24) dedicated to transmitting contrast media is dimensioned and adapted to conform to the shape of a kidney in a main shaft of the catheter and conform to the shape of a crescent in a distal end of the catheter. The geometric shaping of the contrast media lumen (24) enables wall thickness to be maintained within acceptable ranges to sustain desirable mechanical characteristics while allowing for enhanced contrast media flow. A method for employing the balloon catheter (1) is also disclosed.
Owner:COMMAND ENDOSCOPIC TECH
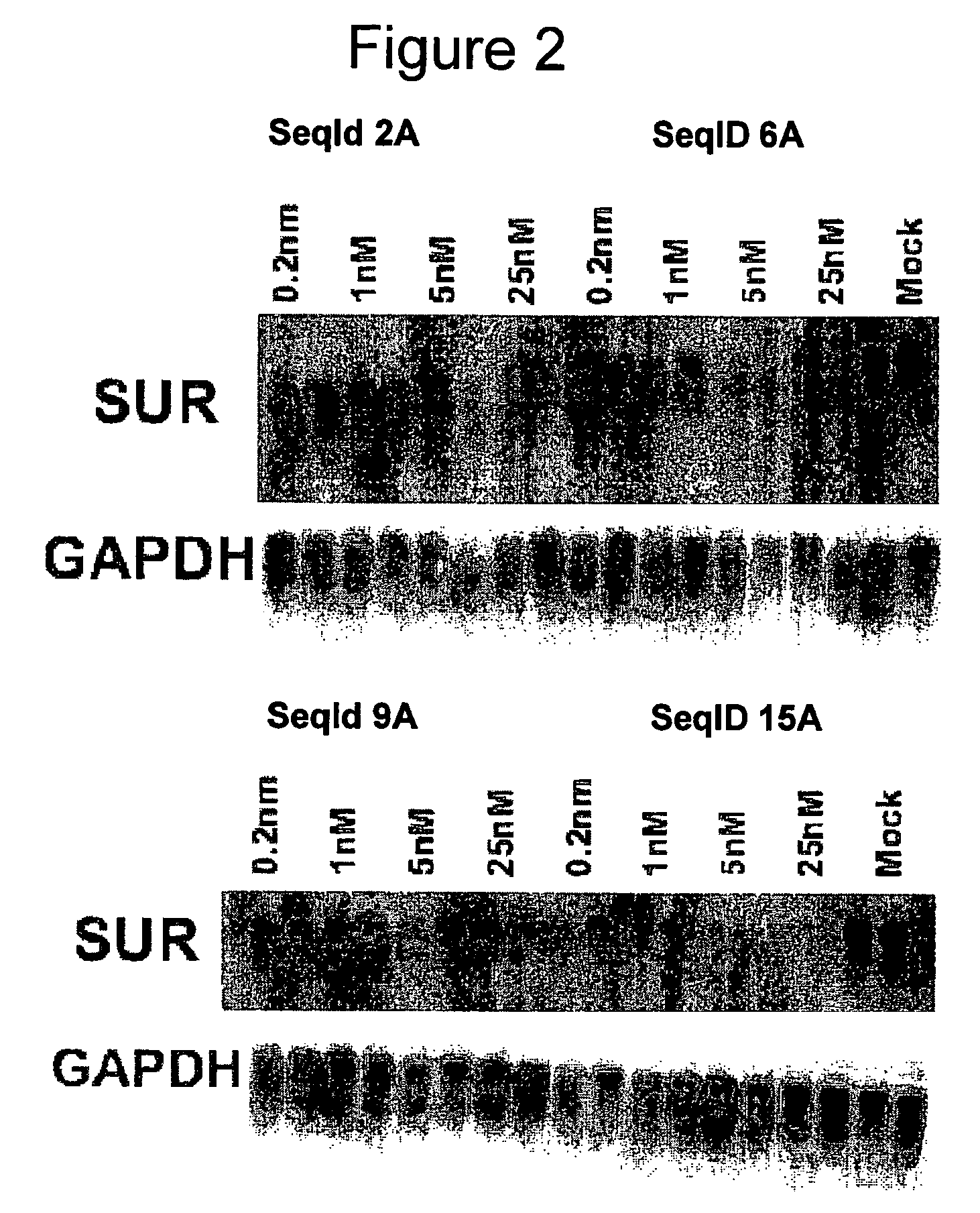
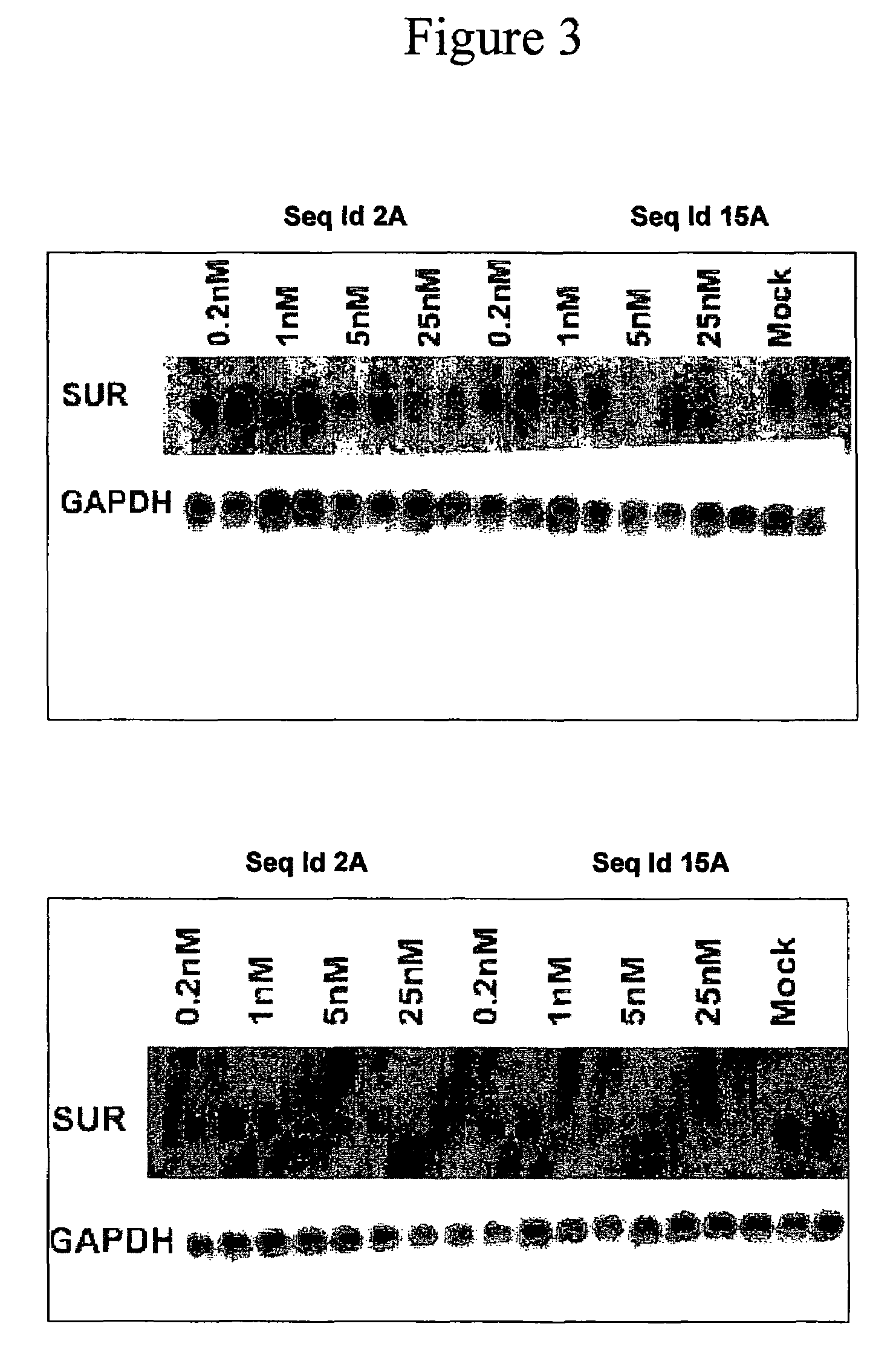
Oligomeric compounds for the modulation of survivin expression
Oligonucleotides directed against the survivin gene are provided for modulating the expression of survivin. The compositions comprise oligonucleotides, particularly antisense oligonucleotides, targeted to nucleic acids encoding the survivin. Methods of using these compounds for modulation of survivin expression and for the treatment of diseases associated with either overexpression of survivin, expression of mutated survivin or both are provided. Examples of diseases are cancer such as lung, breast, colon, prostate, pancreas, lung, liver, thyroid, kidney, brain, testes, stomach, intestine, bowel, spinal cord, sinuses, bladder, urinary tract or ovaries cancers. The oligonucleotides may be composed of deoxyribonucleosides or a nucleic acid analogue such as for example locked nucleic acid or a combination thereof.
Owner:ENZON PHARM INC
Antisense oligonucleotides optimized for kidney targeting
ActiveUS20050113326A1Growth inhibitionPreventing delaying onsetSugar derivativesSpecial deliveryConnective tissue fiberCTGF
The present invention provides antisense compounds and methods for modulating the expression of target genes expressed in the kidney. In particular, this invention provides antisense oligonucleotide compounds optimized for targeting nucleic acid molecules expressed in the kidney. Such compounds are shown herein to efficiently modulate the expression of target genes SGLT2 and connective tissue growth factor (CTGF) in the kidney.
Owner:IONIS PHARMA INC
Artificial kidney dialysis system
ActiveUS20080051696A1Cumbersome to wearImprove the quality of lifeMedical devicesPeritoneal dialysisNephrosisMetabolite
The present disclosure relates to a wearable dialysis system and method for removing uremic waste metabolites and fluid from a patient suffering from renal disease. Uremic waste metabolites can be removed by a wearable peritoneal dialysis device that regenerates the peritoneal dialysis solution without removing positively charged, essential ions from the solution and, consequently, the patient. Fluids can be removed from the blood of the patient by an implantable fluid removing device. Fluids are delivered to the bladder and preferably removed from the body of the patient through urination. The wearable dialysis system may be operated continuously or semi-continuously and be comfortably adapted to the body of the patient while allowing the patient to perform normal activities.
Owner:FRESENIUS MEDICAL CARE HLDG INC
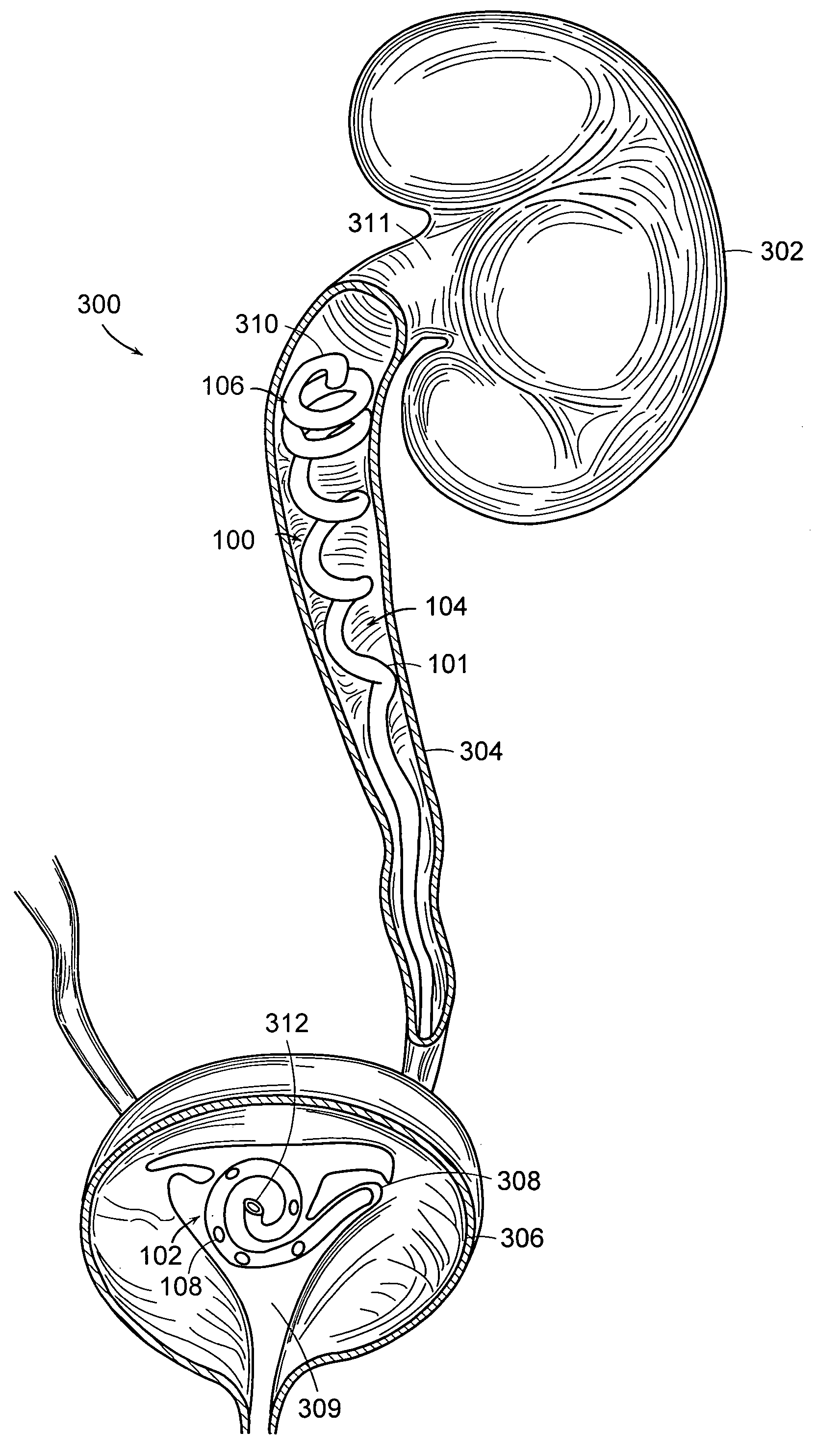
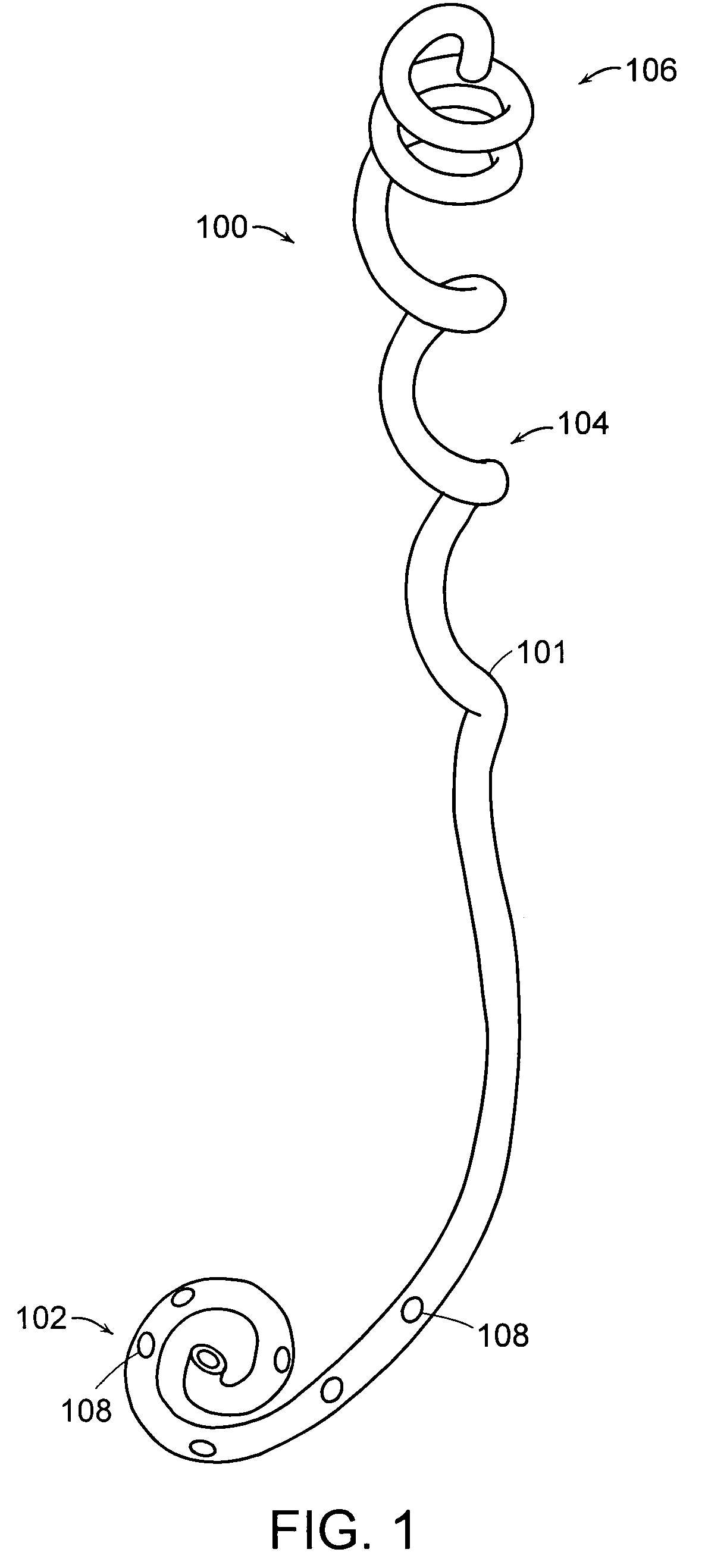
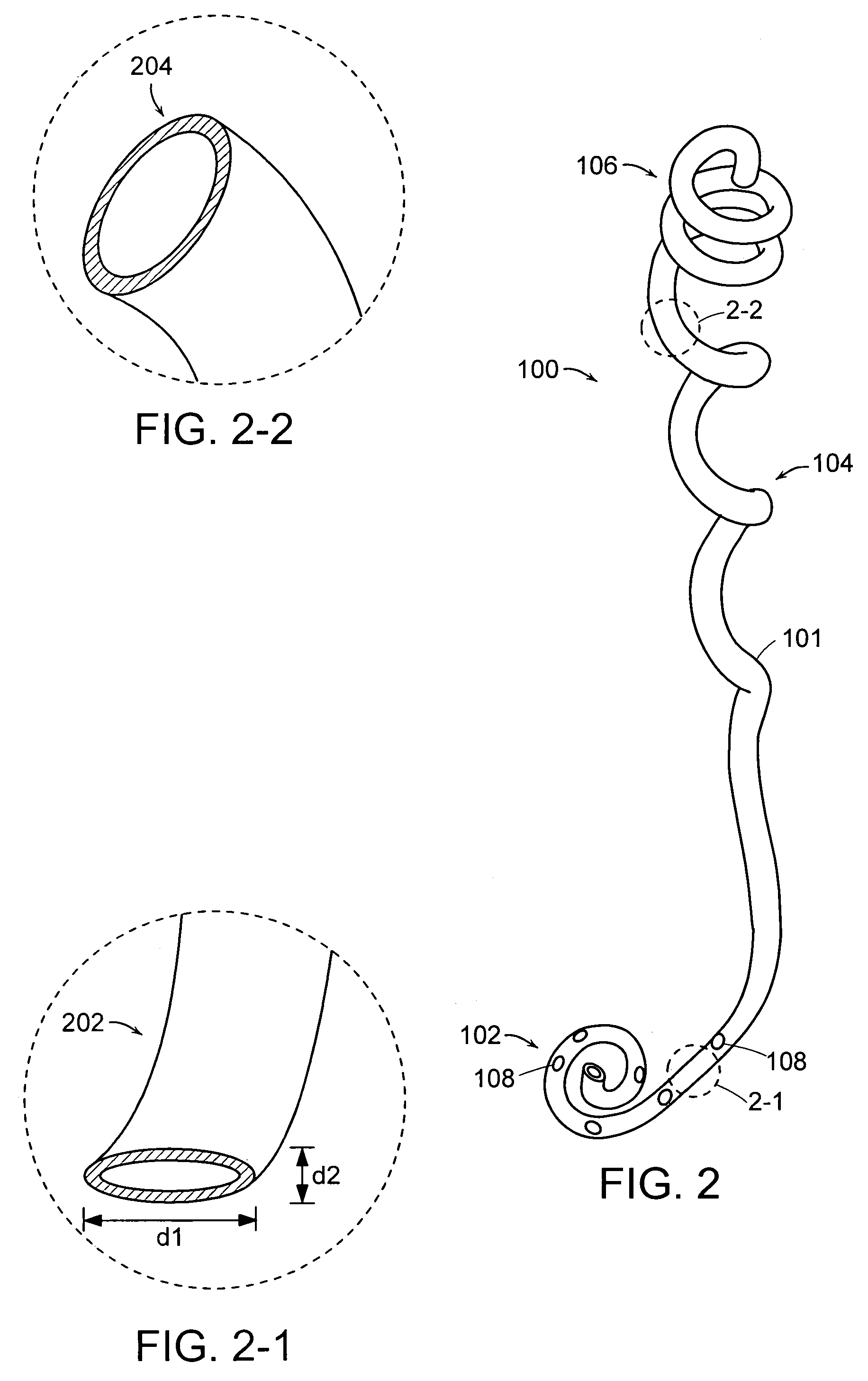
Ureteral stent configured for improved patient comfort and aftercare
A ureteral stent is configured for improved patient comfort and aftercare. The stent can have one or more of the following features: a distal portion with a somewhat flattened, non-circular cross-section that provides reduced irritation and elimination of urine reflux; a proximal portion with a helical coil shape that allows self-anchoring of the stent below the kidney; and a portion along the body of the stent having a coil shape that allows self-adjustment of the stent with ureteral movement.
Owner:BOSTON SCI SCIMED INC
Methods of treating chronic inflammatory diseases using carbonyl trapping agents
InactiveUS6444221B1Improved therapeutic propertyImprove propertiesBiocidePeptide/protein ingredientsEtiologyBenzoic acid
Owner:SECANT PHARMA
Nitrogen-containing fused ring compounds and use thereof
InactiveUS20070010670A1Inhibitory activityWeak CYP inhibitory actionOrganic active ingredientsOrganic chemistryCoronary artery diseaseAcute gouty arthritis
A URAT1 activity inhibitor containing a nitrogen-containing fused ring compound represented by the following formula [1]: wherein each symbol is as defined in the description. The present invention is useful for the prophylaxis or treatment of pathology showing involvement of uric acid, such as hyperuricemia, gouty tophus, acute gouty arthritis, chronic gouty arthritis, gouty kidney, urolithiasis, renal function disorder, coronary artery disease, ischemic heart disease and the like.
Owner:JAPAN TOBACCO INC
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
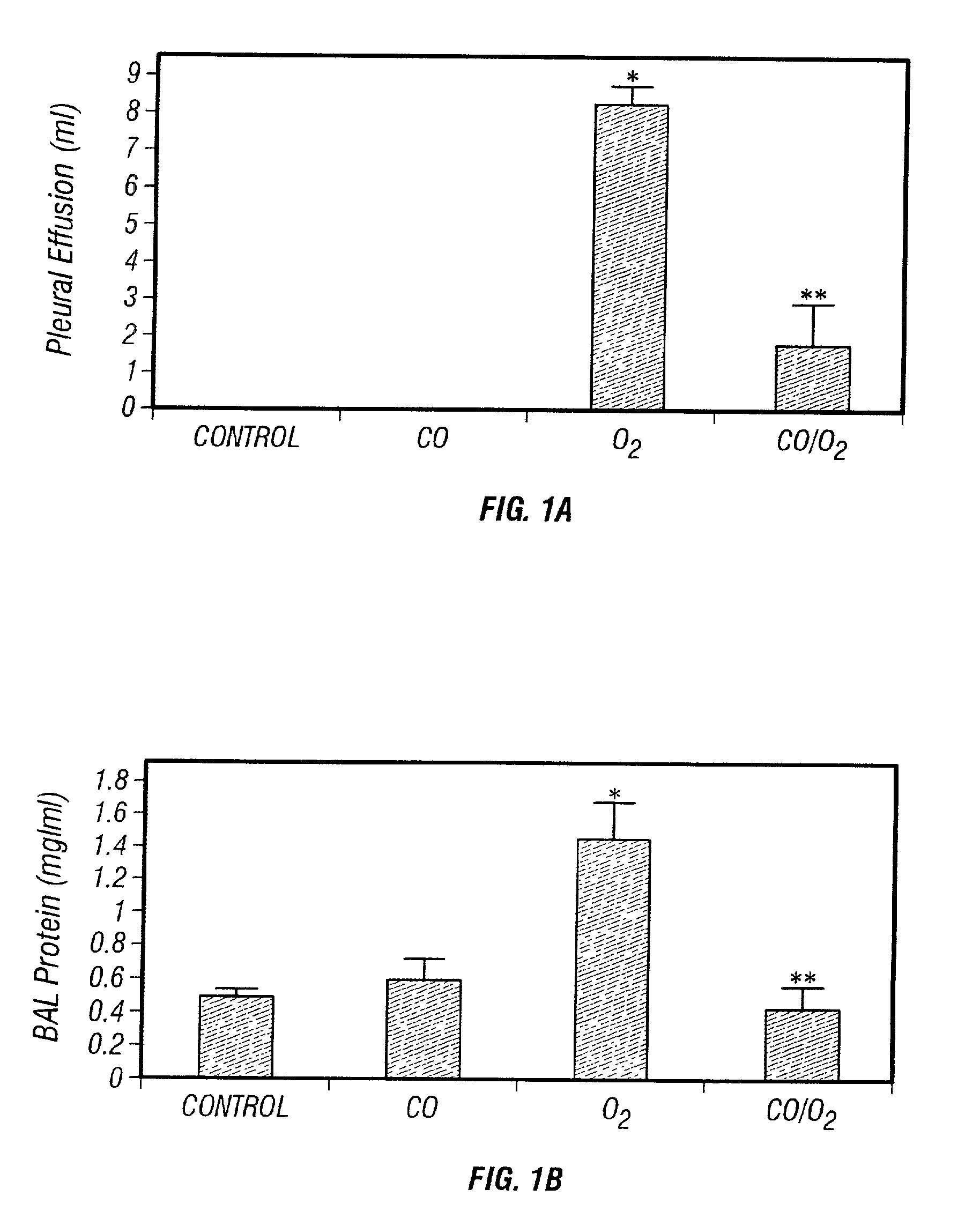
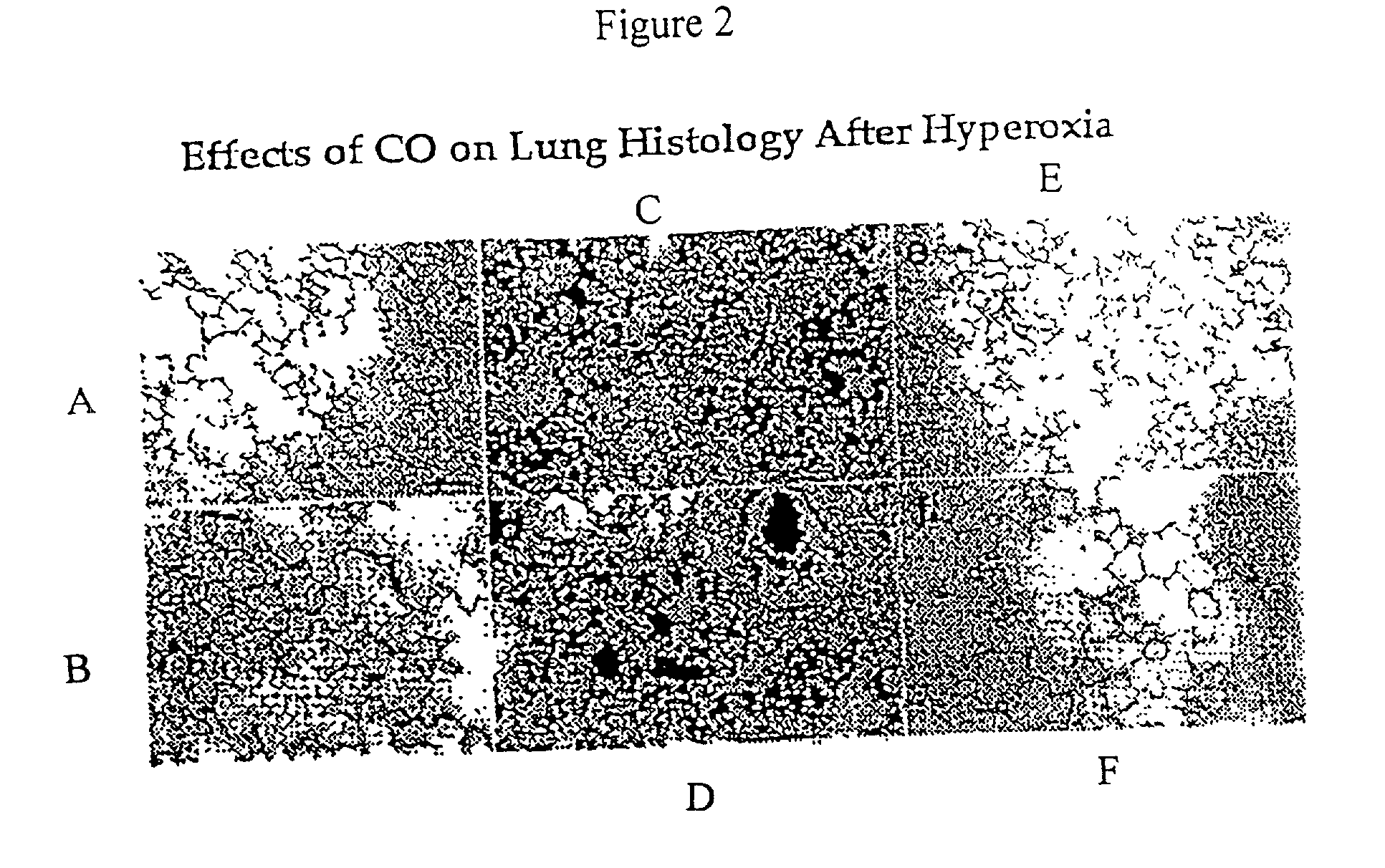
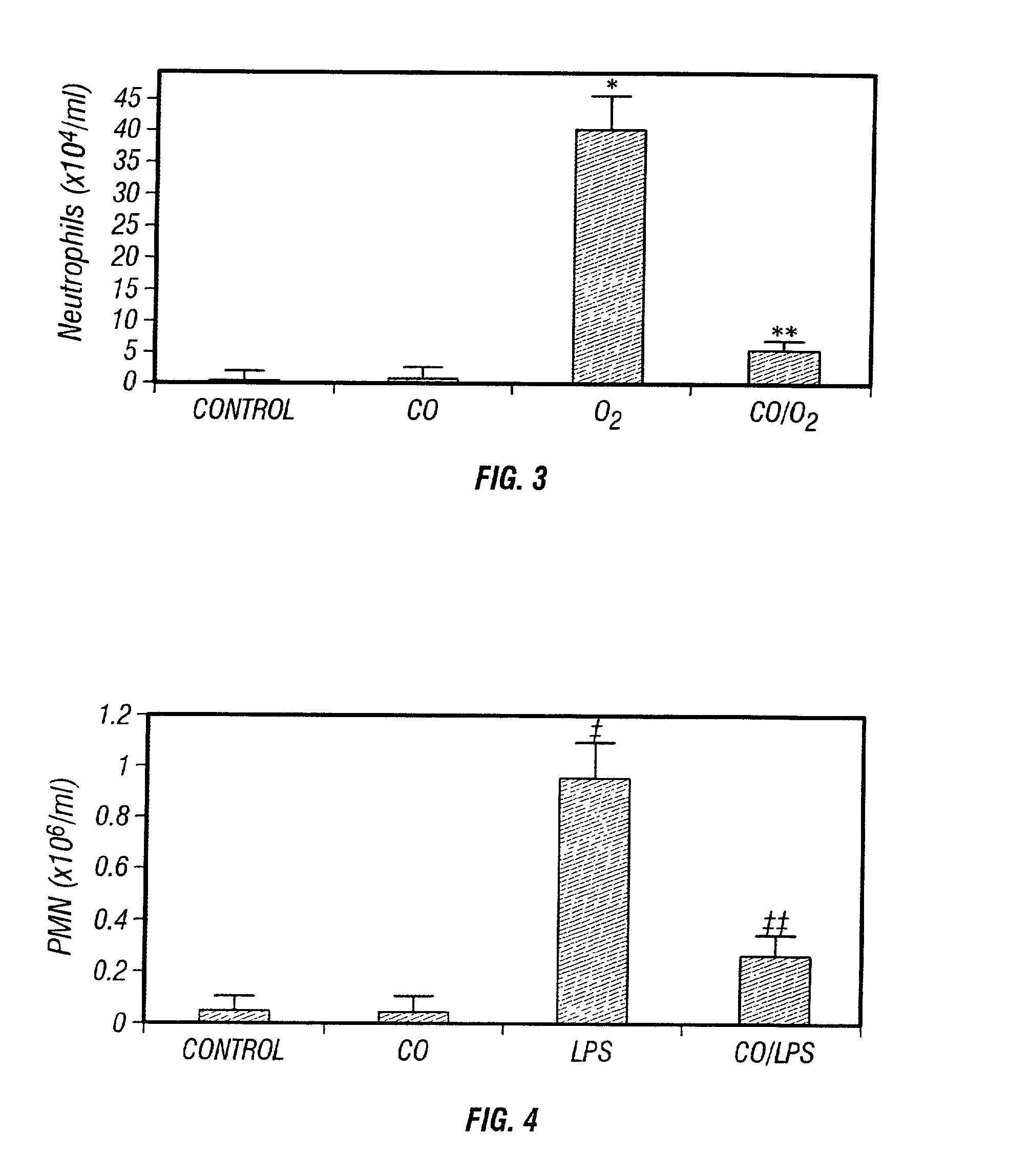
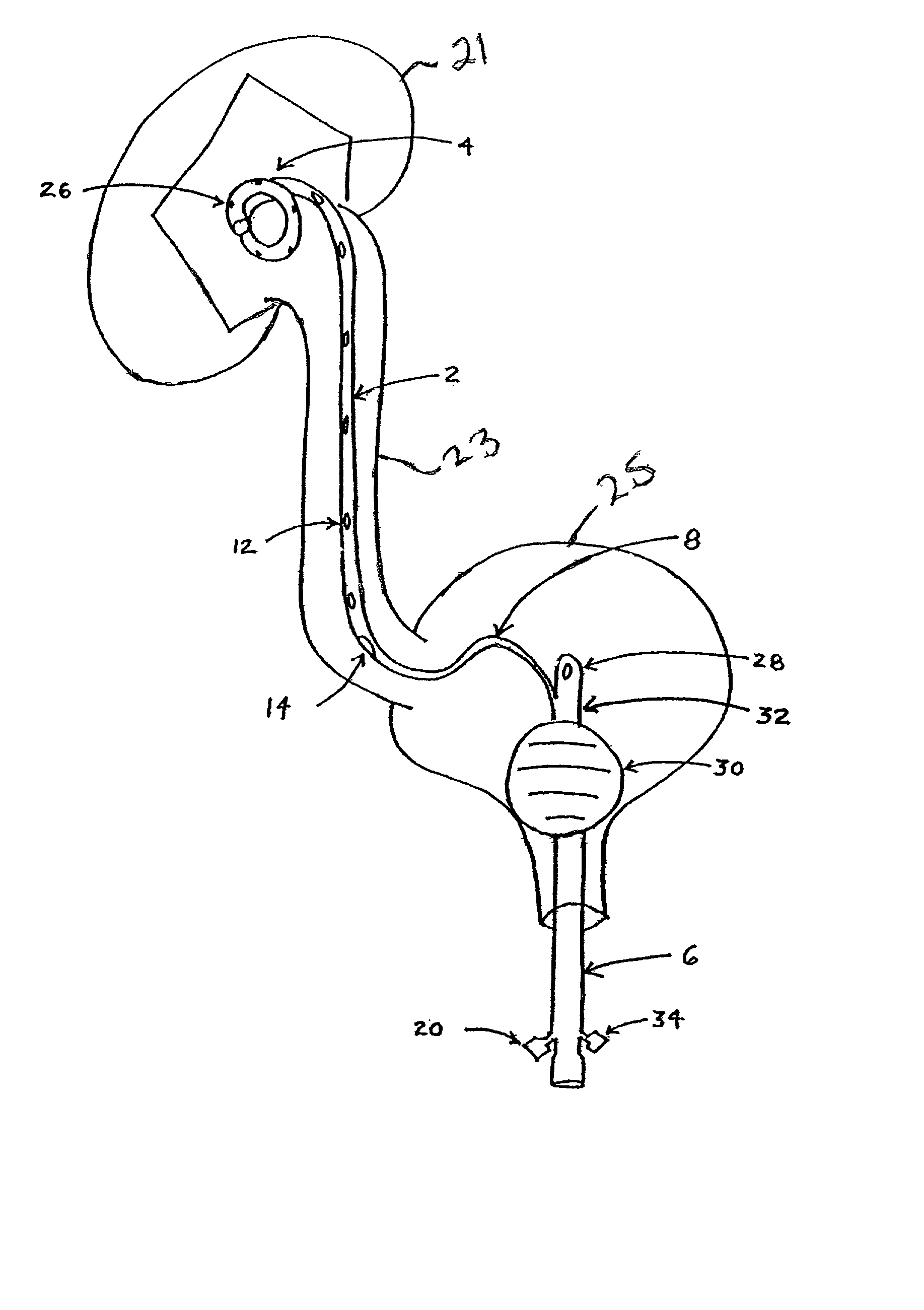
Carbon monoxide as a biomarker and therapeutic agent
InactiveUS20020155166A1Reduce concentrationBiocideInorganic active ingredientsInterstitial lung diseaseRESPIRATORY DISTRESS SYNDROME ADULT
The present invention relates to the use of carbon monoxide (CO) as a biomarker and therapeutic agent of heart, lung, liver, spleen, brain, skin and kidney diseases and other conditions and disease states including, for example, asthma, emphysema, bronchitis, adult respiratory distress syndrome, sepsis, cystic fibrosis, pneumonia, interstitial lung diseases, idiopathic pulmonary diseases, other lung diseases including primary pulmonary hypertension, secondary pulmonary hypertension, cancers, including lung, larynx and throat cancer, arthritis, wound healing, Parkinson's disease, Alzheimer's disease, peripheral vascular disease and pulmonary vascular thrombotic diseases such as pulmonary embolism. CO may be used to provide anti-inflammatory relief in patients suffering from oxidative stress and other conditions especially including sepsis and septic shock. In addition, carbon monoxide may be used as a biomarker or therapeutic agent for reducing respiratory distress in lung transplant patients and to reduce or inhibit oxidative stress and inflammation in transplant patients.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Combination ureteral infusion catheter/drainage stent
A method of treating the upper urinary tract of a mammal having a urethra extending to a bladder and a ureter extending from the bladder to a kidney. The method comprises initially extending a catheter system into the upper urinary tract, the catheter system having a urethral catheter infusion tube section extending through the urethra for infusion of a liquid; a urethral catheter drain tube section extending through the urethra for drainage of a liquid; a ureteral catheter tube section extending through a ureter into the kidney and having a first tube portion for infusion of a liquid connected to the urethral catheter infusion tube section, and a second tube portion for drainage of a liquid. The method then includes flowing a therapeutically effective liquid into the kidney through the urethral catheter infusion tube section and the ureteral catheter first, infusion tube portion, and, simultaneously with the flowing of the fluid into the kidney, draining fluid from the kidney through the urethral catheter drain tube section and the ureteral catheter second, drain tube portion.
Owner:HAYNER GEORGE M +1
Prevention of myocarditis, abortion and intrauterine infection associated with porcine circovirus-2
InactiveUS20050058653A1Avoid spreadingPeptide/protein ingredientsGenetic material ingredientsDiseaseStaining
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6and 8and / or the 10and 13weeks of gestation, can lower viral titer, prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:MERIAL SAS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com