Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1098 results about "Bone growth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
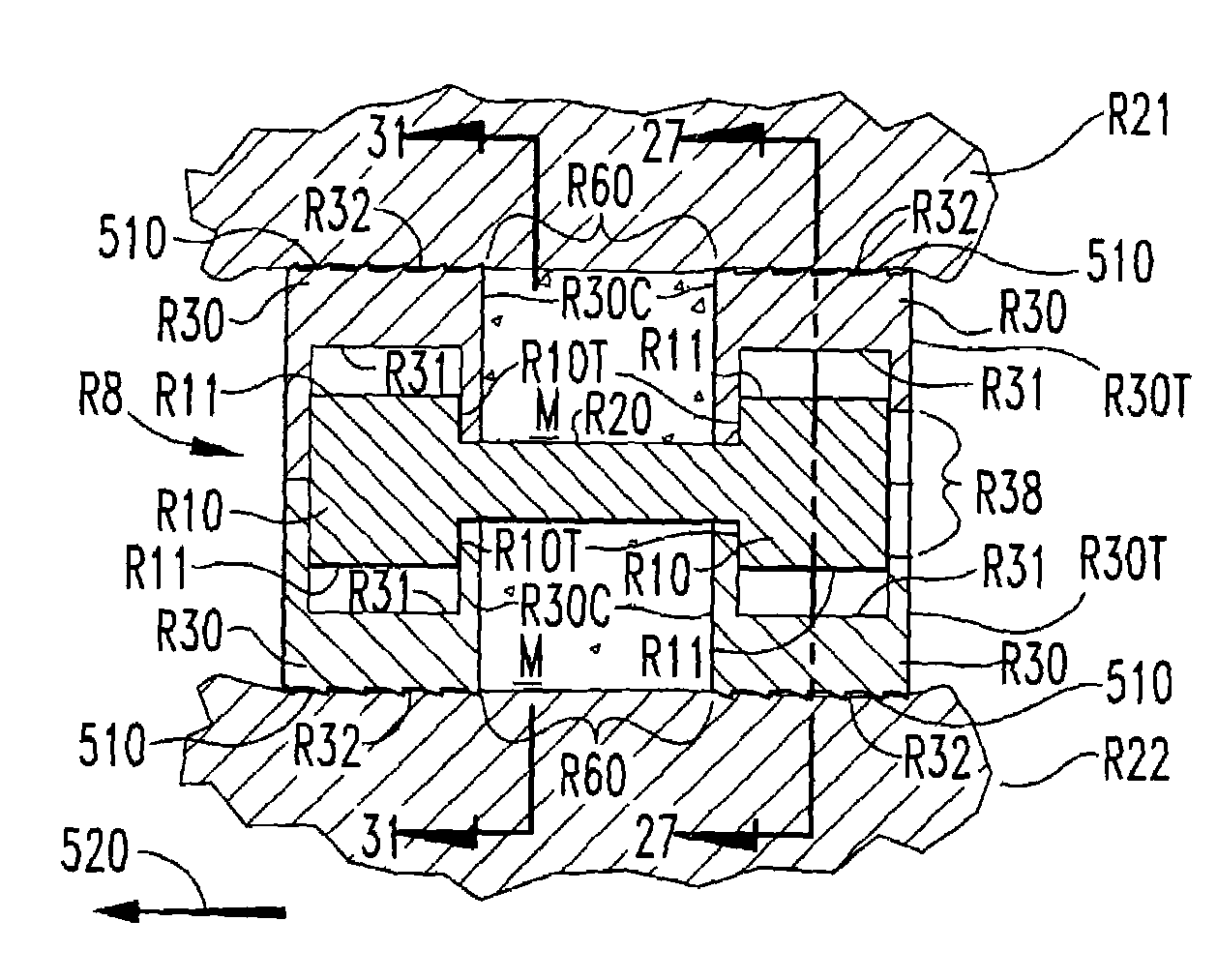
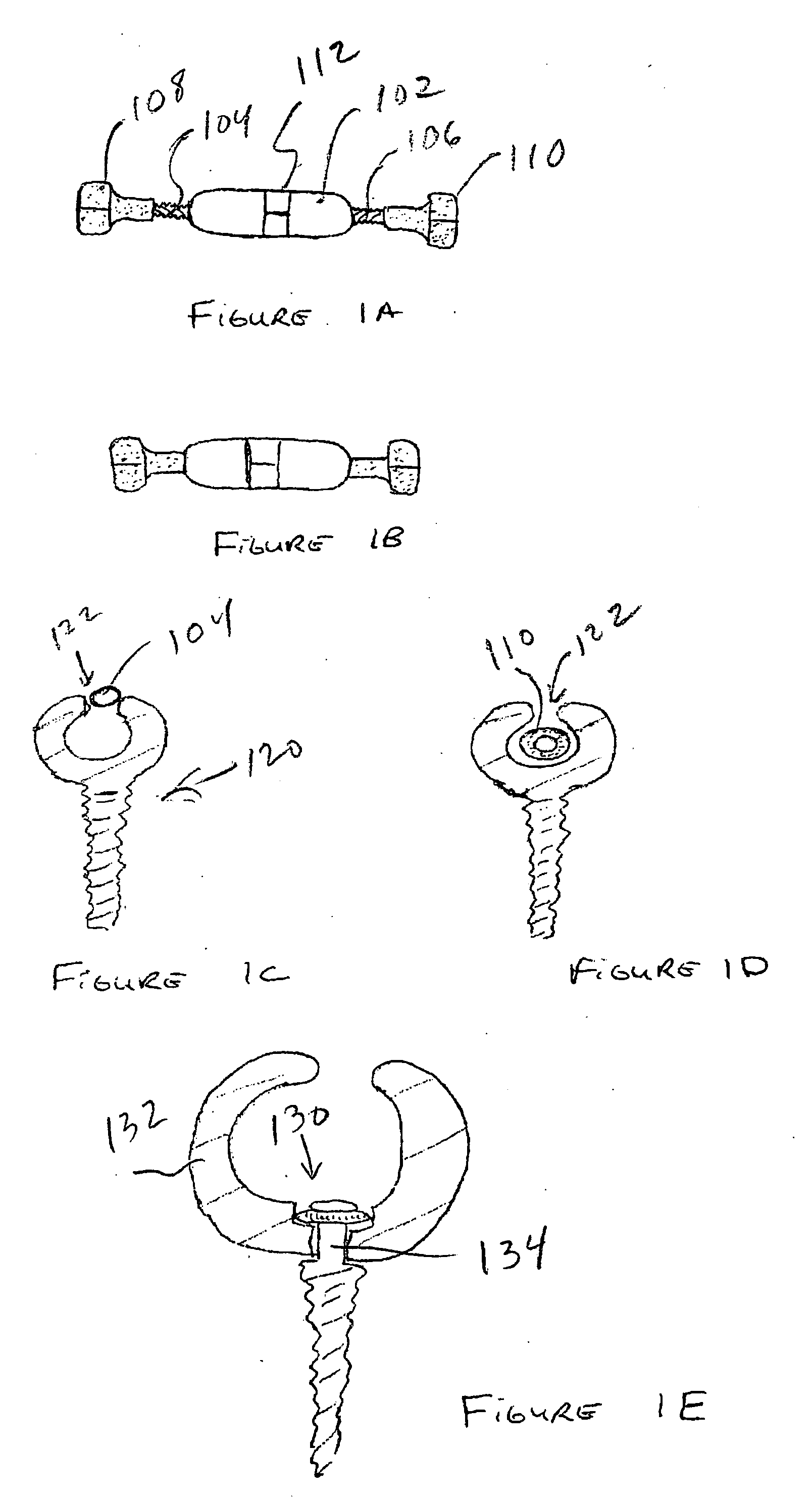
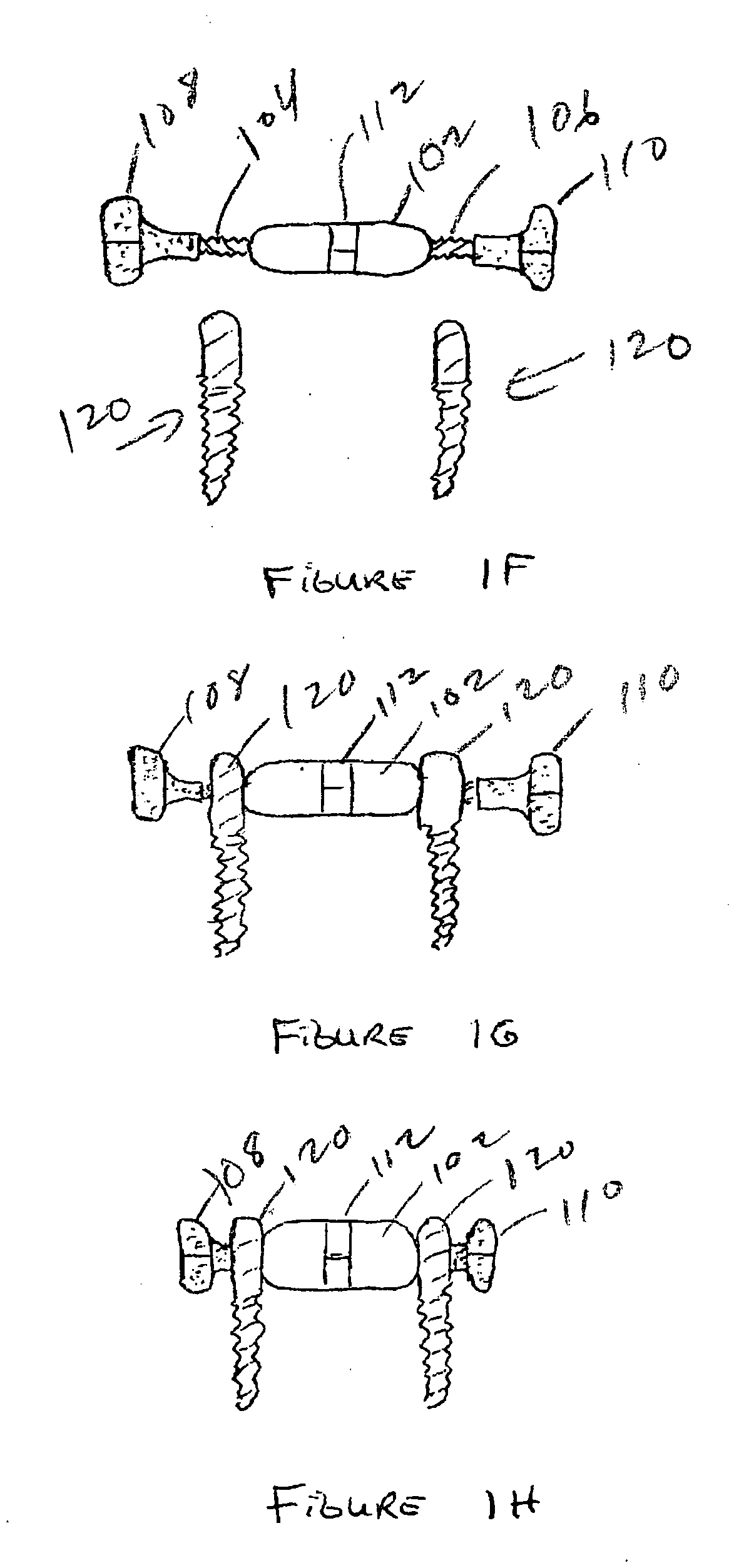
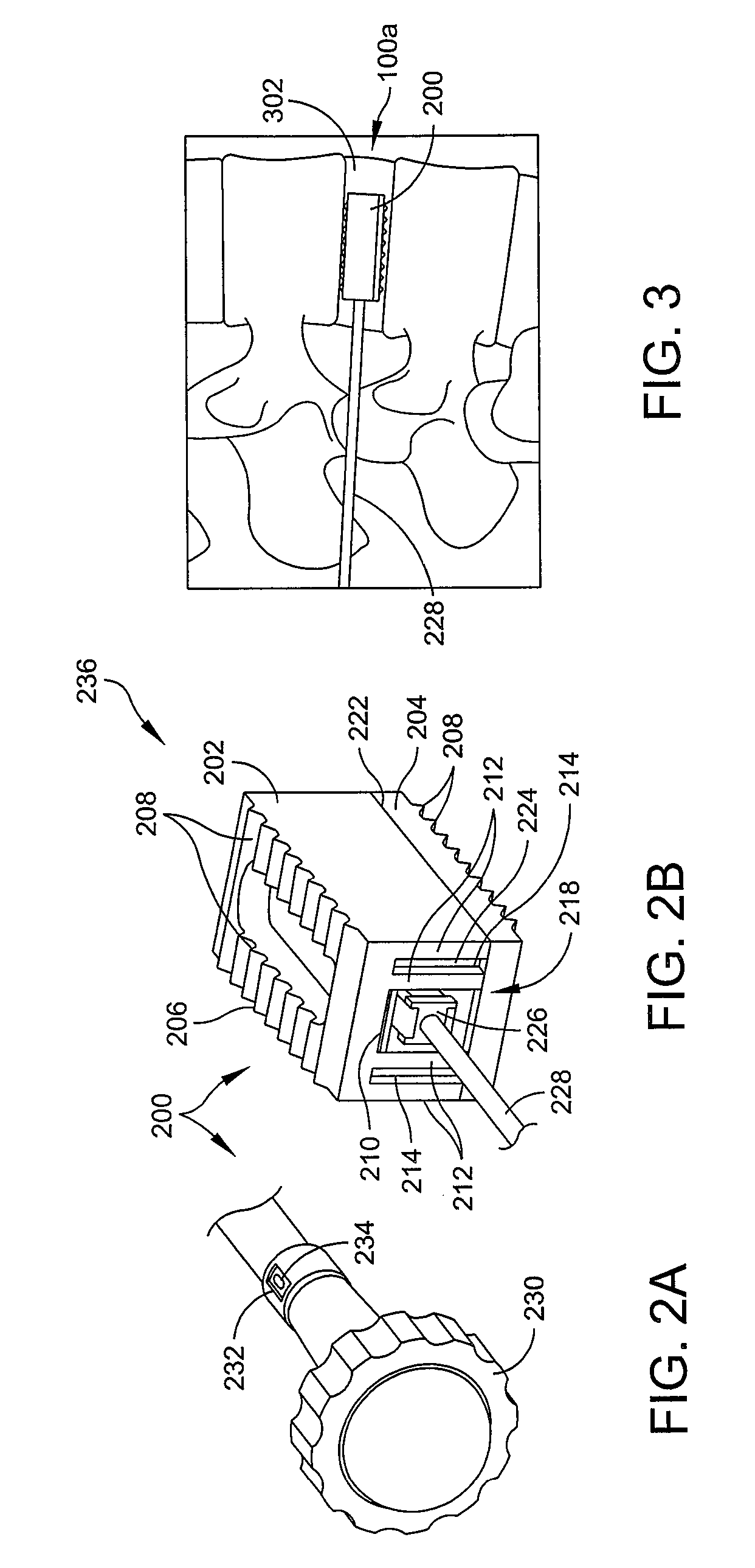
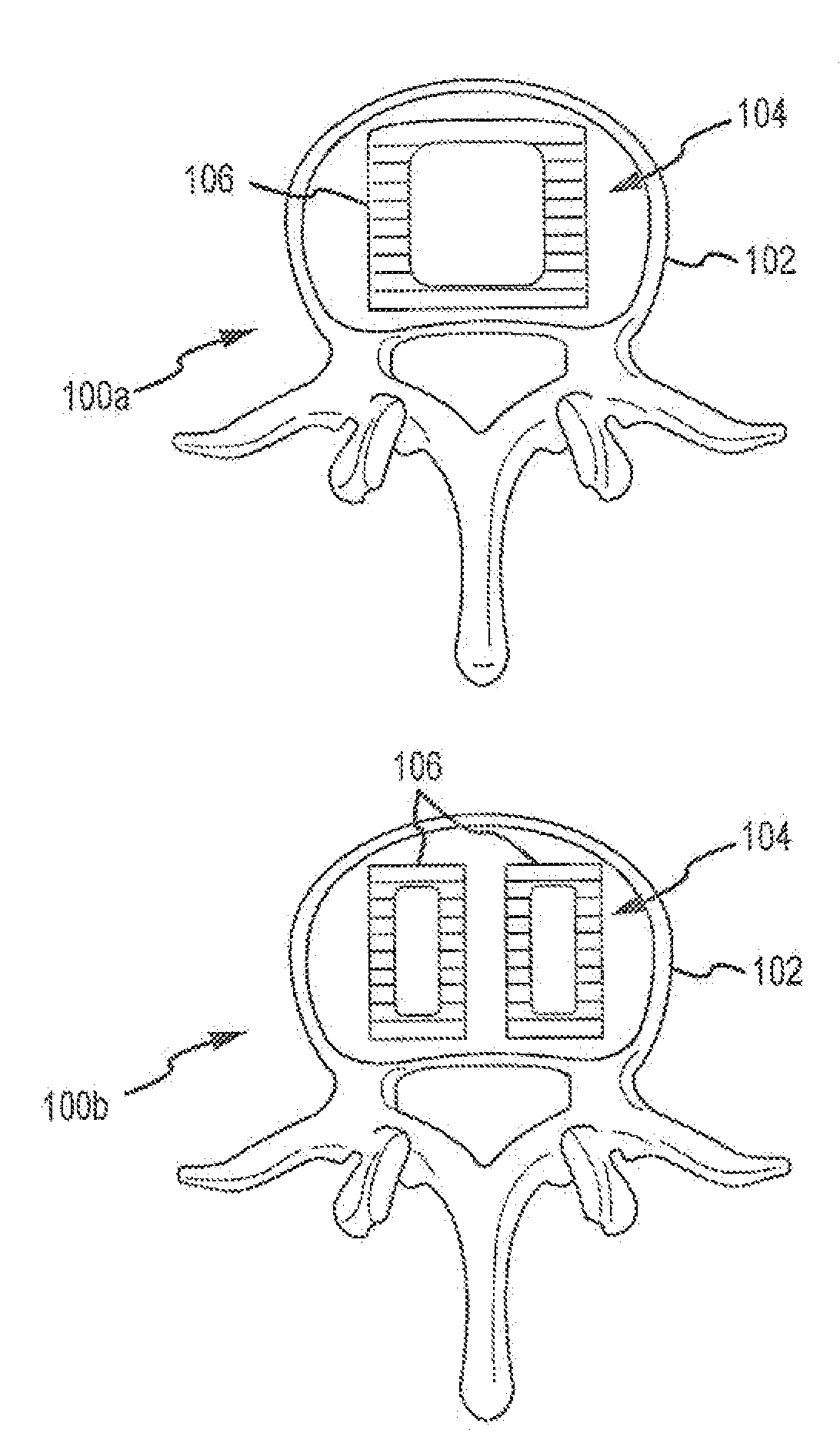
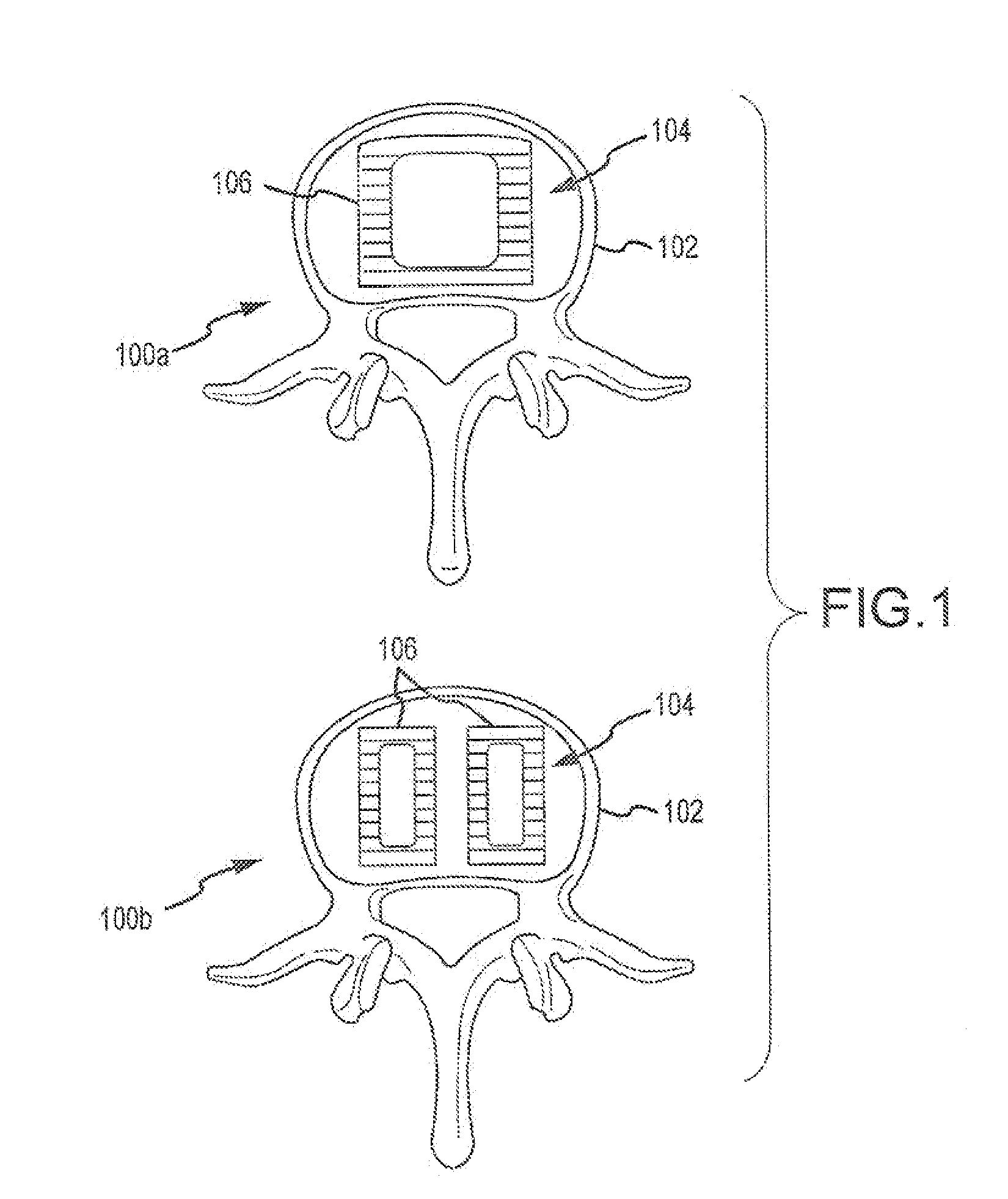
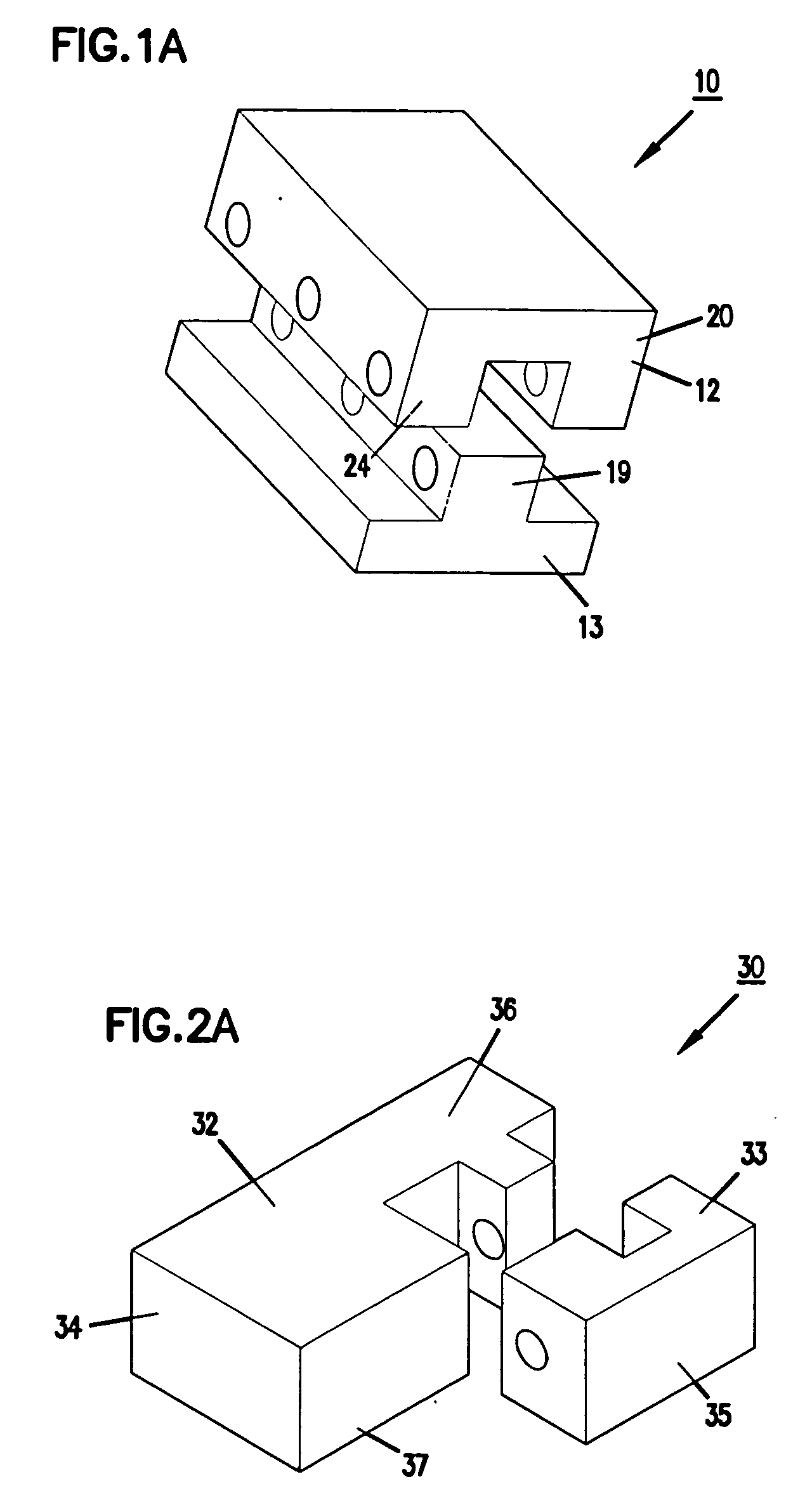
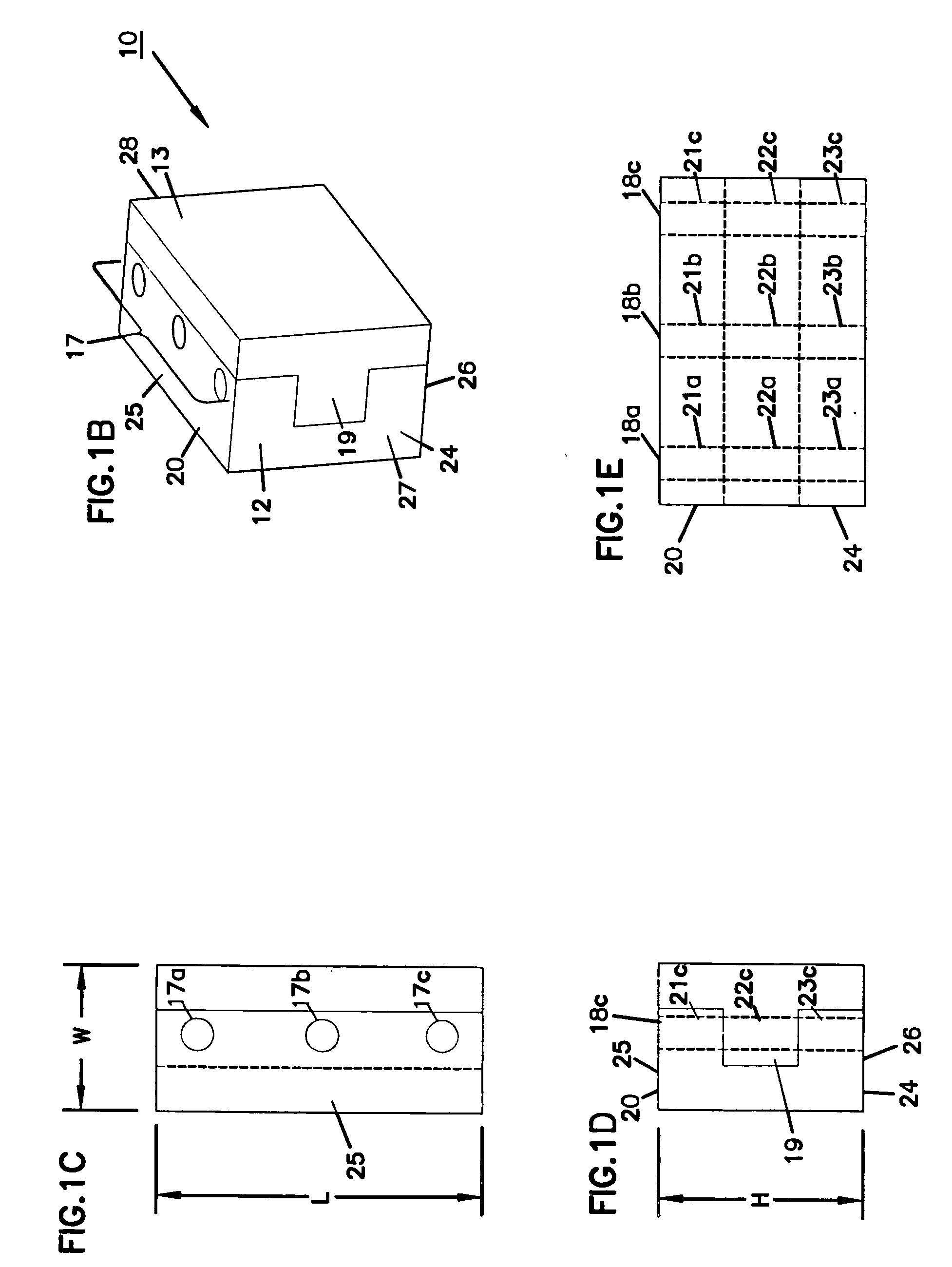
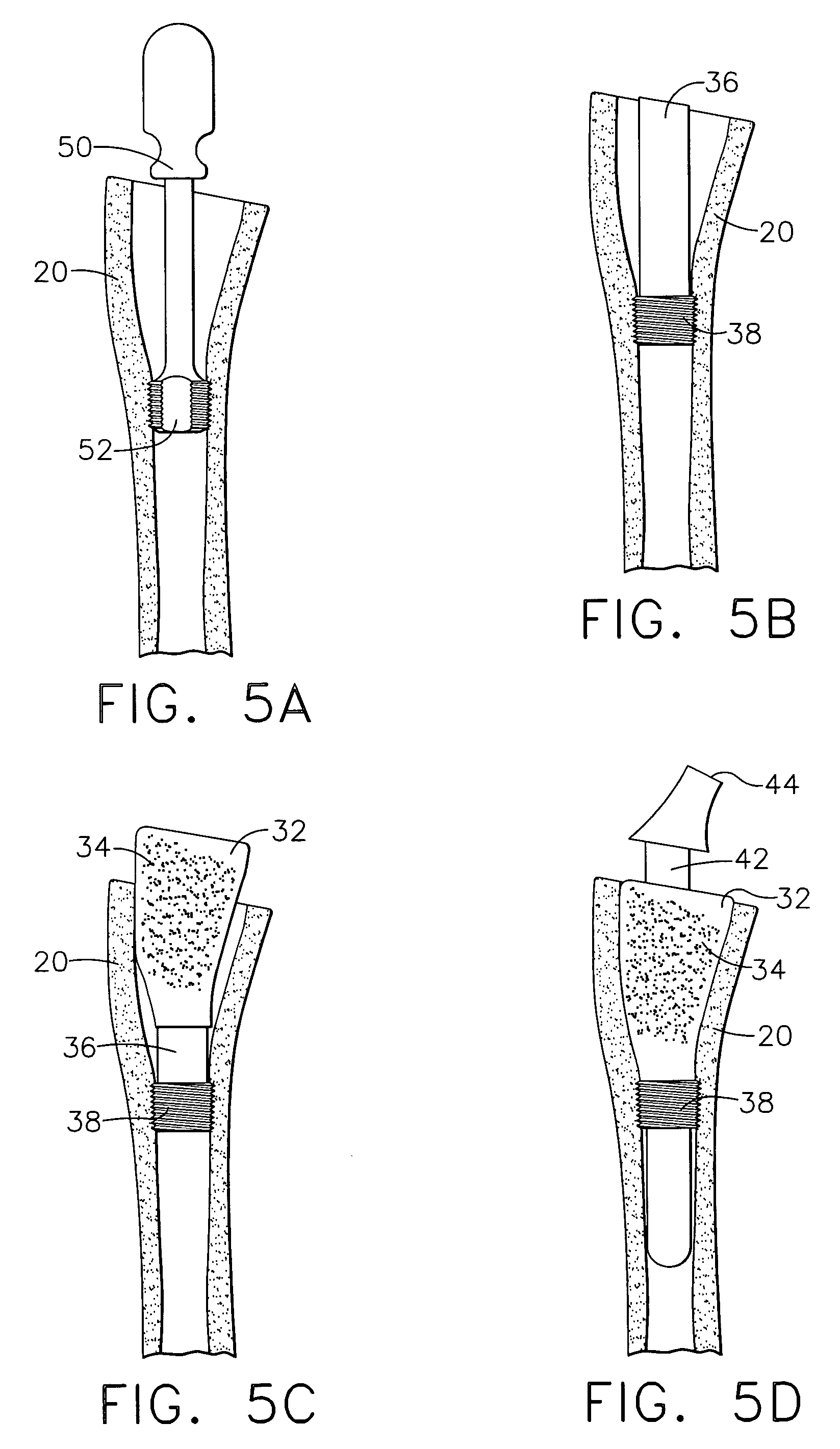
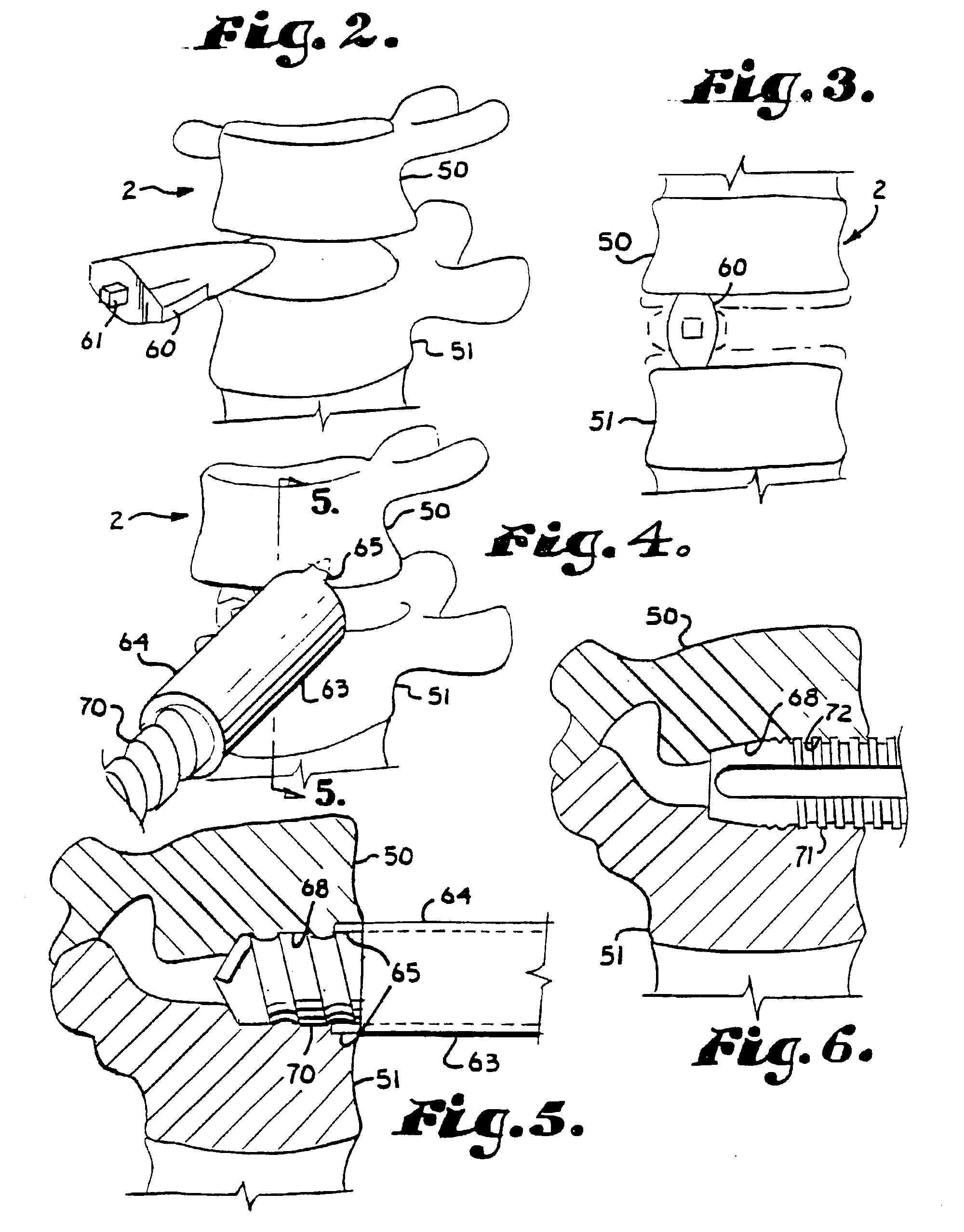
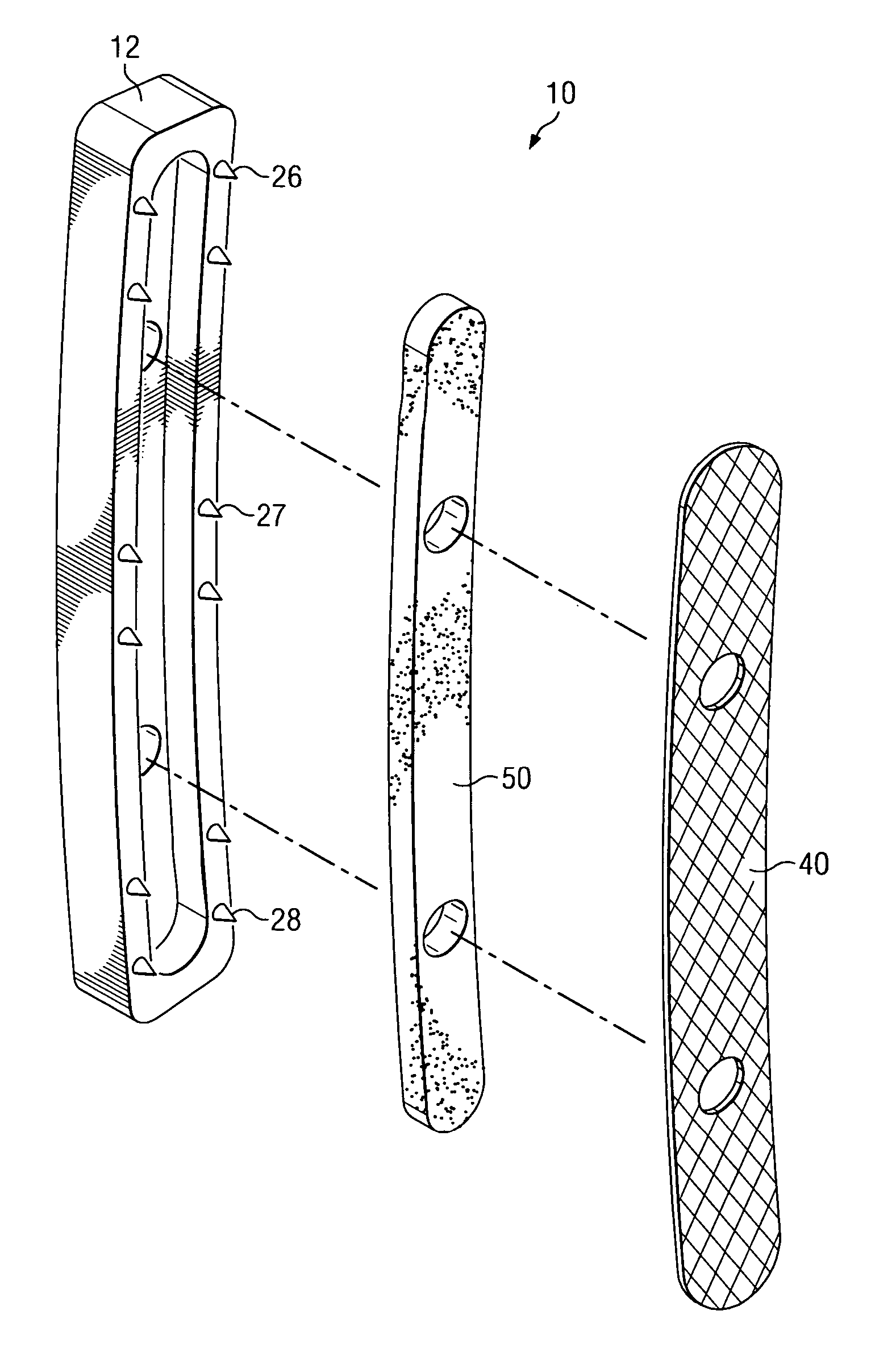
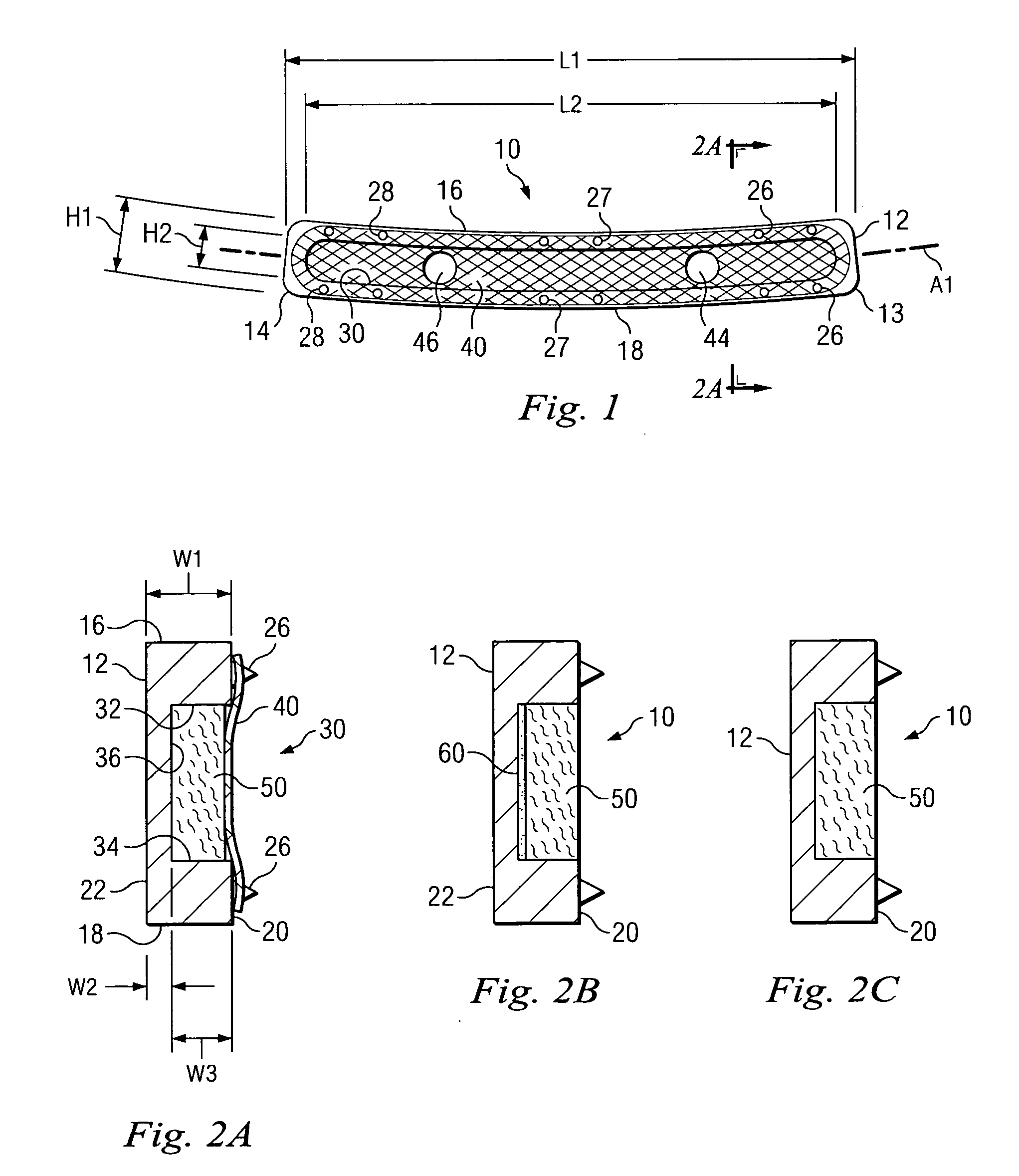
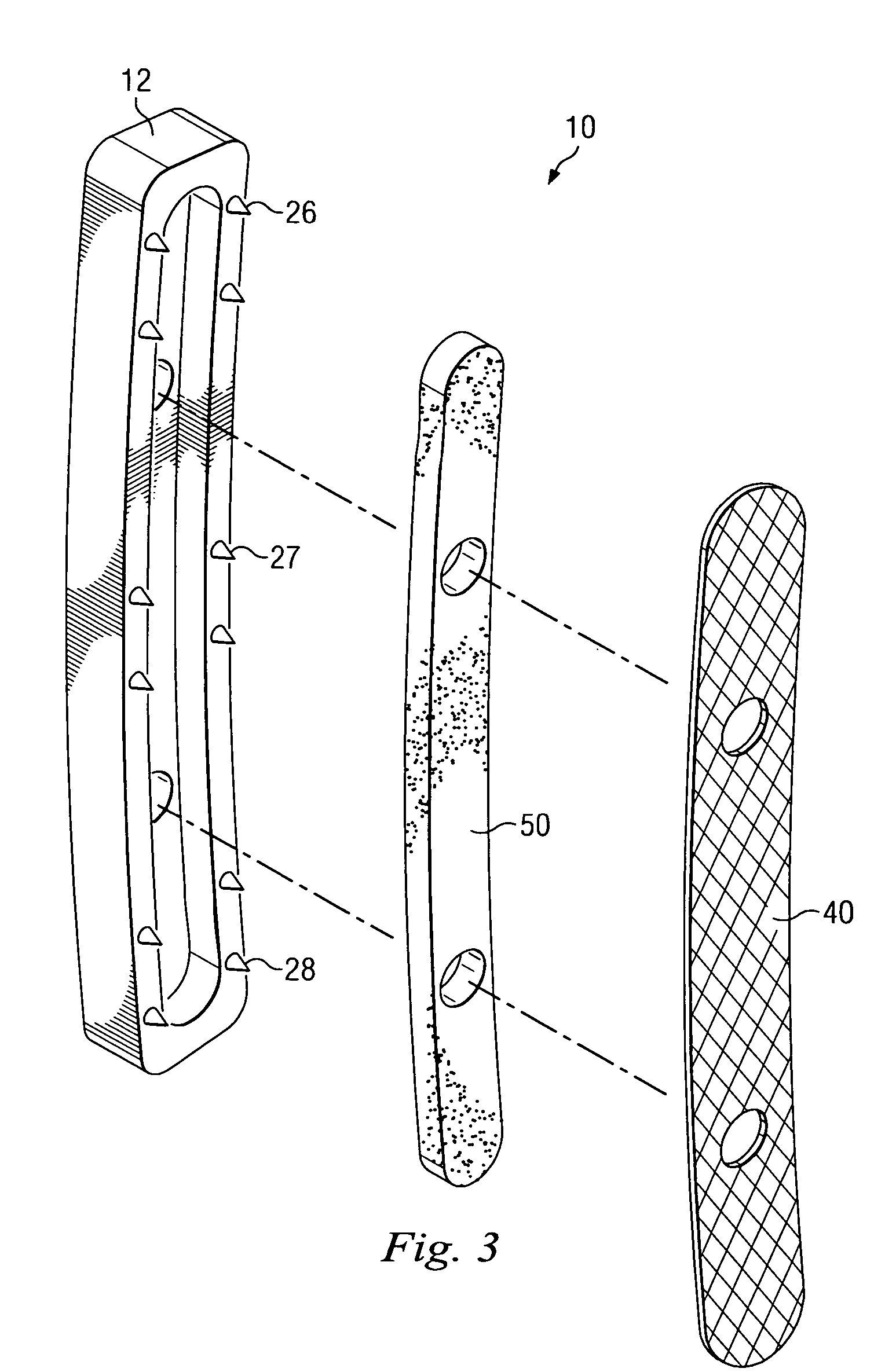
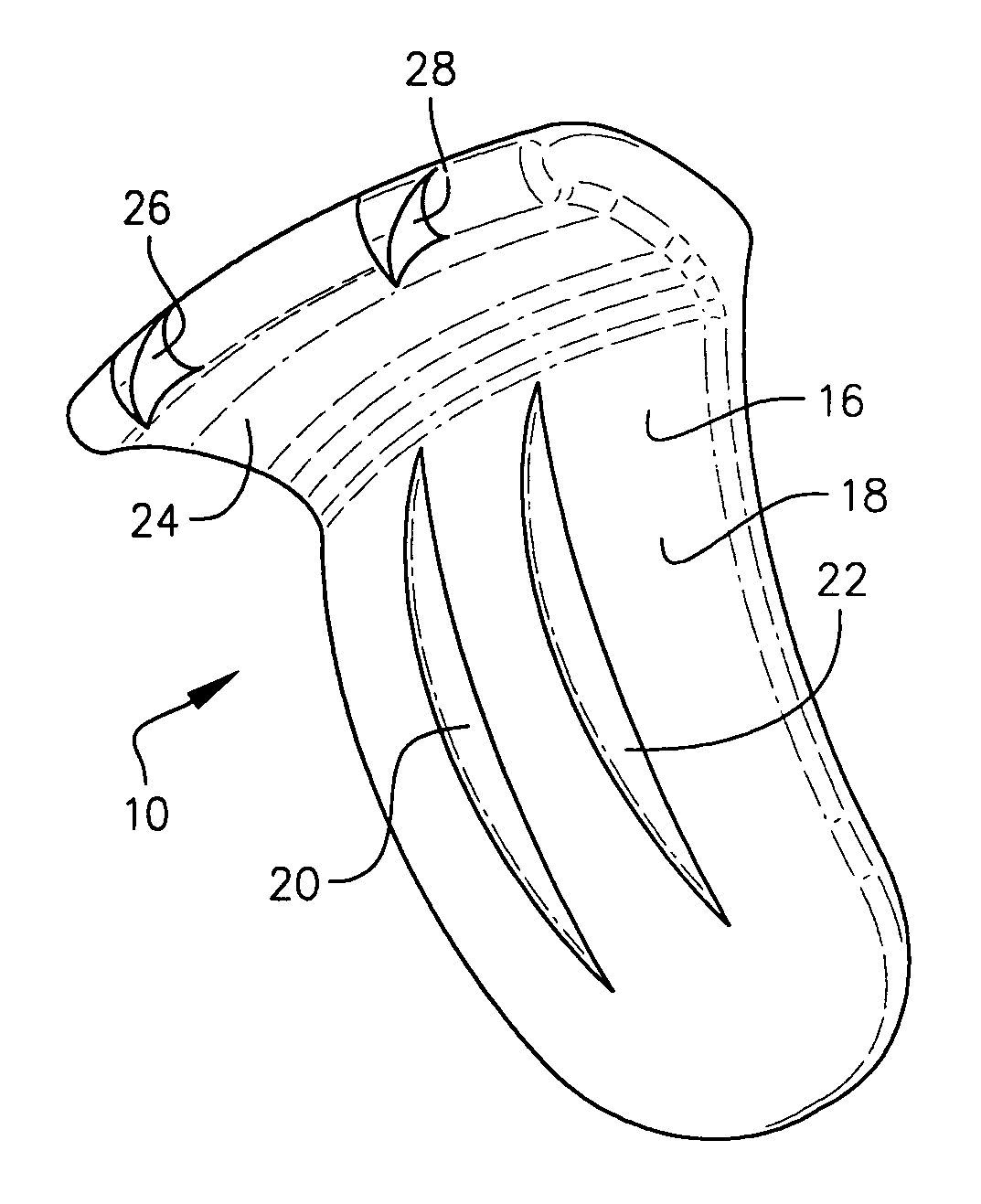
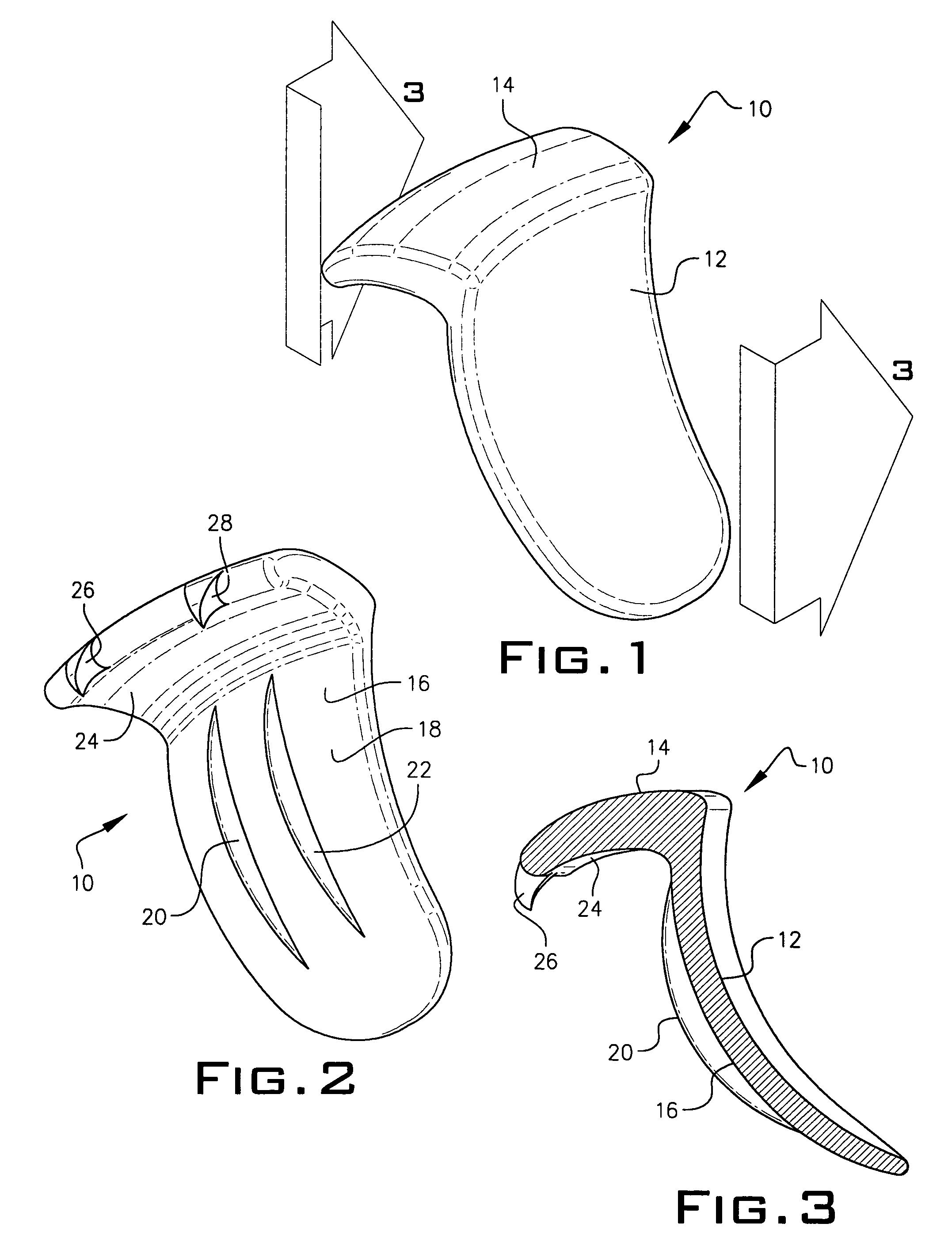
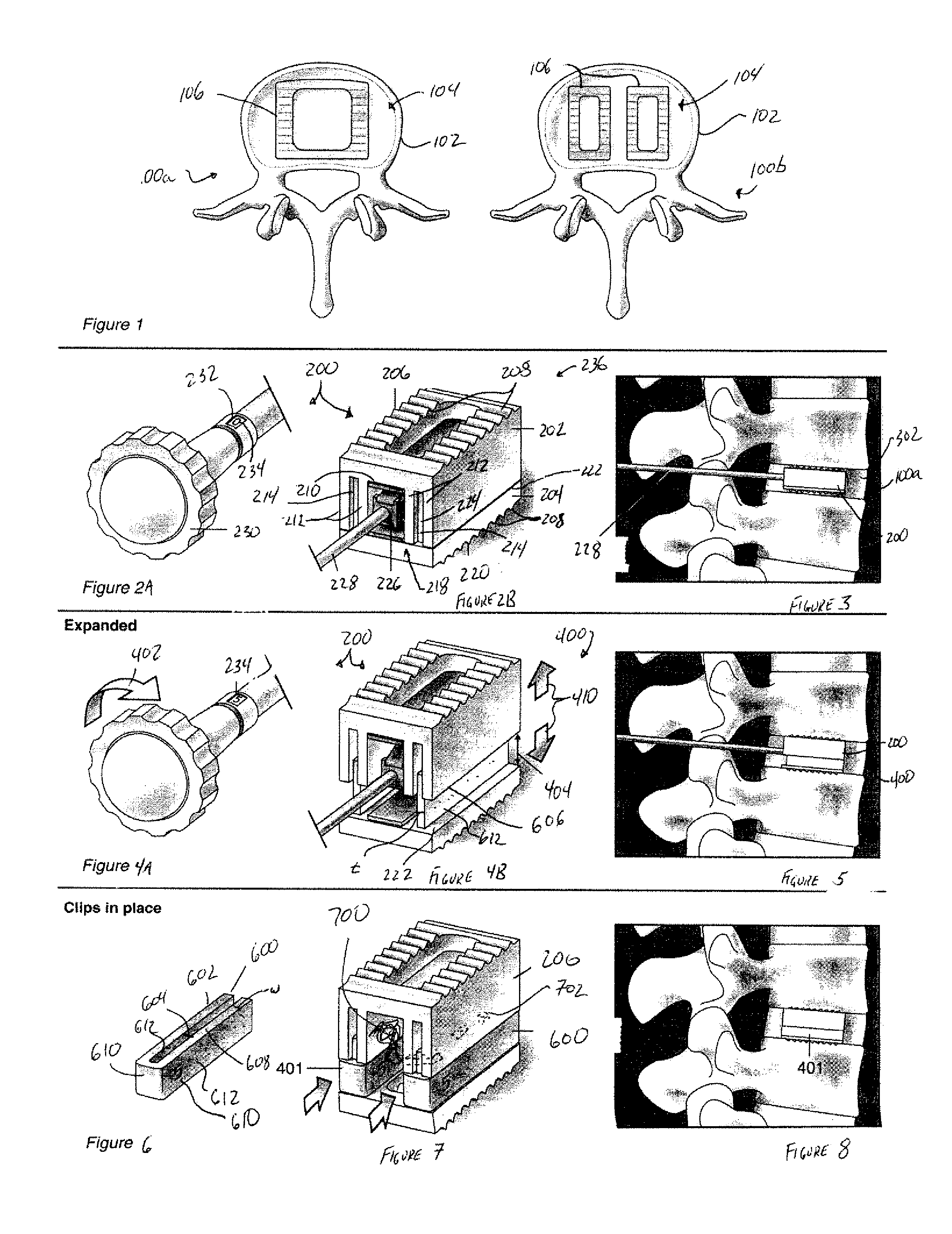
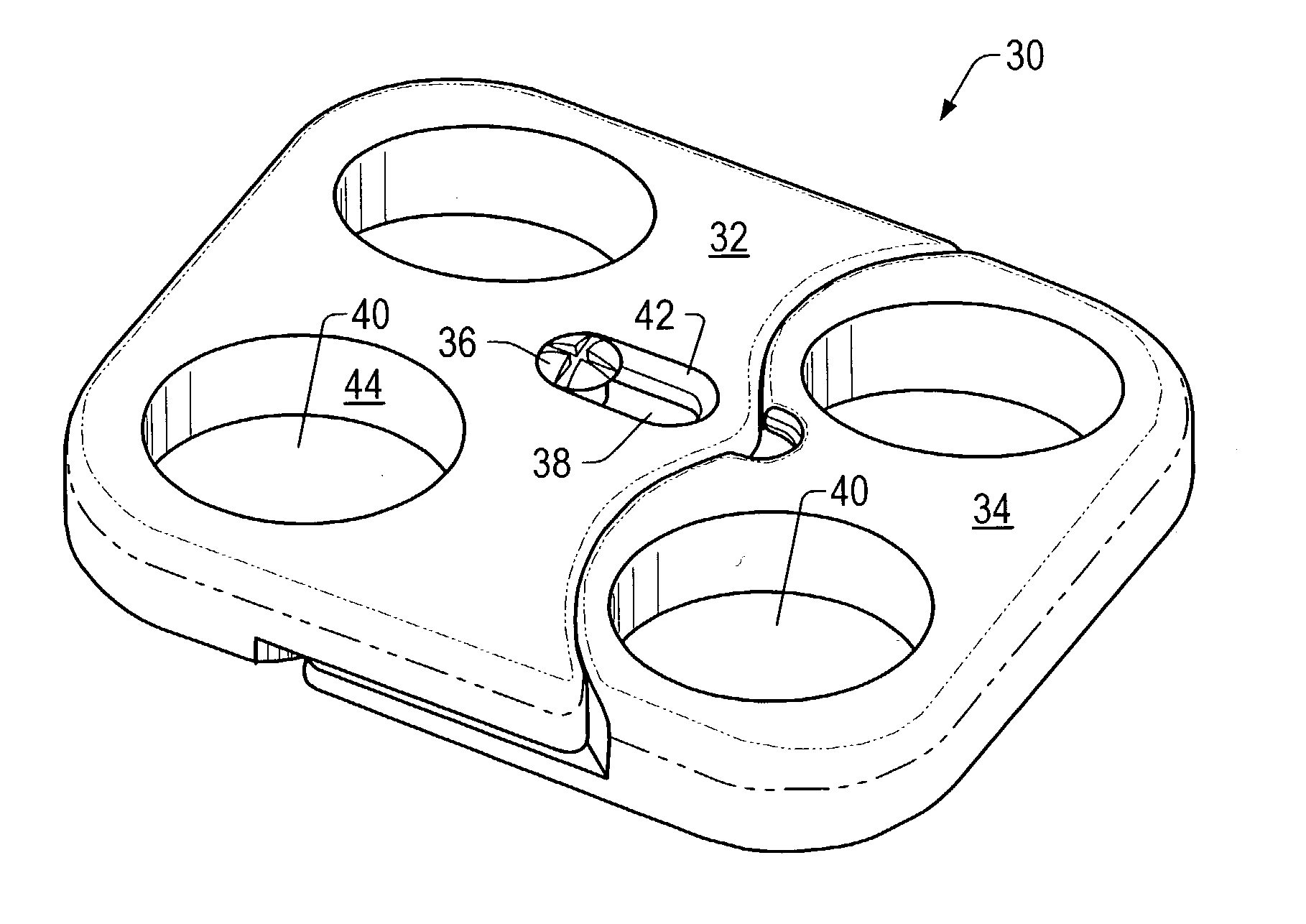
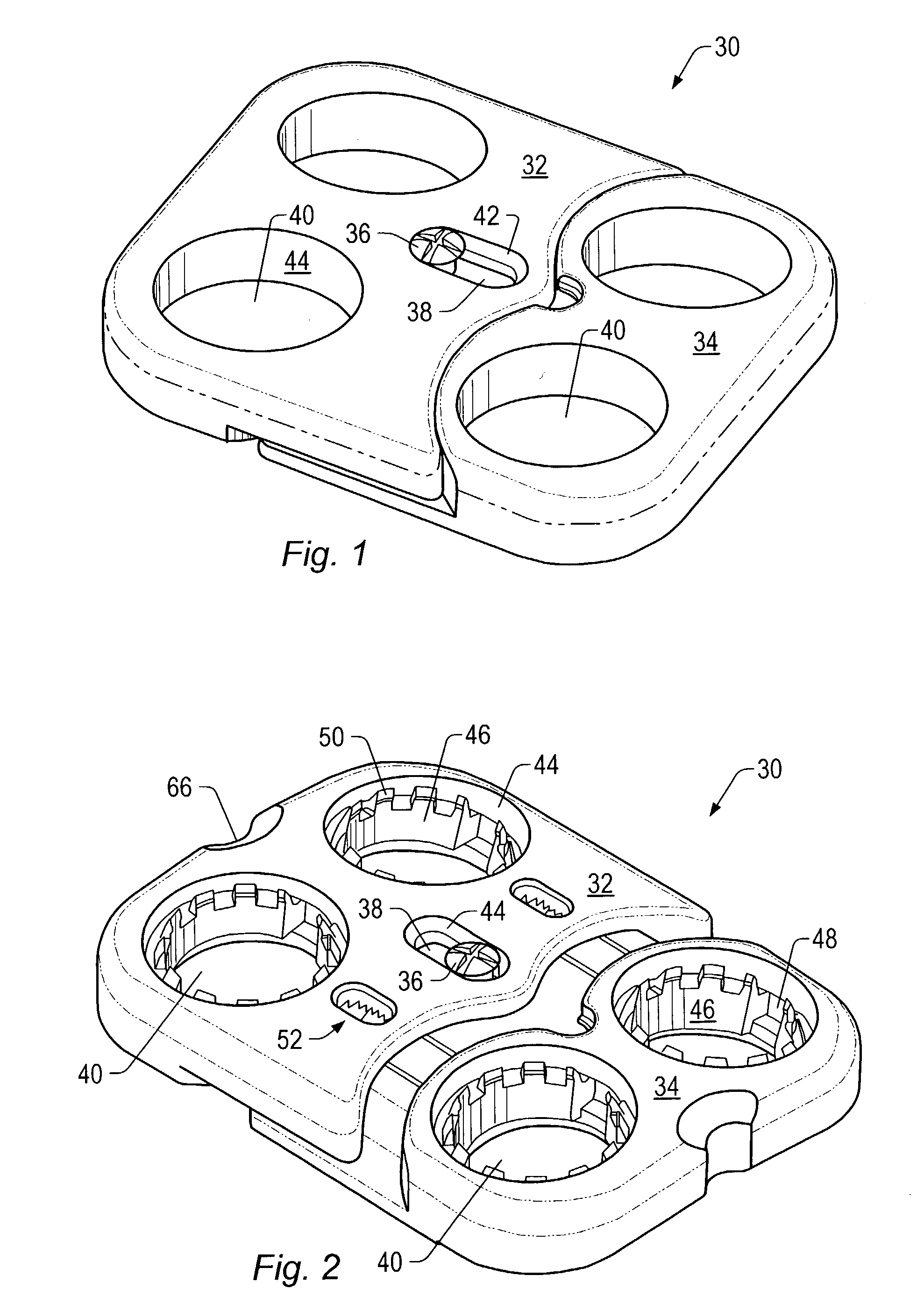
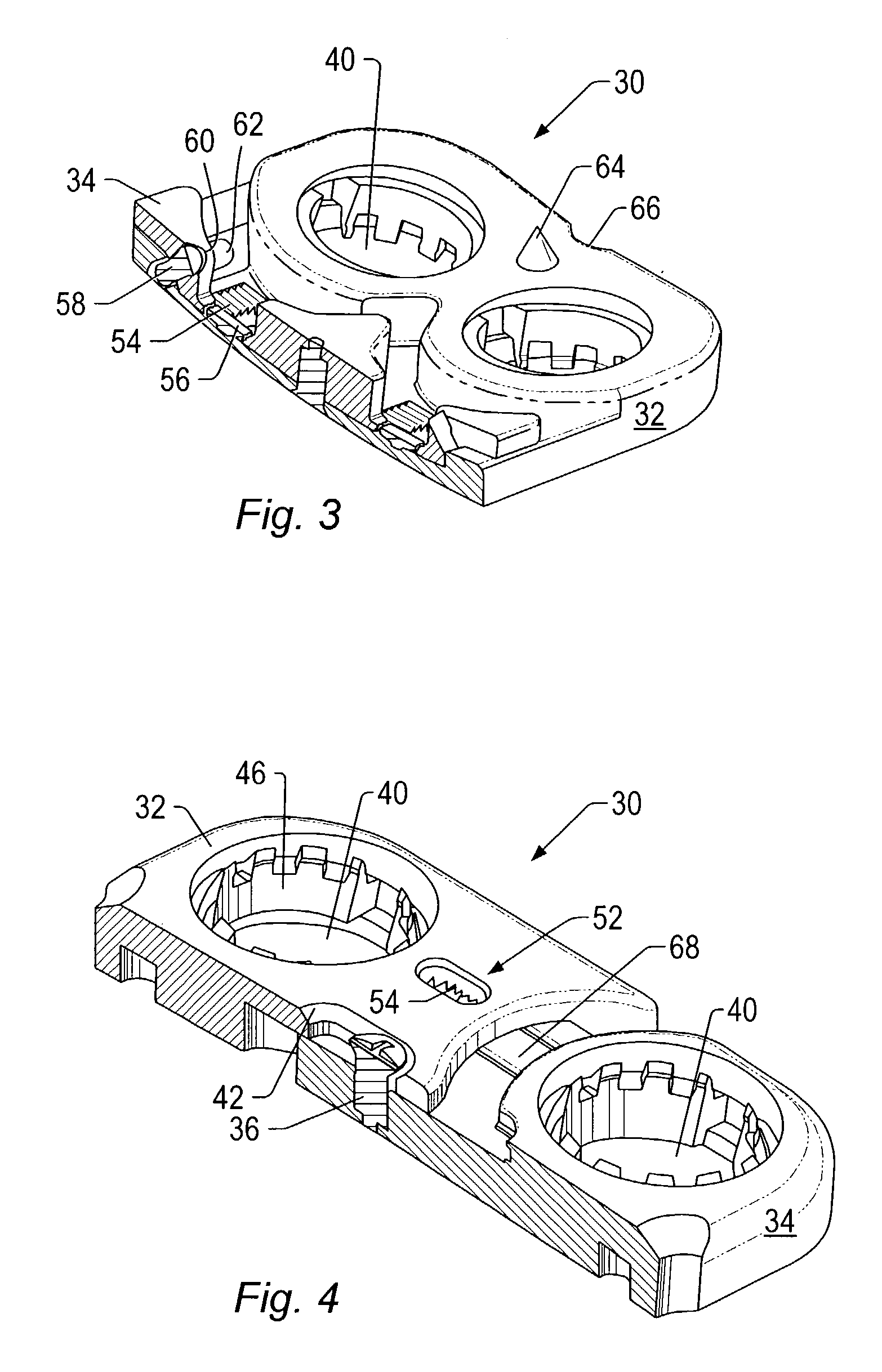
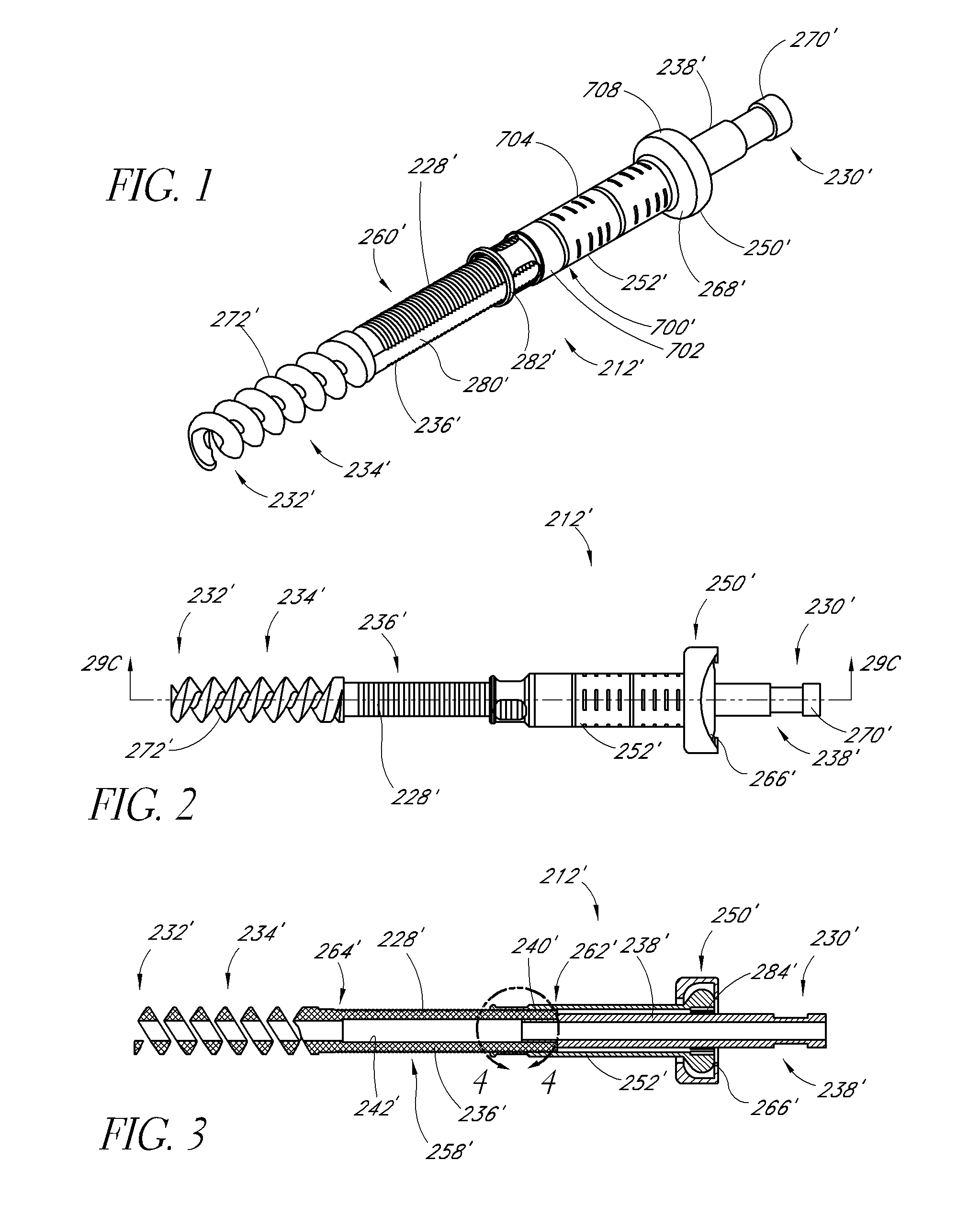
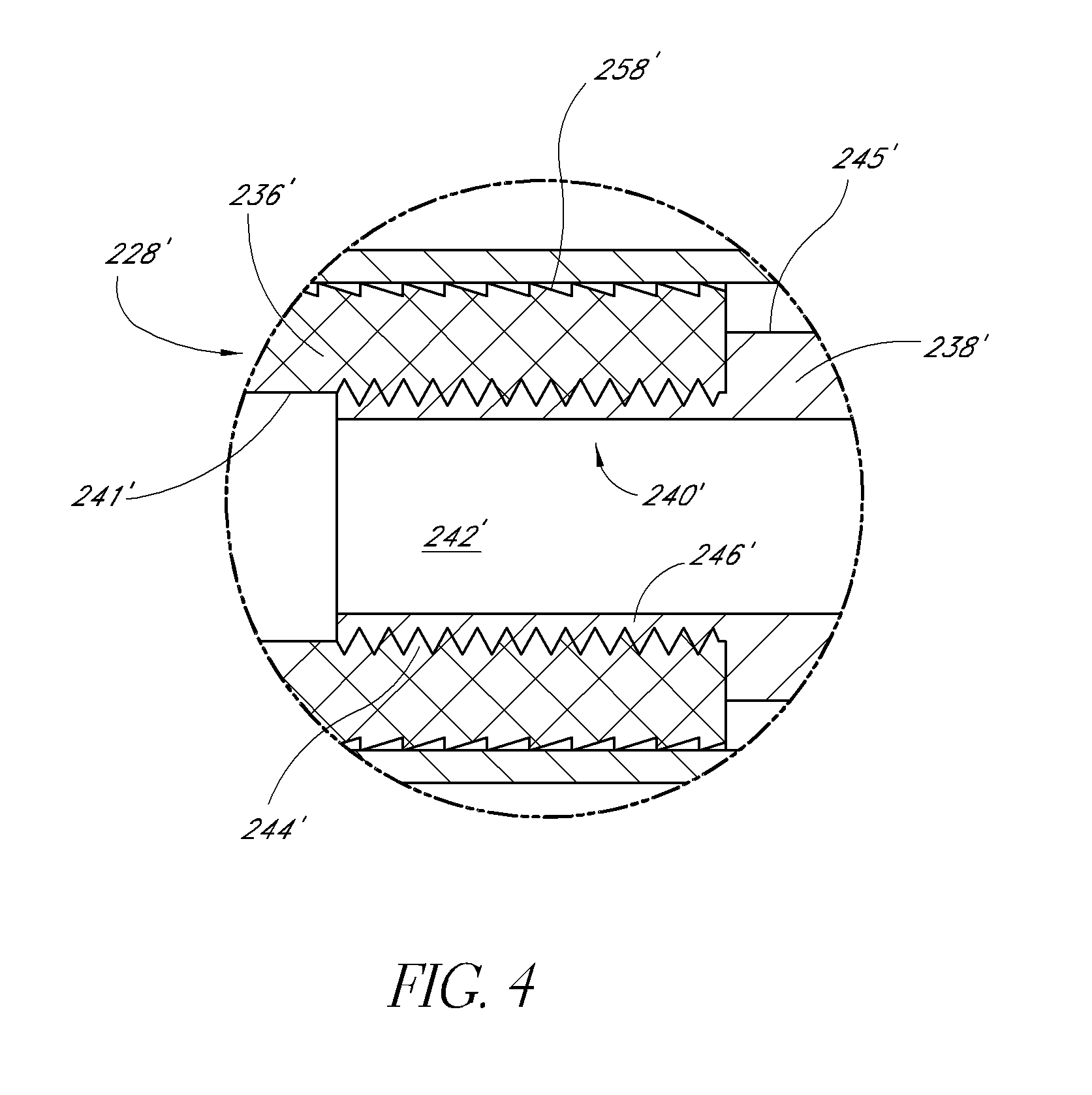
Expandable spinal fusion device and methods of promoting spinal fusion
InactiveUS7018415B1Promoting osteogenic fusionMinimal exposureBone implantJoint implantsSpinal columnBone growth
An intervertebral disc space implant includes spaced-apart bone engagement portions that define an intermediate chamber that holds bone growth inducing material into contact with adjacent vertebral bodies. The implant is expandable to establish and maintain desired intervertebral spacing during fusion. The implant includes a first member and a second member arranged to move relative to each other by action of an expansion member, the first member being engageable with the vertebral body below the disc space.
Owner:WARSAW ORTHOPEDIC INC
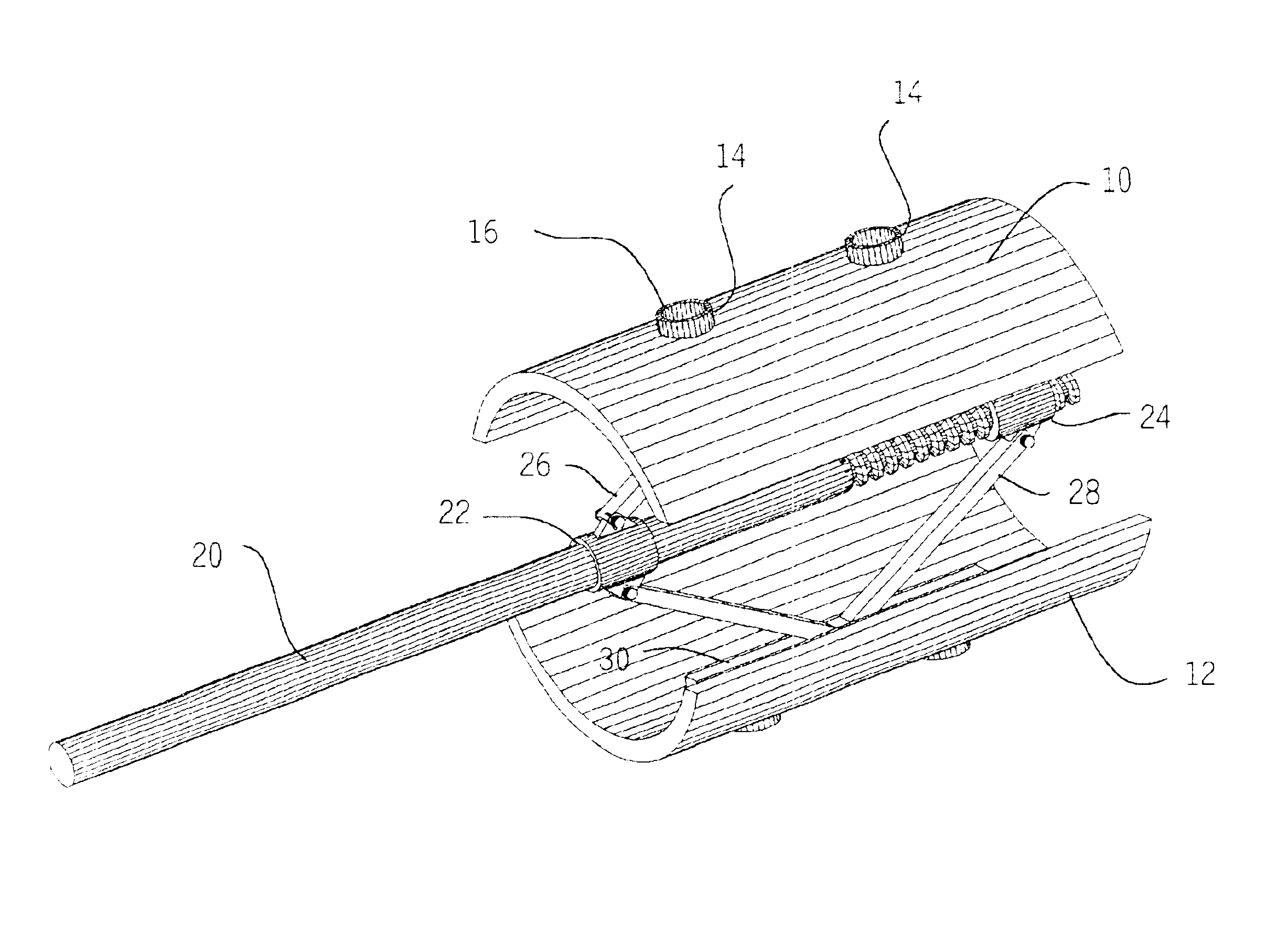
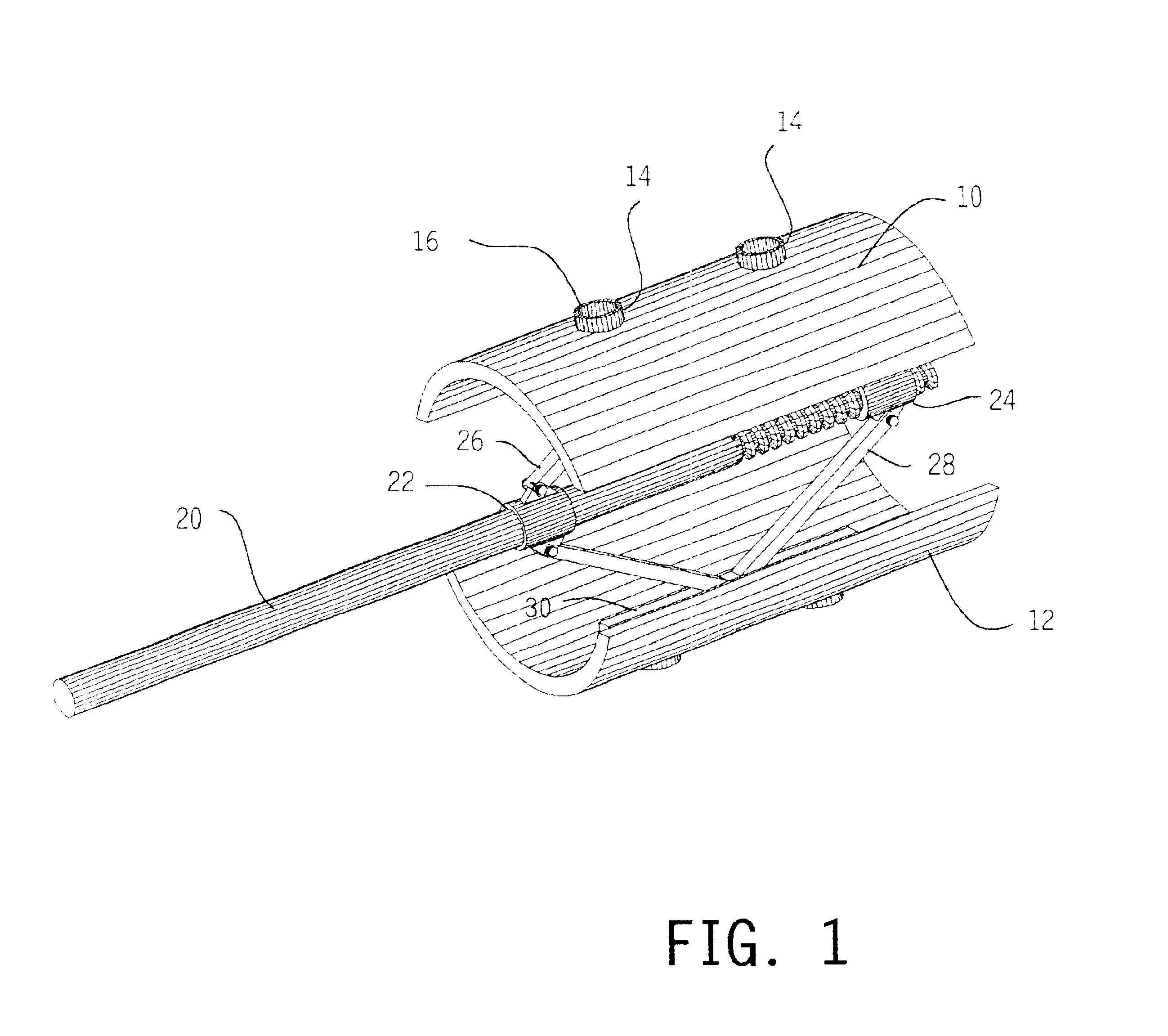
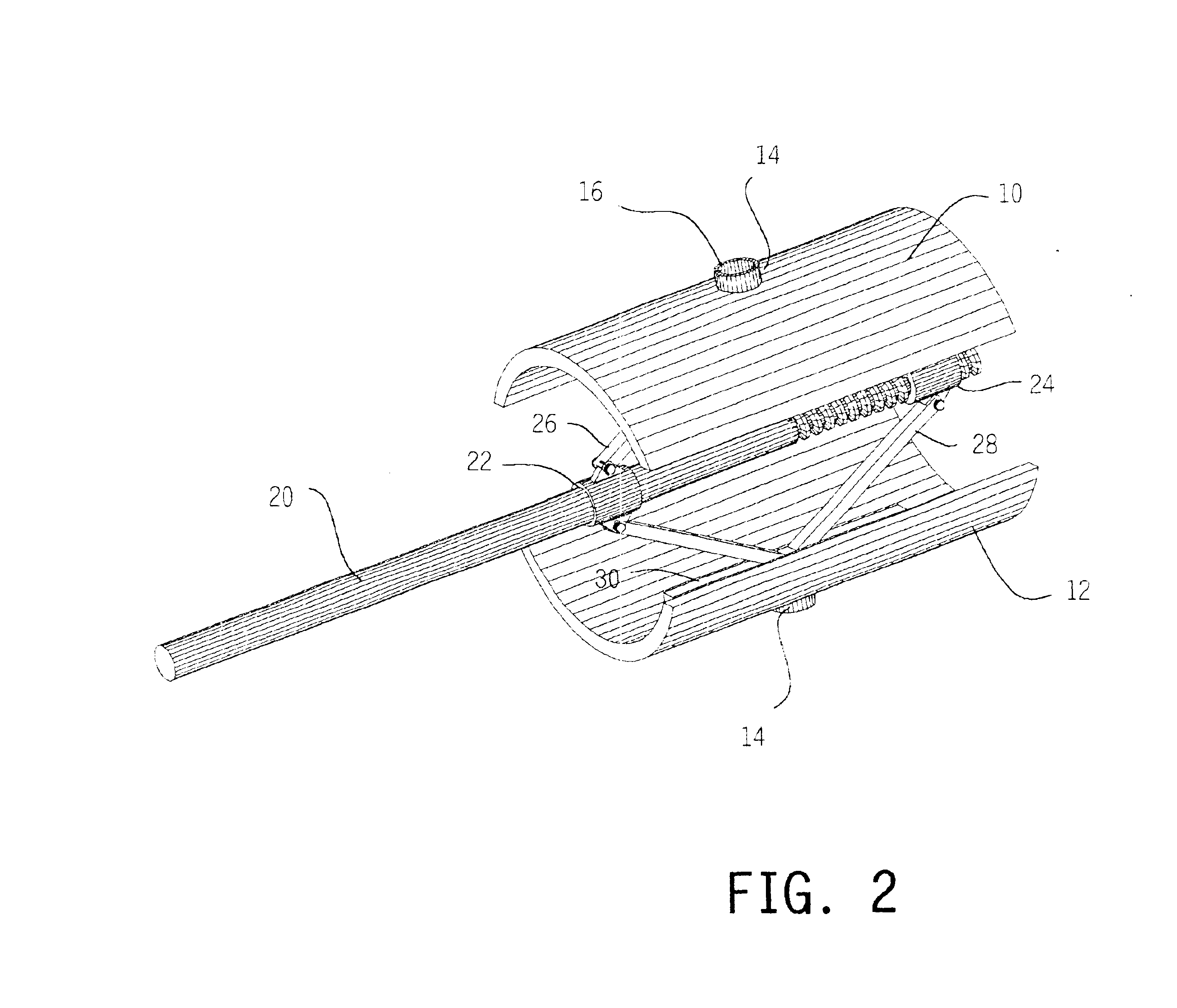
Methods and apparatus for treating spinal stenosis
InactiveUS20060106381A1Effective treatmentPermit flexionInternal osteosythesisJoint implantsSpinal columnDevice form
Surgical implants are configured for placement posteriorly to a spinal canal between vertebral bodies to distract the spine and enlarge the spinal canal. In the preferred embodiments the device permits spinal flexion while limiting spinal extension, thereby providing an effective treatment for treating spinal stenosis without the need for laminectomy. The invention may be used in the cervical, thoracic, or lumbar spine. Numerous embodiments are disclosed, including elongated, length-adjustable components coupled to adjacent vertebral bodies using pedicle screws. The preferred embodiments, however, teach a device configured for placement between adjacent vertebral bodies and adapted to fuse to the lamina, facet, spinous process or other posterior elements of a single vertebra. Various mechanisms, including shape, porosity, tethers, and bone-growth promoting substances may be used to enhance fusion. The tether may be a wire, cable, suture, or other single or multi-filament member. Preferably, the device forms a pseudo-joint in conjunction with the non-fused vertebra. Alternatively, the device could be fused to the caudal vertebra or both the cranial and caudal vertebrae.
Owner:NUVASIVE
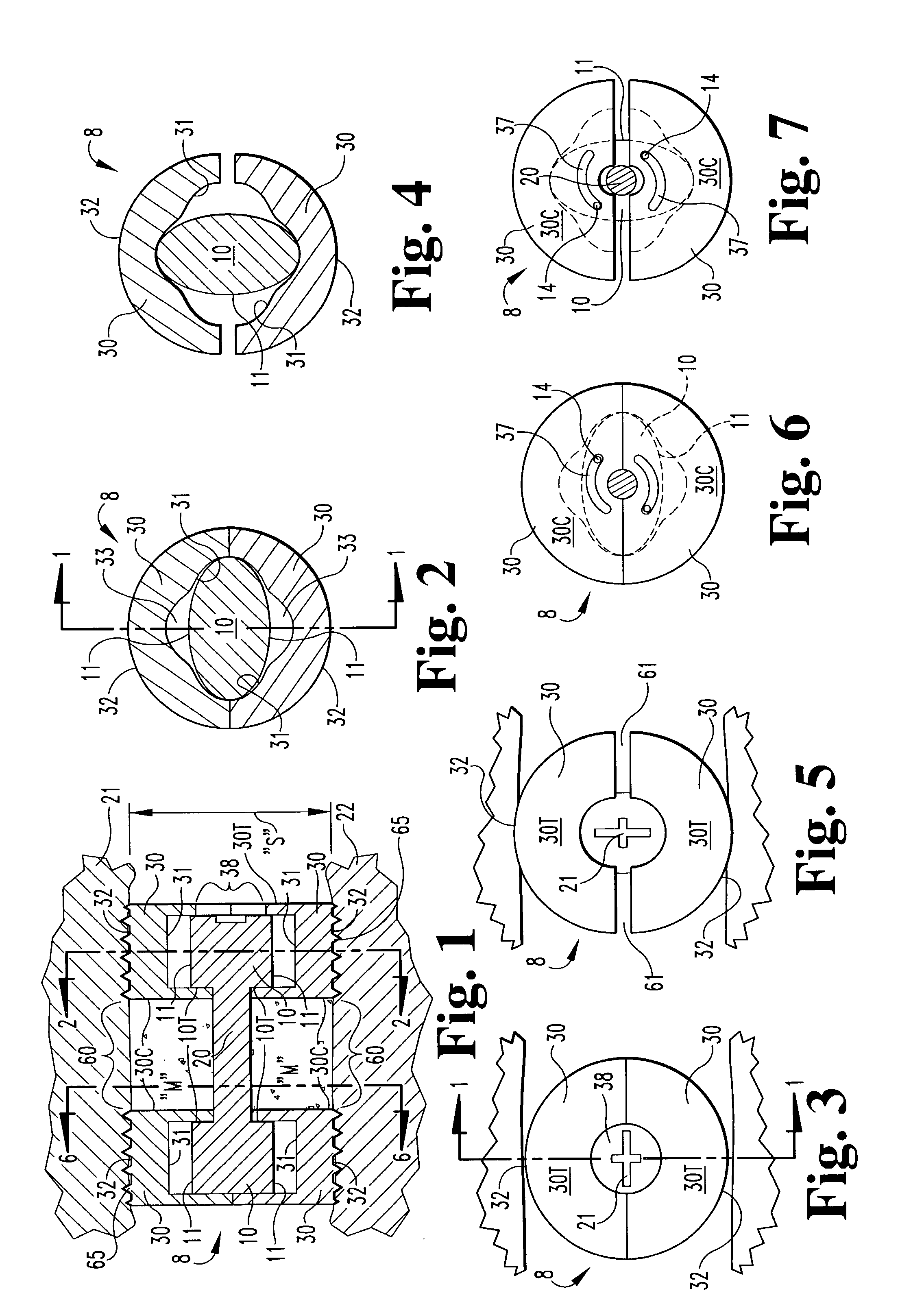
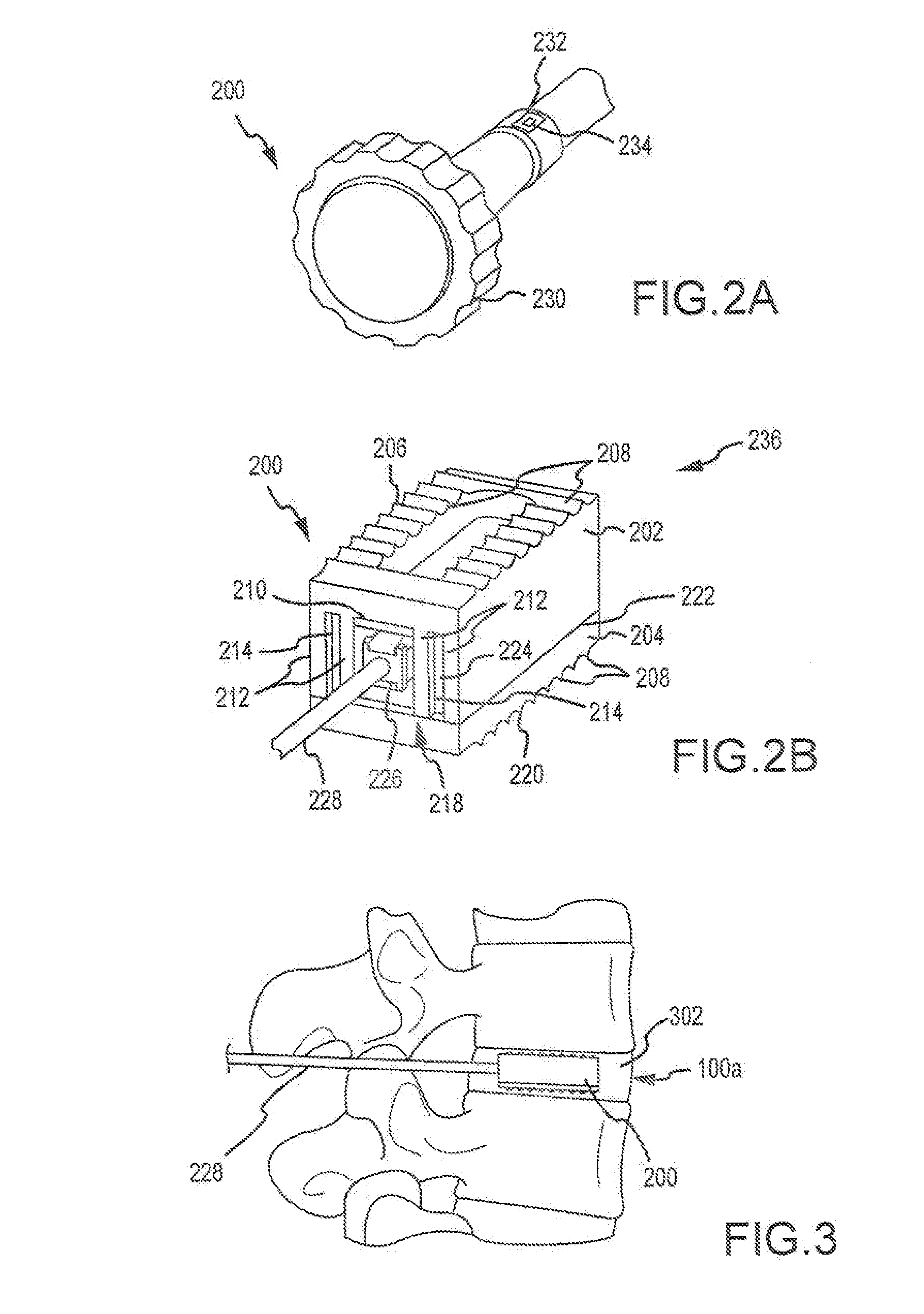
Expandable spinal fusion cage
An expandable spinal fusion device is provided. The expandable device comprises a first part slidingly coupled to a second part. An removable expandable member extends between the first part and the second part and is coupled to a rotating operator such that rotating the operator causes the first part and the second part to move away from each other and distract vertebral bodies. A spacer or clip is used to lock the first part in relation to the second part to allow bone growth the fuse the vertebral bodies.
Owner:ZIMMER BIOMET SPINE INC
Expandable spinal fusion device and methods of promoting spinal fusion
ActiveUS20060149385A1Easy to integrateMinimal exposureBone implantJoint implantsSpinal columnBone growth
An intervertebral disc space implant includes spaced-apart bone engagement portions that define an intermediate chamber that holds bone growth inducing material into contact with adjacent vertebral bodies. The implant is expandable to establish and maintain desired intervertebral spacing during fusion. The implant includes a first member and a second member arranged to move relative to each other by action of an expansion member, the first member being engageable with the vertebral body below the disc space.
Owner:SDGI HLDG
Expandable spinal fusion cage
An expandable spinal fusion device is provided. The expandable device comprises a first part slidingly coupled to a second part. An removable expandable member extends between the first part and the second part and is coupled to a rotating operator such that rotating the operator causes the first part and the second part to move away from each other and distract vertebral bodies. A spacer or clip is used to lock the first part in relation to the second part to allow bone growth the fuse the vertebral bodies.
Owner:LANX INC
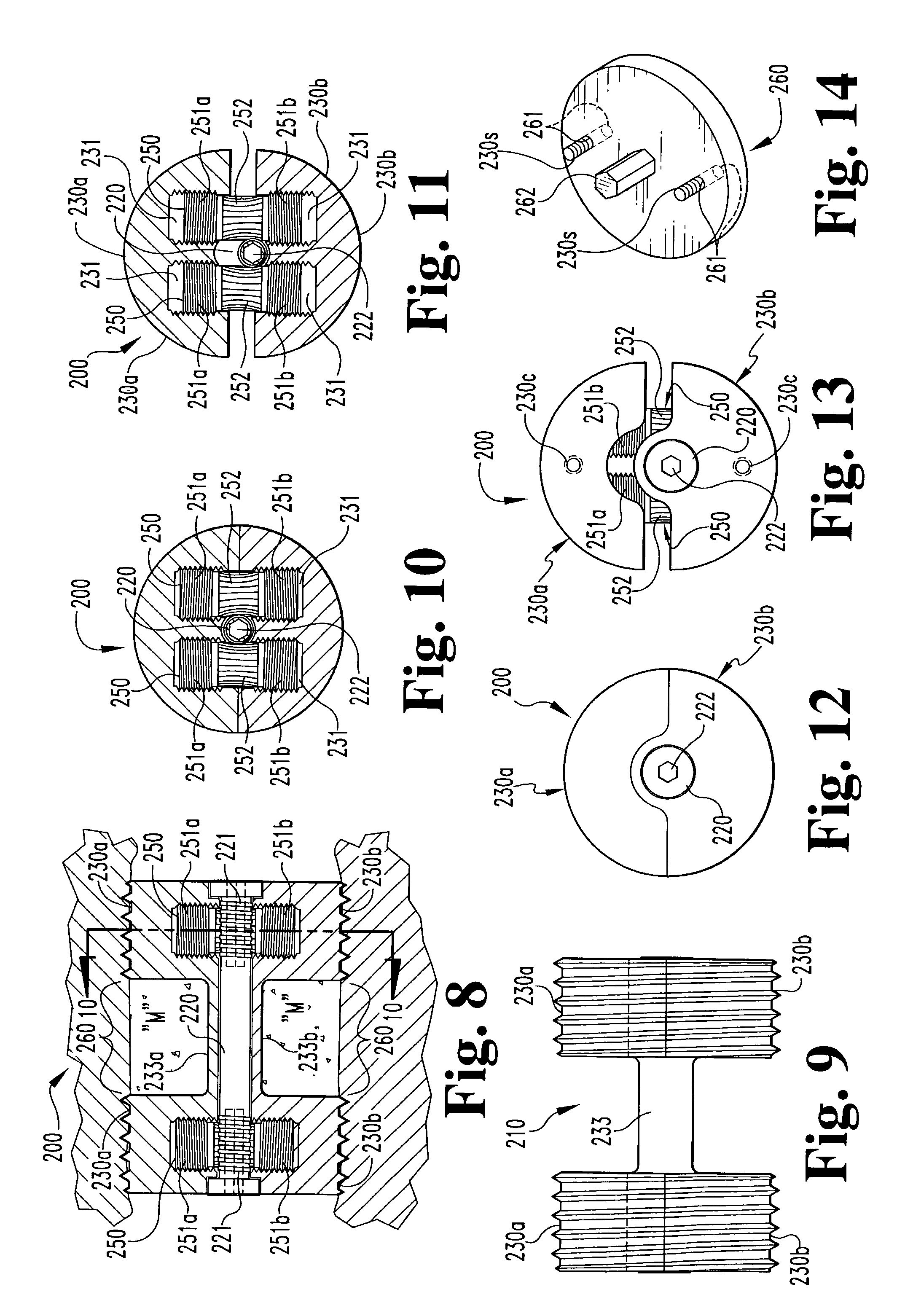
Bone implants and methods
The disclosure provides implants and methods for bone fusion procedures. In some embodiments, the implants are particularly advantageous for use between opposing vertebral bodies to facilitate stabilization or arthrodesis of an intervertebral joint. The implants includes, at least, a support component that provides structural support during fusion. In a typical embodiment, the implants also include a growth component. A growth component provides an environment conductive to new bone growth between the bones being fused. Several unique configuration to enhance fusion, instruments for insertion and methods for insertion use are also disclosed.
Owner:ZIMMER SPINE INC
Biocompatible wires and methods of using same to fill bone void
InactiveUS20050015148A1Reduce compression fractureInternal osteosythesisSpinal implantsWire rodBone structure
Devices, kits, and methods are provided for reducing a bone fracture, e.g., a vertebral compression fracture, is provided. The device comprises a plurality of resilient wires composed of a biocompatible material, such as a biocompatible polymer (e.g., polymethylmethacrylate (PMMA)). The wires can be introduced into the cavity of the bone structure to form a web-like arrangement therein. The web-like arrangement can be stabilized by applying uncured bone cement onto the arrangement to connect the wires at their contacts point. The bone cavity can then be filled with a bone growth enhancing medium.
Owner:BOSTON SCI SCIMED INC
Apparatus for fusing adjacent bone structures
InactiveUS6979353B2Easy to optimizeEasy to integrateBone implantSurgeryBone structuresIntervertebral space
Owner:HOWMEDICA OSTEONICS CORP
Medical and dental implant devices for controlled drug delivery
Implantable devices and methods for use in the treatment of osteonecrosisare provided. The device includes at least one implant device body adapted for insertion into one or more channels or voids in bone tissue; a plurality of discrete reservoirs, which may preferably be microreservoirs, located in the surface of the at least one implant device body; and at least one release system disposed in one or more of the plurality of reservoirs, wherein the release system includes at least one drug selected from the group consisting of bone growth promoters, angiogenesis promoters, analgesics, anesthetics, antibiotics, and combinations thereof. The device body may be formed of a bone graft material, a polymer, a metal, a ceramic, or a combination thereof. The device body may be a monolithic structure, such as one having a cylindrical shape, or it may be in the form of multiple units, such as a plurality of beads.
Owner:MICROCHIPS INC
Method and apparatus for spinal augmentation
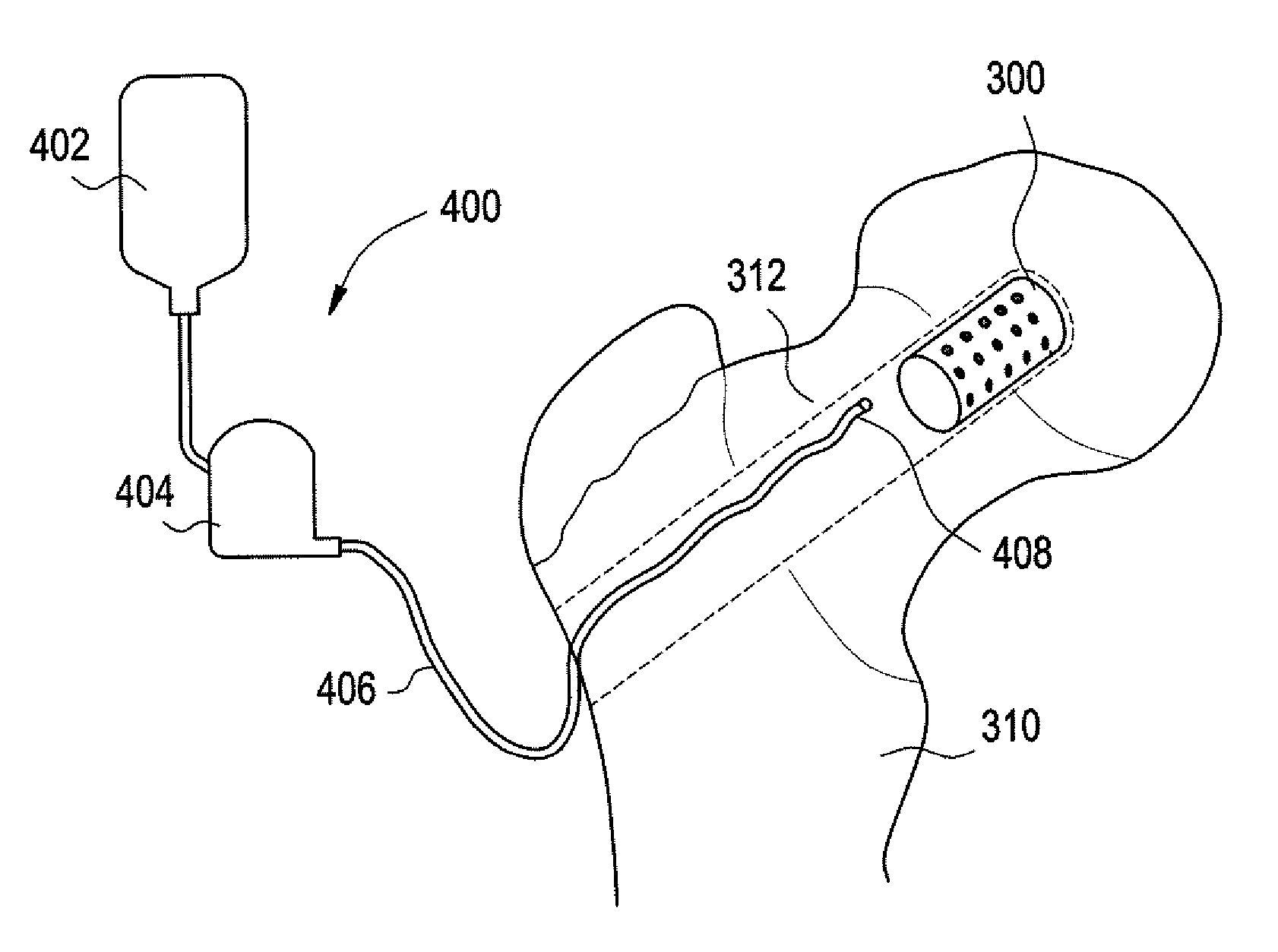
Disclosed are methods and devices for accessing and treating the spine, while minimizing trauma to surrounding tissue. A device is introduced through tissue, to an access point on the spine. The device is thereafter advanced axially within the spine, from the access point across a treatment zone. Spinal fusion cages, other fusion implants and / or bone growth material is introduced into at least one vertebral body and / or at least one disc space.
Owner:MIS IP HLDG LLC
Artificial disc replacements with deployable fixation components
InactiveUS20040030389A1Reduce shear stressInternal osteosythesisDiagnosticsIntervertebral discBone growth
Arthroplasty devices having improved bone in growth to provide a more secure connection within the body. Different embodiments disclosed include devices having threaded intramedullary components, devices configured to receive bone growth promoting substances, devices with resorbable components, and devices configured to reduce shear stress.
Owner:FERREE BRET A
Vertebral body end plate cutter
InactiveUS6840944B2Easy to integrateEasy to manufactureFractureEndoscopic cutting instrumentsProsthesisEngineering
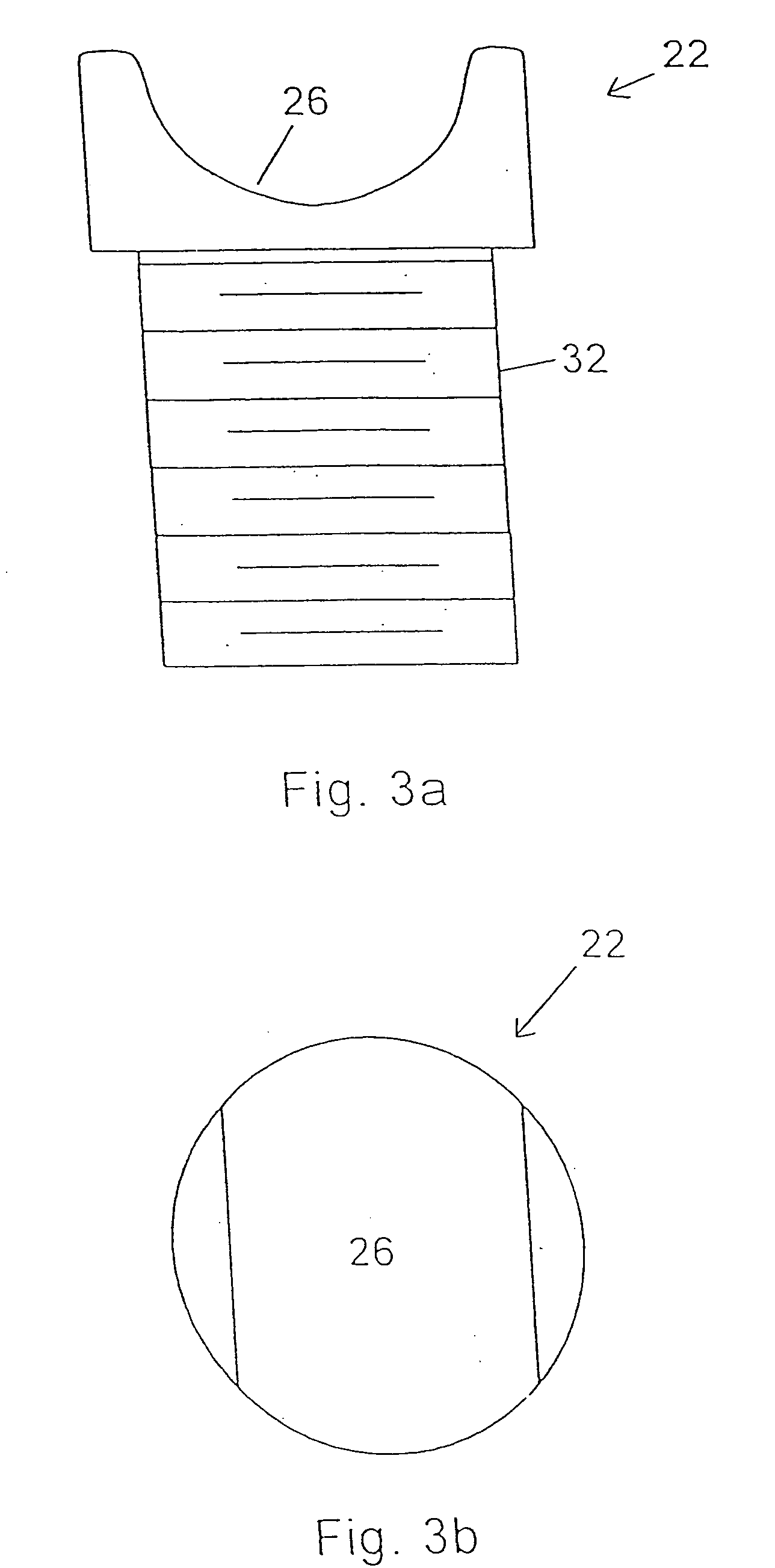
Precision recesses are cut in the end plates of vertebral bodies by inserting into the disc space a cutter having a pair of shell elements provided with cutting edges shaped to match bone growth apertures in a selected prosthesis. The remainder of the end plates are left undisrupted, to maximize bearing strength while promoting bone growth at the apertures.
Owner:SUDDABY LOUBERT
Spine distraction implant and method
Owner:KYPHON
Expandable spinal implant apparatus and method of use
ActiveUS20100286779A1Decrease patient riskIncrease success rateBone implantSpinal implantsEngineeringCam
A spinal implant apparatus that is an expandable spacer including features to minimize or eliminate spacer cant or offset during and after completing the expansion process. The spacer includes a top component, a base component in engagement with the top component, and an expansion mechanism arranged to change the top component's position with respect to the base component. The mechanism for causing expansion may be a screw, a cam, a wedge or other form of distracting device. In one embodiment, the expandable spacer includes a base component with a set of towers and a top component with a set of corresponding silos, where the towers and silos are configured to minimize or eliminate tilt of the top component as it extends upwardly from the base component. In another embodiment, the spacer may include a stepped arrangement around the perimeter of the top component and the base component for engagement during height expansion with minimal canting or slippage. In another embodiment, the spacer may include texturing modification at the opposite ends of the longitudinal axis of the spacer to prevent tilting, slipping, or canting. Additionally, a portion of one or more exterior surfaces of the spacer may be textured, sawtoothed, dovetailed or the like to increase frictional intervertebral contact. The spacer may contain one or more passageways of selectable shape / dimension for bone growth through the spacer.
Owner:STRYKER EURO OPERATIONS HLDG LLC
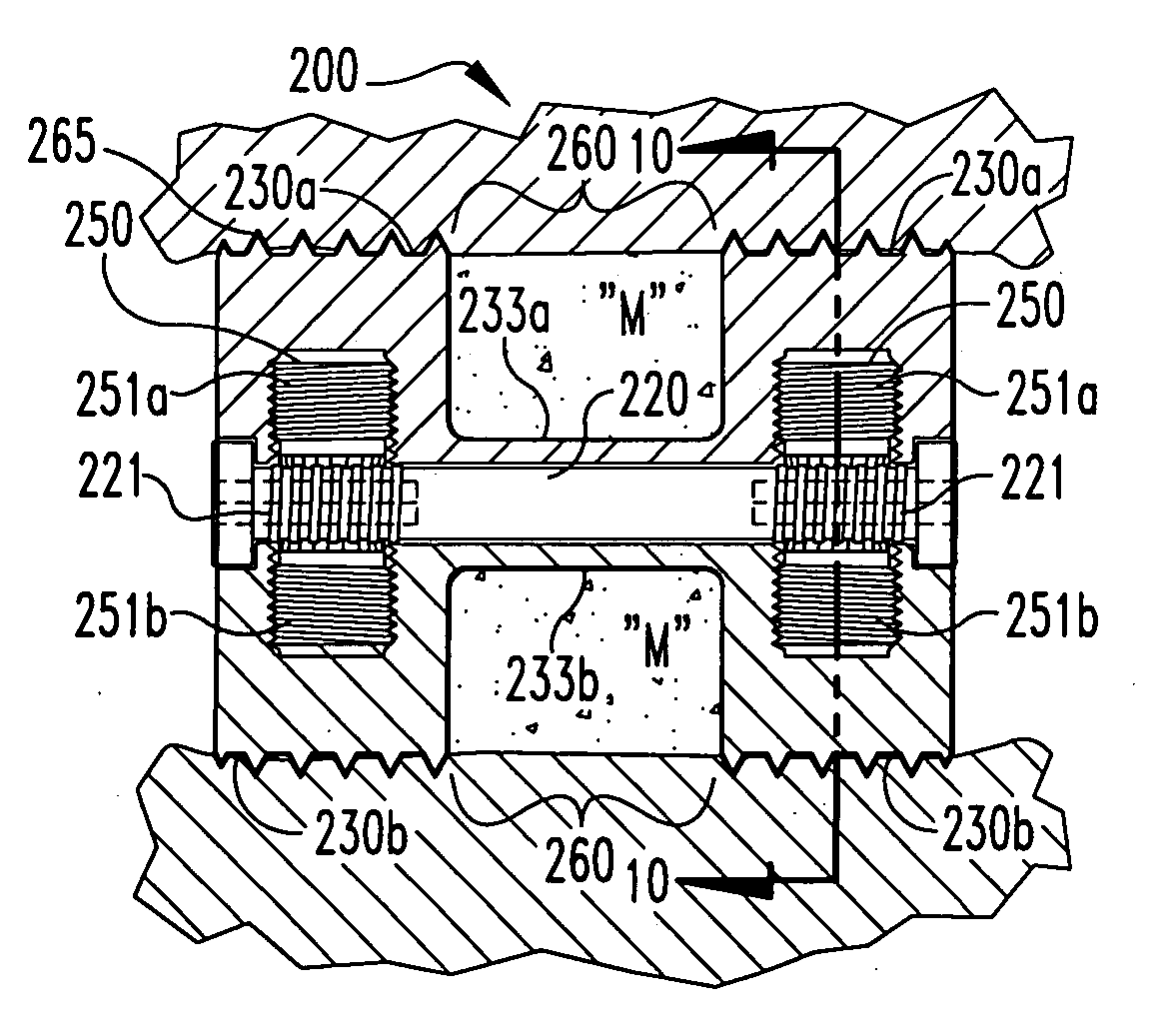
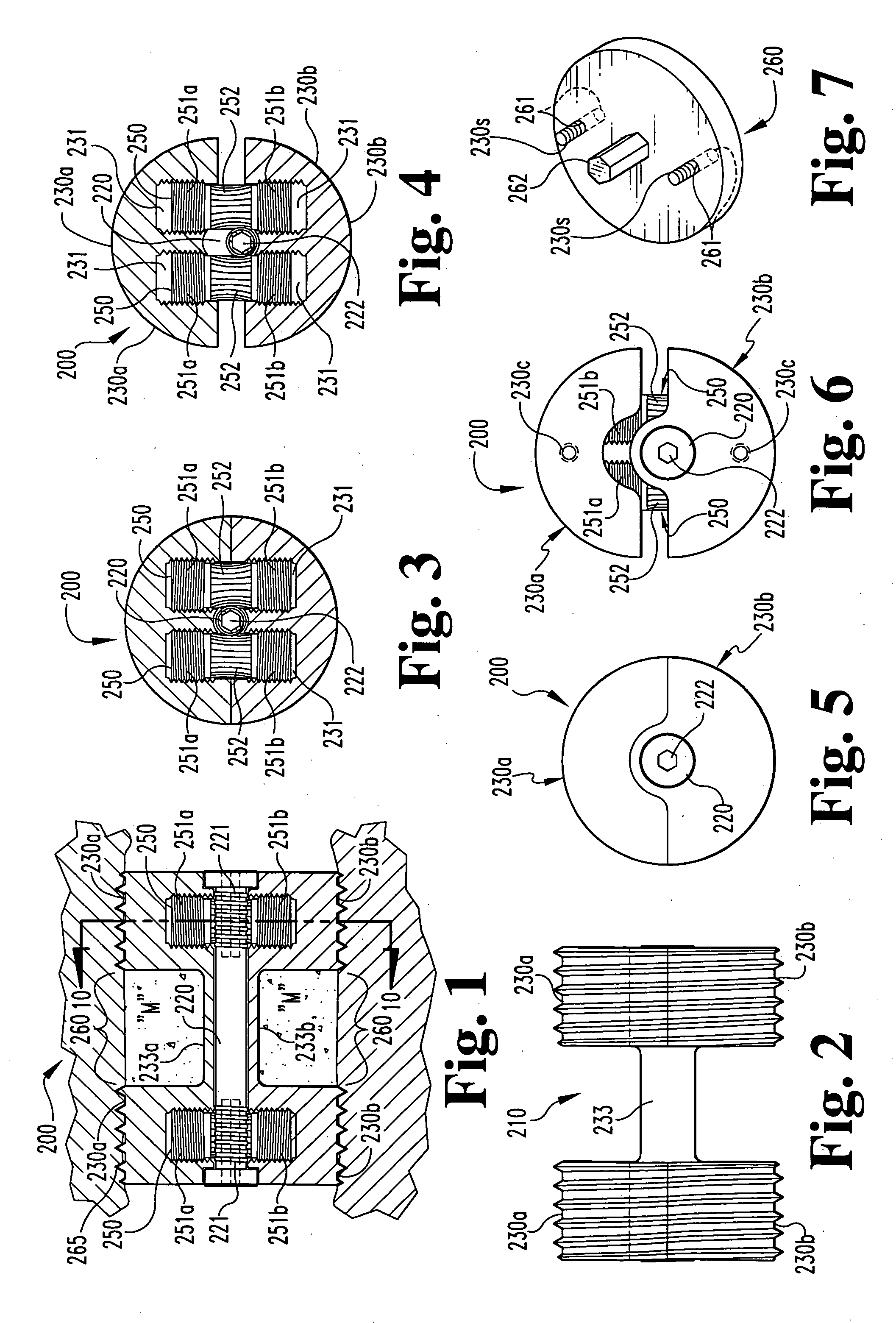
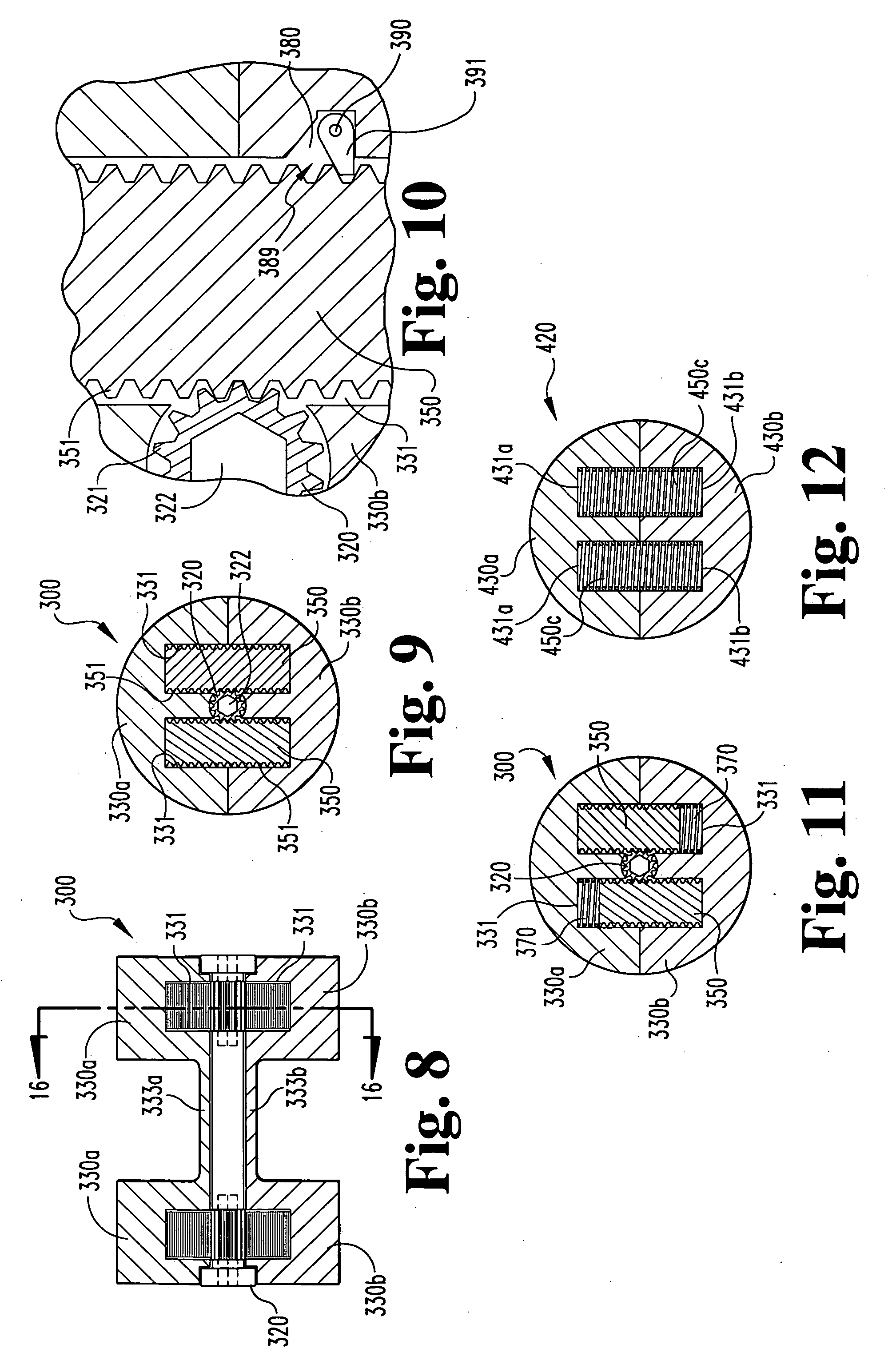
Spinal fusion apparatus and method
InactiveUS6926737B2Relieve stressPhysical recoveryInternal osteosythesisBone implantCushioningVertebral bone
An apparatus for stabilizing and promoting fusion between adjacent vertebrae includes at least a pair of implants to promote bone growth and to fuse with vertebral bone. The implants are joined by a connector. Preferably the implants are inserted into receiving bores in a non-parallel configuration and / or the connector joins the implants so as to bias the implants to a non-parallel configuration. A pair of connecting members also preferably secure the implants to each of the adjacent vertebrae. A method of using the apparatus provides for stabilizing between vertebrae where the original cushioning disc has deteriorated or become damaged. The implants are connected together. Also in the method, the implant receiving bores are non-parallel and / or the implants are biased to non-parallel configurations by joining the implants to the connecting element so as to reduce the inadvertent disturbance of the implants from the receiving bores and to further stabilize the implants overall during the fusion process.
Owner:WARSAW ORTHOPEDIC INC
Spinal implant including a compressible connector
An implant may be formed in a disc space during a spinal fusion procedure. The implant may include implant members and connectors. The connectors may include sections with removed material that allow for some flexibility of the connectors. The connectors may include limiters that limit the amount of flexibility of the connectors. The ability of the connectors to flex may allow stress to be applied to bone growth material positioned in the implant. Stress may promote desirable bone growth.
Owner:ZIMMER SPINE INC
Fixation plate and method of use
A spinous process clamp employs a pair of elongate plates that are positioned on either side of the spinous processes of vertebrae that are to be fused. The plates are joined by fasteners, preferably bolts and nuts. The plates include a recess on the bone facing side of the plate for retaining a bone growth promoting substance. When the bolts and nuts are tightened, the spinous processes are clamped between the plates, thereby pressing the bone growth promoting substance against the bone and encouraging bone growth across the vertebrae fixed by the plates.
Owner:WARSAW ORTHOPEDIC INC
Porous osteoimplant
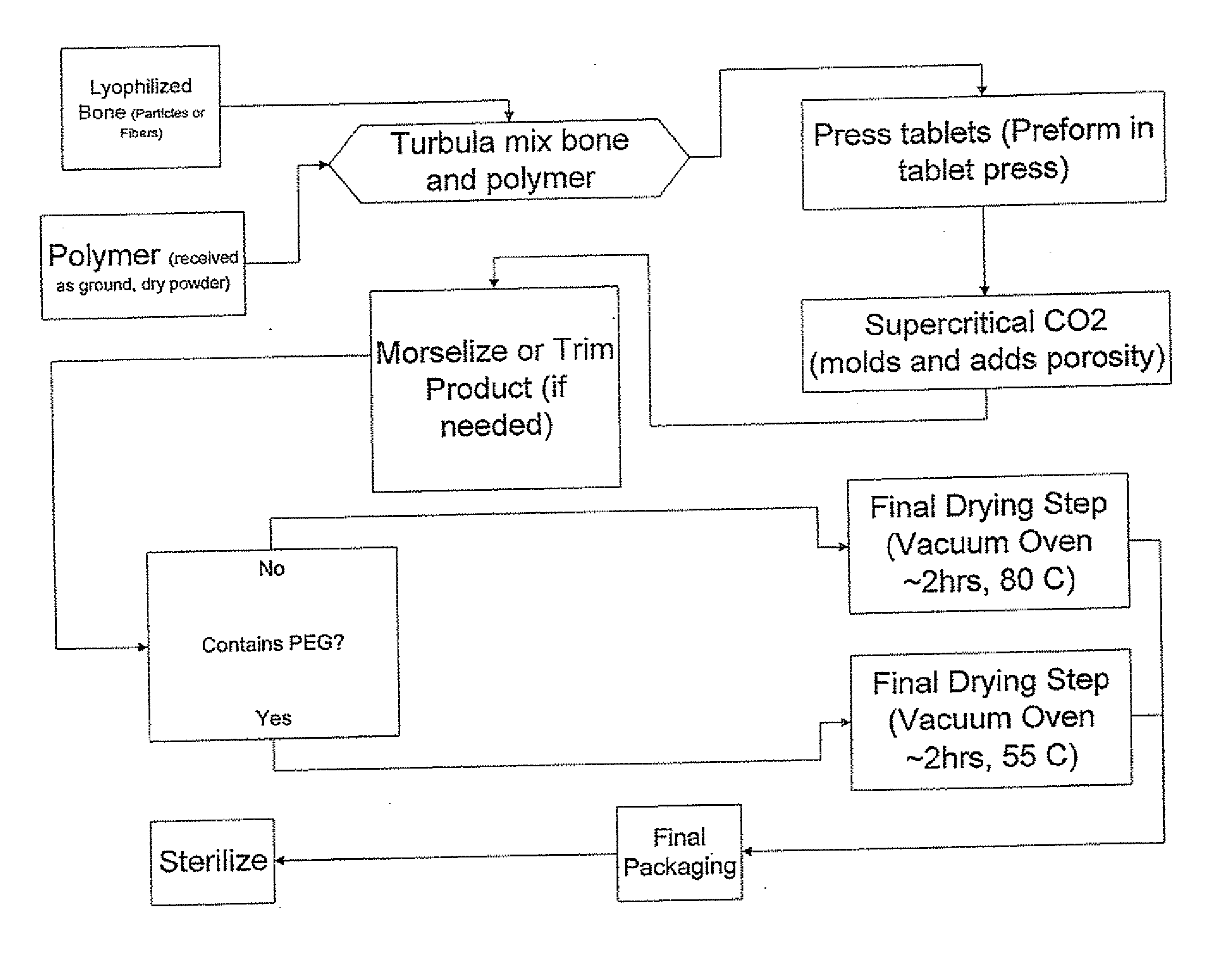
ActiveUS20080069852A1Accelerate the remodeling processImprove permeabilityBone implantSkeletal disorderBone growthBone defect
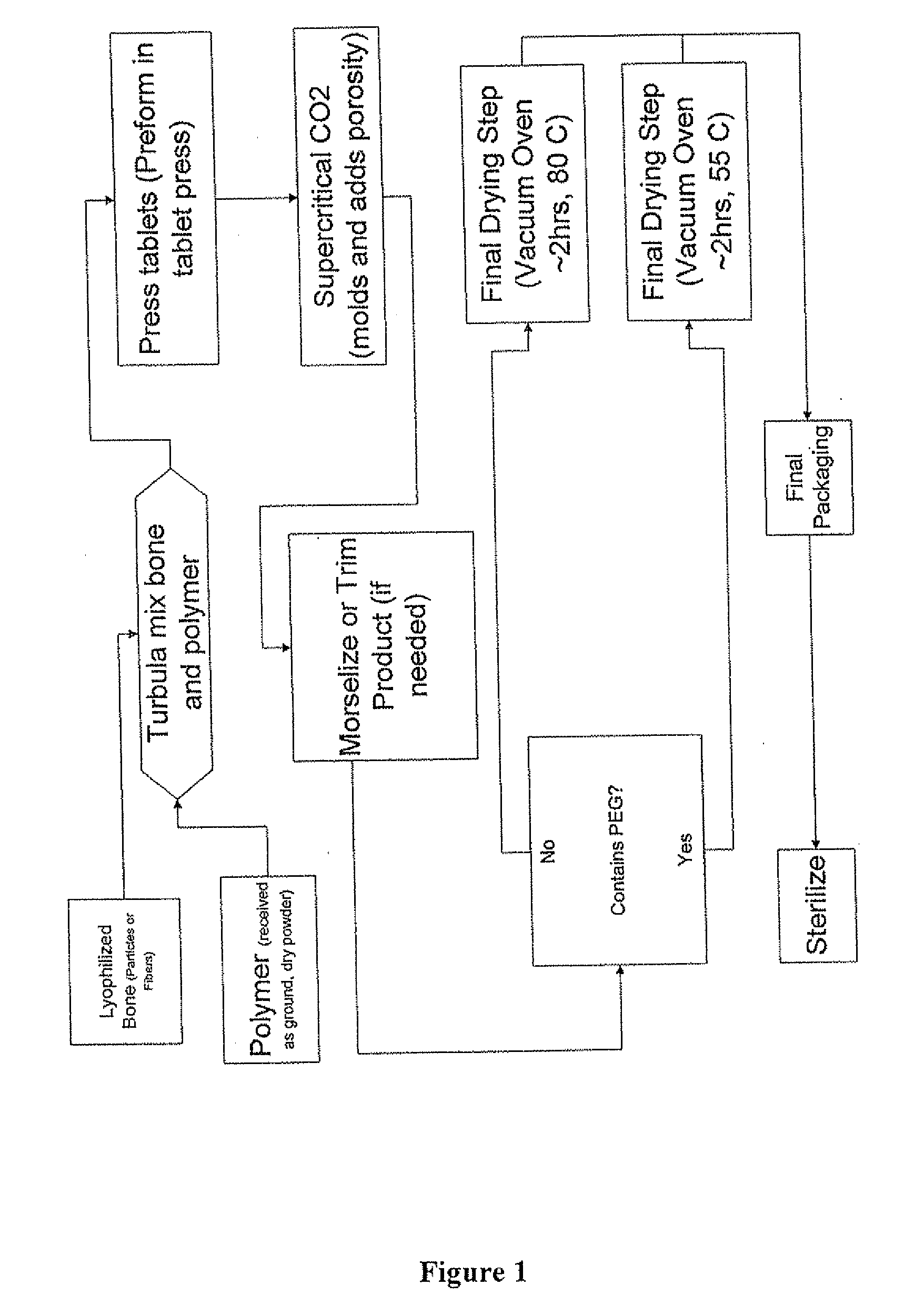
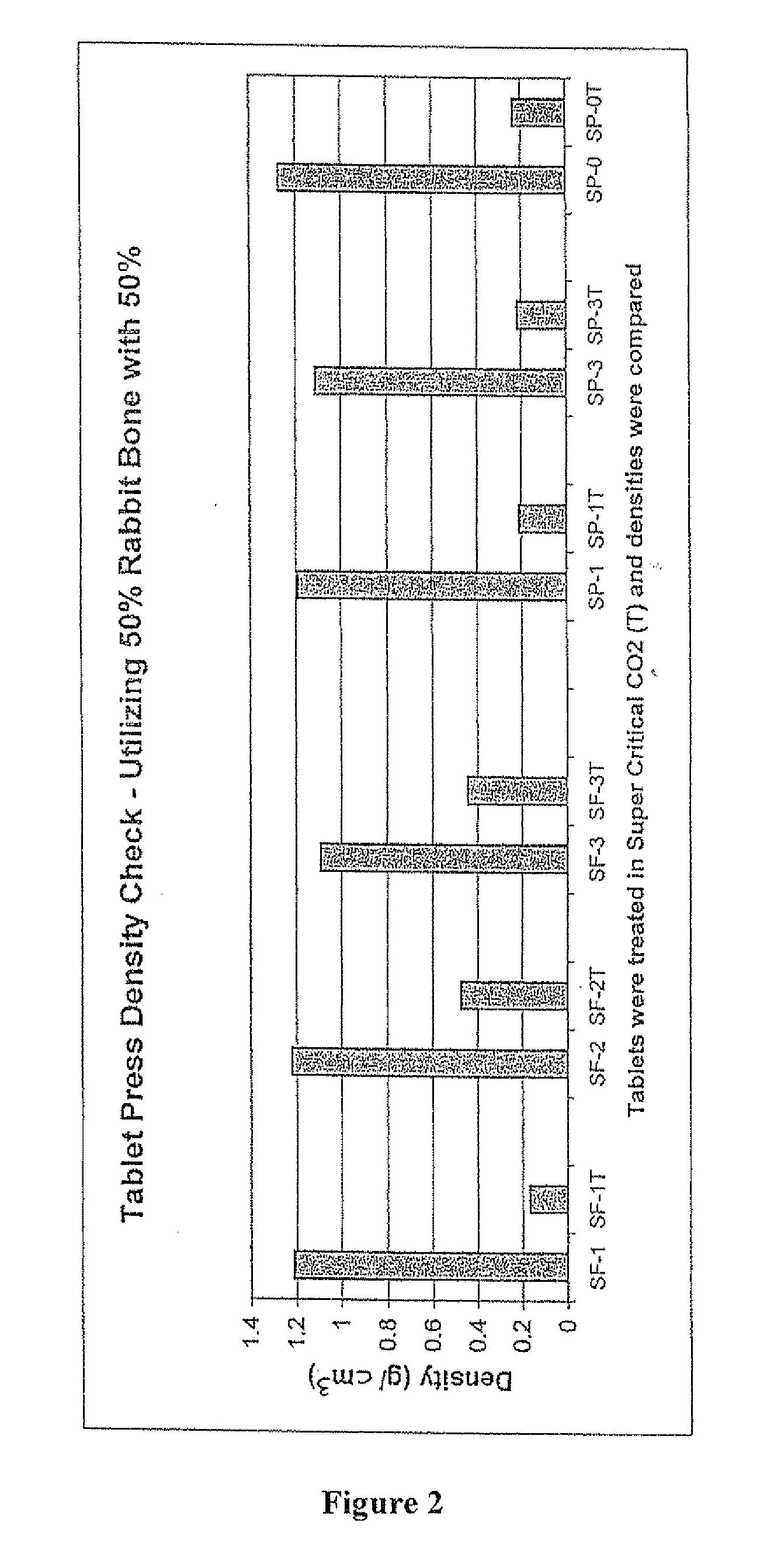
The invention is directed toward porous composites for application to a bone defect site to promote new bone growth. The inventive porous composites comprise a biocompatible polymer and a plurality of particles of bone-derived material, inorganic material, bone substitute material or composite material. In certain embodiments, the porous composites are prepared using a method that includes a supercritical fluid (e.g., supercritical carbon dioxide) treatment. The invention also discloses methods of using these composites as bone void fillers.
Owner:WARSAW ORTHOPEDIC INC
Supplemental spine fixation device and method
InactiveUS20050240182A1Closely positionedInternal osteosythesisJoint implantsBone growthBiomedical engineering
A supplemental spine fixation device and method is used in association with a primary spine fixation device. The supplemental spine fixation device includes a guide and spacer for distracting apart adjacent spinous processes and the device has hook members which hook about the first and second spinous processes. With the spinous processes distracted and the hook members about the spinous processes, the hook members can be rigidly secured to a hub in order to rigidly affix the spinous processes about the spacer. The rigidity between the spinous processes assures that the vertebral bodies will be held rigidly in place in order to promote bone growth and fusion. Further additional freedom of movement between the spacer and hub is accomplished with the spacer being pivotably mounted relative to the hub. The hooks have a tissue distracting lead-in guide for allowing the hooks to be easily urged between spinous processes.
Owner:KYPHON
Articles comprising large-surface-area bio-compatible materials and methods for making and using them
ActiveUS20100303722A1Improve cell adhesionAccelerated cell growth characteristicImmobilised enzymesBioreactor/fermenter combinationsCell culture mediaBone growth
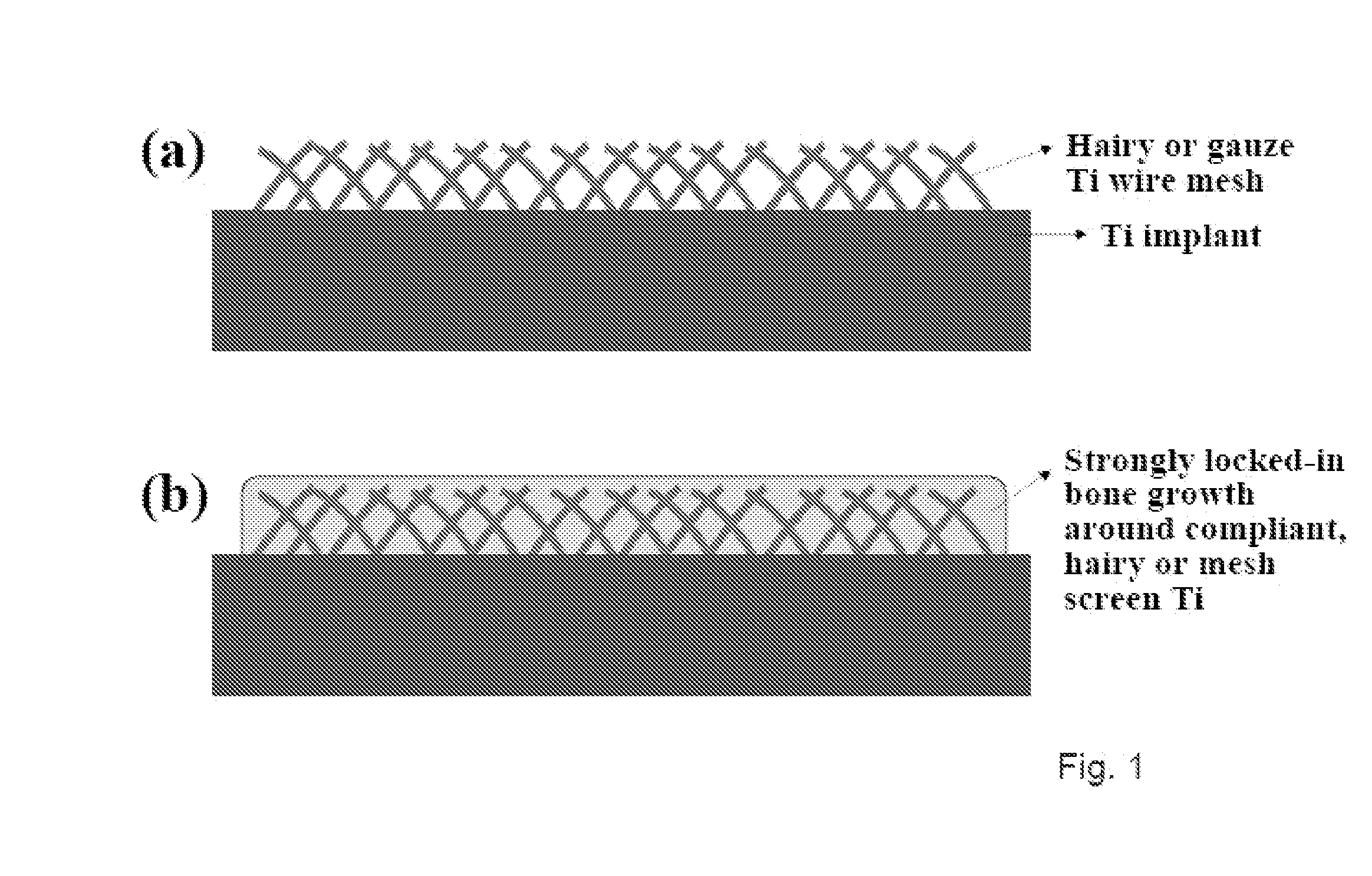
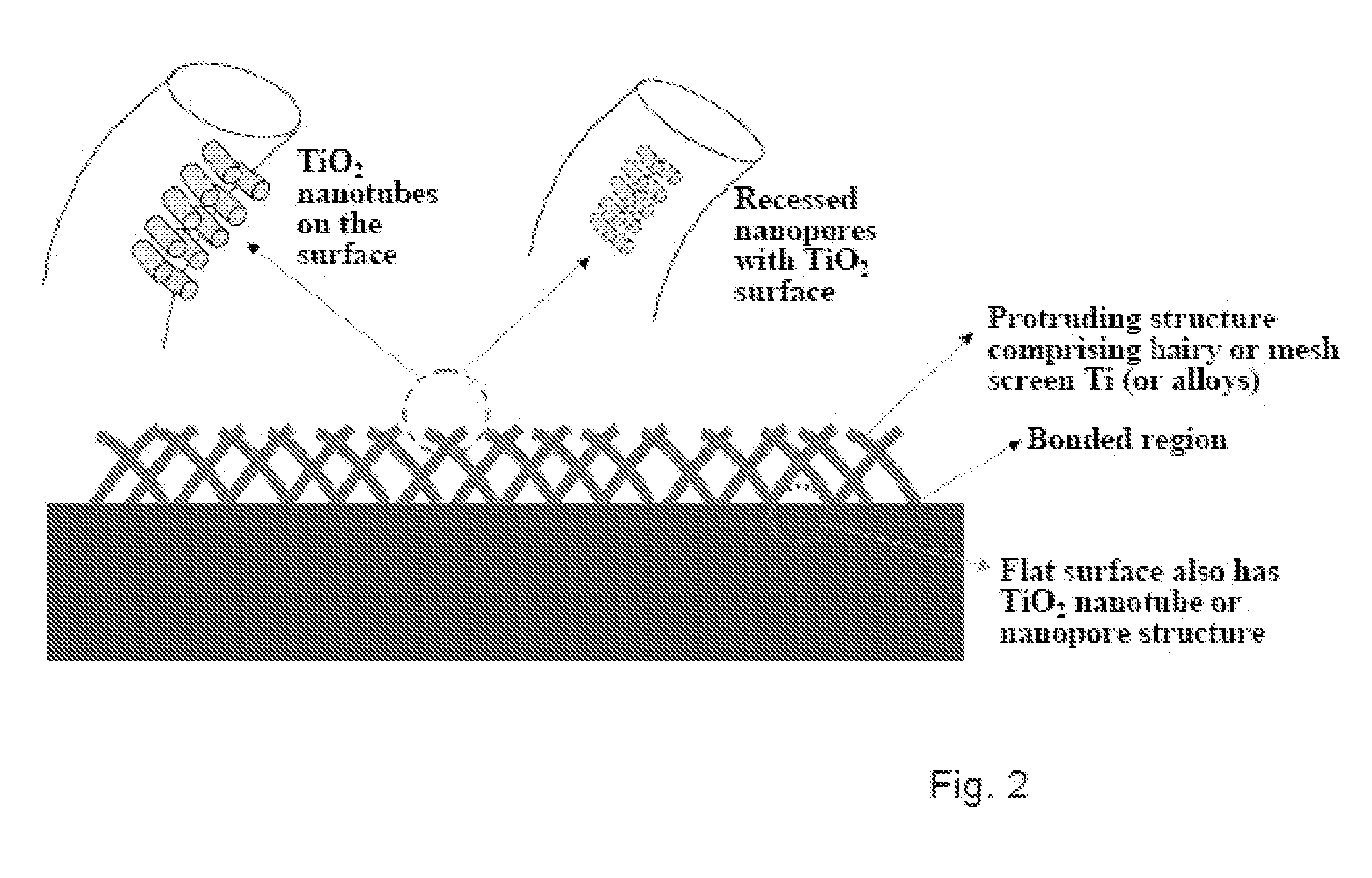
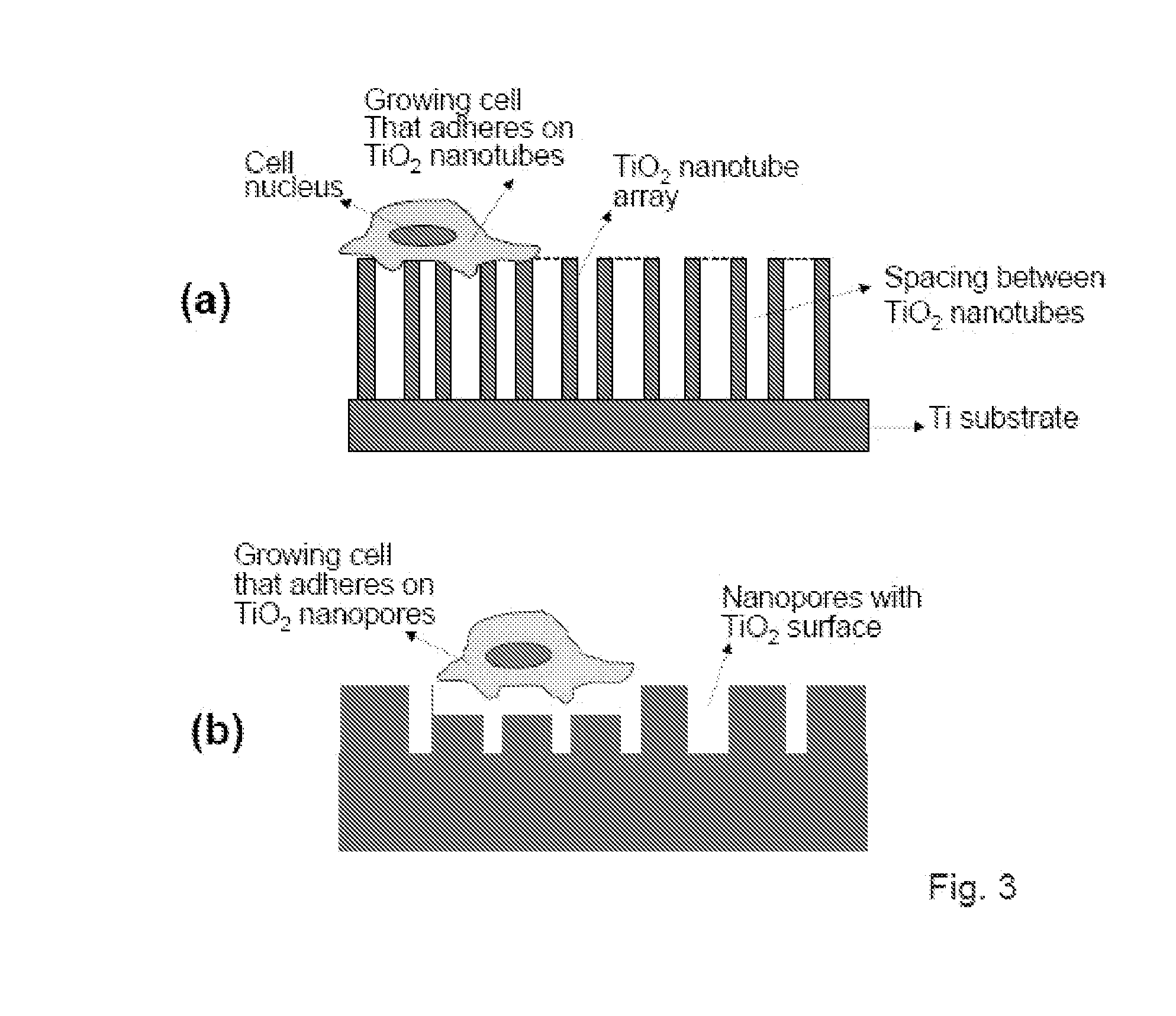
The present invention provides articles of manufacture comprising biocompatible nanostructures comprising significantly increased surface area for, e.g., organ, tissue and / or cell growth, e.g., for bone, tooth, kidney or liver growth, and uses thereof, e.g., for in vitro testing of drugs, chemicals or toxins, or as in vivo implants, including their use in making and using artificial tissues and organs, and related, diagnostic, screening, research and development and therapeutic uses, e.g., as drug delivery devices. The present invention provides biocompatible nanostructures with significantly increased surface area, such as with nanotube and nanopore array on the surface of metallic, ceramic, or polymer materials for enhanced cell and bone growth, for in vitro and in vivo testing, cleansing reaction, implants and therapeutics. The present invention provides optically transparent or translucent cell-culturing substrates. The present invention provides biocompatible and cell-growth-enhancing culture substrates comprising elastically compliant protruding nanostructure substrates coated with Ti, TiO2 or related metal and metal oxide films.
Owner:RGT UNIV OF CALIFORNIA
Implant device used in minimally invasive facet joint hemi-arthroplasty
InactiveUS7517358B2Minimally invasiveLess traumaticInternal osteosythesisJoint implantsHemi arthroplastyImplanted device
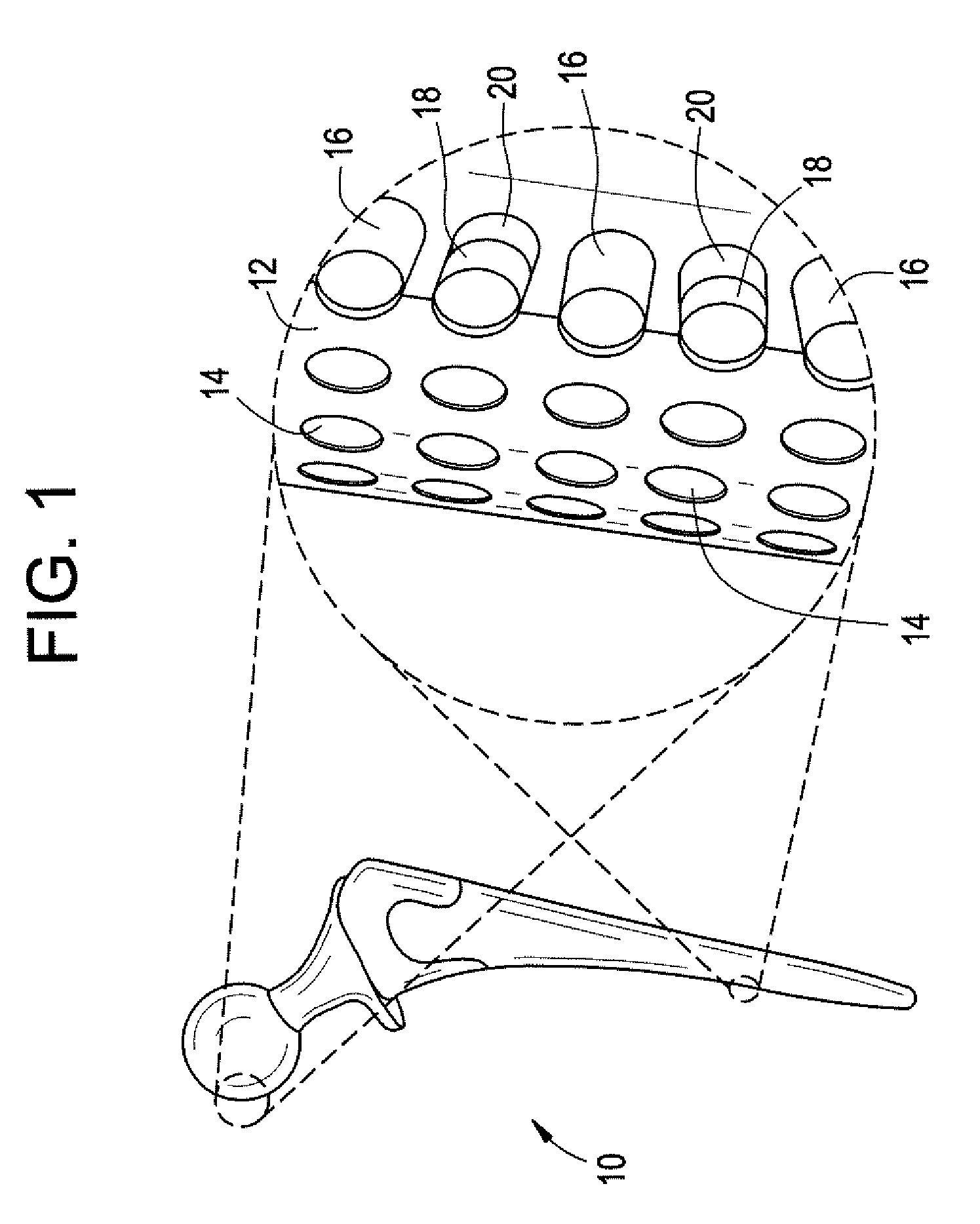
A metallic inverted L-shaped implant is used to resurface the superior facet of the inferior vertebrae limited to the facet joints located on the spine, Occiput-C1 through L5-S1. The metallic implant is highly polished on its exterior and textured on its interior surface. It is mechanically crimped in place without the use of cement or pedicle screws. Permanent fixation occurs when bone in-grows onto a rough, porous surface on the inside of the implant. The implant employed in a hemi-arthroplasty method resurfaces half of the facet joint to provide for smooth, pain free joint articulation in deteriorated or diseased spinal facet joints without the need for major surgery or rehabilitation at considerably less risk to the patient.
Owner:MINSURG INT INC
Expandable spinal fusion cage
An expandable spinal fusion device is provided. The expandable device comprises a first part slidingly coupled to a second part. An removable expandable member extends between the first part and the second part and is coupled to a rotating operator such that rotating the operator causes the first part and the second part to move away from each other and distract vertebral bodies. A spacer or clip is used to lock the first part in relation to the second part to allow bone growth the fuse the vertebral bodies.
Owner:ZIMMER BIOMET SPINE INC
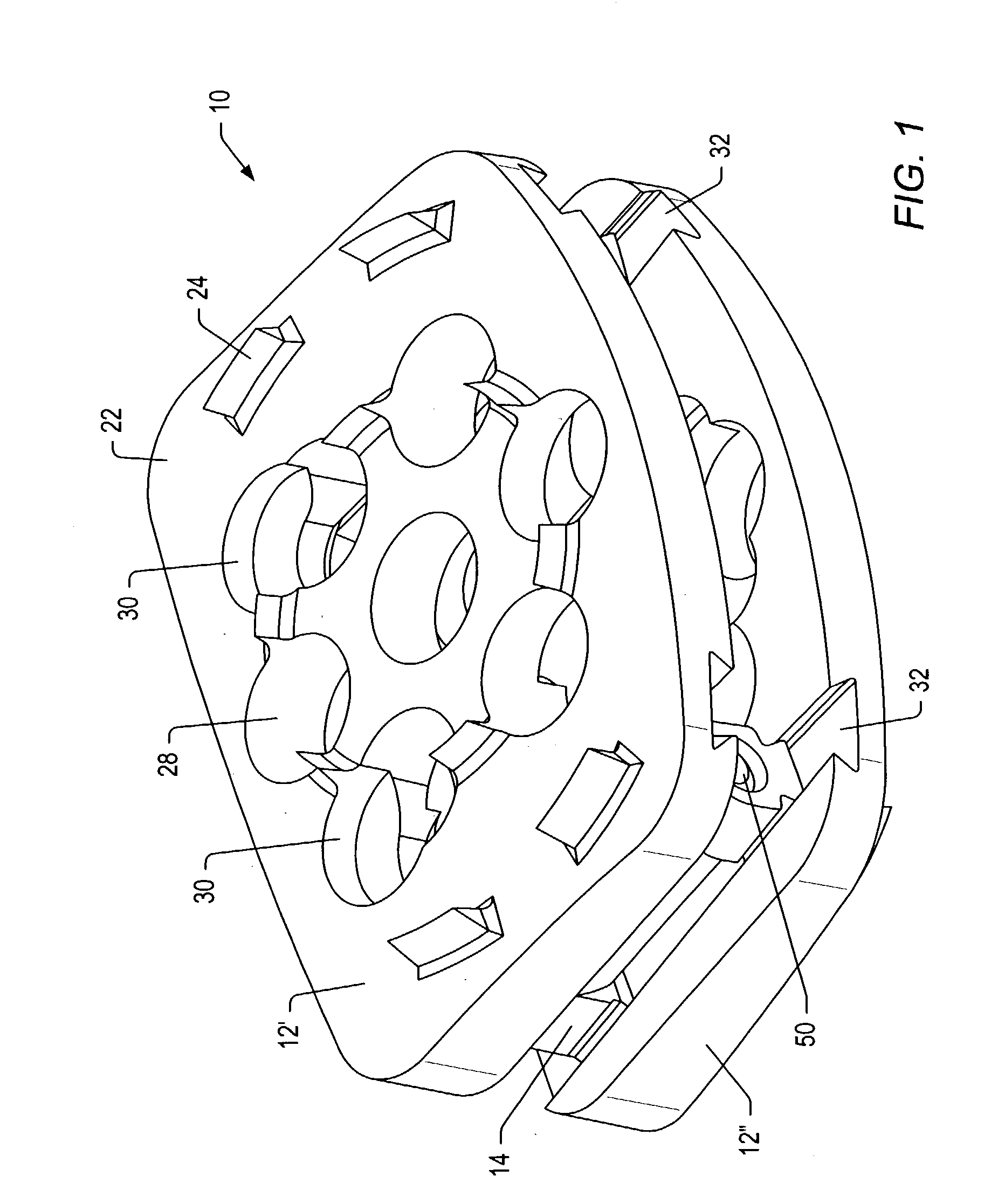
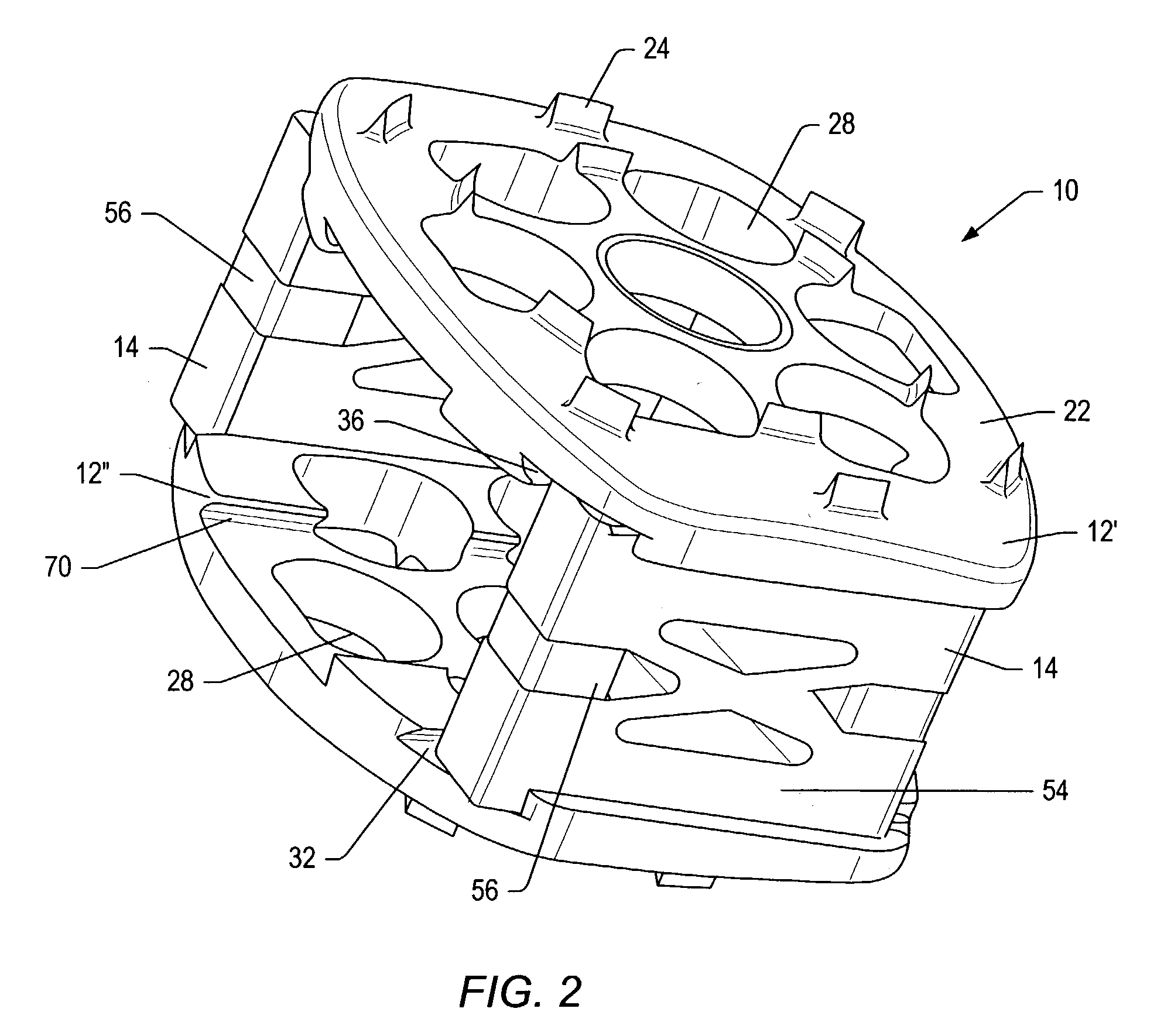
Spinal implants with body and insert
ActiveUS20050251257A1Easy to integrateAvoid passingBone implantJoint implantsBone growthSpinal implant
A spinal implant is provided which maintains intervertebral spacing and stability within the spine. The spinal implant may include a body and an insert. The body of the spinal implant may be formed of a ceramic material. In some embodiments, the body may be formed of beta tricalcium phosphate. The body may include an opening that is complementary to the insert. The insert may fit within the opening. The insert may include a number of passageways. Some of the passageways may intersect to form a scaffold for bone growth. Bone growth promoting material may be introduced into the insert before the insert is positioned in a body and inserted in a patient between two vertebrae.
Owner:ZIMMER BIOMET SPINE INC
Spinal plate system for stabilizing a portion of a spine
A spinal plate system that maintains intervertebral spacing and spinal stability is provided. In an embodiment, a spinal compression plate may include two or more plates coupled together form an adjustable-length plate. Compression of a spinal compression plate movement may mimic natural settling of bones in a spine and / or distribute at least a portion of a vertebral load to an implant positioned between two vertebrae. Maintaining at least a portion of the vertebral load on an insert may increase bone growth and increase fusion between an implant and surrounding vertebrae.
Owner:ZIMMER SPINE INC
Methods for producing metallic implants having roughened surfaces
The invention provides a method of providing a metallic orthopaedic implant with a micron or nanometer-scale surface roughness to facilitate acceptance of tissue and bone growth or apposition after implantation while maintaining the structural integrity of the orthopaedic implant. The invention also provides a metallic orthopaedic implant comprising a metallic body and metallic elements adhered to a portion of the surface of the metallic body to define a three-dimensional porous surface geometry, wherein at least some of the metallic elements have a micron or nanometer-scale surface roughness.
Owner:DEPUY PROD INC
Allograft bone composition having a gelatin binder
InactiveUS7045141B2Low tensile strengthIncrease delayImpression capsSurgical adhesivesCross-linkSolid structure
The invention is directed toward an osteoimplant for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing composition of demineralized allograft bone material mixed with an aqueous phosphate buffered gelatin which when lyophilized to remove water from the composition cross links the gelatin to form a solid structure.
Owner:MUSCULOSKELETAL TRANSPLANT
Systems and methods for implantable leadless bone stimulation
ActiveUS8078283B2Enhance bone healingPromote bone growthElectrotherapyArtificial respirationBone growthElectrical stimulations
Owner:EBR SYST
Composition for filling bone defects
InactiveUS7019192B2Low tensile strengthIncrease delayBiocideSurgical adhesivesSodium phosphatesDemineralized bone
The invention is directed toward a formable bone composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of demineralized lyophilized allograft bone particles. The particle size ranges from about 0.1 mm to about 1.0 cm and is mixed in a hydrogel carrier containing a sodium phosphate saline buffer, the hydrogel component of the carrier ranging from about 1.0 to 5.0% of the composition and a pH between 6.8–7.4 with one or more additives of a cellular material, growth factor, demineralized bone chips or mineralized bone chips.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Composition for promotion of bone growth and maintenance of bone health
InactiveUS20050079232A1Accelerate bone regenerationInhibit bone resorptionBiocideNon-fibrous pulp additionDiseaseBone growth
The present invention relates to a composition for maintenance of bone health or prevention, alleviation and / or treatment of bone disorders. It also relates to the use of the composition in the manufacture of a nutritional product, a supplement, a treat or a medicament; and a method of promoting bone growth or for the maintenance of bone health, which comprises administering an effective amount of the composition.
Owner:NESTEC SA
System and method for spinal fixation
InactiveUS20100331891A1Increase ratingsQuality of the joint formed between fixated bones can be improvedSuture equipmentsInternal osteosythesisSpinal columnDilator
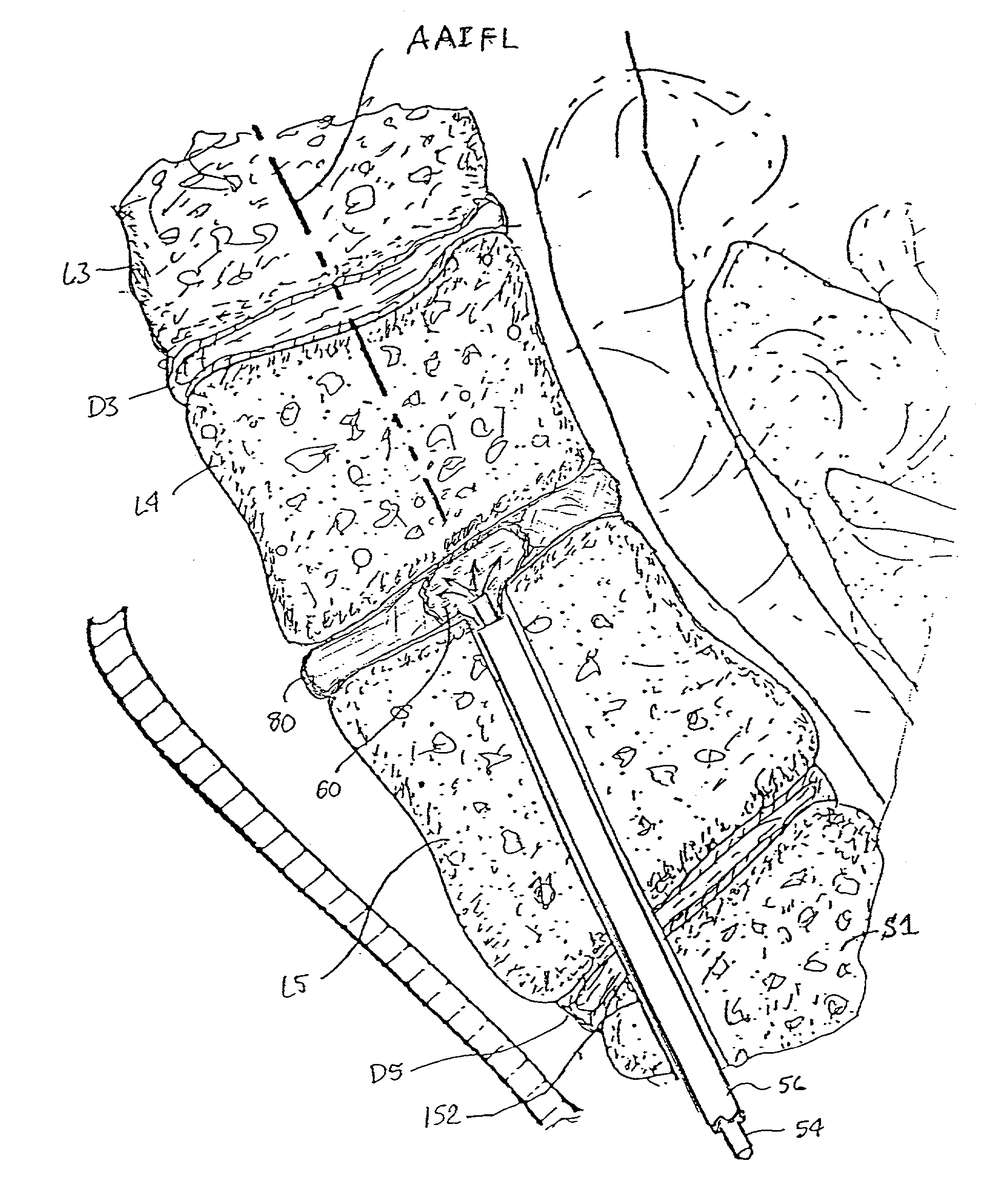
A system and method of bone fixation are provided for improving the bone growth and stability of the fixated bones. For example, a target site for a bone fixation procedure can be accessed at a facet of a first vertebra using a tissue dilator. Bone material can be disrupting from or at the target site, and a bone fixation device can be installed to fix the first vertebra relative to a second vertebra. The disruption and / or removal of the bone material, such as by rasping facets or a facet joint of the first vertebra and the second vertebra, can tend to promote bone growth. Further, it is contemplated that bone graft material can be inserted at the target site, such as into a joint space formed between facets of the first vertebra and the second vertebra.
Owner:INTERVENTIONAL SPINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com