Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
166 results about "Spinal canal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
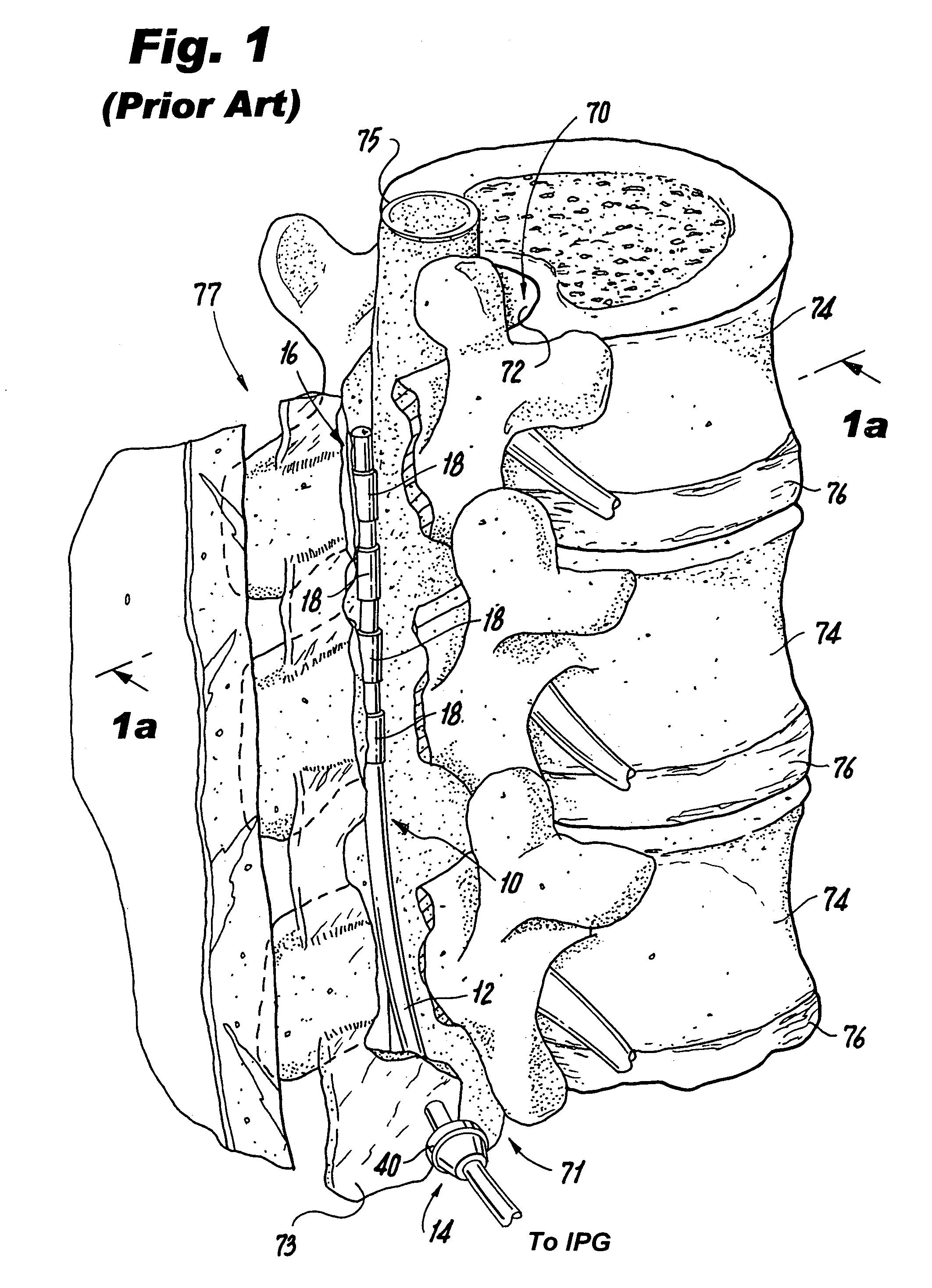
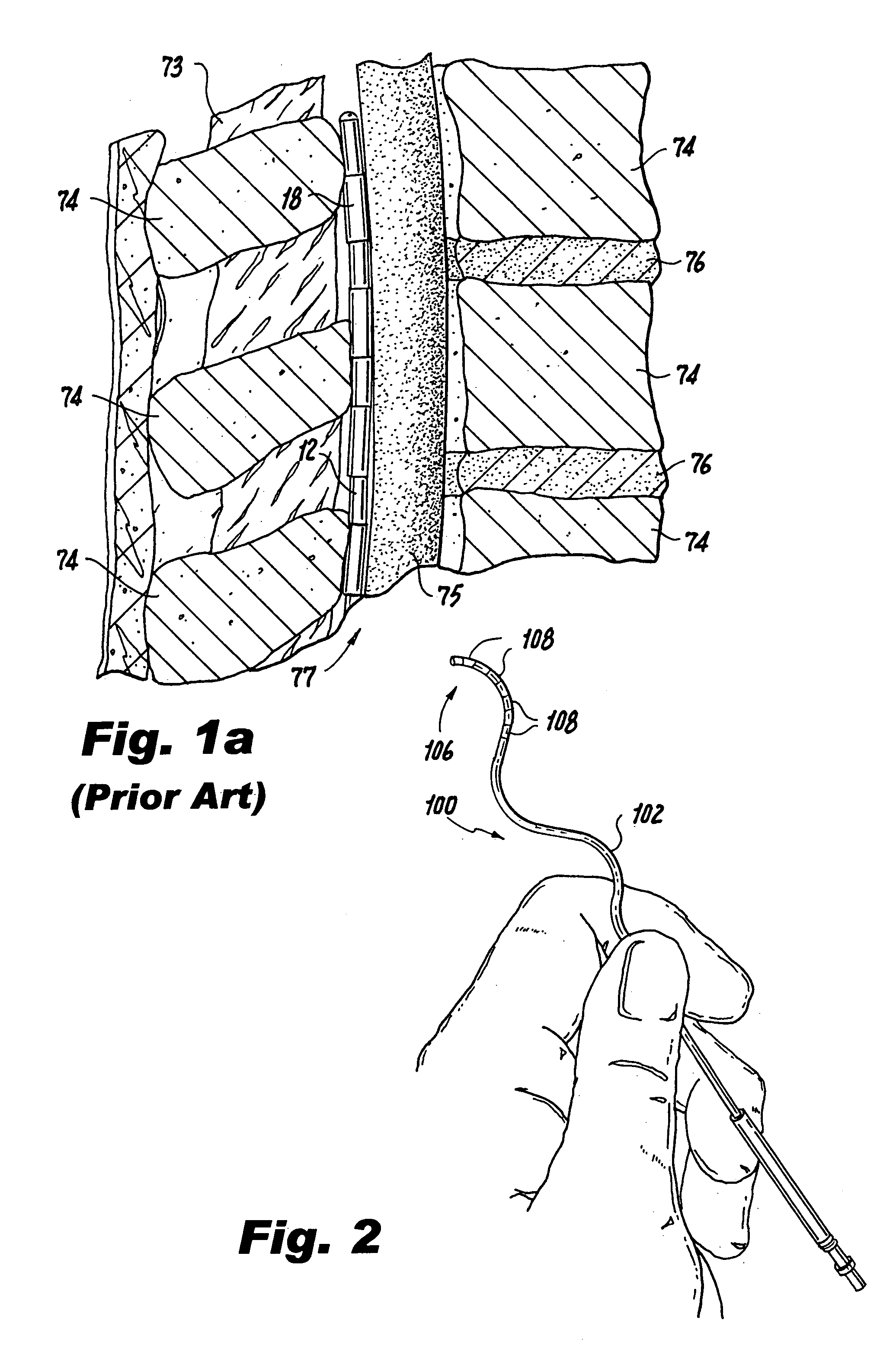
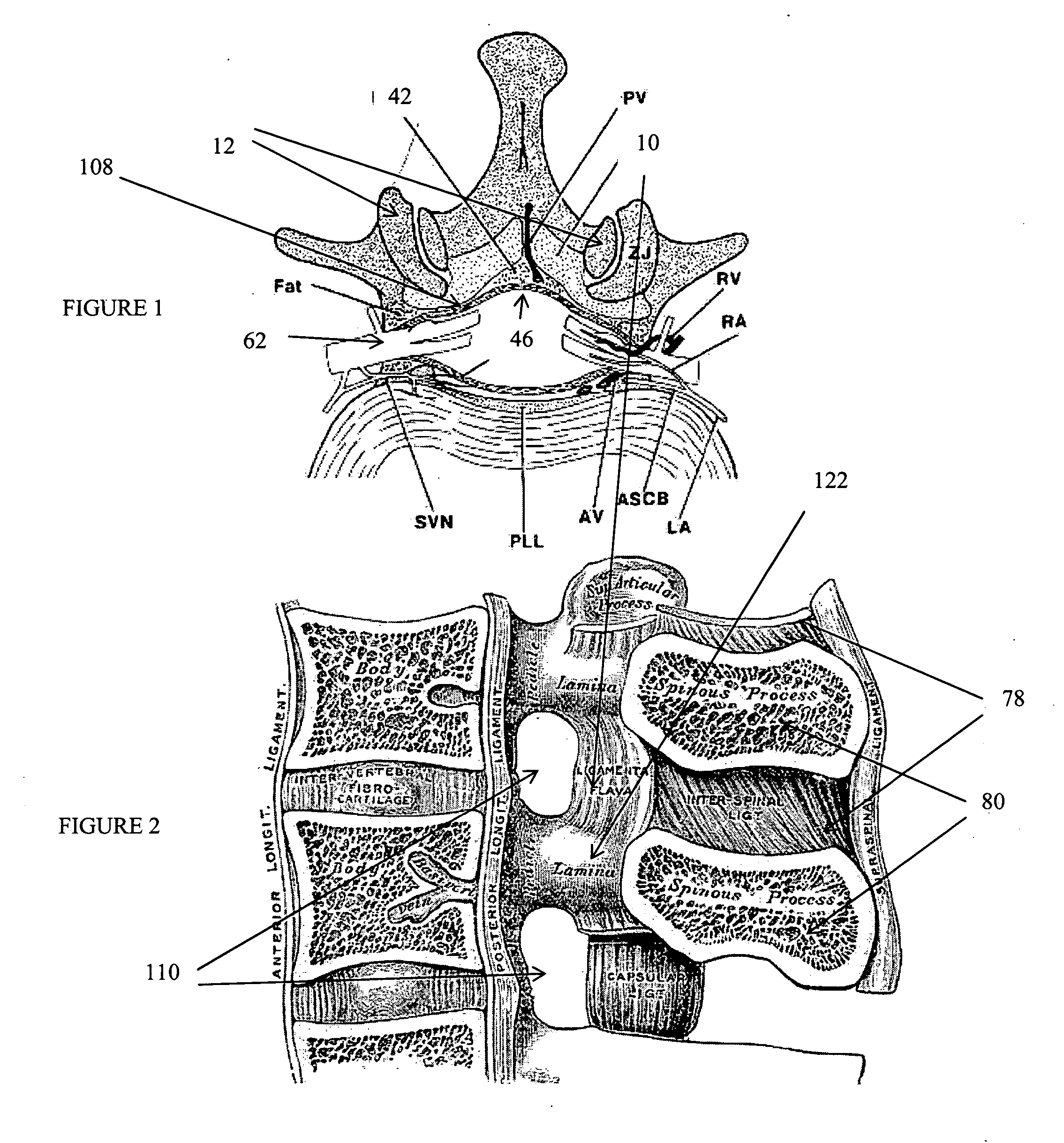
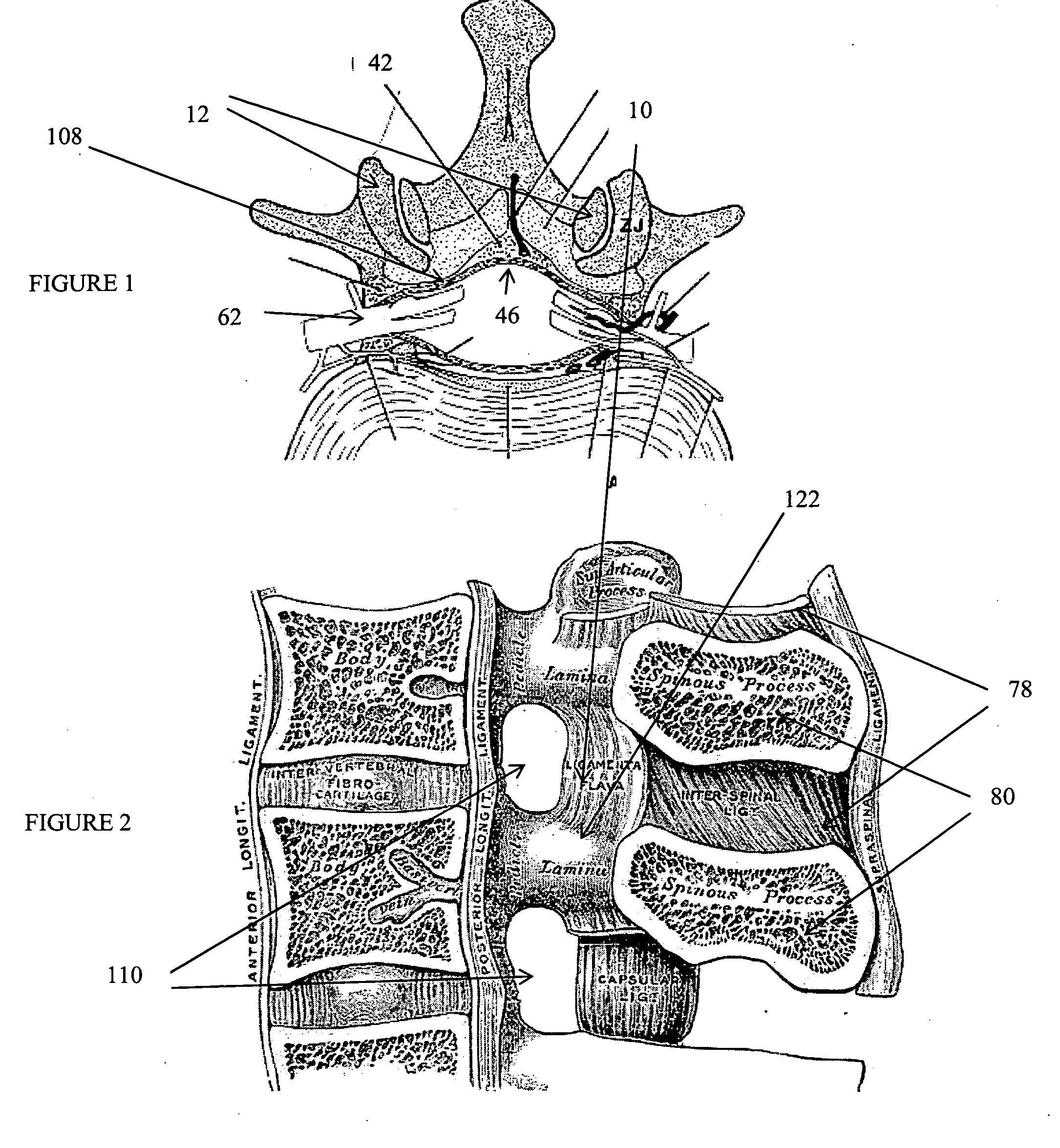
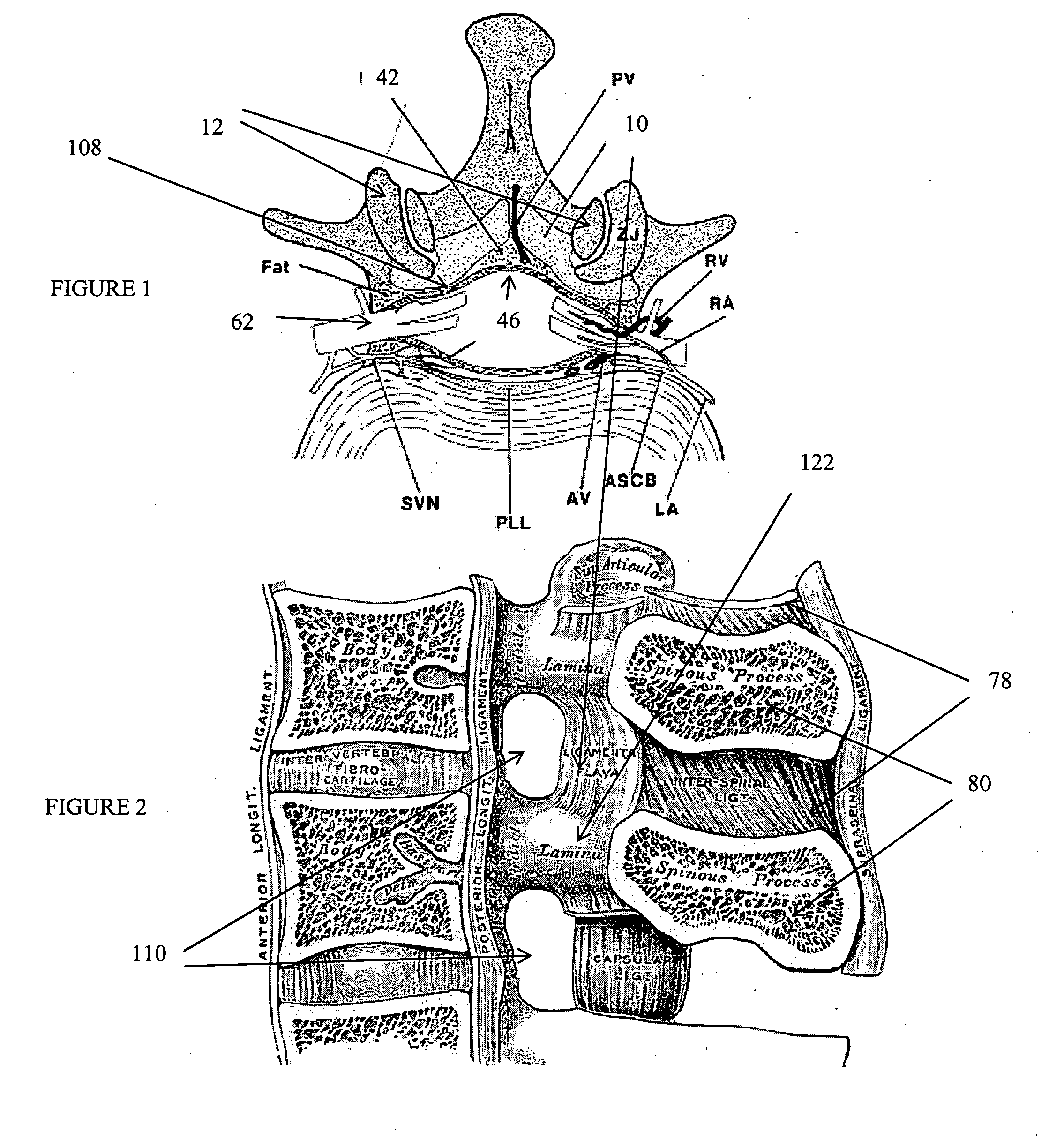
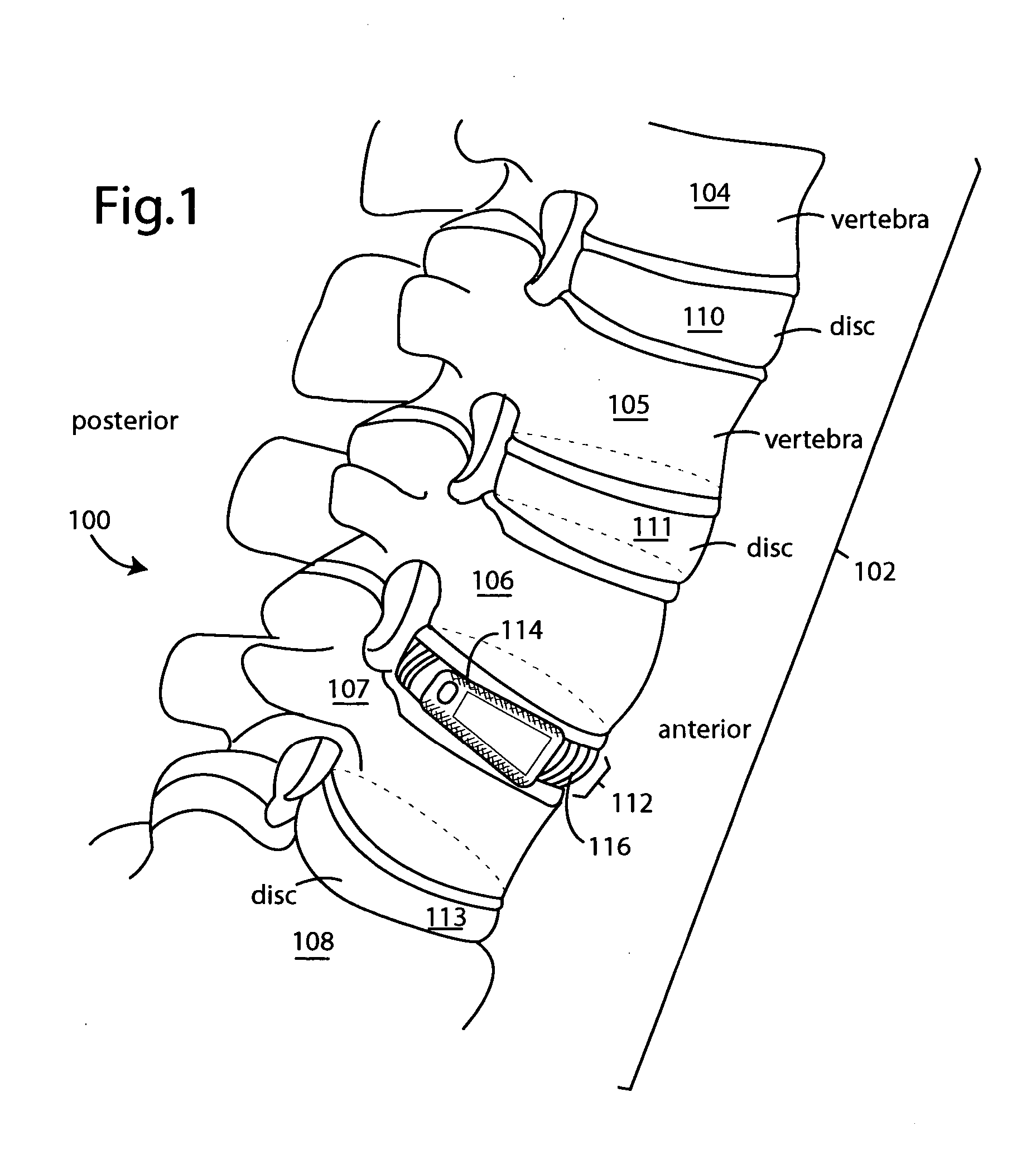
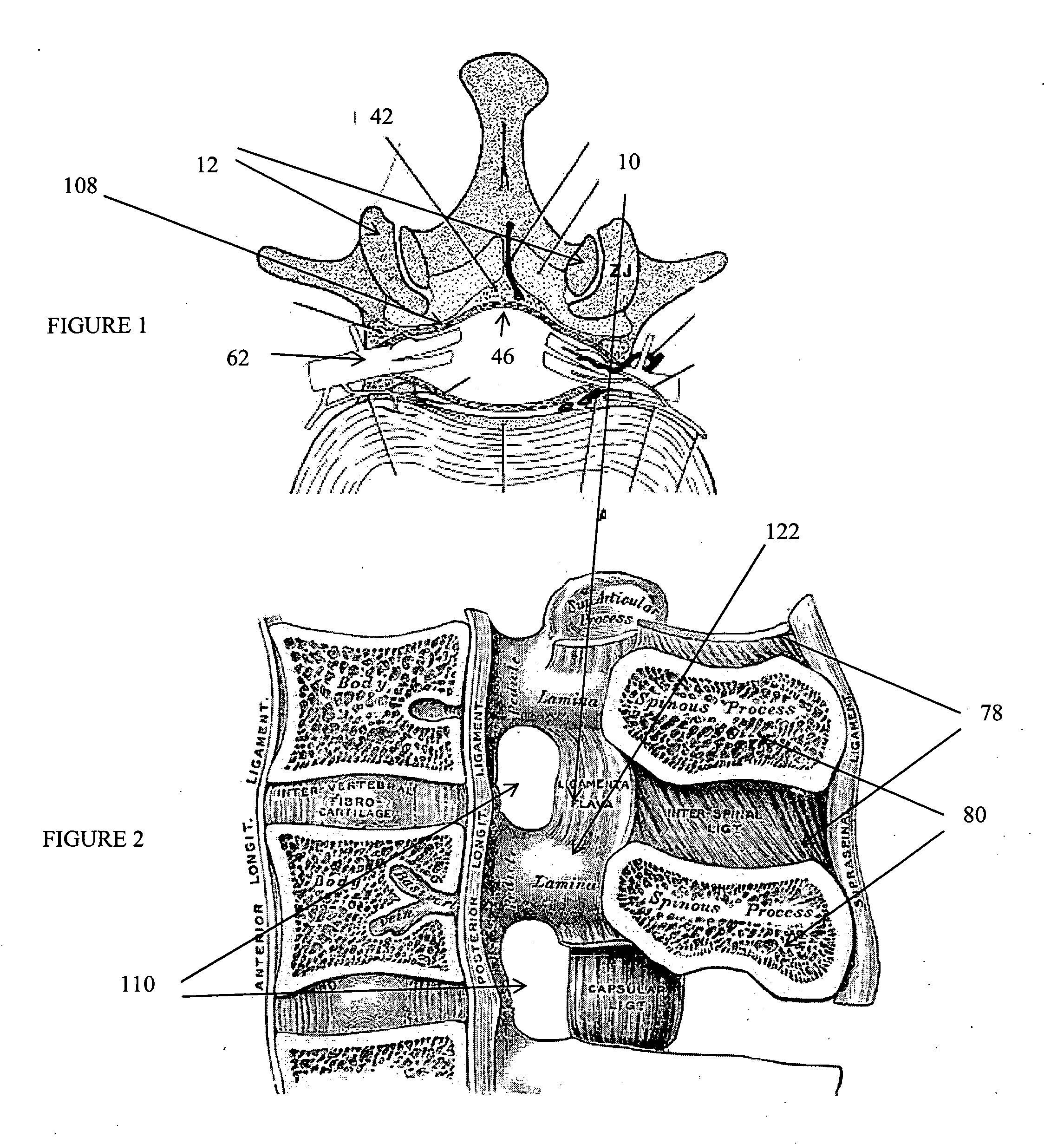
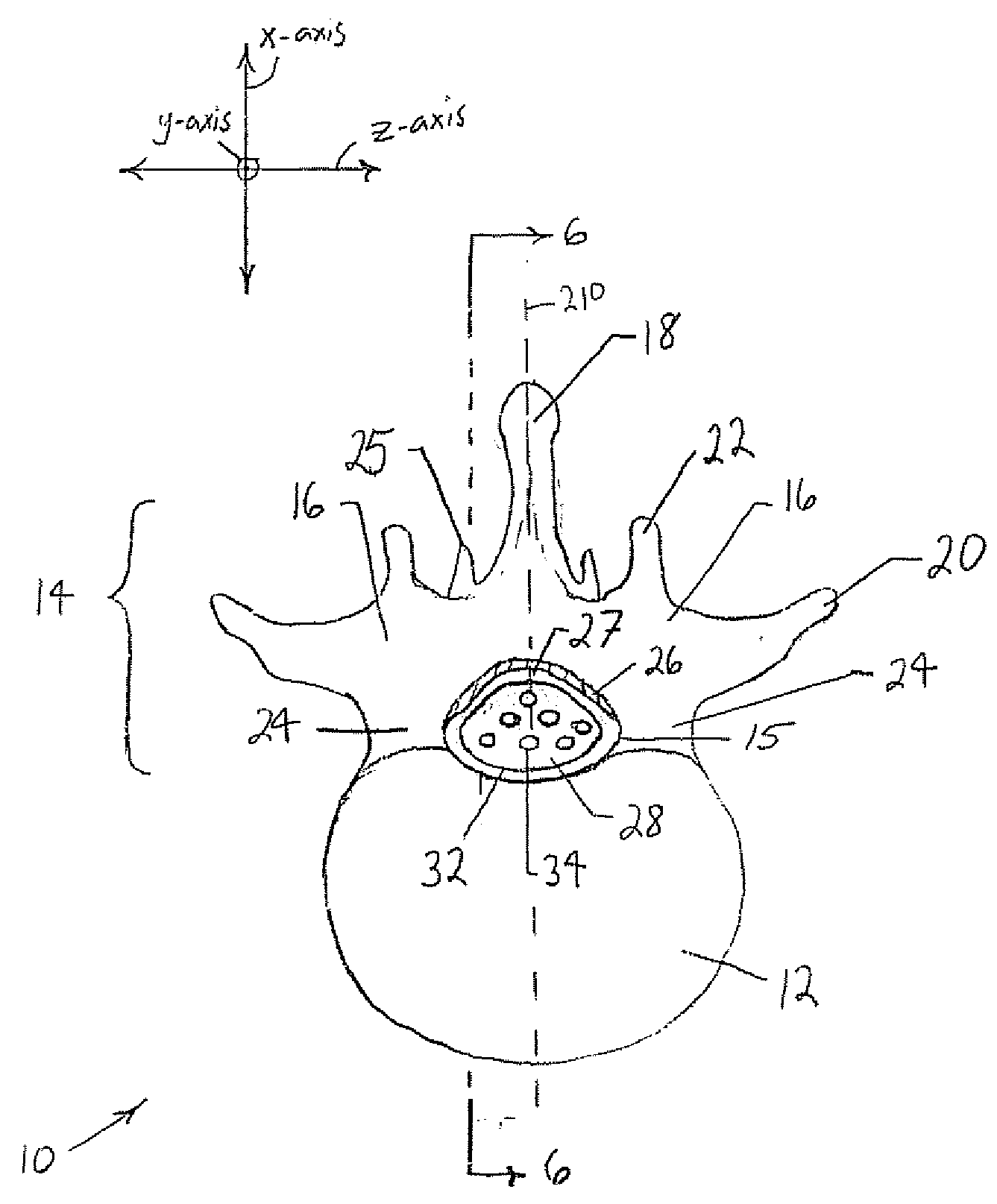
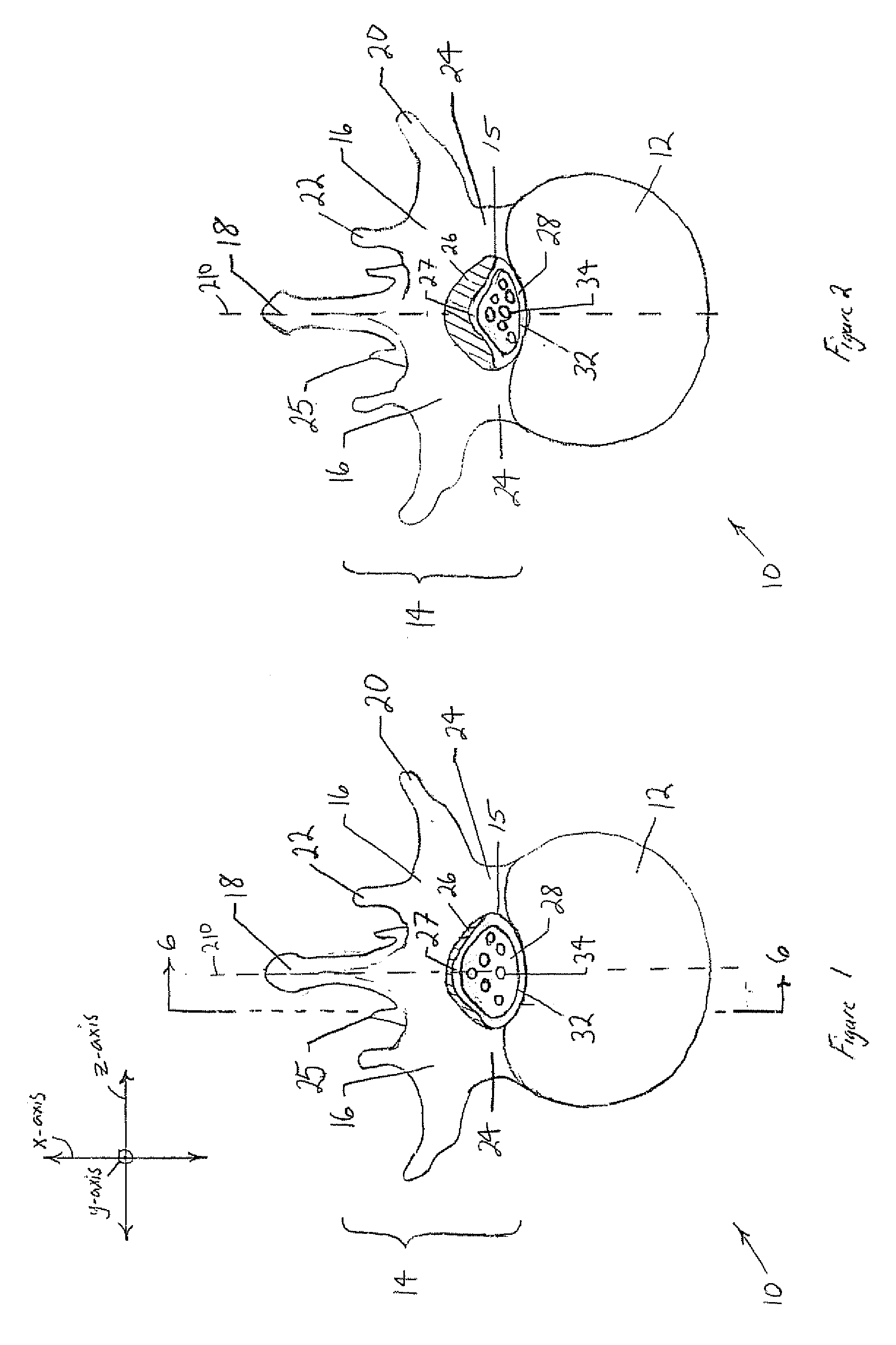
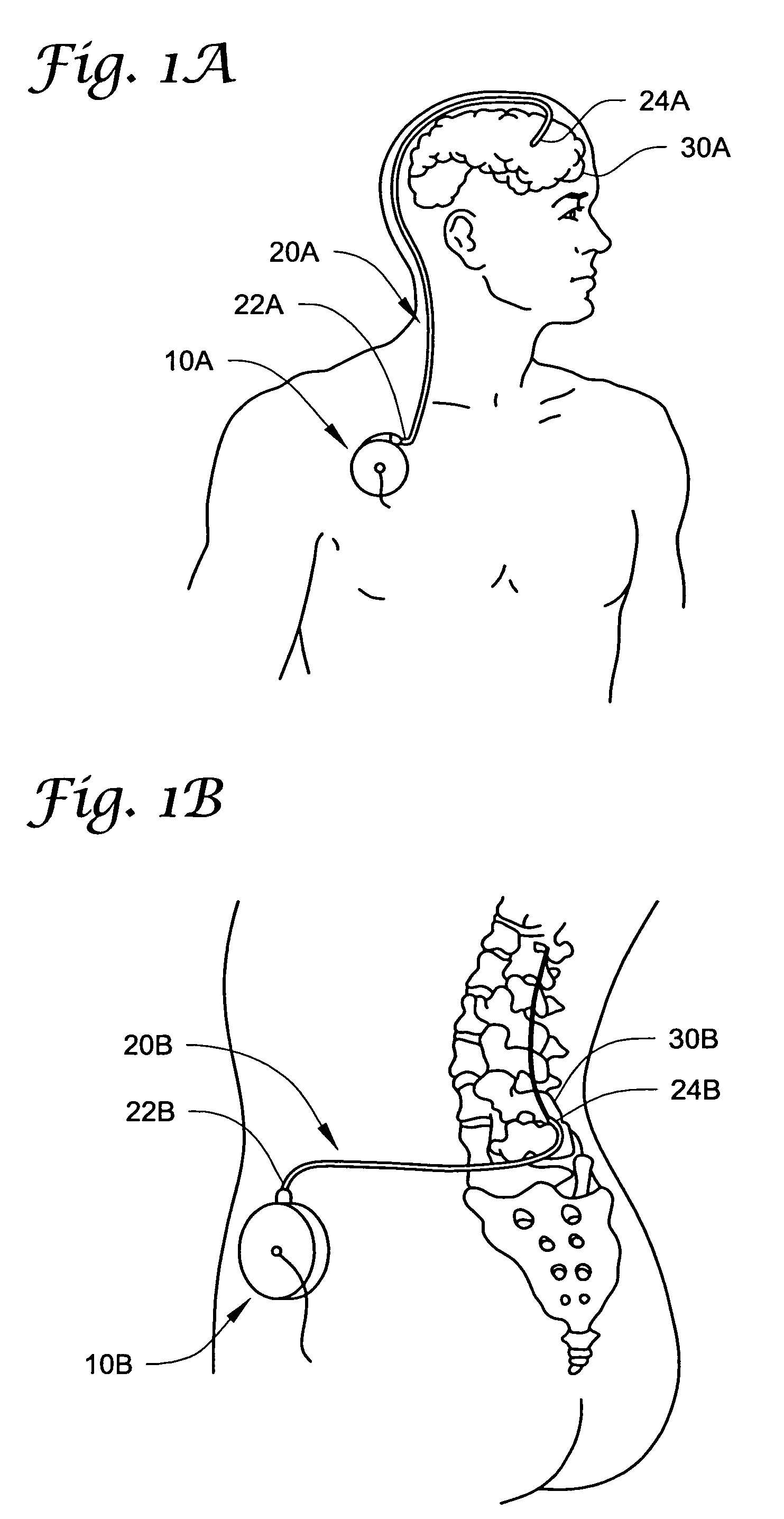
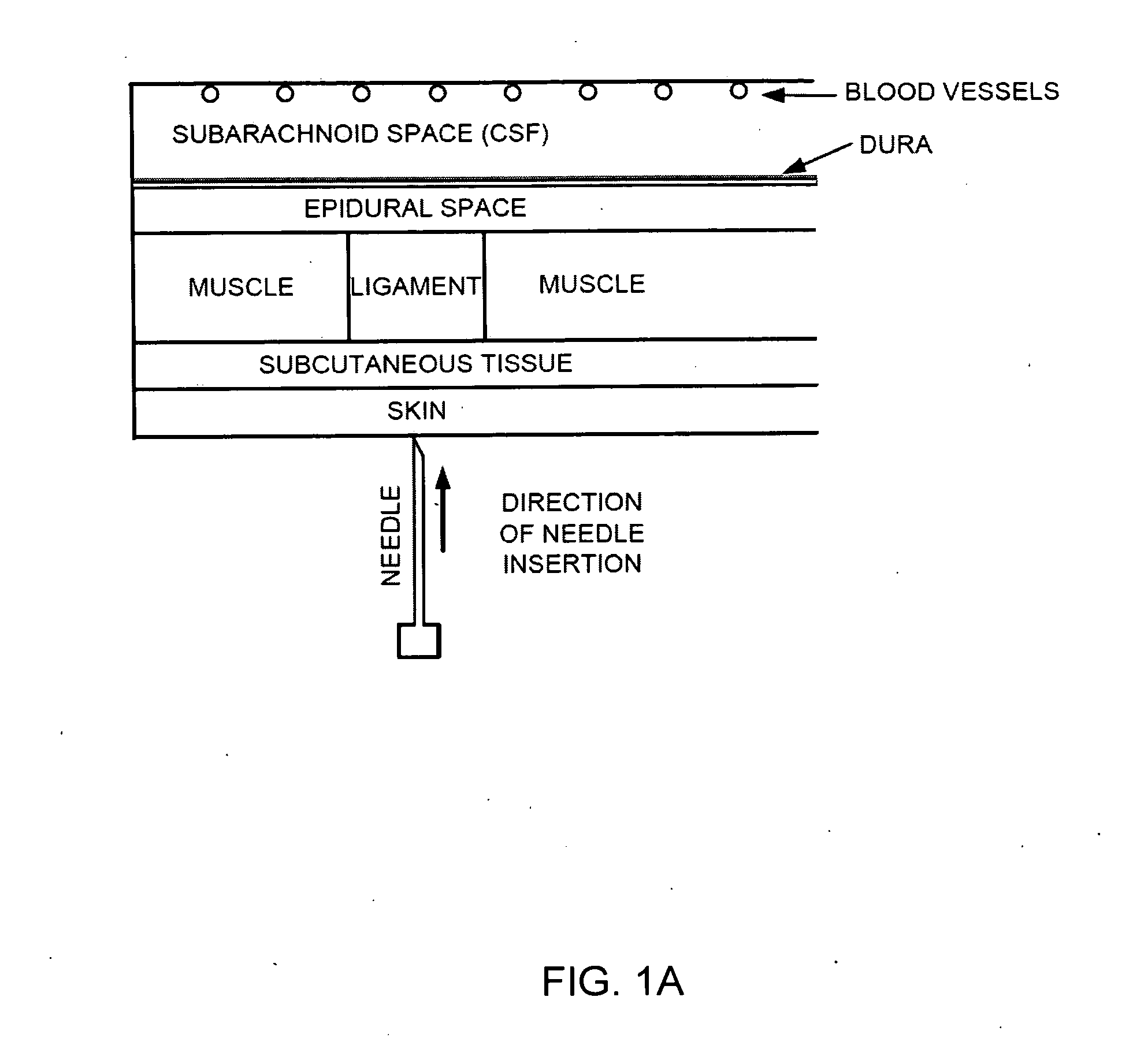
The spinal cavity (or vertebral cavity or spinal canal) is the cavity that contains the spinal cord within the vertebral column, formed by the vertebrae through which the spinal cord passes. It is a process of the dorsal body cavity. This canal is enclosed within the vertebral foramen of the vertebrae. In the intervertebral spaces, the canal is protected by the ligamentum flavum posteriorly and the posterior longitudinal ligament anteriorly.
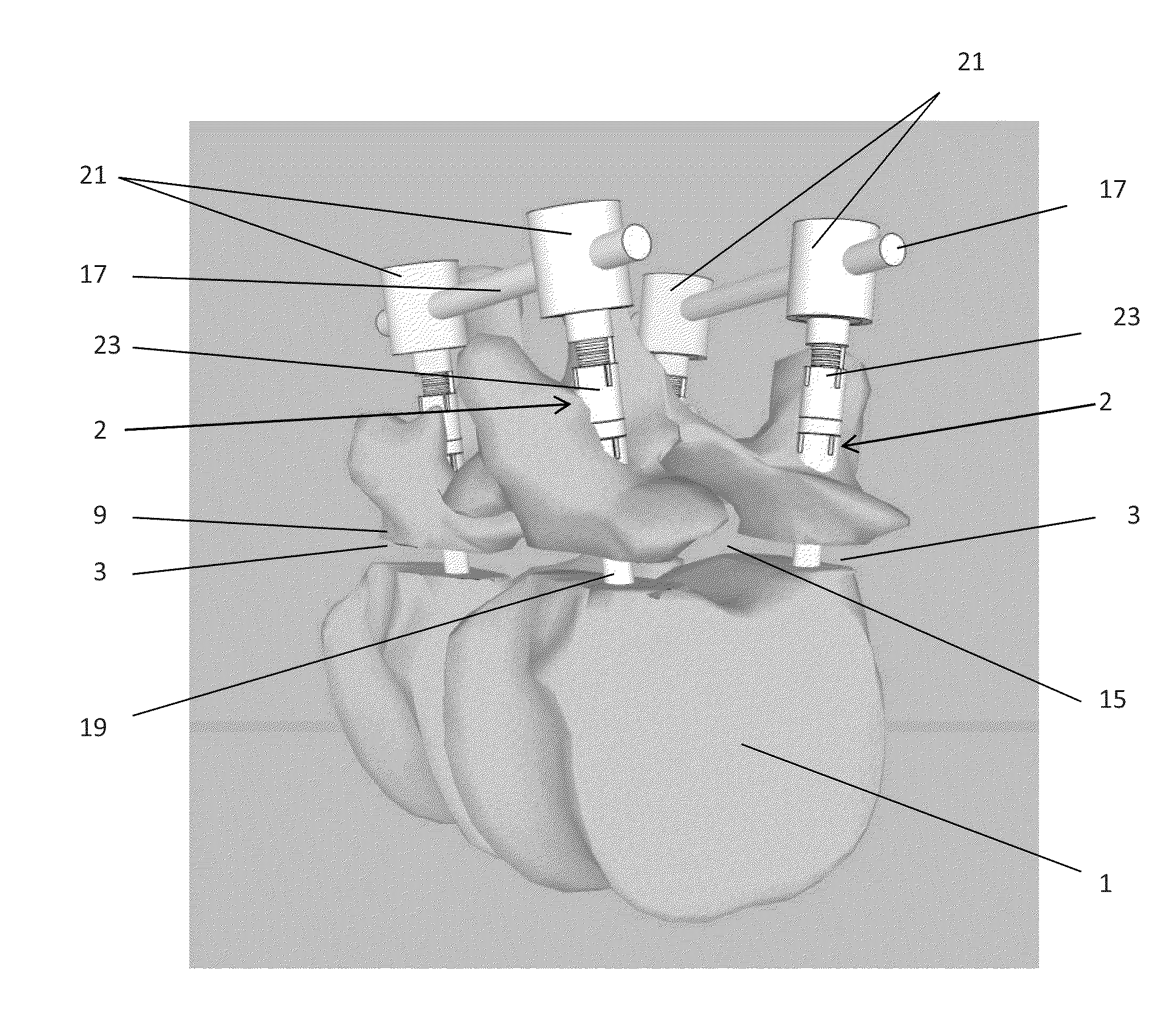
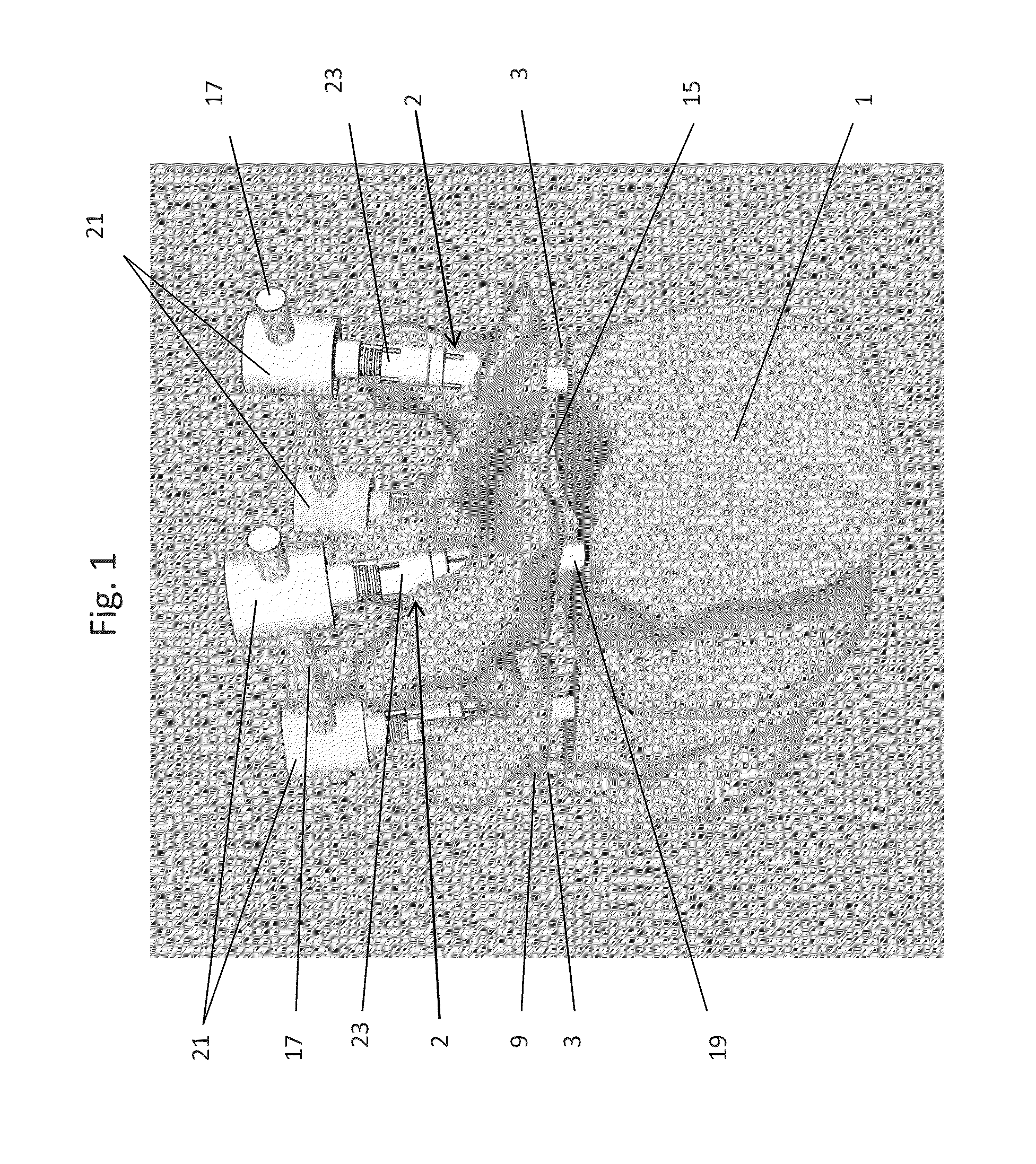
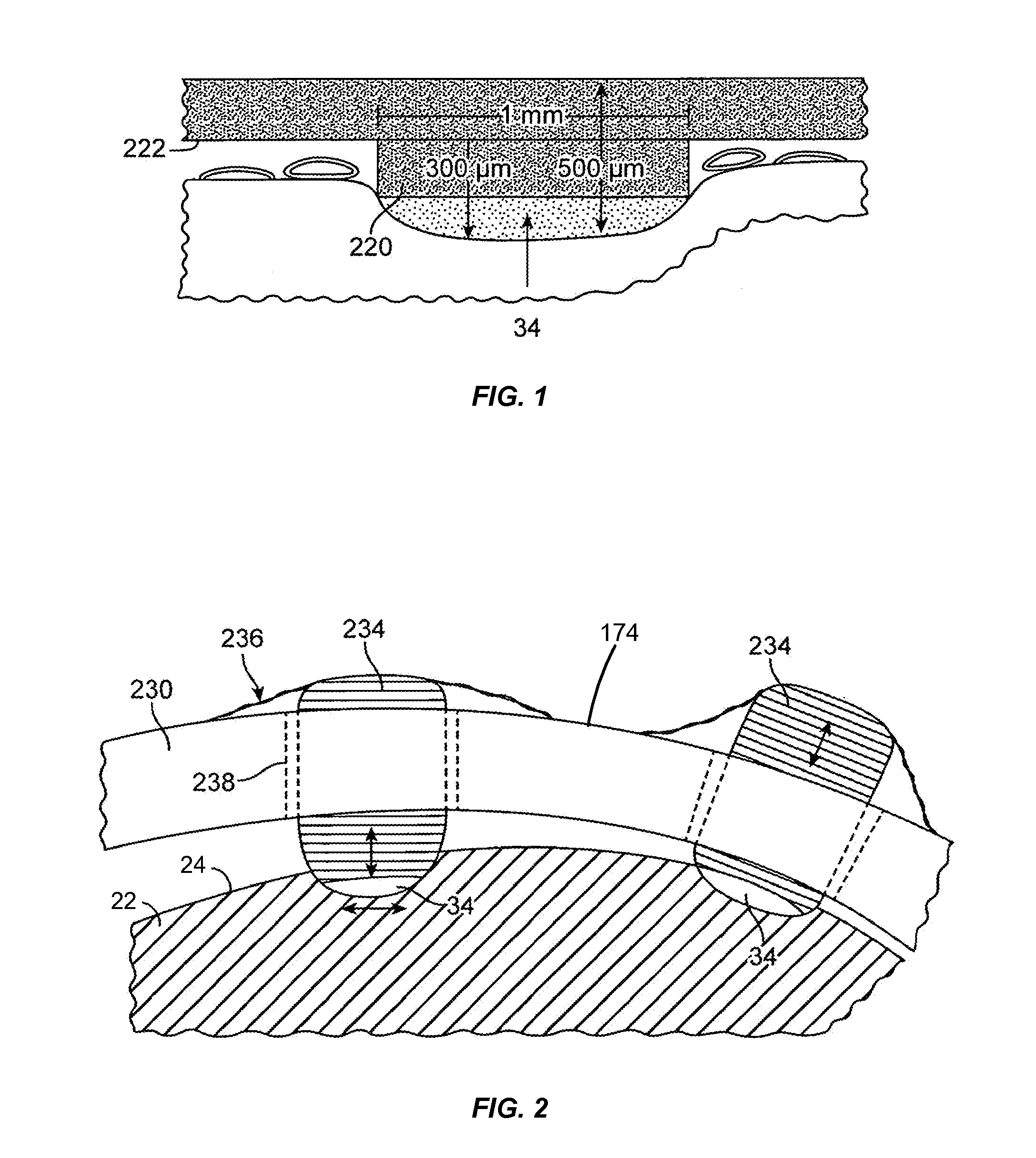
Methods and apparatus for treating spinal stenosis
InactiveUS20060106381A1Effective treatmentPermit flexionInternal osteosythesisJoint implantsSpinal columnDevice form
Surgical implants are configured for placement posteriorly to a spinal canal between vertebral bodies to distract the spine and enlarge the spinal canal. In the preferred embodiments the device permits spinal flexion while limiting spinal extension, thereby providing an effective treatment for treating spinal stenosis without the need for laminectomy. The invention may be used in the cervical, thoracic, or lumbar spine. Numerous embodiments are disclosed, including elongated, length-adjustable components coupled to adjacent vertebral bodies using pedicle screws. The preferred embodiments, however, teach a device configured for placement between adjacent vertebral bodies and adapted to fuse to the lamina, facet, spinous process or other posterior elements of a single vertebra. Various mechanisms, including shape, porosity, tethers, and bone-growth promoting substances may be used to enhance fusion. The tether may be a wire, cable, suture, or other single or multi-filament member. Preferably, the device forms a pseudo-joint in conjunction with the non-fused vertebra. Alternatively, the device could be fused to the caudal vertebra or both the cranial and caudal vertebrae.
Owner:NUVASIVE
Expandable support device and method of use
InactiveUS20080167657A1Increase heightProvide mechanical supportInternal osteosythesisDilatorsSpinal stenosisBiomedical engineering
A device for separating a first bone from a second bone is disclosed. The device can be an expandable orthopedic jack. The device can be used to treat spinal stenosis. The device can be deployed between adjacent spinous processes and then increased in height to reduce pressure on nearby nerves. Methods for using the device are also disclosed.
Owner:STOUT MEDICAL GROUP
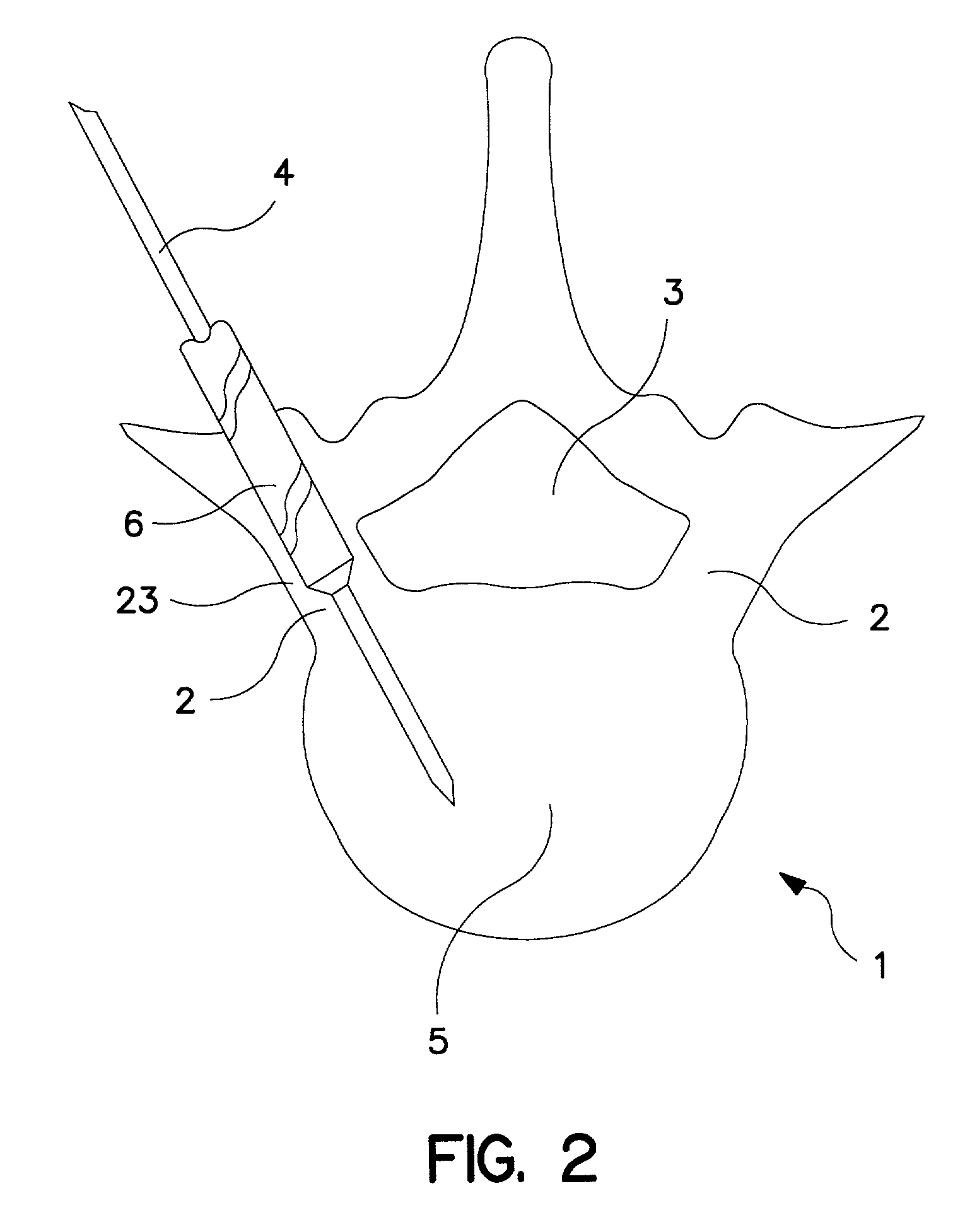
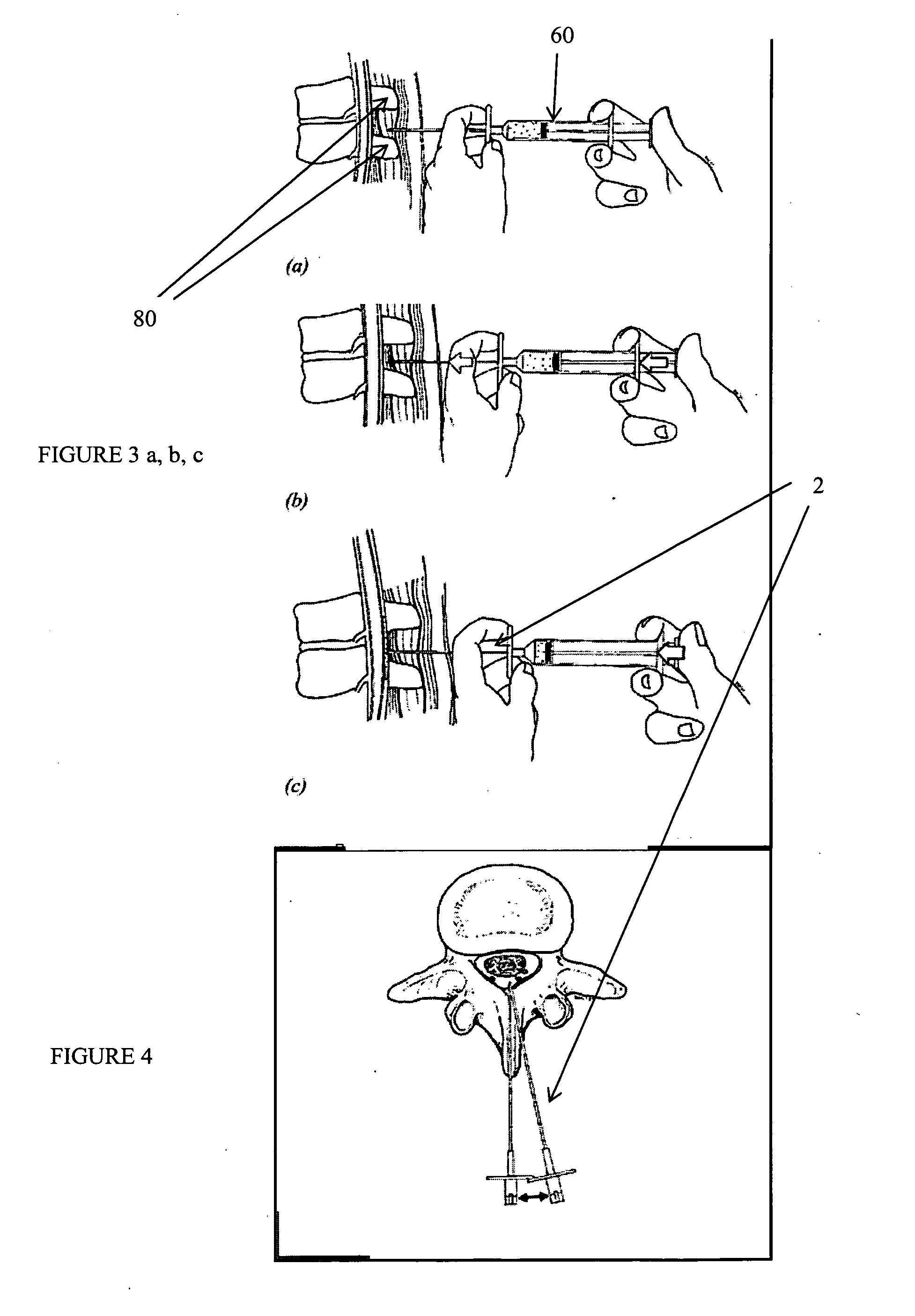
Percutaneous technique and implant for expanding the spinal canal
InactiveUS7166107B2Simple and safe and permanent and minimally invasiveRelieve stressInternal osteosythesisDiagnosticsOsteotomyBiomedical engineering
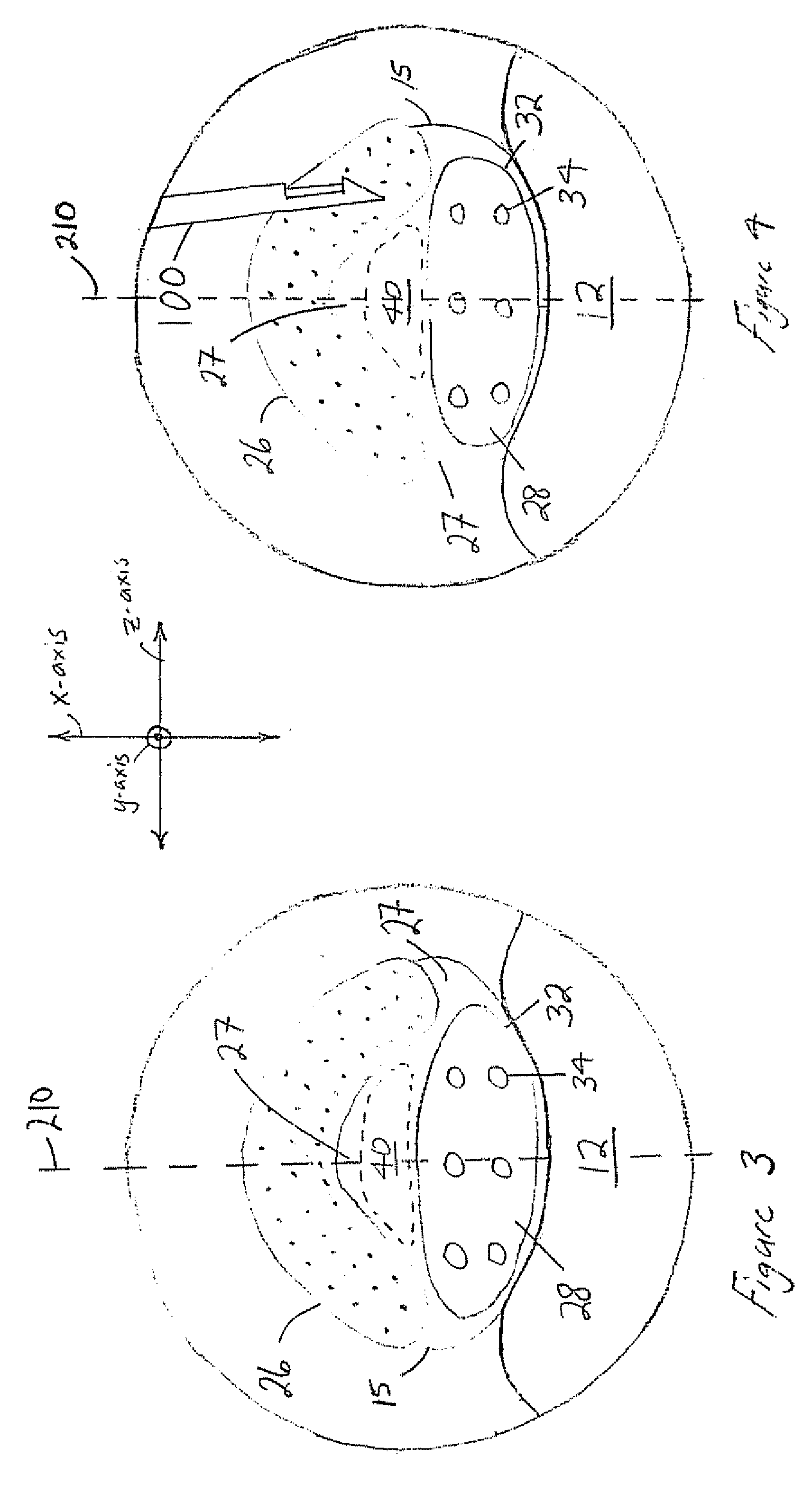
The present invention expands a spinal canal by drilling a cylindrical passage in each pedicle of a vertebra, making a circumferential pedicle cut (osteotomy) through each pedicle from within the passage, separating each pedicle cut by inserting an implant into the passage which distracts the pedicle cut to expand the spinal canal, and securing each pedicle cut, allowing the vertebra to heal with the spinal canal expanded. The implant includes an outer sleeve, an inner bolt, and expandable flanges. The outer sleeve includes an upper portion and a lower portion, with the expandable flanges connected to the lower portion and housed within the upper portion. Rotation of the inner bolt causes the upper and lower portions of the outer sleeve to separate, causing the pedicle cut to widen and the expandable flanges to radially extend into and stabilize the widened pedicle cut to effectuate expansion of the spinal canal.
Owner:DEPUY ACROMED INC +2
Method and apparatus to minimize effects of a cardiac insult
InactiveUS7010345B2Reduced activityImprove heart functionHeart defibrillatorsHeart stimulatorsHeart diseaseElectrical stimulations
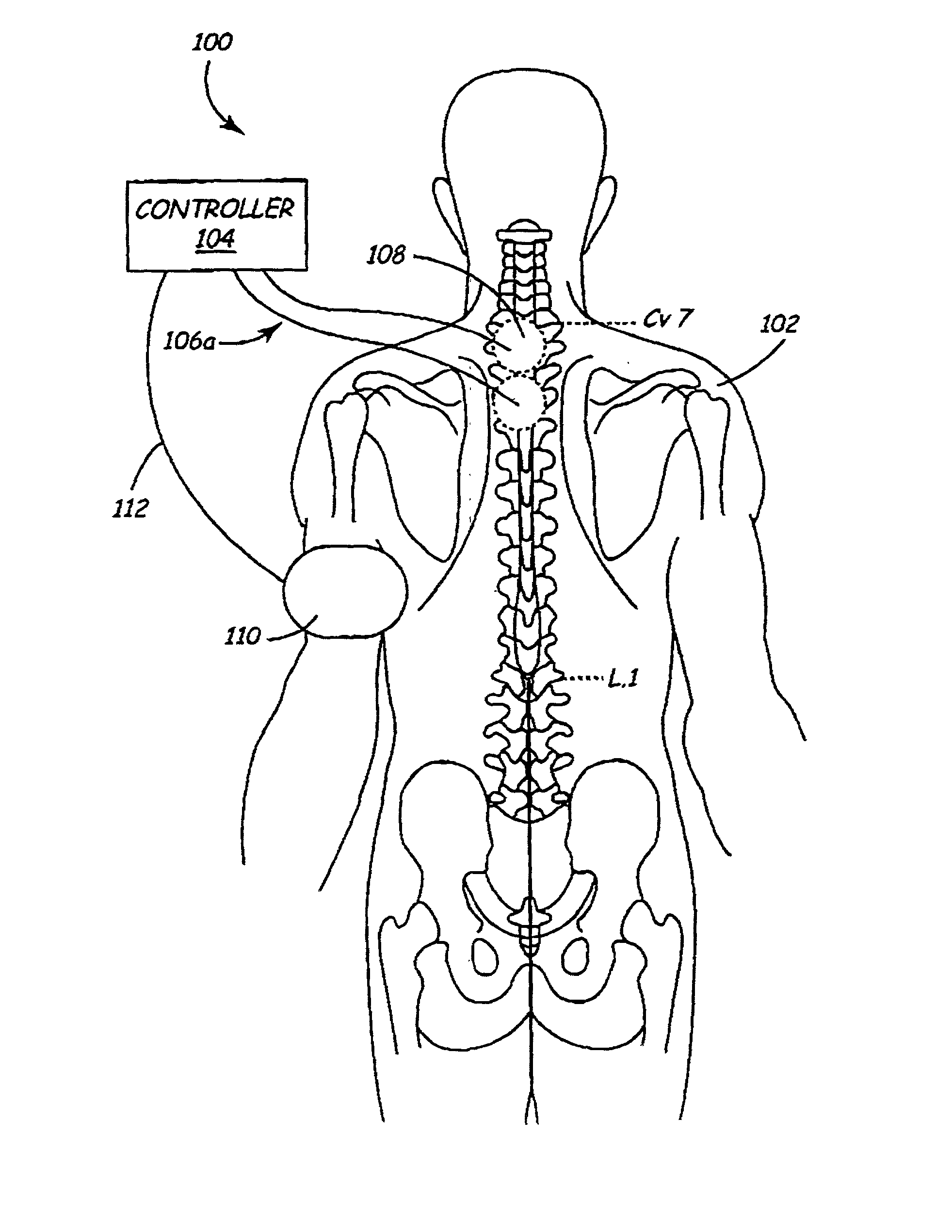
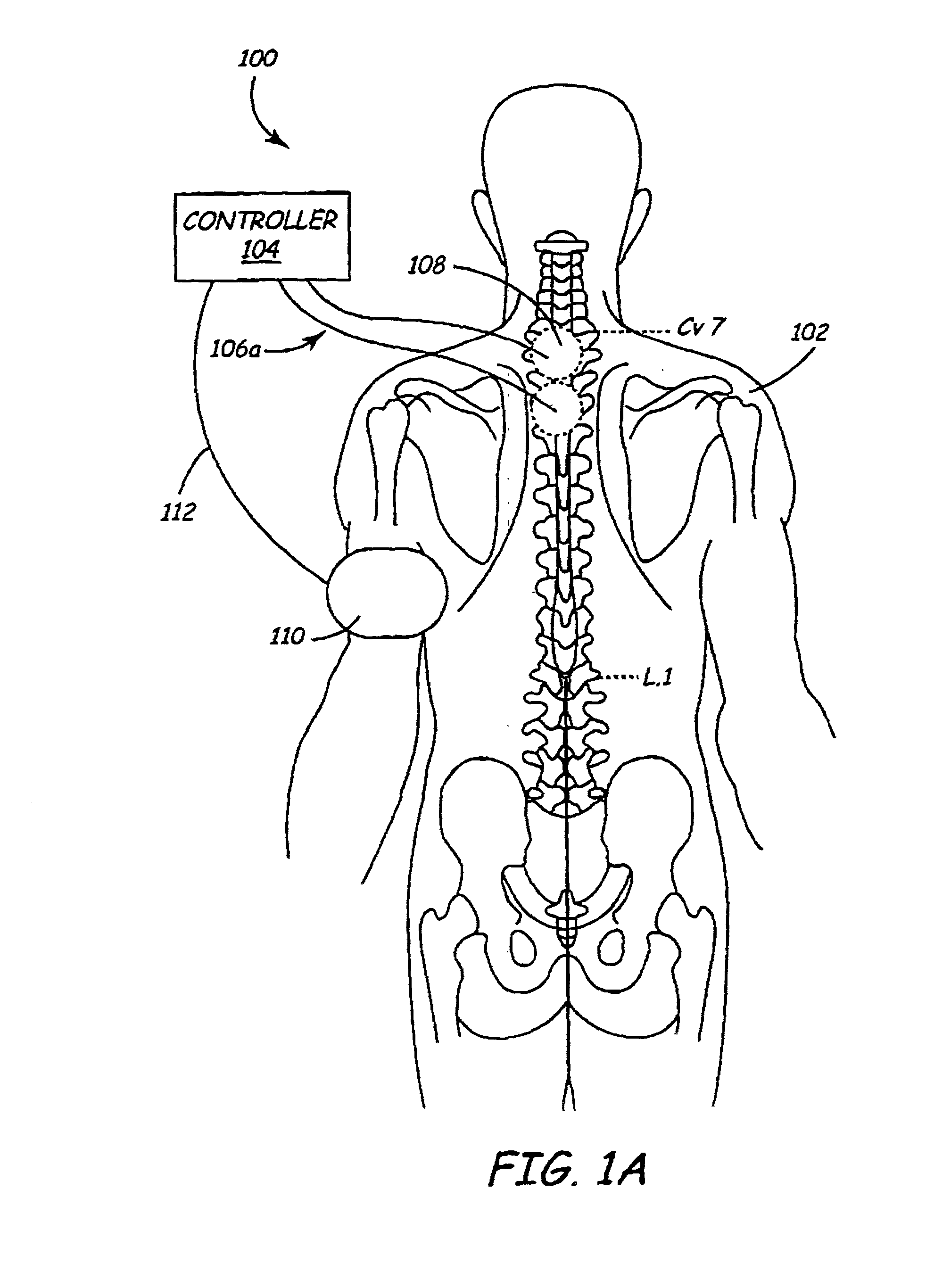
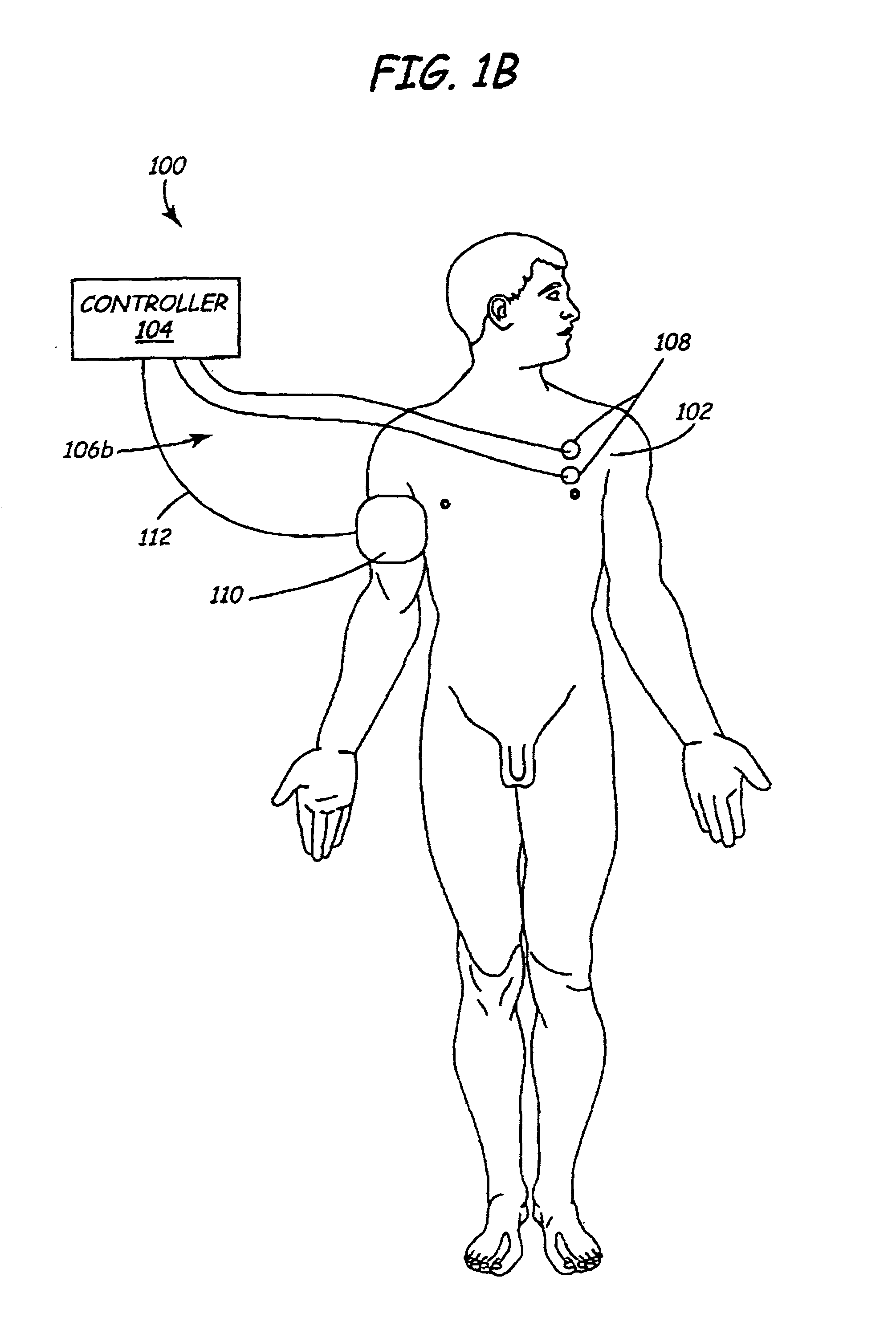
A method and apparatus are provided for protecting cardiac tissue from insult. The method comprises identifying the occurrence of an insult, such as a heart attack, and delivering electrical stimulation to one or more predetermined nerves in a patient's body in response to identifying the occurrence of the insult. The stimulation may be provided at the spinal canal or on the chest wall of the patient through cutaneous electrodes.
Owner:MEDTRONIC INC
Method and apparatus to minimize effects of a cardiac insult
InactiveUS20020143369A1Reduced activityAvoid it happening againHeart defibrillatorsHeart stimulatorsHeart diseaseElectrical stimulations
A method and apparatus are provided for protecting cardiac tissue from insult. The method comprises identifying the occurrence of an insult, such as a heart attack, and delivering electrical stimulation to one or more predetermined nerves in a patient's body in response to identifying the occurrence of the insult. The stimulation may be provided at the spinal canal or on the chest wall of the patient through cutaneous electrodes.
Owner:MEDTRONIC INC
Positive fixation percutaneous epidural neurostimulation lead
InactiveUS20060041295A1Direction easyInhibit migrationSpinal electrodesTransvascular endocardial electrodesLinear configurationSignal generator
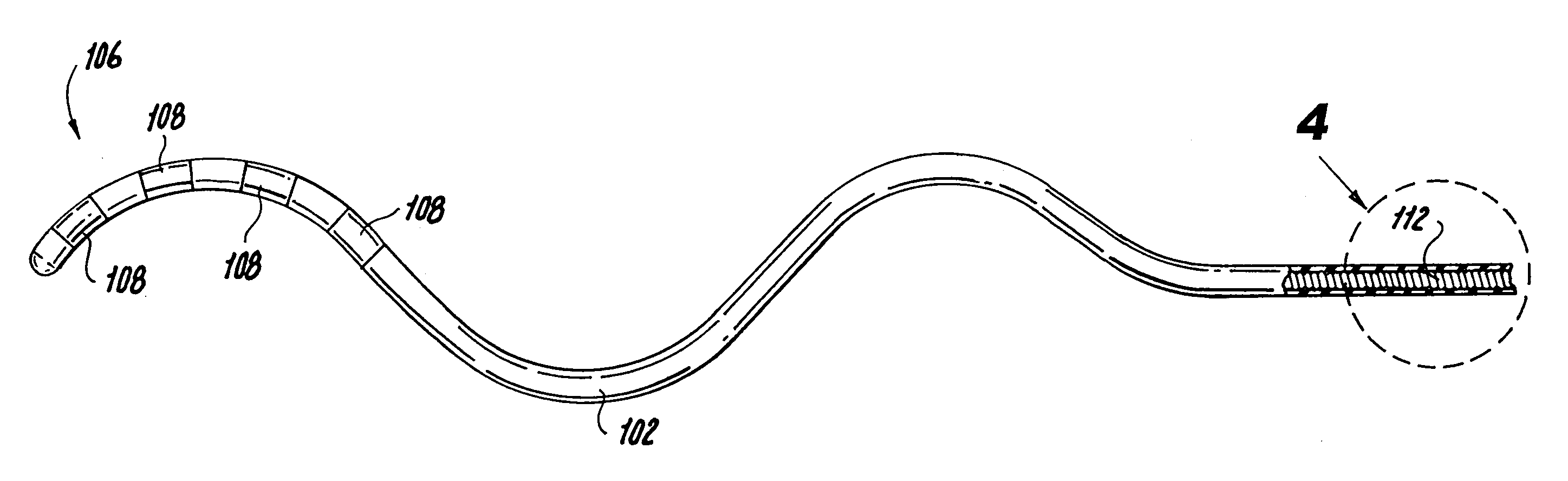
Disclosed is a lead for percutaneous insertion into an epidural space of a spinal canal, which includes an elongated lead body having opposed proximal and distal end portions. At least one electrode for stimulating a patient is operatively associated with the distal end portion of the lead body. Structure for conducting signals extends through the lead body to connect the electrode to a connecting structure operatively associated with the proximal end portion of the lead body. The connecting structure is capable of engaging a signal generator such that signals can be conducted from a signal generator to the electrode. The distal end portion of the lead body is adapted for movement between a first state, in which the distal end portion has a generally linear configuration, and a second state, in which the distal end portion has an undulating configuration.
Owner:NEUROPOINT MEDICAL
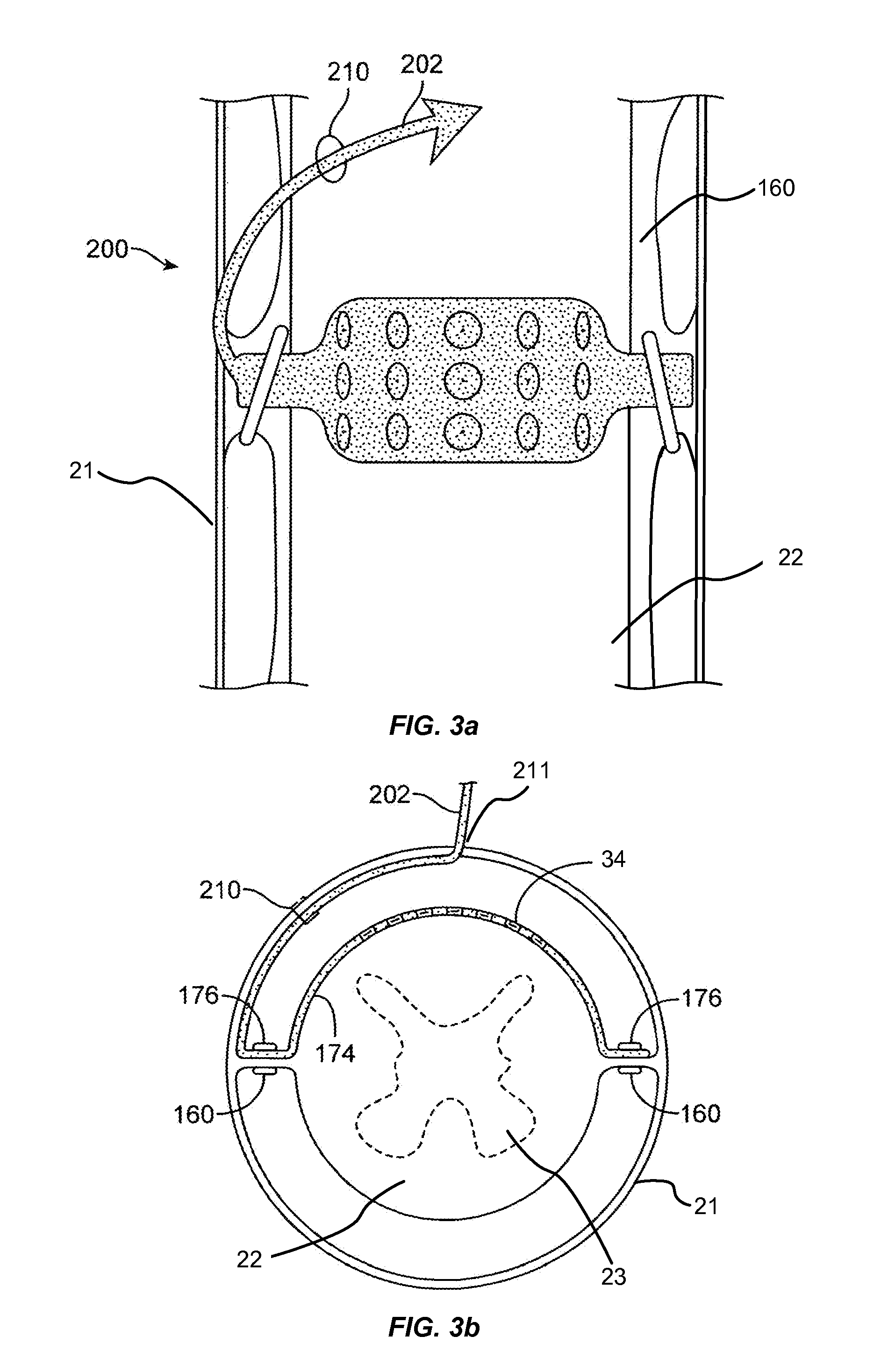
Devices and methods for tissue access
ActiveUS20060089633A1Easy to disassembleEliminate needCannulasAnti-incontinence devicesSurgical departmentNerve stimulation
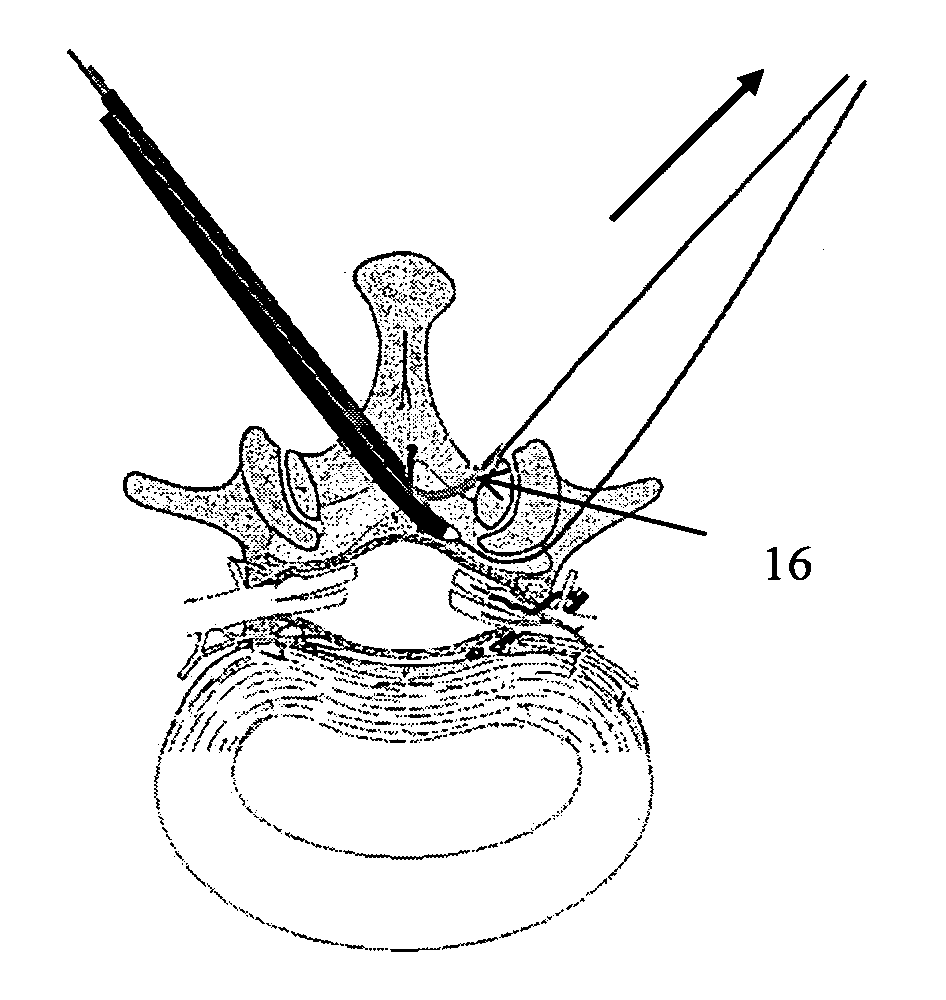
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue abrasion device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the abrasive surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Artificial Disc Replacement Using Posterior Approach
Methods and devices are provided for replacing a spinal disc. In an exemplary embodiment, artificial disc replacements and methods are provided wherein at least a portion of a disc replacement can be implanted using a posterolateral approach. With a posterolateral approach, the spine is accessed more from the side of the spinal canal through an incision formed in the patient's back. A pathway is created from the incision to the disc space between adjacent vertebrae. Portions of the posterolateral annulus, and posterior lip of the vertebral body may be removed to access the disc space, leaving the remaining annulus and the anterior and posterior longitudinal ligaments in tact. The disc implant can be at least partially introduced using a posterolateral approach, yet it has a size that is sufficient to restore height to the adjacent vertebrae, and that is sufficient to maximize contact with the endplates of the adjacent vertebrae.
Owner:DEPUY SPINE INC (US)
Devices and methods for tissue access
InactiveUS20060122458A1Enabling symptomatic reliefApproach can be quite invasiveCannulasDiagnosticsSurgical departmentNerve stimulation
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:BAXANO
Devices and methods for tissue modification
ActiveUS20060095059A1Easy to disassembleEliminate needCannulasAnti-incontinence devicesSurgical departmentNerve stimulation
Owner:MIS IP HLDG LLC +1
Methods and apparatus for preventing the migration of intradiscal devices
Barriers are used to hold prosthetic components, including intradiscal devices, in position while allowing natural movement within a “safe zone.” In the preferred embodiments, the barrier is in the form of a “buttress” plate coupled to a controlled linkage that prevents the device from moving into a dangerous position. The movable member(s) allow an intradiscal device to move through a safe zone while permitting mobile artificial disc replacements (ADRs) or other intradiscal devices to self-center with movement of the vertebrae. The link member(s) broadly serve as a “tether” to prevent extrusion of the intradiscal device into the spinal canal, or outside of the disc space. The intradiscal device may be mobile, or attached to one or both of the vertebral endplates. Other mechanisms to accommodate the movable link members are also disclosed. The plates are preferably constructed of metal, but the invention is not limited in terms of the biocompatible material(s) used for the plate or link members.
Owner:FERREE BRET A
Apparatus and methods for sensing and cooling during application of thermal energy for treating degenerative spinal discs
InactiveUS20050004563A1Reduce the temperatureAvoid motor nerve damageSurgical needlesSurgical instruments for heatingThermal energySpinal nerve
Apparatus and methods for performing spinal disc lesioning procedures while monitoring the temperature near the spinal nerve roots. A needle containing a thermocouple and an injection bore may be placed between the disc and a nerve root. Temperature near the nerve root may be monitored during the lesioning procedure and if raised to a point where damage to the nerve could occur, the procedure may be stopped before damage is done. Coolant may be injected through the injection bore to lower the temperature near the nerve root. Additional dual purpose needles may be placed on the disc adjacent the opposite nerve root or in the spinal canal at the level of the disc for additional control. A hollow, flexible tip electrode needle may be used to repair and lesion the disc tissue. Prior to lesioning, the electrode may be stimulated to assess motor nerve response and avoid motor nerve damage.
Owner:EPIMED INT
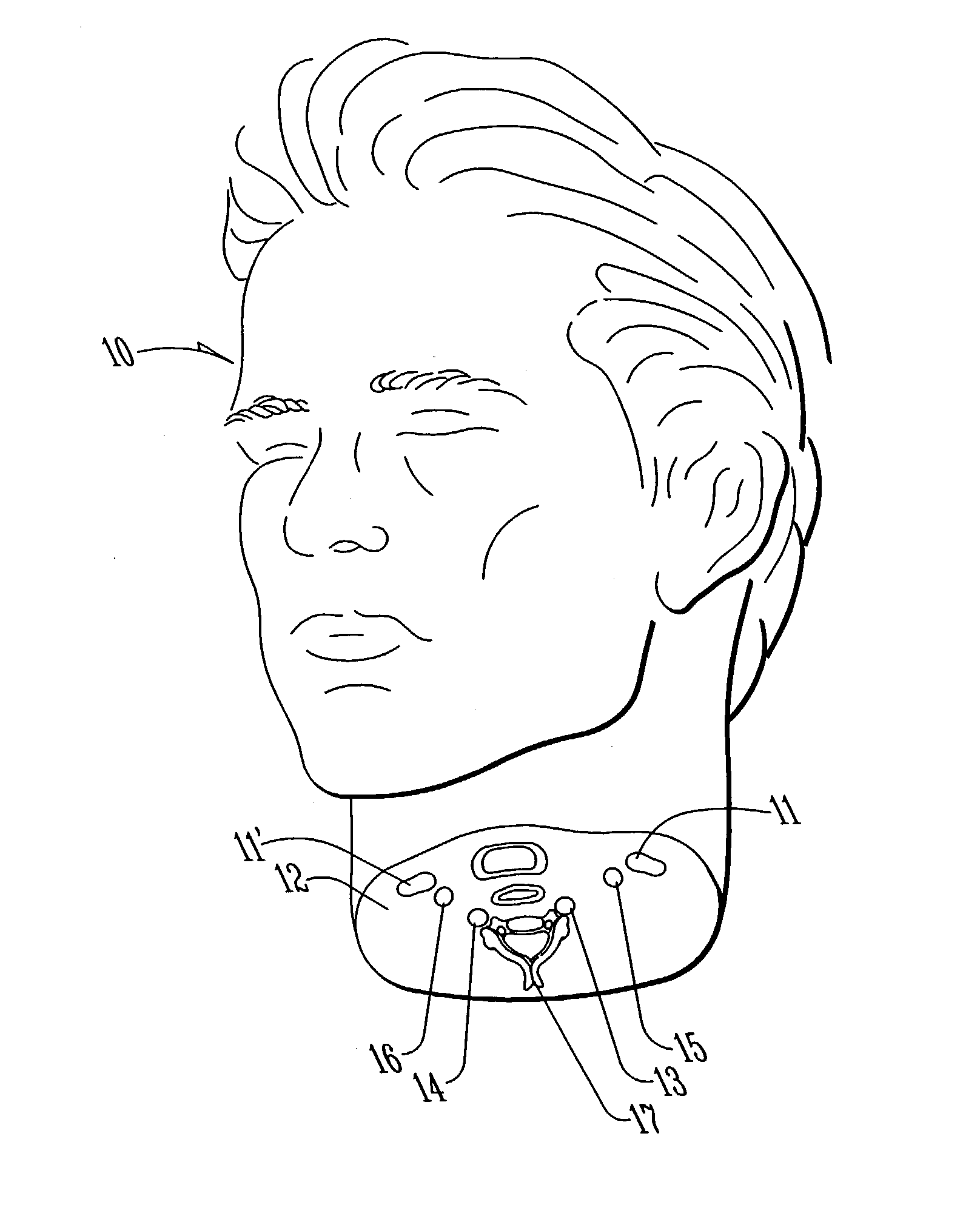
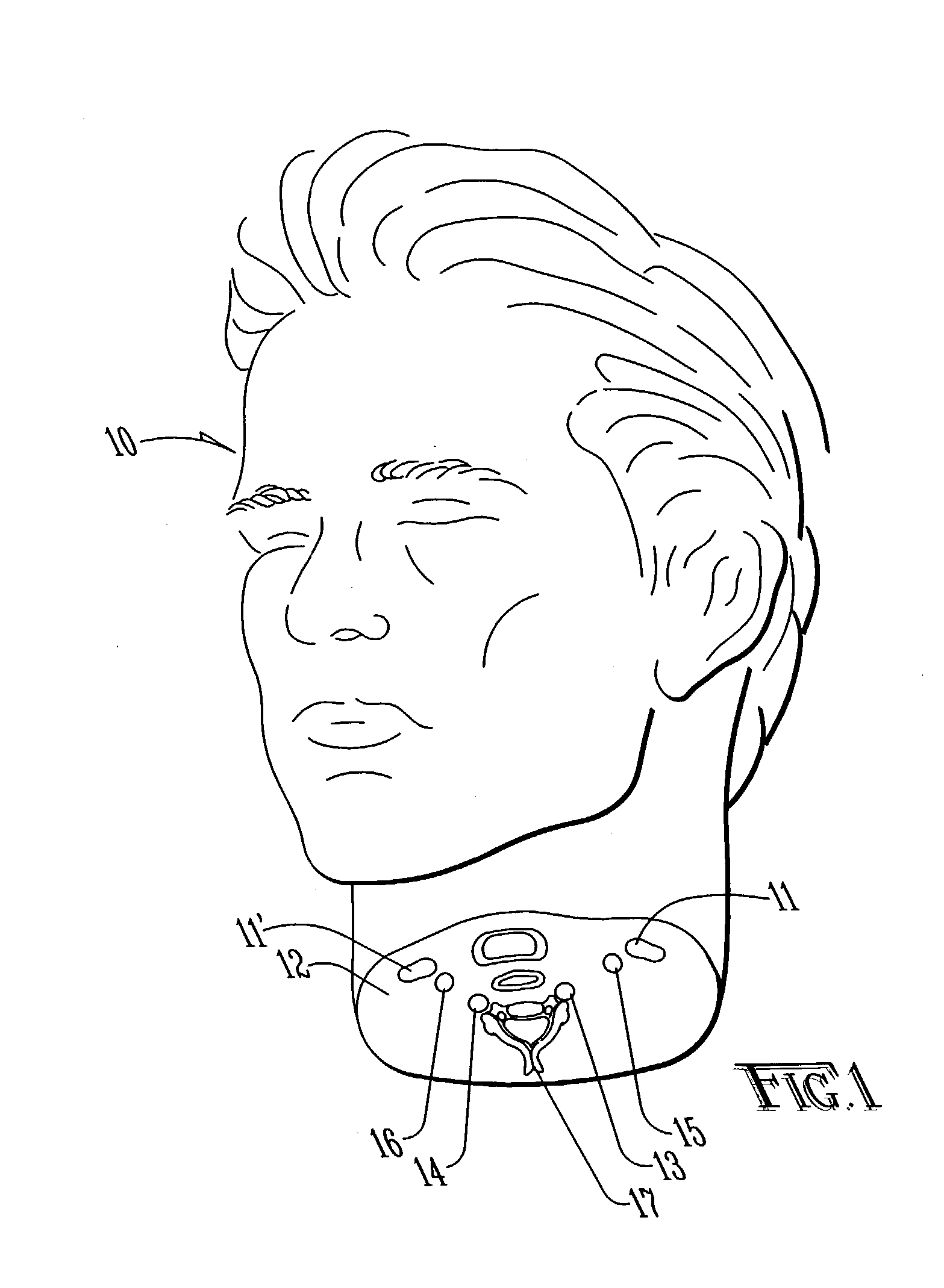
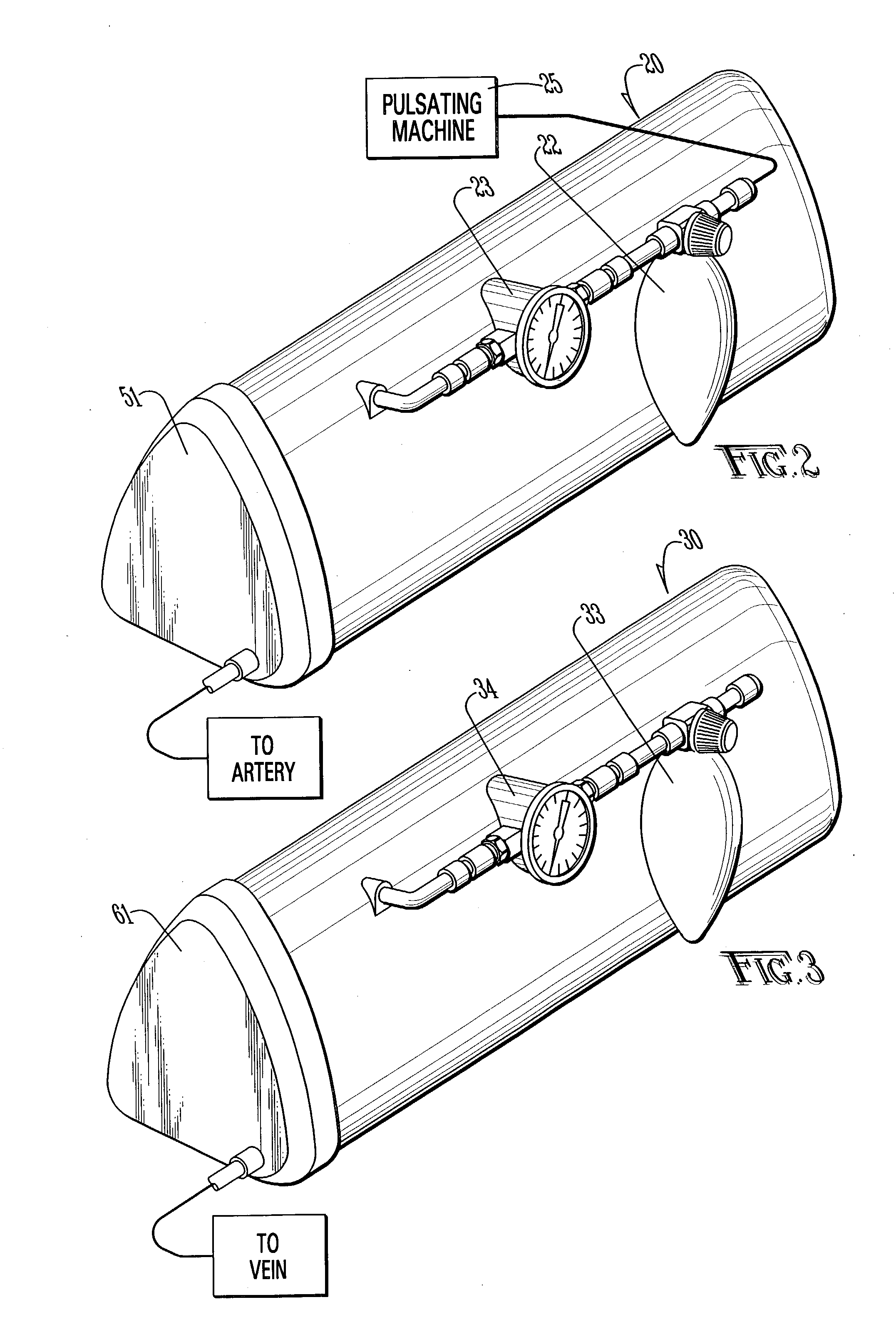
Method and apparatus for surgical training
An apparatus and method for microsurgical training using cadaveric anatomy with filling of the vascular system by fluids under pressure to simulate the appearance and function of live surgery. One or more arteries on the specimen of cadaveric anatomy are cannulated and connected to an arterial reservoir having a flexible container holding an arterial fluid simulating the appearance of blood circulating in the arteries of the living organism from which the cadaveric anatomy is derived. Suitable static pressure simulating the arterial pressure appropriate to that of the living organism is applied to the air in an air-tight space surrounding the flexible container in the arterial reservoir. A pulsating machine provides air pulsations to the space surrounding the flexible fluid container to simulate the normal pulsations of the arterial system. One or more veins on the specimen are also cannulated and connected to a venous reservoir having a flexible container holding a venous fluid simulating the appearance of blood circulating in the veins of the living organism. Suitable static pressure simulating the venous pressure appropriate to that of the living organism is applied to the air in an air-tight space surrounding the flexible container in the venous reservoir. Optionally, if the specimen includes at least a portion of spinal canal, a clear fluid reservoir can be connected to the specimen through the spinal canal to simulate cerebrospinal fluid.
Owner:ABOUD GHAITH +1
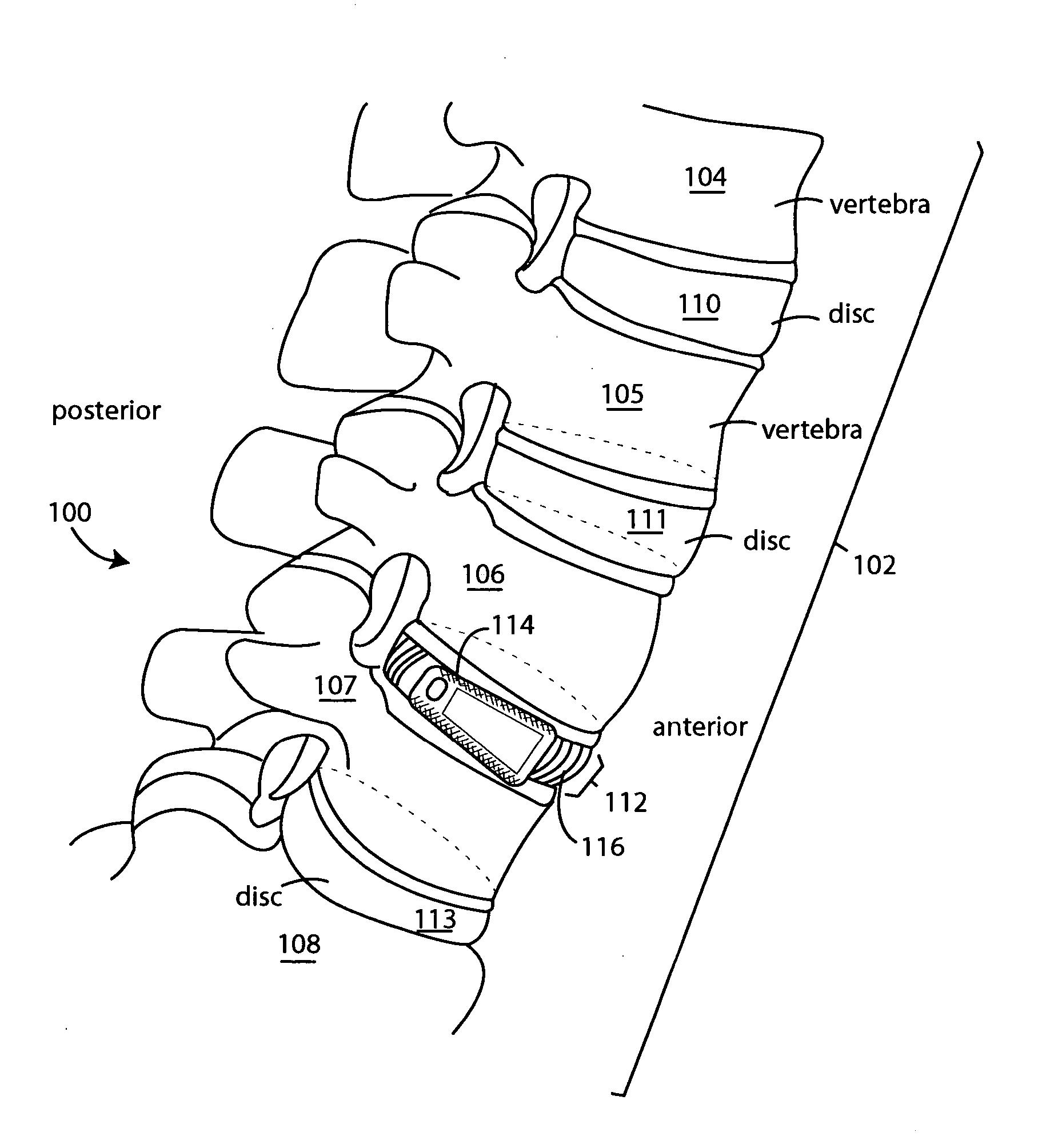
Intervertebral bone fusion device
InactiveUS20050119751A1Preventing subsidence lordosisSupport of loadInternal osteosythesisBone implantSpinal columnIntervertebral space
A surgical spine implant comprises a solid wedge shape device body is such that said polygonal shape with decreased height posterior and several surfaces that contours to the anatomy of the spine intervertebral space without complex machining across radiuses. The diminished height posteriorly provides for the angulations necessary to accommodate and ensure maintenance of the normal spinal alignment resulting in posterior extension angulations. The device provides ease of application of bone grafts. Such as for impaction of cancellous bone grafts after placement of the device and narrow posterior aspect easily directs grafts. This minimizes the problem of bone grafts hanging up behind the device either to the right or the left of the medial or lateral, which prevents the displacement of the device or prominence of the bone grafts near the spinal canal. The recess hole in the device accommodates a holding insertion tool and ease of bone graft packing “fill” to ensure a tight fit and immobile the attachment of the disc to the lower vertebrae.
Owner:LAWSON INC
Devices and methods for selective surgical removal of tissue
ActiveUS20060094976A1Improve securityAvoid injuryCannulasAnti-incontinence devicesTissue remodelingLateral recess
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
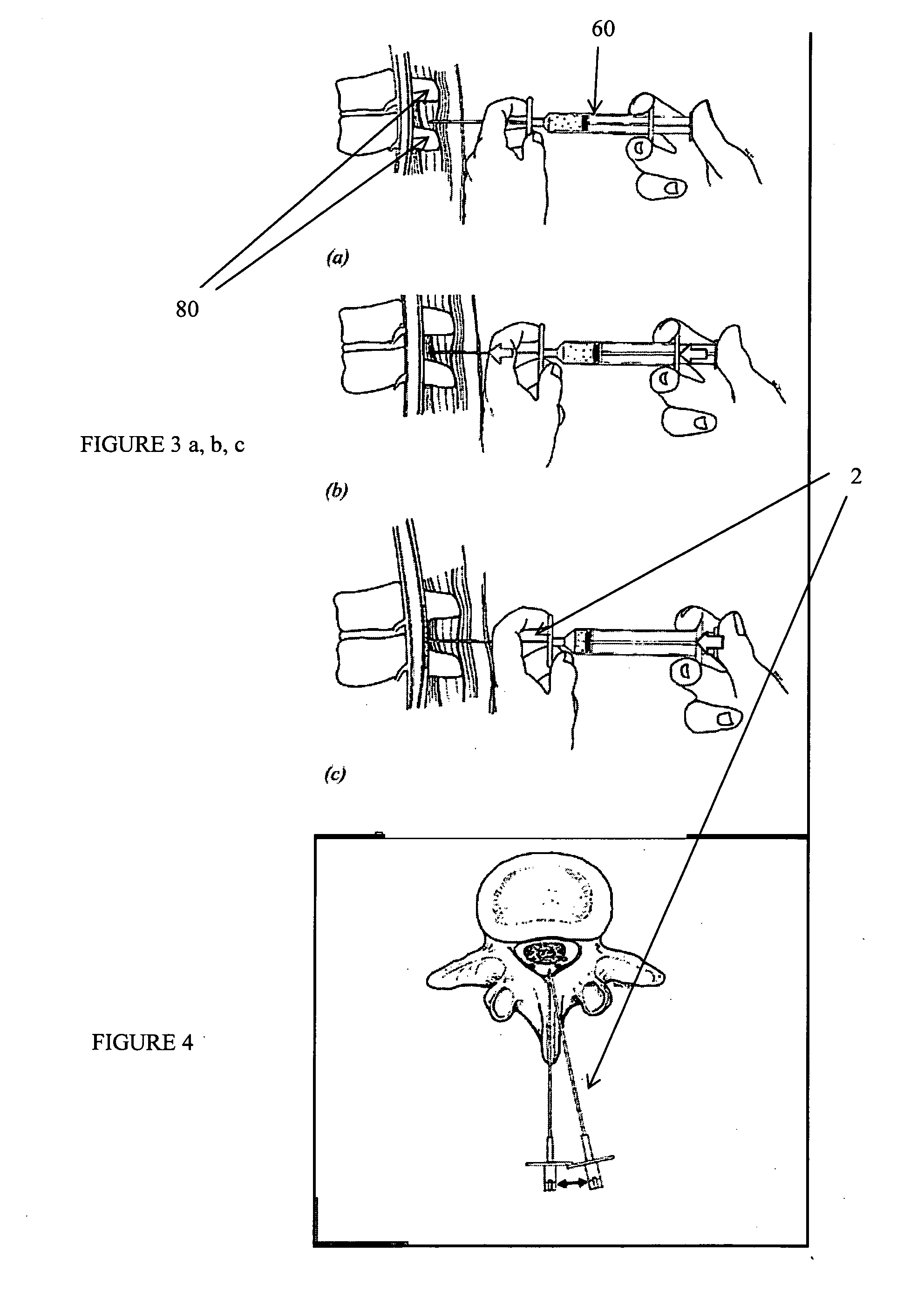
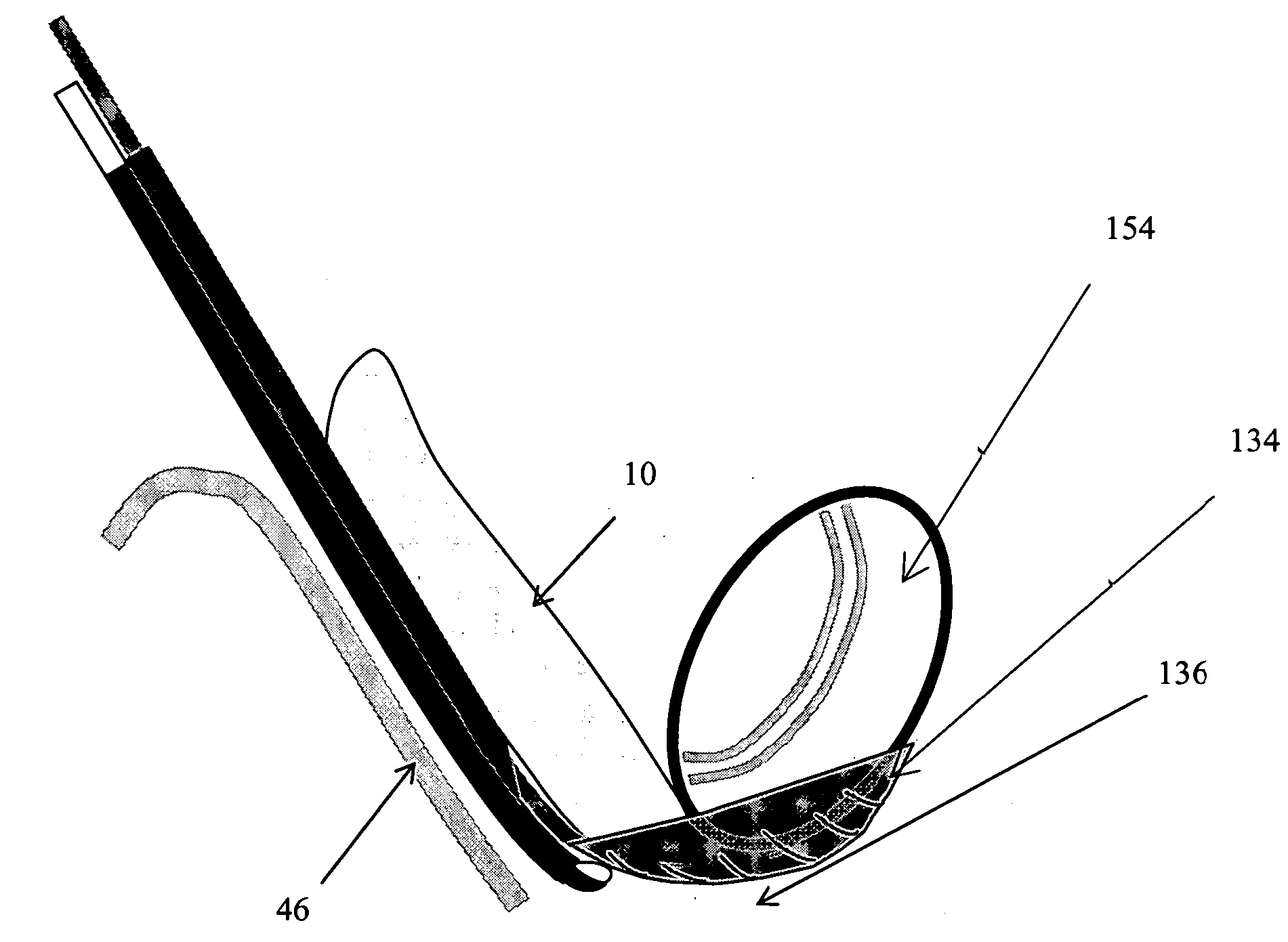
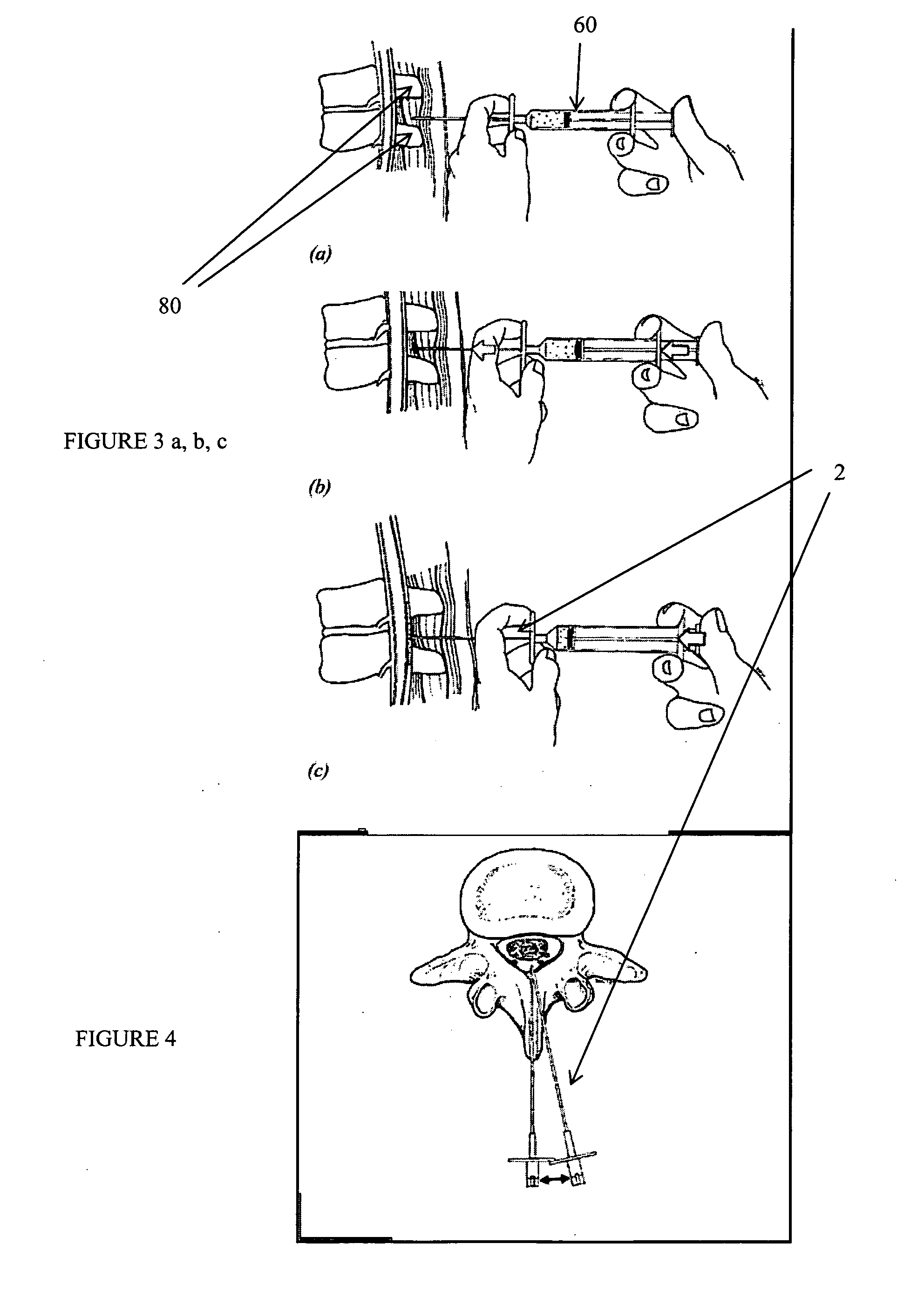
Method, Implant & Instruments for Percutaneous Expansion of the Spinal Canal
InactiveUS20100168751A1Increase heightSufficient gripInternal osteosythesisDilatorsSpinal stenosisDistal portion
A method for correcting spinal stenosis involves cannulating a passage in a vertebra, and placing a distal portion of an implant therein. Then, a circumferential vertebral cut is performed from within the passage, using a proximal end of the distal portion of the implant to align the vertebral cut. Then, a proximal portion of the implant is placed into the passage, positioned against the distal portion at the vertebral cut. Operation of the distal and the proximal portions of the implant relative to one another widens the vertebral cut and expands the spinal canal. The proximal portion can then be secured to the distal portion to stabilize the vertebral cut, allowing vertebral healing with the spinal canal expanded. Flanges may exist that radially extend outward from the implant and into the vertebral cut, or into the passage walls, to assist vertebral cut widening and stabilization.
Owner:INNOVATIVE SURGICAL DESIGNS
Ipsilateral Approach to Minimally Invasive Ligament Decompression Procedure
A method for treating stenosis in a spine of a patient having a median plane, wherein the spine includes a spinal canal having a posterior surface, a dural sac and an epidural space between the posterior surface and dural sac, the location of the stenosis determining a region of interest in the spine. In an embodiment the method comprises the steps of a) generating at least one view of a portion of the spinal canal in the region of interest; b) compressing the dural sac in the region of interest by injecting a fluid to form a safety zone and establish a working zone in the region of interest, the safety zone lying between the working zone and the dural sac; c) percutaneously accessing the epidural space in the region of interest on a first side of the median plane; d) inserting a tissue removal tool into tissue in the working zone on the first side of the median plane; e) using the tissue removal tool to percutaneously reduce the stenosis on the first side of the median plane; and f utilizing the at least one view to position the tissue removal too during at least a part of step d) and at least part of step e)
Owner:VERTOS MEDICAL
Artificial Disc Replacement Using Posterior Approach
Methods and devices are provided for replacing a spinal disc. In an exemplary embodiment, artificial disc replacements and methods are provided wherein at least a portion of a disc replacement can be implanted using a posterolateral approach. With a posterolateral approach, the spine is accessed more from the side of the spinal canal through an incision formed in the patient's back. A pathway is created from the incision to the disc space between adjacent vertebrae. Portions of the posterolateral annulus, and posterior lip of the vertebral body may be removed to access the disc space, leaving the remaining annulus and the anterior and posterior longitudinal ligaments in tact. The disc implant can be at least partially introduced using a posterolateral approach, yet it has a size that is sufficient to restore height to the adjacent vertebrae, and that is sufficient to maximize contact with the endplates of the adjacent vertebrae.
Owner:DEPUY SPINE INC (US)
Intraspinal Drug Delivery Methods and Devices To Alleviate Chronic Pelvic Pain
The disclosure describes methods and systems for alleviating chronic pelvic pain in a subject by intraspinally a therapeutically effective amount of a chronic pelvic pain-alleviating drug. The system includes drug delivery devices and systems for the controlled administration of drugs for alleviation of pelvic pain. The system may deliver drug therapy for pelvic pain in men or women.
Owner:MEDTRONIC INC
Devices and methods for tissue access
InactiveUS20060100651A1Improve securityAvoid injuryEar treatmentCannulasTissue remodelingSpinal column
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
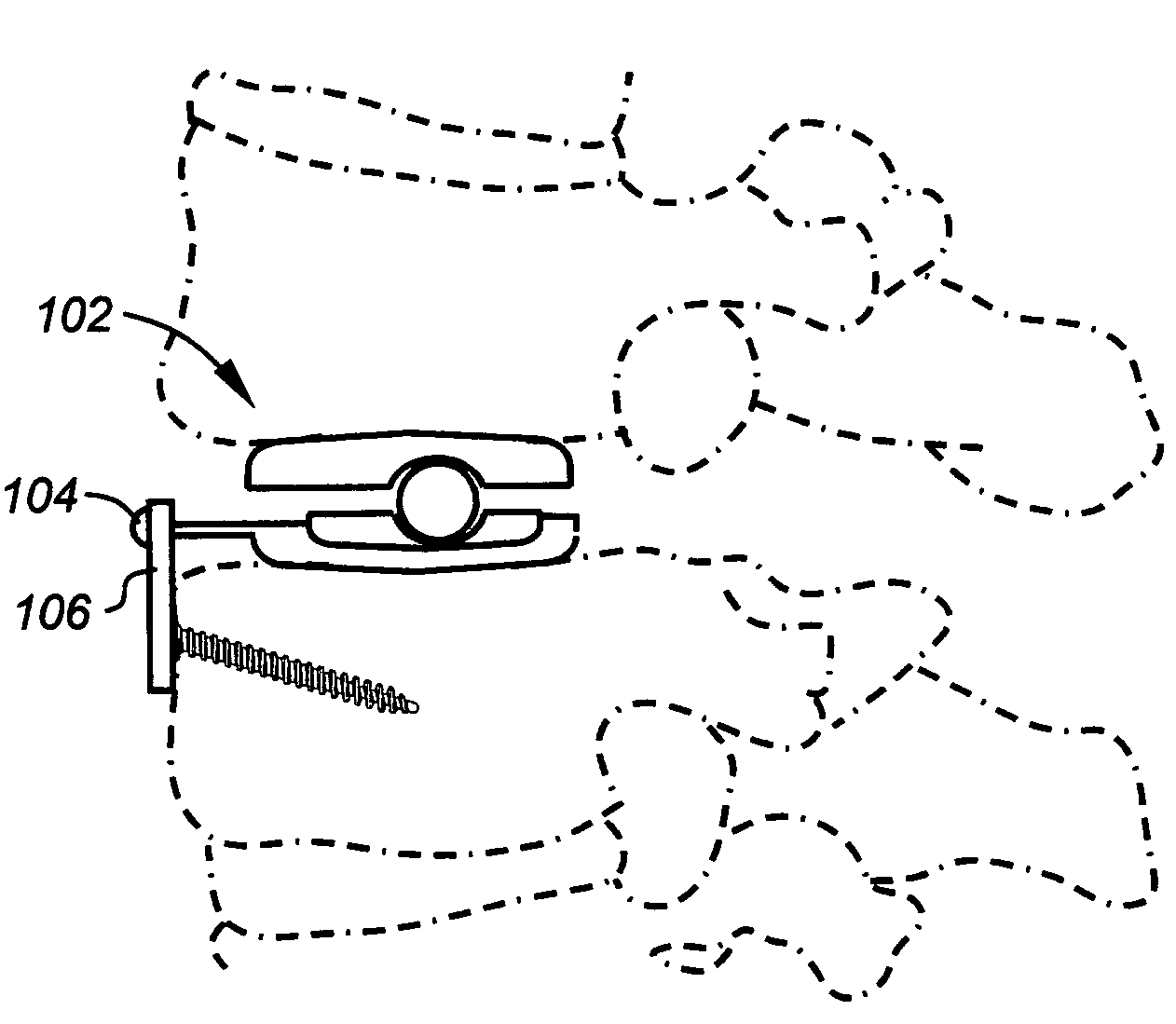
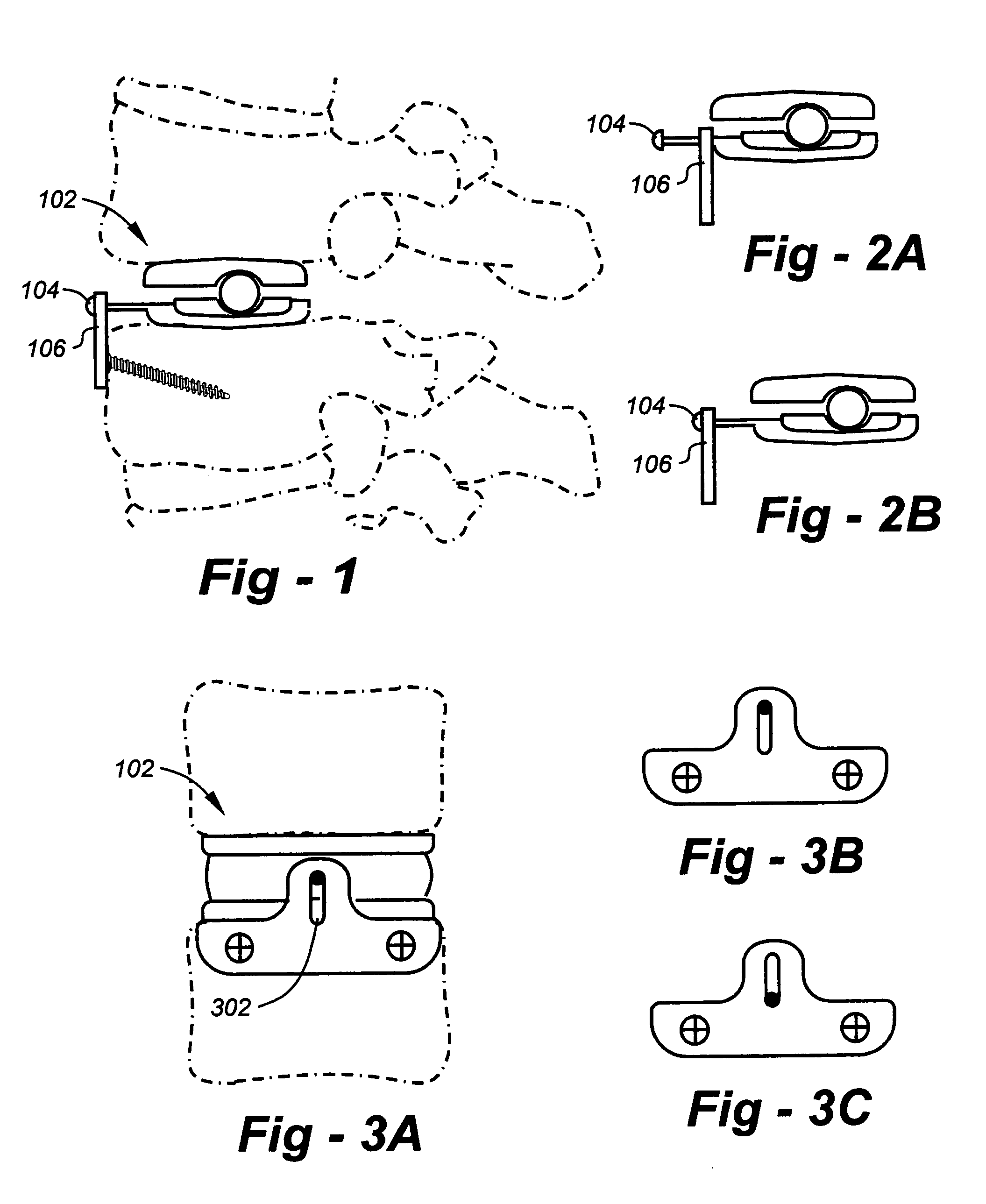
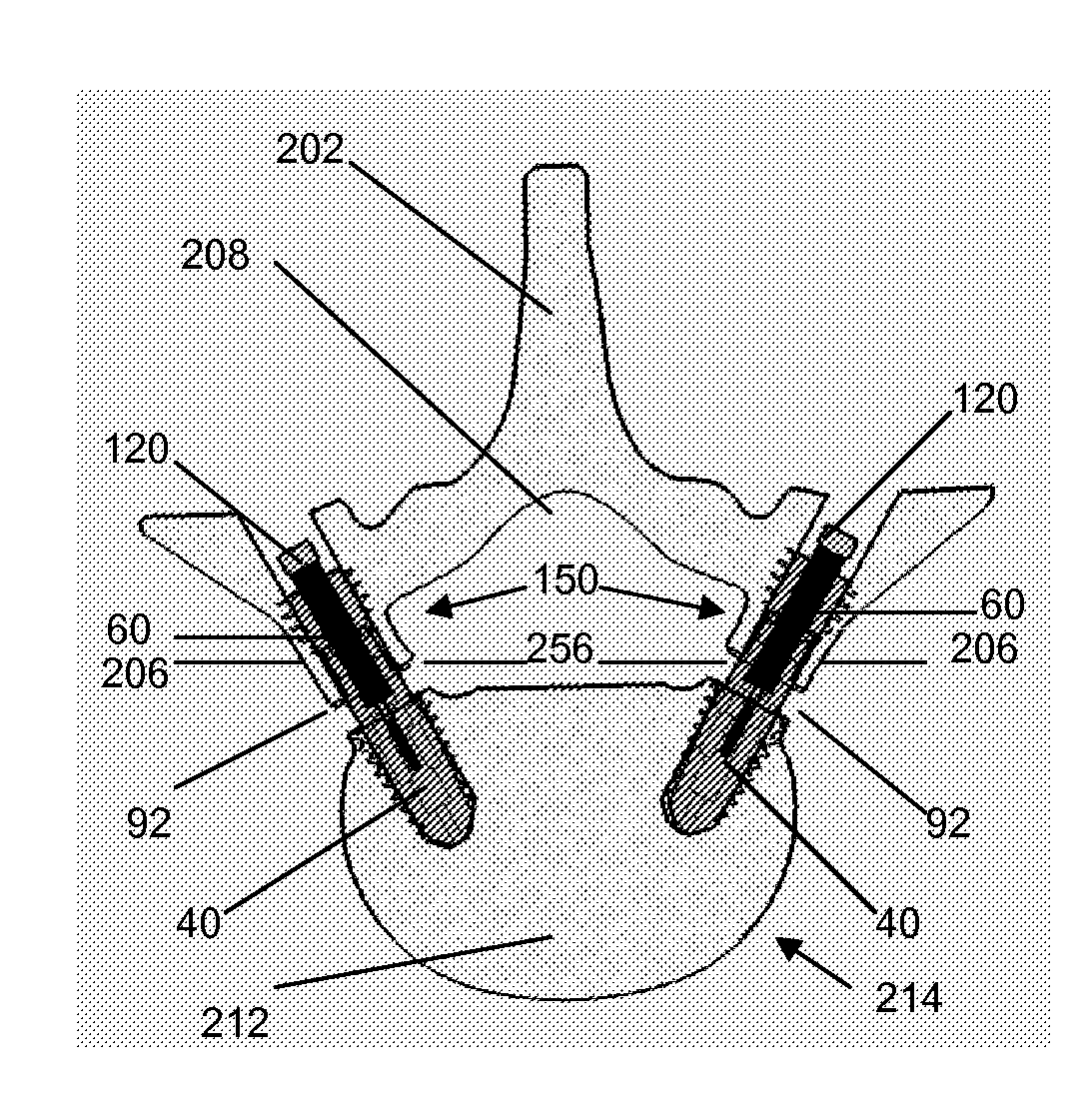
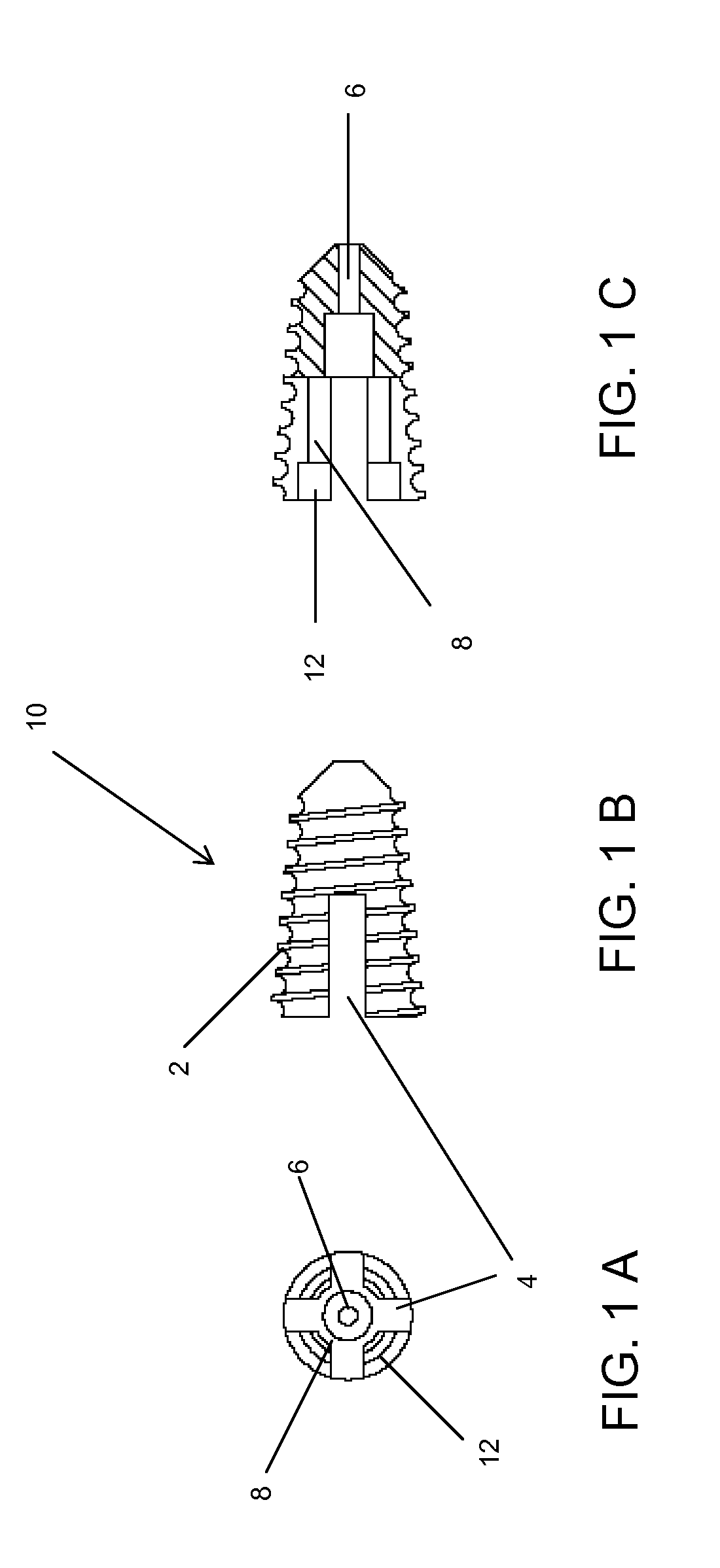
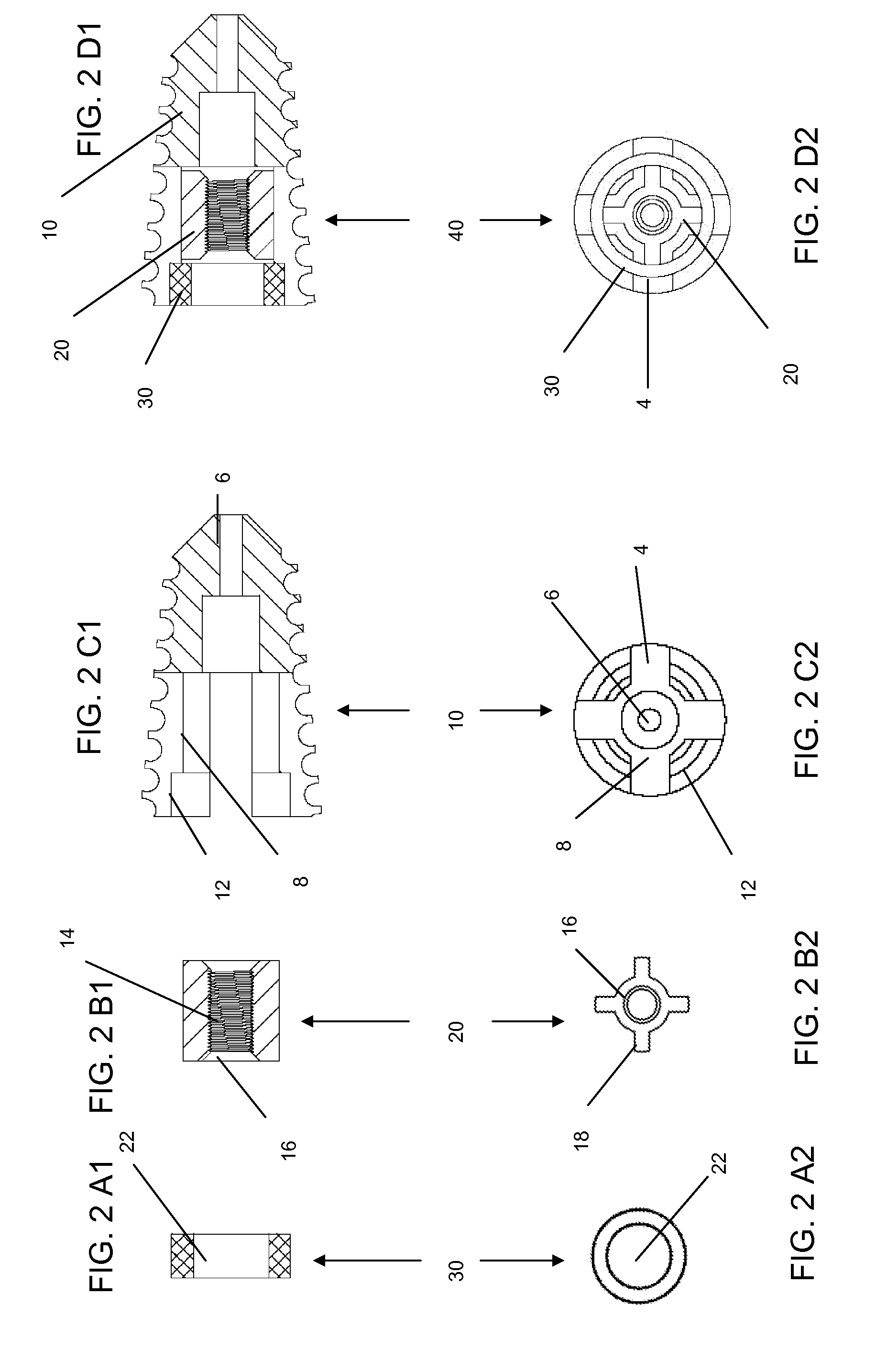
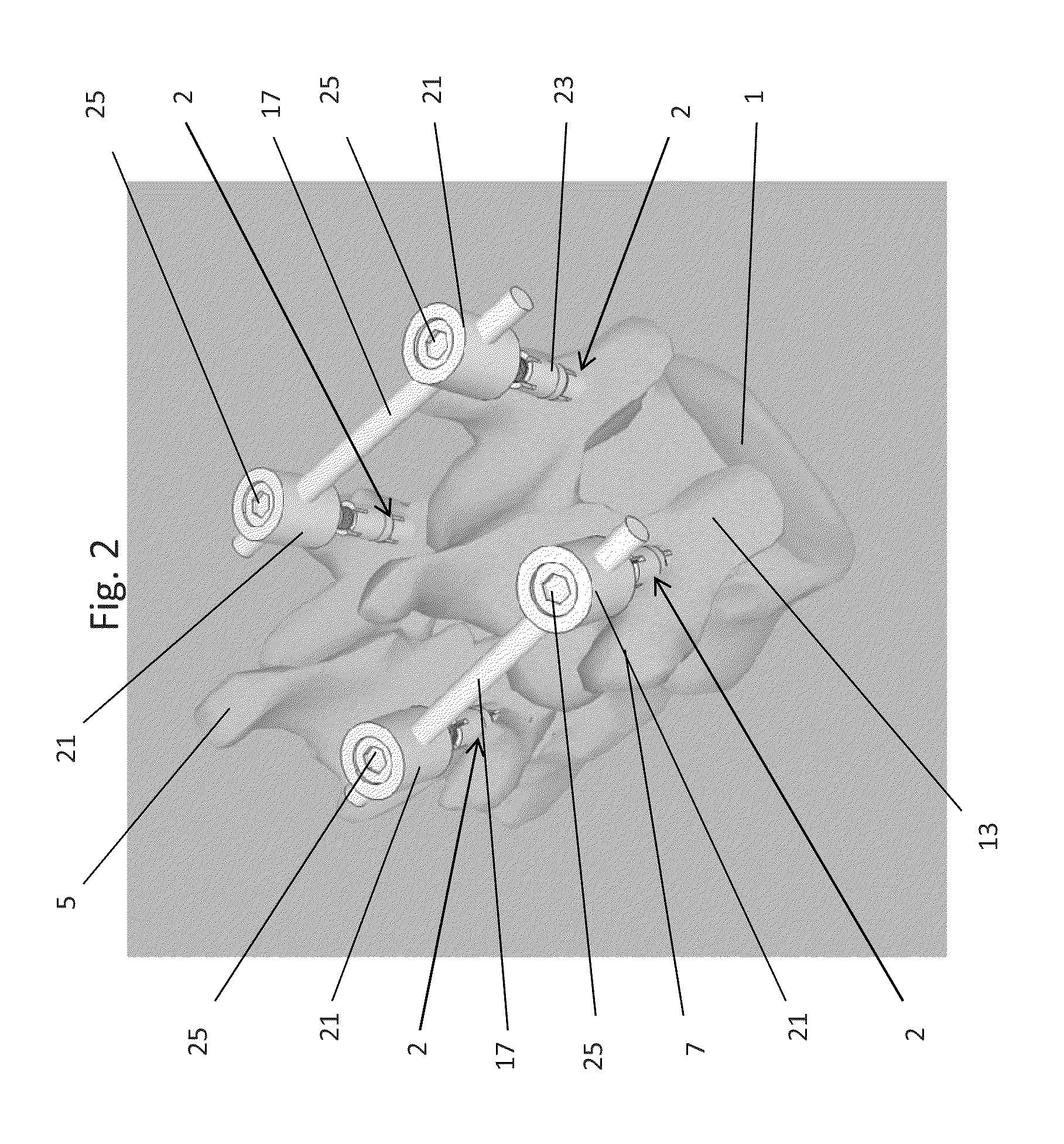
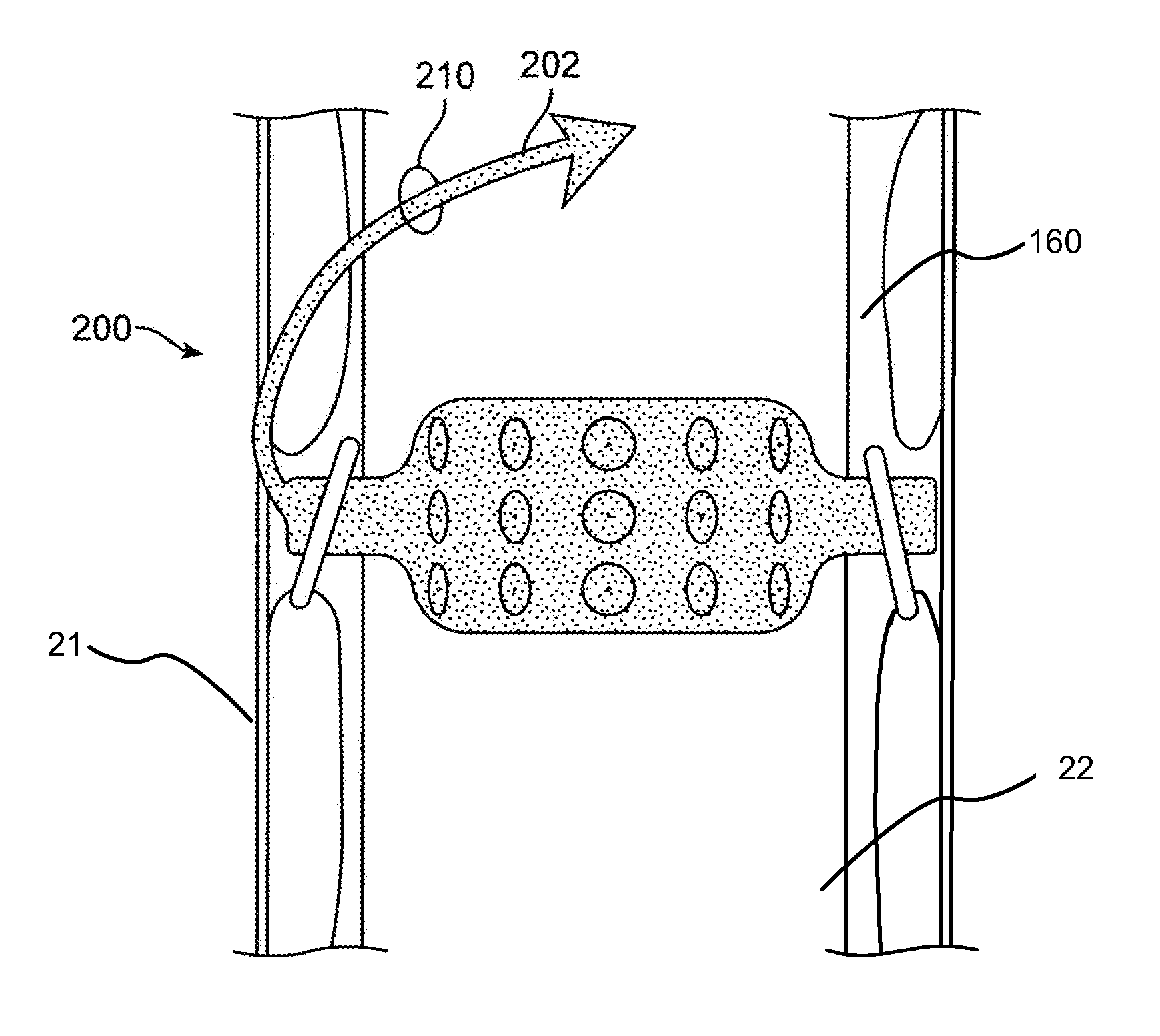
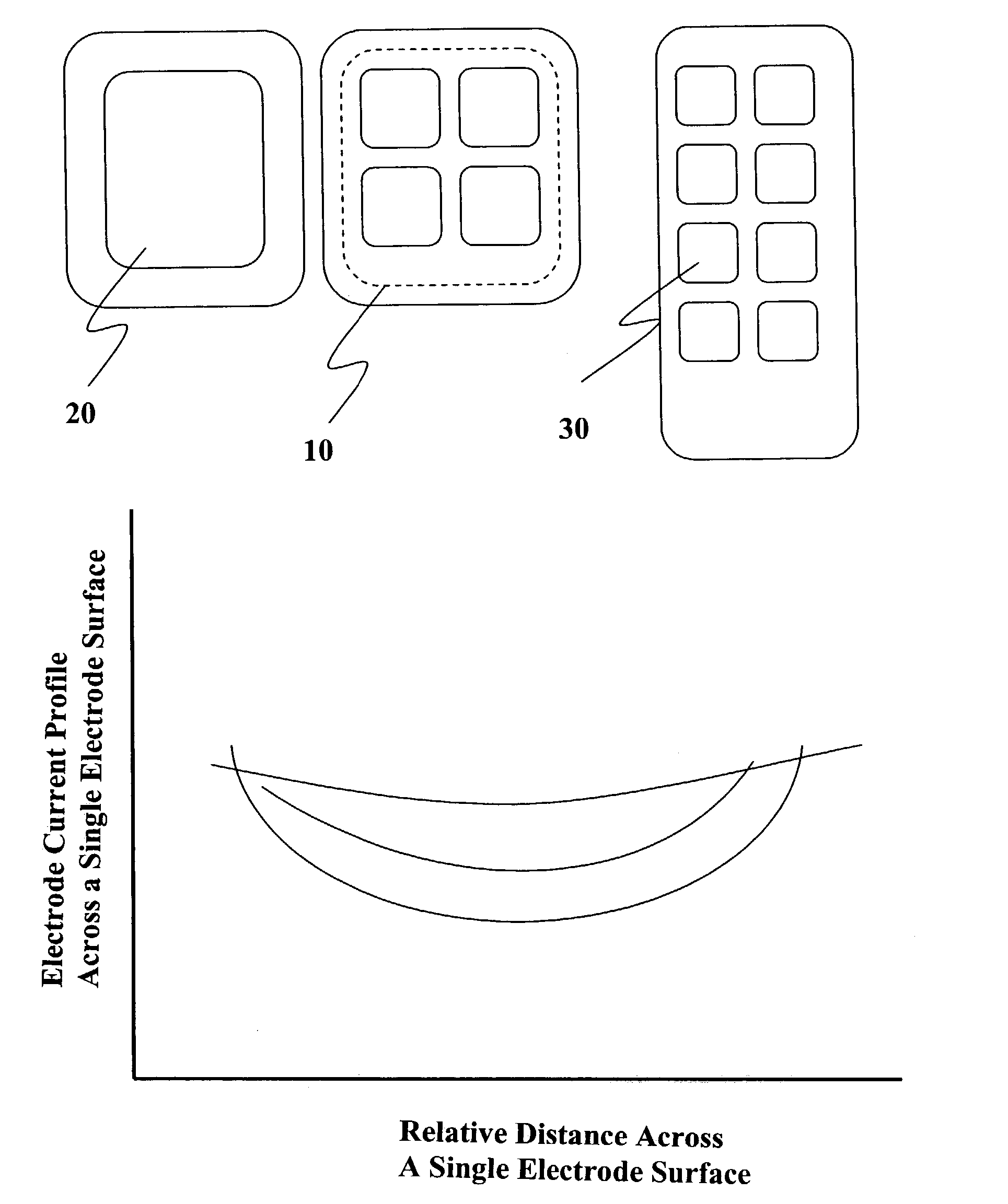
Methods and devices for expanding a spinal canal
ActiveUS20100161056A1Prevent over-insertionExpanding the spinal canalInternal osteosythesisJoint implantsMinimally invasive proceduresImplanted device
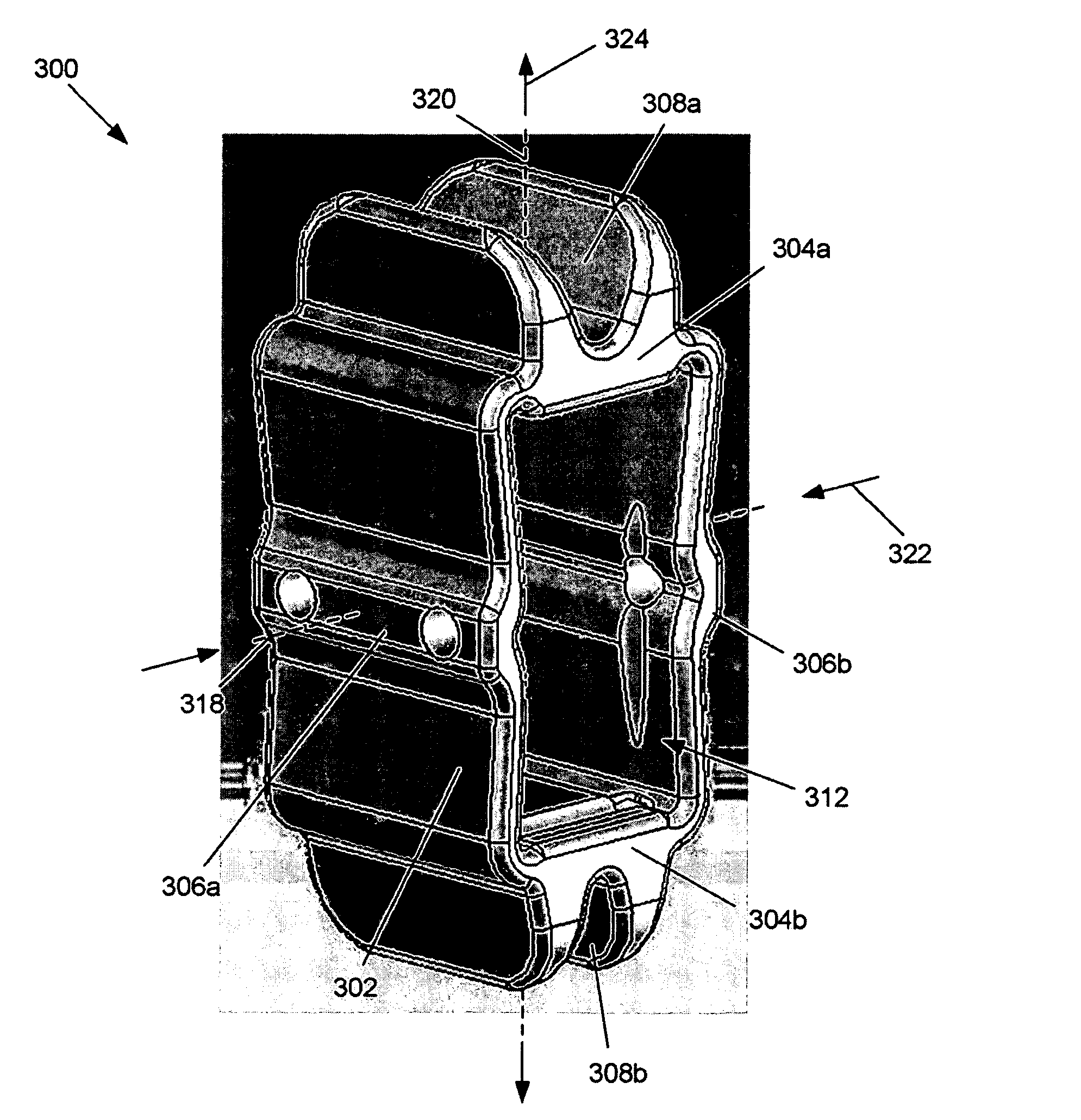
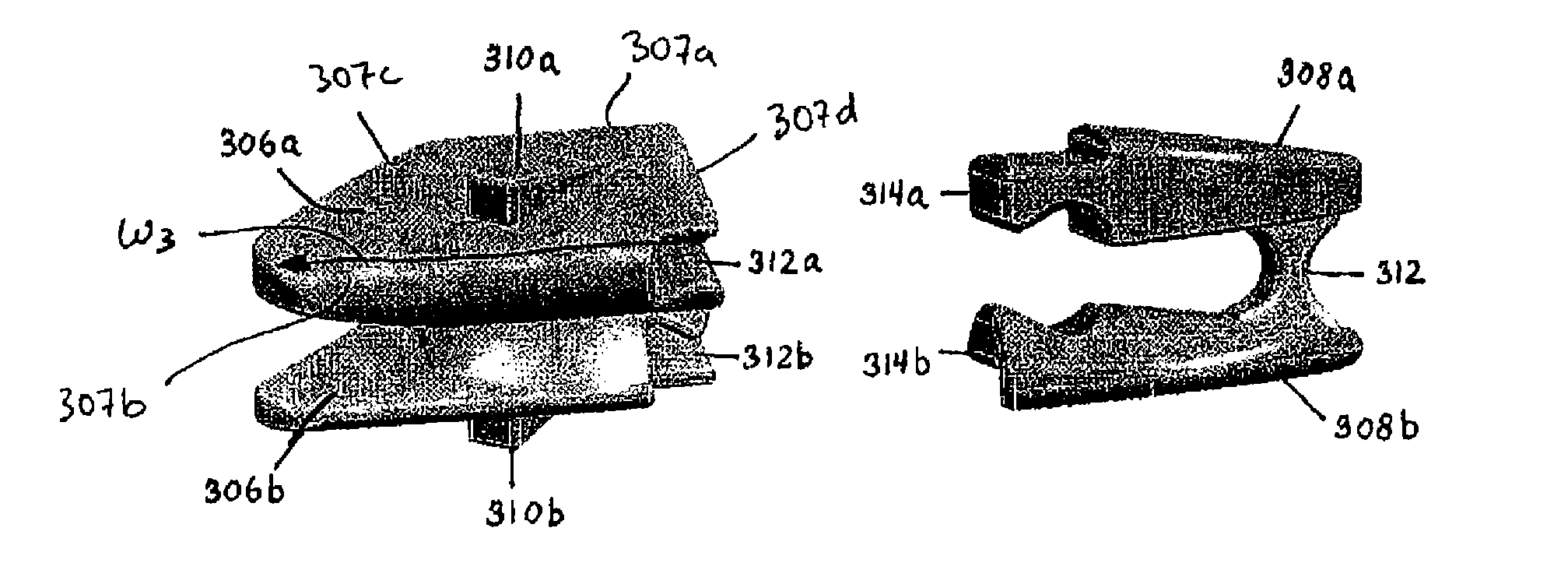
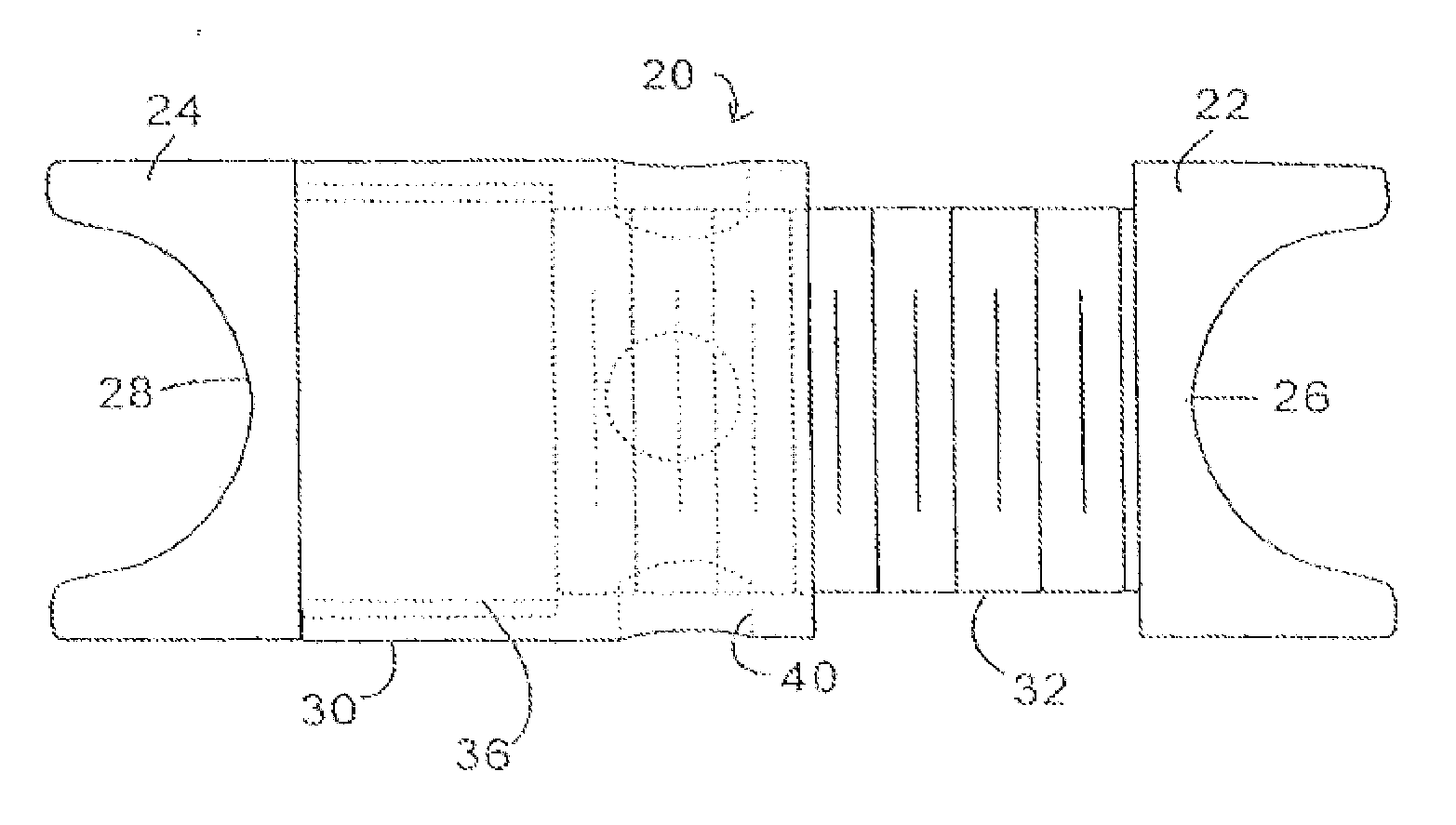
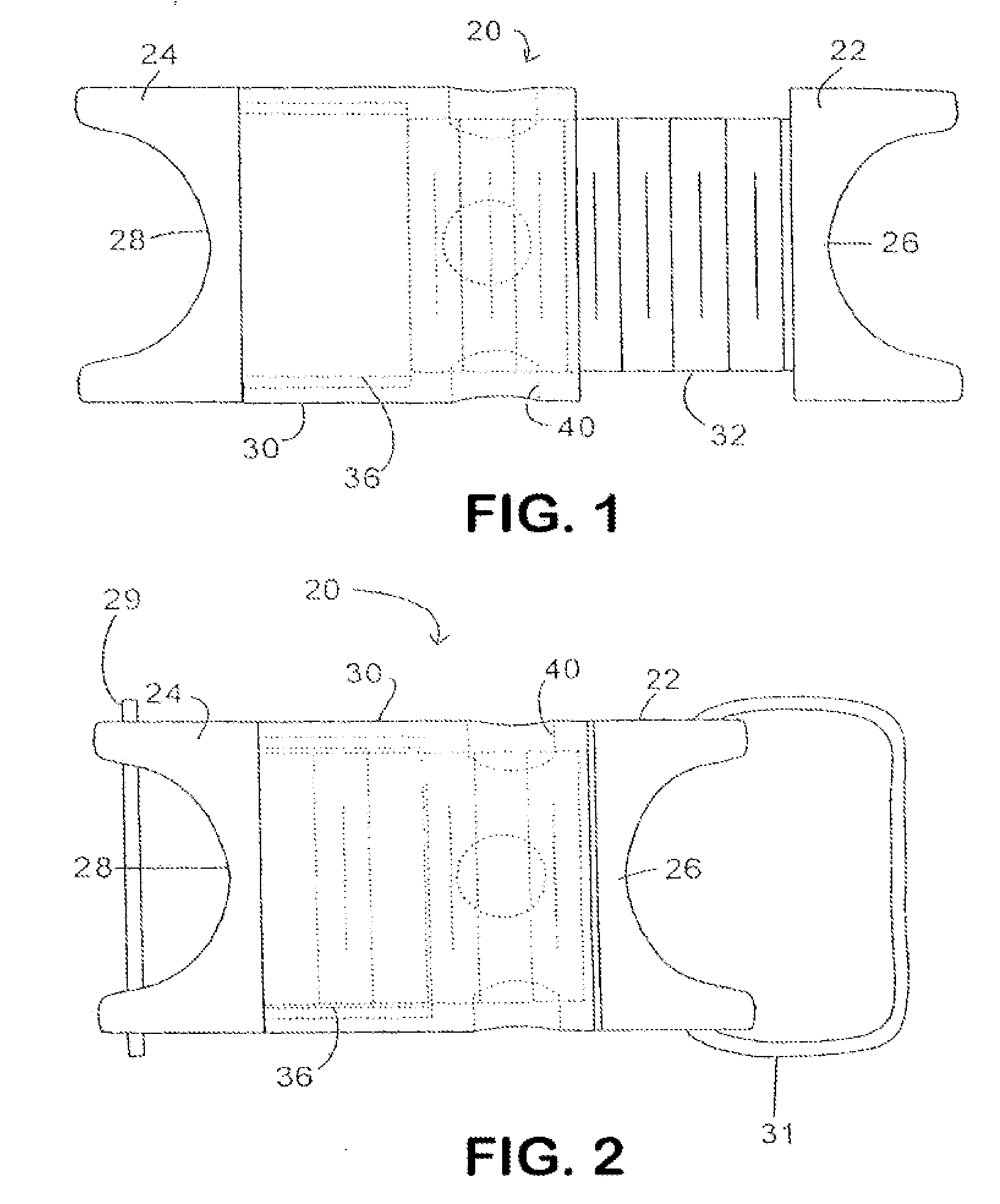
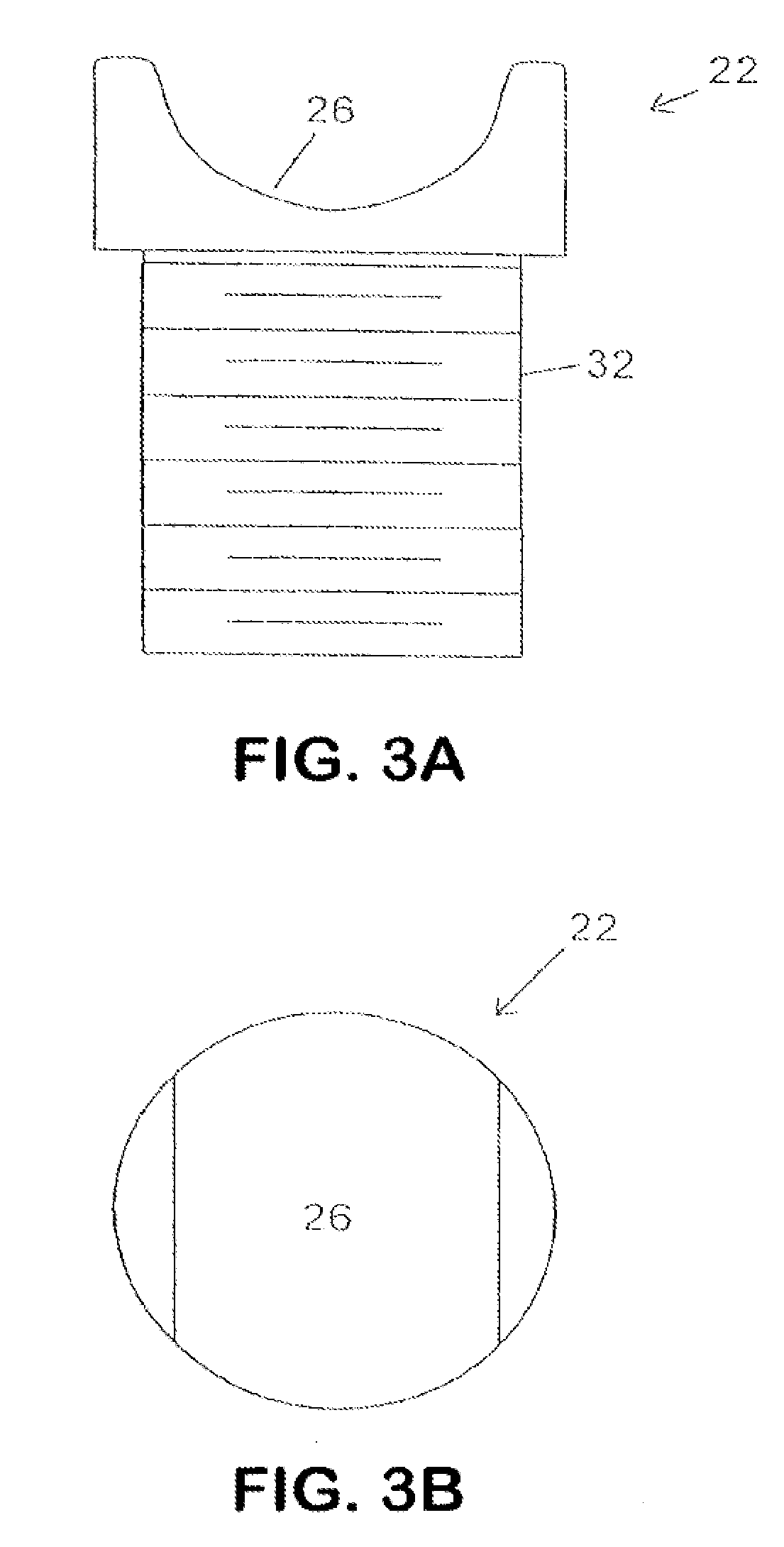
Devices and methods are disclosed for expanding a spinal canal. An implantable device having a shaft with a first cross-sectional dimension distinct from a second cross-sectional dimension can be inserted into an opening in a lamina and rotated 90 degrees to hinge the lamina away from the spinal canal. The implant can have one or more radiused edges, a bulleted tip, one or more lateral extensions for fastening the implantable device to bone, one or more hinged lateral extensions, one or more arcuate protrusions for biting into adjacent bone, an enlarged proximal head to prevent over-insertion, and / or a sleeve disposed therearound to reduce friction. Various embodiments of an insertion apparatus that can be selectively coupled to the implantable device are also disclosed, along with methods of expanding a spinal canal in minimally-invasive procedures using an implantable device and / or an insertion apparatus.
Owner:DEPUY SPINE INC (US)
Spinal Clips For Interspinous Decompression
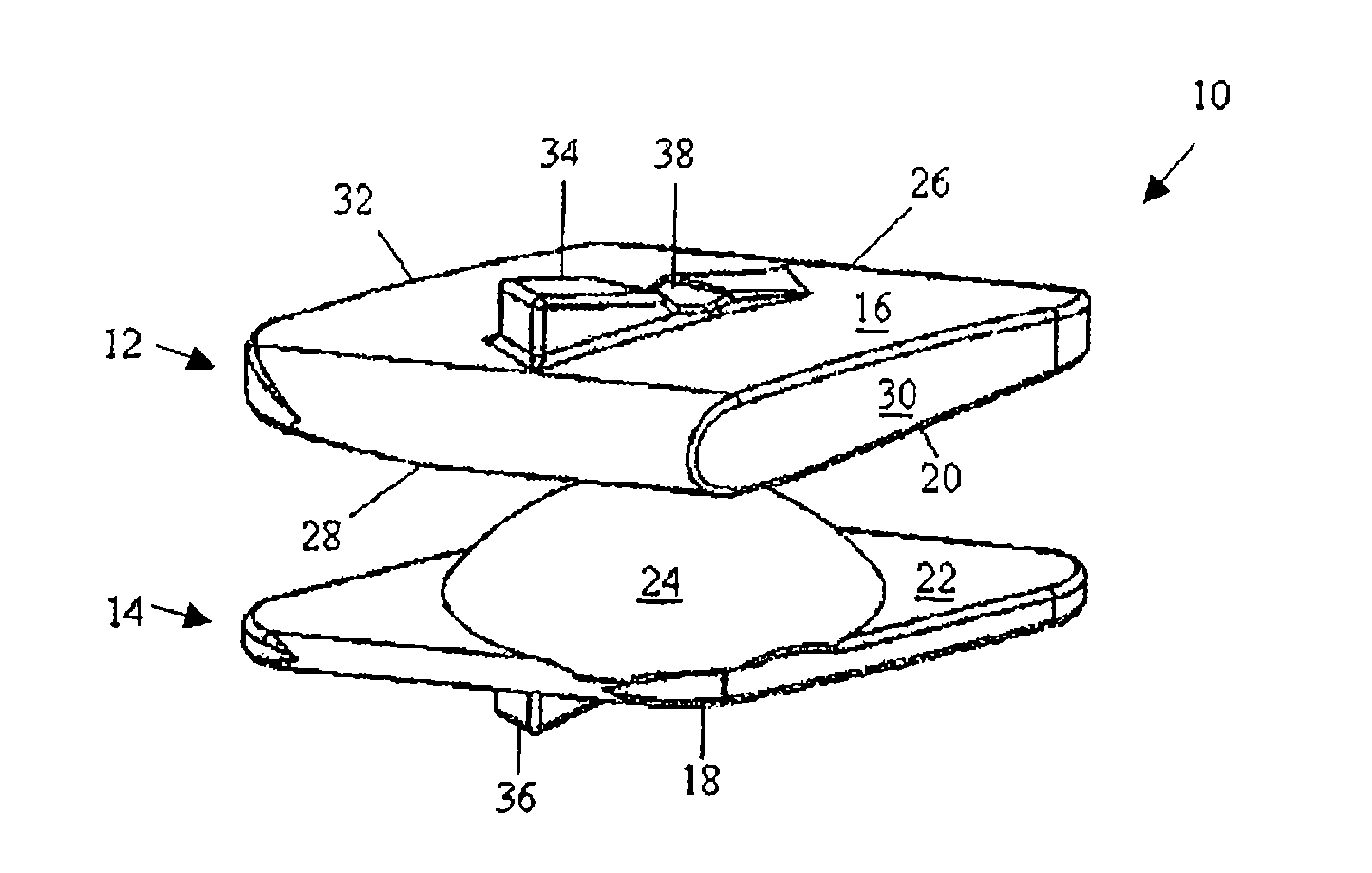
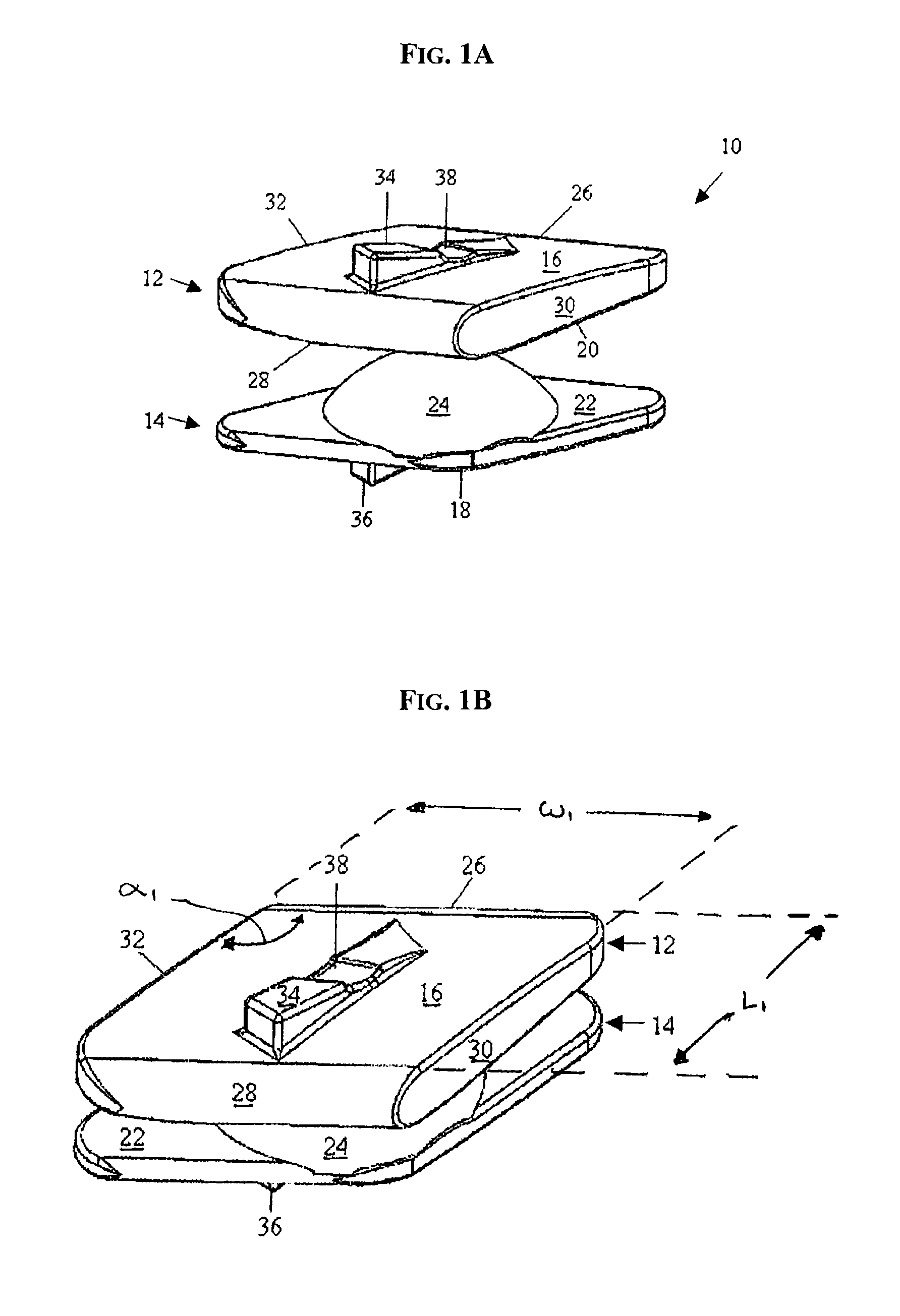
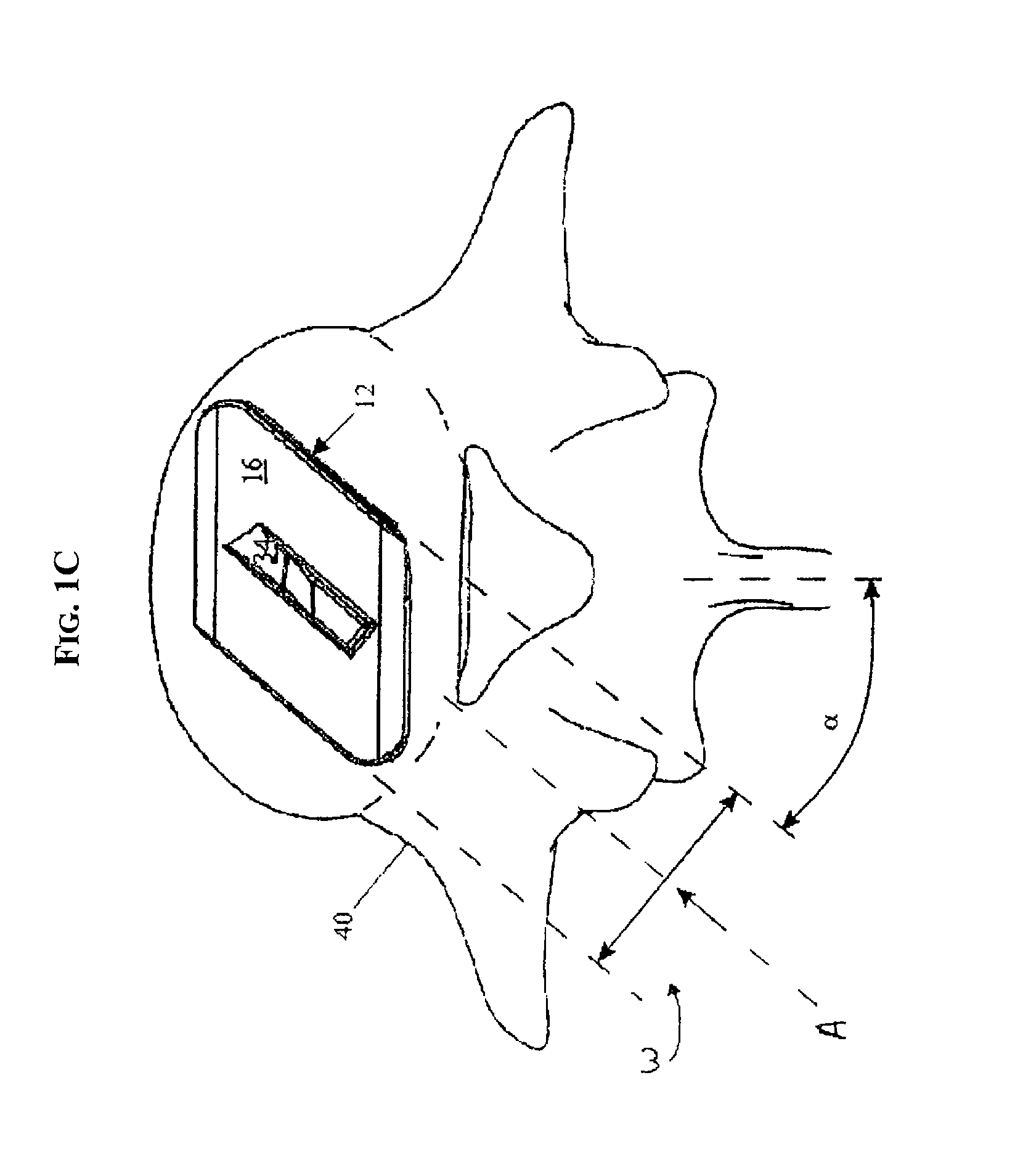
ActiveUS20110313458A1Stabilizing spineLarge amount of adjustmentInternal osteosythesisJoint implantsScrew systemPotential space
A spinal clip for creating a potential space within the spinal canal and thus stabilizing the spine without the need for additional spinal components is embodied in different forms. The spinal clips are configured to provide a clamping or holding force against and / or to the spinous processes, transverse processes and / or the lamina of adjacent vertebrae. In one form, the spinous process clips utilize pivoting to effect clamping or holding. In another form, the spinous process clips utilize rotation to effect clamping or holding. Such rotation may be between clamping or holding members or via a screw system. In yet another form, the spinous process clips utilize ratcheting to effect clamping or holding. In a still further form, the spinous process clips utilize expansion to effect clamping or holding. Depending on the form of clamping or holding, the spinous process clips can provide infinite adjustment of the clamping or holding force within an adjustment range, or provide discrete steps or levels of the clamping or holding force.
Owner:LIFE SPINE INC
Spine distraction implant
InactiveUS20100262243A1Increase volumeReduce restrictionsInternal osteosythesisJoint implantsDistractionDilator
Owner:MEDTRONIC EURO SARL
Method of delivering drug to brain via spinal cord
ActiveUS7662140B2Facilitate entryExtension of timeMedical devicesPharmaceutical delivery mechanismDrugs solutionVolumetric Mass Density
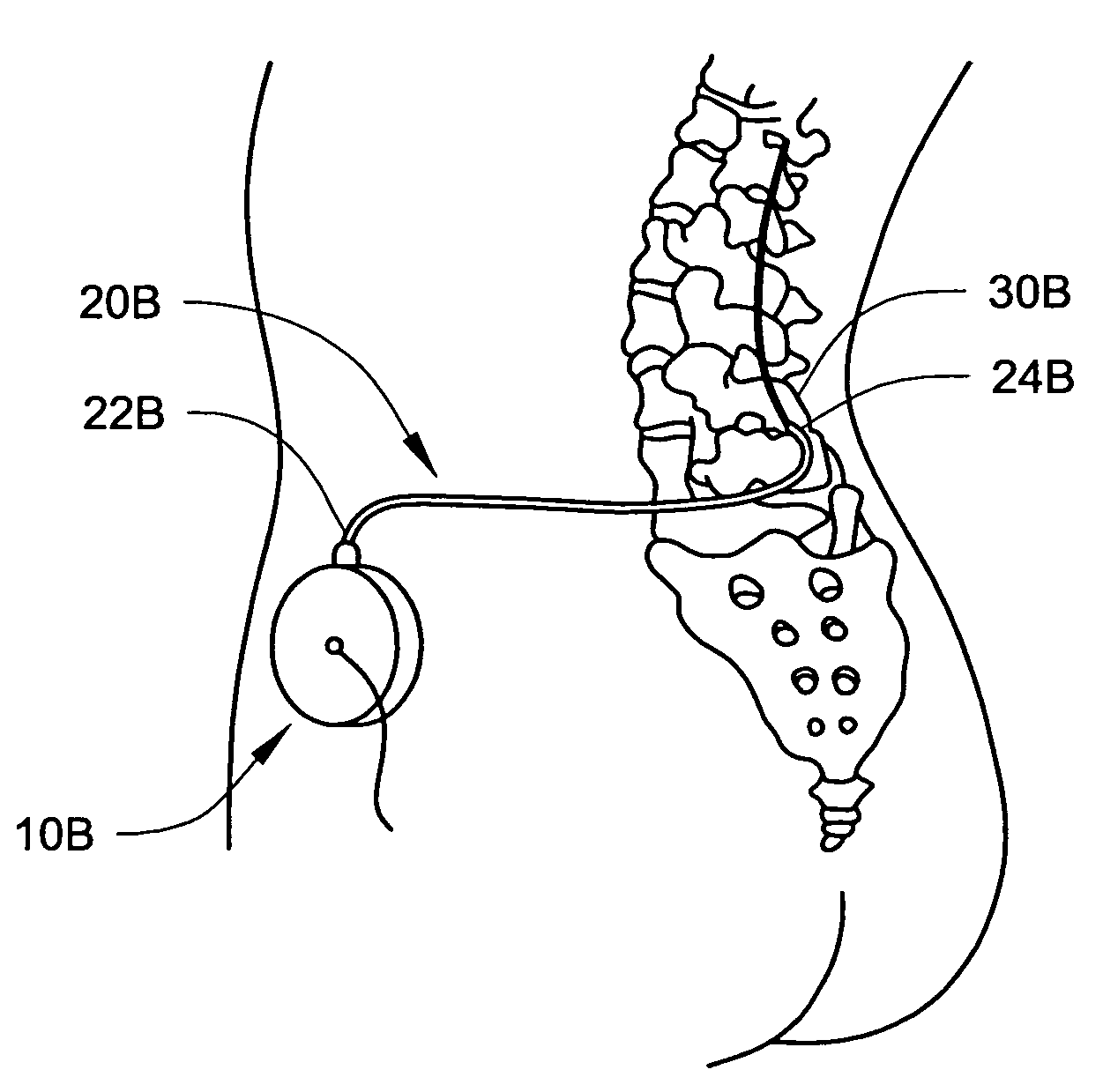
In various aspects, systems and methods involve catheters having infusion sections with permeable membranes that develop significant back pressure to enhance uniform delivery of the drug over an infusion section; catheters that have two or more infusion sections spaced apart along the length of the same catheter, catheters that include two or more infusion sections serviced by independent lumens (such that, e.g., different drug solutions can be delivered to the different infusion sections); implantable drug delivery systems with pumps and multiple reservoirs from which drugs can be delivered; systems that are capable of delivering drug solutions with selected densities; etc. Methods for delivering a drug to a brain through a spinal canal include delivering hypobaric solutions containing the drug.
Owner:MEDTRONIC INC
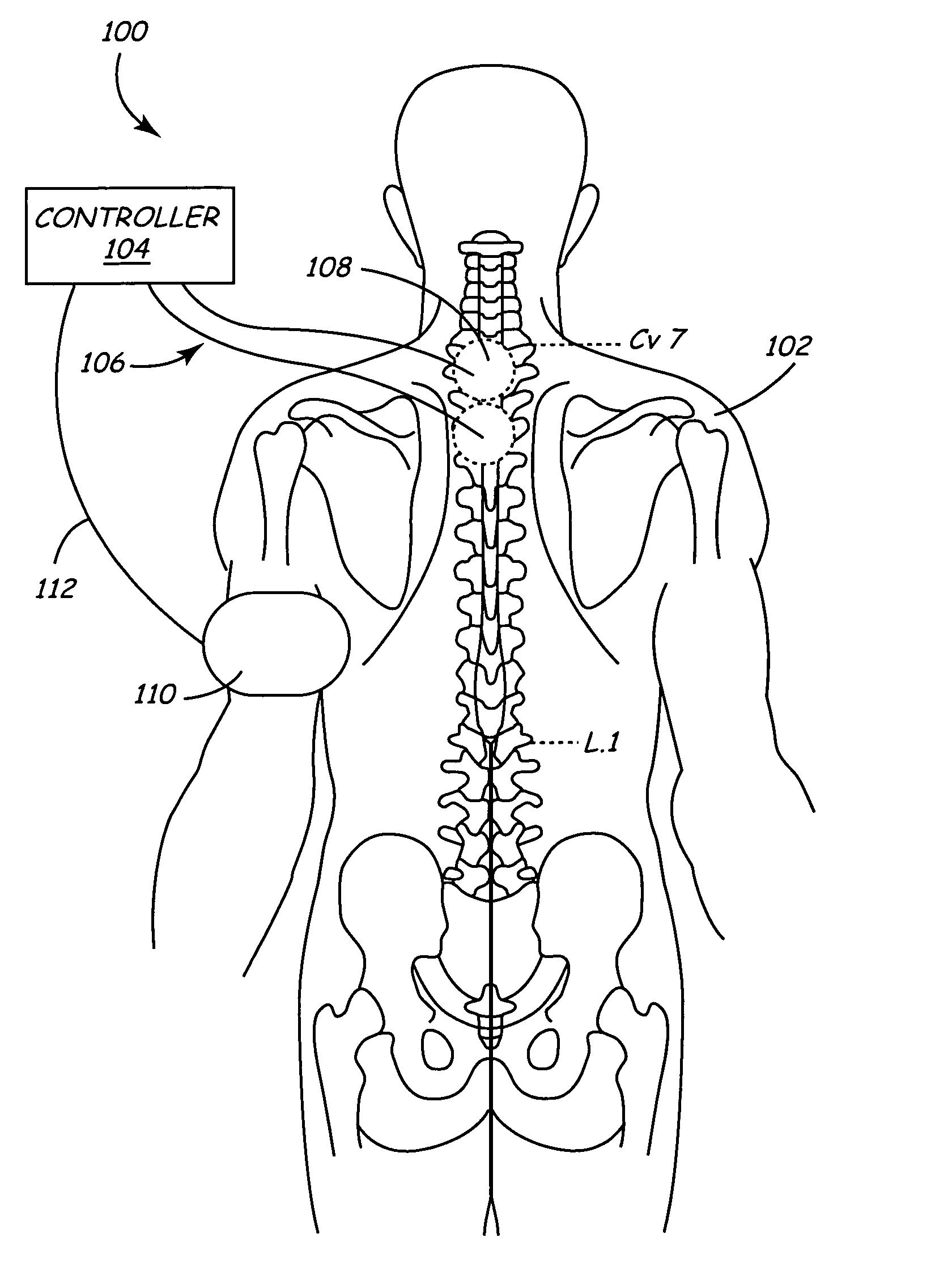
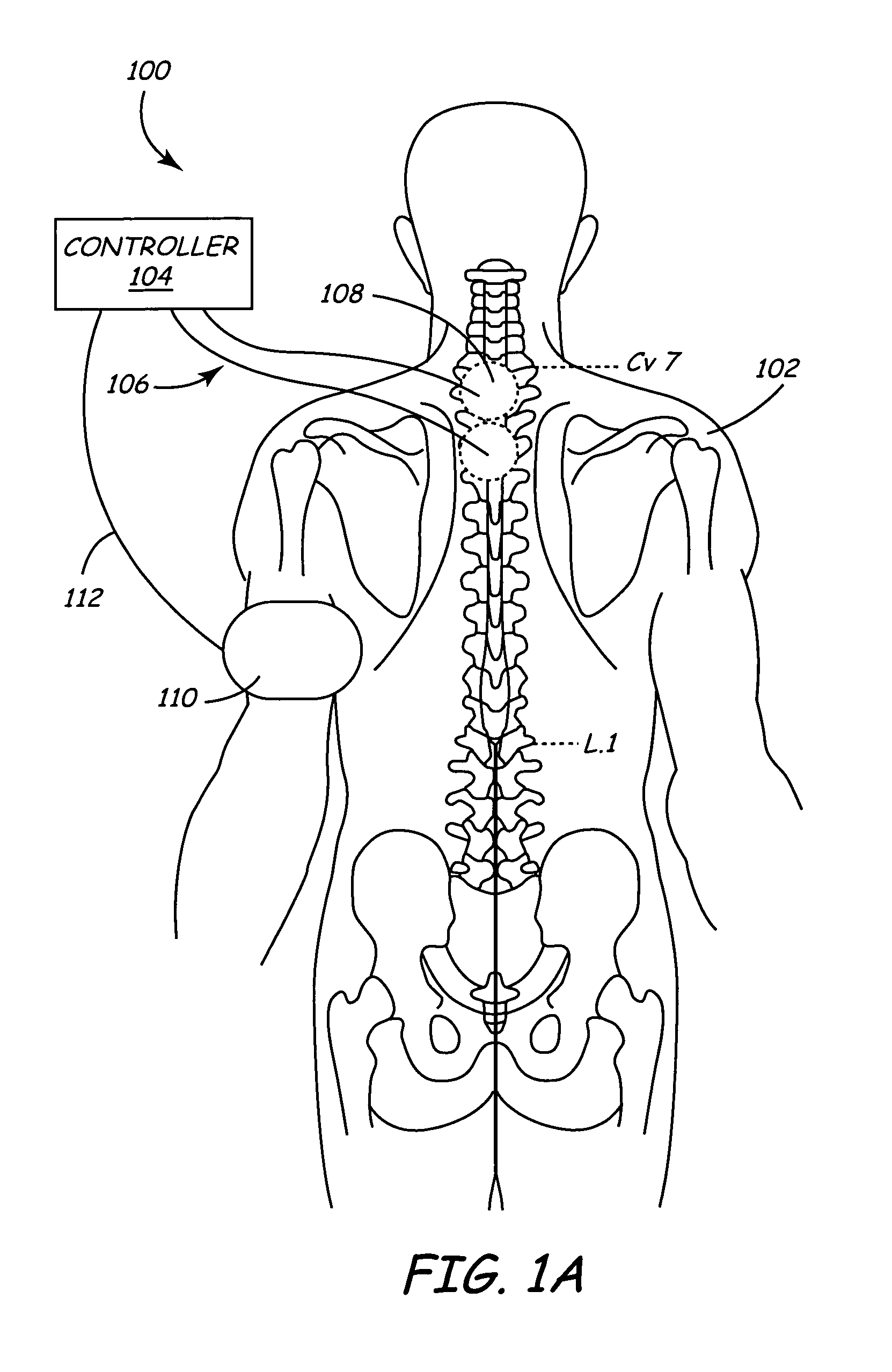
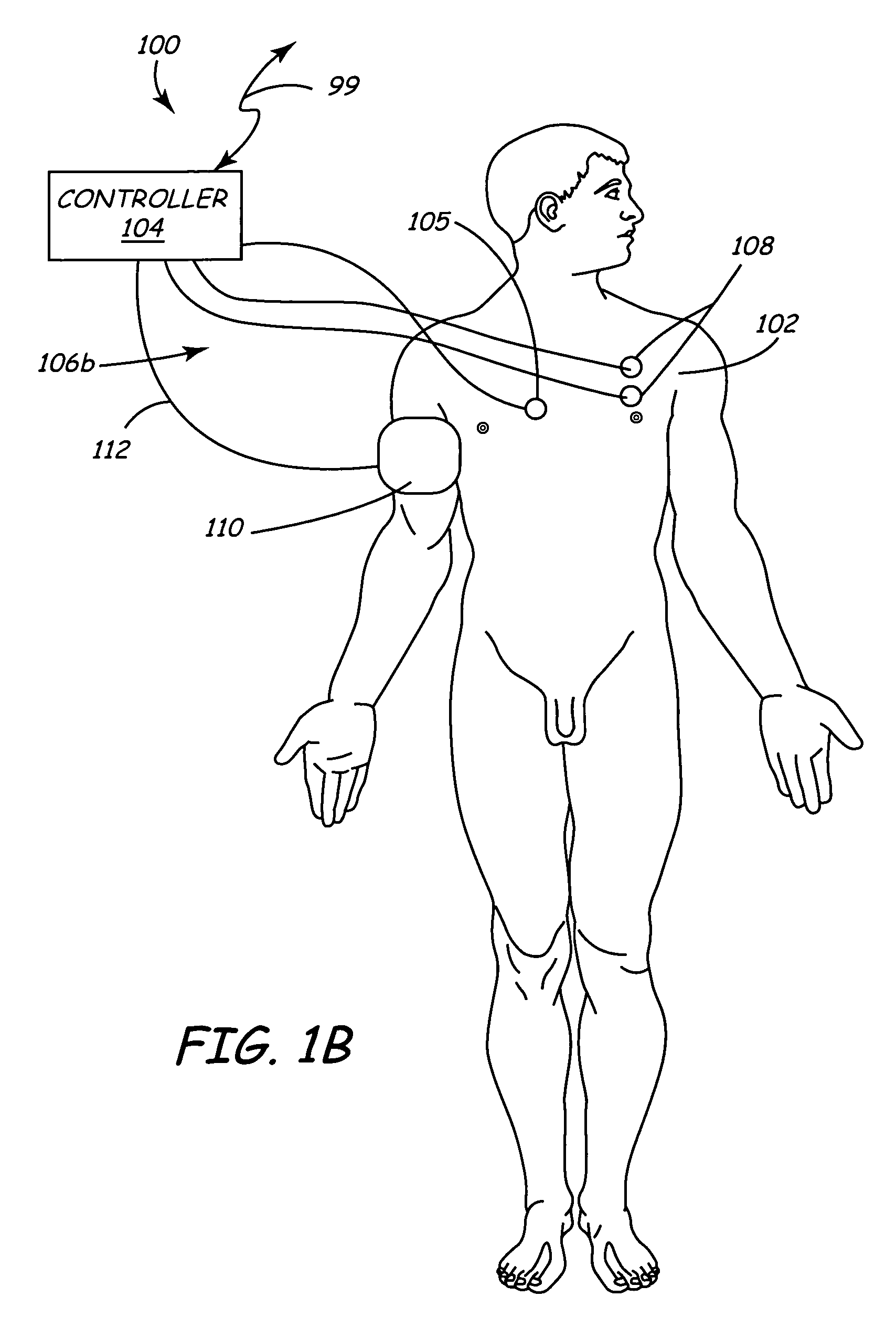
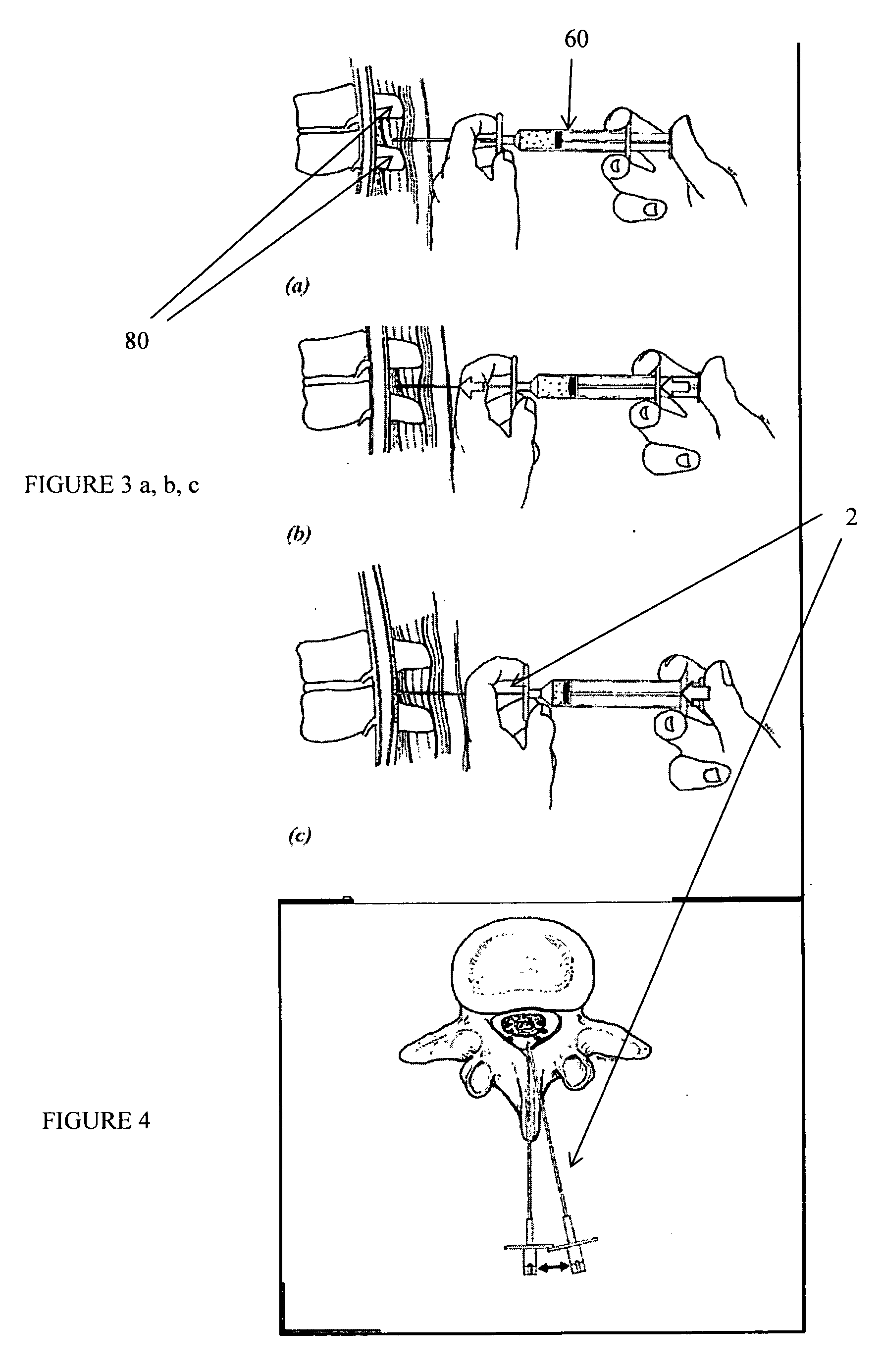
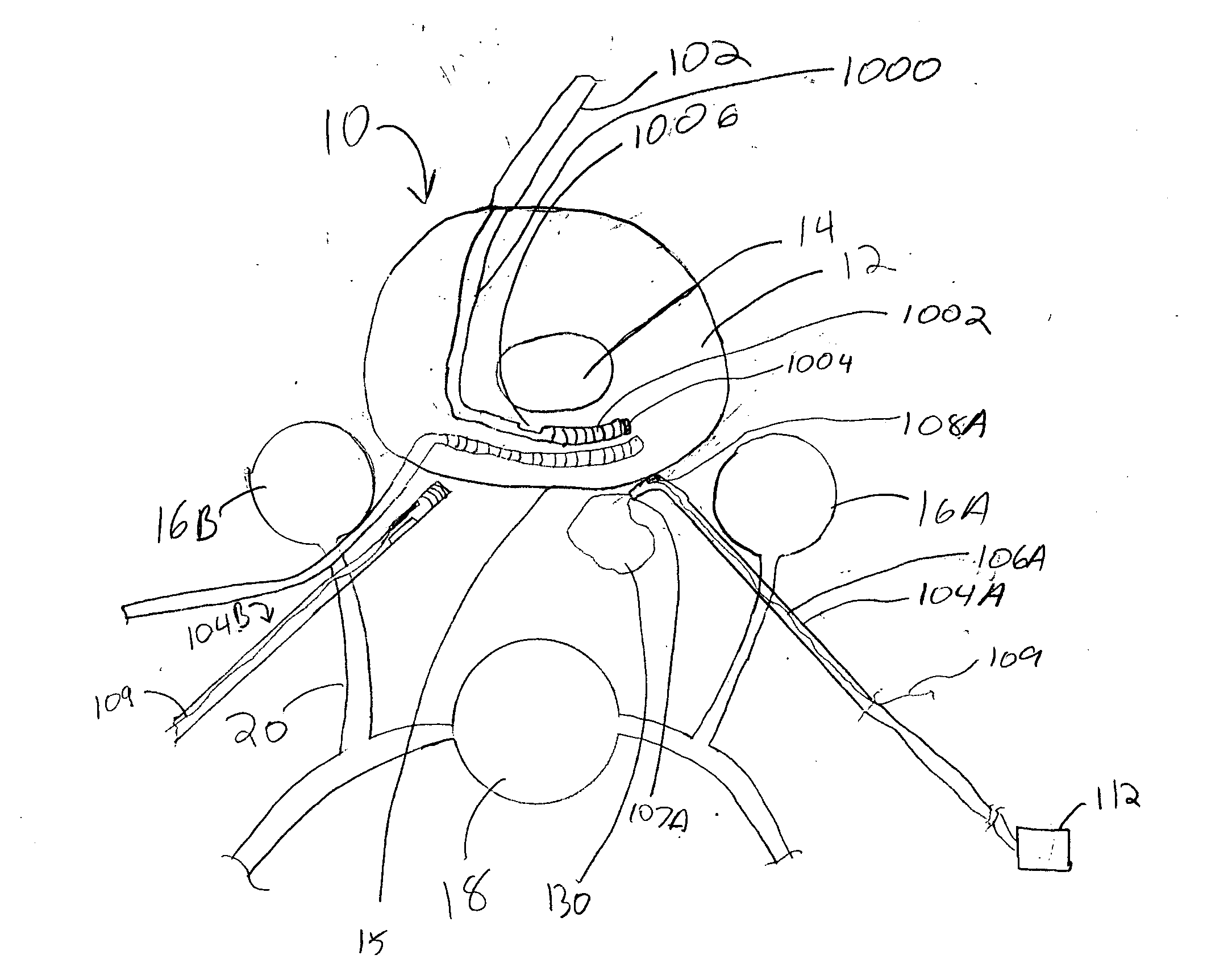
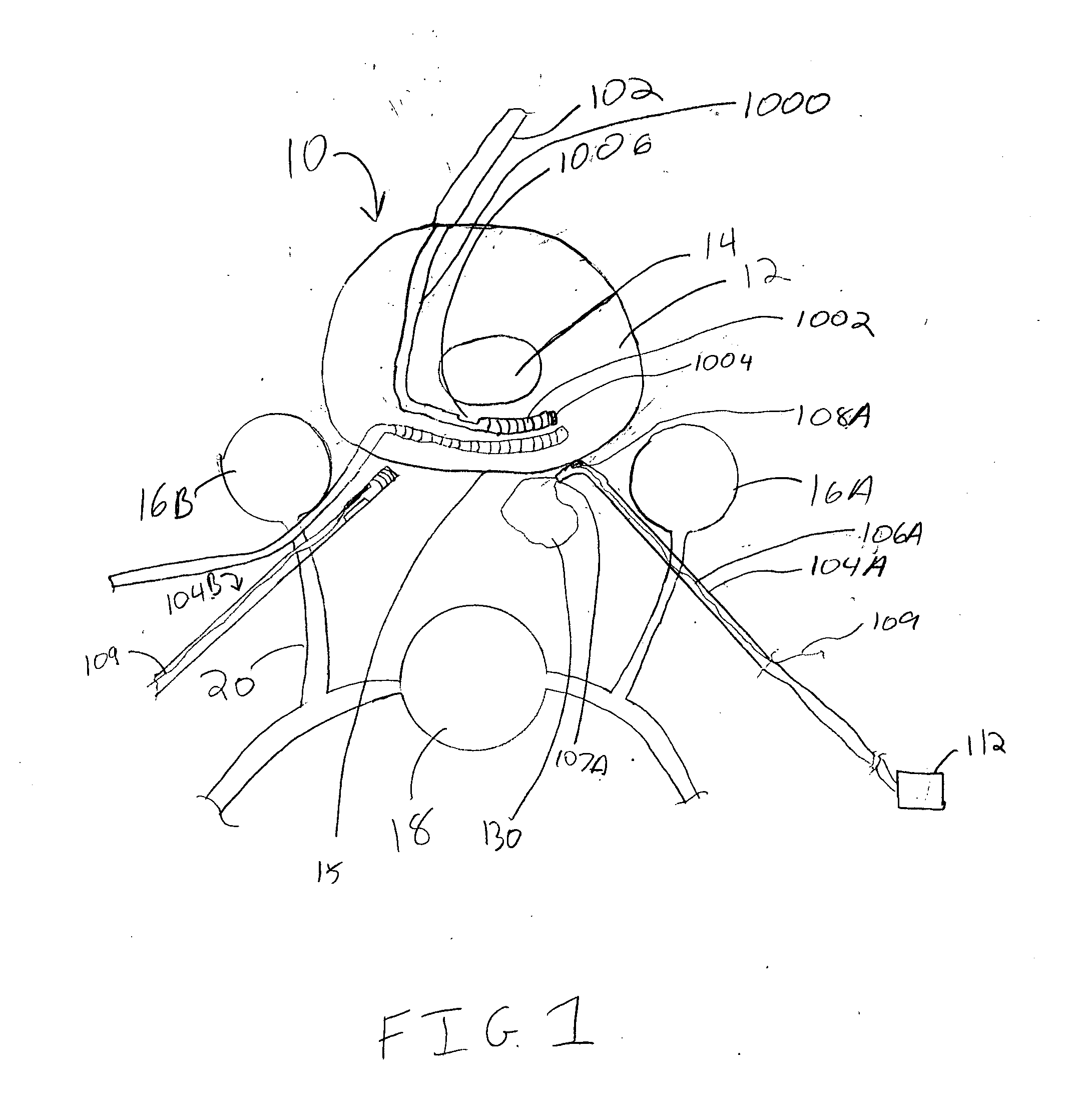
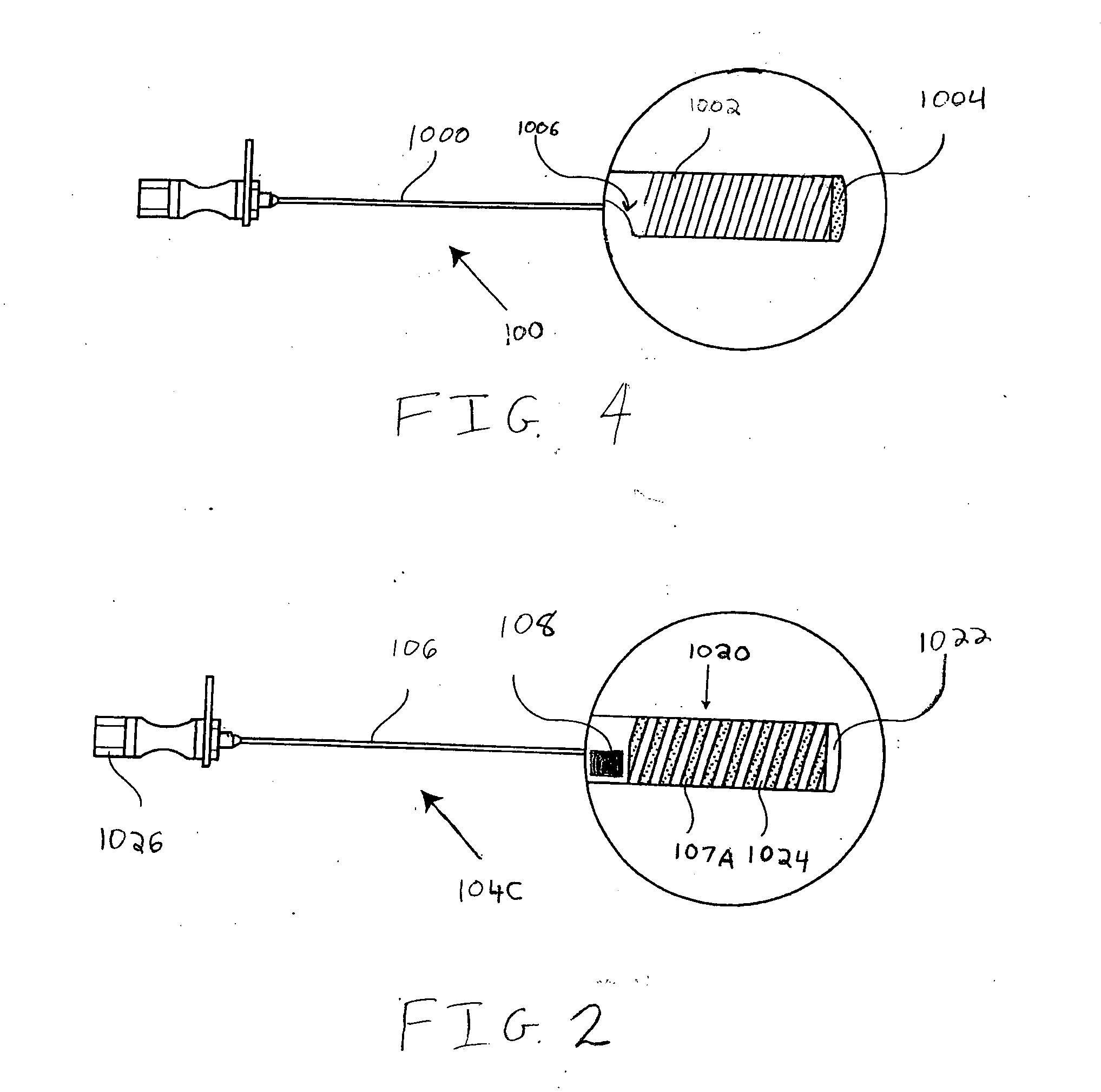
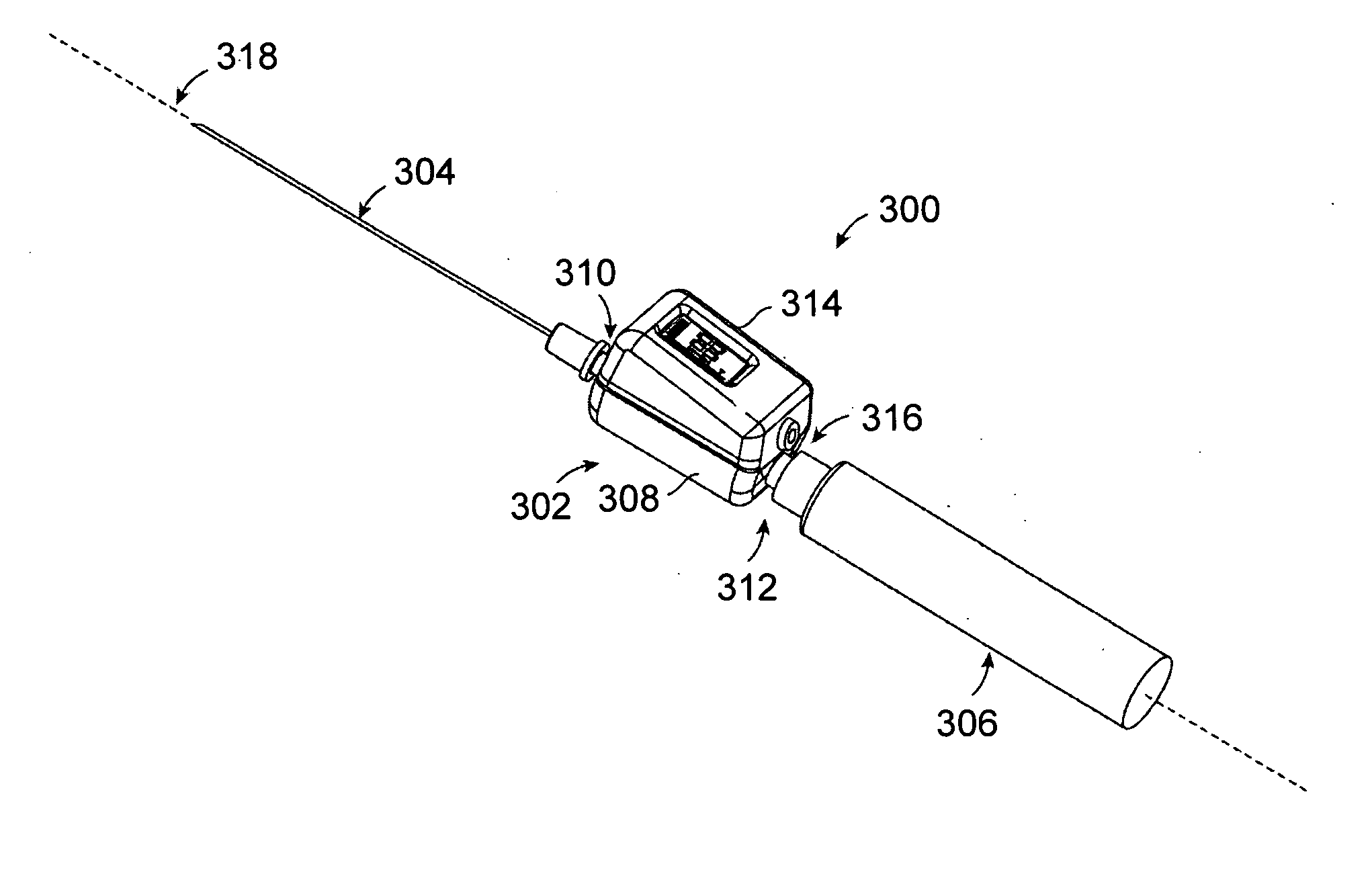
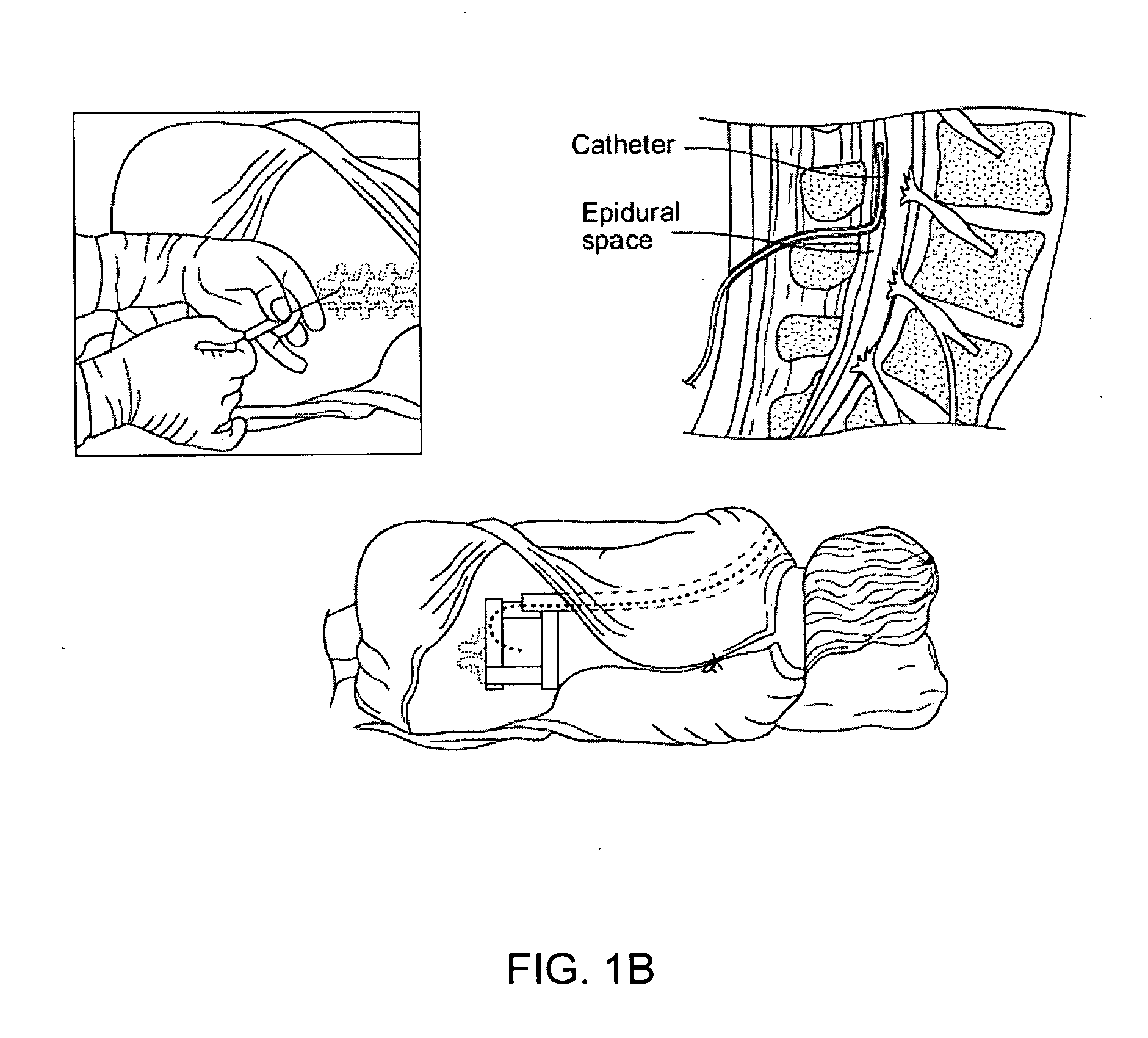
Spinal canal access and probe positioning, devices and methods
Methods and devices for detecting positioning of a probe in a tissue of a patient. A method can include providing a detection device; advancing a device coupled probe through the tissue of the patient and toward the patient's spinal canal; detecting a change in pressure about the distal portion of the coupled probe during advancing, where the detected pressure change indicates probe positioning in the patient's spinal canal; outputting the detected pressure change or indication of probe positioning to a visual display.
Owner:JAMES M PIGOTT
Methods and devices for expanding a spinal canal
ActiveUS8133280B2Prevent over-insertionExpanding the spinal canalInternal osteosythesisJoint implantsMinimally invasive proceduresMedicine
Devices and methods are disclosed for expanding a spinal canal. An implantable device having a shaft with a first cross-sectional dimension distinct from a second cross-sectional dimension can be inserted into an opening in a lamina and rotated 90 degrees to hinge the lamina away from the spinal canal. The implant can have one or more radiused edges, a bulleted tip, one or more lateral extensions for fastening the implantable device to bone, one or more hinged lateral extensions, one or more arcuate protrusions for biting into adjacent bone, an enlarged proximal head to prevent over-insertion, and / or a sleeve disposed therearound to reduce friction. Various embodiments of an insertion apparatus that can be selectively coupled to the implantable device are also disclosed, along with methods of expanding a spinal canal in minimally-invasive procedures using an implantable device and / or an insertion apparatus.
Owner:DEPUY SPINE INC (US)
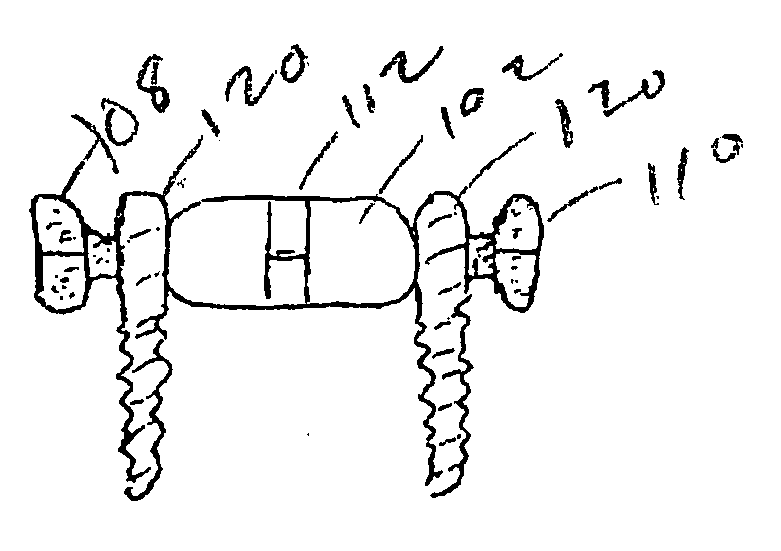
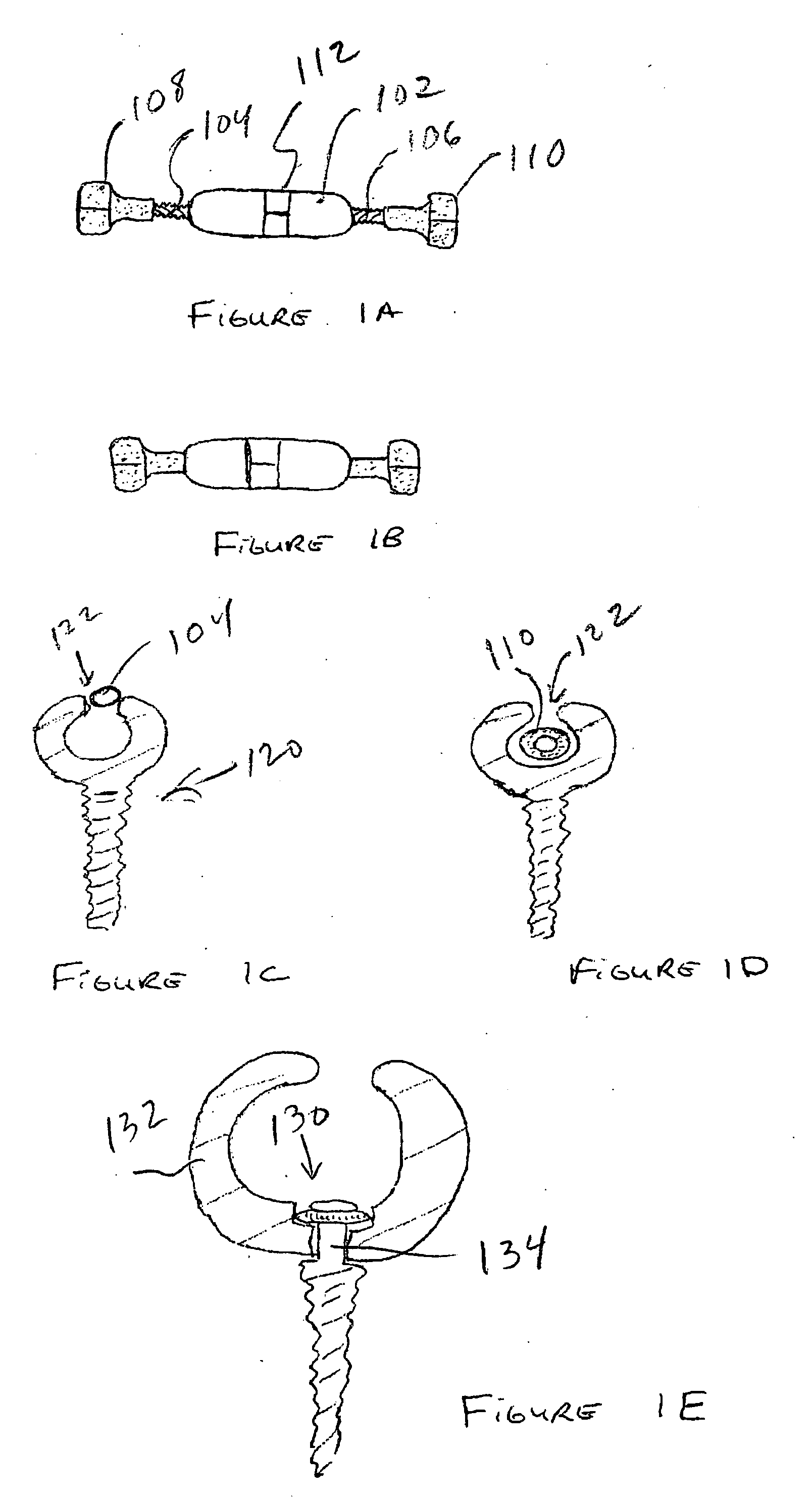
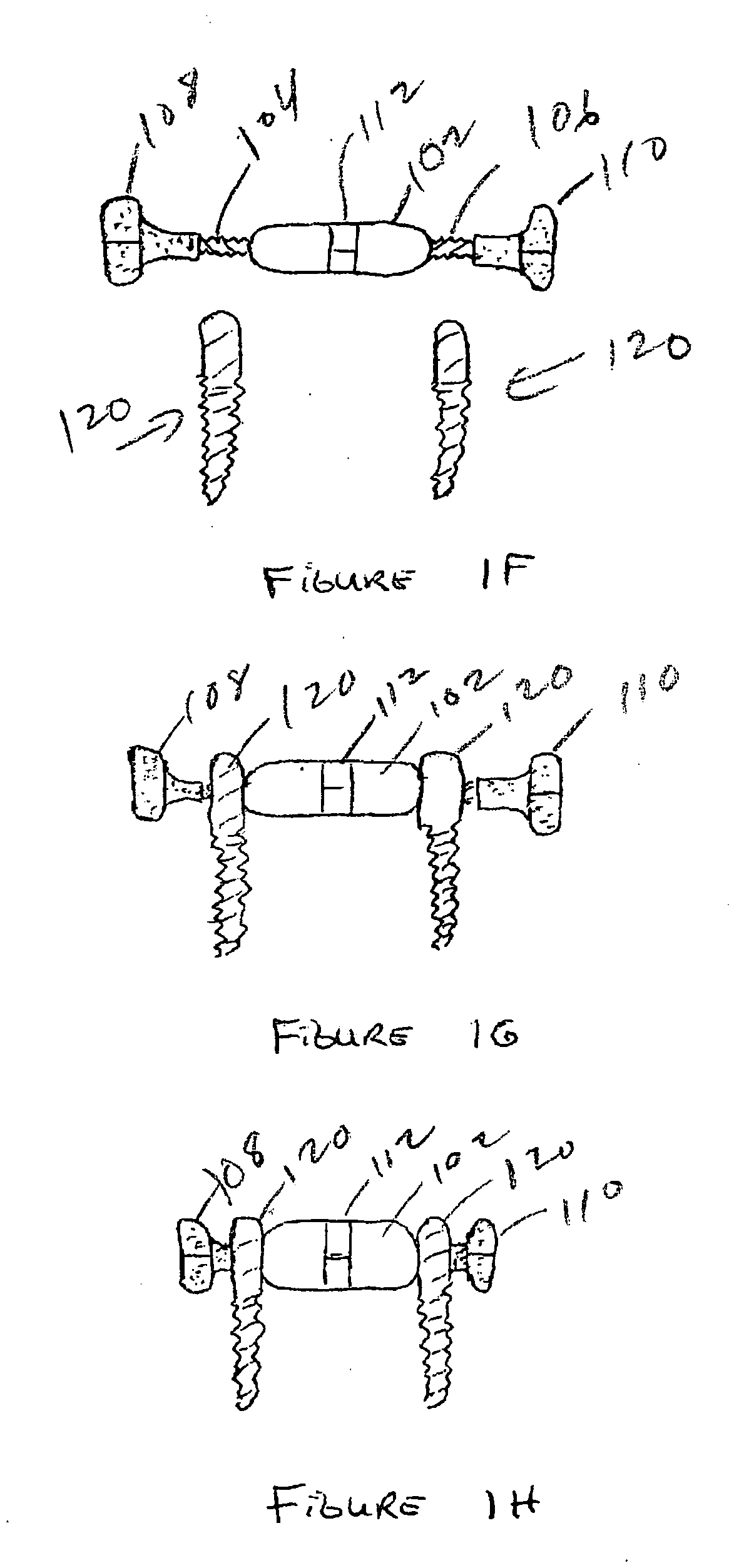
Device and Method for Expanding the Spinal Canal With Spinal Column Stabilization and Spinal Deformity Correction
ActiveUS20110230915A1LengtheningAchieve fixationInternal osteosythesisJoint implantsSpinal columnSpinal deformity correction
Spinal canal expansion through pedicle lengthening in combination with spinal column stabilization, to treat spinal stenosis, spondylolisthesis, kyphosis, scoliosis or a major spinal rotational deformity, with or without a spinal fusion. A device or implant that includes a pedicle lengthening implant to decompress (expand) the spinal canal, and a bridge to connect two or more pedicle lengthening implants and / or pedicle screws or bone anchors to achieve simultaneous spinal stabilization across one or more vertebral segments. The bridge across the vertebral segments can include a longitudinal member, such as a plate or rod. The pedicle lengthening implant is originally made, or can be modified to, connect to the longitudinal member. Spinal deformity can be manipulated, through operation of one or more pedicle lengthening implants in respective vertebral segments, to improve a relative relationship between the respective vertebra segments, the longitudinal member then fixating the vertebral segments into a multi-level spinal construct.
Owner:INNOVATIVE SURGICAL DESIGNS
Managing back pain by applying a high frequency electrical stimulus directly to the spinal cord
ActiveUS20140379043A1Relieve symptomsRisk minimizationSpinal electrodesArtificial respirationImpaired proprioceptionSide effect
This invention provides a new technology for management of back pain by stimulating the spinal cord in a manner that renders it refractory to transmission of deleterious or undesirable sensory input. The electrical stimulus comprises high frequency pulses in a regular or complex pattern or that are stochastically produced under microprocessor control. The stimulus is applied directly to the surface of the spinal cord from within the spinal canal, which provides important benefits over previous technology. The stimulus alleviates symptoms and signs of back pain, while minimizing the risk of side effects such as paresthesia, and potentially minimizing the effects on motor neuron transmission and proprioception.
Owner:UNIV OF IOWA RES FOUND
Apparatus and method for repair of spinal cord injury
InactiveUS6975907B2Lightweight productionDissipate any toxic productSpinal electrodesExternal electrodesPower flowMedicine
An apparatus for stimulating regeneration and repair of damaged spinal nerves, comprising at least two electrodes placed intravertebrally near the site of spinal axon injury and delivering DC current thereto. A method for stimulating regeneration and repair of damaged spinal nervous tissue, comprising placing electrodes intravertebrally near the site of spinal cord injury and applying DC current at a level sufficient to induce regeneration and repair of damaged spinal axons but less than the current level at which tissue toxicity occurs.
Owner:DYNAMED SYST
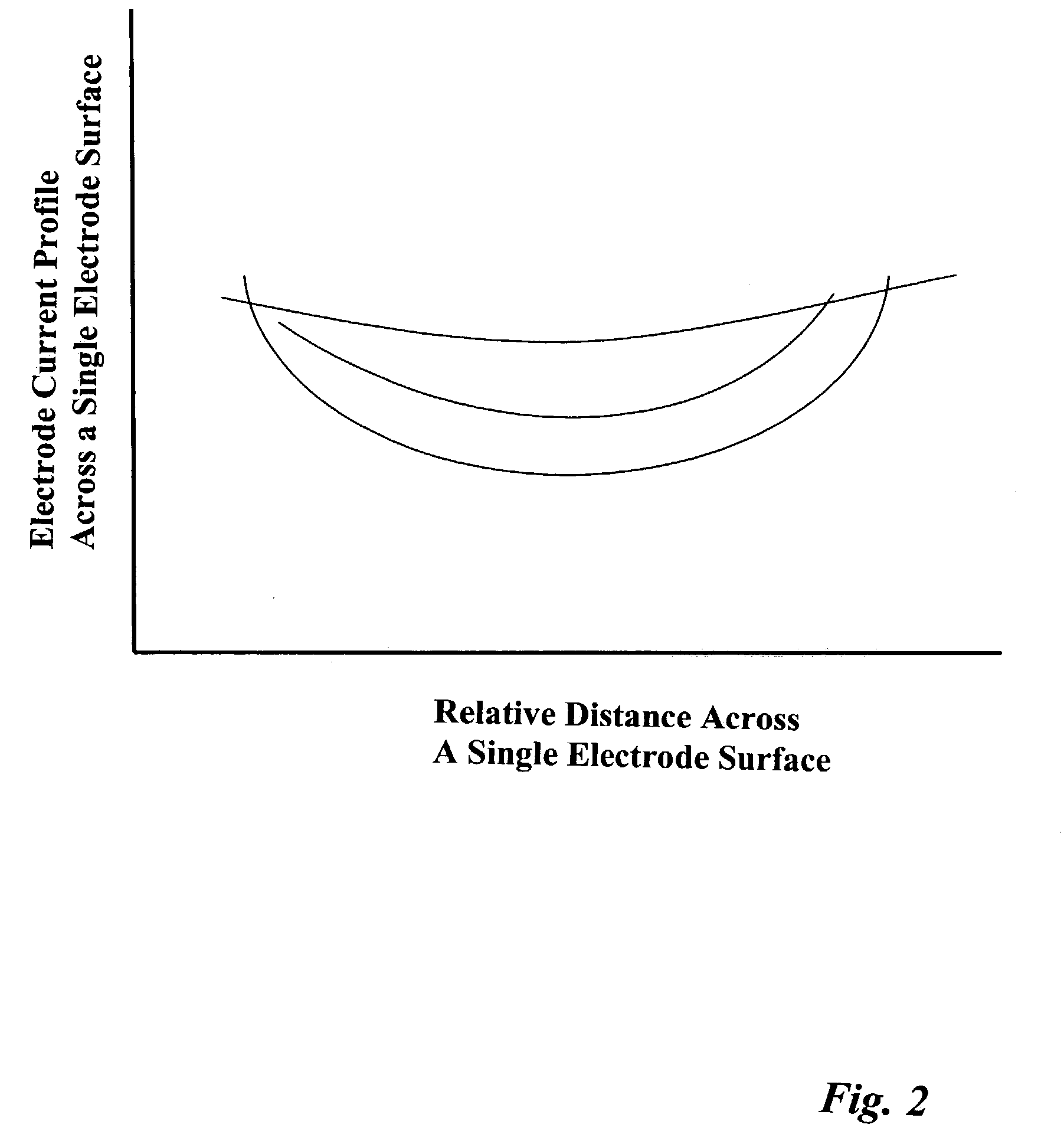
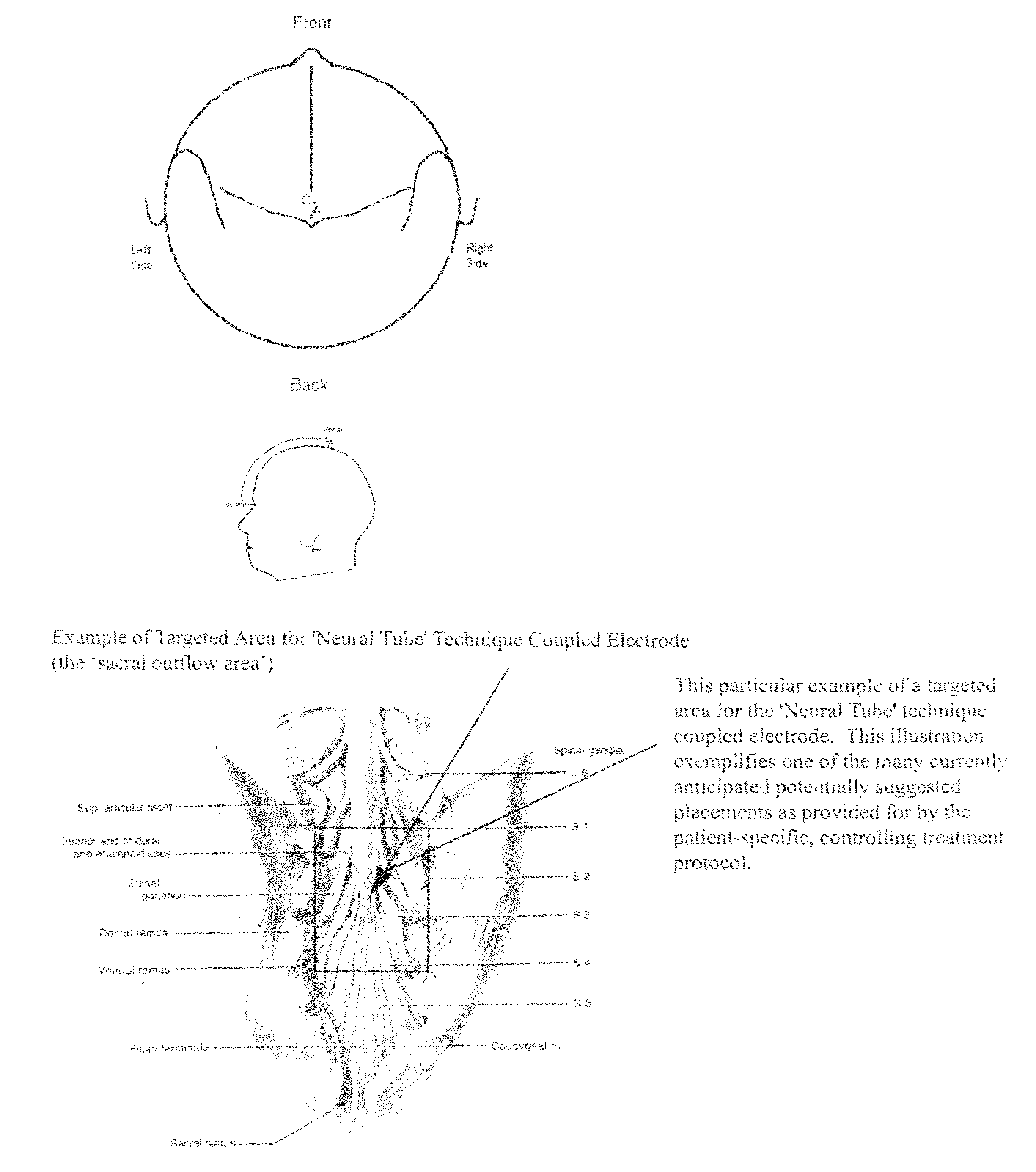
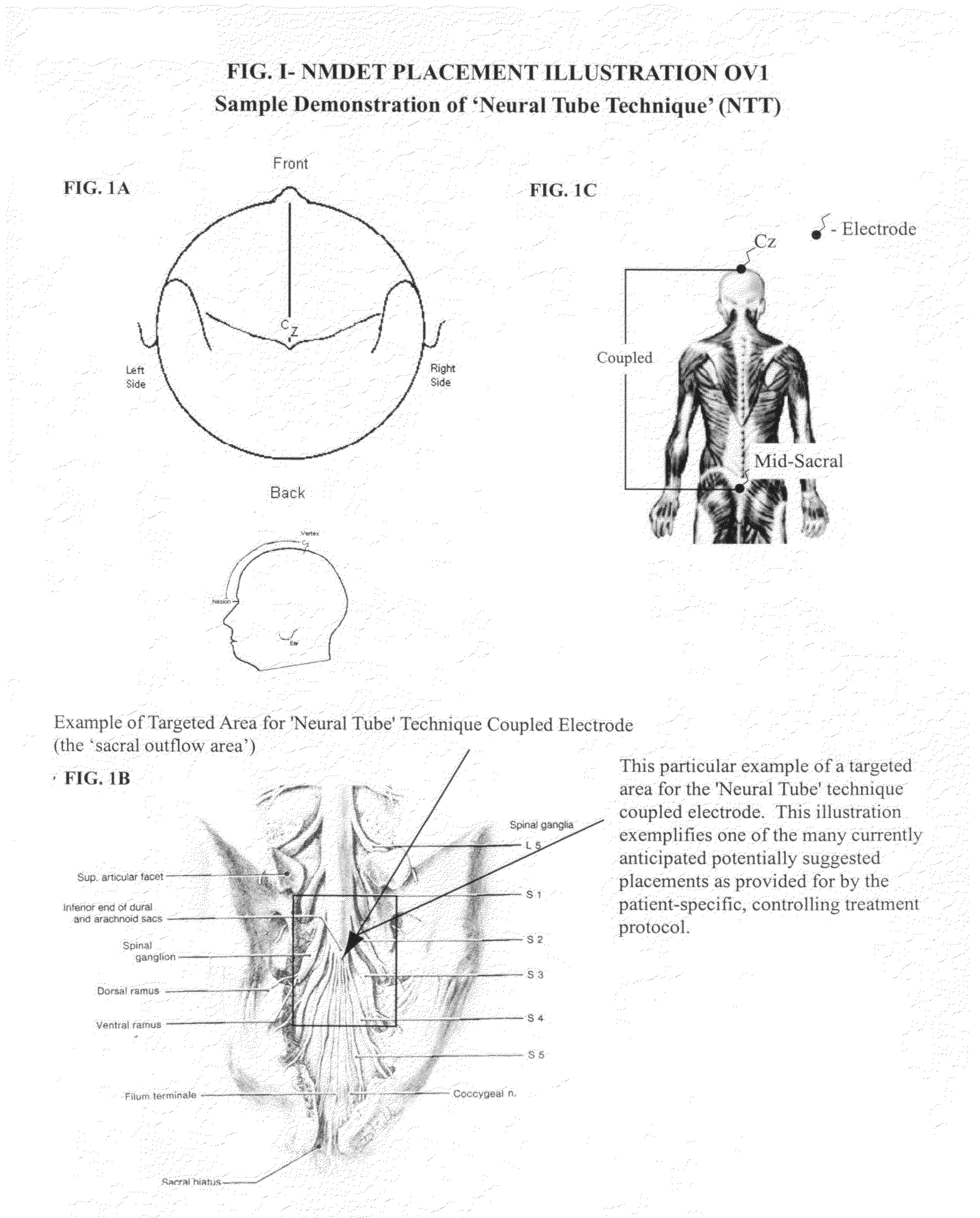
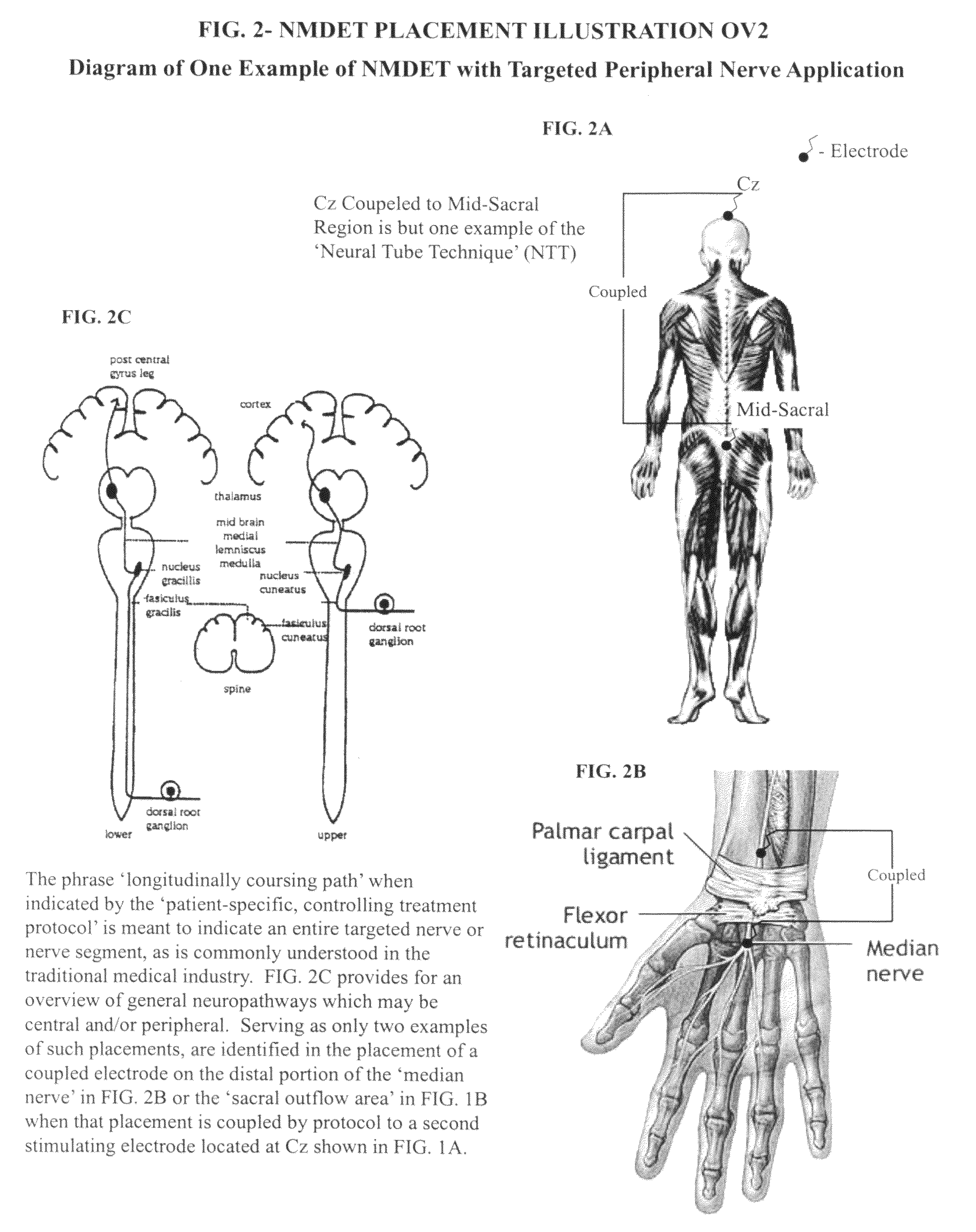
Coupled neuraxial mesoscopic desynchronization electrostimulation therapy (cNMDET) method
ActiveUS8428738B2Add supportIncrease capacityElectrotherapyElectrode placementTranscutaneous electrical nerve stimulation
This novel therapeutic (reparative, etiotropic not merely symptomatolytic) electrostimulatory method hereinafter referred to as Coupled Neuraxial Mesoscopic Desynchronization Electrostimulation Therapy (cNMDET) is comprised of a varied combination of progressively sequenced electrode placements and its clinically targeted areas which are comprised of the CNS [regions of the brain and spinal cord including, but not limited to, the underlying clinicopathologic mechanisms of the ANS] and clinically involved components of the PNS variably coupled in such a manner as to apply this therapeutic electrostimulation (reparative modulation) along the coursing path (longitudinally) of the targeted neural pathway over a segment and / or in its entirety, as well as, the utilization of patterned applications for involved joints, muscles, fascia, ligaments, tendons and areas involved in inflammatory processes of clinical significance accomplished through the application of, preferably, but not limited to, Coupled Neuraxial Transcutaneous Electrical Nerve Stimulation (cNTENS) and Coupled Neuraxial Transcranial Direct Stimulation (ctDCSn).
Owner:VALENCIA ANDREW D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com