Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
219results about How to "Lightweight production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
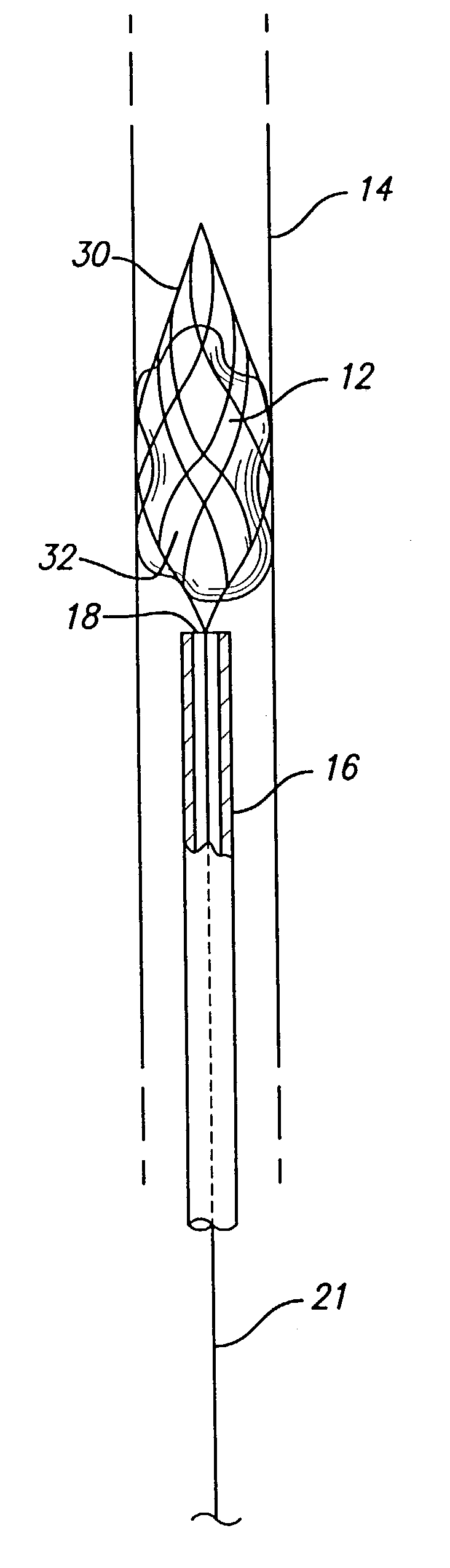
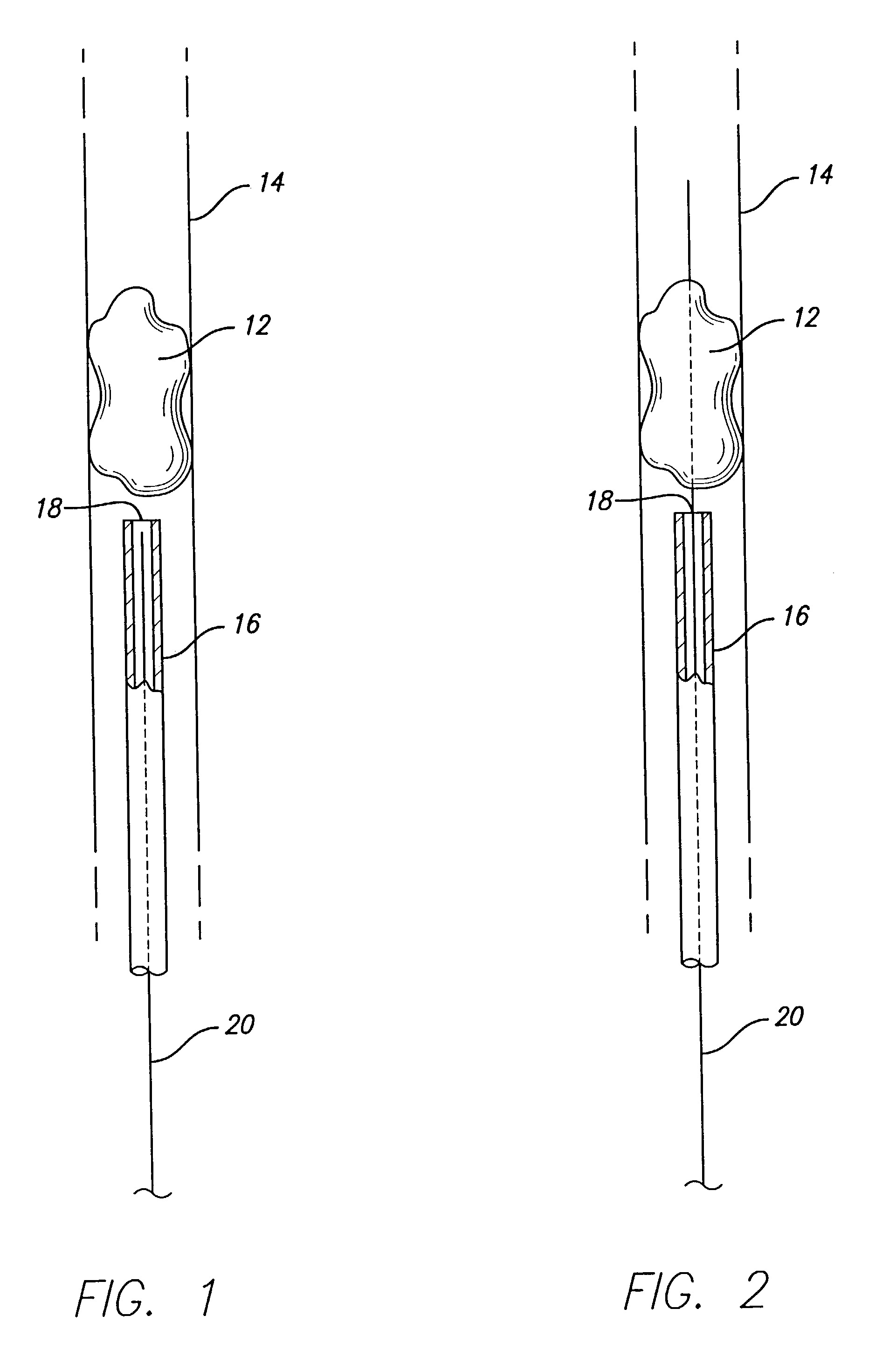
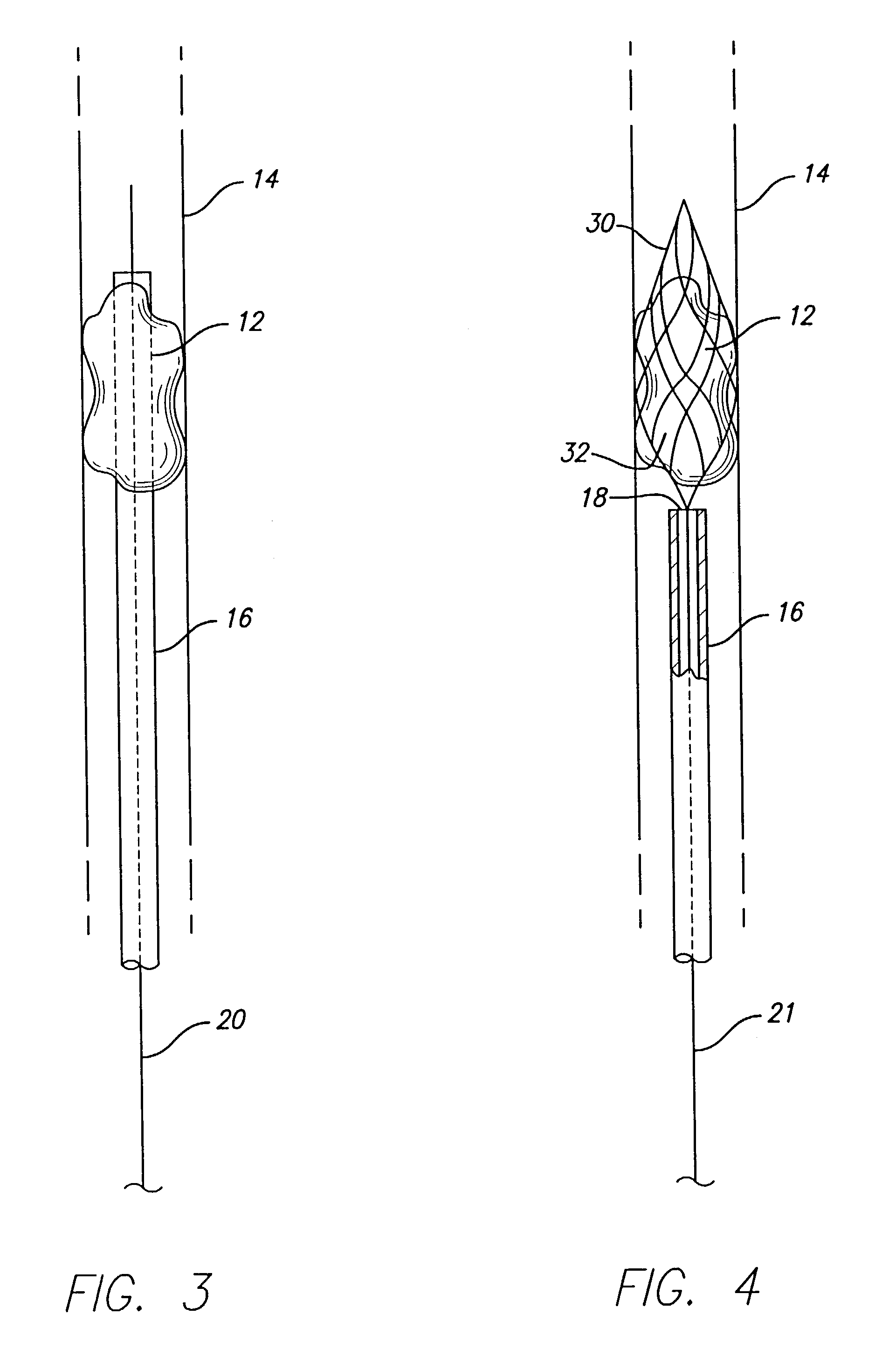
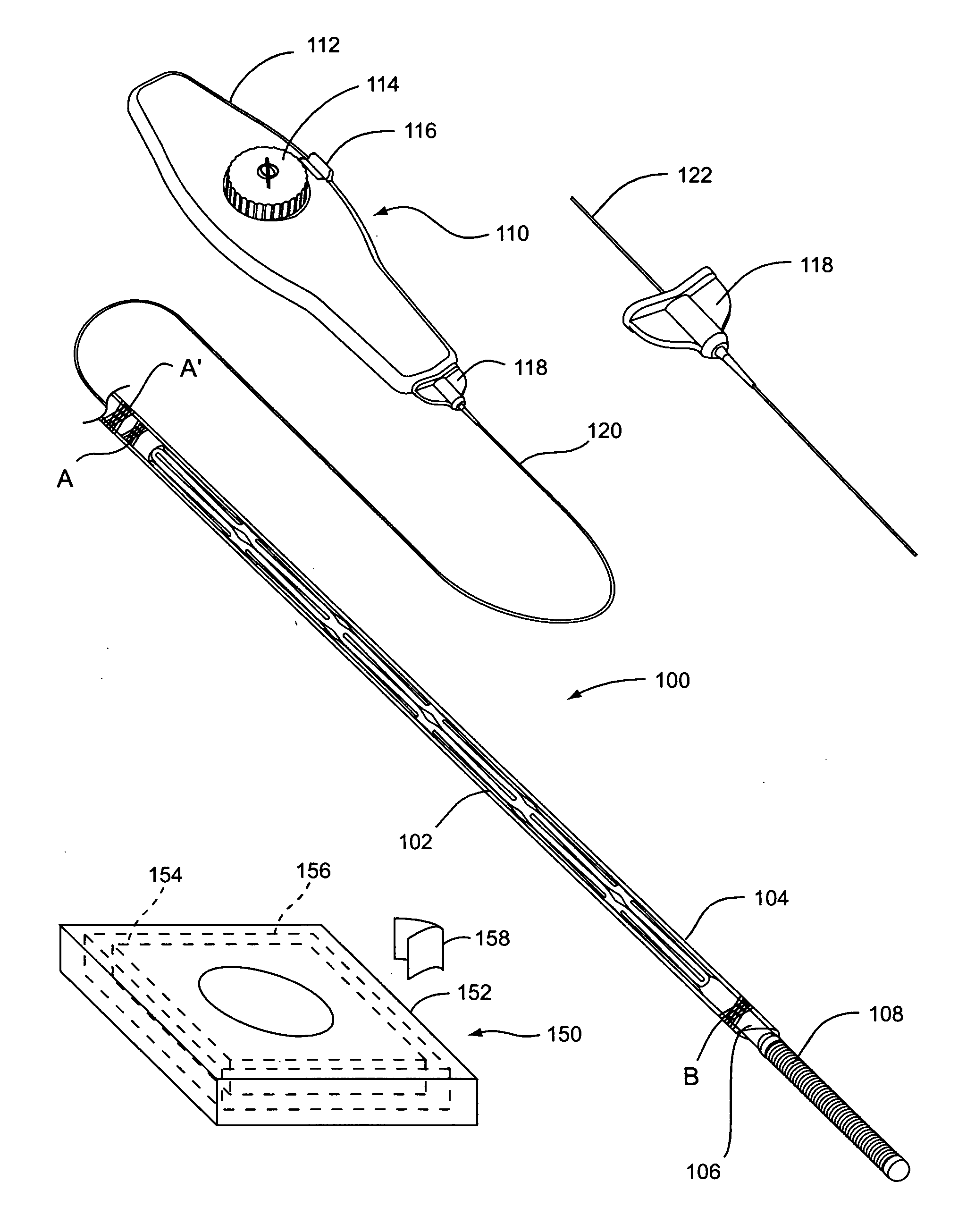
Device for removal of thrombus through physiological adhesion
InactiveUS7004954B1Raise the level of performanceRestoring native blood flowBalloon catheterSurgeryMedicineThrombus
A device that is useful for removing obstructions from vessels. Various embodiments and methods of use are contemplated for the effective removal of obstructions. The disclosed devices utilize a thrombogenic material to promote the formation of fibrin bonds, thus enhancing adhesion. It is further contemplated that the disclosed devices may be used in all vasculature including the cerebral vasculature and the neurovasculature.
Owner:ENDOVASCULAR TECH
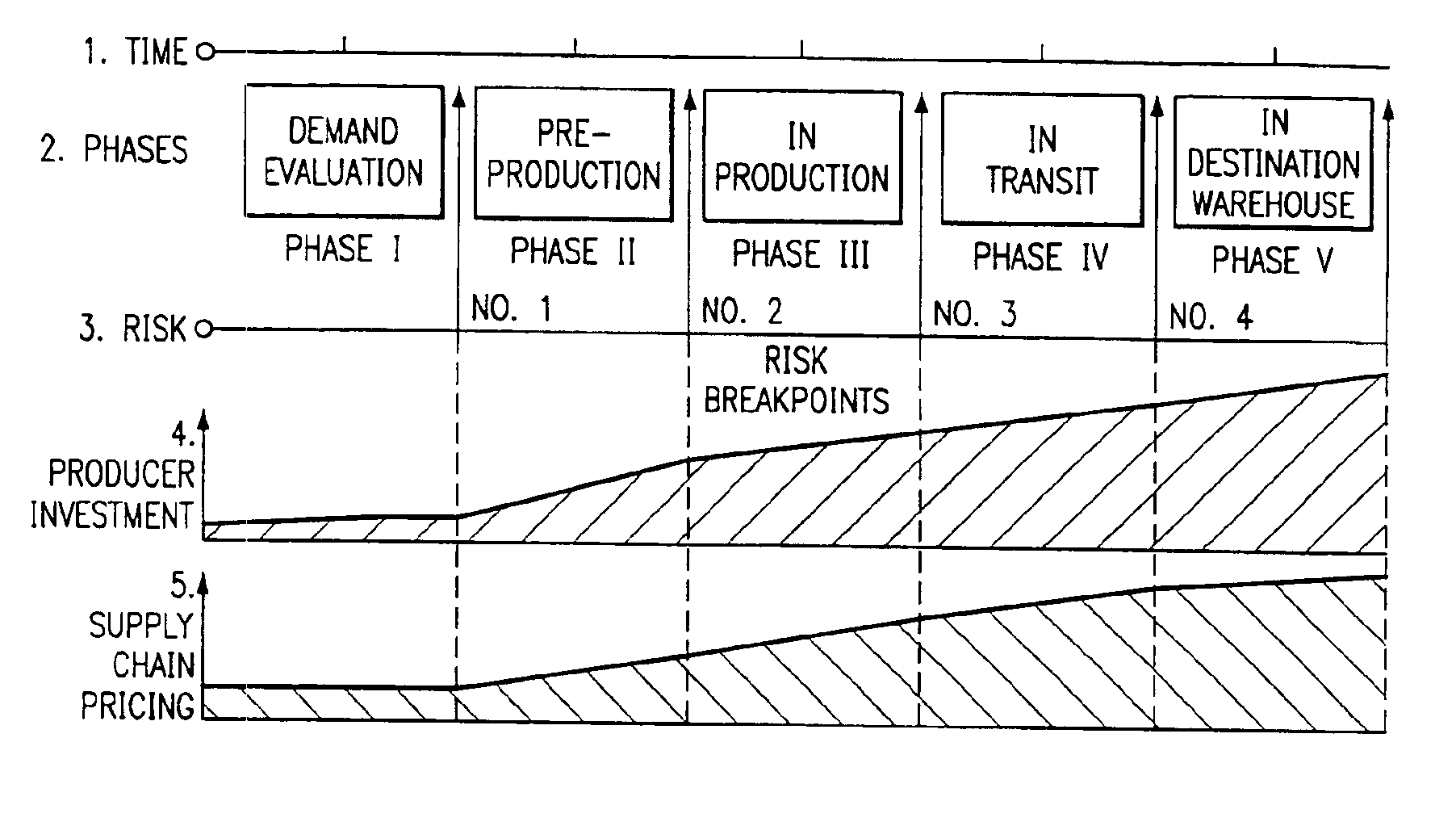
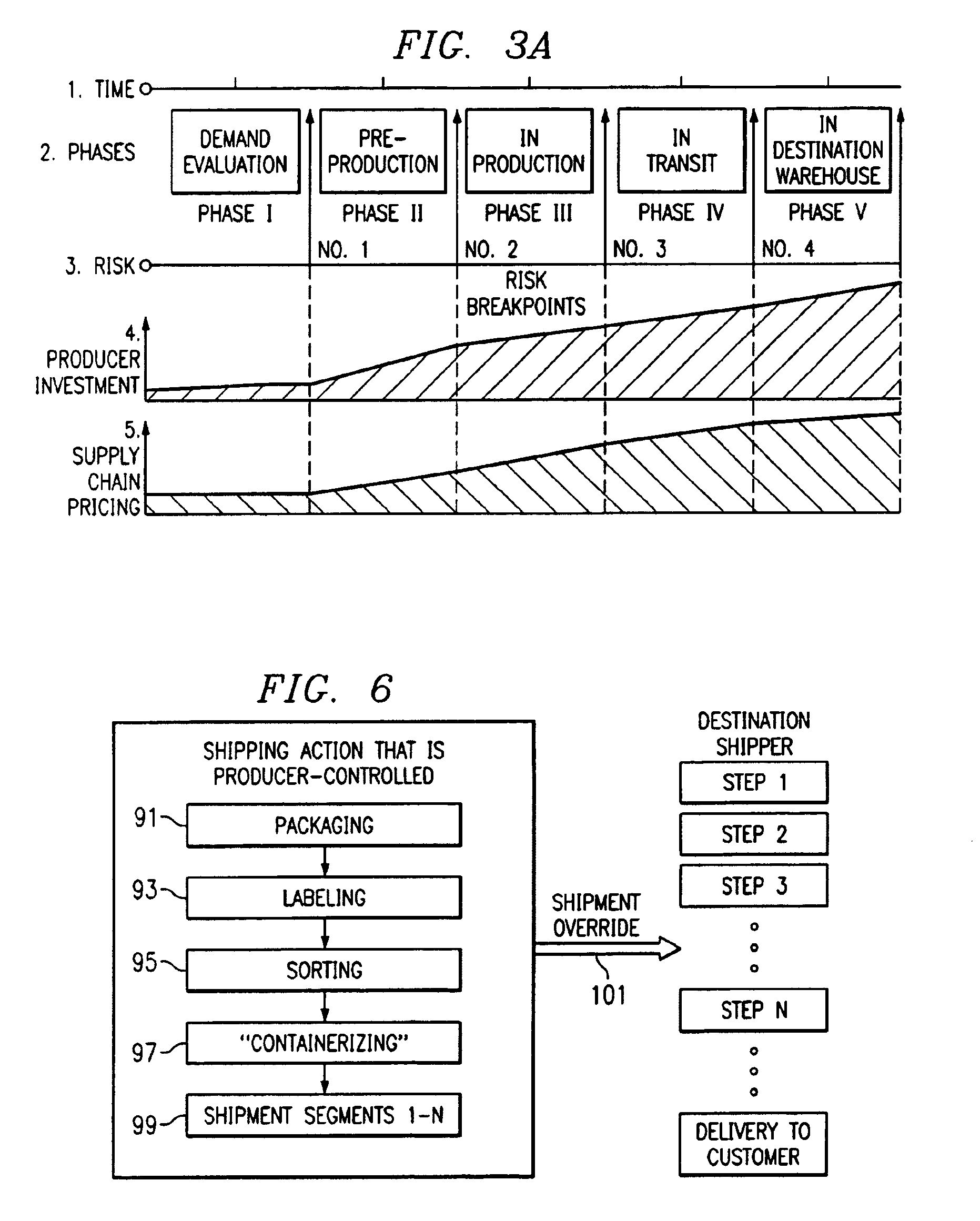
Method of producing, selling, and distributing articles of manufacture
InactiveUS6954734B1Simple preparation conditionsLess riskHand manipulated computer devicesDiscounts/incentivesElectronic communicationMilestone
A method of selling articles of manufacture which utilizes an electronic communication system to identify a plurality of articles of manufacture, from a plurality of manufacturing entities, which are available for purchase by said plurality of potential purchasers. The steps of the method include: Identifying pricing milestones in each of (1) a manufacturing phase and (2) a distribution phase, which correspond to an increase in commercial risk. Determining a separate price for each of said pricing milestones to establish a range of prices for said selected ones of said plurality of articles of manufacture, taking into account a change in said commercial risk as said pricing milestones are experienced. Making conditional offers for sale to potential purchasers at each of the pricing milestones, with the conditional offers specifying at least a minimum number of articles which must be ordered in aggregate before the conditional offer becomes binding upon a manufacturing entity. Communicating with potential purchasers and aggregating commercial commitments from potential purchasers for each pricing milestones and thereby selling articles of manufacture. Such that each pricing milestone corresponds to a period of availability in which costs of future supply chain activities or savings related to avoidance of future supply chain activities are reflected in an offer price. The method may also include the use of a trusted intermediary, which may use a virtual exchange.
Owner:LAKESOUTH HLDG LLC
Method of producing, selling, and distributing articles of manufacture through the automated aggregation of orders and the visual representation of standardized shipping volumes
InactiveUS7136830B1Simple preparation conditionsLess riskBuying/selling/leasing transactionsSpecial data processing applicationsManufactured suppliesIndustrial engineering
The present invention also provides a dynamic on-line order gathering system that enables sellers to offer one or a combination of goods whose availability (production and / or shipping) may be economically linked in some ways with other items and which facilitates aggregation of demand across related items so as to enable the ability to reach critical mass of demand for the related goods by a more efficient means than currently available.
Owner:LAKESOUTH HLDG LLC
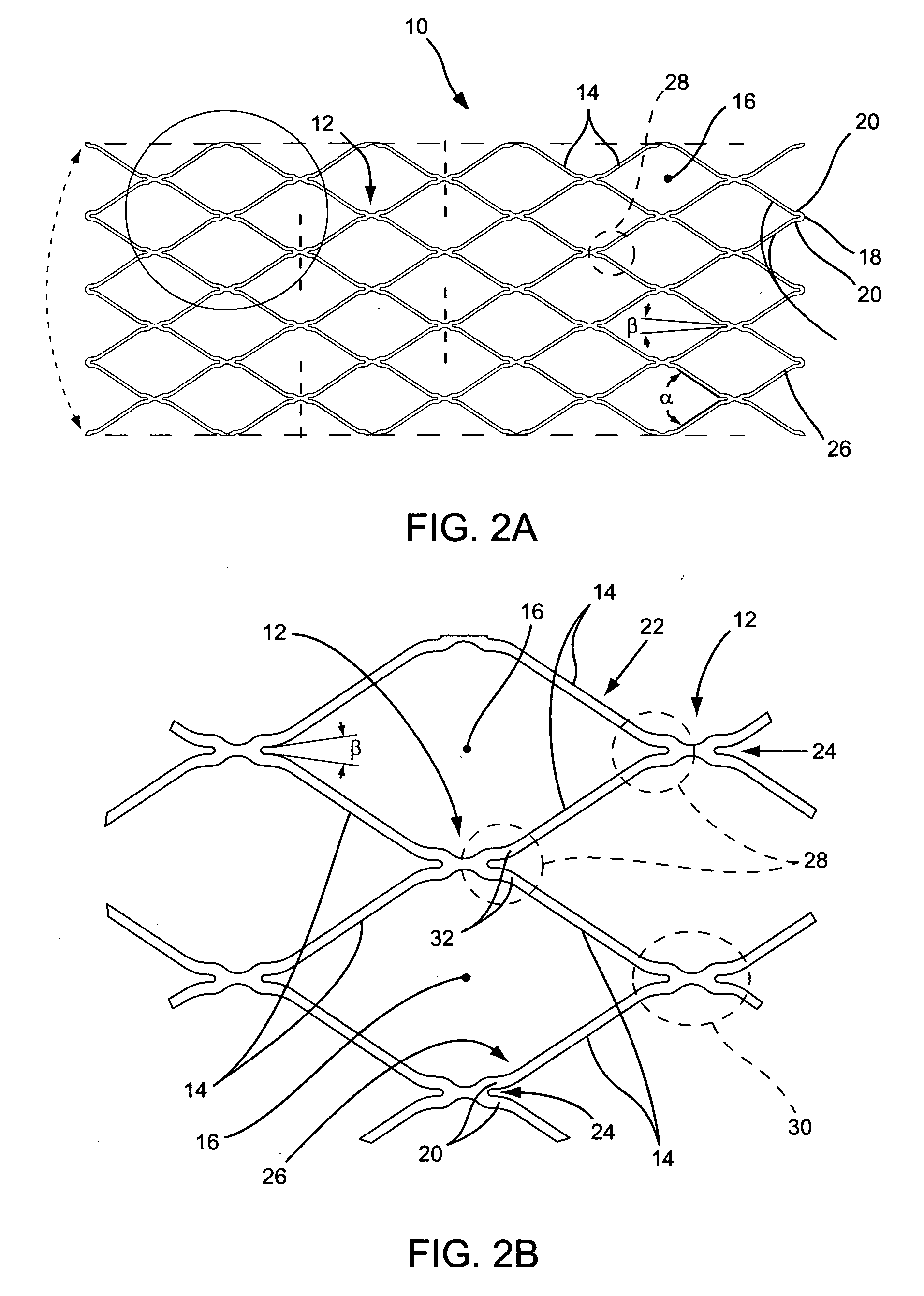
Delivery guide member based stent anti-jumping technologies
InactiveUS20060085057A1Lightweight productionWeaken energyStentsBlood vesselsBody organsSubject matter
Medical device and methods for delivery or implantation of prostheses within hollow body organs and vessels or other luminal anatomy are disclosed. The subject technologies may be used in the treatment of atherosclerosis in stenting procedures or a variety of other procedures.
Owner:BIOSENSORS INT GROUP
Acellular bioabsorbable tissue regeneration matrices
ActiveUS20070202189A1Lightweight productionPromote regenerationBiocideNervous disorderNerves tissuePathology
Owner:GENESIS TECH LTD
Power circuitry for high-frequency applications
InactiveUS20070230222A1Minimal power lossImprove efficiencyDc-dc conversionElectric variable regulationEngineeringCharge and discharge
Owner:BAYER MATERIALSCIENCE AG
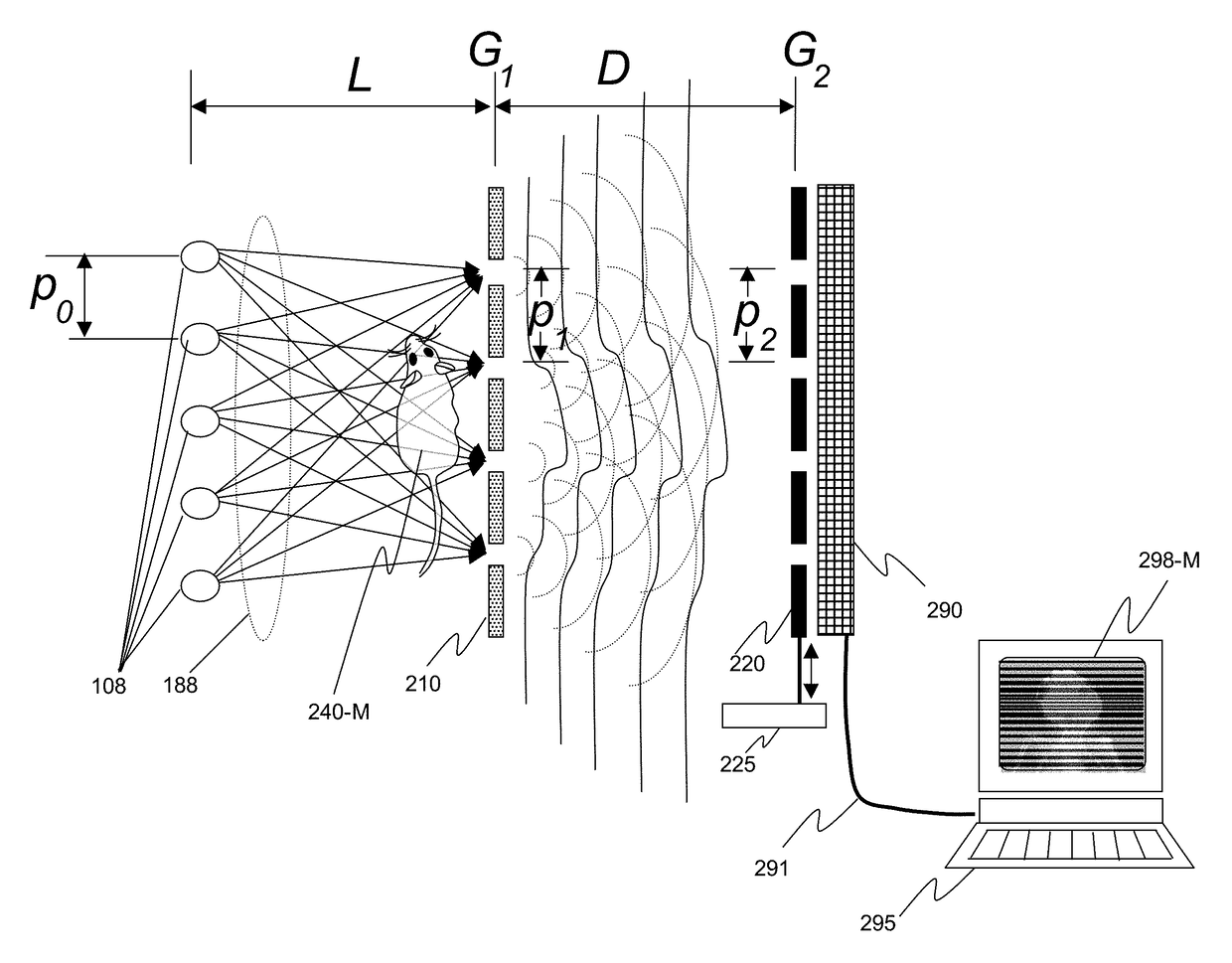
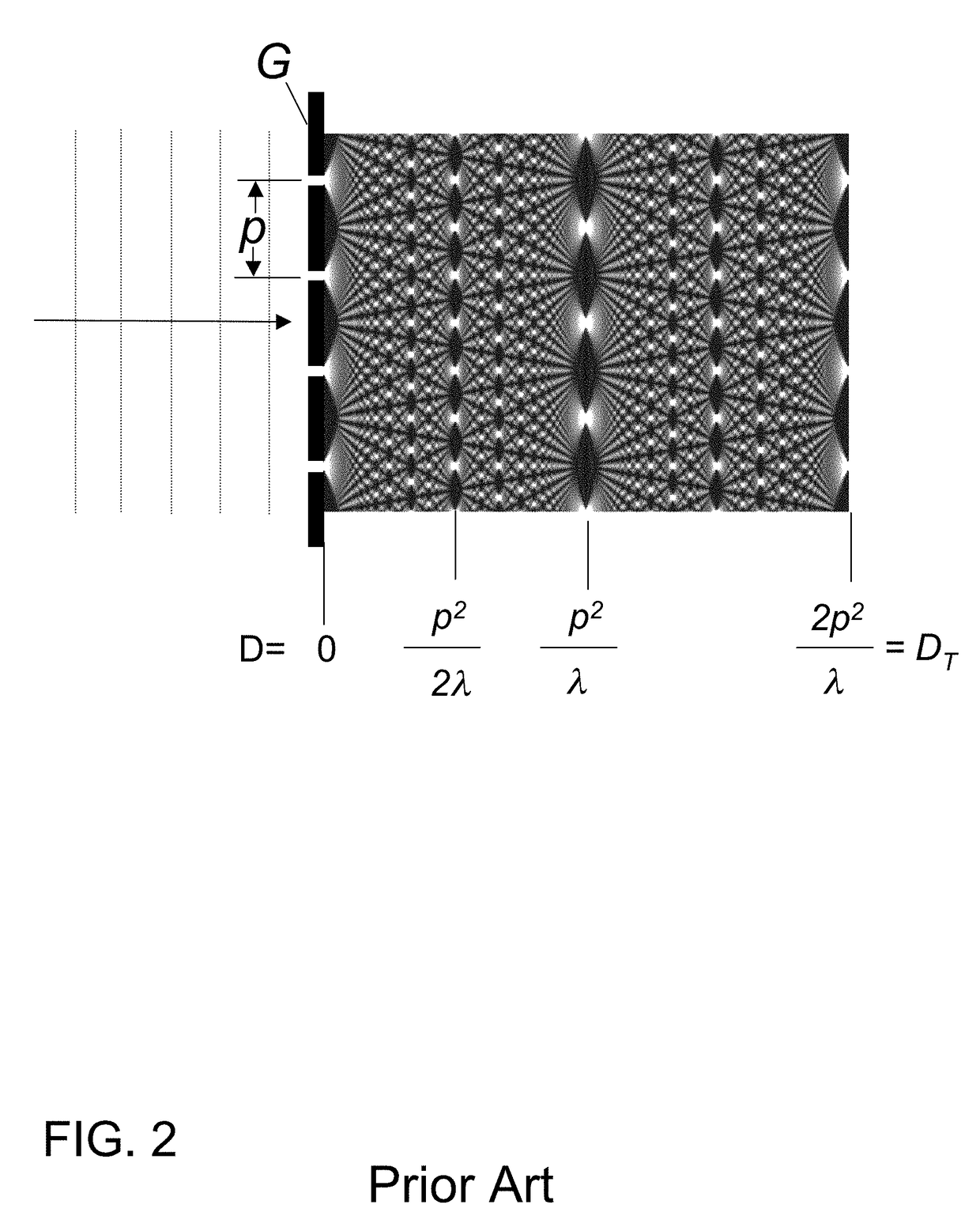
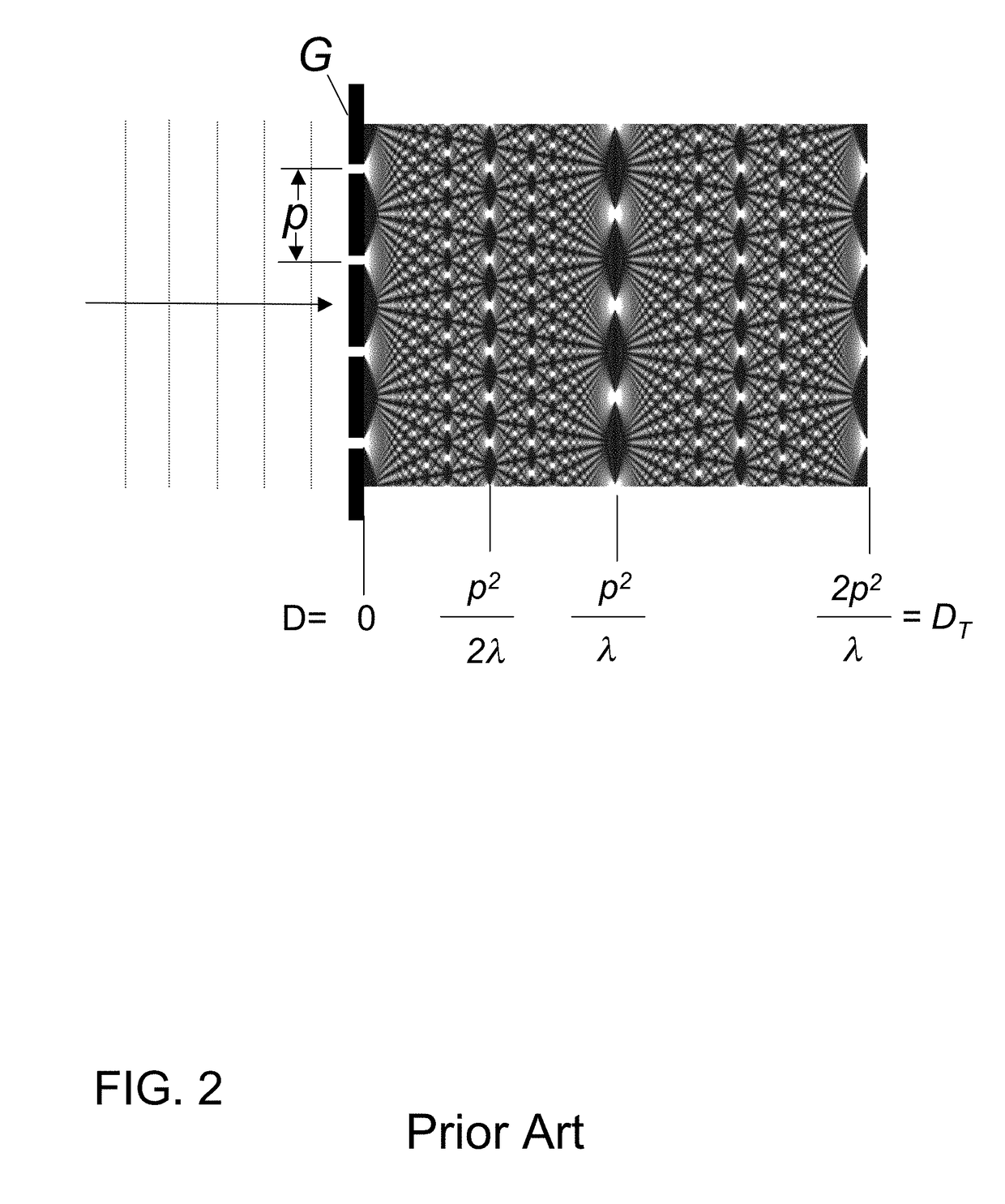
X-ray interferometric imaging system
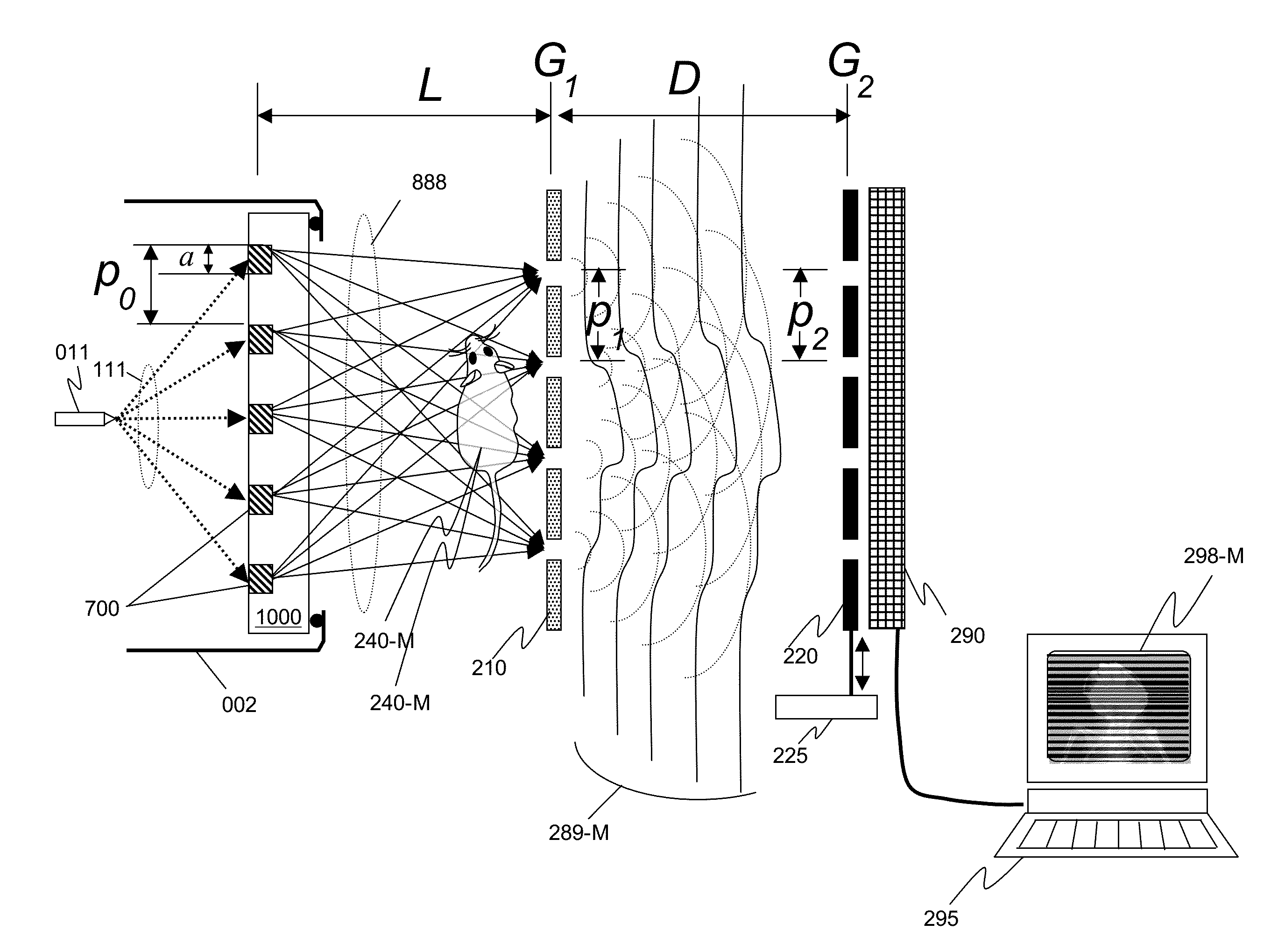
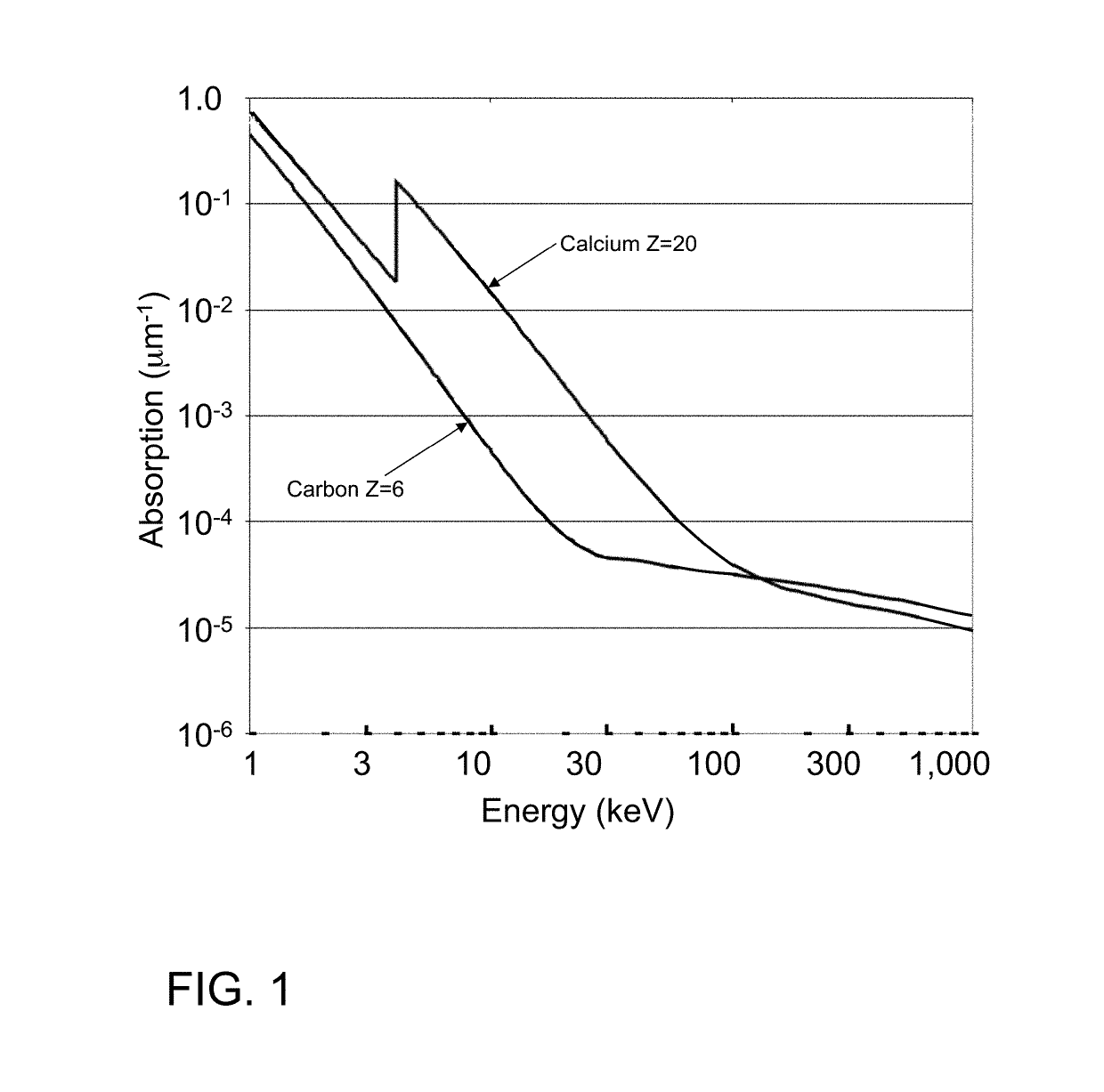
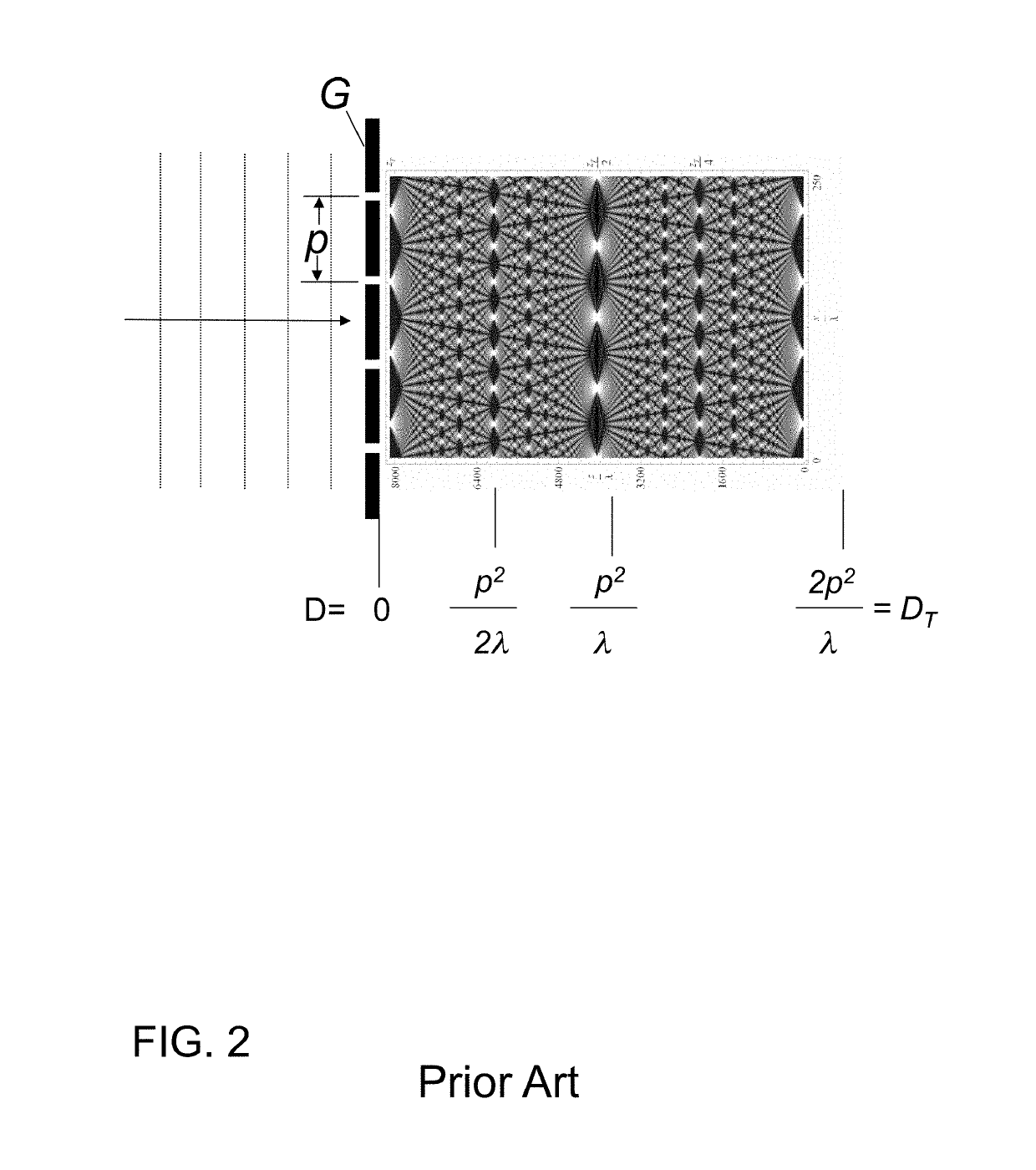
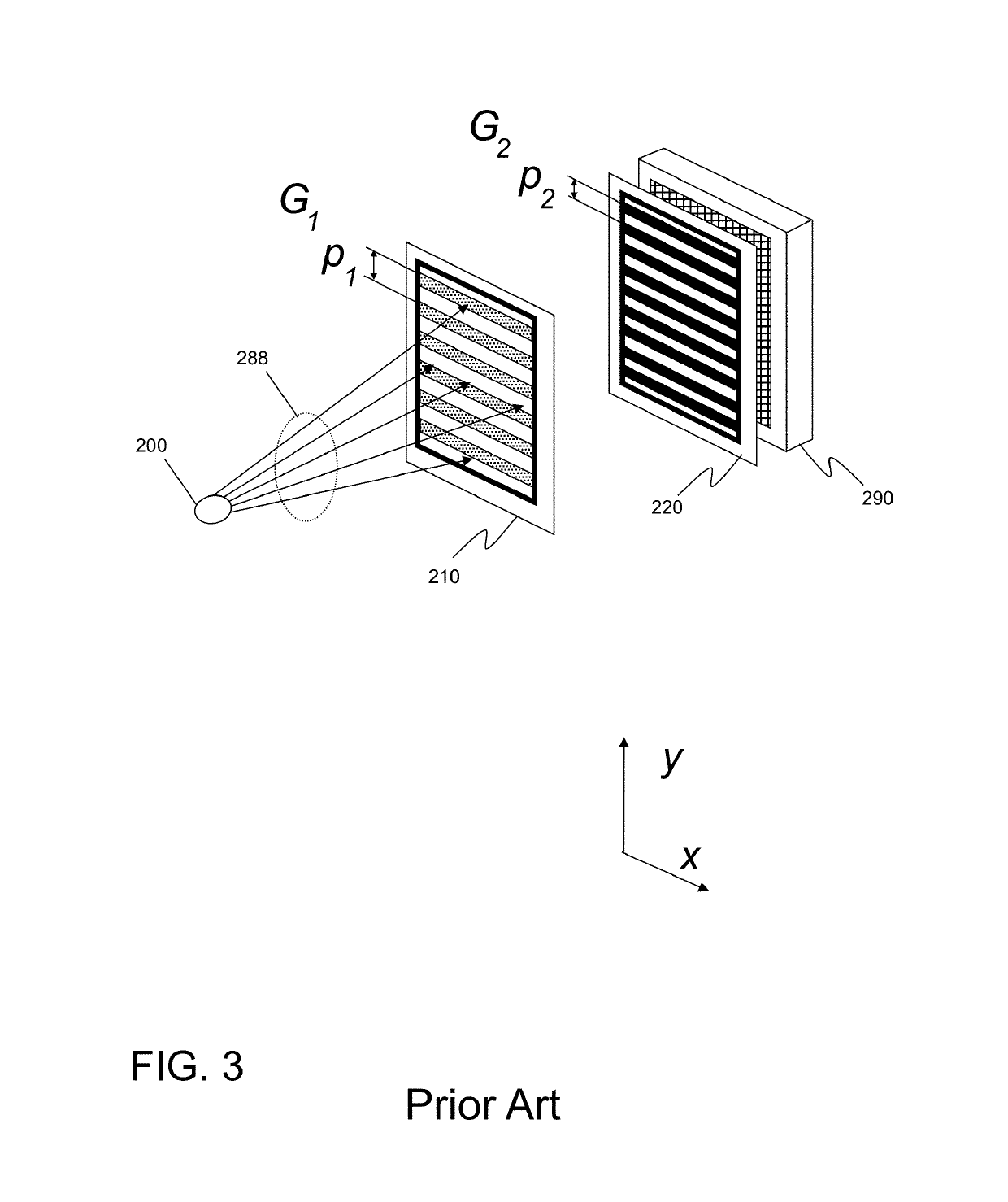
InactiveUS20150117599A1Increase brightnessLarge x-ray powerImaging devicesMaterial analysis using wave/particle radiationSoft x rayGrating
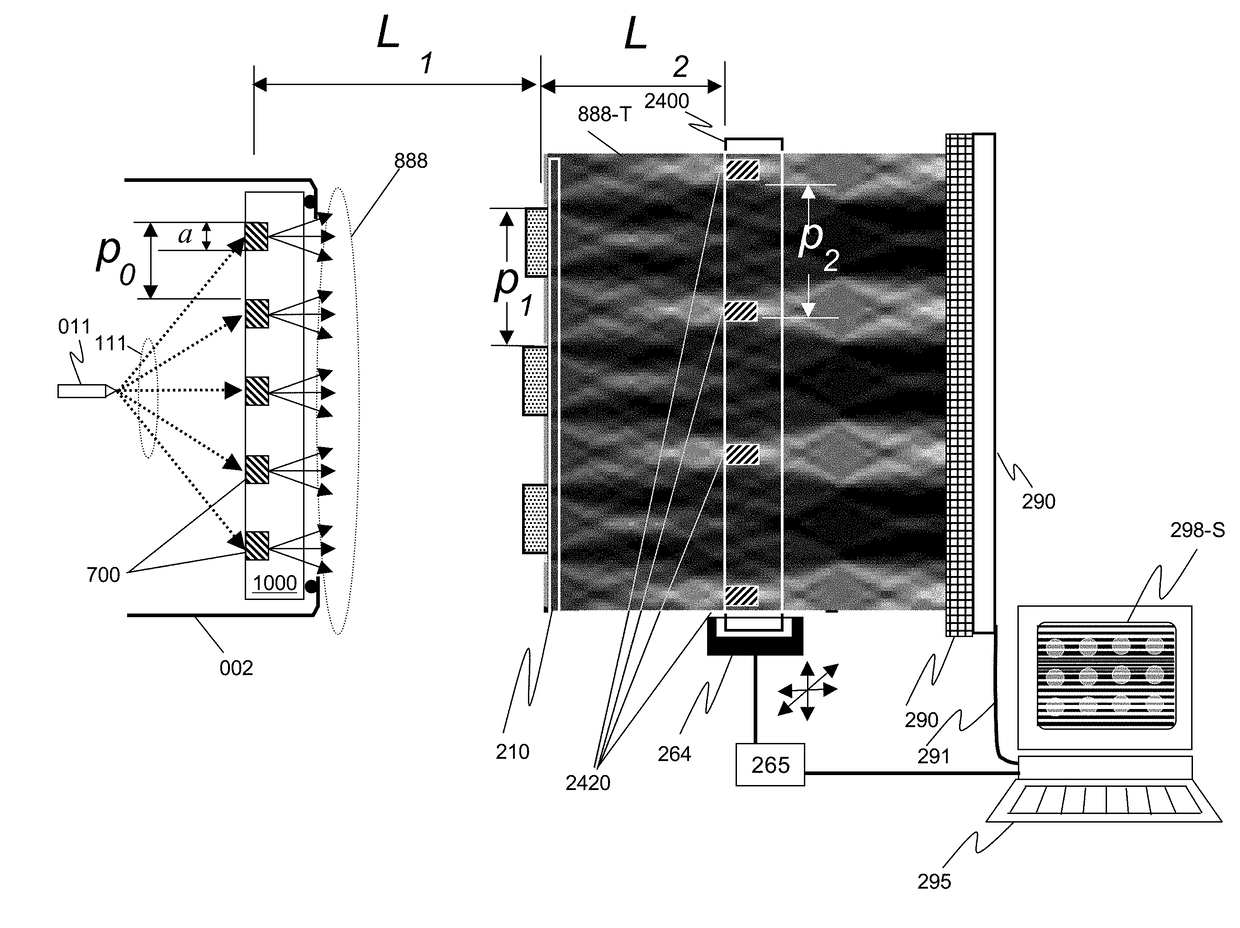
We disclose an x-ray interferometric imaging system in which the x-ray source comprises a target having a plurality of structured coherent sub-sources of x-rays embedded in a thermally conducting substrate. The system additionally comprises a beam-splitting grating G1 that establishes a Talbot interference pattern, which may be a π phase-shifting grating, and an x-ray detector to convert two-dimensional x-ray intensities into electronic signals. The system may also comprise a second analyzer grating G2 that may be placed in front of the detector to form additional interference fringes, and a means to translate the second grating G2 relative to the detector.In some embodiments, the structures are microstructures with lateral dimensions measured on the order of microns, and with a thickness on the order of one half of the electron penetration depth within the substrate. In some embodiments, the structures are formed within a regular array.
Owner:SIGRAY INC
Absorbent fibrous granules
InactiveUS6092302ALightweight productionDrying solid materials with heatOther chemical processesFiberParticulates
There is provided a cellulosic-based fiber granule with added non self-associating particulates or fibers. The resulting granule is free-flowing with a densified outer surface and is capable of removing substantially all oil or other fluids from a flat surface such as a floor. The granule is also capable of being incinerated by being formed substantially of organic materials. Cellulosic plant fibers form at least 10 percent up to 99 percent of the granule. There is also provided a method for forming the incineratable absorbent, free-flowing granules.
Owner:3M INNOVATIVE PROPERTIES CO
X-ray method for the measurement, characterization, and analysis of periodic structures
ActiveUS20150260663A1Lightweight productionIncrease brightnessImaging devicesX-ray tube electrodesSoft x rayGrating
Periodic spatial patterns of x-ray illumination are used to gather information about periodic objects. The structured illumination may be created using the interaction of a coherent or partially coherent x-ray source with a beam splitting grating to create a Talbot interference pattern with periodic structure. The object having periodic structures to be measured is then placed into the structured illumination, and the ensemble of signals from the multiple illumination spots is analyzed to determine various properties of the object and its structures. Applications to x-ray absorption / transmission, small angle x-ray scattering, x-ray fluorescence, x-ray reflectance, and x-ray diffraction are all possible using the method of the invention.
Owner:SIGRAY INC
X-ray interferometric imaging system
ActiveUS9719947B2Lightweight productionIncrease brightnessImaging devicesX-ray tube electrodesSoft x rayGrating
Owner:SIGRAY INC
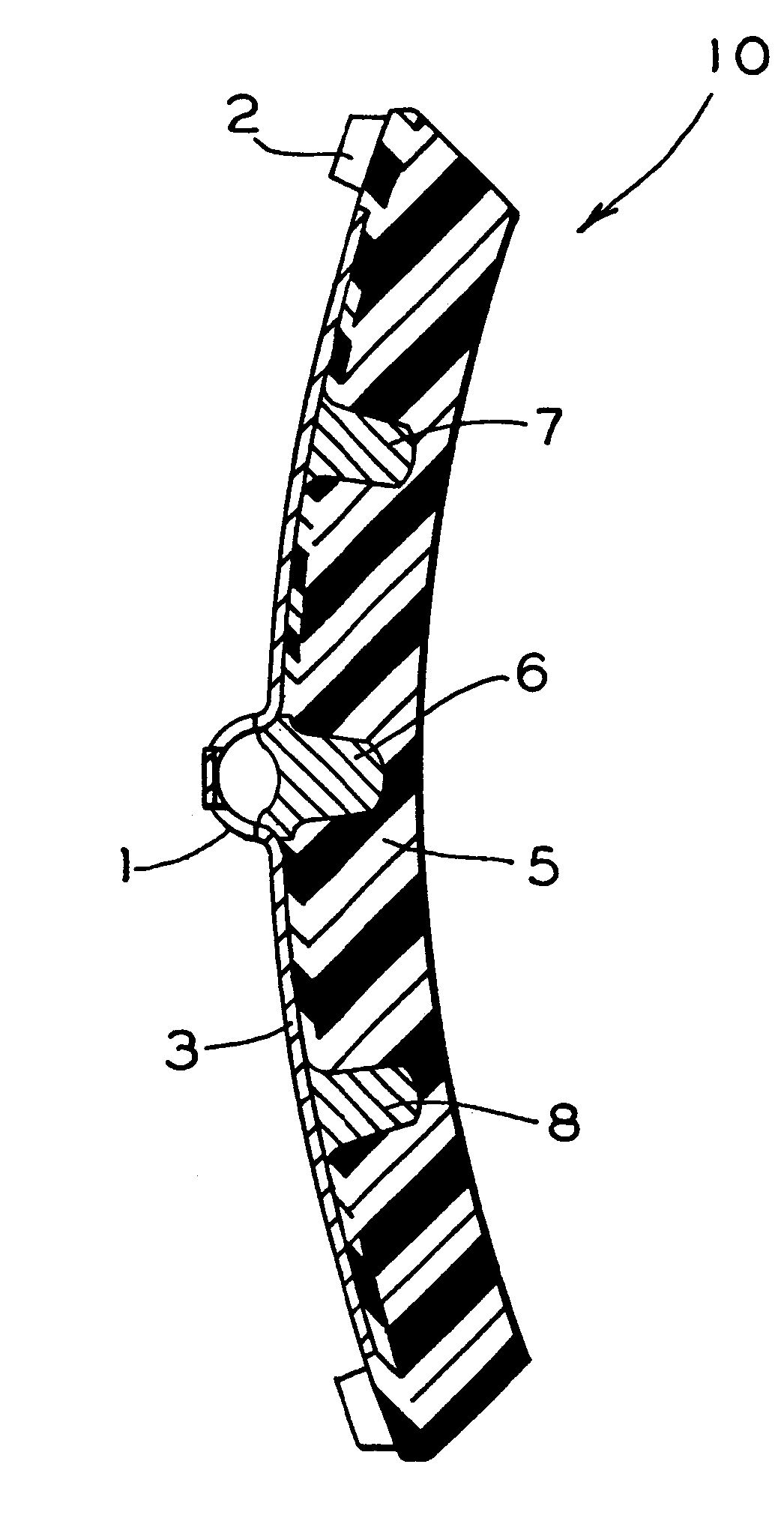
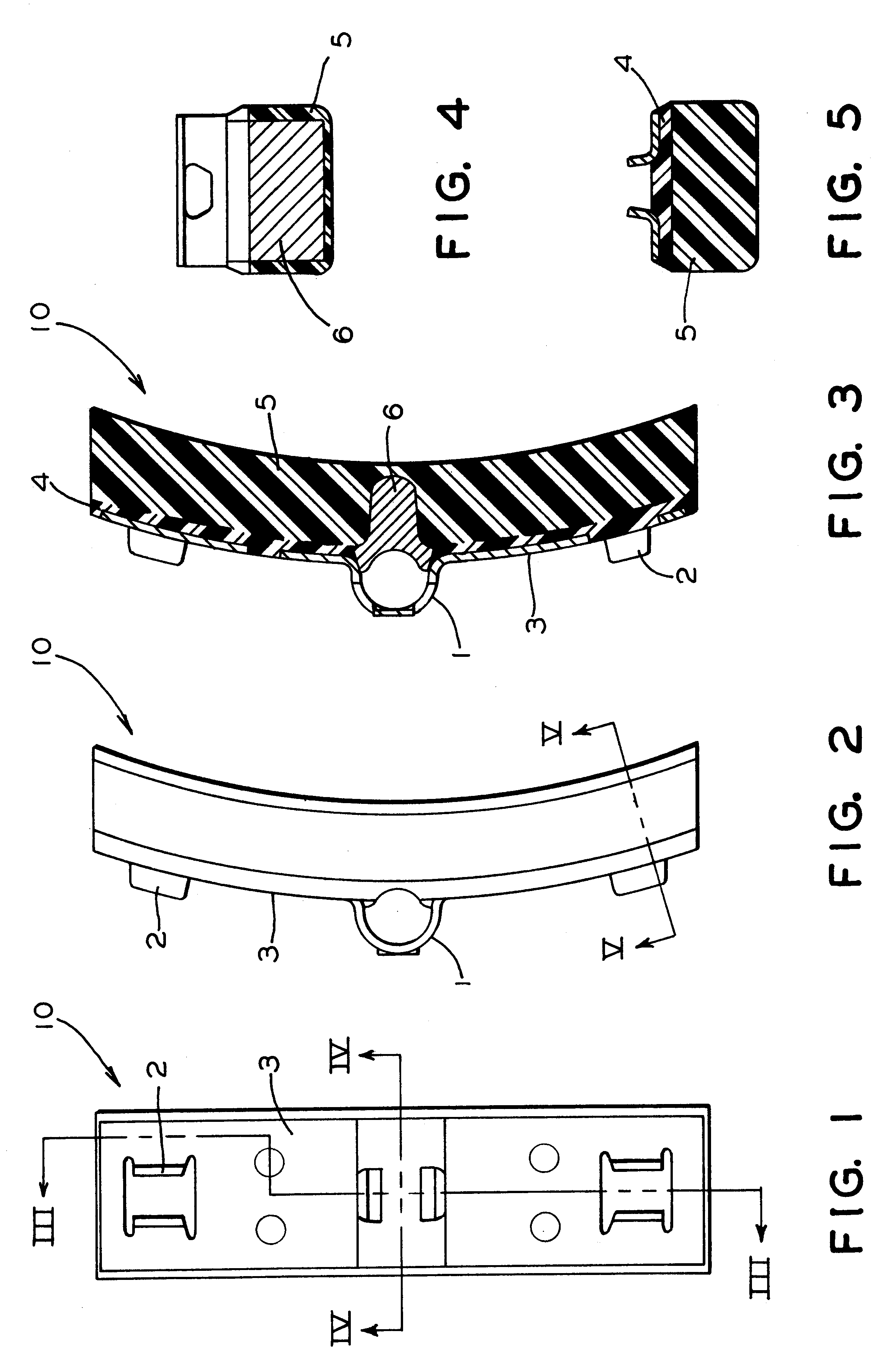
Brake shoe with insert bonded to backing plate
InactiveUS6241058B1Reduce defectsLightweight productionBraking membersFriction liningBrake shoeTread
A composition brake shoe for use on a railway vehicle for reconditioning a wheel tread surface during a normal braking application on such railway vehicle is provided. The composition brake shoe includes a backing plate having a stirrup and a brake surface having a predetermined configuration and a predetermined surface area. It further includes a first friction type composition material extending over the surface area of such brake surface of such composition brake shoe. The composition brake shoe further contains a second friction type material, formed as at least one discrete insert, having a predetermined shape and molded into such first friction type composition material. Such second friction type material initially being completely embedded within such first friction type composition material. One surface of such at least one discrete insert being incrementally exposed as such first friction type composition material is eroded away due to frictional engagement with such wheel tread surface during normal braking operations, such second friction type material exhibiting greater abrasive properties than such first friction type composition material. Such at least one discrete insert of such second friction type material is bonded to such backing plate.
Owner:RFPC HLDG CORP
Method and apparatus for production of uniformly sized nanoparticles
ActiveUS20130001833A1Reduce in quantityLightweight productionMetal-working apparatusNanotechnologyTarget surfaceNanoparticle
An apparatus and process for creating uniformly sized, spherical nanoparticles from a solid target. The solid target surface is ablated to create an ejecta event containing nanoparticles moving away from the surface. Ablation may be caused by laser or electrostatic discharge. At least one electromagnetic field is placed in front of the solid target surface being ablated. The electromagnetic field manipulates at least a portion of the nanoparticles as they move away from the target surface through the electromagnetic field to increase size and spherical shape uniformity of the nanoparticles. The manipulated nanoparticles are collected.
Owner:EVOQ NANO INC
Nitric oxide generator and inhaler
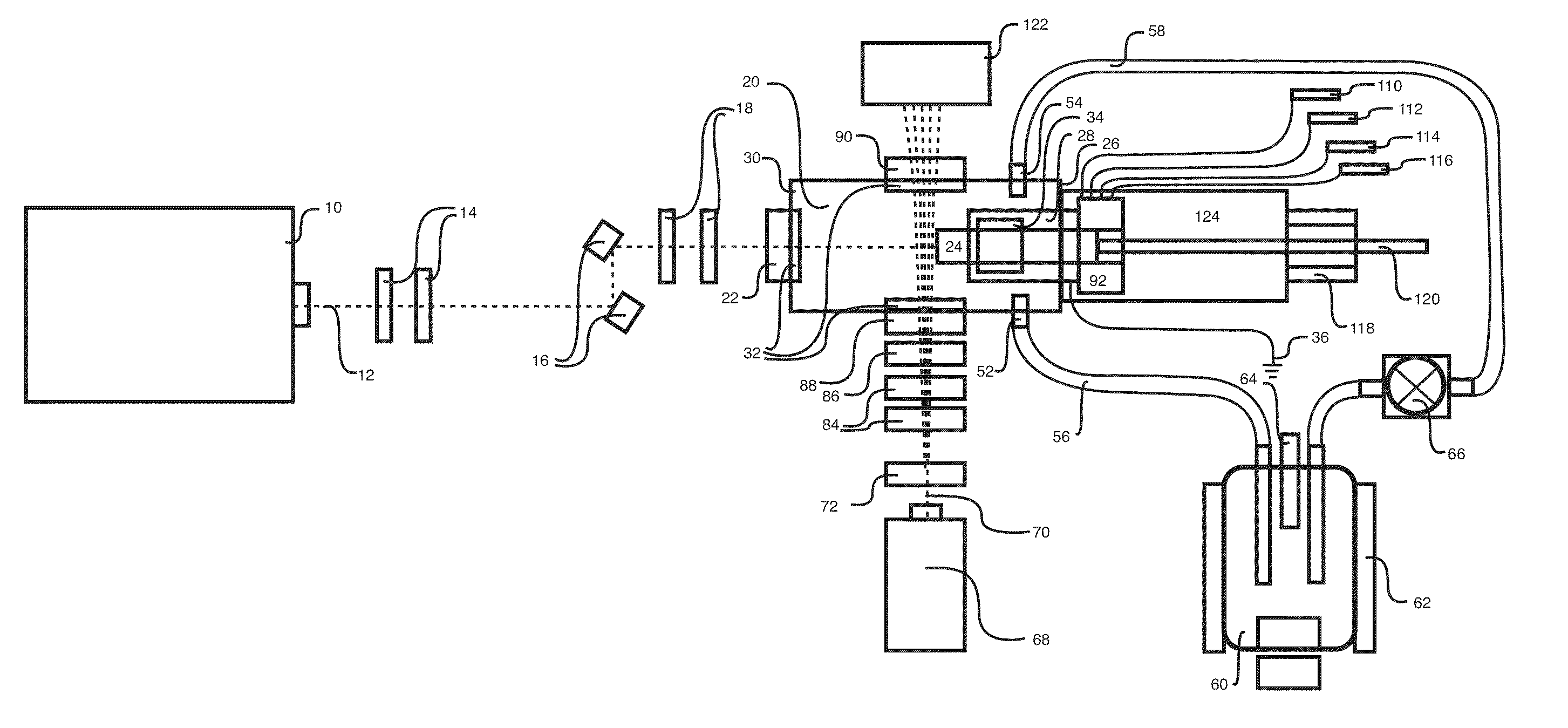
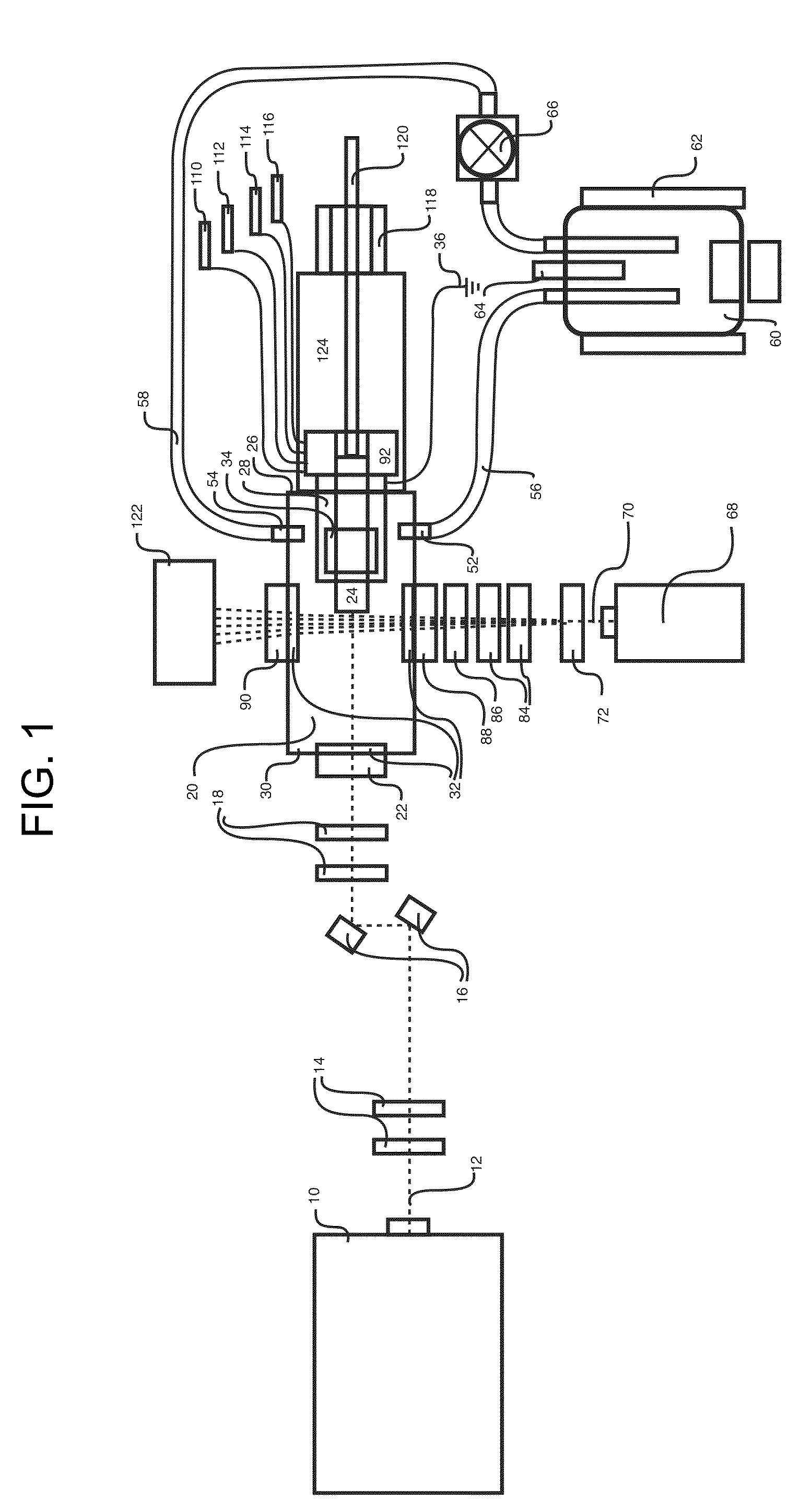
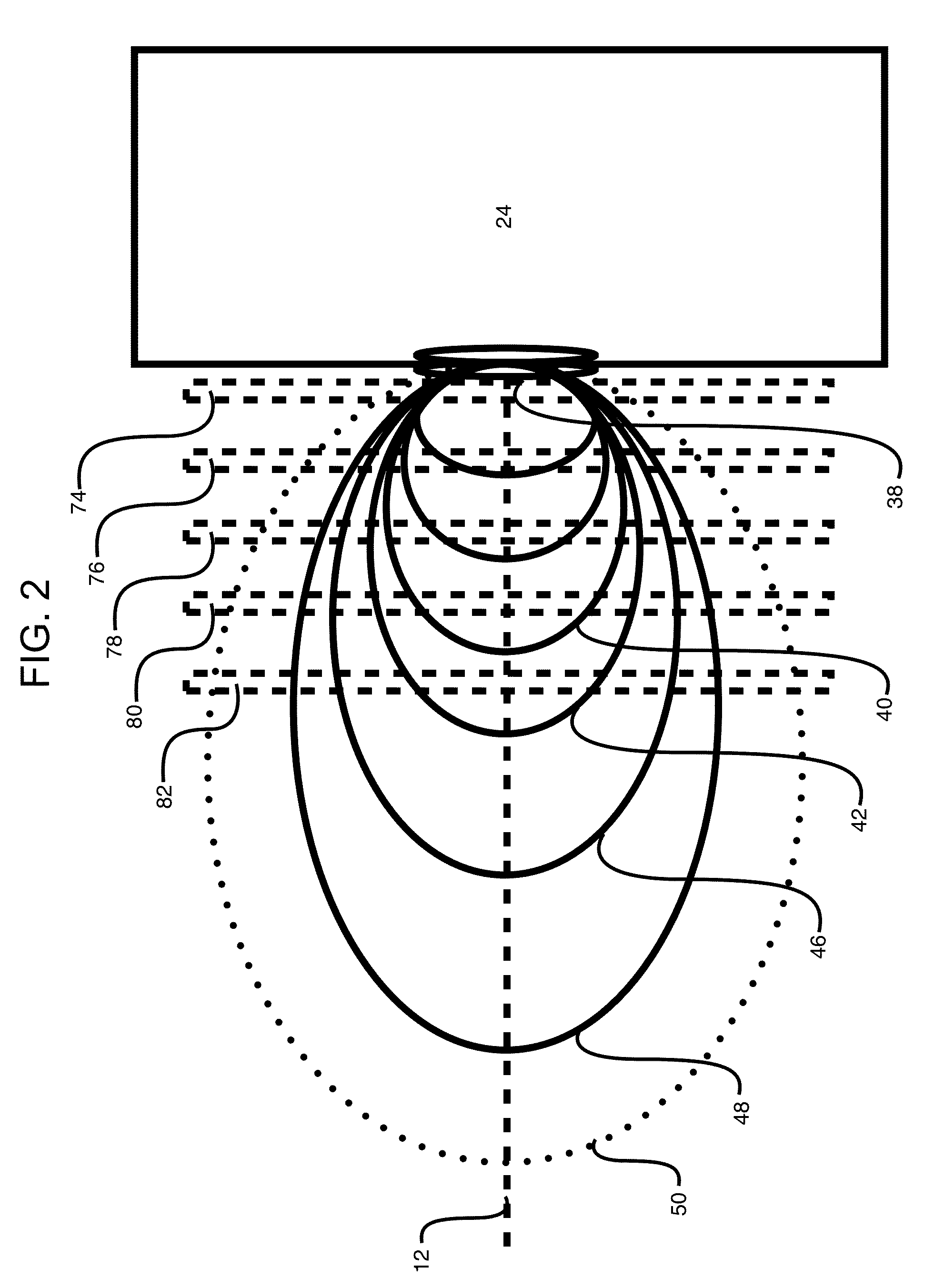
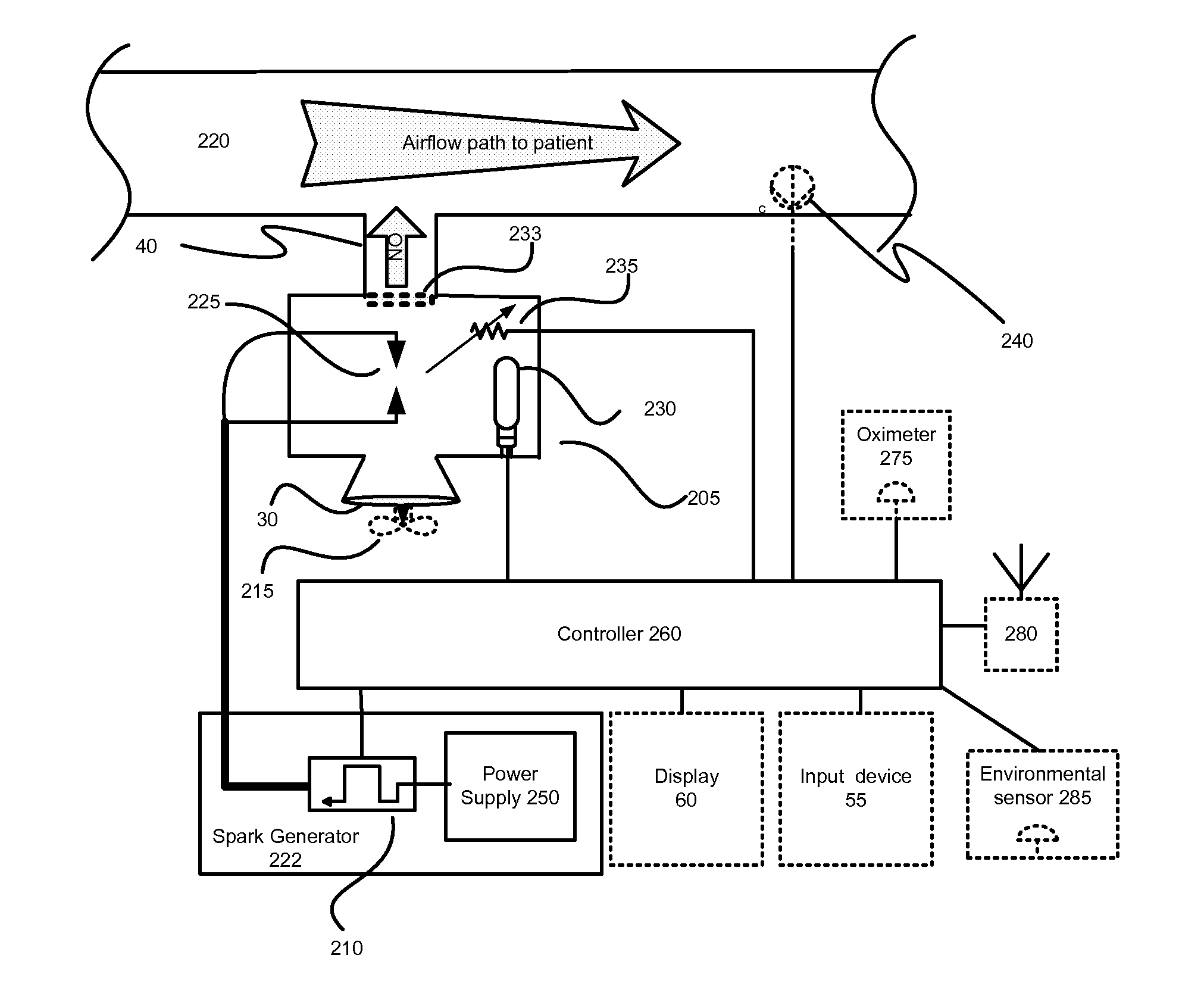
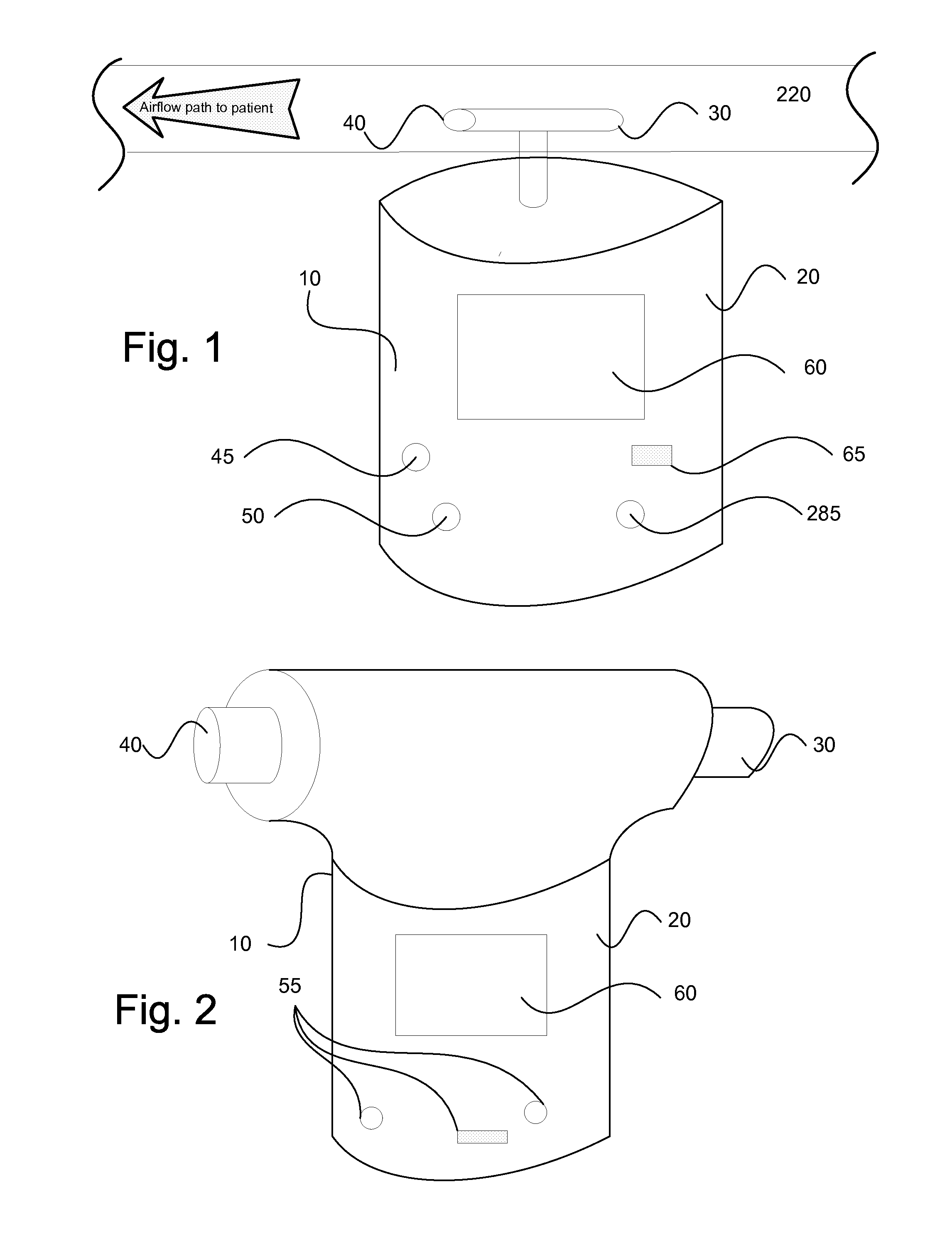
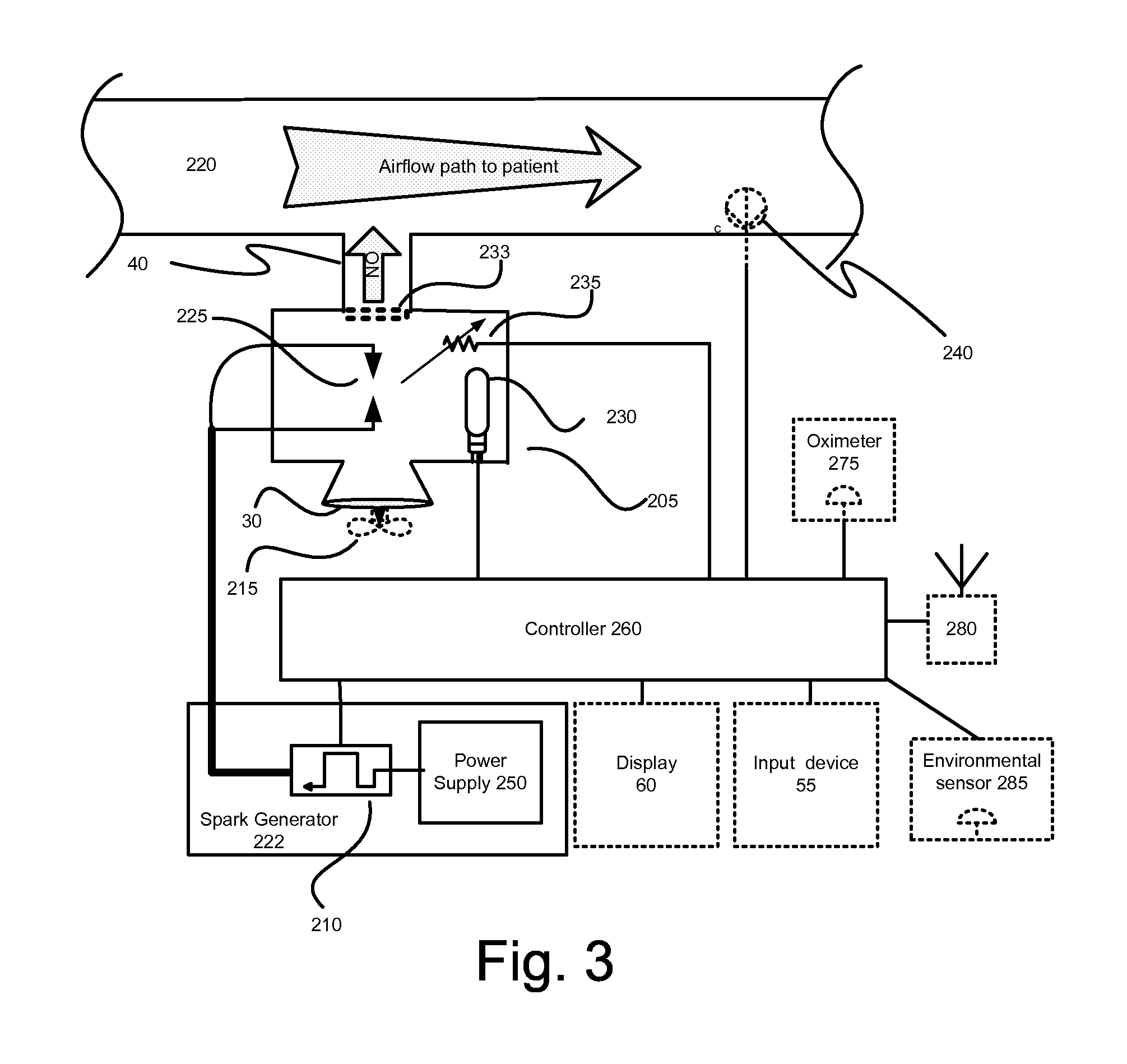
InactiveUS20150090261A1Little and no consumables useAffordable and reliableGas treatmentRespiratory masksNitrogen dioxideNitric oxide
Several embodiments of a Nitric Oxide Inhaler that uses an electrical spark to produce Nitric Oxide from Air, optimized to maximize the production of Nitric Oxide and minimize the production of Nitrogen Dioxide through hardware and control system. Further disclosed is a system to control such inhalers
Owner:CROSBIE DAVID BRUCE
X-ray method for the measurement, characterization, and analysis of periodic structures
ActiveUS9874531B2Lightweight productionIncrease brightnessImaging devicesX-ray tube electrodesSoft x rayGrating
Owner:SIGRAY INC
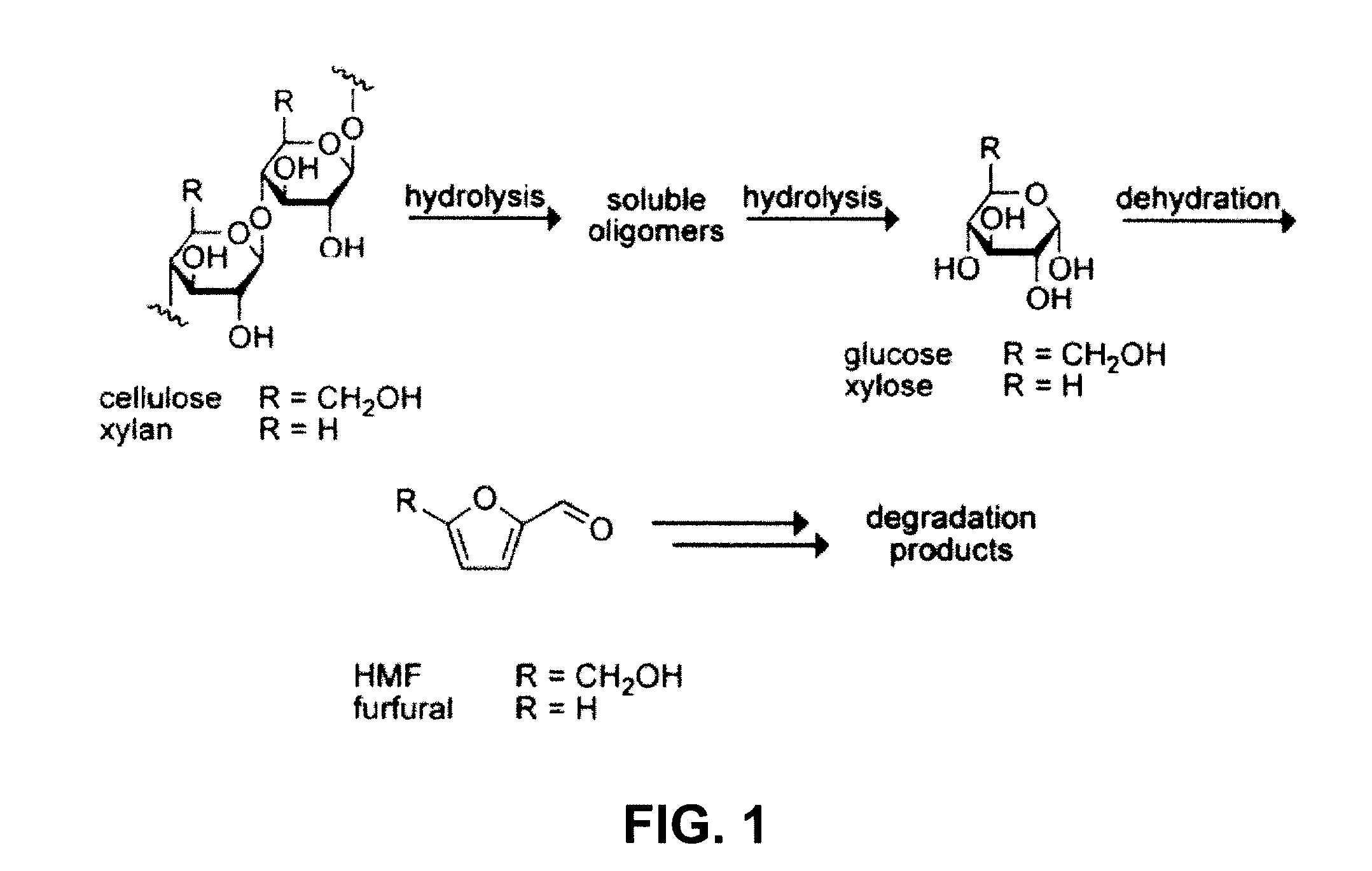
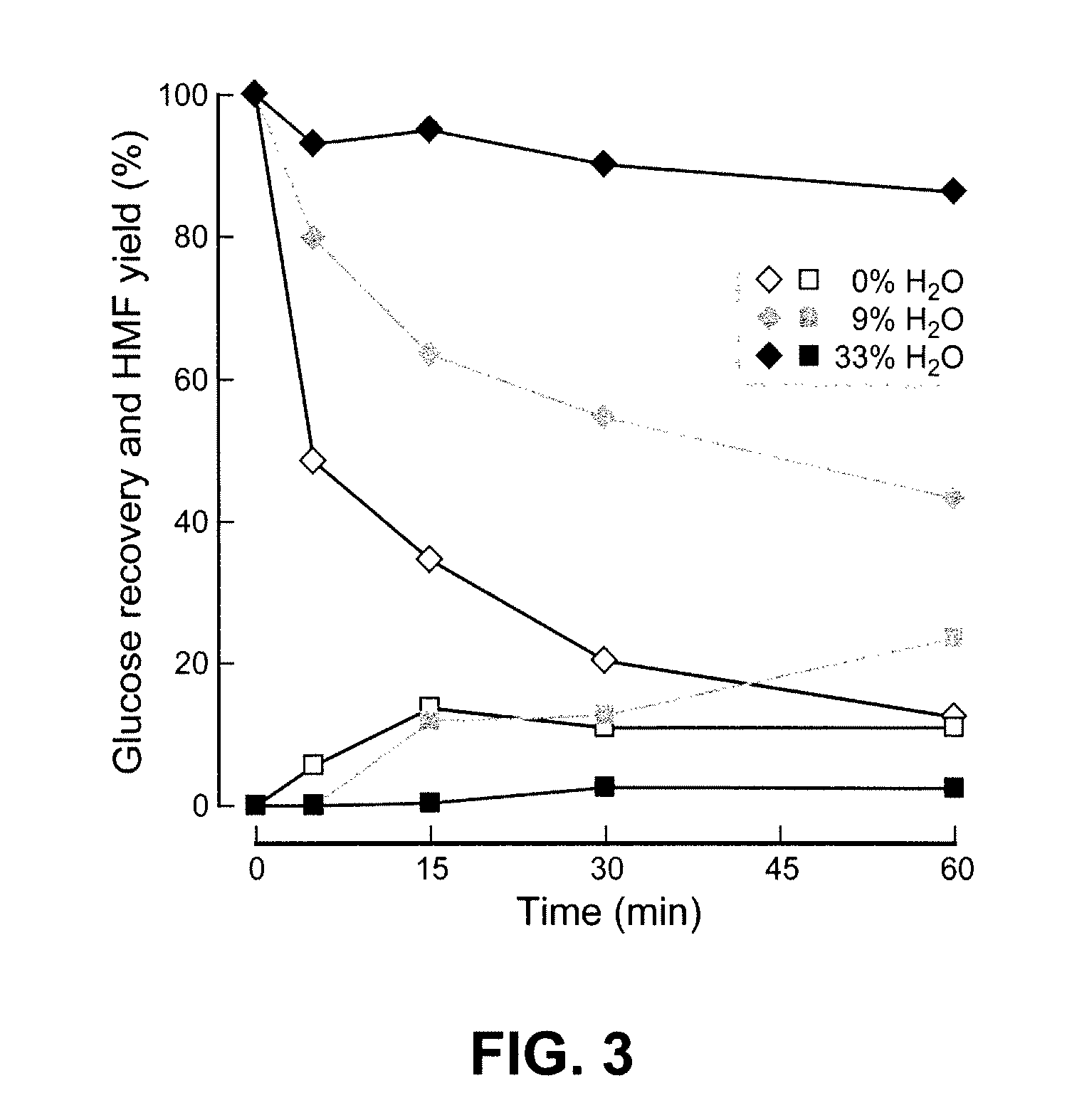
Biomass hydrolysis
ActiveUS20110065159A1Facilitate enhanced yield of glucoseImprove glucose yieldSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsCelluloseHydrolysate
High-yielding method for chemical hydrolysis of lignocellulose into monosaccharides. The process of the invention can additionally be applied to cellulose, xylan and related biomass polysaccharides, such as galactan, mannan, or arabinan. The method is employed for hydrolysis of a biomass polysaccharide substrate. The process is carried out in an ionic liquid in which cellulose is soluble in the presence of catalytic acid at a temperature sufficiently high to initiate hydrolysis. Water is added to the reaction mixture after initiation of hydrolysis at a rate controlled to avoid precipitation yet avoid undesired sugar dehydration products such ad HMF. Hydrolysis product is useful as feedstock for fermentations including fermentation processes for ethanol, butanol and other fuels.
Owner:WISCONSIN ALUMNI RES FOUND
Preparation of CIGS-based solar cells using a buffered electrodeposition bath
InactiveUS7297868B2Easy to handleLightweight productionElectrolytic coatingsSemiconductor/solid-state device manufacturingIndiumSolar cell
A photovoltaic cell exhibiting an overall conversion efficiency of at least 9.0% is prepared from a copper-indium-gallium-diselenide thin film. The thin film is prepared by simultaneously electroplating copper, indium, gallium, and selenium onto a substrate using a buffered electro-deposition bath. The electrodeposition is followed by adding indium to adjust the final stoichiometry of the thin film.
Owner:DAVIS JOSEPH & NEGLEY
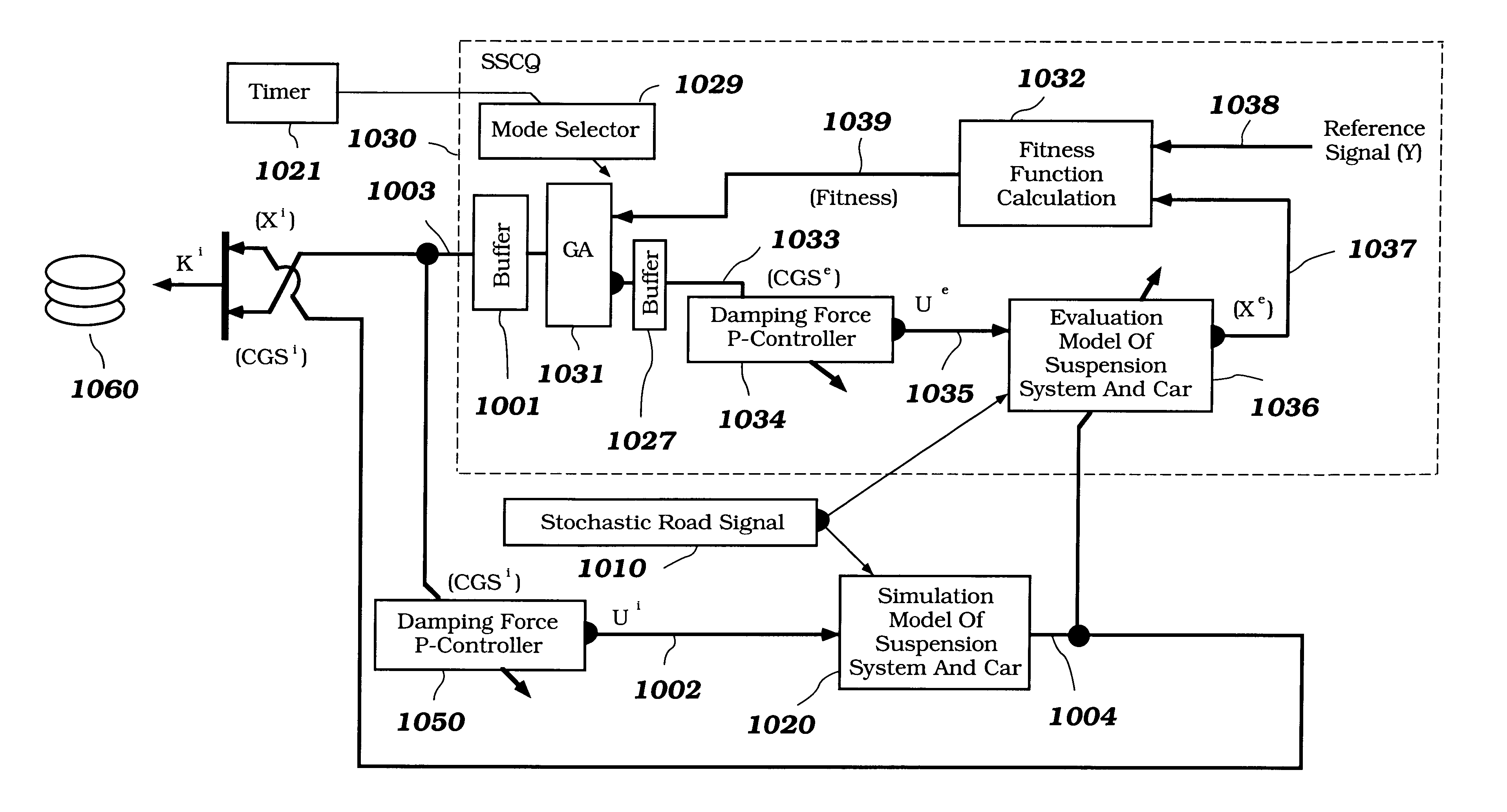
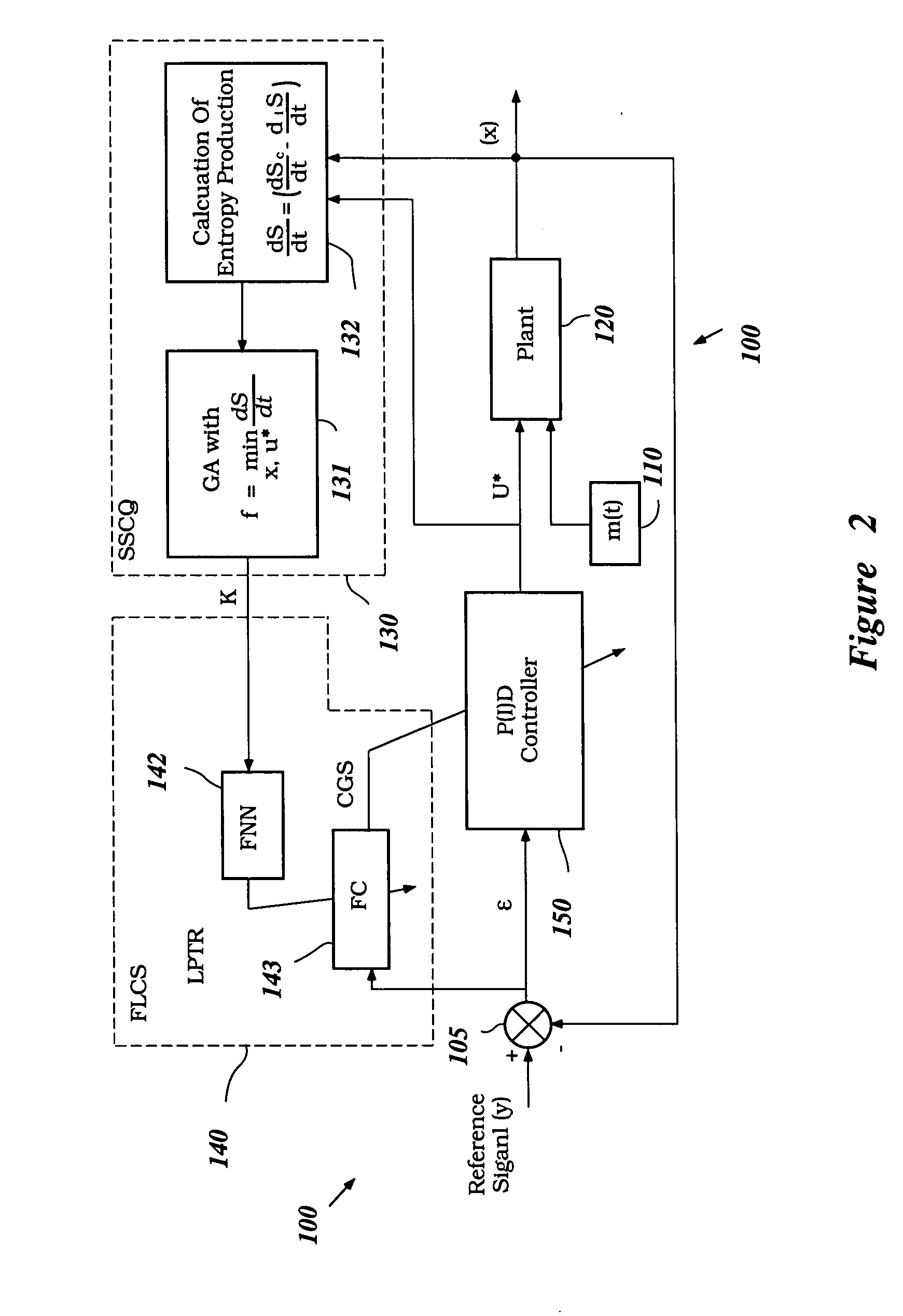
System and method for nonlinear dynamic control based on soft computing with discrete constraints
InactiveUS6950712B2Minimizes entropyMaximizes sensor information contentDigital data processing detailsAnimal undercarriagesNon linear dynamicMinimum entropy
A control system using a genetic analyzer based on discrete constraints is described. In one embodiment, a genetic algorithm with step-coded chromosomes is used to develop a teaching signal that provides good control qualities for a controller with discrete constraints, such as, for example, a step-constrained controller. In one embodiment, the control system uses a fitness (performance) function that is based on the physical laws of minimum entropy. In one embodiment, the genetic analyzer is used in an off-line mode to develop a teaching signal for a fuzzy logic classifier system that develops a knowledge base. The teaching signal can be approximated online by a fuzzy controller that operates using knowledge from the knowledge base. The control system can be used to control complex plants described by nonlinear, unstable, dissipative models. In one embodiment, the step-constrained control system is configured to control stepping motors.
Owner:YAMAHA MOTOR CO LTD
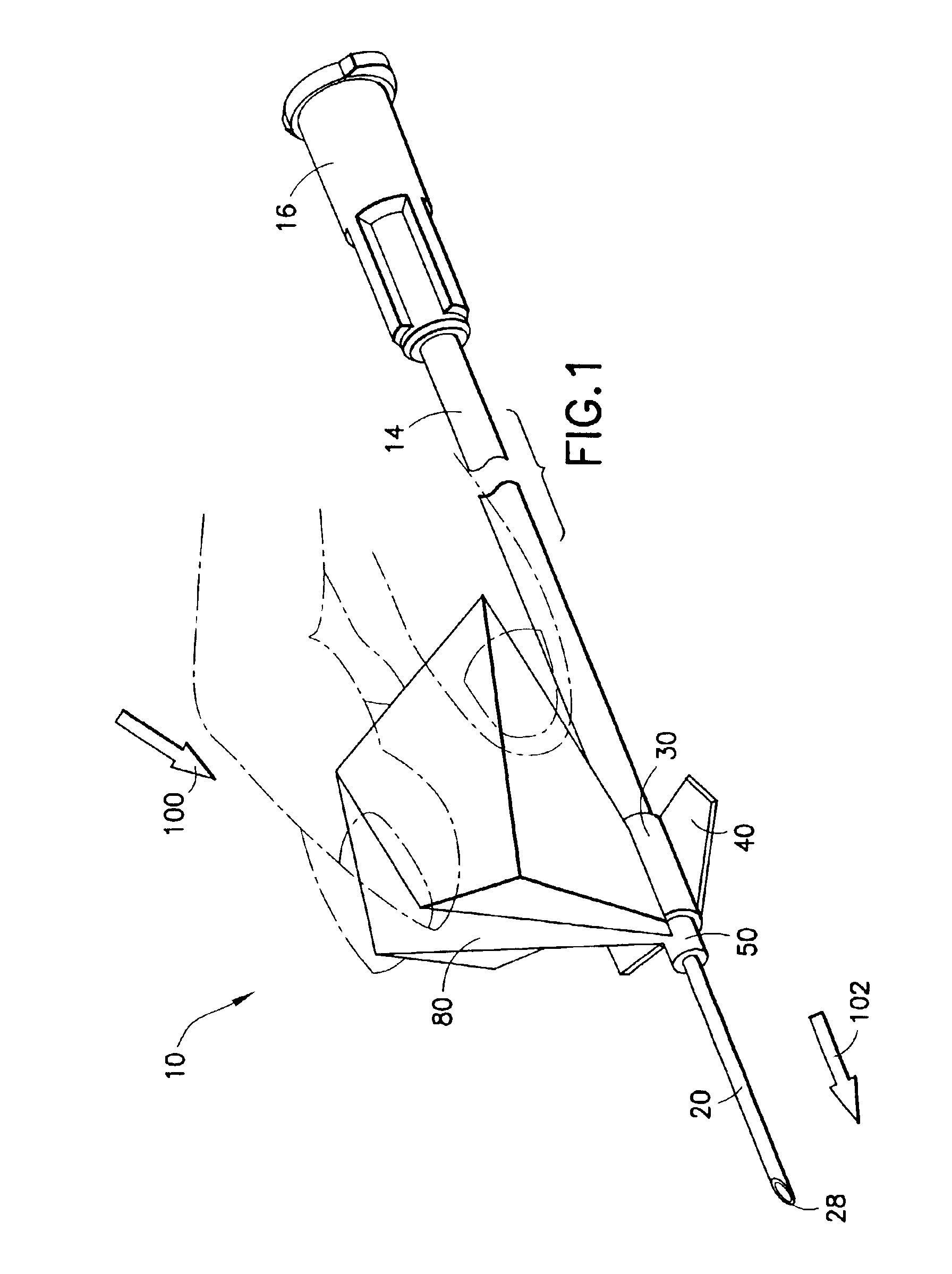
Blood collection agency
InactiveUS6936036B2Secure and effective shieldingSimple and inexpensive to manufactureSensorsPackagingBlood collectionMedical device
The present invention is directed to a low cost shieldable safety needle assembly. The assembly includes a needle cannula and a tip guard axially movable along the needle cannula through a drive mechanism. The drive mechanism may be interconnected between the tip guard and the needle cannula through a hub. The tip guard is axially movable along the needle cannula from a proximal position substantially adjacent a proximal end of the needle cannula at the hub, to a distal position in which the tip guard protectively surrounds the distal end of the needle cannula, thus effectively shielding the puncture tip of the needle. The drive mechanism is a unitary structure which is capable of maintaining a first self-supporting shape for maintaining the tip guard in the proximal position and is deflectable from the first self-supporting shape to a second extended shape in which the tip guard is moved to the distal position. The drive mechanism may be a rigid flexible planar sheet material which includes a plurality of folds for defining the self-supporting shape. The needle assembly may include structure for mating with a hypodermic syringe, a blood collection set, or other medical device.
Owner:BECTON DICKINSON & CO
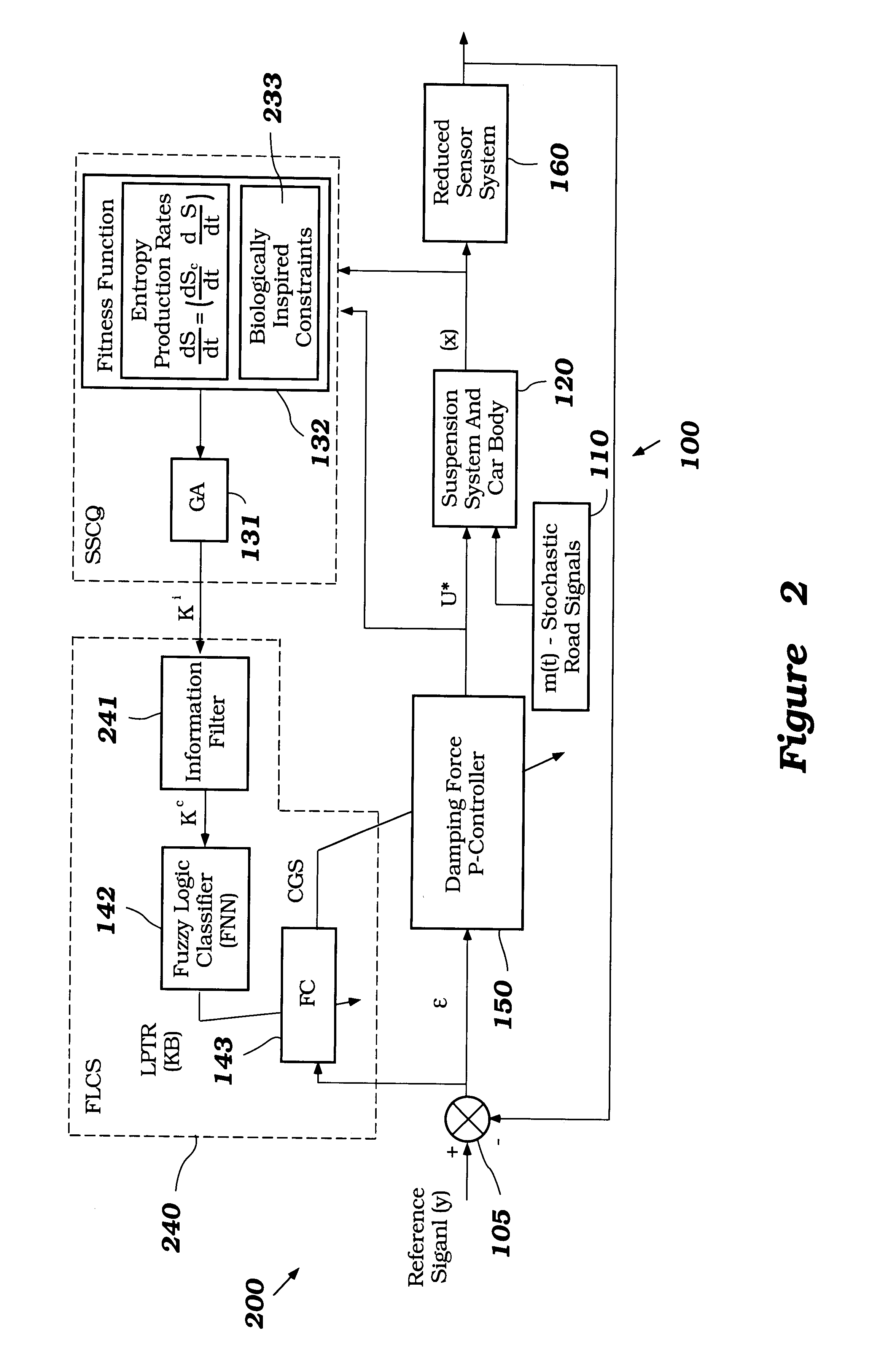
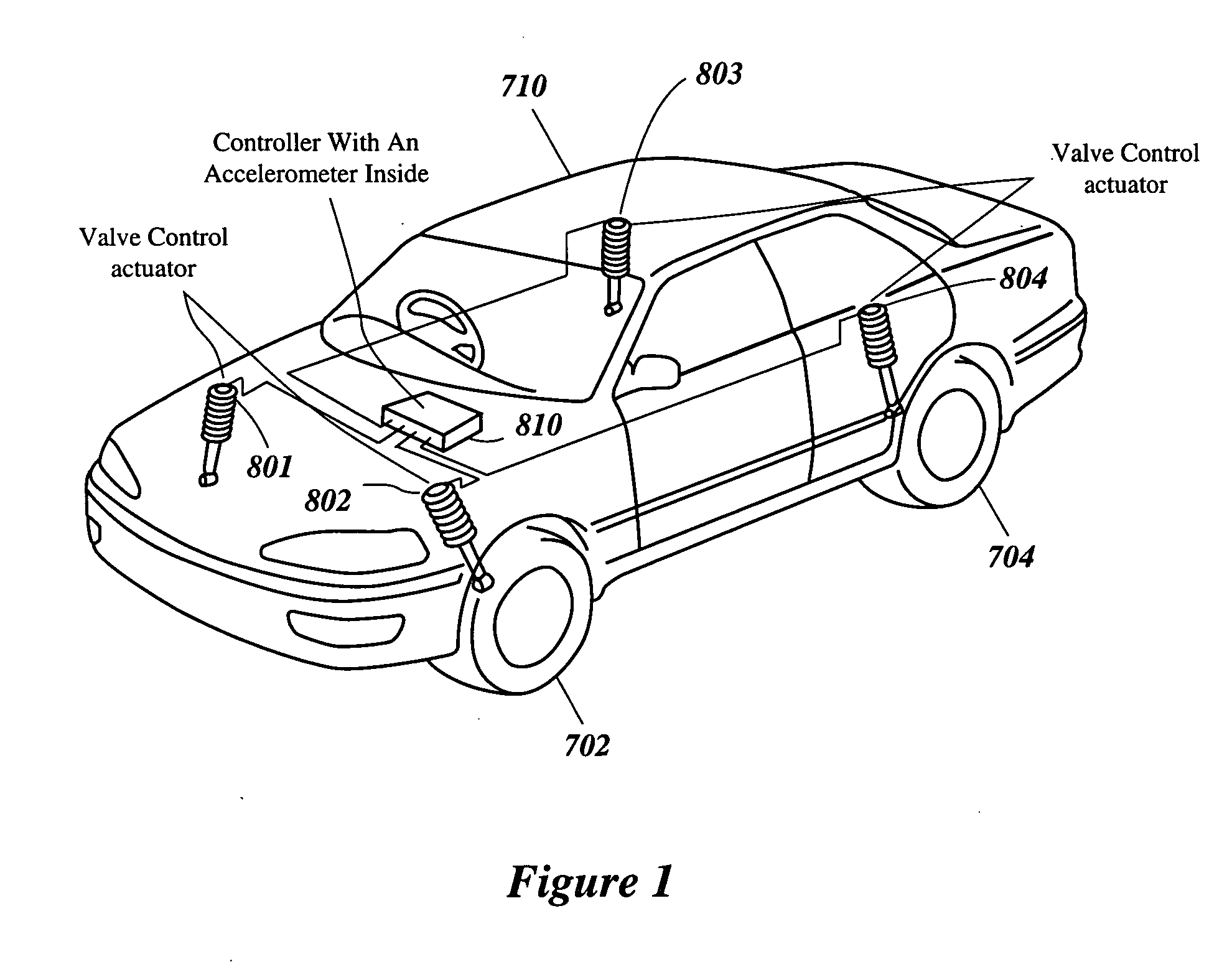
Intelligent mechatronic control suspension system based on soft computing
InactiveUS6701236B2Maximises informationLightweight productionSpringsAnimal undercarriagesControl systemFuzzy control system
A control system for optimizing a shock absorber having a non-linear kinetic characteristic is described. The control system uses a fitness (performance) function that is based on the physical laws of minimum entropy and biologically inspired constraints relating to mechanical constraints and / or rider comfort, driveability, etc. In one embodiment, a genetic analyzer is used in an off-line mode to develop a teaching signal. An information filter is used to filter the teaching signal to produce a compressed teaching signal. The compressed teaching signal can be approximated online by a fuzzy controller that operates using knowledge from a knowledge base. In one embodiment, the control system includes a learning system, such as a neural network that is trained by the compressed training signal. The learning system is used to create a knowledge base for use by an online fuzzy controller. The online fuzzy controller is used to program a linear controller.
Owner:YAMAHA MOTOR CO LTD
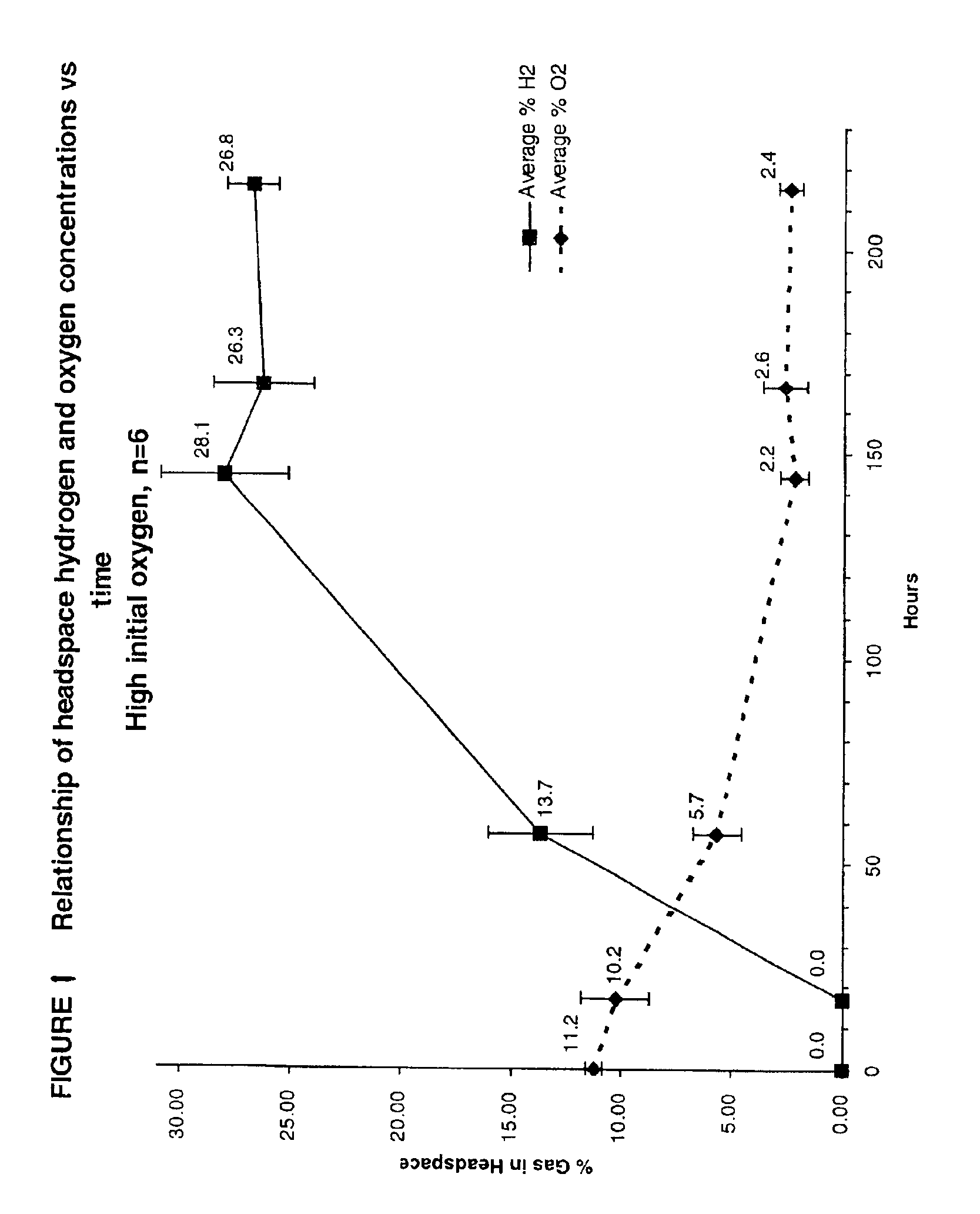
Process for generation of hydrogen gas from various feedstocks using thermophilic bacteria
A method for producing hydrogen gas is provided comprising selecting a bacteria from the Order Thermotogales, subjecting the bacteria to a feedstock and to a suitable growth environment having an oxygen concentration below the oxygen concentration of water in equilibrium with air; and maintaining the environment at a predetermined pH and at a temperature of at least approximately 45° C. for a time sufficient to allow the bacteria to metabolize the feedstock.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
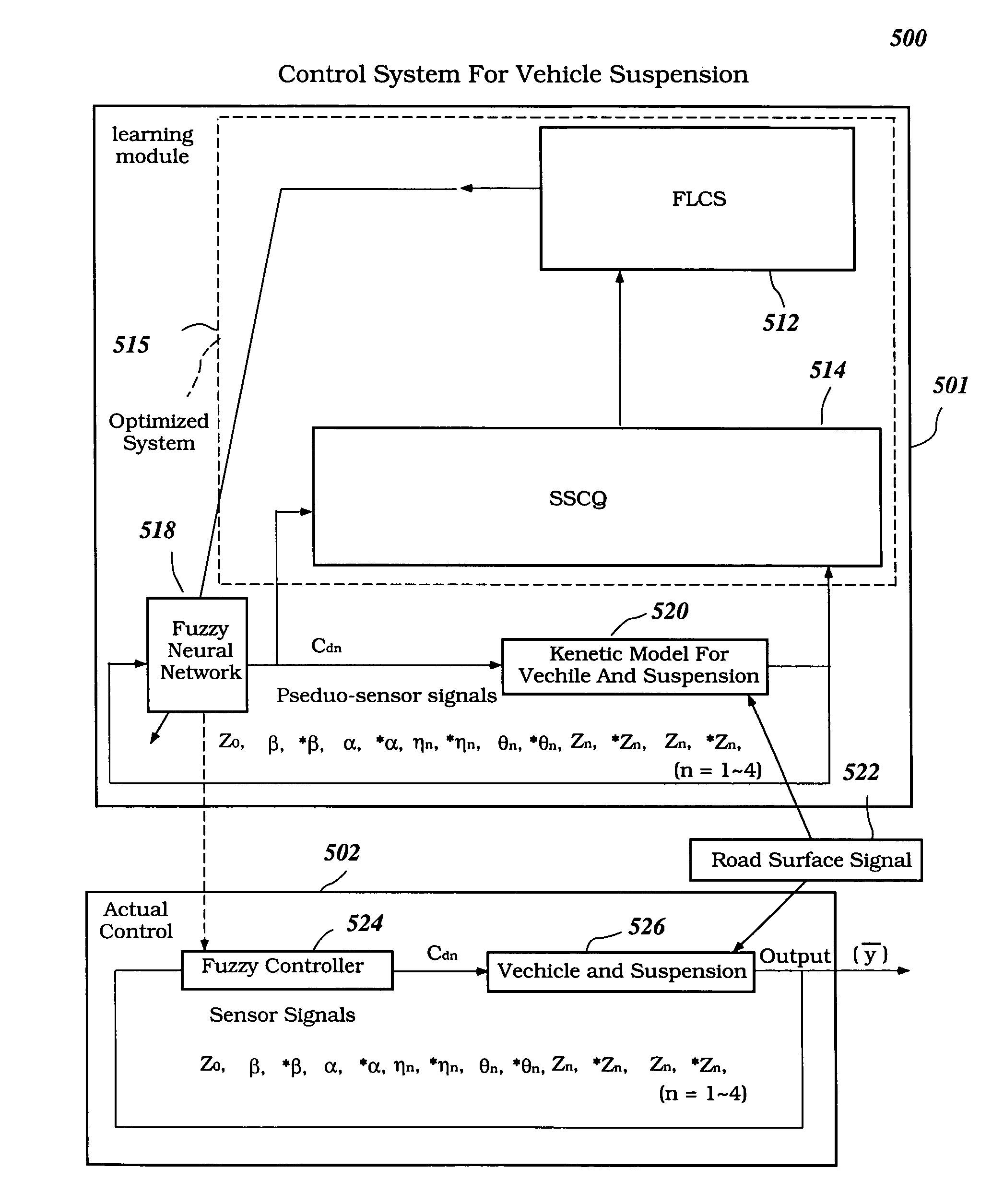
Intelligent electronically-controlled suspension system based on soft computing optimizer
InactiveUS20060293817A1Near-optimal FNNMaximises informationDigital data processing detailsAnimal undercarriagesInput/outputSoft computing
A Soft Computing (SC) optimizer for designing a Knowledge Base (KB) to be used in a control system for controlling a suspension system is described. The SC optimizer includes a fuzzy inference engine based on a Fuzzy Neural Network (FNN). The SC Optimizer provides Fuzzy Inference System (FIS) structure selection, FIS structure optimization method selection, and teaching signal selection and generation. The user selects a fuzzy model, including one or more of: the number of input and / or output variables; the type of fuzzy inference model (e.g., Mamdani, Sugeno, Tsukamoto, etc.); and the preliminary type of membership functions. A Genetic Algorithm (GA) is used to optimize linguistic variable parameters and the input-output training patterns. A GA is also used to optimize the rule base, using the fuzzy model, optimal linguistic variable parameters, and a teaching signal. The GA produces a near-optimal FNN. The near-optimal FNN can be improved using classical derivative-based optimization procedures. The FIS structure found by the GA is optimized with a fitness function based on a response of the actual suspension system model of the controlled suspension system. The SC optimizer produces a robust KB that is typically smaller that the KB produced by prior art methods.
Owner:YAMAHA MOTOR CO LTD
Corrugated carton forming technology
InactiveCN107089036ALightweight productionIncrease productivityMechanical working/deformationBox making operationsPaperboardCarton
The invention discloses a corrugated carton forming technology and belongs to the technical field of agricultural appliances. The corrugated carton forming technology comprises the following steps of producing a corrugated paperboard, wherein corrugation pressing and veneering are conducted on a corrugated medium; conducting die cutting, wherein printing, slotting, corner cutting and creasing are conducted on the corrugated paperboard according to a preset shape; and conducting folding and binding, wherein sticking and binding forming are conducted on the corrugated paperboard through a folding and sticking machine and a binding machine. According to the corrugated carton forming technology, production, die cutting, folding and binding are conducted on the corrugated carton, so that production of the corrugated carton is lightweight, the production efficiency is higher, and the structural strength is improved.
Owner:广东中穗纸品有限公司
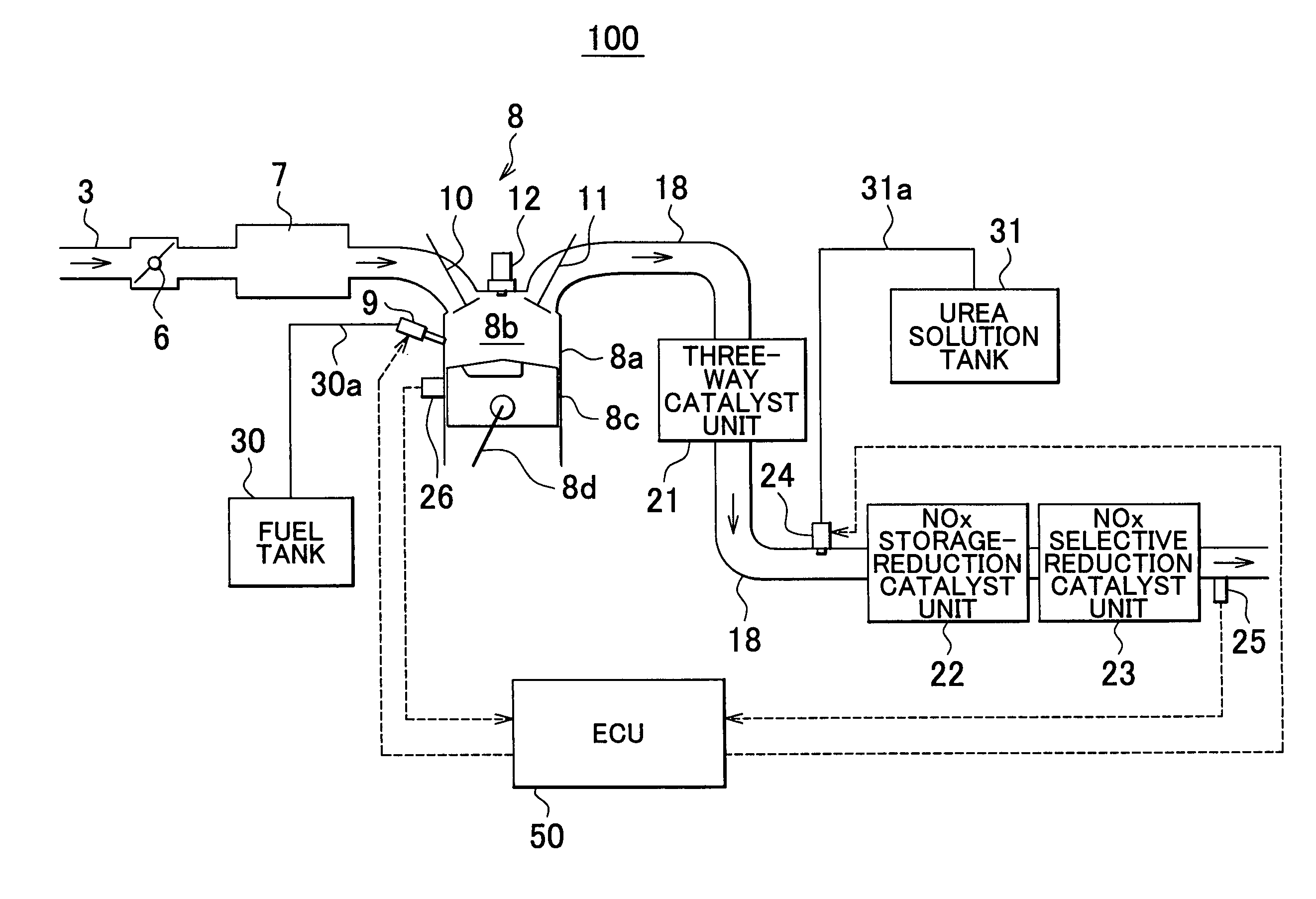
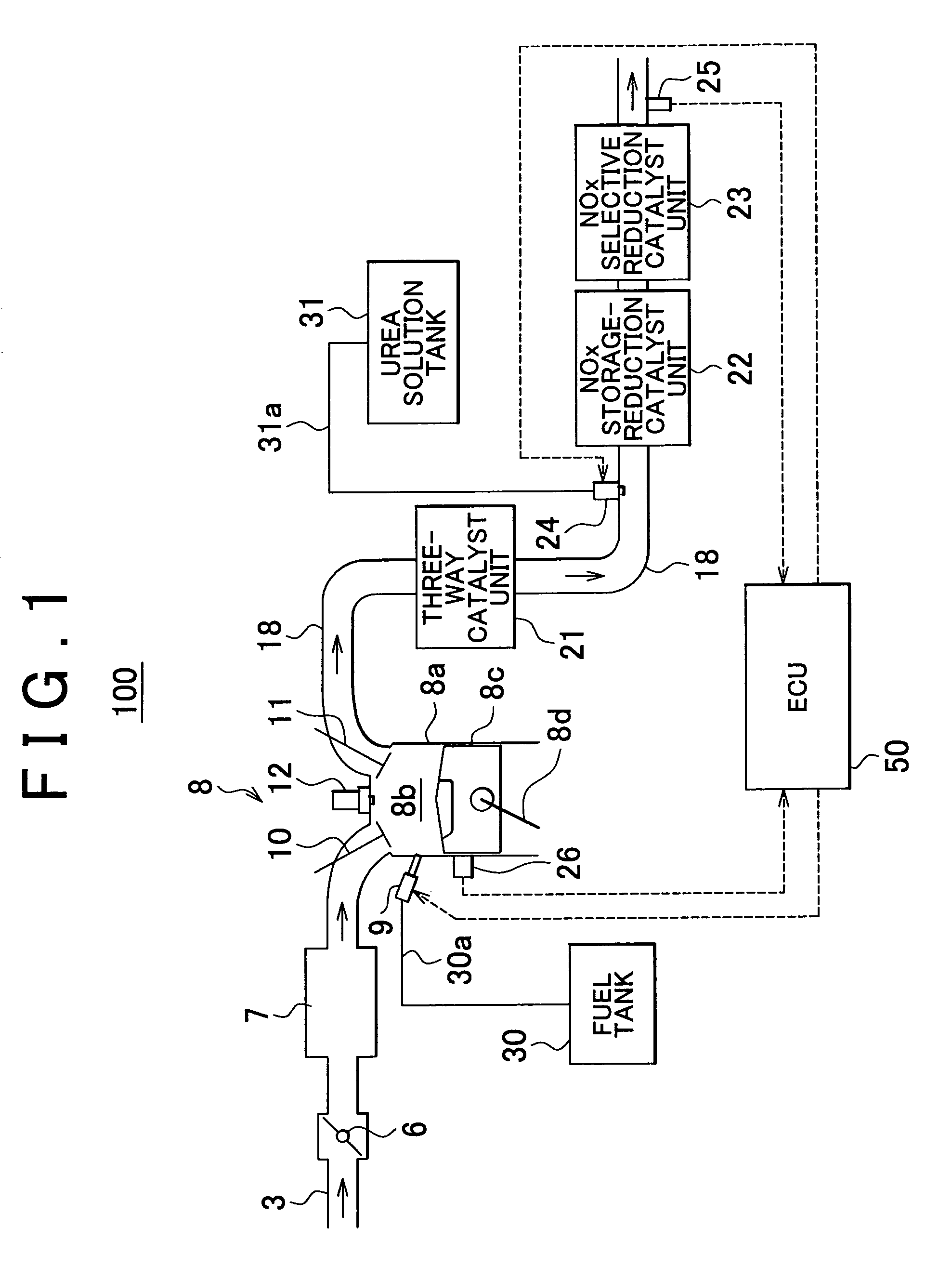
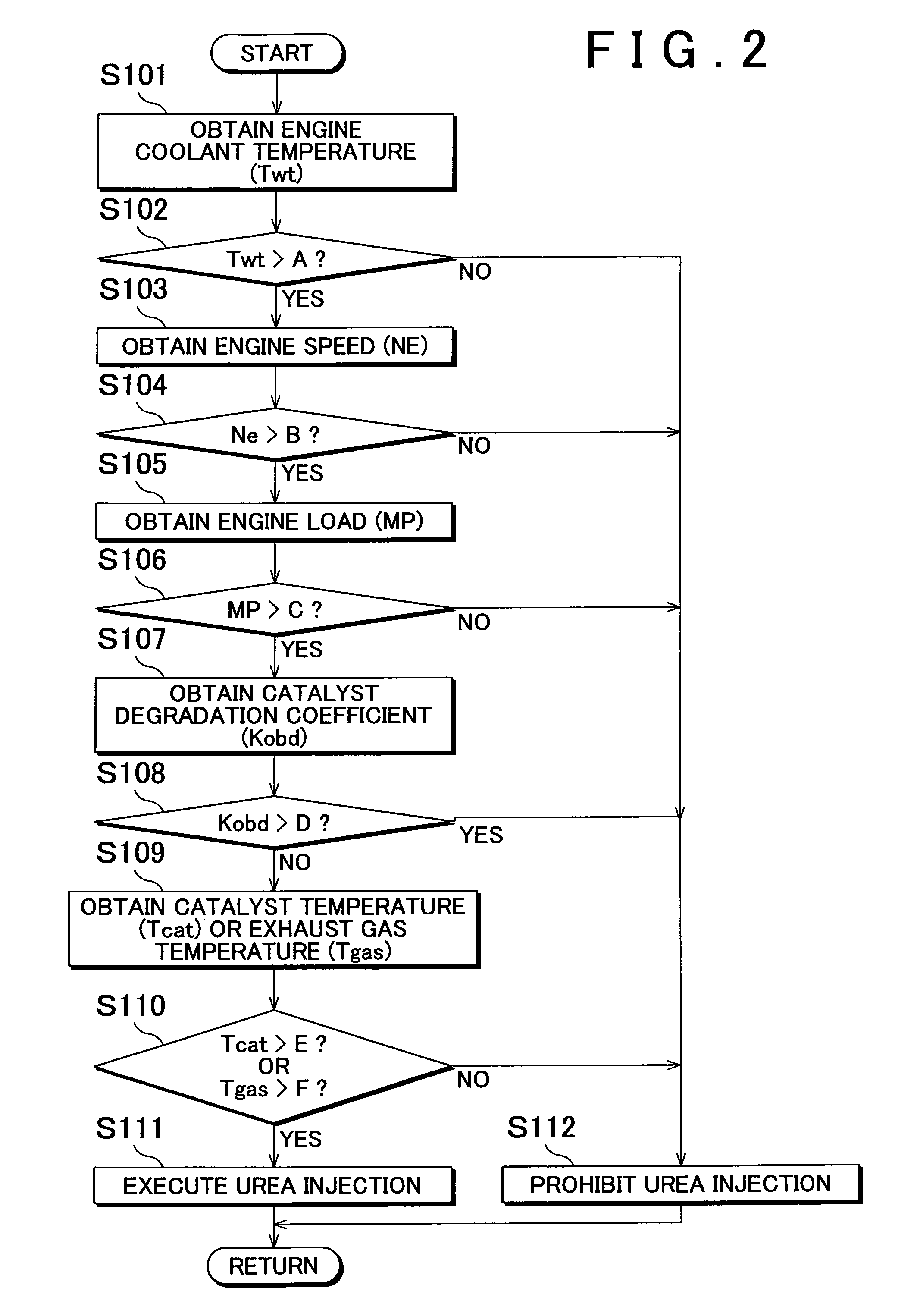
Internal combustion engine exhaust gas purification apparatus and method for controlling same
InactiveUS7892508B2Lightweight productionContainment leakNitrogen compoundsInternal combustion piston enginesInternal combustion engineSelective reduction
An internal combustion engine exhaust gas purification apparatus purifies exhaust gas using a NOx storage-reduction catalyst unit and a NOx selective reduction catalyst unit. The NOx selective reduction catalyst unit is provided downstream of the NOx storage-reduction catalyst unit in an exhaust gas passage. An urea injecting mechanism, for example an injection valve, injects urea into the exhaust gas passage. An urea injection controller prohibits urea injection from the urea injecting mechanism if at least one of the temperatures of the NOx storage-reduction catalyst unit and the NOx selective reduction catalyst unit is equal to or lower than a reference temperature. As such, production of cyanic acid is minimized, and therefore leaks of cyanic acid from the NOx storage-reduction catalyst unit and / or the NOx selective reduction catalyst unit can be suppressed.
Owner:TOYOTA JIDOSHA KK
Apparatus and method for repair of spinal cord injury
InactiveUS6975907B2Lightweight productionDissipate any toxic productSpinal electrodesExternal electrodesPower flowMedicine
An apparatus for stimulating regeneration and repair of damaged spinal nerves, comprising at least two electrodes placed intravertebrally near the site of spinal axon injury and delivering DC current thereto. A method for stimulating regeneration and repair of damaged spinal nervous tissue, comprising placing electrodes intravertebrally near the site of spinal cord injury and applying DC current at a level sufficient to induce regeneration and repair of damaged spinal axons but less than the current level at which tissue toxicity occurs.
Owner:DYNAMED SYST
X-ray interferometric imaging system
ActiveUS20160066870A1Lightweight productionIncrease brightnessImaging devicesMaterial analysis using wave/particle radiationSoft x rayGrating
An x-ray interferometric imaging system in which the x-ray source comprises a target having a plurality of structured coherent sub-sources of x-rays embedded in a thermally conducting substrate. The structures may be microstructures with lateral dimensions measured on the order of microns, and in some embodiments, the structures are arranged in a regular array.The system additionally comprises a beam-splitting grating G1 that establishes a Talbot interference pattern, which may be a π or π / 2 phase-shifting grating, an x-ray detector to convert two-dimensional x-ray intensities into electronic signals, and in some embodiments, also comprises an additional analyzer grating G2 that may be placed in front of the detector to form additional interference fringes. Systems may also include a means to translate and / or rotate the relative positions of the x-ray source and the object under investigation relative to the beam splitting grating and / or the analyzer grating for tomography applications.
Owner:SIGRAY INC
X-ray interferometric imaging system
ActiveUS10349908B2Lightweight productionIncrease brightnessImaging devicesMaterial analysis using wave/particle radiationSoft x rayGrating
An x-ray interferometric imaging system includes an x-ray source with a target having a plurality of discrete structures arranged in a periodic pattern. The system further includes a beam-splitting x-ray grating, a stage configured to hold an object to be imaged, and an x-ray detector having a two-dimensional array of x-ray detecting elements. The object is positioned between the beam-splitting x-ray grating and the x-ray detector, the x-ray detector is positioned to detect the x-rays diffracted by the beam-splitting x-ray grating and perturbed by the object to be imaged.
Owner:SIGRAY INC
Two part antimicrobial boot
ActiveUS20080125728A1Minimize productionEnhance efficacyBiocideTetracycline active ingredientsAntimicrobialBiomedical engineering
A system comprising an implantable medical device and a second polymeric layer configured to be disposed on or about the implantable medical device is described. The device includes a first polymeric layer into which a first therapeutic agent is incorporated. A second therapeutic agent is incorporated into the second polymeric layer. The device is sterilized by a first sterilization method. The second polymeric layer is sterilized by a second sterilization method. A method for making a sterile implantable medical system is also described. The method includes incorporating a first therapeutic agent in a first polymeric material and disposing the first polymeric material on or about an implantable medical device. The first polymeric material and the implantable medical device are sterilized by a first sterilization method. The method further includes incorporating a second therapeutic material in a second polymeric material and disposing the second polymeric material on or about the sterilized first polymeric material and implantable medical device. The second polymeric material is sterilized by a second sterilization method.
Owner:MEDTRONIC INC
Apparatus and method for repair of spinal cord injury
InactiveUS20060167527A1Sufficient durationLightweight productionInternal electrodesExternal electrodesMedicineTissue toxicity
An apparatus for stimulating regeneration and repair of damaged spinal nerves, comprising at least two electrodes placed intravertebrally near the site of spinal neurite injury and delivering direct current thereto. A method for stimulating regeneration and repair of damaged spinal nervous tissue, comprising placing electrodes intravertebrally near the site of spinal cord injury and applying direct current at a level sufficient to induce regeneration and repair of damaged spinal neurites but less than the current level at which tissue toxicity occurs.
Owner:DYNAMED SYST
Process for fluid catalytic cracking of hydrocarbon feedstocks with high levels of basic nitrogen
InactiveUS7744745B2Increase loopKeep in circulationCatalytic crackingTreatment with plural parallel cracking stages onlyNitrogenLow volume
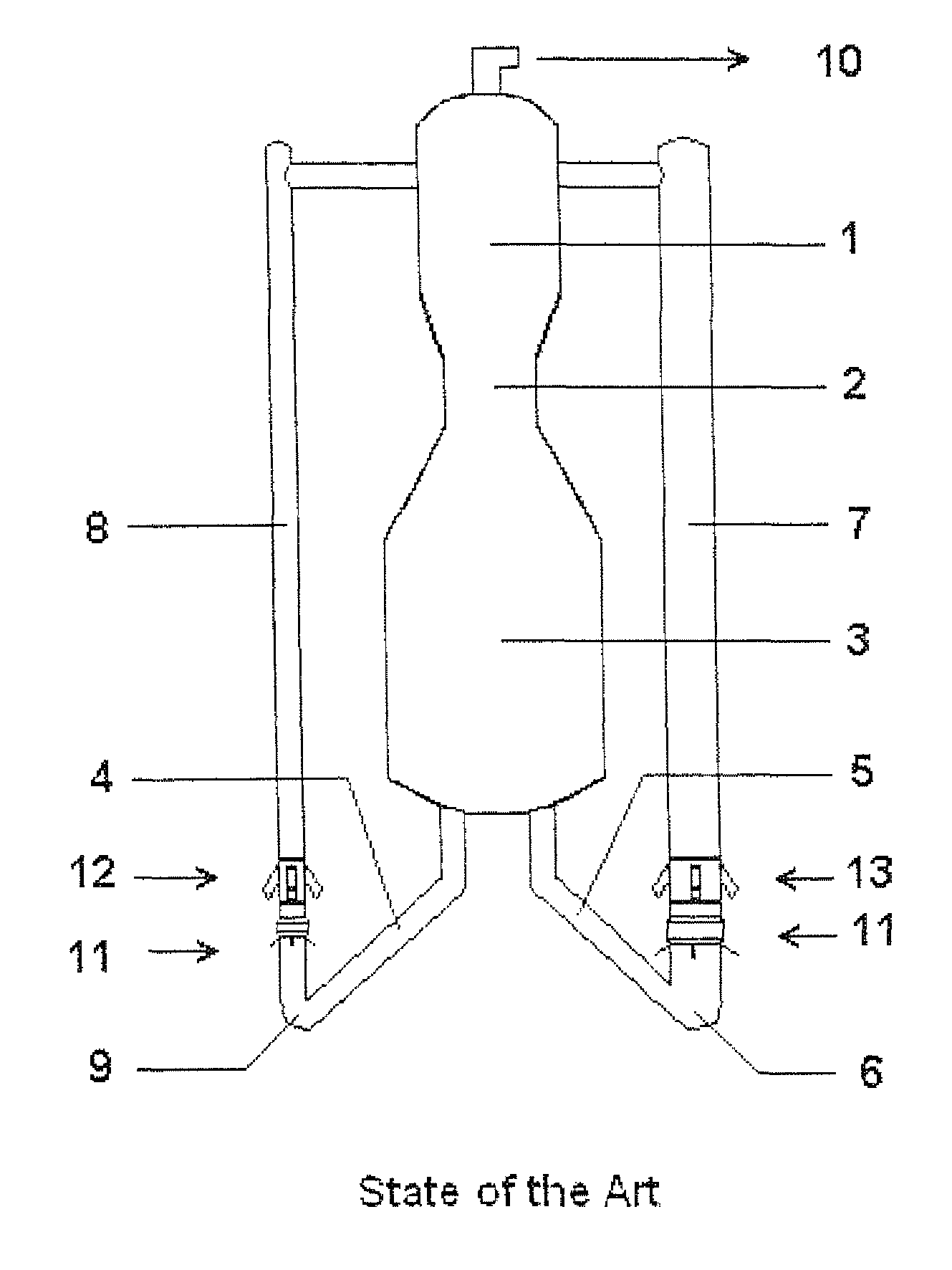
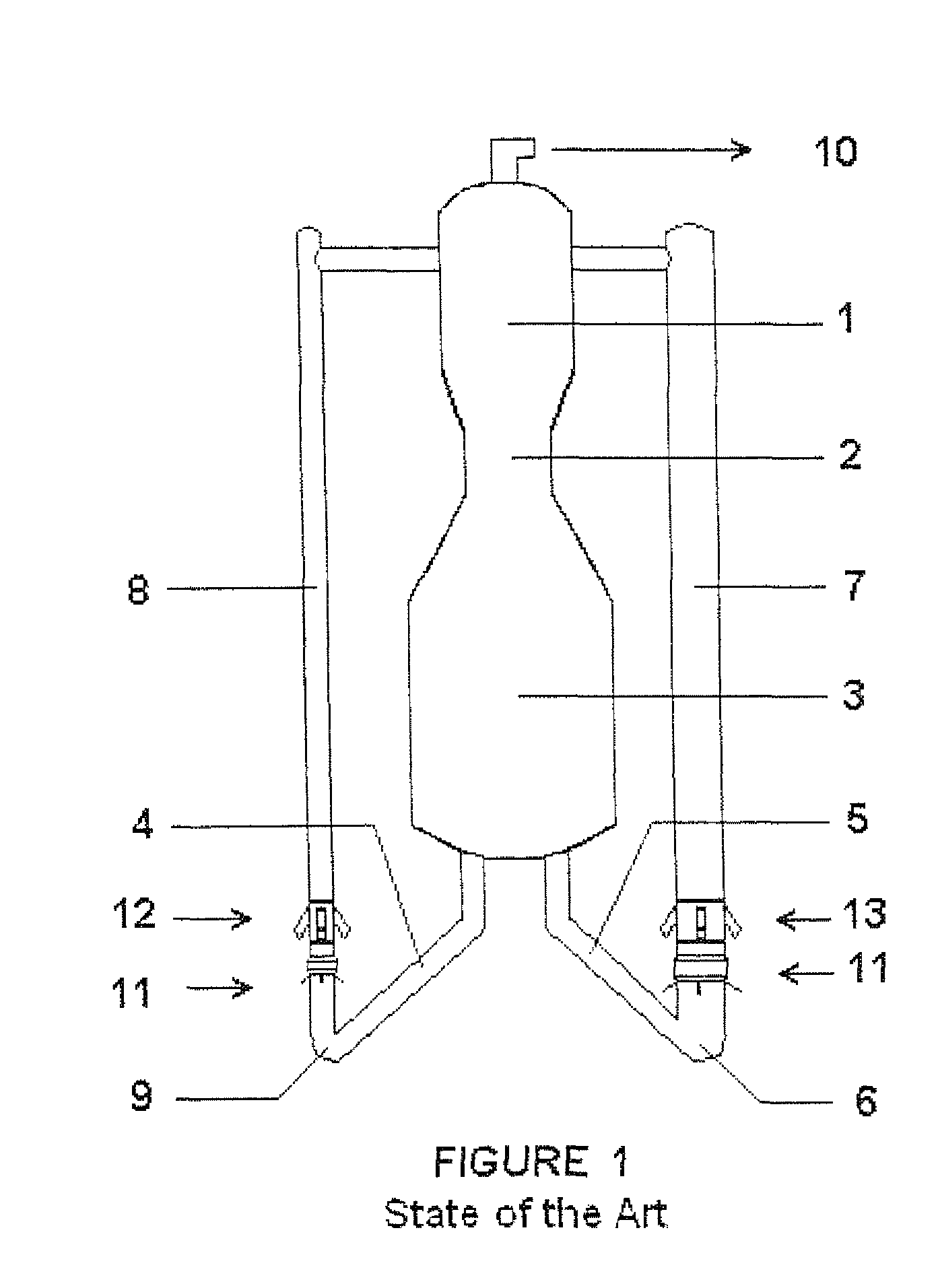
A process is described for fluid catalytic cracking of hydrocarbons with high levels of basic nitrogen, where hydrocarbon feedstocks A and B with different levels of basic nitrogen are injected in a segregated fashion, into different risers of a multiple riser FCCU that possesses at least two risers. The injection of the feedstocks is made in such a way that feedstock A, to be injected in the riser with greater volume—main riser 7—possessing a level of basic nitrogen at least 200 ppm lower than the level of feedstock B to be injected into the riser with lower volume—secondary riser (8).
Owner:PETROLEO BRASILEIRO SA (PETROBRAS)
Method and Catalyst for the Transalkylation/Dealkylation of Organic Compounds
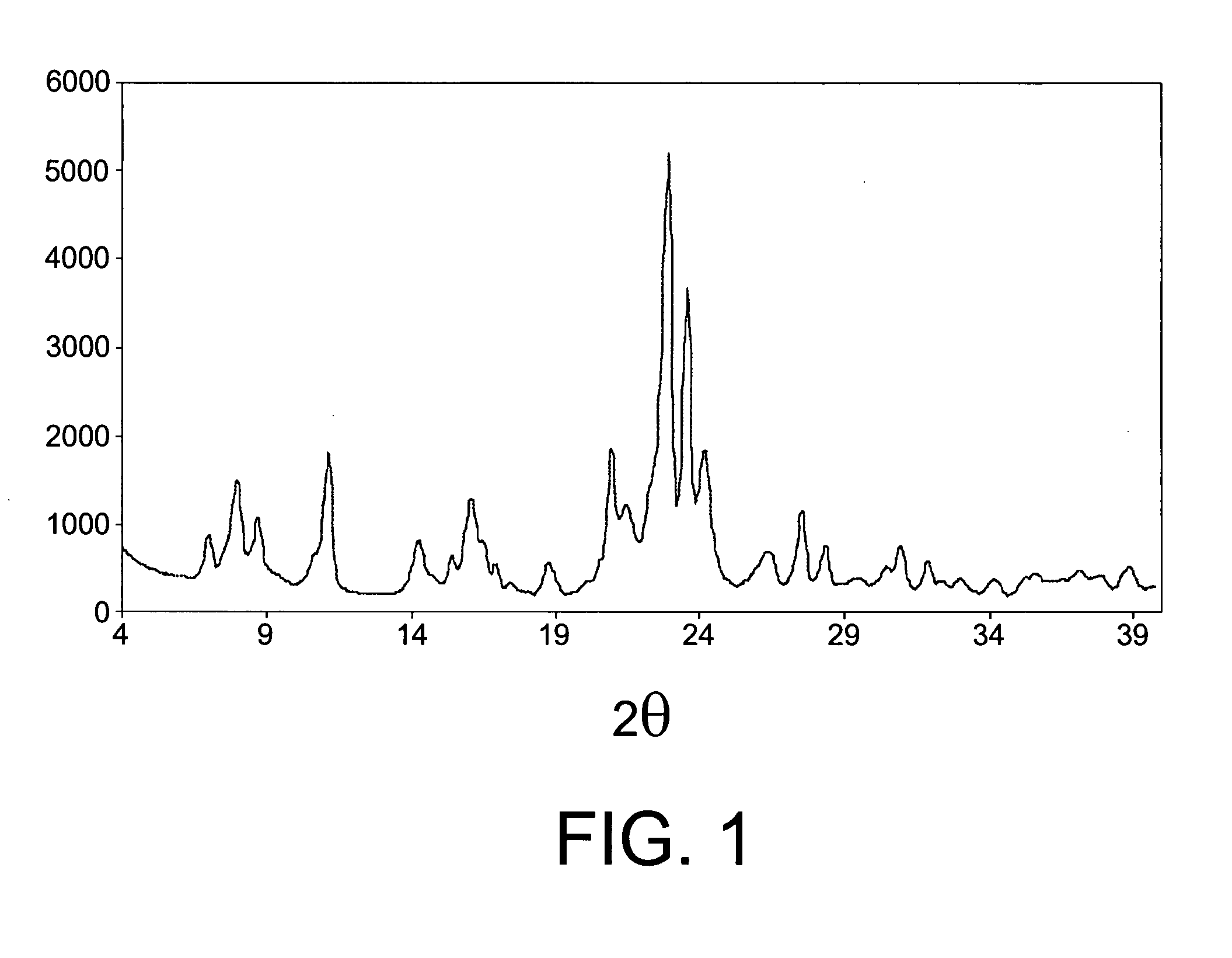
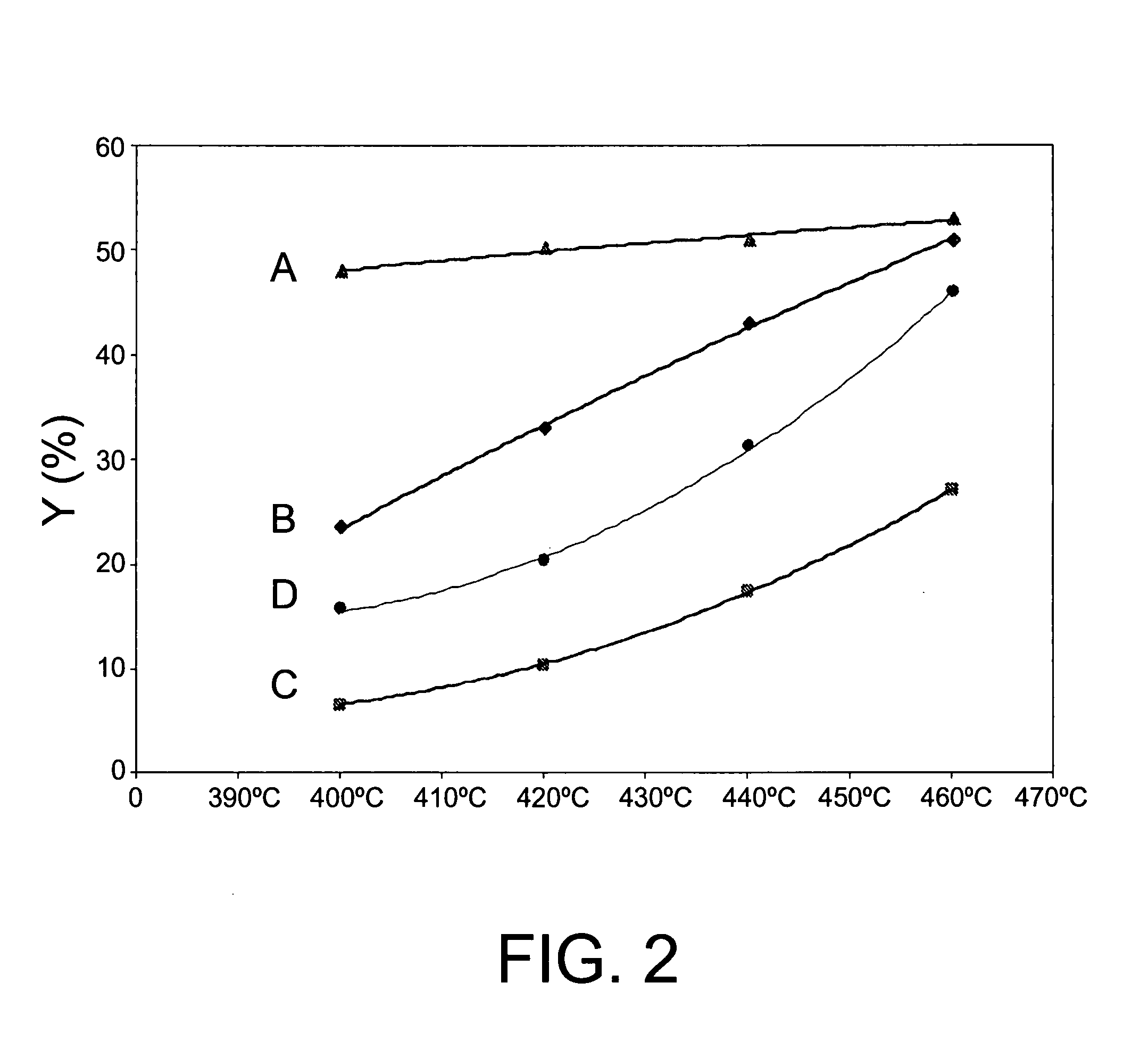
InactiveUS20080021253A1Improve catalytic selectivityMaximize productionHydrocarbon by isomerisationMolecular sieve catalystsCatalytic methodCrystal structure
The invention relates to a catalytic method for the transalkylation / dealkylation of organic compounds, consisting in bringing a supply comprising organic compounds into contact with a catalyst containing a first zeolitic component that is selected from among: a) one or more zeolites having crystalline structure ITQ-13; b) one or more zeolites having crystalline structure ITQ-13, which are modified either by means of selectivation or with the incorporation of one or more metals, or both; and c) a mixture of a) and b). The invention also relates to a catalyst comprising one or more modified zeolites having crystalline structure ITQ-13.
Owner:CORMA CANOS AVELINO +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com