Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4830results about How to "Relieve symptoms" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
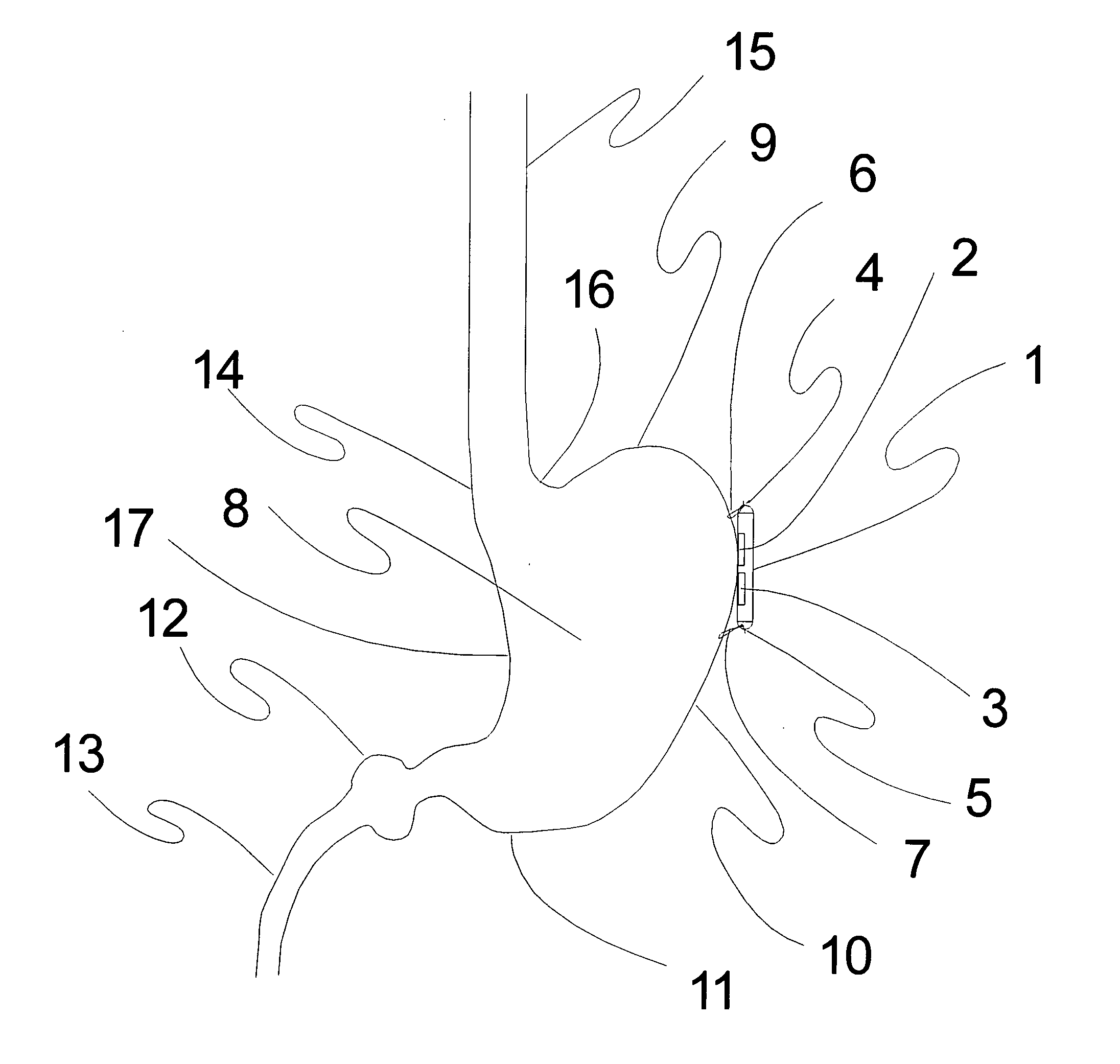
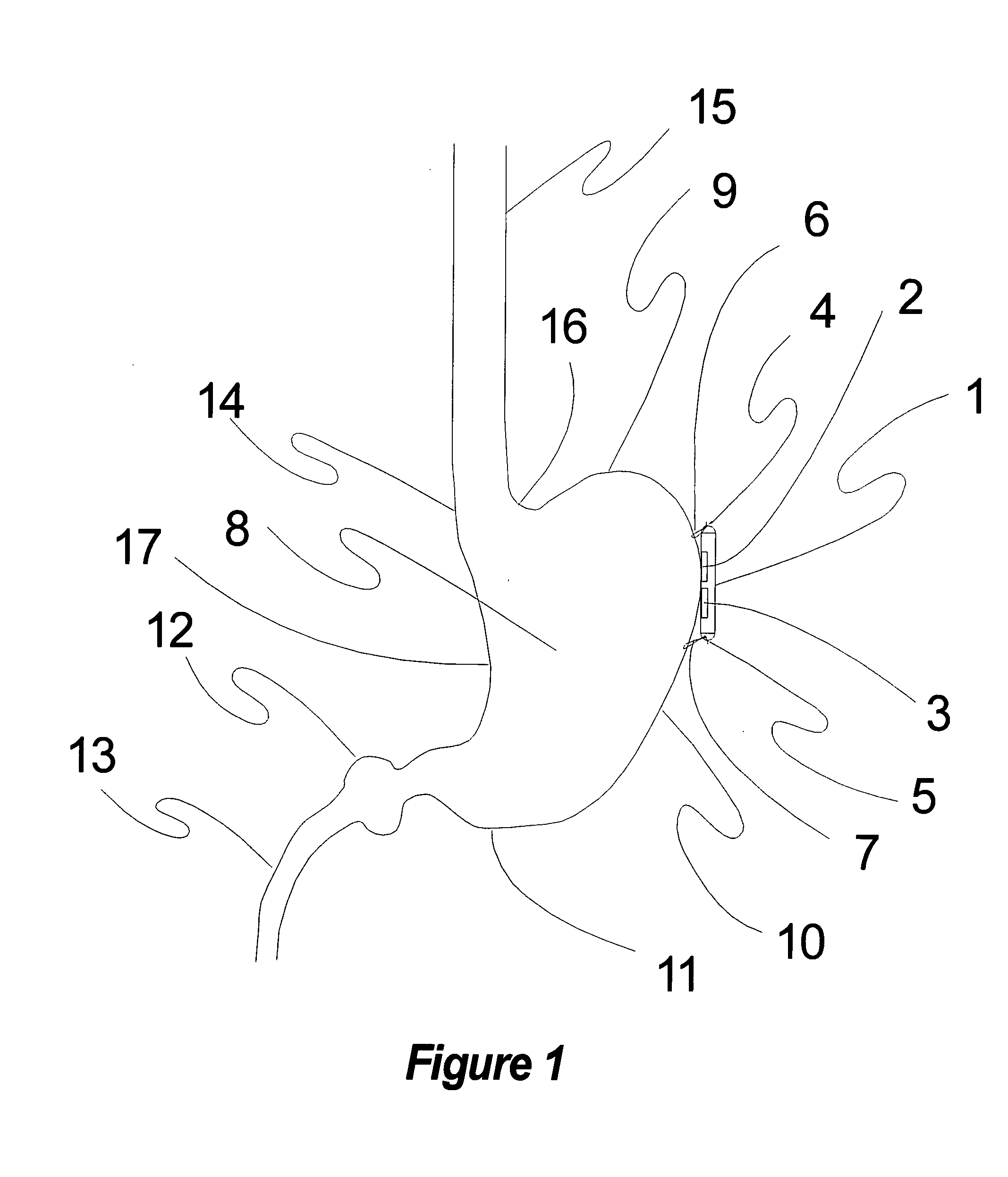
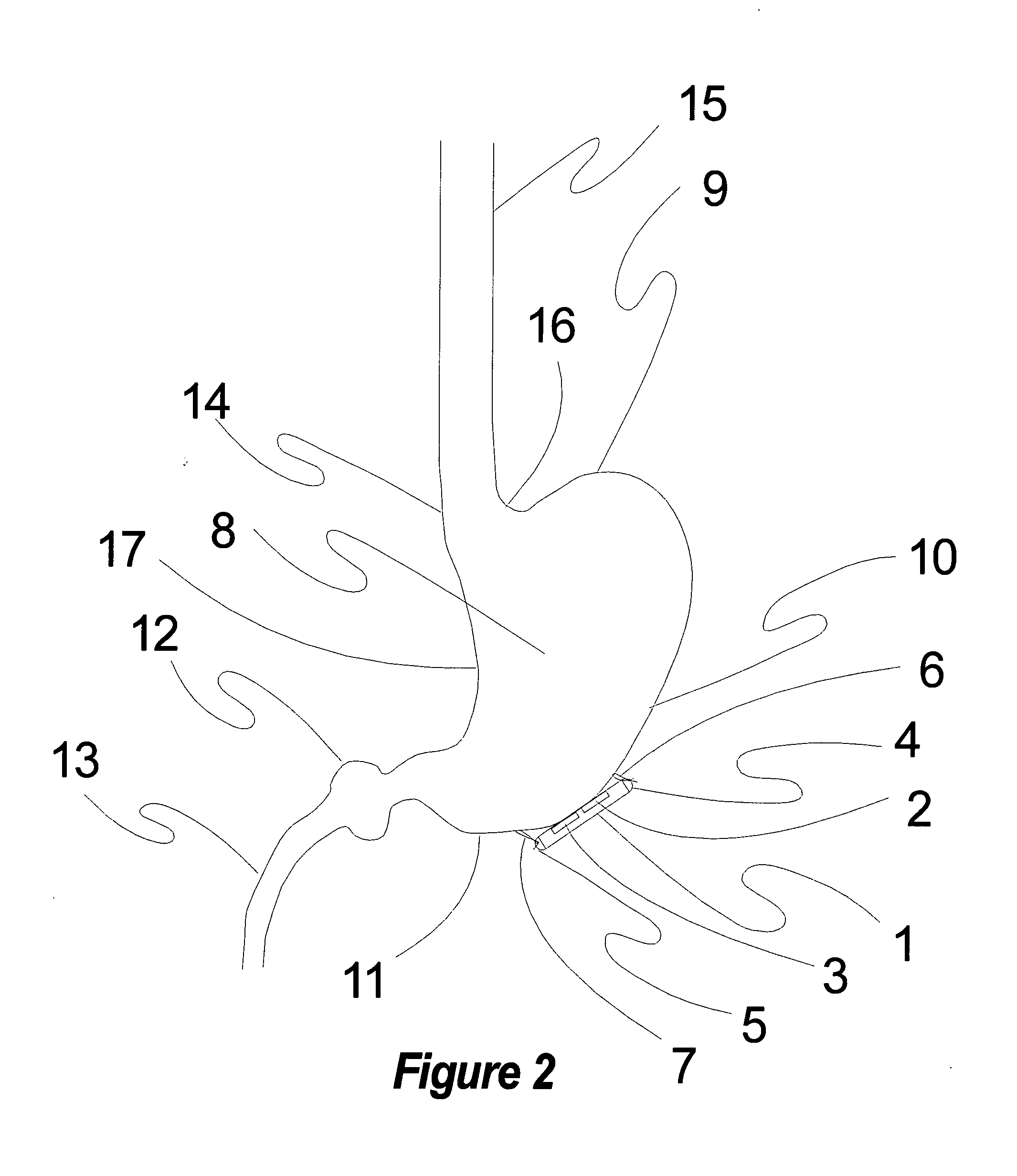
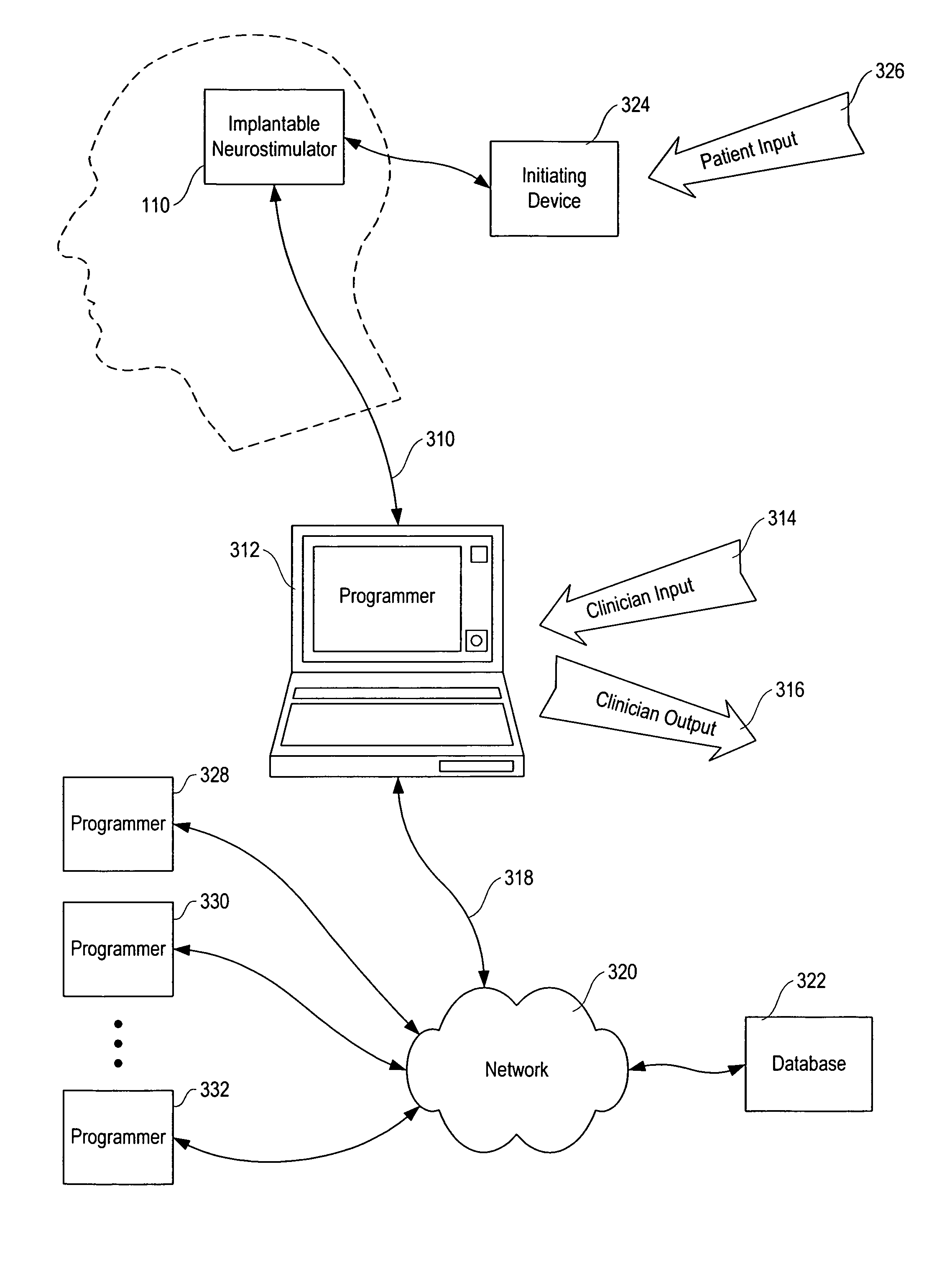
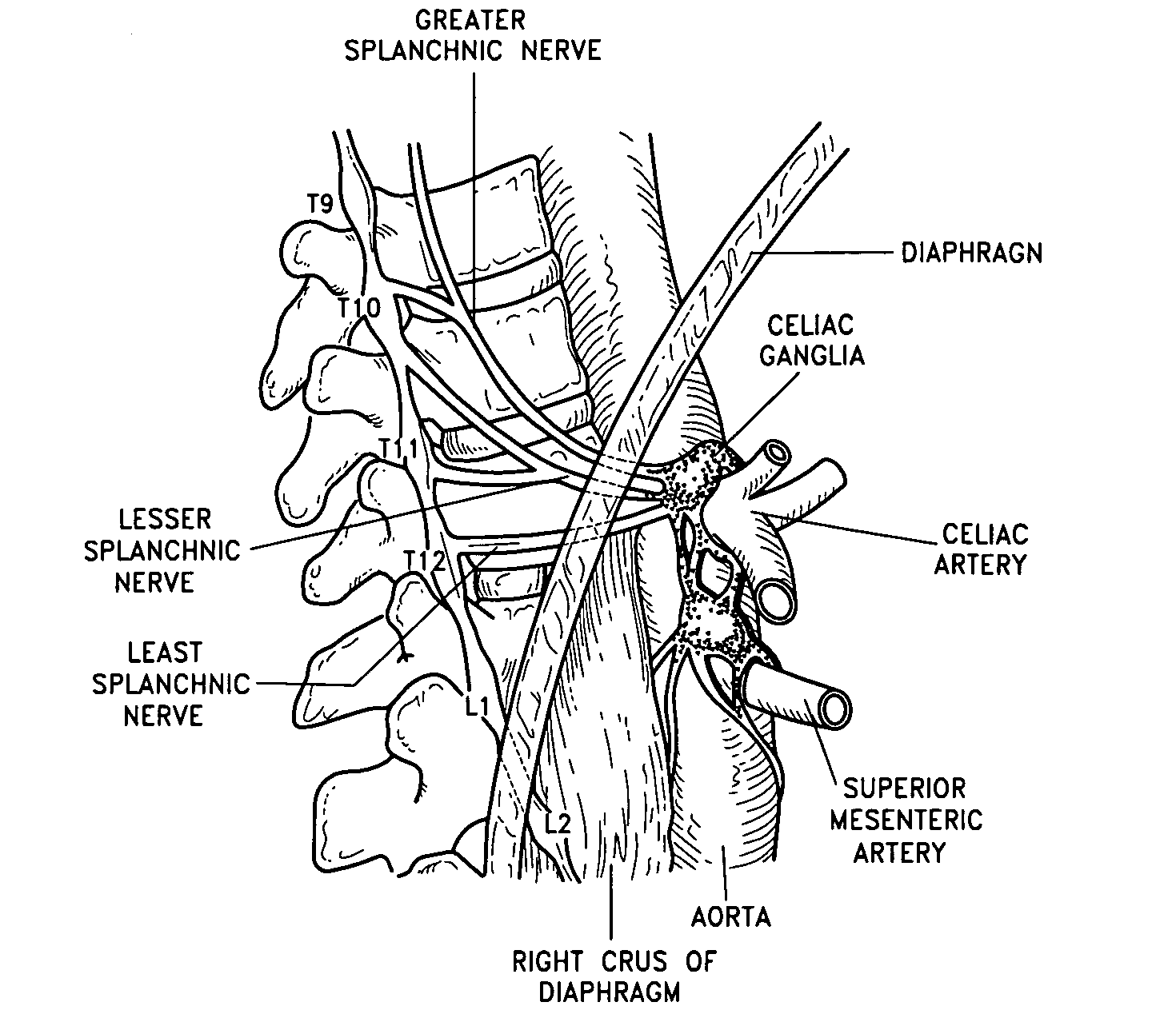
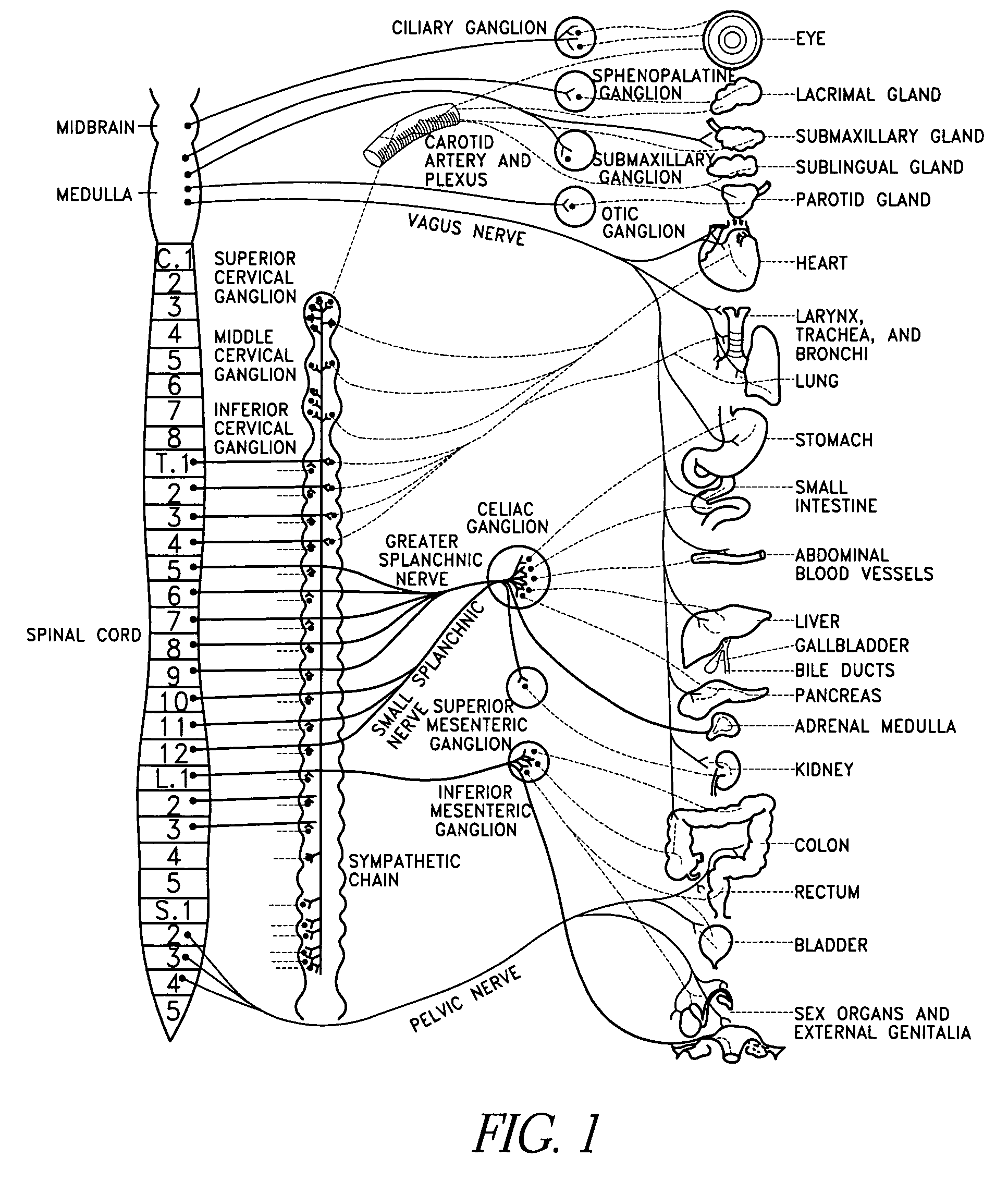
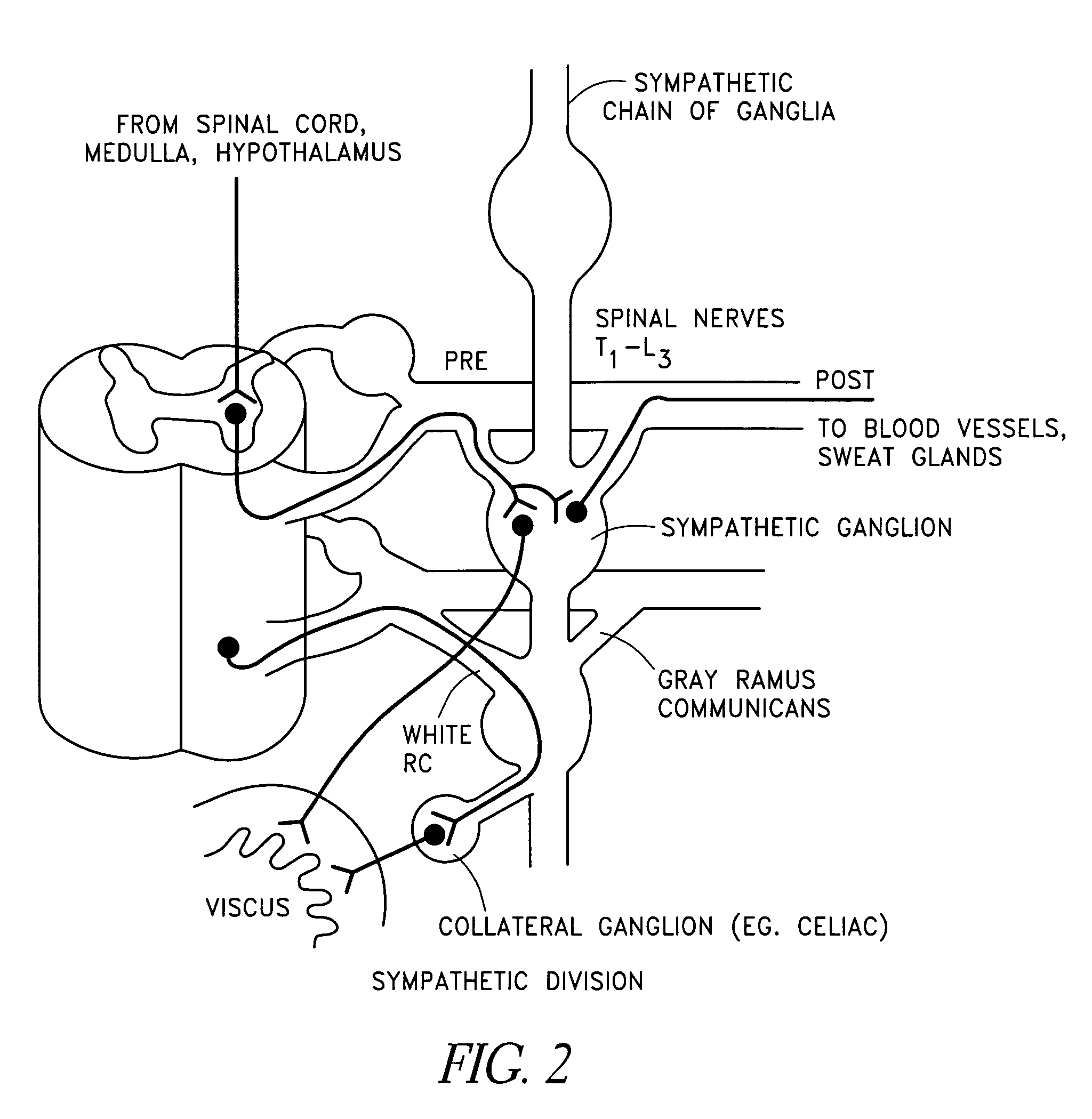
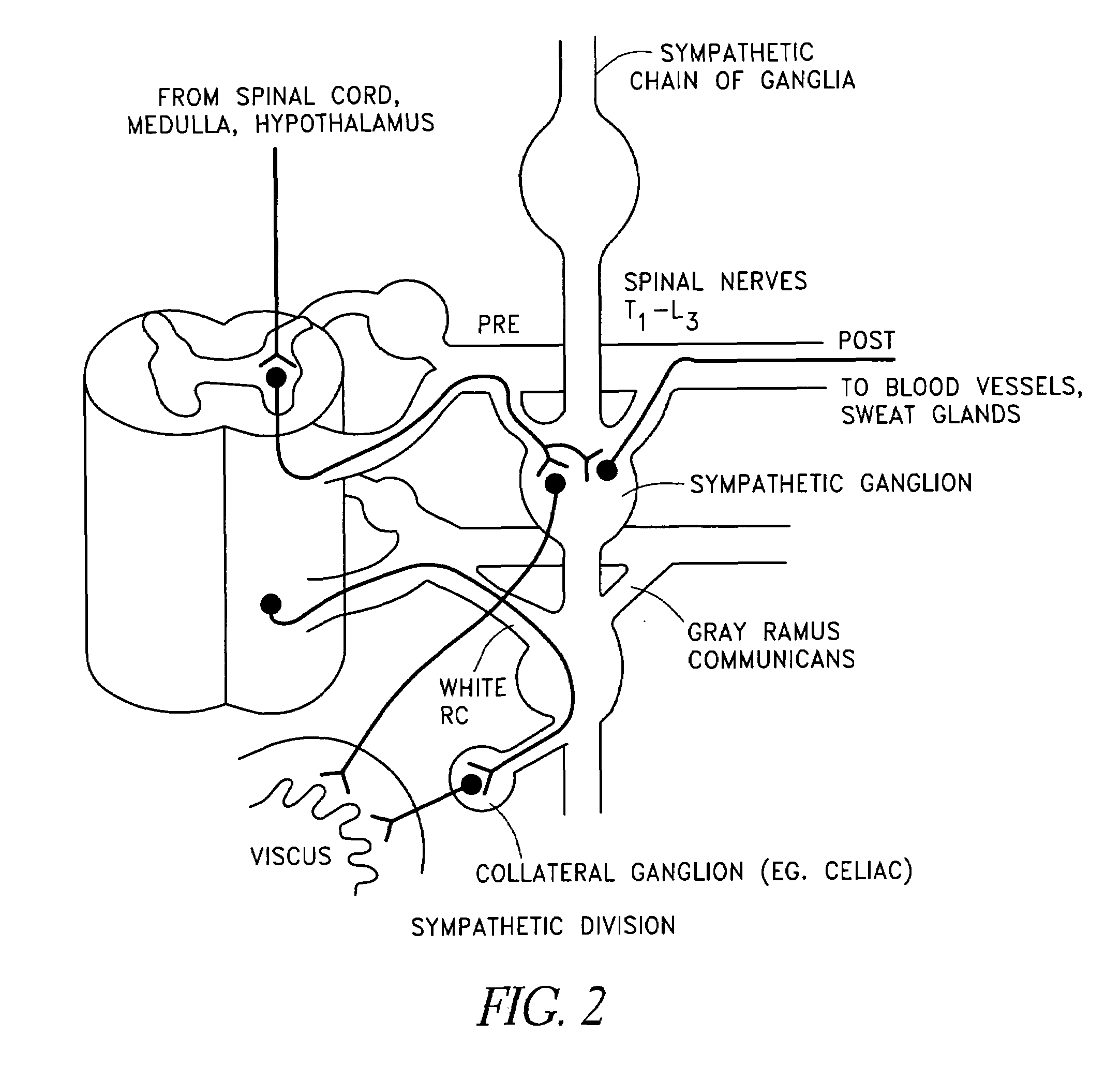
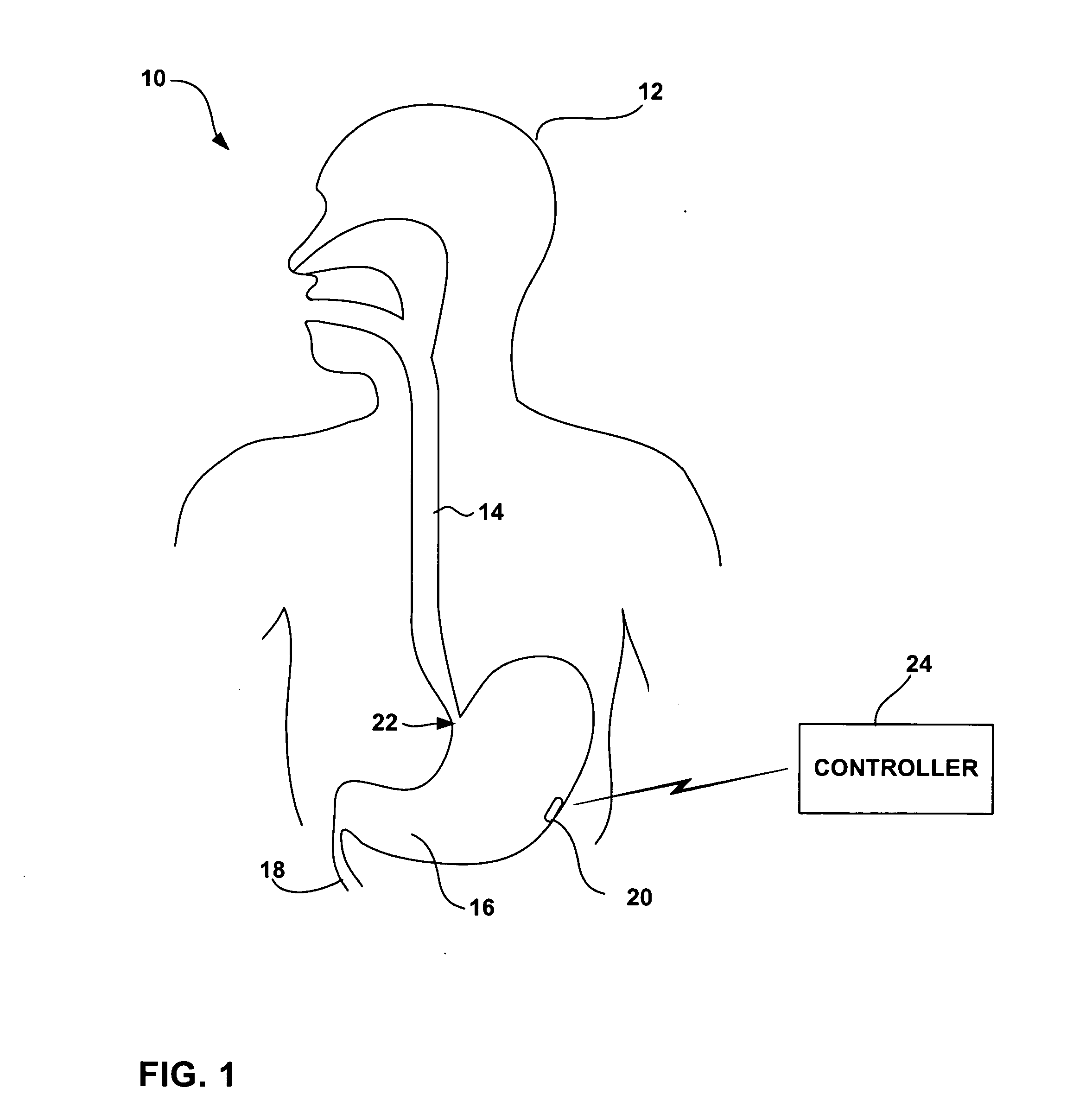
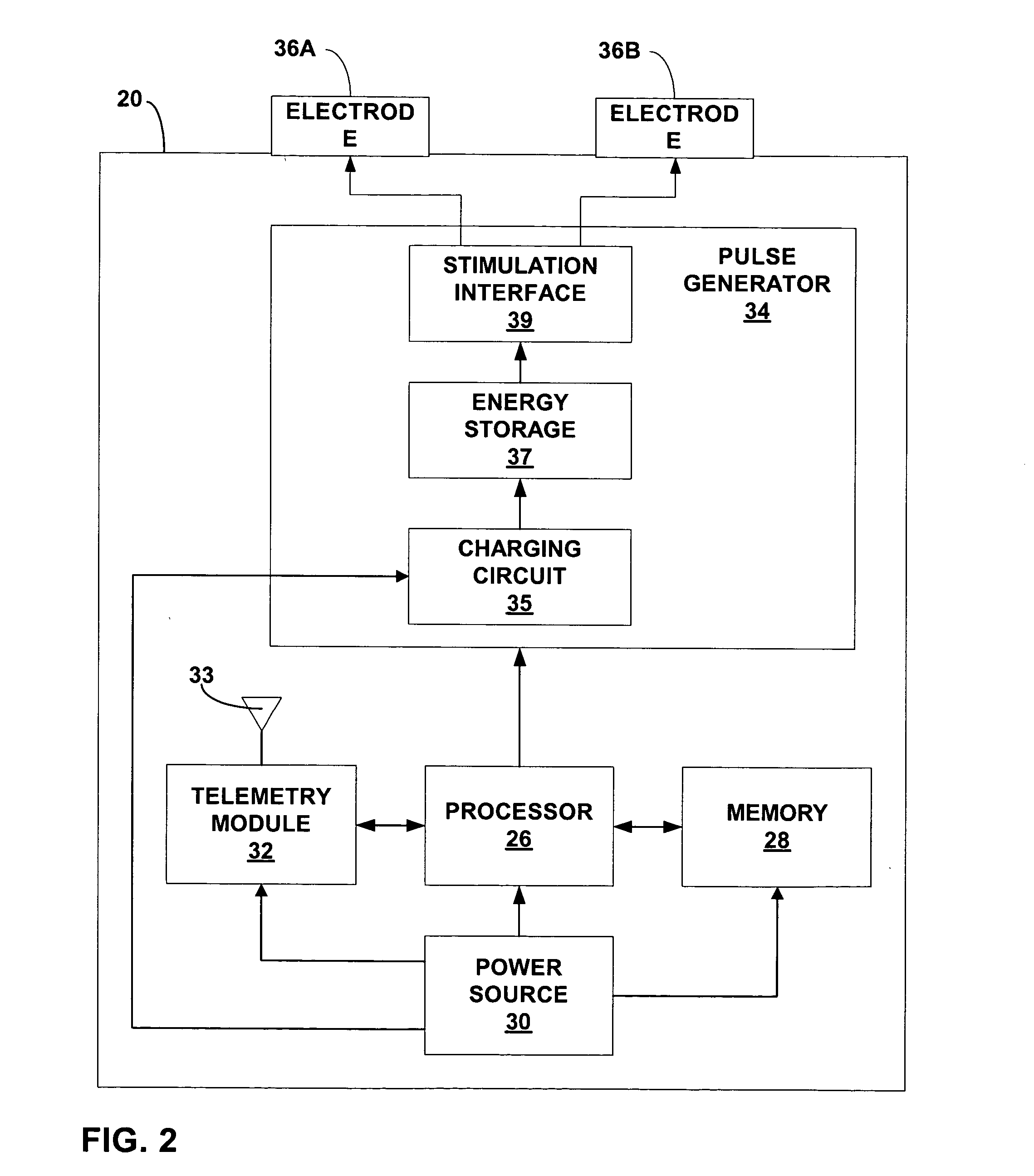
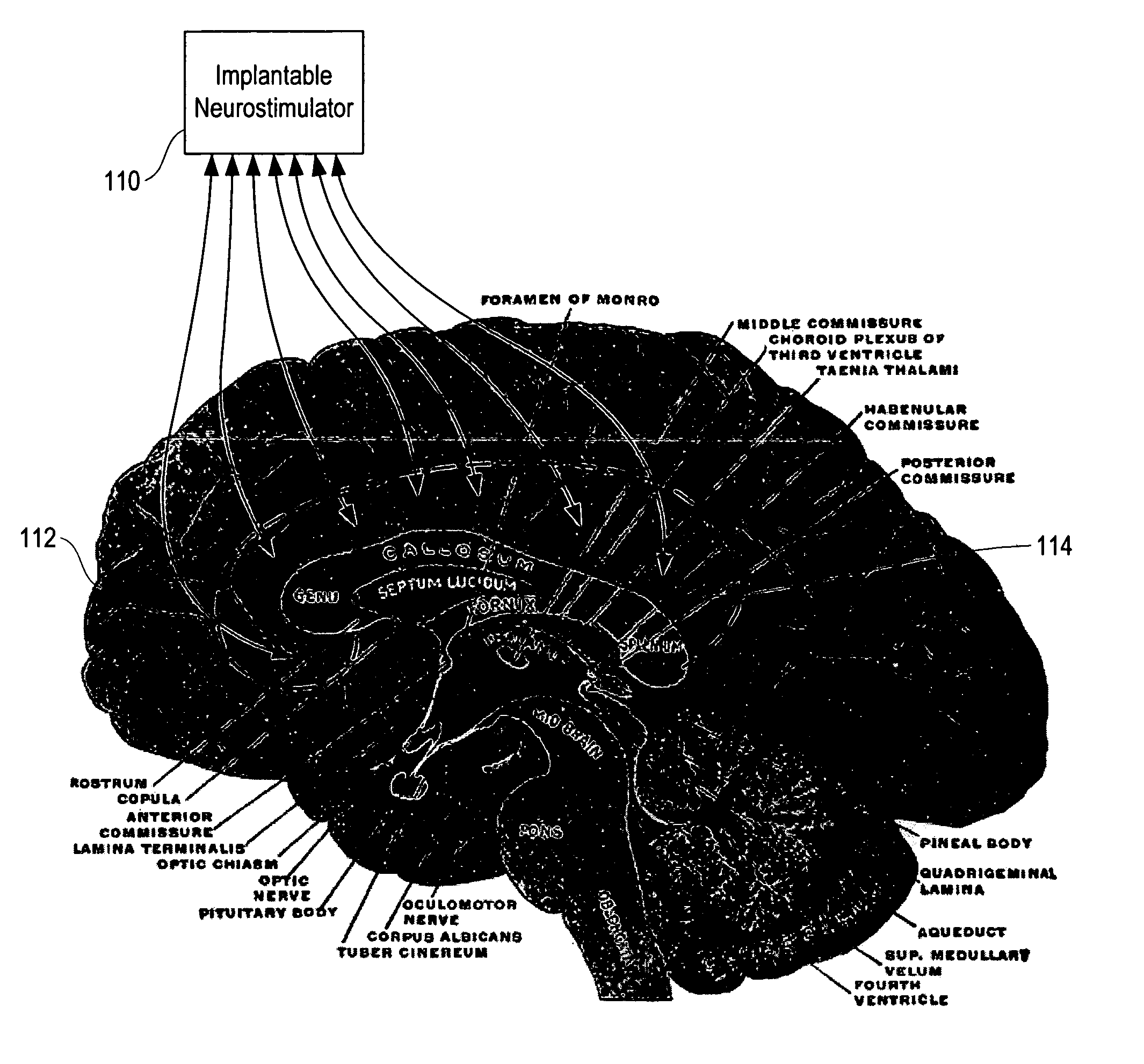
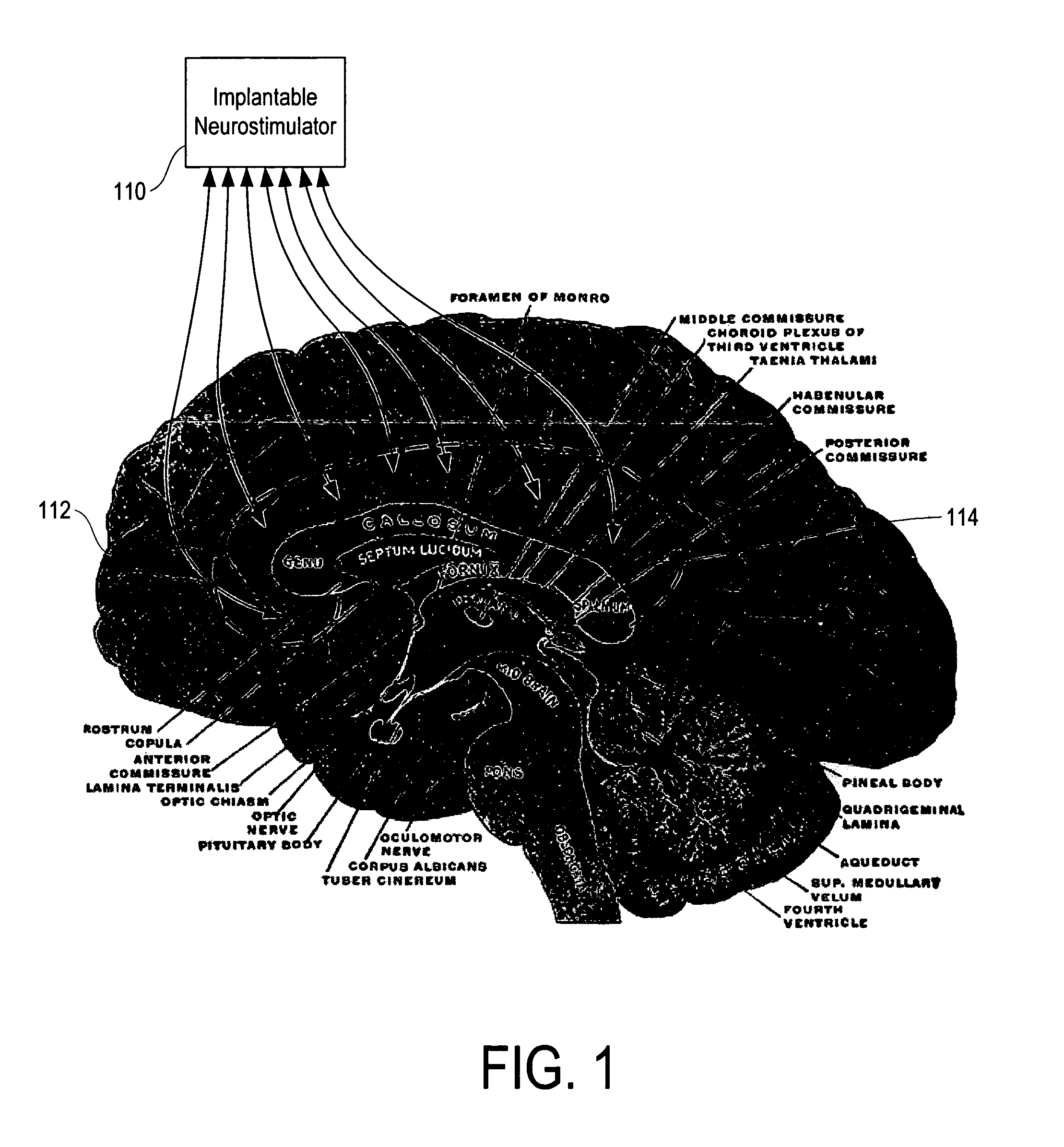
Method, apparatus, and surgical technique for autonomic neuromodulation for the treatment of disease
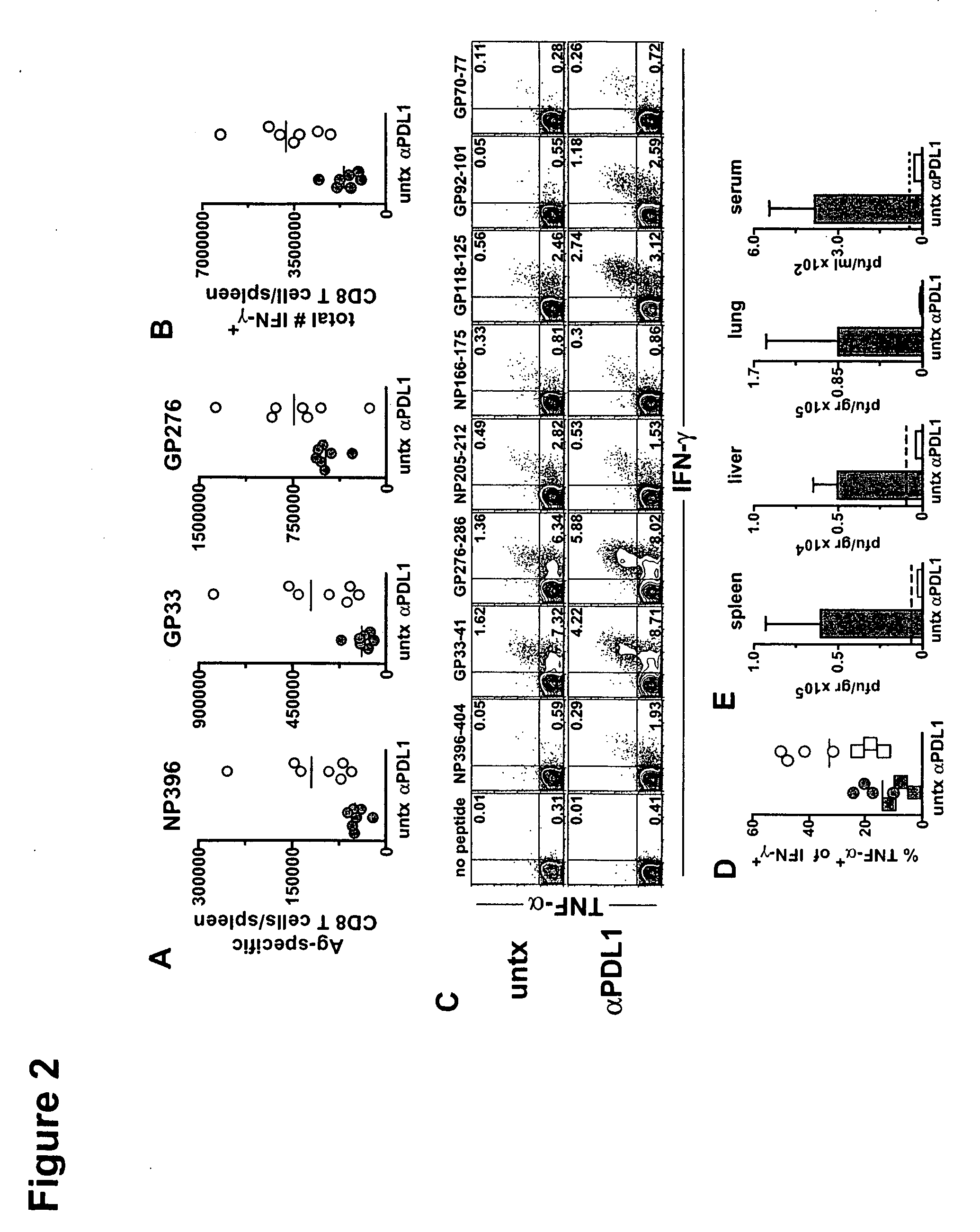
InactiveUS20060167498A1Reduce or prevent conditionReducing and preventing symptomSpinal electrodesSurgical needlesSplanchnic nervesDisease
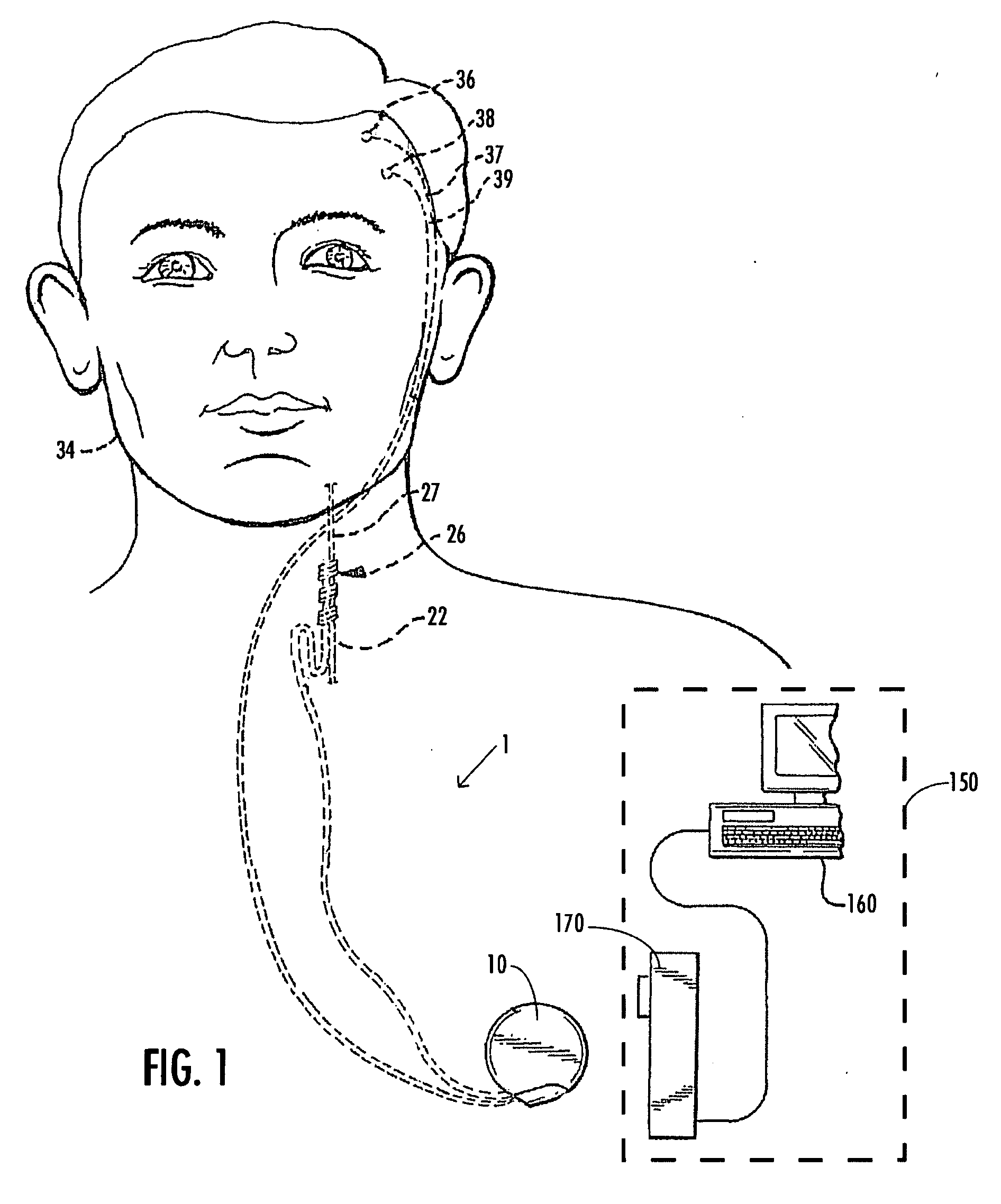
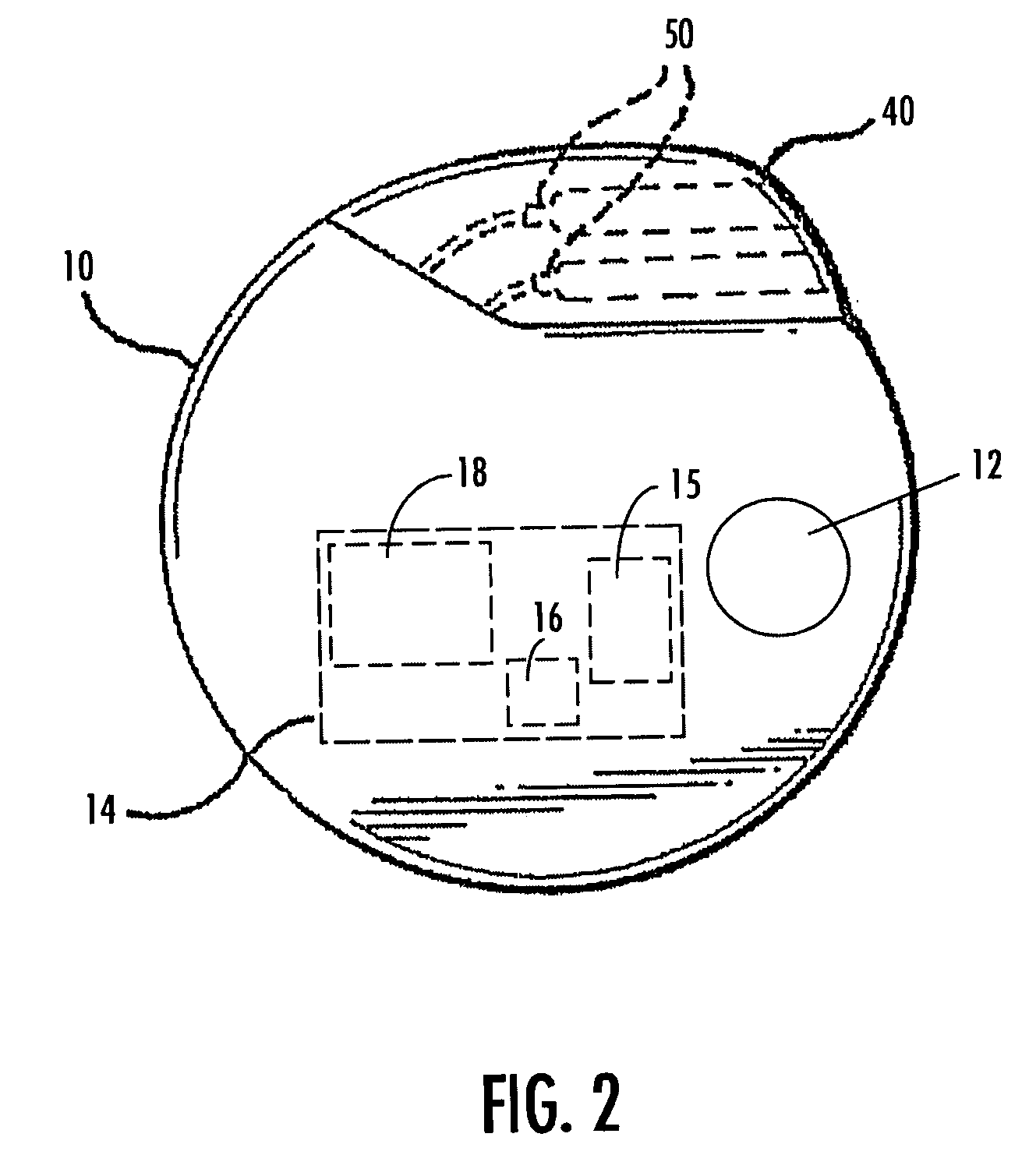
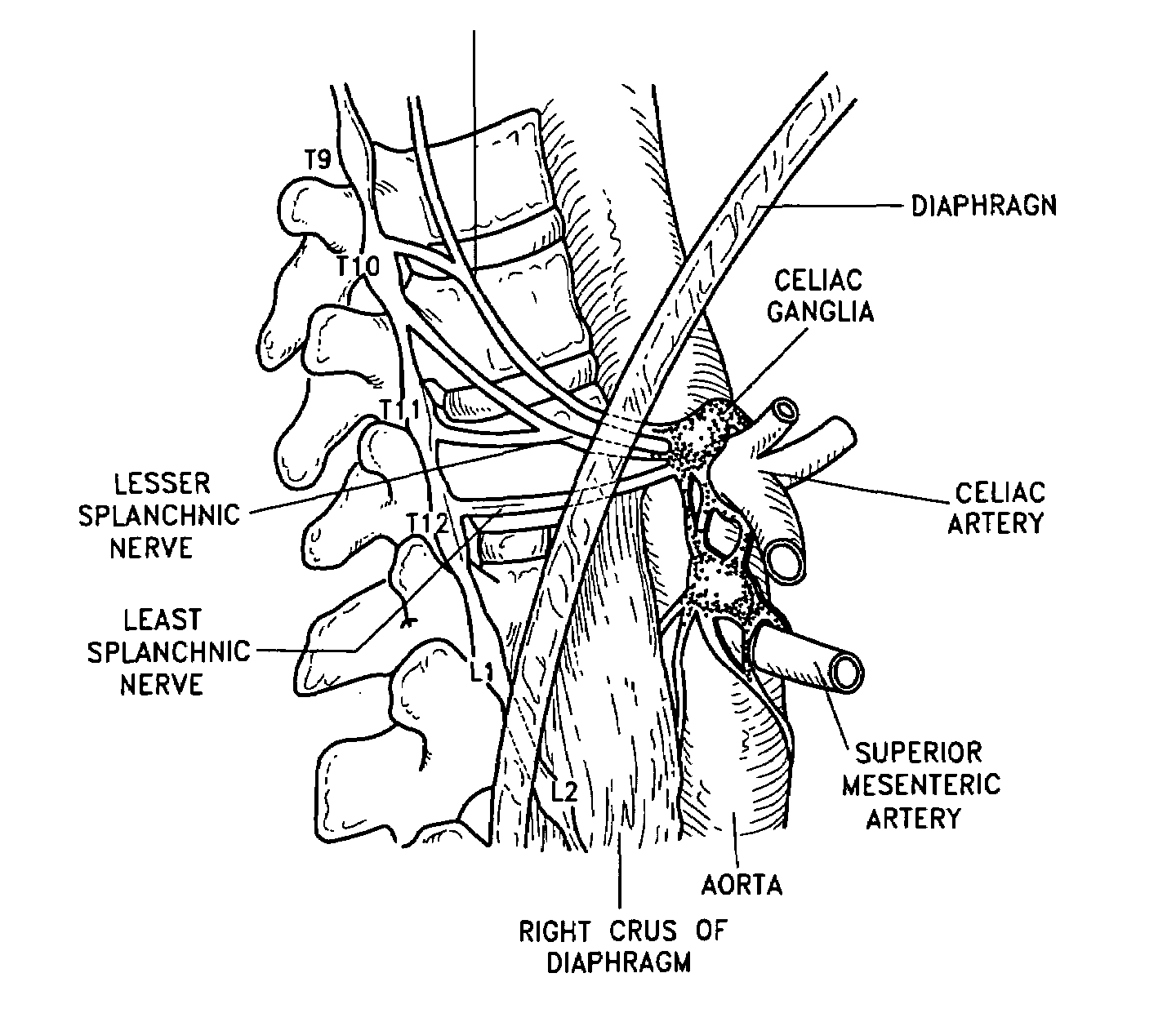
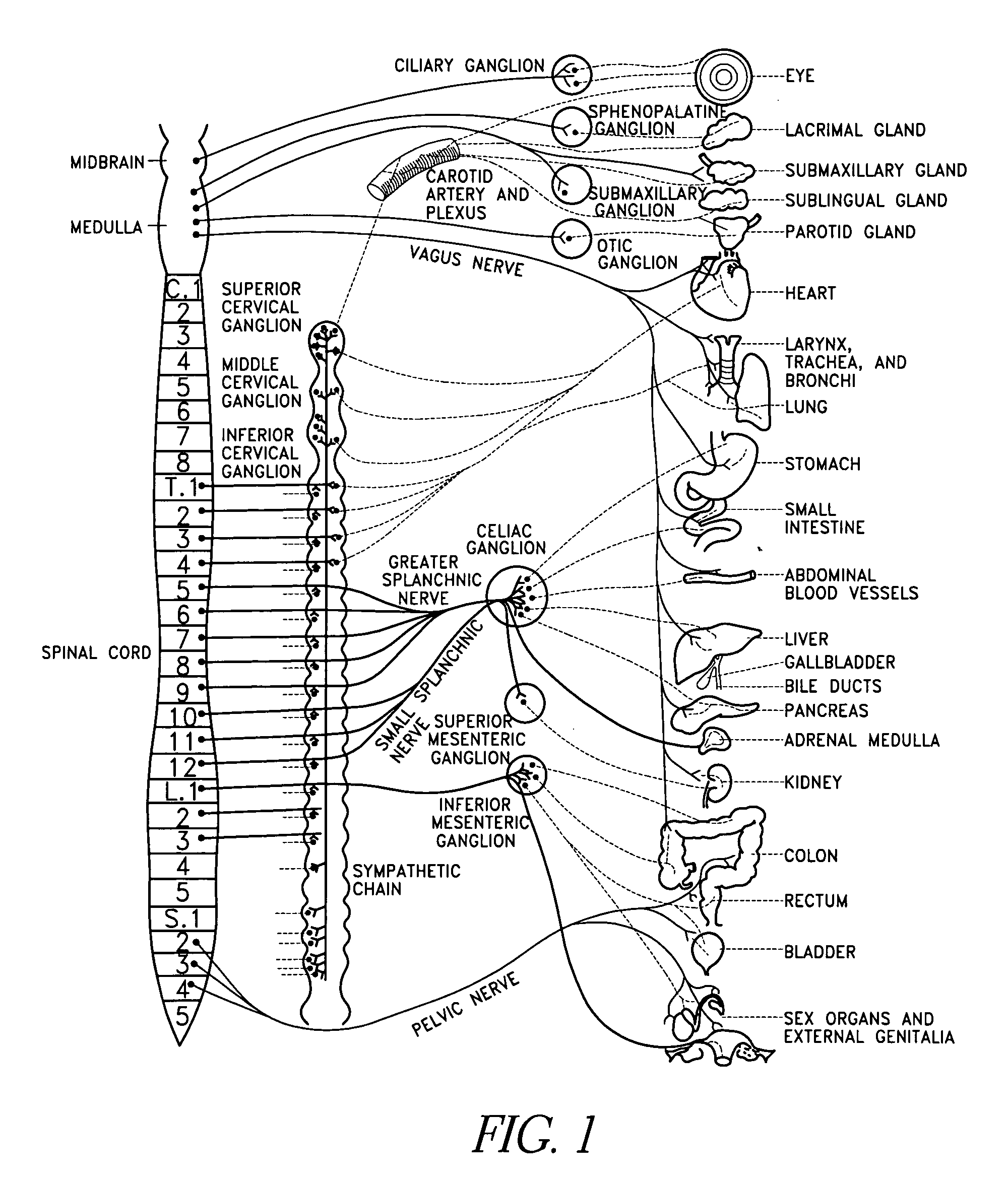
The present invention teaches a method and apparatus for physiological modulation, including neural and gastrointestinal modulation, for the purposes of treating several disorders, including obesity, depression, epilepsy, and diabetes. This includes chronically implanted neural and neuromuscular modulators, used to modulate the afferent neurons of the sympathetic nervous system to induce satiety. Furthermore, this includes neuromuscular stimulation of the stomach to effect baseline and intermittent smooth muscle contraction to increase gastric intraluminal pressure, which induces satiety, and stimulate sympathetic afferent fibers, including those in the sympathetic trunk, splanchnic nerves, and greater curvature of the stomach, to augment the perception of satiety.
Owner:DILORENZO BIOMEDICAL
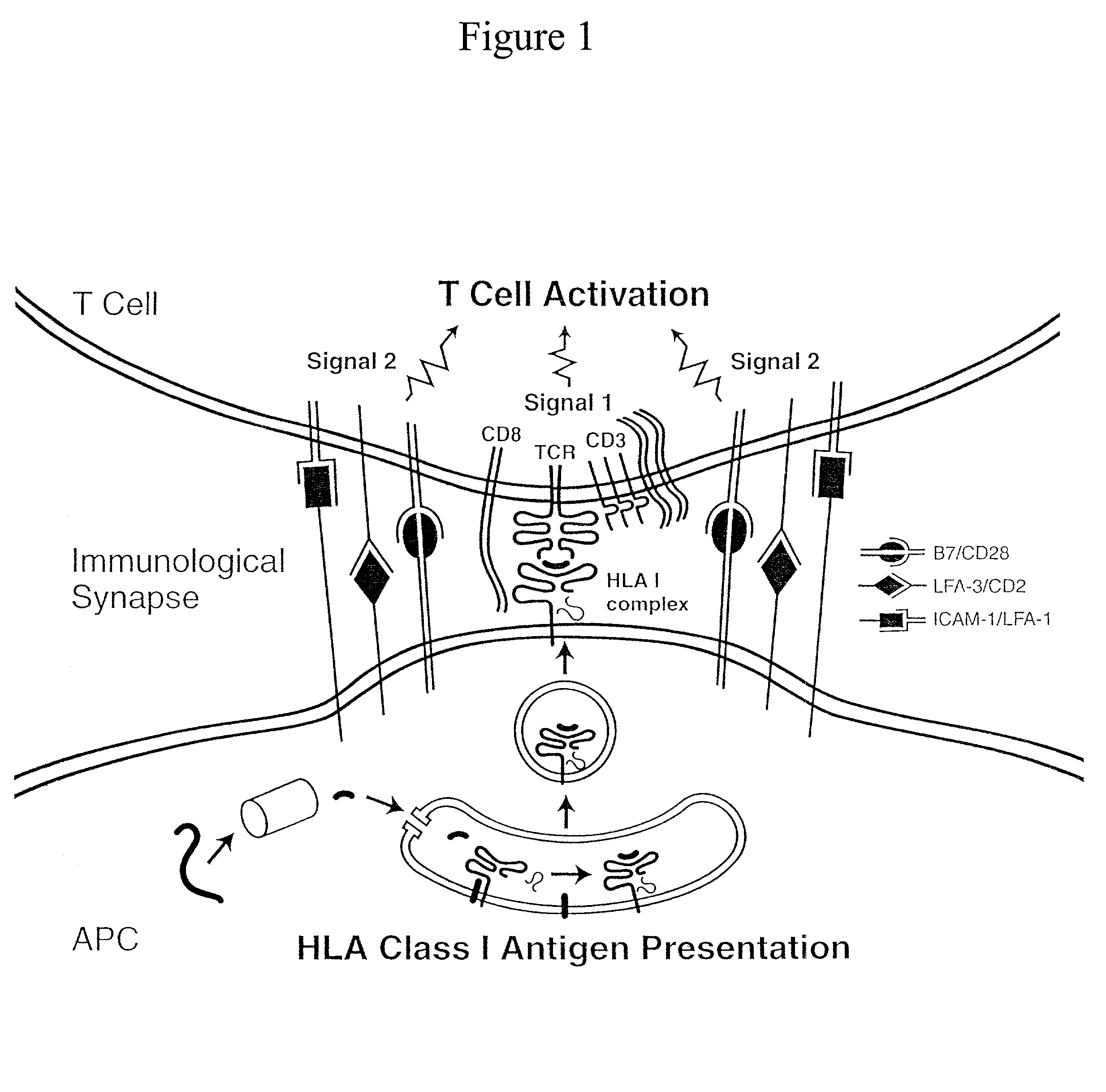
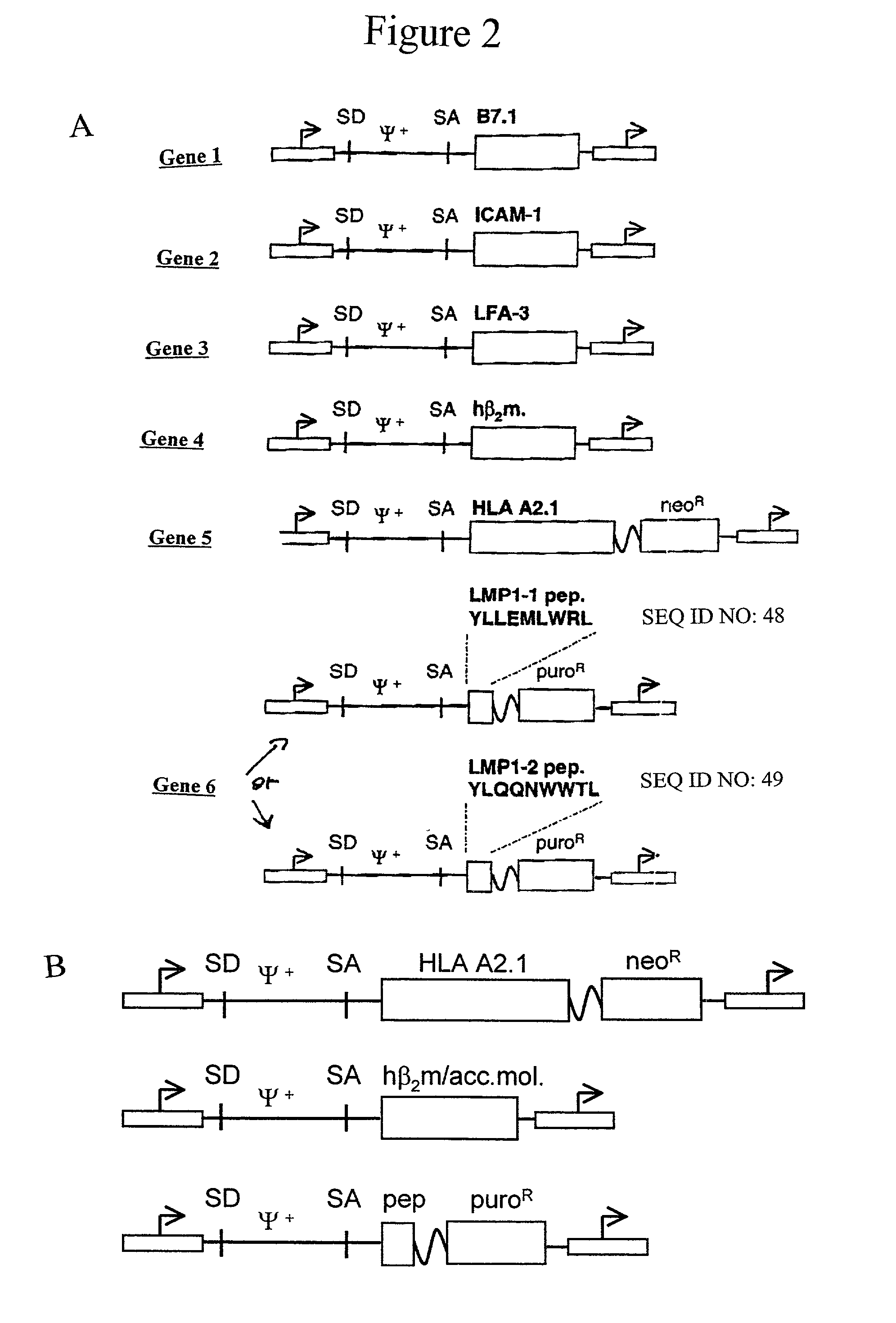
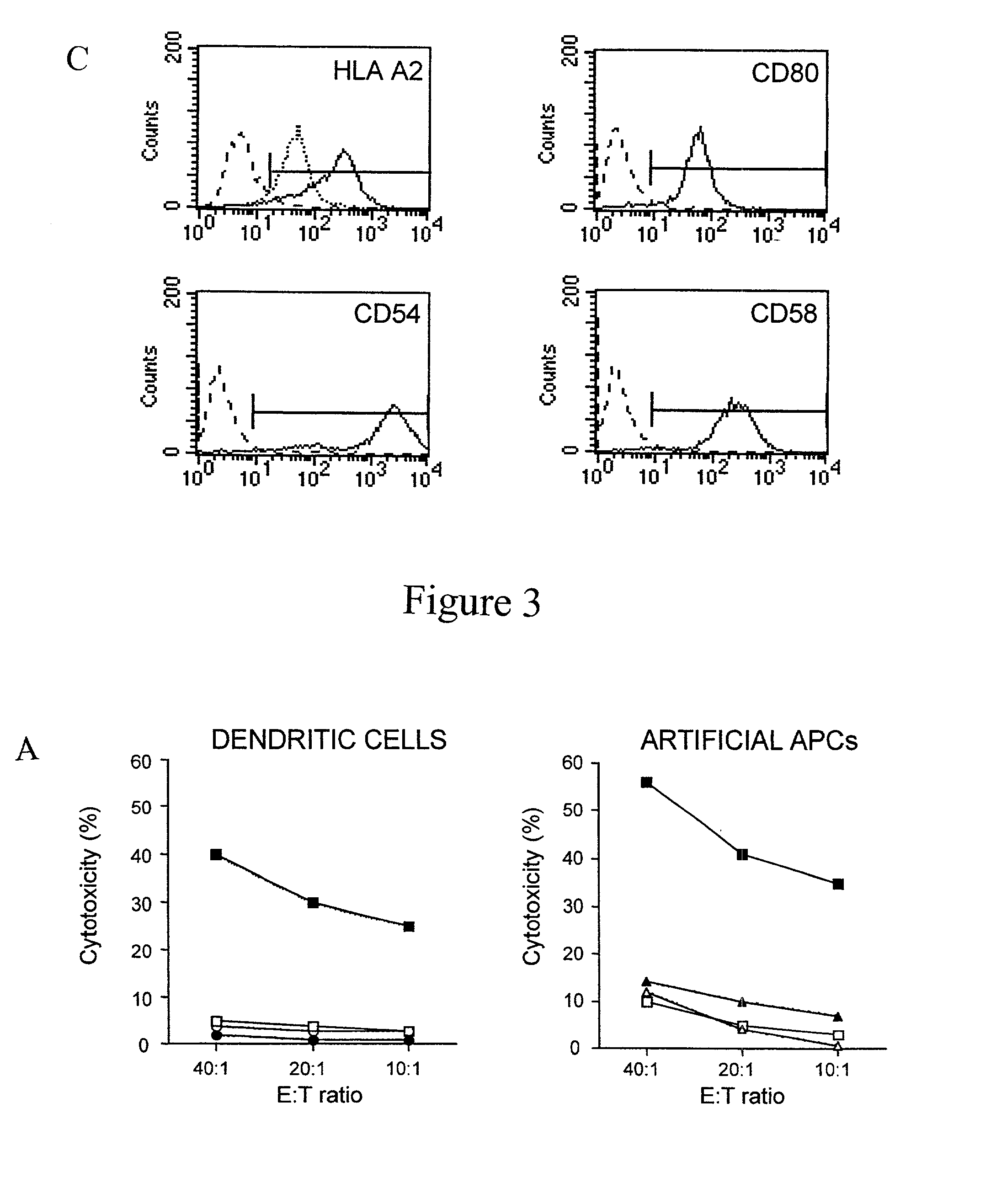
Artificial antigen presenting cells and methods of use thereof
InactiveUS20020131960A1Palliating their conditionReduce riskBiocideCompound screeningEpitopeAccessory molecule
The invention provides an artificial antigen presenting cell (AAPC) comprising a eukaryotic cell expressing an antigen presenting complex comprising a human leukocyte antigen (HLA) molecule of a single type, at least one exogenous accessory molecule and at least one exogenous T cell-specific epitope. Methods of use for activation of T lymphocytes are also provided.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
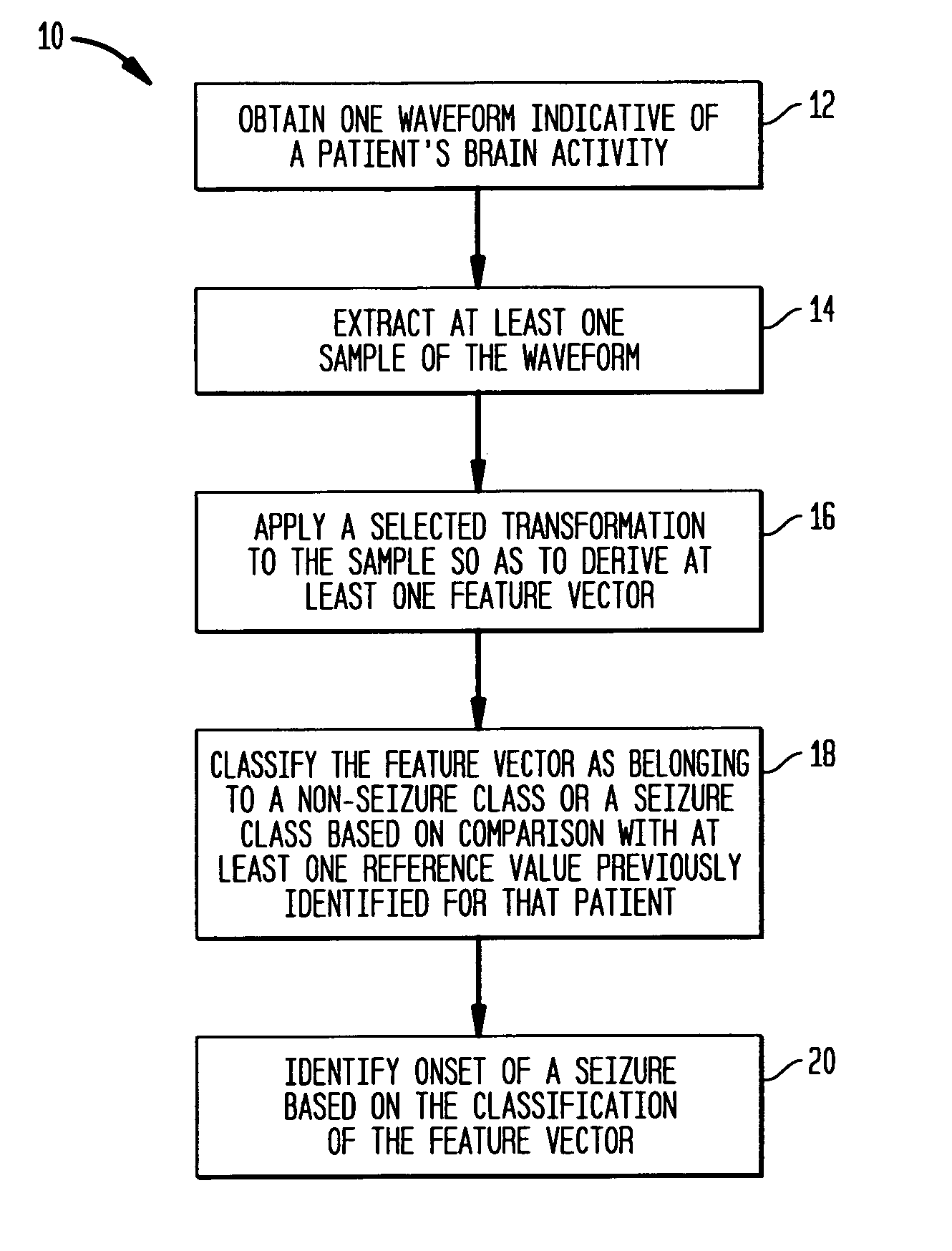
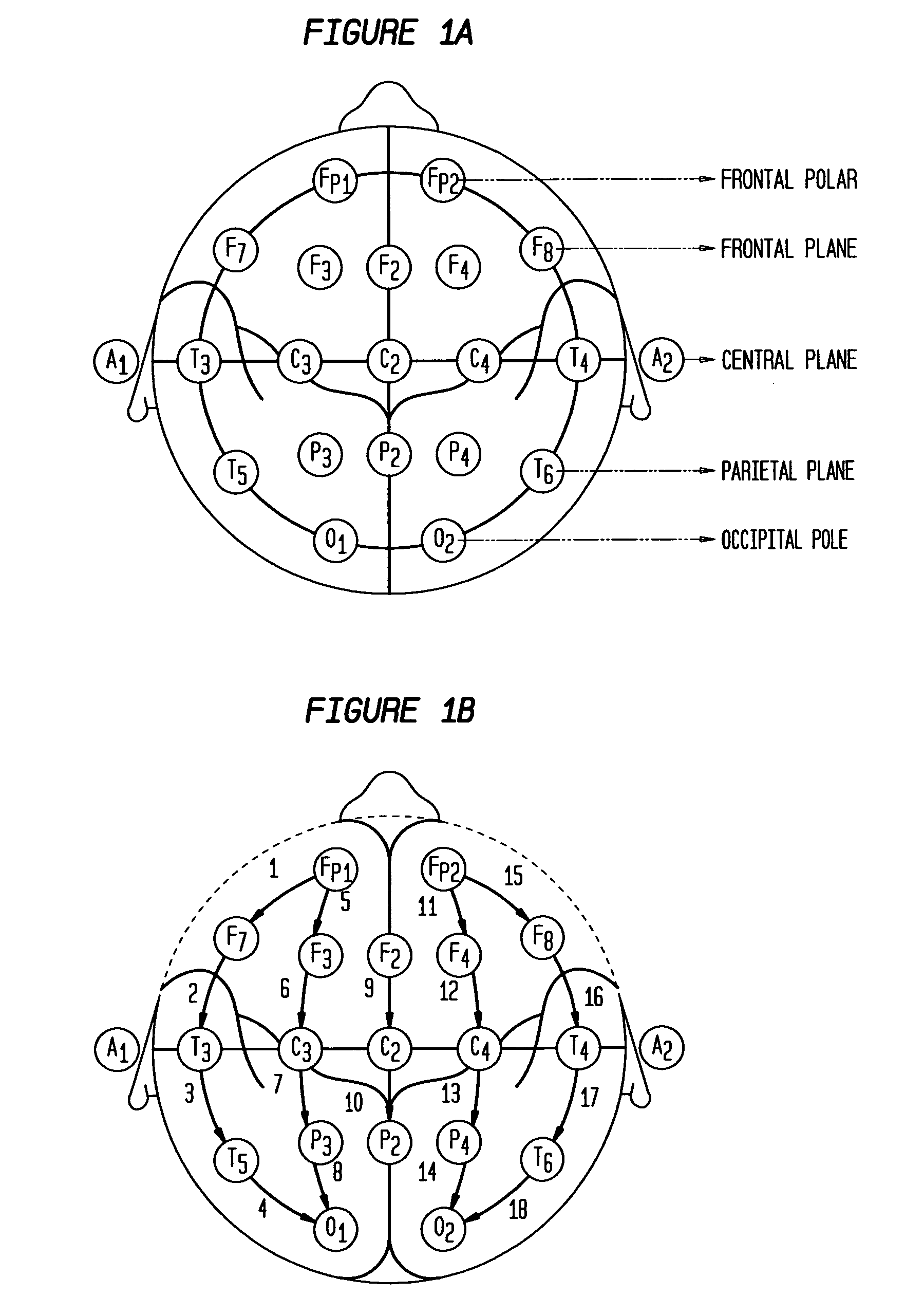
Patient-specific seizure onset detection system
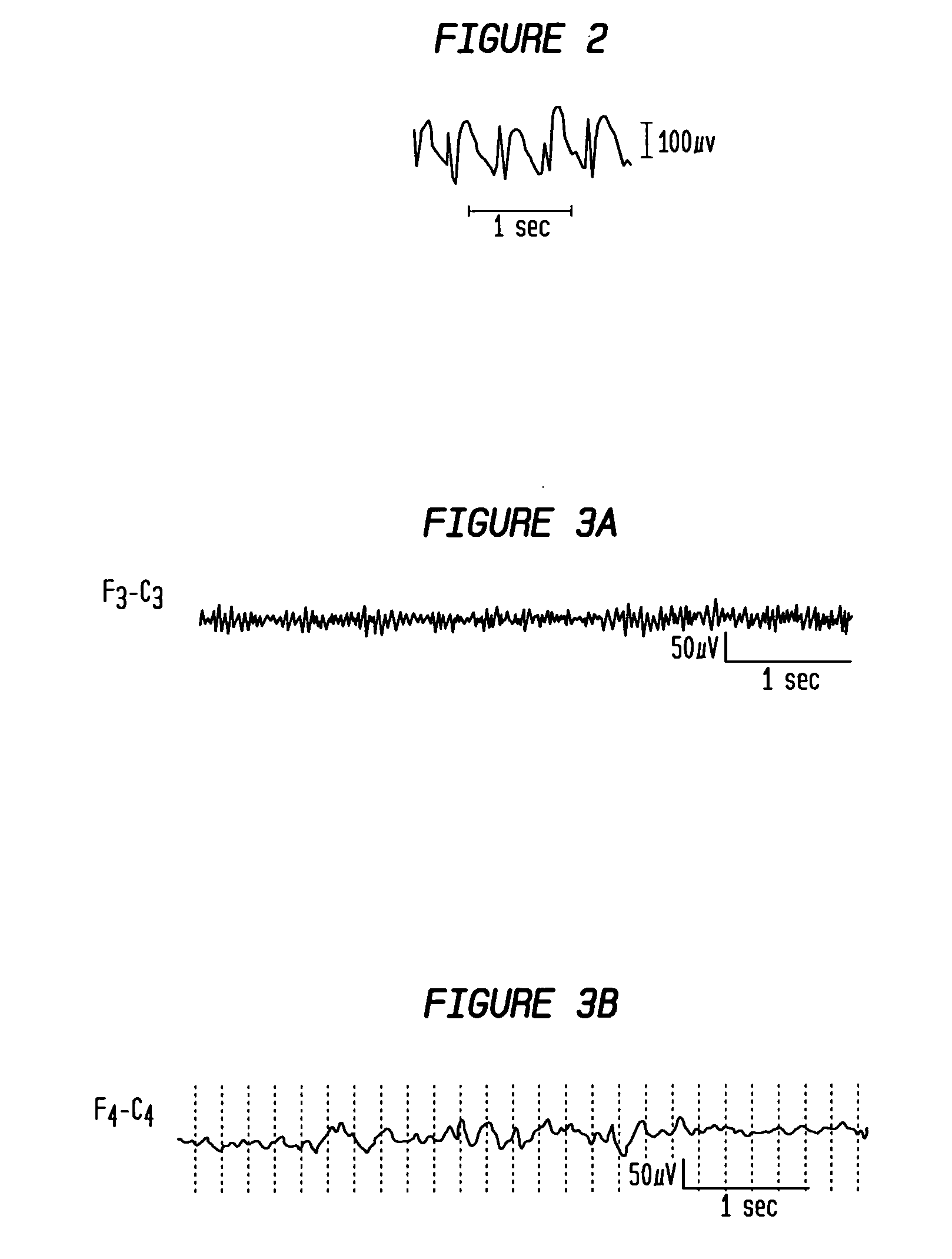
InactiveUS20060111644A1Prevent lessen occurrenceShorten the durationElectroencephalographyMedical data miningFeature vectorAlpha wave
The present invention provides methods and systems for patient-specific seizure onset detection. In one embodiment, at least one EEG waveform of the patient is recorded, and at least one epoch (sample) of the waveform is extracted. The waveform sample is decomposed into one or more subband signals via a wavelet decomposition of the waveform sample, and one or more feature vectors are computed based on the subband signals. A seizure onset can then be identified based on classification of the feature vectors to a seizure or a non-seizure class by comparing the feature vectors with a decision measure previously computed for that patient. The decision measure can be derived based on reference seizure and non-seizure EEG waveforms of the patient. In another aspect, similar methodology is employed for automatic detection of alpha waves. In other aspects, the invention provides diagnostic and imaging systems that incorporate the above seizure-onset and alpha-wave detection methodology.
Owner:CHILDRENS MEDICAL CENT CORP
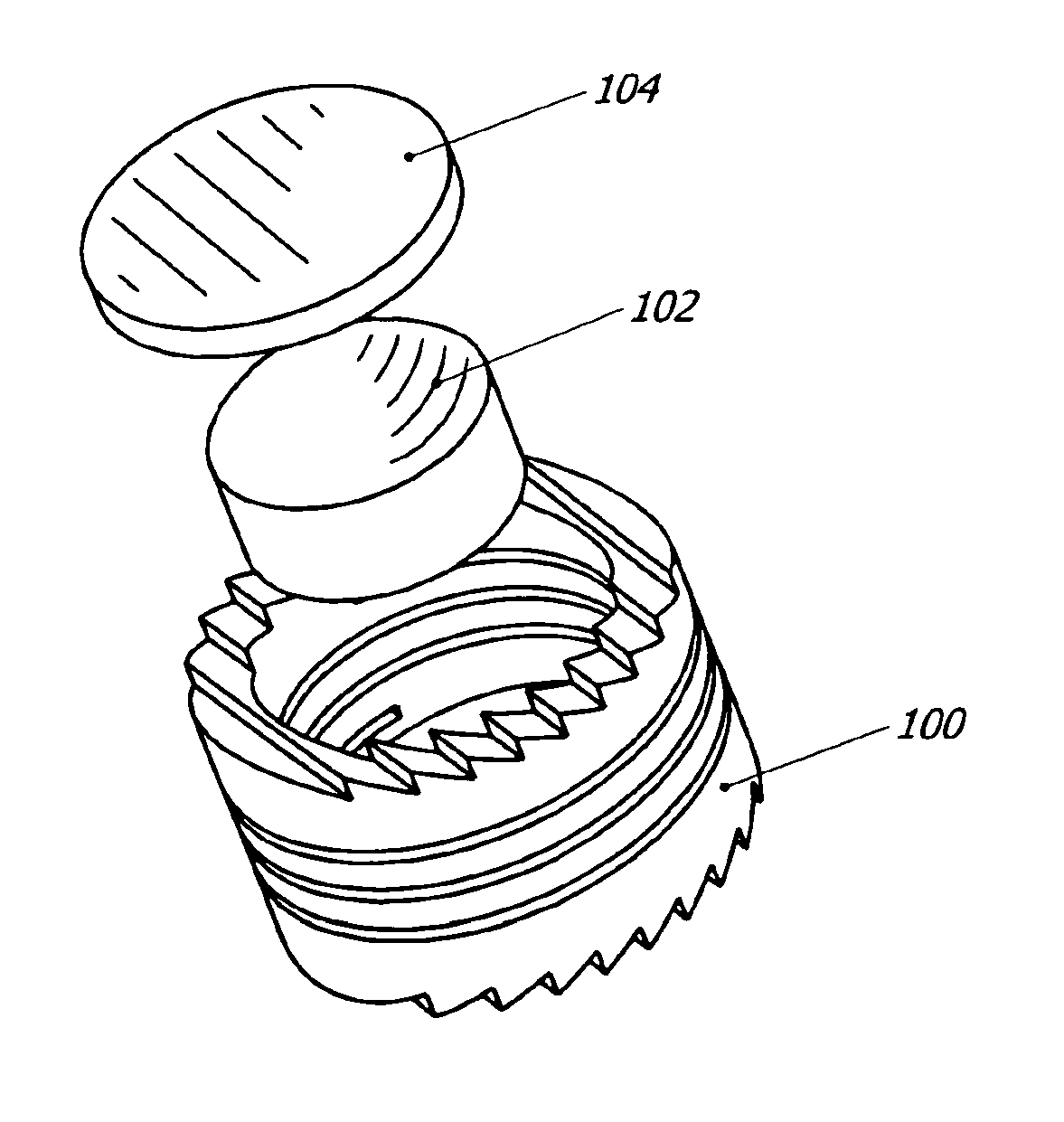
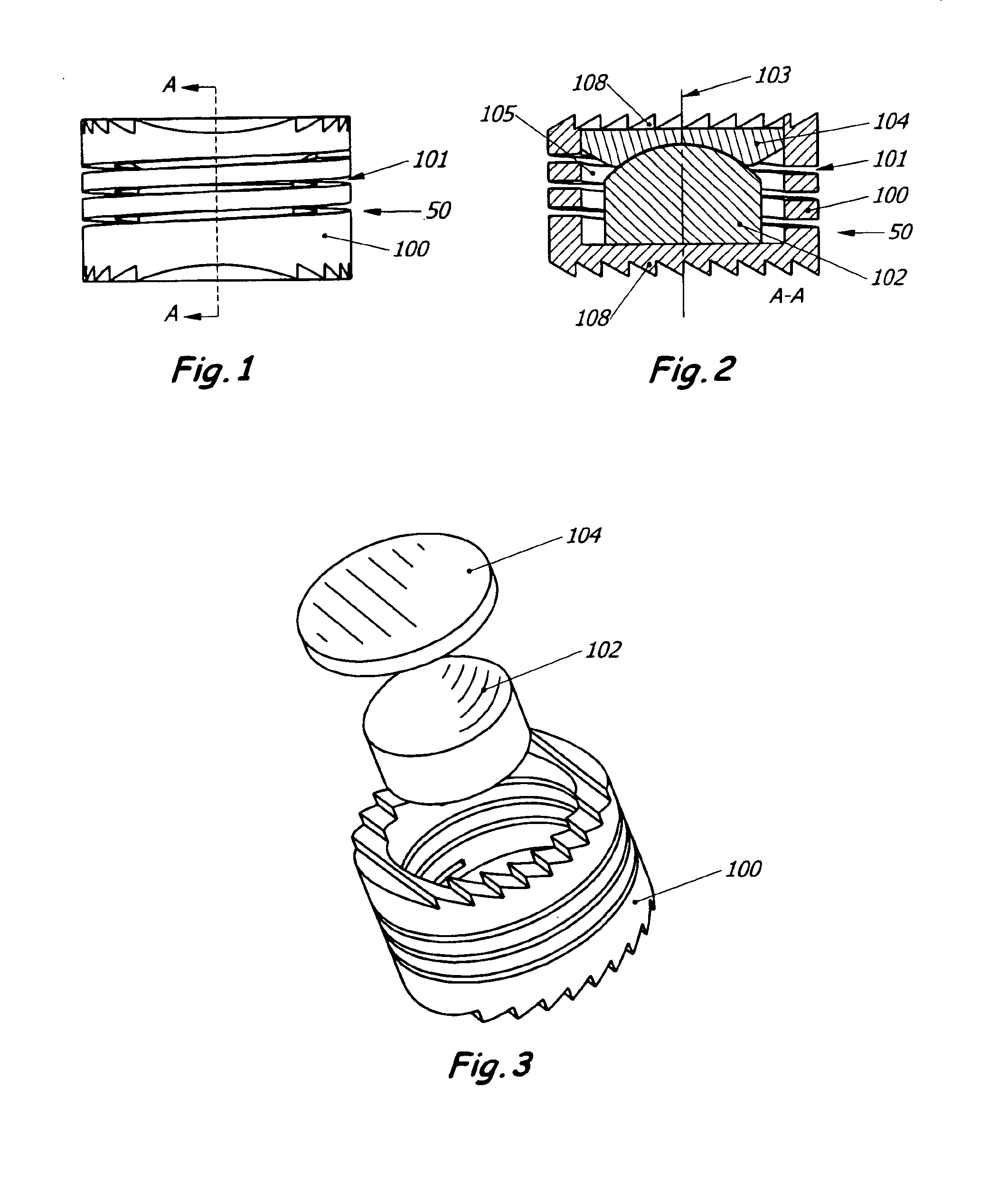
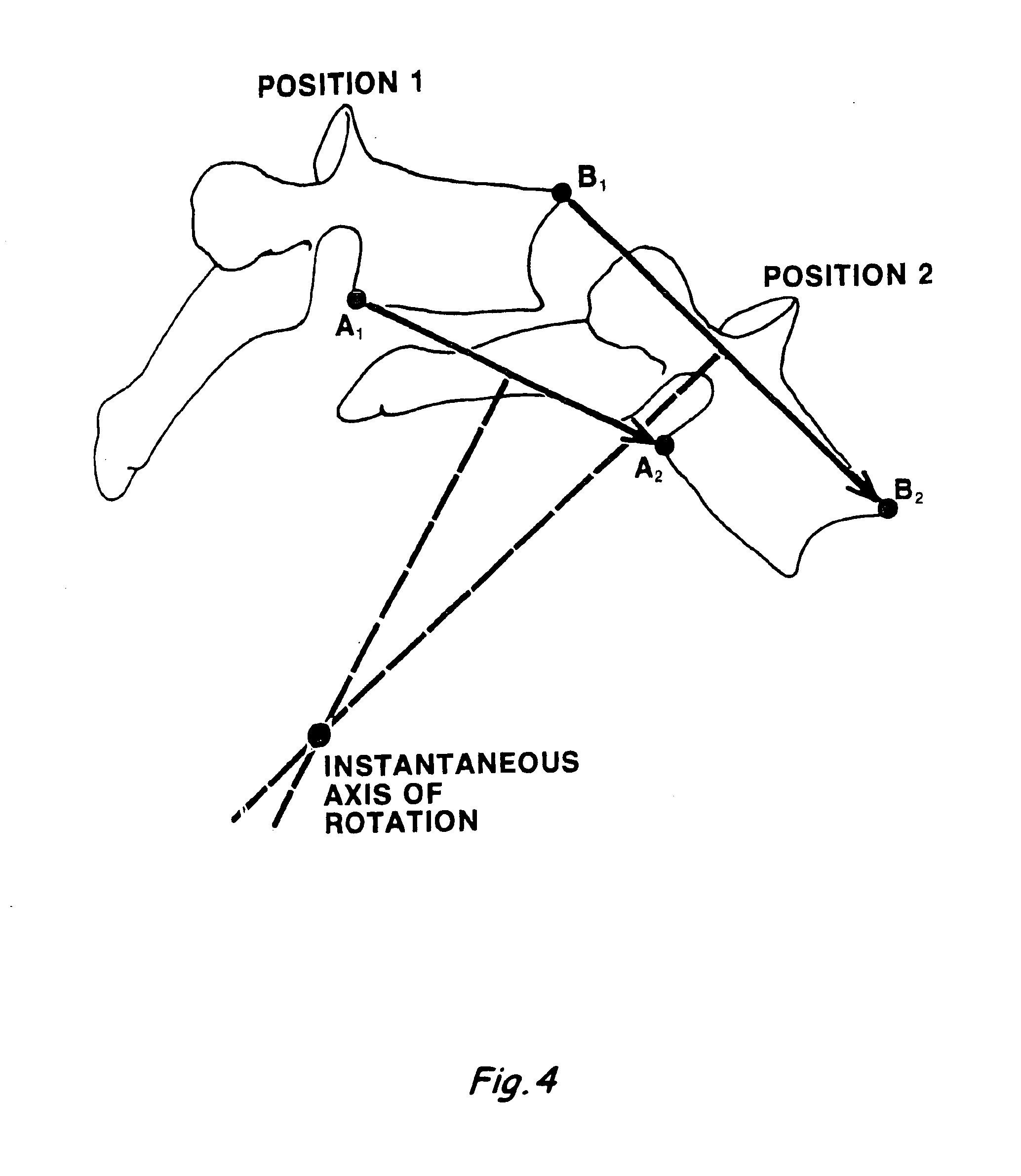
Intervertebral disc replacement prosthesis
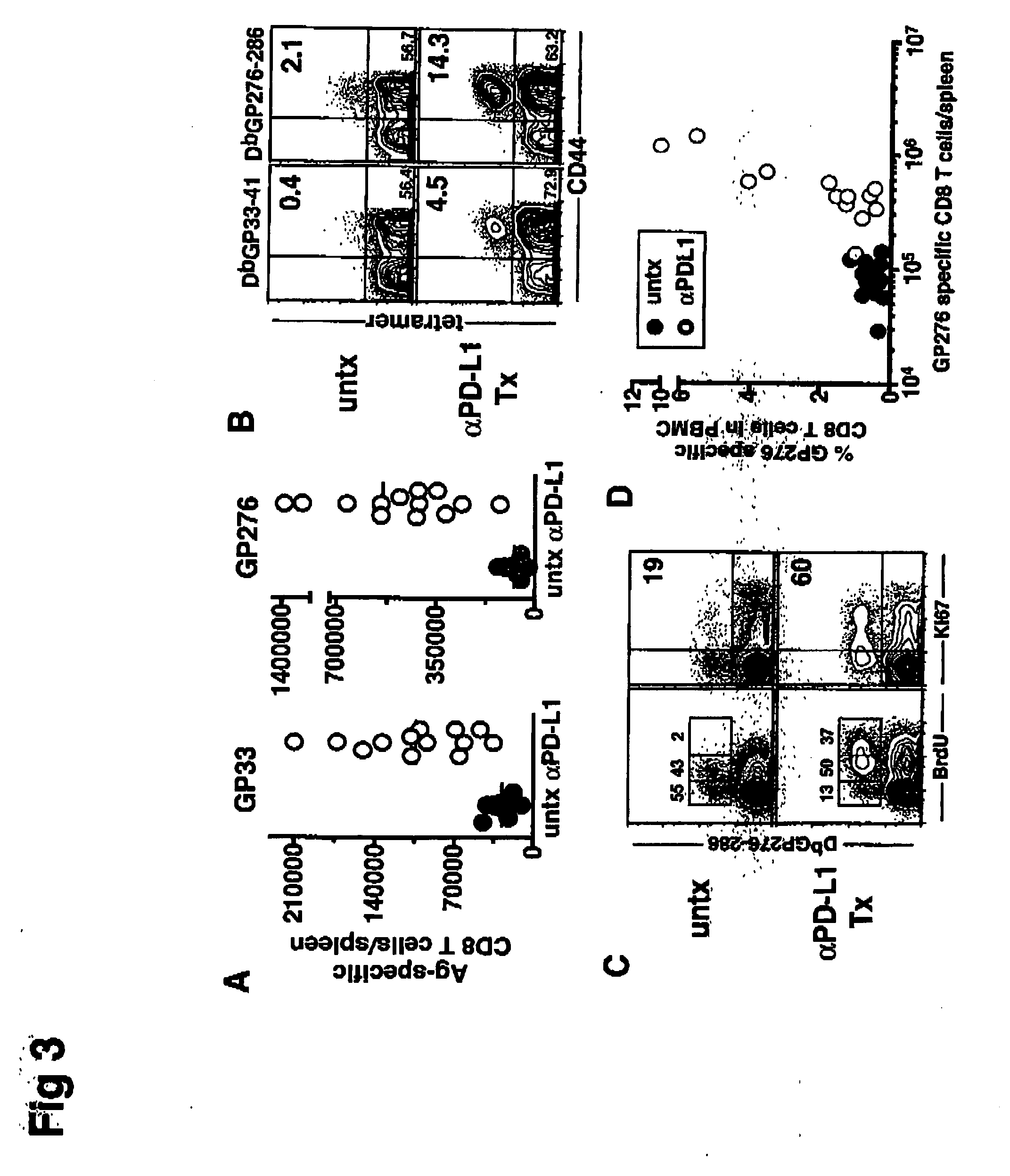
InactiveUS6964686B2Relieve symptomsNot compromising healthJoint implantsSpinal implantsSlice thicknessIntervertebral disk
An intervertebral disc prosthesis that comprises a deformable flexure with an axial cavity, the axial cavity extending along the axis of the flexure, and a slit defined in the perimeter surface of the flexure to provide flexibility to the disc member, the slit having a slit thickness. The slit may be in the form of a coil to impart a spring-like appearance and function. The intervertebral disc prosthesis further comprises a lower disc support housed in the axial cavity and an upper disc support housed in the axial cavity; with the lower and upper disc supports communicating with one another to provide support to the disc. The lower or upper disc support may alternatively be incorporated into the flexure.
Owner:VANDERBILT UNIV
Implantable system enabling responsive therapy for pain
ActiveUS7894905B2Relieve symptomsQuality improvementElectrotherapyFlow monitorsNervous systemNeuropathic pain
An implantable neurostimulator system for treating pain includes scheduled and responsive therapy capabilities including responsive stimulation applied to the brain and peripheral sections of the nervous system. Methods for treating chronic nociceptive, neuropathic, and psychogenic pain employ an inventive system to advantageously reduce multiple symptoms and components of pain and to address underlying causes of pain.
Owner:NEUROPACE
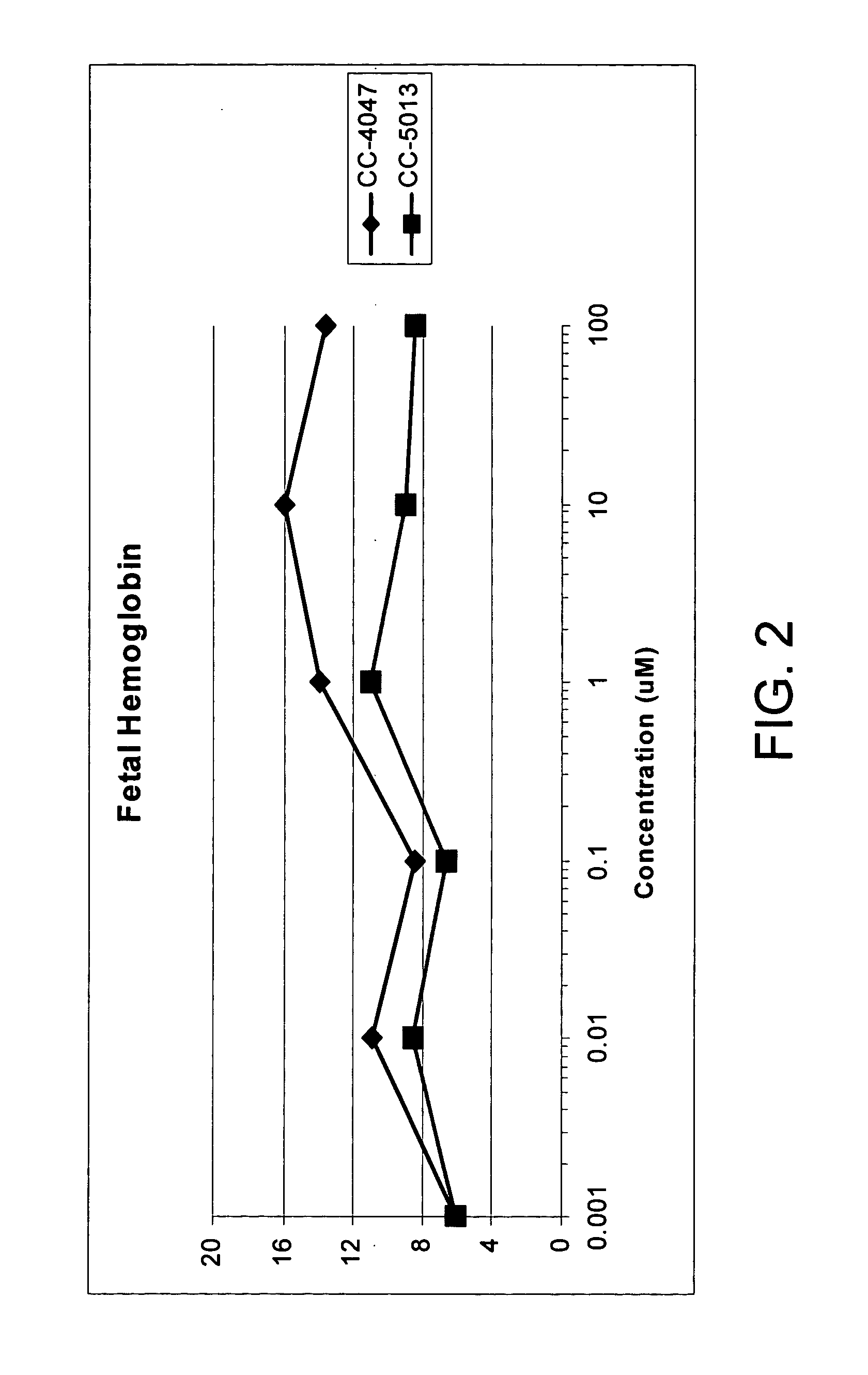
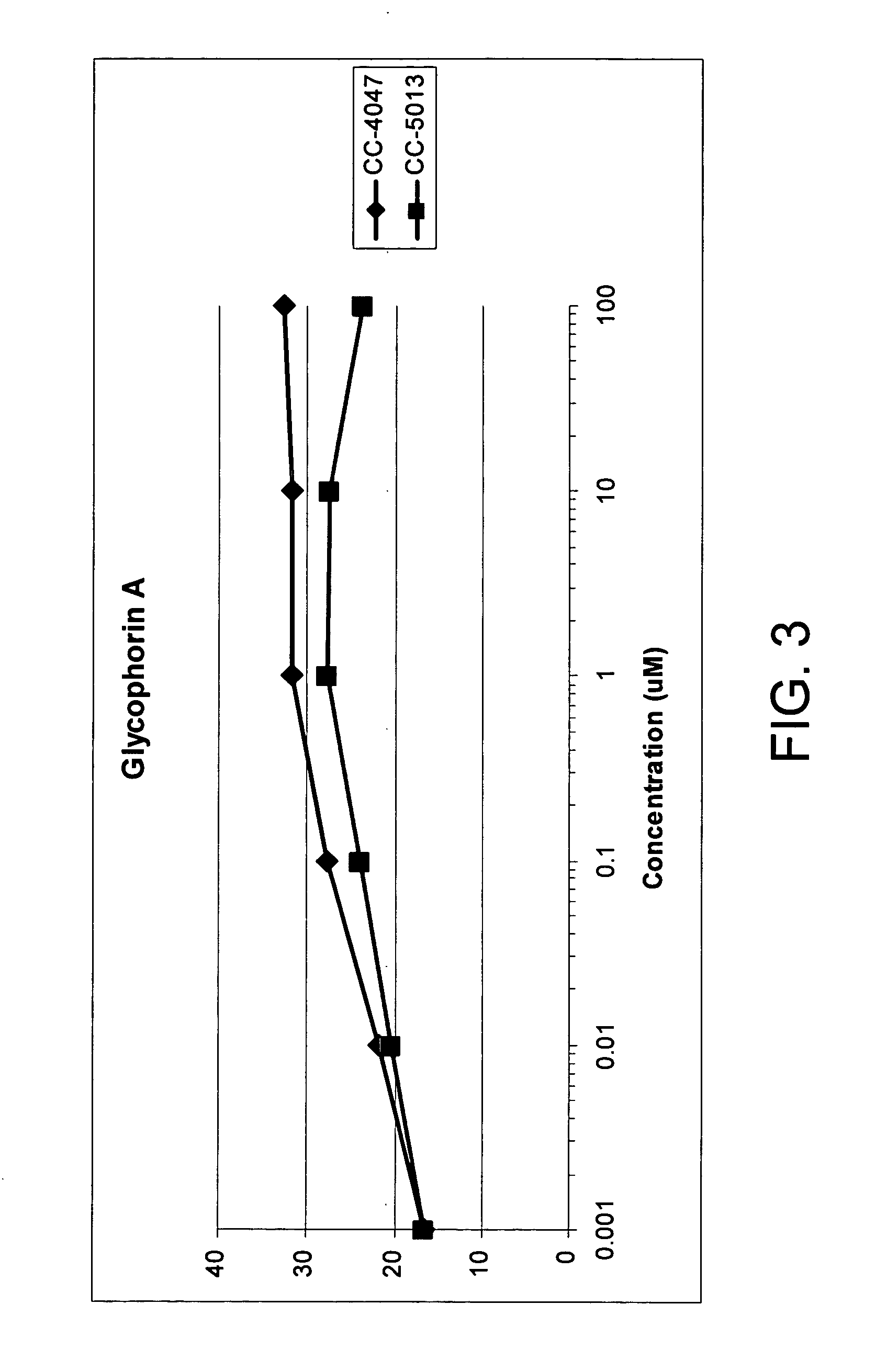
Methods and compositions for the treatment and management of hemoglobinopathy and anemia
InactiveUS20050143420A1Good effectRelieve symptomsBiocidePeptide/protein ingredientsRed blood cellThalassemia
The present invention is directed to the use of immunomodulatory compounds, particularly members of the class of compounds known as IMiDs™, and more specifically the compounds 4-(Amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione and 3-(4-amino-1-oxo-1,3-dihydroisoindol-2-yl)-piperidine-2,6-dione, to induce the expression of fetal hemoglobin genes, genes essential for erythropoiesis, and genes encoding alpha hemoglobin stabilizing protein, within a population of CD34+ cells. These compounds are used to treat hemoglobinopathies such as sickle cell anemia or β-thalassemia, or anemias caused by disease, surgery, accident, or the introduction or ingestion of toxins, poisons or drugs.
Owner:SIGNAL PHARMA LLC
Wireless electric modulation of sympathetic nervous system
InactiveUS7236822B2Increased energy expenditureReducing food intakeInternal electrodesExternal electrodesDiseaseNervous system
A method for the treatment of obesity or other disorders, by wireless electrical activation or inhibition of the sympathetic nervous system. This activation or inhibition can be accomplished by wirelessly stimulating the greater splanchnic nerve or other portion of the sympathetic nervous system using a wireless electrode inductively coupled with a radiofrequency field. The source of radiofrequency energy may be internal or external to the patient. This nerve activation can result in reduced food intake and increased energy expenditure.
Owner:ADVANCED NEUROMODULATION SYST INC
Methods and devices for tissue reconfiguration
InactiveUS20050033328A1Reduce trafficReduce morbiditySuture equipmentsSurgical needlesBody organsEndoscope
Owner:ETHICON ENDO SURGERY INC
Devices and methods for coronary sinus pressure relief
A method and devices for relieving pressure in the left atrium of a patient's heart is disclosed. The method includes using an ablative catheter in a minimally invasive procedure to prepare an opening from the coronary sinus into a left atrium of the patient's heart. Once the opening is prepared, the opening may be enlarged by a technique such as expanding a balloon within the opening. A stent is then placed within the coronary sinus of the patient, with a transverse portion expanding within the opening, allowing blood to flow from the left atrium to the coronary sinus and then to the right atrium. Pressure within the left atrium is thus relieved.
Owner:DC DEVICES
Methods and compositions for the treatment of persistent infections
ActiveUS20070122378A1Reduced activityReduce expressionAntibacterial agentsOrganic active ingredientsMicrobiologyPathology
The present invention provides methods and compositions for the treatment, prevention, or reduction of persistent infections, such as chronic infections, latent infections, and slow infections and cancer. The methods and compositions of the invention are also useful for the alleviation of one or more symptoms associated with such infections and cancer.
Owner:DANA FARBER CANCER INST INC +3
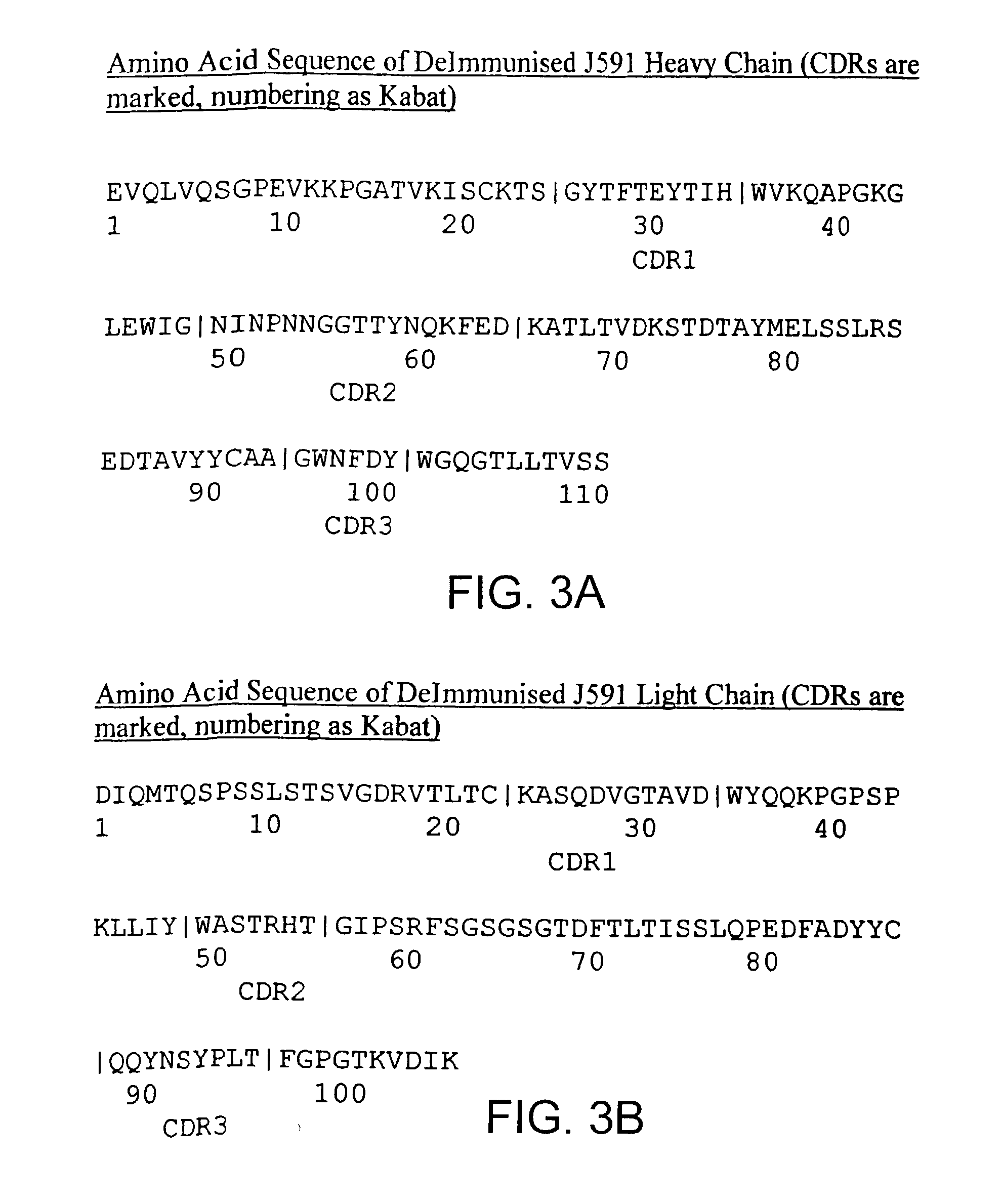
Methods and compositions for treating or preventing skin disorders using binding agents specific for prostate specific membrane antigen
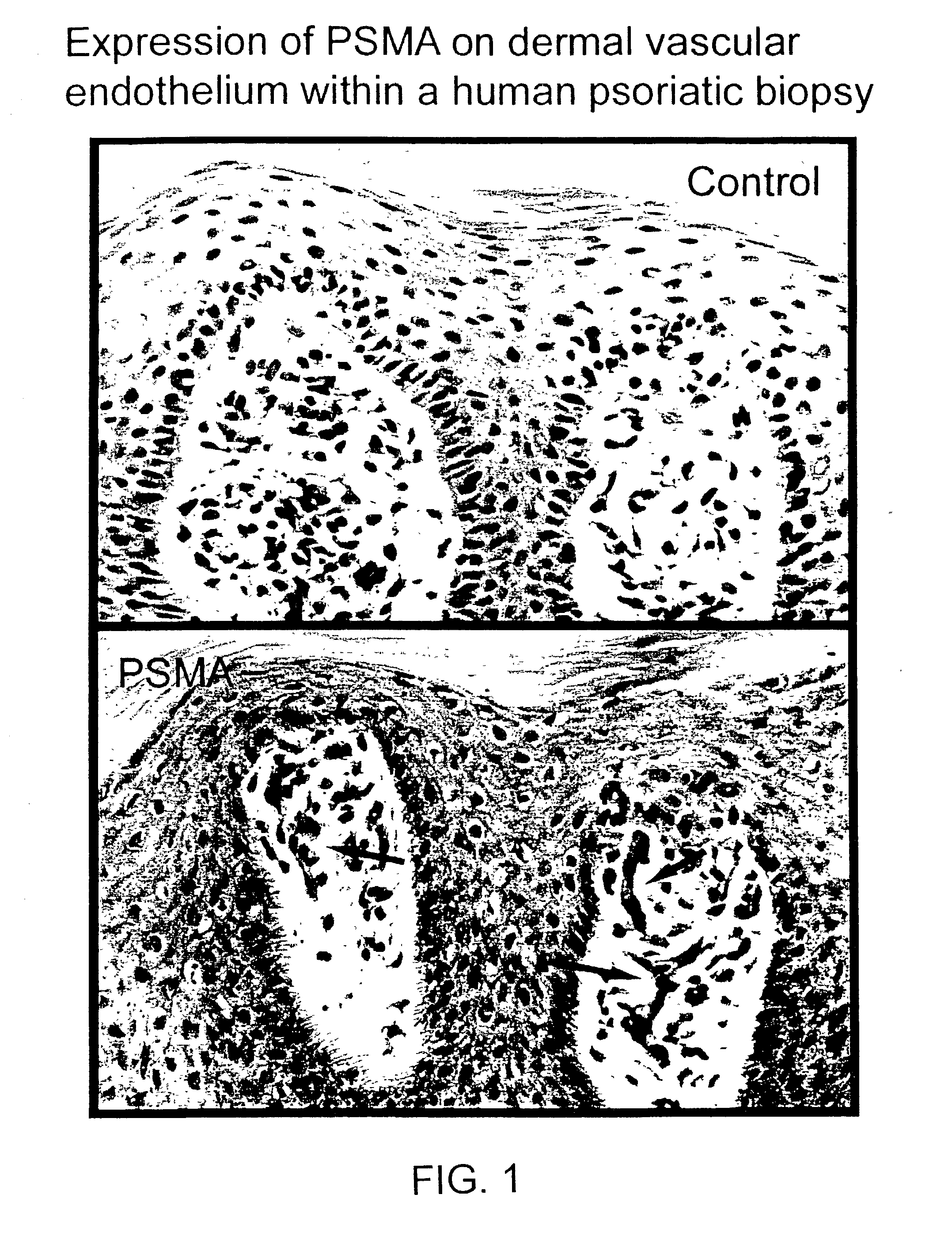
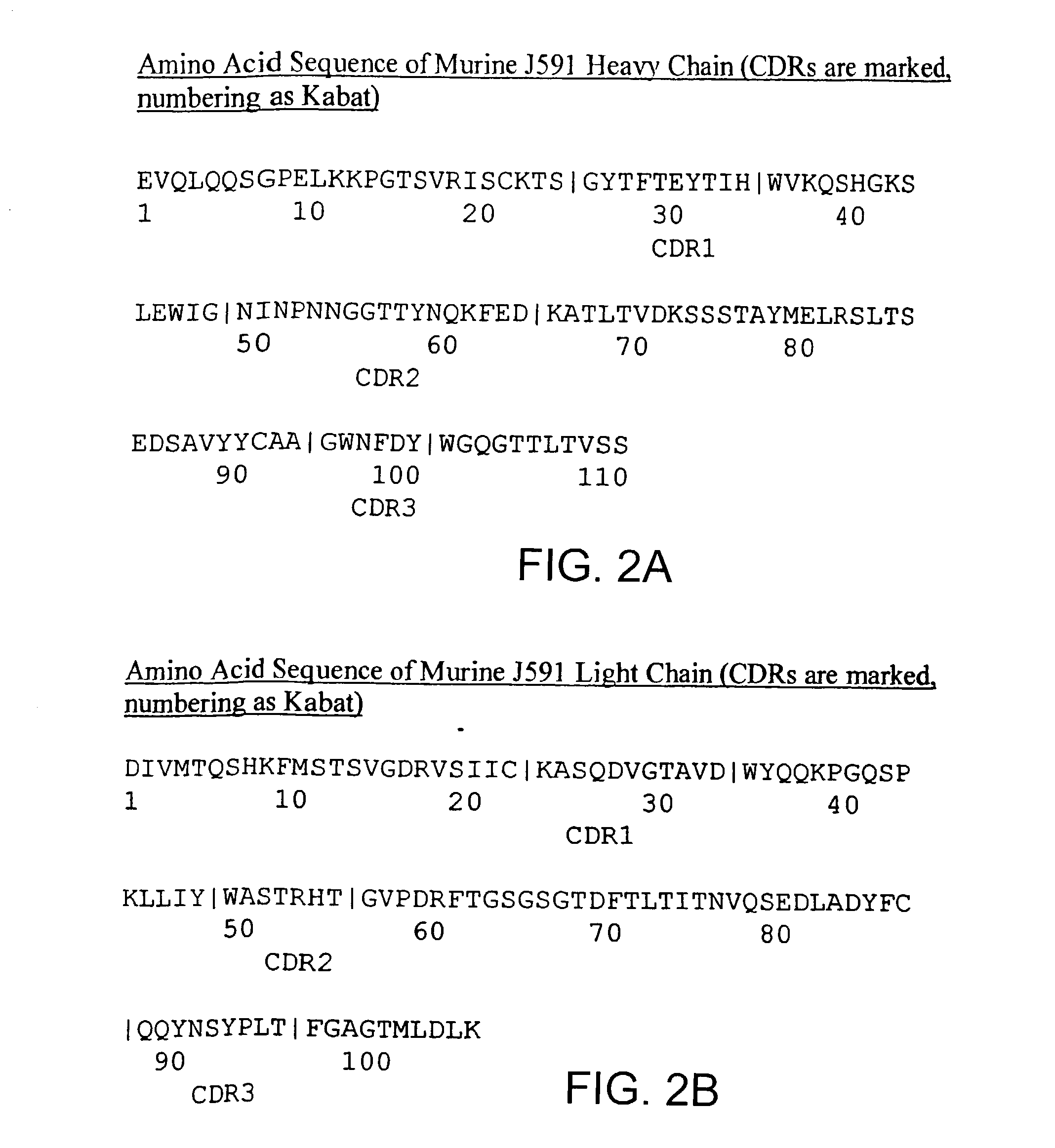
InactiveUS7192586B2Reduce severityHigh expressionOrganic active ingredientsHybrid immunoglobulinsPsoriasisAntigen
Methods and compositions for treating, preventing, or diagnosing epidermal or dermal disorders, e.g., psoriasis, are disclosed. The methods and compositions of the invention use binding agents, e.g., antibodies, specific for the extracellular domain of human prostate specific membrane antigen (PSMA).
Owner:CORNELL RES FOUNDATION INC
Compositions and methods for treatment of viral diseases
InactiveUS20080161324A1Slow and stop replicationReduce loadBiocideMicrobiological testing/measurementSingle-Stranded RNADisease
The present invention features compositions, methods, and kits useful in the treatment of viral diseases. In certain embodiments, the viral disease is caused by a single stranded RNA virus, a flaviviridae virus, or a hepatic virus. In particular embodiments, the viral disease is viral hepatitis (e.g., hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E). Also featured are screening methods for identification of novel compounds that may be used to treat a viral disease.
Owner:EXCRX SINGAPORE PTE +1
Anti-inflammatory supplement compositions and regimens to reduce cardiovascular disease risks
InactiveUS20060172012A1Relieve symptomsPromotes fast digestionOrganic active ingredientsBiocideBlueberry extractApple extract
Disclosed are improvements in human nutrition involving a unique combination of natural products constituting anti-inflammatory compositions which can reduce cardiovascular disease risks as well as play a positive role in other conditions and diseases for which key indicators, especially selected from the group consisting of C-reactive protein (CRP) levels, cyclooxygenase-2 (COX-2), 5-lypoxygenase (5-LOX) expression and prostaglandin E2 (PGE-2) biosynthesis or any combination of these, are indicators. Therapeutic compositions preferably comprise curcumin, bilberry extract, grape seed extract, green tea extract and apple extract, in effective amounts individually and combined to provide a therapeutically significant reduction in one or more key indicators. Another exemplified therapeutic composition comprises: omega-3 rich refined fish oil, resveratrol, blueberry extract, grape seed extract, green tea extract and gamma and / or delta tocopherol, in effective amounts individually for the above benefits.
Owner:A M TODD
Method and apparatus for preventing obstructive sleep apnea
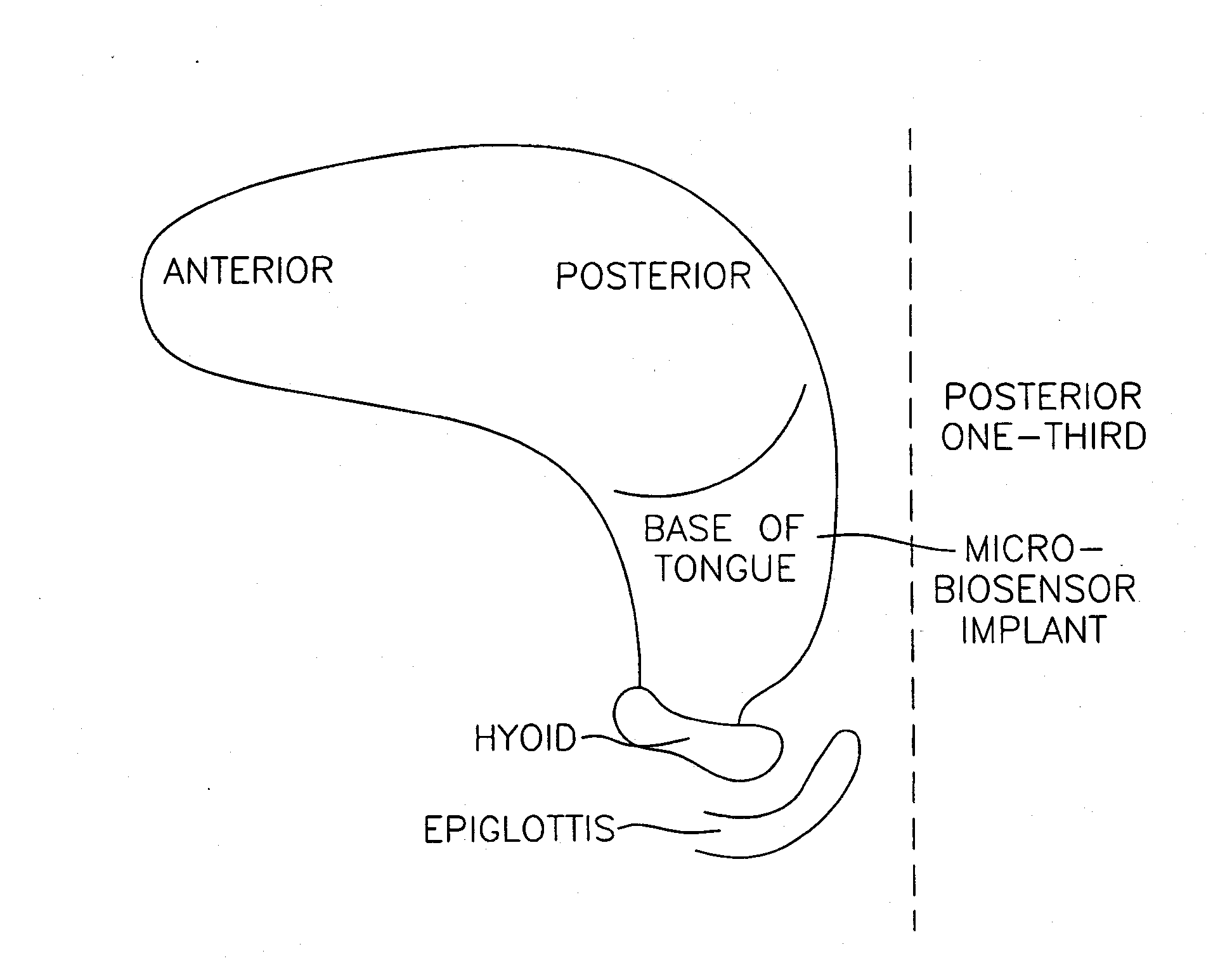
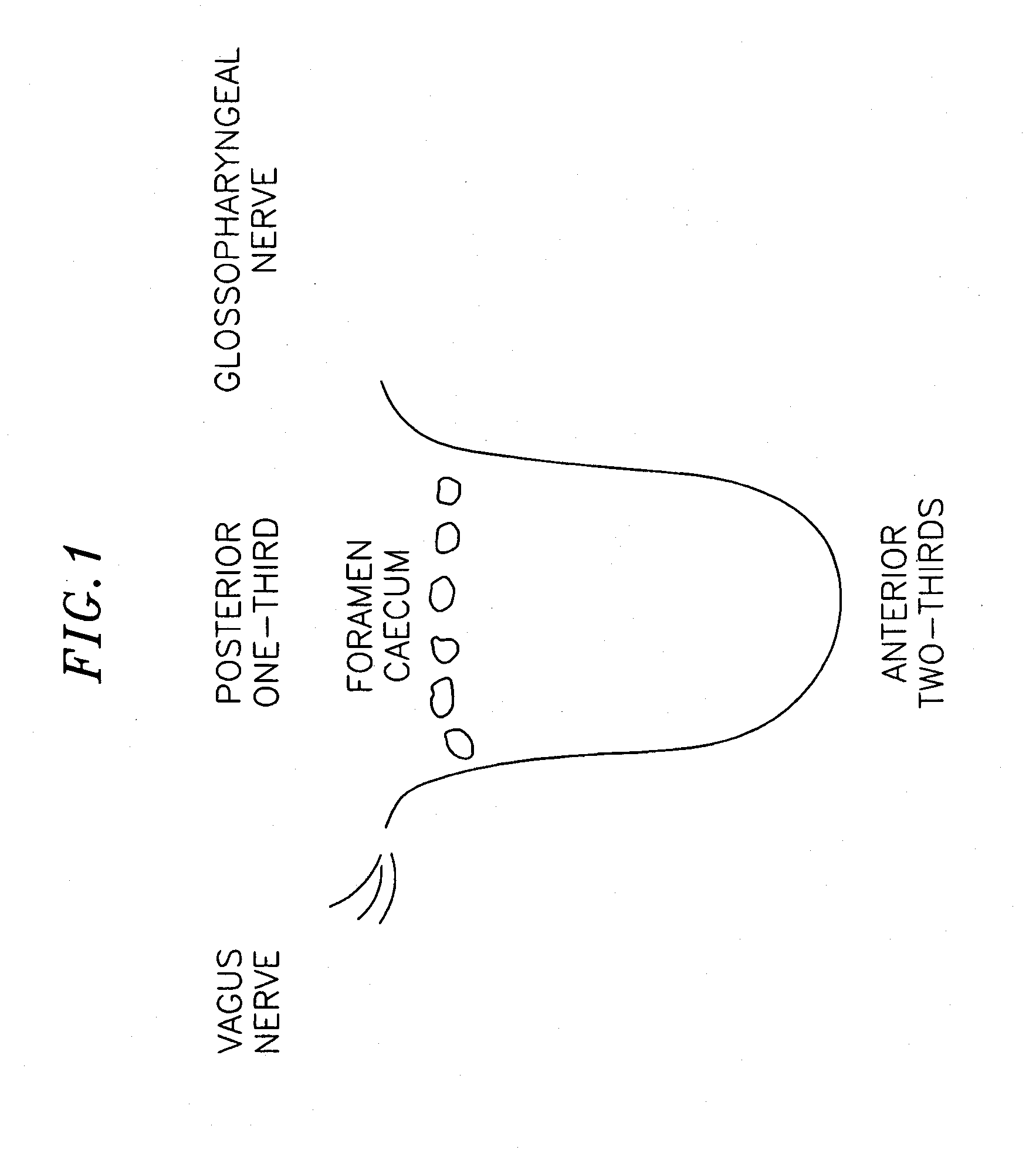
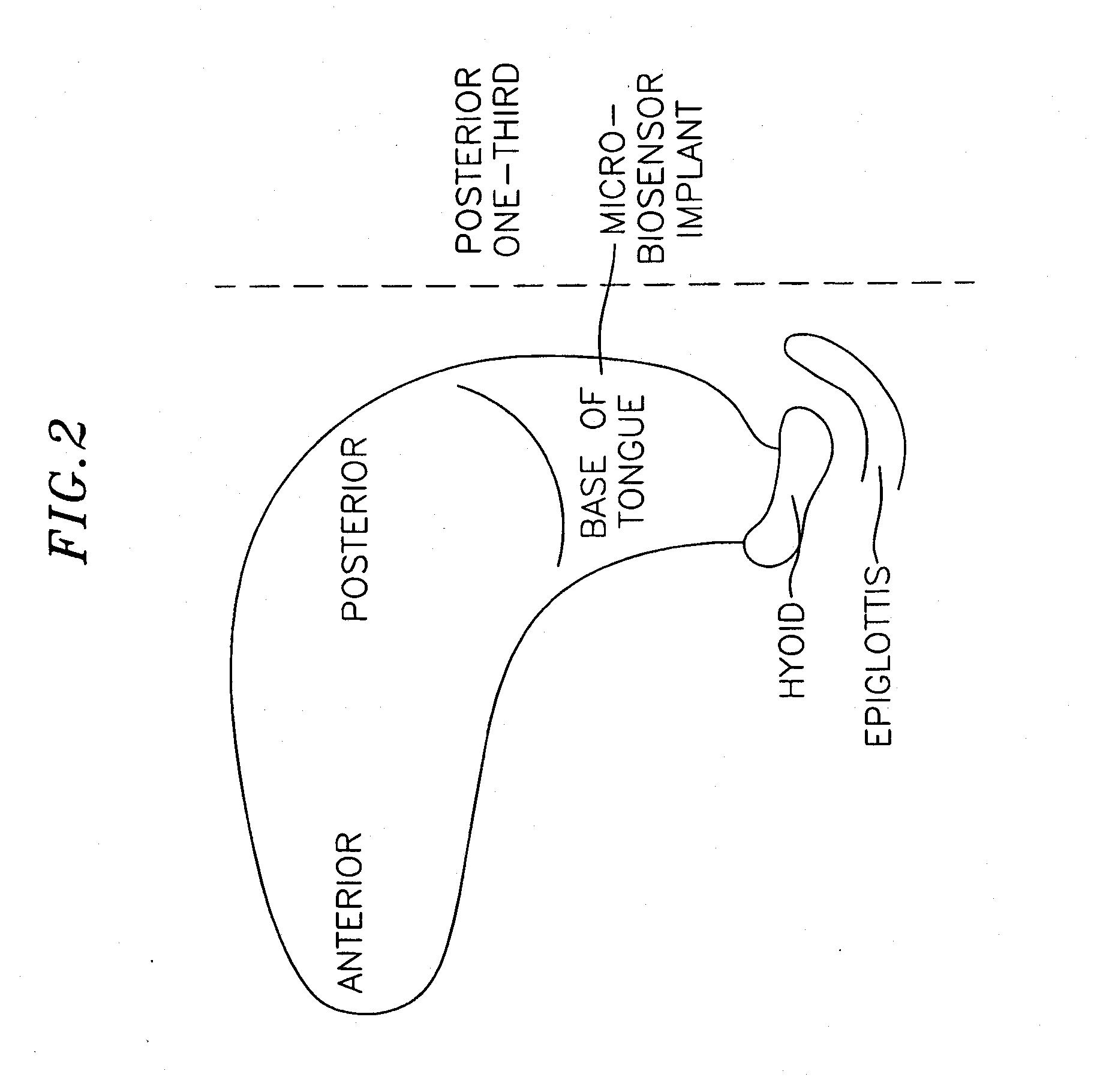
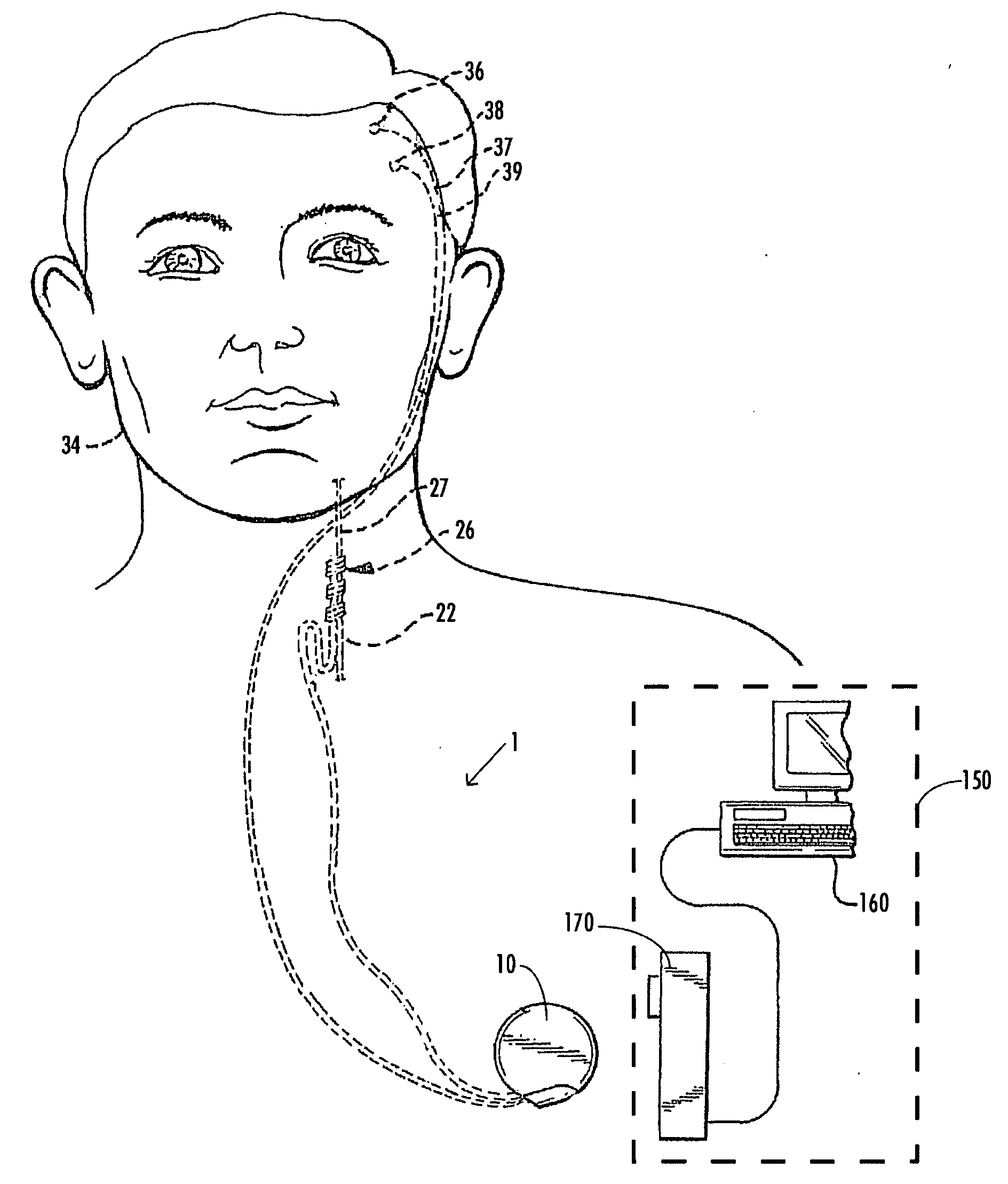
InactiveUS20070173893A1Patient compliance is goodRelieve symptomsElectrotherapyArtificial respirationMuscle toneGenioglossus muscle
A method and device for creating an afferent stimulus for preventing obstructive sleep apnea are disclosed. The device includes at least one electrode and a stimulator, of which at least one electrode stimulates the genioglossus muscle of a patient having obstructive sleep apnea. The electrode is capable of conducting selected electrical stimulation generated by the stimulator, and the system is capable of delivering the selected electrical stimulation during a selected time of day. The electrical stimulation is selected to maintain sufficient muscle tone of the genioglossus muscle to prevent it from obstructing the airway during sleep, preferably at a stimulus intensity low enough to avoid awakening the patient during sleep. A removable mouthpiece having a battery, at least one electrode, and a controller, and optionally a sensor, can be used for providing the stimulation.
Owner:PITTS WALTER C
Selective neurostimulation for treating mood disorders
ActiveUS20070027500A1Relieve symptomsAlter modulation of neuronal activityHead electrodesDiseaseRegimen
A method and device for treating a mood and / or anxiety disorder are disclosed which comprise electrical, chemical or magnetic stimulation of certain areas of the brain to modulate neuronal activity of areas associated with symptoms of mood disorders. In certain embodiments, deep brain stimulation is combined with cranial nerve stimulation to enhance symptomatic relief of the disorder. Certain embodiments also employ a sensing capability to optimize the therapeutic treatment regimen.
Owner:LIVANOVA USA INC
Electric modulation of sympathetic nervous system
InactiveUS7239912B2Increase energy expenditureReduce food intakeInternal electrodesExternal electrodesNervous systemAdrenal tissue
A method for the treatment of obesity or other disorders, by electrical activation or inhibition of the sympathetic nervous system. This activation or inhibition can be accomplished by electrically stimulating the greater splanchnic nerve or other portion of the sympathetic nervous system using an implantable pulse generator. This nerve activation can result in reduced food intake and increased energy expenditure. Reduced food intake may occur through a variety of mechanisms that reduce appetite and cause satiety. Increased adrenal gland hormone levels will result in increased energy expenditure. Fat and carbohydrate metabolism, which are also increased by sympathetic nerve activation, will accompany the increased energy expenditure.
Owner:ADVANCED NEUROMODULATION SYST INC
Uses and compositions for treatment of psoriatic arthritis
InactiveUS20080311043A1Improve the quality of lifeTreatment safetyCompounds screening/testingPeptide/protein ingredientsAntigen bindingPsoriasis arthropathy
The invention provides methods, uses and compositions for the treatment of psoriatic arthritis. The invention describes methods and uses for treating psoriatic arthritis, wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof, is used to psoriatic arthritis in a subject. Also described are methods for determining the efficacy of a TNFα inhibitor for treatment of psoriatic arthritis in a subject.
Owner:HOFFMAN REBECCA S +7
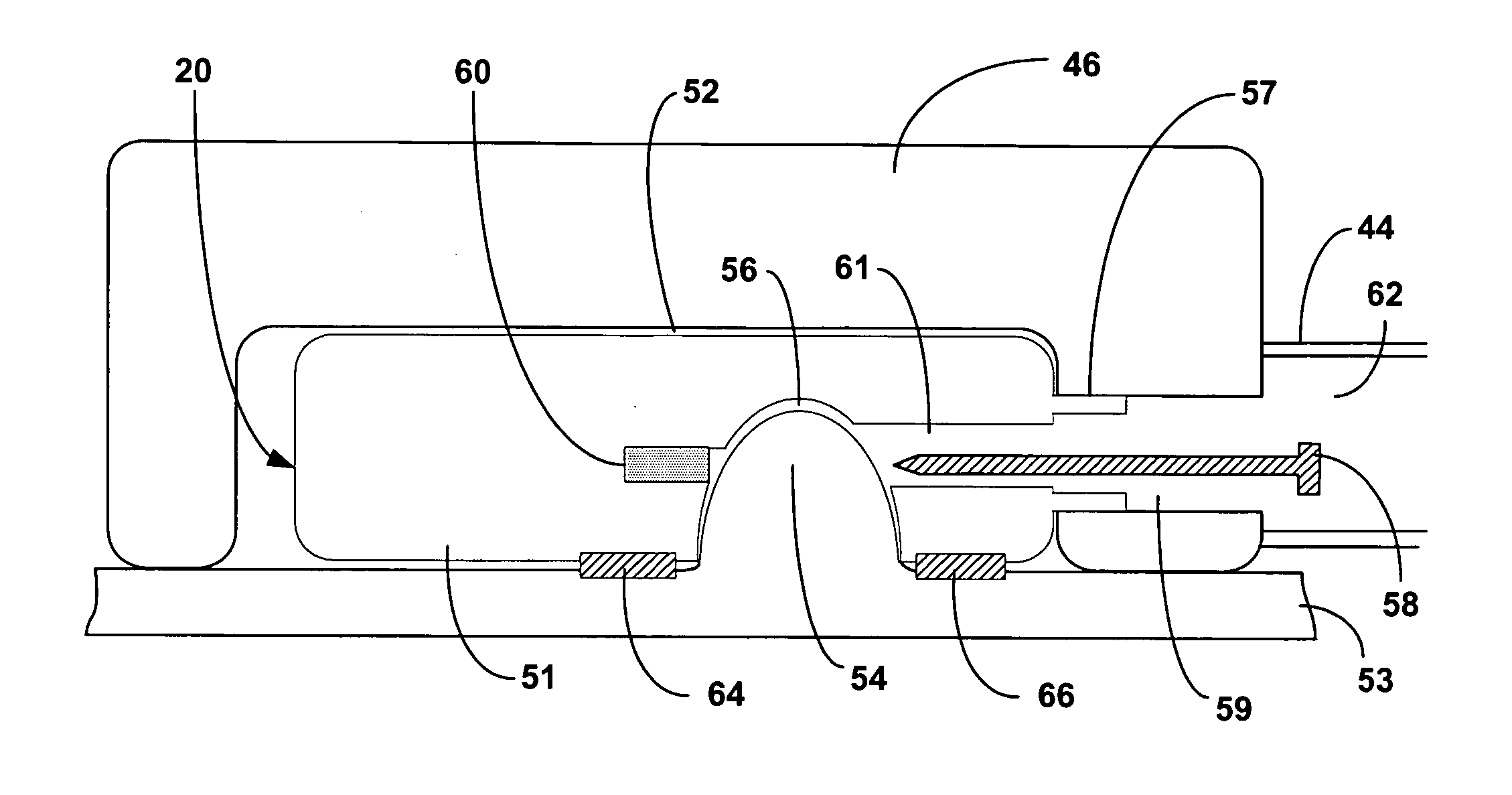
Intra-luminal device for gastrointestinal electrical stimulation
InactiveUS20050209653A1Reduce deliveryRelieve symptomsDiagnostic recording/measuringSensorsShort termsElectrical stimulations
An intra-luminal device for gastrointestinal electrical stimulation is self-powered and self-contained within a capsule-like housing, and is capable of non-surgical implantation within the patient. The device includes an implantable pulse generator and one or more electrodes mounted within a common device housing. The device housing is capable of endoscopic introduction to a desired location within the gastrointestinal tract, such as the stomach, via the esophagus. The device may be appropriate for short-term, mid-term or trial stimulation applications.
Owner:MEDTRONIC INC
Uses and compositions for treatment of CROHN'S disease
InactiveUS20090317399A1Relieve symptomsDecreasing hospitalization riskAntibody ingredientsImmunoglobulinsAntigen bindingCrohn's disease
The invention provides methods, uses and compositions for the treatment of Crohn's disease. The invention describes methods and uses for treating Crohn's disease, wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof, is used to induce and maintain remission of Crohn's disease in a subject. Also described are methods for determining the efficacy of a TNFα inhibitor for treatment of Crohn's disease in a subject.
Owner:ABBVIE BIOTECHNOLOGY LTD
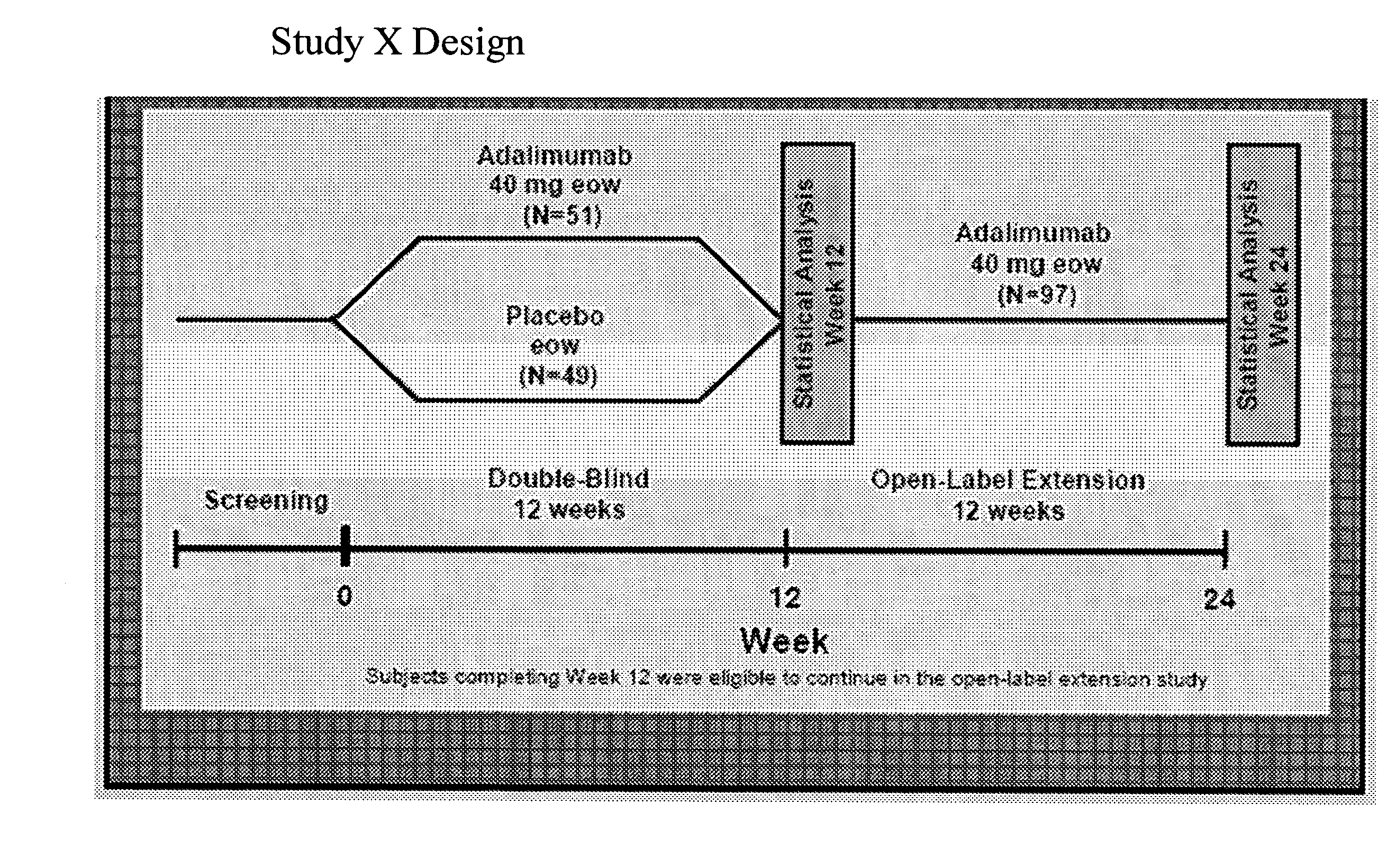
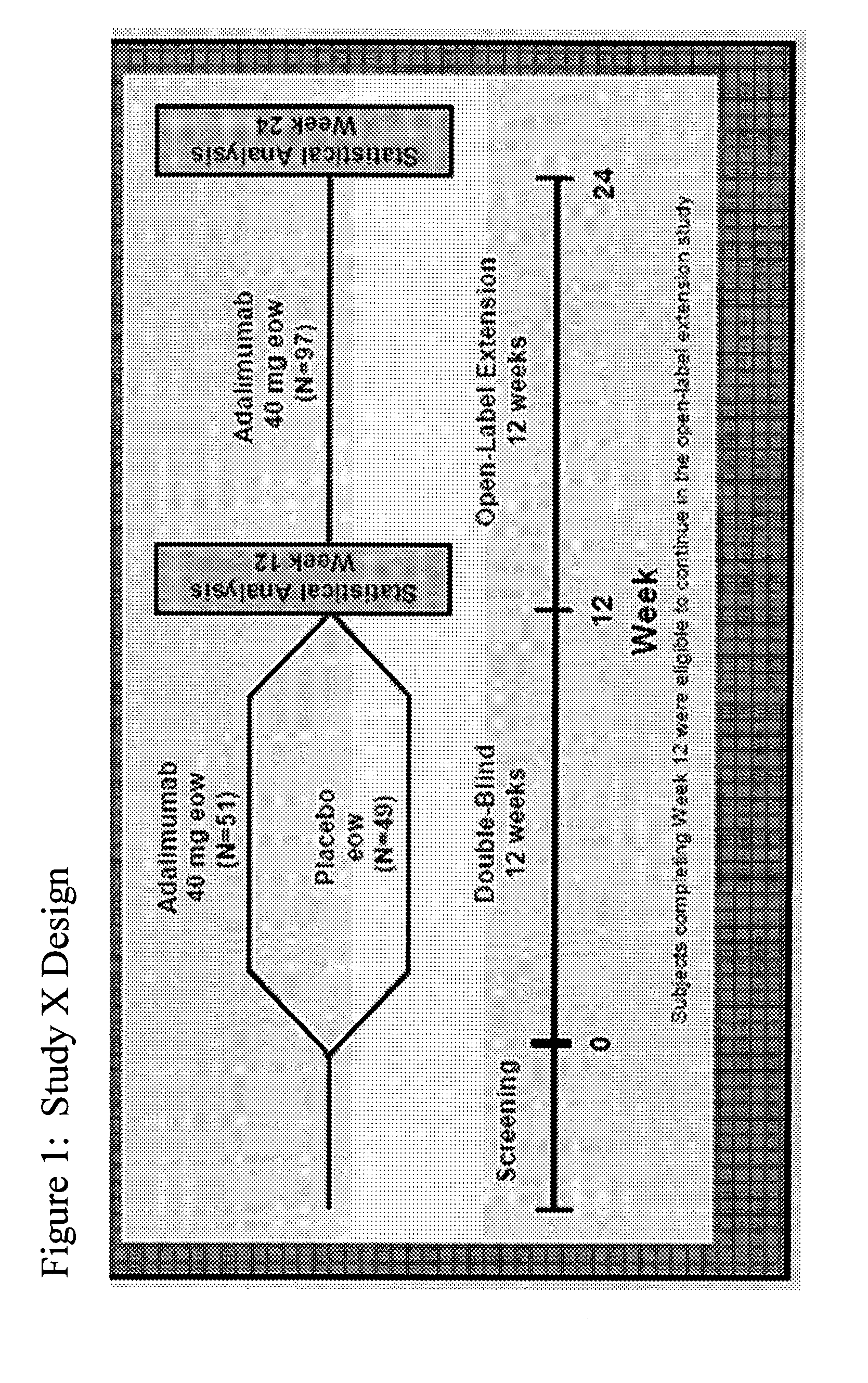
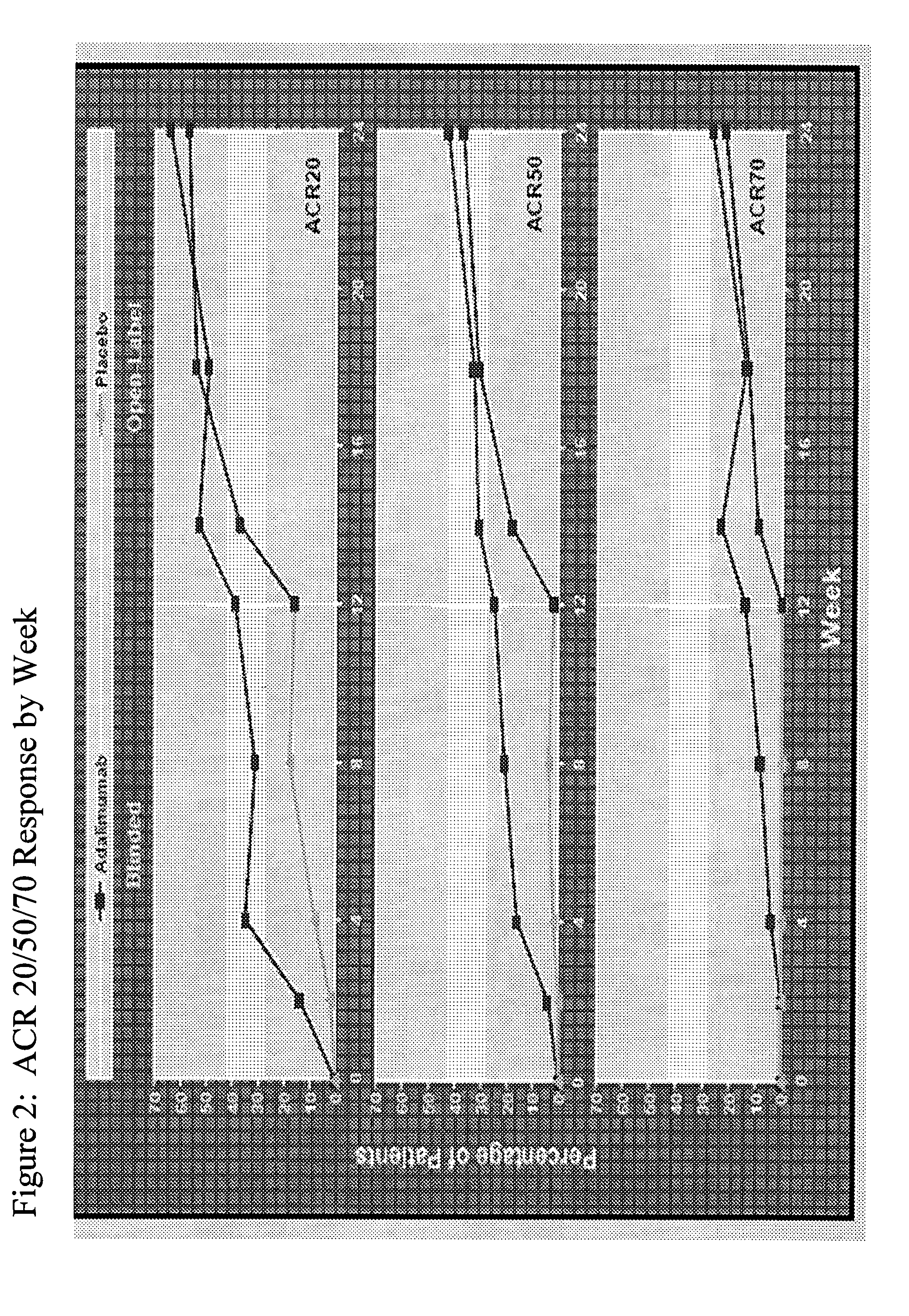
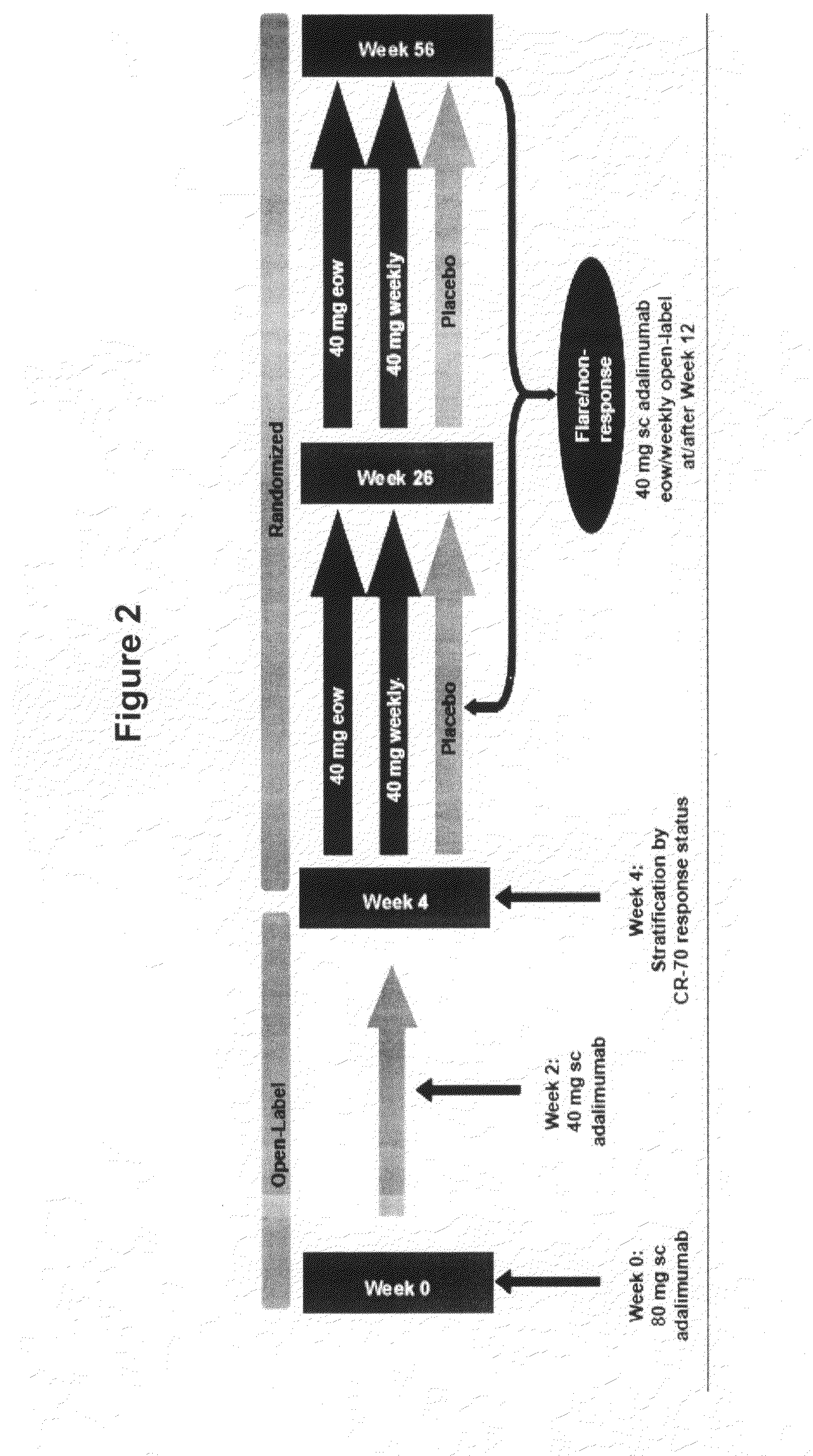
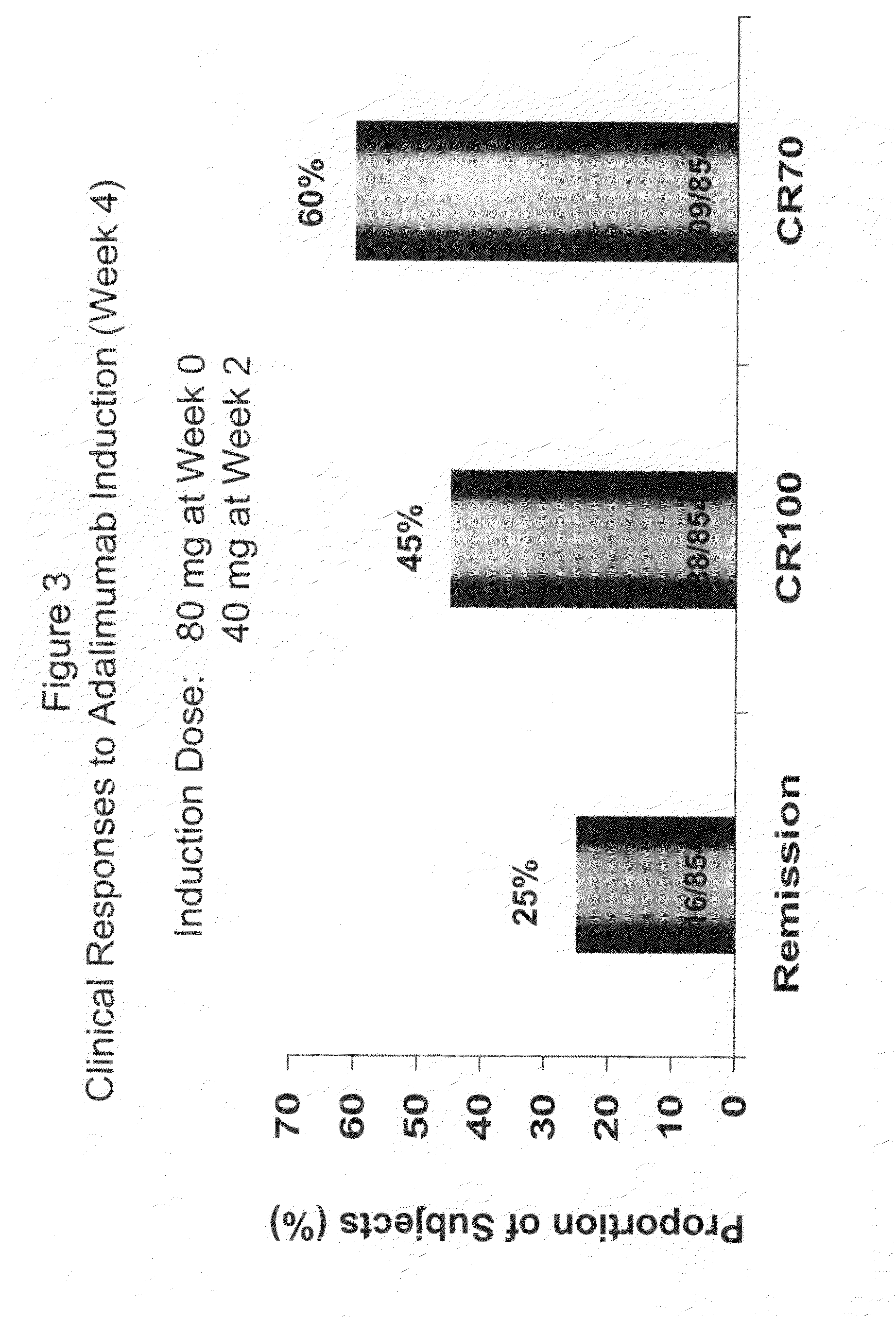
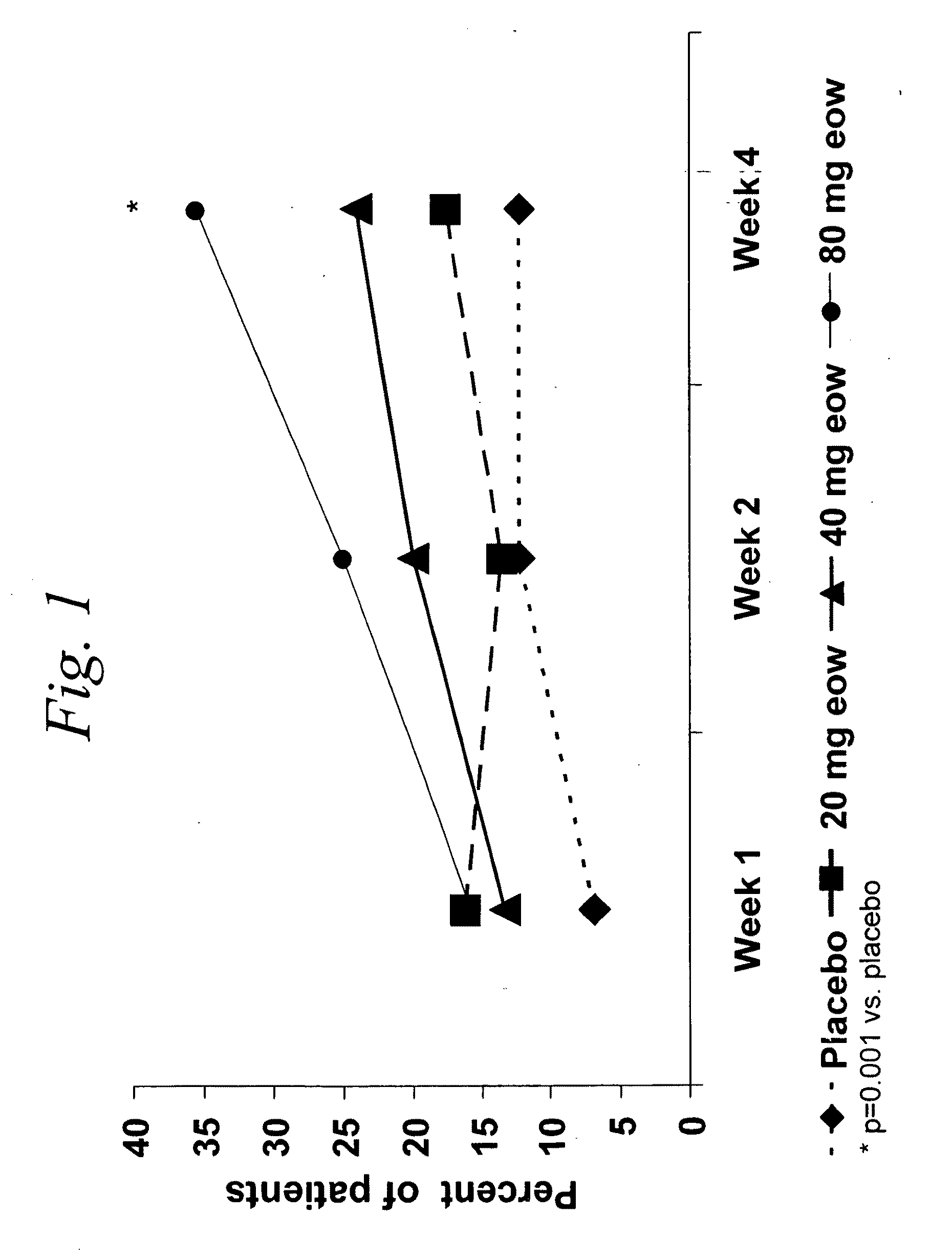
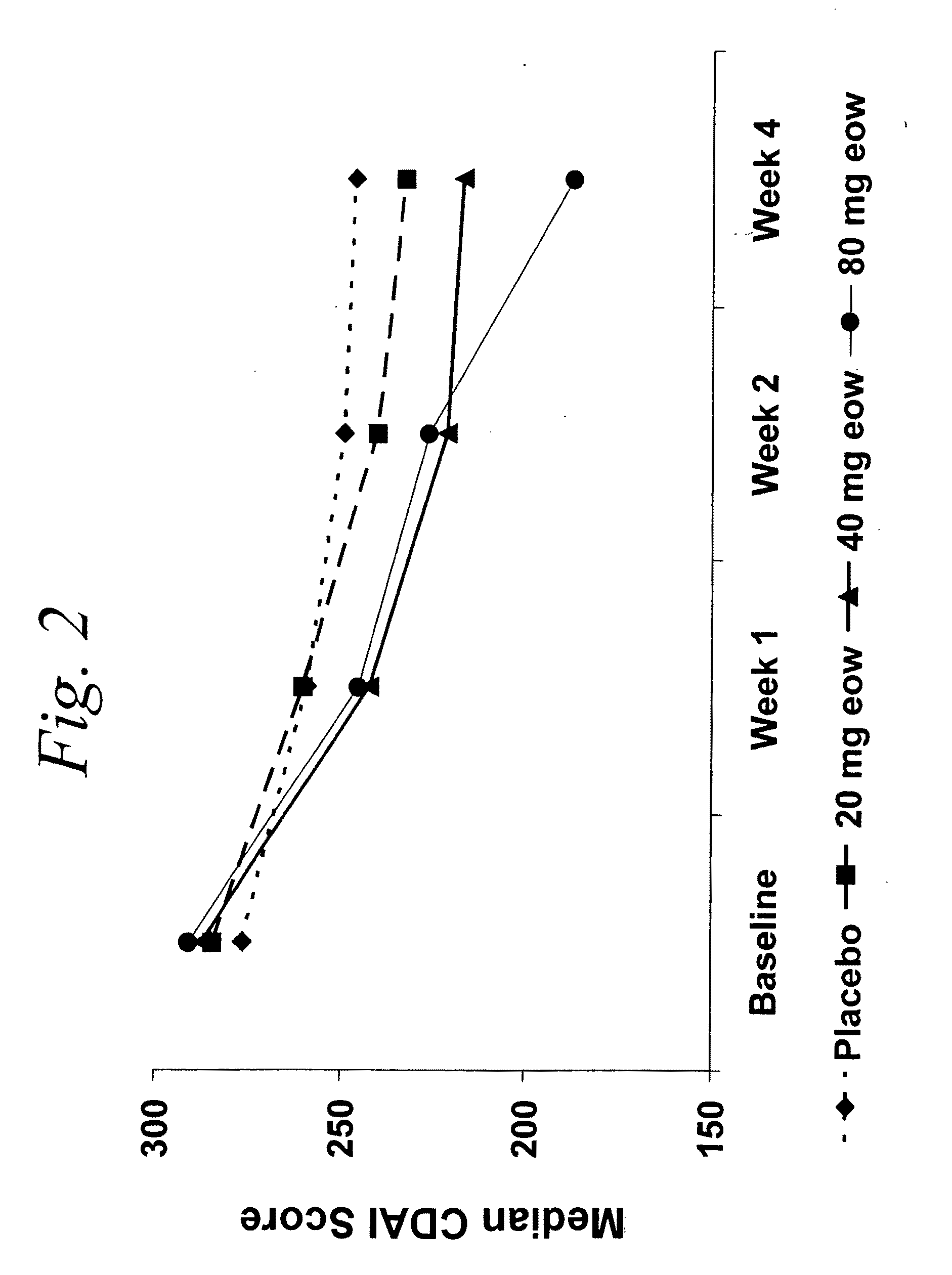
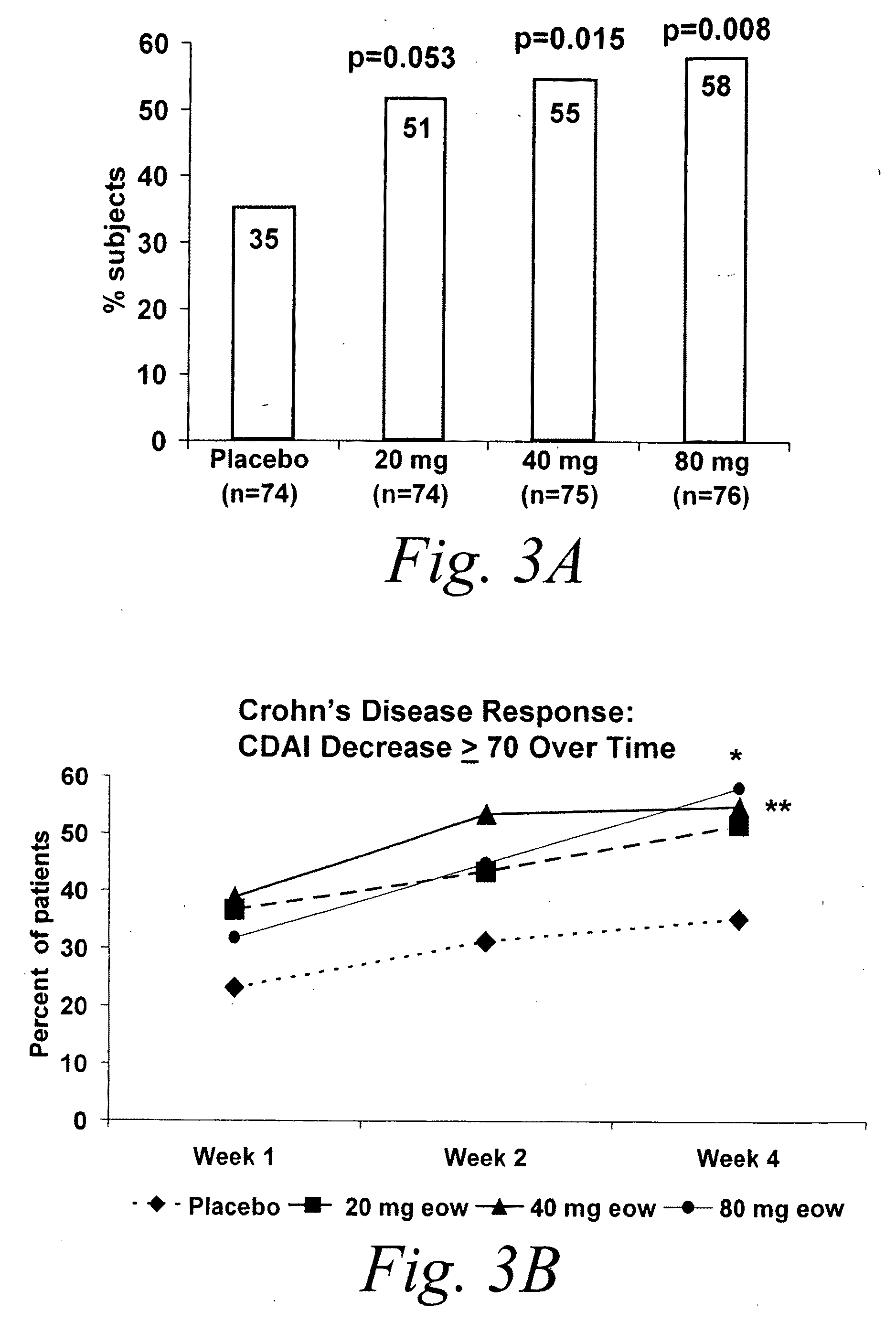
Multiple-variable dose regimen for treating TNFa-related disorders
InactiveUS20090304682A1Reducing remissionReducing signOrganic active ingredientsPeptide/protein ingredientsDosing regimenRegimen
Owner:ABBVIE BIOTECHNOLOGY LTD
Responsive therapy for psychiatric disorders
ActiveUS7353065B2Relieve symptomsHigh energyHead electrodesExternal electrodesDiseaseBipolar mood disorder
An implantable neurostimulator system for treating psychiatric disorders includes scheduled and responsive therapy capabilities including responsive stimulation applied to the cingulate gyrus of the brain. Methods for treating depression, bipolar disorder, anxiety and obsessive-compulsive disorders, post-traumatic stress disorder, addiction, schizophrenia, and autism and other developmental disorders employ an inventive system to advantageously reduce symptoms and address underlying causes of the disorders.
Owner:NEUROPACE
Fenestrated contact lens for treating myopia
An improved corneal contact lens for use in orthokeratology wherein the lens has an anterior surface, posterior surface and includes a tear zone which defines a tear reservoir located on the posterior surface of the lens. The improvement involves providing at least one fenestration surface located in the tear zone which defines an opening extending through the contact lens from the anterior surface to the posterior surface. The openings in the tear zone relieve fluid pressure which was found to cause certain symptoms, such as lens adhesion, tightening and general wearing discomfort. The fenestrations in the tear zone provide enhanced wearing comfort without adversely affecting the cornea-shaping properties of the lens.
Owner:CONTEX
Antagonizing interleukin-21 receptor activity
InactiveUS20060039902A1Reduce riskSufficient amountCompounds screening/testingCompound screeningWhite blood cellFibrosis
Methods and compositions for inhibiting interleukin-21 (IL-21) / IL-21 receptor (MU-1) activity using antagonists of IL-21 or IL-21 receptor (“IL-21R” or “MU-1”), are disclosed. IL-21 / IL-21R antagonists can be used to induce immune suppression in vivo, e.g., for treating, ameliorating or preventing autoimmune or inflammatory disorders, including, e.g., inflammatory bowel disease (IBD), rheumatoid arthritis (RA), transplant / graft rejection, psoriasis, asthma, fibrosis, and systemic lupus erythematosus (SLE).
Owner:WYETH LLC
Phenylepherine containing dosage form
ActiveUS20060057205A1Good treatment effectRelieve symptomsBiocidePill deliveryNorphenylephrineBlood plasma
A pharmaceutical dosage form which comprises phenylepherine or a pharmaceutically acceptable salt thereof and a second drug. The dosage form provides a plasma concentration within the therapeutic range of the second drug over a period which is coextensive with at least about 70% of the period over which the dosage form provides a plasma concentration within the therapeutic range of phenylepherine. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:CAPELLON PHARMA LLC
2'-O-alkylated oligoribonucleotides and phosphorothioate analogs complementary to portions of the HIV genome
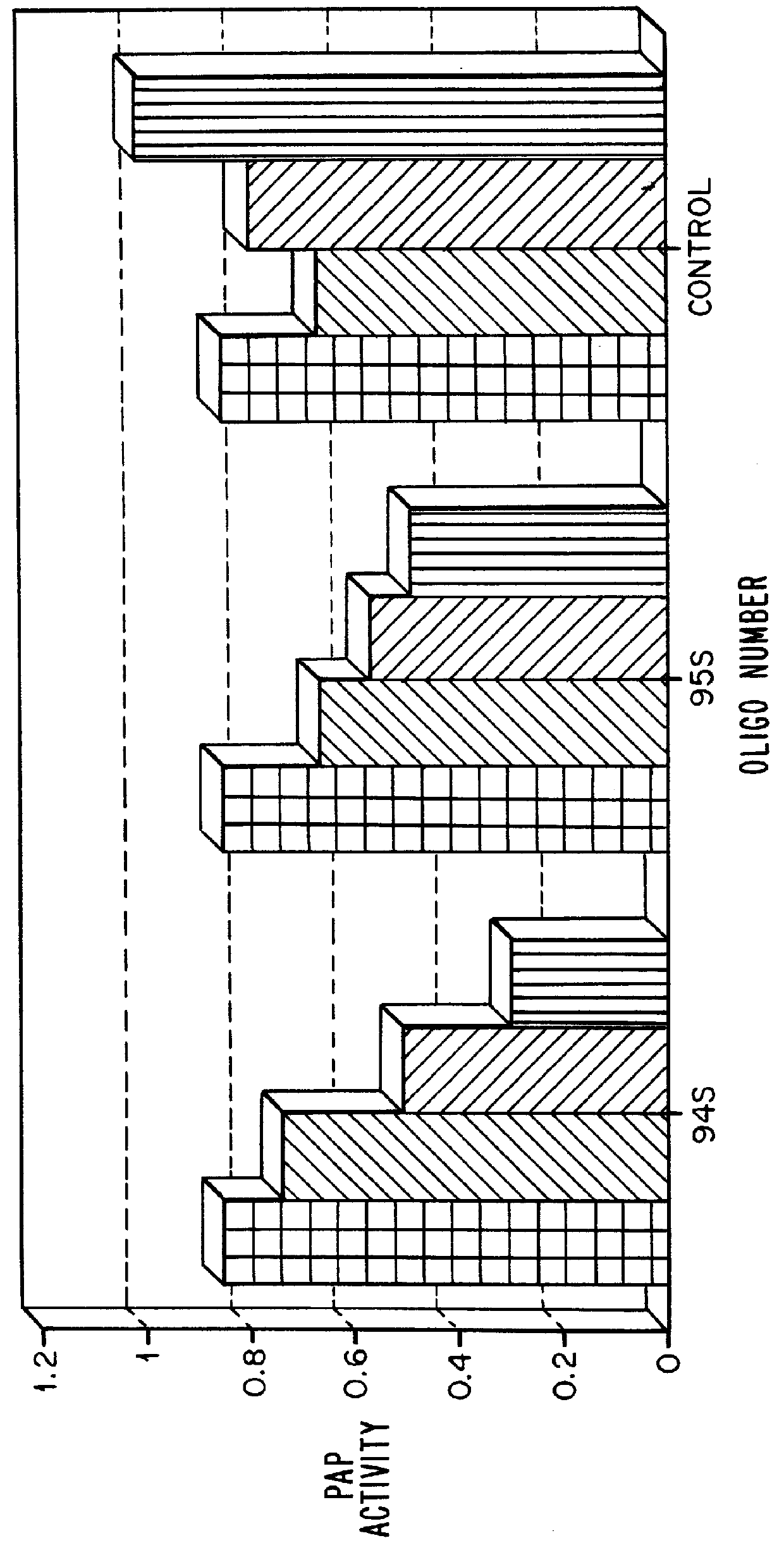
InactiveUS6034233ARelieve symptomsSure easySugar derivativesVirus peptidesOligoribonucleotidesGenome
Owner:IONIS PHARMA INC
AAV vectors for gene delivery to the lung
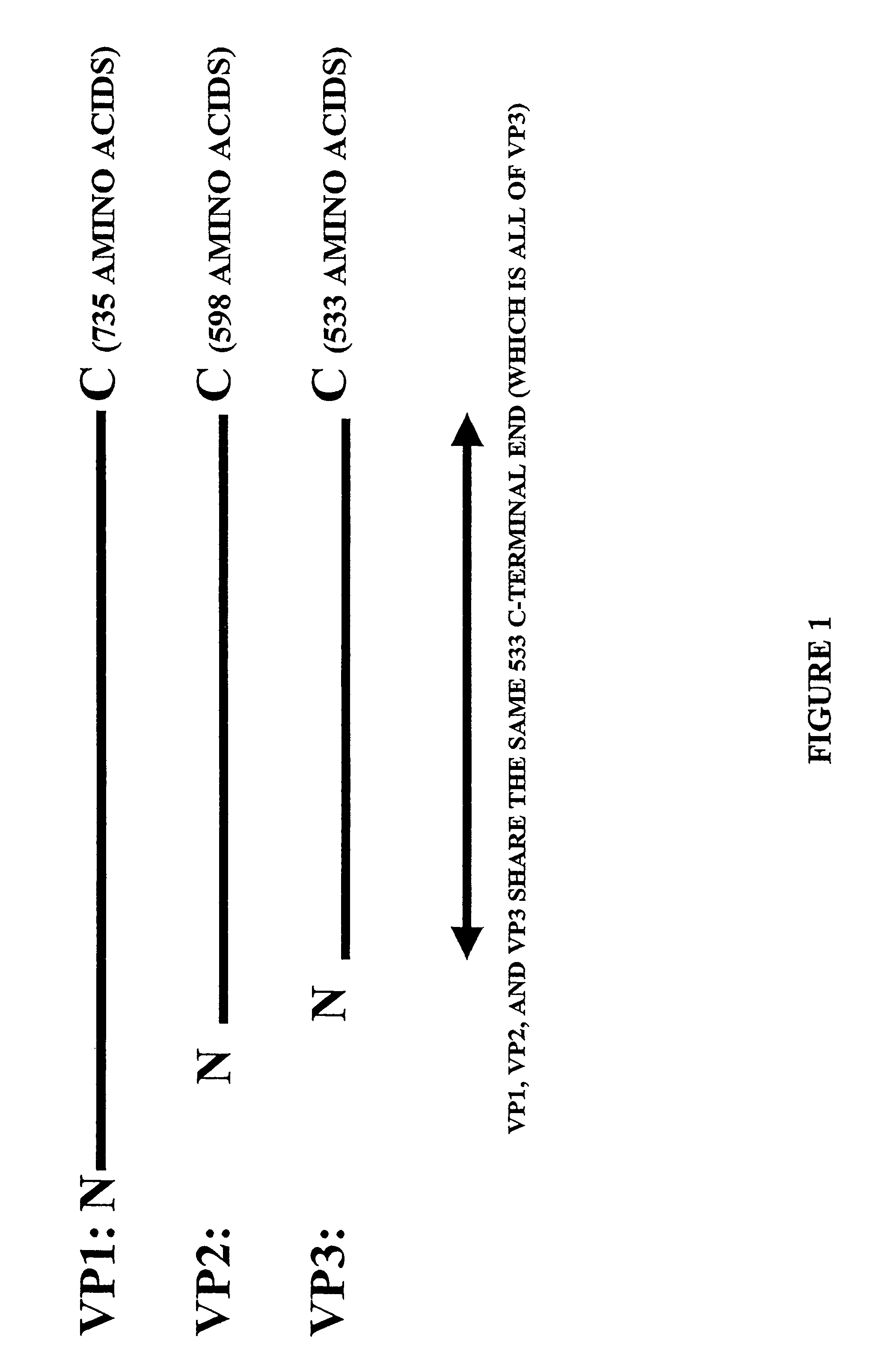
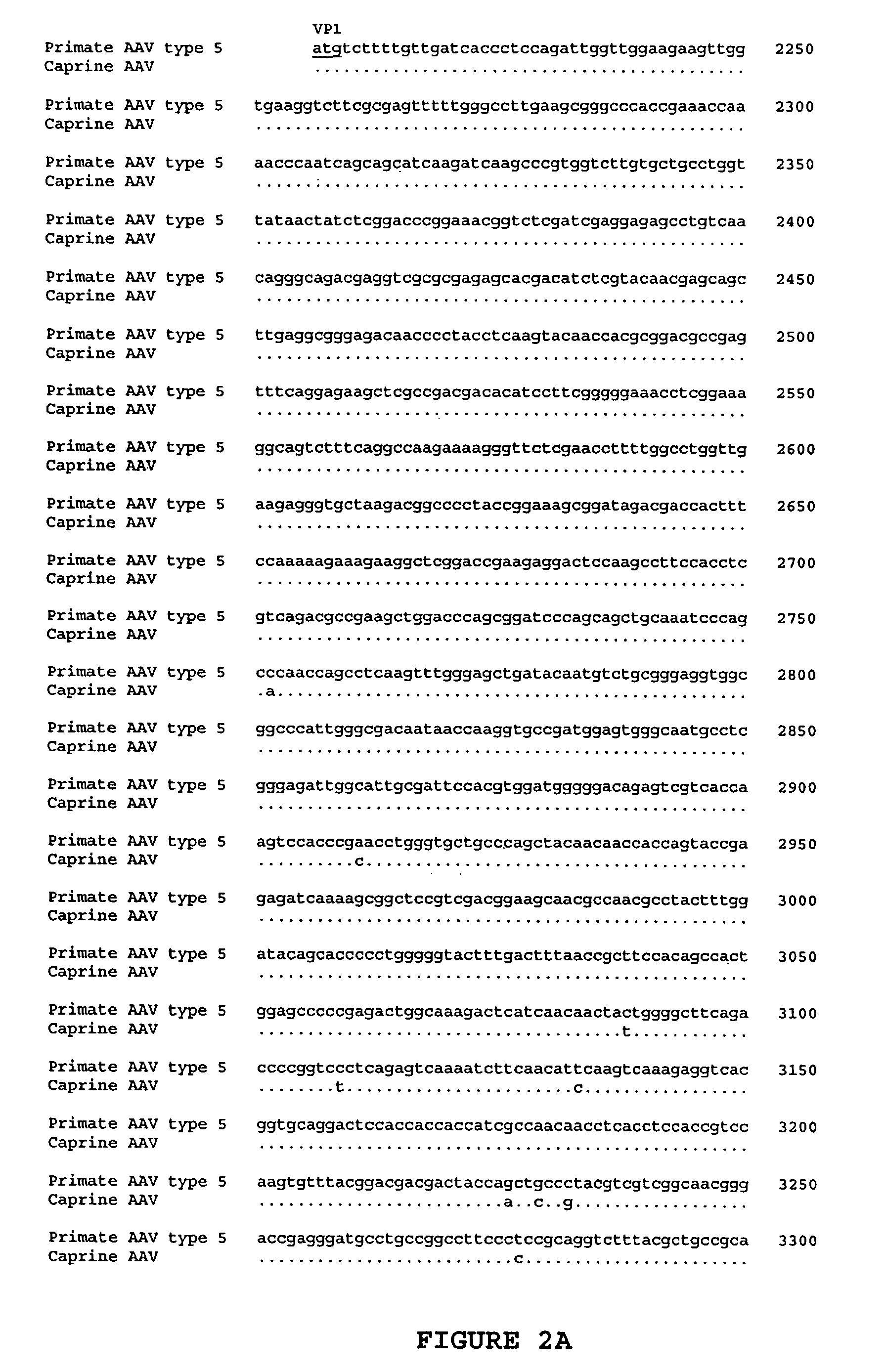
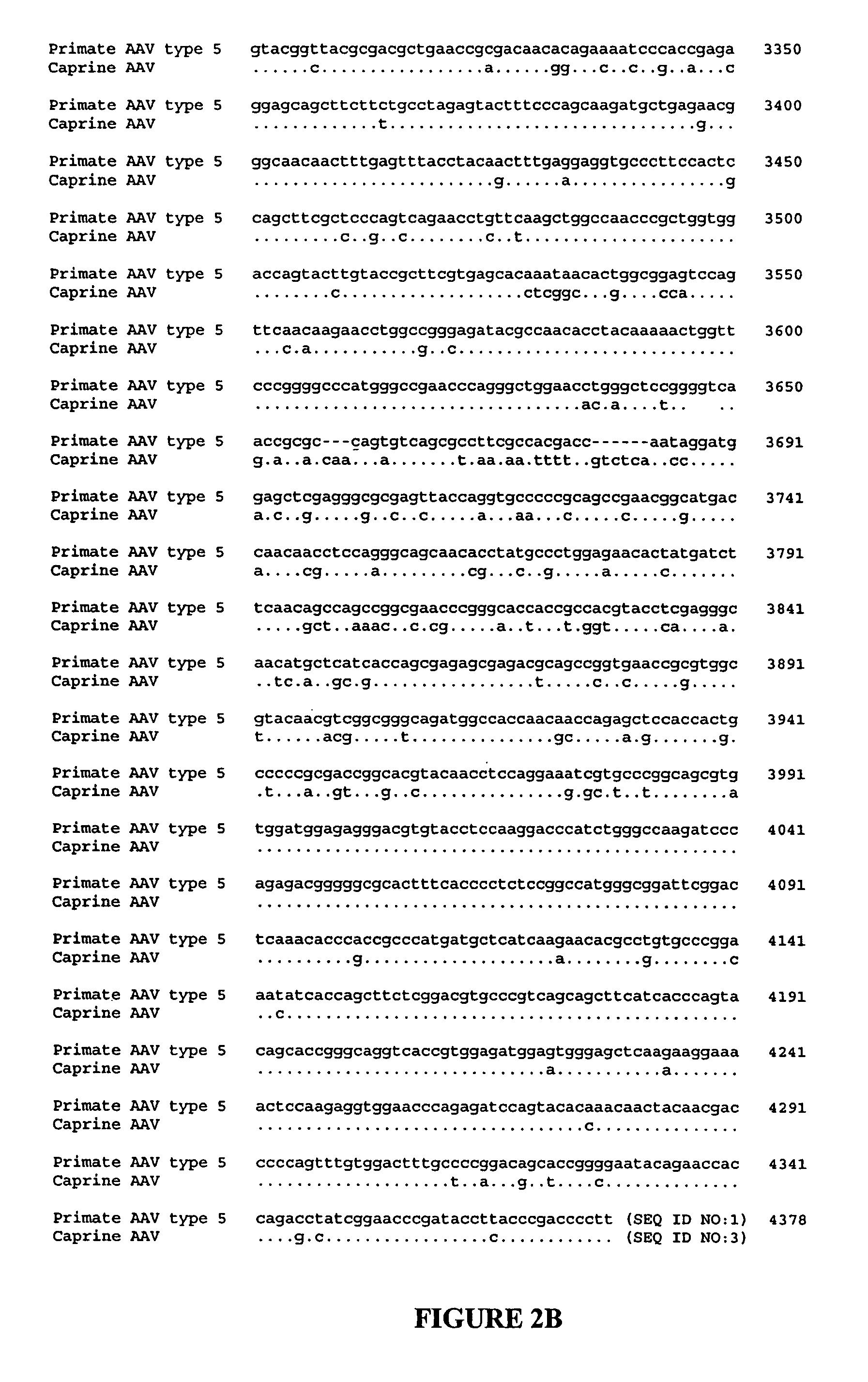
ActiveUS7427396B2Efficient transductionDecreased immunoreactivityPowder deliveryBiocideGene deliveryVirosome
Owner:AVIGEN
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
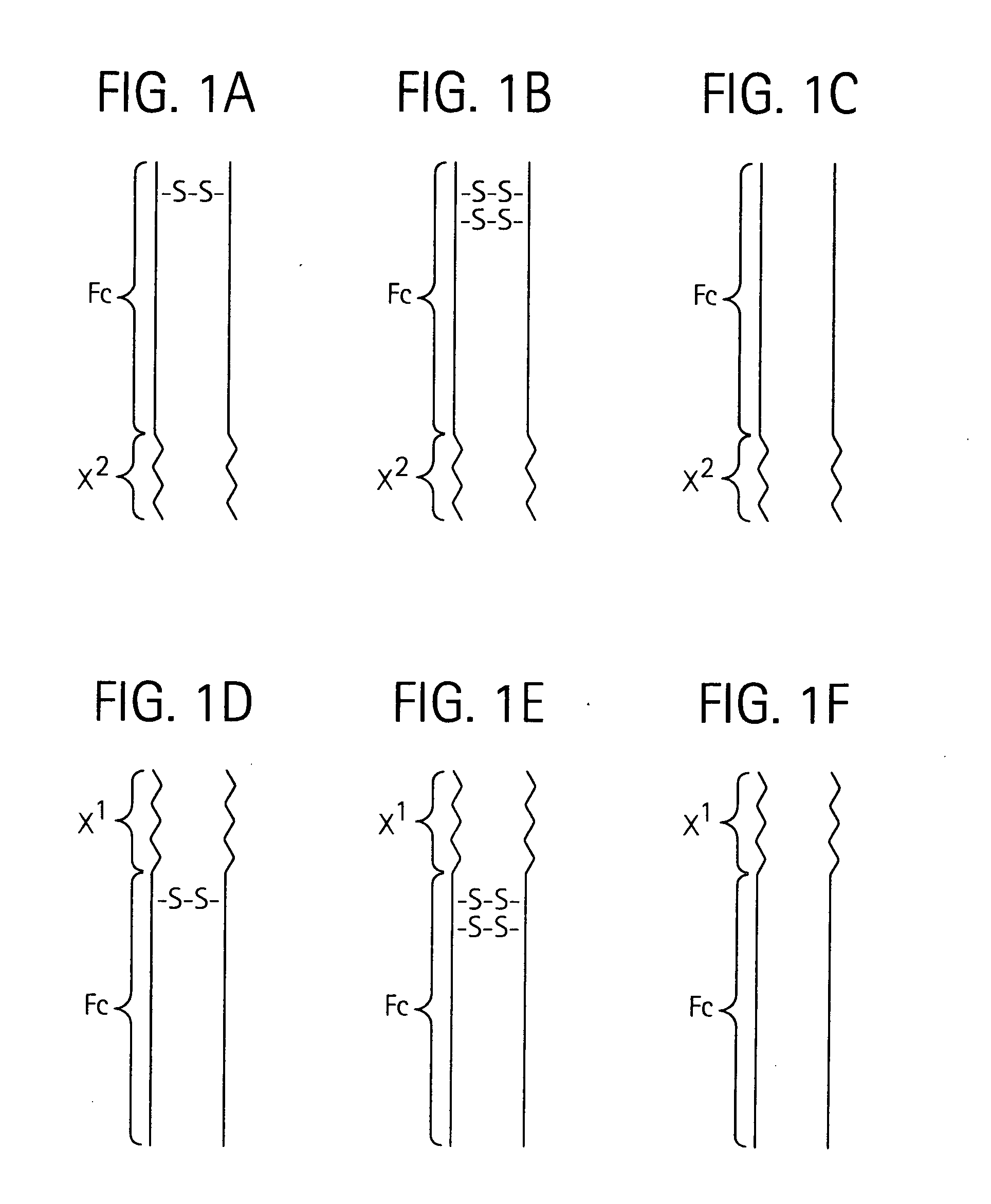
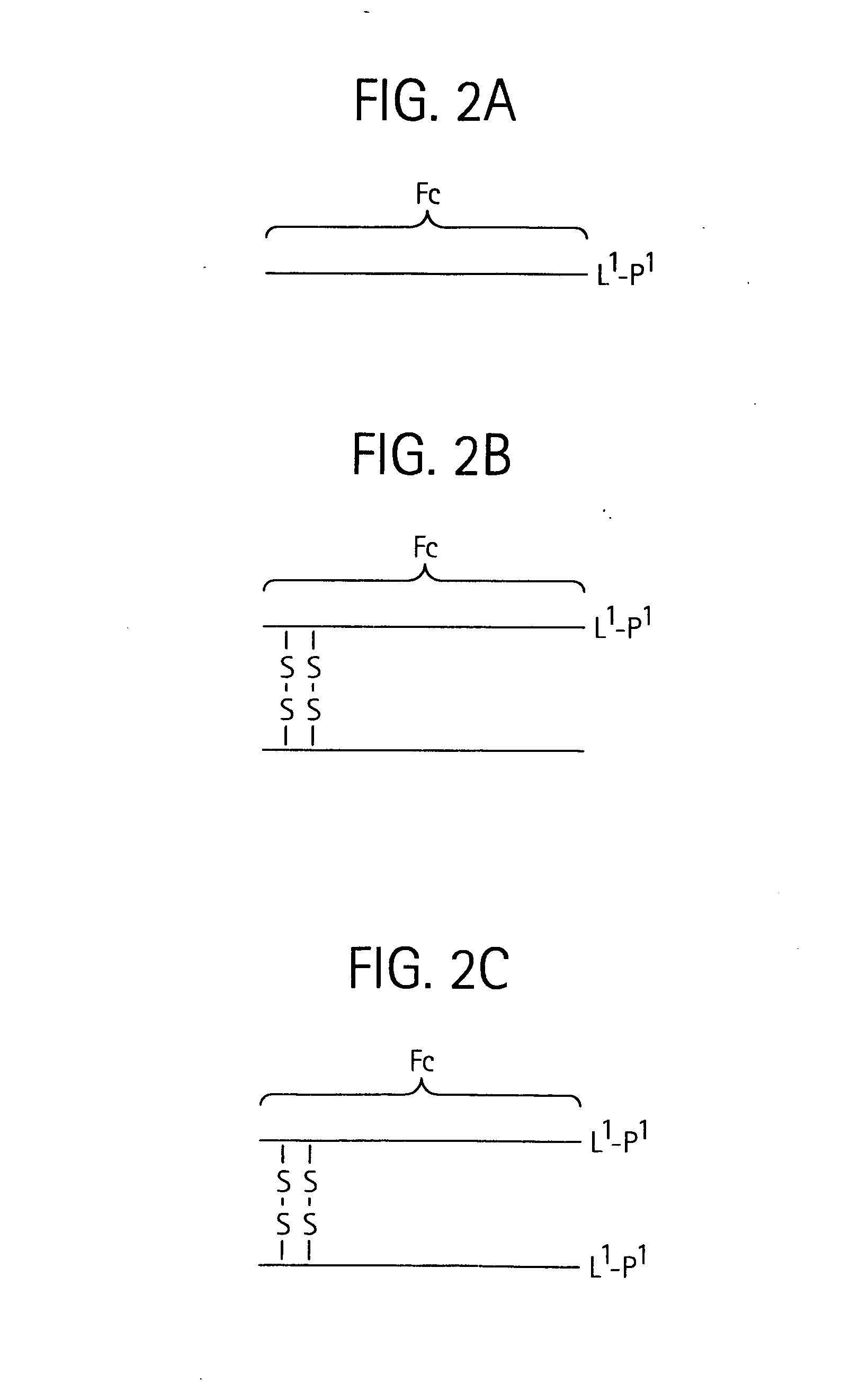
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Immunoglobulin fusion proteins
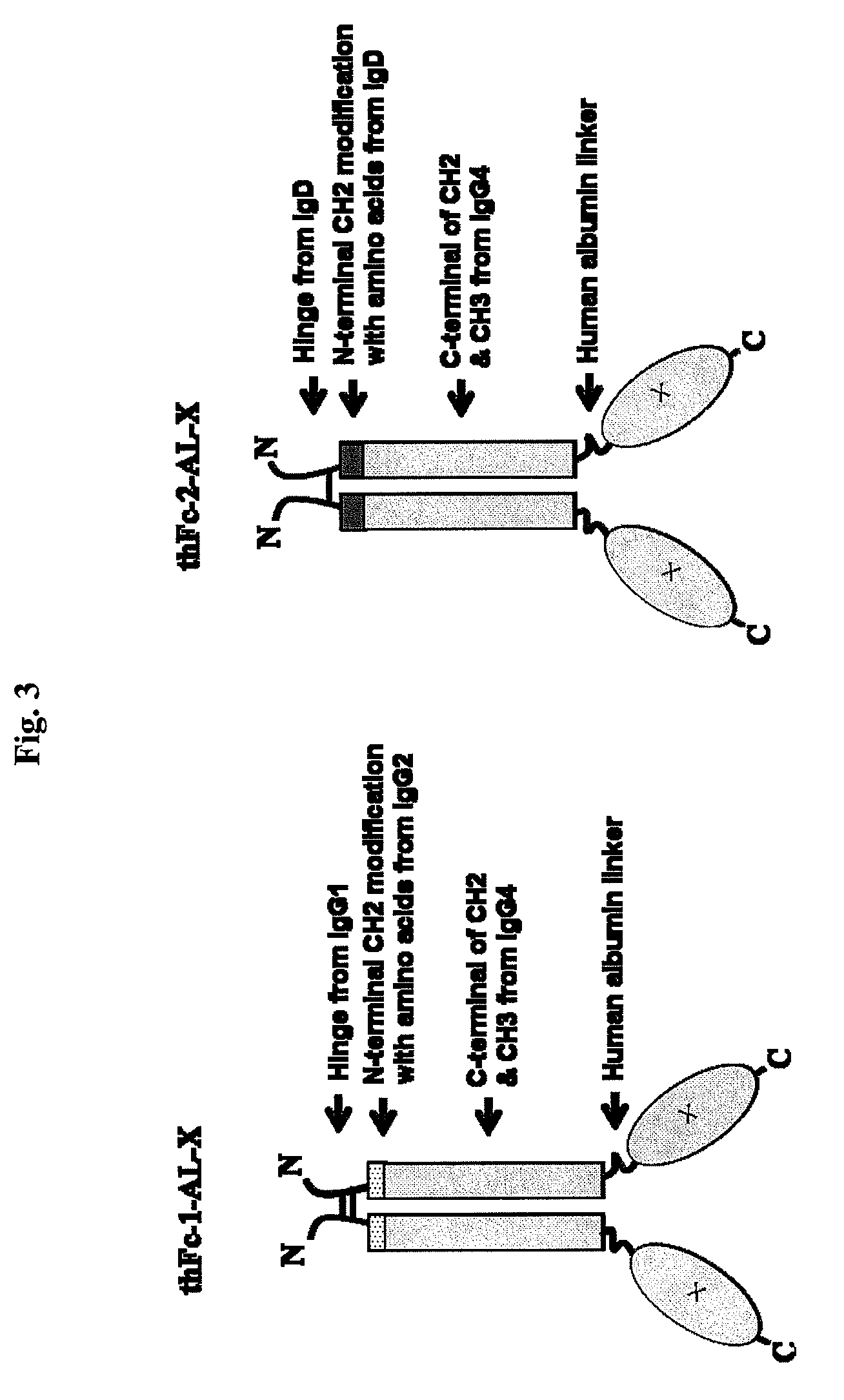
ActiveUS7867491B2Extended half-lifeImprove expression levelPeptide/protein ingredientsAntipyreticFc domainBioactive molecules
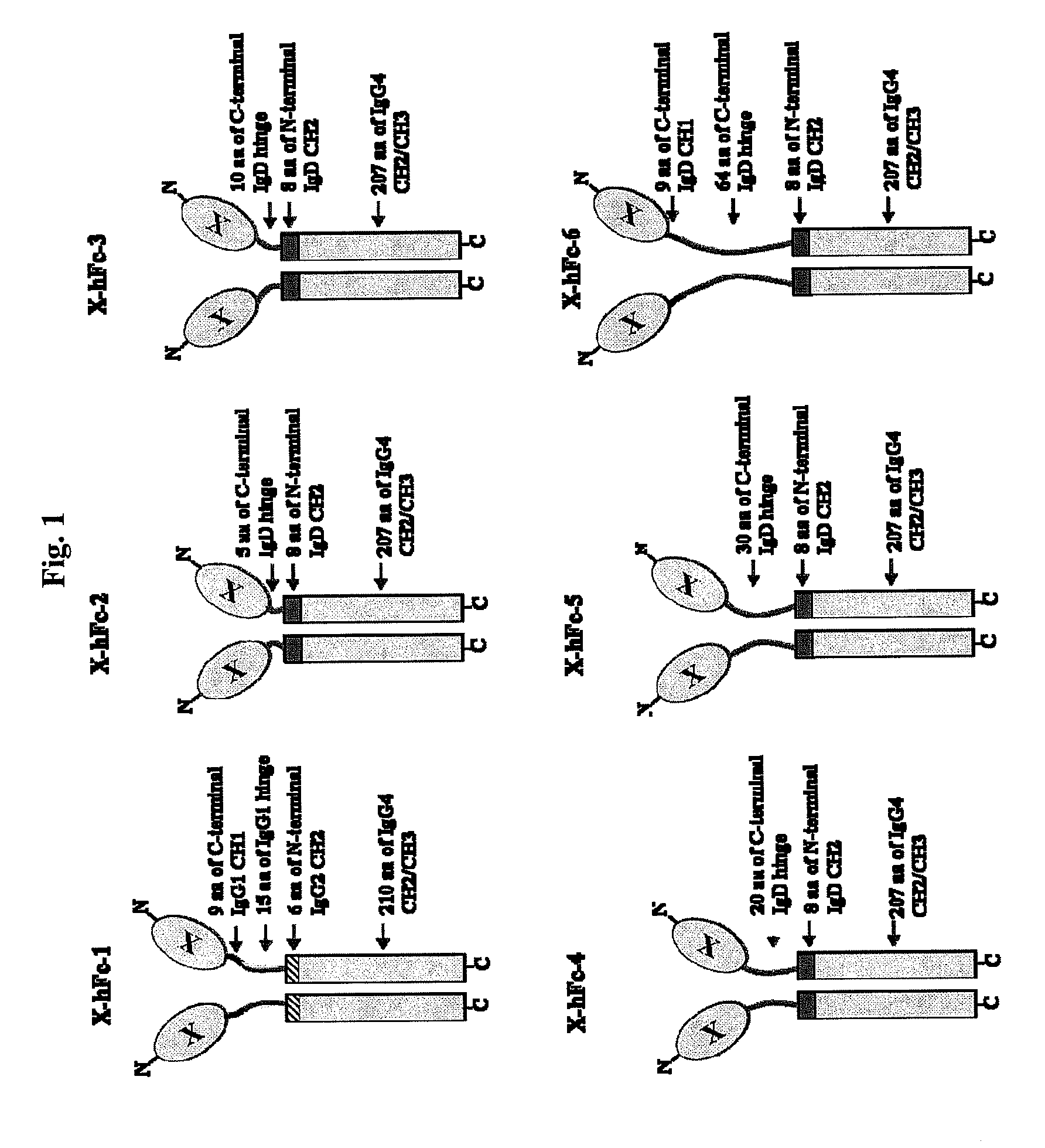
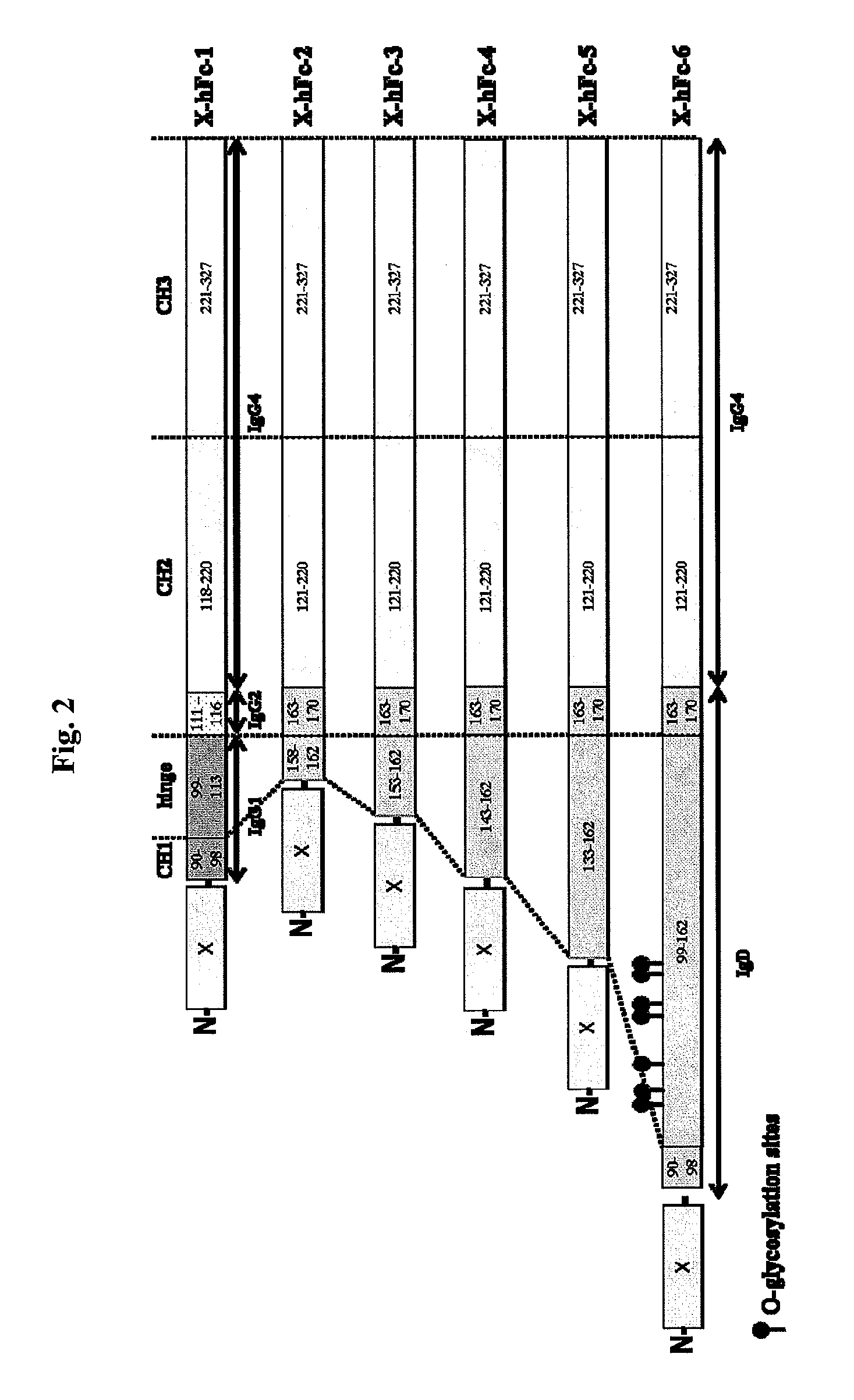
Disclosed are fusion proteins comprising a biologically active molecule and an immunoglobulin (Ig) Fc domain which is linked to the biologically active molecule. The Fc domain is a hybrid human Fc domain of (i) IgG1, IgG2 or IgG4 or (ii) IgG4 and IgD. The hybrid Fc is useful as a carrier of biologically active molecules.
Owner:POSTECH ACADEMY IND FOUND OF POHANG UNIV OF SCI & TECH POSTECH +1
Therapeutic uses for mesenchymal stromal cells
InactiveUS6673606B1Relieve symptomsPrevent degradationBiocideNervous system cellsTay-Sachs diseaseGanglioside
Human mesenchymal stromal cells can be induced to differentiate into oligodendrocytes and neurons, respectively. For these cell types, therefore, MSCs can be a therapeutic source, either in vitro or in vivo, in the context of treating pathologies of the central nervous system which are characterized by neuron loss, such as Parkinson's disease, Alzheimer's disease and stroke, as well as head trauma, or by dysfunction in ganglioside storage or demyelinization, such as Tay-Sachs disease, G1 gangliosidosis, metachromatic leukodystrophy, and multiple sclerosis.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Engineered protein kinases which can utilize modified nucleotide triphosphate substrates
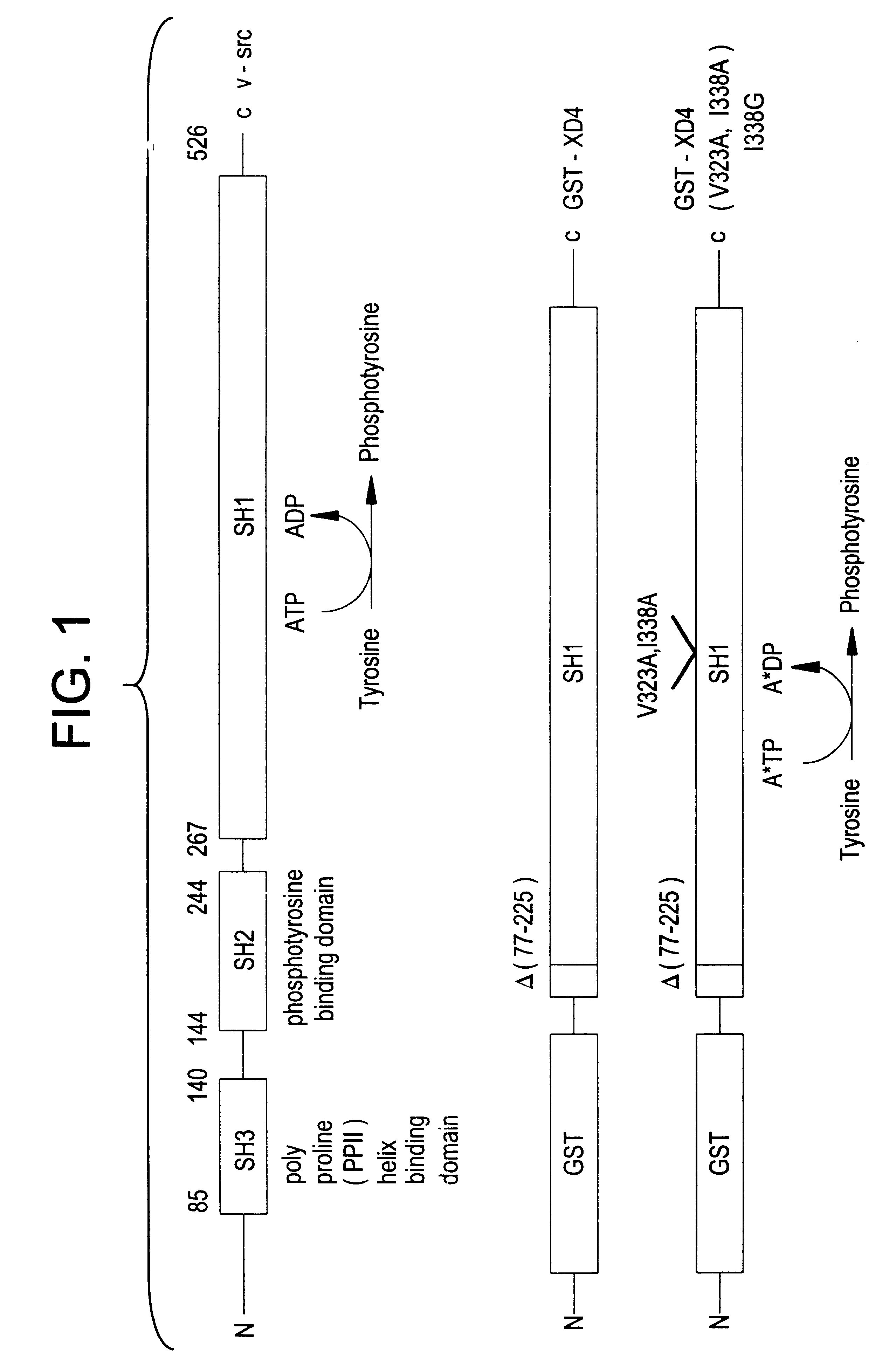
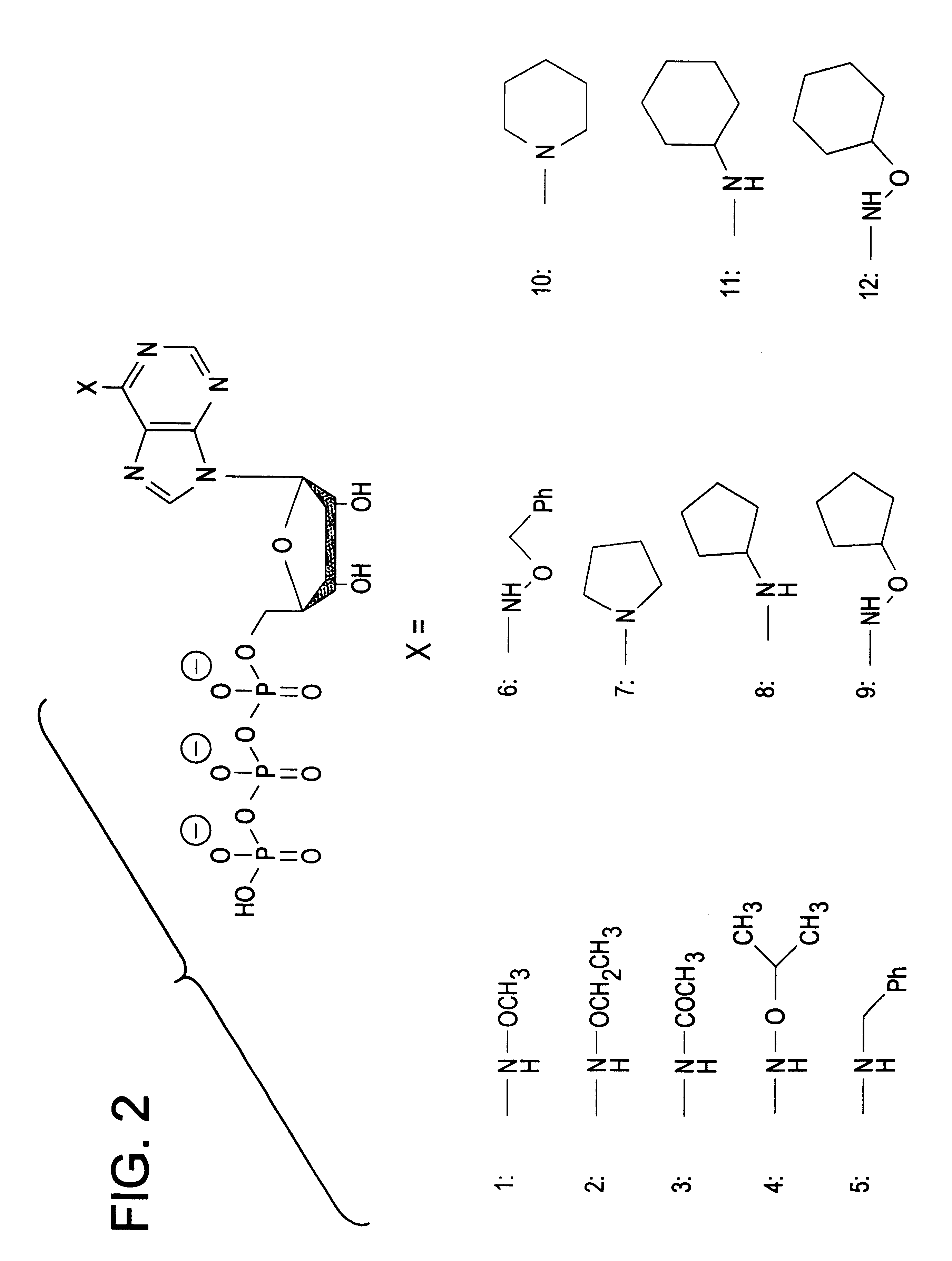
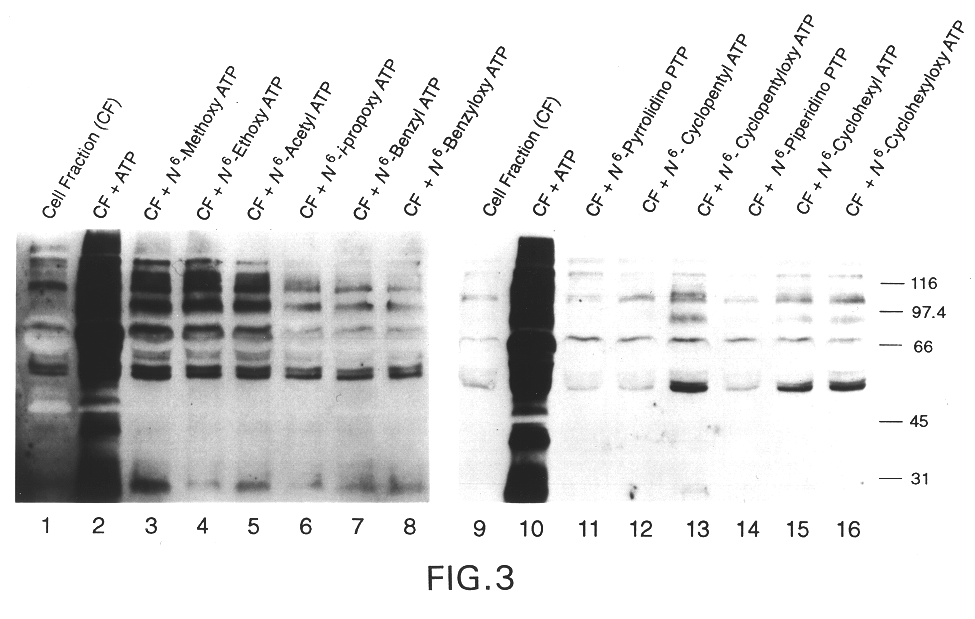
InactiveUS6390821B1Alleviate the causeRelieve symptomsOrganic active ingredientsNervous disorderEnzymeBiology
Engineered protein kinases which can utilize modified nucleotide triphosphate substrates that are not as readily utilized by the wild-type forms of those enzymes, and methods of making and using them. Modified nucleotide triphosphate substrates and methods of making and using them. Methods for using such engineered kinases and such modified substrates to identify which protein substrates the kinases act upon, to measure the extent of such action, and to determine if test compounds can modulate such action. Also engineered forms of multi-substrate enzymes which covalently attach part or all of at least one (donor) substrate to at least one other (recipient) substrate, which engineered forms will accept modified substrates that are not as readily utilized by the wild-type forms of those enzymes. Methods for making and using such engineered enzymes. Modified substrates and methods of making and using them. Methods for using such engineered enzymes and such modified substrates to identify the recipient substrates the enzymes act upon, to measure the extent of such action, and to measure whether test compounds modulate such action.
Owner:PRINCETON UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com