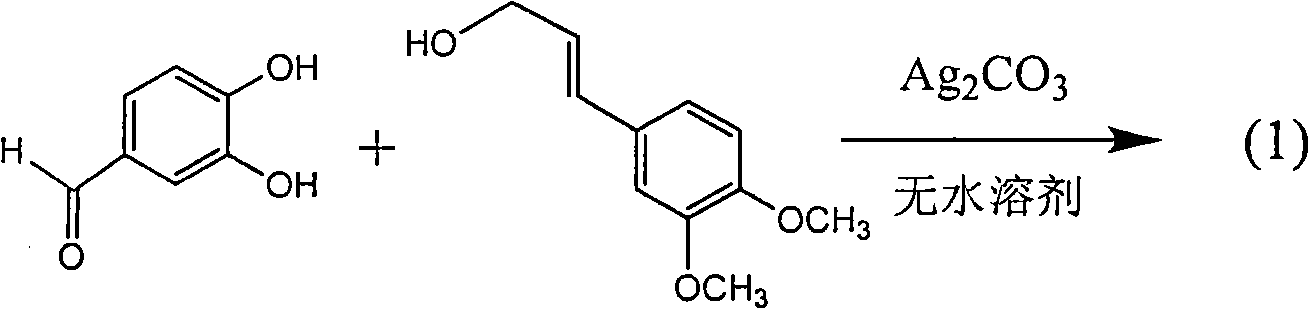
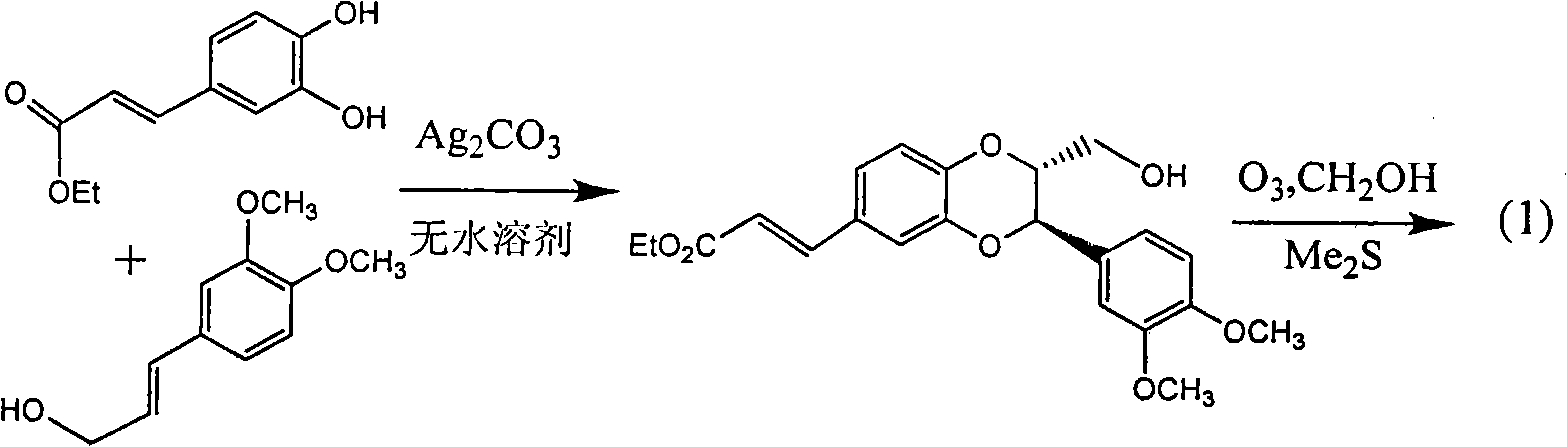
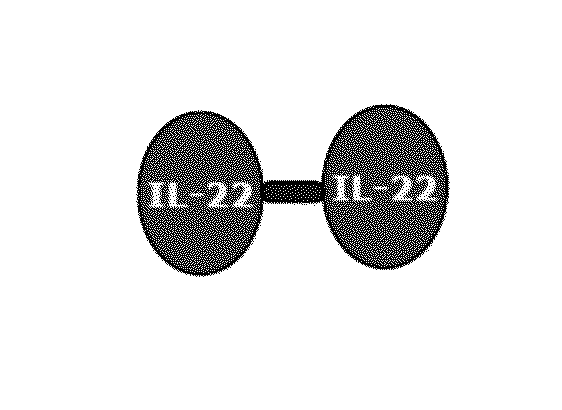Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
621 results about "Viral hepatitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral hepatitis is liver inflammation due to a viral infection. It may present in acute form as a recent infection with relatively rapid onset, or in chronic form. The most common causes of viral hepatitis are the five unrelated hepatotropic viruses hepatitis A, B, C, D, and E. Other viruses can also cause liver inflammation, including cytomegalovirus, Epstein-Barr virus, and yellow fever. There also have been scores of recorded cases of viral hepatitis caused by herpes simplex virus.
Compositions and methods for treatment of viral diseases
InactiveUS20080161324A1Slow and stop replicationReduce loadBiocideMicrobiological testing/measurementSingle-Stranded RNADisease
The present invention features compositions, methods, and kits useful in the treatment of viral diseases. In certain embodiments, the viral disease is caused by a single stranded RNA virus, a flaviviridae virus, or a hepatic virus. In particular embodiments, the viral disease is viral hepatitis (e.g., hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E). Also featured are screening methods for identification of novel compounds that may be used to treat a viral disease.
Owner:EXCRX SINGAPORE PTE +1
Compositions and methods for treatment of viral diseases
InactiveUS20100009970A1Slow and stop replicationReduce loadBiocideNervous disorderSingle-Stranded RNADisease
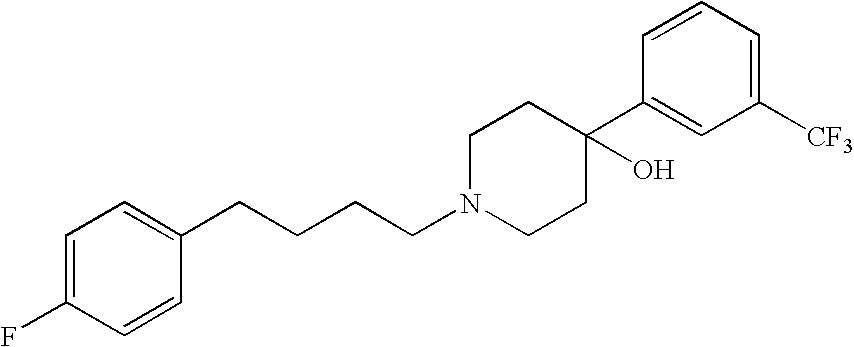
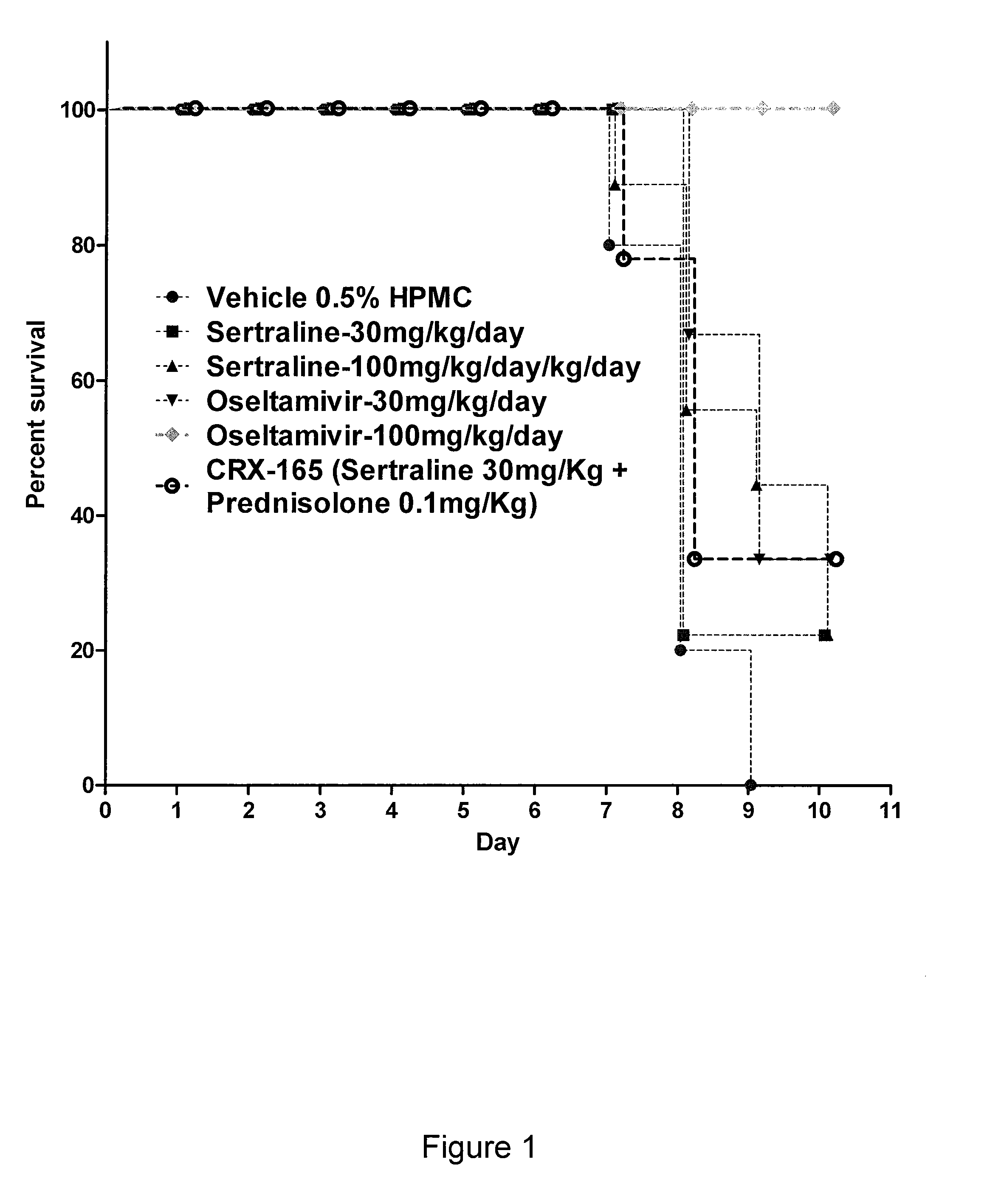
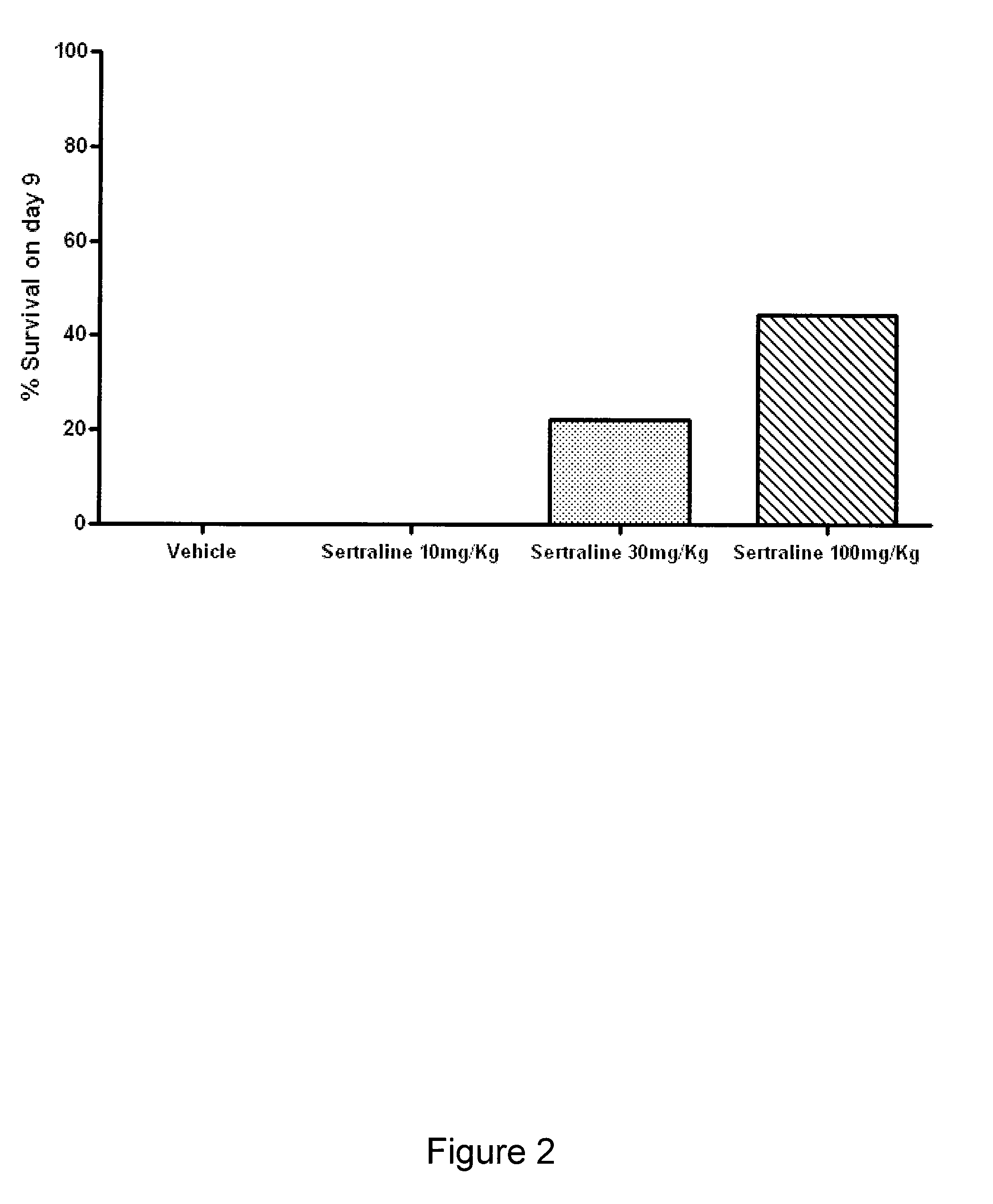
The present invention features compositions, methods, and kits useful in the treatment of viral diseases. In certain embodiments, the viral disease is caused by a single stranded RNA virus, a flaviviridae virus, or a hepatic virus. In particular embodiments, the viral disease is viral hepatitis (e.g., hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E) and the agent or combination of agents includes sertraline, a sertraline analog, UK-416244, or a UK-416244 analog. Also featured are screening methods for identification of novel compounds that may be used to treat a viral disease.
Owner:EXCRX SINGAPORE PTE +1
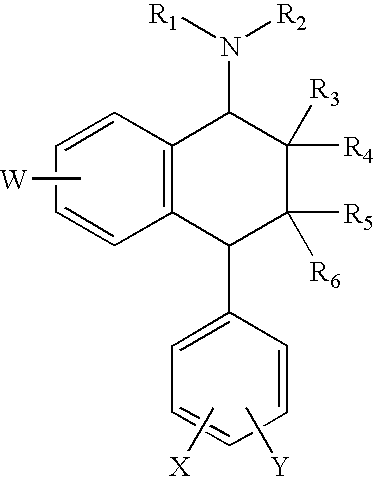
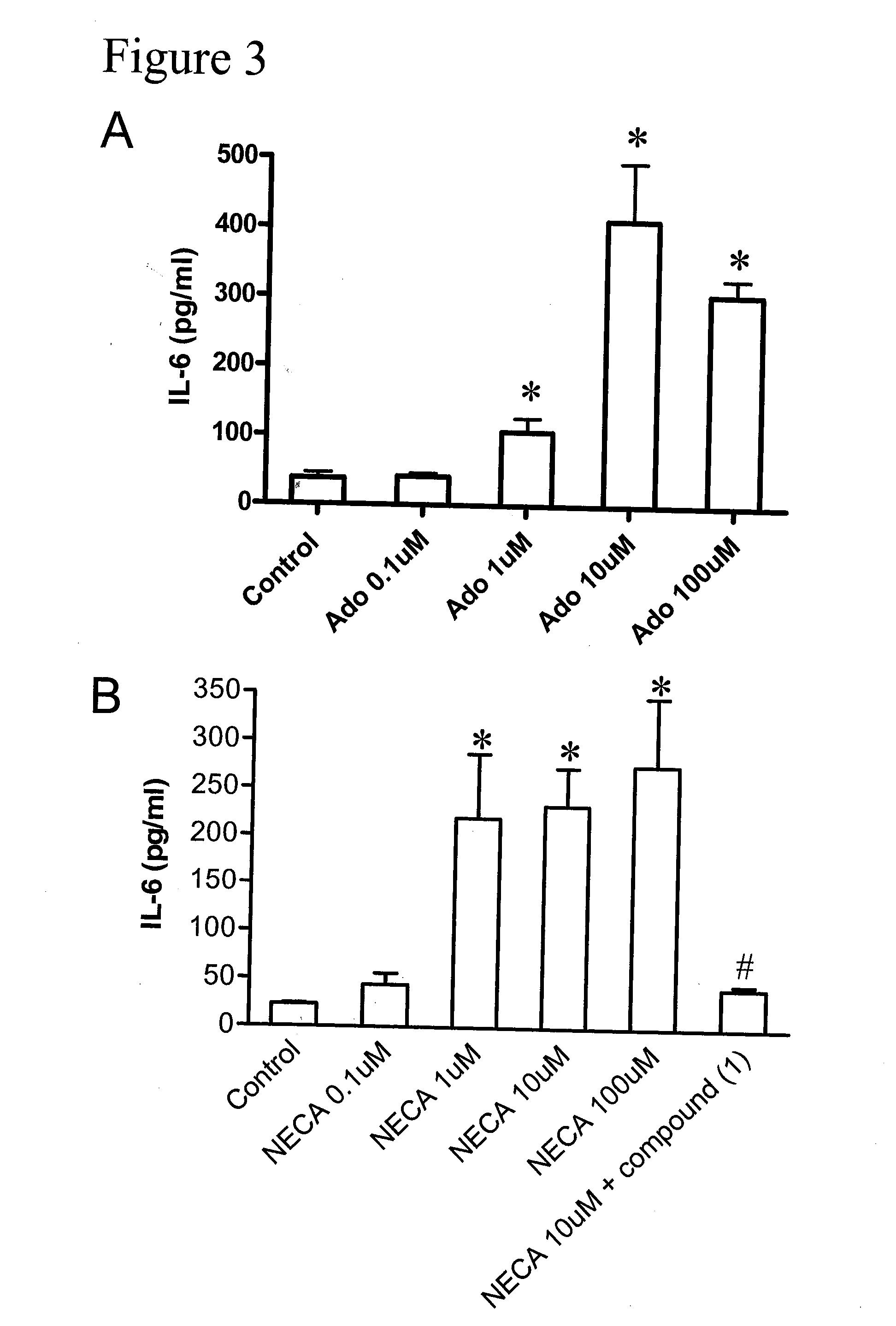
Method of preventing and treating hepatic disease using a2b adenosine receptor antagonists
ActiveUS20070219221A1Decreasing hepatotoxic side effectBiocideOrganic chemistryAlcohol abuseHepatic Diseases
The invention is related to methods of preventing and treating hepatic fibrosis using A2B adenosine receptor antagonists and utility in the treatment and prevention of liver damage caused by alcohol abuse, surgical intervention, viral hepatitis, the ingestion of hepatotoxic drugs, or other hepatic diseases. The invention also relates to pharmaceutical compositions for use in the method.
Owner:GILEAD SCI INC
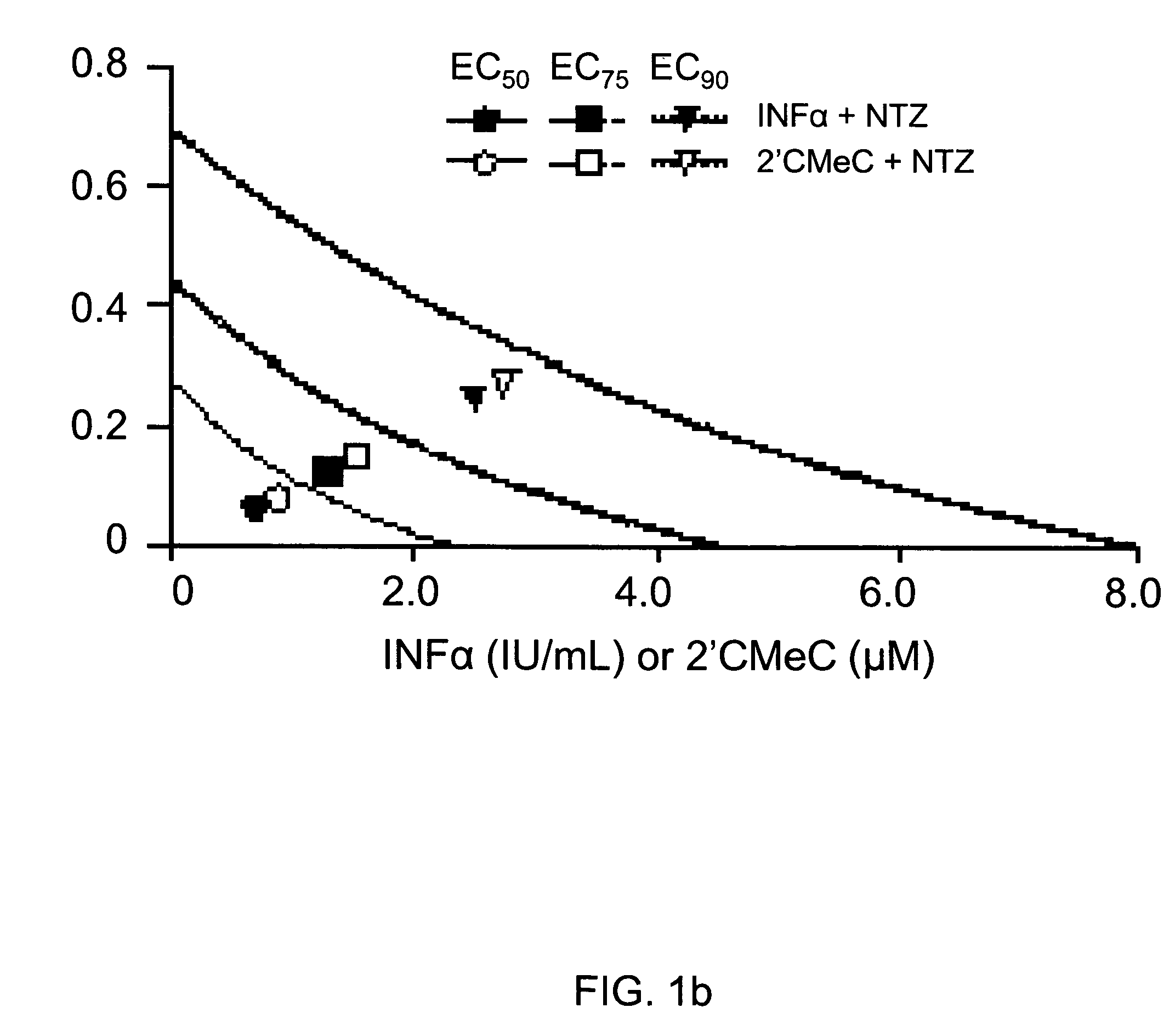
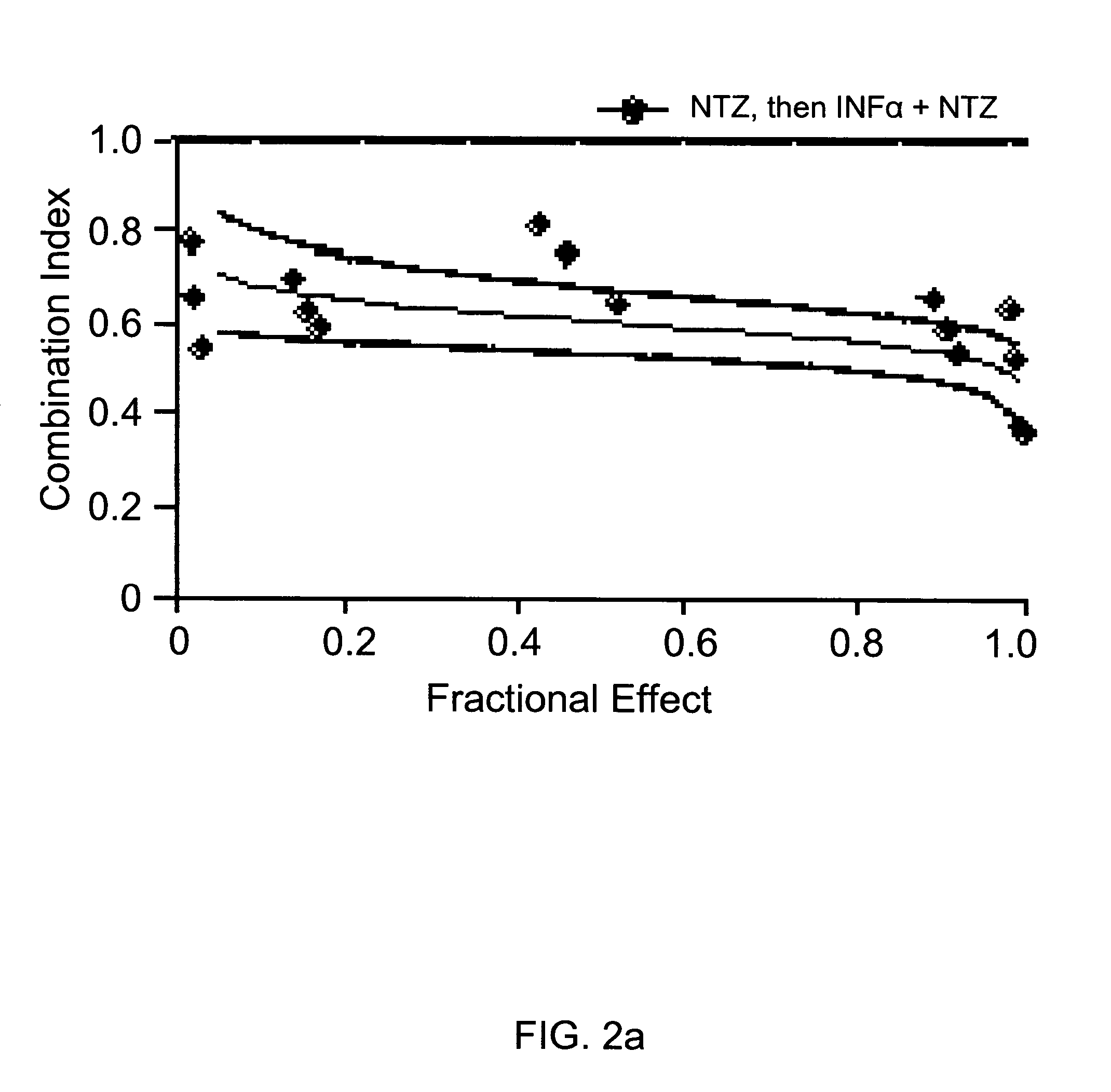
Viral hepatitis treatment
ActiveUS20070167504A1Improve stabilityBiocideOrganic active ingredientsHepatitisPerylene derivatives
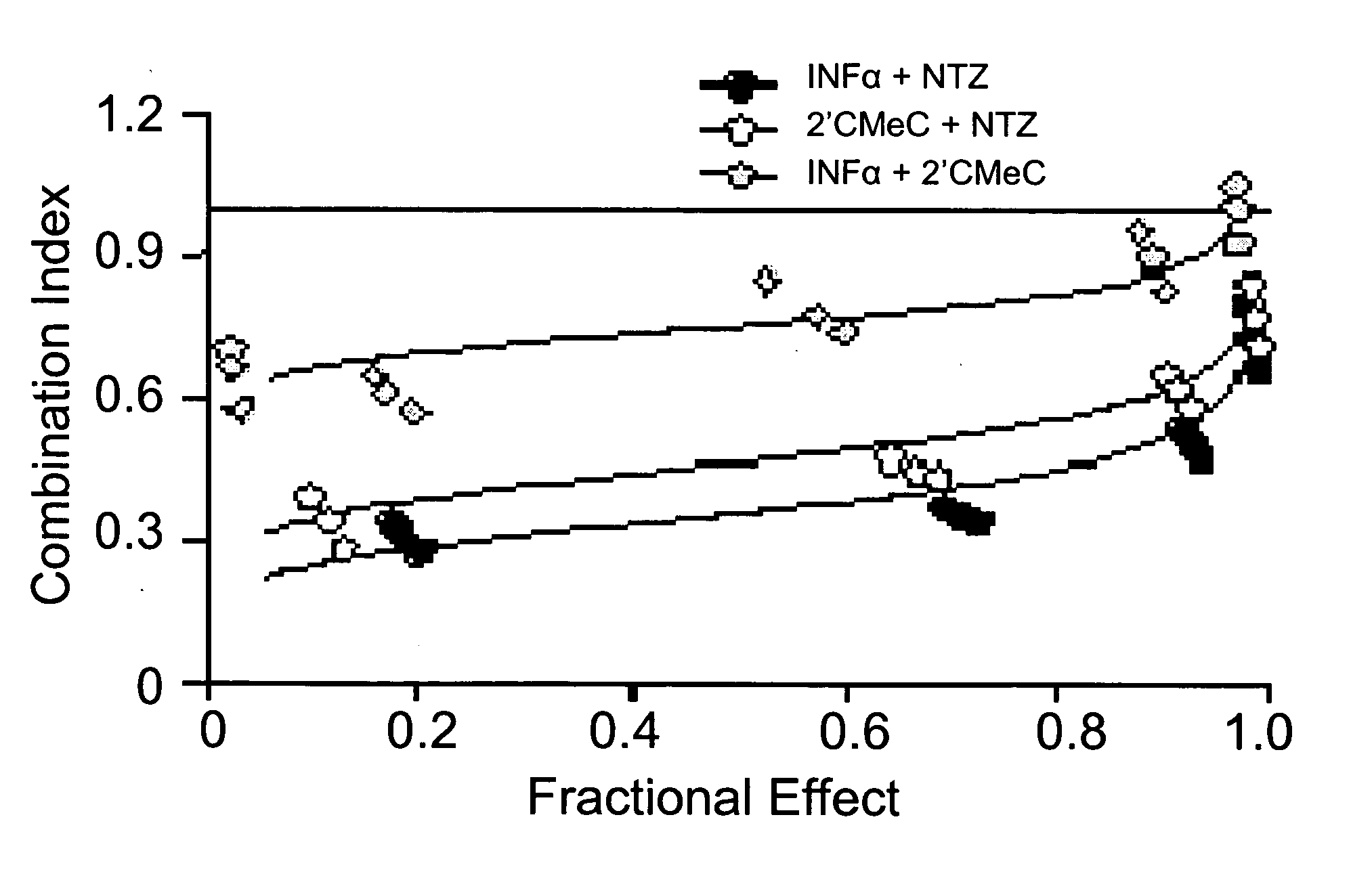
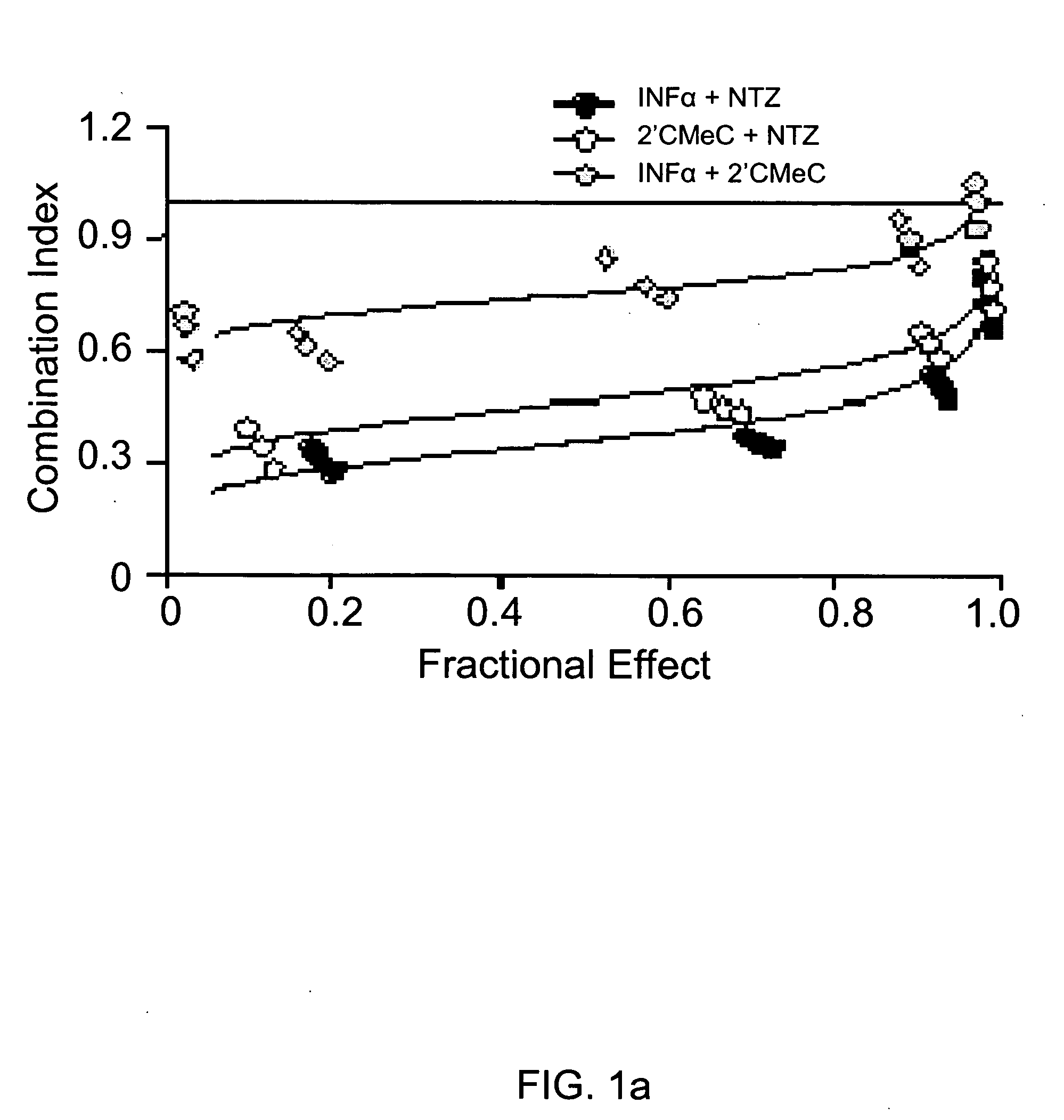
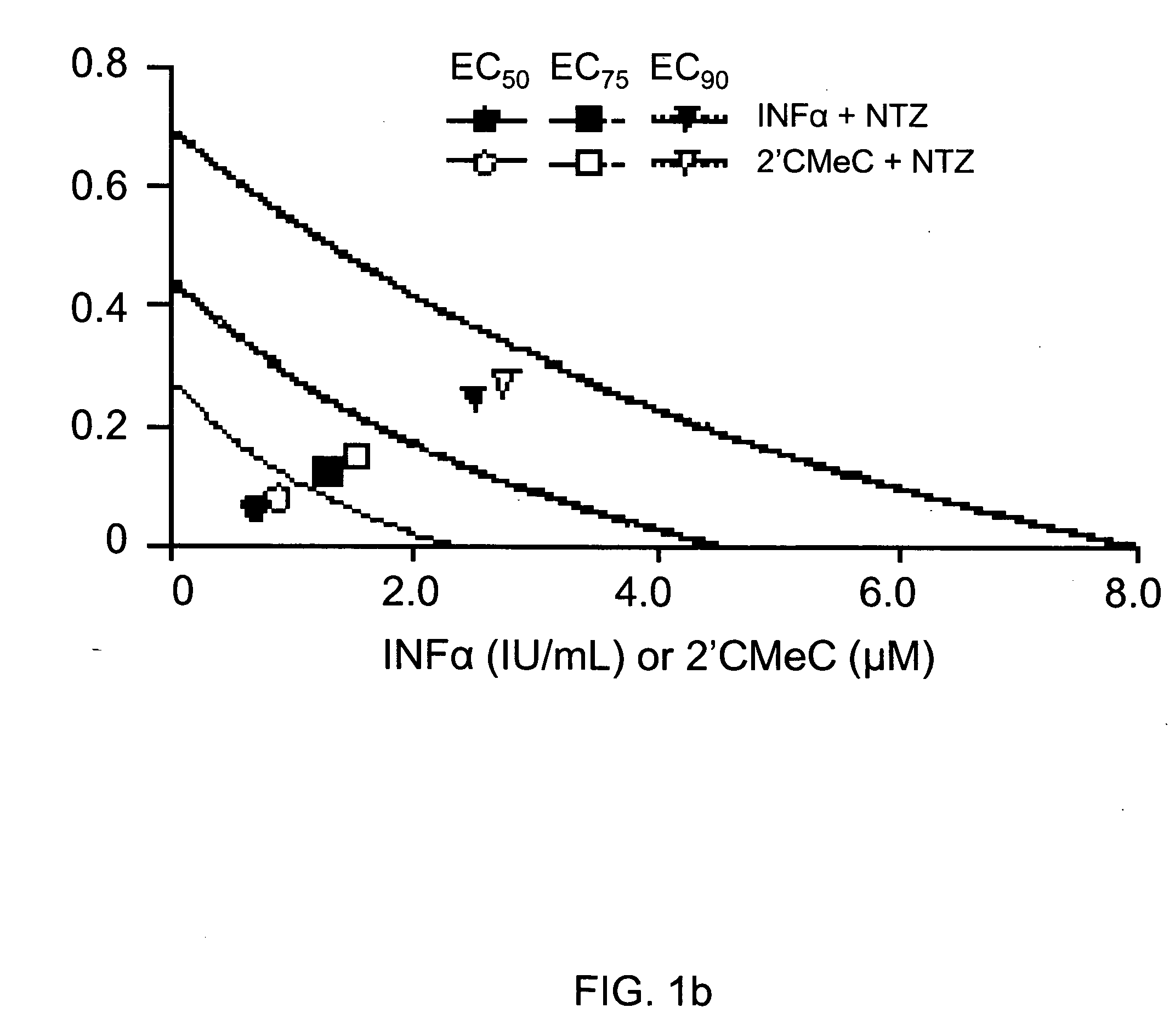
The present disclosure relates to methods for treating viral hepatitis, compounds useful in the treatment of viral hepatitis, and pharmaceutical compositions comprising such compounds. In one embodiment, pharmaceutical compositions comprising nitazoxanide, tizoxanide, or derivatives and / or mixtures thereof are provided, as well as methods of treating hepatitis C using such compositions.
Owner:ROMARK LAB L C
Combination therapy for treating hepatitis viral infection
Disclosed are methods of using proteasome inhibitors (PI) in combinations with other pharmaceutically active agents for treating viral hepatitis infections, for example, for treating therapy-resistant and -refractory viral hepatitis infections. Also disclosed are pharmaceutical compositions and kits of pharmaceutical compositions which can be used for treating viral hepatitis infections, for example, for treating therapy-resistant and refractory viral hepatitis infections.
Owner:SCHUBERT ULRICH
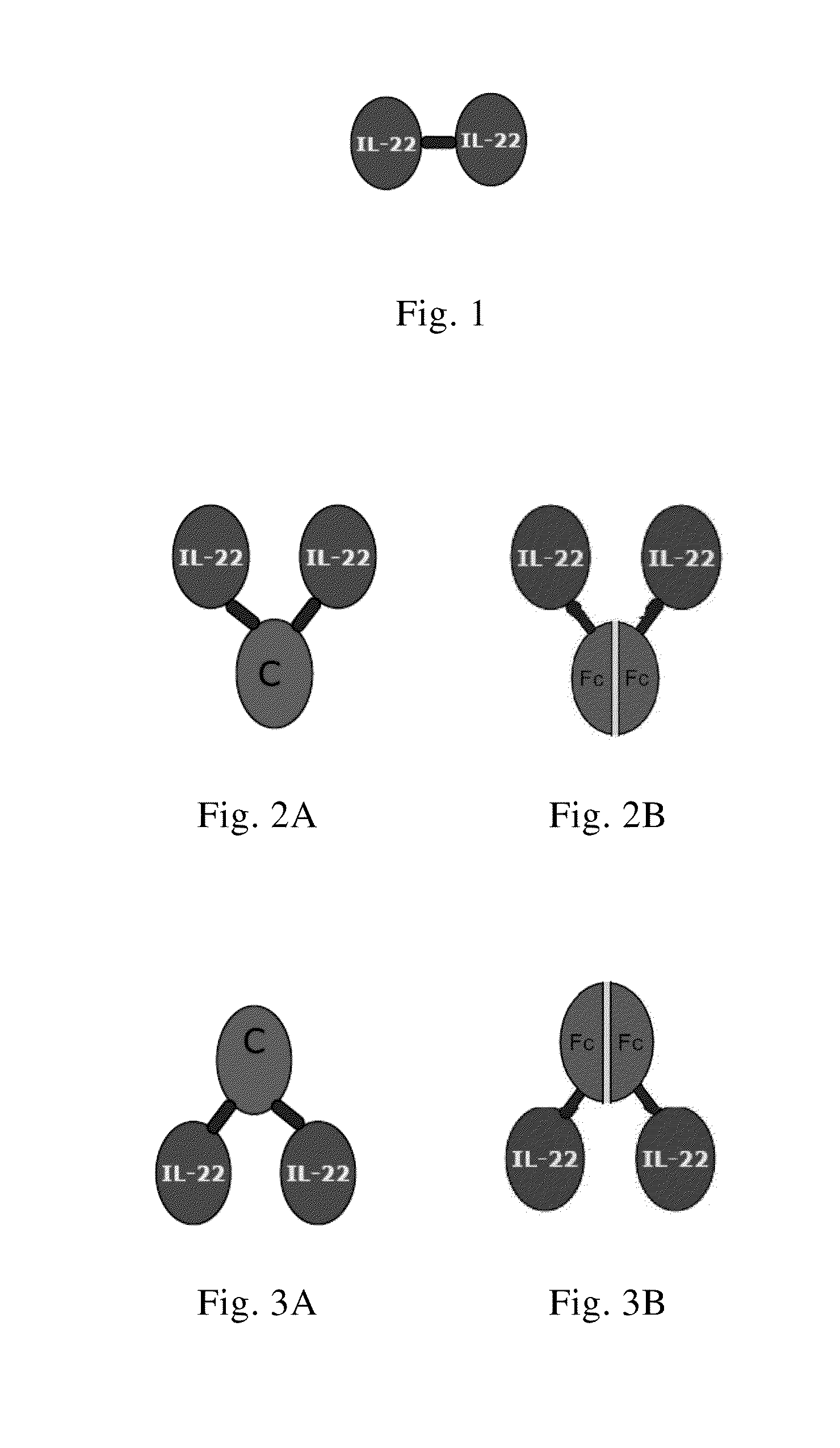
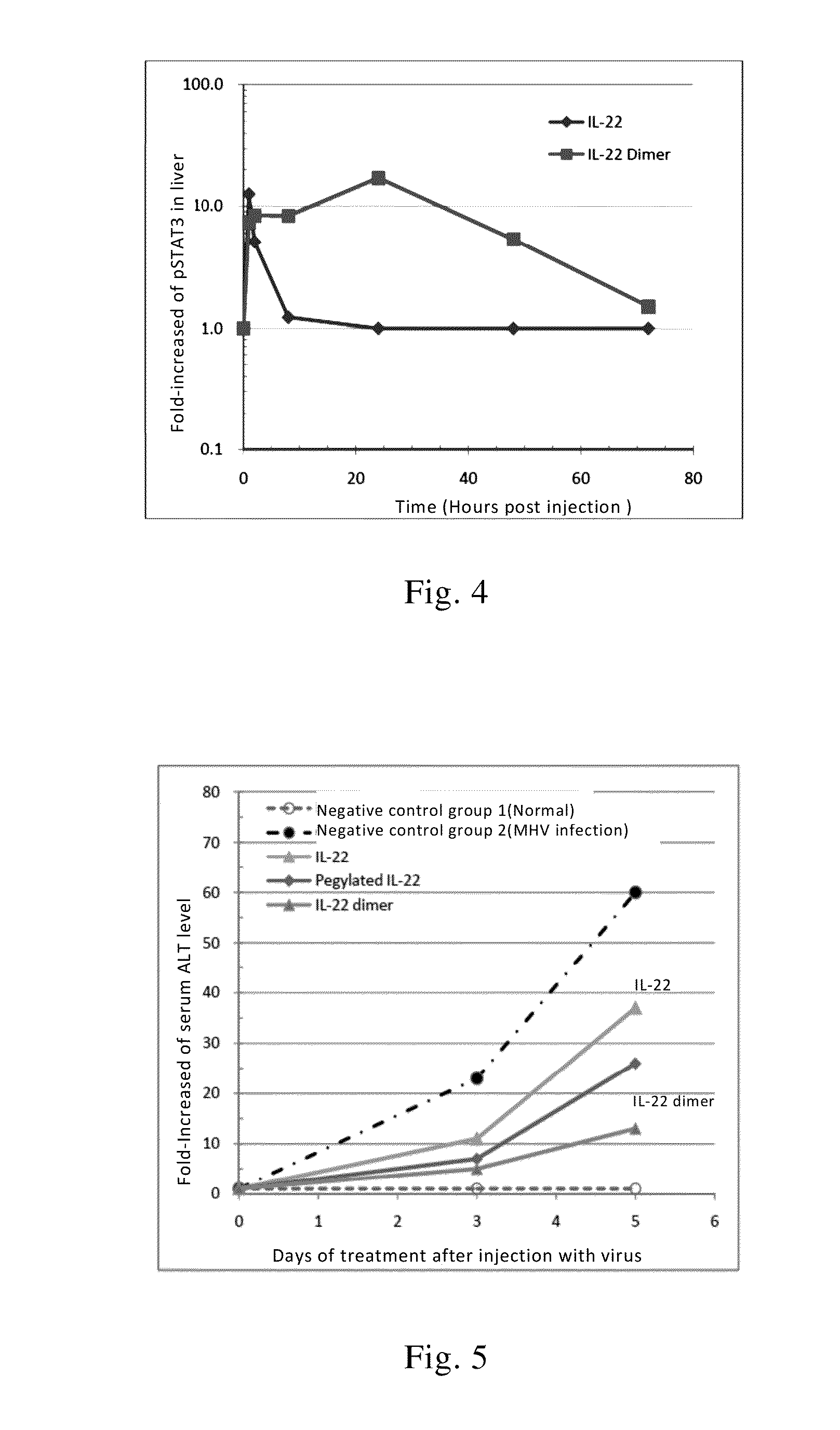
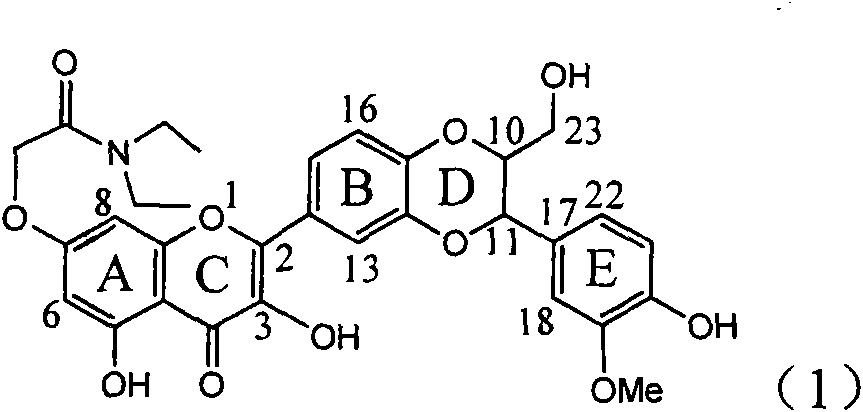
Use of interleukin-22 in treating viral hepatitis
ActiveUS20130171100A1Good curative effectEffective treatmentPeptide/protein ingredientsDigestive systemViral hepatitis bViral hepatitis
This invention relates to a use of IL-22 in the treatment of viral hepatitis. As illustrated in the examples of this invention, IL-22 can significantly reduce liver damage caused by hepatitis virus, and can significantly reduce the increase of transaminase ALT / AST induced by hepatitis virus. In addition, the IL-22 dimer of this invention can effectively treat viral hepatitis.
Owner:EVIVE BIOTECHNOLOGY (SHANGHAI) LTD
Use of acetamide dehydrogenation silibinin as medicament for treating viral hepatitis B
InactiveCN101829091APowerful removalInhibitory activityOrganic active ingredientsDigestive systemAntigenDisease
The invention relates to the use of acetamide dehydrogenation silibinin as a medicament for treating viral hepatitis B, in particular to the use of dehydrogenation silibinin esters flavonoid lignanoid replaced by A ring methoxy formyl amine or pharmaceutically acceptable salt as the medicament for eliminating HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis Be antigen) and restraining copy of HBV DNA. The cetamide dehydrogenation silibinin can obviously restrain the HBsAg and HBeAg activity, and the strengths for eliminating the HBsAg and HBeAg are 90.5% and 63.6% at the concentration of 20 microgramme / milliter and are 5.6 times and 3.8 times more than positive contrast medicament alpha-interferon. Meanwhile, the restraining rate to the HBV DNA is 90.4% at the concentration, is 12% higher than lamivudine, and is 2.4 times more than a- interferon. Therefore, the flavonoid lignanoid or the pharmaceutically acceptable salt can be expected for treating hepatitis B virus infection as the non-nucleoside medicament.
Owner:DALI UNIV
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of flavonoid quercetin dimmer as medicament for treating viral hepatitis B
InactiveCN101829103AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to the application of flavonoid quercetin dimmer as the medicament for treating viral hepatitis B, in particular to the application of flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof to the preparation of the medicament for eliminating HBsAg and HBeAg and inhibiting HBV DNA replication. The flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof has obvious HBsAg and HBeAg inhibiting activity, and at the concentration of 100mcg / ml, the flavonoid quercetin dimmer pharmaceutically acceptable salt thereof has the HBsAg eliminating strength of 65.7% and the HBeAg eliminating strength of 44.8% which are respectively 4.1 times and 2.7 times higher than the positive control medicament of Alpha-interferon and has the HBV DNA inhibiting ratio of 44.8% which is 117% of the HBV DNA inhibiting ratio of the Alpha-interferon at the highest test concentration. Therefore, the flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof can be expectedly used for preparing the non-nucleoside medicament for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating viral hepatitis B.
Owner:DALI UNIV
Preparation of brominated flavanonollignan and application in medicine for treating viral hepatitis B
InactiveCN101955478AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryPositive controlInterferon alpha
The invention relates to the preparation of brominated flavanonollignan and an application in medicines for treating viral hepatitis B, in particular to a B cyclo-dioxane flavanonollignan compound and a preparation method thereof as well as the application of the compound or pharmaceutically acceptable salts thereof in the preparation of medicines for eliminating hepatitis B surface antigens (HBsAg) and hepatitis B e antigens (HBeAg) and medicines for inhibiting HBV DNA replication. The compound has obvious activity of inhibiting HBsAg and HBeAg, and the intensities of the compound for eliminating HBsAg and HBeAg under the concentration of 20 microgram / millimeter are respectively 2.1 times and 1.2 times larger than the corresponding activity of a positive control medicine alpha-interferon; meanwhile, the compound displays high inhibition ratio more than 57% on HBV DNA at the concentration. The results show that the favonolignan or pharmaceutically acceptable salts thereof can be expected to be used for preparing non-nucleoside type medicines for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
InactiveCN101829086AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of aromatic carbamoyl dehydro-silibinin as a medicament for treating viral hepatitis B, in particular to application of todehydro-silibinin flavonolignans with a ring A and a ring E which are substituted by double base aromatic carbamoyl methoxyl and pharmaceutically acceptable salt thereof for preparing medicaments for removing HBsAg and HBeAg and medicaments for inhibiting HBV DNA. The todehydro-silibinin flavonolignans has extremely obvious activity on inhibiting the HBsAG and the HBeAg, has the intensity of 46.2 percent and 68.9 percent for respectively removing the HBsAG and the HBeAg in the presence of the concentration of 100 microgram / milliliter, which is 2.9 times and 4.1 times higher than that of positive control medicament alpha-interferon, and has the inhibition ratio of 96 percent on HBV DNA in the presence of the concentration of 100 microgram / milliliter, which is higher than that of lamivudine and the alpha-interferon. Accordingly, the flavonolignans and the pharmaceutically acceptable salt thereof can be expected to be used for preparing non-nucleoside medicaments applied for removing HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Crystal form of Pifuadefuwei
InactiveCN1396170AOrganic active ingredientsGroup 5/15 element organic compoundsMedicineViral hepatitis
A crystal form of Adefovir dipivoxil, 9-[2-[[bis (neovaleroxy) methyl] phosphoroso] methoxy]ethyl] adenine, its application to treating hepatism, such as viral hepatitis, its medical composition, and the process for preparing it are disclosed.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
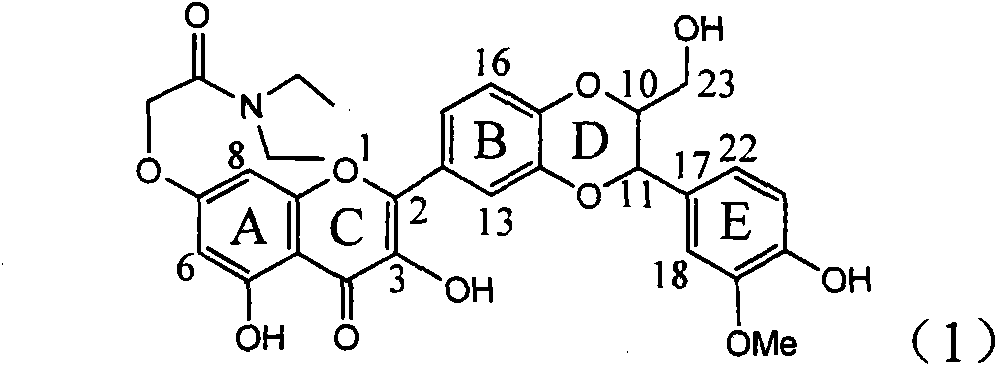
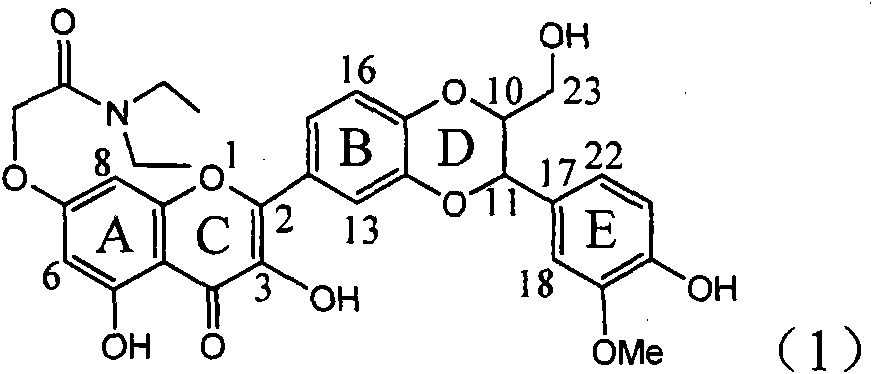
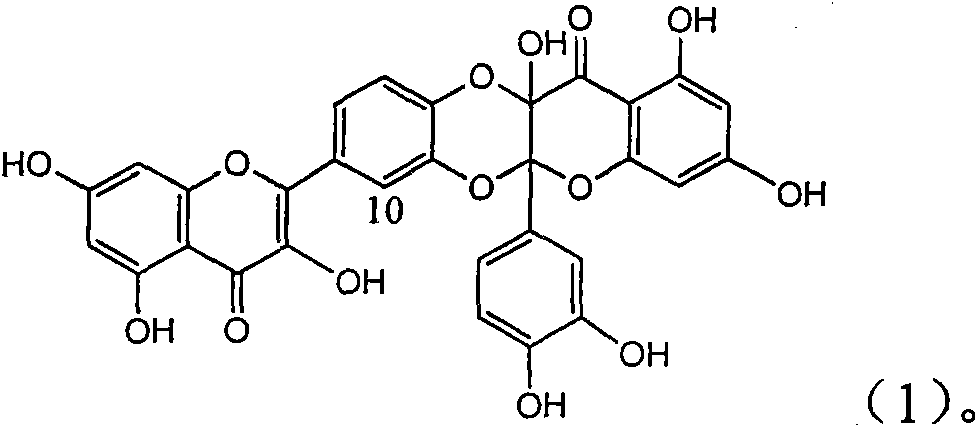
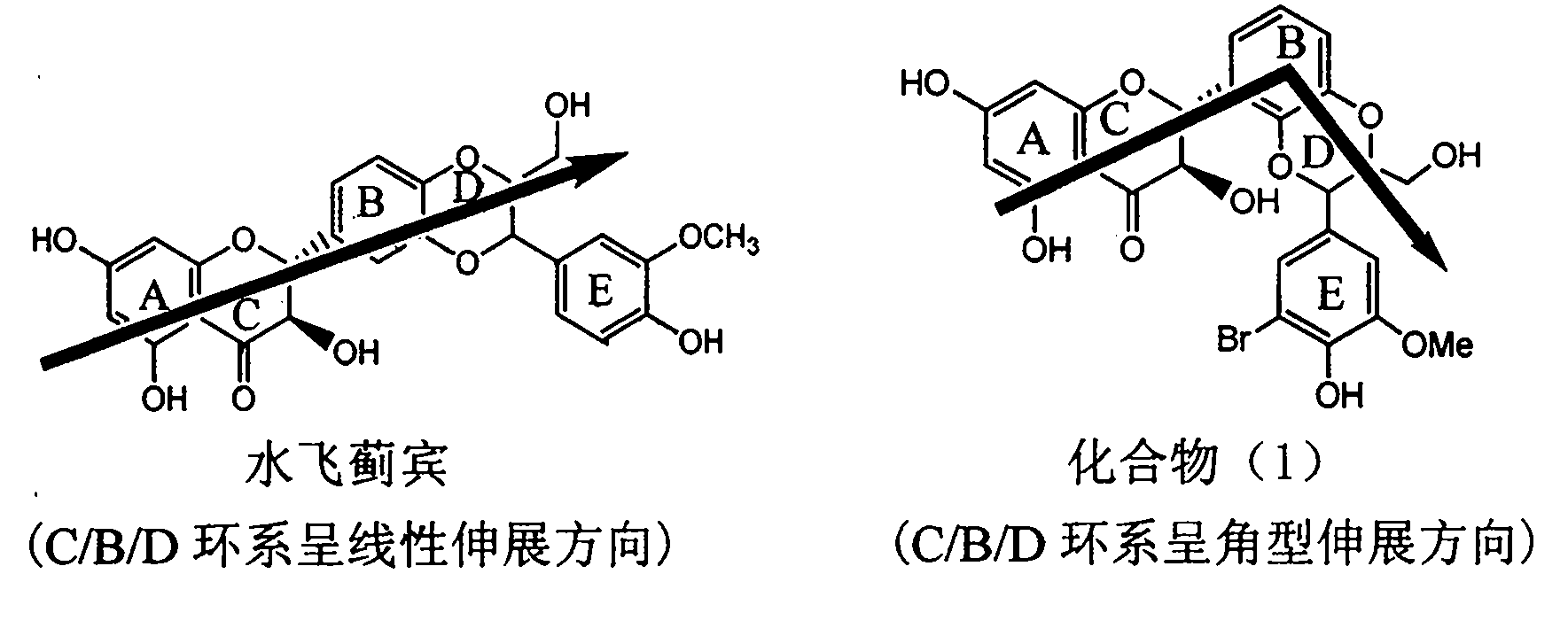
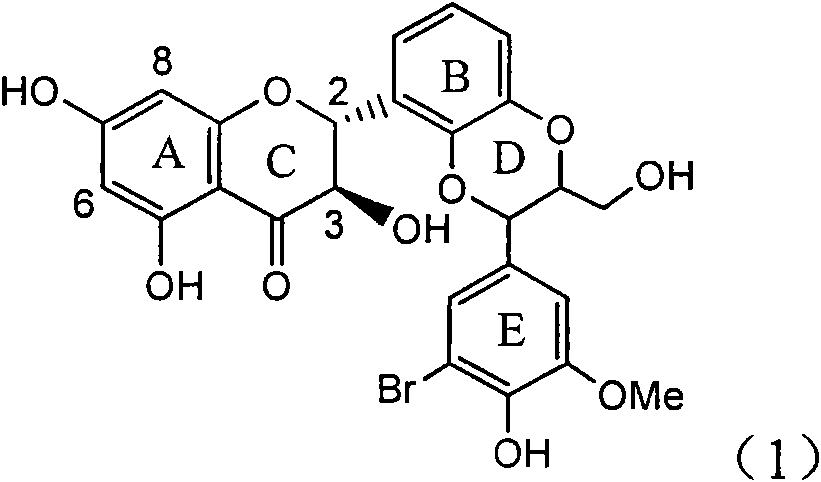
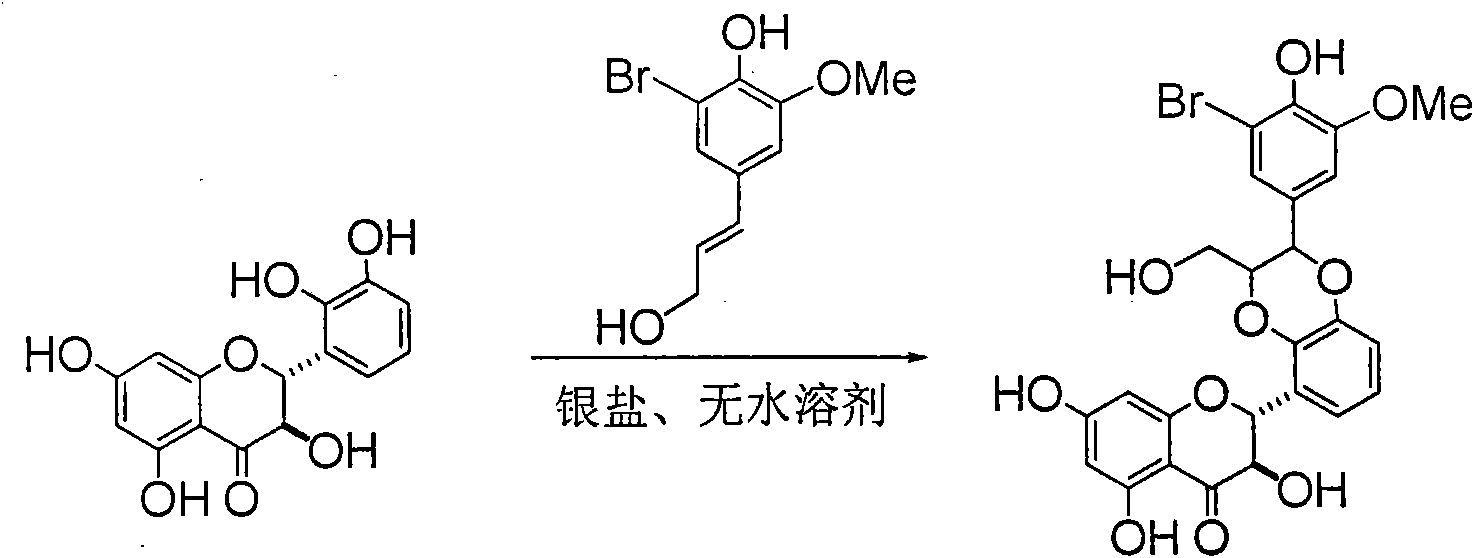
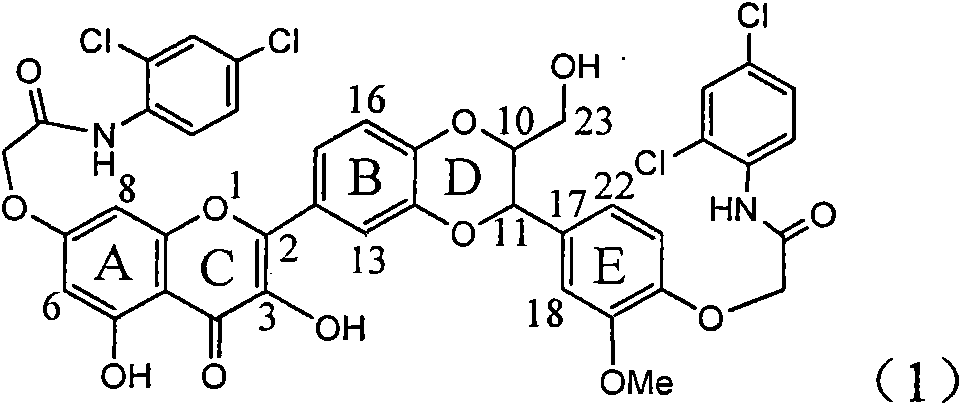
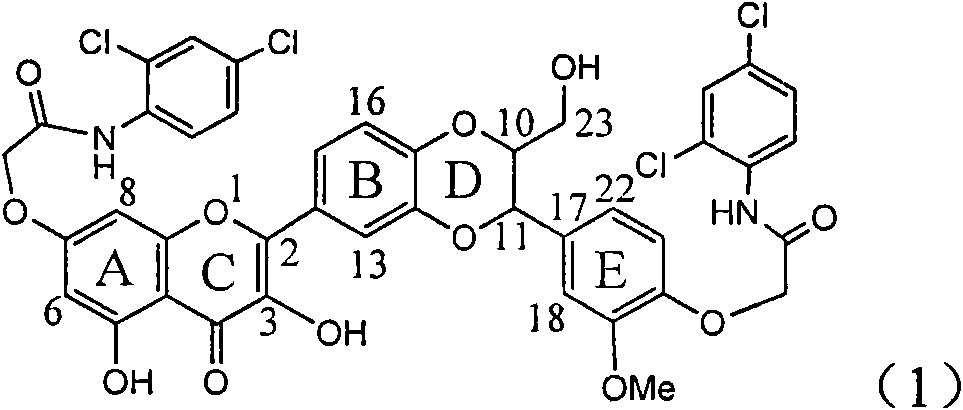
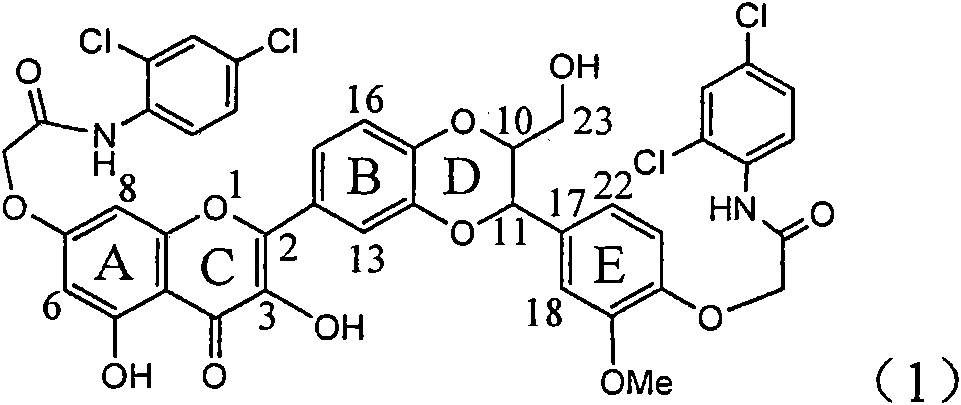
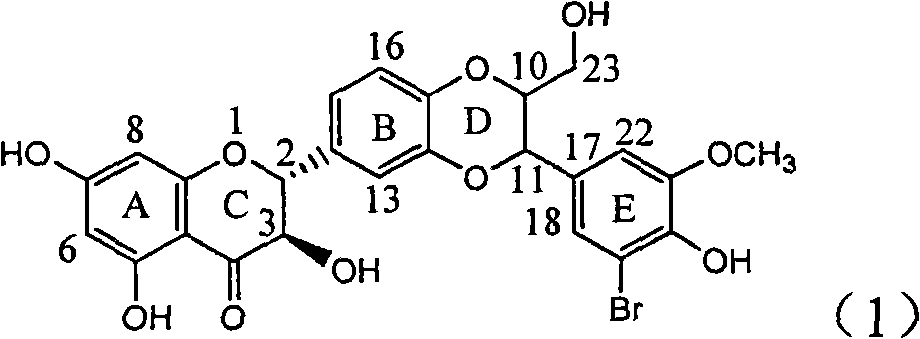
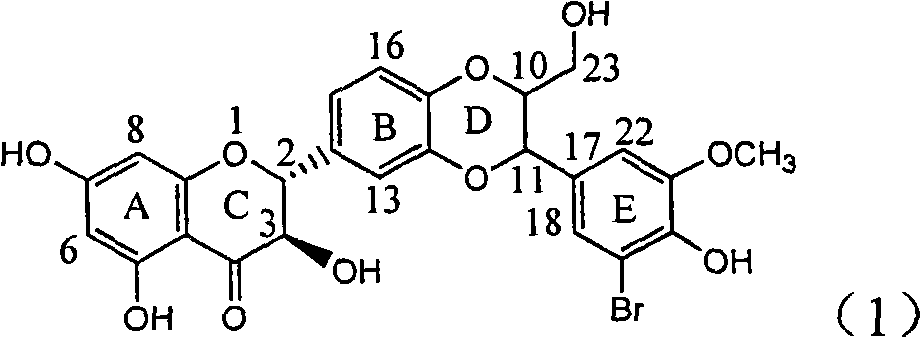
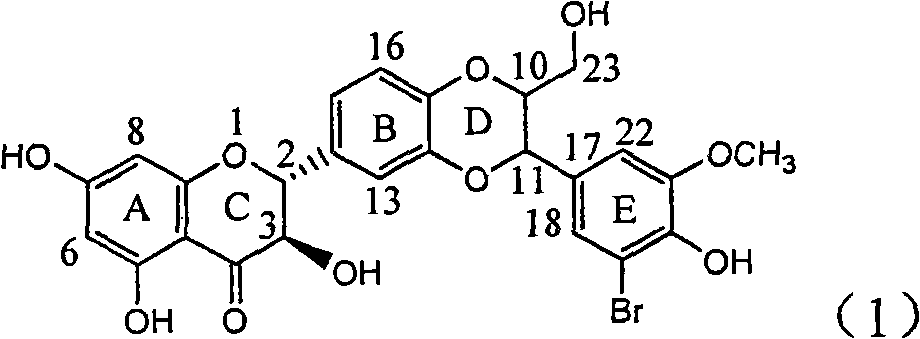
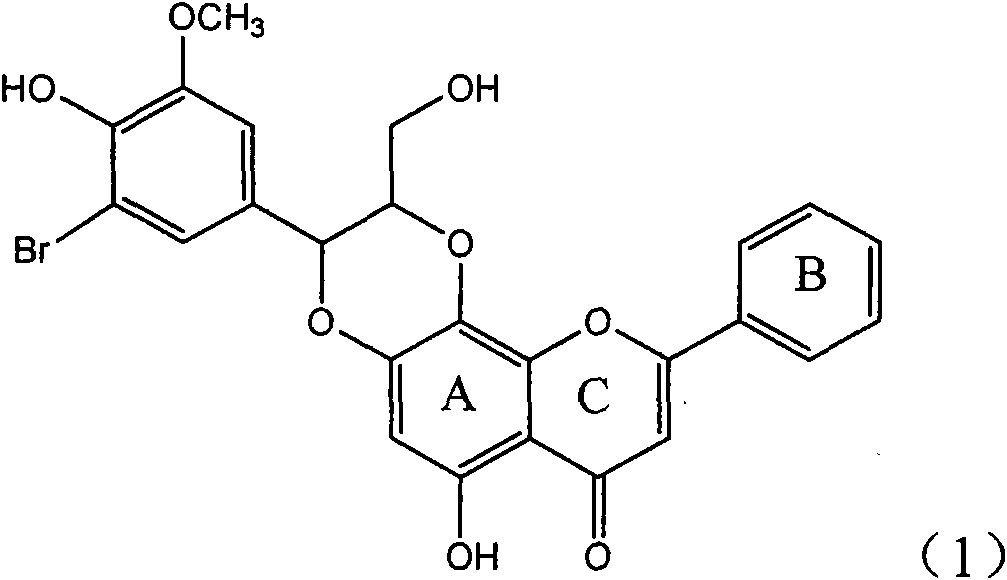
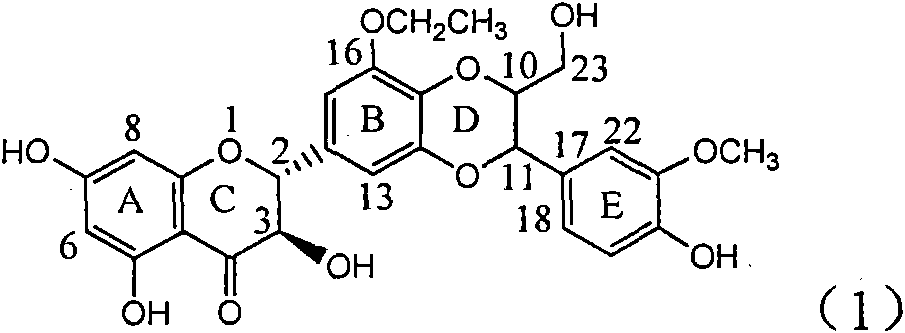
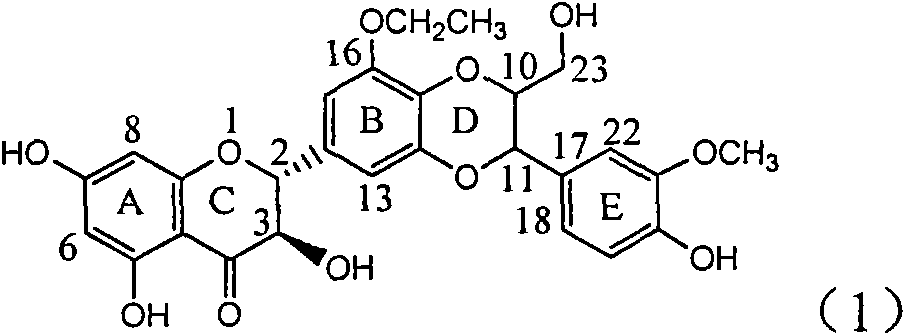
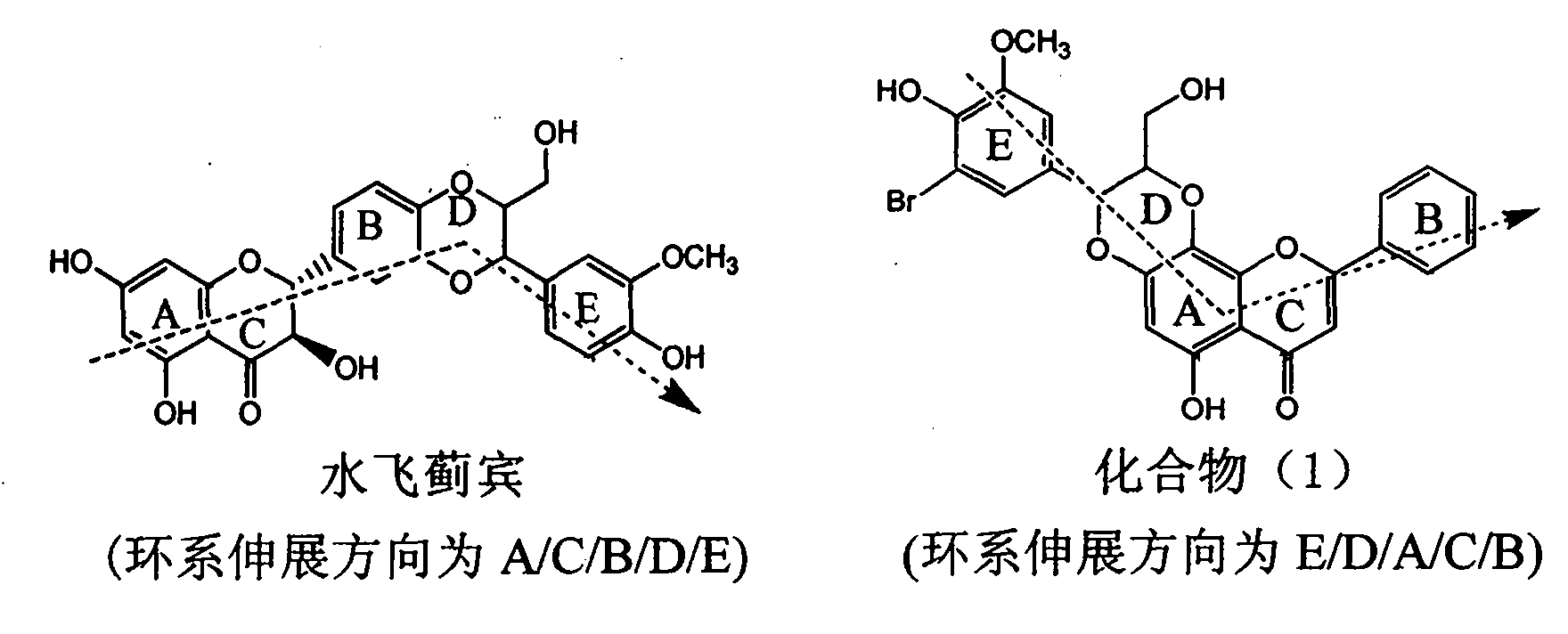
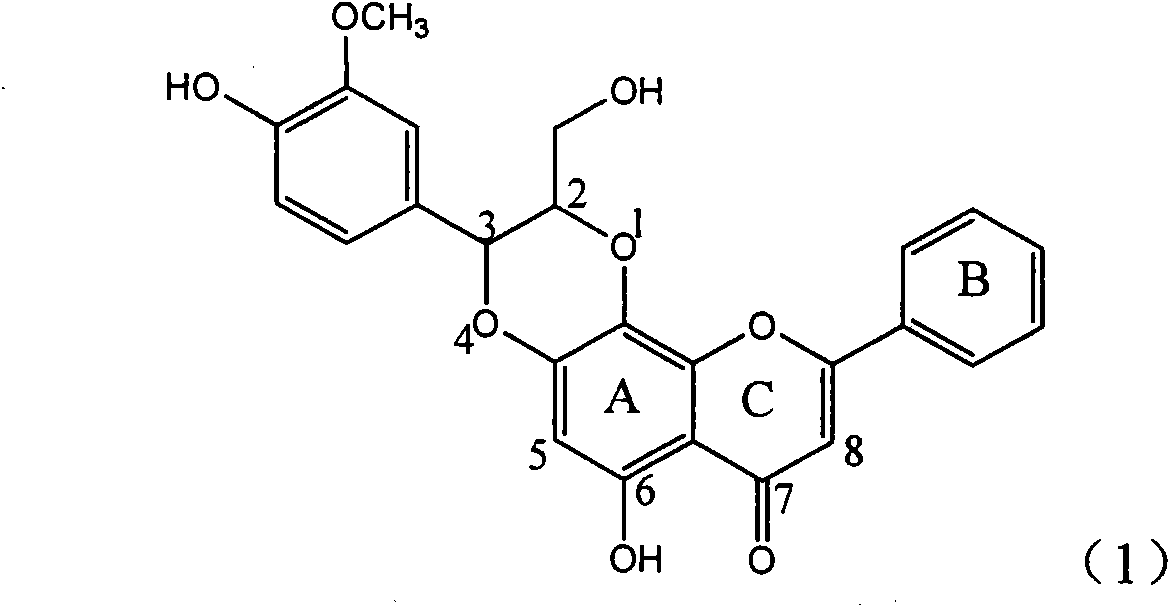
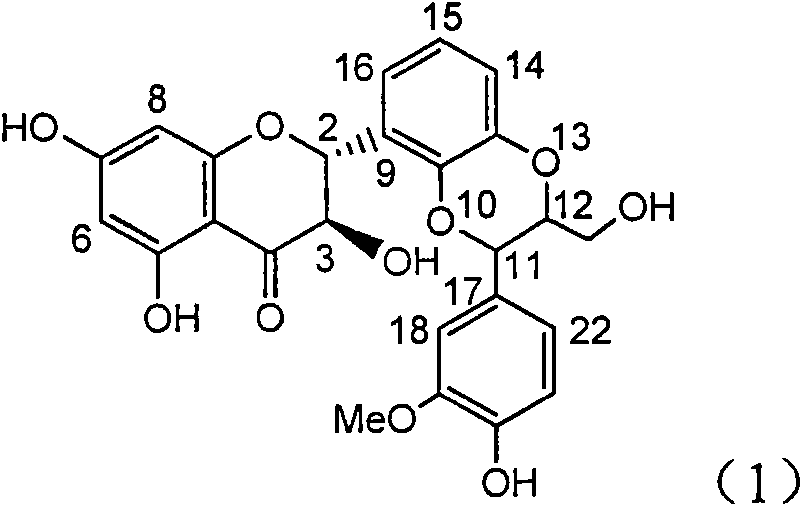
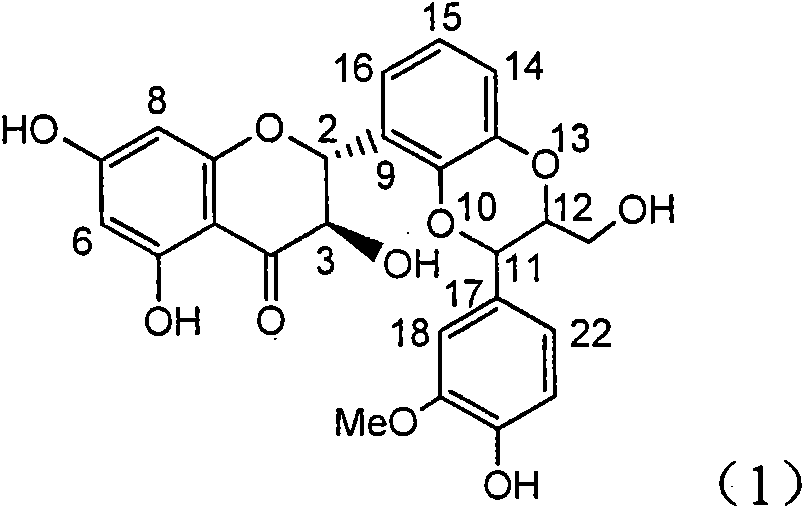
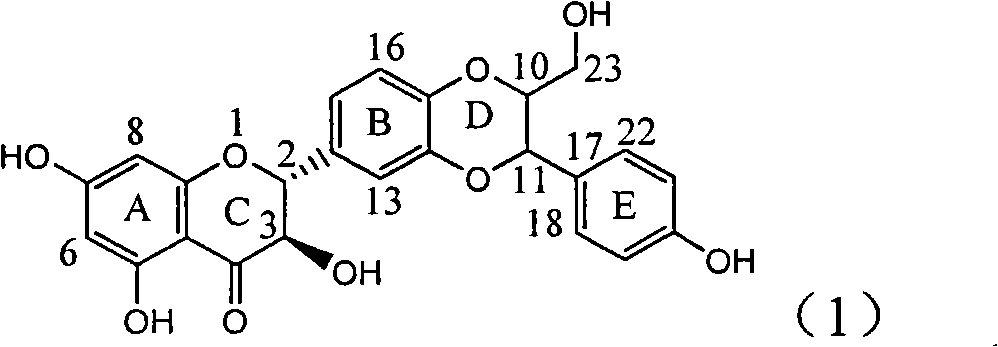
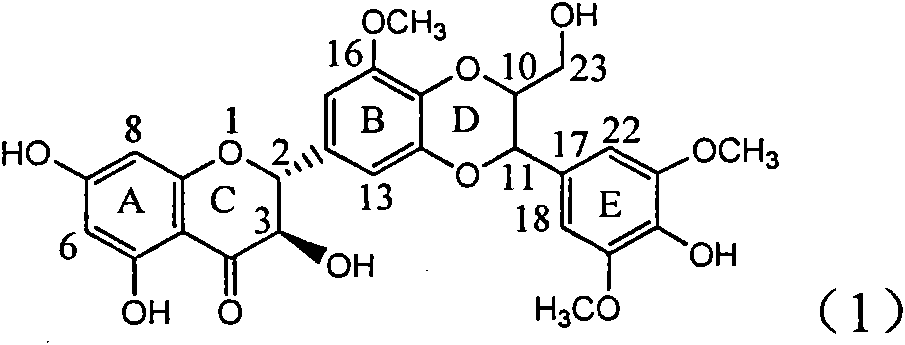
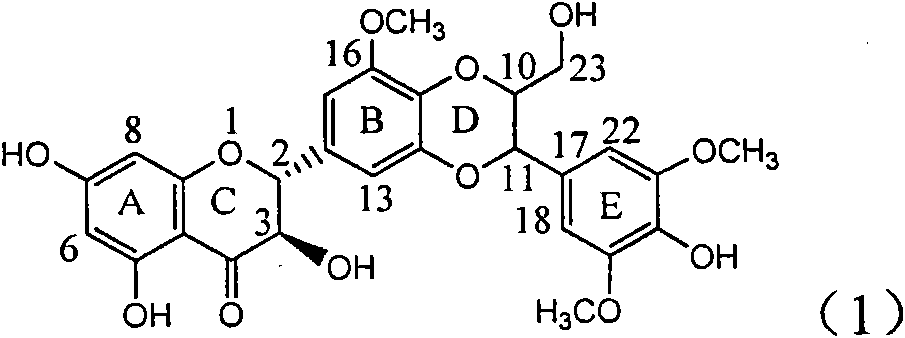
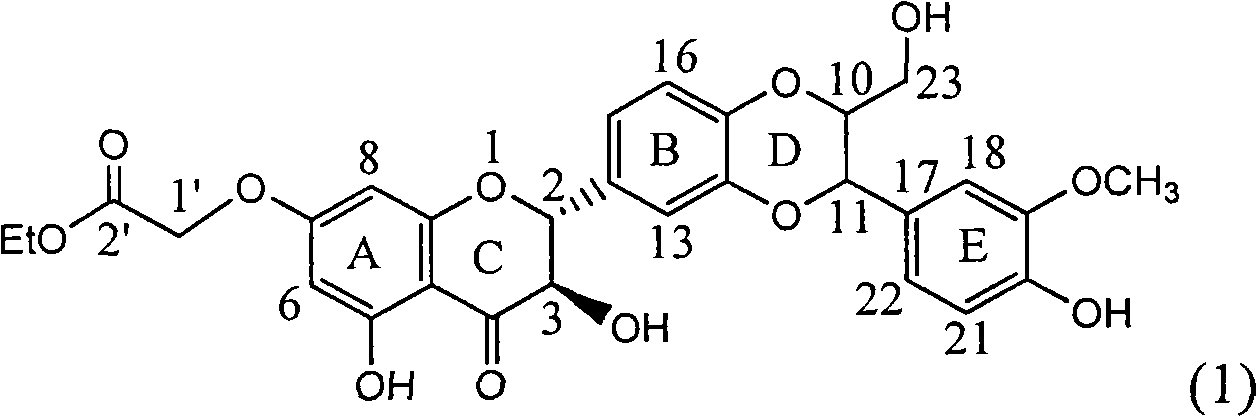
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
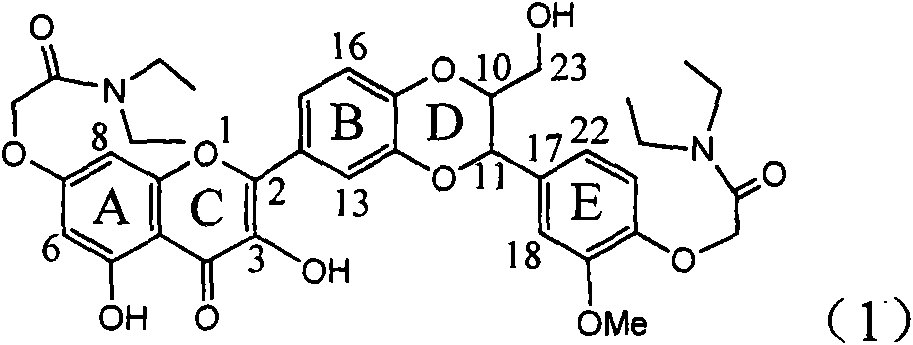
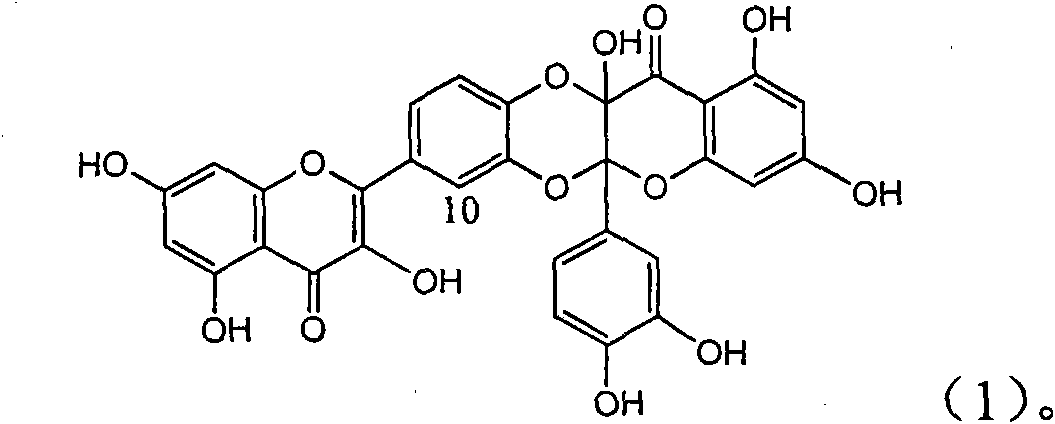
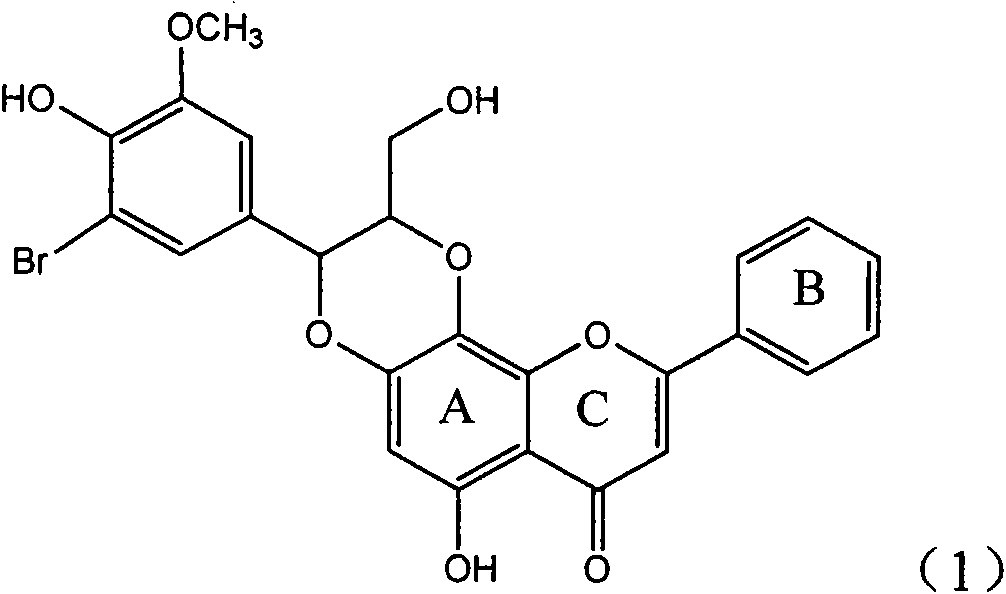
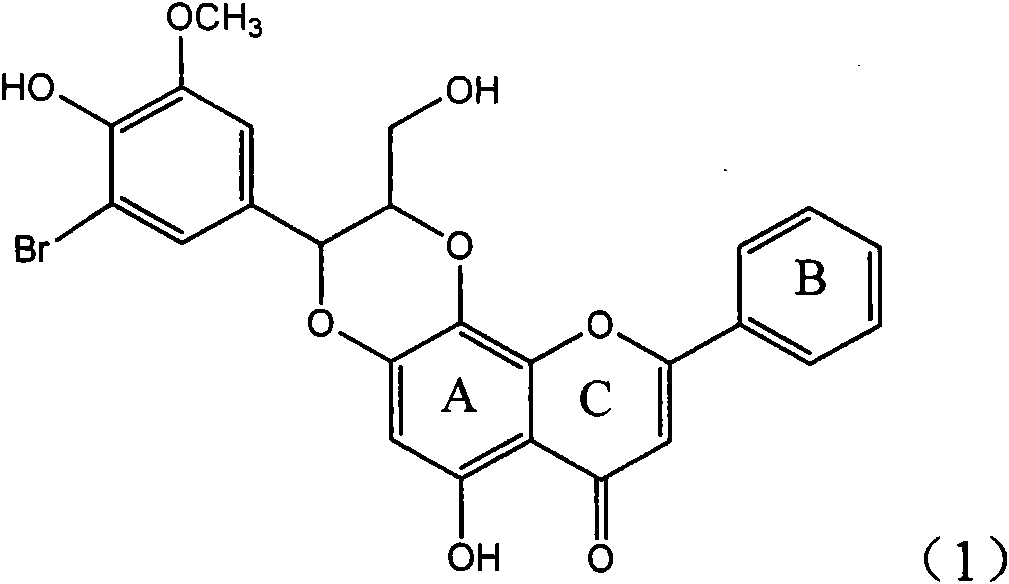
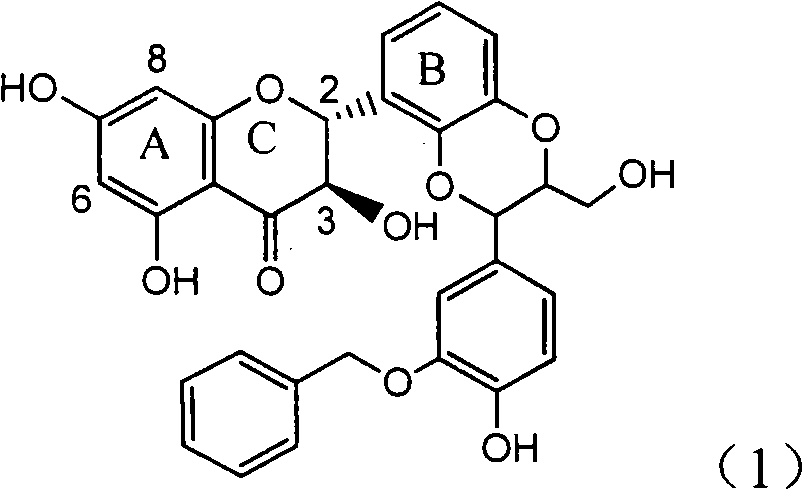
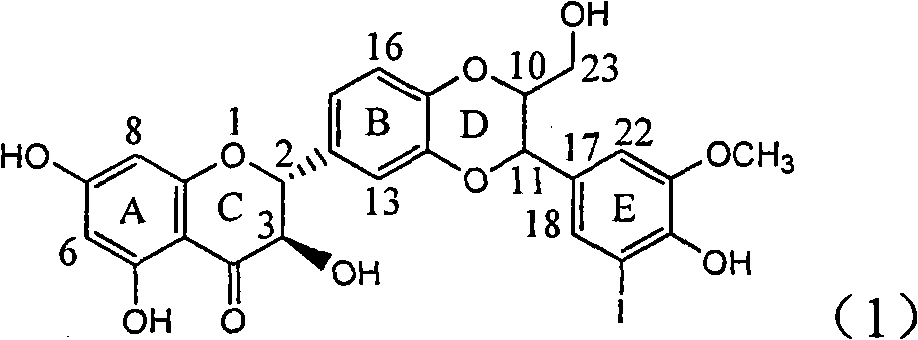
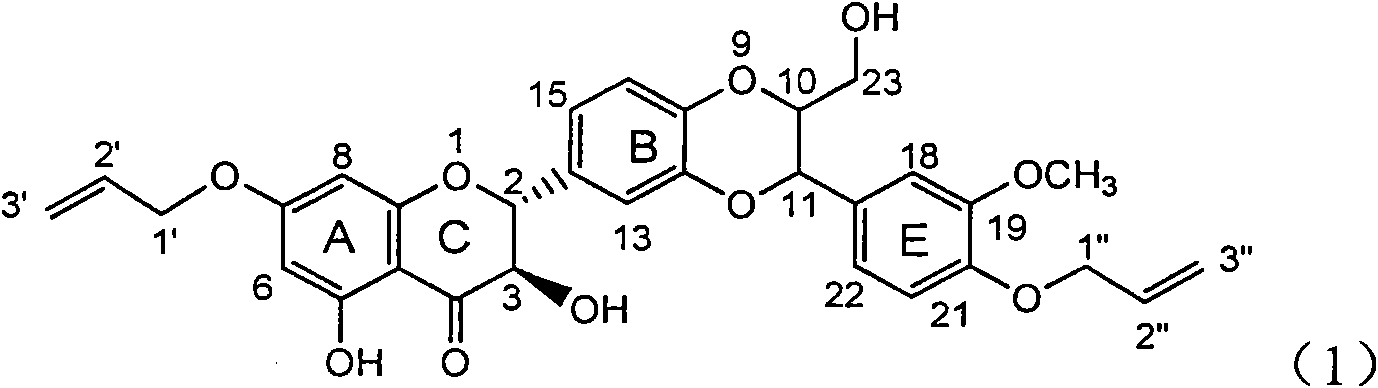
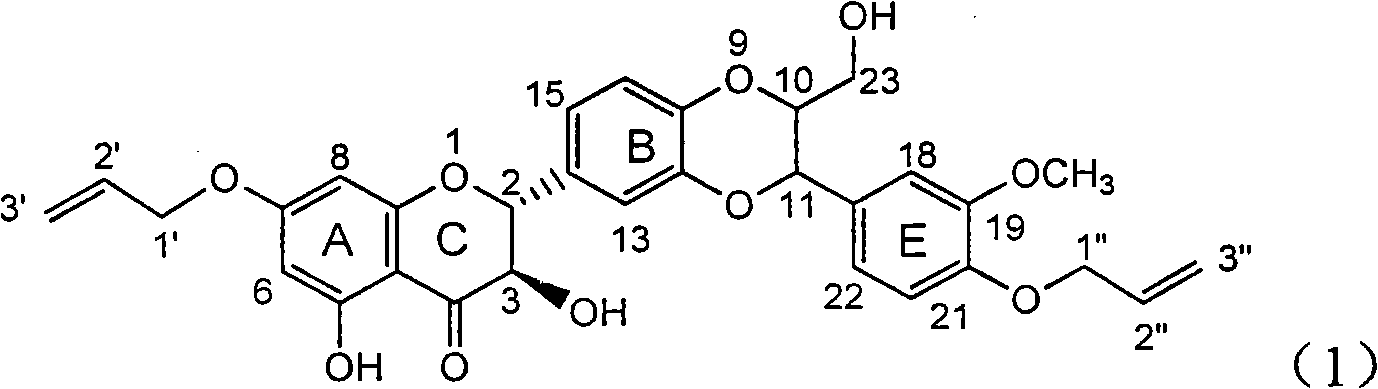
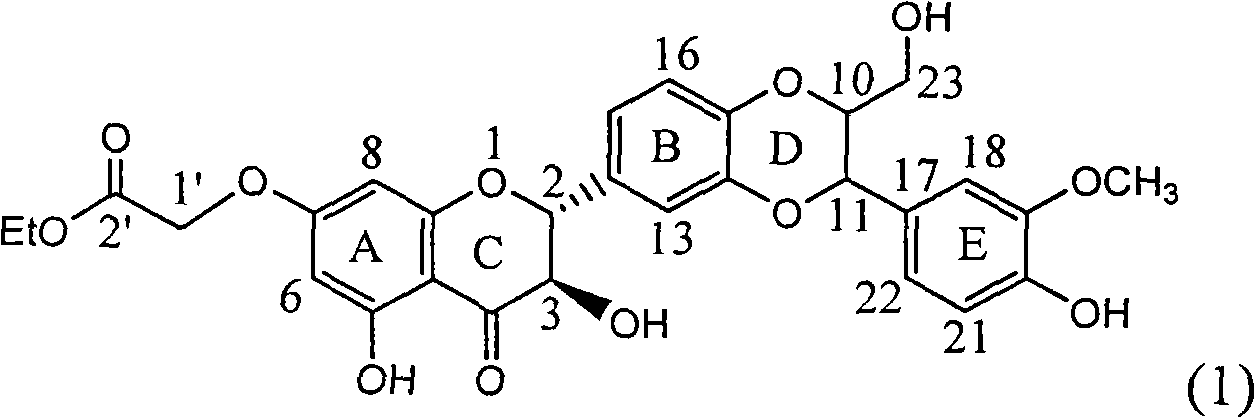
Application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B
InactiveCN101829104AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of the formula (1) or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The intensities of the flavonolignan for clearing away the HBsAg and the HBeAg are respectively 29.4 percent and 29.1 percent in the presence of a concentration of 20 micrograms / milliliter, which is respectively 1.8 times and 1.7 times of the corresponding activity of a positive control medicament (10,000 units / milliliter of alpha-interferon). What is even more exciting is that in the presence of the concentration, the suppression rate of the flavonolignan to the HBV DNA is higher than 83 percent, which is higher than that of Lamivudine which is a positive control and is 2.2 times of that of the alpha-interferon to the HBV DNA. Accordingly, the flavonolignan and the pharmaceutically acceptable salt thereof are indicated to be capable of being expected to be used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
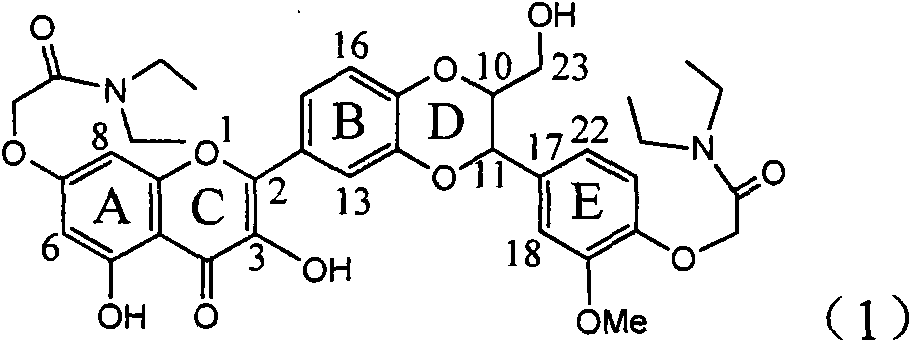
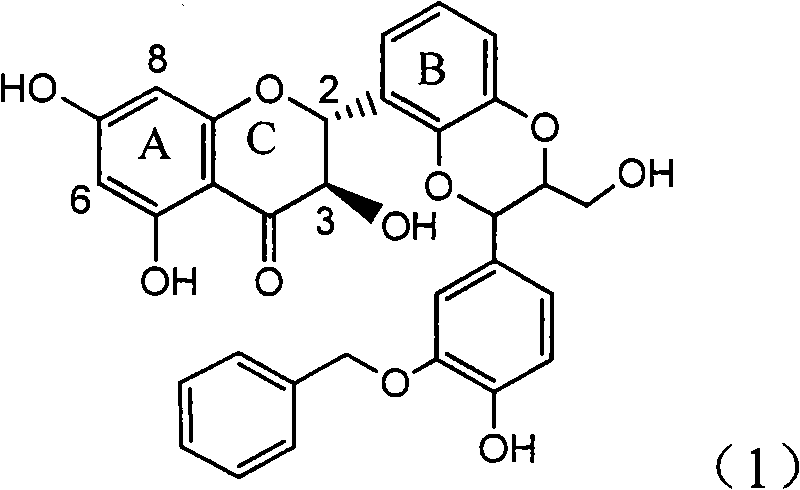
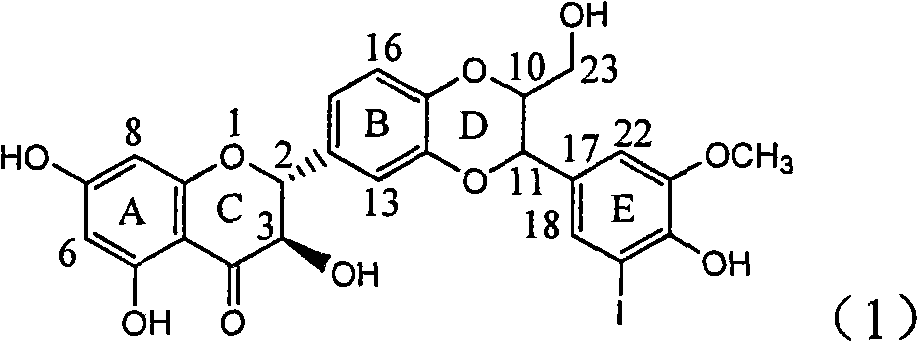
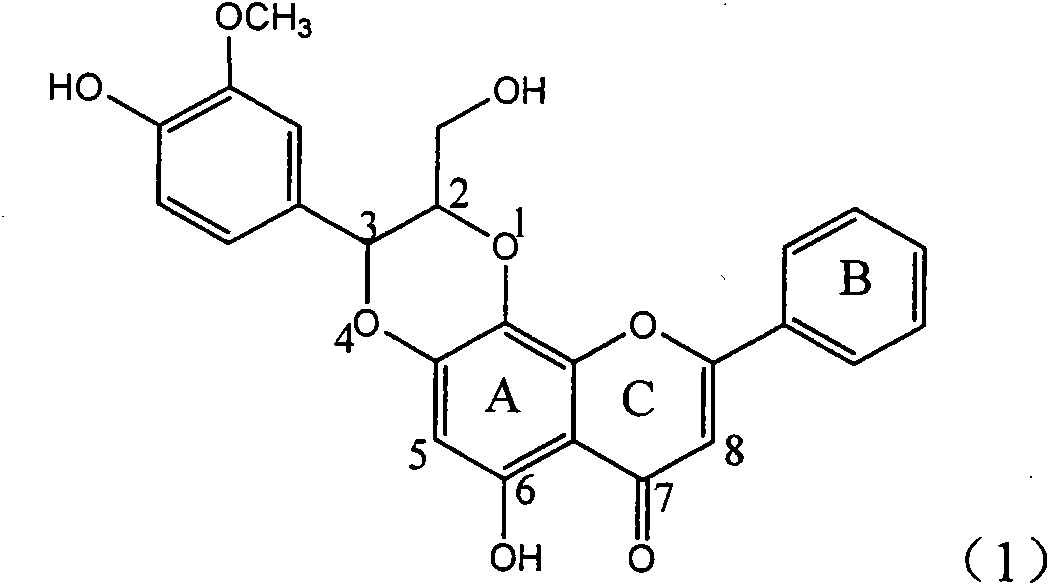
Use of lignanoid containing benzyloxy flavones in preparation of drugs for treating viral hepatitis B
InactiveCN101829095AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to a use of lignanoid containing benzyloxy flavones in the preparation of drugs for treating viral hepatitis B, in particular to the use of a compound as shown in formula (1) or pharmaceutical salts thereof in the preparation of the drugs for eliminating hepatitis B virus surface antigen and hepatitis B e antigen and the drugs for suppressing HBV DNA replication, and the strength of eliminating HBsAg of flavonol lignanoid under the concentration of 20 mu g / ml is 50.8%, which is 3.2 times of the corresponding activity of a positive control drug; the activity of eliminating the HBeAg under the same concentration is equivalent to 10000 units / ml of alpha-interferon; simultaneously, the flavonol lignanoid shows nearly 60% of suppression rate to HBV DNA under the concentration, which is 1.6 times of the corresponding suppression rate of the alpha-interferon. The results show that the lignanoid containing the flavones or the pharmaceutical salts thereof are expected to be used for preparing the non-nucleoside drugs for eliminating the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
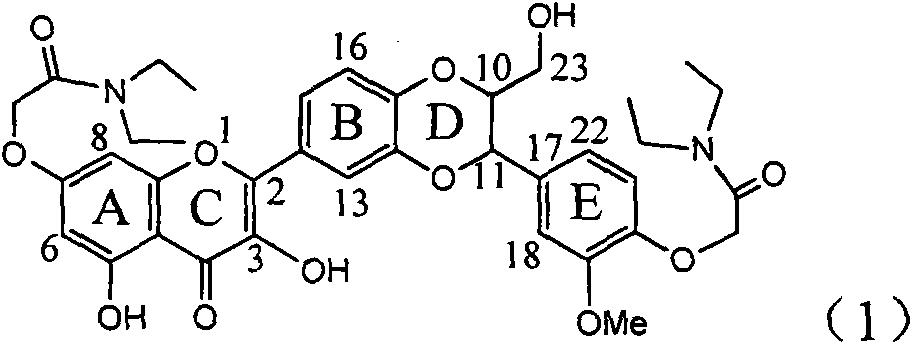
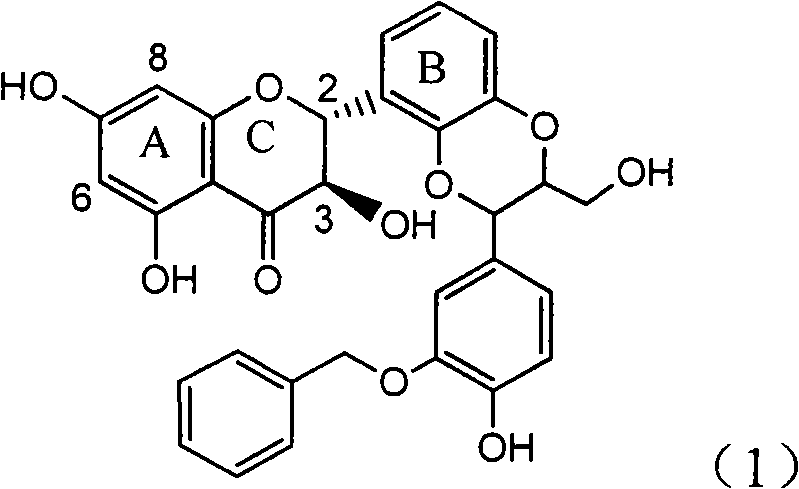
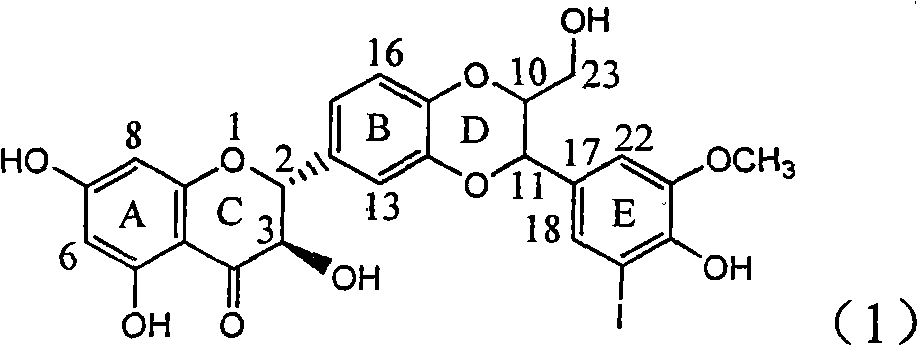
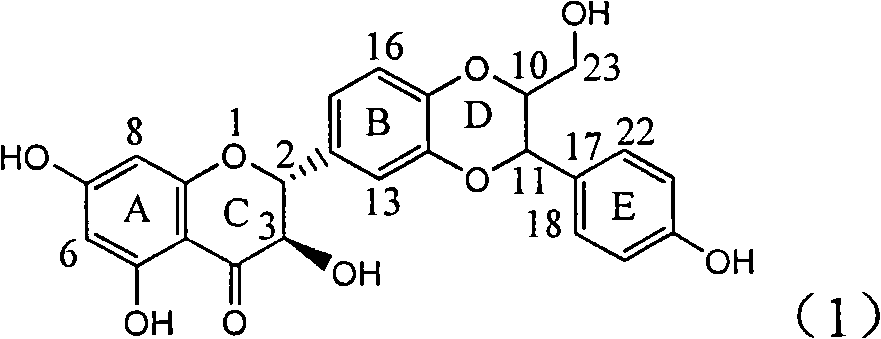
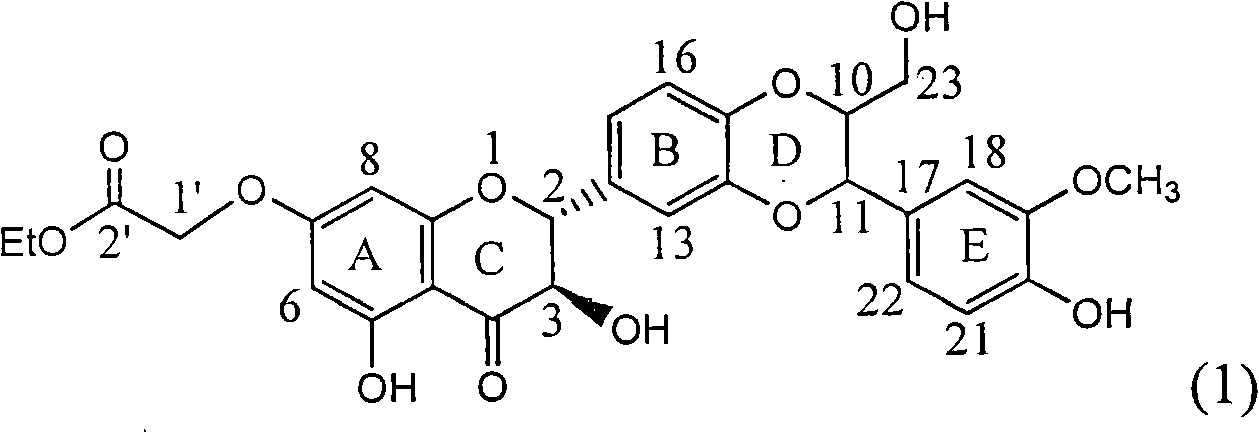
Application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829096AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity of suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 20.0 percent and 29.0 percent which exceed that of a positive control medicament (10,000 units / milliliter of alpha-interferon) by 24 percent and 72 percent. Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 32.6 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
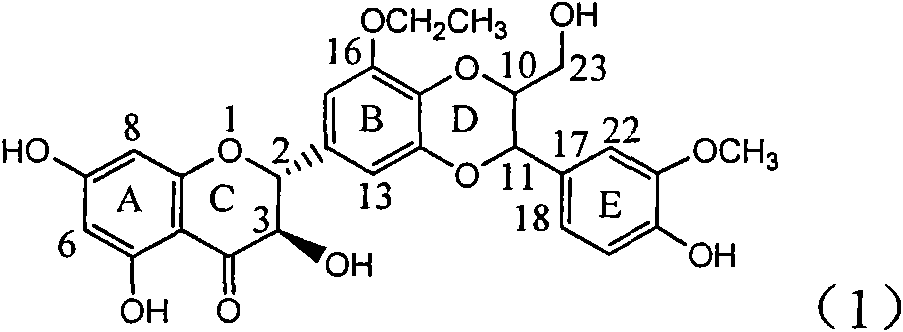
Application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829089AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B, in particular to application of ring B ethyoxyl substituted silybin ester or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAG (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has strong activity on suppressing the HBsAG and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing the HBsAg and the HBeAg are respectively 64.6 percent and 44.8 percent which are 4.0 times and 2.7 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression rate of the compound on the HBV DNA is 58.1 percent which is 1.5 times of the corresponding activity of the alpha-interferon. Accordingly, the flavonolignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have strong efficacy on suppressing the HBsAg, the HBeAg and the HBV DNA and can be expected to be used for preparing non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Application of flavone lignan (+/-) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B
InactiveCN101953827AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseLignan
The invention relates to application of flavone lignan (+ / -) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B, in particular to a compound with the formula (1) or pharmaceutically-acceptable salts thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV (Hepatitis B Virus) DNA replication. In the invention, the intensities of the compound for clearing the HBsAg and the HBeAg under the concentration of 20 micrograms / milliliter respectively reach 81.8 percent and 81.9 percent, which are respectively 5.1 times and 4.8 times as high as the corresponding activity of alpha-interferon used as a positive contrast medicament; and what is more exciting, when the compound has the concentration, the compound performs a suppression ratio higher than 81 percent, and the value is also higher than that of both lamivudine and alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically-acceptable salts can be expectably used for preparing nucleoside medicaments for clearing the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
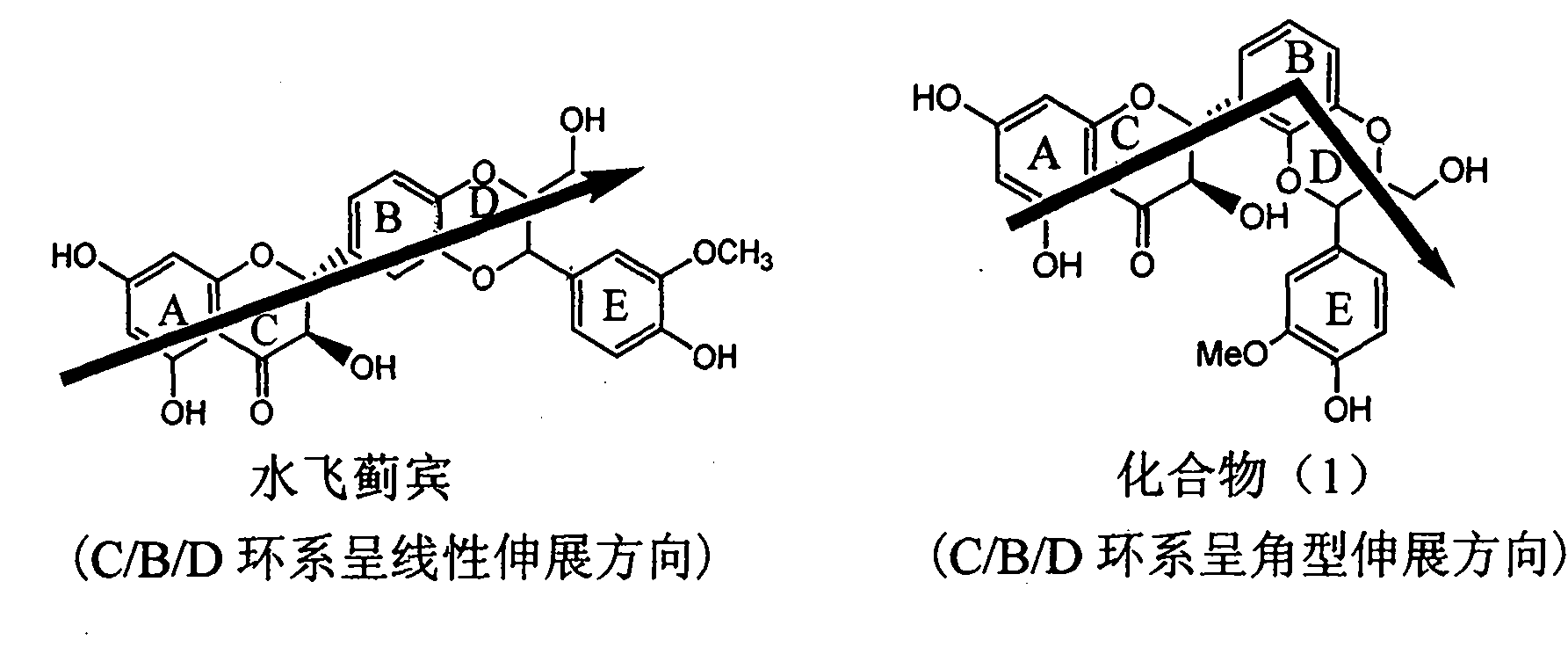
Application of angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B
InactiveCN101953828AInhibition of replicationConvenient sourceOrganic active ingredientsAntiviralsLignanInterferon alpha
The invention relates to application of an angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B, in particular to application of the angle flavonoids lignan or medicinal salts thereof to preparation of medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating HBV infection diseases. The flavonoids lignan can exactly inhibit HBV DNA activity; the inhibition activity of the flavonoids lignan with high dosage (20 microgram / ml) to the HBV DNA replication is 189 percent higher than that of alpha-interferon with the maximum concentration of 10,000 unit / ml; and the flavonoids lignan belongs to a strong-effect non-nucleosides inhibition HBV natural product. The pharmacological results show that the angle flavonoids lignan or the medicinal salts thereof can be expected to be used for preparing the medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
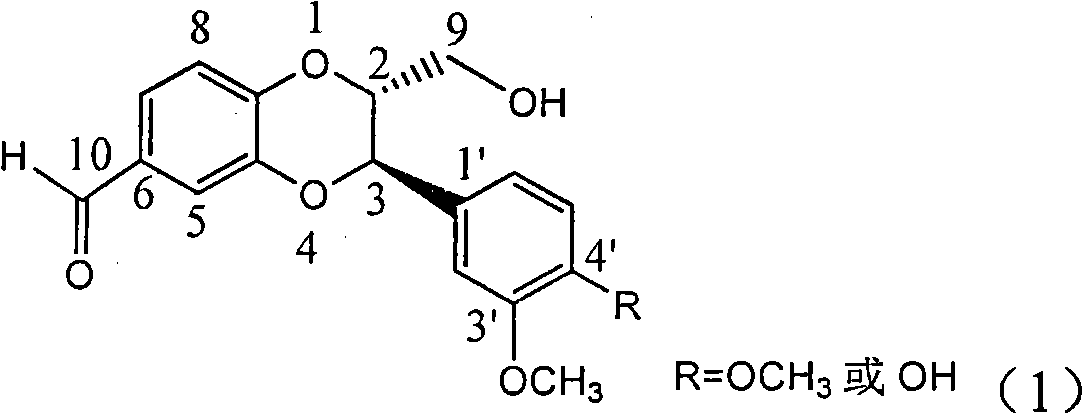
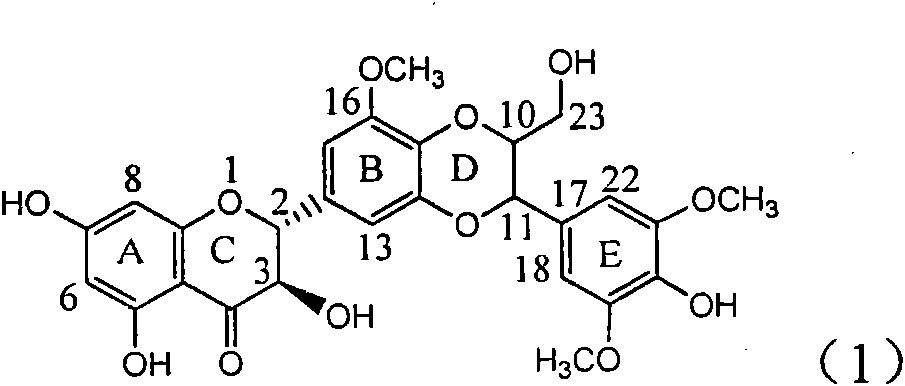
Application of E-ring demethoxy-silibinin for preparing medicament for treating viral hepatitis B
InactiveCN101912383AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of E-ring demethoxy-silibinin for preparing medicaments for treating viral hepatitis B, and particularly to application of compound in formula (1) and pharmaceutically acceptable salt thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV DNA replication. The invention has extremely superactive activity for suppressing the HBsAg and HBeAg; in the presence of the concentration of 20 microgram per millilitre, the intensities for clearing the HBsAg and HBeAg are 95.0% and 34.4% respectively, which are 5.9 and 2.0 times corresponding activity of a positive control medicament alpha-interferon; and it should be noticed that the suppression ratio of the medicament for HBV DNA at the concentration is about 91.5%, which is 13% higher than lamivudine and 2.4 times alpha-interferon suppression activity. In summary, the flavonolignans or pharmaceutically acceptable salt thereof can be prospectively used for preparing non-nucleoside medicaments for clearing the HBsAg and HBeAg, suppressing HBV DNA replication and treating hepatitis B virus infection disease.
Owner:DALI UNIV
Application of benzo-phenylpropanoids in preparing drug for treating viral hepatitis B
InactiveCN101829093AEnhanced inhibitory effectExact originalityOrganic active ingredientsAntiviralsHigh concentrationDisease
The invention relates to an application of benzo-phenylpropanoids in preparing drugs for treating viral hepatitis B, in particular to two benzo-phenylpropanoids or application of pharmaceutically acceptable salt thereof in preparing drugs for inhibiting the replication of hepatitis B virus desoxyribonucleic acid (HBV DNA) and treating hepatitis B virus infection diseases. The two benzo-phenylpropanoids definitely inhibit the activity of the HBV DNA, have the replication inhibition activity on the HBV DNA at high dose (100 microgrammes / milliliter) of 1.3-2.2 times higher than the inhibition activity at the highest concentration (10000 units / milliliter) of an alpha-interferon and belong to an efficient non-nucleoside natural product inhibiting the hepatitis B viruses; pharmacodynamics results show the application of the benzo-phenylpropanoids or the pharmaceutically acceptable salt thereof capable of preparing the drugs for inhibiting the replication of the HBV DNA and treating the hepatitis B virus infection diseases in anticipation.
Owner:DALI UNIV
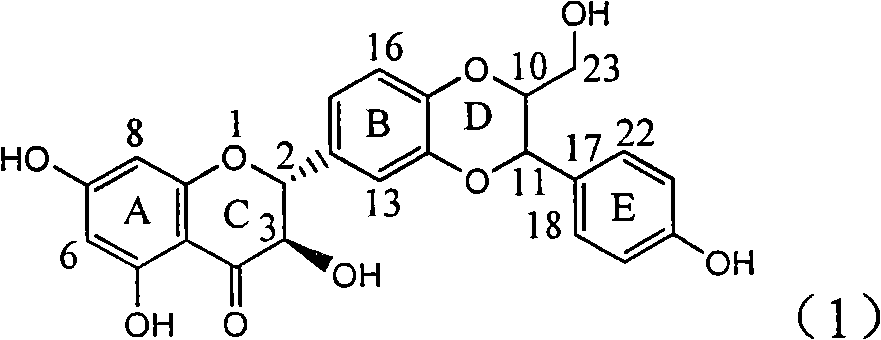
Application of B/E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829088AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of B / E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester substituted by the methoxy on the ring B and the ring E or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The B / E bi-methoxy silybin has strong activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing away the HBsAg and the HBeAg are respectively 43.9 percent and 43.7 percent which are 2.7 times and 2.6 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression ratio on the HBV DNA is 68.6 percent, and the suppression activity is 1.8 times of that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have the effects of strongly suppressing the HBsAG, the HBeAg and the HBV DNA and can be expected to be used for preparing the non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Application of diallyl propyl flavonolignan in preparation of medicament for curing hepatitis B
InactiveCN101829106ALower hepatitis B e antigenInhibition of HBV DNA replicationOrganic active ingredientsOrganic chemistryViral hepatitis bHenipavirus Infections
The invention relates to application of diallyl propyl flavonolignan in preparation a medicament for curing hepatitis B, in particular to application of a flavonolignan or pharmaceutically acceptable salts thereof in preparation of the medicament for curing the hepatitis B by clearing hepatitis B e-antigen and inhibiting HBV DNA replication. The flavonolignan has hepatitis B virus e-antigen (HBeAg) inhibiting activities, and has the inhibition strength higher than a positive control first-line medicament, namely lamivudine and alpha-interferon, at a low concentration of 20 mu g / ml; simultaneously, at the concentration of 5 mu g / ml, the compound has an inhibition rate of the HBV DNA of over 70 percent; and therefore, the flavonolignan can be expected for preparing the medicament for curing the hepatitis B virus infection diseases by clearing the hepatitis B e-antigen and inhibiting the HBV DNA replication.
Owner:DALI UNIV
Application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B
InactiveCN101829101AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester flavonolignan substituted by ethoxycarbonyl methyl on the ring A or a pharmaceutically acceptable salt thereof for preparing medicaments for reducing the hepatitis B virus surface antigen (HBsAg), suppressing the HBV (Hepatitis B Virus) DNA replication and treating HBV infection diseases. The flavonolignan has quite obvious activity on suppressing the HBsAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensity of the flavonolignan for clearing away the HBsAG exceeds that of alpha-interferon which is a positive control medicament by 3.3 times. Meanwhile, in the presence of a concentration of 20 micrograms / milliliter, suppression ratio of the compound to the HBV DNA is close to 60 percent. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for treating the HBV infection diseases.
Owner:DALI UNIV
Chinese medicinal composition for treating hepatitis B virus
InactiveCN1223374CImprove the immunityPromote productionDigestive systemAntiviralsSide effectViral hepatitis b
The Chinese medicine composition for treating viral hepatitis B is prepared with astragalus root, Dangshen, fried white atractylodes rhizome, angelica, white peony root and other 12 kinds of Chinese medicinal materials in certain proportion, and through decoction. It has high viral hepatitis B curing rate and no toxic side effect, and can produces fluent liver Qi and flexible Qi and blood.
Owner:梁兴家
Viral hepatitis treatment
The present disclosure relates to methods for treating viral hepatitis, compounds useful in the treatment of viral hepatitis, and pharmaceutical compositions comprising such compounds. In one embodiment, pharmaceutical compositions comprising nitazoxanide, tizoxanide, or derivatives and / or mixtures thereof are provided, as well as methods of treating hepatitis C using such compositions.
Owner:ROMARK LAB L C
Enoxolone derivative, preparation method and uses
ActiveCN1762967AEasy to makeSolve solubilityAntibacterial agentsOrganic active ingredientsSolubilityInjury brain
The present invention provides one new kind of glycyrrhetic acid derivative with high water solubility, high absorption, no irritation to blood vessel and capacity of being prepared into injection, and its preparation process, medicinal composition and preparation. The glycyrrhetic acid derivative has excellent treating effect on acute and chronic hepatitis, bacterial hepatitis, viral hepatitis, cerebral ischemia / re-perfusion damage, brain injury and myocardial ischemia / re-perfusion damage.
Owner:SHANDONG LUYE PHARMA CO LTD
New applications of antibody of GDF15 (Growth differentiation factor 15) protein
ActiveCN101852804AMicrobiological testing/measurementImmunoglobulins against growth factorsSerodiagnosesDisease
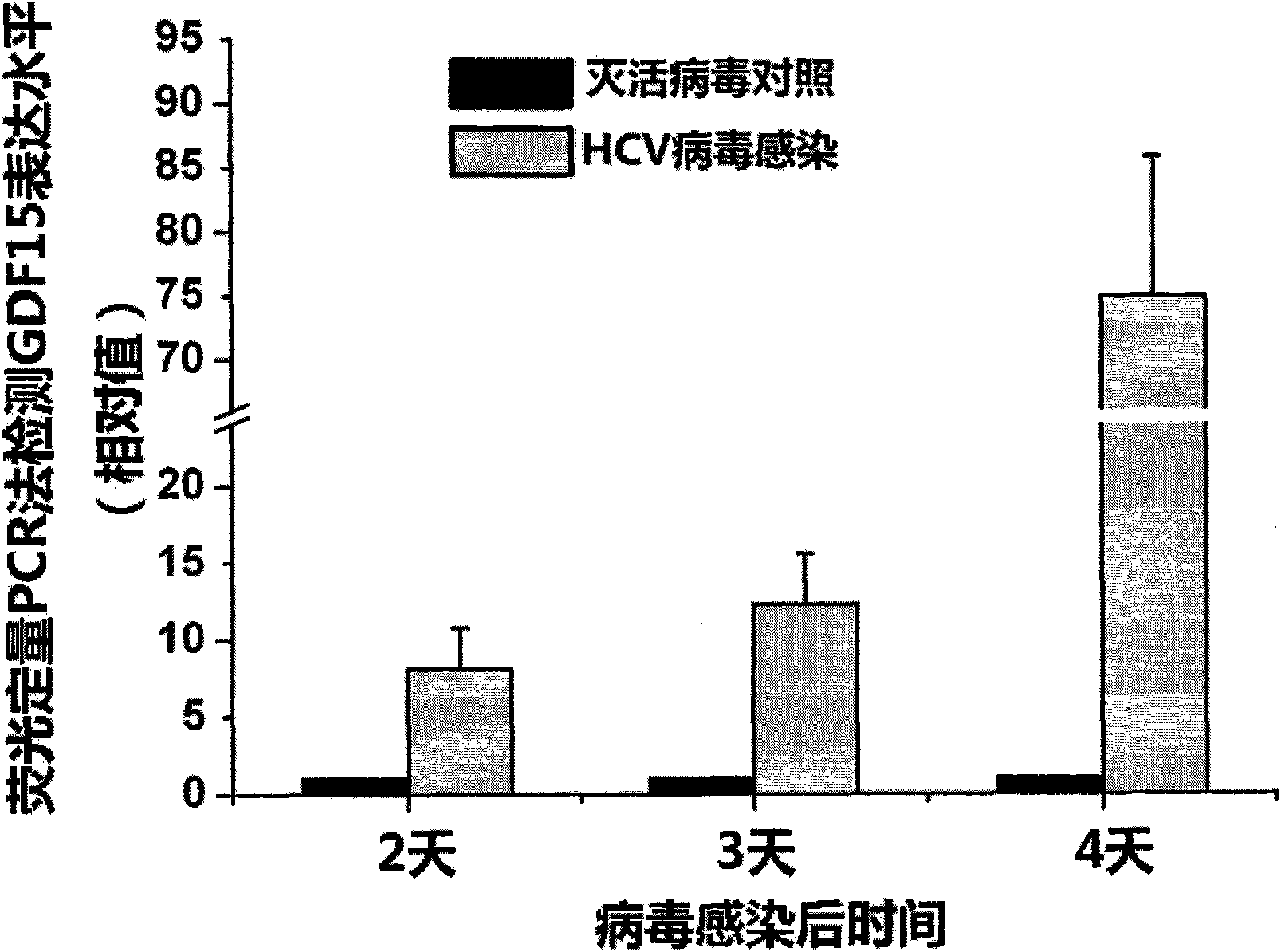
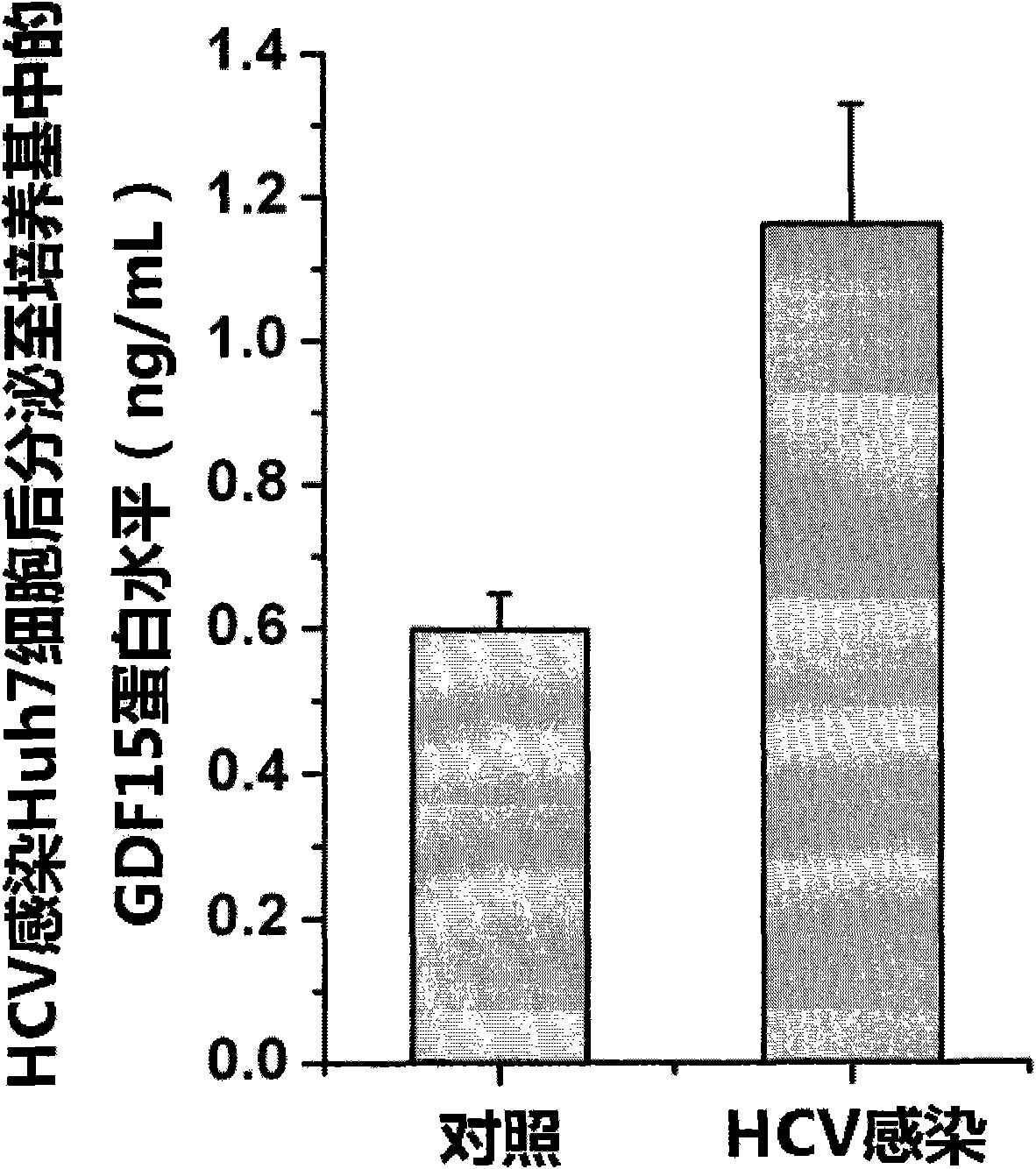
The invention discloses new applications of an antibody of GDF15 (Growth differentiation factor 15) protein. The invention provides the following three applications of the antibody of the GDF15 protein: 1. the application in preparing a reagent for assisting to identify a hepatitis C patient or a hepatitis B patient; 2. the application in preparing a reagent kit for assisting to identify the hepatitis C patient or the hepatitis B patient; and 3. the application in preparing a reagent kit for assisting to identify the hepatitis C progress of the hepatitis C patient, wherein the hepatitis C progress is chronic hepatitis C, hepatitis C cirrhosis or liver cancer. On the basis of the three applications, the antibody of the GDF15 protein can be prepared into three reagent kits. The reagent kit can be used for the serological diagnosis of viral hepatitis (the hepatitis C or the hepatitis B) and judging the disease progress (the chronic hepatitis C, the hepatitis C cirrhosis or the liver cancer) and for prognosis and viral hepatitis treatment monitoring and has great value for the diagnosis and the treatment of the hepatitis C and the hepatitis B.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Treatment and prevention of viral hepatitis infections
InactiveUS20050201952A1Inhibiting HIV- replicationMinimal toxicityBiocideCosmetic preparationsN-Glycolylneuraminic acidViral hepatitis
Methods for treating a viral hepatitis infection in a subject are described that include administering N-glycolylneuraminic acid or a derivative thereof to the subject.
Owner:UNKNOWN
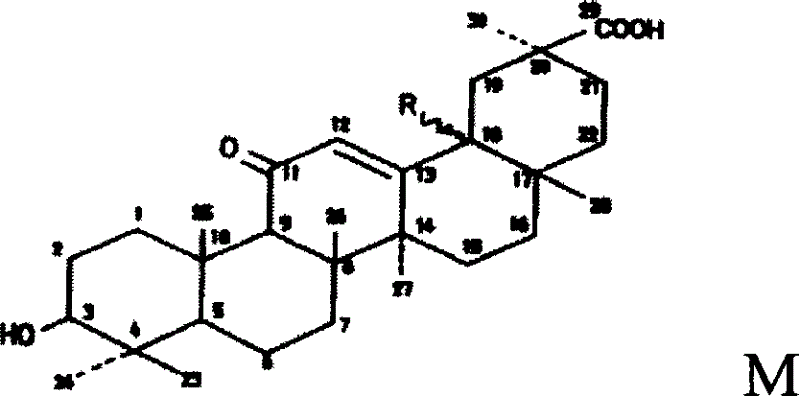
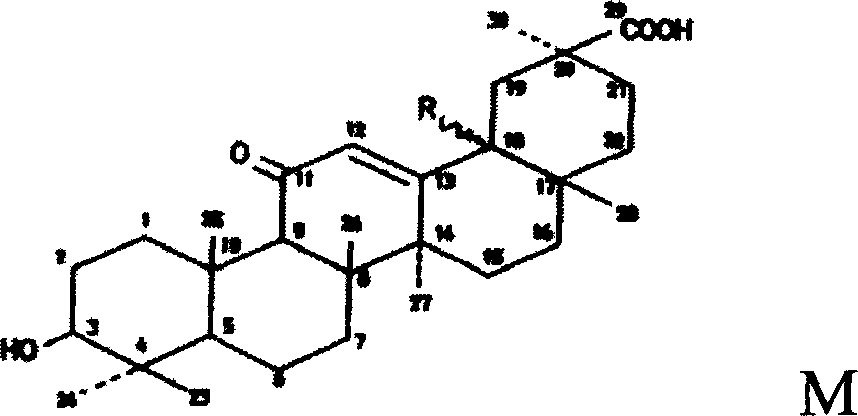
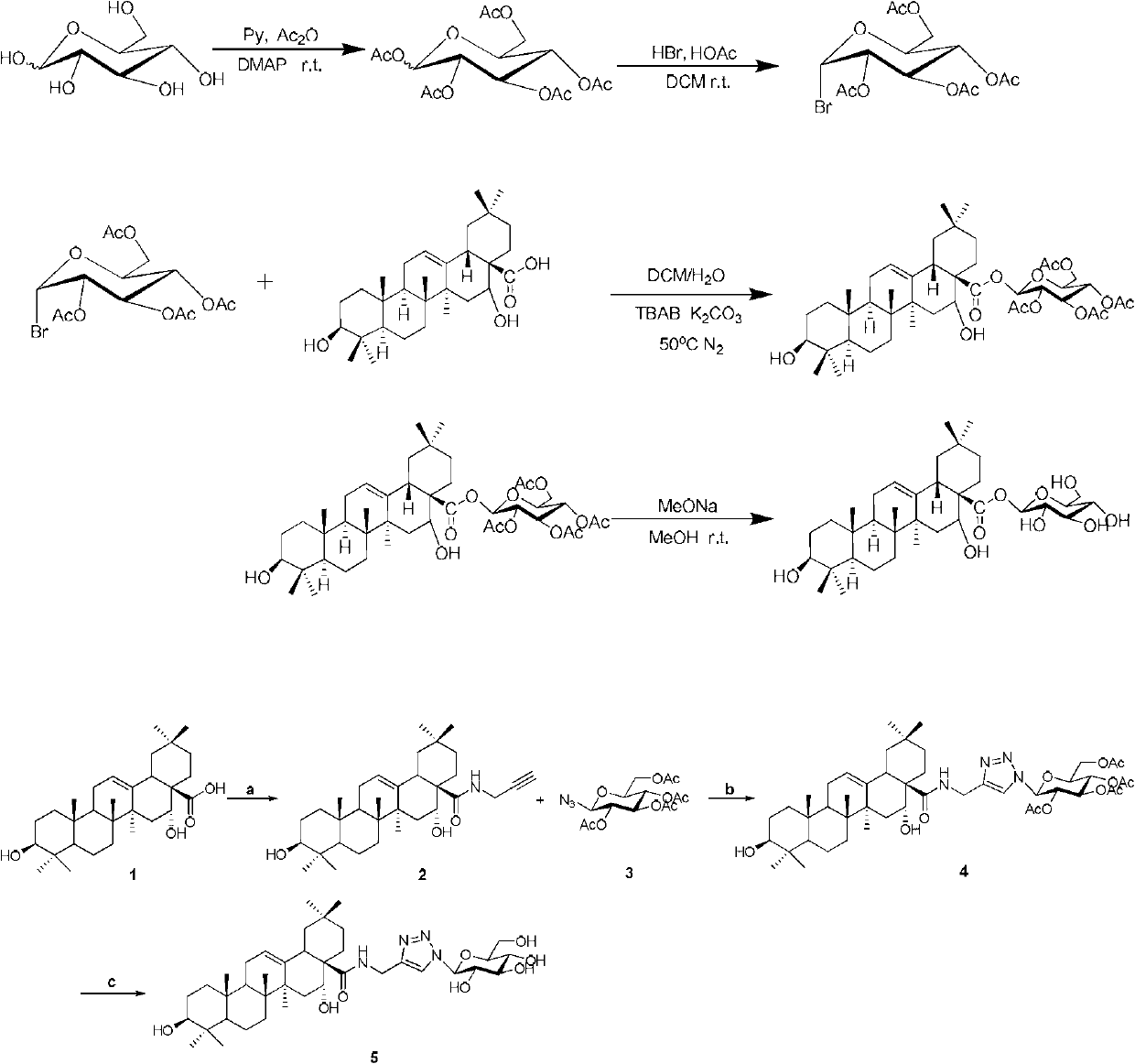
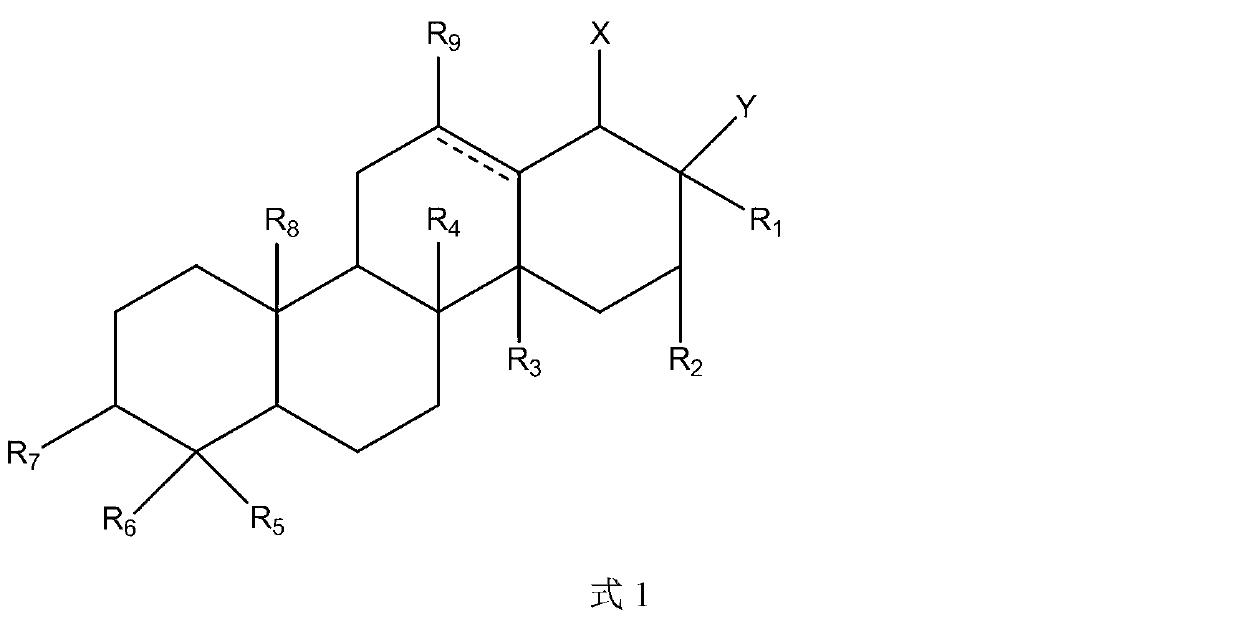
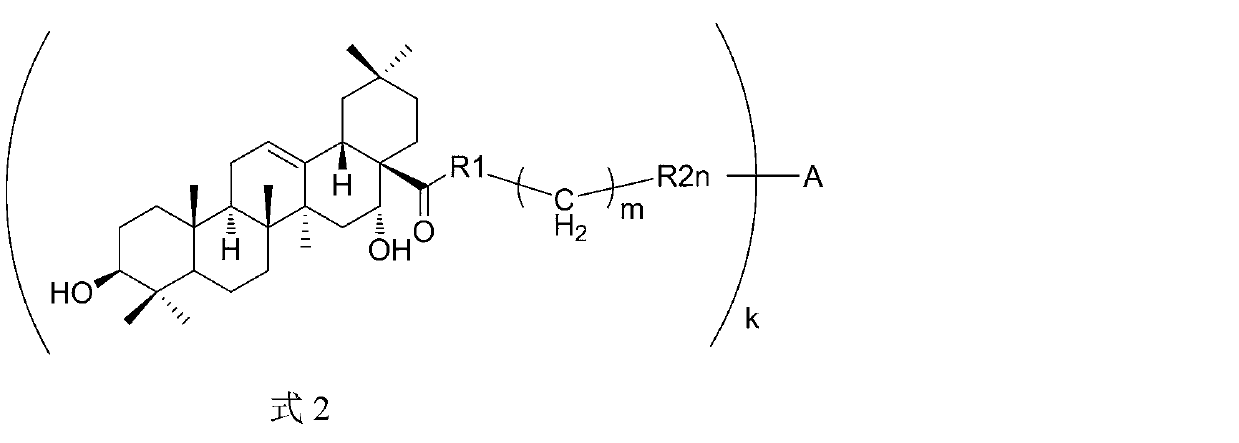
Triterpene derivative and preparation method and application
The invention relates to one type of triterpene, a derivative of the triterpene, a preparation method of the triterpene and the derivative of the triterpene, application in prevention and treatment of viral hepatitis and in particular to the application in treatment and prevention of viral hepatitis C. The triterpene and the derivative of the triterpene are compounds or salts or aquo-complexes of the salts like a structural formula 1 and a structural formula 2 expressed by an instruction book, wherein, the salts or aquo-complexes of the salts can be accepted in pharmacy. A substituent group and symbols are showed in definition of the instruction book.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com