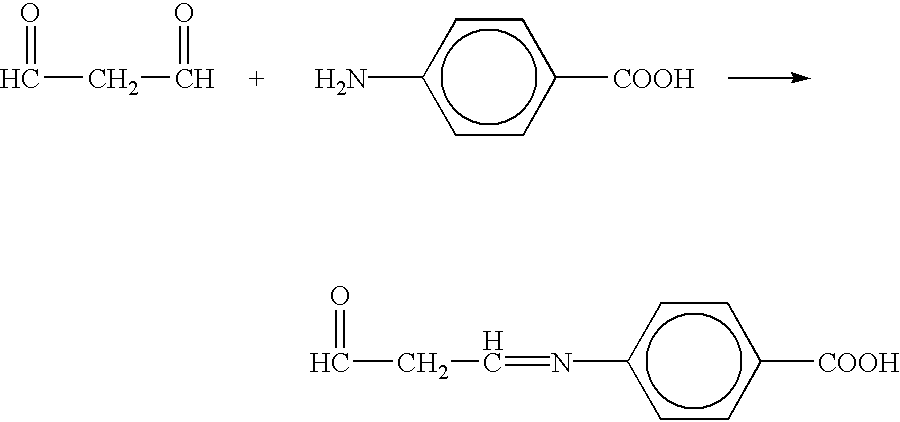
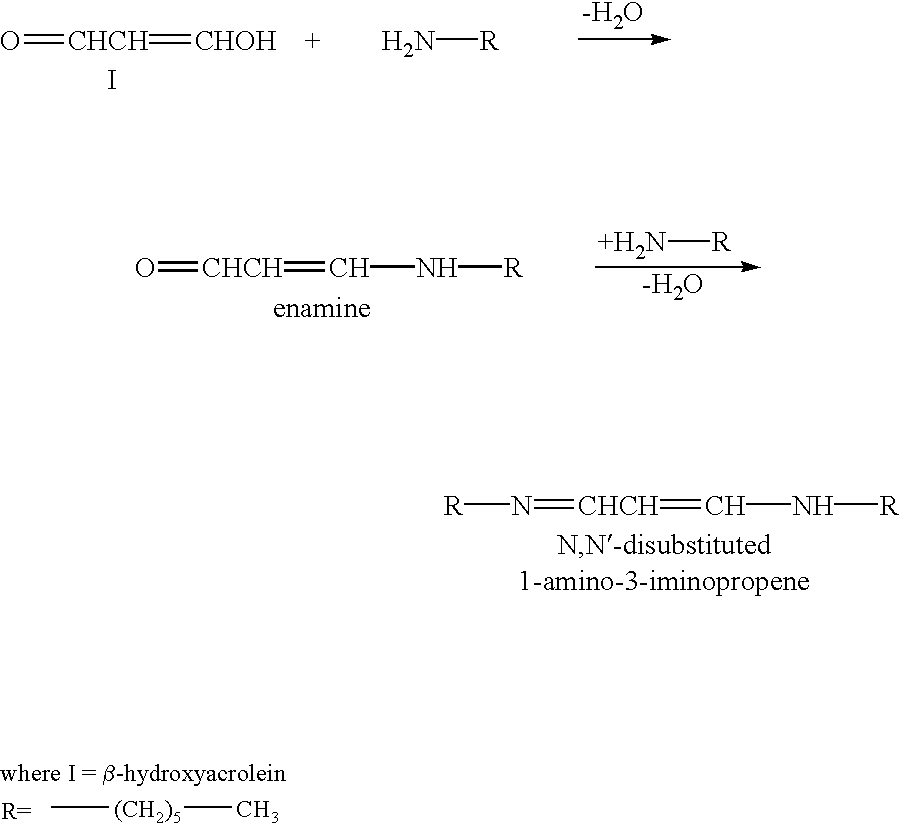
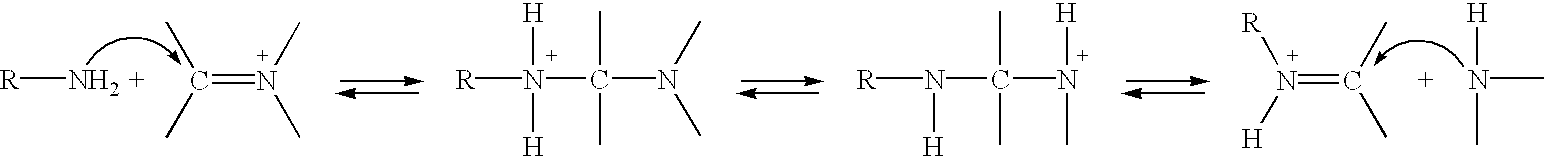Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
420 results about "High dosage" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel dosage form
ActiveUS20060024365A1Effectively control release rateReduce sizePill deliveryEster active ingredientsHigh dosesSolubility
A dosage form comprising of a high dose, high solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of modified release active ingredient per unit is from 500 mg to 1500 mg; a process for preparing the dosage form.
Owner:TORRENT PHARMA LTD
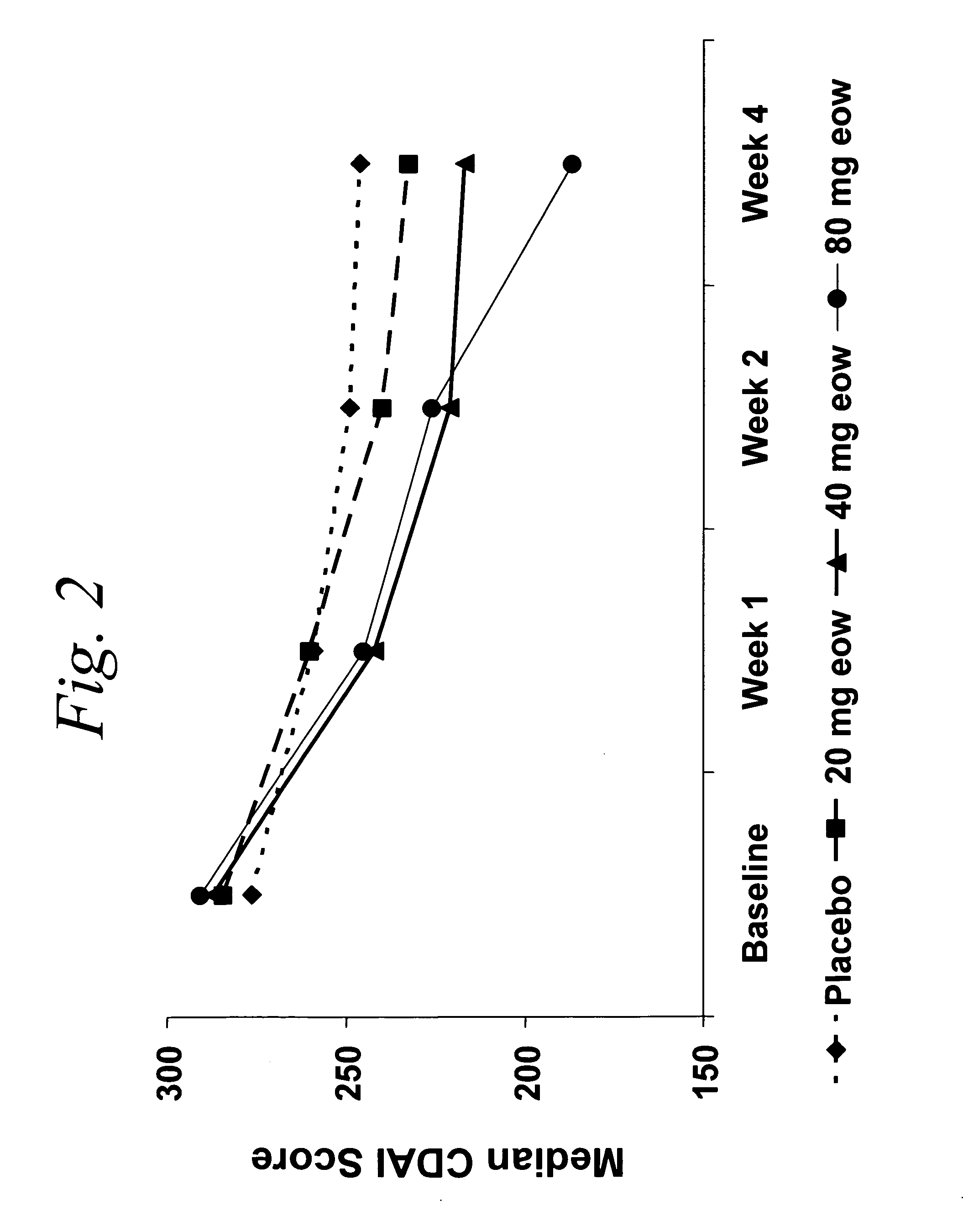
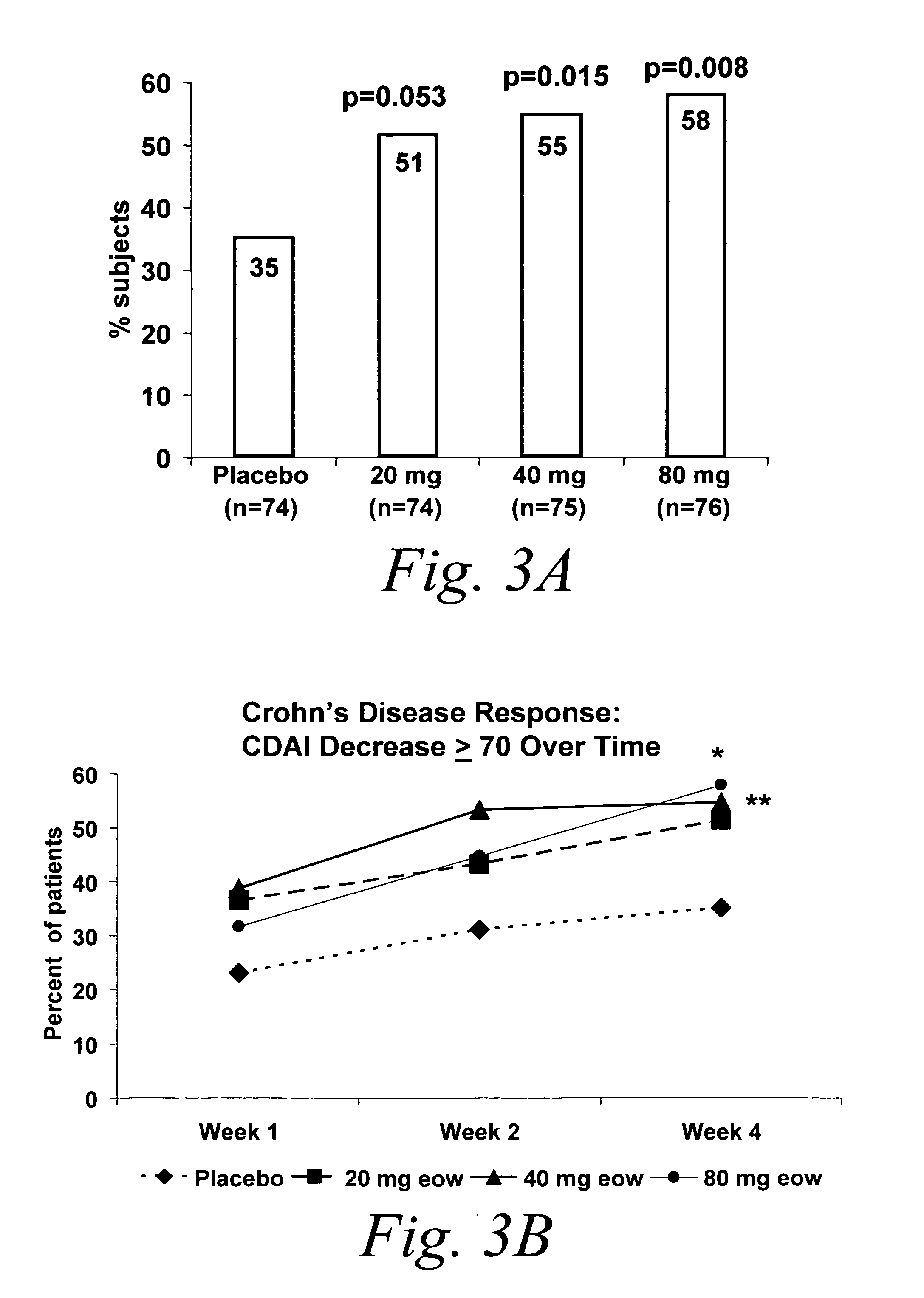
Multiple-variable dose regimen for treating TNFalpha-related disorders
ActiveUS20060009385A1Reduce decreaseIncrease the areaBiocideOrganic active ingredientsDosing regimenRegimen
Multiple-variable dose methods for treating TNFα-related disorders, including Crohn's disease and psoriasis, comprising administering TNFα inhibitors, including TNFα antibodies, are described. Multiple-variable dose methods include administration of a TNF-inhibitor in an induction or loading phase followed by administration of the agent in a maintenance or treatment phase, wherein the TNF-inhibitor is administered in a higher dosage during the induction phase.
Owner:ABBVIE BIOTECHNOLOGY LTD
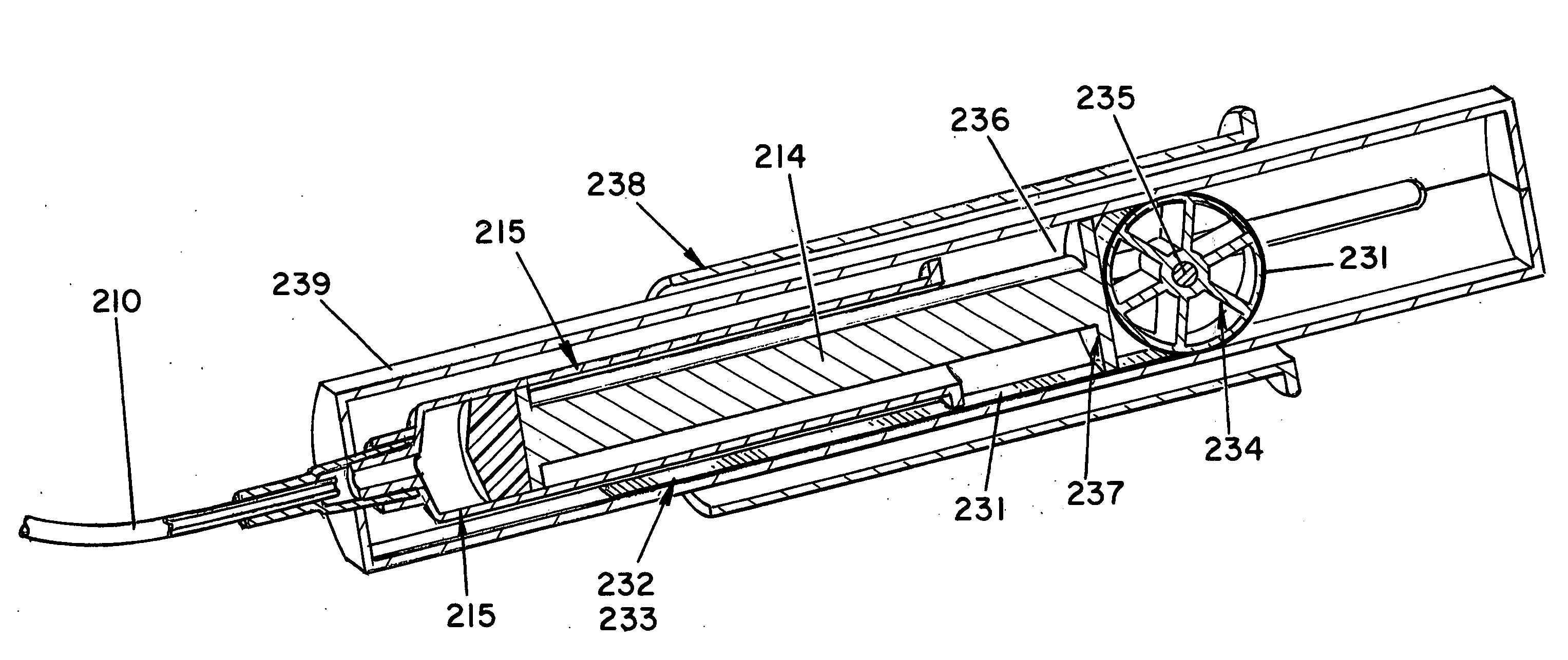
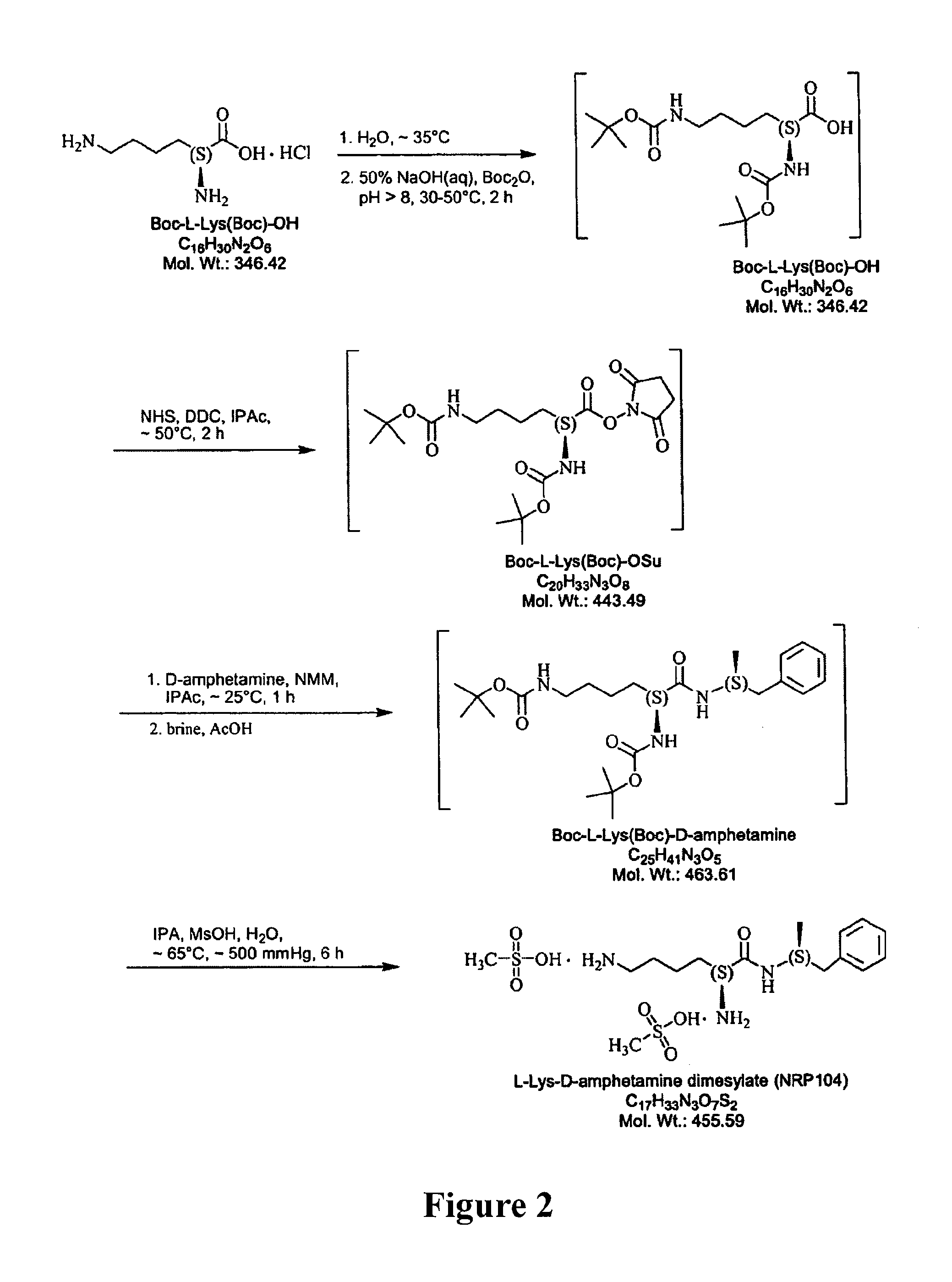
Abuse-resistant amphetamine compounds
InactiveUS7105486B2Reduced activityRelease is diminished and eliminatedOrganic active ingredientsPeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Multiple-variable dose regimen for treating TNFa-related disorders
InactiveUS20090304682A1Reducing remissionReducing signOrganic active ingredientsPeptide/protein ingredientsDosing regimenRegimen
Owner:ABBVIE BIOTECHNOLOGY LTD
Methods of treating chronic inflammatory diseases using carbonyl trapping agents
InactiveUS6444221B1Improved therapeutic propertyImprove propertiesBiocidePeptide/protein ingredientsEtiologyBenzoic acid
Owner:SECANT PHARMA
Novel dosage form
InactiveUS20060153916A1Effectively control release rateSmall sizePill deliveryEster active ingredientsMedicineImmediate release
A dosage form comprising of a high dose, high solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of modified release active ingredient per unit is from 500 mg to 1500 mg; a process for preparing the dosage form.
Owner:TORRENT PHARMA LTD
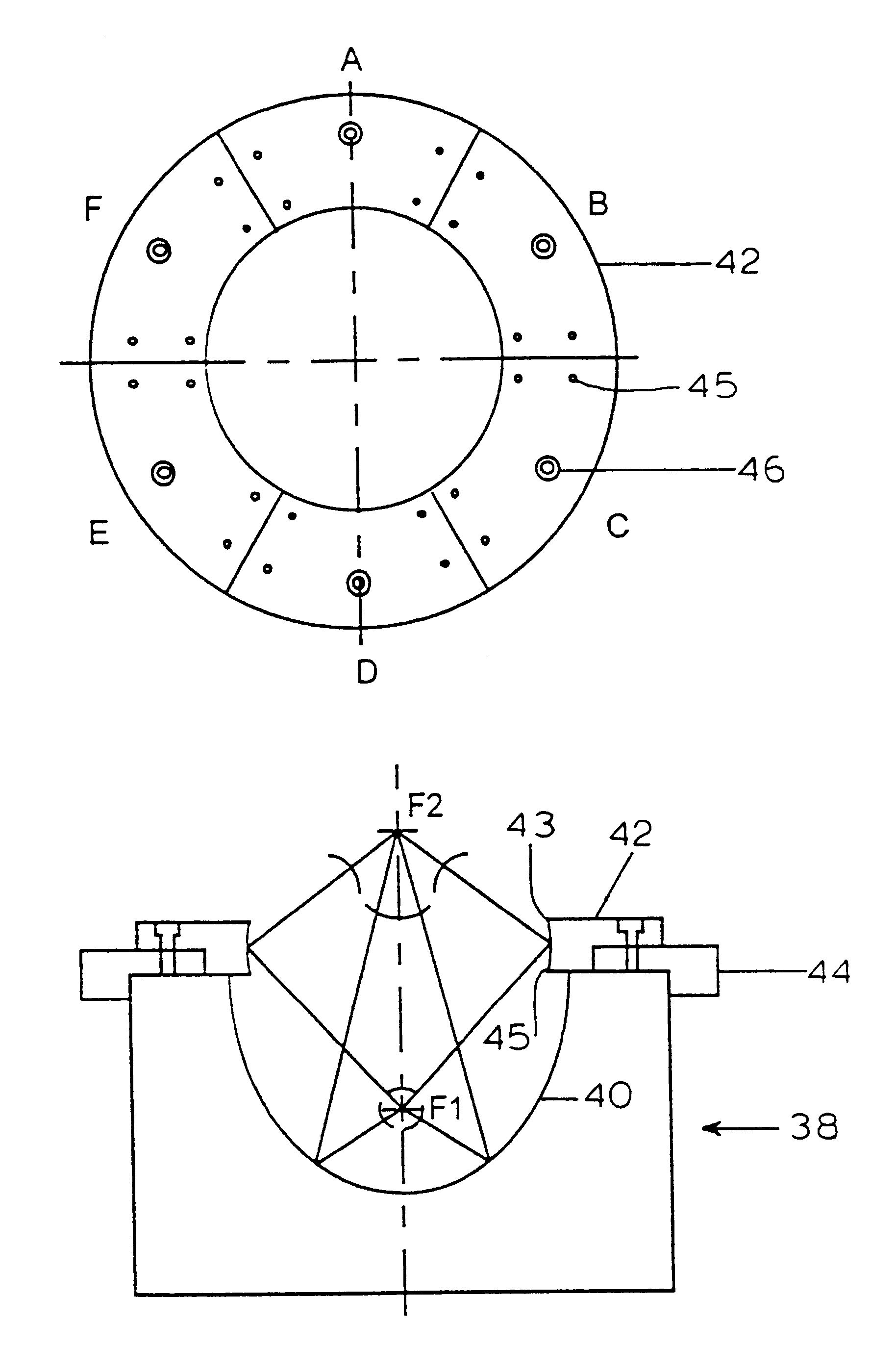
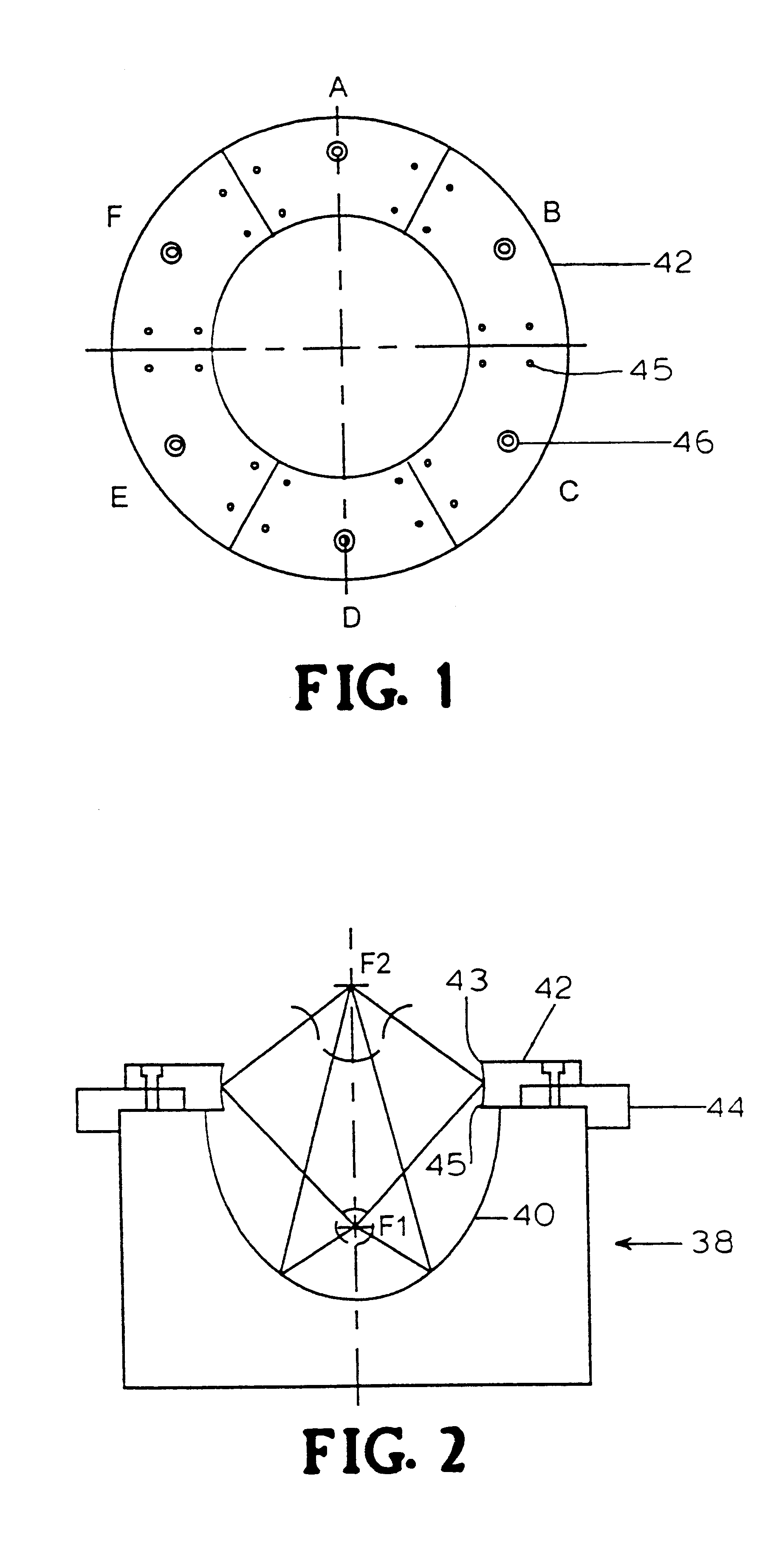
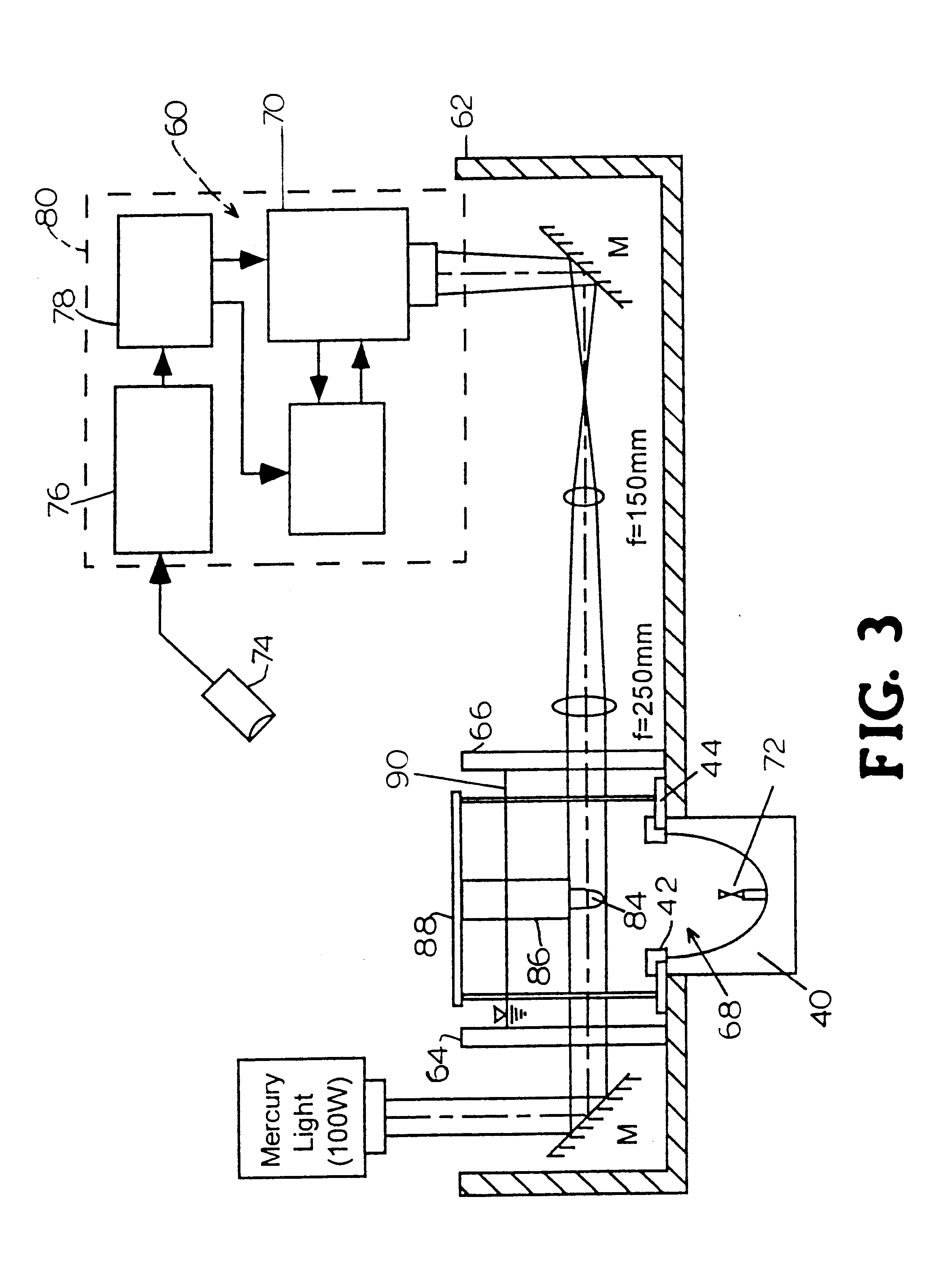
Apparatus and method for macromolecule delivery into living cells
InactiveUS6298264B1Improve efficiencyEasily damagedElectrotherapySurgeryShock waveShock wave lithotripter
This invention discloses an apparatus and method for producing microcavitational activity in aqueous fluids for non-invasive macromolecule delivery into living cells. A standard electrohydraulic shock wave lithotripter is fitted with an adjustable ring reflector that shares the same foci as the standard lithotripter hemi-ellipsoidal reflector. A small portion of the spherical shock wave, generated by the spark discharge at the first focus (F1), is reflected and diffracted by the ring reflector, resulting in a weak preceding shock wave approximately 8.5 mus in front of the lithotripter shock wave reflected and diffracted by the hemi-ellipsoidal reflector. The peak negative pressure of the preceding weak shock wave or pulse at F2 can be adjusted from -0.96 to -1.91 MPa, using an output voltage of 25 kV. Living cells are exposed to the preceding shock wave and the lithotripter shock wave. With optimal pulse combination, the maximum efficiency of shock wave-induced cell membrane permeabilization can be enhanced substantially (up to 91%), by applying to the living cells a low dosage of, for example, 50 shocks. In addition, injury to mouse lymphoid cells is significantly increased at high dosage (up to 50% with shock number >100). The invention thus enables shock wave-inertial microbubble interaction to be used selectively to either enhance the efficiency of shock wave-mediated macromolecule delivery at low dosage or tissue destruction at high dosage.
Owner:DUKE UNIV
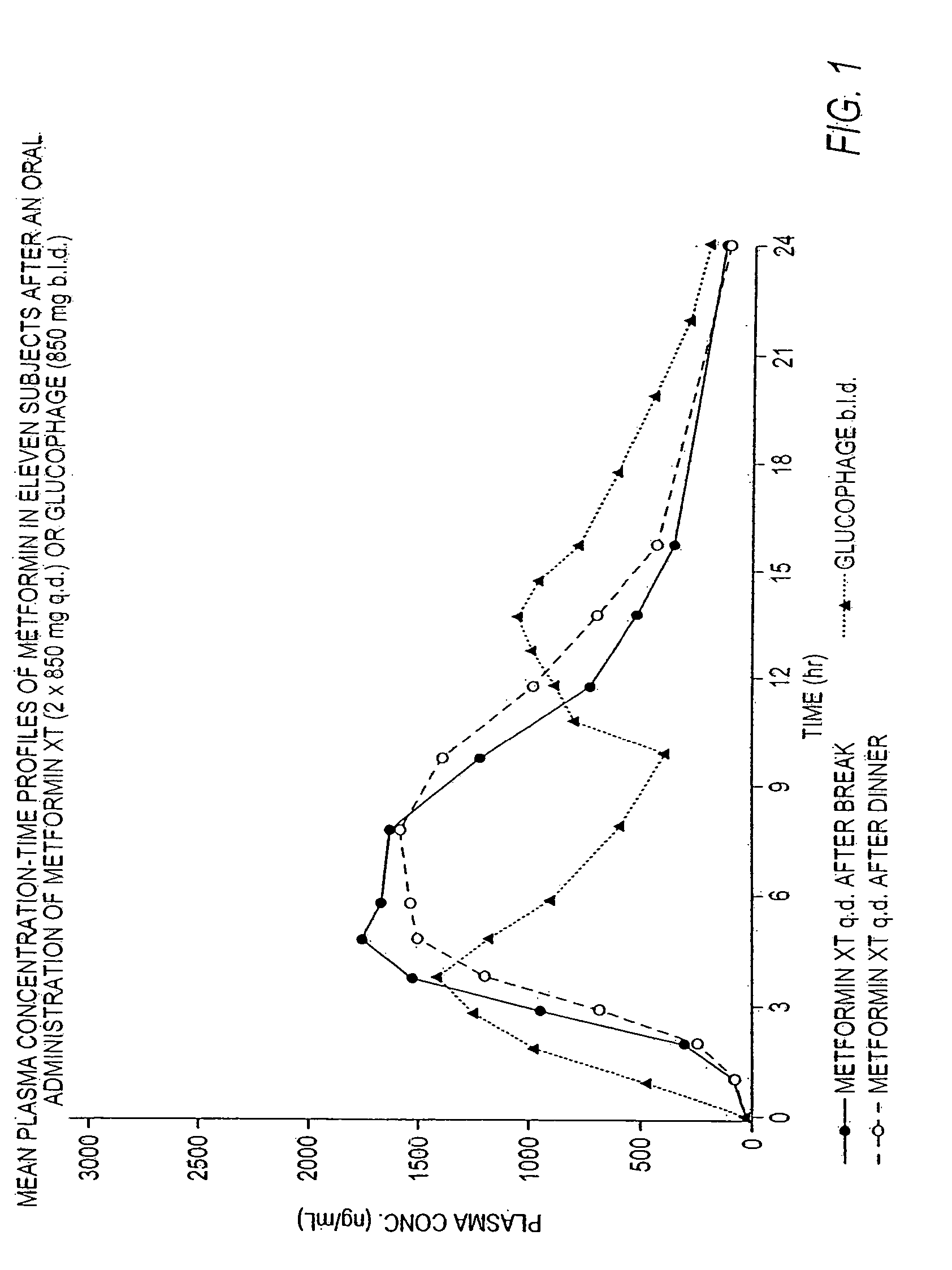
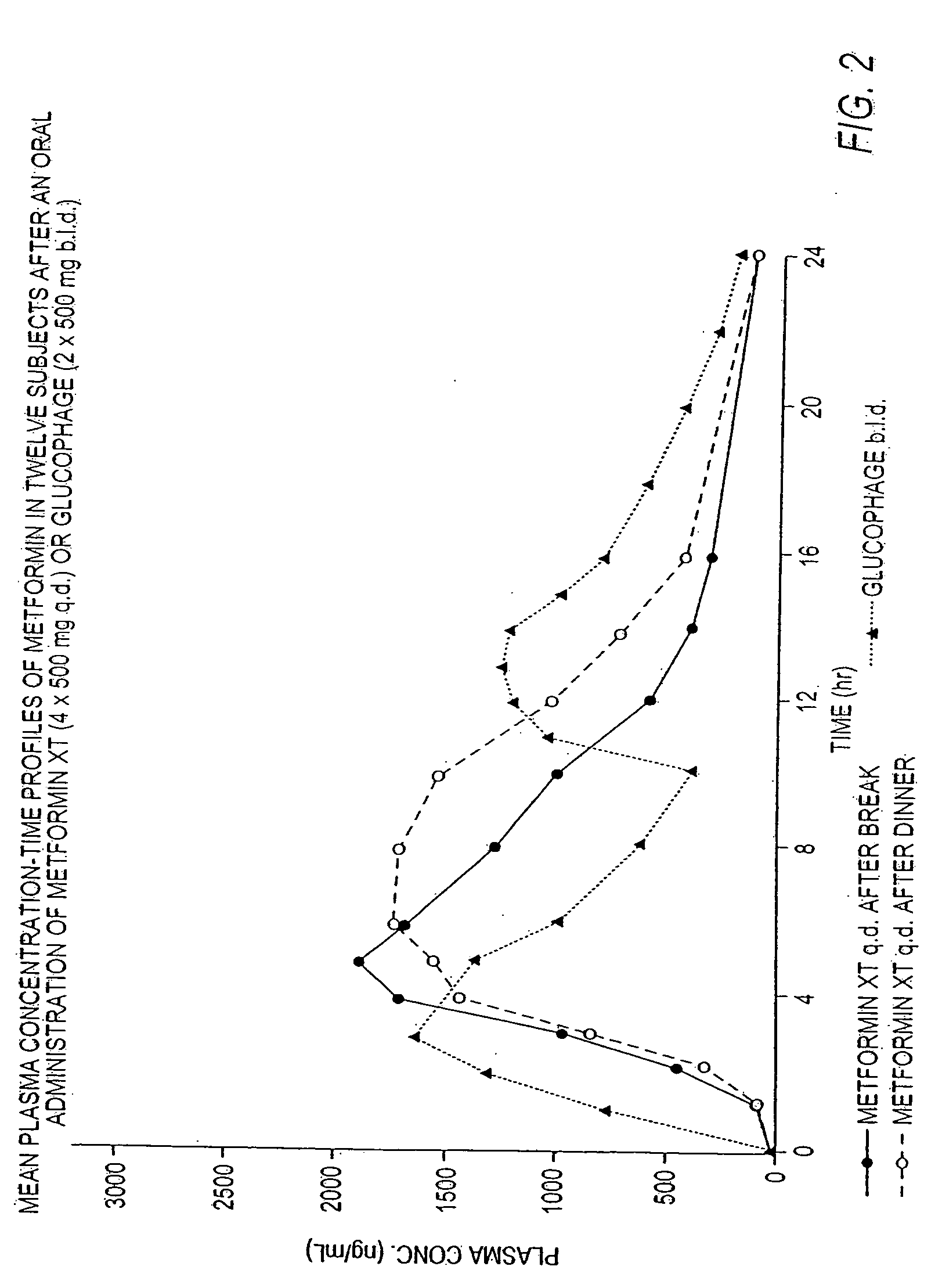
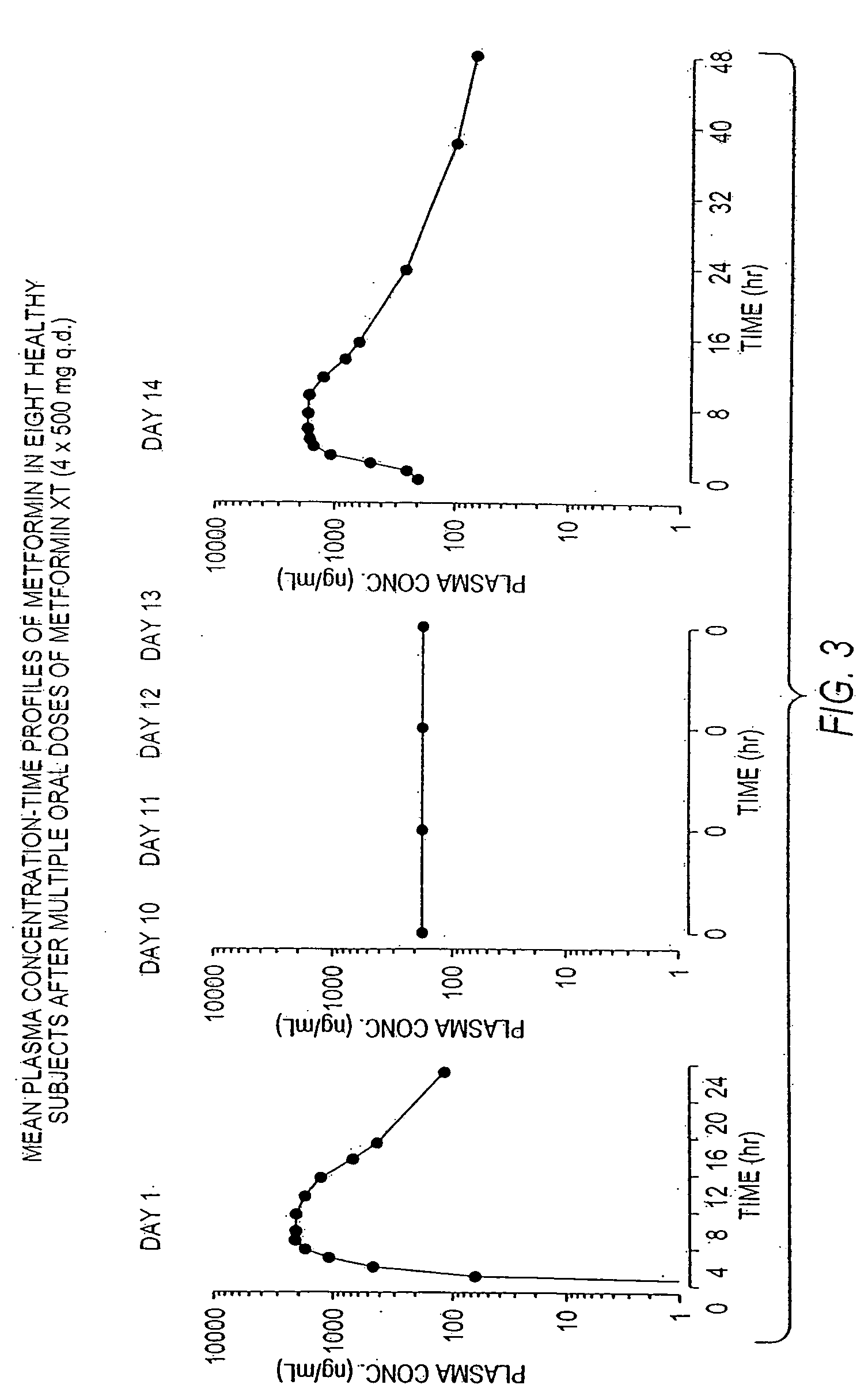
Controlled release metformin compositions
InactiveUS20060034922A1Effective controlImprove bioavailabilityCoatingsOsmotic deliveryControlled releaseHigh doses
Owner:ANDRX LABS
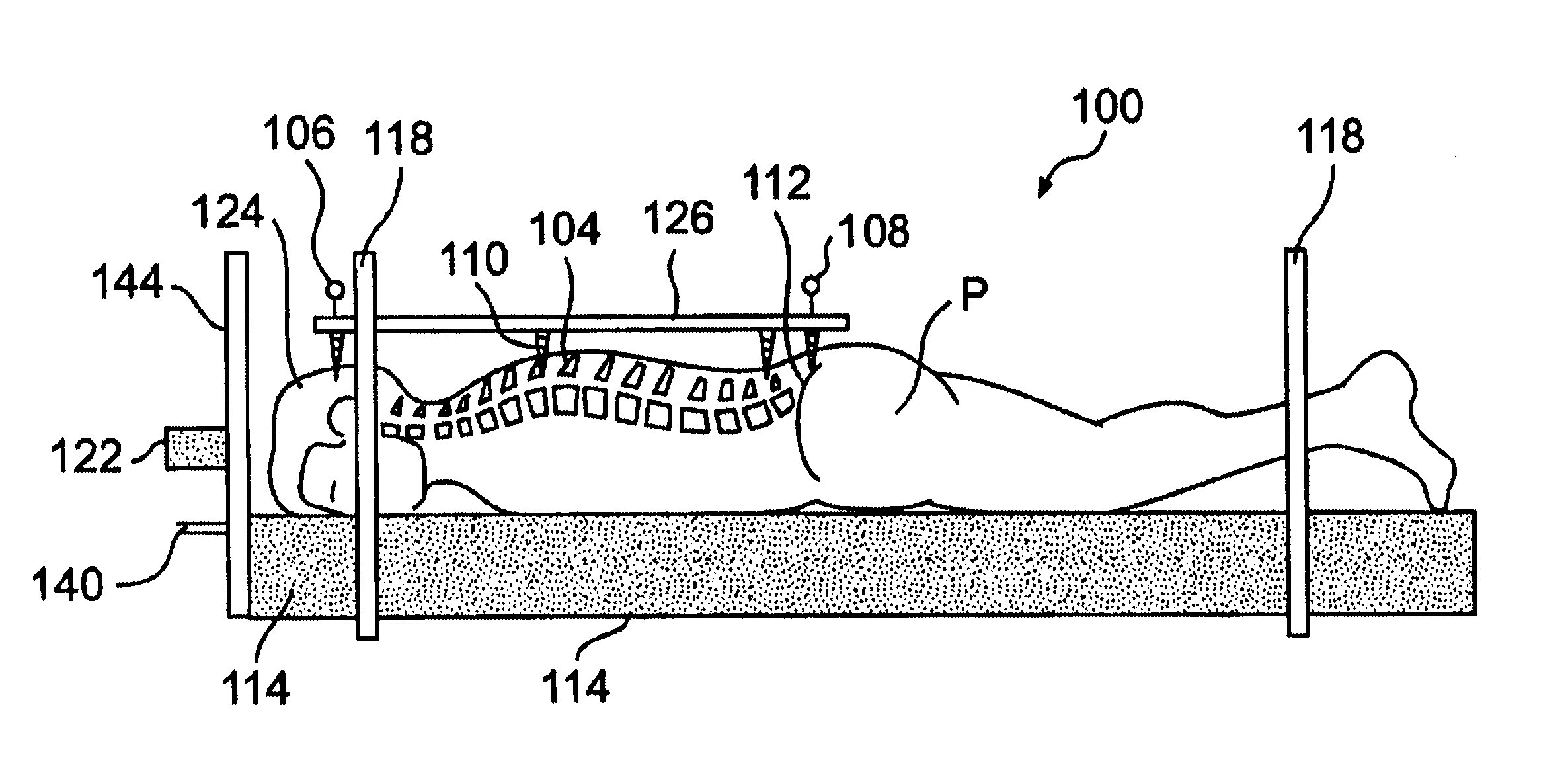
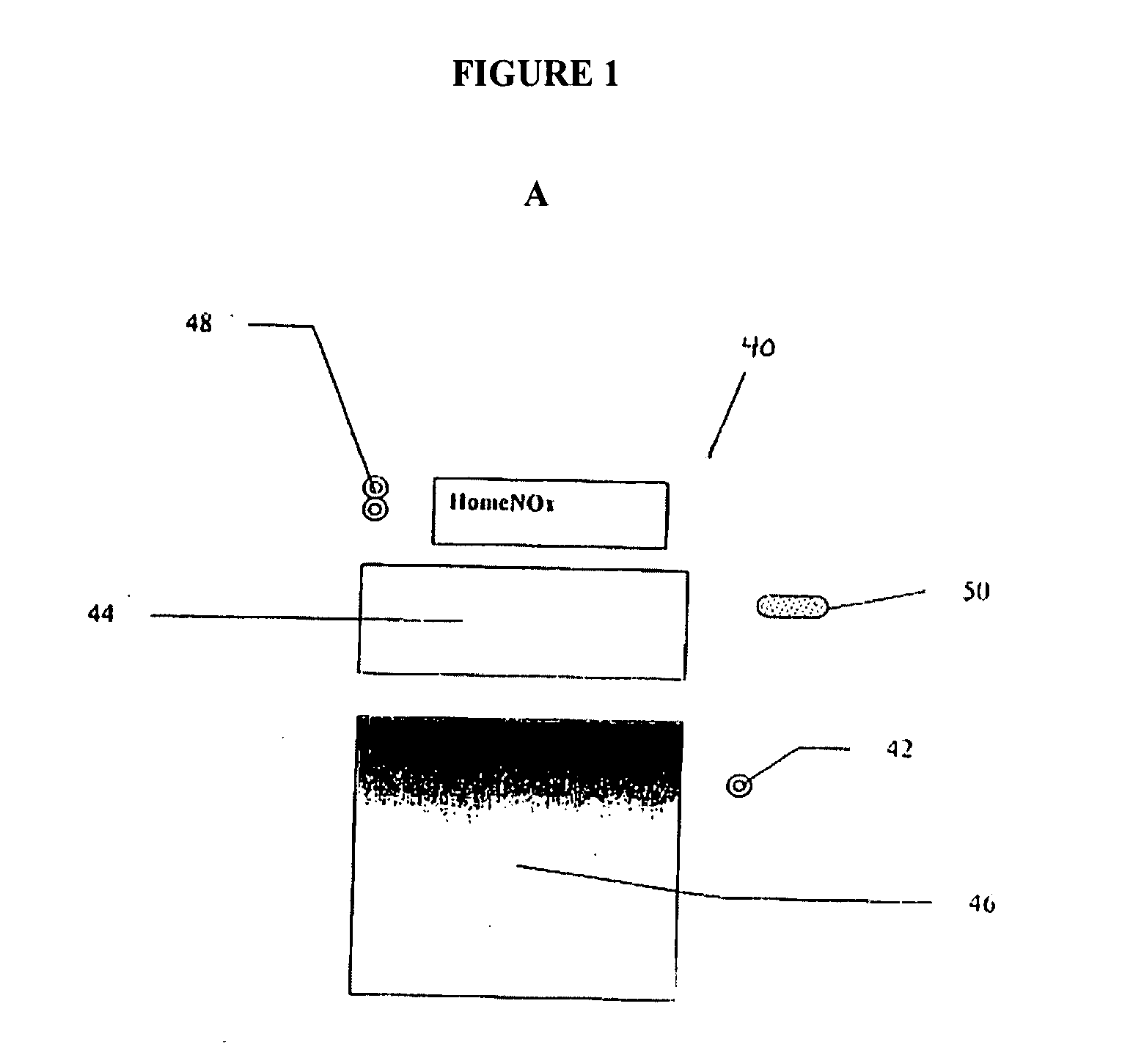
Radiosurgery methods that utilize stereotactic methods to precisely deliver high dosages of radiation especially to the spine
InactiveUS6665555B2Reduce decreaseAvoiding and minimizing deliveryRadiation diagnostic clinical applicationsSurgeryRadiosurgeryStereotaxis
Owner:GEORGETOWN UNIV
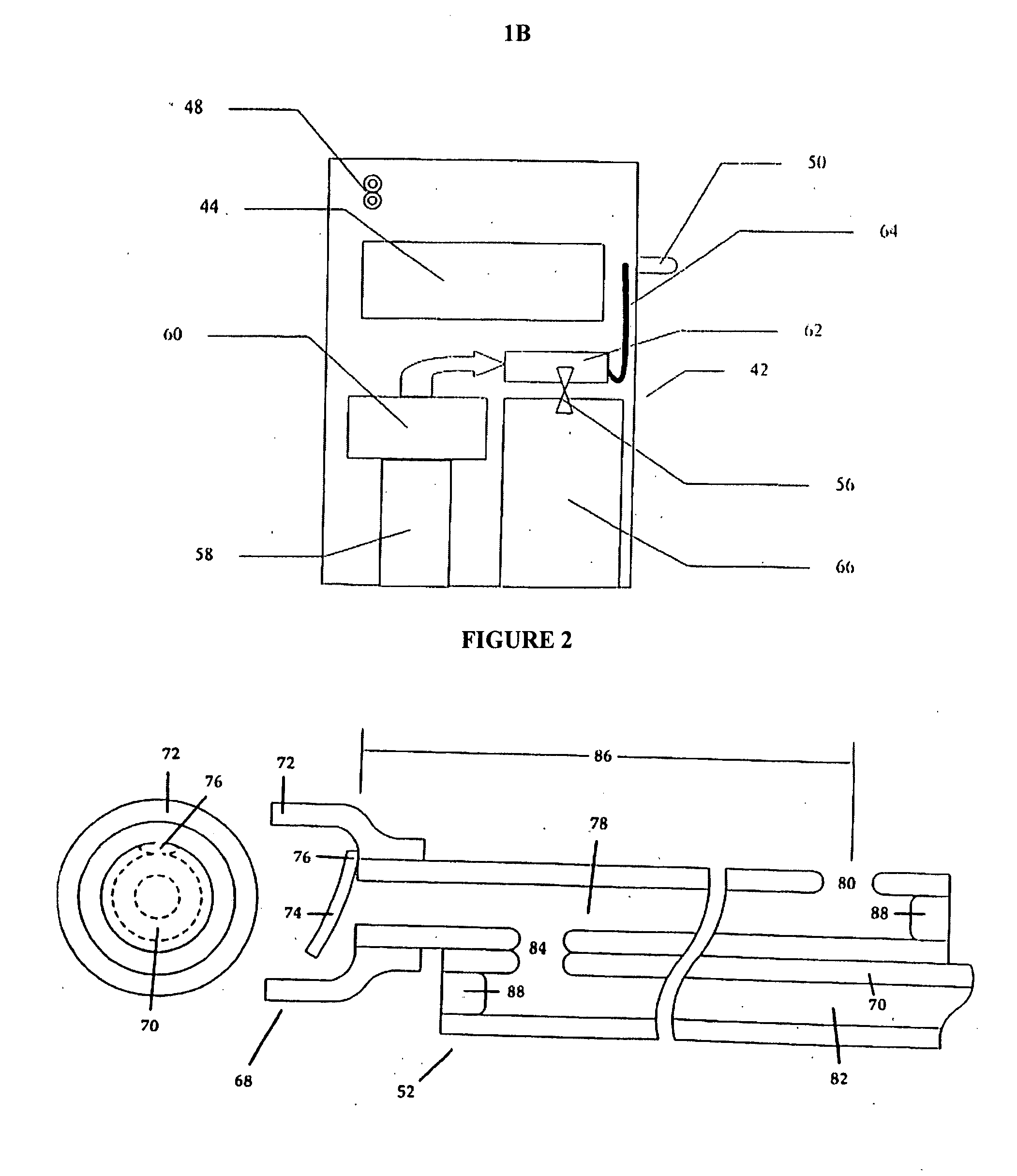
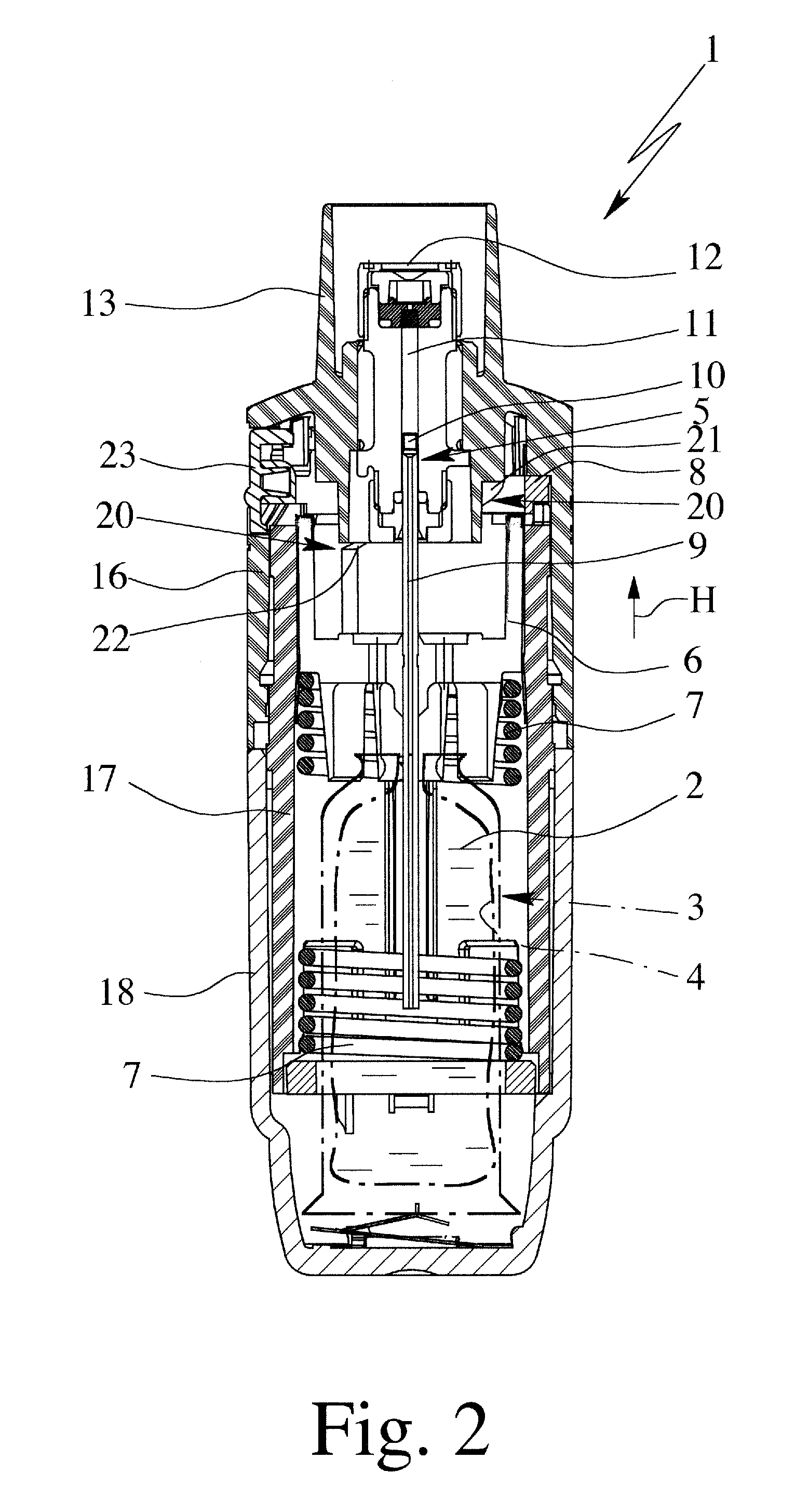
Controlled-volume infusion device
An infusion device capable of administering liquid medication at a continuous flow rate, and upon user demand delivers a controlled volume dosage of liquid medication at a higher dosage flow rate. The dosage reservoir remains empty until the user actuates it by selectively and temporarily removing the pressure source, such as a spring. During actuation, fluid rapidly flows from the medication reservoir to fill the dosage reservoir. After actuation, the pressure source exerts a higher pressure on the dosage reservoir than the medication reservoir pressure, which results in a temporary higher bolus flow rate. Thus, two distinct flow rates are achieved with one flow restrictor element.
Owner:CURLIN MEDICAL INC
Controlled-volume infusion device
An infusion device capable of administering liquid medication at a continuous flow rate, and upon user demand delivers a controlled volume dosage of liquid medication at a higher dosage flow rate. The dosage reservoir remains empty until the user actuates it by selectively and temporarily removing the pressure source, such as a spring. During actuation, fluid rapidly flows from the medication reservoir to fill the dosage reservoir. After actuation, the pressure source exerts a higher pressure on the dosage reservoir than the medication reservoir pressure, which results in a temporary higher bolus flow rate. Thus, two distinct flow rates are achieved with one flow restrictor element.
Owner:CURLIN MEDICAL INC
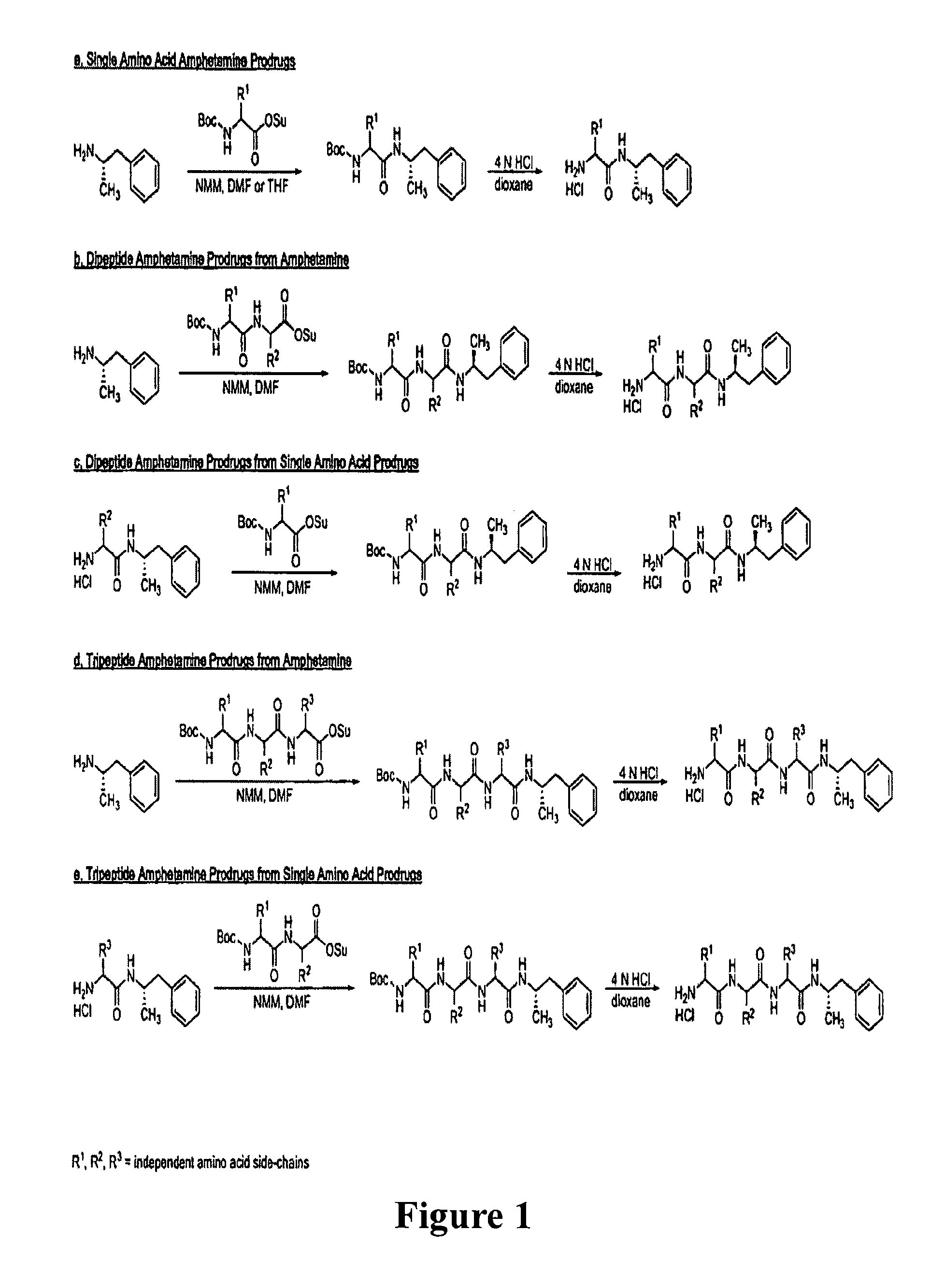
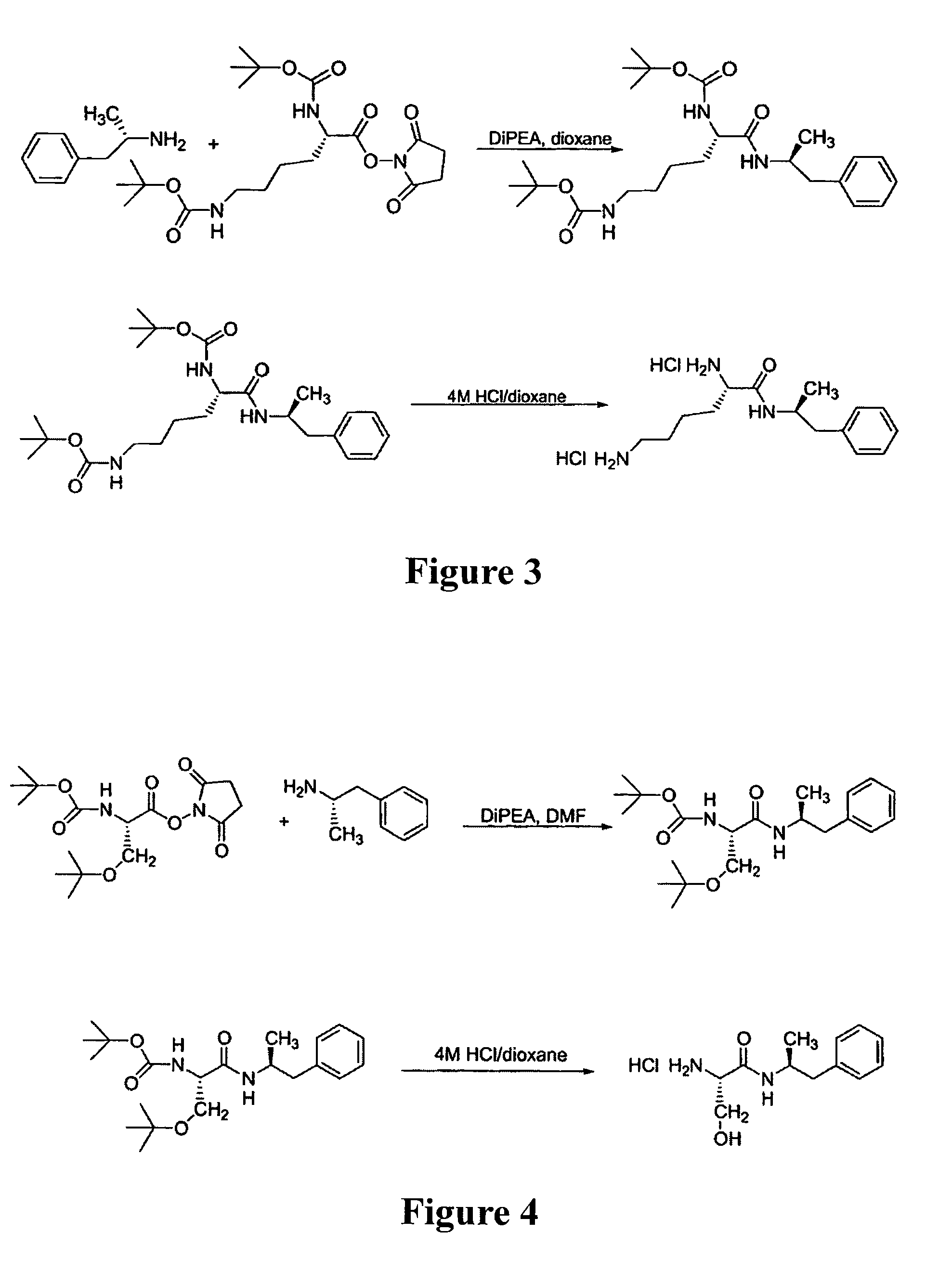
Abuse-resistant amphetamine prodrugs
InactiveUS7659253B2Reducing patient to patient variabilityRisk minimizationBiocideOrganic chemistryDiseaseChemical Moiety
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Axially chiral enantiomers of drug Lesinurad
InactiveCN105399694AEnhanced inhibitory effectAddress nephrotoxicityOrganic active ingredientsSkeletal disorderMedicineEnantiomer
The invention discloses an axially chiral R-enantiomer and an axially chiral S-enantiomer of the drug Lesinurad. The axially chiral R-enantiomer shows excellent URAT1 inhibition effect, overcomes the problems that a high dosage of Lesinurad racemate leads to renal toxicity and a low dosage Lesinurad racemate cannot produce additional drug effect, and can be applied to treatment or prevention of symptoms of abnormal tissue uric acid levels.
Owner:ZHEJIANG JINGXIN PHARMA +1
Co-Administration of an Agent Linked to an Internalization Peptide with an Anti-Inflammatory
ActiveUS20090176713A1Reduce capacityInhibit the inflammatory responseOrganic active ingredientsNervous disorderCo administrationBiotin
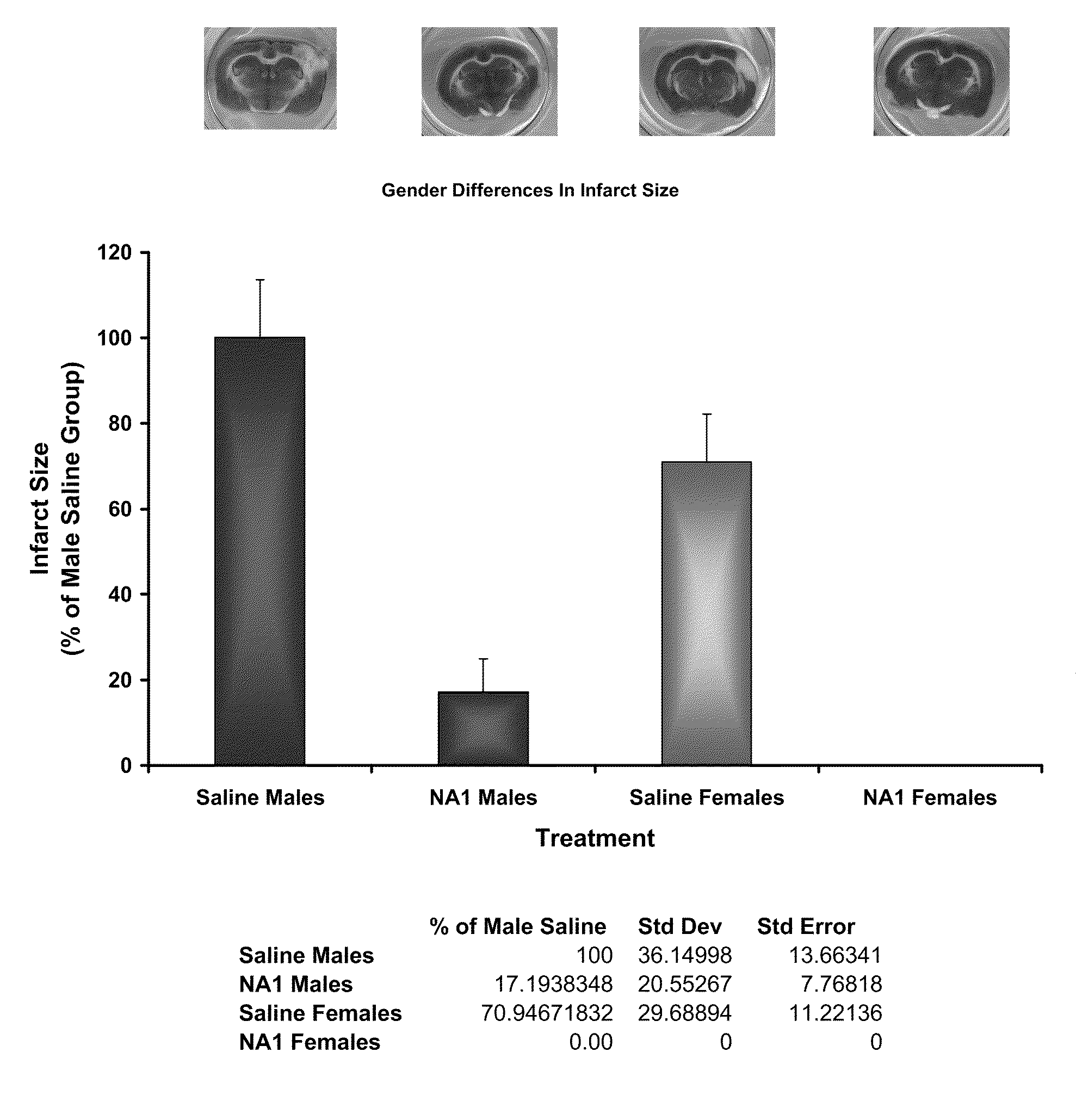
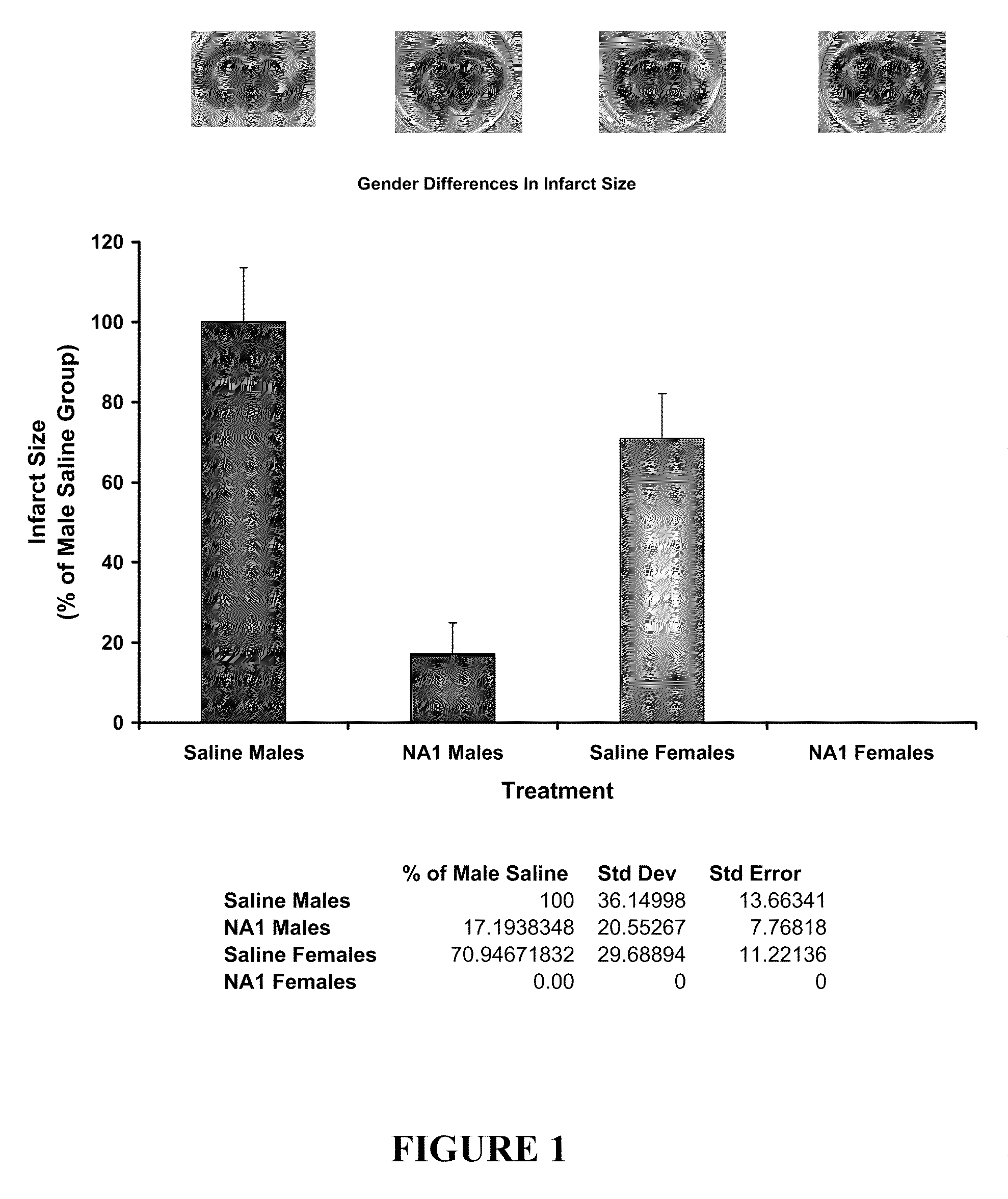
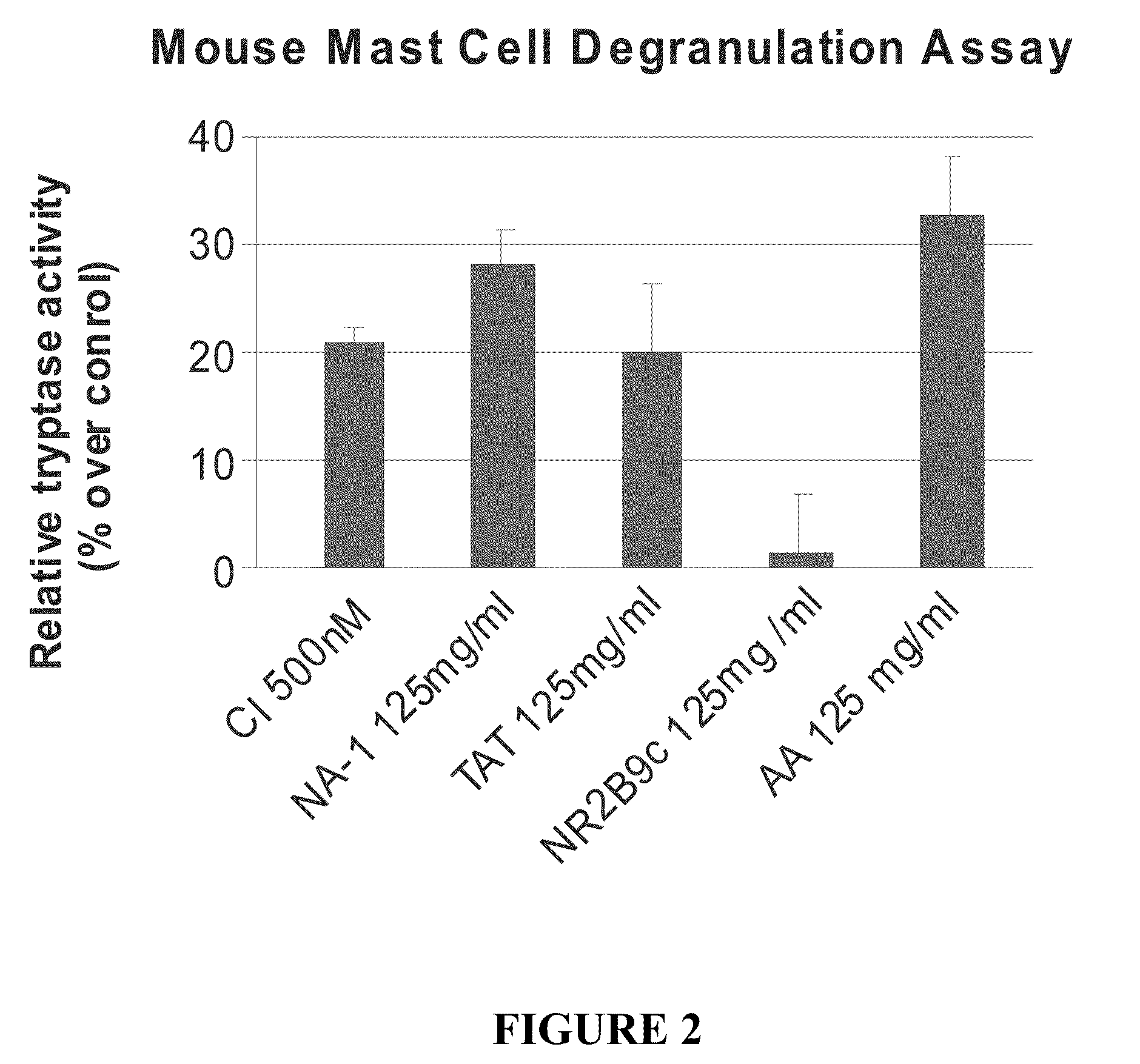
The invention provides methods of delivering pharmacologic agents linked to an internalization peptide, in which an inflammatory response inducible by the internalization peptide is inhibited by co-administration of an anti-inflammatory or by linking the internalization peptide to biotin or similar molecule. Such methods are premised in part on the results described in the examples whereby administration of a pharmacological agent linked to tat high dosages is closely followed by an inflammatory response, which includes mast cell degranulation, histamine release and the typical sequelae of histamine release, such as redness, heat, swelling, and hypotension.
Owner:NONO INC
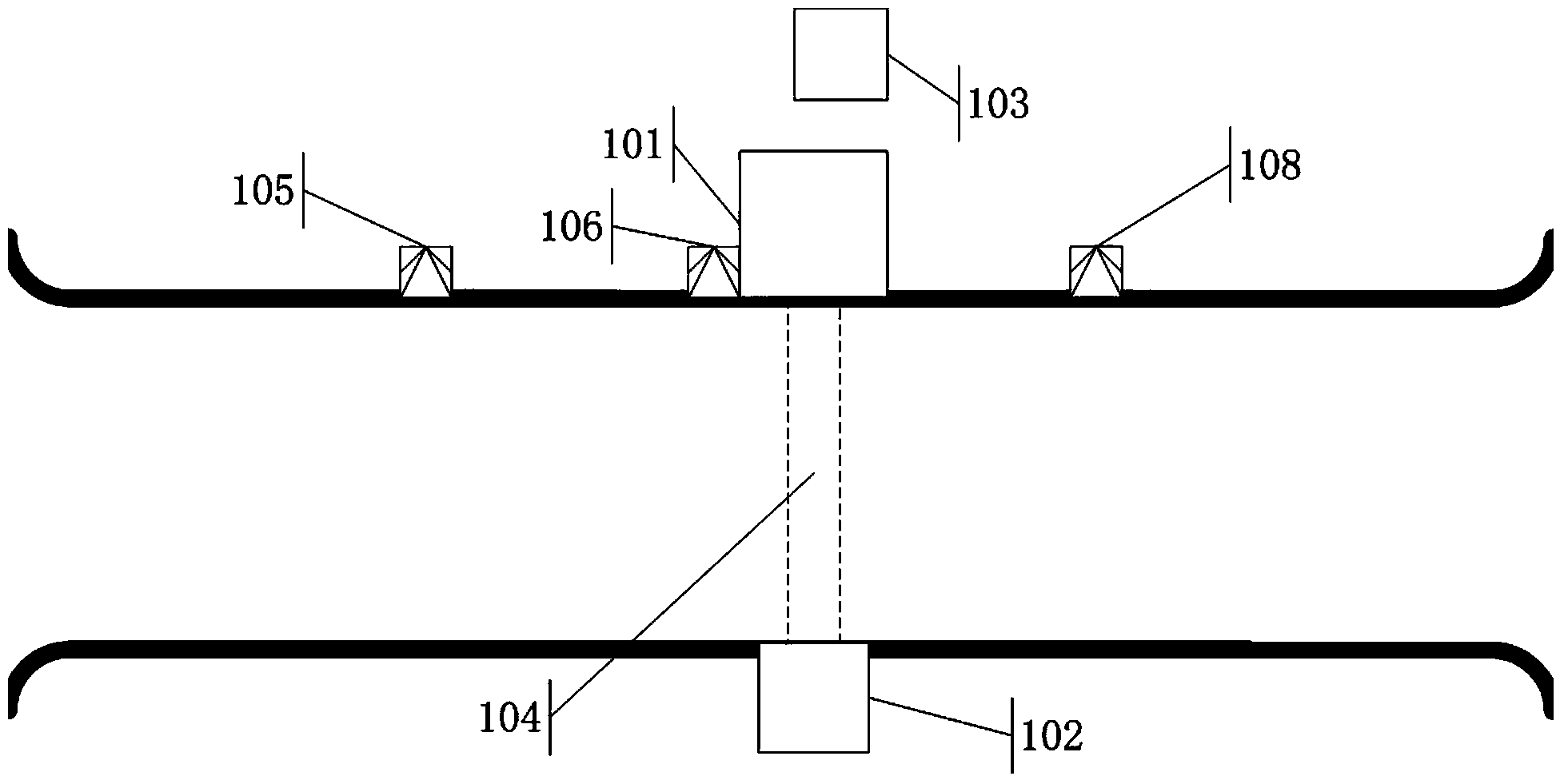
Continuous pass-type radiation scanning system and method
ActiveCN104374785AImprove detection abilityEnsure safetyMaterial analysis by transmitting radiationNuclear radiation detectionHigh dosageLow dose
The invention discloses a continuous pass-type radiation scanning system. The system comprises a radiation source, a collimator, a radiation detector, an imaging device, a first detection unit (105), a second detection unit (108) and a control module; the first detection (105) is used for detecting whether a target object achieves a preset position which is located at the upstream of a scanning region and apart from the upstream side border of the scanning region by a first length L1, wherein the scanning region is a region covered with radiation source rays in a detection channel; the second detection unit (108) is used for detecting whether a part required to be subjected to the scanning of low-dosage rate rays in the target object leaves a scanning region and a part required to be subjected to the canning of high-dosage rate rays in the target object is about to enter the scanning region; and the control module is used for receiving signals from various detection units and controlling the radiation source according to the signals. The system and the method disclosed by the invention can be used for achieving the condition that a large quantity of to-be-detected vehicles can continuously and fast pass through the detection channel to finish the radiation scanning examination.
Owner:พาวเวอร์สแกน ไฮเทค โค แอลทีดี
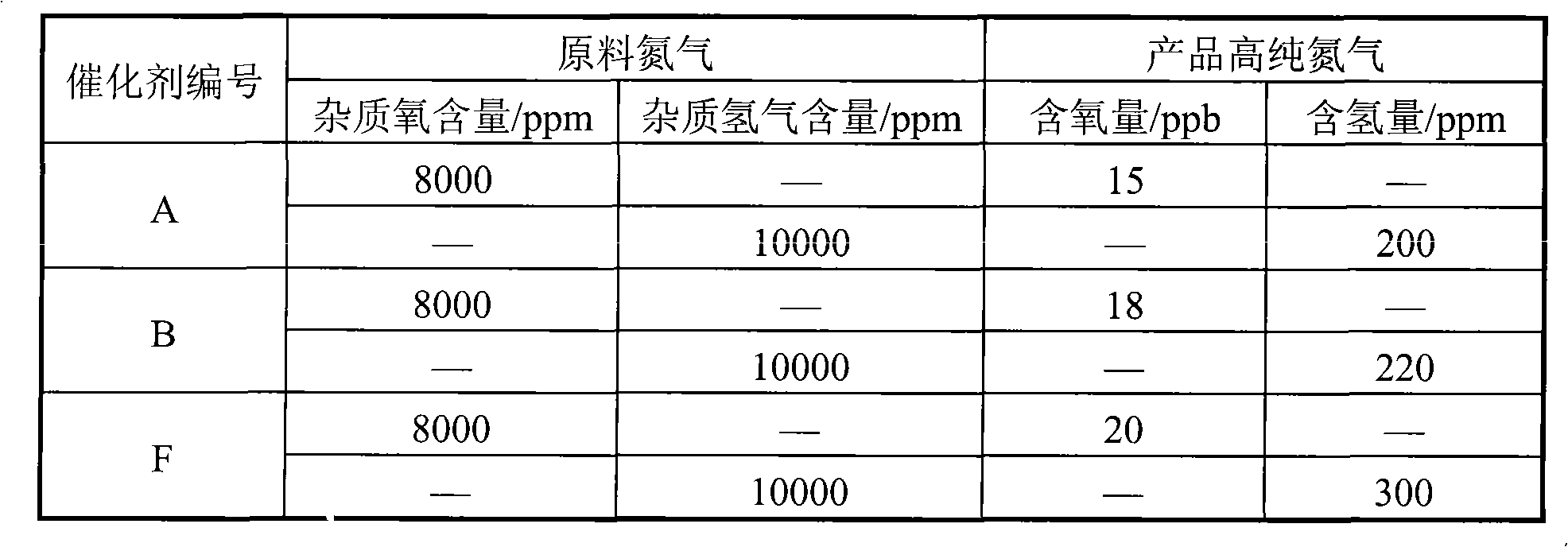
Use of high dose concentrations of gaseous nitric oxide
InactiveUS20080193566A1Efficient procedureBiocideBronchoscopesRESPIRATORY DISTRESS SYNDROME ADULTArteriolar Vasoconstriction
The invention relates to a methods and devices of delivering gaseous nitric oxide to a mammal or surface at a concentration ranging from about 1000 ppm to about 50,000 ppm of gaseous nitric oxide. Different conditions which can be treated by high dosage administration of gNO include but are not limited to: topical treatments with gNO, cosmetic applications of gNO, vasodilation conditions with gNO, inhalation treatments with gNO, treatments of the blood with gNO, treatment of the skin or tissue with gNO, treatment of infections with gNO, treatment of inflammation on or within the body, and treatments of biofilms with gNO. Other conditions, aliments, or symptoms that may be treated with high dosage gNO include bronchoconstriction, reversible pulmonary vasoconstriction, asthma, pulmonary hypertension, adult respiratory distress syndrome (ARDS), and persistent pulmonary hypertension of the newborn (PPHN).
Owner:PULMONOX TECH
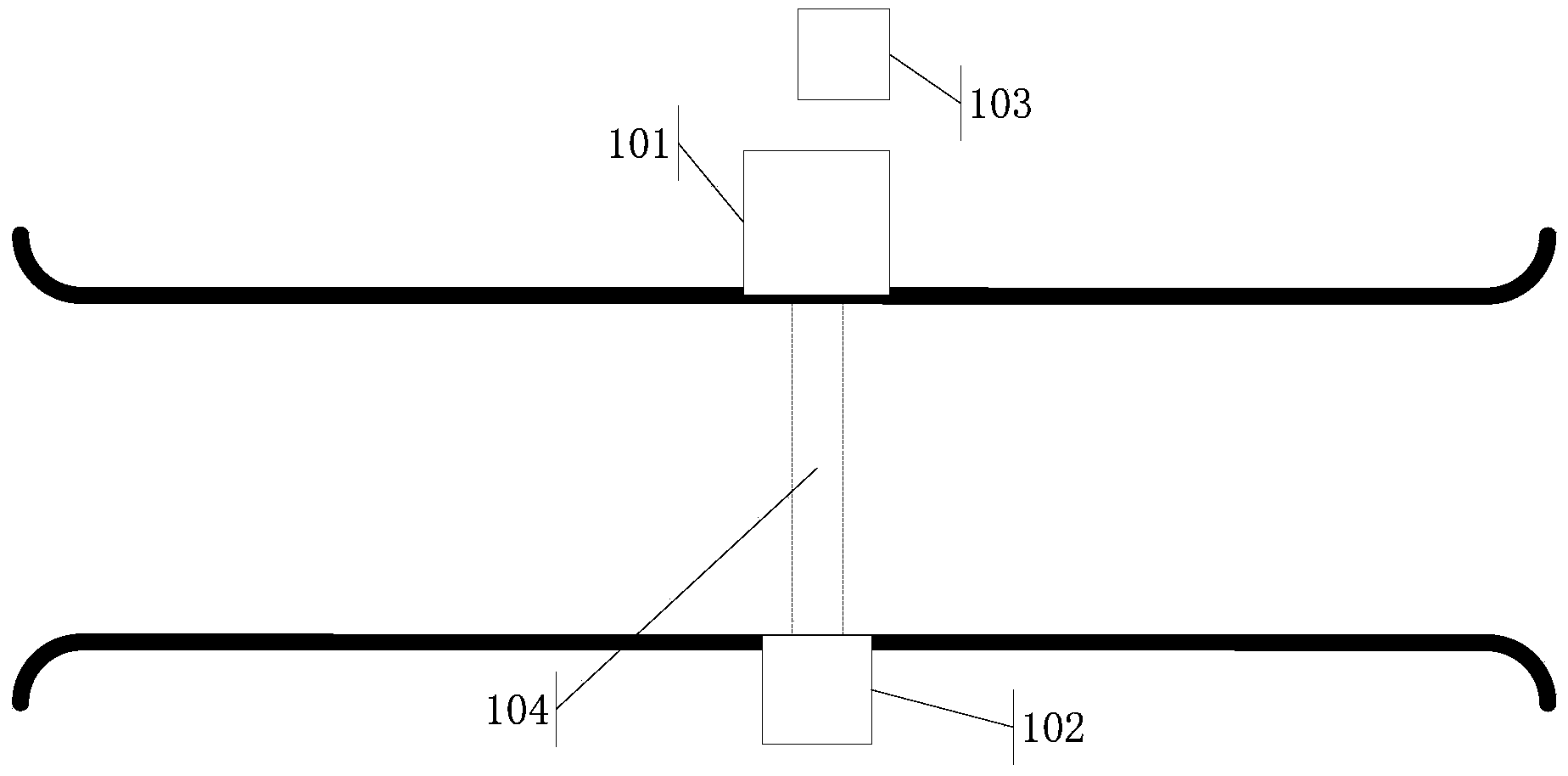
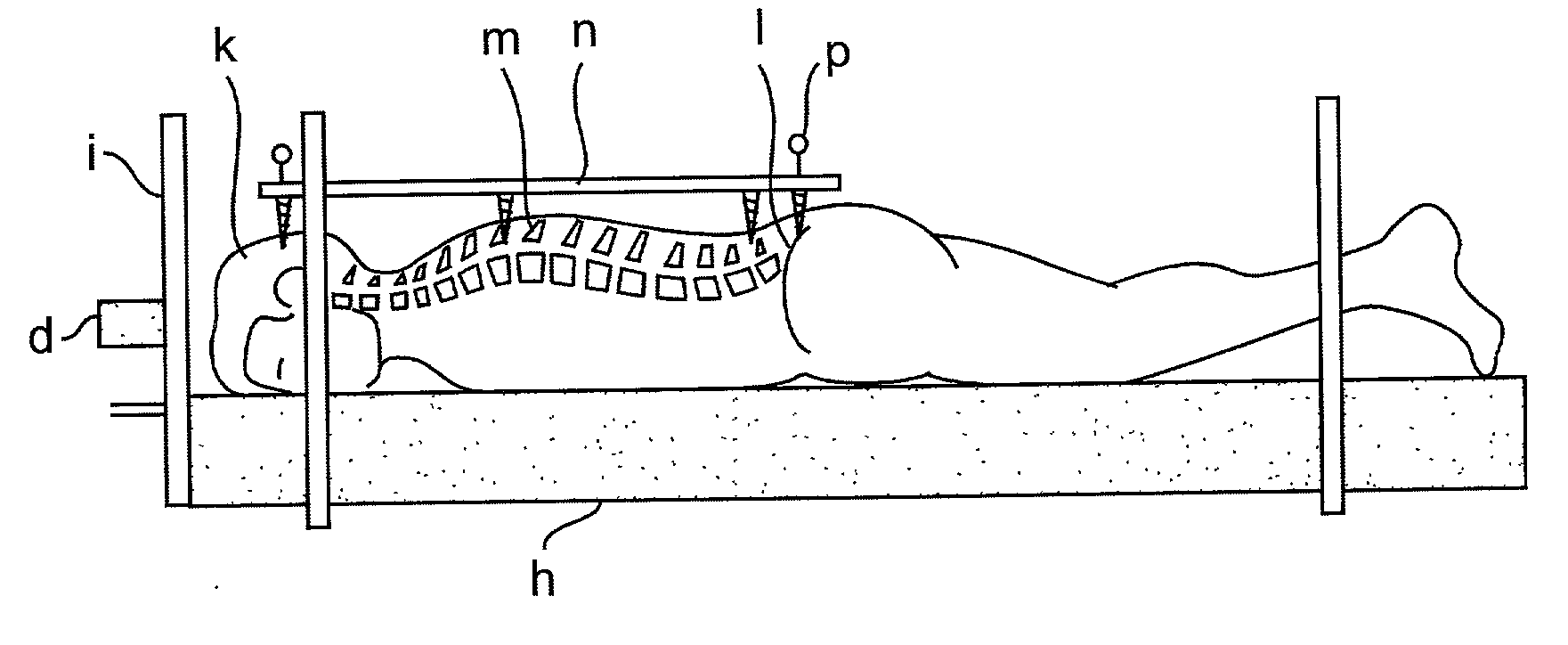
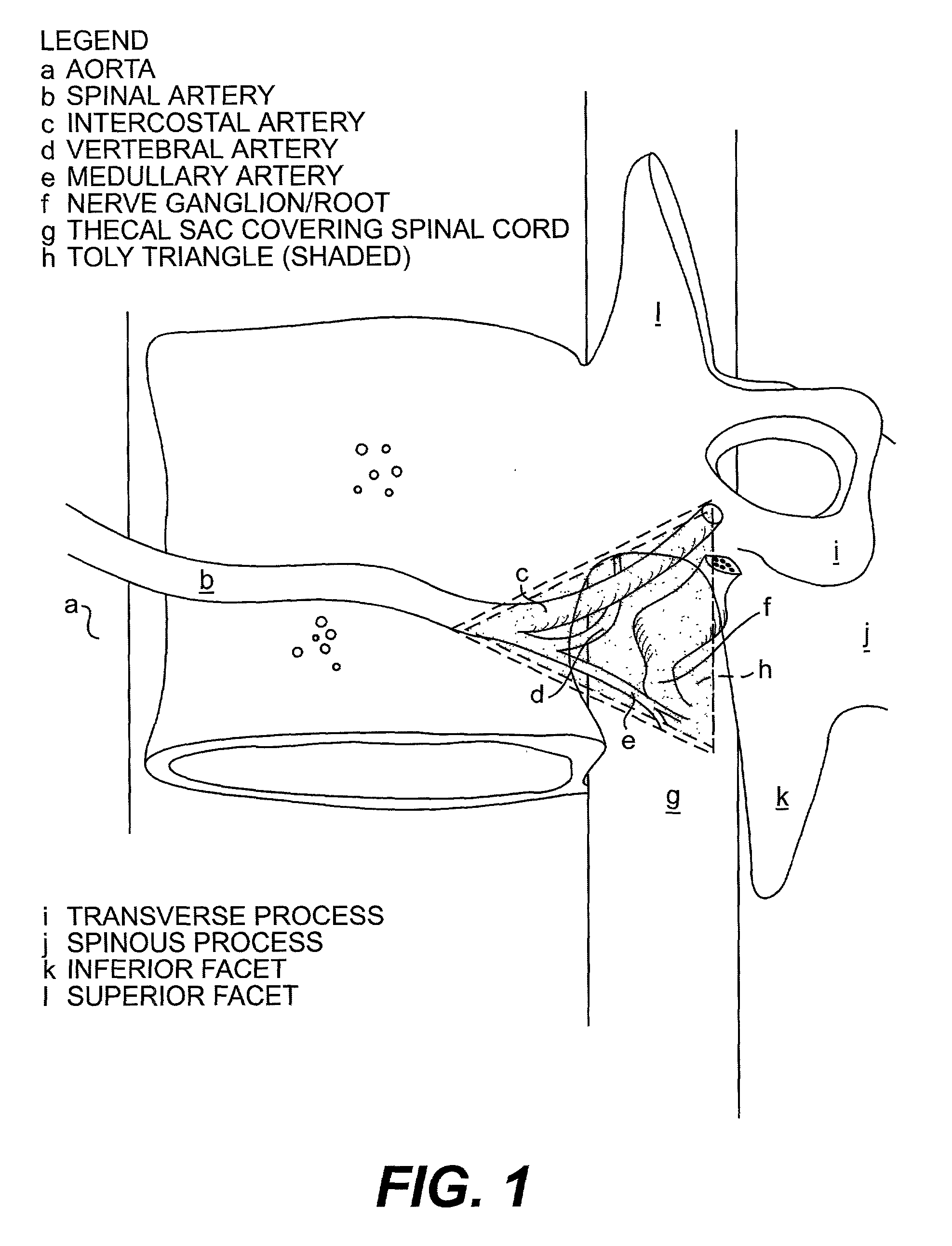
Novel radiosurgery methods that utilize stereotactic methods to precisely deliver high dosages of radiation especially to the spine
InactiveUS20020032378A1Reduce decreaseAvoiding and minimizing deliveryRadiation diagnostic clinical applicationsSurgeryRadiosurgeryStereotaxis
A system for delivery of high dosage of radiation to a targeted spinal area is provided. This is accomplished by a system which provides for precise immobilization and positioning of the treated spinal area during dose planning and treatment via stereotactic radiosurgery. Advantages of the system include convenience to the patient, enhanced efficacy, and reduced risk of radiotoxicity to non-target tissues.
Owner:GEORGETOWN UNIV
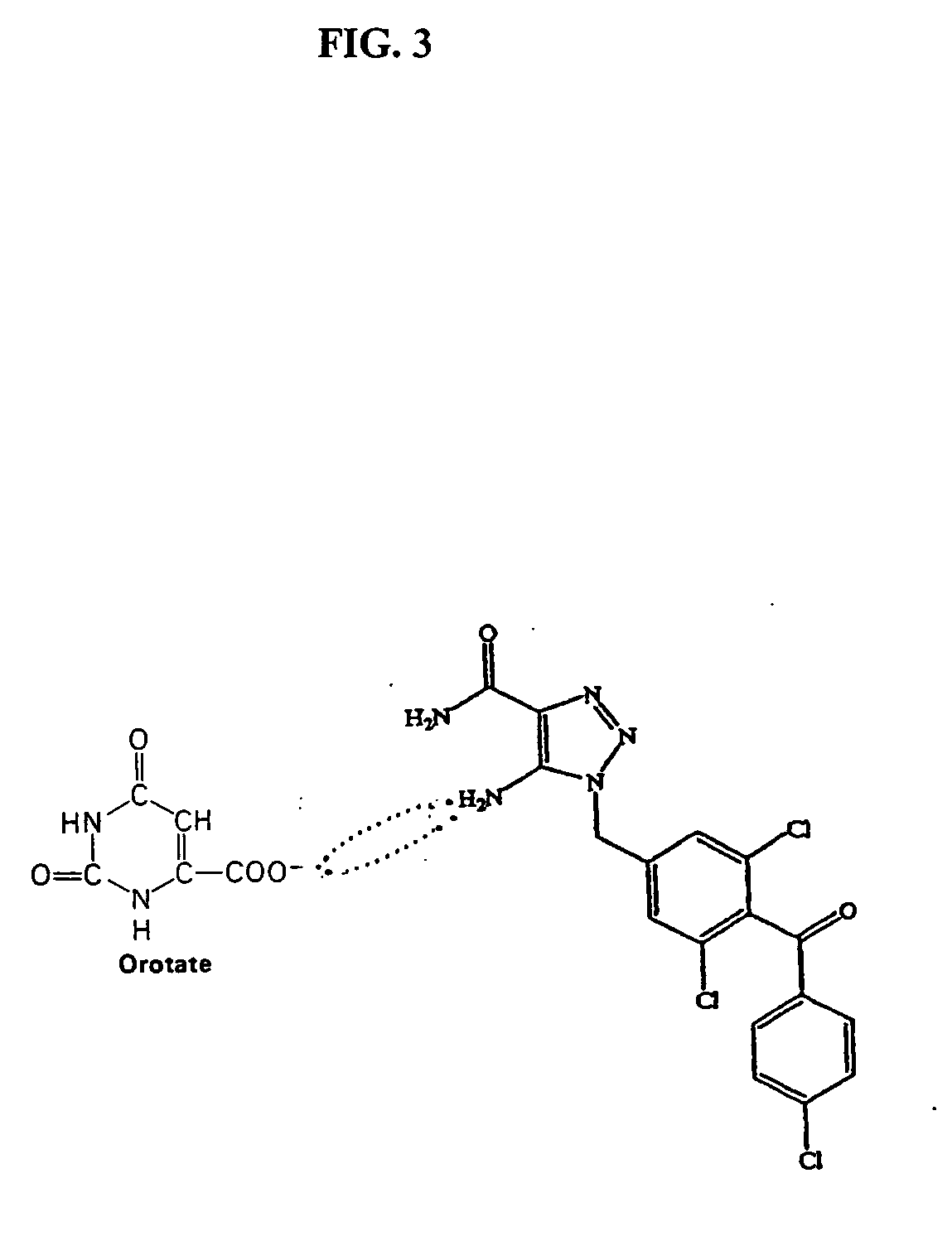
Method of increasing drug oral bioavailability and compositions of less toxic orotate salts
ActiveUS20060189640A1Improve bioavailabilityImprove drug bioavailabilitySalicyclic acid active ingredientsBiocideSide effectDrug interaction
The present invention relates generally to the method of increasing the oral bioavailability, reducing chemotherapy induced toxicity and side effects, and improving the effectiveness of pharmaceutical agents that are poorly absorbed from the gastrointestinal tract. Specifically, the invention relates to poorly absorbed pharmaceutical drugs and converting them to orotate salts. The orotate salts of the drugs can be dosed at lower doses to provide the efficacy benefits of a higher dose, while reducing the drugs' toxic effects at lower doses. Additionally, the orotate salts of pharmaceutical agents have better clearance and reduce the potential for drug-induced hepatic toxicity. Therefore, an especially useful formulation of the orotate salt of the pharmaceutical agent can provide rapid and consistent action using a lower dose while reducing drug interactions and side-effects.
Owner:SAVVIPHARM INC
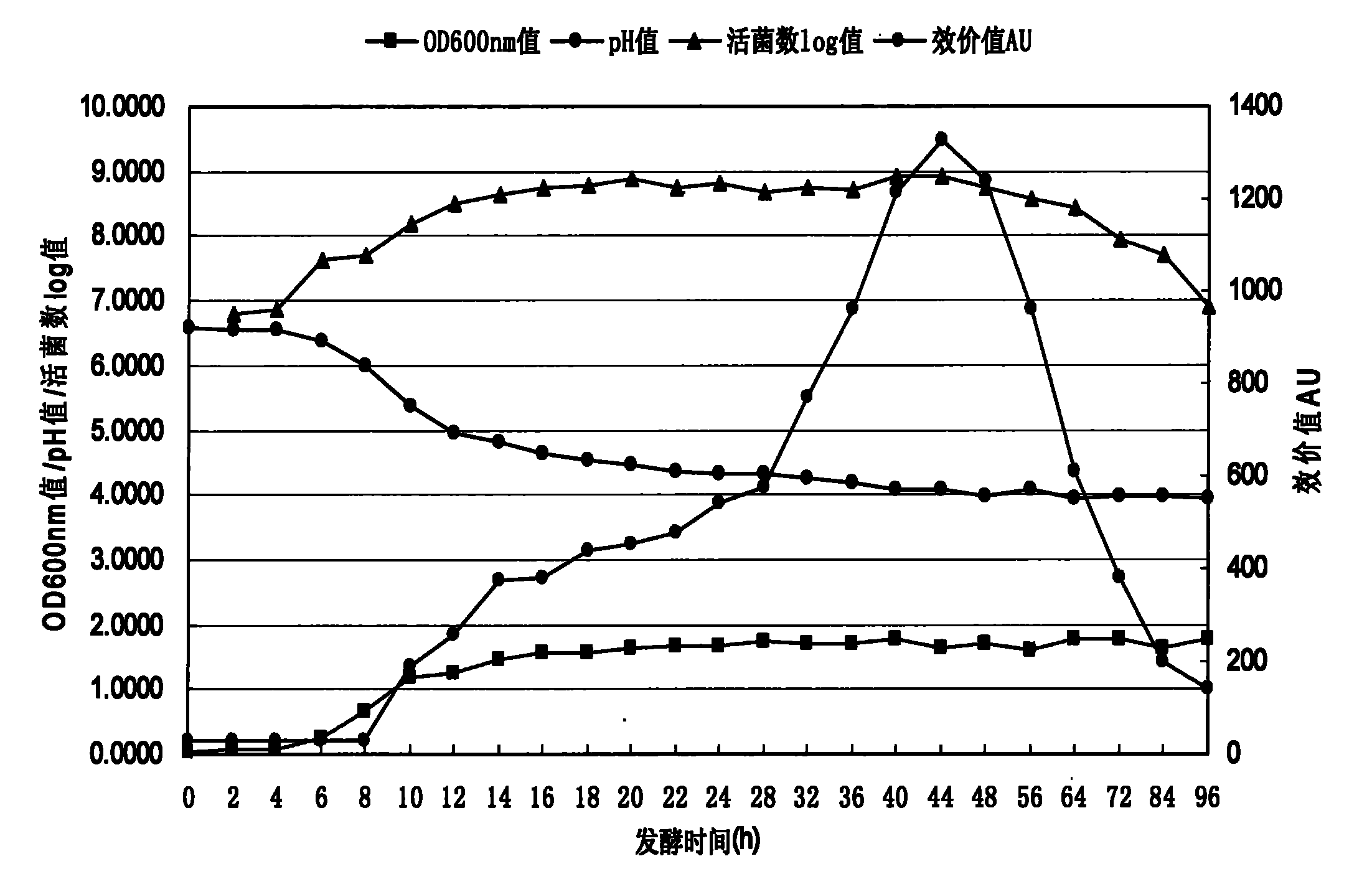
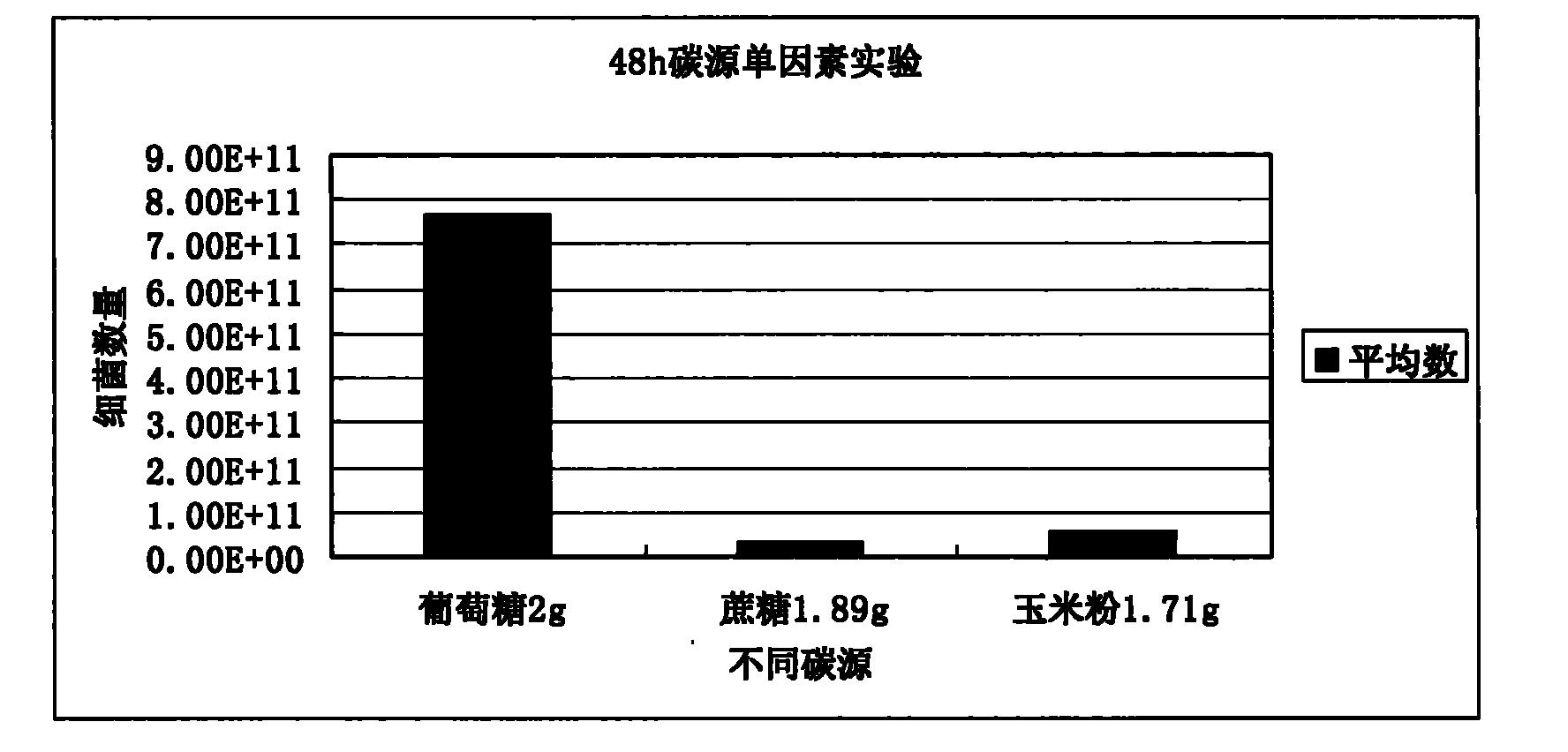
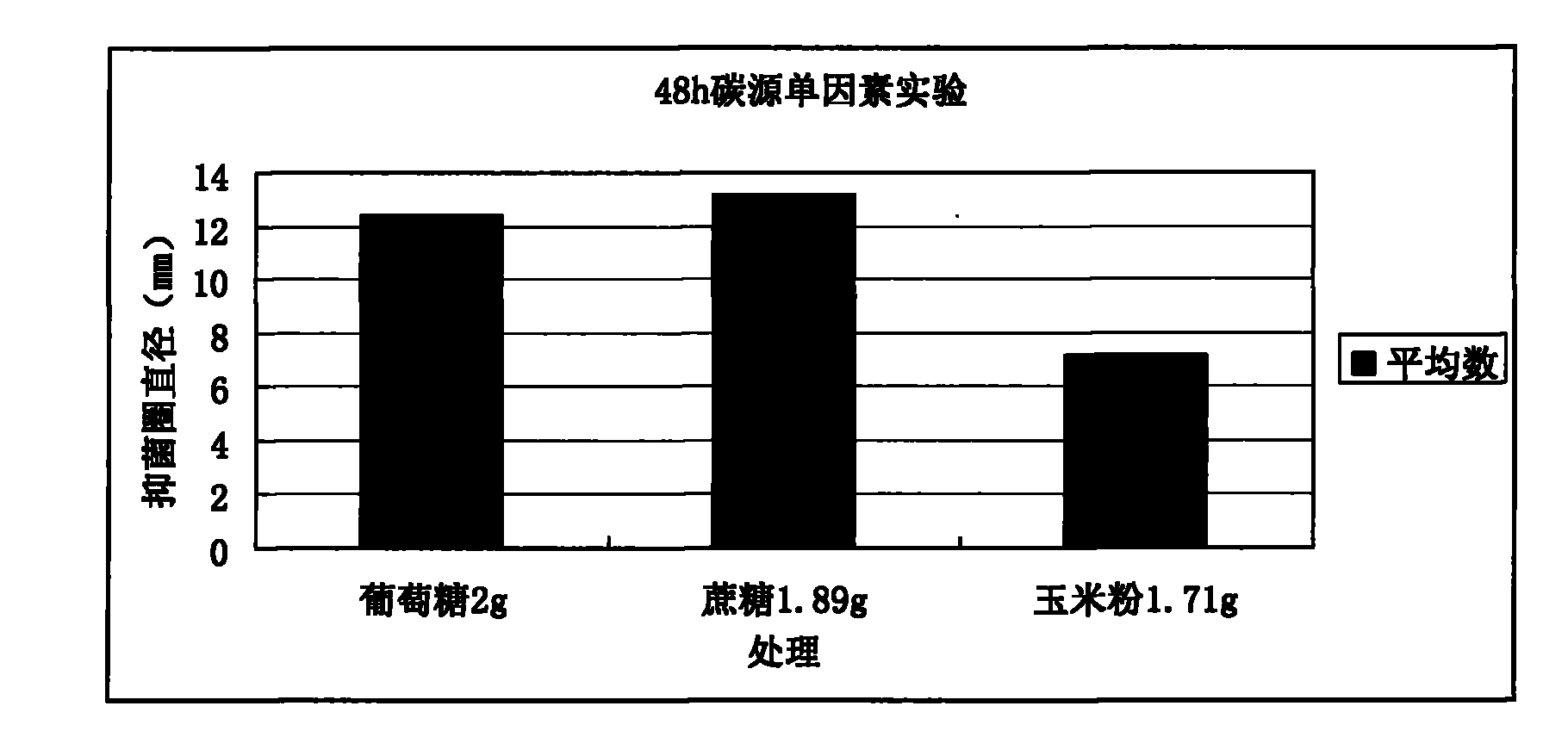
Lactobacillus plantarum, method for fermenting and preparing bacteriocin of Lactobacillus plantarum, and application of Lactobacillus plantarum and bacteriocin
The invention provides a new Lactobacillus plantarum bacterial strain YJG which was preserved in the China General Microbiological Culture Collection Center (CGNCC) with the collection number of CGMCC No. 2994 on march, 30th, 2009. The Lactobacillus plantarum bacterial strain YJG can produce broad-spectrum bacteriostatic activity bacteriocin with high yield, and can express bacteriostasis for staphylococcus aureus, salmonella and E.coli of gram-negative bacterium, L. plantarum, L.brevis, Streptococcus and Bacillus of gram-positive bacterium, and the like. A virus attacking tests with high dosage proves that the Lactobacillus plantarum bacterial strain YJG is safe to animal bodies. The Lactobacillus plantarum bacterial strain YJG and a zymophyte liquid thereof can be used for preparing additives of green animal feeds, and the bacteriocin of the Lactobacillus plantarum bacterial strain YJG can be used for preparing green biological veterinary drugs, environmental-friendly biopreservatives, biocontrol drugs and the like.
Owner:CHINA AGRI UNIV
Preparation method of thin shell shaped noble metal catalyst
InactiveCN101491778AExtended service lifeGood dispersionMolecular sieve catalystsCatalyst activation/preparationAlkaneThin shells
The invention relates to a preparation method of thin-shelled noble metal catalyst, and mainly solves the problems of the prior art of high dosage, poor selectivity and short service life of noble metal. The preparation method better solves the technical problems of the prior art by adopting the technical proposal comprising the following steps of: (a) coating the slurry of a coat porous material on the inner core of an inert carrier, drying the slurry coating and baking the slurry coating at a temperature of between 700 and 1,200 DEG C for 0.5 to 10 hours to obtain a laminar composite carrier; (b) impregnating the surface of the laminar composite carrier with a solution containing noble metal and cocatalyst components, drying the laminar composite carrier, and baking the laminar composite carrier in the air at a temperature of between 200 and 700 DEG C for 1 to 24 hours to obtain a thin-shelled catalyst precursor; and (c) reducing the thin-shelled catalyst precursor in a reducing atmosphere at a temperature between 300 and 800 DEG C for 1 to 24 hours to obtain the thin-shelled noble metal catalyst. The preparation method can be used in the industrial production of gas purification materials which are dehydrogenation-deoxidization catalysts, alkanes and aromatic hydrocarbons for selective hydrogen oxidation in a dehydrogenation process.
Owner:CHINA PETROLEUM & CHEM CORP +1
Atomizer and method of atomizing fluid with a nozzle rinsing mechanism
ActiveUS8733341B2Accurate measurementEasy to operateRespiratorsLiquid surface applicatorsNebulizerBiomedical engineering
Owner:BOEHRINGER INGELHEIM INT GMBH
Methods for treating disorders associated with hyperlipidemia in a mammal
InactiveUS20070093468A1Reduce concentrationExcessive amountBiocideSenses disorderCo administrationSide effect
Owner:AEGERION PHARM INC
Application of angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B
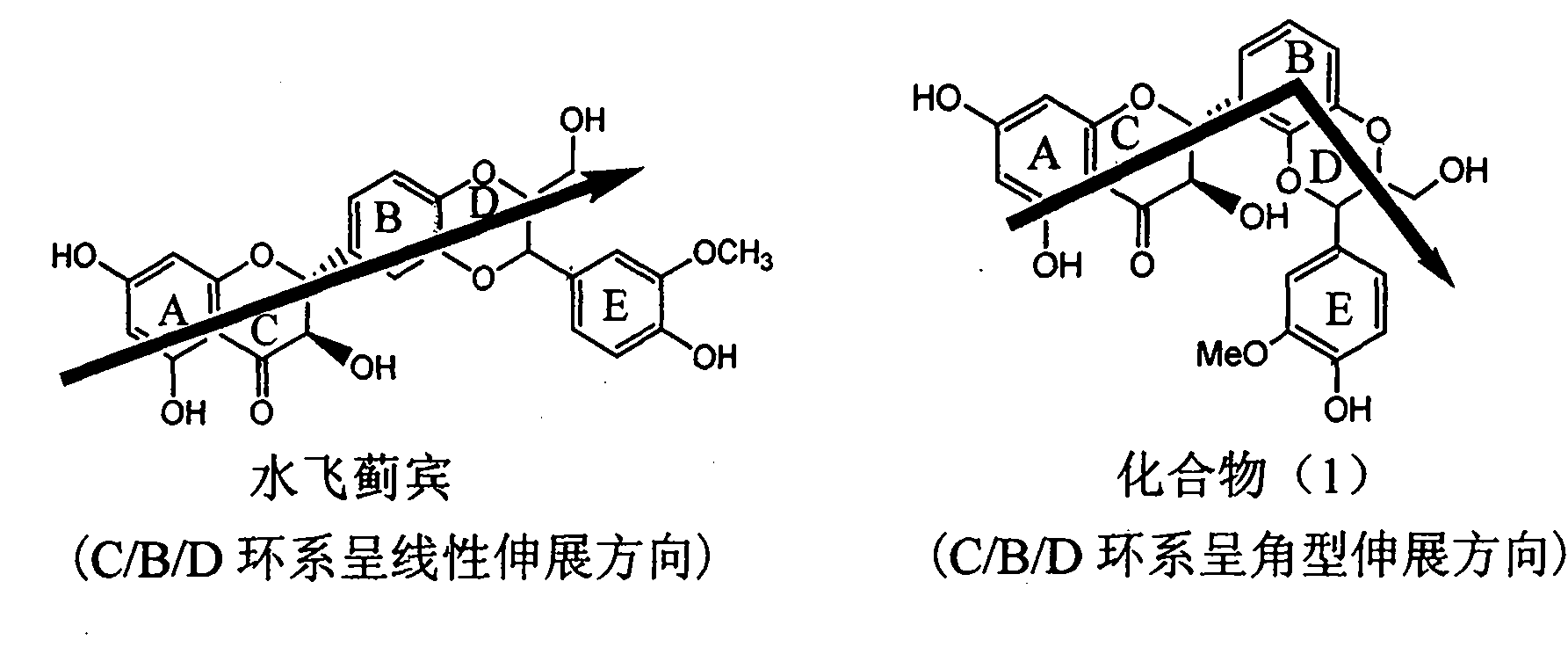
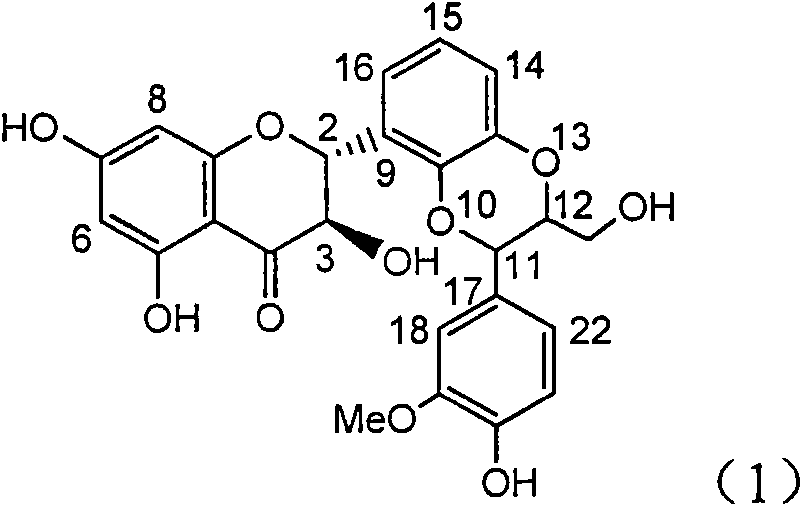
InactiveCN101953828AInhibition of replicationConvenient sourceOrganic active ingredientsAntiviralsLignanInterferon alpha
The invention relates to application of an angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B, in particular to application of the angle flavonoids lignan or medicinal salts thereof to preparation of medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating HBV infection diseases. The flavonoids lignan can exactly inhibit HBV DNA activity; the inhibition activity of the flavonoids lignan with high dosage (20 microgram / ml) to the HBV DNA replication is 189 percent higher than that of alpha-interferon with the maximum concentration of 10,000 unit / ml; and the flavonoids lignan belongs to a strong-effect non-nucleosides inhibition HBV natural product. The pharmacological results show that the angle flavonoids lignan or the medicinal salts thereof can be expected to be used for preparing the medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
Organic selenium crop nutrient and preparation method for same
The invention discloses an organic selenium crop nutrient and a preparation method for the same. The organic selenium crop nutrient comprises the following raw materials in parts by weight: 0.5-1.5 parts of molasses, 5-15 parts of soybean meal amino acid, 6-9 parts of urea, and 0.04-0.1 part of sodium selenite. The preparation method for the organic selenium crop nutrient comprises the following steps of: oscillating all raw materials for 24-72 hours in the conditions of a rotation speed of 120-200 rpm and a temperature of 35-40 DEG C, and sieving to obtain a filtrate, namely, the organic selenium crop nutrient. The content of organic selenium in the organic selenium crop nutrient disclosed by the invention is greater than 0.3 g / L. Additionally, according to the organic selenium crop nutrient and the preparation method disclosed by the invention, harm brought by the too high dosage of an inorganic selenium fertilizer to a human body, and fertilizer damage phenomena of seedling burning, leaf spots and the like due to the inappropriate dosage of the inorganic selenium fertilizer are overcome.
Owner:CHANGSHA UNIVERSITY
High addition fly ash cement and method for producing the same
InactiveCN101172801AIncrease dosageReduce production energy consumptionSolid waste managementPolymer scienceHigh dosage
The invention relates to high-dosage pulverized coal cement and the production method thereof, and belongs to the technical field of cement. The invention aims to realize high-dosage pulverized coal cement with the coal ash quality of over 40 percent and the simple production art. The invention comprises the steps as follows; the coal ash and the cement clinker are taken as the main material, after respectively grinding, a little gypsum and proper water decreasing agent and kindling agent are added, and mixing ball grinding treatment is performed. All properties meet the national coal ash cement standard requirement. The pulverized coal cement produced by the invention has the advantages of large dosage of coal ash, high early intensity, stable product quality, low production cost and small energy consumption. The invention has great significance for adopting coal ash castoff cosmically and saving the cost of cement.
Owner:CHINA UNIV OF MINING & TECH (BEIJING)
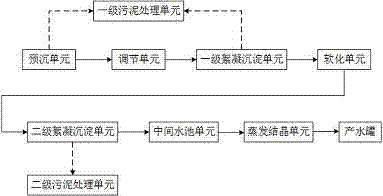
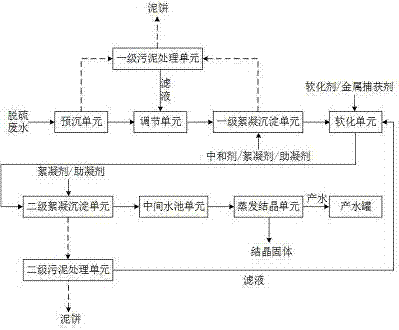
Method and device for realizing zero discharge of desulfurization wastewater
InactiveCN105439358AReduce hardnessEfficient removalSludge treatment by de-watering/drying/thickeningWater contaminantsHigh energySludge
The invention provides a method and a device for realizing zero discharge of desulfurization wastewater and solves problems of the high energy consumption, the high dosage of chemical agents and the high cost in the prior art. The device for realizing zero discharge of the desulfurization wastewater comprises a pre-sedimentation unit, an adjusting unit, a primary flocculation and sedimentation unit, a softening unit, a secondary flocculation and sedimentation unit, an intermediate water tank unit, an evaporation and crystallization unit, two stages of sludge treatment units and a water producing tank, wherein the softening unit and two stages of flocculation and sedimentation units are arranged in front of the evaporation and crystallization unit so as to remove metal ions, F<-> and hardness of the desulfurization wastewater, water inlet requirements of the subsequent evaporation and crystallization unit are met, and the scaling tendency of the evaporation and crystallization unit is relieved. Meanwhile, the content of salt of the desulfurization wastewater is high, the evaporation and crystallization unit enables the salt of the desulfurization wastewater to form valuable industrial salt, and the desulfurization wastewater is recycled. The method and the device have the benefits as follows: the running cost is low, the energy consumption is low, the quality of produced water is good, the design is reasonable, scaling of the evaporation and crystallization unit is effectively prevented, and meanwhile, the desulfurization wastewater can be recycled.
Owner:GO HIGHER ENVIRONMENT GRP CO LTD
Rapid disperse dosage form containing levetiracetam
ActiveUS9339489B2Easy to manageOvercome disadvantagesPowder deliverySheet deliveryDiseaseBiochemistry
Owner:APRECIA PHARMA LLC
Method for regulating metallic silicides source/drain Schottky barrier height
InactiveCN101807526AEasy to integrateCompatibleSemiconductor/solid-state device manufacturingSalicideSchottky barrier
The invention discloses a method for regulating metallic silicides source / drain Schottky barrier height. The method comprises the steps as follows: step 1: using a two-step nickel-self-alignment silicide process to form a nickel silicide film; step 2: injecting low energy high dosage impurities to the nickel silicide film; and step 3: rapidly thermally annealing the nickel silicide film. The invention has the advantages of capability of using the existing process, simple process, easy operation, low cost and easy integration, thereby achieving good compatibility with CMOS process.
Owner:INST OF MICROELECTRONICS CHINESE ACAD OF SCI
Methods for treating disorders associated with hyperlipidemia in a mammal
The invention is directed to methods for treating hyperlipidemia in a mammal. The methods involve combination therapies using a microsomal triglyceride transfer protein (MTP) inhibitor (for example, BMS-201038 and implitapide) and a HMG-CoA reductase inhibitor (for example simvastatin or atorvastatin). Co-administration of the MTP inhibitor with the HMG-CoA reductase inhibitor produces a therapeutic benefit, for example, a reduction in the concentration of cholesterol and / or triglycerides in the blood stream, but with fewer or reduced side effects than when higher dosages of the MTP inhibitor are used during monotherapy to provide the same or similar therapeutic benefit.
Owner:AEGERION PHARM INC
High dose Ambroxol hydrochloride freeze-dried preparation and preparation method
InactiveCN1954808AGood solubilization effectInhibition of precipitation and crystallizationOrganic active ingredientsPowder deliveryFreeze-dryingHigh doses
A freeze-dried powder injection of high-dosage (500mg) ambroxol hydrochloride and its preparing process are disclosed.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com