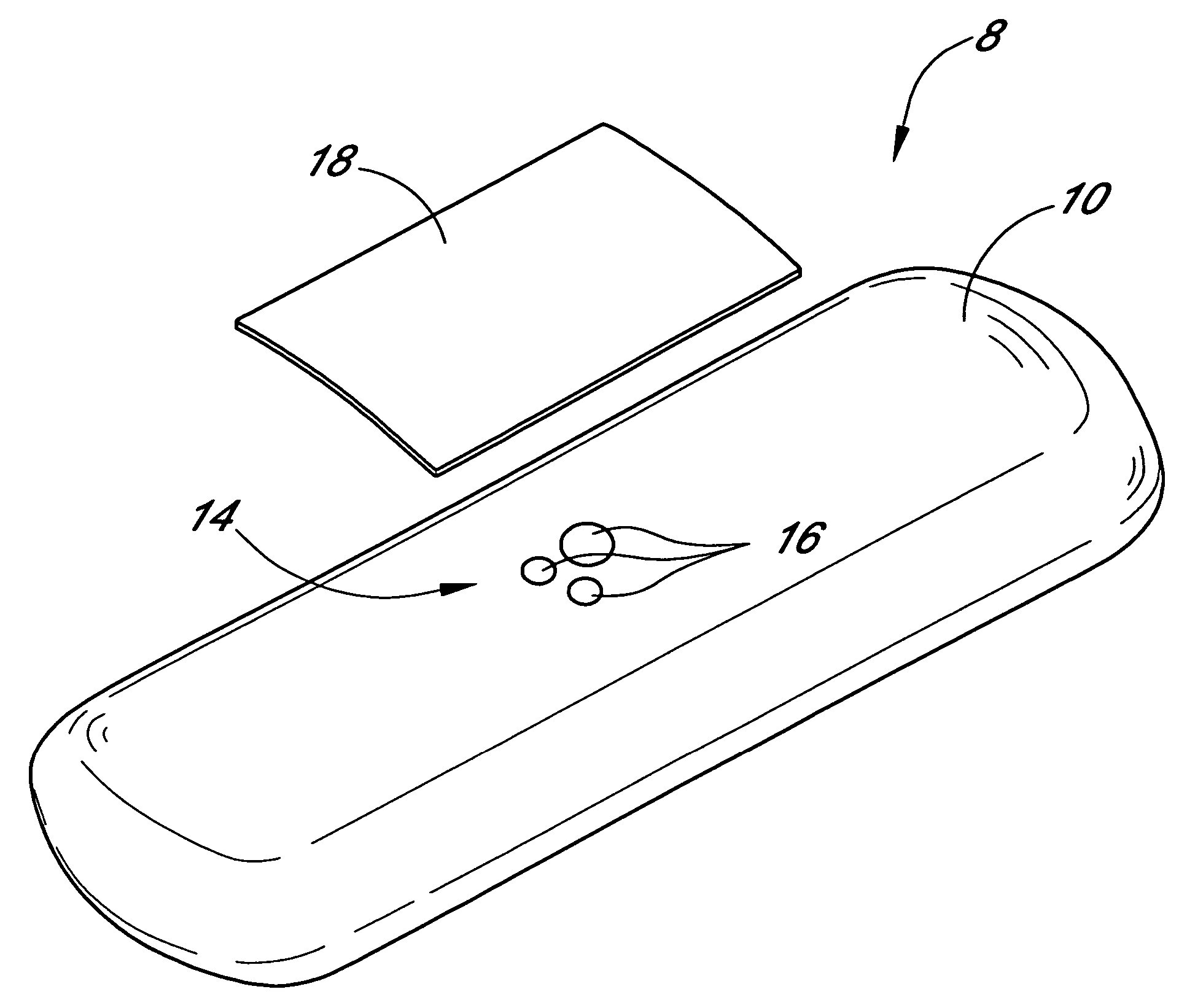
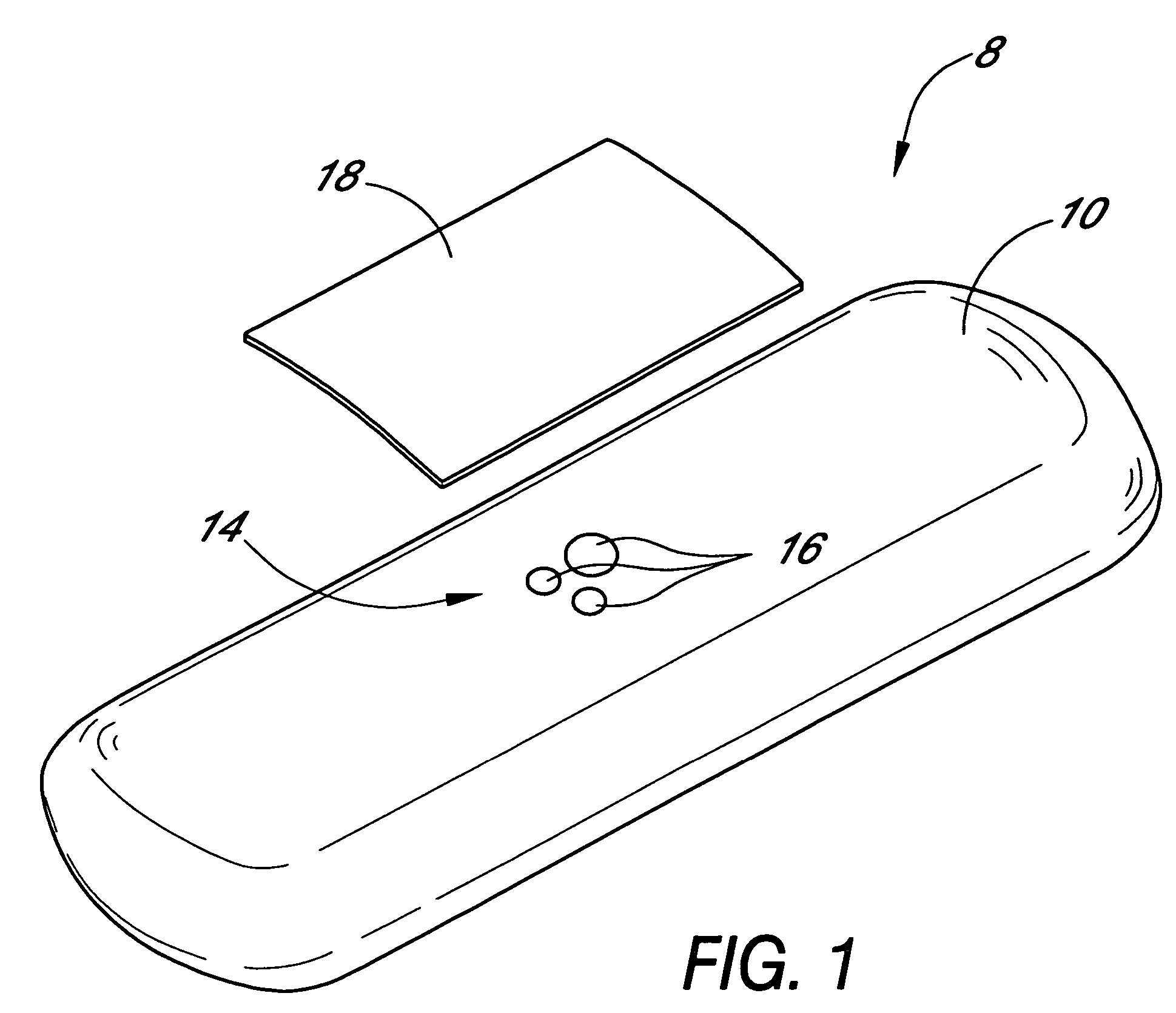
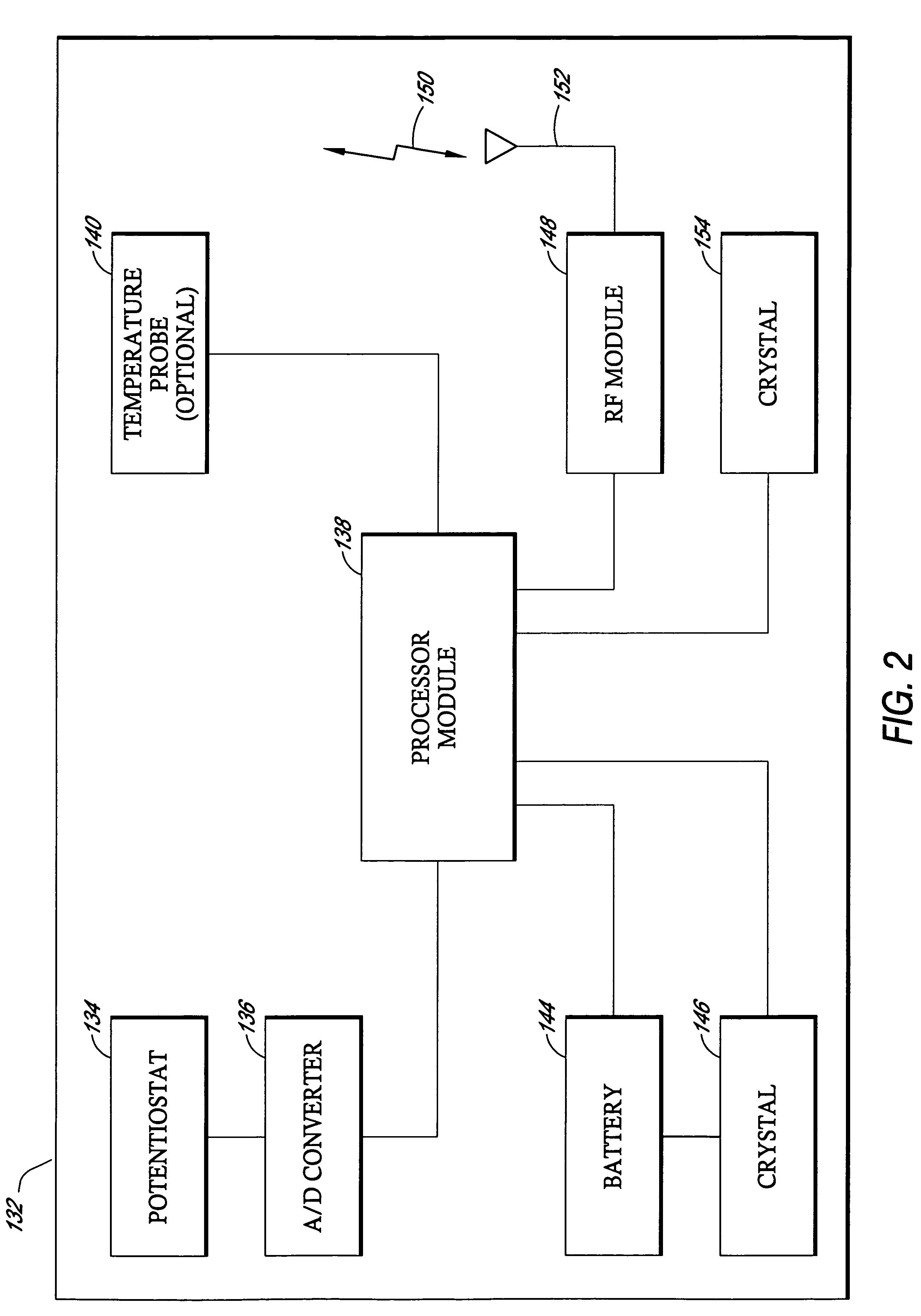
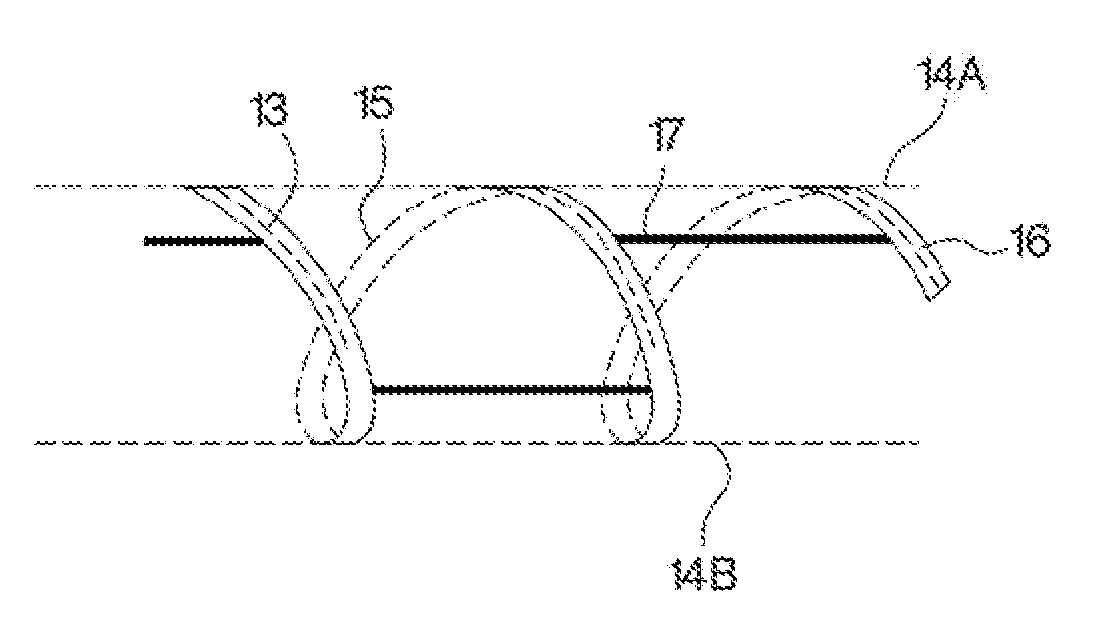
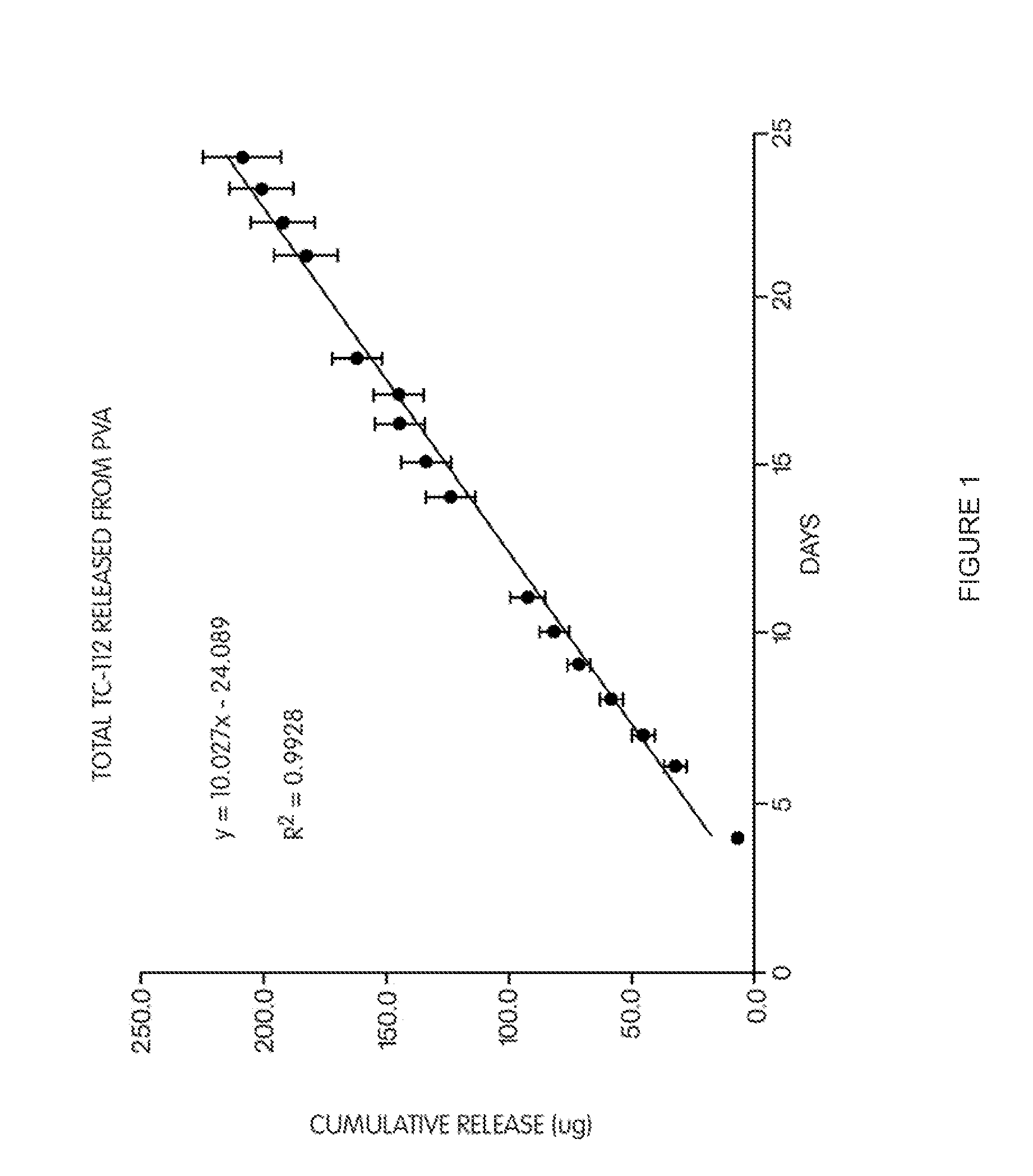
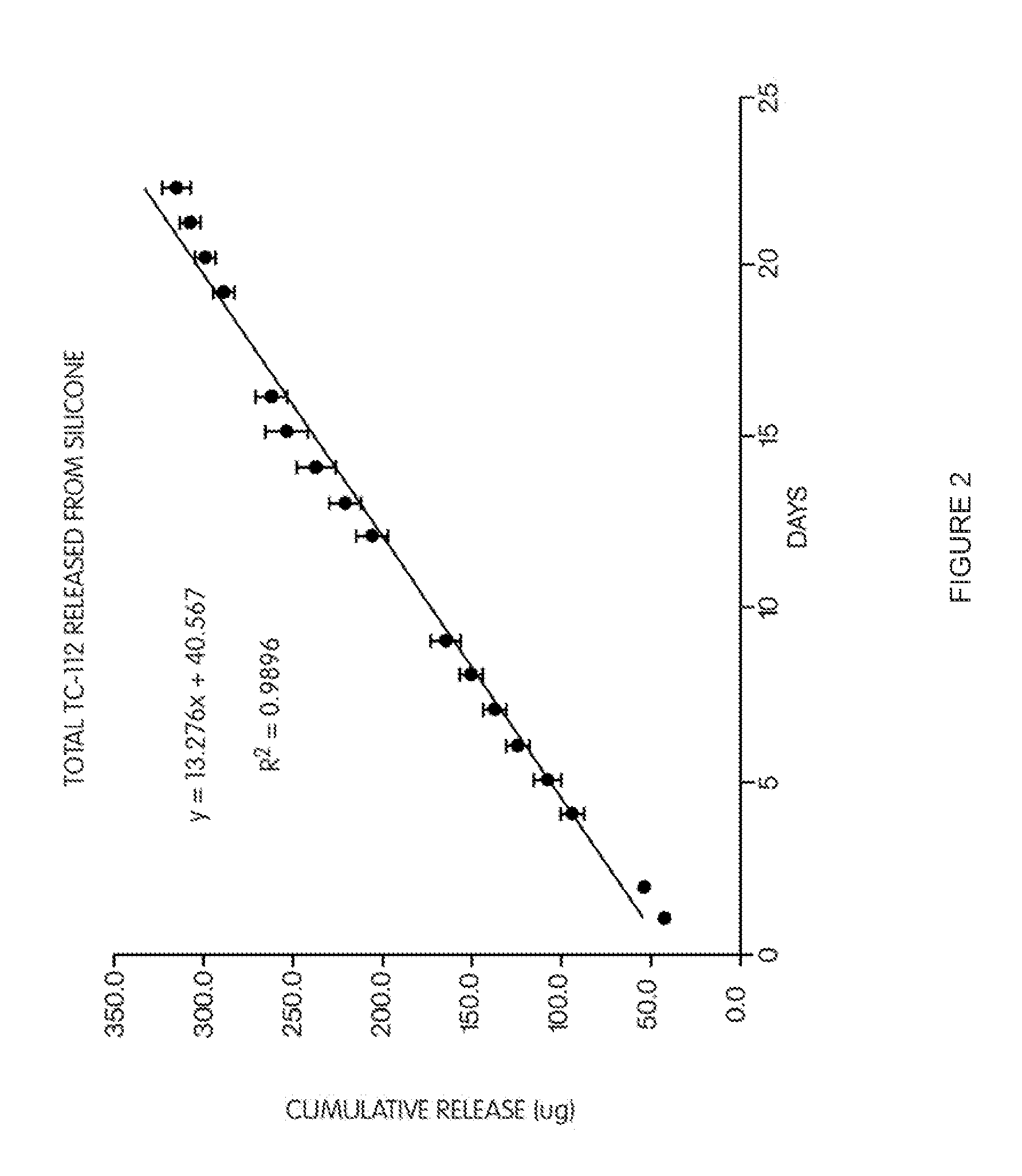
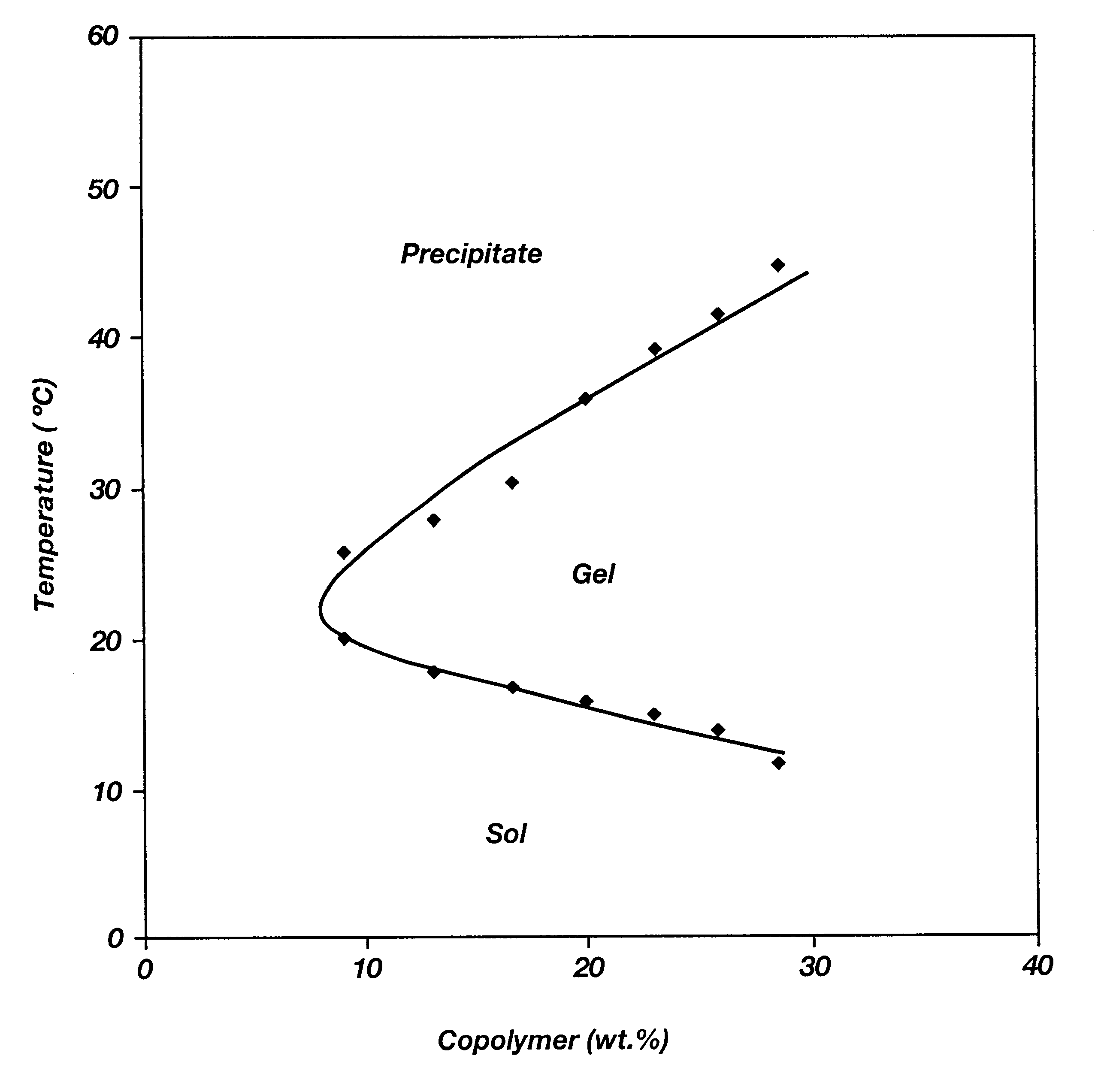
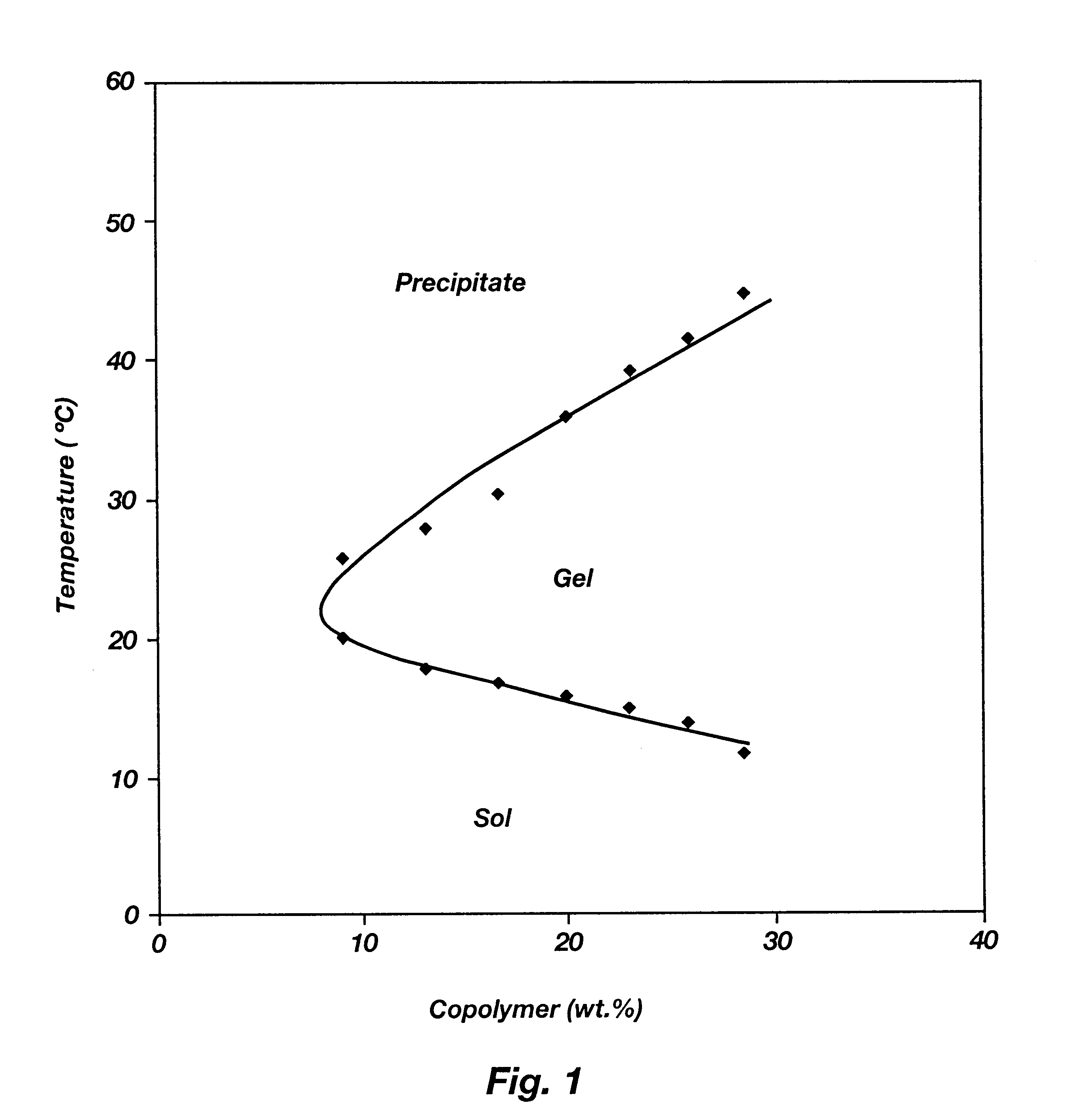
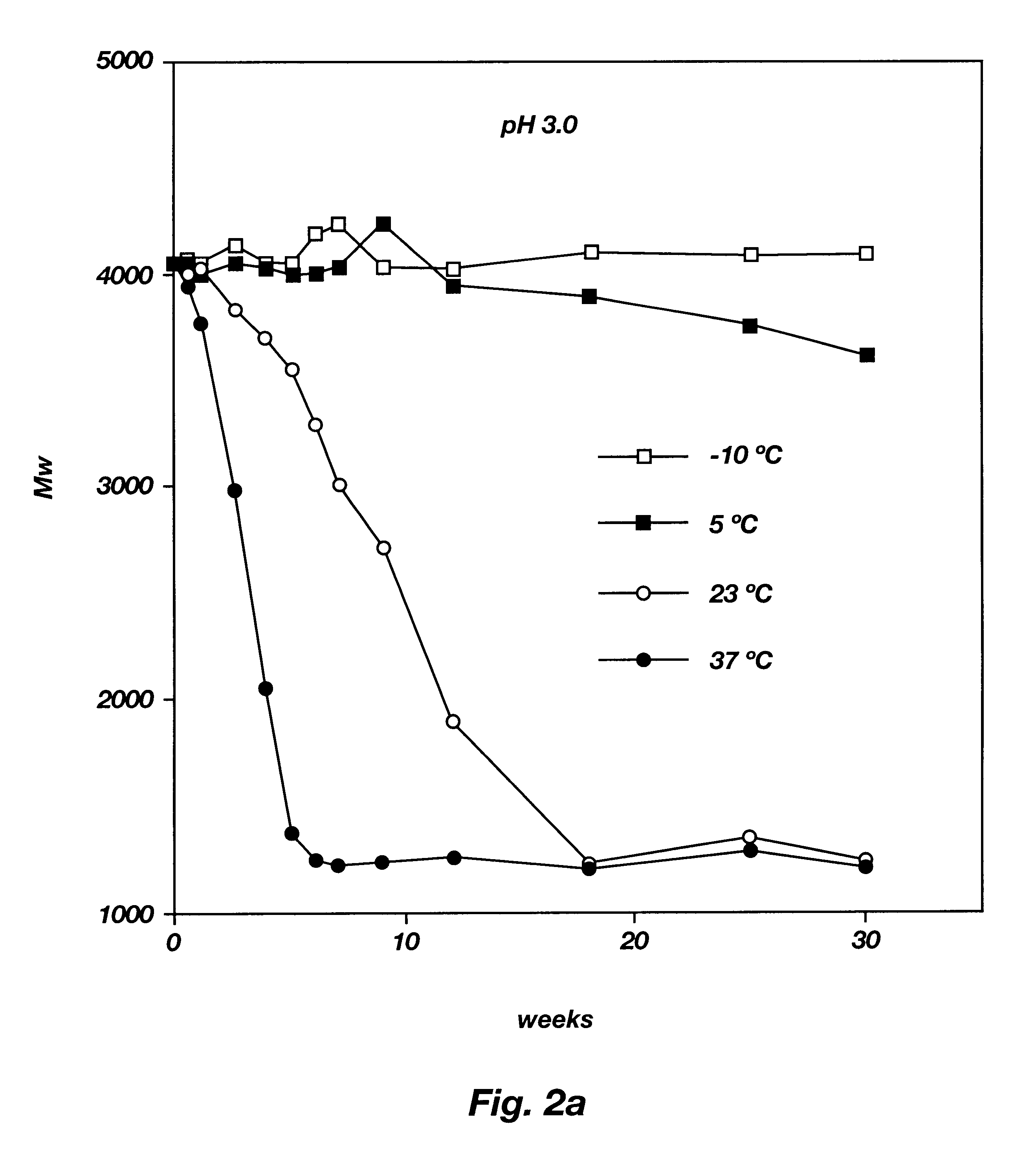
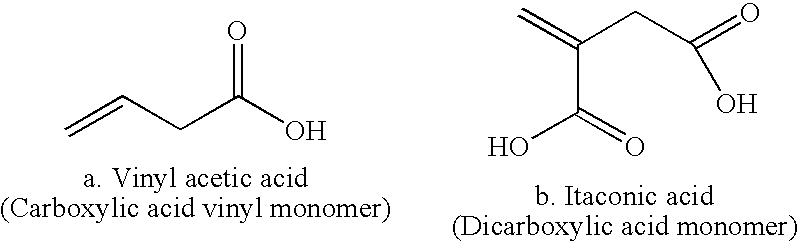
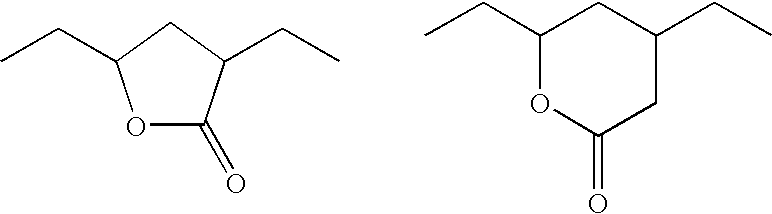
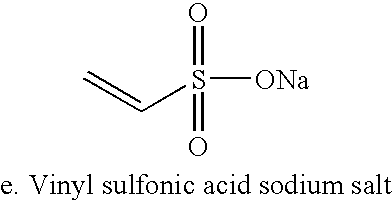
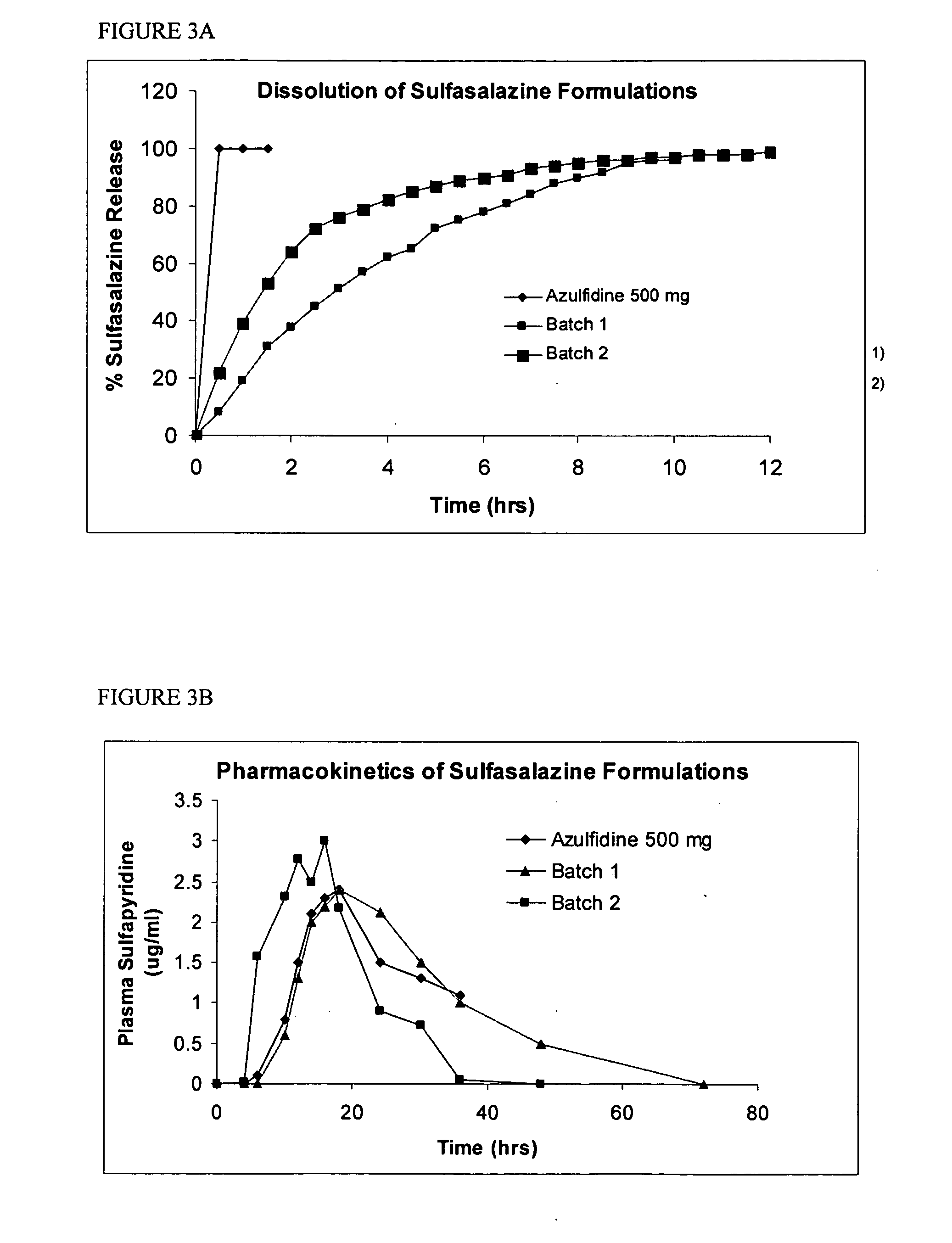
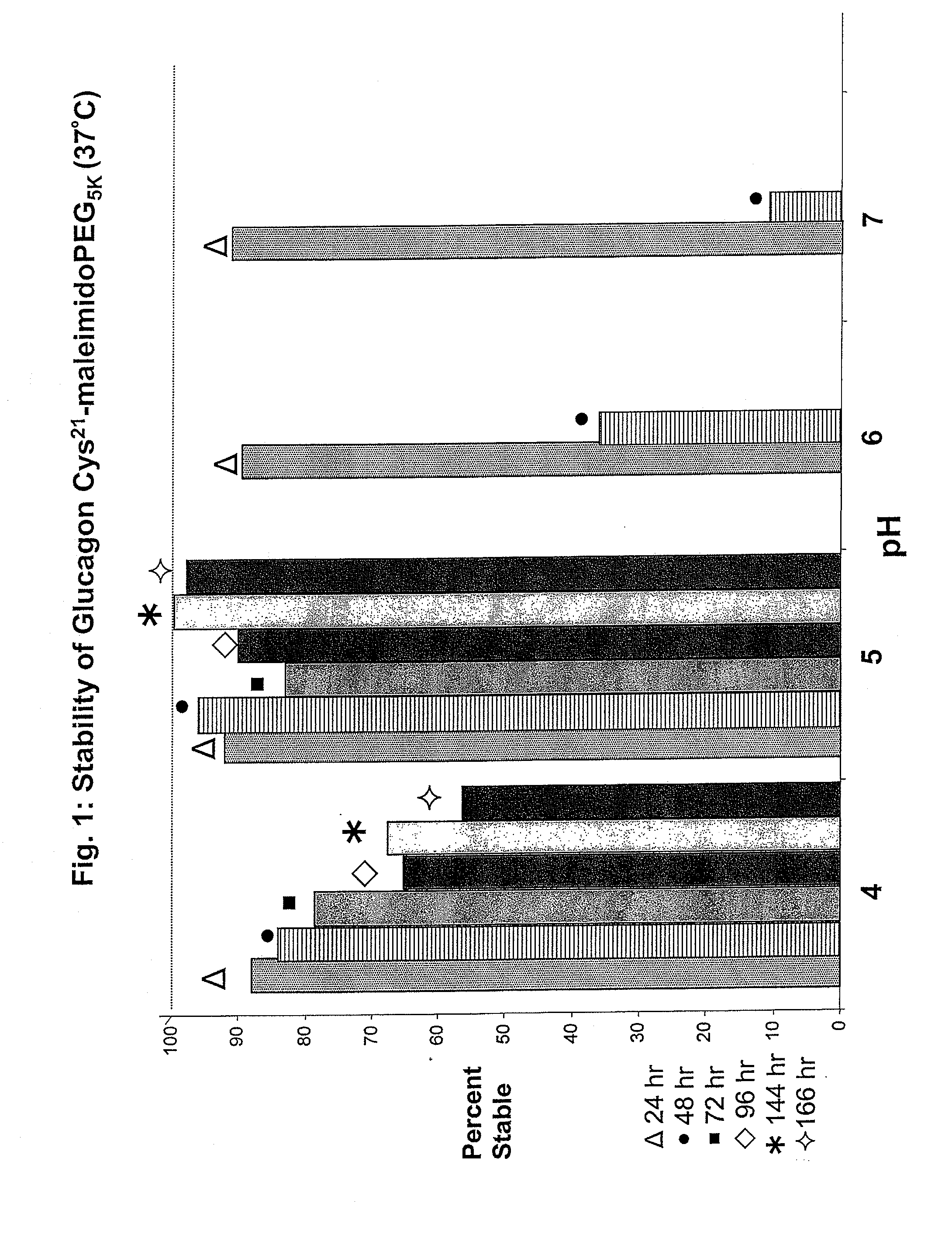
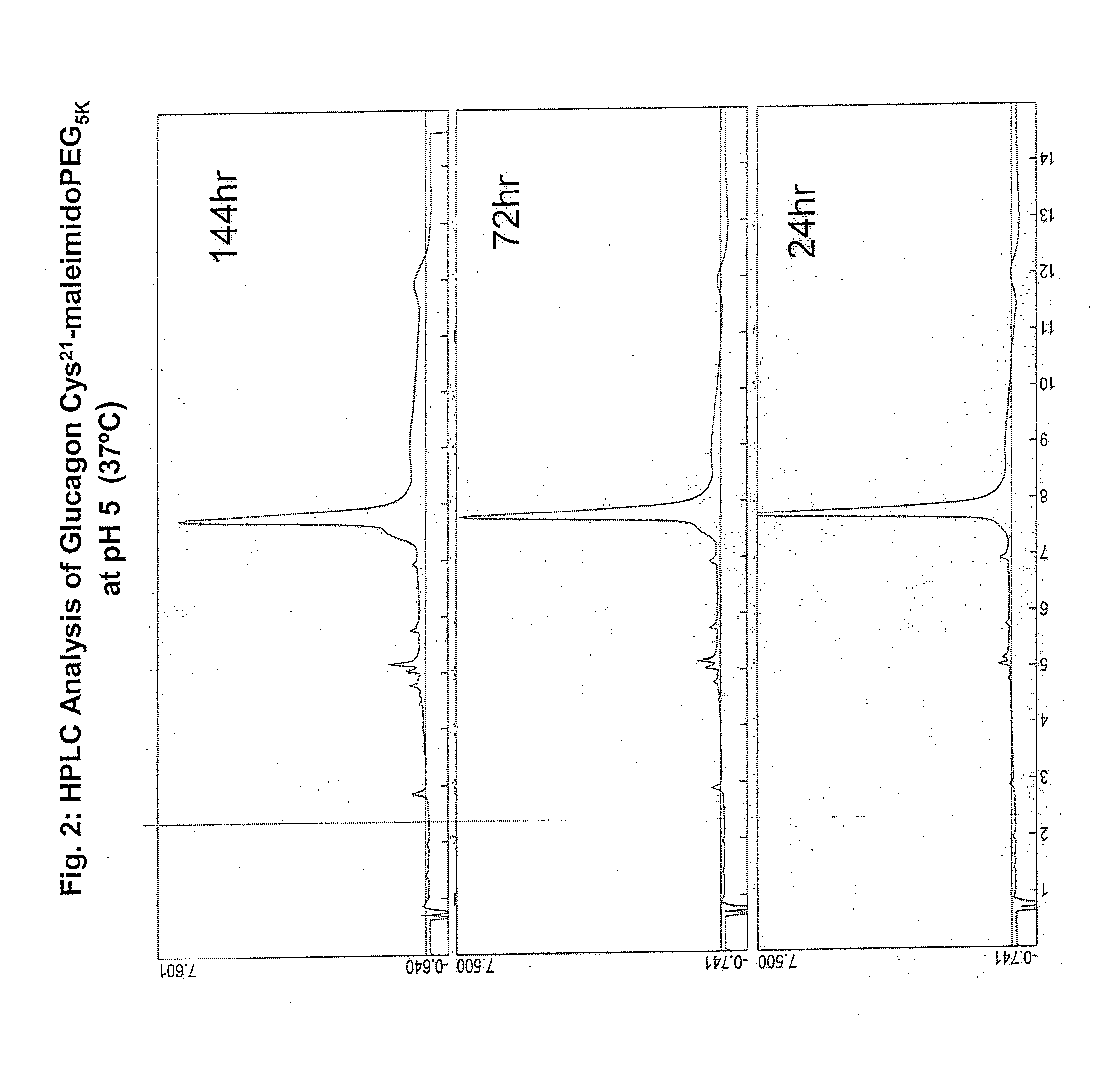
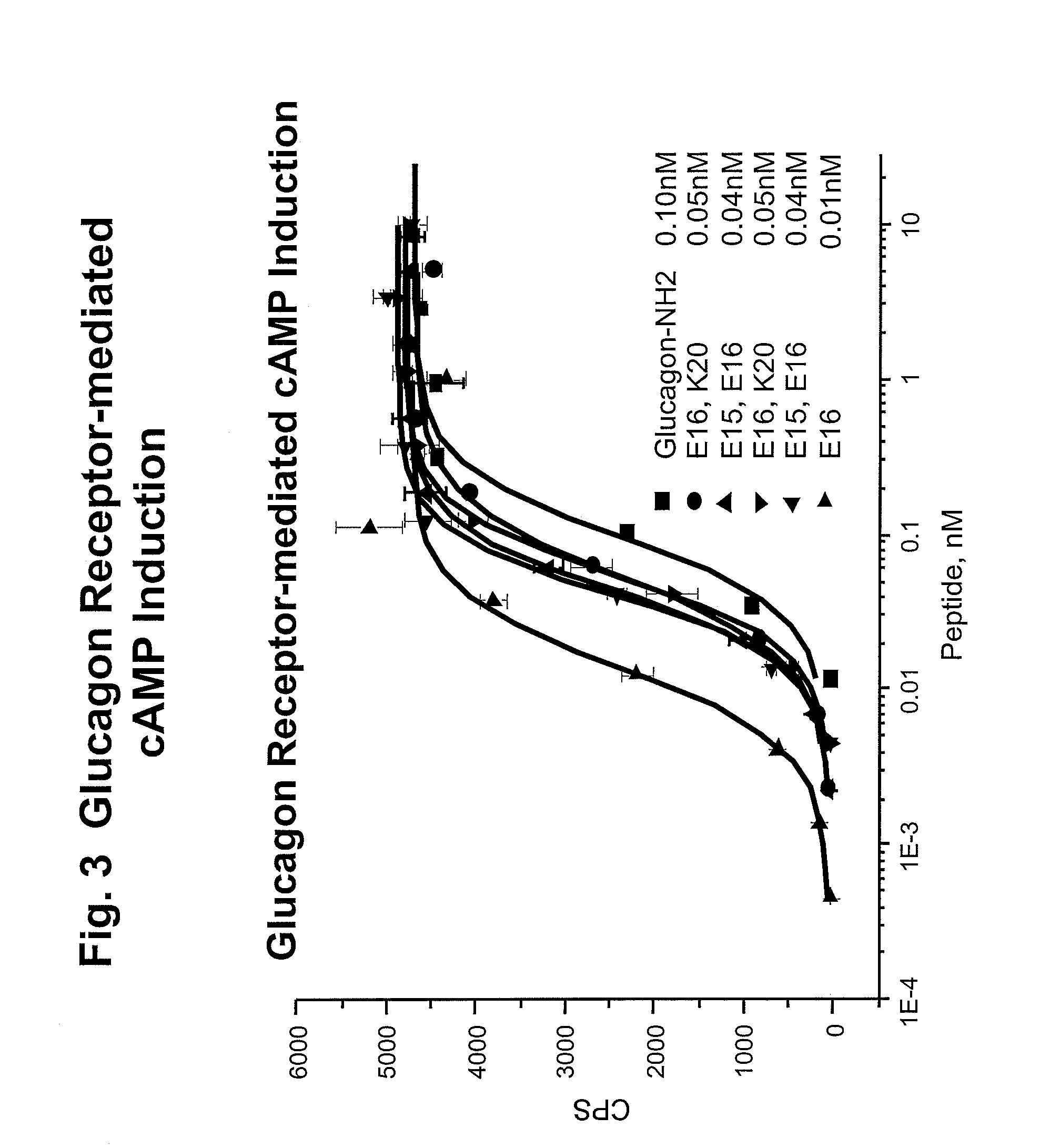
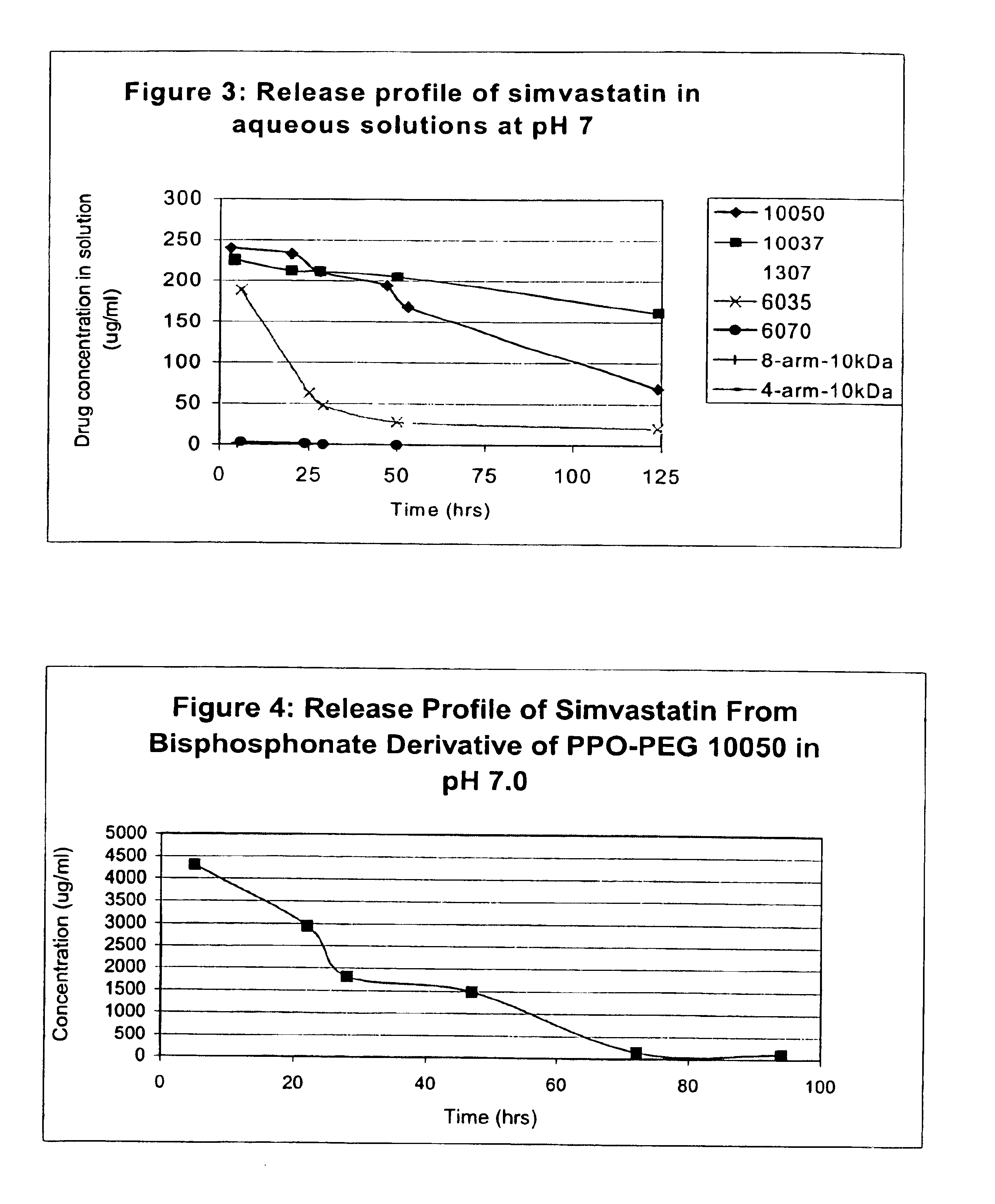
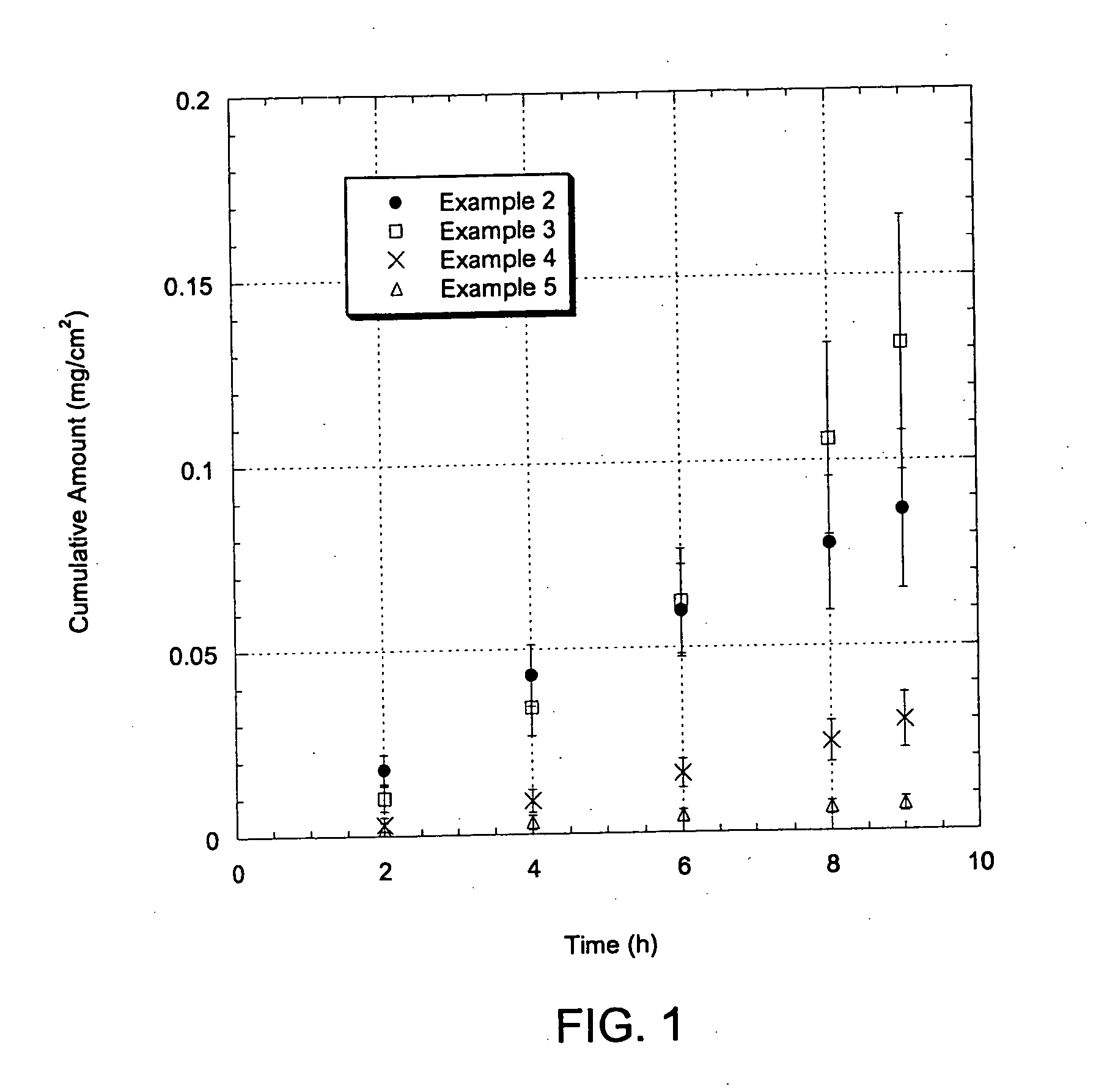
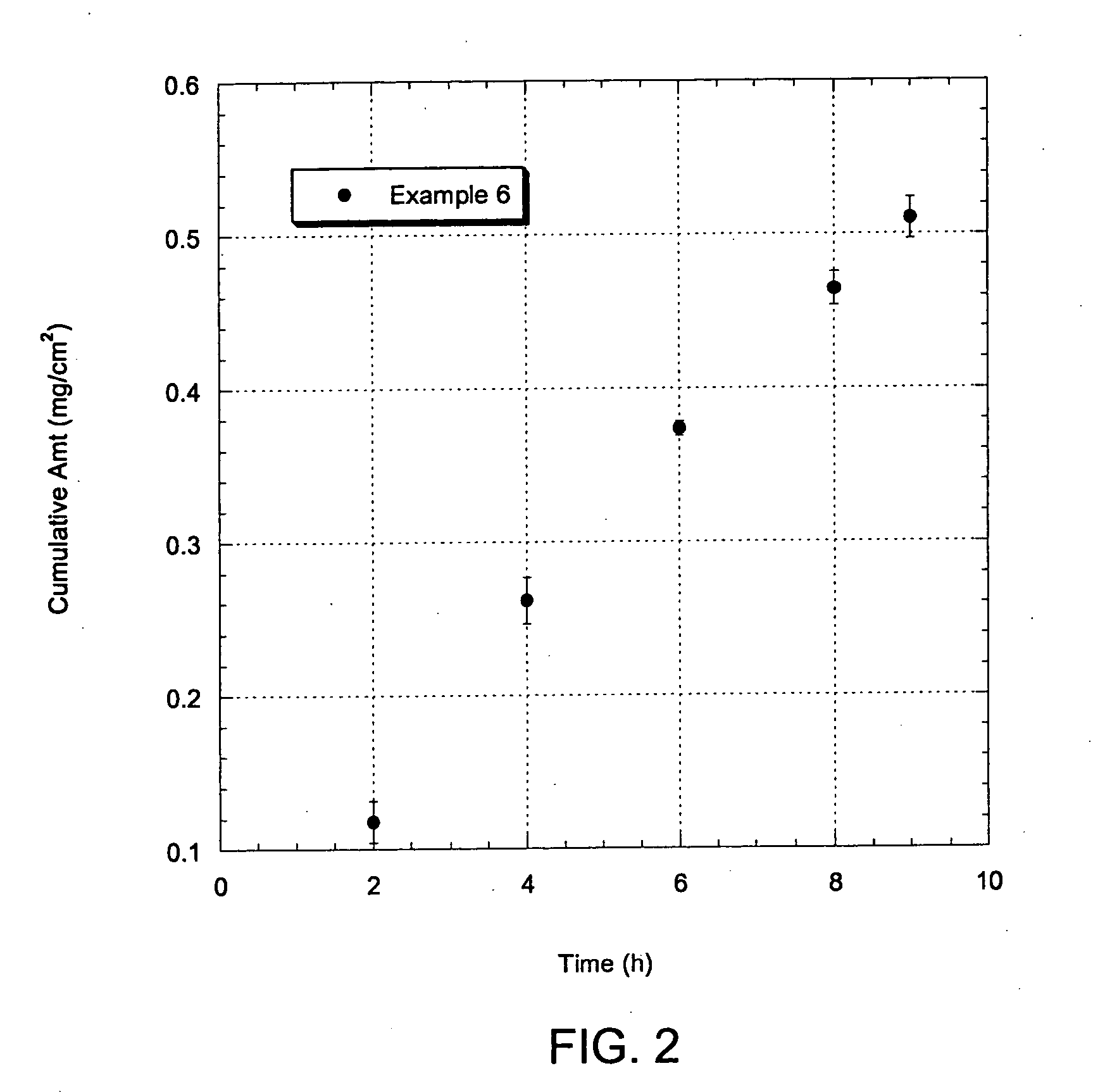
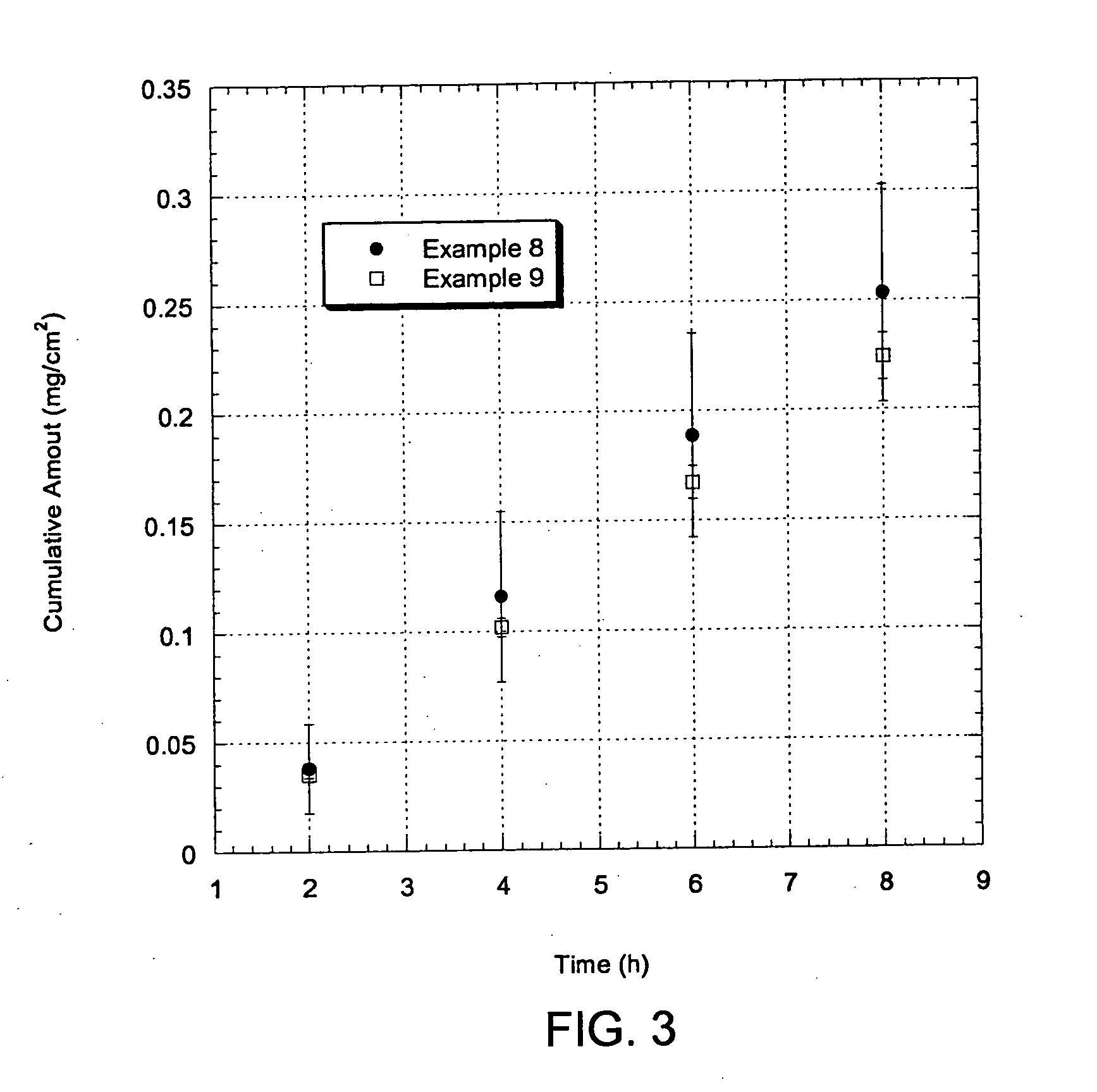
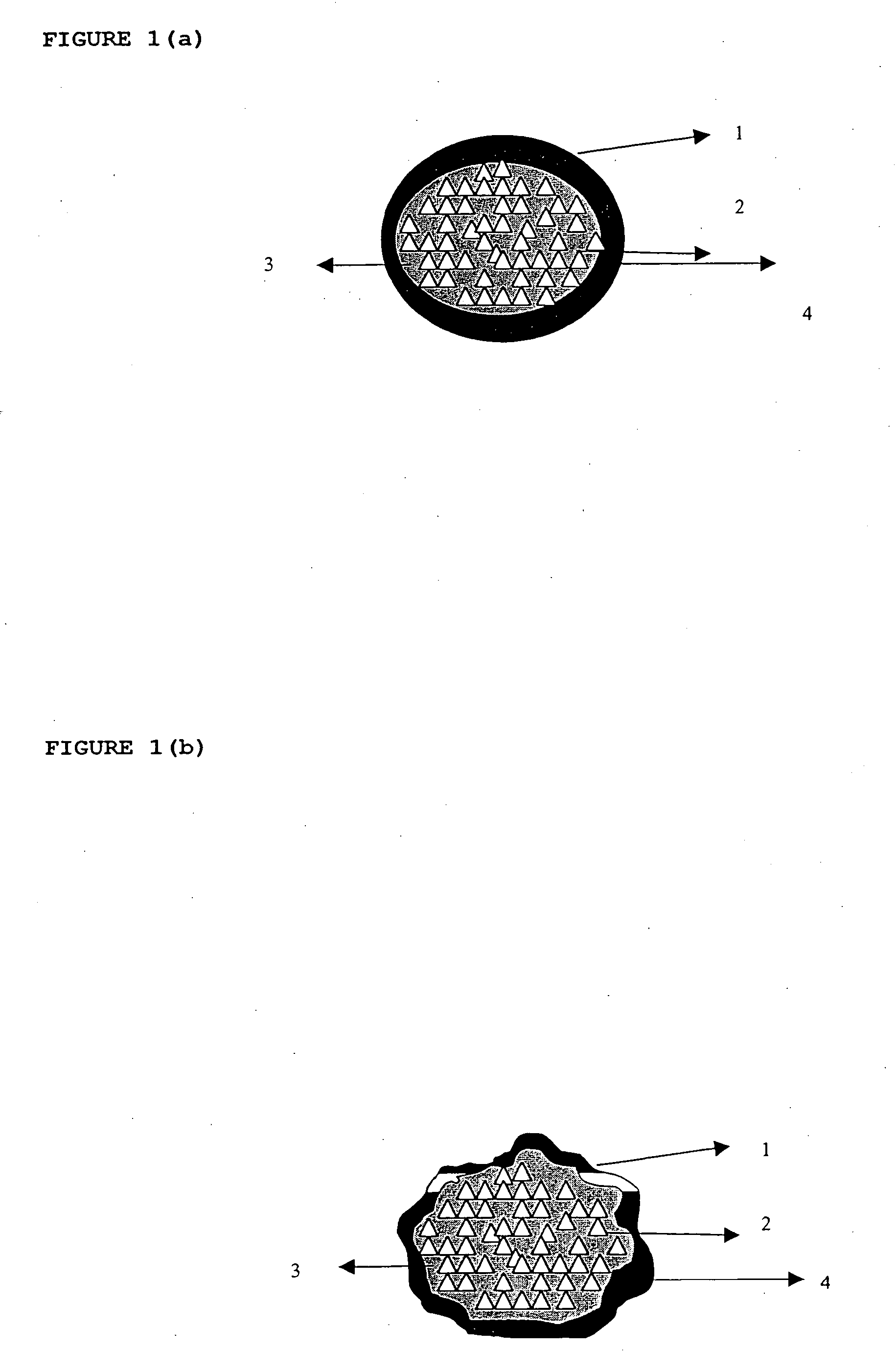
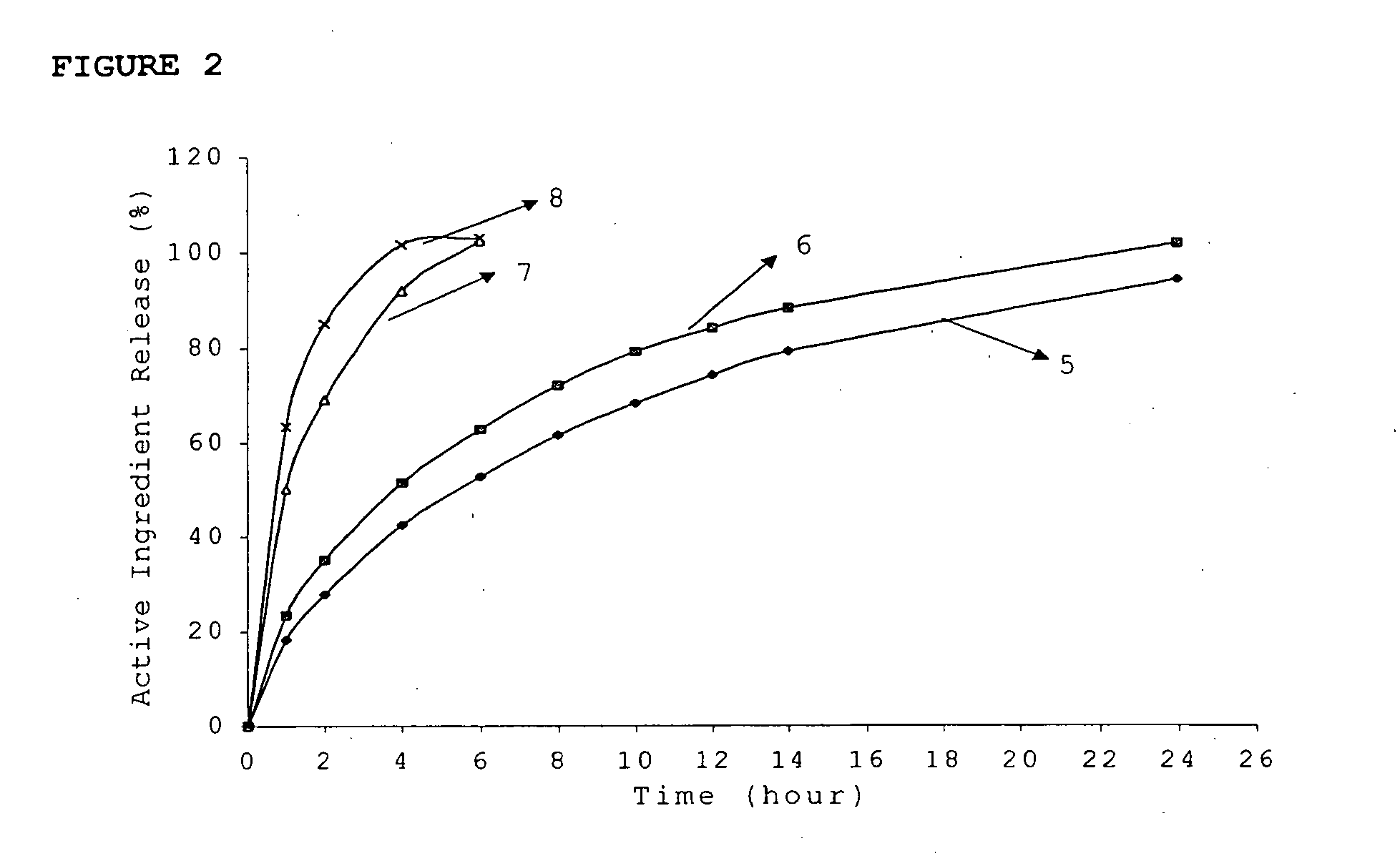
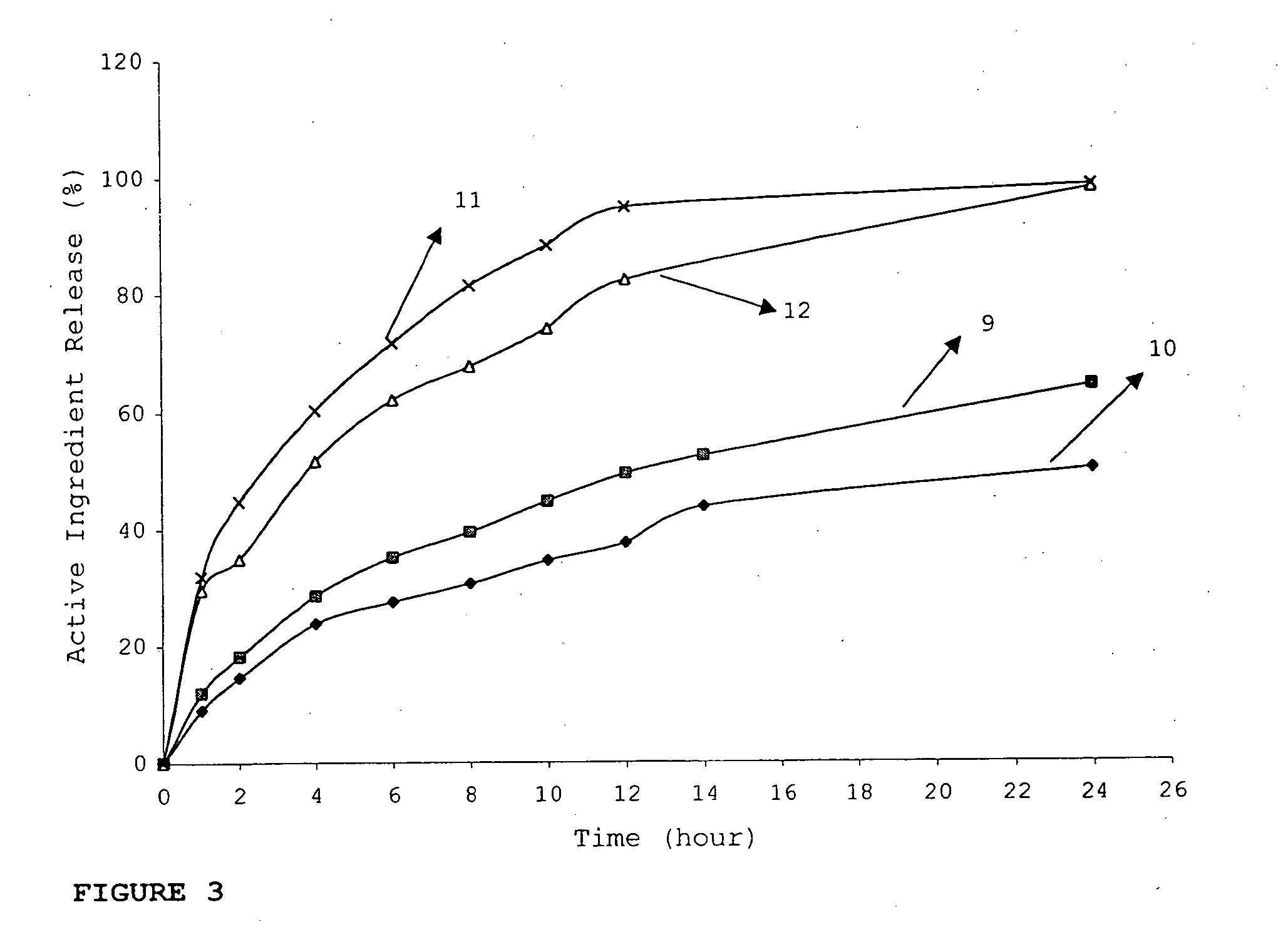
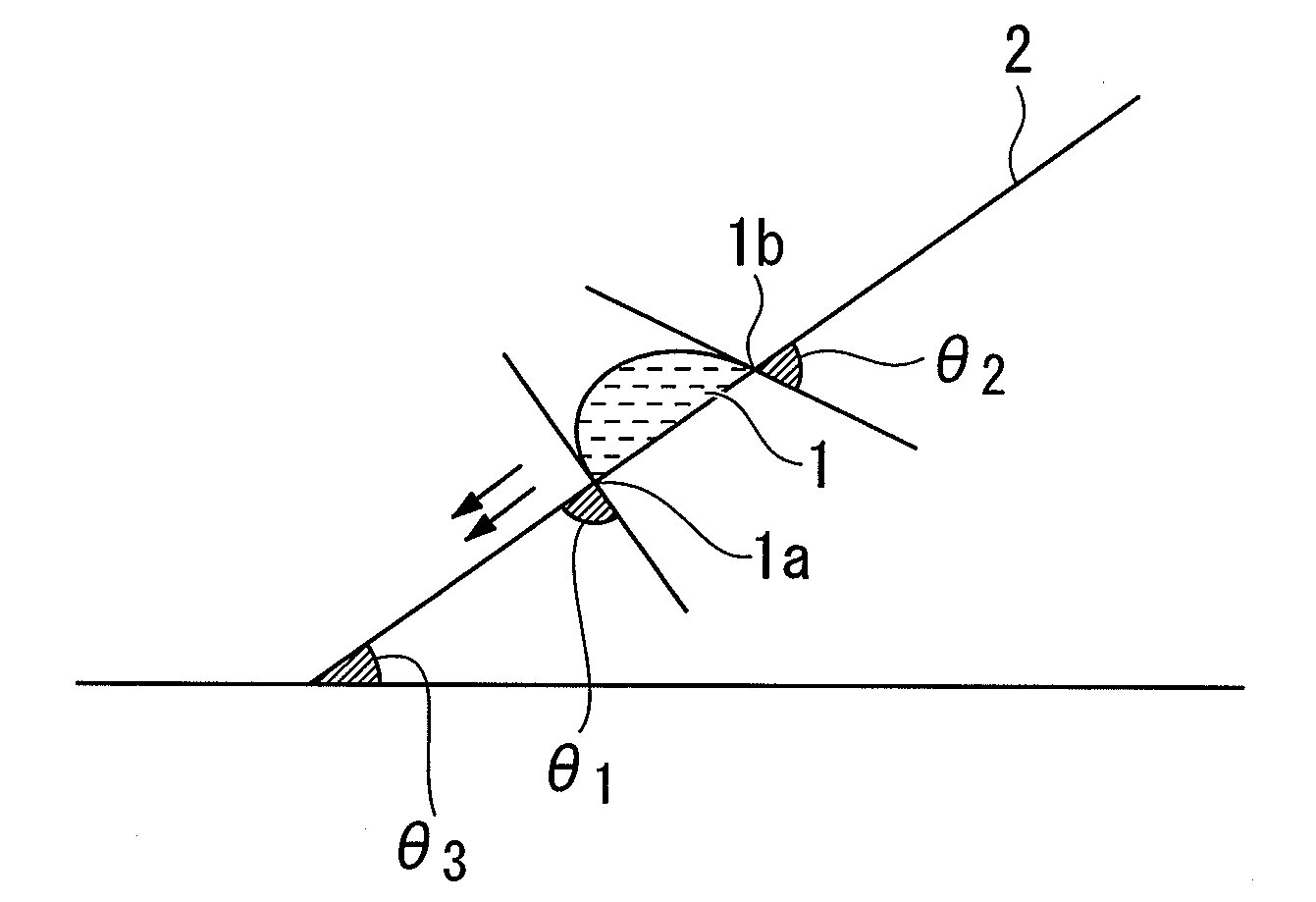
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
28855 results about "Solubility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Solubility is the property of a solid, liquid or gaseous chemical substance called solute to dissolve in a solid, liquid or gaseous solvent. The solubility of a substance fundamentally depends on the physical and chemical properties of the solute and solvent as well as on temperature, pressure and presence of other chemicals (including changes to the pH) of the solution. The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration, where adding more solute does not increase the concentration of the solution and begins to precipitate the excess amount of solute.
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
Silicone based membranes for use in implantable glucose sensors
Membrane systems incorporating silicone polymers are described for use in implantable analyte sensors. Some layers of the membrane system may comprise a blend of a silicone polymer with a hydrophilic polymer, for example, a triblock poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) polymer. Such polymeric blends provide for both high oxygen solubility and aqueous analyte solubility.
Owner:DEXCOM INC
Polymer-based, sustained release drug delivery system
InactiveUS20120016467A1Reduce interactionSuture equipmentsAntibacterial agentsRate limitingSolubility
Disclosed is a sustained release system that includes a polymer and a prodrug having a solubility less than about 1 mg / ml dispersed in the polymer. Advantageously, the polymer is permeable to the prodrug and may be non-release rate limiting with respect to the rate of release of the prodrug from the polymer. This permits improved drug delivery within a body in the vicinity of a surgery via sustained release rate kinetics over a prolonged period of time, while not requiring complicated manufacturing processes.
Owner:PSIVIDA INC
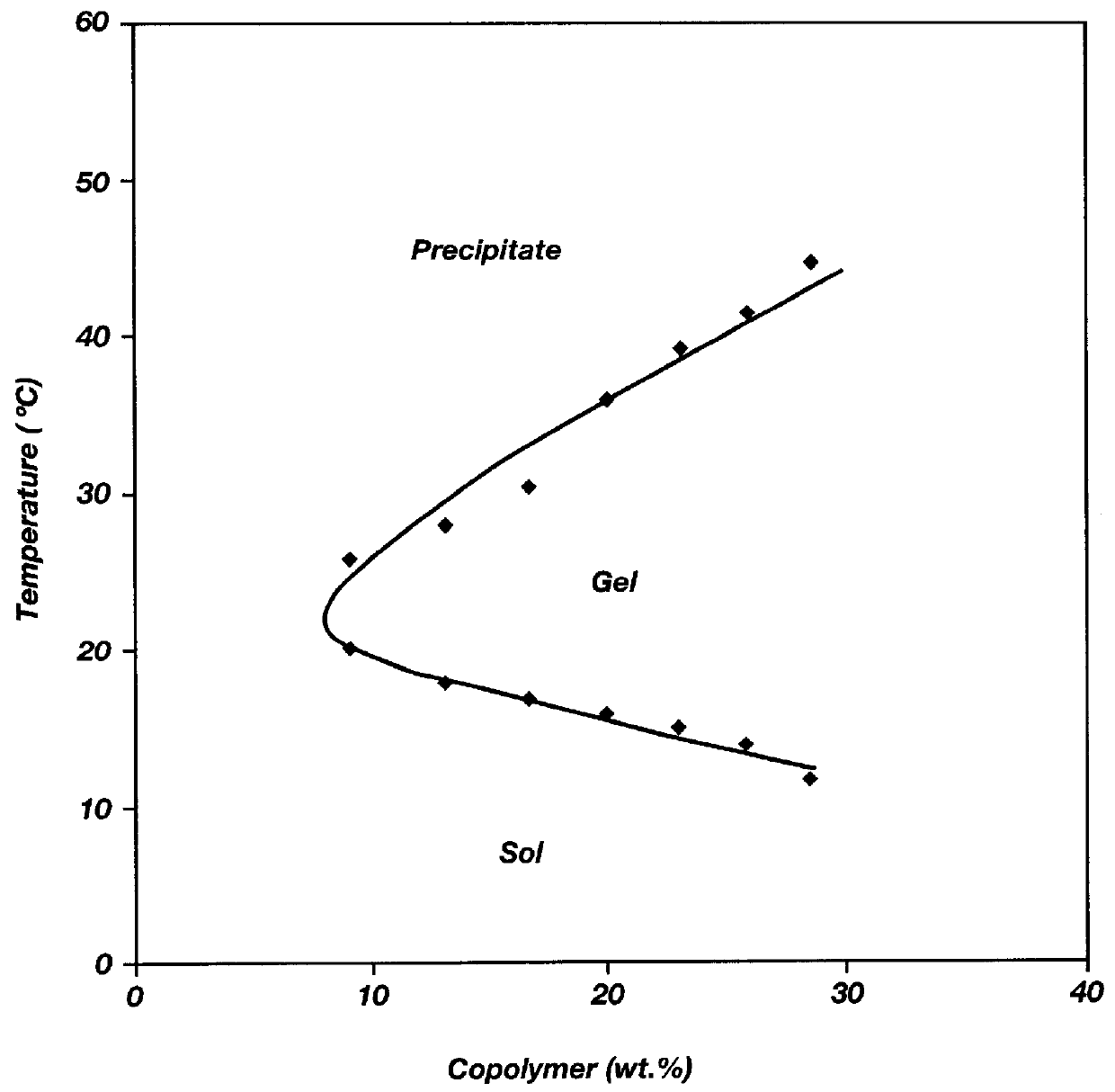
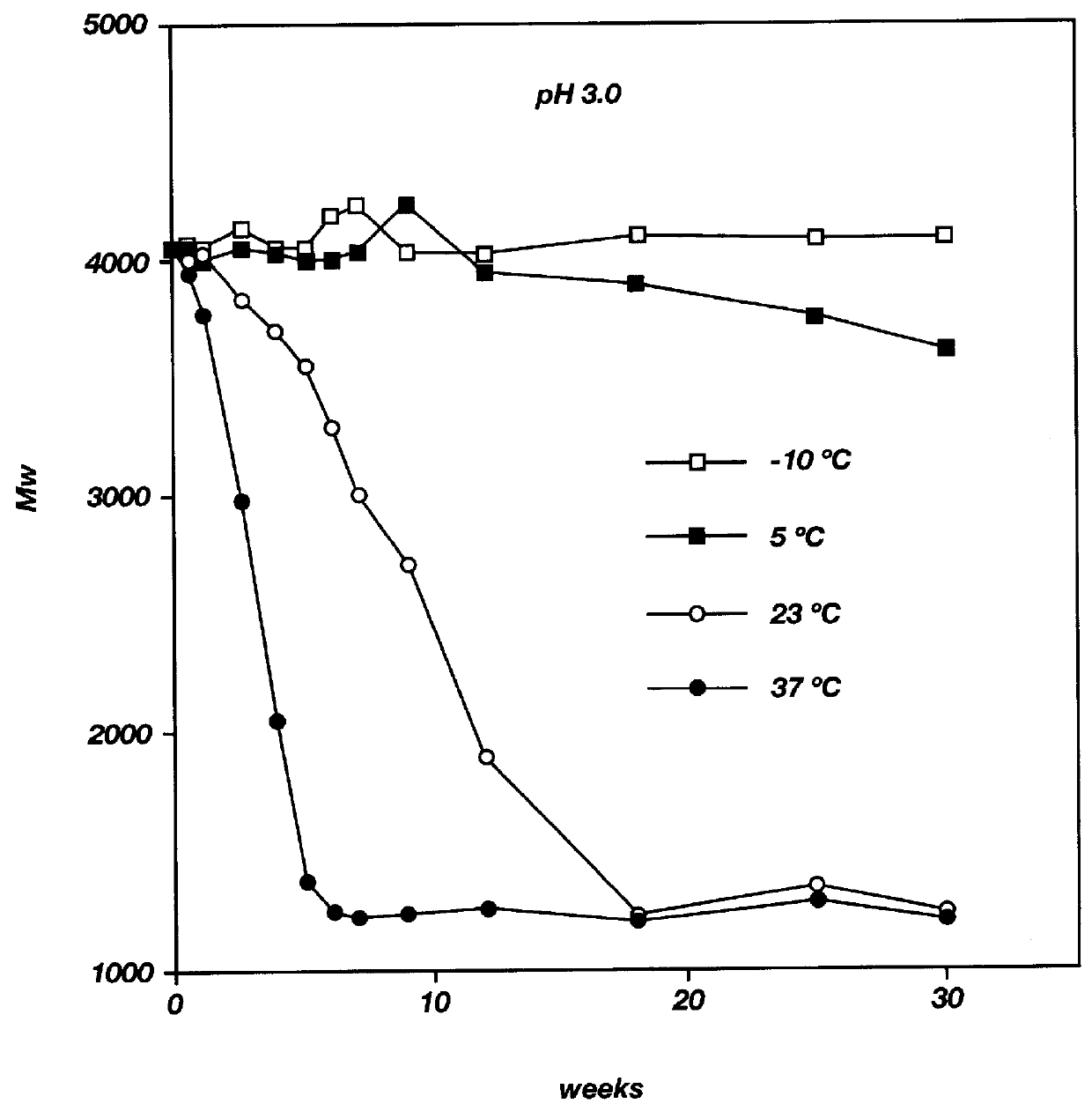
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Water-soluble copolymer film packet
Owner:MONOSOL LLC +1
Pharmaceutical dosage forms for highly hydrophilic materials
Pharmaceutical dosage forms having a highly hydrophilic fill material and a shell encapsulating the fill material are disclosed and described. Generally, the shell has at least one plasticizing agent therein in order to provide the shell with an effective plasticity. In one aspect, the shell may have included therein an amount of plasticizing agent that is sufficient to provide the shell with an effective plasticity upon migration of a portion of the plasticizing agent into the fill material. In another aspect, the plasticizing agent may have a solubility in the fill material of less than about 10% w / w. In yet another aspect, a combination of a plasticizing agent, and a plasticizing agent having a solubility in the fill material of less than about 10% w / w, may be presented in a total amount sufficient to provide the shell with an effective plasticity upon migration of plasticizing agent into the fill material.
Owner:LIPOCINE
Cross-linkers with high reactivity and solubility and their use in the preparation of conjugates for targeted delivery of small molecule drugs
Disclosed is a method of making conjugates of cell binding agents and small molecule drugs comprising reacting a cell binding agent with a bifunctional cross-linking moiety to thereby provide the cell binding agent with a reactive disulfide group and then reacting the modified cell binding agent with a small molecule drug comprising a free thiol group. Bifunctional cross-linking moieties are also disclosed.
Owner:IMMUNOGEN INC
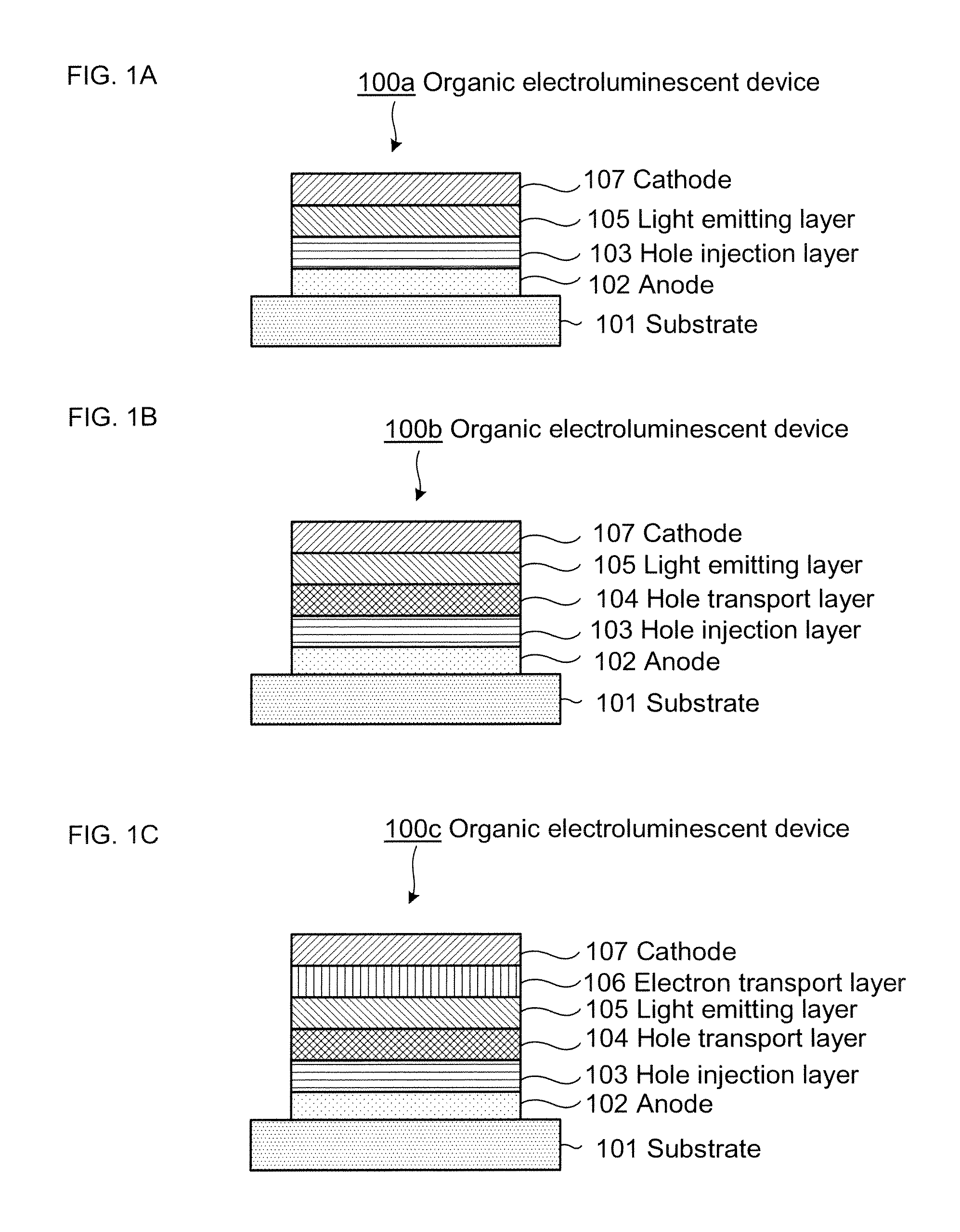
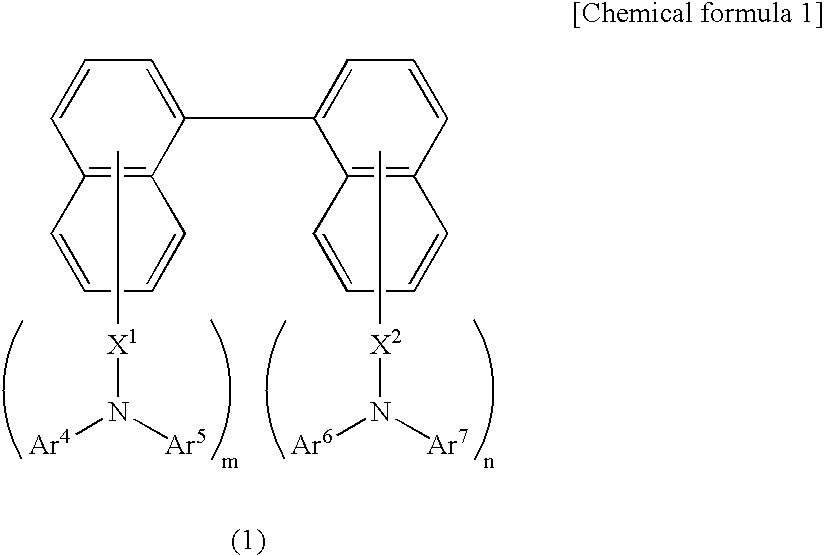
Compositions for organic electroluminescent device and organic electroluminescent device
InactiveUS20060182993A1Reduce inactivationChange propertiesDischarge tube luminescnet screensDuplicating/marking methodsSolubilityHole injection layer
Disclosed are compositions for an organic electroluminescent device favorably used for forming a hole injection layer and a hole transport layer of the organic electroluminescent device by a wet film forming method. The compositions for the organic electroluminescent device, which are composite solutions prepared by dissolving hole transport materials such as aromatic diamine compounds and an electron acceptor such as tri(pentafluorophenyl)boron in a solvent that contains an ether solvent and / or an ester solvent whose water solubility at 25° C. is 1 weight % or less in the solvent, with a concentration of 10 weight % or higher in the compositions.
Owner:MITSUBISHI CHEM CORP
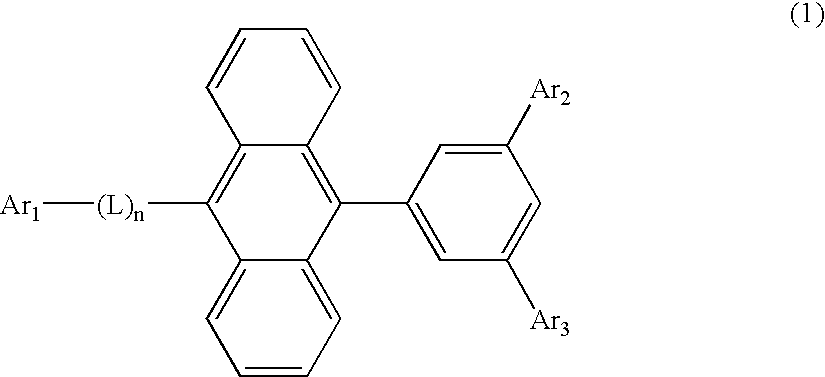
Organic-electroluminescence-material-containing solution, method for forming thin film of organic electroluminescence material, thin film of organic electroluminescence material and organic electroluminescence device
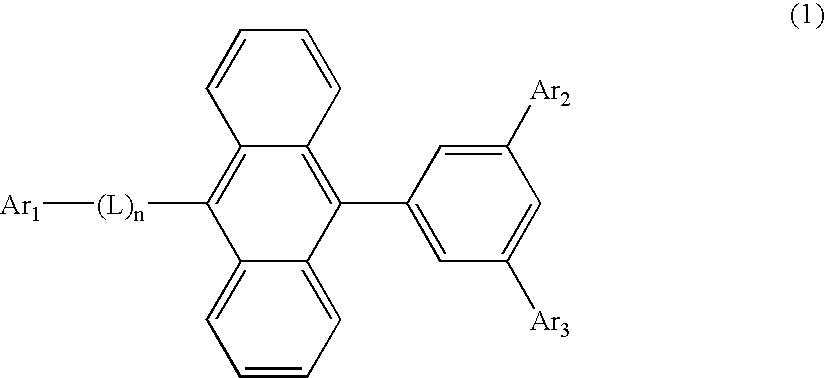
An organic EL material-containing solution contains an organic EL material, a solvent and a viscosity control agent. The organic EL material contains a host and a dopant.The host is a compound shown by Formula (1) below and has a solubility of 2 wt % or higher in the solvent. The solvent is an aromatic solvent, while the viscosity control agent is an alcohol type solution or an alkyl-substituted aromatic solution having 4 or more carbon atoms.
Owner:IDEMITSU KOSAN CO LTD
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
Polymer sacrificial light absorbing structure and method
InactiveUS6876017B2From solid stateSemiconductor/solid-state device detailsSolubilityLithographic artist
Method and structure for optimizing dual damascene patterning with polymeric dielectric materials are disclosed. Certain embodiments of the invention comprise polymeric sacrificial light absorbing materials (“polymer SLAM”) functionalized to have a controllable solubility switch wherein such polymeric materials have substantially the same etch rate as conventionally utilized polymeric dielectric materials, and subsequent to chemical modification of solubility-modifying protecting groups comprising the SLAM materials by thermal treatment or in-situ generation of an acid, such SLAM materials become soluble in weak bases, such as those conventionally utilized to remove materials in lithography treatments.
Owner:INTEL CORP
V-like domain binding molecules
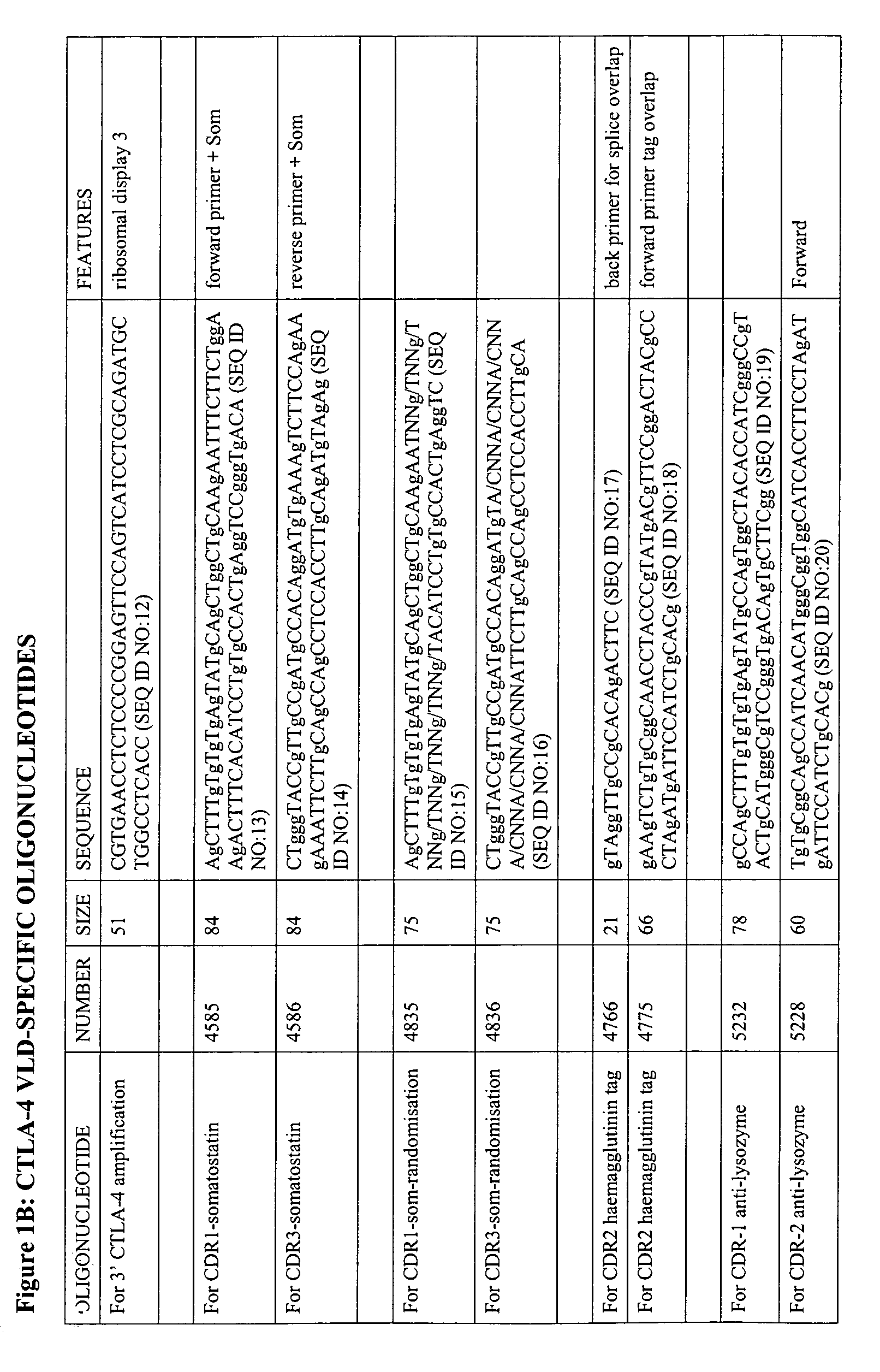
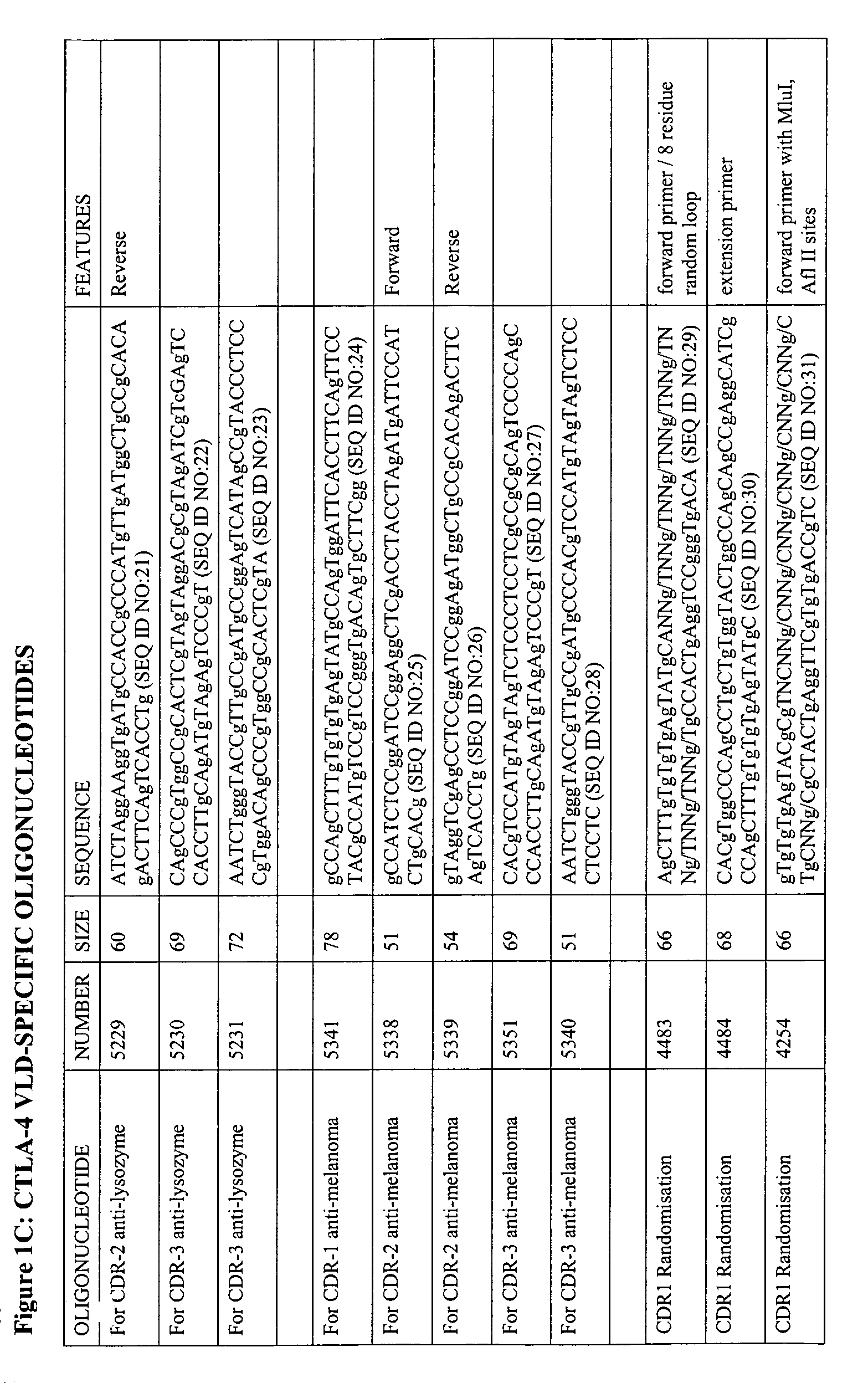
The present invention relates to binding moieties comprising at least one monomeric V-like domain (VLD) derived from a non-antibody ligand, the at least one monomeric V-like domain being characterized in that at least one CDR loop structure or part thereof is modified or replaced such that the solubility of the modified VLD is improved when compared with the unmodified VLD.
Owner:ARANA THERAPEUTICS VIC
High yield method of producing pure rebaudioside A
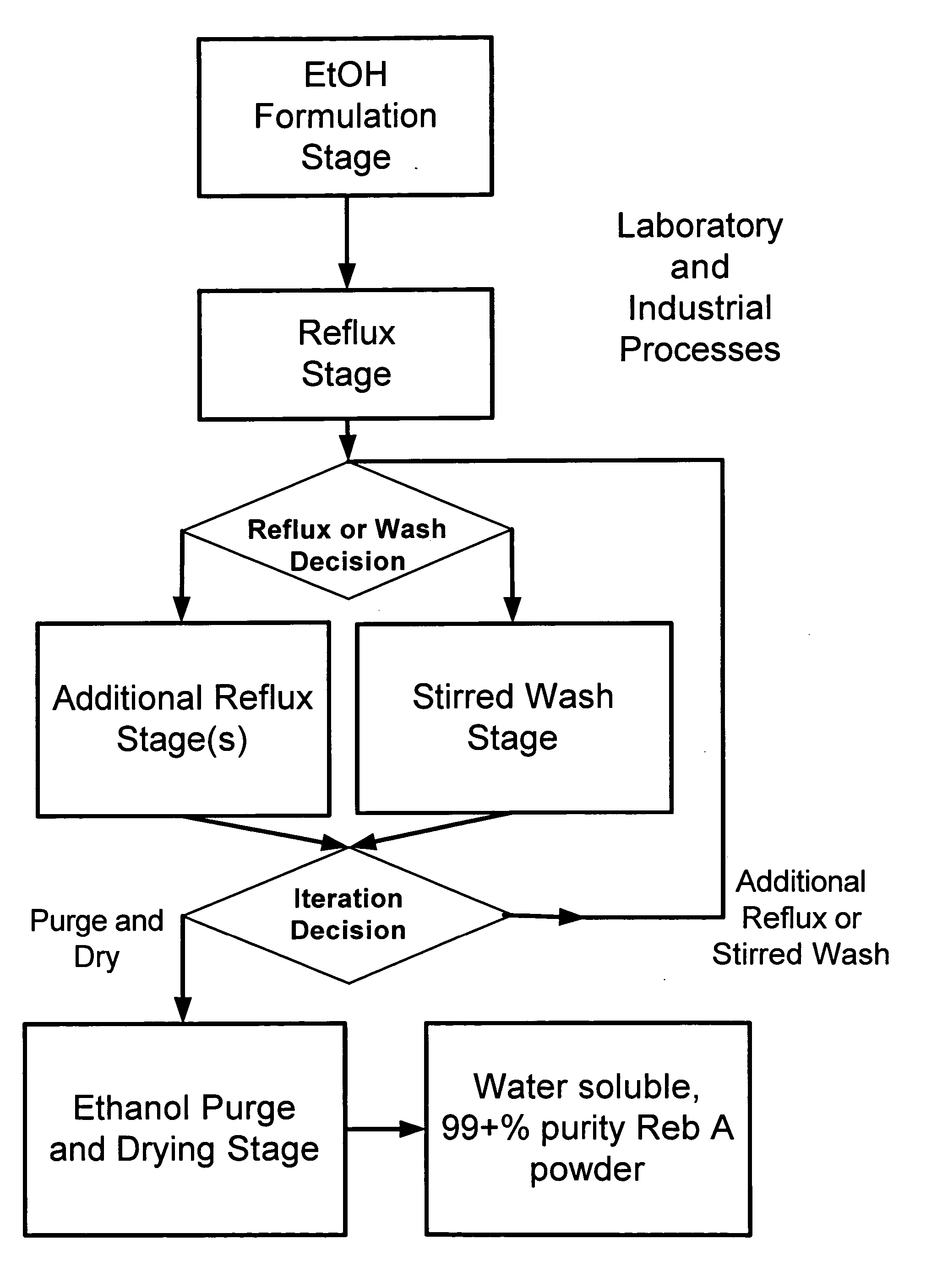
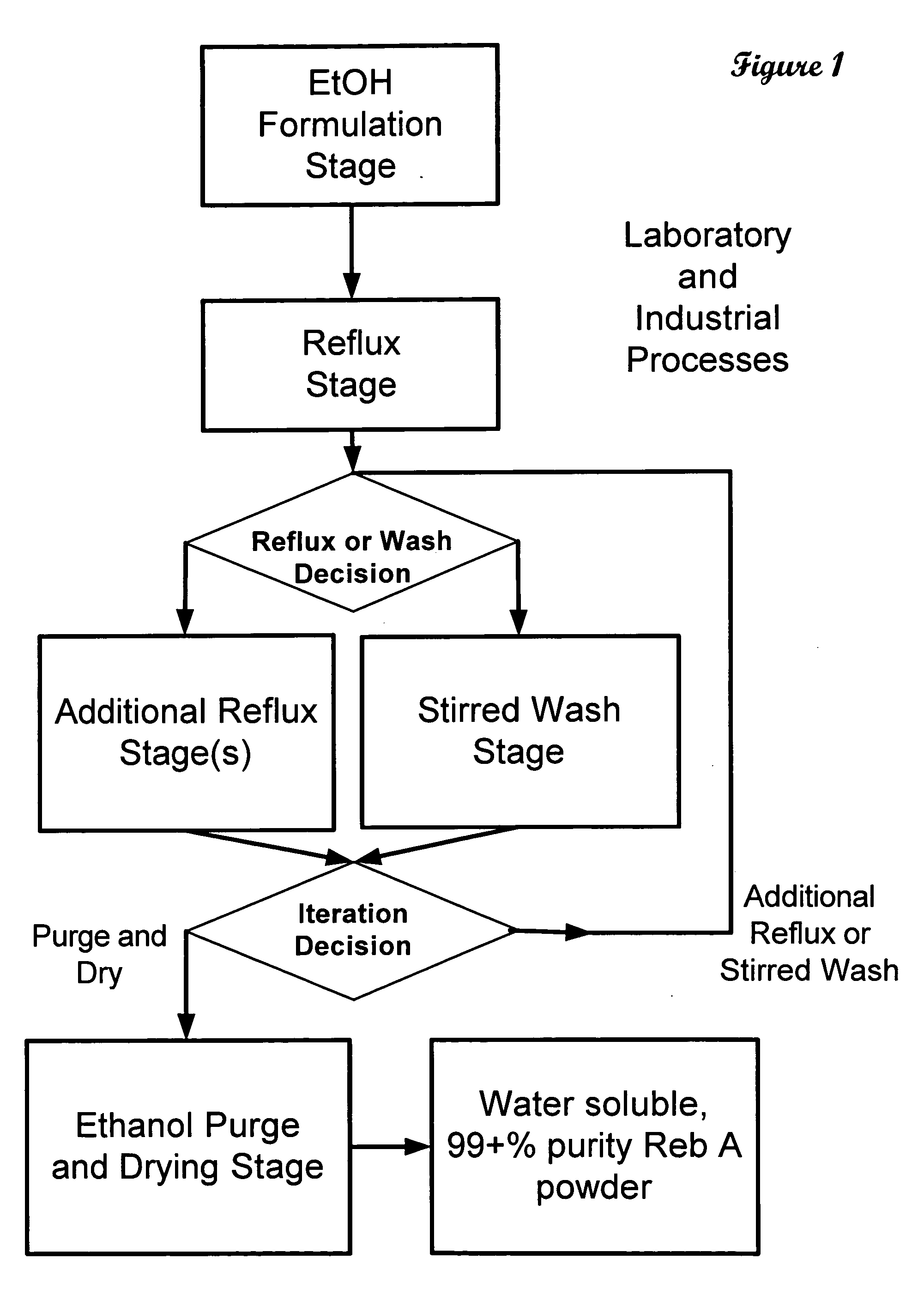
ActiveUS20060083838A1Reduction in yieldQuality improvementSugar derivativesMetabolism disorderSolubilityAdditive ingredient
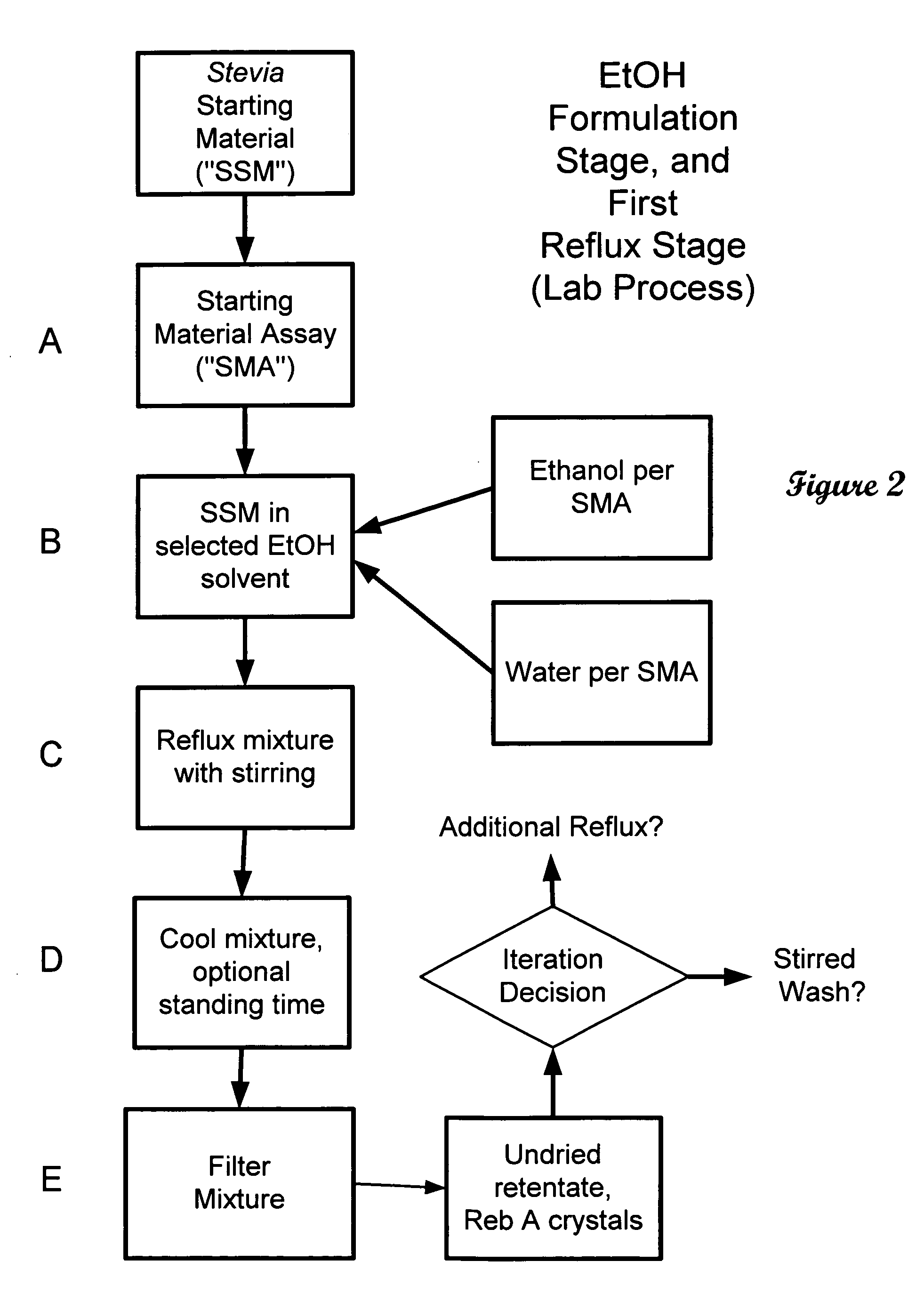
The invention provides a high throughput, high purity, high yield system and method of isolating and purifying rebaudioside A (“Reb A”), with acceptable water solubility for all commercial uses, from commercially available Stevia rebaudiana starting material. The invention also provides a means of maximizing yields of 99+% purity Reb A based on the attributes of a given batch of Stevia starting material. The Reb A produced by the invention is water soluble, devoid of bitterness heretofore associated with rebaudioside sweeteners, non-caloric, and suitable for use as a reagent and as an ingredient in orally consumed products, e.g., as a sweetener, flavor enhancer, and flavor modifier.
Owner:SWEET GREEN FIELDS INT CO LTD
Cleanup additive
InactiveUS6242390B1Low viscosityEasy to disassembleOther chemical processesFluid removalSolubilityHydraulic fracturing
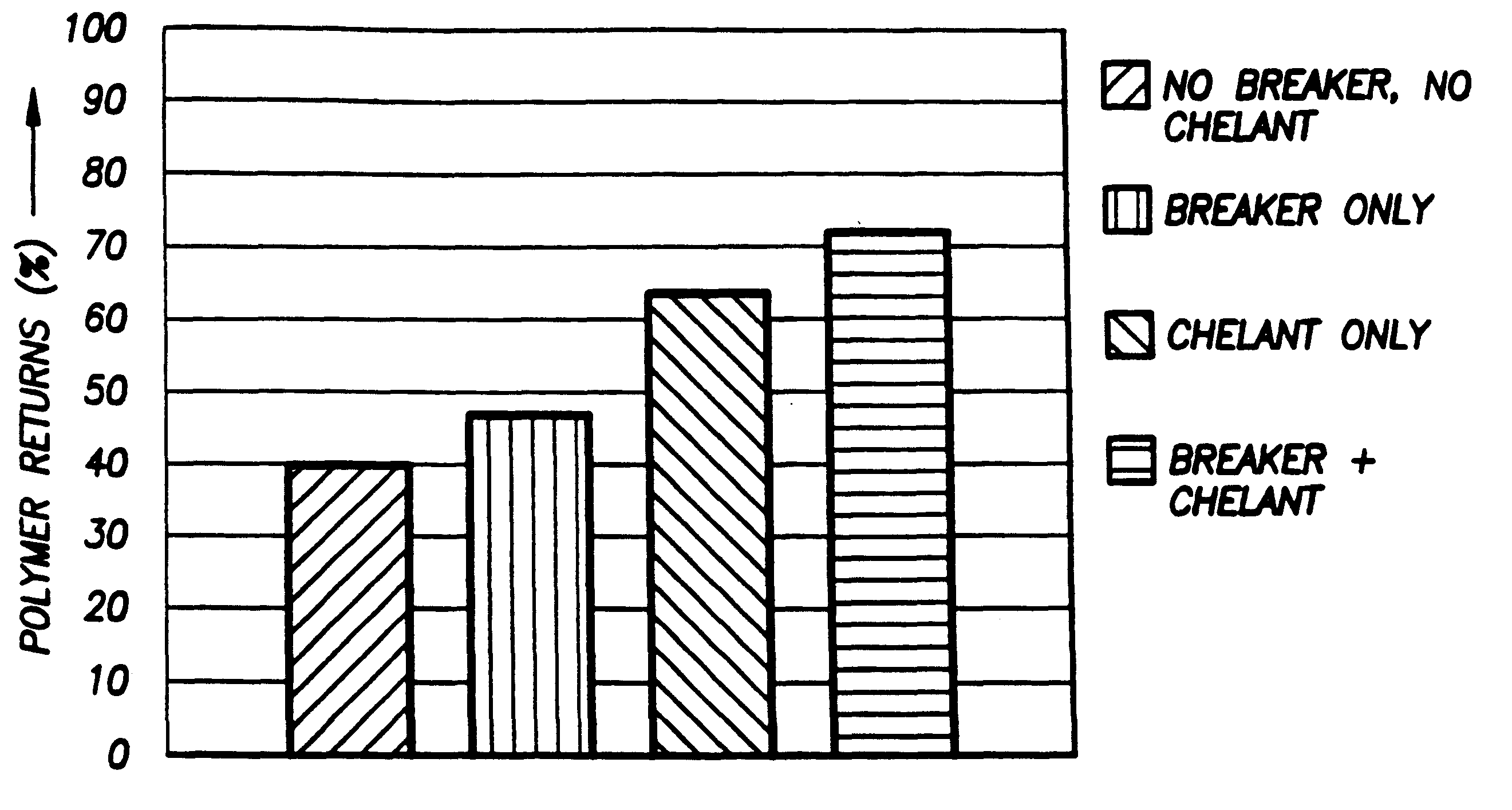
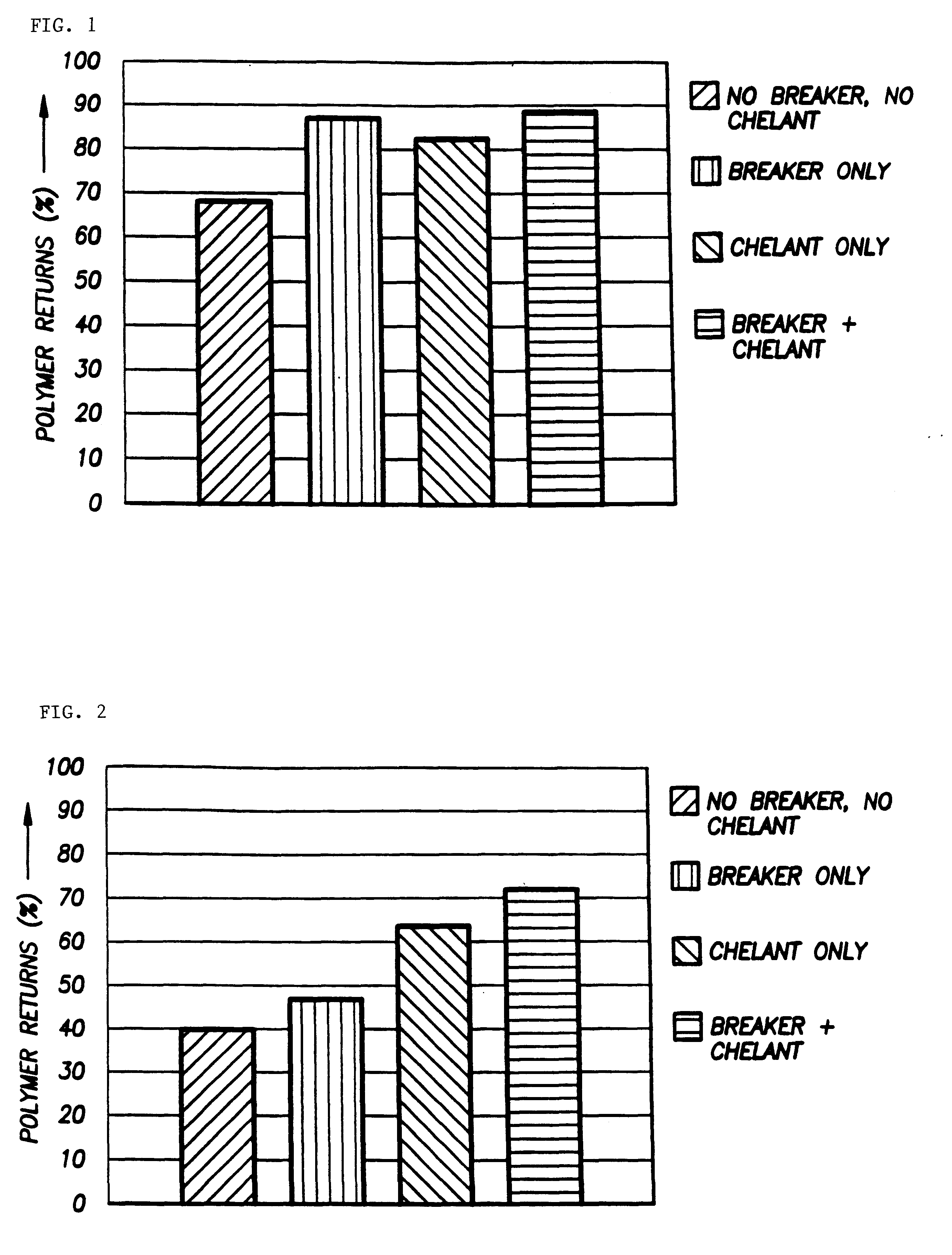
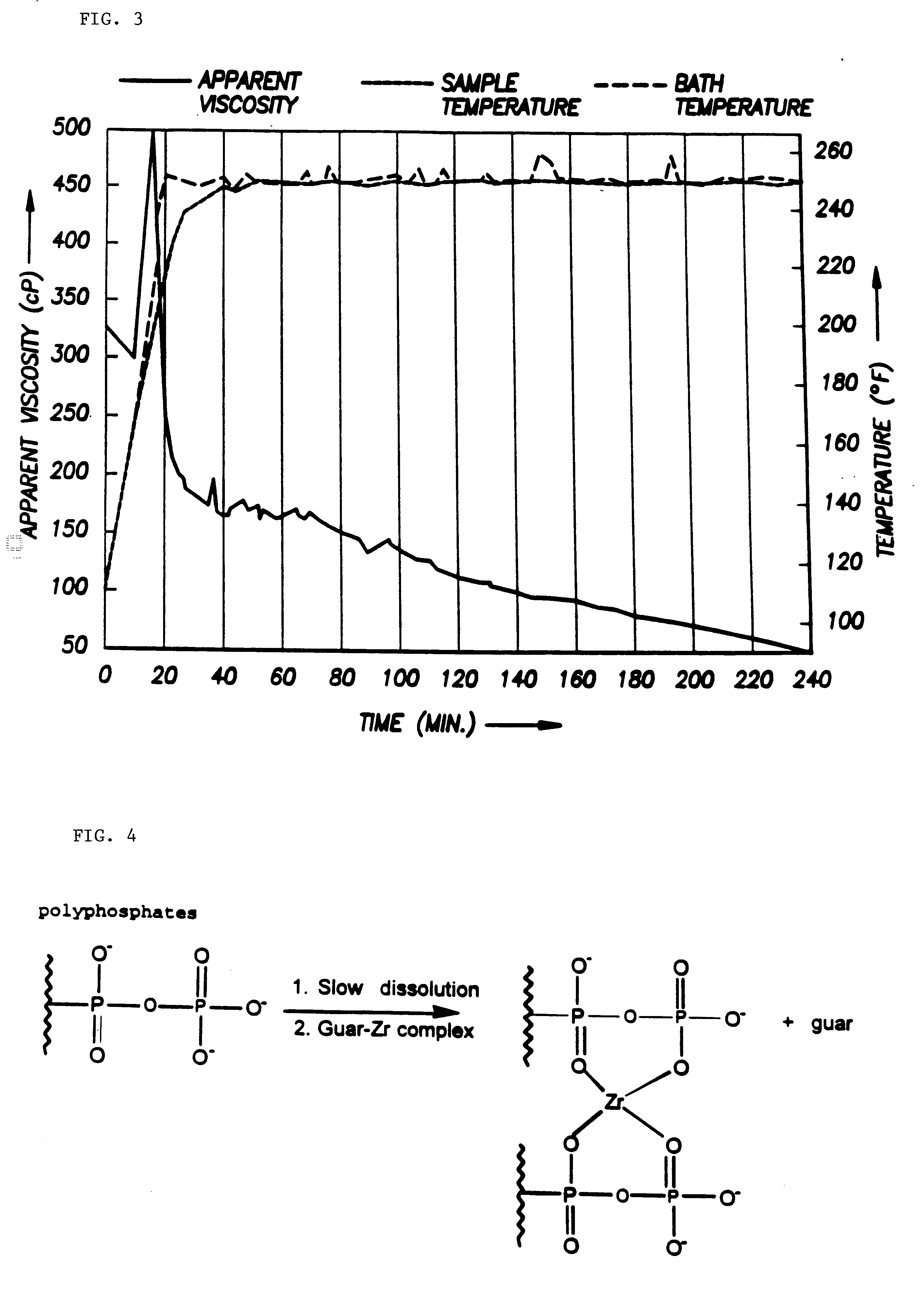
According to the present invention, a composition and method for hydraulically fracturing a subterranean formation is provided. The composition comprises an aqueous mixture of a hydrated polysaccharide, preferably a galactomannan gum, the hydrated polysaccharide having a plurality of bonding sites; a crosslinking agent for crosslinking the hydrated polysaccharide at the bonding sites at the conditions of the subterranean formation with a polyvalent metal ion to form a polyvalent metal crosslink, thereby increasing the viscosity of the hydrated polysaccharide; and a controlled solubility compound for releasing a chelating agent for controllably breaking the polyvalent metal crosslink and bonding with the polyvalent metal ion released by breaking the crosslink, thereby decreasing the viscosity of the hydrated polysaccharide. The method comprises the steps of injecting the above-described composition into the subterranean formation at fracturing pressures; allowing the controlled solubility compound to begin breaking the polyvalent metal crosslink, thereby reducing the viscosity of the hydrated polysaccharide and yielding a lower viscosity fluid; and removing the lower viscosity fluid from the subterranean formation.
Owner:SCHLUMBERGER TECH CORP
Novel drug delivery system
InactiveUS20060018933A1Effectively control release rateSmall sizePill deliveryAnhydride/acid/halide active ingredientsSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Controlled regional oral delivery
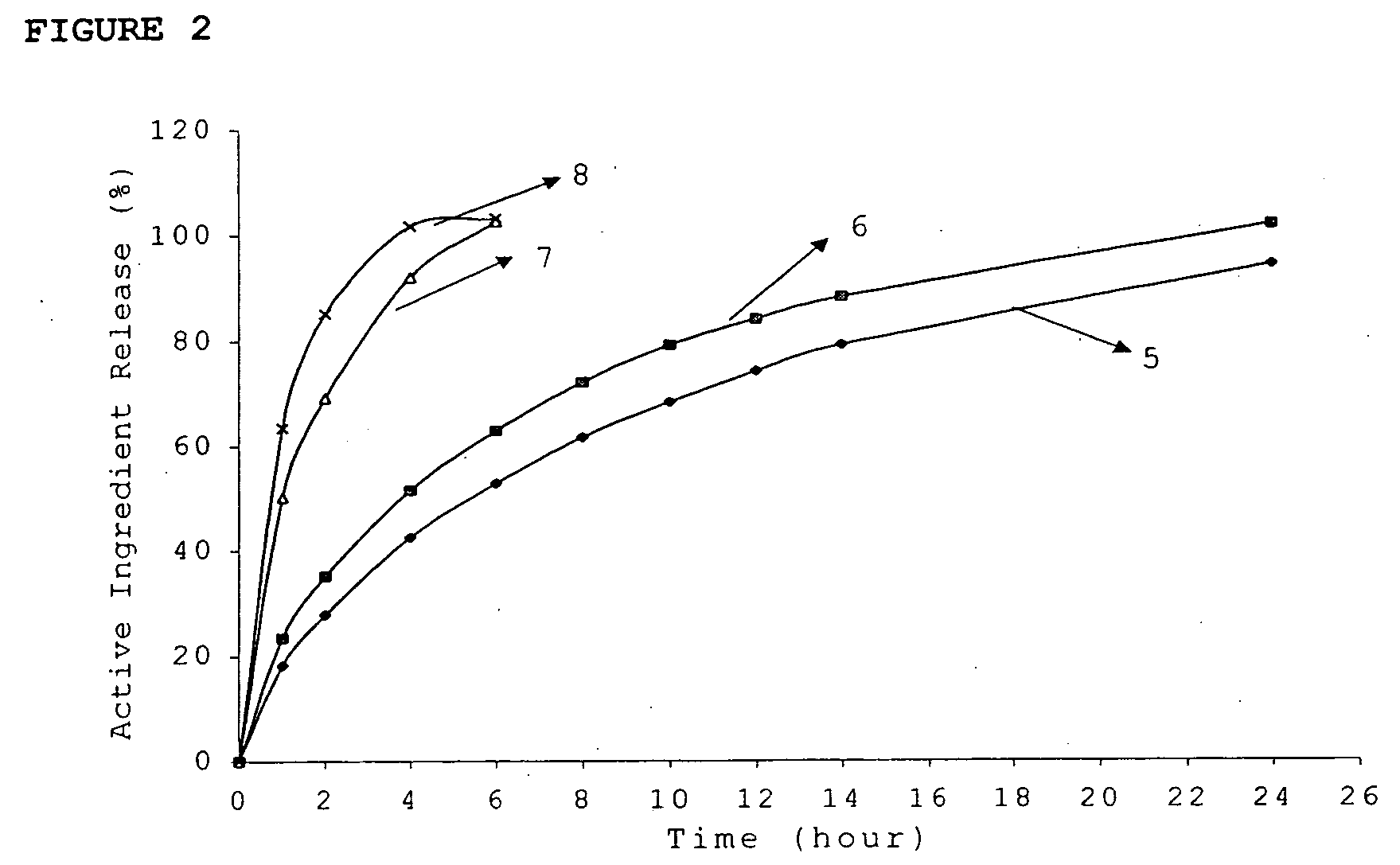
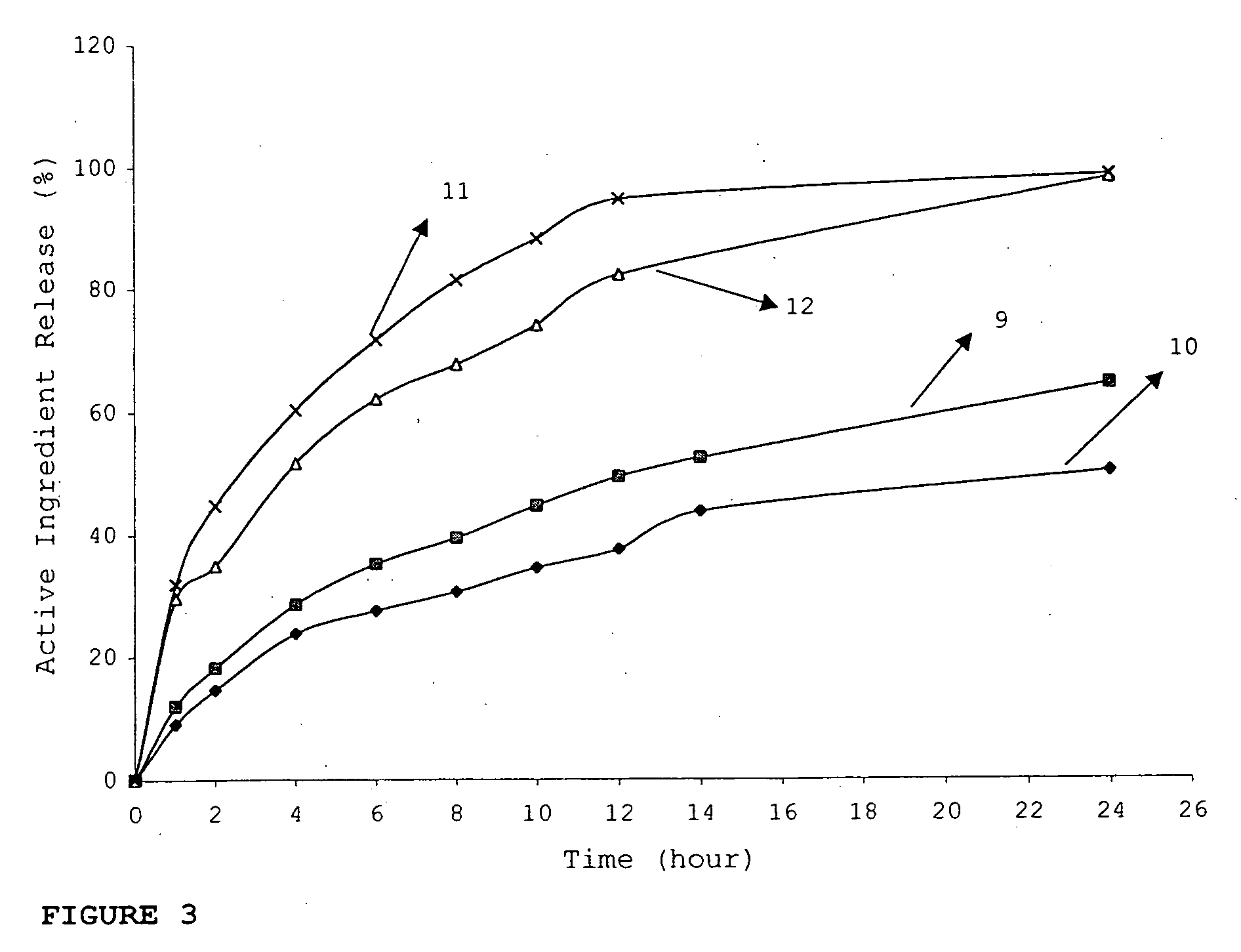
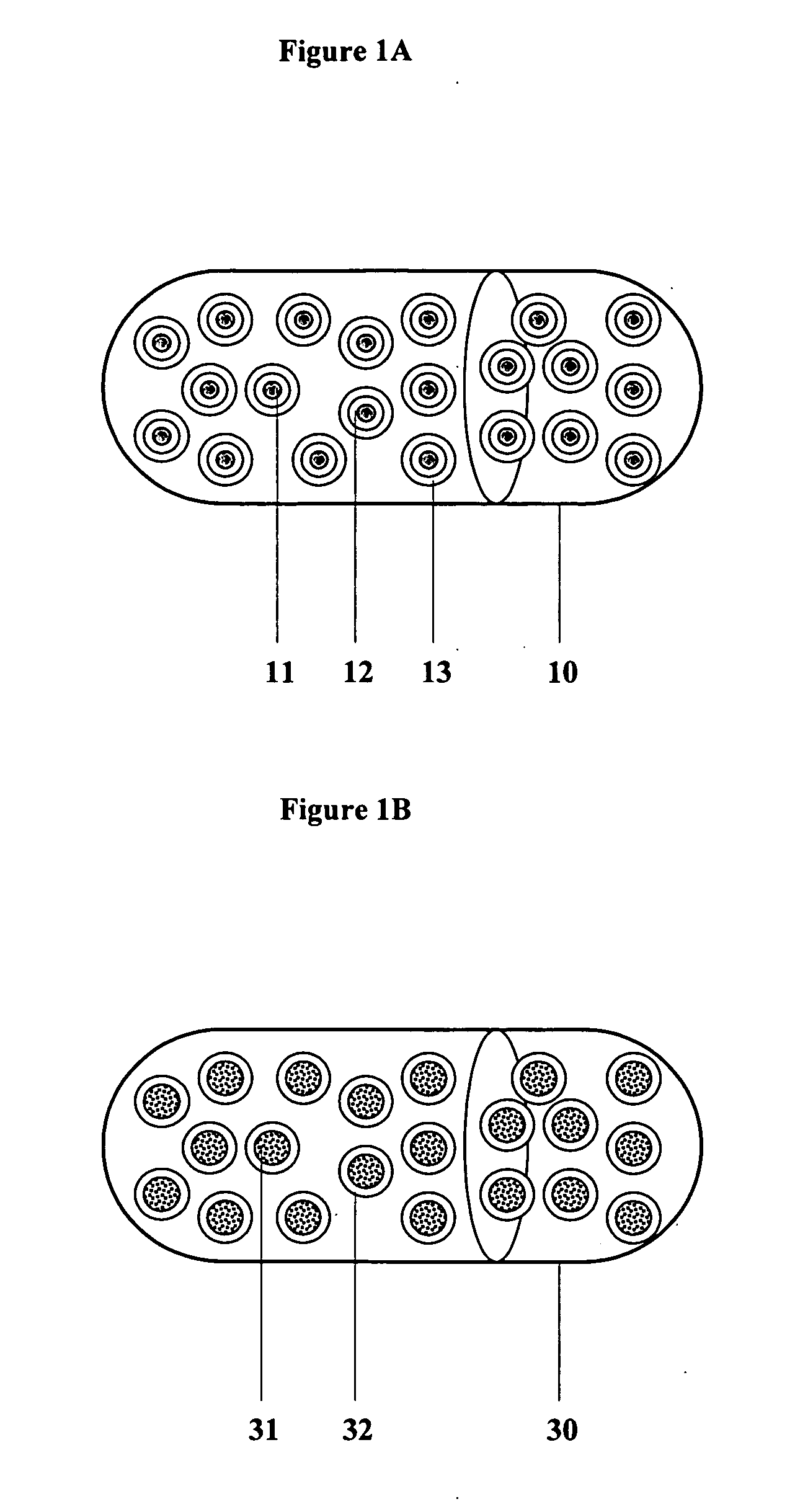
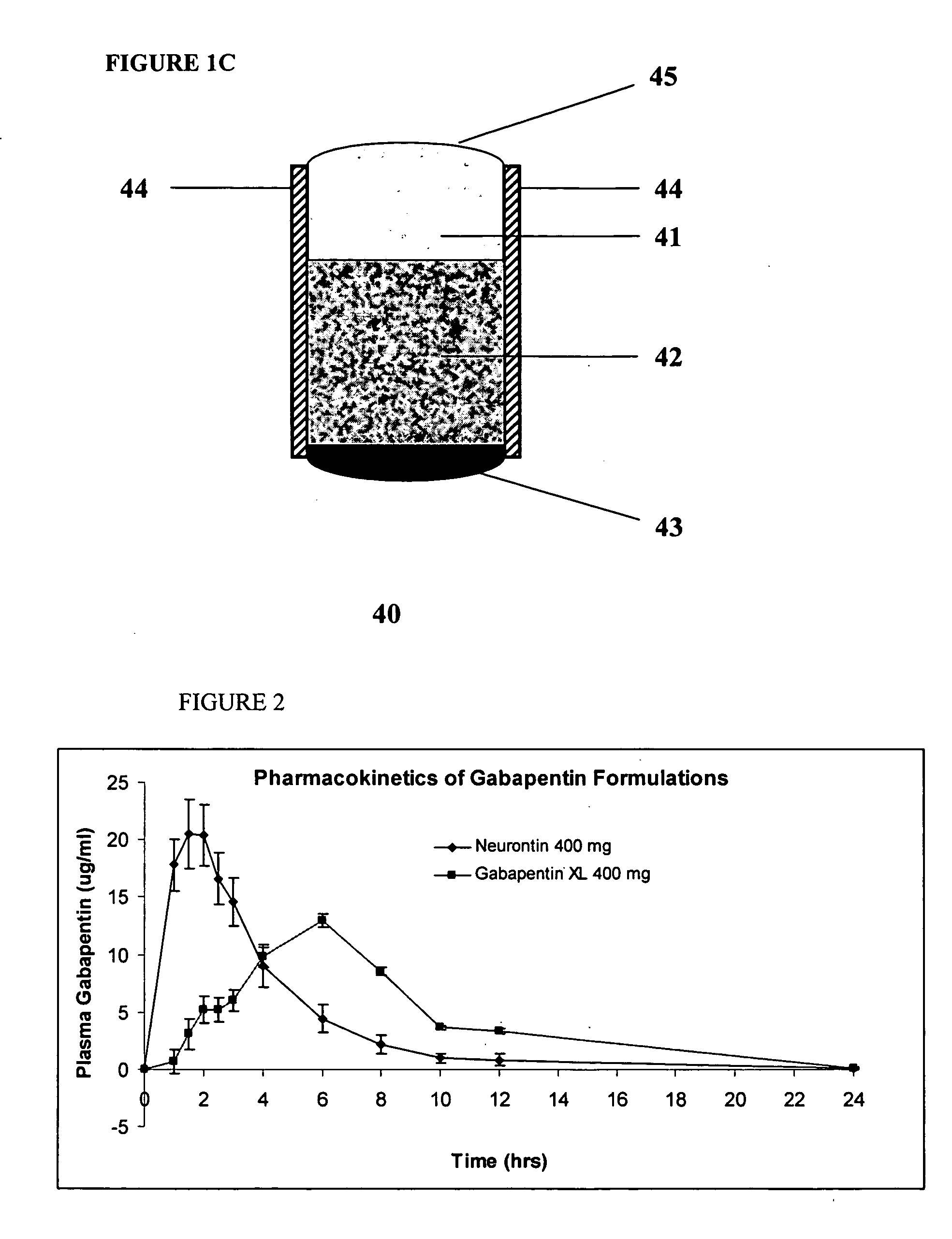
InactiveUS20060045865A1Significant variabilityLow variabilityPill deliveryGranular deliverySolubilityGabapentin
A composite formulation has been developed for selective, high efficacy delivery to specific regions of the mouth and gastrointestinal tract. The formulation is typically in the form of a tablet or capsule, which may include microparticles or beads. The formulation uses bioadhesive and controlled release elements to direct release to specific regions, where the drug is absorbed in enhanced amounts relative to the formulation in the absence of the bioadhesive and / or controlled release elements. This is demonstrated by an example showing delivery of gabapentin with a greater area under the curve (“AUC”) relative to the FDA reference immediate release drug, i.e., the AUC of the composite bioadhesive formulation is greater than 100% of the AUC of the immediate release drug. In the preferred embodiments, the formulation includes drug to be delivered, controlled release elements, and one or more bioadhesive elements. The bioadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. The controlled release elements are selected to determine the site of release. The bioadhesive components are selected to provide retention of the formulation at the desired site of uptake and administration. By selecting for both release and retention at a specific site, typically based on time of transit through the gastrointestinal tract, one obtains enhanced efficacy of uptake of the drug. This is particularly useful for drugs with narrow windows of absorption, and drugs with poor solubility such as the BCE class III and class IV drugs.
Owner:VAUNNEX
Glucagon/glp-1 receptor co-agonists
InactiveUS20100190701A1High activityEnhanced biophysical stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsSolubilityCarboxylic acid
Modified glucagon peptides are disclosed having enhanced potency at the glucagon receptor relative to native glucagon. Further modification of the glucagon peptides by forming lactam bridges or the substitution of the terminal carboxylic acid with an amide group produces peptides exhibiting glucagon / GLP-1 receptor co-agonist activity. The solubility and stability of these high potency glucagon analogs can be further improved by modification of the polypeptides by pegylation, substitution of carboxy terminal amino acids, or the addition of a carboxy terminal peptide selected from the group consisting of SEQ ID NO: 26 (GPSSGAPPPS), SEQ ID NO: 27 (K-RNRNNIA) and SEQ ID NO: 28 (KRNR).
Owner:INDIANA UNIV RES & TECH CORP
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
Well Operating Elements Comprising a Soluble Component and Methods of Use
Well operating elements are described, one embodiment comprising a first component that is substantially non-dissolvable when exposed to a selected wellbore environment and a second component that is soluble in the selected wellbore environment and whose rate and / or location of dissolution is at least partially controlled by structure of the first component. A second embodiment includes the component that is soluble in the selected wellbore environment, and one or more exposure holes or passages in the soluble component to control its solubility. The second embodiment may or may not include a substantially non-dissolvable component. Methods of using the well operating elements in oilfield operations are also described. This abstract allows a searcher or other reader to quickly ascertain the subject matter of the disclosure. It will not be used to interpret or limit the scope or meaning of the claims.
Owner:SCHLUMBERGER TECH CORP
High-throughput formation, identification, and analysis of diverse solid-forms
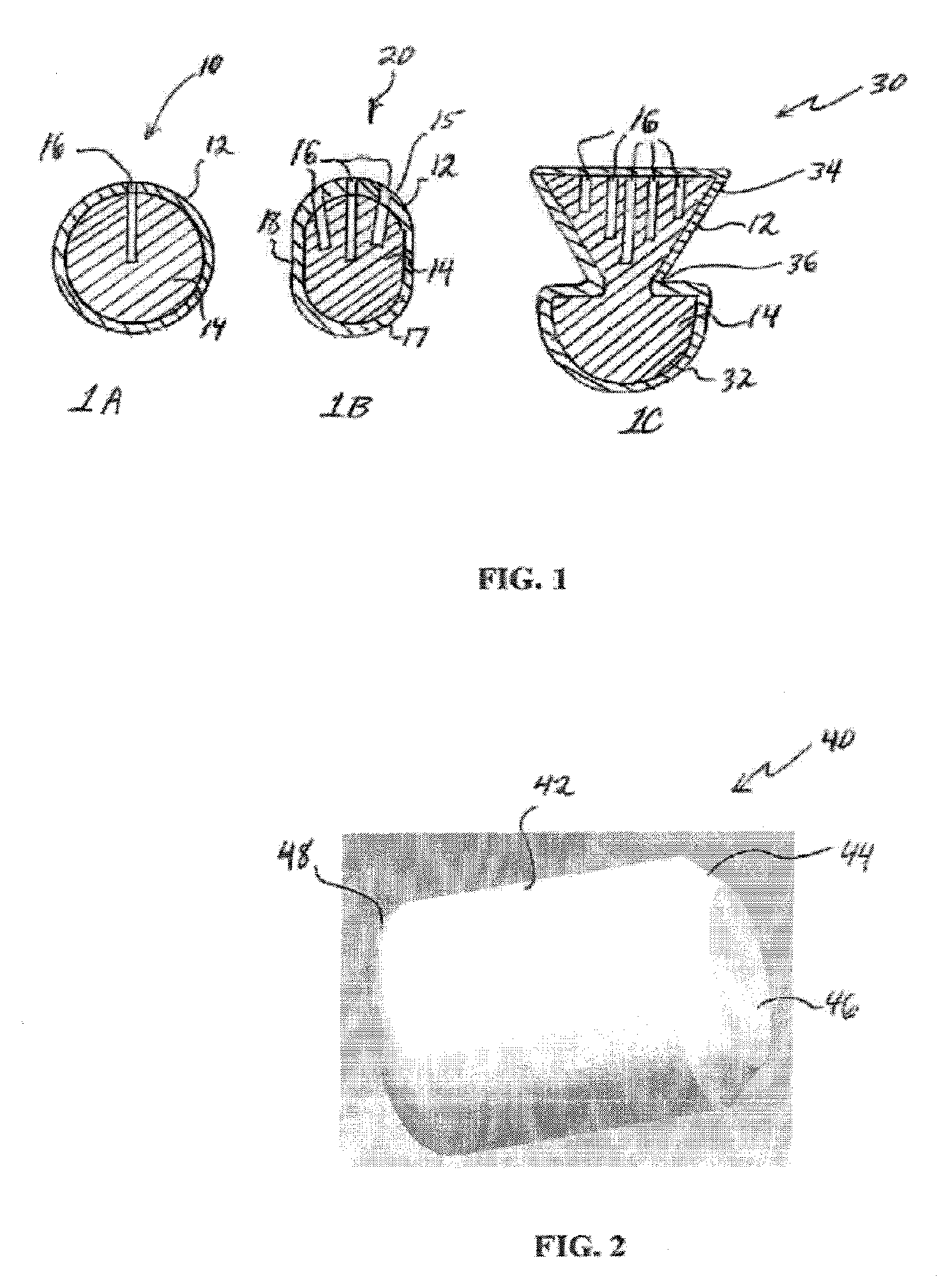
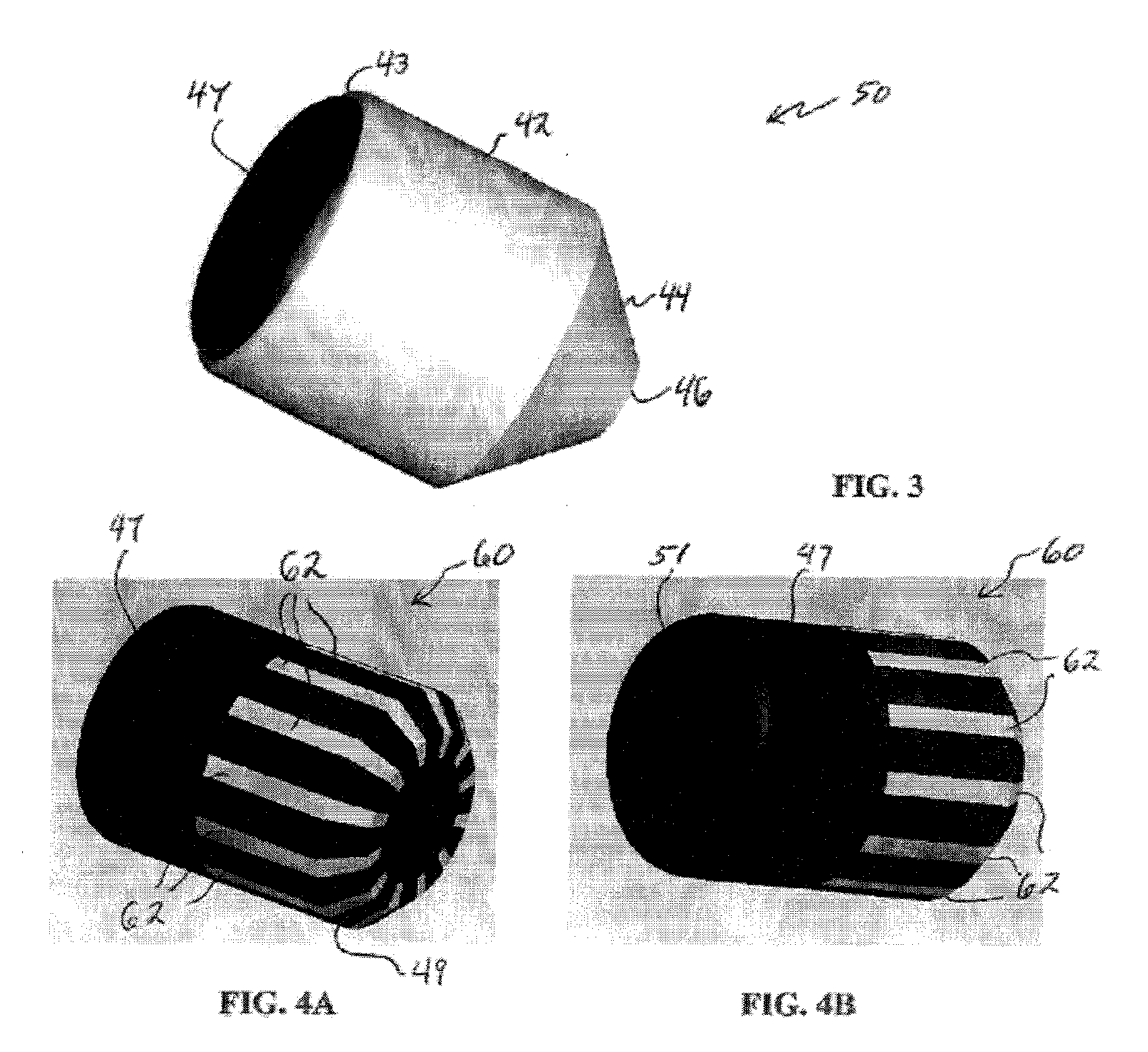
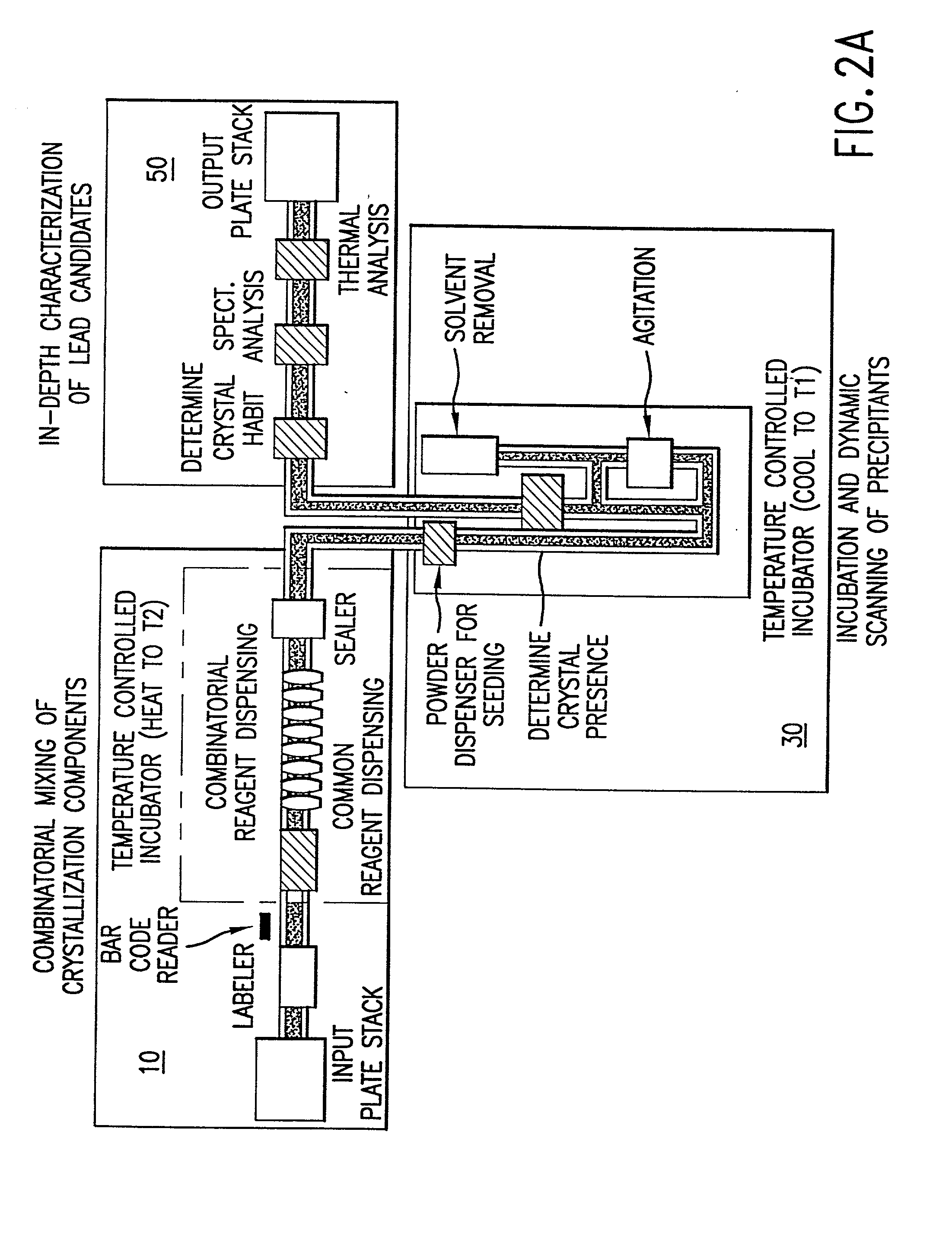
InactiveUS20020048610A1Cost-effectiveImprove bioavailabilitySequential/parallel process reactionsFrom normal temperature solutionsSolubilitySolid mass
The invention concerns arrays of solid-forms of substances, such as compounds and rapid-screening methods therefor to identify solid-forms, particularly of pharmaceuticals, with enhanced properties. Such properties include improved bioavailability, solubility, stability, delivery, and processing and manufacturing characteristics. The invention relates to a practical and cost-effective method to rapidly screen hundreds to thousands of samples in parallel. The invention further provides methods for determining the conditions and / or ranges of conditions required to produce crystals with desired compositions, particle sizes, habits, or polymorphic forms. In a further aspect, the invention provides high-throughput methods to identify sets of conditions and / or combinations of components compatible with particular solid-forms, for example, conditions and / or components that are compatible with advantageous polymorphs of a particular pharmaceutical.
Owner:MILLENNIUM PHARMA INC +1
Oilfield elements having controlled solubility and methods of use
ActiveUS8231947B2Ease of the element traversingEnvelopes/bags making machineryLayered productsSolubilityPolyamide
Oilfield elements are described, one embodiment comprising a combination of a normally insoluble metal with an element selected from a second metal, a semi-metallic material, and non-metallic materials; and one or more solubility-modified high strength and / or high-toughness polymeric materials selected from polyamides, polyethers, and liquid crystal polymers. Methods of using the oilfield elements in oilfield operations are also described. This abstract allows a searcher or other reader to quickly ascertain the subject matter of the disclosure. It will not be used to interpret or limit the scope or meaning of the claims.
Owner:SCHLUMBERGER TECH CORP
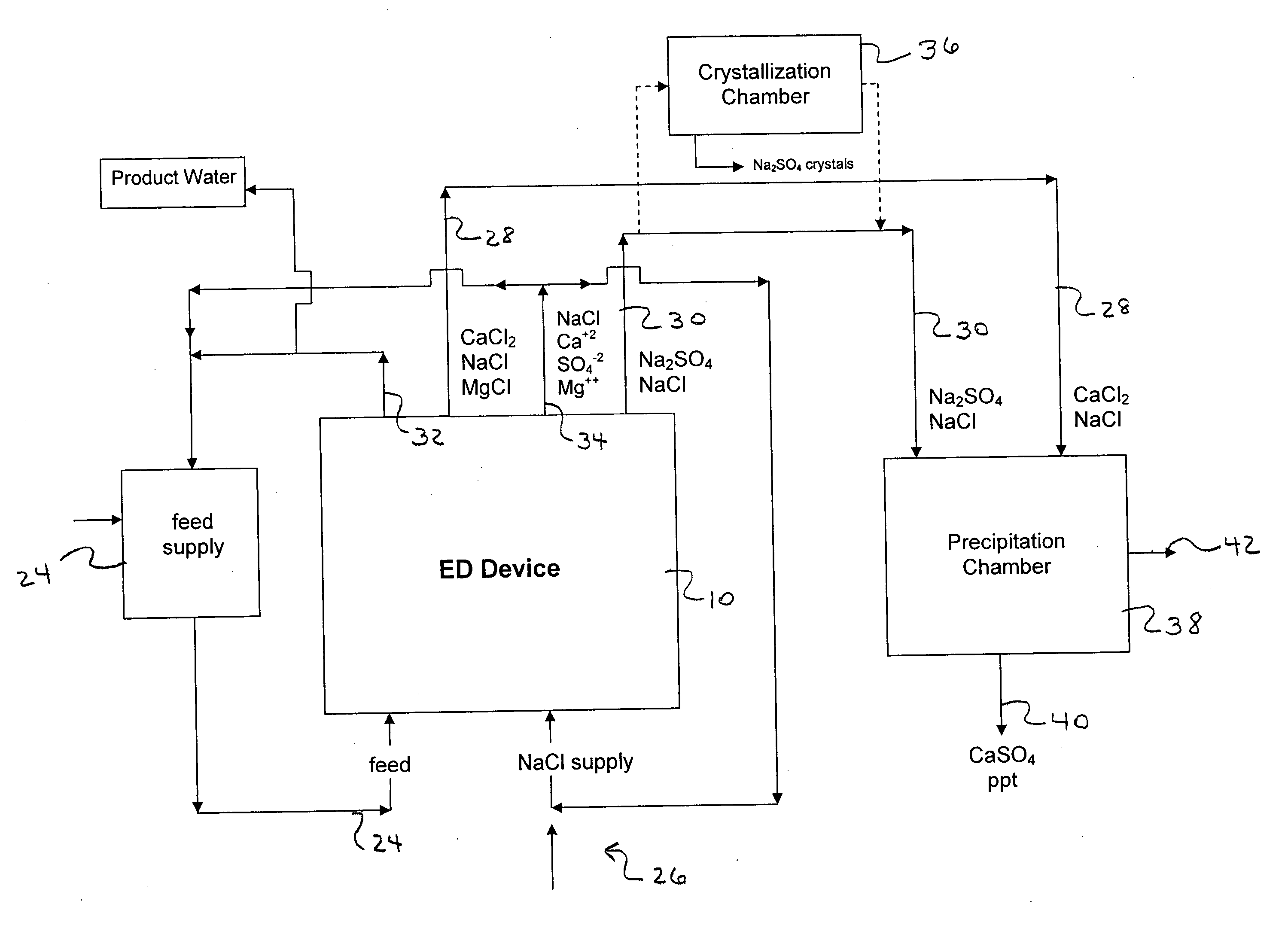
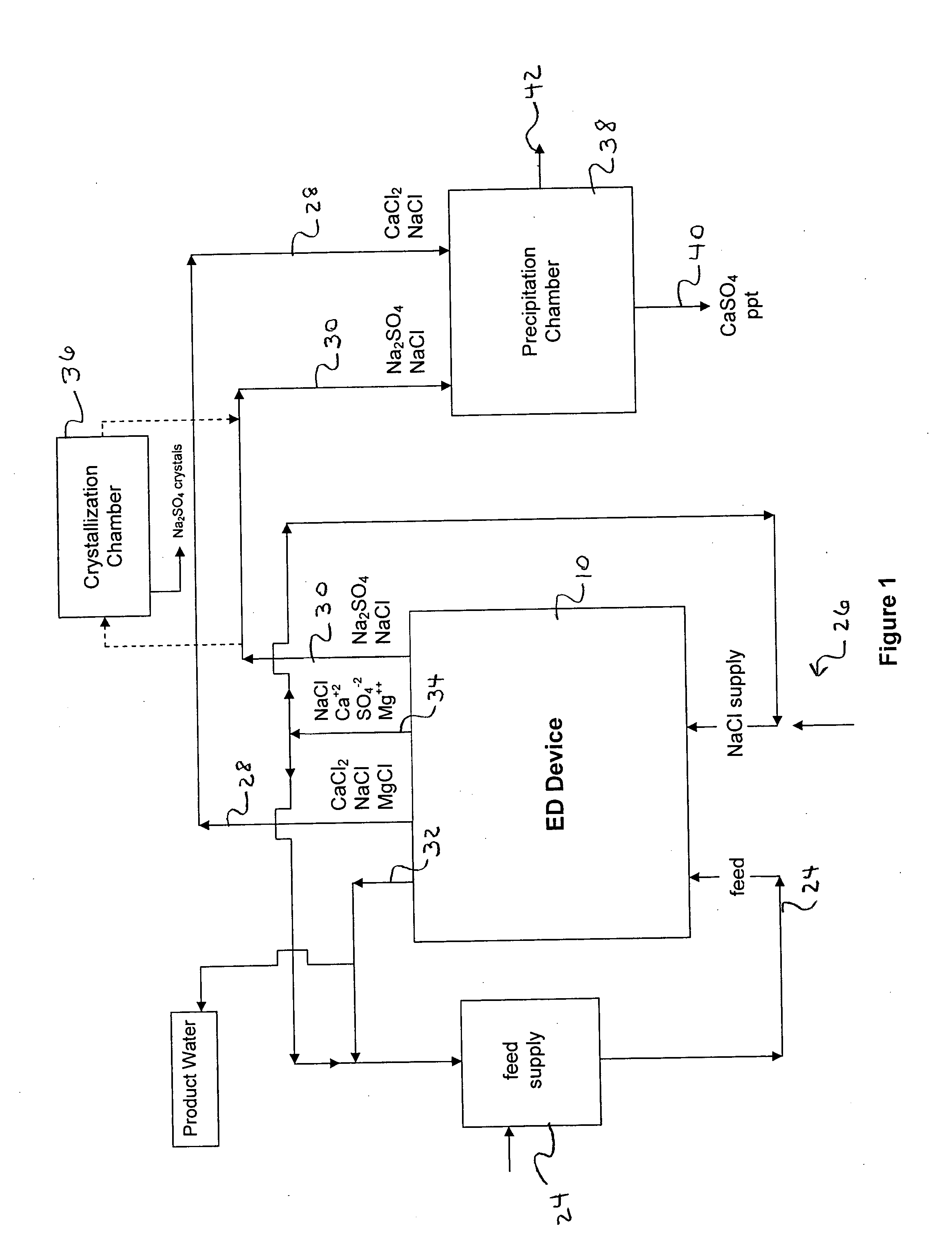
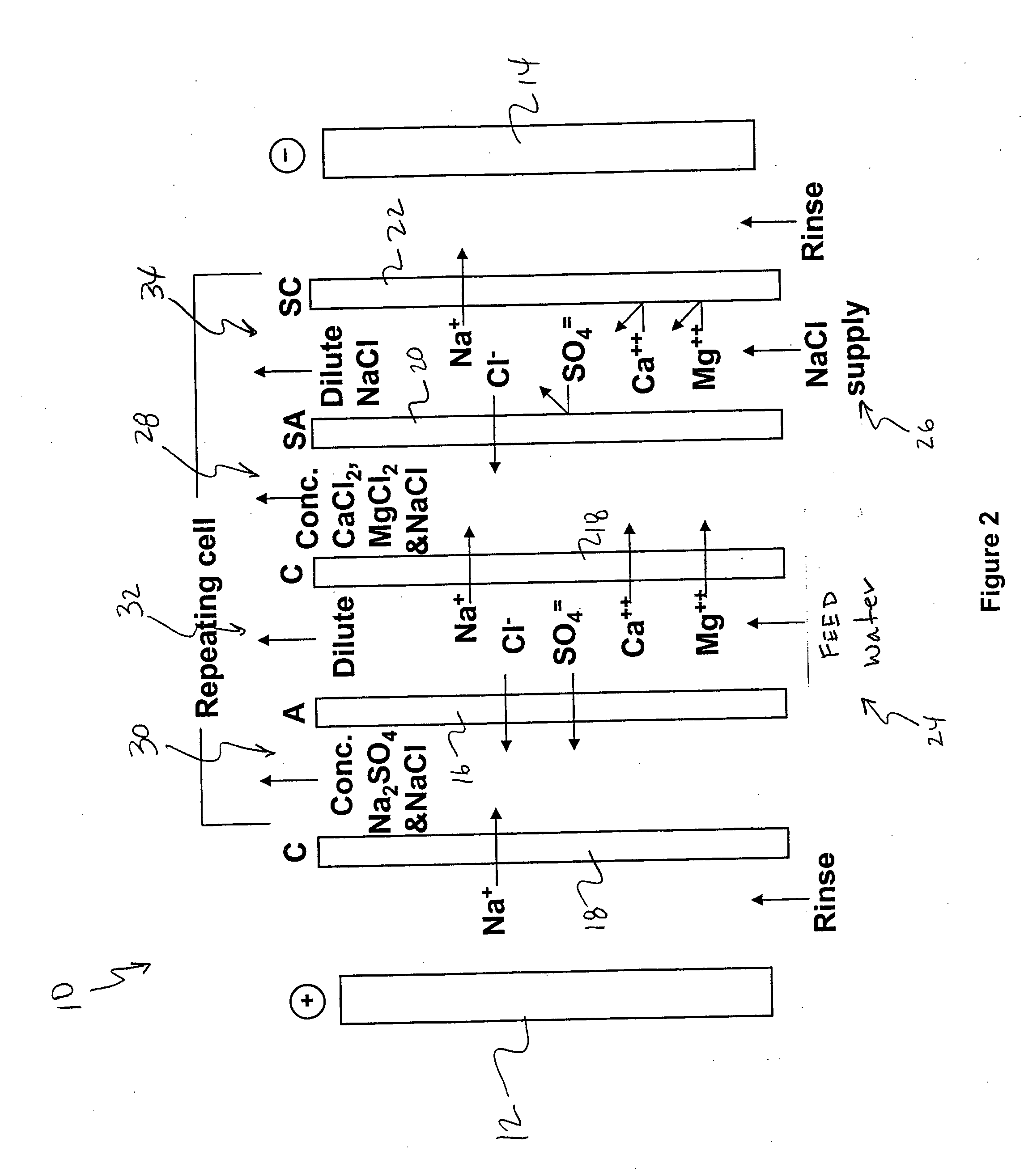
Water desalination process and apparatus
ActiveUS20060060532A1Electrolysis componentsGeneral water supply conservationSolubilityWater desalination
A process and system for purifying water is disclosed. For example, in one embodiment, the process may be used to remove a divalent salt, such as calcium sulfate, from a water source in order to prevent the divalent salt from precipitating during the process. The water source, for instance, may be fed to an ion separating device, such as an electrodialysis device. In the electrodialysis device, an ion exchange takes place between the divalent salt and another salt, such as a monovalent salt to produce two concentrated salt streams that contain salts having greater solubility in water than the divalent salt. In one embodiment, the two salt streams that are produced may then be combined to precipitate the divalent salt in a controlled manner. During the process, various other components contained within the water feed stream may also be removed from the stream and converted into useful products. In one particular embodiment, the process is configured to receive a byproduct stream from a reverse osmosis process.
Owner:SOUTH CAROLINA THE UNIV OF
Silicone based membranes for use in implantable glucose sensors
Membrane systems incorporating silicone polymers are described for use in implantable analyte sensors. Some layers of the membrane system may comprise a blend of a silicone polymer with a hydrophilic polymer, for example, a triblock poly(ethylene oxide) -poly(propylene oxide)-poly(ethylene oxide) polymer. Such polymeric blends provide for both high oxygen solubility and aqueous analyte solubility.
Owner:DEXCOM
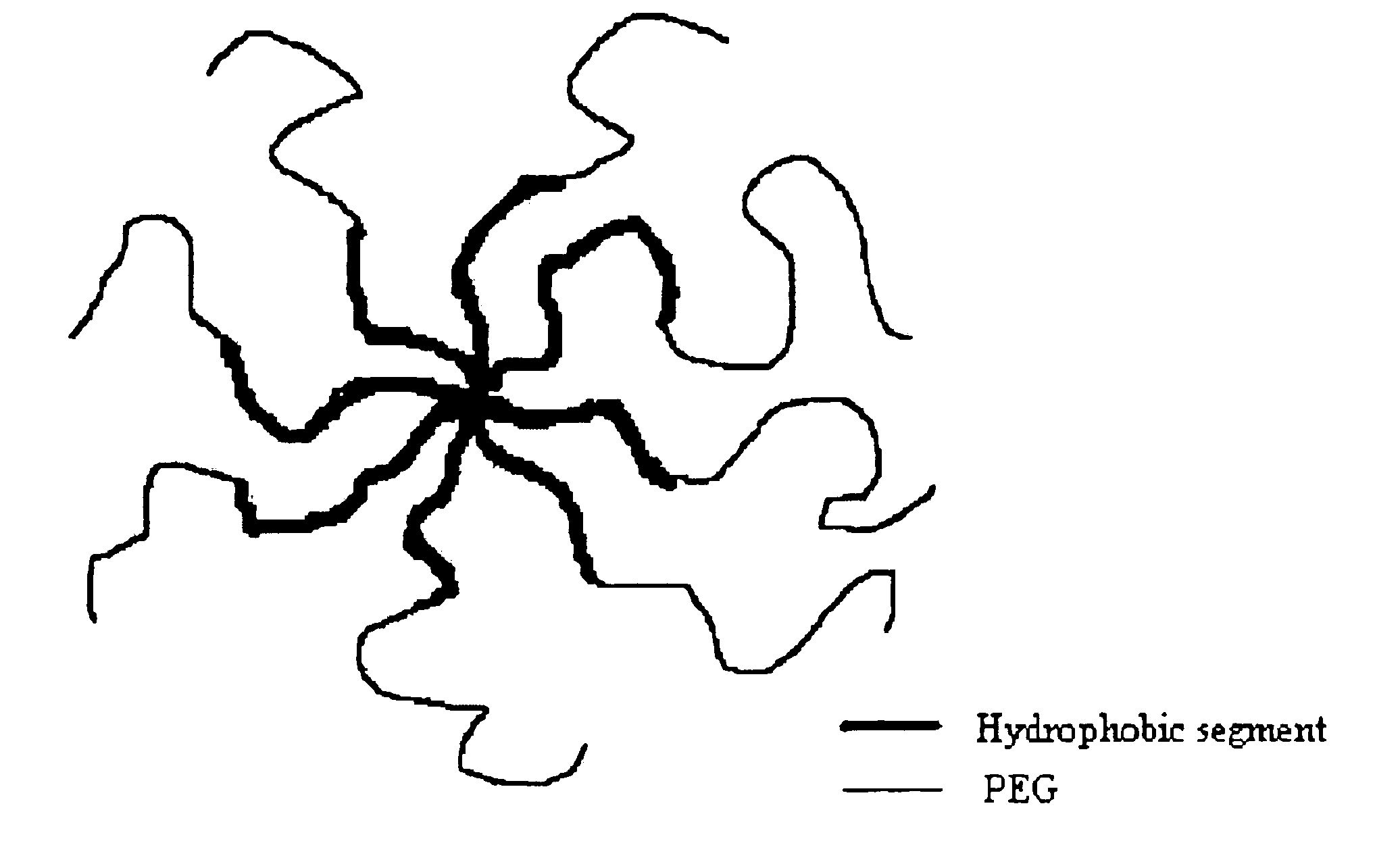
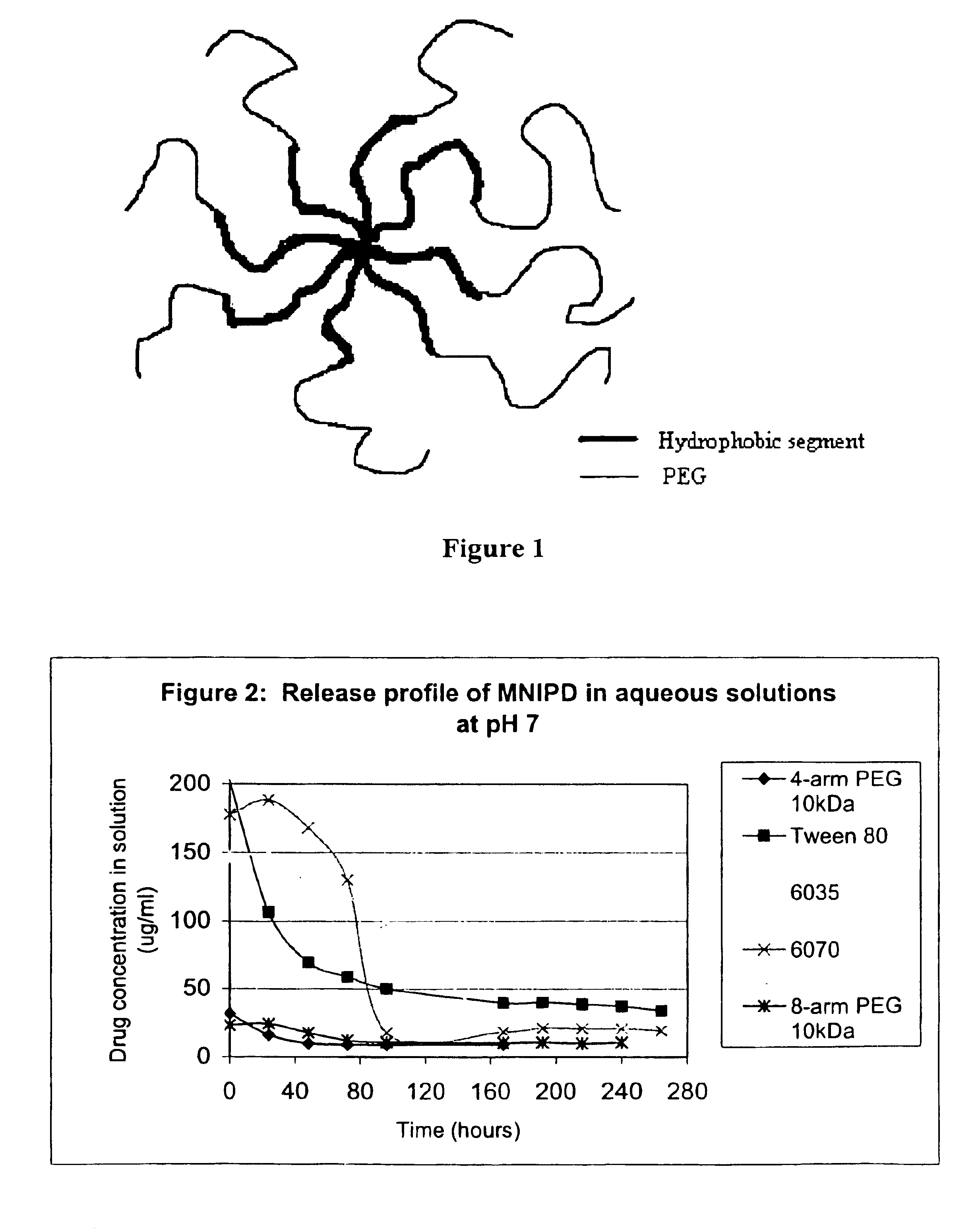
Multi-arm block copolymers as drug delivery vehicles
InactiveUS6838528B2Improve solubilityReduce deliveryPeptide/protein ingredientsNanomedicineSolubilityHydrophilic polymers
The invention provides multi-arm block copolymers useful as drug delivery vehicles comprising a central core molecule, such as a residue of a polyol, and at least three copolymer arms covalently attached to the central core molecule, each copolymer arm comprising an inner hydrophobic polymer segment covalently attached to the central core molecule and an outer hydrophilic polymer segment covalently attached to the hydrophobic polymer segment, wherein the central core molecule and the hydrophobic polymer segment define a hydrophobic core region. The solubility of hydrophobic biologically active agents can be improved by entrapment within the hydrophobic core region of the block copolymer. The invention further includes pharmaceutical compositions including such block copolymers, methods of making such copolymers and pharmaceutical compositions, and methods of using the block copolymers as drug delivery vehicles.
Owner:NEKTAR THERAPEUTICS INC
Heat and corrosion resistant cast CF8C stainless steel with improved high temperature strength and ductility
A CF8C type stainless steel alloy and articles formed therefrom containing about 18.0 weight percent to about 22.0 weight percent chromium and 11.0 weight percent to about 14.0 weight percent nickel; from about 0.05 weight percent to about 0.15 weight percent carbon; from about 2.0 weight percent to about 10.0 weight percent manganese; and from about 0.3 weight percent to about 1.5 weight percent niobium. The present alloys further include less than 0.15 weight percent sulfur which provides high temperature strength both in the matrix and at the grain boundaries without reducing ductility due to cracking along boundaries with continuous or nearly-continuous carbides. The disclosed alloys also have increased nitrogen solubility thereby enhancing strength at all temperatures because nitride precipitates or nitrogen porosity during casting are not observed. The solubility of nitrogen is dramatically enhanced by the presence of manganese, which also retains or improves the solubility of carbon thereby providing additional solid solution strengthening due to the presence of manganese and nitrogen, and combined carbon.
Owner:UT BATTELLE LLC
Adhesive peel-forming formulations for dermal delivery of drugs and methods of using the same
ActiveUS20050276842A1Easily peelableEasily removableOrganic active ingredientsAntimycoticsDrugSolubility
The present invention is drawn to adhesive peel-forming formulations for dermal delivery of a drug. The formulation can include a drug, a solvent vehicle, and a peel-forming agent. The solvent vehicle can include a volatile solvent system having one or more volatile solvent, and a non-volatile solvent system having one or more non-volatile solvent, wherein the non-volatile solvent system has a solubility for the drug that is within a window of operable solubility for the drug such that the drug can be delivered at therapeutically effective rates over a sustained period of time. The formulation can have a viscosity suitable for application to a skin surface prior to evaporation of the volatile solvents system. When applied to the skin, the formulation can form a solidified peelable layer after at least a portion of the volatile solvent system is evaporated.
Owner:CRESCITA THERAPEUTICS INC
Novel drug delivery system
InactiveUS20060018934A1Effectively control release rateSmall sizePill deliveryMicrocapsulesSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Resist composition for immersion exposure, method of forming resist pattern using the same, and fluorine-containing compound
ActiveUS20090197204A1Affect propertyImprove hydrophobicityOrganic chemistryOrganic compound preparationSolubilityResist
A resist composition for immersion exposure, including a base component (A) that exhibits changed solubility in an alkali developing solution under action of acid, an acid generator component (B) that generates acid upon exposure, and a fluorine-containing compound (C) represented by a general formula (c-1) shown below that is decomposable in an alkali developing solution:wherein R1 represents an organic group which may contain a polymerizable group, with the proviso that said polymerizable group has a carbon-carbon multiple bond, and the carbon atoms forming the multiple bond are not directly bonded to the carbon atom within the —C(═O)— group in general formula (c-1); and R2 represents an organic group having a fluorine atom.
Owner:TOKYO OHKA KOGYO CO LTD
Purinone derivative hydrochloride
ActiveUS20140330015A1Good metabolic stabilityImprove solubilityOrganic active ingredientsAntipyreticMast cellSolubility
The purinone derivative 6-amino-9-[(3R)-1-(2-butynoyl)-3-pyrrolidinyl]-7-(4-phenoxyphenyl)-7,9-dihydro-8H-purin-8-one hydrochloride has Btk-selective inhibitory activity and, in addition to having excellent metabolic stability, it is a compound that exhibits a high level of solubility and absorption with respect to the free base and can be crystallized, hence it can serve as a therapeutic agent for diseases involving B cells and mast cells.
Owner:ONO PHARMA CO LTD
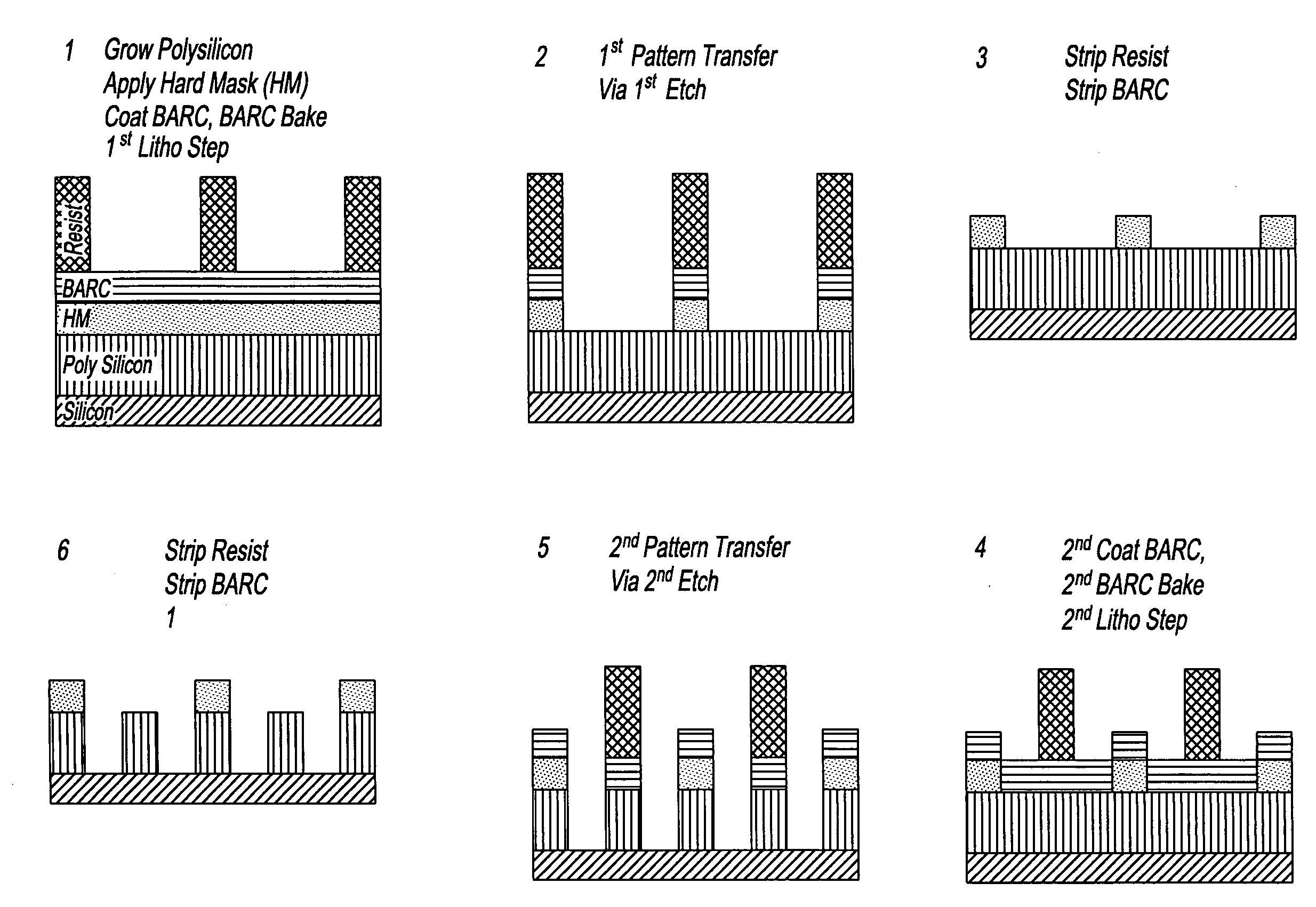
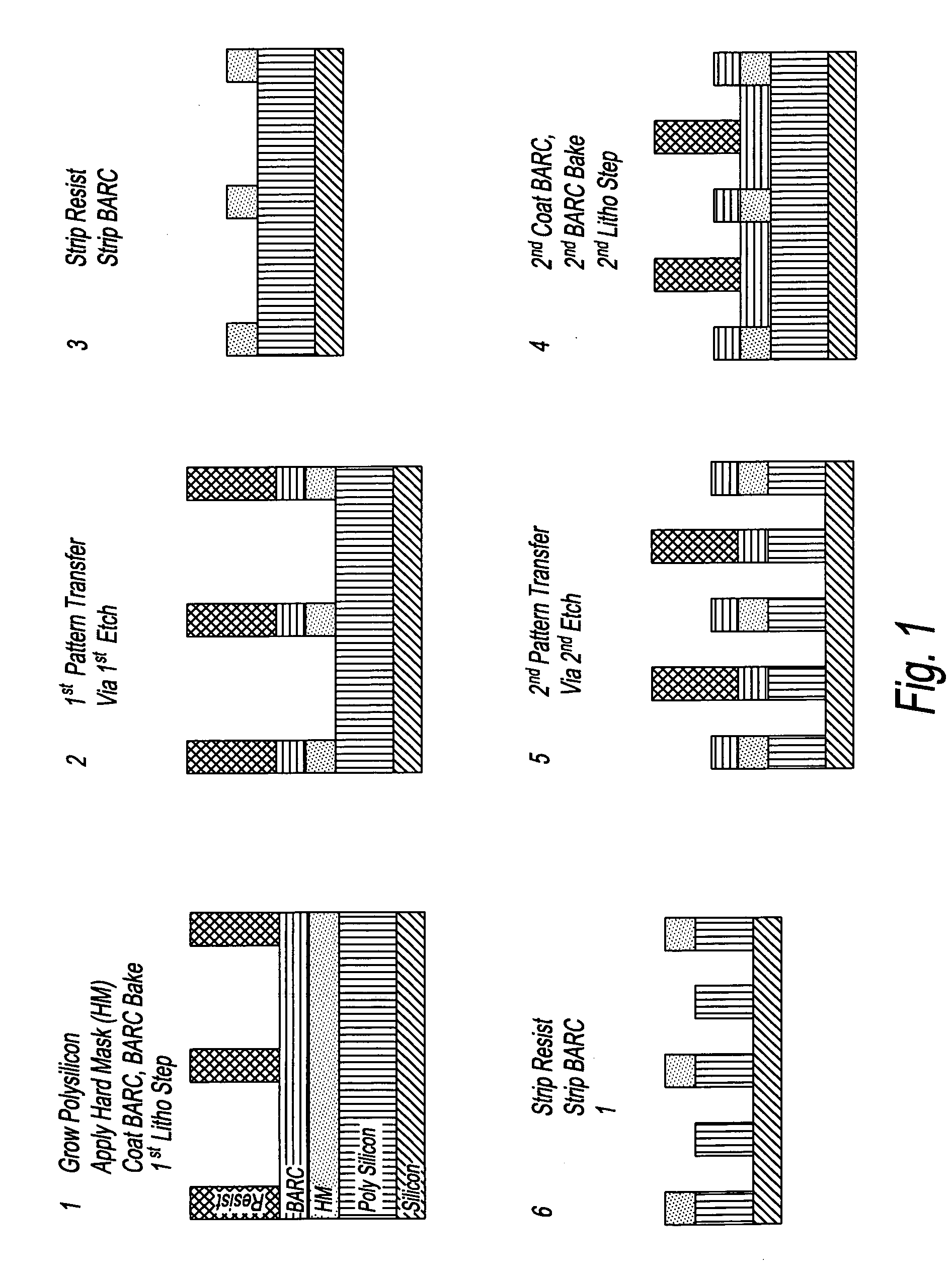
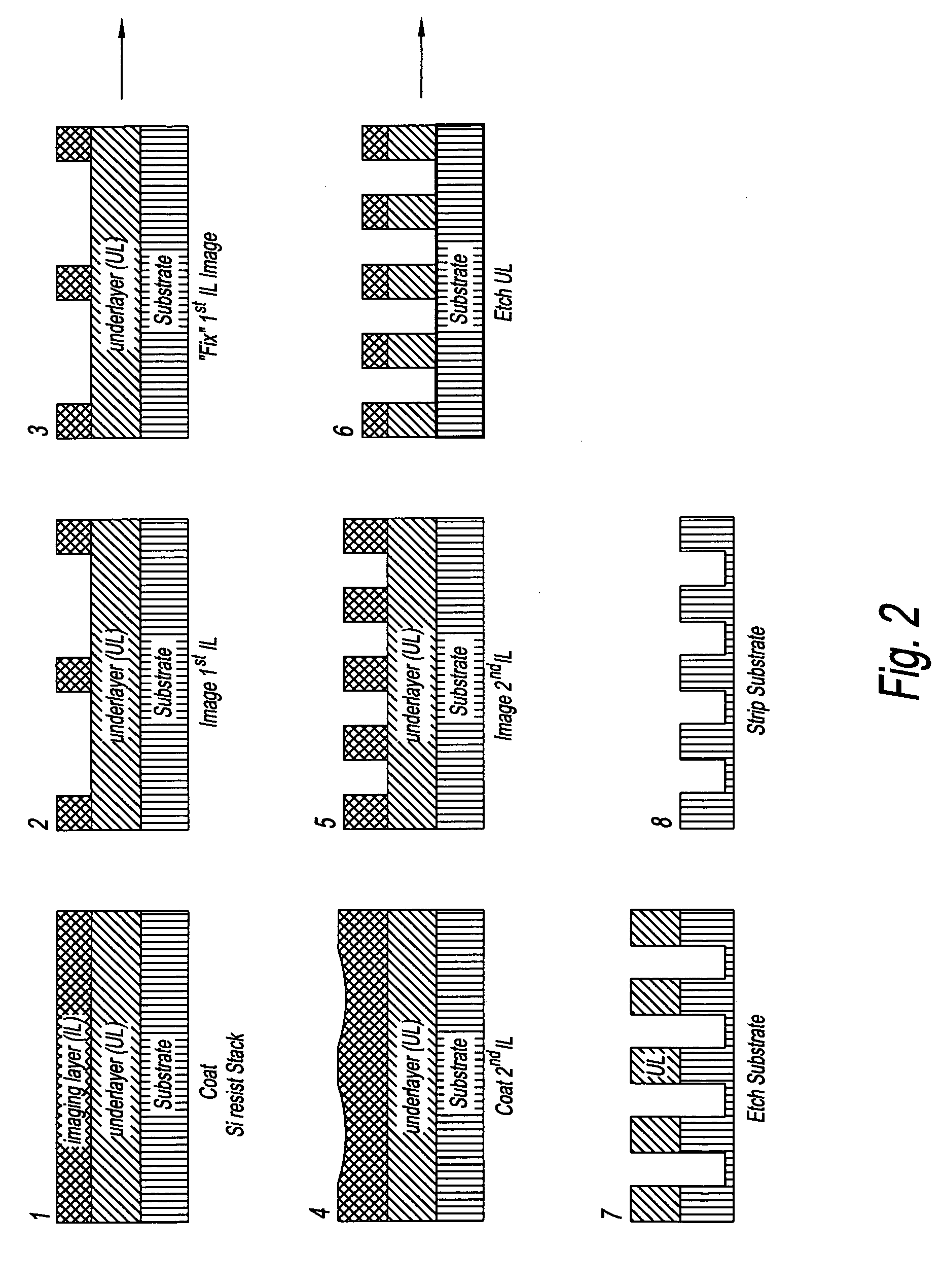
Device manufacturing process utilizing a double patterning process
InactiveUS20080199814A1Photomechanical apparatusSemiconductor/solid-state device manufacturingSolubilityAnti-reflective coating
Manufacturing semiconductor device by steps of:a) providing substrate with antireflective coating or underlayer,b) applying first photosensitive composition over substrate,c) exposing first composition to radiation to produce first pattern,d) developing exposed first composition to produce an imaged bilayer stack,e) rinsing the stack,f) applying fixer to the stack,g) applying optional bake,h) rinsing the stack,i) applying second optional bake,j) applying second photosensitive composition onto the stack to produce multilayer stack,k) exposing second composition to produce second pattern offset from first pattern,l) developing exposed second composition to produce multilayer stack, andm) rinsing multilayer stack;the photosensitive compositions have photoacid generator and substantially aqueous base insoluble polymer whose solubility increases upon treatment with acid and further comprises an anchor group, and the fixer is a polyfunctional compound reactive with anchor group, but does not contain silicon and the substrate stays within a lithographic cell from at least first coating step until at least after final exposure.
Owner:FUJIFILM ELECTRONICS MATERIALS US
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com