Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
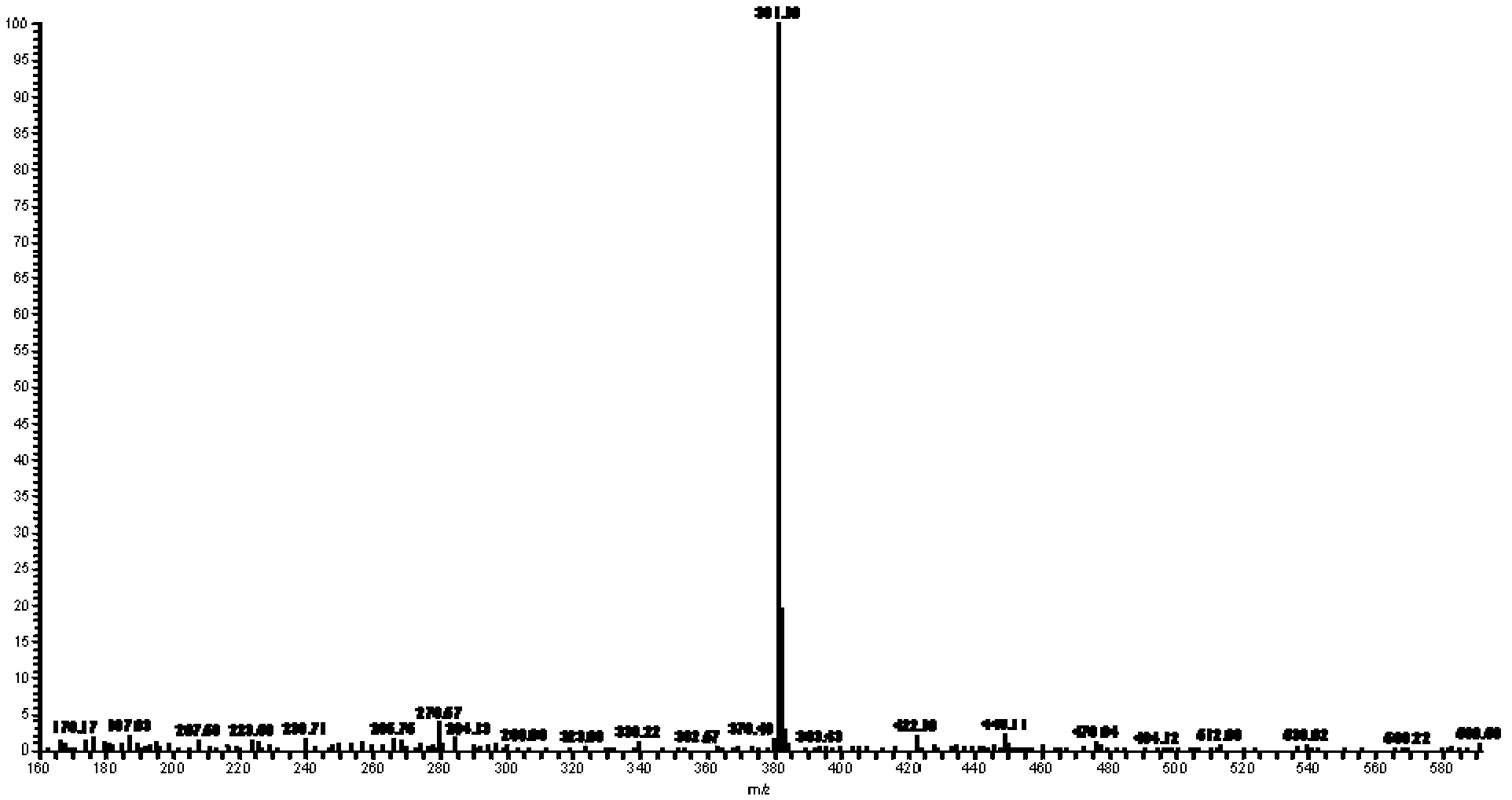
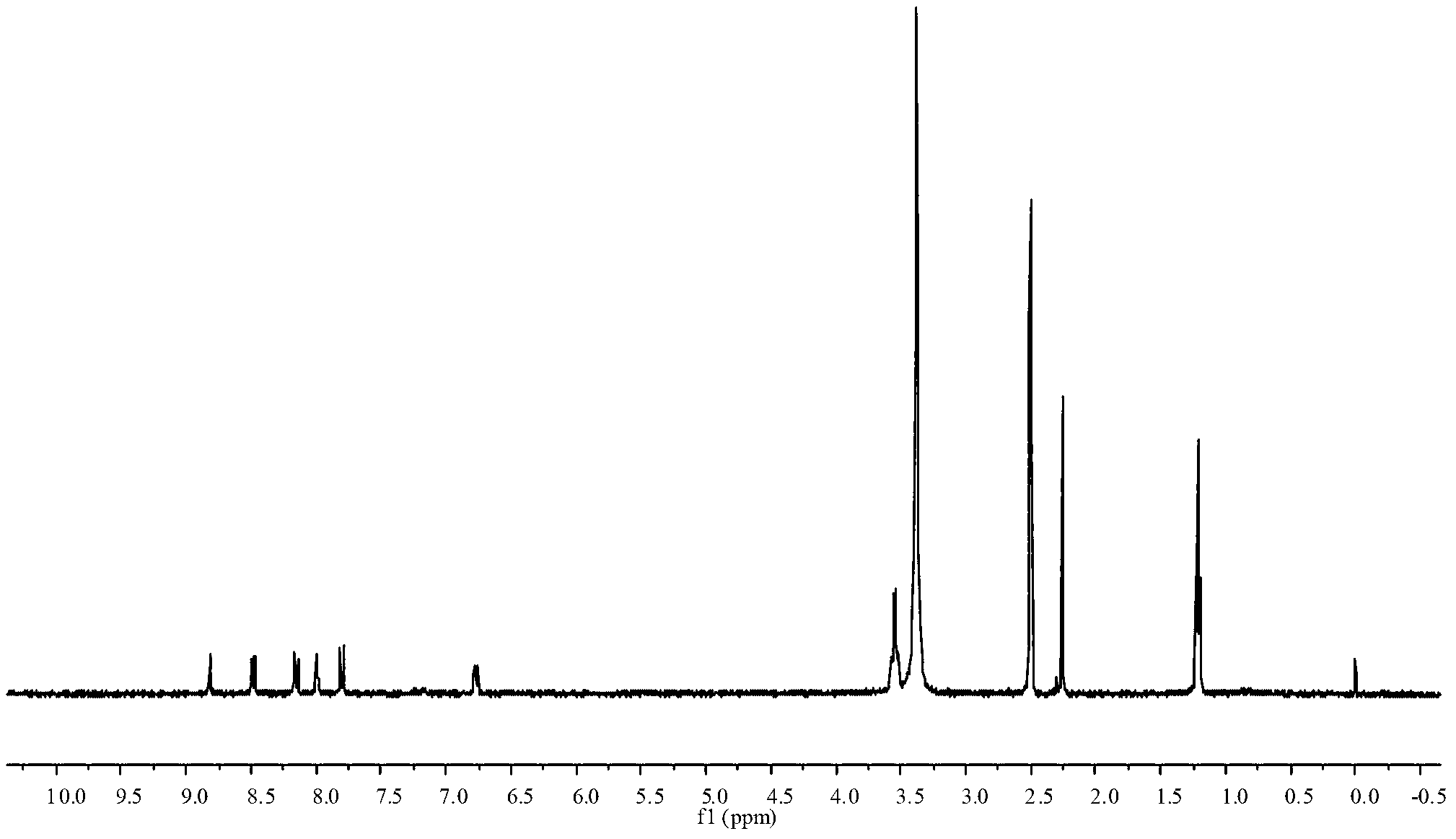
488 results about "Dimethoxyethane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
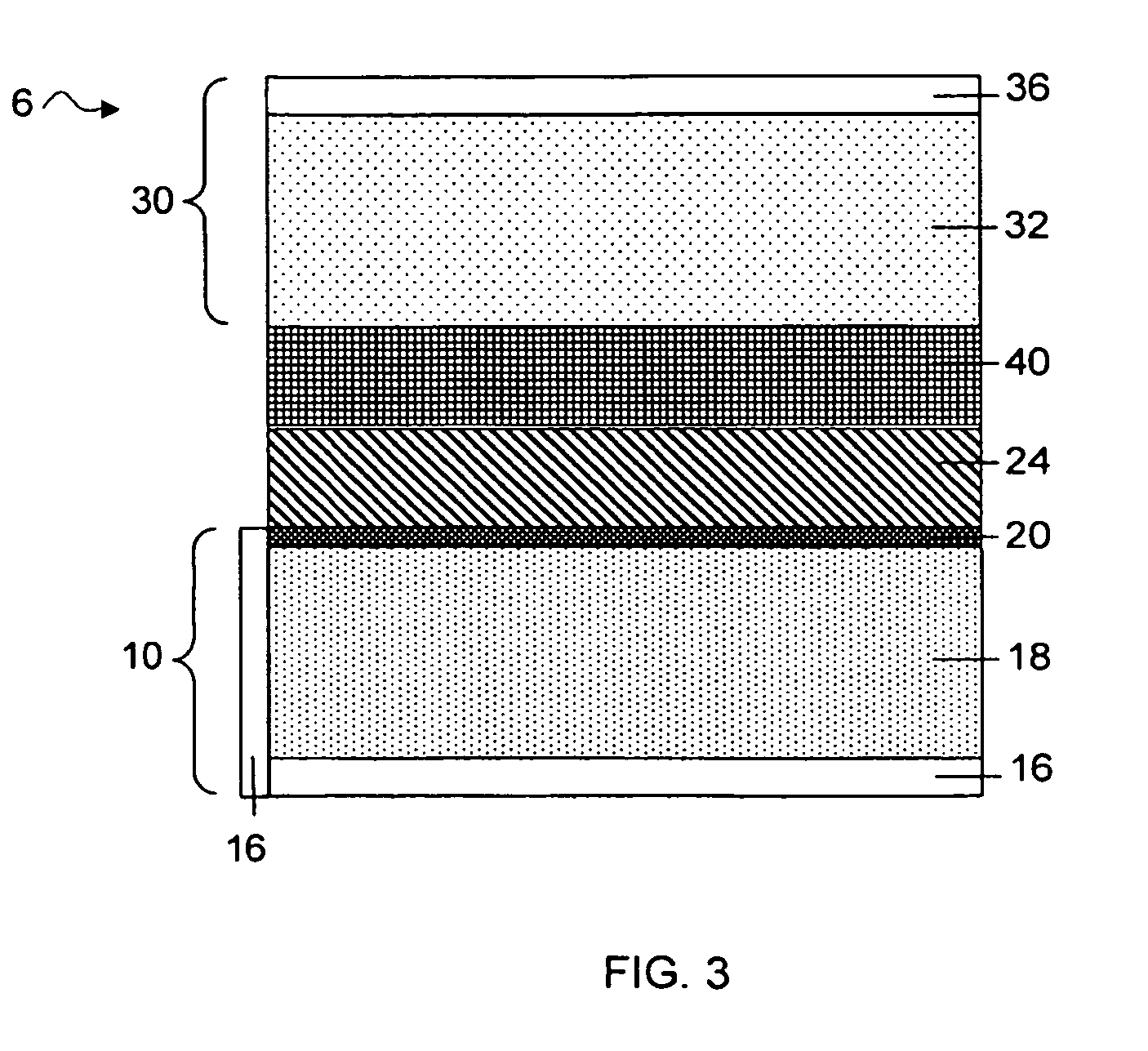
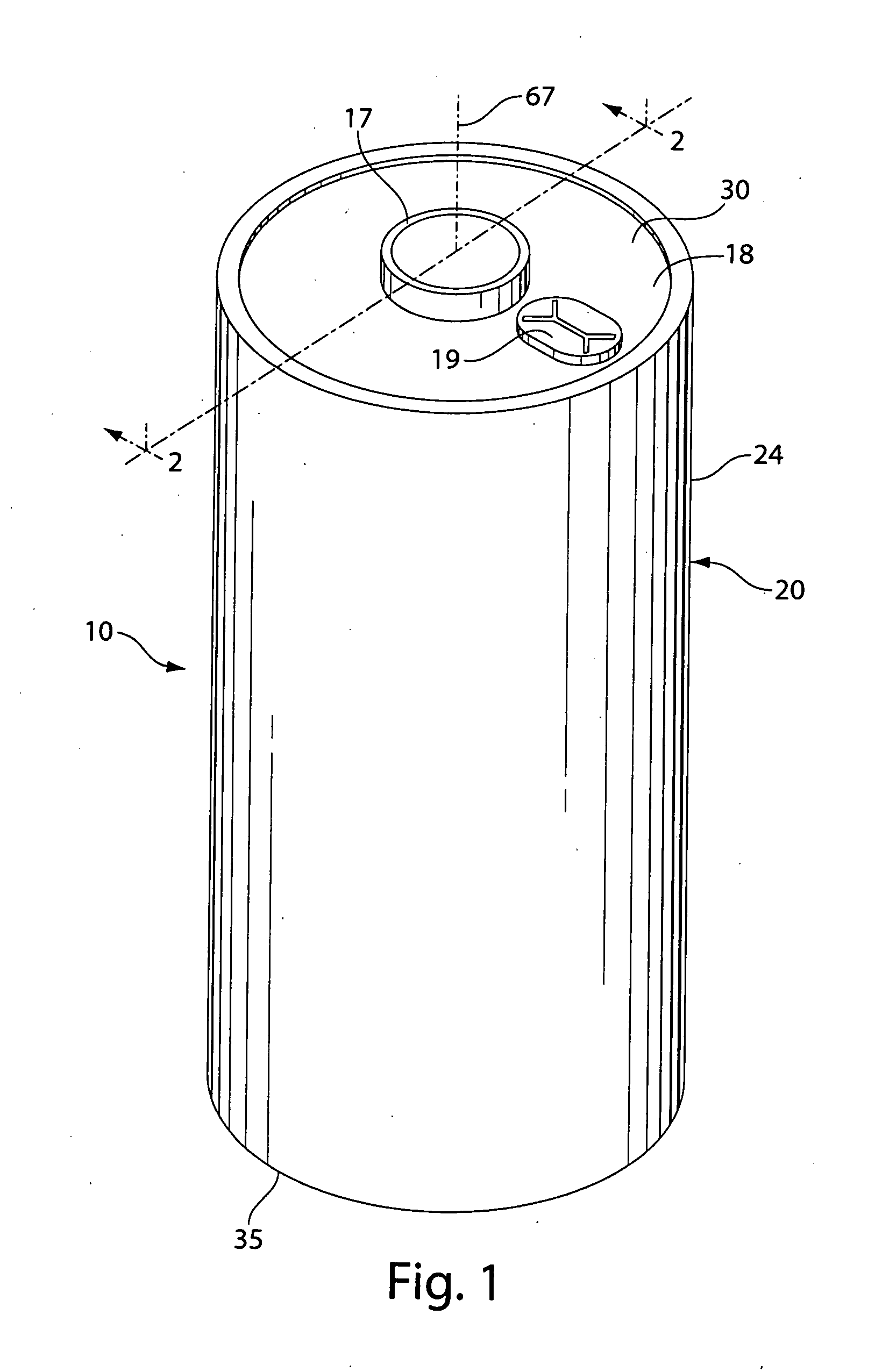
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is miscible with water.
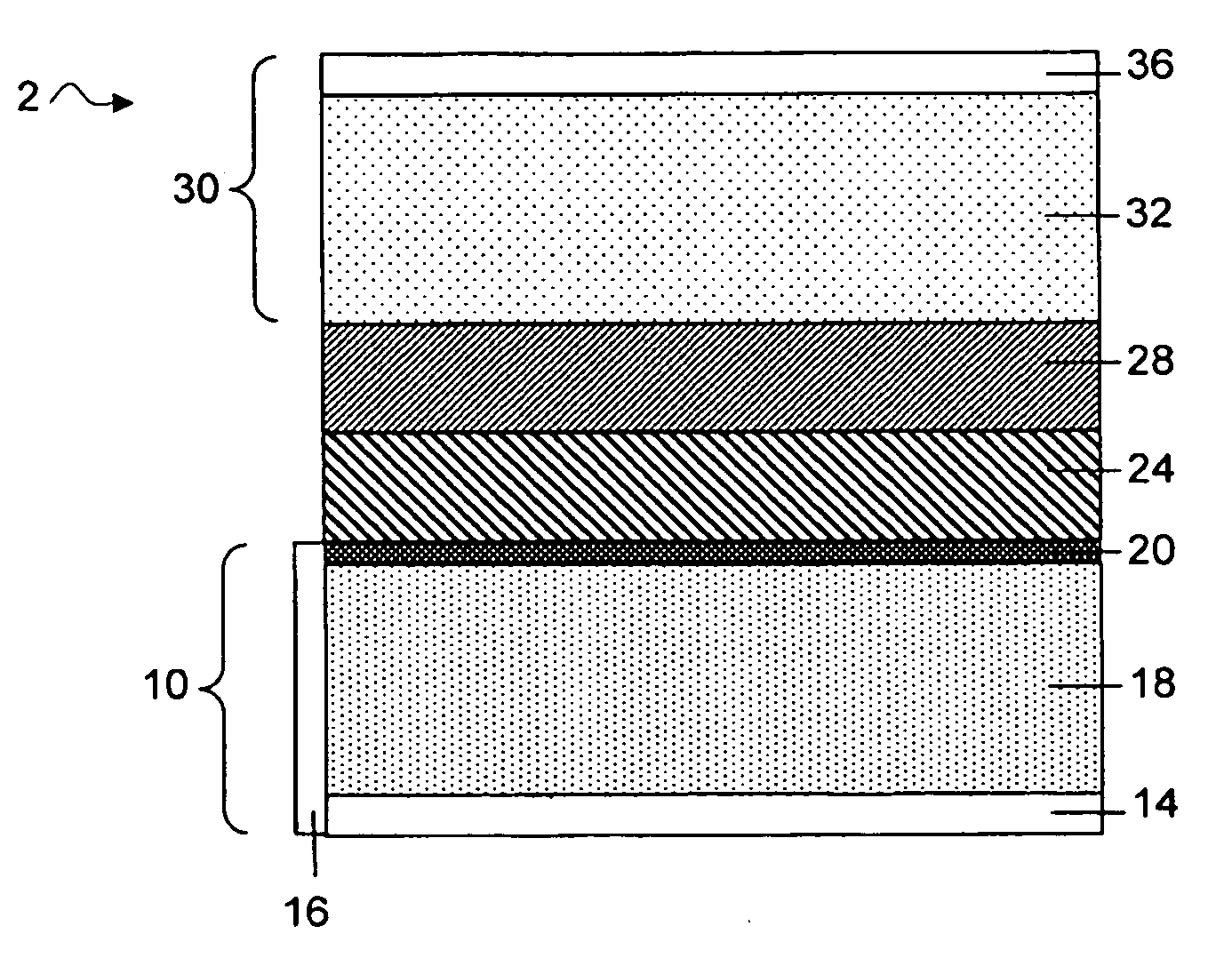
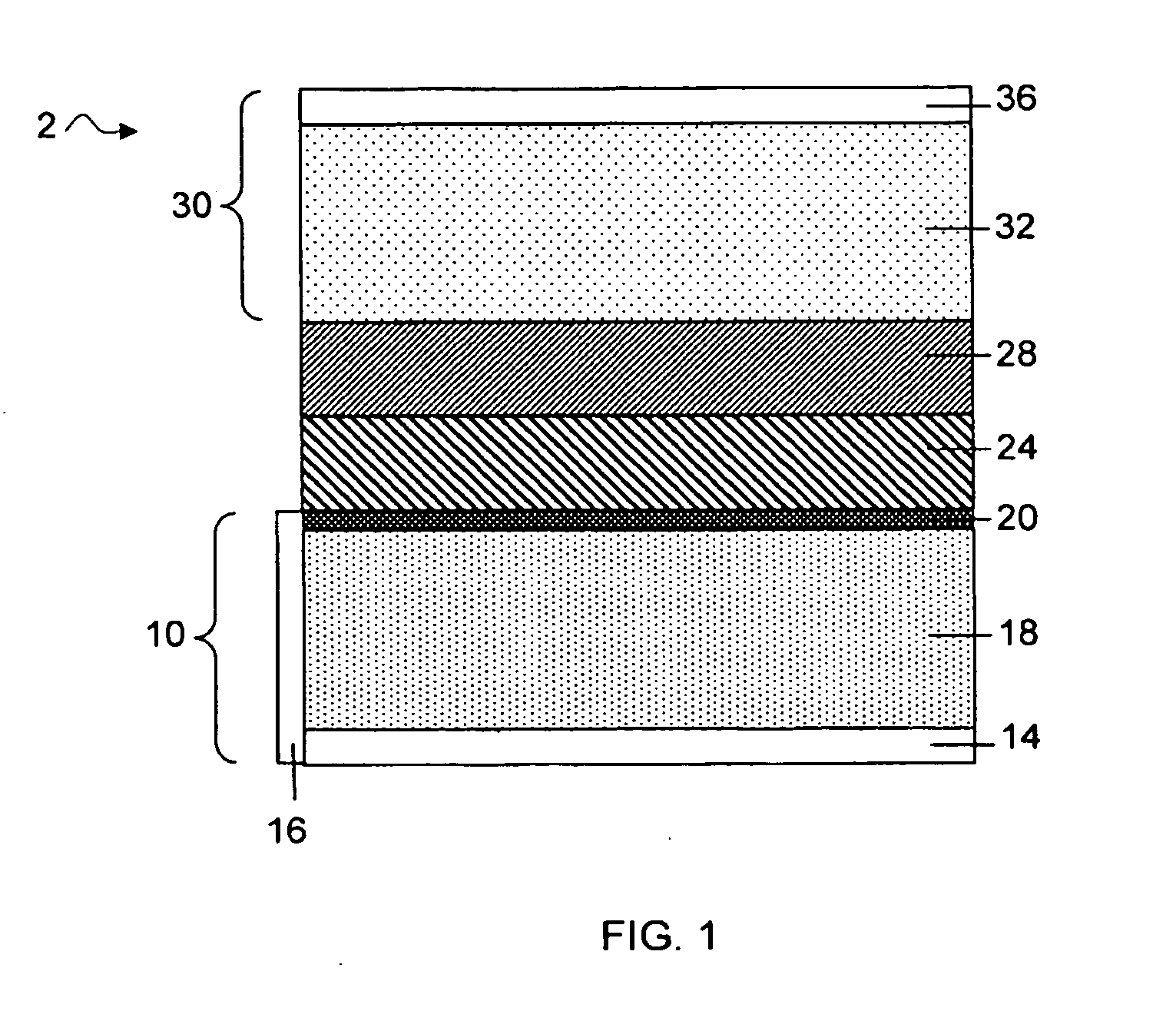
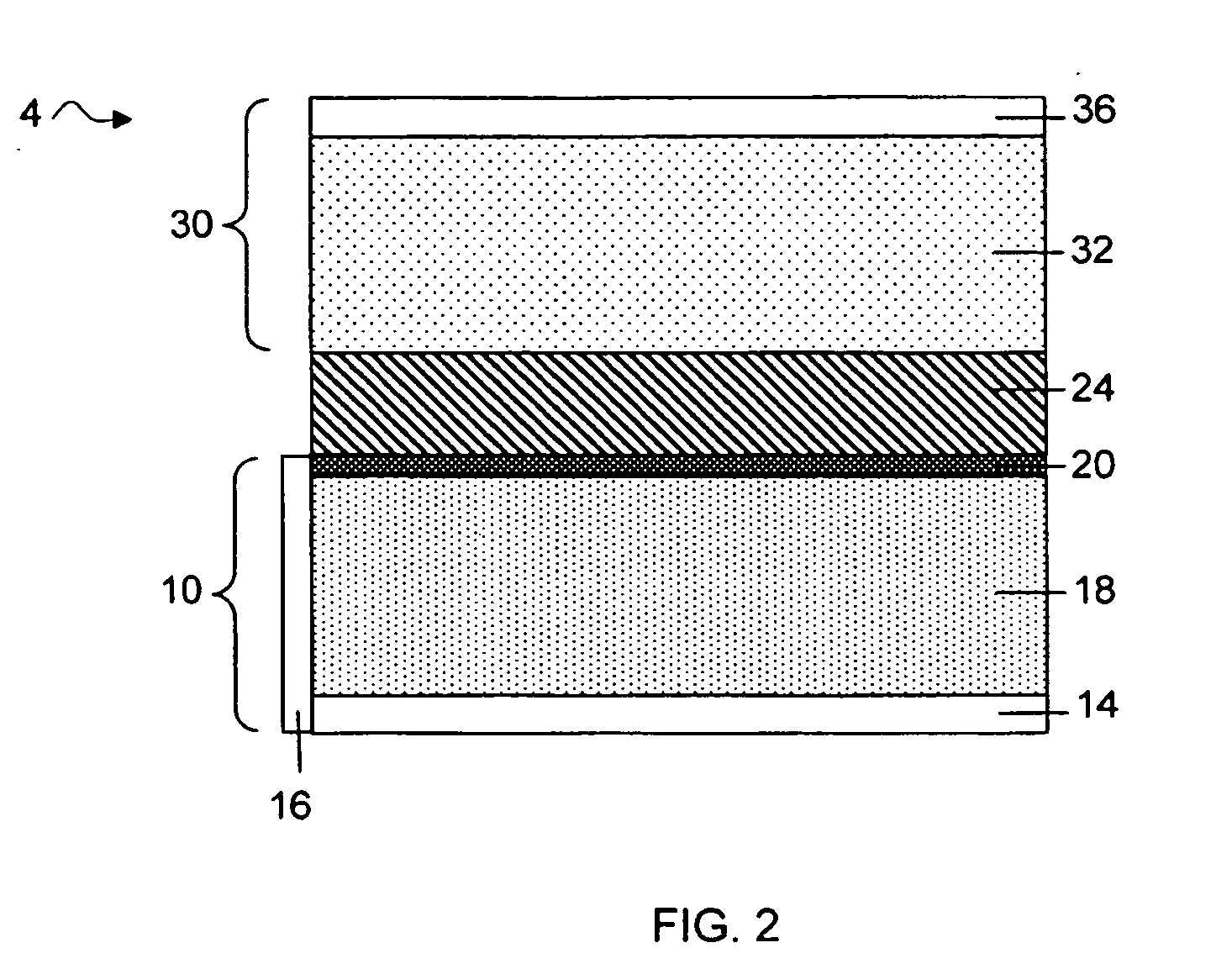
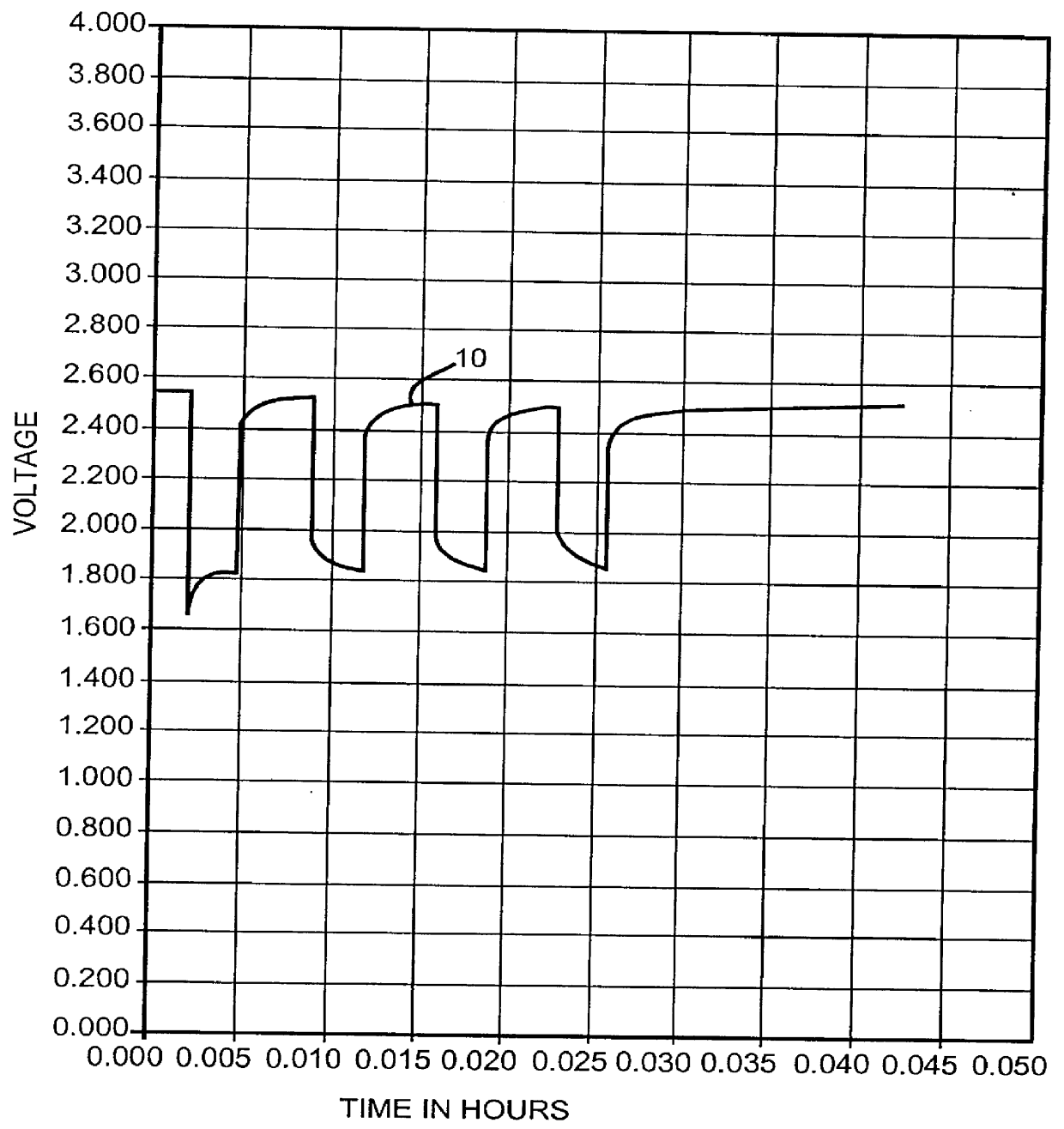
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
Inorganic and organic nitrate additives for nonaqueous electrolyte in alkali metal electrochemical cells
InactiveUS6060184AGood charge and discharge cycleImprove efficiencyOrganic electrolyte cellsSecondary cells charging/dischargingAlkaline earth metalOrganic nitrates
An alkali metal, solid cathode, nonaqueous electrochemical cell capable of delivering high current pulses, rapidly recovering its open circuit voltage and having high current capacity, is described. The stated benefits are realized by the addition of at least one nitrate additive to an electrolyte comprising an alkali metal salt dissolved in a mixture of a low viscosity solvent and a high permittivity solvent. A preferred solvent mixture includes propylene carbonate, dimethoxyethane and an alkali metal nitrate, alkaline earth metal nitrate and / or an organic alkyl nitrate additive.
Owner:WILSON GREATBATCH LTD
Phosphonate additives for nonaqueous electrolyte in alkali metal electrochemical cells
InactiveUS6096447AGood charge and discharge cycleImprove efficiencyOrganic electrolyte cellsHeart stimulatorsPermittivityPropylene carbonate
An alkali metal, solid cathode, nonaqueous electrochemical cell capable of delivering high current pulses, rapidly recovering its open circuit voltage and having high current capacity, is described. The stated benefits are realized by the addition of at least one phosphonate additive to an electrolyte comprising an alkali metal salt dissolved in a mixture of a low viscosity solvent and a high permittivity solvent. A preferred solvent mixture includes propylene carbonate, dimethoxyethane and an alkyl phosphonate additive.
Owner:WILSON GREATBATCH LTD
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
Electrolyte additives for lithium sulfur rechargeable batteries
ActiveUS9160036B2Improve Coulombic efficiencyAvoid reactionLi-accumulatorsLithium–sulfur batteryLithium sulfur
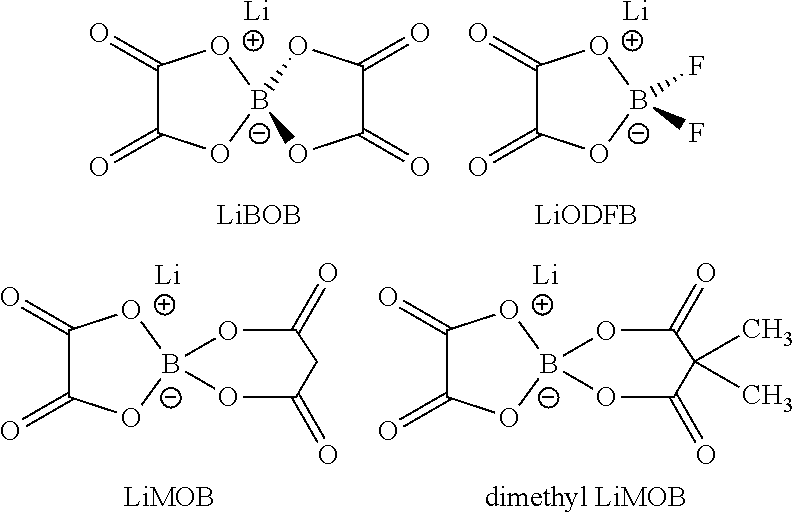
An electrolyte solution for a lithium sulfur battery contains a lithium oxalatoborate compound in a 0.05-2 M solution in conventional lithium sulfur battery electrolyte solvents, optionally with other lithium compounds. Examples of solvents include dimethoxyethane (DME), dioxolane, and triethyleneglycol dimethyl ether (TEGDME). Electrochemical cells contain a lithium anode, a sulfur-containing cathode, and a non-aqueous electrolyte containing the lithium oxalatoborate compound. Lithium sulfur batteries contain a casing enclosing a plurality of the cells.
Owner:GM GLOBAL TECH OPERATIONS LLC
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
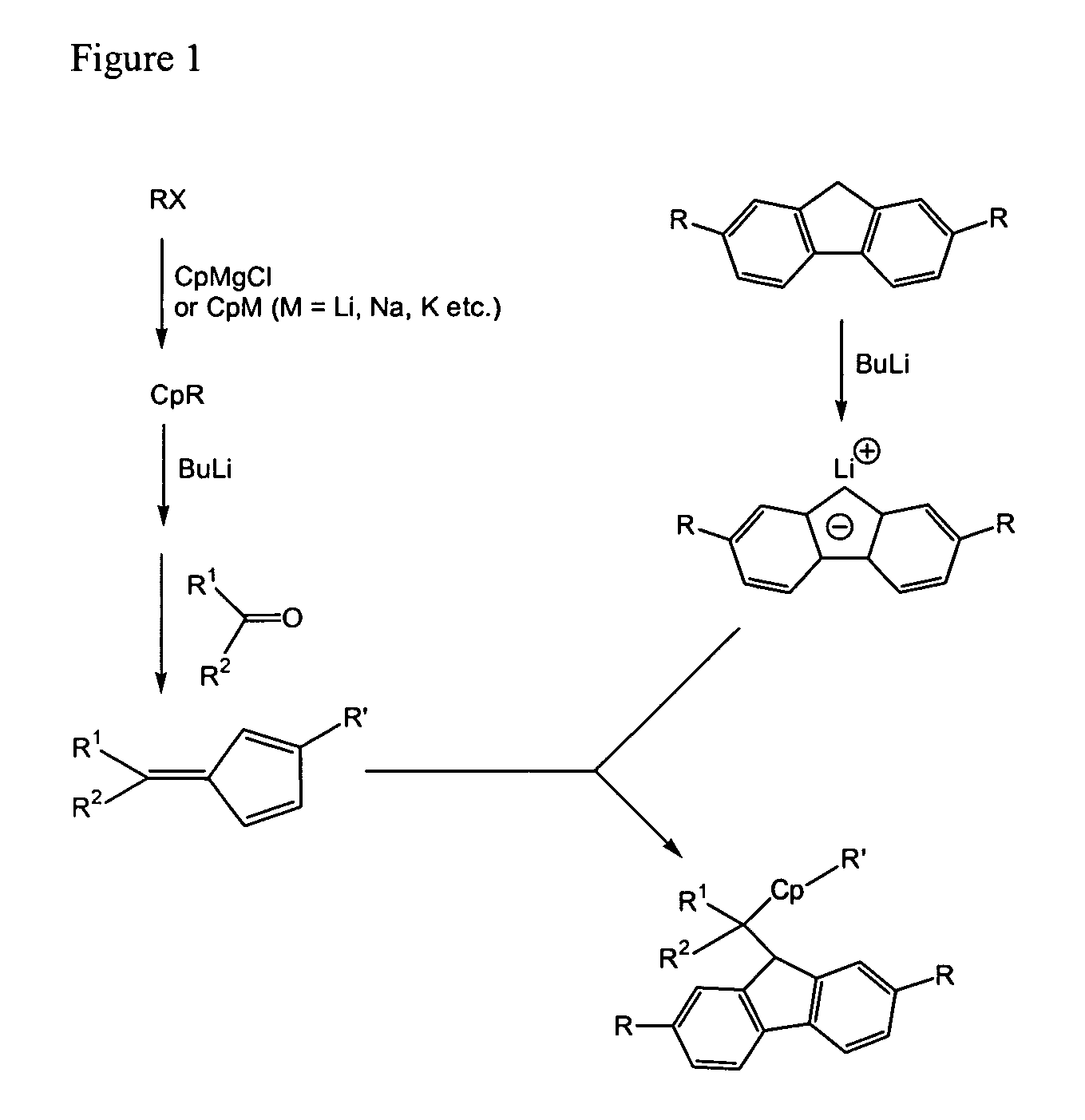
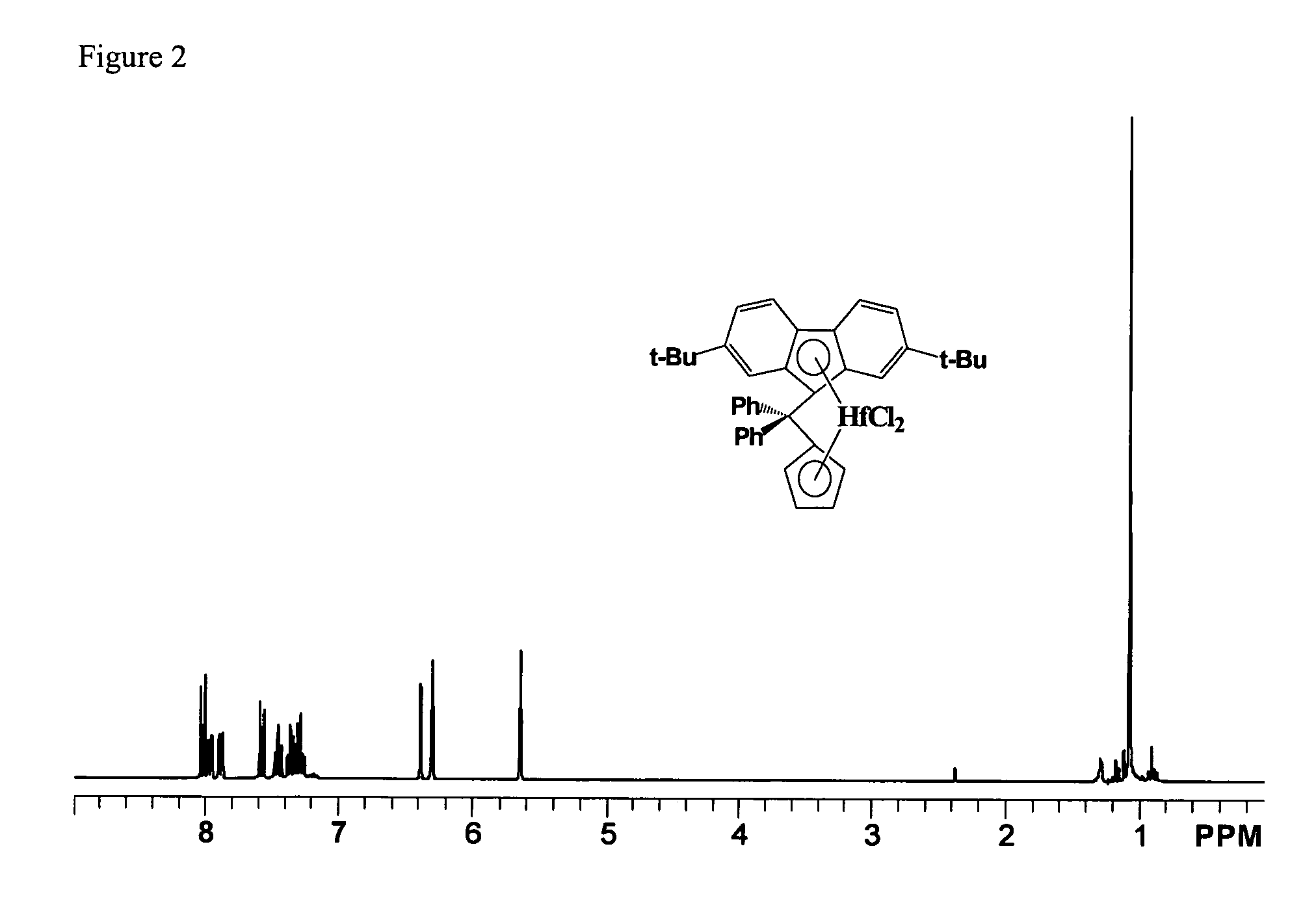
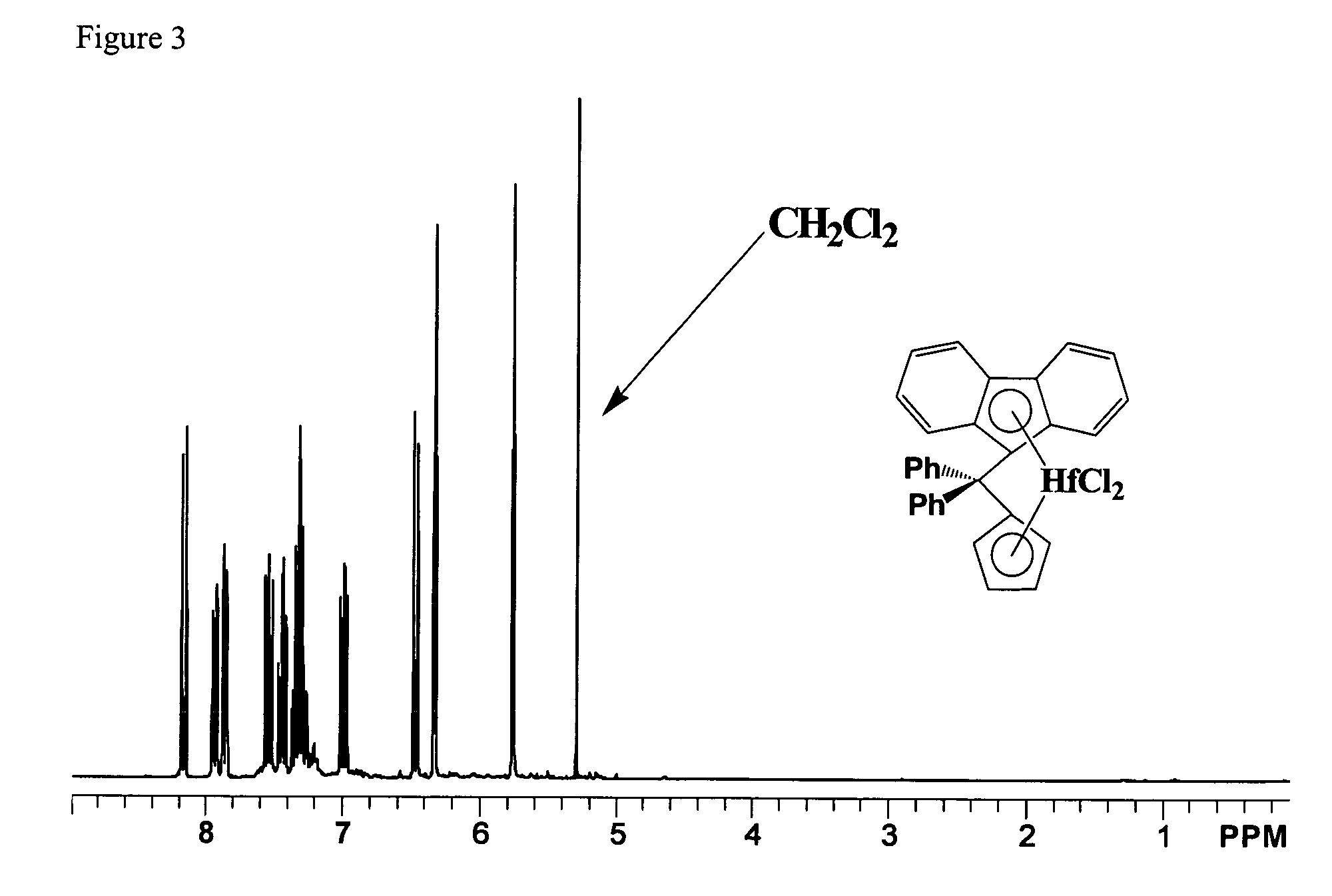
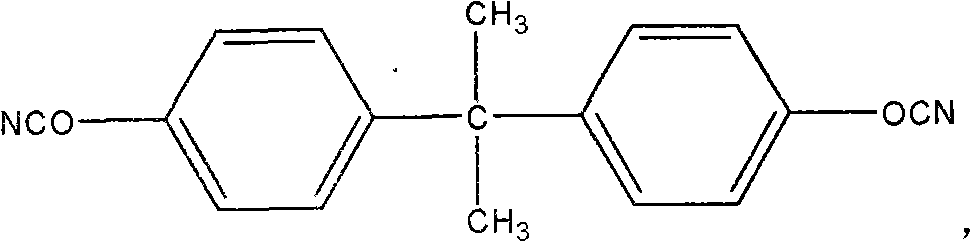
Process for one-pot synthesis of 1,1-diphenyl-1-(3-substituted-cyclopentadienyl)-1-(2,7-di-t-butyl-fluoren-9-yl)methane type ligands
ActiveUS7468452B1Silicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsAnsa-metallocenePhenyl group
The present invention is directed to a method of making a ligand which can be used to form an ansa-metallocene. Further, the present invention is directed to a method of making the ansa-metallocene. In both methods the process steps employed to form the ligand are conducted in the presence of tetrahydrofuran, a substituted tetrahydrofuran, tetrahydropyran, a substituted tetrahydropyran or ethylene glycol dimethyl ether.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Organic nitrite additives for nonaqueous electrolyte in alkali metal electrochemical cells
InactiveUS6027827AGood charge and discharge cycleImprove efficiencyElectrotherapyPrimary cell maintainance/servicingPermittivitySolvent
Owner:WILSON GREATBATCH LTD
Highly conductive and stable nonaqueous electrolyte for lithium electrochemical cells
InactiveUS6844115B2Increase electrolyte conductivityImprove electrochemical stabilitySilver accumulatorsLead-acid accumulatorsPhysical chemistryPropylene carbonate
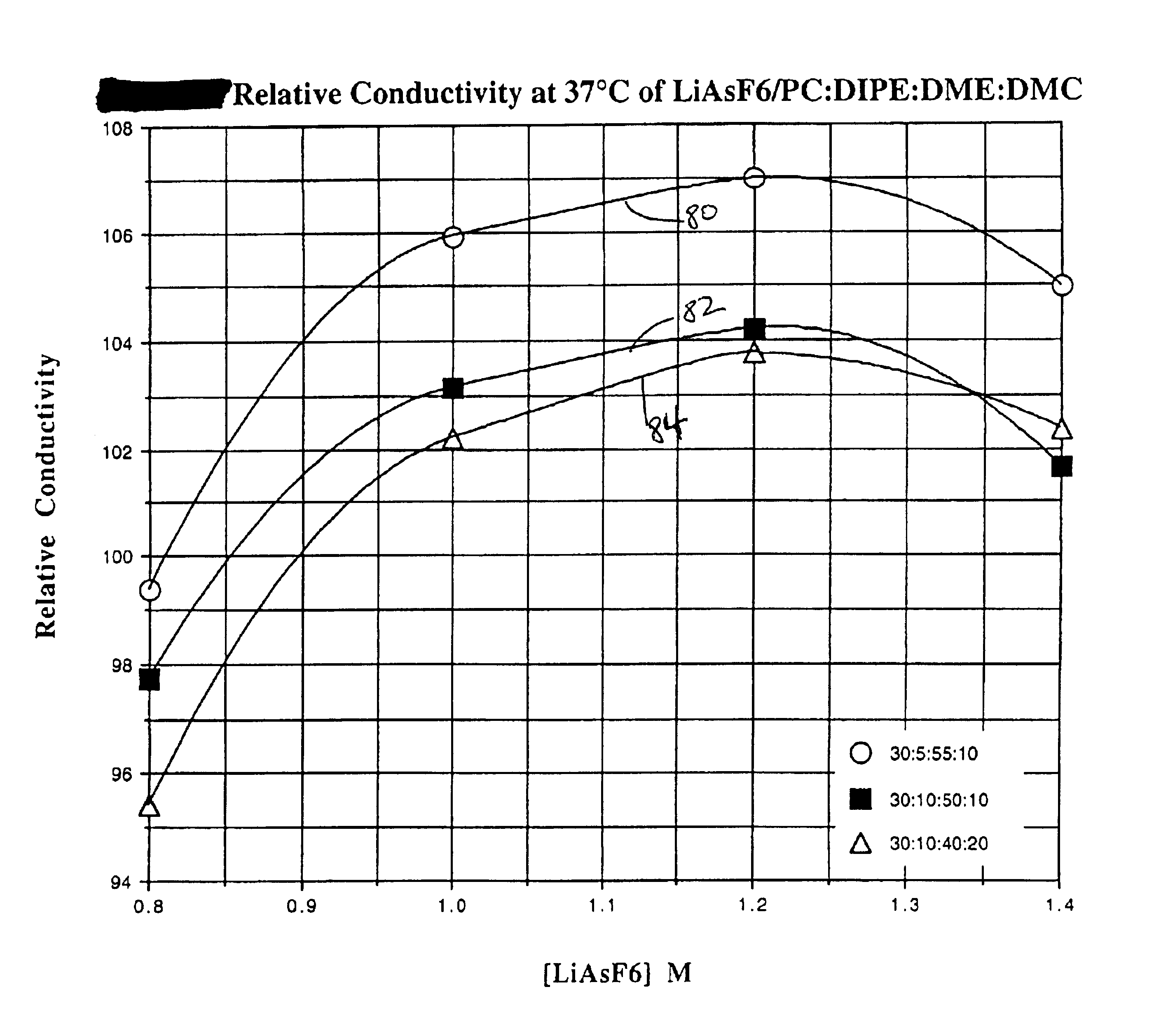
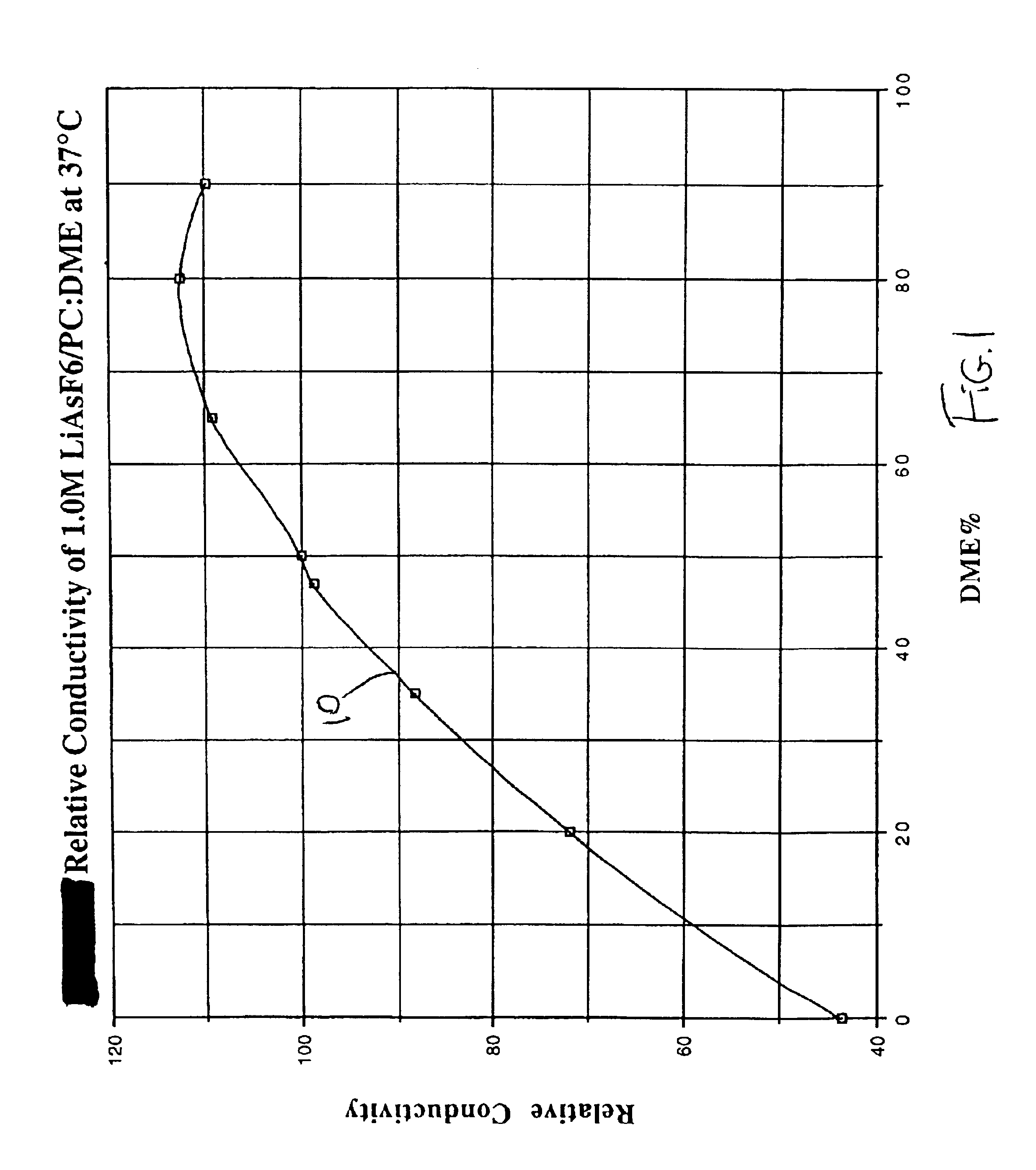
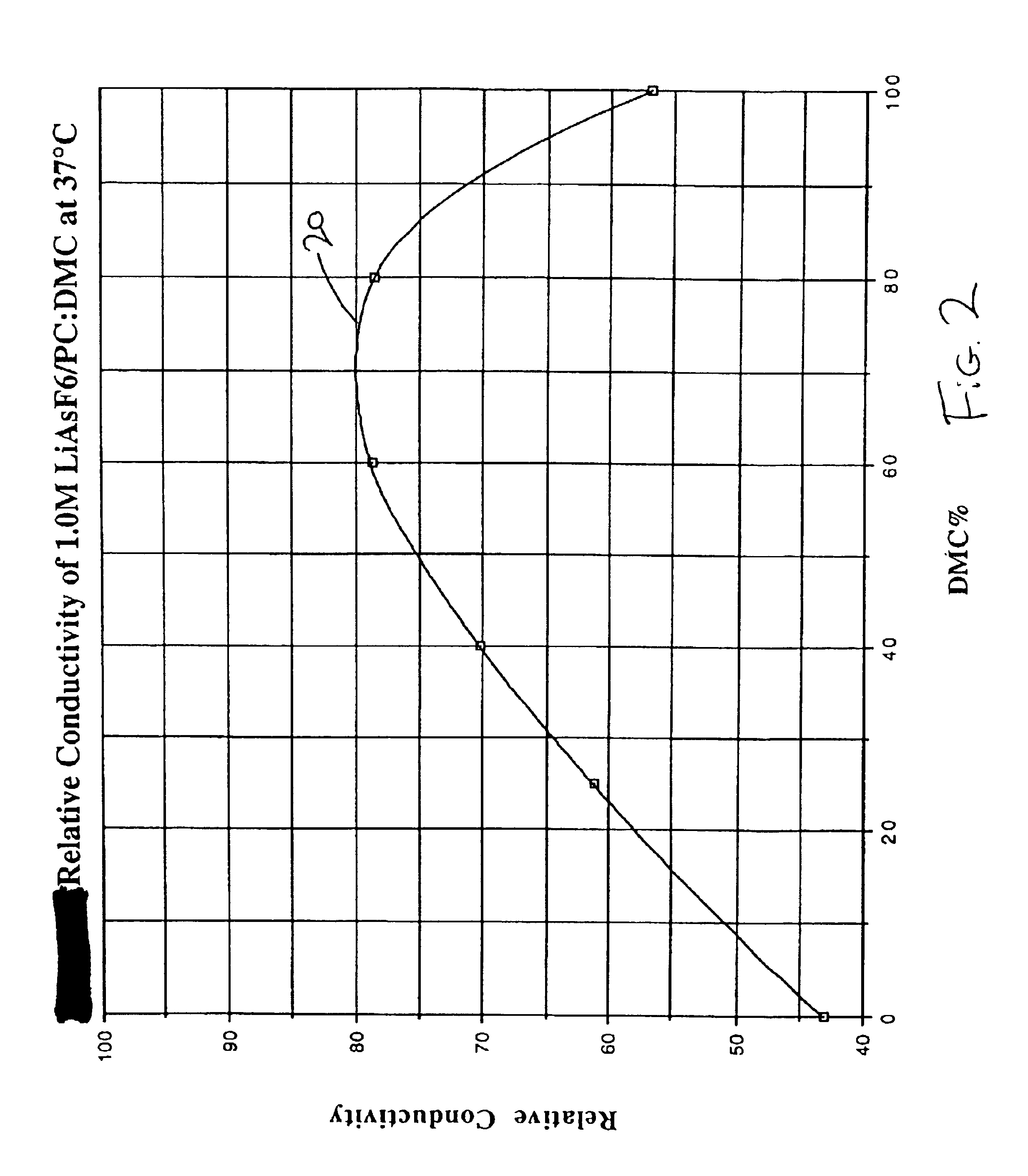
The present invention is directed to at least partially replacing PC and / or DME with a linear carbonate, preferably dimethyl carbonate, and a linear mono-ether, the most preferred being diisopropyl ether, in electrolytes useful for activating alkali metal-containing cells. This electrolyte has improved conductivity and provides electrochemical cells with enhanced discharge performance. A most preferred electrolyte comprises 1,2-dimethoxyethane, propylene carbonate, dimethyl carbonate and diisopropyl ether.
Owner:WILSON GREATBATCH LTD
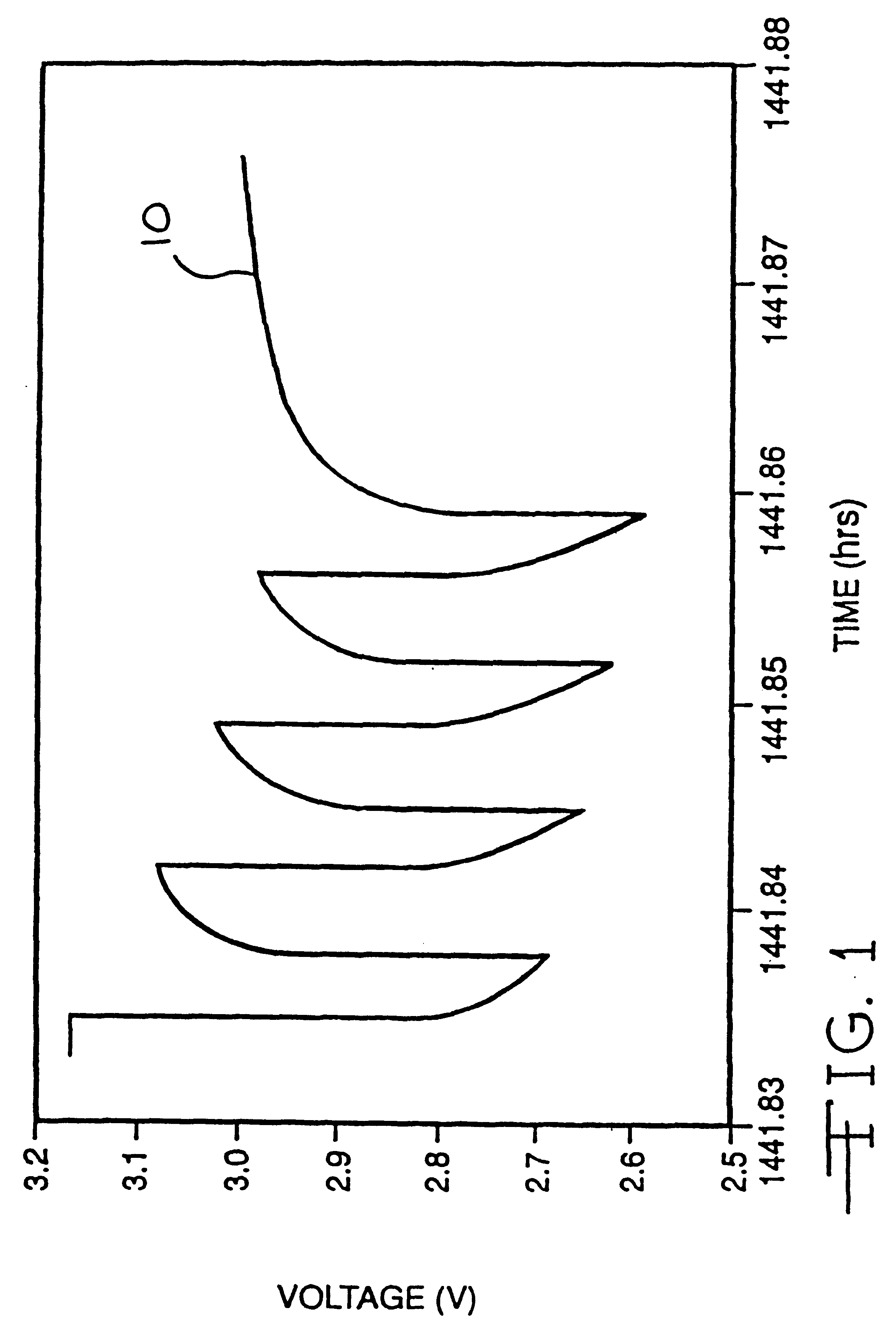
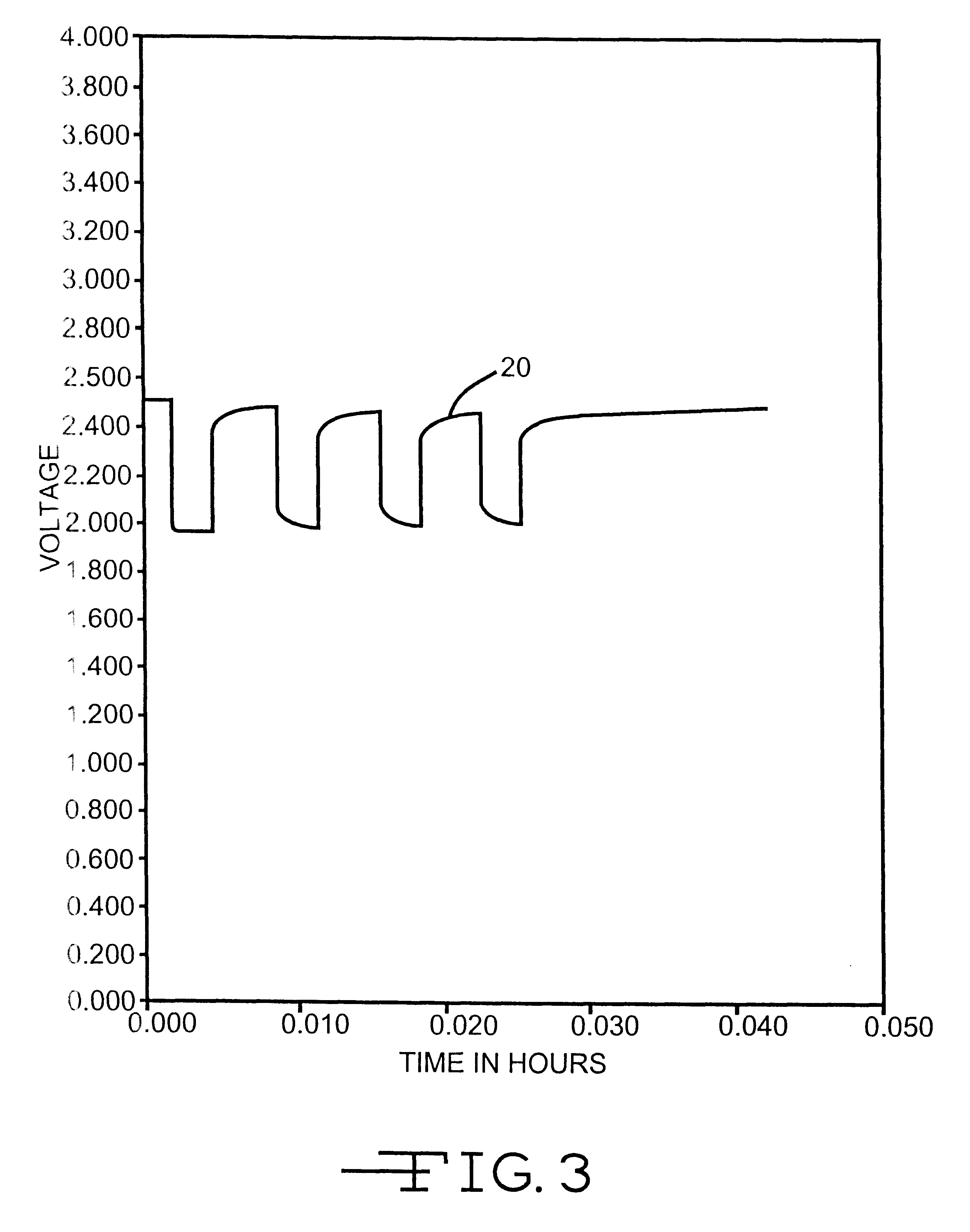
Method for reducing voltage delay in an alkali metal electrochemical cell activated with a nonaqueous electrolyte having a sulfate additive
An alkali metal, solid cathode, nonaqueous electrochemical cell capable of delivering high current pulses, rapidly recovering its open circuit voltage and having high current capacity, is described. The stated benefits are realized by the addition of at least one organic sulfate additive to an electrolyte comprising an alkali metal salt dissolved in a mixture of a low viscosity solvent and a high permittivity solvent. A preferred solvent mixture includes propylene carbonate, dimethoxyethane and a sulfate additive having at least one unsaturated hydrocarbon containing a C(sp2 or sp3 )-C(sp3) bond unit having the C(sp3) carbon directly connected to the -OSO3- functional group, or a silyl sulfate or a tin sulfate.
Owner:WILSON GREATBATCH LTD
Lithium cell
ActiveUS20090208849A1Reduce passive layer resistanceSlow build ratesPrimary cell maintainance/servicingElectrode carriers/collectorsSulfolaneAlloy
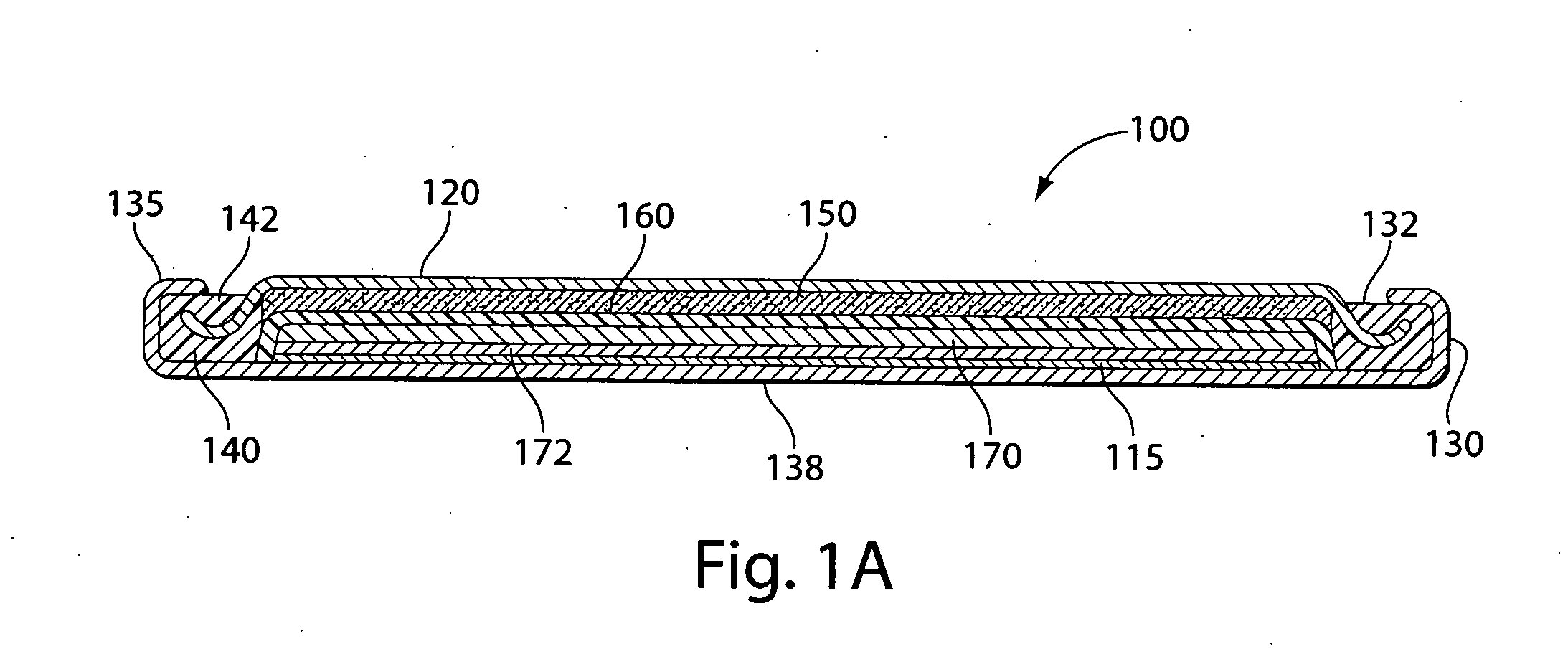
A primary cell having an anode comprising lithium or lithium alloy and a cathode comprising iron disulfide (FeS2) and carbon particles. The electrolyte comprises a lithium salt preferably lithium iodide (LiI) dissolved in an organic solvent mixture. The solvent mixture preferably comprises dioxolane, dimethoxyethane and sulfolane. The electrolyte typically contains between about 100 and 2000 parts by weight water per million parts by weight (ppm) electrolyte therein. A cathode slurry is prepared comprising iron disulfide powder, carbon, binder, and a liquid solvent. The mixture is coated onto a conductive substrate and solvent evaporated leaving a dry cathode coating on the substrate. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added.
Owner:DURACELL U S OPERATIONS
Special MDEA formula solution activated by functional ion liquid for CO2 gas absorption separation
InactiveCN101804292AReduce lossOvercome the disadvantage of high energy consumptionDispersed particle separationBy chemical separationSulfolanePoly(ethylene glycol) dimethyl ether
The invention relates to a special N-methyldiethanolamine formula solution activated by ion liquid for CO2 gas absorption separation, which consists of the following ingredients in mass percent: 35 to 50 percent of N-methyldiethanolamine, 5 to 20 percent of low-viscosity kalescent functional ion liquid, 15 to 30 percent of dimethyl ether of polyethlene glycol and / or sulfolane and 15 to 30 percent of water, wherein cations of the low-viscosity kalescent functional ion liquid are tetraalkylammonium ions, and anions of the low-viscosity kalescent functional ion liquid are amino acid radicals or organic carboxylate anions. The formula solution of the invention has the advantages that the high mass transfer performance of the absorption-desorption process is improved, the material consumption in the use process is low, the defect of high energy consumption because a large amount of water vapor is brought away during the absorbing agent regeneration, and the invention belongs to an energy-saving formula with high green degree. The regeneration temperature of the solution is lower than that of the traditional absorbing liquid, the grade of a heat source required to be provided in the regeneration process is reduced, energy sources can be saved, the stability of the absorbing agent solution in the operation is high, the consumption of each absorption-desorption circulation is low, and in addition, the cost is low.
Owner:NANJING UNIV
Electrolyte additives for lithium sulfur rechargeable batteries
ActiveUS20140272603A1Improve Coulombic efficiencyAvoid reactionOrganic electrolyte cellsLi-accumulatorsLithium–sulfur batteryLithium sulfur
An electrolyte solution for a lithium sulfur battery contains a lithium oxalatoborate compound in a 0.05-2 M solution in conventional lithium sulfur battery electrolyte solvents, optionally with other lithium compounds. Examples of solvents include dimethoxyethane (DME), dioxolane, and triethyleneglycol dimethyl ether (TEGDME). Electrochemical cells contain a lithium anode, a sulfur-containing cathode, and a non-aqueous electrolyte containing the lithium oxalatoborate compound. Lithium sulfur batteries contain a casing enclosing a plurality of the cells.
Owner:GM GLOBAL TECH OPERATIONS LLC
Alkali metal electrochemical cell activated with a nonaqueous electrolyte having a sulfate additive
InactiveUS6265106B1Improve discharge performanceIncrease delaySilver accumulatorsPrimary cell maintainance/servicingSulfatePermittivity
An alkali metal, solid cathode, non-aqueous electrochemical cell capable of delivering high current pulses, rapidly recovering its open circuit voltage and having high current capacity, is described. The stated benefits are realized by the addition of at least one organic sulfate additive to an electrolyte comprising an alkali metal salt dissolved in a mixture of a low viscosity solvent and a high permittivity solvent. A preferred solvent mixture includes propylene carbonate, dimethoxyethane and a sulfate additive.
Owner:WILSON GREATBATCH LTD
Modified diaphragm special for Li-S battery, preparation method of modified diaphragm and Li-S battery
ActiveCN105280867AIncrease capacityPromote circulationCell seperators/membranes/diaphragms/spacersSecondary cellsCalcinationDimethyl ether
In a modified diaphragm special for a Li-S battery, the surface at one side, close to a positive electrode, of a general diaphragm is coated with a Ketjen black coated metal oxide modified coating layer added with a conductive agent. A preparation method of the modified diaphragm special for the Li-S battery comprises the following steps of sequentially mixing Ketjen black and an aqueous solution of a metal oxide inorganic salt, carrying out dispersion, drying and calcinations to prepare a paste, and applying the paste at one side, close to the positive electrode, of a commercial diaphragm to obtain the modified diaphragm. The invention also discloses the Li-S battery applying the modified diaphragm special for the Li-S battery. The Li-S battery is prepared by taking metal lithium as a negative electrode, applying a Ketjen balck-sulfur composite material onto an aluminum foil to serve as the positive electrode, taking a mixture of LiTFSI, lithium nitrate, 1,3-dioxolane and glycol dimethyl ether as an electrolyte and assembling the modified diaphragm special for the Li-S battery. With the modified diaphragm disclosed by the invention, a "shuttle effect" of polysulfide lithium in the Li-S battery can be prevented, the electrochemical performance and the capacity of the Li-S battery are improved, the cycle lifetime of the Li-S battery is prolonged, and the modified diaphragm is suitably used for production at a large scale.
Owner:CHANGSHA RES INST OF MINING & METALLURGY
Organic sulfate additives for nonaqueous electrolyte in alkali metal electrochemical cells
An alkali metal, solid cathode, nonaqueous electrochemical cell capable of delivering high current pulses, rapidly recovering its open circuit voltage and having high current capacity, is described. The stated benefits are realized by the addition of at least one organic sulfate additive to an electrolyte comprising an alkali metal salt dissolved in a mixture of a low viscosity solvent and a high permittivity solvent. A preferred solvent mixture includes propylene carbonate, dimethoxyethane and a dialkyl sulfate additive.
Owner:WILSON GREATBATCH LTD
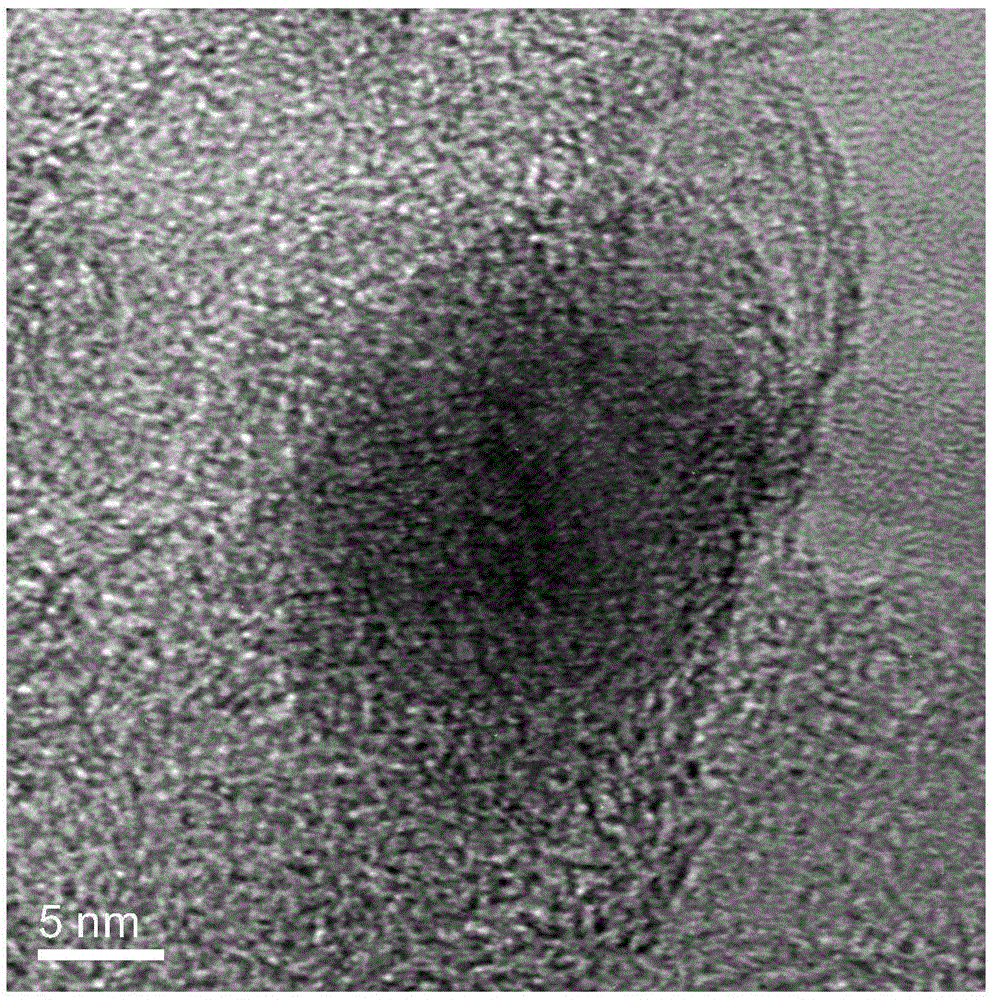
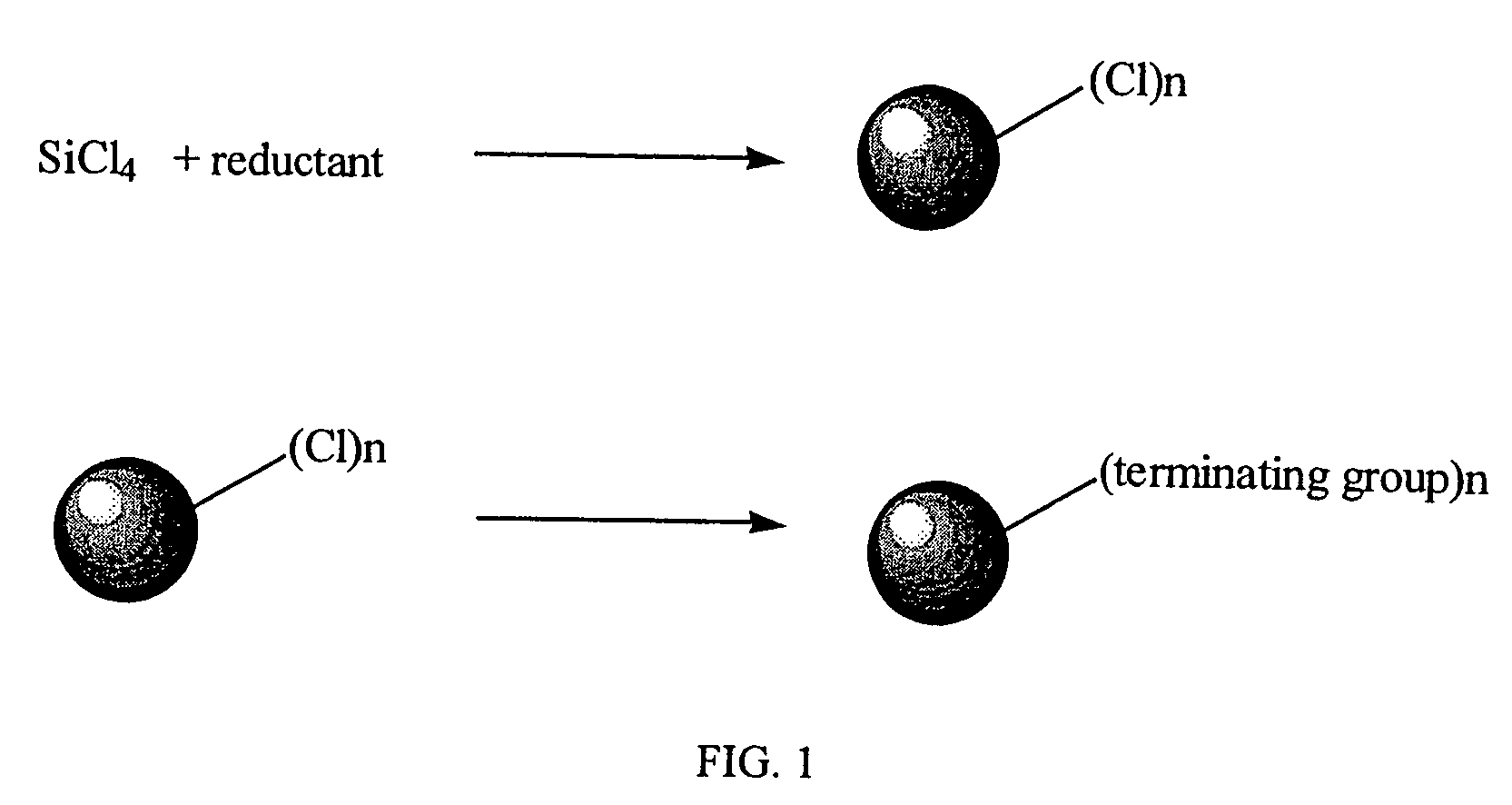
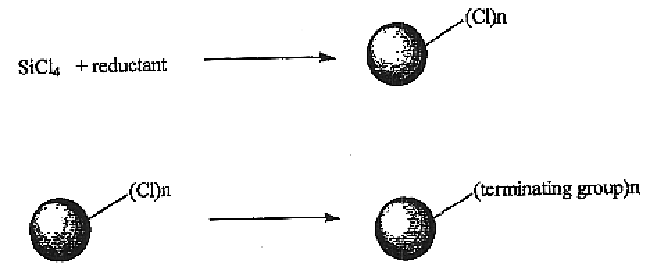
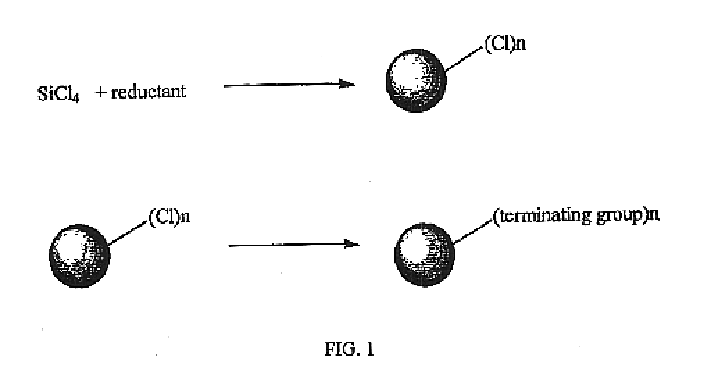
Method for preparing group IV nanocrystals with chemically accessible surfaces
Group IV nanocrystals, such as, for example, silicon nanocrysals and germanium nanocrystals, with chemically accessible surfaces are produced in solution reactions. Group IV halides can be reduced in organic solvents such as 1,2-dimethoxyethane (glyme), with soluable reducing agents to give halide-terminated group IV nanocrystals, which can then be easily functionalized with alkyl lithium, Grignard or other reagents to synthesize group IV nanocrystals having air and moisture stable surfaces. Synthesis can occur at ambient temperature and pressure.
Owner:EVERGREEN SOLAR
Hydrogen fluoride additive for nonaqueous electrolyte in alkali metal electrochemical cells
InactiveUS6117591AImprove discharge performanceIncrease delayOrganic electrolyte cellsSecondary cellsHydrogen fluoridePermittivity
An alkali metal, solid cathode, nonaqueous electrochemical cell capable of delivering high current pulses, rapidly recovering its open circuit voltage and having high current capacity, is described. The stated benefits are realized by the addition of hydrogen fluoride to the nonaqueous electrolyte comprising an alkali metal salt dissolved in a mixture of a low viscosity solvent and a high permittivity solvent. A preferred solvent mixture includes propylene carbonate, dimethoxyethane and hydrogen fluoride having LiAsF6 or LiPF6 dissolved therein.
Owner:WILSON GREATBATCH LTD
Preparation method of Cf/BN-SiC composite material
Owner:NAT UNIV OF DEFENSE TECH
Method for purifying ethylenediamine
ActiveCN101723837AHigh purityAvoid corrosionAmino compound purification/separationEthylenediamineDiethylene glycol monobutyl ether
The invention discloses a method for purifying ethylenediamine, comprising the following steps: (1) heating extractant, feeding the extractant in to an extracting tower from the upper part of the extracting tower, gasifying 60.0-92.0% water-bearing ethylenediamine, feeding the ethylenediamine into the lower part of the extracting tower, making the ethylenediamine reversely contact the extractant on the upper part of the extracting tower in the extracting tower, discharging water from the top of the extracting tower and separating the mixture of the ethylenediamine and the extractant at the bottom of the extracting tower, wherein the extractant is diethylene glycol monomethyl ether, diethylene glycol dimethyl ether, triethylene glycol dimethyl ether or diethylene glycol monobutyl ether; and (2) heating the mixture of the ethylenediamine and the extractant, feeding the mixture of the ethylenediamine and the extractant to the middle part of the extracting tower, rectifying the mixture ofthe ethylenediamine and the extractant in a rectifying tower, separating the ethylenediamine on the top of the rectifying tower and discharging the extractant from the bottom of the rectifying tower.The method is mainly used for purifying the ethylenediamine.
Owner:山西玉龙化工有限公司
Non-aqueous electrolyte and electrochemical energy storage device using the same
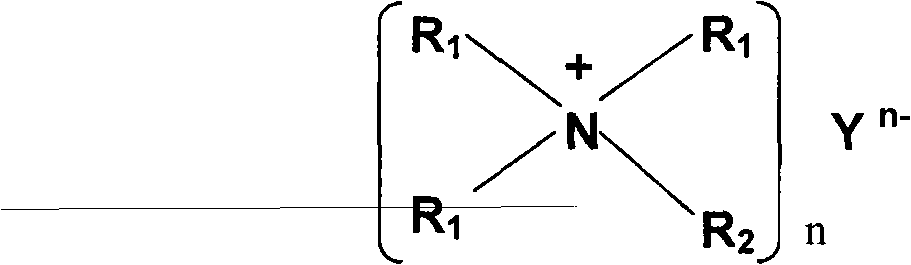
InactiveUS20090130565A1Improve antioxidant capacityReduce resistanceHybrid capacitor electrolytesNon-aqueous electrolyte accumulatorsQuaternary ammonium cationCarbonate
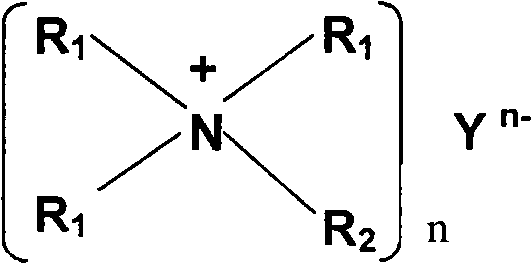
A non-aqueous electrolyte of the present invention contains a lithium salt (A), a quaternary ammonium salt (B) containing a straight chain alkyl group having carbon atoms of 4 or less and a solvent (C) composed of at least one compound selected from the group consisting of ethylene carbonate, propylene carbonate, butylene carbonate, γ-butyrolactone, dimethyl carbonate, ethyl methyl carbonate, diethyl carbonate, dimethoxyethane, ethoxymethoxyethane and diethoxyethane. The molar ratio C / A of the solvent (C) to the lithium salt (A) or the molar ratio C / B of the solvent (C) to the ammonium salt (B) is 6 or less. The non-aqueous electrolyte has a single phase. Consequently, there can be obtained a non-aqueous electrolyte of a high ion concentration having an excellent oxidation resistance and reduction resistance.
Owner:PANASONIC CORP
Liquid metal anode material, room-temperature liquid metal battery, and preparation method and application
ActiveCN105098140ALow costAbundant resourcesElectrode manufacturing processesNon-aqueous electrolyte accumulator electrodesPotassiumLiquid metal
The invention discloses a liquid metal anode material, a room-temperature liquid metal battery, and a preparation method and an application. The anode material is a black green liquid which is formed by mixing alkali metal, an aromatic compound and an ether solvent, wherein the alkali metal is any one of metal sodium, metal lithium or metal potassium; and the aromatic compound is any one of biphenyl, derivatives of the biphenyl, naphthaline, derivatives of the naphthaline and anthracene or derivatives of the anthracene; and the ether solvent comprises one or more of glycol dimethyl ether, diethylene glycol dimethyl ether or tetraethylene glycol dimethylether and the like.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Fiber-reinforced fireproof insulation board and preparation process thereof
InactiveCN103979918AOvercome defectsAvoid defectsSolid waste managementClimate change adaptationCalcium formateFire retardant
The invention discloses a fiber-reinforced fireproof insulation board. The fiber-reinforced fireproof insulation board is prepared from the raw materials in parts by weight: 80-100 parts of fly ash, 30-50 parts of expanded perlite, 10-20 parts of ceramsite, 10-20 parts of plant fiber, 5-10 parts of floating bead, 5-8 parts of meerschaum, 3-5 parts of sodium fluosilicate, 3-5 parts of calcium formate, 3-5 parts of flame retardant, 0.1-0.5 part of oleic triisopropanolamine and 0.1-0.3 part of glycol dimethyl ether. According to the fiber-reinforced fireproof insulation board, industrial production wastes are adopted as main raw materials, so that the production cost is reduced; through the organic combination and generated synergism of the fly ash, lightweight aggregate and additives, the defects in the existing building insulation boards can be effectively overcome.
Owner:秦菊霞
Method for preparing azo dye with alkalescent arylamine serving as diazotization ingredient
ActiveCN103265820AReduce manufacturing costReduce pollutionMonoazo dyesDisperse dyePolyethylene glycol
The invention provides a method for preparing an azo dye with alkalescent arylamine serving as a diazotization ingredient. The method is characterized in that in a diazotization reaction, ethyl acetate, propyl acetate, butyl acetate, acetone, glycol dimethyl ether or polyethylene glycol 200 is used as a diazotization reaction solvent instead of concentrated sulfuric acid, fluoroboric acid or naphthalene disulfonic acid is used as a diazo salt stabilizer instead of concentrated sulfuric acid, a diazo salt is filtered and separated, filtrate is directly applied to a next diazotization reaction of the alkalescent arylamine, and the diazo salt is applied to a coupling reaction so as to synthesize disperse dyes. Compared with the traditional preparation methods, the method has the advantages that the dosage of the concentrated sulfuric acid can be reduced greatly during the diazotization reaction of the alkalescent arylamine, so that the production cost of the azo dye with the alkalescent arylamine serving as the diazotization ingredient is reduced greatly, the environmental pollution can be reduced from the source, the pollution retreatment of a production end is avoided, and thus, the method has broad application prospects.
Owner:DALIAN UNIV OF TECH
High yield method for preparing silicon nanocrystals with chemically accessible surfaces
InactiveUS6855204B2Material nanotechnologyPolycrystalline material growthSilicon nanocrystalMoisture
Silicon nanocrystals with chemically accessible surfaces are produced in solution in high yield. Silicon tetrahalide such as silicon tetrachloride (SiCl4) can be reduced in organic solvents, such as 1,2-dimethoxyethane (glyme), with soluble reducing agents, such as sodium naphthalenide, to give halide-terminated (e.g., chloride-terminated) silicon nanocrystals, which can then be easily functionalized with alkyl lithium, Grignard or other reagents to give easily processed silicon nanocrystals with an air and moisture stable surface. The synthesis can be used to prepare alkyl-terminated nanocrystals at ambient temperature and pressure in high yield. The two-step process allows a wide range of surface functionality.
Owner:EVERGREEN SOLAR
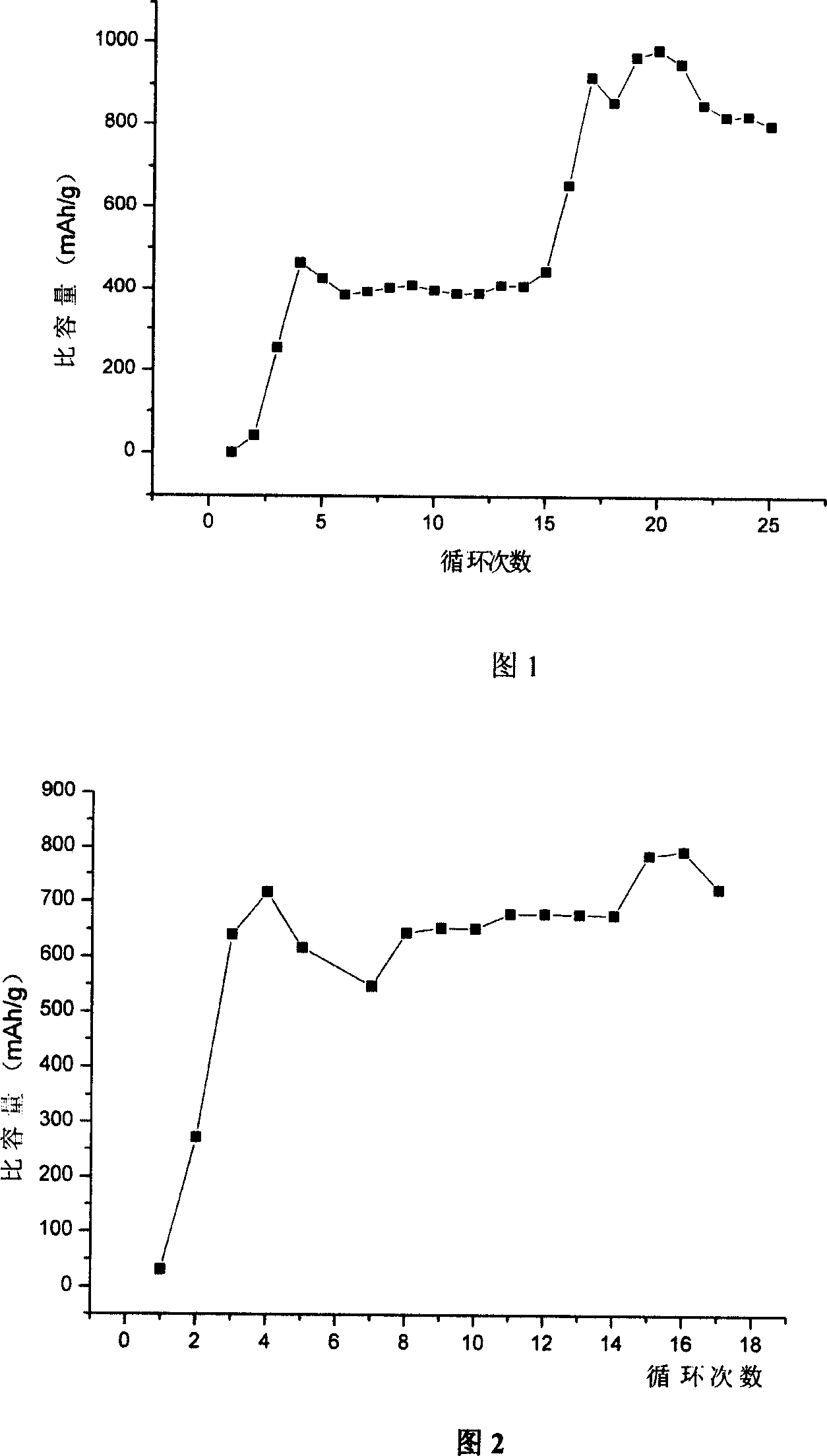
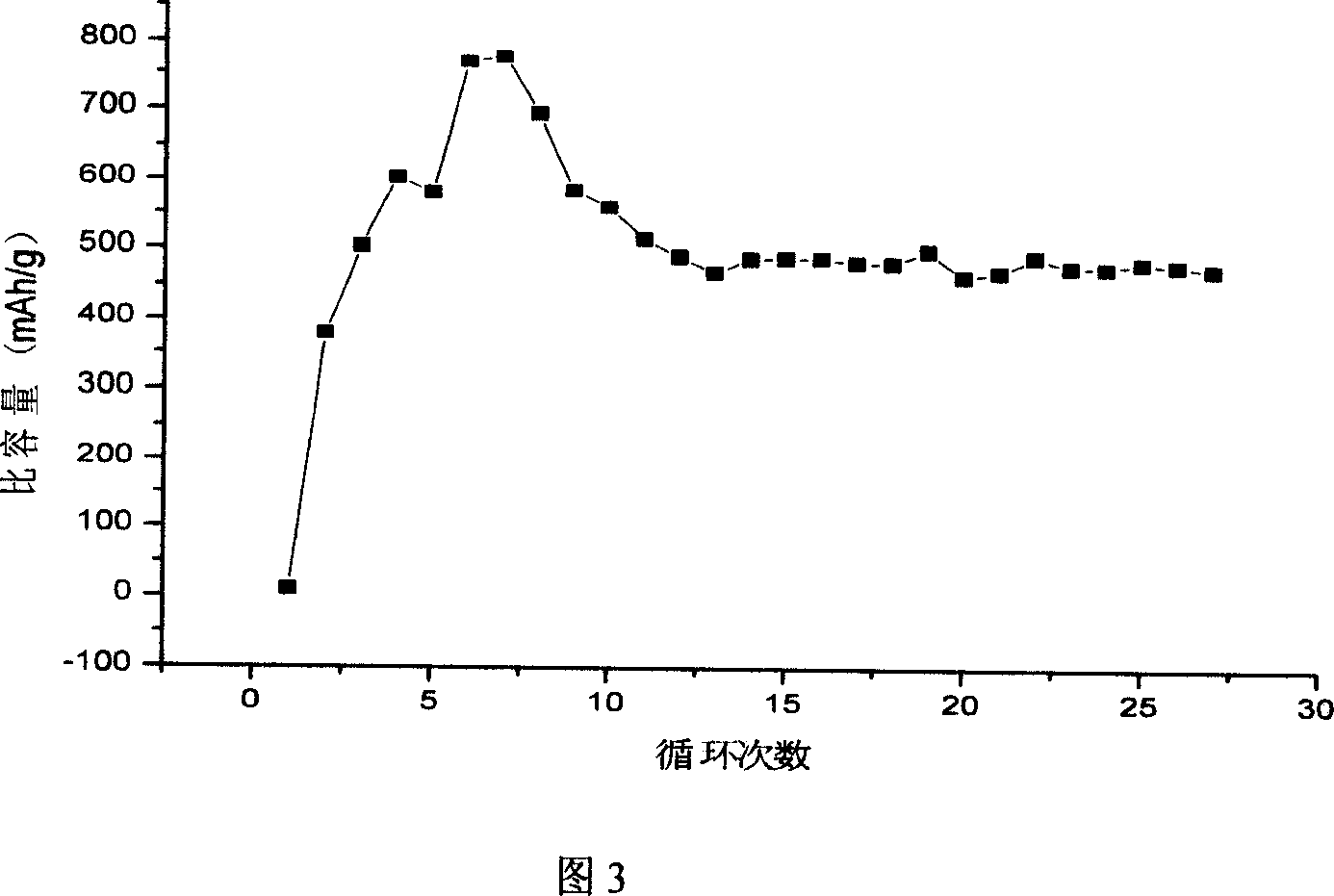
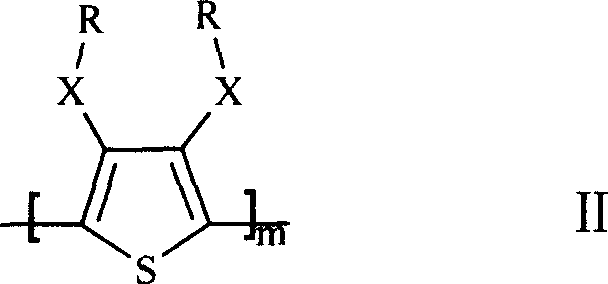
Lithium cell positive material thiofuran polymer and lithium battery preparation method
ActiveCN101007866AHigh capacity densityIncrease energy densityCell electrodesThiophene derivativesElectrolyte
The invention discloses an anode material trihalide polymer for lithium battery and the preparation method for lithium battery. Carrying out oxidative polymerization with thiofuran derivatives such as thiofuran, alkoxy thiofuran, alkyl thiofuran, hydro sulfo-thiofuran, 3, 4- sulfo thiofuran to produce poly thiofuran, poly alkoxy thiofuran, poly alkyl thiofuran, poly hydro sulfo-thiofuran, poly 3, 4- sulfo thiofuran; preparing anode with thiofuran polymer, carbon black and politef, and assembling lithium secondary battery by taking (CxF2x+1SO2)2NLi, CxF2x+1SO3Li, (CxF2x+1SO2)(CyF2y+1)NLi as electrolyte, employing dioxohexacyclic ring, dioxolane and glycol dimethyl ether as solvent, and taking metal lithium slice as negative pole. The discharge specific capacity of assembled lithium secondary battery is 400 Ah / kg to 1200 Ah / kg, and the cycling stability is sound.
Owner:SUZHOU YOULION BATTERY INC
Lithium cell with iron disulfide cathode
InactiveUS20100203370A1Advantage in cell performanceAdvantages in cell performanceCell electrodesOrganic electrolyte cellsLithium metalAlloy
A primary cell having an anode comprising lithium or lithium alloy and a cathode comprising iron disulfide (FeS2) and carbon particles. The electrolyte comprises a lithium salt preferably lithium iodide (LiI) dissolved in an organic solvent mixture. The solvent mixture preferably comprises dioxolane and dimethoxyethane. The electrolyte typically contains between about 100 and 2000 parts by weight water per million parts by weight (ppm) electrolyte therein. The anode may be lithium metal or preferably is a lithium alloy. A cathode slurry is prepared comprising iron disulfide powder, carbon, binder, and a liquid solvent. The mixture is coated onto a conductive substrate and solvent evaporated leaving a dry cathode coating on the substrate. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added.
Owner:THE GILLETTE CO
Electrolyte of the lithium ion battery for ultra-low temperature discharge and its lithium ion battery
InactiveCN101017918AImprove performanceExcellent cycle indexOrganic electrolyte cellsSecondary cellsDimethoxyethaneEthyl carbonate
The related Li-ion cell fit to discharge at ultra-low temperature comprises an anode, a cathode, a membrane, and the electrolyte. Wherein, the electrolyte comprises: lithium hexafluorophosphate and lithium tetrafluoroborate with weight ratio as 1:5-10:1, and the solvent (including ethylene carbonate, dimethyl carbonate, methyl- ethyl carbonate, and dimethoxyethane). Wherein, the dimethoxyethane is 0.5-10wt%. Compared with prior art, this product can work well at -40deg.
Owner:HANGZHOU SKYRICH POWER CO LTD
Method for preparing core-shell structure functional coating nano aluminium-nickel powder
A preparing method of core-casing structure function coating nano aluminum-nickel powder comprises: using aluminum powder with a granule size of 60-100 nanometer as core, using nickel acetylacetonate as coating agent; adding nanometer aluminium powder into glycol dimethyl ether liquid, dispersing them evenly by mixing; adding nickel acetylacetonate into glycol dimethyl ether liquid, dissolving them completely by mixing to form homogeneously mixed liquid; mixing above two liquids, making the nickel acetylacetonate to absorpt spontaneously on nanometer aluminium powder in glycol dimethyl ether liquid; naturally volatilizing and drying the nanometer aluminium powder after completing absorption and then taking it out, and core-casing structure function coating nano aluminum-nickel powder is produced. The advantages of the invention are: function coating nano aluminum-nickel powder has good heat-releasing feature and thermostability, nickel and aluminum absorb with each other effectively while activity is retained so that the aluminum powder coated by metal nickel is effective; the reaction condition is controlled easily, operation thereof is convenient and easily, and the raw material is sufficient.
Owner:CHINA NAT ACAD NANOTECH & ENG
Method for preparing bimalieimide resin modified cyanate preimpregnation material
The invention provides a preparation method for bismaleimide resin modified cyanate prepreg, Imide-thioether with end capped by maleic anhydrided and cyanate dissolve in one or the mixture of dioxane, ethylene glycol monomethyl ether and glycol dimethyl ether to be heated and stirred to obtain a resin solution used for impregnating fibers. The preparation method has the advantages that bismaleimide resin and cyanate copolymer with good forming processes are adopted, and low boiling point solvents are adopted to be propitious to the preparation of high performance composites; the tensile strength of the composites which are enhanced by glass fibers is more than 300MPa, and the elongation at break is more than 10 percent. Compared with the ordinary bismaleimide resin modified cyanate, the composite of the invention which is prepared by the bismaleimide resin modified cyanate has higher toughness.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com