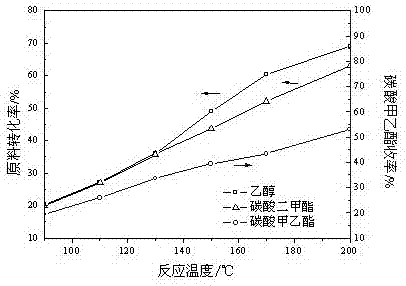
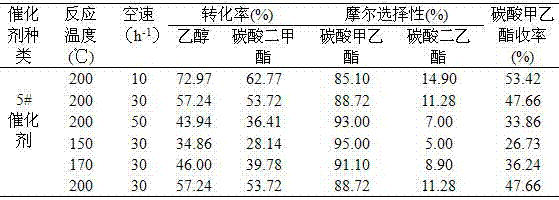
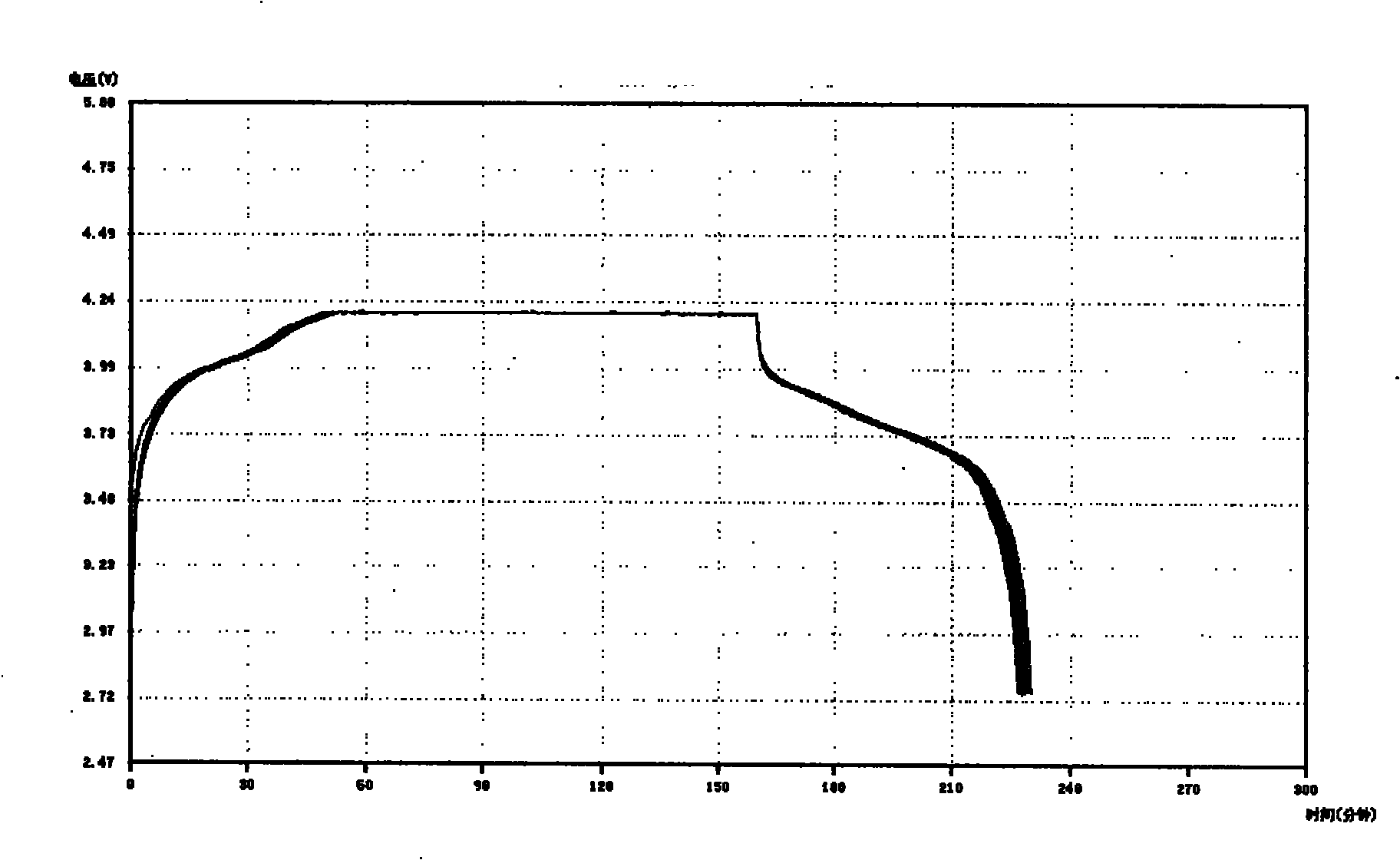
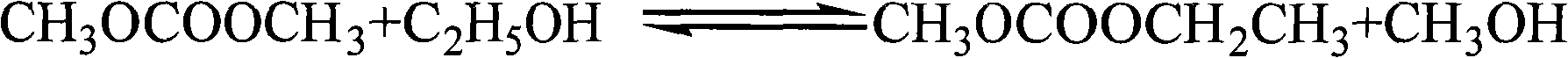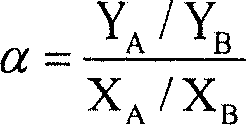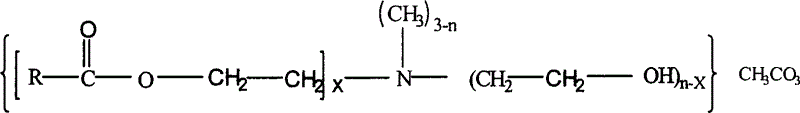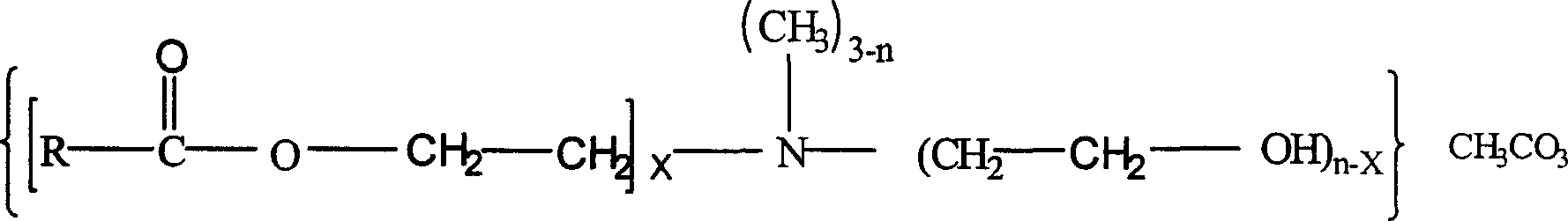Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1340 results about "Methyl carbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dimethyl carbonate (DMC) is an organic compound with the formula OC(OCH3)2. It is a colourless, flammable liquid. It is classified as a carbonate ester. This compound has found use as a methylating agent and more recently as a solvent that is exempt from the restrictions placed on most volatile organic compounds (VOCs) in the US.
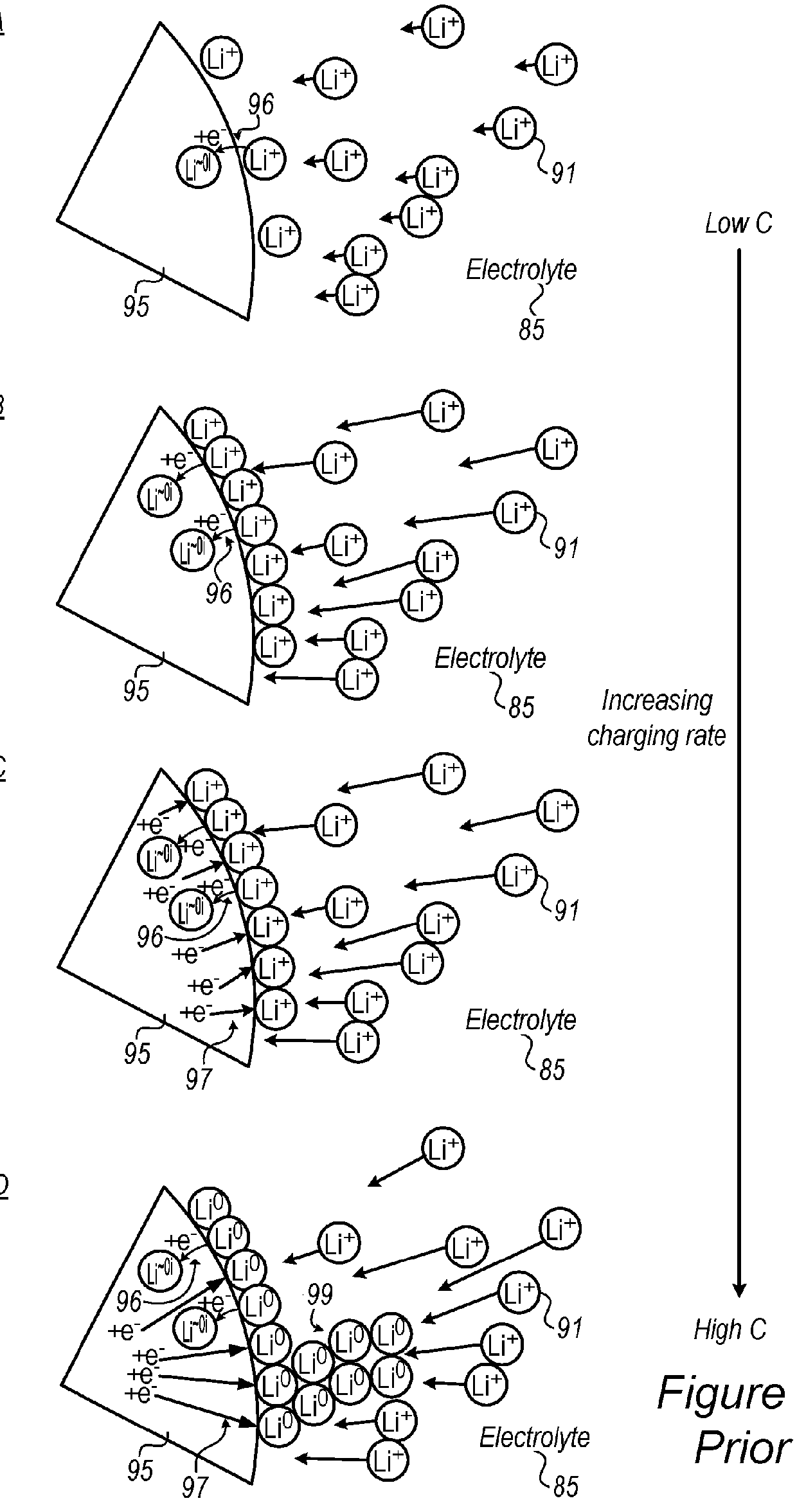
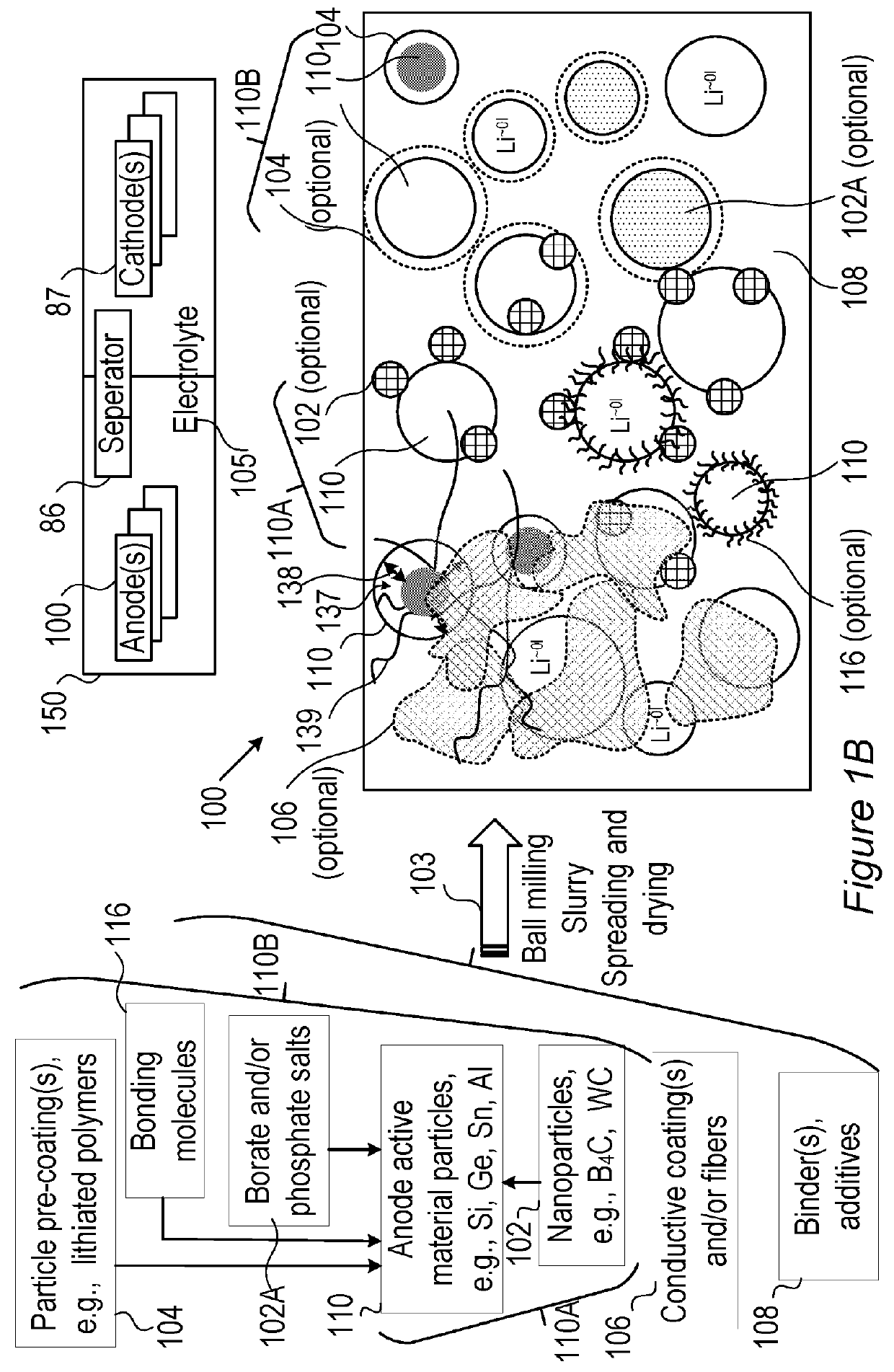
Lithium ion battery with high voltage electrolytes and additives
ActiveUS20110136019A1Final product manufactureElectrode carriers/collectorsMethyl carbonateHigh pressure
Desirable electrolyte compositions are described that are suitable for high voltage lithium ion batteries with a rated charge voltage at least about 4.45 volts. The electrolyte compositions can comprise ethylene carbonate and solvent composition selected from the group consisting of dimethyl carbonate, methyl ethyl carbonate, γ-butyrolactone, γ-valerolactone or a combination thereof. The electrolyte can further comprise a stabilization additive. The electrolytes can be effectively used with lithium rich positive electrode active materials.
Owner:IONBLOX INC
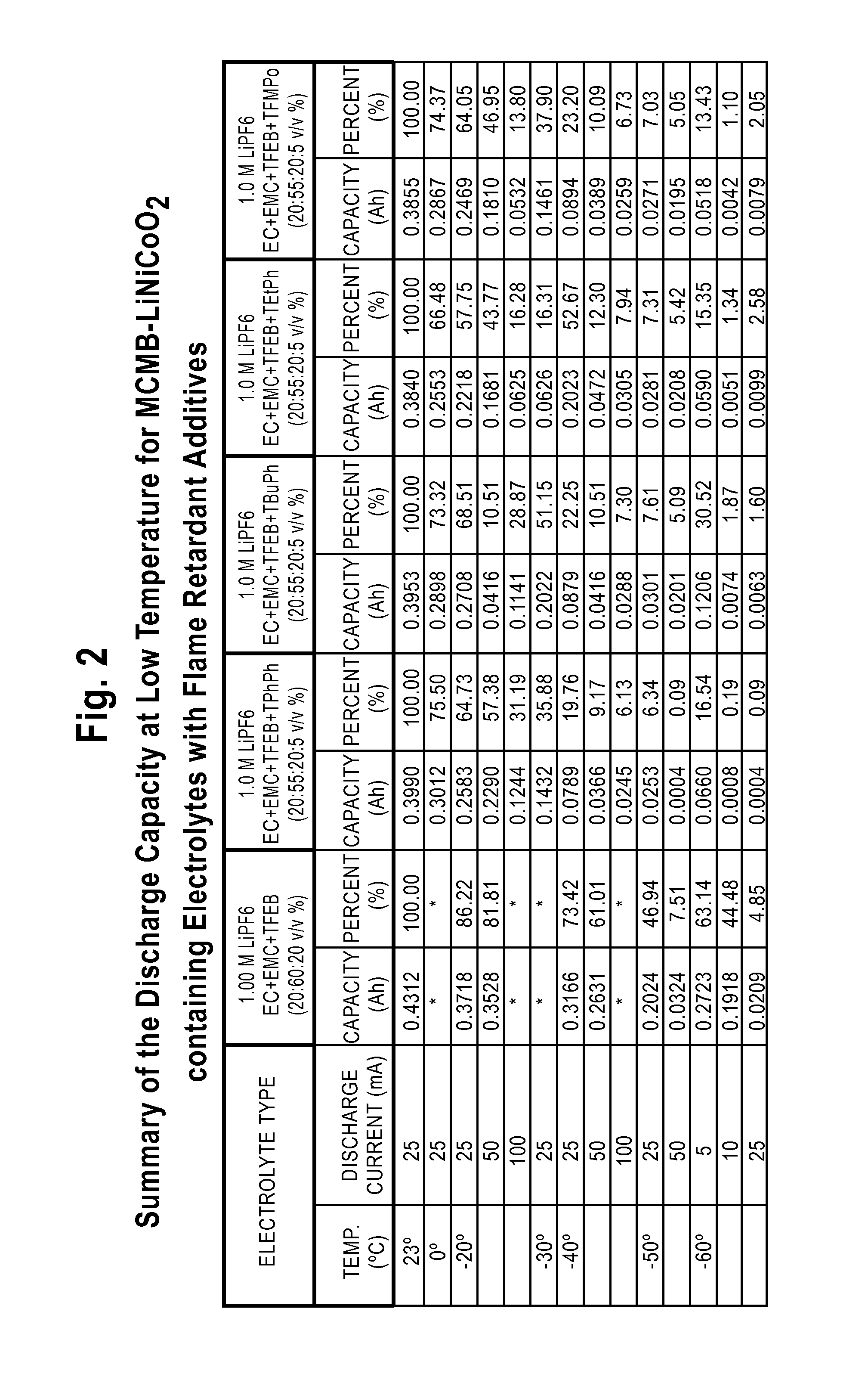
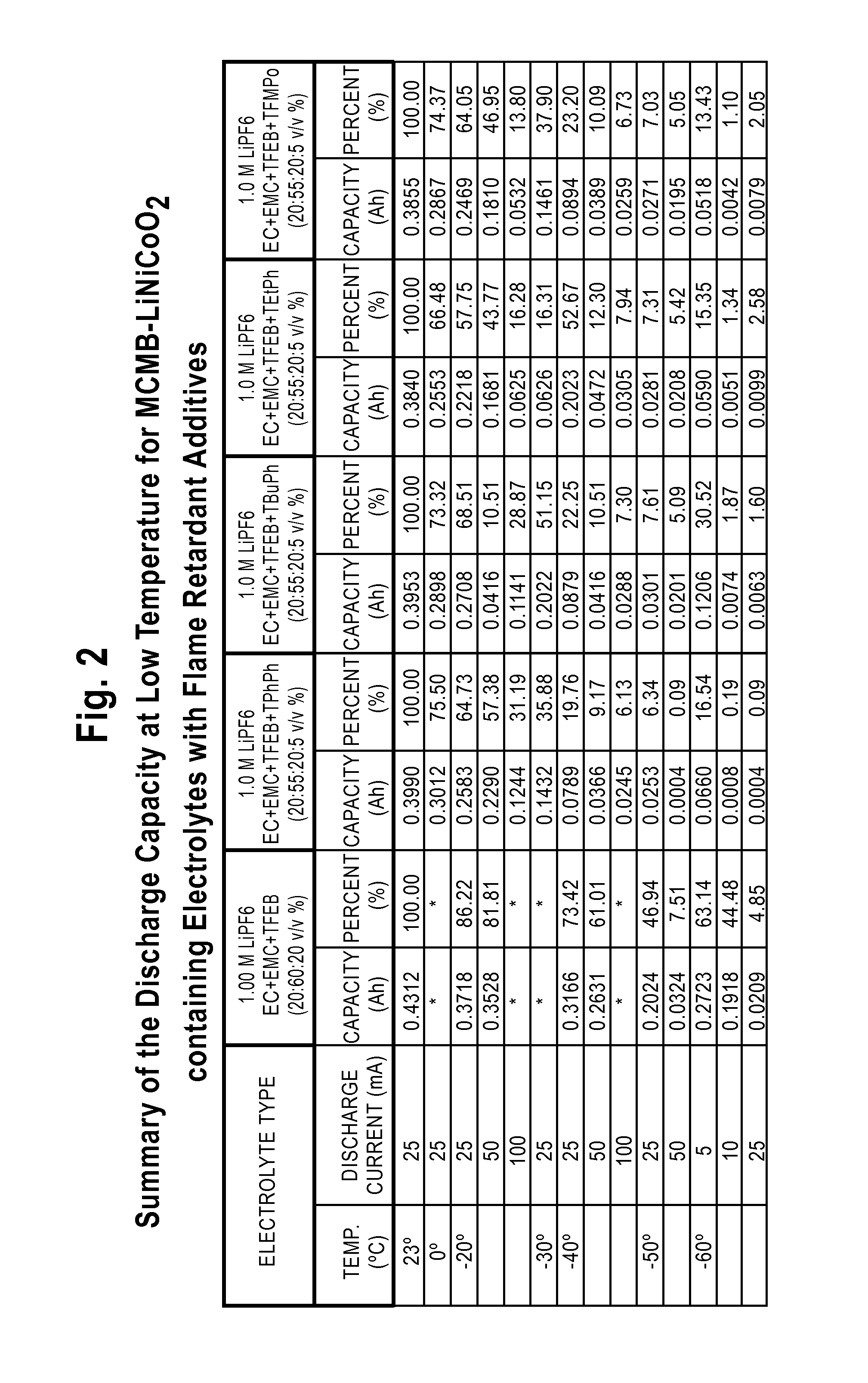
Lithium-Ion Electrolytes Containing Flame Retardant Additives for Increased Safety Characteristics
InactiveUS20100047695A1Improve performanceImprove security featuresOrganic electrolyte cellsSecondary cellsMethyl carbonatePhysical chemistry
The invention discloses various embodiments of Li-ion electrolytes containing flame retardant additives that have delivered good performance over a wide temperature range, good cycle life characteristics, and improved safety characteristics, namely, reduced flammability. In one embodiment of the invention there is provided an electrolyte for use in a lithium-ion electrochemical cell, the electrolyte comprising a mixture of an ethylene carbonate (EC), an ethyl methyl carbonate (EMC), a fluorinated co-solvent, a flame retardant additive, and a lithium salt. In another embodiment of the invention there is provided an electrolyte for use in a lithium-ion electrochemical cell, the electrolyte comprising a mixture of an ethylene carbonate (EC), an ethyl methyl carbonate (EMC), a flame retardant additive, a solid electrolyte interface (SEI) film forming agent, and a lithium salt.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Non-aqueous electrolyte for high-voltage lithium ion batteries
ActiveCN103268956ASimple compositionPromote circulationSecondary cellsHigh voltage batteryPropylene carbonate
The invention relates to a non-aqueous electrolyte for high-voltage lithium ion batteries, which is prepared from the following raw materials in percentage by weight: 70-85% of carbonate, 3-20% of functional additive and 11-17% of lithium hexafluorophosphate. The carbonate is one or mixture of more of ethylene carbonate, propylene carbonate, butylene carbonate, dimethyl carbonate, diethyl carbonate, dipropyl carbonate, methylethyl carbonate, methyl propyl carbonate and methyl butyl carbonate; and the functional additive is one or mixture of more of 0.5-10% of negative pole film-forming additive, 0.5-10% of high-temperature additive, 0.5-10% of positive pole film-forming additive, 0.5-10% of high-voltage additive and 0.001-2% of stability additive. The invention solves the problem of adaptation of the lithium ion battery electrolyte to the 4.35V high-voltage battery positive / negative pole, and provides an electrolyte for high-voltage batteries, which has the advantages of high cycle life, low inflation rate and favorable high-temperature properties.
Owner:广东金光高科股份有限公司
Lithium-ion secondary battery and electrolyte thereof
InactiveCN103078141AFacilitated DiffusionImproved magnification performanceSecondary cellsNon-aqueous electrolyte accumulator electrodesMethyl carbonateCarbonate
The invention discloses a lithium-ion secondary battery and an electrolyte thereof. The electrolyte comprises a solvent, lithium salt and a film forming additive, wherein the solvent comprises a first solvent and a second solvent; the first solvent comprises linear carboxylic ester and ethylene carbonate; the second solvent is one or more of ethyl methyl carbonate, diethyl carbonate, dimethyl carbonate and propylene carbonate; and the film forming additive is one or more of fluoroethylene carbonate, vinylene carbonate, 1,3-propane suhone, succinonitrile, adiponitrile, lithium bis(oxalato)borate, and lithium oxalyldifluoroborate. Due to the collocation of linear carboxylic ester and ethylene carbonate, a solvent system with a higher dielectric constant and low viscosity is obtained; the film forming additive improves poor compatibility between linear carboxylic ester and graphite; and finally the lithium-ion secondary battery adopting the electrolyte presents high-power discharge capacity, excellent high-temperature cycling stability and low-temperature charge and discharge properties.
Owner:NINGDE AMPEREX TECH
Load type solid body base catalyst of synthesizing dimethyl carbonate and method of preparing the same
InactiveCN101249452AEasy to makeEasy to preparePhysical/chemical process catalystsPreparation from organic carbonatesMethyl carbonatePotassium fluoride
The invention relates to a carrier type solid base catalyst for synthesizing dimethyl carbonate, which belongs to the technical field of catalytic material. The catalyst's formula is KF / M<2+>-N<3+>-(O), wherein M<2+>-N<3+>-(O) represents the composite metal oxide, M<2+> can be divalent metal ion (Mg<2+> or Ca<2+> ) and N<3+> can be trivalent metal ion (Fe<3+> and / or Al<3+>). The catalyst is prepared from potassium fluoride and composite metal oxide at a certain weight ratio by immersing the potassium fluoride into the composite metal oxide, wherein the composite metal oxide is prepared from hydrotalcite precursor and in nucleation / crystallization isolation method by calcining at a certain temperature. The catalyst has the advantages of nanoscaled particle size, high specific surface area and high activity, selectivity in catalytic reaction and simple preparation, and can be reused as it can be centrifugally separated after reacting.
Owner:BEIJING UNIV OF CHEM TECH
Rechargeable lithium electrochemical cell
InactiveUS6942949B2Organic electrolyte cellsNon-aqueous electrolyte accumulator electrodesMethyl carbonatePhosphate
A secondary battery is comprised of a positive electrode, a negative electrode formed from a lithium storage material, and a non-aqueous electrolyte. The non-aqueous electrolyte includes a lithium salt, non-aqueous aprotic solvent(s), such as ethylen carbonate, propylene carbonate, dimethyl carbonate, ethymethyl carbonate and diethyl carbonate, and a small percentage of at least one organic additive. The negative electrode may comprise a carbon such as graphite, and the positive electrode may comprise a lithiated metal oxide or phosphate, such as LiCoO2, LiNiO2, LiMn2O4, LiFePO4, or mixtures thereof. The organic additives have one or more unsaturated bonds activated with respect to oxidation by electron-pushing alkyl groups. They are in most cases known to be able to undergo polymerization reactions, such as an anodically induced polymerization especially under certain conditions. The additives are oxidized at the cathode at a potential of more than 4.3 V vs. Li / Li+. With these additives in amounts of 0.001 to 10%, a passivation layer is formed on the cathodes, and the sensitiveness of the battery against overcharge is reduced. The electrolyte mixtures do not deteriorate the properties of the battery anodes.
Owner:LG ENERGY SOLUTION LTD
Lithium-Ion Electrolytes With Improved Safety Tolerance To High Voltage Systems
ActiveUS20120141883A1Improved safety toleranceExcellent rate performanceOrganic electrolyte cellsLi-accumulatorsMethyl carbonateHigh voltage cathode
The invention discloses various embodiments of electrolytes for use in lithium-ion batteries, the electrolytes having improved safety and the ability to operate with high capacity anodes and high voltage cathodes. In one embodiment there is provided an electrolyte for use in a lithium-ion battery comprising an anode and a high voltage cathode. The electrolyte has a mixture of a cyclic carbonate of ethylene carbonate (EC) or mono-fluoroethylene carbonate (FEC) co-solvent, ethyl methyl carbonate (EMC), a flame retardant additive, a lithium salt, and an electrolyte additive that improves compatibility and performance of the lithium-ion battery with a high voltage cathode. The lithium-ion battery is charged to a voltage in a range of from about 2.0 V (Volts) to about 5.0 V (Volts).
Owner:UNIV OF SOUTHERN CALIFORNIA +1
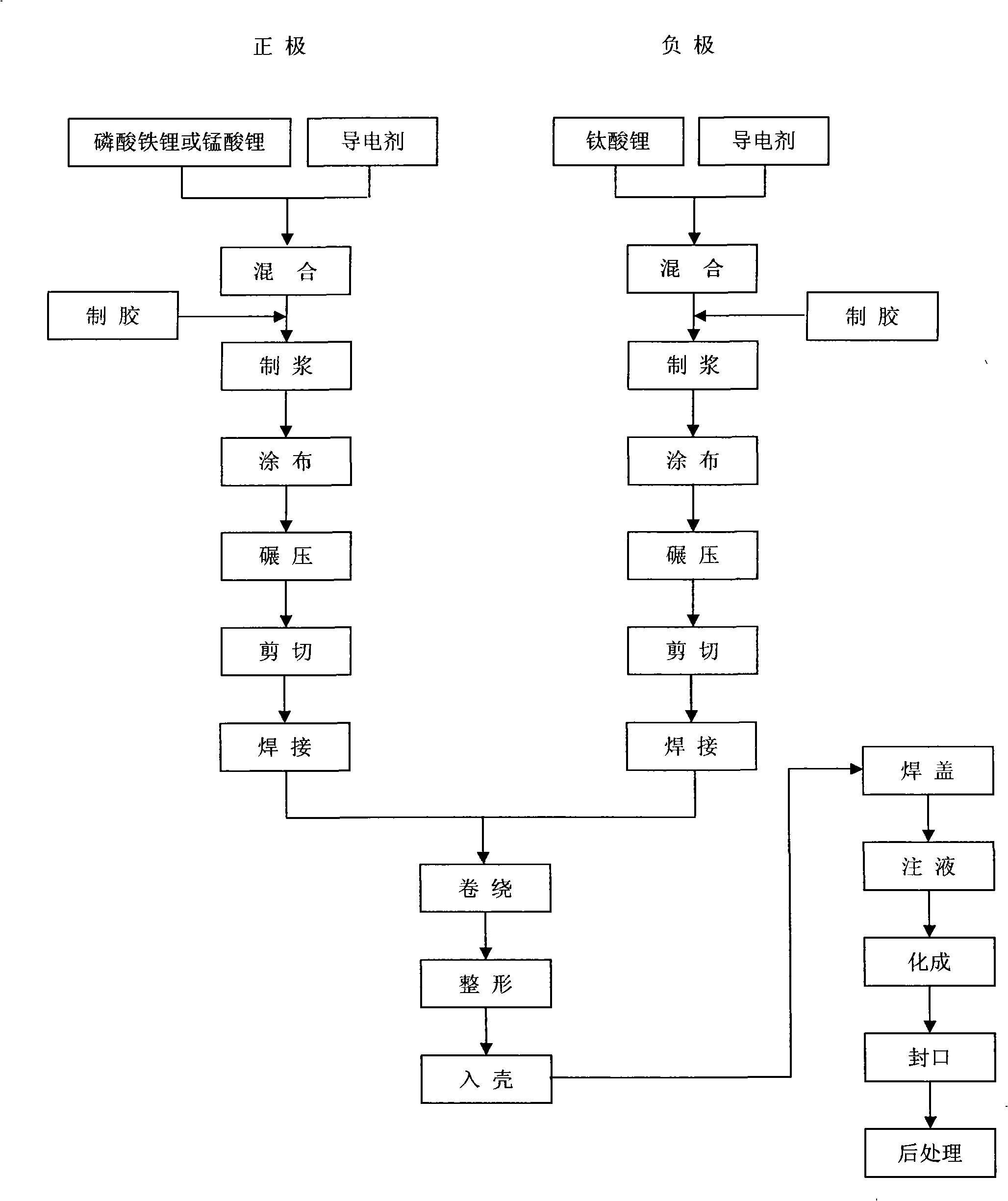
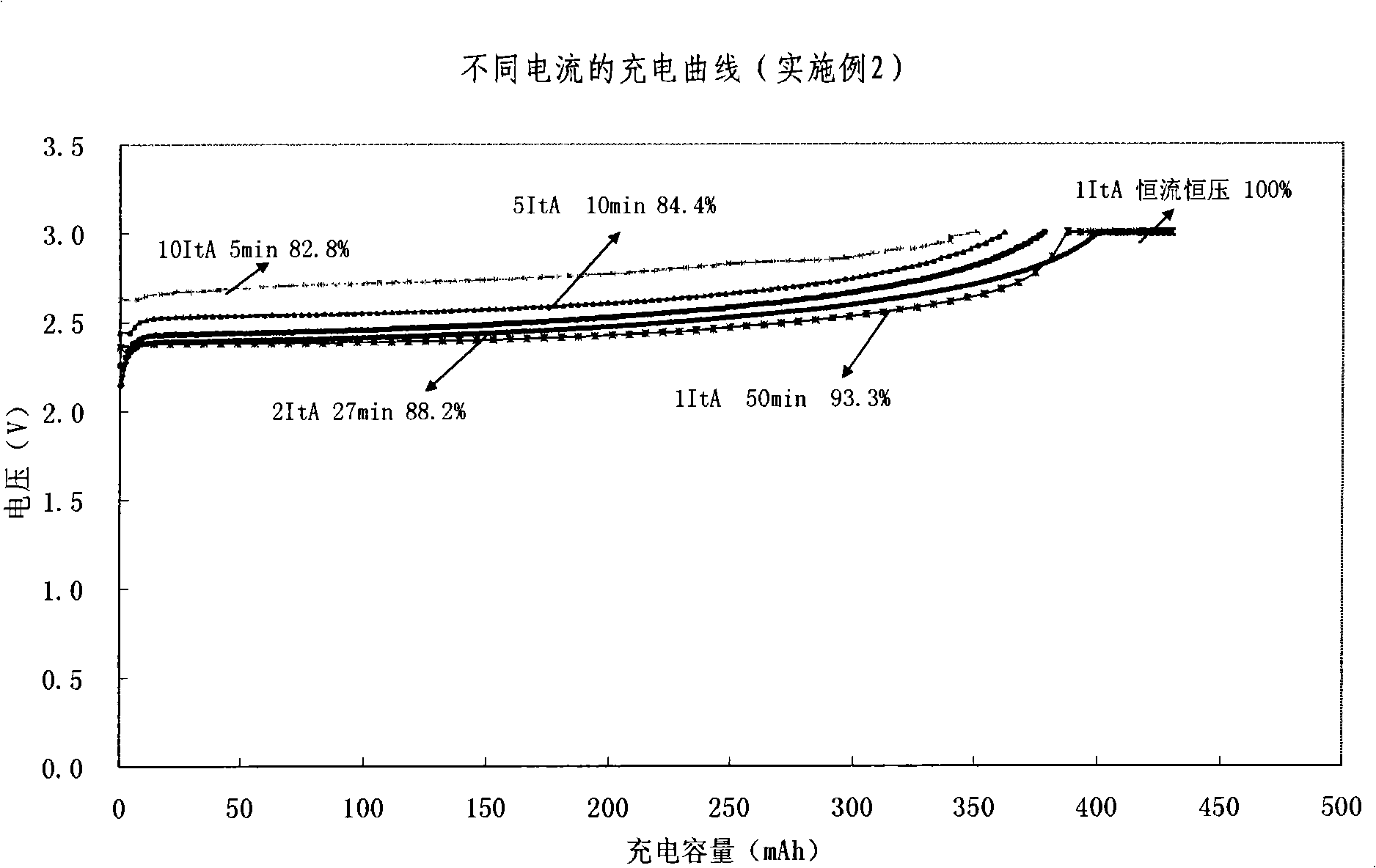
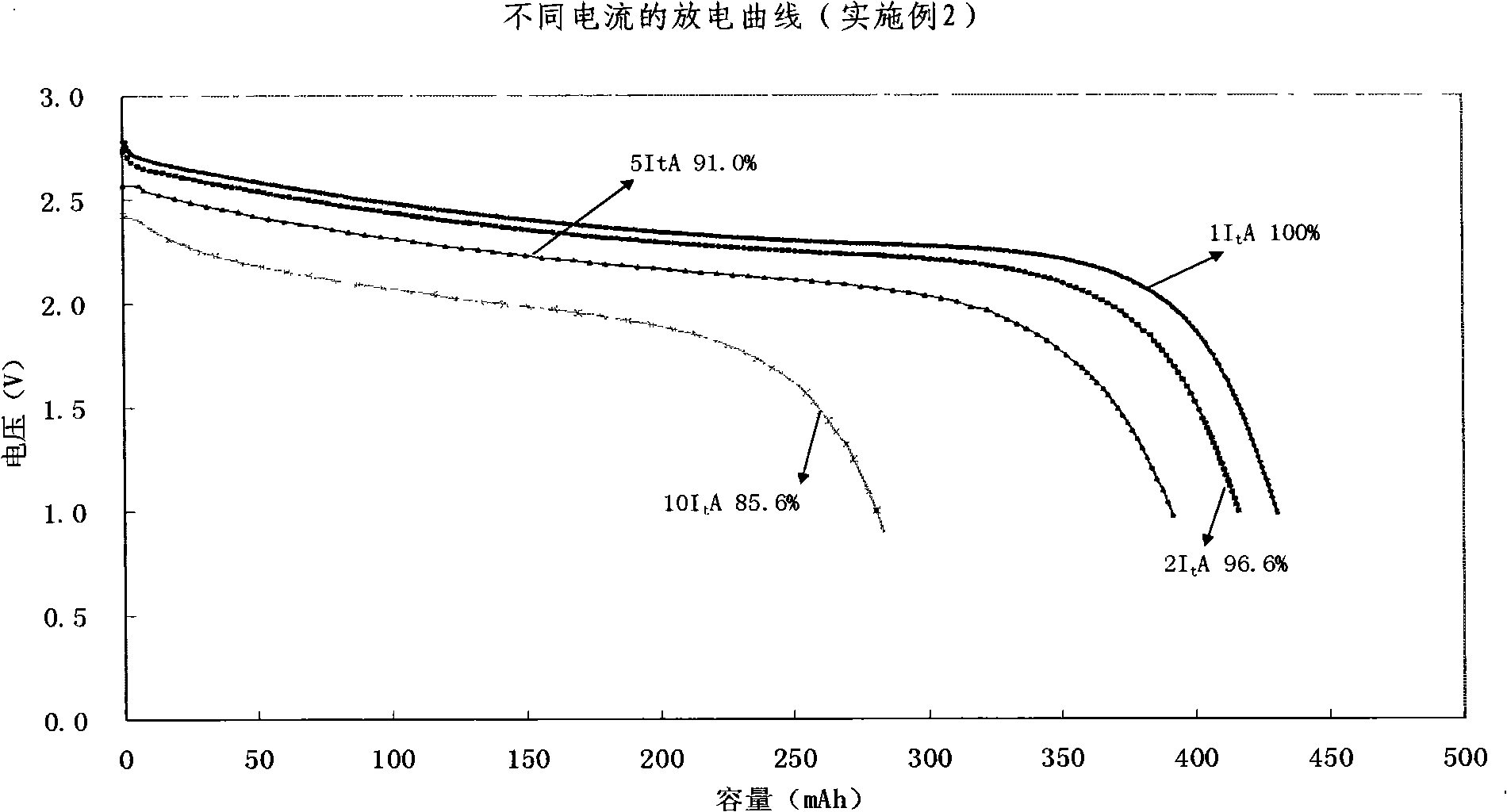
Quickly chargeable lithium ion battery and its making method
InactiveCN101262078AExcellent fast charge and discharge performanceCell electrodesFinal product manufactureMethyl carbonateManganate
The invention discloses a lithium-ion battery with fast charge property, which comprises an anode, a cathode, a diaphragm sandwiched between the anode and the cathode and an organic electrolyte, wherein, sub micrometer lithium titanate is used as the active substance of the cathode; one or several materials of lithium manganate (LiMn2O4), lithium iron phosphate (LiFePO4), lithium nickel cobalt oxide (LiNixCoyMzO2), ternary substance(LiNixMnxCo1-2xO2) are used as active materials of the anode, or a mixture of lithium cobalt (LiCoO2) and one of lithium manganate (LiMn2O4), lithium iron phosphate (LiFePO4), lithium nickel cobalt oxide (LiNixCoyMzO2), ternary substance(LiNixMnxCo1-2xO2) is adopted as the active materials of the anode; lithium hexafluorophosphate (LiPF6) is adopted as the electrolyte, and a multicomponent mixture of ethylene carbonate (EC), dimethylcarbonate(DMC), Ethyl Methyl Carbonate (EMC) is used as a solution. The invention also discloses a preparation method of the lithium-ion battery with fast charged property. The lithium-ion battery of the invention has excellent fast charging and discharging performance.
Owner:TIANJIN B&M SCI & TECH
Non-aqueous electrolyte for lithium iron phosphate battery
The invention discloses a non-aqueous electrolyte for a lithium iron phosphate battery. The non-aqueous electrolyte comprises 70 to 85 weight percent of carbonic ester compound, 3 to 20 weight percent of various function additives and 11 to 17 weight percent of lithium hexafluorophosphate, wherein the carbonic ester compound is one of ethylene carbonate, propylene carbonate, butylene carbonate, dimethyl carbonate and diethyl carbonate or a mixture of more of the ethylene carbonate, the propylene carbonate, the butylene carbonate, the dimethyl carbonate and the diethyl carbonate; and the additives comprise one of 0.5 to 10 percent of film-forming additive, 0.5 to 10 percent of high-temperature additive, 0.5 to 10 percent of low-temperature additive, 0.5 to 10 percent of overcharge-preventing additive and 0.001 to 2 percent of stability additive, and a mixture of more of the additives. The non-aqueous electrolyte for the lithium iron phosphate battery has the advantages that the solubility and dissociation of the lithium hexafluorophosphate are improved, and electric conductivity is improved; the low temperature resistance of a solid electrolyte interphase (SEI) is reduced; the overall stability of the battery is improved, the overall service life of the battery is prolonged, the compatibility of an electrolyte and a cathode is improved, circulation of the battery is improved, and the service life is prolonged; and the non-aqueous electrolyte can have high performance at high temperature.
Owner:广东金光高科股份有限公司
Lithium ion electrolytes and lithium ion cells with good low temperature performance
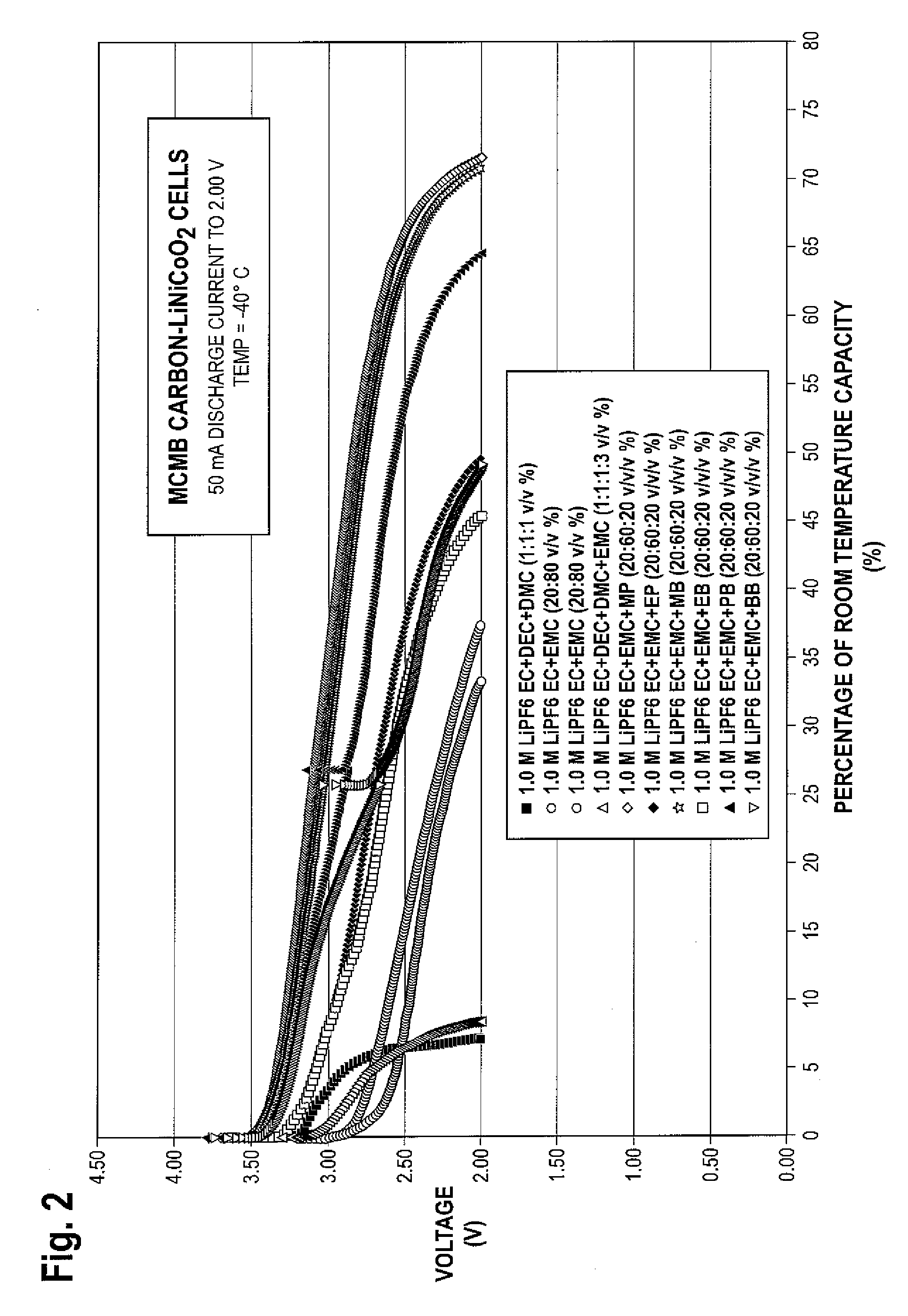
ActiveUS20090253046A1Improve low temperature performanceImprove stabilityOrganic electrolyte cellsNegative electrodesCelsius DegreeMethyl carbonate
There is provided in one embodiment of the invention an electrolyte for use in a lithium ion electrochemical cell. The electrolyte comprises a mixture of an ethylene carbonate (EC), an ethyl methyl carbonate (EMC), an ester cosolvent, and a lithium salt. The ester cosolvent comprises methyl propionate (MP), ethyl propionate (EP), methyl butyrate (MB), ethyl butyrate (EB), propyl butyrate (PB), or butyl butyrate (BB). The electrochemical cell operates in a temperature range of from about −60 degrees Celsius to about 60 degrees Celsius. In another embodiment there is provided a lithium ion electrochemical cell using the electrolyte of the invention.
Owner:CALIFORNIA INST OF TECH
Metal oxide catalyst for synthesizing methyl carbonate by urea process and its prepn
InactiveCN1416949ASolve key problems such as difficult separation and recyclingHigh yieldOrganic compound preparationMetal/metal-oxides/metal-hydroxide catalystsMethyl carbonateCoprecipitation
The metal oxide catalyst for urea alcoholysis process of synthesizing methyl carbonate consists of 1-3 kinds oxides of Li, Mg, Ni, Zn, Pb, Al, Fe, Mo, Zr and La, and has ZnO as main component accounting for 35-95 wt%. When it consists of three kinds of metal oxide, the other two except ZnO have the same weight percentage. It is prepared through thermal decomposition process, precipitation process, or coprecipitation process. The catalyst is easy to prepare, separate, recover and regenerate, and has high catalytic activity for urea alcoholysis process of synthesizing methyl carbonate, yield up to 49.7%, no corrosion of equipment and no environmental pollution.
Owner:HEBEI UNIV OF TECH
Lithium-ion electrolytes containing flame retardant additives for increased safety characteristics
InactiveUS8795903B2Improve featuresImprove performanceElectrolytic capacitorsOrganic electrolyte cellsMethyl carbonatePhysical chemistry
The invention discloses various embodiments of Li-ion electrolytes containing flame retardant additives that have delivered good performance over a wide temperature range, good cycle life characteristics, and improved safety characteristics, namely, reduced flammability. In one embodiment of the invention there is provided an electrolyte for use in a lithium-ion electrochemical cell, the electrolyte comprising a mixture of an ethylene carbonate (EC), an ethyl methyl carbonate (EMC), a fluorinated co-solvent, a flame retardant additive, and a lithium salt. In another embodiment of the invention there is provided an electrolyte for use in a lithium-ion electrochemical cell, the electrolyte comprising a mixture of an ethylene carbonate (EC), an ethyl methyl carbonate (EMC), a flame retardant additive, a solid electrolyte interface (SEI) film forming agent, and a lithium salt.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Method for separating dimethyl carbonate and methanol azeotrope
ActiveCN101143803AAchieve separationNo secondary pollutionDistillationHydroxy compound separation/purificationHigh concentrationVacuum pumping
The utility model relates to a method which utilizes a vapour permeation membrane to separate dimethyl carbonate from methanol azeotrope. Saturated vapour at the temperature of 64 to 120 DEG C and with the pressure of 0.10 to 0.6 MPa is sent into a membrane separator and is contacted with a separation membrane, the downstream side of the membrane separator keeps the vacuum degree at 4 to 200 mmHg by vacuum pumping, the dimethyl carbonate first passes through a concentration membrance filter at the permeation side of the membrane and is condensed into liquid under the condition of vacuum and low pressure, the liquid mixture directly enters into an atmospheric rectifying column, dimethyl carbonate with more than or equal to ninety nine point five percent of content is produced from the bottom of the column, and high-concentration methanol is produced from the interception side of the membrane separator and enters into next technique unit. The separation membrane is an organic composite membrane, an organic and mineral composite membrane or a mineral membrane which can preferentially permeate dimethyl carbonate, and the method has the advantages of simple technique and low energy consumption; the throughput of the permeation of the membrane is increased, and the area of the membrane is reduced; safety and reliability are increased, and the purity of dimethyl carbonate is increased as well.
Owner:PETROCHINA CO LTD +1
Method for synthesizing dimethyl carbonate by using urea and methanol and adopting heterogeneous catalyst
InactiveCN1428329ALow costReact SafeOrganic compound preparationCarbonic/haloformic acid esters preparationAlkaline earth metalMethyl carbonate
The method for synthesizing dimethyl carbonate by using urea and methanol and adopting heterogeneous catalyst includes the following steps: under the condition of 600-1300 deg.c 10X10 to the power 5-1X10 to the power 5 pa and air or oxygen gas atmosphere calcining carbonate or hydroxide of alkali metal and alkaline earth metal for 1-10 hr. to obtain catalyst, adding urea and methanol into high-pressure reaction still, and adding catalyst, heating to 120-180 deg.c under the condition of magnetic stirring, low-temp. resistion time is 2 hr-10 hr. more heating to 180-240 deg.C, high-temp. reaction time 2 hr-20 hr, cooling to room temp. and making separation so as to obtain the invented roduct.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Method for preparing methyl ethyl carbonate
InactiveCN101289395AHigh yieldPreparation from organic carbonatesTransesterificationMethyl carbonate
The invention relates to a preparation method of methyl ethyl carbonate, which takes methyl carbonate and ethanol as raw material and carries on transesterification with the existence of the catalyst with the reaction temperature of 50 DEG C to 60 DEG C. The temperature of the reaction materials rises from room temperature with a heating rate of five to ten DEG C per hour. The method also includes a water removing treatment to the ethanol so that the water content of the ethanol is less than or equal to 0.1 percent of the gross weight of the ethanol. By adopting the preparation method of the invention, the purity of the methyl ethyl carbonate is as high as 99.8 percent and the yield is higher than 70 percent. The method has the advantages of mild reaction condition, simple separation and purification of reaction products and low cost.
Owner:BYD CO LTD
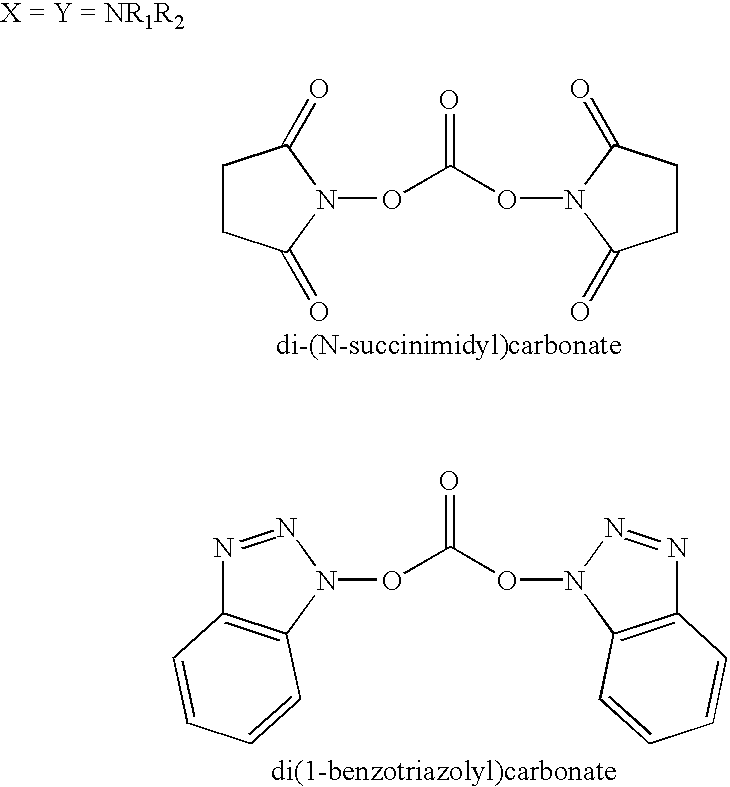
Organic carbonate additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS20030129500A1Improve oxidation stabilityImprove dynamic stabilityElectrode carriers/collectorsOrganic electrolyte cellsMethyl carbonateSolvent
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one carbonate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture including ethylene carbonate, dimethyl carbonate, ethyl methyl carbonate and diethyl carbonate. The preferred additive is either a linear or cyclic carbonate containing covalent O-X and O-Y bonds on opposite sides of a carbonyl group wherein at least one of the O-X and the O-Y bonds has a dissociation energy less than about 80 kcal / mole.
Owner:WILSON GREATBATCH LTD
Bimetal composite oxide catalyst for change of methyl carbonate and phenol ester
ActiveCN1803282AHigh catalytic activityEasy to separatePreparation from organic carbonatesMetal/metal-oxides/metal-hydroxide catalystsMethyl carbonateCopper oxide
The invention discloses a two-metal composite oxide catalyst of synthetic biphenyl carbonate through ester exchange, which consists of vanadium oxide and copper oxide, wherein the mole ratio is that V:Cuú¢100í1-100; The sintering temperature is between 250 and 850 deg C; the parent body can be vanadium salt or vanadium oxide and copper salt or copper oxide; the making method adapts coprecipitation method or mechanic abrasion method. The invention improves the total productivity of biphenyl carbonate, which is easy to separate and recover the biphenyl carbonate.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI +1
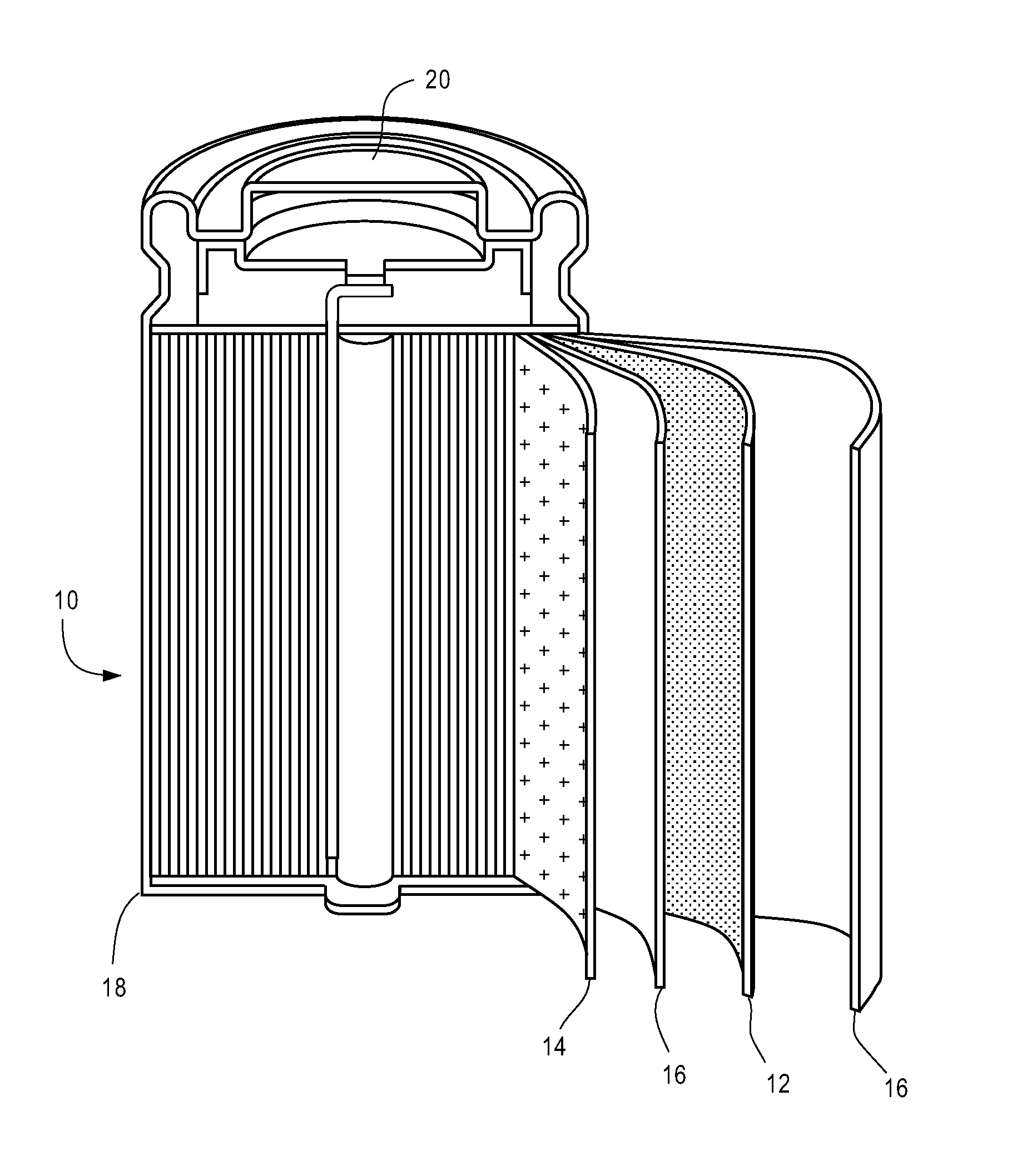
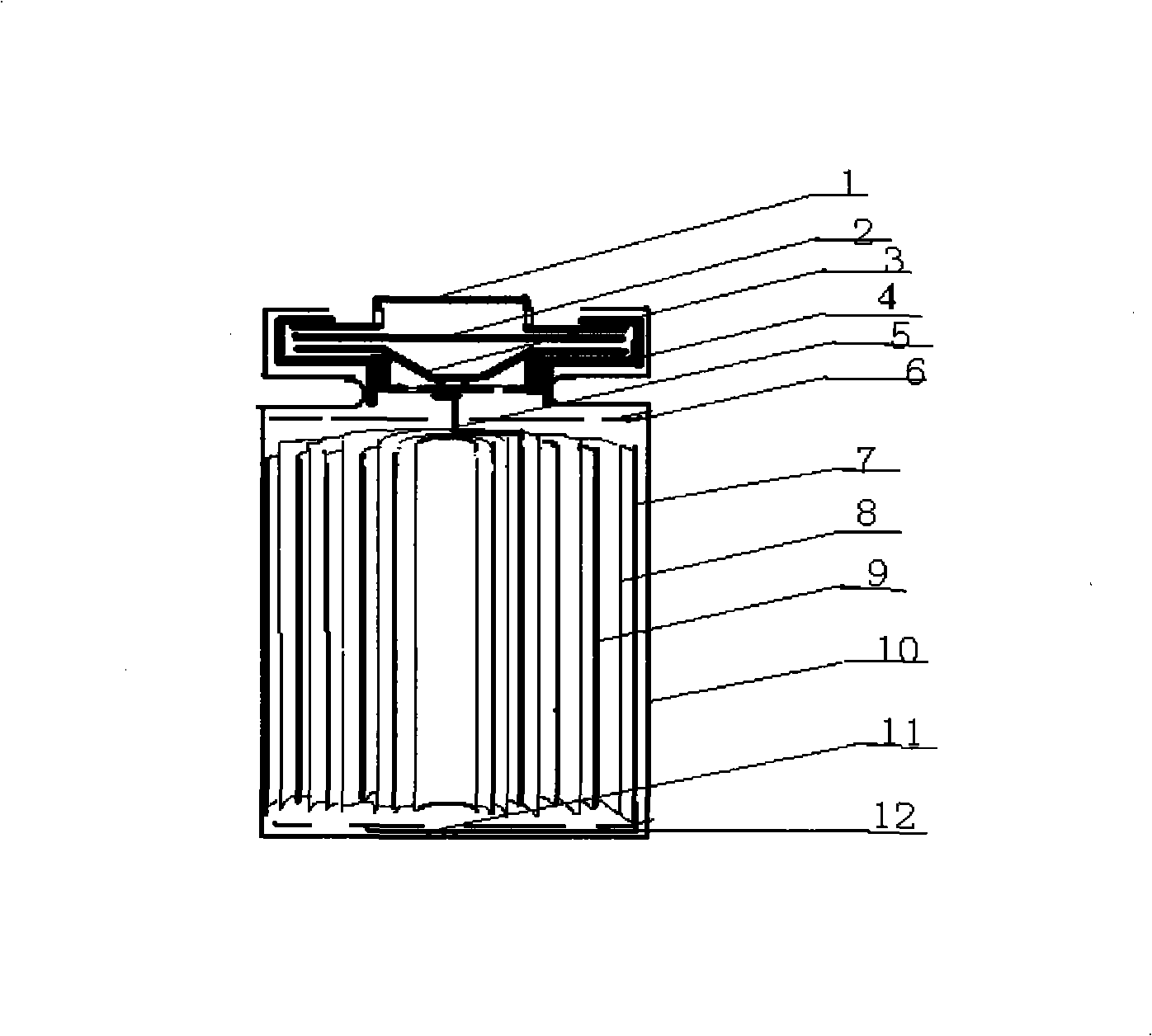
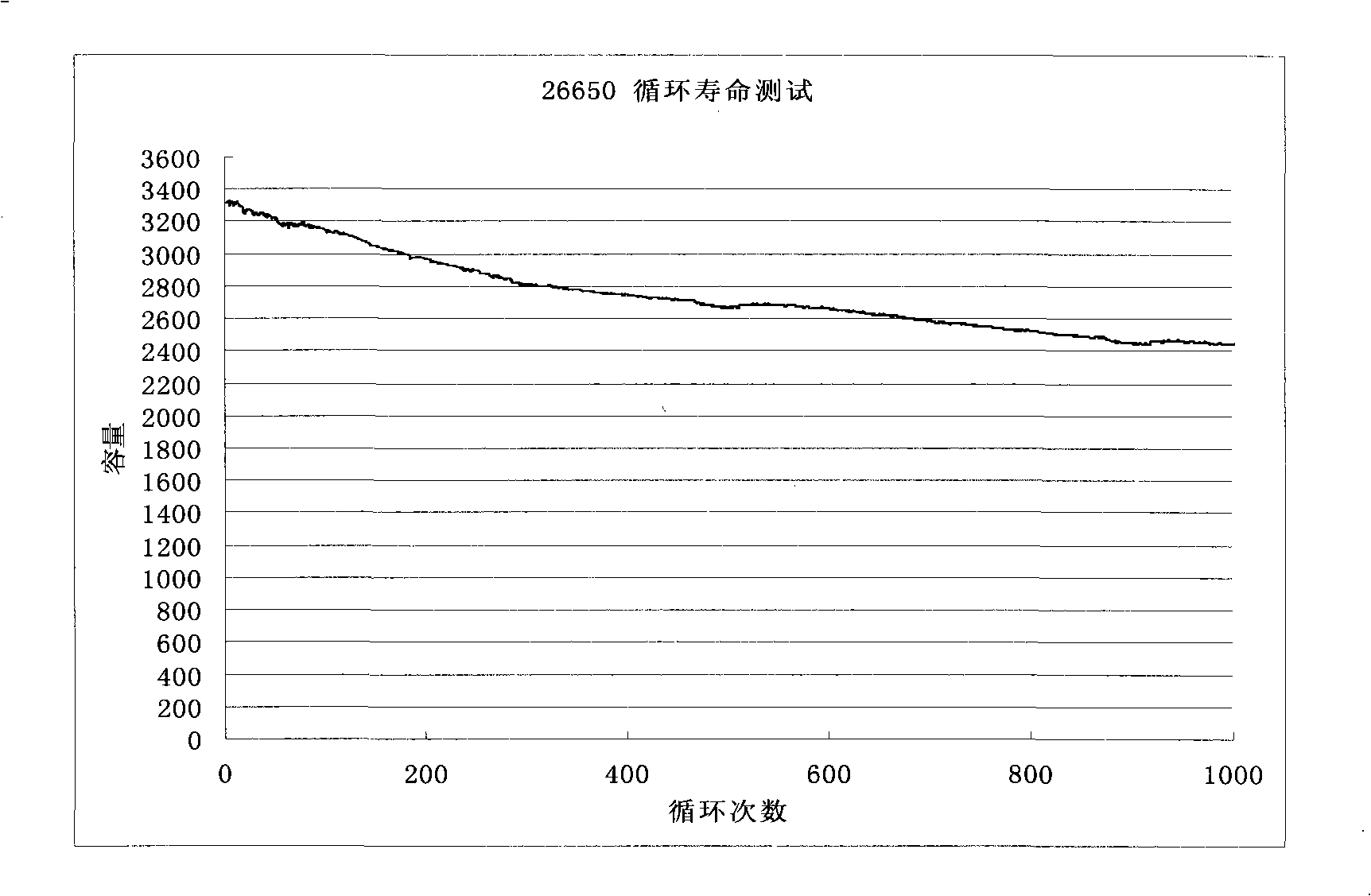
A high-capacity secure 26650 lithium ion battery and its making method
InactiveCN101262077AHigh feasibilityFinal product manufactureCell electrodesMethyl carbonatePhosphate
The invention relates to the technical field of a secondary power (a chargeable battery) which can be reused, in particular to a 26650 type lithium ion battery which is safe and has high capacity and a preparation method thereof. Lithium ferrous phosphate is used as cathode material; graphite materials are adopted as anode materials; mixture of EC (ethylene carbonate), DMC (Di Methyl Carbonate), EMC (methyl ethyl carbonate) (based on volume ratio of 1: 1: 1) is used as electrolyte solution, and LiPF6 (lithium hexafluorophosphate ) with molar ration 1.0 to 1.4 mol / L is adopted as electrolyte; multi-porous PP (polypropylene) and PE (polyethylene) are used as separating membrane, and a metal tank is used as a shell, thus the 26650 type battery is made with the diameter of 26mm and the height of 65mm; the capacity of the battery can reach 3200mAh, the internal resistance is within 30mOmega, and the cycle life thereof is more than 1000 times. The 26650 type lithium ion battery mainly solves the limitation problem of the existing lithium ion battery in the respect of safe and large size, and develops towards large size, has longer service life and higher safety and belongs to an environment protection chemical power with wider application.
Owner:上海德朗能电池有限公司
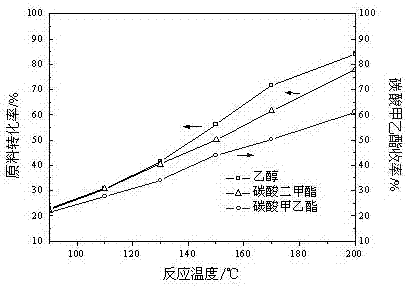
Method for synthesizing methylethyl carbonate
ActiveCN102850223AHigh catalytic activityLow costOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingMethyl carbonateReaction temperature
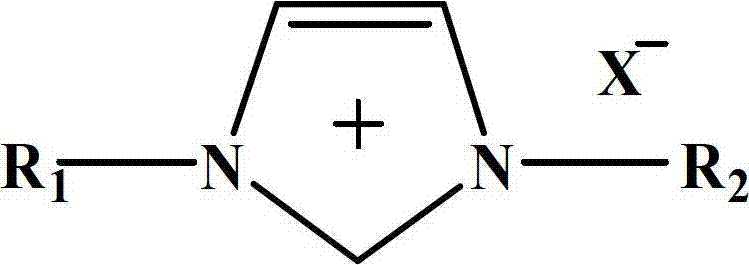
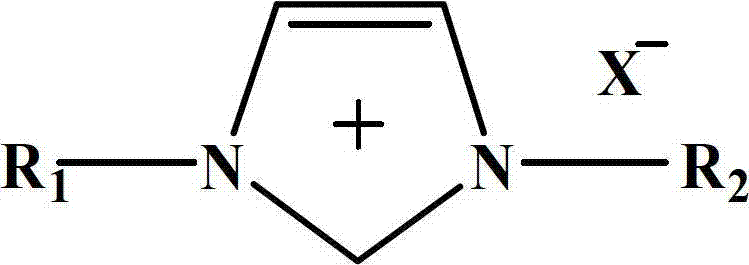
The invention relates to a method for synthesizing methylethyl carbonate. According to the method, under ordinary pressure, dimethyl carbonate and ethanol which are used as raw materials are subjected to synthetic reaction of methylethyl carbonate at certain reaction temperature for some time by using an imidazole ionic liquid as a catalyst. The invention has the following advantages: the catalyst consumption is only 0.5-5 wt% of the dimethyl carbonate; the ionic liquid catalyst has high catalytic activity in the reaction process, the maximum yield of methylethyl carbonate is up to 71.22%, and the selectivity is up to 88.52%; and the catalyst can be recycled for cyclic utilization after being subjected to simple treatment after reaction, has the advantages of long service life and no pollution, and greatly lowers the preparation cost of methylethyl carbonate.
Owner:辽阳东昌化工股份有限公司
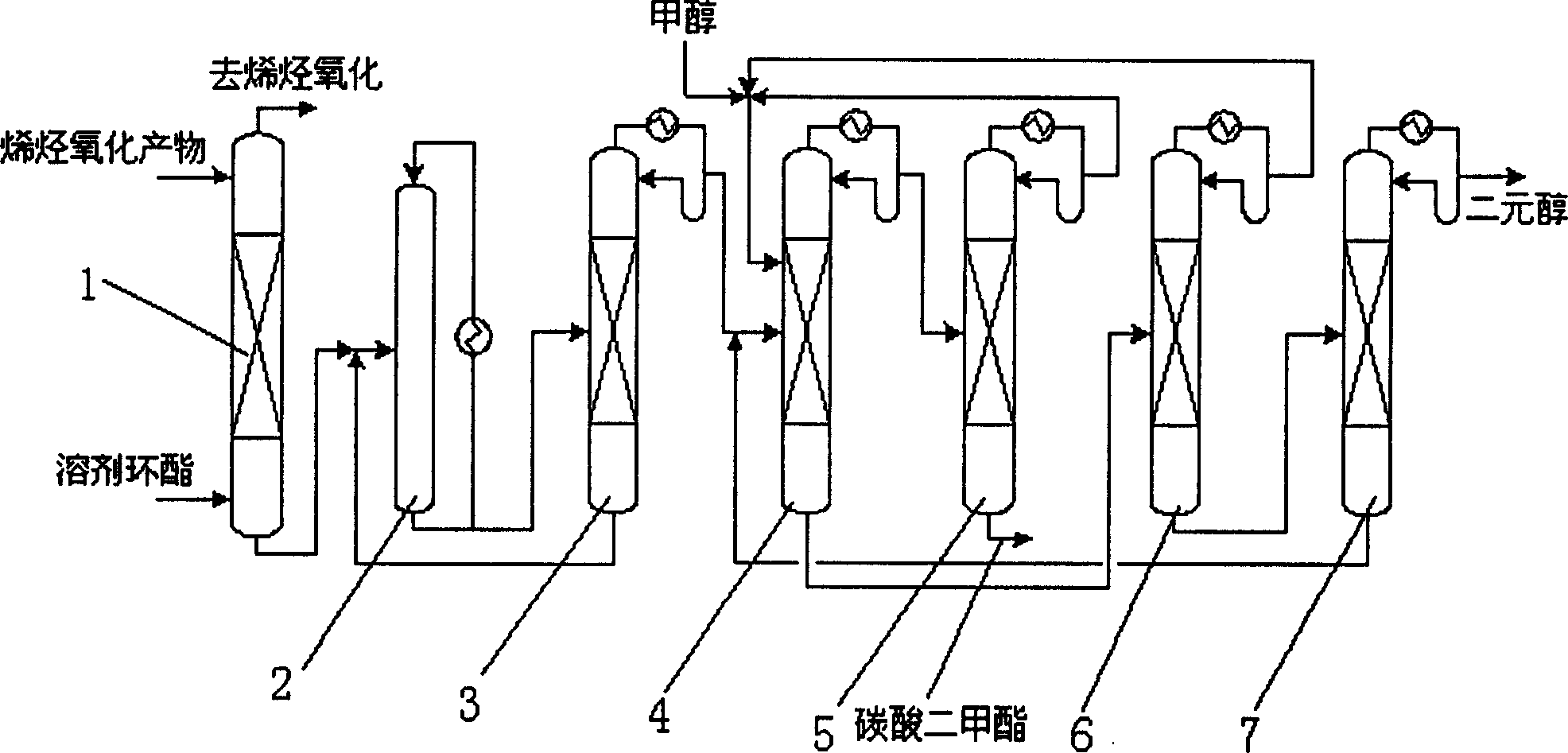
Method of distillation and ester exchange reaction for producing dimethyl carbonate and dihydroxyl alcohols
InactiveCN1733696AIncrease conversion rate per passReduce energy consumptionPreparation from organic carbonatesMentholAlcohol
The invention discloses a method of distillation and ester exchange reaction for producing dimethyl carbonate and dihydroxyl alcohols comprising the following steps, counterflow contacting cyclic carbonates absorbent with mixed gas containing alkylene oxide and carbon dioxide in an absorption column, reacting alkylene oxide with carbon dioxide to obtain cyclic carbonate, the cyclic carbonate synthesized liquid entering a rectifying tower, the cyclic carbonate on top of the tower entering an reaction rectifying tower through pipe lines, carrying out ester exchange reaction with menthol at the presence of ester exchange catalyst, obtaining dimethyl carbonate and dibastic alcohol.
Owner:上海衡和环保新材料科技有限公司
Process for synthesizing methyl carbonate by alcoholysis of urea with aliphatic diol as cyclic agent
InactiveCN1569810AEasy to separateNo pollutionOrganic compound preparationCarbonic/haloformic acid esters preparationAlcoholMethyl carbonate
The invention discloses a process for synthesizing methyl carbonate by alcoholysis of urea with aliphatic diol as cyclic agent consisting two steps, (1) reacting urea with aliphatic diatomic alcohol (ethylene glycol or 1,2-methyl ethylene glycol) to synthesize ethylene carbonate ester or propylene carbonate ester, (2) subjecting ethylene carbonate ester or propylene carbonate ester with methanol ester to synthesize methylcarbonate.
Owner:HEBEI UNIV OF TECH
Method for preparing ethyl methyl carbonate through ester exchange method
InactiveCN107473968AGood dispersionImprove mass transfer effectPhysical/chemical process catalystsPreparation from organic carbonatesDispersityMethyl carbonate
The invention provides a method for preparing ethyl methyl carbonate through an ester exchange method, and relates to a method for preparing a chemical raw material. A first catalyst prepared by the method simultaneously has macropore and micropore structures, wherein the macropores can obviously improve the mass transfer effect; and the micropores can obviously improve the specific surface area of a carrier and simultaneously improve the dispersity of active centers. Meanwhile, the prepared first catalyst simultaneously has an alkali active center and a Lewis acid catalytic active center. The prepared 15%MgO-5%MgCl2-2%La2O3 / Al2O3-SiO2 is used in a dimethyl carbonate and ethanol ester exchange fixed bed continuous reaction; when the reaction temperature is 200 DEG C and the space velocity is 30h<-1>, the catalyst is not inactivated after 5000h of continuous reaction, the dimethyl carbonate conversion rate can be kept at 70%, the ethanol conversion rate can be kept at 80%, and the yield of the product ethyl methyl carbonate is 56%; and after the reaction, the catalyst can be reused through simple filtration treatment, and the activity of the catalyst can still be kept unchanged after the catalyst is reused for multiple times.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Process for synthesizing methyl carbonate by alcoholysis of methanol and urea
InactiveCN1569809AThe reaction process is simpleEasy to operateOrganic compound preparationCarbonic/haloformic acid esters preparationChemical synthesisAlcohol
The invention discloses a process for synthesizing methyl carbonate from alcoholysis of methanol and urea in a high pressure reactor, wherein the catalyst is a amine salt type ionic liquid having a molecular formula of R1R2R3NHX-MaXb, the raw material methanol and urea are subject to alcoholysis reaction and methyl carbonate is synthesized in one step.
Owner:EAST CHINA NORMAL UNIVERSITY
Permeating gasification film used for separation of methanol/dimethyl carbonate azeotropic liquid, and its preparing method
InactiveCN101003002ALow priceReduce energy consumptionDistillationCarbonic/haloformic acid esters purification/separationMethyl carbonateALLYL SUCROSE
A gasifying osmotic film for separating the azeotropic methanol / dimethyl carbonate solution is prepared from polyvinyl alcohol and polyacrylic acid. Its preparing process is also disclosed.
Owner:山东蓝景膜技术工程有限公司
Lithium-ion secondary battery and manufacturing method thereof
InactiveCN101887990AImprove securityMeet environmental protection requirementsCell electrodesFinal product manufactureMethyl carbonateManganate
The invention discloses a lithium-ion secondary battery and a manufacturing method thereof. The lithium-ion secondary battery comprises an anode material, a cathode material, a diaphragm and electrolyte, wherein, the anode material and the cathode material are coated in a metal casing, the diaphragm is arranged between the anode material and the cathode material, and the electrolyte is filled in the diaphragm; the anode material is prepared by mixing a Ni-Co-Mn ternary material with a lithium manganate material base on a proportion of 1-9:9-1 by weight parts; the cathode material is made of graphite; the electrolyte is prepared by mixing ethylene carbonate, dimethyl carbonate with methyl ethyl carbonate based on the volume ratio of 1:1:1; and the diaphragm is porous polypropylene, polyethylene or a modified polymer of the porous polypropylene and the polyethylene. The invention has the advantages of excellent safety performance, least production cost and larger specific capacity, and through safety tests such as overcharge, over-discharge, short circuit, needling, extrusion, heavy impact, hot box and the like, the safety performance meets the standard testing requirements of UL1642, IEC60949-3 and GB / T18287-2004, and products meet the requirements and reach RoHS instruction requirement.
Owner:濮阳市星驰电源制造有限公司
Preparation method for double metal cyanide catalyst for polycarbonate synthesis
ActiveCN102179262AAvoid synergistic effectsImprove performanceOrganic-compounds/hydrides/coordination-complexes catalystsCyanideMethyl carbonate
The invention discloses a preparation method for a double metal cyanide catalyst for polycarbonate synthesis. The preparation method comprises the following steps of: firstly dissolving soluble metallic salt in organic ligand, and then adding deionized water to serve as a solution 1; dissolving water-soluble metallic cyanide salt in the deionized water to serve as a solution 2, wherein the volume ratio of the solution 1 to the solution 2 is 0.1: 1-5: 1; and then dripping the solution 2 to the solution 1 to obtain a solid catalyst after stirring and drying. In the invention, the influence of the deionized water to the synergic action between the organic ligand and the soluble metal salt is avoided; the synergic action between the organic ligand and the soluble metal salt in the DMC (Di Methyl Carbonate) catalyst is exerted at the greatest degree; and the reaction time when the catalyst catalyzes a copolymerization of propylene oxide and CO2 does not exceed 8h, the activity reaches to above 30Kg polymer / g catalyst, and the CO2 unit content in the polymer reaches up to 45.0%.
Owner:HEBEI UNIV OF TECH +1
Application of cleavable polyethylene glycol (PEG) lipid derivative to preparation
InactiveCN102068701AEasy to prepareNo radioactive contaminationPowder deliveryPharmaceutical non-active ingredientsLiposome VesicleMethyl carbonate
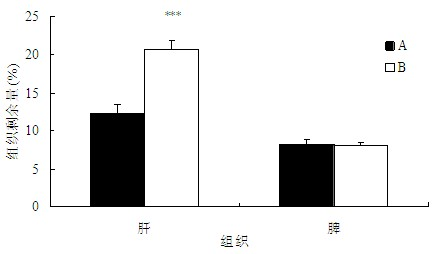
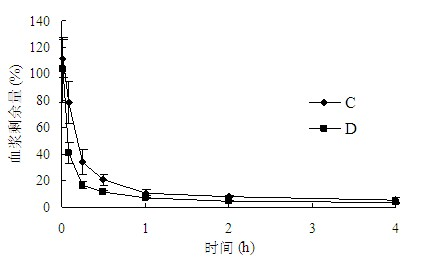
The invention belongs to the technical field of medicaments, and provides application of a cleavable polyethylene glycol (PEG) lipid derivative to preparation of a PEGylated preparation for relieving or avoiding accelerated blood clearance. In the application, liquid microparticle preparations such as liposome, vesicles, emulsions, microemulsion, micelles, nanoparticles and the like are modified by the cleavable PEG lipid derivative such as PEG-cholesteryl hemisuccinate, PEG-cholesteryl methyl carbonate, PEG-alpha tocopheryl hemisuccinate and the like; and the measurement of variation of preparation elimination in tissues such as animal blood plasma, liver, spleen and the like after a cleavable PEG lipid derivative-modified medicinal preparation is repeatedly injected proves that repeated injection of cleavable PEG lipid derivative-modified microparticle preparations only causes light accelerated blood clearance or avoids the accelerated blood clearance, namely the accelerated blood clearance can be relieved or avoided. The invention discloses new application of the cleavable PEG lipid derivative.
Owner:SHENYANG PHARMA UNIVERSITY
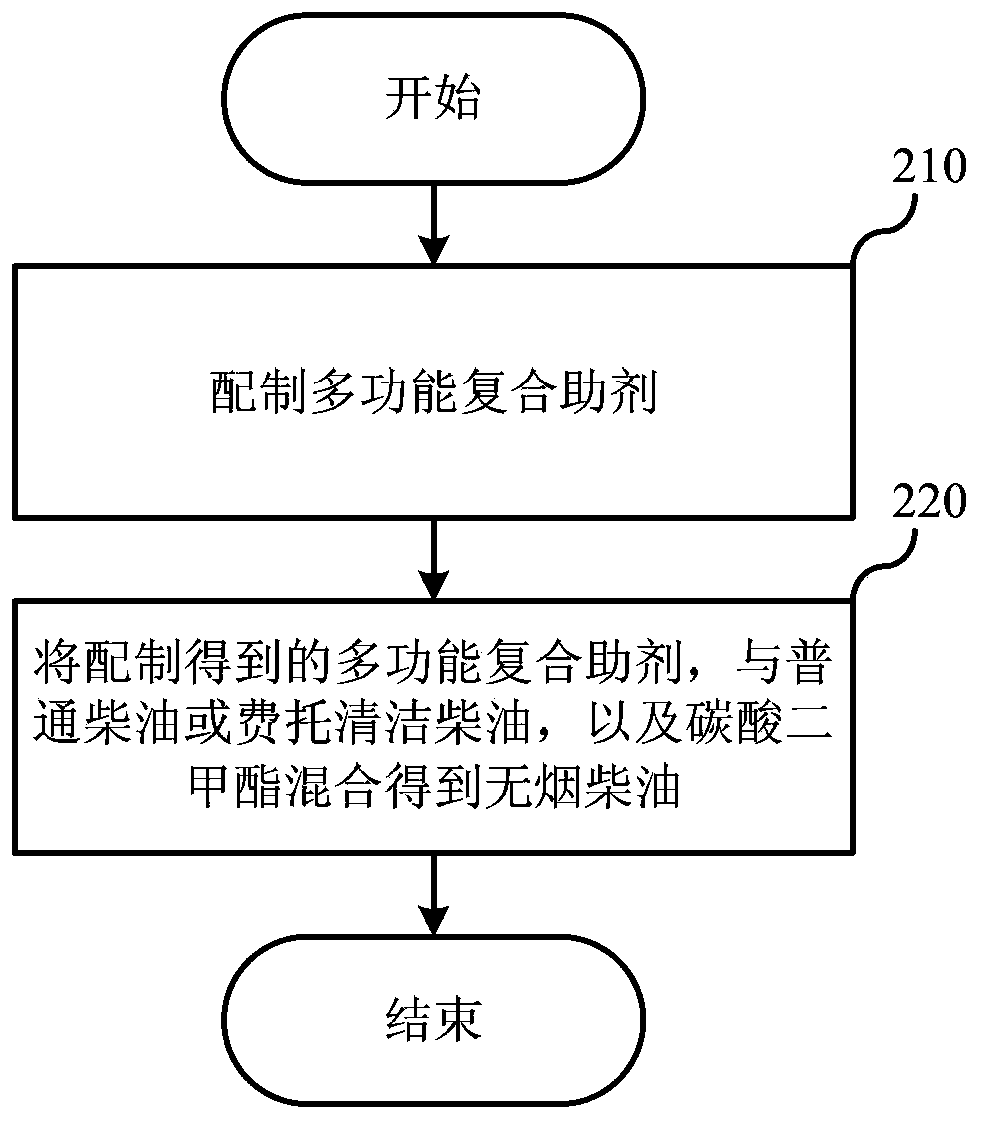
Smokeless diesel oil and preparation method thereof
ActiveCN103805297AEliminate emissionsImprove combustion effectLiquid carbonaceous fuelsParticulatesMethyl carbonate
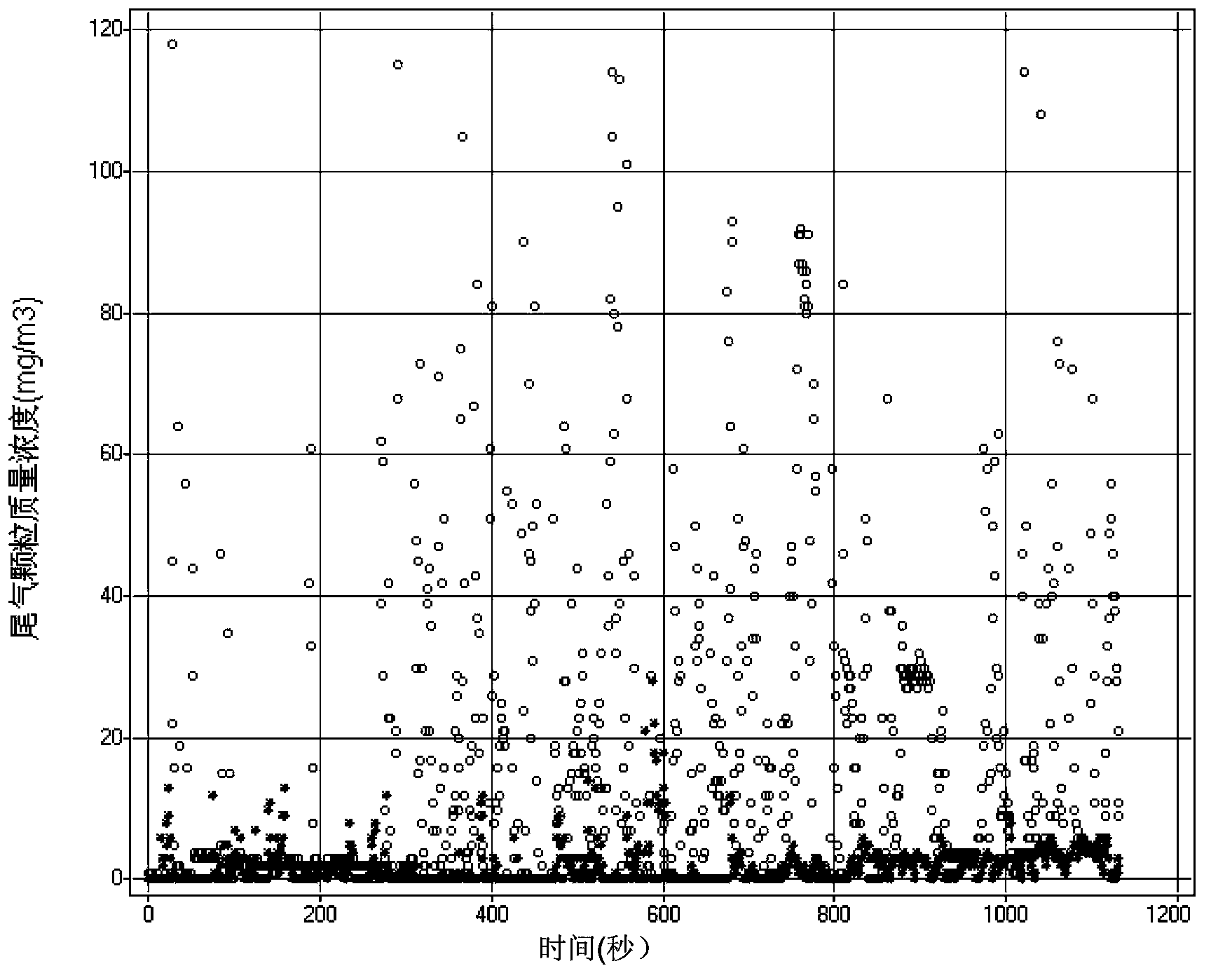
The invention relates to the field of fuels, and discloses smokeless diesel oil and a preparation method thereof. The smokeless diesel oil comprises 70-95wt% of common diesel oil, 2-25wt% of dimethyl carbonate and 0-10wt% of a multifunctional compound additive. According to the smokeless diesel oil provided by the invention, particulate matter emission in the using process of means of conveyance (comprising various motor vehicles and ships) taking diesel oil as a fuel and machinery for engineering, mining and agriculture and forestry taking diesel engines as power can be greatly reduced or even eliminated without affecting other emission indexes. The smokeless diesel oil is clean and environment-friendly diesel oil, and is simple and convenient and feasible. The fuel system of the diesel engine needs not to be changed.
Owner:YASHENTECH CORP
Electrolytes for lithium ion batteries
Electrolytes, lithium ion cells and corresponding methods are provided, for extending the cycle life of fast charging lithium ion batteries. The electrolytes are based on fluoroethylene carbonate (FEC) and / or vinylene carbonate (VC) as the cyclic carbonate component, and possibly on ethyl acetate (EA) and / or ethyl methyl carbonate (EMC) as the linear component. Proposed electrolytes extend the cycle life by factors of two or more, as indicated by several complementary measurements.
Owner:STOREDOT
Quaternary ammonium salt in ester-amines and synthetic method
InactiveCN1562960ARaise the gradePromote degradationOrganic compound preparationAmino-hyroxy compound preparationQuaternary ammonium cationMethyl carbonate
In this invention, fatly acid and ethanolamine are proportioned and under ester-aminated, then reacting with dimethyl carbonate to produce ester-amine type quaternary ammonium salt. Said invention p rocess of synthesis has high transforming rate, excellent selectivity, high content of active matter in said product, utilizing no pollution type raw material of dimethyl carbonate, so, no remaining of poison in product, lowering quaternary ammonium salt pollution to environment and to human health.
Owner:CHINA RES INST OF DAILY CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com