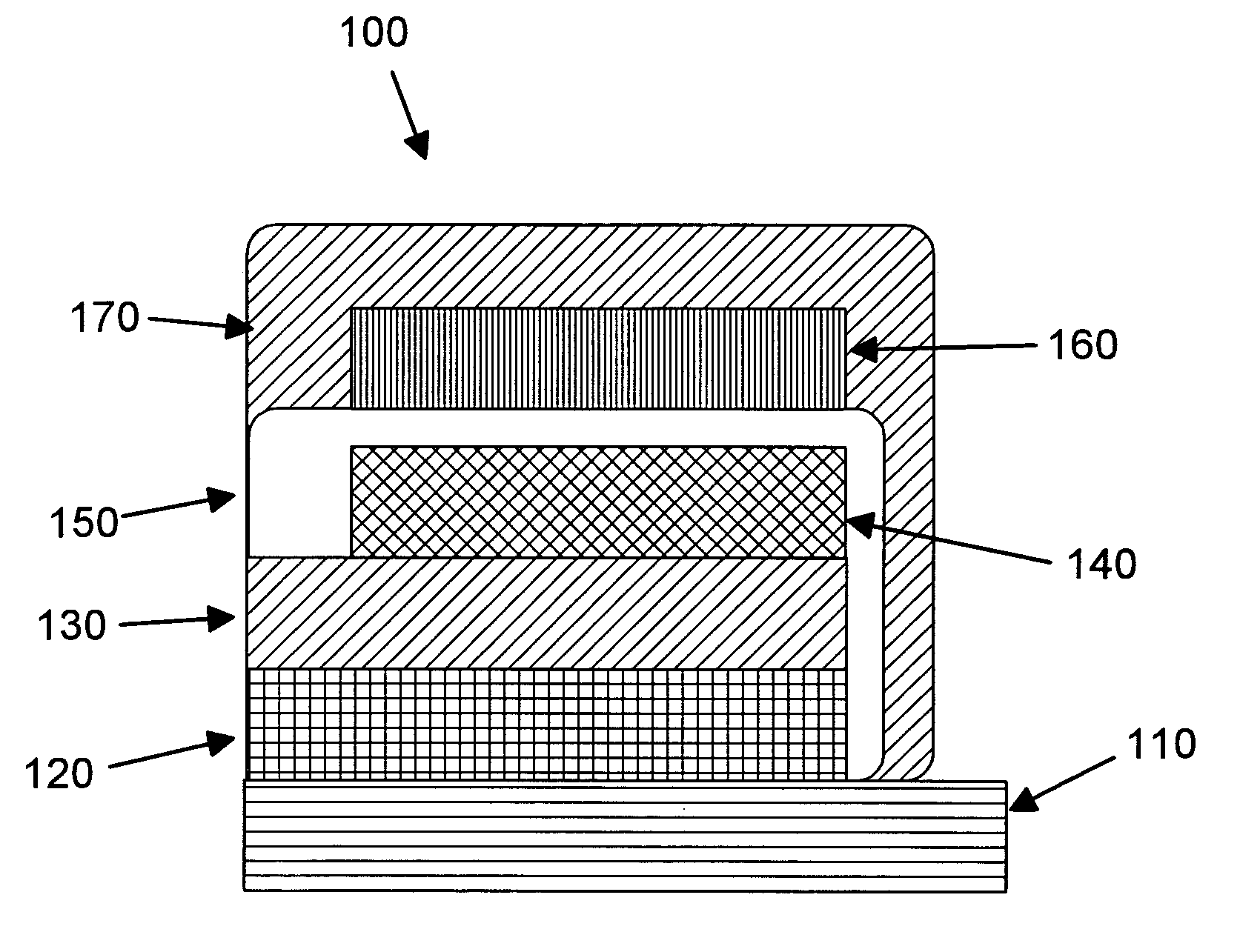
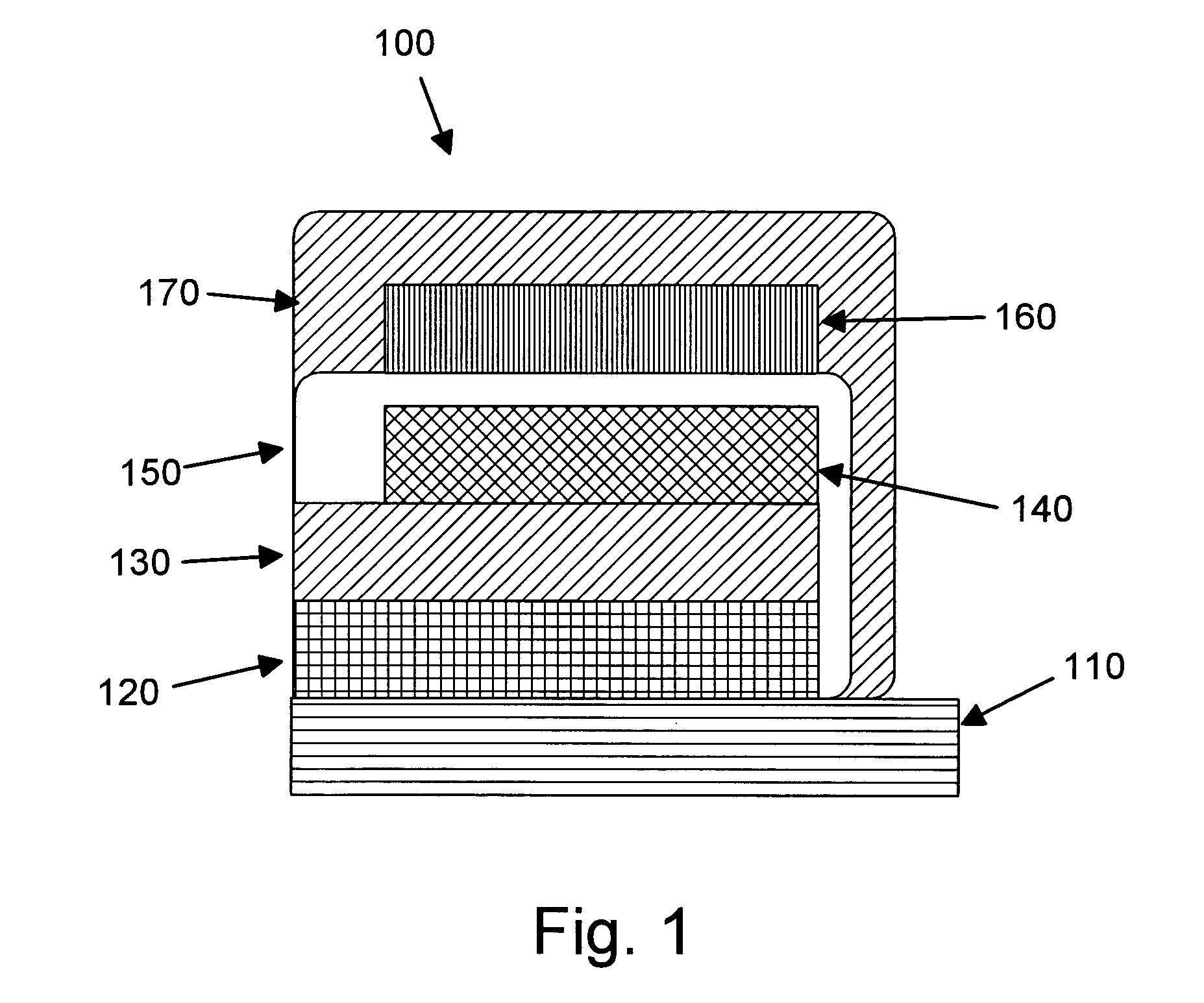
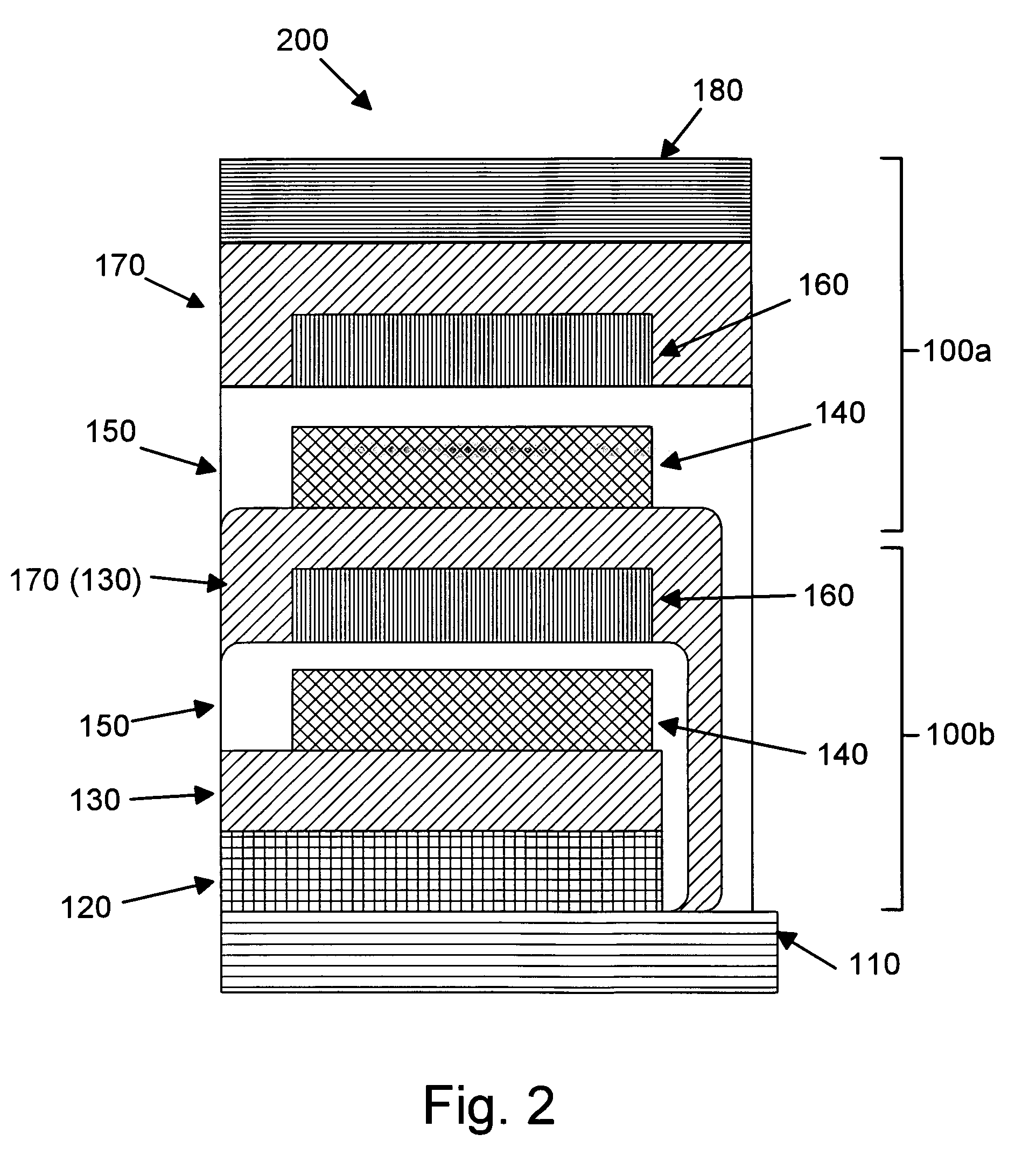
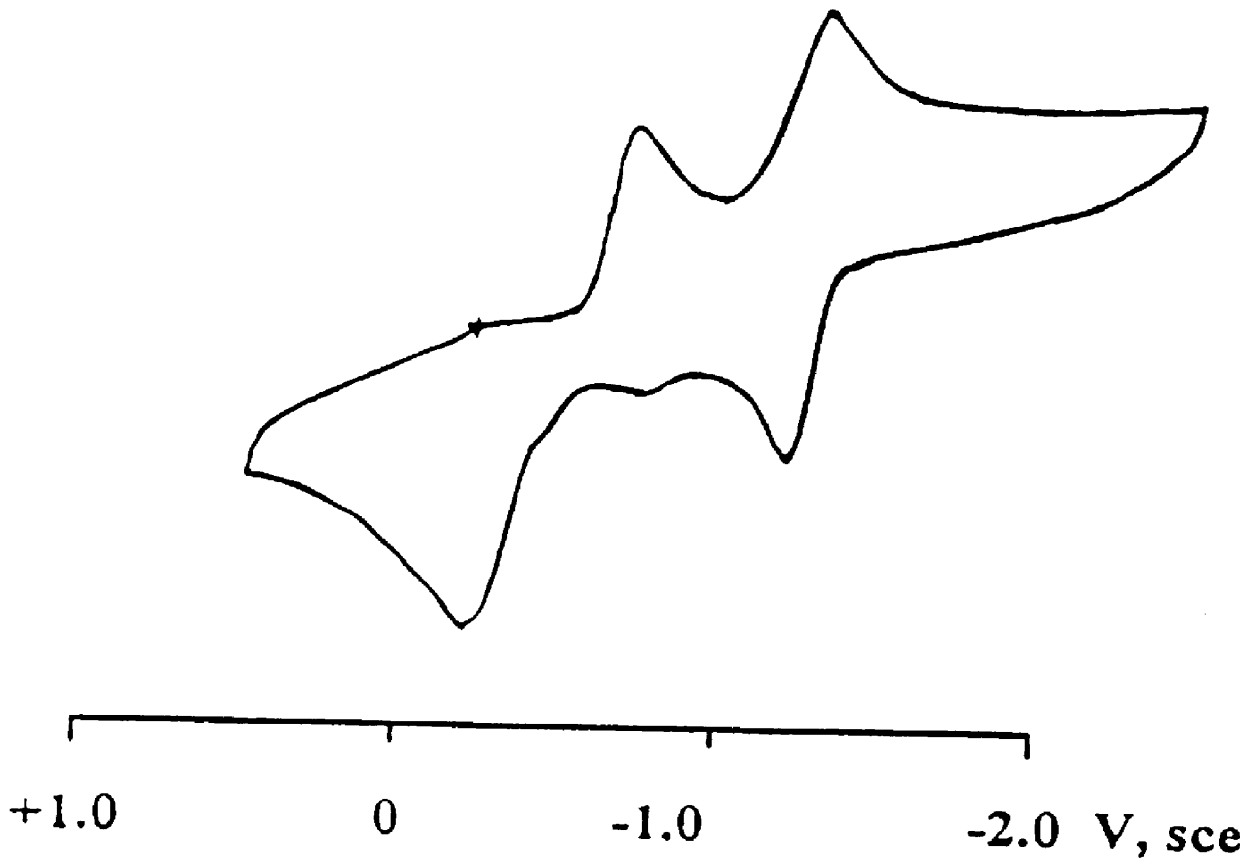
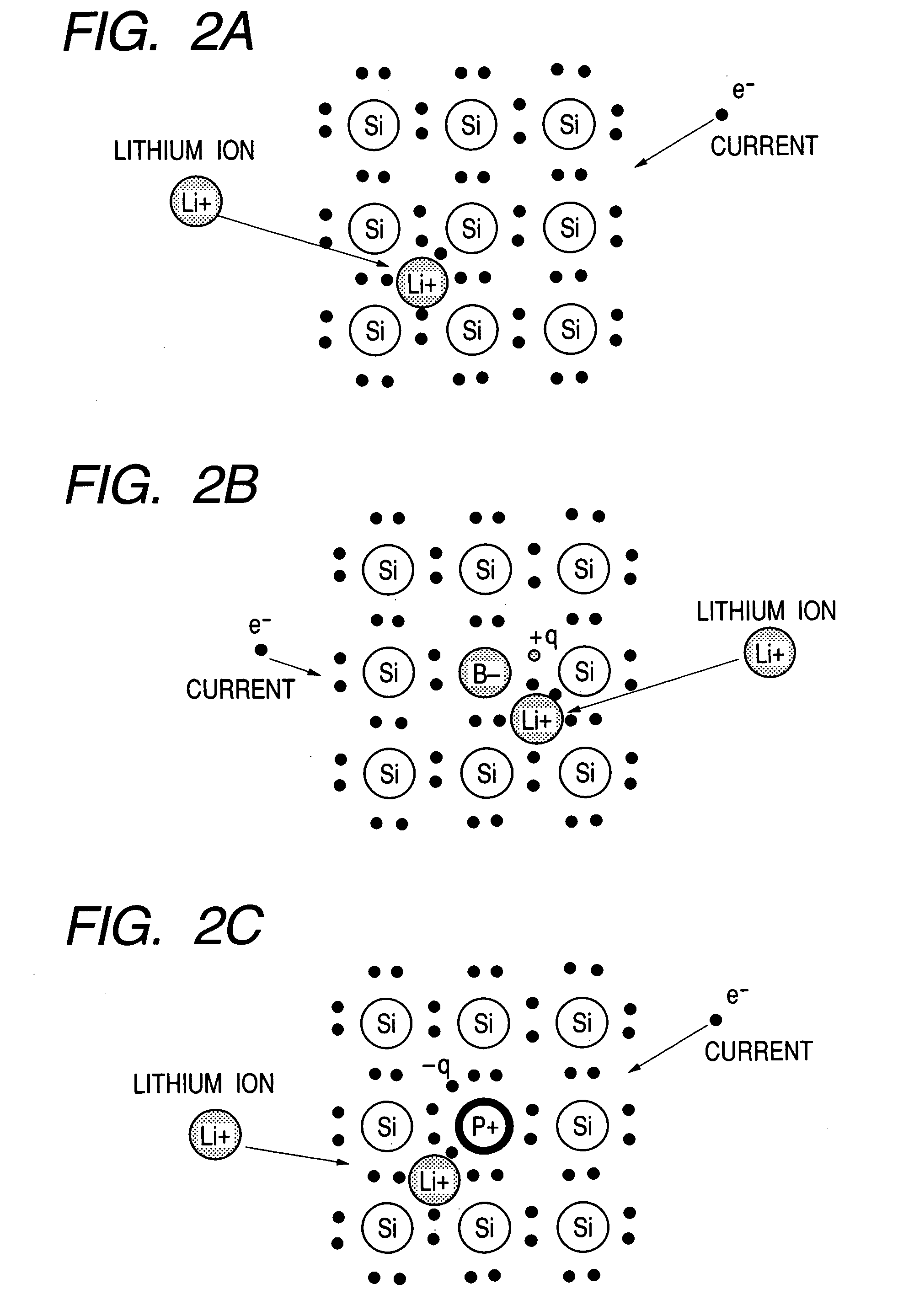
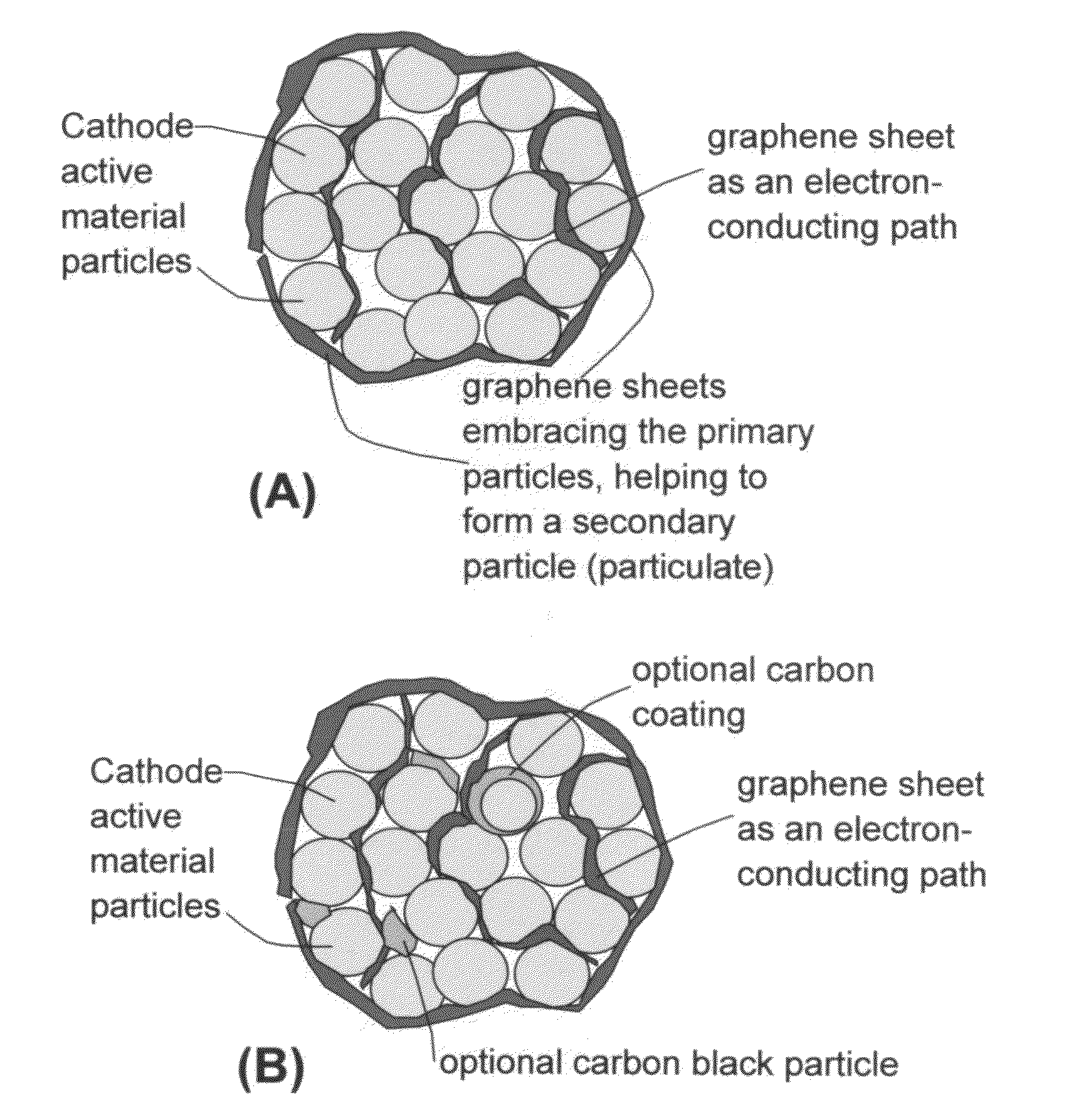
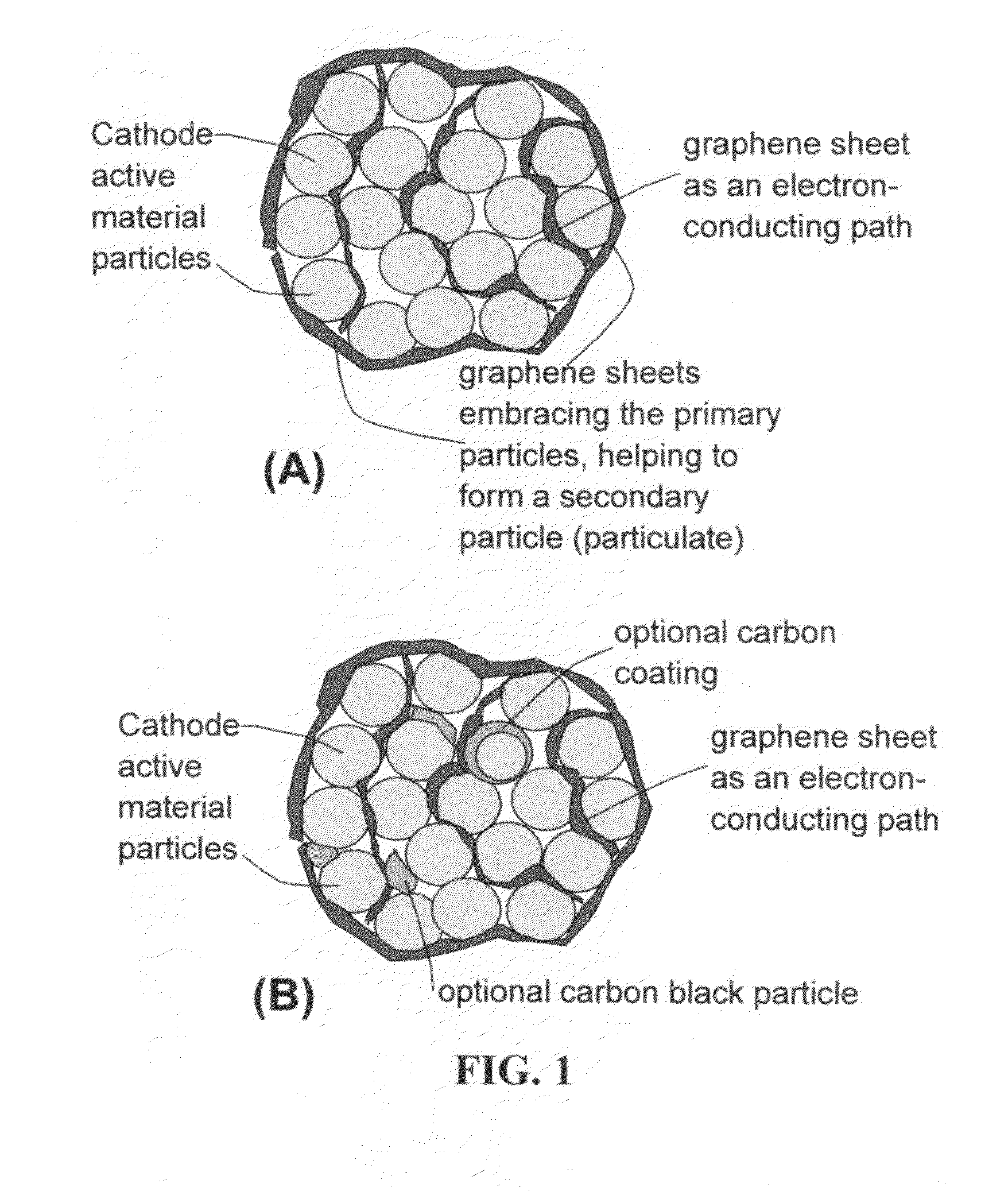
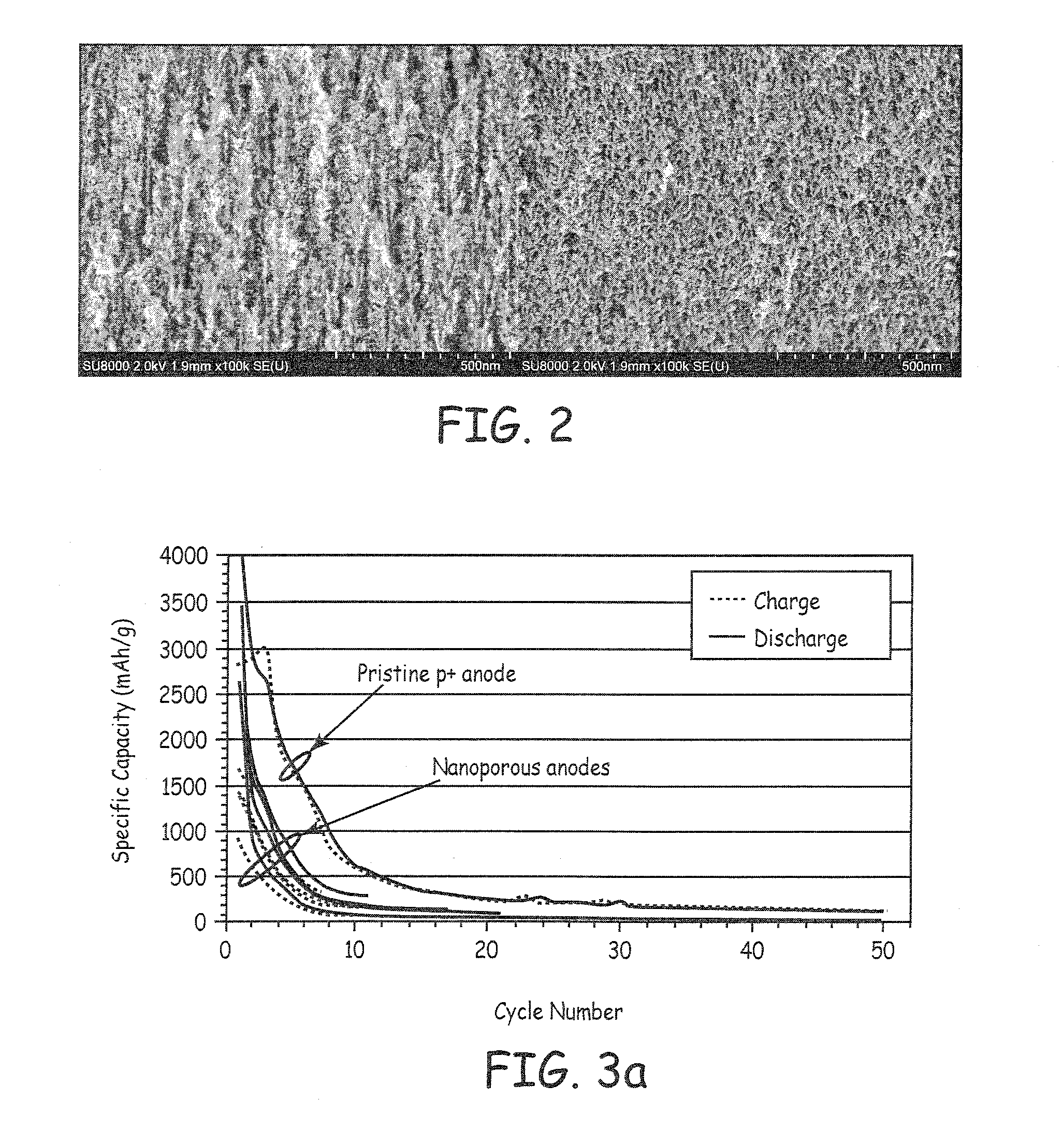
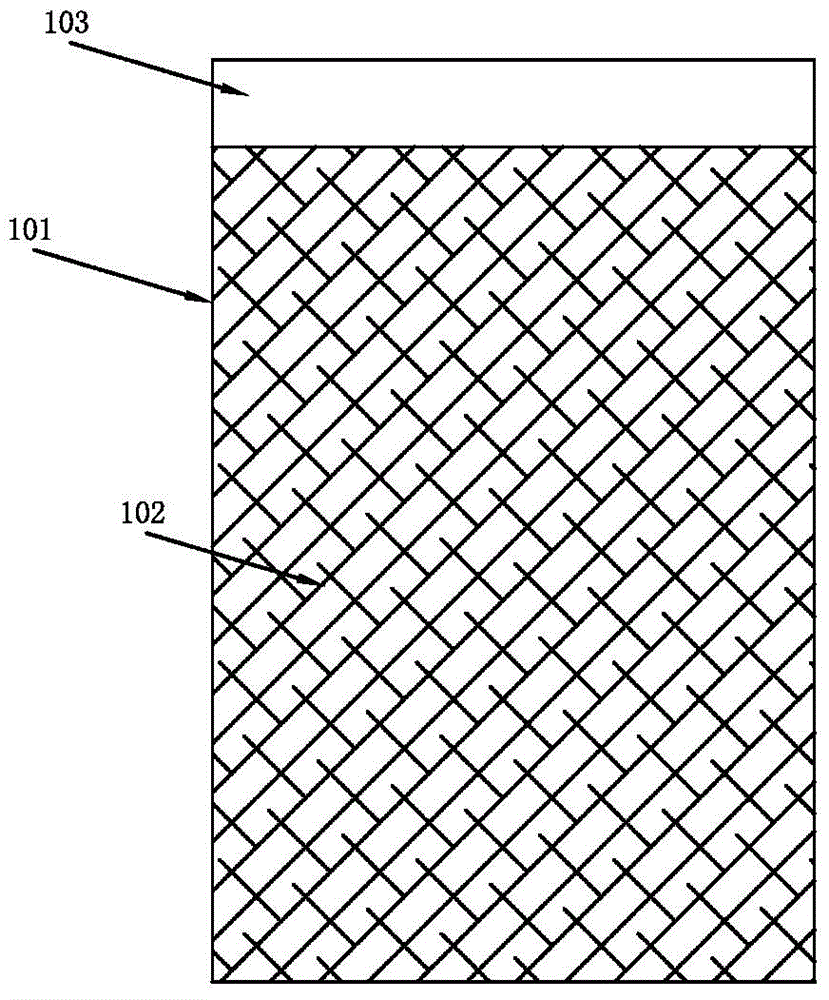
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
17912results about "Non-aqueous electrolyte accumulator electrodes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
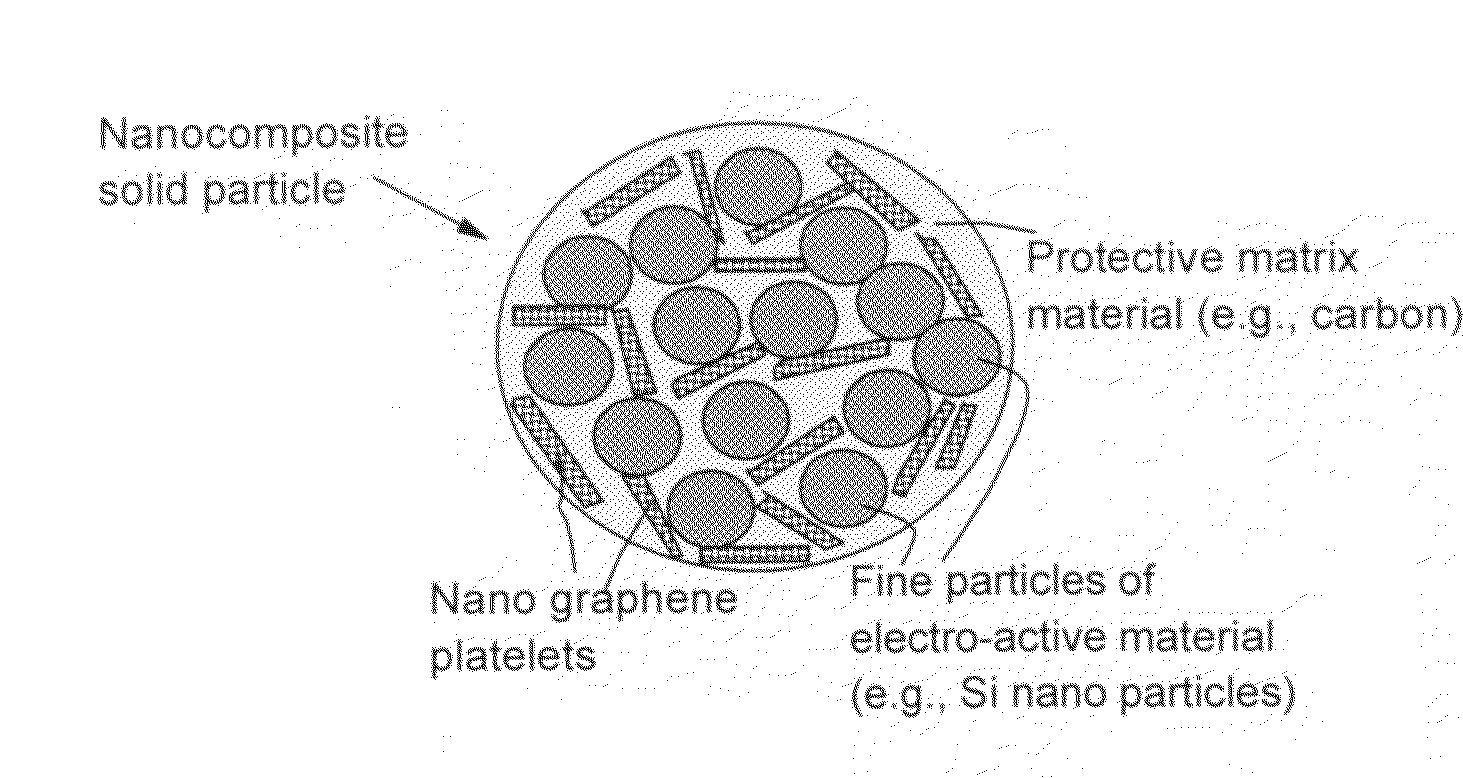
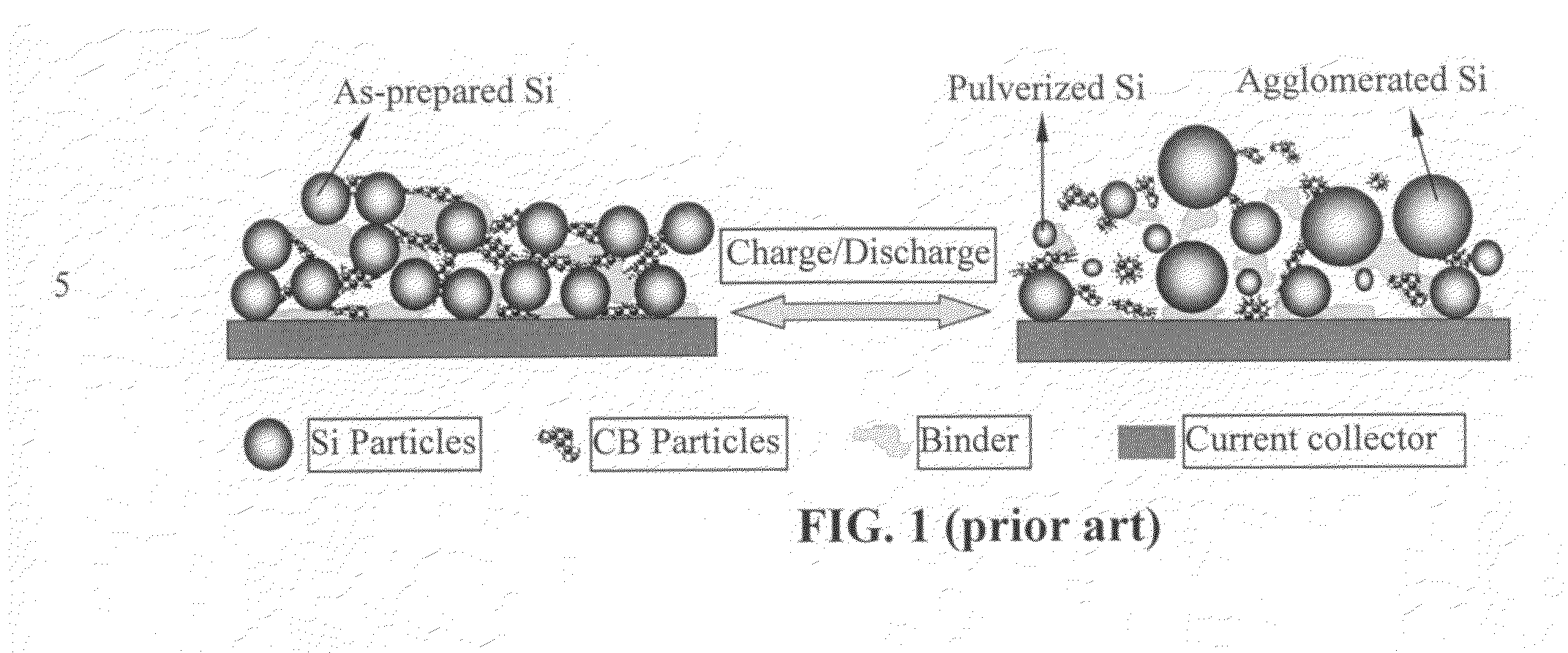
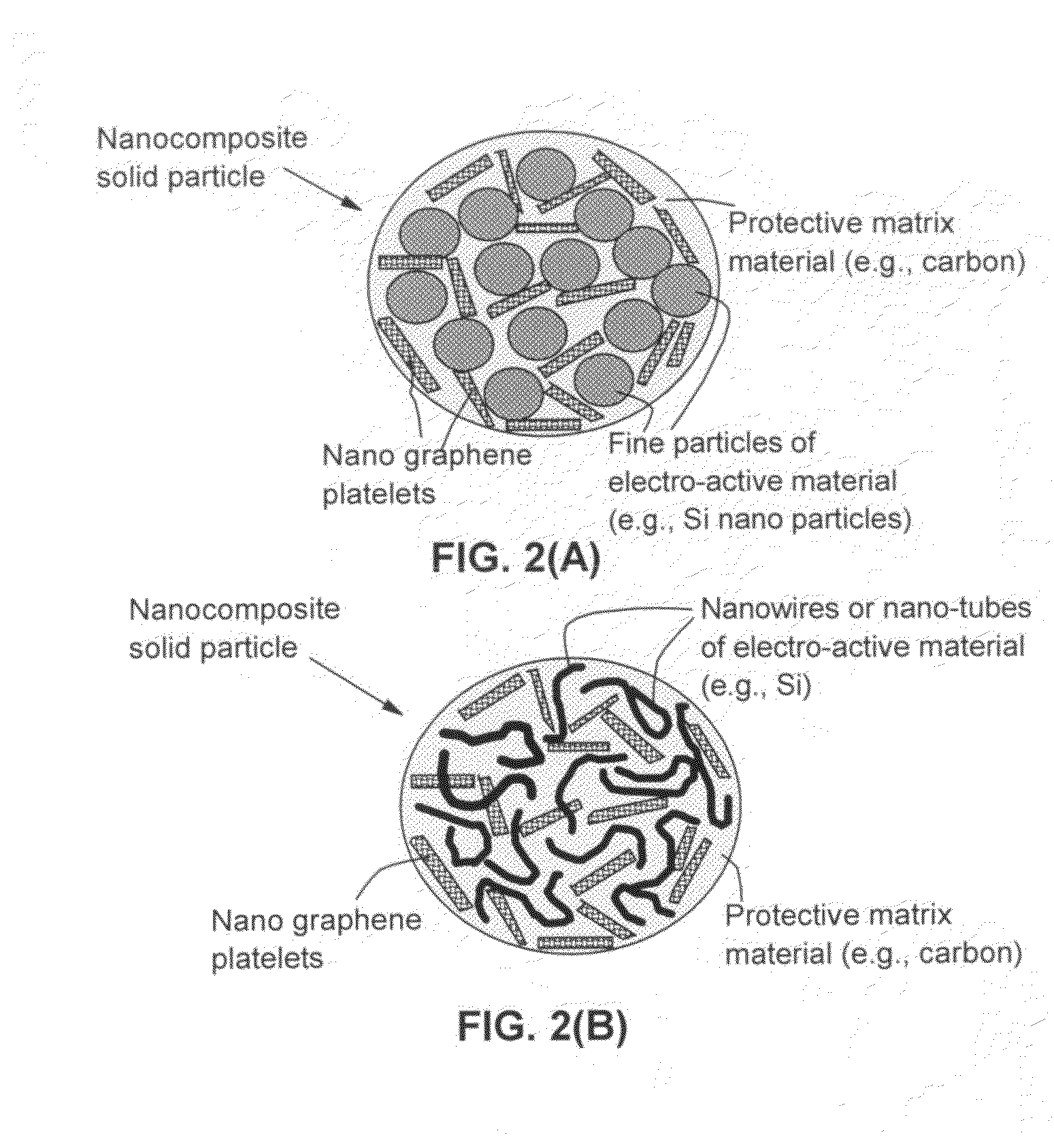
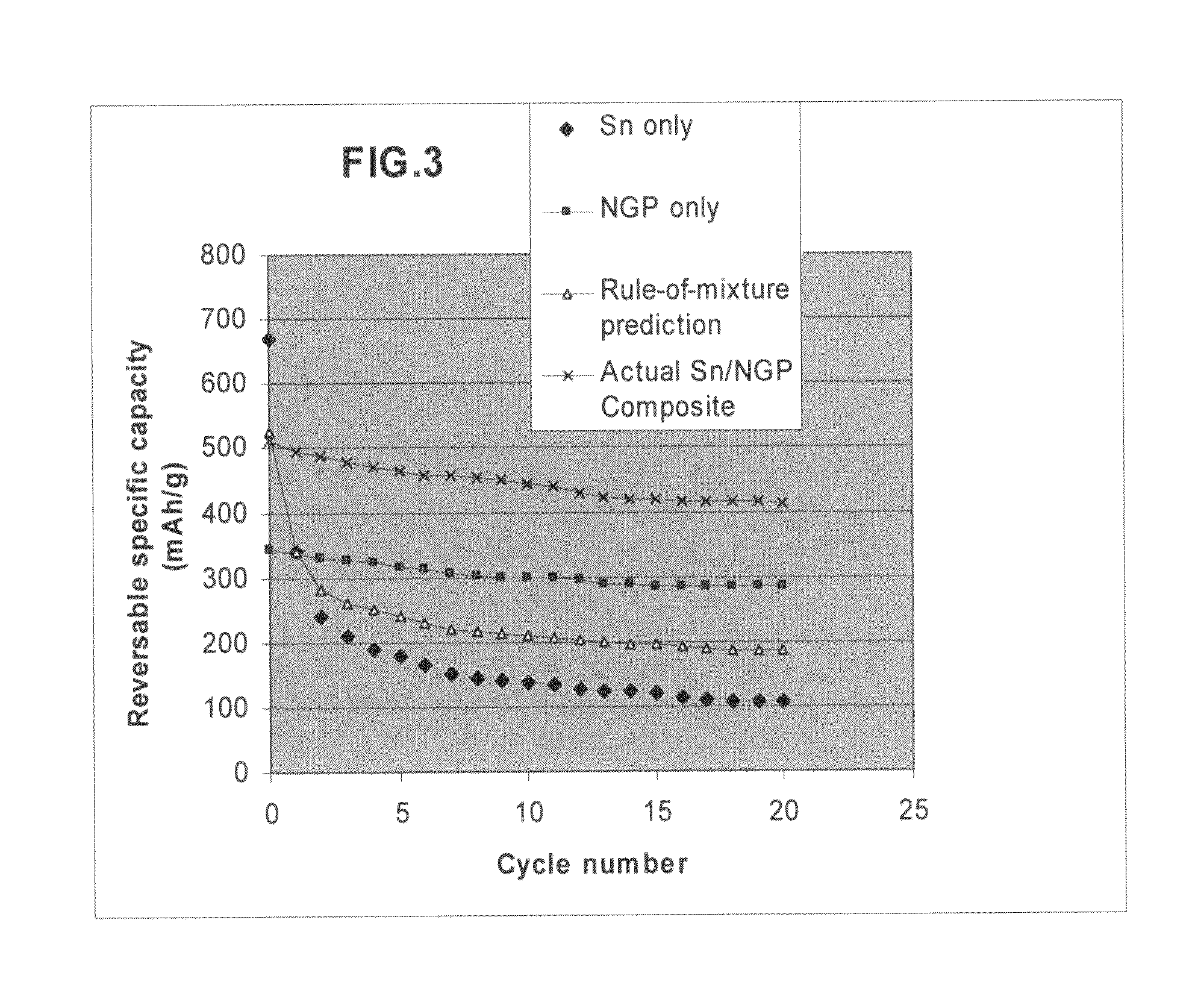
Nano graphene reinforced nanocomposite particles for lithium battery electrodes
ActiveUS20100143798A1Increase stiffnessHigh strengthActive material electrodesSecondary cellsLithium metalNanocomposite
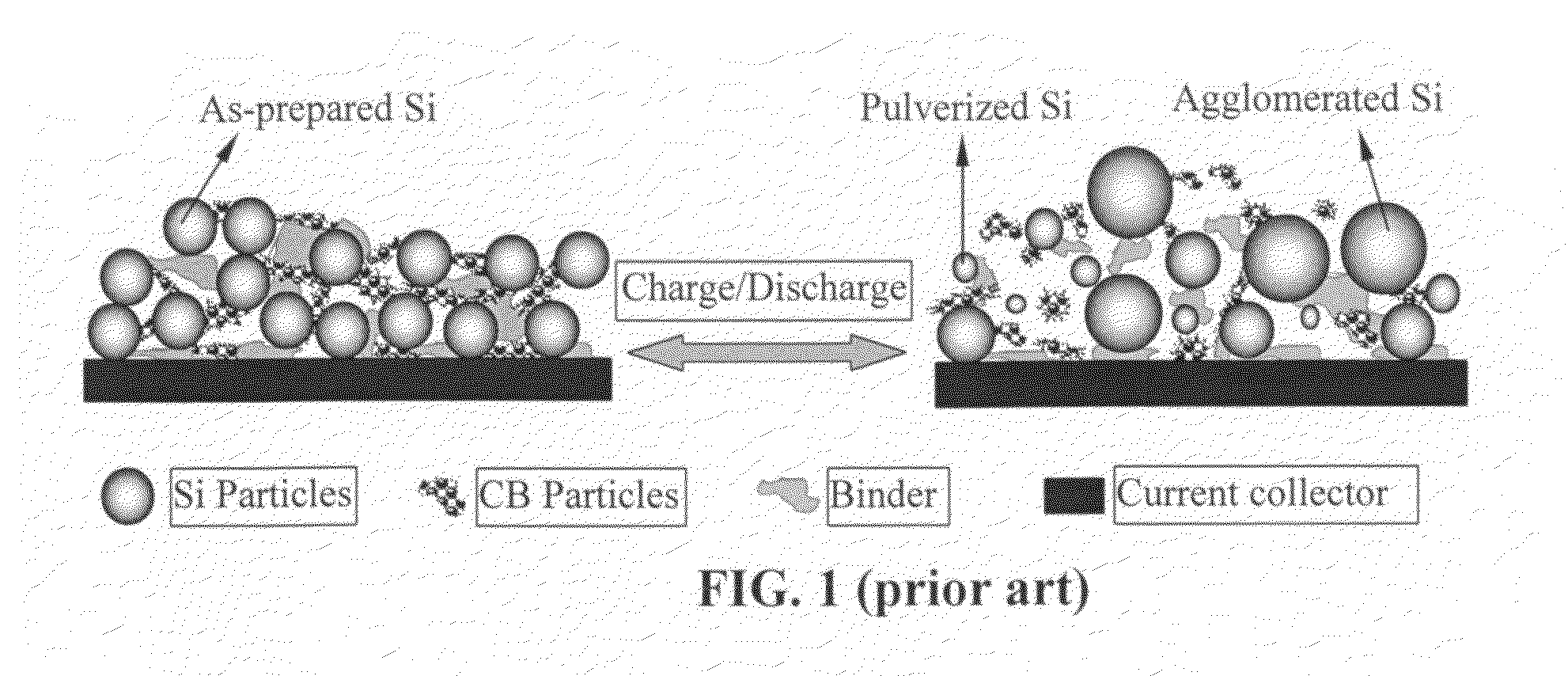
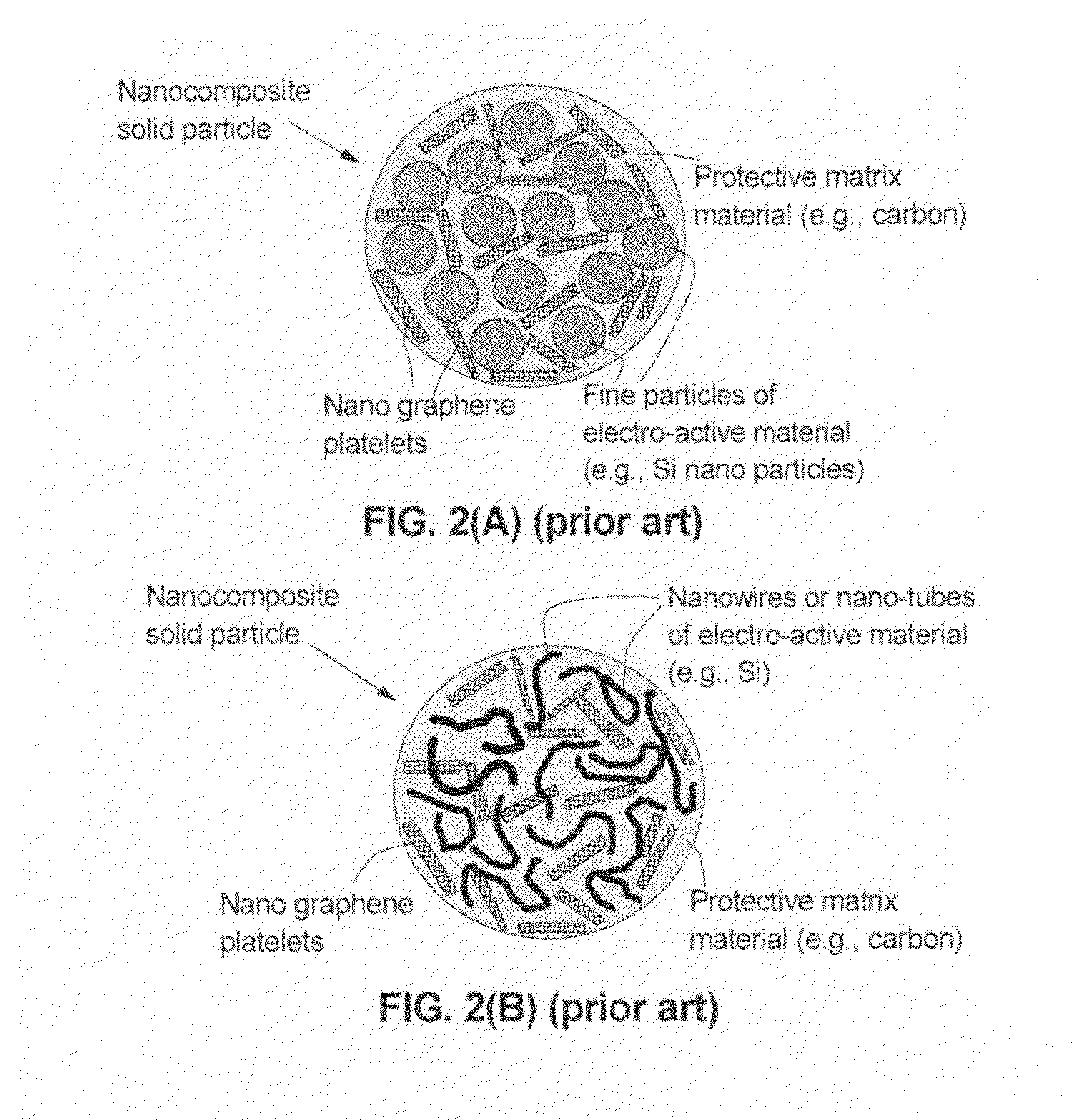
A solid nanocomposite particle composition for lithium metal or lithium ion battery electrode applications. The composition comprises: (A) an electrode active material in a form of fine particles, rods, wires, fibers, or tubes with a dimension smaller than 1 μm; (B) nano graphene platelets (NGPs); and (C) a protective matrix material reinforced by the NGPs; wherein the graphene platelets and the electrode active material are dispersed in the matrix material and the NGPs occupy a weight fraction wg of 1% to 90% of the total nanocomposite weight, the electrode active material occupies a weight fraction wa of 1% to 90% of the total nanocomposite weight, and the matrix material occupies a weight fraction wm of at least 2% of the total nanocomposite weight with wg+wa+wm=1. For a lithium ion battery anode application, the matrix material is preferably amorphous carbon, polymeric carbon, or meso-phase carbon. Such a solid nanocomposite composition provides a high anode capacity and good cycling stability. For a cathode application, the resulting lithium metal or lithium ion battery exhibits an exceptionally high cycle life.
Owner:SAMSUNG ELECTRONICS CO LTD
Separator for a high energy rechargeable lithium battery
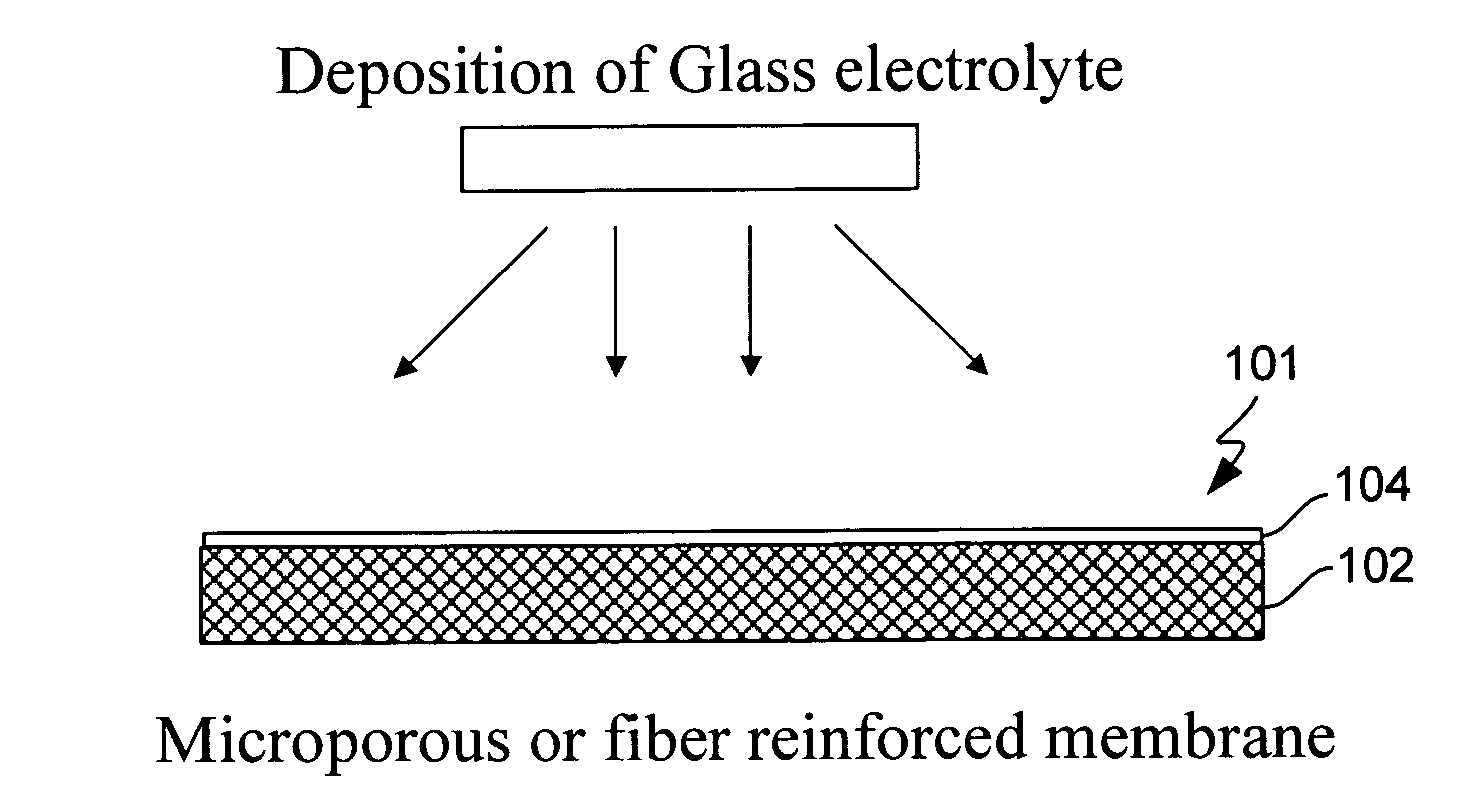
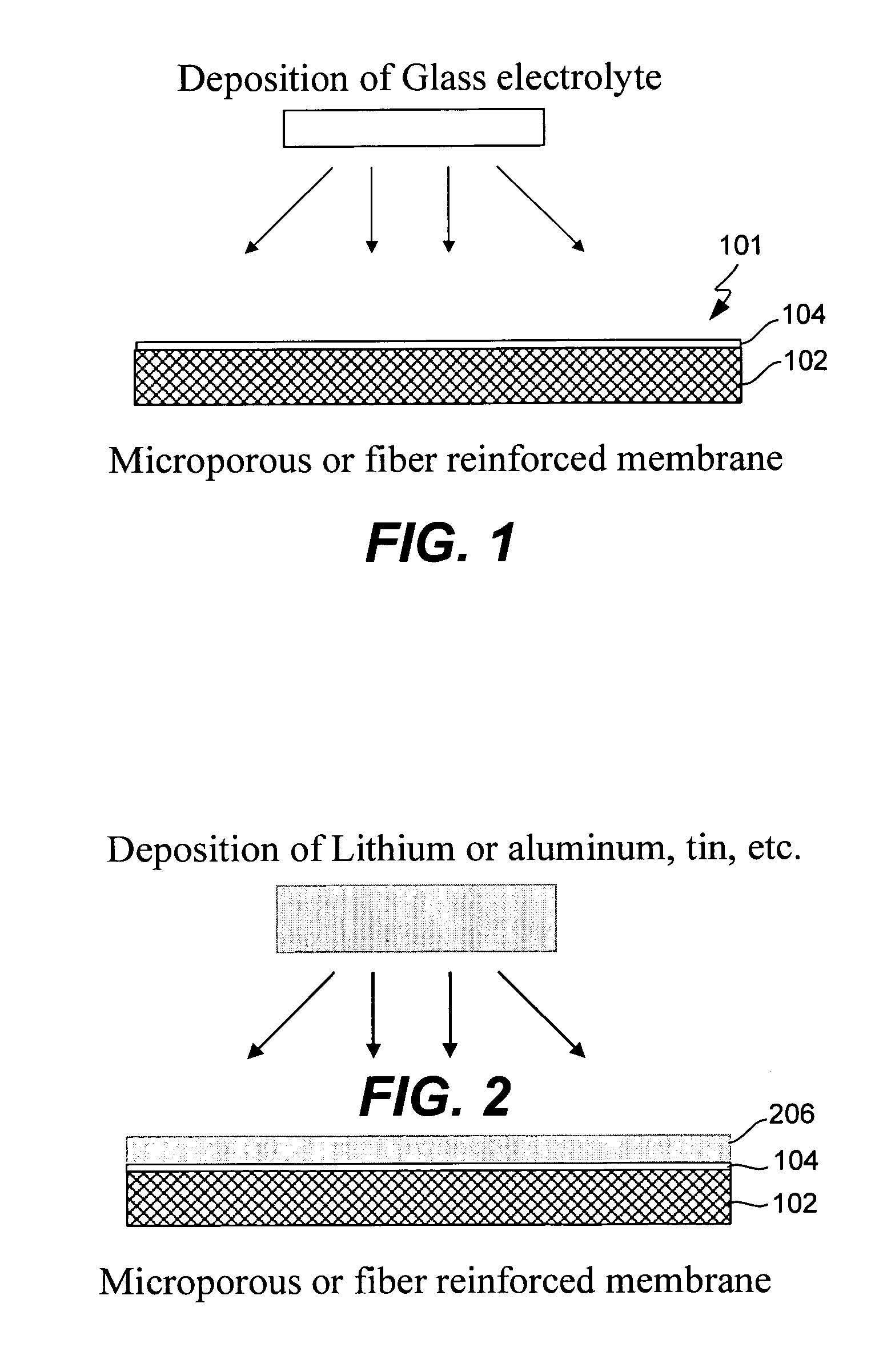
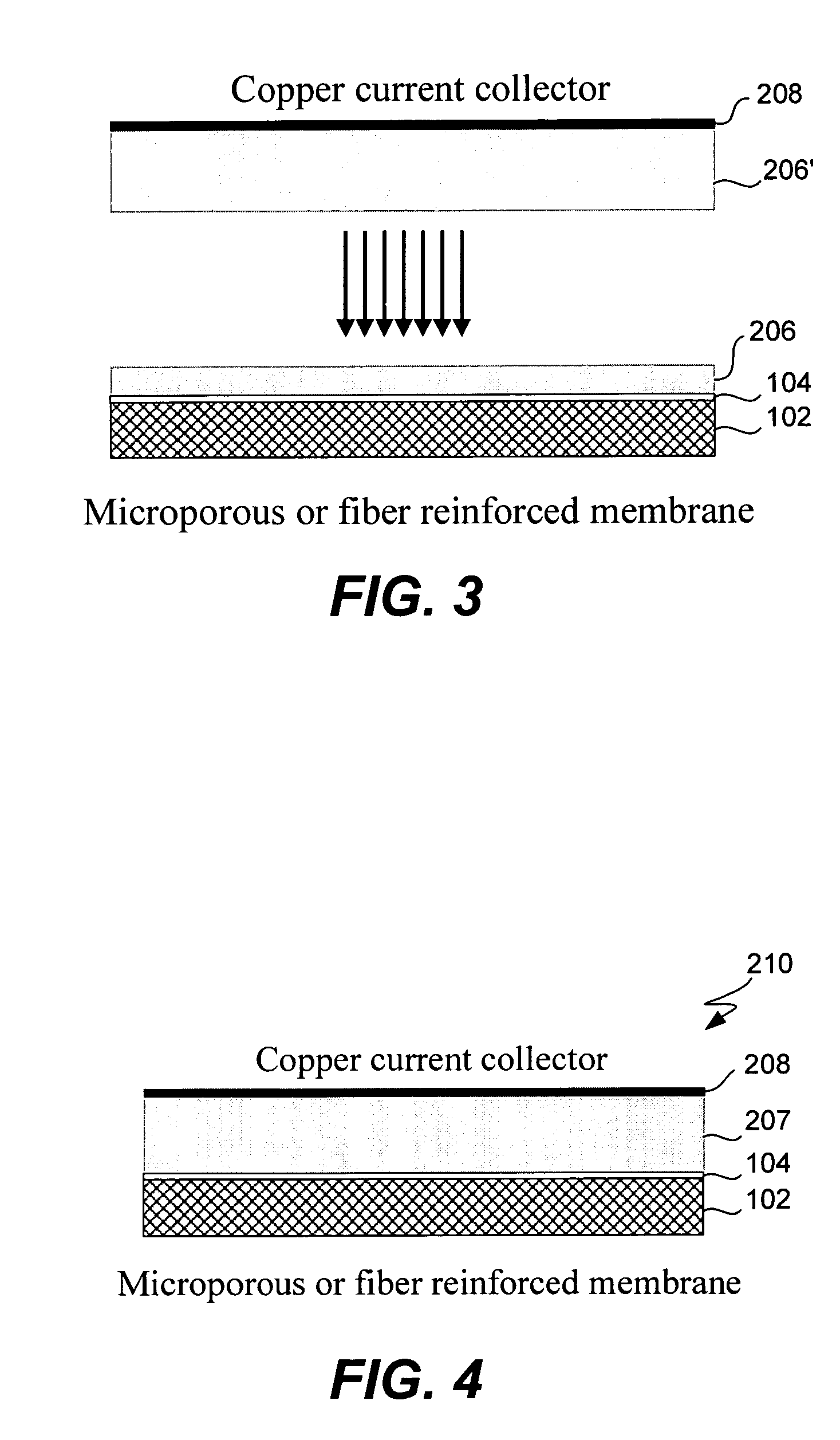
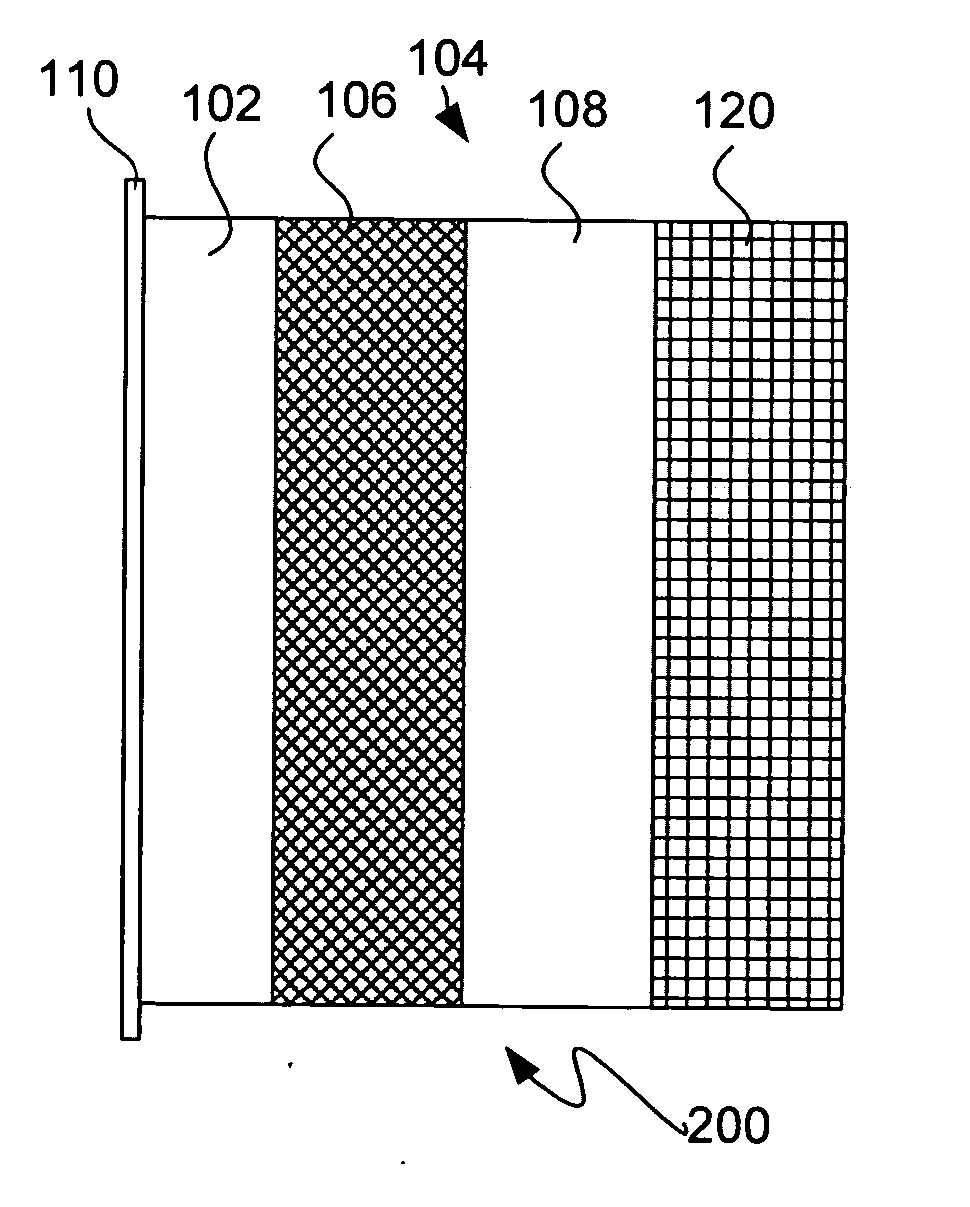
InactiveUS6432586B1Cell seperators/membranes/diaphragms/spacersNon-aqueous electrolyte accumulator electrodesCeramic compositeMetallurgy
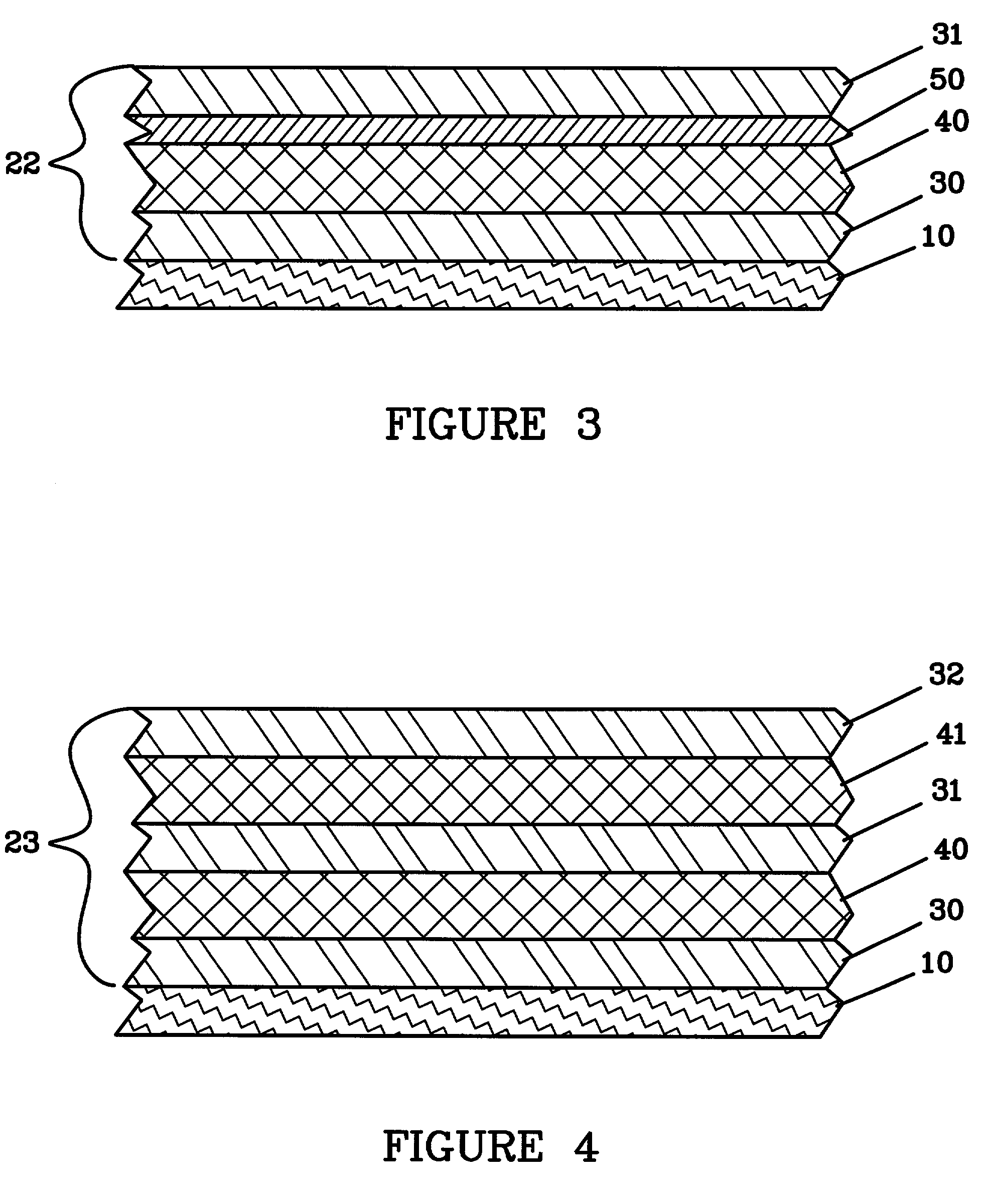
The instant invention is directed to a separator for a high energy rechargeable lithium battery and the corresponding battery. The separator includes a ceramic composite layer and a polymeric microporous layer. The ceramic layers includes a mixture of inorganic particles and a matrix material. The ceramic layer is adapted, at least, to block dendrite growth and to prevent electronic shorting. The polymeric layer is adapted, at least, to block ionic flow between the anode and the cathode in the event of thermal runaway.
Owner:CELGARD LLC
Nano graphene platelet-based composite anode compositions for lithium ion batteries
ActiveUS20090117467A1Improve conductivityLower internal resistanceElectrolytic capacitorsSecondary cellsGraphene flakeGraphite
The present invention provides a nano-scaled graphene platelet-based composite material composition for use as an electrode, particularly as an anode of a lithium ion battery. The composition comprises: (a) micron- or nanometer-scaled particles or coating which are capable of absorbing and desorbing lithium ions; and (b) a plurality of nano-scaled graphene platelets (NGPs), wherein a platelet comprises a graphene sheet or a stack of graphene sheets having a platelet thickness less than 100 nm; wherein at least one of the particles or coating is physically attached or chemically bonded to at least one of the graphene platelets and the amount of platelets is in the range of 2% to 90% by weight and the amount of particles or coating in the range of 98% to 10% by weight. Also provided is a lithium secondary battery comprising such a negative electrode (anode). The battery exhibits an exceptional specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:SAMSUNG ELECTRONICS CO LTD
Conductive nanocomposite-based electrodes for lithium batteries
ActiveUS20090305135A1High specific capacityHigh reversible capacityMaterial nanotechnologyNon-aqueous electrolyte accumulator electrodesCarbon layerComposite electrode
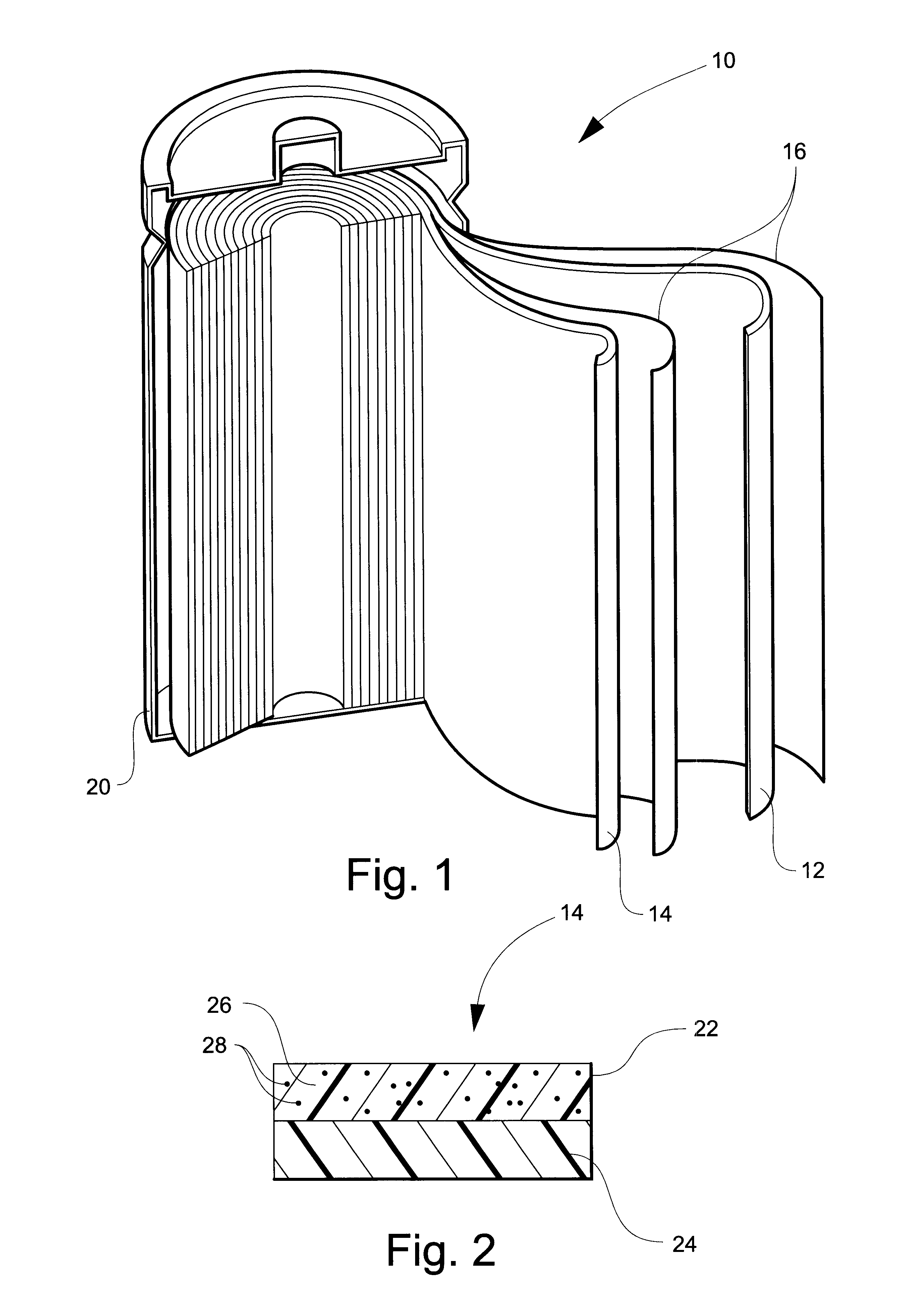
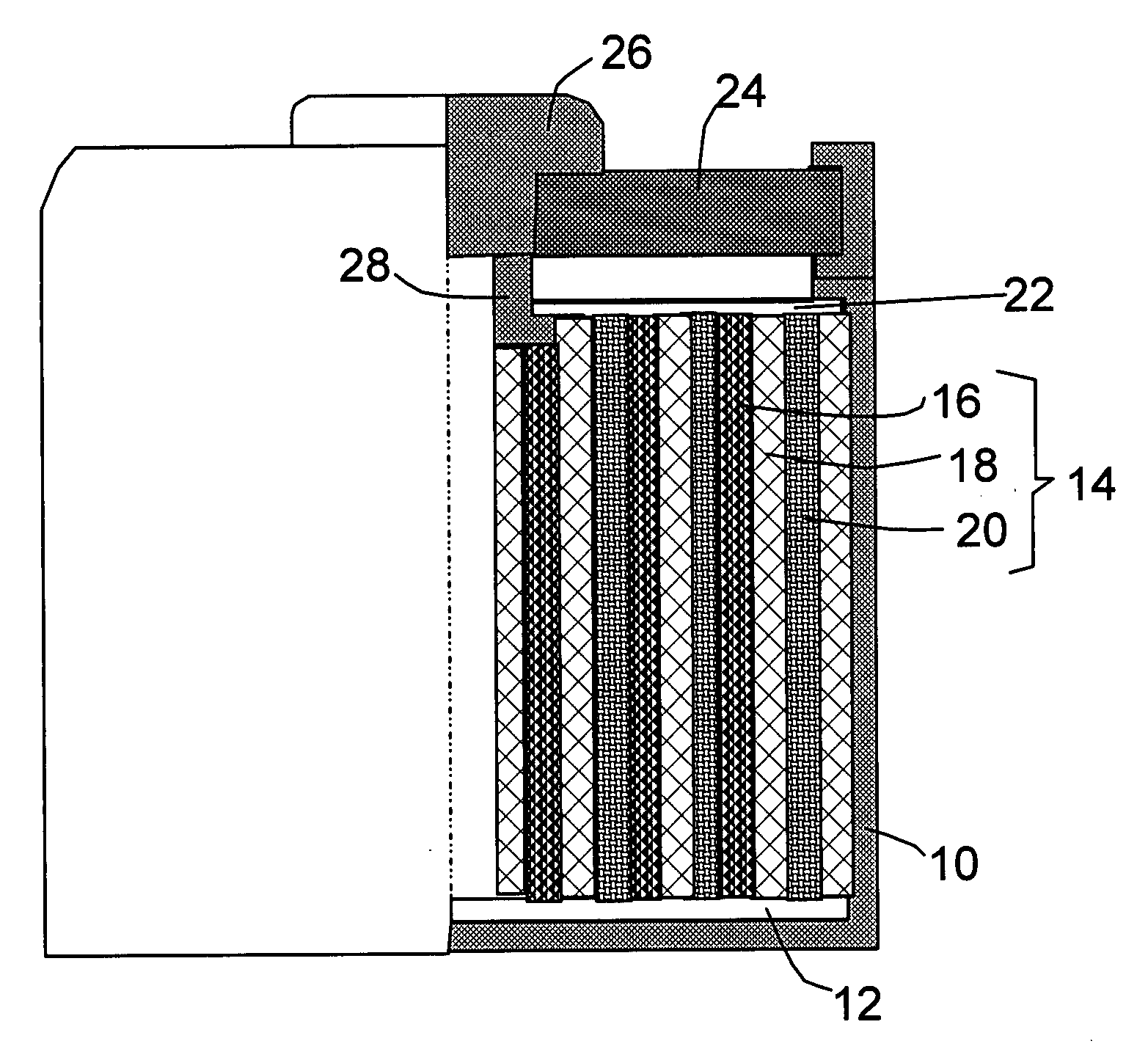
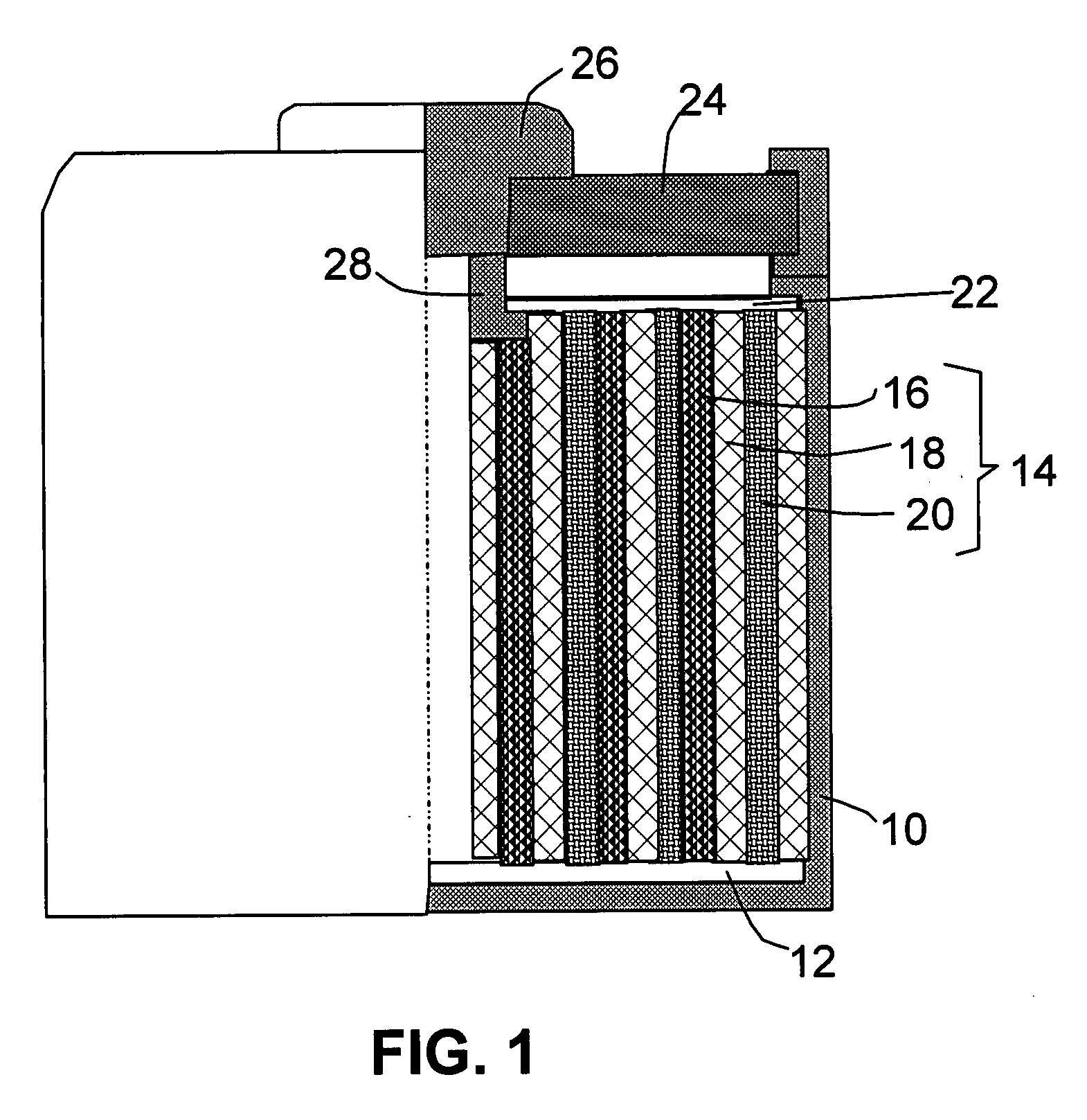
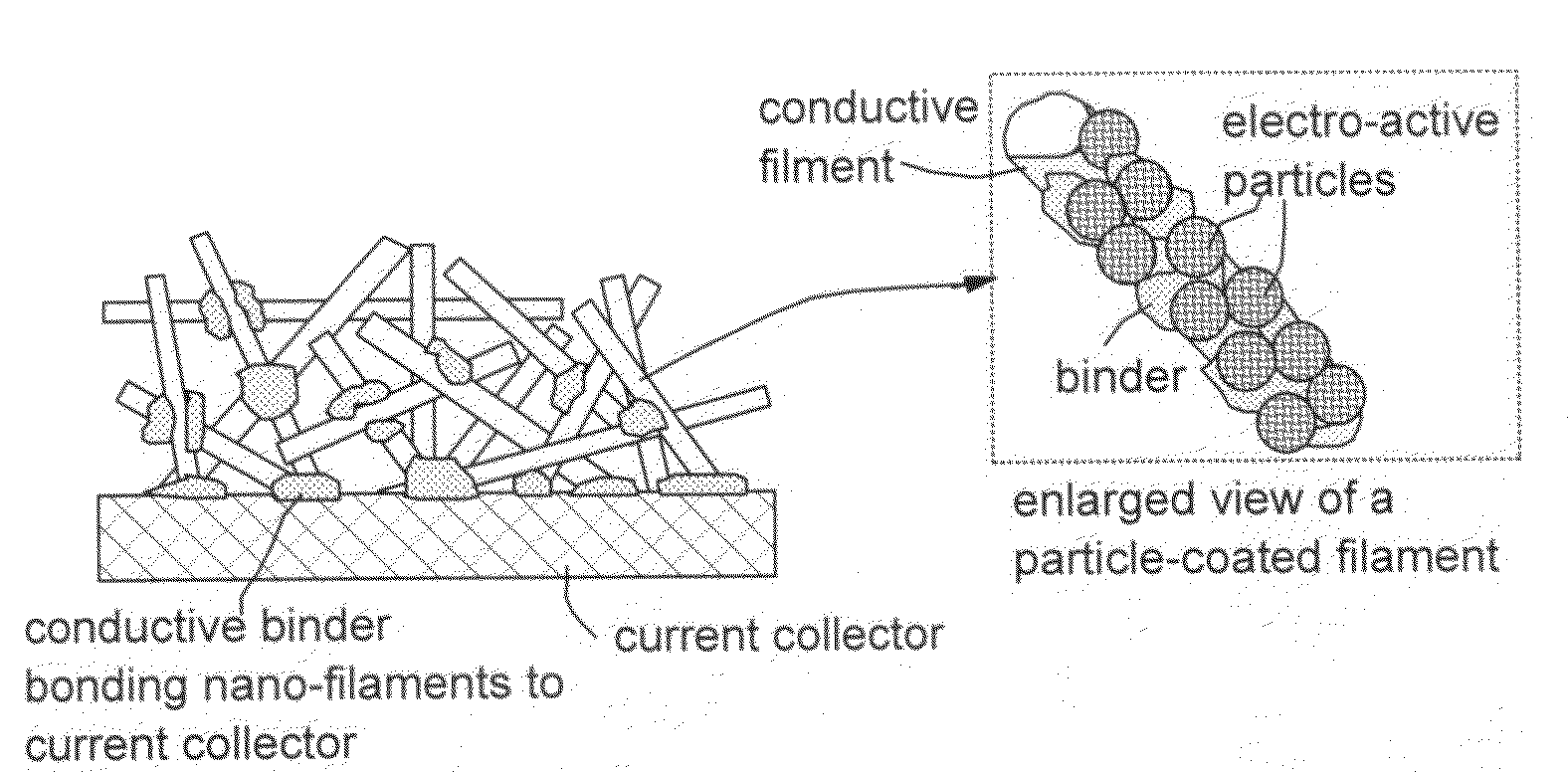
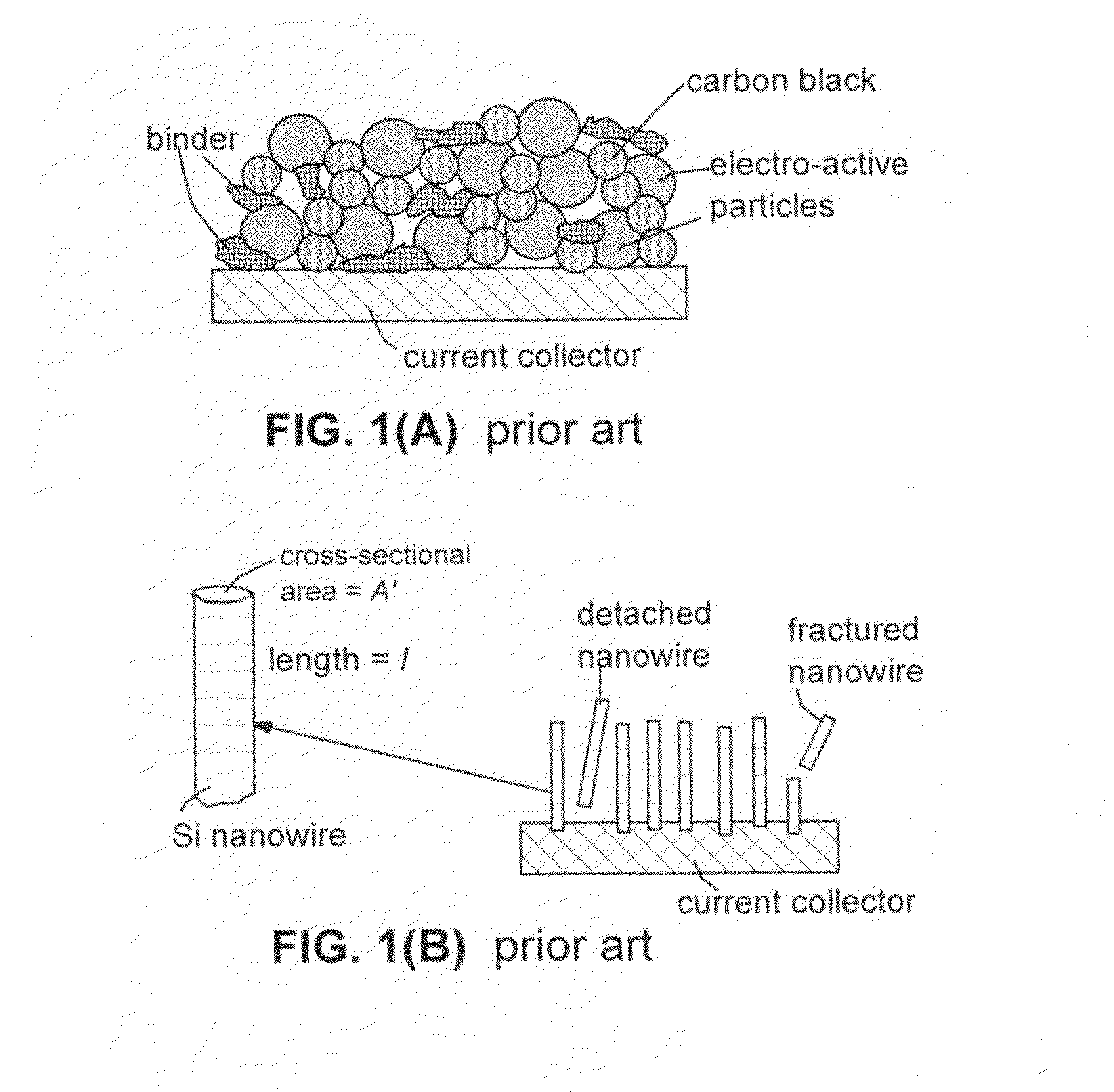
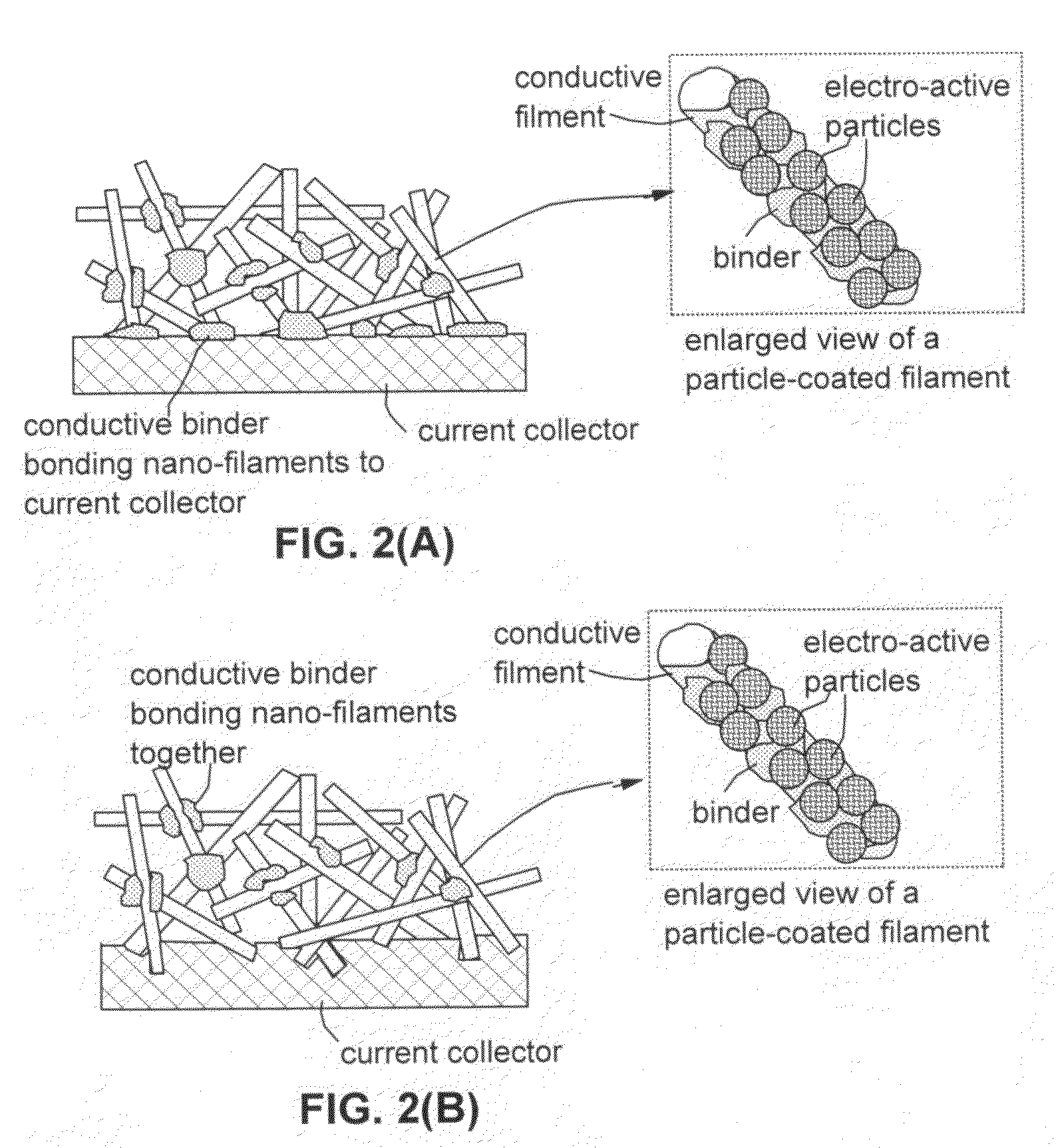
This invention provides a nanocomposite-based lithium battery electrode comprising: (a) A porous aggregate of electrically conductive nano-filaments that are substantially interconnected, intersected, physically contacted, or chemically bonded to form a three-dimensional network of electron-conducting paths, wherein the nano-filaments have a diameter or thickness less than 1 μm (preferably less than 500 nm); and (b) Sub-micron or nanometer-scale electro-active particles that are bonded to a surface of the nano-filaments with a conductive binder material, wherein the particles comprise an electro-active material capable of absorbing and desorbing lithium ions and wherein the electro-active material content is no less than 25% by weight based on the total weight of the particles, the binder material, and the filaments. Preferably, these electro-active particles are coated with a thin carbon layer. This electrode can be an anode or a cathode. The battery featuring such an anode or cathode exhibits an exceptionally high specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
Lithium anodes for electrochemical cells
InactiveUS7247408B2Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
Graphene-enhanced anode particulates for lithium ion batteries
ActiveUS20120064409A1Enhanced Li-ion insertionIncrease capacityNon-metal conductorsMaterial nanotechnologyParticulatesMicroparticle
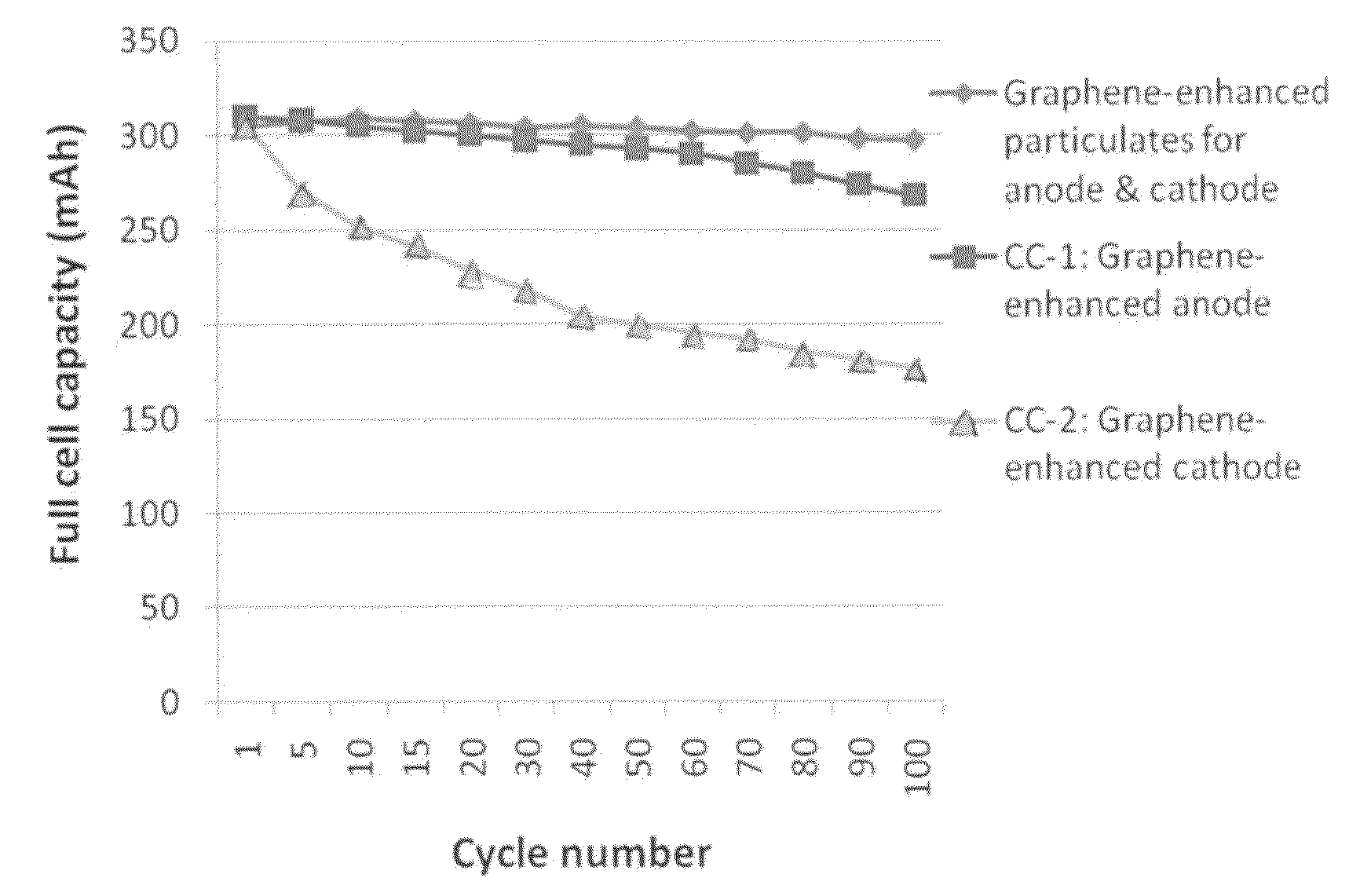
A nano graphene-enhanced particulate for use as a lithium-ion battery anode active material, wherein the particulate is formed of a single sheet of graphene or a plurality of graphene sheets and a plurality of fine anode active material particles with a size smaller than 10 μm. The graphene sheets and the particles are mutually bonded or agglomerated into the particulate with at least a graphene sheet embracing the anode active material particles. The amount of graphene is at least 0.01% by weight and the amount of the anode active material is at least 0.1% by weight, all based on the total weight of the particulate. A lithium-ion battery having an anode containing these graphene-enhanced particulates exhibits a stable charge and discharge cycling response, a high specific capacity per unit mass, a high first-cycle efficiency, a high capacity per electrode volume, and a long cycle life.
Owner:SAMSUNG ELECTRONICS CO LTD +1
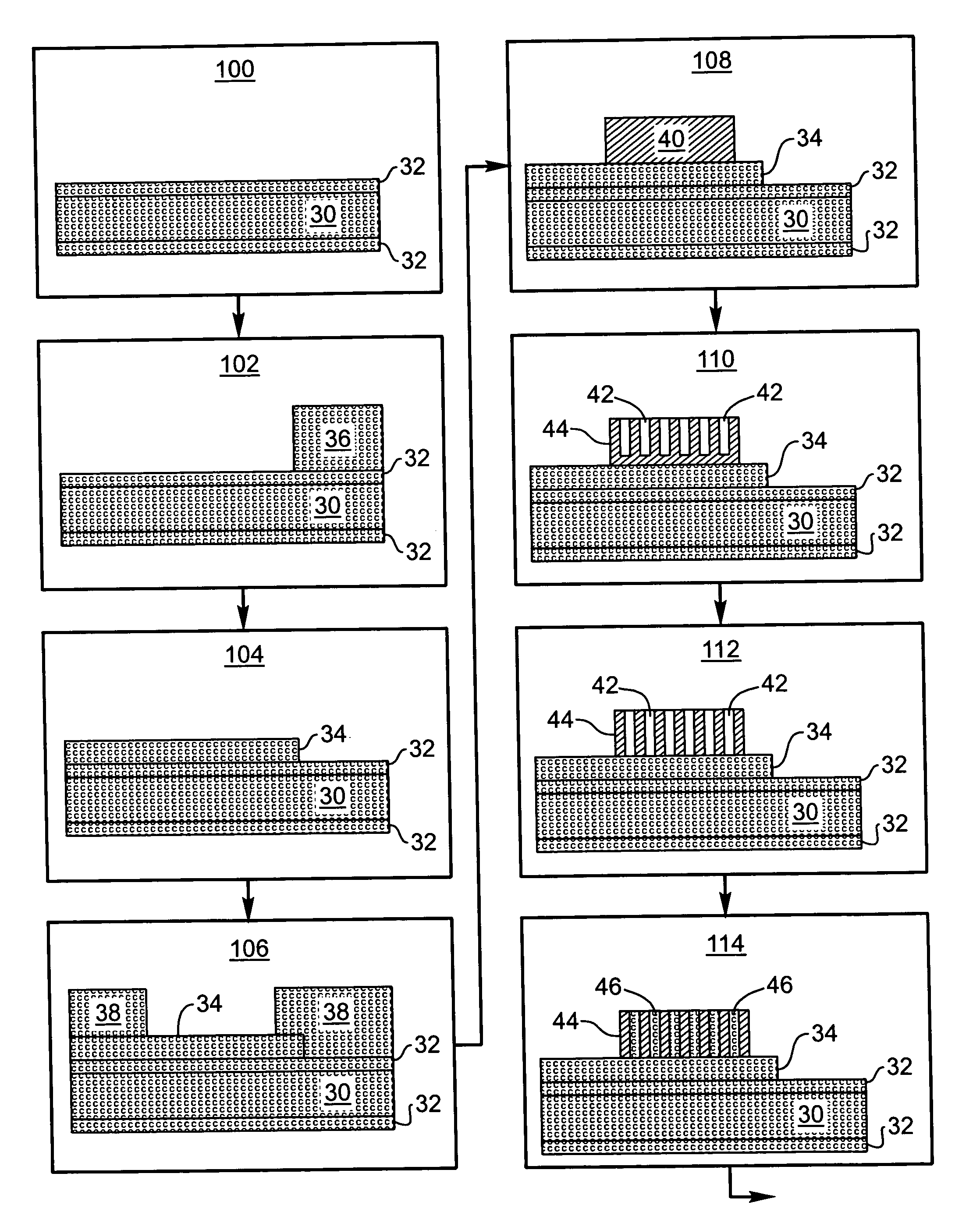
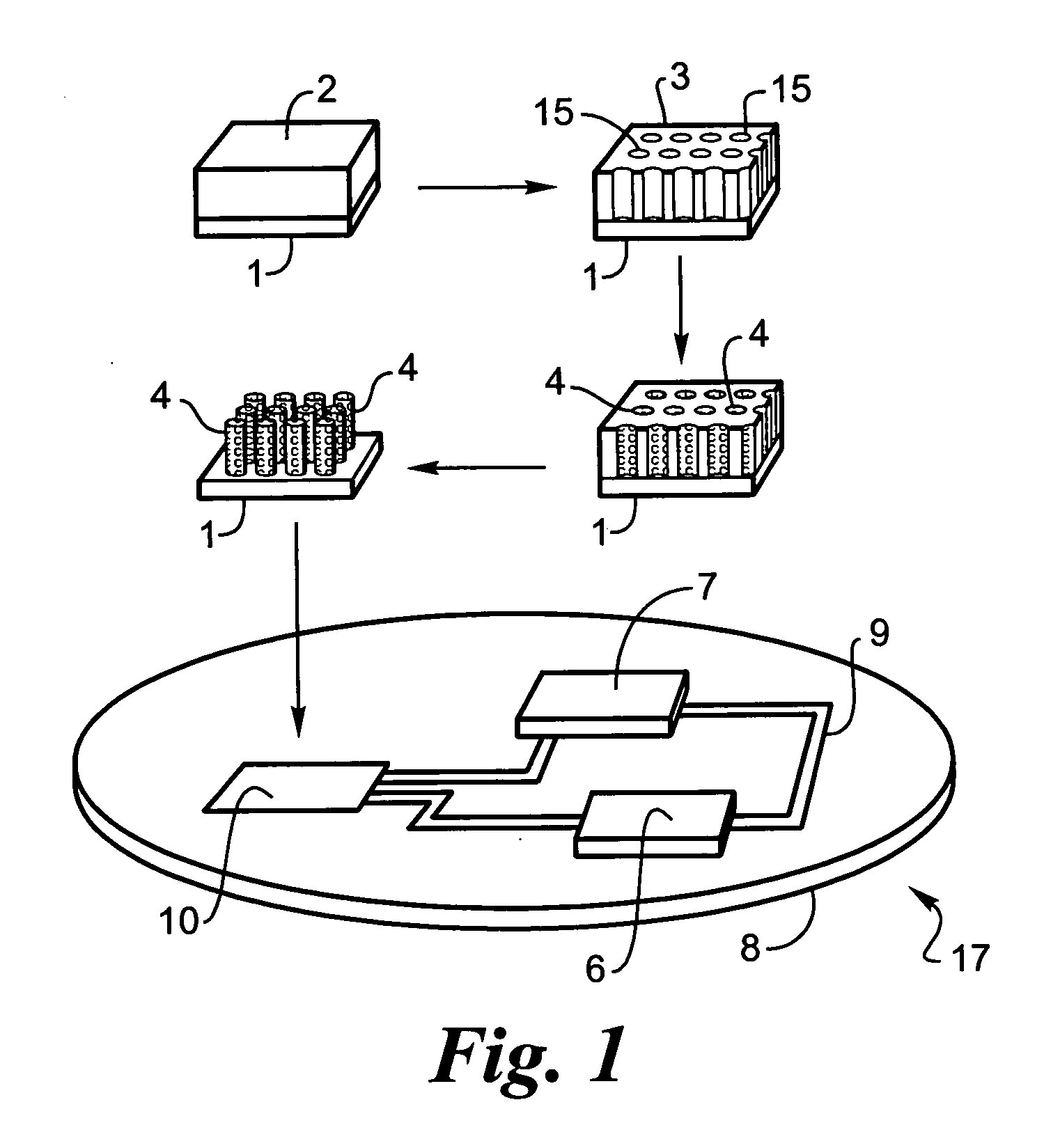
Rechargeable thin film battery and method for making the same
InactiveUS6982132B1Improve lithium ion mobilityHigh voltageElectrode thermal treatmentFinal product manufactureElectrical batteryHigh energy
A rechargeable, stackable, thin film, solid-state lithium electrochemical cell, thin film lithium battery and method for making the same is disclosed. The cell and battery provide for a variety configurations, voltage and current capacities. An innovative low temperature ion beam assisted deposition method for fabricating thin film, solid-state anodes, cathodes and electrolytes is disclosed wherein a source of energetic ions and evaporants combine to form thin film cell components having preferred crystallinity, structure and orientation. The disclosed batteries are particularly useful as power sources for portable electronic devices and electric vehicle applications where high energy density, high reversible charge capacity, high discharge current and long battery lifetimes are required.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Electroactive high storage capacity polyacetylene-co-polysulfur materials and electrolytic cells containing same
InactiveUS6117590AHigh storage capacity per unit weightFacilitates electron transportElectrode manufacturing processesNon-aqueous electrolyte accumulatorsElectrochemical cellElectrode material
The present invention relates to novel electroactive energy storing polyacetylene-co-polysulfur (PAS) materials of general formula (C2Sx)n wherein x is greater than 1 to about 100, and n is equal to or greater than 2. This invention also relates to novel rechargeable electrochemical cells containing positive electrode materials comprised of said polyacetylene-co-polysulfur materials with improved storage capacity and cycle life at ambient and sub-ambient temperatures.
Owner:THE BANK OF NEW YORK +1
Mesoporous network electrode for electrochemical cell
InactiveUS20040131934A1Simplifies production of cellFull penetrationMaterial nanotechnologyElectrode manufacturing processesLithiumNanoparticle
A high kinetics rate electrochemical cell in which at least one of the electrodes is composed of a mesostructural electroactive material comprising nanoparticles forming a three-dimensional framework structure of mesoporous texture having a bicontinuous junction of large specific surface area with the electrolyte. A low temperature method of preparation of the electrodes employs a high-speed deposition of the electrically active material in the form of a thin film. The application of said electrodes in high power lithium ion insertion batteries, photovoltaic cells, supercapacitors and fast electrochromic devices is disclosed.
Owner:FRANCOIS SUGNAUX
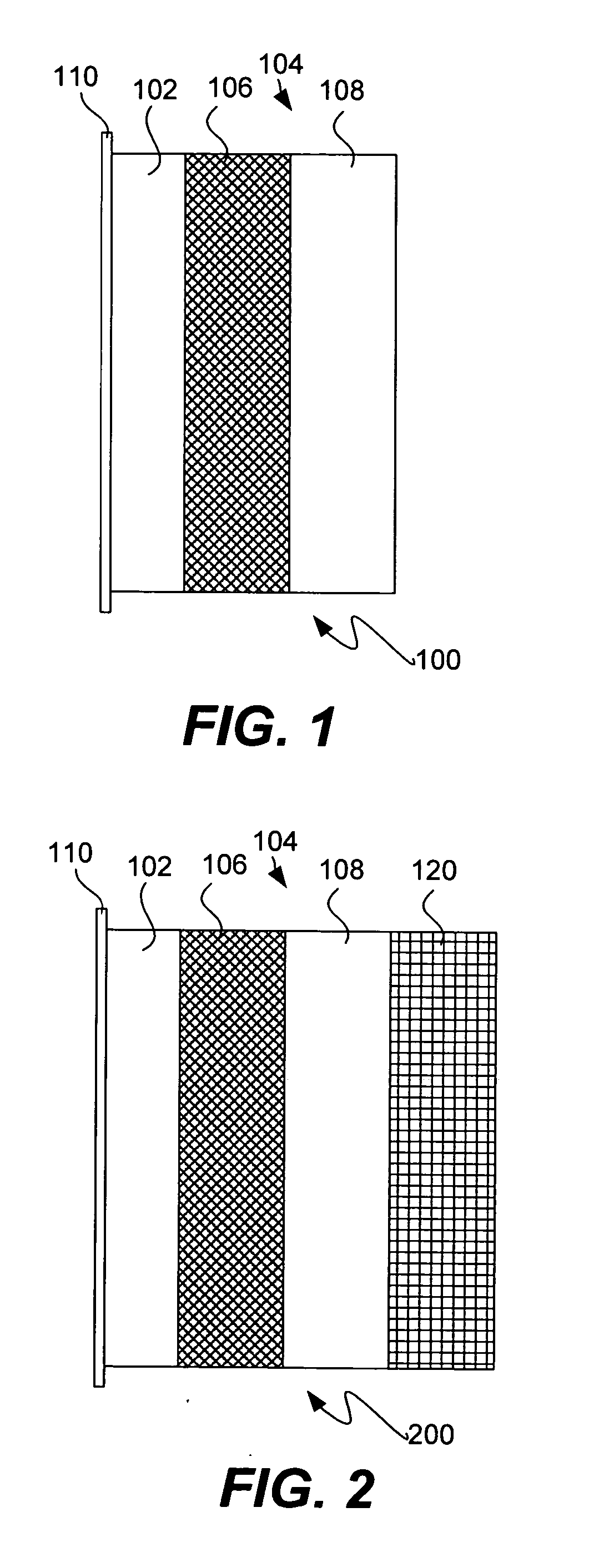
Protected active metal electrode and battery cell structures with non-aqueous interlayer architecture
ActiveUS7282295B2Avoid harmful reactionsFinal product manufactureElectrode carriers/collectorsMetal electrodesBattery cell
Active metal and active metal intercalation electrode structures and battery cells having ionically conductive protective architecture including an active metal (e.g., lithium) conductive impervious layer separated from the electrode (anode) by a porous separator impregnated with a non-aqueous electrolyte (anolyte). This protective architecture prevents the active metal from deleterious reaction with the environment on the other (cathode) side of the impervious layer, which may include aqueous or non-aqueous liquid electrolytes (catholytes) and / or a variety electrochemically active materials, including liquid, solid and gaseous oxidizers. Safety additives and designs that facilitate manufacture are also provided.
Owner:POLYPLUS BATTERY CO INC
Nanostructured Materials for Battery Applications
The present invention relates to nanostructured materials (including nanowires) for use in batteries. Exemplary materials include carbon-comprising, Si-based nanostructures, nanostructured materials disposed on carbon-based substrates, and nanostructures comprising nanoscale scaffolds. The present invention also provides methods of preparing battery electrodes, and batteries, using the nanostructured materials.
Owner:ONED MATERIAL INC
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6017651AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
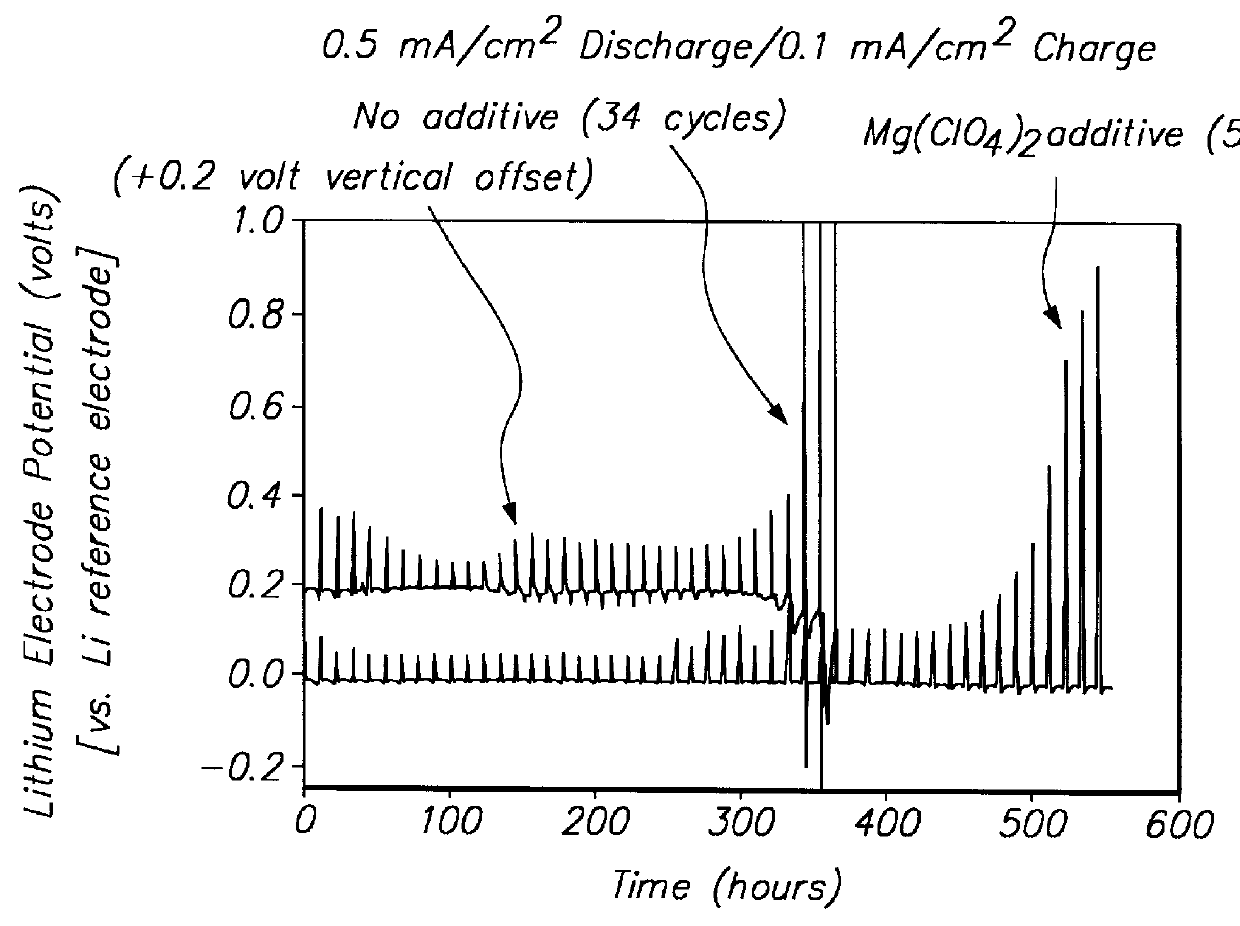
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Electrode Including Nanostructures for Rechargeable Cells
InactiveUS20100285358A1Lower resistanceNanostructure manufactureMicroscopic fiber electrodesRechargeable cellSilicon nanowires
A lithium ion battery electrode includes silicon nanowires used for insertion of lithium ions and including a conductivity enhancement, the nanowires growth-rooted to the conductive substrate.
Owner:AMPRIUS INC
Conductive lithium storage electrode
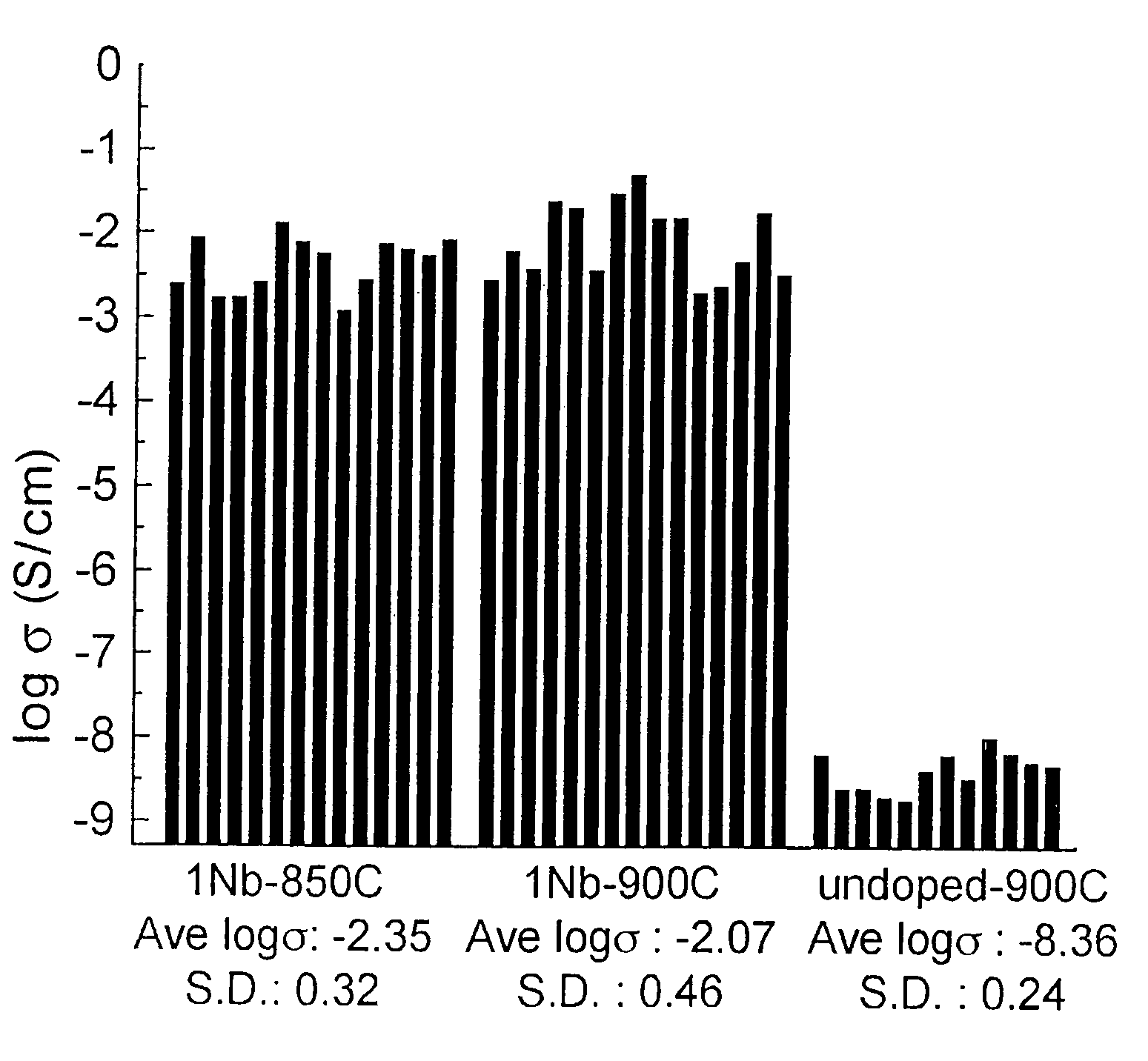
A compound comprising a composition Ax(M′1-aM″a)y(XD4)z, Ax(M′1-aM″a)y(DXD4)z, or Ax(M′1-aM″a)y(X2D7)z, and have values such that x, plus y(1-a) times a formal valence or valences of M′, plus ya times a formal valence or valence of M″, is equal to z times a formal valence of the XD4, X2D7, or DXD4 group; or a compound comprising a composition (A1-aM″a)xM′y(XD4)z, (A1-aM″a)xM′y(DXD4)z(A1-aM″a)xM′y(X2D7)z and have values such that (1-a)x plus the quantity ax times the formal valence or valences of M″ plus y times the formal valence or valences of M′ is equal to z times the formal valence of the XD4, X2D7 or DXD4 group. In the compound, A is at least one of an alkali metal and hydrogen, M′ is a first-row transition metal, X is at least one of phosphorus, sulfur, arsenic, molybdenum, and tungsten, M″ any of a Group IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA, IB, IIB, IIIB, IVB, VB, and VIB metal, D is at least one of oxygen, nitrogen, carbon, or a halogen, 0.0001<a≦0.1, and x, y, and z are greater than zero. The compound can have a conductivity at 27° C. of at least about 10−8 S / cm. The compound can be a doped lithium phosphate that can intercalate lithium or hydrogen. The compound can be used in an electrochemical device including electrodes and storage batteries and can have a gravimetric capacity of at least about 80 mAh / g while being charged / discharged at greater than about C rate of the compound.
Owner:MASSACHUSETTS INST OF TECH
Lithium-ion rechargeable battery based on nanostructures
A nanowire-based Li-ion rechargeable battery having superior performance with little capacity fade for use in applications including consumer electronics and medical devices is made by incorporating nanowire construction of the cathode. The nanowire-based battery system includes a nanostructured high surface area cathode structure fabricated by electrodeposition using alumina nanopore templates.
Owner:ENABLE IPC
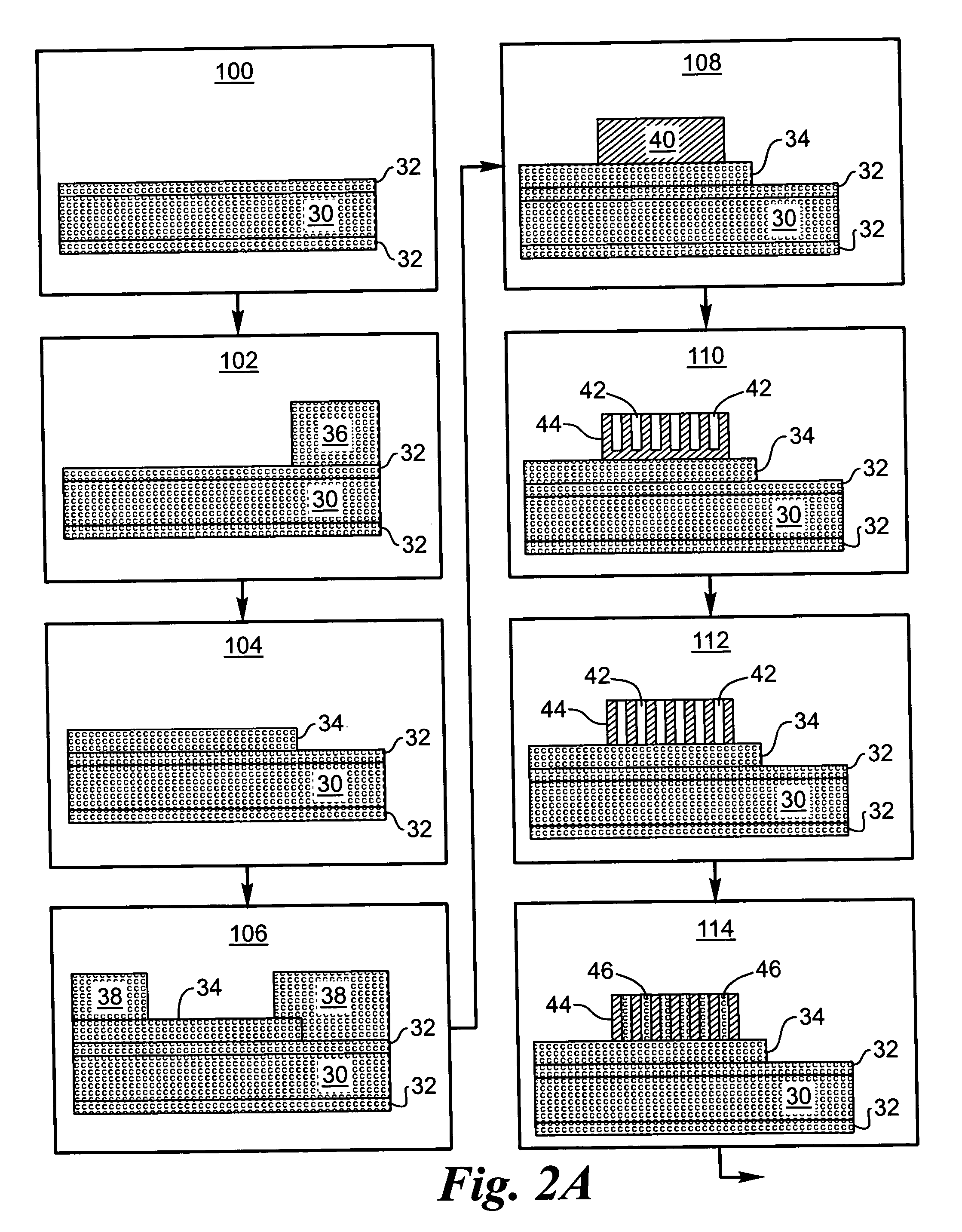
Electrode material for lithium secondary battery and electrode structure having the electrode material
InactiveUS20060040182A1Reduce conductivityImprove conductivityLi-accumulatorsNon-aqueous electrolyte accumulator electrodesLithiumLiquid state
The electrode material for a lithium secondary battery according to the present invention includes particles of a solid state alloy having silicon as a main component, wherein the particles of the solid state alloy have a microcrystal or amorphous material including an element other than silicon, dispersed in microcrystalline silicon or amorphized silicon. The solid state alloy preferably contains a pure metal or a solid solution. The composition of the alloy preferably has an element composition in which the alloy is completely mixed in a melted liquid state, whereby the alloy has a single phase in a melted liquid state without presence of two or more phases. The element composition can be determined by the kind of elements constituting the alloy and an atomic ratio of the elements.
Owner:CANON KK
Graphene-Enhanced cathode materials for lithium batteries
ActiveUS20120058397A1Short timeEasy dischargeNon-metal conductorsMaterial nanotechnologyParticulatesCvd graphene
A nano graphene-enhanced particulate for use as a lithium battery cathode active material, wherein the particulate is formed of a single or a plurality of graphene sheets and a plurality of fine cathode active material particles with a size smaller than 10 μm (preferably sub-micron or nano-scaled), and the graphene sheets and the particles are mutually bonded or agglomerated into an individual discrete particulate with at least a graphene sheet embracing the cathode active material particles, and wherein the particulate has an electrical conductivity no less than 10−4 S / cm and the graphene is in an amount of from 0.01% to 30% by weight based on the total weight of graphene and the cathode active material combined.
Owner:GLOBAL GRAPHENE GRP INC
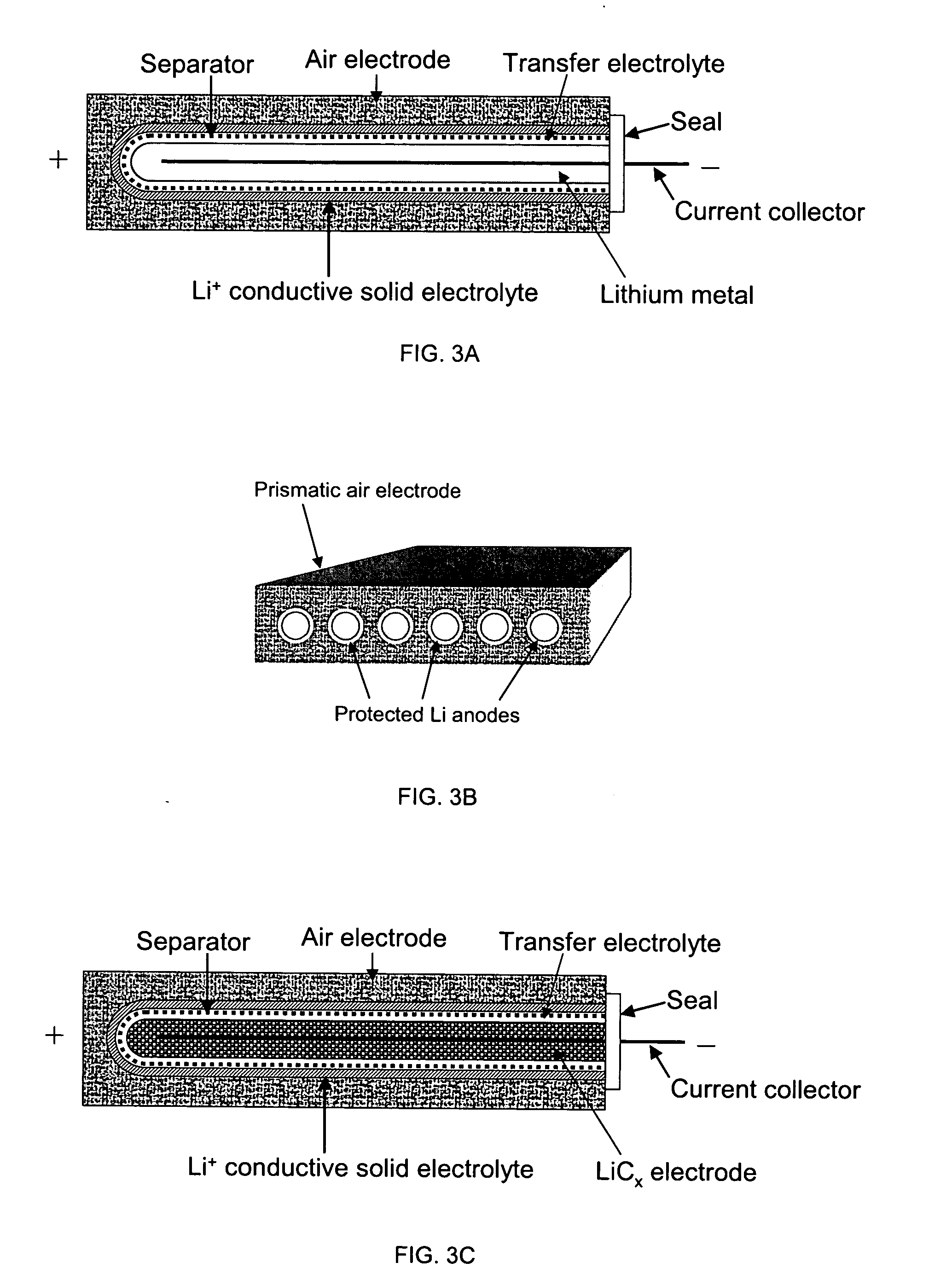
Rechargeable lithium/water, lithium/air batteries
InactiveUS20070221265A1Improve protectionEasy to controlFinal product manufacturePV power plantsHigh energyOptoelectronics
Electrochemical cells, and more specifically, rechargeable batteries comprising lithium anodes for use in water and / or air environments, as well as non-aqueous and non-air environments, are presented. In one embodiment, an electrochemical cell includes an anode comprising lithium and a multi-layered structure positioned between the anode and an electrolyte of the cell. A multi-layered structure can include at least a first single-ion conductive material layer (e.g., a lithiated metal layer), and at least a first polymeric layer positioned between the anode and the single-ion conductive material. The invention also can provide an electrode stabilization layer positioned within the electrode, i.e., between one portion and another portion of an electrode, to control depletion and re-plating of electrode material upon charge and discharge of a battery. Advantageously, electrochemical cells comprising combinations of structures described herein are not only compatible with environments that are typically unsuitable for lithium, but the cells may be also capable of displaying long cycle life, high lithium cycling efficiency, and high energy density.
Owner:SION POWER CORP
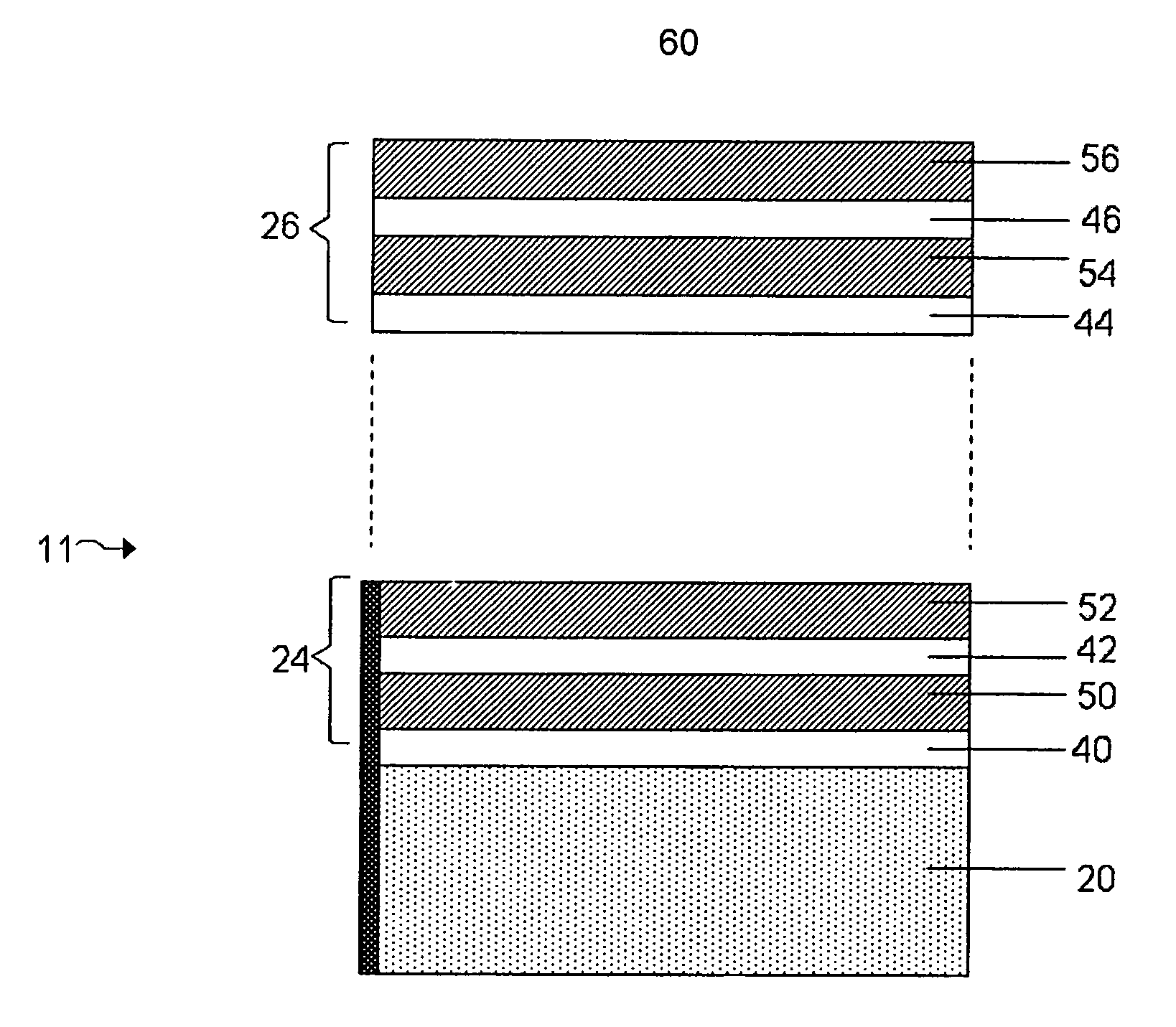
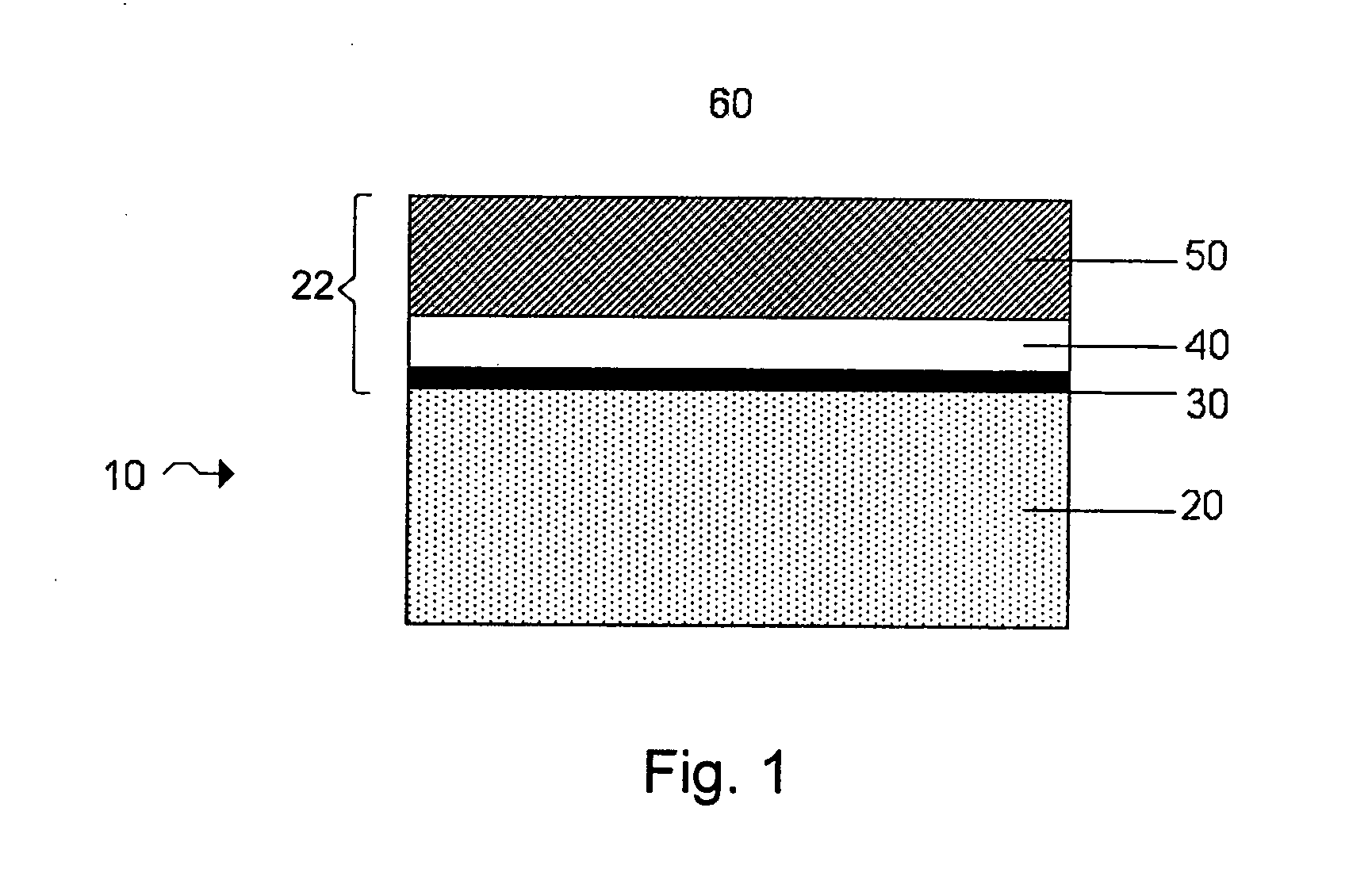
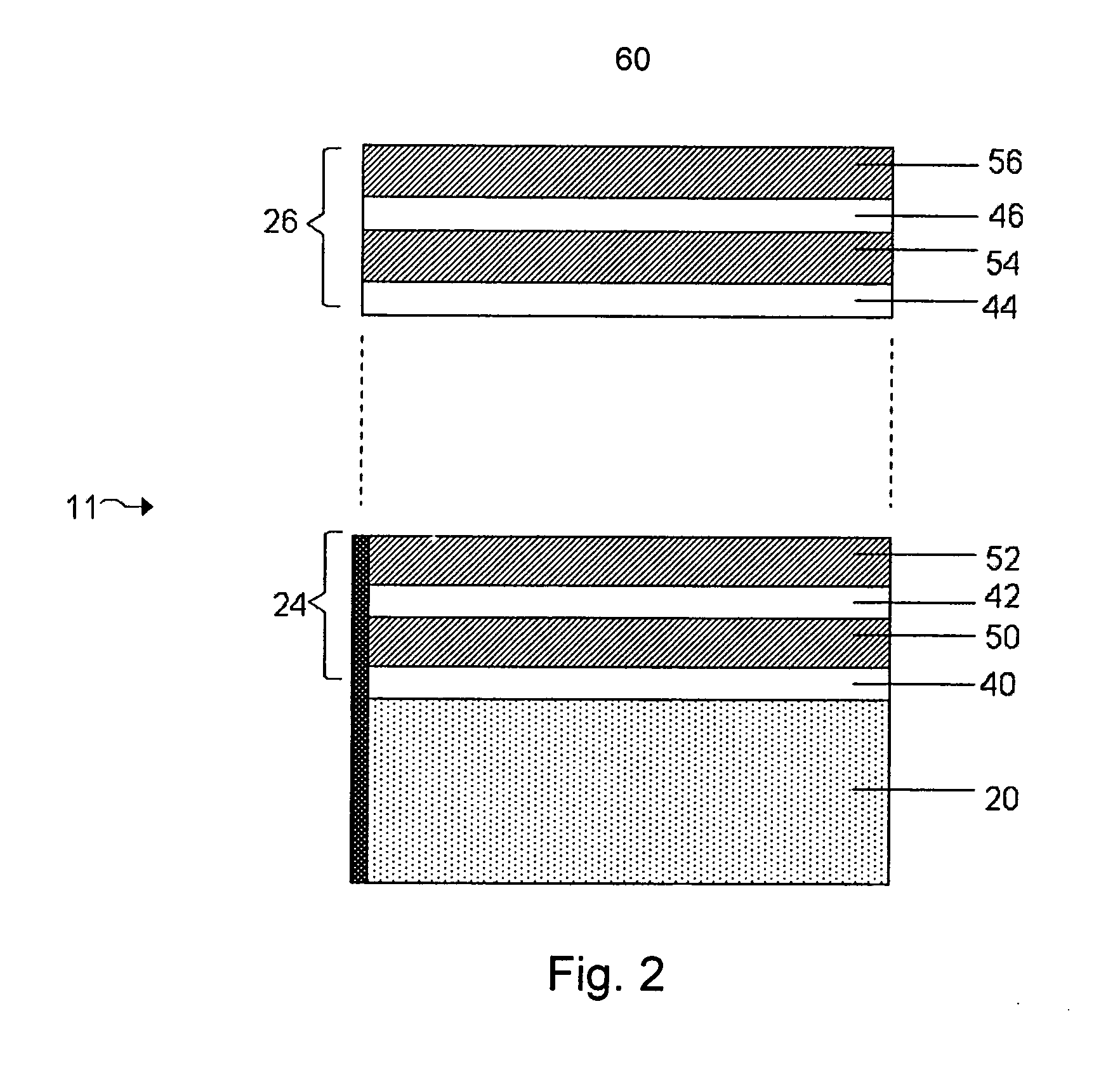
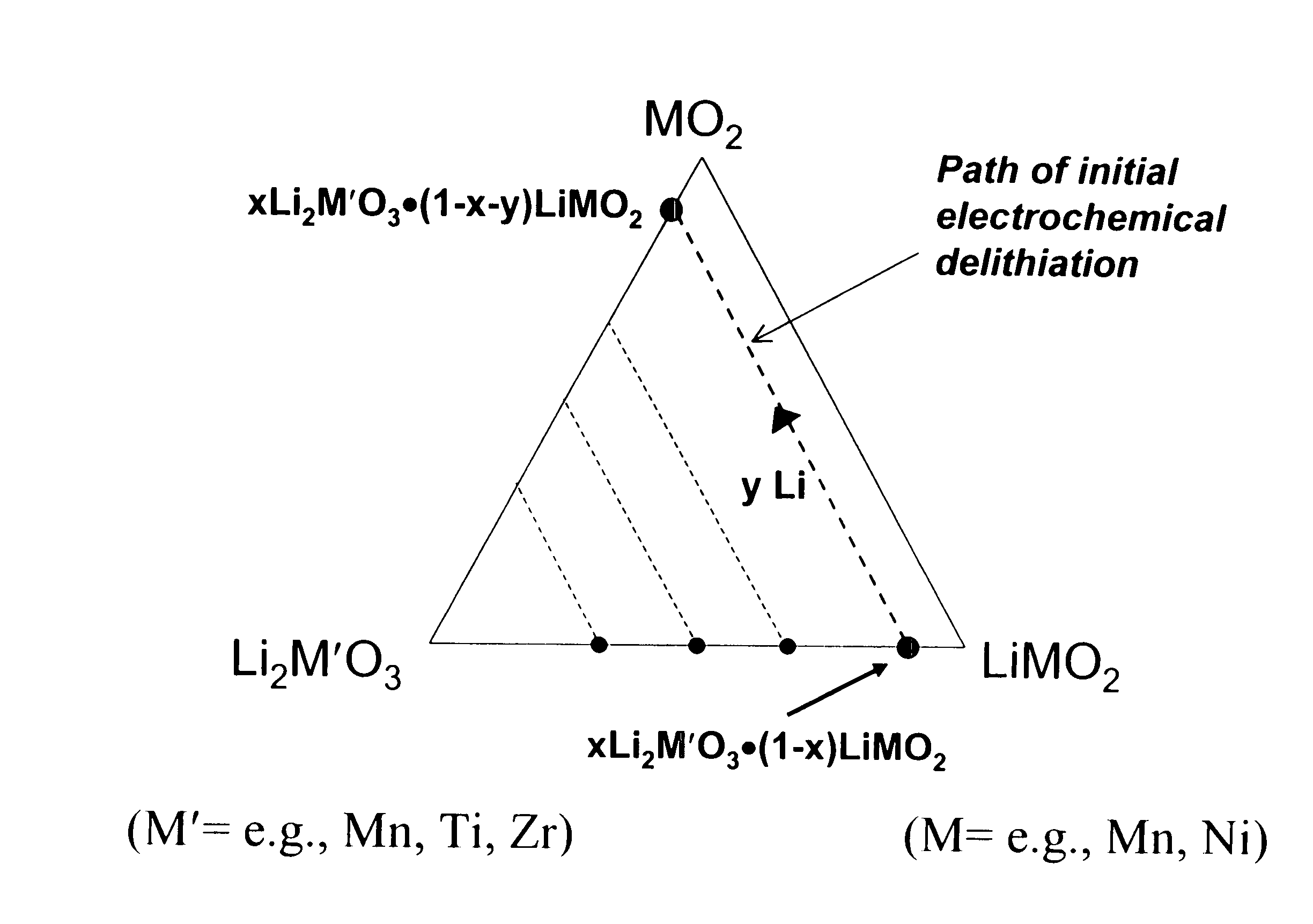
Lithium metal oxide electrodes for lithium cells and batteries
InactiveUS7135252B2Improve structural stabilityImprove stabilityZirconium compoundsSecondary cellsLithium metalOxidation state
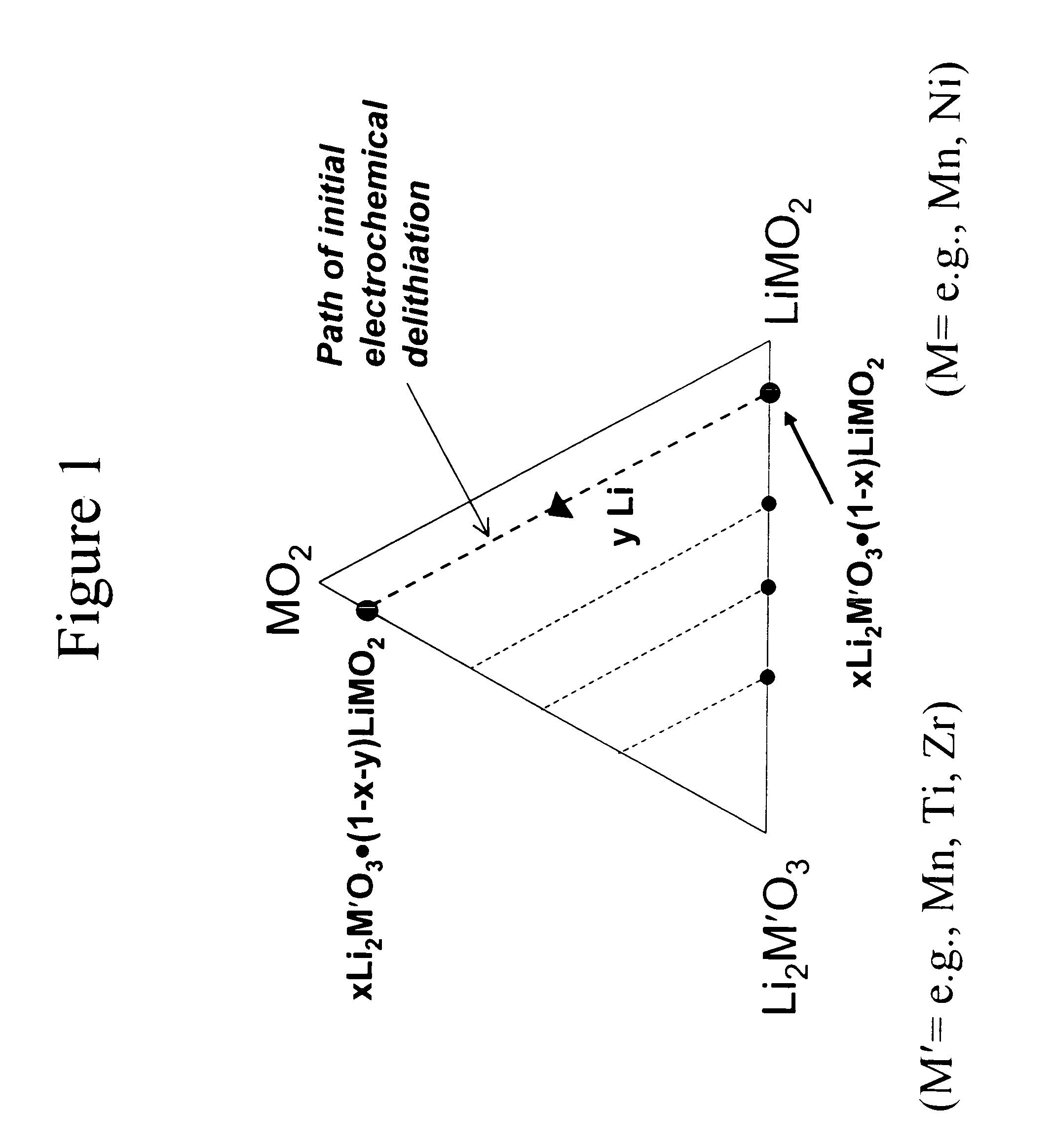
A lithium metal oxide positive electrode for a non-aqueous lithium cell is disclosed. The cell is prepared in its initial discharged state and has a general formula xLiMO2.(1-x)Li2M′O3 in which 0<x<1, and where M is more than one ion with an average trivalent oxidation state and with at least one ion being Ni, and where M′ is one or more ions with an average tetravalent oxidation state. Complete cells or batteries are disclosed with anode, cathode and electrolyte as are batteries of several cells connected in parallel or series or both.
Owner:CHICAGO UNIV OF THE +1
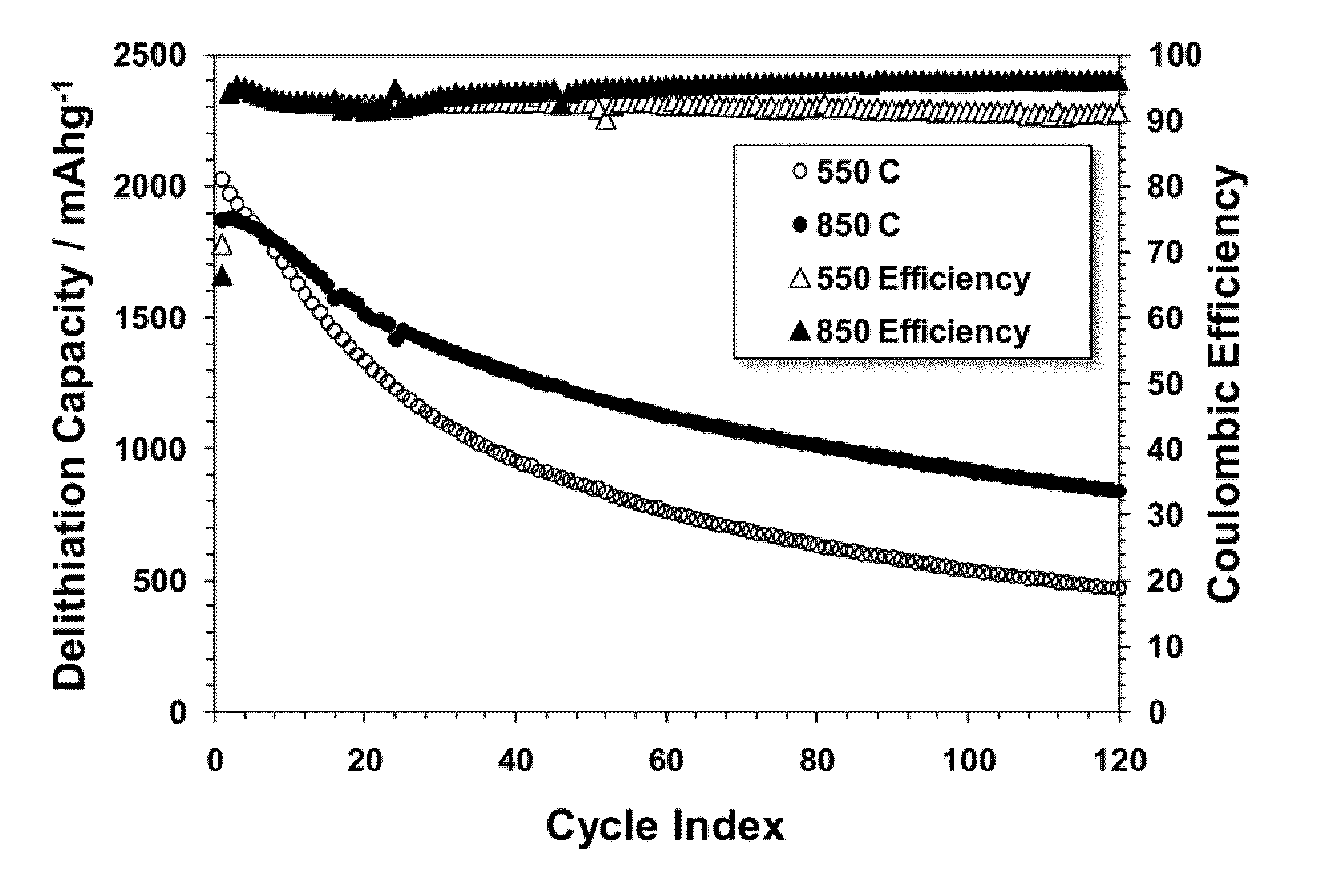
High Capacity Anode Materials for Lithium Ion Batteries
High capacity silicon based anode active materials are described for lithium ion batteries. These materials are shown to be effective in combination with high capacity lithium rich cathode active materials. Supplemental lithium is shown to improve the cycling performance and reduce irreversible capacity loss for at least certain silicon based active materials. In particular silicon based active materials can be formed in composites with electrically conductive coatings, such as pyrolytic carbon coatings or metal coatings, and composites can also be formed with other electrically conductive carbon components, such as carbon nanofibers and carbon nanoparticles. Additional alloys with silicon are explored.
Owner:IONBLOX INC
Laminated lithium ion battery, battery pack comprising same and pole piece of laminated lithium ion battery
InactiveCN104882635AImprove cooling effectReduce welding processFinal product manufactureElectrode carriers/collectorsInternal resistanceElectrical battery
Disclosed are a laminated lithium ion battery, a battery pack comprising the same and a pole piece of the laminated lithium ion battery. The pole piece comprises a current collector and an active material layer, wherein the current collector is coated with the active material layer. A section of continuous uncoated area is arranged at the tail end of a first width end portion of the pole piece, the top face and the bottom face of the uncoated area are not coated with the active material layer, the current collector is exposed in the uncoated area, and the exposed current collector serves as a pole lug of the pole piece. By the pole piece, internal resistance of the lithium ion battery is reduced and heat dissipation performance of the battery is improved.
Owner:SHENZHEN GREPOW BATTERY CO LTD
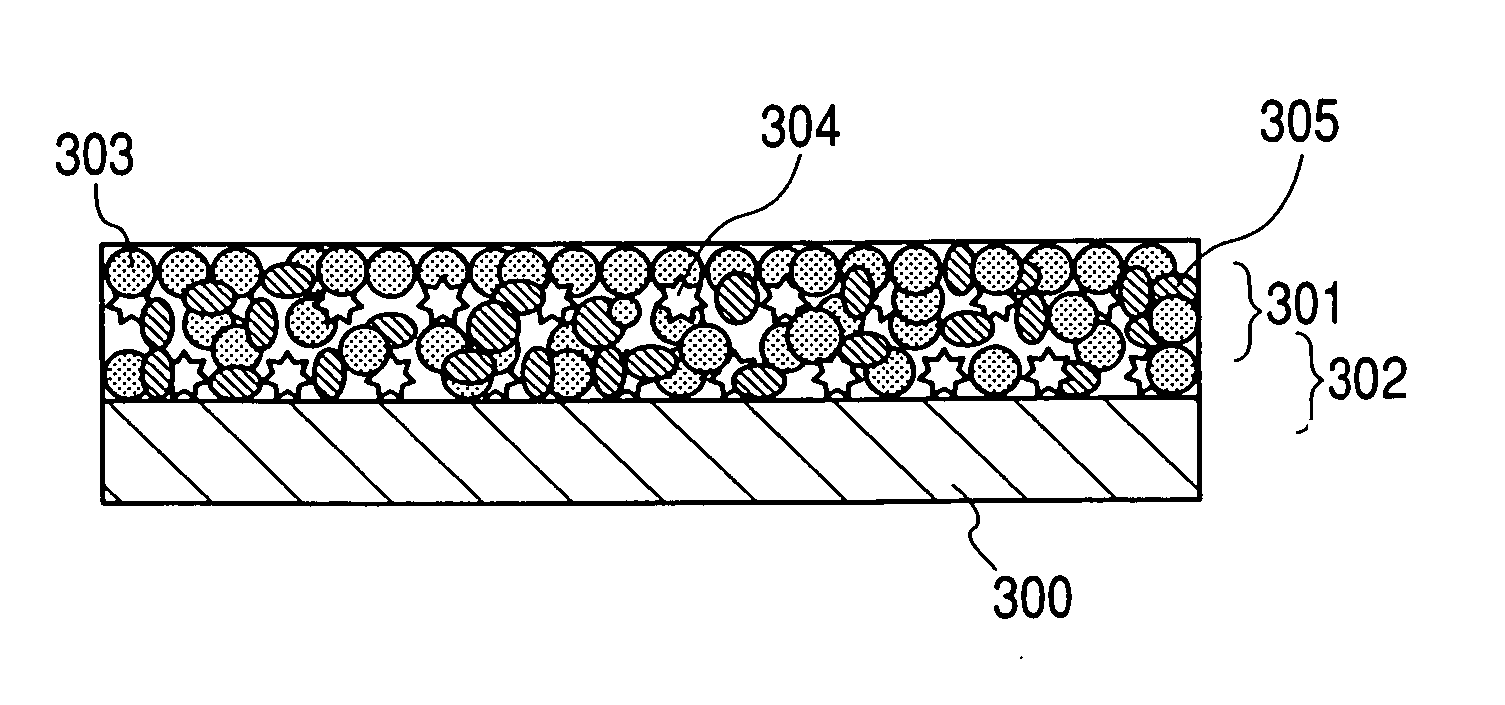
Porous silicon particulates for lithium batteries
An anode structure for lithium batteries includes nanofeatured silicon particulates dispersed in a conductive network. The particulates are preferably made from metallurgical grade silicon powder via HF / HNO3 acid treatment, yielding crystallite sizes from about 1 to 20 nm and pore sizes from about 1 to 100 nm. Surfaces of the particles may be terminated with selected chemical species to further modify the anode performance characteristics. The conductive network is preferably a carbonaceous material or composite, but it may alternatively contain conductive ceramics such as TiN or B4C. The anode structure may further contain a current collector of copper or nickel mesh or foil.
Owner:TIEGS TERRY N
Electrochemical device separator structures with barrier layer on non-swelling membrane
InactiveUS7070632B1Avoid harmful reactionsElectrode carriers/collectorsSolid electrolyte cellsElectrochemistryFluid electrolytes
Disclosed are electrochemical device separator structures which include a substantially impervious active metal ion conducting barrier layer material, such as an ion conducting glass, is formed on an active metal ion conducting membrane in which elongation due to swelling on contact with liquid electrolyte is constrained in at least two of three orthogonal dimensions of the membrane. The non-swelling character of the membrane prevents elongation in the x-y (or lateral, relative to the layers of the composite) orthogonal dimensions of the membrane when it is contacted with liquid electrolyte that would otherwise cause the barrier layer to rupture. Substantial swelling of the membrane, if any, is limited to the z (or vertical, relative to the layers of the composite) dimension.
Owner:POLYPLUS BATTERY CO INC
Protected active metal electrode and battery cell structures with non-aqueous interlayer architecture
ActiveUS20050175894A1Avoid harmful reactionsHybrid capacitor separatorsHybrid capacitor electrolytesMetal electrodesBattery cell
Active metal and active metal intercalation electrode structures and battery cells having ionically conductive protective architecture including an active metal (e.g., lithium) conductive impervious layer separated from the electrode (anode) by a porous separator impregnated with a non-aqueous electrolyte (anolyte). This protective architecture prevents the active metal from deleterious reaction with the environment on the other (cathode) side of the impervious layer, which may include aqueous or non-aqueous liquid electrolytes (catholytes) and / or a variety electrochemically active materials, including liquid, solid and gaseous oxidizers. Safety additives and designs that facilitate manufacture are also provided.
Owner:POLYPLUS BATTERY CO INC
Electrode material comprising graphene composite materials in a graphite network formed from reconstituted graphene sheets
ActiveUS20110111303A1Weight increaseIncrease storage capacityConductive materialNon-conductive material with dispersed conductive materialGraphiteCvd graphene
A durable electrode material suitable for use in Li ion batteries is provided. The material is comprised of a continuous network of graphite regions integrated with, and in good electrical contact with a composite comprising graphene sheets and an electrically active material, such as silicon, wherein the electrically active material is dispersed between, and supported by, the graphene sheets.
Owner:NORTHWESTERN UNIV
Ionically conductive membranes for protection of active metal anodes and battery cells
InactiveUS20040191617A1Improve propertiesImprove ionic conductivitySolid electrolytesSolid electrolyte cellsChemical stabilityBattery cell
Disclosed are ionically conductive membranes for protection of active metal anodes and methods for their fabrication. The membranes may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the membrane has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The membrane is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the membrane is incorporated.
Owner:POLYPLUS BATTERY CO INC
Method of depositing silicon on carbon materials and forming an anode for use in lithium ion batteries
ActiveUS20080261116A1Increase rangeMaterial nanotechnologyNanoinformaticsPhysical chemistryCarbon nanofiber
A method of modifying the surface of carbon materials such as vapor grown carbon nanofibers is provided in which silicon is deposited on vapor grown carbon nanofibers using a chemical vapor deposition process. The resulting silicon-carbon alloy may be used as an anode in a rechargeable lithium ion battery.
Owner:APPLIED SCI
Nano graphene platelet-base composite anode compositions for lithium ion batteries
ActiveUS7745047B2Improve conductivityLower internal resistanceAlkaline accumulatorsElectrolytic capacitorsGraphiteGraphene
The present invention provides a nano-scaled graphene platelet-based composite material composition for use as an electrode, particularly as an anode of a lithium ion battery. The composition comprises: (a) micron- or nanometer-scaled particles or coating which are capable of absorbing and desorbing lithium ions; and (b) a plurality of nano-scaled graphene platelets (NGPs), wherein a platelet comprises a graphene sheet or a stack of graphene sheets having a platelet thickness less than 100 nm; wherein at least one of the particles or coating is physically attached or chemically bonded to at least one of the graphene platelets and the amount of platelets is in the range of 2% to 90% by weight and the amount of particles or coating in the range of 98% to 10% by weight. Also provided is a lithium secondary battery comprising such a negative electrode (anode). The battery exhibits an exceptional specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:SAMSUNG ELECTRONICS CO LTD
Nanocomposite of graphene and metal oxide materials
ActiveUS20100081057A1Easy to distinguishMaterial nanotechnologyRecord information storageDischarge rateCvd graphene
Nanocomposite materials comprising a metal oxide bonded to at least one graphene material. The nanocomposite materials exhibit a specific capacity of at least twice that of the metal oxide material without the graphene at a charge / discharge rate greater than about 10C.
Owner:THE TRUSTEES FOR PRINCETON UNIV +1
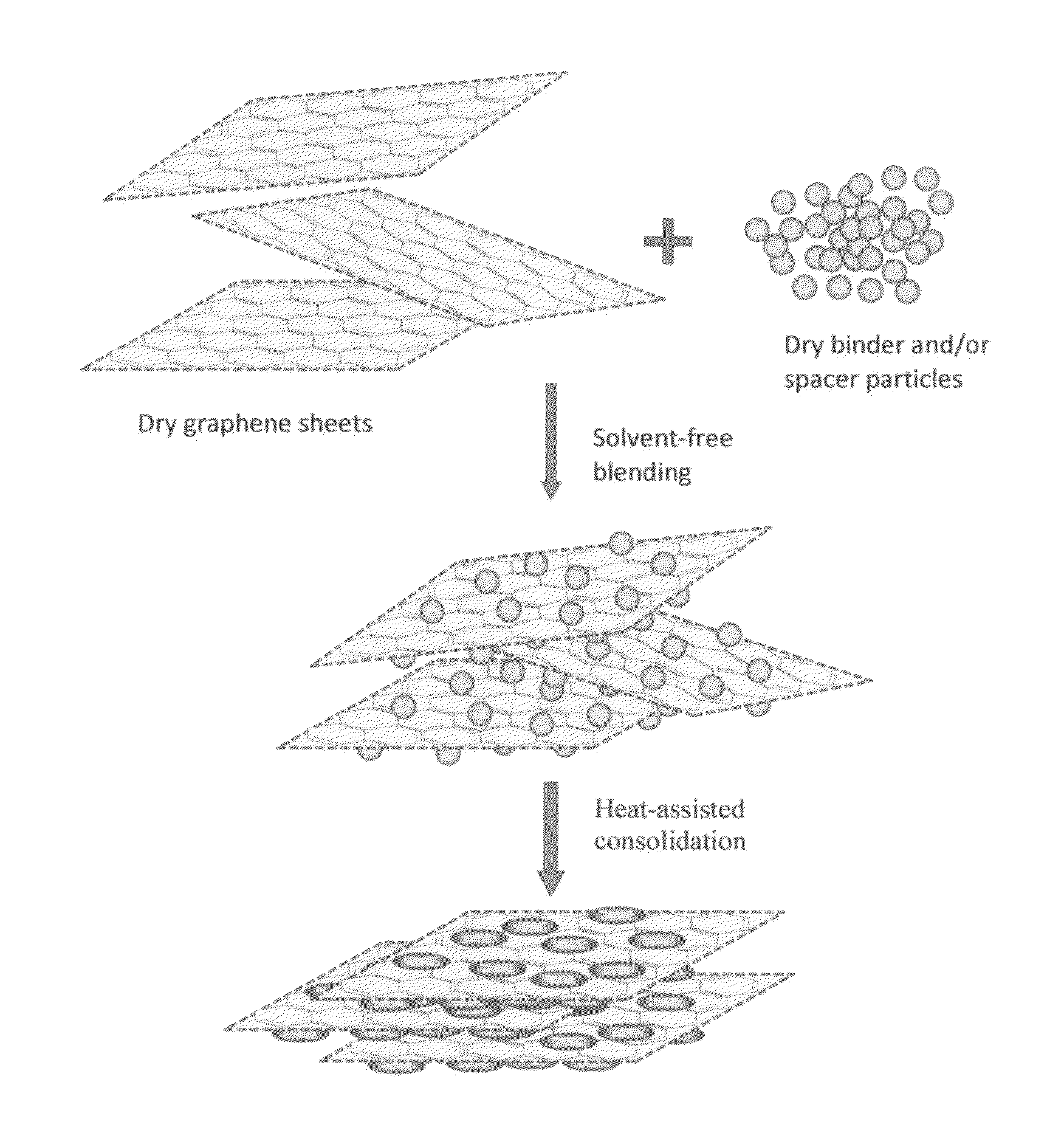
Solvent-free process based graphene electrode for energy storage devices
PendingUS20140030590A1Inexpensive and durable and highly reliableHigh capacitanceMaterial nanotechnologyHybrid capacitor electrodesGraphene flakeSolvent free
Disclosed is an electrode for an electrochemical energy storage device, the electrode comprising a self-supporting layer of a mixture of graphene sheets and spacer particles and / or binder particles, wherein the electrode is prepared without using water, solvent, or liquid chemical. The graphene electrode prepared by the solvent-free process exhibits many desirable features and advantages as compared to the corresponding electrode prepared by a known wet process. These advantages include a higher electrode specific surface area, higher energy storage capacity, improved or higher packing density or tap density, lower amount of binder required, lower internal electrode resistance, more consistent and uniform dispersion of graphene sheets and binder, reduction or elimination of undesirable effect of electrolyte oxidation or decomposition due to the presence of water, solvent, or chemical, etc.
Owner:GLOBAL GRAPHENE GRP INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com