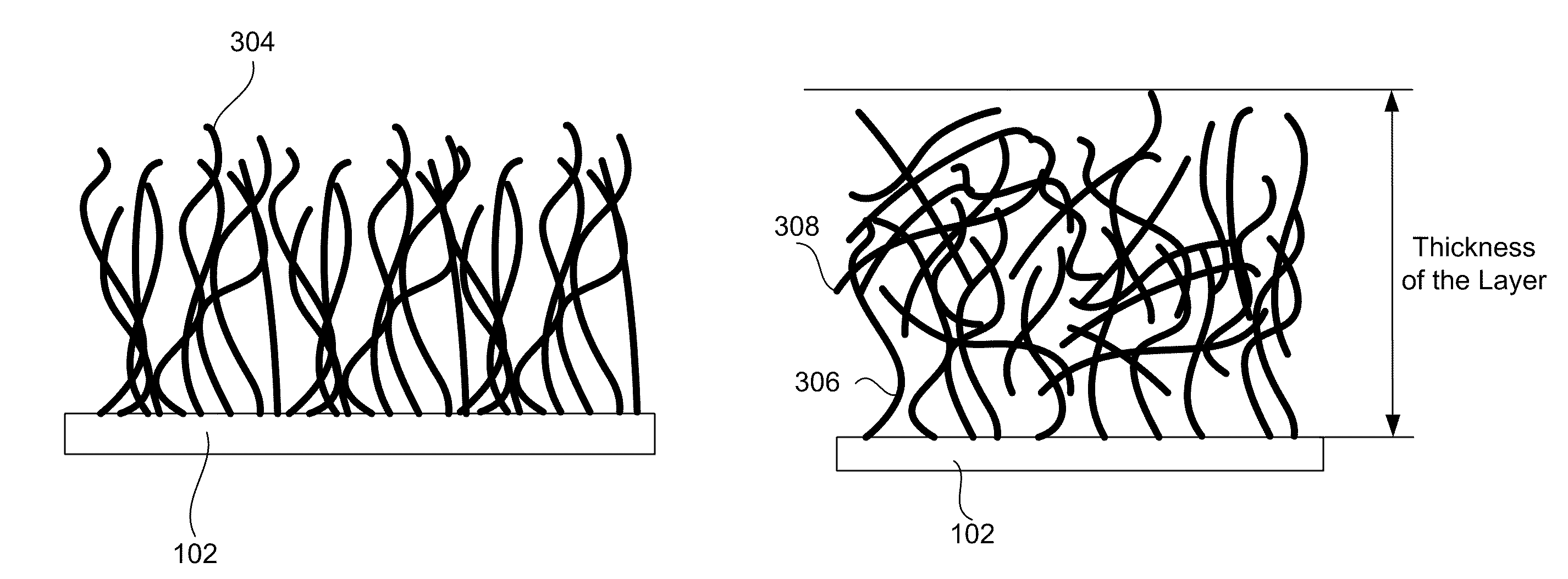
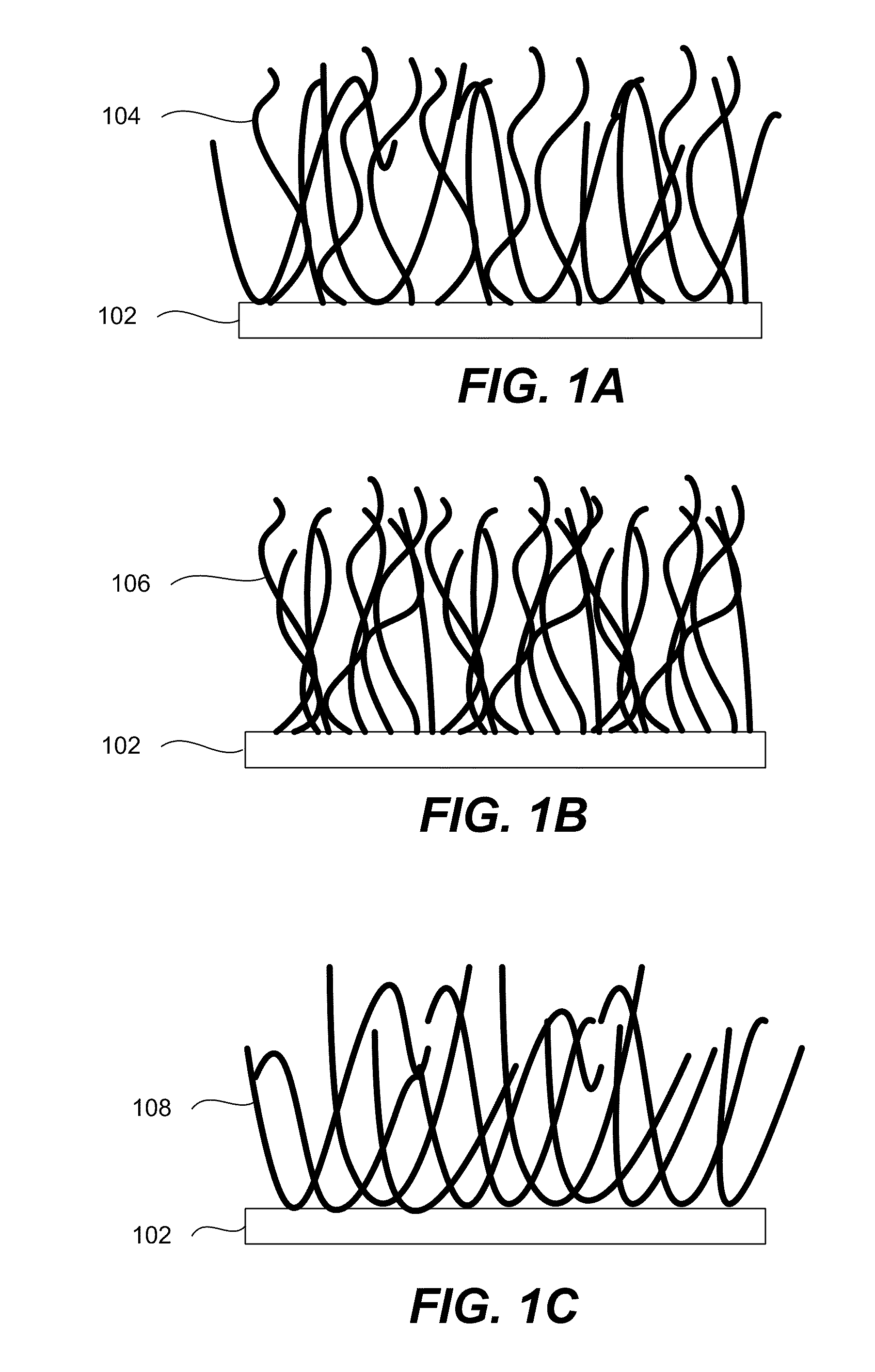
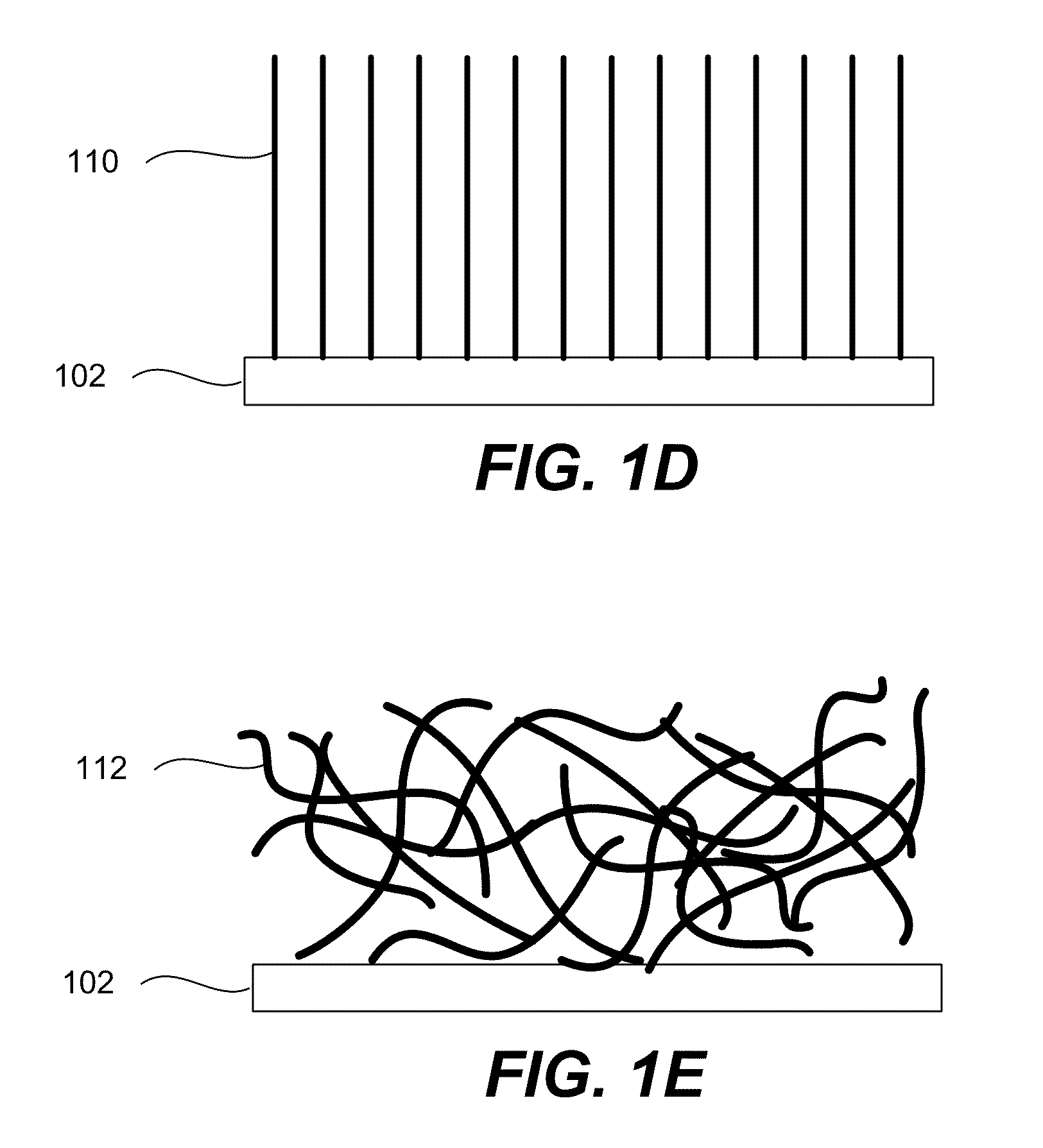
Electrode Including Nanostructures for Rechargeable Cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example
[0114]Half cells were constructed with silicon nanowires grown on a stainless steel substrate as one electrode and lithium foil as the other electrode. The electrodes were arranged in the glass fixtures with 1.0 M LiPF6 electrolyte mixed in equal parts of ethylene carbonate (EC) and diethyl carbonate (DEC) as solvent. The half cells were then tested to determine capacity retention after cycling. The results demonstrated that a capacity was about 3000 mAh / g after 20 cycles. Another set of half cells was subjected to longer cycling. The capacity of these cells was about 1000 mAh / g after 180 cycles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com