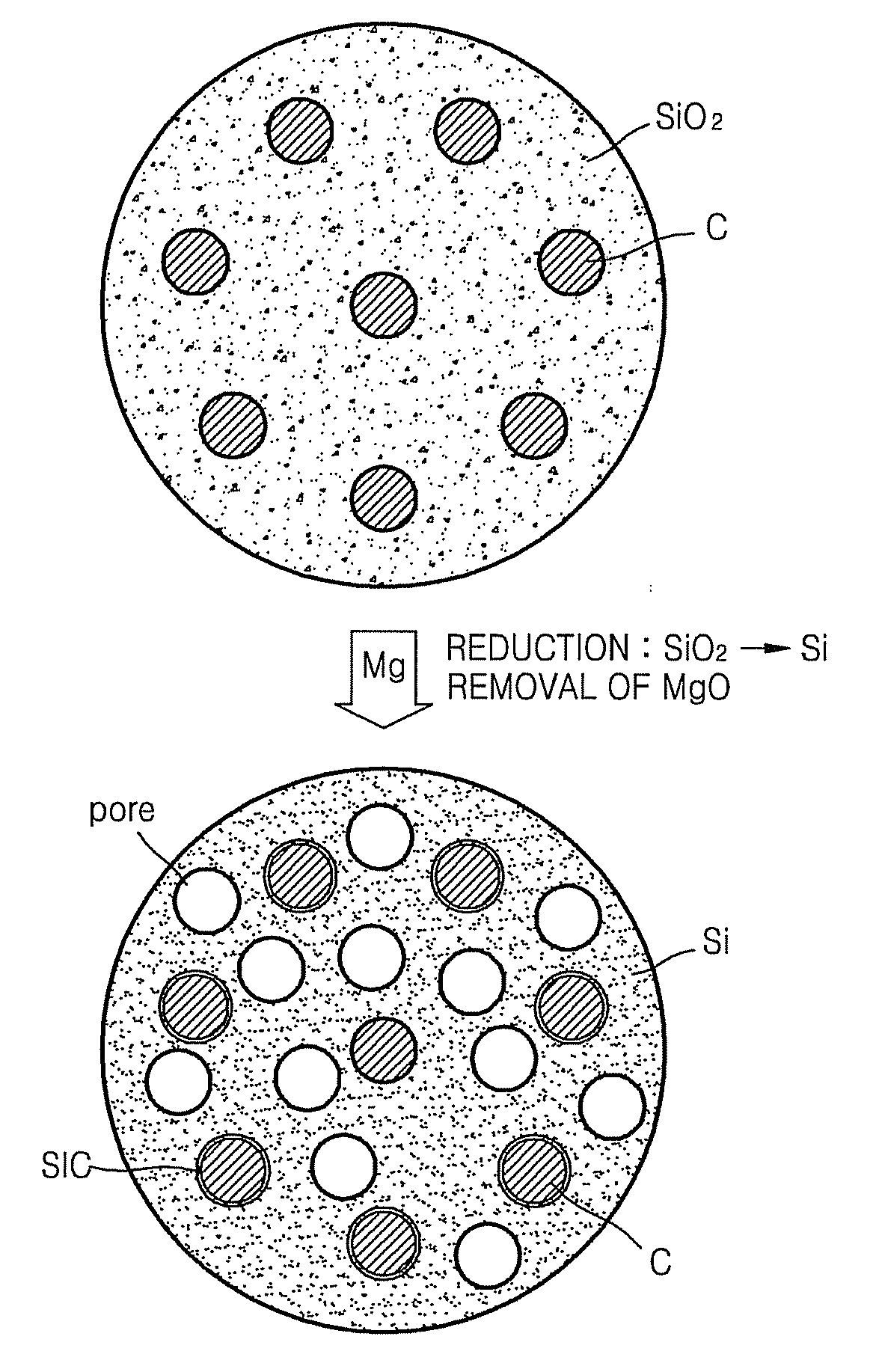
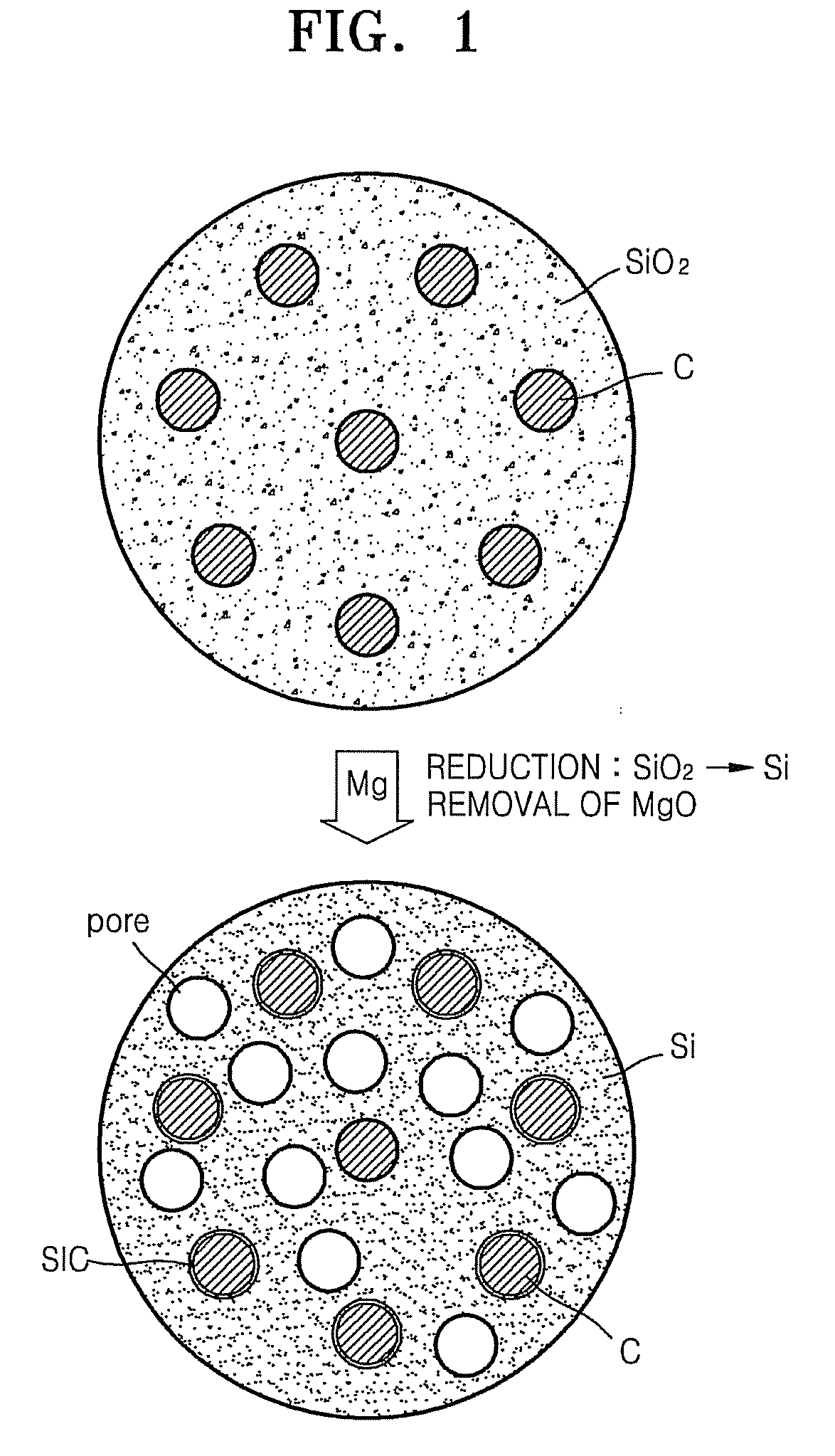
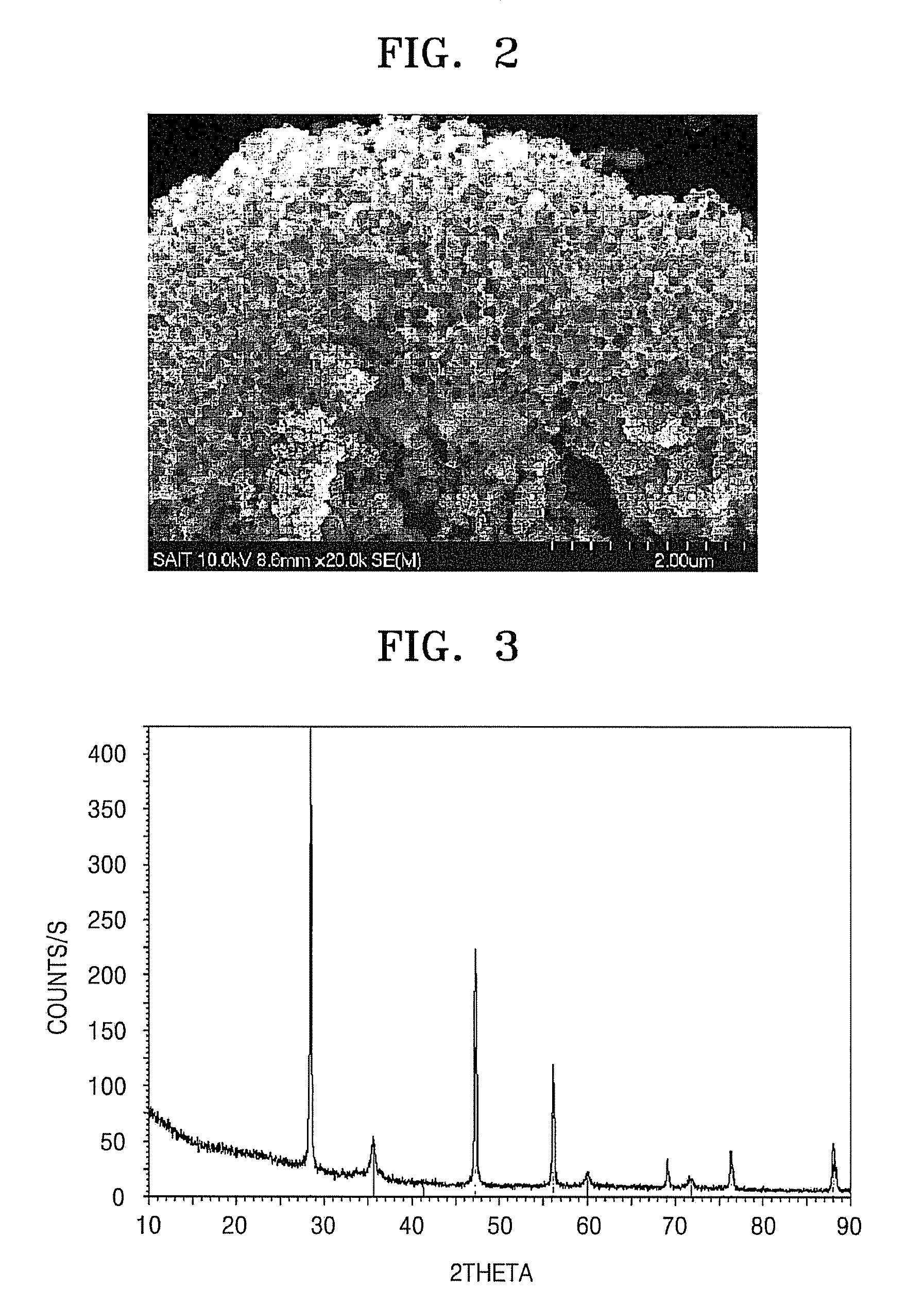
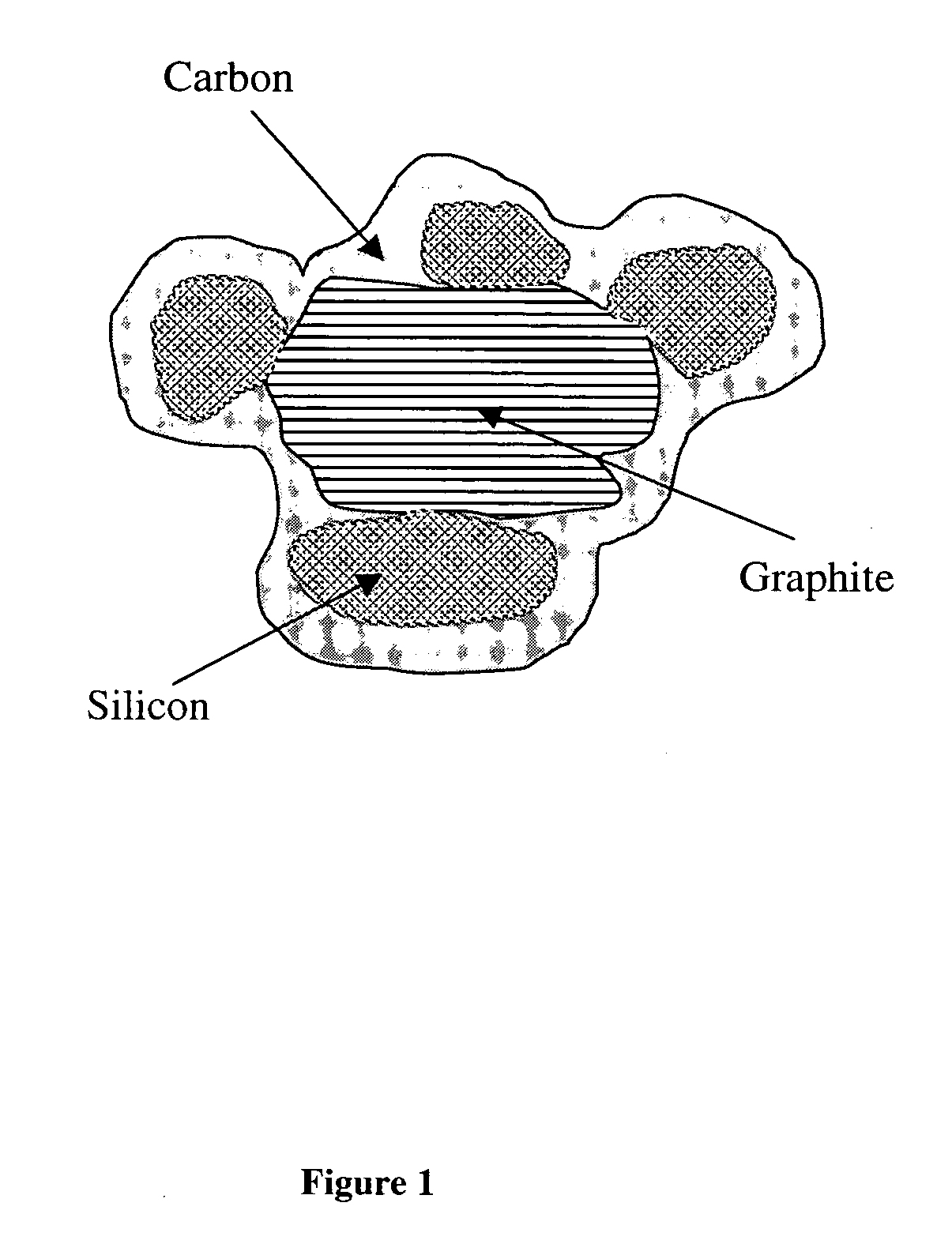
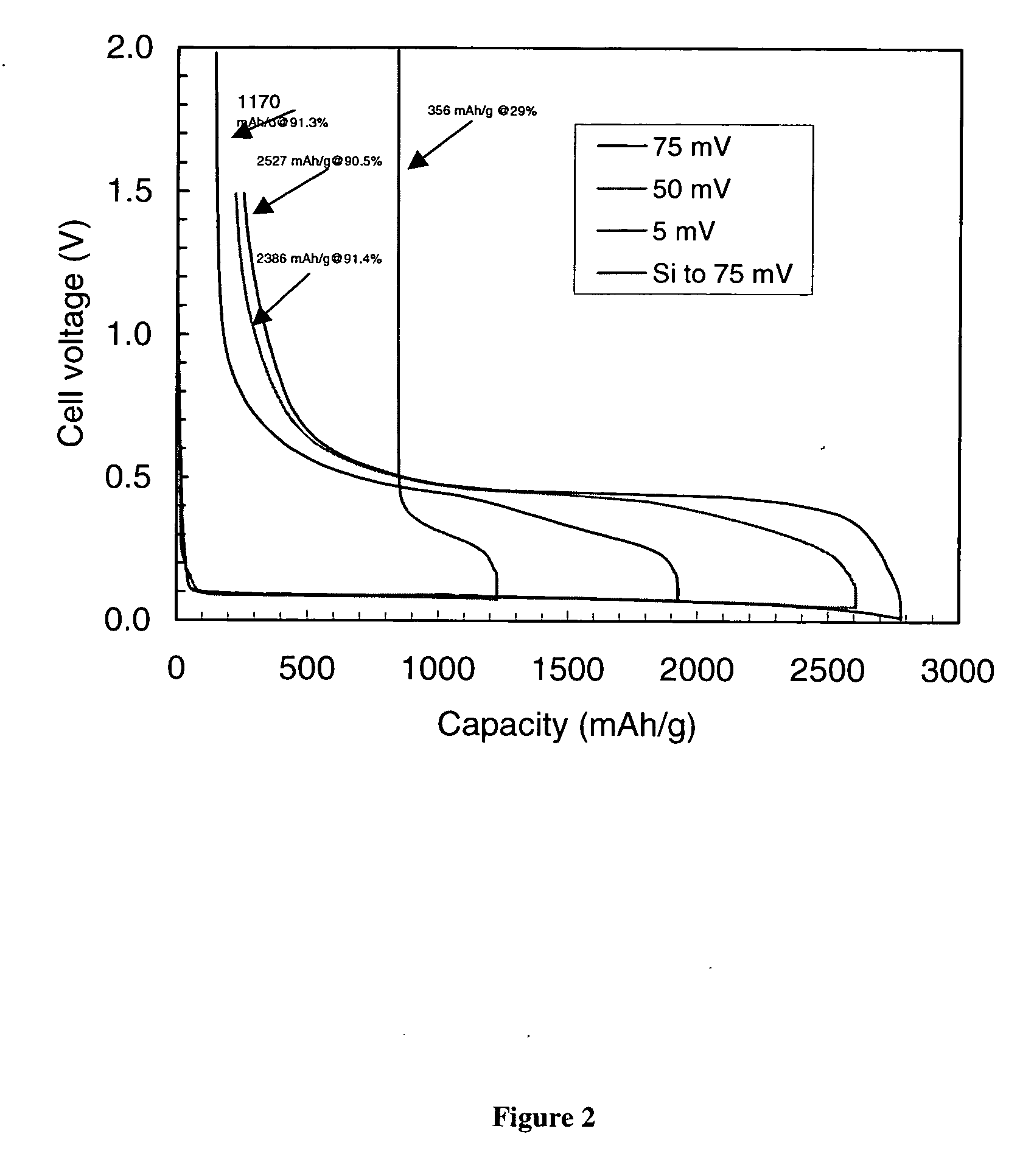
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
15424results about "Electrode carriers/collectors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
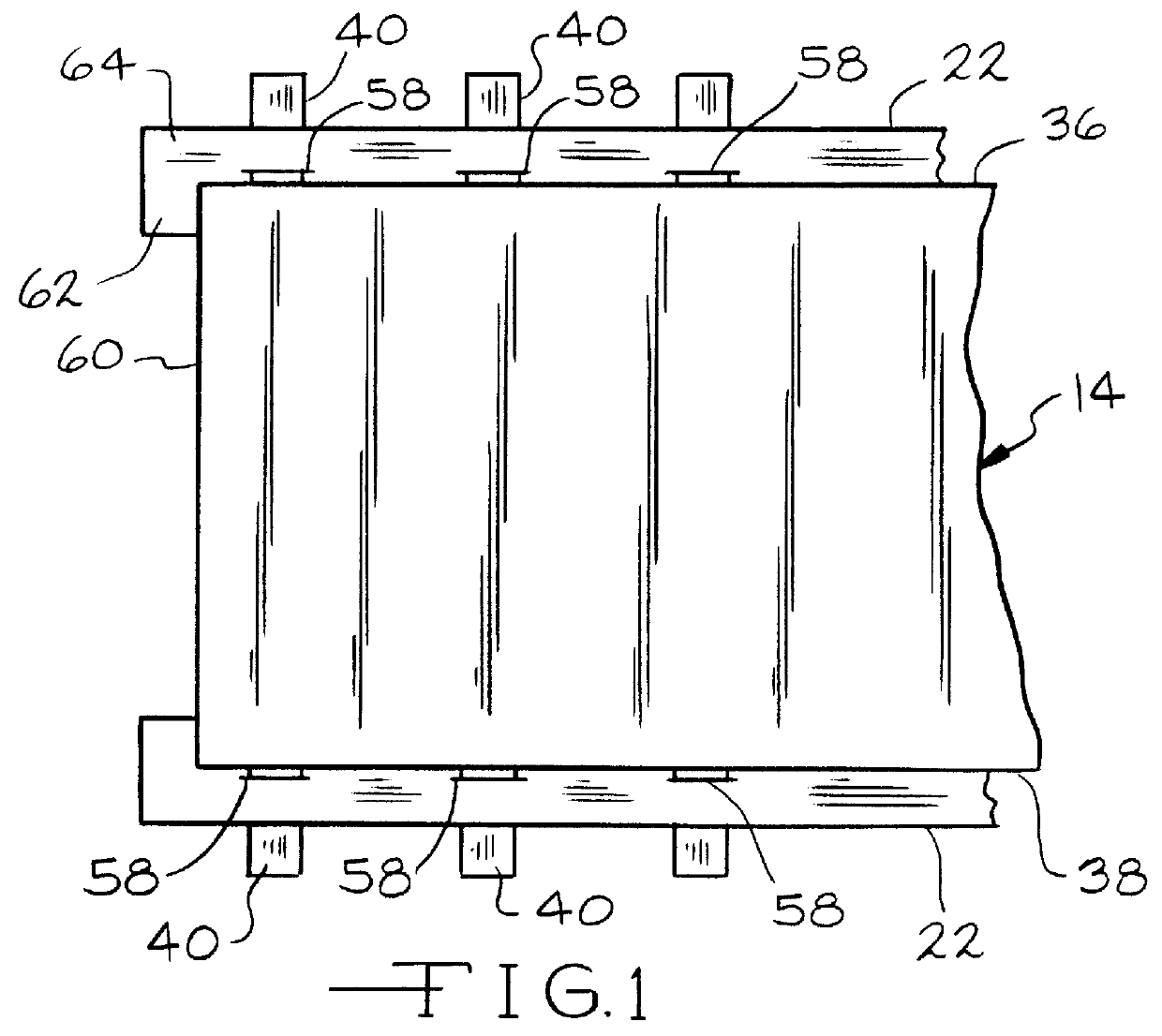
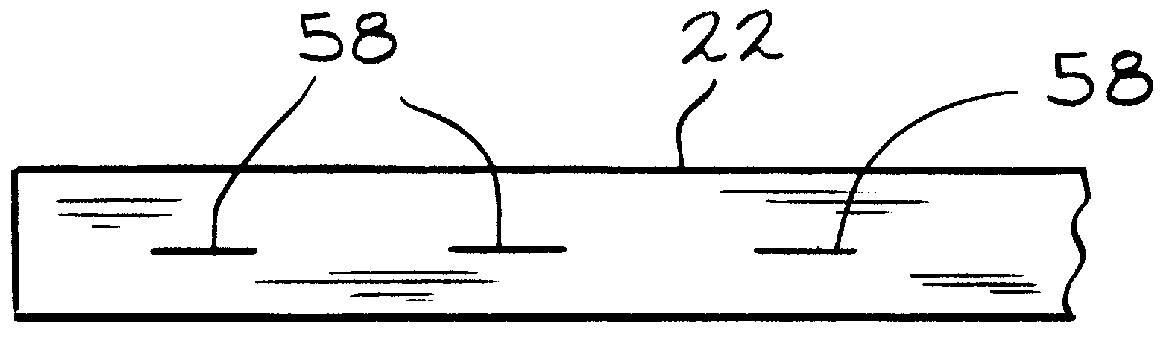
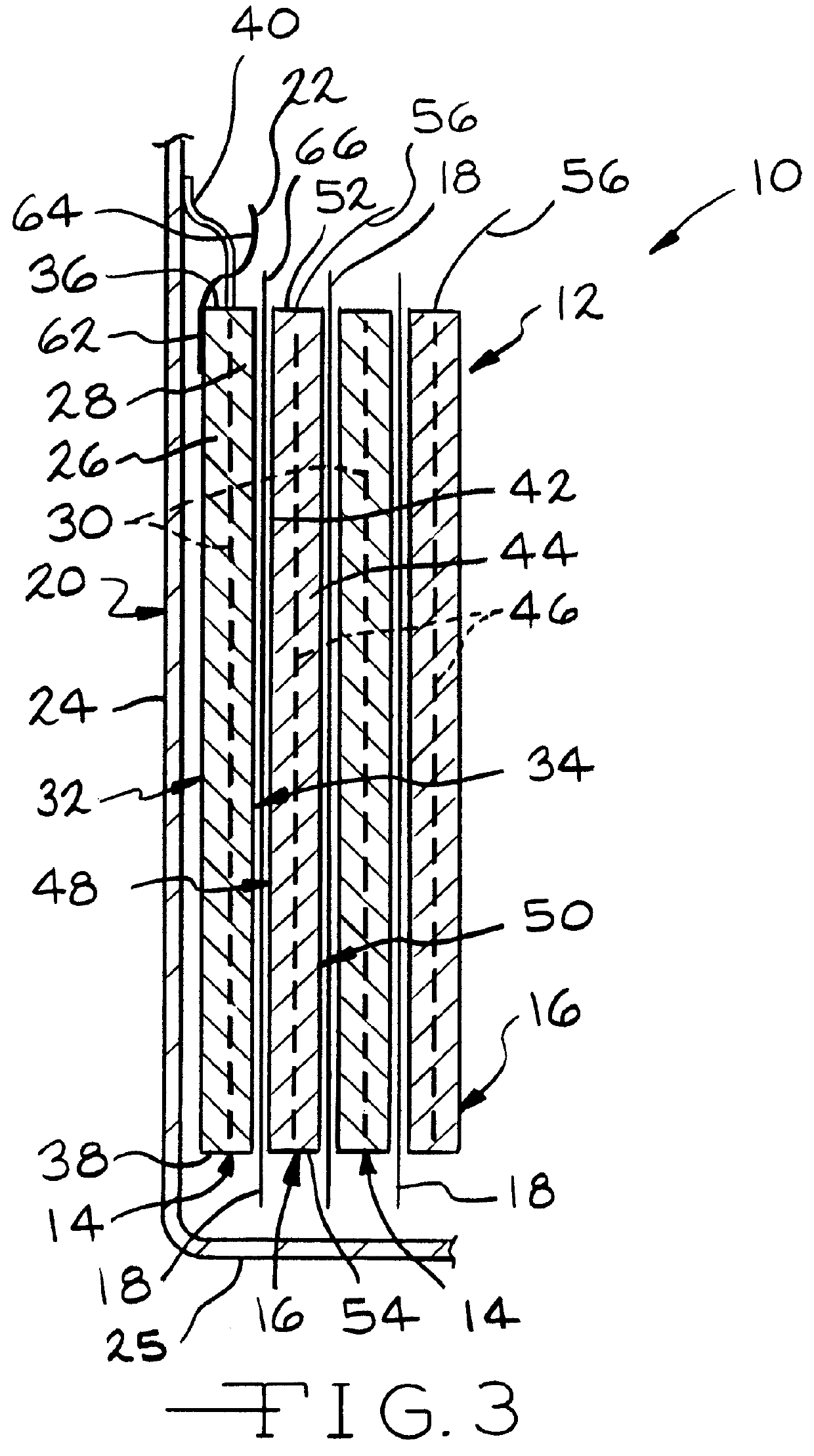
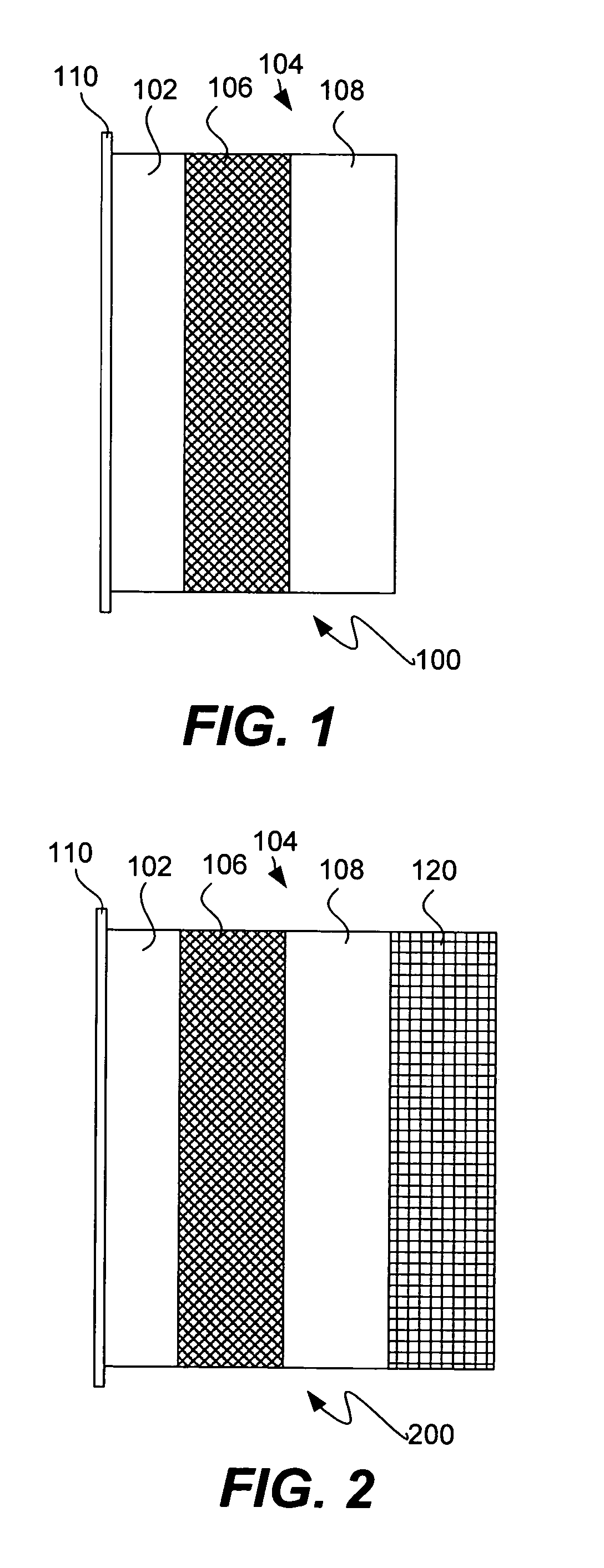
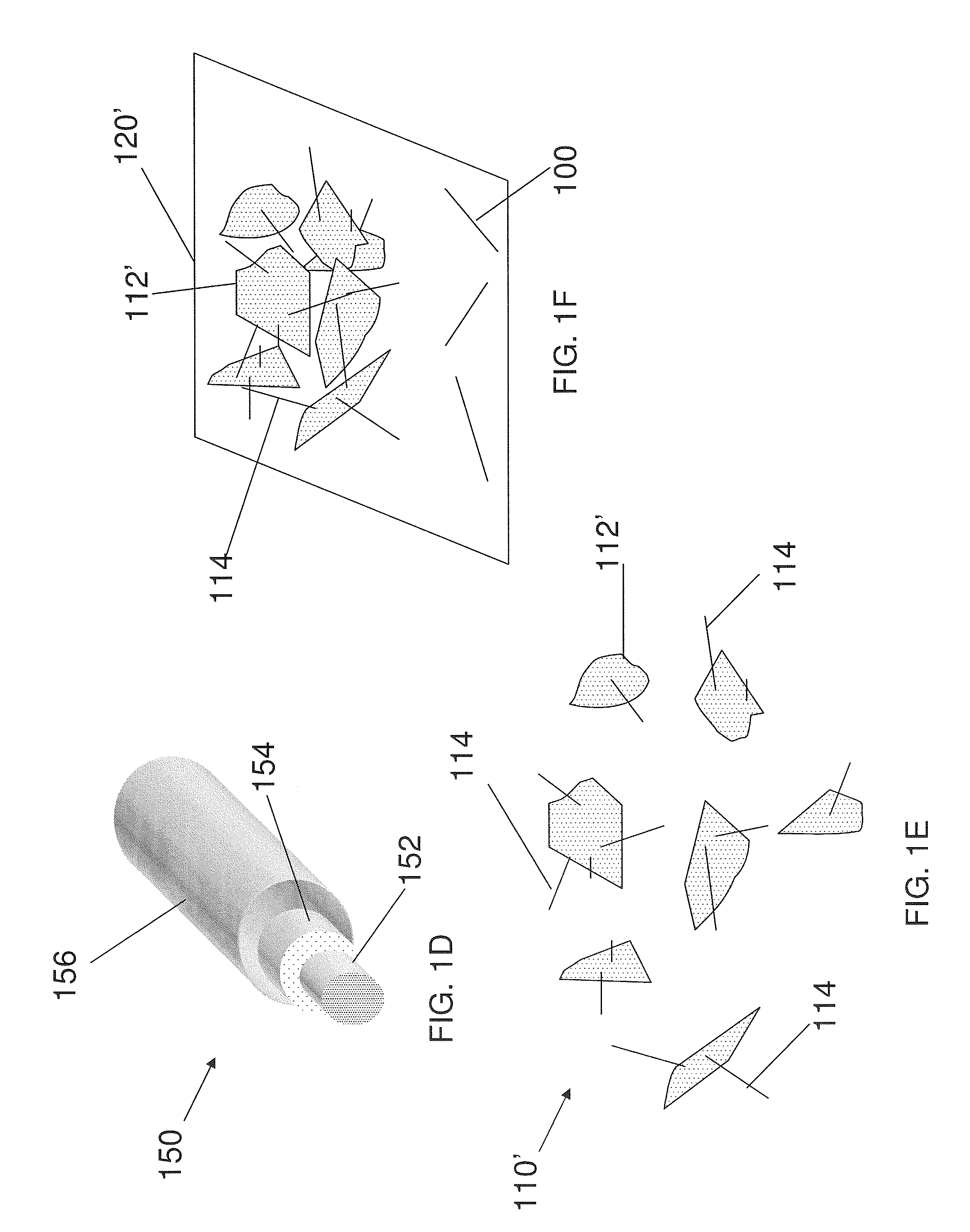
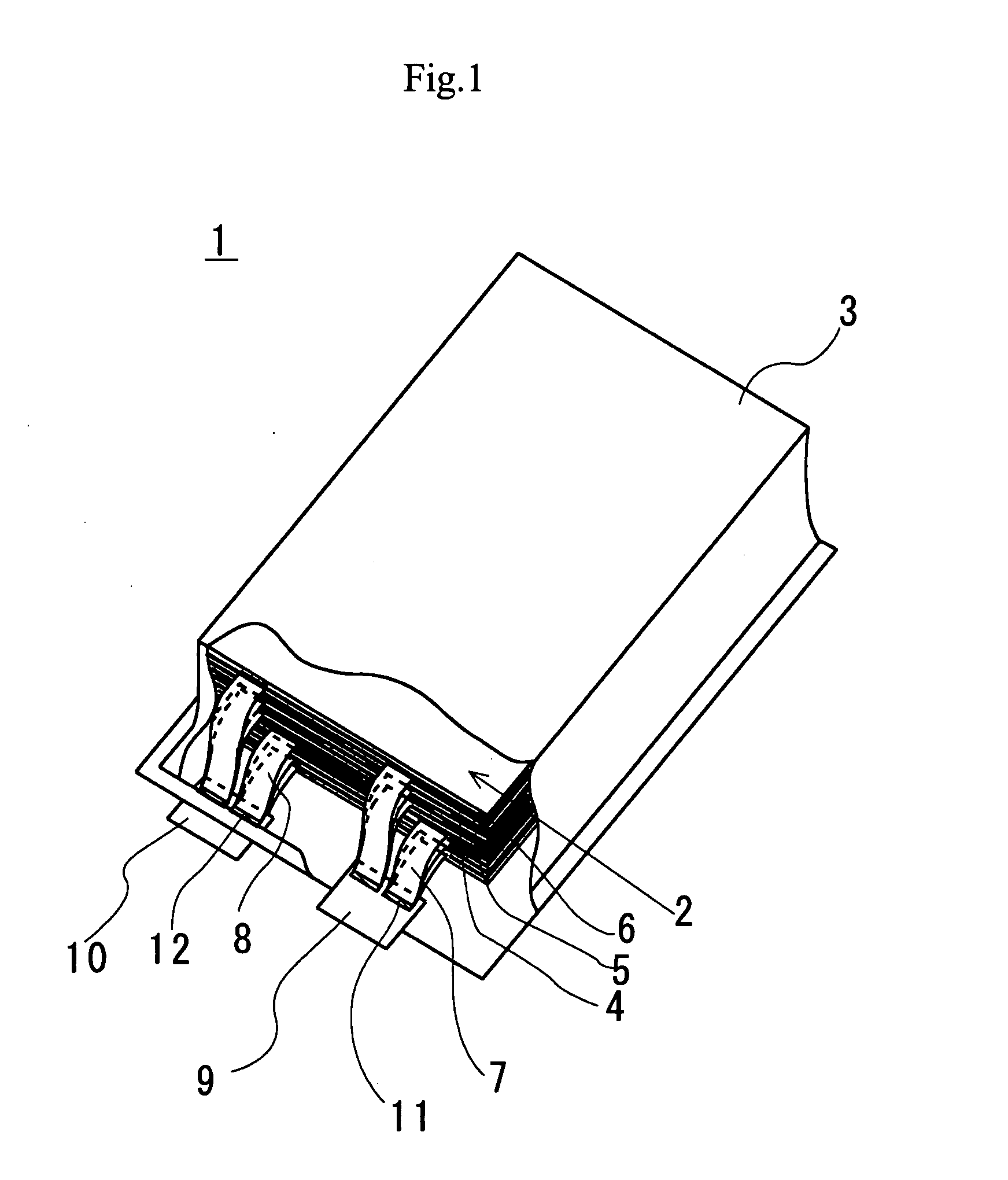
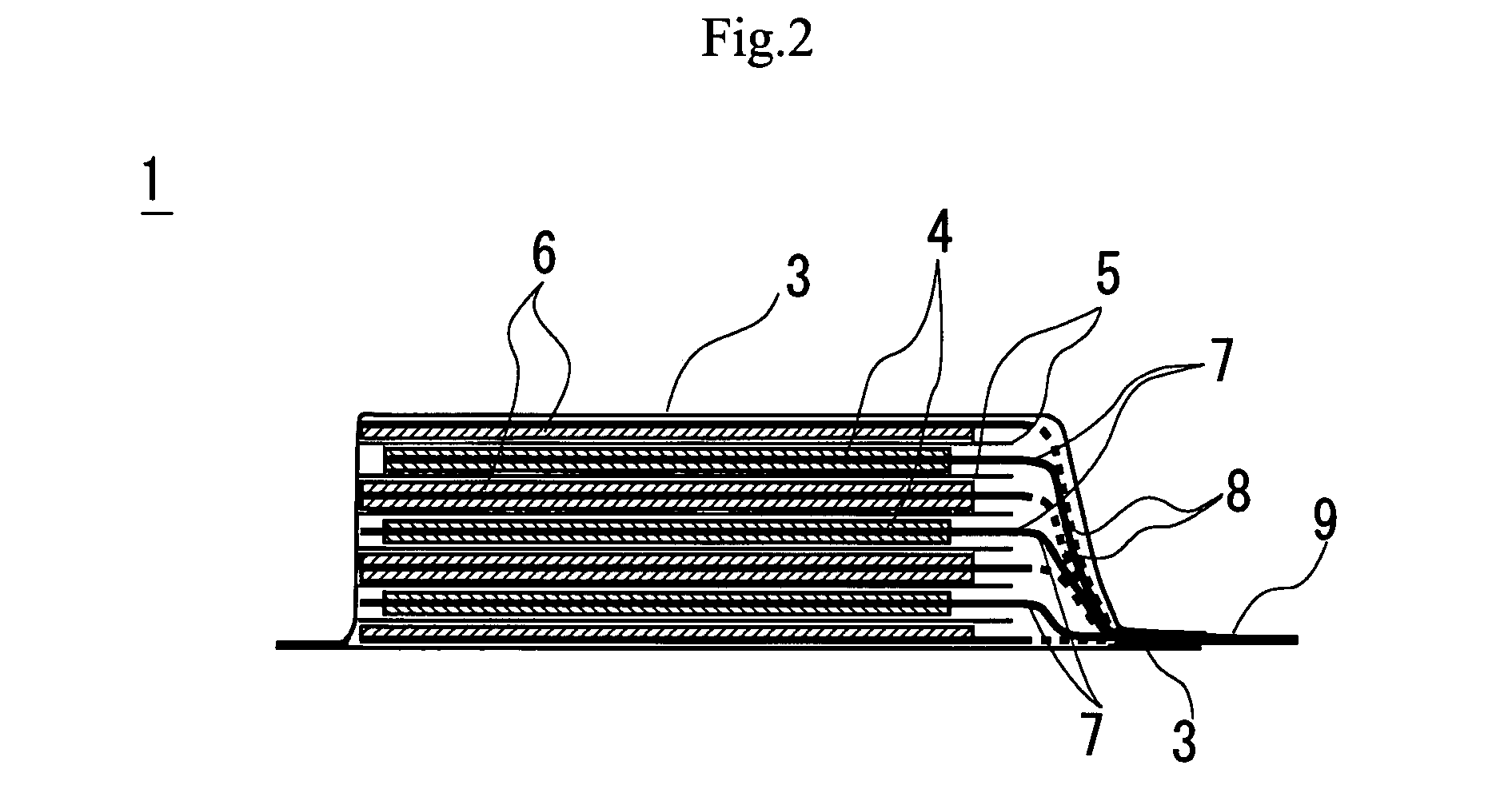
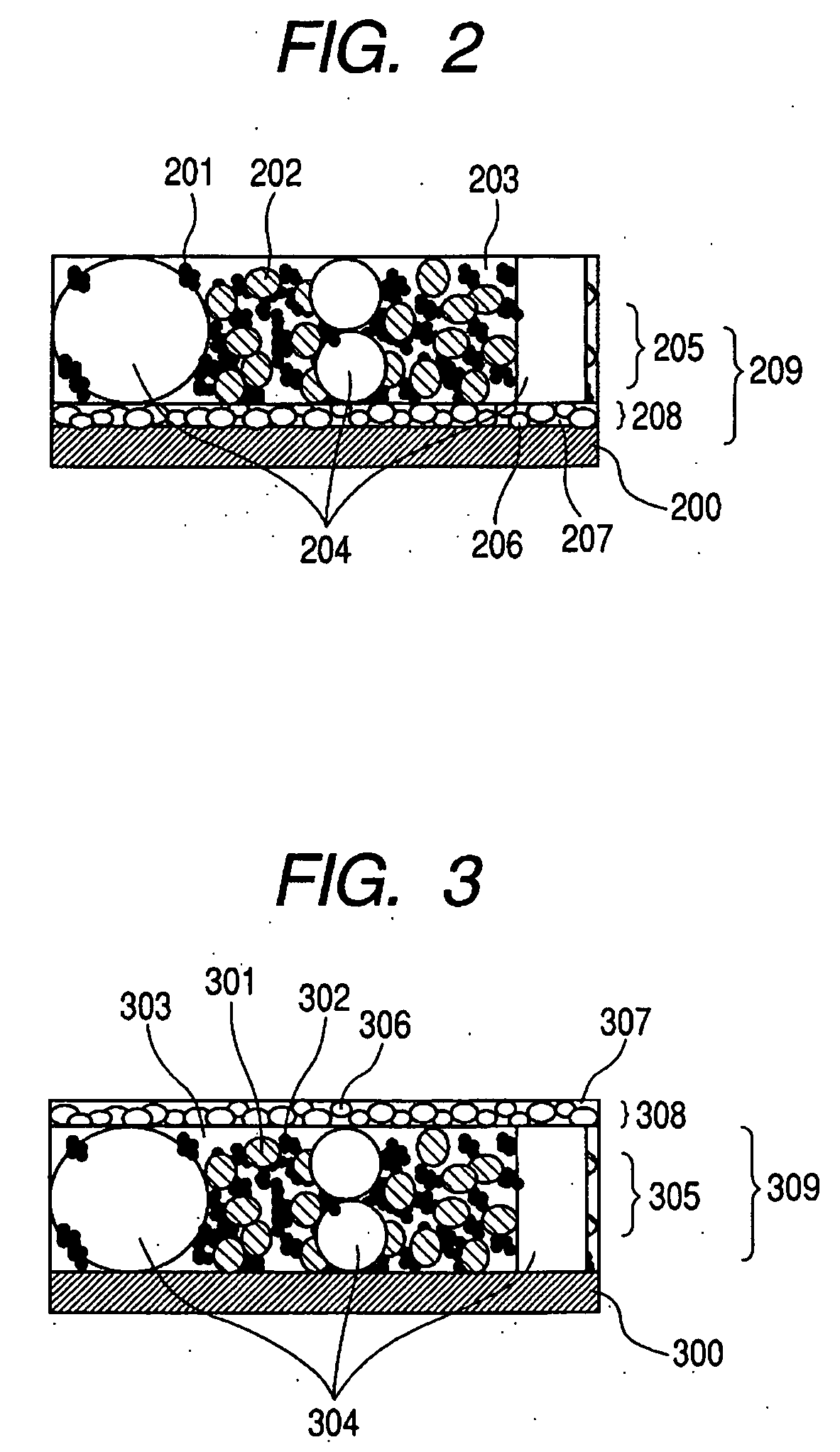
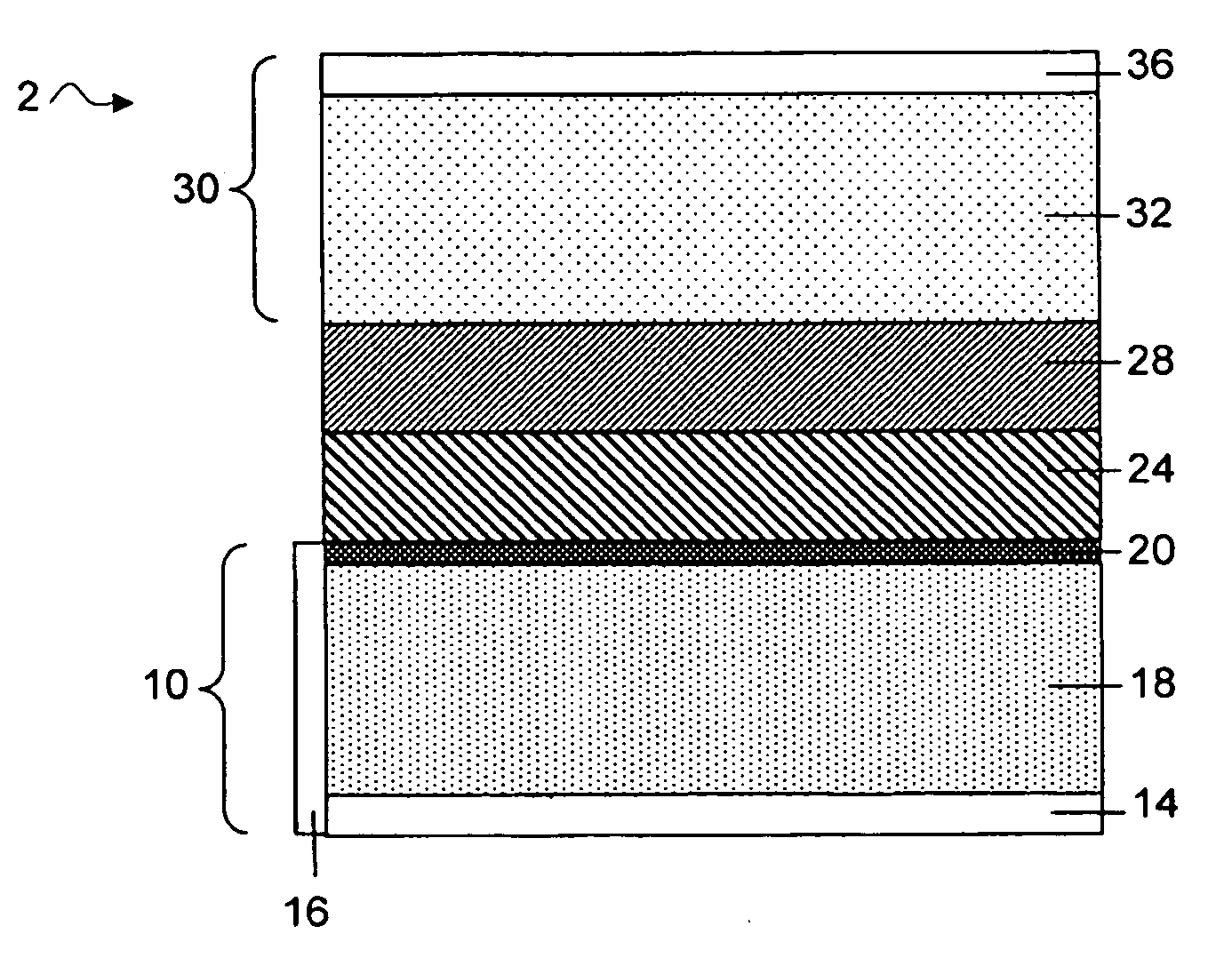
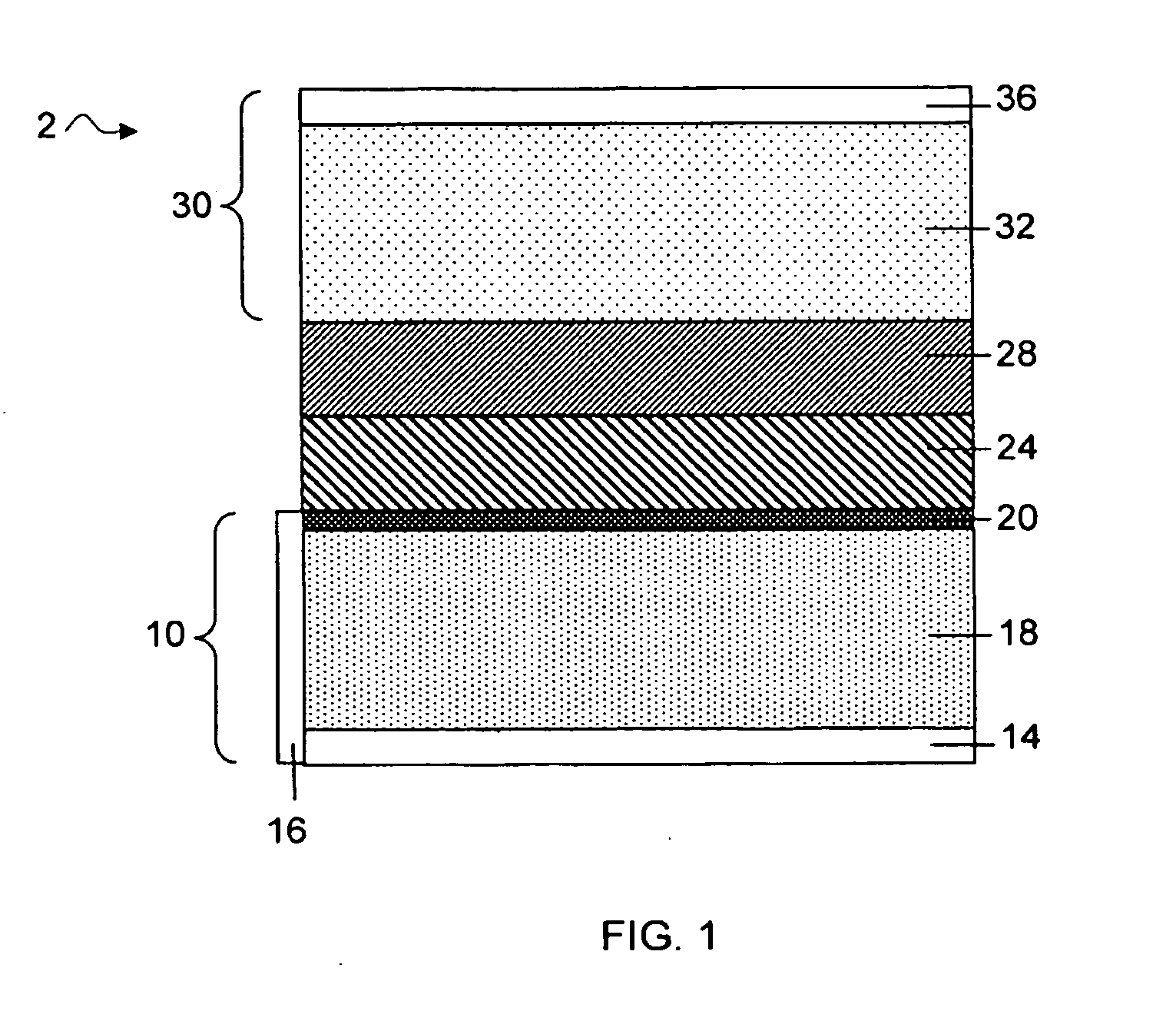
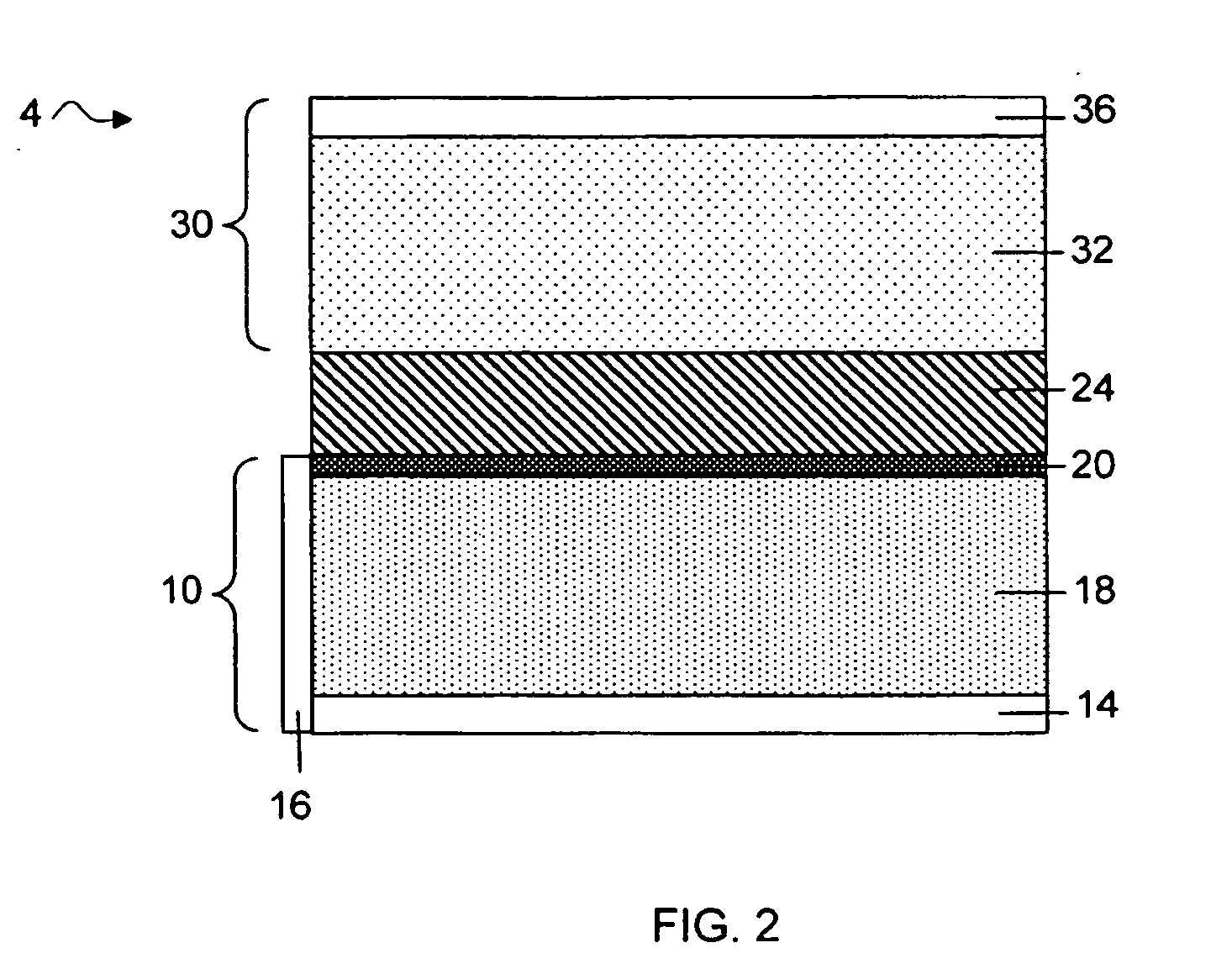
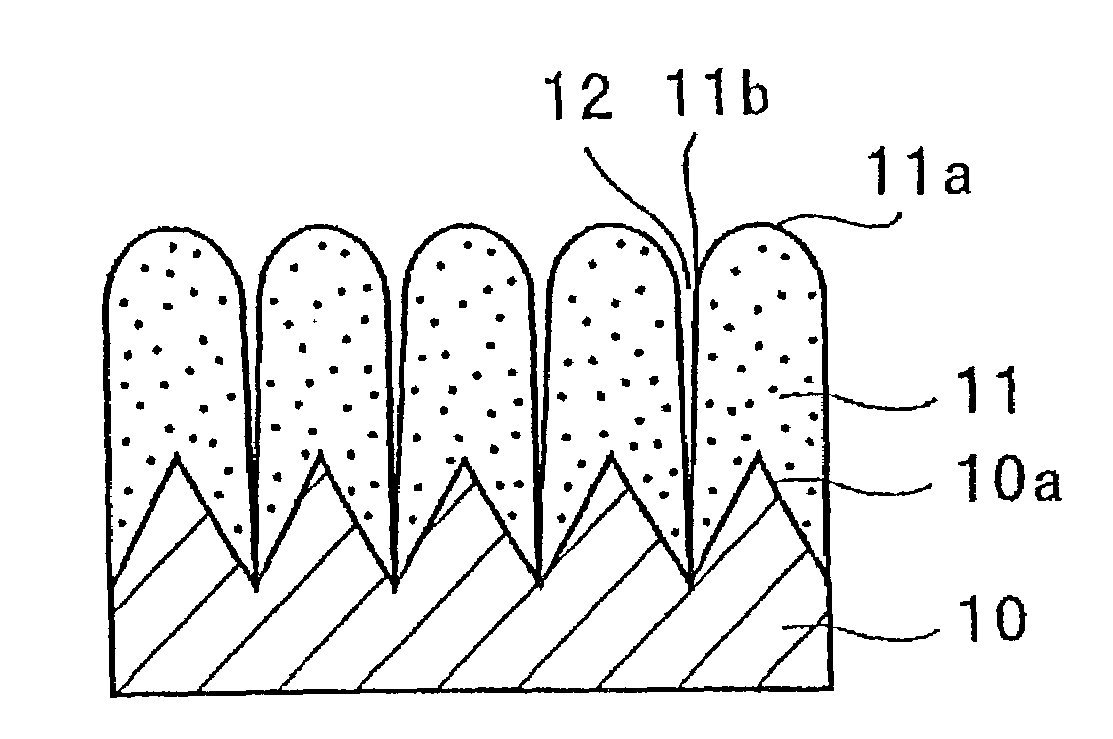
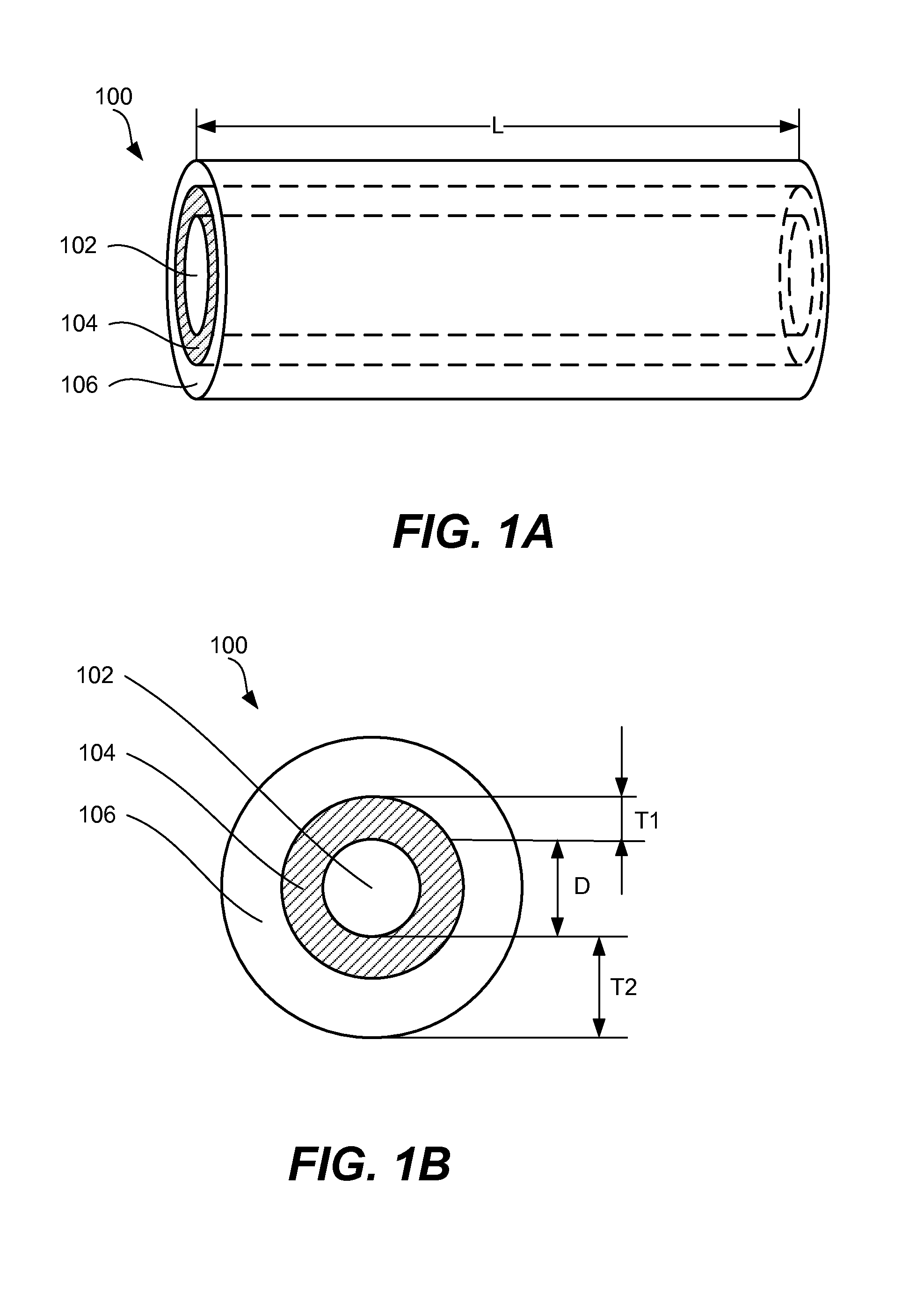
Slotted insulator for unsealed electrode edges in electrochemical cells
InactiveUS6013113AFinal product manufactureElectrode carriers/collectorsMushroomElectrochemical cell
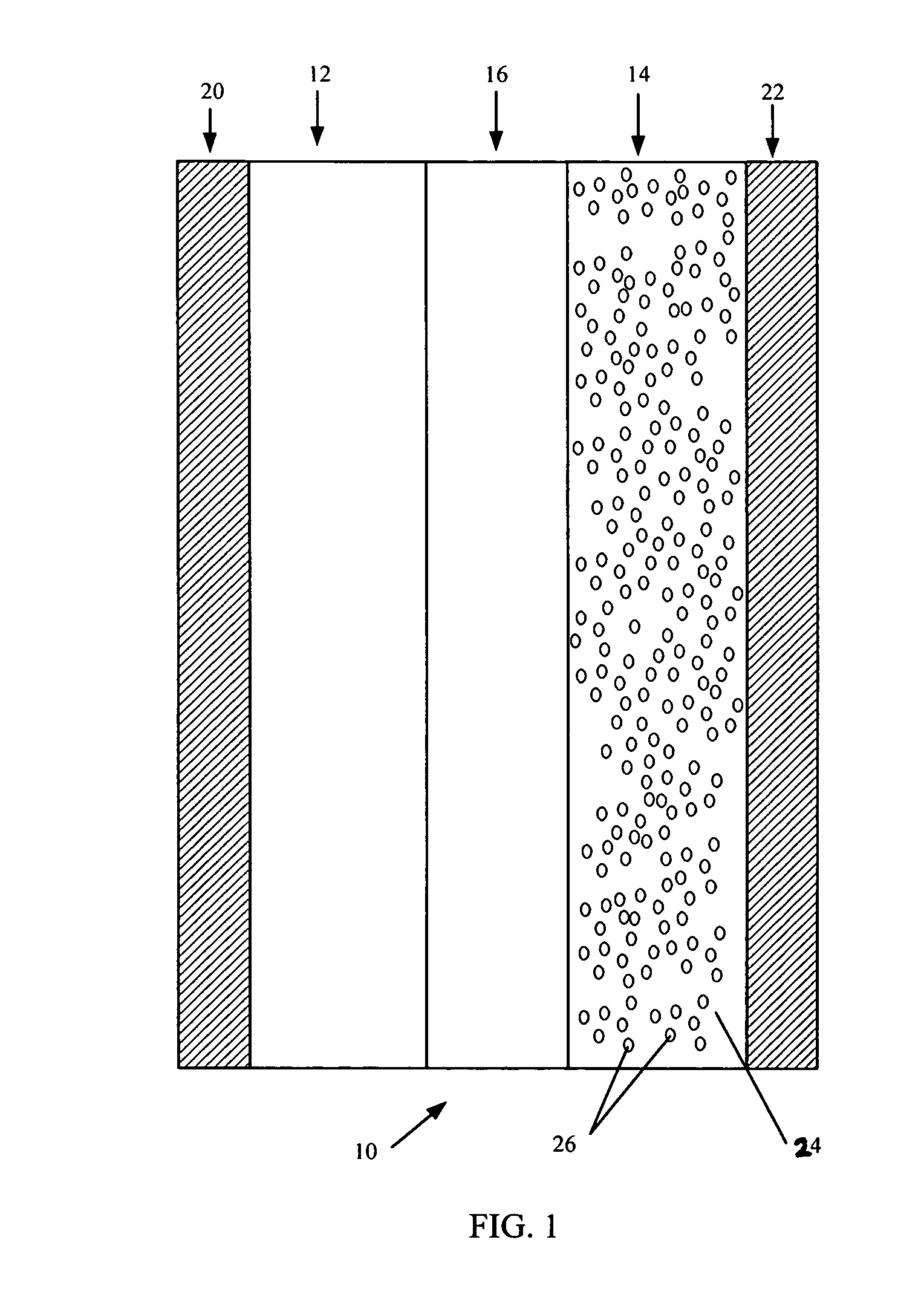
In fabrication of conventional spirally wound cells, a length of separator is provided at least twice as long as one of the electrodes, for example, the cathode, and then folded to cover both sides of the electrode. The separator is also somewhat wider than the covered electrode to extend beyond the upper and lower edges thereof. The cathode assembly is then placed along side a strip of anode material and rolled into a jellyroll configuration. The separator sheet is not sealed at the opposed upper and lower edges of the cathode, and during high shock and vibration conditions the edges tend to mushroom which can lead to short circuit conditions. The insulator of the present invention is a slotted member that covers the upper and lower edges of the other electrode not covered by the separator, for example the anode with the anode leads extending through the slots to shield them from short circuit conditions with the cell casing or other leads if the cell should be subjected to severe shock forces and the like.
Owner:WILSON GREATBATCH LTD
Lithium anodes for electrochemical cells
InactiveUS7247408B2Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
Rechargeable thin film battery and method for making the same
InactiveUS6982132B1Improve lithium ion mobilityHigh voltageElectrode thermal treatmentFinal product manufactureElectrical batteryHigh energy
A rechargeable, stackable, thin film, solid-state lithium electrochemical cell, thin film lithium battery and method for making the same is disclosed. The cell and battery provide for a variety configurations, voltage and current capacities. An innovative low temperature ion beam assisted deposition method for fabricating thin film, solid-state anodes, cathodes and electrolytes is disclosed wherein a source of energetic ions and evaporants combine to form thin film cell components having preferred crystallinity, structure and orientation. The disclosed batteries are particularly useful as power sources for portable electronic devices and electric vehicle applications where high energy density, high reversible charge capacity, high discharge current and long battery lifetimes are required.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Electroactive high storage capacity polyacetylene-co-polysulfur materials and electrolytic cells containing same
InactiveUS6117590AHigh storage capacity per unit weightFacilitates electron transportElectrode manufacturing processesNon-aqueous electrolyte accumulatorsElectrochemical cellElectrode material
The present invention relates to novel electroactive energy storing polyacetylene-co-polysulfur (PAS) materials of general formula (C2Sx)n wherein x is greater than 1 to about 100, and n is equal to or greater than 2. This invention also relates to novel rechargeable electrochemical cells containing positive electrode materials comprised of said polyacetylene-co-polysulfur materials with improved storage capacity and cycle life at ambient and sub-ambient temperatures.
Owner:THE BANK OF NEW YORK +1
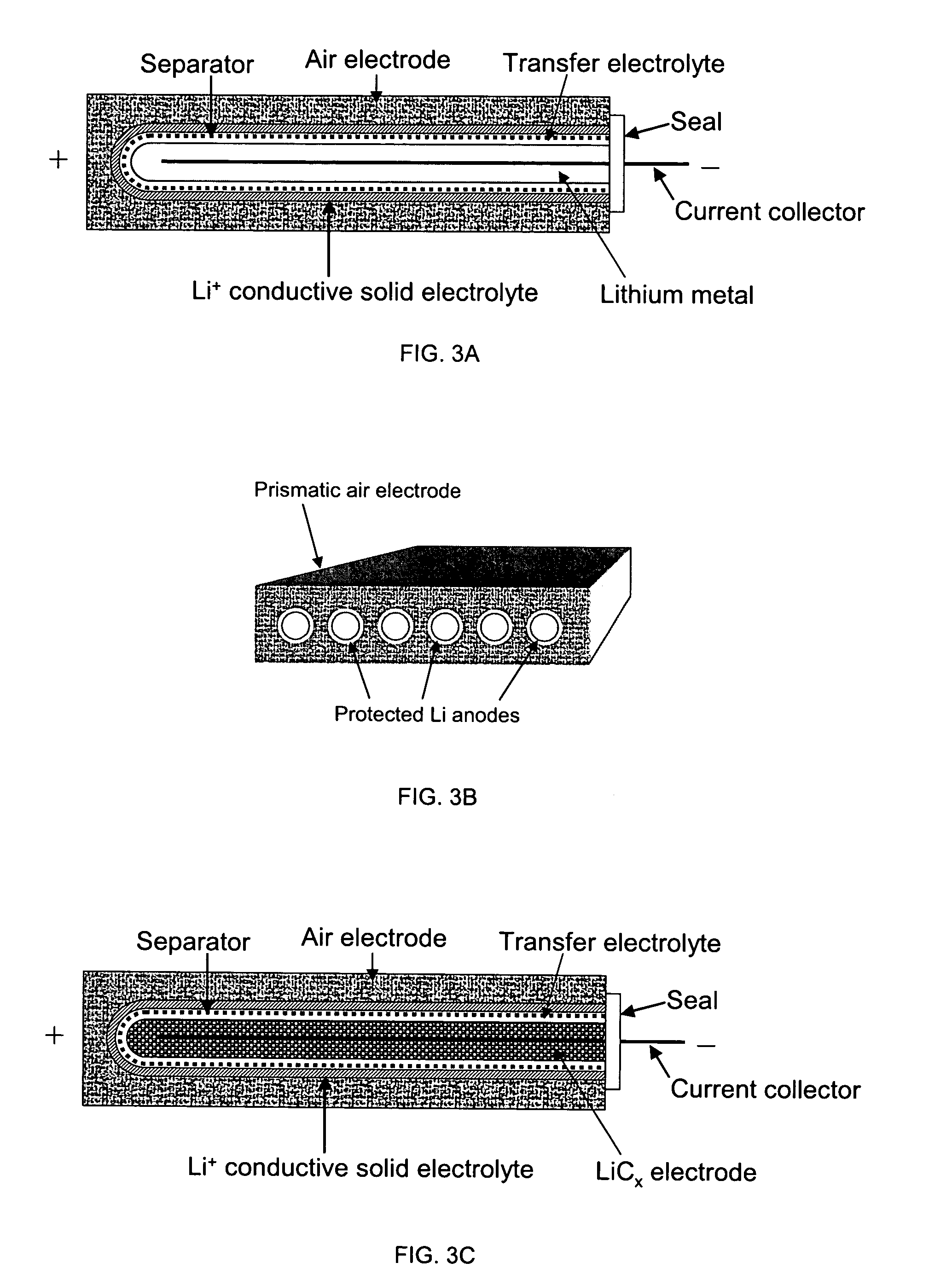
Protected active metal electrode and battery cell structures with non-aqueous interlayer architecture
ActiveUS7282295B2Avoid harmful reactionsFinal product manufactureElectrode carriers/collectorsMetal electrodesBattery cell
Active metal and active metal intercalation electrode structures and battery cells having ionically conductive protective architecture including an active metal (e.g., lithium) conductive impervious layer separated from the electrode (anode) by a porous separator impregnated with a non-aqueous electrolyte (anolyte). This protective architecture prevents the active metal from deleterious reaction with the environment on the other (cathode) side of the impervious layer, which may include aqueous or non-aqueous liquid electrolytes (catholytes) and / or a variety electrochemically active materials, including liquid, solid and gaseous oxidizers. Safety additives and designs that facilitate manufacture are also provided.
Owner:POLYPLUS BATTERY CO INC
Nanostructured Materials for Battery Applications
The present invention relates to nanostructured materials (including nanowires) for use in batteries. Exemplary materials include carbon-comprising, Si-based nanostructures, nanostructured materials disposed on carbon-based substrates, and nanostructures comprising nanoscale scaffolds. The present invention also provides methods of preparing battery electrodes, and batteries, using the nanostructured materials.
Owner:ONED MATERIAL INC
Electrode Including Nanostructures for Rechargeable Cells
InactiveUS20100285358A1Lower resistanceNanostructure manufactureMicroscopic fiber electrodesRechargeable cellSilicon nanowires
A lithium ion battery electrode includes silicon nanowires used for insertion of lithium ions and including a conductivity enhancement, the nanowires growth-rooted to the conductive substrate.
Owner:AMPRIUS INC
Conductive lithium storage electrode
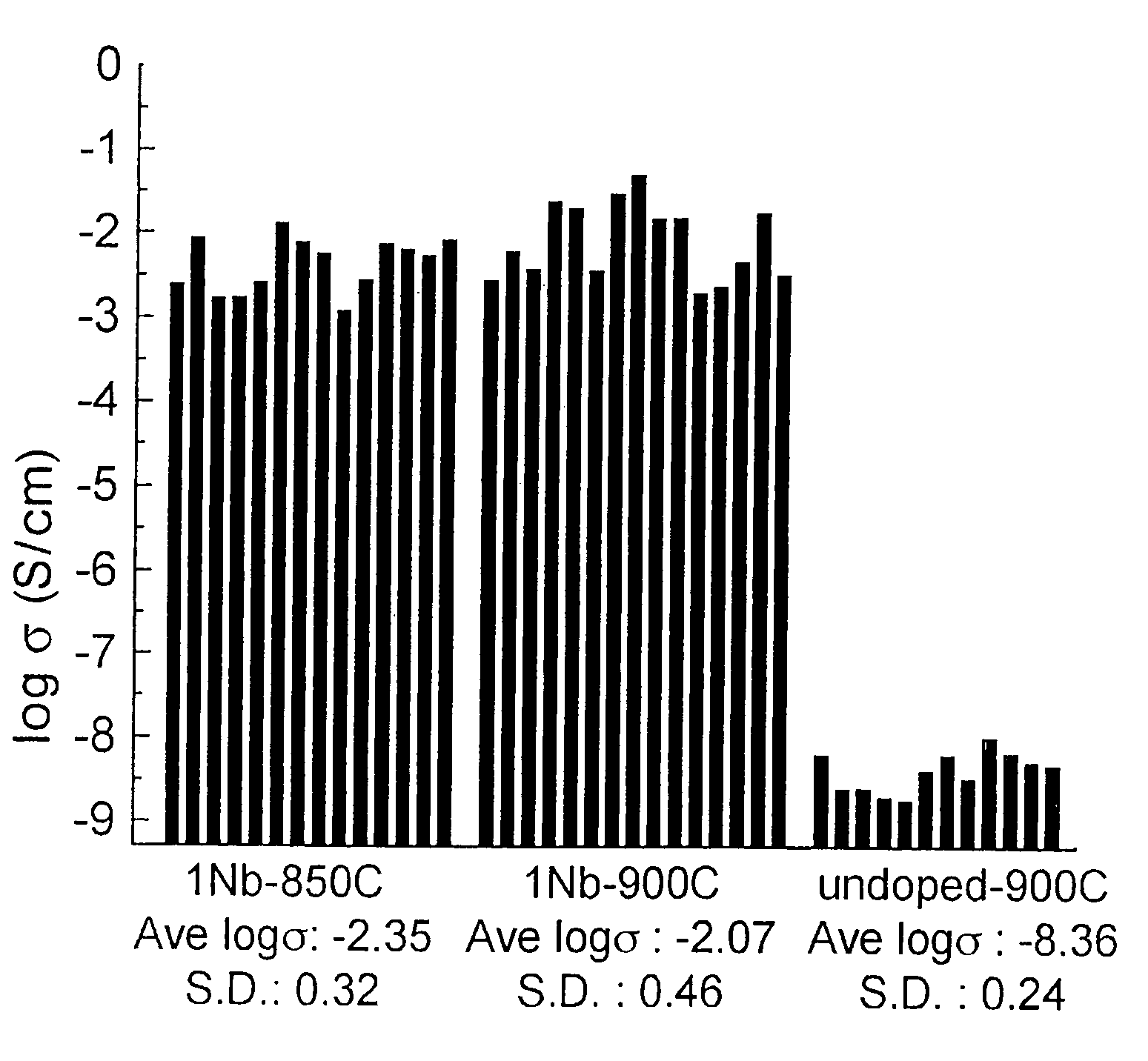
A compound comprising a composition Ax(M′1-aM″a)y(XD4)z, Ax(M′1-aM″a)y(DXD4)z, or Ax(M′1-aM″a)y(X2D7)z, and have values such that x, plus y(1-a) times a formal valence or valences of M′, plus ya times a formal valence or valence of M″, is equal to z times a formal valence of the XD4, X2D7, or DXD4 group; or a compound comprising a composition (A1-aM″a)xM′y(XD4)z, (A1-aM″a)xM′y(DXD4)z(A1-aM″a)xM′y(X2D7)z and have values such that (1-a)x plus the quantity ax times the formal valence or valences of M″ plus y times the formal valence or valences of M′ is equal to z times the formal valence of the XD4, X2D7 or DXD4 group. In the compound, A is at least one of an alkali metal and hydrogen, M′ is a first-row transition metal, X is at least one of phosphorus, sulfur, arsenic, molybdenum, and tungsten, M″ any of a Group IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA, IB, IIB, IIIB, IVB, VB, and VIB metal, D is at least one of oxygen, nitrogen, carbon, or a halogen, 0.0001<a≦0.1, and x, y, and z are greater than zero. The compound can have a conductivity at 27° C. of at least about 10−8 S / cm. The compound can be a doped lithium phosphate that can intercalate lithium or hydrogen. The compound can be used in an electrochemical device including electrodes and storage batteries and can have a gravimetric capacity of at least about 80 mAh / g while being charged / discharged at greater than about C rate of the compound.
Owner:MASSACHUSETTS INST OF TECH
Rechargeable lithium/water, lithium/air batteries
InactiveUS20070221265A1Improve protectionEasy to controlFinal product manufacturePV power plantsHigh energyOptoelectronics
Electrochemical cells, and more specifically, rechargeable batteries comprising lithium anodes for use in water and / or air environments, as well as non-aqueous and non-air environments, are presented. In one embodiment, an electrochemical cell includes an anode comprising lithium and a multi-layered structure positioned between the anode and an electrolyte of the cell. A multi-layered structure can include at least a first single-ion conductive material layer (e.g., a lithiated metal layer), and at least a first polymeric layer positioned between the anode and the single-ion conductive material. The invention also can provide an electrode stabilization layer positioned within the electrode, i.e., between one portion and another portion of an electrode, to control depletion and re-plating of electrode material upon charge and discharge of a battery. Advantageously, electrochemical cells comprising combinations of structures described herein are not only compatible with environments that are typically unsuitable for lithium, but the cells may be also capable of displaying long cycle life, high lithium cycling efficiency, and high energy density.
Owner:SION POWER CORP
Depositing thin layer of material on permeable substrate
InactiveUS20120213947A1Avoid excessive heightSpecial surfacesChemical vapor deposition coatingThin layerChemistry
Owner:VEECO ALD
Laminated lithium ion battery, battery pack comprising same and pole piece of laminated lithium ion battery
InactiveCN104882635AImprove cooling effectReduce welding processFinal product manufactureElectrode carriers/collectorsInternal resistanceElectrical battery
Disclosed are a laminated lithium ion battery, a battery pack comprising the same and a pole piece of the laminated lithium ion battery. The pole piece comprises a current collector and an active material layer, wherein the current collector is coated with the active material layer. A section of continuous uncoated area is arranged at the tail end of a first width end portion of the pole piece, the top face and the bottom face of the uncoated area are not coated with the active material layer, the current collector is exposed in the uncoated area, and the exposed current collector serves as a pole lug of the pole piece. By the pole piece, internal resistance of the lithium ion battery is reduced and heat dissipation performance of the battery is improved.
Owner:SHENZHEN GREPOW BATTERY CO LTD
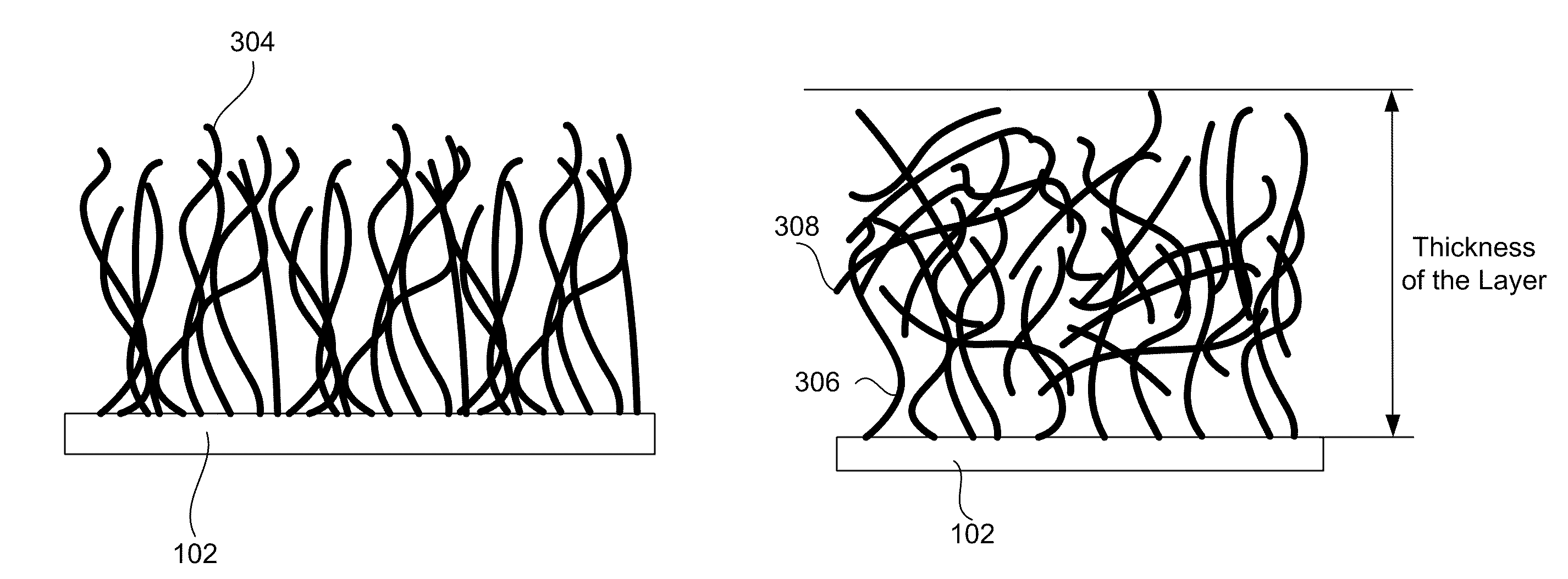
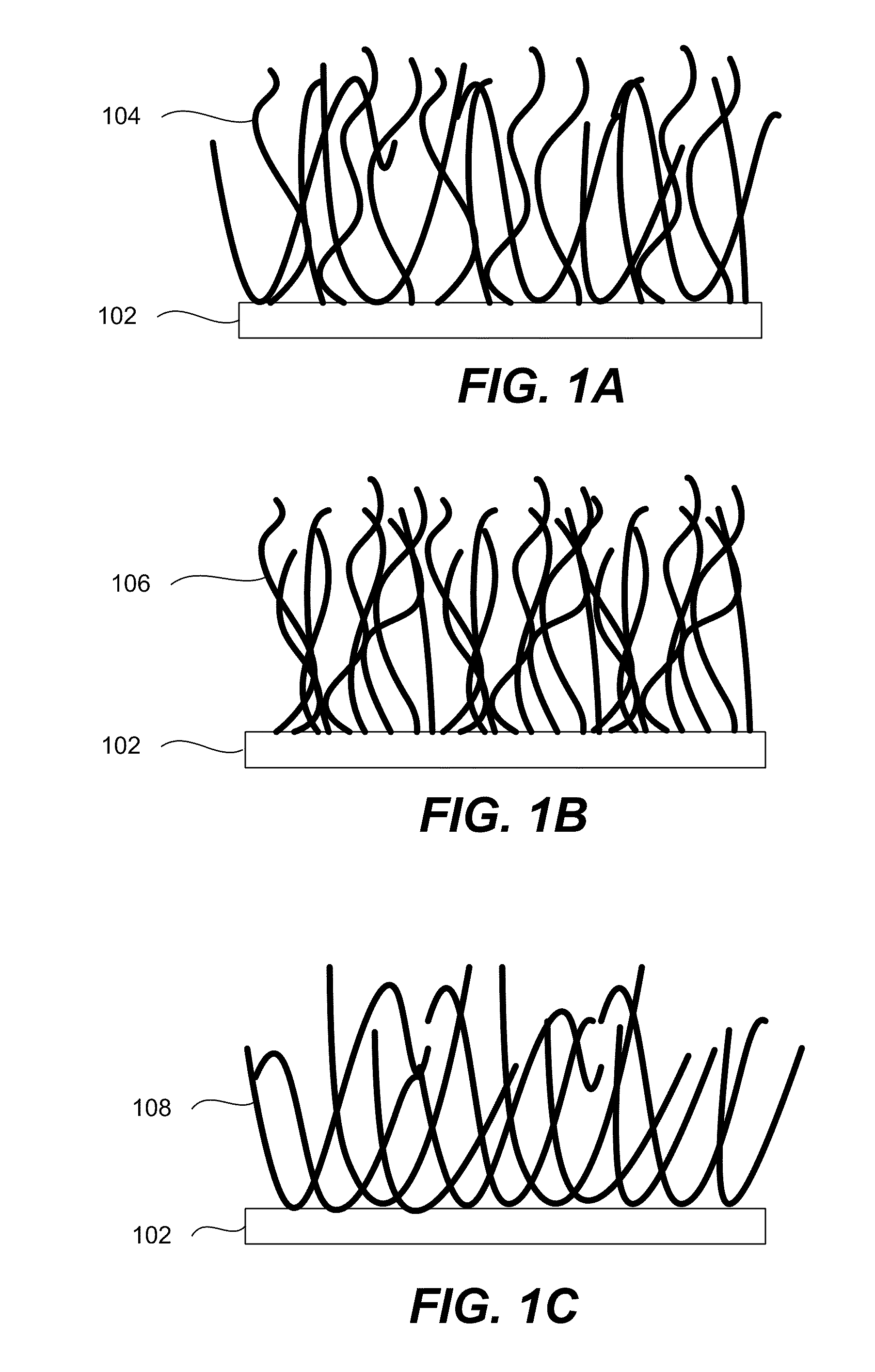
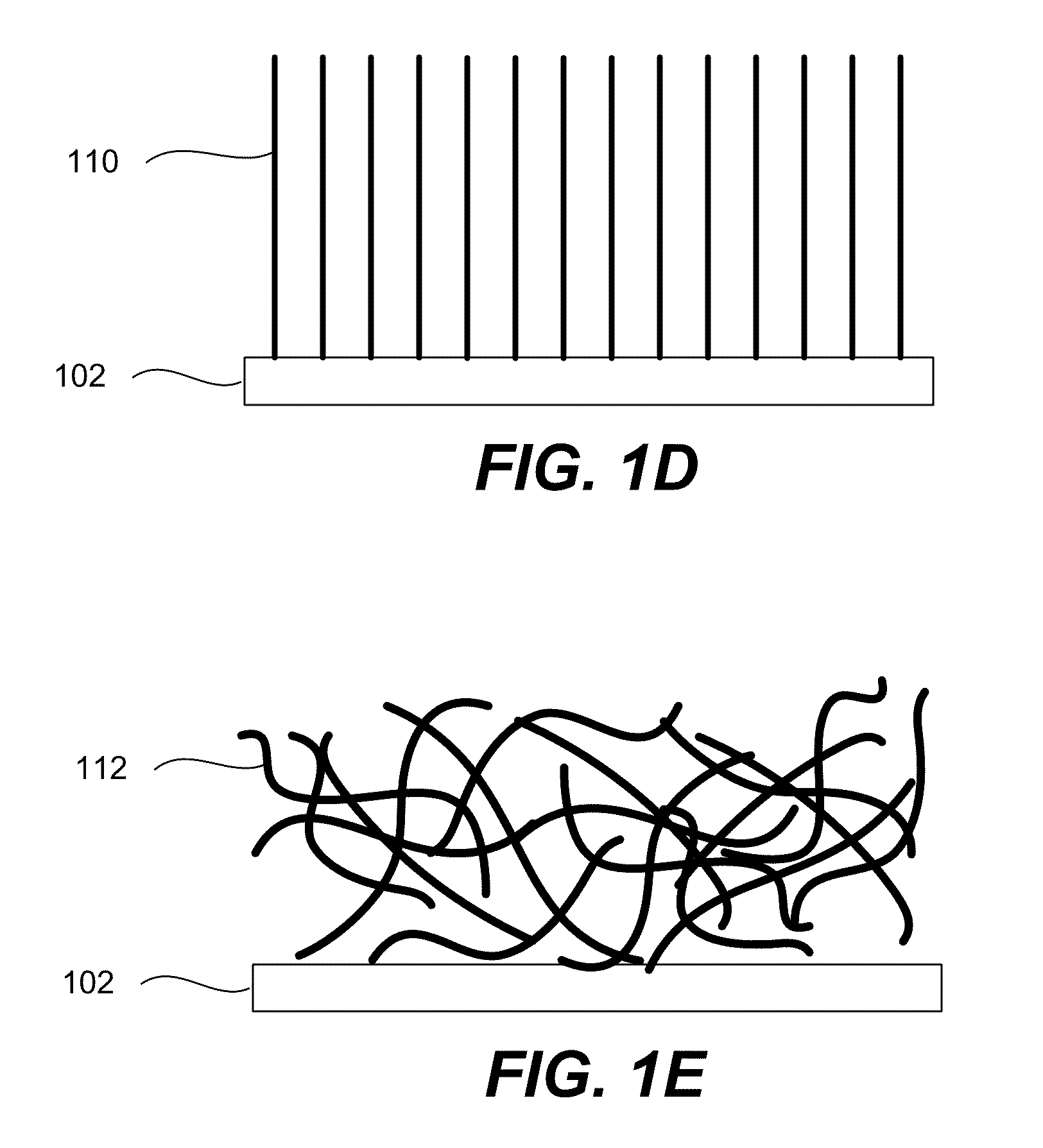
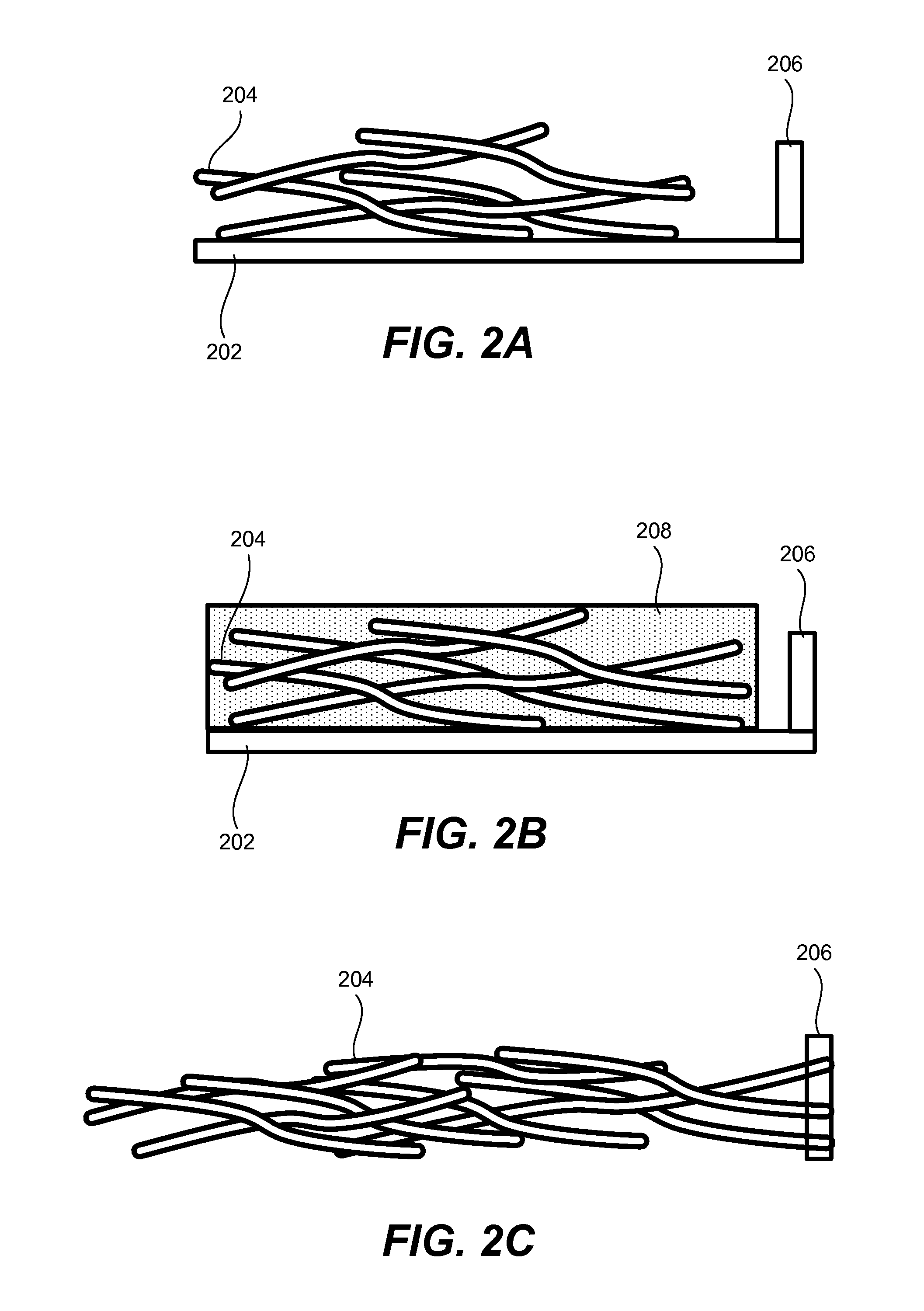
Porous silicon particulates for lithium batteries
An anode structure for lithium batteries includes nanofeatured silicon particulates dispersed in a conductive network. The particulates are preferably made from metallurgical grade silicon powder via HF / HNO3 acid treatment, yielding crystallite sizes from about 1 to 20 nm and pore sizes from about 1 to 100 nm. Surfaces of the particles may be terminated with selected chemical species to further modify the anode performance characteristics. The conductive network is preferably a carbonaceous material or composite, but it may alternatively contain conductive ceramics such as TiN or B4C. The anode structure may further contain a current collector of copper or nickel mesh or foil.
Owner:TIEGS TERRY N
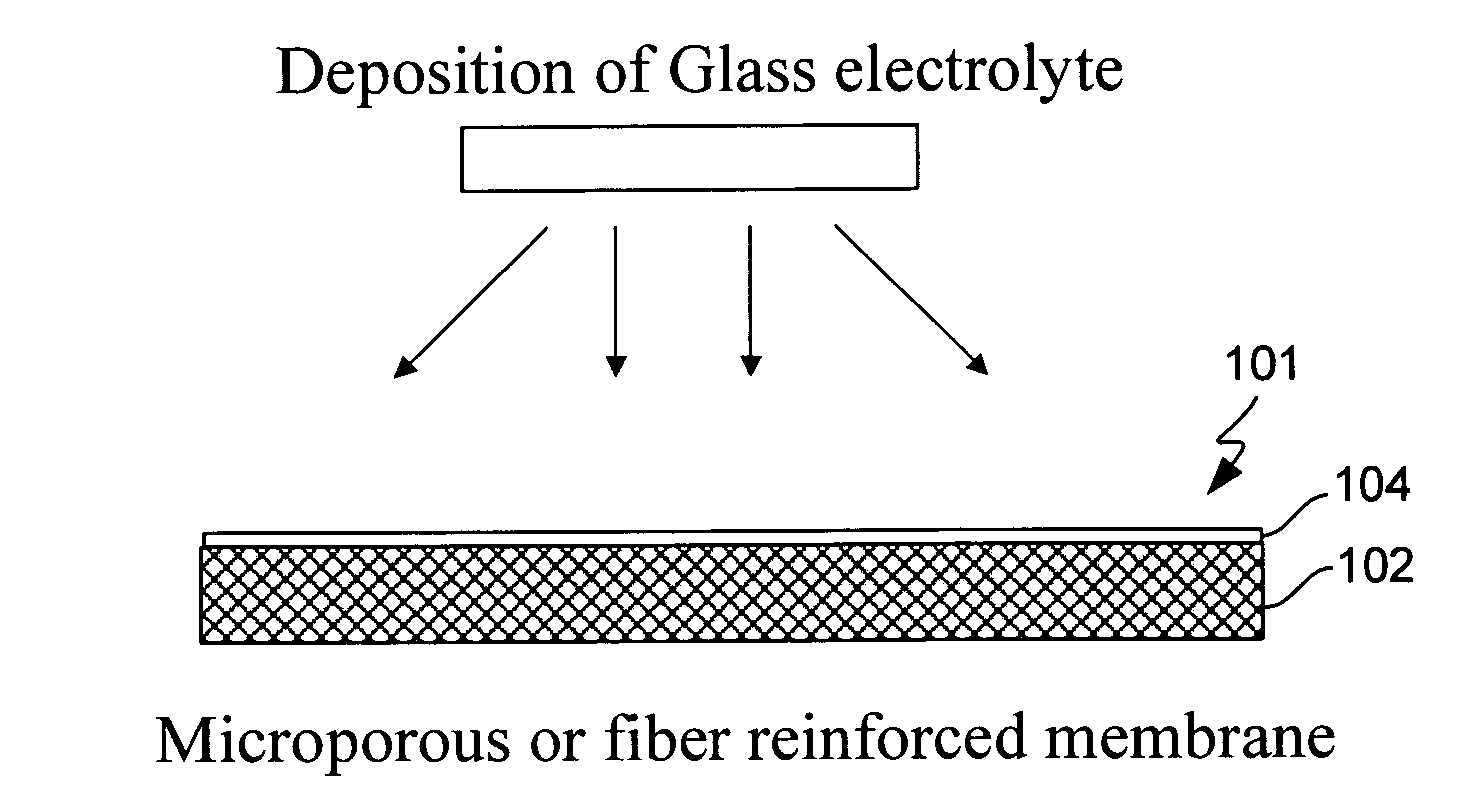
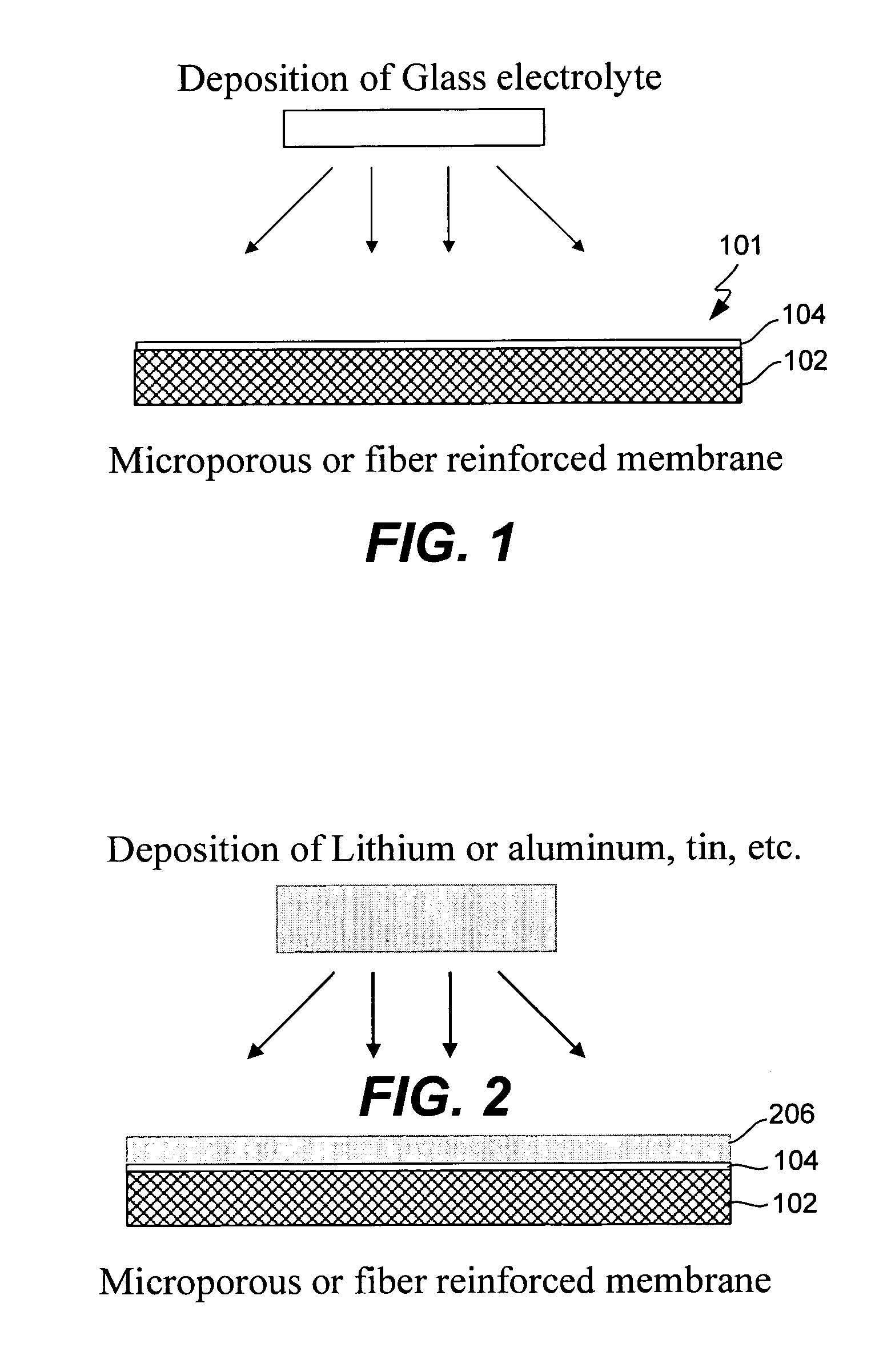
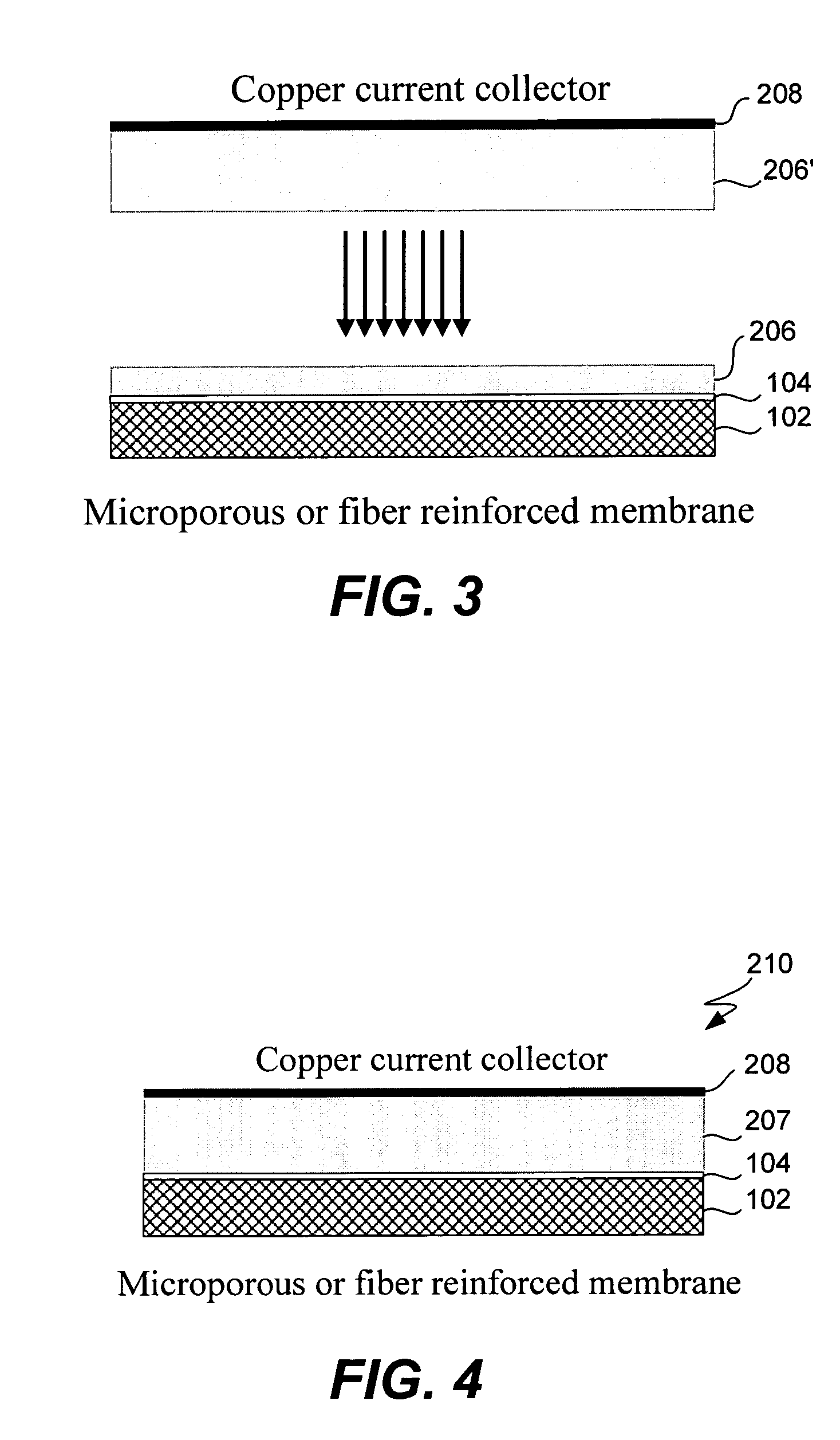
Electrochemical device separator structures with barrier layer on non-swelling membrane
InactiveUS7070632B1Avoid harmful reactionsElectrode carriers/collectorsSolid electrolyte cellsElectrochemistryFluid electrolytes
Disclosed are electrochemical device separator structures which include a substantially impervious active metal ion conducting barrier layer material, such as an ion conducting glass, is formed on an active metal ion conducting membrane in which elongation due to swelling on contact with liquid electrolyte is constrained in at least two of three orthogonal dimensions of the membrane. The non-swelling character of the membrane prevents elongation in the x-y (or lateral, relative to the layers of the composite) orthogonal dimensions of the membrane when it is contacted with liquid electrolyte that would otherwise cause the barrier layer to rupture. Substantial swelling of the membrane, if any, is limited to the z (or vertical, relative to the layers of the composite) dimension.
Owner:POLYPLUS BATTERY CO INC
Method for production of stacked battery
ActiveUS20080060189A1Improve reliabilityShorten the lengthNon-aqueous electrolyte accumulatorsLarge-sized flat cells/batteriesEngineeringElectrical and Electronics engineering
A method for production of a stacked battery having a plurality of positive electrode current collection tabs and a plurality of negative electrode current collection tabs drawn out from a stacked member formed by laying positive electrodes and negative electrodes alternately one on the other with separators interposed between them and bonded respectively to a positive lead terminal and a negative lead terminal comprises a step of determining in advance a simultaneously bondable number, or the number of positive electrode current collection tabs and the number of negative electrode current collection tabs that can be laid one on the other and collectively bonded, and bonding conditions for bonding them and a step of forming groups of current collection tabs, each group being formed by laying a number of current collection tabs not exceeding the simultaneously bondable number one on the other, displacing the bonding positions of the groups of positive electrode current collection tabs or those of negative electrode current collection tabs relative to each other in the direction of drawing out the positive electrode current collection tabs or the negative electrode current collection tabs, whichever appropriate, or in a direction perpendicular to the direction on the surface of the positive lead terminal or the negative lead terminal, whichever appropriate, and collectively bonding the positive electrode current collection tabs or the negative electrode current collection tabs of each group under the bonding conditions.
Owner:ENVISION AESC ENERGY DEVICES LTD
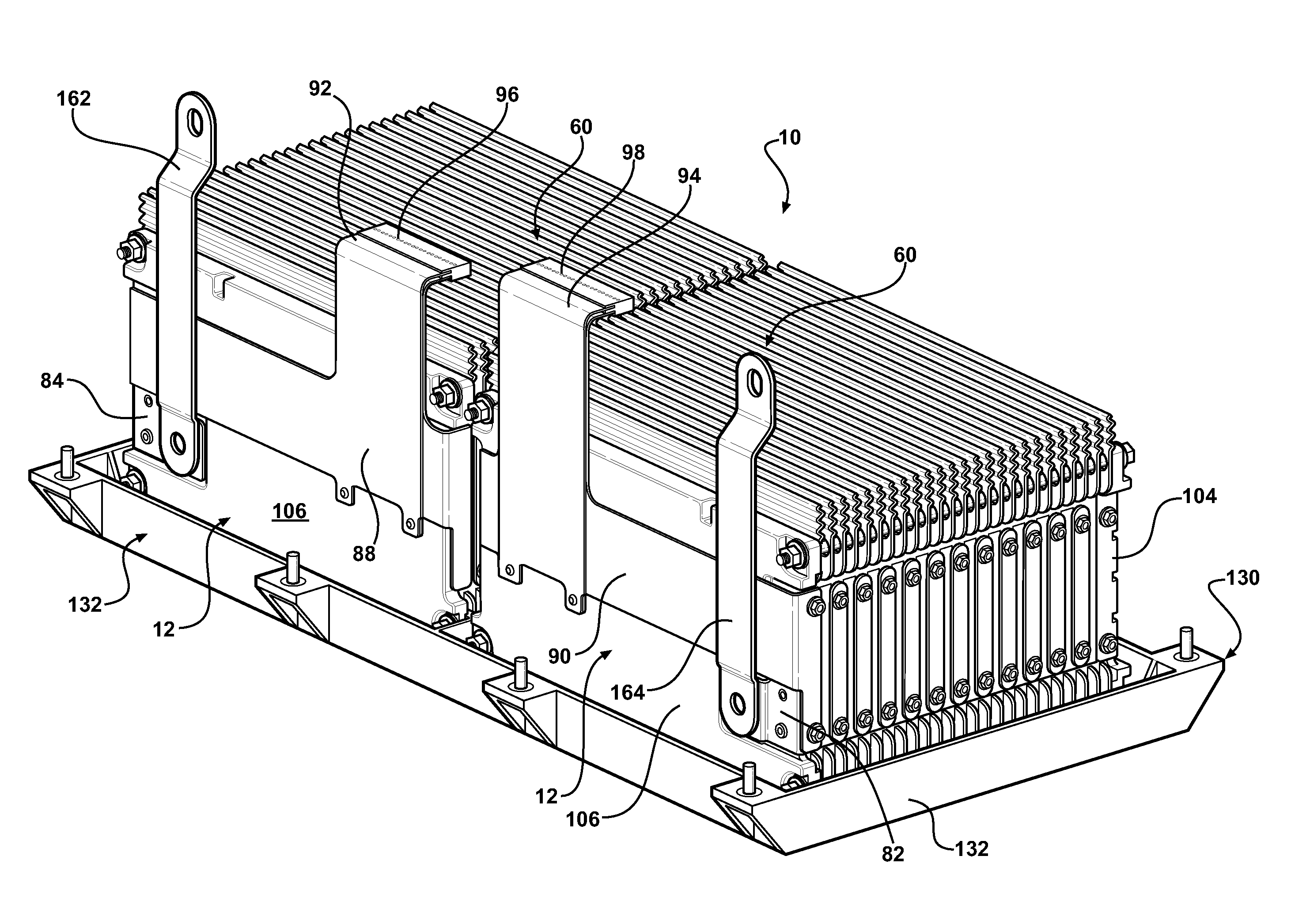
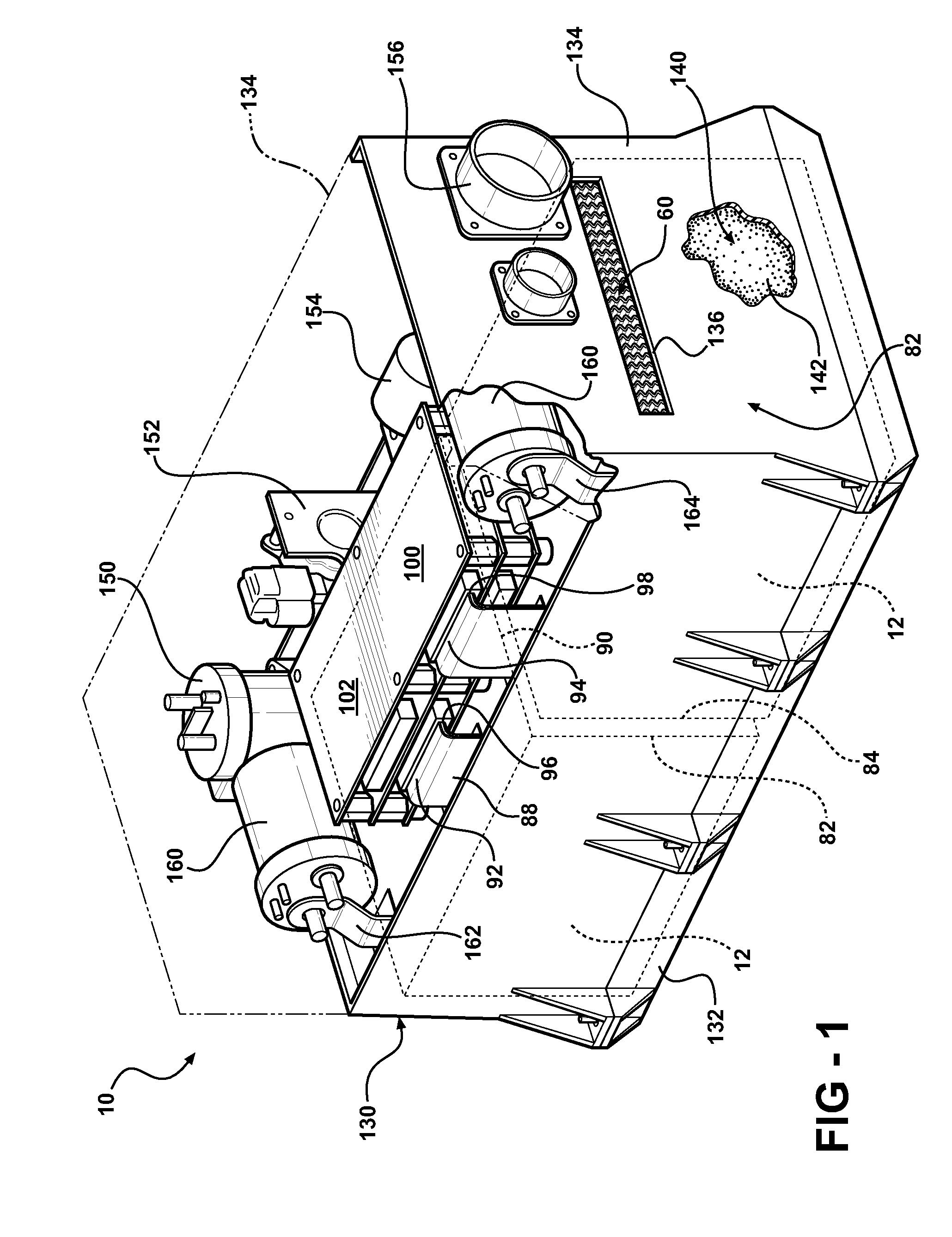
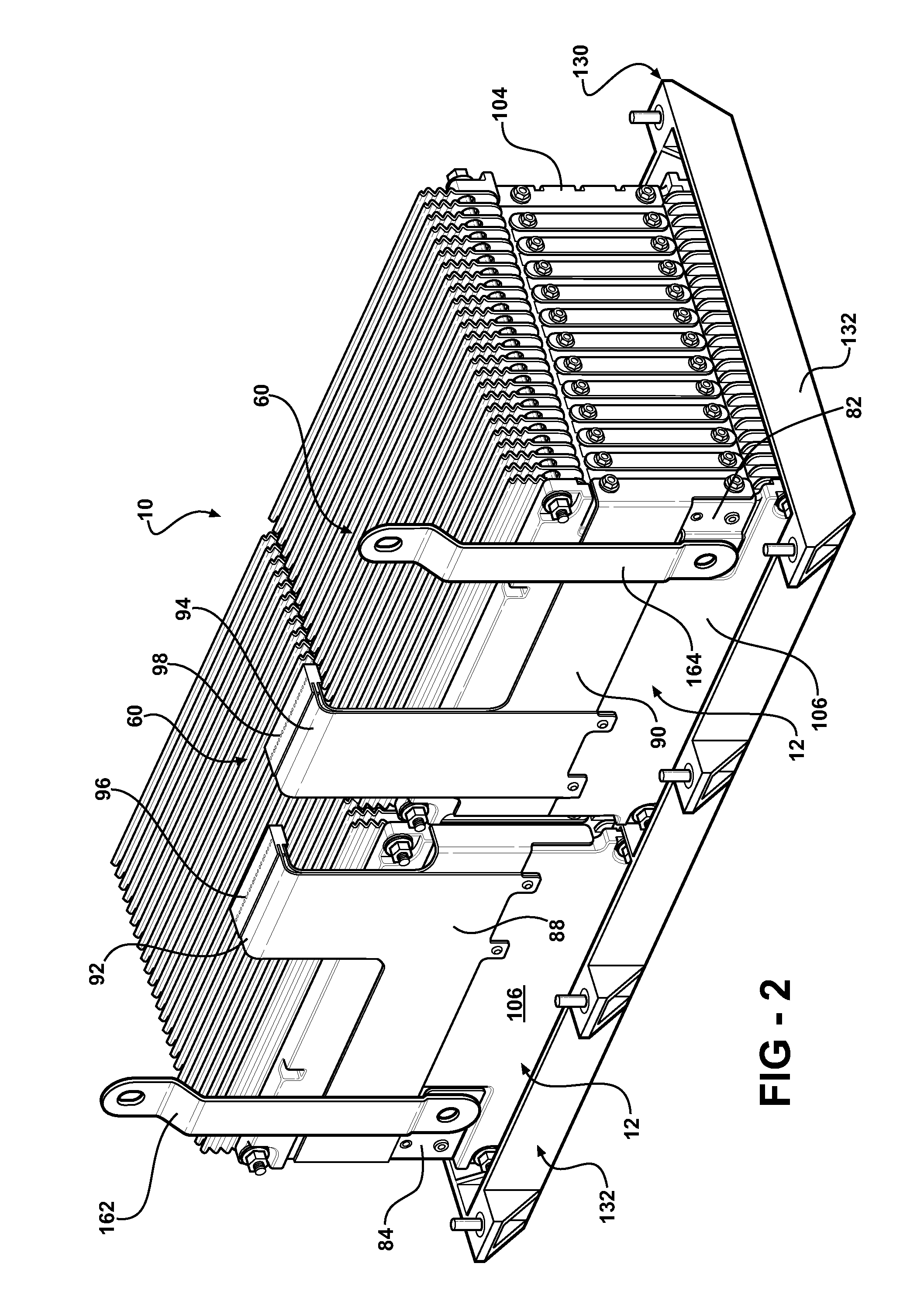
Battery pack with integral cooling and bussing devices
ActiveUS20080090137A1High energy density characteristicImproving packaging characteristicFinal product manufacturePrimary cellsMobile vehicleEngineering
A battery module of the present invention is adaptable to be utilized in various configurations including and not limited to an overlapping battery cell packaging configuration and a vertical stack battery cell packaging configuration used in an automotive vehicle. The battery module has a plurality of battery heatsink assemblies with the cells disposed therebetween. A plurality of rods extend through the each heatsink assemblies to secure the heatsink assemblies and the cell with one another to form the battery module.
Owner:ENERDEL
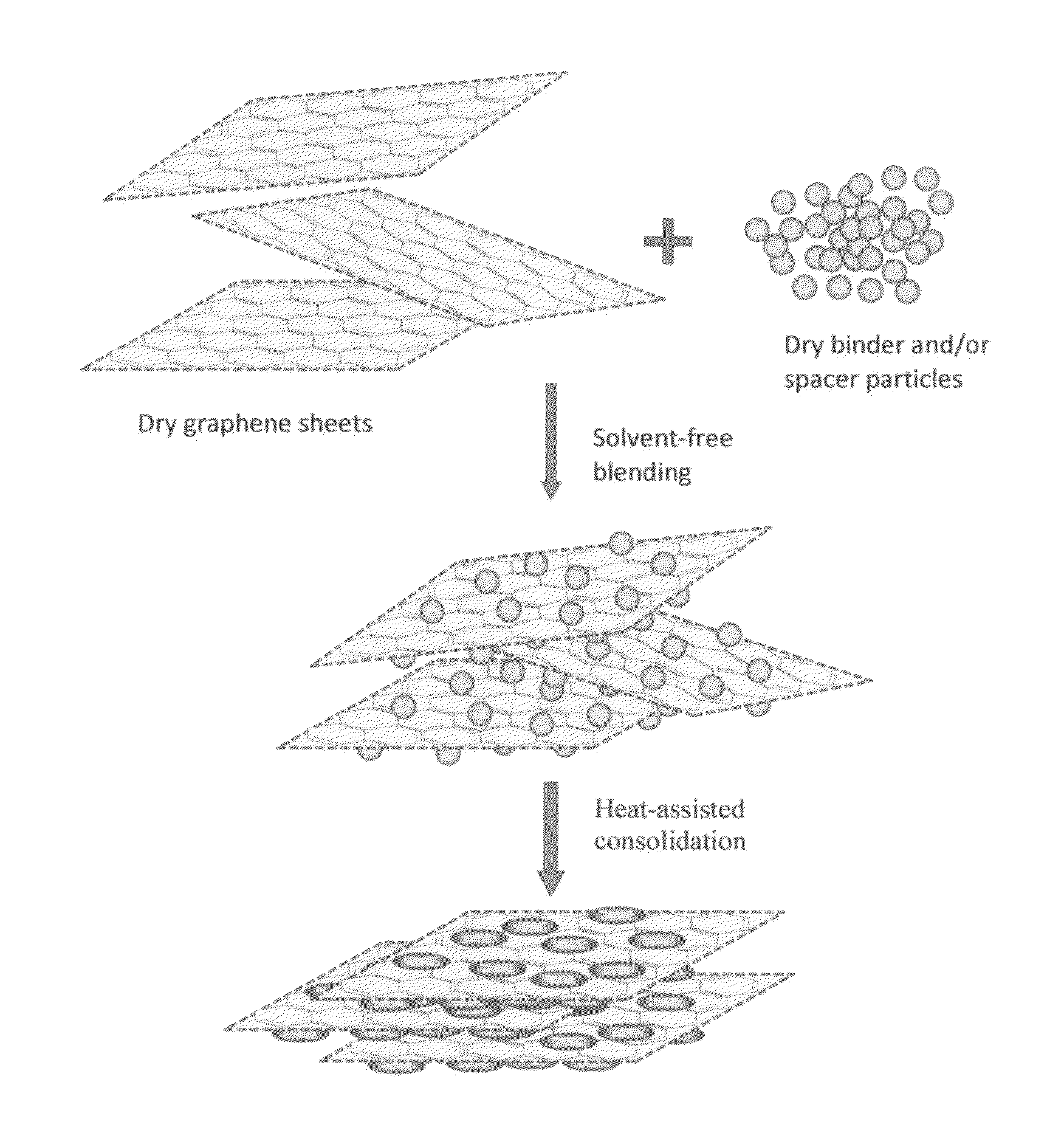
Solvent-free process based graphene electrode for energy storage devices
PendingUS20140030590A1Inexpensive and durable and highly reliableHigh capacitanceMaterial nanotechnologyHybrid capacitor electrodesGraphene flakeSolvent free
Disclosed is an electrode for an electrochemical energy storage device, the electrode comprising a self-supporting layer of a mixture of graphene sheets and spacer particles and / or binder particles, wherein the electrode is prepared without using water, solvent, or liquid chemical. The graphene electrode prepared by the solvent-free process exhibits many desirable features and advantages as compared to the corresponding electrode prepared by a known wet process. These advantages include a higher electrode specific surface area, higher energy storage capacity, improved or higher packing density or tap density, lower amount of binder required, lower internal electrode resistance, more consistent and uniform dispersion of graphene sheets and binder, reduction or elimination of undesirable effect of electrolyte oxidation or decomposition due to the presence of water, solvent, or chemical, etc.
Owner:GLOBAL GRAPHENE GRP INC
Si/c composite, anode active materials, and lithium battery including the same
ActiveUS20090029256A1Improve initial Coulombic efficiencyGood capacity retentionLiquid surface applicatorsElectrode manufacturing processesLithium-ion batteryPorous silicon
An Si / C composite includes carbon (C) dispersed in porous silicon (Si) particles. The Si / C composite may be used to form an anode active material to provide a lithium battery having a high capacity and excellent capacity retention.
Owner:SAMSUNG SDI CO LTD
Carbon-coated silicon particle power as the anode material for lithium batteries and the method of making the same
InactiveUS20050136330A1Large capacityImprove efficiencyElectrode thermal treatmentElectrode carriers/collectorsCarbon compositesSilicon particle
A process for the production of coated silicon / carbon particles comprising: providing a carbon residue forming material; providing silicon particles; coating said silicon particles with said carbon residue forming material to form coated silicon particles; providing particles of a carbonaceous material; coating said particles of carbonaceous material with said carbon residue forming material to form coated carbonaceous particles; embedding said coated silicon particles onto said coated carbonaceous particles to form silicon / carbon composite particles; coating said silicon / carbon composite particles with said carbon residue forming material to form coated silicon / carbon composite particles; and stabilizing the coated composite particles by subjecting said coated composite particles to an oxidation reaction. The coated composite particles will have a substantially smooth coating. The particles may be coated with multiple layers of carbon residue forming material /
Owner:PYROTECK INC
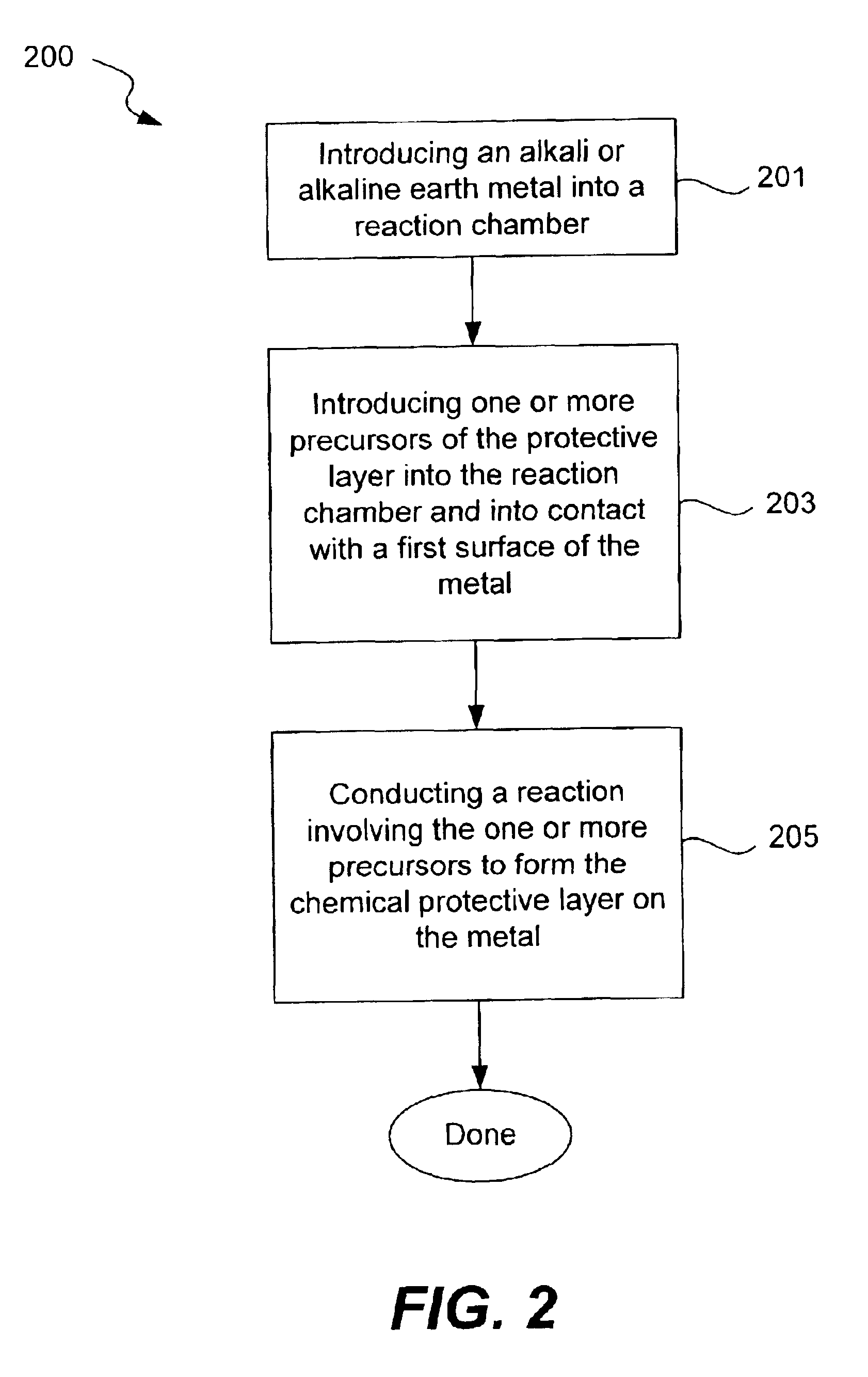
Chemical protection of a lithium surface
InactiveUS6911280B1Easy to produceSimple processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process in the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
Ionically conductive composites for protection of active metal anodes
ActiveUS7282296B2Easy to manufactureImprove battery performanceFinal product manufactureElectrode carriers/collectorsChemical stabilityIonic conductivity
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
Lithium metal dispersion in electrodes
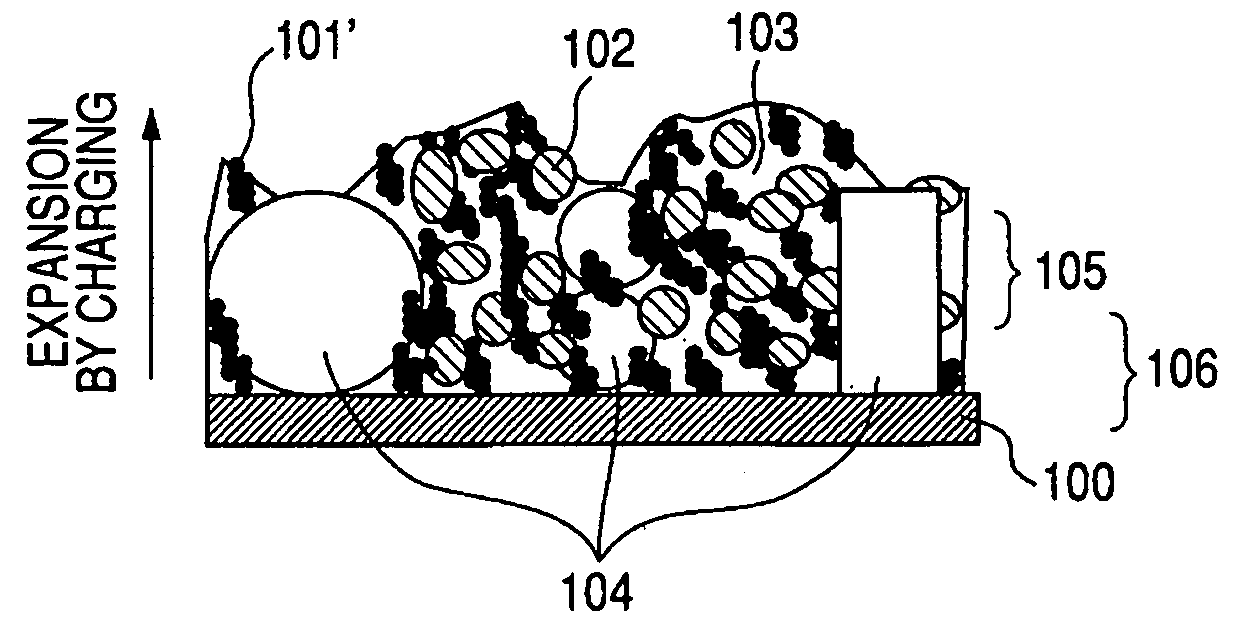
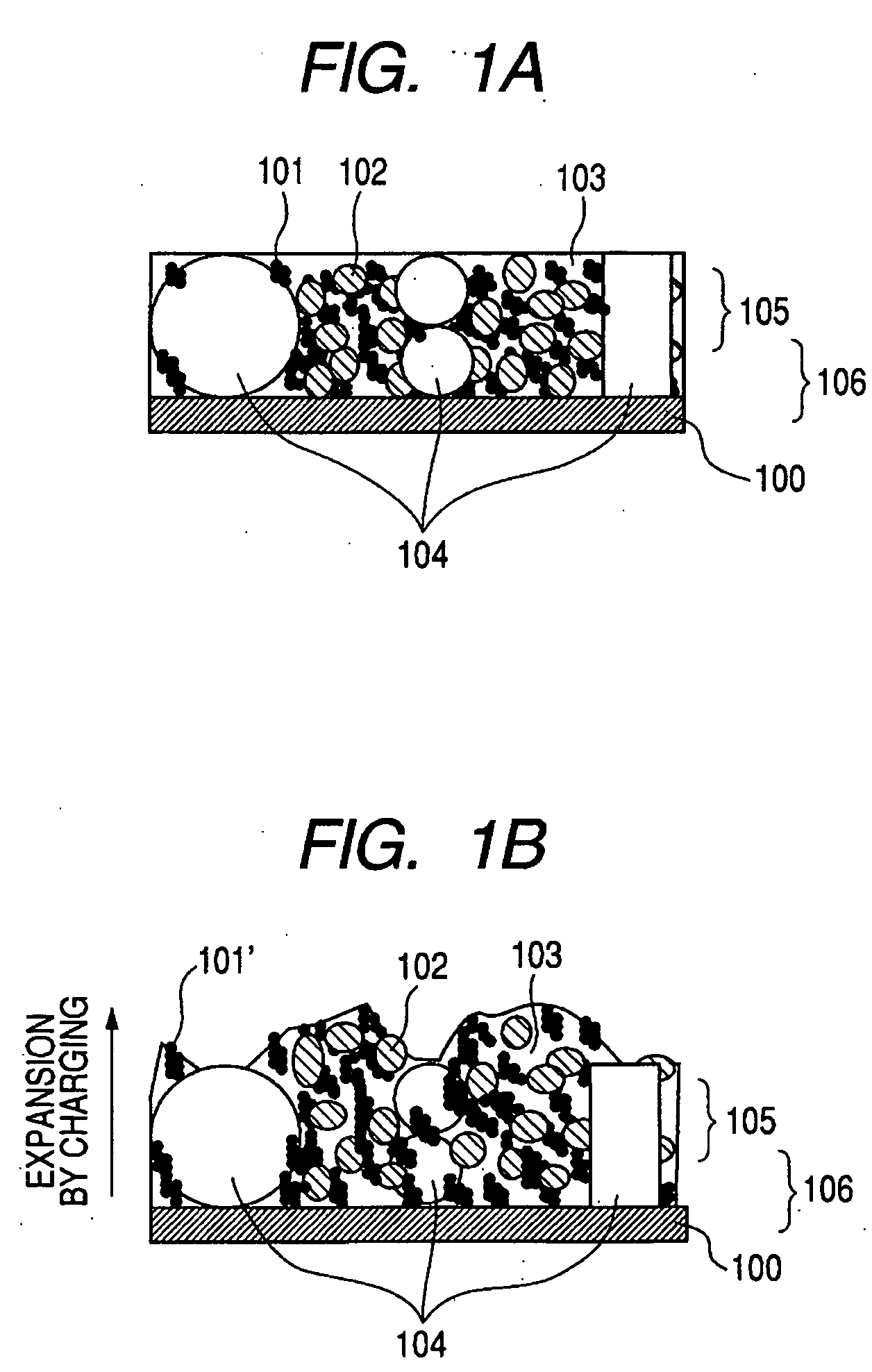
InactiveUS20050130043A1High specific capacityPromote circulationElectrode rolling/calenderingElectrode thermal treatmentLithium metalHost material
Electrodes, such as anodes and cathodes, can include a host material that is prelithiated or undergoes lithiation upon electrolyte introduction into a battery. Lithiation of the host material can occur by the agitation of lithium metal and a host material, the agitation of a lithium metal powder and a host material at a temperature greater than room temperature, the application of pressure to a lithium metal and host material mixture, contact of the host material with molten lithium metal, the lamination of lithium foil or lithium mesh onto an electrode containing the host material, or by lamination of lithium metal or mesh onto an electrode at elevated temperatures.
Owner:AEA TECH BATTERY SYST +1
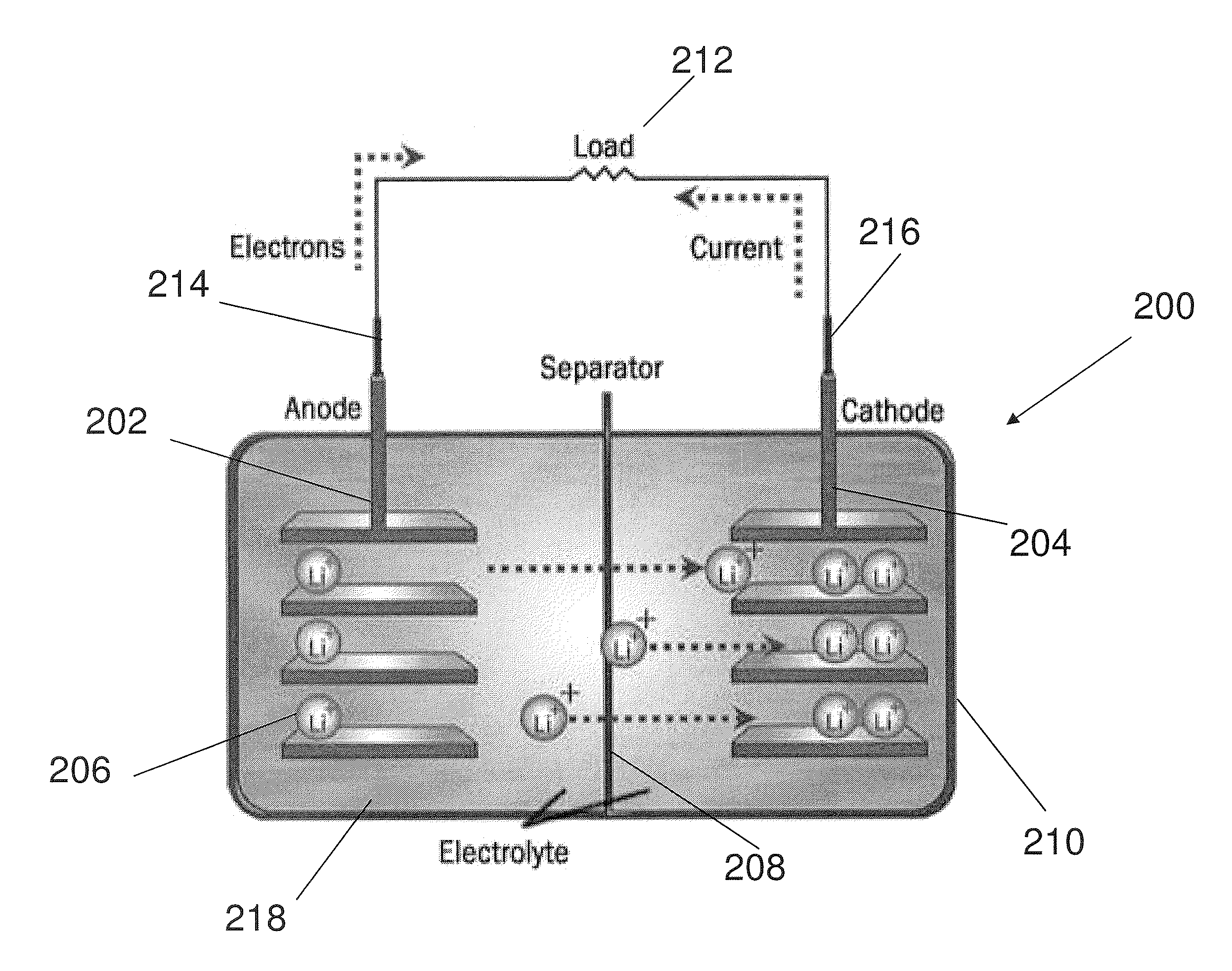
Electrode structure for lithium secondary battery and secondary battery having such electrode structure
InactiveUS20060127773A1Small capacity reductionLarge capacityElectrode manufacturing processesFinal product manufactureElectrochemical responseConductive polymer
In an electrode structure for a lithium secondary battery including: a main active material layer formed from a metal powder selected from silicon, tin and an alloy thereof that can store and discharge and capable of lithium by electrochemical reaction, and a binder of an organic polymer; and a current collector, wherein the main active material layer is formed at least by a powder of a support material for supporting the electron conduction of the main active material layer in addition to the metal powder and the powder of the support material are particles having a spherical, pseudo-spherical or pillar shape with an average particle size of 0.3 to 1.35 times the thickness of the main active material layer. The support material is one or more materials selected from a group consisting of graphite, oxides of transition metals and metals that do not electrochemically form alloy with lithium. Organic polymer compounded with a conductive polymer is used for the binder. There are provided an electrode structure for a lithium secondary battery having a high capacity and a long lifetime, and a lithium secondary battery using the electrode structure and having a high capacity, a high energy density and a long lifetime.
Owner:CANON KK
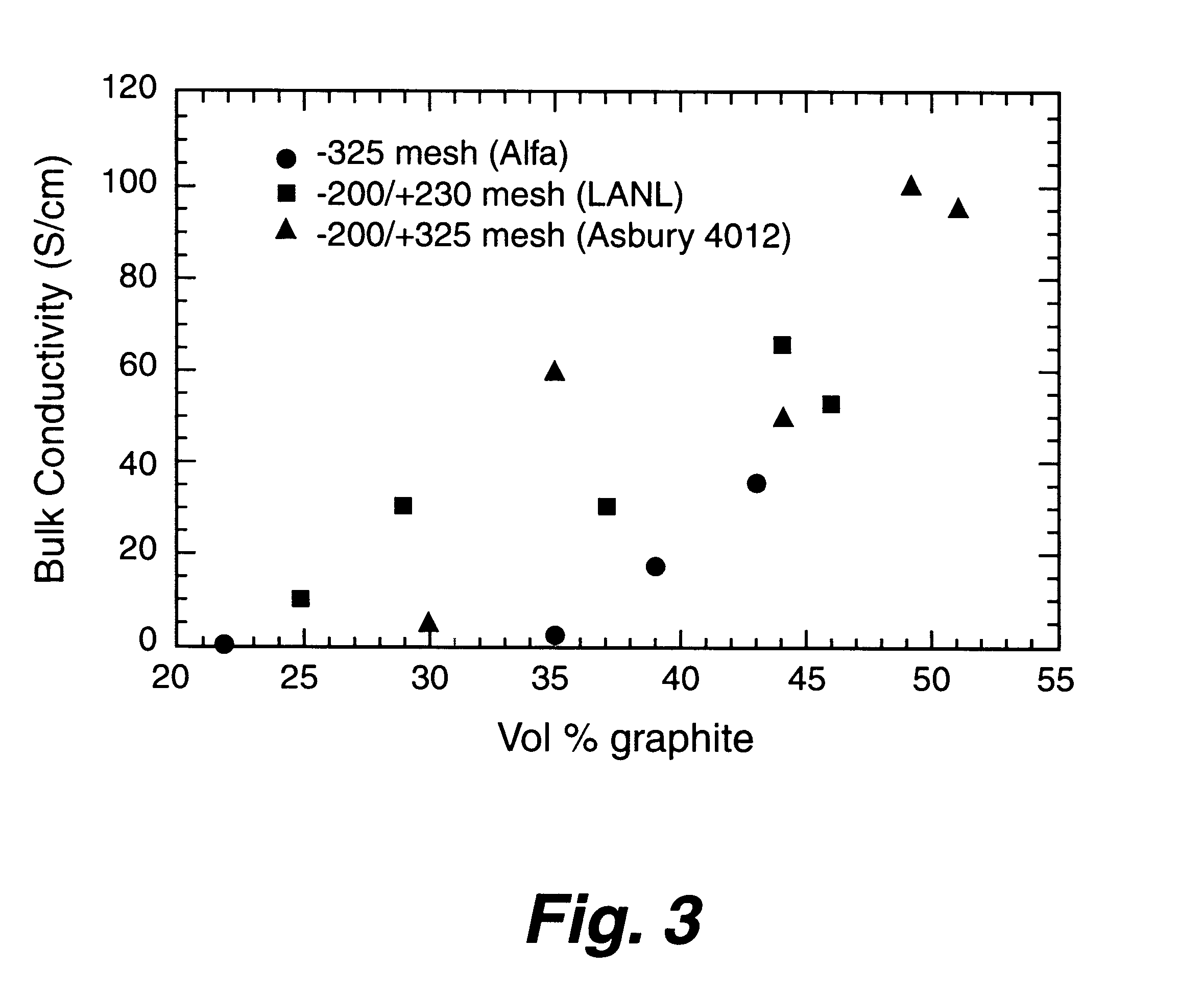
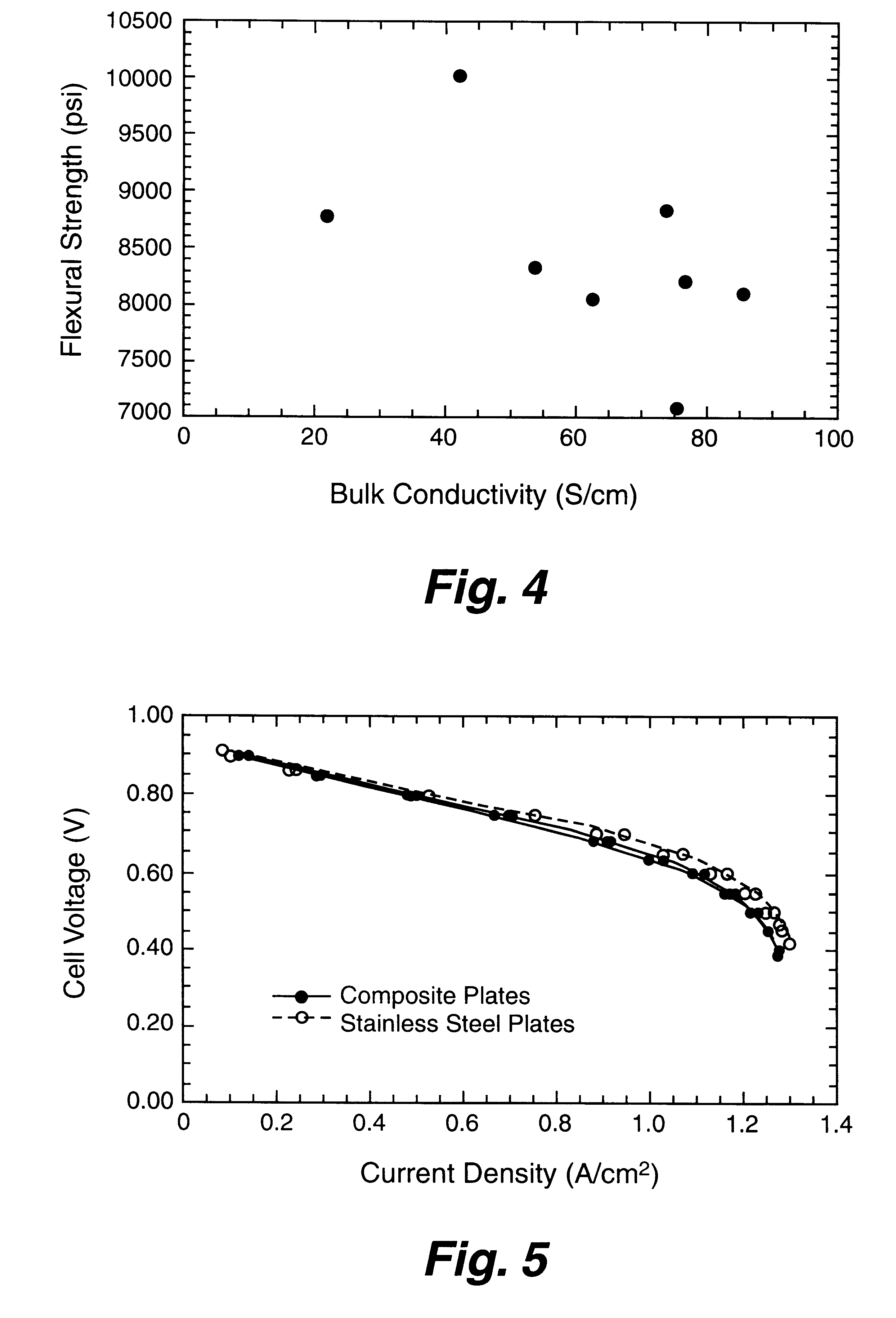
Composite bipolar plate for electrochemical cells
A bipolar separator plate for fuel cells consists of a molded mixture of a vinyl ester resin and graphite powder. The plate serves as a current collector and may contain fluid flow fields for the distribution of reactant gases. The material is inexpensive, electrically conductive, lightweight, strong, corrosion resistant, easily mass produced, and relatively impermeable to hydrogen gas. The addition of certain fiber reinforcements and other additives can improve the properties of the composite material without significantly increasing its overall cost.
Owner:TRIAD NAT SECURITY LLC
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
Long cycle-life alkali metal battery
InactiveUS6203947B1Improve cycle lifeFast charging rateElectrochemical processing of electrodesElectrode carriers/collectorsHigh pressureElectrochemical cell
The present invention provides a cathode for use in a secondary electrochemical cell, such cathode being coated with a very thin, protective film, permeable to ions. The protective film of the cathode usually has a thickness of up to about 0.1 mum and it provides protection against high voltage charging and overdiscbarging. The present invention further provides a secondary electrochemical cell comprising such a cathode.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
Compliant seal structures for protected active metal anodes
ActiveUS20070037058A1Reduced ionic contact areaReduce the total massPrimary cell to battery groupingElectrode manufacturing processesOptoelectronicsAnodic protection
Protected anode architectures have ionically conductive protective membrane architectures that, in conjunction with compliant seal structures and anode backplanes, effectively enclose an active metal anode inside the interior of an anode compartment. This enclosure prevents the active metal from deleterious reaction with the environment external to the anode compartment, which may include aqueous, ambient moisture, and / or other materials corrosive to the active metal. The compliant seal structures are substantially impervious to anolytes, catholyes, dissolved species in electrolytes, and moisture and compliant to changes in anode volume such that physical continuity between the anode protective architecture and backplane are maintained. The protected anode architectures can be used in arrays of protected anode architectures and battery cells of various configurations incorporating the protected anode architectures or arrays.
Owner:POLYPLUS BATTERY CO INC
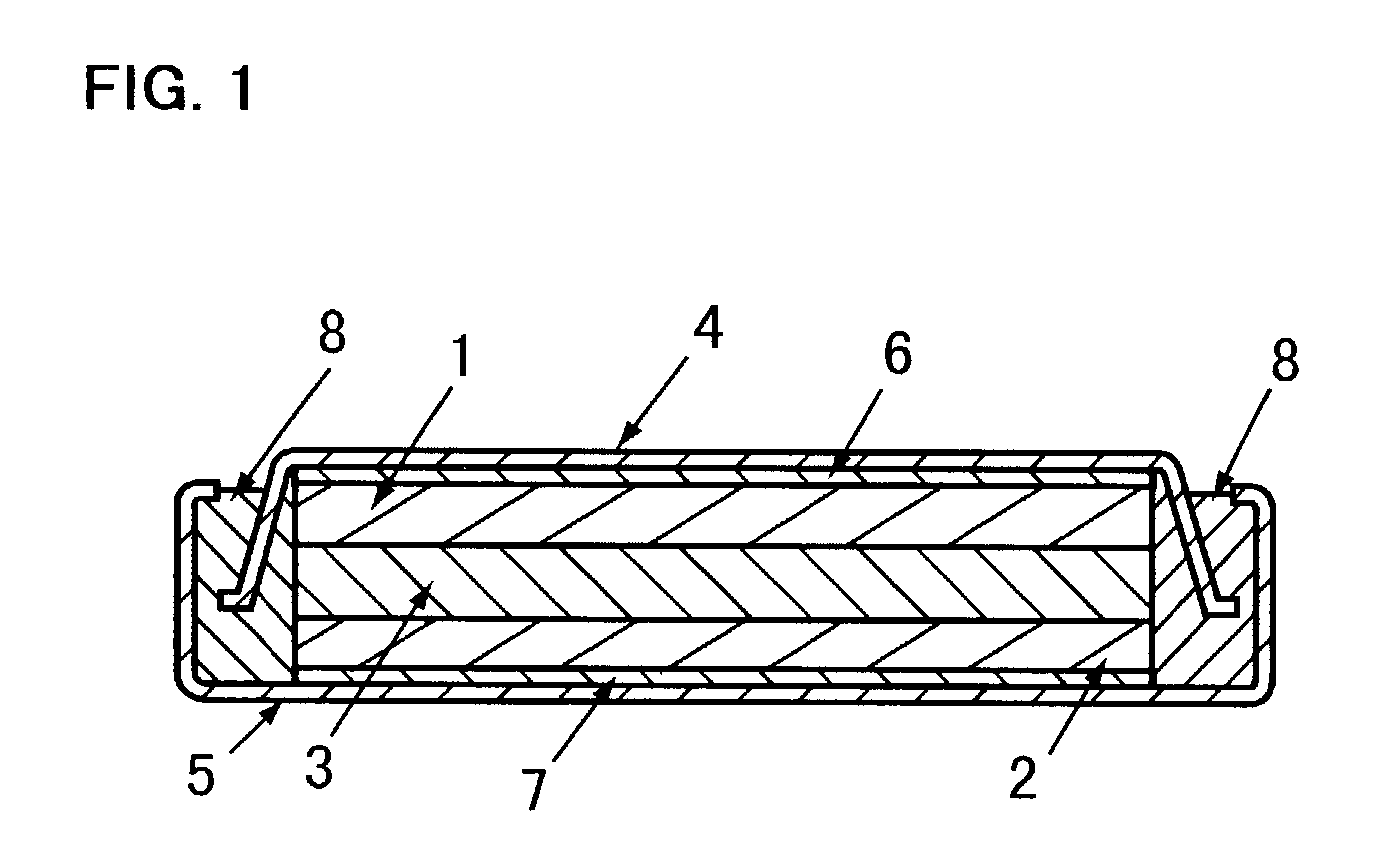
Lithium ion secondary battery
InactiveUS7335448B2Improve securityFinal product manufactureElectrode carriers/collectorsLithiumEngineering
A lithium ion secondary battery includes: (a) a positive electrode plate comprising an active material part and a current collector carrying the active material part, the active material part comprising a positive electrode active material capable of absorbing or desorbing a lithium ion during charge and discharge; (b) a negative electrode plate comprising an active material part and a current collector carrying the active material part, the active material part comprising a negative electrode active material capable of absorbing or desorbing a lithium ion during charge and discharge; (c) a separator interposed between the positive and negative electrode plates; (d) an electrolyte; and (e) a battery case accommodating the positive and negative electrode plates, the separator, and the electrolyte. The positive and negative electrode plates are wound with the separator interposed therebetween, thereby to form an electrode plate assembly. The electrode plate assembly is so configured that each lengthwise edge of the positive electrode current collector is positioned on an outer side of each lengthwise edge of the negative electrode active material part.
Owner:GK BRIDGE 1
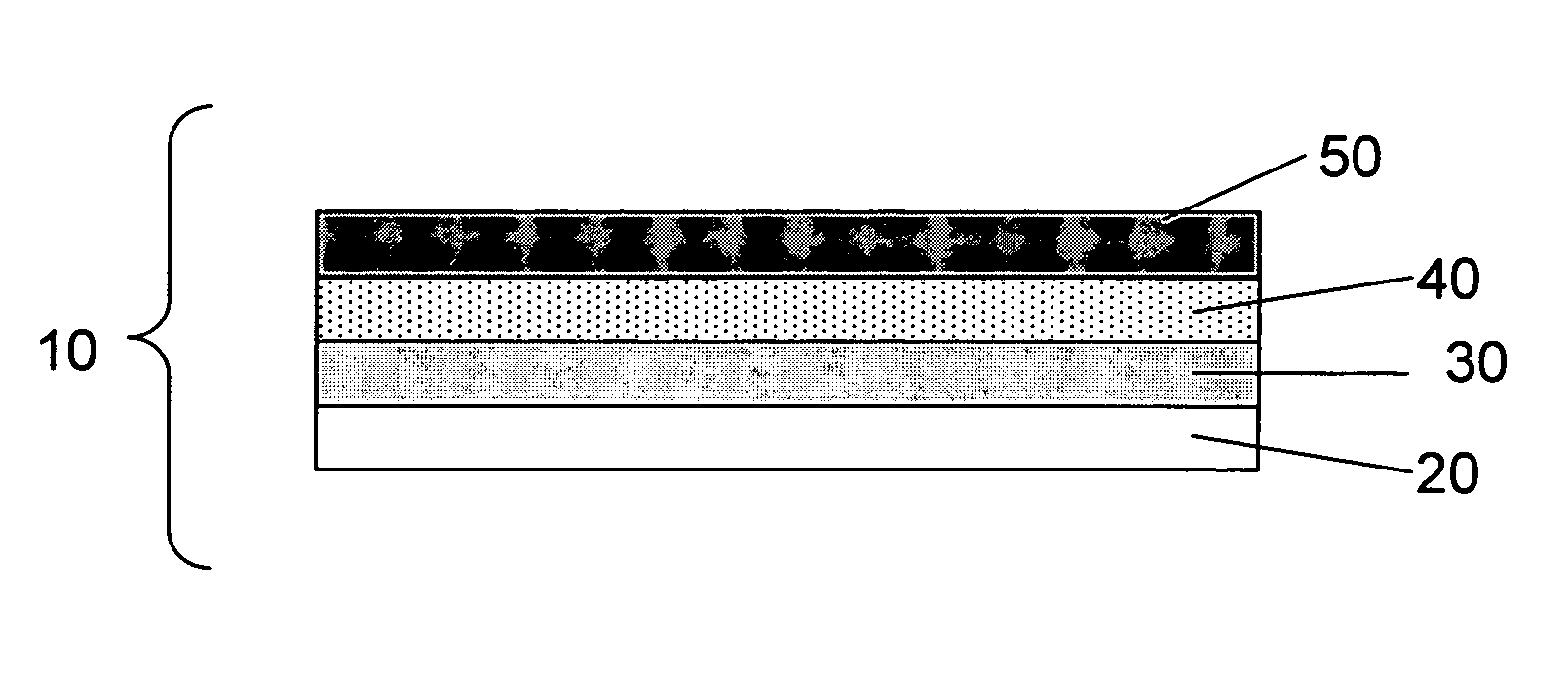
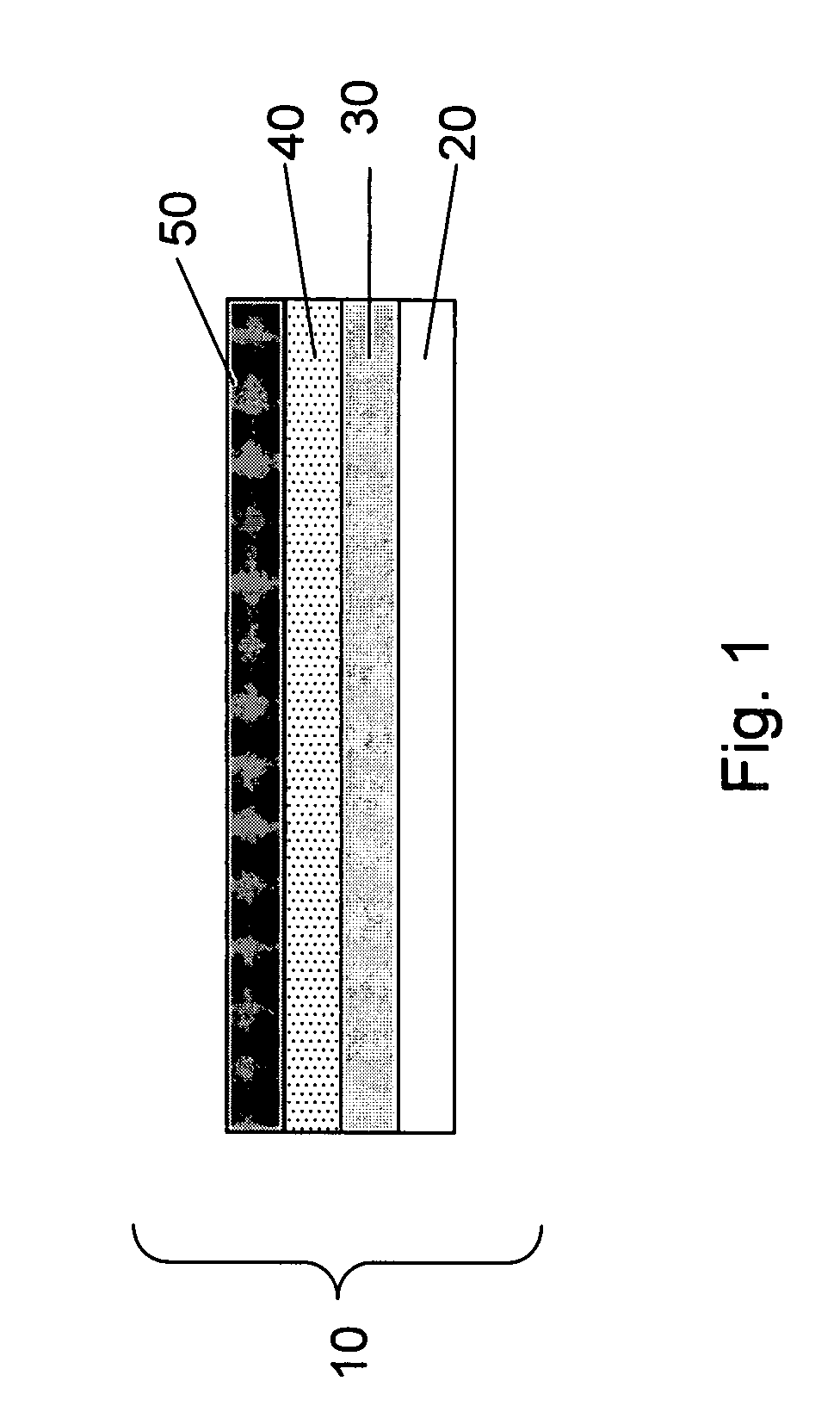
Electrode for rechargeable lithium battery and rechargeable lithium battery
InactiveUS7192673B1Improve charge and discharge cycle characteristicsInhibition formationElectrode manufacturing processesSmall-sized cells cases/jacketsAmorphous siliconMaterials science
An electrode for a rechargeable lithium battery which includes a thin film composed of active material that expands and shrinks as it stores and releases lithium, e.g., a microcrystalline or amorphous silicon thin film, deposited on a current collector, characterized in that said current collector exhibits a tensile strength (=tensile strength (N / mm2) per sectional area of the current collector material×thickness (mm) of the current collector) of not less than 3.82 N / mm.
Owner:SANYO ELECTRIC CO LTD
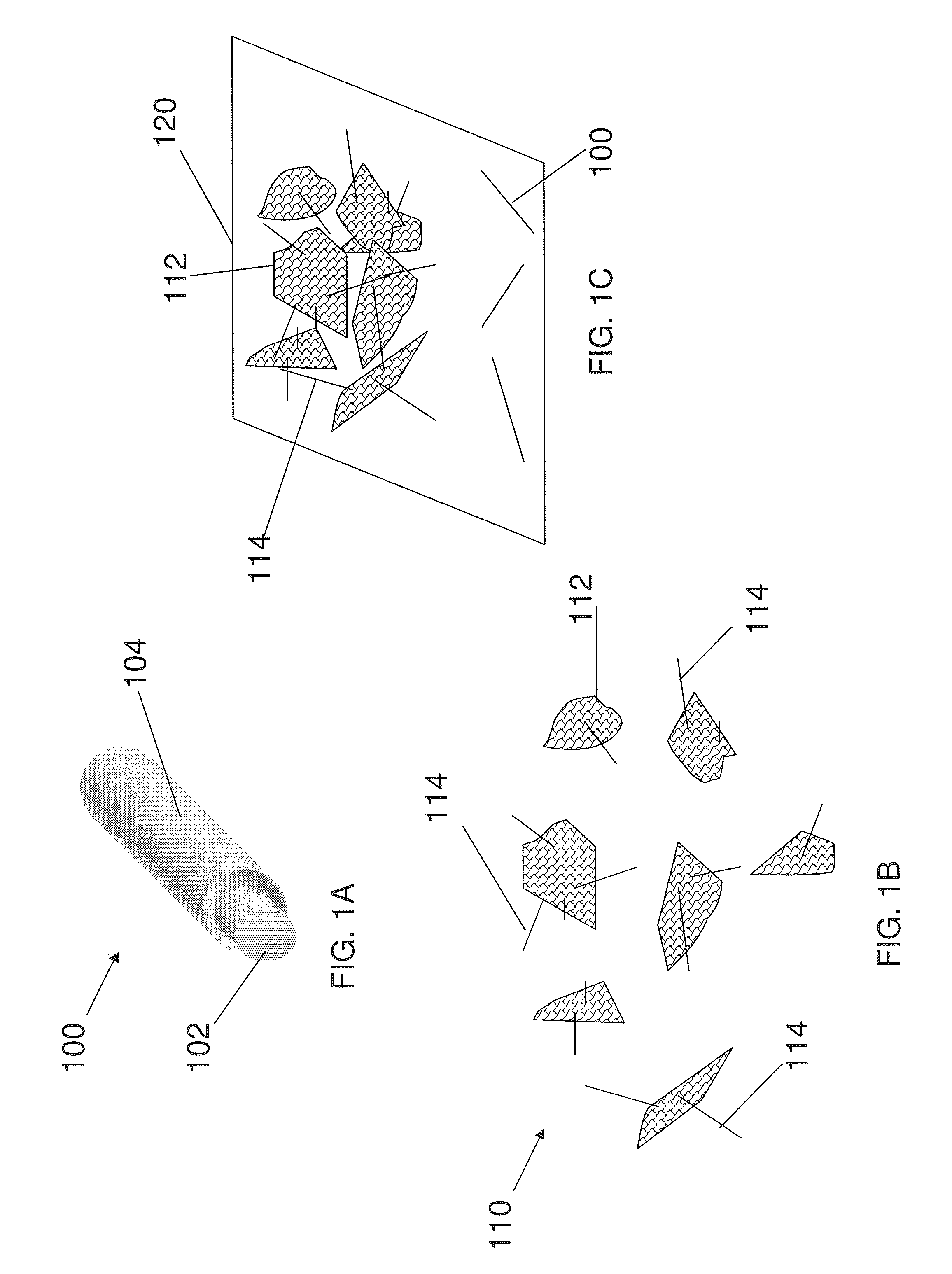
Core-shell high capacity nanowires for battery electrodes
InactiveUS20100330421A1Large capacityInhibition formationMaterial nanotechnologyNanostructure manufactureNanowireElectrochemistry
Provided are nanostructures containing electrochemically active materials, battery electrodes containing these nanostructures for use in electrochemical batteries, such as lithium ion batteries, and methods of forming the nanostructures and battery electrodes. The nanostructures include conductive cores, inner shells containing active materials, and outer shells partially coating the inner shells. The high capacity active materials having a stable capacity of at least about 1000 mAh / g can be used. Some examples include silicon, tin, and / or germanium. The outer shells may be configured to substantially prevent formation of Solid Electrolyte lnterphase (SEI) layers directly on the inner shells. The conductive cores and / or outer shells may include carbon containing materials. The nanostructures are used to form battery electrodes, in which the nanostructures that are in electronic communication with conductive substrates of the electrodes.
Owner:AMPRIUS INC
Lithium alloy/sulfur batteries
Electrochemical cells including anode compositions that may enhance charge-discharge cycling efficiency and uniformity are presented. In some embodiments, alloys are incorporated into one or more components of an electrochemical cell, which may enhance the performance of the cell. For example, an alloy may be incorporated into an electroactive component of the cell (e.g., electrodes) and may advantageously increase the efficiency of cell performance. Some electrochemical cells (e.g., rechargeable batteries) may undergo a charge / discharge cycle involving deposition of metal (e.g., lithium metal) on the surface of the anode upon charging and reaction of the metal on the anode surface, wherein the metal diffuses from the anode surface, upon discharging. In some cases, the efficiency and uniformity of such processes may affect cell performance. The use of materials such as alloys in an electroactive component of the cell have been found to increase the efficiency of such processes and to increase the cycling lifetime of the cell. For example, the use of alloys may reduce the formation of dendrites on the anode surface and / or limit surface development.
Owner:SION POWER CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com