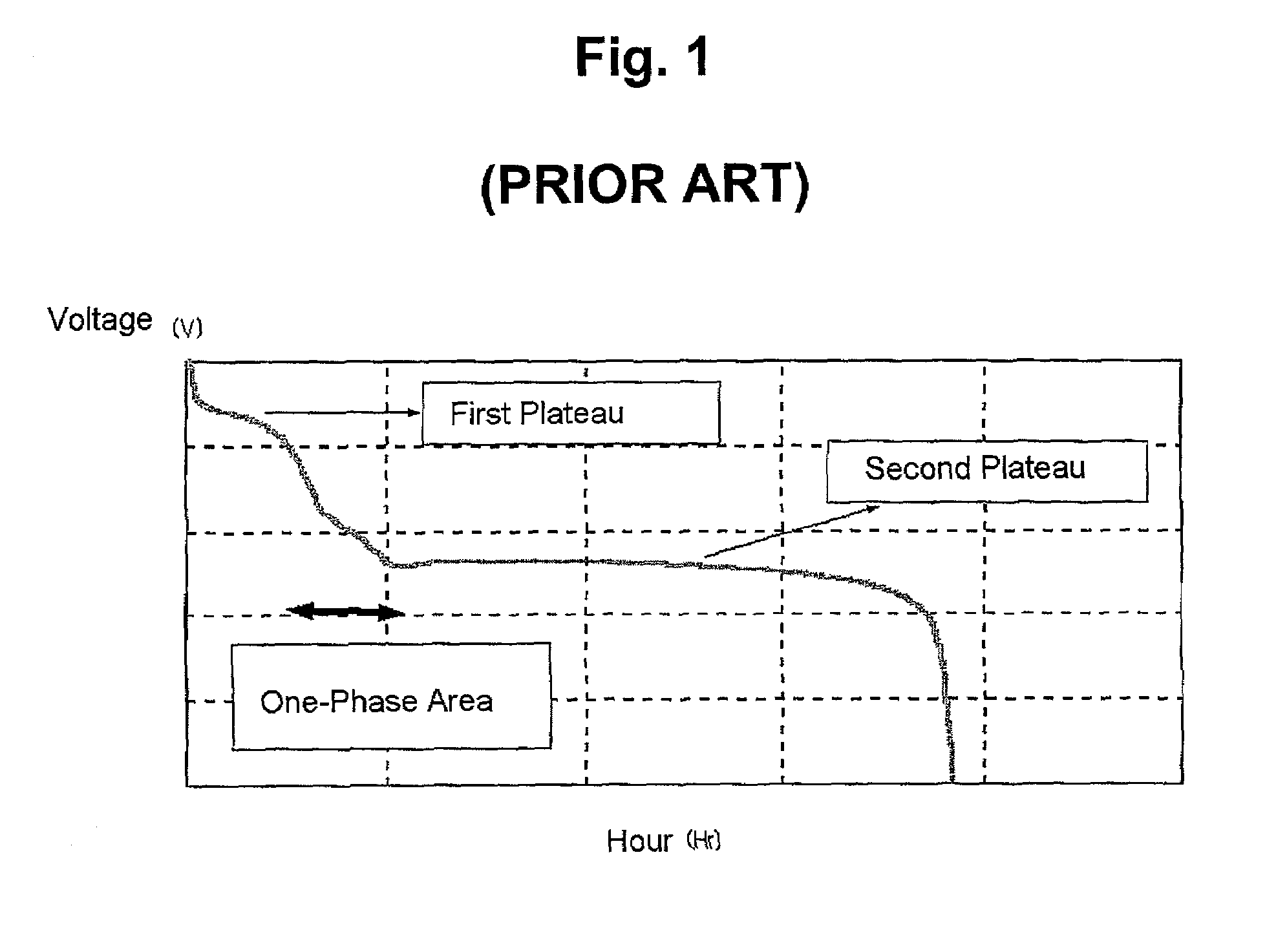
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4505 results about "Electrochemical response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
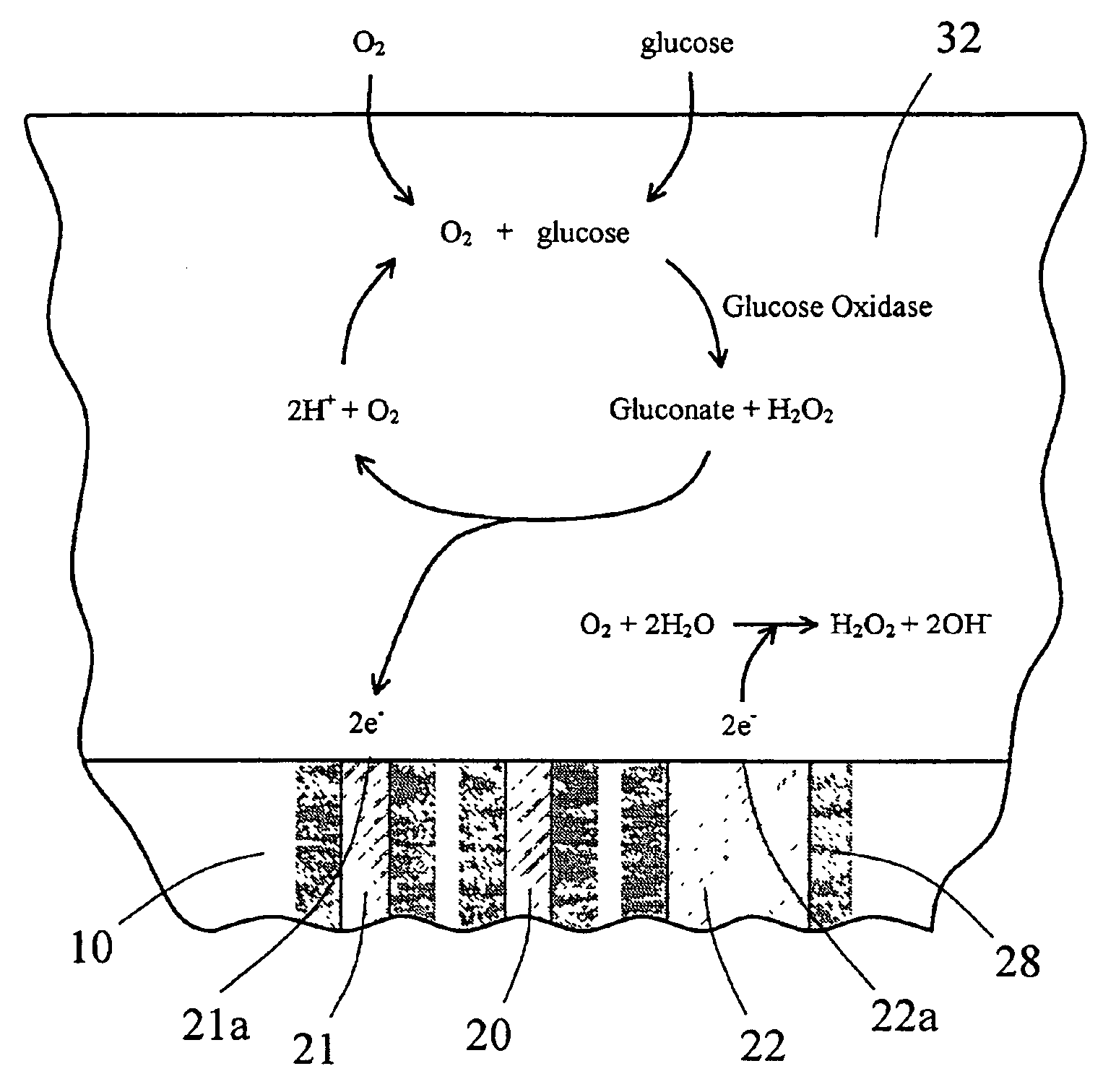
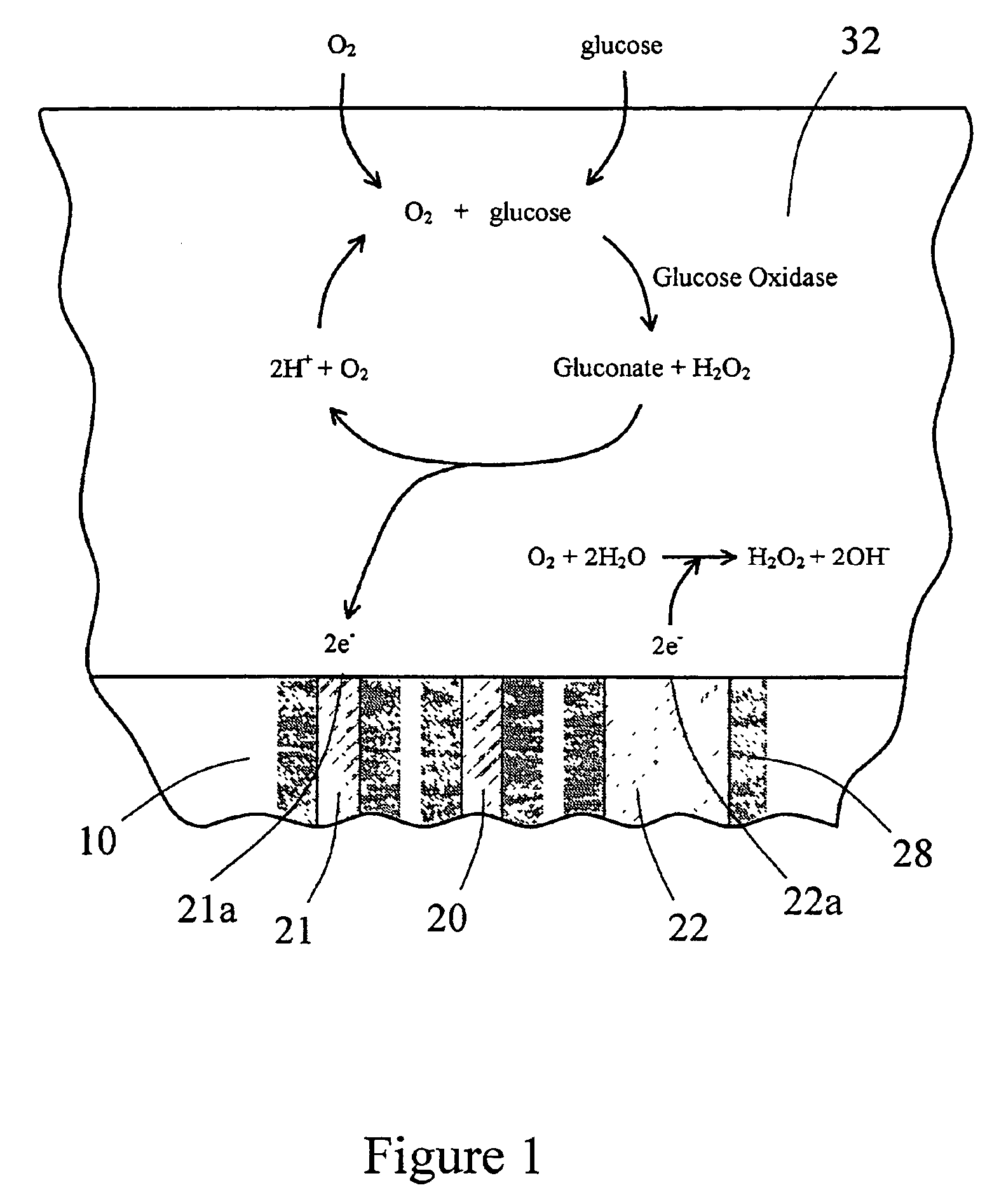
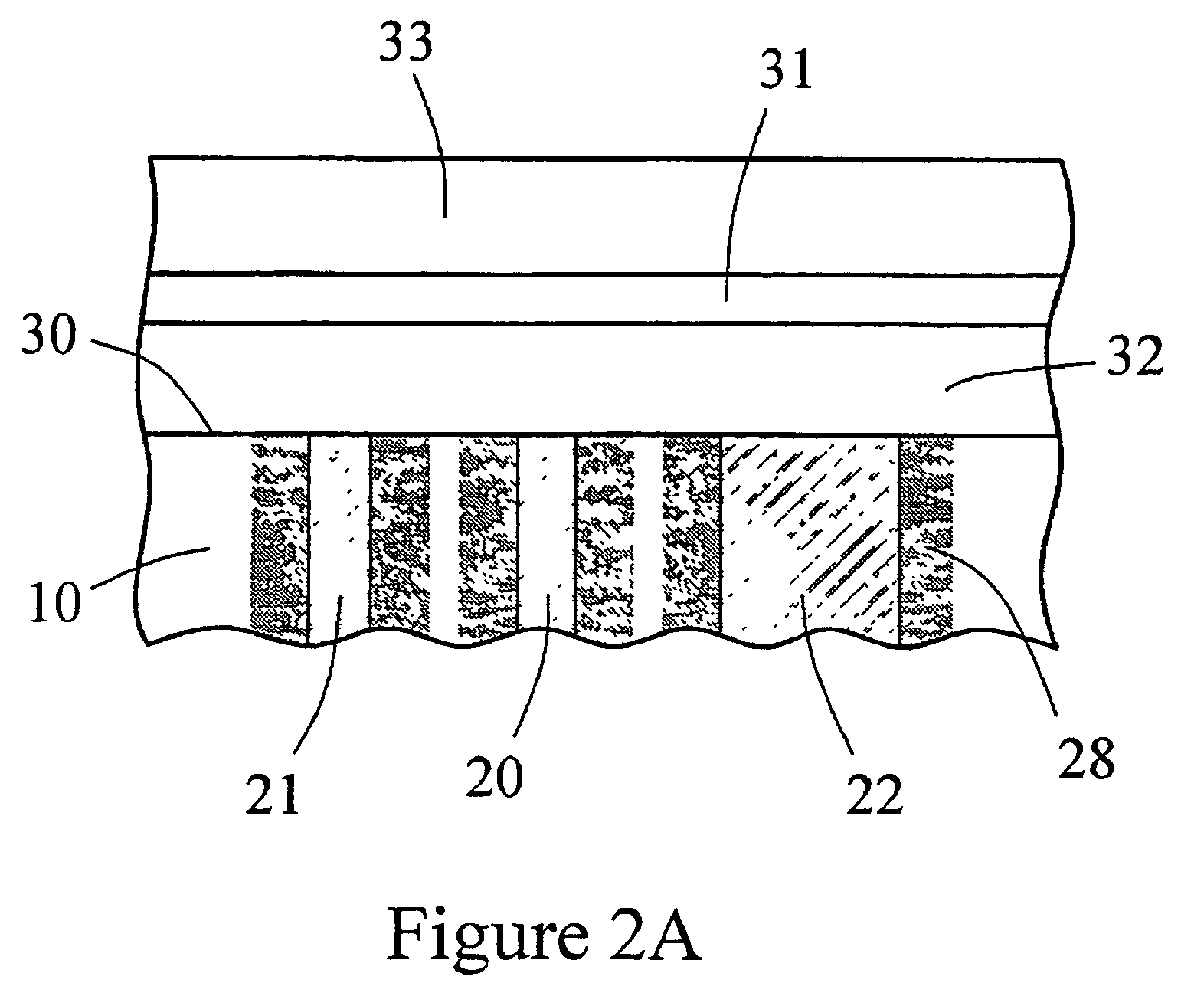
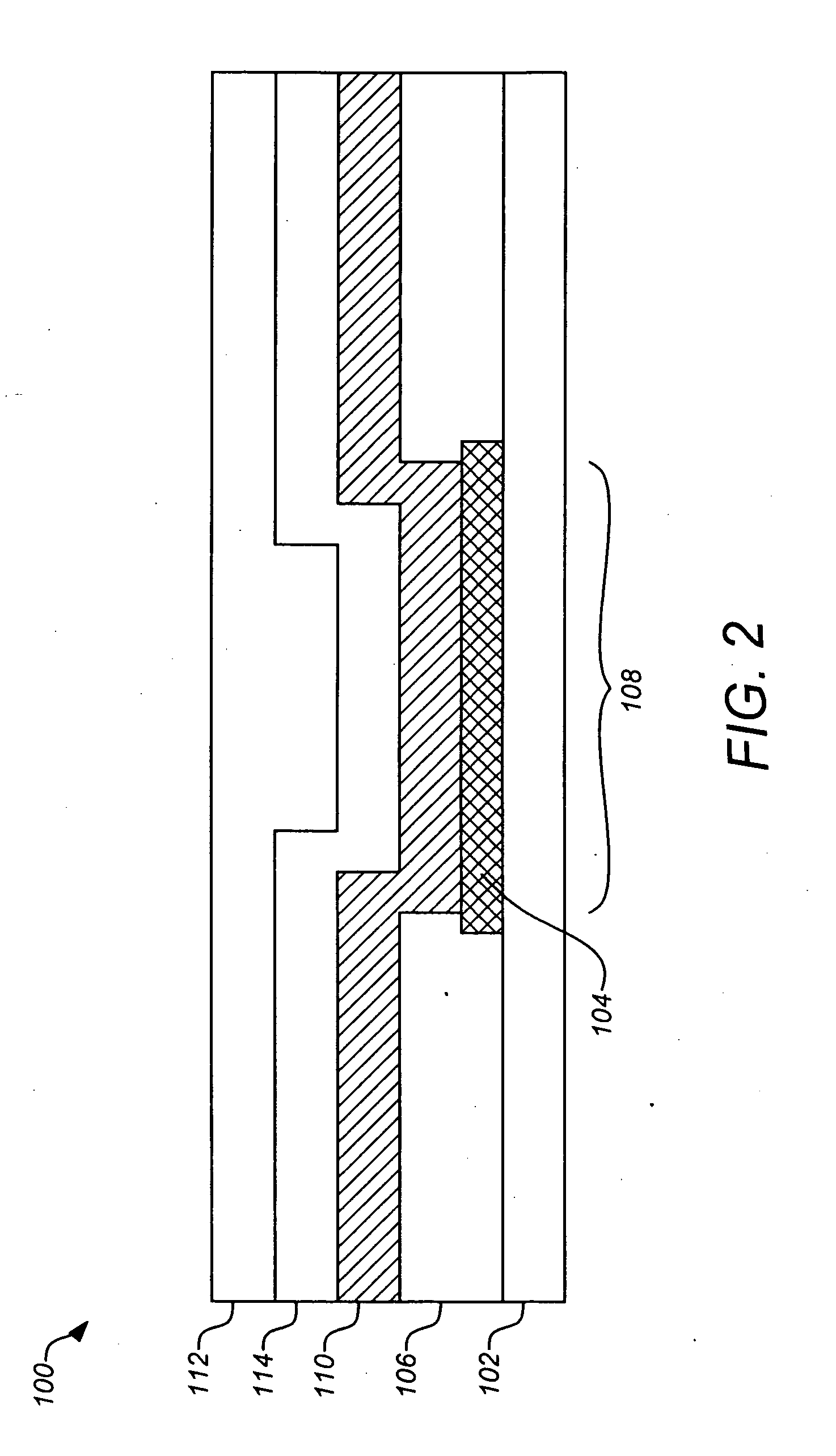
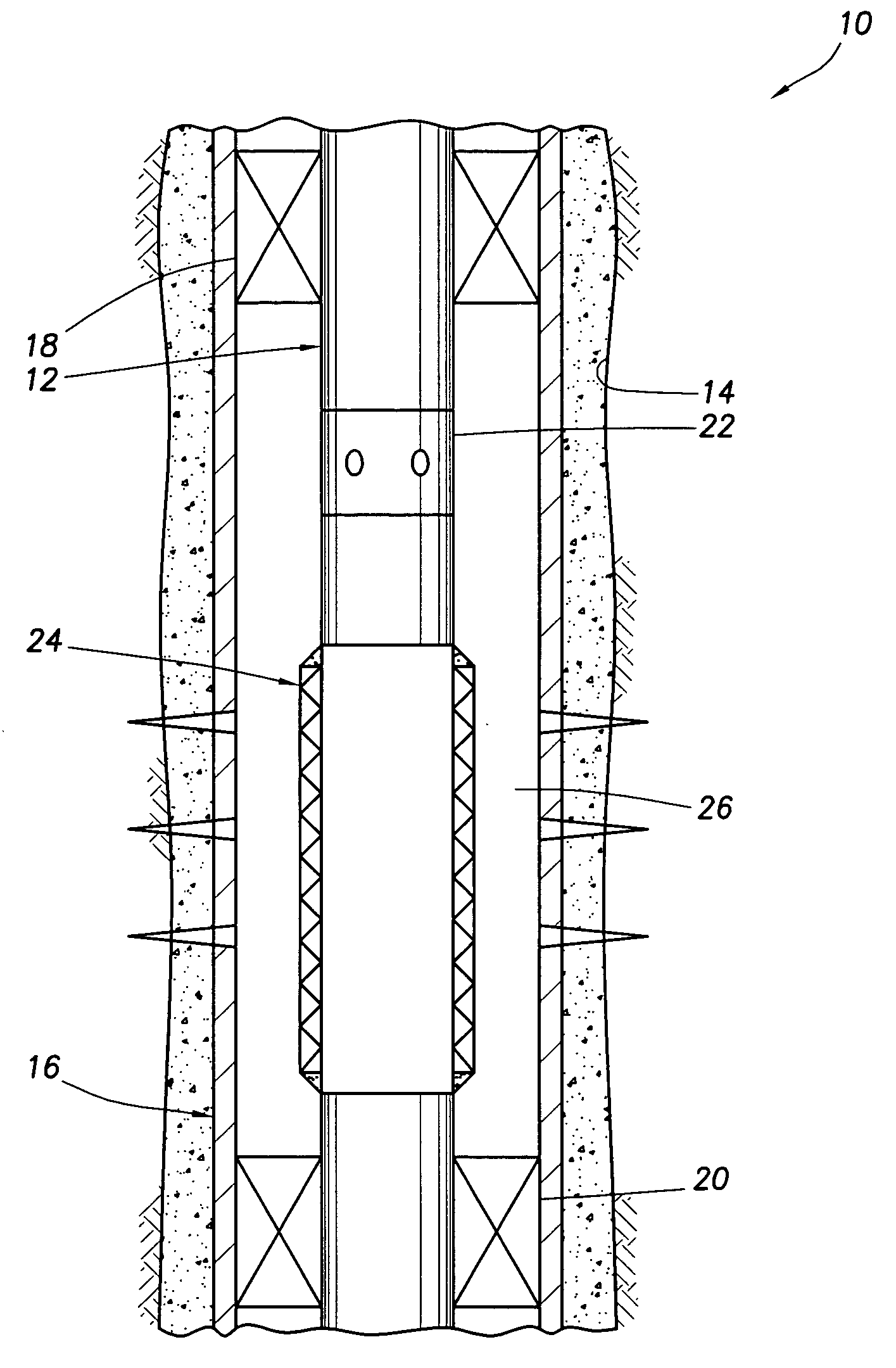
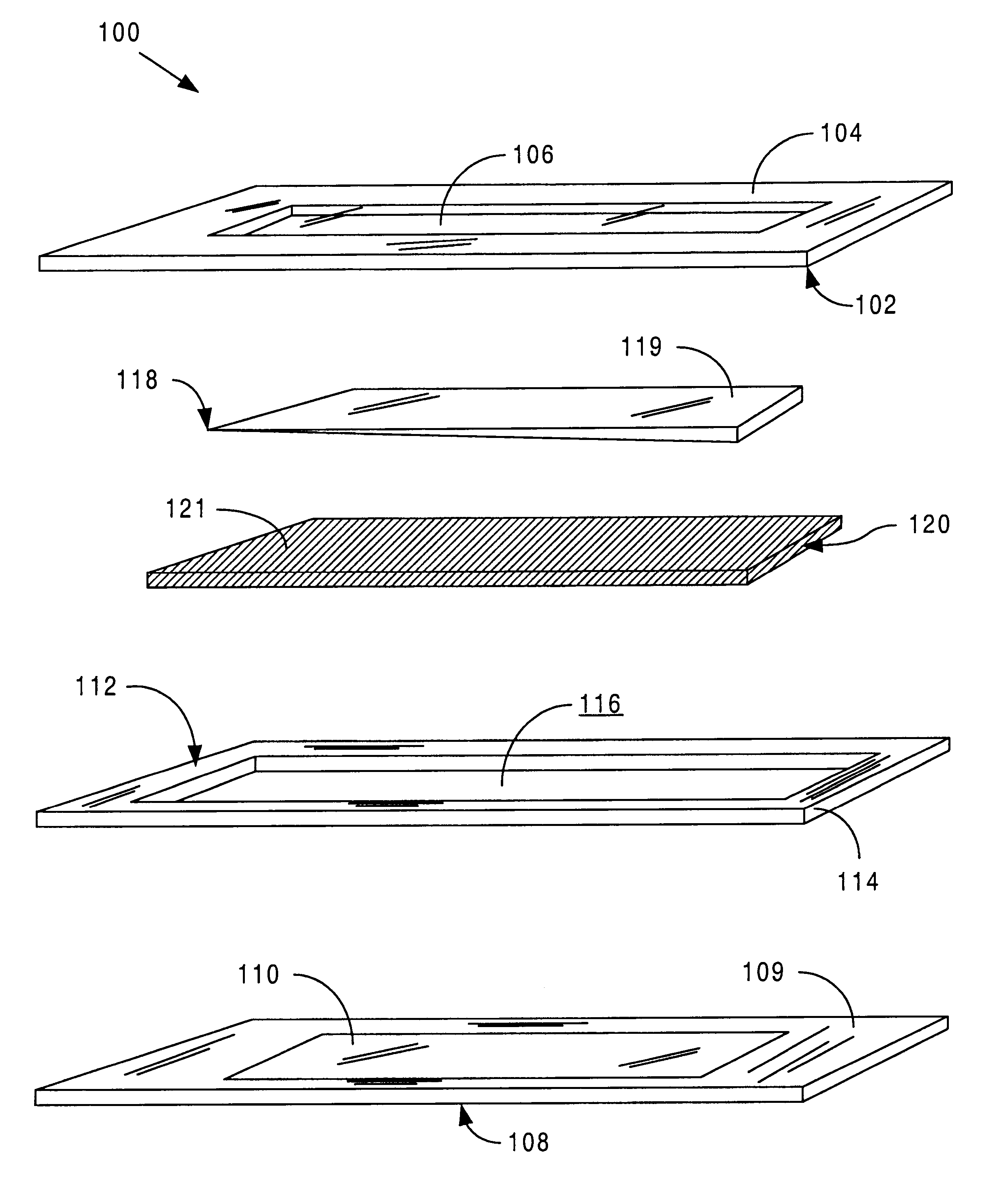
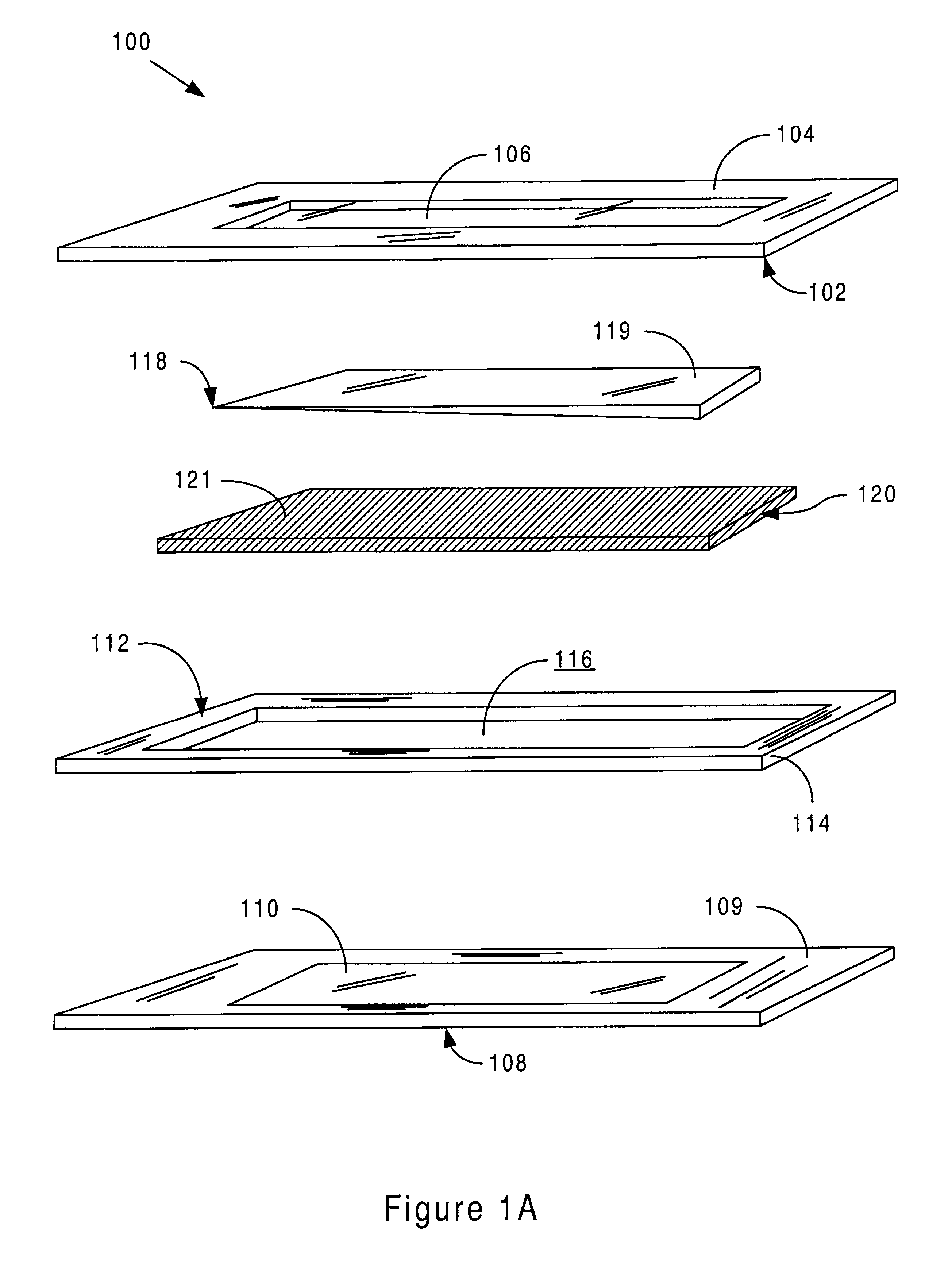
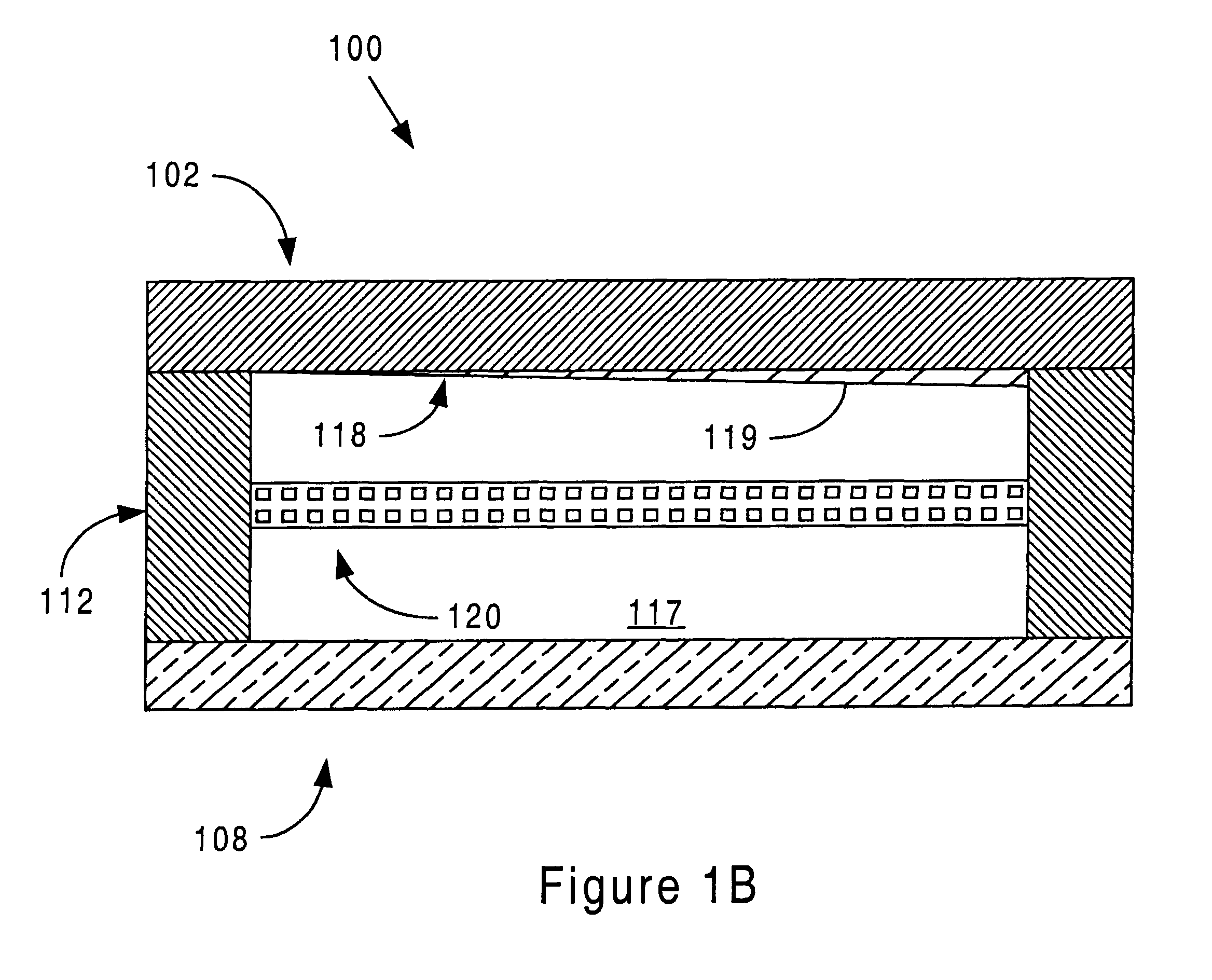
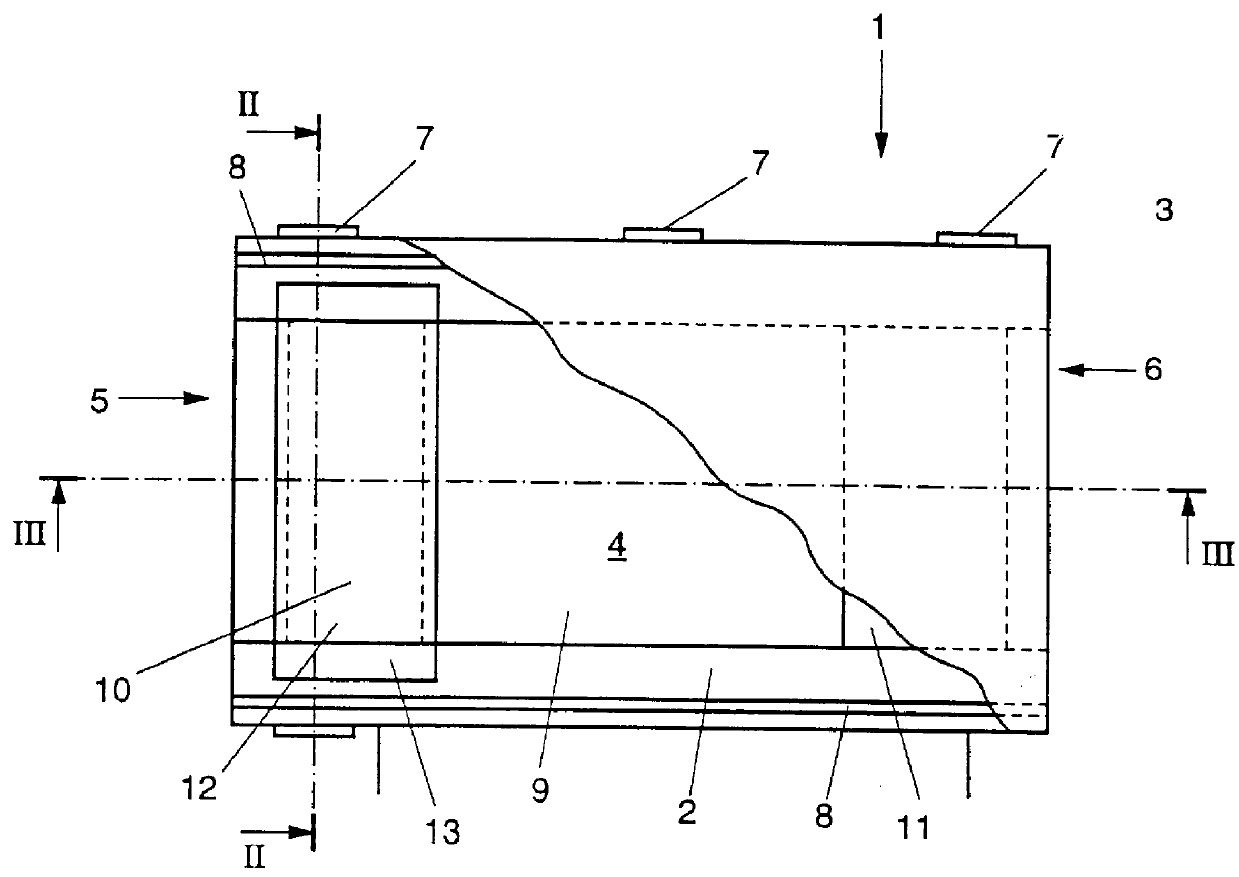
Sensor head for use with implantable devices
InactiveUS7471972B2Minimizing negative potential extremesImprove solubilityMicrobiological testing/measurementMaterial analysis by electric/magnetic meansElectrochemical responseAnalyte
The present invention provides a sensor head for use in an implantable device that measures the concentration of an analyte in a biological fluid which includes: a non-conductive body; a working electrode, a reference electrode and a counter electrode, wherein the electrodes pass through the non-conductive body forming an electrochemically reactive surface at one location on the body and forming an electronic connection at another location on the body, further wherein the electrochemically reactive surface of the counter electrode is greater than the surface area of the working electrode; and a multi-region membrane affixed to the nonconductive body and covering the working electrode, reference electrode and counter electrode. In addition, the present invention provides an implantable device including at least one of the sensor heads of the invention and methods of monitoring glucose levels in a host utilizing the implantable device of the invention.
Owner:DEXCOM INC
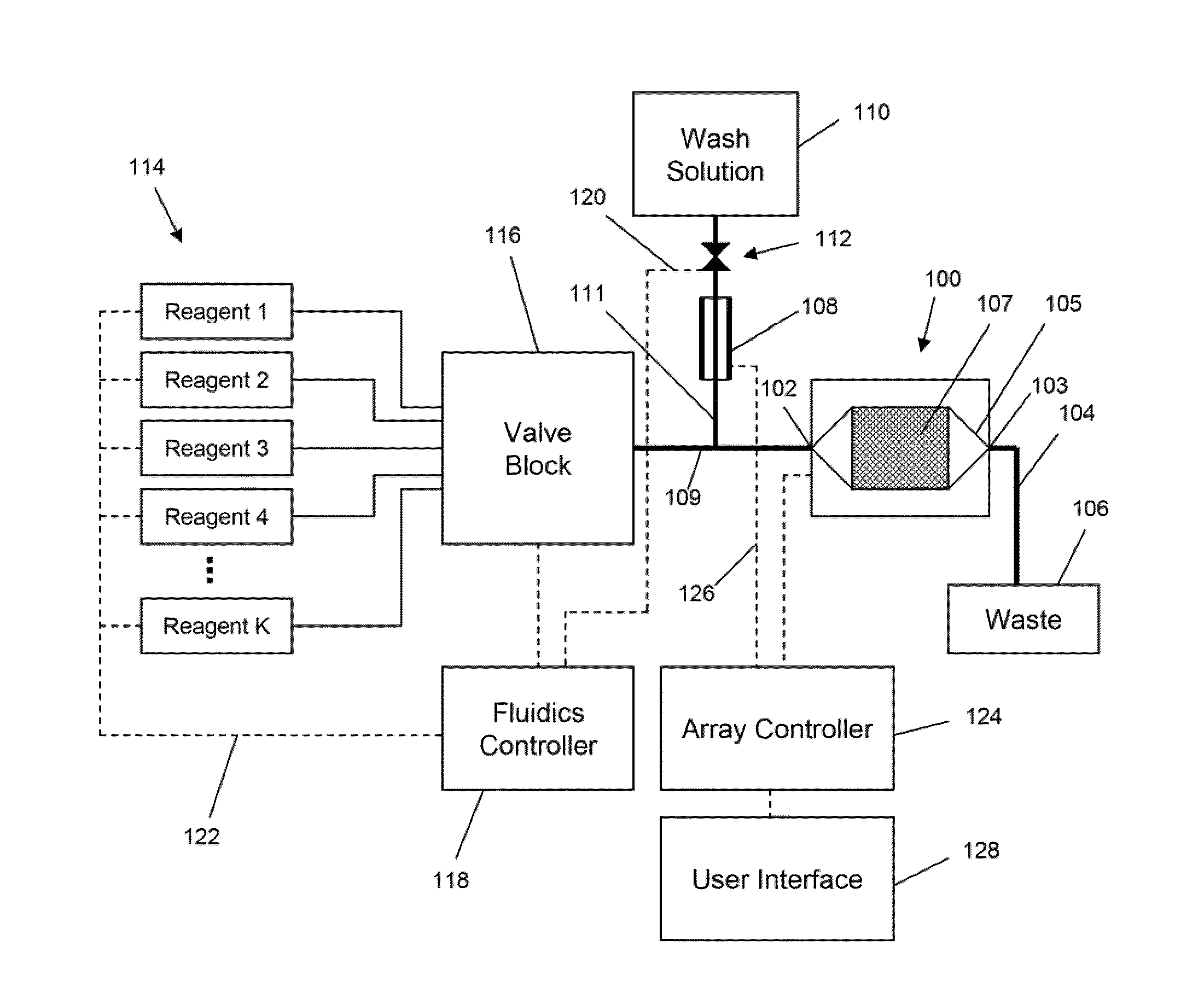
Apparatus and methods for performing electrochemical reactions
ActiveUS20100300895A1Reduce noiseWeather/light/corrosion resistanceVolume/mass flow measurementElectrochemical responseSignal-to-noise ratio (imaging)
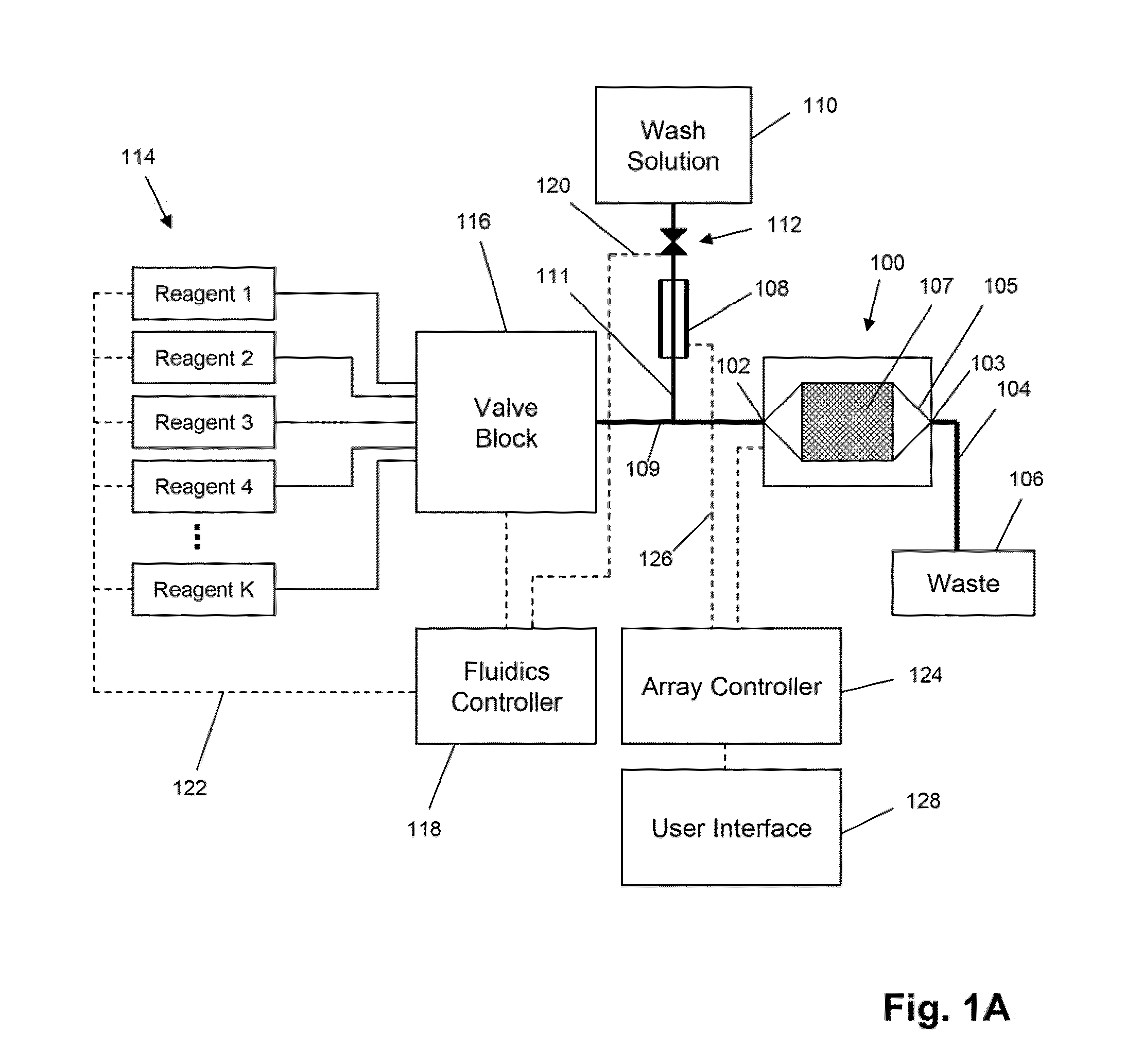
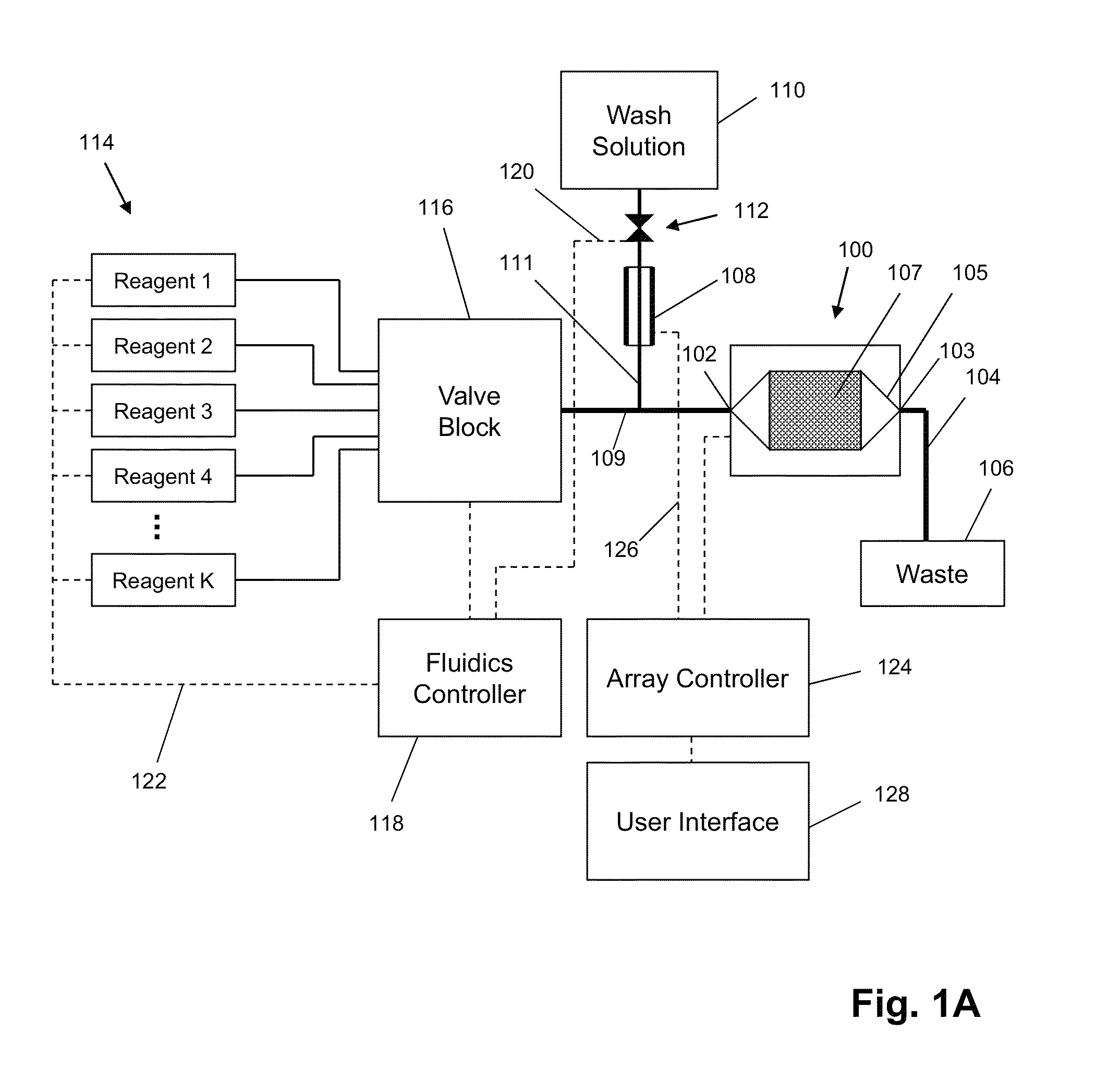
The invention is directed to apparatus and methods for delivering multiple reagents to, and monitoring, a plurality of analytical reactions carried out on a large-scale array of electronic sensors underminimal noise conditions. In one aspect, the invention provides method of improving signal-to-noise ratios of output signals from the electronic sensors sensing analytes or reaction byproducts by subtracting an average of output signals measured from neighboring sensors where analyte or reaction byproducts are absent. In other aspects, the invention provides an array of electronic sensors integrated with a microwell array for confining analytes and / or particles for analytical reactions and a method for identifying microwells containing analytes and / or particles by passing a sensor-active reagent over the array and correlating sensor response times to the presence or absence of analytes or particles. Such detection of analyte- or particle-containing microwells may be used as a step in additional noise reduction methods.
Owner:LIFE TECH CORP
Methods and materials for controlling the electrochemistry of analyte sensors
InactiveUS20070227907A1Increase surface areaImmobilised enzymesBioreactor/fermenter combinationsElectrochemical responseAnalyte
Embodiments of the invention provide electrochemical analyte sensors having elements designed to modulate their electrochemical reactions as well as methods for making and using such sensors.
Owner:MEDTRONIC MIMIMED INC
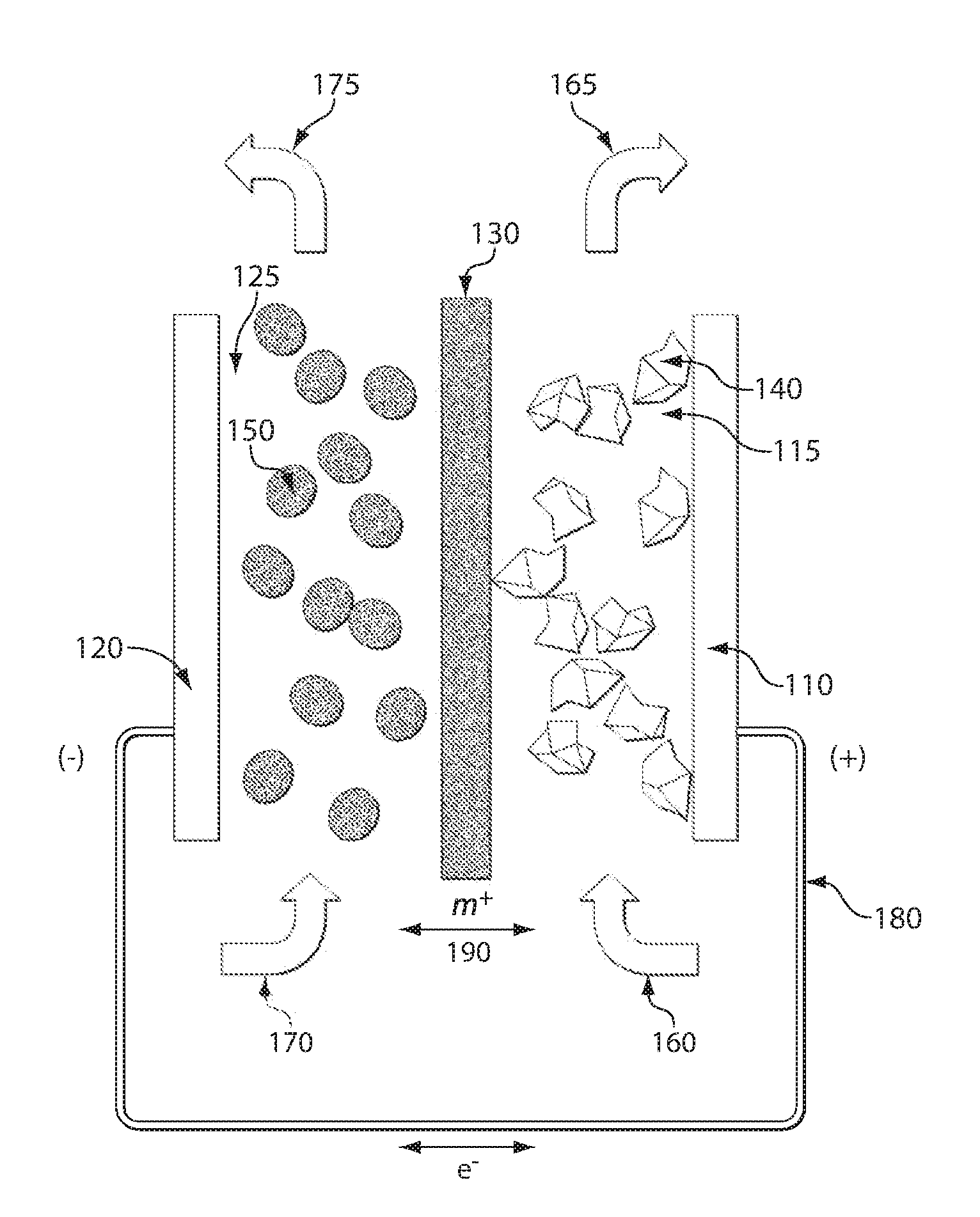
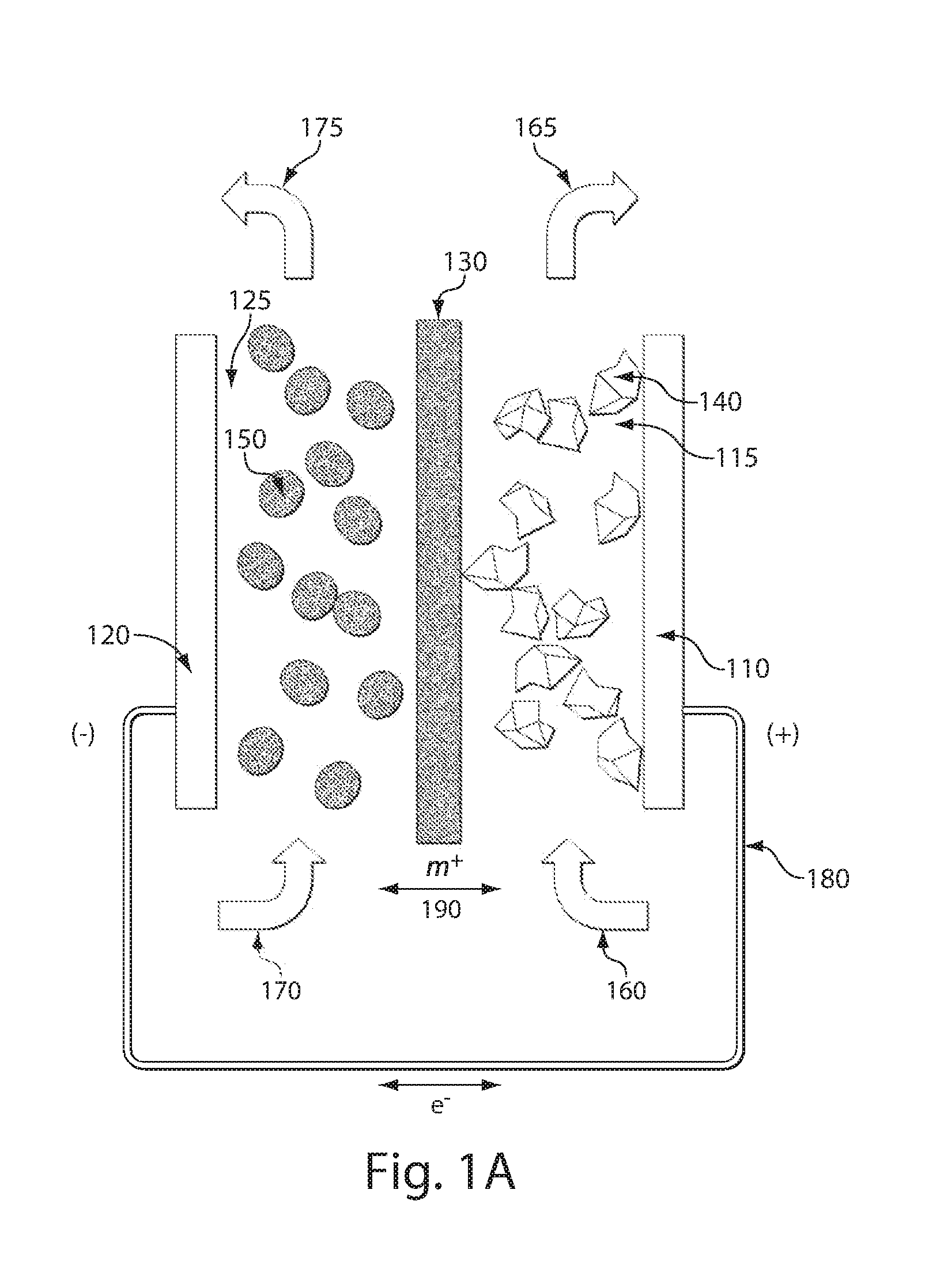
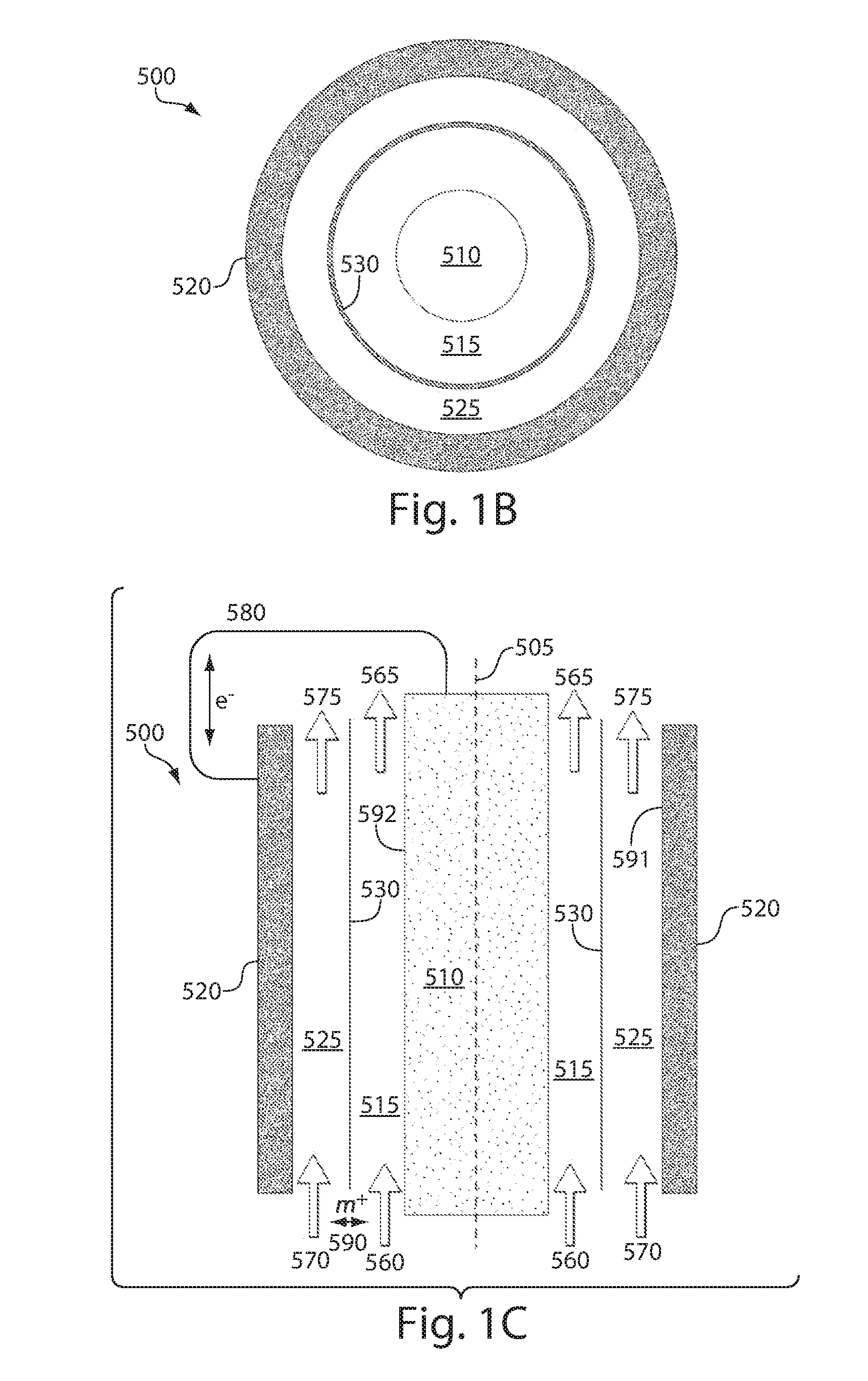
High energy density redox flow device
ActiveUS20110200848A1Avoid accumulationHigh enough specific energyOrganic chemistryFlow propertiesElectrochemical responseHigh energy
Redox flow devices are described in which at least one of the positive electrode or negative electrode-active materials is a semi-solid or is a condensed ion-storing electroactive material, and in which at least one of the electrode-active materials is transported to and from an assembly at which the electrochemical reaction occurs, producing electrical energy. The electronic conductivity of the semi-solid is increased by the addition of conductive particles to suspensions and / or via the surface modification of the solid in semi-solids (e.g., by coating the solid with a more electron conductive coating material to increase the power of the device). High energy density and high power redox flow devices are disclosed. The redox flow devices described herein can also include one or more inventive design features. In addition, inventive chemistries for use in redox flow devices are also described.
Owner:MASSACHUSETTS INST OF TECH +2
Biosensor apparatus and methods of use
InactiveUS20060134713A1Increase in the amount of probe boundReduce the amount requiredBioreactor/fermenter combinationsBiological substance pretreatmentsElectrochemical responseAnalyte
Disclosed herein are methods and devices for detecting the presence of an analyte of interest. A biosensor device can include a reaction chamber and an electrochemical detection chamber. The reaction chamber can include at least one immobilized binding site and a probe conjugate adapted to bind to at least one of the target analyte and the immobilized binding site, while the detection chamber can include electrodes for detecting an electrochemical reaction. If present, the target analyte in the fluid sample results in a change in the amount of probe conjugate bound in the reaction chamber, which can be detected electrochemically in the detection chamber.
Owner:UNIVERSAL BIOSENSORS
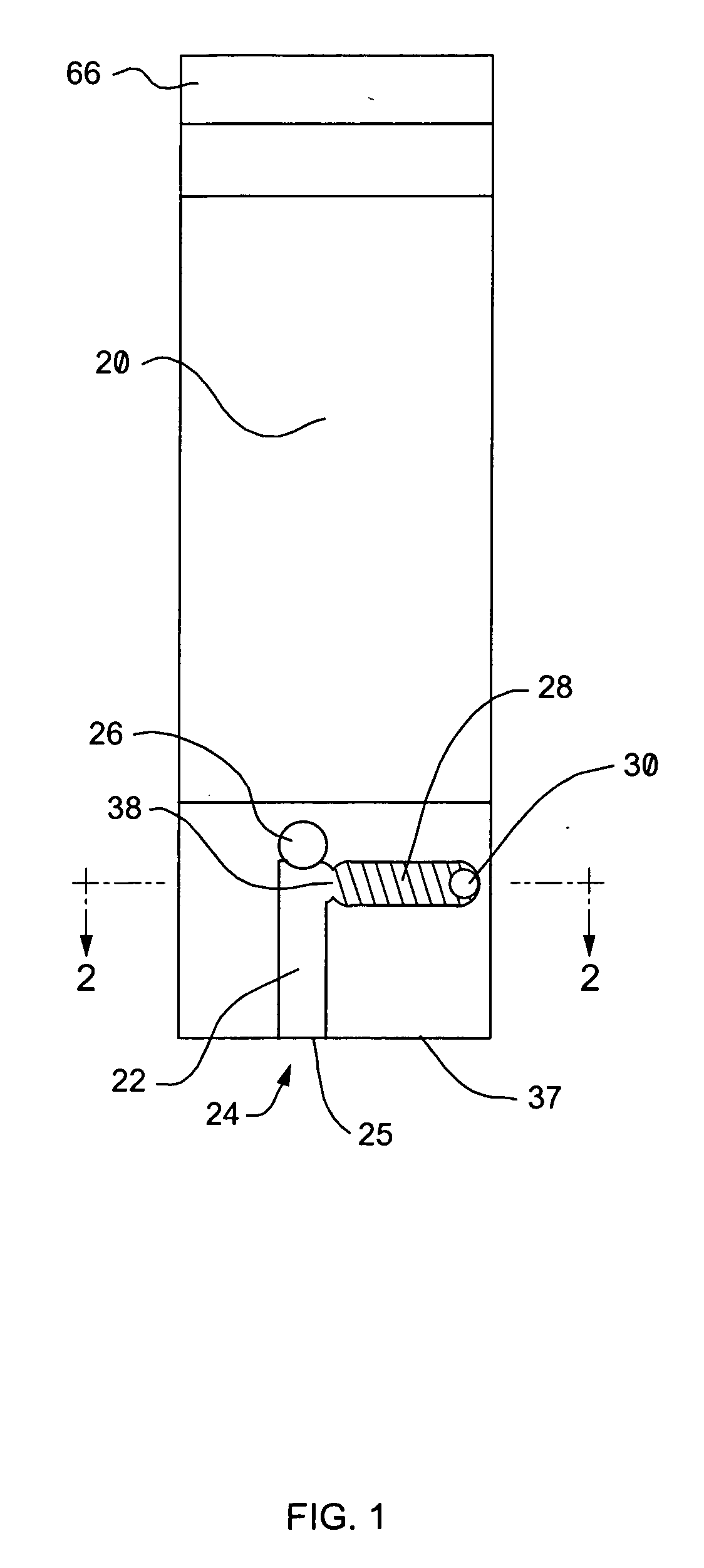
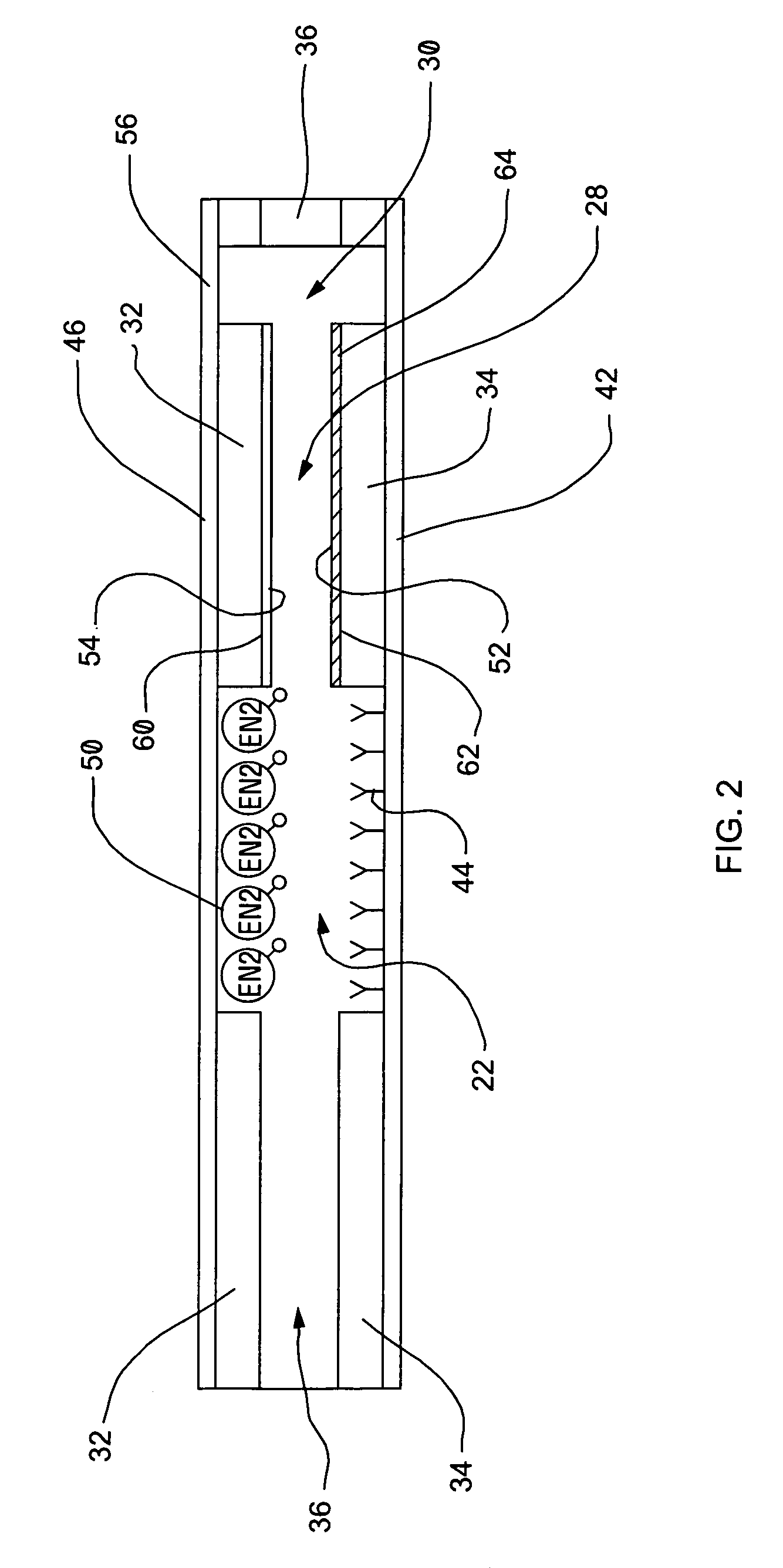
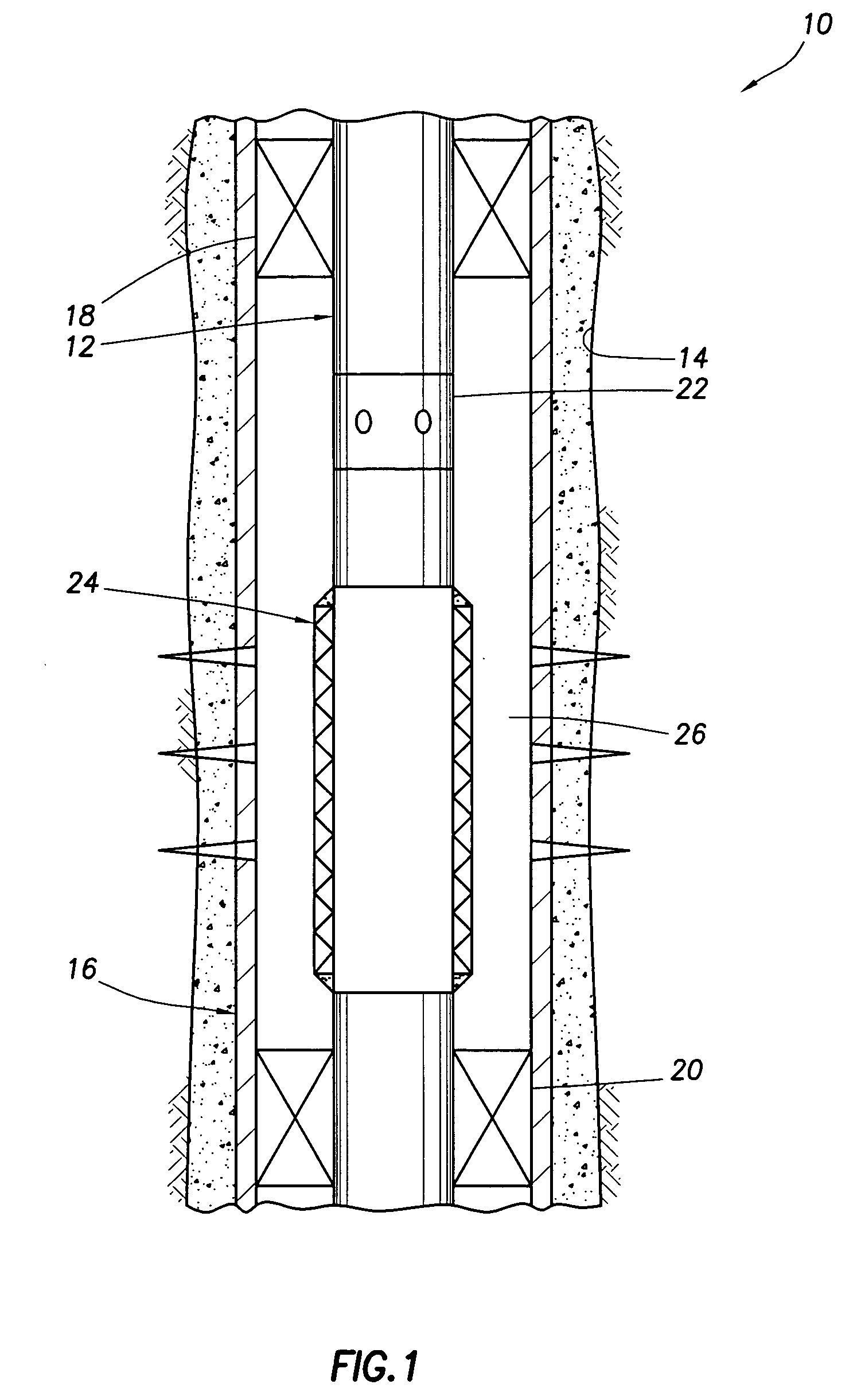
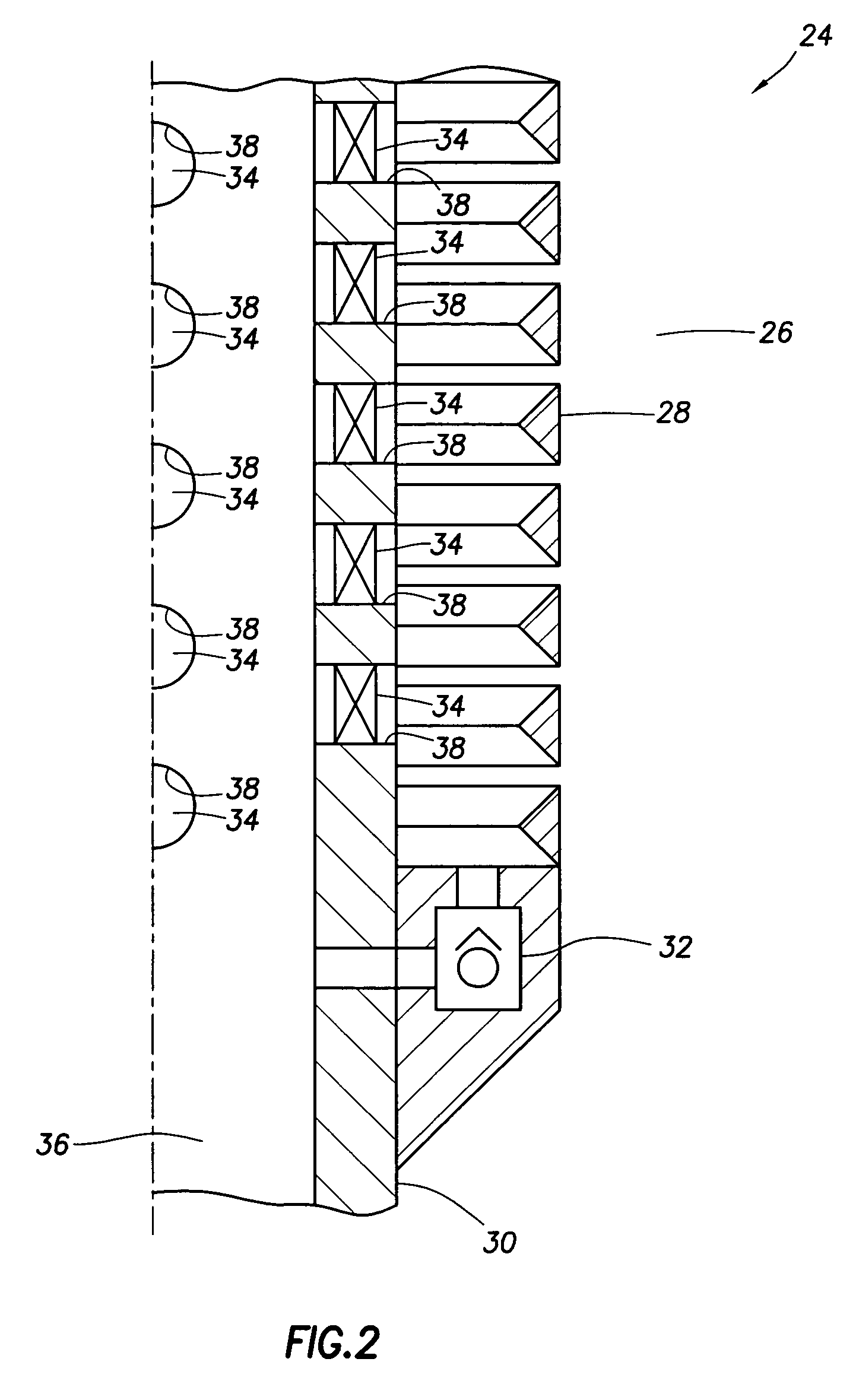
Well system having galvanic time release plug
ActiveUS20080135249A1Speed up circulationFluid removalSealing/packingElectrical batteryElectrochemical response
A well system having a galvanic time release plug. A well system includes a flow passage and a flow blocking device which selectively obstructs flow through the passage, the device including an electrode in a galvanic cell. A flow blocking device for use in conjunction with a subterranean well includes a portion which delays an electrochemical reaction in a galvanic cell. A method of controlling fluid flow in a well system includes the steps of: obstructing flow through a passage using a flow blocking device which includes an electrode of a galvanic cell; and increasing flow through the passage by operation of the galvanic cell.
Owner:HALLIBURTON ENERGY SERVICES INC
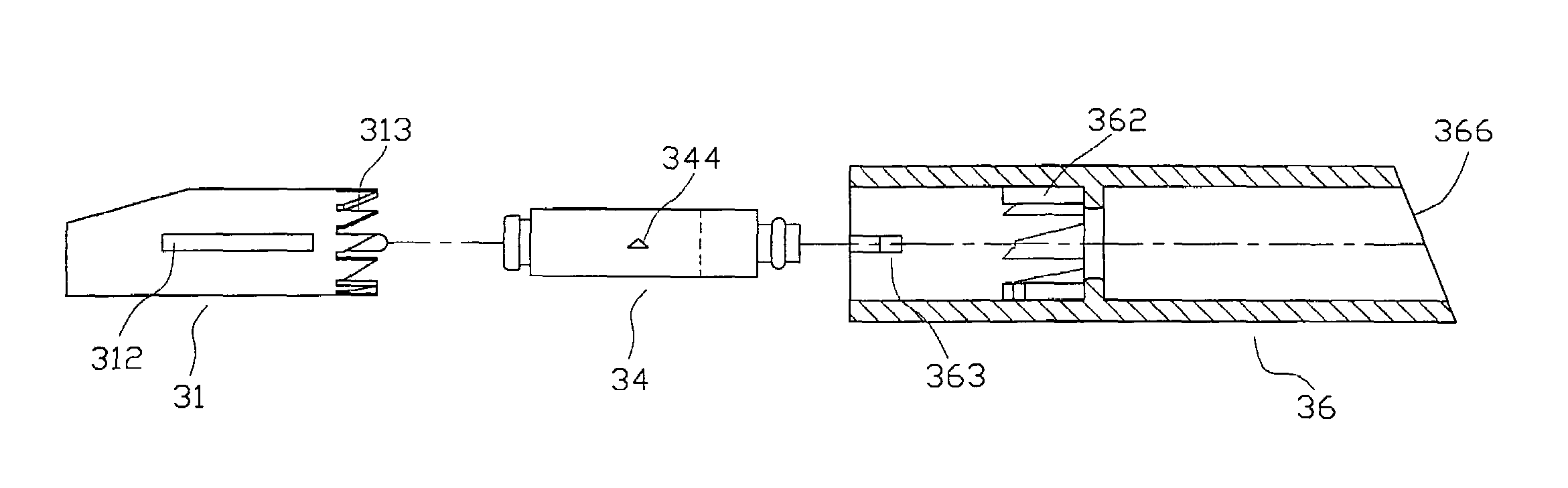
Electrode structure for lithium secondary battery and secondary battery having such electrode structure
InactiveUS20060127773A1Small capacity reductionLarge capacityElectrode manufacturing processesFinal product manufactureElectrochemical responseConductive polymer
In an electrode structure for a lithium secondary battery including: a main active material layer formed from a metal powder selected from silicon, tin and an alloy thereof that can store and discharge and capable of lithium by electrochemical reaction, and a binder of an organic polymer; and a current collector, wherein the main active material layer is formed at least by a powder of a support material for supporting the electron conduction of the main active material layer in addition to the metal powder and the powder of the support material are particles having a spherical, pseudo-spherical or pillar shape with an average particle size of 0.3 to 1.35 times the thickness of the main active material layer. The support material is one or more materials selected from a group consisting of graphite, oxides of transition metals and metals that do not electrochemically form alloy with lithium. Organic polymer compounded with a conductive polymer is used for the binder. There are provided an electrode structure for a lithium secondary battery having a high capacity and a long lifetime, and a lithium secondary battery using the electrode structure and having a high capacity, a high energy density and a long lifetime.
Owner:CANON KK
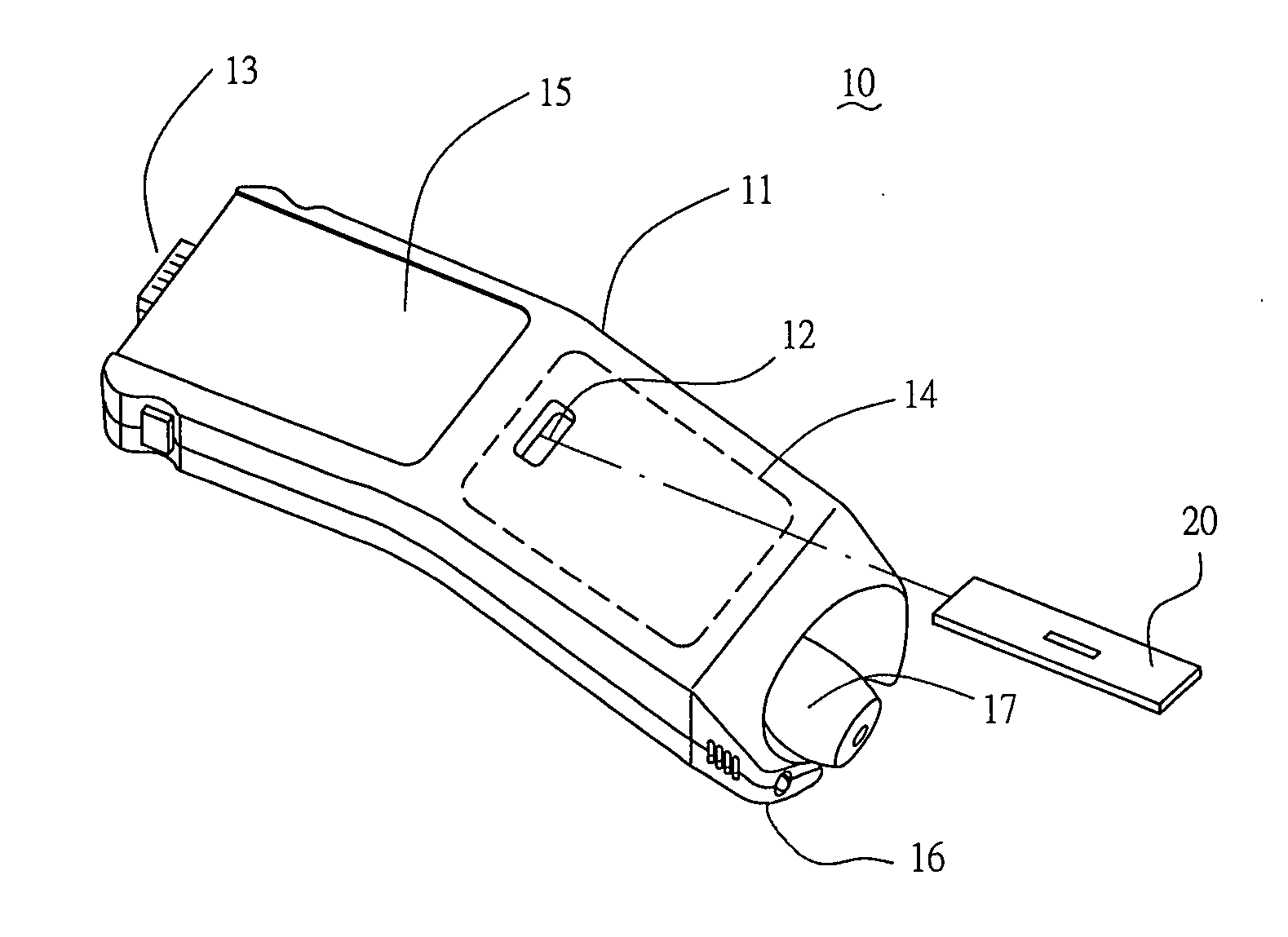
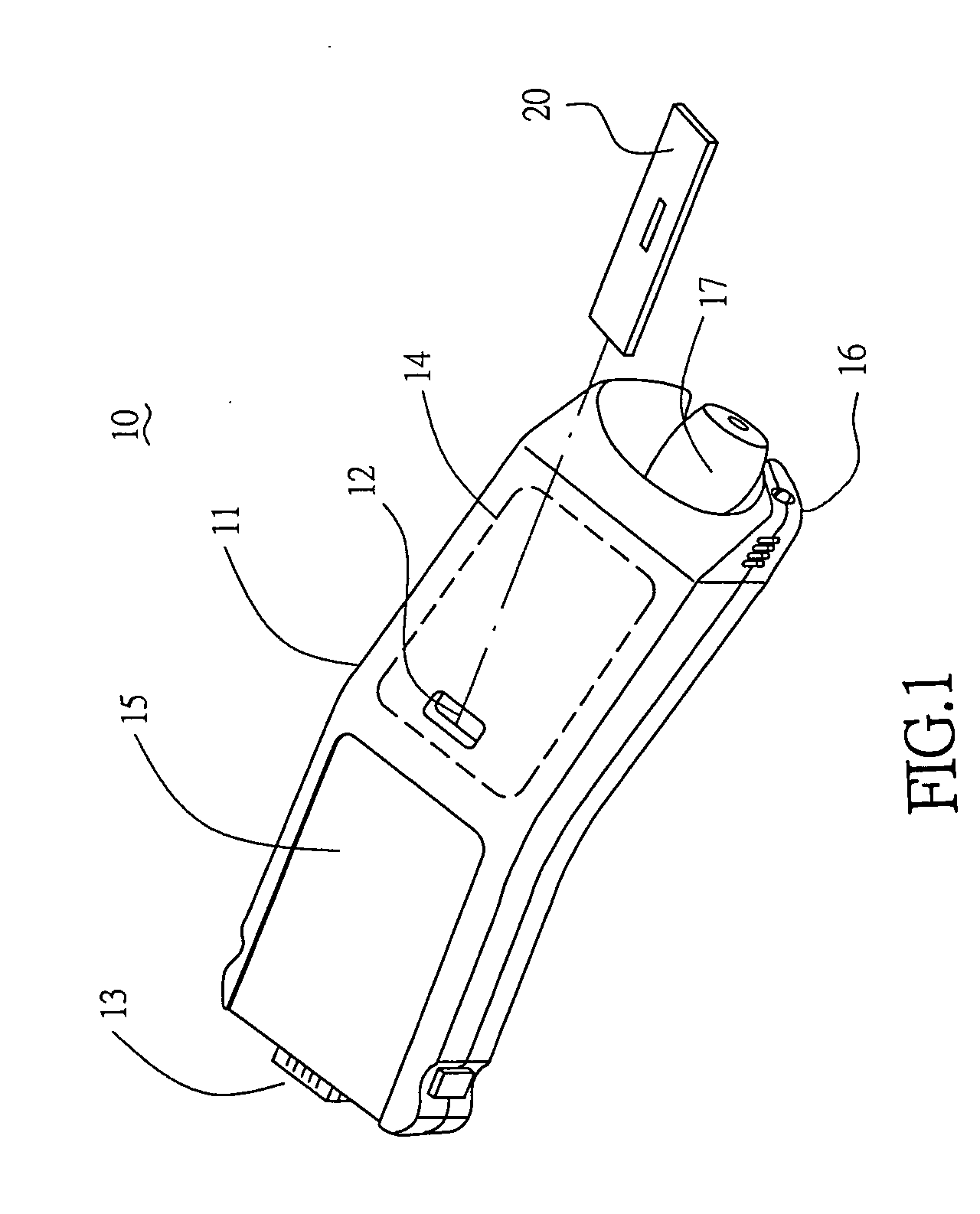
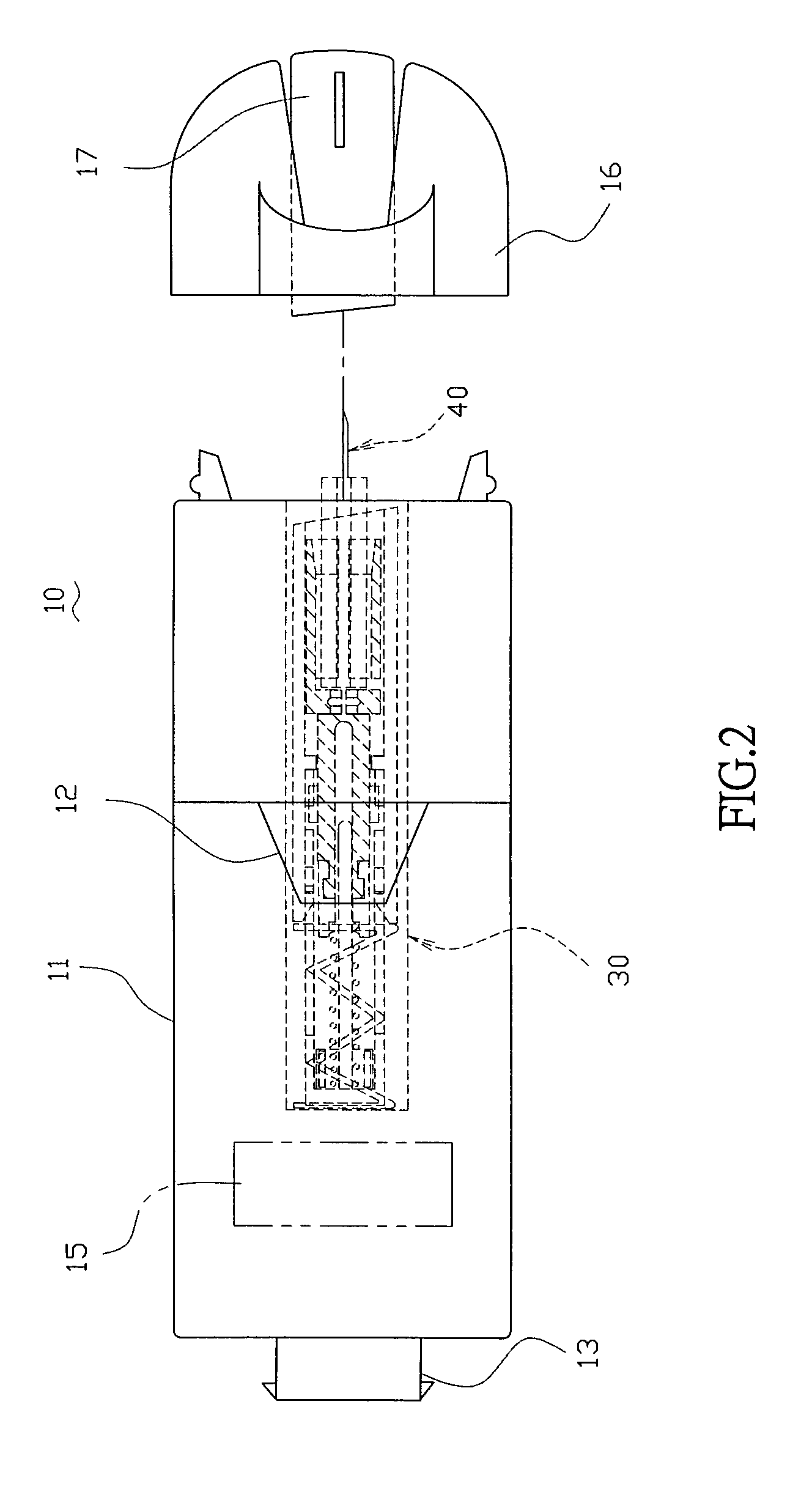
Handheld diagnostic device with renewable biosensor
A handheld diagnostic device having a test head and a handle is equipped with an open test channel having sensors and liquid reagent dispensing opening for the diagnostic testing of body fluids. The test channel can draw in fluid sample by capillary force and be closed by a channel cover for mixing the fluid sample with liquid reagent for electrochemical reactions for providing measurement signals for diagnostic analysis by a microprocessor included in the handle. A vibration means is added for stimulating the production of the body fluid sample and for assisting mixing of the sample solution. A renewable biosensor having a reusable electrode system and a dispensing means for providing a new dose of liquid reagent is included in the test head for repeated uses of the test channel and the biosensor. A dual-dispensers system having two reagent cartridges and two dispensing lines is included for simultaneous or selective dispensing of reagents for multiple diagnostic testing. The handheld device can be used for the self-diagnostic testing of saliva, body fluid, blood and vagina fluid for home healthcare and for monitoring predetermined components in a pourable fluid. For vagina fluid applications, a handheld diagnostic device may include cream or foam dispenser for dispensing vagina medication material, lubricant, or spermicide.
Owner:KUO YOUTI
Electrochemical timer
InactiveUS6198701B1Easy to useReduce manufacturing costMeasurement devicesTime interval measurement without driving mechanismElectrochemical responseReactive material
An electrochemical timer is described that is compact, lightweight, inexpensive to manufacture, and simple to use in which the consumption of reactive materials in an electrochemical reaction provides a visual indication of the passage of time. In one embodiment, the electrochemical timer includes a first electrode, an electrolyte, and a second electrode. The electrodes and electrolyte are chosen such that the first electrode is consumed at a predetermined rate upon electrochemical contact of the electrodes and electrolyte. The electrodes and electrolyte are further configured such that the consumption of the first electrode can be monitored to provide an indication of the passage of time. In one embodiment, the electrochemical timers provided herein are used to monitor the shelf-life of perishables.
Owner:POLYPLUS BATTERY CO INC
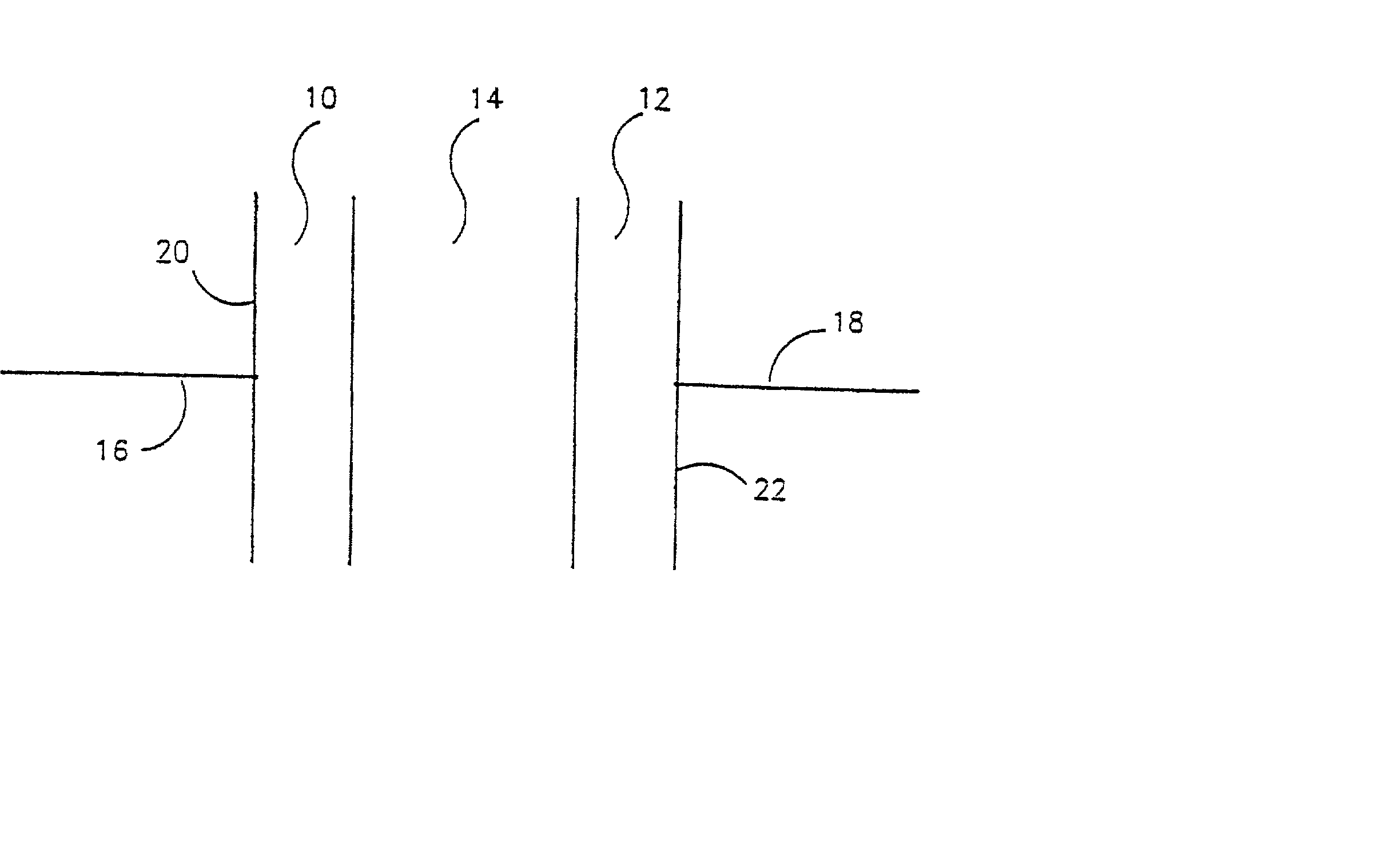
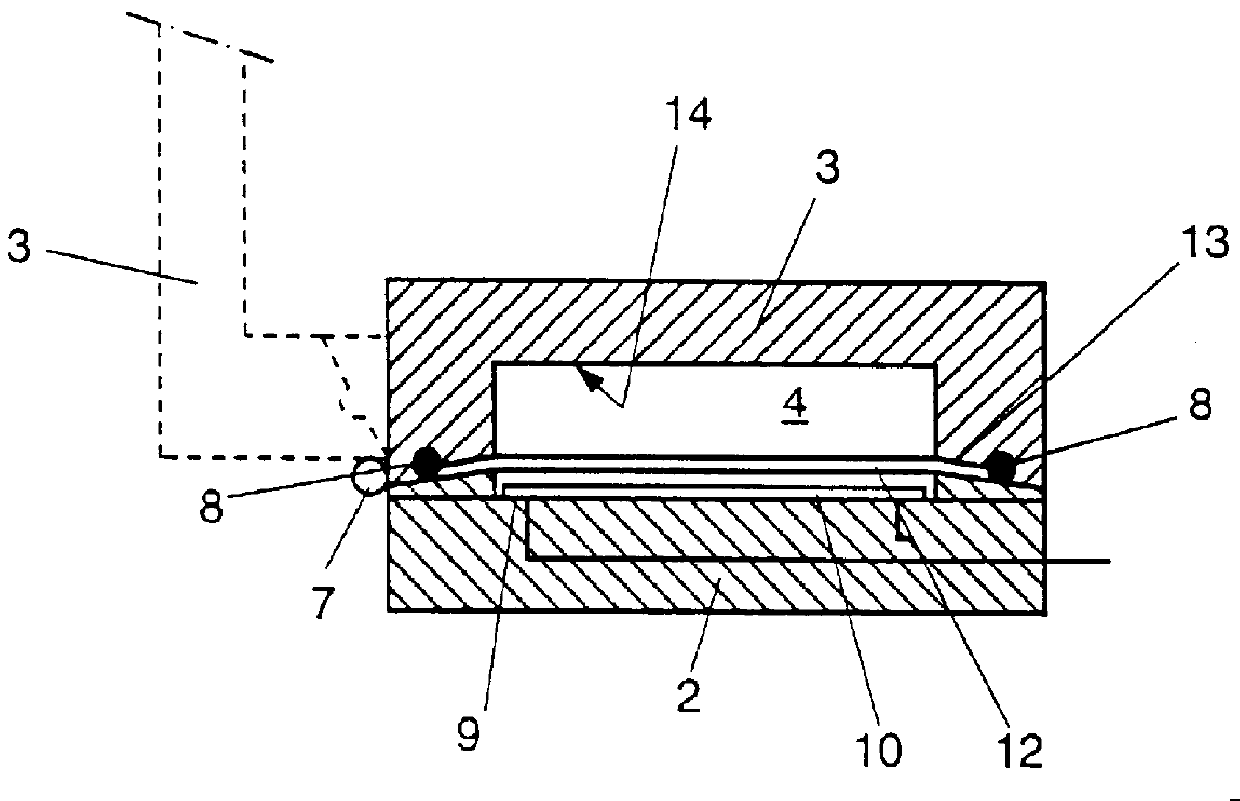
Electrochemical cell with a non-liquid electrolyte
InactiveUS20020122980A1High ambient temperature proton conductivityNon-corrosive materialSilver accumulatorsSolid electrolytesElectrochemical responseElectrochemical cell
A non-liquid electrolyte containing electrochemical cell which operates efficiently at room temperature. The cell includes: (a) a non-liquid electrolyte (14) in which protons are mobile; (b) an anode (10) including an active material based on the organic compound which is a source of protons during cell discharge; and (c) a solid cathode (12) including a compound which forms an electrochemical couple with the anode. The active materials can be chosen so that the cell has the feature that the electrochemical reactions at the anode and cathode are at least partially reversible. An important feature of the cell is that no thermal activation is required for its operation. Therefore, the cell efficiently operates under ambient temperatures.
Owner:FLEISCHER NILES A +4
Electrochemical method of producing nano-scaled graphene platelets
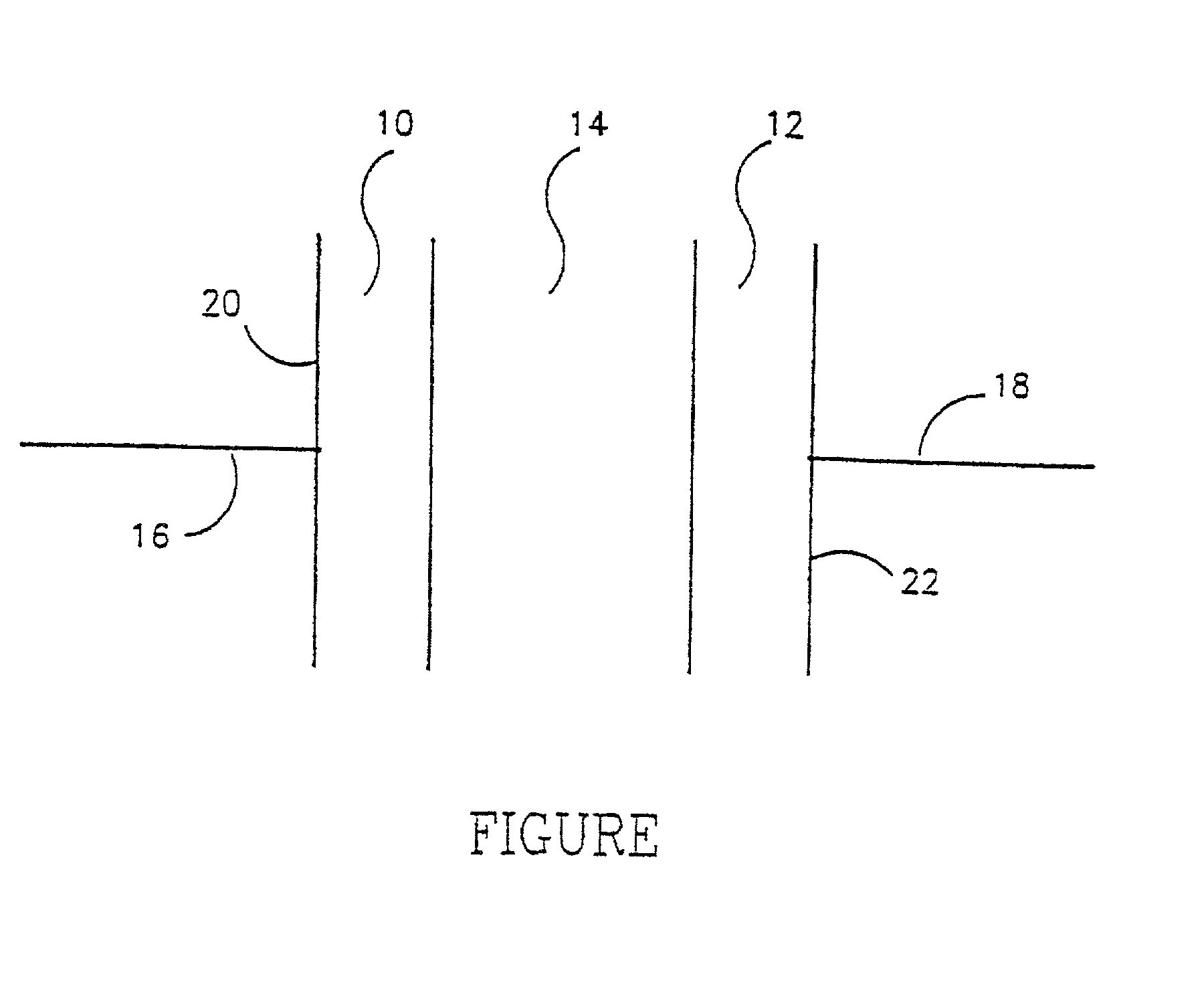
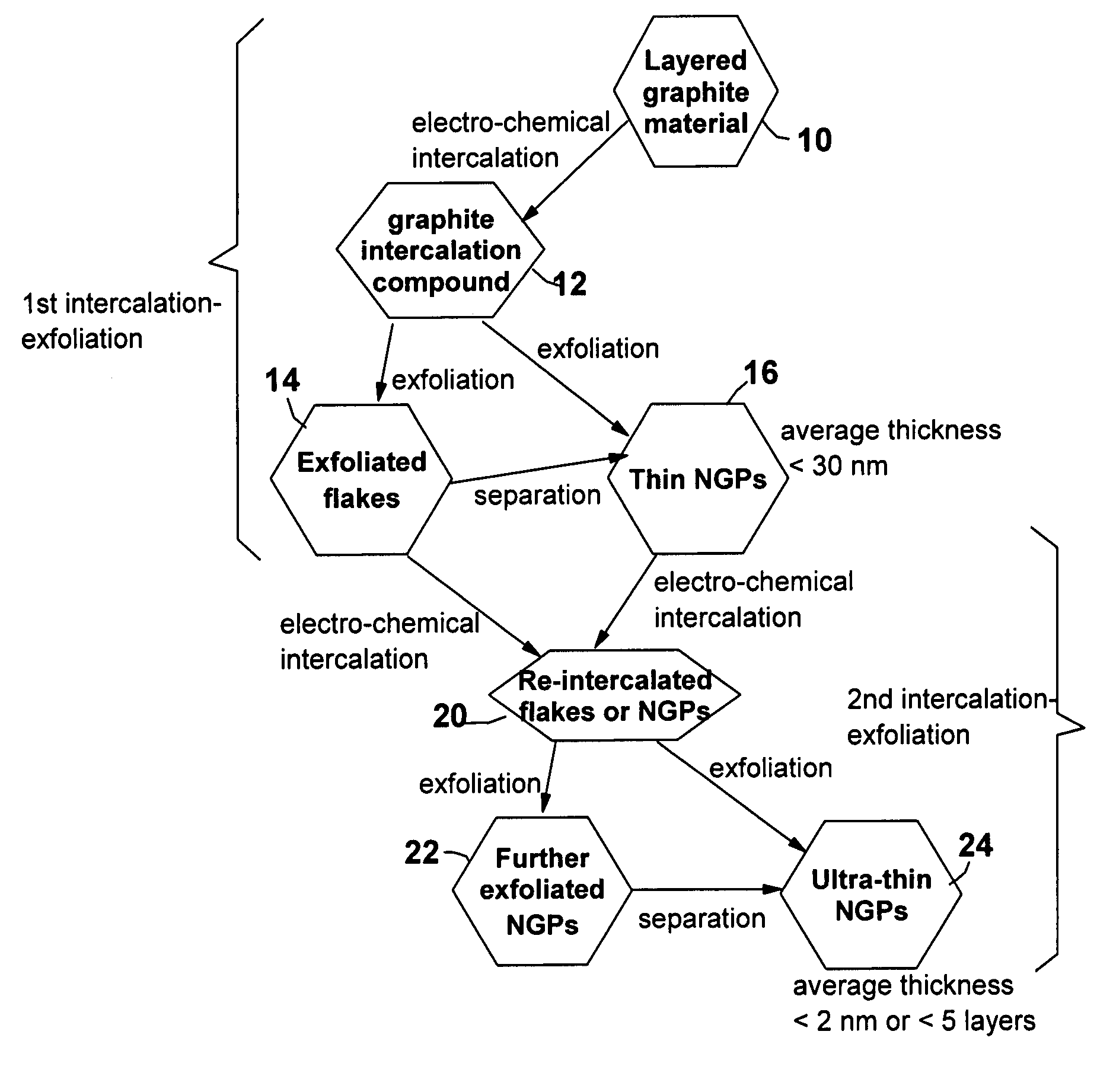
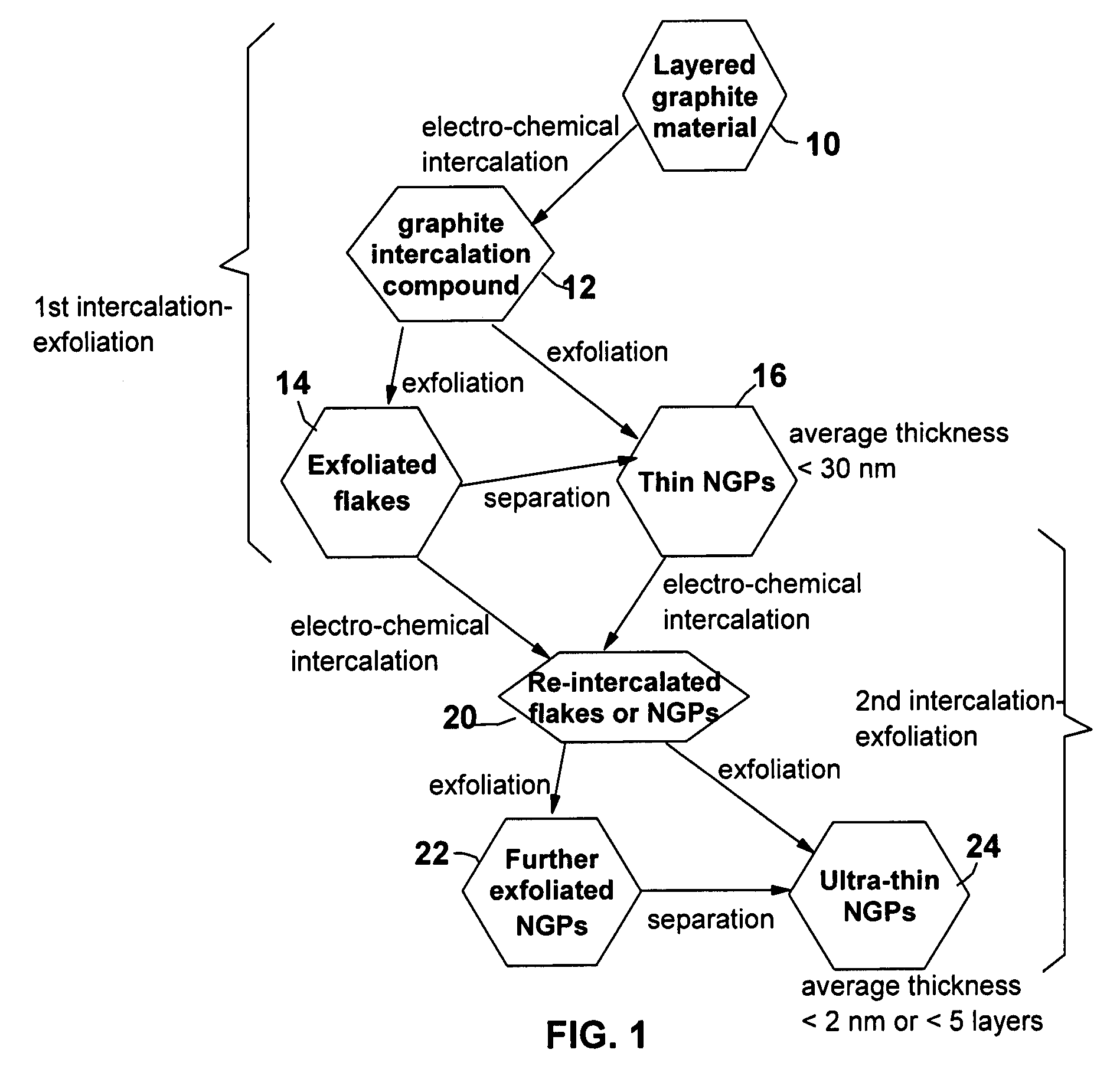
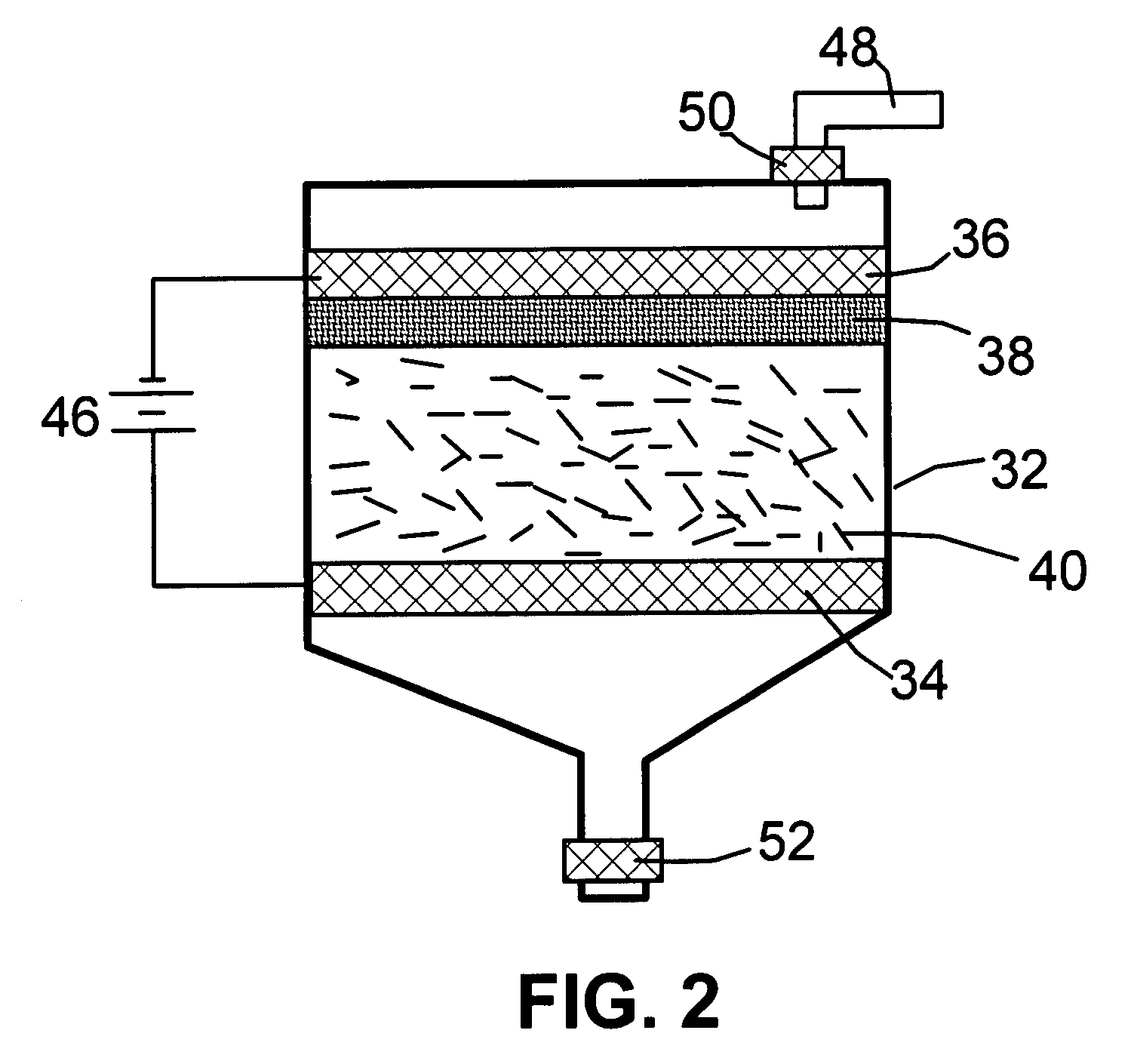
A method of producing nano-scaled graphene platelets with an average thickness smaller than 30 nm from a layered graphite material. The method comprises (a) forming a carboxylic acid-intercalated graphite compound by an electrochemical reaction which uses a carboxylic acid as both an electrolyte and an intercalate source, the layered graphite material as an anode material, and a metal or graphite as a cathode material, and wherein a current is imposed upon the cathode and the anode at a current density for a duration of time sufficient for effecting the electrochemical reaction; (b) exposing the intercalated graphite compound to a thermal shock to produce exfoliated graphite; and (c) subjecting the exfoliated graphite to a mechanical shearing treatment to produce the nano-scaled graphene platelets. Preferred carboxylic acids are formic acid and acetic acid. The exfoliation step in the instant invention does not involve the evolution of undesirable species, such as NOx and SOx, which are common by-products of exfoliating conventional sulfuric or nitric acid-intercalated graphite compounds. The nano-scaled platelets are candidate reinforcement fillers for polymer nanocomposites. Nano-scaled graphene platelets are much lower-cost alternatives to carbon nano-tubes or carbon nano-fibers.
Owner:GLOBAL GRAPHENE GRP INC
Apparatus and methods for performing electrochemical reactions
ActiveUS8673627B2Reduce noiseBioreactor/fermenter combinationsBiological substance pretreatmentsElectrochemical responseAnalyte
The invention is directed to apparatus and methods for delivering multiple reagents to, and monitoring, a plurality of analytical reactions carried out on a large-scale array of electronic sensors underminimal noise conditions. In one aspect, the invention provides method of improving signal-to-noise ratios of output signals from the electronic sensors sensing analytes or reaction byproducts by subtracting an average of output signals measured from neighboring sensors where analyte or reaction byproducts are absent. In other aspects, the invention provides an array of electronic sensors integrated with a microwell array for confining analytes and / or particles for analytical reactions and a method for identifying microwells containing analytes and / or particles by passing a sensor-active reagent over the array and correlating sensor response times to the presence or absence of analytes or particles. Such detection of analyte- or particle-containing microwells may be used as a step in additional noise reduction methods.
Owner:LIFE TECH CORP
Electrolytic production of hydrogen
InactiveUS6890419B2Oxide/hydroxide preparationOrganic-compounds/hydrides/coordination-complexes catalystsElectrochemical responseElectrolysis
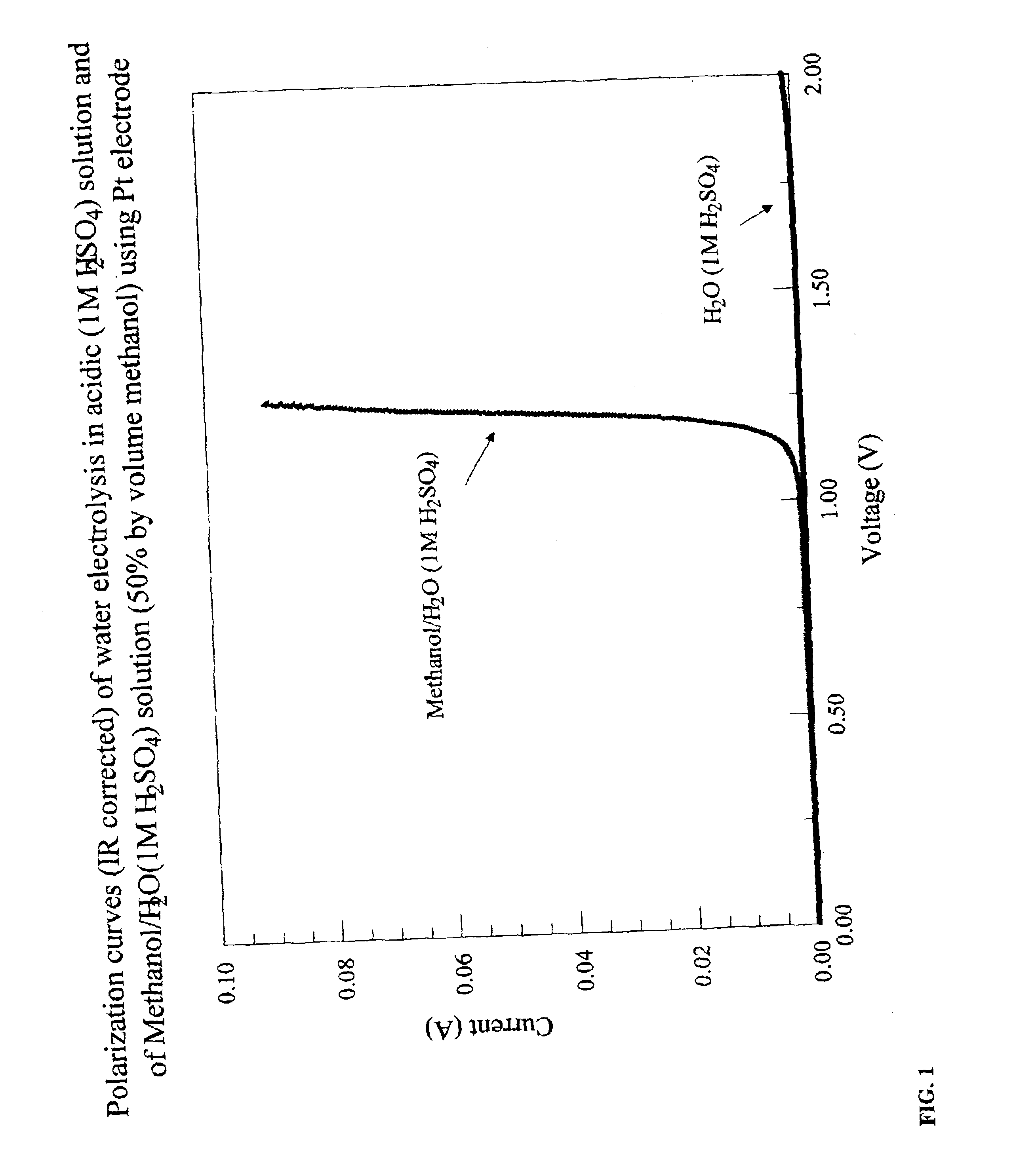
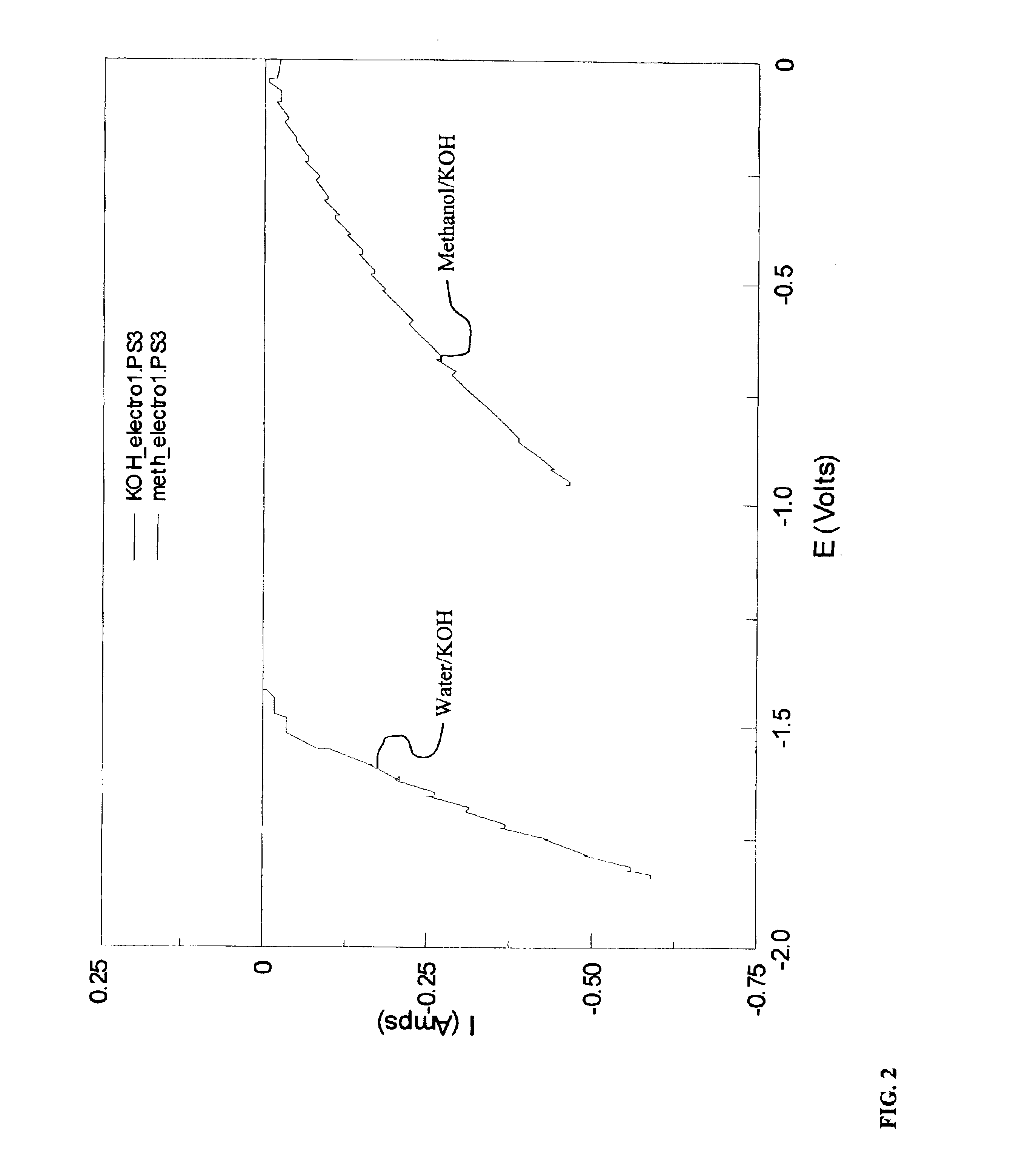
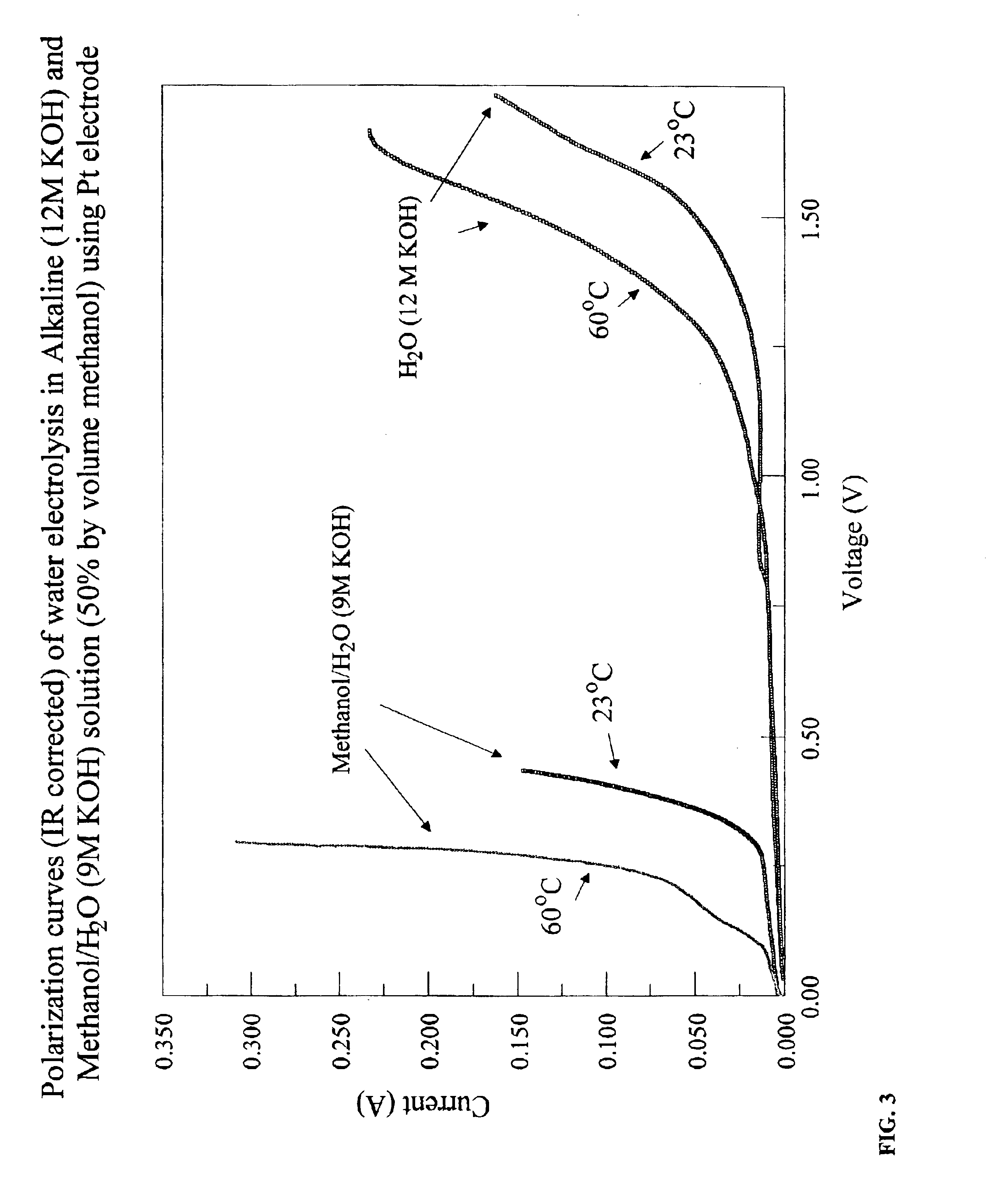
Methods for producing hydrogen gas from organic substances. According to the methods, hydrogen is produced from an electrochemical reaction of an organic substance with water or a base. The instant methods permit the production of hydrogen at lower operating voltages or lower operating temperatures relative to water electrolysis. Operable organic substances include alcohols, ethers, carboxylic acids, aldehydes, and ketones. In a preferred embodiment, hydrogen gas is produced from an electrochemical reaction of methanol in the presence of a base such as NaOH or KOH.
Owner:TACTICAL FUEL CELLS
Compact structure of a new biosensor monitor
The invention discloses a new two-in-one biosensor monitor includes a housing to accommodate a printed circuit board for data analysis from the electrochemical reaction on the inserted test strip, a communication port to transmit signals, a LCD screen to show test result, and a lancer for blood inoculation which requires only one finger to activate the release of needle, unlike all other conventional lancers which needs two hands to operate. This new monitor has both the function of regular biosensor monitor and the lancer to make it more compact in size and facilitates portability.
Owner:CHANG YU HONG
Compact structure of a new biosensor monitor
Owner:CHANG YU HONG
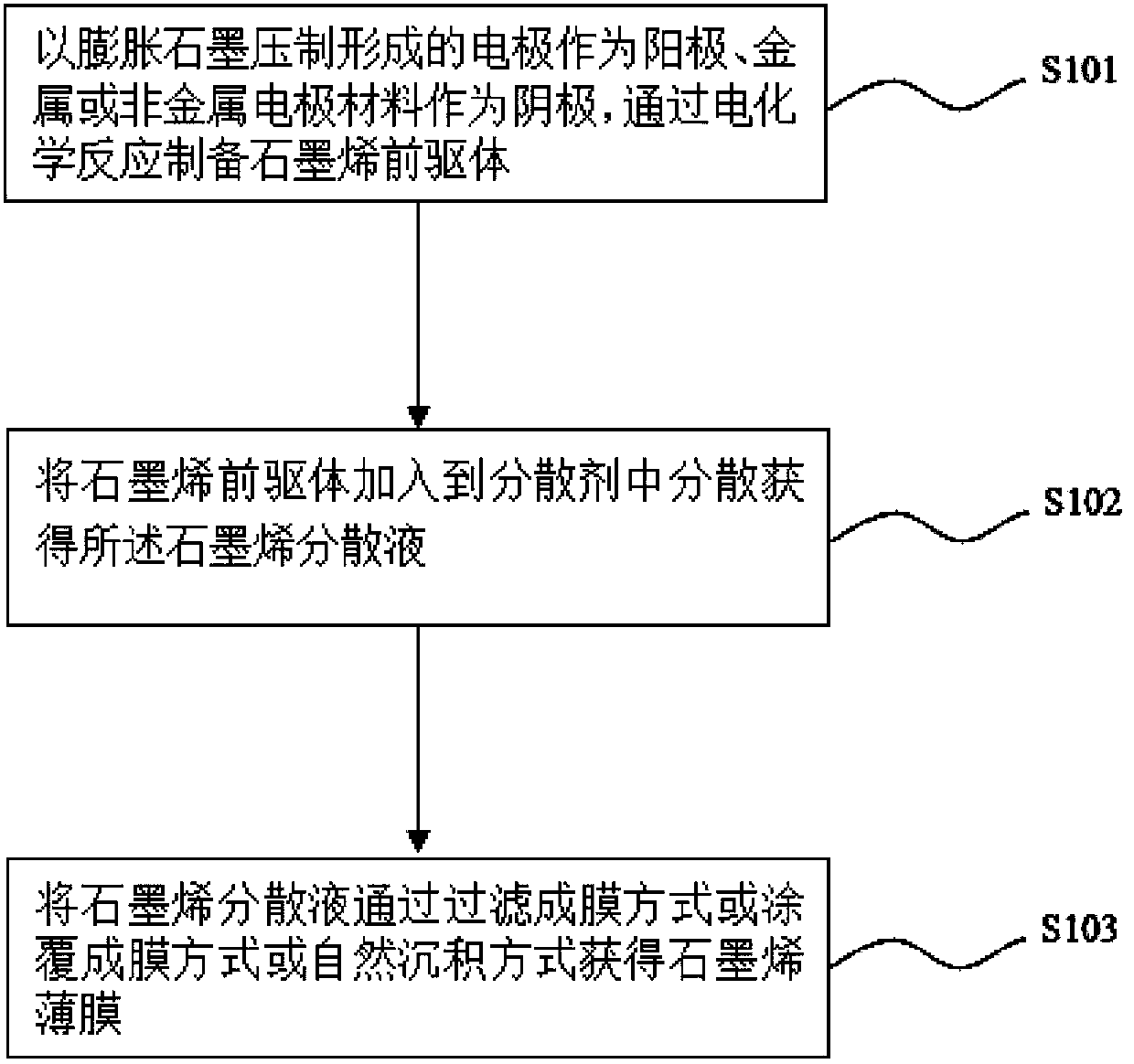
Preparation method of graphene dispersion liquid, and preparation method of graphene film
ActiveCN103466603AIncrease layer spacingIncrease concentrationMaterial nanotechnologyGrapheneElectrochemical responseMetallic electrode
The invention discloses a preparation method of a graphene dispersion liquid. The preparation method of the graphene dispersion liquid comprises the following steps: placing an expanded graphite compacted electrode and a metallic or non-metallic electrode material in an electrolyte solution as an anode and a cathode respectively, and applying a voltage of 1-20V and / or a current having a density of 1-200mA / cm<2> between the anode and the cathode for carrying out an electrochemical reaction for 1-120min; and washing an anode product obtained after the electrochemical reaction, adding to a dispersant, and carrying out ultrasonic or / and mechanical stirring dispersion to obtain the graphene dispersion liquid. The invention also discloses a preparation method of a graphene film. The graphene dispersion liquid forms the graphene film on a substrate in a filter film forming mode or a coating film forming mode or a natural deposition mode. The preparation methods have the advantages of simple process, easy operation, high controllability, low cost and mild reaction condition, and are suitable for the industrialized large-scale production.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Electrode material for anode of rechargeable lithium battery, electrode structural body using said electrode material, rechargeable lithium battery using said electrode structural body, process for producing said electrode structural body, and process for producing said rechargeable lithium battery
InactiveUS6949312B1Improve featuresLarge capacitySilver accumulatorsFinal product manufactureElectrochemical responseElectrode material
An electrode material for an anode of a rechargeable lithium battery, containing a particulate comprising an amorphous Sn.A.X alloy with a substantially non-stoichiometric ratio composition. For said formula Sn.A.X, A indicates at least one kind of an element selected from a group consisting of transition metal elements, X indicates at least one kind of an element selected from a group consisting of O, F, N, Mg, Ba, Sr, Ca, La, Ce, Si, Ge, C, P, B, Pb, Bi, Sb, Al, Ga, In, Tl, Zn, Be, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, As, Se, Te, Li and S, where the element X is not always necessary to be contained. The content of the constituent element Sn of the amorphous Sn.A.X alloy is Sn / (Sn+A+X)=20 to 80 atomic %. An electrode structural body for a rechargeable lithium battery, comprising said electrode material for an anode and a collector comprising a material incapable of being alloyed with lithium in electrochemical reaction, and a rechargeable lithium battery having an anode comprising said electrode structural body.
Owner:CANON KK
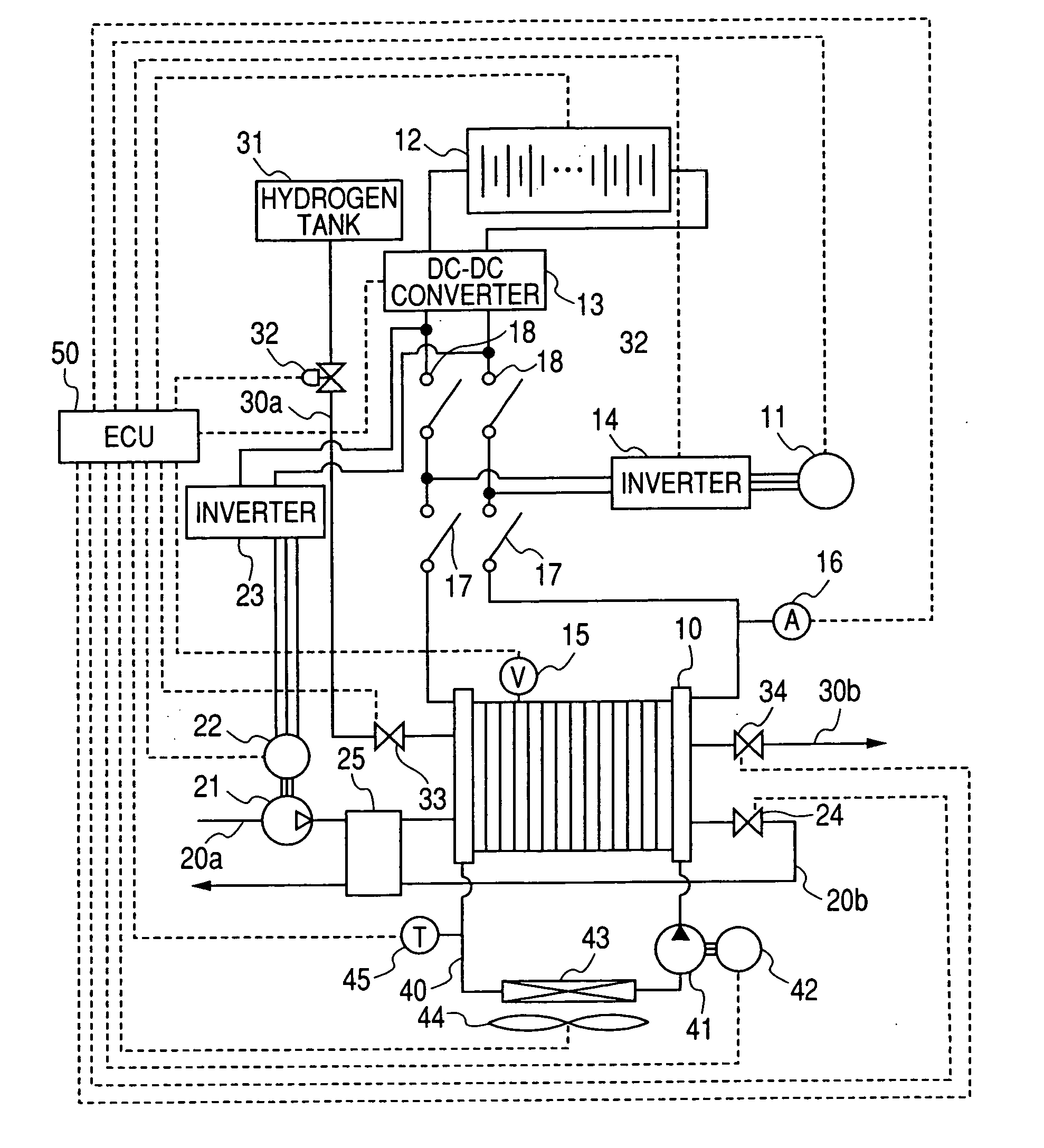
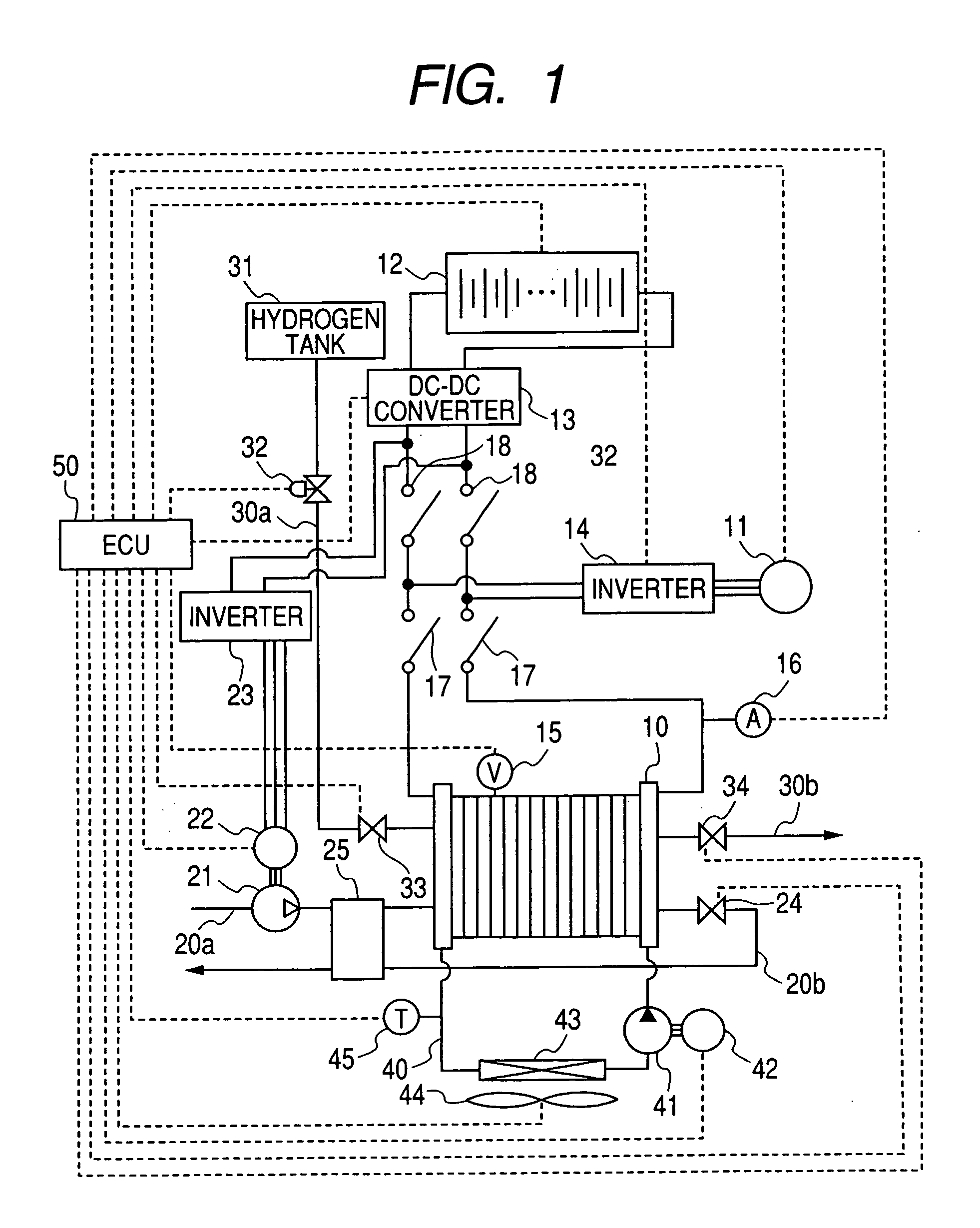
Electric power generation system for vehicle
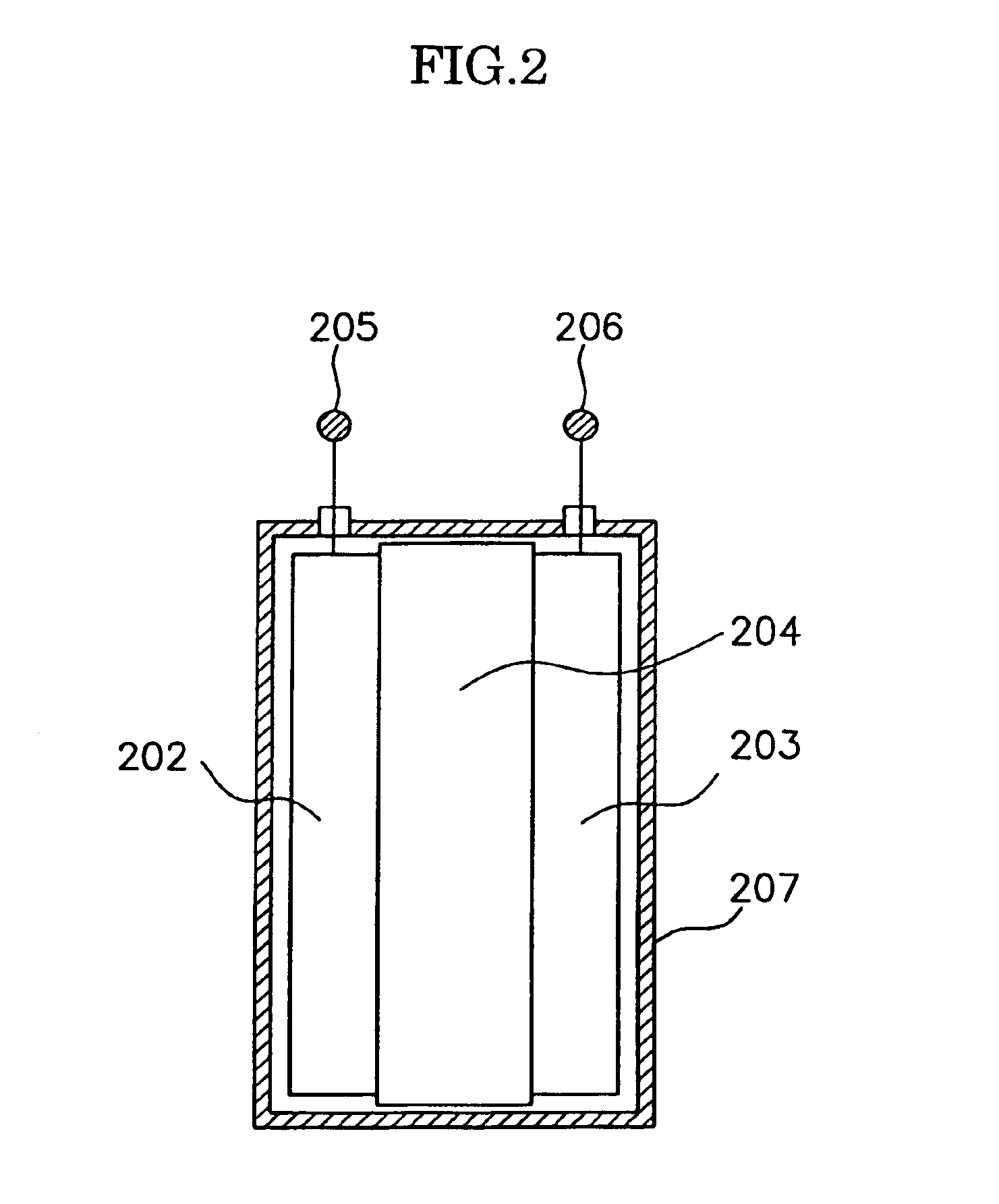
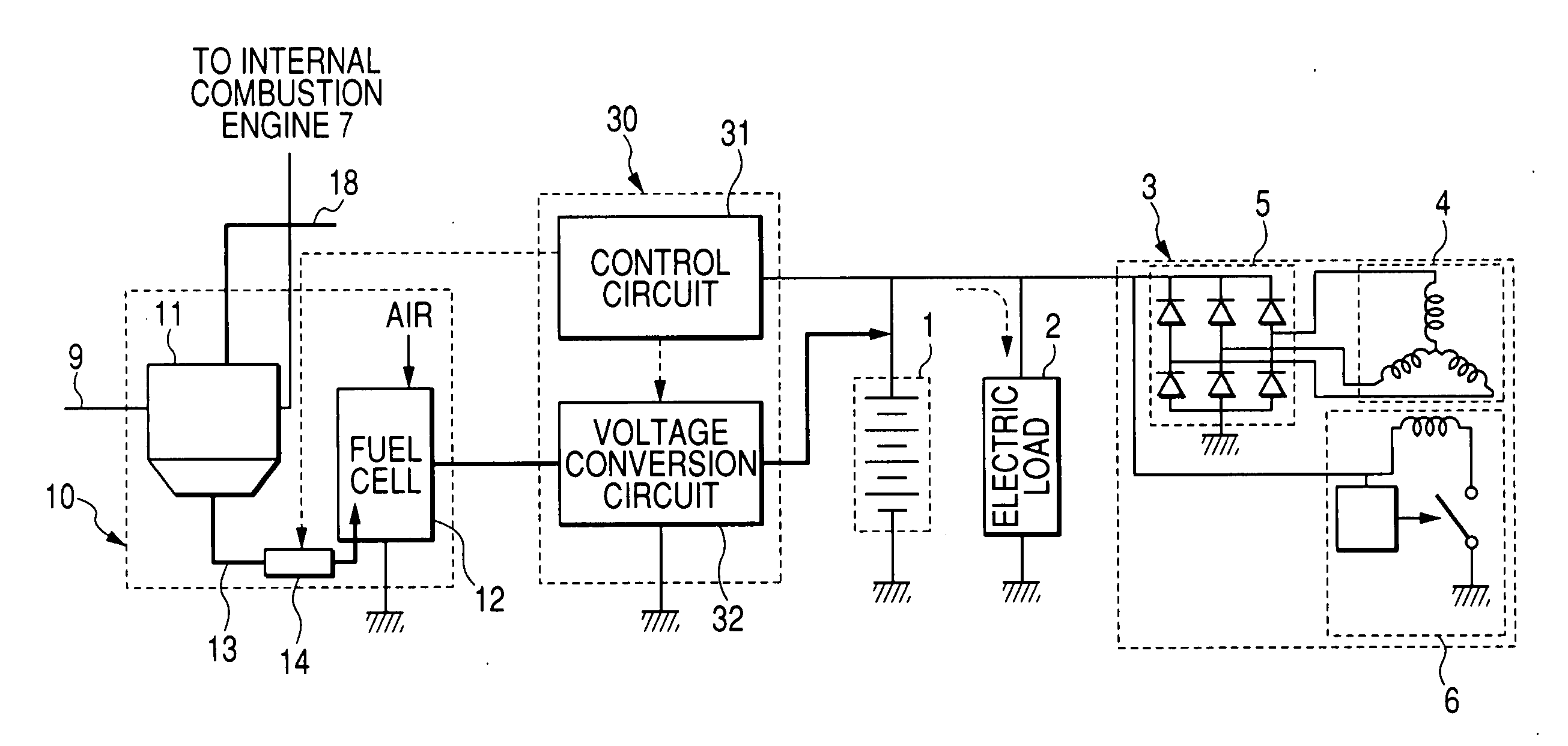
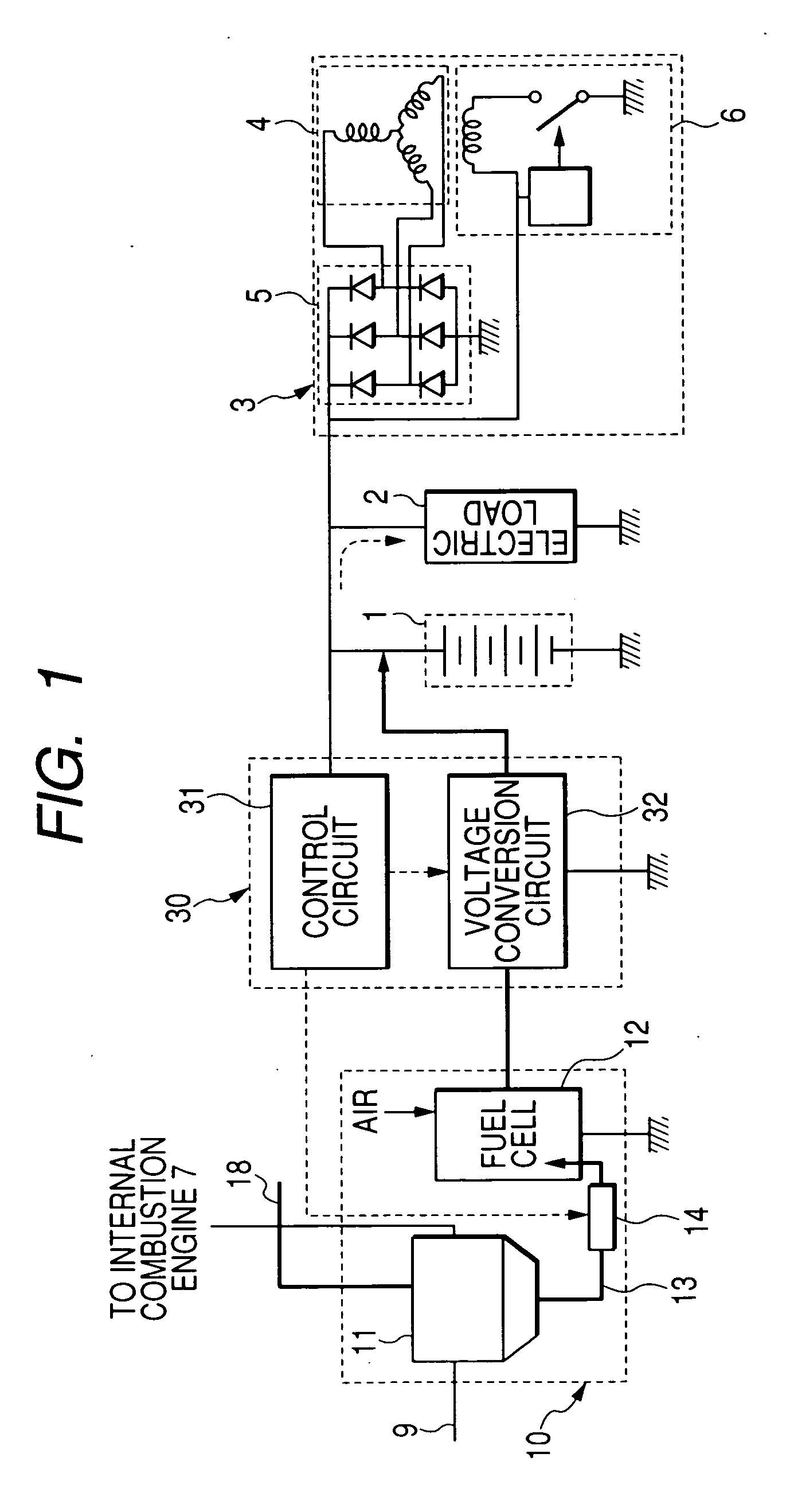
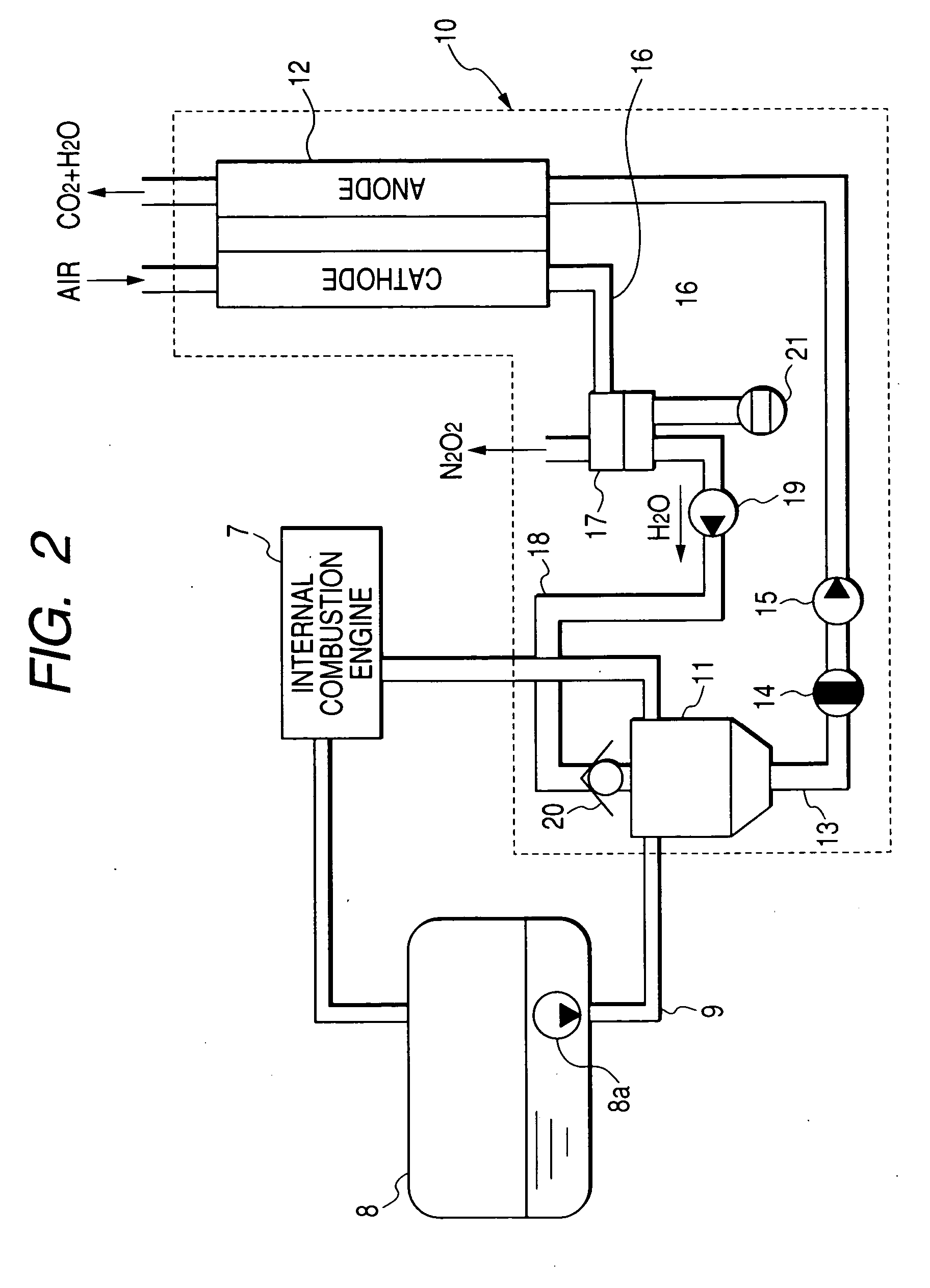
An electric power generation system is mounted on a vehicle having a secondary battery, an electric load, and an internal combustion engine as a driving source consuming gasoline in a mixed fuel which mainly consists of the gasoline and ethanol. The electric power generation system has a fuel cell for generating electric power by electric chemical reaction and a fuel storage unit having an ethanol selection permeable membrane for separating the ethanol from the mixed fuel involving the ethanol and gasoline. On satisfying a given condition, the fuel cell receives the ethanol from the permeable membrane and initiates the generation of electric power, and supplies the generated electric power at least one of the secondary battery and the electric load.
Owner:DENSO CORP
Lithium-sulfur batteries
InactiveUS7250233B2Improved cycle life characteristicsElectrochemical processing of electrodesFinal product manufactureElectrochemical responseLithium–sulfur battery
A lithium-sulfur battery having a positive electrode including a positive active material including an active sulfur, where the positive electrode comprises an electron-conductive path and an ion-conductive path, and includes active pores of the average size of up to 20 μm having both electron-conductive and ion-conductive properties, and are filled with the active sulfur during an electrochemical reaction of the battery.
Owner:SAMSUNG SDI CO LTD
Portable multi-functional electrochemical biosensor system
InactiveUS20040050694A1Immobilised enzymesBioreactor/fermenter combinationsElectrochemical responseElectrochemical biosensor
A portable multi-functional electrochemical biosensor system includes a plurality of sample cells, pluggable information memories and a multi-functional signal analysis processor. The biosensor system uses a set of sample cell and pluggable information memory to detect the concentration of a corresponding selected analyte. Each sample cell has a reaction zone on which a chemical substance is placed to react with the corresponding analyte and has at least two independent electrodes. During detection, each corresponding pluggable information memory can provide parameters used for analysis. The multi-functional signal analysis processor has a microprocessor, an electrically erasable programmable read / write memory and an environmental temperature sensor. The concentration of the selected analyte is calculated by using the electrochemical reaction signal output from the sample cell and the parameters with cooperation of the environmental temperature sensor, and then an analysis result is output.
Owner:APEX TECH CO LTD
Gas component sensor for gas oxides
InactiveUS7001495B2Additional sensorExcellent chemical stability and thermal compatibilityWeather/light/corrosion resistanceElectrolytic capacitorsElectrochemical responseGas composition
The present invention is a gas component sensor comprising novel electrolyte compositions. The electrolyte compositions in bulk, sintered or thin film embodiments are capable of forming with different-metal sensing and reference electrodes a highly stable gas oxide sensors. The novel electrolyte composition changes electrochemical reactions at the sensing and reference electrodes and the overall reaction of the electrodes and electrolyte. The novel electrolyte compositions have: (1) excellent chemical stability and thermal compatibility as to the electrodes and a preferred ceramic substrate, (2) excellent chemical stability with the environment as to the reference and sensing electrodes, which need not be sealed against the atmosphere to be sensed, (3) effective adherence to the substrate and electrode metals.
Owner:AIR Q
Electrochemical assay device and related methods
ActiveUS20050258036A1Stable stateLess timeImmobilised enzymesBioreactor/fermenter combinationsElectrochemical responseEngineering
An electrochemical test device is provided having a base layer with a first electrode thereon and a top layer with a second electrode thereon. The two electrodes are separated by a spacer layer having an opening therein, such that a sample-receiving space is defined with one electrode on the top surface, the other electrodes on the bottom surface and side walls formed from edges of the opening in the spacer. Reagents for performing the electrochemical reaction are deposited on one of the electrodes and on the side walls of the sample-receiving space.
Owner:AGAMATRIX INC
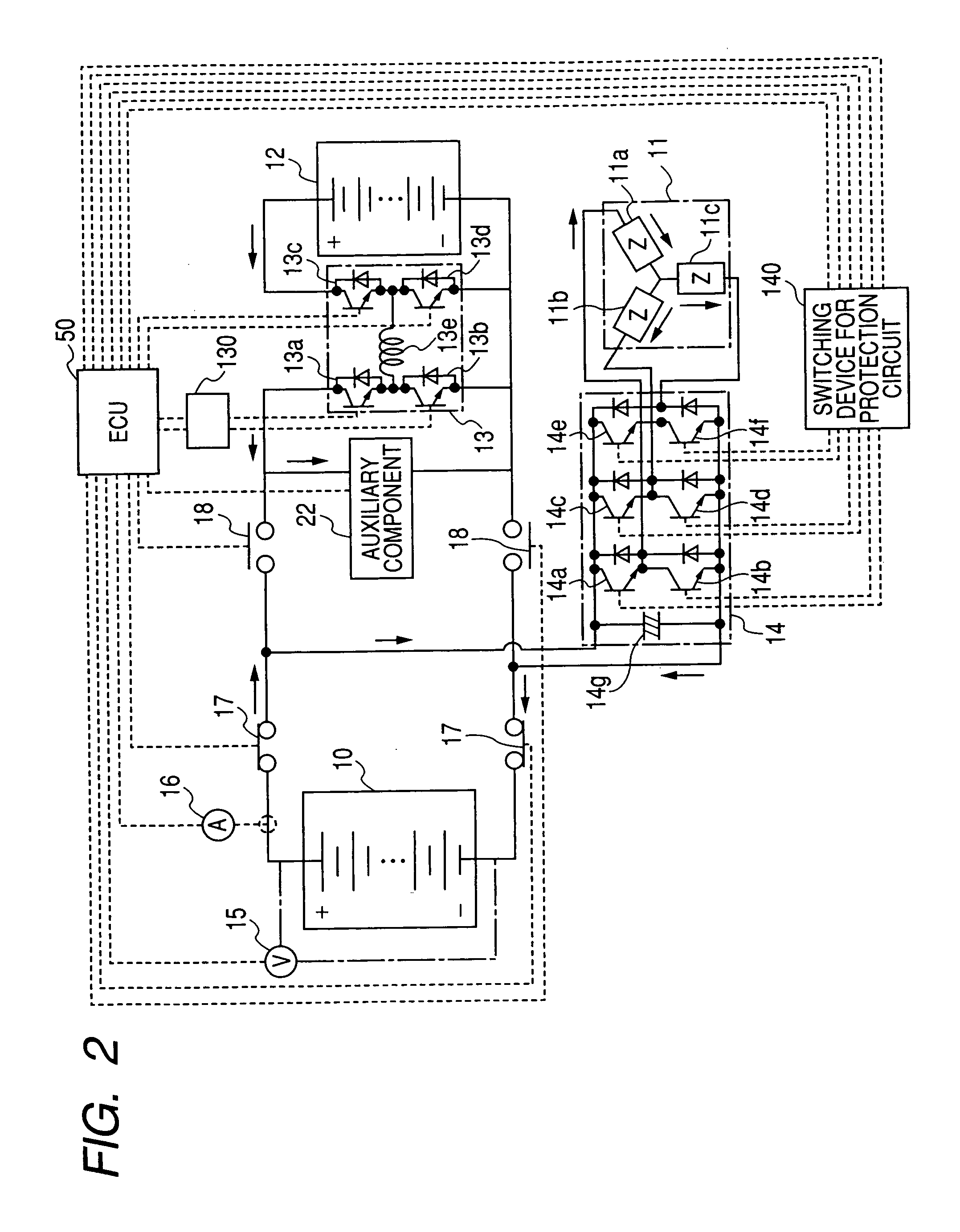
Fuel cell system
InactiveUS20060280977A1Additional heat energyGenerating efficiency is reducedFuel cell heat exchangeBattery/fuel cell control arrangementThermal energyElectrochemical response
A fuel cell system equipped with a fuel cell that generates electrical power in electrochemical reaction of hydrogen and oxygen. The system has the improved cold starting capability that increases heat energy generated in the fuel cell in order to rise the temperature of the fuel cell rapidly in a cold temperature environment. The system has an inverter having plural switching elements connected in series and a control section for controlling ON / OFF operation of the plural switching elements. The control section controls the amount of current output from the fuel cell by performing the ON / OFF control of the switching elements. On commencing the cold starting process of the fuel cell, the control means changes the current path in a drive motor by performing the ON / OFF control of the switching elements, in which both the inverter and the drive motor are used as a variable resistance to the fuel cell.
Owner:DENSO CORP
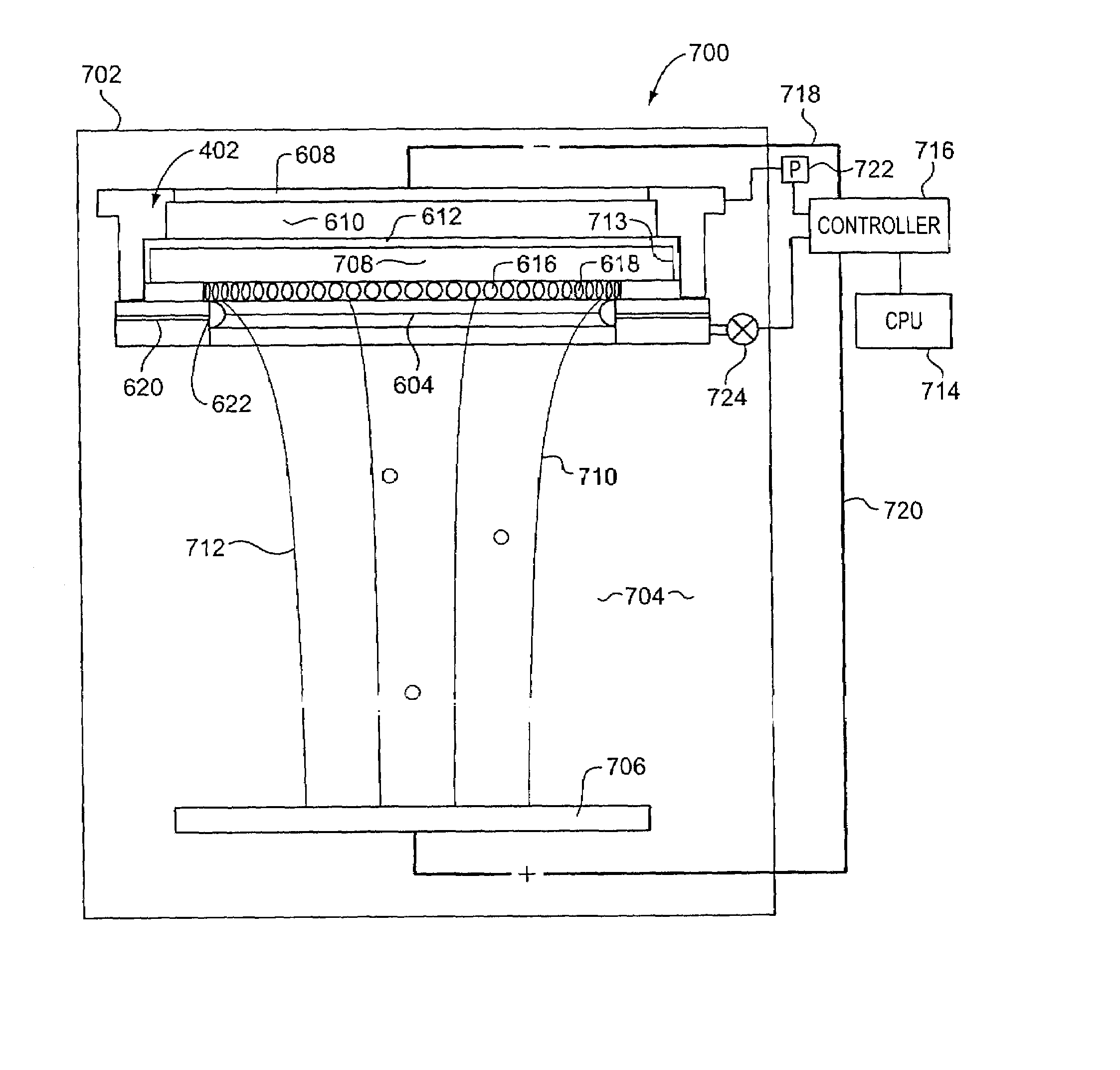
Dynamically variable field shaping element
InactiveUS7070686B2Uniform current distributionUniform currentAnodisationMachining electric circuitsElectrical resistance and conductanceElectrochemical response
In an electrochemical reactor used for electrochemical treatment of a substrate, for example, for electroplating or electropolishing the substrate, one or more of the surface area of a field-shaping shield, the shield's distance between the anode and cathode, and the shield's angular orientation is varied during electrochemical treatment to screen the applied field and to compensate for potential drop along the radius of a wafer. The shield establishes an inverse potential drop in the electrolytic fluid to overcome the resistance of a thin film of conductive metal on the wafer.
Owner:NOVELLUS SYSTEMS
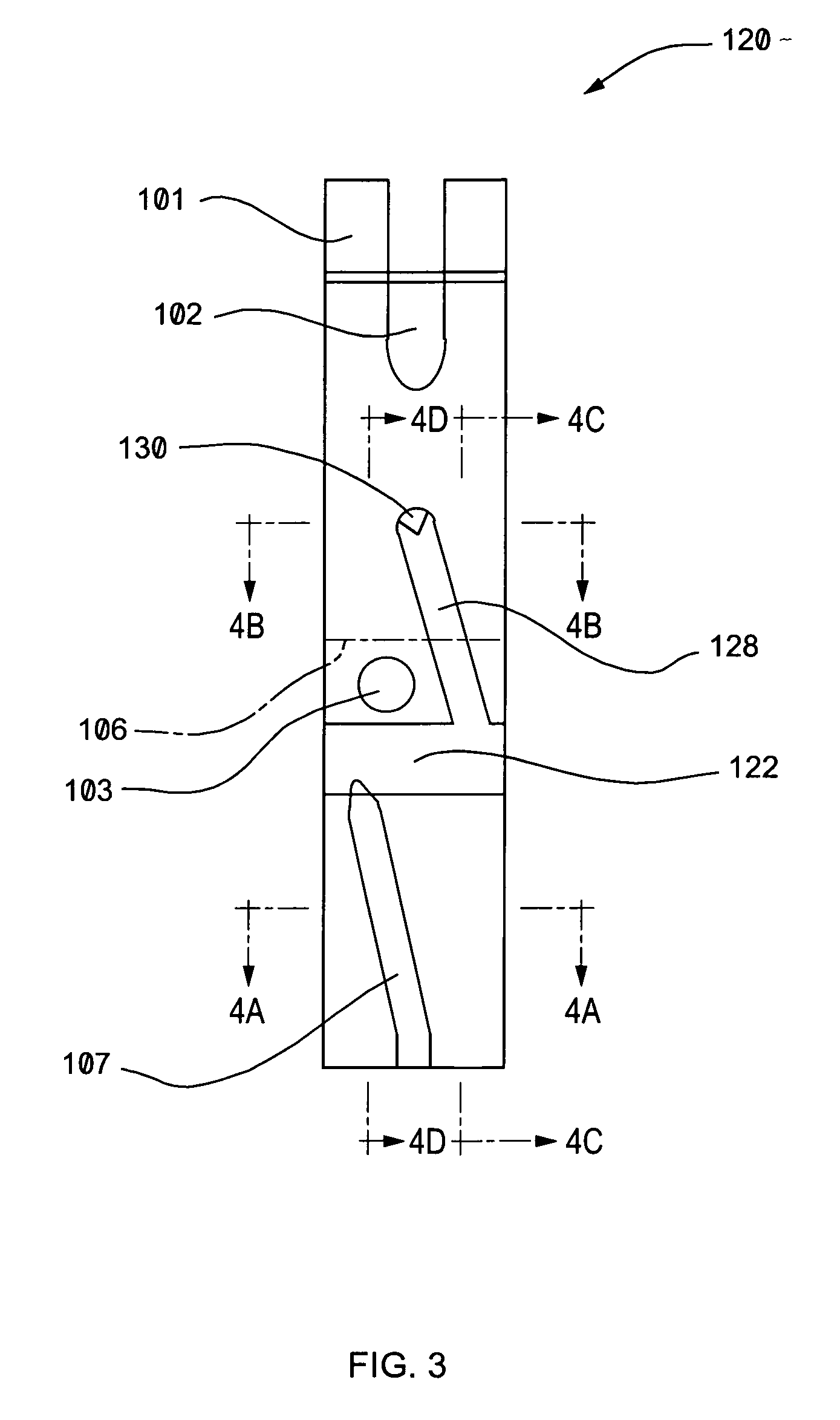
Devices and methods for use in assessing a flow condition of a fluid
InactiveUS20050249606A1Immobilised enzymesBioreactor/fermenter combinationsMicrocontrollerElectrochemical response
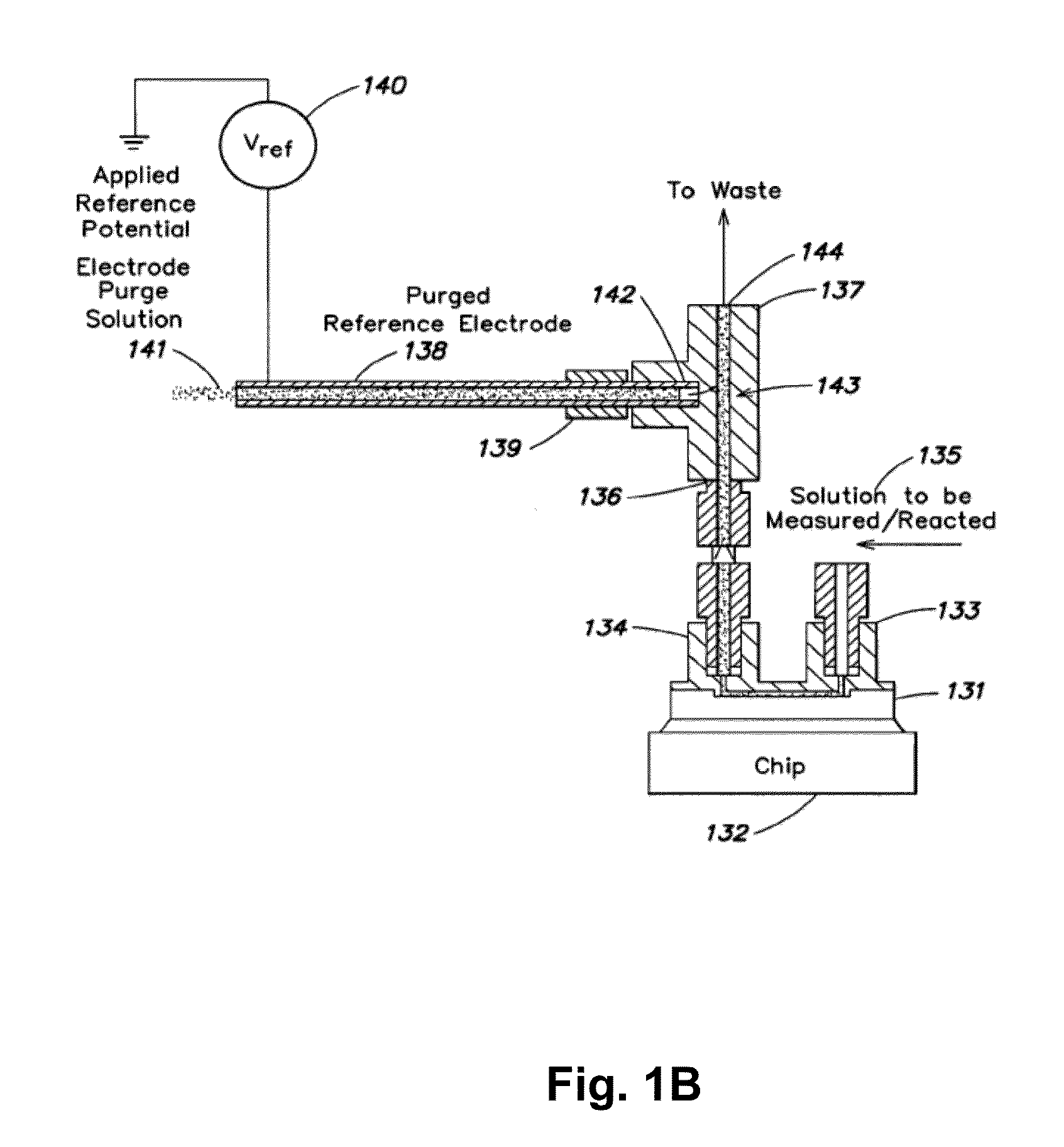
A device for use in assessing a flow condition of a fluid, or a fluid in a flow path, is provided. The device comprises at least one electrochemical cell, comprising a working electrode and at least one other electrode, sufficient for communication with the fluid, or sufficient for communication with a flow path, such that when sufficient fluid is in the flow path, the cell is in communication with the fluid. The fluid comprises a component sufficient to affect a mass-transport limited electrochemical reaction at the working electrode. The device also comprises at least one microcontroller operably connected to the at least one electrochemical cell for providing a potential or a current to the working electrode and for assessing the electrochemical reaction. A method of assessing a flow condition of a fluid, or a fluid in a flow path, is also provided. The device and the method of the present invention may be used in connection with the delivery of a fluid-borne or fluidized drug or medicament to a subject.
Owner:THERASENSE
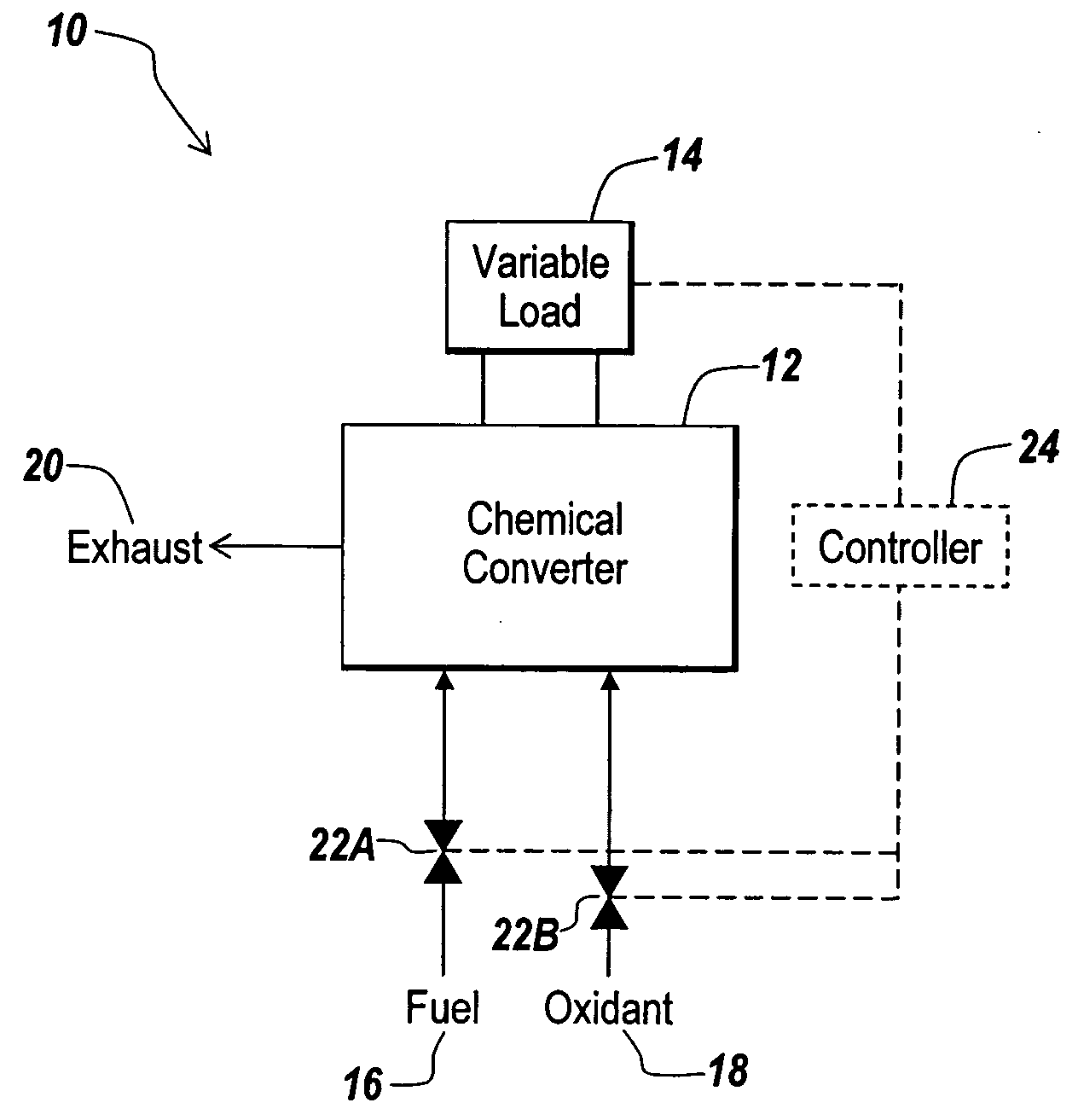
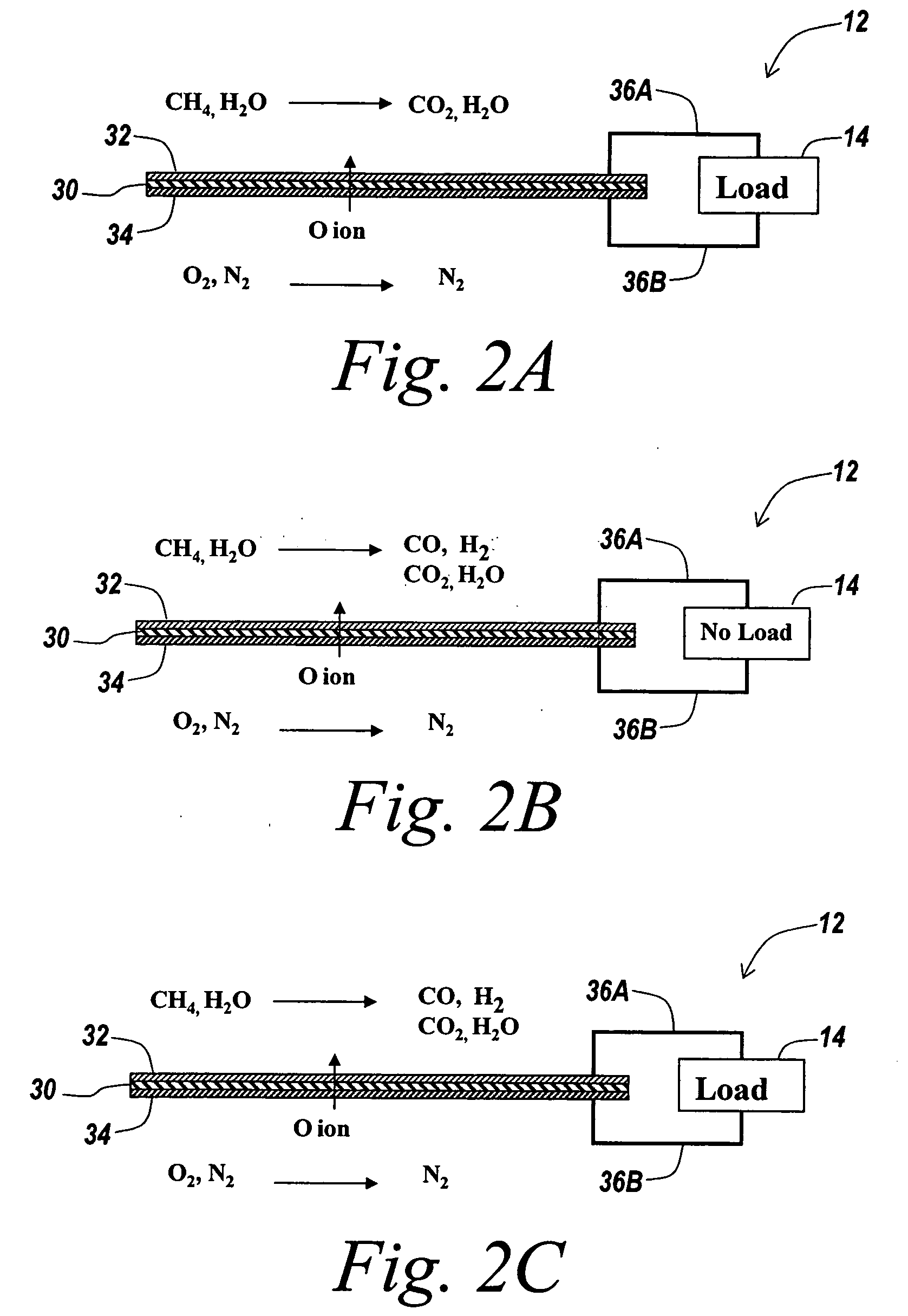
Fuel cell for hydrogen production, electricity generation and co-production
InactiveUS20050112425A1Amount be controlFuel cell combinationsHydrogenElectricityElectrochemical response
A hydrogen-electricity co-production (HECP) system utilizes a fuel cell to produce hydrogen, electricity, or a combination of both hydrogen and electricity. In a first mode, the fuel cell performs an electrochemical reaction by reacting a hydrogen-containing fuel with oxygen to produce electricity, water and heat. In a second mode, the fuel cell utilizes heat released by an electrochemical reaction of the fuel cell to reform a hydrogen-containing fuel to produce hydrogen rich gas. In a third mode, both hydrogen and electricity are co-produced by the fuel cell. The HECP system can control an amount of hydrogen and / or electricity produced and switch between modes by varying an electrical load on the system.
Owner:ZTEK
Method and apparatus for electrochemical analysis
InactiveUS20070205103A1Immobilised enzymesBioreactor/fermenter combinationsElectrochemical responseElectricity
Electrochemical sensors for investigating a physiological sample and methods of manufacture are disclosed. The sensor includes an electrochemical reaction cell, having electrodes and a reagent, and laterally spaced electrical contact points for electrically communication with a meter. Further described herein is a multi-chambered sensor having an electrochemical reaction cell and an immunological cell. The multi-chambered cell can also include a pre-chamber.
Owner:UNIVERSAL BIOSENSORS
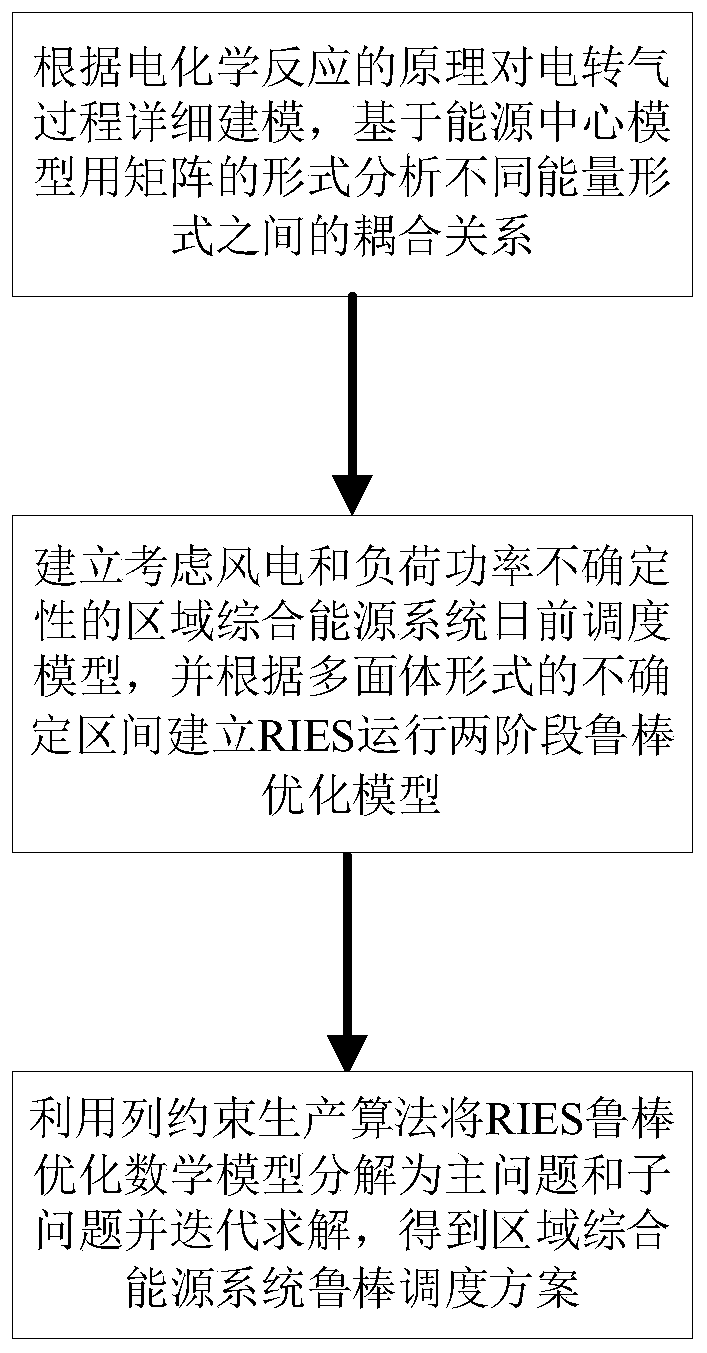
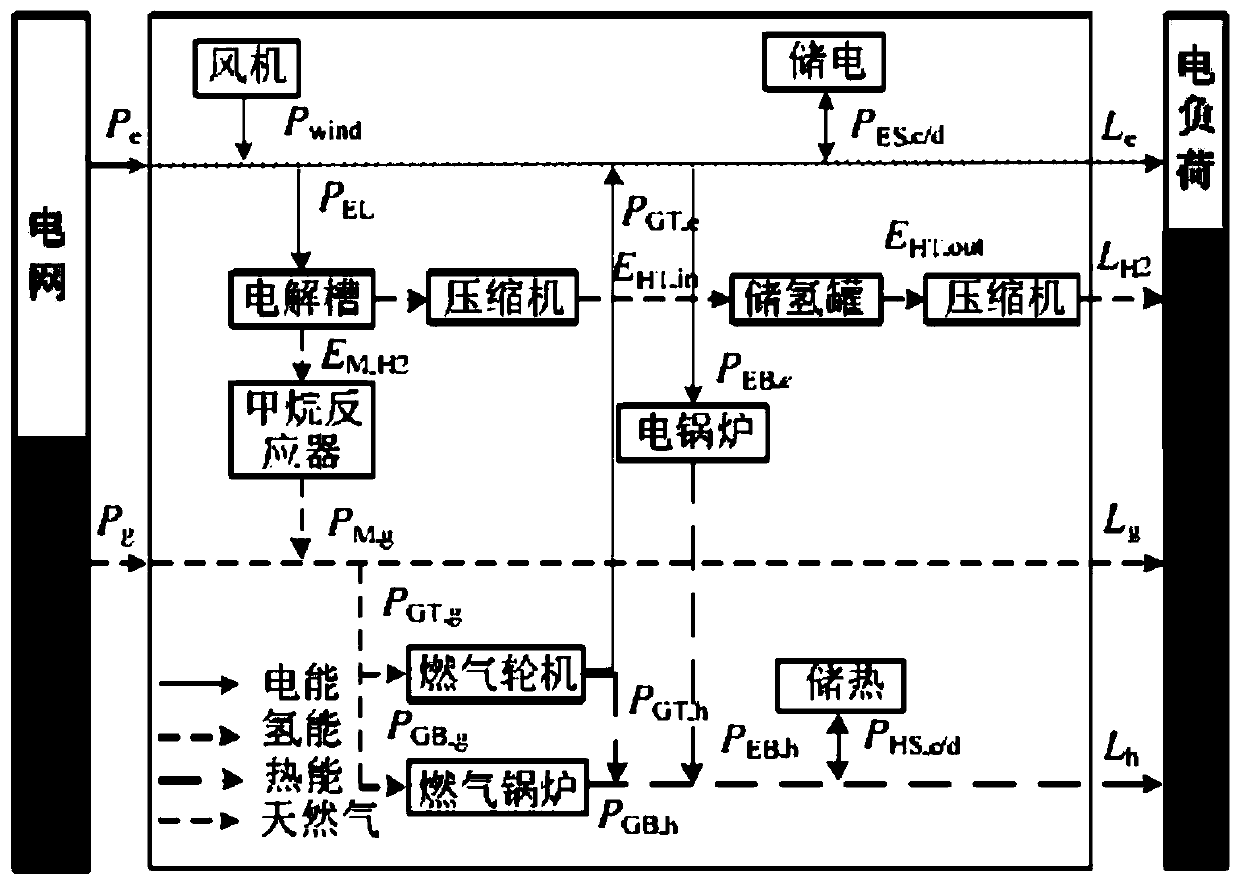
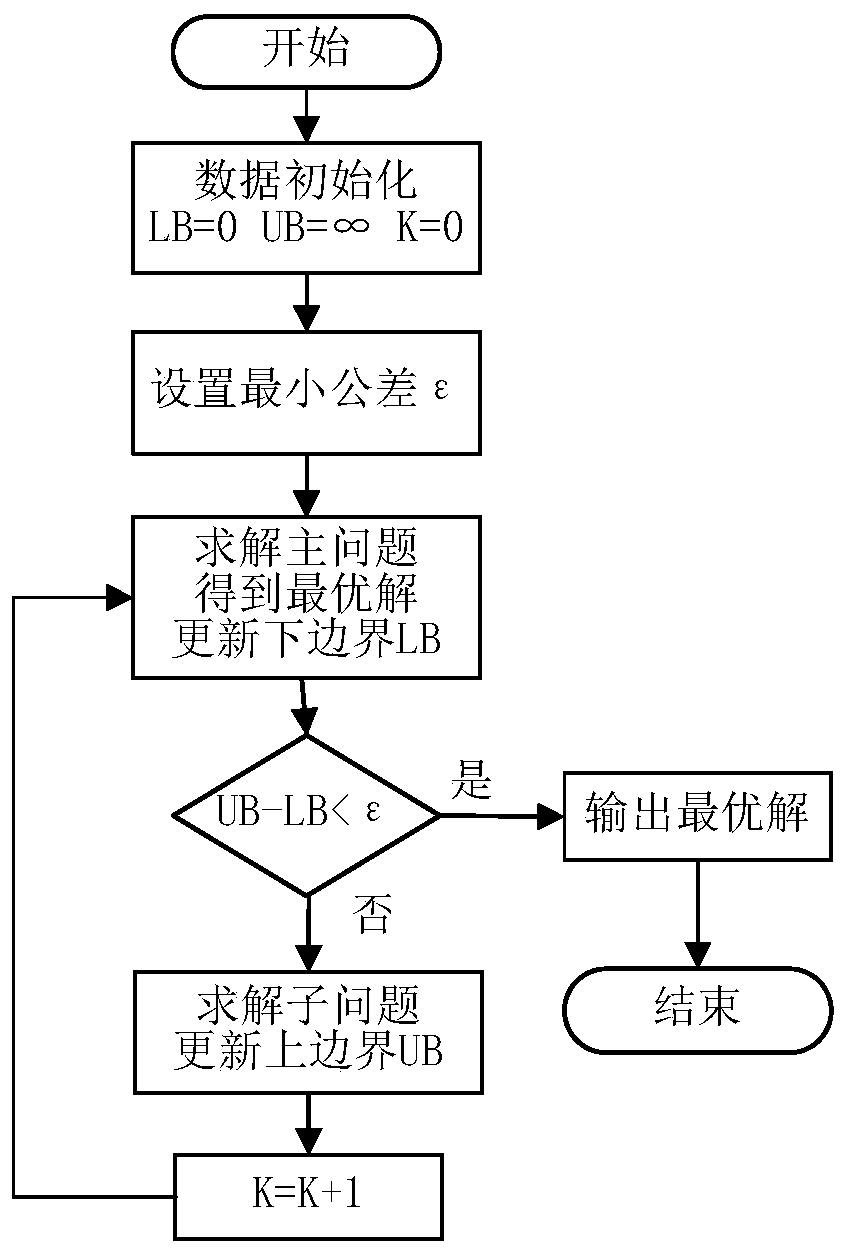
Regional integrated energy system operation robust optimization method considering electricity-to-gas conversion and uncertainty
ActiveCN110009152AImprove economyIncrease flexibilityForecastingResourcesElectrochemical responseForms of energy
The invention discloses a regional integrated energy system operation robust optimization method considering electricity-to-gas conversion and uncertainty, and the method comprises the steps: carryingout the detailed modeling of an electricity-to-gas conversion process according to the principle of an electrochemical reaction, and analyzing the coupling relation between different energy forms ina matrix mode based on an energy center model; establishing a regional comprehensive energy system operation optimization model considering the uncertainty of various load prediction powers, and establishing a two-stage robust optimization model according to a polyhedral uncertain interval; and decomposing the two-stage robust optimization model into a main problem and a sub-problem, converting the sub-problem into an optimization problem of a single target, and carrying out iterative solution to obtain a comprehensive energy system robust optimization scheme. According to the method, the potential of complementary mutual relief and flexible scheduling among multiple energy forms is fully excavated, wind curtailment can be reduced, and the operation economy and flexibility of the comprehensive energy system are improved; and the contradiction between the operation risk and the cost can be coordinated according to the actual condition of the regional integrated energy system.
Owner:SOUTHEAST UNIV
Detection of analytes using electrochemistry
InactiveUS6100045ALow costReduce lossesImmobilised enzymesBioreactor/fermenter combinationsElectrochemical responseMatrix solution
The present invention relates to diagnostic assays whereby the detection means is based on electrochemical reactions. This means that the label to be detected provides an electric signal. Preferred labels are enzymes giving such a signal. Provided is a flow cell whereby a solid phase is provided in a flow stream of the sample, in close proximity to a working electrode to detect any electrical signal. In a typical embodiment, a sample is mixed with molecule having specific binding affinity for an analyte of which the presence in the sample is to be detected, whereby said specific binding molecule is provided with a label. The conjugate of labelled specific binding molecule and analyte is then immobilized on the solid phase in the vicinity of the working electrode, the flow cell is rinsed with a solution and afterwards a substrate solution for the label (an enzyme) is provided upon which an electrical signal is generated and can be detected by the working electrode. The methods and devices of the present invention are particular useful for liquids which comprise many substances that may disturb measurement in conventional assays. The design of the flow cell allows for removal of said interfering substances before measurement. In a preferred embodiment at least part of the solid phase is provided in the form of magnetic beads. In this embodiment the solid phase can be mixed with the sample thereby creating a longer reaction time, a better sensitivity and a higher speed of the assay.
Owner:DSM NV
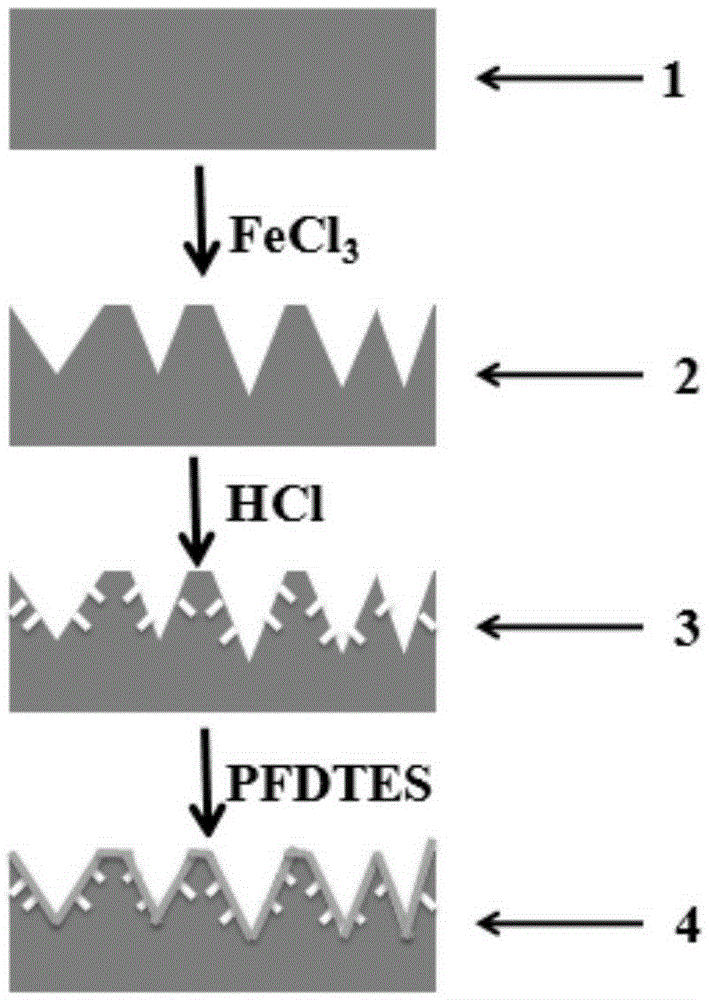
Preparation method for super-hydrophobic surface with bionic micro-nano composite structure
InactiveCN105413994AExcellent surface superhydrophobicityLow costPretreated surfacesSpecial surfacesMicro nanoElectrochemical response
The invention belongs to the technical field of preparation of super-hydrophobic surfaces, and relates to a preparation method for a super-hydrophobic surface with a bionic micro-nano composite structure. From the angle of the super-hydrophobic surface, the super-hydrophobic surface is prepared by virtue of a mesoscale two-step etching method; etching treatment is carried out on a substrate surface twice; a micro-nano composite structure similar to a lotus leaf surface is constructed by utilizing a chemical or an electrochemical reaction etching substrate, and surface modification is carried out by virtue of a surface modifier to reduce surface free energy. The size of the micro-nano structure can be controlled by virtue of reaction time, a reaction temperature and concentration. A contact angle, on the surface of the micro-nano composite structure, of water drops is as high as 170 degrees while a rolling angle of the water drops is smaller than 5 degrees. According to electrochemical test results, corrosion resistance of a super-hydrophobic stainless steel sheet is 22 times that of common stainless steel. The method provided by the invention does not need special equipment, is low in cost, good in stability, excellent in super-hydrophobic property, good in corrosion resistance and can be applied to metal corrosion-resistant protection.
Owner:DALIAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com