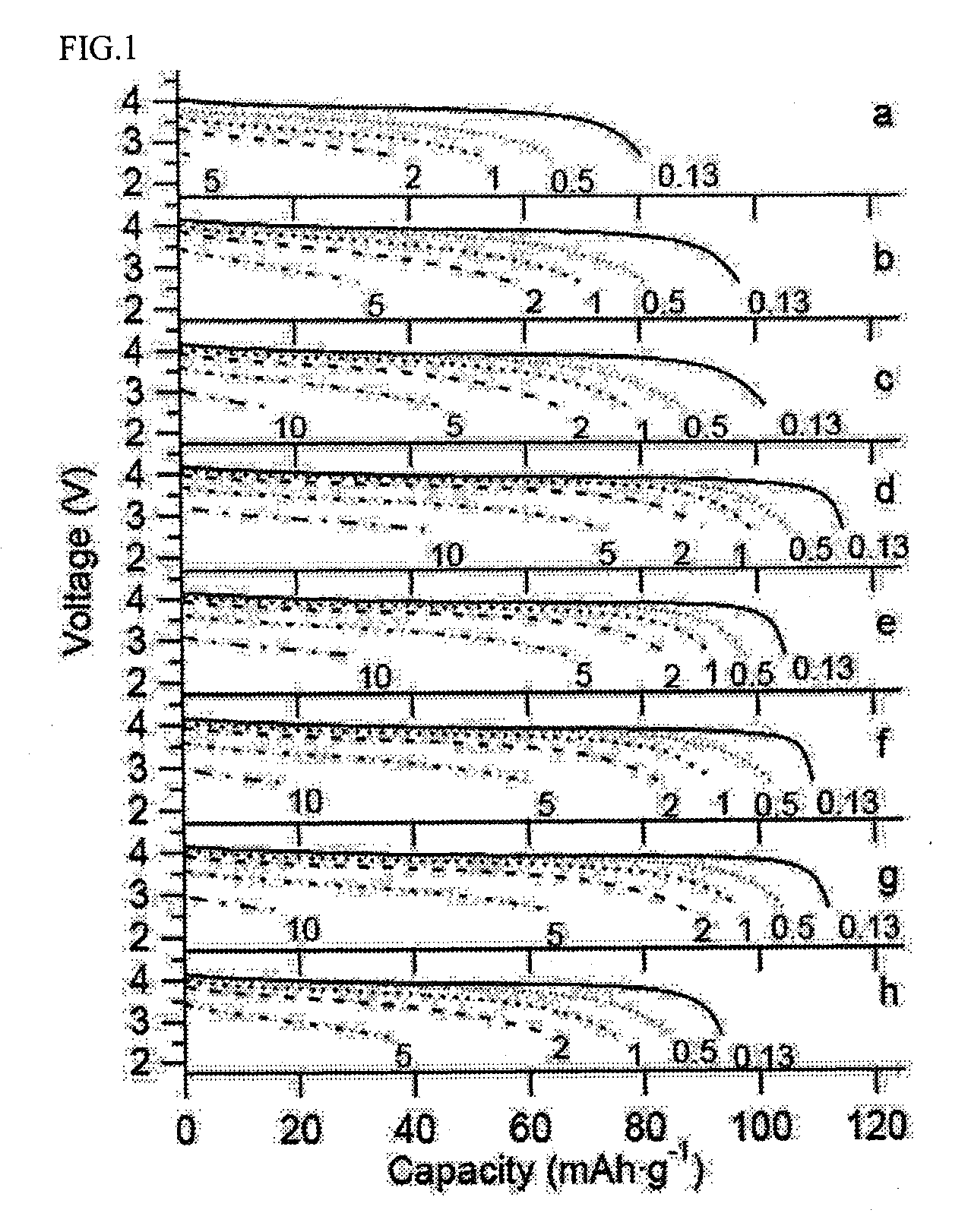
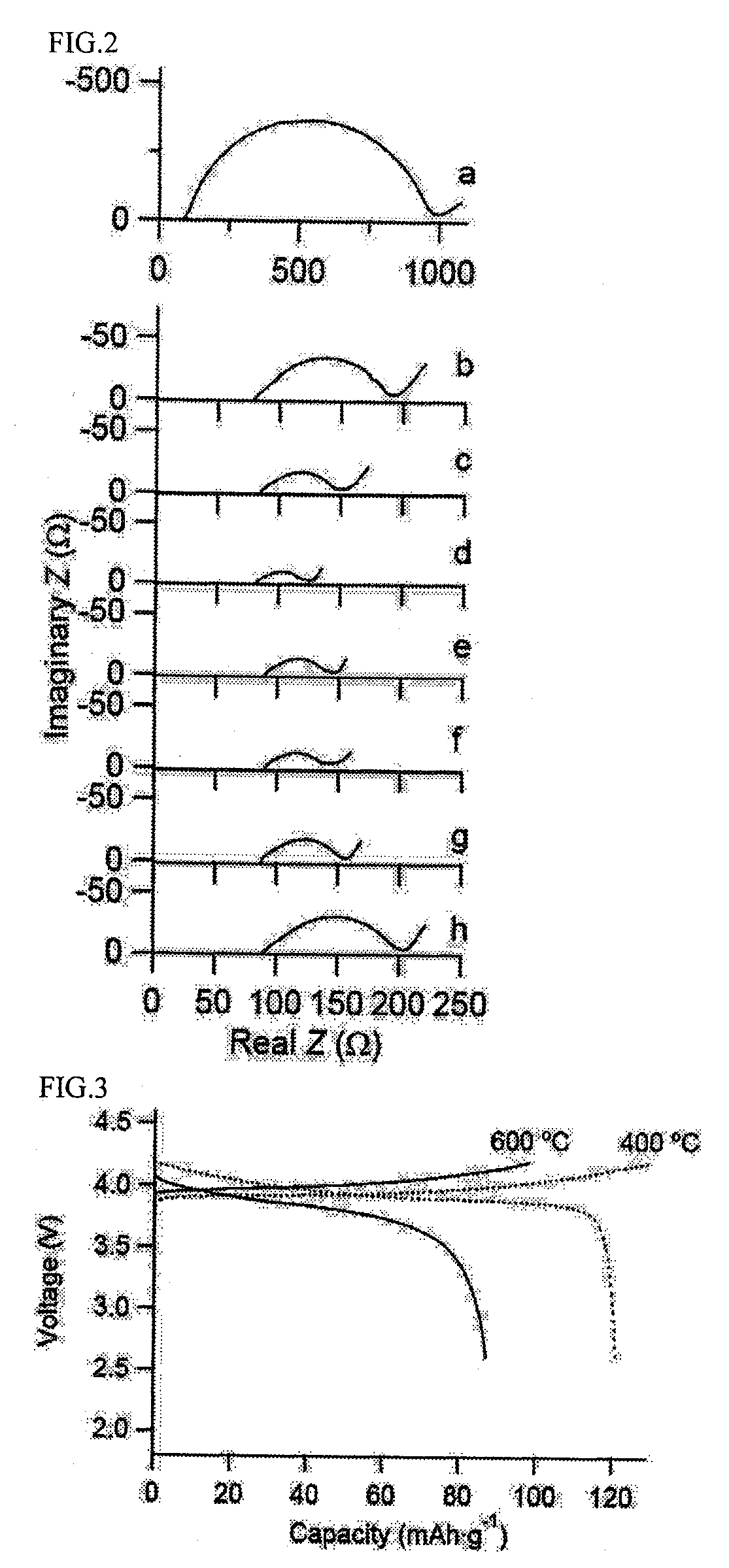
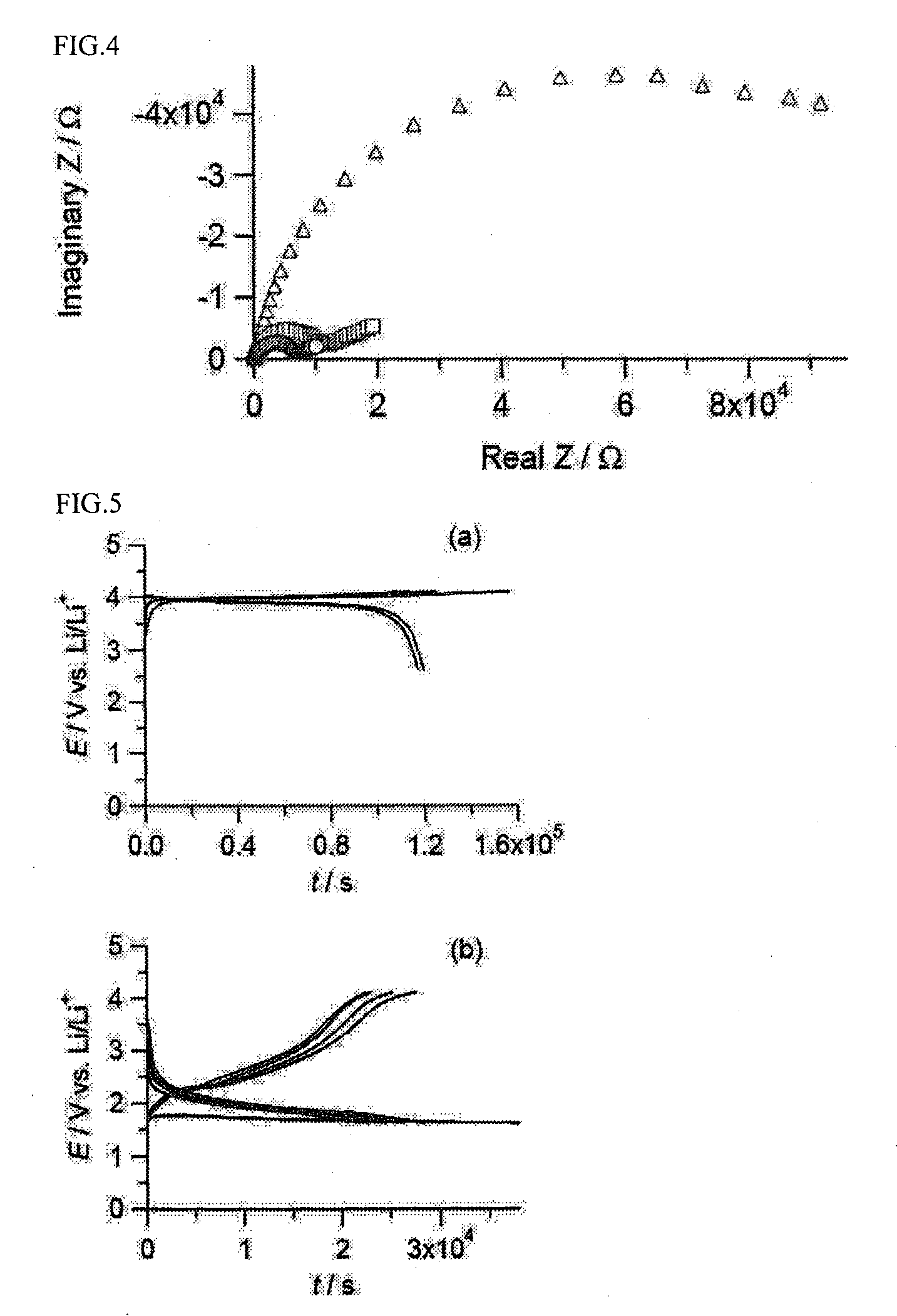
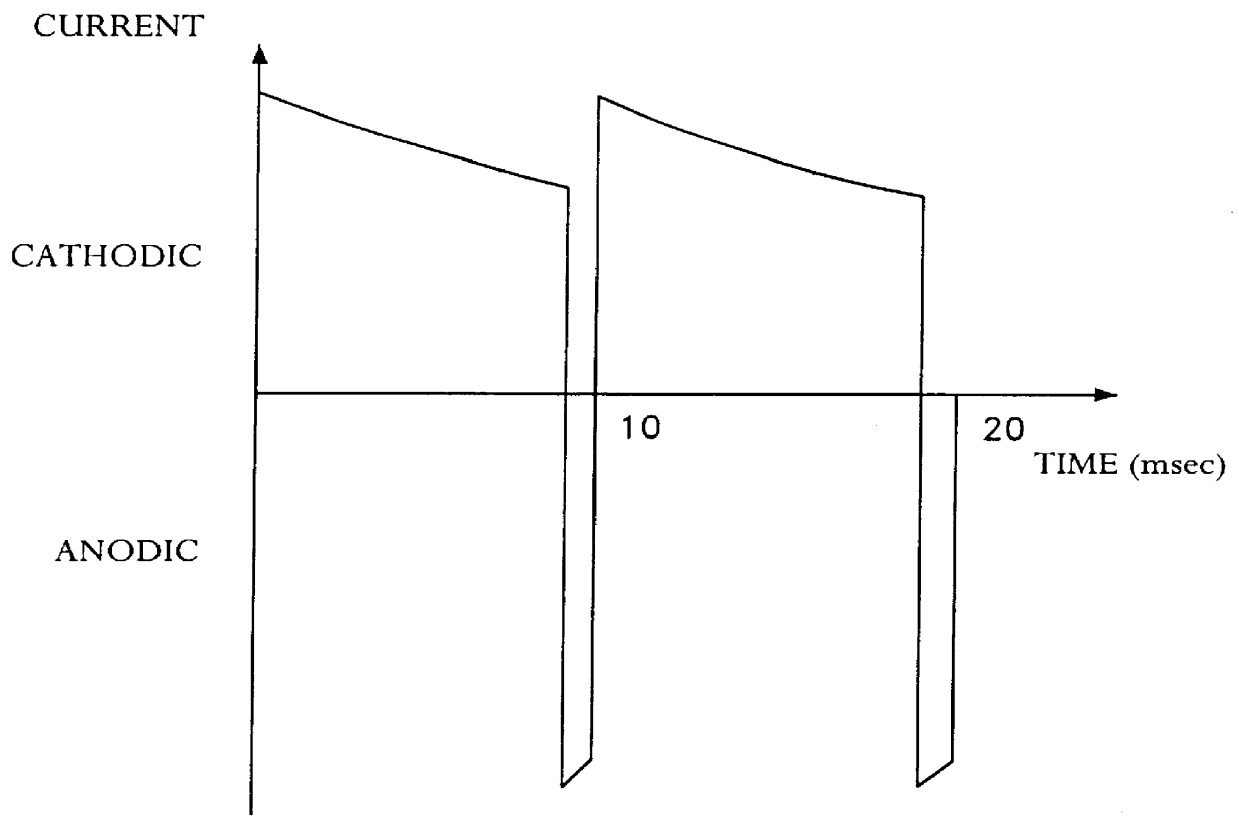
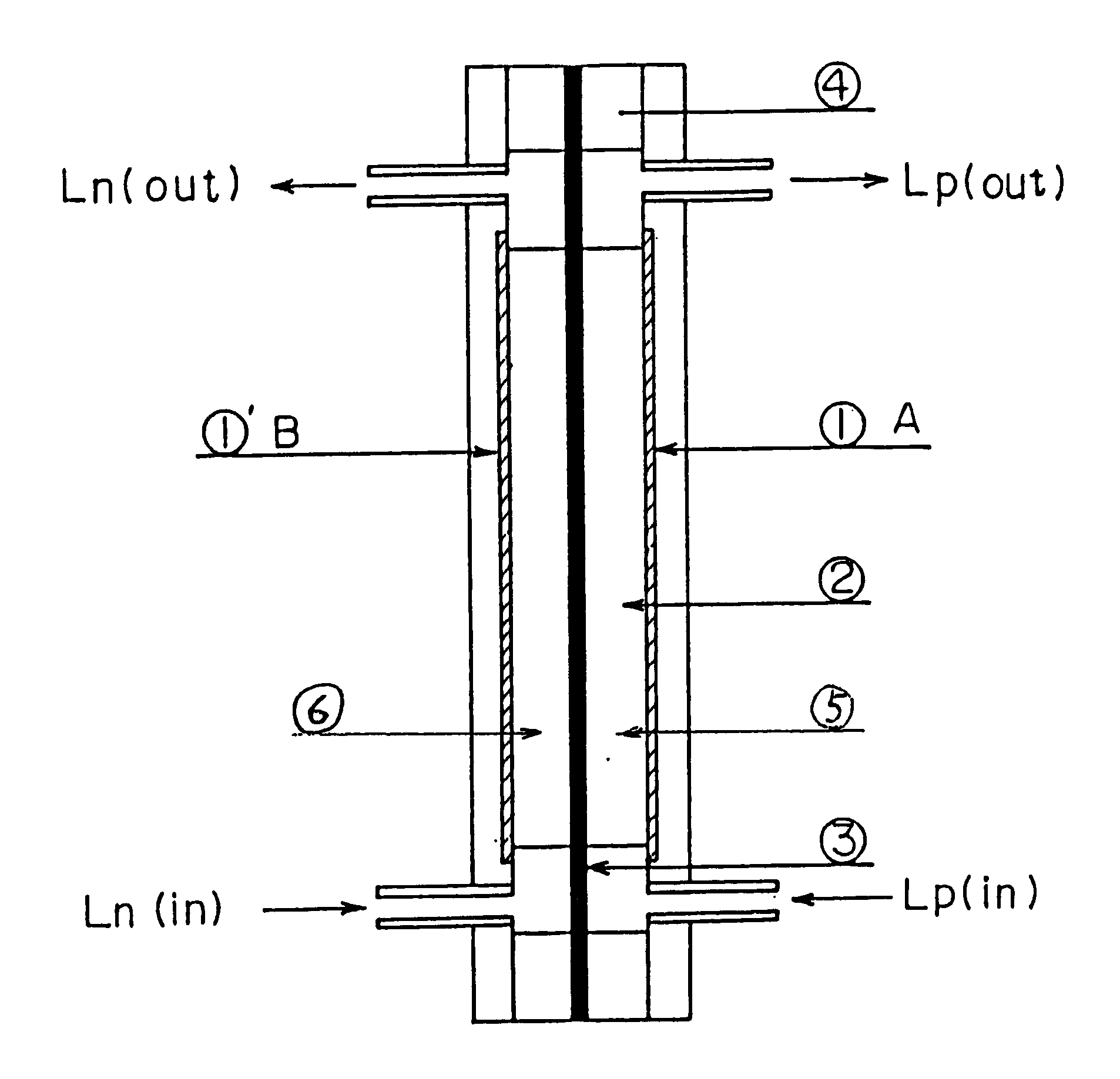
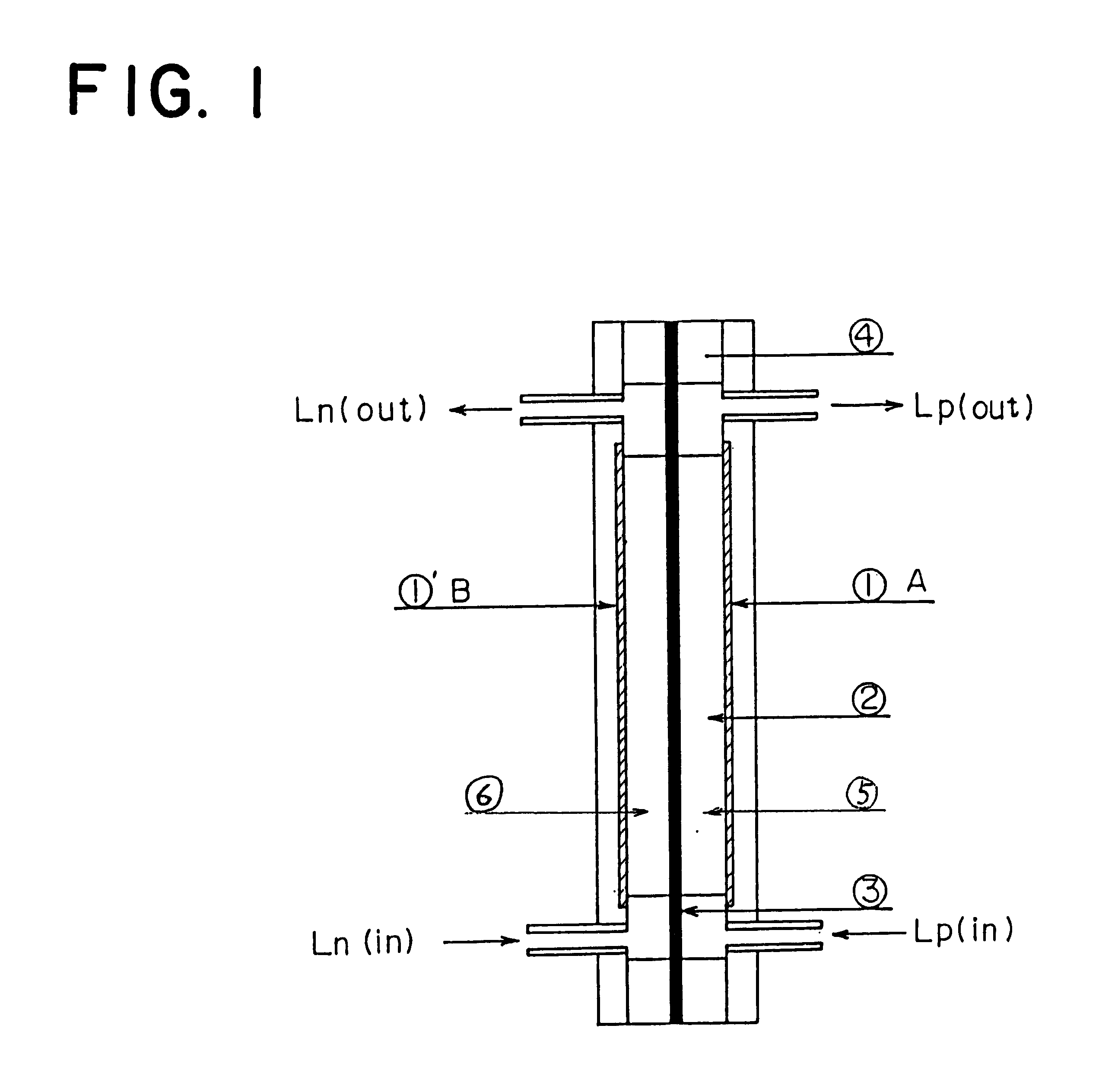
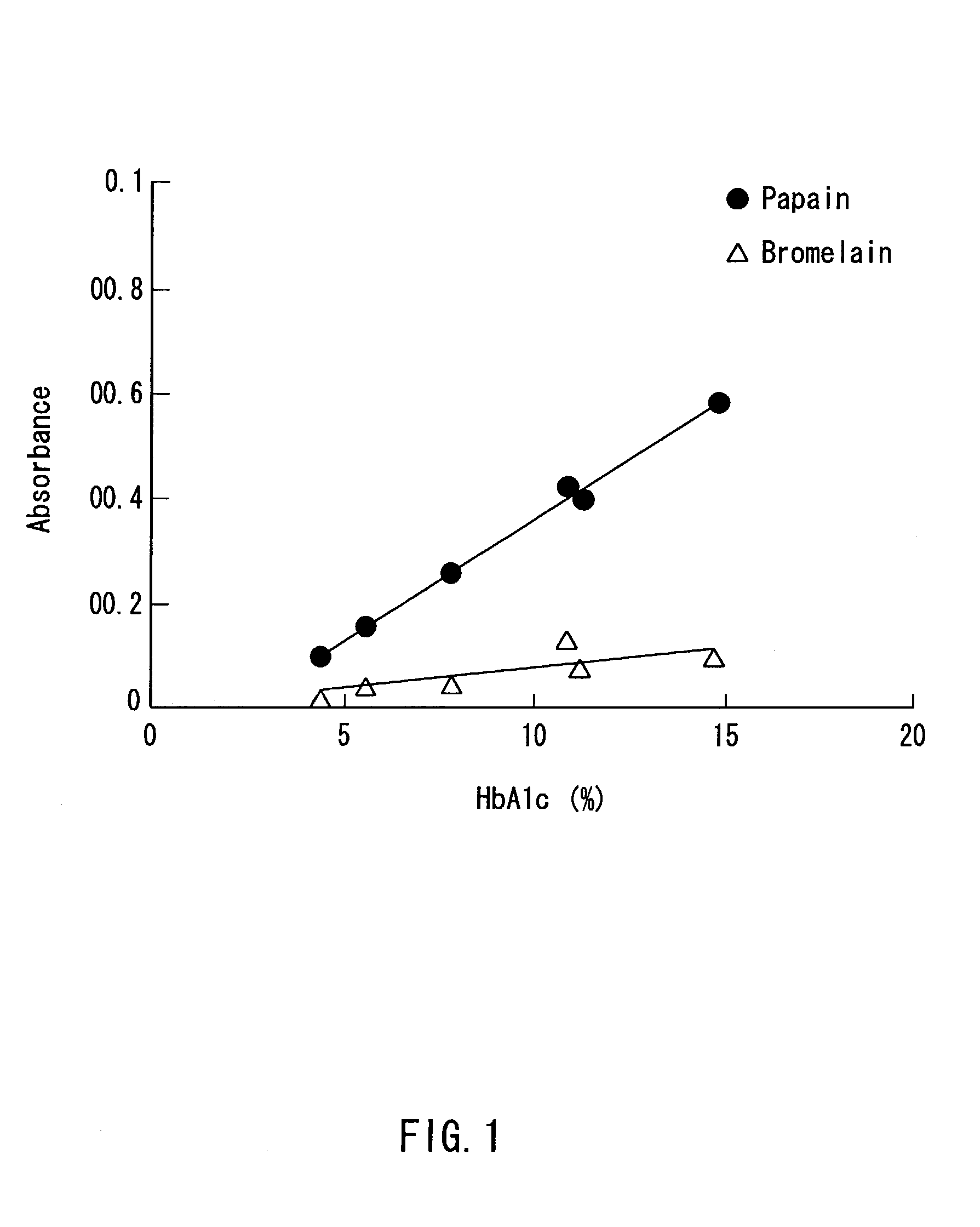
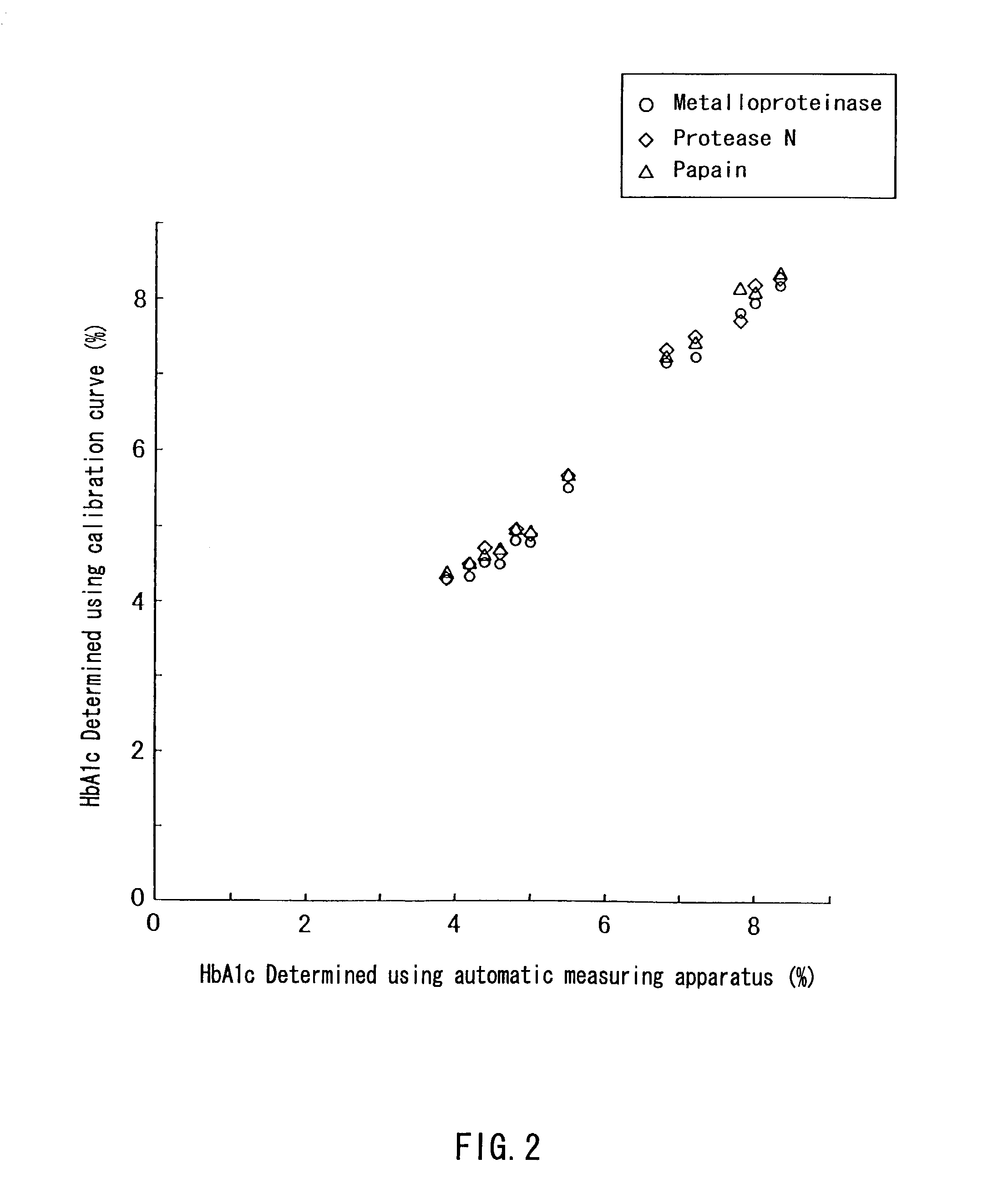
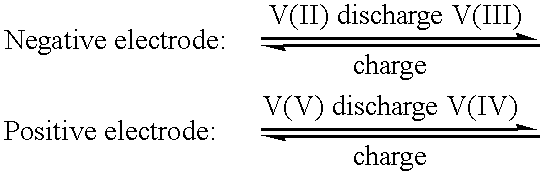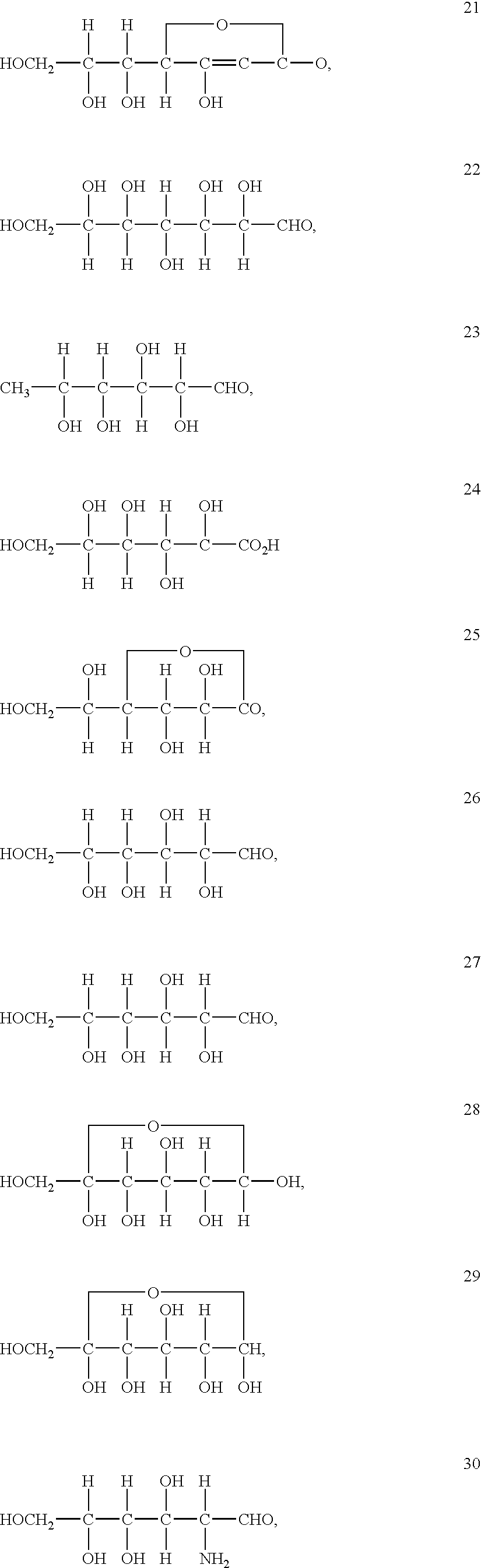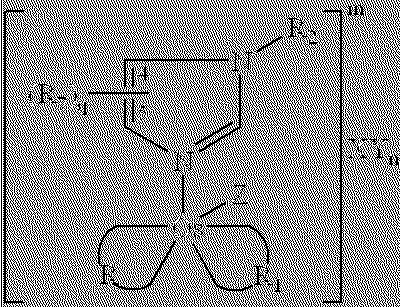Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6961 results about "Redox" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
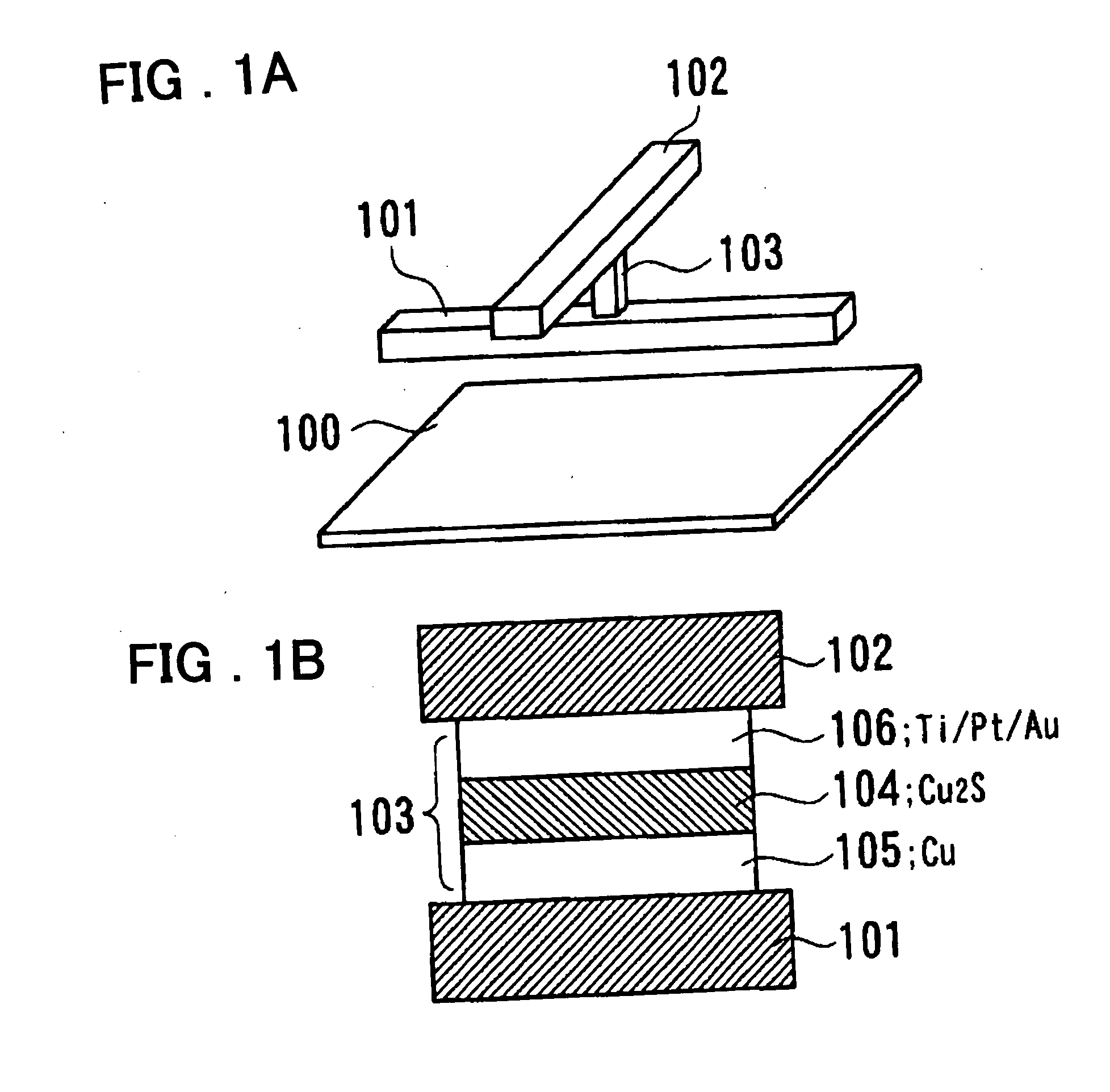
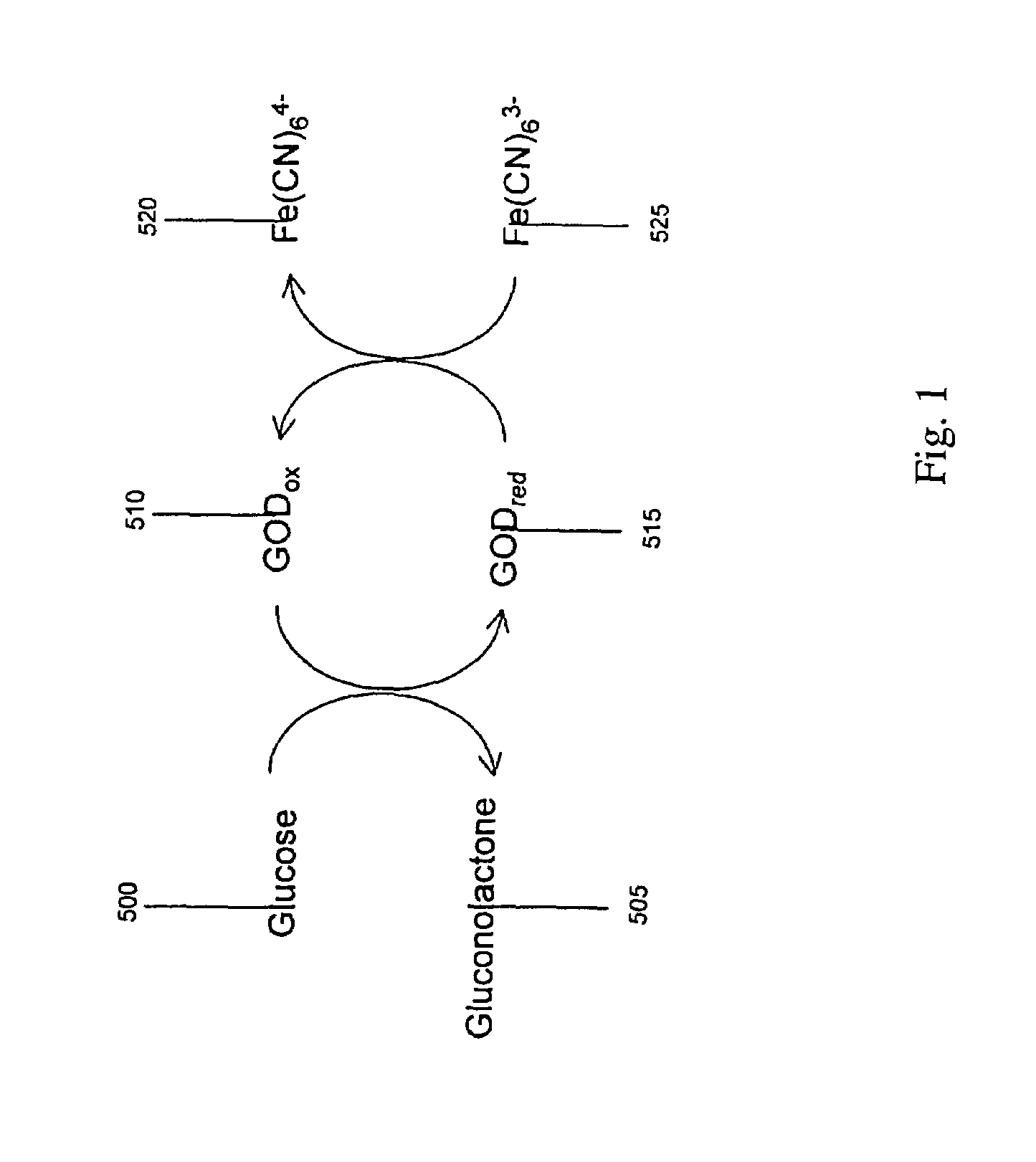
Redox (short for reduction–oxidation reaction) (pronunciation: /ˈrɛdɒks/ redoks or /ˈriːdɒks/ reedoks) is a type of chemical reaction in which the oxidation states of atoms are changed. Redox reactions are characterized by the transfer of electrons between chemical species, most often with one species (the reducing agent) undergoing oxidation (losing electrons) while another species (the oxidizing agent) undergoes reduction (gains electrons). The chemical species from which the electron is stripped is said to have been oxidized, while the chemical species to which the electron is added is said to have been reduced. In other words...
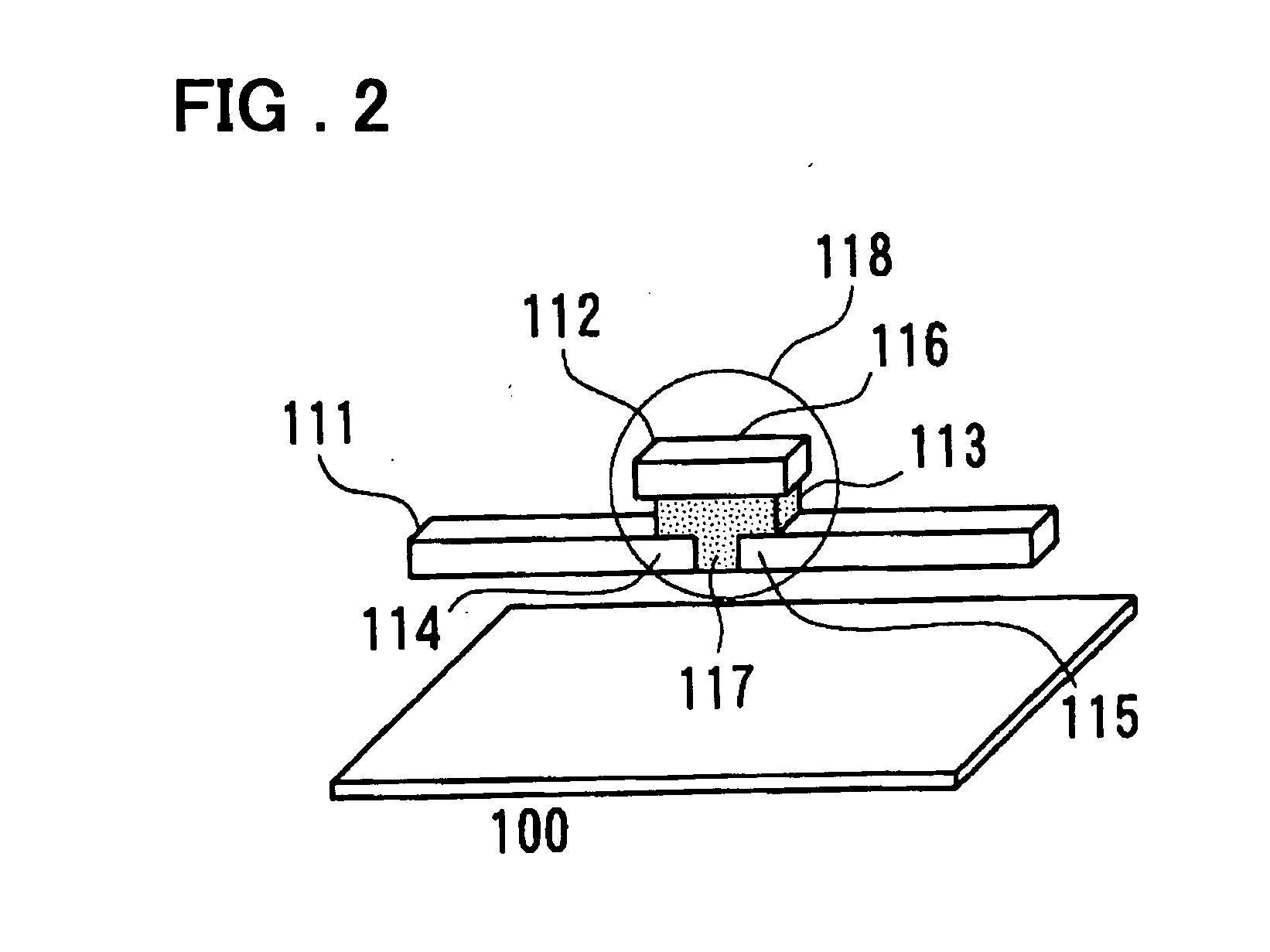
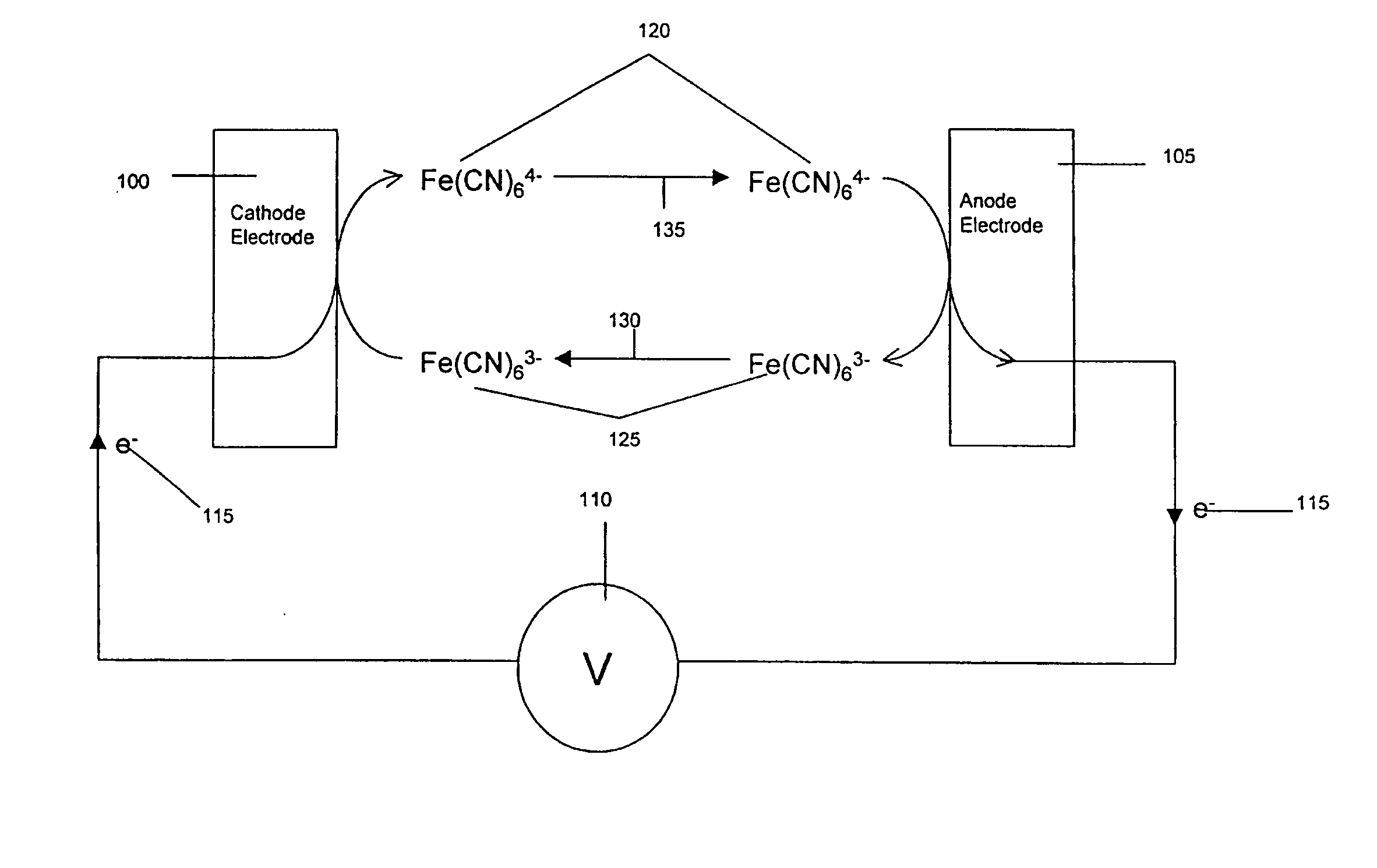
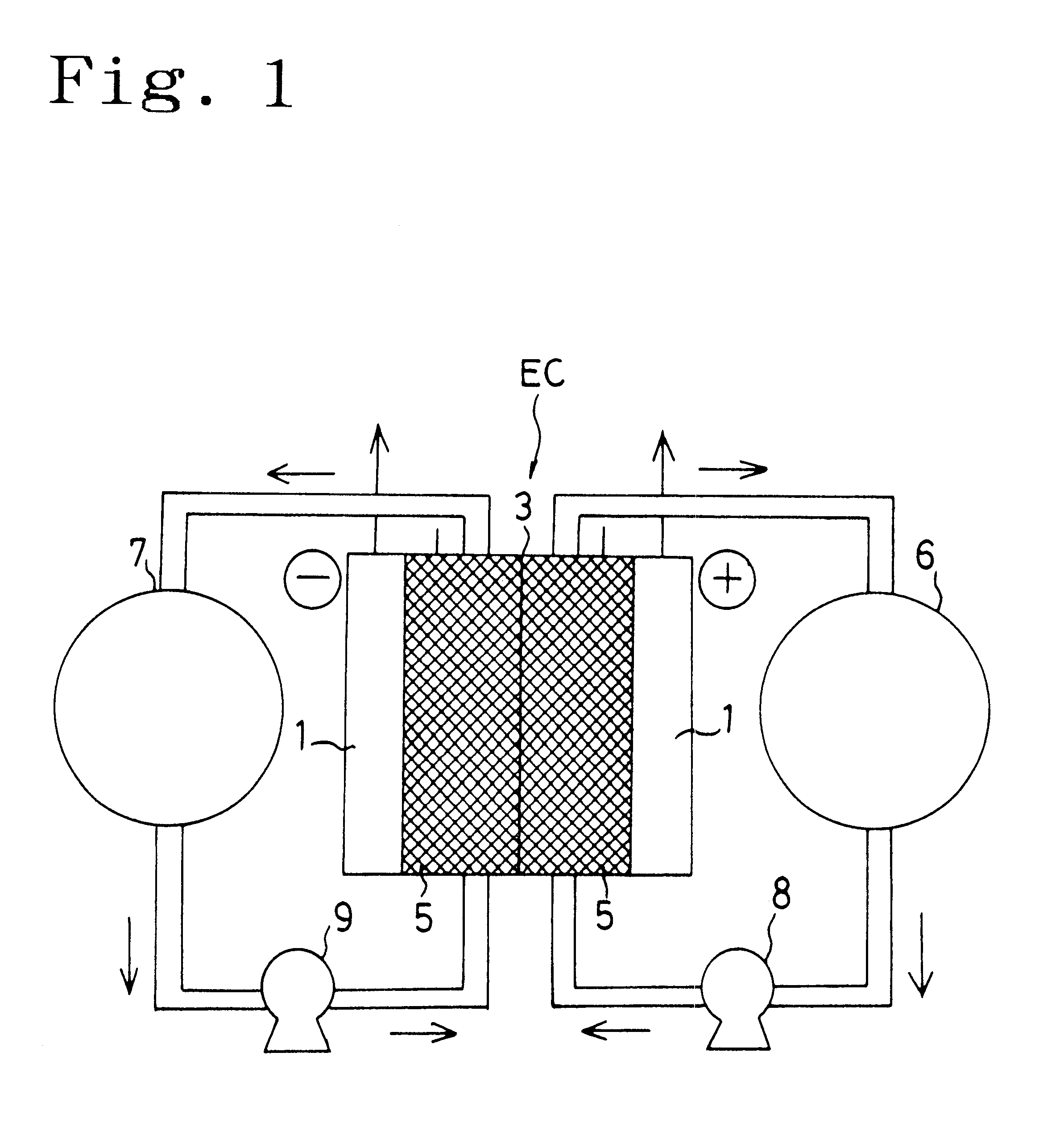
Electrochemical cell
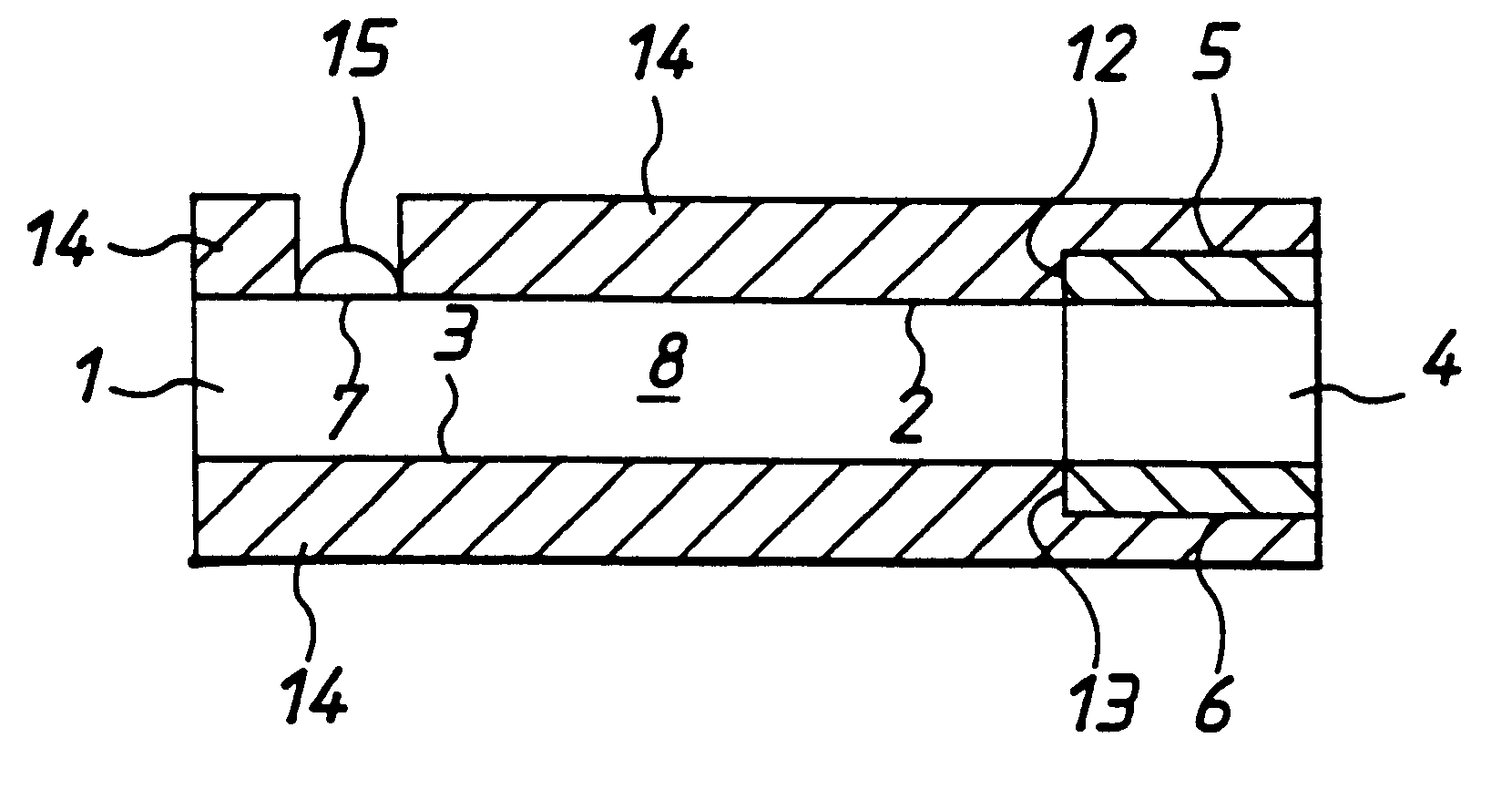
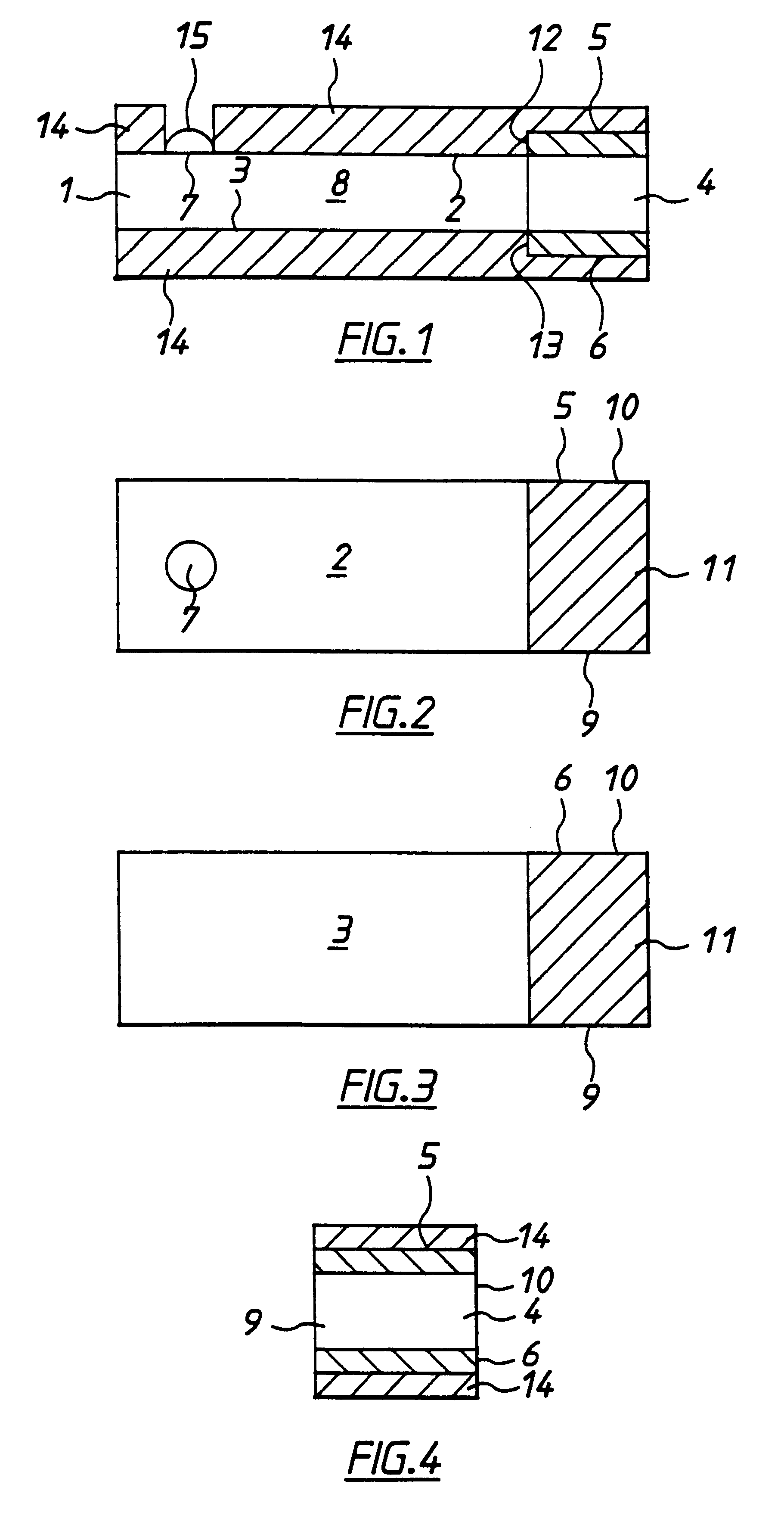
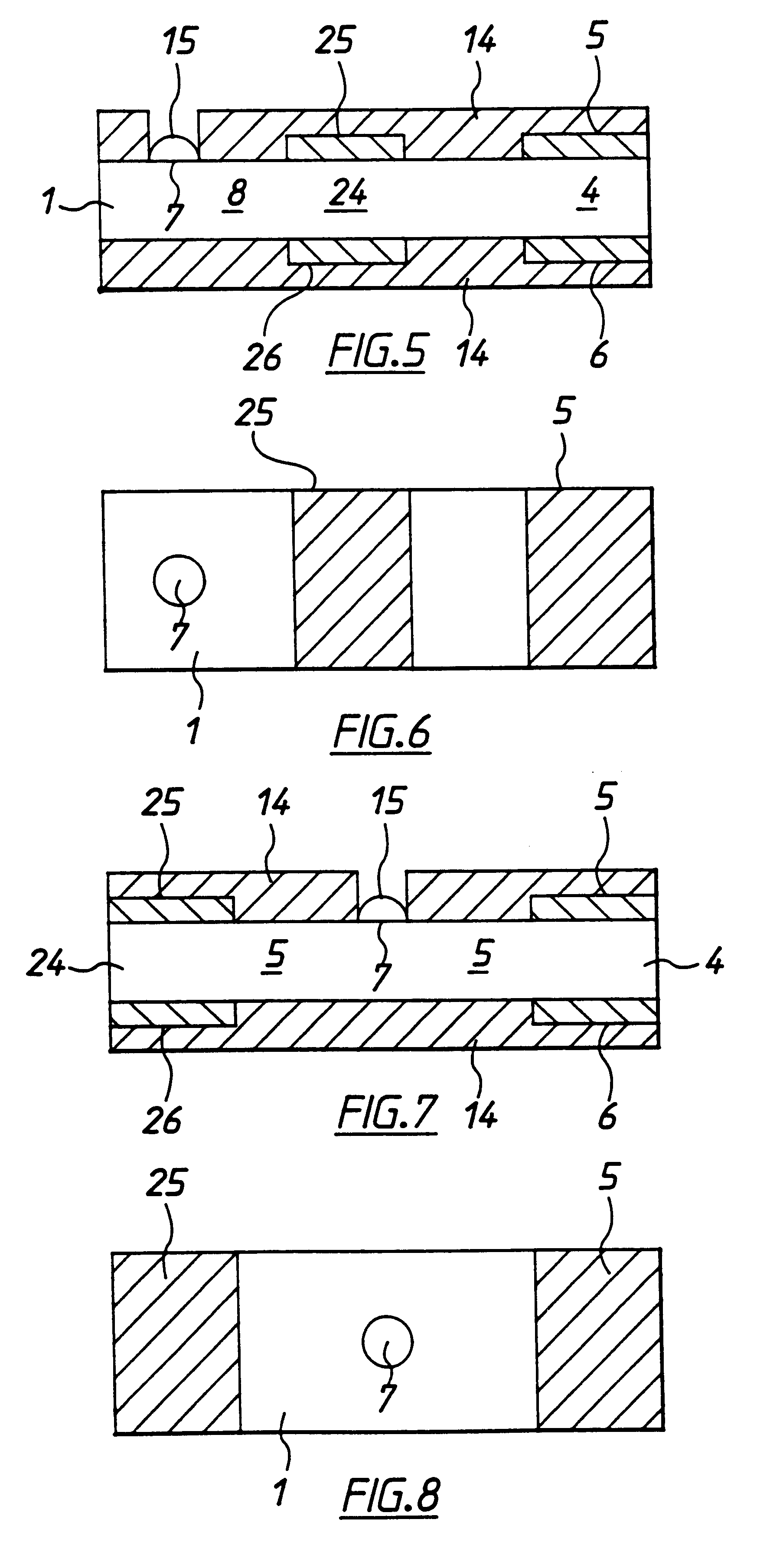
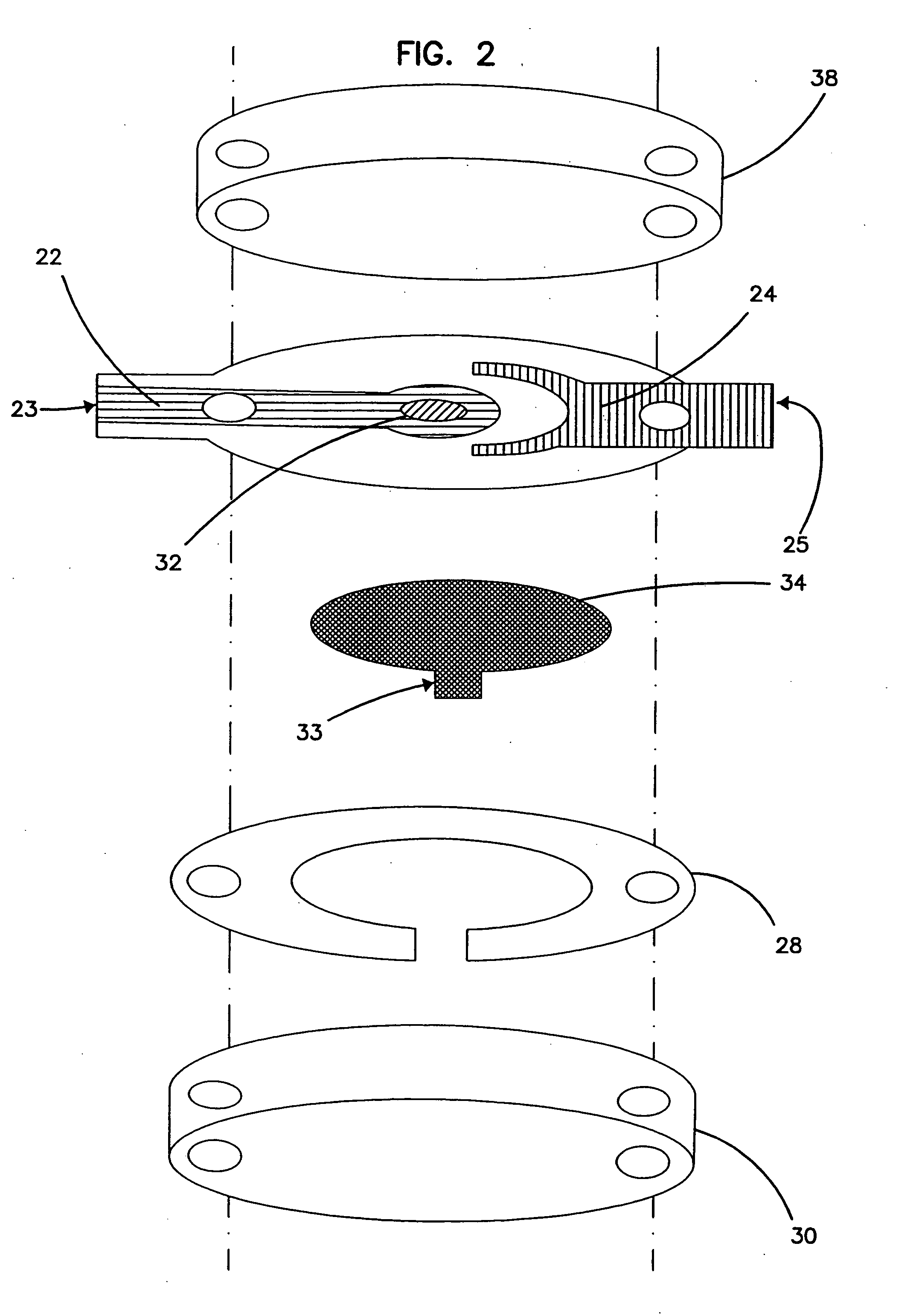
InactiveUS6284125B1Improve accuracyImprove reliabilityImmobilised enzymesBioreactor/fermenter combinationsElectricityDiffusion
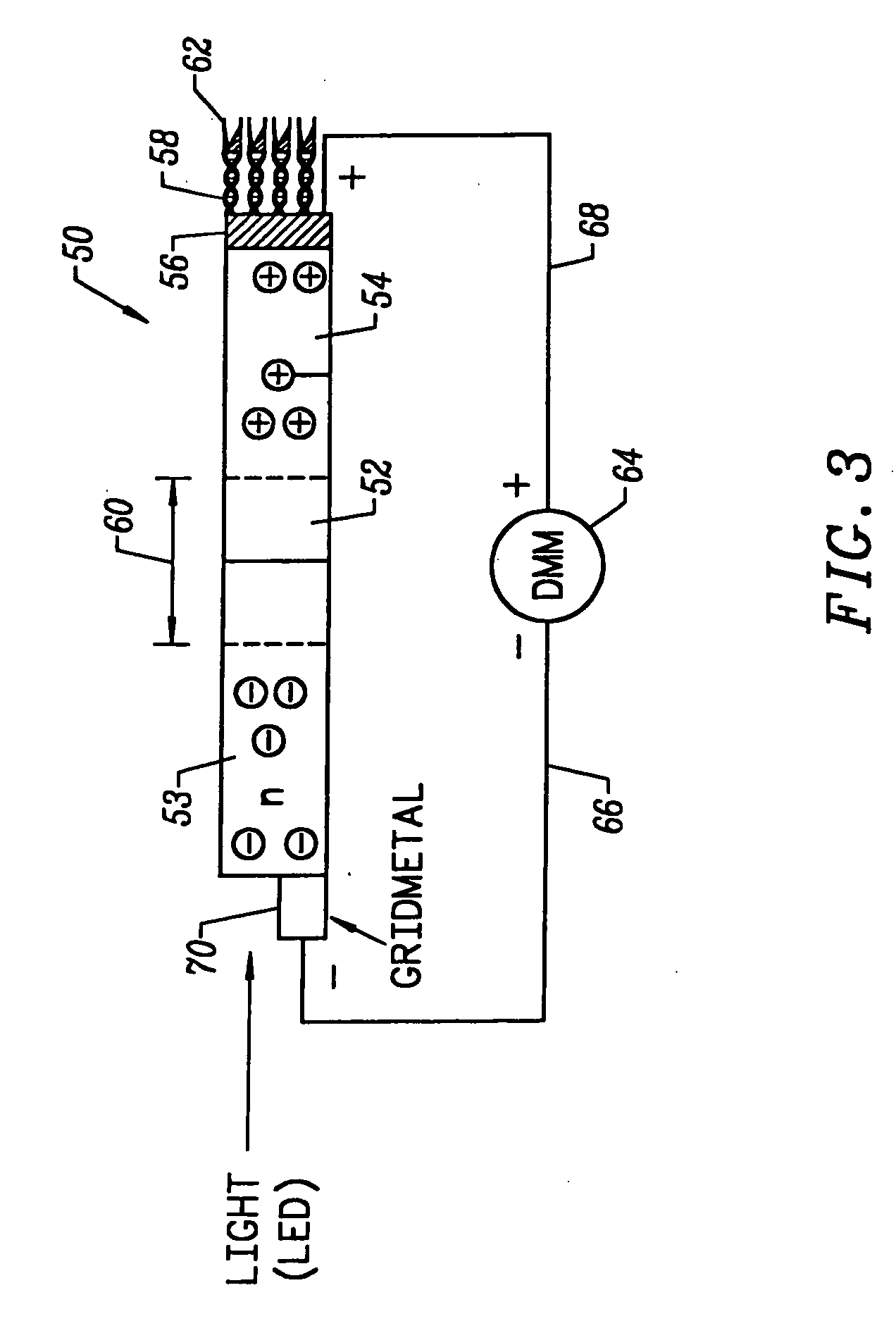
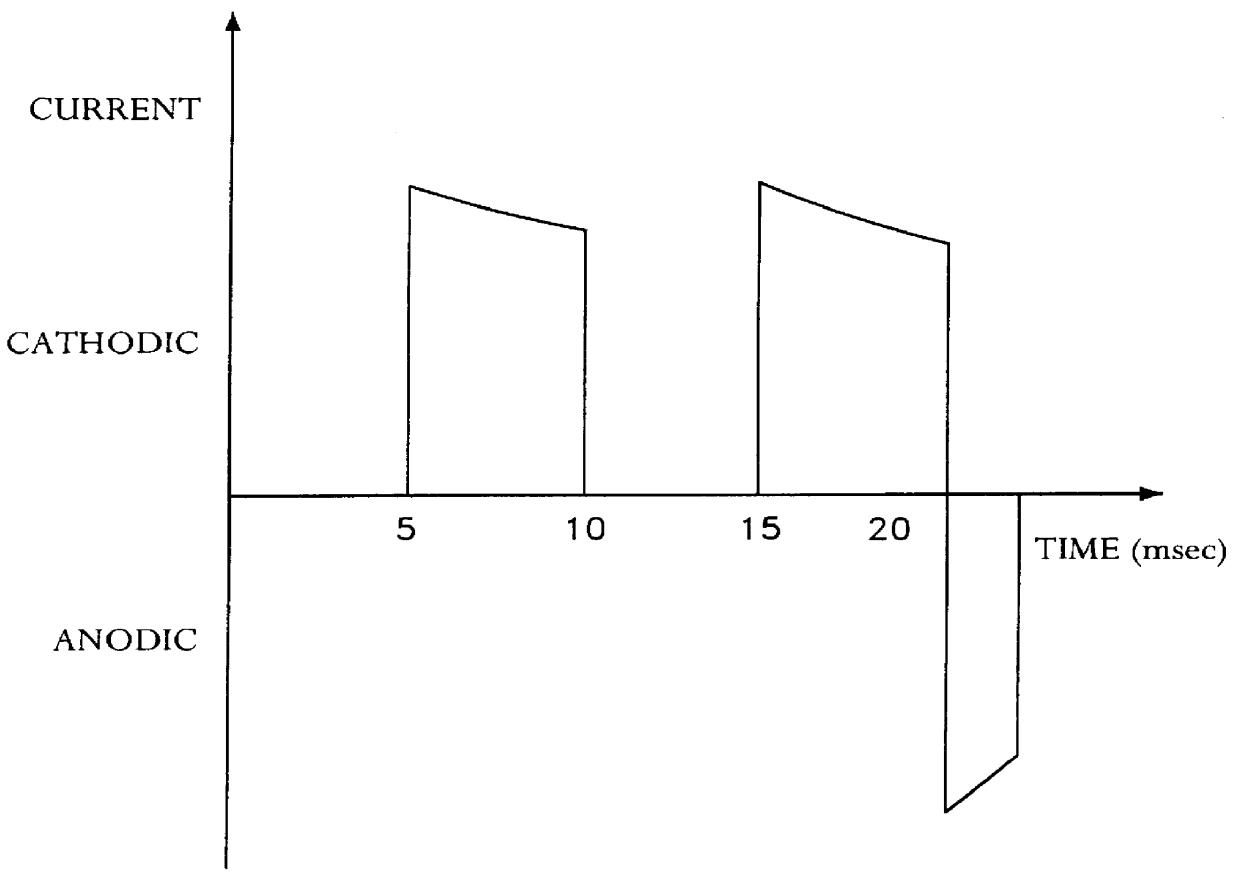
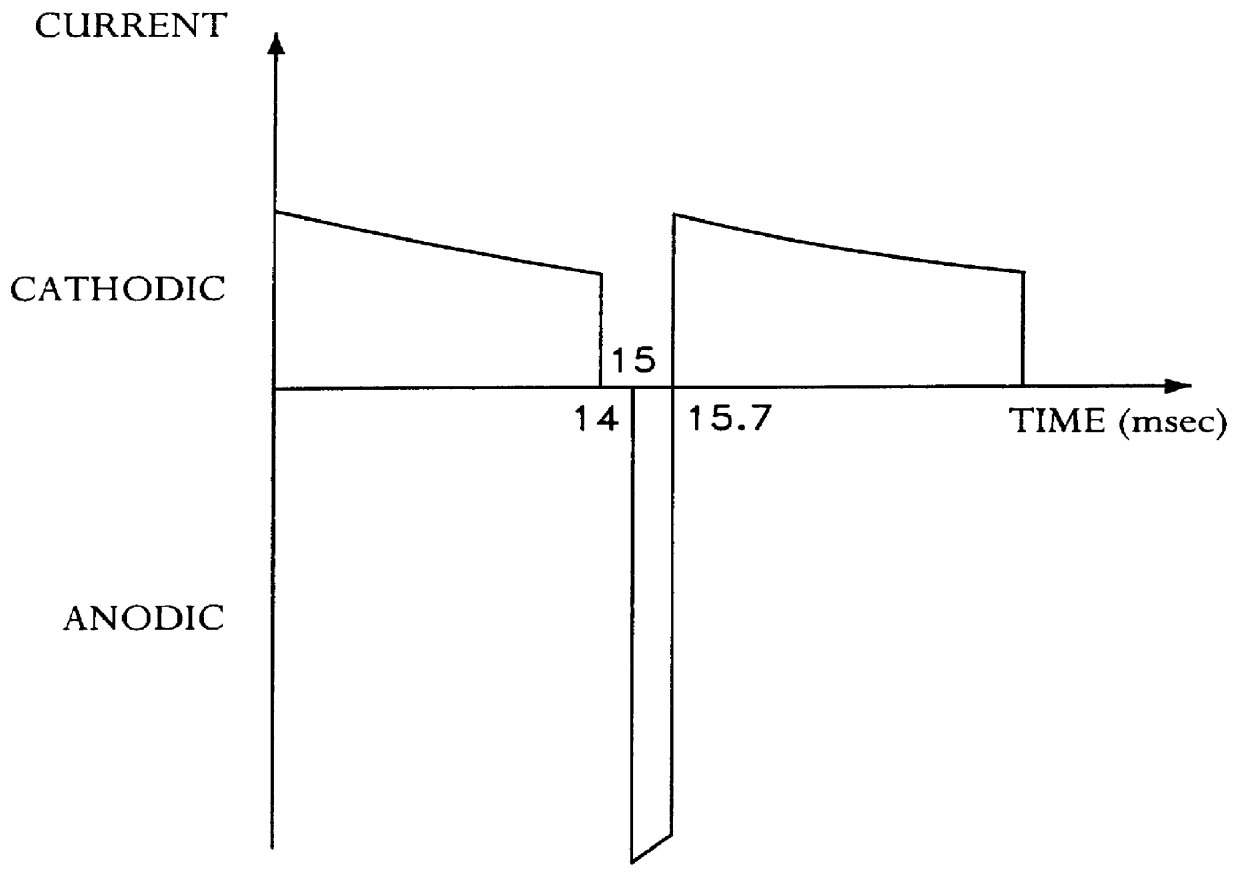
A method for determining the concentration of a reduced (or oxidised) form of a redox species in an electrochemical cell of the kind comprising a working electrode and a counter electrode spaced from the working electrode by a predetermined distance, said method comprising the steps of: (1) applying an electric potential difference between the electrodes; (2) selecting the potential of the working electrode such that the rate of electro-oxidation of the reduced form (or electro-reduction of the oxidised form) of the species is diffusion controlled, (3) selecting the spacing between the working electrode and the counter electrode so that reaction products from the counter electrode arrive at the working electrode; (4) determining current as a function of time after application of the potential and prior to achievement of a steady state; (5) estimating the magnitude of the steady state current, and (6) obtaining from the change in current with time and the magnitude of the steady state current, a value indicative of the diffusion coefficient and / or of the concentration of the reduced form (or the oxidised form) of the species. Also disclosed is an apparatus for determining the concentration of a redox species in an electrochemical cell comprising: an electrochemical cell having a working electrode and a counter (or counter / reference) electrode, means for applying and electric potential difference between said electrodes, means for measuring the change in current with time, and characterised in that the working electrode is spaced from the counter electrode by less than 500 mum.
Owner:LIFESCAN INC
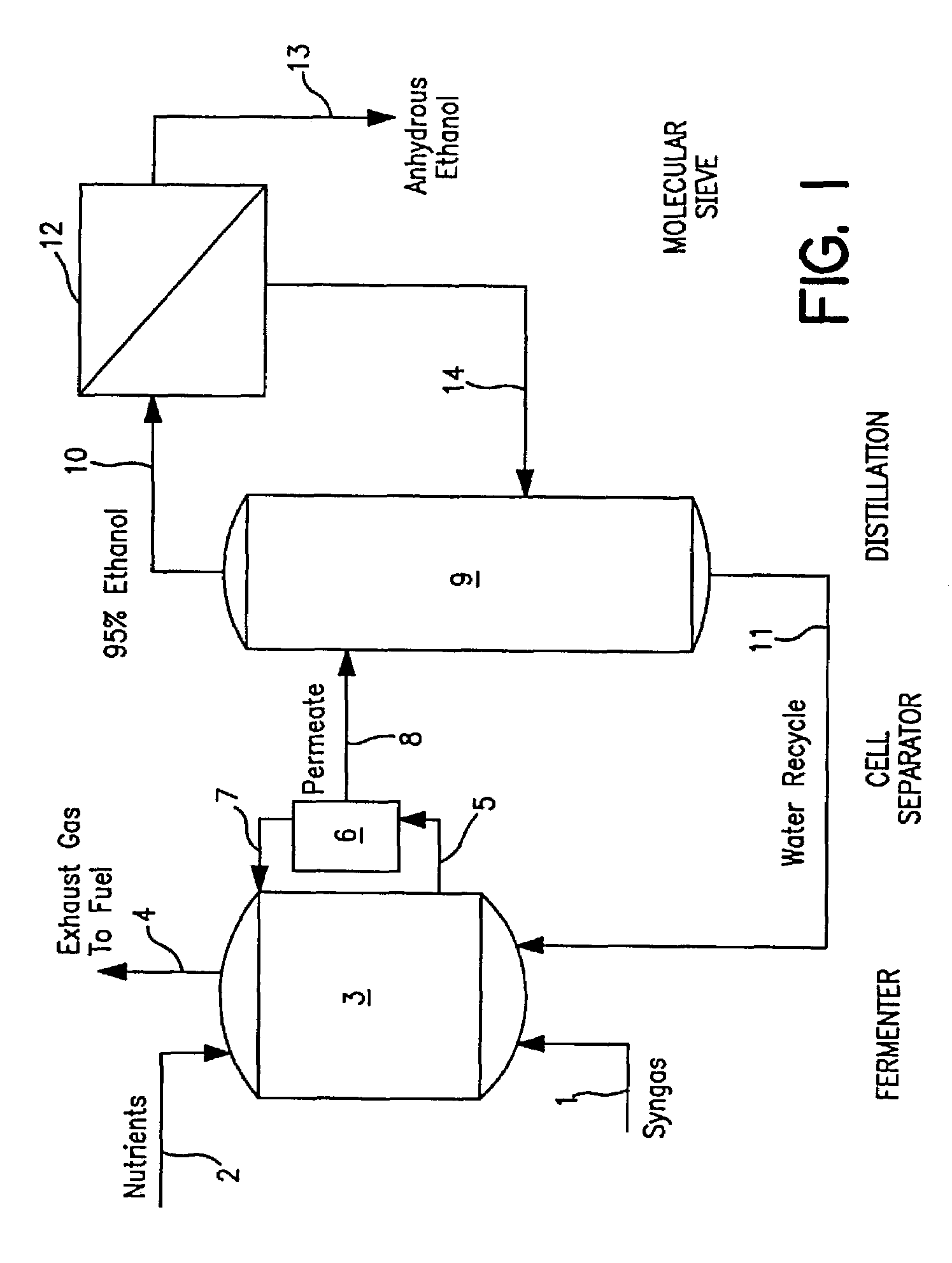
Methods for increasing the production of ethanol from microbial fermentation
InactiveUS7285402B2Good culture stabilityPermit growthBioreactor/fermenter combinationsSolid waste disposalBioreactorNutrient
A stable continuous method for producing ethanol from the anaerobic bacterial fermentation of a gaseous substrate containing at least one reducing gas involves culturing a fermentation bioreactor anaerobic, acetogenic bacteria in a liquid nutrient medium; supplying the gaseous substrate to the bioreactor; and manipulating the bacteria in the bioreactor by reducing the redox potential, or increasing the NAD(P)H TO NAD(P) ratio, in the fermentation broth after the bacteria achieves a steady state and stable cell concentration in the bioreactor. The free acetic acid concentration in the bioreactor is maintained at less than 5 g / L free acid. This method allows ethanol to be produced in the fermentation broth in the bioreactor at a productivity greater than 10 g / L per day. Both ethanol and acetate are produced in a ratio of ethanol to acetate ranging from 1:1 to 20:1.
Owner:JUPENG BIO HK LTD
Low-carbon-doped silicon oxide film and damascene structure using same
ActiveUS20050260850A1Reduce electrical circuit lifetimeEnhance moisture intakeSemiconductor/solid-state device detailsSolid-state devicesMetal interconnectDevice material
A method of forming an interconnect for a semiconductor device using triple hard layers, comprises: forming a first hard layer serving as an etch stop layer on a metal interconnect-formed dielectric layer; forming a second hard layer on the first hard layer; forming a dielectric layer on the second hard layer; forming a third hard layer on the dielectric layer; forming a hole through the third and second hard layers, the dielectric layer, and the first hard layer; and filling the hole with metal to establish an interconnect. The second and third hard layers are each made of carbon-doped silicon oxide formed from a source gas and a redox gas, while controlling the carbon content in the second hard layer as a function of a flow rate of the redox gas.
Owner:ASM JAPAN
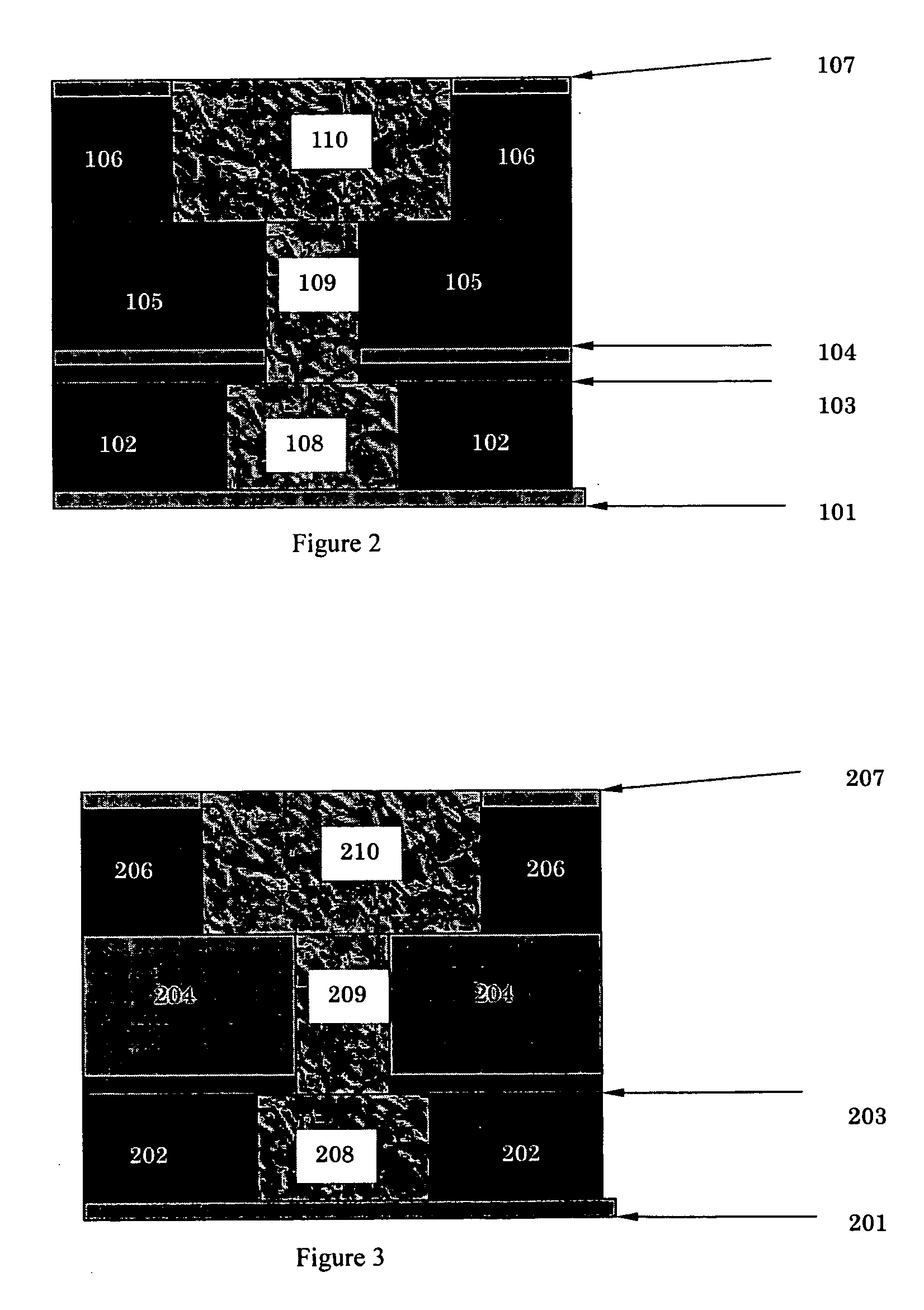
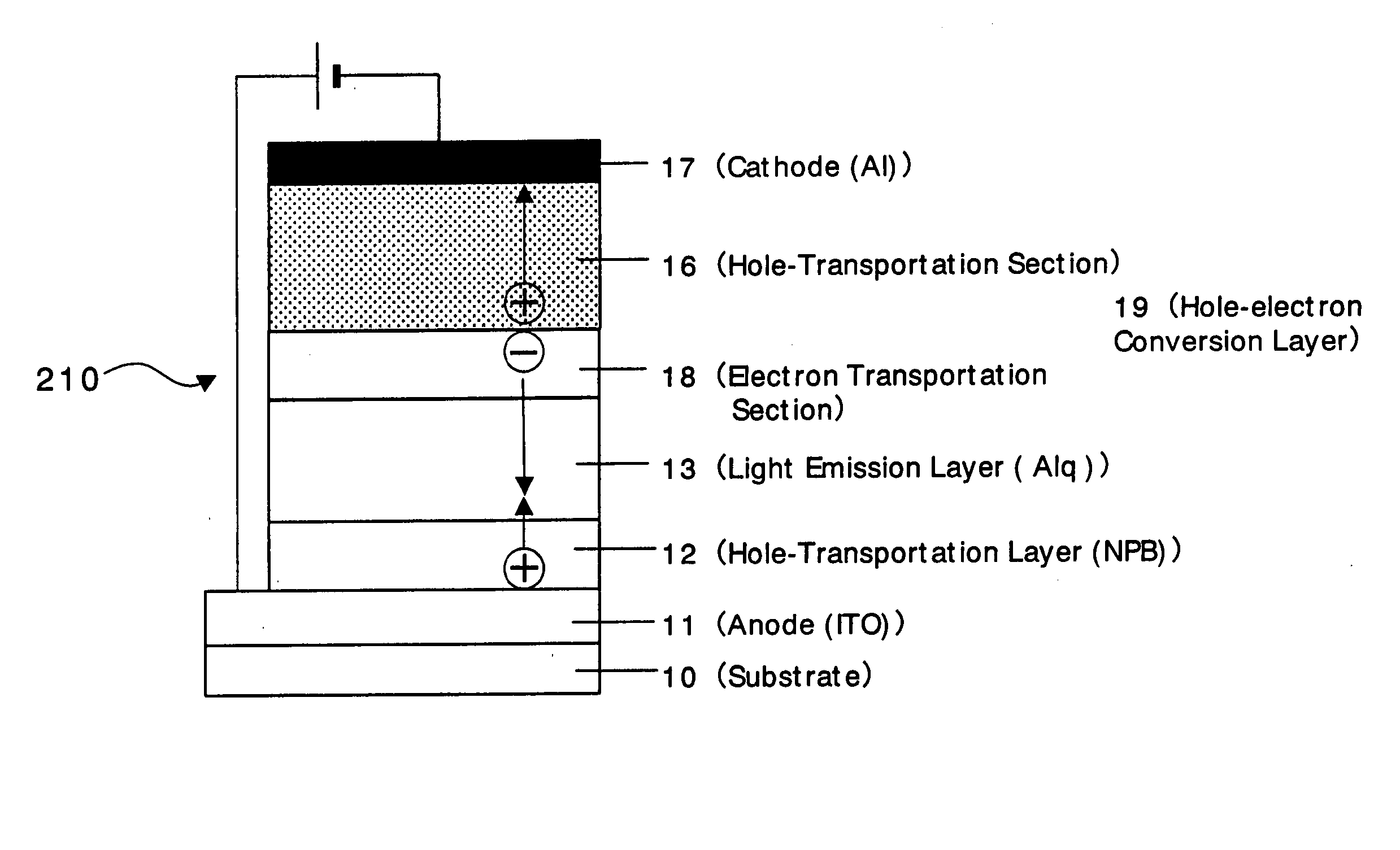
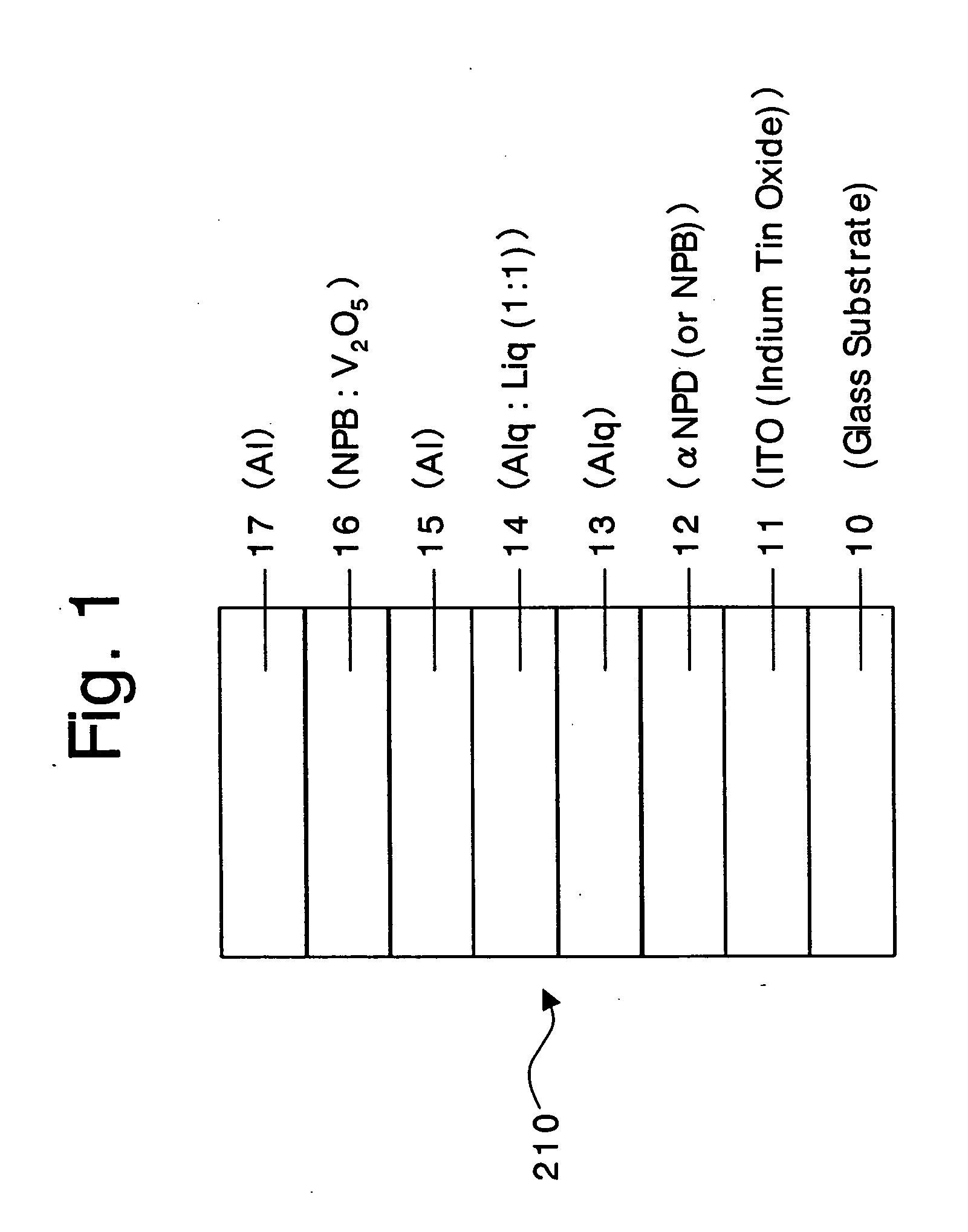
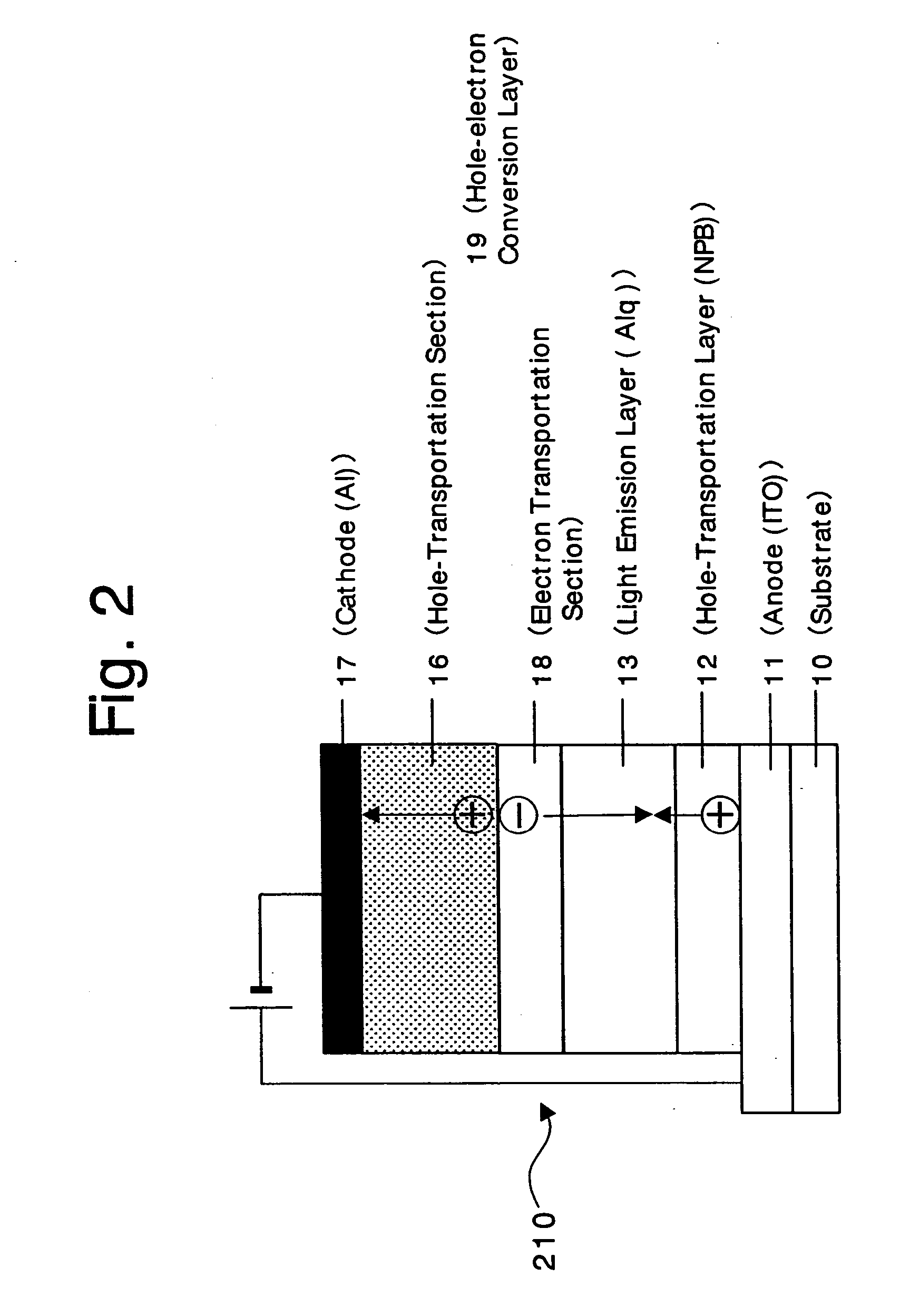
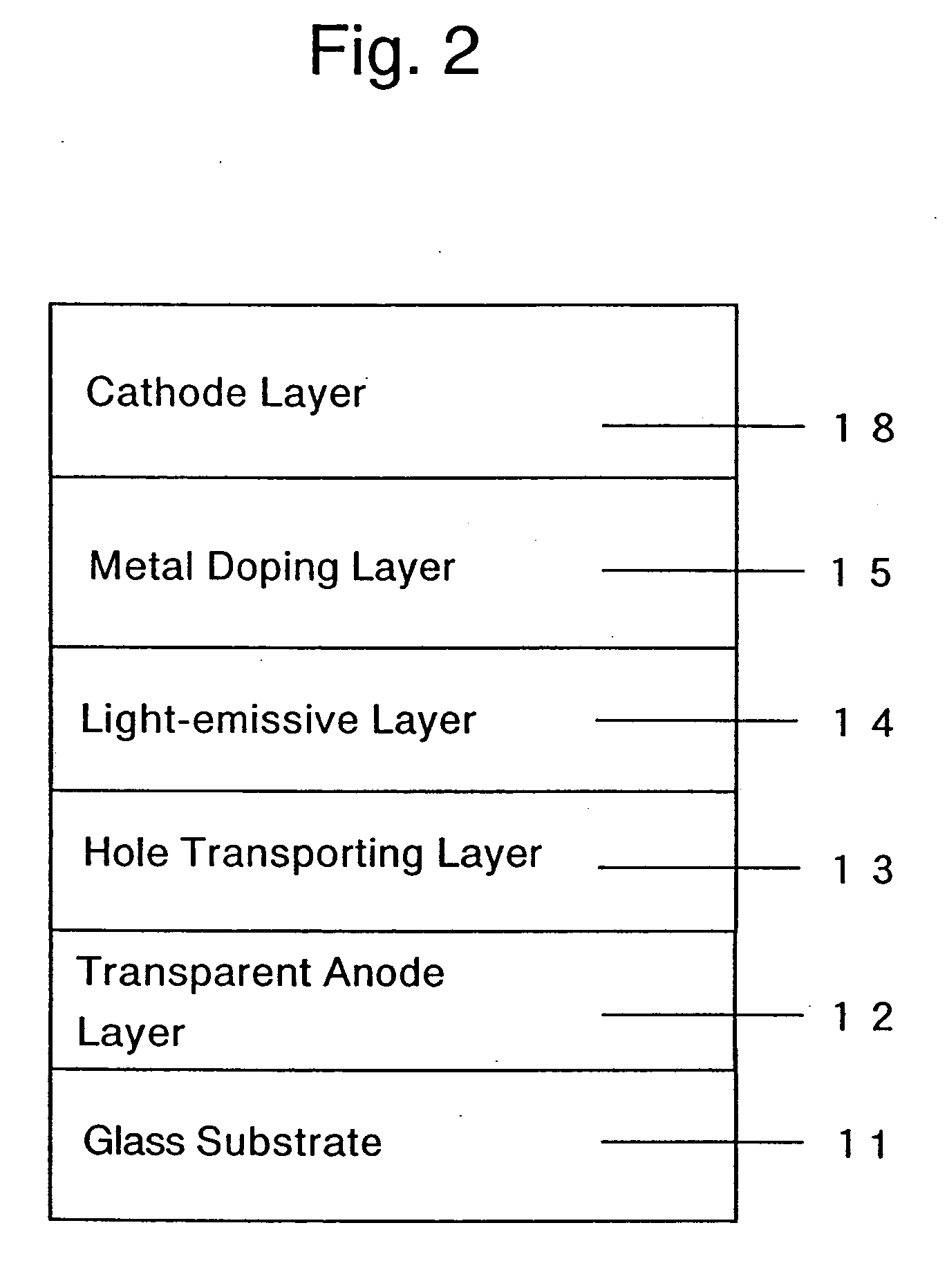
Organic devices, organic electroluminescent devices, organic solar cells, organic FET structures and production method of organic devices
ActiveUS20050098207A1TransistorDischarge tube luminescnet screensElectronic transmissionOrganic solar cell
An organic device has a hole current-electron current conversion layer which comprises a laminate of an electron transportation section and a hole transportation section. The electron transportation section includes a charge transfer complex formed upon an oxidation-reduction reaction between a reduced low work function metal and an electron-accepting organic compound, the reduced metal being produced upon an in-situ thermal reduction reaction caused upon contact, through lamination or mixing by co-deposition, of an organic metal complex compound or an inorganic compound containing at least one metal ion selected from ions of low work function metals having a work function of not more than 4.0 eV, and a thermally reducible metal capable of reducing a metal ion contained in the organic metal complex compound or the inorganic compound in vacuum to the corresponding metal state, and the electron transportation section having the electron-accepting organic compound in the state of radical anions. The hole transportation section includes an organic compound having an ionization potential of less than 5.7 eV and an electron-donating property and an inorganic or organic substance capable of forming a charge transfer complex upon its oxidation-reduction reaction with the organic compound, the organic compound and the inorganic or organic substance being contacted through lamination or mixing, and the electron-donating organic compound is in the state of radical cations.
Owner:MITSUBISHI HEAVY IND LTD +1
Semiconductor device
InactiveUS20050045919A1Increase the areaFine granularitySemiconductor/solid-state device detailsSolid-state devicesDevice materialEngineering
A programmable semiconductor device has a switch element in an interconnection layer, wherein in at least one of the inside of a via, interconnecting a wire of a first interconnection layer and a wire of a second interconnection layer, a contact part of the via with the wire of the first interconnection layer and a contact part of the via with the wire of the second interconnection layer, there is provided a variable electrical conductivity member, such as a member of an electrolyte material. The via is used as a variable electrical conductivity type switch element or as a variable resistance device having a contact part with the wire of the first interconnection layer as a first terminal and having a contact part with the wire of the second interconnection layer as a second terminal. By varying the electrical conductivity of the switch element, the state of connection of the via with the wire of the first interconnection layer and the state of connection of the via with the wire of the second interconnection layer may be variably set to a shorted state, an open-circuited state or to an intermediate state A two-state switch element includes an ion conductor for conducting metal ions interposed between the first and second electrodes. The second electrode is formed of a material lower in reactivity than the first electrode. The electrical conductivity across the first and second electrodes is changed by the oxidation-reduction reaction of the metal ions. There are provided first and second transistors of opposite polarities, connected to the first electrode, and third and fourth transistors of opposite polarities, connected to the second electrode.
Owner:NEC CORP
Electrode materials with high surface conductivity
InactiveUS6855273B2Electrode manufacturing processesDouble layer capacitorsSurface conductivityIon exchange
The present invention concerns electrode materials capable of redox reactions by electrons and alkaline ions exchange with an electrolyte. The applications are in the field of primary (batteries) or secondary electrochemical generators, super capacitors and light modulating system of the super capacitor type.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Electrochromic media for producing a preselected color
InactiveUS6141137AHigh proportionSignificant contributionOrganic chemistryTenebresent compositionsElectricityRedox
Electrochromic compositions suitable for use in electrochromic media in electrochromic devices contain minimally two electrochromic compounds of the same redox type, whose redox potentials are greater than 30 mV. The lower redox potential electrochromic compound makes a large contribution to the absorbancy of the electrochromic medium despite being present in only minor concentration.
Owner:GENTEX CORP
High energy density redox flow device
ActiveUS20110200848A1Avoid accumulationHigh enough specific energyOrganic chemistryFlow propertiesElectrochemical responseHigh energy
Redox flow devices are described in which at least one of the positive electrode or negative electrode-active materials is a semi-solid or is a condensed ion-storing electroactive material, and in which at least one of the electrode-active materials is transported to and from an assembly at which the electrochemical reaction occurs, producing electrical energy. The electronic conductivity of the semi-solid is increased by the addition of conductive particles to suspensions and / or via the surface modification of the solid in semi-solids (e.g., by coating the solid with a more electron conductive coating material to increase the power of the device). High energy density and high power redox flow devices are disclosed. The redox flow devices described herein can also include one or more inventive design features. In addition, inventive chemistries for use in redox flow devices are also described.
Owner:MASSACHUSETTS INST OF TECH +2
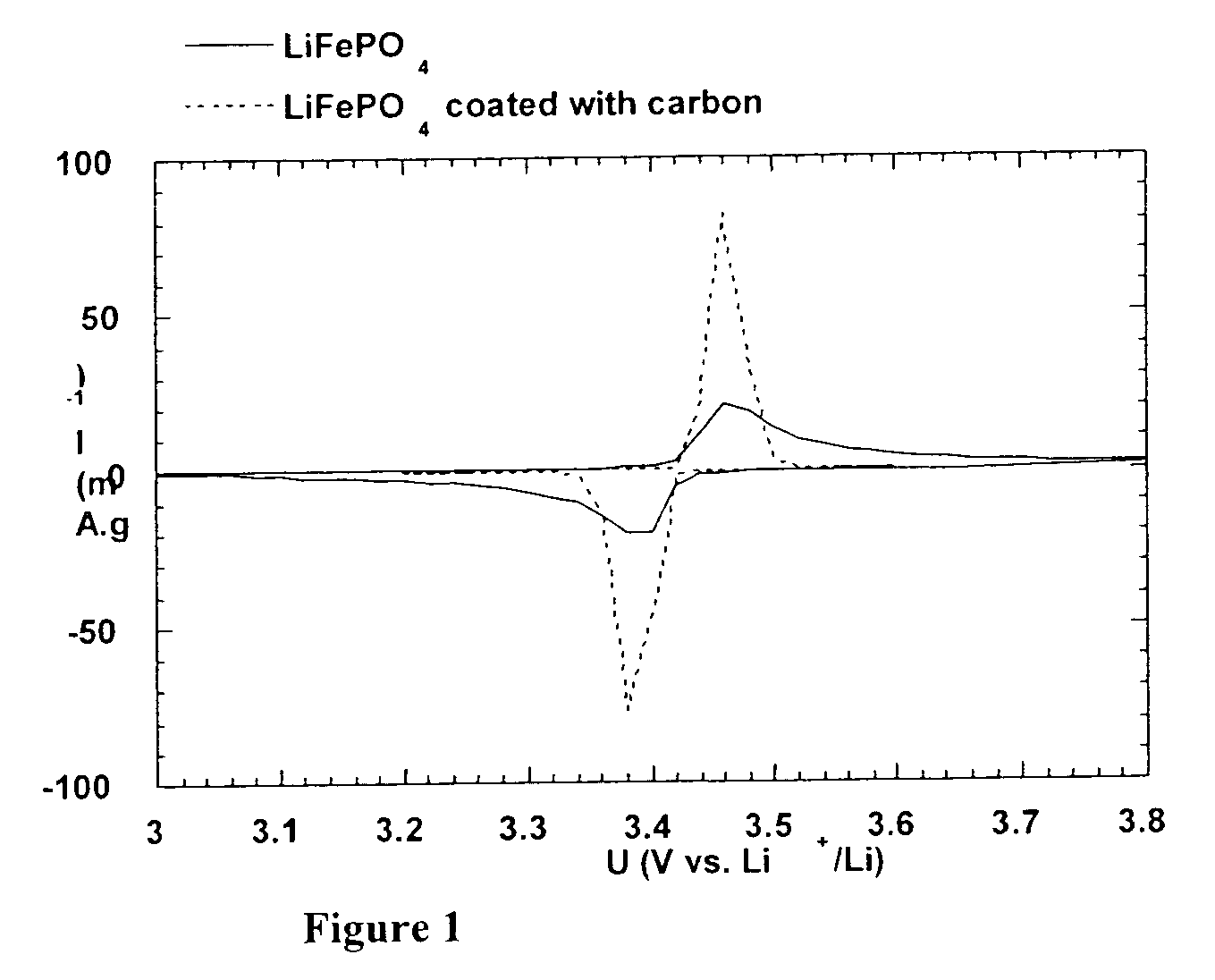
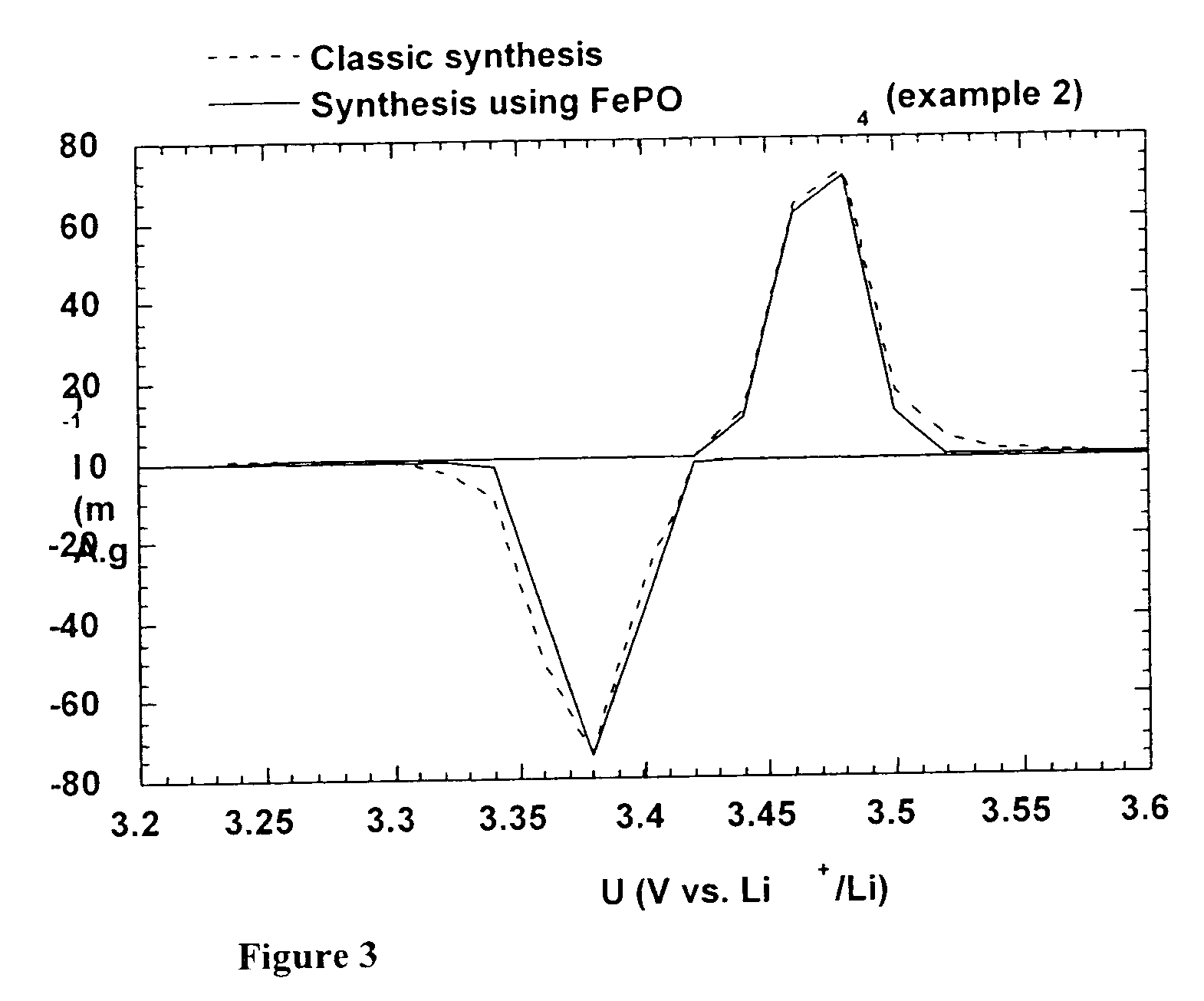
Method for synthesis of carbon-coated redox materials with controlled size
ActiveUS20040033360A1Low costReduce the numberMaterial nanotechnologyHybrid capacitorsCross-linkRedox
A method for the synthesis of compounds of the formula C-LixM1-yM'y(XO4)n, where C represents carbon cross-linked with the compound LixM1-yM'y(XO4)n, in which x, y and n are numbers such as 0<=x<=2, 0<=y<=0.6, and 1<=n<=1.5, M is a transition metal or a mixture of transition metals from the first period of the periodic table, M' is an element with fixed valency selected among Mg<2+>, Ca<2+>, Al<3+>, Zn<2+> or a combination of these same elements and X is chosen among S, P and Si, by bringing into equilibrium, in the required proportions, the mixture of precursors, with a gaseous atmosphere, the synthesis taking place by reaction and bringing into equilibrium, in the required proportions, the mixture of the precursors, the procedure comprising at least one pyrolysis step of the carbon source compound in such a way as to obtain a compound in which the electronic conductivity measured on a sample of powder compressed at a pressure of 3750 Kg.cm<-2 >is greater than 10<-8 >S.cm<-1>. The materials obtained have excellent electrical conductivity, as well a very improved chemical activity.
Owner:CENT NAT DE LA RECHERCHE SCI +2
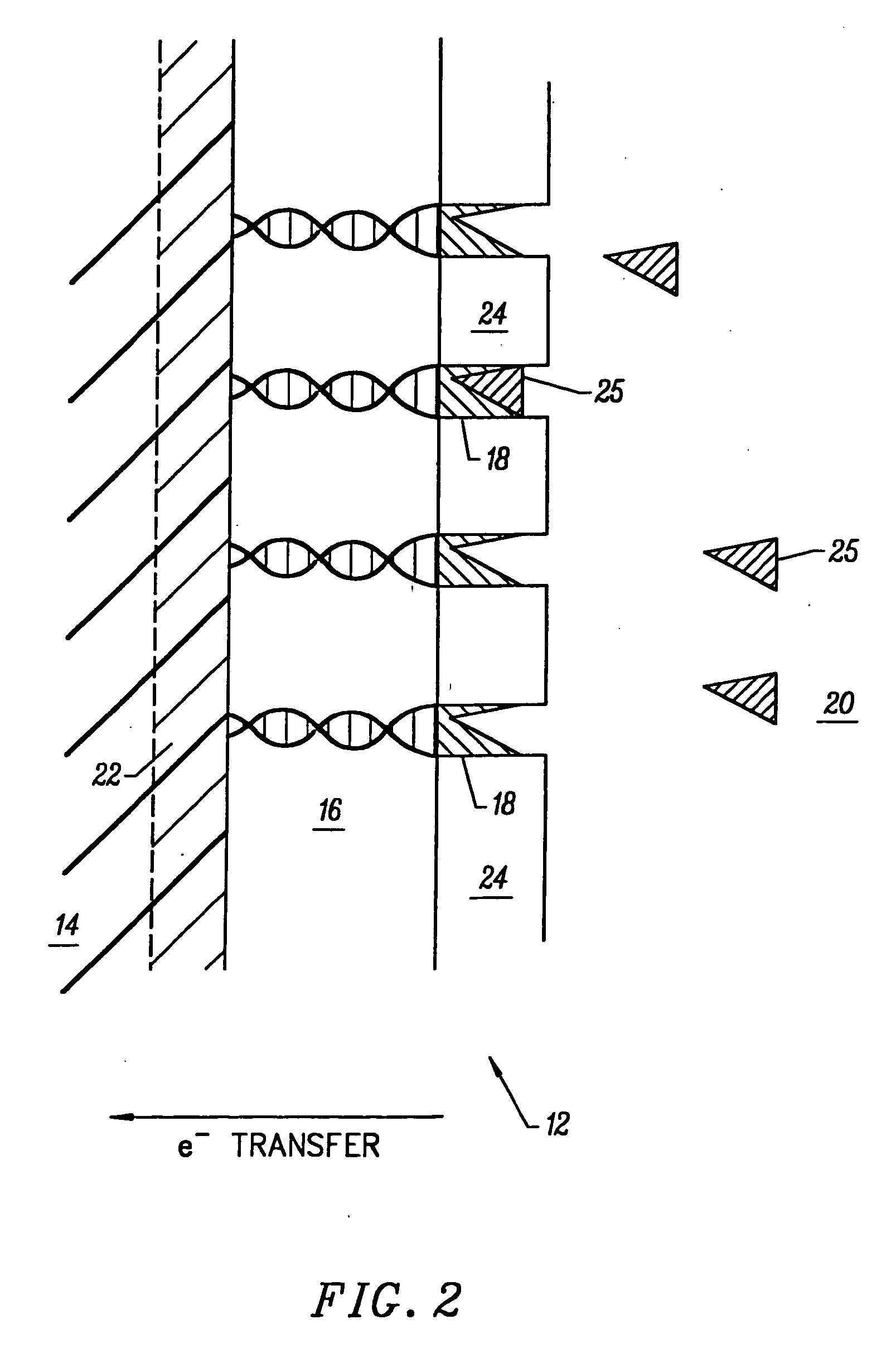
Molecular wire injection sensors
InactiveUS20060115857A1Rapid responseHigh sensitivityImmobilised enzymesBioreactor/fermenter combinationsConductive polymerMolecular wire
Disclosed is a sensor for sensing the presence of an analyte component without relying on redox mediators. This sensor includes (a) a plurality of conductive polymer strands each having at least a first end and a second end and each aligned in a substantially common orientation; (b) a plurality of molecular recognition headgroups having an affinity for the analyte component and being attached to the first ends of the conductive polymer strands; and (c) an electrode substrate attached to the conductive polymer strands at the second ends. The electrode substrate is capable of reporting to an electronic circuit reception of mobile charge carriers (electrons or holes) from the conductive polymer strands. The electrode substrate may be a photovoltaic diode.
Owner:KEENSENSE
High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions
InactiveUS20010028977A1Effective amountEasy to modifyCharging stationsCell electrodesElectricityVanadium redox battery
Disclosed is a method for preparing a high energy density (HED) electrolyte solution for use in an all-vanadium redox cells, a high energy density electrolyte solution, in particular an all-vanadium high energy density electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the high energy density electrolyte solution, a redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the HED electrolyte, a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell. A method for stabilising an electrolyte solution for use in a redox cell, in particular for stabilising an electrolyte solution for use in an all-vanadium redox cell, a stabilised electrolyte solution, in particular an all-vanadium stabilised electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the stabilised electrolyte solution, a redox battery, in particular an all-vanadium redox battery comprising the stabilised electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the stabilised electrolyte solution, and a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the stabilised electrolyte solution are disclosed. Also disclosed are a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell.
Owner:JD HLDG INC
All-solid lithium battery
ActiveUS20090081554A1Improve output performanceLayer formationSolid electrolytesElectrode carriers/collectorsRedoxSulfide
Owner:NAT INST FOR MATERIALS SCI
Process for the electrolytic deposition of metal layers
InactiveUS6099711AOptimize allocationImpairing propertyCellsElectrolysisHigh current densityMetal coating
PCT No. PCT / EP96 / 05140 Sec. 371 Date Apr. 23, 1998 Sec. 102(e) Date Apr. 23, 1998 PCT Filed Nov. 21, 1996 PCT Pub. No. WO97 / 19206 PCT Pub. Date May 29, 1997The invention relates to a method for the electrolytic deposition of metal coatings, in particular of copper coatings with certain physical-mechanical and optical properties and uniform coating thickness. According to known methods using soluble anodes and applying direct current, only uneven metal distribution can be attained on complex shaped workpieces. By using a pulse current or pulse voltage method, the problem of the coatings being of varying thickness at various places on the workpiece surfaces can indeed be reduced. However, the further problem of the geometric ratios being changed continuously during the depositing process by dissolving of the anodes is not resolved thus. This can be avoided by using insoluble anodes. In order to guarantee sufficient stability of the anodes and a bright coating even at those points on the workpiece surfaces, onto which the metal is deposited with high current density, it is essential to add compounds of an electrochemically reversible redox system to the depositing solution.
Owner:ATOTECH DEUT GMBH
Stabilized vanadium electrolyte solutions for all-vanadium redox cells and batteries
InactiveUS6562514B1Effective amountEasy to modifyFinal product manufactureRegenerative fuel cellsRedoxPhysical chemistry
Owner:JD HLDG INC
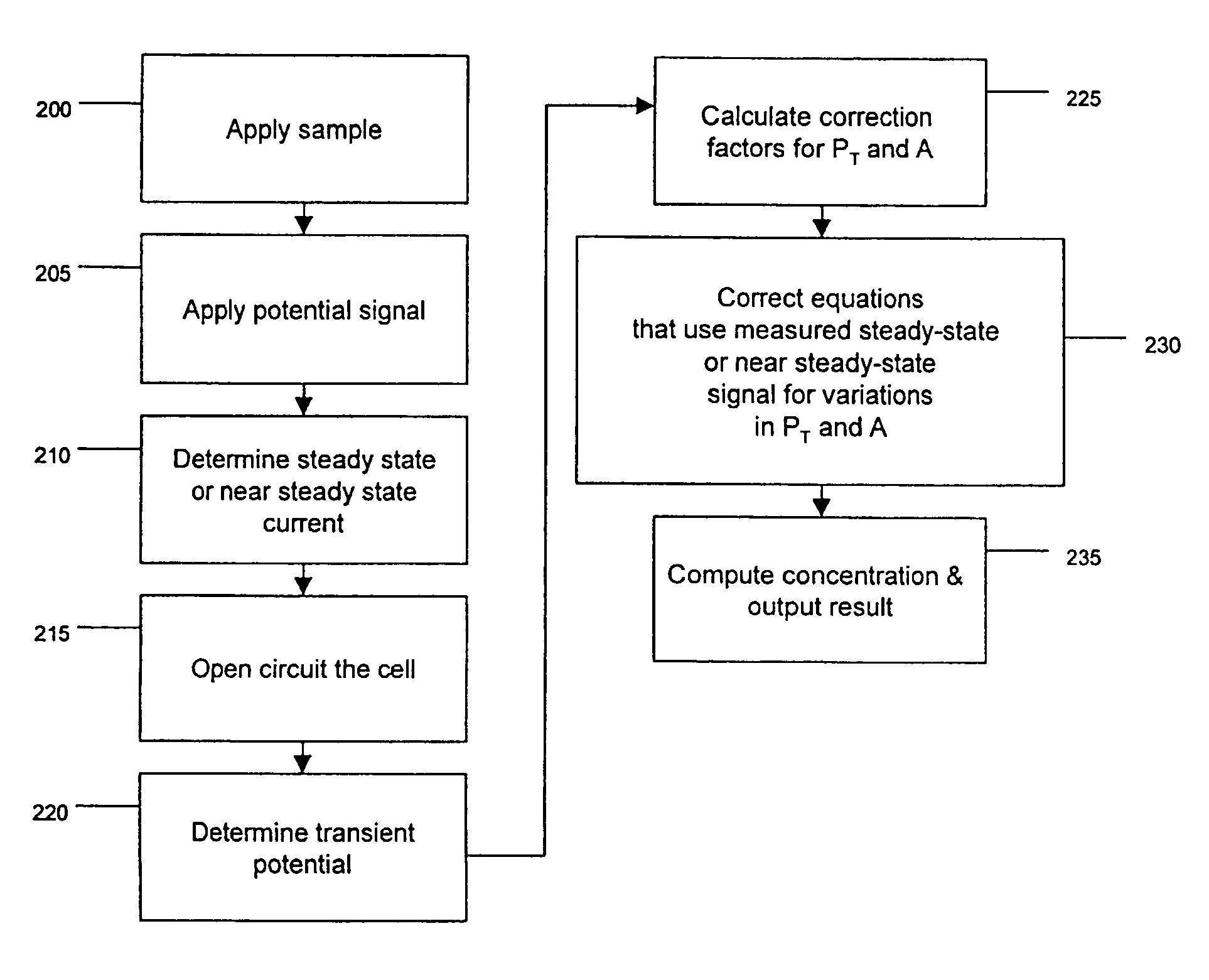
Method and apparatus for assay of electrochemical properties
ActiveUS20050109637A1Easy to measureImprove accuracyWeather/light/corrosion resistanceMicrobiological testing/measurementHand heldApplied potential
The presence of a select analyte in the sample is evaluated in an an electrochemical system using a conduction cell-type apparatus. A potential or current is generated between the two electrodes of the cell sufficient to bring about oxidation or reduction of the analyte or of a mediator in an analyte-detection redox system, thereby forming a chemical potential gradient of the analyte or mediator between the two electrodes After the gradient is established, the applied potential or current is discontinued and an analyte-independent signal is obtained from the relaxation of the chemical potential gradient. The analyte-independent signal is used to correct the analyte-dependent signal obtained during application of the potential or current. This correction allows an improved measurement of analyte concentration because it corrects for device-specific and test specific factors such as transport (mobility) of analyte and / or mediator, effective electrode area, and electrode spacing (and as a result, sample volume), without need for separate calibration values. The analysis can be performed using disposable test strips in a hand held meter, for example for glucose testing.
Owner:AGAMATRIX INC
Method of measuring blood component, sensor used in the method, and measuring device
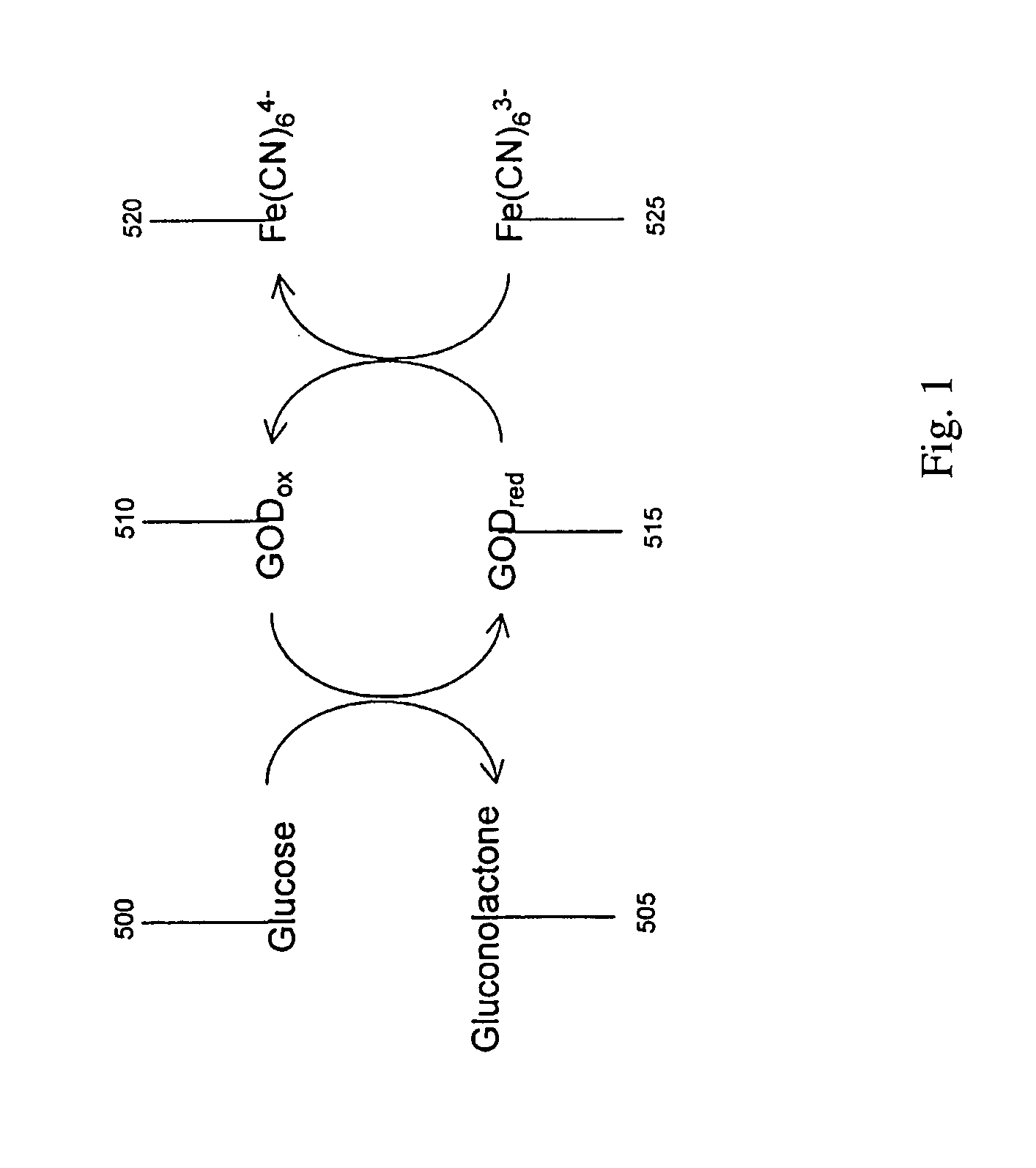
ActiveUS20070131565A1Easy to measureImprove accuracyImmobilised enzymesBioreactor/fermenter combinationsOxidoreductasePhysics
The present invention provides a method of measuring a component in blood, by which an amount of the component can be corrected accurately by measuring a hematocrit (Hct) value of the blood with high accuracy and high reliability and also provides a sensor used in the method. The sensor for measuring a component in blood has a first analysis portion and a second analysis portion. The first analysis portion has a first electrode system (11,12) and a reagent layer (14), and the reagent layer (14) has an oxidoreductase that acts on the component and a mediator. In the first analysis portion, the component in the blood is measured by causing a redox reaction of the component with the oxidoreductase in the presence of the mediator and detecting a redox current caused when a voltage is applied by the first electrode (11,12). The second analysis portion has a working electrode and a counter electrode, and a mediator is provided on the counter electrode but not on the working electrode. In the second analysis portion, a Hct value of the blood is measured by supplying the blood to the electrode system, applying a voltage to cause a current to flow, and detecting a value of the current. Using this Hct value, the amount of the component is corrected.
Owner:PHC HLDG CORP
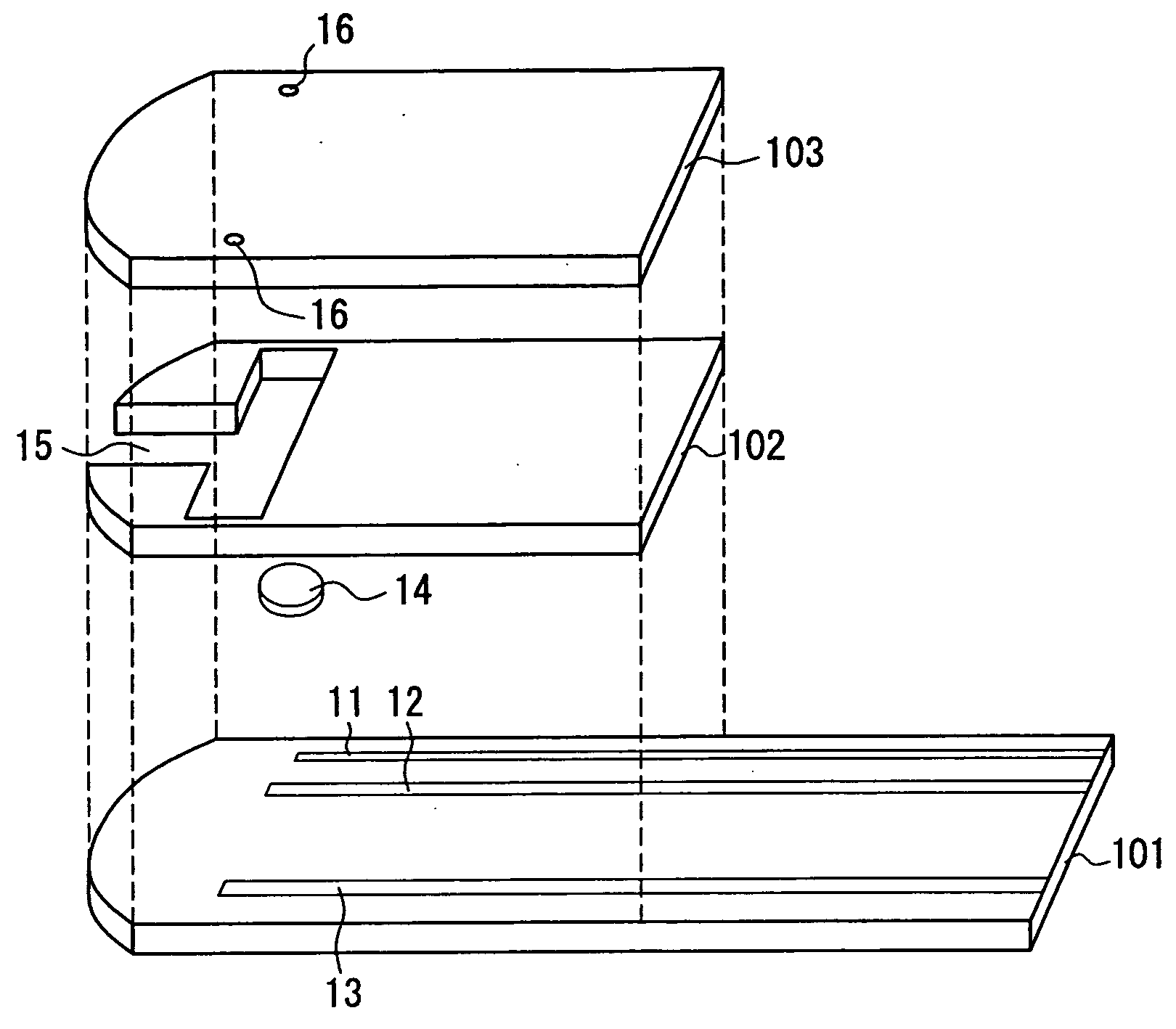
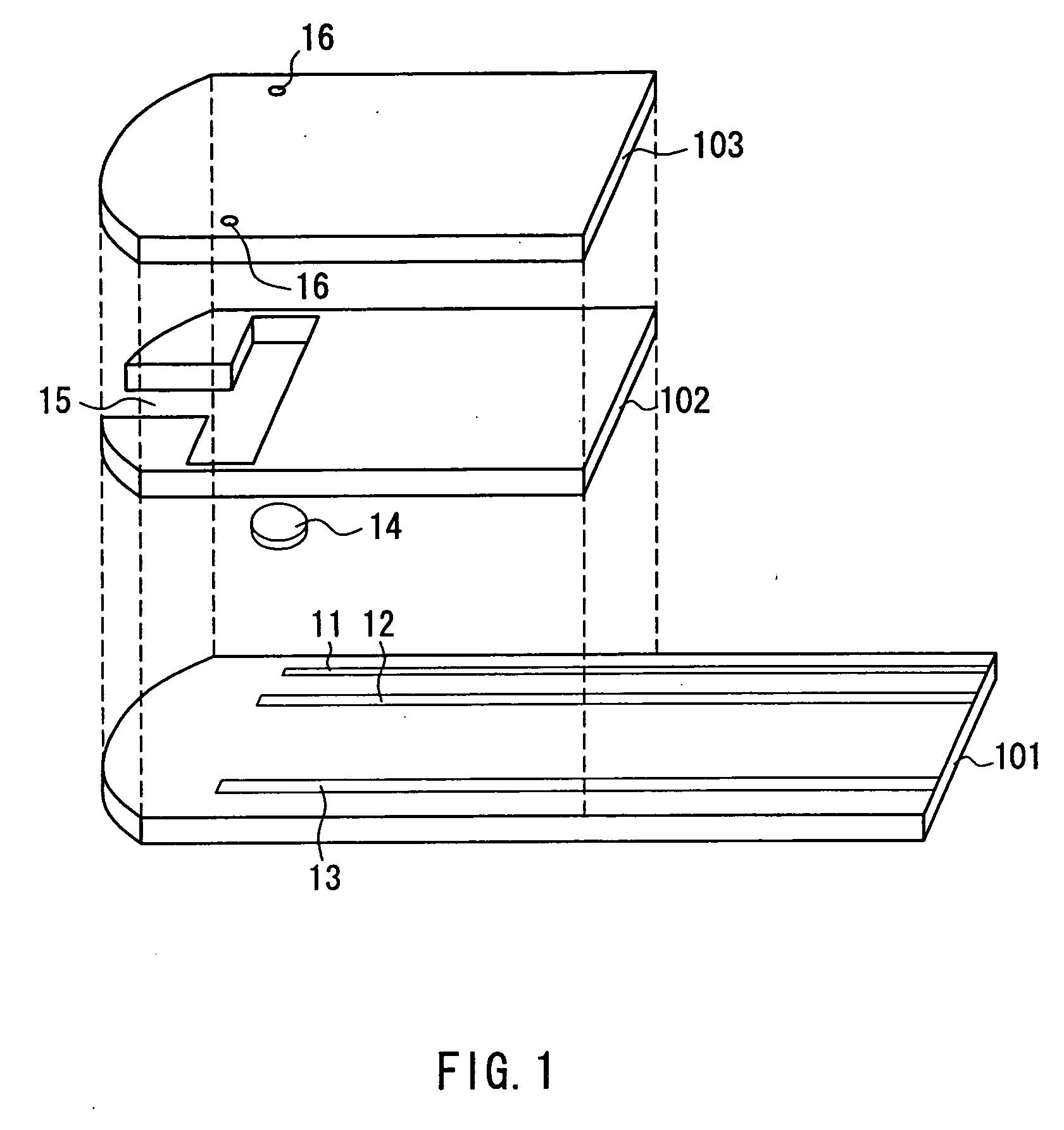
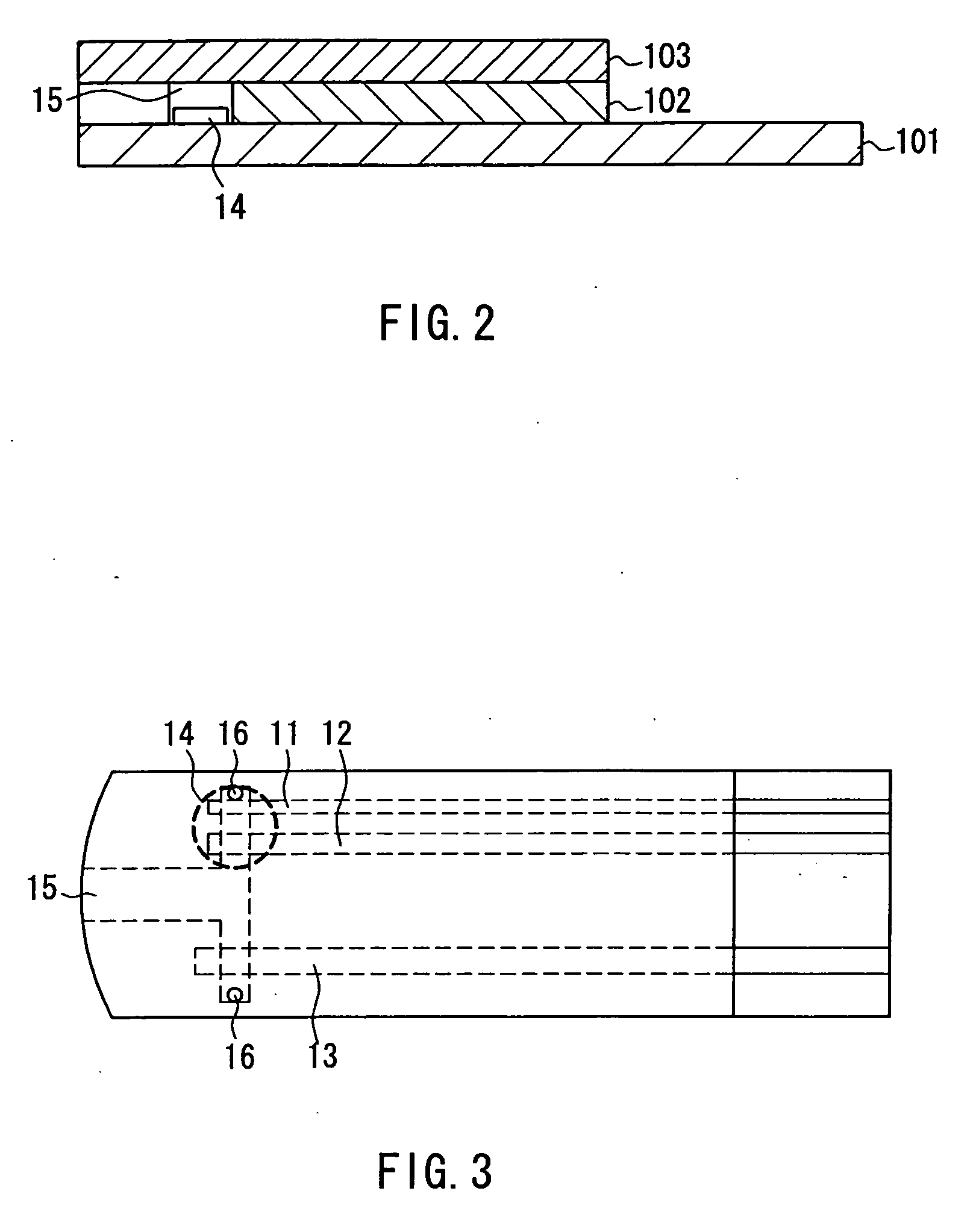
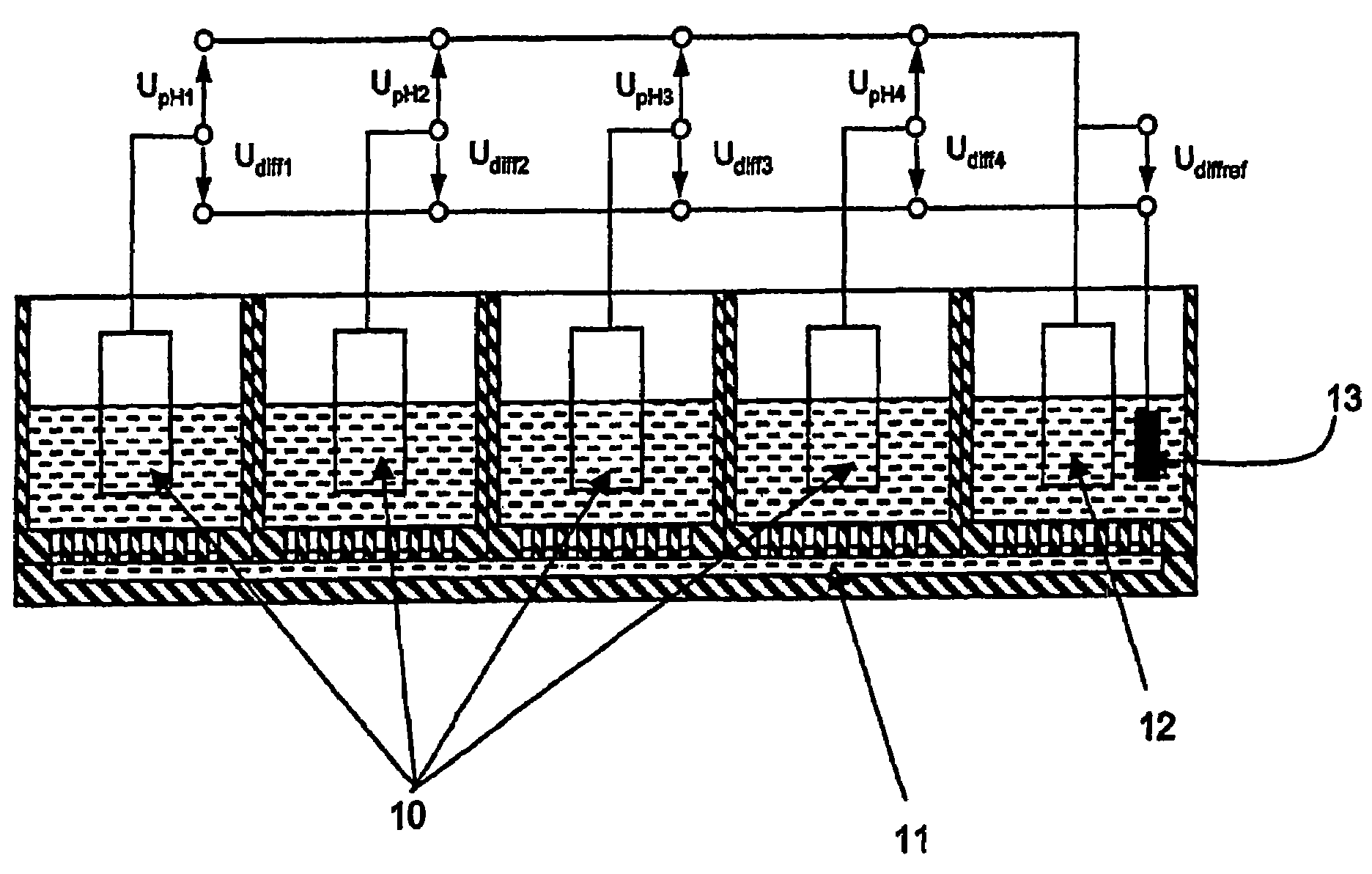
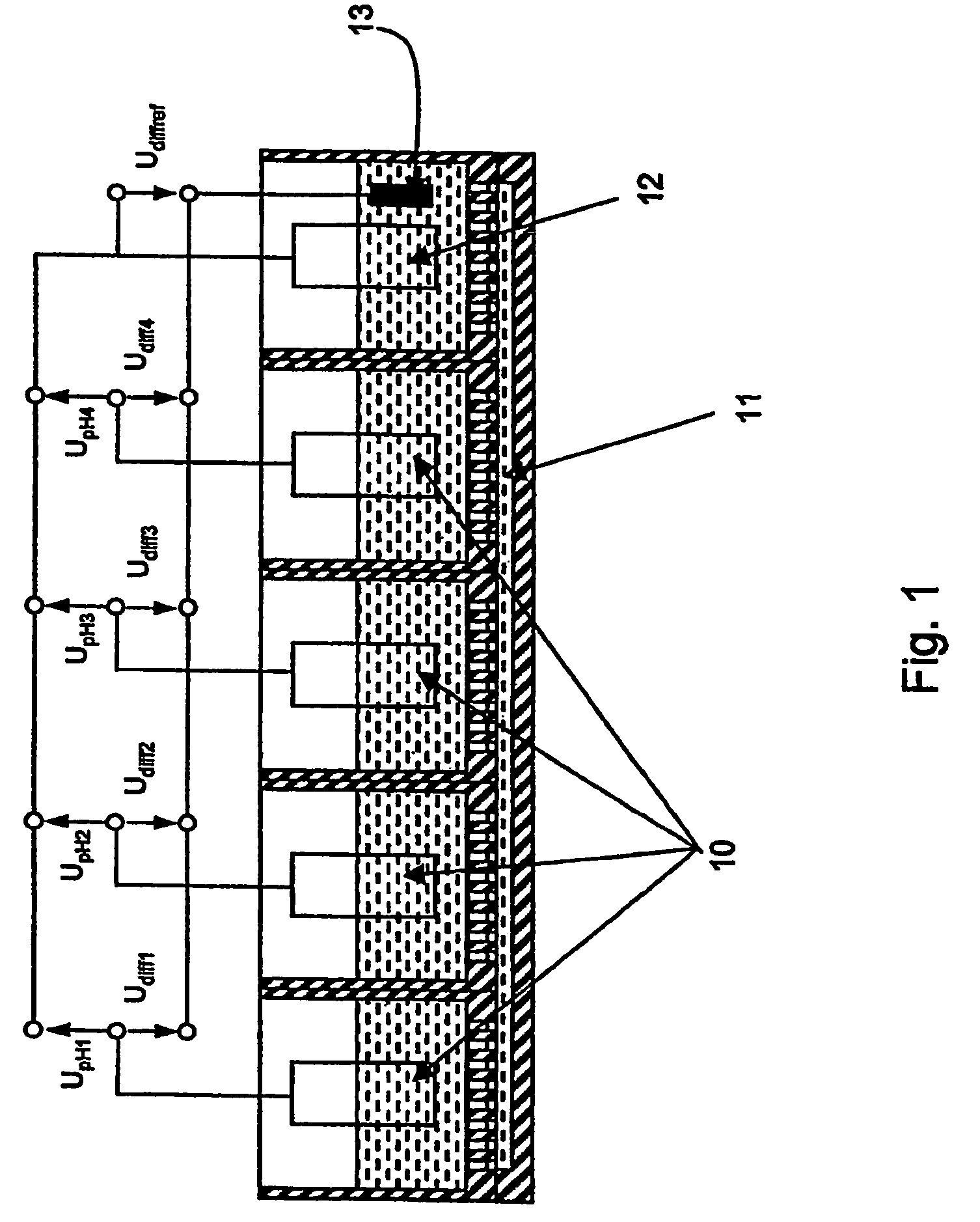
Sensor arrangement with a plurality of potentiometric sensors
InactiveUS7394263B2Improve sealingSafely accommodatedResistance/reactance/impedenceMicrobiological testing/measurementPotential differenceRedox
The sensor arrangement includes: a least two sample chambers; at least two potentiometric FET-sensors, especially ISFET-sensors or ChemFET-sensors, having, in each case, a sensitive surface section, wherein each sensitive surface section lies in flow connection with its one of the sample chambers; and a reference cell having a reference medium for providing a reference potential, wherein the sample chambers are connected with the reference medium via an electrolyte bridge. The reference cell has, preferably, a potentiometric reference-FET-sensor for providing a reference potential, which is registered against the pseudo-reference-potential of a redox electrode. The potentials Udiff1, Udiff2, . . . UdiffN of N FET-sensors in the sample chambers are determined against the pseudo-reference-potential, and the measured-variable-relevant, potential differences are determined, in each case, by difference formation between the pertinent potential and the reference potential—thus, in the case of pH, according to the formulasUpH1. . . N=Udiff1. . . N−Udiffref.
Owner:ENDRESS HAUSER CONDUCTA GESELLSCHAFT FUER MESS UND REGELTECHNIK MBH CO KG
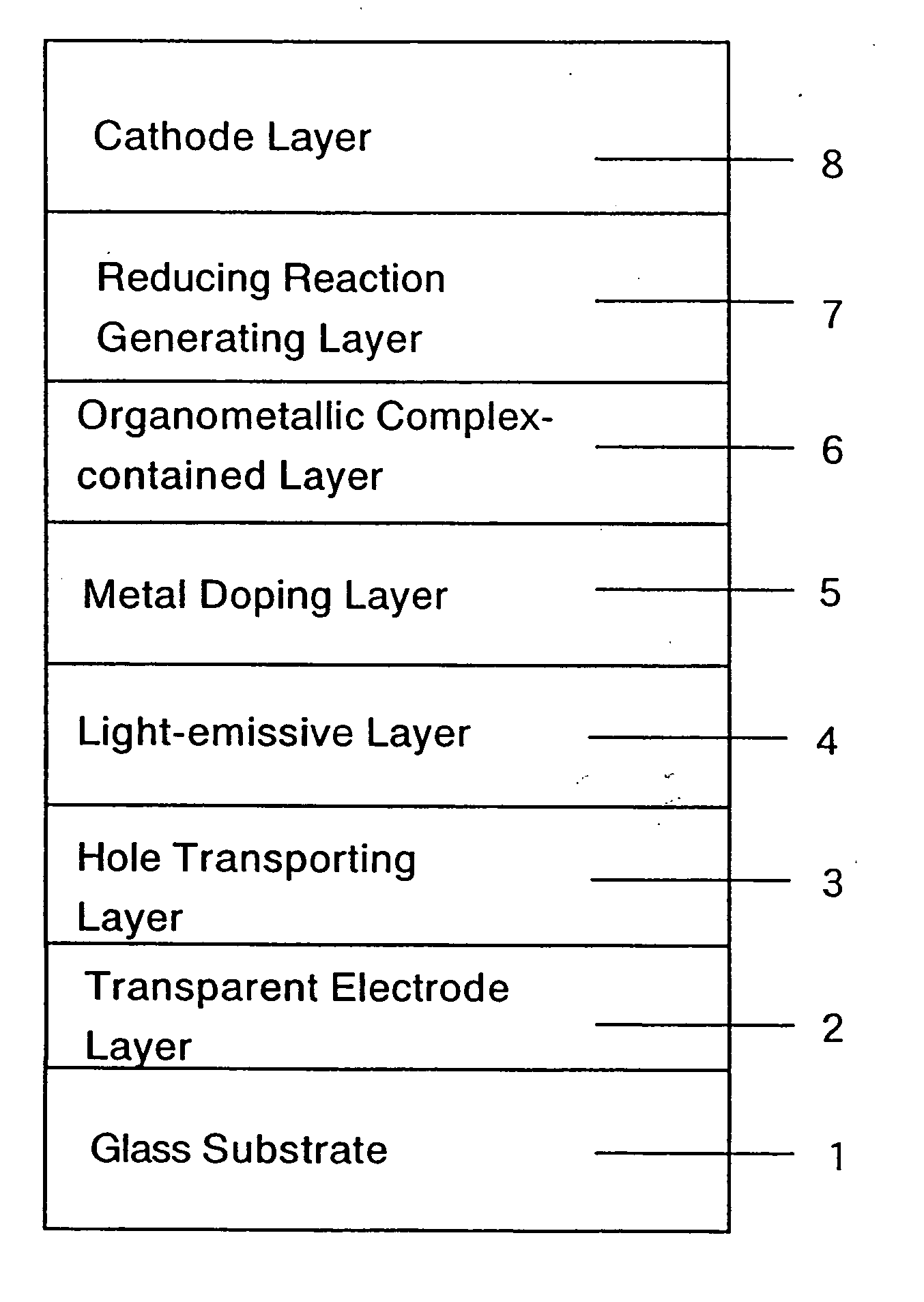
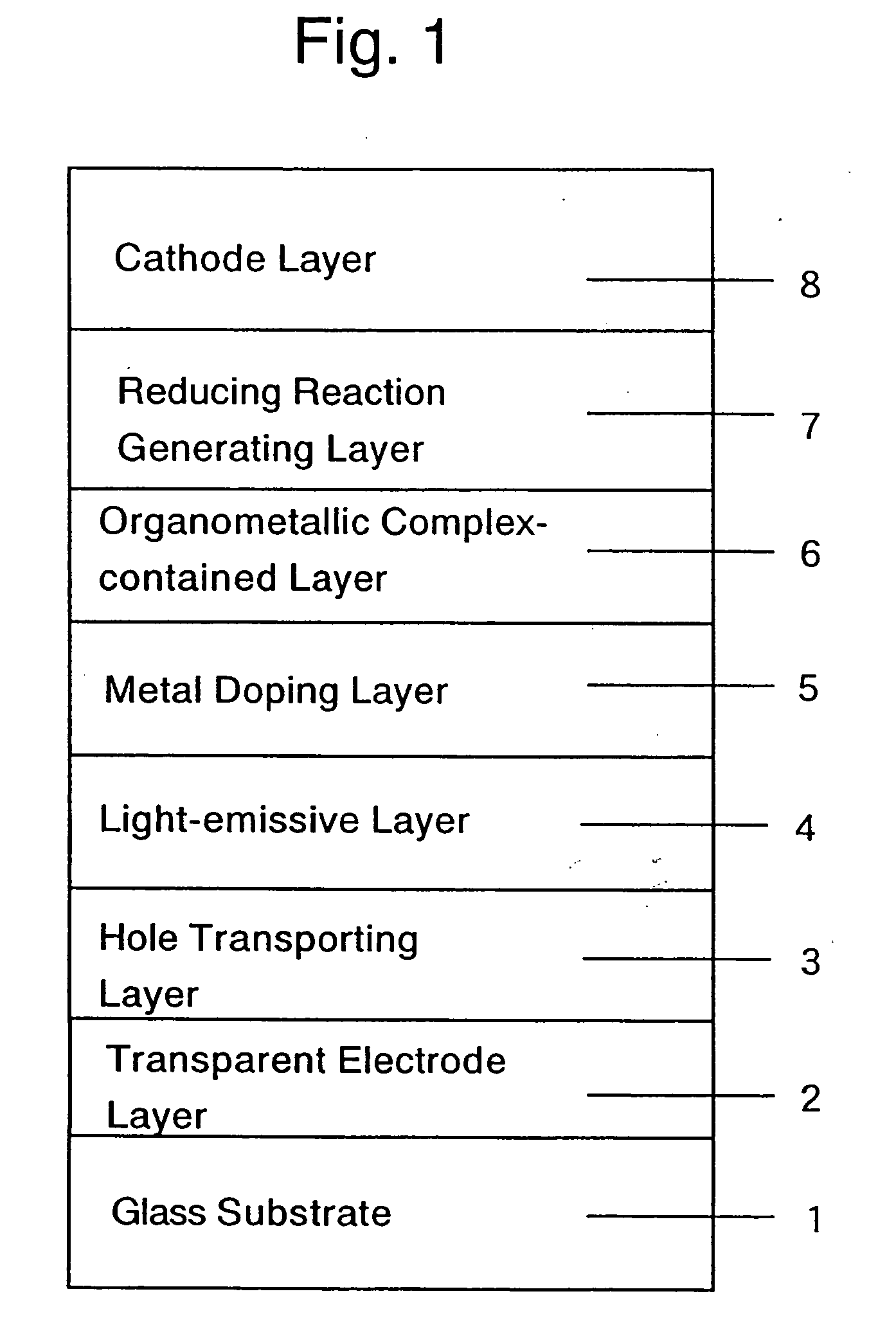
Organic electroluminescent device and production process thereof
ActiveUS20050084713A1Stable device propertyIncrease the driving voltageDischarge tube luminescnet screensElectroluminescent light sourcesOrganic structureAlkaline earth metal
Owner:ROHM CO LTD +1
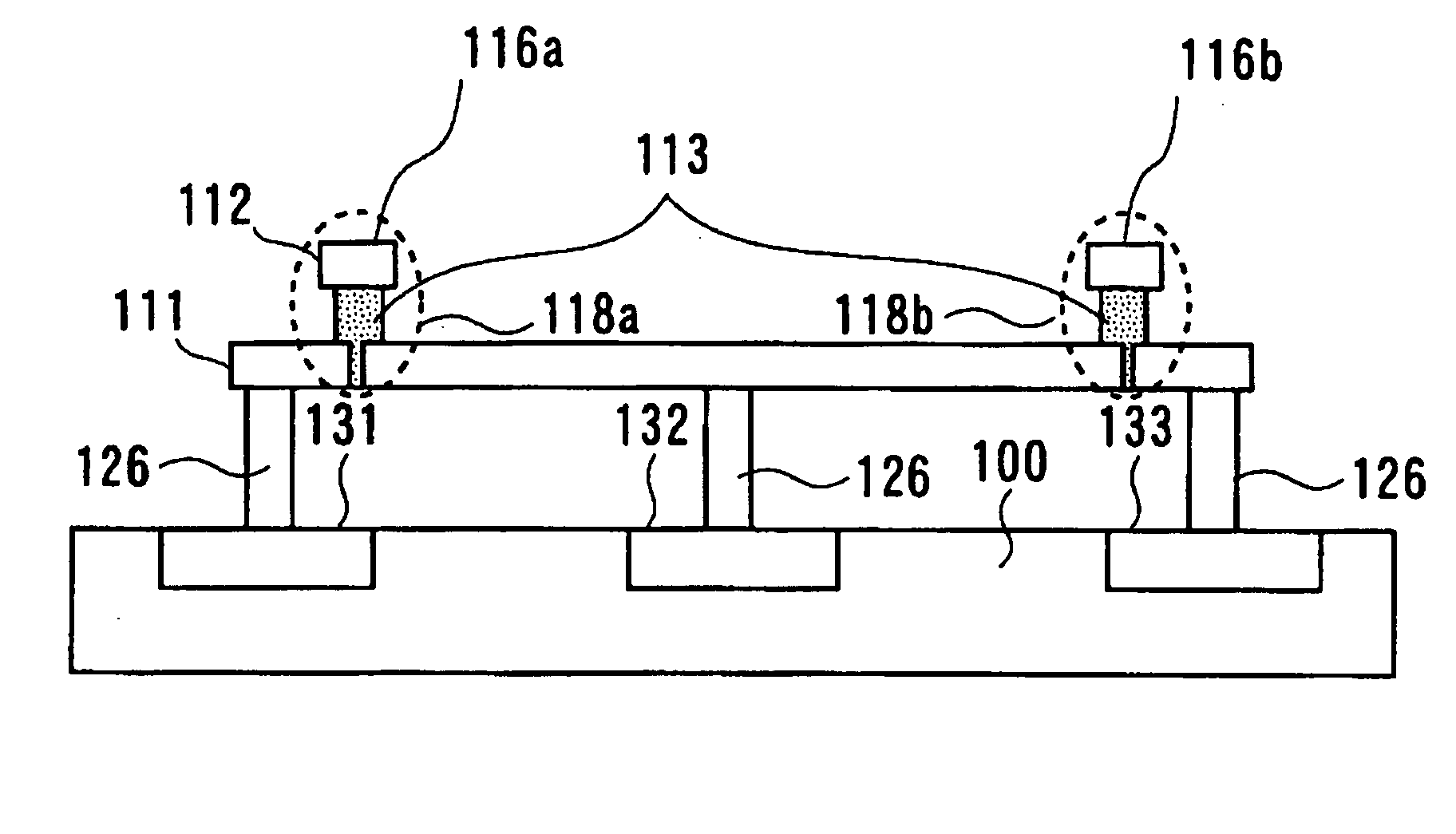
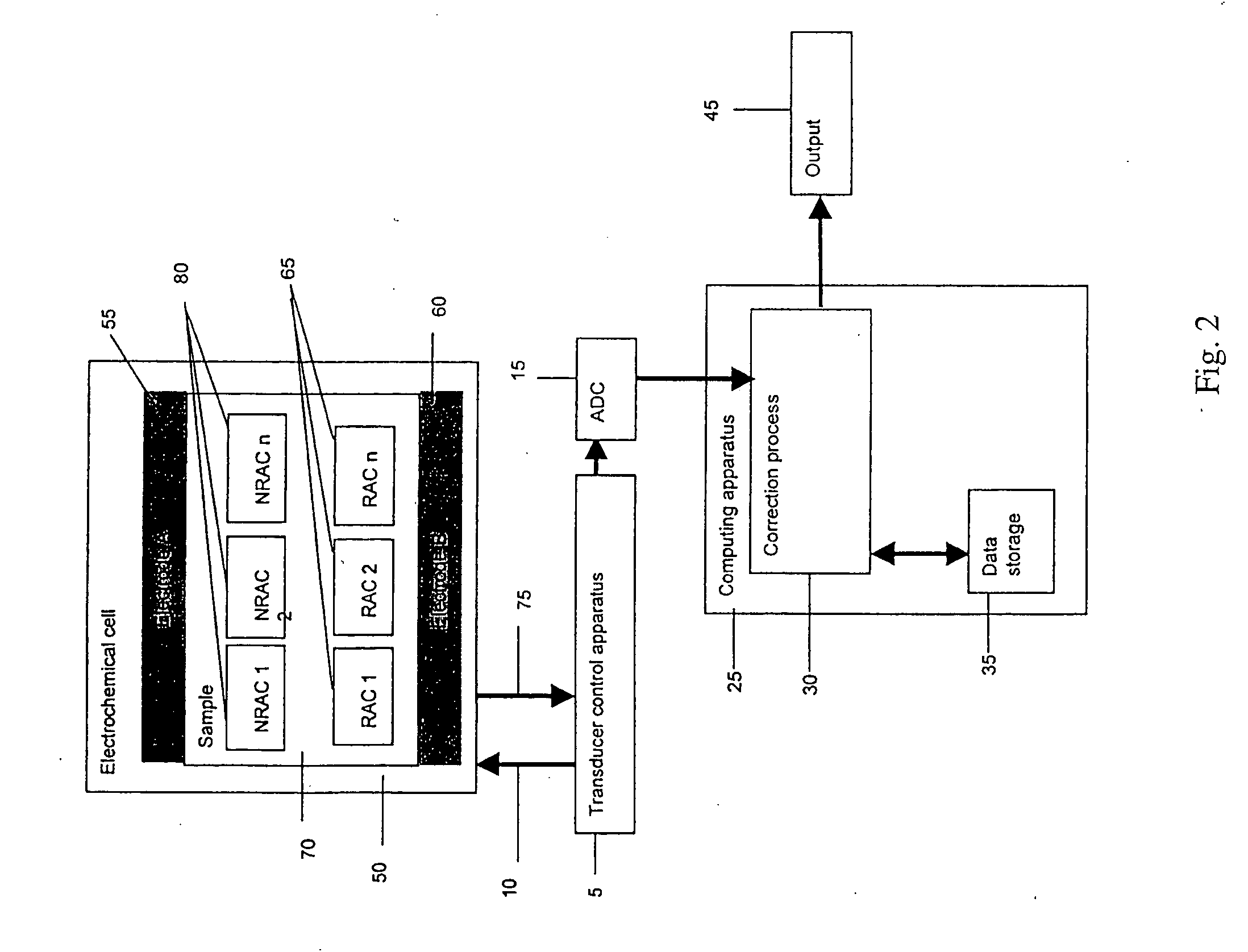
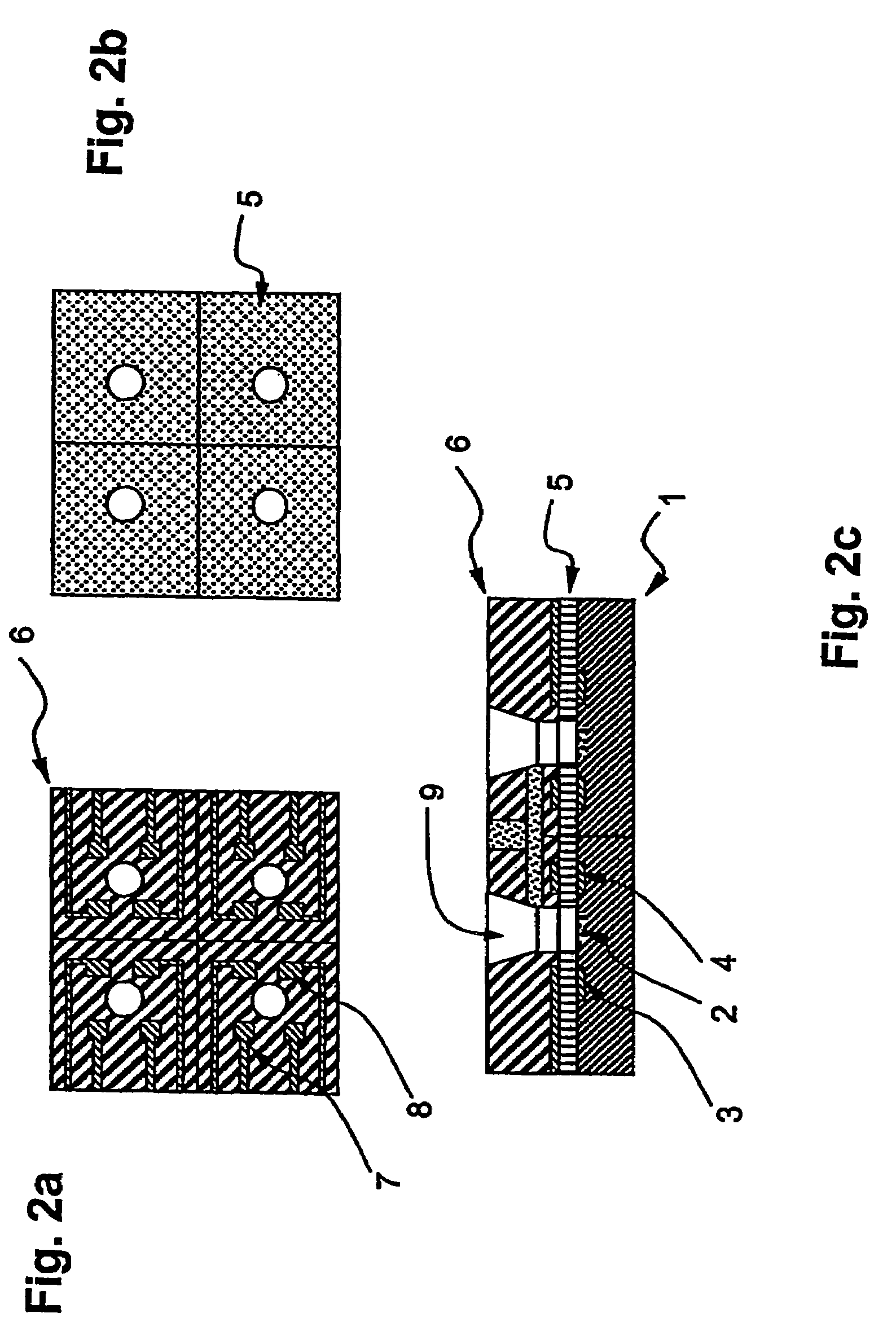
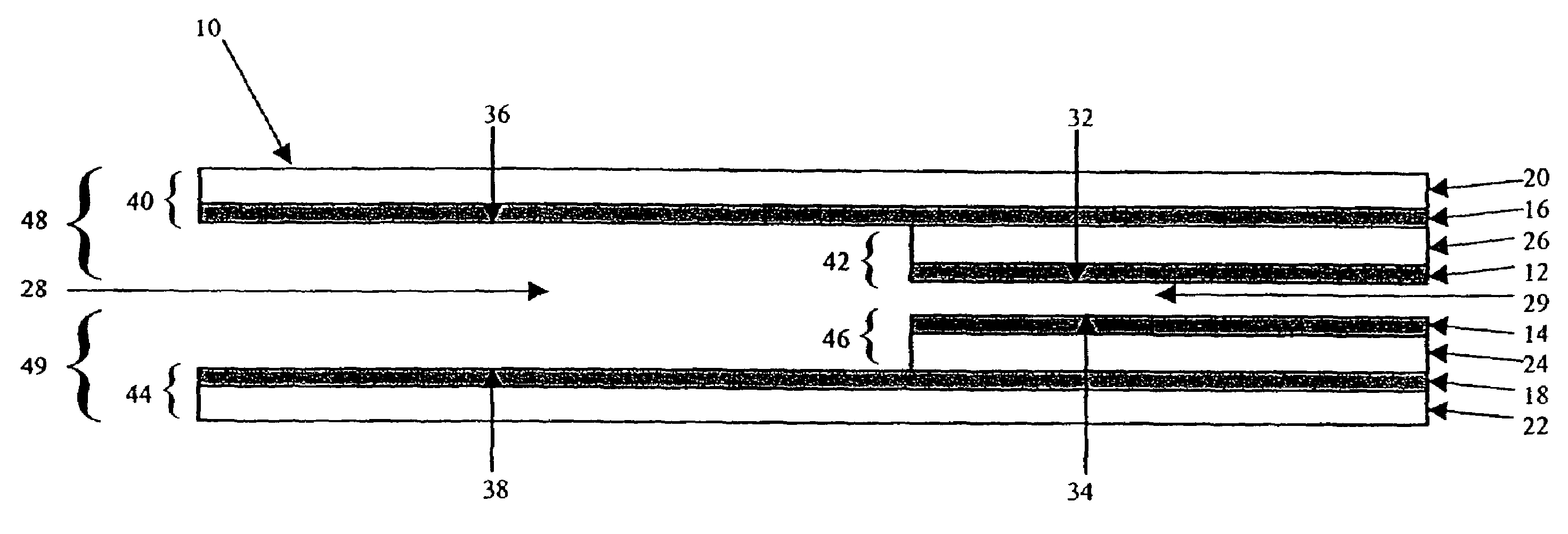
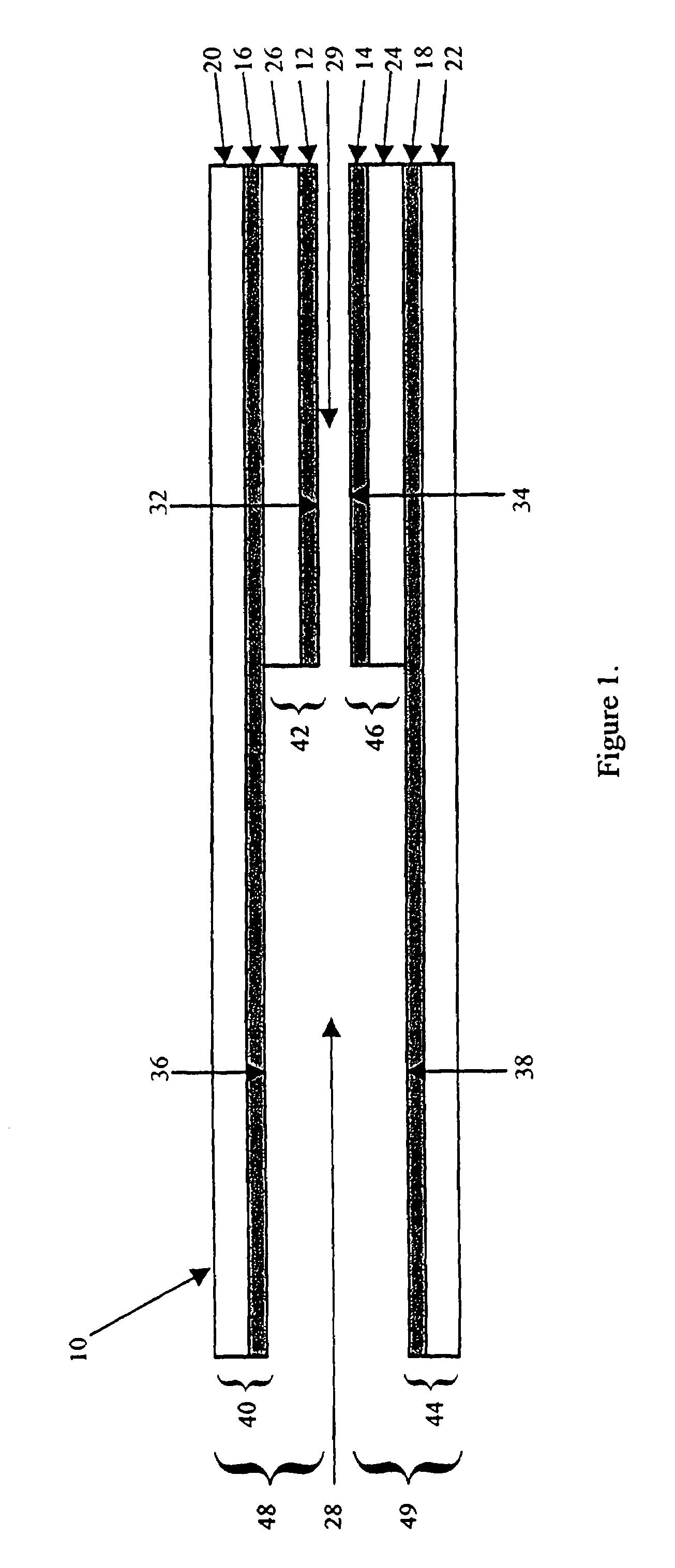
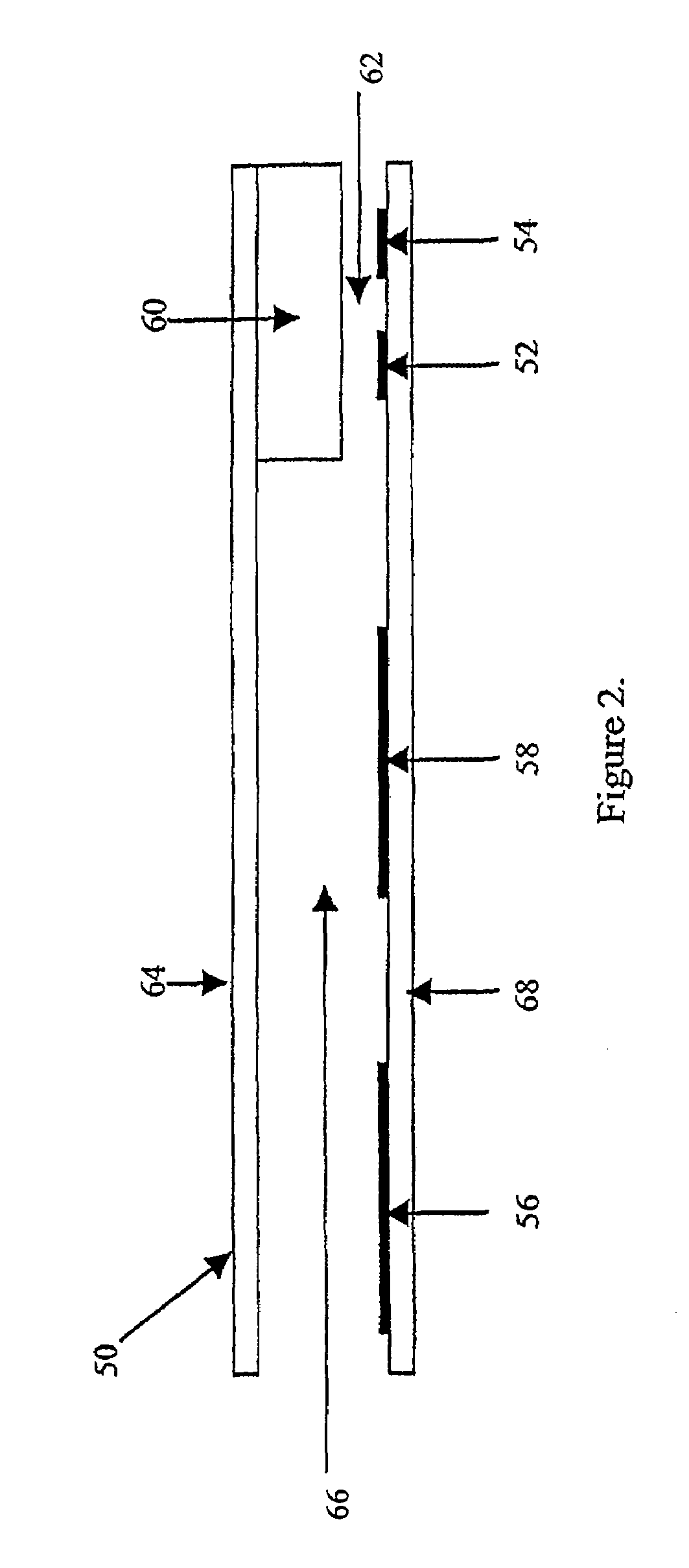
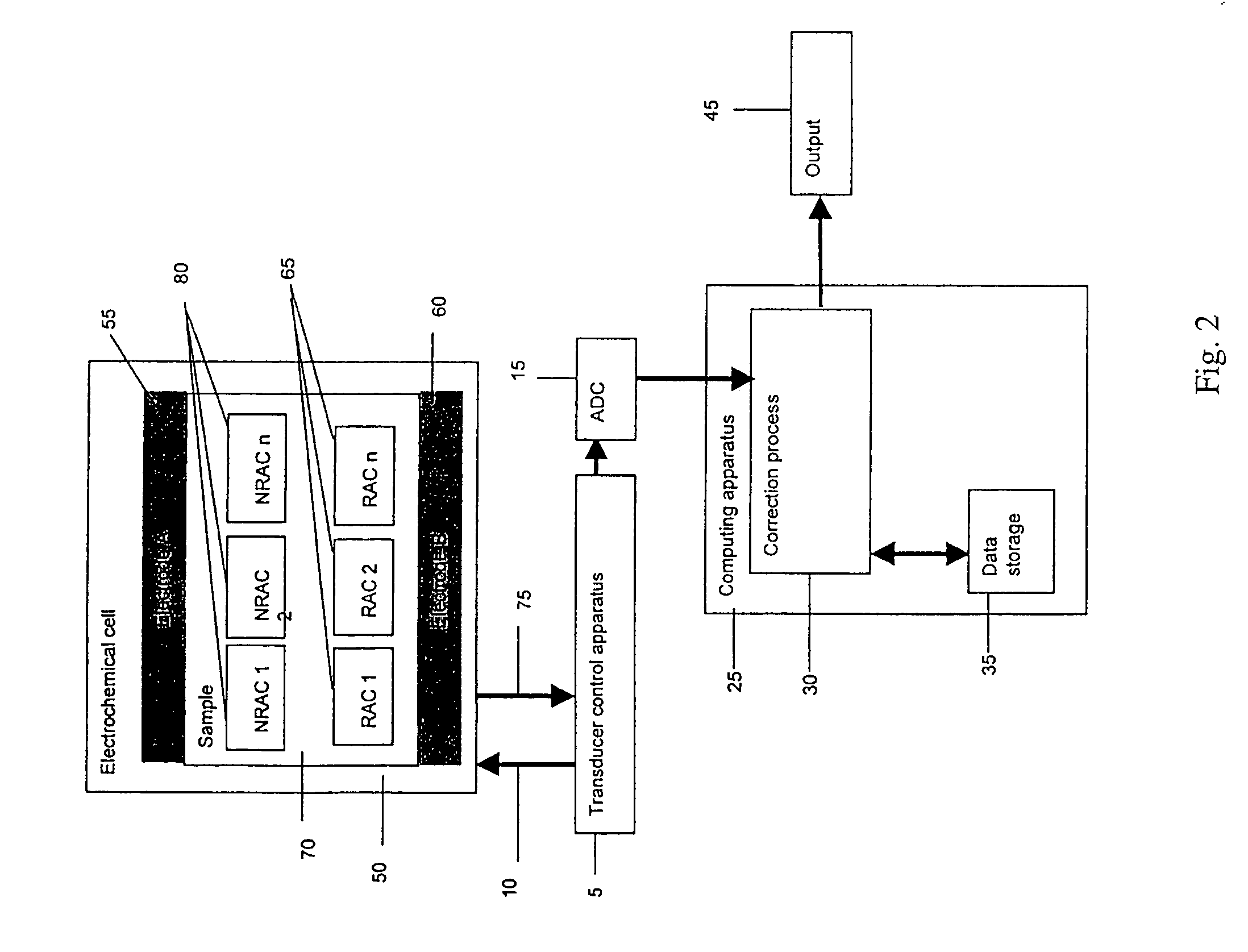
Electrochemical cell
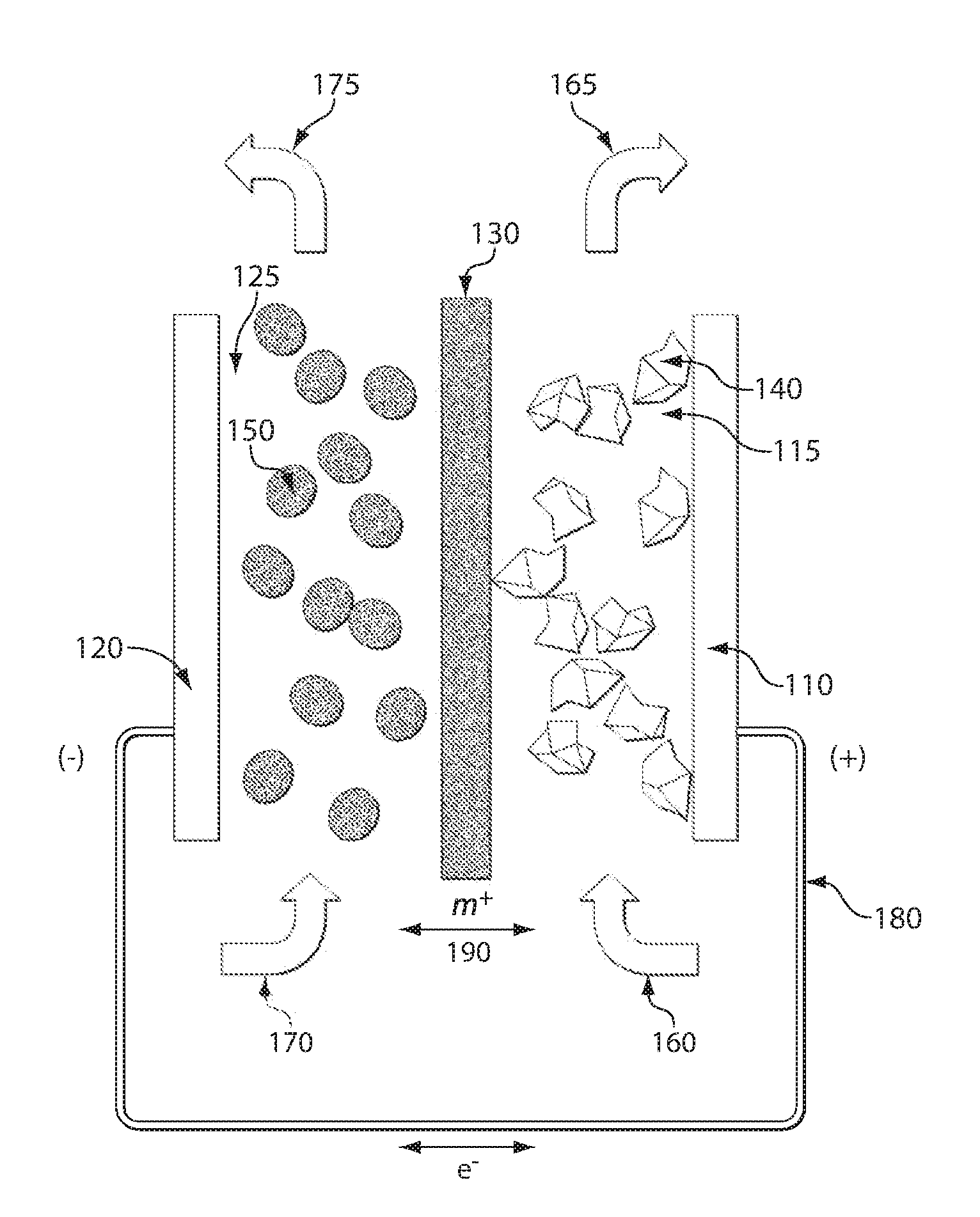
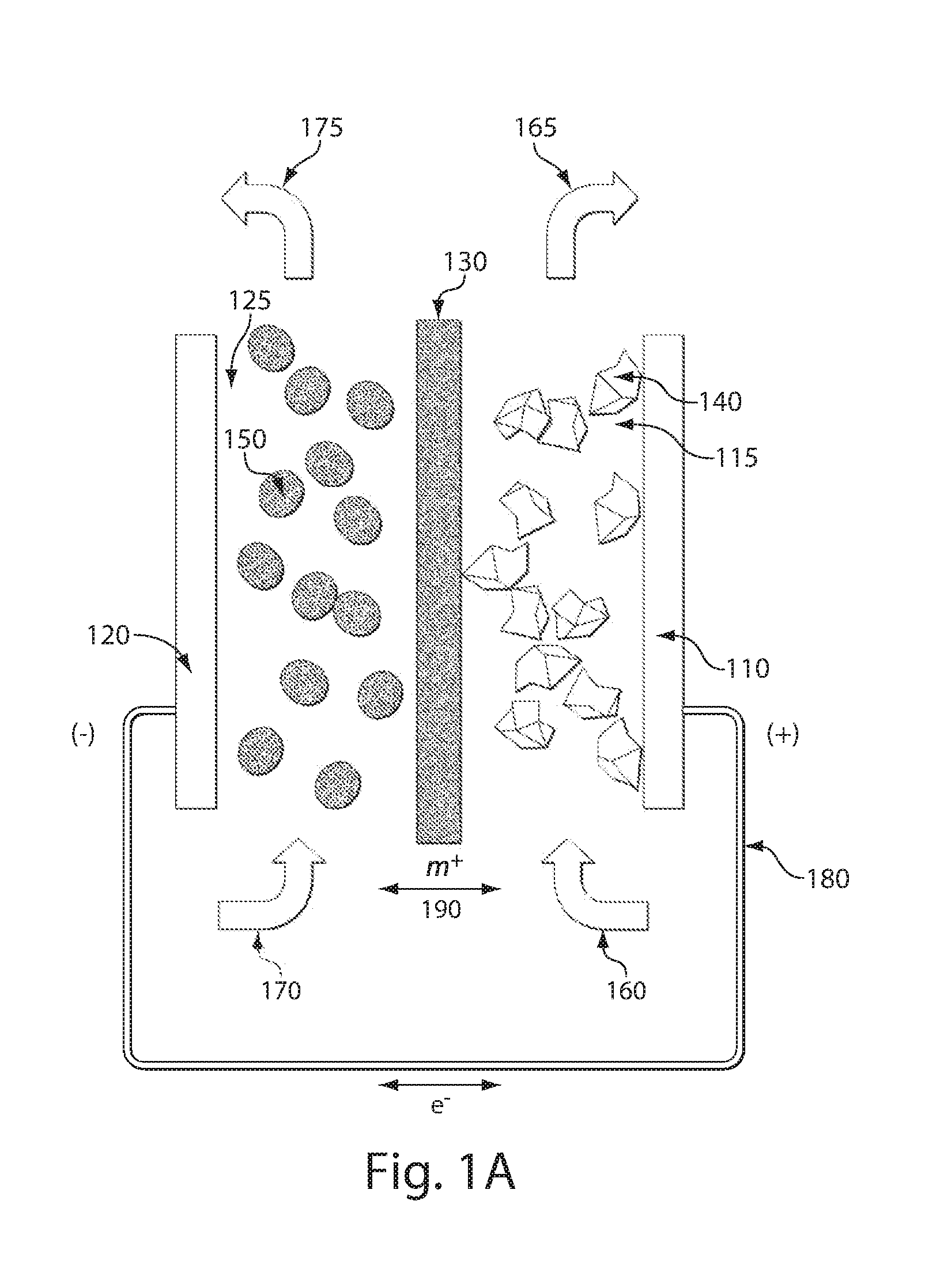
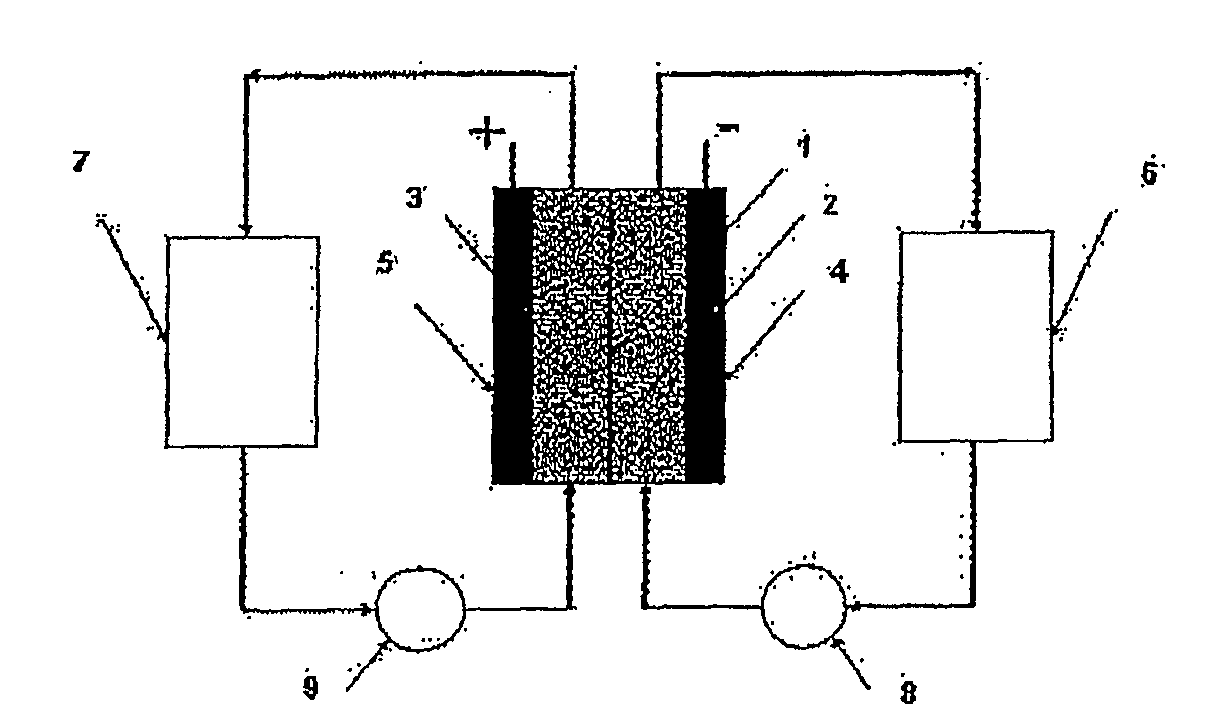
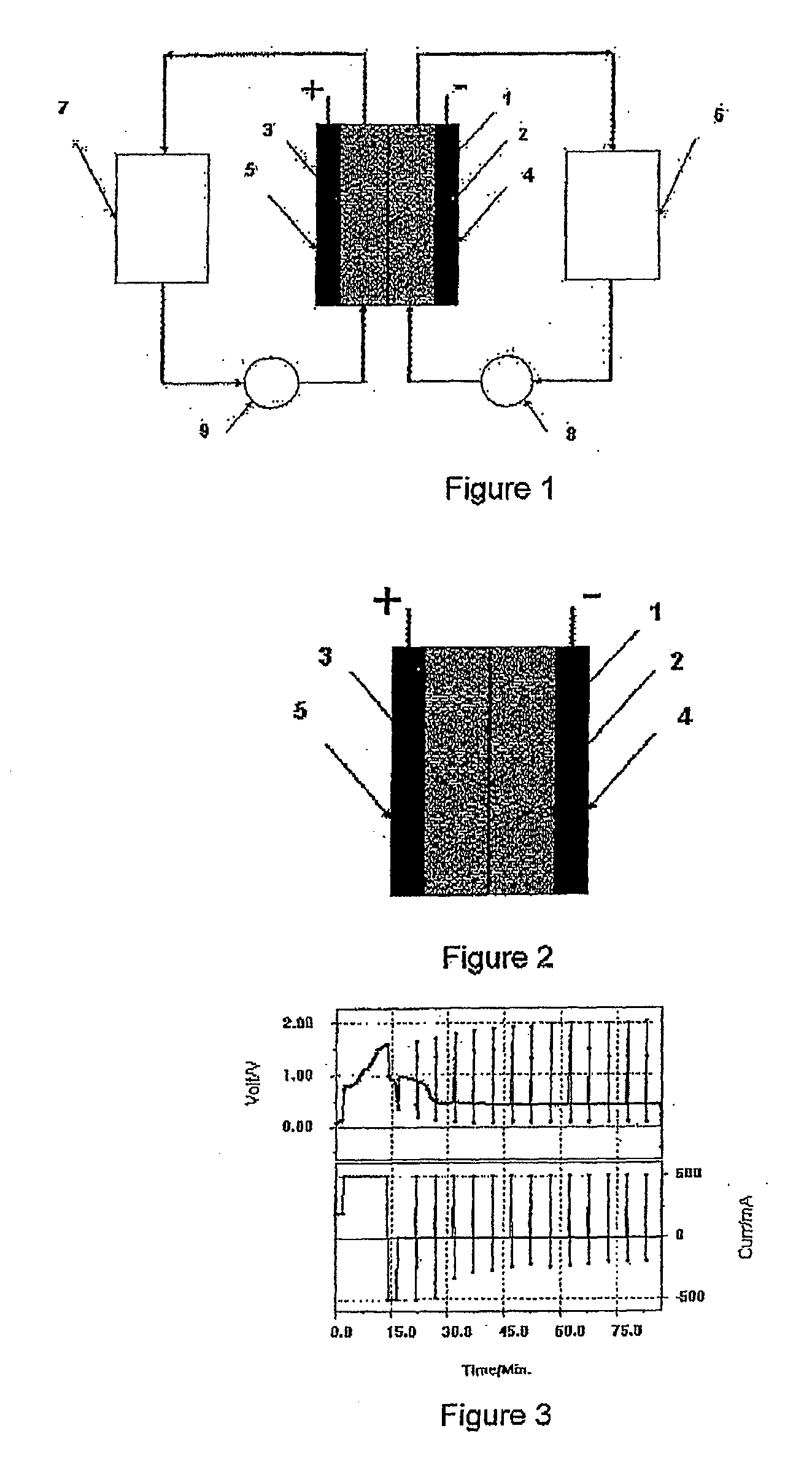
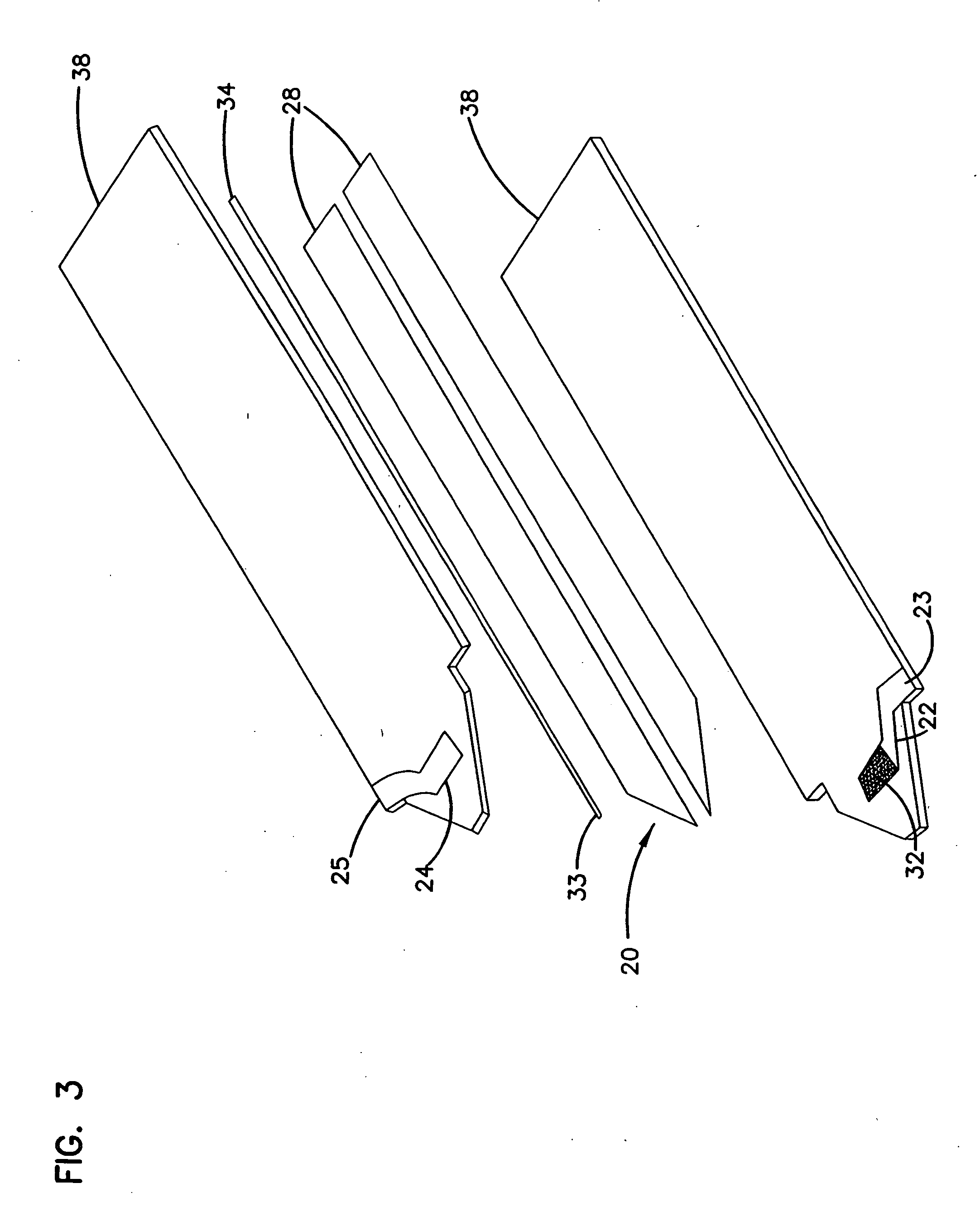
ActiveUS7431820B2Control rateImprove accuracyImmobilised enzymesBioreactor/fermenter combinationsRedoxElectrochemical cell
The present invention relates to electrochemical cells including a first working electrode 32, a first counter electrode 34, a second working electrode 36, and a second counter electrode 38, wherein the electrodes are spaced such that reaction products from the first counter electrode 34 arrive at the first working electrode 32, and reaction products from the first and second counter electrodes 34, 38 do not reach the second working electrode 36. Also provided is a method of using such electrochemical cells for determining the concentration of a reduced or oxidized form of a redox species with greater accuracy than can be obtained using an electrochemical cell having a single working and counter electrode.
Owner:LIFESCAN IP HLDG LLC
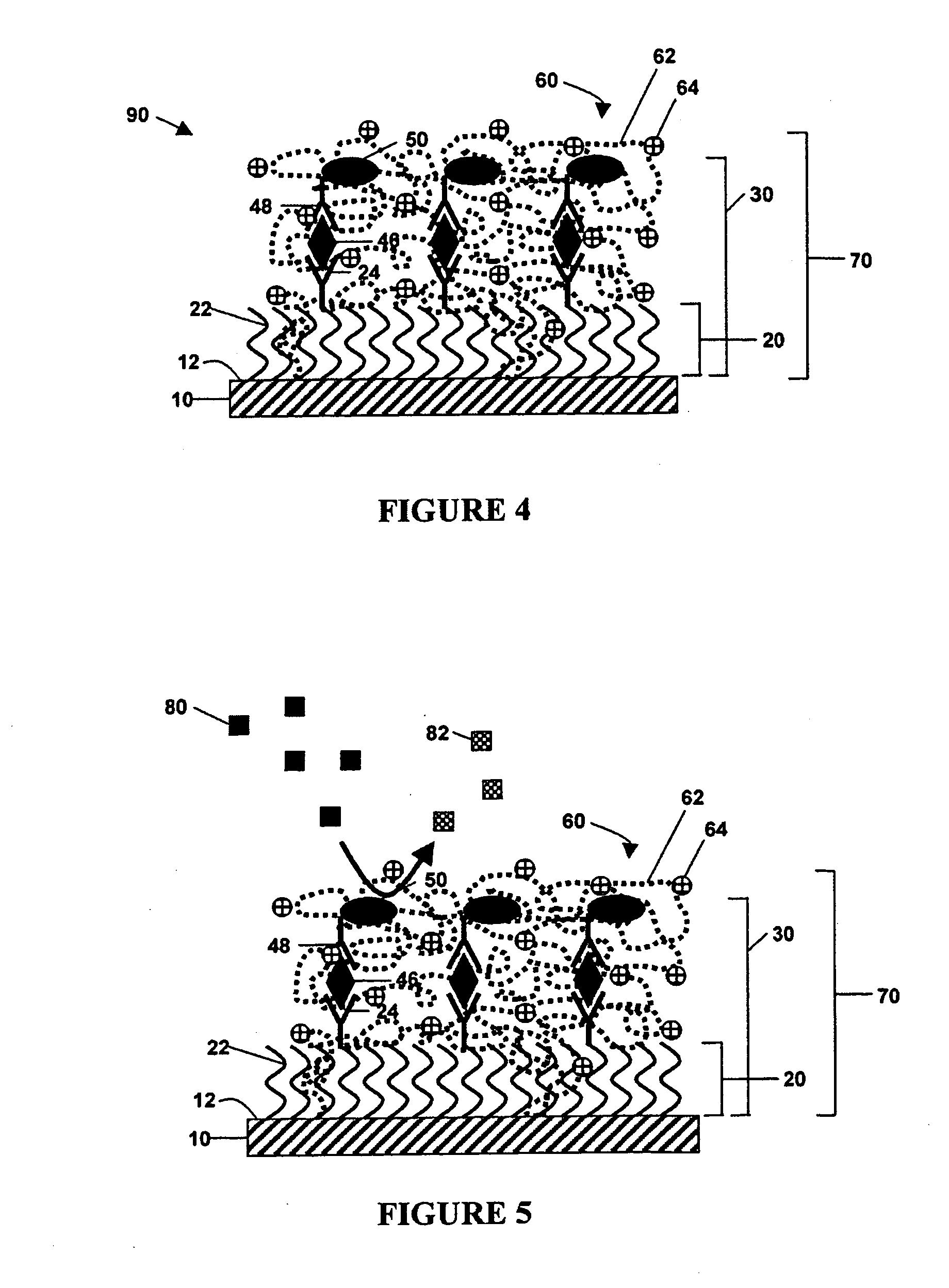
Enzyme-catalyzed metal deposition for the enhanced detection of analytes of interest
ActiveUS20050100976A1Rapidly and accurately determinedHigh detection sensitivitySugar derivativesMicrobiological testing/measurementTarget analysisAnalyte
The invention is directed to enhanced methods for detecting an analyte of interest in situ, by immunoassay, or by hybridization comprising binding an enzyme-labeled conjugate molecule to an analyte of interest in the presence of a redox-inactive reductive species and a soluble metal ion. The enzyme catalyzes the conversion of the inactive reductive species to an active reducing agent, which in turn reduces the metal ion to a metal atom thereby providing an enhanced means of detecting the analyte via metal deposition.
Owner:VENTANA MEDICAL SYST INC
Enzymatic electrochemical detection assay using protective monolayer and device therefor
InactiveUS20060160100A1Material nanotechnologyMicrobiological testing/measurementOxidation-Reduction AgentRedox
There is provided an electrochemical assay method for detecting a target molecule, for example a protein, in a sample, which involves the use of a protective monolayer and a redox polymer to form a bilayer immobilized on an electrode. The monolayer protects the electrode from non-specific adherence of reagents, particular proteins, to the electrode while simultaneously providing a surface that can be functionalized to immobilize a capture molecule and that can interact with the redox polymer.
Owner:AGENCY FOR SCI TECH & RES
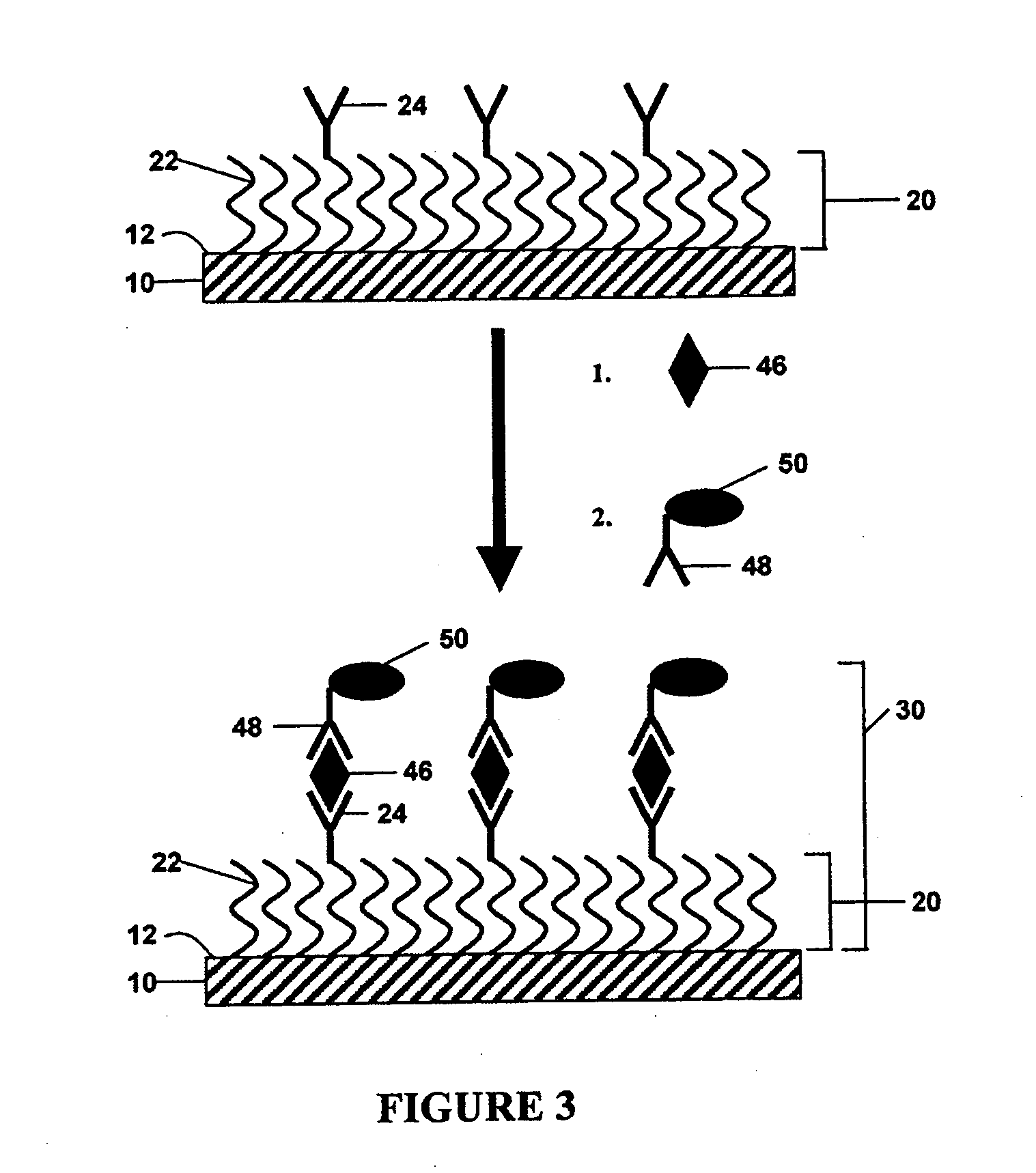
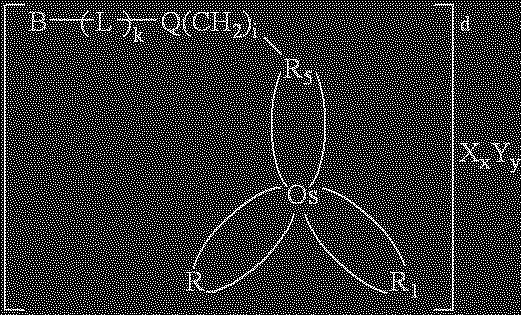
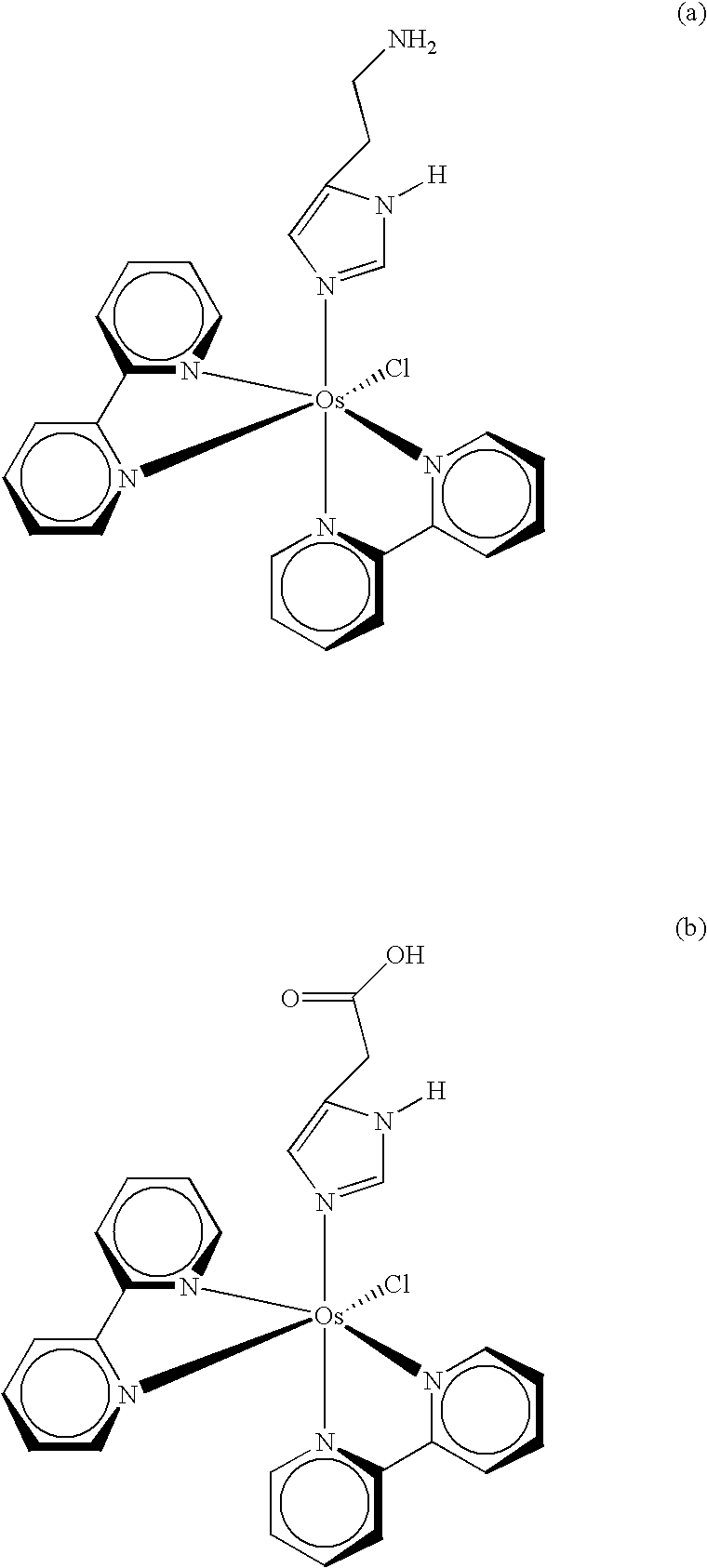
Redox reversible bipyridyl-osmium complex conjugates
InactiveUS7045310B2High measurement accuracyImprove accuracyBioreactor/fermenter combinationsBiological substance pretreatmentsRedoxElectrochemistry
Novel bipyridyl-osmium complex conjugates and their use in electrochemical assays are described. The redox reversible-osmium complexes can be prepared to exhibit unique reversible redox potentials and can thus be used in combination with other electroactive redox reversible species having redox potentials differing by at least 50 millivolts in electrochemical assays designed for use of multiple electroactive species in the same cell and in the same sample without interference between the two or more redox coupled conjugate systems.
Owner:ROCHE DIABETES CARE INC
Method and apparatus for assay of electrochemical properties
ActiveUS7501052B2Easy to measureImprove accuracy and precisionImmobilised enzymesBioreactor/fermenter combinationsHand heldApplied potential
The presence of a select analyte in the sample is evaluated in an an electrochemical system using a conduction cell-type apparatus. A potential or current is generated between the two electrodes of the cell sufficient to bring about oxidation or reduction of the analyte or of a mediator in an analyte-detection redox system, thereby forming a chemical potential gradient of the analyte or mediator between the two electrodes After the gradient is established, the applied potential or current is discontinued and an analyte-independent signal is obtained from the relaxation of the chemical potential gradient. The analyte-independent signal is used to correct the analyte-dependent signal obtained during application of the potential or current. This correction allows an improved measurement of analyte concentration because it corrects for device-specific and test specific factors such as transport (mobility) of analyte and / or mediator, effective electrode area, and electrode spacing (and as a result, sample volume), without need for separate calibration values. The analysis can be performed using disposable test strips in a hand held meter, for example for glucose testing.
Owner:AGAMATRIX INC
Perfluorinated Membranes and Improved Electrolytes for Redox Cells and Batteries
ActiveUS20080292964A1Improve performanceReduce resistanceFinal product manufactureSecondary cellsSupporting electrolyteVanadyl ion
A vanadium redox cell having a positive half cell containing a positive half cell solution comprising a supporting electrolyte selected from H2SO4, HBr / HCl mixtures and one or more ions selected from the group vanadium (EI), Vanadium (IV), Vanadium (V), Br3 and Br2Cl; a negative half cell containing a negative half cell solution comprising a supporting electrolyte selected from H2SO4, HBr and HBr / HCl mixtures and one or more vanadium ions selected from the group Vanadium (II), Vanadium (III) and Vanadium (FV) and a perfluorinated cast cation exchange membrane or separator disposed between the positive and negative half cells and in contact with the positive and negative half cell solutions.
Owner:NEWSOUTH INNOVATIONS PTY LTD
Small volume in vitro analyte sensor
InactiveUS20050164322A1Lower the volumeAccurate and efficient measurementBioreactor/fermenter combinationsBiological substance pretreatmentsElectron transferPolymer
A sensor designed to determine the amount and concentration of analyte in a sample having a volume of less than about 1 μL. The sensor has a working electrode coated with a non-leachable redox mediator. The redox mediator acts as an electron transfer agent between the analyte and the electrode. In addition, a second electron transfer agent, such as an enzyme, can be added to facilitate the electrooxidation or electroreduction of the analyte. The redox mediator is typically a redox compound bound to a polymer. The preferred redox mediators are air-oxidizable. The amount of analyte can be determined by coulometry. One particular coulometric technique includes the measurement of the current between the working electrode and a counter or reference electrode at two or more times. The charge passed by this current to or from the analyte is correlated with the amount of analyte in the sample. Other electrochemical detection methods, such as amperometric, voltammetric, and potentiometric techniques, can also be used. The invention can be used to determine the concentration of a biomolecule, such as glucose or lactate, in a biological fluid, such as blood or serum. An enzyme capable of catalyzing the electrooxidation or electroreduction of the biomolecule is provided as a second electron transfer agent.
Owner:ABBOTT DIABETES CARE INC
Redox flow cell
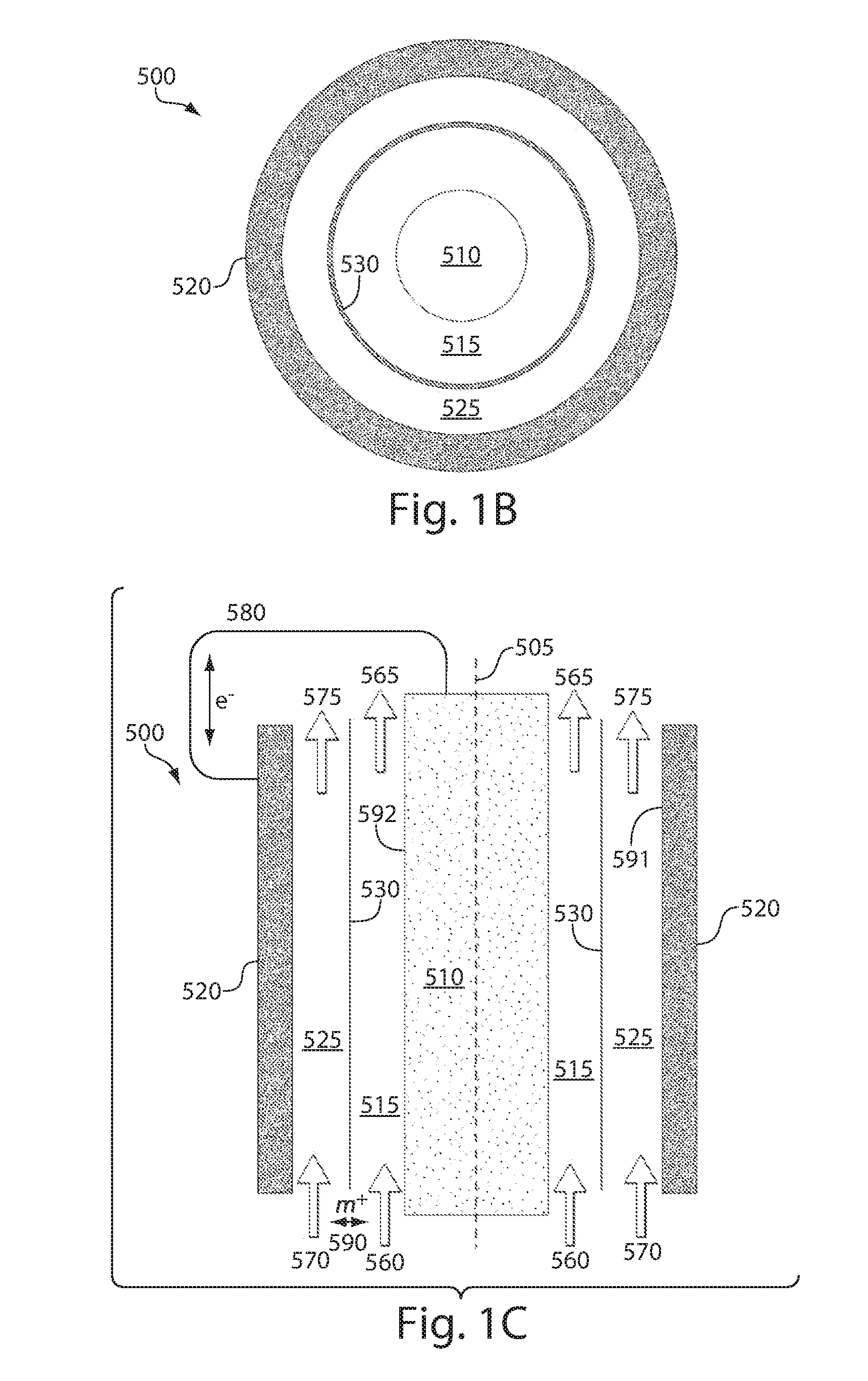
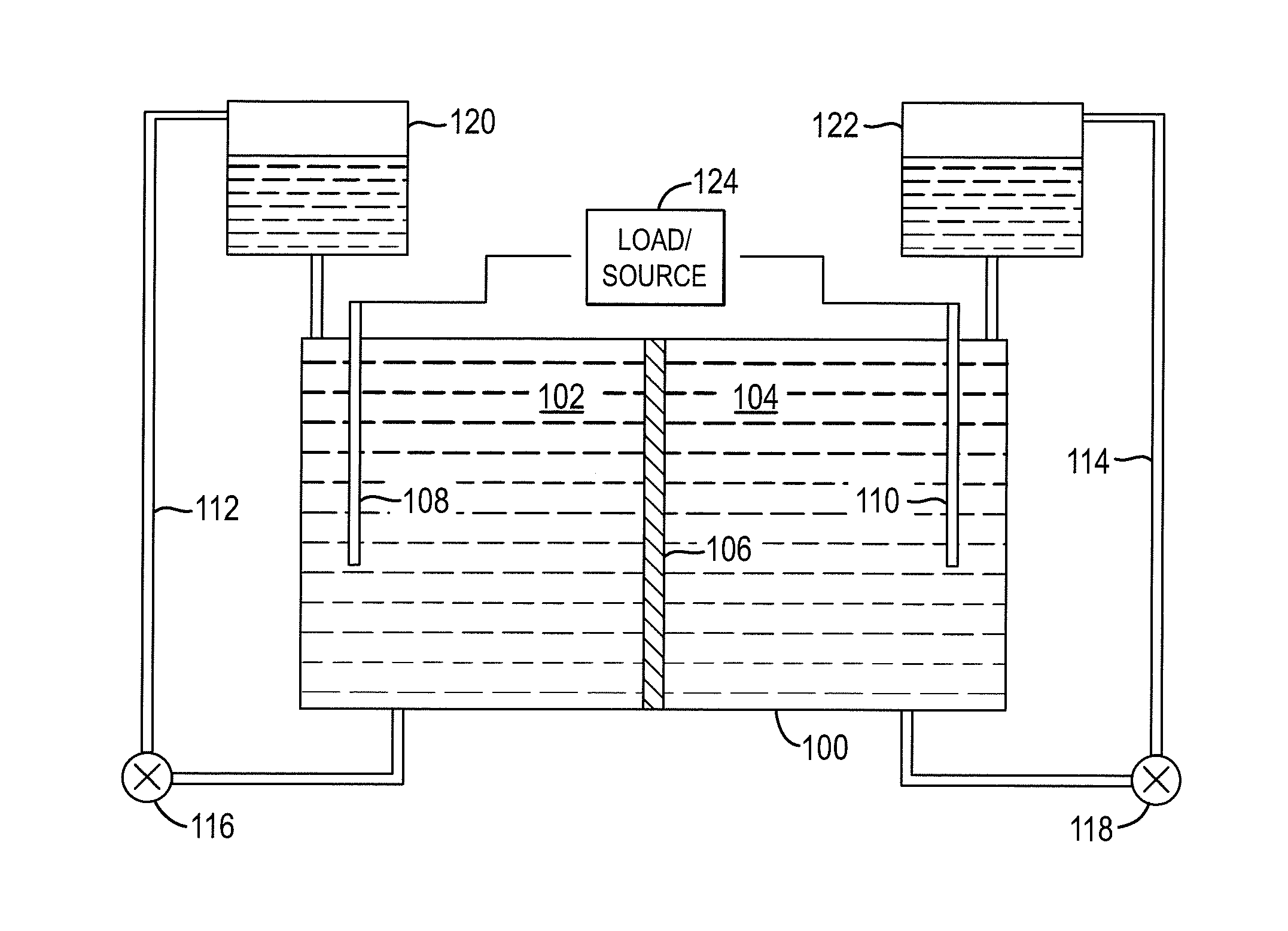
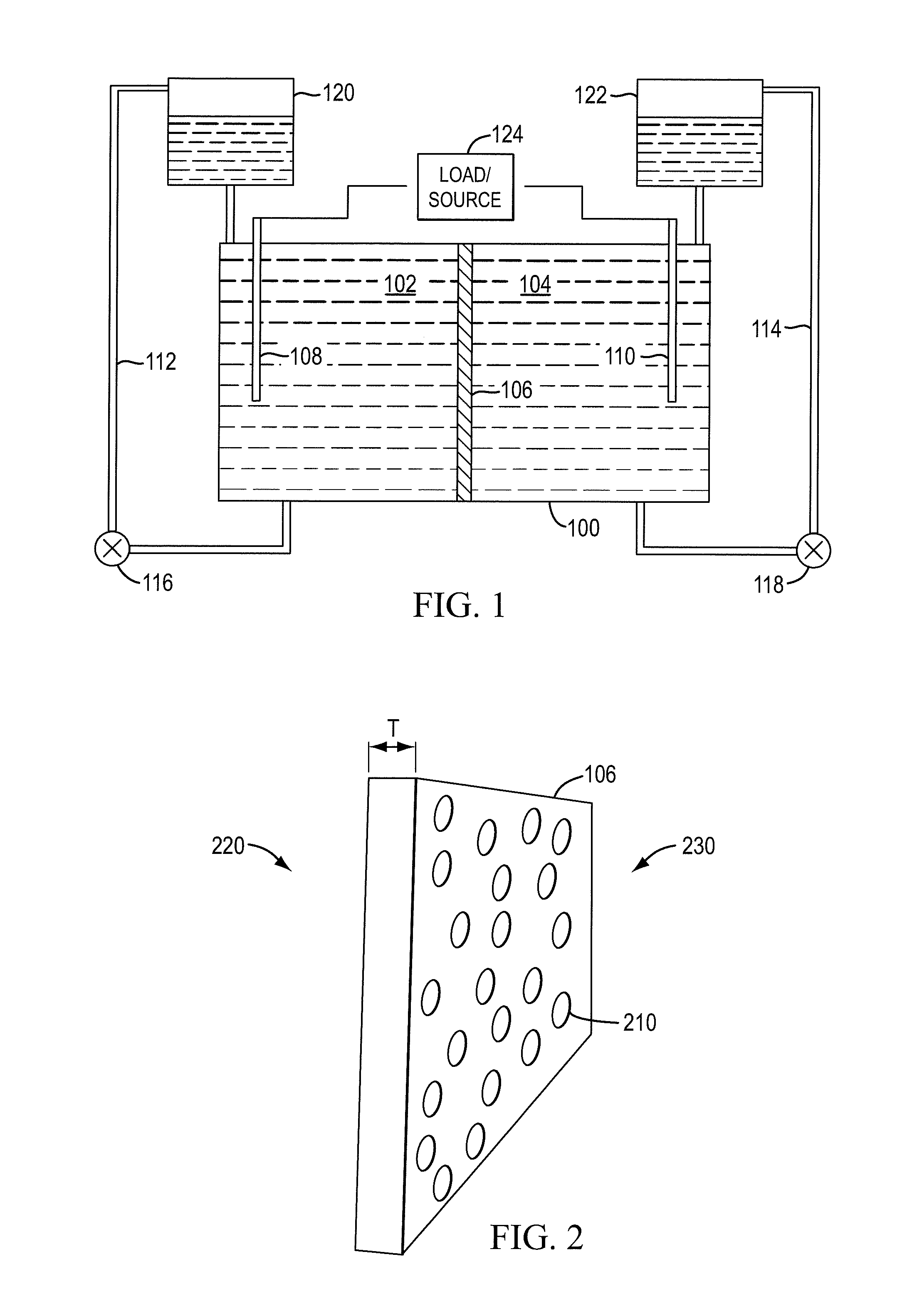
InactiveUS20100003586A1Facilitate ion exchangeCell electrodesFuel cell auxillariesFlow cellPorous membrane
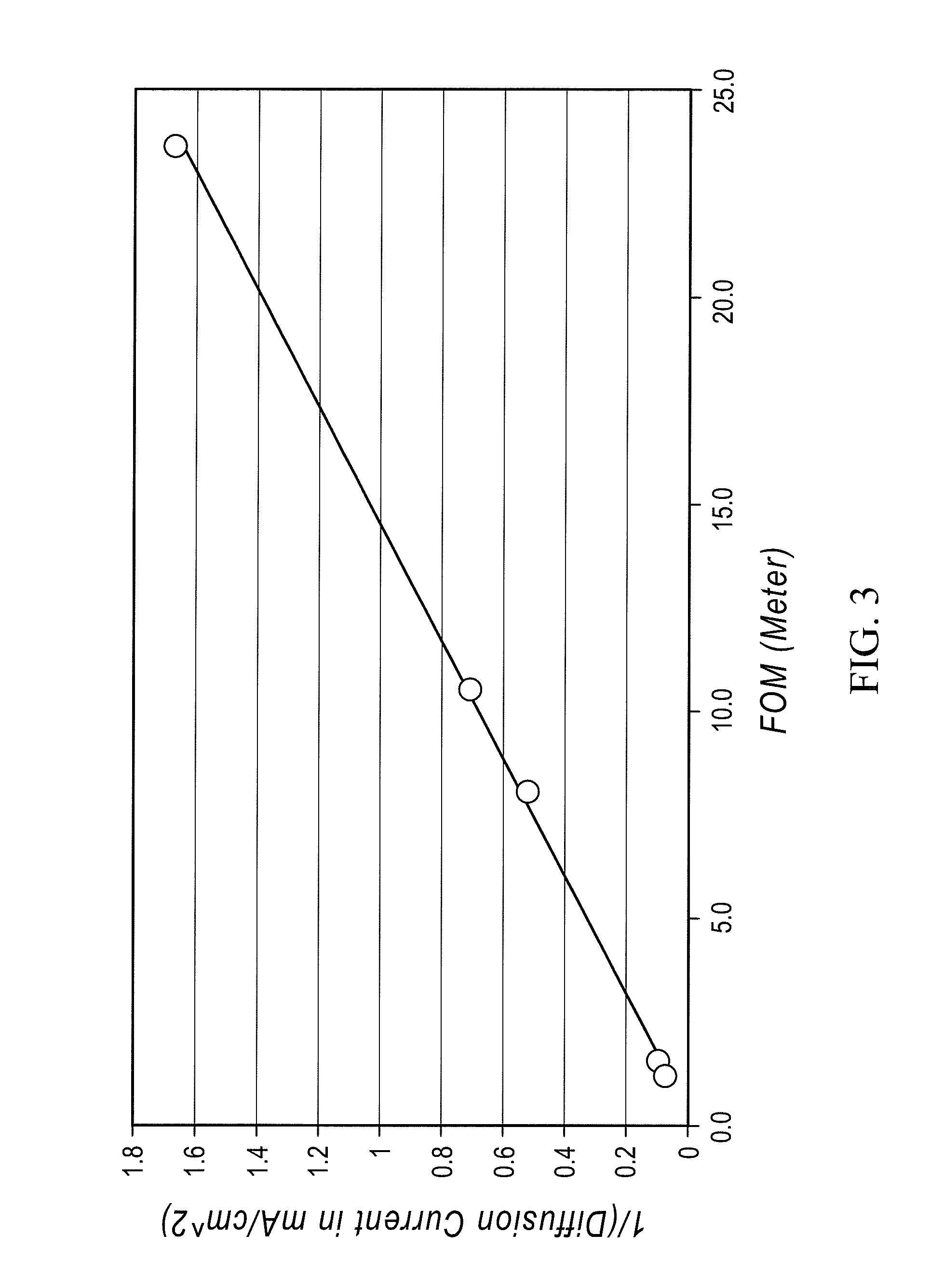
A redox flow cell is presented that utilizes a porous membrane separating a first half cell and a second half cell. The porous membrane is chosen to have a figure of merit (FOM) is at least a minimum FOM. A method of providing a porous membrane for a flow cell can include determining a figure of merit; determining a first parameter from a pore size or a thickness for the porous membrane; determining a second parameter from the pore size or the thickness that is not the first parameter for the porous membrane, based on the figure of merit; and constructing a porous membrane having the pore size and the thickness.
Owner:IMERGY POWER SYST
Carbon electrode material for a vanadium-based redox-flow battery
The carbon electrode material of the present invention is used for a vanadium redox-flow cell. The carbon electrode material has quasi-graphite crystal structure in which <002> spacing obtained by X-ray wide angle analysis is 3.43 to 3.60 Å, size of a crystallite in c axial direction is 15 to 33 Å and size of crystallite in a axial direction is 30 to 70 Å. In addition, an amount of surface acidic functional groups obtained by XPS surface analysis is 0.1 to 1.2% and total number of surface bound-nitrogen atoms is 5% or smaller relative to total number of surface carbon atoms. The carbon electrode materials formed of a non-woven fabric of a carbonaceouss fiber is preferable.
Owner:TOYO TOYOBO CO LTD
Transition metal complexes with (pyridyl)imidazole ligands
InactiveUS7074308B2Rapid electron exchangeFast dynamicsImmobilised enzymesBioreactor/fermenter combinationsOxidation-Reduction AgentRedox
Novel transition metal complexes of iron, cobalt, ruthenium, osmium, and vanadium are described. The transition metal complexes can be used as redox mediators in enzyme-based electrochemical sensors. The transition metal complexes include substituted or unsubstituted (pyridyl)imidazole ligands. Transition metal complexes attached to polymeric backbones are also described.
Owner:ABBOTT DIABETES CARE INC
Redox flow battery
InactiveUS6764789B1Reduce capacityReduce frequencyElectrolyte holding meansCell electrodesRedoxAqueous solution
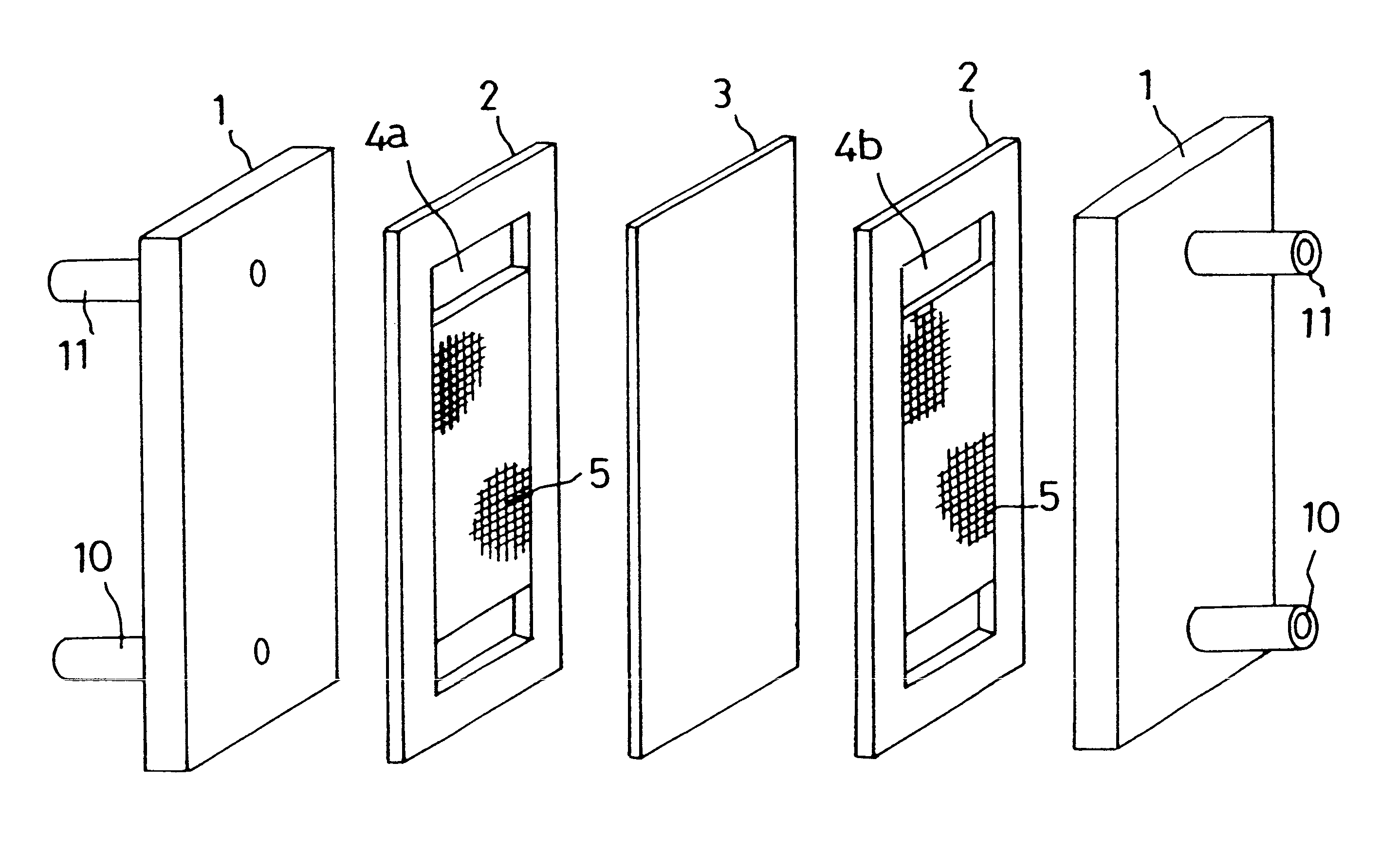
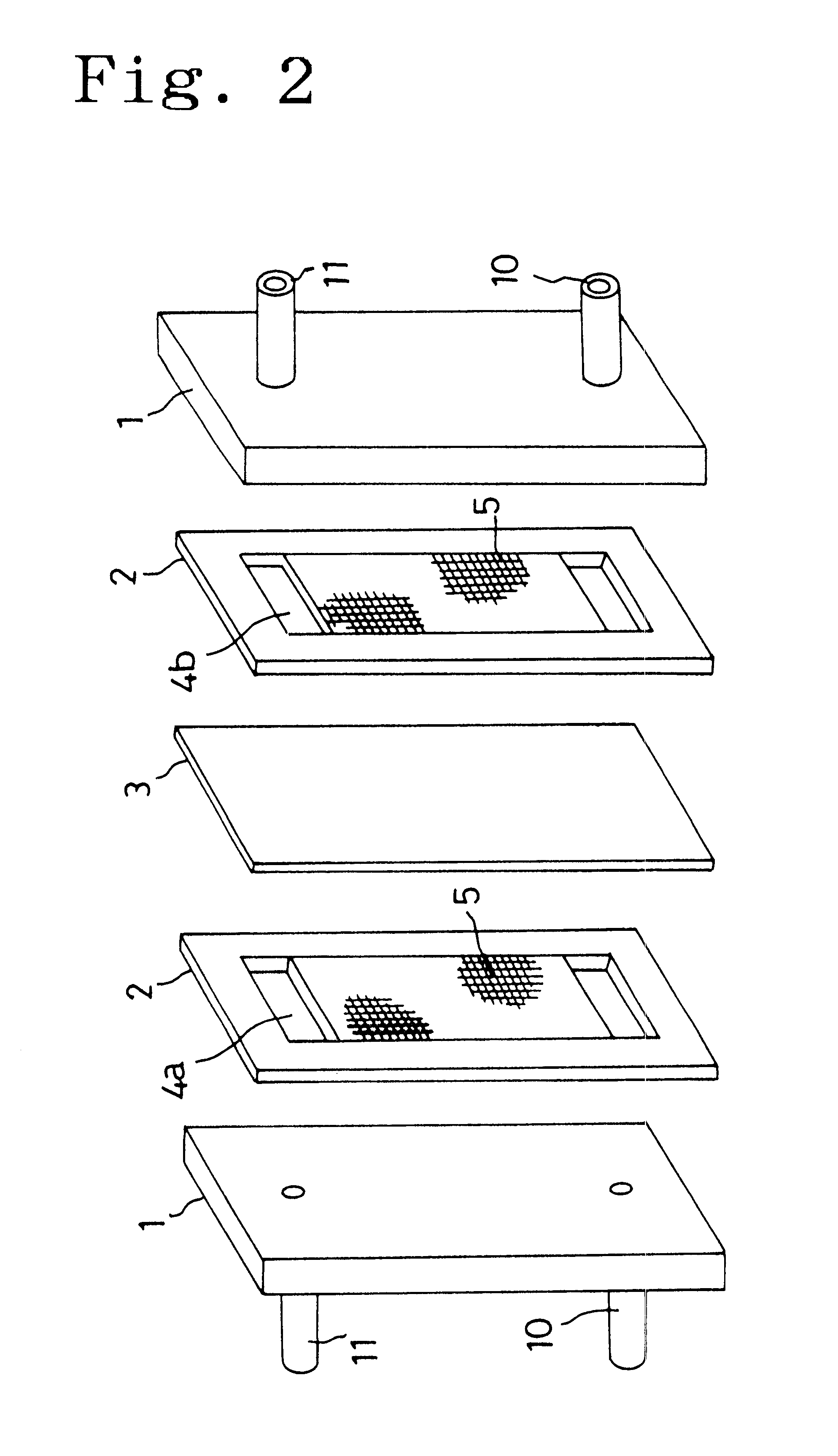
The present invention provides a redox flow type battery which a liquid-circulating battery comprising a battery cell and storage tanks for positive and negative electrolytes, wherein the battery cell is separated by a membrane to provide a positive cell and a negative cell, each cell having a liquid-permeable porous electrode disposed therein, wherein the positive and negative electrolytes are sulfuric acid aqueous solutions with vanadium ion concentrations of 0.5 mol / l to 8 mol / l and the electrolyte which migrates through the membrane over cycles of charge and discharge is returned from the storage tank where the liquid increases to the storage tank where the liquid decreases in order to keep the change in the amounts of the positive and negative electrolytes in a certain range while charge and discharge are carried out.
Owner:SUMITOMO ELECTRIC IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com