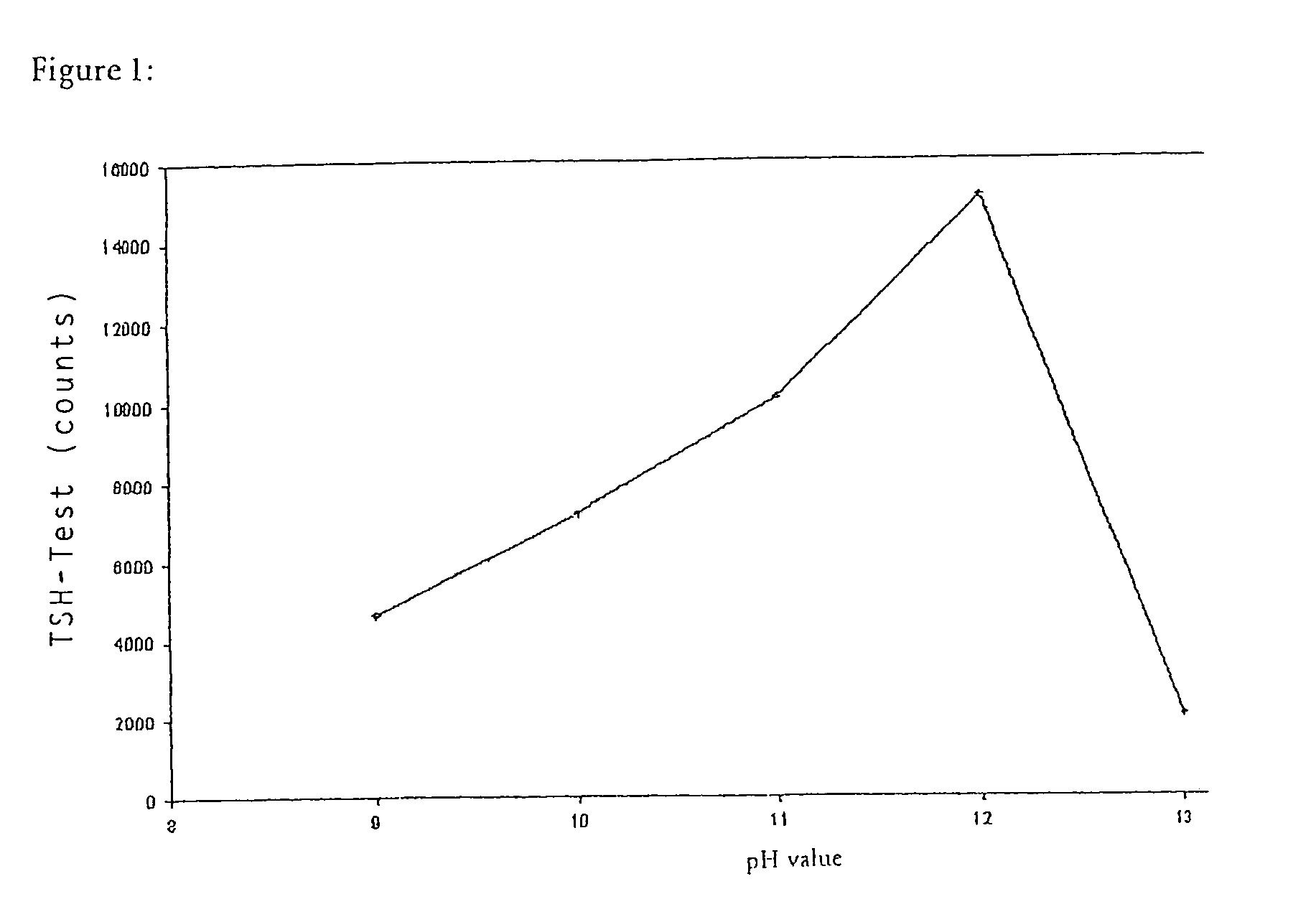
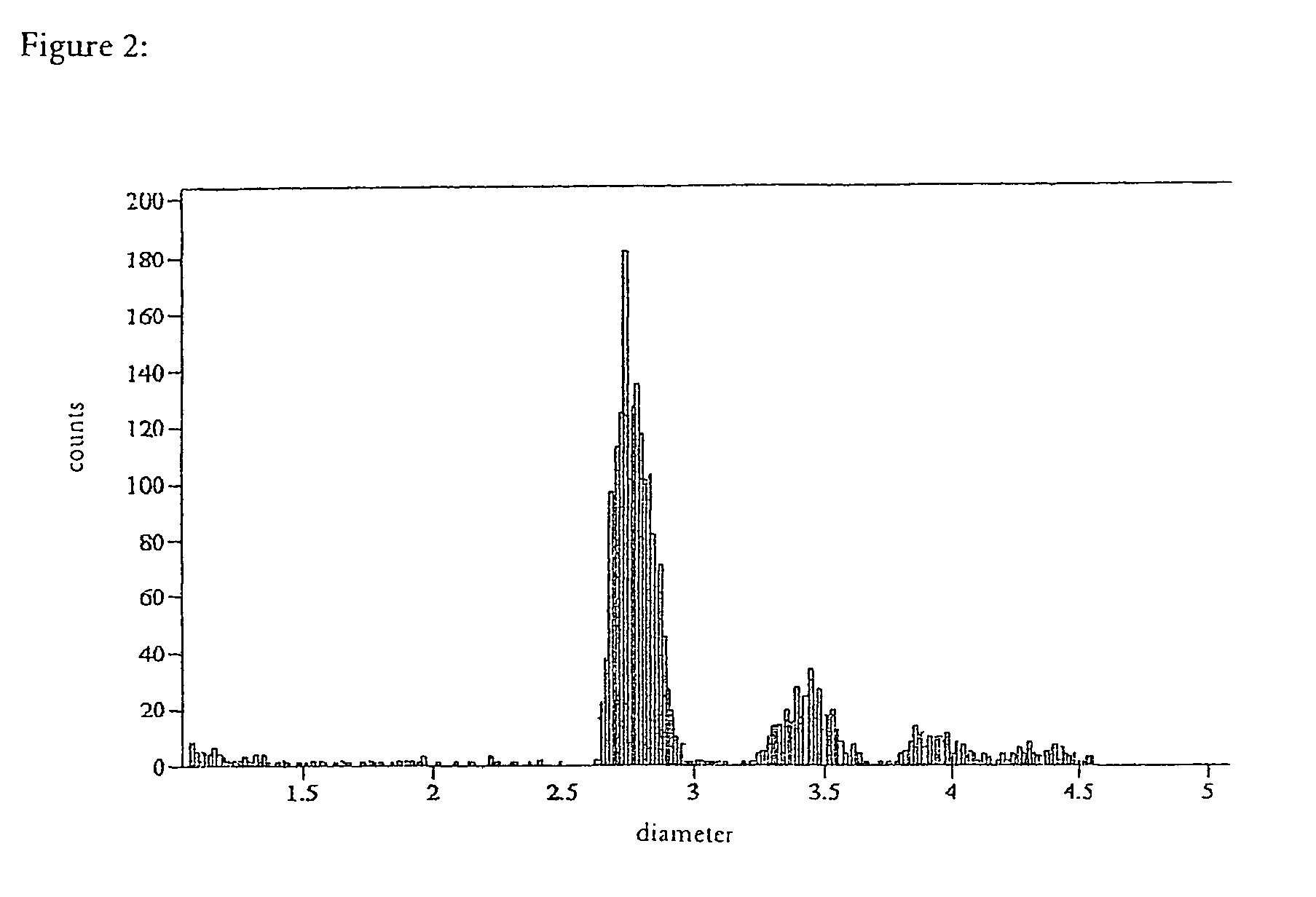
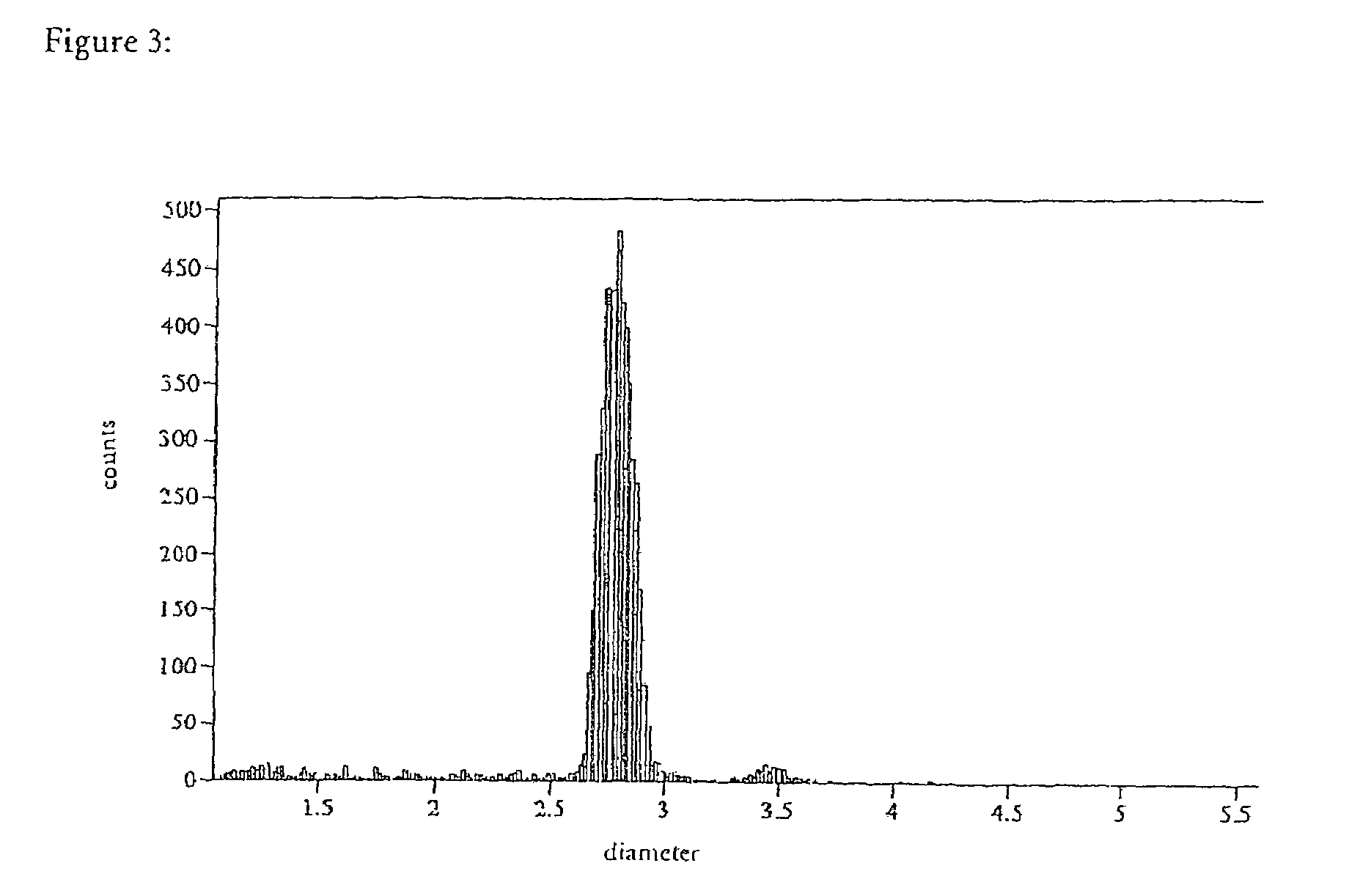
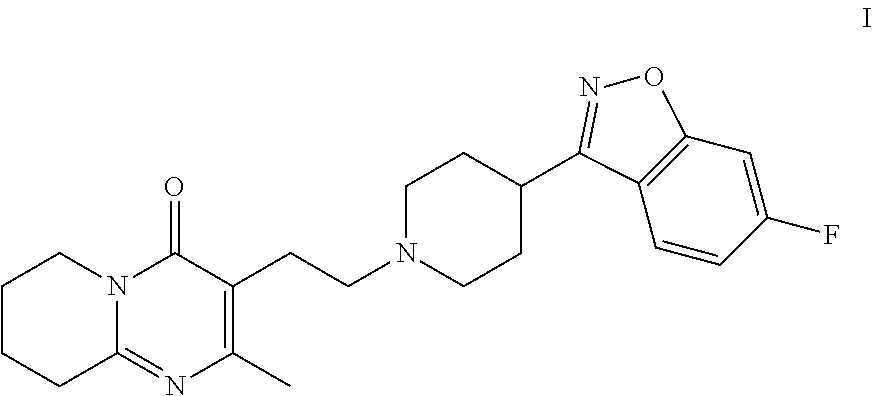
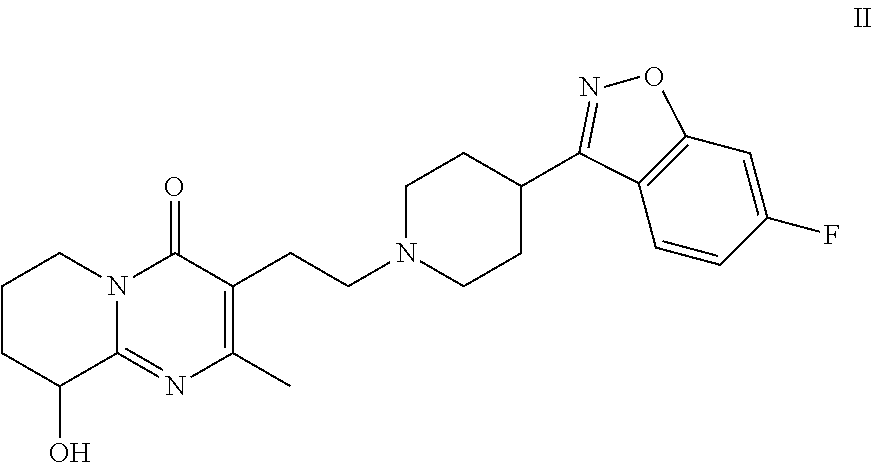
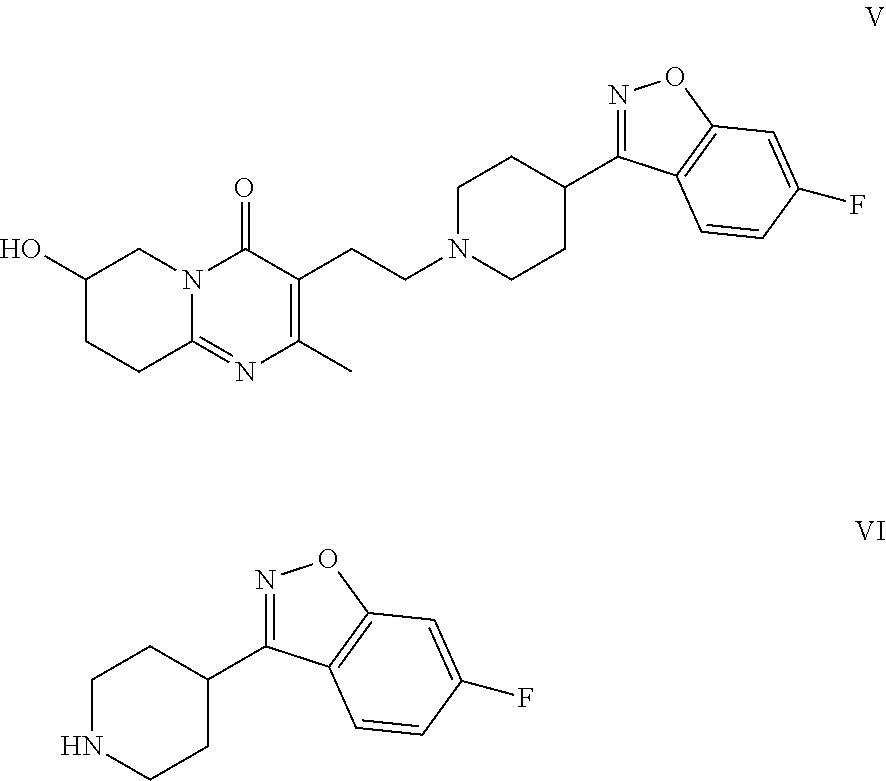
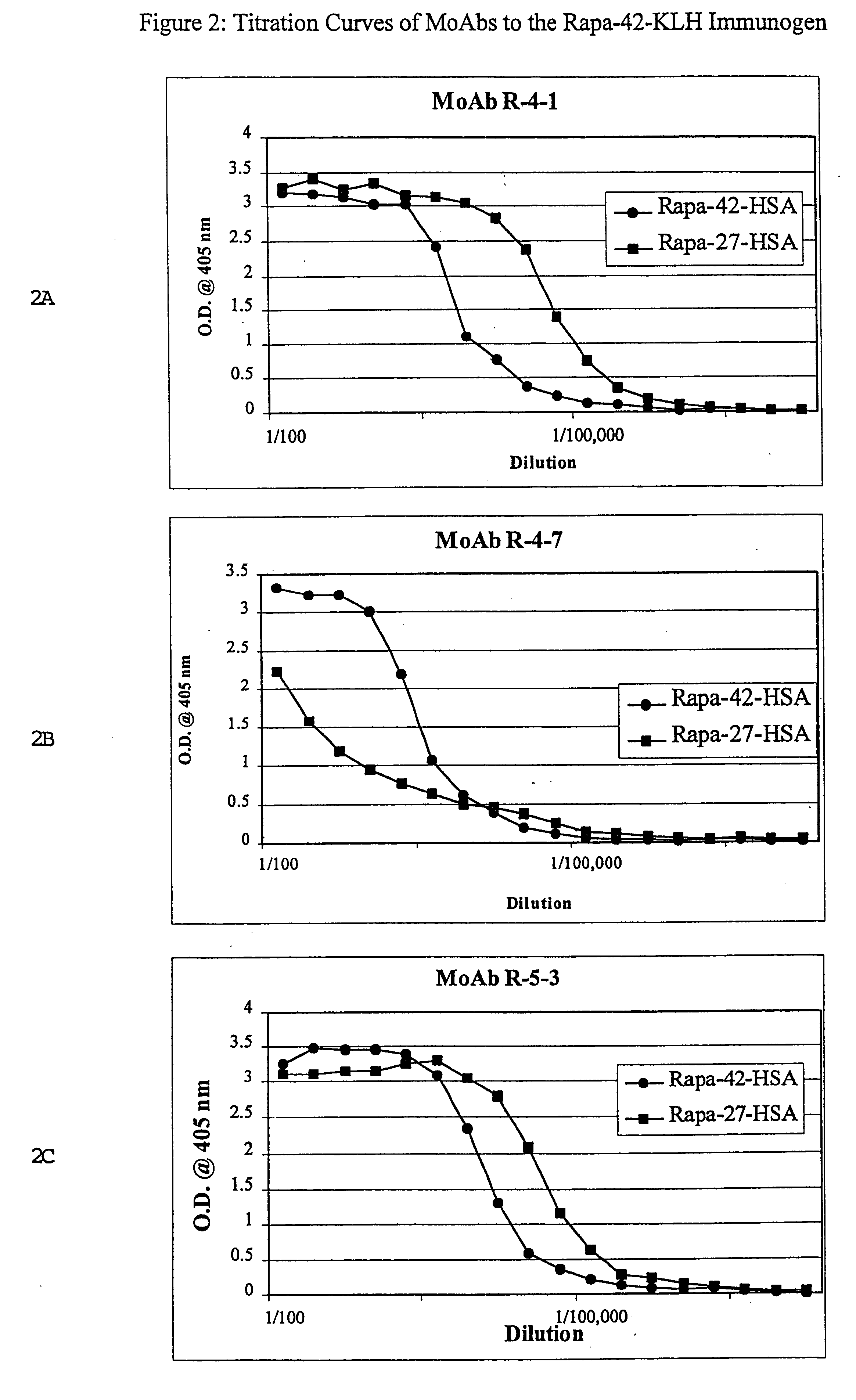
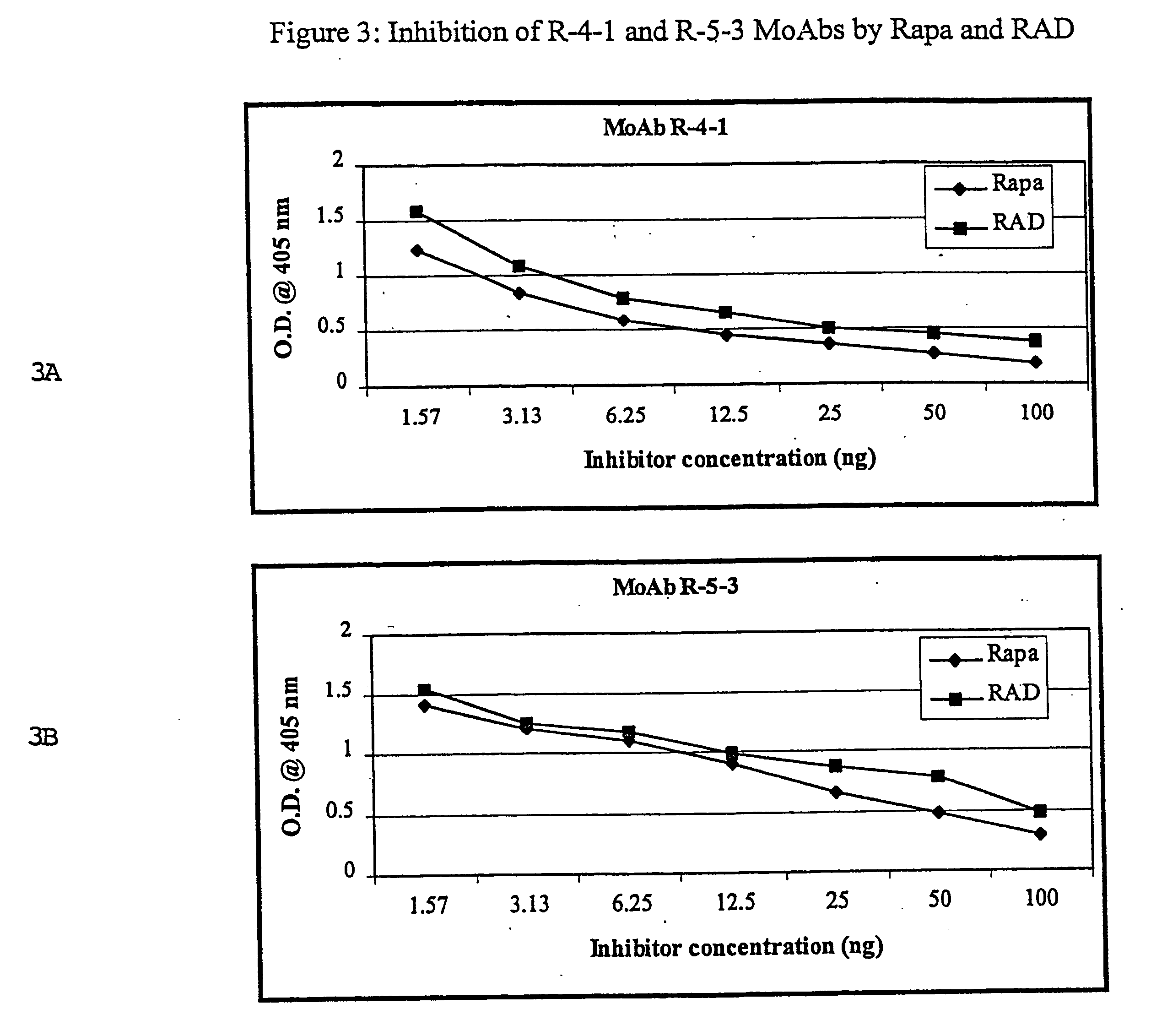
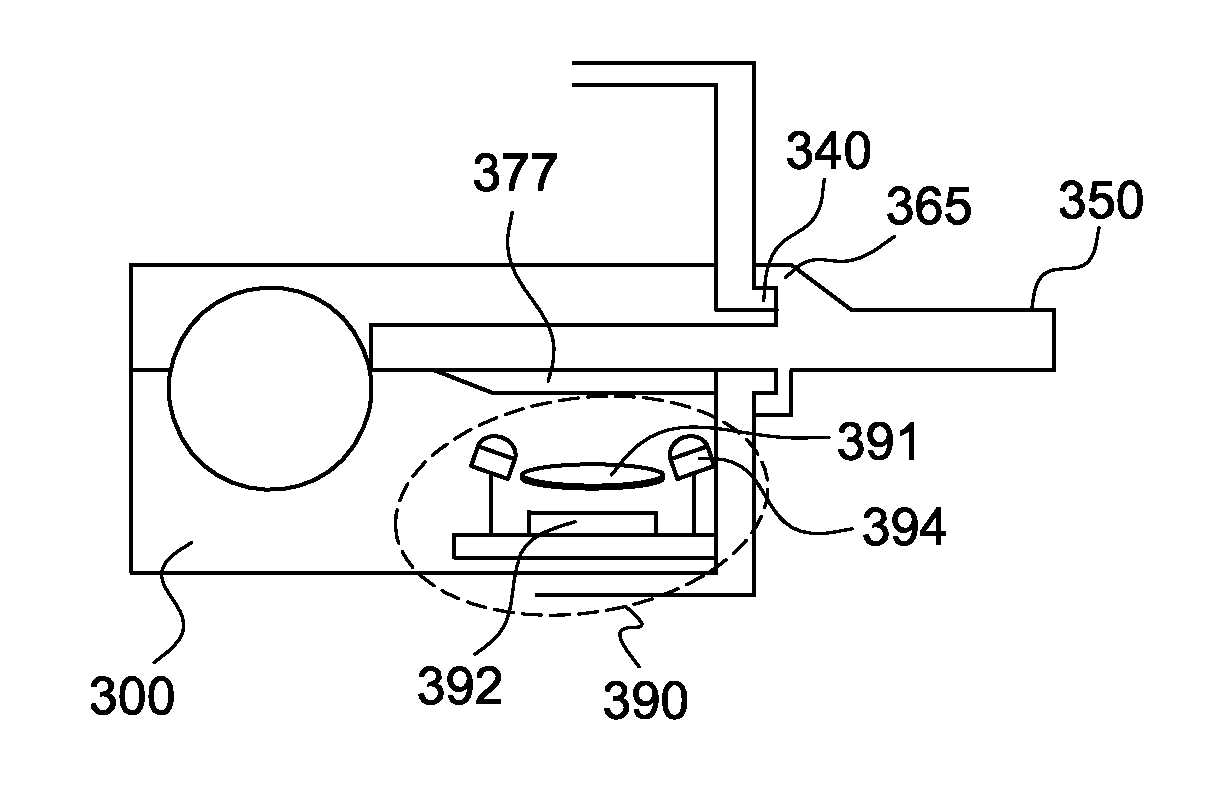
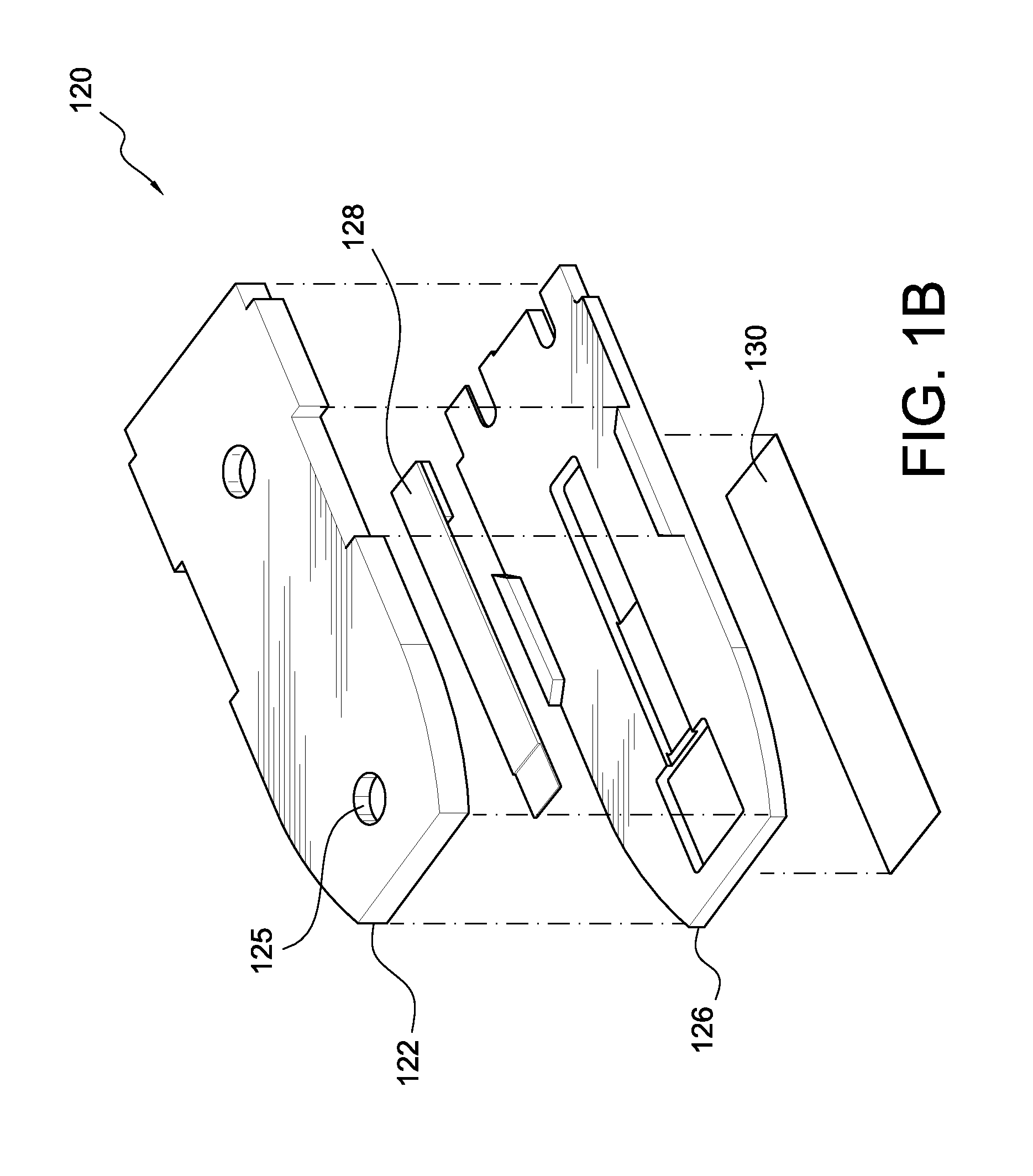
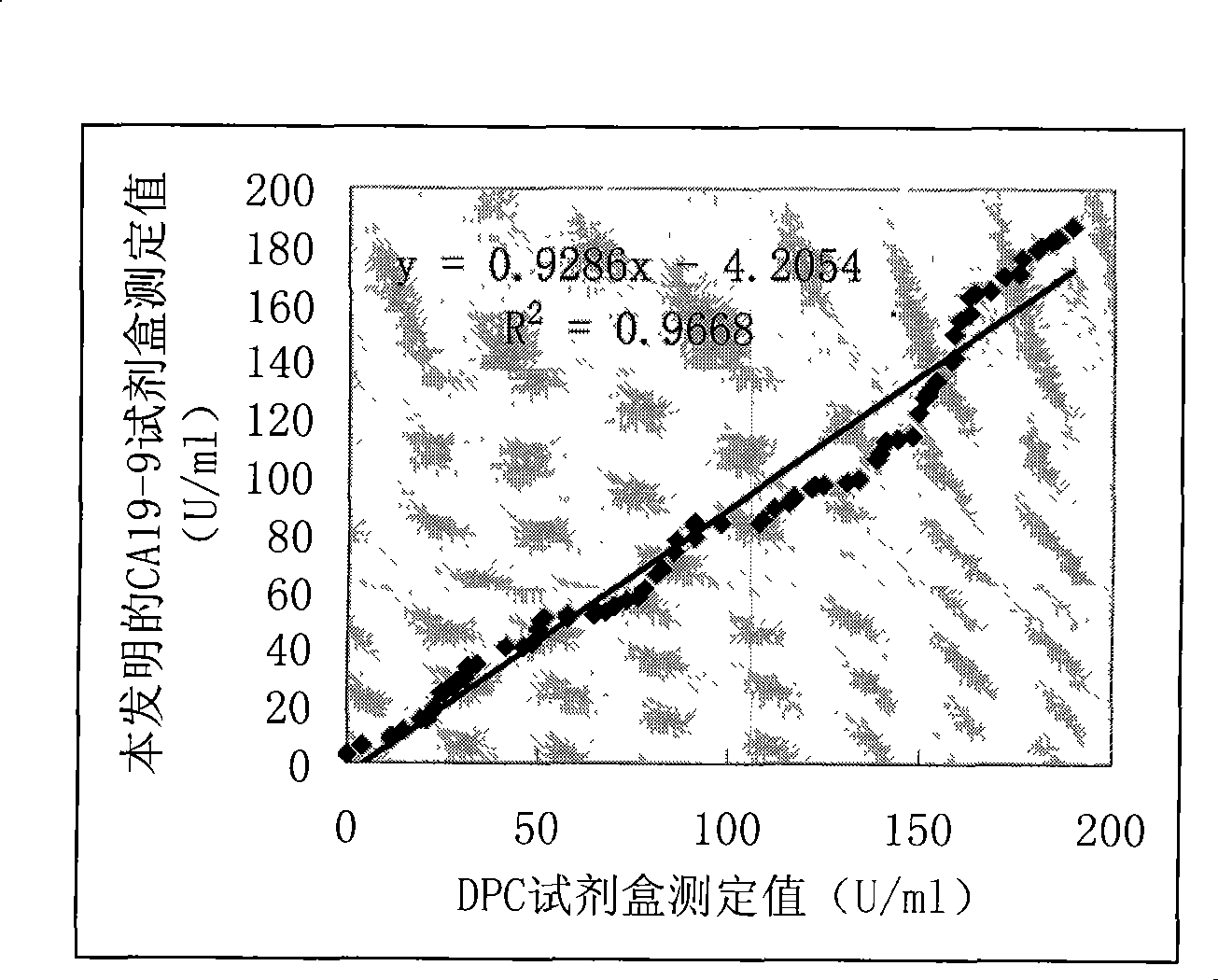
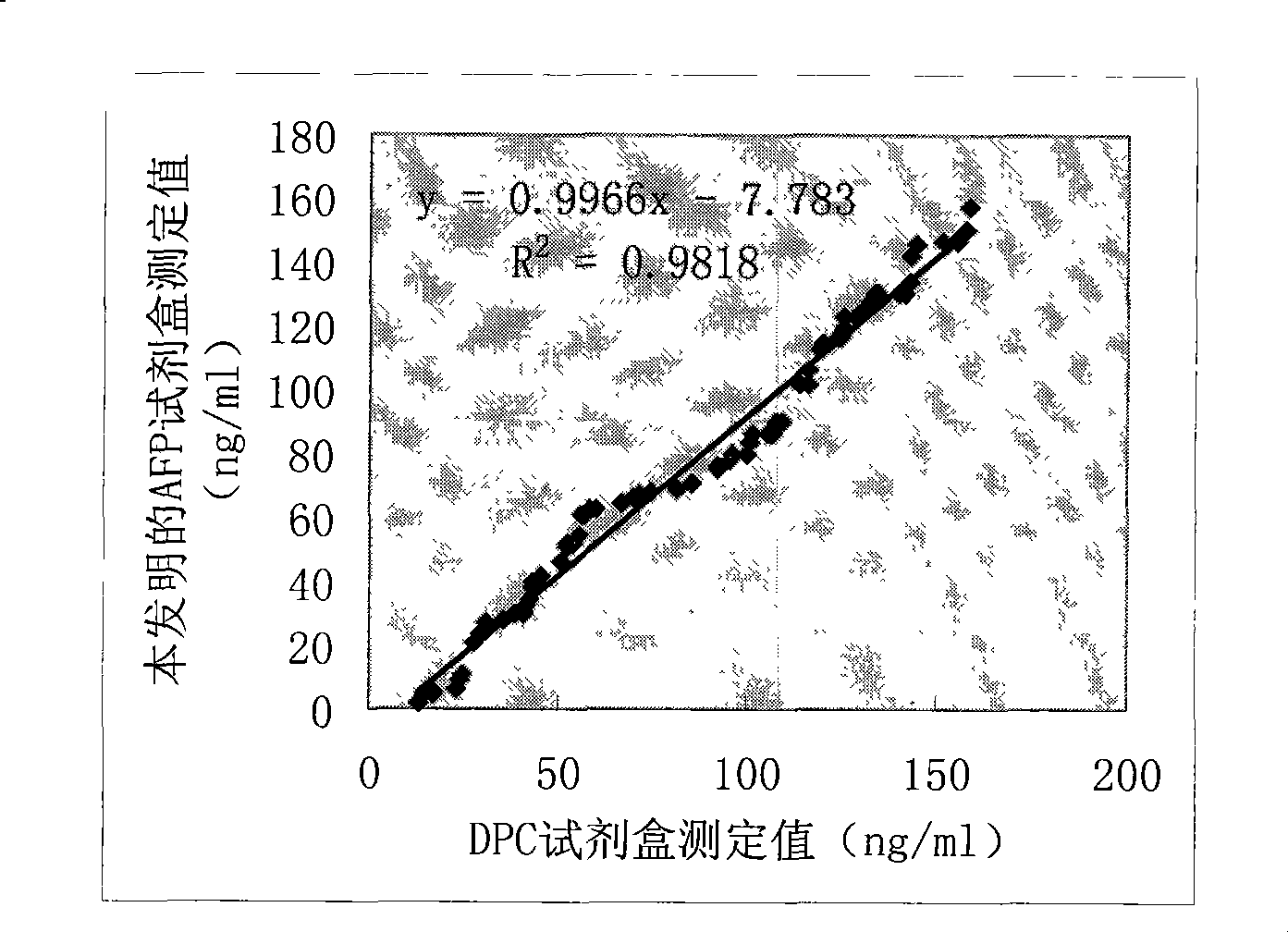
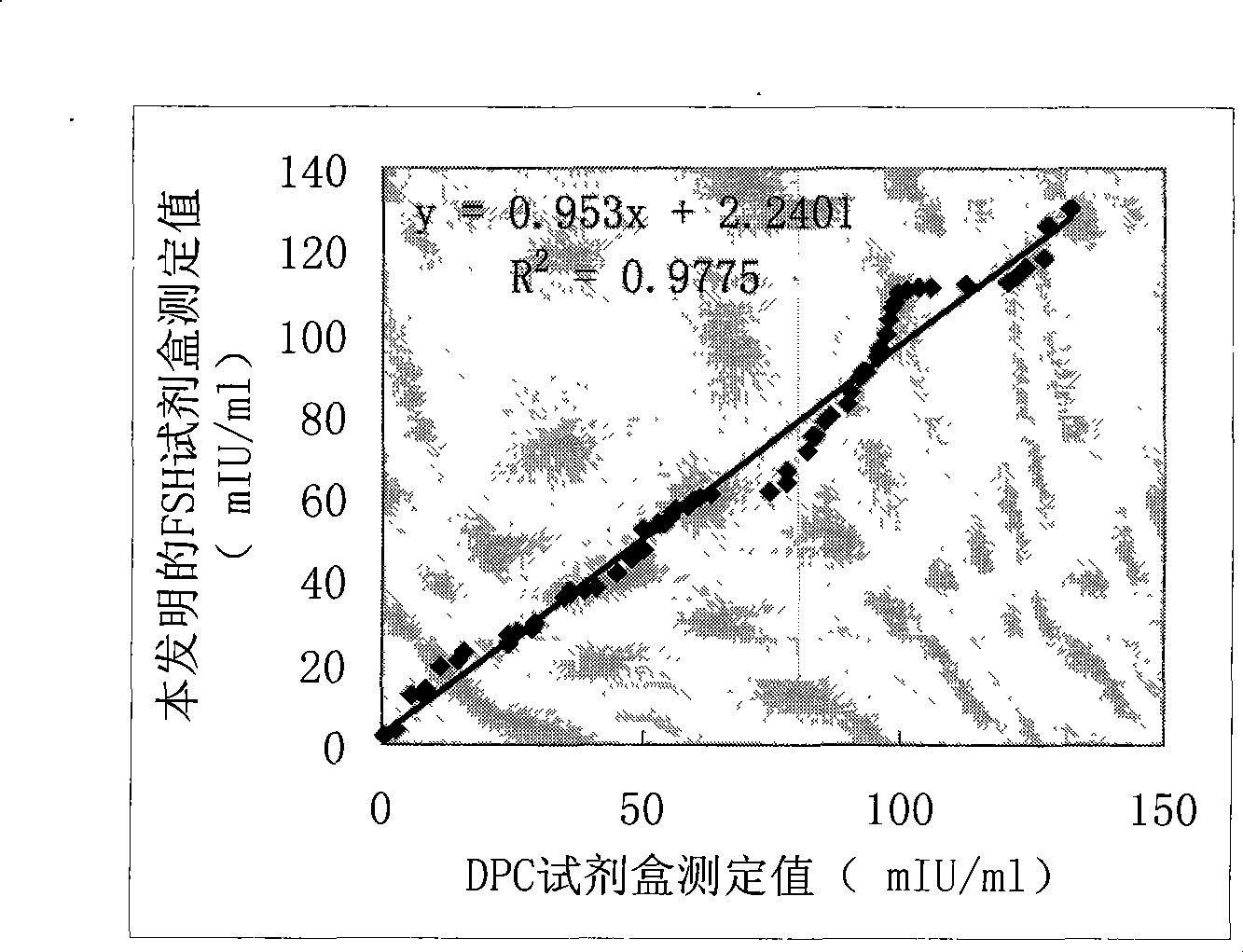
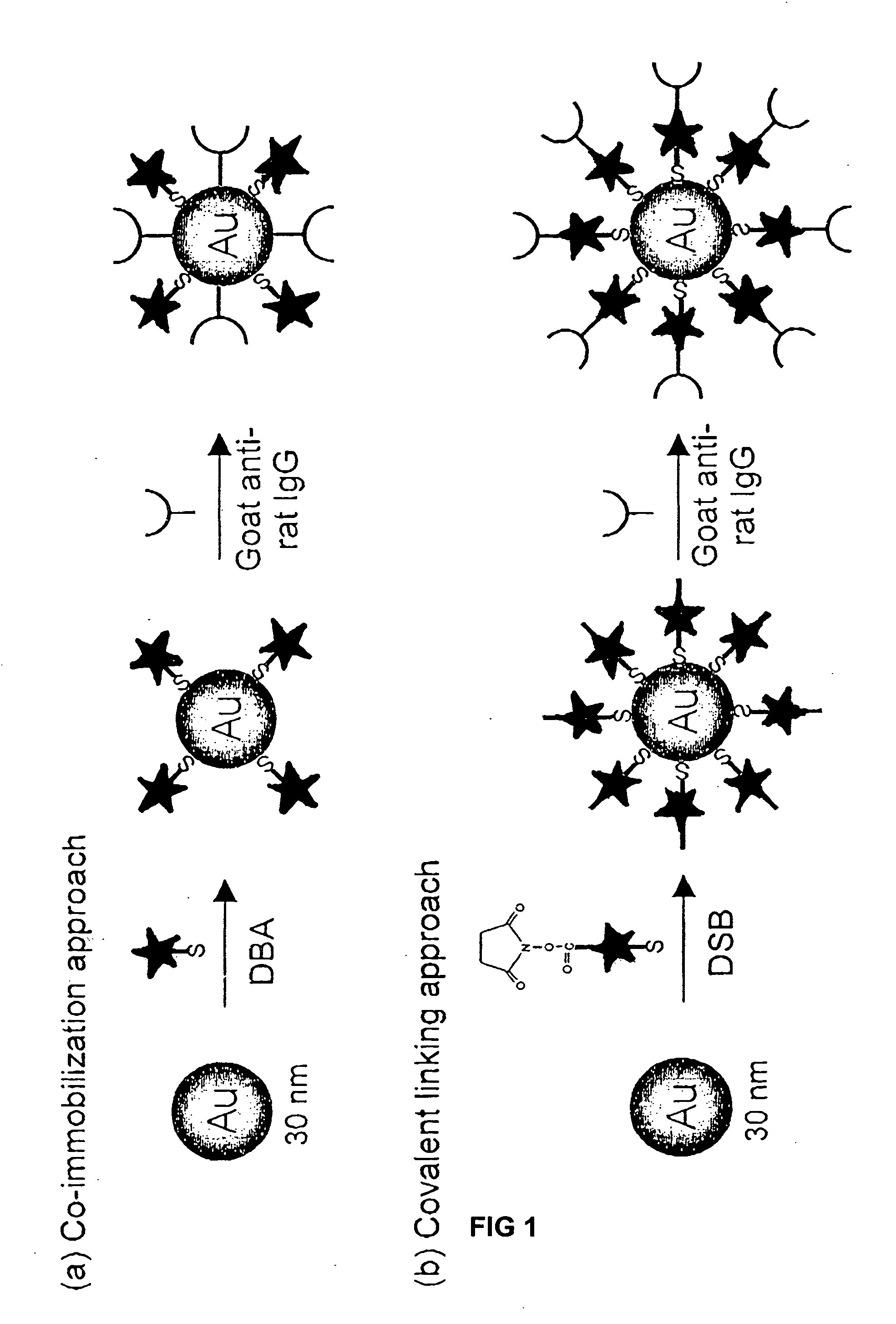
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2948 results about "Immunoassay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An immunoassay is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different size and types, as long as the proper antibodies that have the adequate properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.
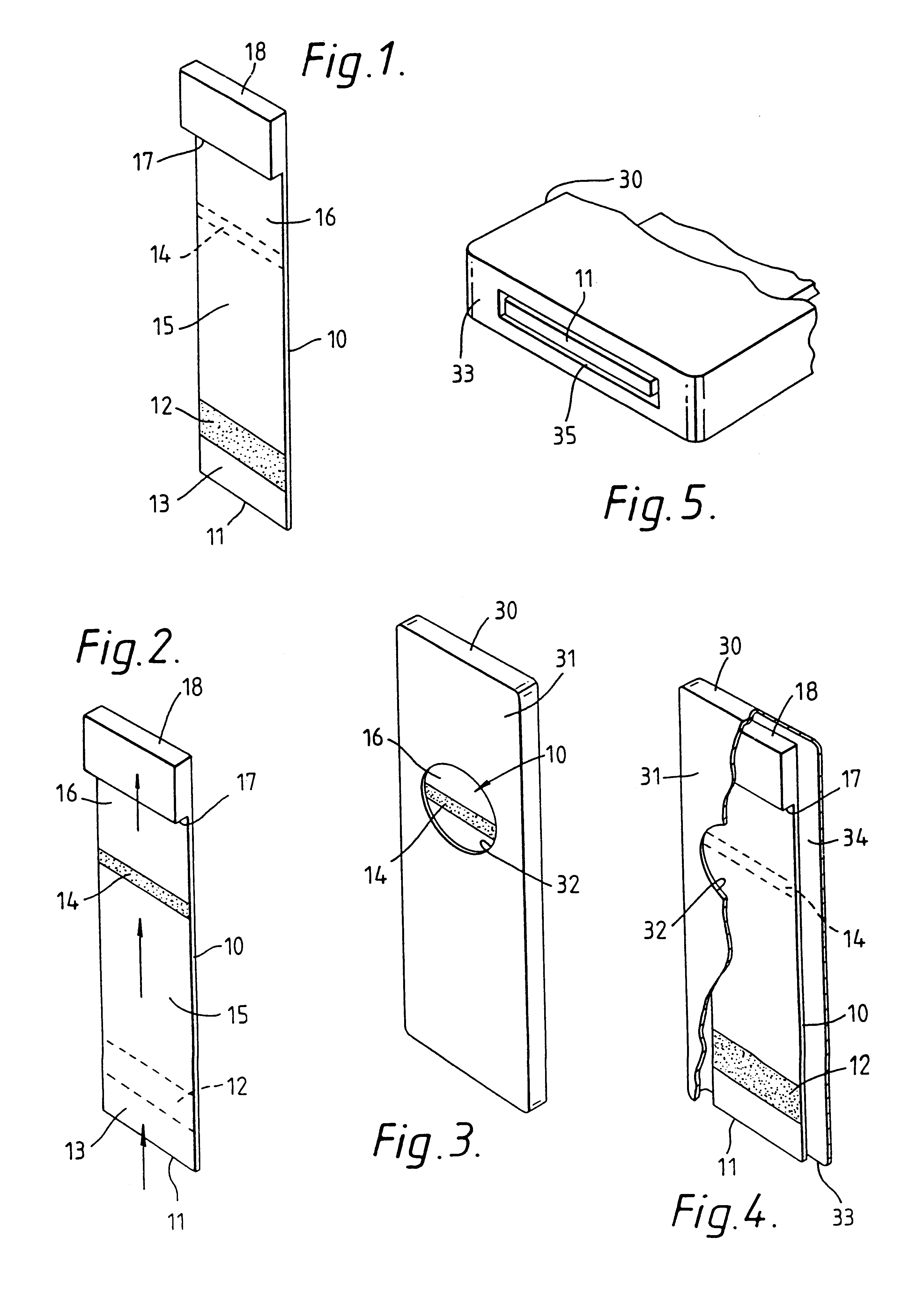
Method for producing microparticles loaded with proteins
InactiveUS7335401B2Improve bindingLow degreePowder deliveryLiquid surface applicatorsMicroparticleChemistry
The present invention concerns a method for producing microparticles loaded with proteins which is characterized in that the microparticles are loaded in suspension under strongly alkaline conditions. The invention also concerns microparticles which can be produced using this method and their use in a binding test e.g. in an immunoassay.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Capillary immunoassay and device therefor comprising mobilizable particulate labelled reagents
InactiveUS6228660B1Improve completenessAnalysis using chemical indicatorsComponent separationParticulatesAnalyte
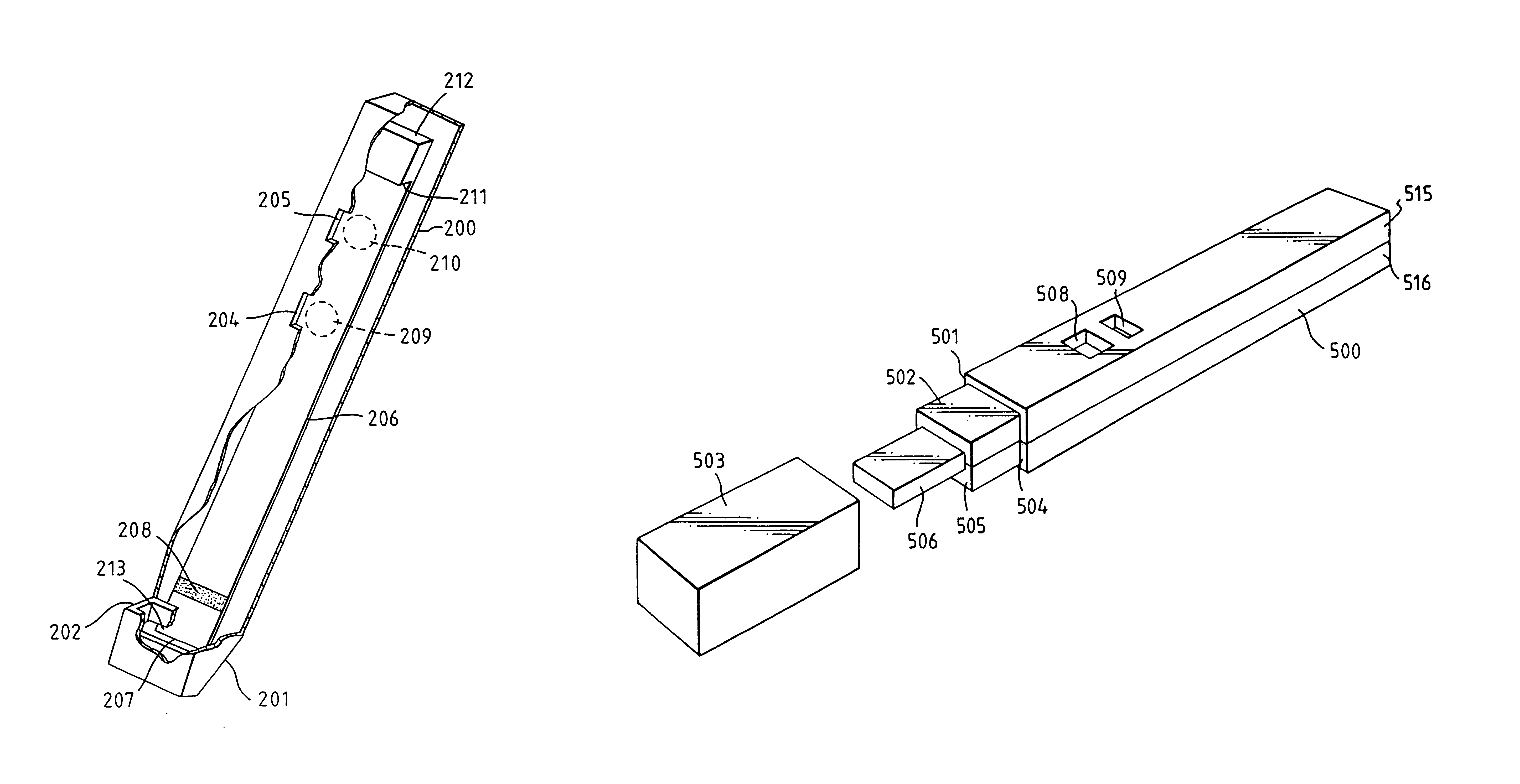
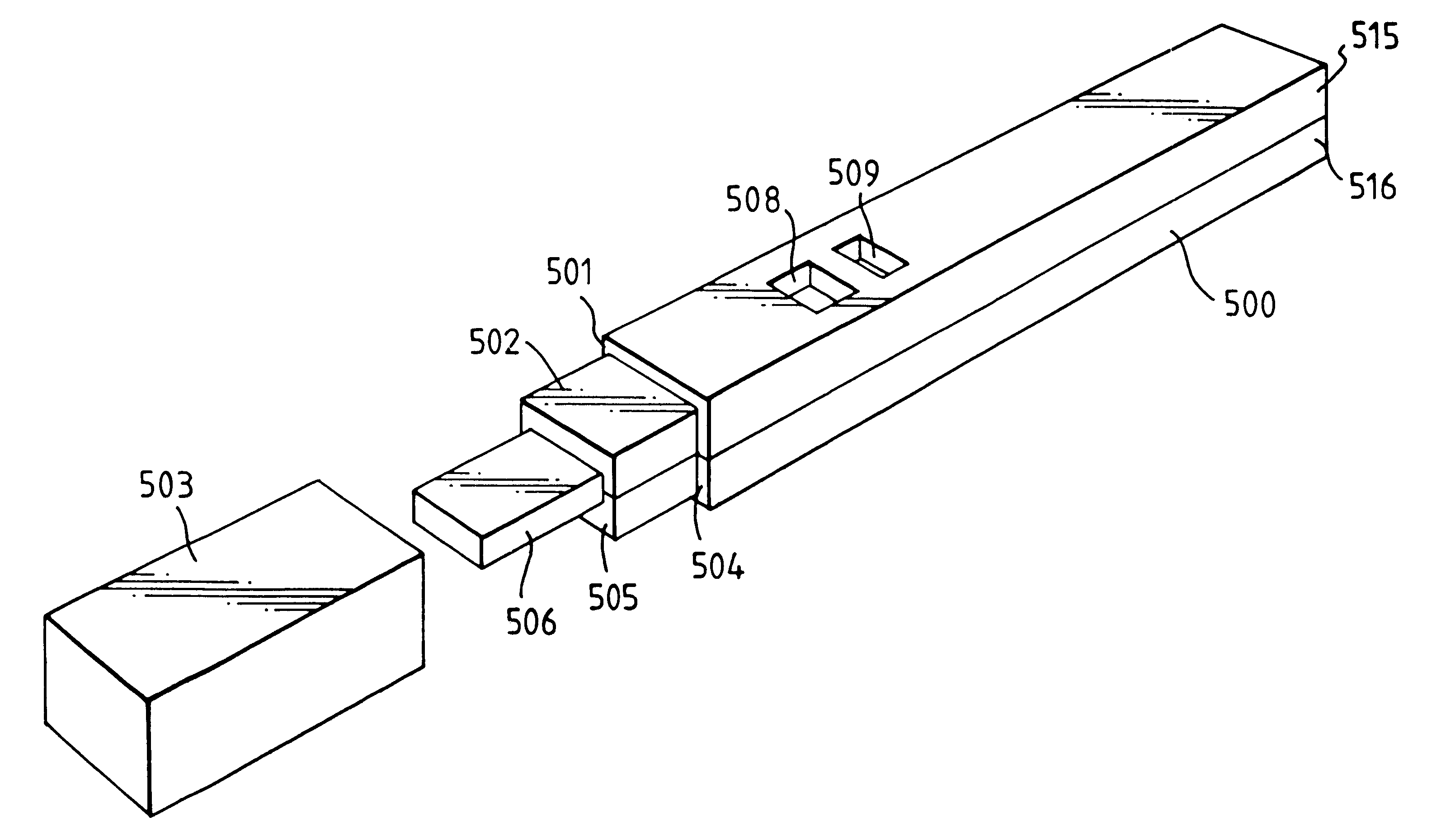
An analytical test device useful for example in pregnancy testing, comprises a hollow casing (500) constructed of moisture-impervious solid material, such as plastics materials, containing a dry porous carrier (510) which communicates indirectly with the exterior of the casing via a bibulous sample receiving member (506) which protrudes from the casing such that a liquid test sample can be applied to the receiving member and permeate therefrom to the porous carrier, the carrier containing in a first zone a labelled specific binding reagent is freely mobile within the porous carrier when in the moist state, and in a second zone spatially distinct from the first zone unlabelled specific binding reagent for the same analyte which unlabelled reagent is permanently immobilized on the carrier material and is therefore not mobile in the moist state, the two zones being arranged such that liquid sample applied to the porous carrier can permeate via the first zone into the second zone, and the device incorporating means, such as an aperture (508) in the casing, enabling the extent (if any) to which the labelled reagent becomes bound in the second zone to be observed. Preferably the device includes a removable cap for the protruding bibulous member.
Owner:CONOPCO INC D B A UNILEVER
Immunoassay device with improved sample closure
ActiveUS20050054078A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCapillary TubingEngineering
An apparatus and method for sealing a fluid sample collection device, comprising: loading a fluid sample collection device with a fluid sample, said device comprising a housing having at least one substantially planar surface that includes an orifice in fluid communication with an internal fluid sample holding chamber which terminates at an internal capillary stop; and slidably moving a sealing element over at least a portion of said substantially planar surface in a way that displaces any excess fluid sample away from the orifice, seals the fluid sample within said holding chamber, and inhibits the fluid sample from prematurely breaking through the internal capillary stop.
Owner:ABBOTT POINT CARE
Capillary immunoassay and device therefor comprising mobilizable particulate labelled reagents
InactiveUS6187598B1Improve completenessBioreactor/fermenter combinationsBiological substance pretreatmentsPlastic materialsCapillary Tubing
An analytical test device useful for example in pregnancy testing, comprises a hollow casing (500) constructed of moisture-impervious solid material, such as plastics materials, containing a dry porous carrier (510) which communicates indirectly with the exterior of the casing via a bibulous sample receiving member (506) which protrudes from the casing such that a liquid test sample can be applied to the receiving member and permeate therefrom to the porous carrier, the carrier containing in a first zone a labelled specific binding reagent is freely mobile within the porous carrier when in the moist state, and in a second zone spatially distinct from the first zone unlabelled specific binding reagent for the same analyte which unlabelled reagent is permanently immobilised on the carrier material and is therefore not mobile in the moist state, the two zones being arranged such that liquid sample applied to the porous carrier can permeate via the first zone into the second zone, and the device incorporating means, such as an aperture (508) in the casing, enabling the extent (if any) to which the labelled reagent becomes bound in the second zone to be observed. Preferably the device includes a removable cap for the protruding bibulous member.
Owner:INVERNESS SWITZERLAND GMBH
Apparatus and methods for analyte measurement and immunoassay
ActiveUS7419821B2Bioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careEngineering
Owner:ABBOTT POINT CARE
Immunoassay that provides for both collection of saliva and assay of saliva for one or more analytes with visual readout
InactiveUS6248598B1Eliminate riskBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteSaliva sample
A device that provides for both the collection of saliva and detection of at least one analyte therein, e.g., a drug, is provided. This device provides for rapid analysis of saliva samples, while also providing a convenient assay method that does not require the addition of extraneous reagents, or other materials. Thereby, this device can be used by non-laboratory personnel without risk of user introduced errors.
Owner:BOGEMA STUART C
Methods and devices for microfluidic point-of-care immunoassays
ActiveUS20090181411A1Endpoint detectionReduce incubation timeBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careSystems design
Microfluidic methods and devices for heterogeneous binding and agglutination assays are disclosed, with improvements relating to mixing and to reagent and sample manipulation in systems designed for safe handling of clinical test samples.
Owner:PERKINELMER HEALTH SCIENCES INC
Immunoassay device with immuno-reference electrode
InactiveUS7723099B2Increase ionic strengthReduce distractionsBioreactor/fermenter combinationsBiological substance pretreatmentsProtein insertionNon specific
An electrochemical immunosensor system with reduced interference, comprising: a first immunosensor that generates an electrochemical signal based on the formation of a sandwich between an immobilized antibody, a target analyte and a labeled antibody, wherein a portion of the signal arises from non-specific binding of the labeled antibody in the region of the first immunosensor, anda second immunosensor that acts as an immuno-reference sensor and generates a signal that is the same as or predictably related to the degree of non-specific binding which occurs in the region of the first immunosensor, and has an immunocomplex between an immobilized antibody and an endogenous or exogenous protein that is in the sample and that is not the target analyte.
Owner:ABBOTT POINT CARE
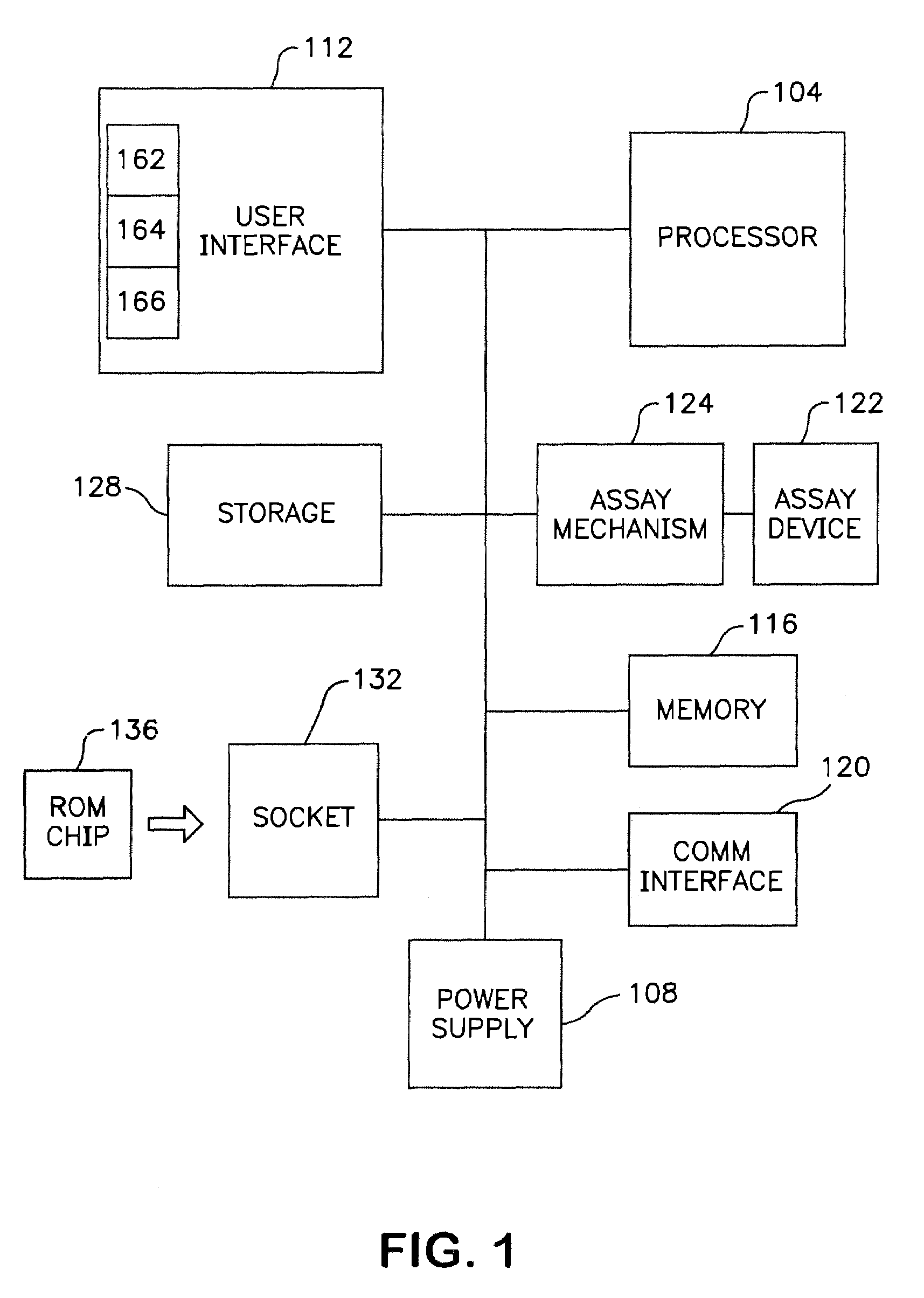
Immunoassay fluorometer
InactiveUS7416700B2Easy accessChance for errorChemiluminescene/bioluminescenceAnalysis by electrical excitationCommunication interfaceData set
A fluorometer for sensing the fluorescence of a sample utilizes an optical energy source for exciting a sample to be tested and an optical energy detector for detecting the emitted energy from the excited sample. Drive electronics are used for positioning the sample with respect to the optical components allowing a plurality of sample regions to be tested. A processor is utilized to control the operation of the test in accordance with test instructions and for processing the emitted energy detected from the sample to determine test results. A ROM chip socket accepts a plurality of ROM chips, wherein each ROM chip stores test data sets for one or more test types to be performed. ROM chips can be swapped to allow the fluorometer to be configured and reconfigured to perform a plurality of different tests. A communications interface facilitates the sharing of test information between the fluorometer and external entities.
Owner:BIOSITE INC
Automated analyzer for clinical laboratory
InactiveUS20090117620A1Manual loadingBioreactor/fermenter combinationsBiological substance pretreatmentsClinical chemistryMicro perforated plate
A laboratory automation system that is capable of carrying out clinical chemistry assays, immunoassays, amplification of nucleic acid assays, and any combination of the foregoing, said laboratory automation system employing at least one of micro-well plates and deep multi-well plates as reaction vessels. The use of micro-well plates as reaction vessels enables the laboratory automation system to assume a variety of arrangements, i.e., the laboratory automation system can comprise a variety of functional modules that can be arranged in various ways. In order to effectively carry out immunoassays by means of micro-well plates, a technique known as inverse magnetic particle processing can be used to transfer the product(s) of immunoassays from one micro-well of a micro-well plate to another.
Owner:ABBOTT LAB INC
Electrochemcial immunoassay for tumor marker and small size immunoassay chip
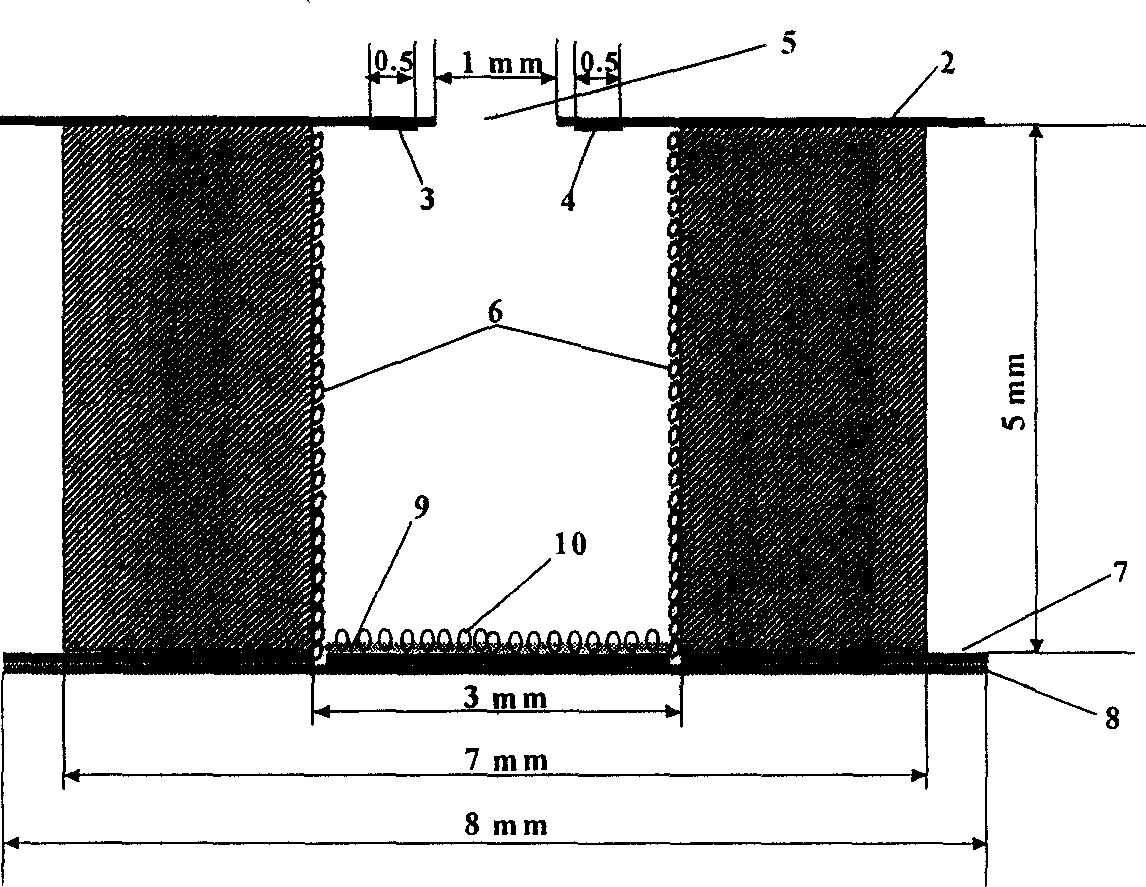
InactiveCN1614405ARapid separation-free assayAvoid pollutionMaterial analysis by electric/magnetic meansBiological testingAntigenContact free
An electrochemical method for determining immunity of tumor marker includes forming microreaction cell, placing reference and counter electrode above it and fixing antigen molecular functioning film at pool bottom, connecting enzyme labelled antibody to pool bottom at time of only proper quantity of horseradish peroxidase to label tumor marker antibody, obtaining cataltyic current on working electrode by contacting free immune matter with electronic media and having positive ratio of the current to antigen. The microvolume immune determining chip is also disclosed.
Owner:NANJING UNIV +1
Chemiluminescence-based microfluidic biochip
InactiveUS6949377B2Accurate and reproducible resultSimple and rapid and POCT applicationBioreactor/fermenter combinationsBiological substance pretreatmentsPositive pressureBiochip
The disclosure describes how to use luminescence detection mechanism, move microfluid, and control multiple-step biochemical reactions in closed confined microfluidic biochip platform. More particularly, a self-contained disposable biochip with patterned microchannels and compartments having storage means for storing a plurality of samples, reagents, and luminescent substrates. At least one external microactuator in the biochip system produces positive pressure and automates multiple-step reactions in microfluidic platforms for clinical chemistry, cell biology, immunoassay and nucleic acid analysis. The method comprises the steps of transferring sequentially at least one of samples, reagents, and then luminescent substrate from compartments through microchannels to reaction sites. The luminescent substrates react with probes to form a probe complex resulting into luminescence, which is detected by an optical detector.
Owner:HO WINSTON Z
Optical disk-based assay devices and methods
InactiveUS6342349B1Strong specificityReduce nonspecific bindingSequential/parallel process reactionsSugar derivativesAnalyteLaser light
Optical disk-based assay devices and methods are described, in which analyte-specific signal elements are disposed on an optical disk substrate. In preferred embodiments, the analyte-specific signal elements are disposed readably with the disk's tracking features. Also described are cleavable signal elements particularly suitable for use in the assay device and methods. Binding of the chosen analyte simultaneously to a first and a second analyte-specific side member of the cleavable signal element tethers the signal-responsive moiety to the signal element's substrate-attaching end, despite subsequent cleavage at the cleavage site that lies intermediate the first and second side members. The signal responsive moiety reflects, absorbs, or refracts incident laser light. Described are nucleic acid hybridization assays, nucleic acid sequencing, immunoassays, cell counting assays, and chemical detection. Adaptation of the assay device substrate to function as an optical waveguide permits assay geometries suitable for continuous monitoring applications.
Owner:VINDUR TECH
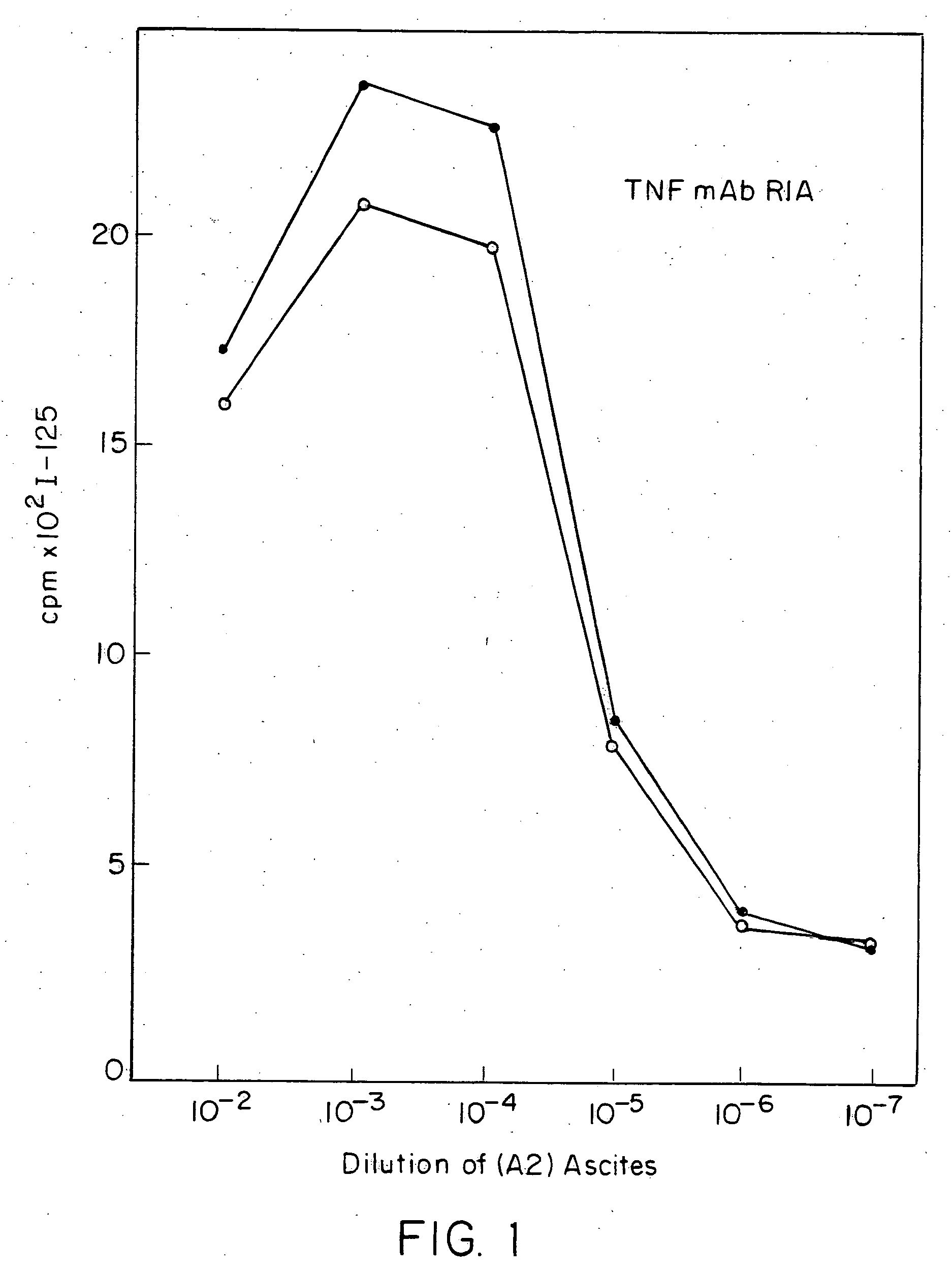
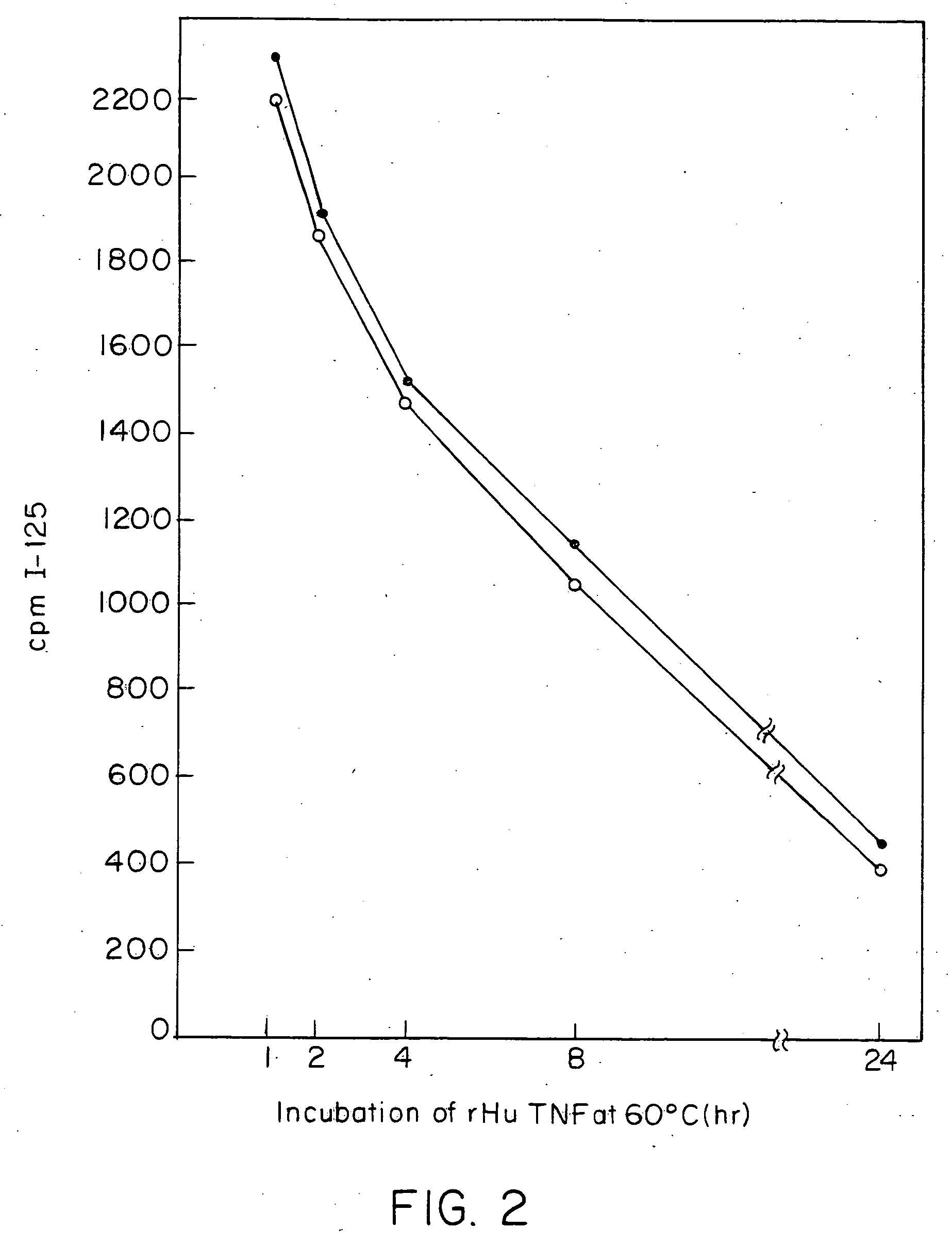
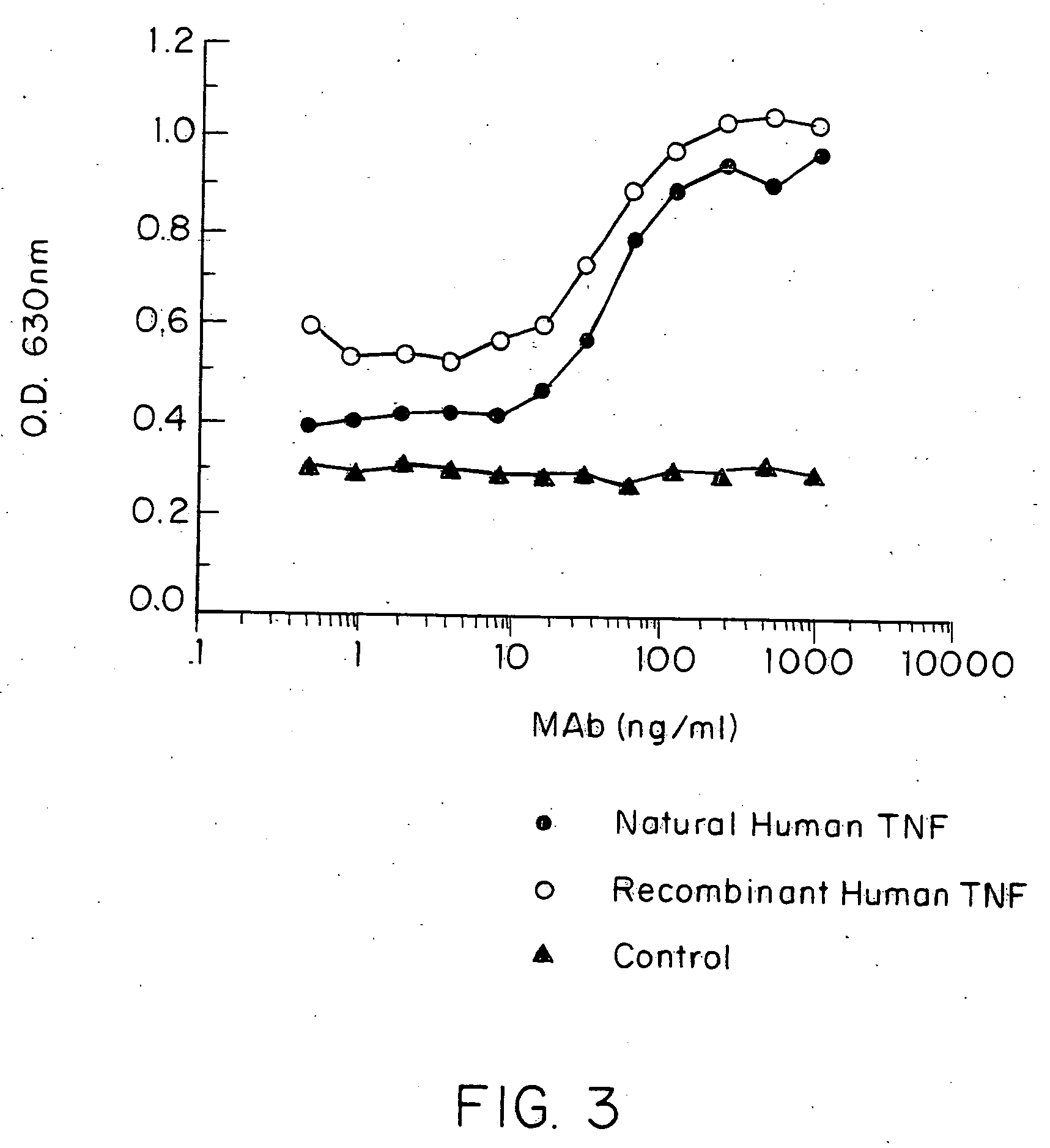
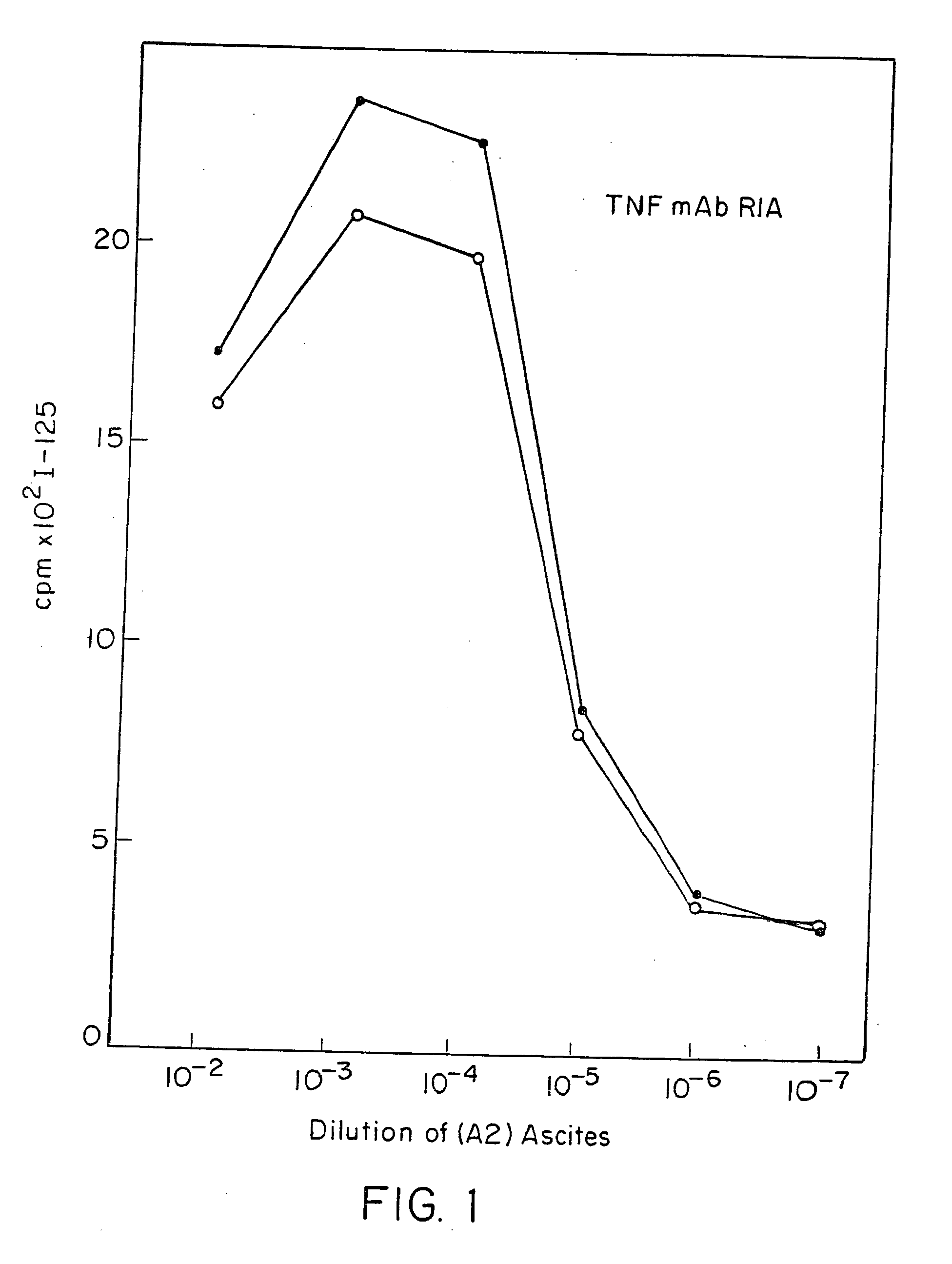
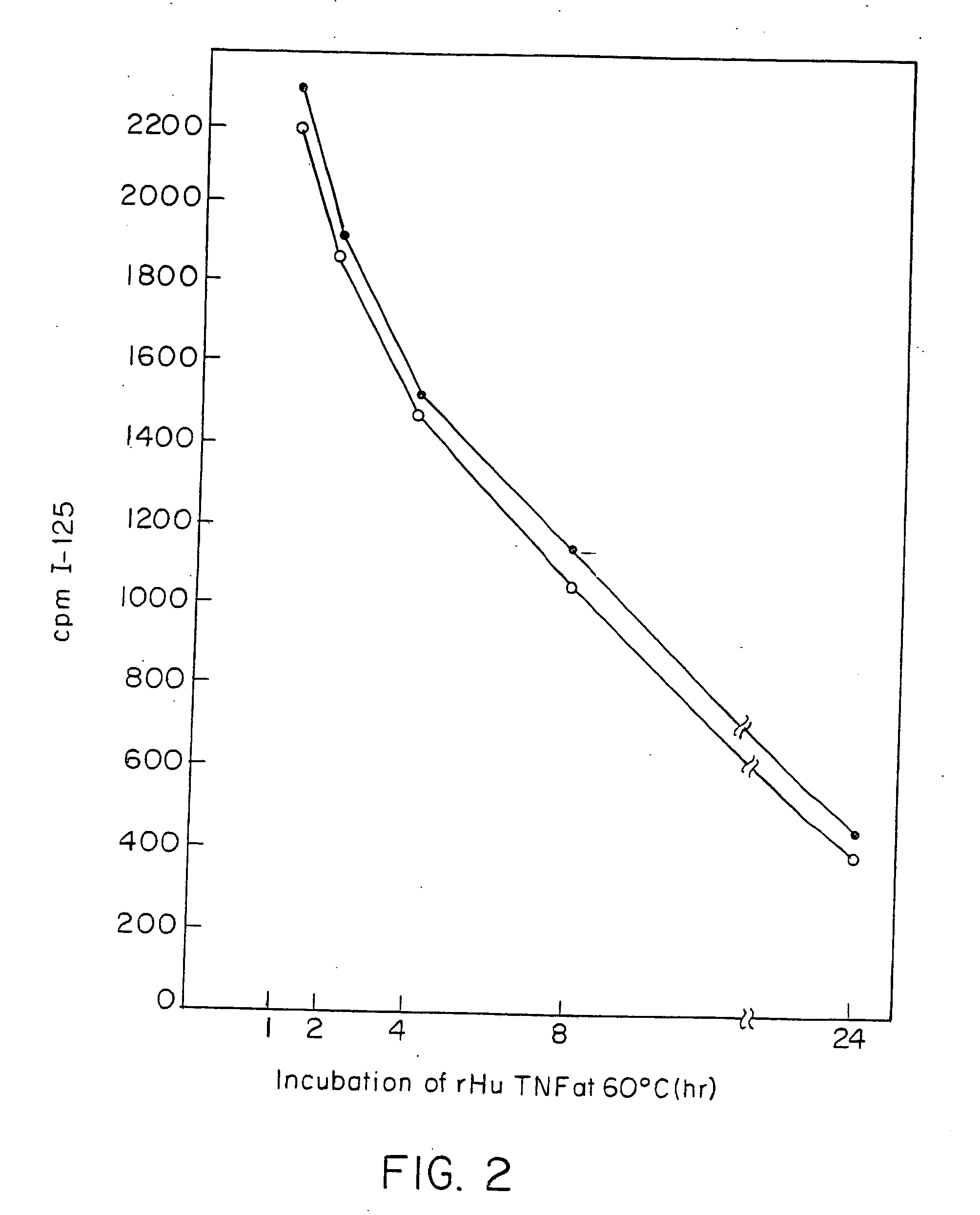
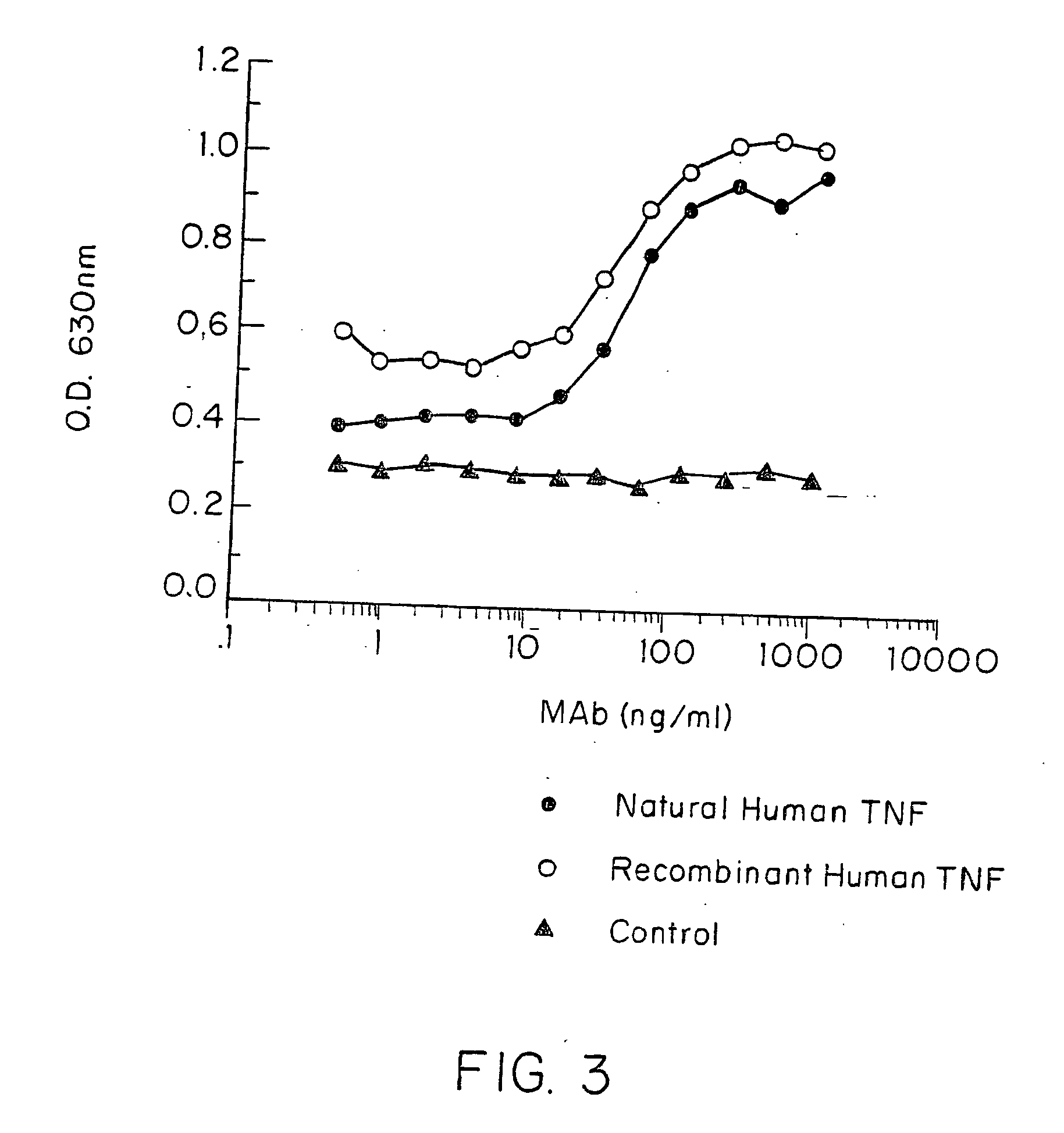
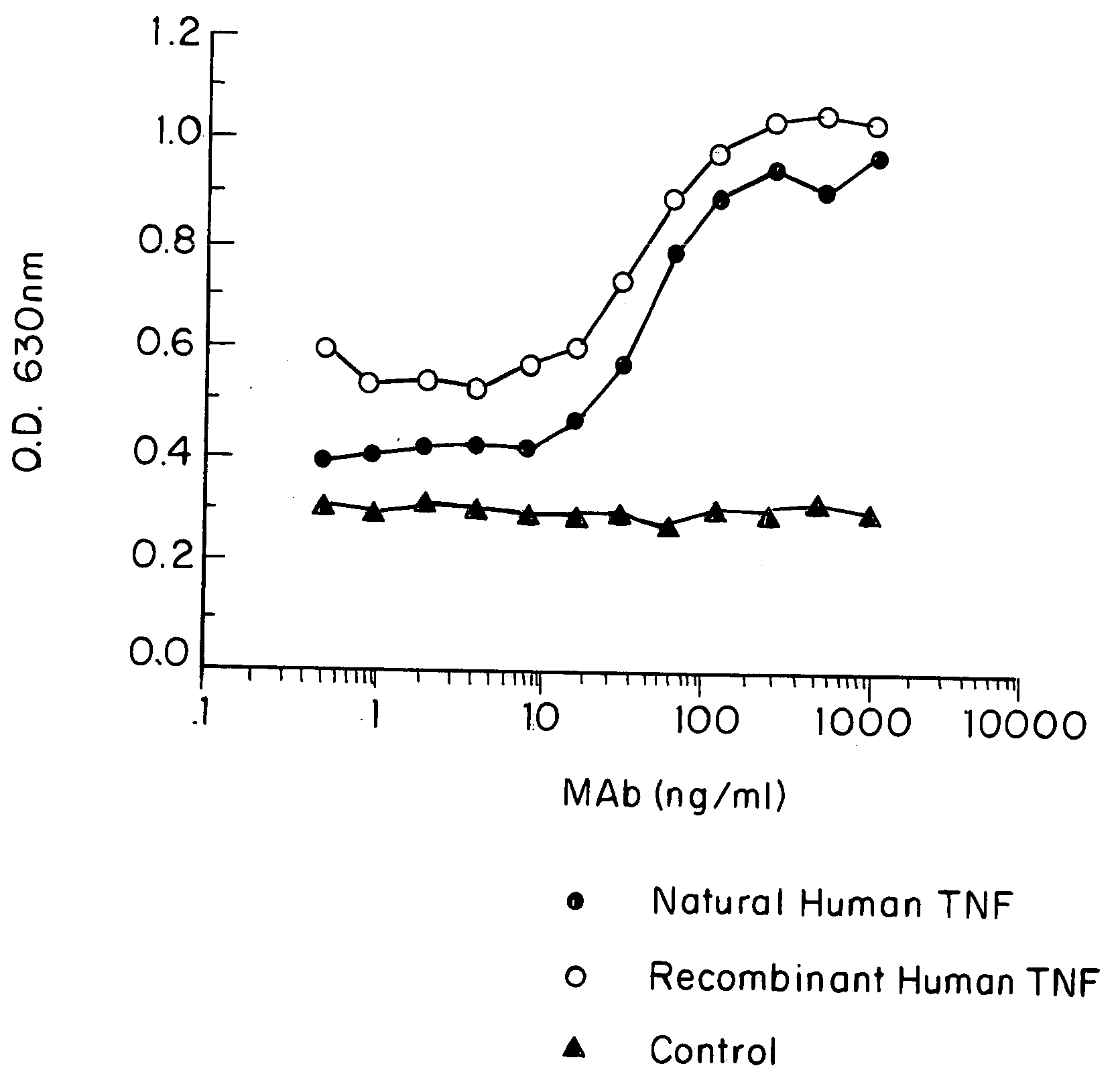
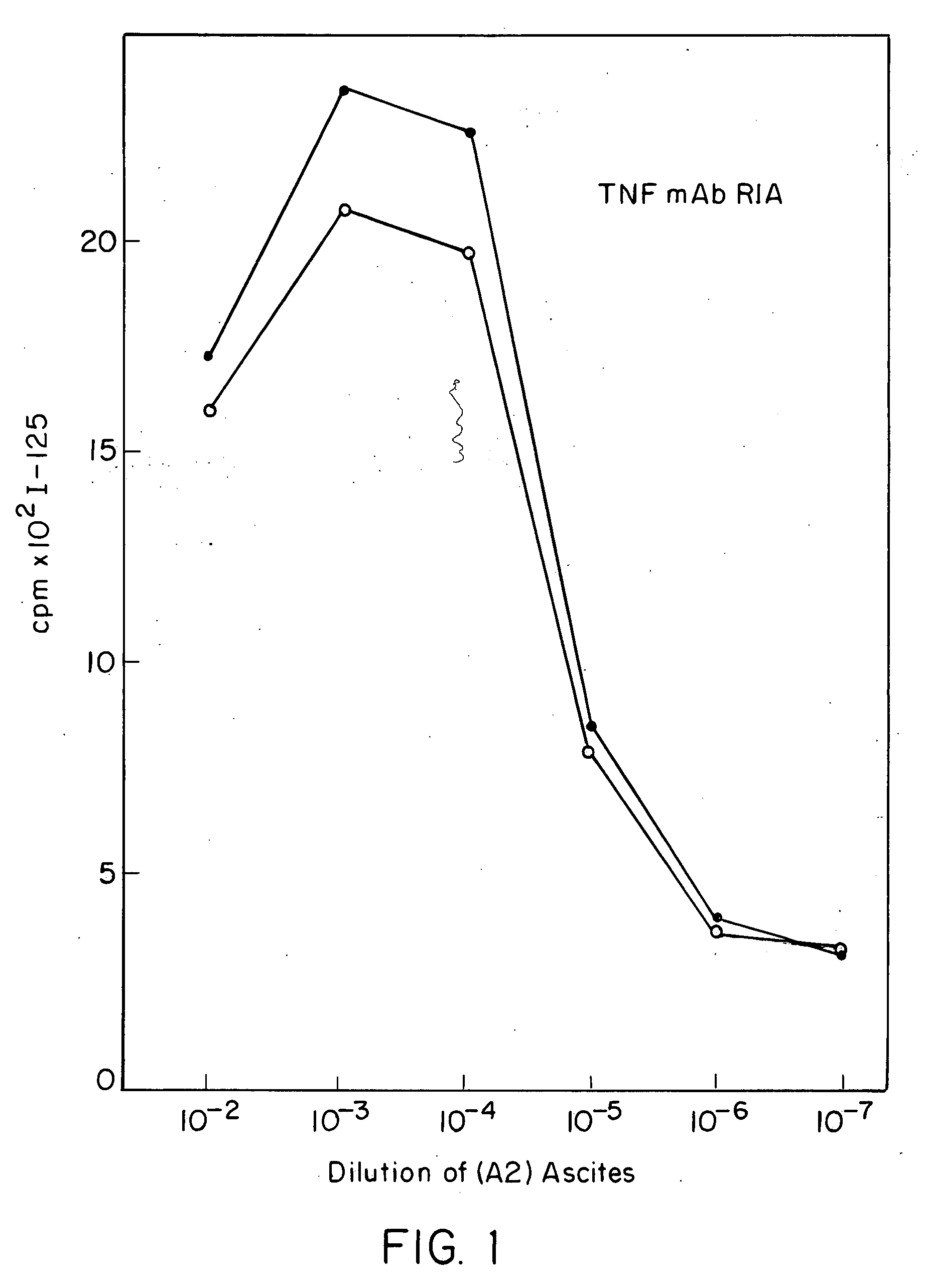
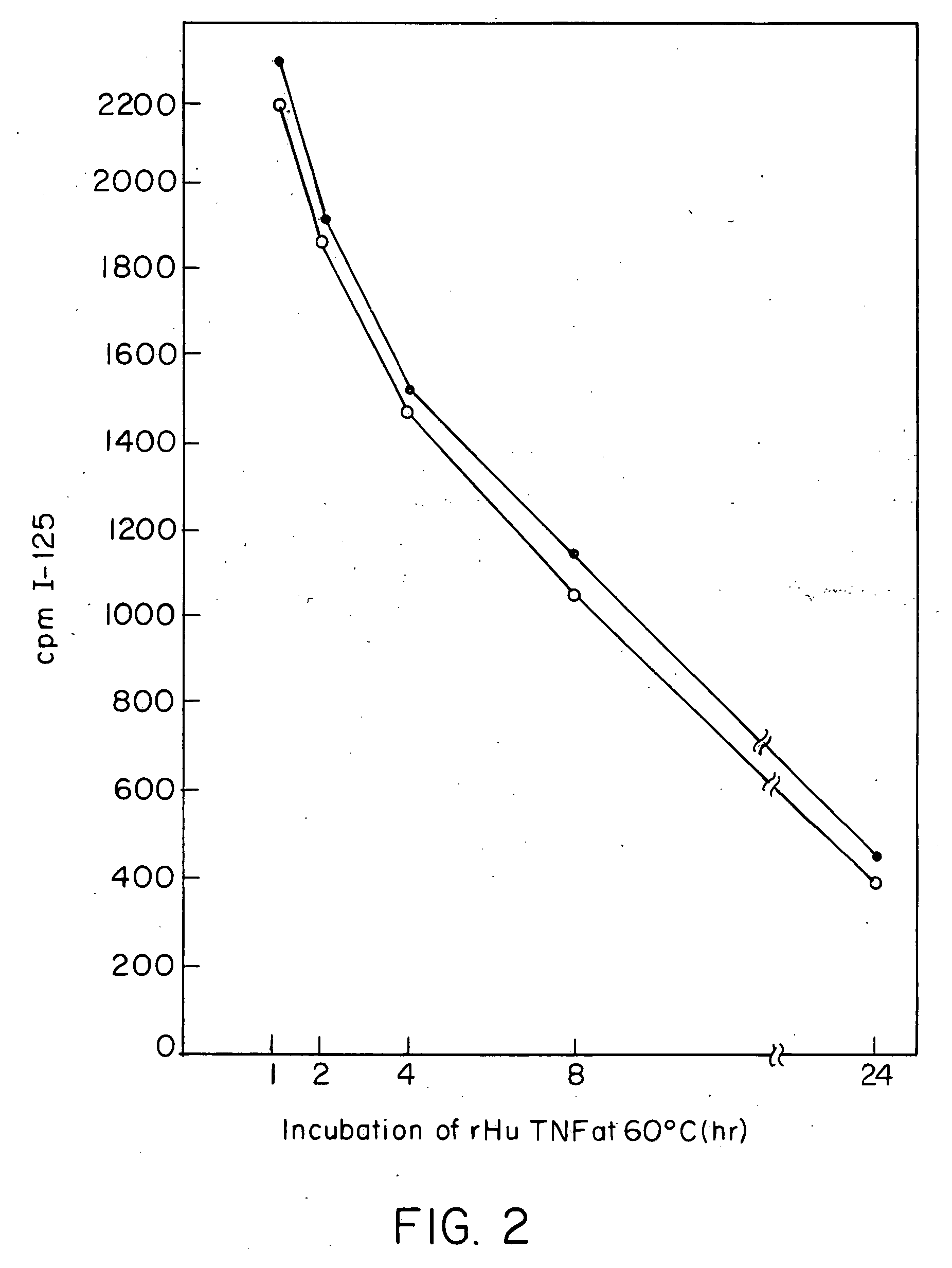
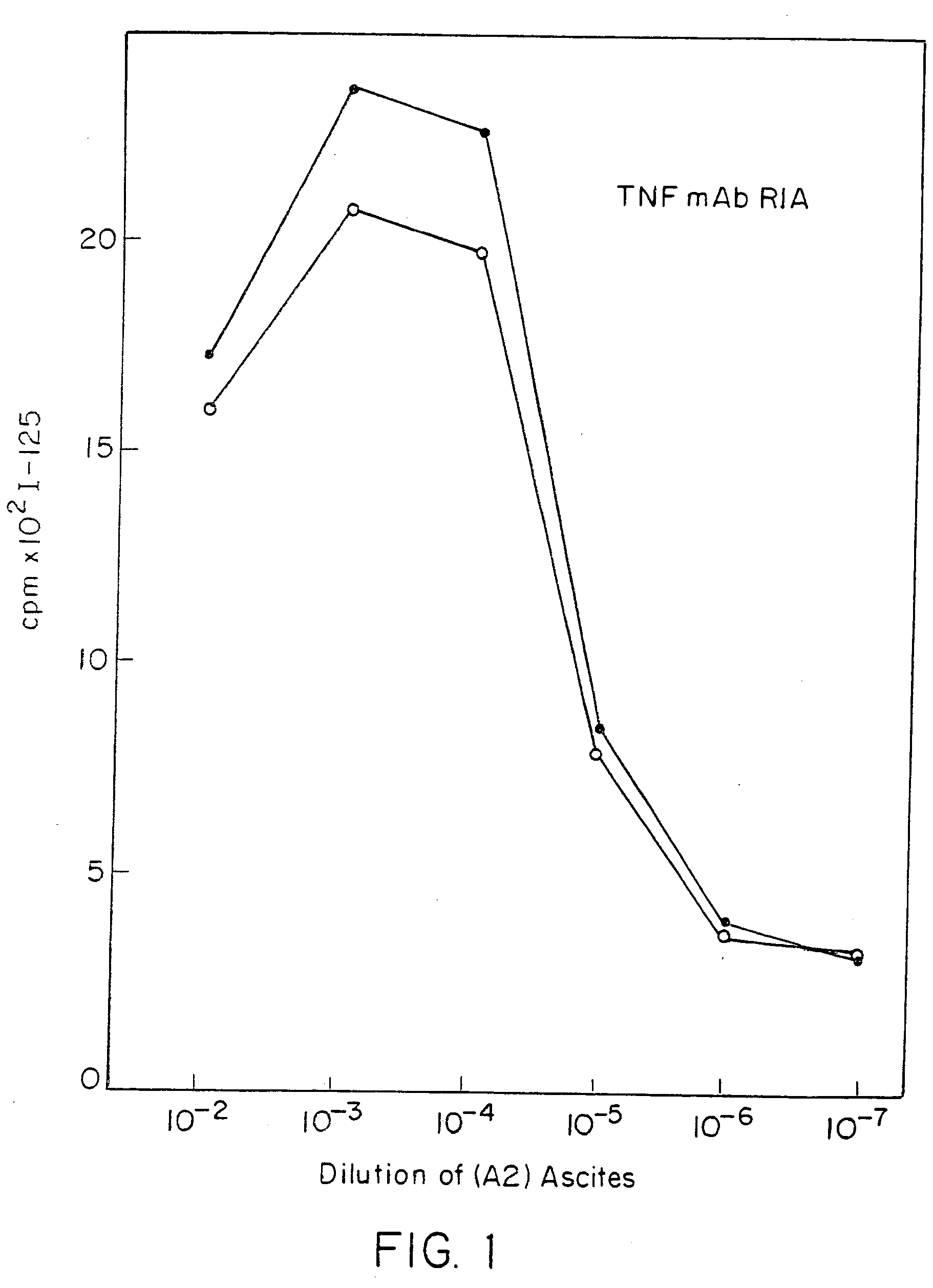
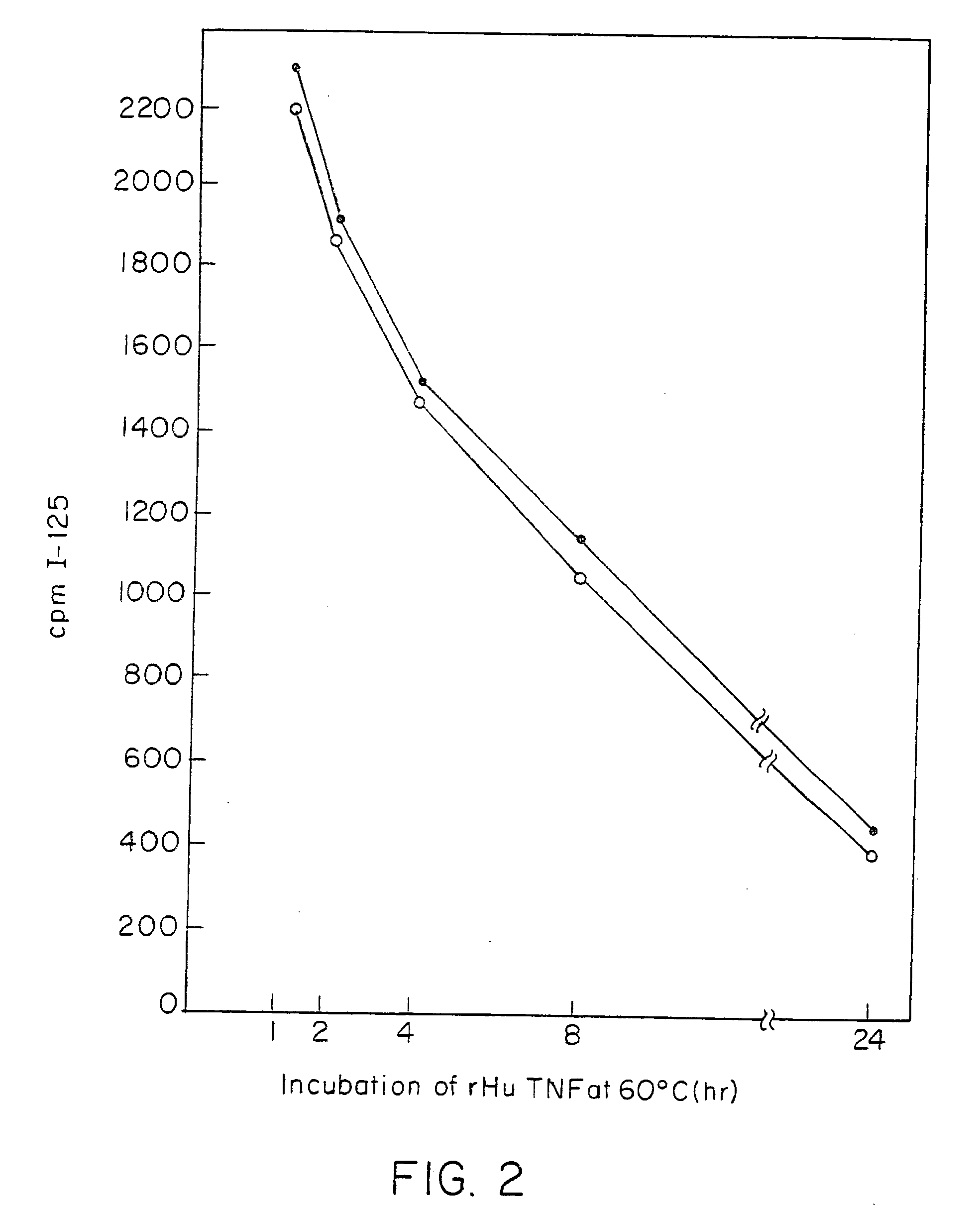
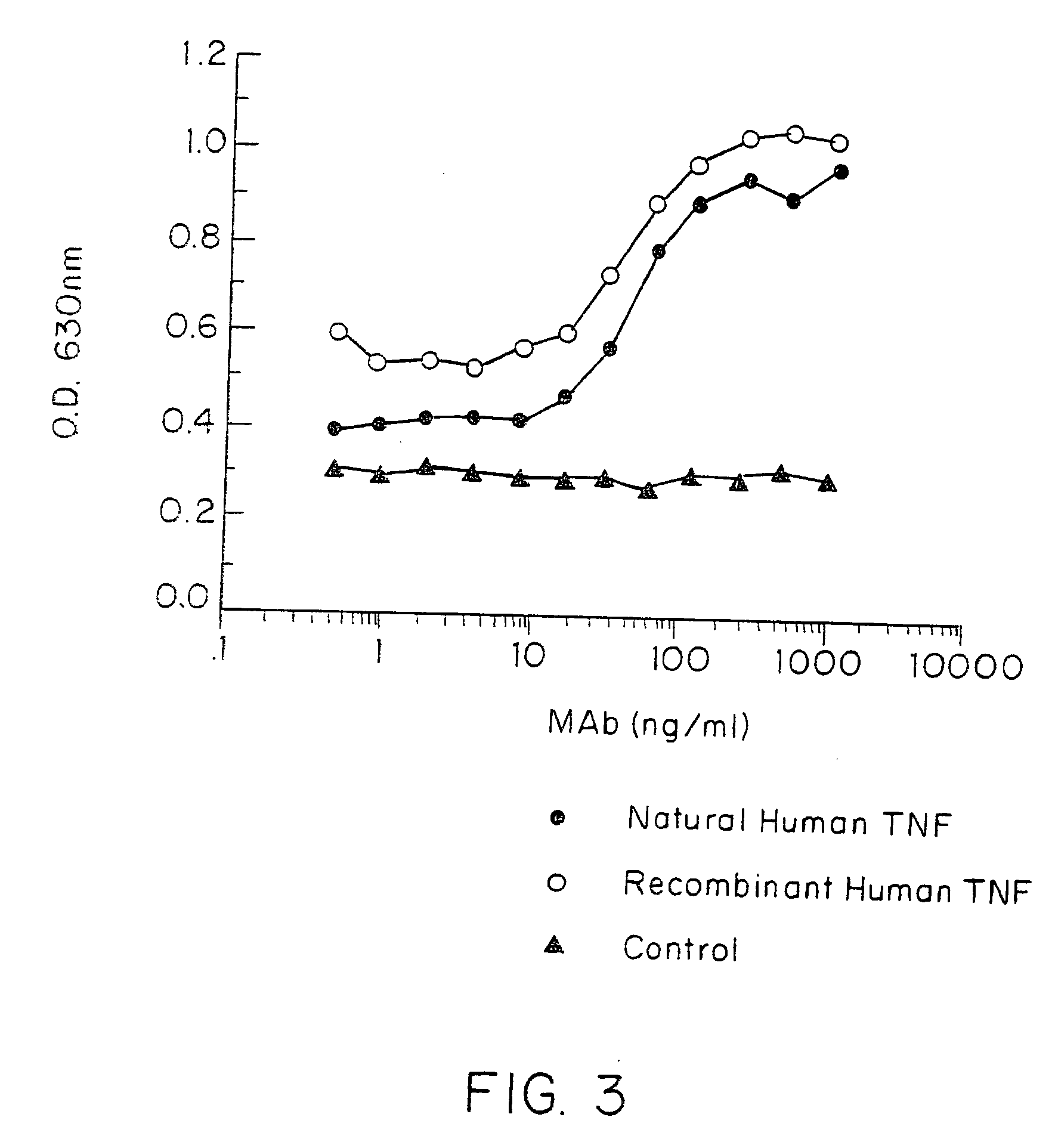
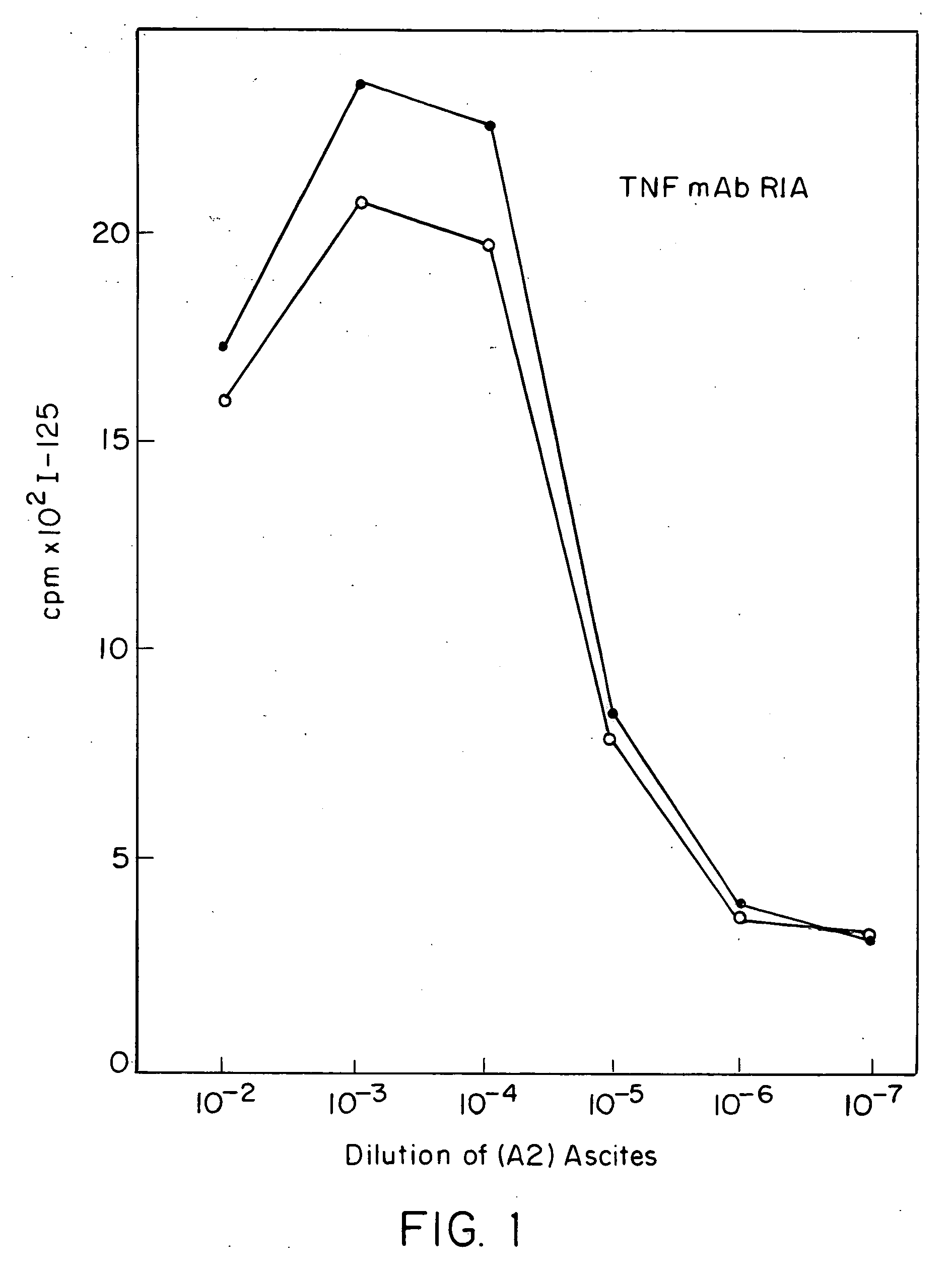
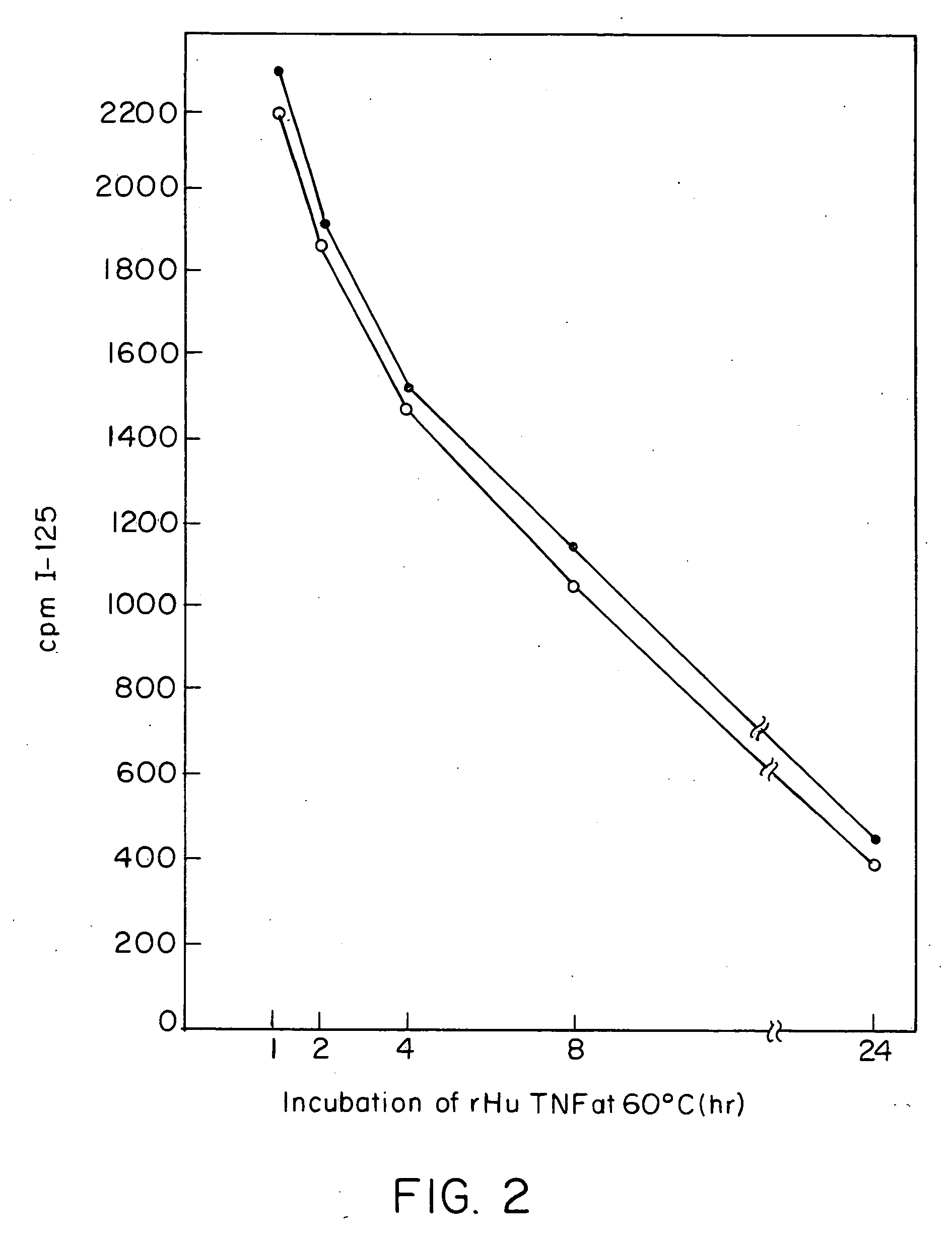
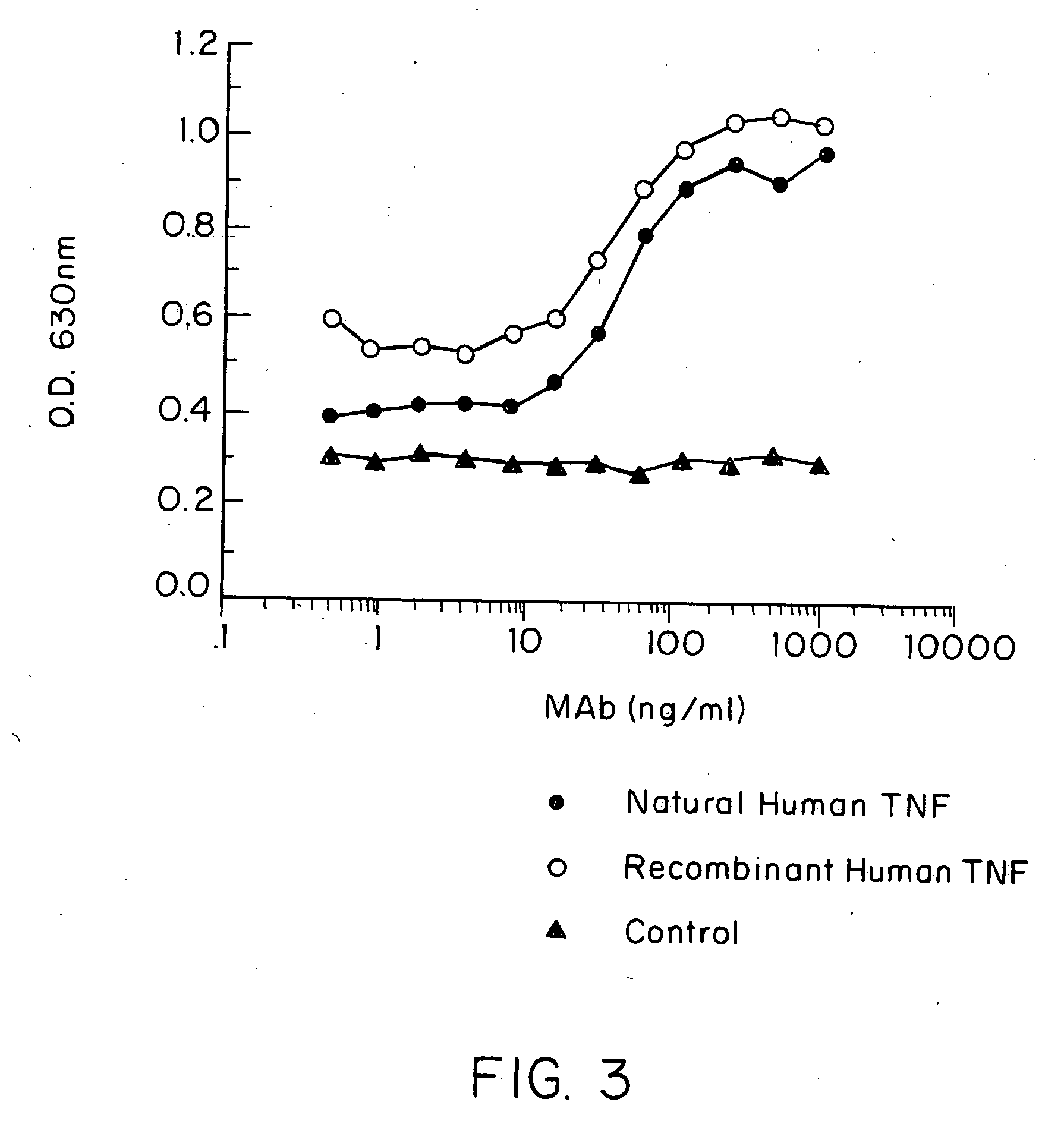
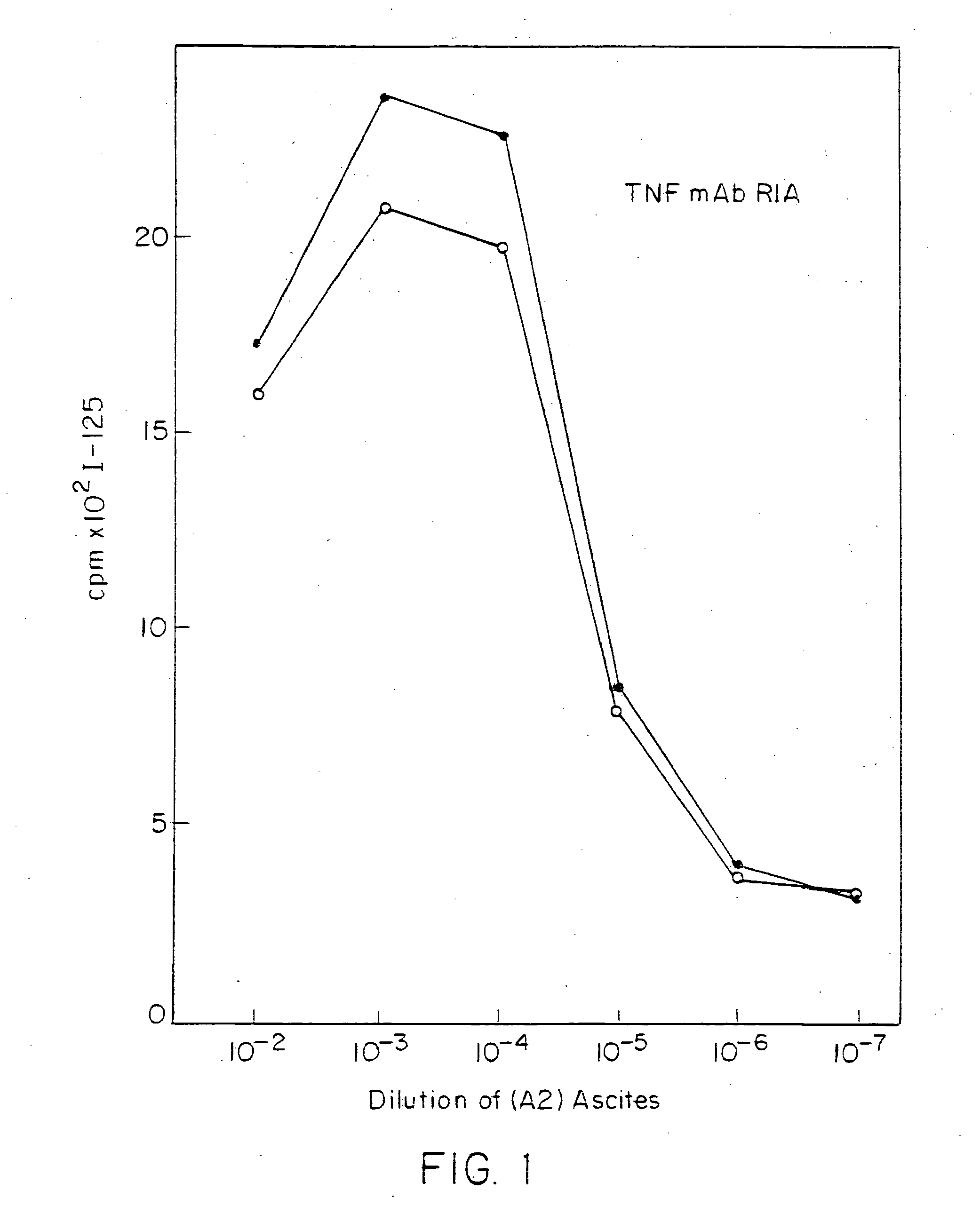
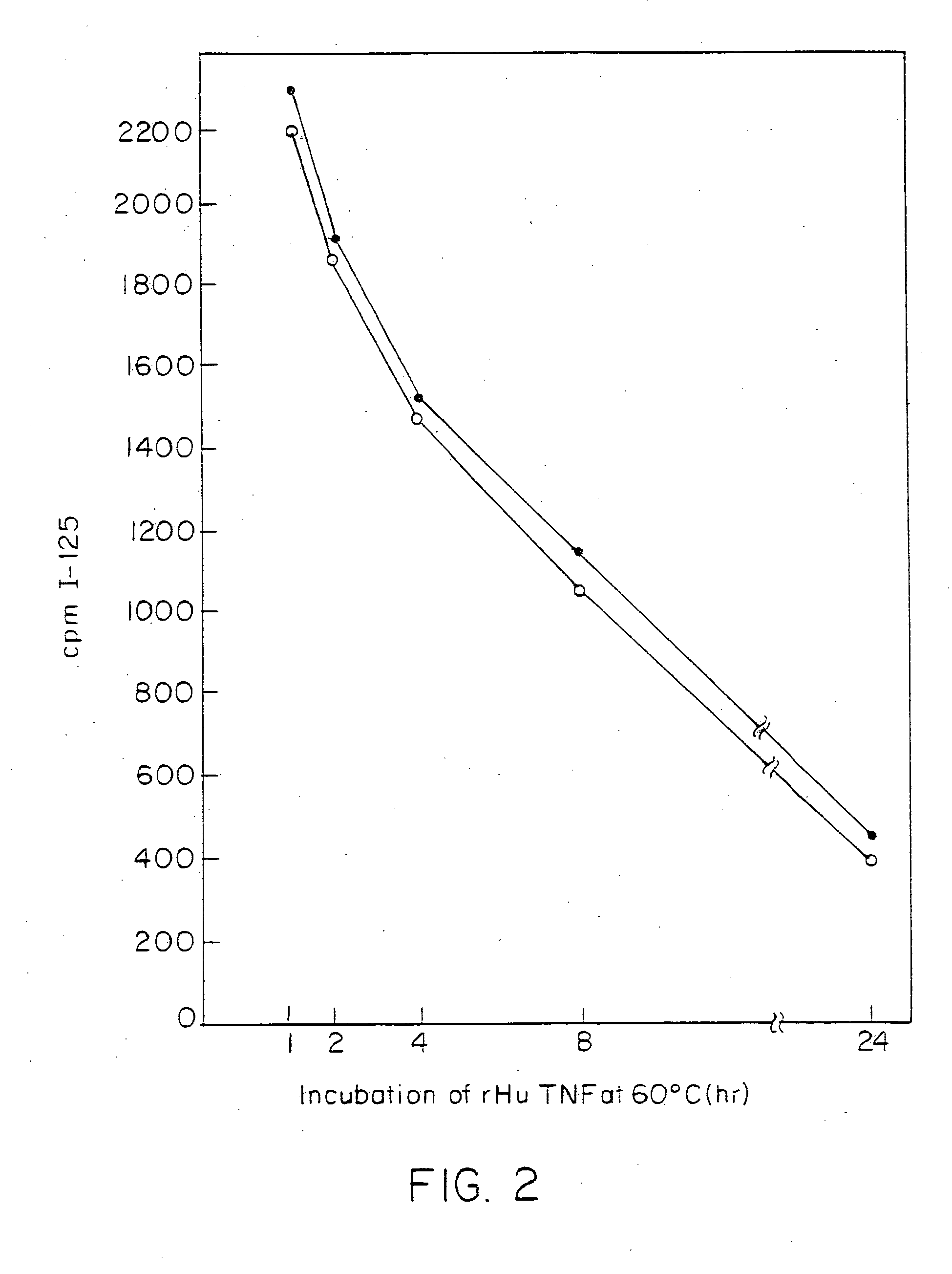
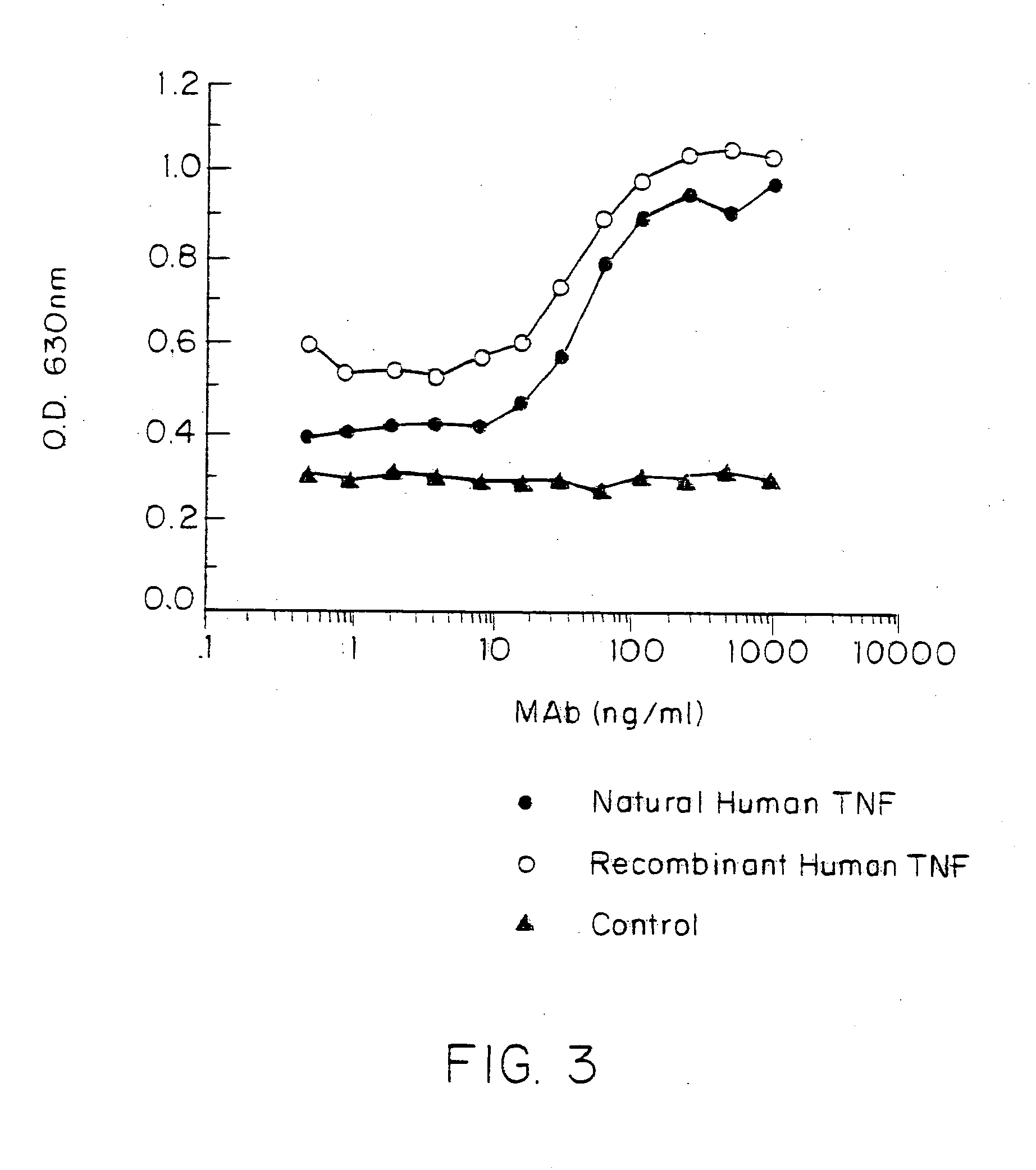
Anti-TNF antibodies and peptides of human tumor necrosis factor
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:CENTOCOR
Diagnostic device and method
InactiveUS6222619B1Fast resultsImprove the level ofRadiation pyrometrySpectrum investigationDiagnostic programReaction rate
A method and apparatus of diagnosing a cardiac disease state in as little as two minutes involving the utilization of an evanescent wave assay system in conjunction with a data acquisition and analysis procedure that monitors the precision of assay results in real time (i.e., while data is being acquired). The method includes diagnosing a disease state using a diagnostic procedure (e.g., an immunoassay) wherein the testing device informs the person conducting the test of the results of the test as soon as reliable test data is obtained (generally, <5% variation in the reaction rate of the assay). After which point, the diagnostic procedure may be terminated.
Owner:UNIV OF UTAH RES FOUND
Methods of treating ankylosing spondylitis using anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20050249735A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV +1
Methods of treating ankylosing spondylitis using anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20080025976A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:LE JUNMING +6
Enzyme-catalyzed metal deposition for the enhanced detection of analytes of interest
ActiveUS20050100976A1Rapidly and accurately determinedHigh detection sensitivitySugar derivativesMicrobiological testing/measurementTarget analysisAnalyte
The invention is directed to enhanced methods for detecting an analyte of interest in situ, by immunoassay, or by hybridization comprising binding an enzyme-labeled conjugate molecule to an analyte of interest in the presence of a redox-inactive reductive species and a soluble metal ion. The enzyme catalyzes the conversion of the inactive reductive species to an active reducing agent, which in turn reduces the metal ion to a metal atom thereby providing an enhanced means of detecting the analyte via metal deposition.
Owner:VENTANA MEDICAL SYST INC
Anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20060018907A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV +1
Methods of treating seronegative arthropathy with anti-TNF antibodies
InactiveUS20070298040A1Inhibit biological activityHigh affinityAntibody ingredientsImmunoglobulinsSeronegative arthropathyHuman tumor
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFαx-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV
Anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20070196373A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:LE JUNMING +6
Risperidone immunoassay
Novel conjugates and immunogens derived from risperidone and antibodies generated by these immunogens are useful in immunoassays for the quantification and monitoring of risperidone and paliperidone in biological fluids.
Owner:SALADAX BIOMEDICAL INC
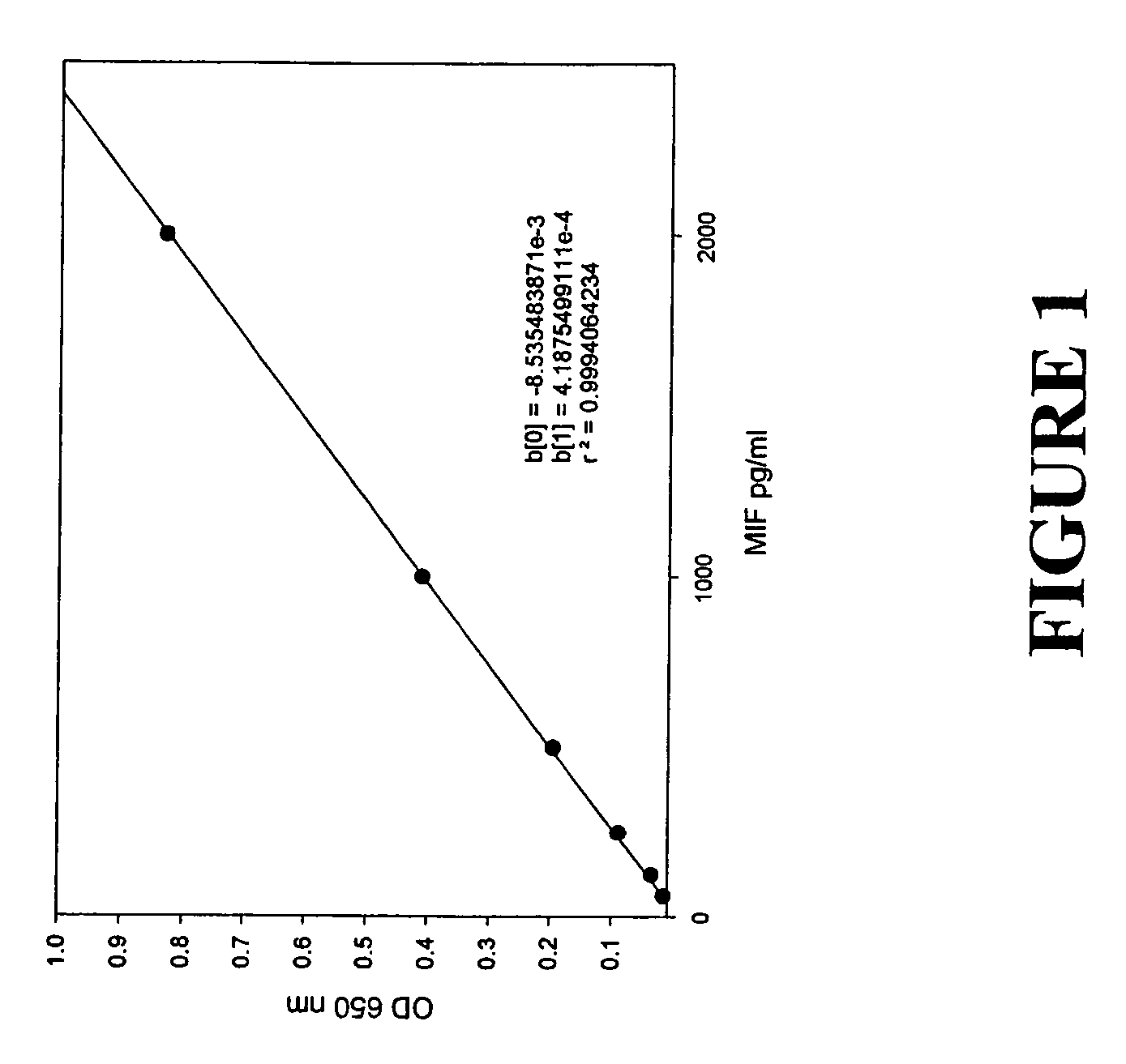
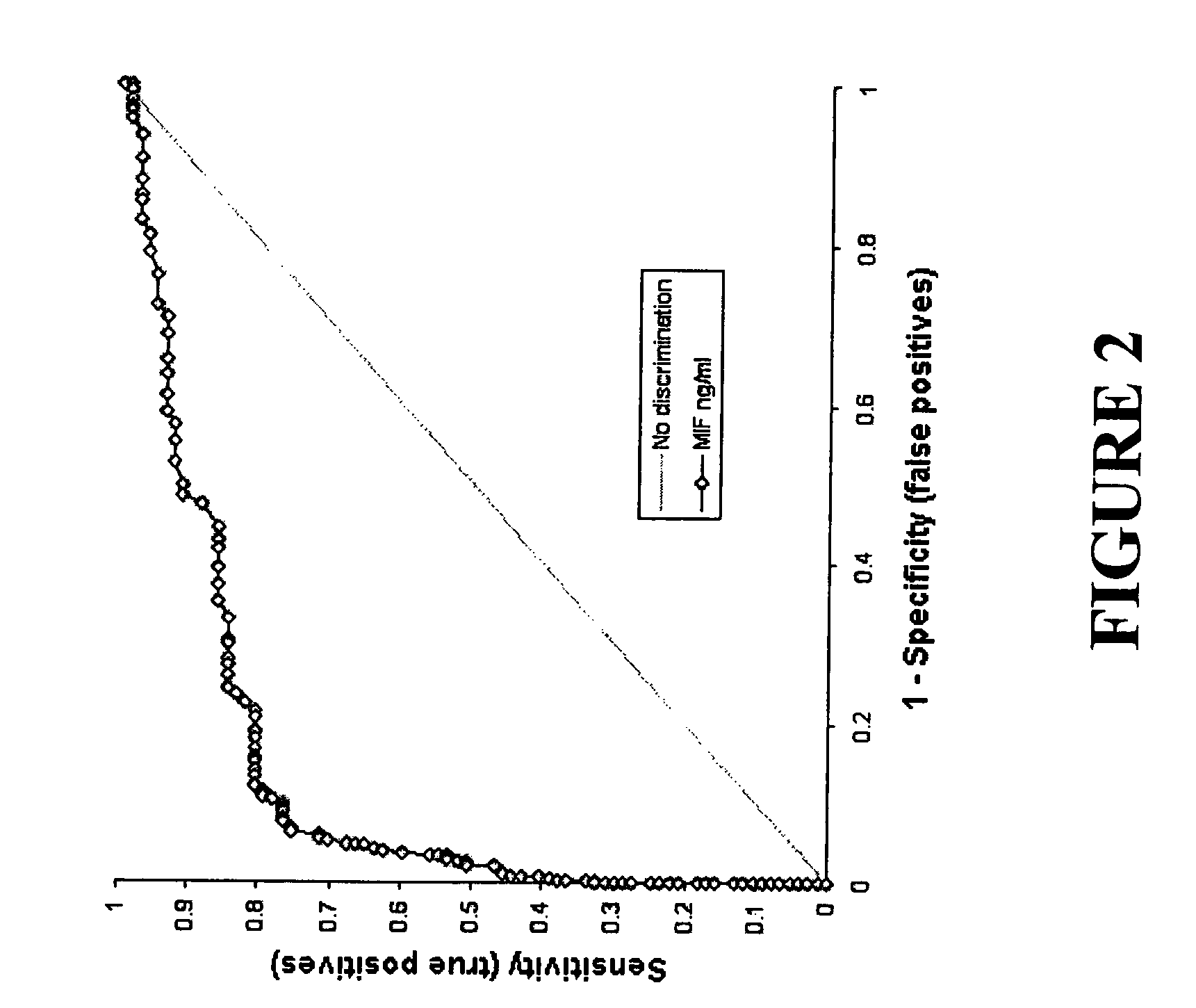
Serum macrophage migration inhibitory factor (MIF) as marker for prostate cancer
InactiveUS7361474B2Aggressive diseaseAggressive treatmentMicrobiological testing/measurementPeptide preparation methodsSerum igeGenetic Change
The present invention provides methods for detecting, diagnosing or prognosticating prostate cancer by measuring the levels of macrophage migration inhibitory factor (MIF) in the serum of an individual. The assay for MIF can be an immunoassay, such as ELISA, or a nucleic assay, such as Nouthern blot. Genetic changes within MIF gene can predict patients that express high levels of MIF.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
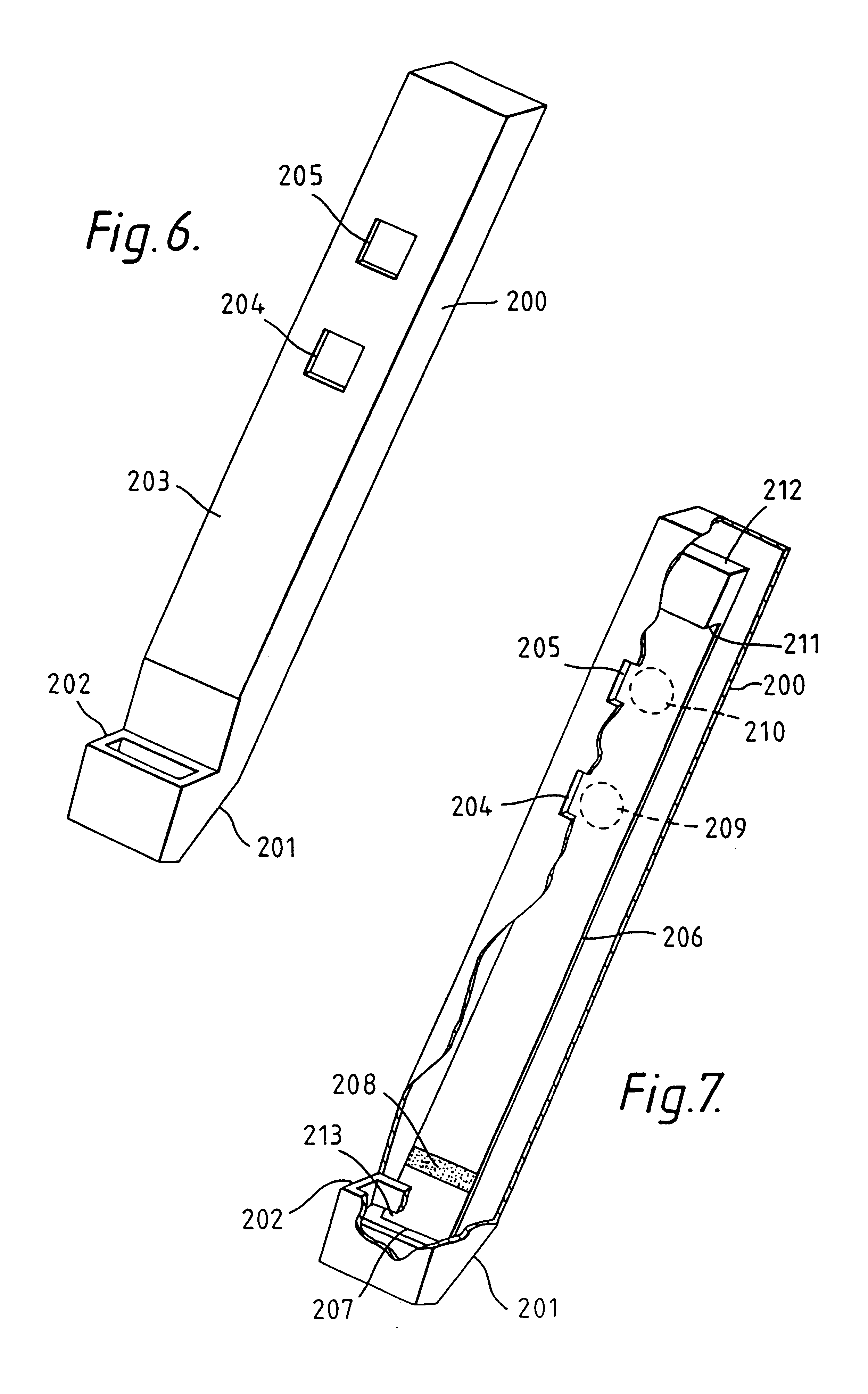
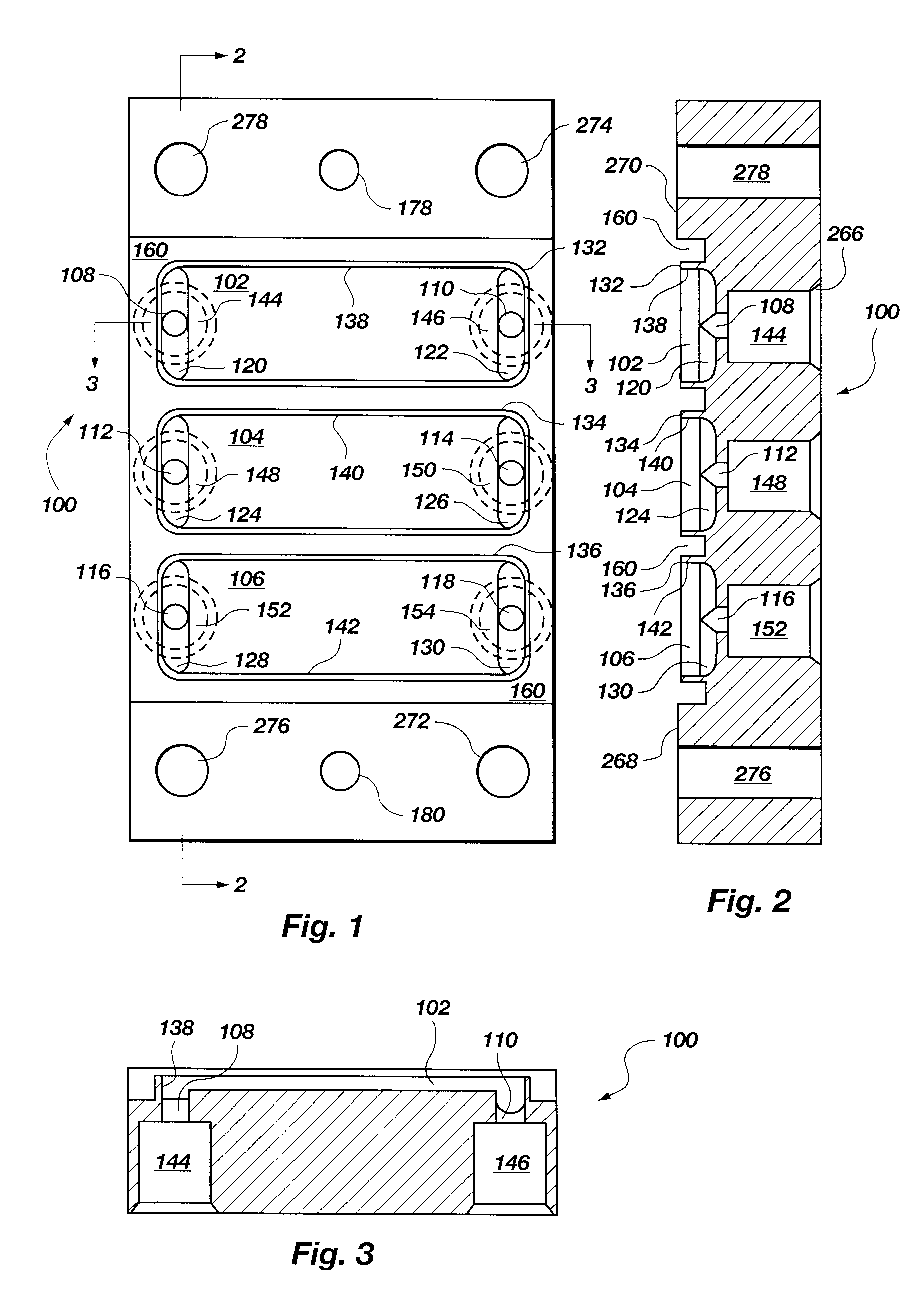
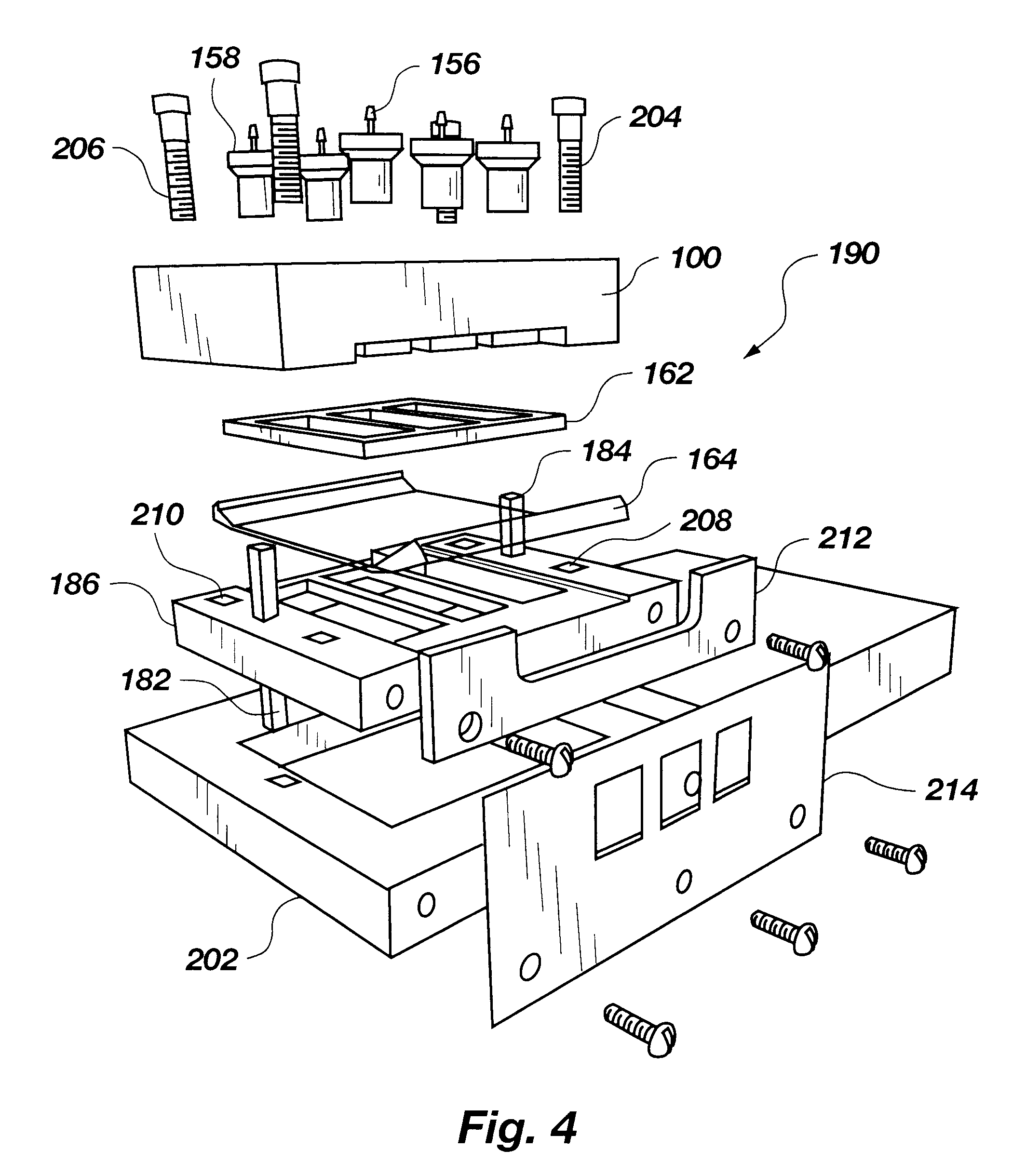
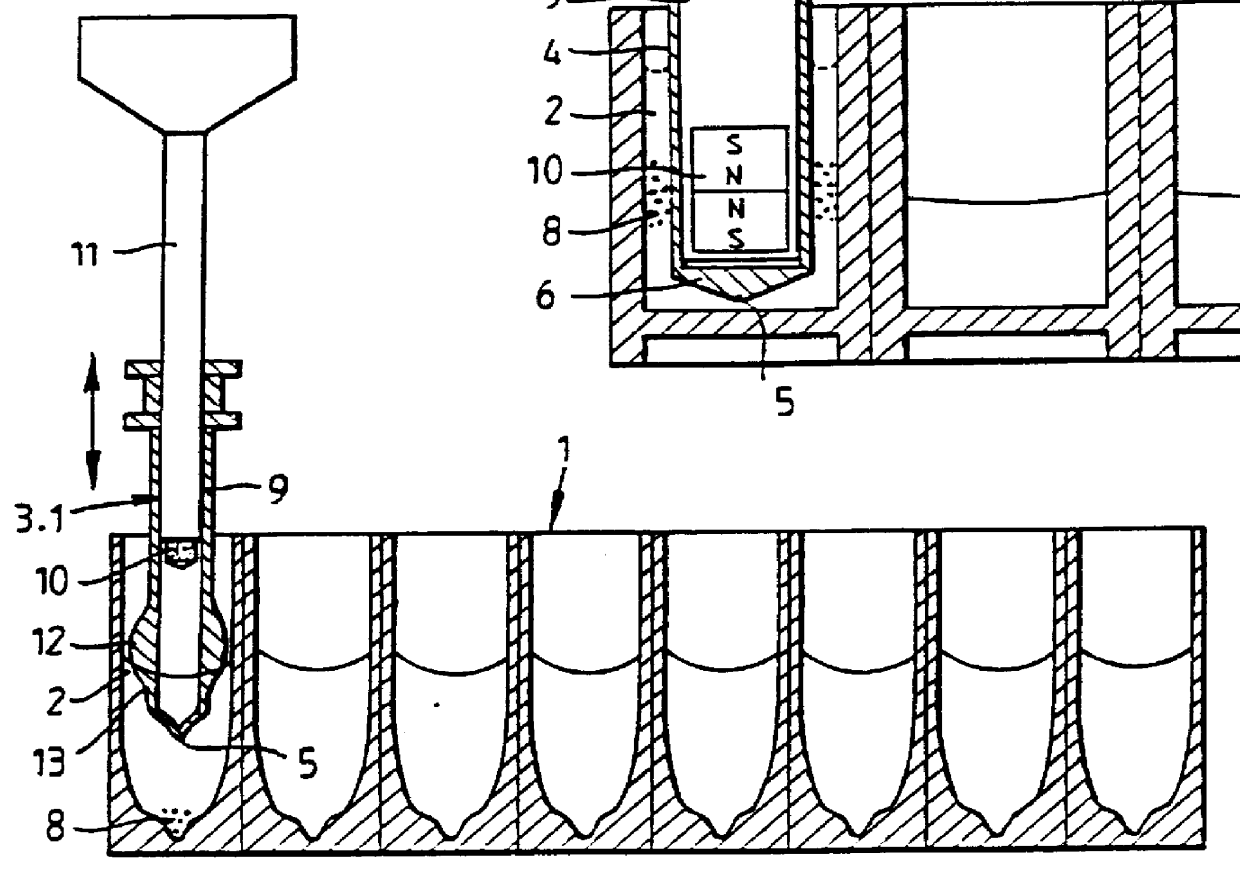
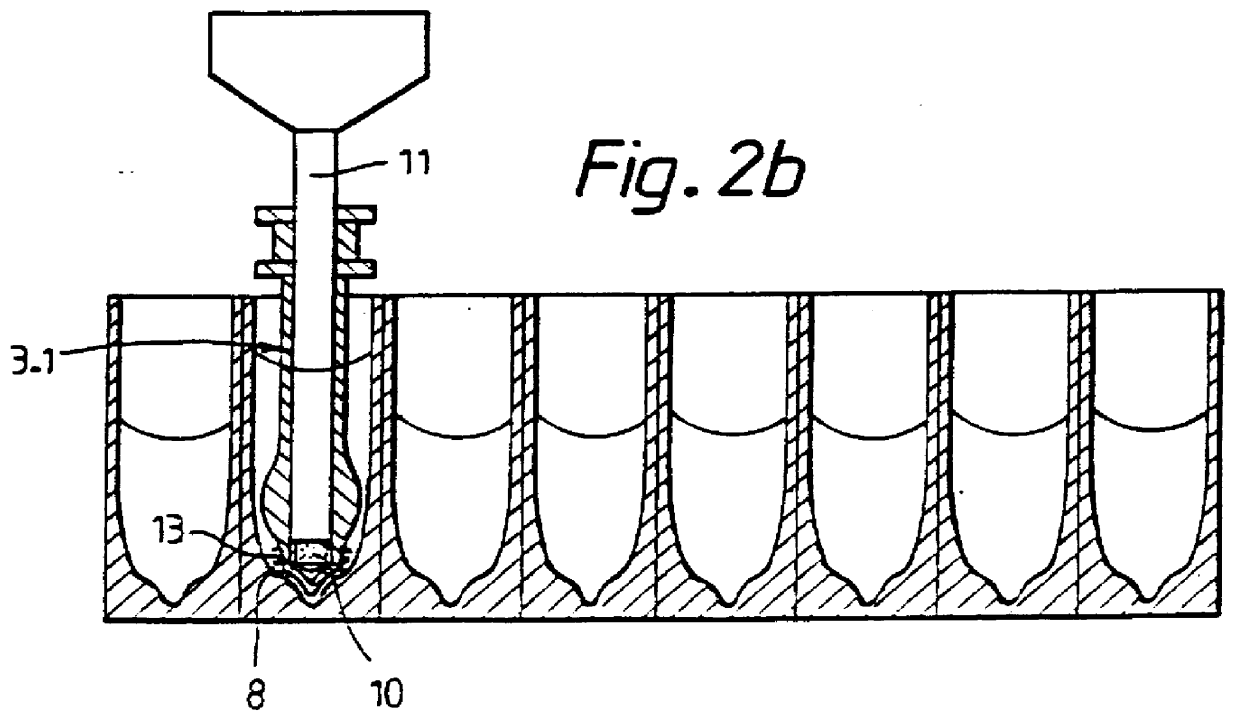
Method and means for magnetic particle specific binding assay
InactiveUS6040192ABioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringImmunoassay
A system and method for handling magnetic particles, as in an immunoassay. A remover having a magnetic body and covering sleeve is inserted in a vessel containing magnetic particles and a liquid. The remover is moved up and down with the particles adhered to agitate the liquid and improve adherence of the particles.
Owner:LABSYSTEMS OY
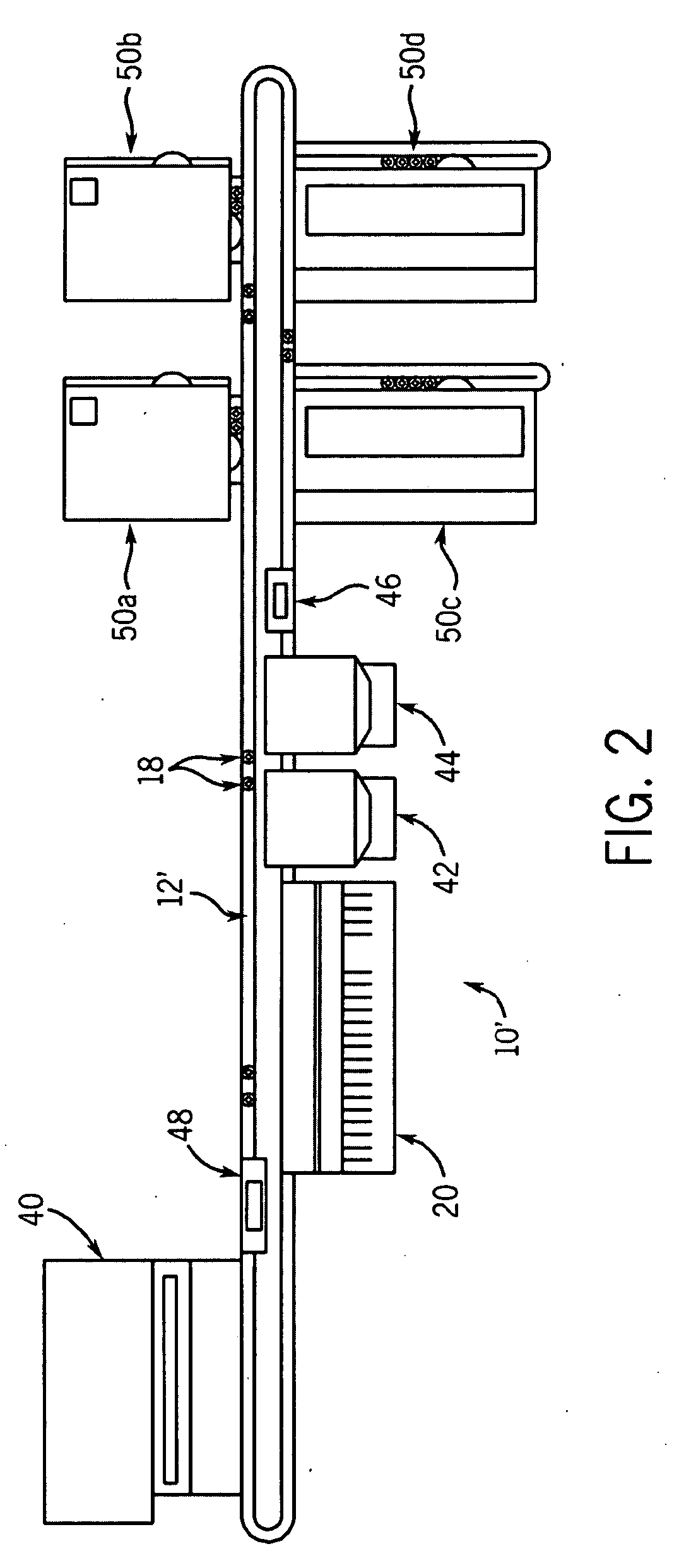
Bead Incubation and Washing on a Droplet Actuator
The present invention relates to bead incubating and washing on a droplet actuator. Methods for incubating magnetically responsive beads that are labeled with primary antibody, a sample (i.e., analyte), and secondary reporter antibodies on a magnet, on and off a magnet, and completely off a magnet are provided. Also provided are methods for washing magnetically responsive beads using shape-assisted merging of droplets. Also provided are methods for shape-mediated splitting, transporting, and dispensing of a sample droplet that contains magnetically responsive beads. The apparatuses and methods of the invention provide for rapid time to result and optimum detection of an analyte in an immunoassay.
Owner:ADVANCED LIQUID LOGIC
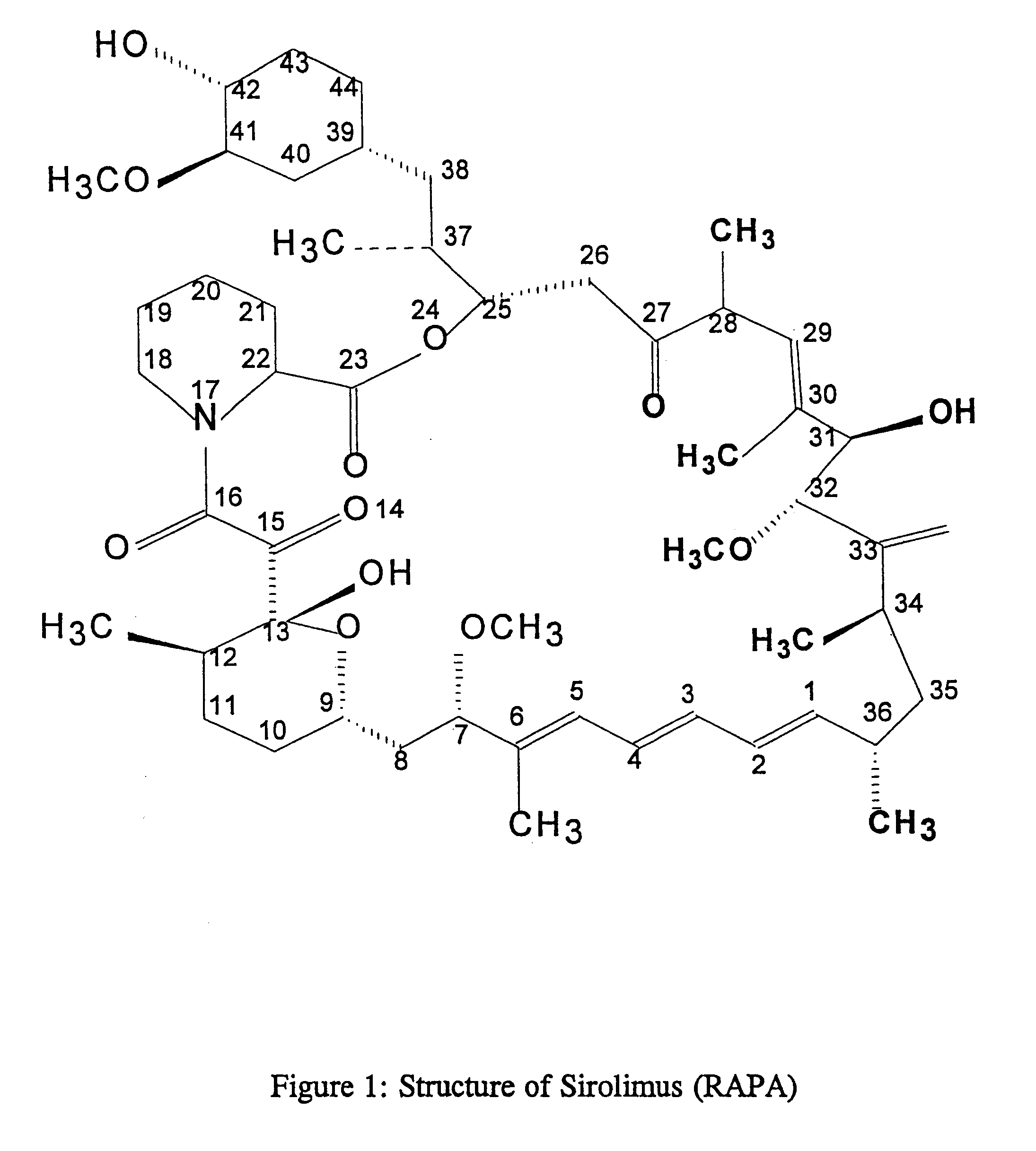
Method for production of antibodies to specific sites of rapamycin
InactiveUS6709873B1Immunoglobulins against fungi/algae/lichensBiological testingPolyclonal antibodiesDivinyl sulfone
Owner:ISODIAGNOSTIKA
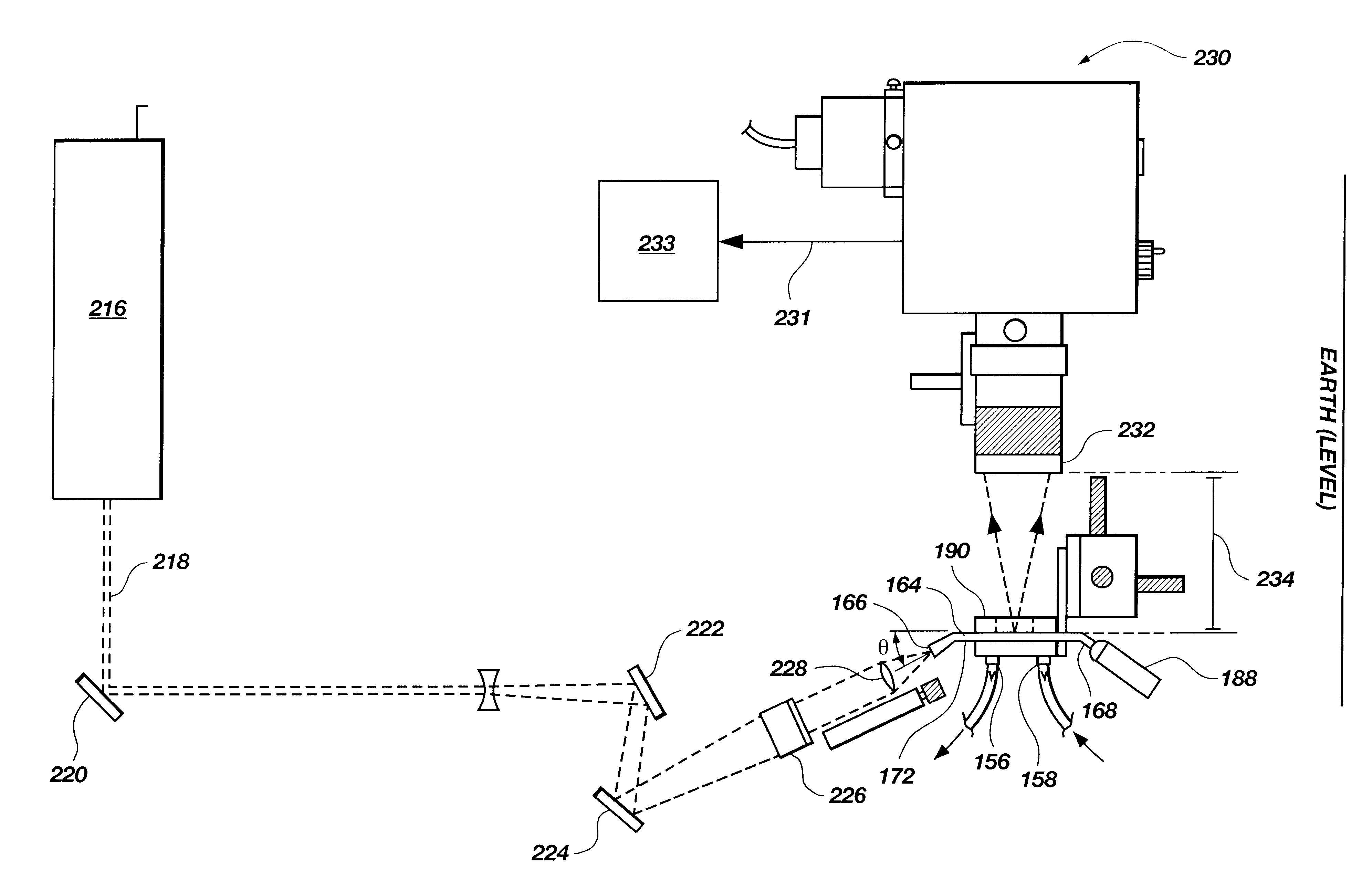
Reader Devices for Optical and Electrochemical Test Devices
ActiveUS20130162981A1Immobilised enzymesBioreactor/fermenter combinationsElectrochemical detectorPoint of care
Owner:ABBOTT POINT CARE
Magnetic microparticle separating chemiluminescence immune analysis determination reagent kit for detecting related sign object and preparing method thereof
ActiveCN101377490AEnsure sensitivityEnsure effectivenessChemiluminescene/bioluminescenceBiological testingDiseaseMicroparticle
The invention relates to the immunoassay medical field, particularly provides a magnetic particle separation chemiluminescence immunoassay assay kit and a preparation method thereof used for detecting disease related markers. The kit of the invention comprises: 1) a calibrator; 2) magnetic particles which are coated with streptavidin; 3) disease related marker antibodies of enzyme markers and biotin markers; and 4) a chemiluminescence substrate. Further, the method for preparing the kit according to the invention includes the following steps: 1) pure raw materials are used to prepare the calibrator; 2) the streptavidin is used to coat the magnetic particles; 3) the mixed liquid of the enzyme and the biotin markers are prepared; 4) the calibrator, the chemiluminescence substrate as well as the mixed liquid of the enzyme and the biotin markers are packaged in a separated way; and 5) a finished product is packaged. The kit has the advantages of convenience, rapidness, sensitivity, stability, and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
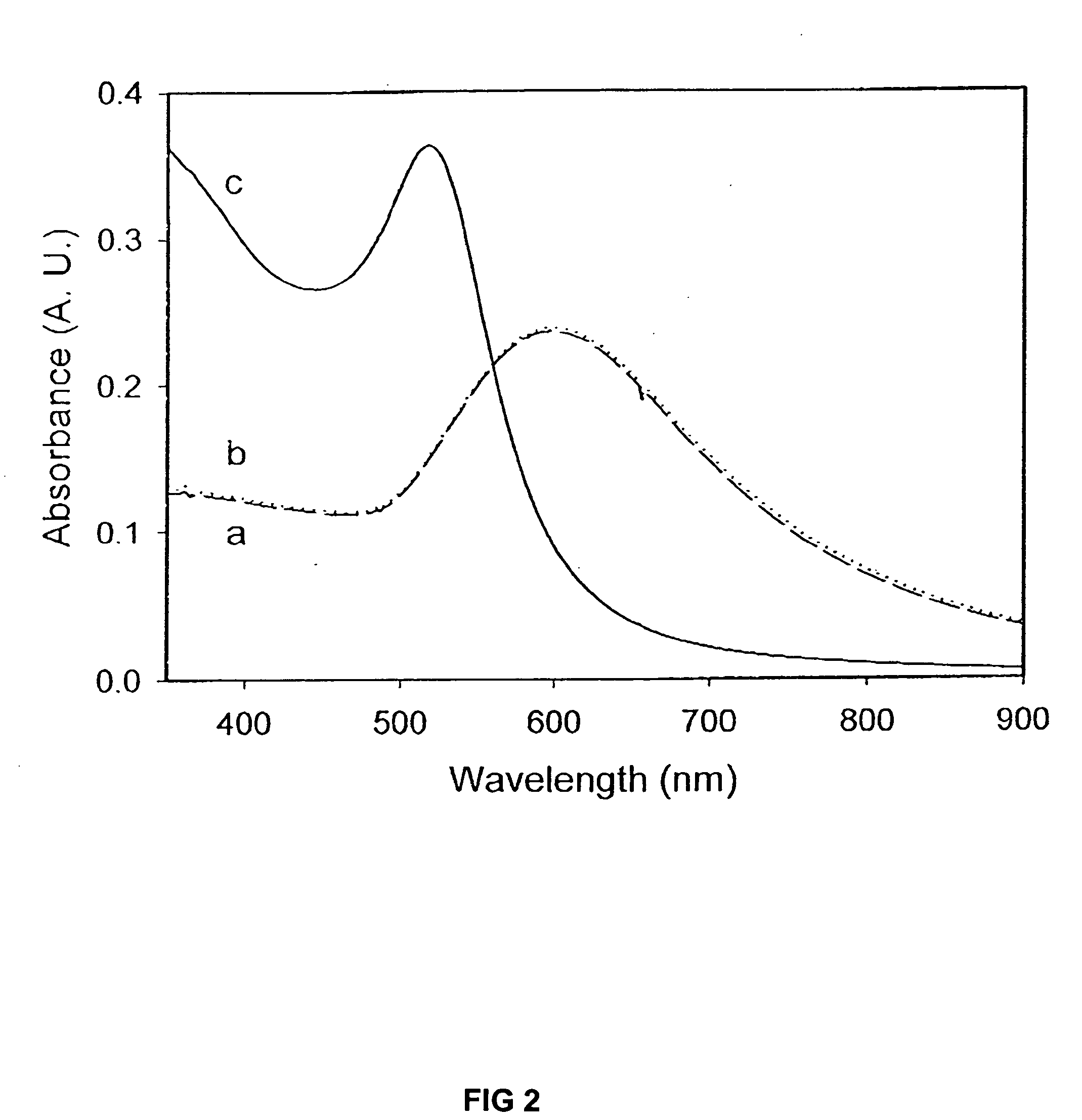
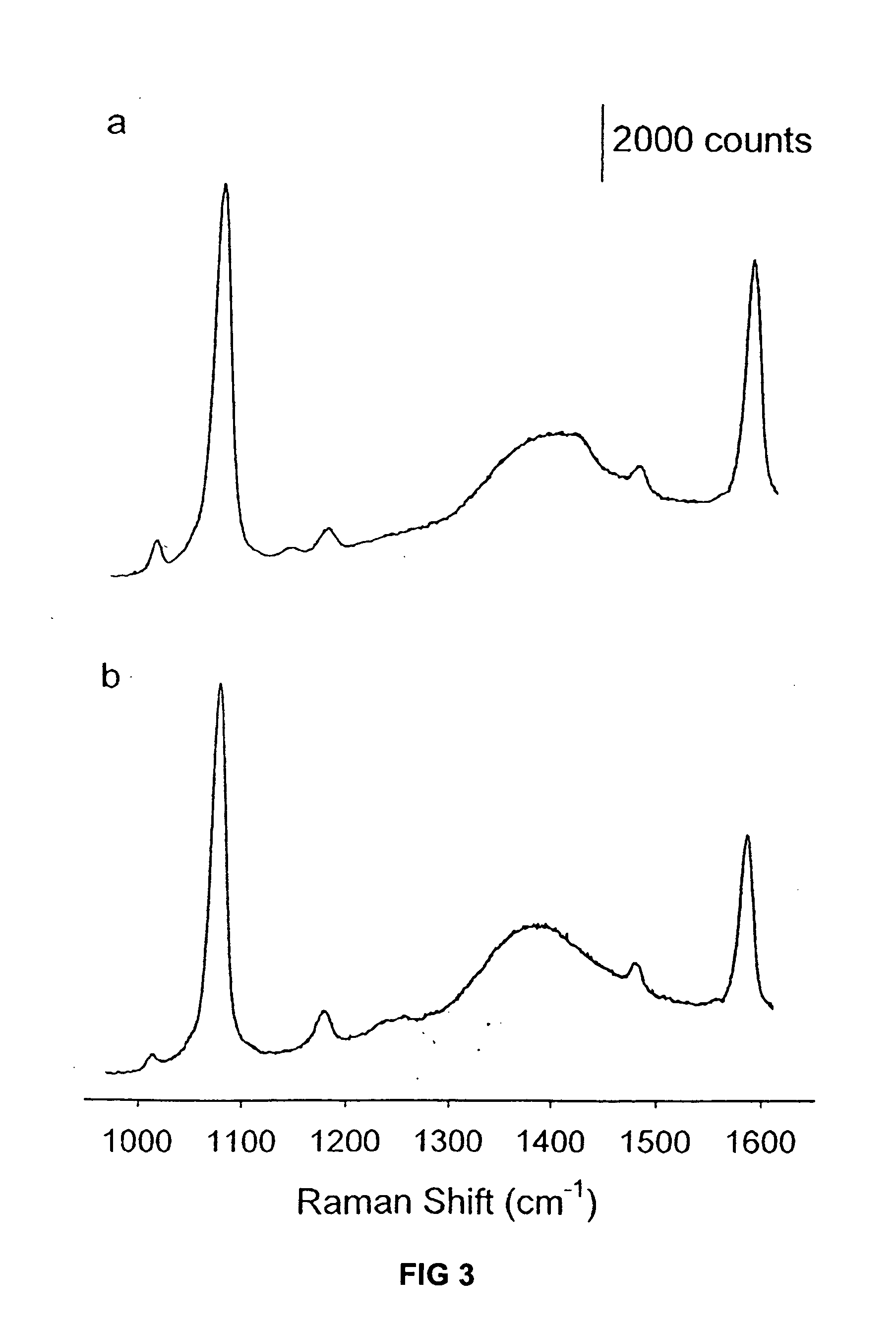
Raman-active reagents and the use thereof
InactiveUS20050089901A1Minimizes separationMaximizing numberHybrid immunoglobulinsSugar derivativesAntigenBiological materials
The present invention provides a new class of Raman-active reagents for use in biological and other applications, as well as methods and kits for their use and manufacture. Each reagent includes a Raman-active reporter molecule, a binding molecule, and a surface enhancing particle capable of causing surface enhanced Raman scattering (SERS). The Raman-active reporter molecule and the binding molecule are affixed to the particle to give both a strong SERS signal and to provide biological functionality, i.e. antigen or drug recognition. The Raman-active reagents can function as an alternative to fluorescence-labeled reagents, with advantages in detection including signal stability, sensitivity, and the ability to simultaneously detect several biological materials. The Raman-active reagents also have a wide range of applications, especially in clinical fields (e.g., immunoassays, imaging, and drug screening).
Owner:PORTER MARC D
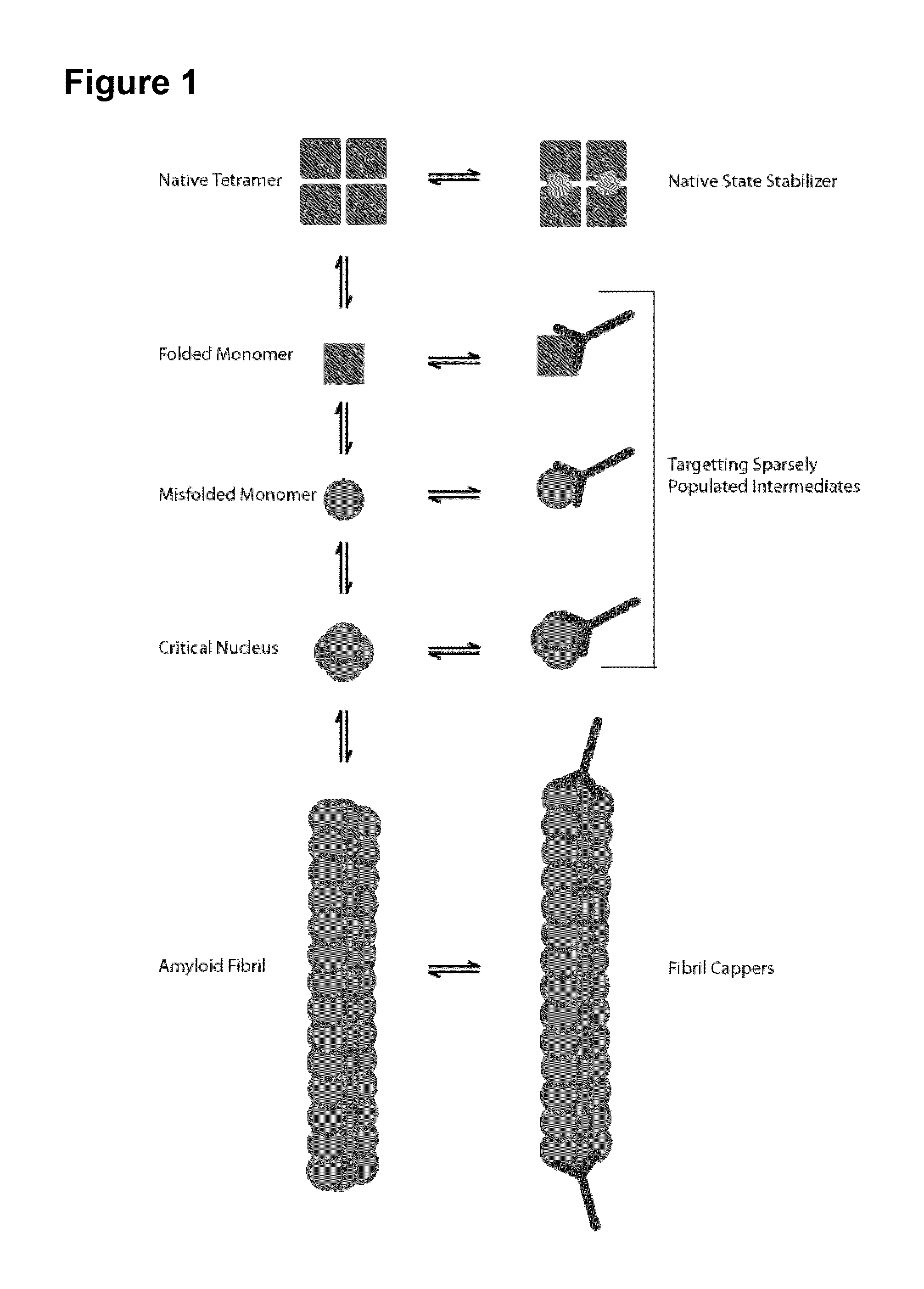
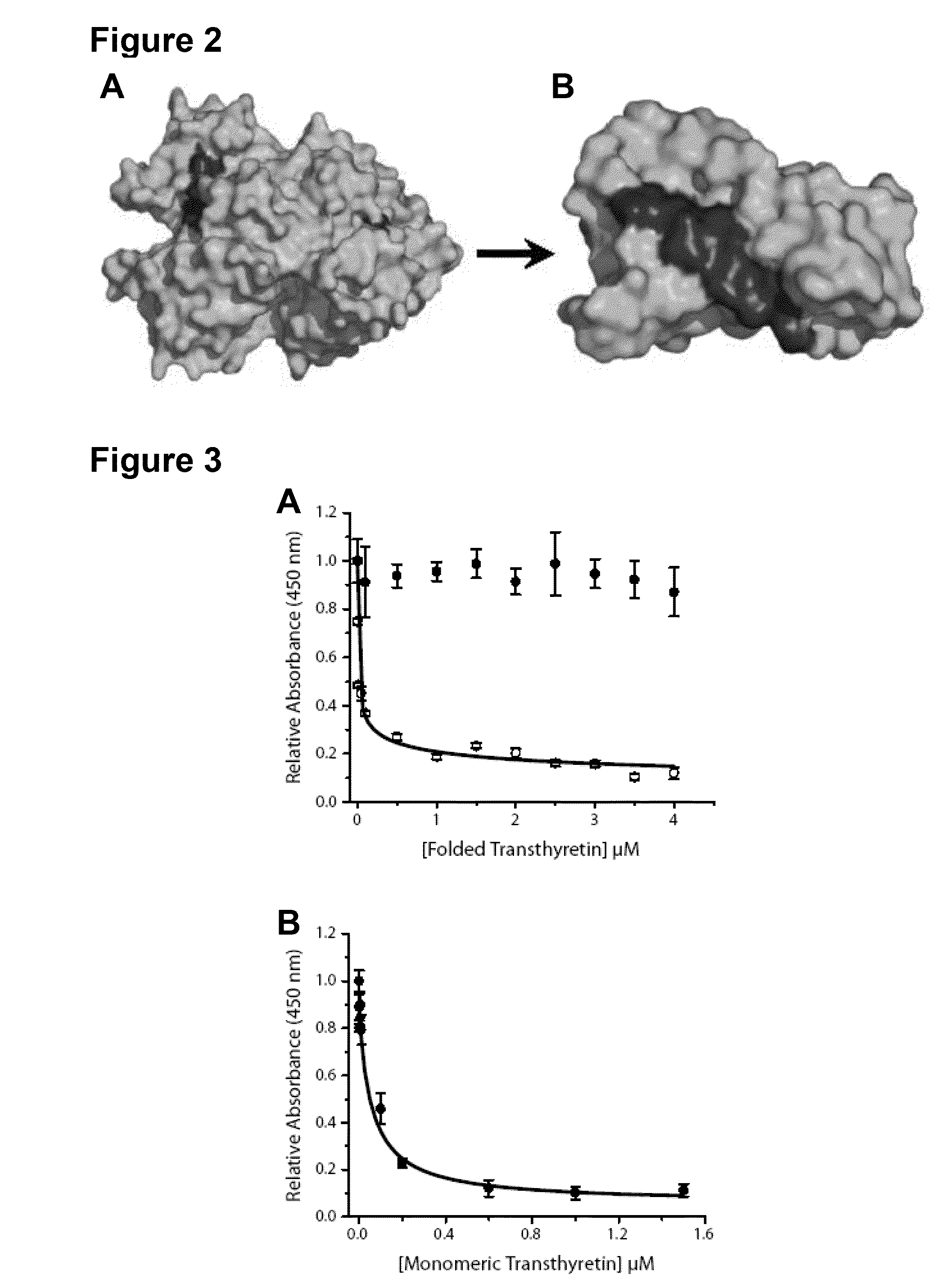
Antibodies to TTR and Methods of Use
ActiveUS20140056904A1Disrupting and reducing formationReduce formationBioreactor/fermenter combinationsBiological substance pretreatmentsReagentPeptide
Owner:UNIV HEALTH NETWORK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com