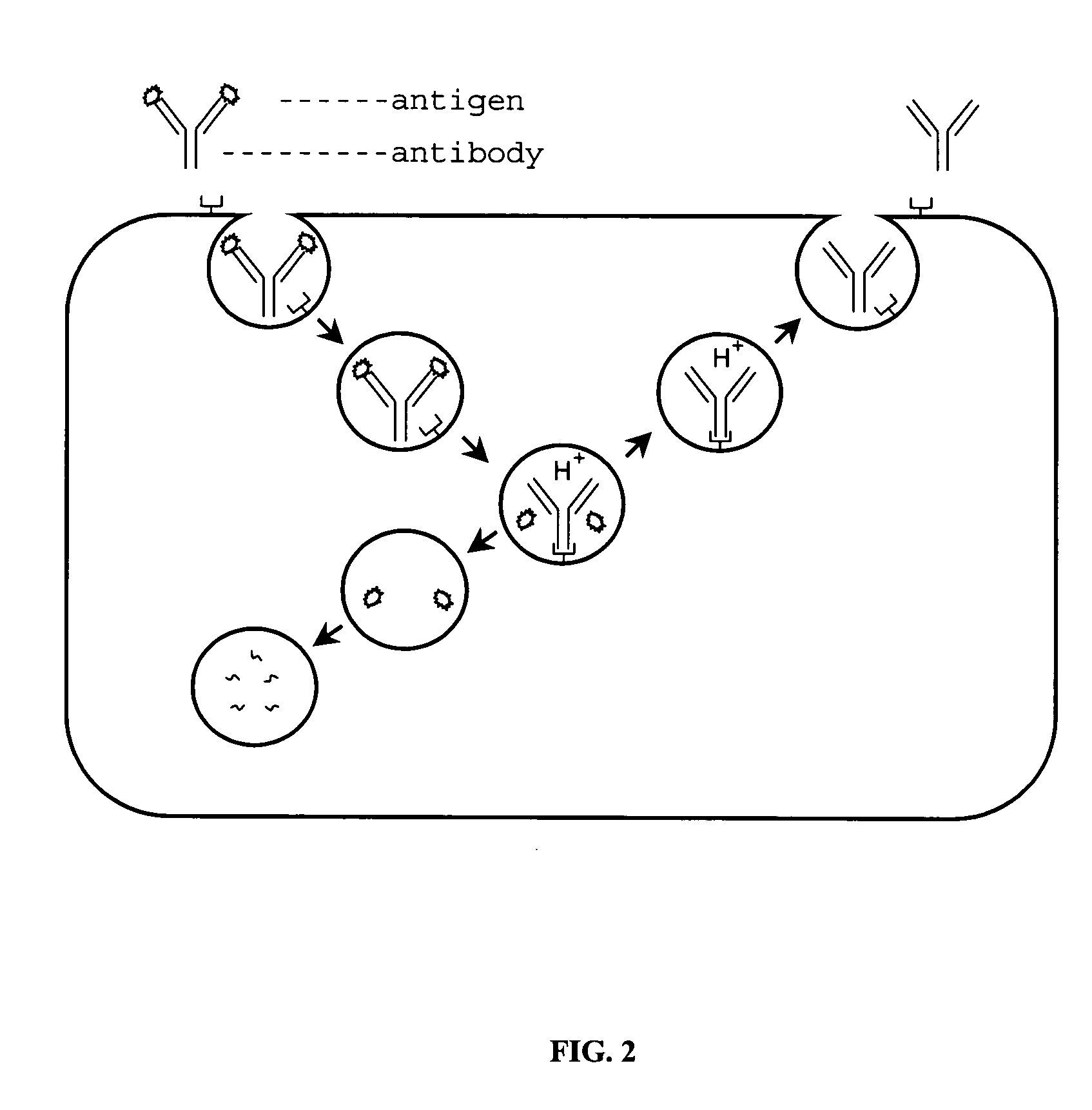
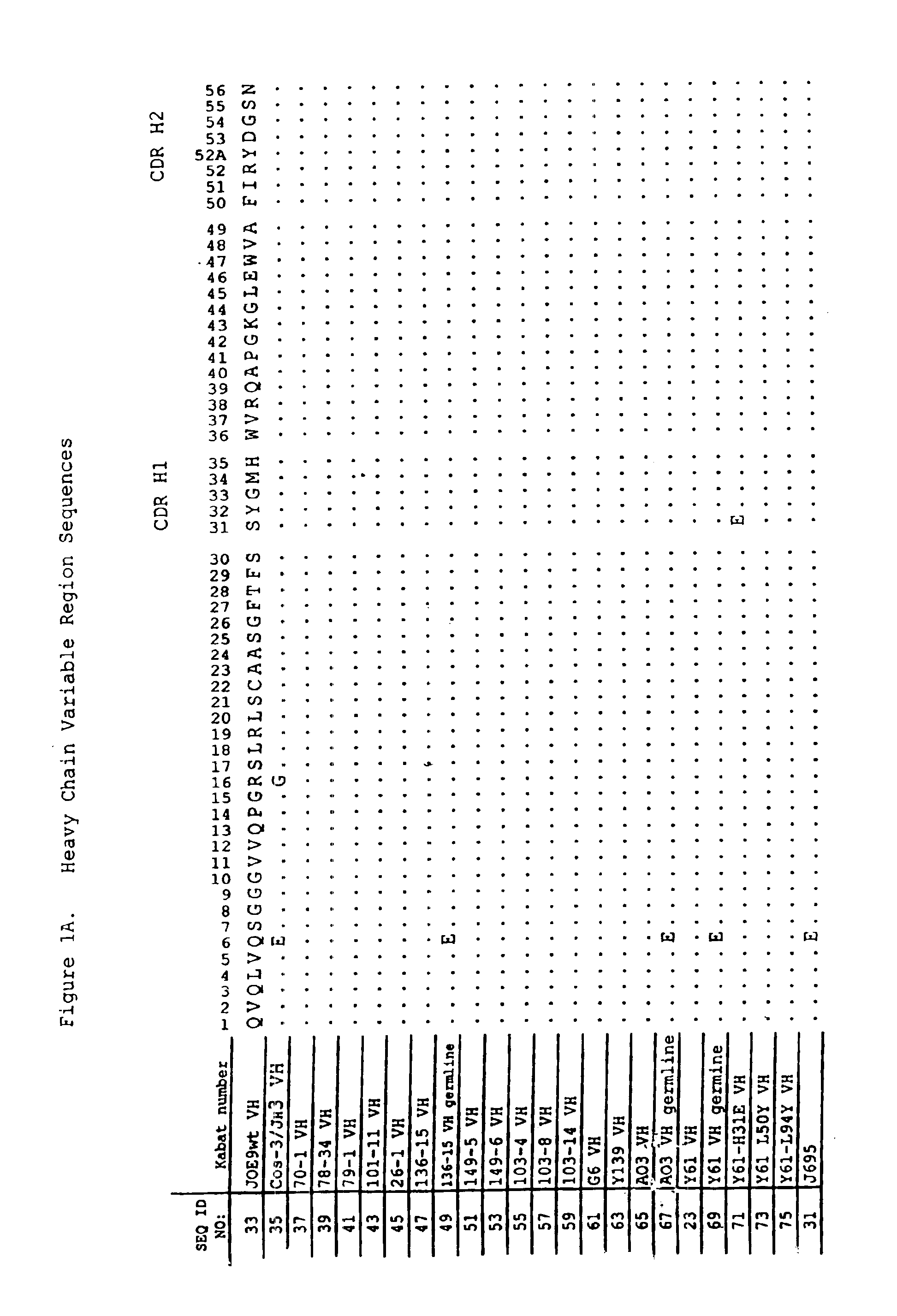
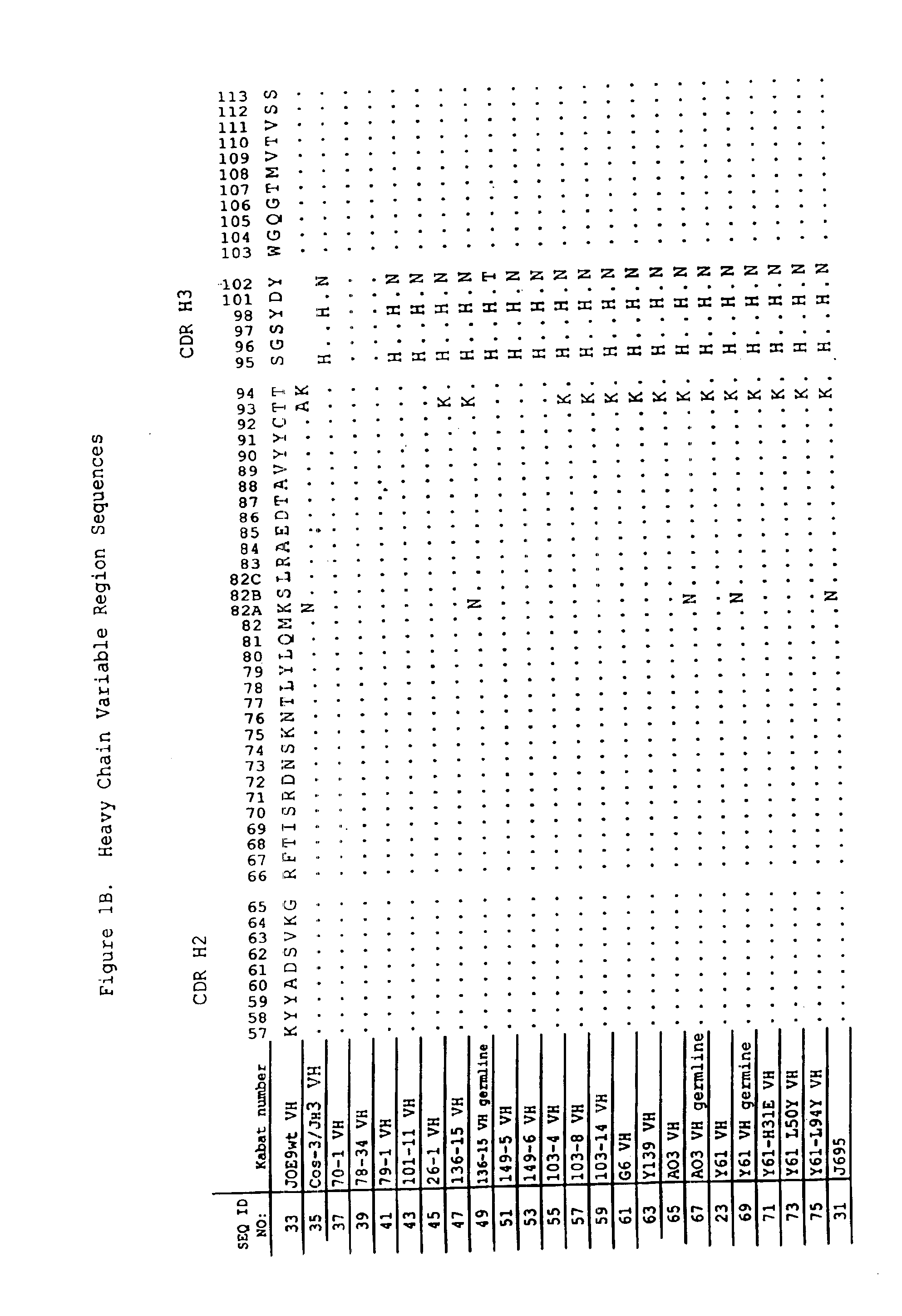
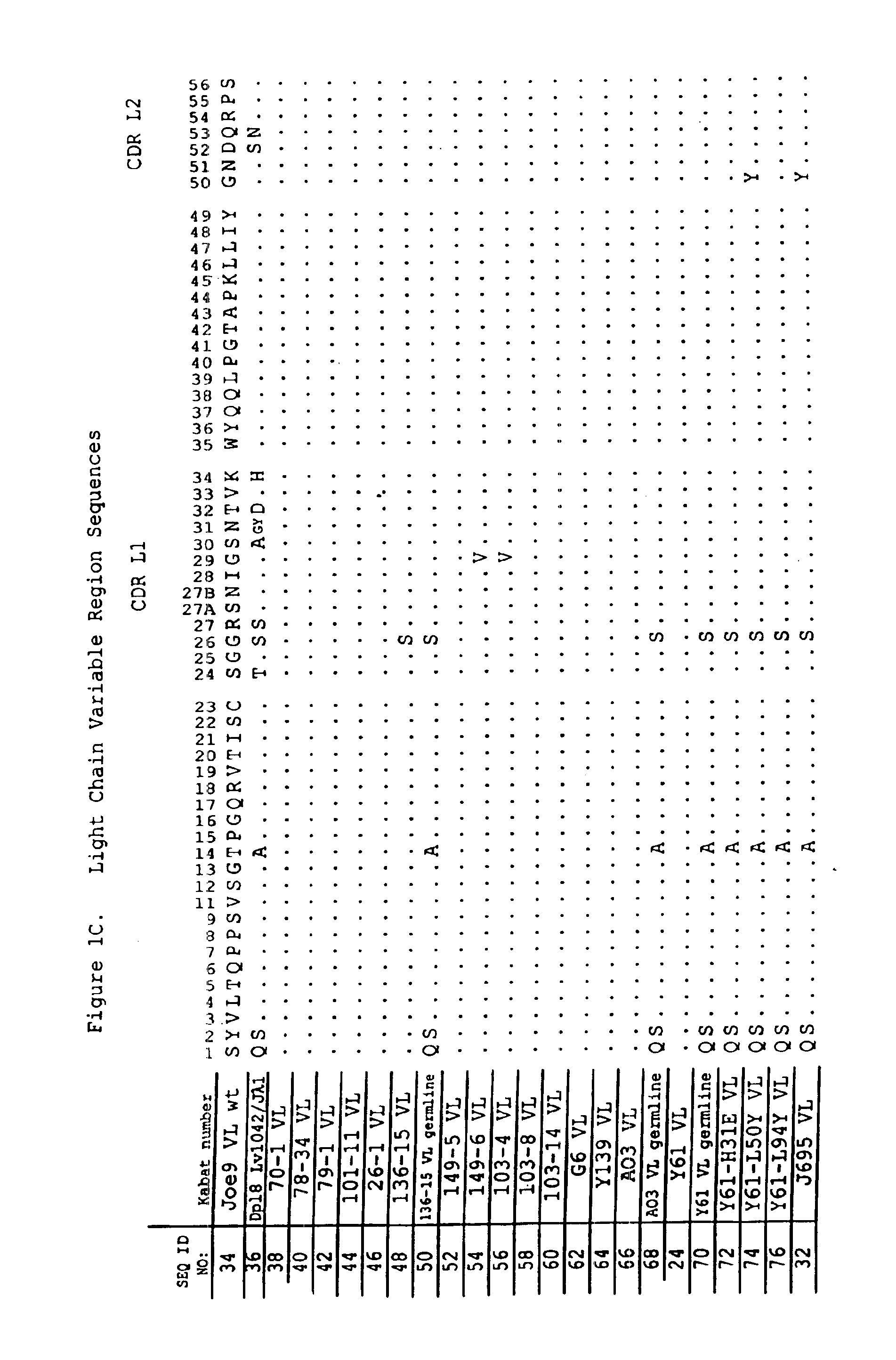
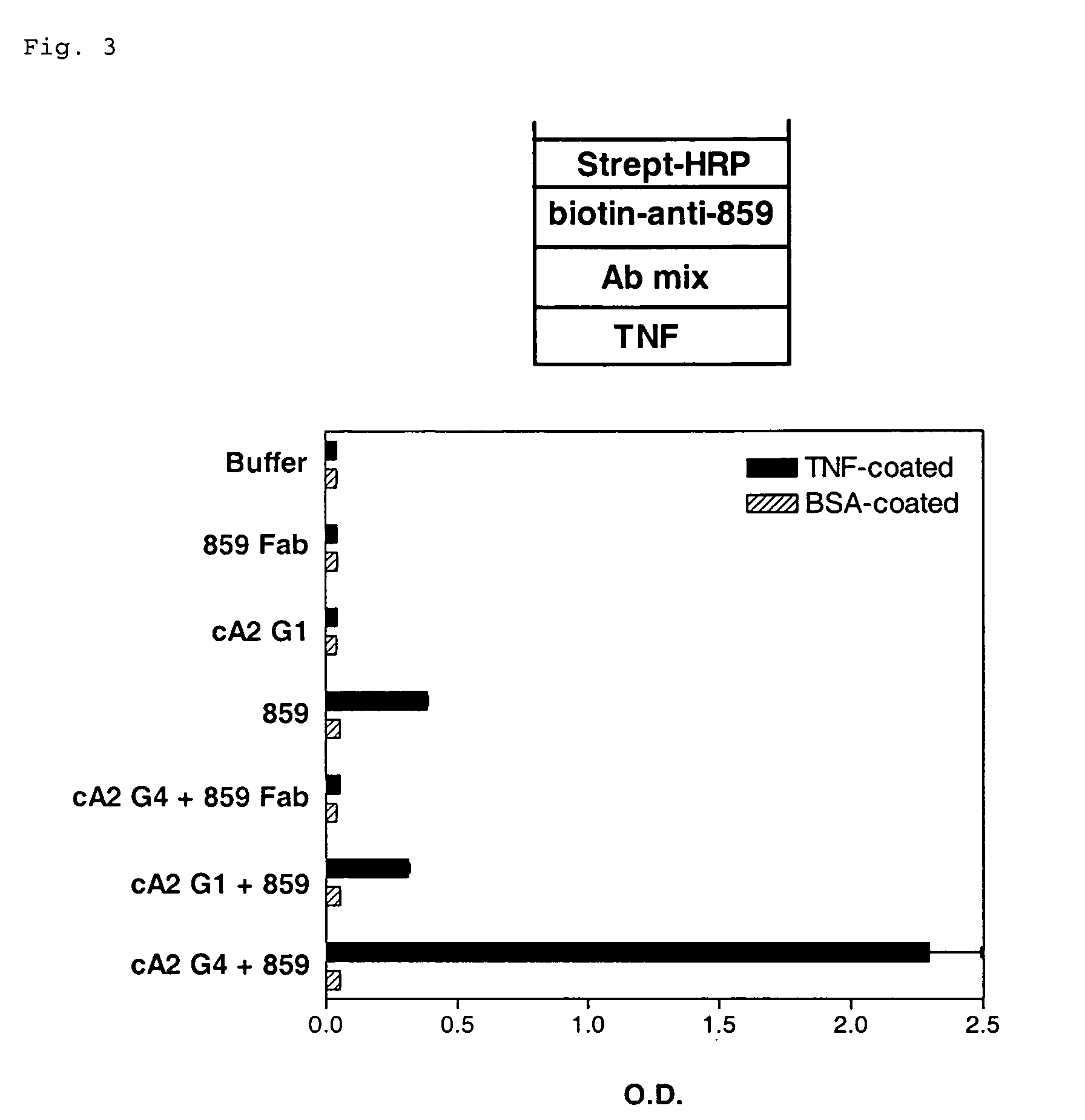
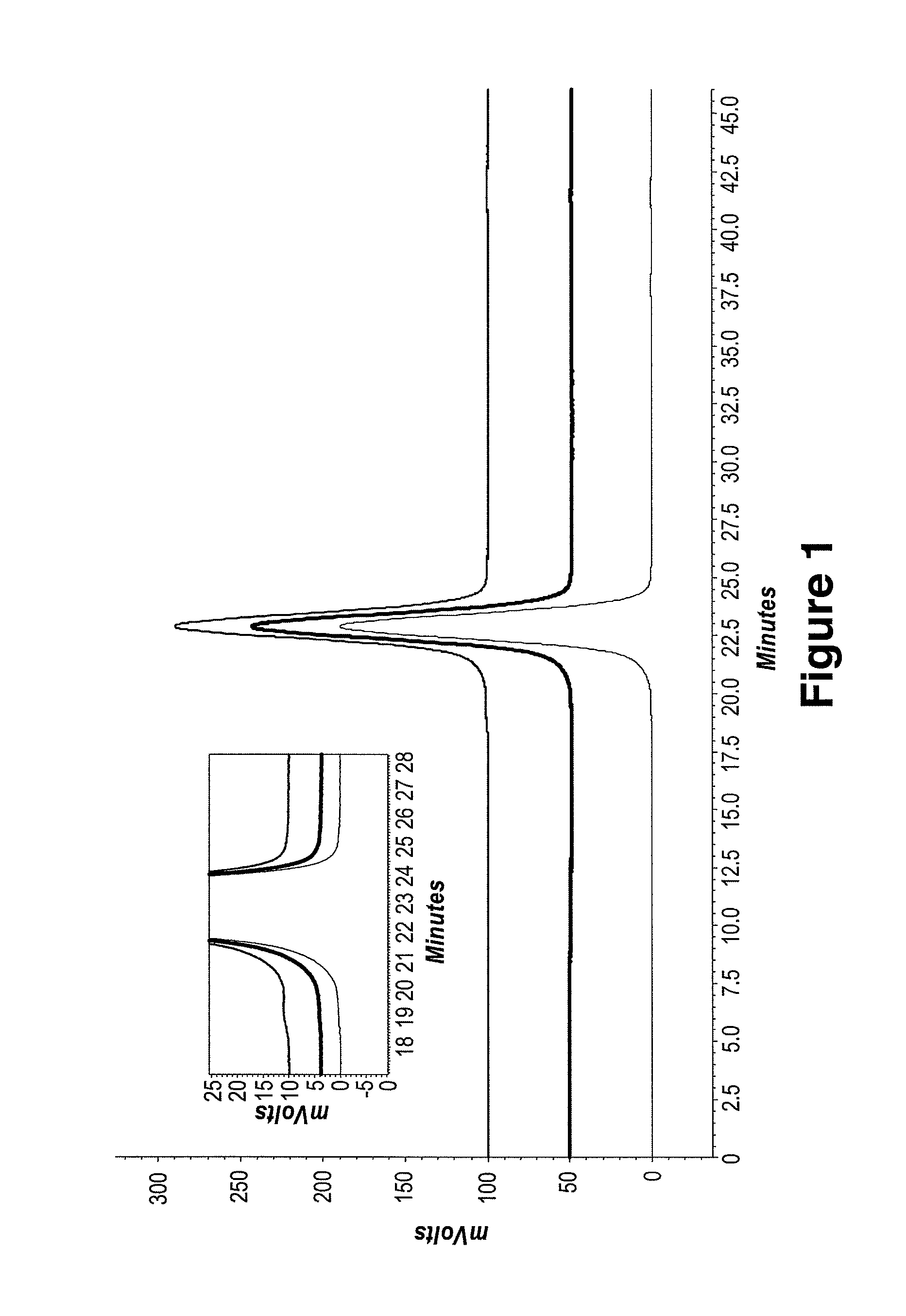
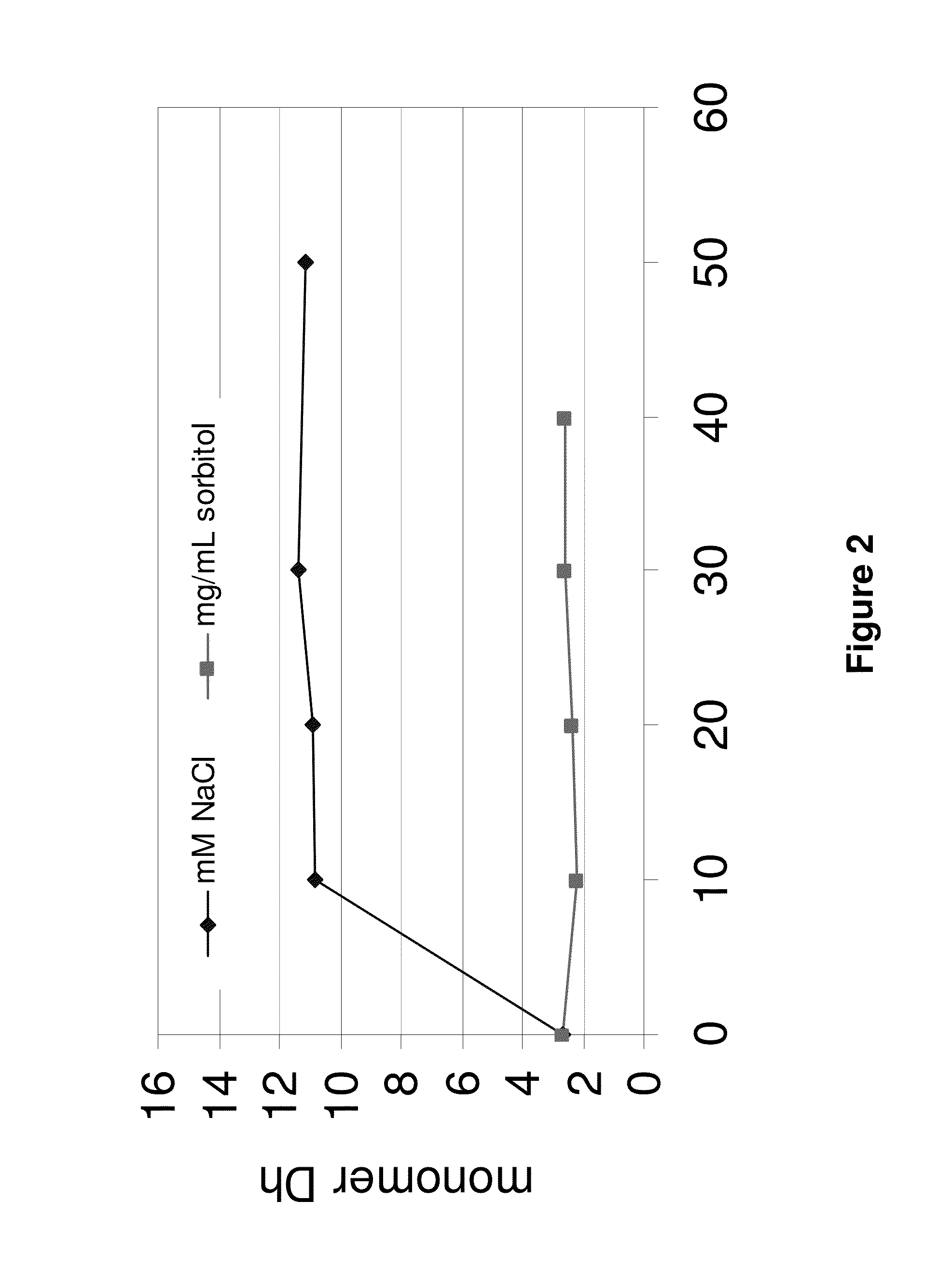
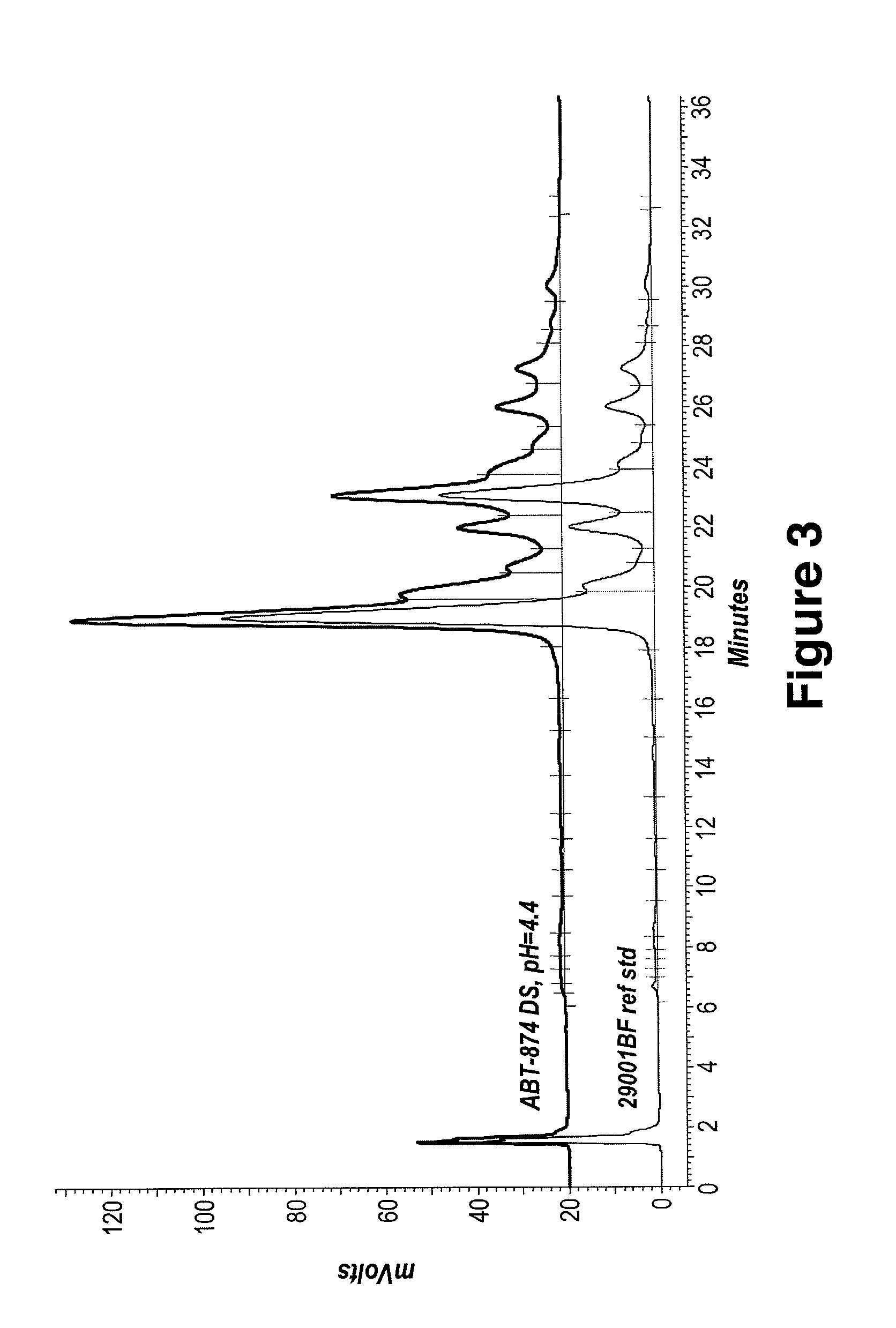
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2718results about "Immunoglobulins against cytokines/lymphokines/interferons" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
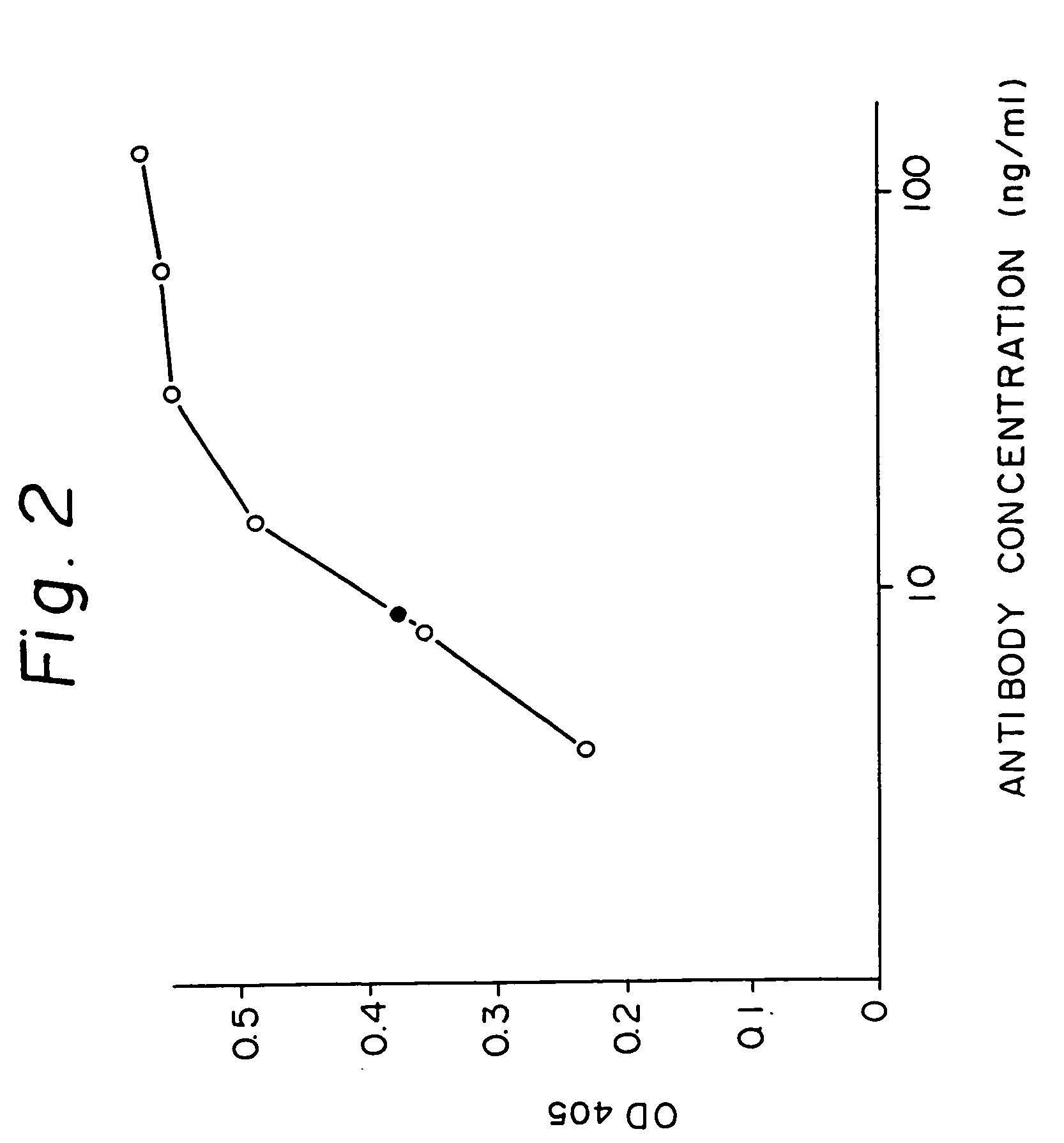
Human antibodies derived from immunized xenomice
Fully human antibodies against a specific antigen can be prepared by administering the antigen to a transgenic animal which has been modified to produce such antibodies in response to antigenic challenge, but whose endogenous loci have been disabled. Various subsequent manipulations can be performed to obtain either antibodies per se or analogs thereof.
Owner:AMGEN FREMONT INC
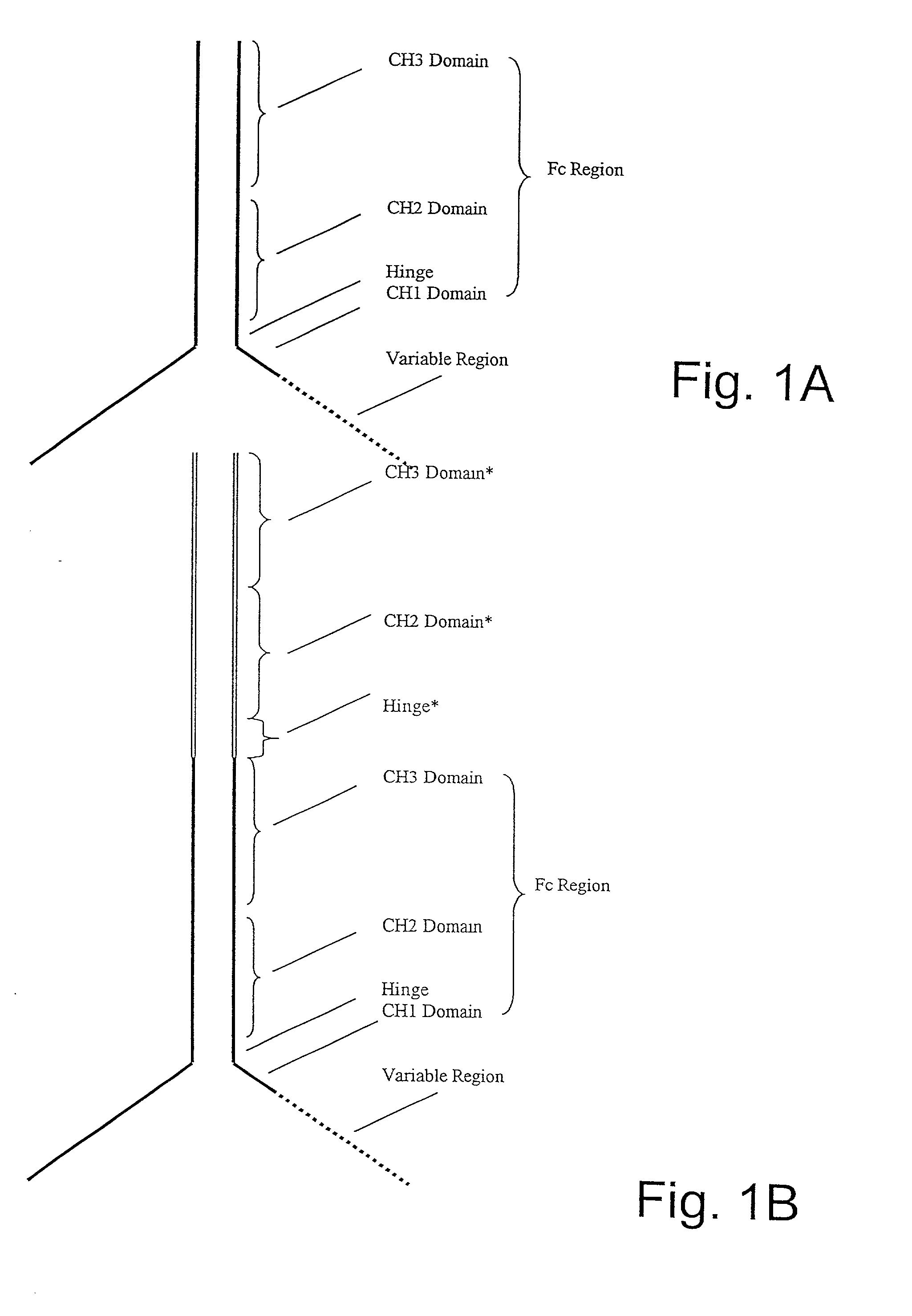
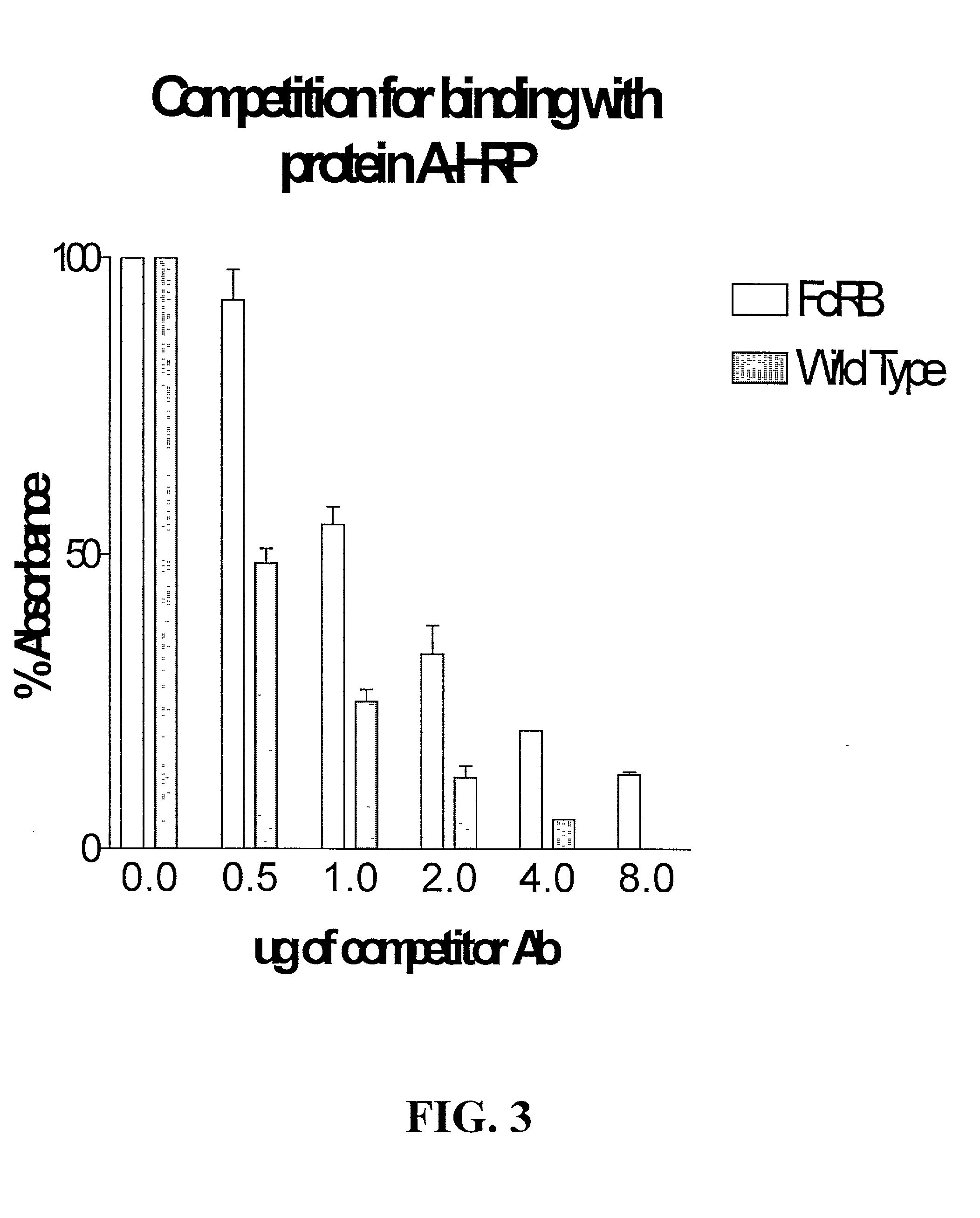
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050014934A1Reducing FcRn binding affinityReduced half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesHalf-lifeAntibody
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Human antibodies that bind human IL-12 and methods for producing
InactiveUS6914128B1Avoid interferencePreservationNervous disorderPeptide/protein ingredientsAntigen bindingIn vivo
Human antibodies, preferably recombinant human antibodies, that specifically bind to human interleukin-12 (hIL-12) are disclosed. Preferred antibodies have high affinity for hIL-12 and neutralize hIL-12 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and for inhibiting hIL-12 activity, e.g., in a human subject suffering from a disorder in which hIL-12 activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS7217797B2Function increaseExtended half-lifeAnimal cellsSugar derivativesAntiendomysial antibodiesBiochemistry
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Humanized antibodies to gamma-interferon
The invention provides humanized immunoglobulins that bind to and neutralize gamma-interferon. The antibodies are useful for treatment of diseases of the immune system, particularly autoimmune diseases.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Fc variants with optimized properties
InactiveUS20060235208A1Improve bindingStrong cytotoxicityImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against growth factorsChemistry
Owner:XENCOR
Protein scaffolds for antibody mimics and other binding proteins
InactiveUS7115396B2Easy to foldImprove stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsWAS PROTEINAntibody
Disclosed herein are proteins that include an immunoglobulin fold and that can be used as scaffolds. Also disclosed herein are nucleic acids encoding such proteins and the use of such proteins in diagnostic methods and in methods for evolving novel compound-binding species and their ligands.
Owner:BRISTOL MYERS SQUIBB CO
Monoclonal antibodies detection methods for enzymes that confer resistance to 2,4-dichlorophenoxyacetic acid in plants
ActiveUS8460891B2Animal cellsImmunoglobulins against cytokines/lymphokines/interferonsMonoclonal antibodyDioxygenase
Owner:CORTEVA AGRISCIENCE LLC
Polypeptide variants with altered effector function
InactiveUS20060067930A1High affinity bindingWeaker binding affinityCompound screeningNervous disorderEffector functionsMolecular biology
The invention provides polypeptides having IgG Fc regions with amino acid modifications that result in the polypeptides exhibiting altered Fc effector functions.
Owner:GENENTECH INC
Tumour necrosis factor antibodies
InactiveUS6451983B2Enhance or inhibit TNF alpha activityInduction of endothelial procoagulant activityPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman tumorSingle-Chain Antibodies
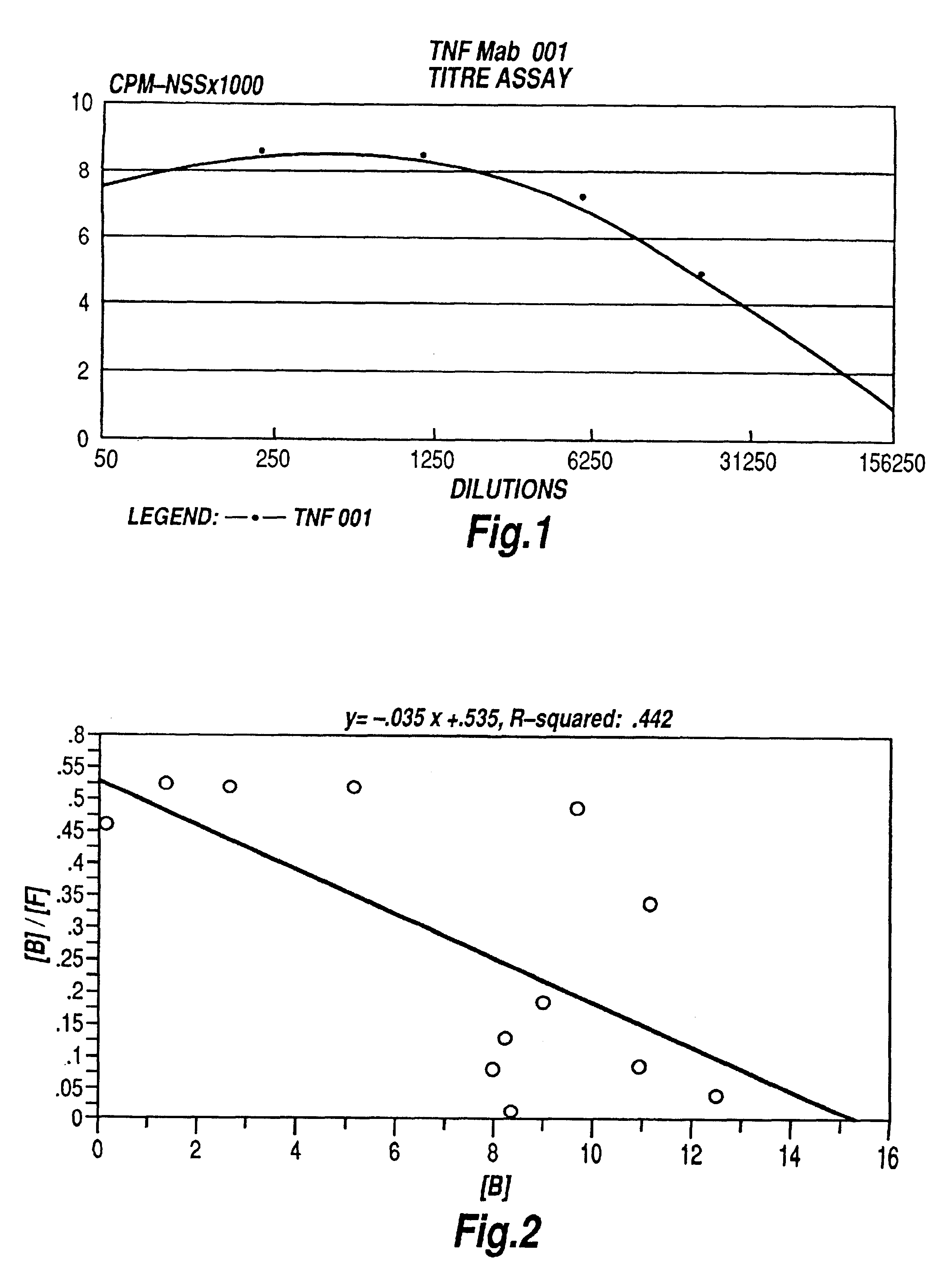
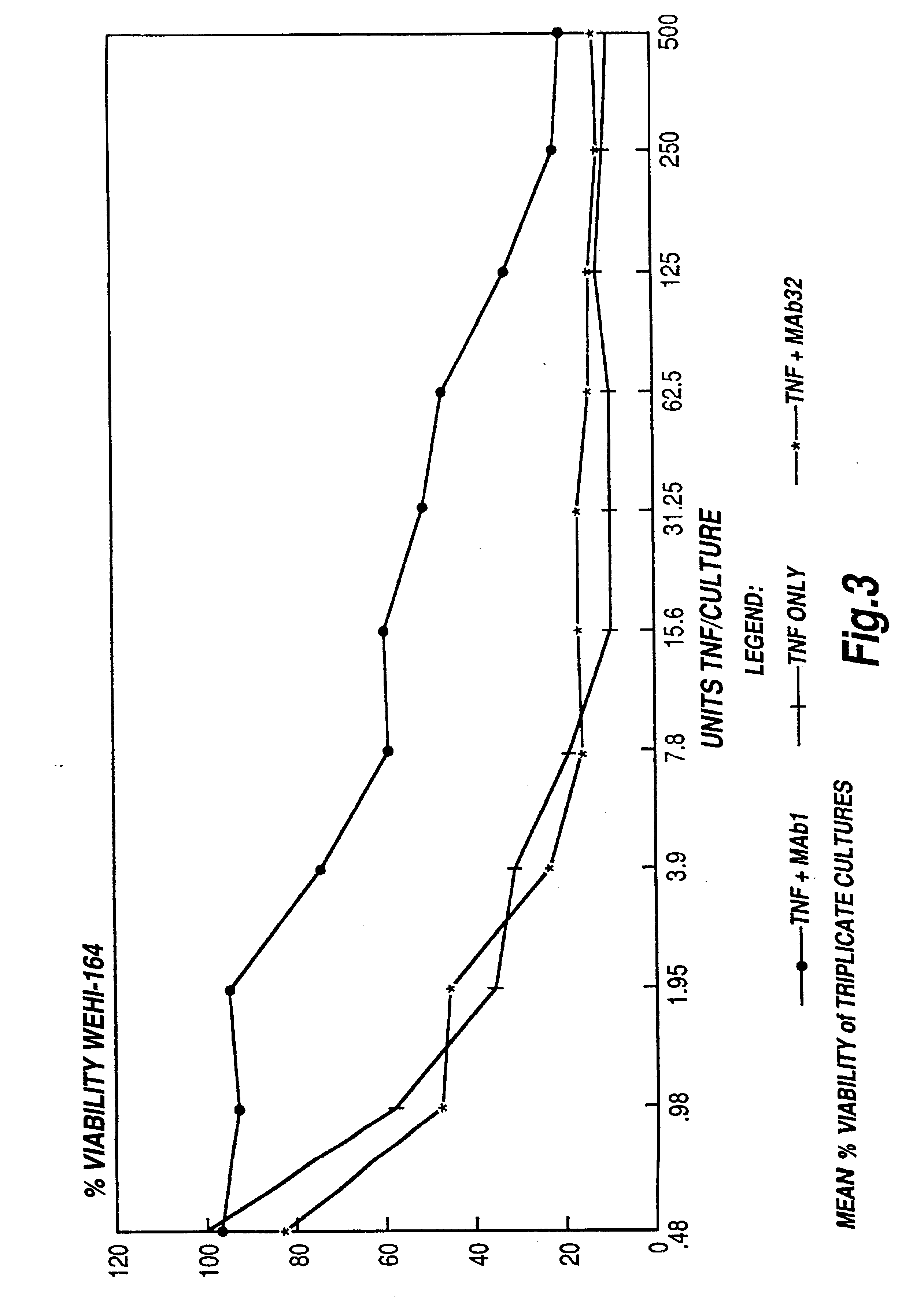
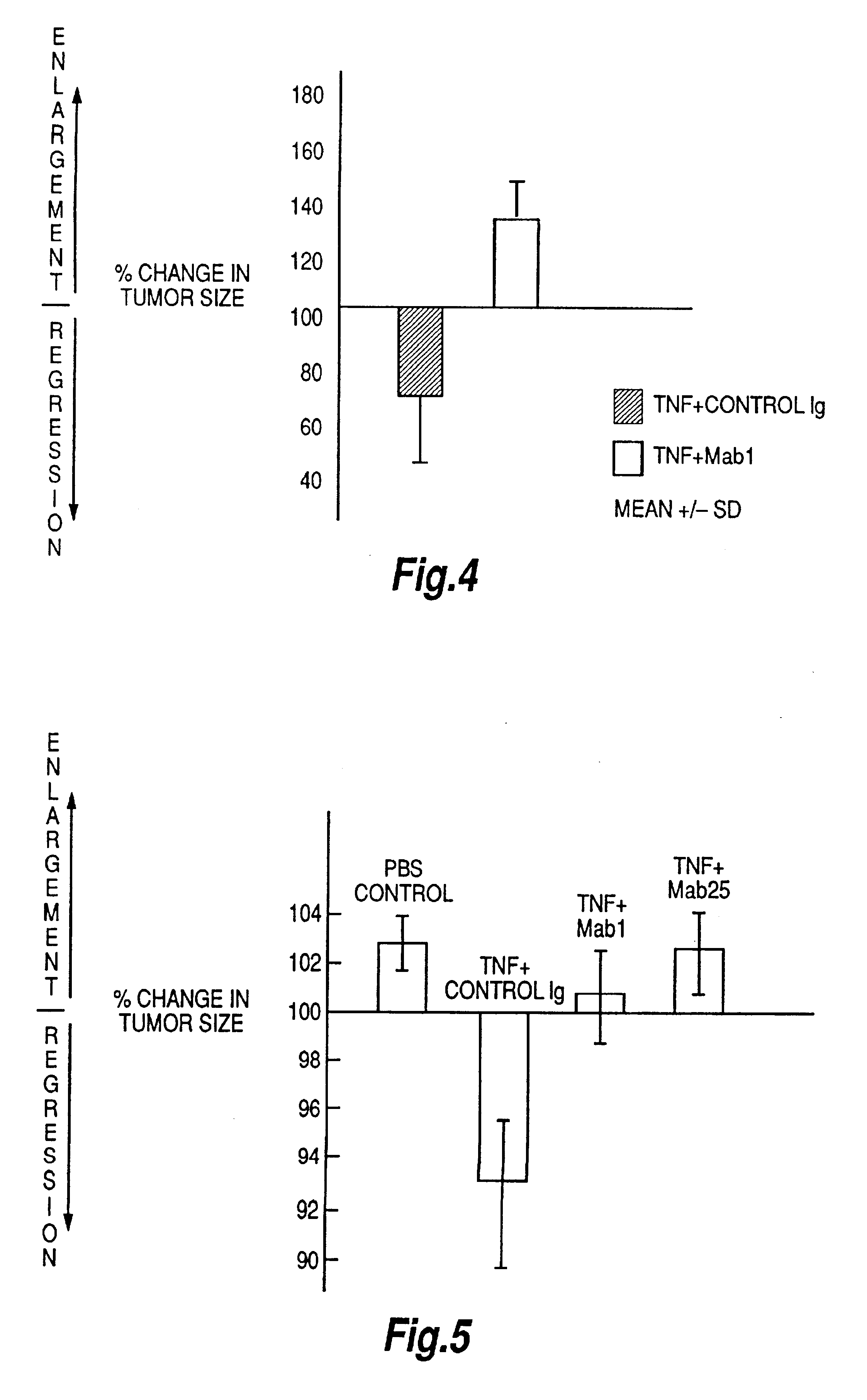
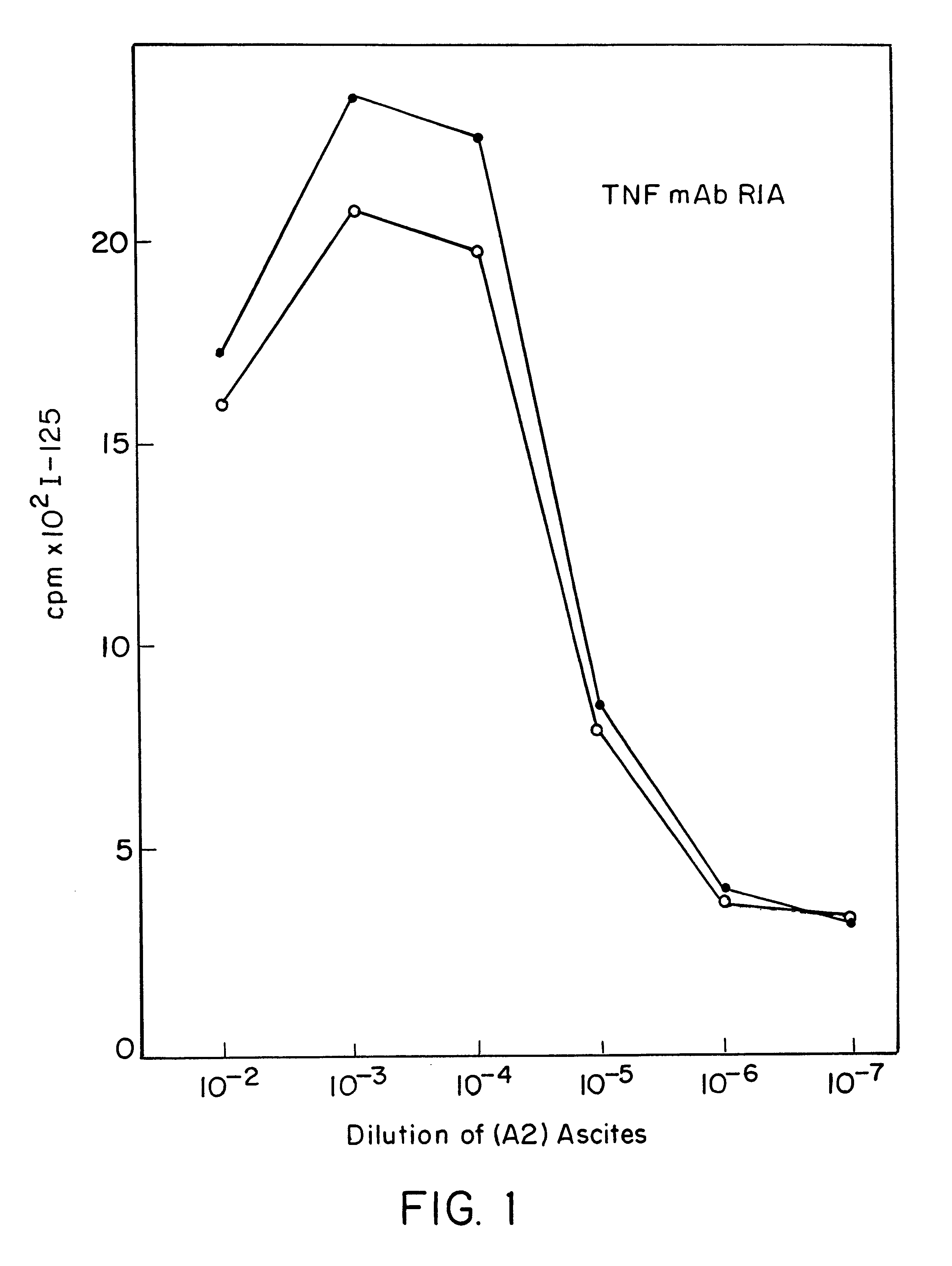
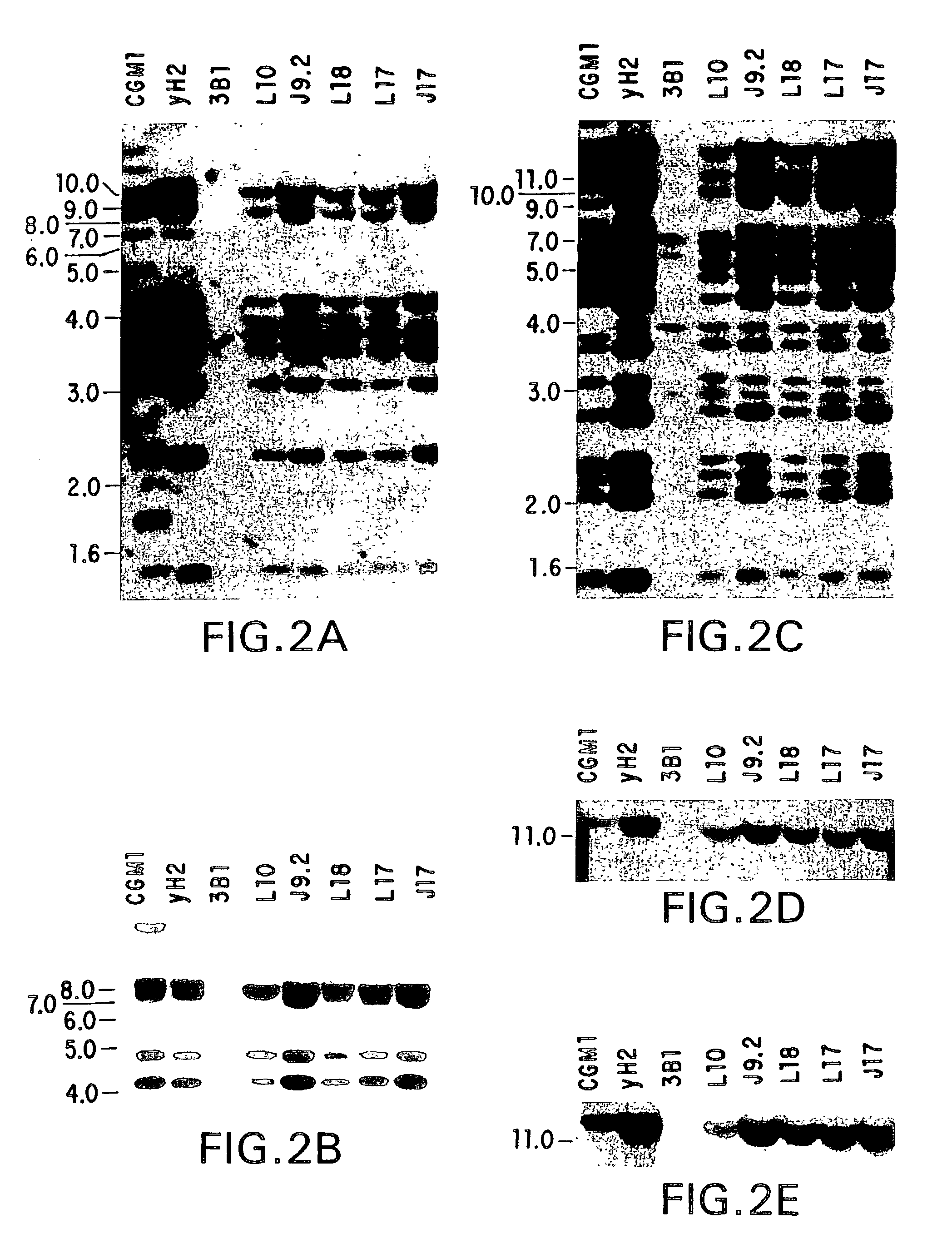
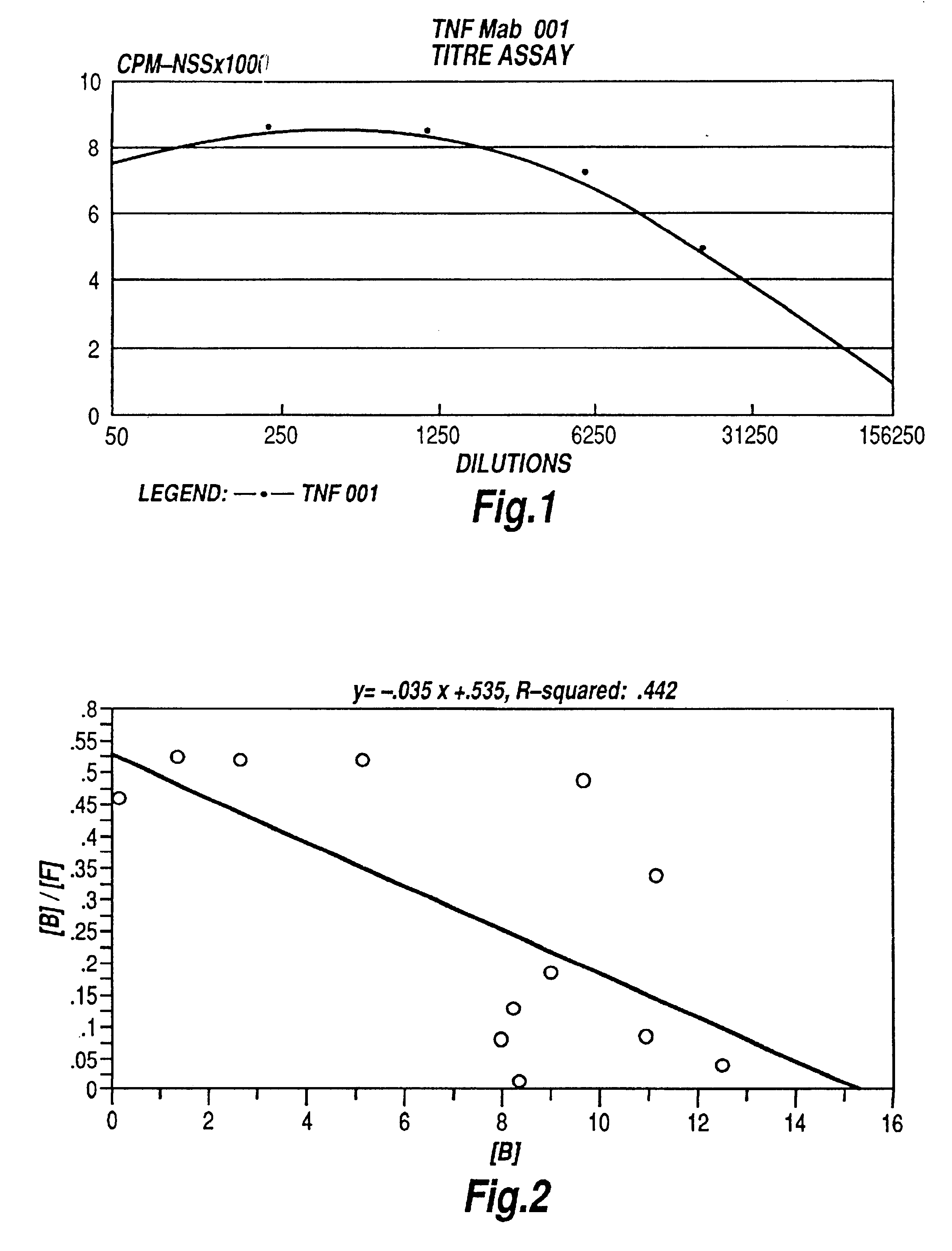
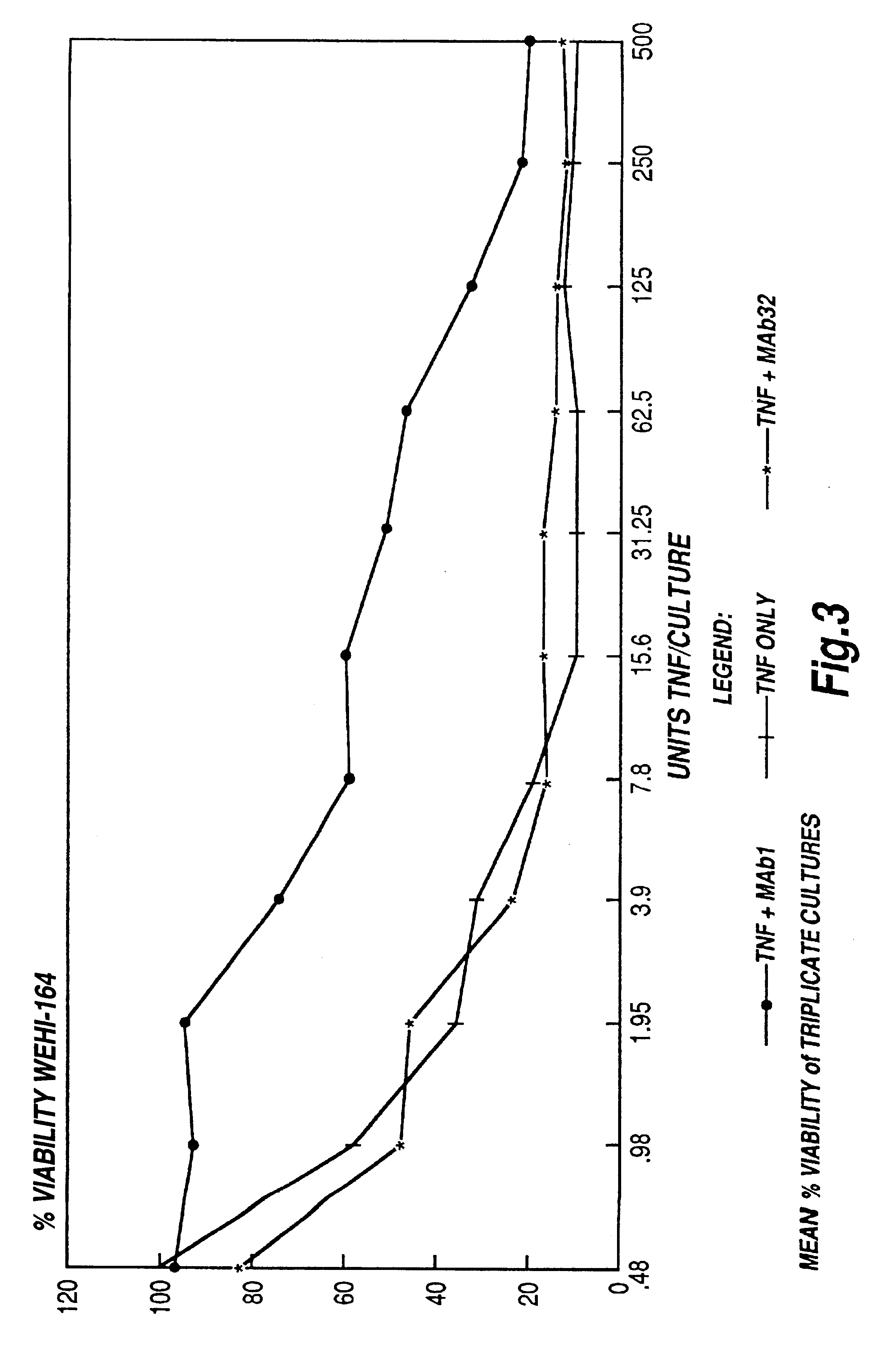
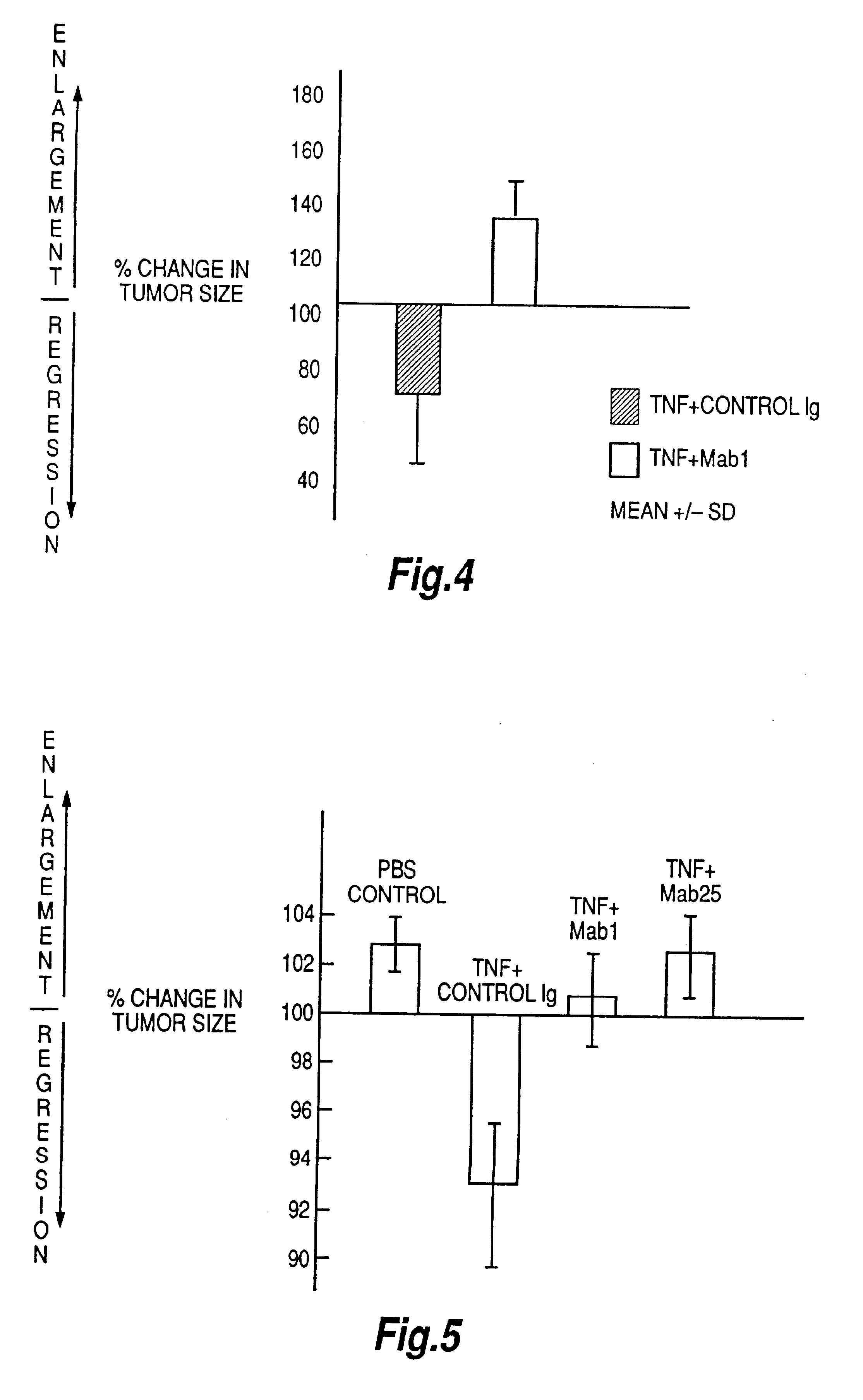
The present invention relates to ligands which bind to human tumor necrosis factor alpha (TNF) in a manner such that upon binding of these ligands to TNF the biological activity of TNF is modified. In preferred forms the ligand binds to TNF in a manner such that the induction of endothelial procoagulant activity of the TNF is inhibited; the binding of TNF to receptors on endothelial cells is inhibited; the induction of fibrin deposition in the tumor and tumor regression activities of the TNF are enhanced; and the cytotoxicity and receptor binding activities of the TNF are unaffected or enhanced on tumor cells. The ligand is preferably an antibody, F(ab) fragment, single domain antibody (dABs) single chain antibody or a serum binding protein. It is preferred, however, that the ligand is a monoclonal antibody or F(ab) fragment thereof.
Owner:CEPHALON AUSTRALIA
Anti-TNF antibodies and peptides of human tumor necrosis factor
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-alpha (TNFalpha) and are useful in vivo diagnosis and therapy of a number of TNFalpha-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV +1
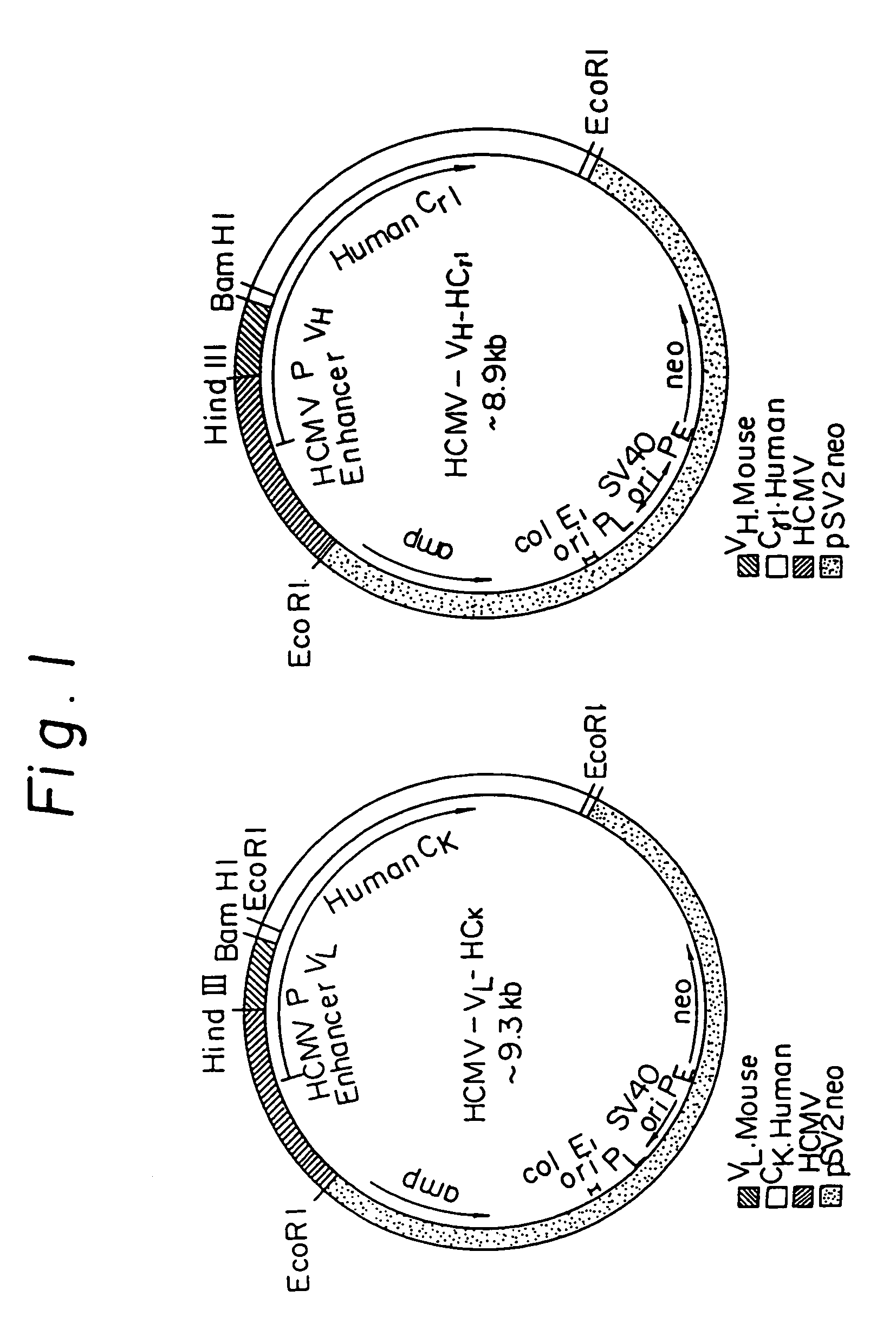
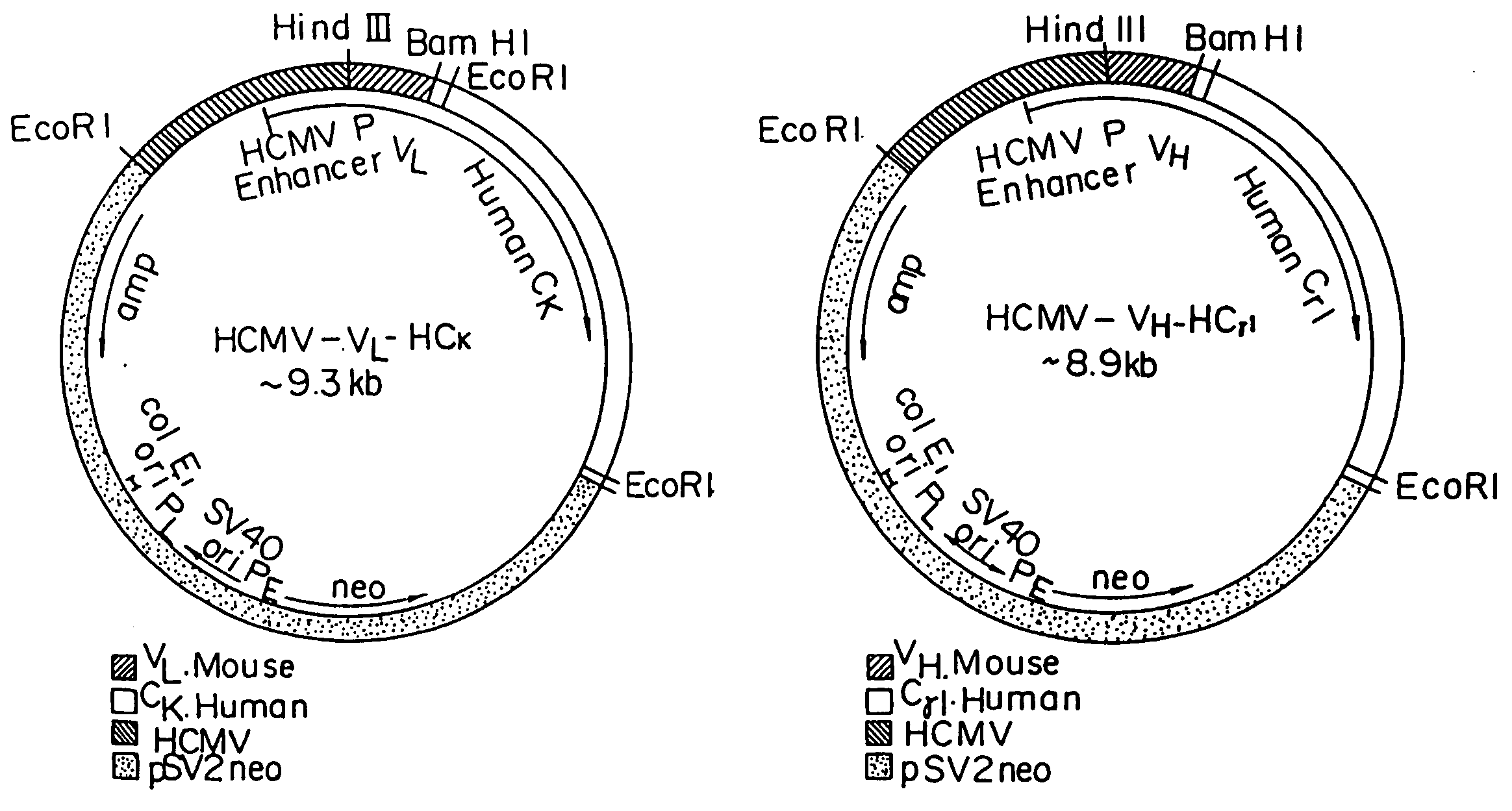
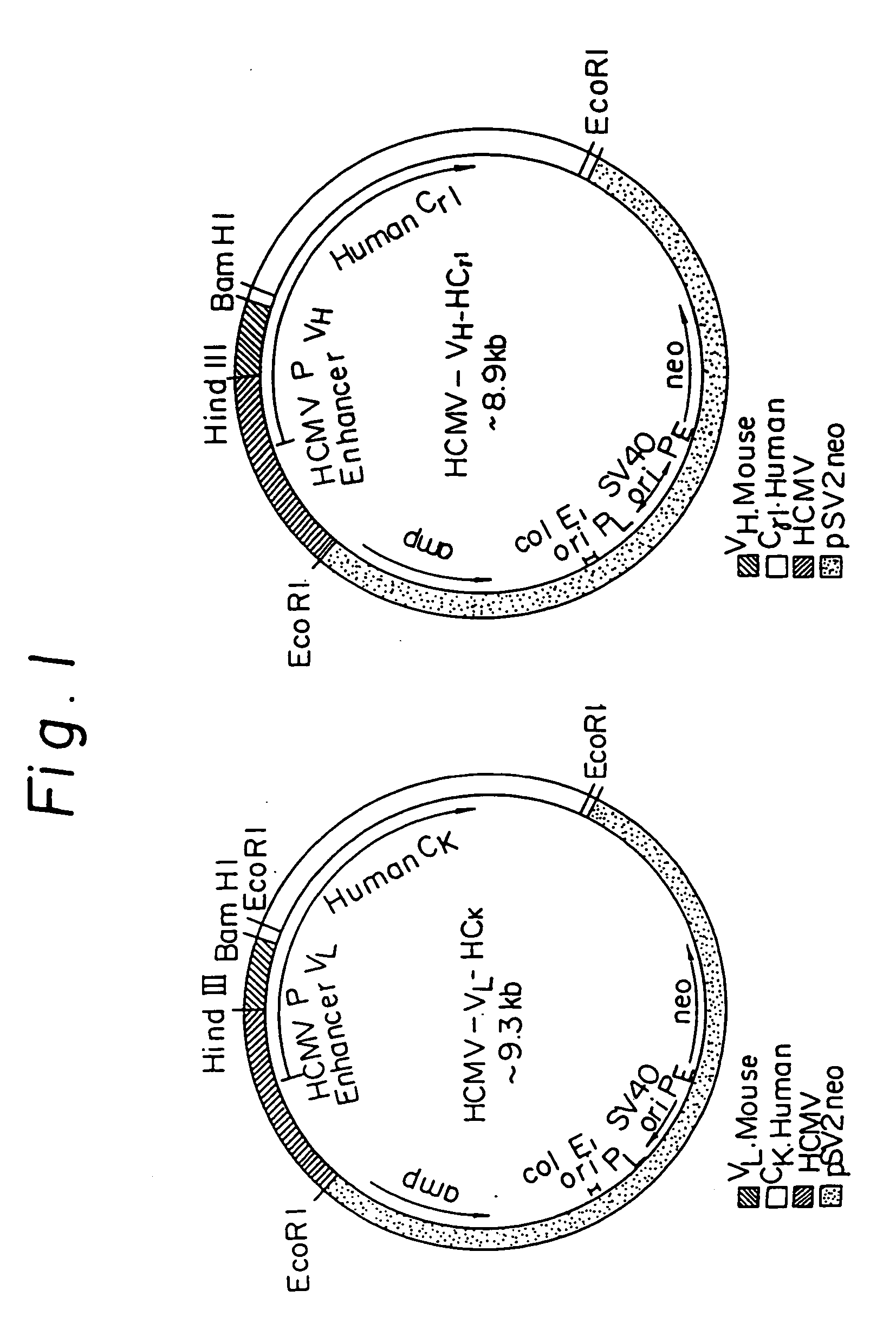
Transgenic mammals having human Ig loci including plural VH and VK regions and antibodies produced therefrom
InactiveUS7064244B2Reduced development and maturation of B-cellsEfficient productionAntipyreticAnalgesicsHuman animalMammal
The present invention relates to transgenic non-human animals that are engineered to contain human immunoglobulin gene loci. In particular, animals in accordance with the invention possess human Ig loci that include plural variable (VH and Vκ) gene regions. Advantageously, the inclusion of plural variable region genes enhances the specificity and diversity of human antibodies produced by the animal. Further, the inclusion of such regions enhances and reconstitutes B-cell development to the animals, such that the animals possess abundant mature B-cells secreting extremely high affinity antibodies.
Owner:ABQENIX INC
Tumor necrosis factor peptide binding antibodies
InactiveUS6593458B1Enhance or inhibit TNF alpha activityInduction of endothelial procoagulant activityPeptide/protein ingredientsAntibody mimetics/scaffoldsDrug biological activityAntibody
Provided are isolated antibodies or fragments thereof which bind a peptide consisting of residues Leu63-Phe64-Lys65-Gly66-Gln67-Gly68-Cys69-Pro70-Ser71-Thr72-His73-Val74-Leu75-Leu76-Thr77-His78-Thr79-Ile80-Ser81-Arg82-Ile83 (peptide 304) of mature human TNF-alpha. The antibodies and fragments thereof may be used to identify antibodies capable of binding in the region of mature human TNF-alpha of amino acid resides 63-83. Antibodies or fragments thereof which bind particular regions of mature human TNF-alpha are shown to elicit particular biological activities dependent upon the particular region wherein binding occurs.
Owner:ARANA THERAPEUTIC LTD
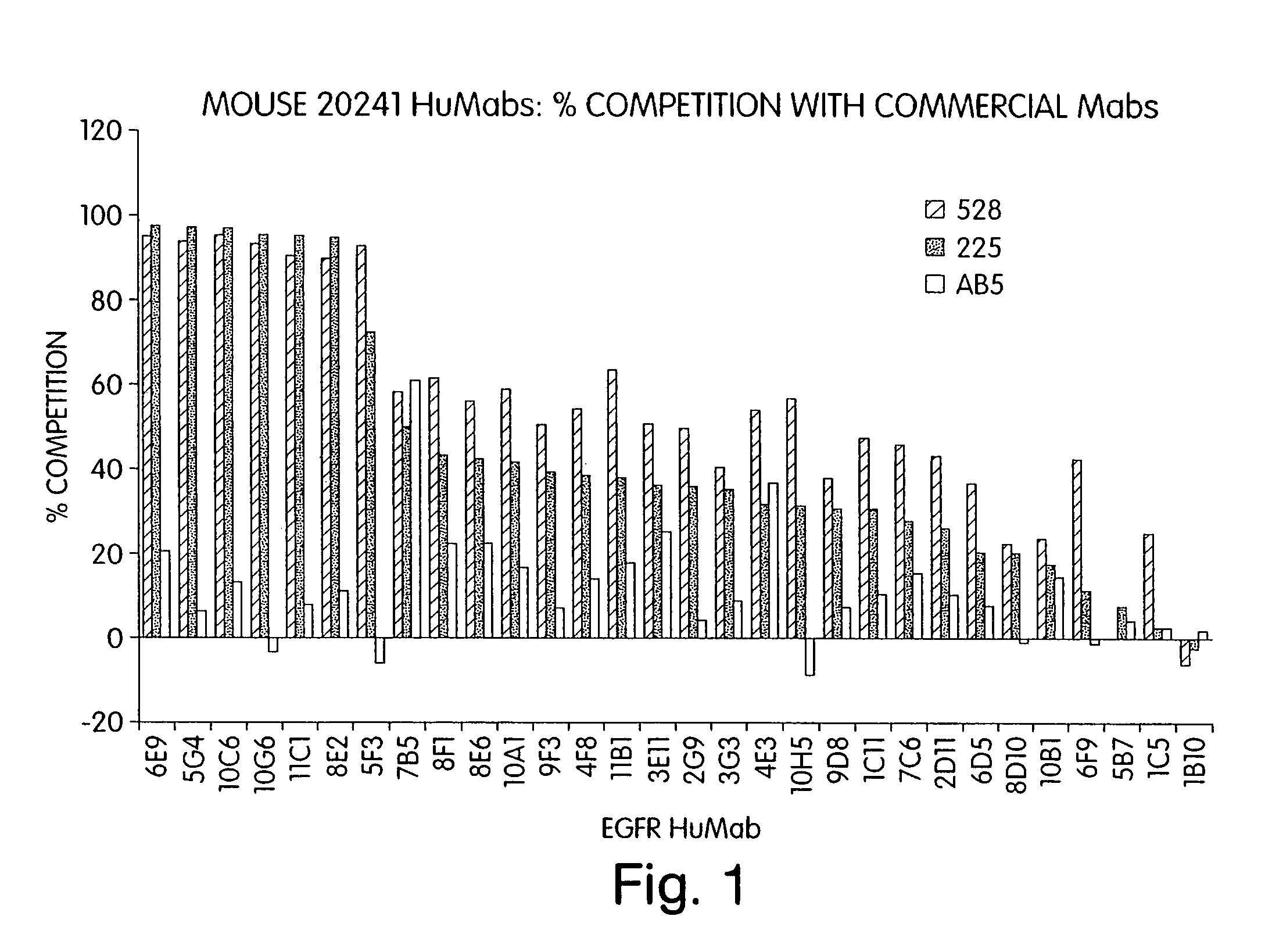
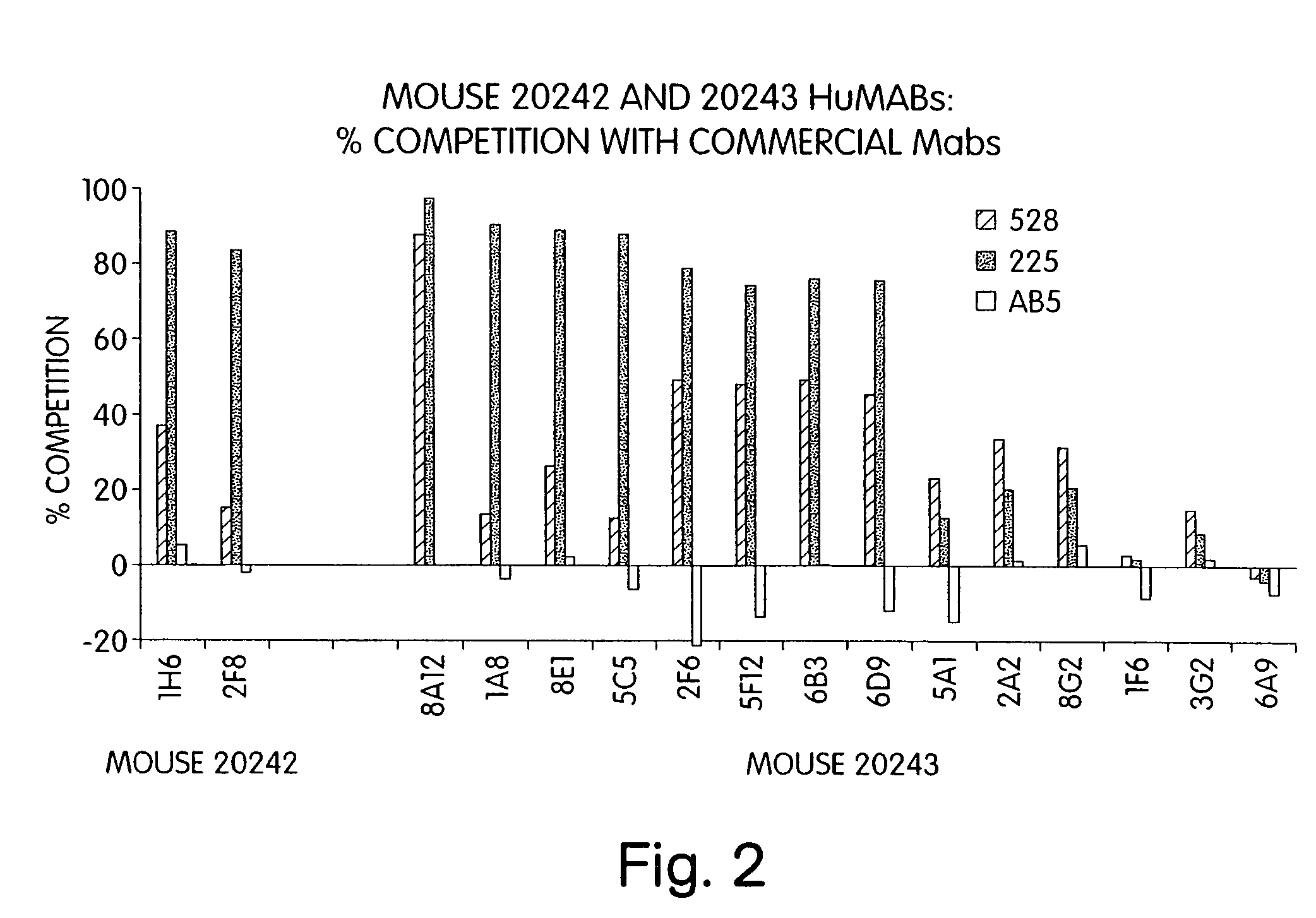
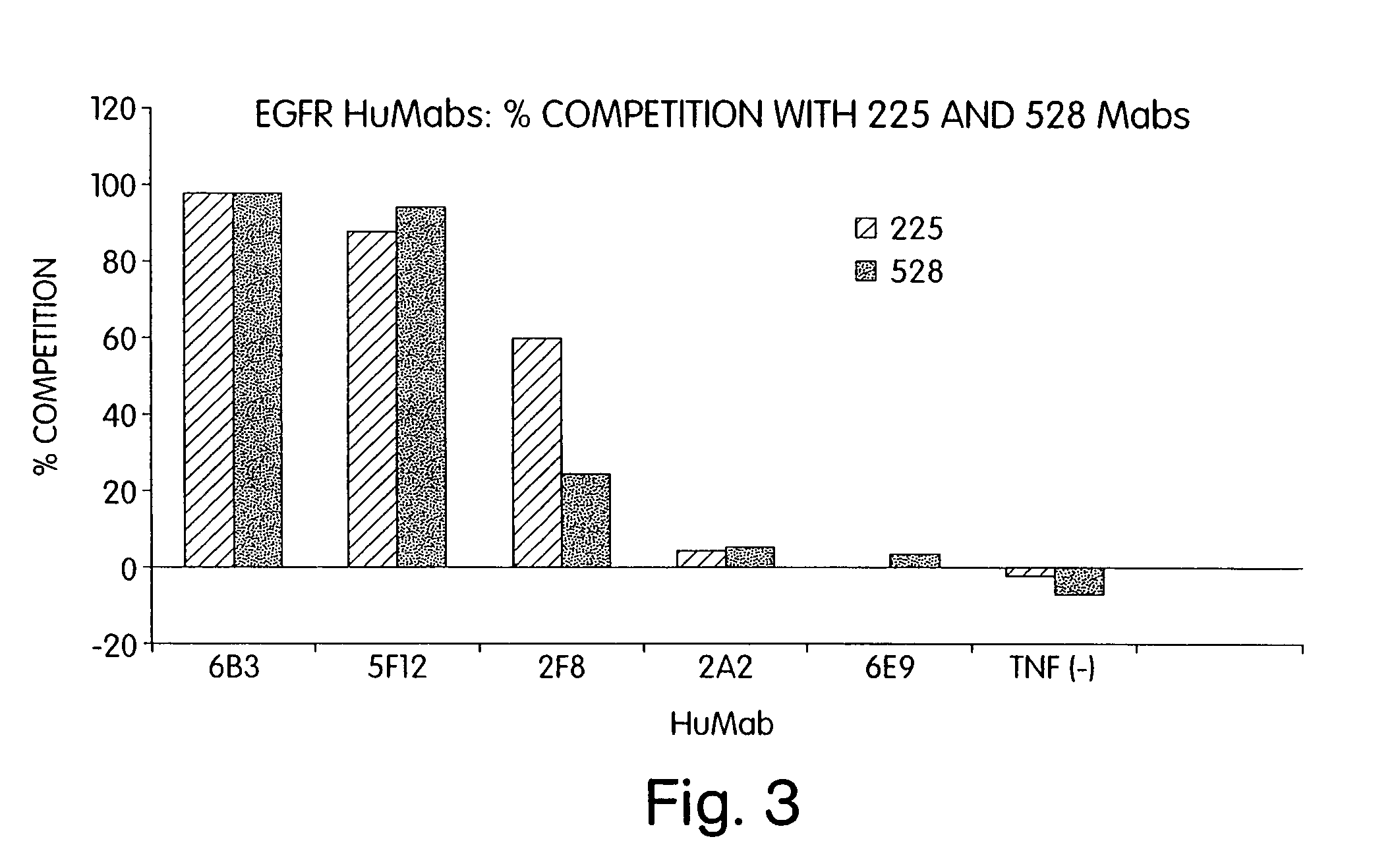
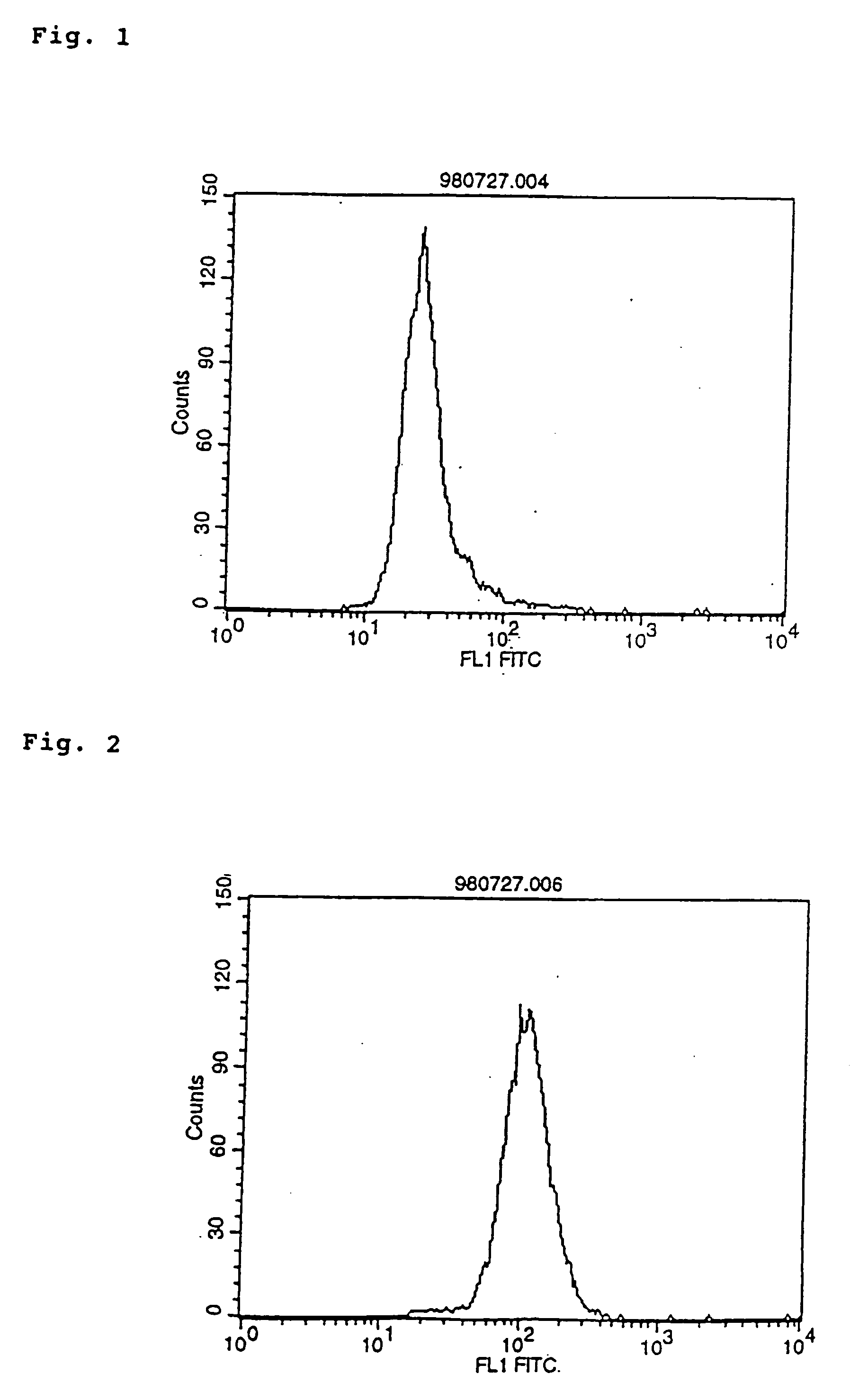
Human monoclonal antibodies to epidermal growth factor receptor (EGFR)
InactiveUS7247301B2Less immunogenicReduce adverse side effectsInorganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsV(D)J recombinationHuman epidermal growth factor receptor
Isolated human monoclonal antibodies which specifically bind to human EGFR, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB INC
Degraded agonist antibody
InactiveUS20040242847A1Excellent antigen-binding propertyExcellent agonist activityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseAntiendomysial antibodies
The invention relates to a modified antibody which contains two or more H chain V regions and two or more L chain V regions of monoclonal antibody and can transduce a signal into cells by crosslinking a cell surface molecule(s) to thereby serve as an agonist. The modified antibody can be used as a signal transduction agonist and, therefore, useful as a preventive and / or remedy for various diseases such as cancer, inflammation, hormone disorders and blood diseases.
Owner:CHUGAI PHARMA CO LTD
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20020012665A1Avoiding and decreasing and resistanceOrganic active ingredientsIn-vivo radioactive preparationsFactor iiBiological activation
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN(R).
Owner:BIOGEN INC
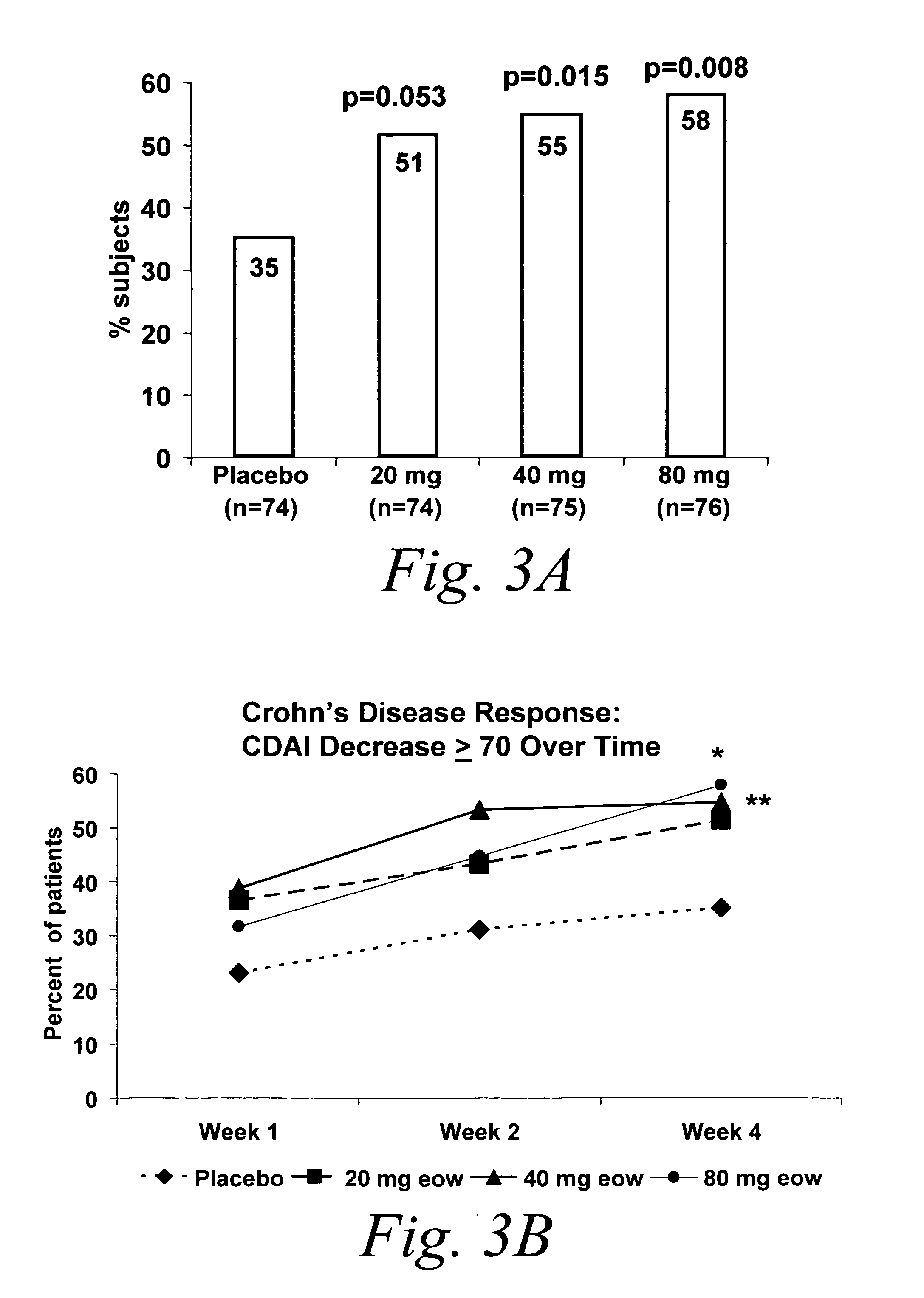
Multiple-variable dose regimen for treating TNFalpha-related disorders
ActiveUS20060009385A1Reduce decreaseIncrease the areaBiocideOrganic active ingredientsDosing regimenRegimen
Multiple-variable dose methods for treating TNFα-related disorders, including Crohn's disease and psoriasis, comprising administering TNFα inhibitors, including TNFα antibodies, are described. Multiple-variable dose methods include administration of a TNF-inhibitor in an induction or loading phase followed by administration of the agent in a maintenance or treatment phase, wherein the TNF-inhibitor is administered in a higher dosage during the induction phase.
Owner:ABBVIE BIOTECHNOLOGY LTD
Reshaped human antibody to human interleukin-6 receptor
InactiveUS7479543B2Peptide/protein ingredientsAntibody mimetics/scaffoldsComplementarity determining regionV region
A reshaped human antibody to the human IL-6R, comprising:(A) an L chain comprising,(1) a human L chain C region, and(2) an L chain V region comprising human L chain framework regions (FRs), and mouse L chain complementary determination regions (CDRs) of a momoclonal antibody to the IL-6 receptor (IL-6R); and(B) an H chain comprising,(1) a human H chain C region, and(2) an H chain V region comprising human H chain FRS, and mouse H chain CDRs of a monoclonal antibody to the IL-6R.Since major portion of the reshaped human antibody is derived from a human antibody and the mouse CDRs which are less immunogenic, the present reshaped human antibody is less immunogenic to human, and therefor is promised for therapeutic uses.
Owner:CHUGAI PHARMA CO LTD
Generation of modified molecules with increased serum half-lives
InactiveUS20020142374A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsSerum igeHalf-life
In accordance with the present invention, there are provided methods for the extension of serum half-lives of proteinaceous molecules, particularly antibody molecules, and compositions of molecules modified in accordance with the methods of the invention. In accordance with a first aspect of the present invention, there is provided a method of modifying the half-life of an antibody through providing an antibody containing an FcRn binding domain or the genes encoding such antibody and physically linking the antibody or the antibody as encoded to a second FcRn binding domain. In accordance with a second aspect of the present invention, there is provided a molecule that contains at least two distinct FcRn binding moieties.
Owner:ABQENIX INC
Compositions and methods for immunomodulation in an organism using IL-15 and soluble IL-15Ra
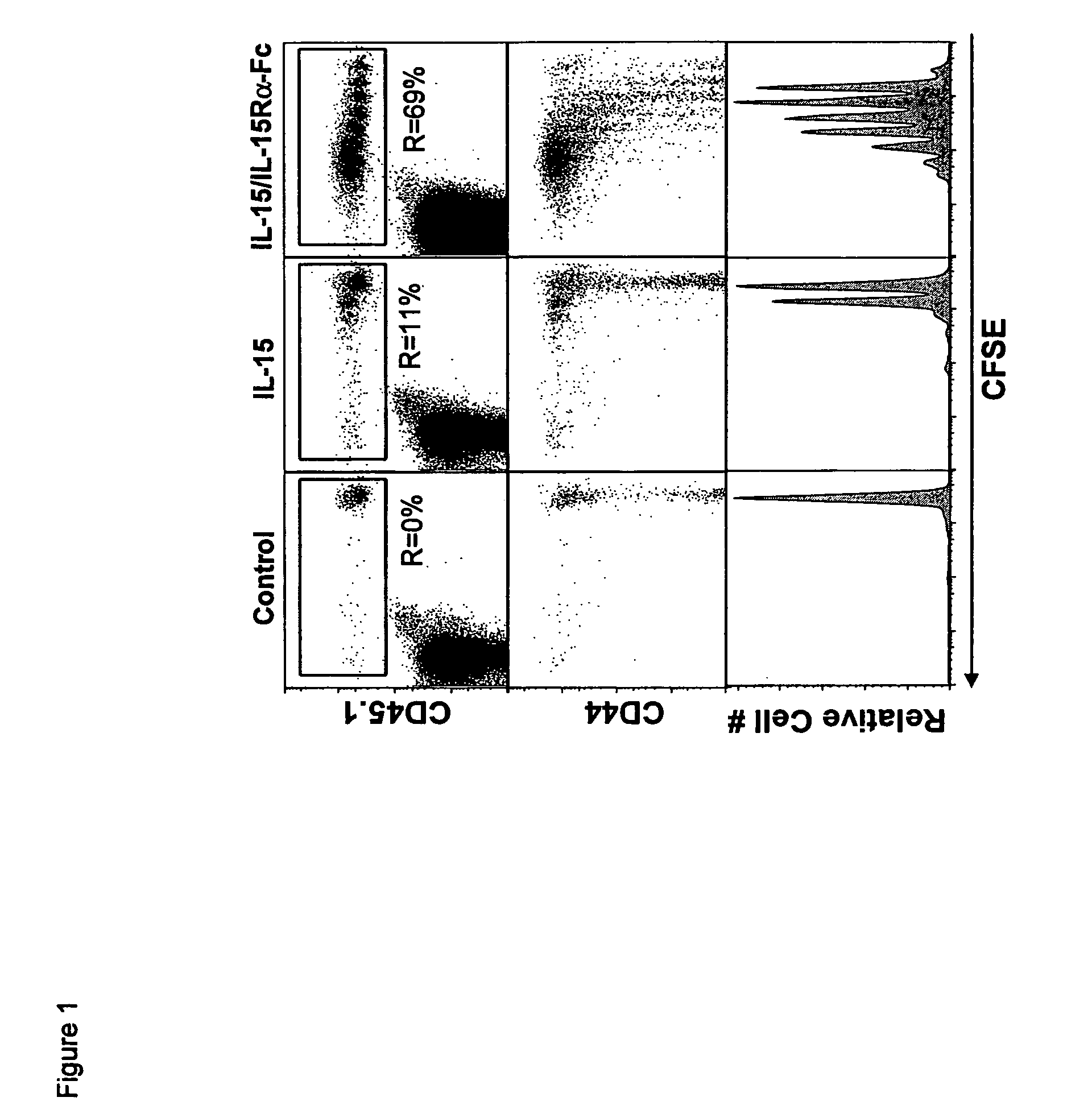
ActiveUS8124084B2Extended half-lifeImprove bioavailabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBiological bodyVaccination
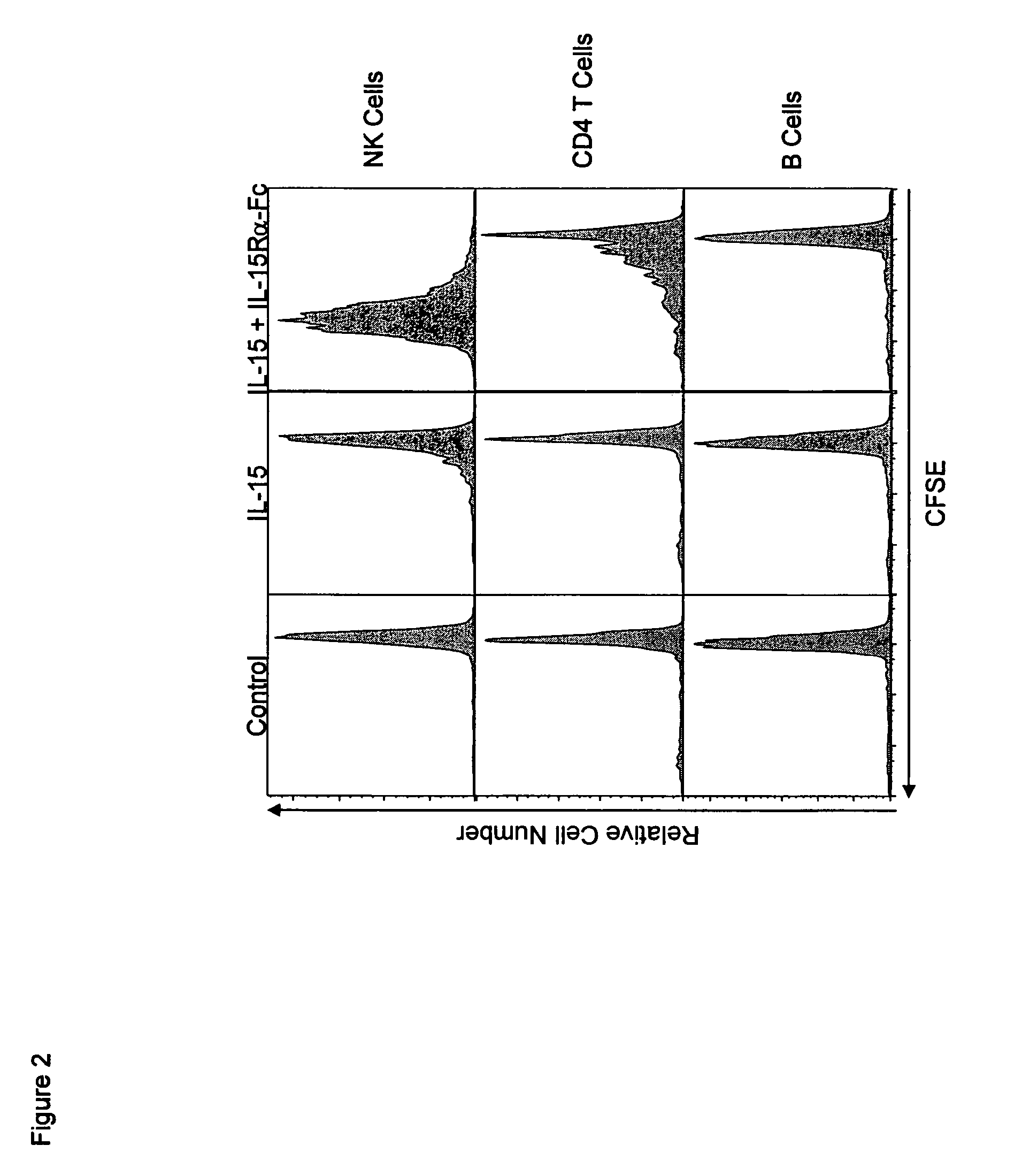
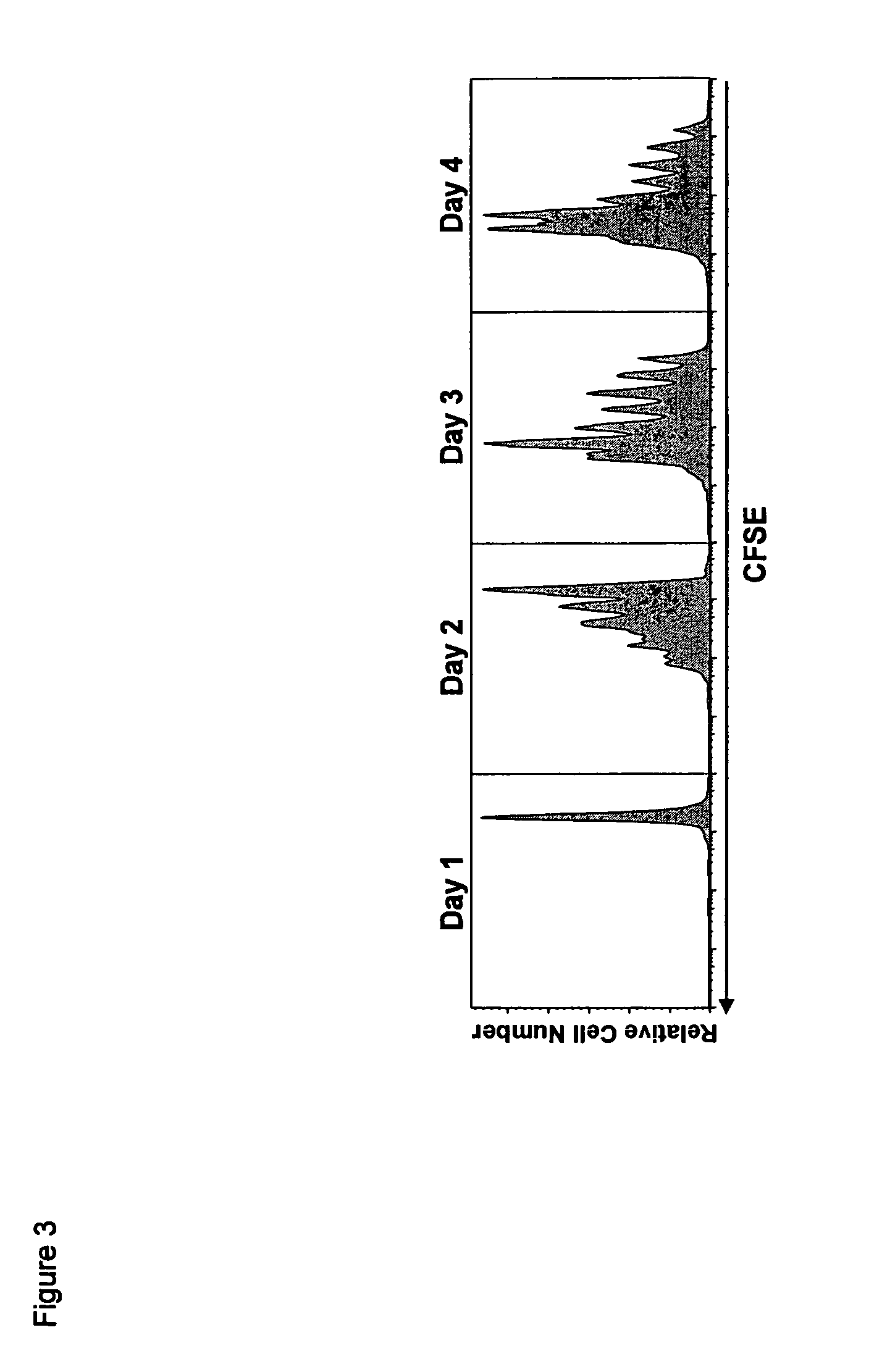
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
Treatment of metabolic disorders using TNFalpha inhibitors
Methods for treating metabolic disorders, including diabetes and obesity, using TNFalpha inhibitors are described.
Owner:ABBOTT BIOTECHNOLOGY LTD
Transgenic animals for producing specific isotypes of human antibodies via non-cognate switch regions
InactiveUS7049426B2Immunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenExon
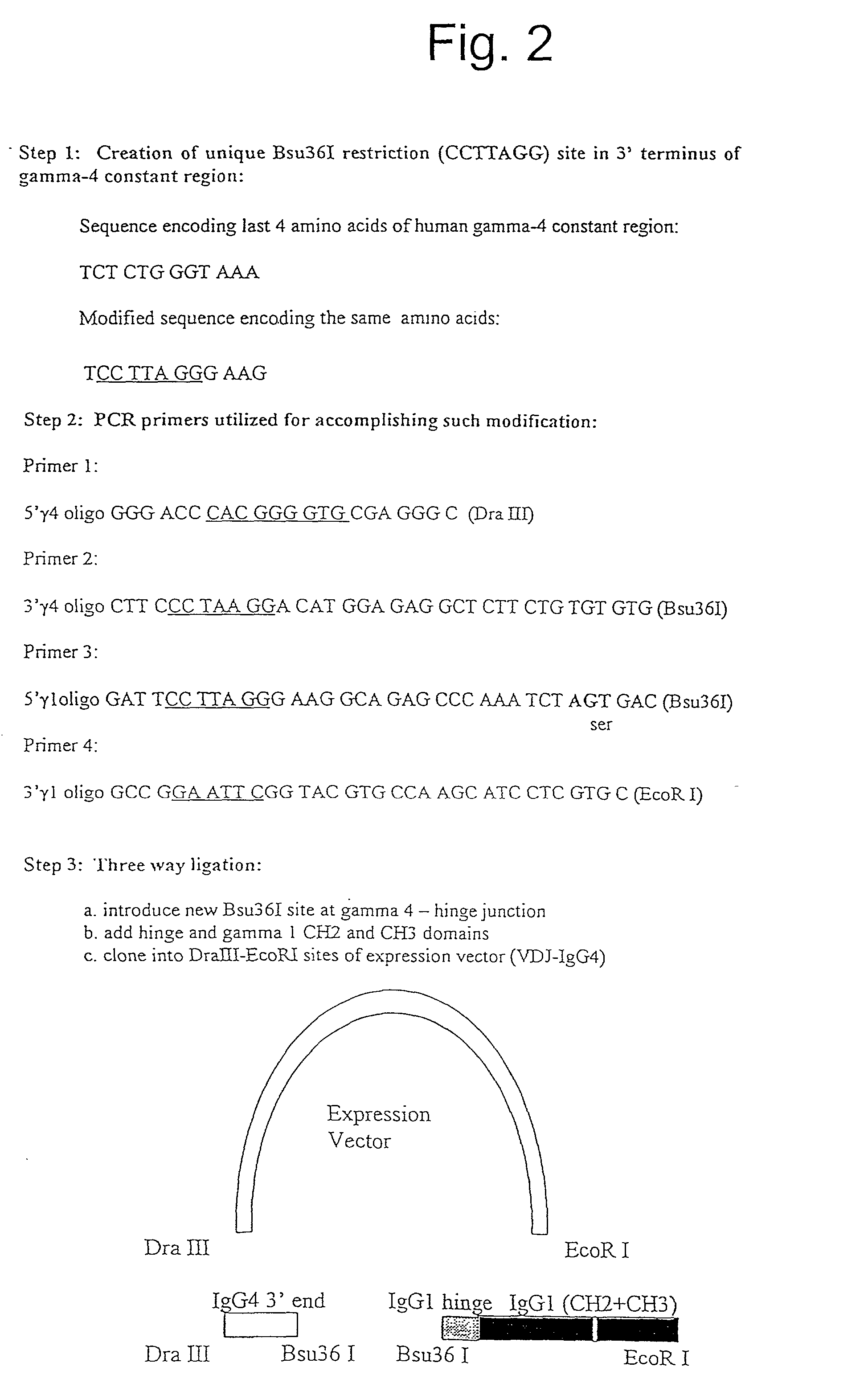
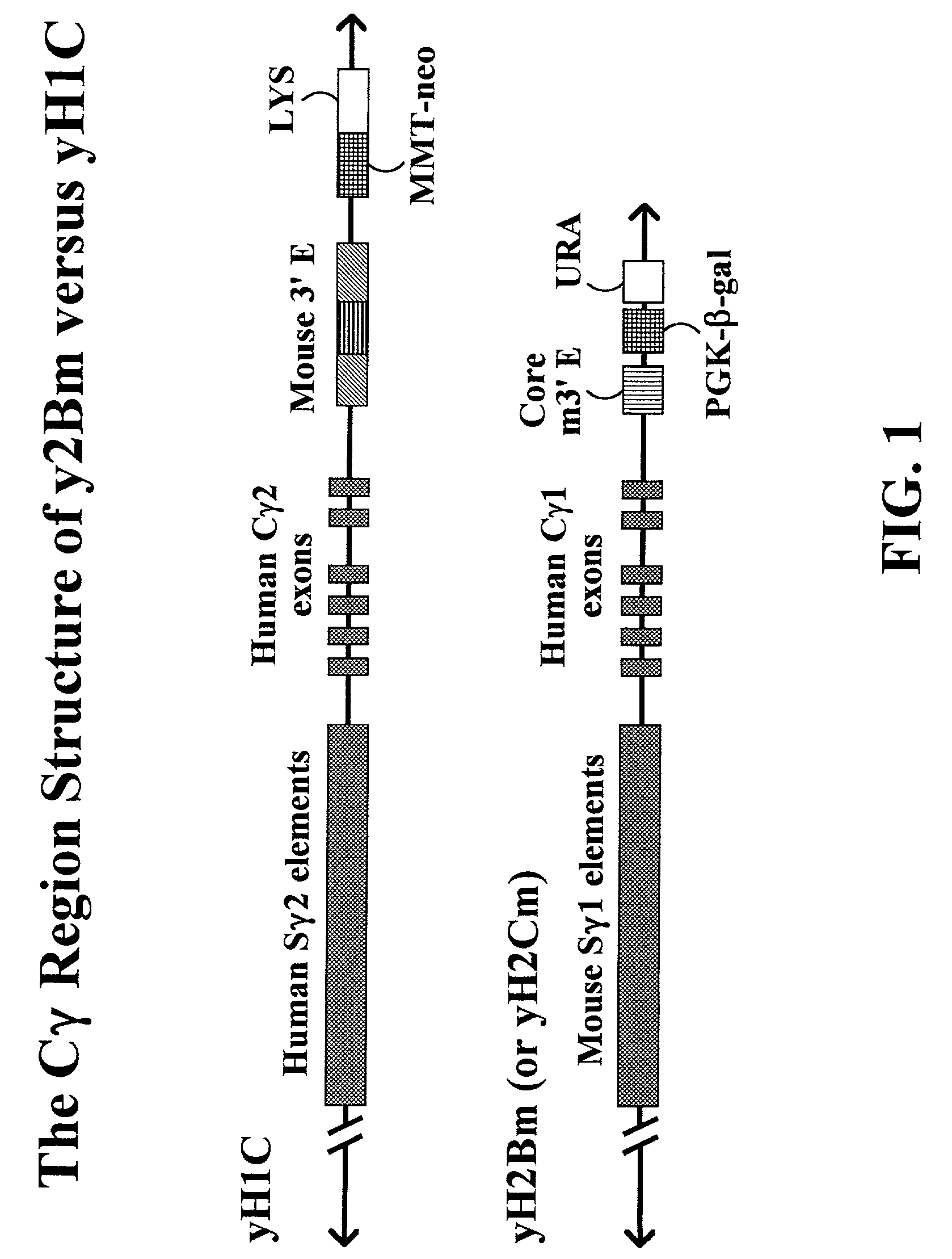
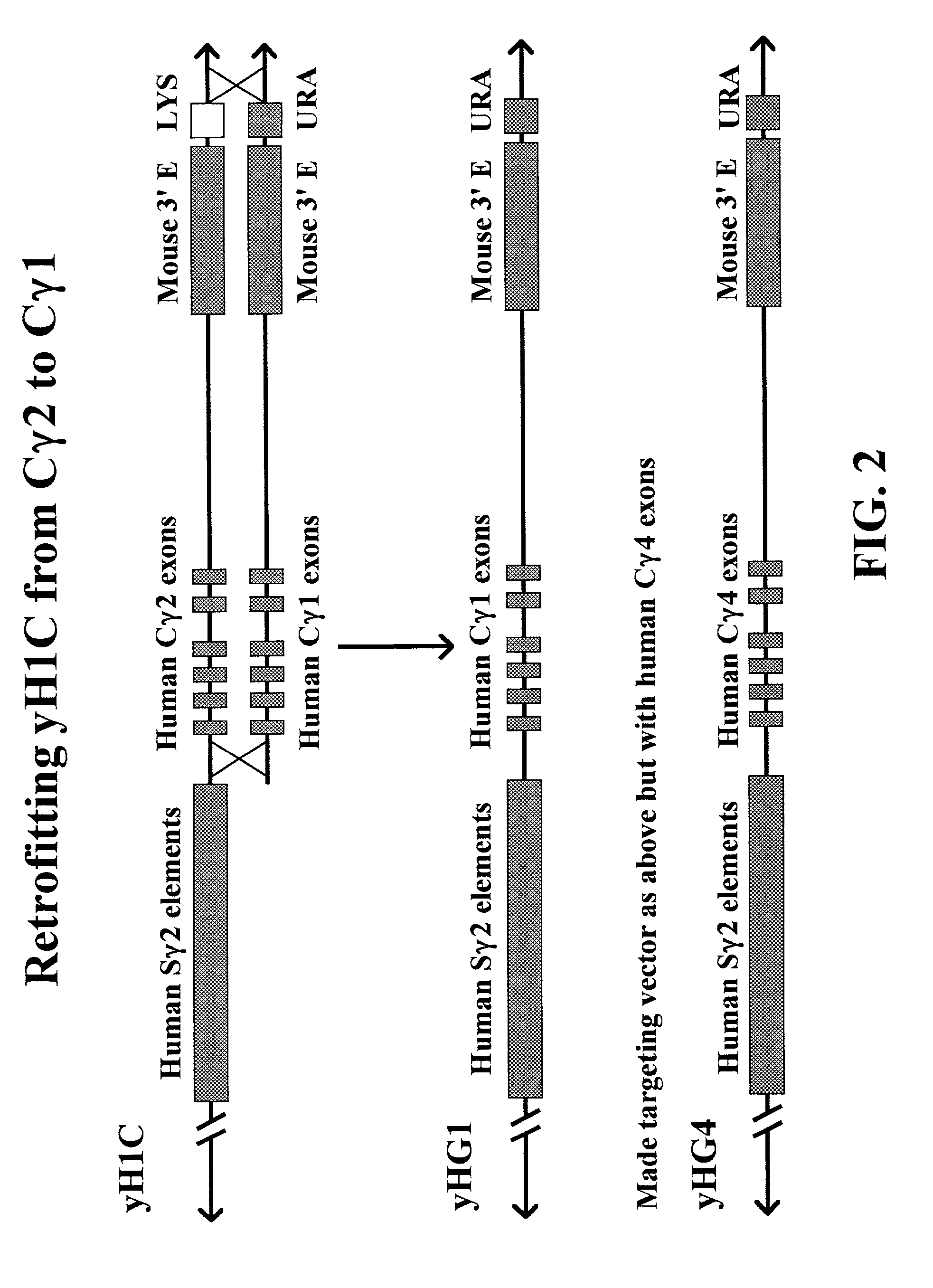
The present invention provides fully human antibodies in a transgenic animal of a desired isotype in response to immunization with any virtually any desired antigen. The human immunoglobulin heavy chain transgene in the foregoing animals comprises a human constant region gene segment comprising exons encoding the desired heavy chain isotype, operably linked to switch segments from a constant region of a different heavy chain isotype, i.e., a non-cognate switch region. Said additional constant region segment comprises a switch region and human constant region coding segment, wherein the constant region coding segment is operably linked to a switch region that it is not normally associated with, i.e., a non-cognate switch region. In the transgenes of the invention, the non-cognate switch region may be a switch region from a different species than the constant region coding segment. The switch region and membrane exons of the invention may comprise a human gamma-2 constant region and the secreted constant region exons are from a human gamma-1 or a human gamma-4 constant region.
Owner:ABQENIX INC
Protein scaffolds for antibody mimics and other binding proteins
InactiveUS20050255548A1Easy to foldImprove stabilityAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsWAS PROTEINAntibody
Disclosed herein are proteins that include a fibronectin type III domain having at least one randomized loop. Also disclosed herein are nucleic acids encoding such proteins and the use of such proteins in diagnostic methods and in methods for evolving novel compound-binding species and their ligands.
Owner:BRISTOL MYERS SQUIBB CO
Reshaped human antibody to human interleukin-6 receptor
InactiveUS20050142635A1Less immunogenicPeptide/protein ingredientsHydrolasesComplementarity determining regionV region
A reshaped human antibody to the human IL-6R, comprising: (A) an L chain comprising, (1) a human L chain C region, and (2) an L chain V region comprising human L chain framework regions (FRs), and mouse L chain complementary determination regions (CDRs) of a momoclonal antibody to the IL-6 receptor (IL-6R); and (B) an H chain comprising, (1) a human H chain C region, and (2) an H chain V region comprising human H chain FRS, and mouse H chain CDRs of a monoclonal antibody to the IL-6R. Since major portion of the reshaped human antibody is derived from a human antibody and the mouse CDRs which are less immunogenic, the present reshaped human antibody is less immunogenic to human, and therefor is promised for therapeutic uses.
Owner:CHUGAI PHARMA CO LTD
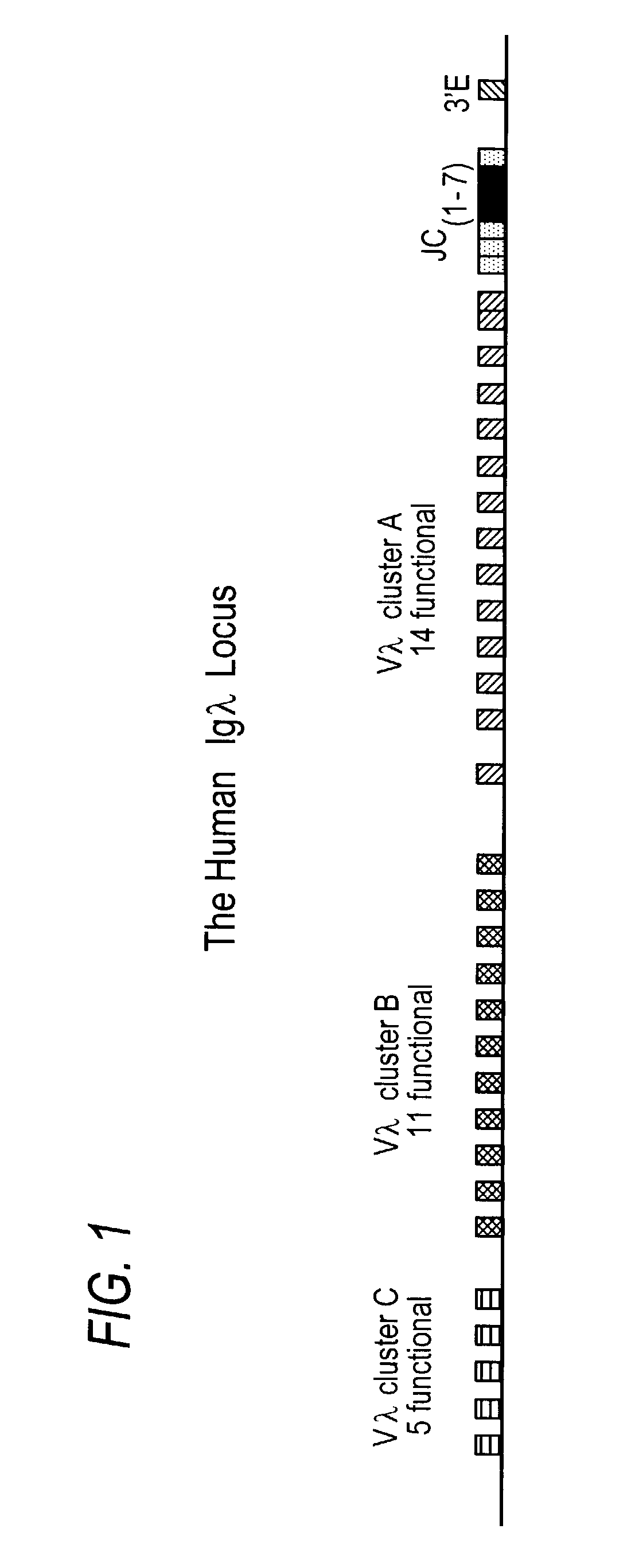
Transgenic animals bearing human Iglambda light chain genes
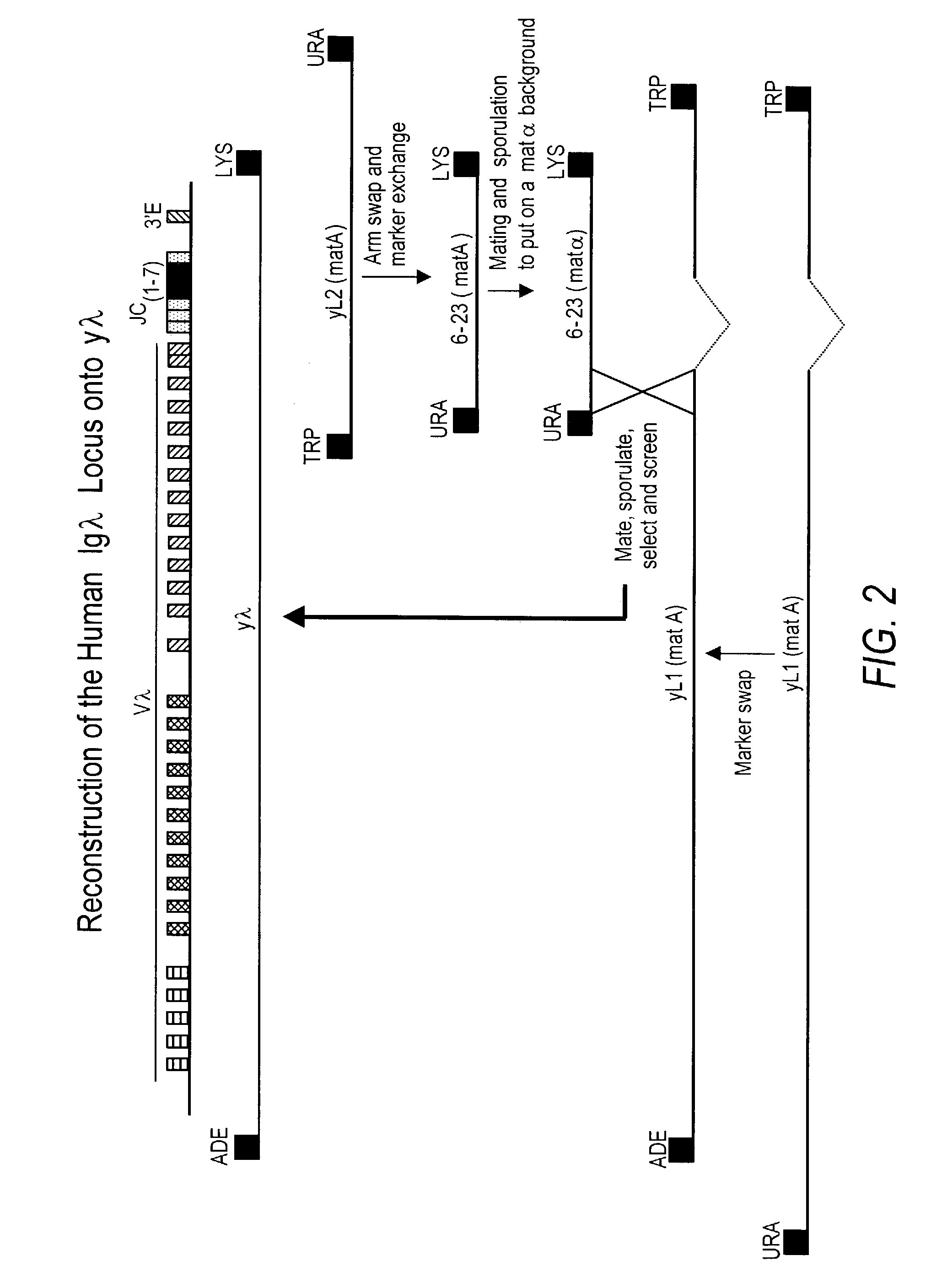
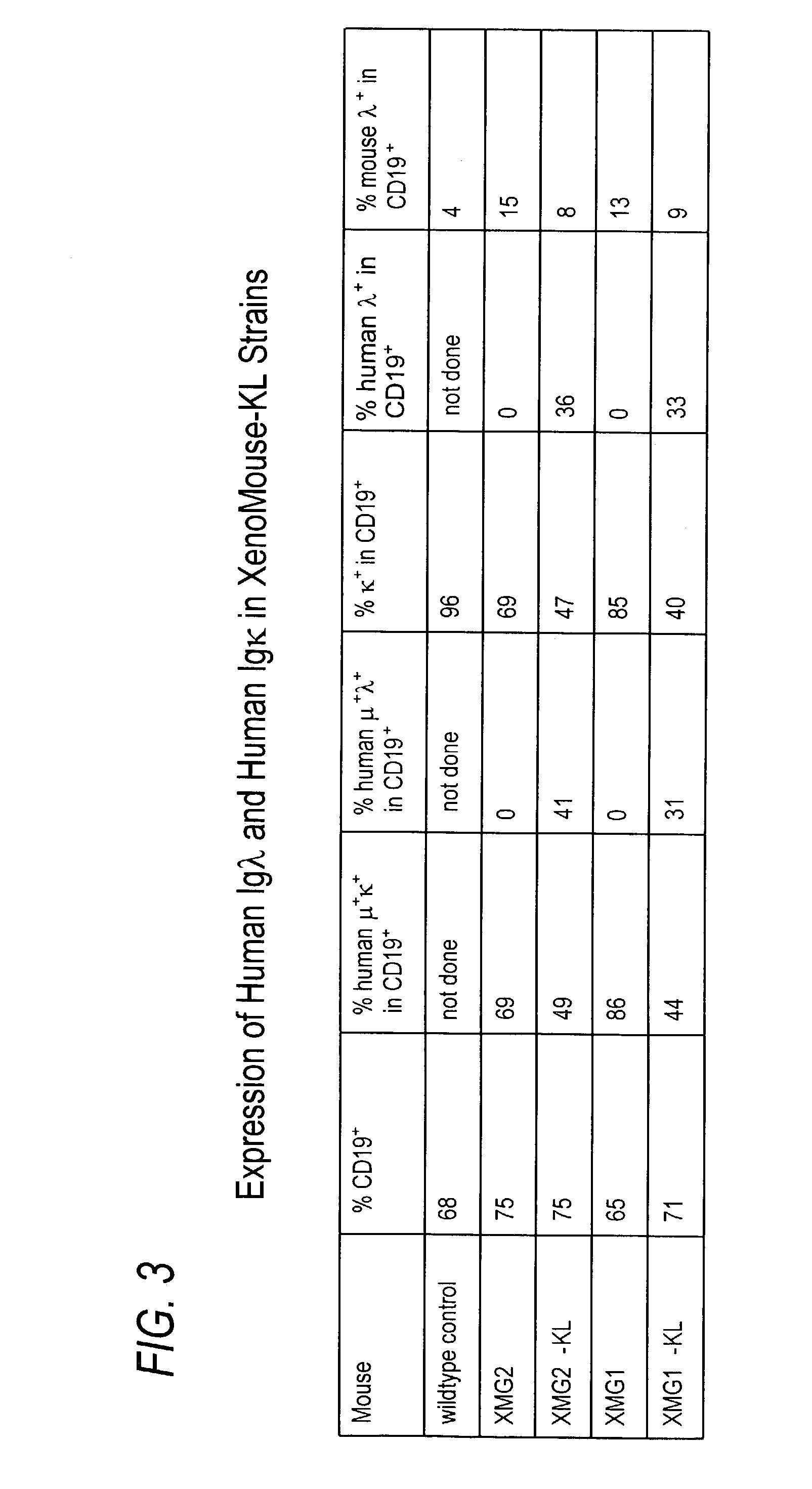
ActiveUS7435871B2Restore normal B-cell developmentAnimal cellsSerum immunoglobulinsPhysiologyLambda Light Chain Gene
Owner:AMGEN FREMONT INC
Methods for generating multimeric molecules
InactiveUS20050136051A1Immunoglobulins against blood coagulation factorsHybrid immunoglobulinsBispecific antibodyIn vivo
Owner:SCALLON BERNARD
Formulation of human antibodies for treating tnf-alpha associated disorders
ActiveUS20060153846A1Enhance stabilityImprove stabilityAntibacterial agentsPowder deliveryDrugAntibody
A liquid aqueous pharmaceutical formulation is described which has a high protein concentration, a pH of between about 4 and about 8, and enhanced stability.
Owner:ABBVIE BIOTECHNOLOGY LTD
Antibody formulations and methods of making same
ActiveUS8420081B2Minimal aggregationLow levelPeptide/protein ingredientsAntipyreticExcipientNuclear chemistry
Owner:ABBVIE BIOTECHNOLOGY LTD
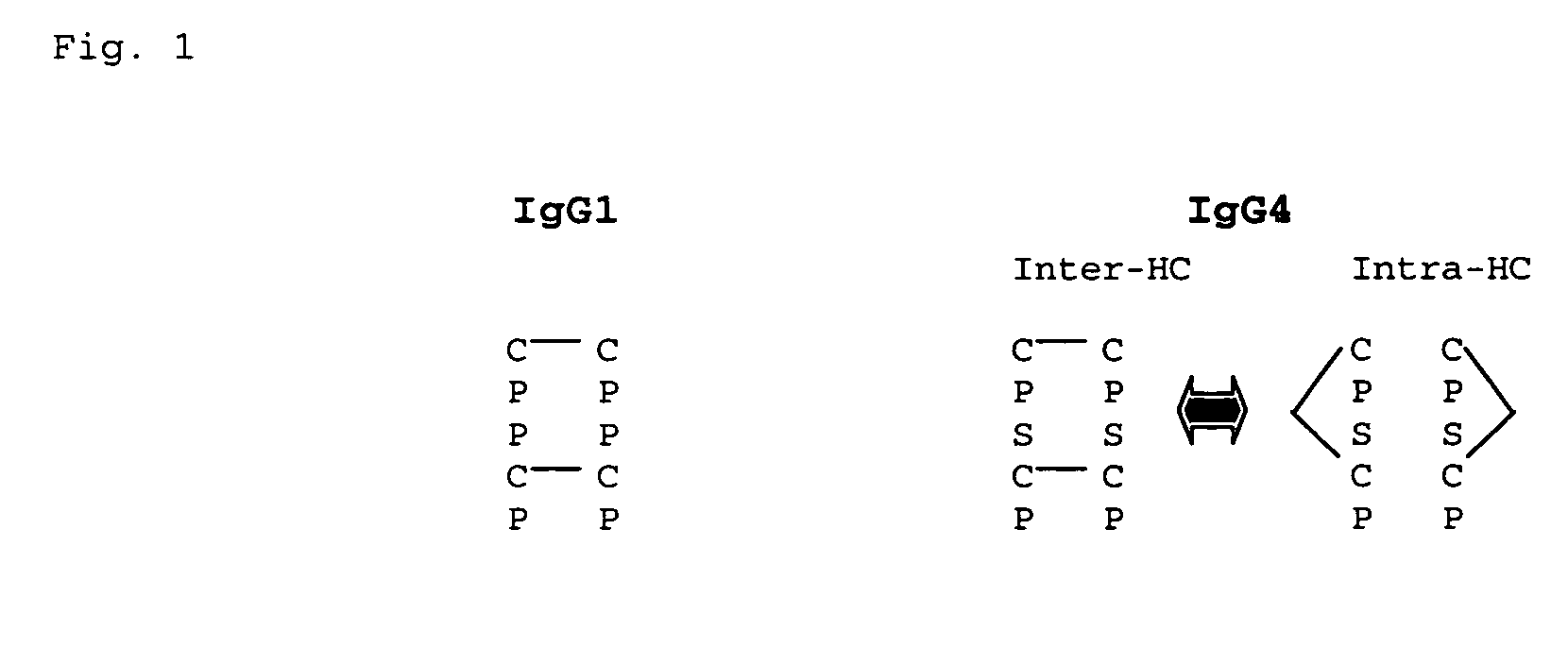
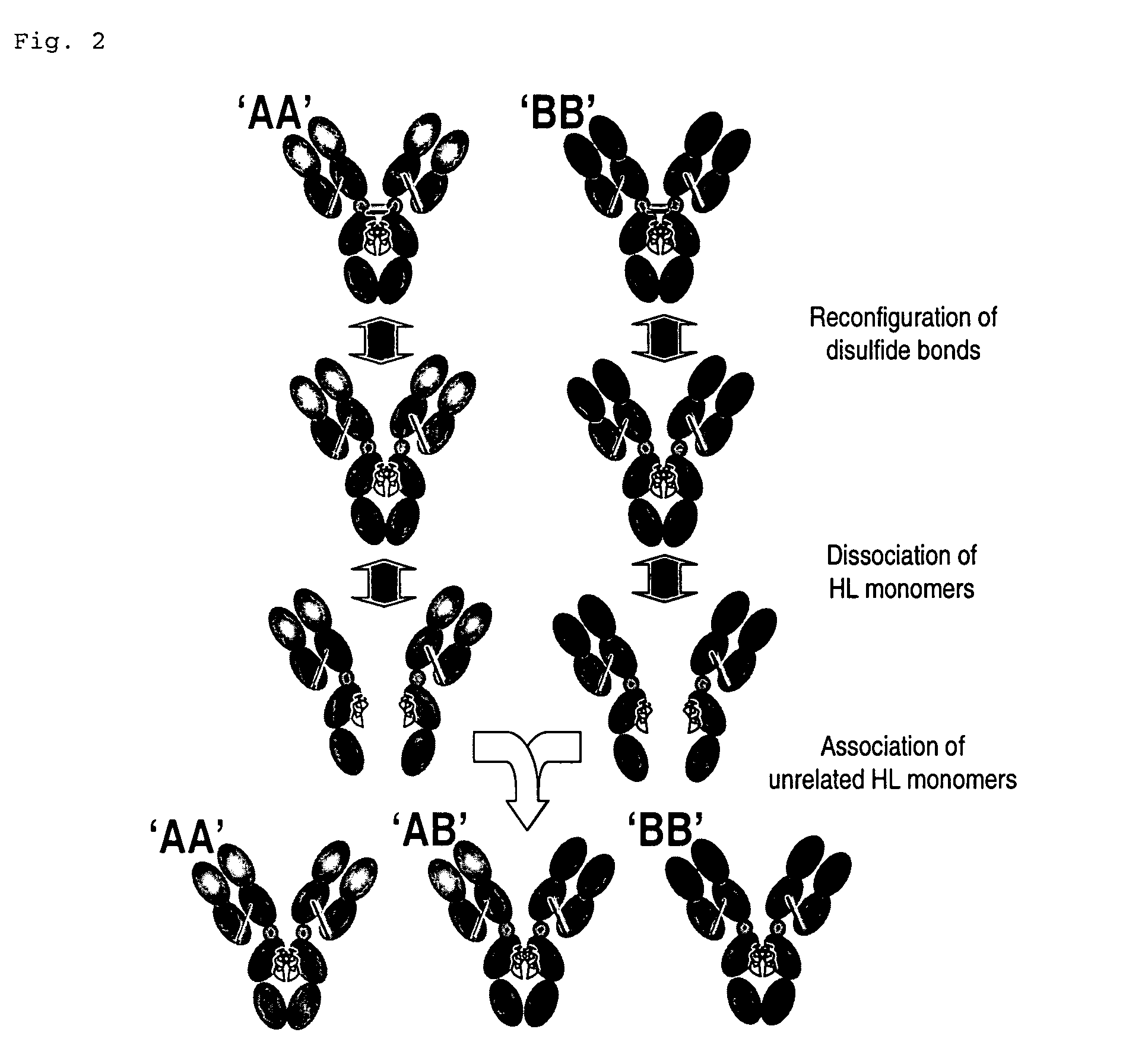
Modified Antibody Constant Region
ActiveUS20100298542A1Improving immunogenicityImprove propertiesAntipyreticAnalgesicsHigh concentrationHinge region
The present inventors succeeded in improving the antibody constant region to have increased stability under acid conditions, reduced heterogeneity originated from disulfide bonds in the hinge region, reduced heterogeneity originated from the H chain C terminus, and increased stability at high concentrations as well as in discovering novel constant region sequences having reduced Fcγ receptor-binding, while minimizing the generation of novel T-cell epitope peptides. As a result, the present inventors successfully discovered antibody constant regions with improved physicochemical properties (stability and homogeneity), immunogenicity, safety, and pharmacokinetics.
Owner:CHUGAI PHARMA CO LTD
Human antibodies derived from immunized xenomice
Antibodies with fully human variable regions against a specific antigen can be prepared by administering the antigen to a transgenic animal which has been modified to produce such antibodies in response to antigenic challenge, but whose endogenous loci have been disabled. Various subsequent manipulations can be performed to obtain either antibodies per se or analogs thereof.
Owner:KUCHERLAPATI RAJU +4
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com