Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
296 results about "Hinge region" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
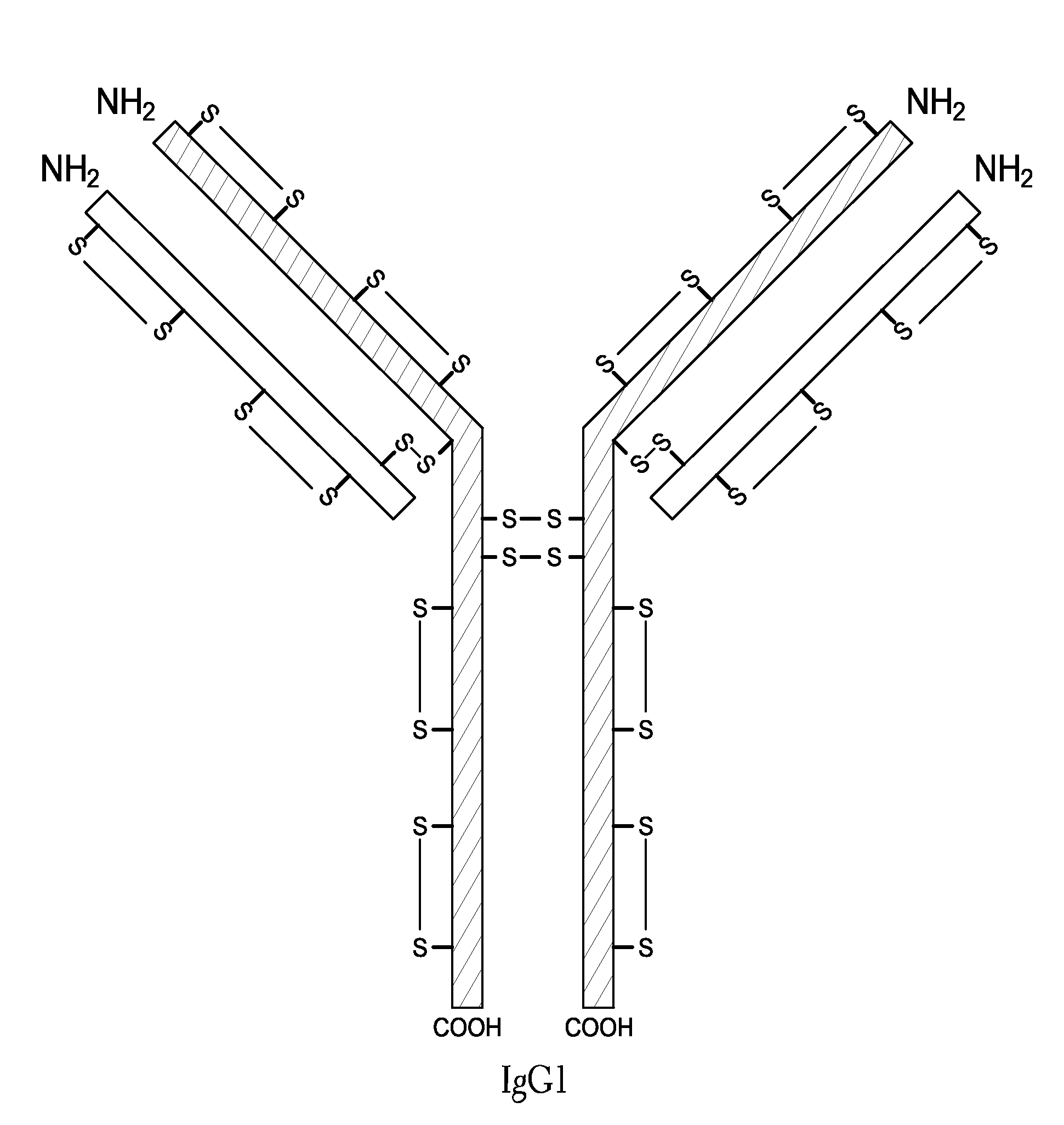
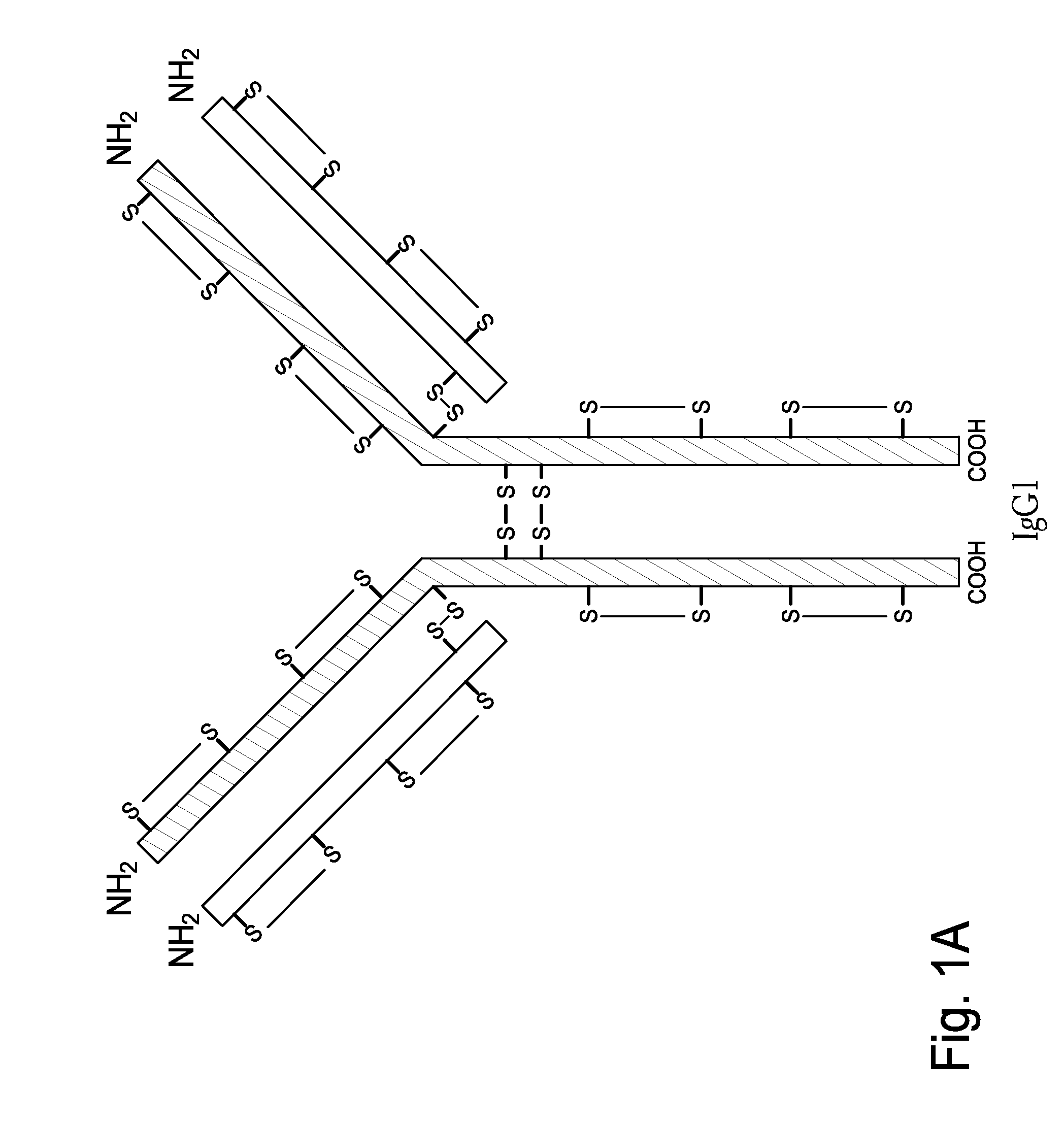
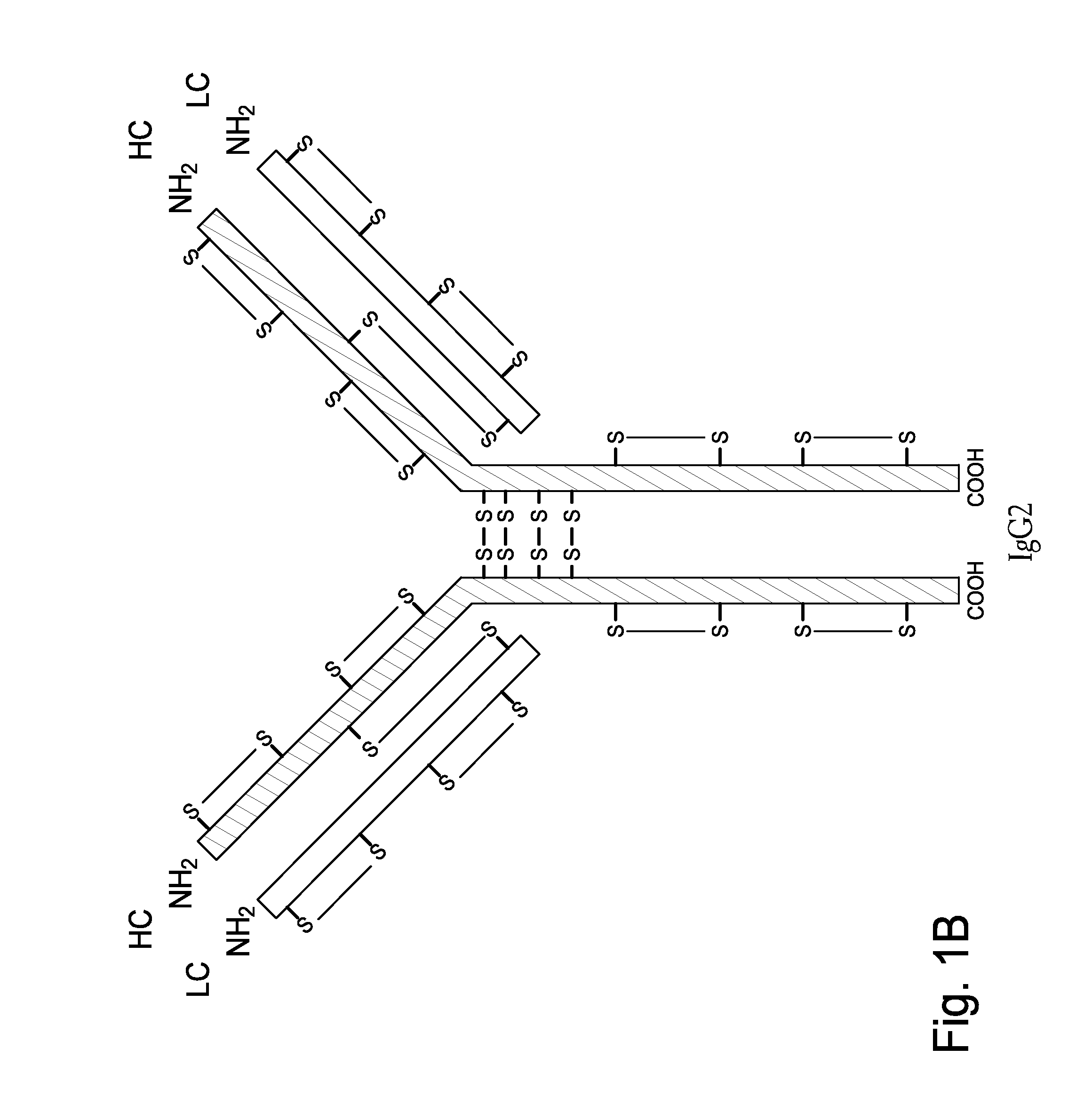
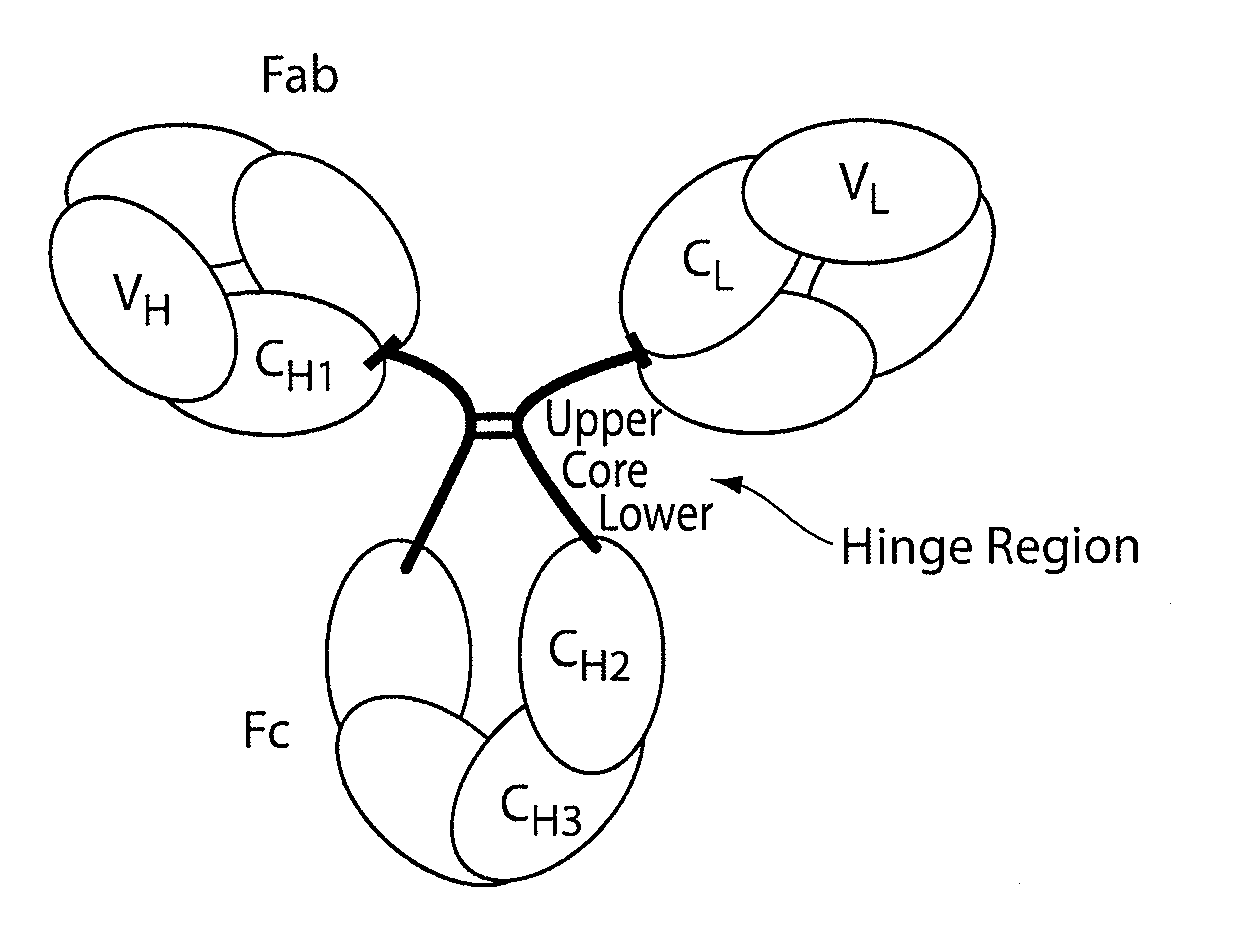
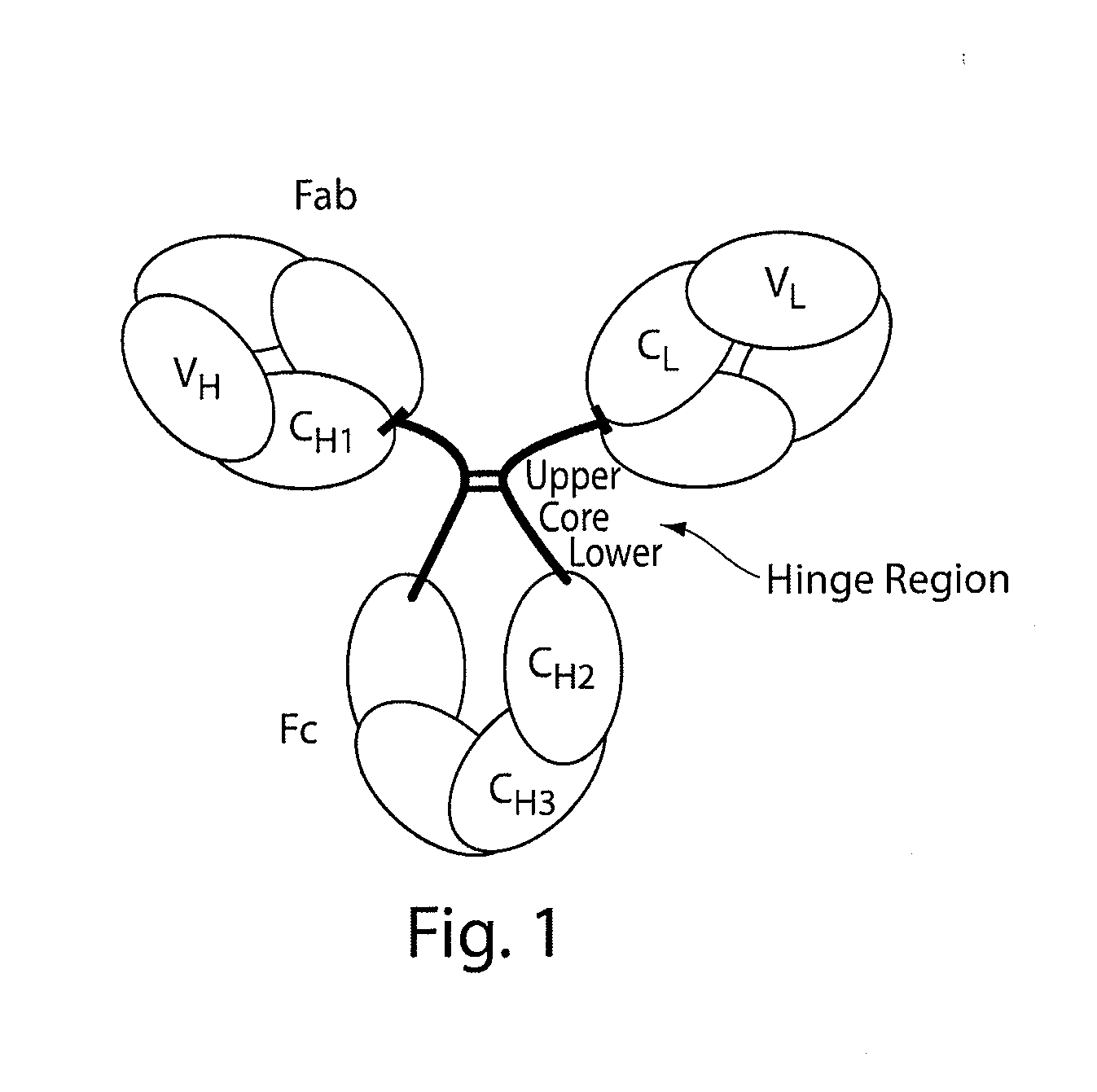
Hinge region. a proline-rich portion of an immunoglobulin heavy chain between the Fc and Fab regions that confers mobility on the two Fab arms of the antibody molecule thereby allowing it to combine better with the two epitopes.
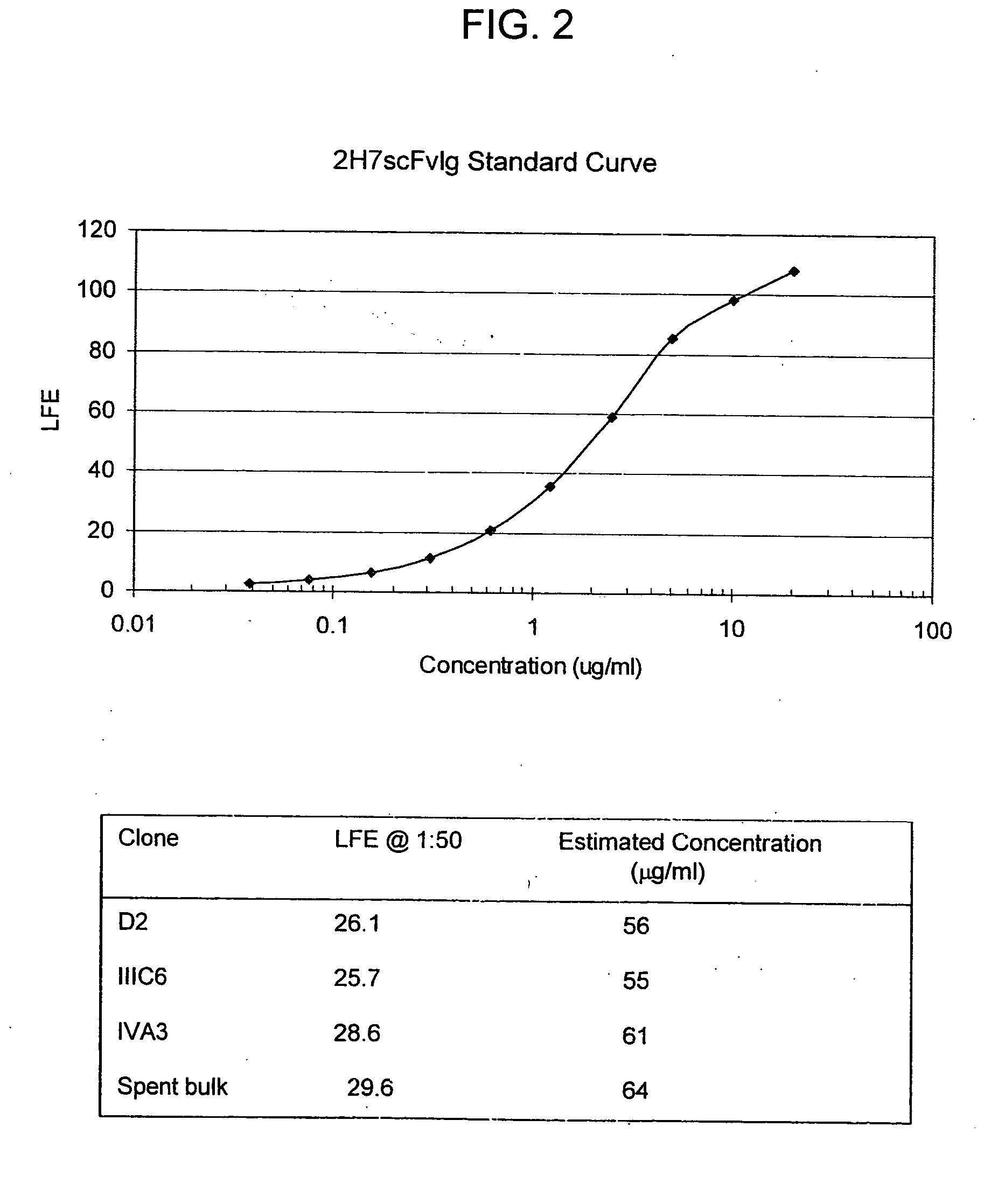
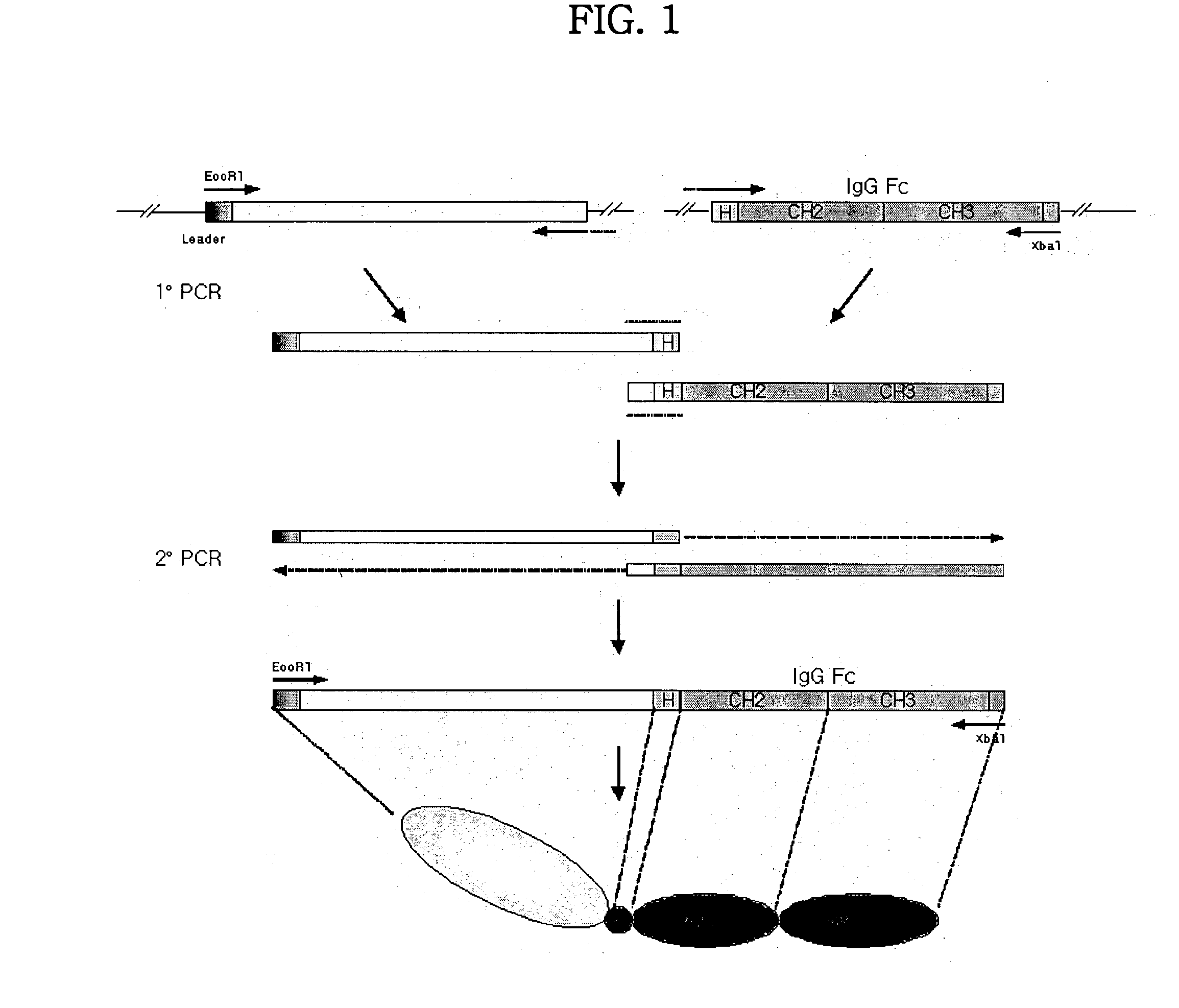
Binding domain-immunoglobulin fusion proteins
InactiveUS20050175614A1Reduced ability to dimerizeHybrid immunoglobulinsAntipyreticCrystallographyAntigen
The invention relates to novel binding domain-immunoglobulin fusion proteins that feature a binding domain for a cognate structure such as an antigen, a counterreceptor or the like, a hinge region polypeptide having either zero or one cysteine residue, and immunoglobulin CH2 and CH3 domains, and that are capable of ADCC and / or CDC while occurring predominantly as monomeric polypeptides. The fusion proteins can be recombinantly produced at high expression levels. Also provided are related compositions and methods, including immunotherapeutic applications.
Owner:TRUBION PHARM INC
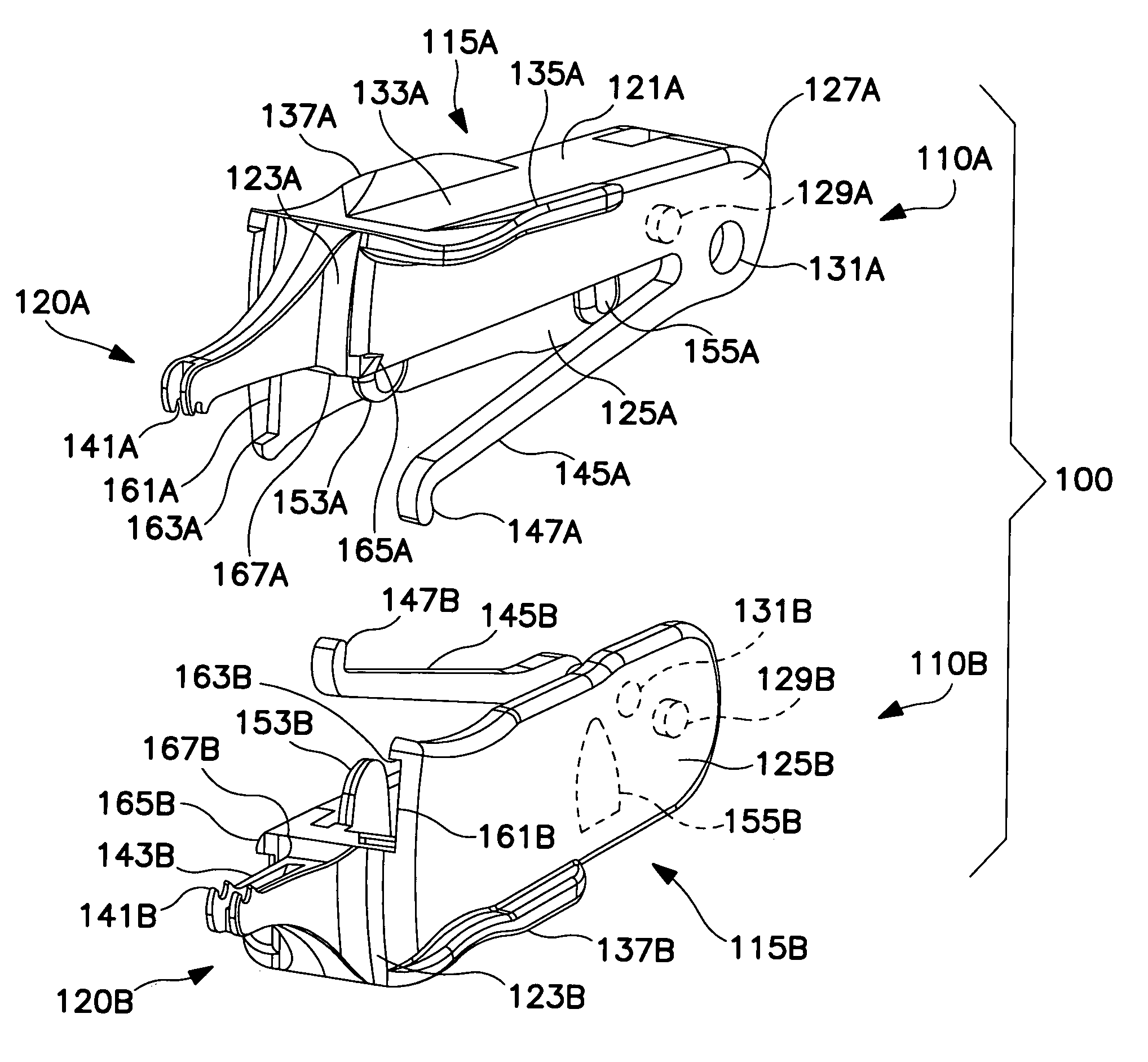
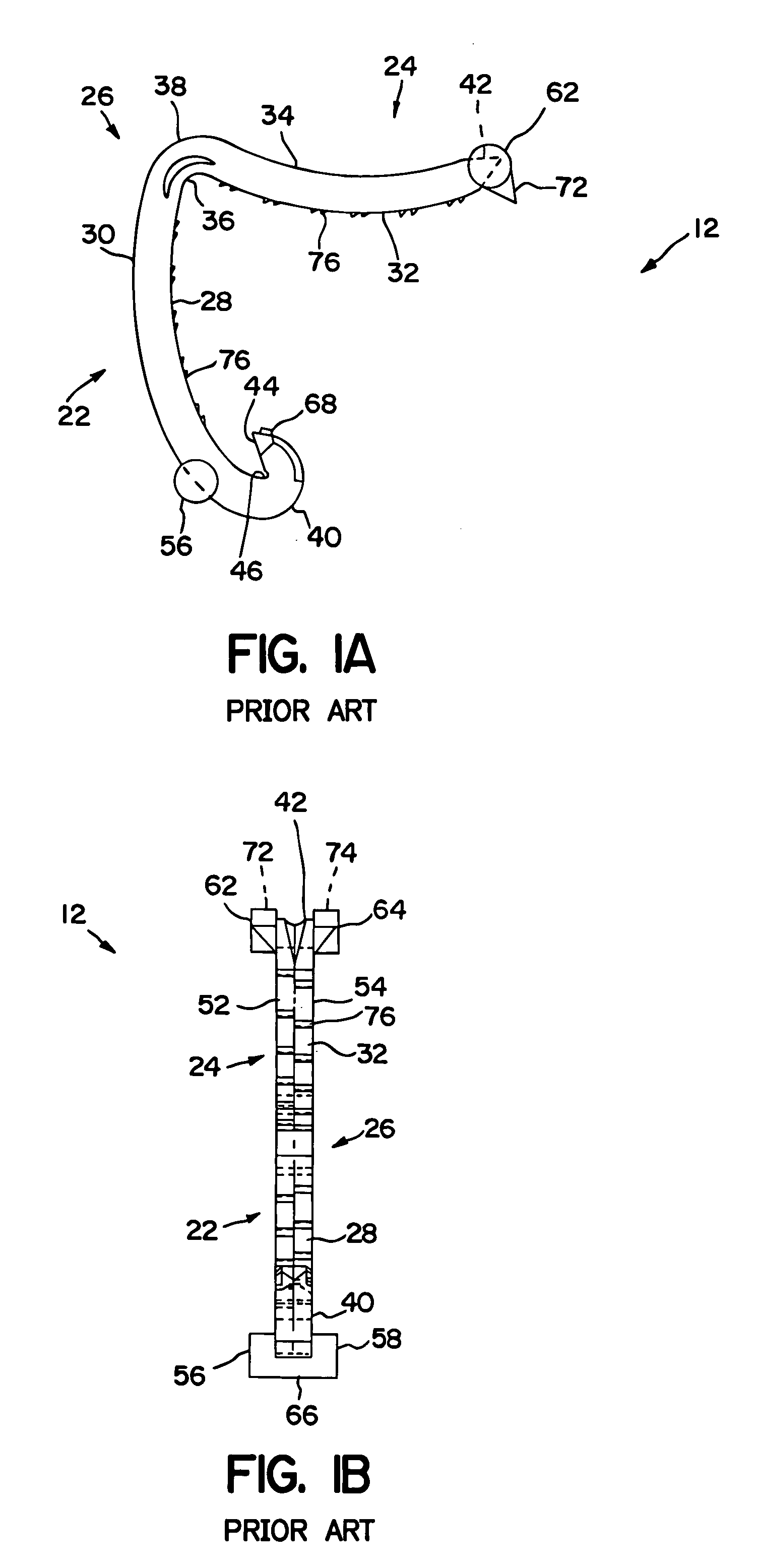
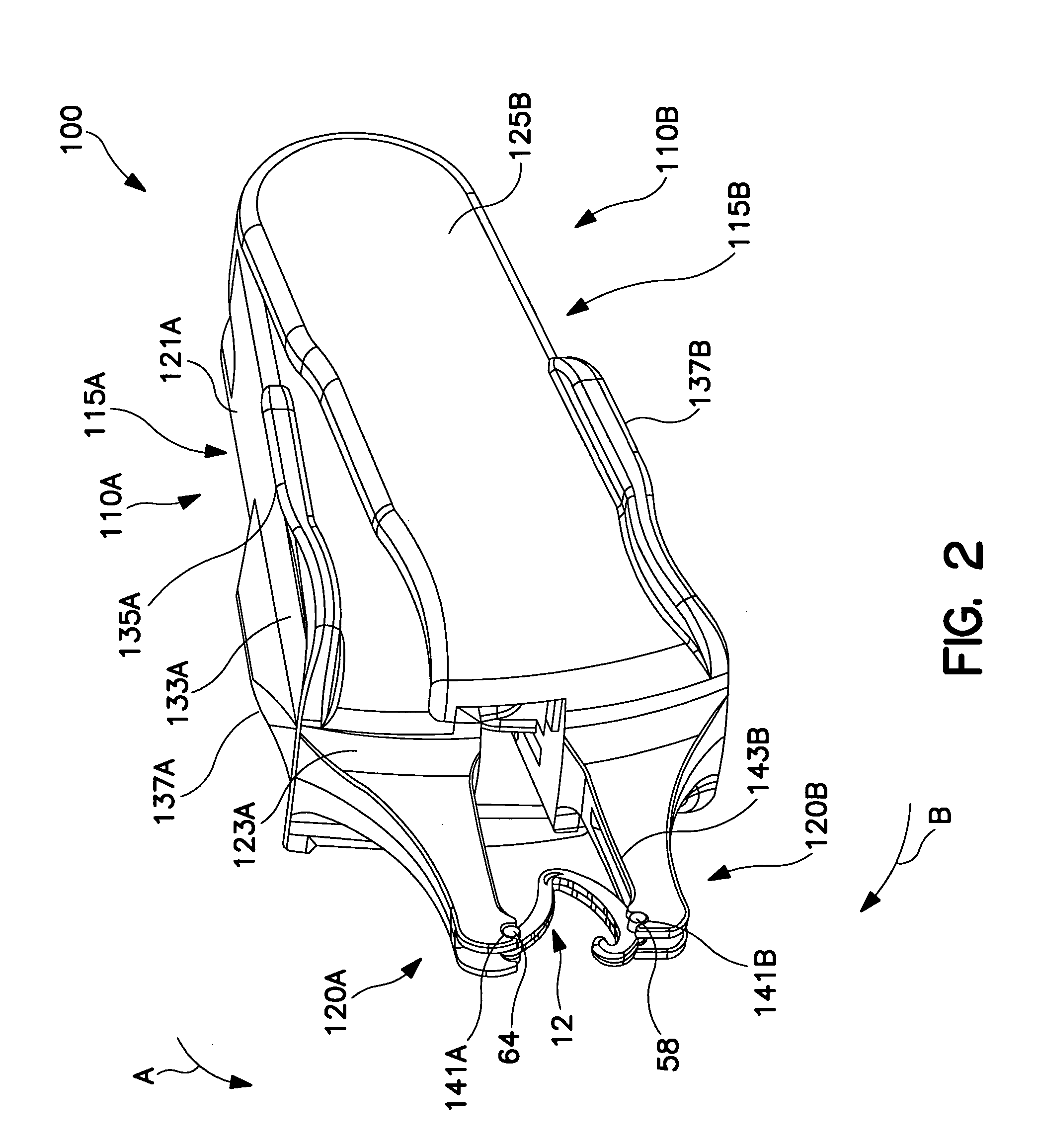
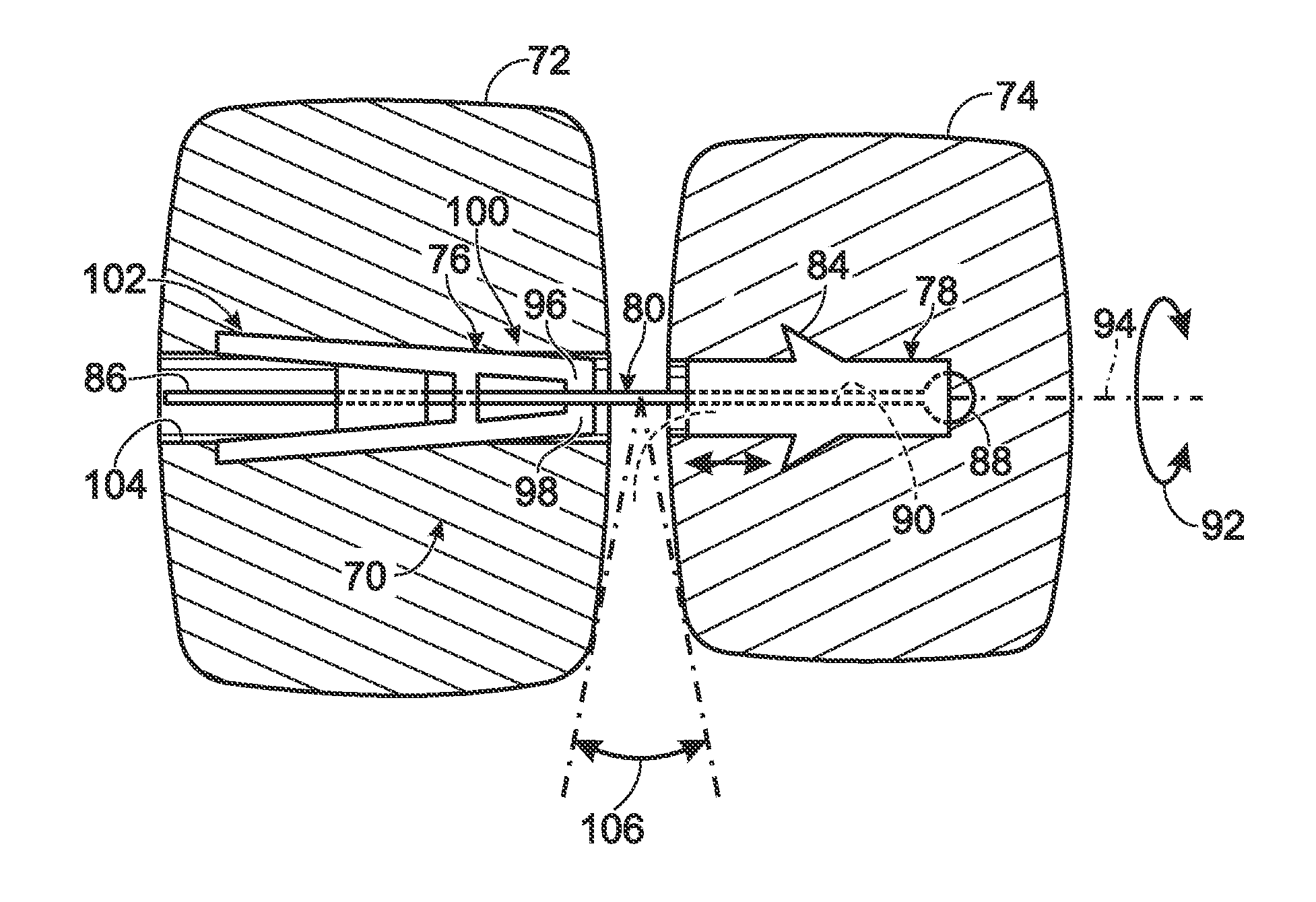
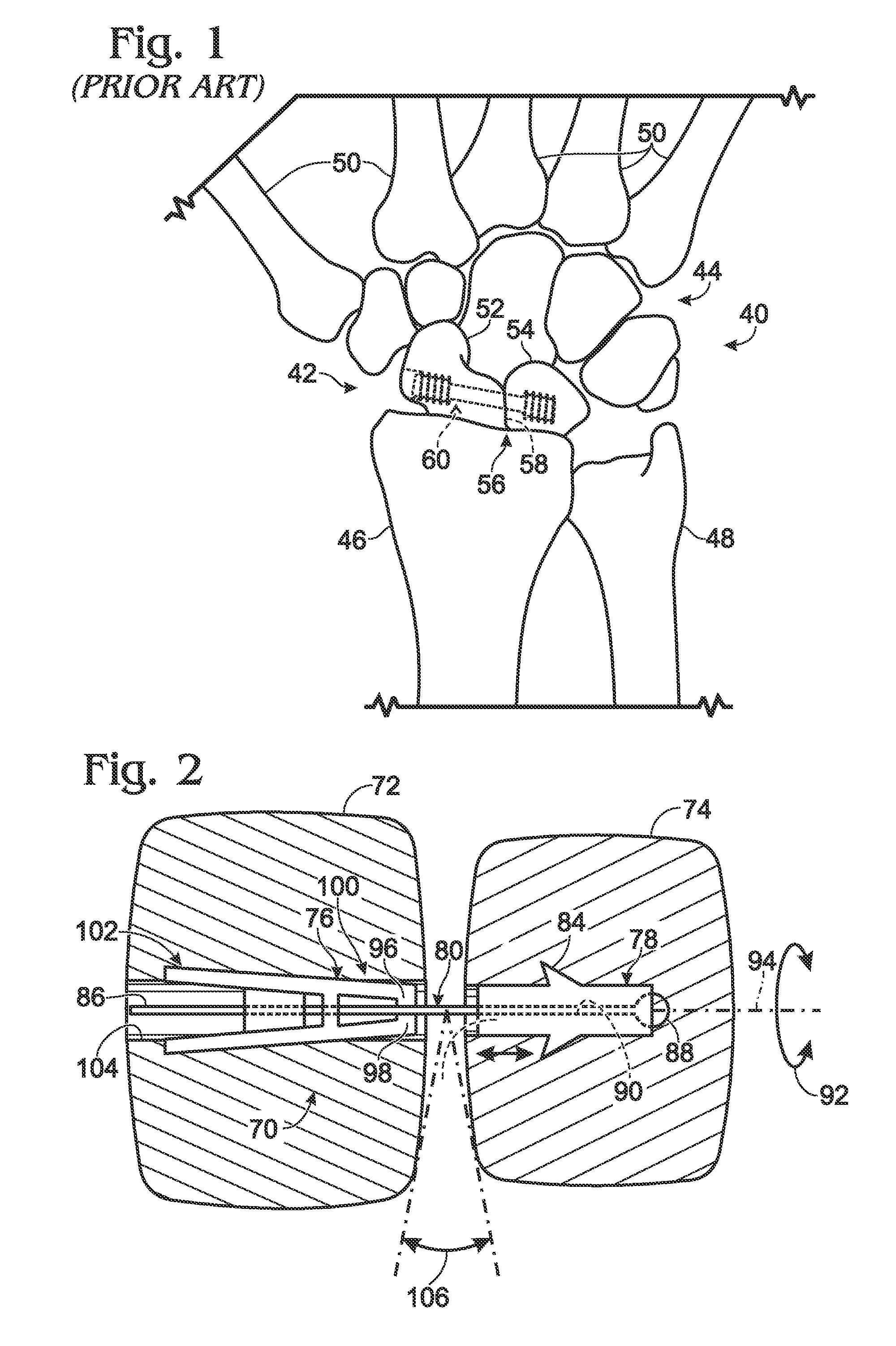
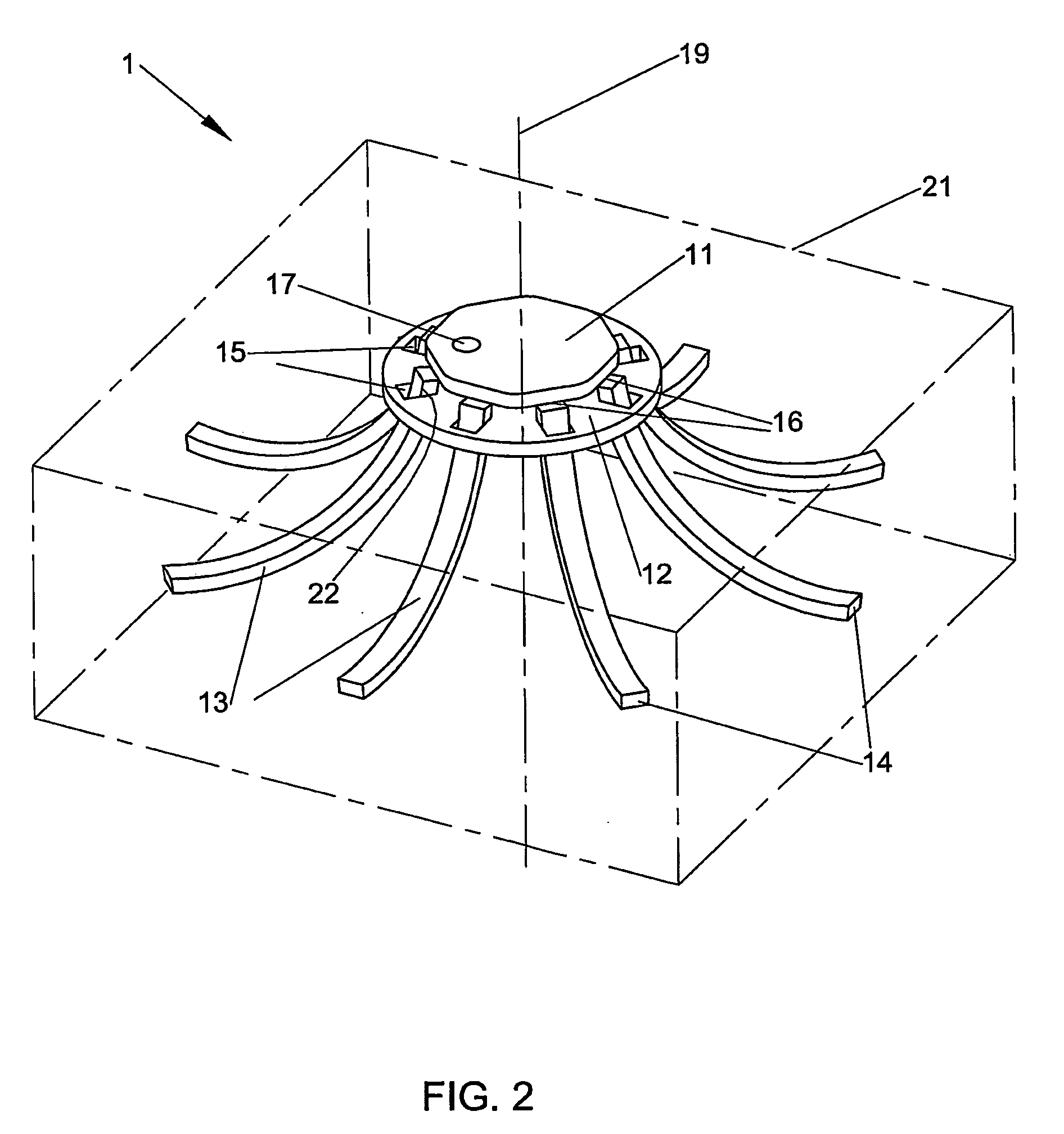
Fingertip-actuated surgical clip applier and related methods
A fingertip-actuated surgical clip applier includes a first body and a second body substantially structurally identical to the first body. The first body includes a main section and a first jaw extending in a distal direction from the main section. The main section includes a hinge region and a first longitudinal wall extending between the first jaw and the hinge region. The first longitudinal wall includes a first outside surface adapted for contacting a first fingertip. The second body includes a second jaw and a second longitudinal wall. The second longitudinal wall includes a second outside surface adapted for contacting a second fingertip. The second body is inverted in relation to the first body and is pivotably connected to the hinge region. The first and second jaws are pivotable toward each other to a closed position and away from each other to an open position.
Owner:PILLING WECK INC
Binding domain-immunoglobulin fusion proteins
The invention relates to novel binding domain-immunoglobulin fusion proteins that feature a binding domain for a cognate structure such as an antigen, a counterreceptor or the like, a hinge region polypeptide having either zero or one cysteine residue, and immunoglobulin CH2 and CH3 domains, and that are capable of ADCC and / or CDC while occurring predominantly as monomeric polypeptides. The fusion proteins can be recombinantly produced at high expression levels. Also provided are related compositions and methods, including immunotherapeutic applications.
Owner:TRUBION PHARM INC
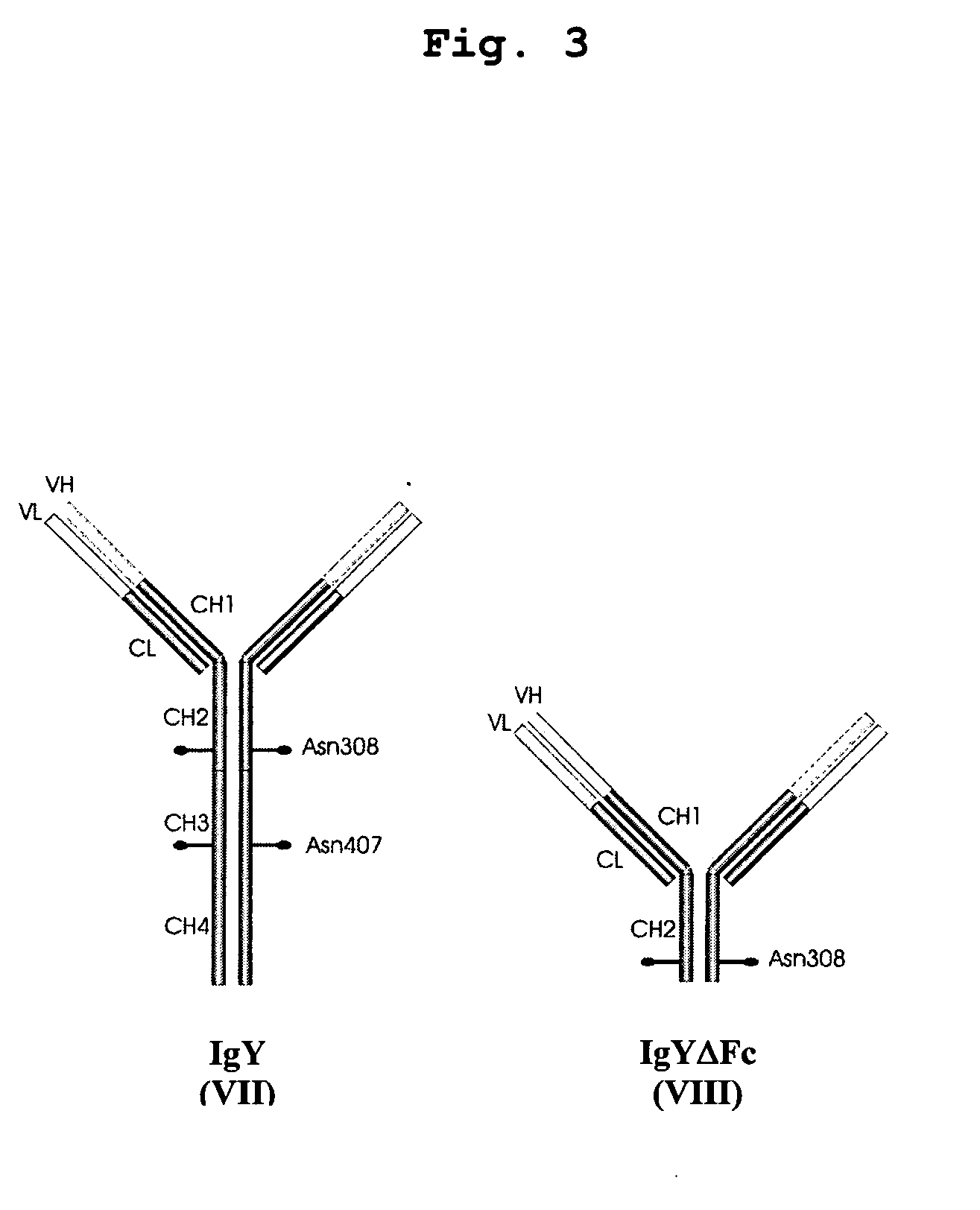
Bivalent IgY antibody constructs for diagnostic and therapeutic applications
InactiveUS20070141049A1Improve the immunityIncrease contentImmunoglobulins against virusesAntibody ingredientsHeavy chainMammal
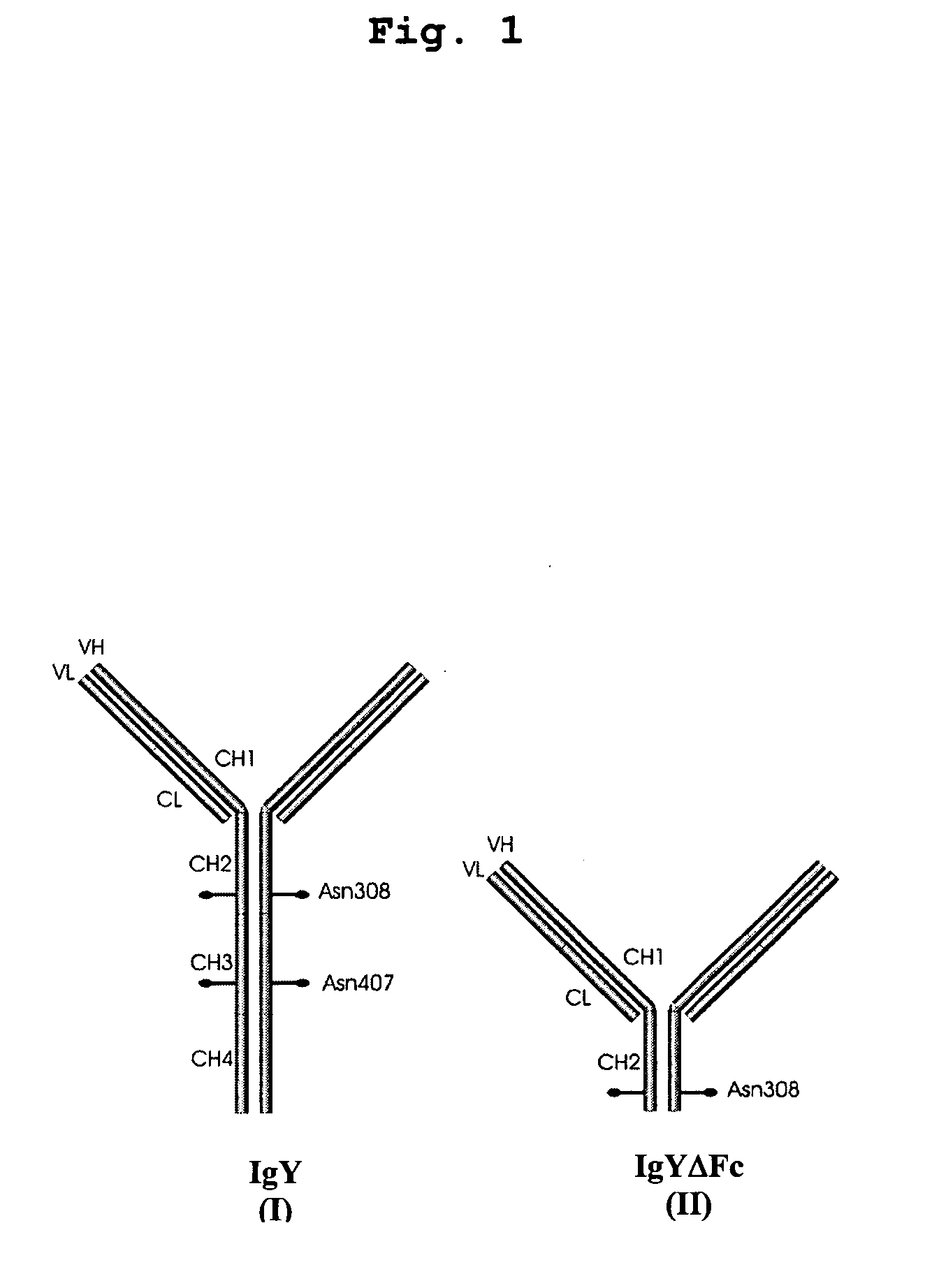
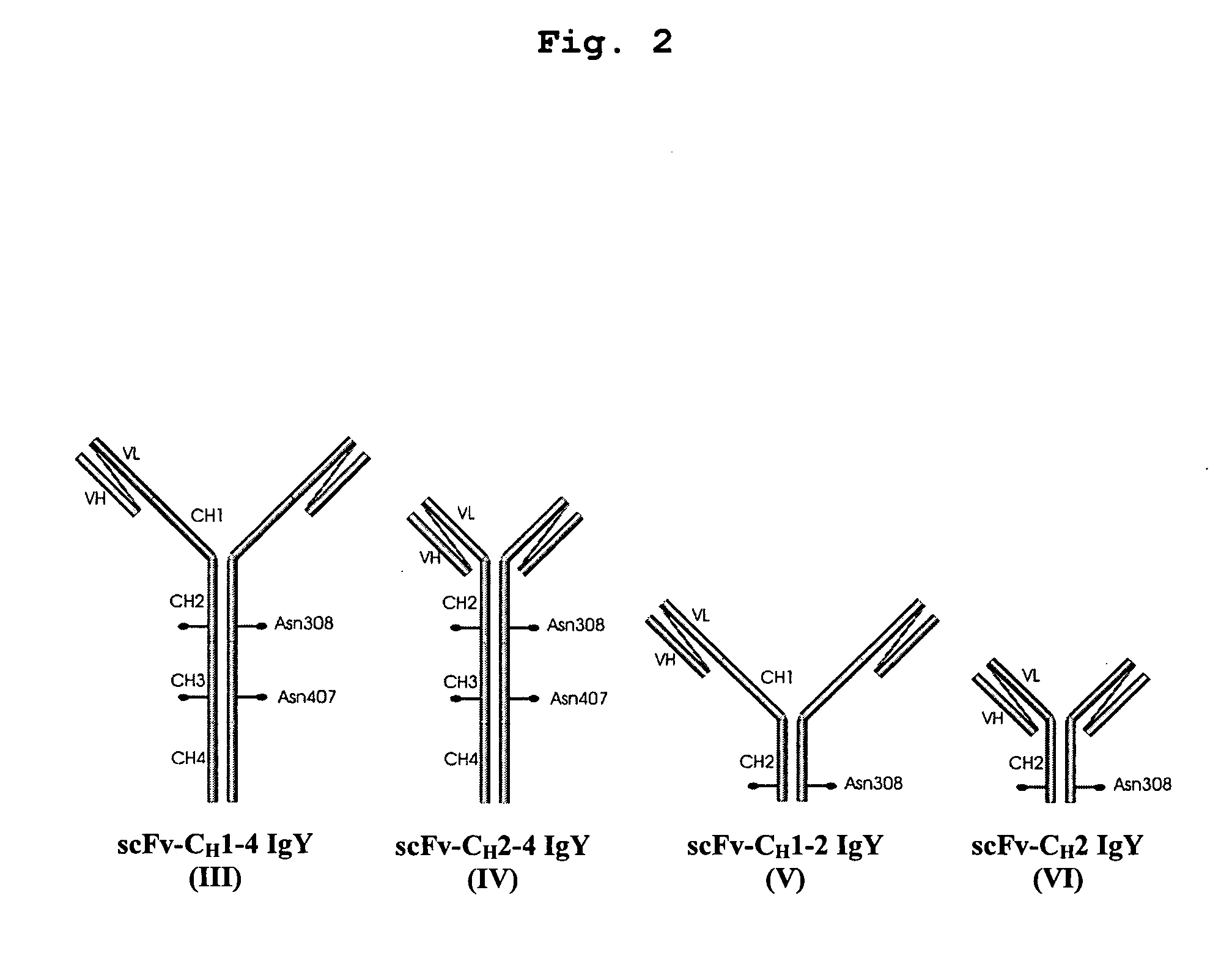
This invention relates to the field of recombinant antibody technology. It provides novel recombinant IgY antibody constructs for diagnostic and therapeutical applications. The bivalent antibody constructs display a heterotetrameric or homodimeric format stabilized by disulfide bonds. The constant heavy chain domains CH2-CH4 are partly or completely of avian origin, whereas the VH, VL, CL, and CH1 domains as well as the hinge region may be of avian origin or derived from any other species. The invention allows to combine the advantages of IgY antibodies with those of established mammalian monoclonal antibodies. IgY antibody constructs comprising nonglycosylated IgY constant heavy chain domains allow to reduce unwanted interactions with C-type lectins, e.g., in human sera. Furthermore, chimeric IgY antibody containing mammalian VH, VL, CL, and CH1 domains as well as a mammalian hinge region provide a higher molecular stability than IgY antibodies in acidic conditions and, thereby, are especially suited for peroral therapeutic applications.
Owner:PLS DESIGN
Modified Antibody Constant Region
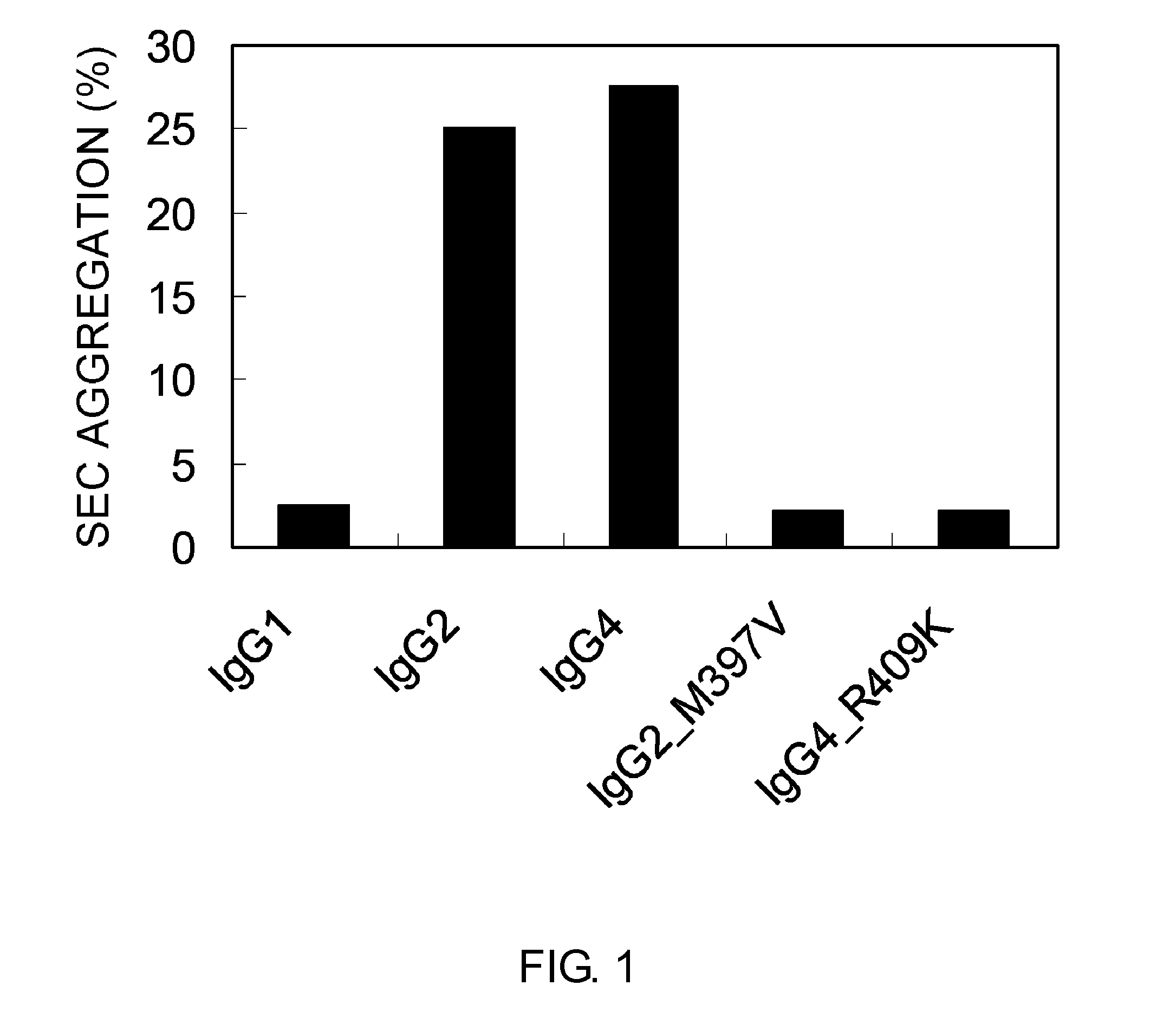
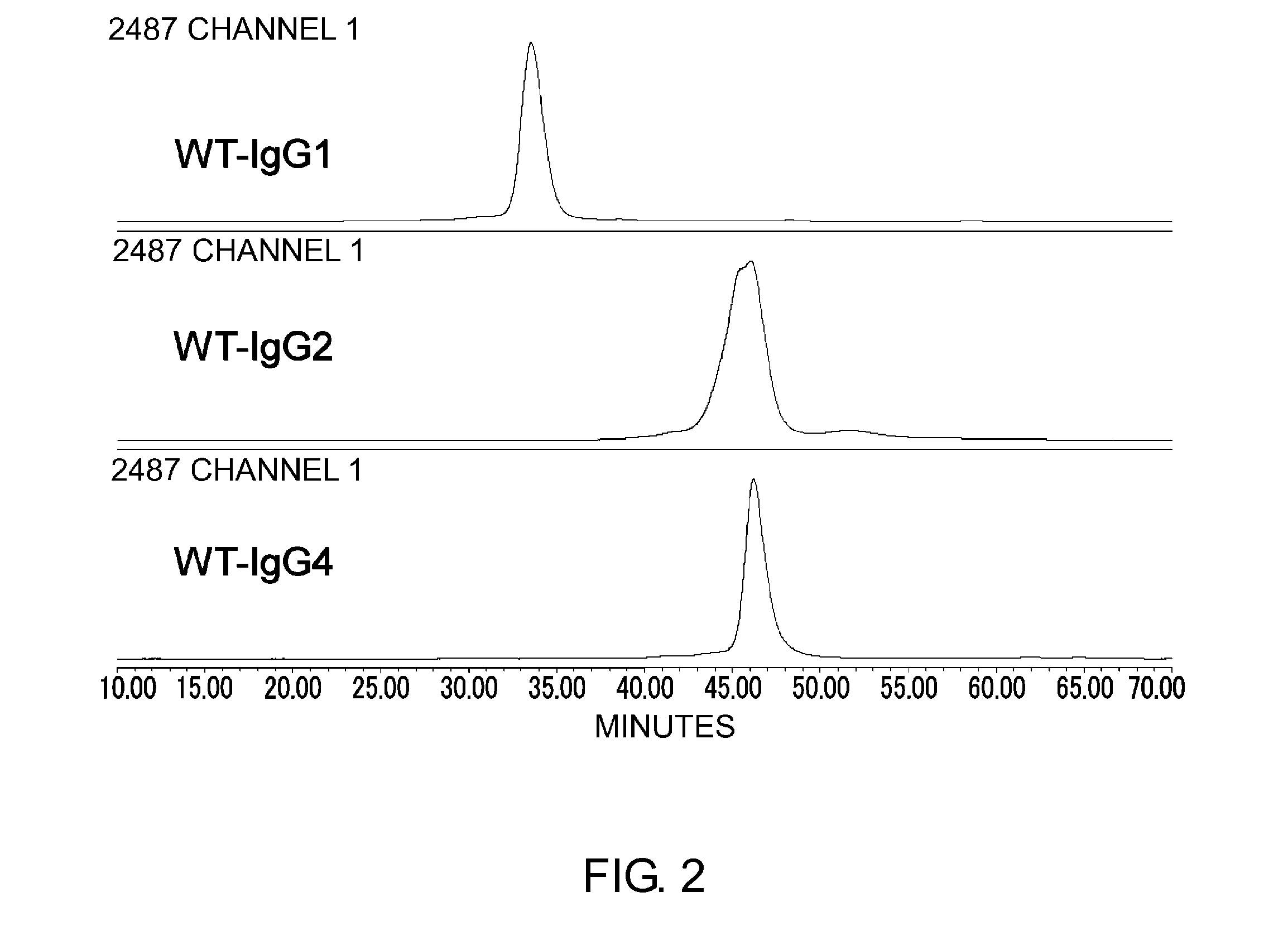
ActiveUS20100298542A1Improving immunogenicityImprove propertiesAntipyreticAnalgesicsHigh concentrationHinge region
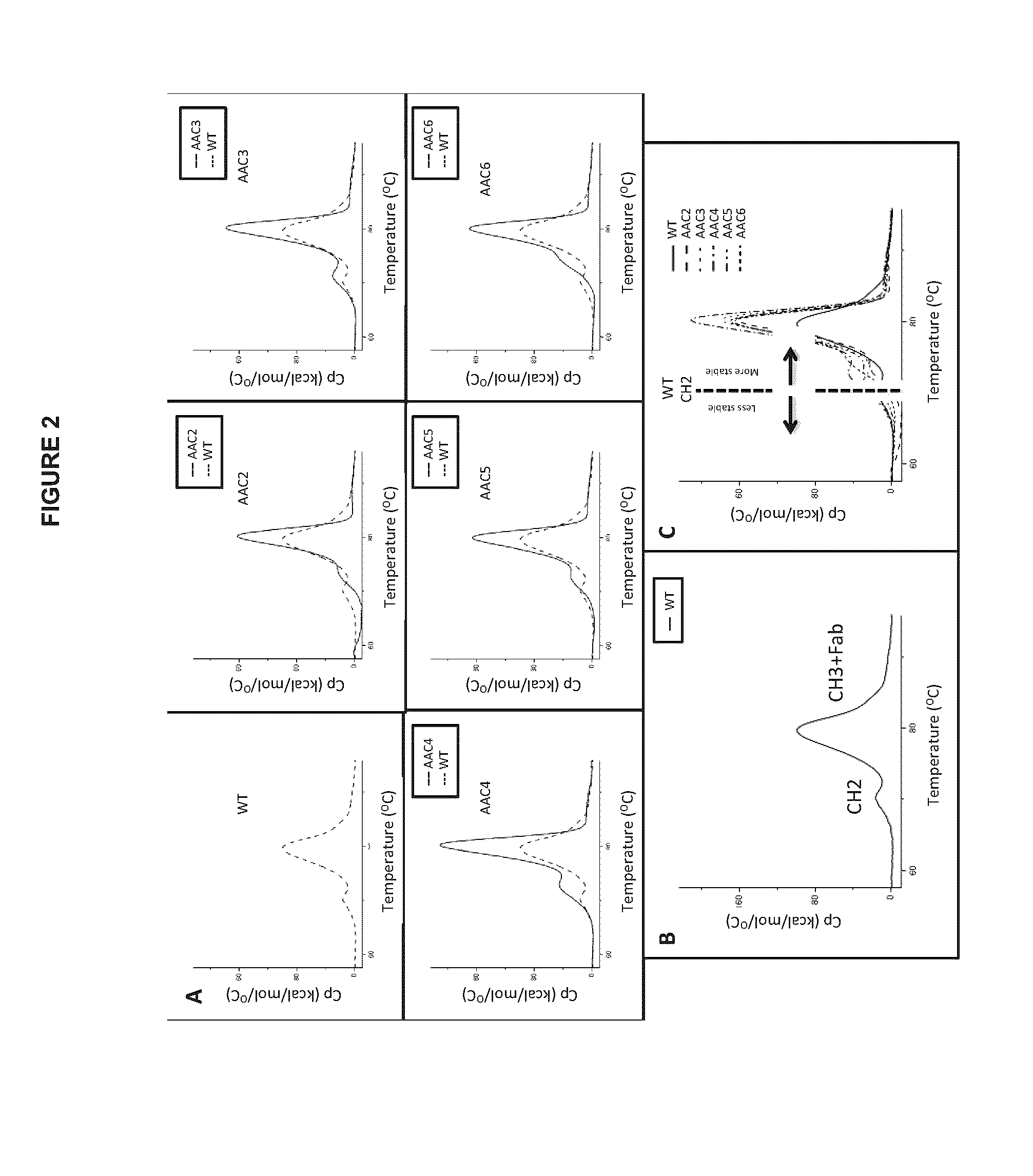
The present inventors succeeded in improving the antibody constant region to have increased stability under acid conditions, reduced heterogeneity originated from disulfide bonds in the hinge region, reduced heterogeneity originated from the H chain C terminus, and increased stability at high concentrations as well as in discovering novel constant region sequences having reduced Fcγ receptor-binding, while minimizing the generation of novel T-cell epitope peptides. As a result, the present inventors successfully discovered antibody constant regions with improved physicochemical properties (stability and homogeneity), immunogenicity, safety, and pharmacokinetics.
Owner:CHUGAI PHARMA CO LTD
Orthopedic connector system
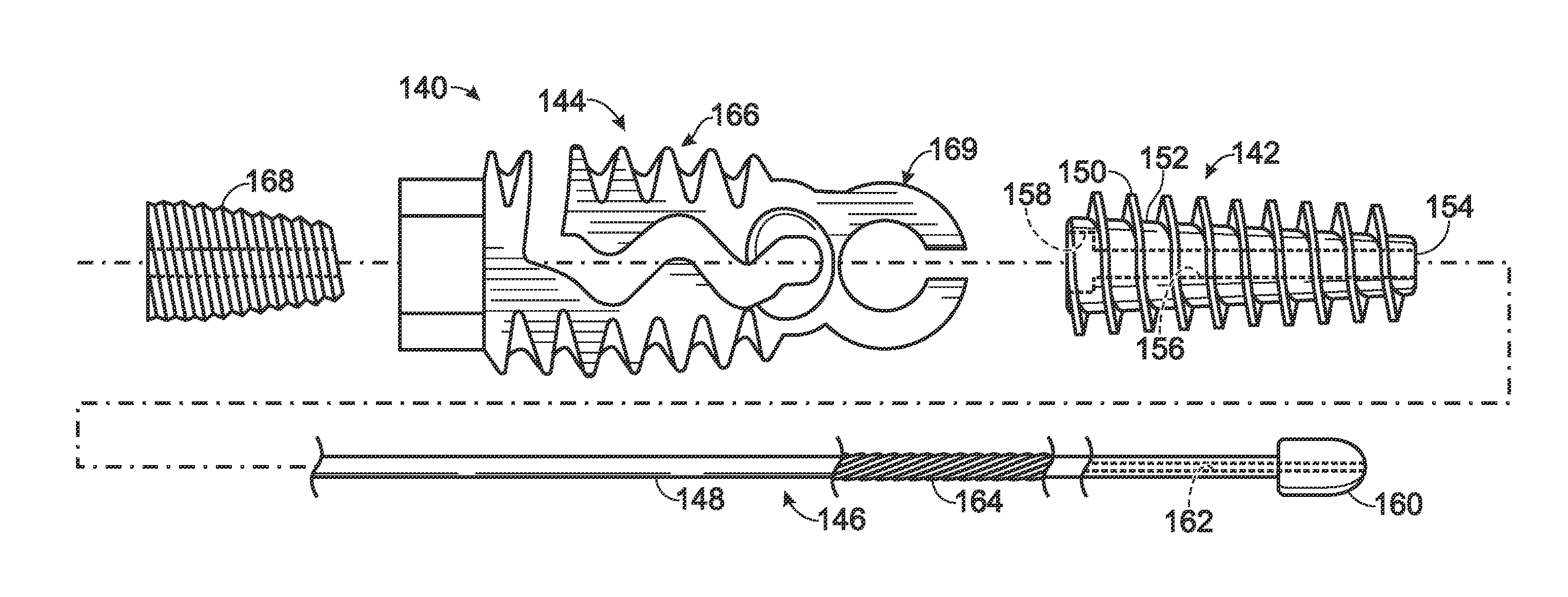
System, including methods, apparatus and components thereof, and kits, for connecting to bone using an anchor element configured to be anchored in bone and having a selectively bendable hinge region. In some embodiments, the system may connect the anchor element to connective tissue, a bridge member, and / or to another anchor element by bending the hinge region while the anchor element is disposed in bone, thereby connecting bone to the connective tissue or bridge member, and / or connecting bone members.
Owner:ACUMED
Anti-IL-6 Receptor Antibody
InactiveUS20110245473A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
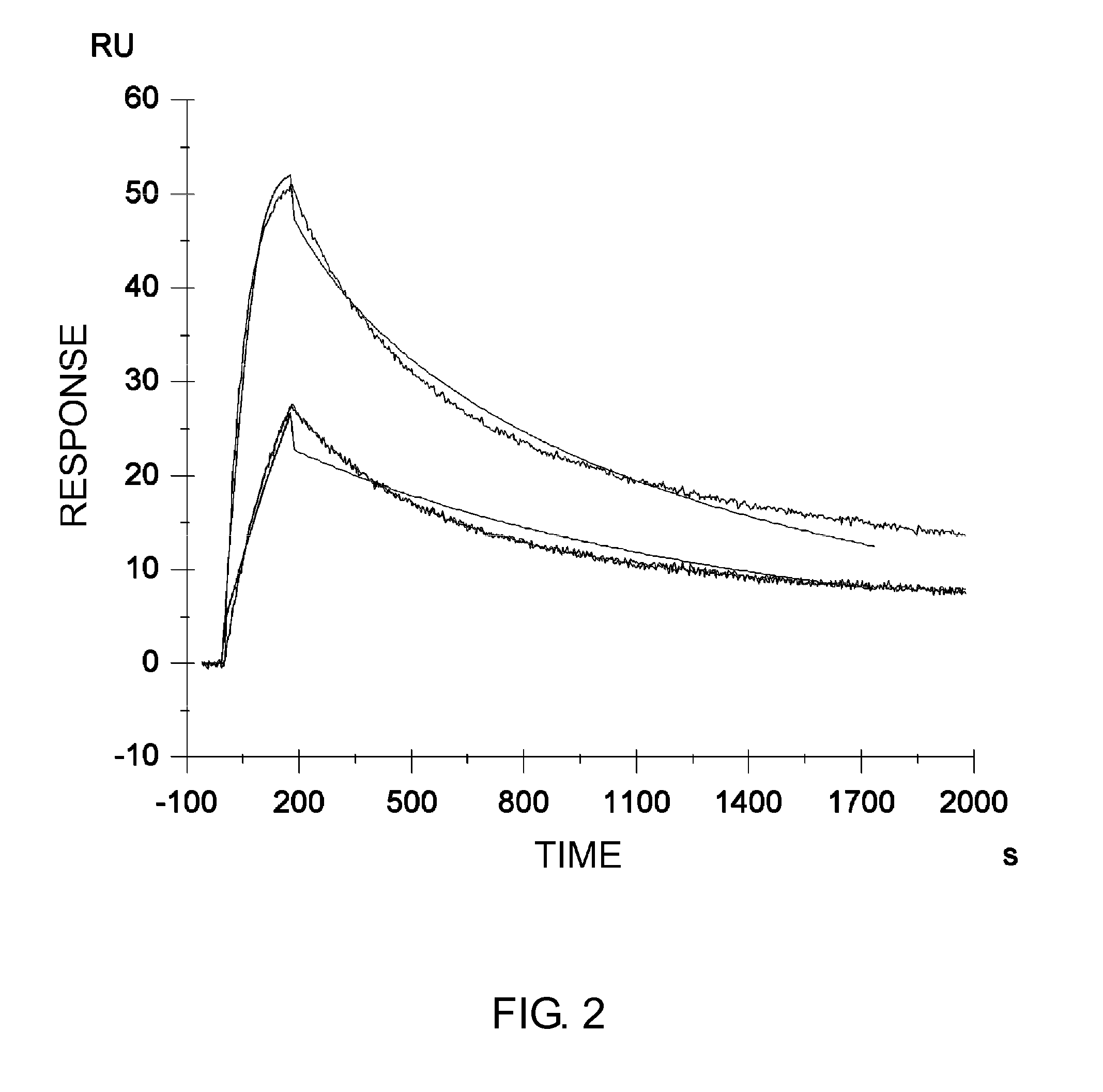
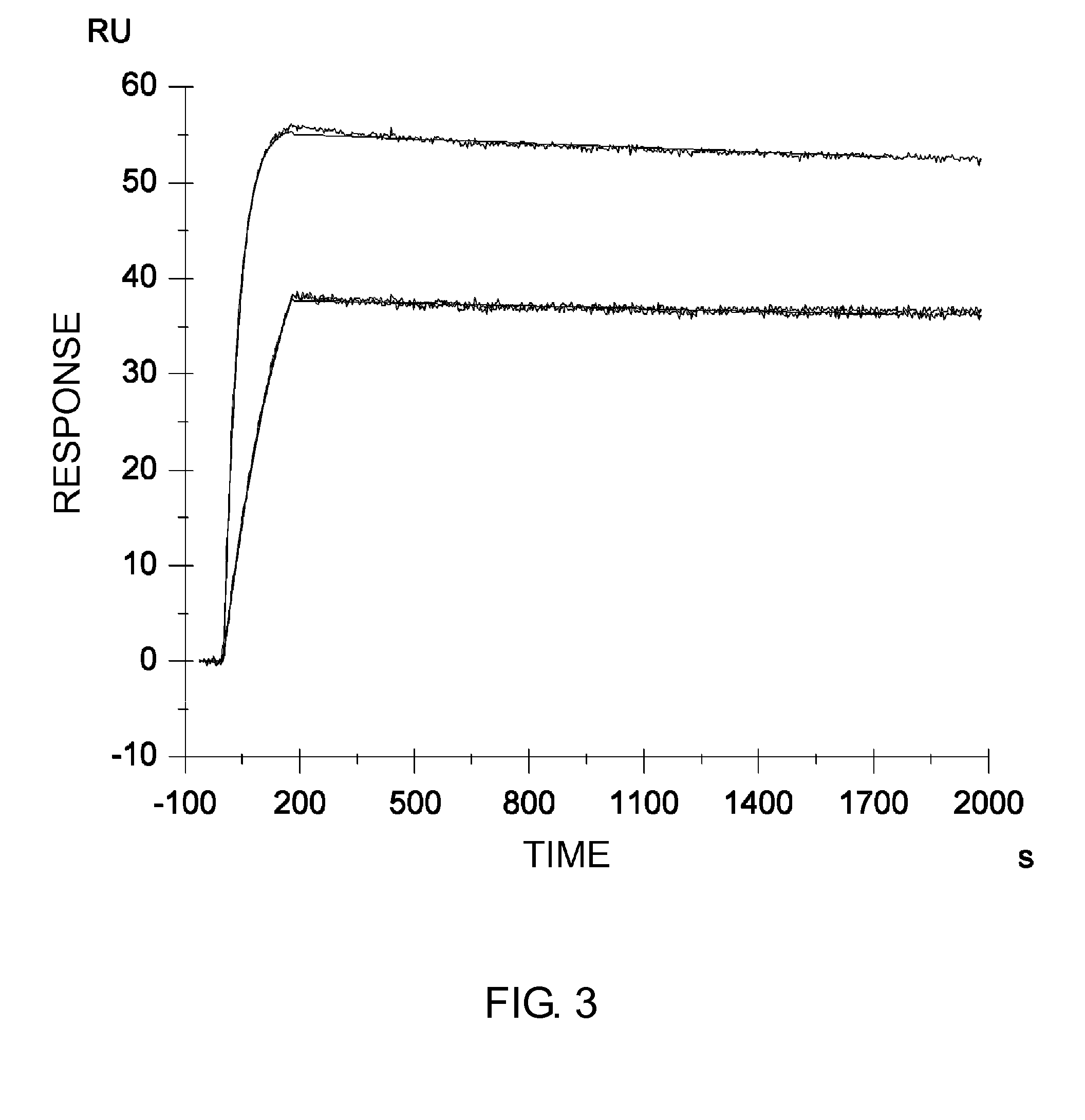
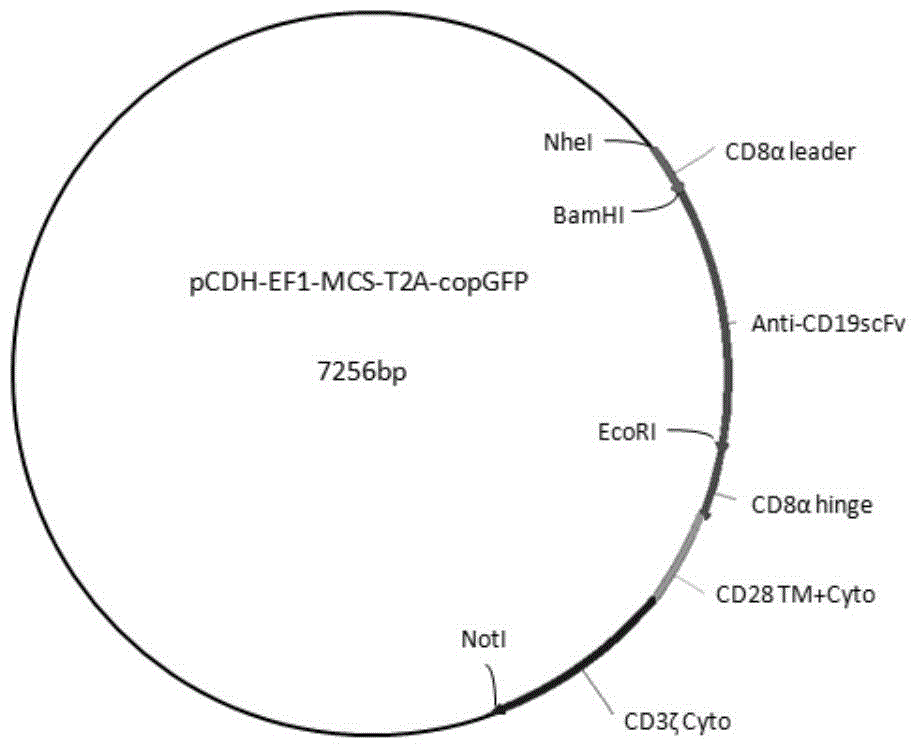
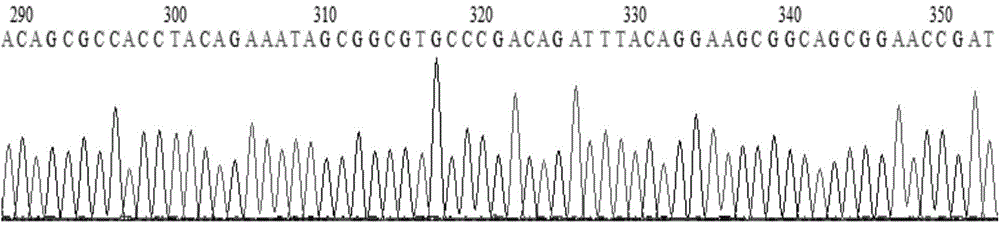
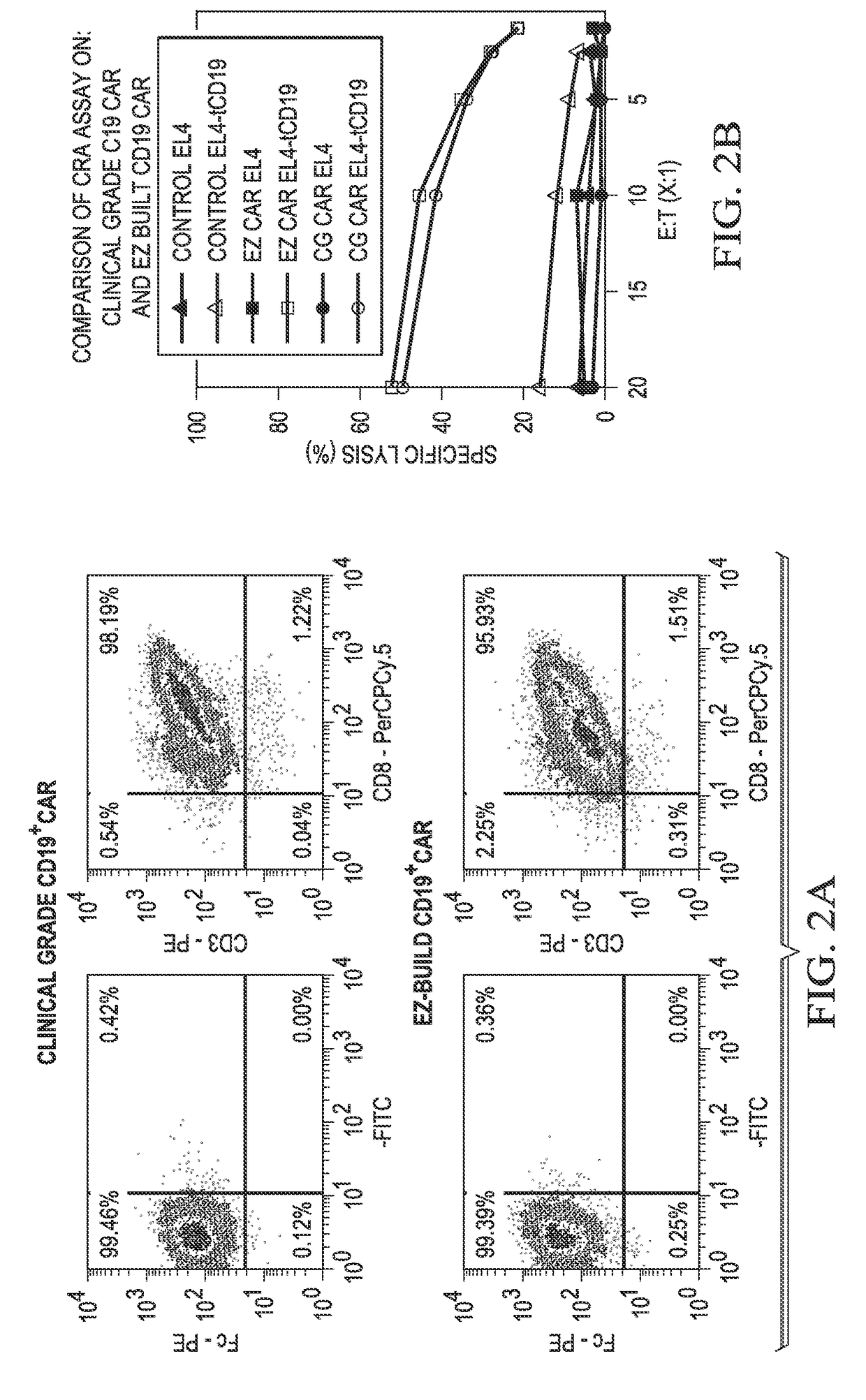
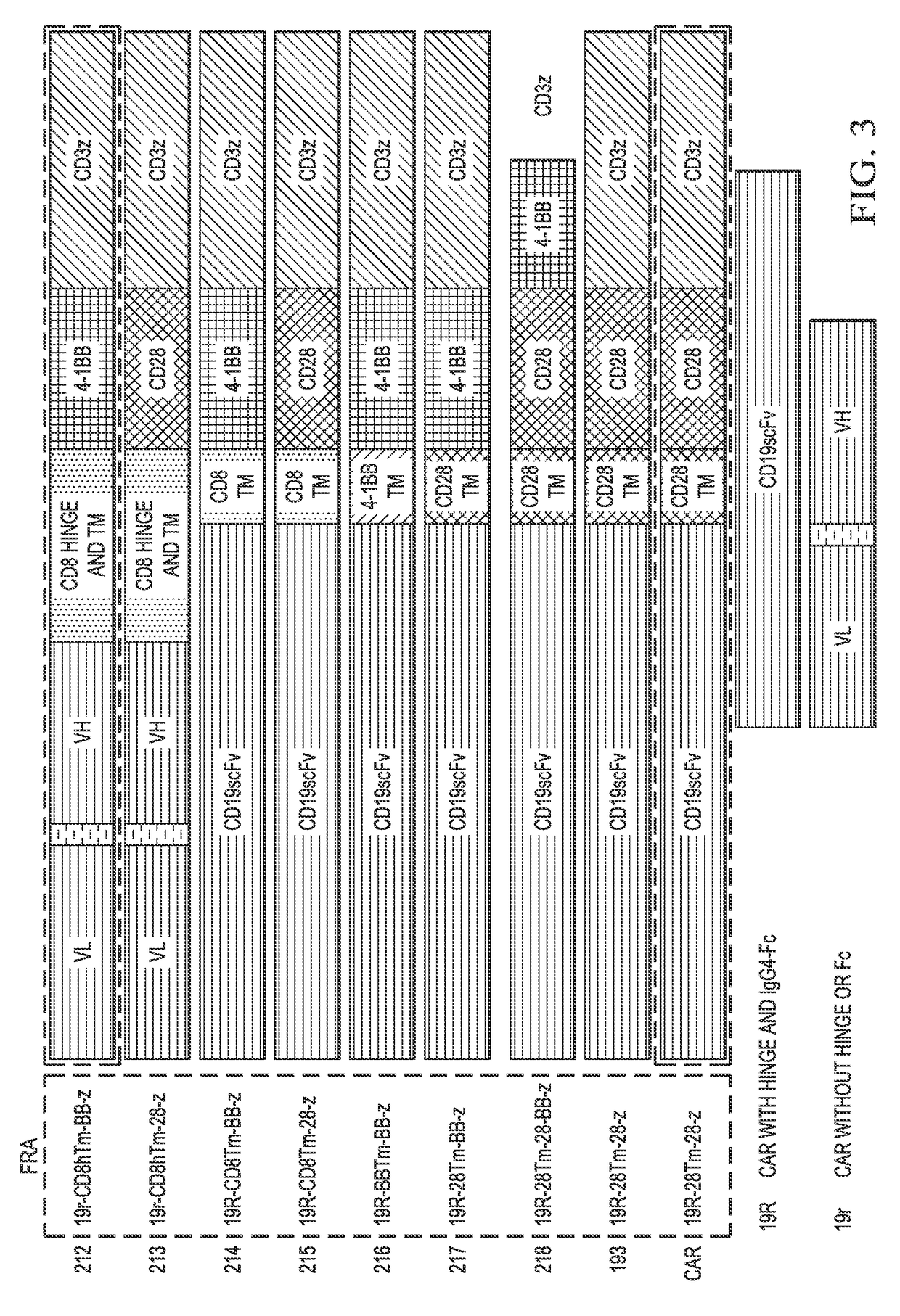
Chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof
ActiveCN104788573AConfirmed specific killing effectMammal material medical ingredientsHybrid peptidesAntigen receptorHeavy chain
The invention discloses a chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof. The chimeric antigen receptor is serially connected by an anti-human CD19 monoclonal antibody H119a light chain and heavy chain variable region (hCD19scFv), human CD8a hinge region, a human CD28 transmembrane region, an intracellular region and a human CD3zata intracellular region. The chimeric antigen receptor is used for modifying T lymphocyte, and the modified T lymphocyte (CAR-T cell) can be used for treating the tumor with positive surface CD19.
Owner:JUVENTAS CELL THERAPY LTD
Cd123-specific chimeric antigen receptor redirected t cells and methods of their use
ActiveUS20140271582A1BiocideAntibody mimetics/scaffoldsCD19-specific chimeric antigen receptorT-Cell Specificity
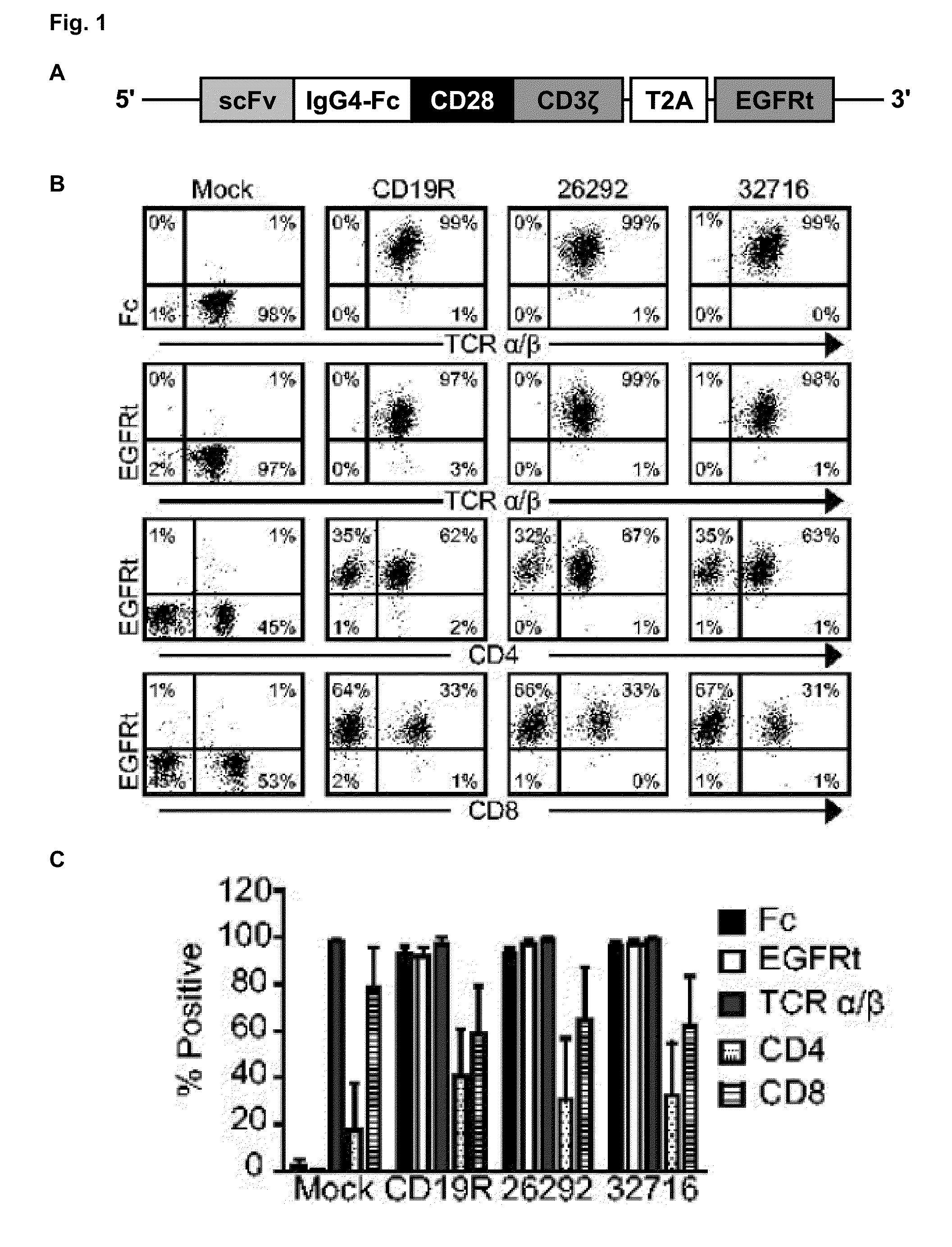
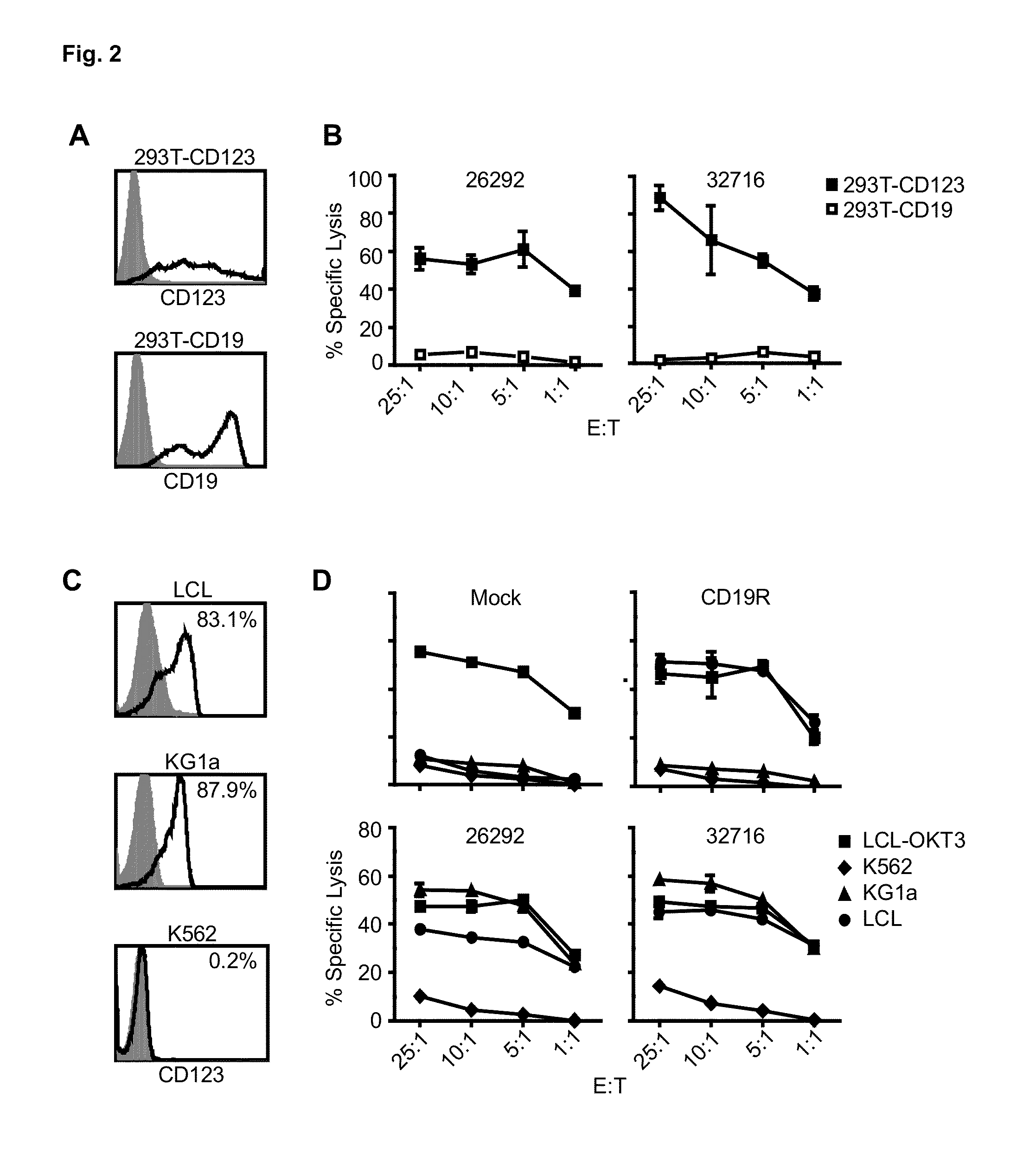
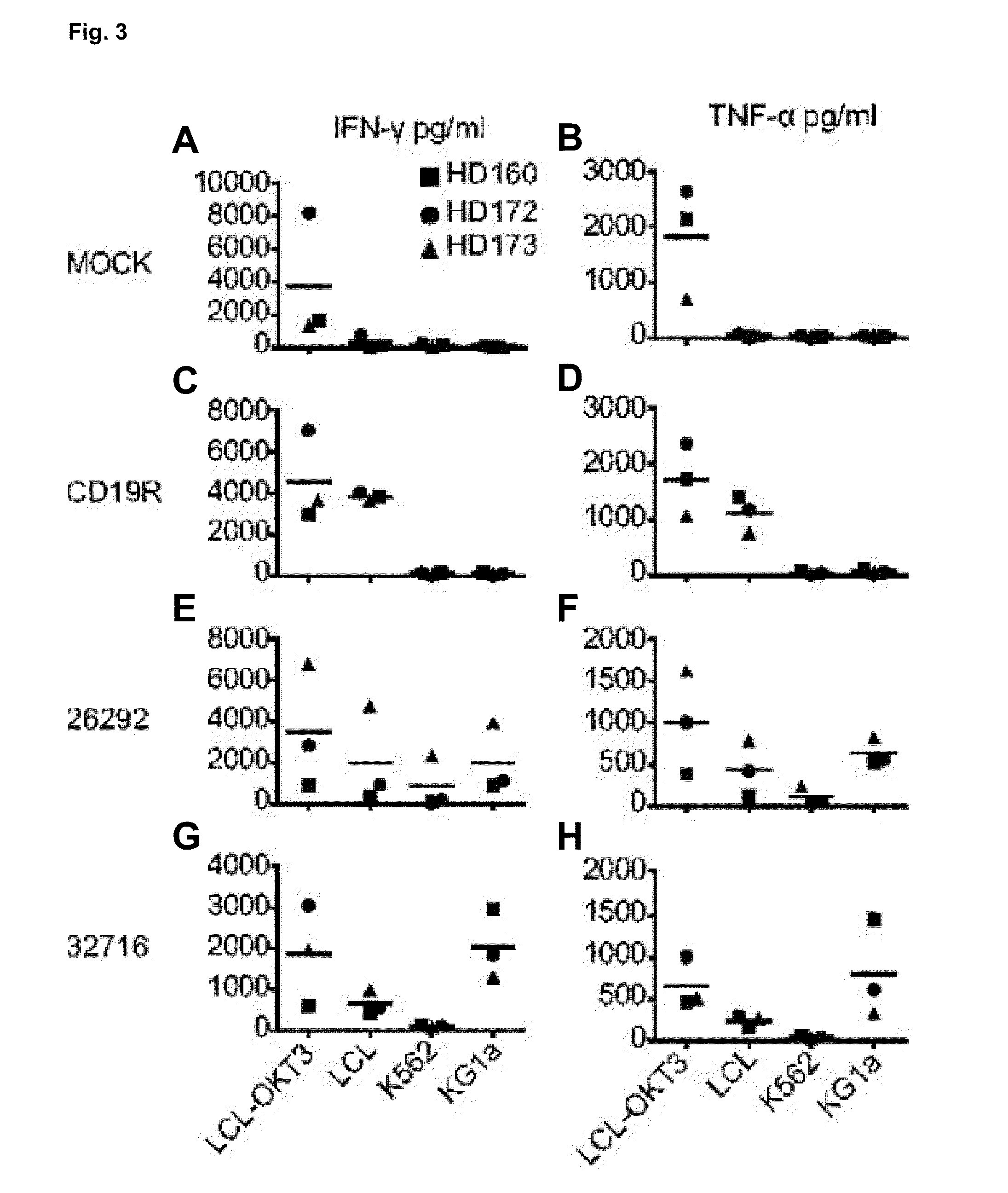
A family of chimeric antigen receptors (CARs) containing a CD123 specific scFv was developed to target different epitopes on CD123. In some embodiments, such a CD123 chimeric antigen receptor (CD123CAR) gene includes an anti-CD123 scFv region fused in frame to a modified IgG4 hinge region comprising an S228P substitution, an L235E substitution, and optionally an N297Q substitution; a costimulatory signaling domain; and a T cell receptor (TCR) zeta chain signaling domain. When expressed in healthy donor T cells (CD4 / CD8), the CD123CARs redirect T cell specificity and mediated potent effector activity against CD123+ cell lines as well as primary AML patient samples. Further, T cells obtained from patients with active AML can be modified to express CD123CAR genes and are able to lyse autologous AML blasts in vitro. Finally, a single dose of 5.0×106 CAR123 T cells results in significantly delayed leukemic progression in mice. These results suggest that CD123CAR-transduced T cells may be used as an immunotherapy for the treatment of high risk AML.
Owner:CITY OF HOPE
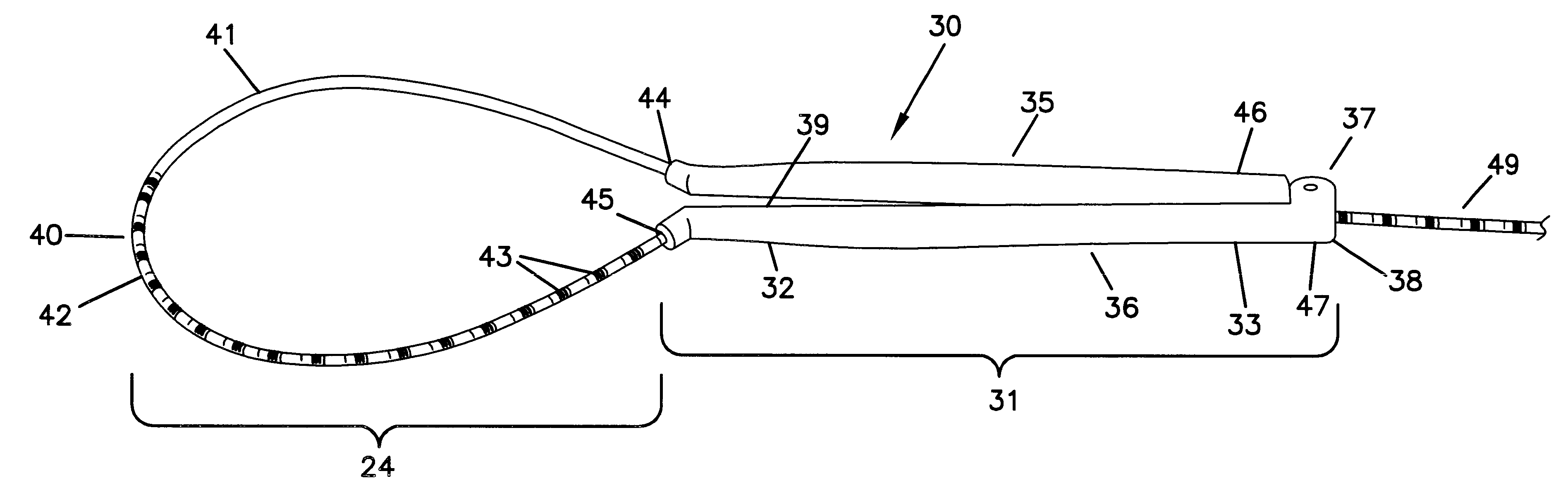
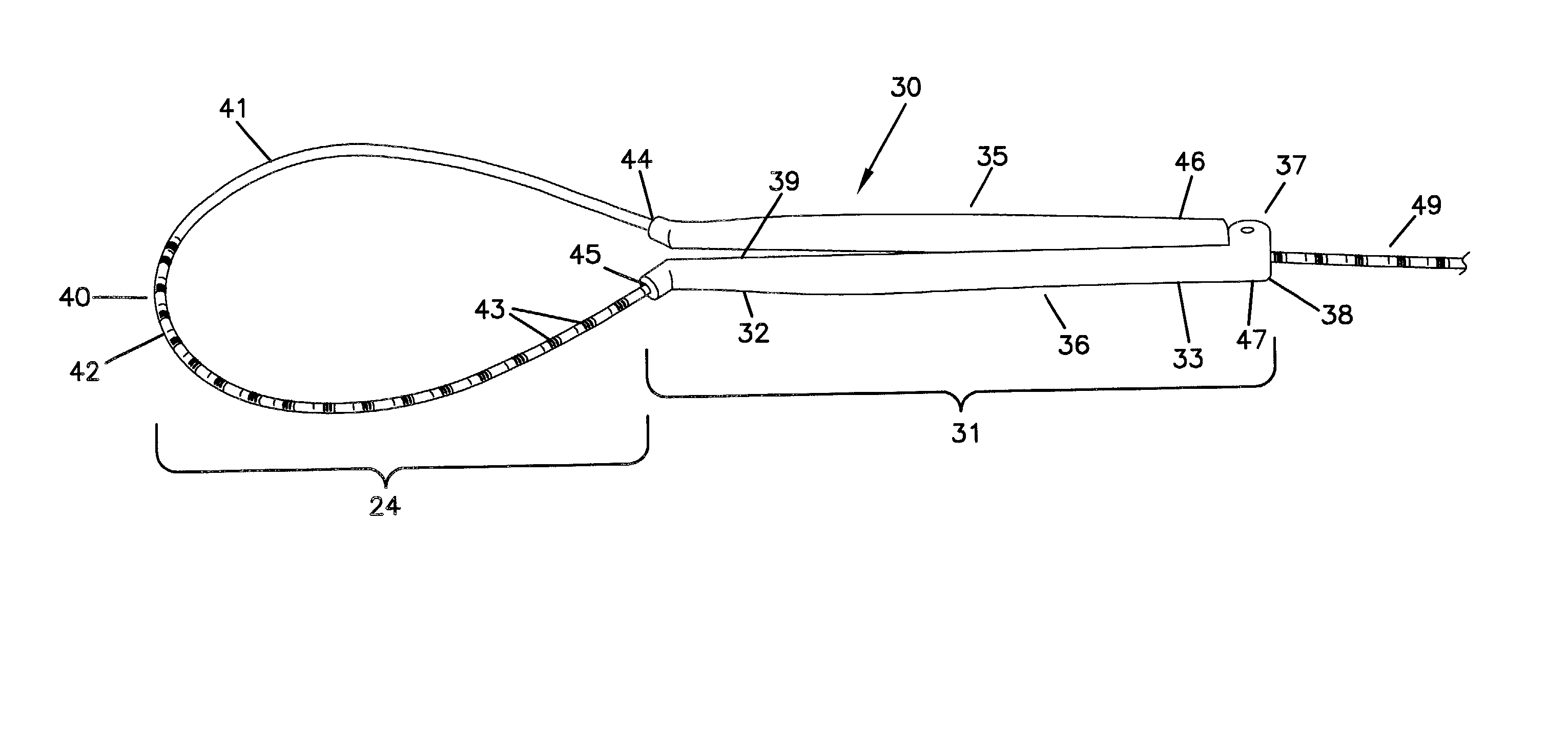
Device for heart measurement
A surgical tool for measuring a heart that includes a first and second handle member having proximal and distal ends and internal passageways that run lengthwise from the proximal end to the distal end, a flexible member that is cylindrically shaped with a marked proximal end that is larger in diameter than the unmarked distal end, a hinged region with a connection receiving portion on the first handle member and a connection portion on the second handle member, wherein the distal end of the flexible member passes through the internal passageway of the first handle member from the proximal end of the first handle member to the distal end of the first handle member, extends out, then enters into the distal end of the second handle member, passes through the internal passageway of the second handle member from the proximal end to the distal end of the second handle member.
Owner:MARDIL
Mutants of anti-cd40 antibody
ActiveUS20070148163A1High therapeutic effectImmunoglobulins against bacteriaImmunoglobulins against virusesHinge regionMutant
A mutant of a potentially therapeutic anti-CD40 antibody is provided which mutant has reduced ADCC and CDC activities designed to be optimized as a pharmaceutical agent. A mutant of an agonistic anti-CD40 antibody, comprising mutation and / or substitution of at least one amino acid in the constant region to reduce the ADCC and / or CDC activities therein, and a mutant of an antagonistic anti-CD40 antibody, comprising at least one mutation or substitution in the constant region to reduce the ADCC and / or CDC activities therein, both mutants having at least a hinge region derived from a human IgG2.
Owner:KYOWA HAKKO KIRIN CO LTD
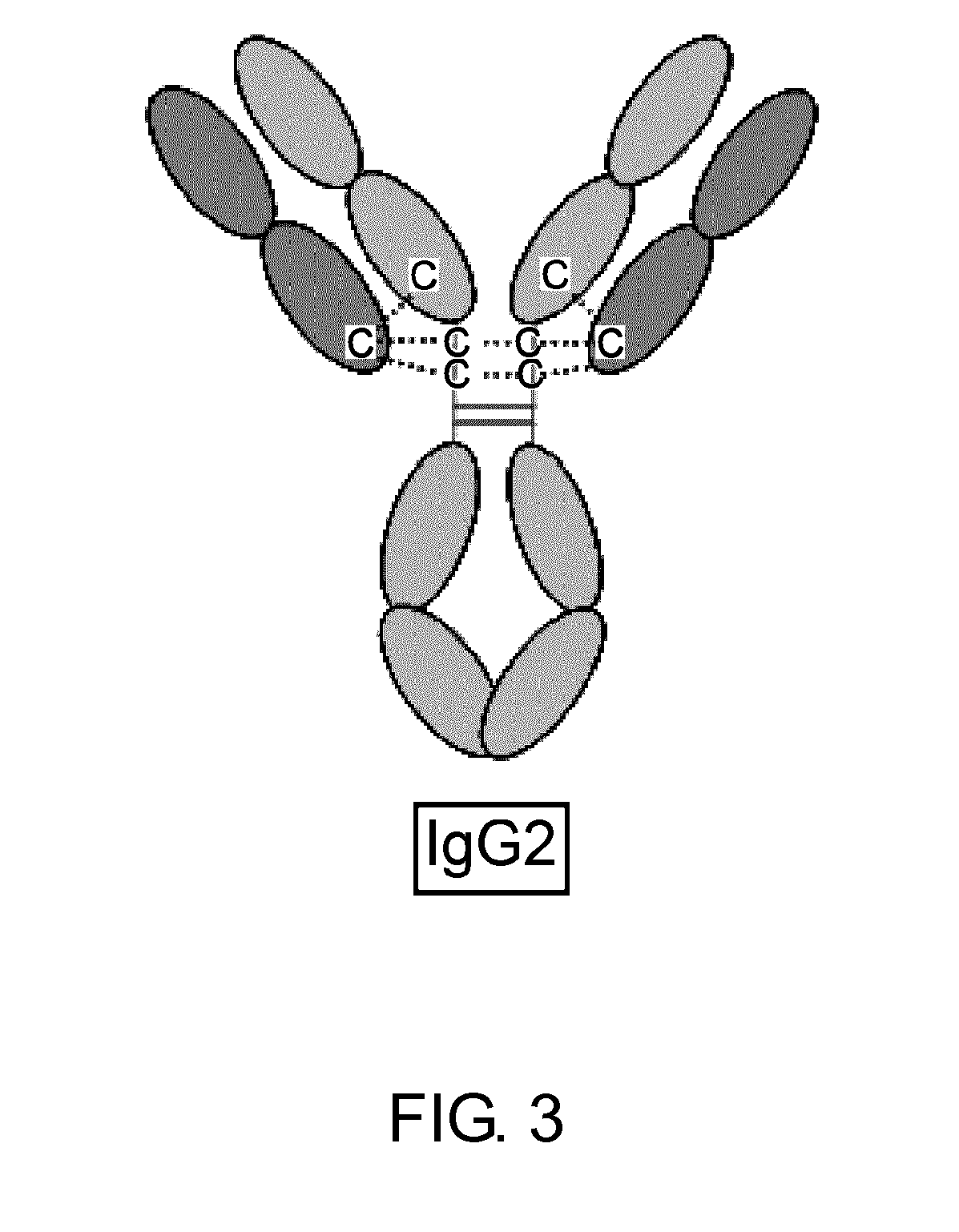
Homogeneous Antibody Populations
InactiveUS20100226925A1Efficient and economic productionImprove propertiesNervous disorderAntipyreticMonoclonal antibodyHinge region
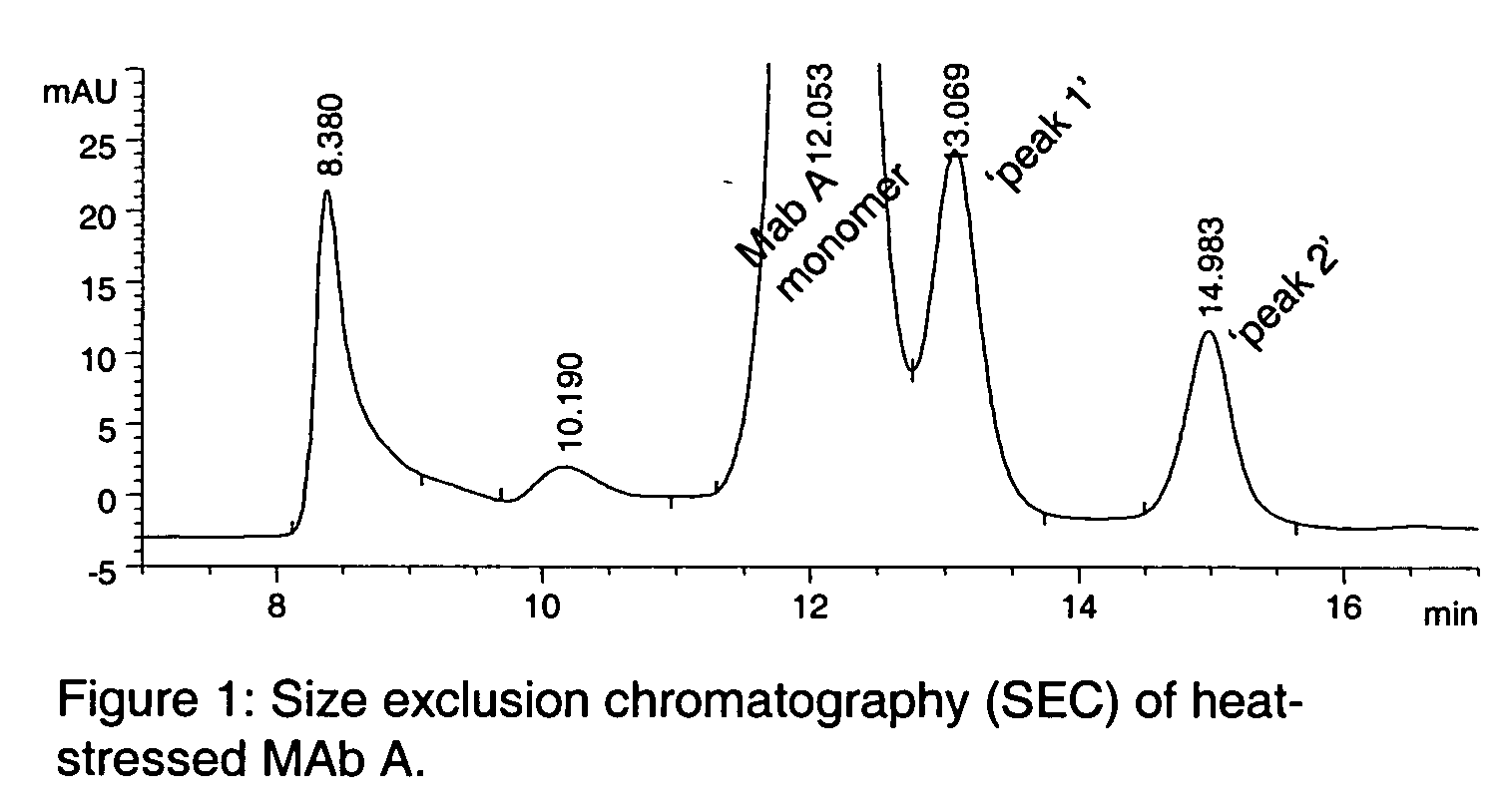
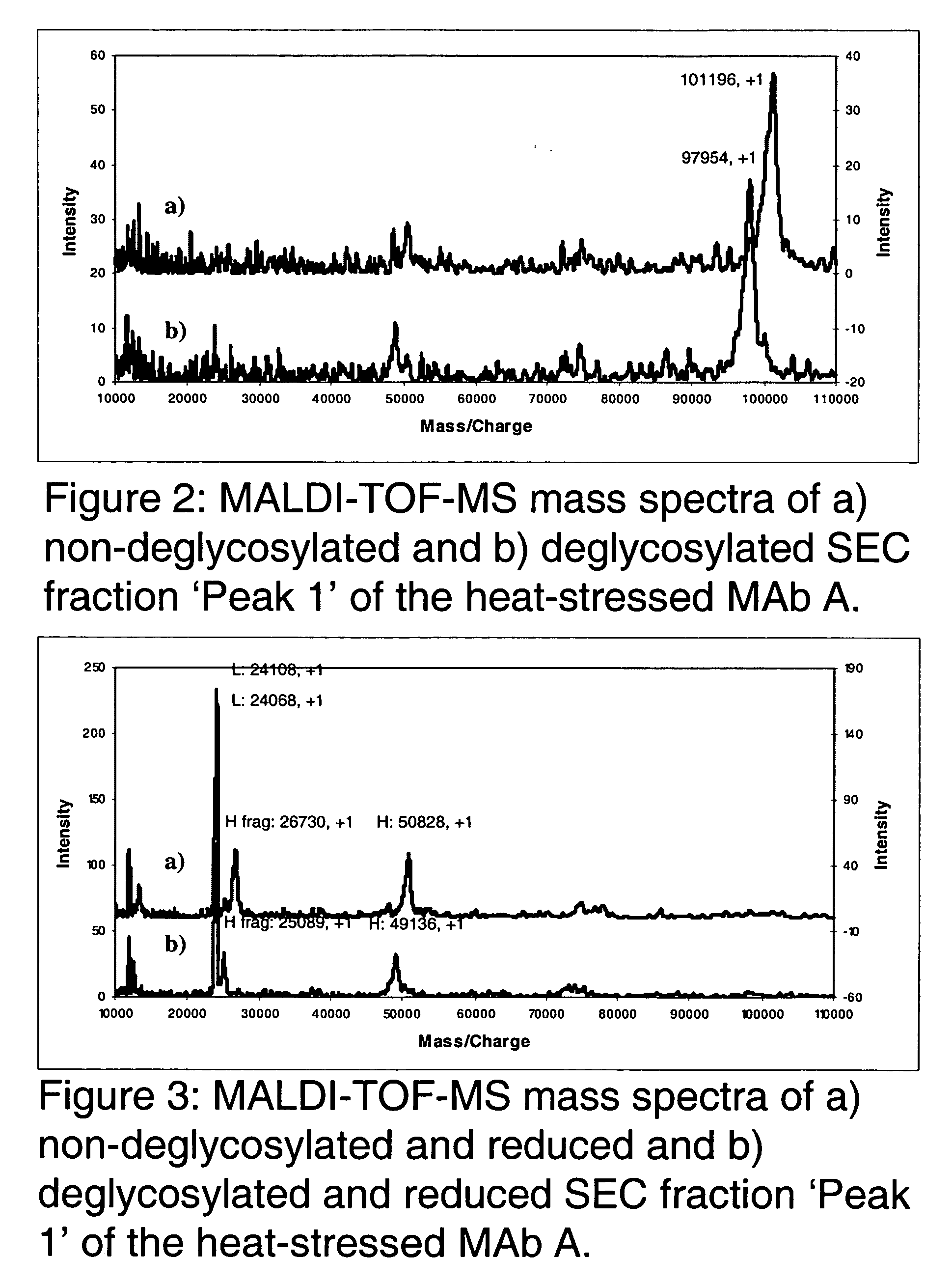
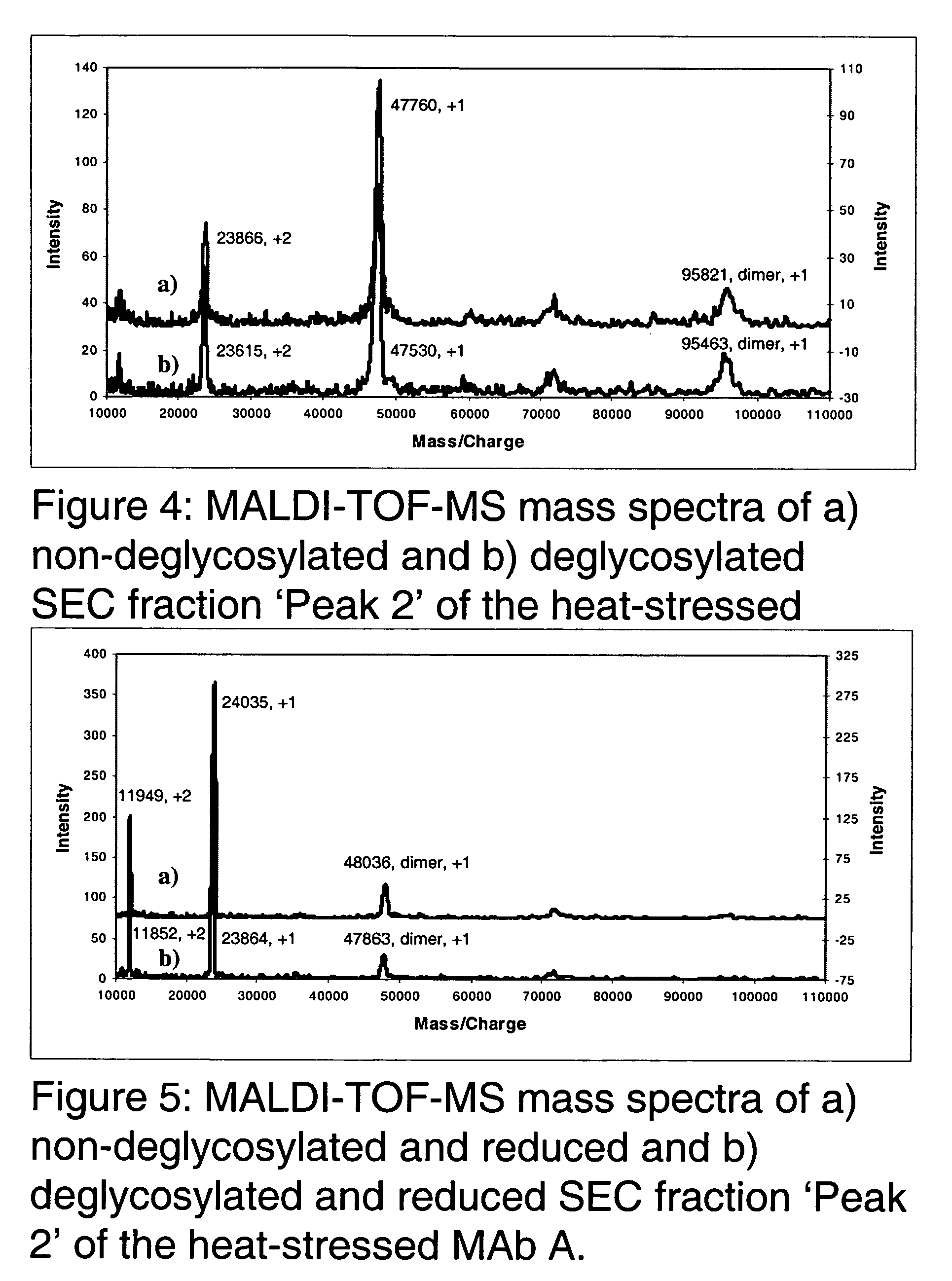
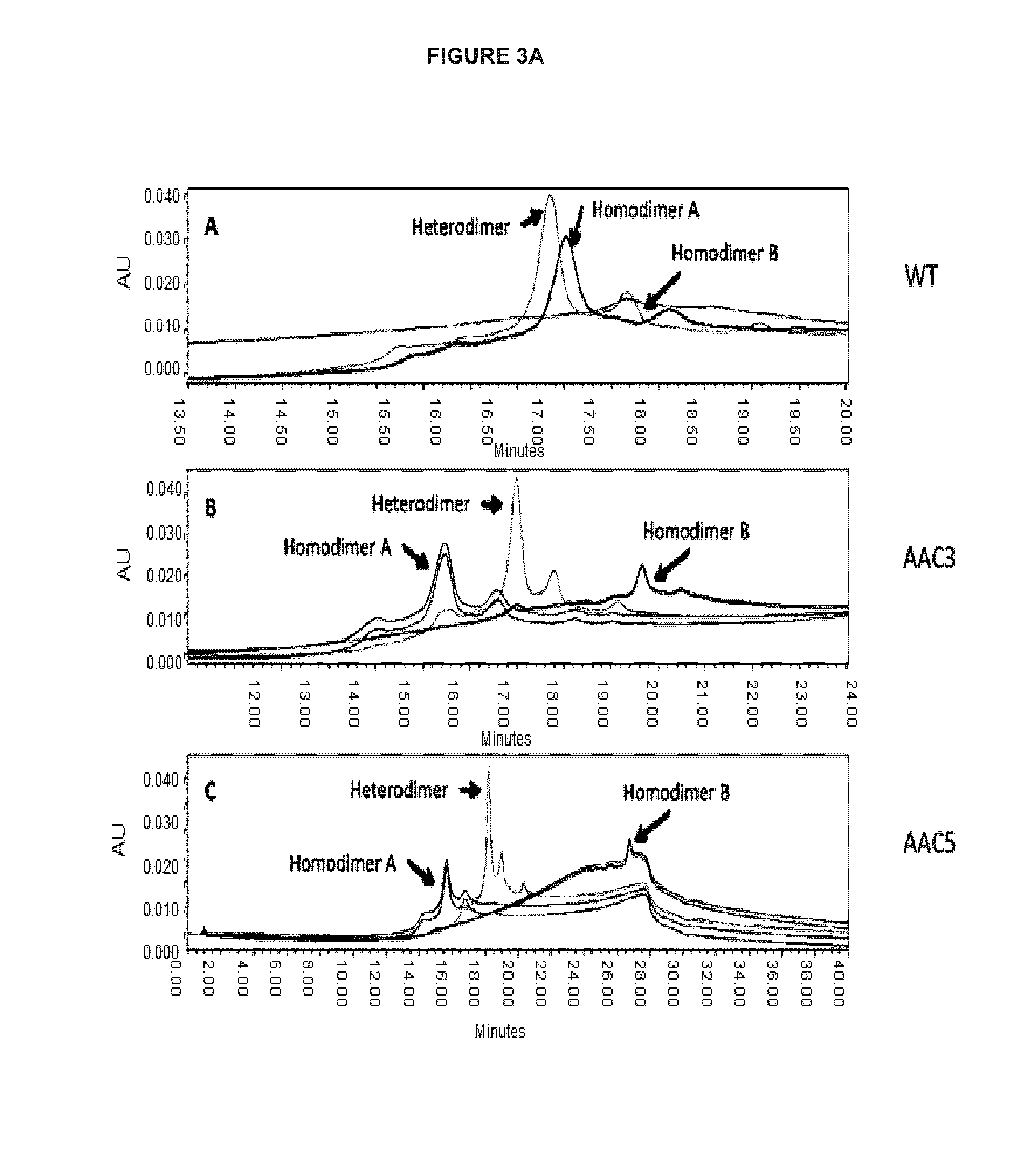
The present invention is generally directed to methods of producing an increase in the enrichment and / or recovery of preferred forms of monoclonal antibodies. More particularly, the invention relates to methods for eliminating disulfide heterogeneity in the hinge region of recombinant IgG2 antibody proteins.
Owner:AMGEN INC
Stabilized glycoproteins
ActiveUS20040191265A1Increased in half lifeGood storage stabilitySnake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainGlycoprotein i
The present invention provides stabilized immunoglobulin molecules that have increased storage stability and / or in vivo half-lives due to the mutation of one or more amino acids that would otherwise render the immunoglobulin molecules susceptible to degradation. In a preferred embodiment, the stabilized immunoglobulins of the invention have mutations at the heavy chain constant domain hinge region. Such stabilized immunoglobulin molecules, i.e., immunoglobulin molecules with increased storage stability have one or more of the following advantages they are more readily transported and / storable for longer periods and / or less stringent conditions than non-stabilized counterparts; that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such stabilized molecules.
Owner:MEDIMMUNE LLC
Orthopedic connector system
System, including methods, apparatus and components thereof, and kits, for connecting to bone using an anchor element configured to be anchored in bone and having a selectively bendable hinge region. In some embodiments, the system may connect the anchor element to connective tissue, a bridge member, and / or to another anchor element by bending the hinge region while the anchor element is disposed in bone, thereby connecting bone to the connective tissue or bridge member, and / or connecting bone members.
Owner:ACUMED
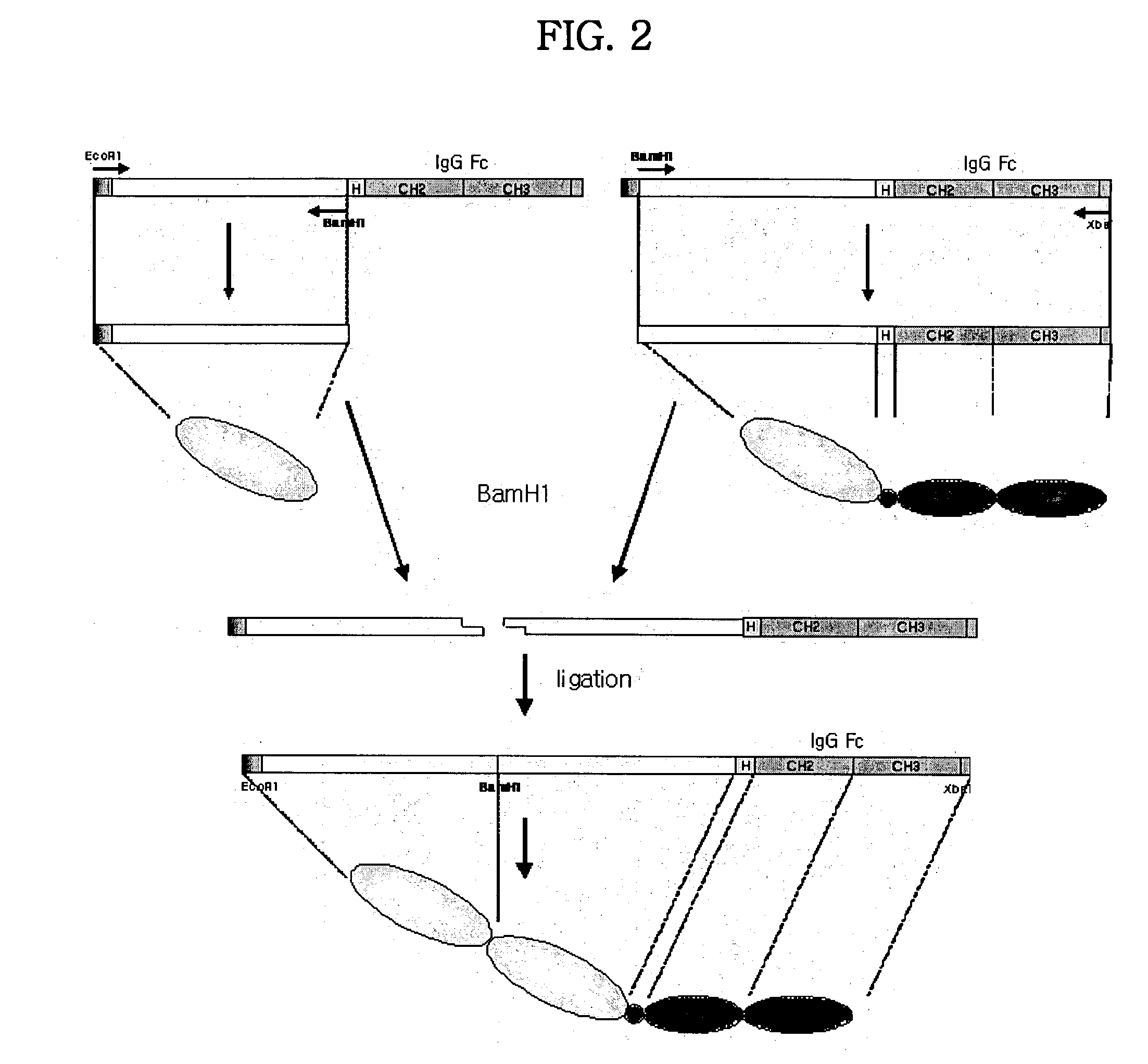
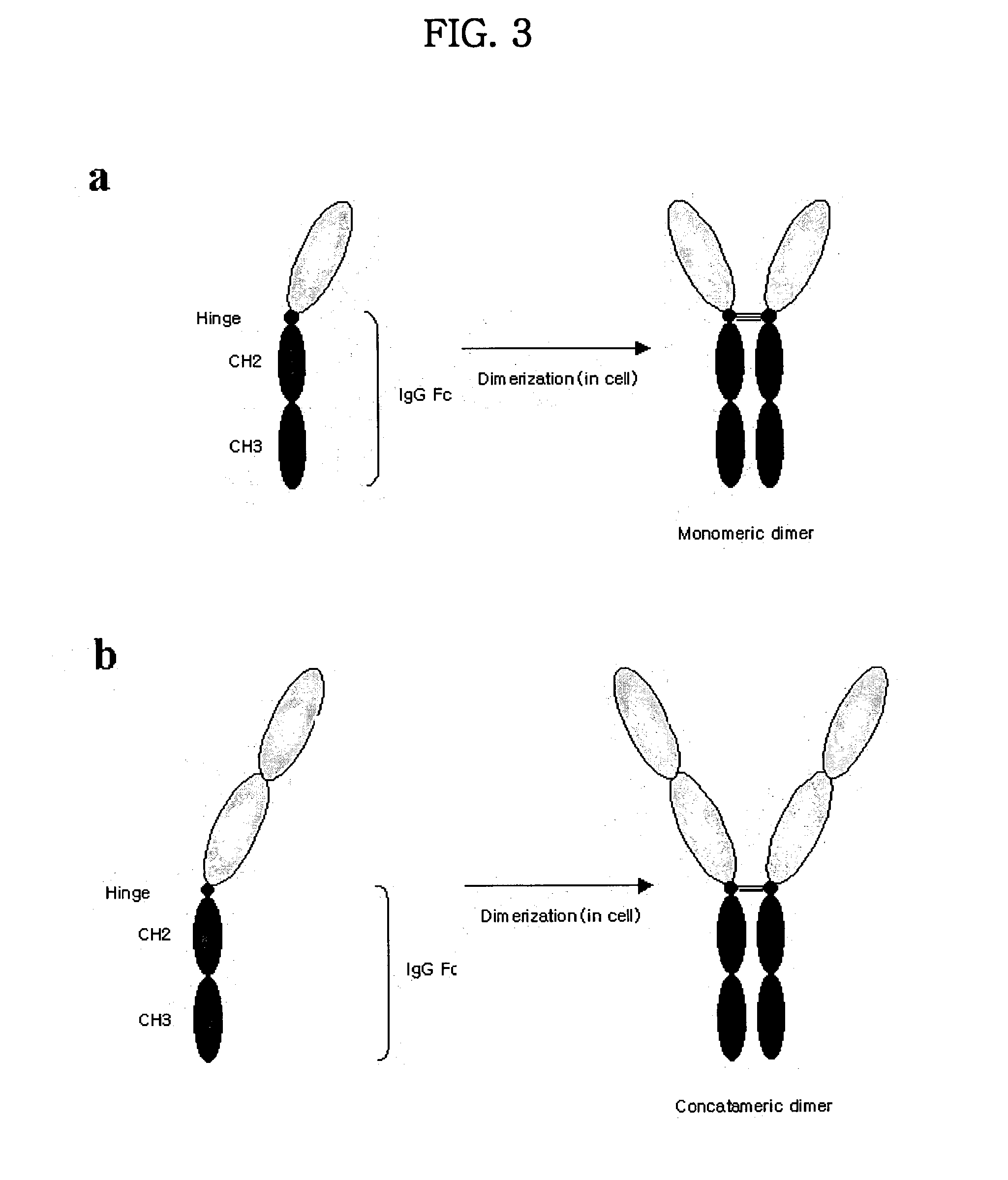
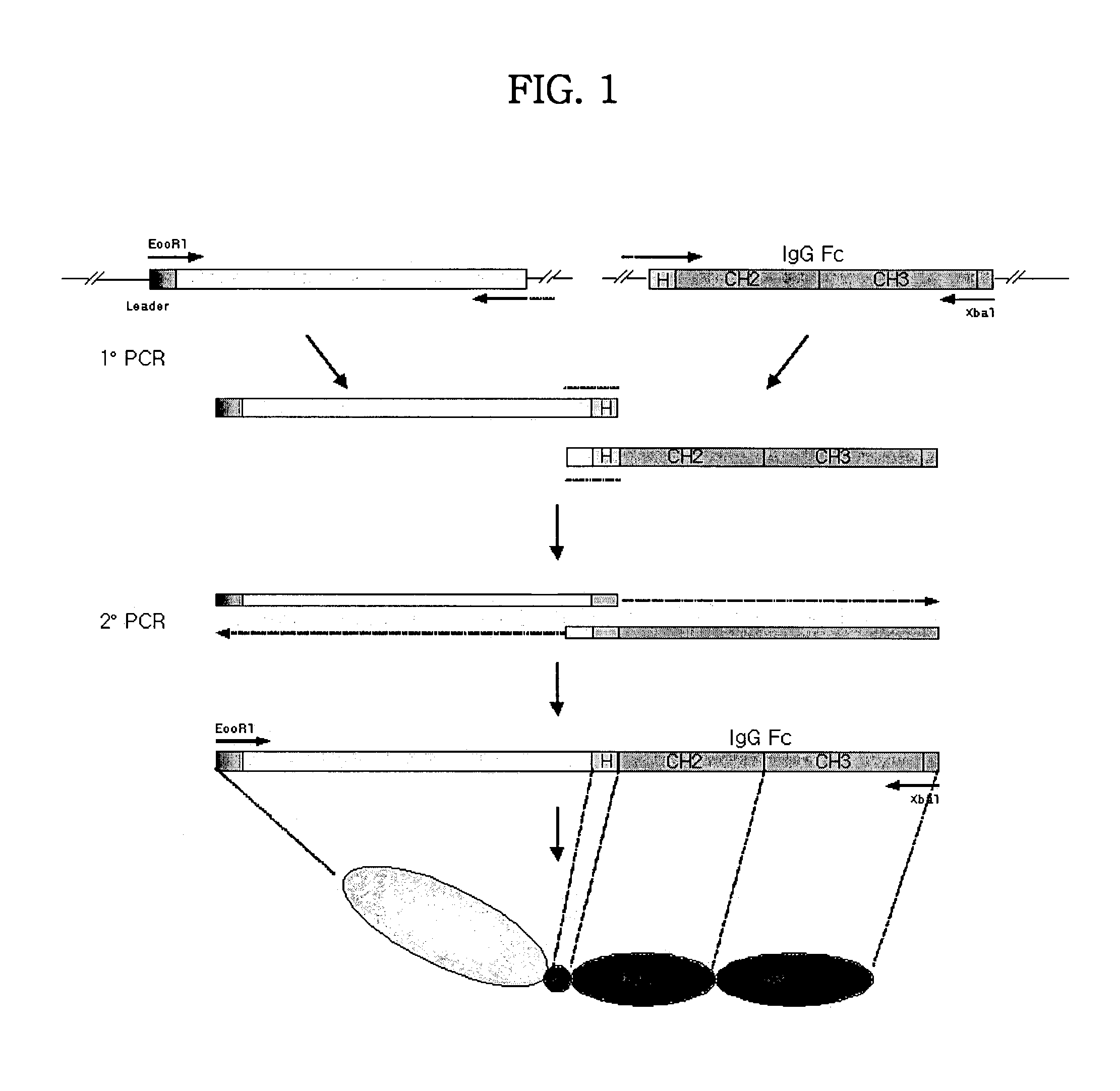
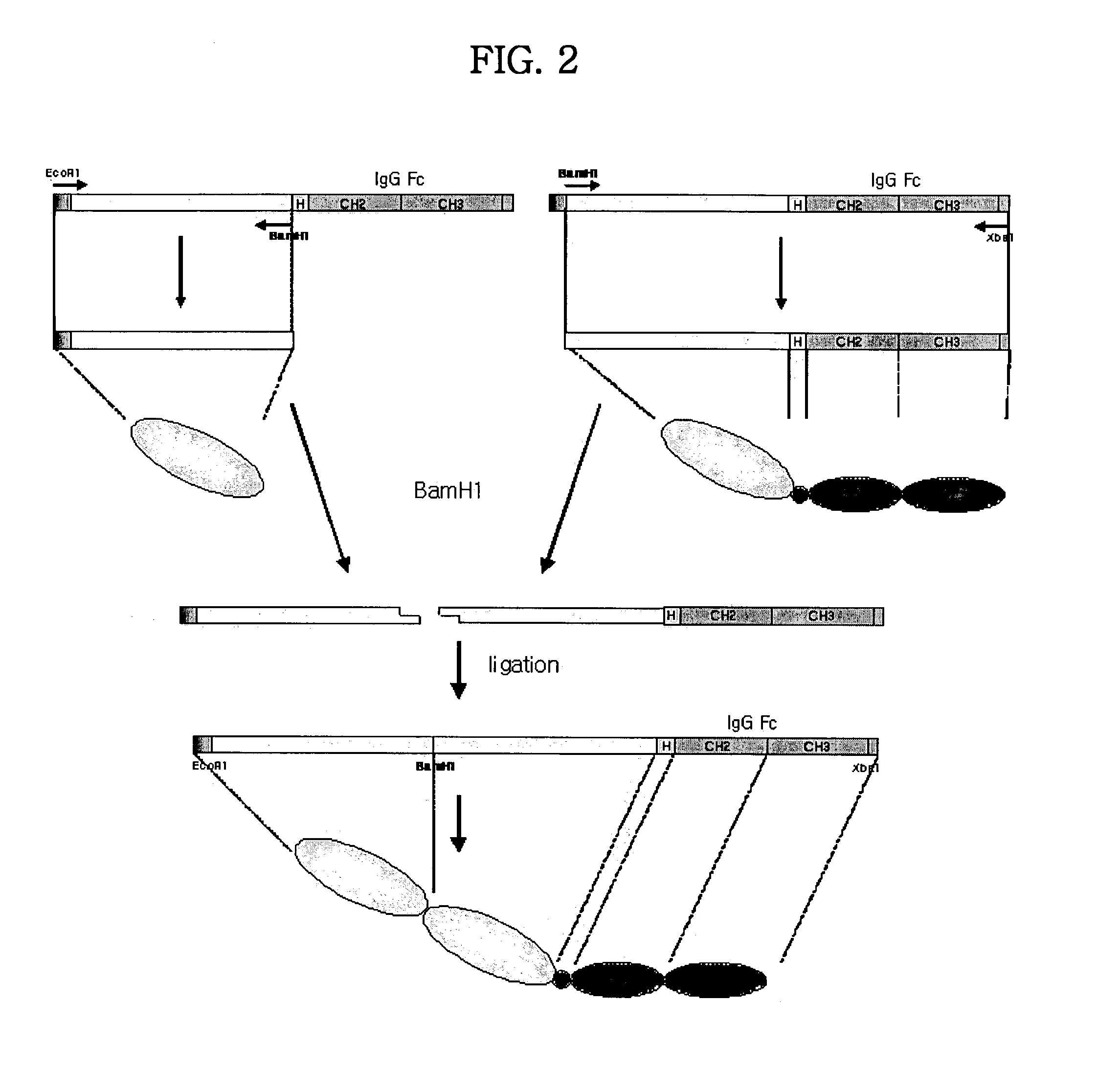
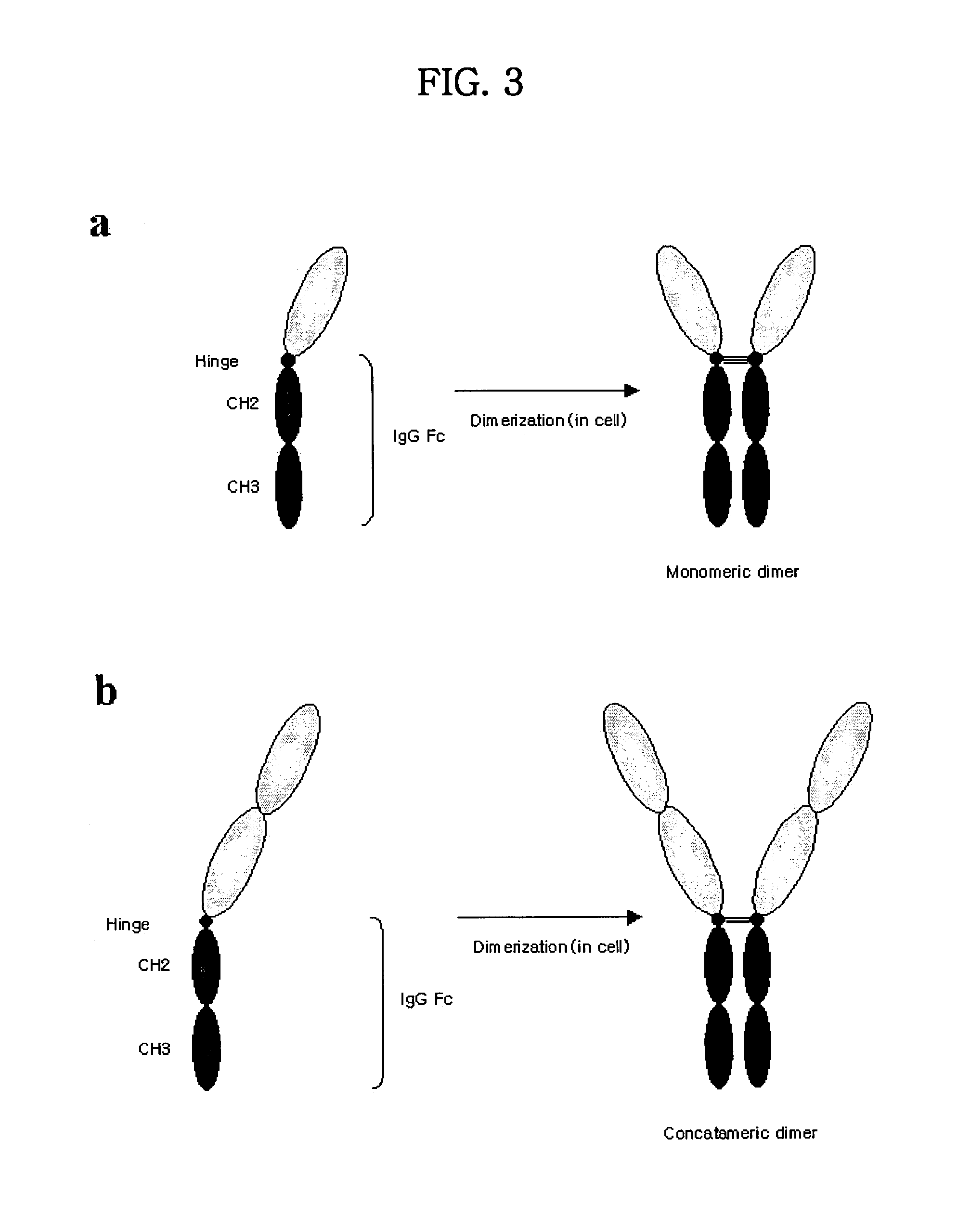
Concatameric immunoadhesion
ActiveUS20030195338A1Improve efficacyImprove stabilityAntipyreticAntibody mimetics/scaffoldsDNA constructActive protein
Disclosed are concatameric proteins comprising two soluble domains, in which the C-terminus of a soluble domain of a biologically active protein is linked to the N-terminus of an identical soluble domain or a distinct soluble domain of a biologically active protein. Also, the present invention discloses dimeric proteins formed by formation of intermolecular disulfide bonds at the hinge region of two monomeric proteins formed by linkage of a concatamer of two identical soluble extracellular regions of proteins involving immune response to an Fc fragment of an immunoglobulin molecule, their glycosylated proteins, DNA constructs encoding the monomeric proteins, recombinant expression plasmids containing the DNA construct, host cells transformed or transfected with the recombinant expression plasmids, and a method of preparing the dimeric proteins by culturing the host cells. Further, the present invention discloses pharmaceutical or diagnostic compositions comprising the dimeric protein or its glycosylated form.
Owner:MEDEXGEN
Vascular prosthesis
InactiveUS6916336B2Easy to trackImprove consistencyStentsBlood vesselsHinge regionVascular prosthesis
Owner:ALTAI MEDICAL TECH
Stable antibody compositions and methods of stabilizing same
InactiveUS20100172862A1Improve propertiesMaintain stabilityOrganic active ingredientsNervous disorderFractionationAntigen binding
The invention provides compositions and methods for inhibiting fractionation of immunoglobulins comprising a lambda light chain based on the observation that iron, in the presence of histidine, results in increased fragmentation of a recombinant fully human IgG molecule containing a lambda light chain due to cleavage in the hinge region. The invention further provides an aqueous pharmaceutical formulation comprising an antibody, or antigen-binding portion thereof, that binds the p40 subunit of IL-12 / IL-23 and a buffer system comprising histidine, wherein the formulation has enhanced stability, including enhanced resistance to fragmentation.
Owner:ABBVIE INC
LED illuminated novelty glasses
Illuminated novelty eye glasses include a frame having a body with portions that define lens openings and adjacent hinge regions, and a bridge region between the portions defining the lens openings. The body has a channel formed therein traversing around the lens openings and across the bridge region. The body is formed from a transparent or translucent material. Ear pieces are connected to the frame at the hinge regions. The ear pieces each have a hinge region for hingedly connecting the ear pieces to the body such that the ear pieces fold inwardly toward the body. The ear pieces each include a compartment and a channel extending generally from the compartment to the hinge region. An LED assembly is formed from a plurality of light emitting diodes mounted to a plurality of conductors. A power source is operably connected to the LED assembly and is carried in one of the ear piece compartments and a switch operably connects the power source to the LED assembly and is carried in the other ear piece compartment. The LED assembly is disposed in the channel in the frame and in the ear pieces and the light emitting diodes are illuminated by actuation of the switch. Light from the light emitting diodes is transmitted by the diodes and is visible through the frame and ear pieces.
Owner:CHEM LIGHT
System and method for protecting neurovascular structures
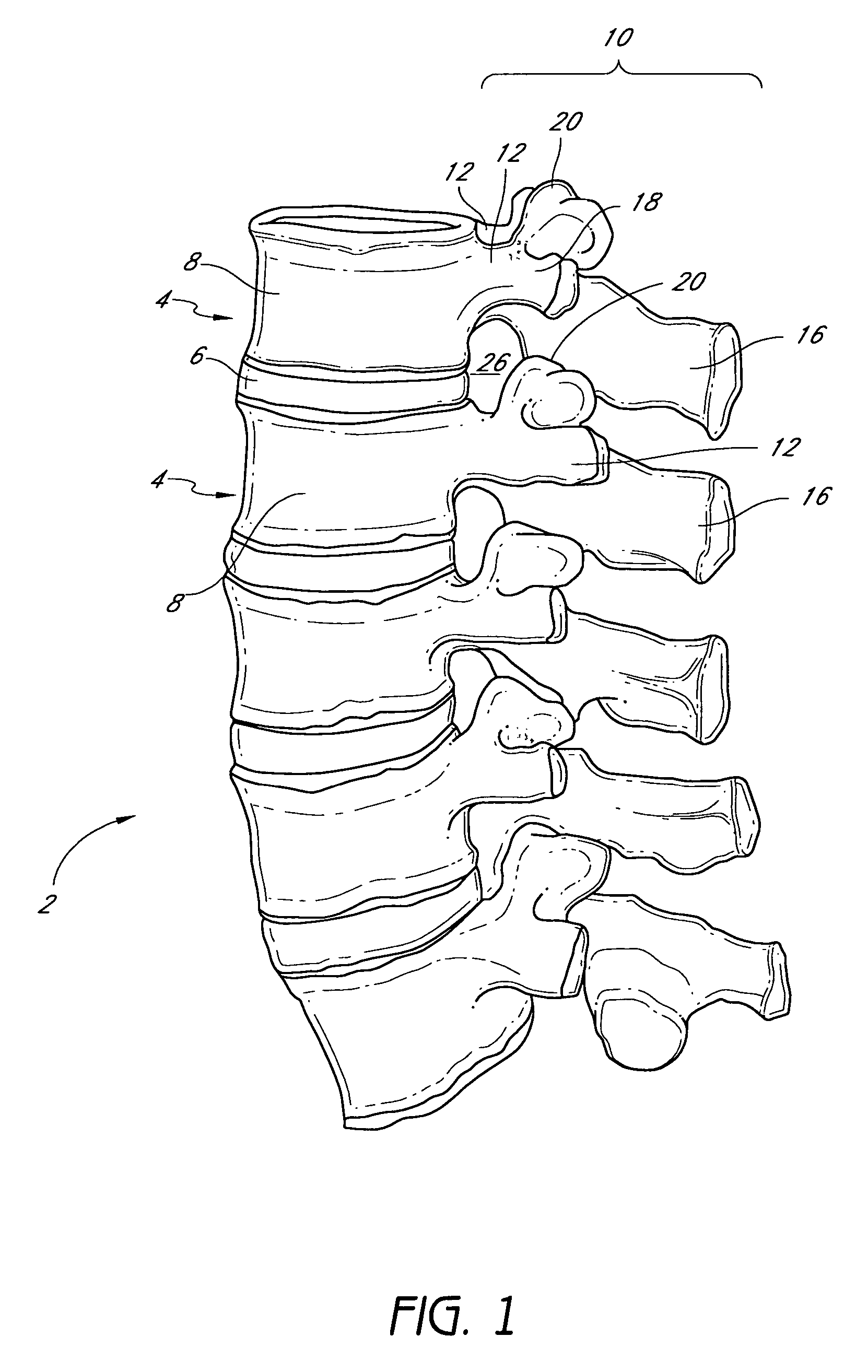
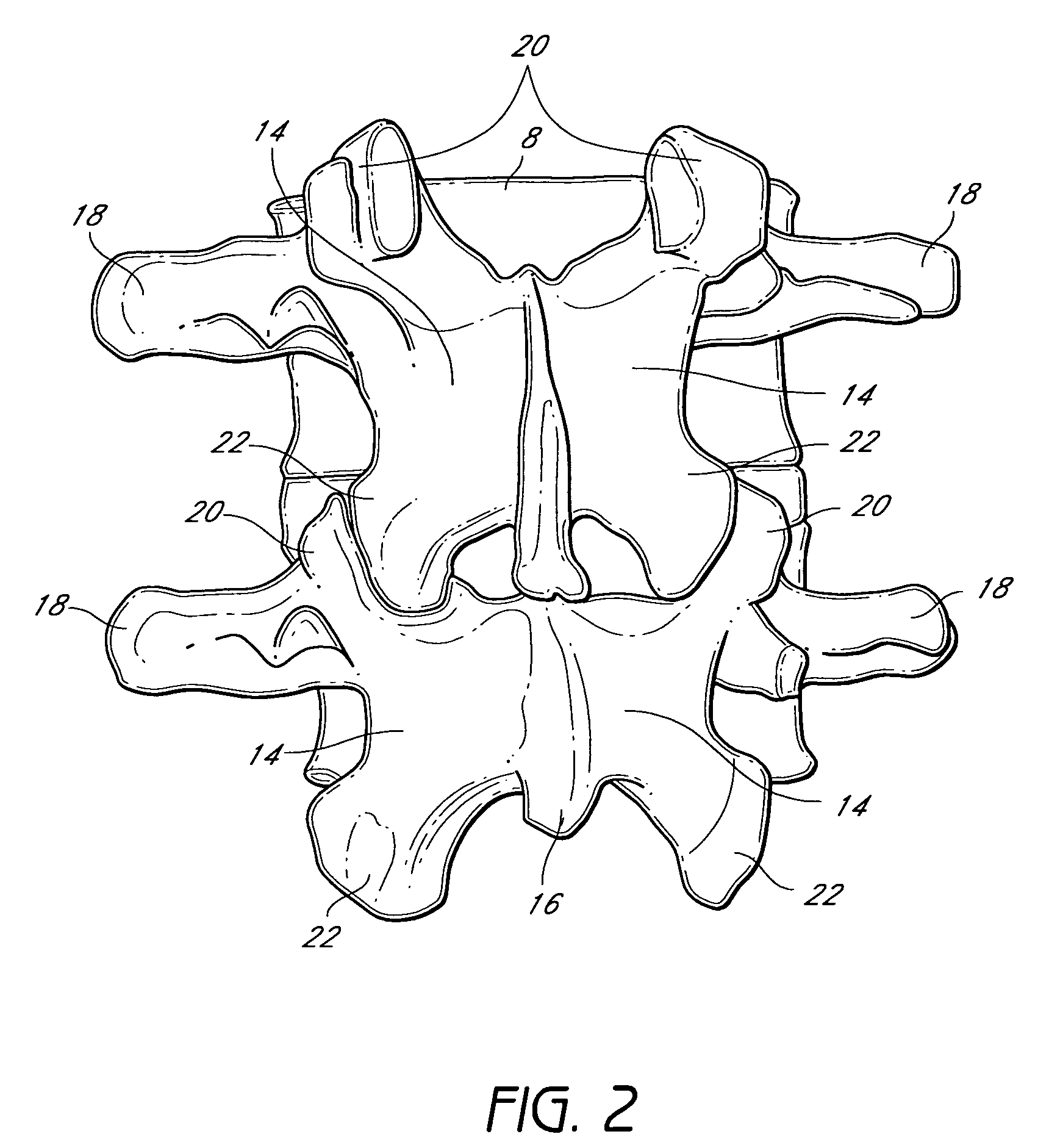
Devices and methods for protecting the neurovascular structures about the vertebral column are provided. One embodiment of the invention comprises a neuroprotective stent or device adapted for placement in an intervertebral foramen of a vertebral column and configured to resist compression or impingement from surrounding structures or forces. The stent or device may further comprise a flange or hinge region to facilitate attachment of the device to the vertebrae or to facilitate insertion of the device in the foramen, respectively.
Owner:SPINAL ELEMENTS INC
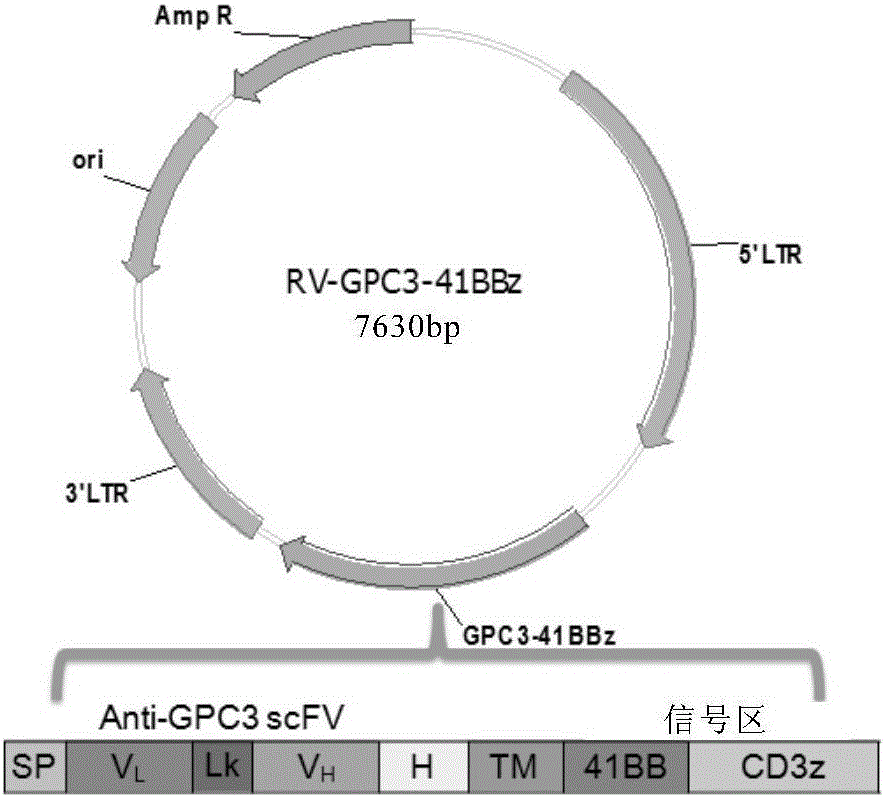
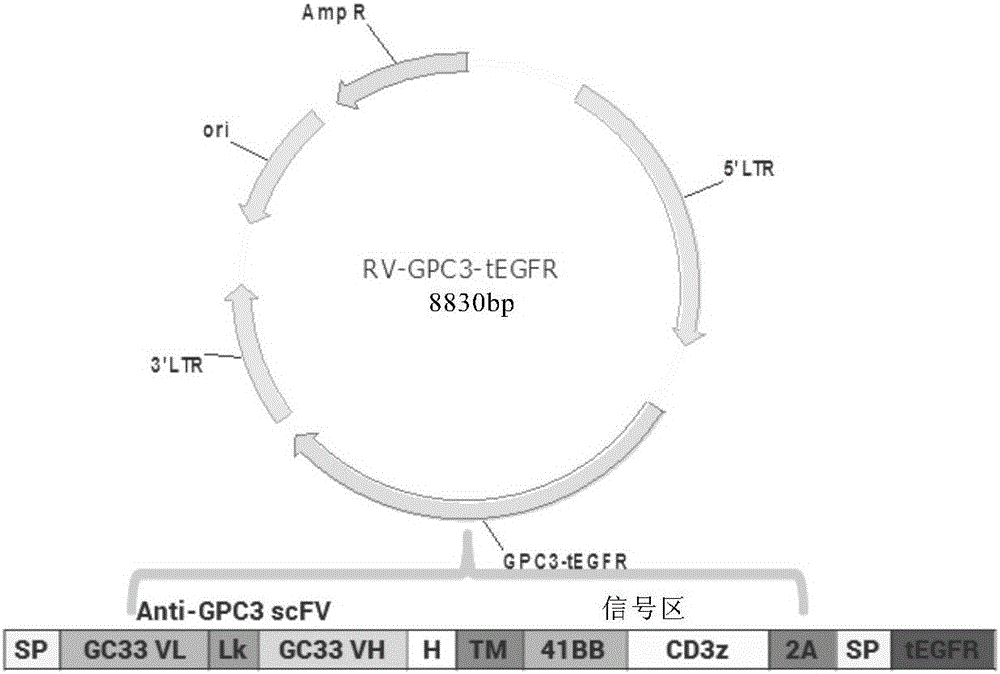
Chimeric antigen receptor of targeted GPC3 (Glypican 3) and application thereof
The invention relates to a chimeric antigen receptor of a targeted GPC3 (Glypican 3) and an application thereof. Specifically, the invention provides a polynucleotide sequence, wherein the polynucleotide sequence is selected from: polynucleotide sequences (1) containing a coding sequence of a CD8 antigen leading peptide, a coding sequence of an anti-GPC3 single-chain antibody, a coding sequence of a human CD8 alpha hinge region, a coding sequence of a human CD8 transmembrane region, a coding sequence of a human 41BB intracellular region, and a coding sequence of a human CD3 zeta intracellular region, and a coding sequence of an optional fragment of an EGFR (Epidermal Growth Factor Receptor) sequence containing an extracellular domain III and an extracellular domain IV, wherein the coding sequences are sequentially connected; and complementary sequences (2) of the polynucleotide sequences (1). The invention provides a CAR (chimeric antigen receptor) coded by the polynucleotide sequences and a T cell expressing the CAR. The prepared CAR-T cell has a function of strongly killing a specific tumor cell, and the kill effectiveness is more than 90% under the condition that the effector-target ratio is 20:1.
Owner:HRAIN BIOTECHNOLOGY CO LTD
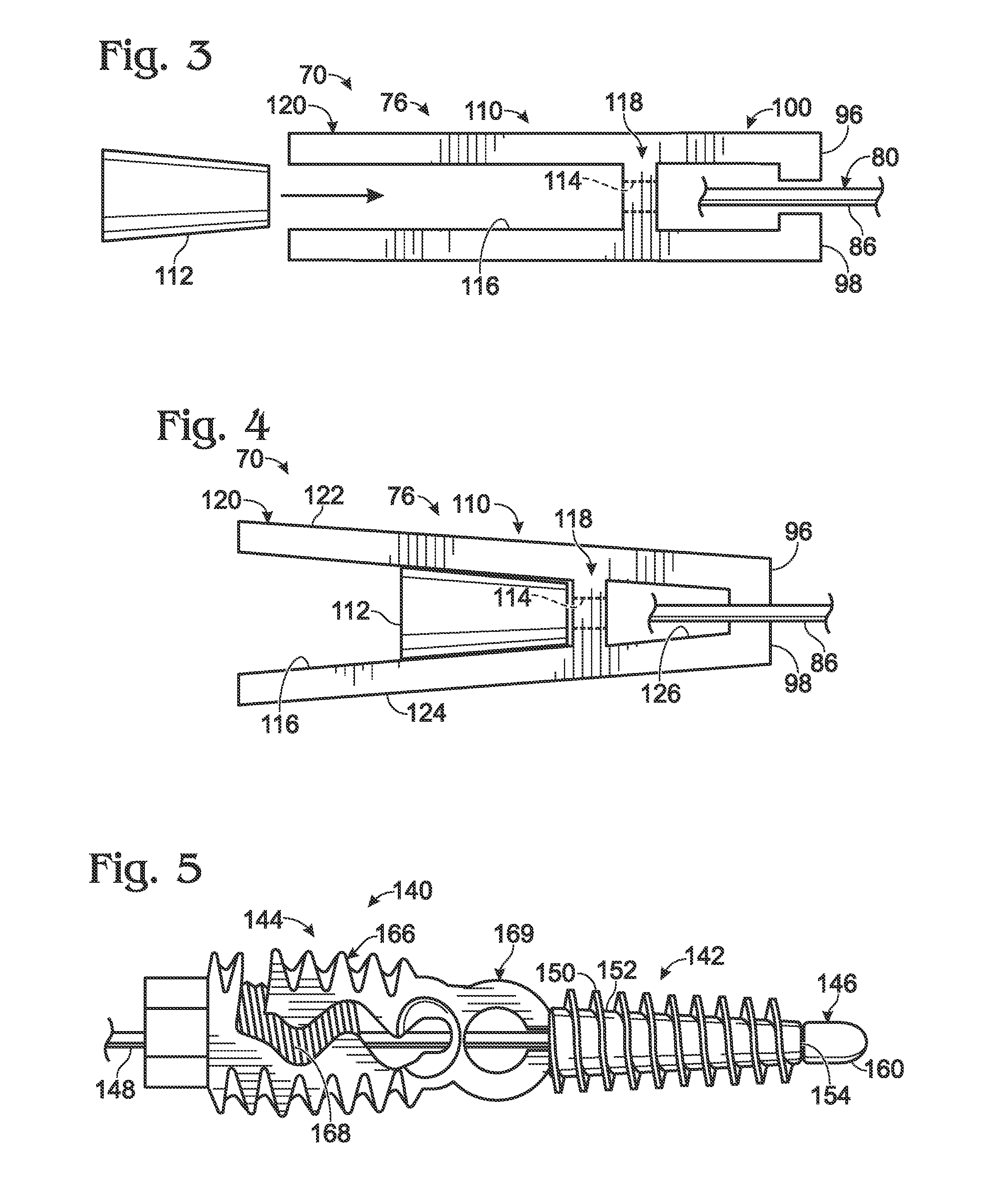
Device for heart measurement
A surgical tool for measuring a heart that includes two handle members, and a flexible member. The handle members are configured with internal passageways to allow the flexible members to pass through the handle members. The flexible member or the first handle member are configured to anchor the flexible member in a first handle member. This can either be accomplished by configuring the flexible member with an enlarged portion on one end or by configuring the flexible member in concert with the first handle member to anchor the elongate flexible member within an expanded region of the first handle member. The handle members can be configured for the construction of a hinged region that is easily hinged and unhinged. The surgical tool is assembled by extending the flexible member into the proximal end of a first handle member, passing it through an internal passageway of a first handle member, out the distal end of a first handle member and into the distal end of a second handle member, passing it through the second handle member and through the proximal end of a second handle member. Measurements are taken by extending the flexible member along the surface to be measured and noting the marking on the flexible member that intersects with the proximal end of said second handle member.
Owner:MARDIL
Tetravalent etanercept
ActiveUS7229962B2Improve efficacyImprove stabilityAntibody mimetics/scaffoldsAntipyreticDNA constructEtanercept
Disclosed are concatameric proteins comprising two soluble domains, in which the C-terminus of a soluble domain of a biologically active protein is linked to the N-terminus of an identical soluble domain or a distinct soluble domain of a biologically active protein. Also, the present invention discloses dimeric proteins formed by formation of intermolecular disulfide bonds at the hinge region of two monomeric proteins formed by linkage of a concatamer of two identical soluble extracellular regions of proteins involving immune response to an Fc fragment of an immunoglobulin molecule, their glycosylated proteins, DNA constructs encoding the monomeric proteins, recombinant expression plasmids containing the DNA construct, host cells transformed or transfected with the recombinant expression plasmids, and a method of preparing the dimeric proteins by culturing the host cells. Further, the present invention discloses pharmaceutical or diagnostic compositions comprising the dimeric protein or its glycosylated form.
Owner:MEDEXGEN
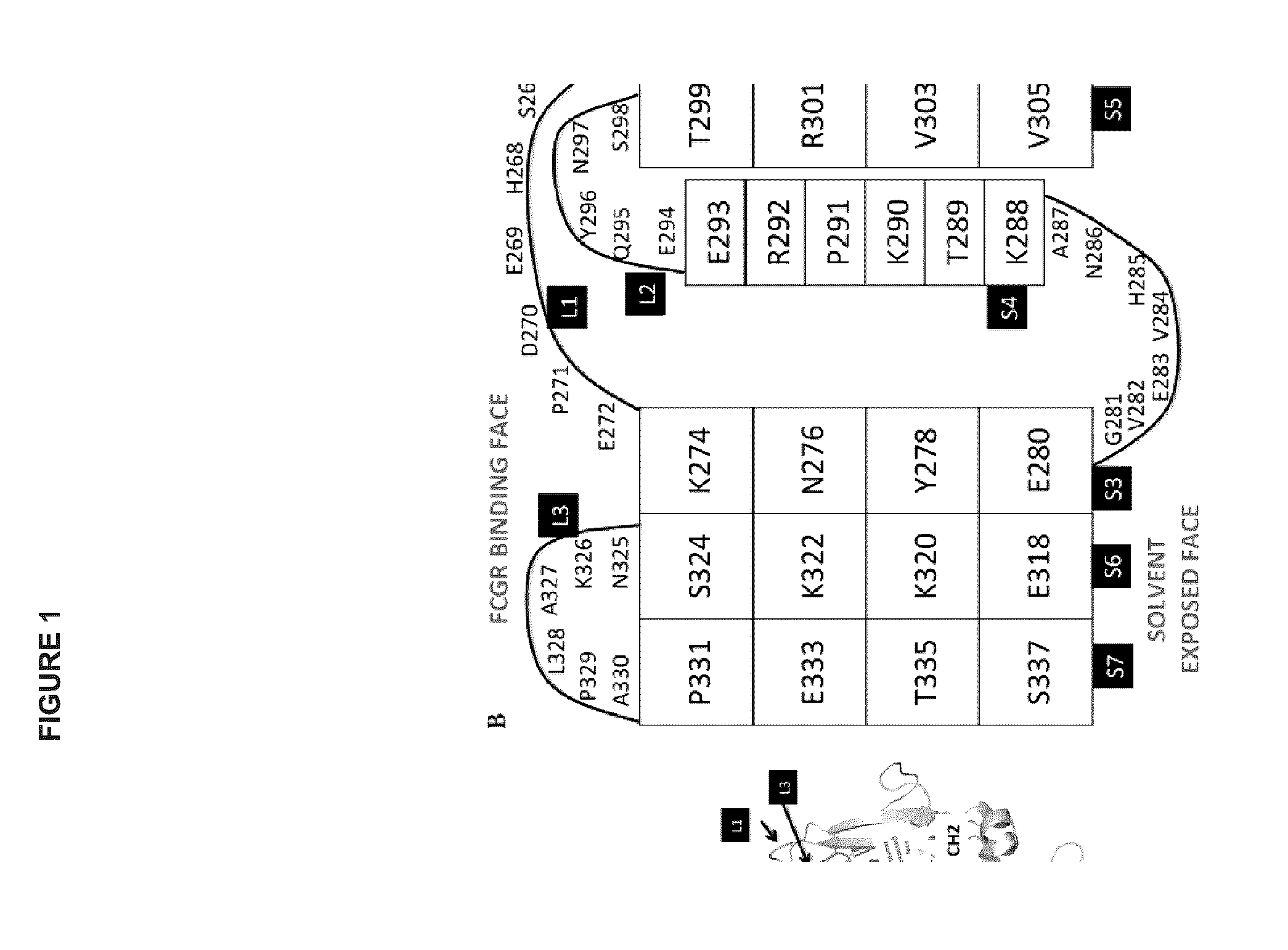
Heteromultimers with reduced or silenced effector function
ActiveUS20160102135A1Reduce the binding forceReduced effector functionNervous disorderBacteriaHinge regionProtein L
Provided herein are heteromultimer constructs with reduced or silenced effector function. In an embodiment is provided a heteromultimer construct comprising an IgG Fc construct having a first and a second Fc polypeptide, each Fc polypeptide comprising a modified lower hinge region wherein: the modified lower hinge region of said first Fc polypeptide comprises at least one amino acid modification, the modified lower hinge region of said second Fc polypeptide comprises at least one amino acid modification which is different from at least one amino acid modification of said first Fc polypeptide, and the IgG Fc construct displays reduced binding to all Fcγ receptors and to C1q protein as compared to a corresponding parent IgG Fc construct. Also provided are methods of producing such heteromultimer constructs, and methods of reducing ADCC for an antibody construct by reducing effector function.
Owner:ZYMEWORKS INC
Surgical Fasteners and Fastening Devices
ActiveUS20090182352A1High strengthAttached to delicate soft tissueSuture equipmentsProsthesisHinge regionEngineering
The invention provides a surgical fastener. The fastener has a first element defining an axis of the fastener, and or more prongs attached to the first element at hinge regions. Deploying the fastener involves bending the prongs at the hinges so as to increase the distance of the prong tips from the axis and locking the fastener in this deployed configuration. The invention also provides a surgical fastening device and a method for attaching a surgical fastener to a site of a body tissue.
Owner:I B I ISRAEL BIOMEDICAL INNOVATIONS
CD19 targeting chimeric antigen receptor and NKT cell, and preparation method thereof and applications thereof
ActiveCN105418765AEnhance specific killing activityProlong survival timePeptide/protein ingredientsGenetic material ingredientsAntigenHinge region
The present invention discloses a chimeric antigen receptor, a gene and a recombinant expression vector thereof, an engineered CD19 targeting NKT cell and applications thereof. The chimeric antigen receptor is CD19ScFv-CD8-CD137-CD3 zeta, and consists of a hinge region and a transmembrane region of the CD19ScFv and CD8, an intracellular signal structural domain of CD137, and an intracellular signal structural domain of CD3 zeta, and the the hinge region, the transmembrane region, the intracellular signal structural domain of CD137 and the intracellular signal structural domain of CD3 zeta are connected in series. When used to treat advanced stage CD19-positive B-cell acute lymphocytic leukemia, the NKT cell modified by the chimeric antigen receptor CD19ScFv-CD8-CD137-CD3 zeta provided by the present invention has good specific killing activity on leukemic cells, and has certain therapeutic effect on advanced stage CD19-positive B-cell acute lymphocytic leukemia patients who are repeatedly subjected to therapy such as radiotherapy, chemotherapy and symptomatic therapy by other drugs but cannot recover obviously.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI +1
Chimeric antigen receptors and methods of making
ActiveUS20170183407A1Improved therapeutic potentialConvenient treatmentAntibacterial agentsTumor rejection antigen precursorsAntigen receptorsBinding domain
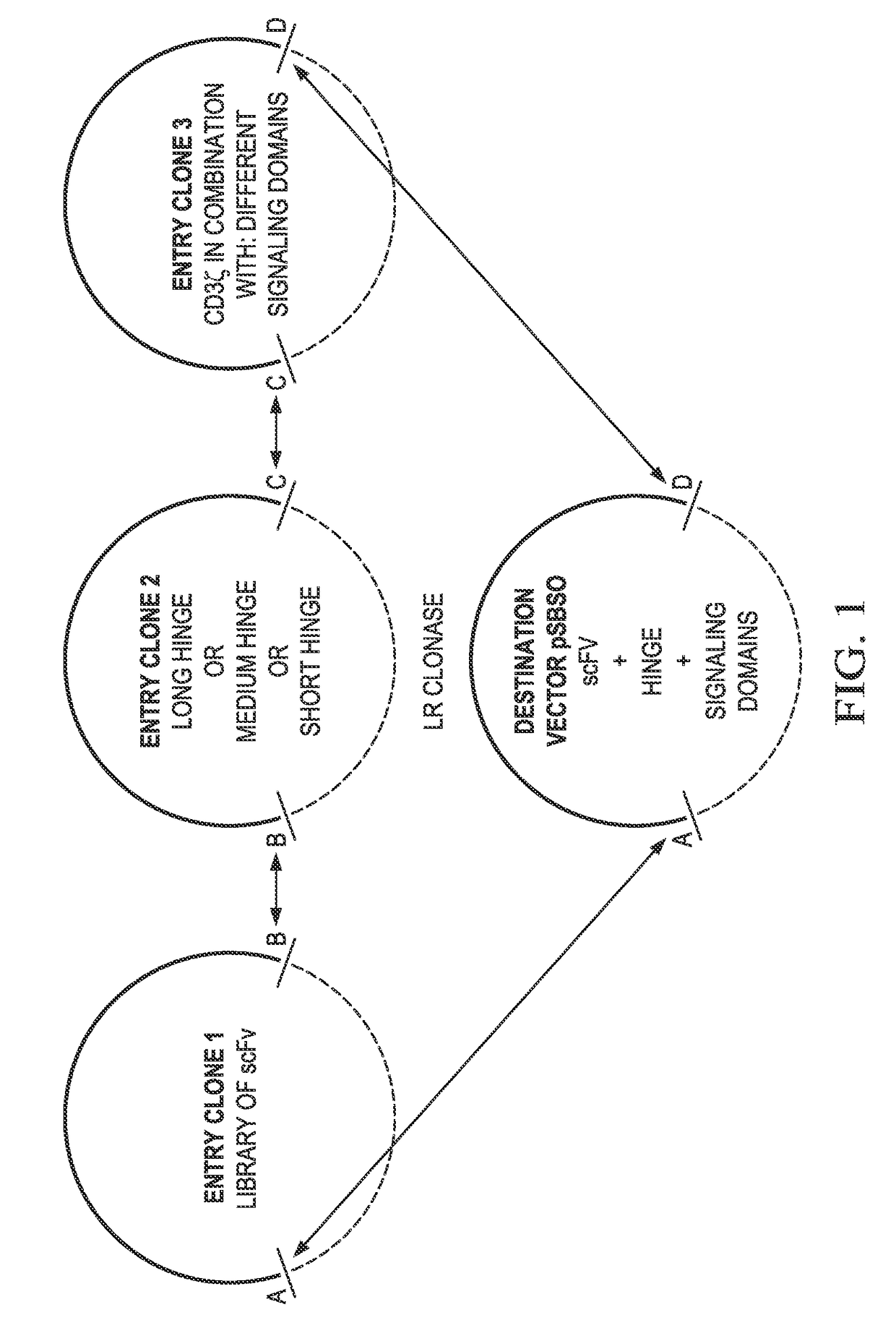
Provided are methods of generating chimeric antigen receptors (CAR). In some embodiments, library screening of CAR is performed by generating a vector encoding the CAR from random attachment of vectors from libraries of vectors encoding antigen-binding domains (e.g., scFv regions), hinge regions, and endodomains. In some embodiments, the vectors contain a transposon.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Chimeric antigen receptor of anti-human CD19 antigen and application thereof
ActiveCN107226867ANo side effectsIncreased proliferationPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCancer cellSide effect
The invention belongs to the field of gene engineering, and relates to chimeric antigen receptor of anti-human CD19 antigen and application thereof. The chimeric antigen receptor of anti-human CD19 antigen comprises polypeptide (scFv) for recognizing human CD19 antigen, a hinge region, a transmembrane region and an intracellular signal domain which are connected in sequence, wherein an amino acid sequence of the polypeptide (scFv) for recognizing human CD19 antigen is as shown in SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3 or SEQ ID NO.4. The chimeric antigen receptor can be more stably expressed in T lymphocyte, has the capacity for properly removing cancer cell, can not only maintain the positive rate of CD19 targeted chimeric antigen receptor during the cell culture of a patient, but also enhance the capacity for CAR-T multiplication and tumor killing, does not have toxic and / or side effects on antigen-negative cell, and can be applied to the targeted therapy of tumor.
Owner:CHONGQING PRECISION BIOTECH CO LTD
Bispecific antibodies and methods for production thereof
ActiveUS9212230B2Promote exchangeHybrid immunoglobulinsAntibody mimetics/scaffoldsIsomerizationBispecific antibody
The invention relates to an ex vivo method for the generation of a bispecific antibody, comprising the steps of: a) providing a first antibody having a first binding specificity, wherein said first antibody comprises an IgG4-like CH3 region, b) providing a second antibody having a second binding specificity which differs from said first binding specificity, wherein said second antibody comprises an IgG4-like CH3 region, c) incubating said first and second antibodies together under reducing conditions which allow the cysteines in the core hinge region to undergo disulfidebond isomerization, and d) obtaining a bispecific antibody. The invention furthermore relates to bispecific antibodies obtainable by the method of the invention.
Owner:GENMAB AS
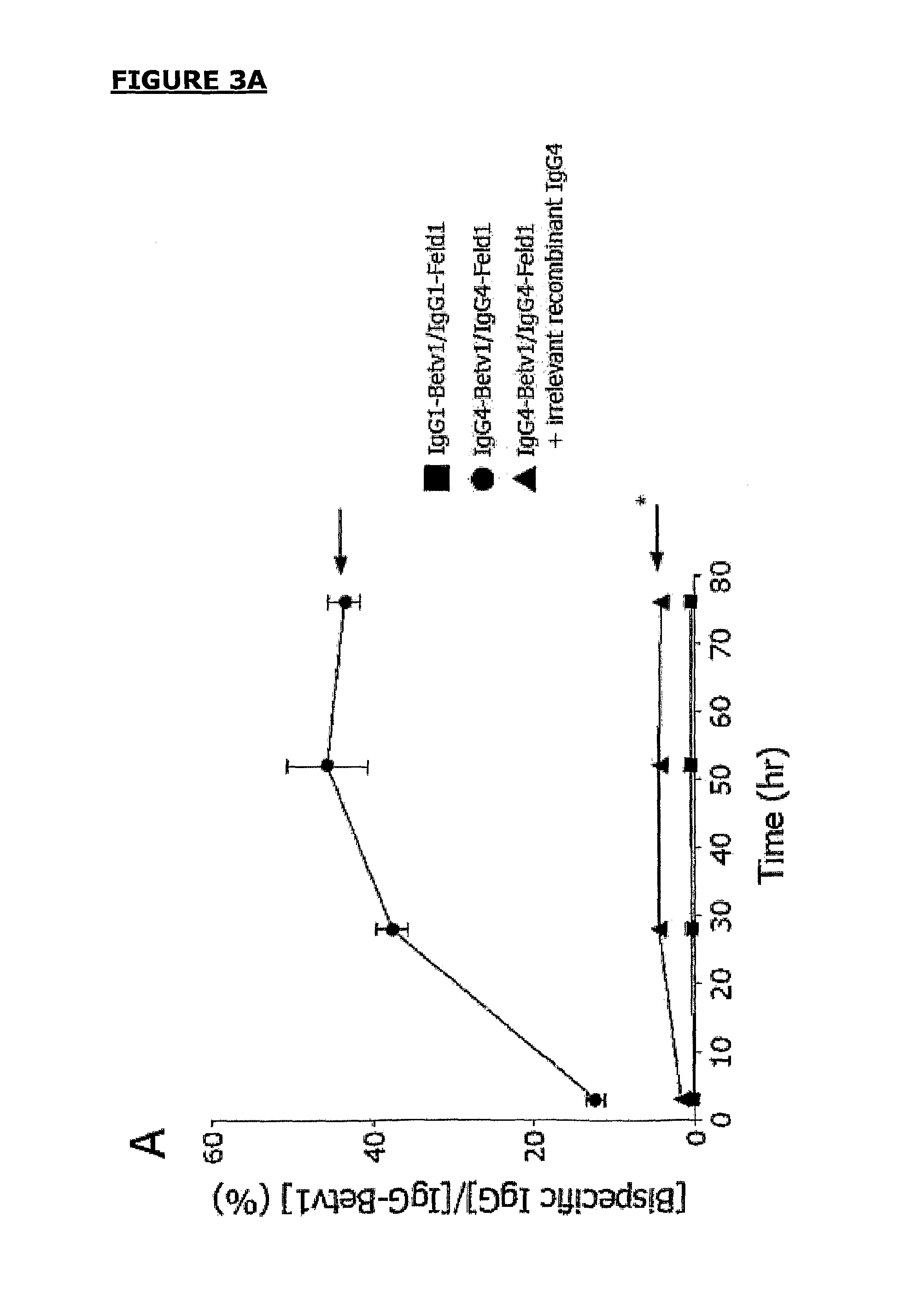
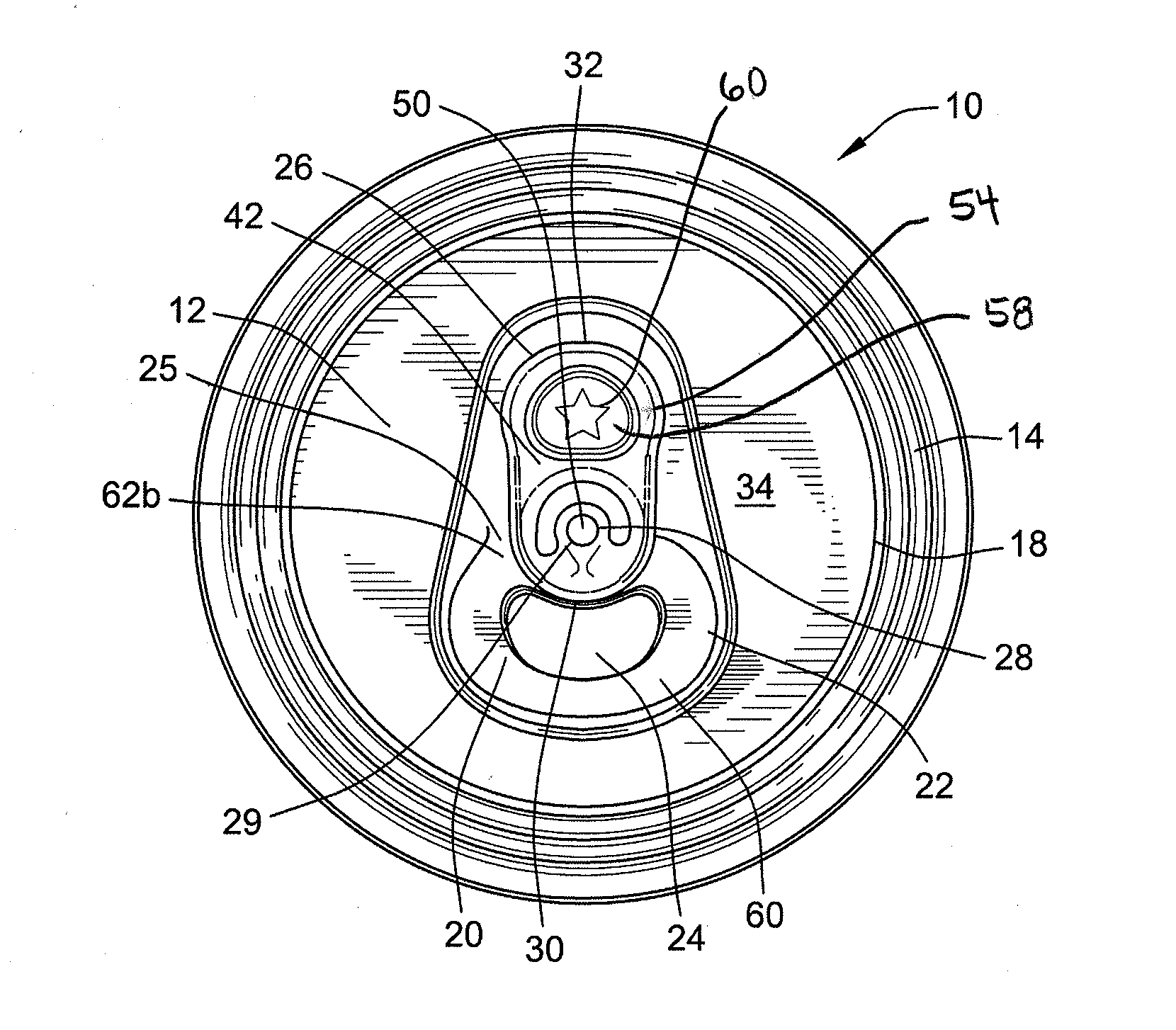
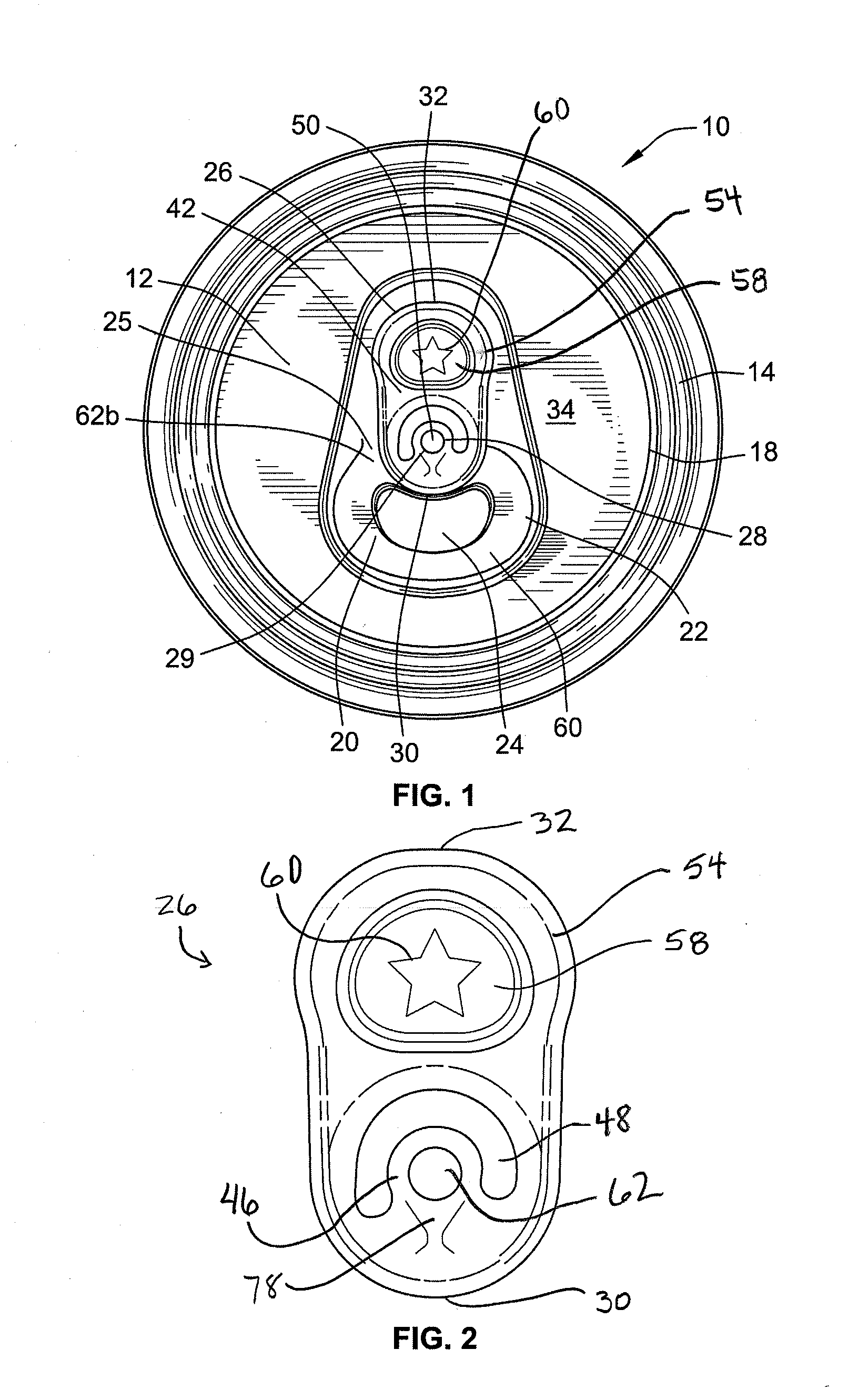
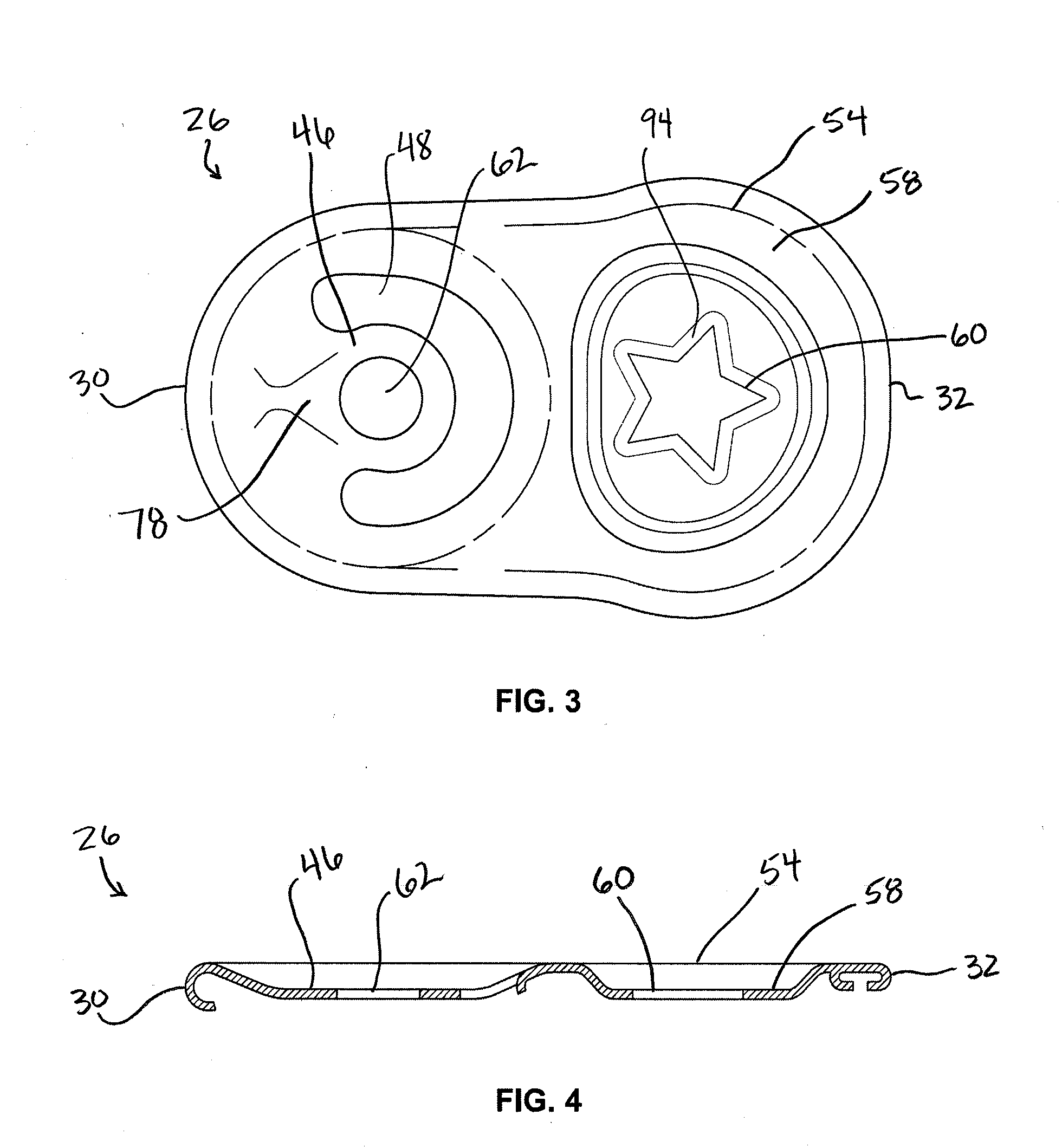
Stay-on tab for a beverage container
A non-detachable pull tab for a beverage container has a nose end, a lift end opposite the nose end, and a central webbing of a metallic material between the nose and lift end. The central webbing has a hinge region, a rivet island surrounding a rivet hole, a void region at least partially surrounding the rivet island, and a grab portion between the first void region and the lift end. The grab portion has an upper surface, a lower surface, and an aperture therethrough. The aperture is defined by a circumferential wall of the grab portion extending from the upper surface to the lower surface and includes a first segment of a reworked portion of a burr.
Owner:REXAM BEVERAGE CAN
Recombinant human EPO-Fc fusion proteins with prolonged half-life and enhanced erythropoietic activity in vivo
ActiveUS7625564B2Enhanced erythropoietic bioactivityExtended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeRed blood cell
A recombinant fusion protein comprising a human erythropoietin peptide portion linked to an immunoglobulin peptide portion is described. The fusion protein has a prolonged half-life in vivo in comparison to naturally occurring or recombinant native human erythropoietin. In one embodiment of the invention, the protein has a half-life in vivo at least three fold higher than native human erythropoietin. The fusion protein also exhibits enhanced erythropoietic bioactivity in comparison to native human erythropoietin. In one embodiment, the fusion protein comprises the complete peptide sequence of a human erythropoietin (EPO) molecule and the peptide sequence of an Fc fragment of human immunoglobulin IgG1. The Fc fragment in the fusion protein includes the hinge region, CH2 and CH3 domains of human immunoglobulin IgG1. The EPO molecule may be linked directly to the Fc fragment to avoid extraneous peptide linkers and lessen the risk of an immunogenic response when administered in vivo. In one embodiment the hinge region is a human Fc fragment variant having a non-cysteine residue at amino acid 6. The invention also relates to nucleic acid and amino acid sequences encoding the fusion protein and transfected cell lines and methods for producing the fusion protein. The invention further includes pharmaceutical compositions comprising the fusion protein and methods of using the fusion protein and / or the pharmaceutical compositions, for example to stimulate erythropoiesis in subjects in need of therapy.
Owner:NOVAGEN HLDG CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com