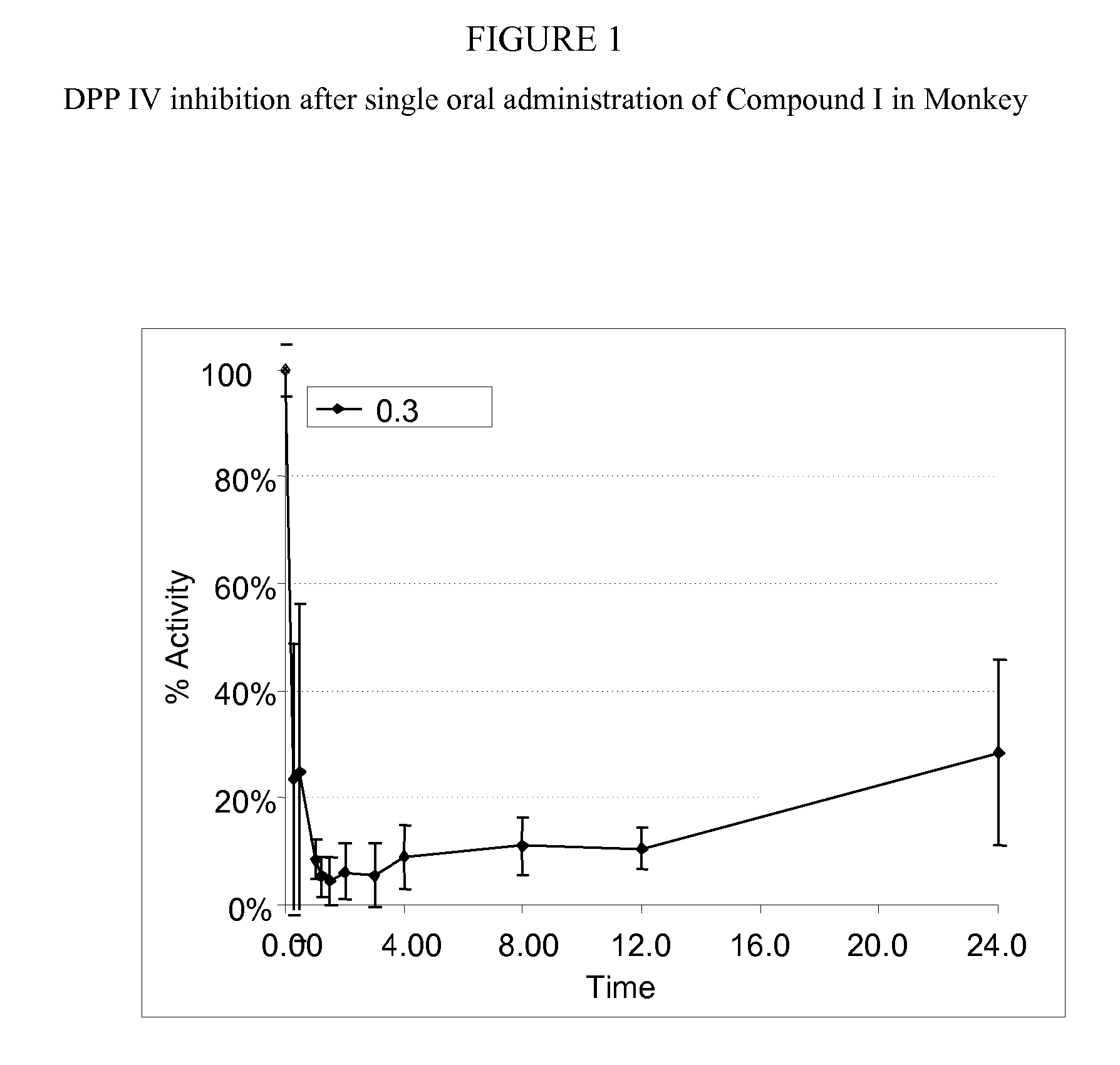Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6331results about How to "Convenient treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
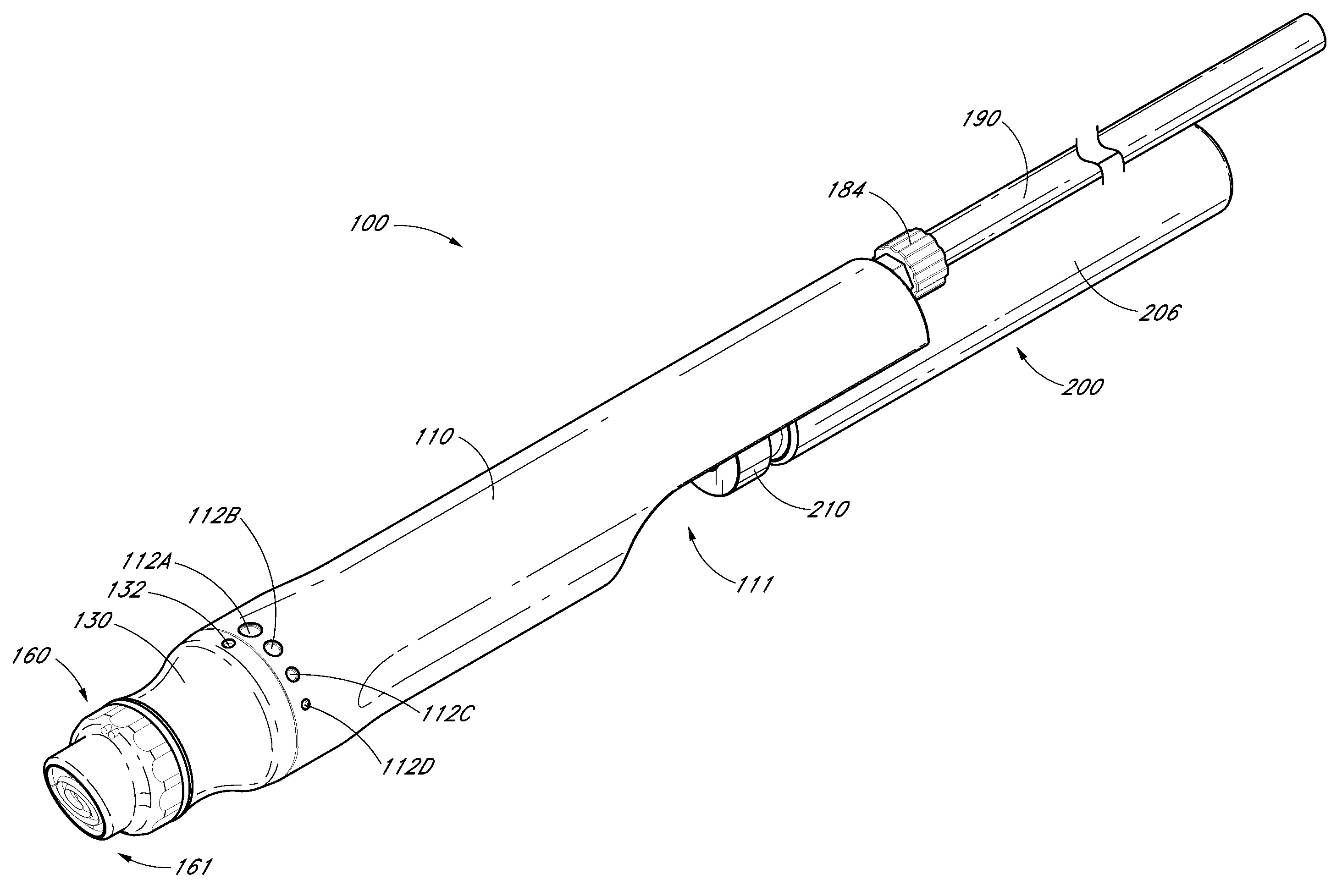
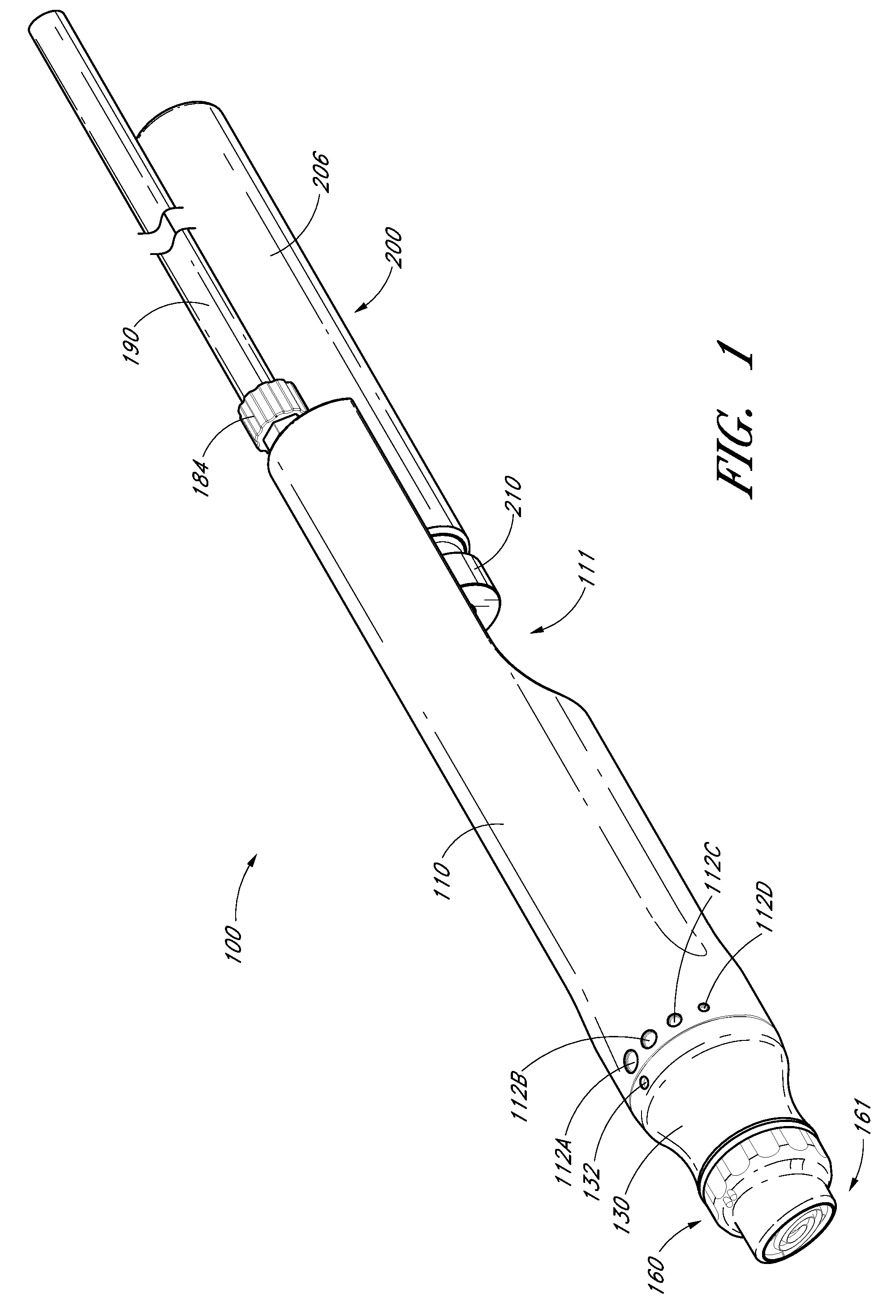
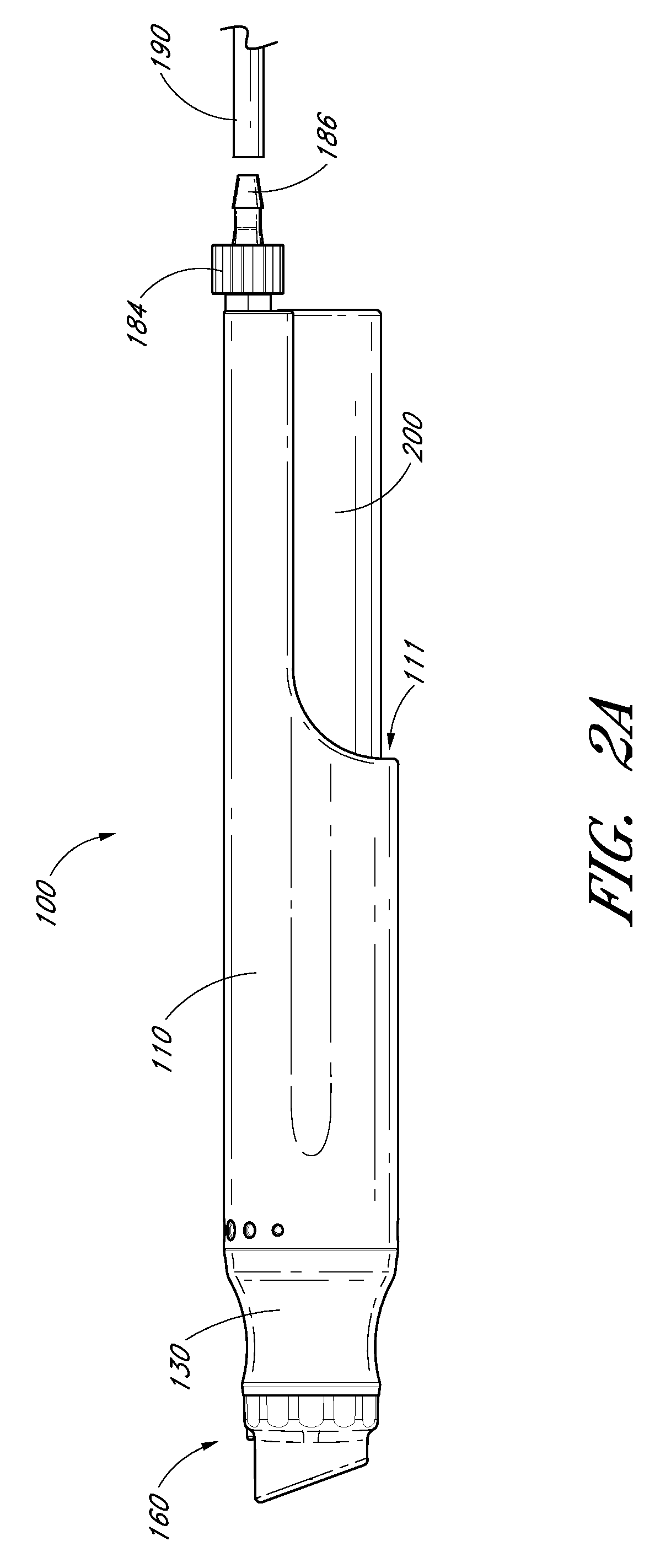
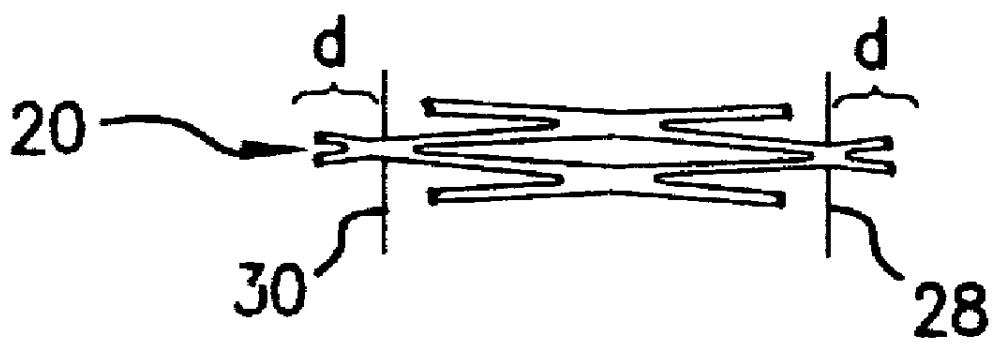
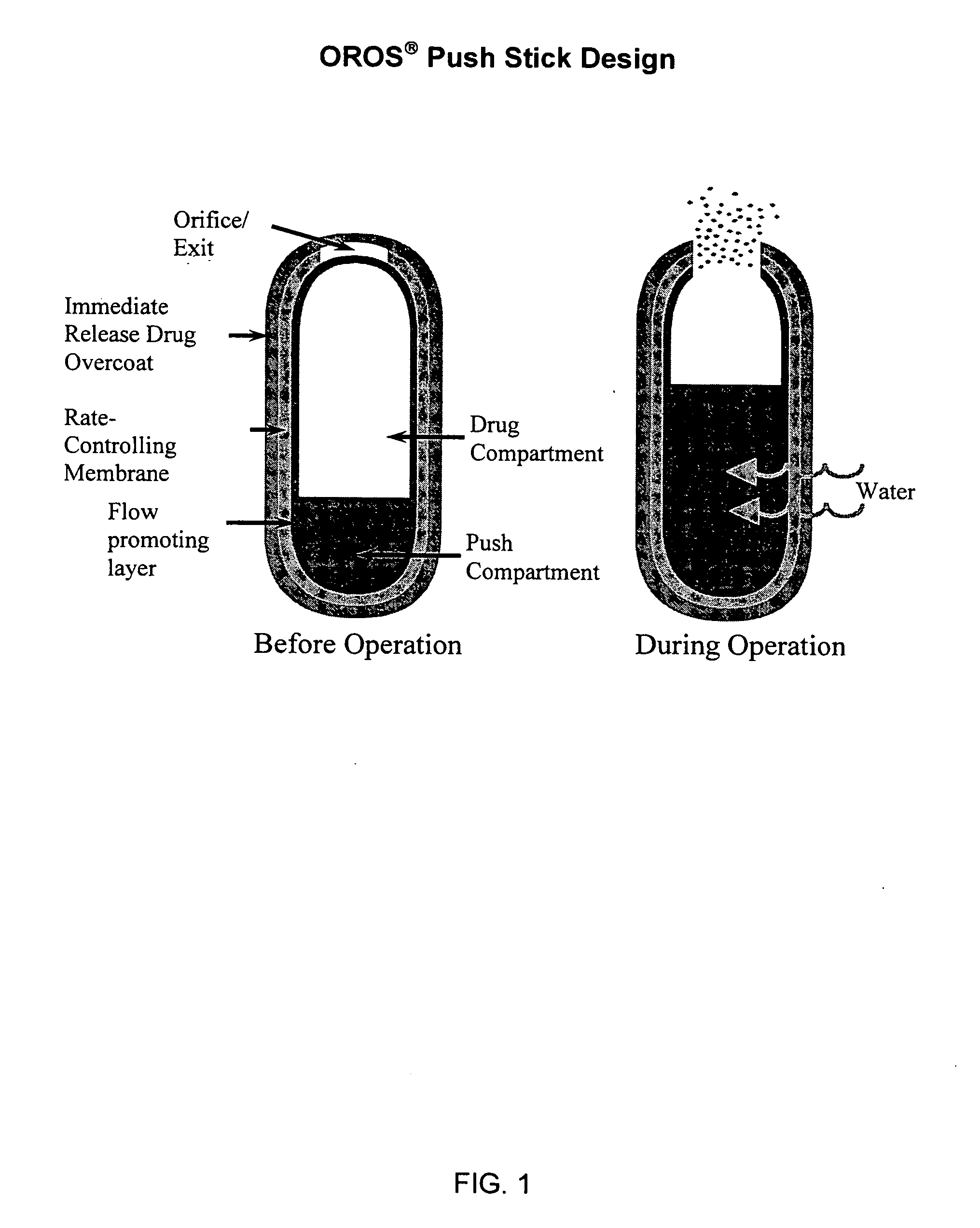
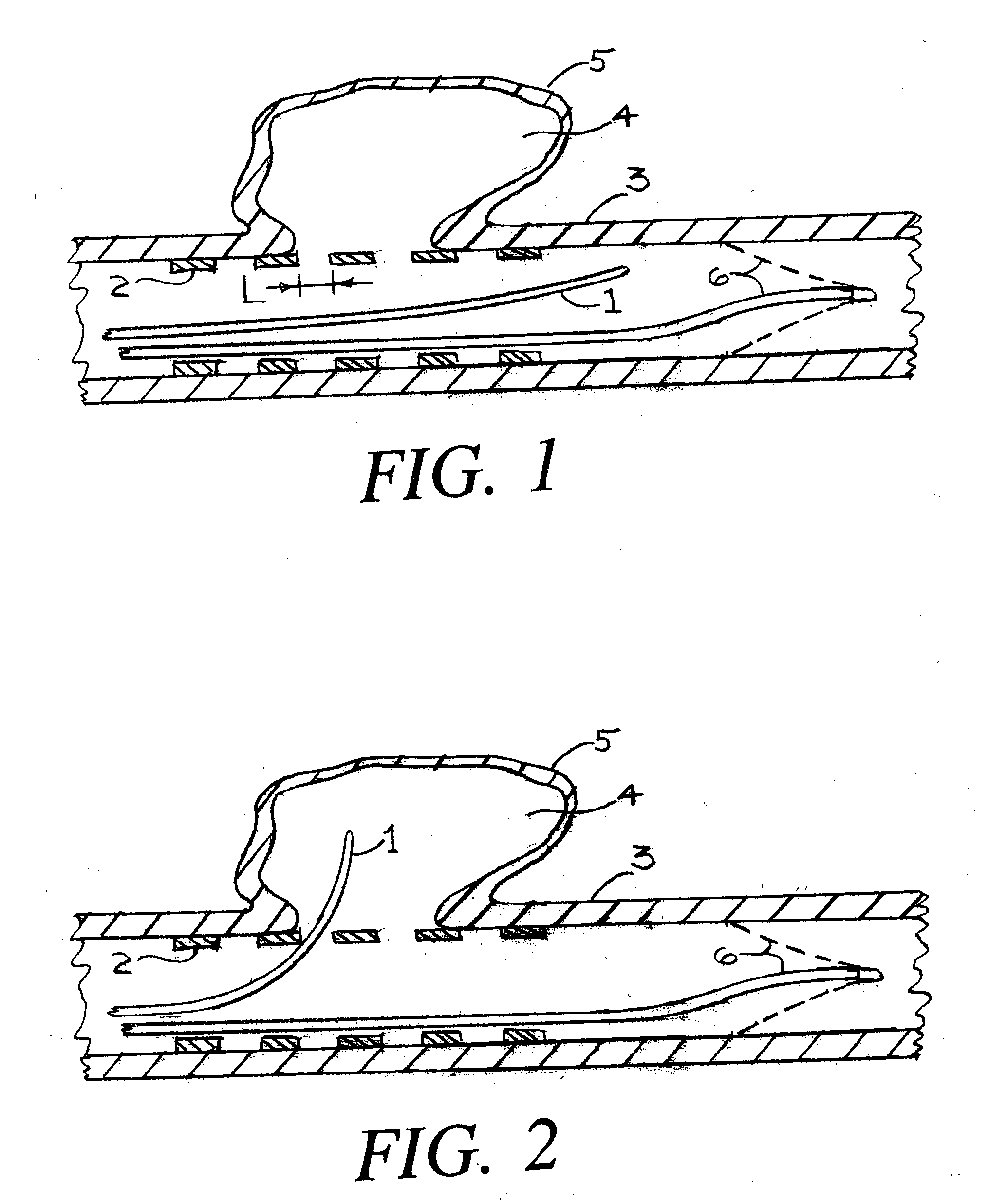
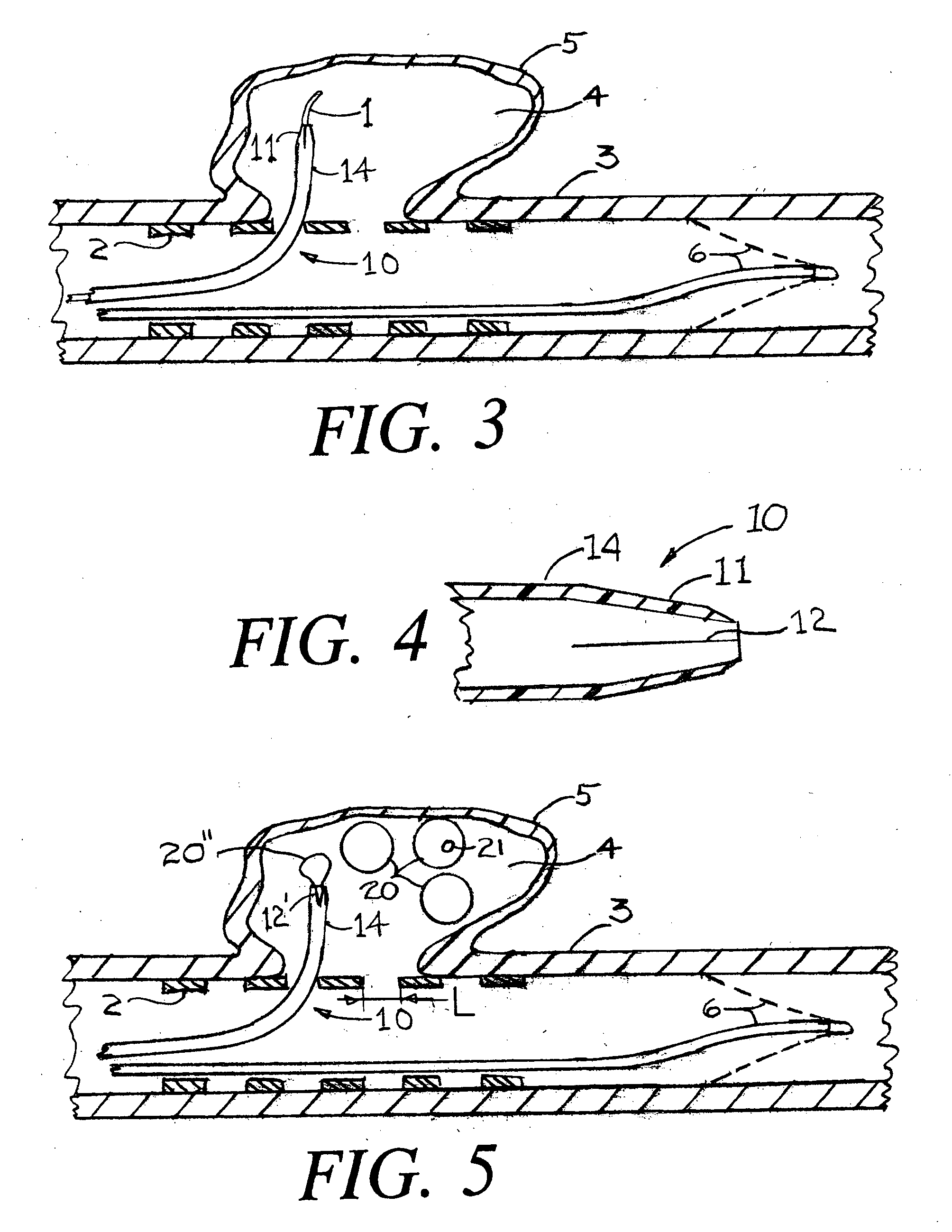
Devices, systems and methods for treating the skin using time-release substances
ActiveUS8814836B2Facilitate treatment procedureConvenient treatmentChiropractic devicesVibration massageLiquid wasteSkin surface
According to some embodiments, a microdermabrasion device for treating skin comprises a handpiece assembly having a distal end and a proximal end. The handpiece assembly includes at least one delivery conduit and at least one waste conduit. The microdermabrasion device additionally comprises a tip configured to be positioned along the distal end of the handpiece assembly, wherein the tip is adapted to contact skin surface. In several embodiments, the tip comprises a lip, a first opening in fluid communication with the fluid delivery conduit and a second opening in fluid communication with the waste conduit. In one embodiment, the device includes one or more abrasive elements positioned along a distal end of the tip, wherein the abrasive elements are configured to selectively remove skin as the tip is moved relative to a skin surface. In some embodiments, the delivery conduit is configured to selectively deliver at least one time-release material to the skin surface being treated.
Owner:HYDRAFACIAL LLC
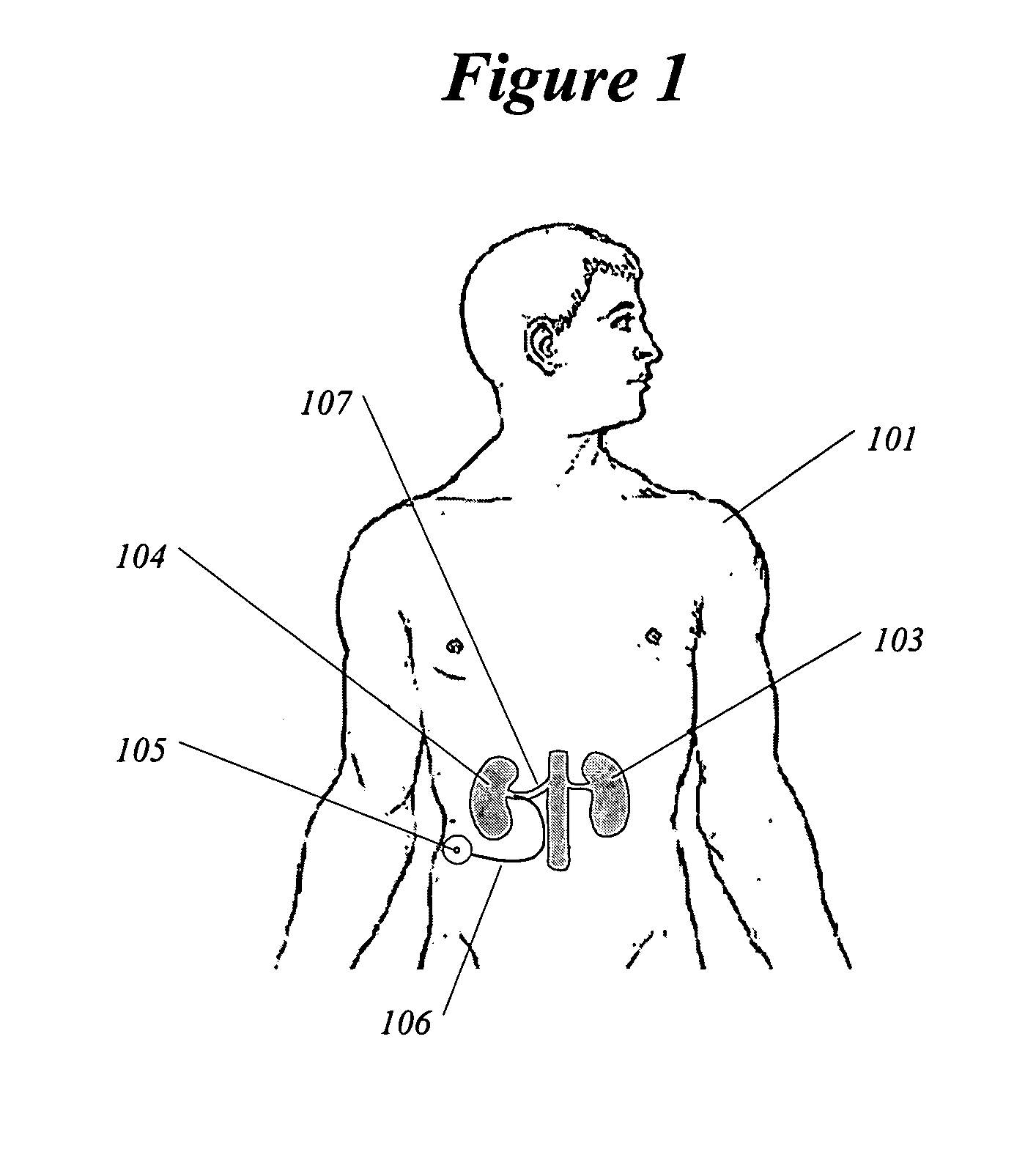
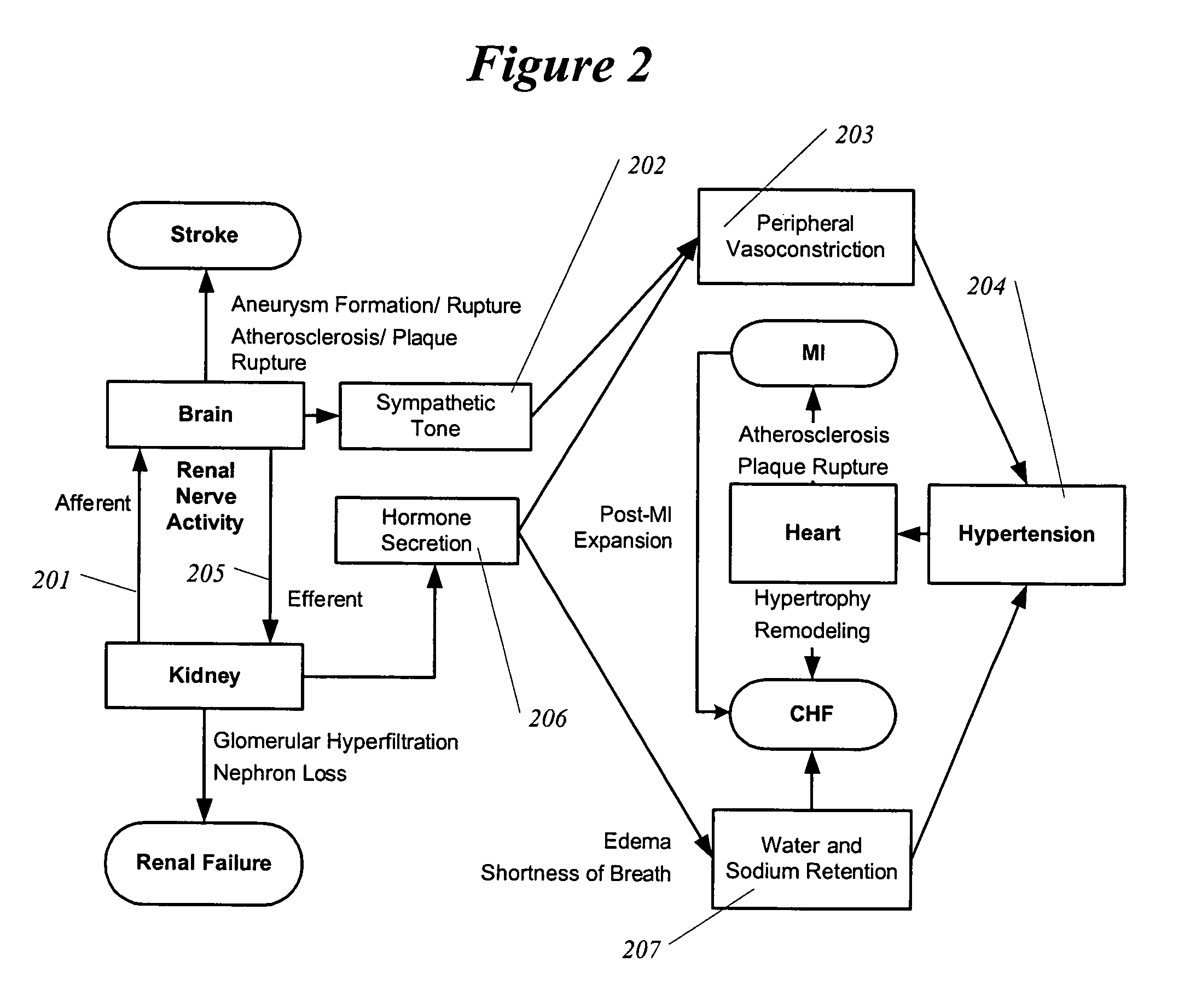
Methods and devices for renal nerve blocking
InactiveUS6978174B2Shorten the progressResolution of overloadPharmaceutical delivery mechanismImplantable neurostimulatorsDiseaseNephropathy
A method and apparatus for treatment of cardiac and renal diseases associated with the elevated sympathetic renal nerve activity by implanting a device to block the renal nerve signals to and from the kidney. The device can be a drug pump eluting implant for targeted delivery of a nerve-blocking agent to the periarterial space of the renal artery.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Non-foreshortening intraluminal prosthesis
InactiveUS6106548ADifferent degree of flexibilityVaried flexibilityStentsBlood vesselsProsthesisEngineering
An intraluminal prosthesis is provided with a plurality of annular elements. Each annular element includes a plurality of struts and apices connected to form an annular configuration. Each annular element has a compressed state and an expanded state, and has a longitudinal dimension which is smaller in the expanded state than in the compressed state. A plurality of connecting members connect the apices of adjacent annular elements. The connecting members have a plurality of alternating segments that function to compensate for the smaller longitudinal dimension of each annular element in the expanded state. The stent may be provided with varying flexibility along its length and / or circumference, and may include segments that have different diameters.
Owner:ENDOSYST
Method, apparatus, and surgical technique for autonomic neuromodulation for the treatment of disease
InactiveUS20060167498A1Reduce or prevent conditionReducing and preventing symptomSpinal electrodesSurgical needlesSplanchnic nervesDisease
The present invention teaches a method and apparatus for physiological modulation, including neural and gastrointestinal modulation, for the purposes of treating several disorders, including obesity, depression, epilepsy, and diabetes. This includes chronically implanted neural and neuromuscular modulators, used to modulate the afferent neurons of the sympathetic nervous system to induce satiety. Furthermore, this includes neuromuscular stimulation of the stomach to effect baseline and intermittent smooth muscle contraction to increase gastric intraluminal pressure, which induces satiety, and stimulate sympathetic afferent fibers, including those in the sympathetic trunk, splanchnic nerves, and greater curvature of the stomach, to augment the perception of satiety.
Owner:DILORENZO BIOMEDICAL
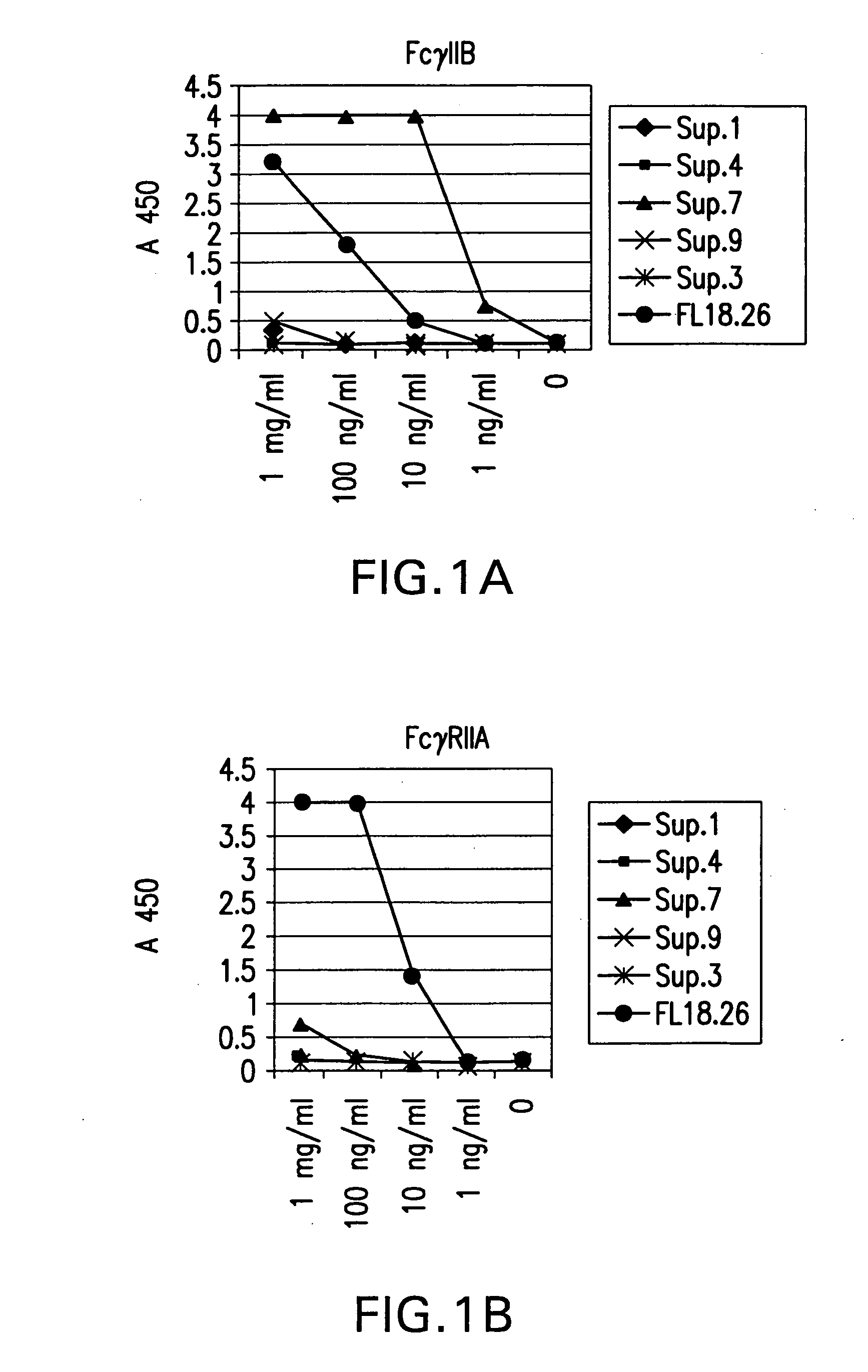
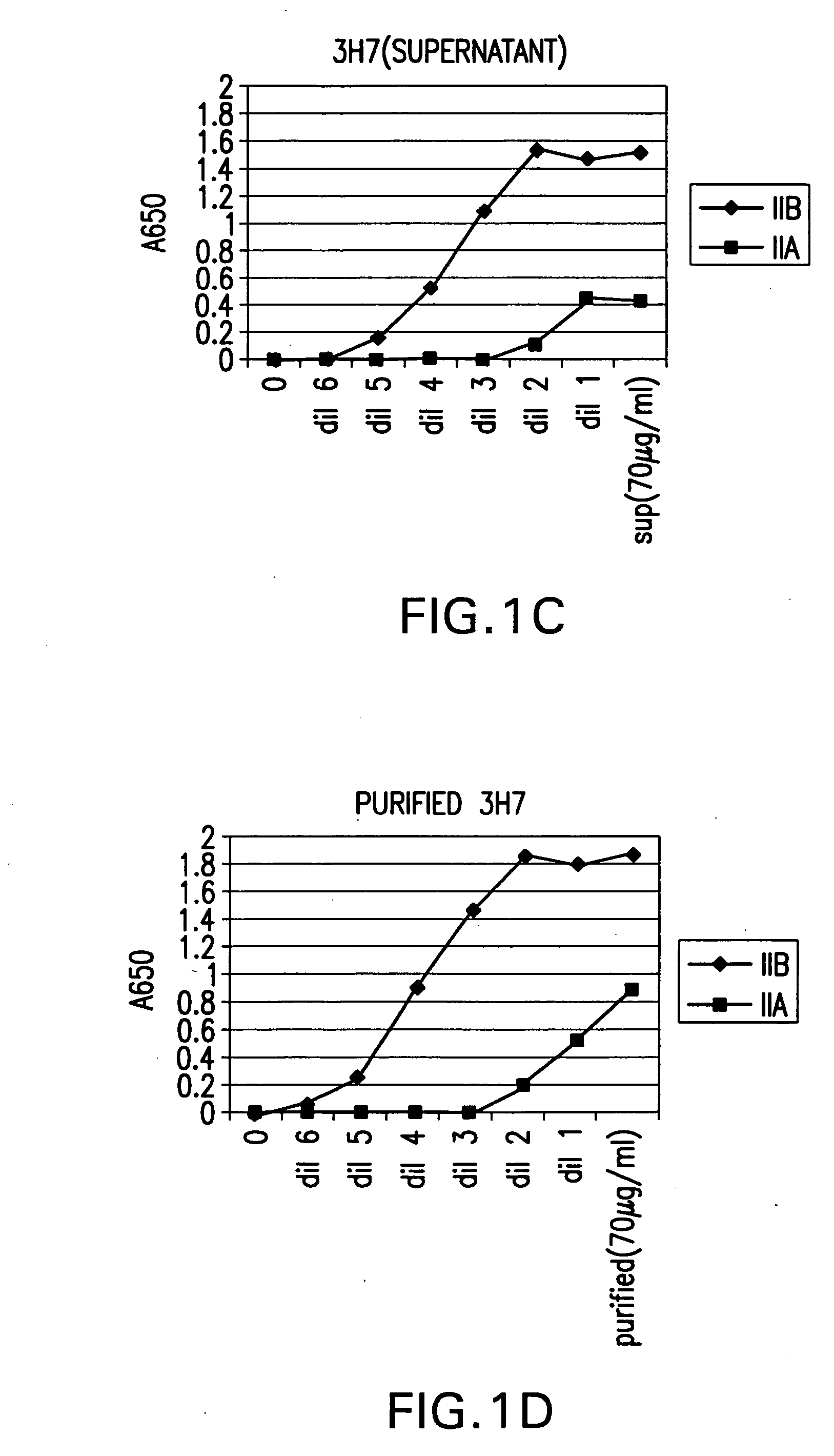
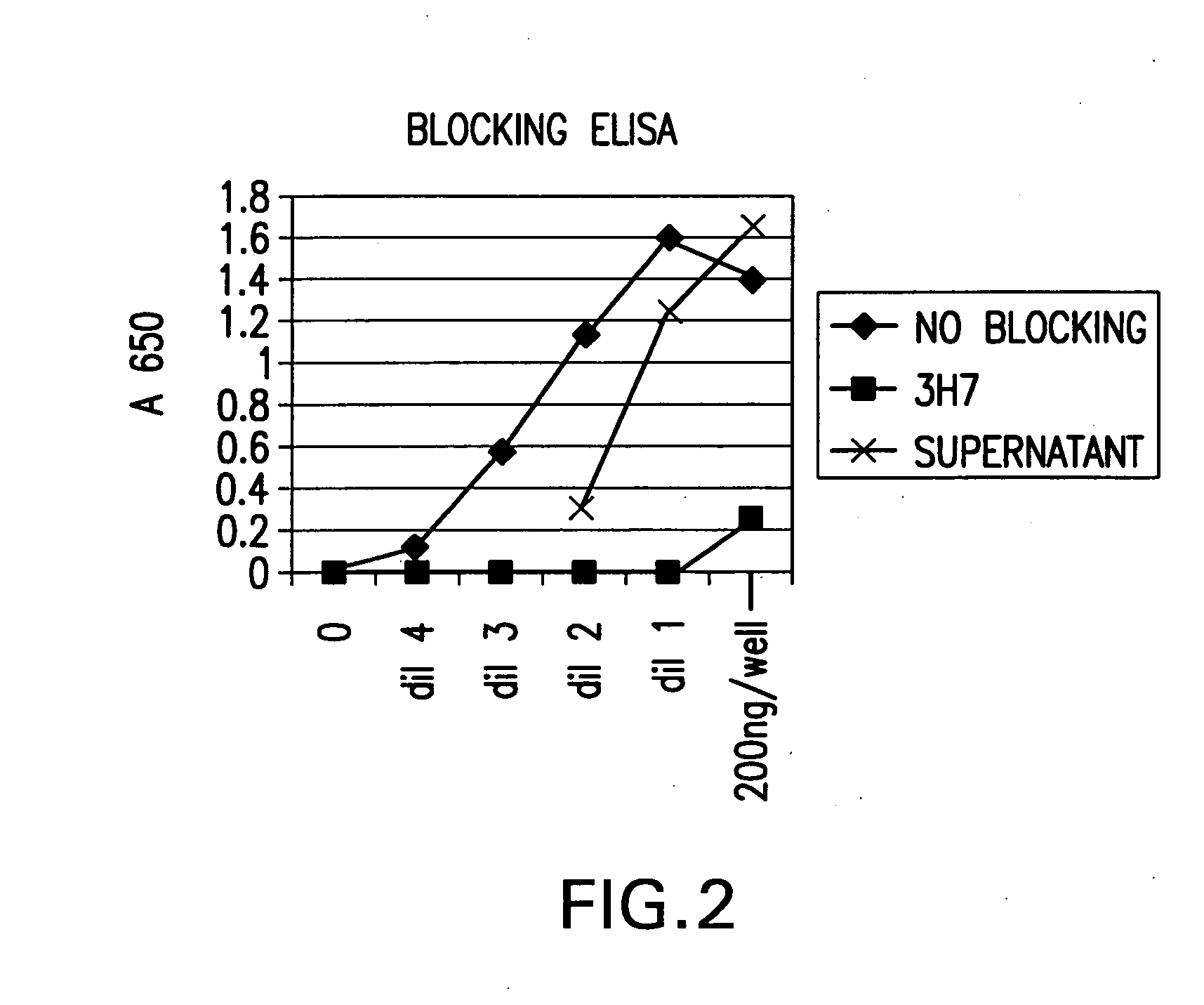
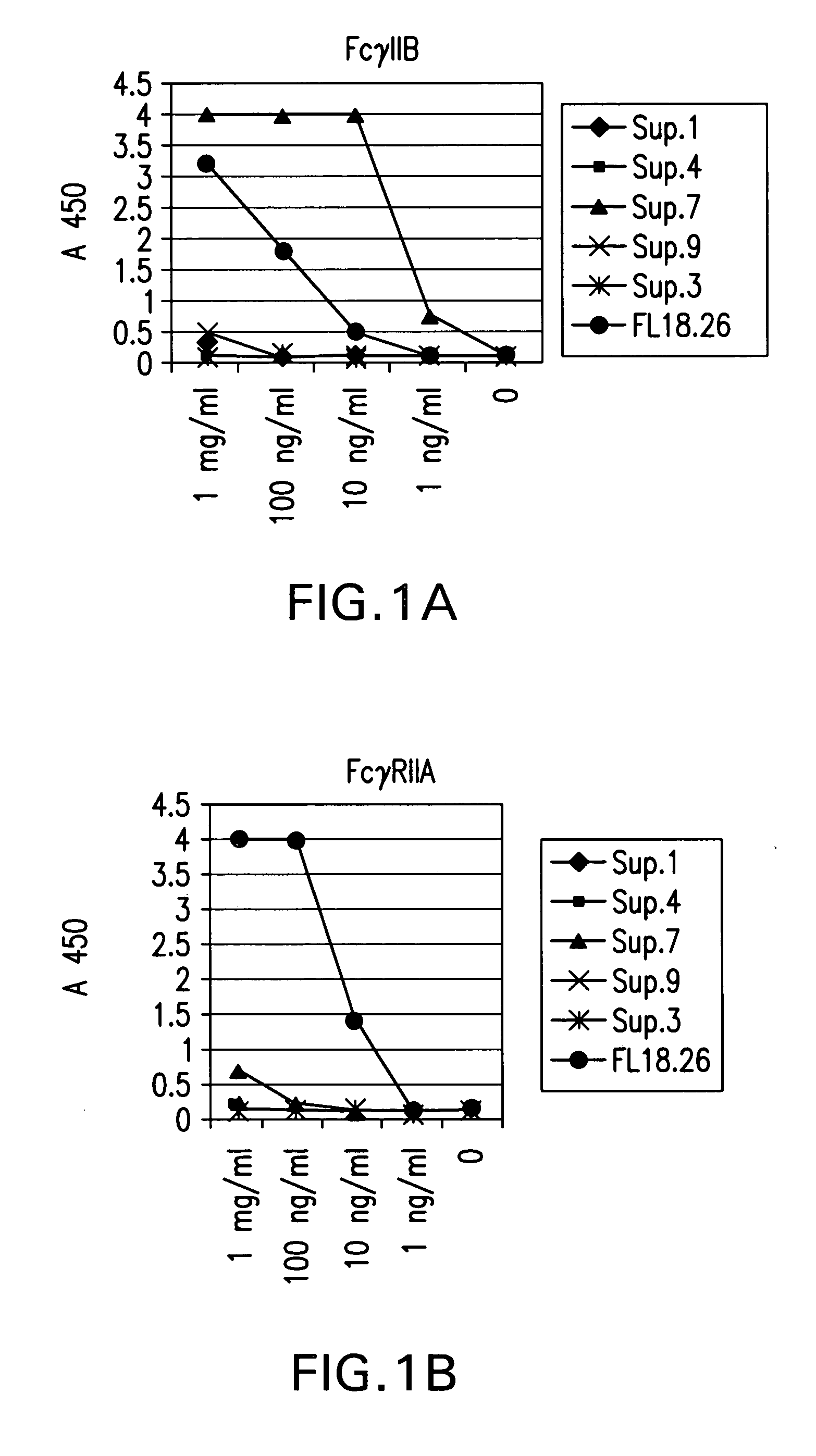
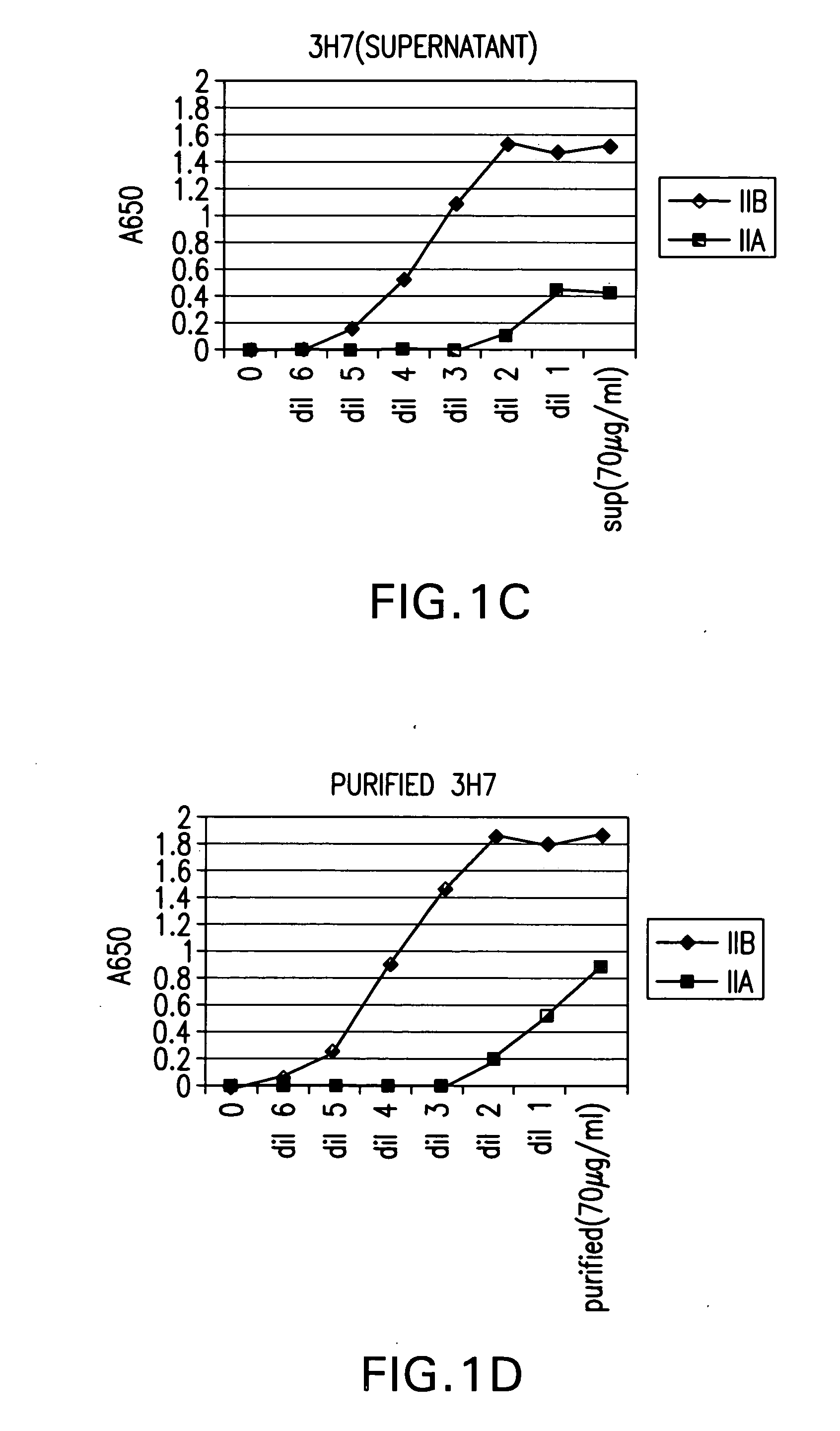
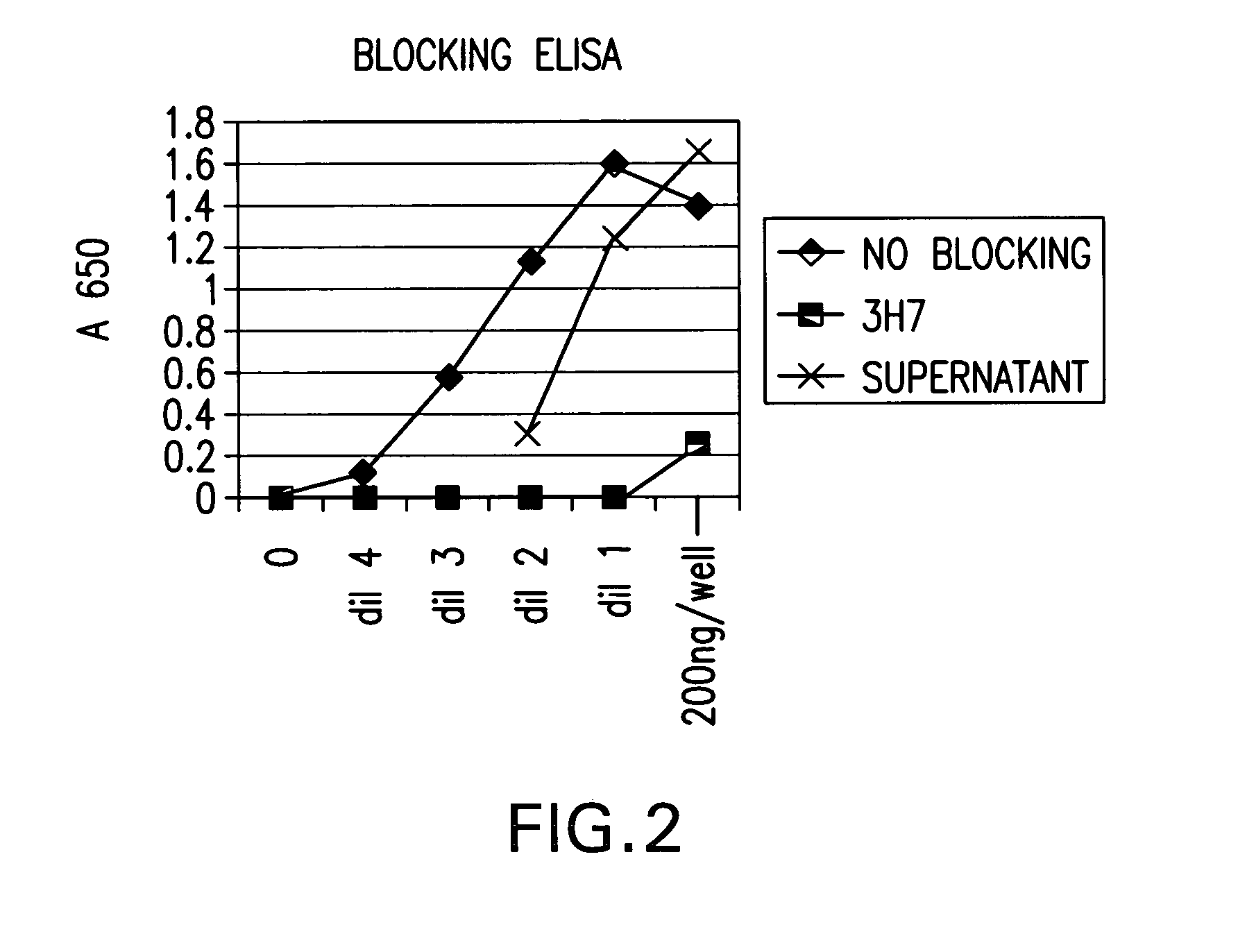
FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20040185045A1Strong therapeutic activityEnhancing antibody-mediated effector functionSenses disorderAntipyreticTherapeutic antibodyTreatment effect
The present invention relates to antibodies or fragments thereof that specifically bind FcgammaRIIB, particularly human FcgammaRIIB, with greater affinity than said antibodies or fragments thereof bind FcgammaRIIA, particularly human FcgammaRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
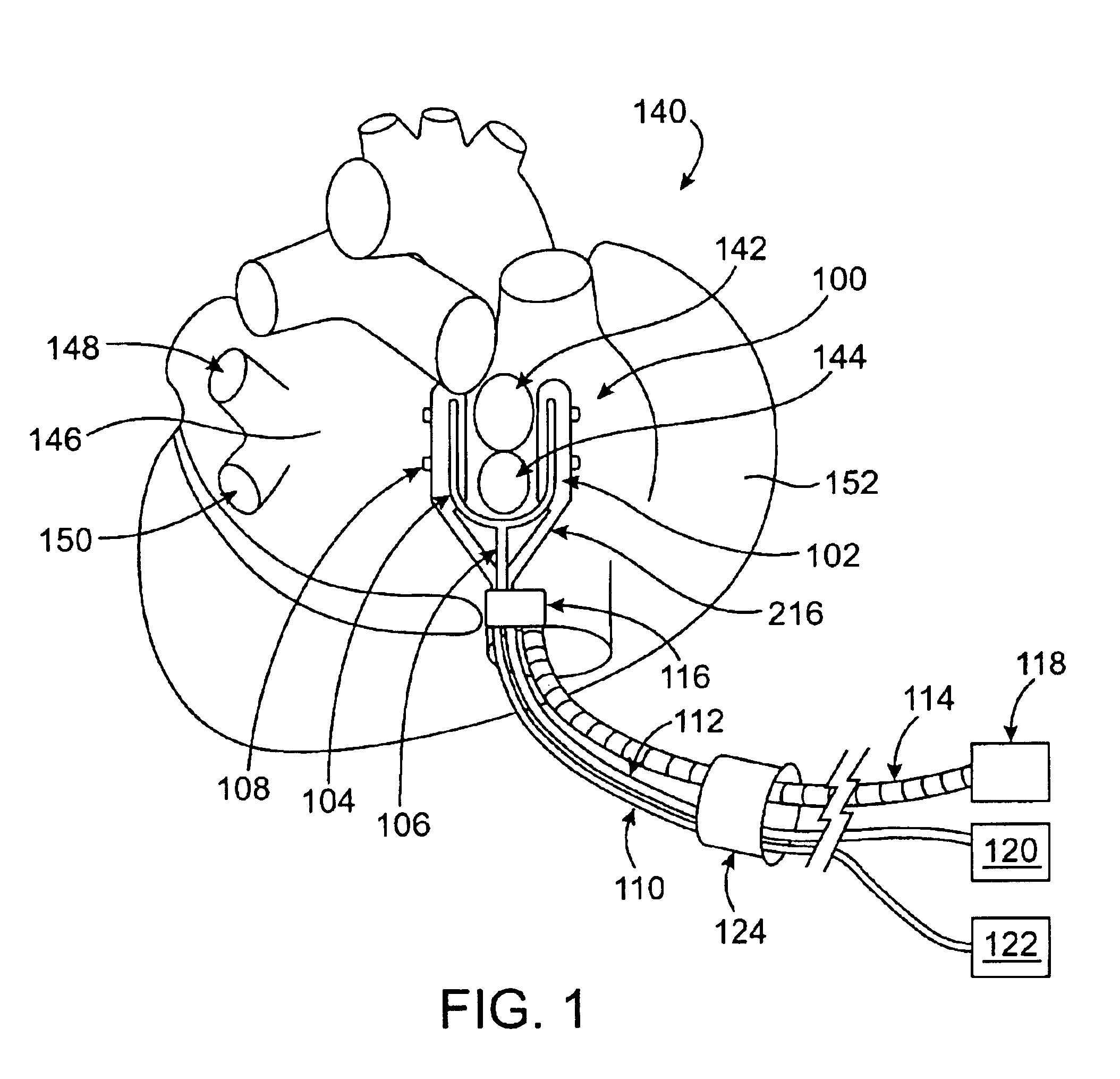
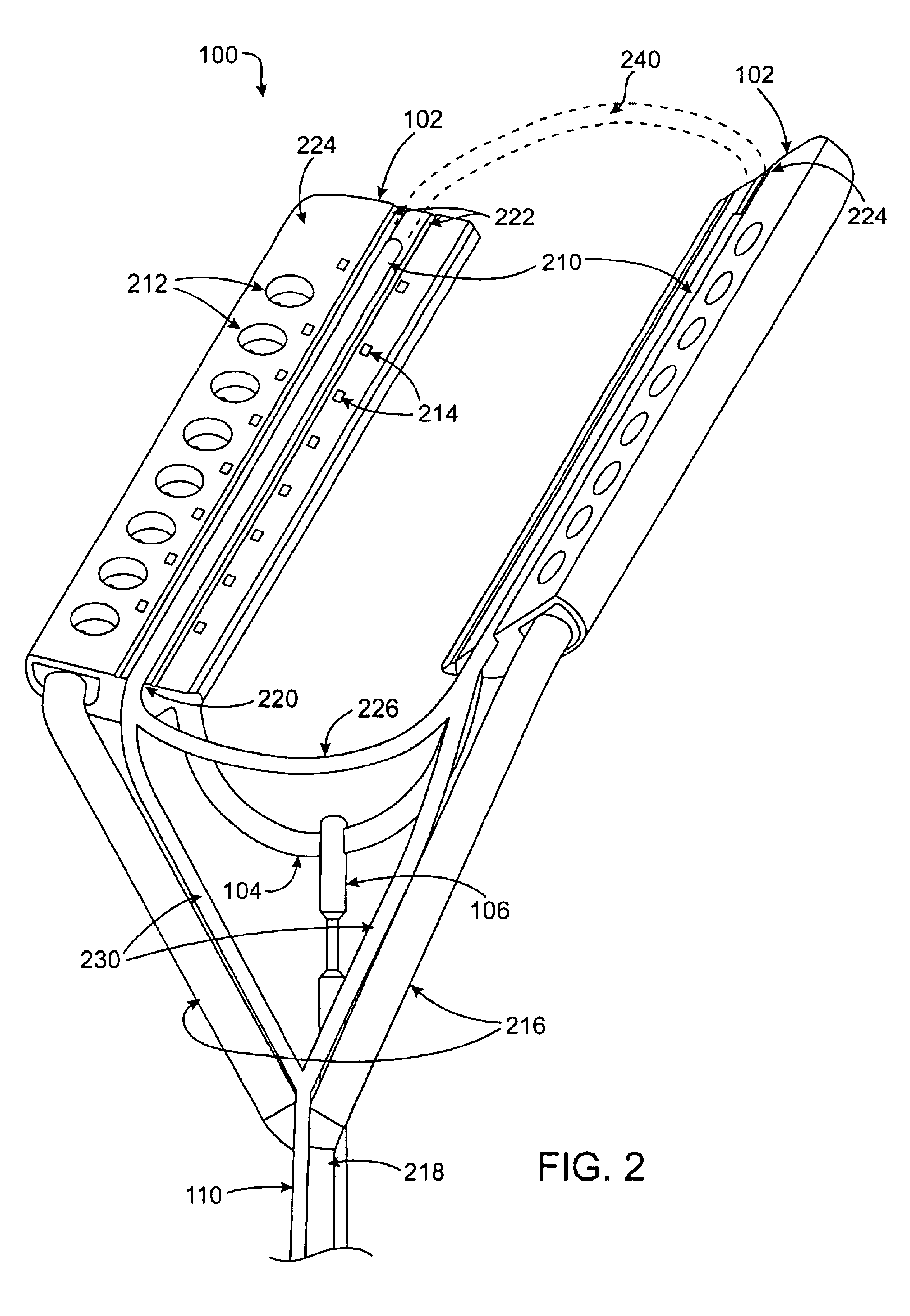
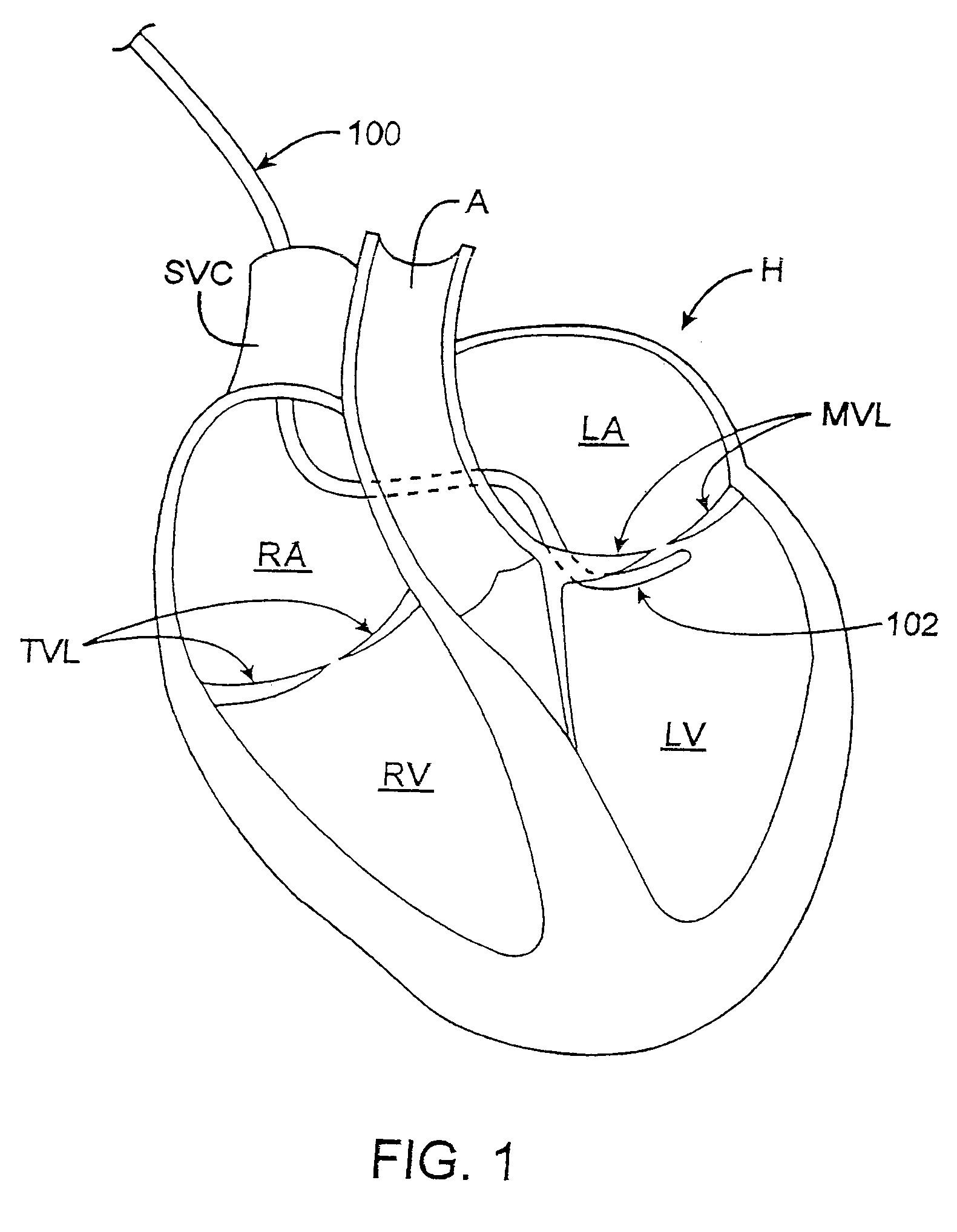
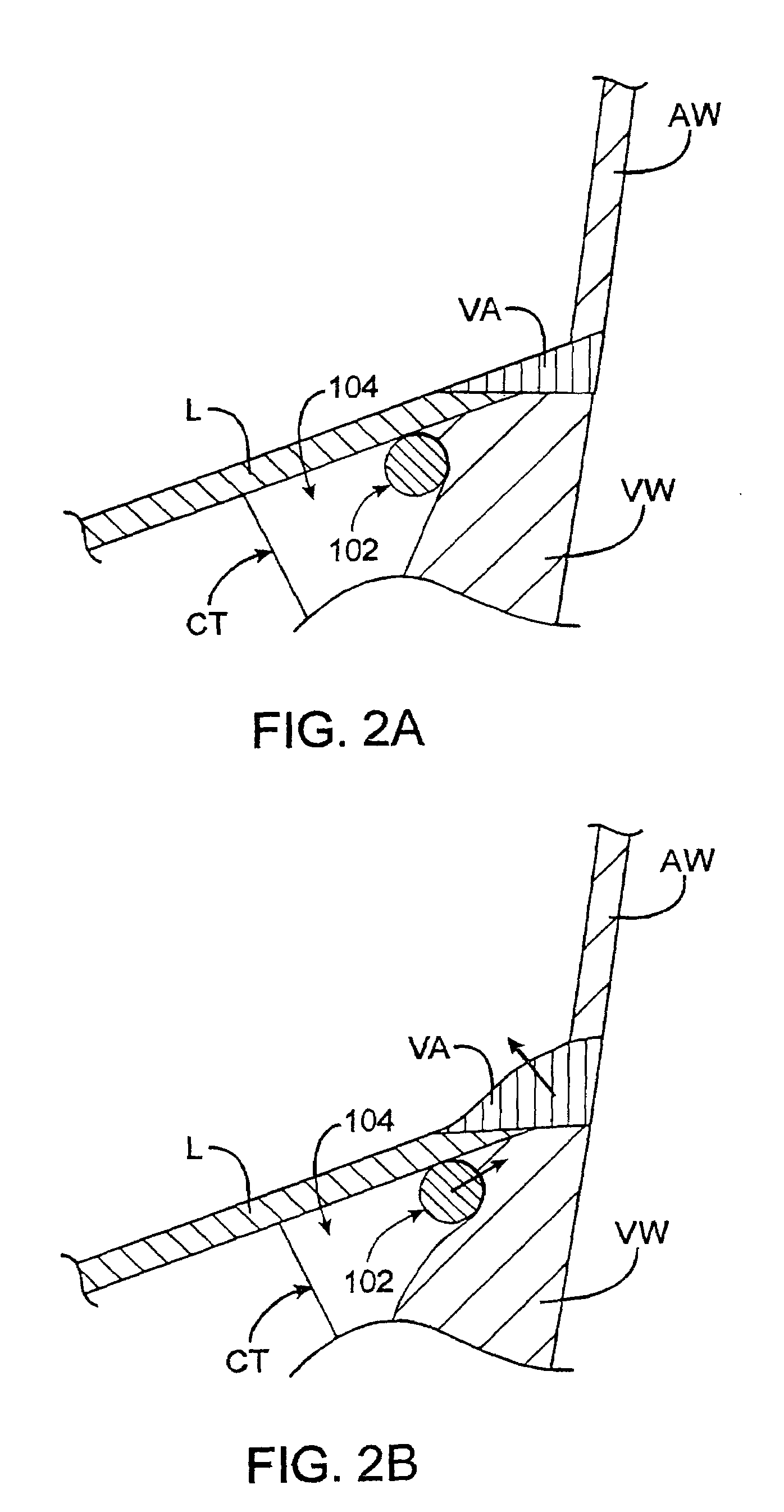
Cardiac ablation devices and methods
InactiveUS6849075B2Improve treatmentReduce and eliminate cardiac arrhythmiaElectrotherapyEndoscopesAtrial cavityBiomedical engineering
Devices and methods provide for ablation of cardiac tissue for treating cardiac arrhythmias such as atrial fibrillation. Although the devices and methods are often be used to ablate epicardial tissue in the vicinity of at least one pulmonary vein, various embodiments may be used to ablate other cardiac tissues in other locations on a heart. Devices generally include at least one tissue contacting member for contacting epicardial tissue and securing the ablation device to the epicardial tissue, and at least one ablation member for ablating the tissue. Various embodiments include features, such as suction apertures, which enable the device to attach to the epicardial surface with sufficient strength to allow the tissue to be stabilized via the device. For example, some embodiments may be used to stabilize a beating heart to enable a beating heart ablation procedure. Many of the devices may be introduced into a patient via minimally invasive introducer devices and the like. Although devices and methods of the invention may be used to ablate epicardial tissue to treat atrial fibrillation, they may also be used in veterinary or research contexts, to treat various heart conditions other than atrial fibrillation and / or to ablate cardiac tissue other than the epicardium.
Owner:ATRICURE +1
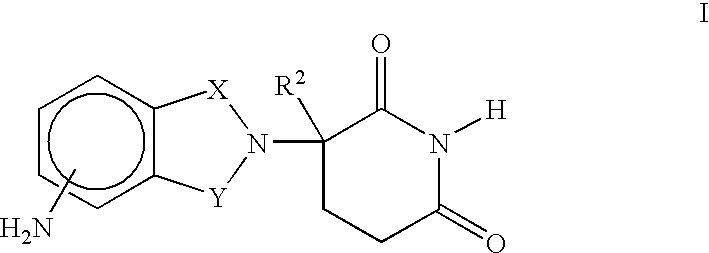
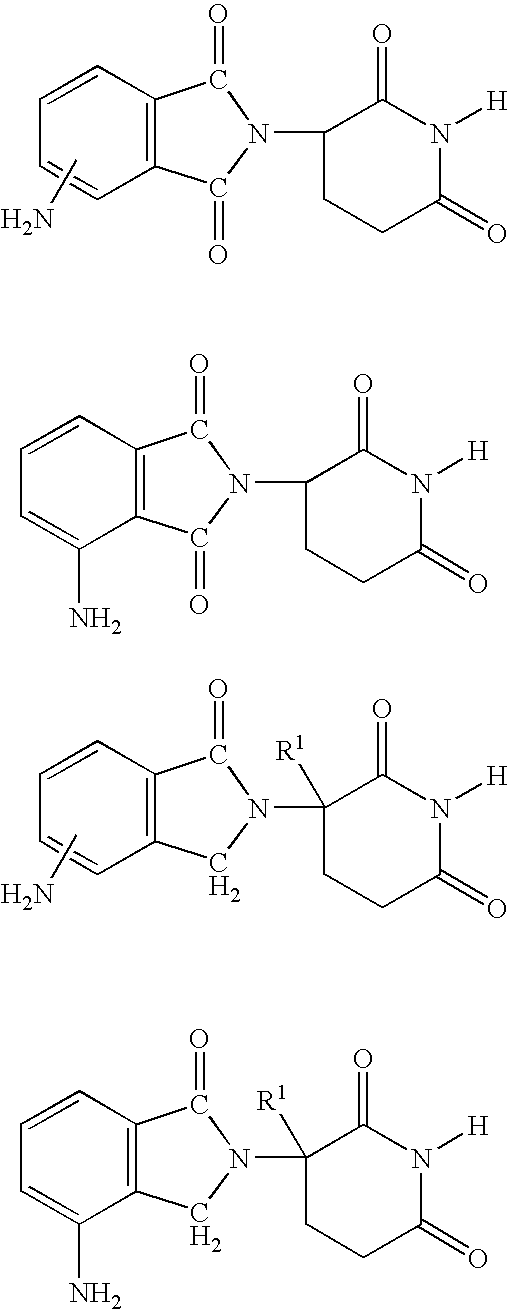
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of myeloproliferative diseases
InactiveUS20040087546A1Reduce adverse effectsImprove toleranceBiocideNervous disorderActive agentMyeloproliferative disease
Methods of treating, preventing and / or managing a myeloproliferative disease are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent, and / or the transplantation of blood or cells. Particular second active agents are capable of suppressing the overproduction of hematopoietic stem cells or ameliorating one or more of the symptoms of a myeloproliferative disease. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Glaucoma implant with therapeutic agents
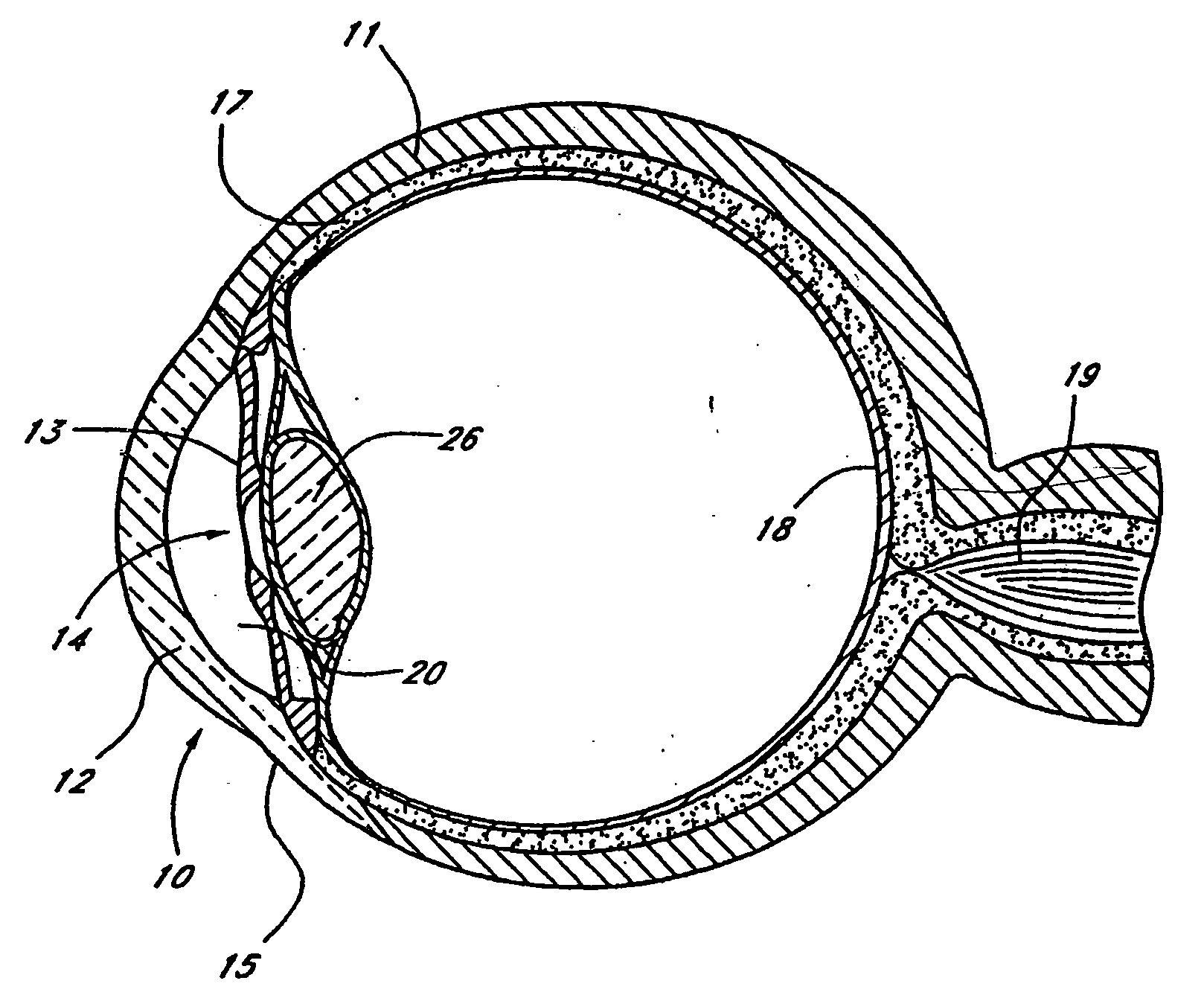
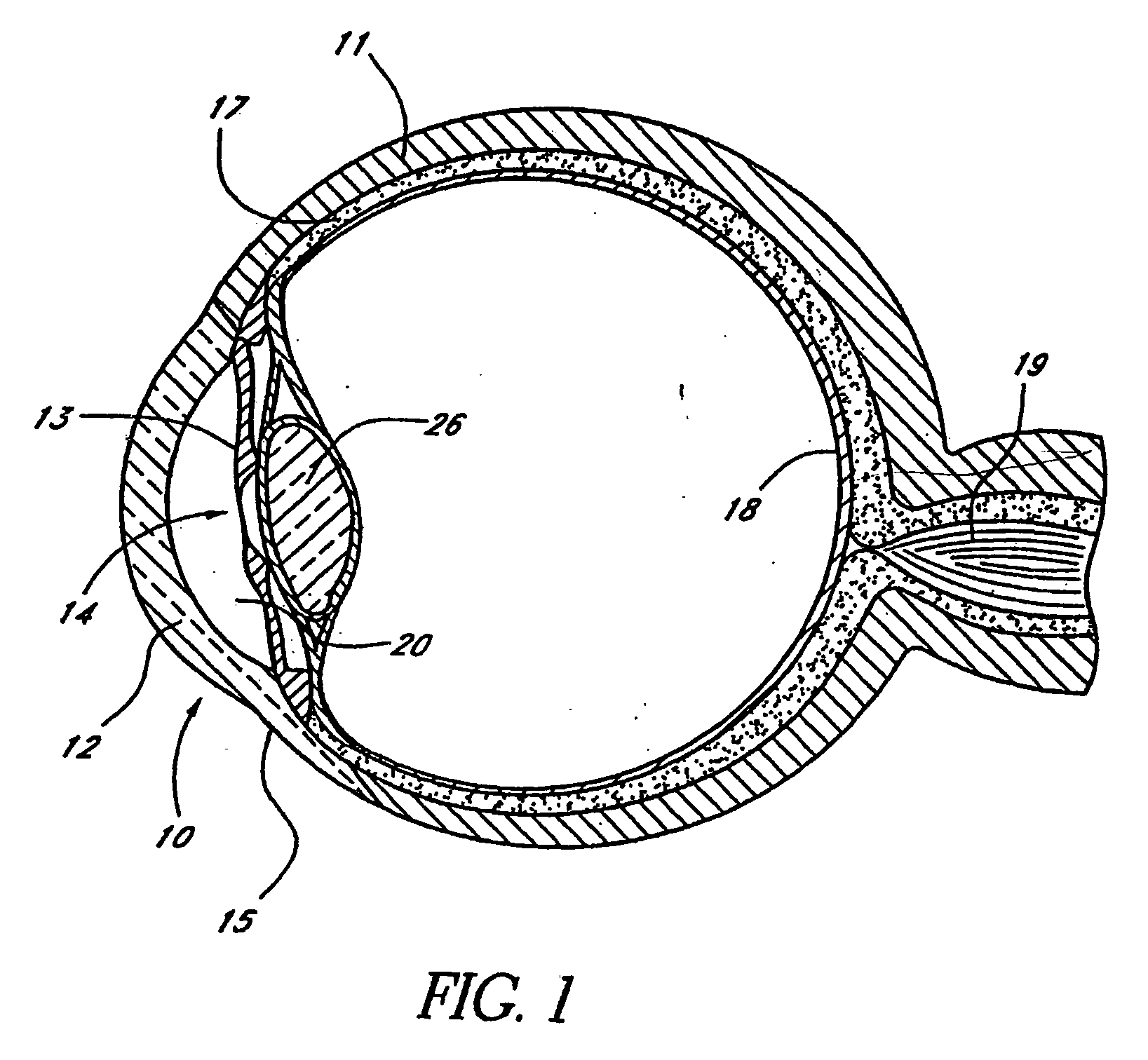
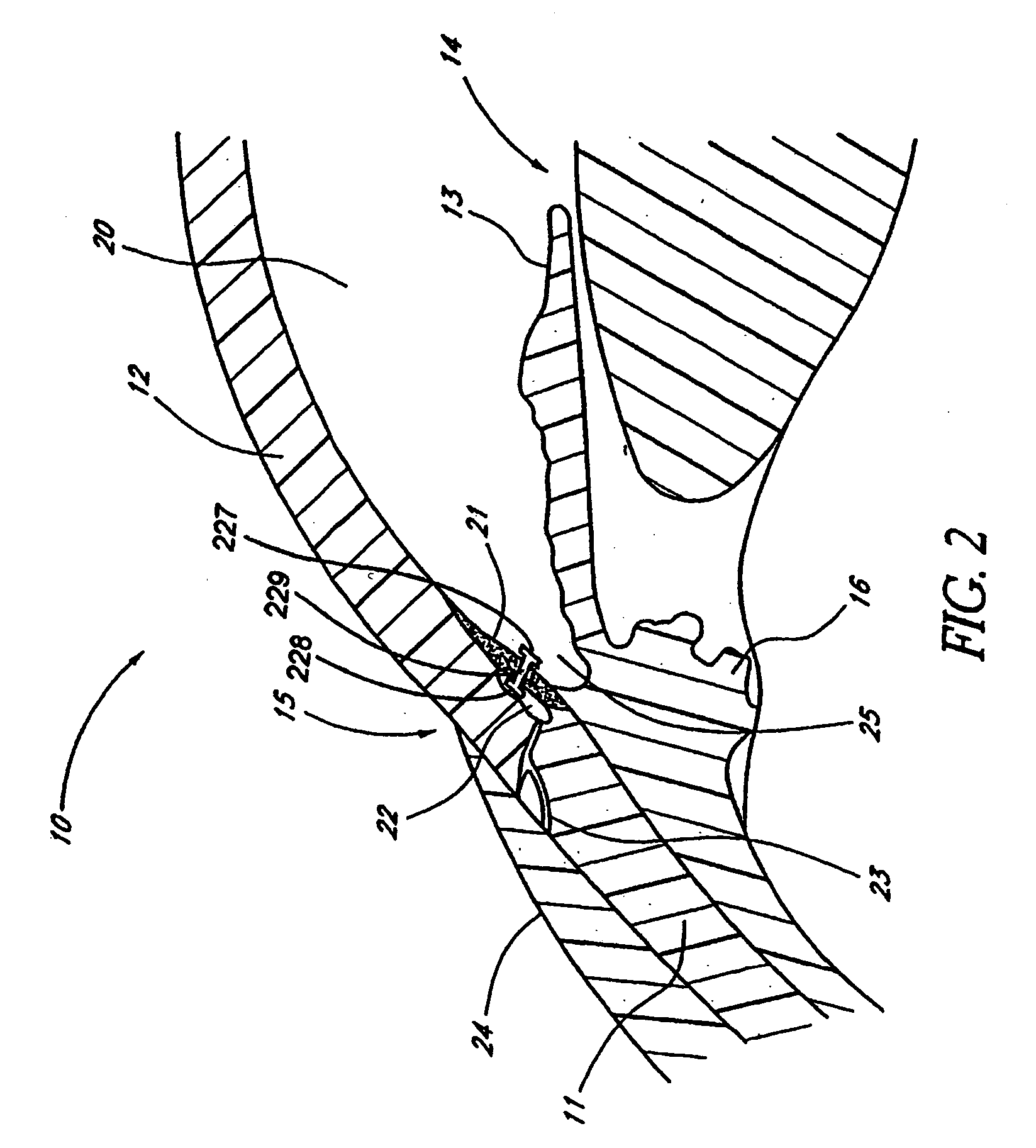
InactiveUS20040127843A1Convenient treatmentReduce and inhibit and slow effectEye surgeryWound drainsAqueous humorSchlemm's canal
Devices and methods are provided for the treatment of glaucoma. An ocular implant is adapted such that aqueous humor flows controllably from the anterior chamber of the eye to Schlemm's canal, bypassing the trabecular meshwork. The implant may utilize one or more bioactive agents effective in treating glaucoma or other pathology.
Owner:DOSE MEDICAL CORP
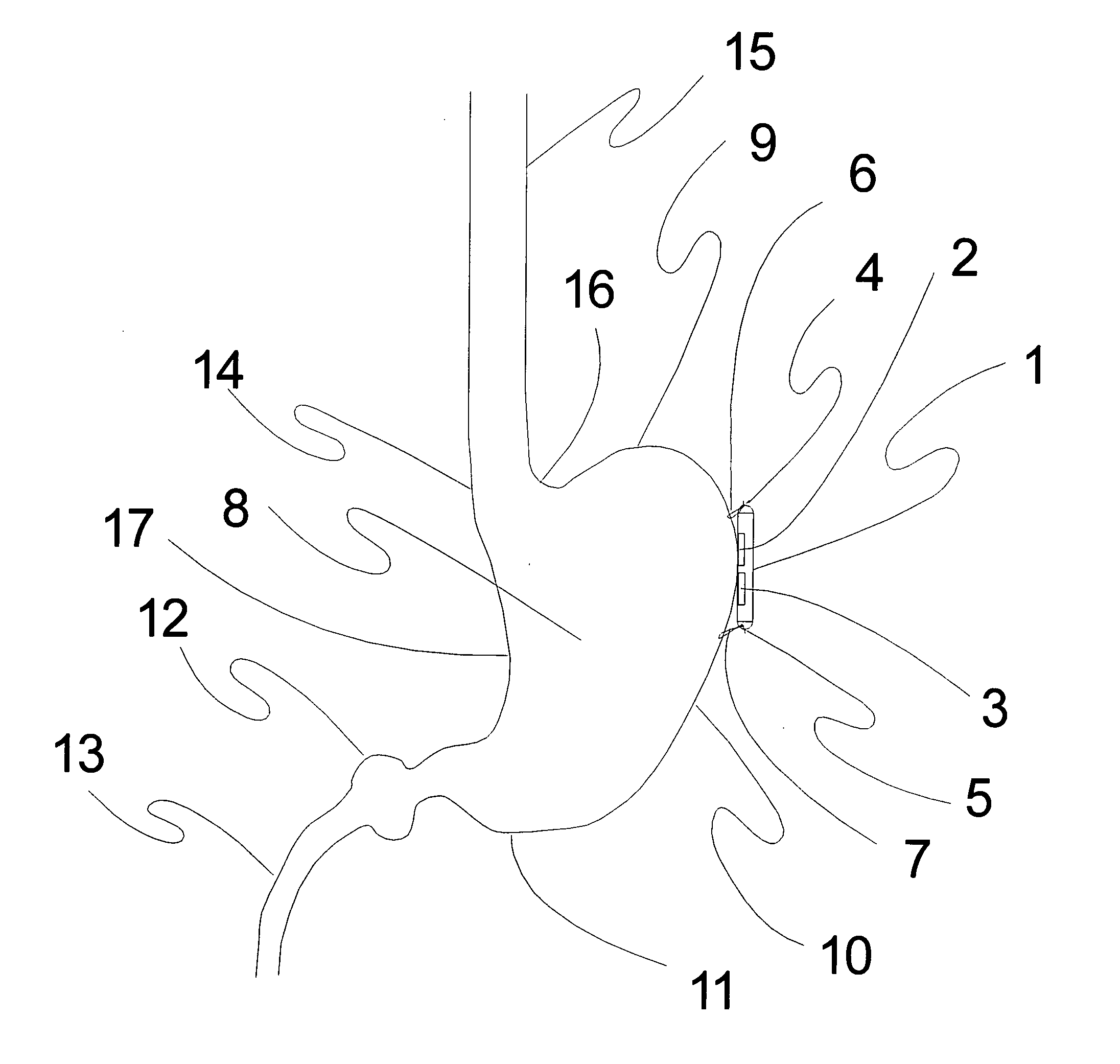
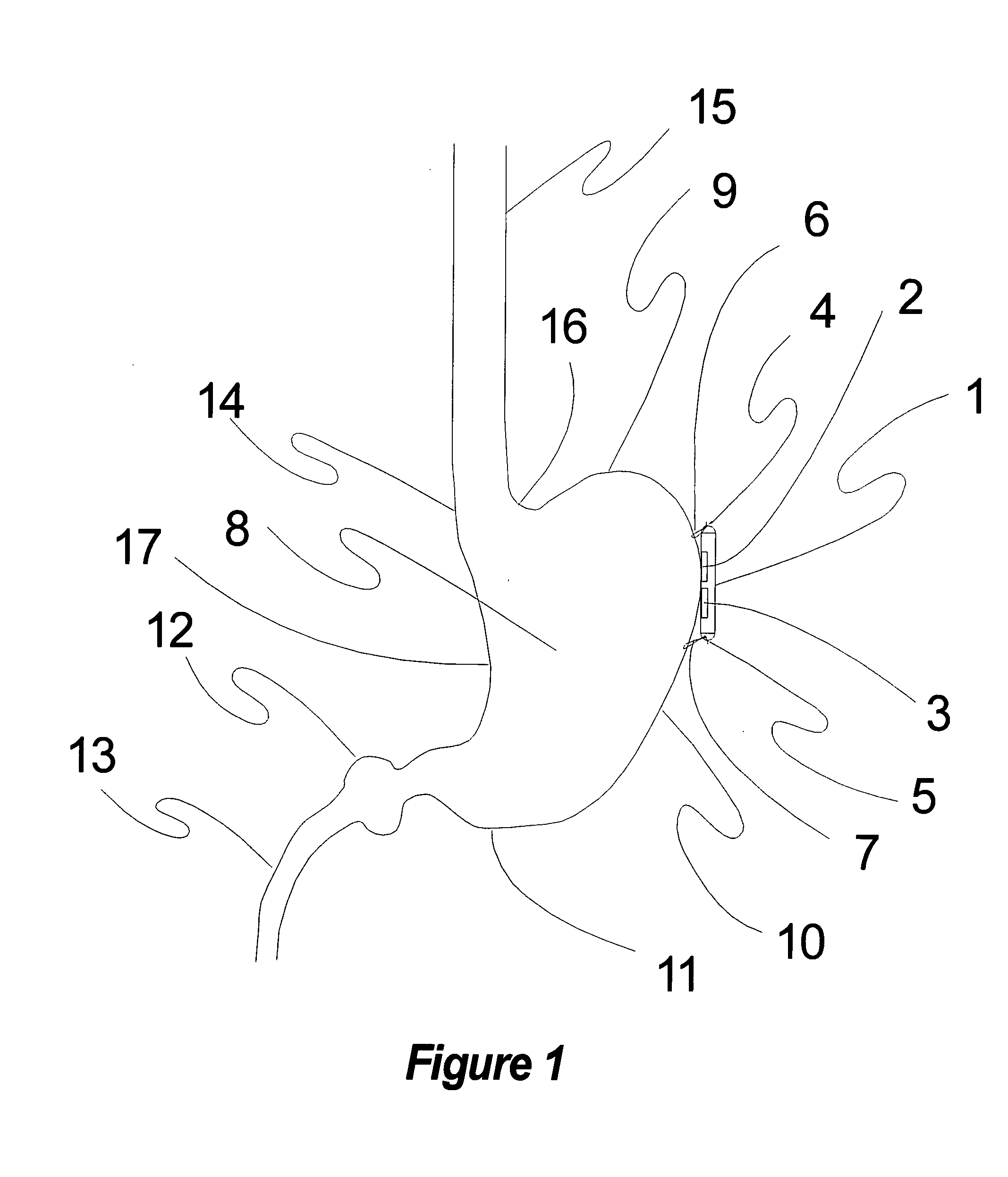
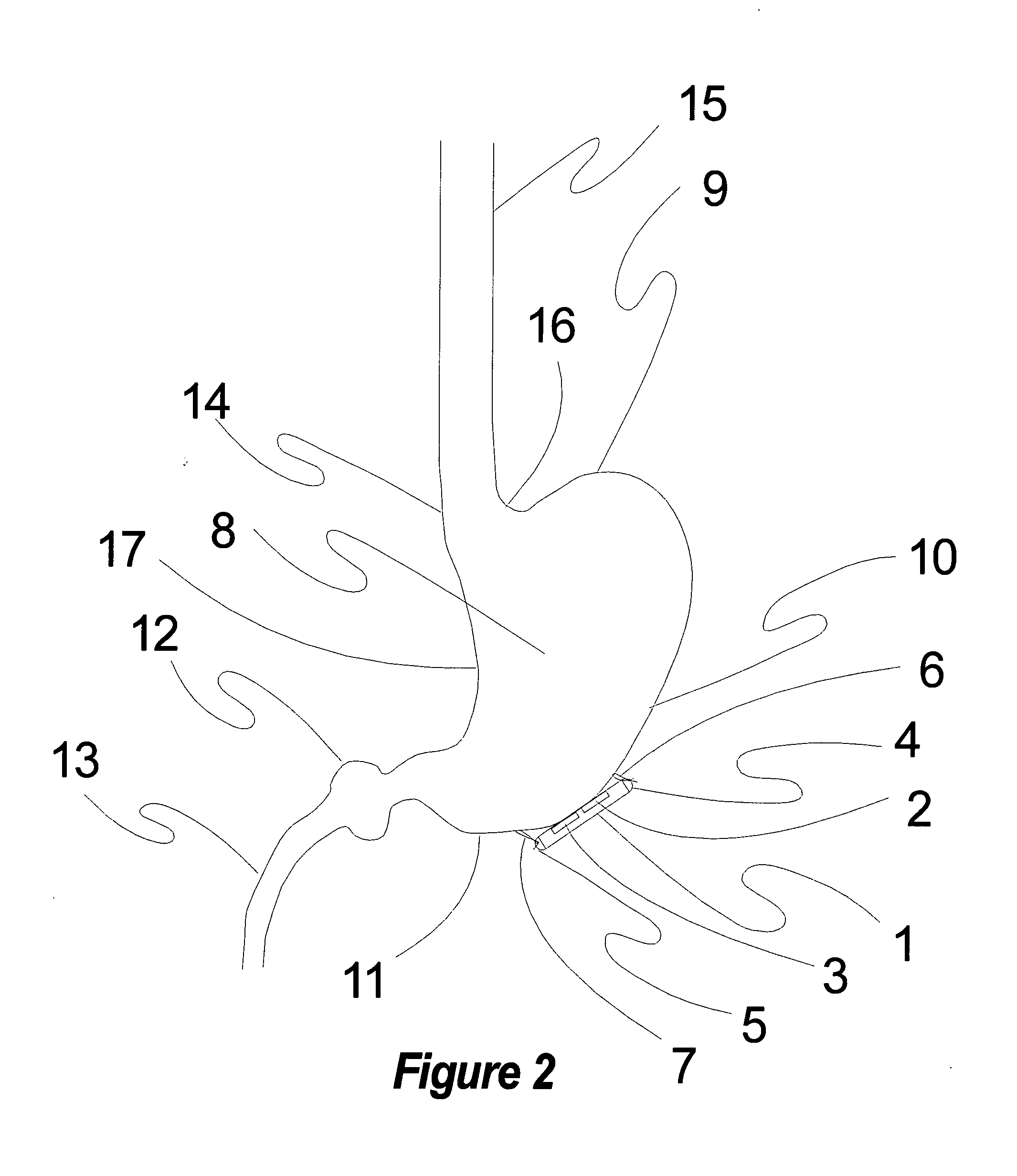
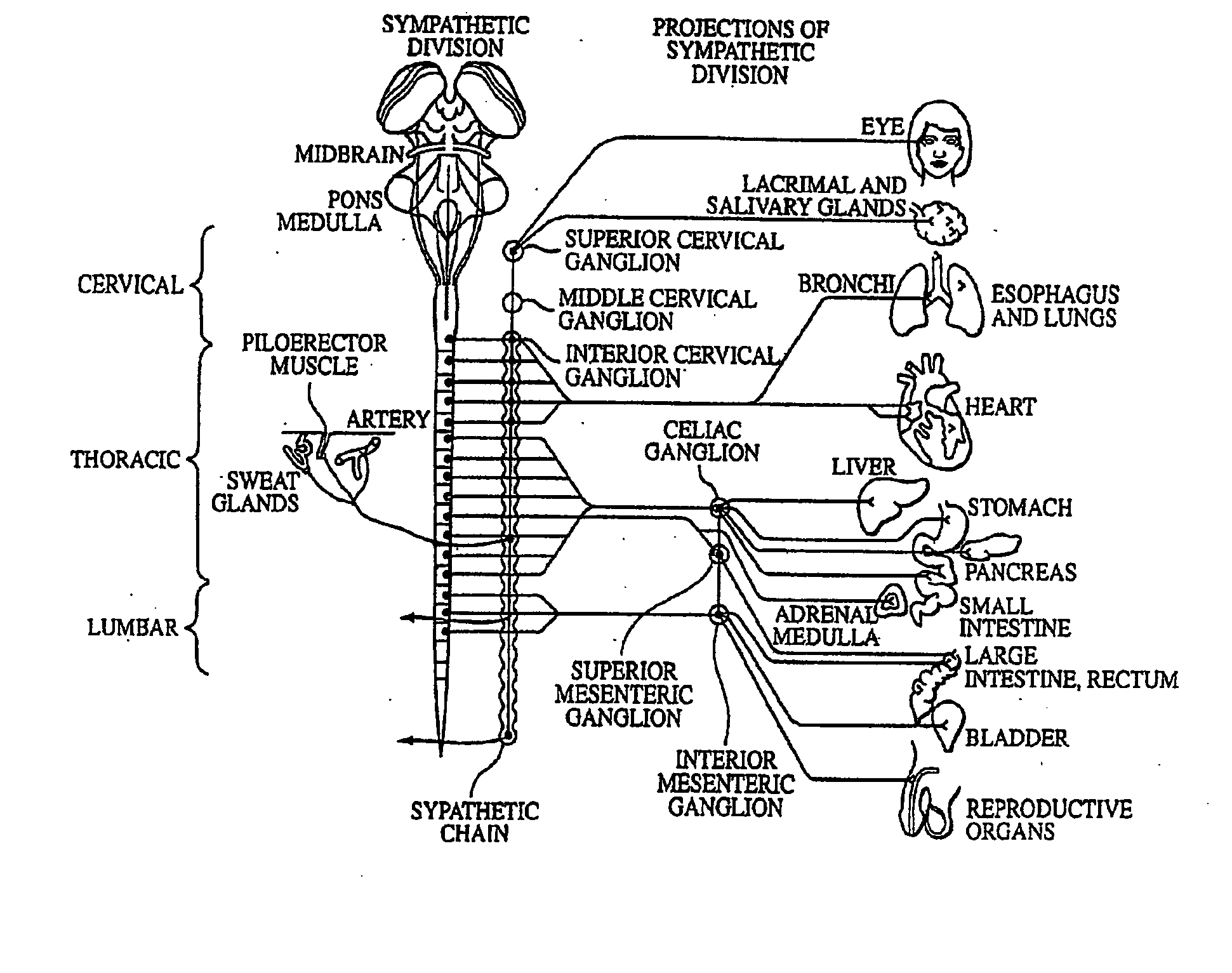
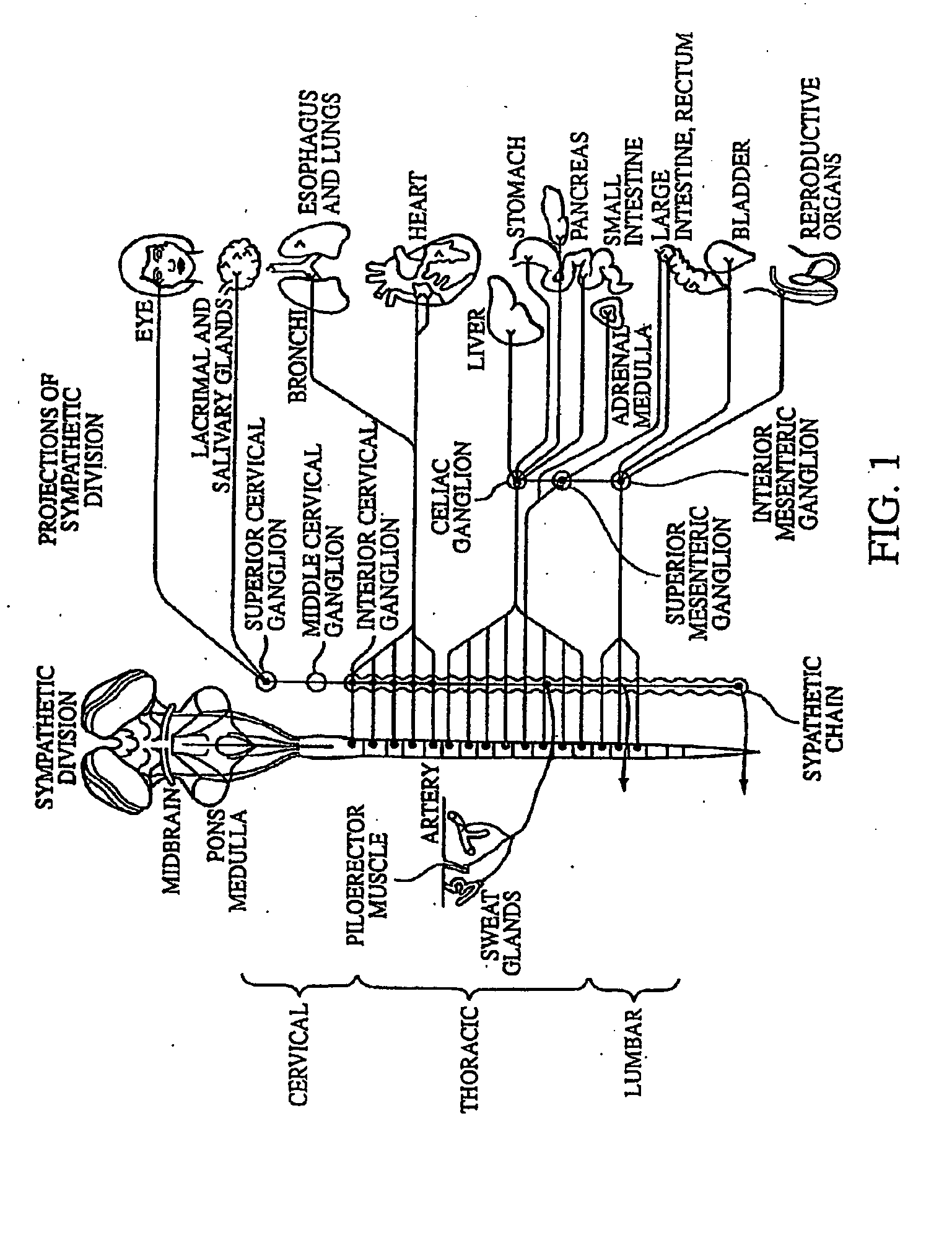
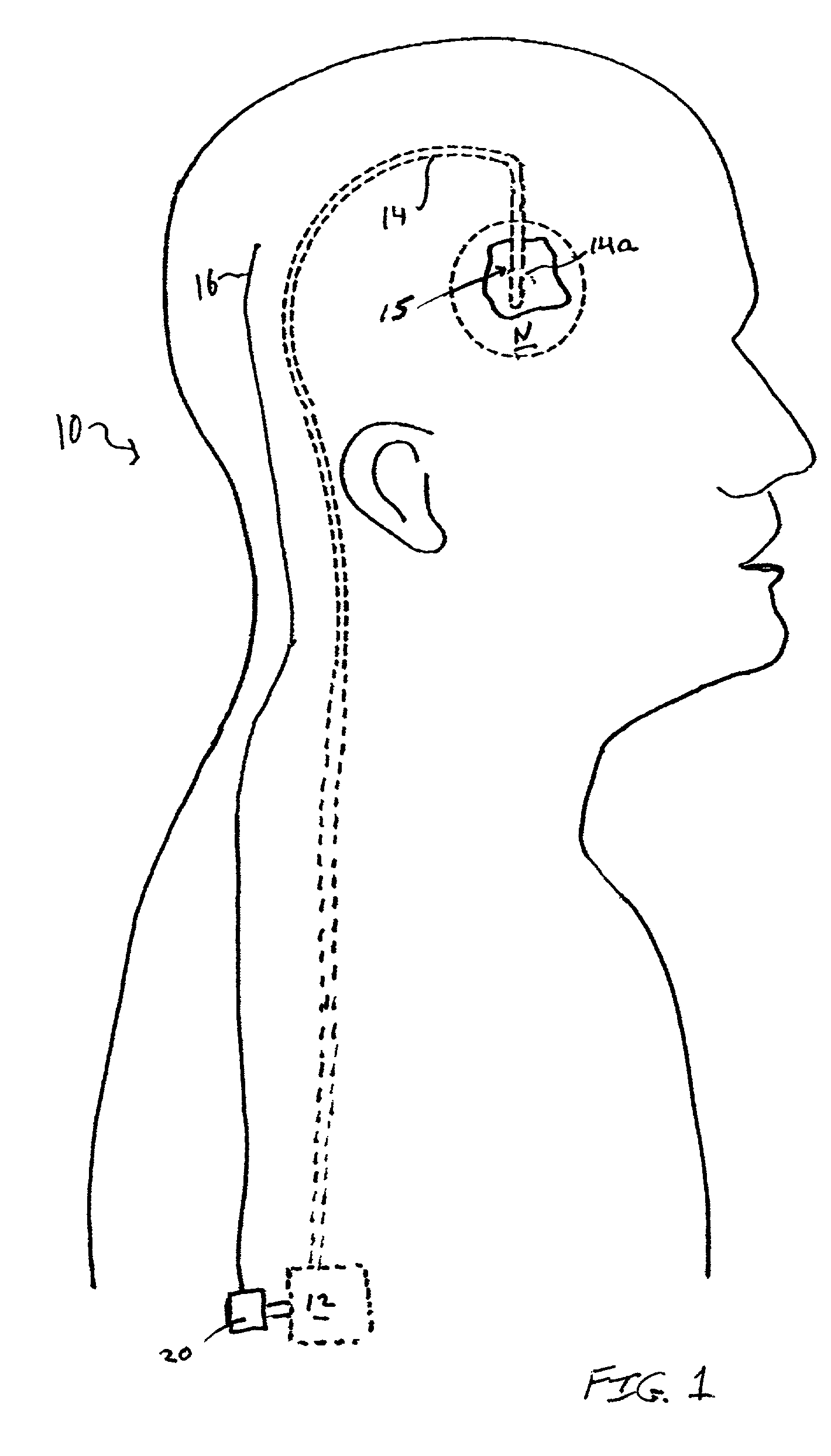
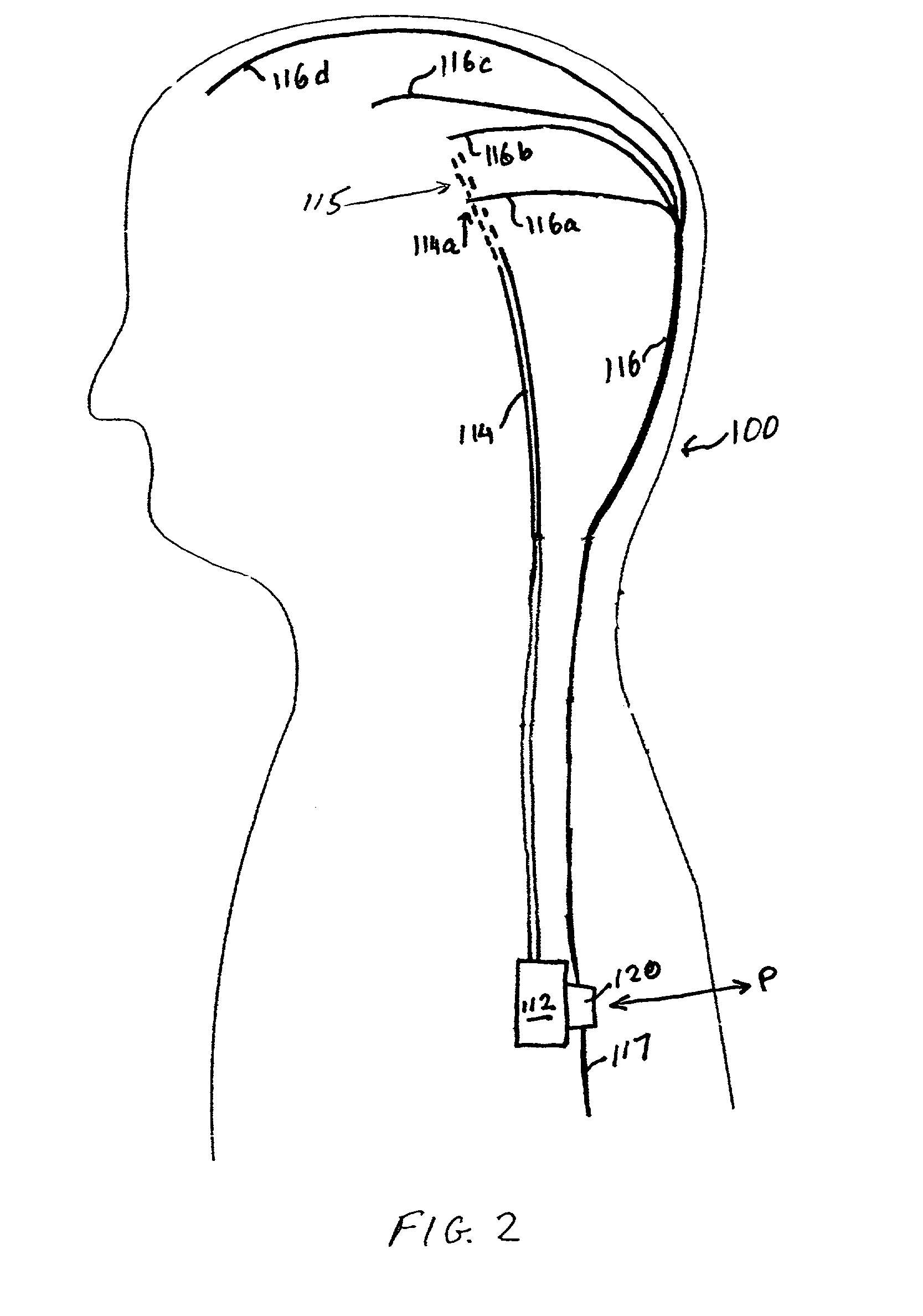
Electrical stimulation of the sympathetic nerve chain
InactiveUS20050065562A1Convenient treatmentModulating levelSpinal electrodesExternal electrodesSympathetic nerveSacral sympathetic chain
The present invention provides a method of affecting physiological disorders by stimulating a specific location along the sympathetic nerve chain. Preferably, the present invention provides a method of affecting a variety of physiological disorders or pathological conditions by placing an electrode adjacent to or in communication with at least one ganglion along the sympathetic nerve chain and stimulating the at least one ganglion until the physiological disorder or pathological condition has been affected.
Owner:THE CLEVELAND CLINIC FOUND
Closed-loop drug delivery system
InactiveUS7108680B2Safety and well be enhanceAdvantageousMedical devicesPressure infusionNervous systemImplantable Pump
A delivery system for a drug or bioactive agent includes an implantable pump and a delivery conduit that may be implanted in an organ or other tissue (e.g., the central or the peripheral nervous system) of a subject. A sensor is also implanted, and a controller unit receives the sensor output and directs drug delivery from within the patient pump accordingly. The sensor directly measures a primary biochemical material or state in the tissue or organ system, and the monitoring unit effects closed loop feedback control of the pump to achieve a desired end. The end may be the regulation of metabolism or maintenance of a stable metabolic or other state, or may be treatment regimen, e.g., by delivery of a dosage level or distribution of a drug in specific brain or nervous an system tissue. The sensed material may be the agent itself, a metabolite, or a related native material, tissue state or condition.
Owner:CODMAN & SHURTLEFF INC
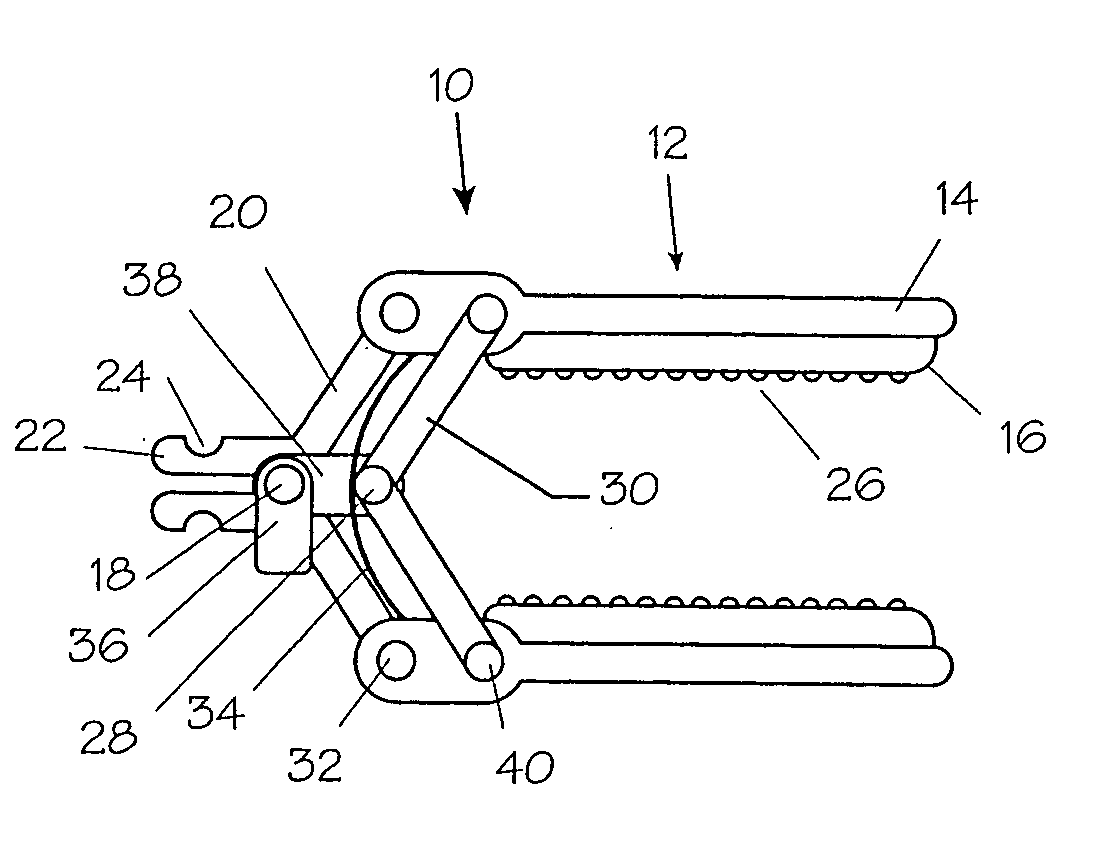
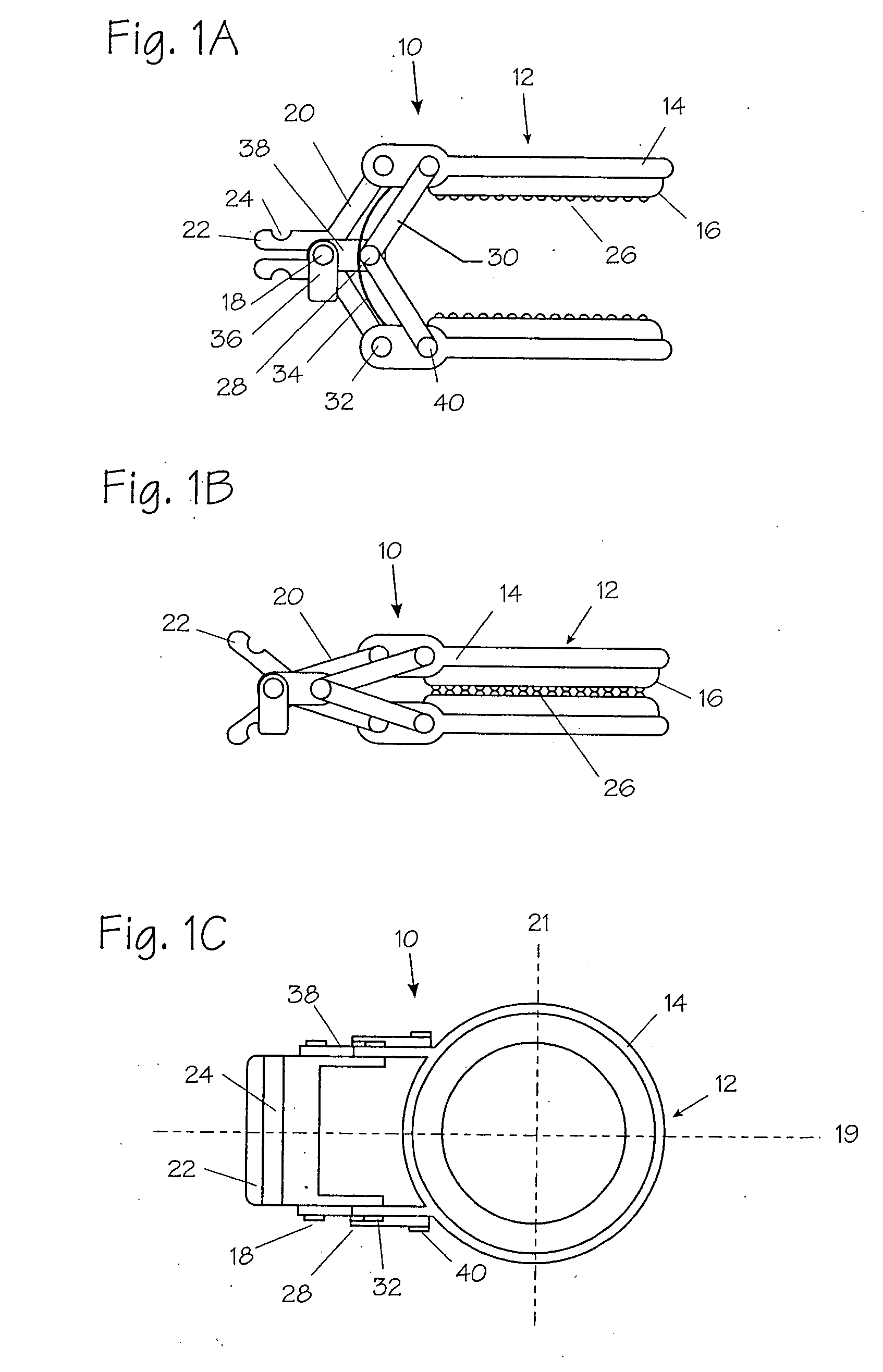
Method and apparatus for vascular and visceral clipping
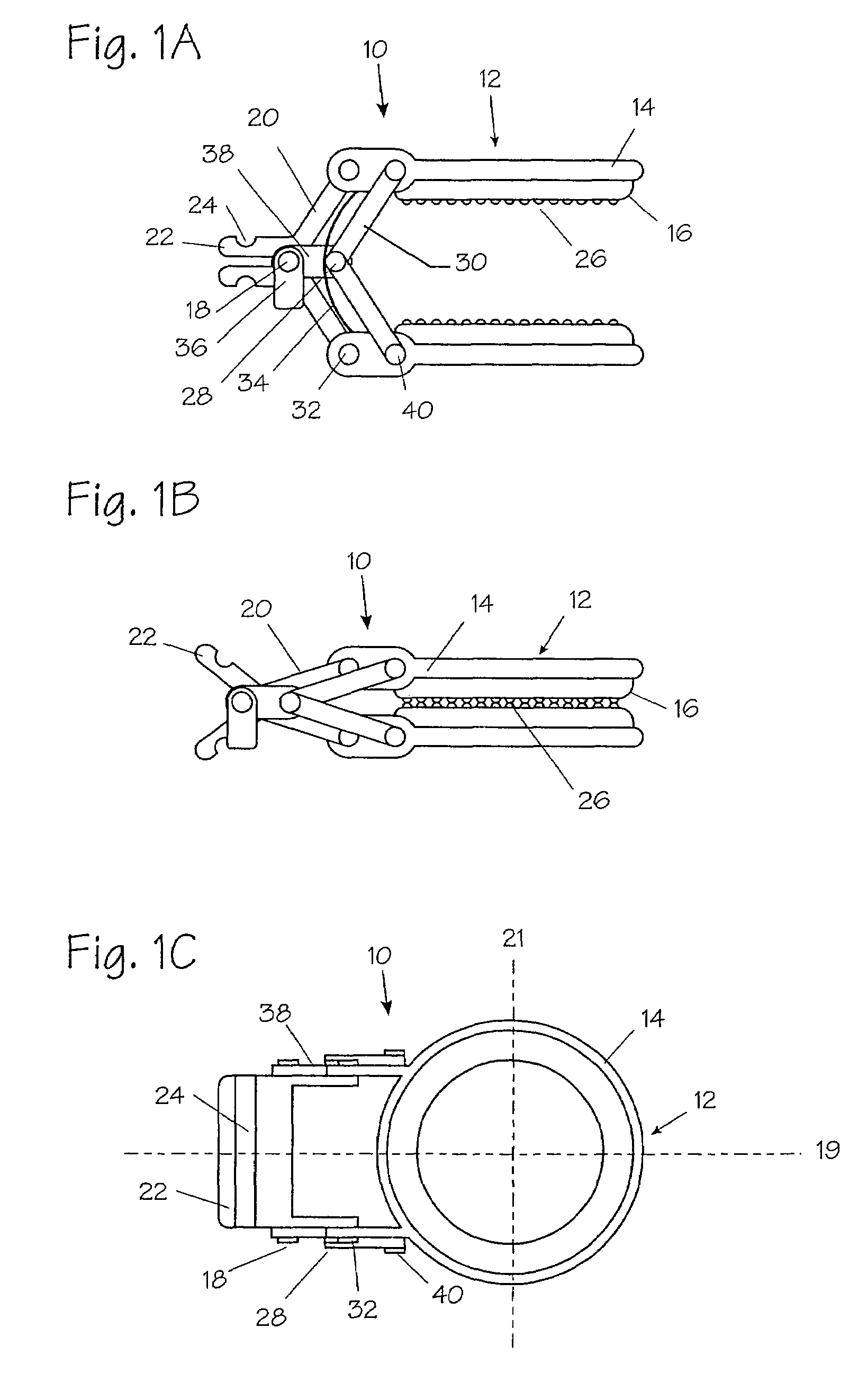
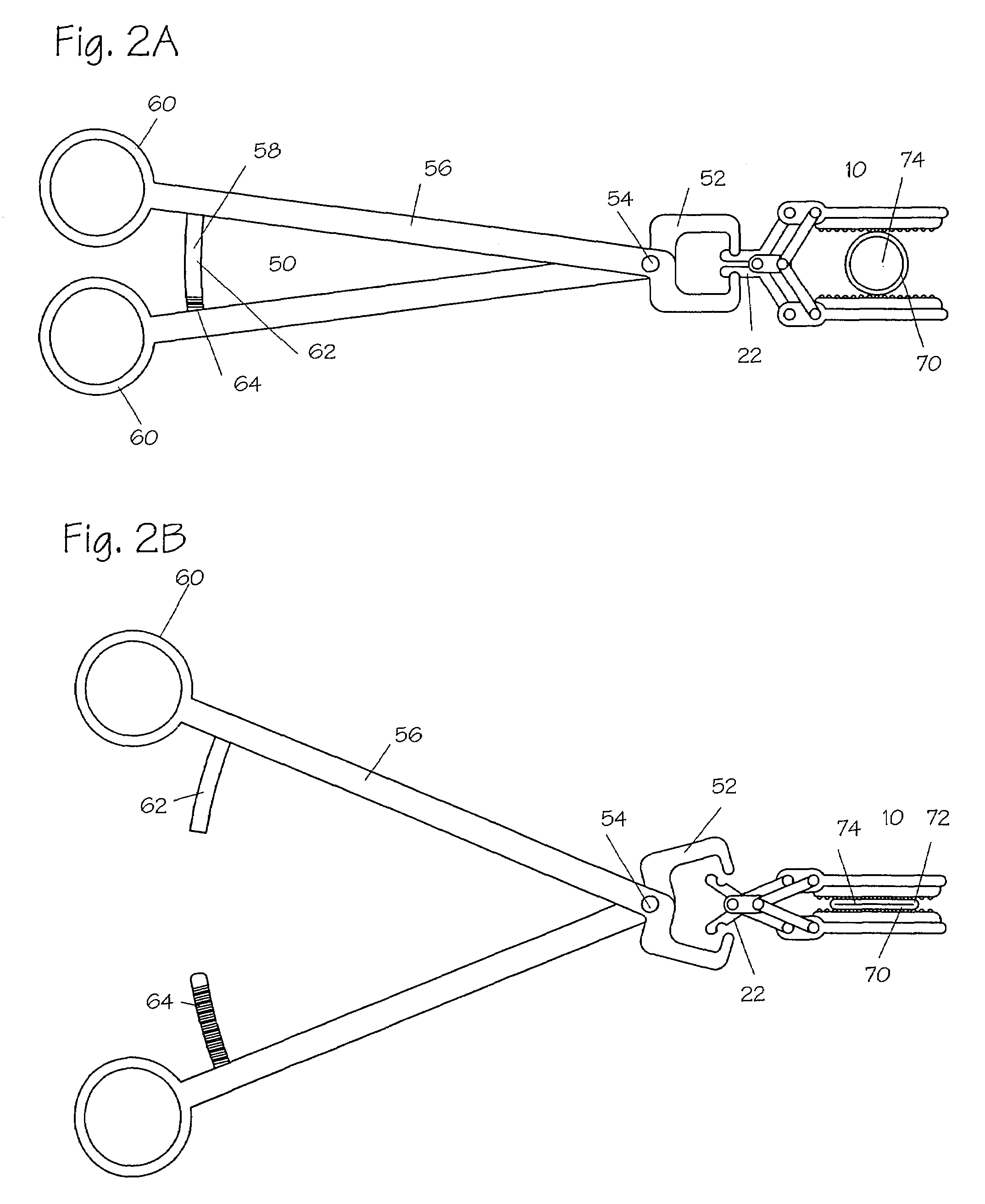
InactiveUS20050251183A1Convenient treatmentMinimizing chanceSnap fastenersClothes buttonsTrauma surgeryLarge intestine
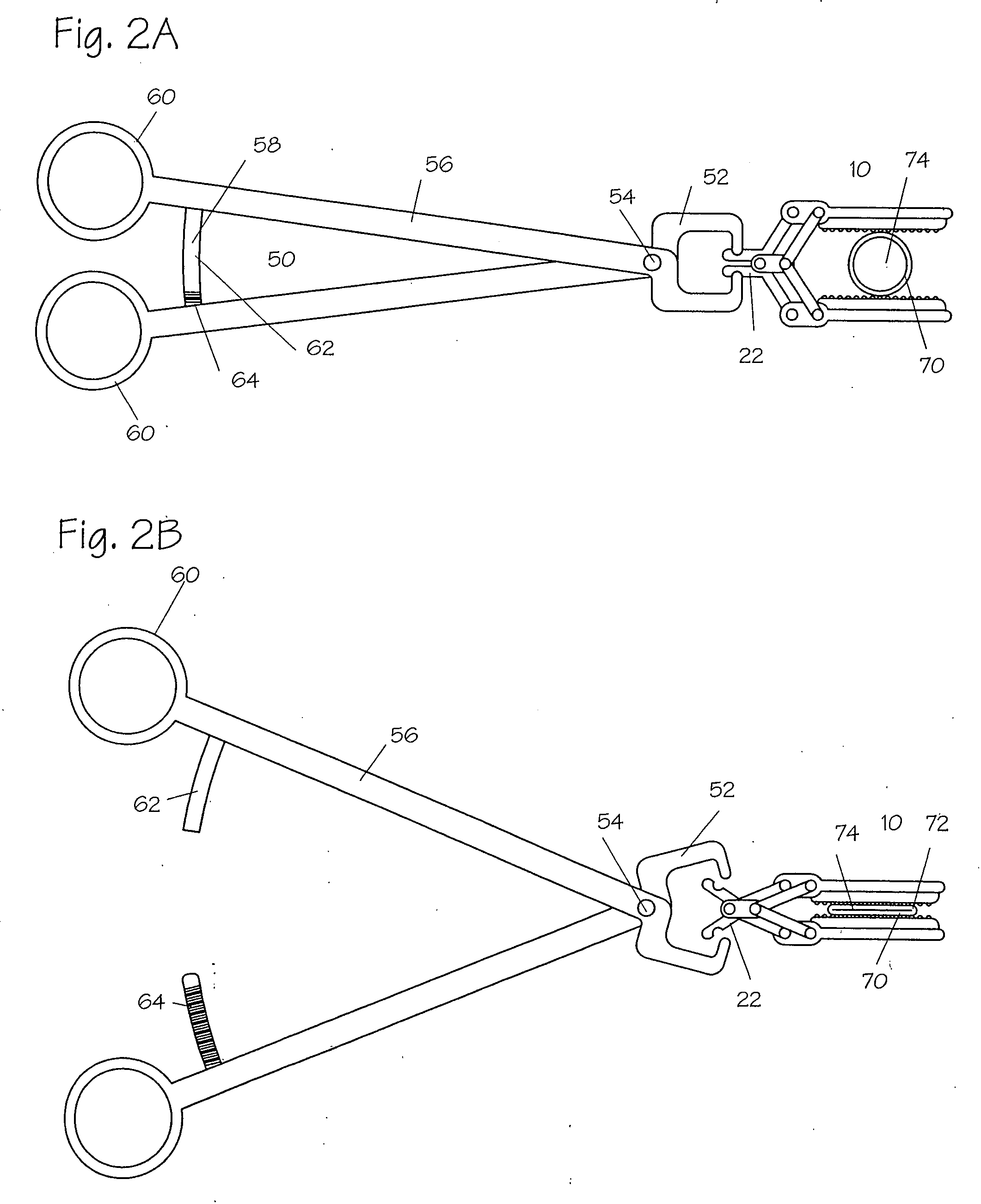
Devices and methods for achieving hemostasis and leakage control in hollow body vessels such as the small and large intestines, arteries and veins as well as ducts leading to the gall bladder and other organs. The devices and methods disclosed herein are especially useful in the emergency, trauma surgery or military setting, and most especially during damage control procedures. In such cases, the patient may have received trauma to the abdomen, extremities, neck or thoracic region. The devices utilize removable or permanently implanted, broad, soft, parallel jaw clips with minimal projections to maintain vessel contents without damage to the tissue comprising the vessel. These clips are applied using either standard instruments or custom devices that are subsequently removed leaving the clips implanted, on a temporary or permanent basis, to provide for hemostasis or leakage prevention, or both. These clips overcome the limitations of clips and sutures that are currently used for the same purposes. The clips come in a variety of shapes and sizes. The clips may be placed and removed by open surgery or laparoscopic access.
Owner:DAMAGE CONTROL SURGICAL TECH
Sensor based gastrointestinal electrical stimulation for the treatment of obesity or motility disorders
InactiveUS20050222638A1Reduce riskAvoid stimulationElectrotherapyArtificial respirationGastroparesisMotility
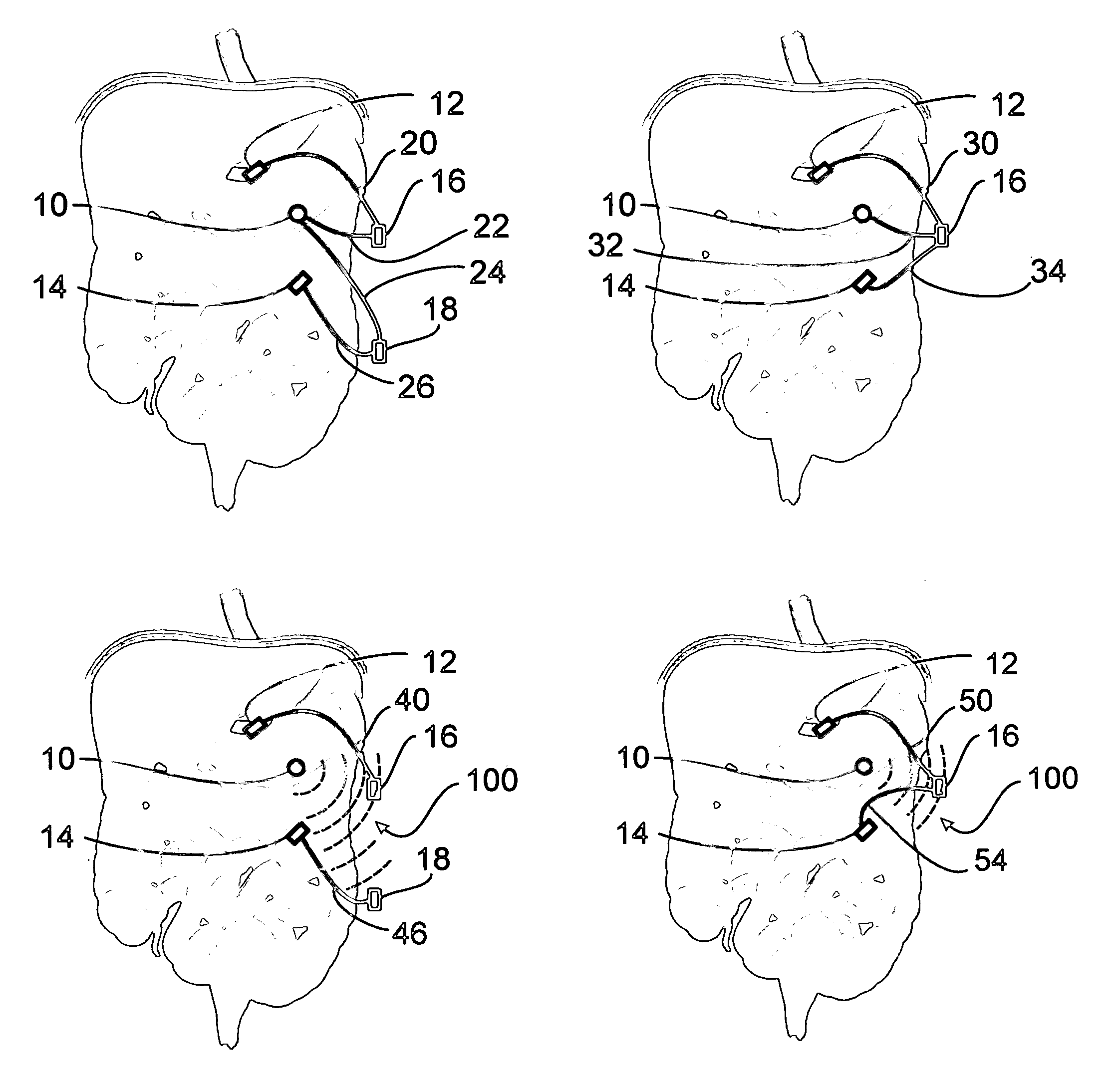
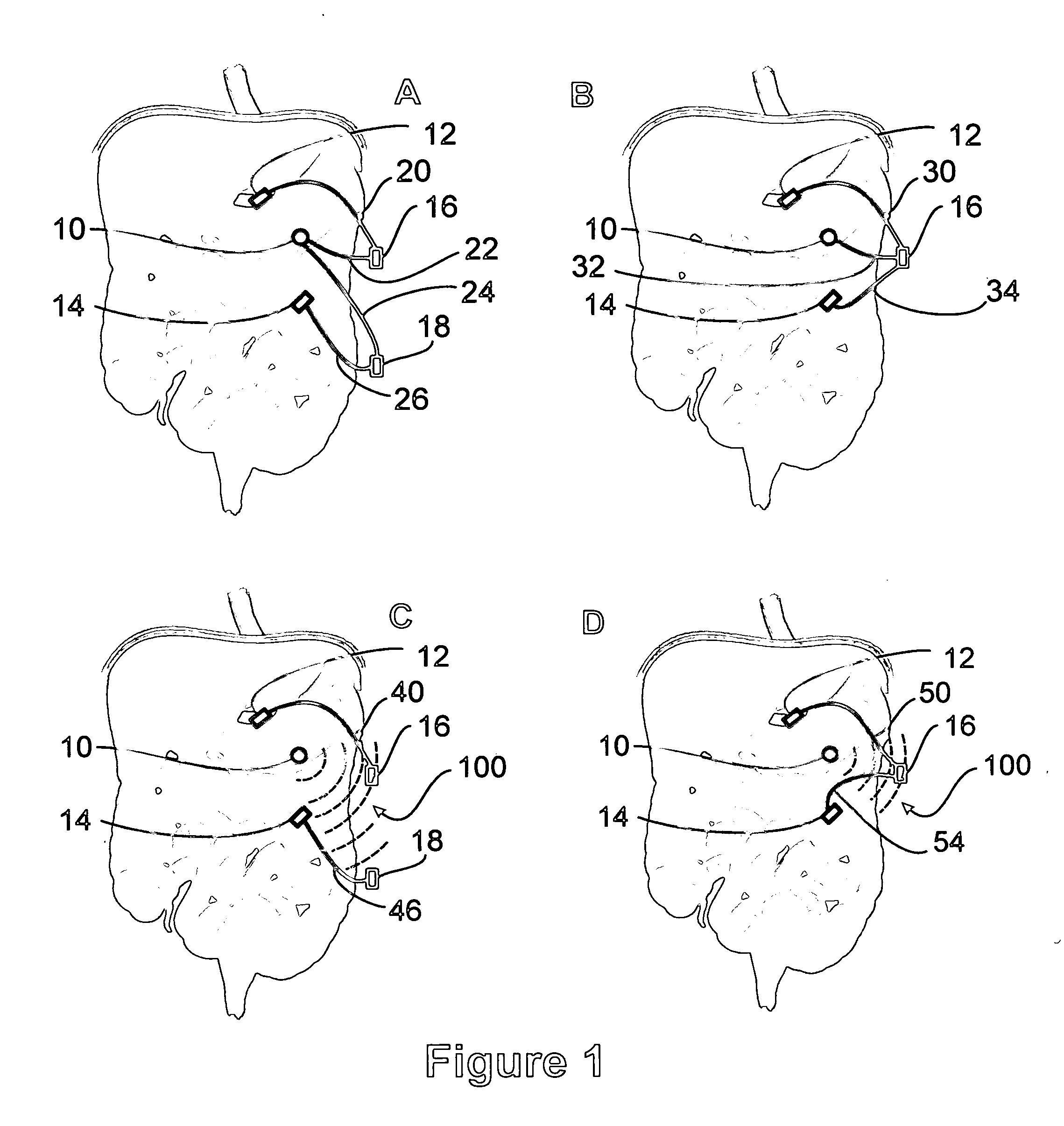
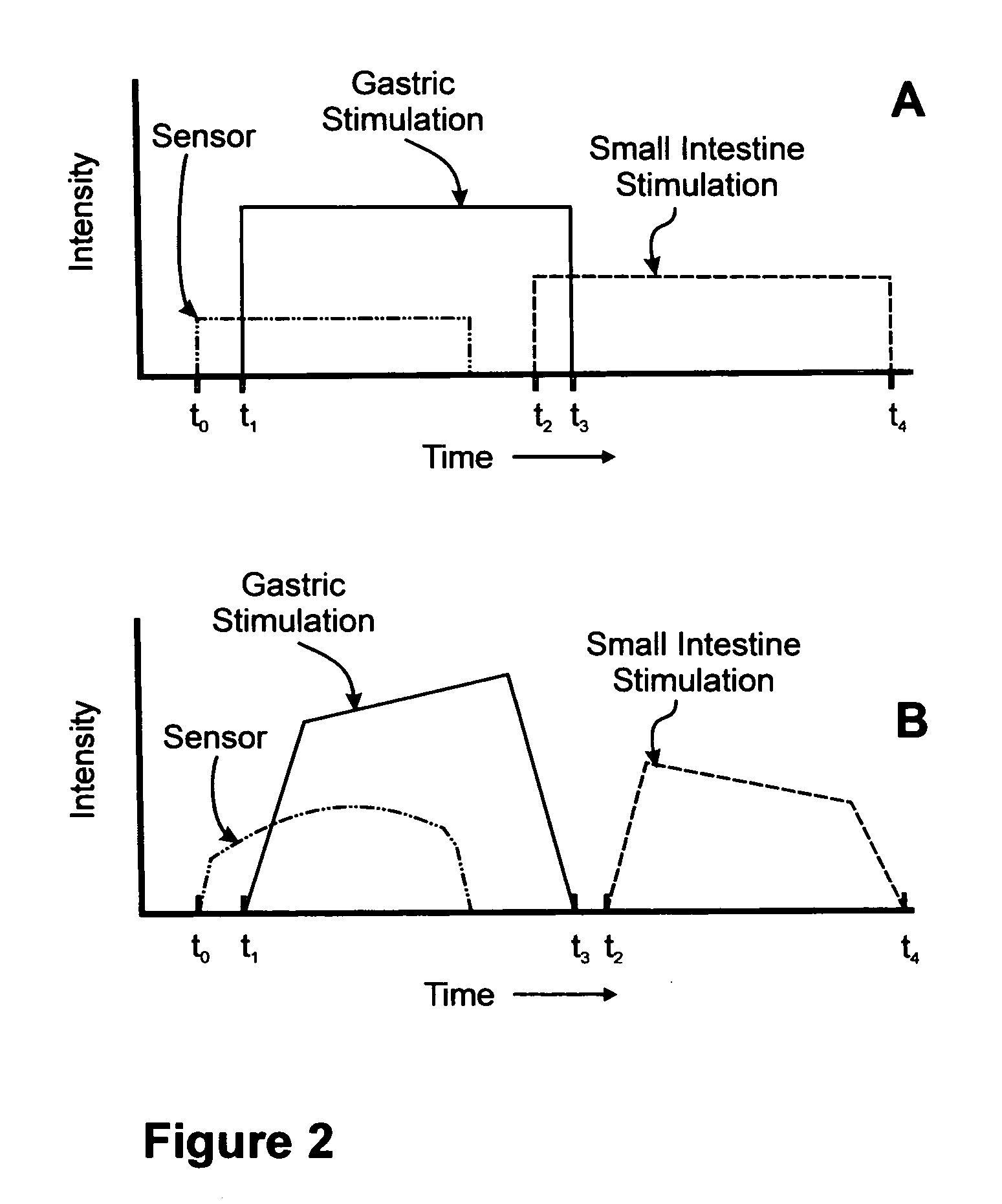
A method for treatment of obesity, especially morbid obesity, gastroparesis and other syndromes related to motor disorders of the stomach. The method of this invention utilizes a sensor to detect food entering the patient's stomach, thereby the sensor communicates with and activates at least one electrical stimulation device attached to either the stomach or the small intestine.
Owner:MEDTRONIC TRANSNEURONIX
Methods for remodeling cardiac tissue
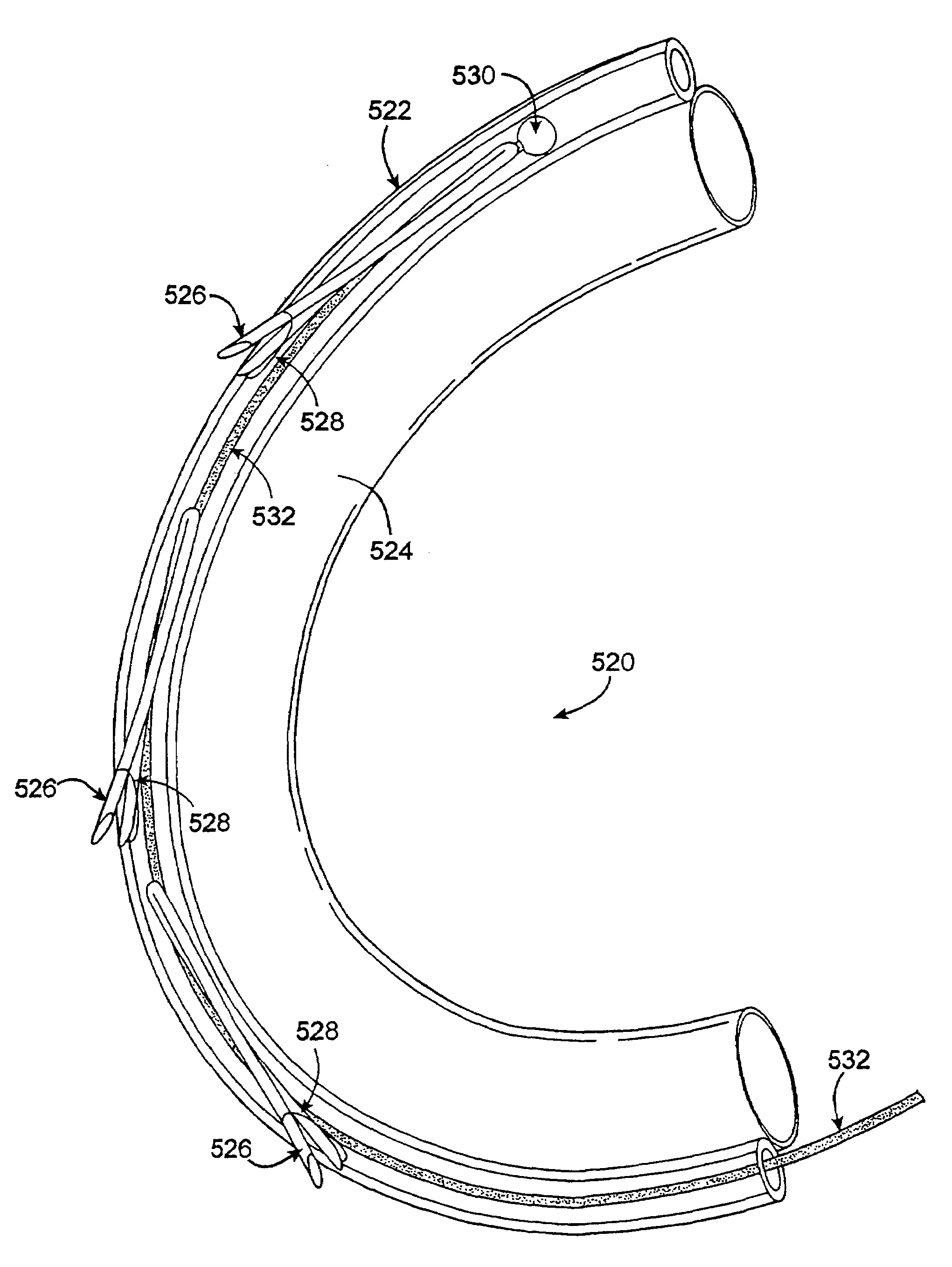
InactiveUS7588582B2Convenient treatmentConstricting the valve annulusSuture equipmentsSurgical needlesTethered CordVALVE PORT
Described herein are methods of remodeling the base of a ventricle. In particular, methods of remodeling a valve annulus by forming a new fibrous annulus are described. These methods may result in a remodeled annulus that corrects valve leaflet function without substantially inhibiting the mobility of the leaflet. The methods of remodeling the base of the ventricle include the steps of securing a plurality of anchors to the valve annulus beneath one or more leaflets of the valve, constricting the valve annulus by cinching a tether connecting the anchors, and securing the anchors in the cinched conformation to allow the growth of fibrous tissue. The annulus may be cinched (e.g., while visualizing the annulus) so that the mobility of the valve leaflets is not significantly restricted. The remodeled annulus is typically constricted to shorten the diameter of the annulus to correct for valve dysfunction (e.g., regurgitation).
Owner:ANCORA HEART INC
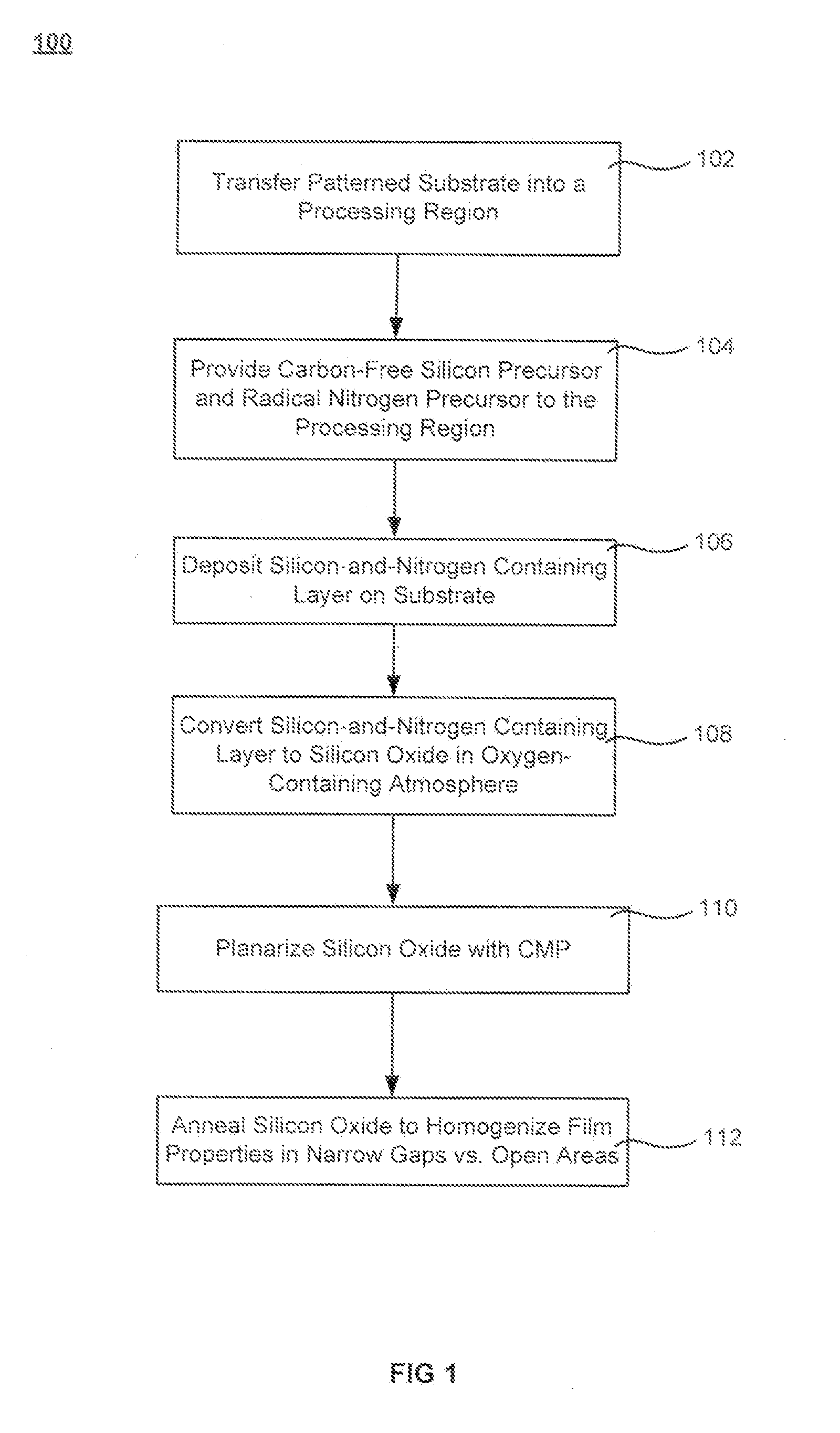
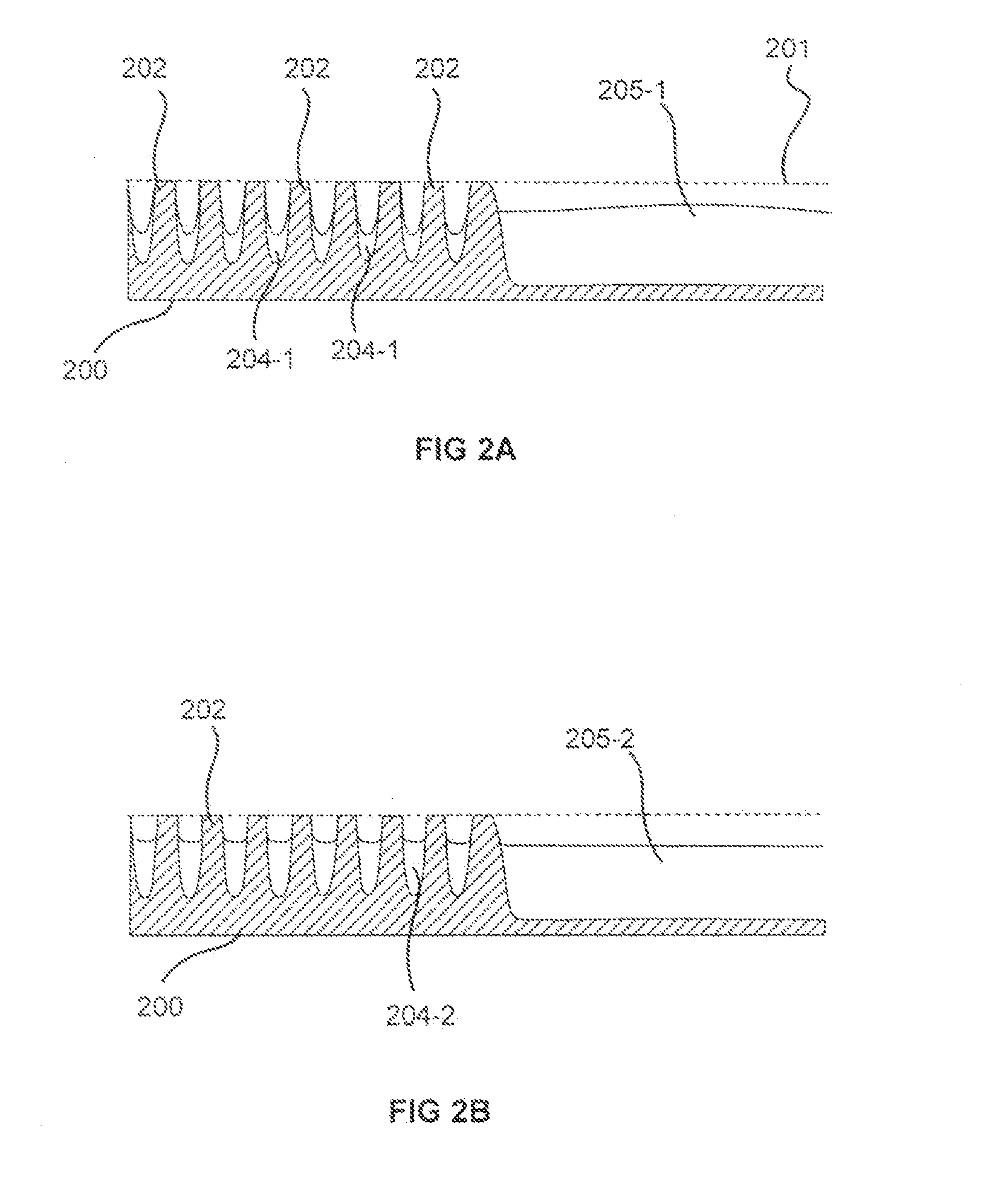
Post-planarization densification
InactiveUS20110081782A1High densityReduce Pattern Loading EffectSemiconductor/solid-state device manufacturingChemical vapor deposition coatingHigh densitySilicon oxide
Processes for forming high density gap-filling silicon oxide on a patterned substrate are described. The processes increase the density of gap-filling silicon oxide particularly in narrow trenches. The density may also be increased in wide trenches and recessed open areas. The densities of the gap-filling silicon oxide in the narrow and wide trenches / open areas become more similar following the treatment which allows the etch rates to match more closely. This effect may also be described as a reduction in the pattern loading effect. The process involves forming then planarizing silicon oxide. Planarization exposes a new dielectric interface disposed closer to the narrow trenches. The newly exposed interface facilitates a densification treatment by annealing and / or exposing the planarized surface to a plasma.
Owner:APPLIED MATERIALS INC
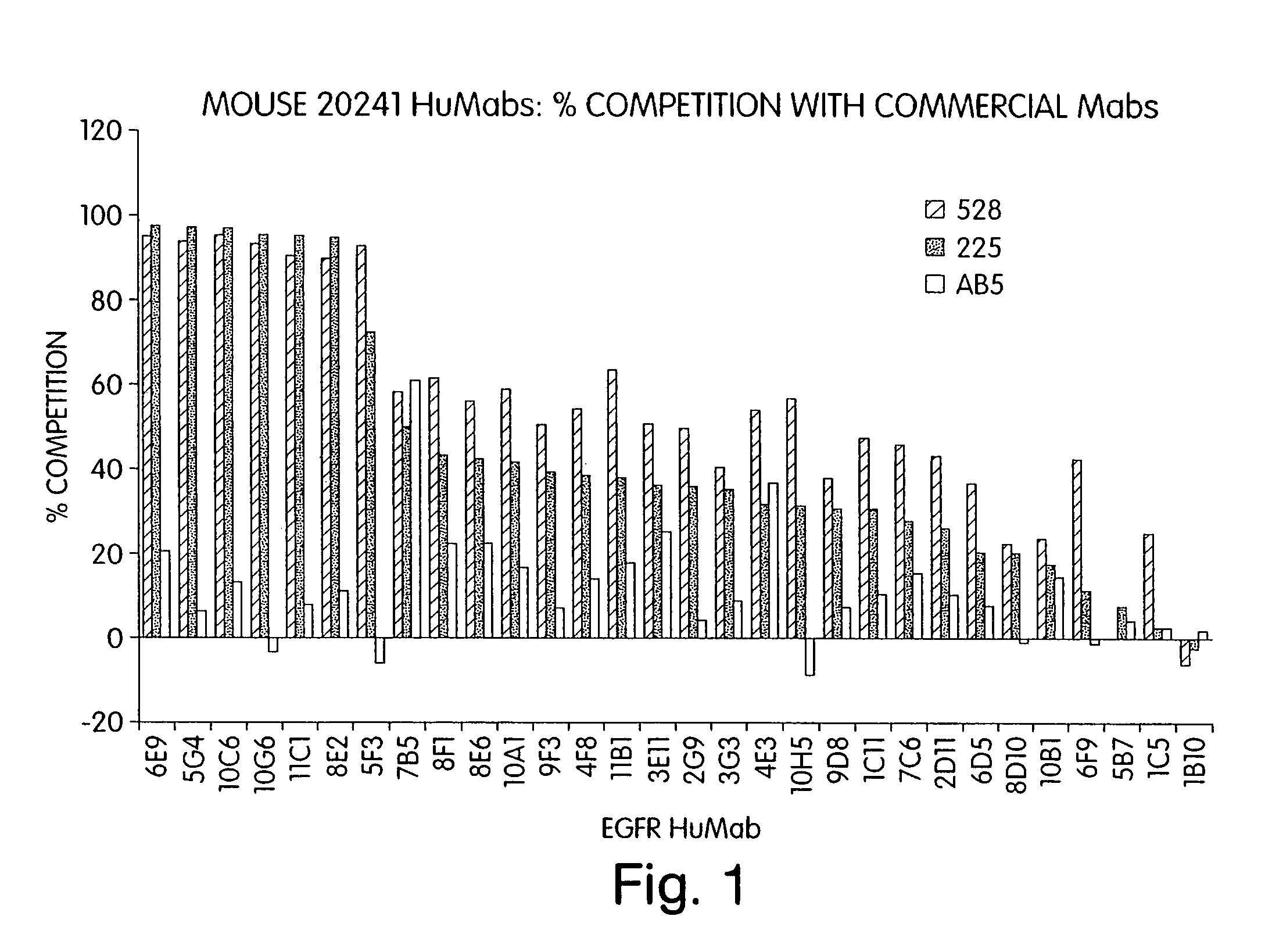
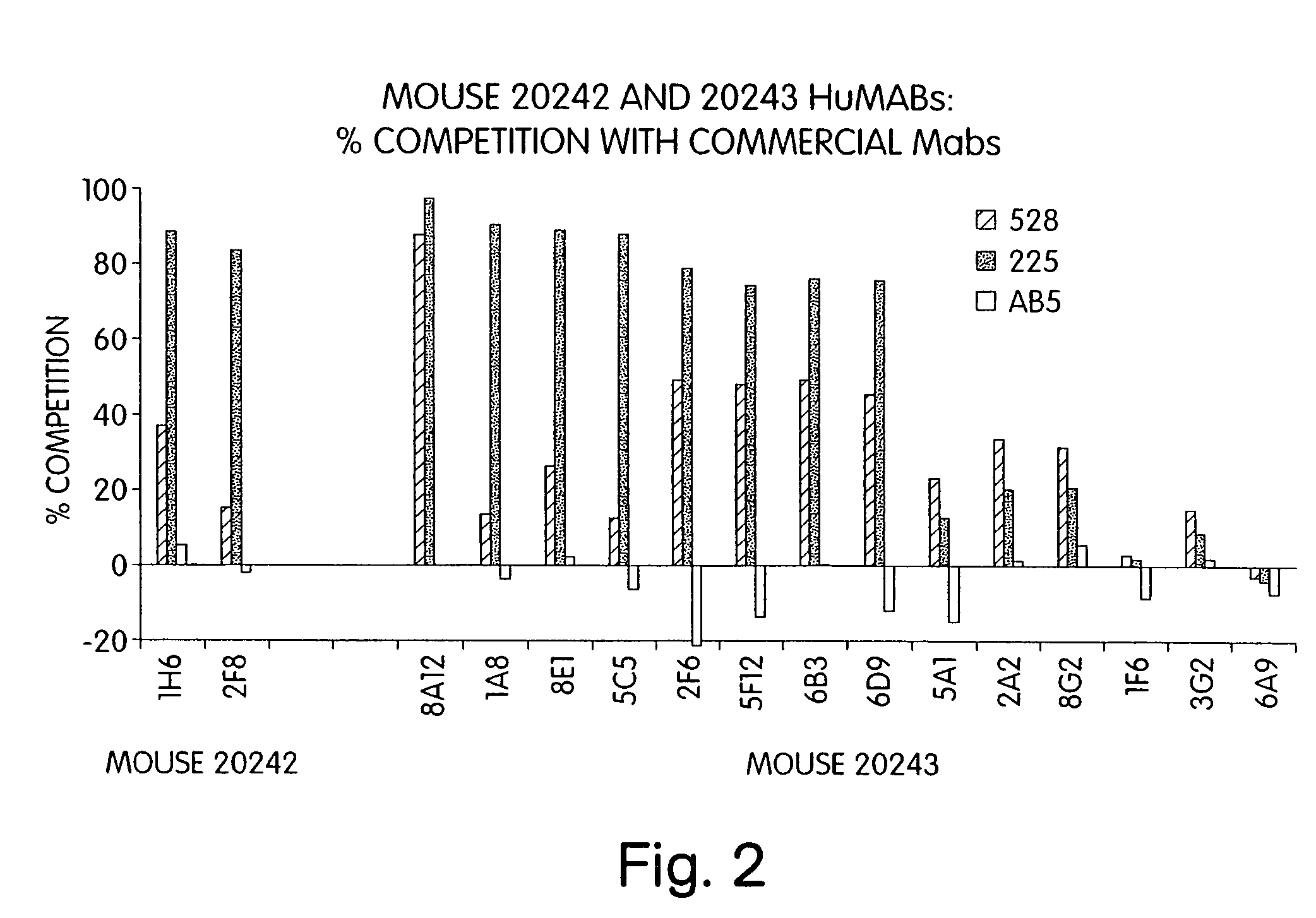
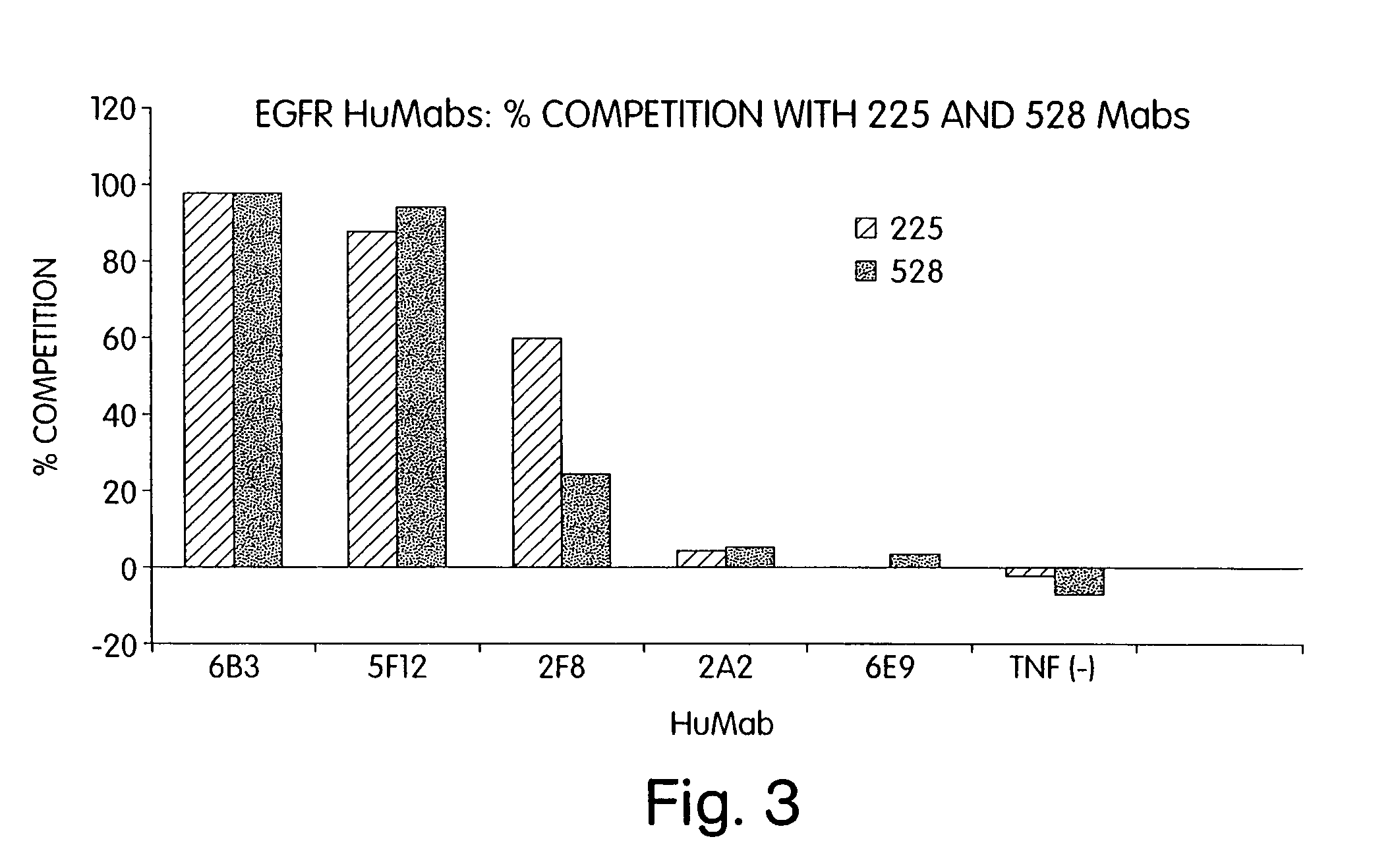
Human monoclonal antibodies to epidermal growth factor receptor (EGFR)
InactiveUS7247301B2Less immunogenicReduce adverse side effectsInorganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsV(D)J recombinationHuman epidermal growth factor receptor
Isolated human monoclonal antibodies which specifically bind to human EGFR, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB INC
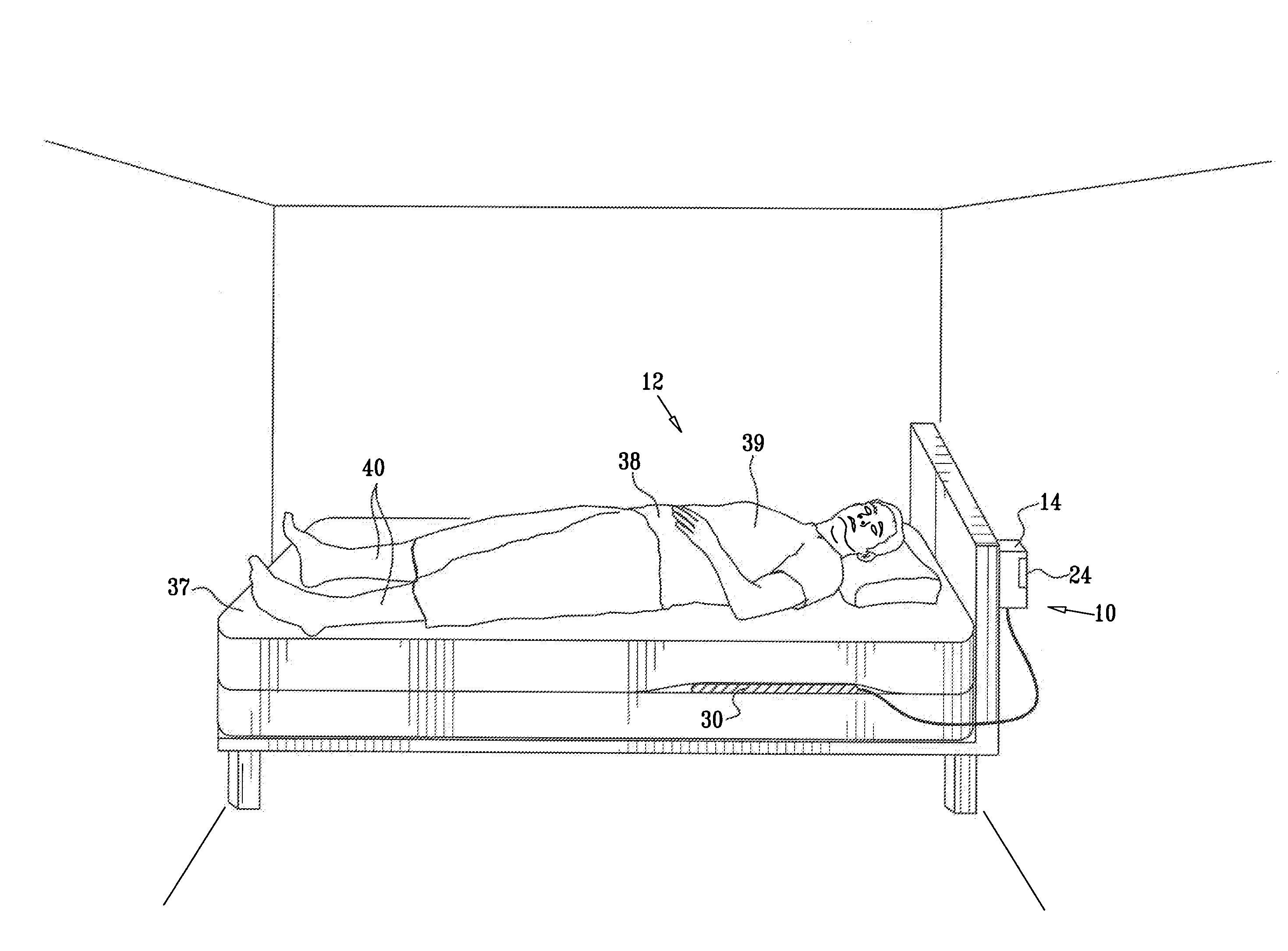
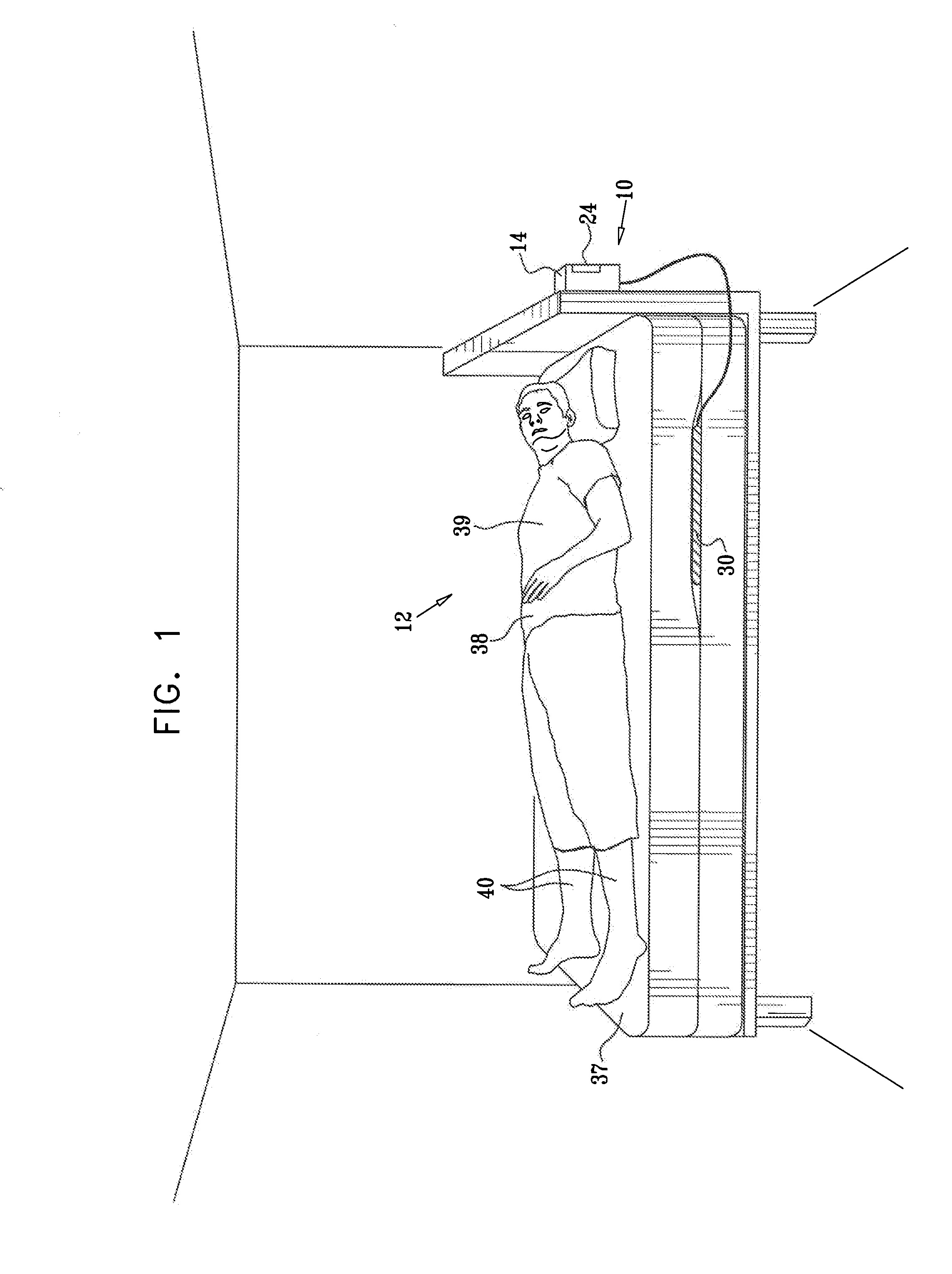
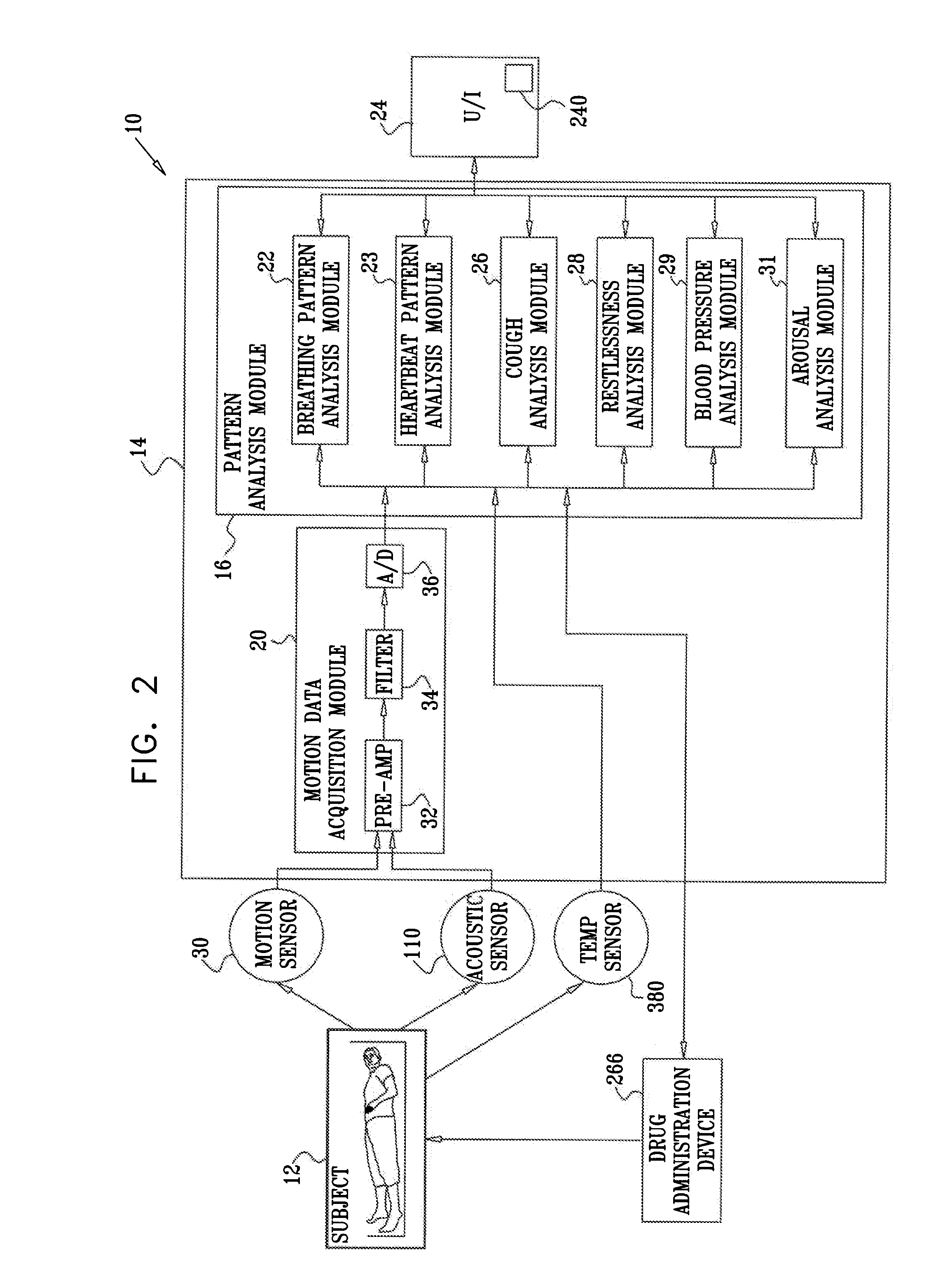
Prediction and monitoring of clinical episodes
ActiveUS20080269625A1Convenient treatmentReduces required dosageElectrotherapyElectrocardiographyMedicineAsthma attack
A method is provided for predicting an onset of an asthma attack. The method includes sensing at least one parameter of a subject without contacting or viewing the subject or clothes the subject is wearing, and predicting the onset of the asthma attack at least in part responsively to the sensed parameter. Also provided is a method for predicting an onset of an episode associated with congestive heart failure (CHF), including sensing at least one parameter of a subject without contacting or viewing the subject or clothes the subject is wearing, and predicting the onset of the episode at least in part responsively to the sensed parameter. Other embodiments are also described.
Owner:HILL ROM SERVICES
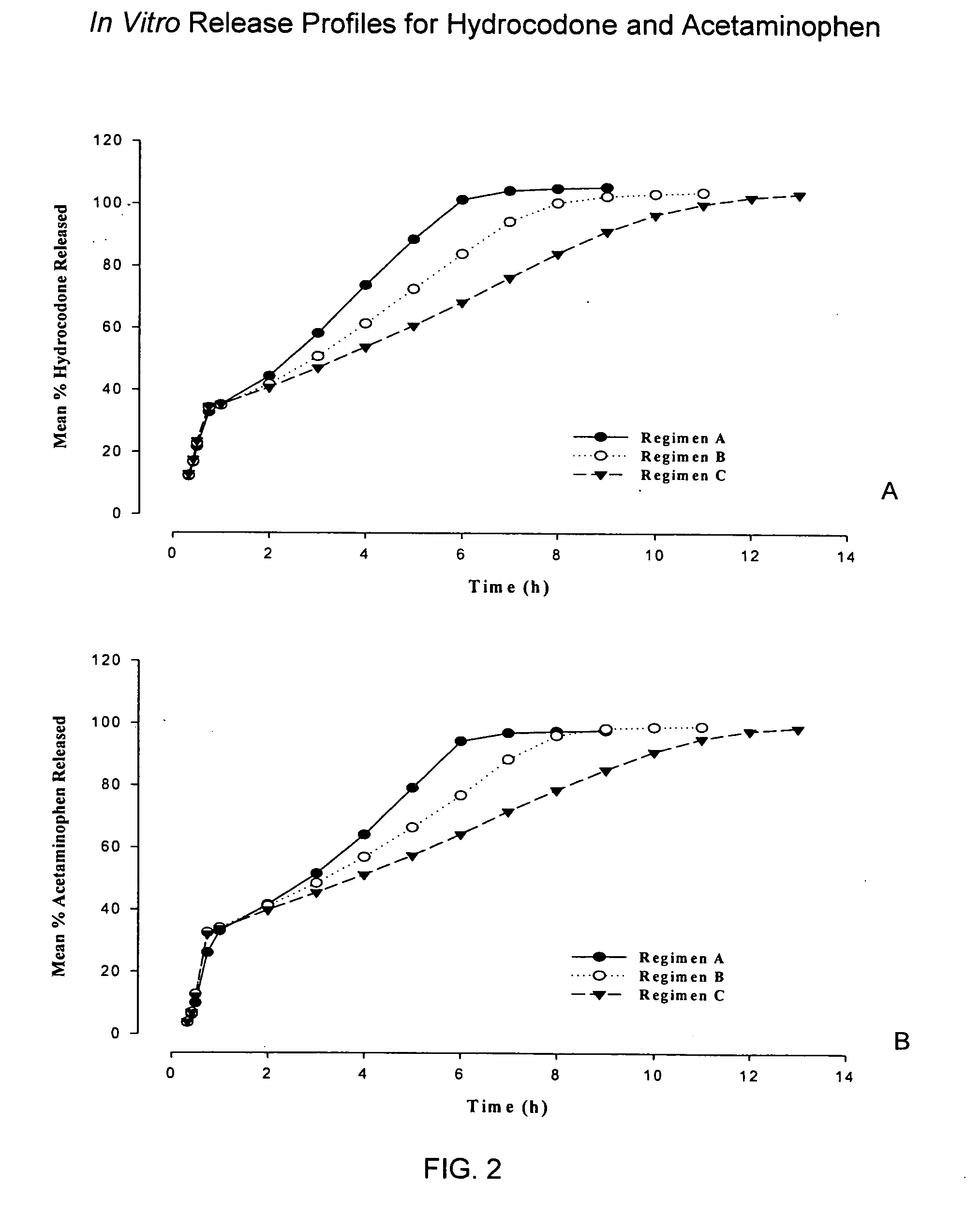
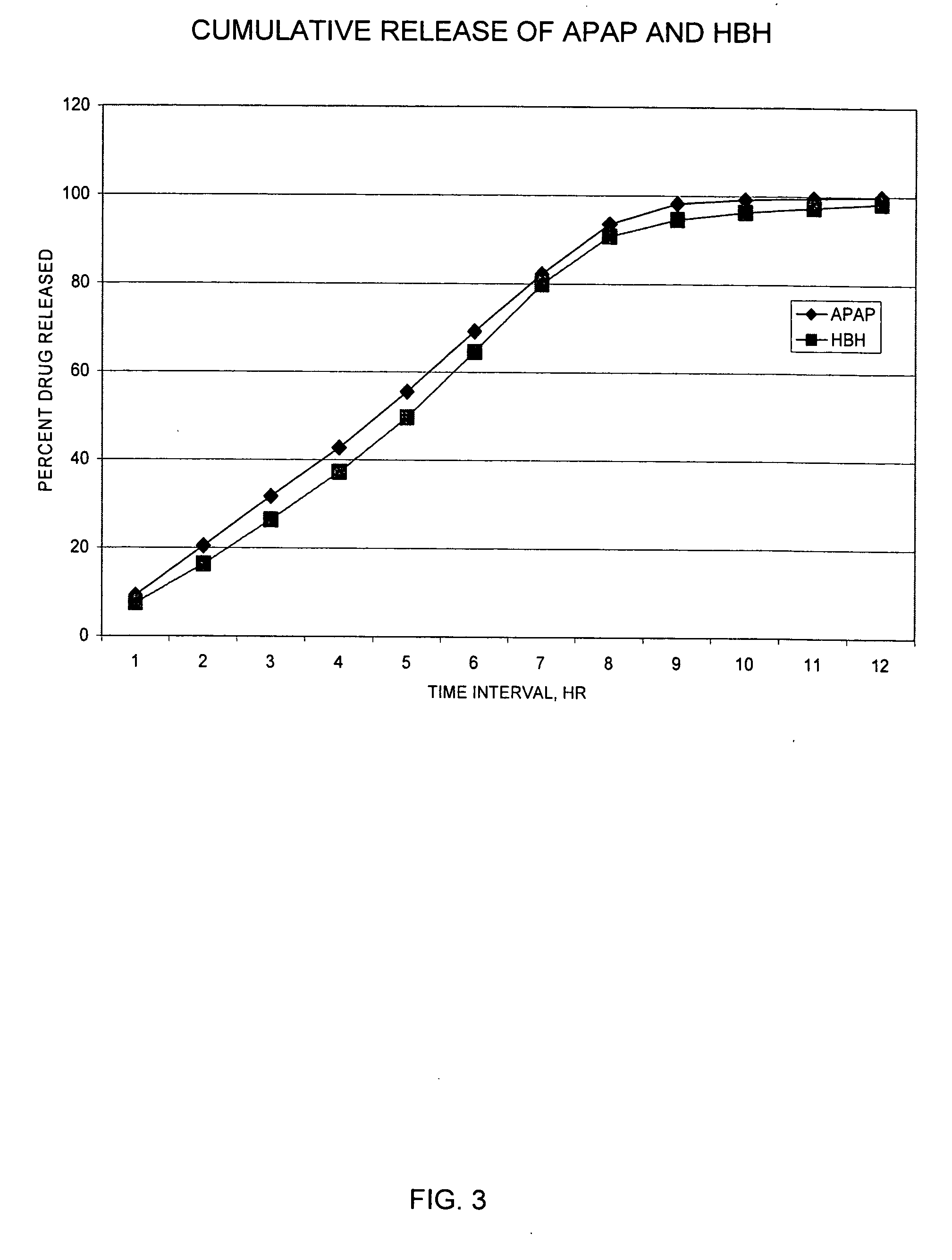
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
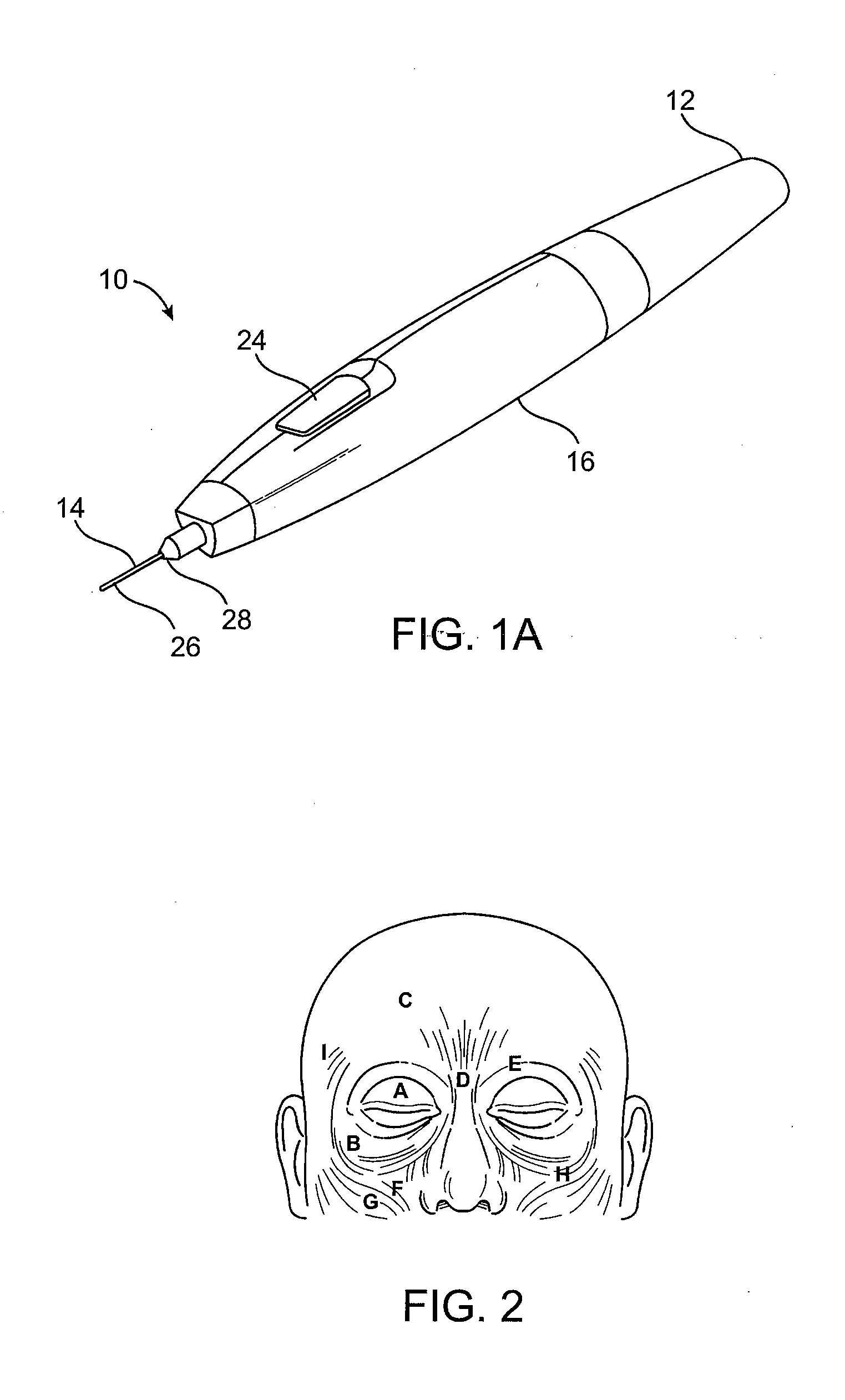
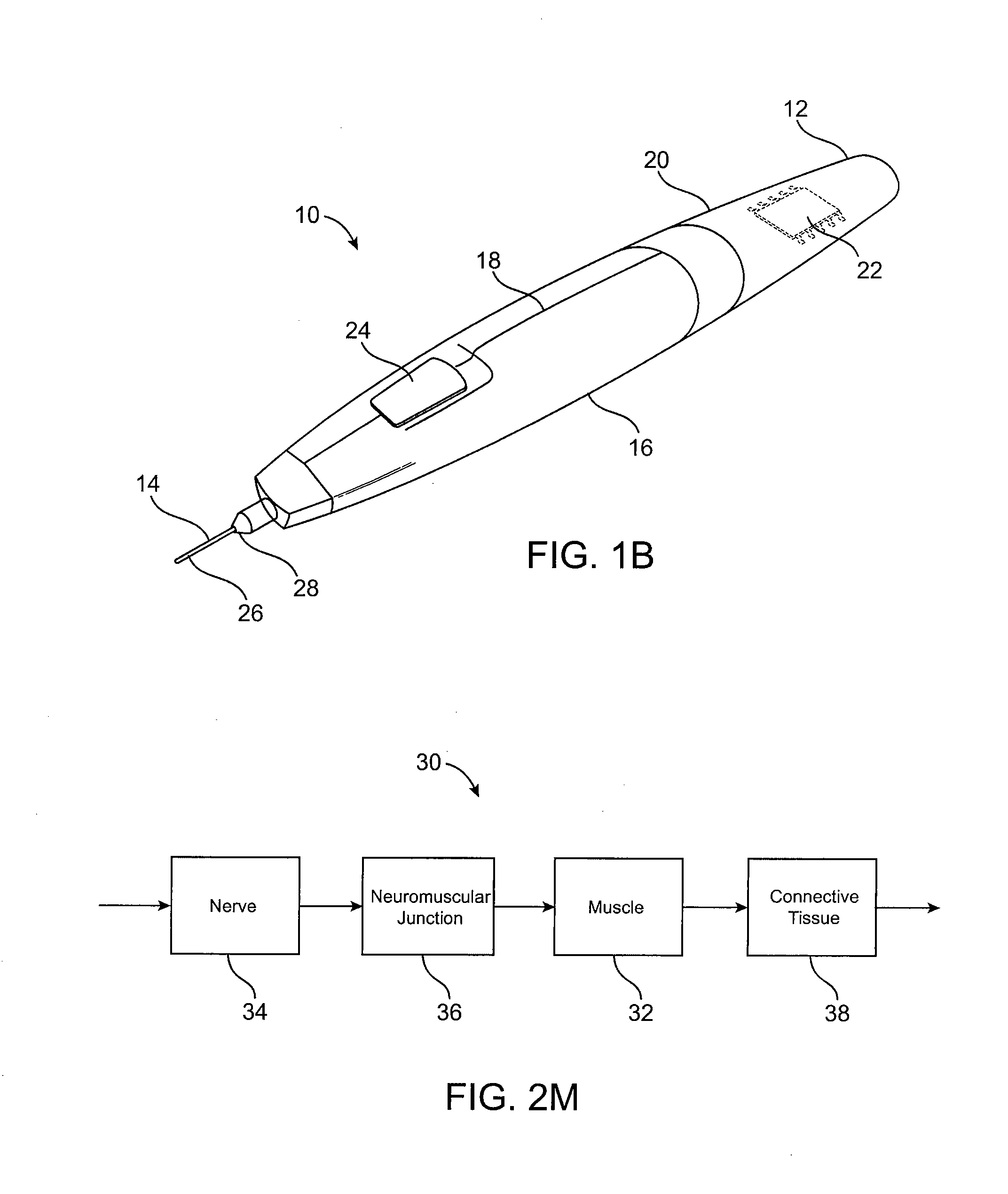
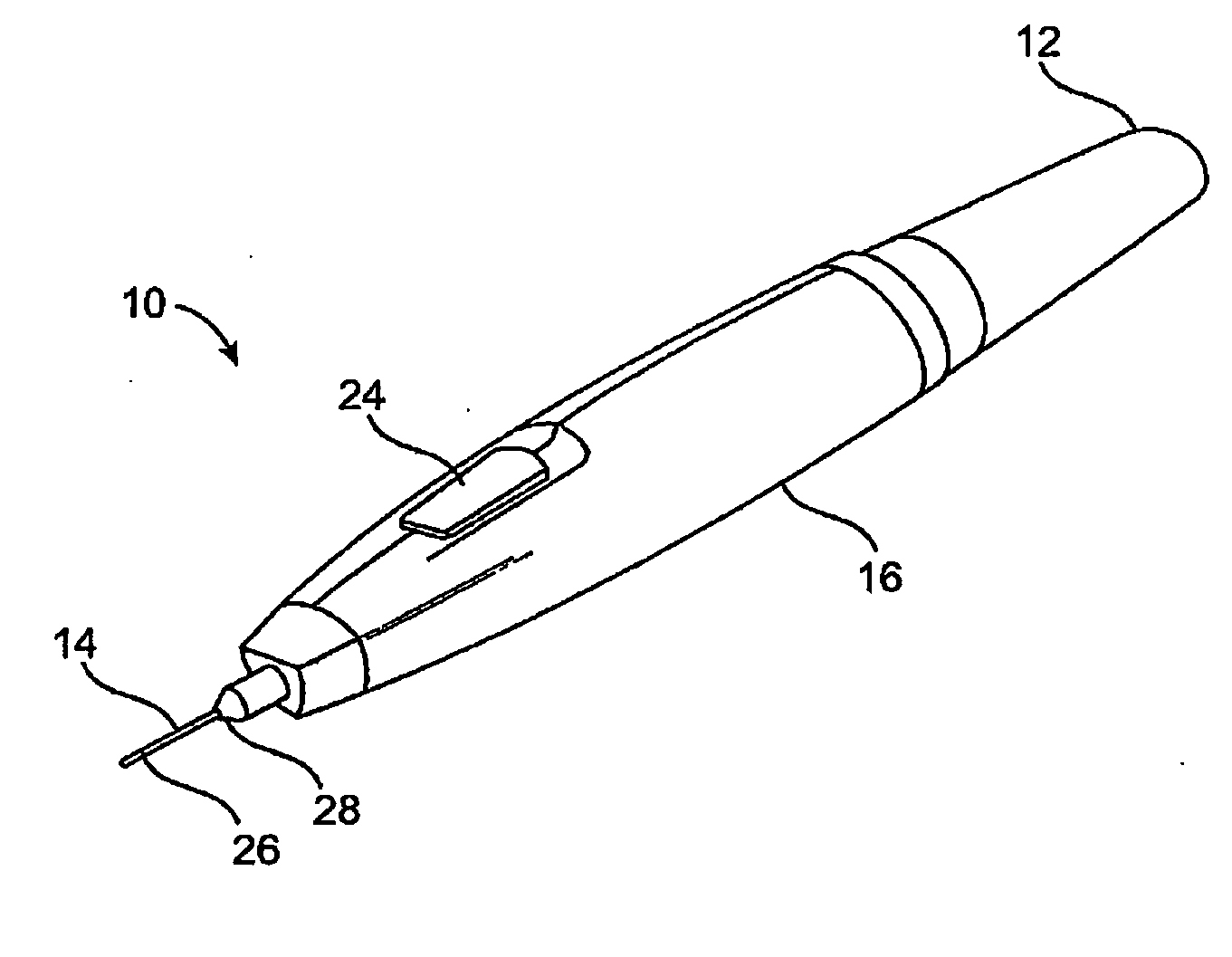
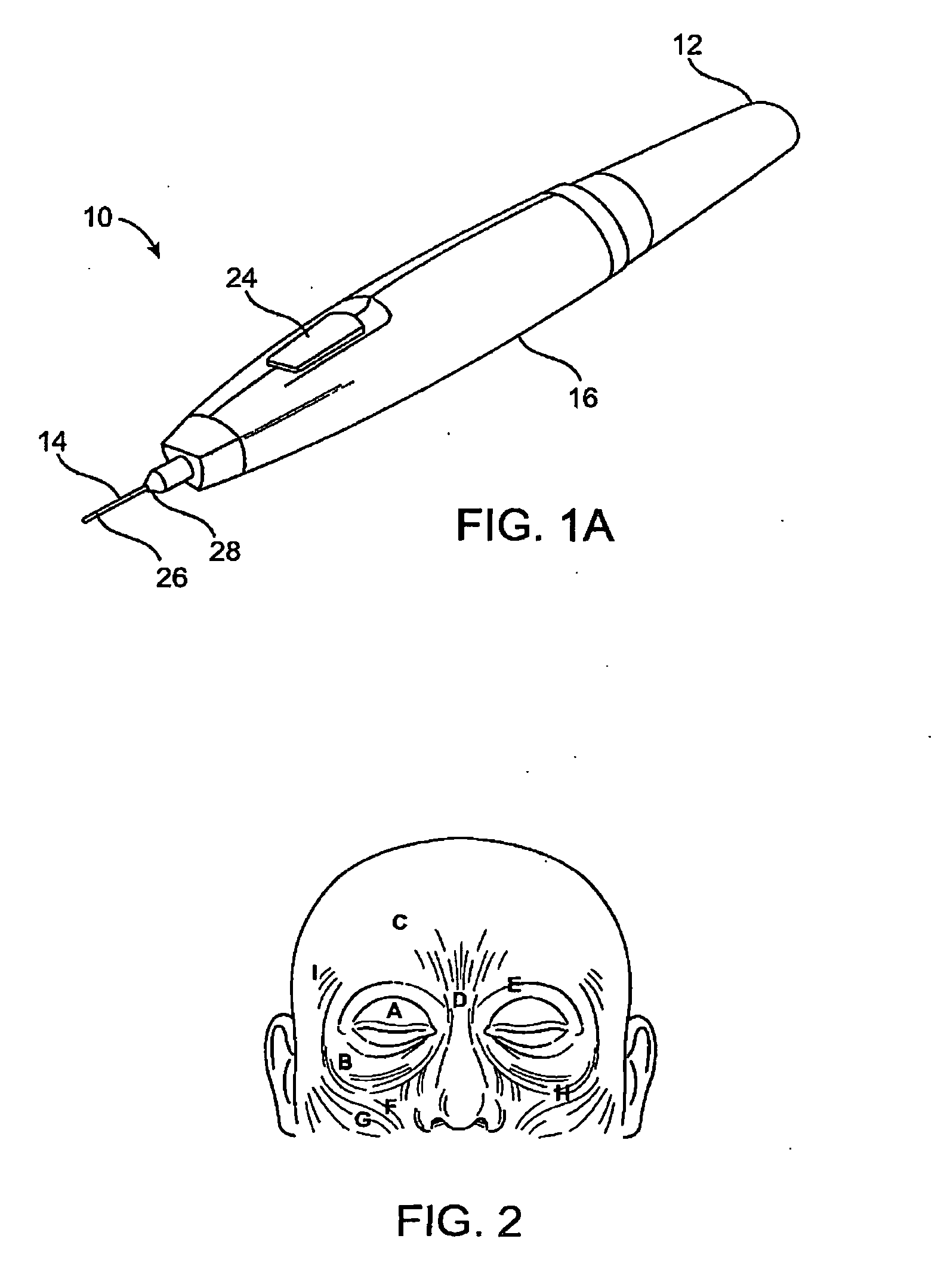
Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (FAT)
ActiveUS20080183164A1Reduce wrinklesAlter shapeDiagnostic recording/measuringSurgical instruments for heatingWrinkle skinMuscle contraction
Devices, systems, and methods treat cosmetic defects, and often apply cooling with at least one tissue-penetrating probe inserted through of the skin of a patient. The cooling may remodel one or more target tissue so as to effect a desired change in a composition of the target tissue and / or a change in its behavior. Exemplary embodiments of the cooling treatments will interfere with the nerve / muscle contractile function chain so as to mitigate wrinkles of the skin. Related treatments may be used therapeutically for treatment of back and other muscle spasms, chronic pain, and the like. Some embodiments may remodel subcutaneous adipose tissue so as to alter a shape or appearance of the skin surface.
Owner:PACIRA CRYOTECH INC
Decellularized extracellular matrix of conditioned body tissues and uses thereof
InactiveUS20050013870A1Increase in sizeHigh strengthGastrointestinal cellsGenetically modified cellsCell-Extracellular MatrixECM Protein
The present invention relates generally to decellularized extracellular matrix of conditioned body tissues. The decellularized extracellular matrix contains a biological material, preferably vascular endothelial growth factor (VEGF), produced by the conditioned body tissue that is in an amount different than the amount of the biological material that the body tissue would produce absent the conditioning. The invention also relates to methods of making and methods of using said decellularized extracellular matrix. Specifically, the invention relates to treating defective, diseased, damaged or ischemic cells, tissues or organs in a subject by administering, injecting or implanting the decellularized extracellular matrix of the invention into a subject in need thereof. The invention is further directed to a tissue regeneration scaffold for implantation into a subject inflicted with a disease or condition that requires tissue or organ repair, regeneration and / or strengthening. Additionally, the invention is directed to a medical device, preferably a stent or an artificial heart, having a surface coated or covered with the decellularized extracellular matrix of the invention or having a component comprising the decellularized extracellular matrix of the invention for implantation into a subject, preferably a human. Methods for making the tissue regeneration scaffold and methods for manufacturing a coated or covered medical device having a component comprising decellularized extracellular matrix of conditioned body tissues are also provided.
Owner:BOSTON SCI SCIMED INC
Methods and compositions for metal nanoparticle treated surfaces
ActiveUS20070207335A1Extended shelf lifeConvenient coatingMaterial nanotechnologyBiocidePolyamideSolvent
The present invention comprises methods and compositions comprising metal nanoparticles. The invention comprises metal nanoparticles and surfaces treated with a metal nanoparticle coating. The present invention further comprises compositions for preparing nanoparticles comprising at least one stabilizing agent, one or more metal compounds, at least one reducing agent and a solvent. In one aspect, the stabilizing agent comprises a surfactant or a polymer. The polymer may comprise polymers such as polyacrylamides, polyurethanes, and polyamides. In one aspect, the metal compound comprises a salt comprising a metal cation and an anion. The anion may comprise saccharinate derivatives, long chain fatty acids, and alkyl dicarboxylates.
Owner:AVENT INC
Systems and methods for collecting and transmitting assay results
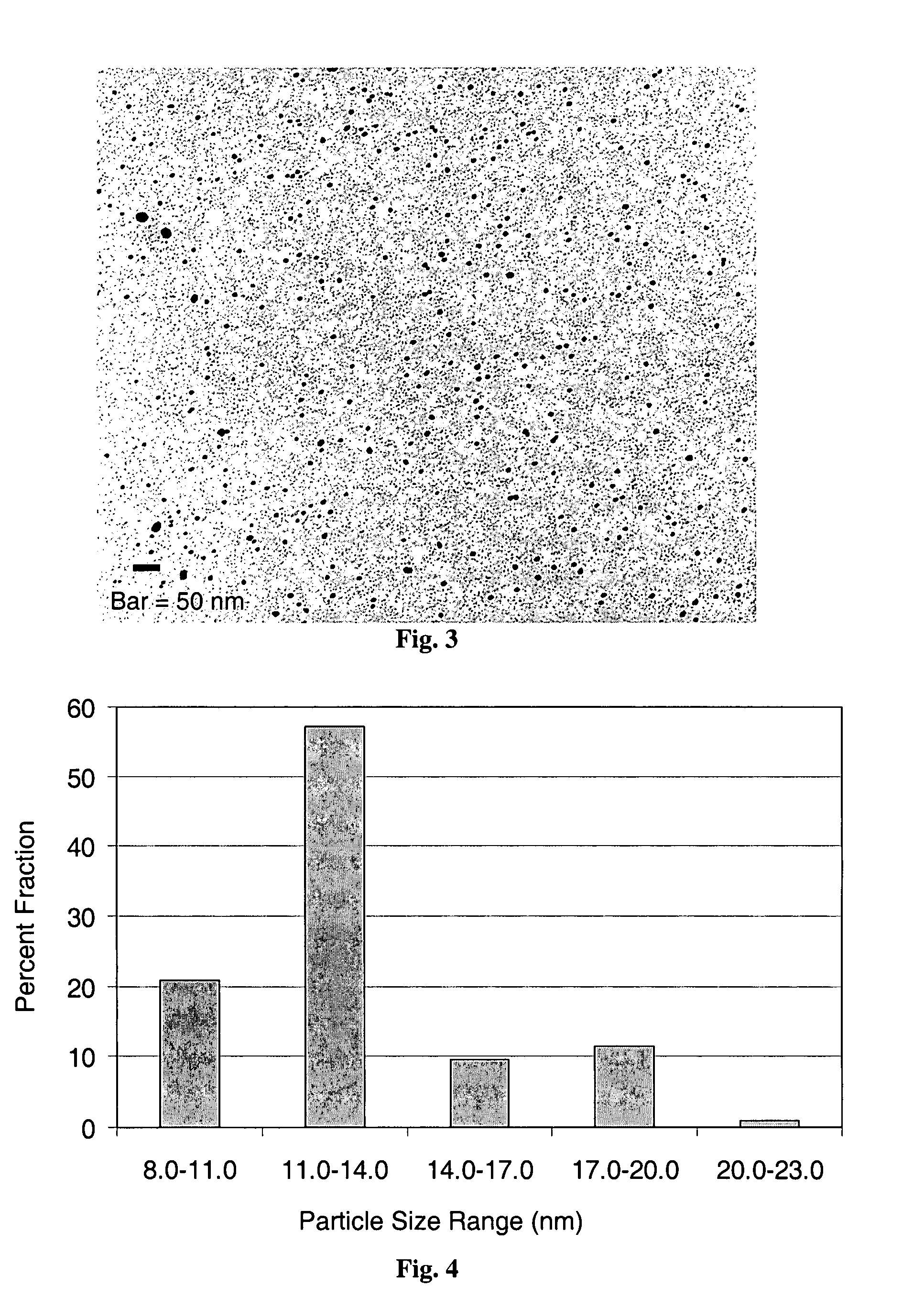
ActiveUS8380541B1Quality improvementLittle variabilityImage analysisData processing applicationsSample collectionDisease
Systems and methods are provided for collecting, preparing, and / or analyzing a biological sample. A sample collection site may be utilized with one or more sample processing device. The sample processing device may be configured to accept a sample from a subject. The sample processing device may perform one or more sample preparation step and / or chemical reaction involving the sample. Data related to the sample may be sent from the device to a laboratory. The laboratory may be a certified laboratory that may generate a report that is transmitted to a health care professional. The health care professional may rely on the report for diagnosing, treating, and / or preventing a disease in the subject.
Owner:LABRADOR DIAGNOSTICS LLC
Internal medical devices for delivery of therapeutic agent in conjunction with a source of electrical power
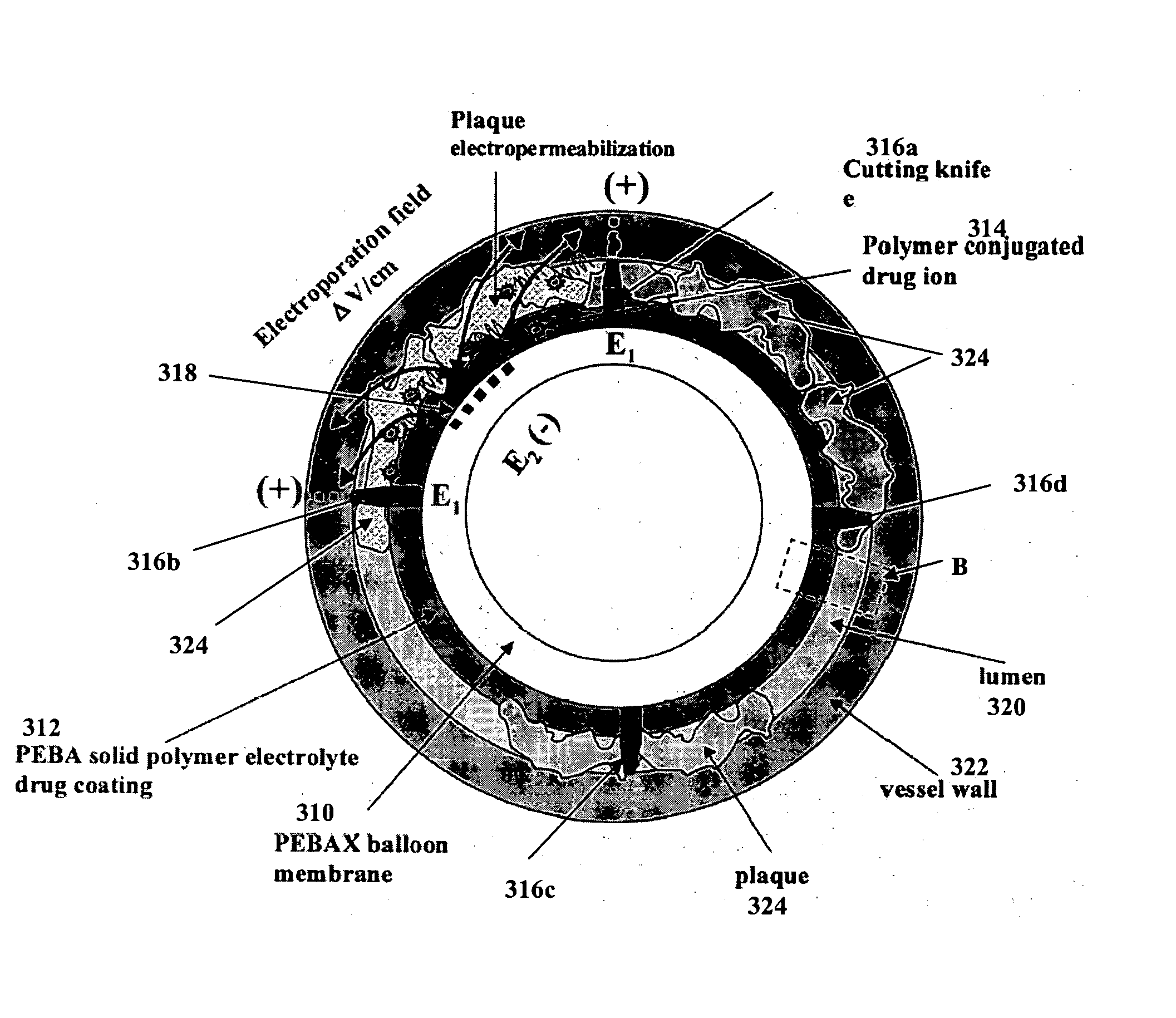
InactiveUS20060184092A1Convenient treatmentReduce deliveryStentsInternal electrodesConductive polymerElectroporation
The invention generally relates to internal (e.g., implantable, insertable, etc.) drug delivery devices which contain the following: (a) one or more sources of one or more therapeutic agents; (b) one or more first electrodes, (c) one or more second electrodes and (d) one or more power sources for applying voltages across the first and second electrodes. The power sources may be adapted, for example, to promote electrically assisted therapeutic agent delivery within a subject, including electroporation and / or iontophoresis. In one aspect of the invention, the first and second electrodes are adapted to have tissue of a subject positioned between them upon deployment of the medical device within the subject, such that an electric field may be generated, which is directed into the tissue. Furthermore, the therapeutic agent sources are adapted to introduce the therapeutic agents into the electric field. In another aspect, the therapeutic agent sources are polymeric regions that contain one or more types of ion-conductive polymers and one or more types of charged therapeutic agents. In yet another aspect, the therapeutic agent sources are polymeric regions that contain one or more types of electrically conductive polymers and one or more types of charged therapeutic agents.
Owner:BOSTON SCI SCIMED INC
Method and apparatus for vascular and visceral clipping
InactiveUS7322995B2Convenient treatmentMinimizing chanceSnap fastenersClothes buttonsTrauma surgeryChest region
Devices and methods for achieving hemostasis and leakage control in hollow body vessels such as the small and large intestines, arteries and veins as well as ducts leading to the gall bladder and other organs. The devices and methods disclosed herein are especially useful in the emergency, trauma surgery or military setting, and most especially during damage control procedures. In such cases, the patient may have received trauma to the abdomen, extremities, neck or thoracic region. The devices utilize removable or permanently implanted, broad, soft, parallel jaw clips with minimal projections to maintain vessel contents without damage to the tissue comprising the vessel. These clips are applied using either standard instruments or custom devices that are subsequently removed leaving the clips implanted, on a temporary or permanent basis, to provide for hemostasis or leakage prevention, or both. These clips overcome the limitations of clips and sutures that are currently used for the same purposes. The clips come in a variety of shapes and sizes. The clips may be placed and removed by open surgery or laparoscopic access.
Owner:DAMAGE CONTROL SURGICAL TECH
Systems and methods for implantable leadless nerve stimulation
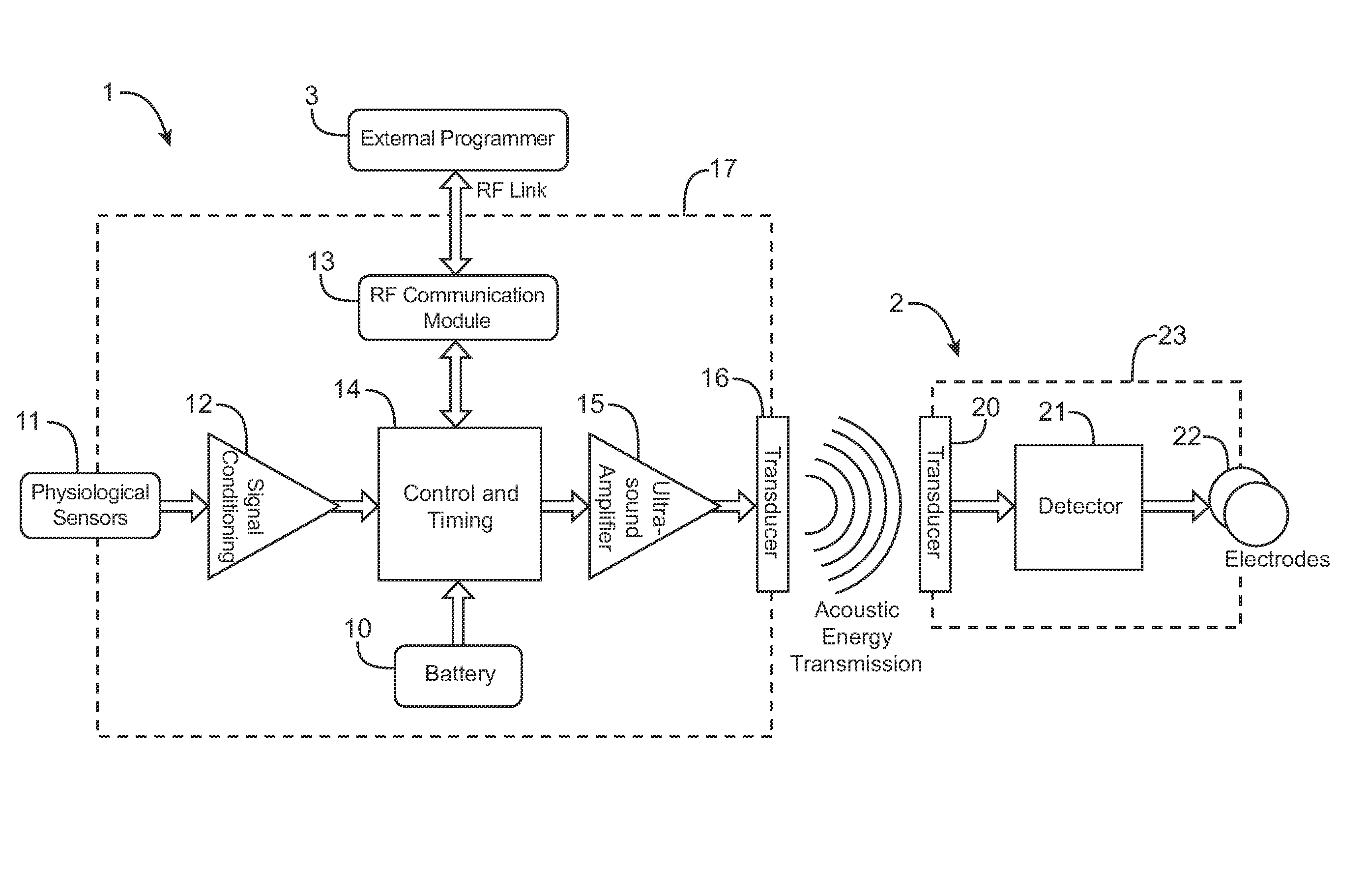
ActiveUS7894907B2Convenient treatmentEliminate requirementsElectrotherapyElectricityImplanted device
Systems and methods are disclosed to stimulate nerves to treat medical conditions such as pain, and other conditions, such as, CHF, obesity, incontinence, etc., that could be controlled by the stimulation of the vagal nerves. The invention uses electrical stimulation of the nerve, where vibrational energy from a source is received by an implanted device and converted to electrical energy and the converted electrical energy is used by implanted electrodes to stimulate the pre-determined nerve site. The vibrational energy is generated by a controller-transmitter, which could be implanted or located externally. The vibrational energy is received by a receiver-stimulator, which could be located in the various regions on or around the nerve that needs to be stimulated. The implantable receiver-stimulator stimulates different nerves and regions of a nerve to provide therapeutic benefit.
Owner:EBR SYST
Non-foreshortening intraluminal prosthesis
InactiveUS6475236B1Different degree of flexibilitySame lengthStentsBlood vesselsProsthesisEngineering
An intraluminal prosthesis is provided with a plurality of annular elements. Each annular element includes a plurality of struts and apices connected to form an annular configuration. Each annular element has a compressed state and an expanded state, and has a longitudinal dimension which is smaller in the expanded state than in the compressed state. A plurality of connecting members connect the apices of adjacent annular elements. The connecting members have a plurality of alternating segments that function to compensate for the smaller longitudinal dimension of each annular element in the expanded state. The stent may be provided with varying flexibility along its length and / or circumference, and may include segments that have different diameters.
Owner:ENDOSYST
Fcgamma riib specific antibodies and methods of use thereof
InactiveUS20050215767A1Enhance immune responseImprove responseSenses disorderAntipyreticTherapeutic antibodyAntiendomysial antibodies
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Means and method for the treatment of cerebral aneurysms
Disclosed is a system for the treatment of cerebral aneurysms using a stent and a aneurysm pocket fill structure delivery system. One embodiment of the present invention uses a highly radiopaque, drug eluting stent that is deployed with its sidewall over the ostium of the aneurysm pocket. A fill structure delivery catheter is then advanced through the patient's vascular system until the catheter's distal end is situated within the aneurysm pocket. Compressed aneurysm pocket filling structures are then pushed through the fill structure delivery catheter. As the aneurysm pocket filling structures emerge from an opening in the catheter's distal end, they promptly expand so that their minimum dimension is sufficiently large so that they cannot pass through the spaces between the struts of the stent that cover the ostium of the aneurysm pocket.
Owner:FISCHELL ROBERT E +1
Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (FAT)
ActiveUS20070129714A1Reduce wrinklesAlter shapeMicroneedlesSurgical instruments for heatingWrinkle skinMuscle contraction
Devices, systems, and methods treat cosmetic defects, and often apply cooling with at least one tissue-penetrating probe inserted through of the skin of a patient. The cooling may remodel one or more target tissue so as to effect a desired change in a composition of the target tissue and / or a change in its behavior. Exemplary embodiments of the cooling treatments will interfere with the nerve / muscle contractile function chain so as to mitigate wrinkles of the skin. Related treatments may be used therapeutically for treatment of back and other muscle spasms, chronic pain, and the like. Some embodiments may remodel subcutaneous adipose tissue so as to alter a shape or appearance of the skin surface.
Owner:PACIRA CRYOTECH INC
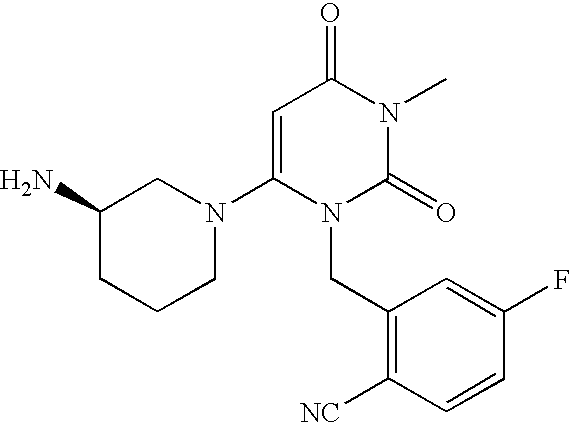
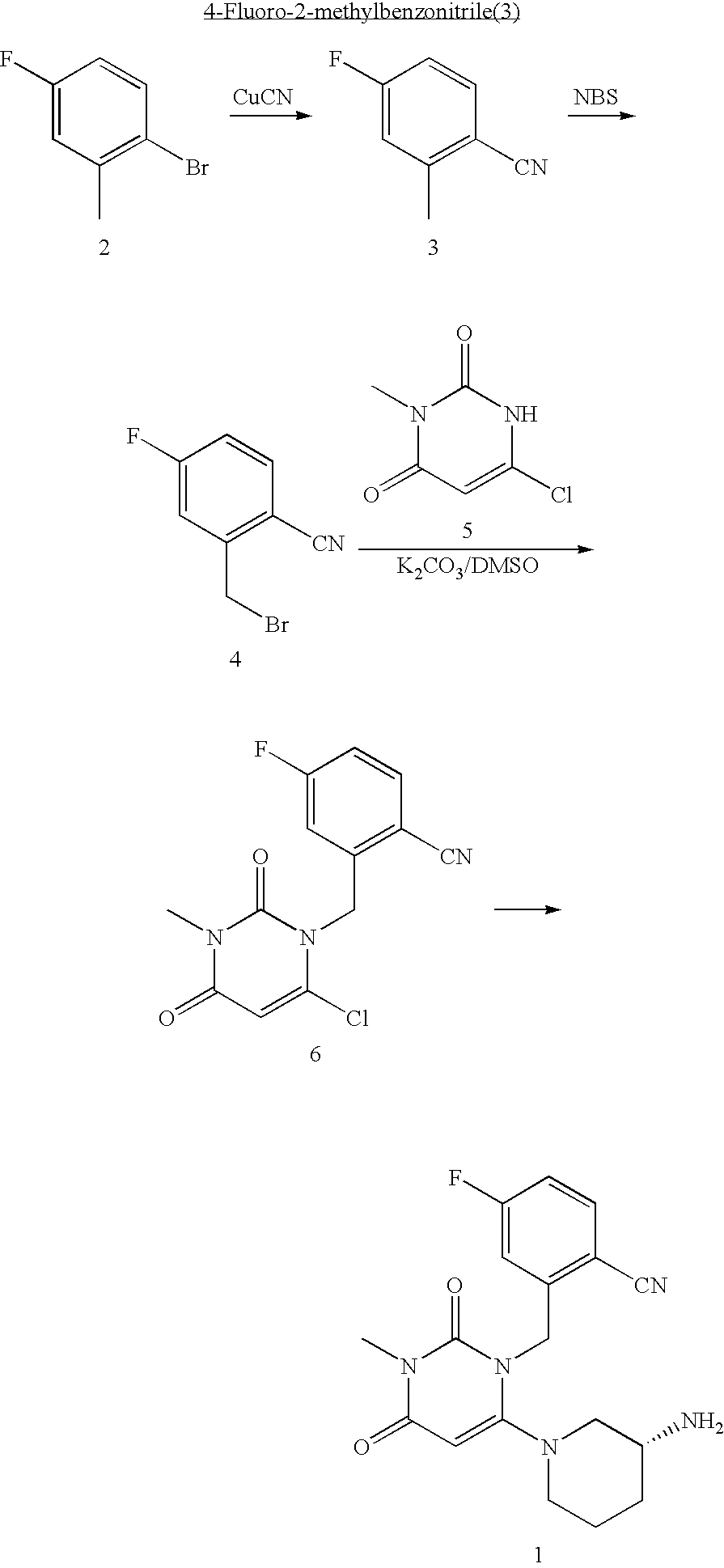
Administration of dipeptidyl peptidase inhibitors
InactiveUS20070060530A1Convenient treatmentEliminate side effectsBiocidePeptide/protein ingredientsDipeptidyl peptidaseBenzonitrile
Pharmaceutical compositions comprising 2-[6-(3-Amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl]-4-fluoro-benzonitrile and pharmaceutically acceptable salts thereof are provided as well as kits and articles of manufacture comprising the pharmaceutical compositions as well as methods of using the pharmaceutical compositions.
Owner:TAKEDA PHARMA CO LTD
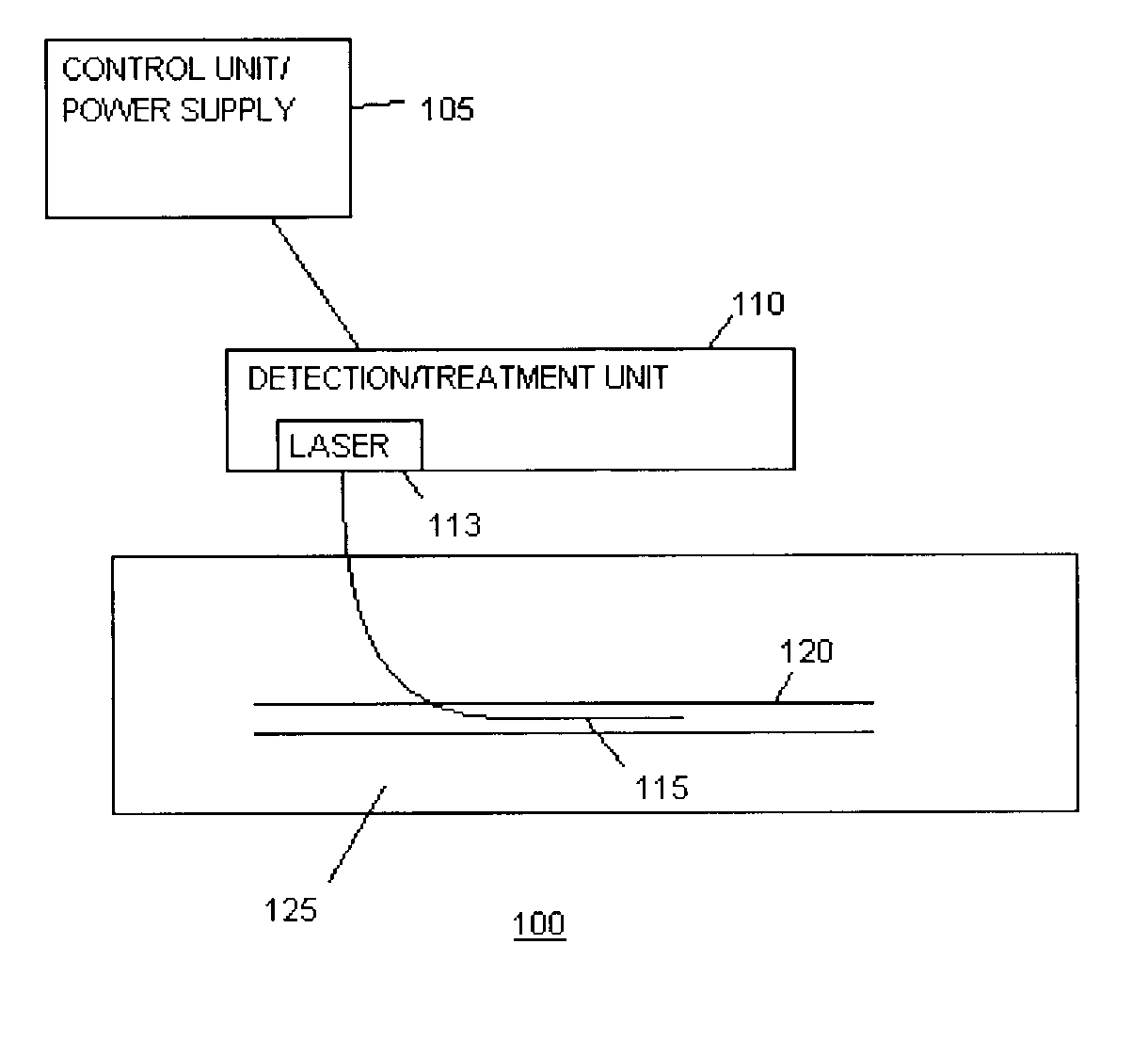
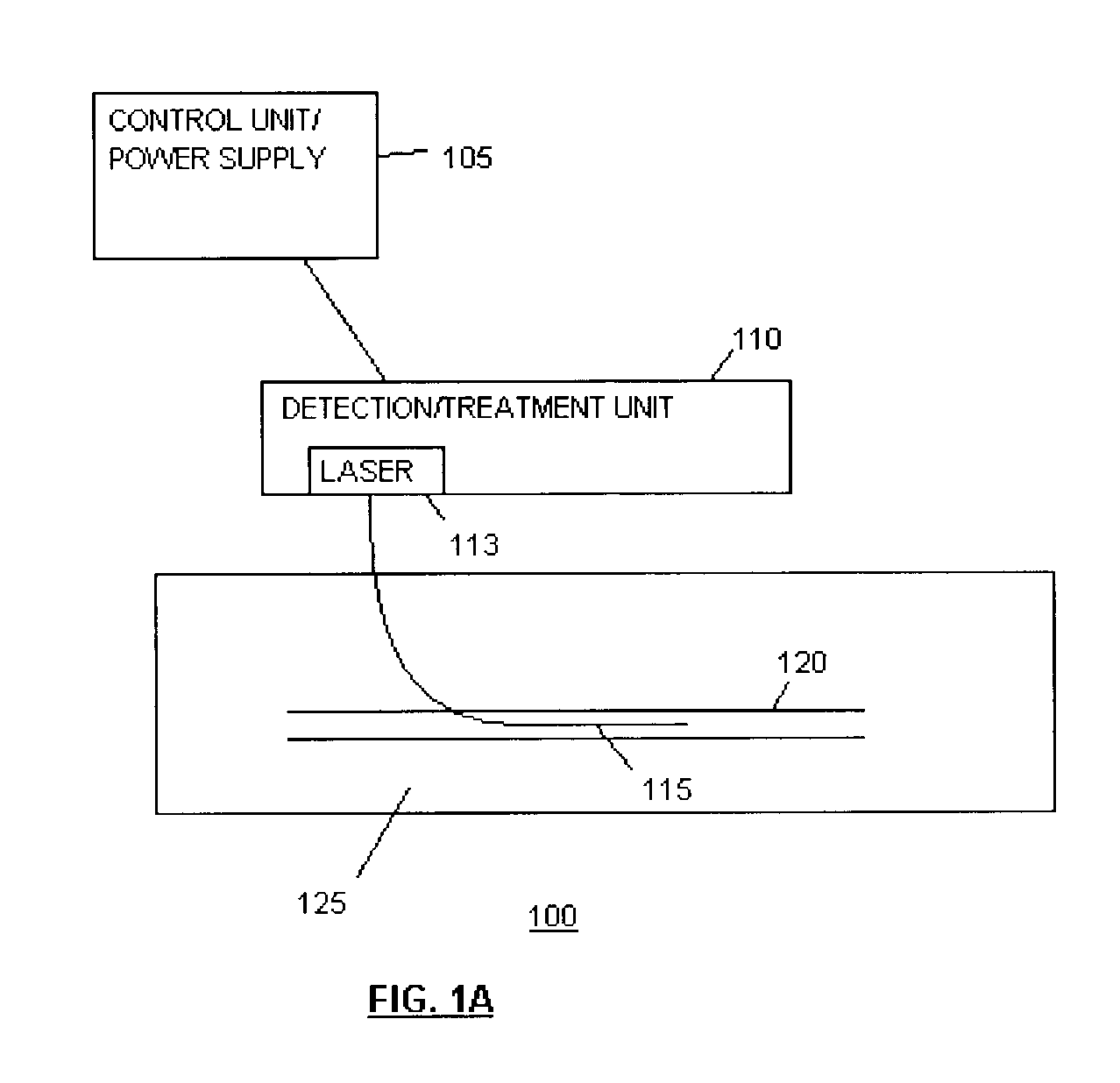
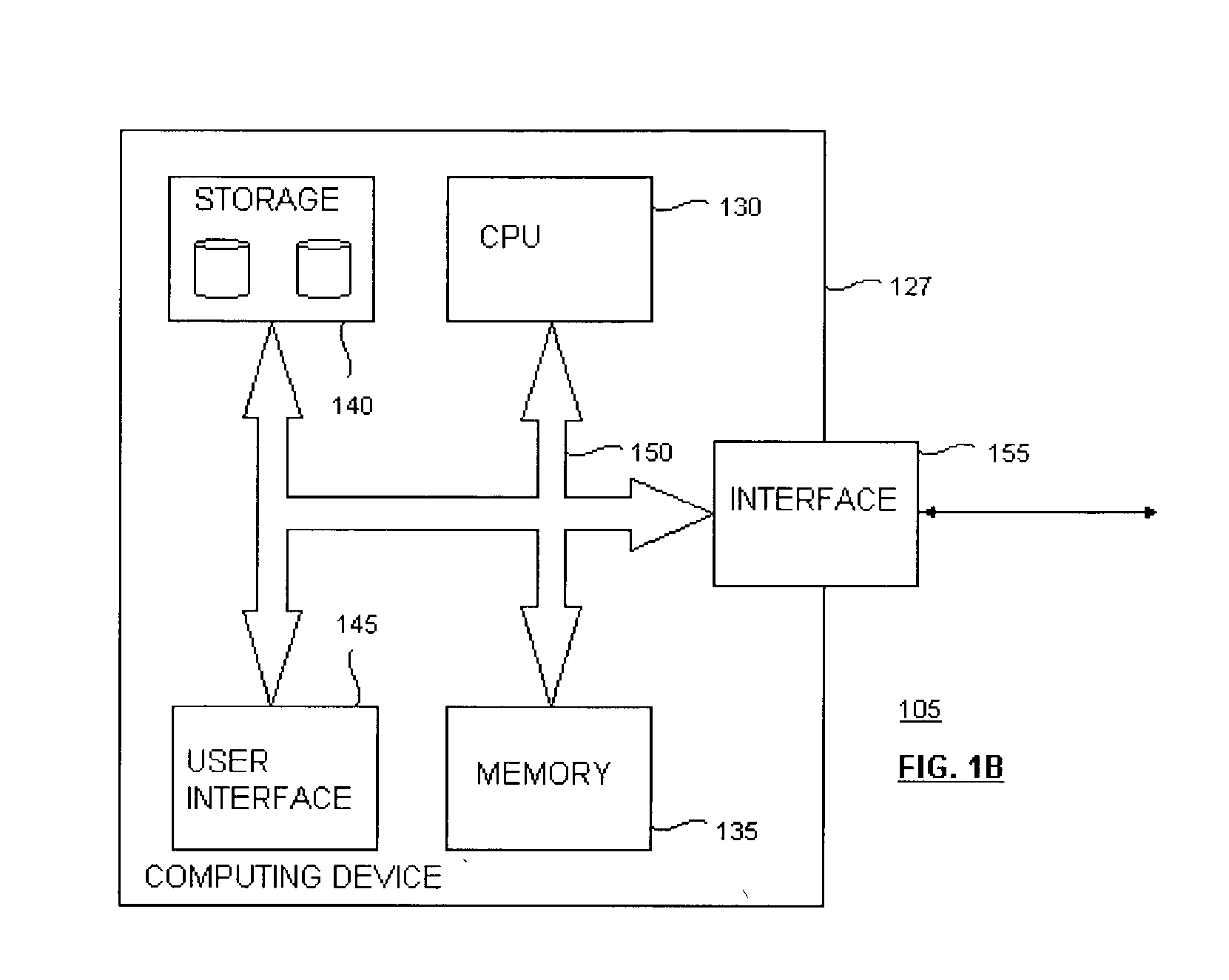
Methods and devices for detection and therapy of atheromatous plaque
InactiveUS20030082105A1Easy to detectHigh sensitivityUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsVulnerable plaqueFluorescence
The present invention relates to devices for detection and therapy of active atheromatous plaque and / or thin-capped fibro-atheroma ("vulnerable plaque"), using selectively targeted fluorescent, radiolabeled, or fluorescent and radiolabeled compositions. The present invention further relates to methods and devices for detection and theraphy of active atheromatous plaques and / or vulnerable plaques, using selectively targeted beta-emitting compositions, optionally comprising fluorescent compositions.
Owner:THE GENERAL HOSPITAL CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com