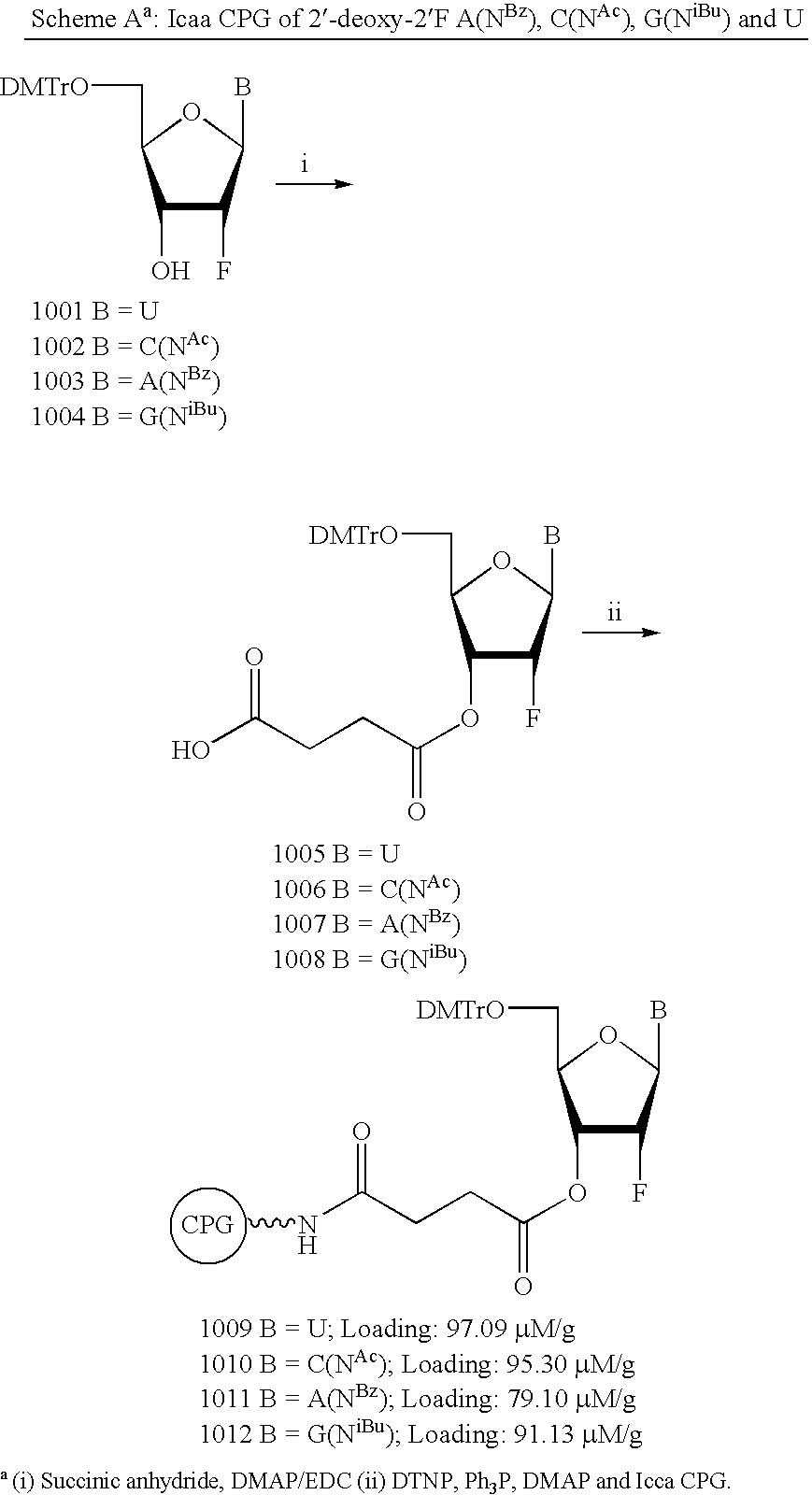
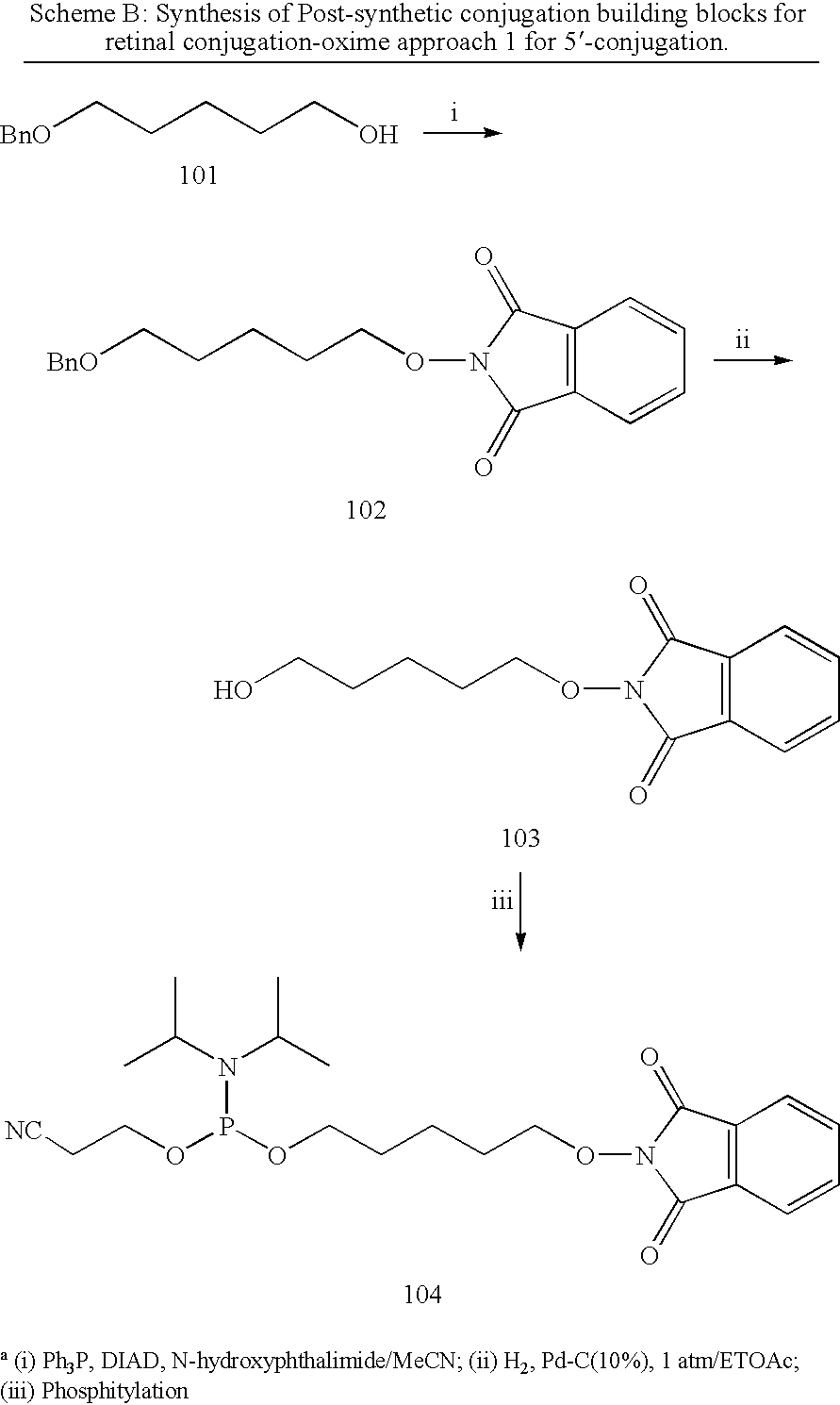
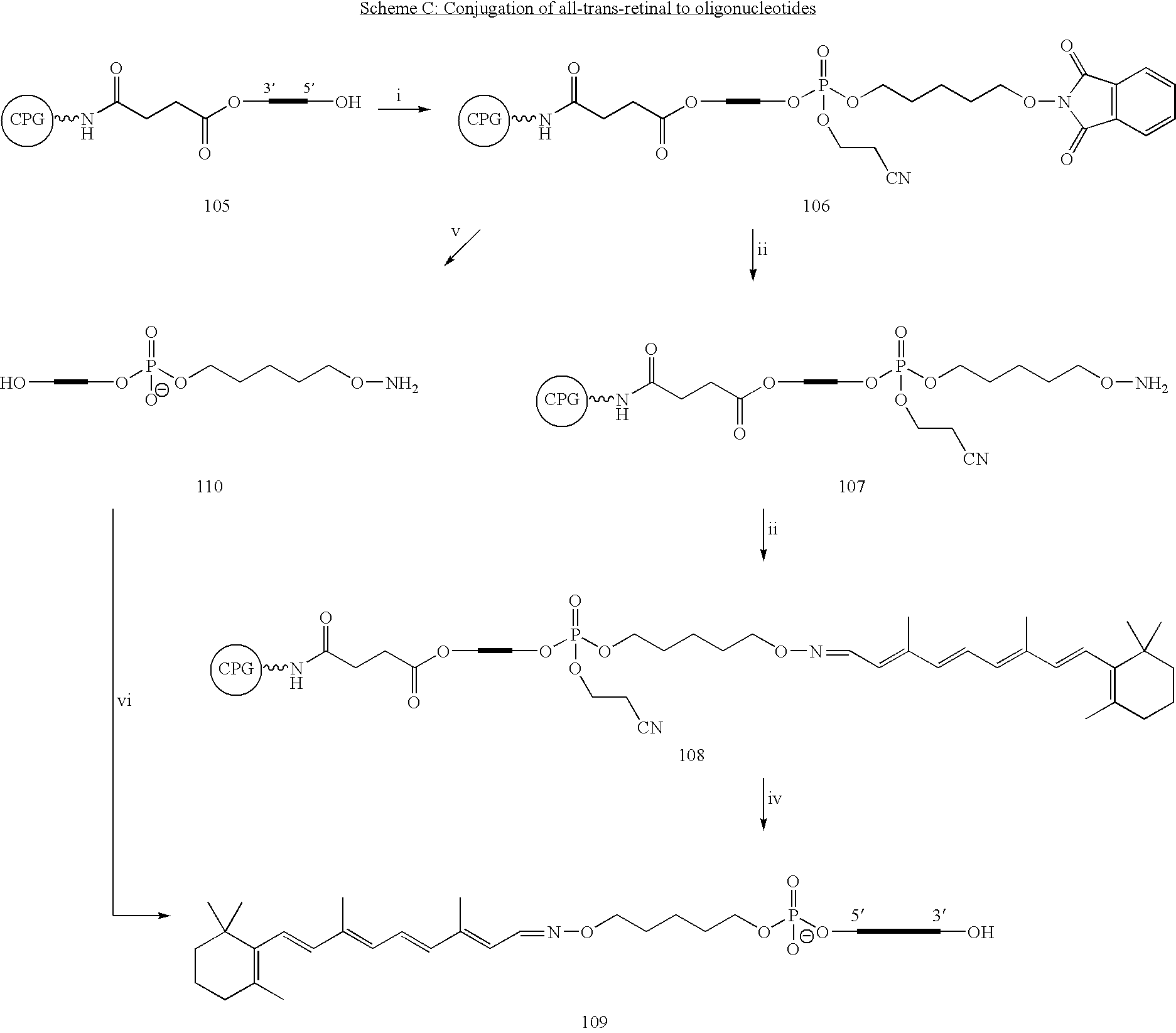
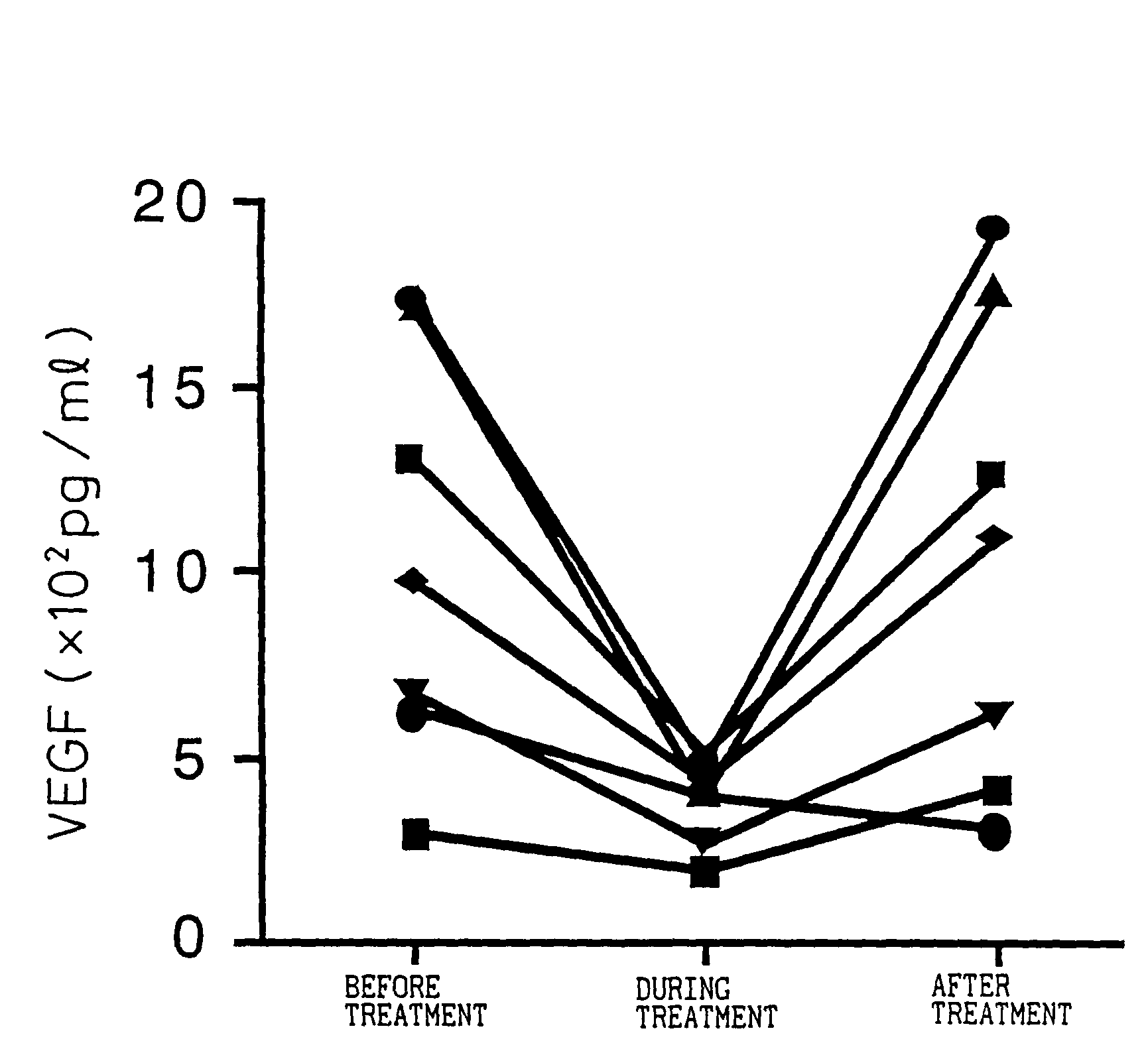
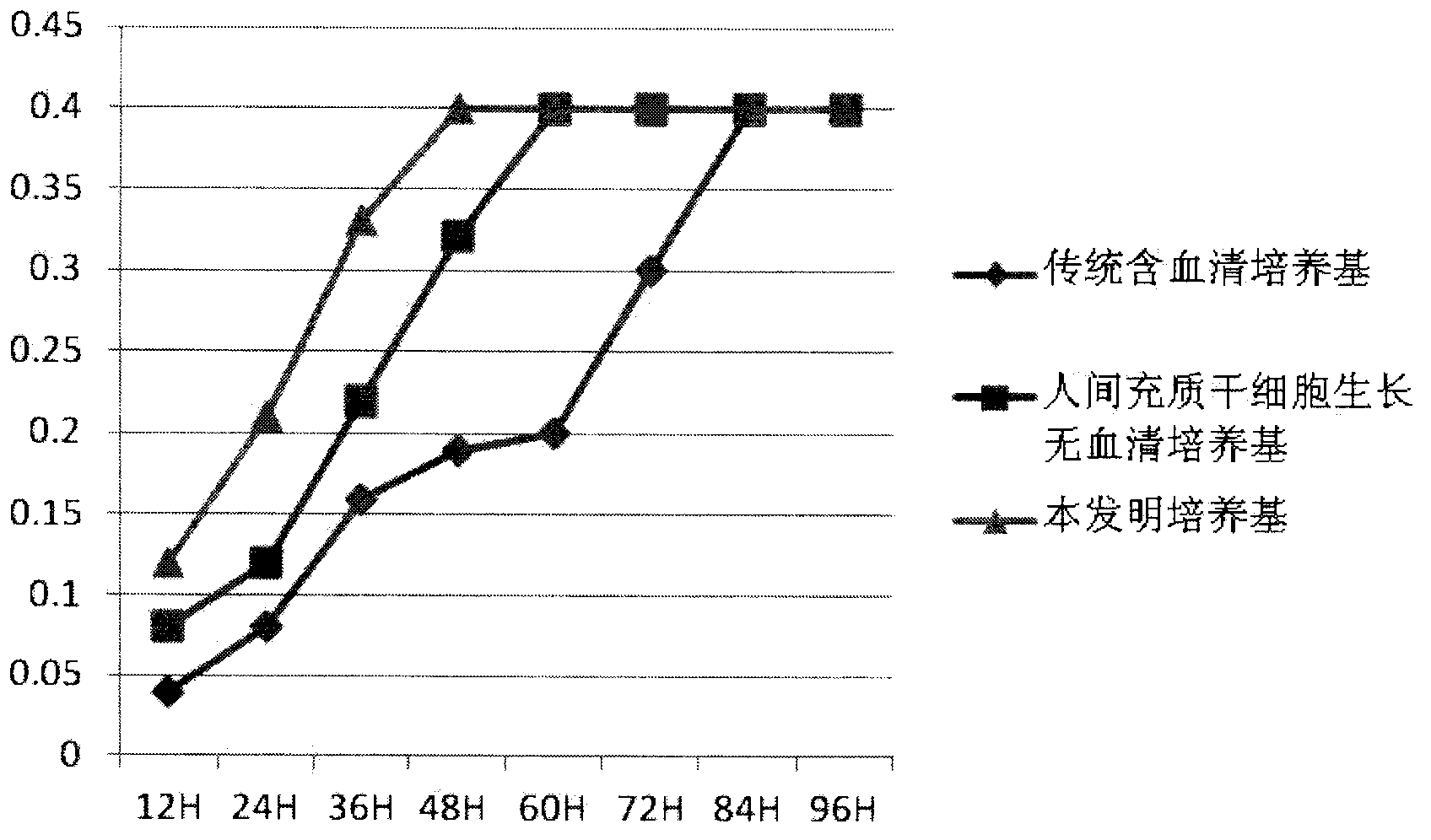
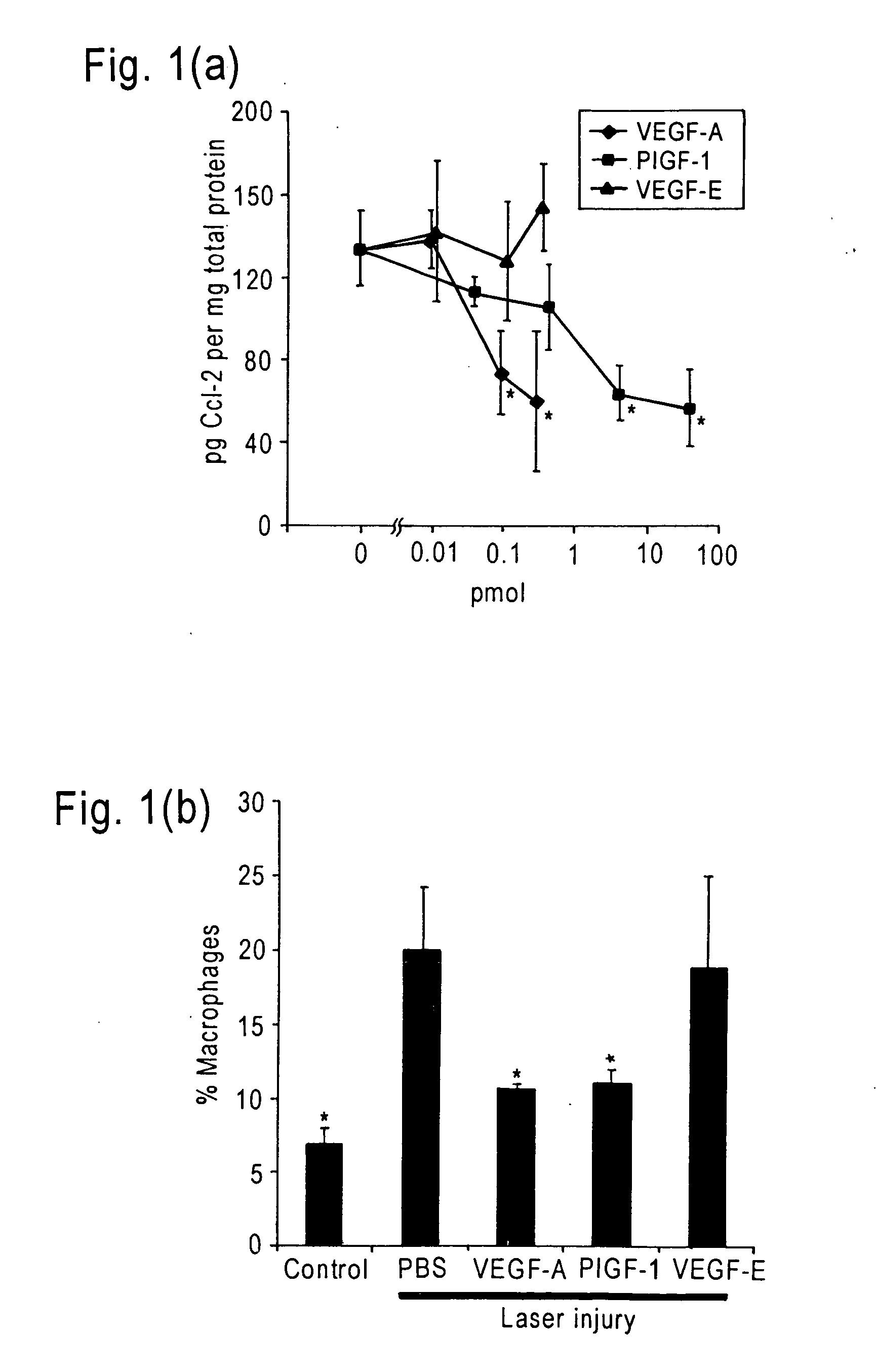
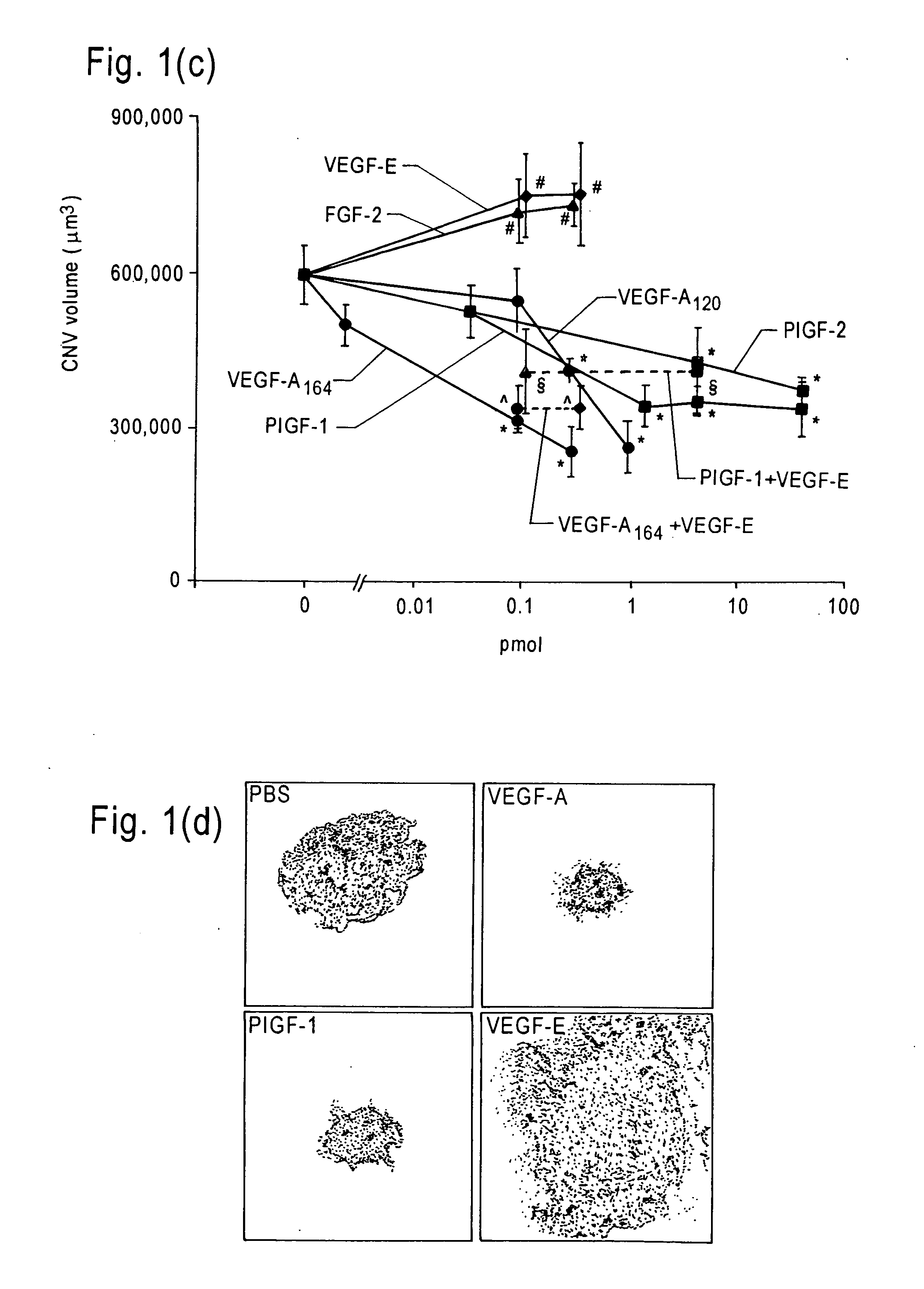
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
583 results about "Vascular endothelial growth factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vascular endothelial growth factor (VEGF), originally known as vascular permeability factor (VPF), is a signal protein produced by cells that stimulates the formation of blood vessels. To be specific, VEGF is a sub-family of growth factors, the platelet-derived growth factor family of cystine-knot growth factors. They are important signaling proteins involved in both vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature).
Decellularized extracellular matrix of conditioned body tissues and uses thereof
InactiveUS20050013870A1Increase in sizeHigh strengthGastrointestinal cellsGenetically modified cellsCell-Extracellular MatrixECM Protein
The present invention relates generally to decellularized extracellular matrix of conditioned body tissues. The decellularized extracellular matrix contains a biological material, preferably vascular endothelial growth factor (VEGF), produced by the conditioned body tissue that is in an amount different than the amount of the biological material that the body tissue would produce absent the conditioning. The invention also relates to methods of making and methods of using said decellularized extracellular matrix. Specifically, the invention relates to treating defective, diseased, damaged or ischemic cells, tissues or organs in a subject by administering, injecting or implanting the decellularized extracellular matrix of the invention into a subject in need thereof. The invention is further directed to a tissue regeneration scaffold for implantation into a subject inflicted with a disease or condition that requires tissue or organ repair, regeneration and / or strengthening. Additionally, the invention is directed to a medical device, preferably a stent or an artificial heart, having a surface coated or covered with the decellularized extracellular matrix of the invention or having a component comprising the decellularized extracellular matrix of the invention for implantation into a subject, preferably a human. Methods for making the tissue regeneration scaffold and methods for manufacturing a coated or covered medical device having a component comprising decellularized extracellular matrix of conditioned body tissues are also provided.
Owner:BOSTON SCI SCIMED INC
Ligands that have binding specificity for VEGF and/or EGFR and methods of use therefor
InactiveUS20070003549A1Not effectiveExtended half-lifeNervous disorderAntipyreticVascular endothelial growth factorCancer therapy
Disclosed are ligands that have binding specificity for vascular endothelial growth factor (VEGF), for epidermal growth factor receptor (EGFR), or for VEGF and EGFR. Also disclosed are methods of using these ligands. In particular, the use of these ligands for cancer therapy is described.
Owner:DORMANTIS LTD
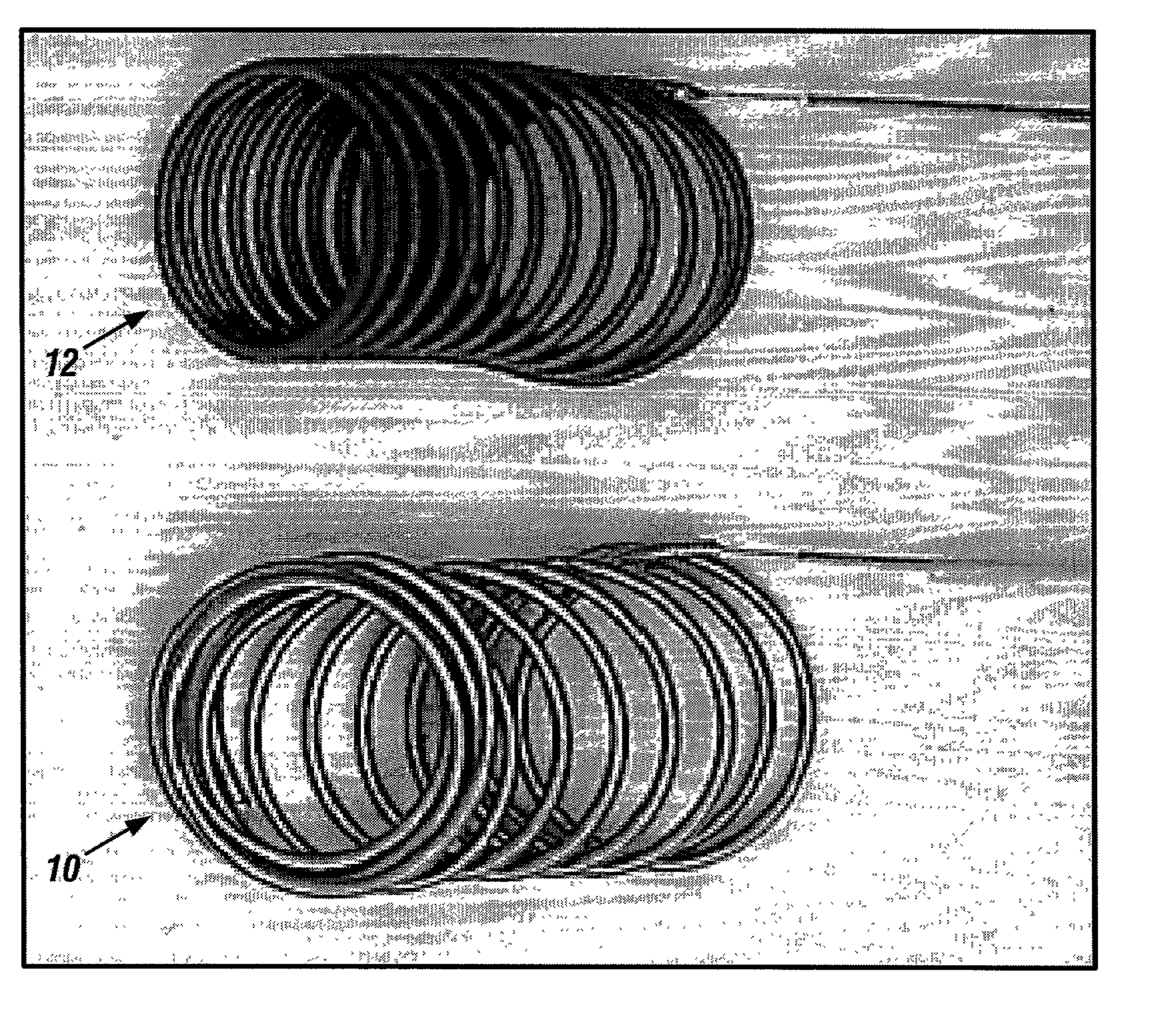
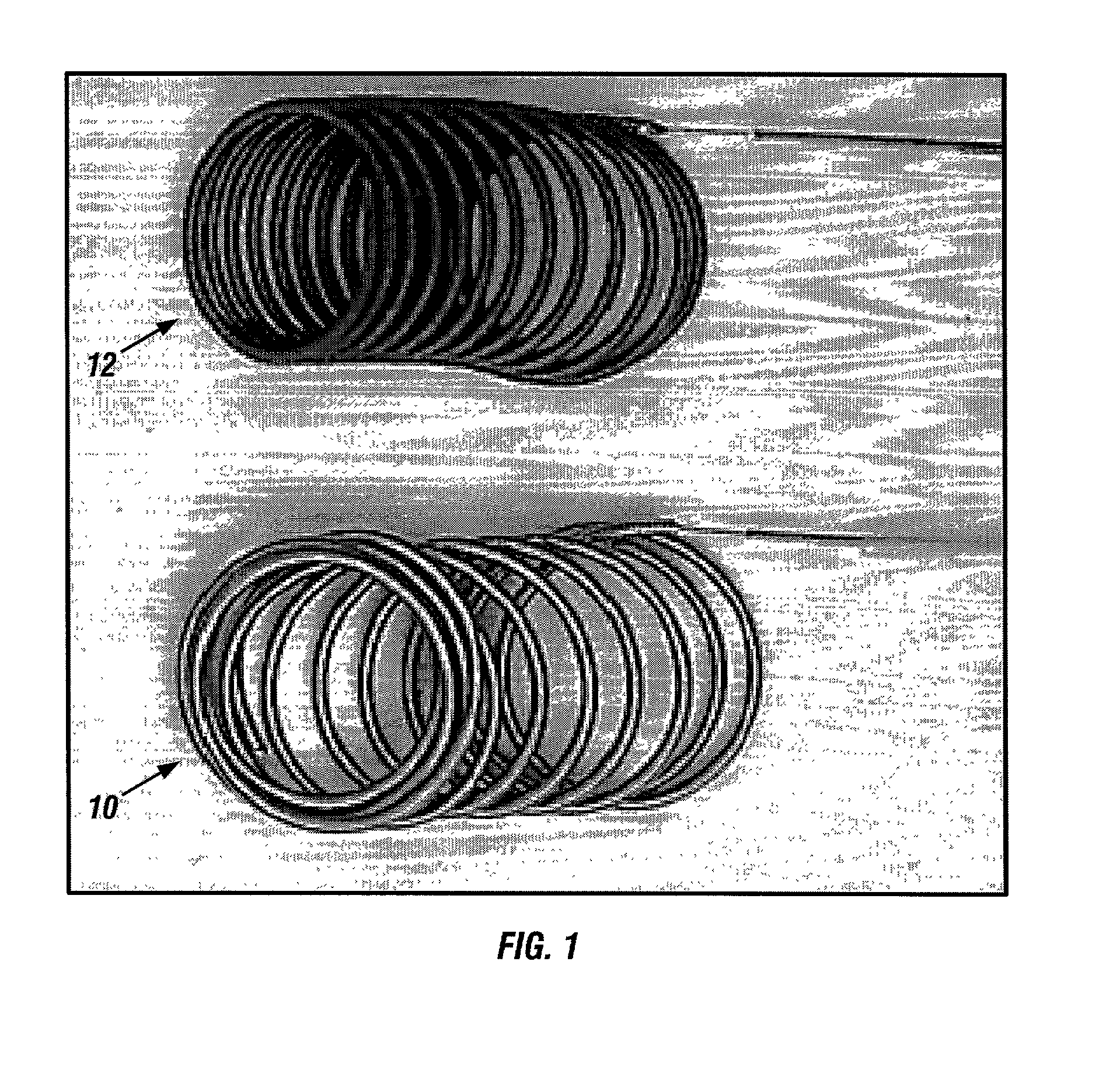
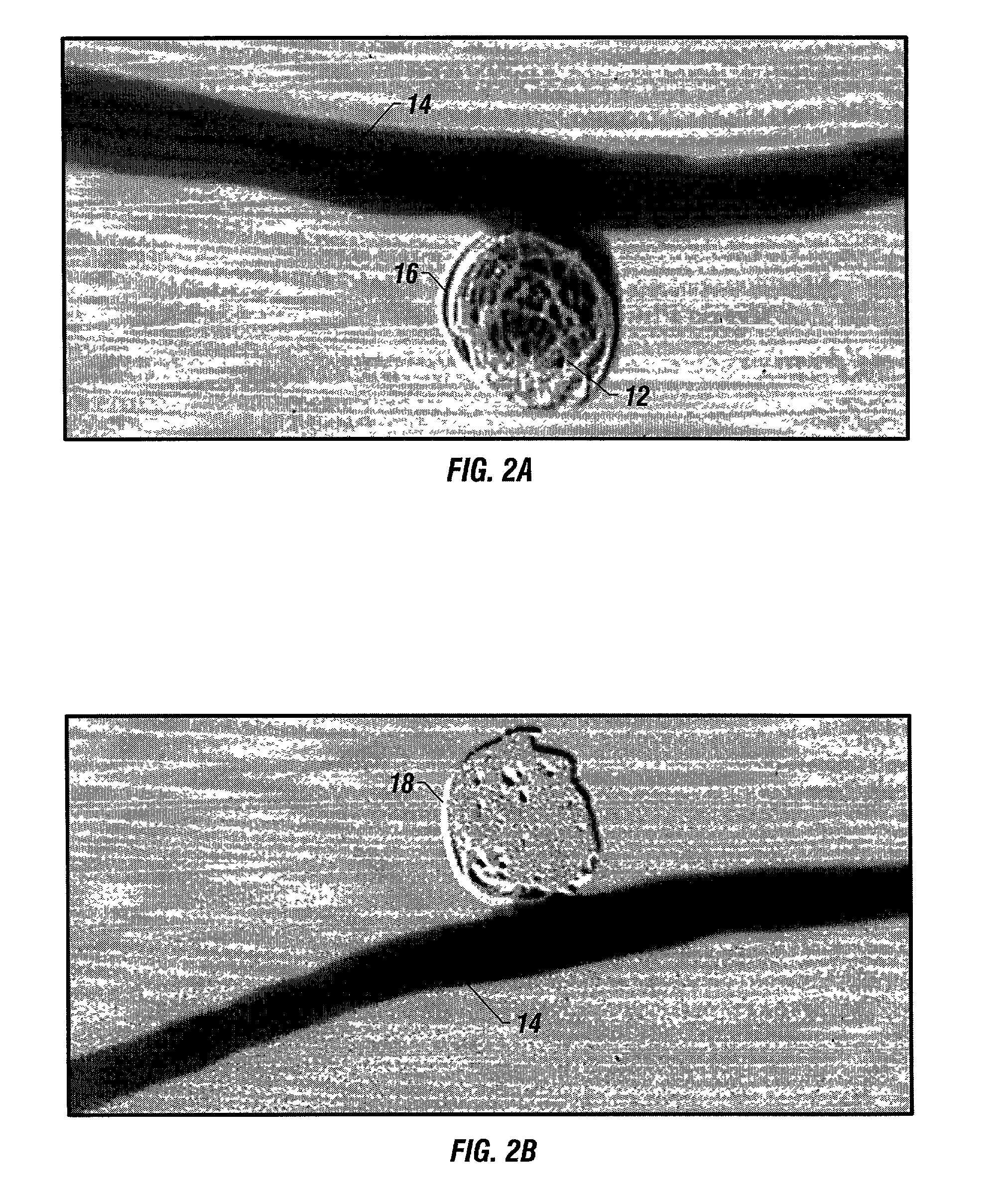
Biodegradable polymer coils for intraluminal implants
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
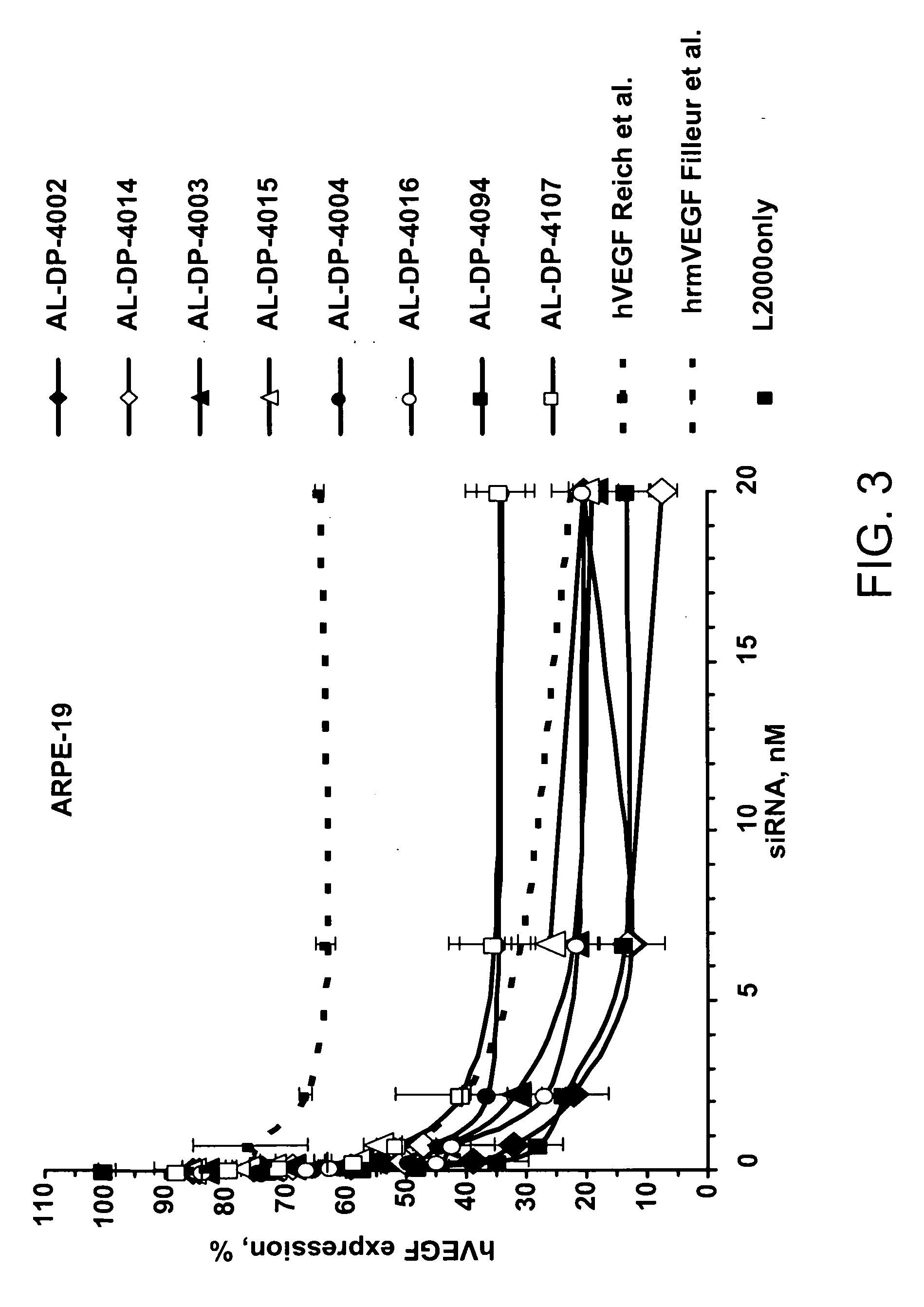
iRNA agents targeting VEGF
ActiveUS20060094032A1Improve propertiesImprove the immunityOrganic active ingredientsSenses disorderVascular endothelial growth factorEndothelium
The features of the present invention relate to compounds, compositions and methods useful for modulating the expression of vascular endothelial growth factor (VEGF), such as by the mechanism of RNA interference (RNAi). The compounds and compositions include iRNA agents that can be unmodified or chemically-modified.
Owner:ALNYLAM PHARM INC
Drug delivery systems and use thereof
InactiveUS20050175708A1Reduced inhibitory activityTreat and inhibit diseasePowder deliveryBiocideVascular endothelial growth factorDelivery system
The invention provides a microsphere formulation for the sustained delivery of an aptamer, for example, an anti-Vascular Endothelial Growth Factor aptamer, to a preselected locus in a mammal, such as the eye. In addition, the invention provides methods for making such formulations, and methods of using such formulations to deliver an aptamer to a preselected locus in a mammal. In particular, the invention provides a method for delivering the aptamer to an eye for the treatment of an ocular disorder, for example, age-related macular degeneration.
Owner:MASSACHUSETTS EYE & EAR INFARY
Stimulation for delivery of molecular therapy
The present invention provides a novel stimulatory device for the controlled production of angiogenic growth factors. More specifically, the present invention provides a subthreshold pulse generator for the local production of vascular endothelial growth factor.
Owner:MEDTRONIC INC
Compositions and methods for treatment of neovascular diseases
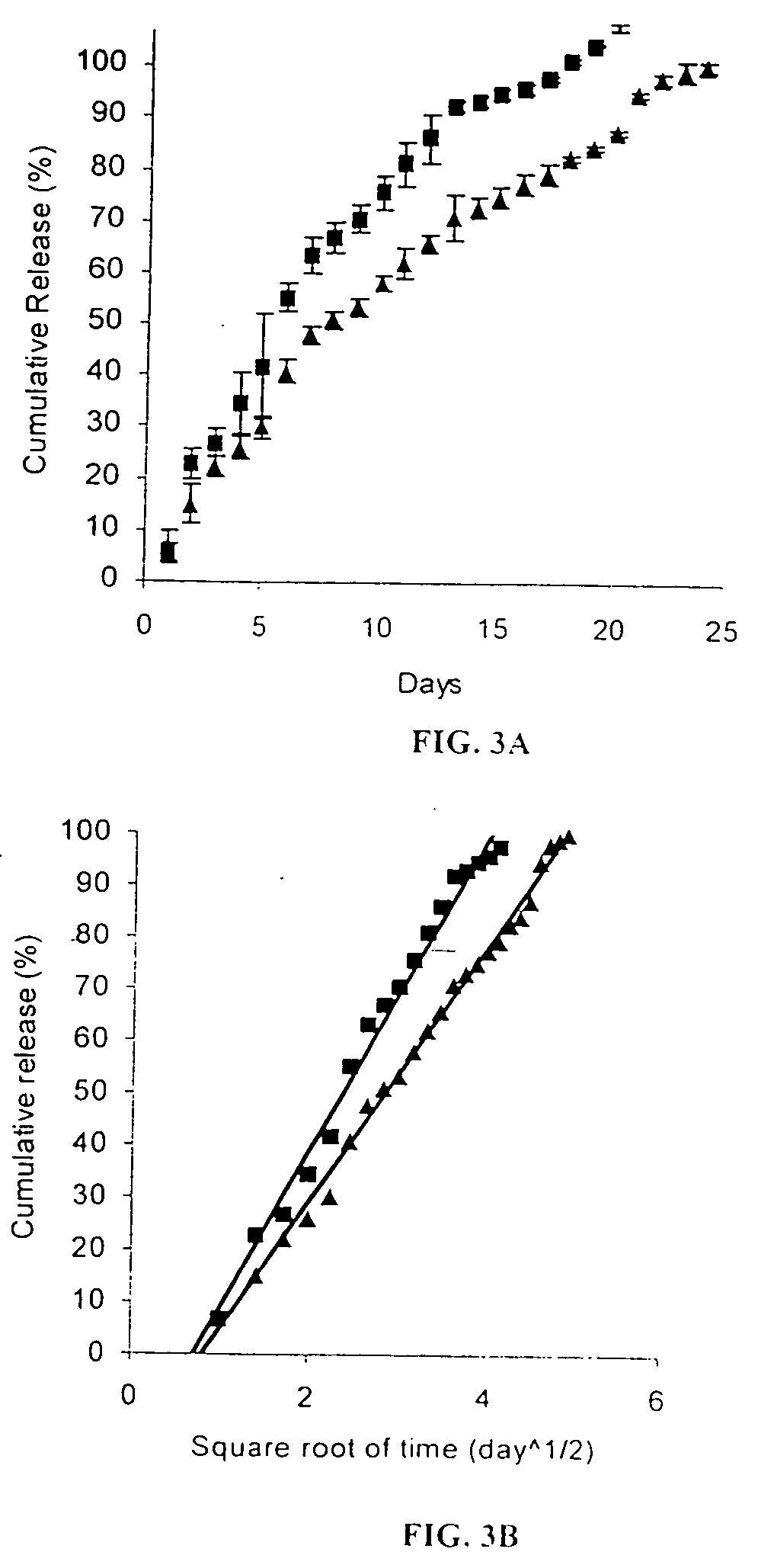
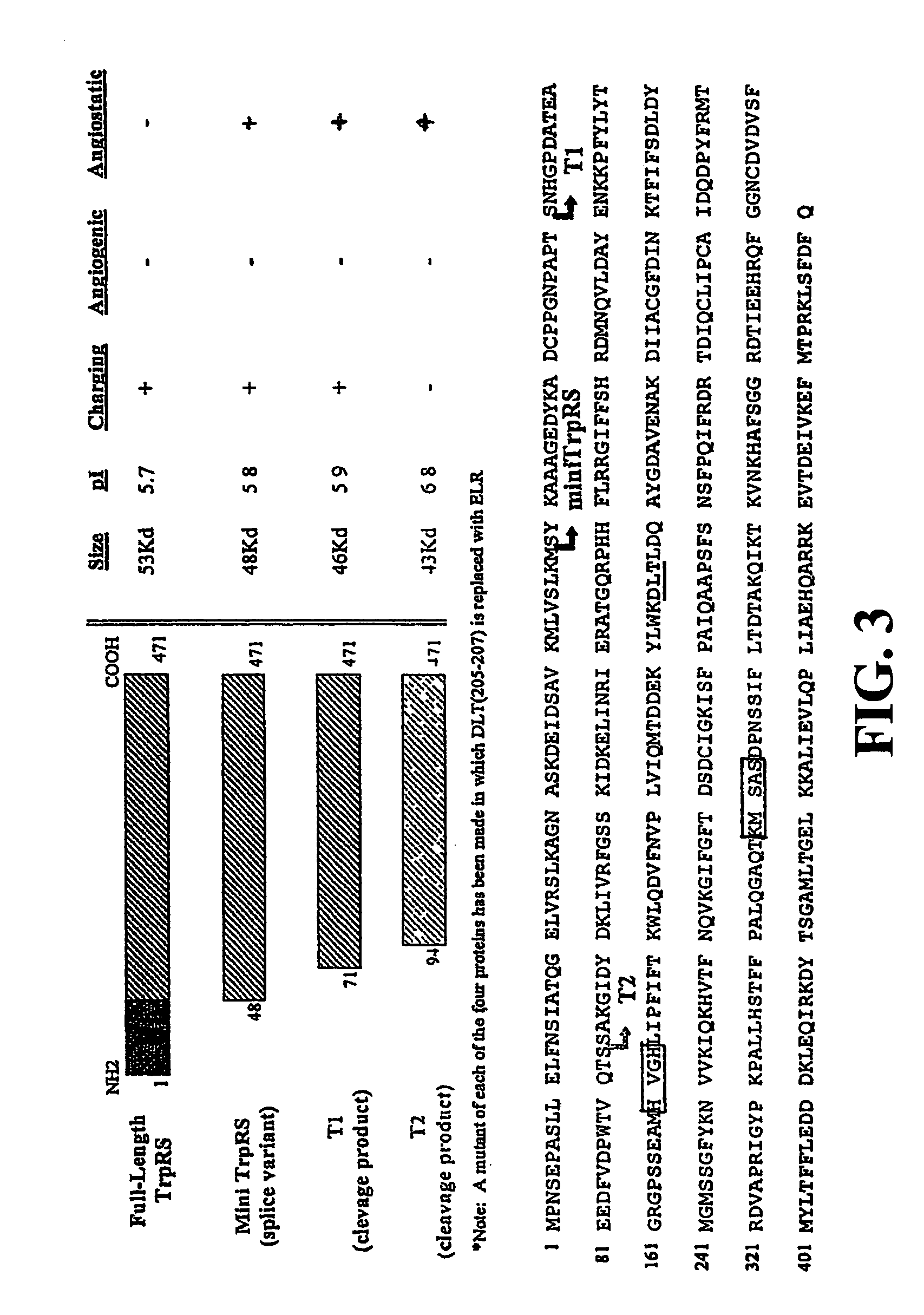
The present invention provides compositions and methods of treating neovascular diseases, such as a retinal neovascular diseases and tumors, by administering to a patient suffering from a neovascular disease or tumor a vascular development inhibiting amount of a combination of the angiogenesis suppressing drugs comprising an angiostatic fragment of tryptophanyl-tRNA synthetase (TrpRS) and at least one compound selected from the group consisting of a vascular endothelial growth factor (VEGF) signaling inhibitor and an integrin signaling inhibitor. Compositions for use in the methods include an admixture of an angiostatic fragment of tryptophanyl-tRNA synthetase (TrpRS) and at least one of a vascular endothelial growth factor (VEGF) signaling inhibitor and an integrin signaling inhibitor, together with a pharmaceutically acceptable excipient.
Owner:THE SCRIPPS RES INST
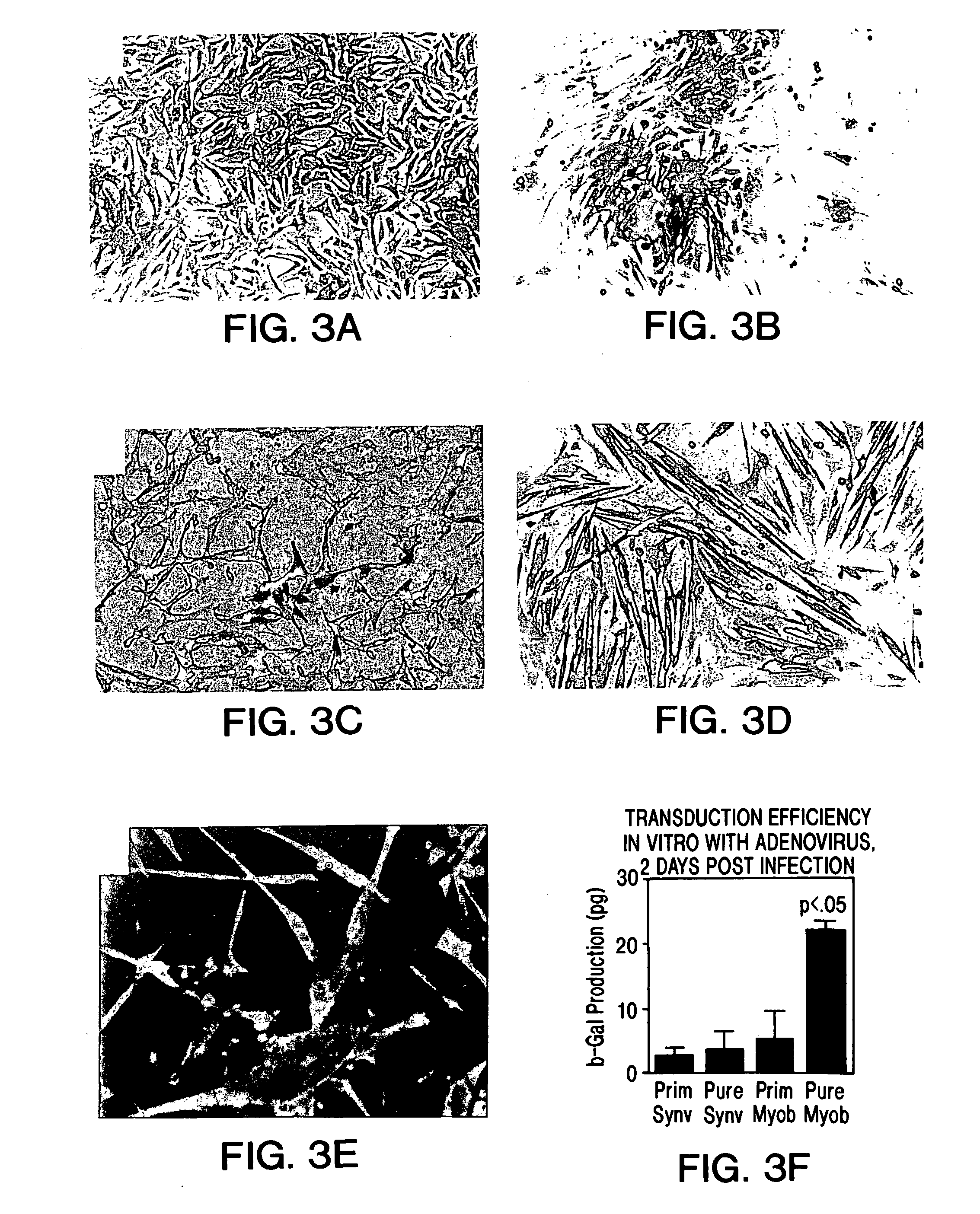
Muscle-derived cells (MDCs) for treating muscle- or bone-related injury or dysfunction
The present invention provides muscle-derived cells, preferably myoblasts and muscle-derived stem cells, genetically engineered to contain and express one or more heterologous genes or functional segments of such genes, for delivery of the encoded gene products at or near sites of musculoskeletal, bone, ligament, meniscus, cartilage or genitourinary disease, injury, defect, or dysfunction. Ex vivo myoblast mediated gene delivery of human inducible nitric oxide synthase, and the resulting production of nitric oxide at and around the site of injury, are particularly provided by the invention as a treatment for lower genitourinary tract dysfunctions. Ex vivo gene transfer for the musculoskeletal system includes genes encoding acidic fibroblast growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor, platelet derived growth factor, transforming growth factor-β, transforming growth factor-α, nerve growth factor and interleukin-1 receptor antagonist protein (IRAP), bone morphogenetic protein (BMPs), cartilage derived morphogenetic protein (CDMPs), vascular endothelial growth factor (VEGF), and sonic hedgehog proteins.
Owner:UNIVERSITY OF PITTSBURGH
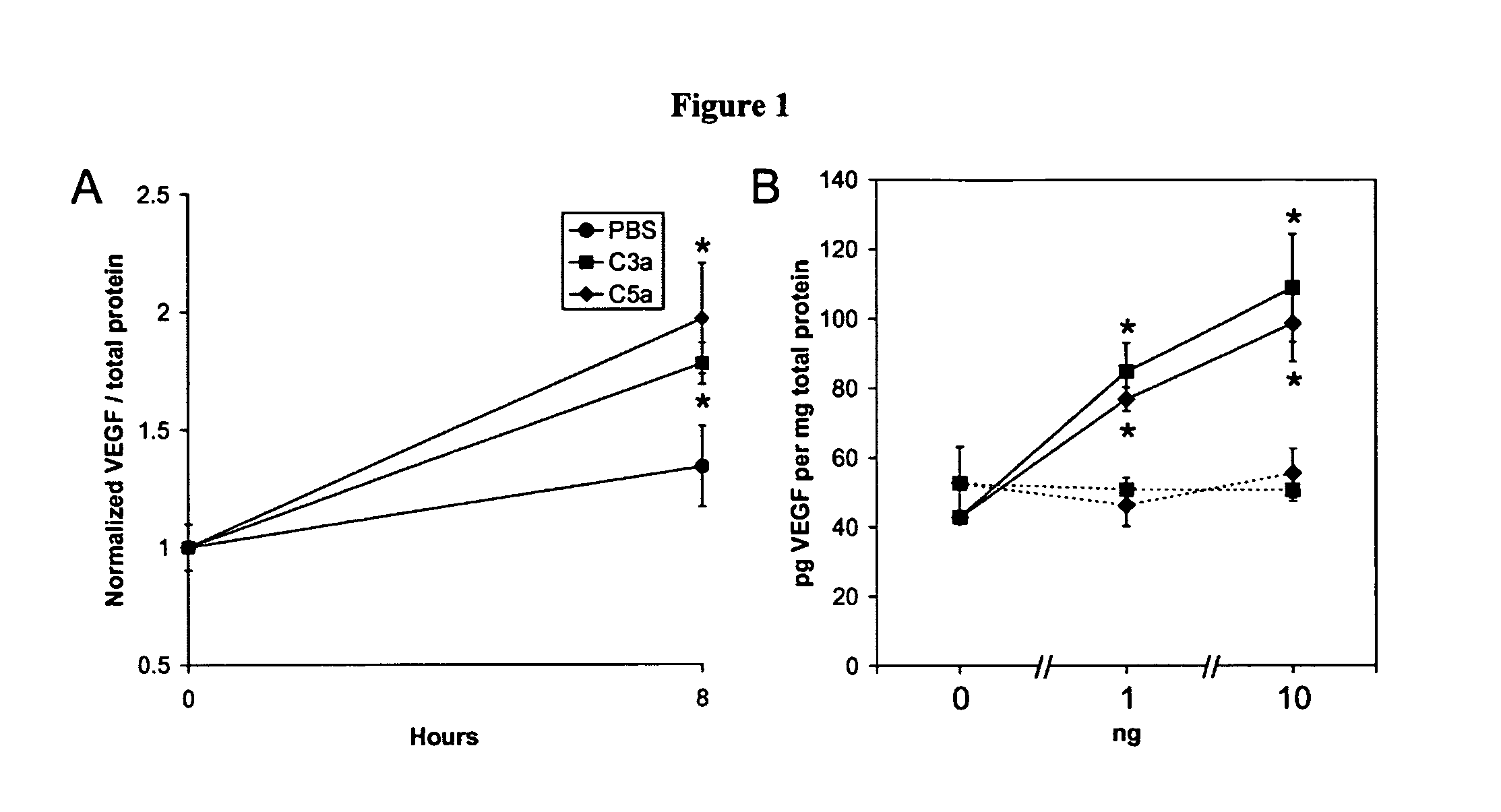
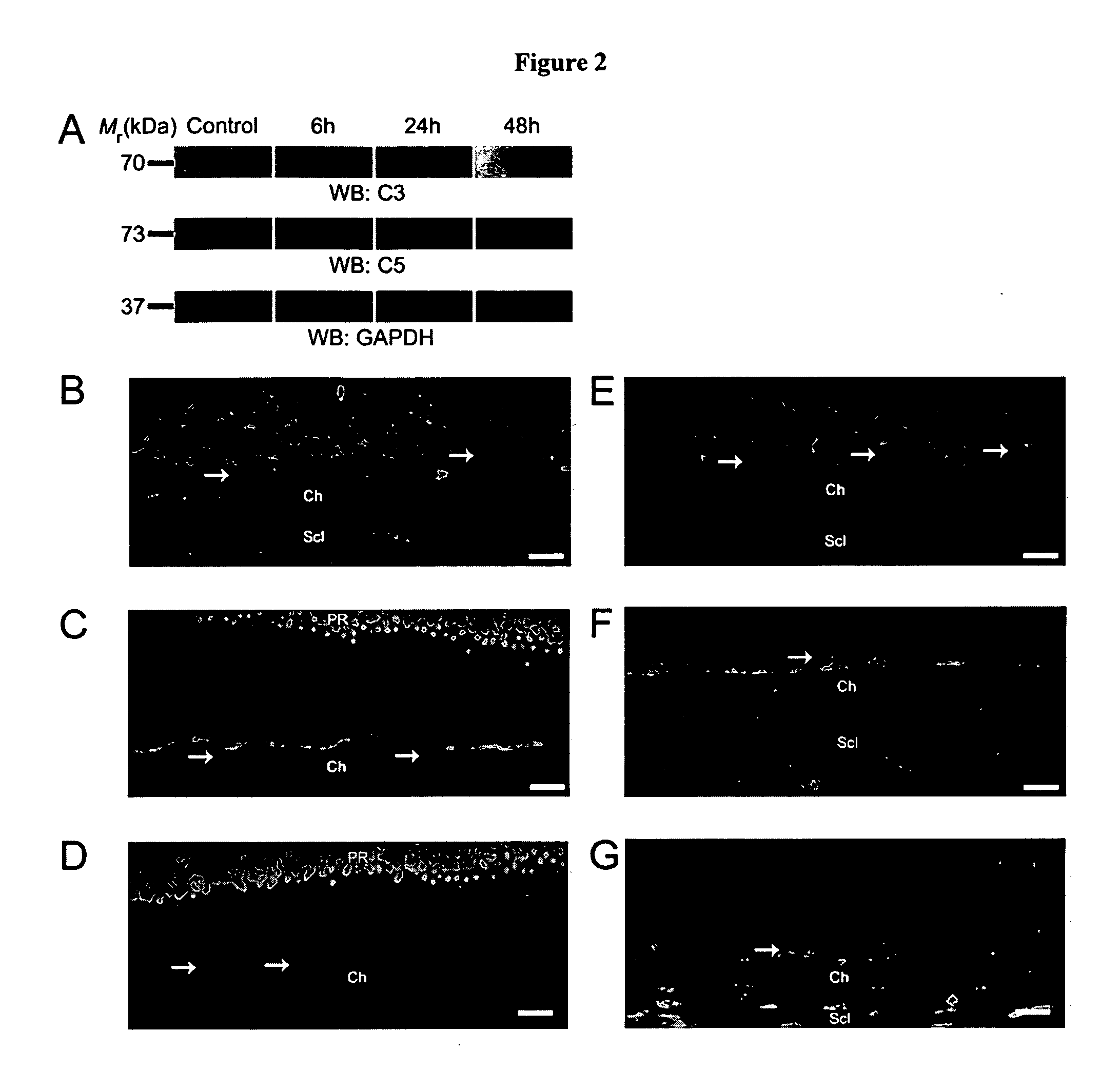
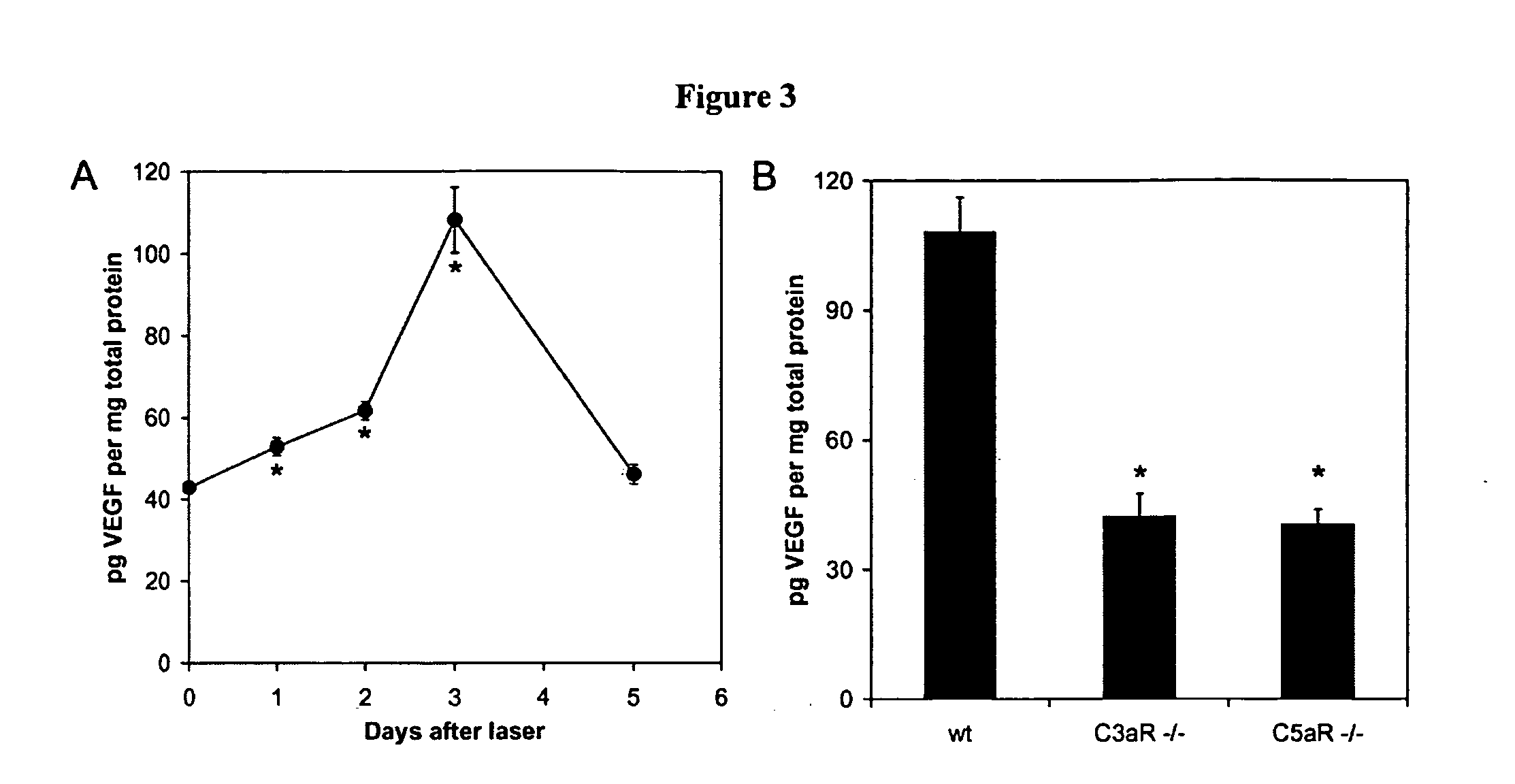
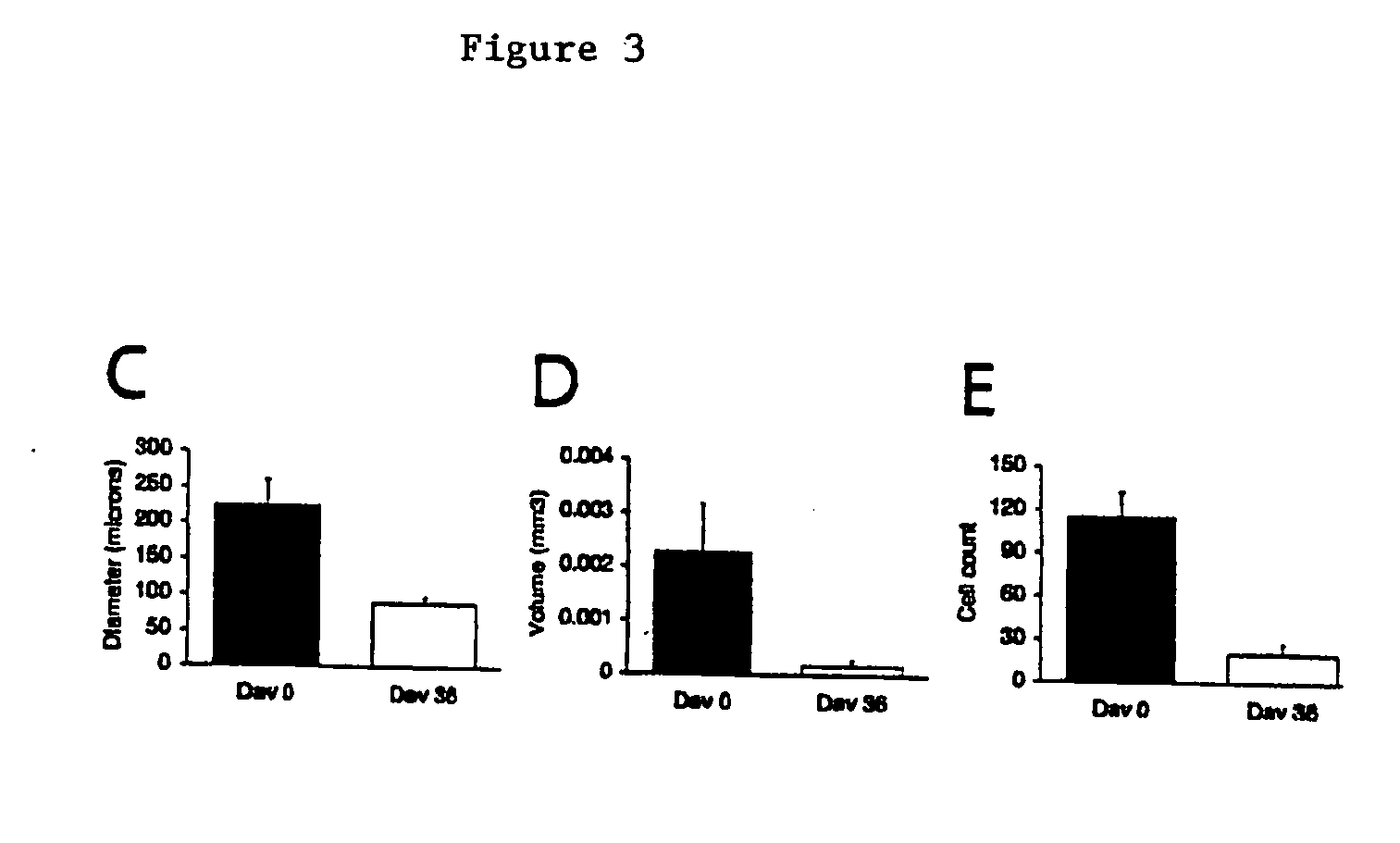
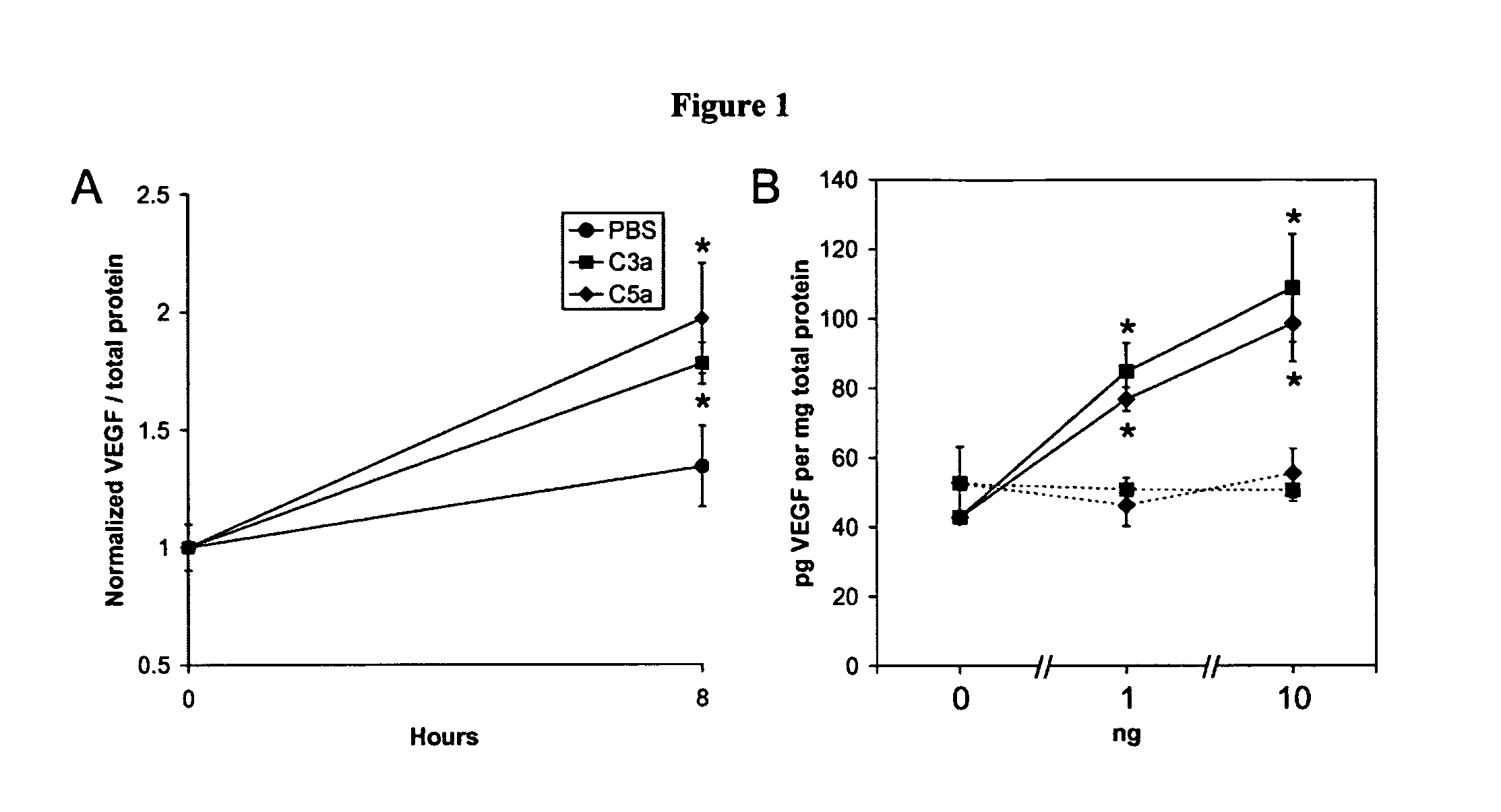
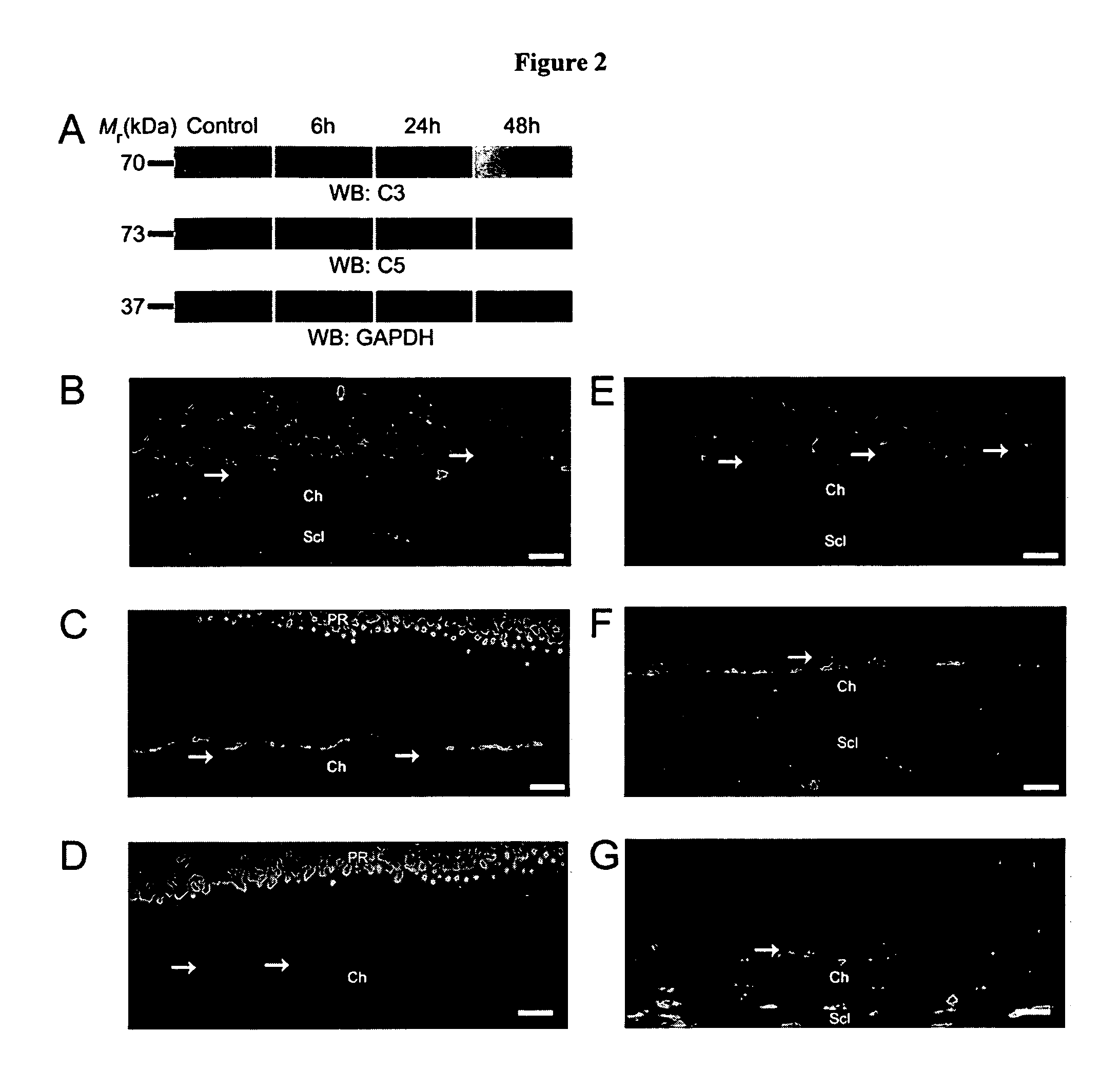
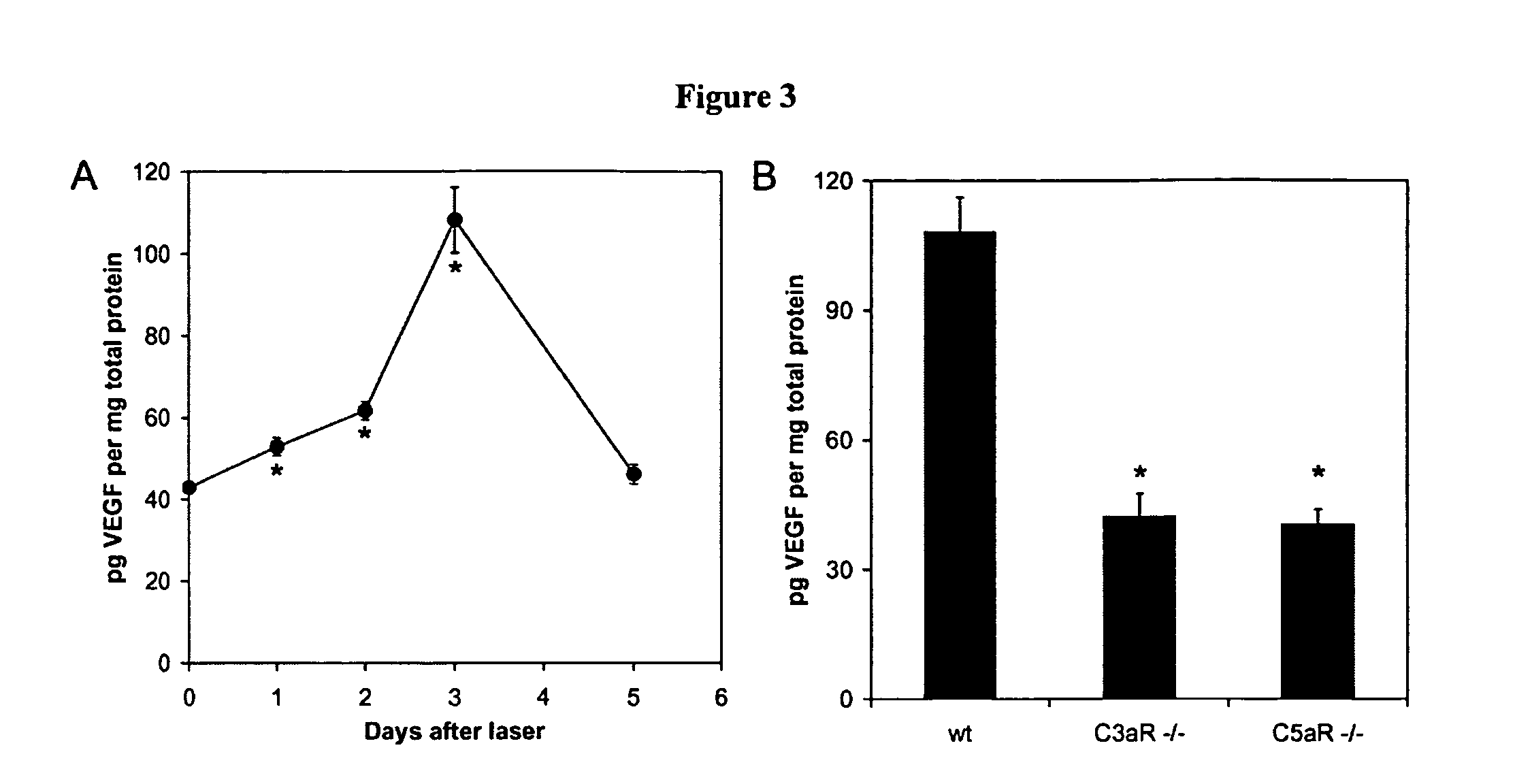
Compositions and methods for inhibiting drusen complement components C3a and C5a for the treatment of age-related macular degeneration
InactiveUS20060067935A1Reduces VEGF expressionReduce expressionGenetic material ingredientsAntibody ingredientsIn vivoBiology
Activated C3 (C3a) and its receptor (C3aR) and activated C5 (C5a) and its receptor (C5aR) have been shown to induce vascular endothelial growth factor (VEGF) expression in vitro and in vivo. Compositions and methods for inhibiting C3a, C3aR, C5a and C5aR for the treatment and / or prevention of neovascular disease are provided. Also provided are Novel therapeutic targets and diagnostic markers for choroidal neovascularization.
Owner:KENTUCKY UNIVERISTY OF
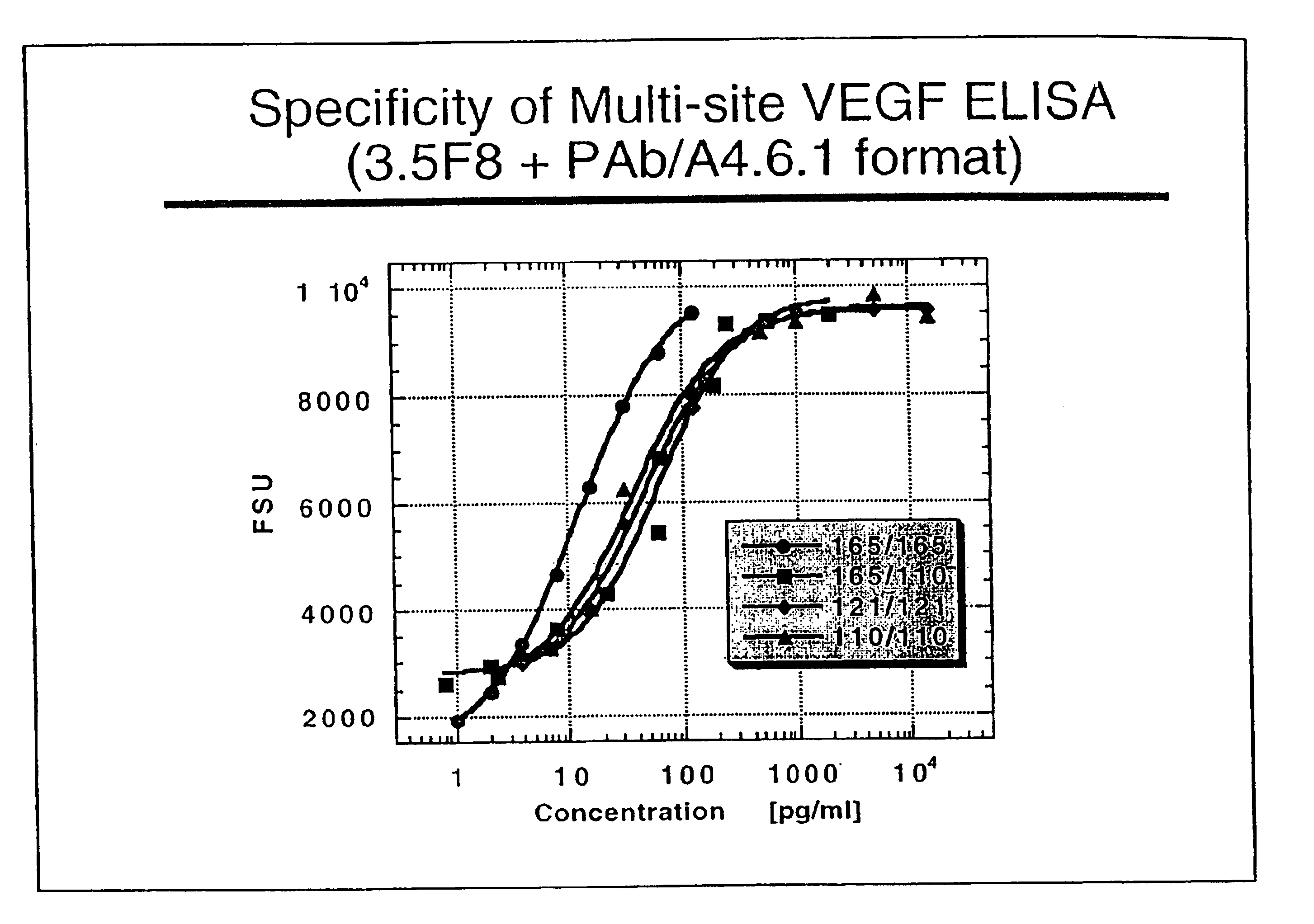
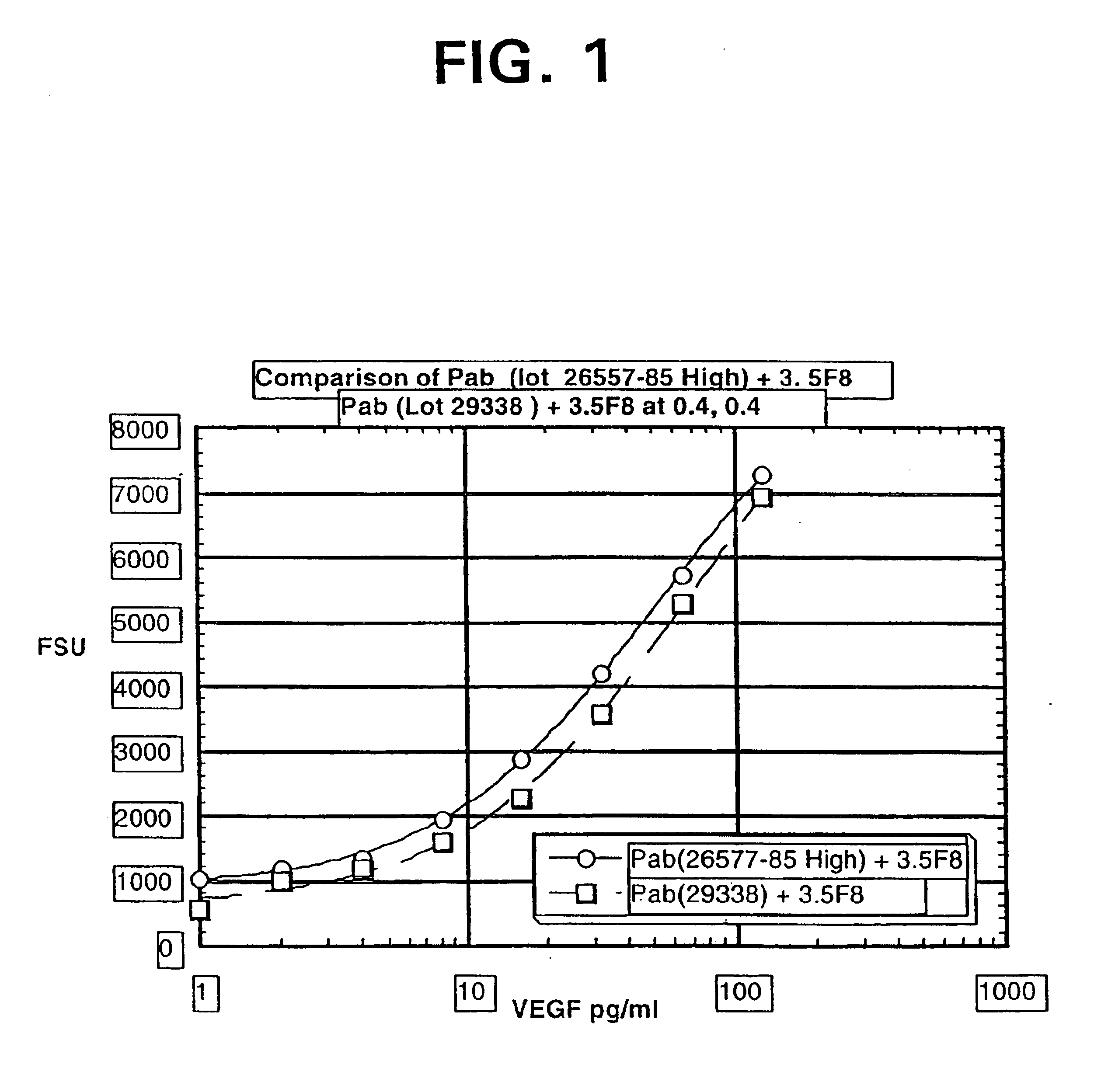
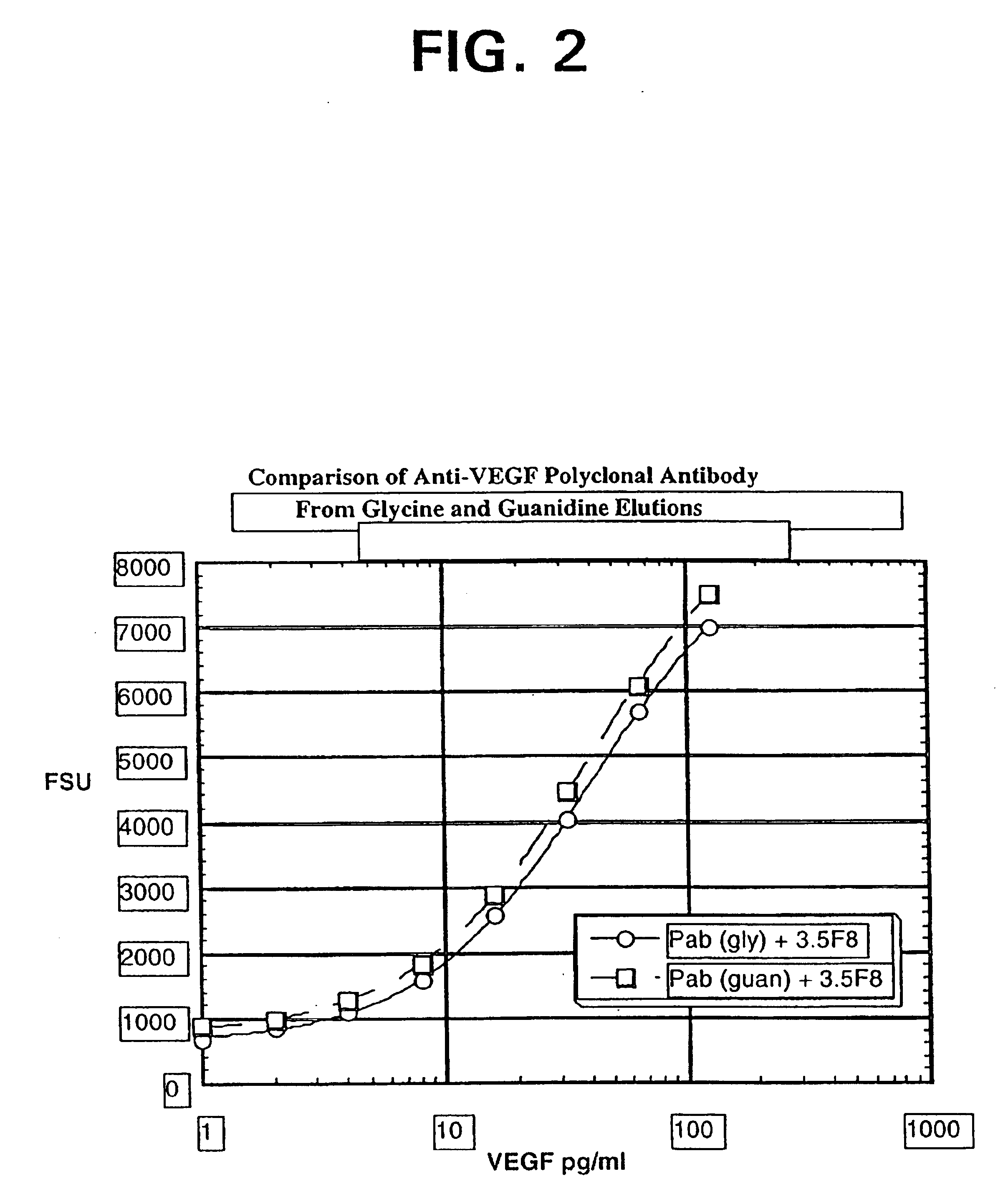
ELISA for VEGF
InactiveUS6855508B2Sensitivity of assay is lostLose sensitivityAnimal cellsMaterial analysis by observing effect on chemical indicatorDiabetes mellitusAntigen
The vascular endothelial growth factor (VEGF) activity in a patient's bloodstream or other biological sample can serve as a diagnostic and prognostic index for cancer, diabetes, heart conditions, and other pathologies. Antibody-sandwich ELISA method and kits for VEGF as an antigen were developed to detect VEGF levels in biological samples from animal models and human patients and are used as a diagnostic / prognostic index.
Owner:GENENTECH INC
Bioabsorbable polymeric implants and a method of using the same to create occlusions
InactiveUS20020040239A1Peptide/protein ingredientsPharmaceutical containersPoly-L-lactideVascular compartment
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
Methods and compositions for treating ocular disorders
InactiveUS20060293270A1Efficient ConcentrationEffective maintenanceGenetic material ingredientsGene therapyCompound (substance)Vascular endothelial growth factor
This invention relates to methods of treating ocular disease. The method of the invention is directed to the administration of an anti-vascular endothelial growth factor (anti-VEGF) compound to treat such disease.
Owner:EYETECH
High throughput multi-antigen microfluidic fluorescence immunoassays
InactiveUS20060263818A1Reduce total integrated backgroundIncreased signal noiseBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenPoint of care
The development of a high-throughput multi-antigen microfluidic fluorescence immunoassay system is illustrated in a 100-chamber PDMS (polydimethylsiloxane) chip which performs up to 5 tests for each of 10 samples. Specificity of detection is demonstrated and calibration curves produced for C-Reactive Protein (CRP), Prostate Specific Antigen (PSA), ferritin, and Vascular Endothelial Growth Factor (VEGF). The measurements show sensitivity at and below levels that are significant in current clinical laboratory practice (with SIN>8 at as low as 10 pM antigen concentration). The chip uses 100 nL per sample for all four tests and provides an improved instrument for use in scientific research and “point-of-care” testing in medicine.
Owner:SCHERER AXEL +3
Method of treating cancer, especially soft tissue sarcoma utilizing gemcitabine in combination with docetaxel and anti-VEGF therapy (bevacizumab)
InactiveUS20070065449A1Great likelihoodLong median survivalBiocideGenetic material ingredientsAbnormal tissue growthLymphatic Spread
The present invention relates to a pharmaceutical cocktail, in particular, effective amounts of gemcitabine, in combination with effective amounts of docetaxel and angiogenesis inhibitor, especially a vascular endothelial growth factor (VEGF) inhibitor, such as bevacizumab for the treatment of cancer, in particular sarcoma, especially soft tissue sarcoma. Pharmaceutical compositions and methods of treating cancer, including sarcoma, especially soft tissue sarcoma (prolonging the patient's life, eliminating the tumor, improving the patient's quality of life, shrinking the tumor, prolonging survival and / or preventing the tumor's metastases) are additional aspects of the present invention.
Owner:STC UNM
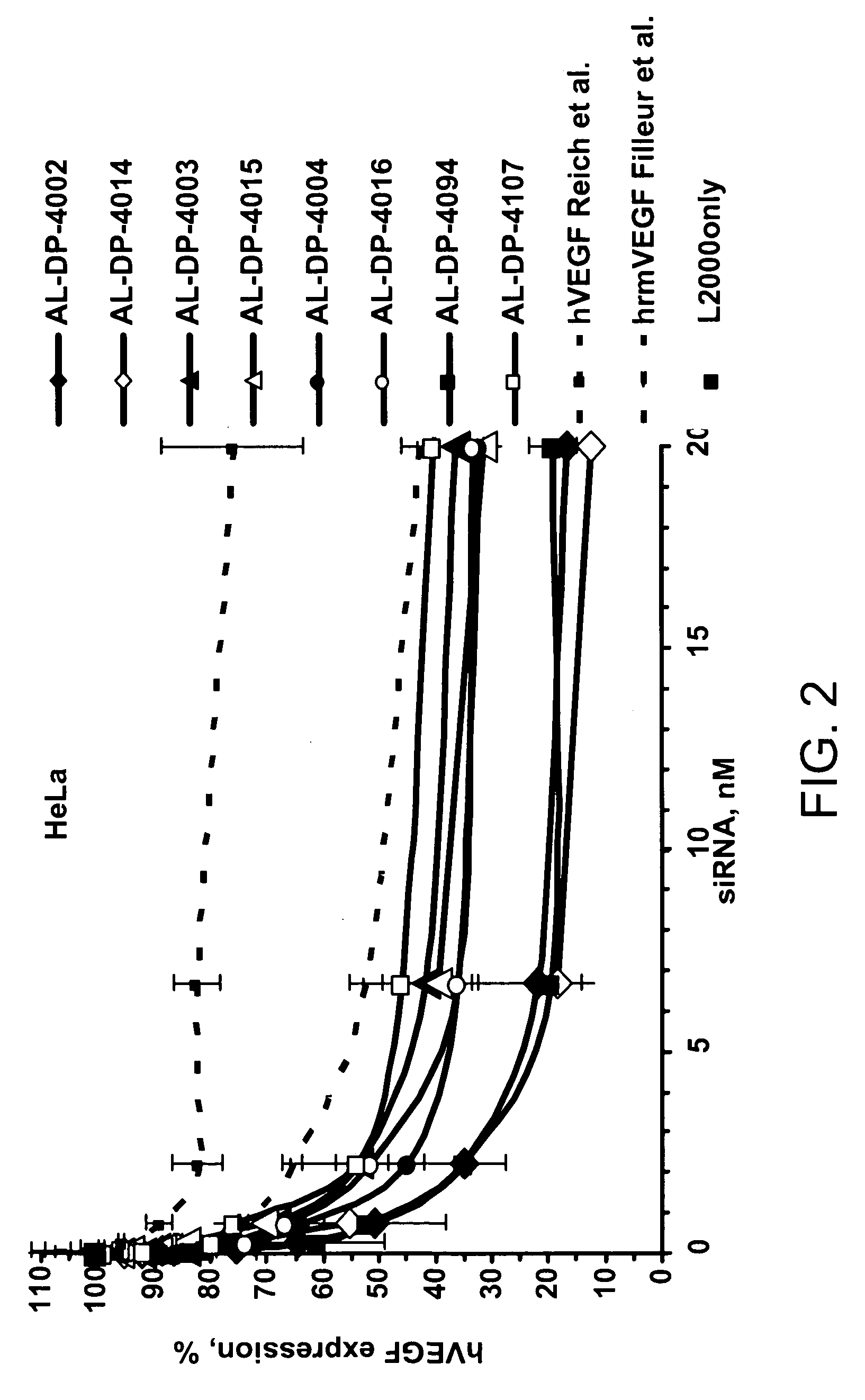
IRNA agents targeting VEGF
ActiveUS20060223770A1Improve propertiesImprove the immunityOrganic active ingredientsSenses disorderVascular endothelial growth factorEndothelium
The features of the present invention relate to compounds, compositions and methods useful for modulating the expression of vascular endothelial growth factor (VEGF), such as by the mechanism of RNA interference (RNAi). The compounds and compositions include iRNA agents that can be unmodified or chemically-modified.
Owner:ALNYLAM PHARMA INC
Method and compositions for highly sensitive detection of molecules
InactiveUS20090234202A1Detection moreImprove the level ofElectrocardiographyMicrobiological testing/measurementVascular endothelial growth factorPhysiological markers
The present invention discloses methods for the detection and monitoring of a condition in a subject using highly sensitive detection of molecules. The invention provides a method for detecting or monitoring a condition in a subject, comprising detecting a first marker in a first sample from the subject and detecting a second marker, wherein the first marker comprises a biomarker, e.g., Cardiac Troponin-I (cTnI) or Vascular Endothelial Growth Factor (VEGF), and wherein the limit of detection of the first marker is less than about 10 pg / ml. The second marker can be a biomarker, physiological marker, a molecular marker or a genetic marker.
Owner:SINGULEX
Methods and compositions for modulating hair growth or regrowth
InactiveUS20070036742A1Inhibit synthesisIncreased vascularizationBiocideCosmetic preparationsAstaxanthinRed Clover
The present invention relates to compositions and methods for modulating hair growth or regrowth. The compositions of the present invention comprise extracts of one or more of the following: Boswellia serrata, Undaria pinnatifida, green tea (e.g., Camellia sinensis), shiso, Pureraria mirifica, luteolin (e.g. Perilla ocymoides leaf extract), astilbin, vitamin E, amentoflavone, tetrahydropiperine, licochalcone, astaxanthin, red clover, Brassica juncea, unfermented green rooibos, enzyme CoQ10, salvia, ximenynic acid, hops oleoresin, apple, soy, saw palmetto, or ellagic extract, or any derivative thereof. In particular, the compositions and methods of the present invention can be used to stimulate or increase hair growth and / or prevent or slow the loss of hair by having one or more of the following functions: (a) inhibiting synthesis of DHT; (b) inhibiting proteasomal activity; (c) inhibiting IL-1 activity; (d) increasing vascularization; (e) increasing expression of vascular endothelial growth factor; (f) increasing expression of keratinocyte growth factor; (g) inhibiting inflammation; or (h) acting as an antibacterial.
Owner:ACCESS BUSINESS GRP INT LLC
Blood VEGF level-lowering agent containing IL-6 antagonist as the active ingredient
InactiveUS8173126B2Inhibit angiogenesisLower Level RequirementsPeptide/protein ingredientsDigestive systemInterleukin 6Vascular endothelial growth factor
A blood vascular endothelial growth factor (VEGF) level-lowering agent comprising an interleukin-6 (IL-6) antagonist as an active ingredient.
Owner:CHUGAI PHARMA CO LTD
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
VEGF-A as an inhibitor of angiogenesis and methods of using same
InactiveUS20060135423A1Inhibit angiogenesisModulate angiogenesisSenses disorderPeptide/protein ingredientsOcular angiogenesisAngiogenesis growth factor
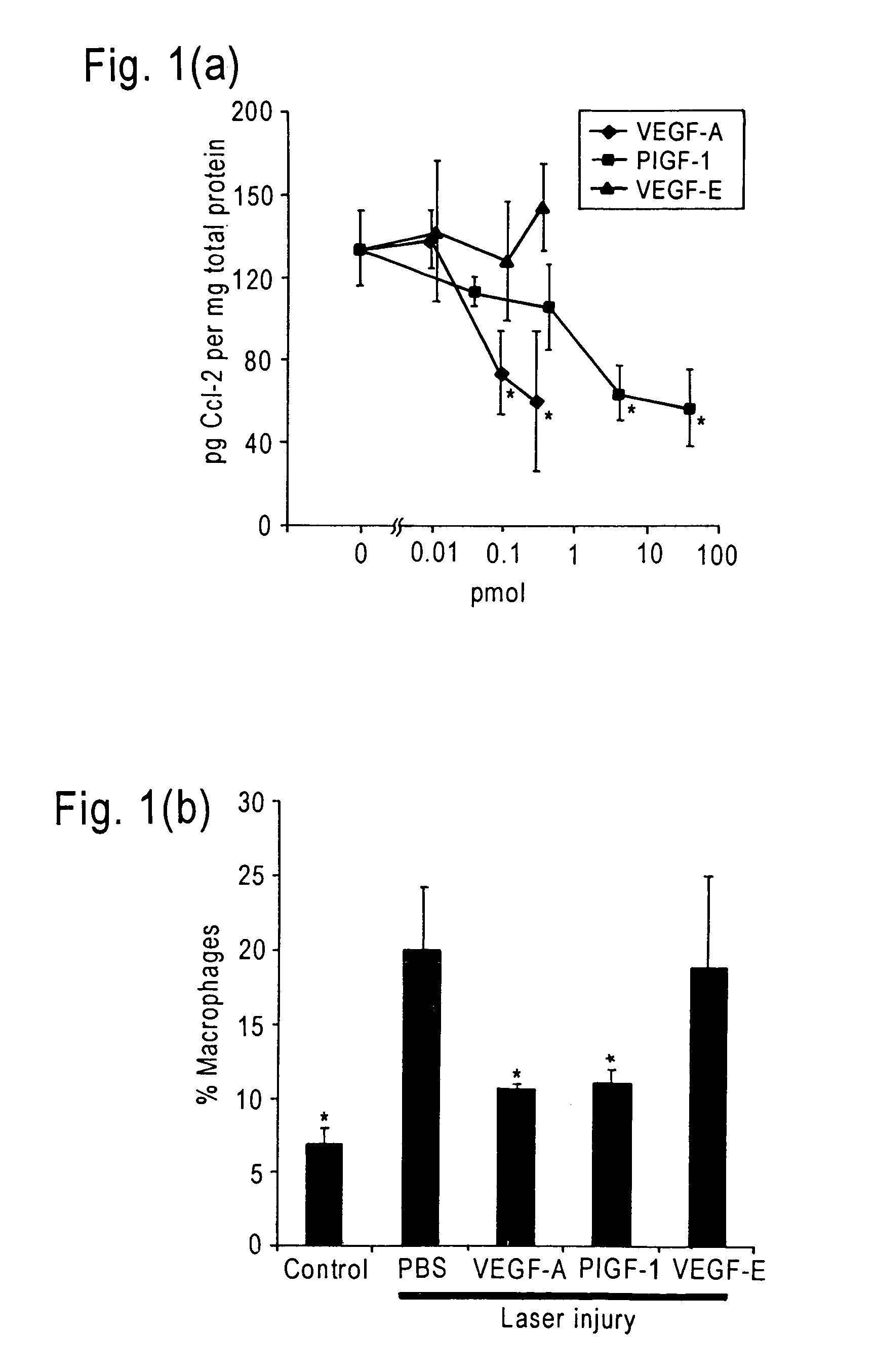
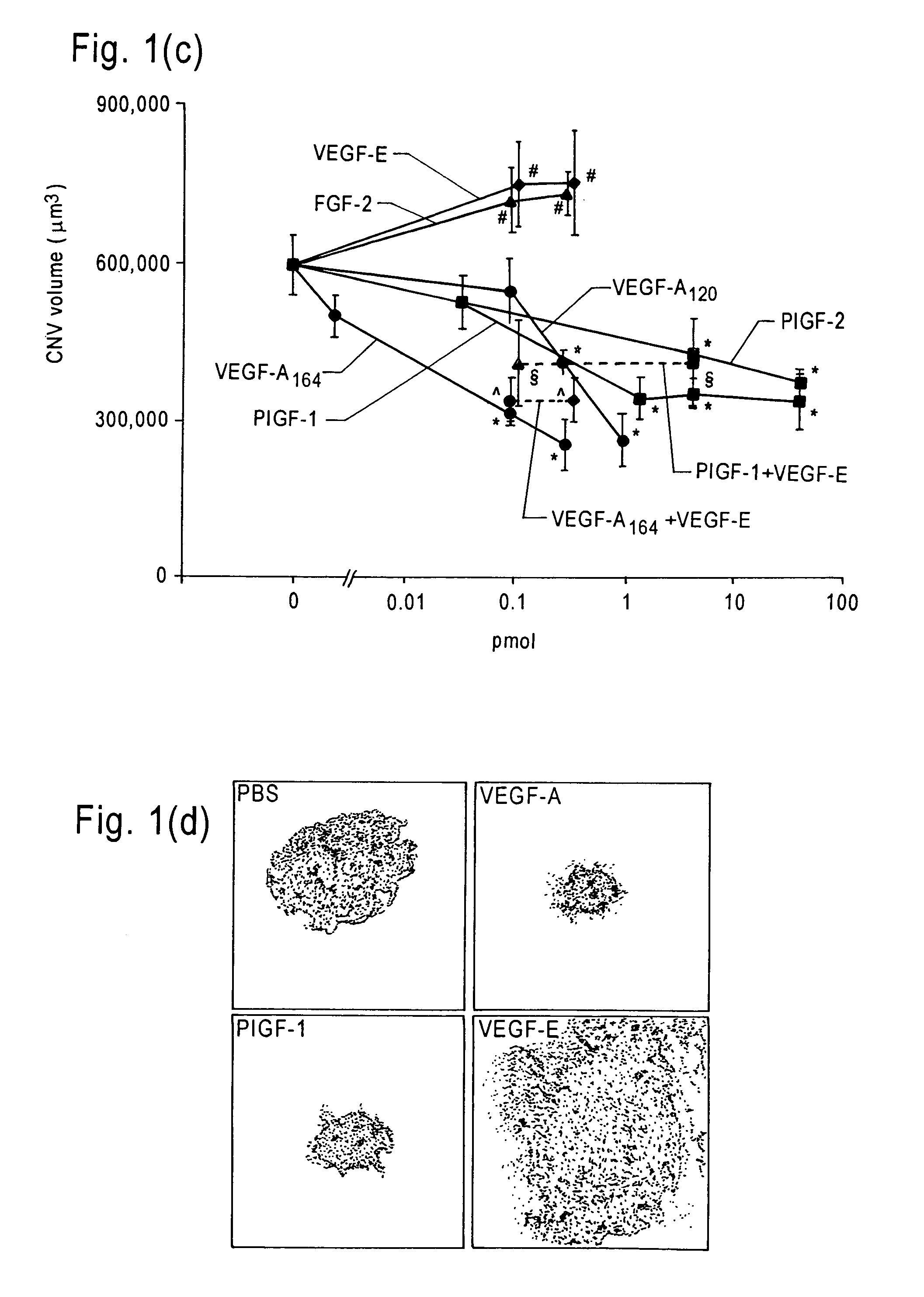
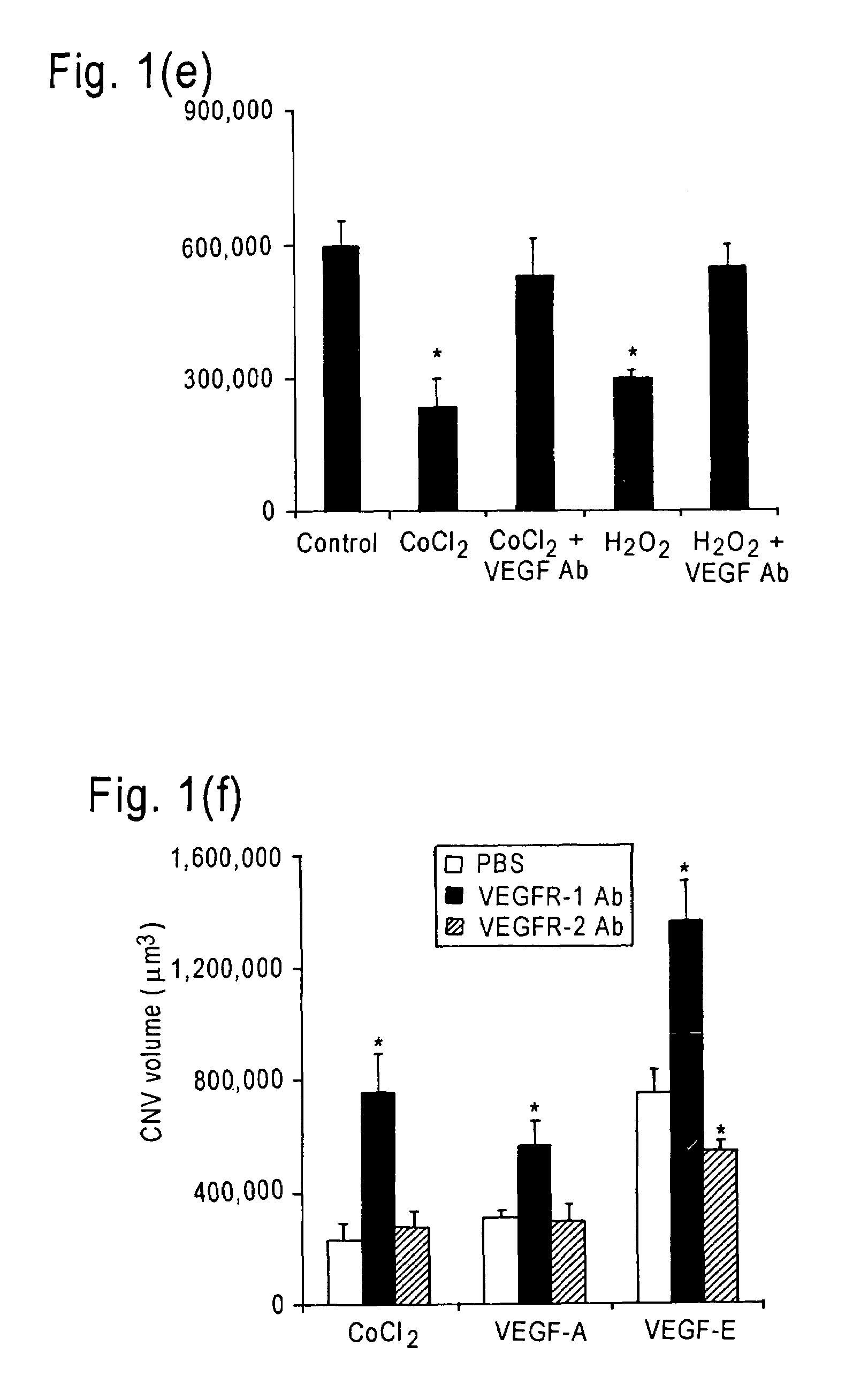
The invention relates to methods and compositions for the treatment or prevention of ocular angiogenesis and neovascularization associated with neovascular disease. Administration of vascular endothelial growth factor (VEGF)-A into the eye when macrophage infiltration is reduced inhibits ocular angiogenesis.
Owner:UNIV OF KENTUCKY RES FOUND
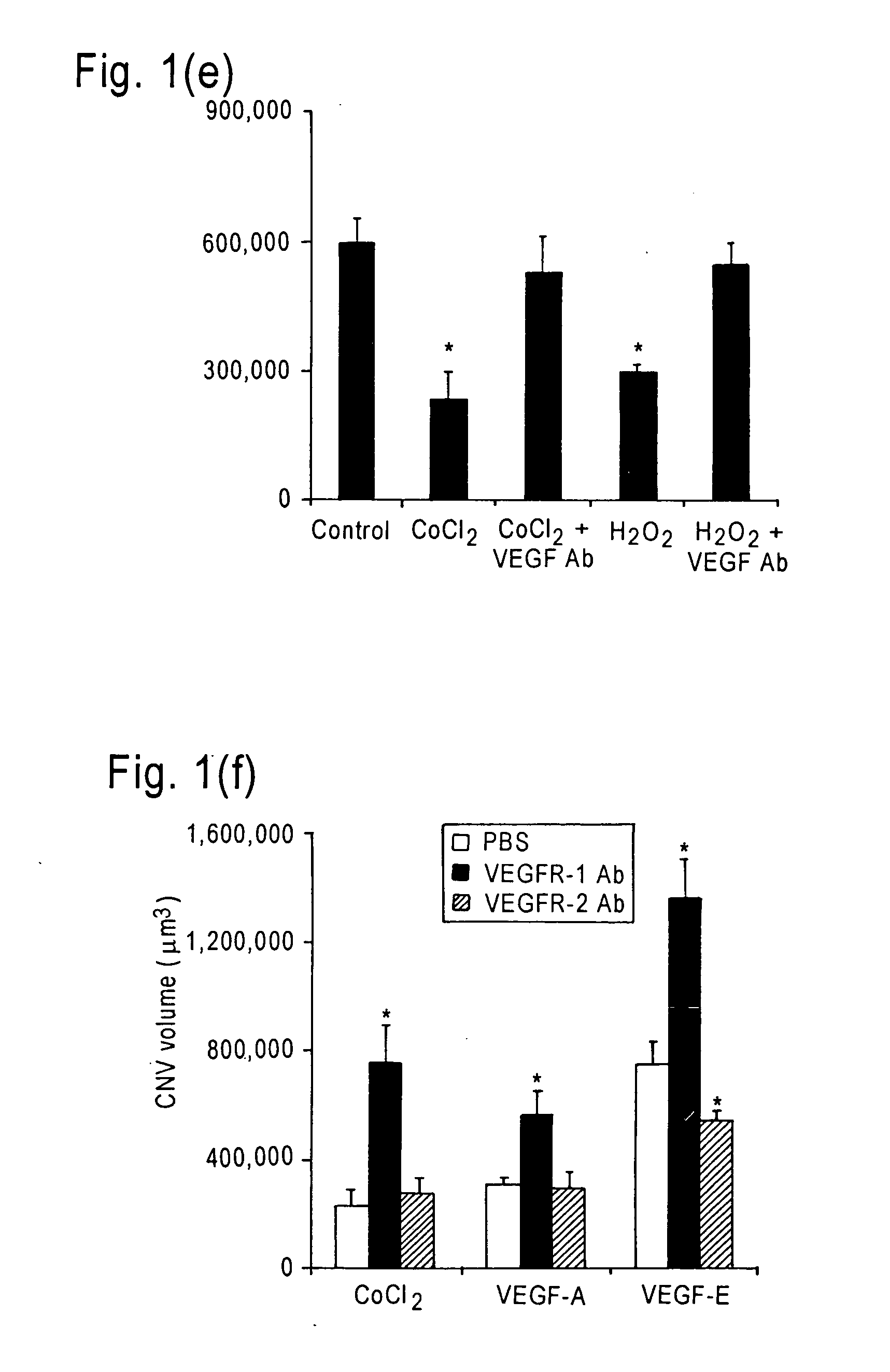
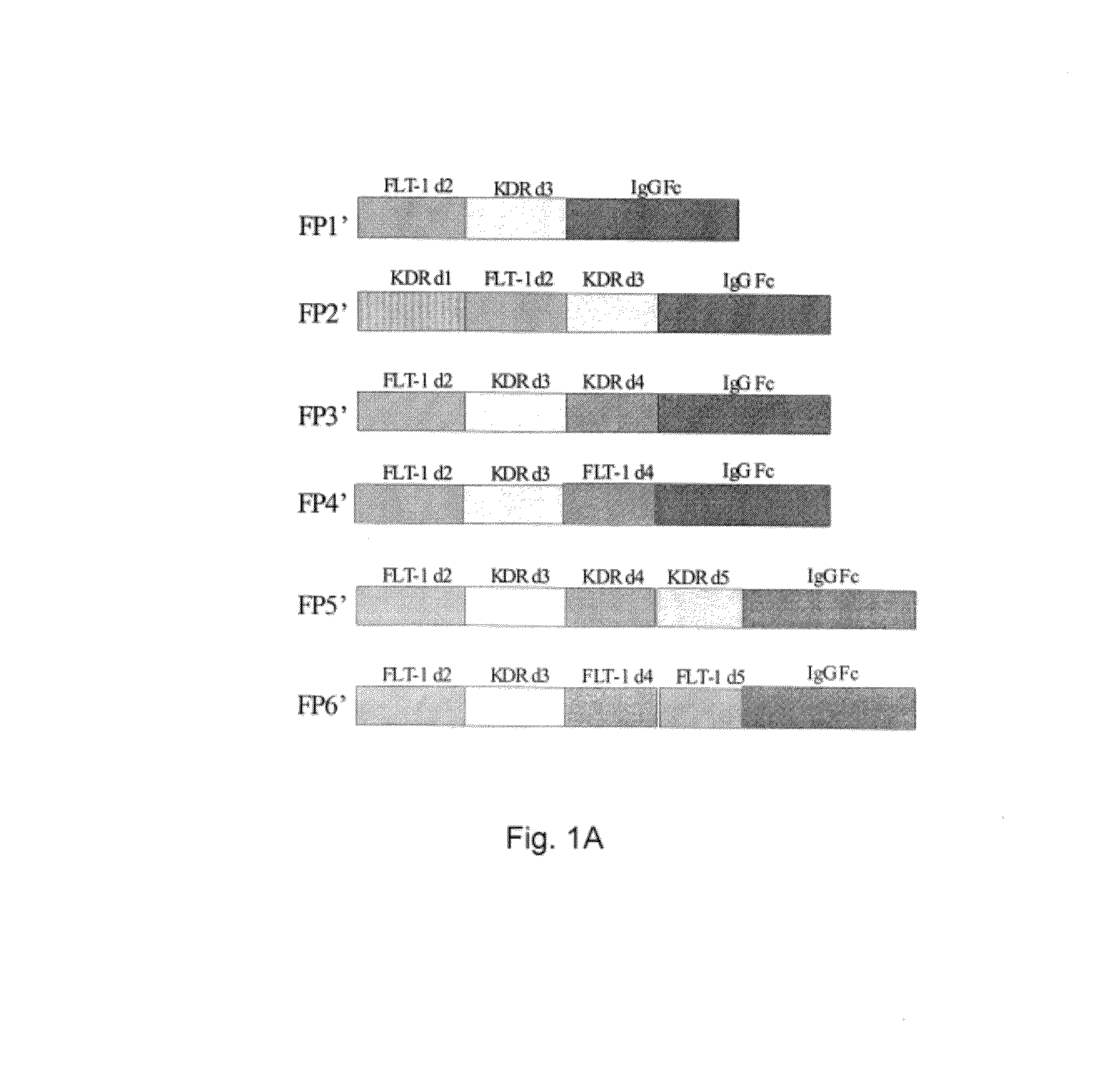
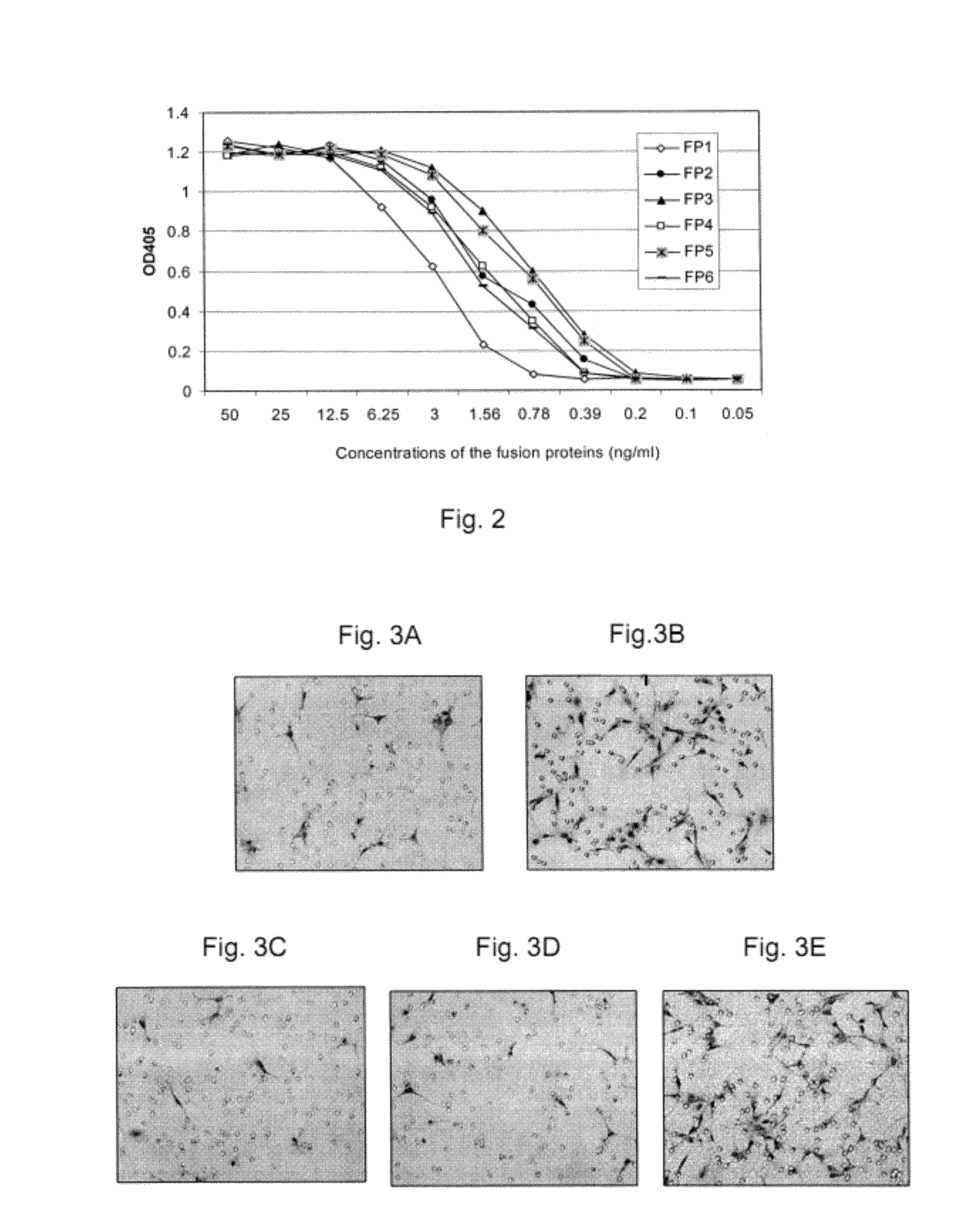
Inhibition of neovascularization with a soluble chimeric protein comprising VEGF FLT-1 and KDR domains
ActiveUS8216575B2Reduce neovascularizationImprove acuitySenses disorderPeptide/protein ingredientsChimerin ProteinsEndothelial NOS
Described herein are novel soluble chimeric fusion proteins comprising amino acid sequences derived from the vascular endothelial growth factor (VEGF) receptors flt-1 and KDR, including domain 4 of KDR. The claimed chimeric fusion proteins antagonize the endothelial cell proliferative and angiogenic activity of VEGF and are useful in the treatment of neovascularization-related disease.
Owner:CHENGDU KANGHONG BIOTECH
Method and apparatus for detecting and regulating vascular endothelial growth factor (VEGF) by forming a homeostatic loop employing a half-antibody biosensor
ActiveUS20100260679A1Maximize detection surface areaPeptide librariesBiological material analysisAbnormal tissue growthIn vivo
A biosensor for detection of vascular endothelial growth factor (VEGF) hybridization uses an array of parallel capacitors to detect electrochemical binding of circulating VEGF to immobilized anti-VEGF monoclonal half-antibodies (a-VEGF mhAb). Binding of a-VEGF mhAb modulates the threshold voltage of a circuit, changing the impedance of the circuit. An electrode coated with a p-Si substrate enhances the affinity between the VEGF molecules. A fluid cell delivers VEGF samples onto the active surface of the chip. An array of parallel capacitors arranged in an interdigitated pattern detects the VEGF in the fluid. The detector provides an accurately measured and quantifiable rate of change of the VEGF molecules in vivo, providing real time feedback which is used to measure response of the tumor to delivered chemotherapeutic agents and biological response modifiers (BRMs) for the purpose of determining tumor burden and efficacy of the chemotherapy as part of a homeostatic loop for chemotherapy.
Owner:SENSOR KINESIS
Method of tumor regression with VEGF inhibitors
ActiveUS20040265309A1Peptide/protein ingredientsAntibody mimetics/scaffoldsLymphatic SpreadFactor ii
Methods of regressing or inhibiting a tumor in a subject by administering an agent capable of blocking, inhibiting, or ameliorating vascular endothelial growth factor (VEGF)-mediated activity to a subject in need thereof such that the tumor is regressed or inhibited. The method of the invention results in a reduction of tumor size and inhibition of tumor metastases. This method is particularly useful for patients suffering from bulky, metastatic cancers.
Owner:REGENERON PHARM INC +1
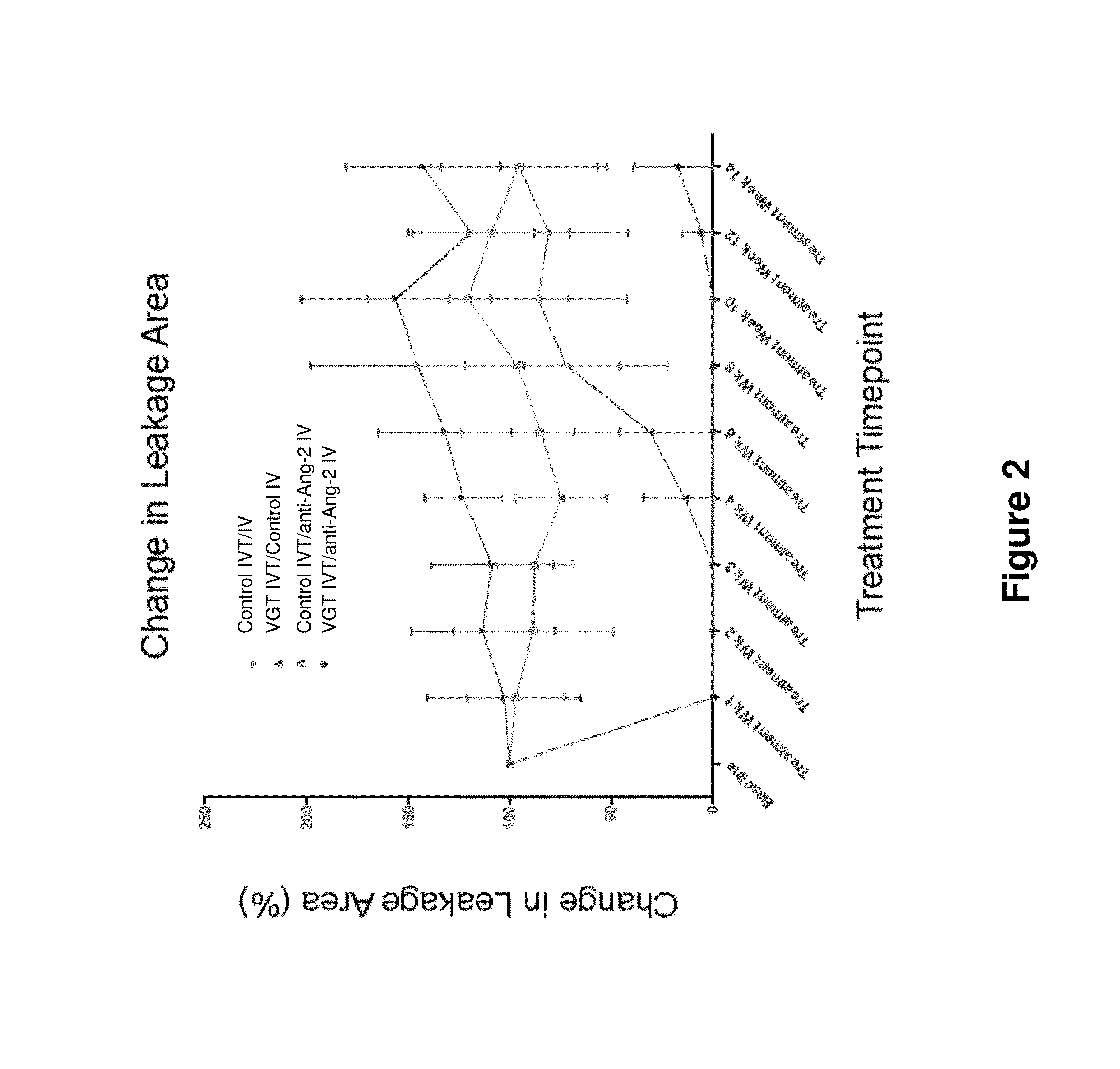
Methods and formulations for treating vascular eye diseases
InactiveUS20160144025A1Reducing dependence and treatment burdenReduce managementSenses disorderHybrid immunoglobulinsVascular diseaseVascular endothelial growth factor
The present invention provides methods for treating, preventing or reducing the severity of an eye disease. The methods of the present invention comprise administering to a subject in need thereof a therapeutic composition comprising an angiopoietin-2 (Ang-2) inhibitor such as an anti-Ang-2 antibody in combination with a vascular endothelial growth factor (VEGF) antagonist (e.g., aflibercept).
Owner:REGENERON PHARM INC
Compositions and methods for inhibiting drusen complement components C3a and C5a for the treatment of age-related macular degeneration
Activated C3 (C3a) and its receptor (C3aR) and activated C5 (C5a) and its receptor (C5aR) have been shown to induce vascular endothelial growth factor (VEGF) expression in vitro and in vivo. Compositions and methods for inhibiting C3a, C3aR, C5a and C5aR for the treatment and / or prevention of neovascular disease are provided. Also provided are Novel therapeutic targets and diagnostic markers for choroidal neovascularization.
Owner:KENTUCKY UNIVERISTY OF
Use of VEGF-C or VEGF-D gene or protein to prevent restenosis
InactiveUS20050256075A1Prevent restenosisReduce amount/severityBiocidePeptide/protein ingredientsRestenosisVascular Endothelial Growth Factor D
The present invention provides materials and methods for preventing stenosis or restenosis of a blood vessel using Vascular Endothelial Growth Factor C (VEGF-C) and / or Vascular Endothelial Growth Factor D (VEGF-D) genes or proteins.
Owner:VEGENICS PTY LTD
VEGF receptor fusion proteins, their pharmaceutical compositions and therapeutic applications for the eye diseases
InactiveUS20090264358A1Improve stabilityImprove securitySenses disorderPeptide/protein ingredientsDiabetic retinopathyVEGF receptors
Vascular endothelial growth factor (VEGF) receptor fusion protein comprising Ig domain 2 of Flt-1 and Ig domains 3, or Ig domain 2 of Flt-1 and Ig domain 3 and 4 of KDR, the gene encoding the fusion protein, the pharmaceutical composition containing the fusion protein and the pharmaceutical use of the fusion protein are provided. The fusion protein can be used for treatment of eye disorders involving angiogenesis such as diabetic retinopathy.
Owner:CHENGDU KANGHONG BIOTECH
VEGF-A as an inhibitor of angiogenesis and methods of using same
The invention relates to methods and compositions for the treatment or prevention of ocular angiogenesis and neovascularization associated with neovascular disease. Administration of vascular endothelial growth factor (VEGF)-A into the eye when macrophage infiltration is reduced inhibits ocular angiogenesis.
Owner:UNIV OF KENTUCKY RES FOUND
COMPOSITIONS AND METHODS FOR siRNA INHIBITION OF ANGIOGENESIS
RNA interference using small interfering RNAs which are specific for the vascular endothelial growth factor (VEGF) gene and the VEGF receptor genes Flt-1 and Flk-1 / KDR inhibit expression of these genes. Diseases which involve angiogenesis stimulated by overexpression of VEGF, such as diabetic retinopathy, age related macular degeneration and many types of cancer, can be treated by administering the small interfering RNAs.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
COMPOSITIONS AND METHODS FOR siRNA INHIBITION OF ANGIOGENESIS
RNA interference using small interfering RNAs which are specific for the vascular endothelial growth factor (VEGF) gene and the VEGF receptor genes Flt-1 and Flk-1 / KDR inhibit expression of these genes. Diseases which involve angiogenesis stimulated by overexpression of VEGF, such as diabetic retinopathy, age related macular degeneration and many types of cancer, can be treated by administering the small interfering RNAs.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com