Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Chimerin Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A family of GTPASE-ACTIVATING PROTEINS that are specific for RAC GTP-BINDING PROTEINS.
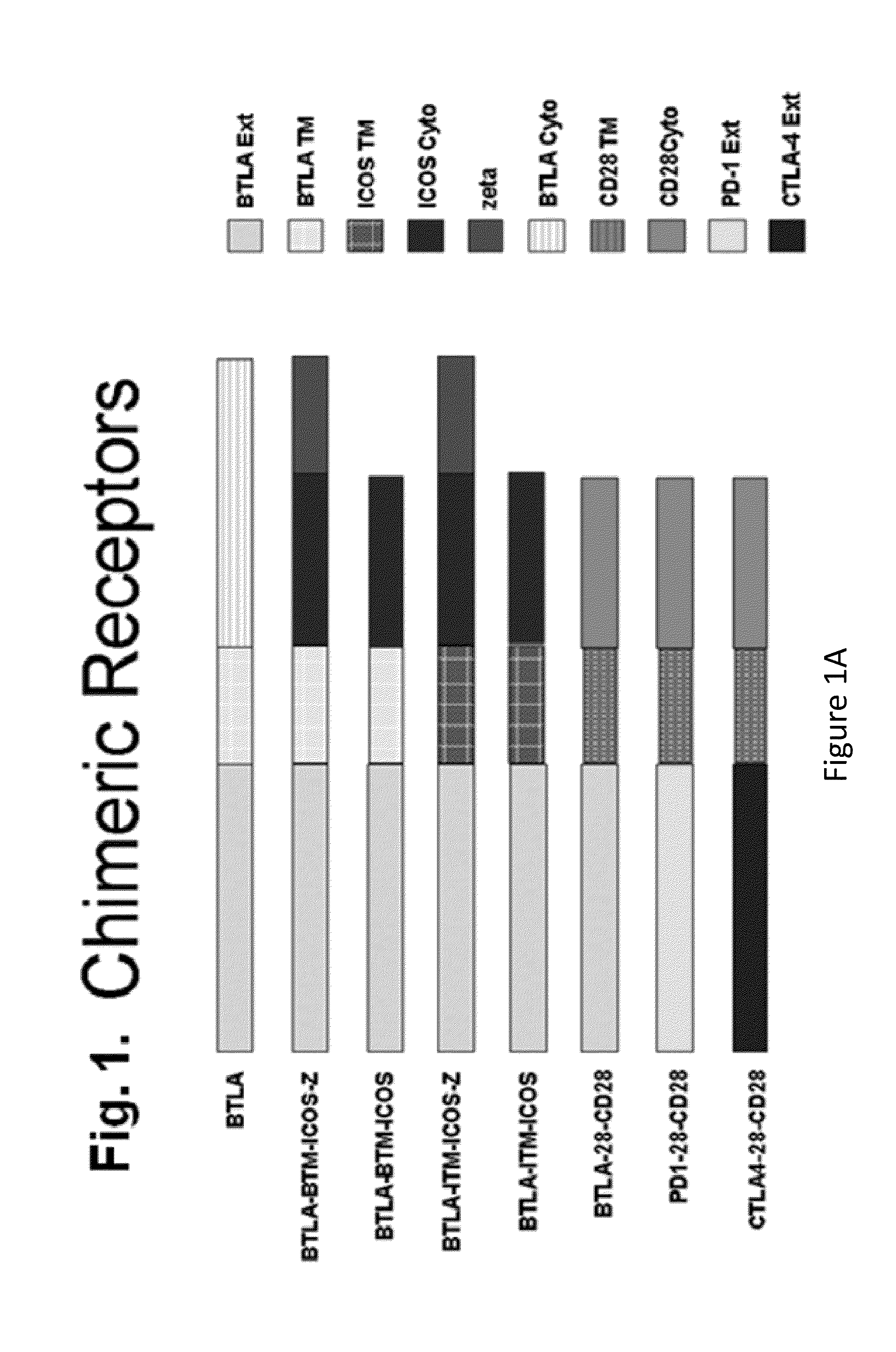
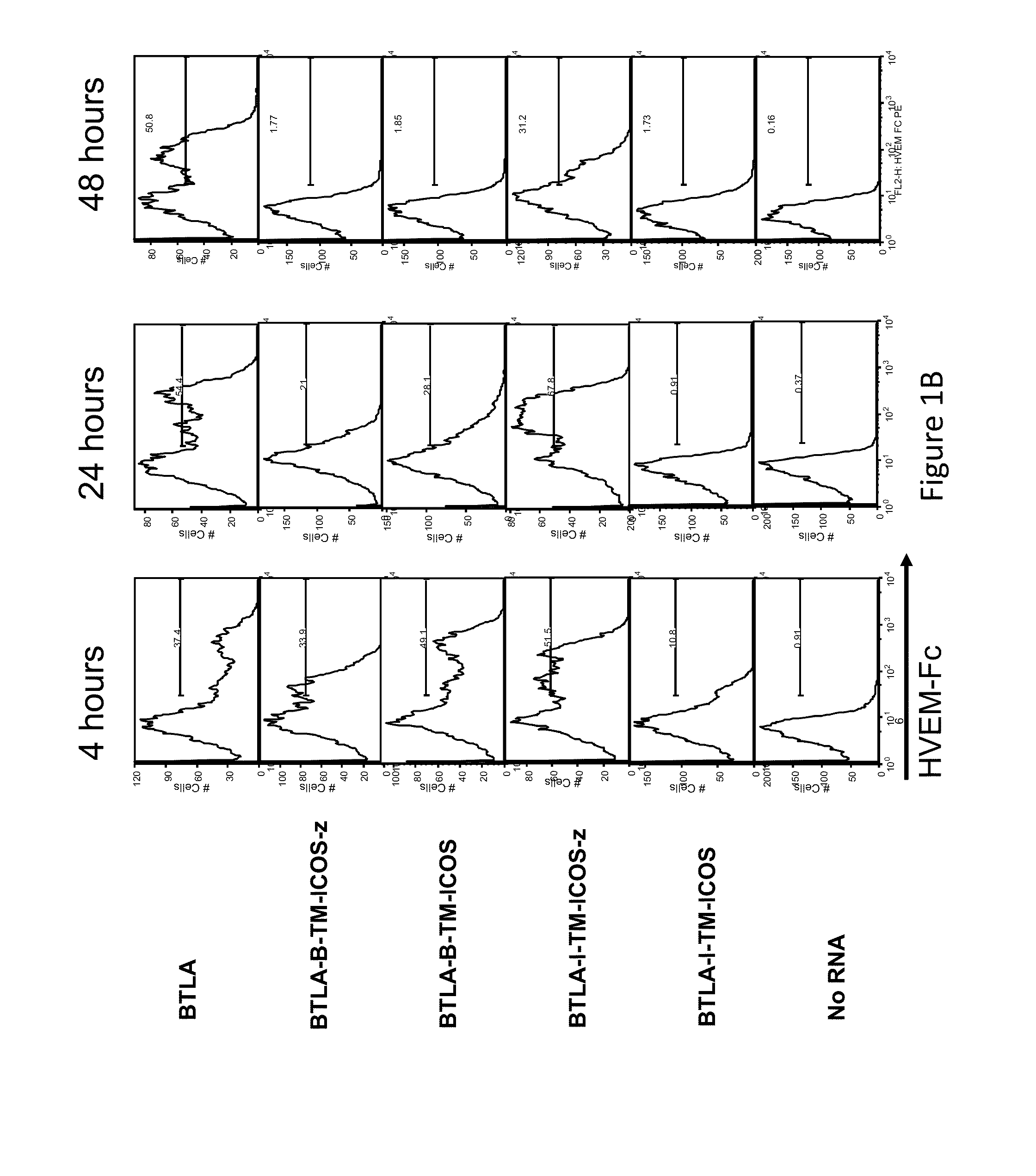
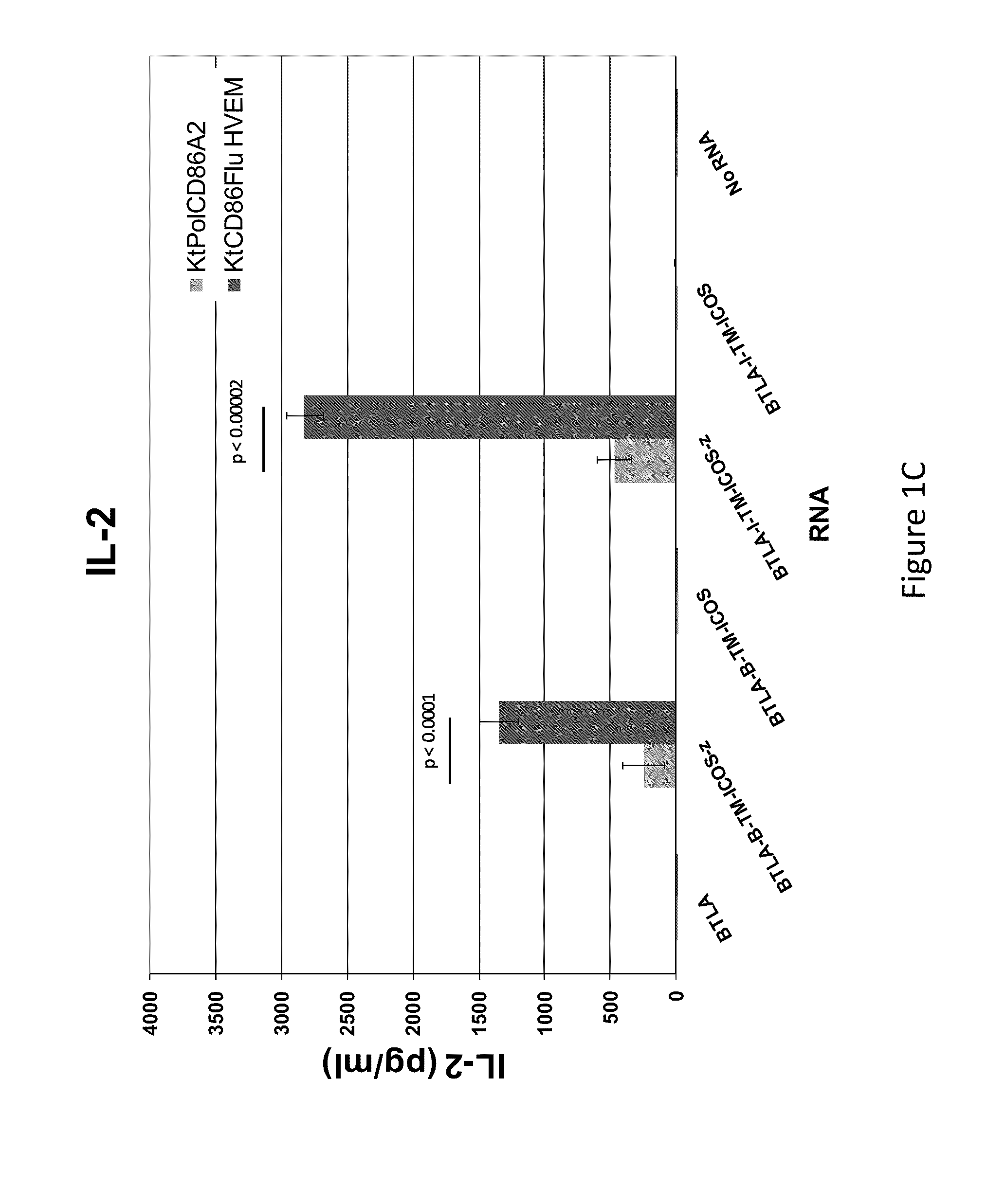
Switch costimulatory receptors
ActiveUS20140219975A1BiocidePeptide/protein ingredientsChimerin ProteinsReceptor for activated C kinase 1
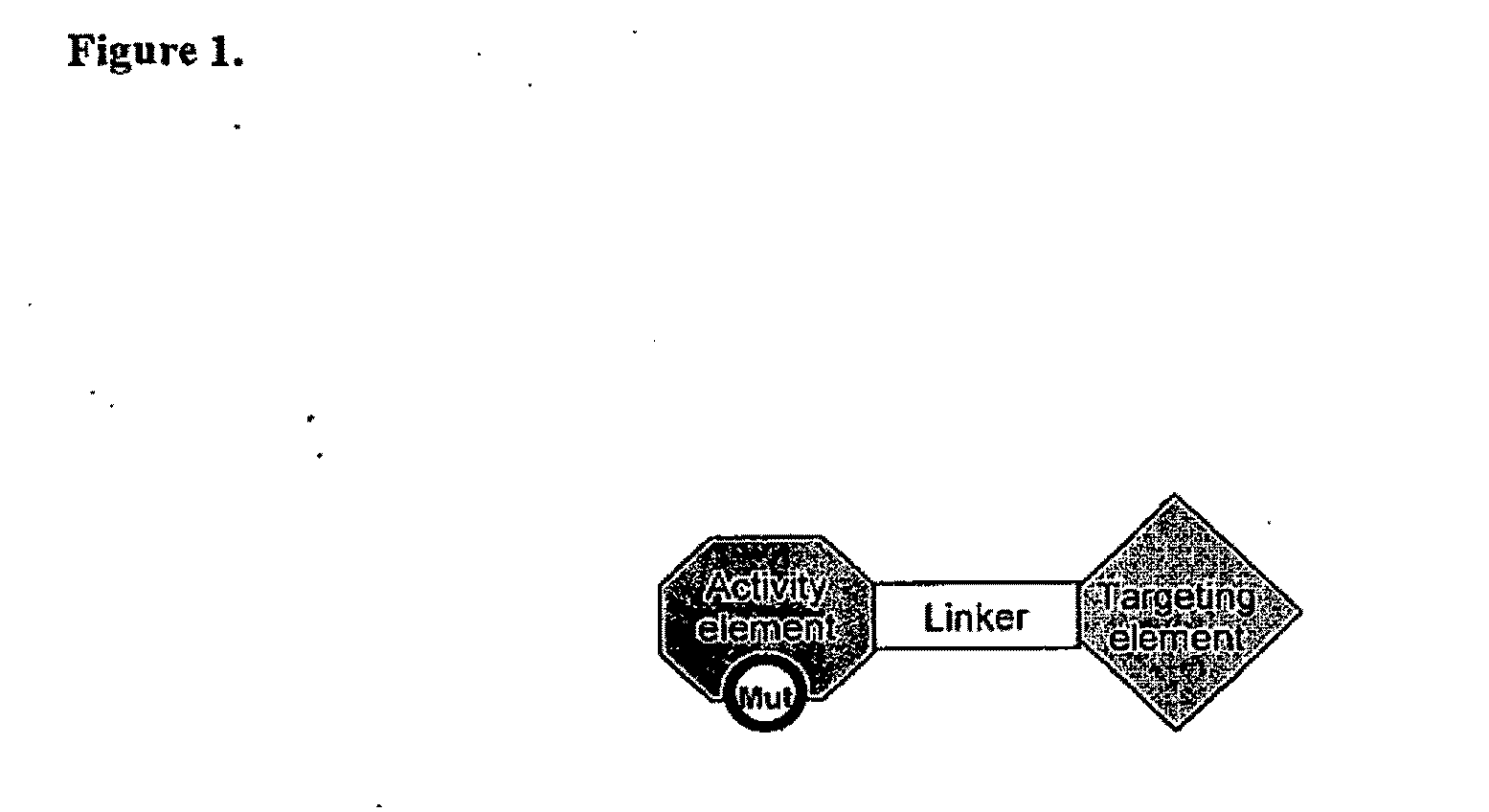
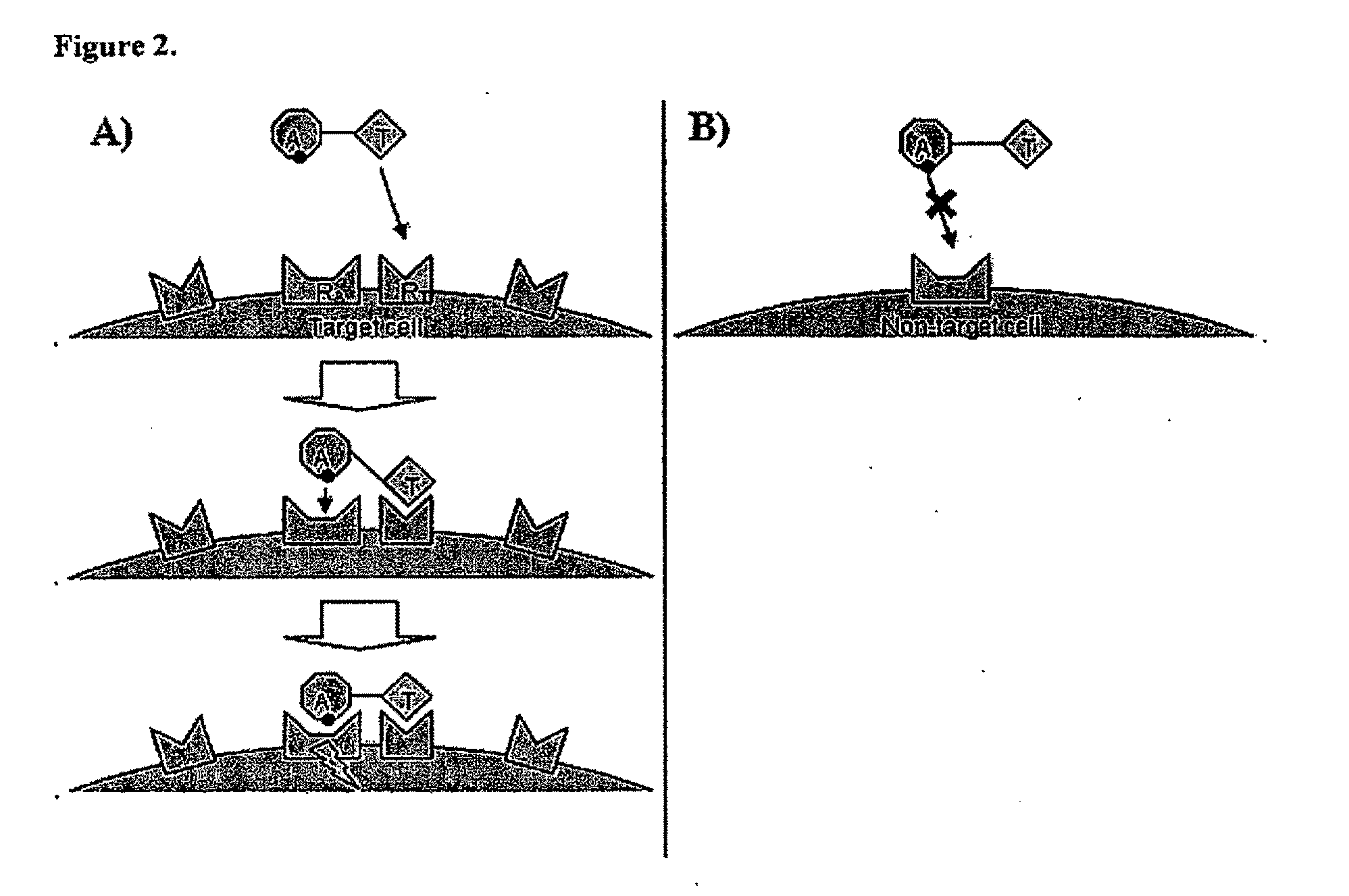
The present invention relates generally to a fusion protein that when displayed on a cell can convert a negative signal into a positive signal in the cell. The fusion protein is a chimeric protein in that the protein comprises at least two domains, wherein the first domain is a polypeptide that is associated with a negative signal and the second domain is a polypeptide that is associated with a positive signal. Thus, the invention encompasses switch receptors that are able to switch negative signals to positive signals for enhancement of an immune response.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
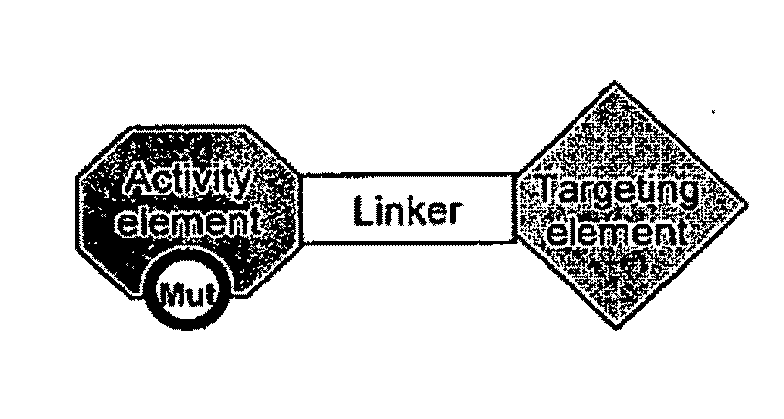
Chimeric activators: quantitatively designed protein therapeutics and uses thereof
ActiveUS20110274658A1Reduced cell-activating propertyAvoid and reduce unwanted side effectObesity gene productsPeptide/protein ingredientsChimerin ProteinsApoptosis
Aspects of the invention provide methods for harnessing the potential of proteins that occur naturally (e.g., in humans) and that have serious but finite toxicity. Aspects of the invention relate to a quantitative systems-biological and structural approach to design a class Mof chimeric proteins that avoid the toxicity of protein drugs while retaining their desired activities. In particular, chimeric proteins containing a variant form of a natural protein fused to a targeting moiety may be administered to a subject to target a signal (e.g., induction of apoptosis) to particular cells without having a generalized toxic effect
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Inhibition of neovascularization with a soluble chimeric protein comprising VEGF FLT-1 and KDR domains
ActiveUS8216575B2Reduce neovascularizationImprove acuitySenses disorderPeptide/protein ingredientsChimerin ProteinsEndothelial NOS
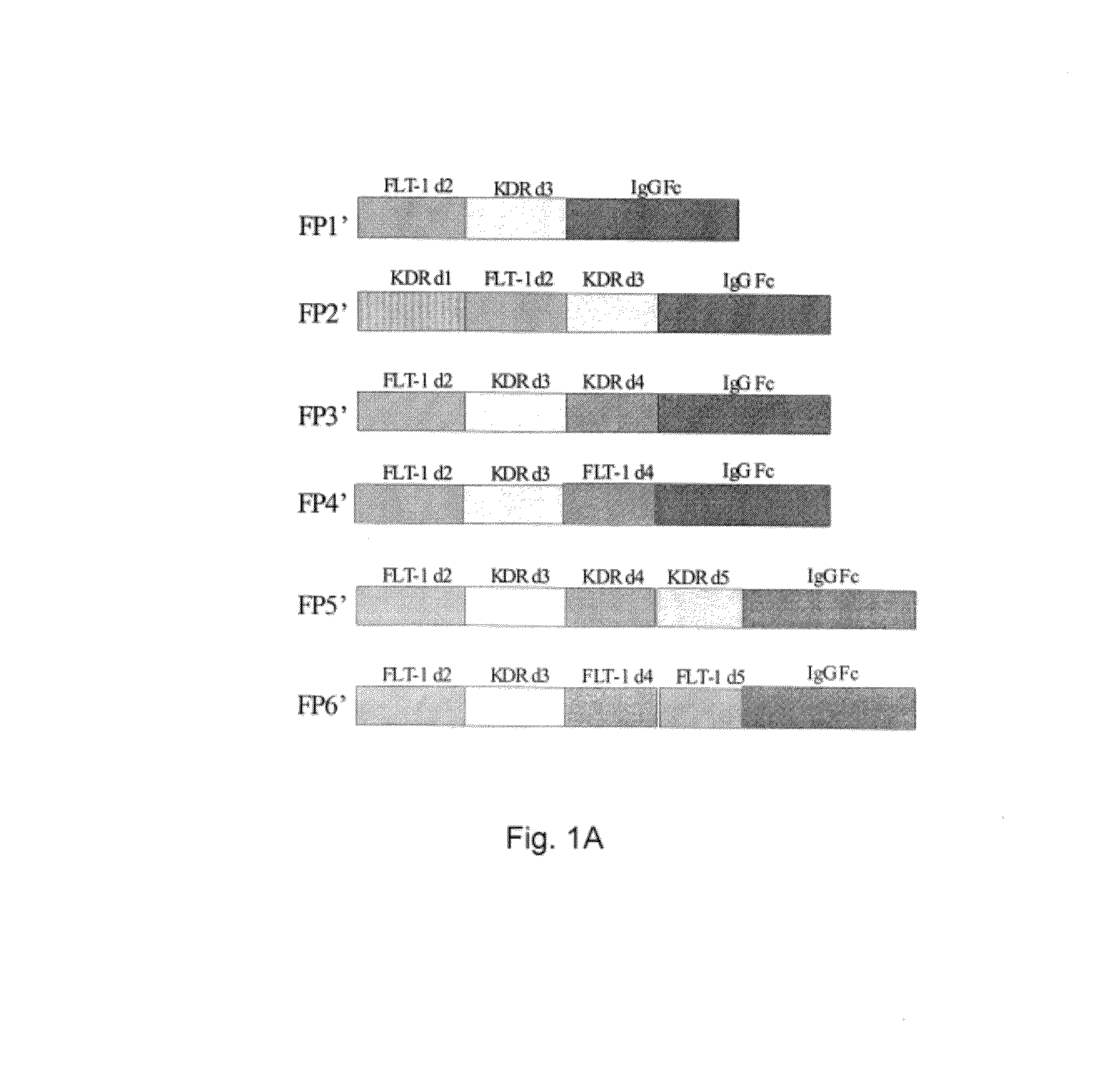
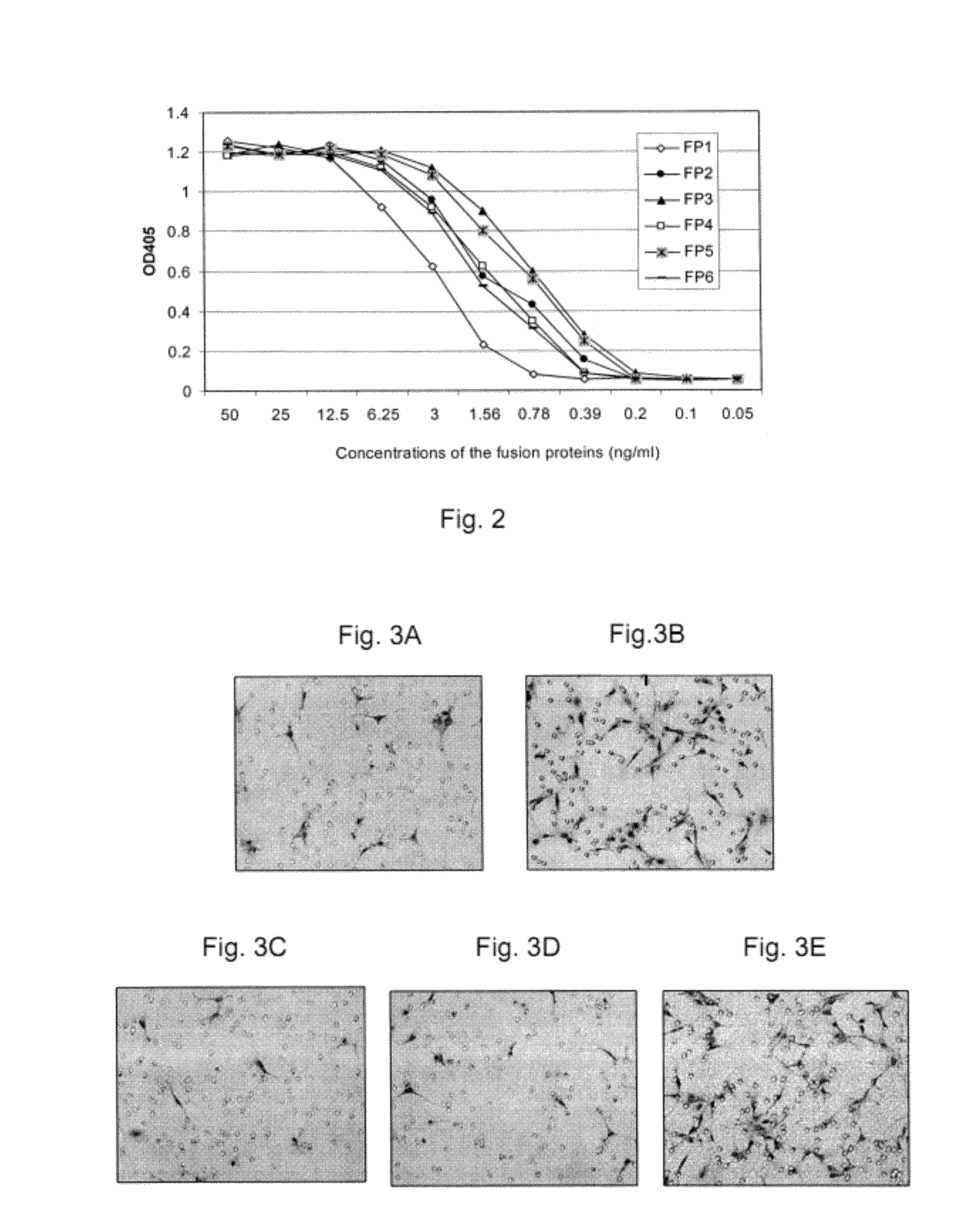
Described herein are novel soluble chimeric fusion proteins comprising amino acid sequences derived from the vascular endothelial growth factor (VEGF) receptors flt-1 and KDR, including domain 4 of KDR. The claimed chimeric fusion proteins antagonize the endothelial cell proliferative and angiogenic activity of VEGF and are useful in the treatment of neovascularization-related disease.
Owner:CHENGDU KANGHONG BIOTECH
Protease inhibitor: protease sensitivity expression system composition and methods improving the therapeutic activity and specificity of proteins delivered by bacteria
ActiveUS9068187B1Direct cytotoxicDirect inhibitoryBiocidePeptide/protein ingredientsBacteroidesSurface display
Bacteria which co-express protease inhibitors and protease sensitive therapeutic agents, which are surface displayed, secreted and / or released and result in their localized production and maintenance within a target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. The bacteria may be attenuated, non-pathogenic, low pathogenic or a probiotic. Protease sensitivity may be further accomplished by engineering protease degradation sites within the therapeutic agents, further enhancing the inactivation outside of the target tissue while retaining activity within the target tissue through co-expression of a protease inhibitor. Novel chimeric proteins secreted by bacteria, including chimeric toxins targeted to neoplastic cells, tumor matrix cells and cells of the immune system, and combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria limiting exchange of genetic material, and antibody resistant bacteria are also provided.
Owner:BERMUDES DAVID GORDON
Novel chimeric proteins
InactiveUS20110041190A1Extended half-lifeEnhanced advantageCompounds screening/testingBiocideTrans signalingDisease
Novel chimeric proteins are disclosed. The proteins comprise at least two portions. The first portion binds to a first cell and decreases the cell's ability to send a trans signal to a second cell; the second portion sends its own trans signal to the second cell. Methods for making and using these proteins in the treatment of cancer, viral infections, autoimmune and alloimmune diseases are also disclosed, as are pharmaceutical formulations comprising the novel chimeric proteins and genes. Either the proteins themselves or a genetic sequence encoding the protein can be administered. Other methods are also disclosed in which two molecular components result in decrement of a first trans signal from a first cell and the conferring of a second trans signal to a second cell.
Owner:TYKOCINSKI MARK L +1
Novel Chimeric Proteins and Methods for Using The Same
InactiveUS20130243697A1Enhanced advantageImprove propertiesCompounds screening/testingPeptide/protein ingredientsTrans signalingDisease
Novel chimeric proteins are disclosed. The proteins comprise at least two portions. The first portion binds to a first cell and decreases the cell's ability to send a trans signal to a second cell; the second portion sends its own trans signal to the second cell. Methods for making and using these proteins in the treatment of cancer, viral infections, autoimmune and alloimmune diseases are also disclosed, as are pharmaceutical formulations comprising the novel chimeric proteins and genes. Either the proteins themselves or a genetic sequence encoding the protein can be administered. Other methods are also disclosed in which two molecular components result in decrement of a first trans signal from a first cell and the conferring of a second trans signal to a second cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
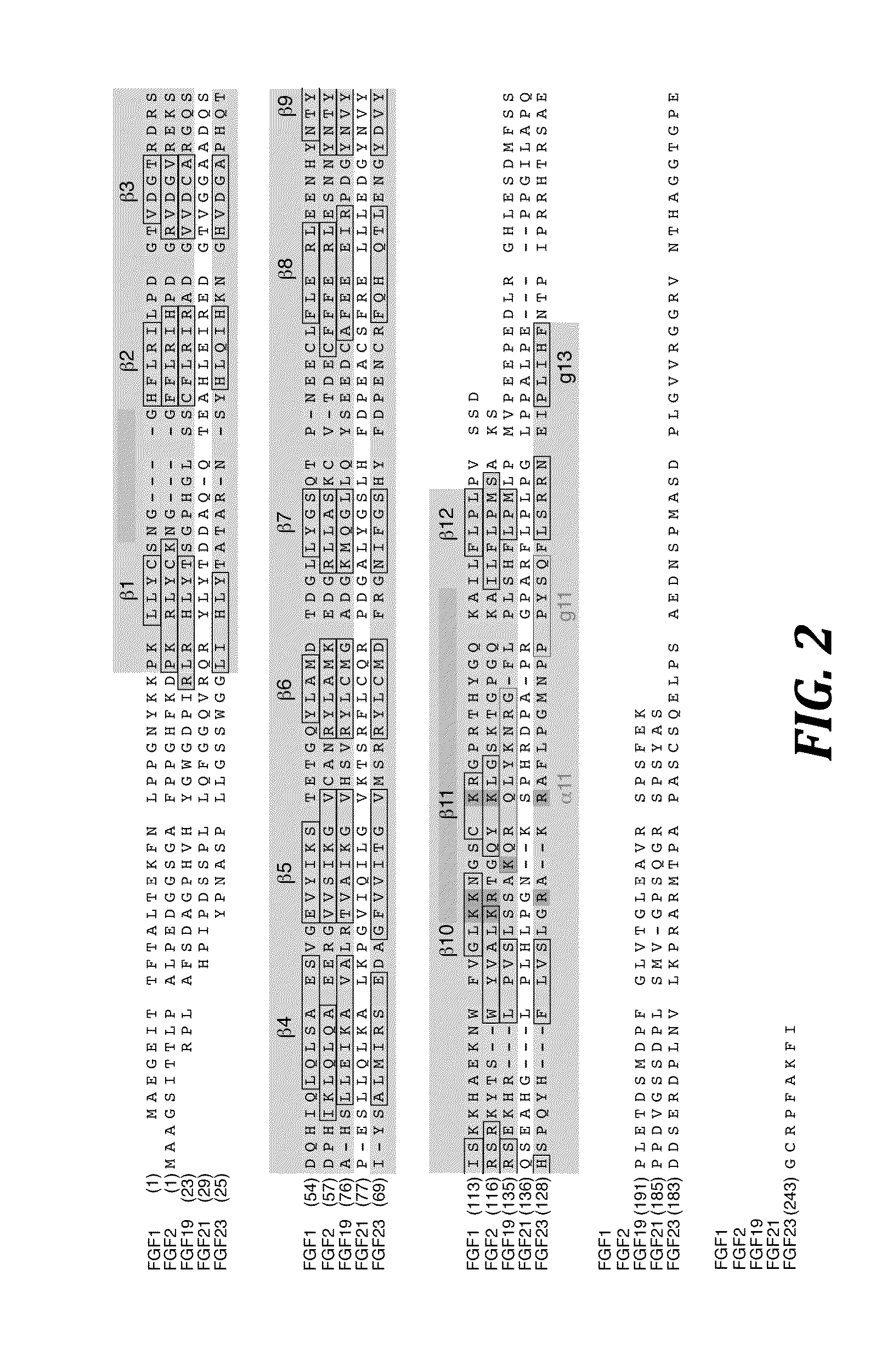
Chimeric fibroblast growth factor 19 proteins and methods of use
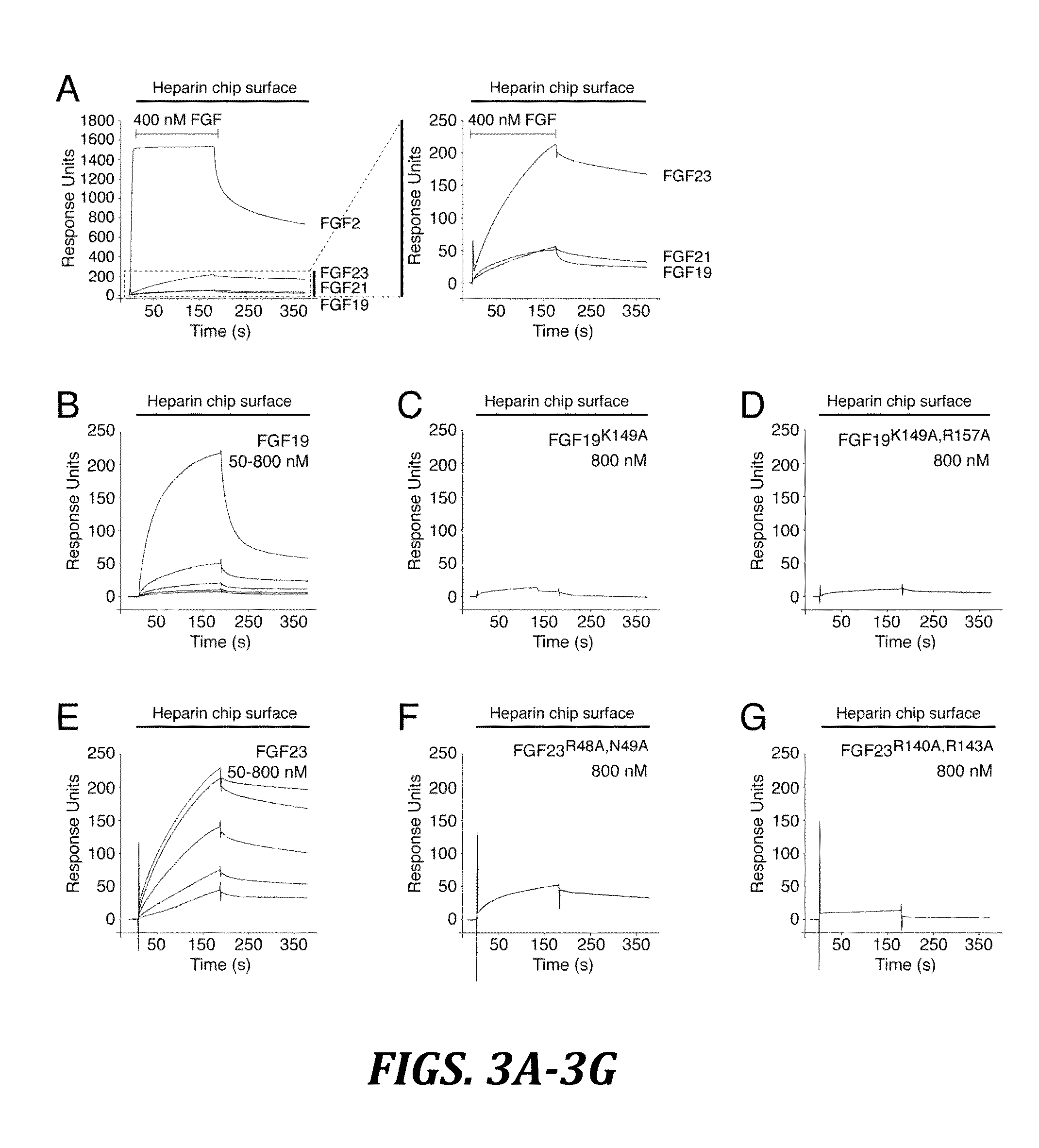
The present invention relates to a chimeric protein that includes an N-terminus coupled to a C-terminus, where the N-terminus includes a portion of a paracrine fibroblast growth factor (“FGF”) and the C-terminus includes a C-terminal portion of an FGF19 molecule. The portion of the paracrine FGF is modified to decrease binding affinity for heparin and / or heparan sulfate compared to the portion without the modification. The present invention also relates to pharmaceutical compositions including chimeric proteins according to the present invention, methods for treating a subject suffering from diabetes, obesity, or metabolic syndrome, and methods of screening for compounds with enhanced binding affinity for the βKlotho-FGF receptor complex involving the use of chimeric proteins of the present invention.
Owner:NEW YORK UNIV
Chimeric vaccines
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Chimeric proteins and uses thereof
The present invention provides a chimeric protein capable of killing or modifying a cell expressing abnormally high levels of a ligand of a receptor of the TNF / NGF family, comprising the amino acid sequence of at least one polypeptide consisting of an extracellular portion of said receptor connected to an effector molecule. In addition the invention provides pharmaceutical compositions comprising said chimeric protein and use thereof.
Owner:YEDA RES & DEV CO LTD
Tool for the transfer and production of proteins using the pseudomonas type III secretion system
ActiveUS7939319B2Easy to retouchPolypeptide with localisation/targeting motifBacteriaNucleotidePseudomonas
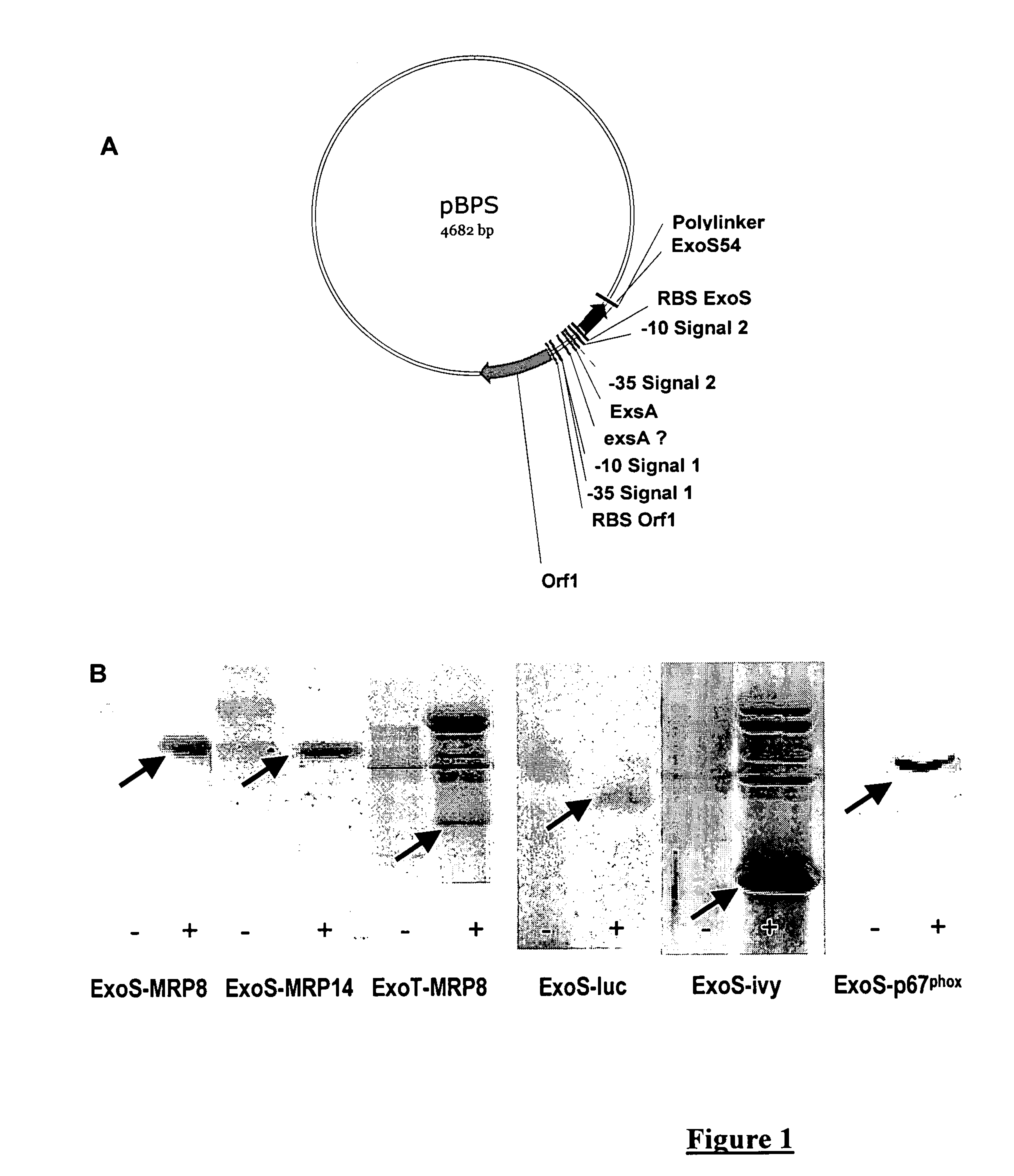
The present invention provides a vector for expression of a chimeric protein which is the result of the fusion between the N-terminal end of ExoS of Pseudomonas aeruginosa and an amino acid sequence of interest, the N-terminal end of ExoS corresponding to the N-terminal end of the chimeric protein. The vector contains the following from 5′ to 3′:a promoter,a nucleic acid comprising a nucleotide sequence encoding at least the 48 (SEQ ID 4) and at the most the 96 (SEQ ID 6) amino acids of the N-terminal end of ExoS; or any sequence of the same size that is at least 70% identical thereto and that has a secretion activity at the same level as that of ExoS,a nucleic acid comprising a nucleotide sequence encoding the amino acid sequence of interest.
Owner:UNIV GRENOBLE ALPES
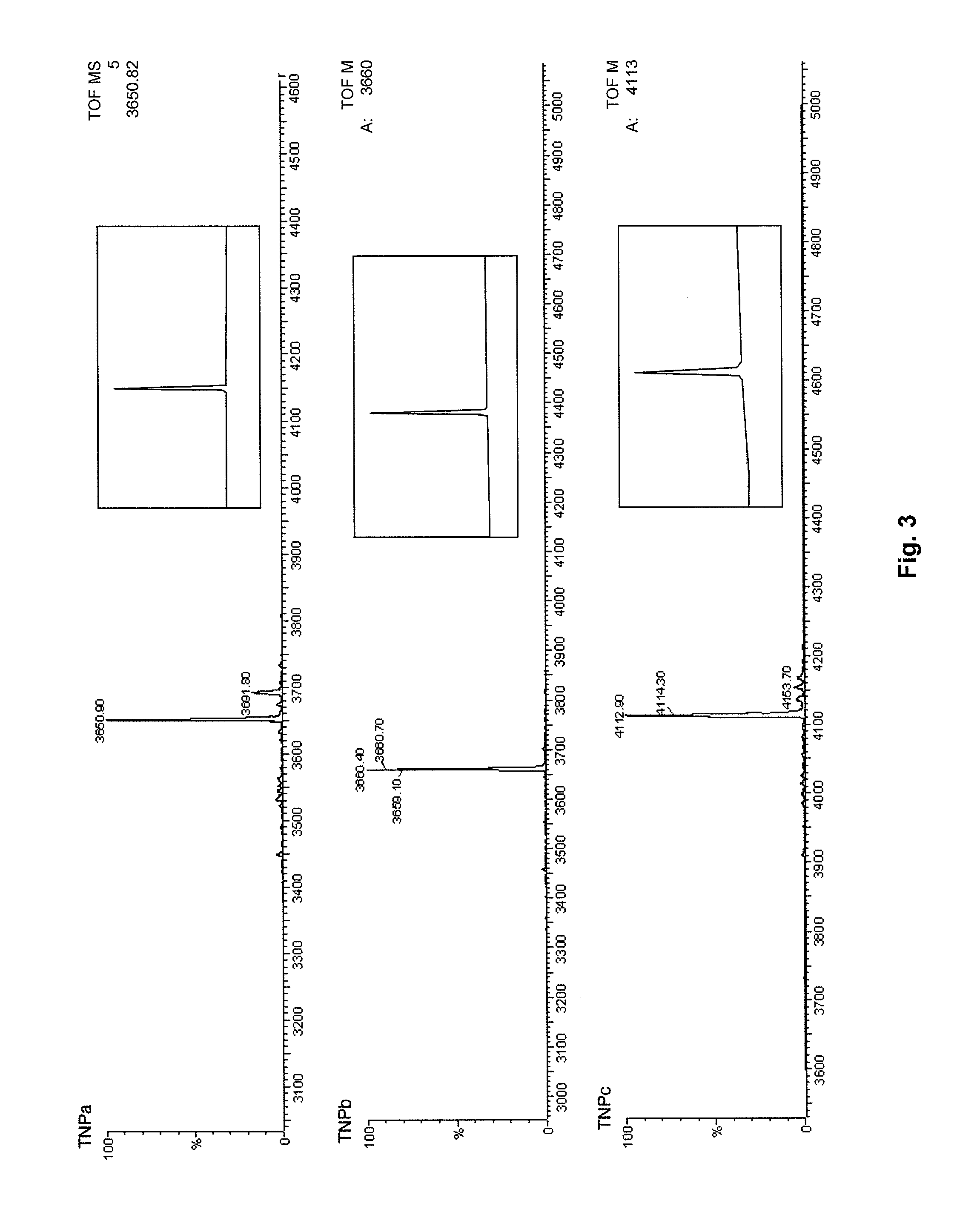
Chimeric proteins with natriuretic activity
ActiveUS8058242B2Useful profiles of bioactivityAntibacterial agentsAntipyreticBone growthDrug biological activity
The present invention discloses proteinaceous compounds that comprise at least a biologically active portion of a taipan natriuretic peptide (TNP) or a variant or derivative thereof. The invention also relates to the use of these compounds in methods for stimulating vasodilation, natriuresis, diuresis, renin-suppression, bactericidal activity, weight-loss or bone growth in a mammalian host. In specific embodiments, the compounds are useful in the treatment of congestive heart failure.
Owner:QUEENSLAND THE UNIV OF +1
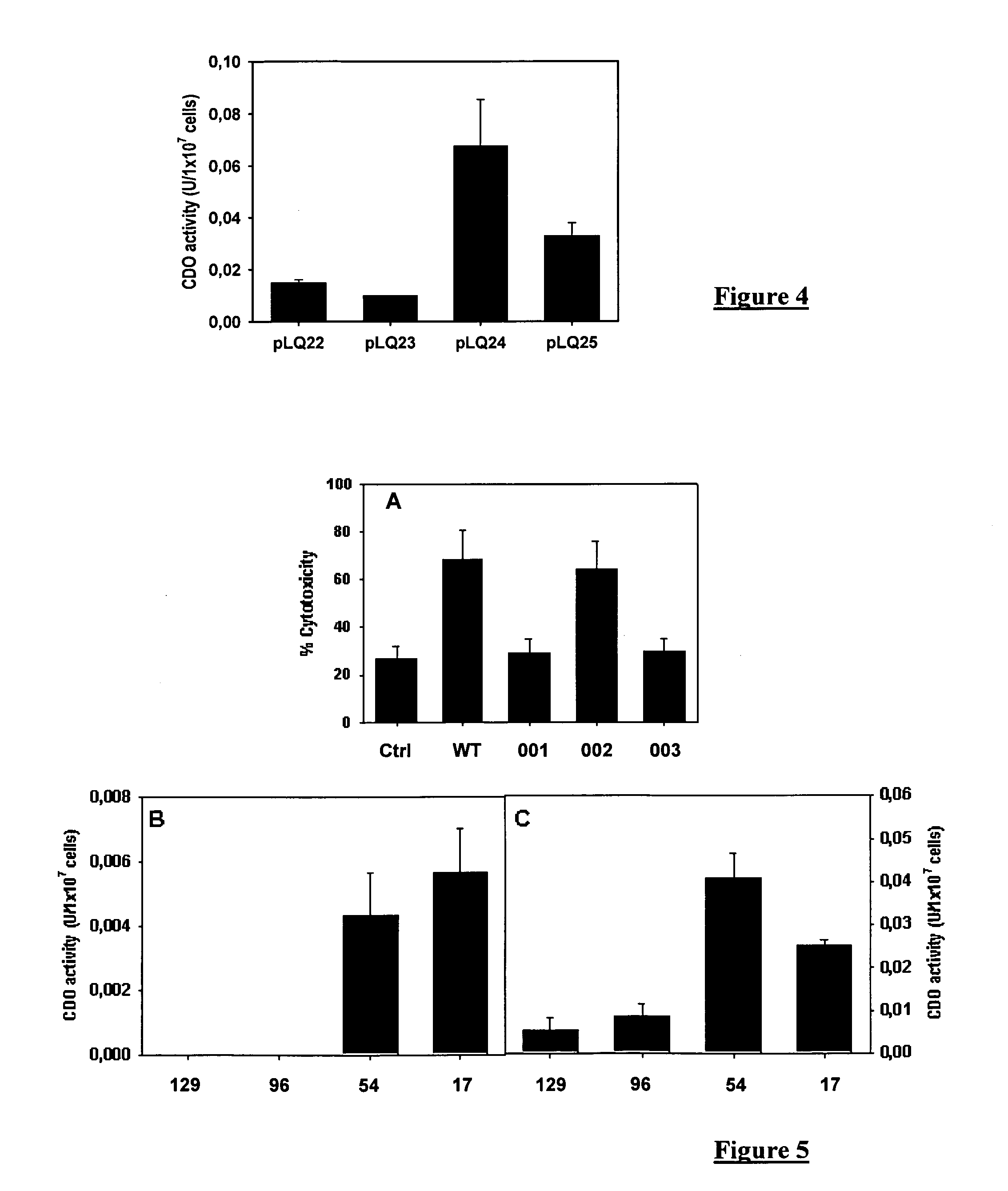
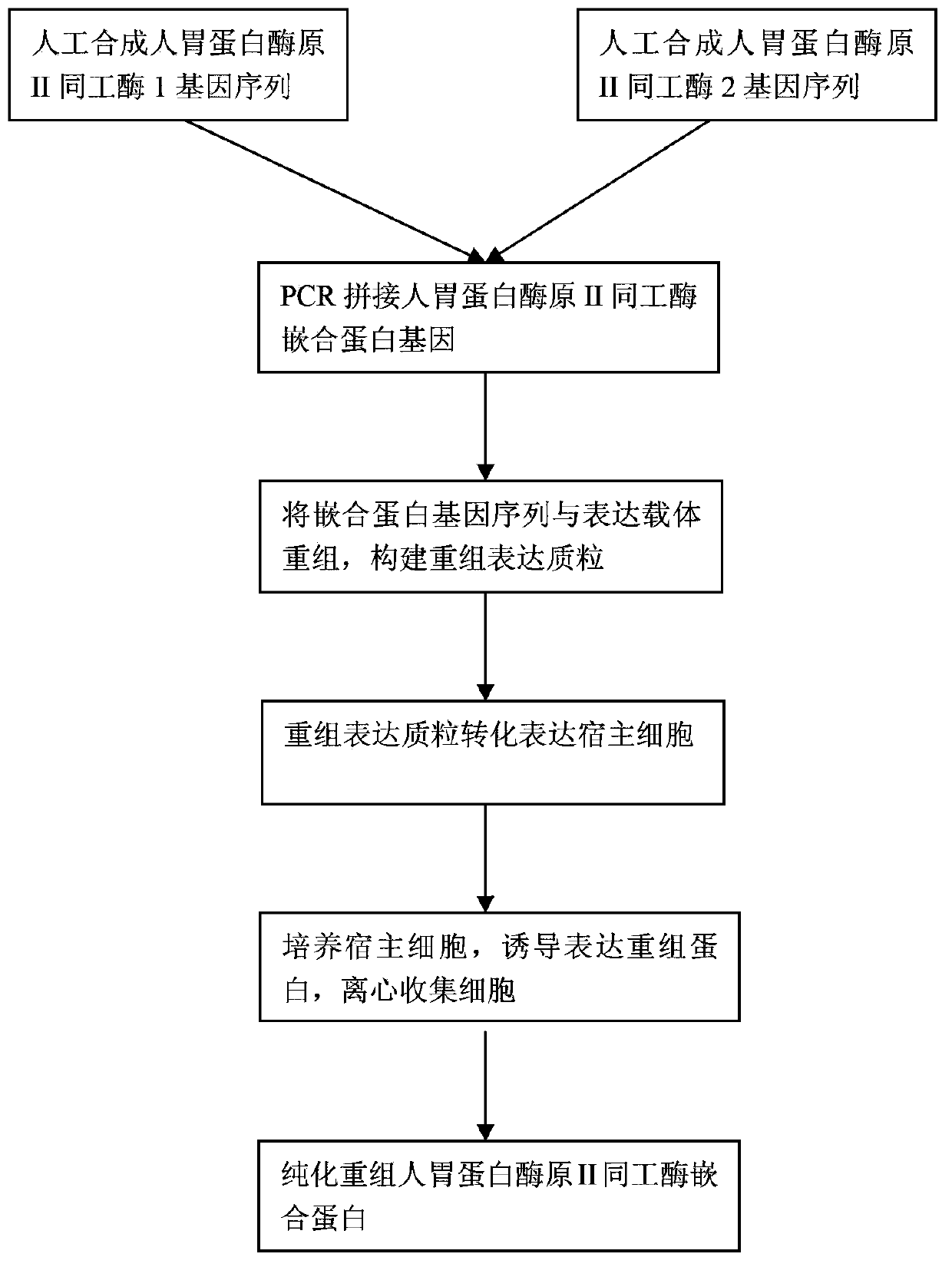
Recombinant human pepsinogen II isozyme chimeric protein, and preparation method and applications thereof
ActiveCN103387971AHigh sensitivityIncrease catch rateHydrolasesSerum immunoglobulinsPepsinogen IIIsozyme
The invention discloses a recombinant human pepsinogen II isozyme chimeric protein, and a preparation method and applications thereof. The preparation method comprises the steps as follows: by taking gene sequences of two isozymes of recombinant human pepsinogen II as a template, carrying out PCR (polymerase chain reaction) splicing to obtain a chimeric protein coding gene sequence, constructing recombinant expression plasmids, transforming the screened positive clone plasmids into expression host cells, screening an efficiently-expressed recombinant chimeric protein strain, carrying out enlargement culture on the cells and inducing expression chimeric protein, and purifying to obtain the recombinant human pepsinogen II isozyme chimeric protein. The invention further discloses the applications of the recombinant human pepsinogen II isozyme chimeric protein in preparing monoclonal antibodies and multiresistant serums or pepsinogen II kit calibration products. A great amount of stably expressed recombinant human pepsinogen II isozyme chimeric protein can be produced by utilizing a genetic engineering technology, and one protein has two chimeric protein sequences of the human pepsinogen II.
Owner:常州爱复康生物科技有限公司
Cancer Vaccines Against Mucosal Antigens and Methods of Making and Using the Same
Nucleic acid molecules comprising a nucleotide sequence that encodes a chimeric protein are disclosed. The chimeric proteins comprise at least one epitope of a mucosally restricted antigen, at least one CD4+ helper epitope, and, optionally, a secretion sequence. Chimeric proteins that comprise at least one epitope of a mucosally restricted antigen, at least one CD4+helper epitope and, optionally a secretion sequence are also disclosed. Compositions including pharmaceutical compositions and injectables comprising nucleic acid molecule and proteins are disclosed. Methods of treating individuals diagnosed with cancer of a mucosal tissue and methods of preventing cancer of a mucosal tissue are disclosed.
Owner:THOMAS JEFFERSON UNIV
Vaccine for chimeric virus-like particles and preparation method thereof
ActiveCN102153656AStrong immune-inducing activityInactivation/attenuationAntiviralsEpitopeChimerin Proteins
The invention discloses chimeric protein. The chimeric protein is obtained by inserting an epitope E7 of a human papillomavirus type 16 at a ring position of human papillomavirus type 16 (HPV 16) protein L1, namely between the 140th amino acid and the 141st amino acid, wherein the epitope E7 of the human papillomavirus type 16 has genes of protein (a) with the following codes: the protein is formed by an amino acid sequence shown by an MLDLQPETT epitope (12-20E7 for short), a RAHYNIVTF epitope(49-57E7 for short), an LLMGTLGIV epitope (82-90E7 for short) or a TLGIVCPI epitope (86-93E7 for short). The invention also discloses virus-like particles formed by aggregating the chimeric protein, a mixture formed by the virus-like particles and application of the virus-like particles and the mixture of the virus-like particles to the preparation of the vaccine for HPV 16 L1-virus-like particles.
Owner:SOUTH CHINA UNITED VACCINE INST
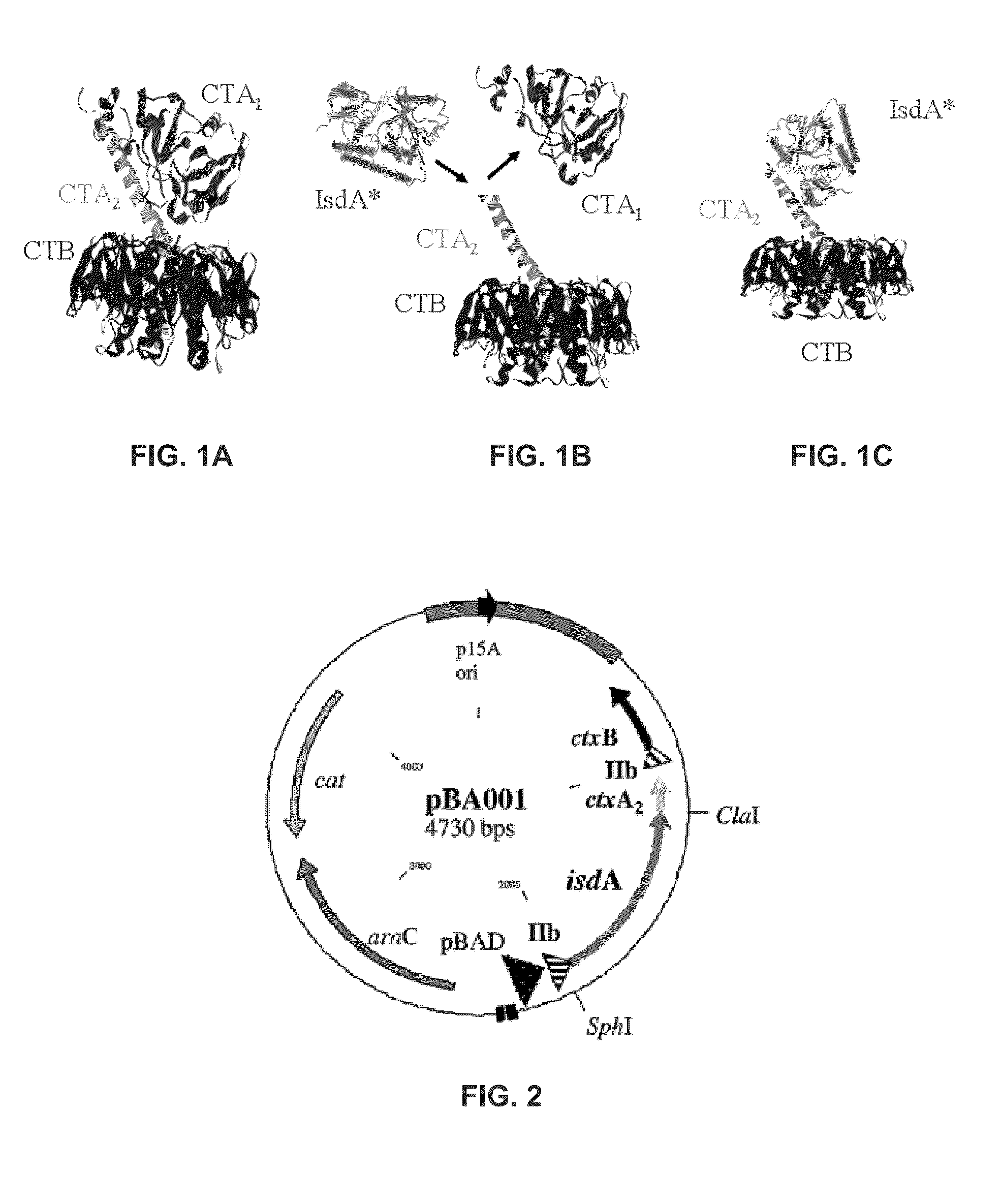
Cholera Toxin Chimera and its Use as a Staph Vaccine
The present invention relates to chimeric protein vaccines and methods of use thereof in the treatment of Staphylococcus aureus. One embodiment of the present invention provides a method of generating an immune response in a mammal, that includes administering to the mammal, a composition having a chimeric protein having at least one of: a portion of a cholera toxin, a portion of a heat-labile toxin, and a portion of a shiga toxin; and an antigen having at least one of an antigenic material from S. aureus and an antigenic material from a S. aureus-specific polypeptide.
Owner:BOISE STATE UNIVERSITY
Establishing and expressing method for chimeric vector of EV71 neutralization epitope and norovirus P structure domain
PendingCN106220738AHigh purityEasy to makePeptide preparation methodsHybrid peptidesEnterovirusAntigen
The invention aims at providing chimeric protein of enterovirus EV71 neutralization epitope and a human norovirus P structure domain and an establishing method of a chimeric vector. By means of immunoblotting, it is verified that the chimeric protein of the enterovirus EV71 neutralization epitope and the norovirus P structure domain can be used for research and development of detection kits of EV71 and norovirus and novel bivalent subunit vaccines.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Purification of chimeric protein
InactiveUS20140128577A1Inhibitory activityReduce impactAntibody mimetics/scaffoldsImmunoglobulinsChimerin ProteinsBuffer solution
Provided is a method for purification of TNFR:Fc fusion protein comprising hydrophobic interaction chromatography, wherein the buffer solution used in the said chromatography does not contain any additives.
Owner:DR REDDYS LAB LTD
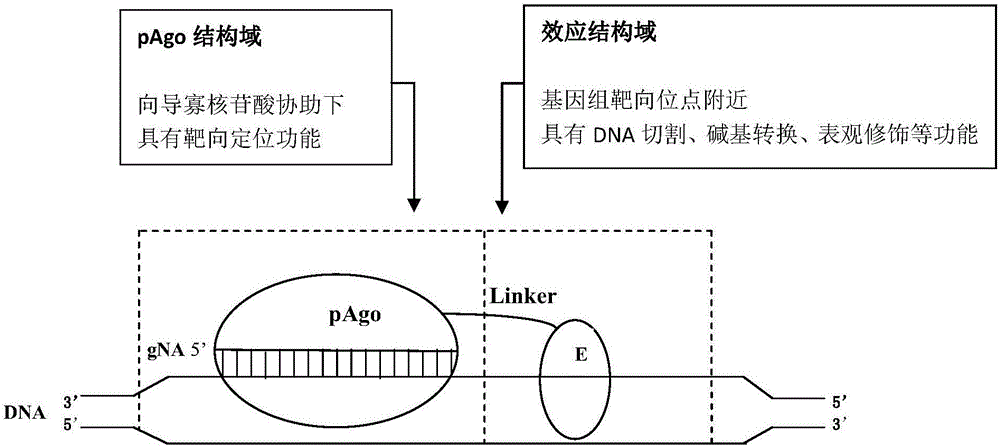
Chimeric protein pAgoE, construction method and applications thereof, chimeric protein pAgoE using guide, and construction method and applications thereof
ActiveCN106589134ATo achieve synchronous generationReduce off-target effectsTransferasesBacteria peptidesChimerin ProteinsGenome
The invention relates to biological technology, specifically relates to a chimeric protein pAgoE, a construction method and applications thereof, and at the same time, relates to a chimeric protein pAgoE using a guide, a construction method and applications thereof, and a chimeric protein, which is based on the chimeric protein pAgoE and has a genome targeting editing function. The chimeric protein pAgoE contains a pAgo structural domain with a genome targeting positioning function and an effect structural domain E, which is connected to the pAgo structural domain and has a genome cutting or modifying activity. The pAgo structural domain with a genome targeting positioning function and the effect structural domain E with a genome cutting or modifying activity are fused to establish the chimeric protein pAgoE; the chimeric protein pAgoE maintains the original targeting binding performance of the genome (DNA) having a targeting positioning function in the pAgo structural domain and the genome catalytic or modifying performance of the effect structural domain E at the same time, and an sufficient space is provided for the structural domain E to fully exert the effect.
Owner:仪宏
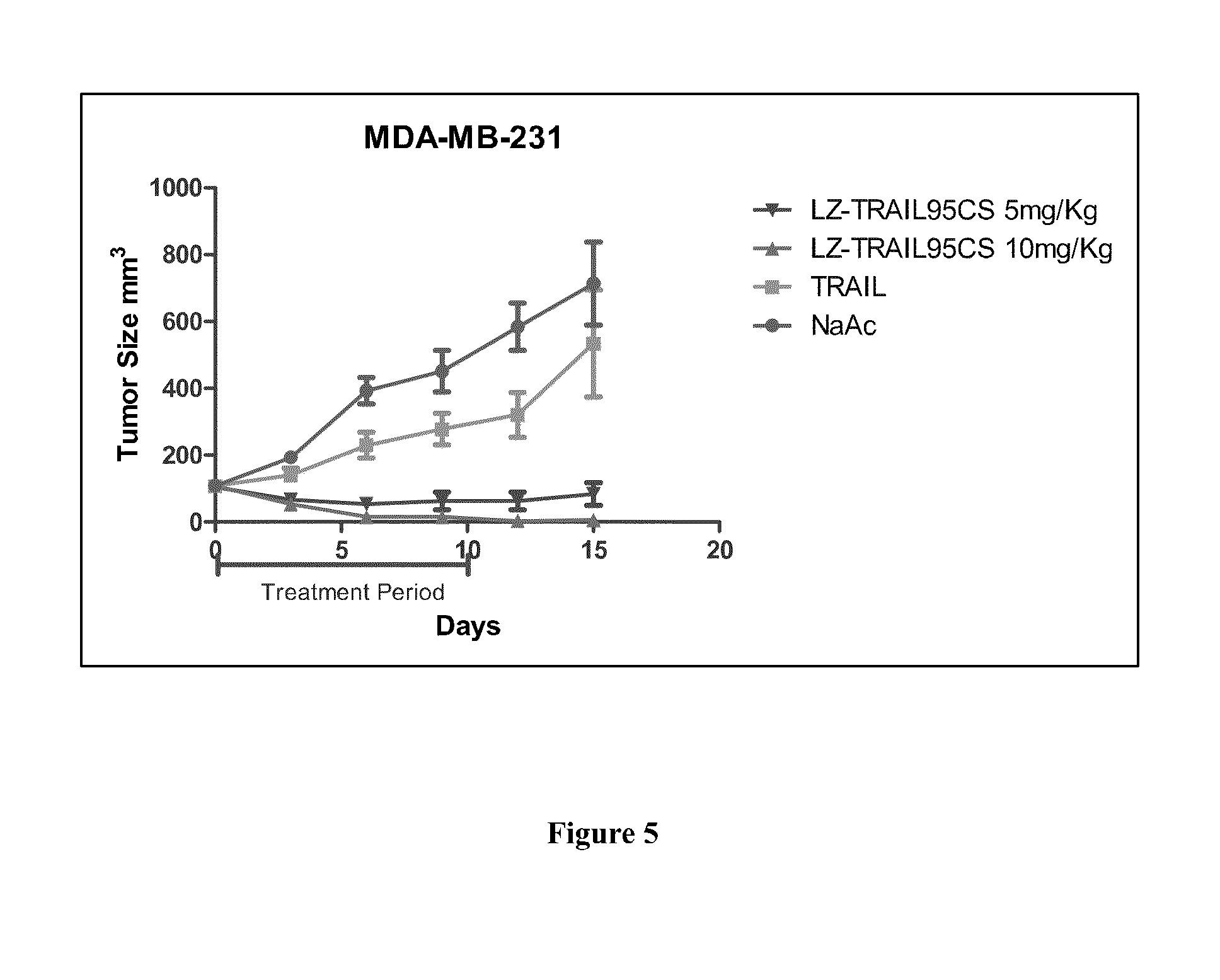
Therapeutic TRAIL Fusion Protein and Preparation and Use Thereof
InactiveUS20140206843A1Good for healthLow toxicityBacteriaPeptide/protein ingredientsChimerin ProteinsHalf-life
This invention relates to the biotechnology area, and provides a kind of TRAIL chimeric protein, DNA sequence encoding this chimeric protein, vectors comprising this DNA, host cells or transgenic animals that contain one of the vectors, and preparation methods for the said chimeric protein and its applications. The said TRAIL fusion protein from the N to C terminal comprises human leucine zipper sequence, human TRAIL protein, human TRAIL extracellular domain or a fragment thereof. The chimeric protein has significantly enhanced stability, prolonged half-life in animals, increased efficacy and thus has broad application future.
Owner:MAGELLAN PHARMA
VLPs derived from cells that do not express a viral matrix or core protein
ActiveUS8697088B2SsRNA viruses negative-sensePeptide/protein ingredientsChimerin ProteinsVirus-like particle
Owner:NOVAVAX
EGF-like protein and construction method thereof, and chimeric protein as well as preparation method and application thereof
ActiveCN110078835ASignificant pro-growth effectBiologically activePolypeptide with localisation/targeting motifNervous disorderBALB/c3T3 cells
The invention belongs to the technical field of bioengineering, and particularly relates to EGF-like protein and a construction method thereof, and chimeric protein as well as a preparation method andapplication thereof. The invention provides the EGF-like protein; the EGF-like protein is EGF-CTA2-TAT protein; the nucleotide sequence of the EGF-CTA2-TAT protein is shown in SEQ ID NO: 1. The EGF-CTA2-TAT protein is chimeric with EGF protein with a repair effect, cell-penetrating peptide assisting transdermal absorption, and a cholera toxin A2 subunit (CTA2) with the ability of penetrating cellmembranes; it is shown by a cell activity experiment test result that the EGF-CTA2-TAT protein has obvious effect of promoting the growth of BALB / c 3T3 cell strains, and has bioactivity.
Owner:广东格烯生物科技股份有限公司
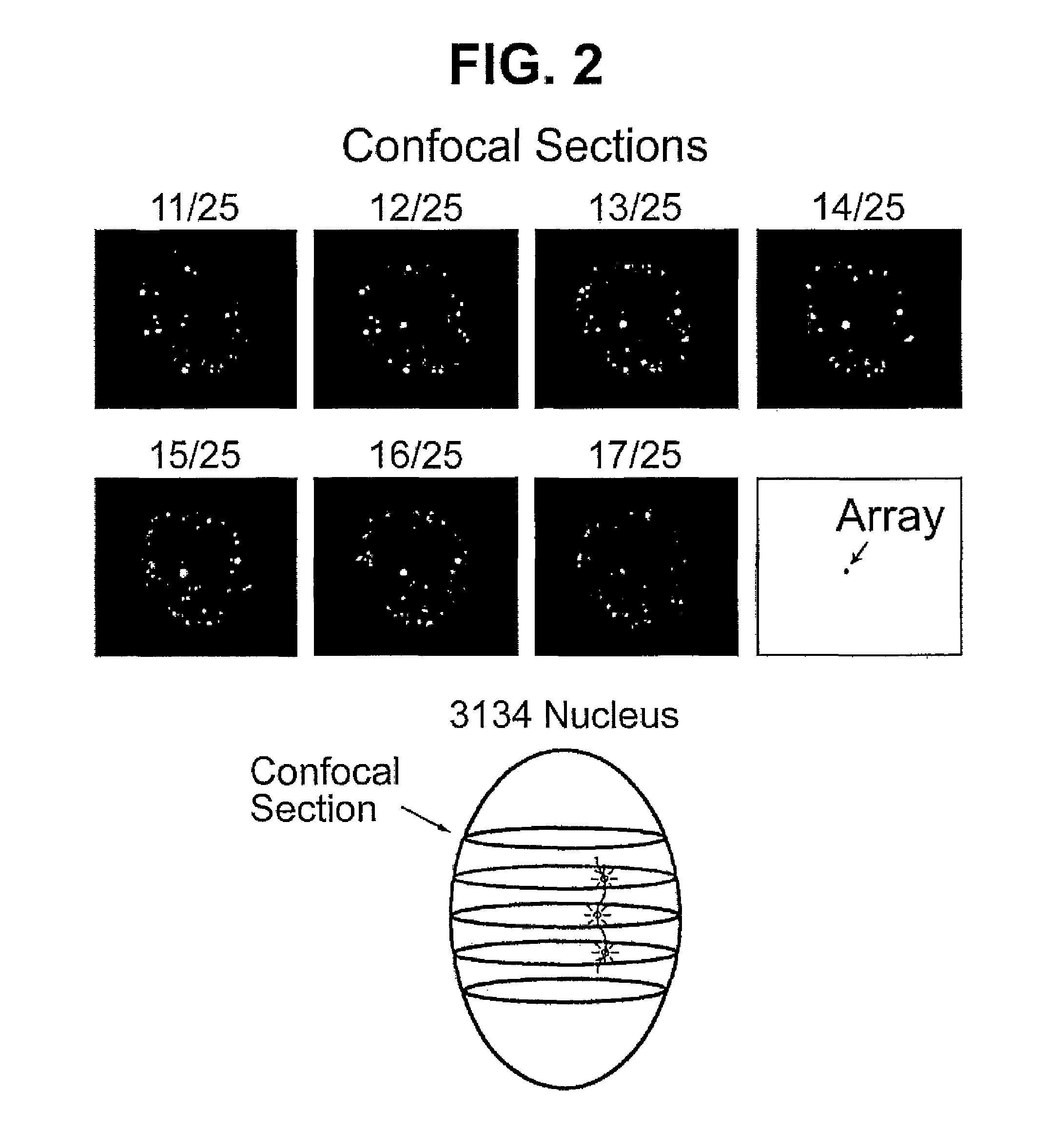
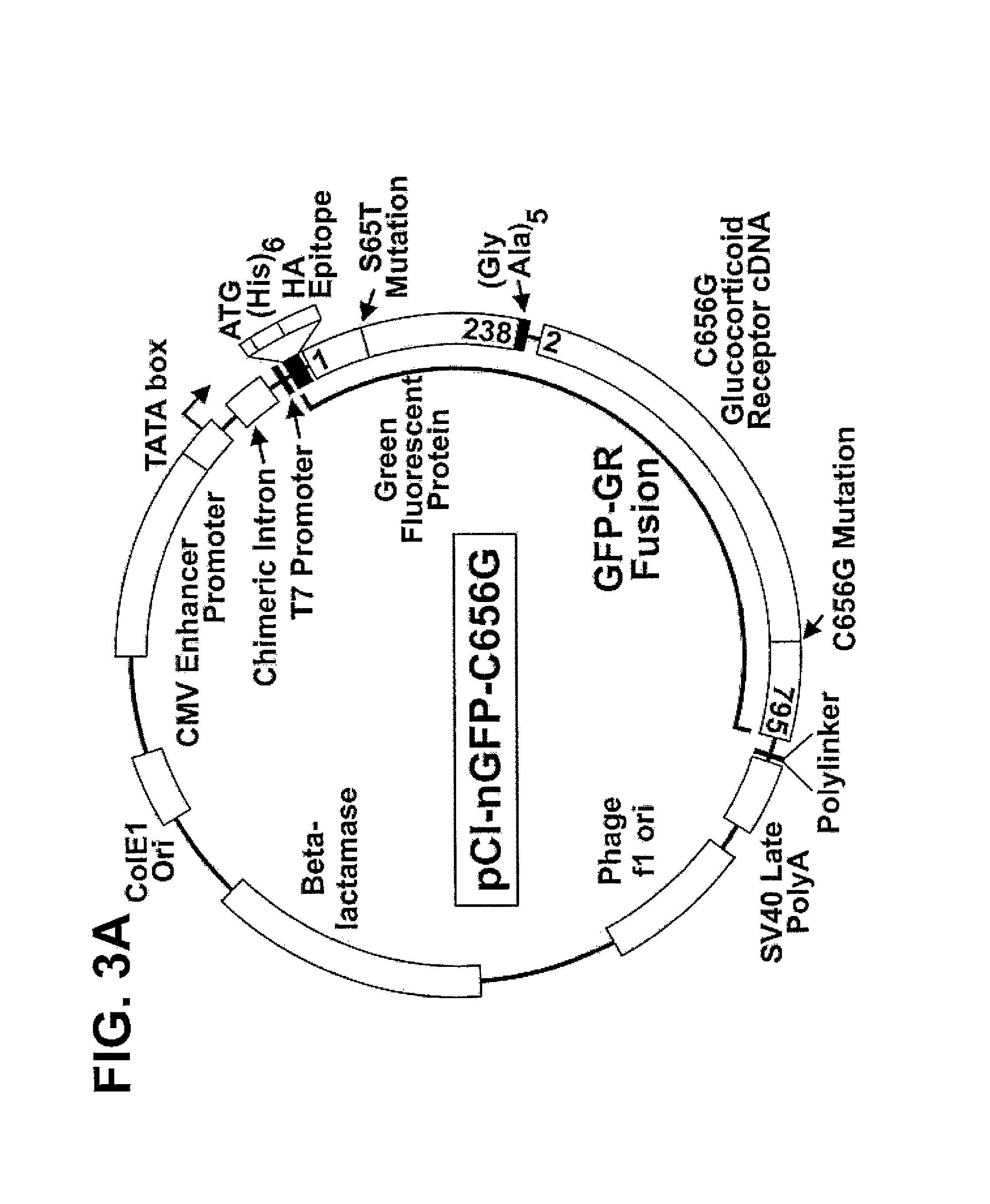
Chimeric protein comprising a green fluorescent ptotein fused to a transcription factor
The present invention provides a method of screening for a compound that binds to a selected nucleic acid comprising contacting compound fluorescently labeled by a fluorescent protein with a cell having a plurality of copies of the nucleic acid in an array such that the nucleic acid can be directly detected when bound by fluorescently labeled compound; and directly detecting the location of fluorescence within the cell, fluorescence aggregated at the site of the nucleic acid array indicating a compound that binds to the selected nucleic acid. In particular compounds such a transcription factors can be screened. Reagents for such method are provided including a mammalian cell having a plurality of steroid receptor response elements in an array such that the response element can be directly detected when bound by fluorescently labeled steroid receptor and a chimeric protein comprising a fluorescent protein fused to a steroid receptor.
Owner:HTUN HAN +1
Bovine rotavirus VP8* subunit recombinant chimeric protein and application thereof
ActiveCN104479028AImprove immune efficiencyReduce economic lossViral antigen ingredientsAntiviralsBovine rotavirusGenotype
The invention provides a bovine rotavirus VP8* subunit recombinant chimeric protein. According to the bovine rotavirus VP8* subunit recombinant chimeric protein, a T cell epitope polypeptide P2 in a tetanus toxin is introduced into an epitope vaccine of a bovine rotavirus VP8* subunit multi-copy chimeric gene recombinant protein, so that the immune efficacy of the epitope vaccine can be greatly enhanced, cross neutralizing immune protection can be induced, higher neutralizing antibody titer can be induced, and a high-titer anti-P[5] and P[11] genotype-specific rotavirus neutralizing antibody can be induced. The bovine rotavirus VP8* subunit recombinant chimeric protein provided by the invention can be used for preventing and controlling both G6 and G10 type rotaviruses which are mainly pandemic within a world range, and is suitable for preparing a safe and low-cost bovine rotavirus vaccine; in addition, the bovine rotavirus vaccine can be used for effectively reducing the economic loss brought by rotavirus gastroenteritis to cattle rearing by being matched with other rotavirus vaccines researched and developed at present, and has wide market prospects.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Serogroup B meningococcus recombinant chimeric protein vaccine and preparation method thereof
ActiveCN107823638AImprove the effect of preventionEffective protectionAntibacterial agentsNervous disorderPericarditisSalmonella serotype typhi
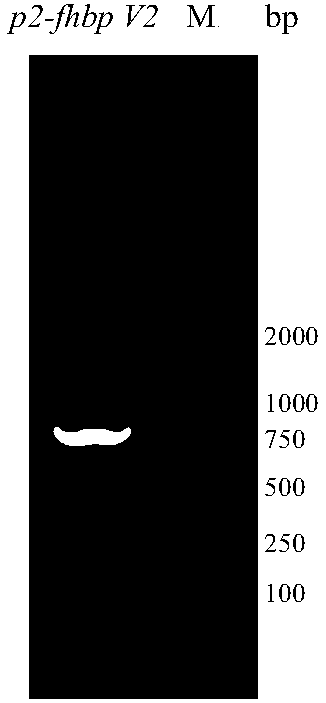
The invention provides a serogroup B meningococcus recombinant chimeric protein vaccine and a preparation method thereof. The vaccine contains three recombinant chimeric proteins of P2-fHBP V1, P2-fHBP V2 and P2-fHBP V3, wherein amino acid sequences of the recombinant chimeric proteins are SEQ ID NO.1, SEQ ID NO.2 and SEQ ID NO.3 respectively; the contents of all the proteins are 30 to 50mu g / dose; optimally, a vaccine preparation also contains a cryoprotectant or vaccine adjuvant. The invention also provides the preparation method of the three recombinant chimeric proteins. By using the recombinant chimeric proteins formed by linking P2 with serogroup B meningococcus fHBP protein, humoral immune response can be effectively induced, and immunogenicity of fHBP is obviously improved. The vaccine provided by the invention can effectively cover all serogroup B meningococcus strains, thereby providing a broad-spectrum preventing effect on invasive diseases such as cerebrospinal meningitis,bacteremia, pneumonia and pericarditis caused by the serogroup B meningococcus.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
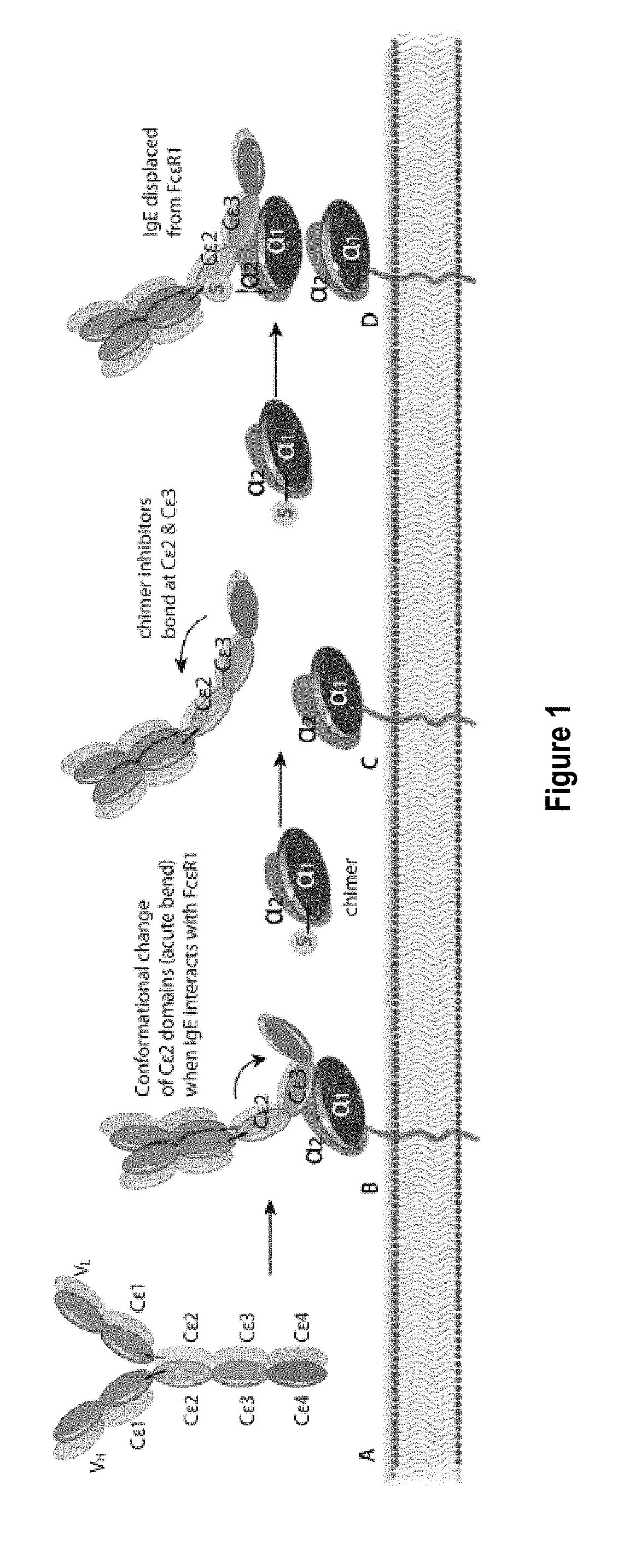
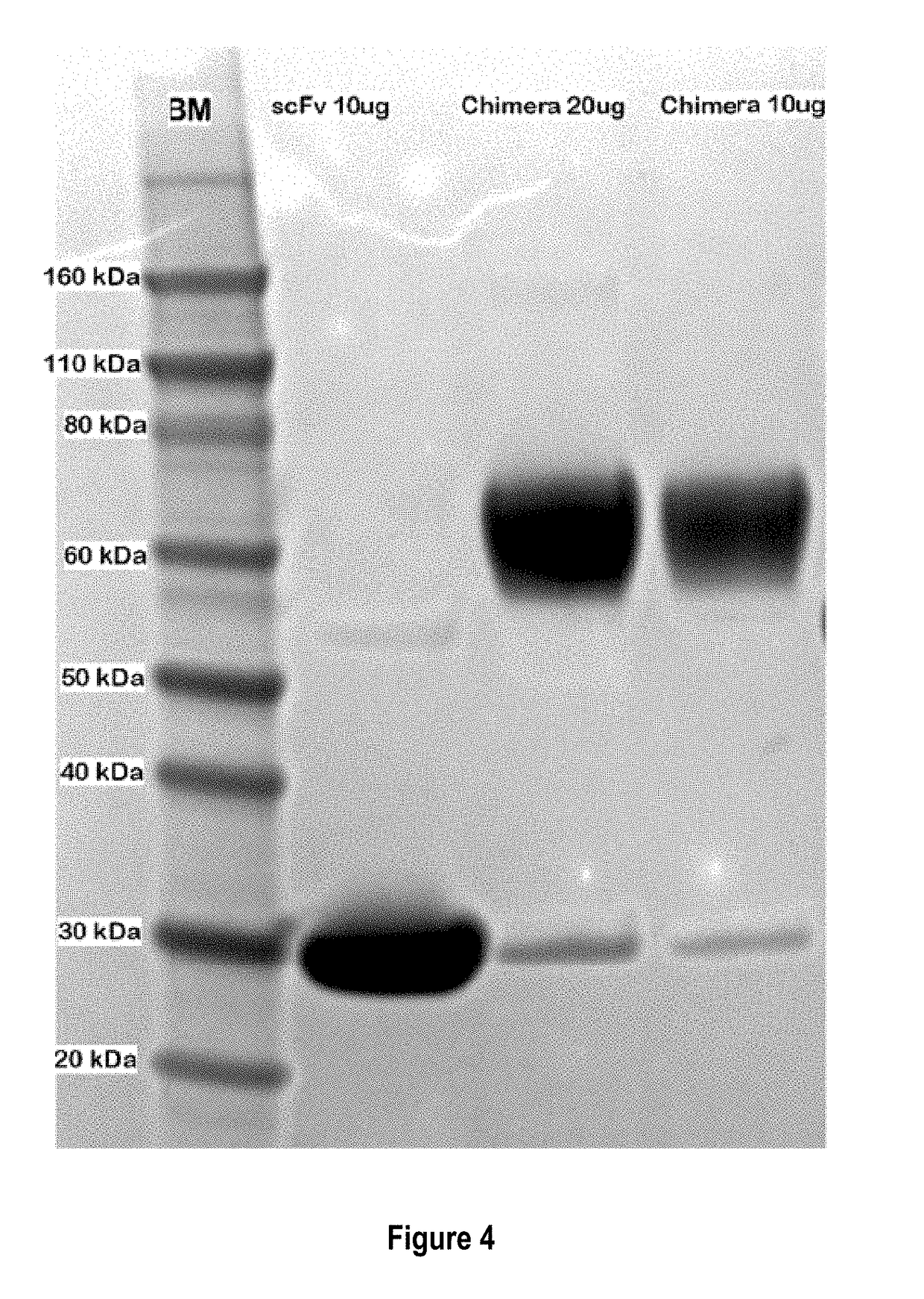
Treatment of allergic diseases with chimeric protein
ActiveUS20180230236A1Reduce free serum IgE levelDecreasing IgE levelSenses disorderAntibody mimetics/scaffoldsLinker peptideDisease cause
The present invention provides a chimeric protein comprising: a) a single chain variable fragment (scFv) that binds to a mammalian IgE at an epitope within the amino acid sequence VDGQKATNIFPYTAPGTK (SEQ ID NO:1) of canine IgE or at an epitope within the corresponding amino acid sequence of a different mammalian species; b) a linker peptide; and c) an amino acid sequence comprising an IgE high affinity receptor alpha chain, and methods of use thereof.
Owner:NORTH CAROLINA STATE UNIV
Broadly protective Shigella vaccine based on type III secretion apparatus proteins
InactiveUS9492523B2Bacterial antigen ingredientsPeptide preparation methodsShigella sonneiChimerin Proteins
Broad-based, cross-protective, O-serotype independent vaccines against Shigella and related pathogenic organisms are provided. The vaccines comprise one or more of the type III secretion (TTS) apparatus needle proteins IpaD and IpaB, and / or the IpaB cognate chaperone protein IpgC. Chimeric proteins of IpaD and IpaB, and / or IpgC are also encompassed.
Owner:BOARD OF REGENTS FOR OKLAHOMA STATE UNIVERSITY
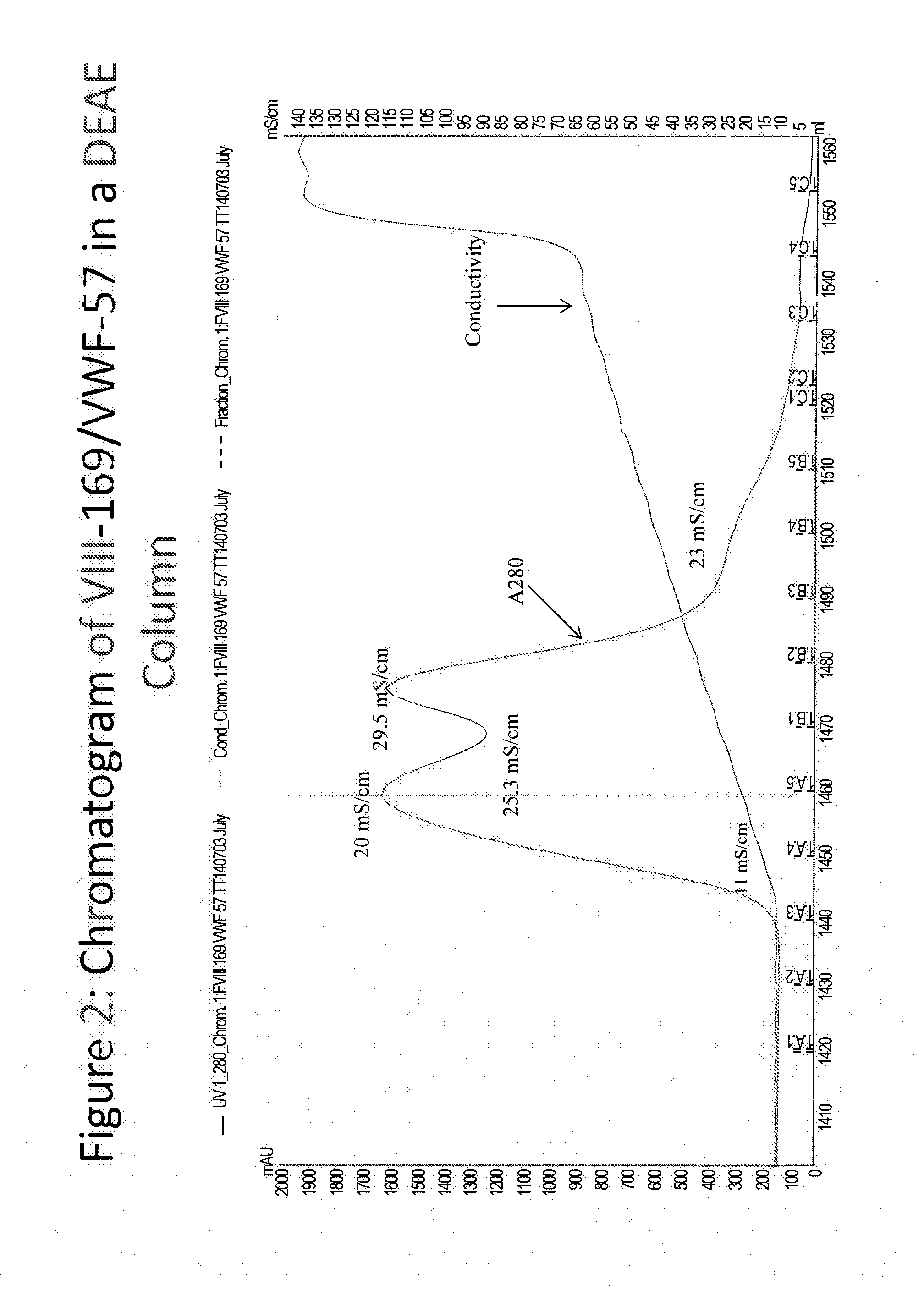
Purification of chimeric fviii molecules
ActiveUS20160185817A1Factor VIIMammal material medical ingredientsFactor VIII ActivityPresent method
The invention is directed to methods of purifying a chimeric protein comprising subjecting the chimeric protein to a factor VIII-specific affinity chromatography, and subjecting the chimeric protein to an AEX chromatography; wherein the chimeric protein comprises a factor VIII protein or a fragment thereof. The chimeric protein purified by the present methods shows improved factor VIII activity.
Owner:BIOVERATIV THERAPEUTICS INC
Chimeric proteins
This invention relates to modular proteins that interact with one or more target molecules. The chimeric proteins comprise two or more repeat domains, such as tetratricopeptide repeat domains; inter-repeat loops linking the repeat domains; and one or more peptide ligands. Each peptide ligand is located in an inter-repeat loop or at the N or C terminus of the chimeric protein. The peptide ligands may include heterologous peptidyl binding motifs, such as short linear motifs (SLiMs). Chimeric proteins with various configurations and methods for their production and use are provided.
Owner:CAMBRIDGE ENTERPRISE LTD
Chimeric protein vaccine containing HPV16 type L1 and E7 target antigens expressed in yeast cells and its preparation method
InactiveCN102614505AImprove the possibility of applicationAchieving large-scale fermentationViral antigen ingredientsMicroorganism based processesNeutralizing antibodyFermentation
The invention provides an L1E7 chimeric protein vaccine composed of L1 capsid protein and E7 polypeptide and a preparation method thereof. In the invention, optimized induction expression conditions are employed, and expression of a chimeric protein through large scale fermentation of yeast cells can significantly reduce the cost. Moreover, the purification process adopted in the invention is relatively simple and has low cost, thus being suitable for mass production of HPV16L1 / E7 protein. The HPV16L1 / E7 protein containing a late structural gene and an early gene can also induce the body to generate strong E7-specific C TL reaction and antitumor activity besides enabling the body to generate a neutralizing antibody. Therefore, the chimeric protein vaccine provided in the invention is considered to be the most promising vaccine for cervical cancer prevention and treatment, and can be used for HPV16 infected patients with or without clinical symptoms.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Polyvalent chimeric ospc vaccinogen and diagnostic antigen
InactiveUS20130266606A1Broad protectionAntibacterial agentsAntibody mimetics/scaffoldsEpitopeChimerin Proteins
A chimeric polyvalent recombinant protein for use as a vaccine and diagnostic for Lyme disease is provided. The chimeric protein comprises epitopes of the loop 5 region and / or the alpha helix 5 region of outer surface protein C (OspC) types. The OspC types may be associated with mammalian Borrelia infections.
Owner:VIRGINIA COMMONWEALTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com