Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "Rotavirus vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rotavirus vaccine is a vaccine used to protect against rotavirus infections, which are the leading cause of severe diarrhea among young children. The vaccines prevent 15–34% of severe diarrhea in the developing world and 37–96% of severe diarrhea in the developed world. The vaccines decrease the risk of death among young children due to diarrhea. Immunizing babies decreases rates of disease among older people and those who have not been immunized.
Live attenuated rotavirus vaccine for oral administration
ActiveUS8192747B2Maintain immunogenicityStable over a long shelf-lifeBacterial antigen ingredientsViral antigen ingredientsDiseaseOral medication
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Prepn process of inactivated rotavirus vaccine
ActiveCN101020053APrevent infectious diseasesViral antigen ingredientsAntiviralsRotavirus RNAResearch Object
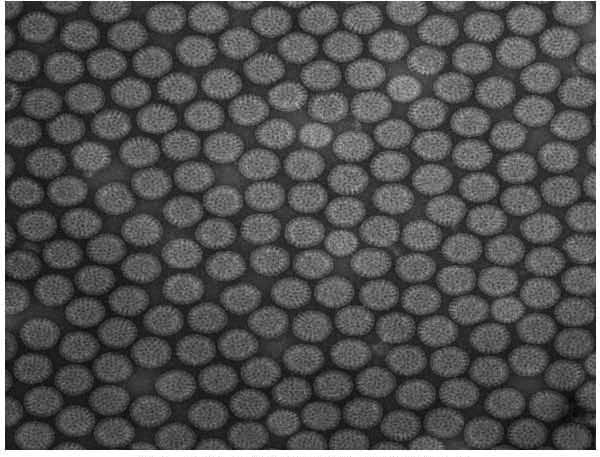
The present invention discloses preparation process of inactivated rotavirus vaccine. Rotavirus strains G1, G2, G3 and G4 are made to adapt human diploid cell line and propagate on human diploid cell line to obtain the producing strain of inactivated rotavirus vaccine, with the human diploid cell line being KMB17. The human diploid cell line used as the cell matrix for producing inactivated rotavirus vaccine is safer compared with available cell matrixes. Therefore, by means of the established technological platform, it is possible to develop inactivated rotavirus vaccine with other serotypes of rotavirus as the target.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Composition Useful as a Vaccine
The present invention relates to a composition comprising a viral antigen, a first protein and a second protein. Optionally, the composition also comprises three different disaccharaides, or, optionally, the composition comprises a primary sugar and at least one, preferably two secondary sugars. The present invention also relates to the use of a viral antigen, a first protein and a second protein for the manufacture of a composition, preferably a vaccine. The present invention furthermore relates to a method of treatment or prevention of virus associates diseases in humans. Moreover, the present invention relates to a method of adapting a virus to a suitable cell-line. The invention is also useful for the production of virus suspensions suitable for making stable, live / inactivated, monovalent and / or polyvalent, liquid / lyophilized rotavirus vaccine compositions for oral and / or nasal or any other suitable route of administration in human.
Owner:BHARAT BIOTECH INTERNATIONAL
Human rotavirus Delta VP8* subunit recombinant protein and application thereof
ActiveCN103319604AImprove immune efficiencyFast titerBacteriaViral antigen ingredientsCross neutralizationRotavirus RNA
The invention relates to human rotavirus Delta VP8* subunit recombinant protein and application thereof. The human rotavirus Delta VP8* subunit recombinant protein comprises a T cell epitope P2 in tetanus toxin and a rotavirus Delta VP8* subunit. By the recombinant protein disclosed by the invention, the immune efficacy of a Delta VP8* subunit vaccine can be greatly improved; faster and stronger neutralization antibody titer can be induced; moreover, anti-p[4] genotype specific rotavirus cross neutralization antibody of high titer can be induced; simultaneously, the potential risk of inducing intussusception by taking attenuated rotavirus vaccine orally can be overcome; therefore, the recombinant protein is applicable to preparing a rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Preparation of tetravalent wheel shaped virus inactivated vaccine and application
ActiveCN1686540APrevent infectious diseasesViral antigen ingredientsAntiviralsBacteroidesRotavirus RNA
A deactivated tetravalent rotavirus vaccine for preventing the infantile rotavirus infections diseases is prepared from the calf kidney cells digested and dispersed by pancreatin or cultured Vero cells through inoculating rotaviruses G1, G2, G3 and G4, culturing in non-serum culture liquid D-MEM, concentrating, purifying, deactivating, mixing and adding aluminium hydroxide.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Method for preparing rotavirus vaccine stock solution by using serum-free Vero cells and serum-free rotavirus vaccine product
InactiveCN106676076ARemove inhibitionHigh titerViral antigen ingredientsMicroorganism based processesRotavirus RNAFiltration
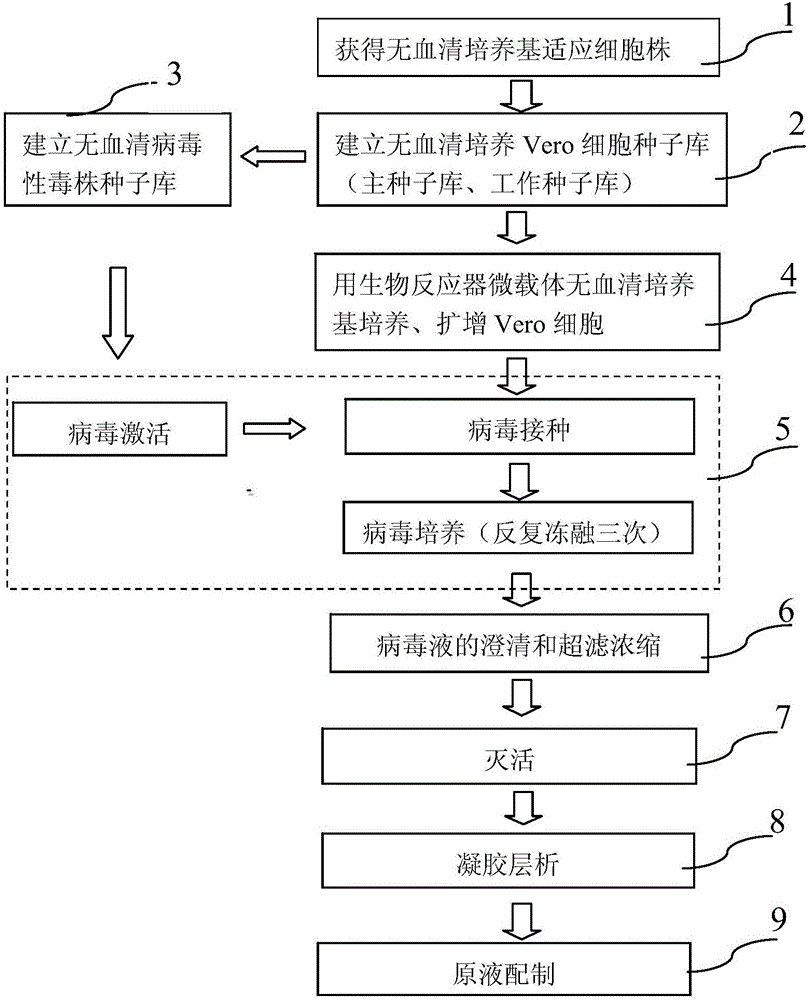
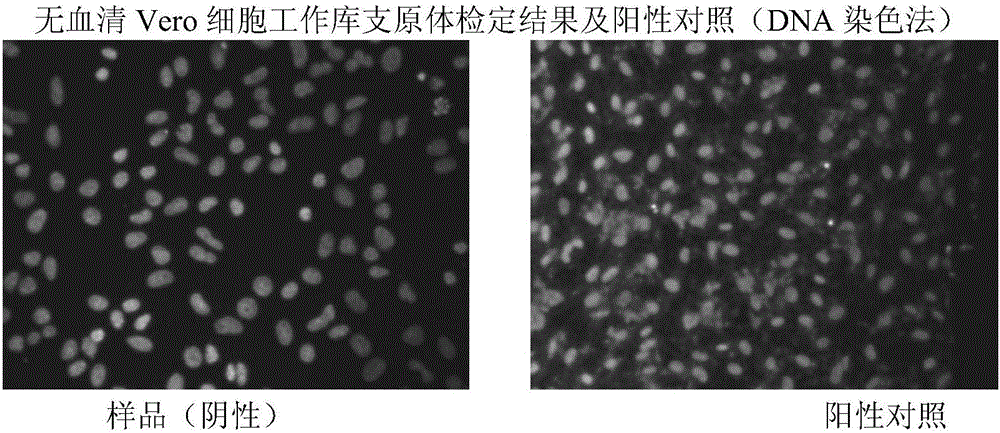
The invention provides a method for preparing a rotavirus vaccine stock solution by using serum-free Vero cells. The method comprises the following steps: culturing Vero cells by using a serum-free culture medium, thus obtaining serum-free culture medium adapted cell strains; establishing a serum-free Vero cell seed bank by utilizing the obtained serum-free culture medium adapted cell strains; establishing a serum-free rotavirus strain working seed bank by utilizing the obtained serum-free culture medium adapted cell strains; carrying out reviving, culturing, passage and amplification on cells in a Vero cell working seed bank by utilizing the serum-free culture medium, using the cells in the Vero cell working seed bank as basic cells cultured in a bioreactor, and carrying out continuous perfusion culture on high-density Vero cells by applying the bioreactor and a microcarrier and using the serum-free culture medium after cell amplification; after inoculating virus seeds in the rotavirus strain working seed bank, carrying out bioreactor-microcarrier serum-free culture, obtaining a virus solution when virus is amplified to the summit, obtaining liquid virus titer, and carrying out clarification and ultra-filtration concentration, thus obtaining a serum-free rotavirus stock solution for human.
Owner:AB&B BIO TECH CO LTD JS
Human rotavirus strain and separation, culture and identification method thereof
InactiveCN102618506AMicrobiological testing/measurementInactivation/attenuationMicroorganismHuman rotavirus
The invention discloses a human rotavirus strain. The human rotavirus strain is characterized in that the strain is a Rotavirus A ZTR-5 strain, and is collected to CGMCC (China General Microbiological Culture Collection Center of Microbiological Culture Management Committee) on 27th, June 2011, and the number is CGMCCNo.4977. The strain is a wild type single purified strain of human rotaviruses, and is strong in virus replication capability and good in genetic stability; and the virus seed can be applied as a production virus seed for developing human rotavirus vaccines, and is applicable to attenuated type rotavirus oral vaccines and inactivated human rotavirus inject vaccines. The invention provides a separation, culture and identification method of the human rotavirus strain.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
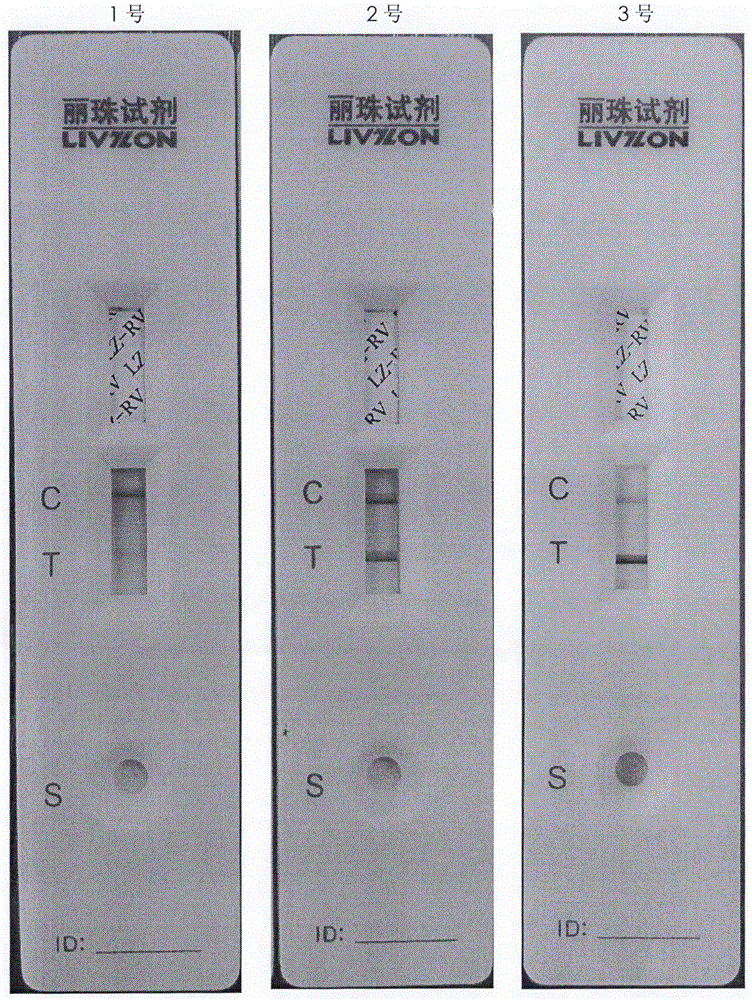
Method for producing rotavirus vaccines in large scale by utilizing bioreactor
ActiveCN106047821AIncrease productionQuality improvementViral antigen ingredientsAntiviralsMicrobiologyVero cell
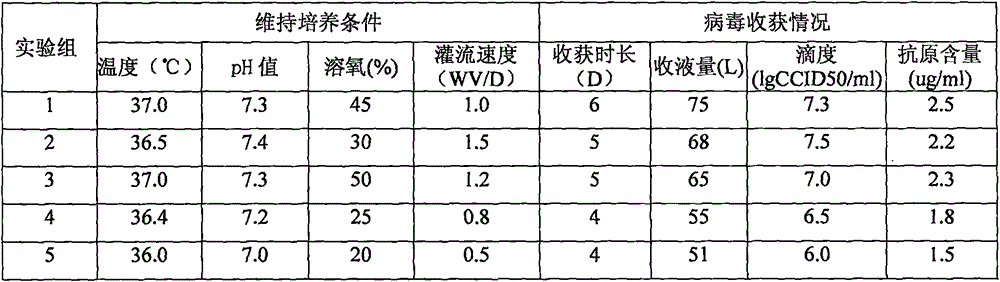
The invention relates to a method for producing rotavirus vaccines in large scale by utilizing a bioreactor, and in particular relates to a method for culturing rotavirus inactivated vaccines in large scale by utilizing a 75L bioreactor. The method comprises the following steps: (1) carrying out reviving and passage on Vero cells; (2) collecting the cells and inoculating the cells into the bioreactor; (3) culturing the cells in the bioreactor; (4) changing the liquid and washing the cells; (5) activating and infecting rotaviruses; and (6) carrying out maintenance culture and harvesting on the rotaviruses. The method aims at overcoming the defect that in the large-scale production, the rotaviruses can not release cells easily, so that the obtained viruses are low in toxicity titer, and the inactivated rotavirus vaccines with high titer and high yield are obtained; the method also aims at overcoming the defect that in the large-scale production, the adsorption force and infection force of the rotaviruses are poor, and thus the yield and quality of the rotaviruses are greatly improved.
Owner:LIVZON GROUP VACCINE ENG
Food grade lactic acid bacteria active carrier Group A rotavirus vaccine and preparation method thereof
InactiveCN103656633AWill not spreadNo horizontal transferViral antigen ingredientsDigestive systemEscherichia coliSerotype
The invention discloses food grade lactic acid bacteria active carrier Group A rotavirus vaccine and a preparation method thereof. The food grade lactic acid bacteria active carrier Group A rotavirus vaccine is characterized in that VP6 antigen protein from Group A virus, common serotype VP7 antigen protein (P serotype) and VP4 antigen protein (G serotype-expressed separately in the form of VP5* and VP8* protein subunit), vaccine adjuvant escherichia coli thermal unstable toxin B (LTB) and cholera toxin subunit B (CTB) are expressed and secreted by thyA gene deletion lactic acid bacteria cell or shown by the cell wall. Expressions of antigen protein and vaccine adjuvant protein are controlled by inducible or constitutive promoter, protein expression cassette is integrated onto the chromosome of the expression host lactic acid bacteria strain, and external antibiotics resistance gene introduced in gene manipulation is removed. The lactic acid bacteria active carrier rotavirus vaccine has the advantages of having wide serotype covering range, being easy to produce in large scale, and being safe and convenient to use without a refrigerator and a needle tubing.
Owner:刘占良 +2
Attenuated human rotavirus vaccine
InactiveUS7150984B2Enhance immune responseMaximum efficacyViral antigen ingredientsInactivation/attenuationRotavirus RNACold adapted
The present invention provides vaccine compositions of attenuated human rotavirus. More particularly, the attenuated human rotavirus is produced by cold passage and thus contains attenuating mutations which produce virus having a cold-adapted (ca) and temperature sensitive (ts) phenotype. The attenuated strains are used in methods for stimulating the immune system of an individual to induce protection against human rotavirus by administration of the ca attenuated rotavirus.
Owner:GOVERNMENT UNITED STATES OF AMERICA THE
Composition useful as rotavirus vaccine and a method therefor
ActiveUS20120107356A1Extended shelf lifeDrawback can be obviatedViral antigen ingredientsInactivation/attenuationRotavirus gastroenteritisRotavirus RNA
Compositions and methods related to live or live attenuated pre-conditioned and typical viruses such as rotaviruses are disclosed. The live attenuated rotaviruses exhibit better stability characteristics and are useful for the prevention of a rotavirus infection and / or rotavirus gastroenteritis in children.
Owner:BHARAT BIOTECH INTERNATIONAL
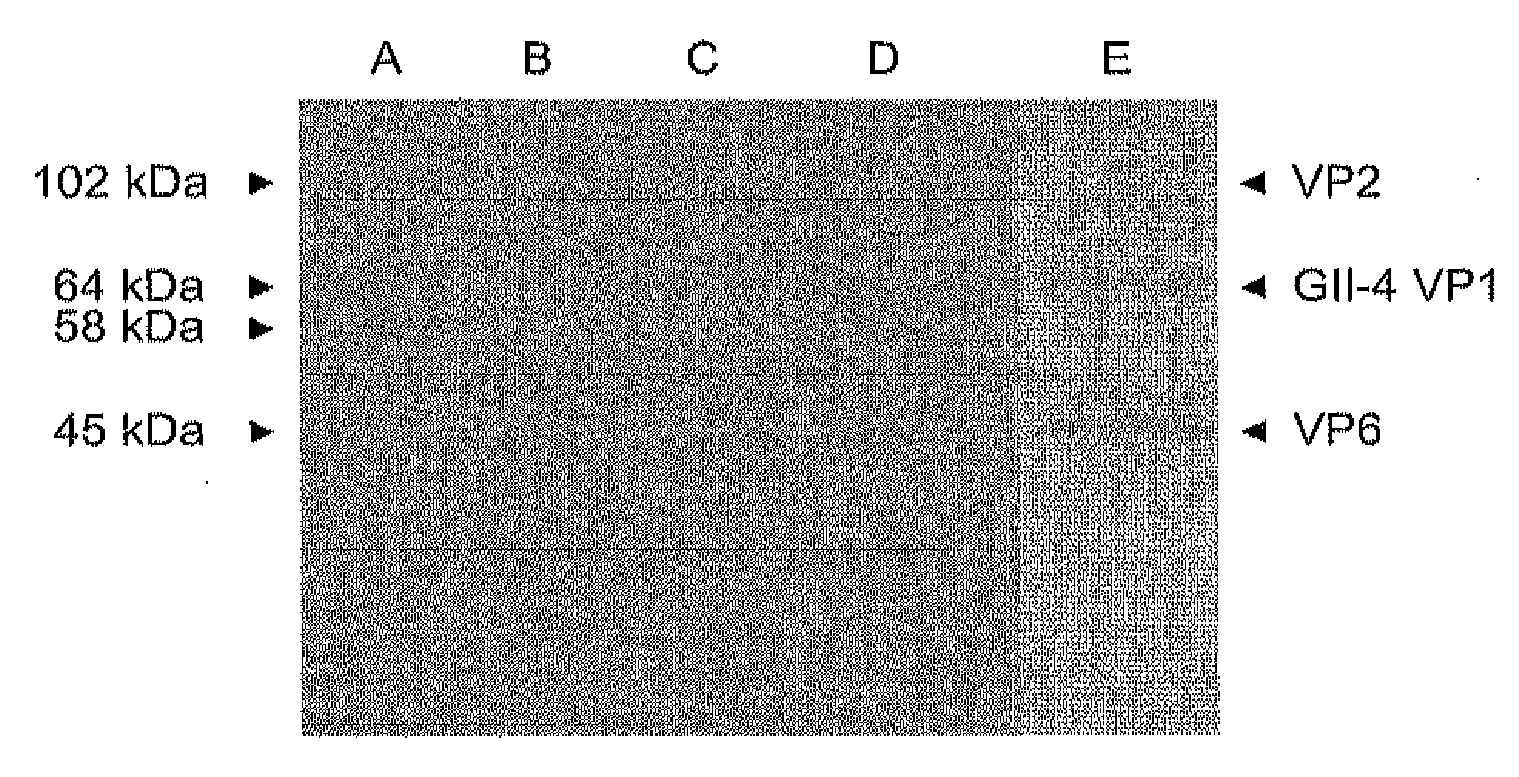
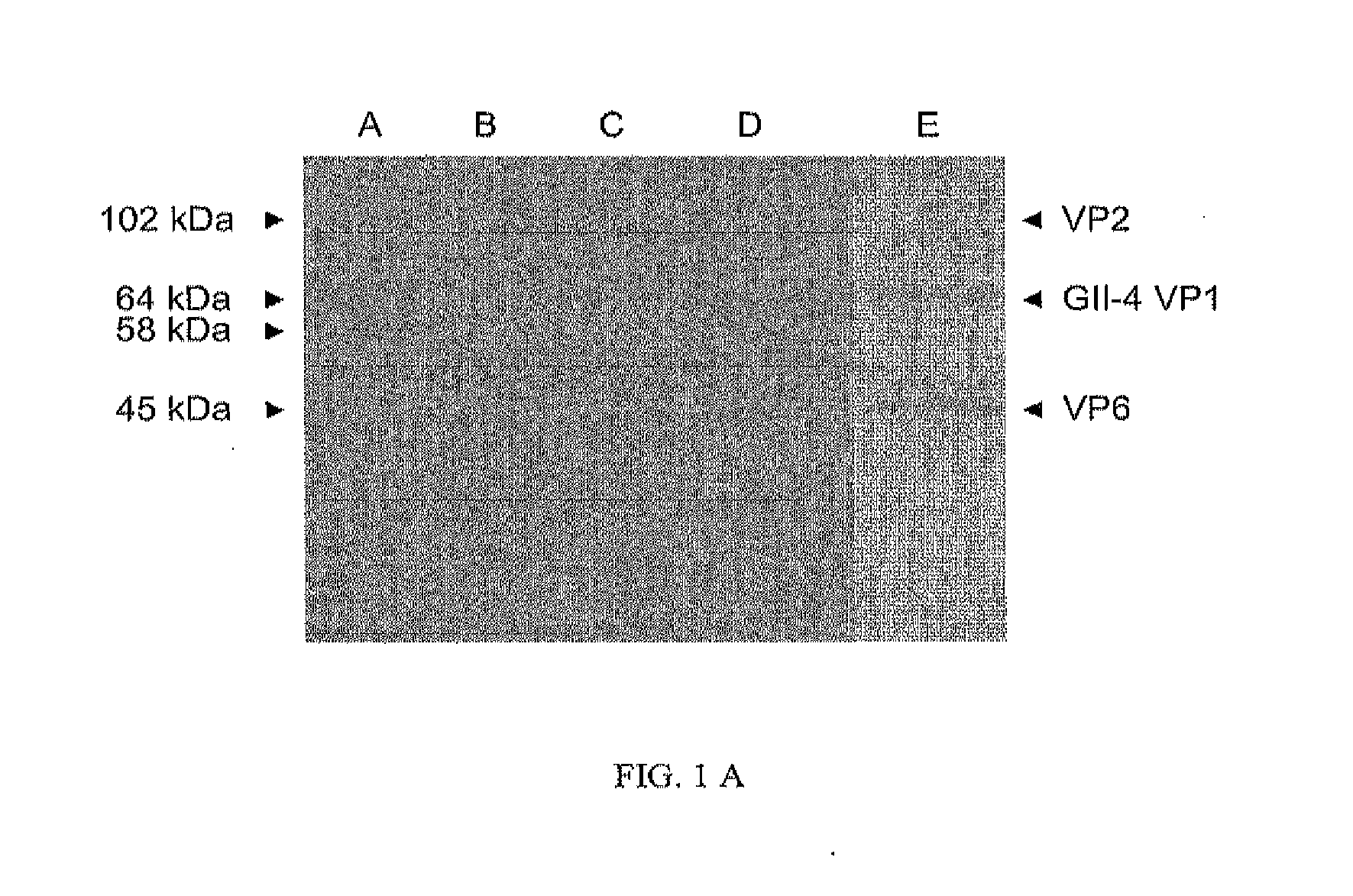
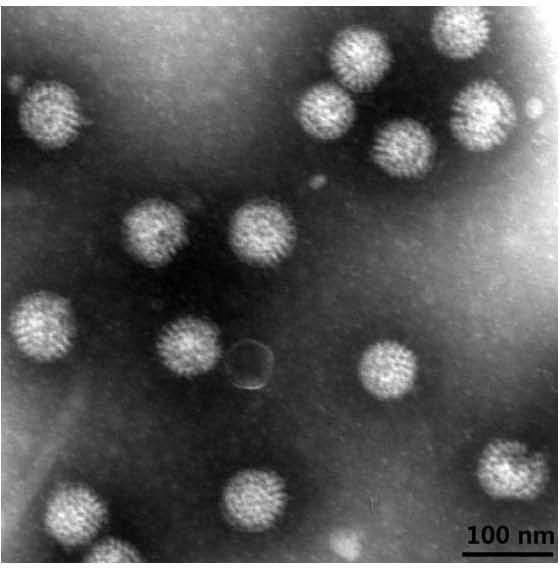
Norovirus capsid and rotavirus vp6 protein for use as combined vaccine
The present invention relates to a combined norovirus and rotavirus vaccine for prevention of norovirus and rotavirus infection and / or viral-induced diarrheal and vomiting diseases in man. More specifically, the invention comprises a method of preparing combination vaccine compositions comprising norovirus and rotavirus antigens, in particular mixtures of norovirus VLPs and rotavirus recombinant VP6 protein or double-layered VP2 / VP6 VLPs. In addition, the invention relates to methods of inducing an immune response.
Owner:VESIKARI TIMO +1
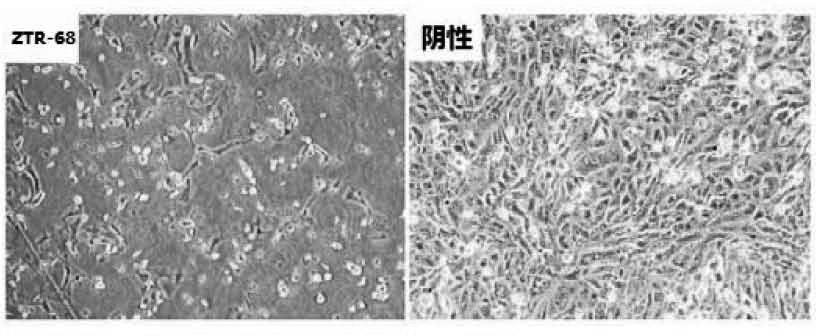
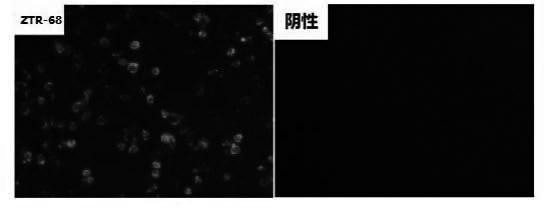
Human rotavirus-A seed strain ZTR-68, and isolation, culture and identification thereof
ActiveCN102618505AMicrobiological testing/measurementInactivation/attenuationBiotechnologyMicroorganism
A human rotavirus-A seed strain ZTR-68 is submitted to CGMCC (china general microbiological culture collection center) for preservation in September 22th, 2011, and encoded as CGMCC No.5265. The strain is a human rotavirus wide-type single purified strain, which is high in virus replication and fine in genetic stability. The human rotavirus-A seed strain ZTR-68 is applicable to developing and producing virus seeds for human rotavirus vaccines, and applicable to attenuated rotavirus oral vaccines and inactivated human rotavirus injection vaccines. The invention further provides a method for isolation, culture and identification of the human rotavirus-A seed strain ZTR-68.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Live Attenuated Rotavirus Vaccine for Oral Administration
ActiveUS20080166372A1Maintain immunogenicityStable over a long shelf-lifeBacterial antigen ingredientsViral antigen ingredientsDiseaseRotavirus RNA
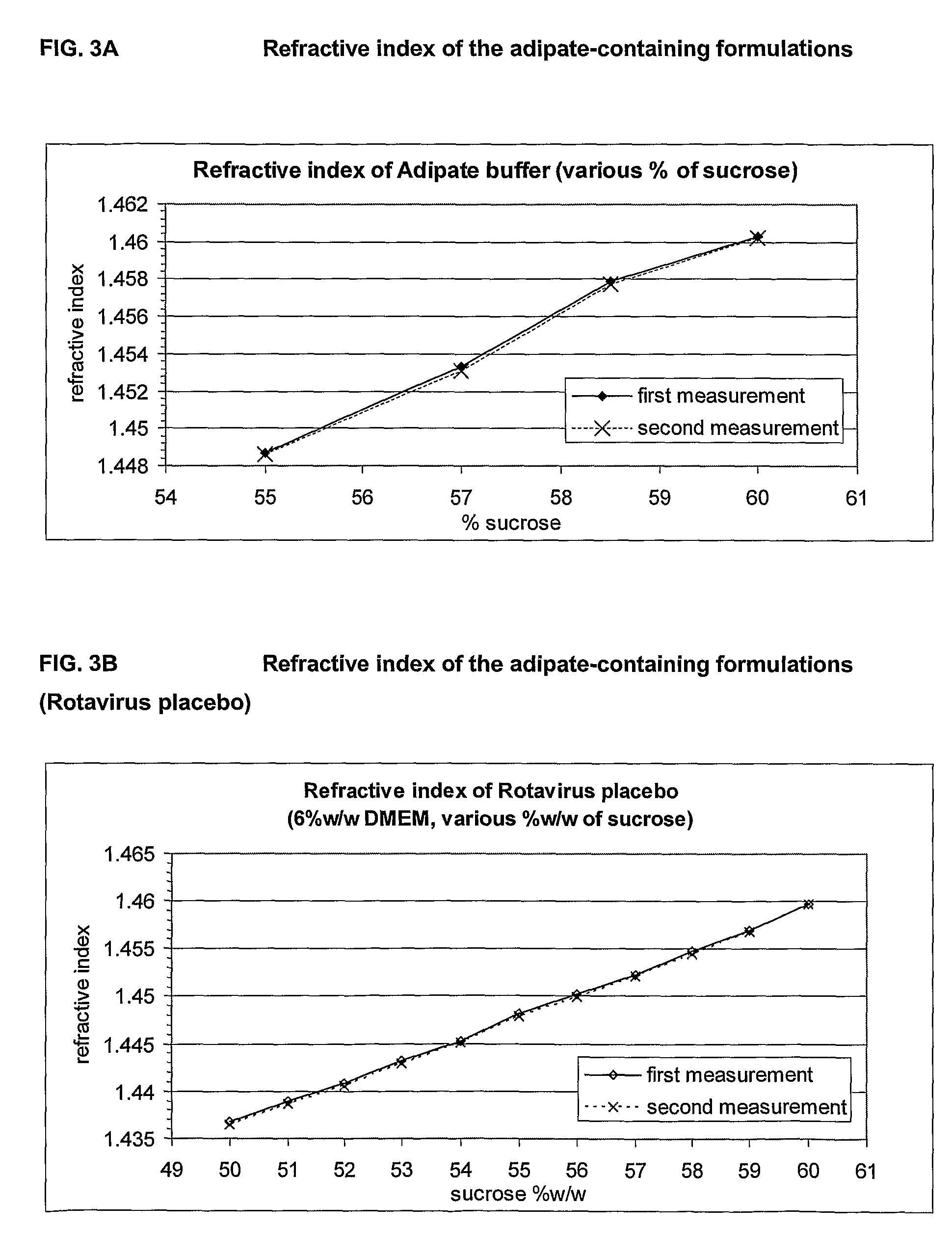
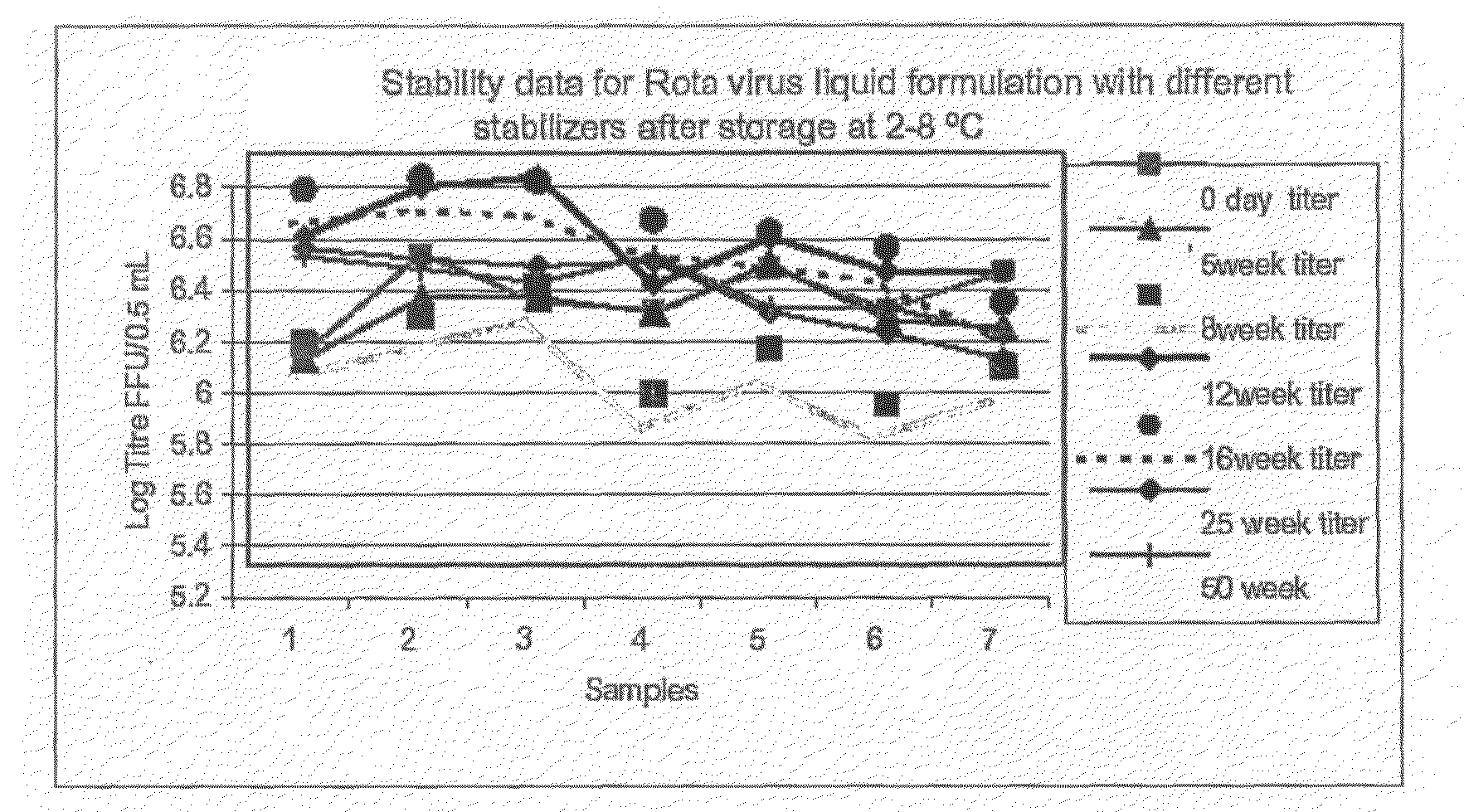
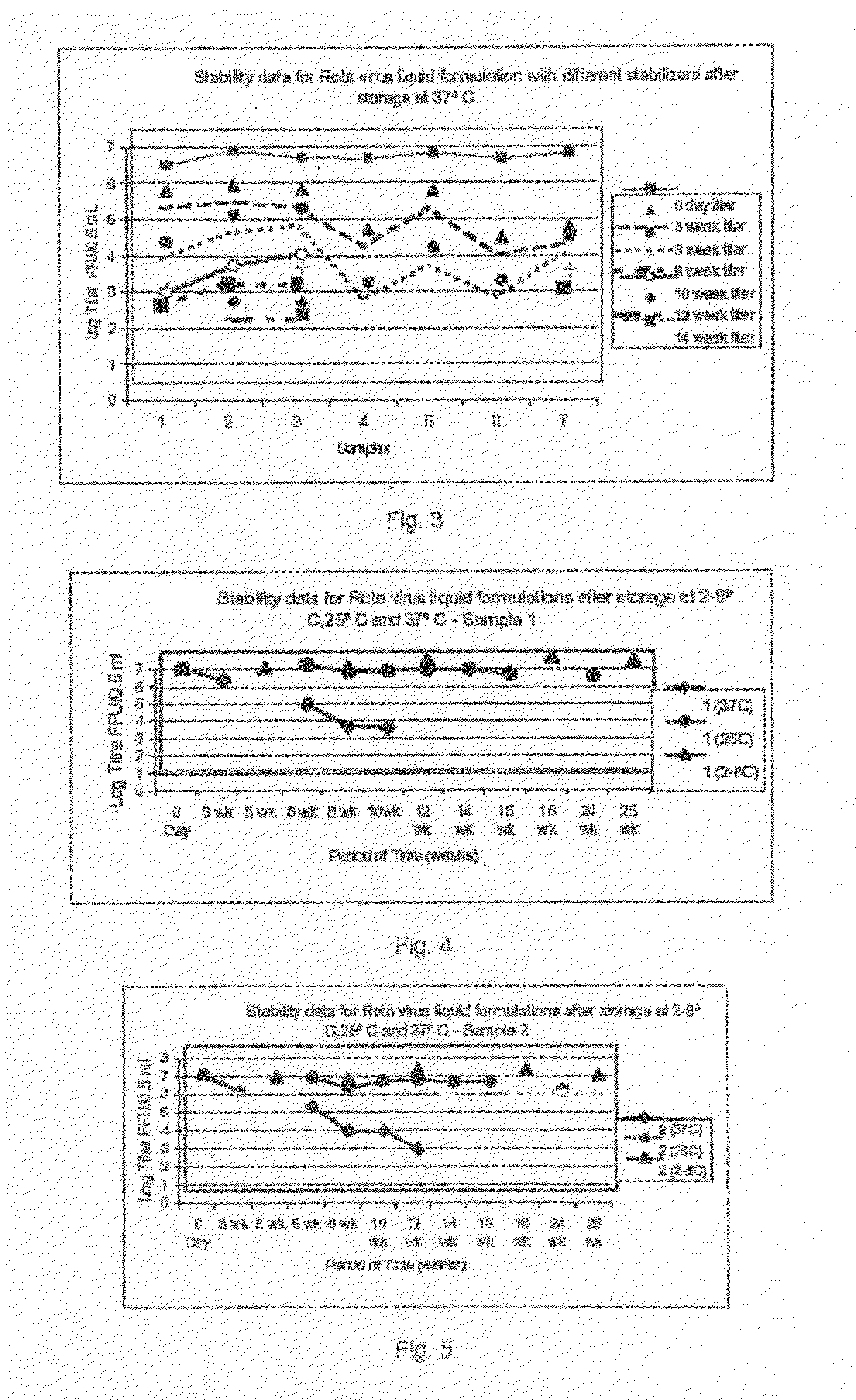
This invention provides liquid rotavirus formulations that are suitable for oral administration to human infants. In particular, the invention provides pharmaceutical compositions and vaccines, comprising a rotavirus antigen, a sugar and a carboxylate, wherein said formulation has a pH of between pH 5.0 and pH 8.0 and comprises no phosphate or less than 5 mM phosphate. The invention also provides methods of preparing said rotavirus formulations and use thereof in the prevention or treatment or rotavirus associated diseases in humans.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Large-scale production method of rotavirus vaccine
ActiveCN105969737AIncrease productionQuality improvementViral antigen ingredientsAntiviralsRotavirus RNABiological activation
The invention relates to a large-scale production method of a rotavirus vaccine, particularly a large-scale culture method of a rotavirus inactivated vaccine by using a 14L bioreactor. The method comprises the following steps: 1) revival and subculture of Vero cells; 2) cell collection and bioreactor inoculation; 3) bioreactor culture of cells; 4) solution exchange and cell washing; 5) rotavirus activation and infection; and 6) sustaining culture and collection of rotavirus. The invention aims to overcome the defect of low infectivity titer in the obtained virus since the rotavirus can not easily release cells in rotavirus vaccine large-scale production, thereby obtaining the high-titer rotavirus, and greatly enhancing the rotavirus yield. The invention also aims to overcome the defect of low adsorption infectivity of rotavirus in the mass production process, thereby greatly enhancing the rotavirus quality and further enhancing the antigenicity and immunogenicity of the rotavirus inactivated vaccine.
Owner:LIVZON GROUP VACCINE ENG
Construction of carriers for prokaryotic expression and eukaryotic expression of candidate gene of porcine rota virus vaccines
InactiveCN102041266ABiologically activeNormal biological activityViral antigen ingredientsMicroorganism based processesCandidate Gene Association StudyPorcine rotavirus vaccine
The invention discloses a construction method for carriers for prokaryotic expression and eukaryotic expression of candidate gene of porcine rotavirus vaccines VP4 and VP7, comprising: cloning and prokaryotic expression of genes of porcine rotavirus VP4, construction and transfection of the recombinant vector of genes of rotavirus VP4 and VP7 and the expression carriers of insect cell sf9, and the construction of a recombinant vector of genes of rotavirus VP4 and VP7 and the expression vector of yeast cells GS115.
Owner:SHANGHAI ACAD OF AGRI SCI
Human rotavirus VP8 recombinant protein and human rotavirus vaccine using same
The invention provides a human rotavirus VP8 recombinant protein. The amino acid sequences of the VP8 recombinant protein include an amino acid sequence of a human rotavirus VP8 protein (hereinafter referred to as VP8 protein) and an amino acid sequence of exogenous polypeptide. The isoelectric point of the exogenous polypeptide is less than or equal to 5. The invention also provides a human rotavirus vaccine prepared based on the VP8 recombinant protein. Compared with the prior art, the VP8 recombinant protein provided by the invention can be expressed by fusing the VP8 protein with negativecharge-containing amino acid polypeptide fragments in tetanus toxin and / or diphtheria toxin proteins, and the water solubility and yield of the rotavirus VP8 protein expressed by escherichia coli areimproved; and moreover, the obtained fused protein has good immune ability, and the prepared vaccine has good effect.
Owner:CHENGDU MAXVAX BIOTECHNOLOGY LLC
Human rotavirus Delta VP8* subunit recombinant protein and application thereof
ActiveCN103319604BImprove immune efficiencyFast titerBacteriaViral antigen ingredientsAntiendomysial antibodiesTetanus
The invention relates to human rotavirus Delta VP8* subunit recombinant protein and application thereof. The human rotavirus Delta VP8* subunit recombinant protein comprises a T cell epitope P2 in tetanus toxin and a rotavirus Delta VP8* subunit. By the recombinant protein disclosed by the invention, the immune efficacy of a Delta VP8* subunit vaccine can be greatly improved; faster and stronger neutralization antibody titer can be induced; moreover, anti-p[4] genotype specific rotavirus cross neutralization antibody of high titer can be induced; simultaneously, the potential risk of inducing intussusception by taking attenuated rotavirus vaccine orally can be overcome; therefore, the recombinant protein is applicable to preparing a rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
One coding gene of rotavirus VP6 protein and its application
InactiveCN1772902AHigh expressionPrevention of Acute DiarrheaViral antigen ingredientsDigestive systemAcute diarrheaRotavirus RNA
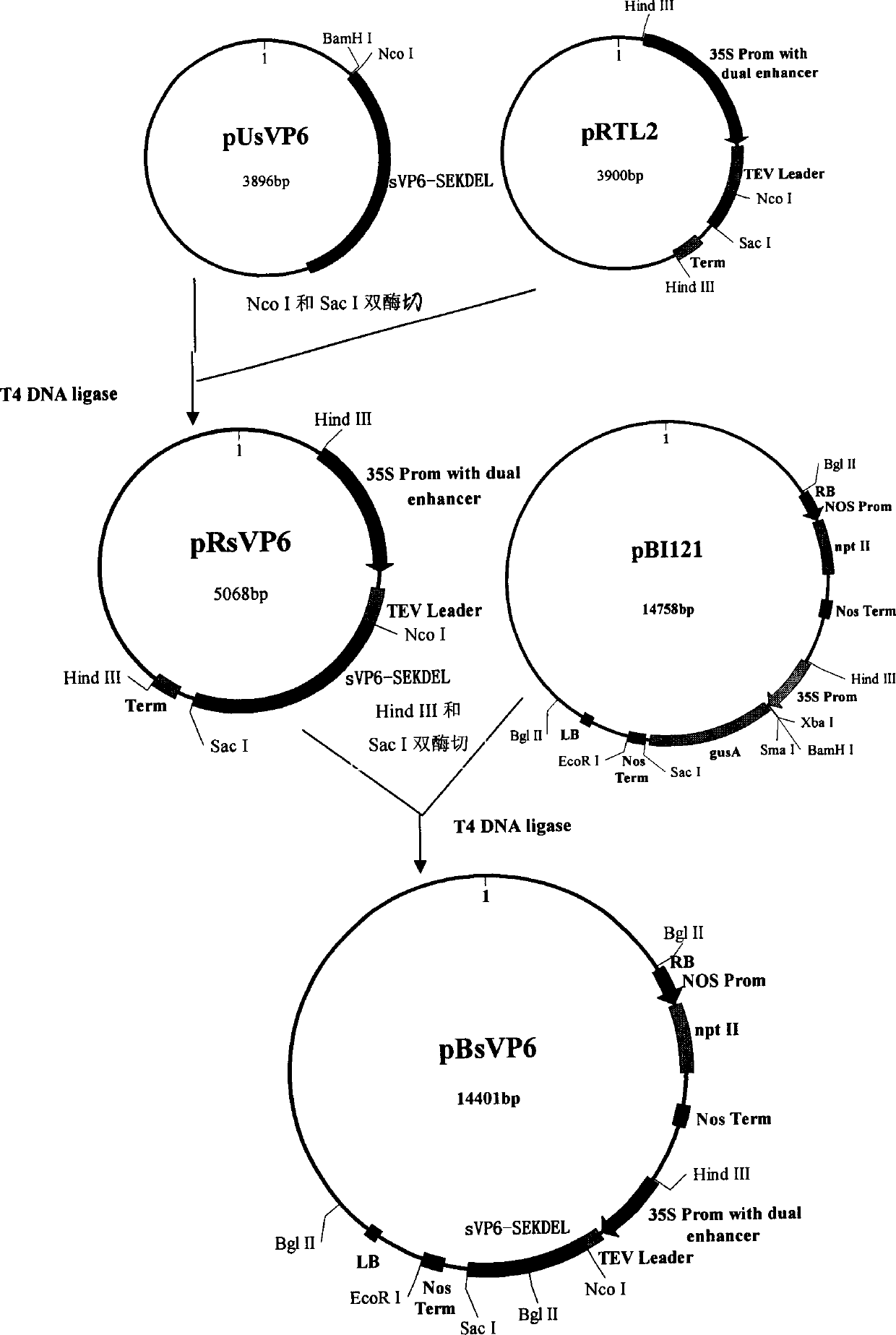
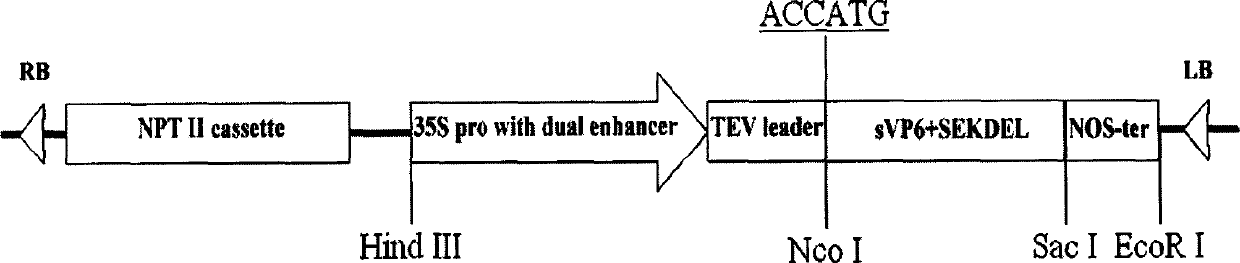
The present invention discloses one coding gene of rotavirus VP6 protein and its application, and aims at providing one coding gene of rotavirus VP6 protein with high expression amount in dicotyledonous plant, especially clover, method of producing oral rotavirus vaccine with the coding gene and the obtained oral vaccine. The rotavirus VP6 protein coding gene has one of the following nucleotide sequences: 1. DNA sequence of SEQ ID No. 1 in the sequence list; 2. DNA sequence of SEQ ID No. 2 in the sequence list; 3. the nucleotide sequence capable of hybridizing in high strict condition with the DNA sequence limited by SEQ ID No. 1 or SEQ ID No. 2; and 4. DNA sequence possessing over 80 % homology with the SEQ ID No. 1 or SEQ ID No. 2 limited DNA sequence and high expression amount in dicotyledonous plant. The oral rotavirus vaccine is used in preventing various rotavirus infection caused acute diarrhea of infant and young animal.
Owner:CHINA AGRI UNIV
VP7 gene of human rotavirus and composition for diagnosis of human rotavirus infection comprising primer or probe specific to thereof
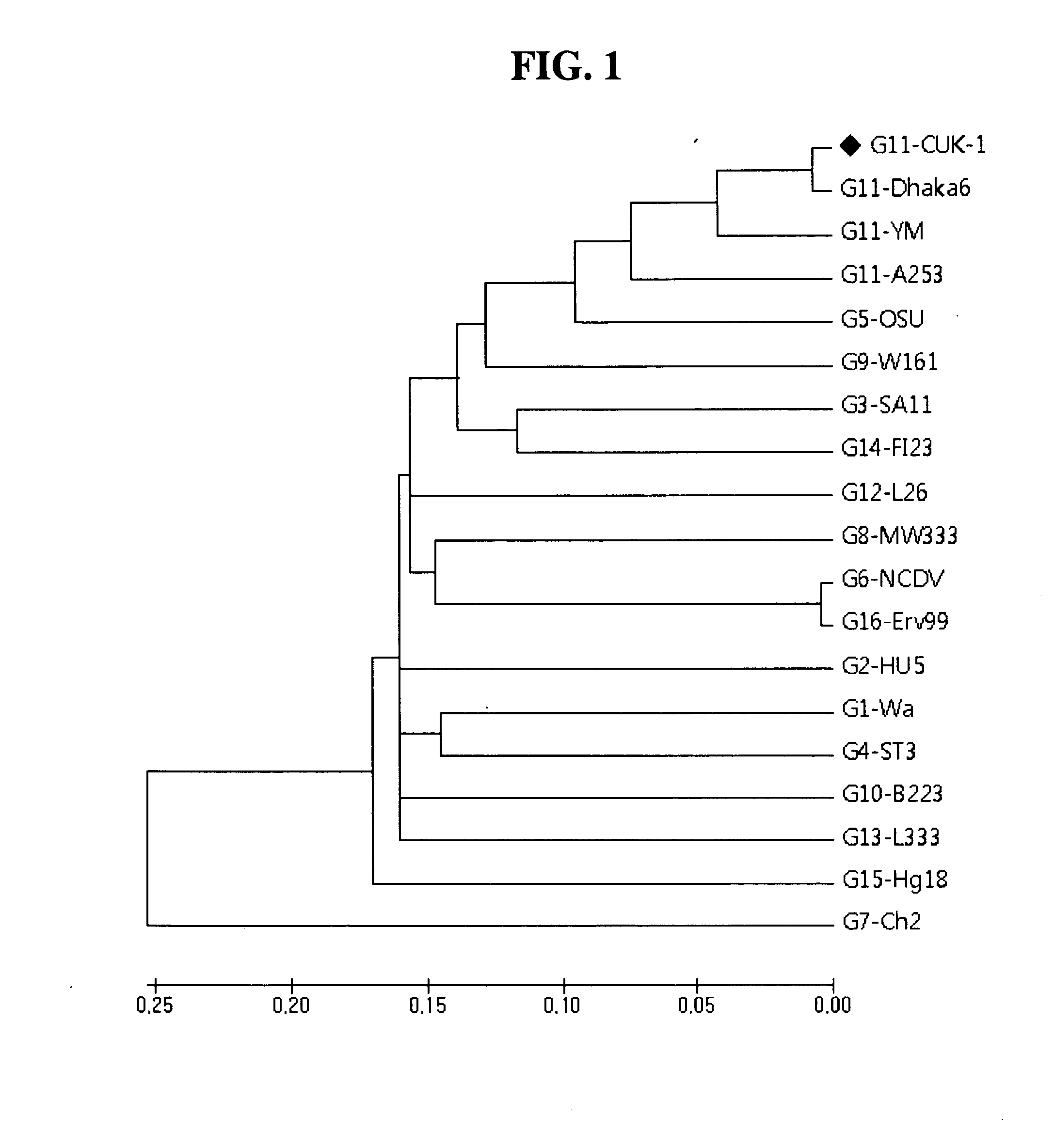
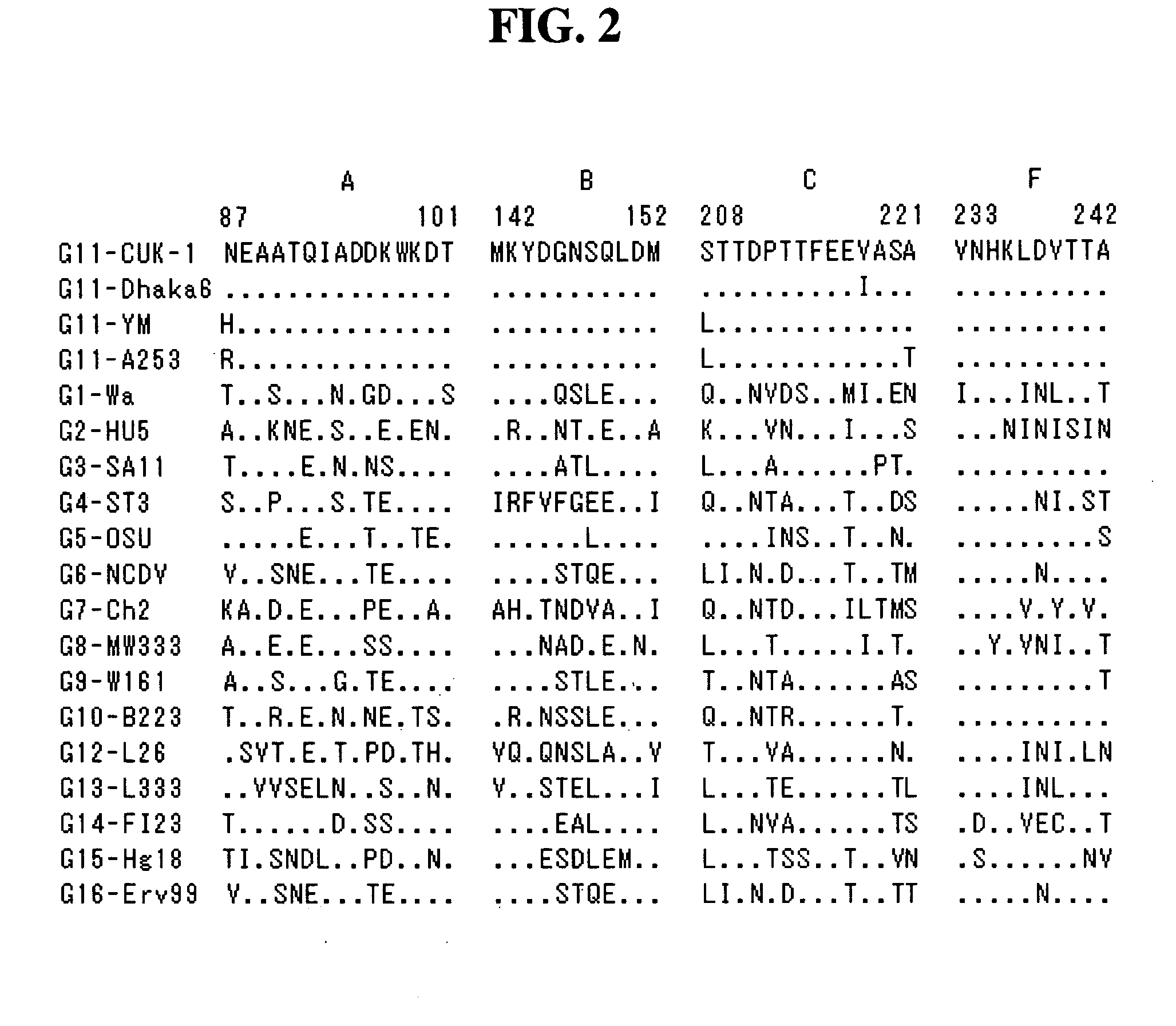
The present invention relates to a VP7 gene of human rotavirus and a composition for diagnosis of human rotavirus infection comprising primer or probe specific to thereof, and more particularly to a VP7 gene encoding the amino acid sequence set forth in SEQ ID NO:2 and a composition for diagnosis of human rotavirus infection comprising primer or probe specific to thereof. The human rotavirus VP7 gene according to the present invention will be useful for diagnosis of novel G11 type human rotavirus infection, and will be used for development of rotavirus vaccine.
Owner:MSD KOREA
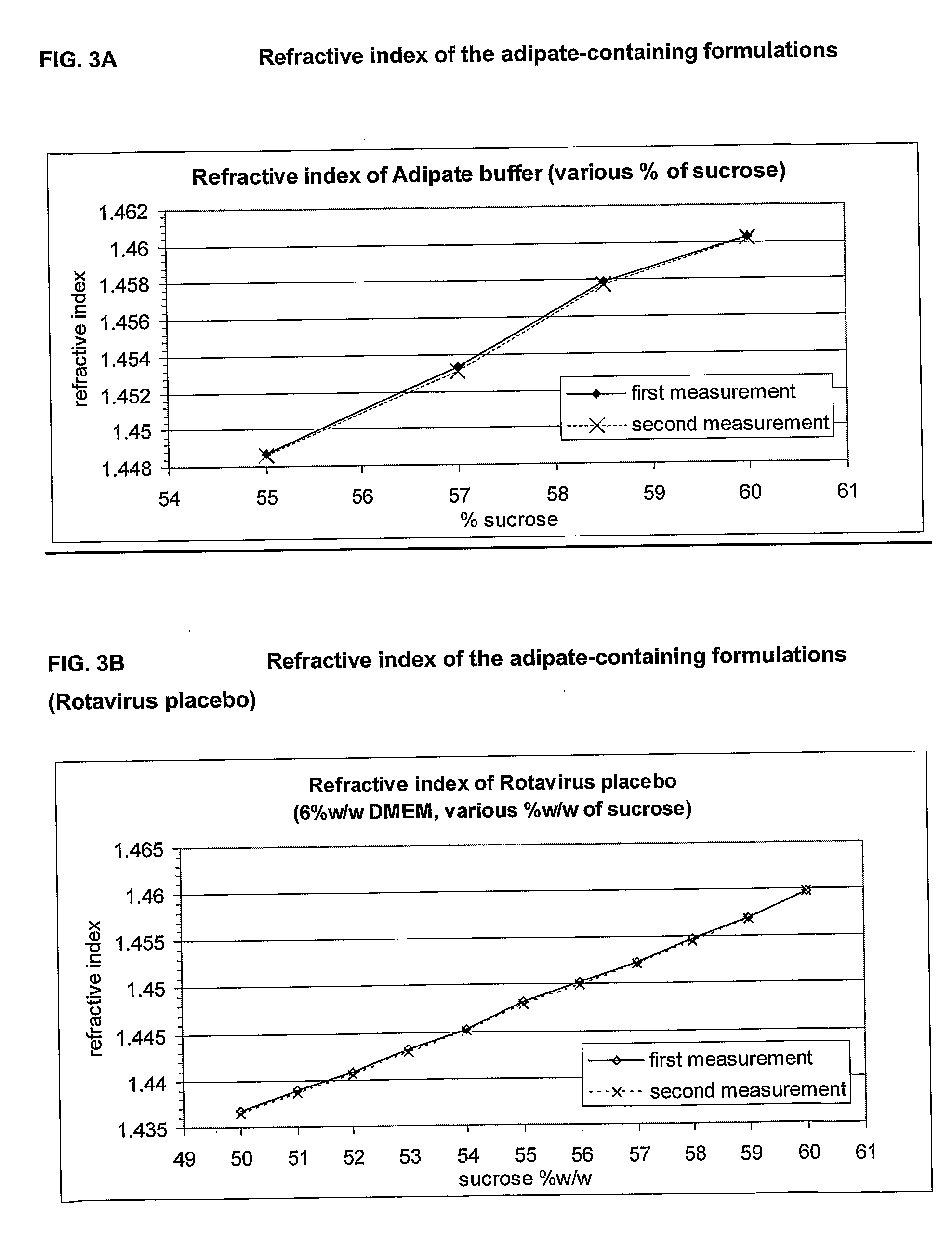
Stable, dried rotavirus vaccine, compositions and process for preparation thereof
The present invention provides novel lyophilized rotavirus vaccine formulations and methods of their preparation. The formulations include vaccine stabilizers, resulting in a vaccine formulation with enhanced stability and minimal loss of potency. The rotavirus vaccine formulations comprise an advantageous ratio of a disaccharide (such as sucrose) to an amino acid (such as glycine). The lyophilization results in a virus formulation with 100% virus preservation and residual moisture from about 0.8% to 1.4%.
Owner:SERUM INST OF INDIA PTE LTD
Multivalent huma-bovine retavirus vaccine
The present invention provides vaccine compositions for protection against human rotaviral disease without significant reactogenicity. Human×bovine reassortant rotavirus comprising each of the four clinically most important VP7 serotypes of human rotavirus are combined in a multivalent formulation which provides a high degree of infectivity and immunogenicity without producing a transient febrile condition. Methods for producing an immunogenic response without producing a transient febrile condition are also provided.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF HEALTH & HUMAN SERVICES THE
Thermostable freeze dried rotavirus vaccine formulation and process to prepare thereof
ActiveUS10532093B2Minimizes potency lossesDecreasing refrigeration “Powder deliveryViral antigen ingredientsFreeze-dryingMedicine
Owner:MSD WELLCOME TRUST HILLEMAN LAB PVT LTD
Composition useful as rotavirus vaccine and a method therefor
Compositions and methods related to live or live attenuated pre-conditioned and typical viruses such as rotaviruses are disclosed. The live attenuated rotaviruses exhibit better stability characteristics and are useful for the prevention of a rotavirus infection and / or rotavirus gastroenteritis in children.
Owner:BHARAT BIOTECH INTERNATIONAL
Porcine rotavirus vaccine, antigen for preparing vaccine and coding sequence of antigen
ActiveCN111569056AGood immune effectReduce manufacturing costViral antigen ingredientsVirus peptidesEscherichia coliNucleotide
The invention relates to the technical field of immunology, in particular to a porcine rotavirus vaccine, an antigen for preparing the vaccine and a coding sequence of the antigen. The invention provides the antigen with an amino acid sequence as shown in SEQ ID NO:1, and the antigen comprises a nucleotide sequence for coding the antigen. The invention further provides a method for preparing the antigen. The antigen for preparing the porcine rotavirus vaccine is relatively good in immune effect, the soluble expression quantity of the antigen in escherichia coli is obviously increased by modifying the nucleotide sequence encoding the antigen, the preparation process is simple, and the method is suitable for low-cost large-scale industrial production.
Owner:山东信得动物疫苗有限公司
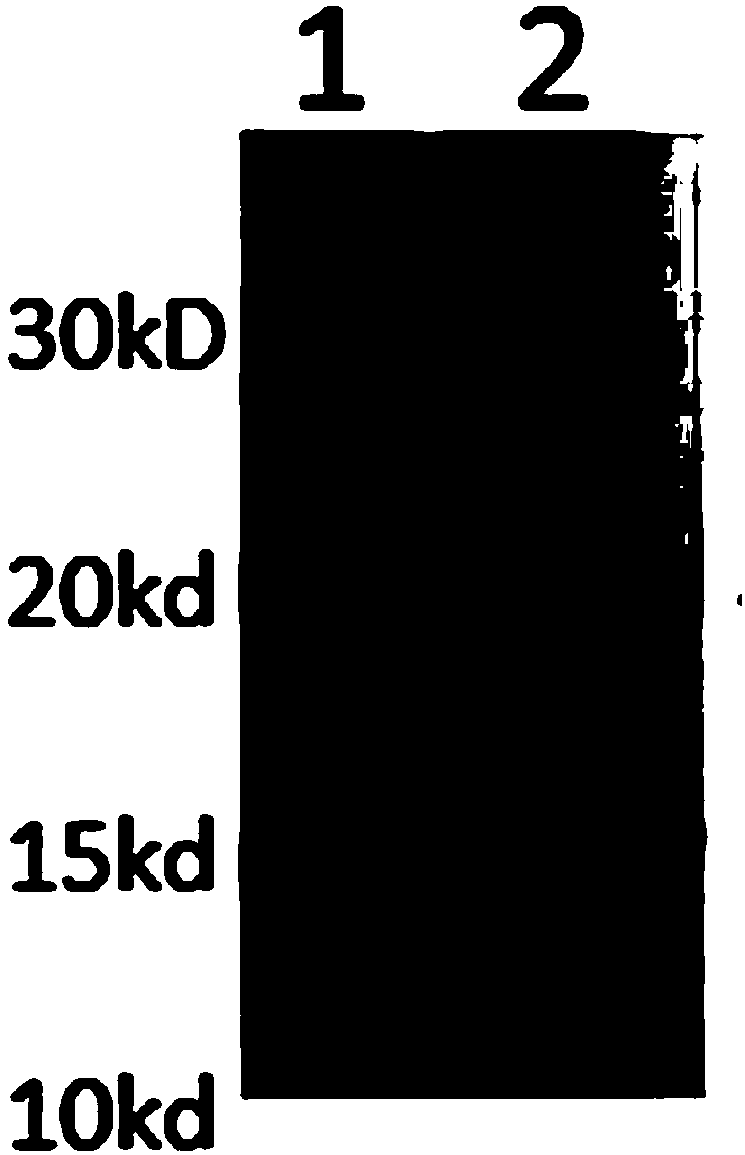
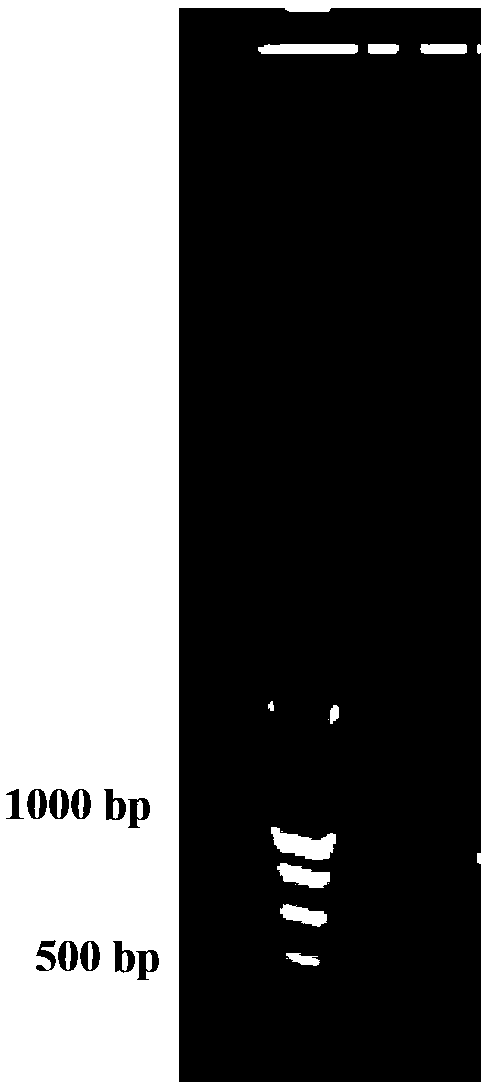
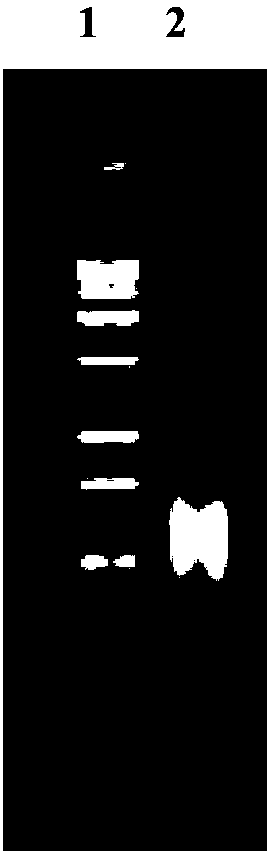
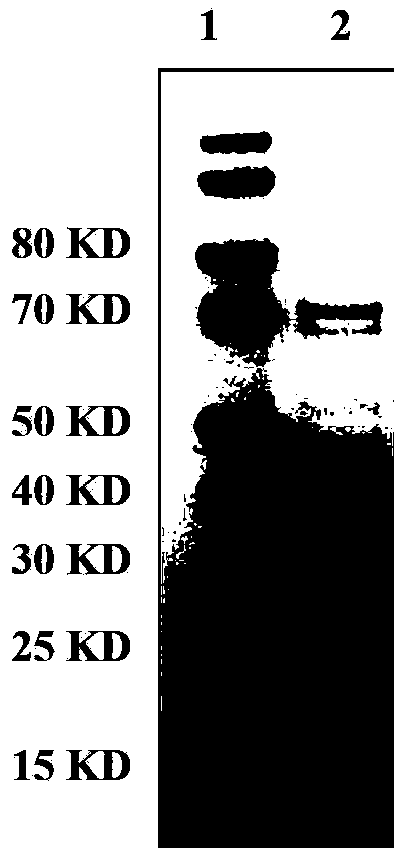
Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof
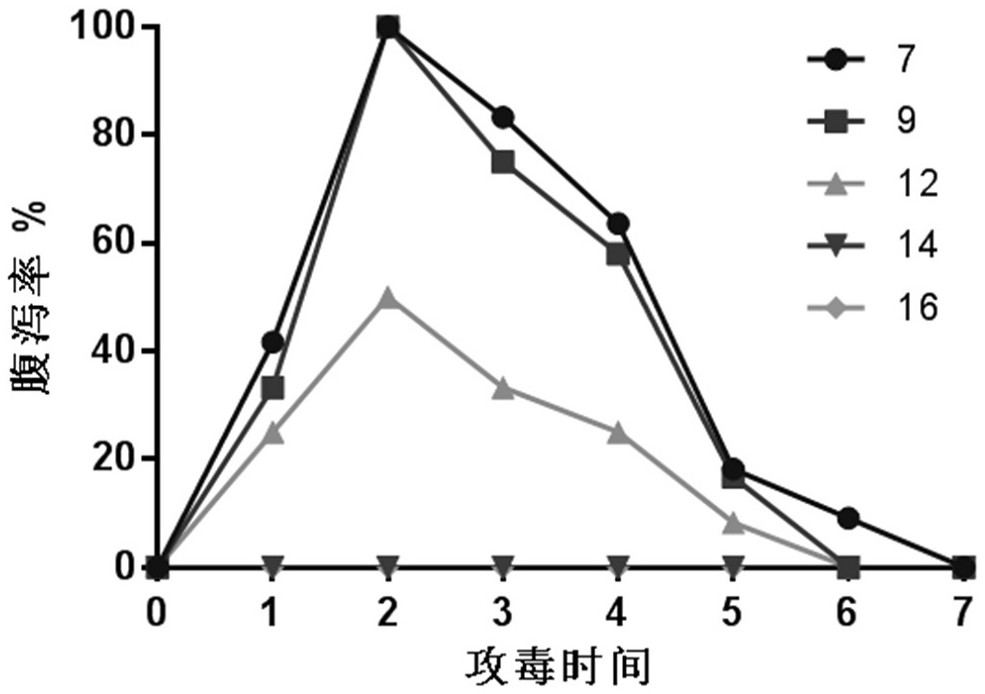
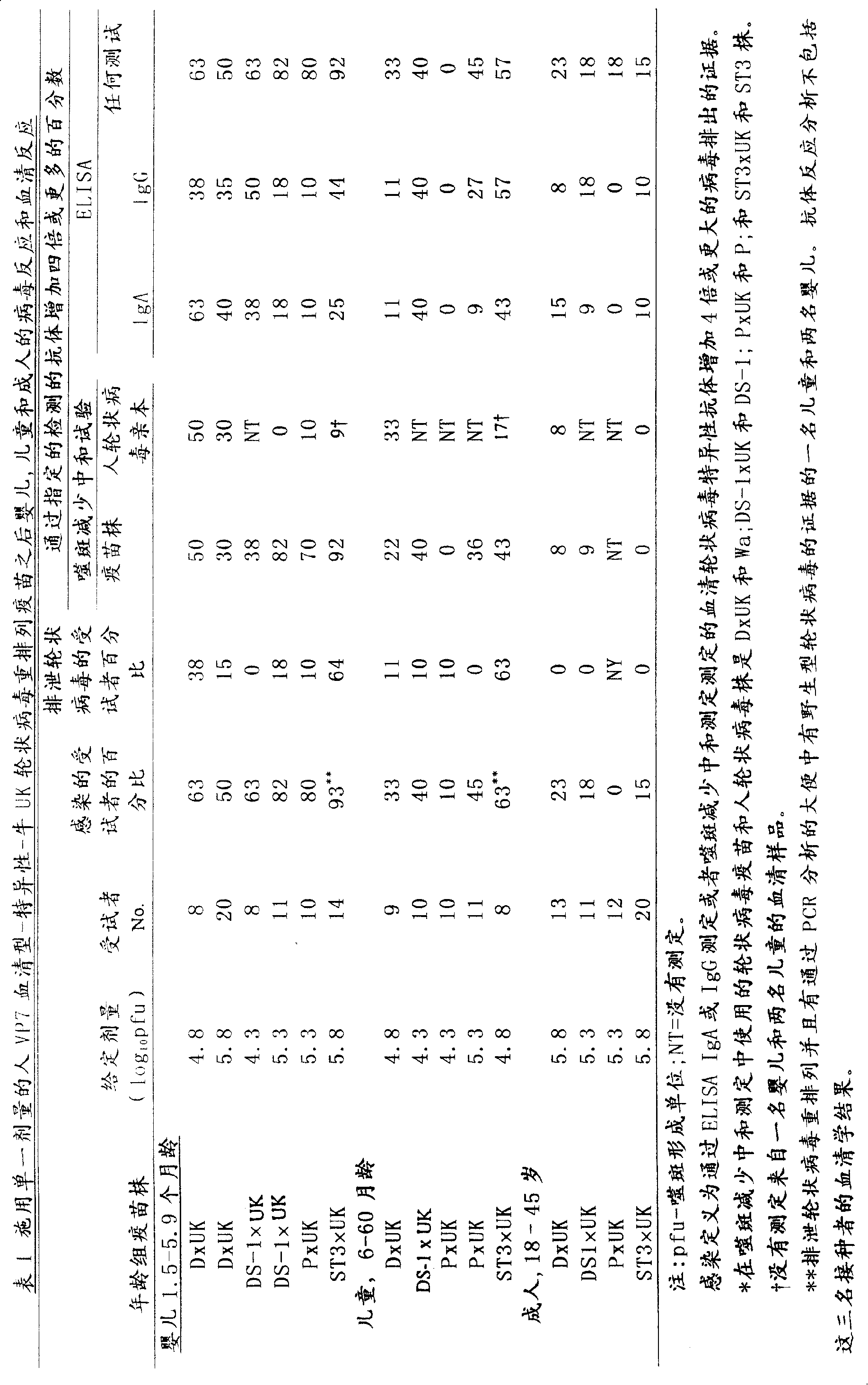
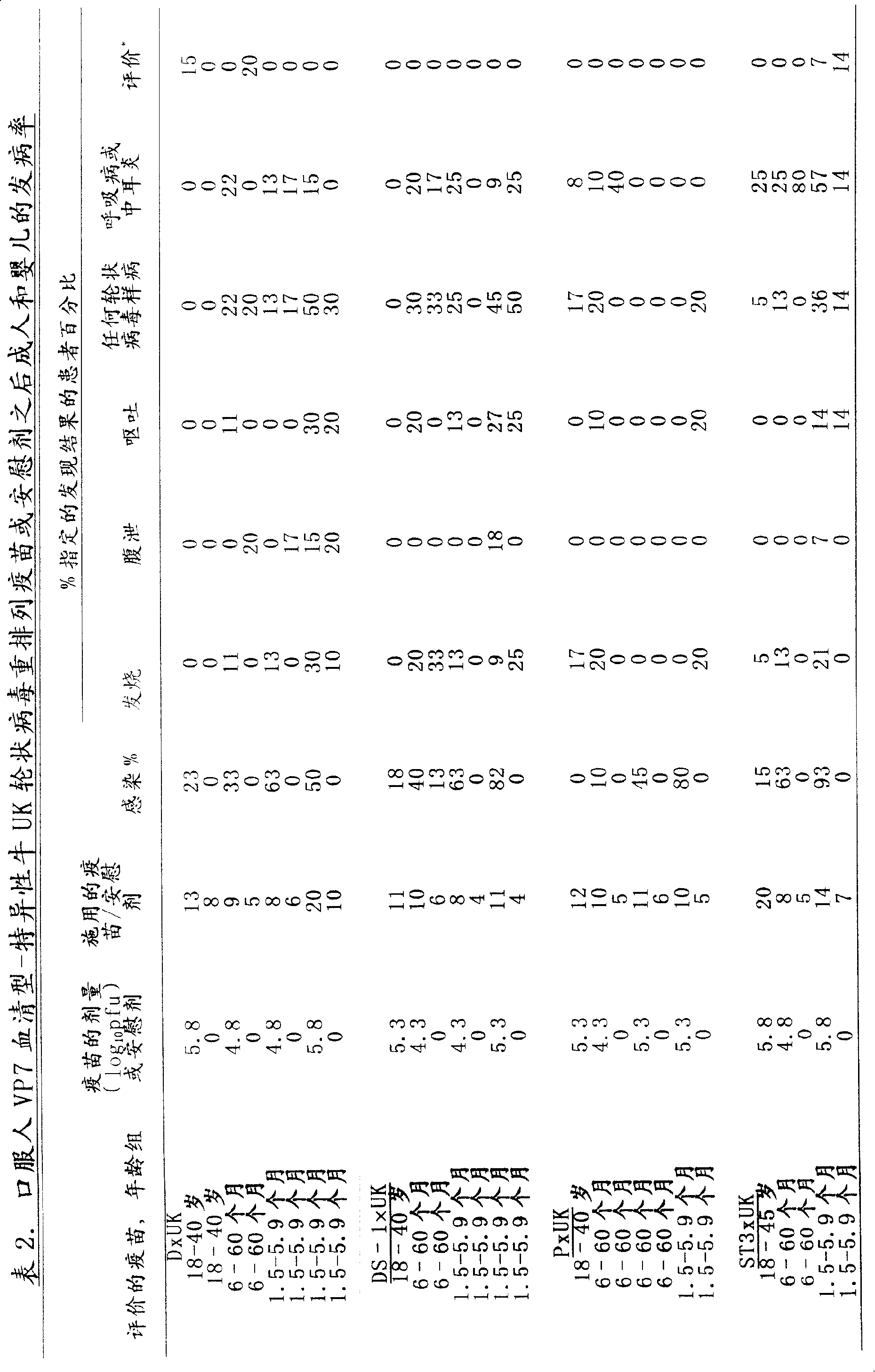
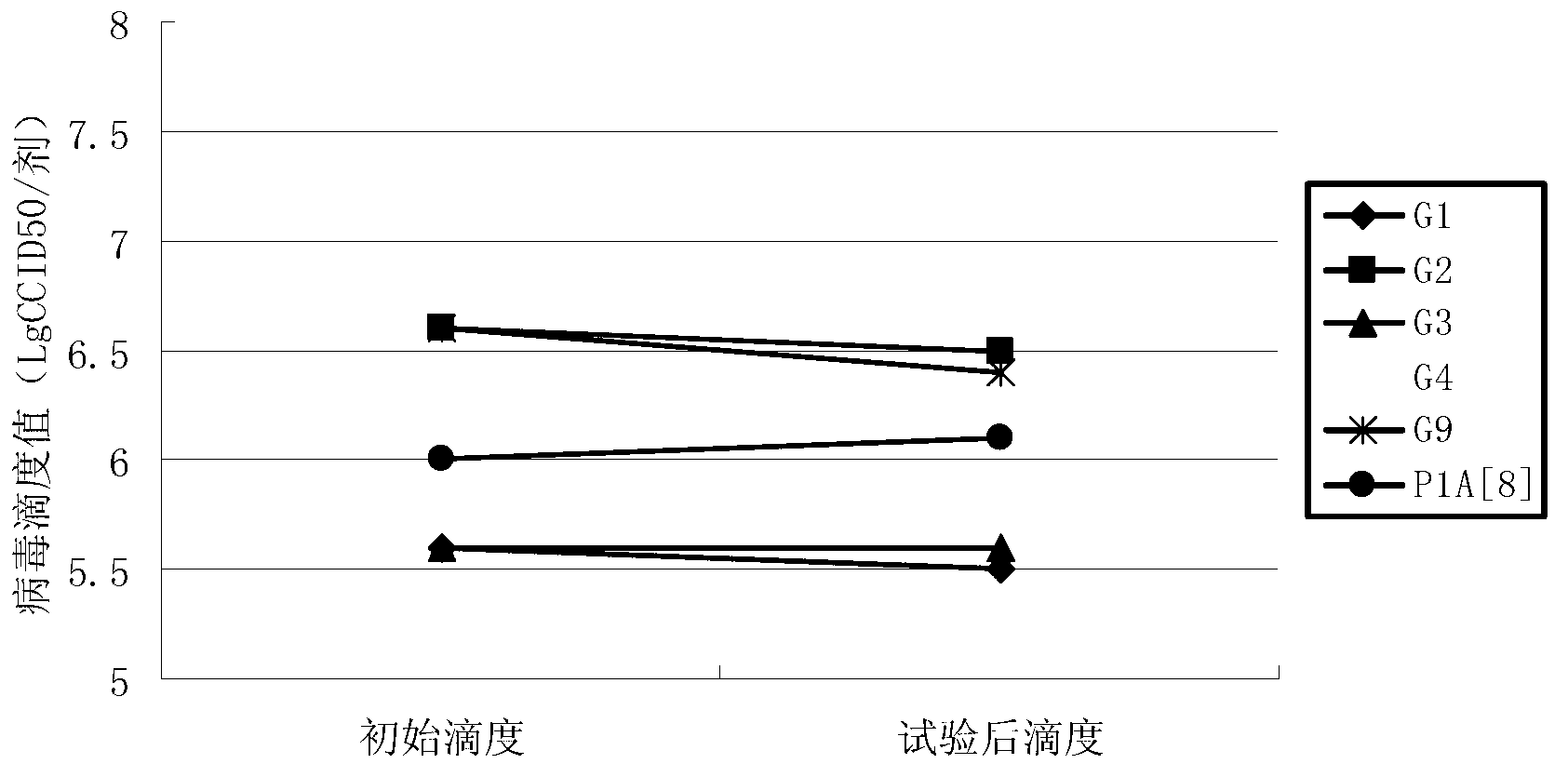
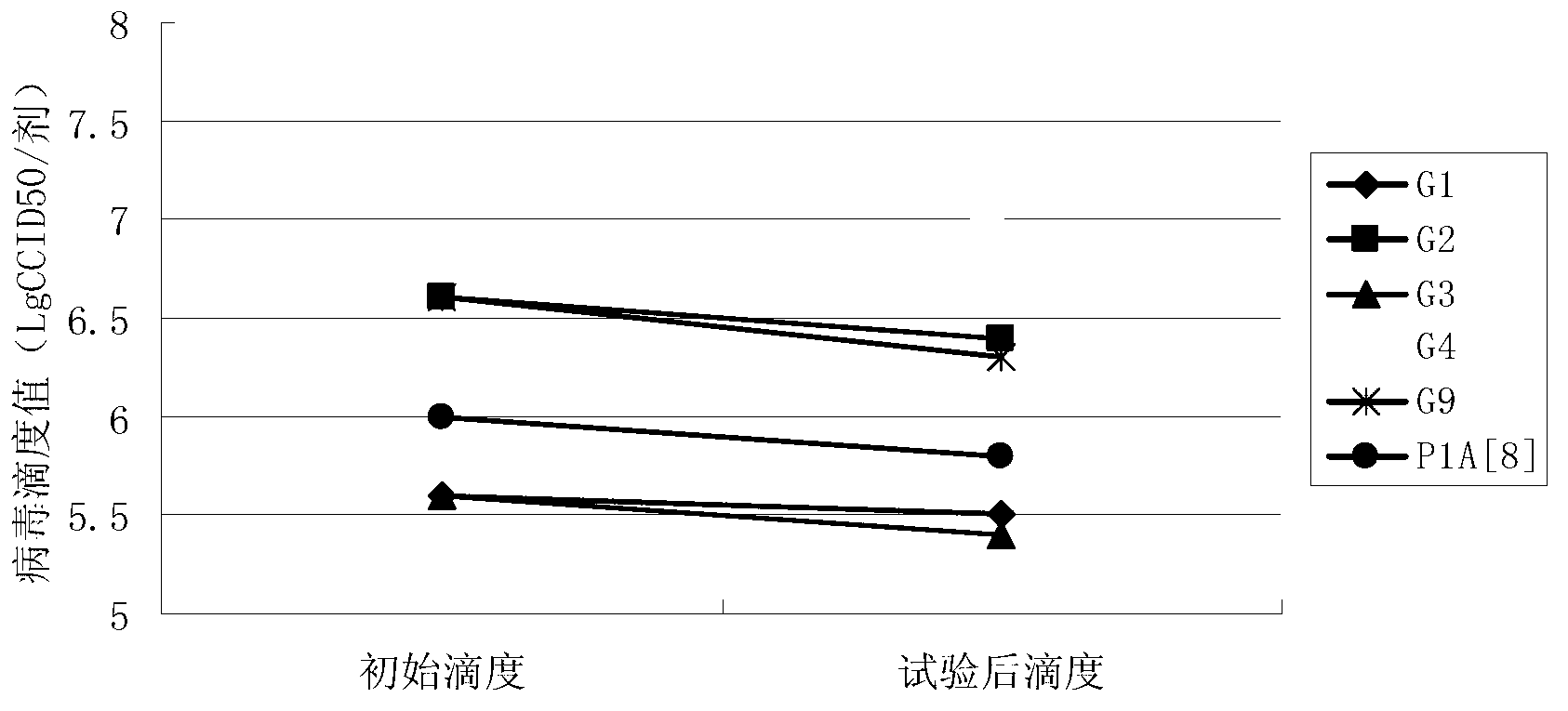
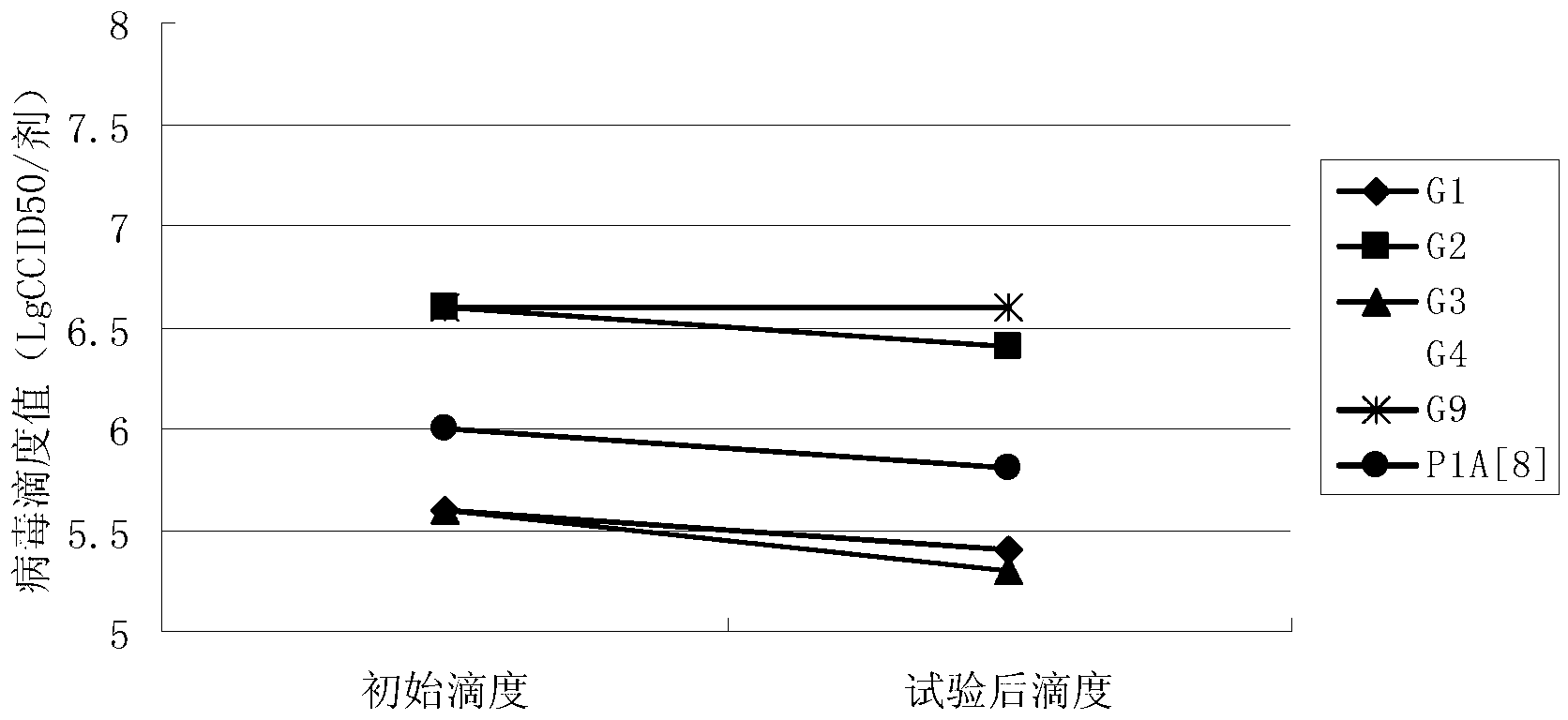
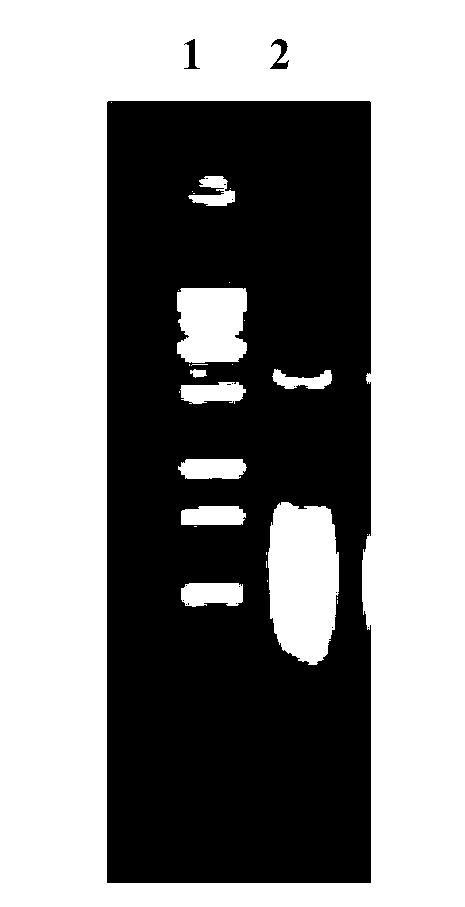
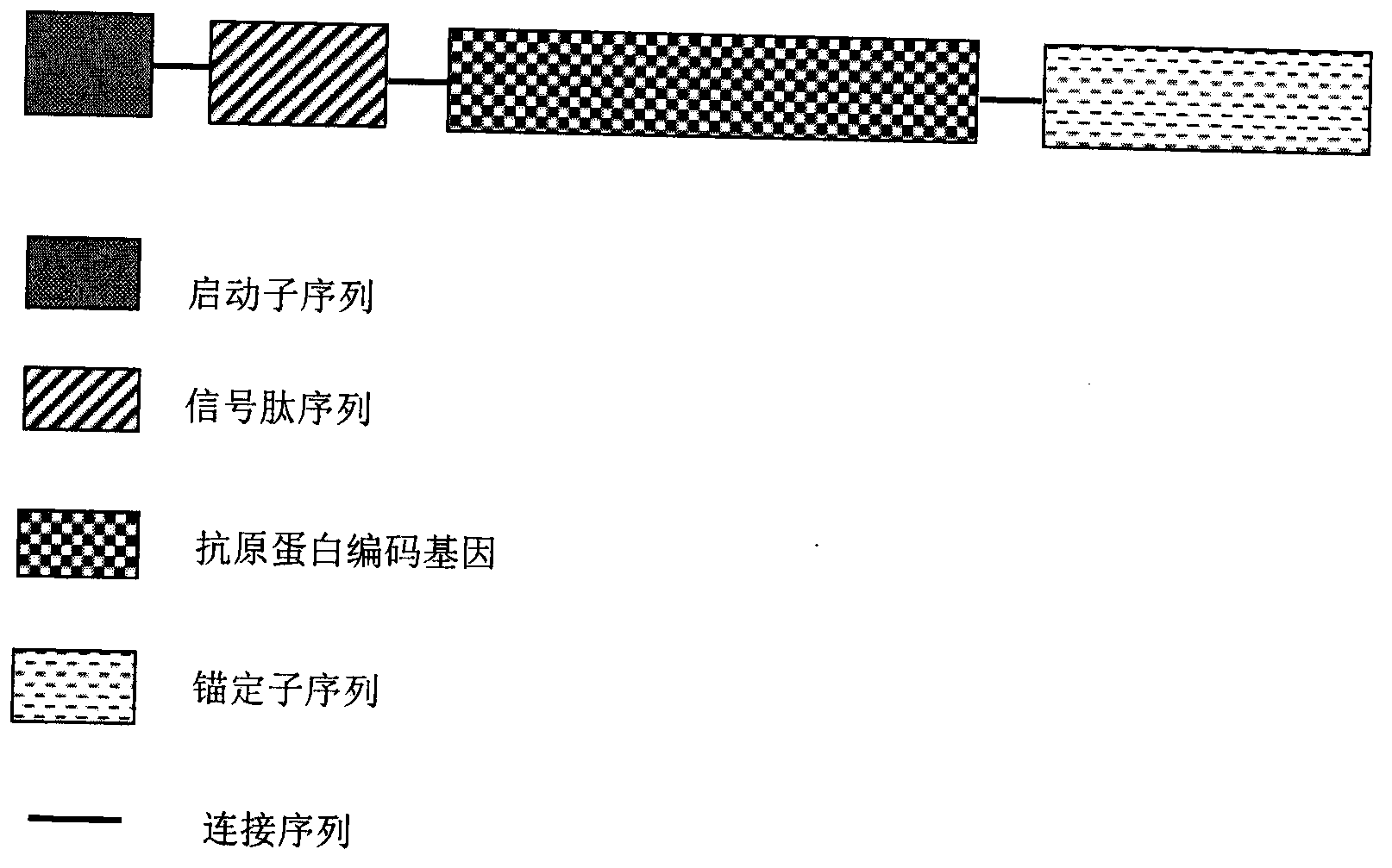
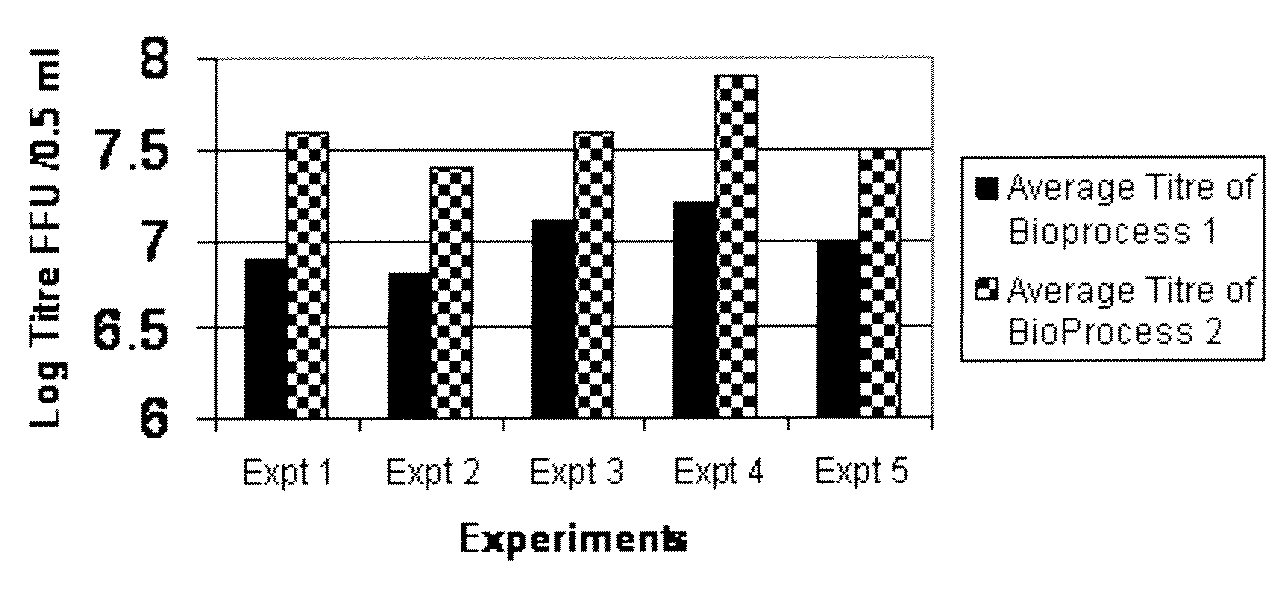
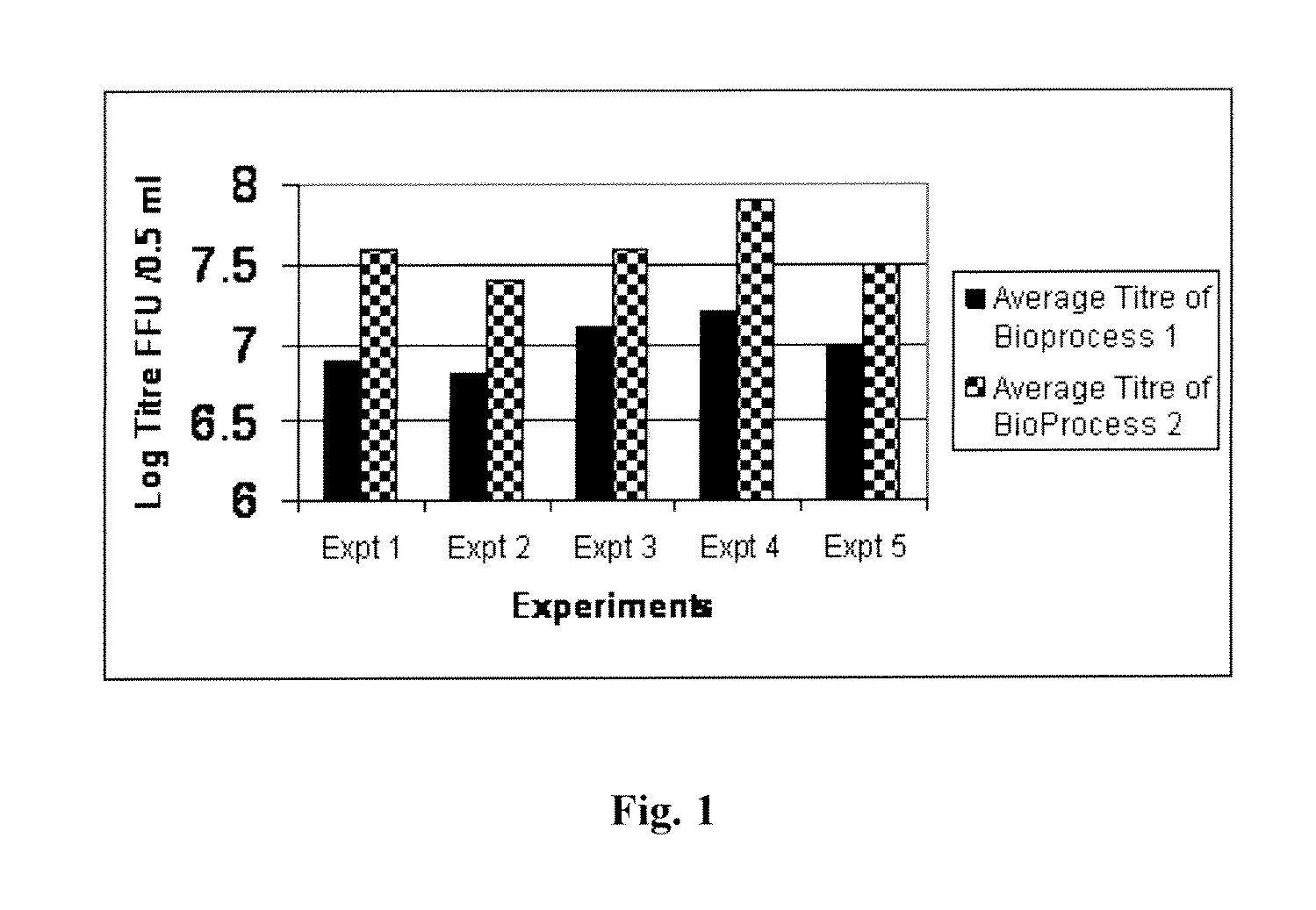
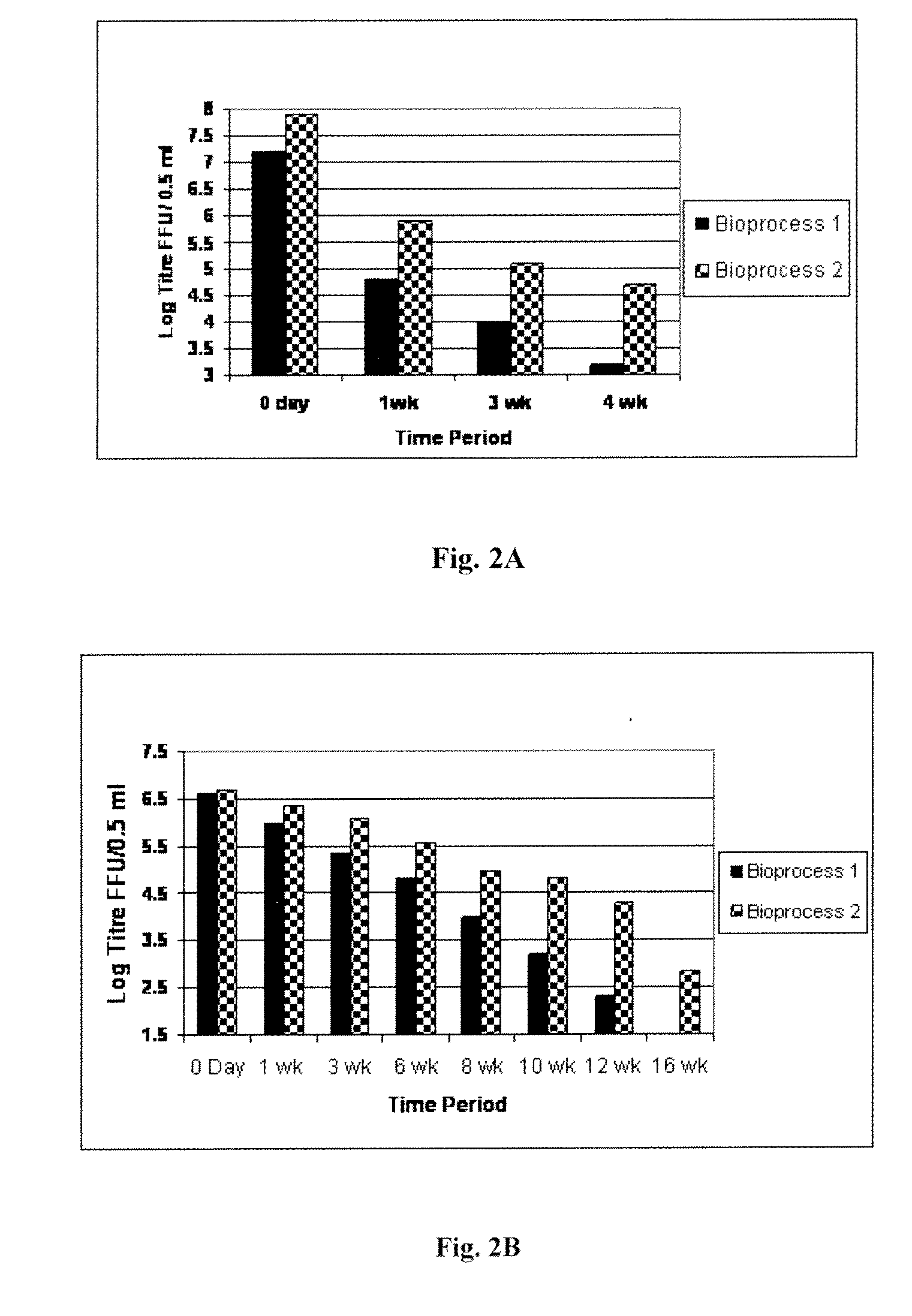
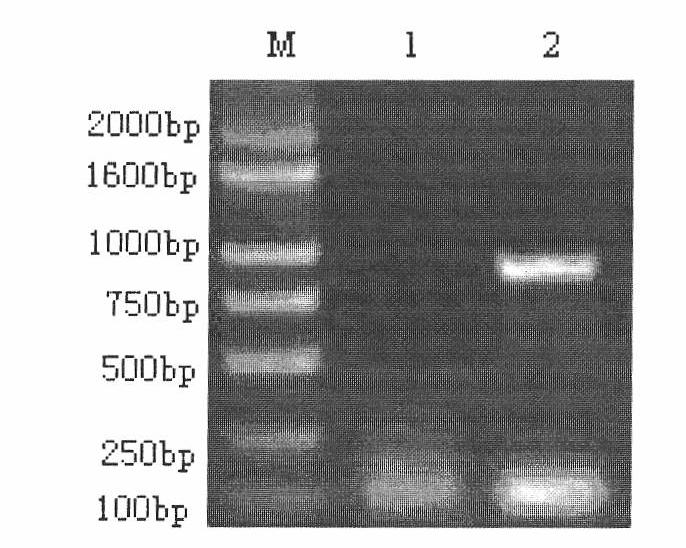
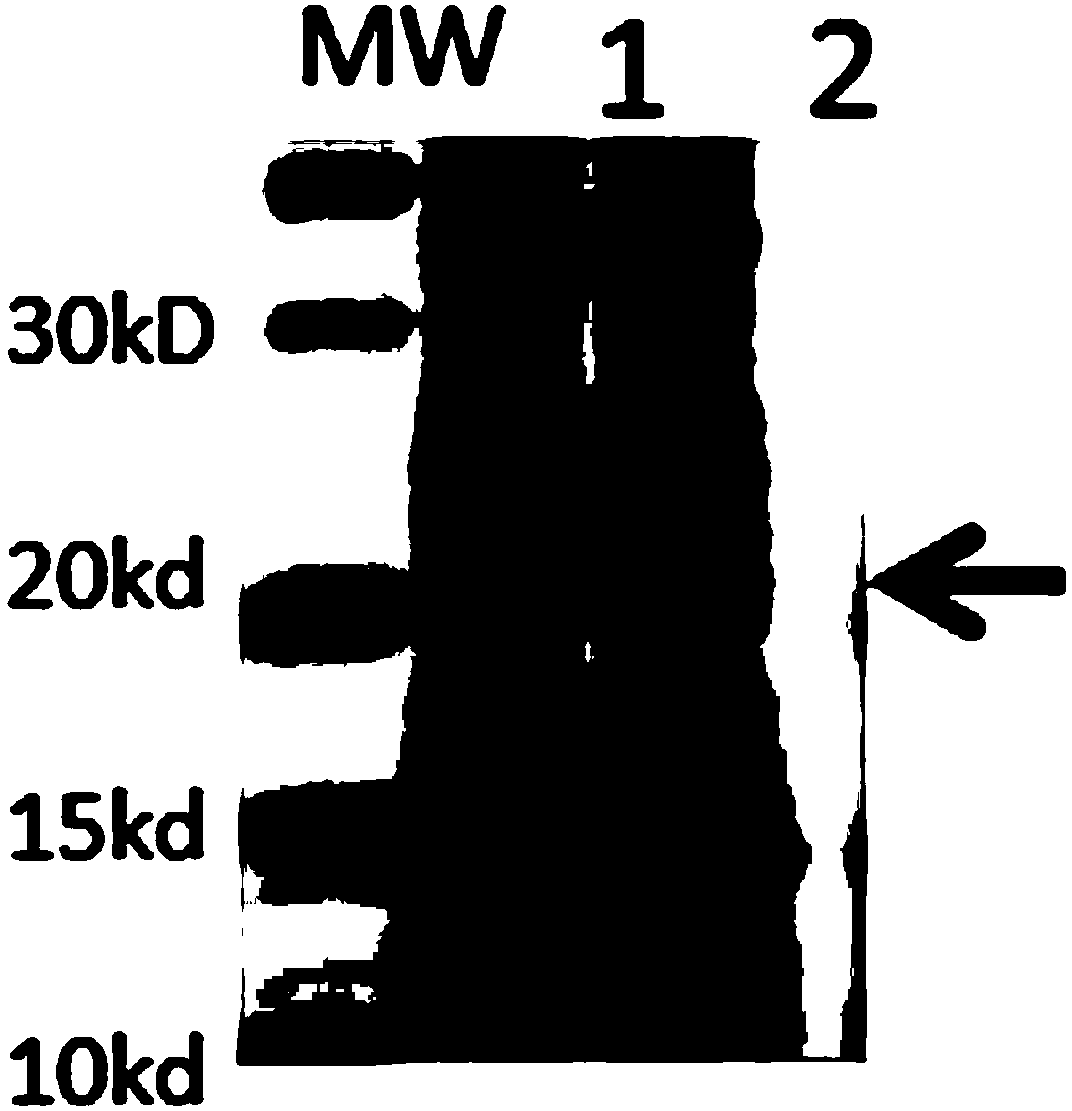
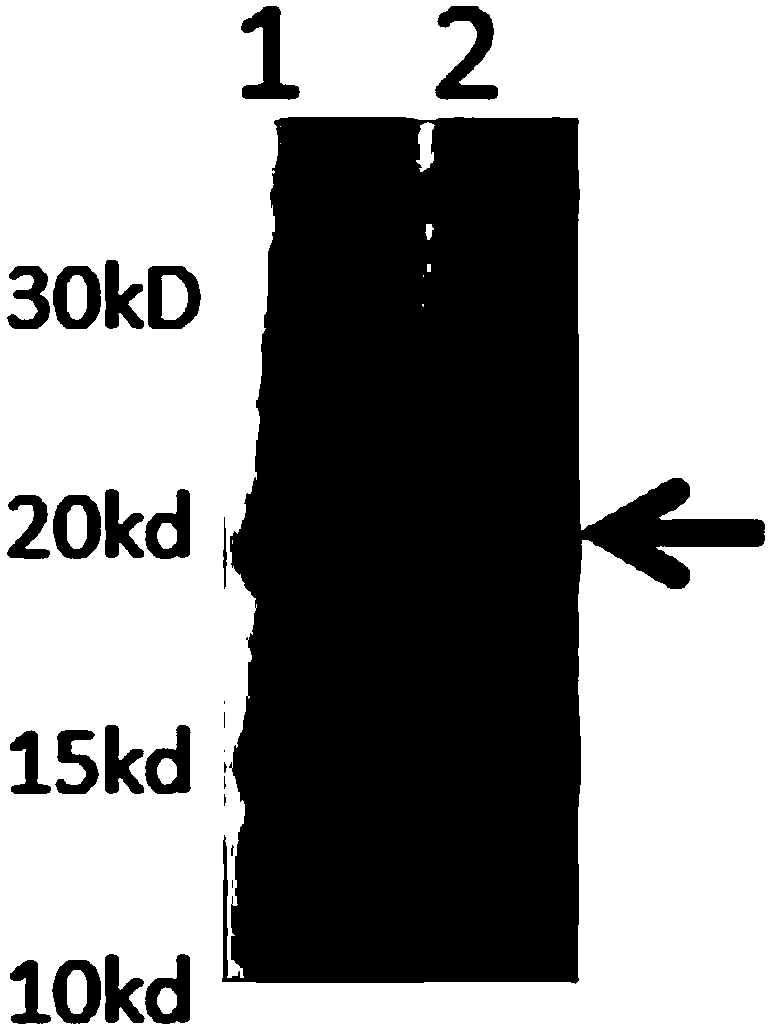
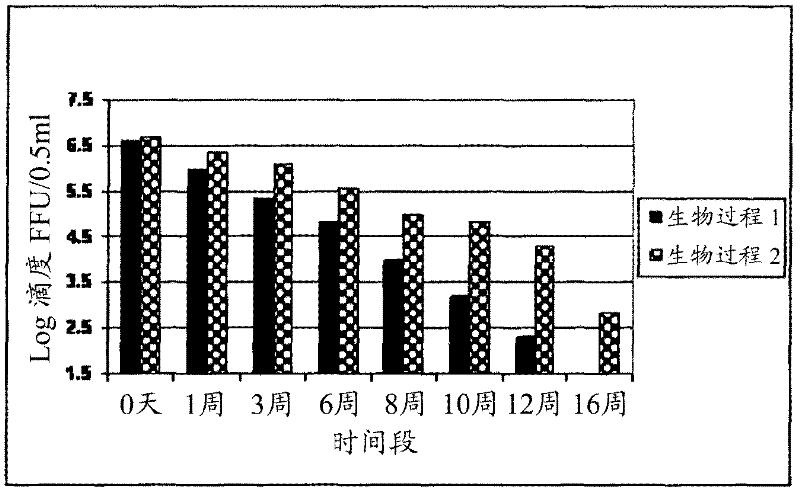
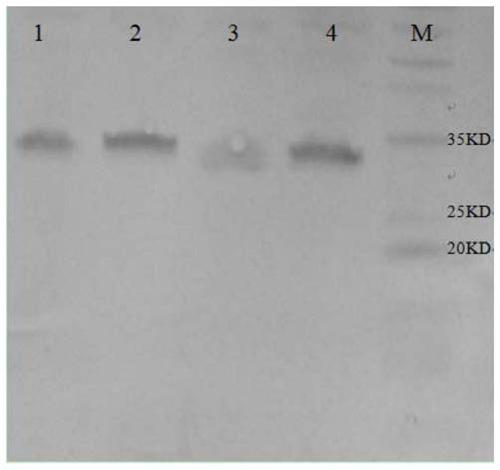
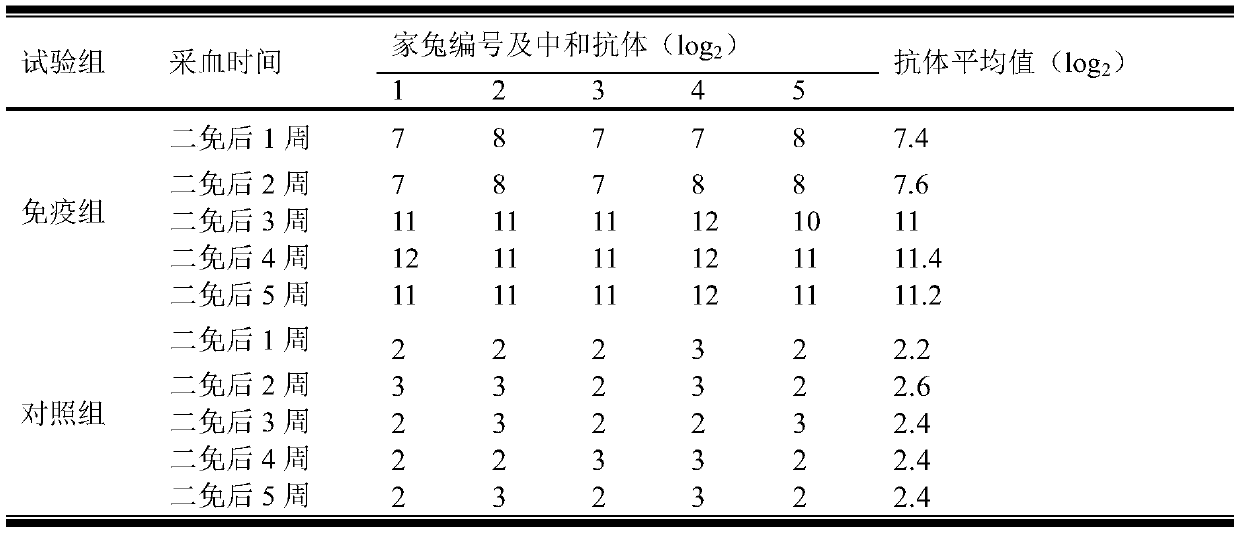
InactiveCN103304671AOvercoming the potential risk of intussusceptionImprove immune efficiencyViral antigen ingredientsMicroorganism based processesViral VaccineGenotype
The invention relates to a human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein, wherein the amino acid sequence of the protein is shown in SEQ ID NO.2. Furthermore, the invention provides a coding gene and a preparation method of the protein as well as an application of the protein in preparation of a rotavirus vaccine. Two segments P[8]deltaVP8* and P[6]deltaVP8* are connected in serial to be expressed into the human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein; the protein can simultaneously induce neutralizing antibodies of major P genotypes P [8], P[4] and P[6] of the human rotavirus, overcome the shortcoming of an existing vaccine which can induce the generation of an antibody only capable of resisting one genotype and greatly enhance immune efficiency; and meanwhile, the protein can avoid a potential risk that oral administration of attenuated vaccine rotavirus possibly induces intestinal invagination, and is applicable to preparation of rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Rotavirus challenge animal model and establishment method thereof
ActiveCN113994925AImprove stabilityCompounds screening/testingAnimal husbandryBiotechnologySodium bicarbonate
The invention relates to a rotavirus challenge animal model and an establishment method thereof, and belongs to the technical field of biology. The invention provides a rotavirus challenge animal model. The rotavirus challenge animal model is a suckling mouse filled with an anti-acid protective agent (such as disodium adipate, sodium bicarbonate, sodium succinate, sodium citrate, sodium malate and sodium acetate) and rotavirus. Researches find that before or when the rotavirus is used for counteracting toxic substances, the rotavirus morbidity and the model stability of the large-day-old suckling rats can be effectively improved by irrigating the large-day-old suckling rats with the anti-acid protective agent; the large-day-old suckling mouse can complete the complete immune process of the vaccine; therefore, besides the virulence evaluation of the rotavirus strain, the rotavirus challenge animal model also has an application prospect in the evaluation of the effectiveness of the rotavirus vaccine.
Owner:BEIJING CELL FUSION BIOTECHNOLOGY CO LTD +1
Bovine rotavirus VP8* subunit recombinant chimeric protein and application thereof
ActiveCN104479028AImprove immune efficiencyReduce economic lossViral antigen ingredientsAntiviralsBovine rotavirusGenotype
The invention provides a bovine rotavirus VP8* subunit recombinant chimeric protein. According to the bovine rotavirus VP8* subunit recombinant chimeric protein, a T cell epitope polypeptide P2 in a tetanus toxin is introduced into an epitope vaccine of a bovine rotavirus VP8* subunit multi-copy chimeric gene recombinant protein, so that the immune efficacy of the epitope vaccine can be greatly enhanced, cross neutralizing immune protection can be induced, higher neutralizing antibody titer can be induced, and a high-titer anti-P[5] and P[11] genotype-specific rotavirus neutralizing antibody can be induced. The bovine rotavirus VP8* subunit recombinant chimeric protein provided by the invention can be used for preventing and controlling both G6 and G10 type rotaviruses which are mainly pandemic within a world range, and is suitable for preparing a safe and low-cost bovine rotavirus vaccine; in addition, the bovine rotavirus vaccine can be used for effectively reducing the economic loss brought by rotavirus gastroenteritis to cattle rearing by being matched with other rotavirus vaccines researched and developed at present, and has wide market prospects.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Multivalent huma-bovine retavirus vaccine
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF HEALTH & HUMAN SERVICES THE
Rotavirus vaccine for oral administration
ActiveCN103316336AImprove stabilityImprove securityViral antigen ingredientsAntiviralsAntigenOral medication
The invention provides a rotavirus vaccine for oral administration. The rotavirus vaccine contains rotavirus antigen, carbohydrate, phosphate and carboxylate. The rotavirus vaccine for oral administration can be adopted to raise stability of vaccine antigenic components, has good stability at 37 DEG C, 2-8 DEG C and 25 DEG C, has stable antigen activity, can enhance stomach acid resistance after oral administration of the vaccine finished product, and has good immune response stimulating capability and good safety. The rotavirus vaccine contains no other humanized or animal-based protein, has better safety, requires lower cost, and is suitable for industrial production of the product.
Owner:SINOVAC RES & DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com













![Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b30dd372-e6d6-40c8-84cc-007c9162170e/HDA00003309229100011.PNG)
![Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b30dd372-e6d6-40c8-84cc-007c9162170e/HDA00003309229100012.PNG)
![Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b30dd372-e6d6-40c8-84cc-007c9162170e/HDA00003309229100013.PNG)