Composition useful as rotavirus vaccine and a method therefor
一种组合物、病毒的技术,应用在生产这类病毒,制备这类制剂,预防和治疗领域,能够解决有限储存稳定性、不是商业上可行的等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
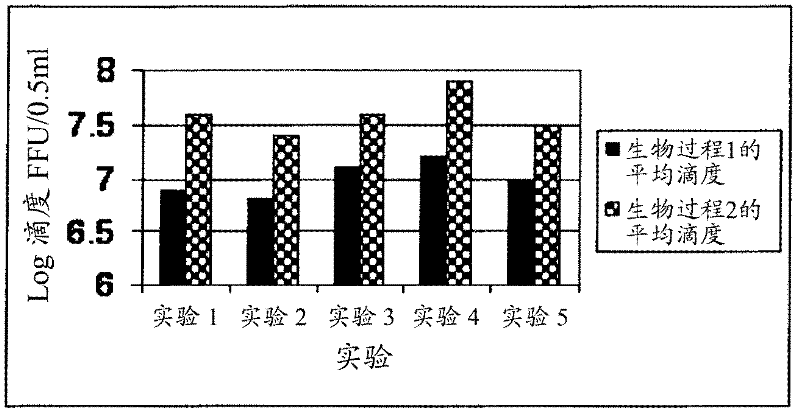
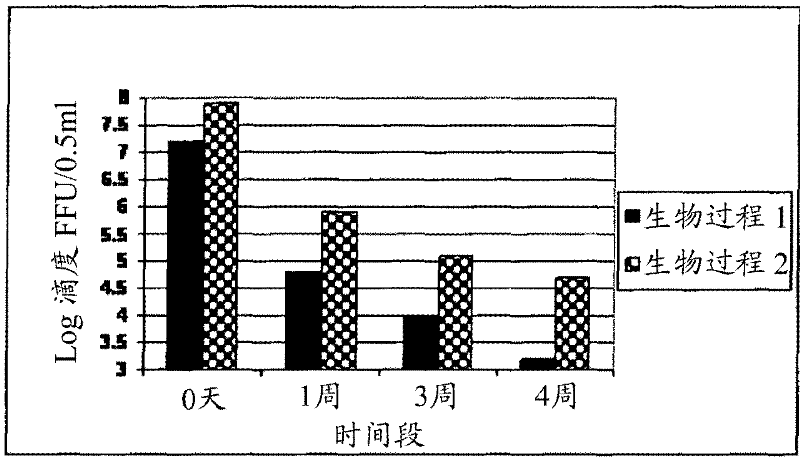
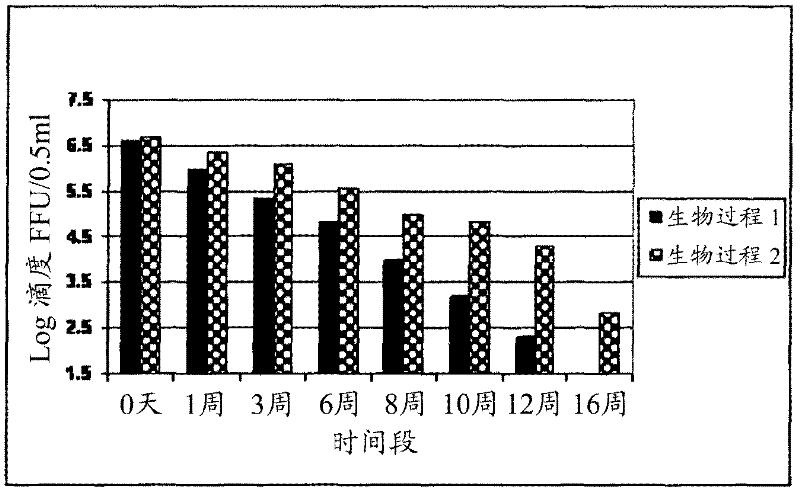
[0105] Example 1. Mass production of typical and pretreated rotavirus populations and their stability properties:
[0106] Bharat Biotech International Ltd (BBIL) obtained human rotavirus strains 116E and I321 from NIH under a material transfer agreement with the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH), Bethesda, USA. Raw 116E (G9[P11]) and I321 (G10P[11]) were passed through primary African green monkey kidney (AGMK) cells, then MA 104 cell substrates and then serially passaged AGMK (SPAGMK ) cells to adapt to growth in cell culture. MA 104 and SPAGMK cell substrates are not approved by the National Regulatory Agency (NRA) for use in commercial vaccine production. These two human rotavirus vaccine strains (116E and 1321) were therefore adapted to Vero cells, grown individually in these cells to generate a viral bulk population and prepared individually as monovalent, live, attenuated 1. Trial production batche...
Embodiment 2
[0115] Example 2. Typical formulations of rotavirus and pretreated rotavirus in liquid and lyophilized form and the effect of each formulation on the stability profile:
[0116] Store pooled stocks at 2-8°C or -70°C based on 10 3 FFU / 0.5mL to 10 8.5 A target titer of FFU / 0.5 mL was formulated into a semi-finished product and filled as a vaccine. Based on the titer of the pooled stock solution, a calculated volume of the pooled stock solution was withdrawn and added to a predetermined volume of work in progress containing stabilizers, antibiotics and buffers. The formulated semi-finished product is filled into vials as a vaccine. Various stabilizers used in different combinations and concentrations in the formulation are lactalbumin hydrolyzate (LAH), trehalose, sucrose, starch, lactose, maltose, soy protein, rHSA (excluding Any residual rHSA that was carried out with the pretreated virus harvested by the process). Aliquots of 0.5 ml of the virus-containing formulation were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com