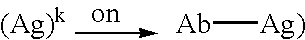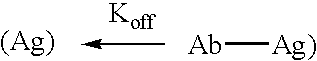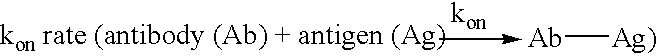Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2082 results about "Titer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Titer (or titre) is a way of expressing concentration. Titer testing employs serial dilution to obtain approximate quantitative information from an analytical procedure that inherently only evaluates as positive or negative. The titer corresponds to the highest dilution factor that still yields a positive reading. For example, positive readings in the first 8 serial twofold dilutions translate into a titer of 1:256 (i.e., 2⁻⁸). Titers are sometimes expressed by the denominator only, for example 1:256 is written 256.
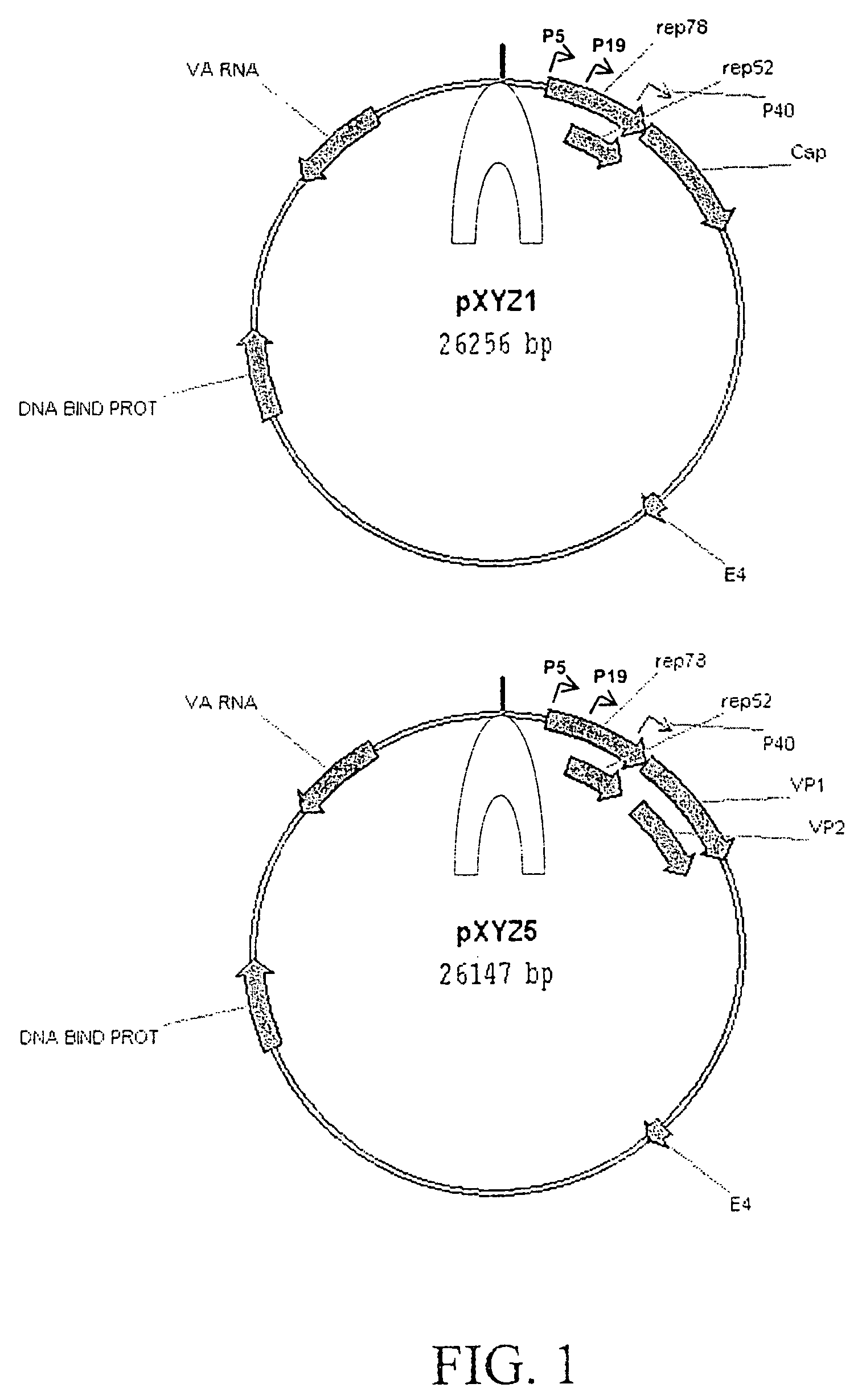
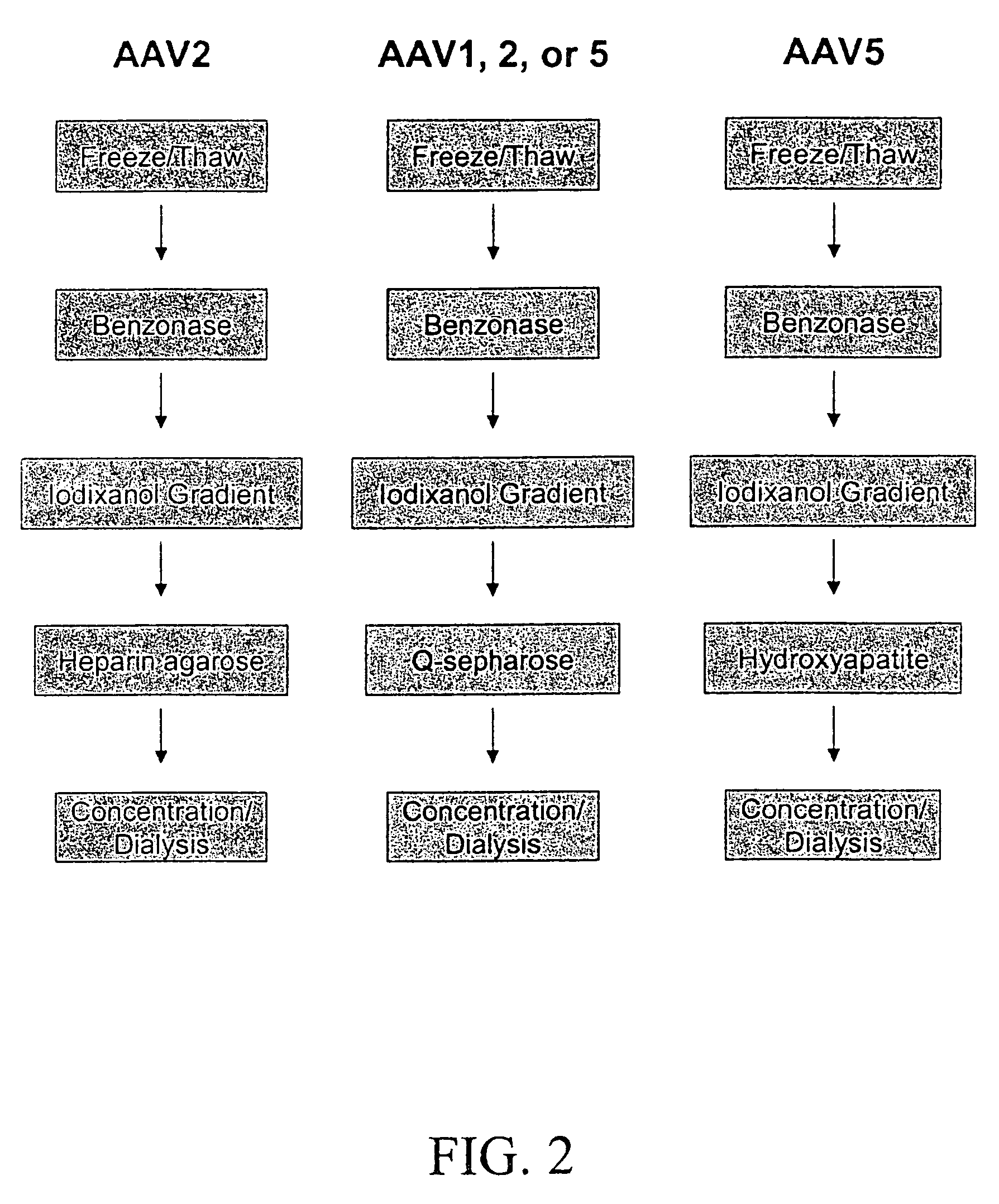
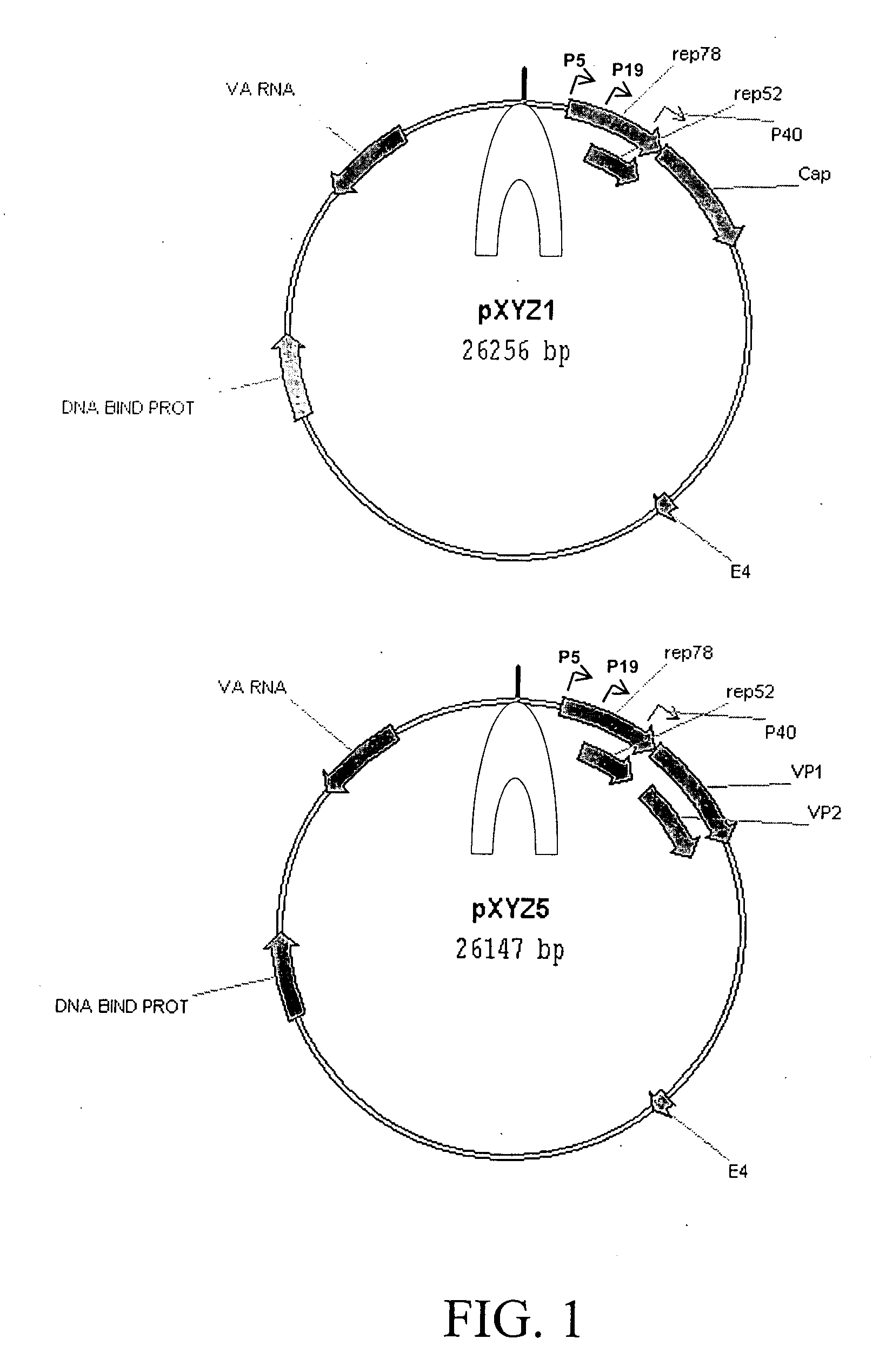
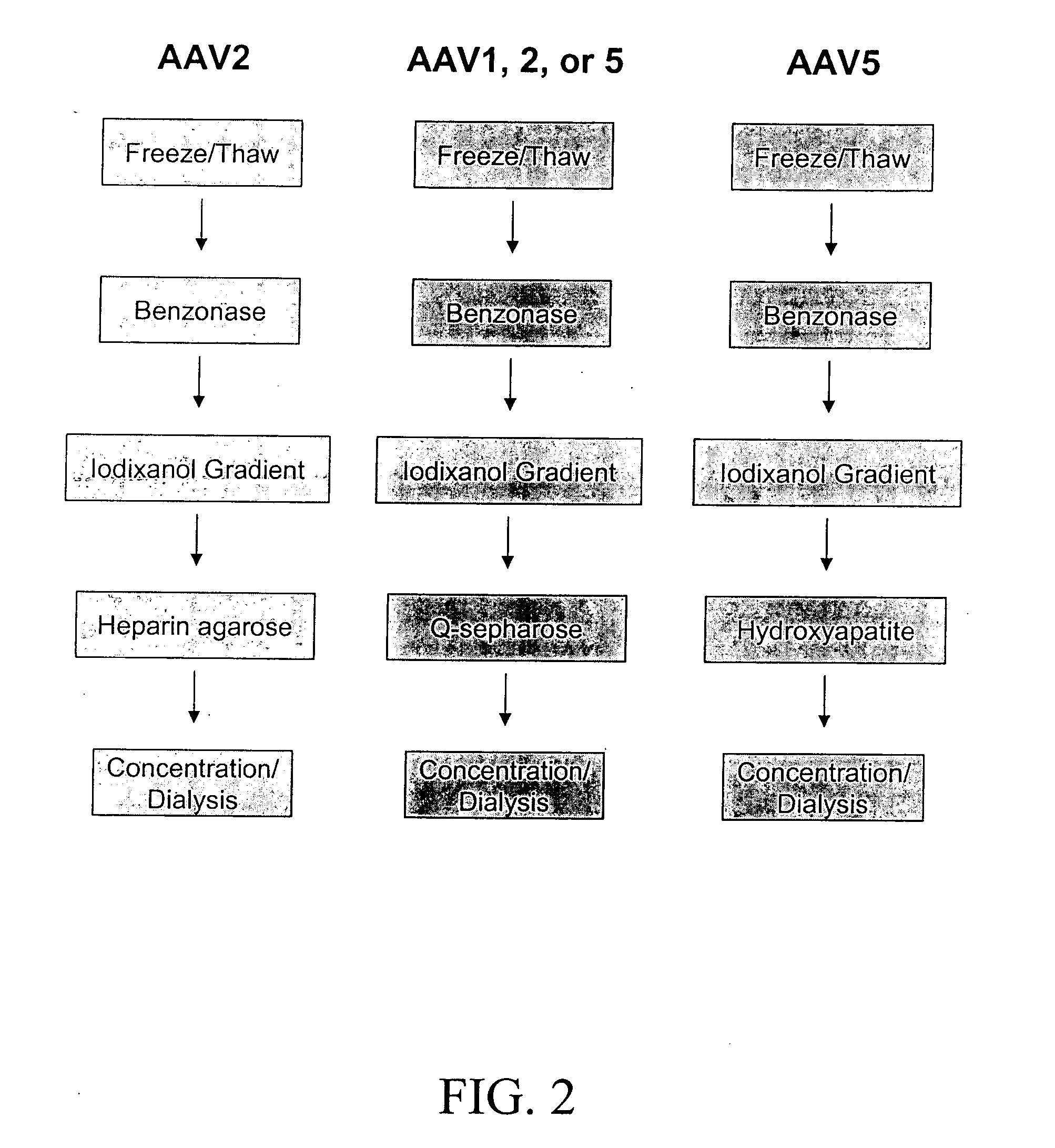
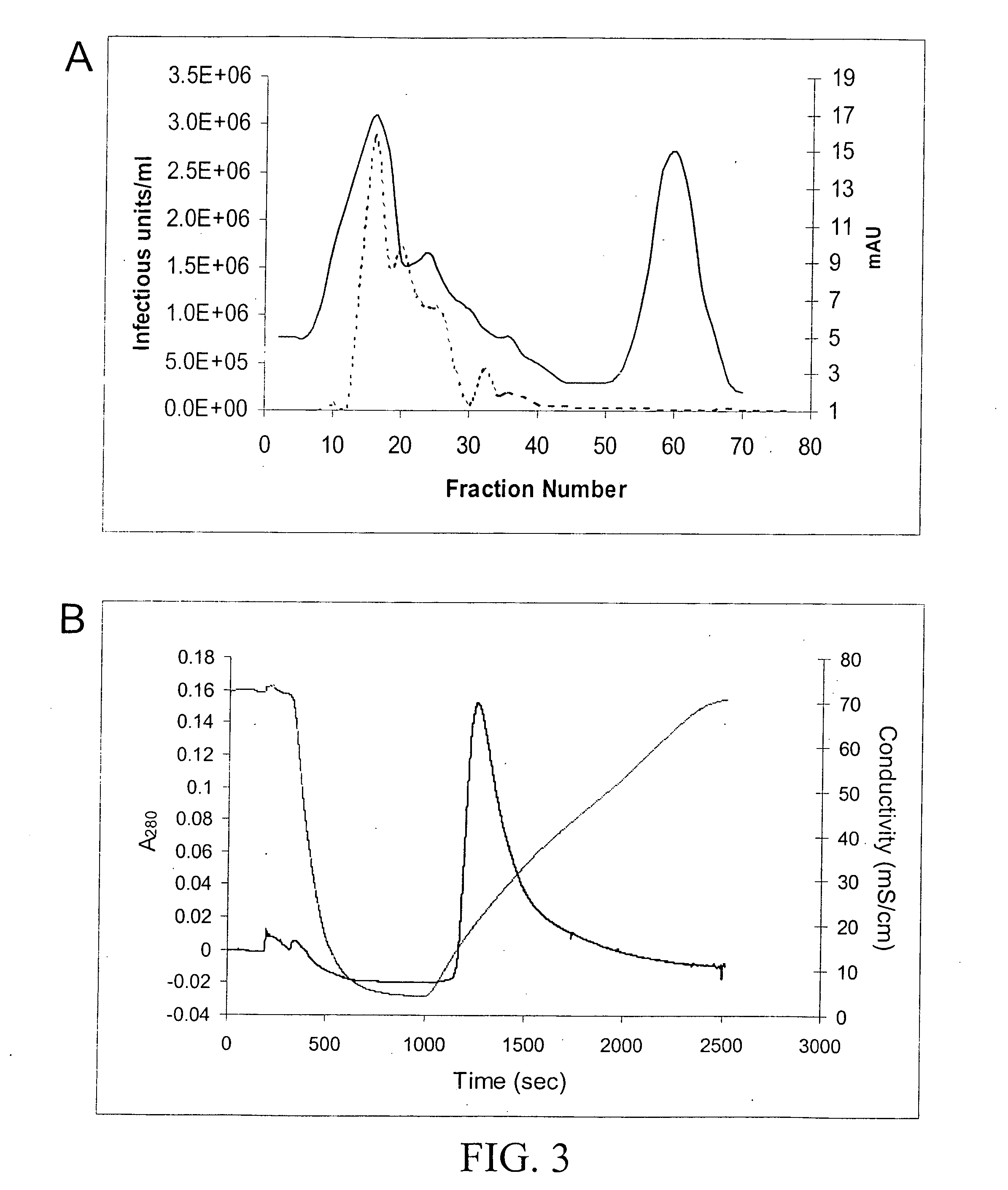
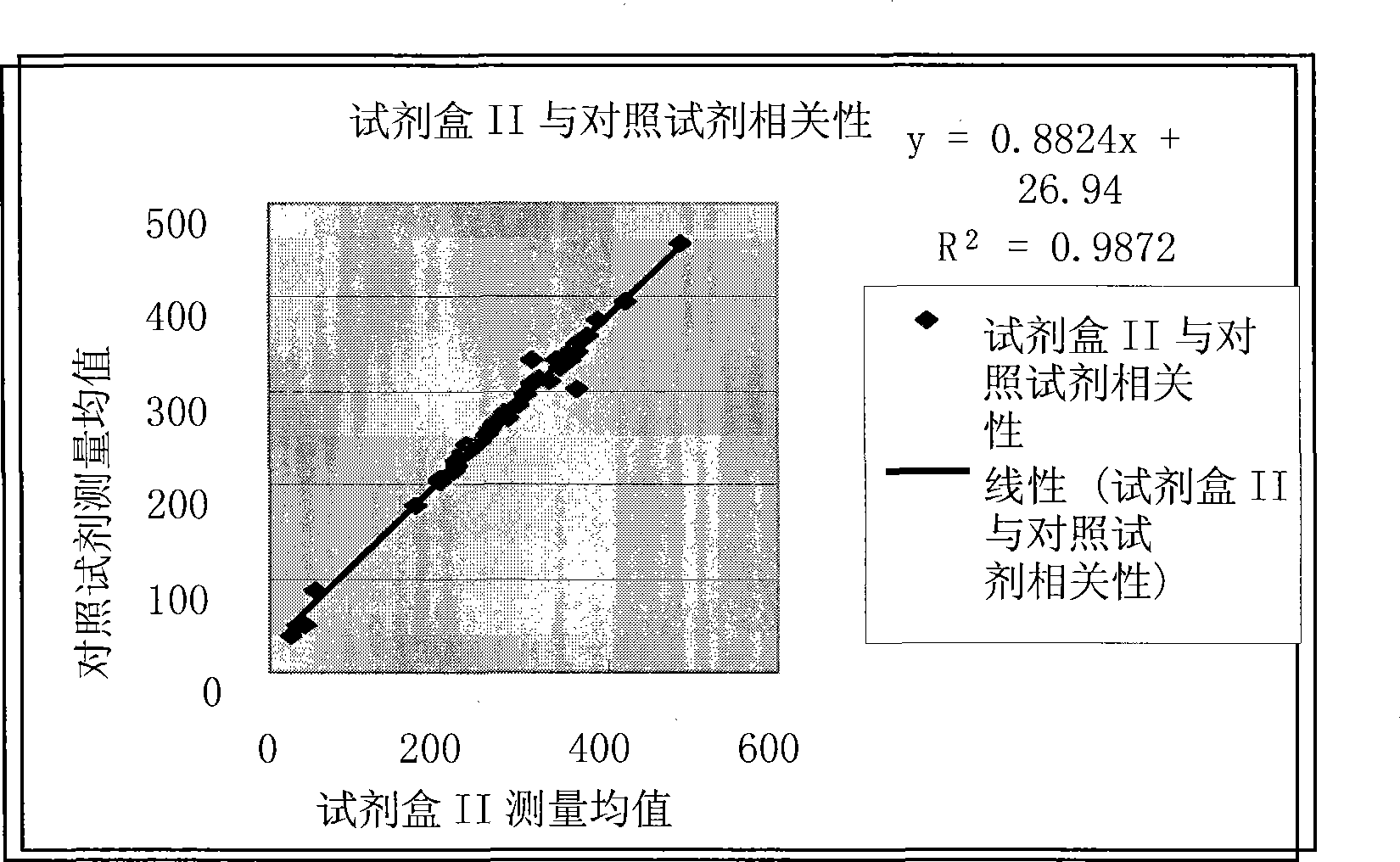
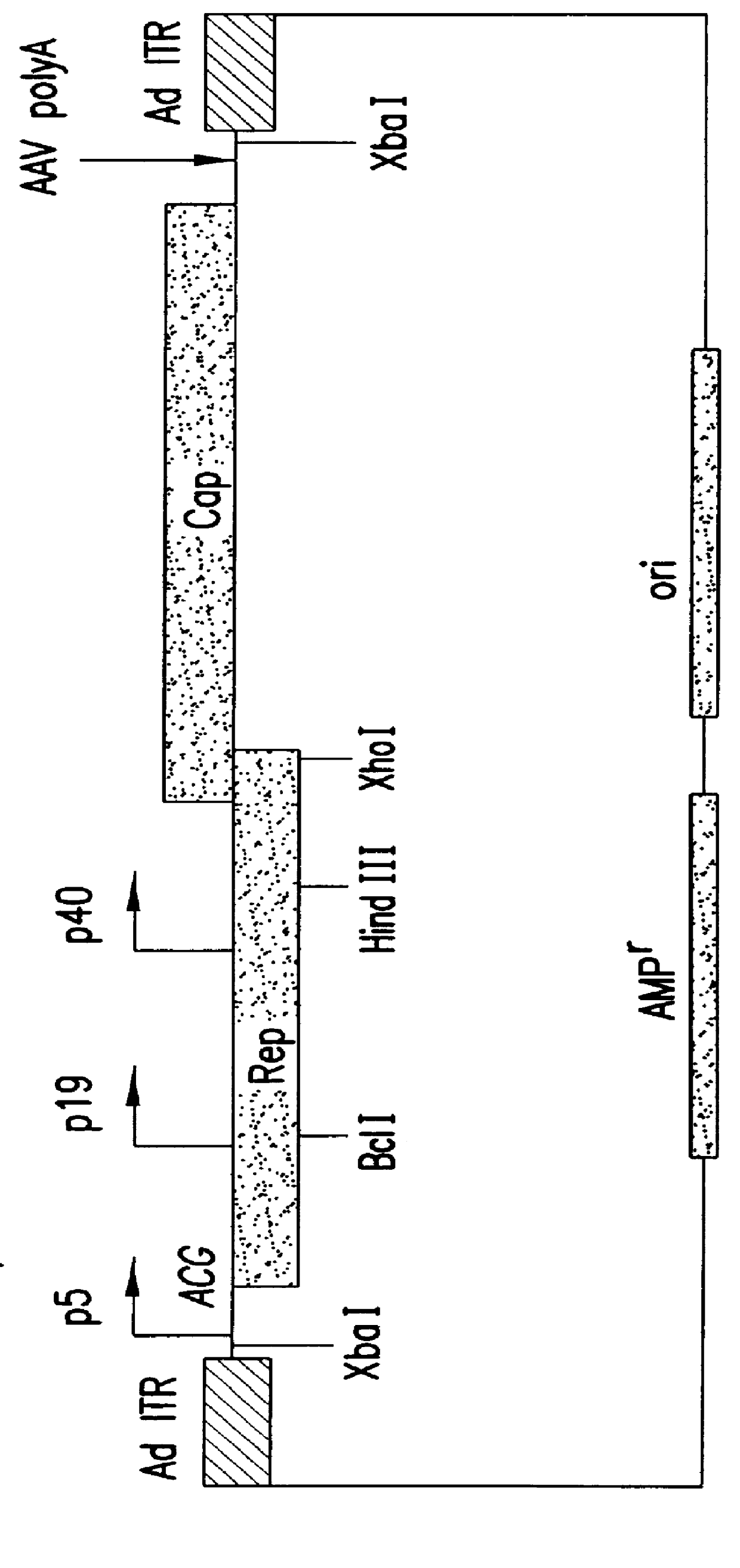
Production of pseudotyped recombinant AAV virions
InactiveUS7094604B2Highly purified and concentratedEfficient and large-scale productionVectorsSugar derivativesPurification methodsSerotype
Vectors that encode Adeno-Associated Virus (AAV) Rep and Cap proteins of different serotypes and Adenovirus transcription products that provide helper functions were used to produce pseudotyped recombinant AAV (rAAV) virions. Purification methods generated pseudotyped rAAV virion stocks that were 99% pure with titers of 1×1012–1×1013 vector genomes / ml.
Owner:UNIVIRSITY OF FLORIDA RES FOUND INC
Stable digestive enzyme compositions
InactiveUS20090117180A1Minimal loss of activityComposition is stablePowder deliveryHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 9% or less or 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 25%, about 20%, about 15% or about 10% after six months of accelerated stability testing and the titer level of a viral contaminant present in the pancreatin is at least about 1000 times less than the titer level of the viral contaminant present in a preparation from which the pancreatin is obtained.
Owner:APTALIS PHARMA
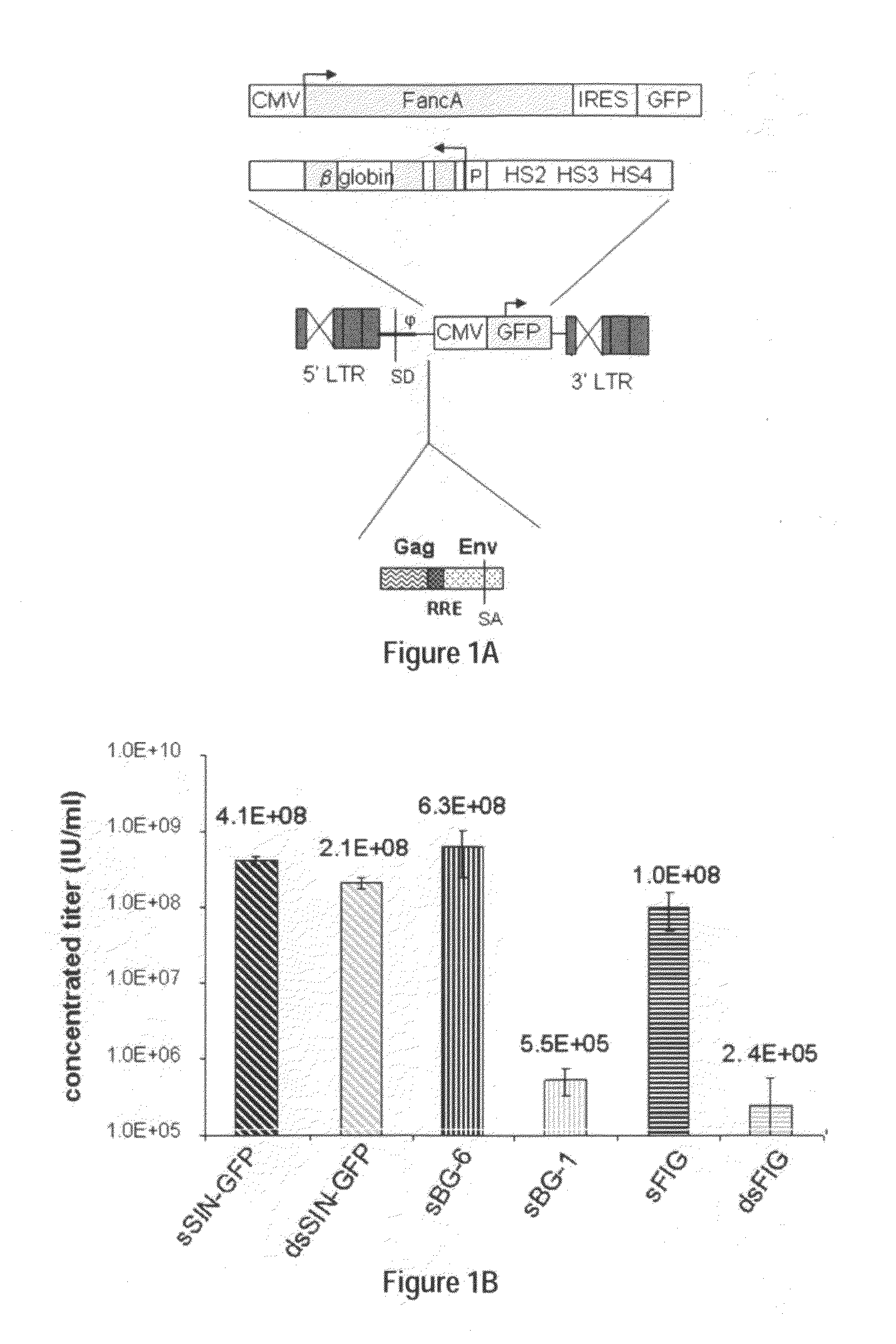
Optimization of determinants for successful genetic correction of diseases, mediated by hematopoietic stem cells
InactiveUS20110294114A1Improve stabilityImprove securityVectorsSugar derivativesNervous systemSickle cell anemia
Methods and compositions disclosed herein generally relates to methods of determining minimum hematopoietic stem cell (HSC) chimerism and gene dosage for correction of a hematopoietic disease; in particular, in in vivo models. The invention also relates to modified lentiviral expression vectors for increase a viral titer and various methods for increasing such titers as well as expression vectors capable of enhancing such titers. The invention also relates to CHS4 chromatin insulator-derived functional insulator sequences. The invention further relates to methods for genetic correction of diseases or reducing symptoms thereof, such as sickle cell anemia, a lysosomal storage disease. The invention further relates to a method of improving and / or correcting one or more central nervous system (CNS) abnormalities caused by one or more lysosomal storage disease. The invention further relates to methods of improving titer in transfection-based bioreactor culture production or transfection-based production systems using eukaryotic cells.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
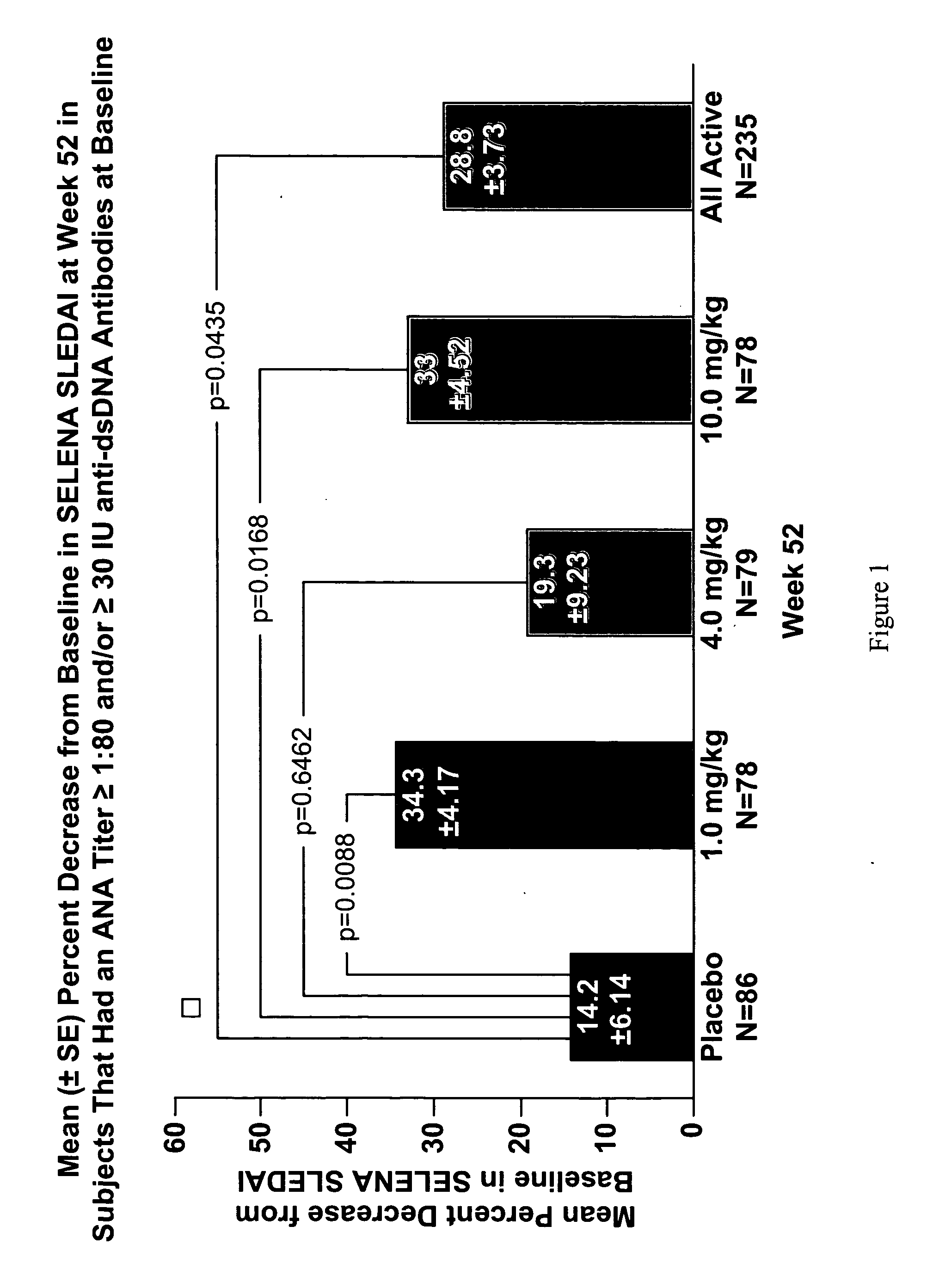
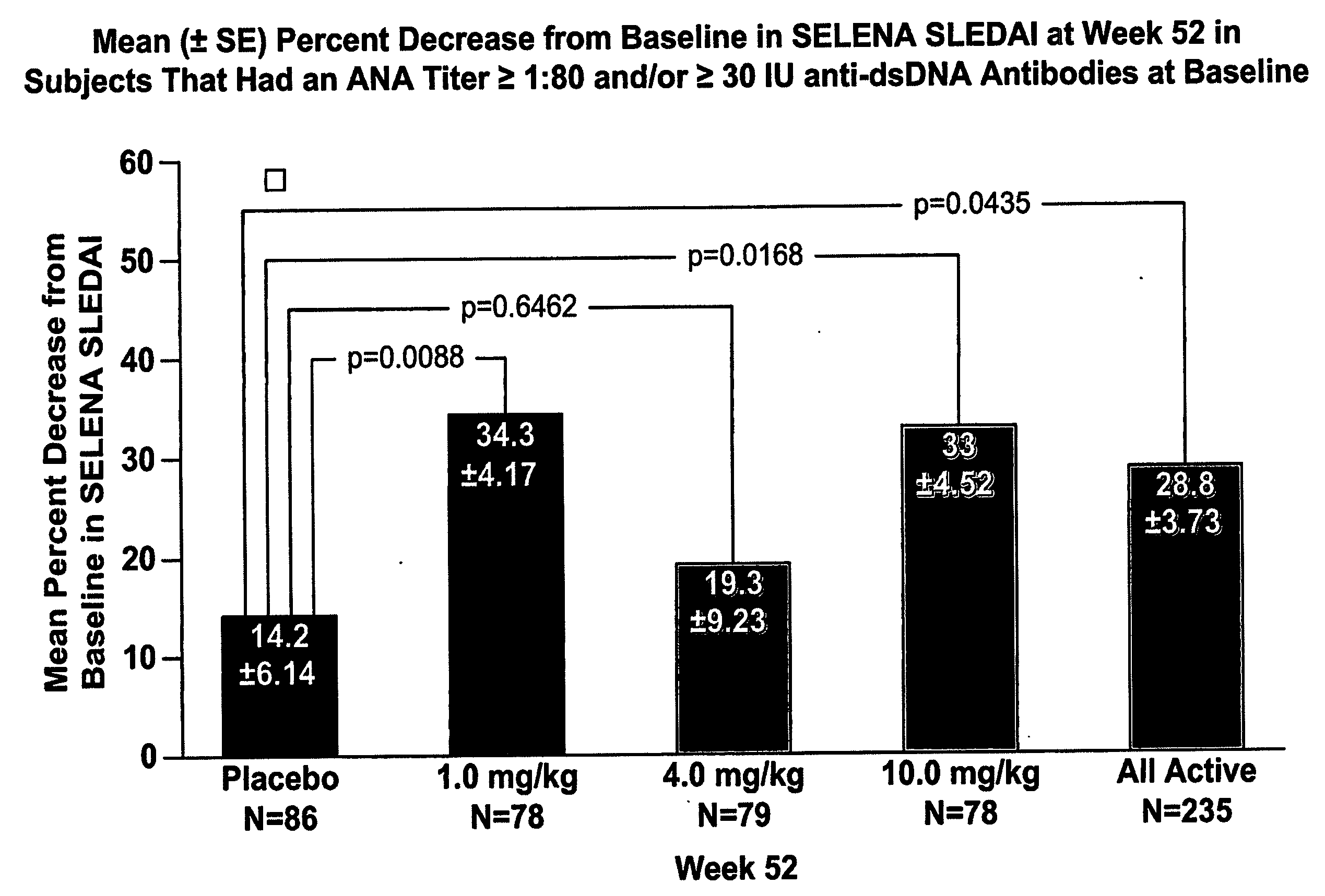
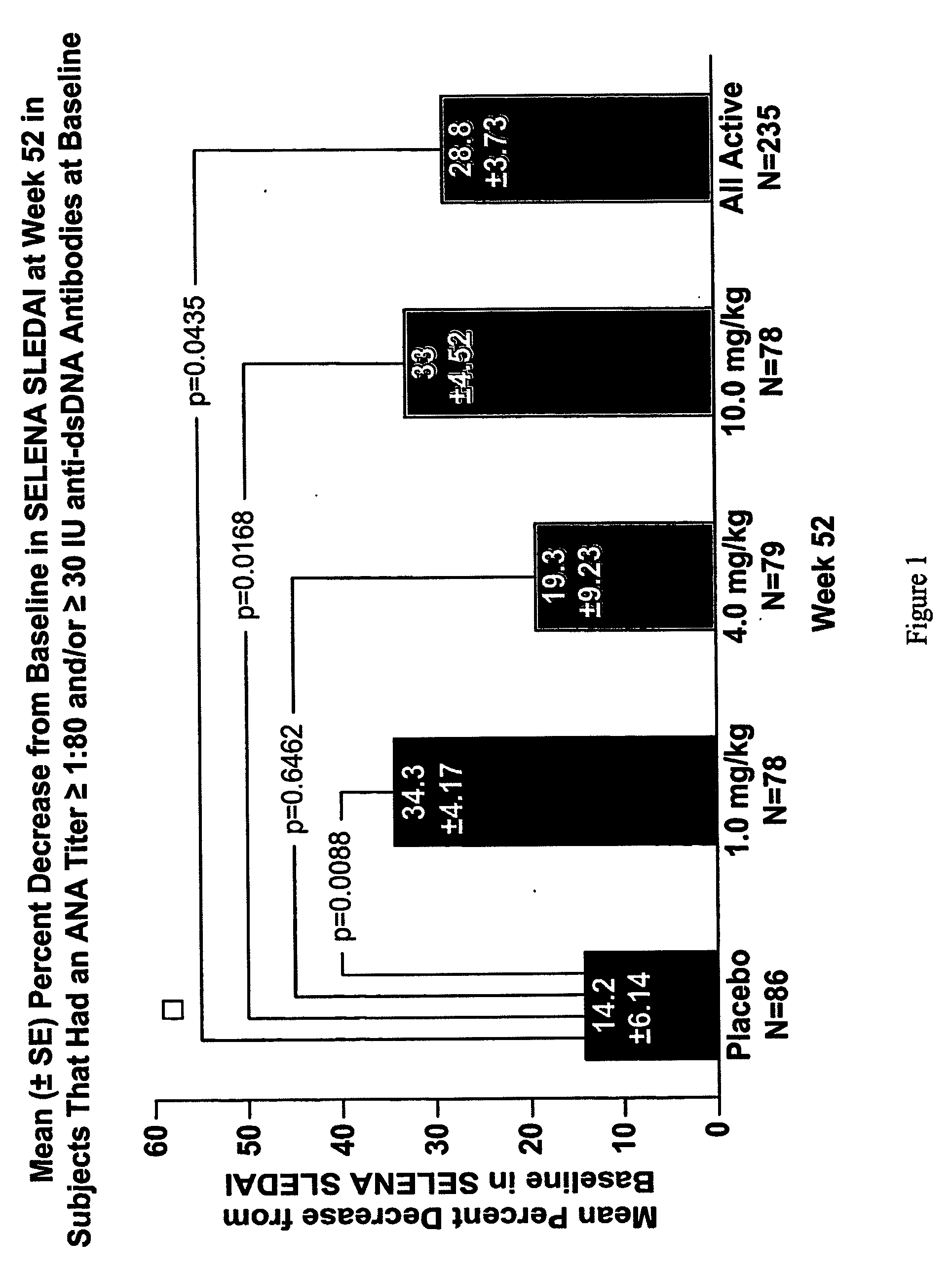
Methods and compositions for use in treatment of patients with autoantibody positive disease
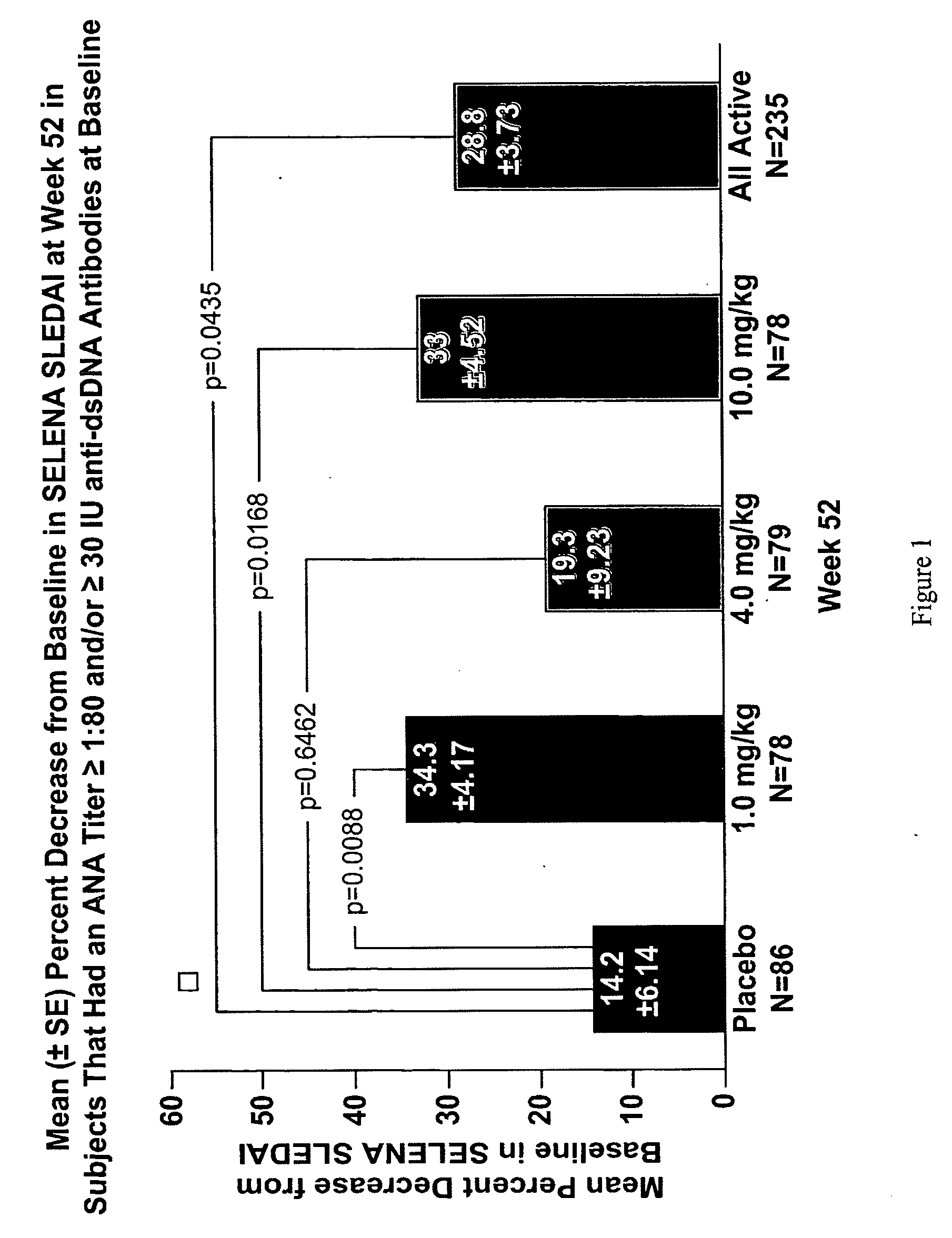
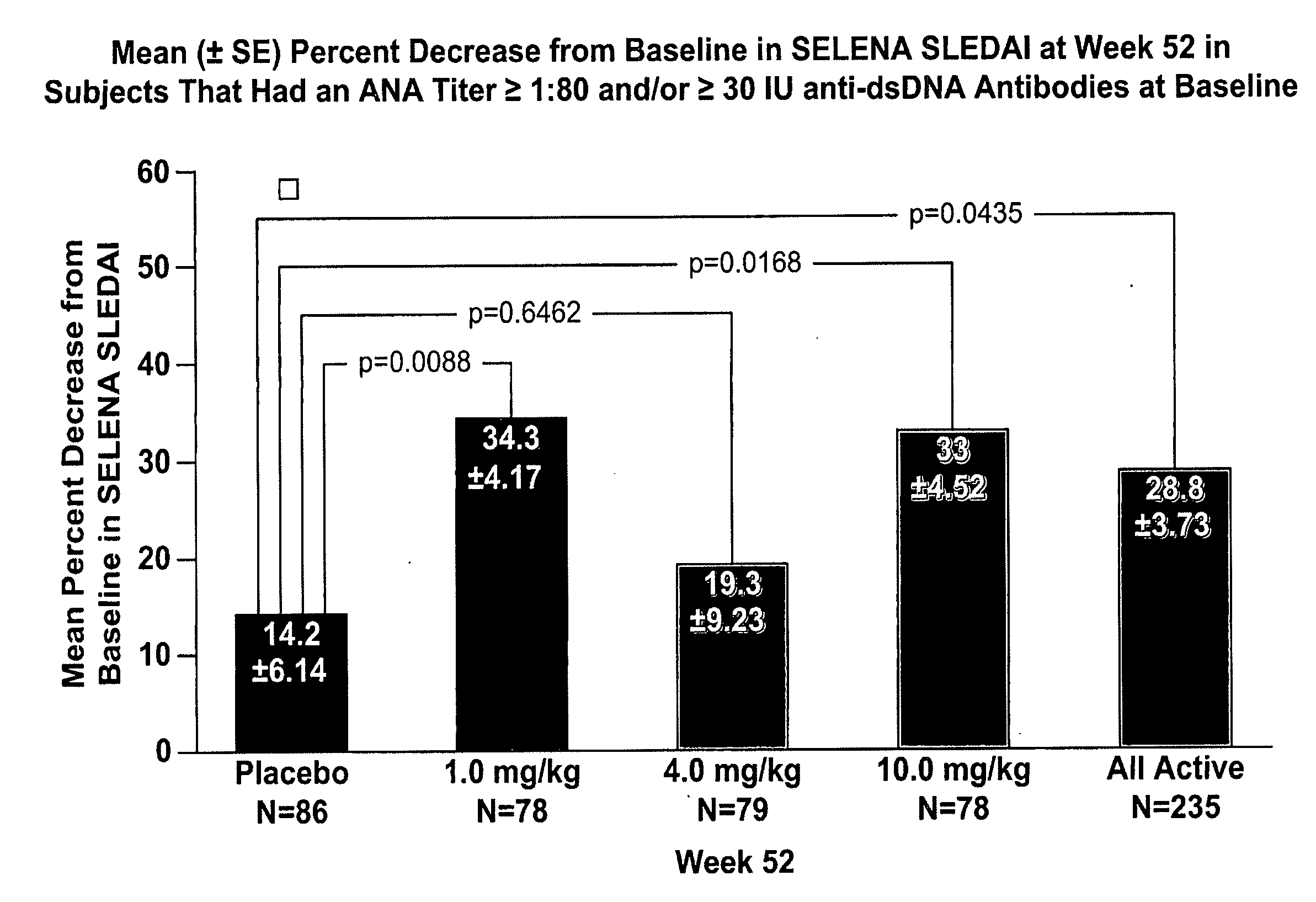
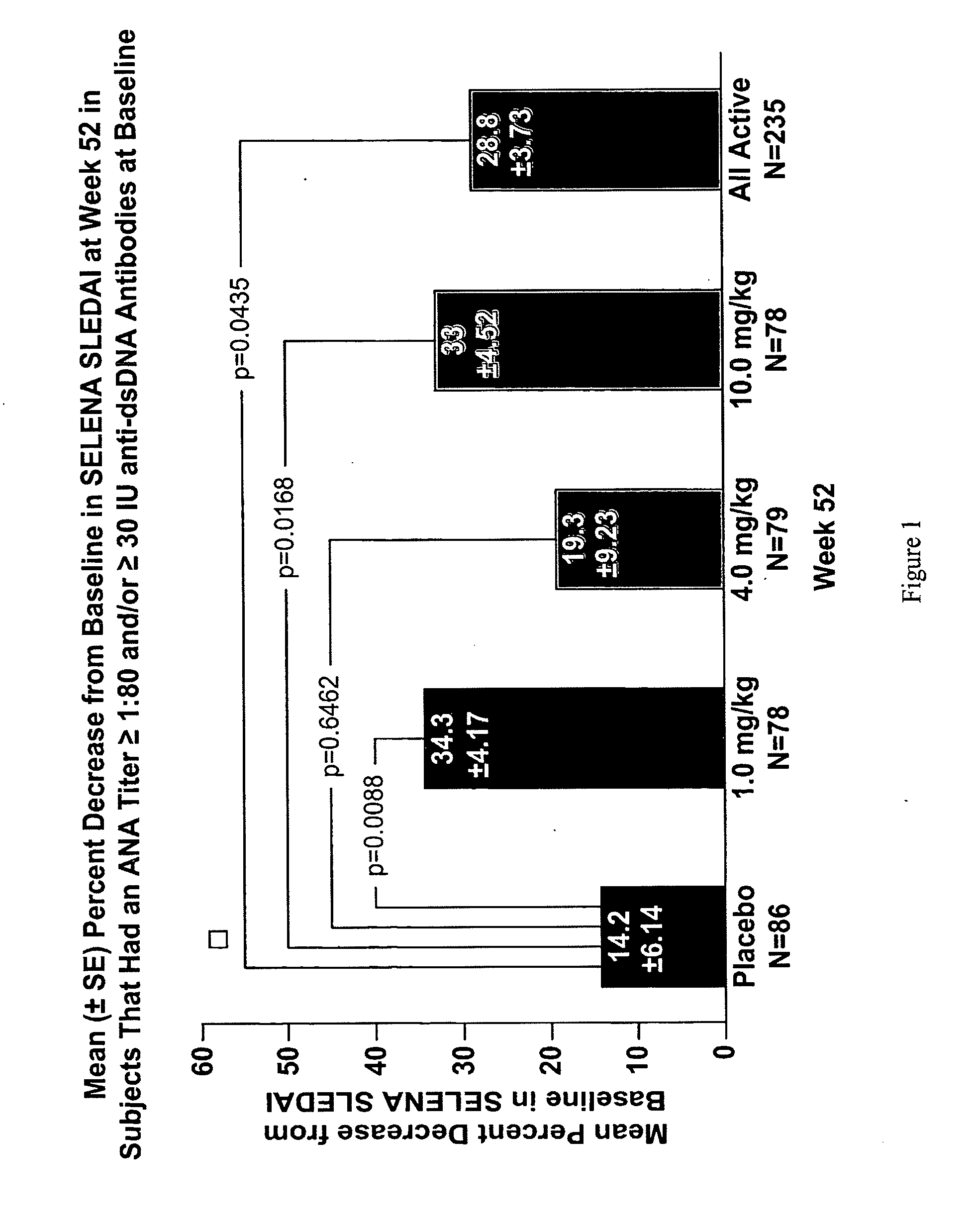
InactiveUS20070086979A1Reduce frequencyReduce in quantityPeptide/protein ingredientsAntipyreticAnti-dsDNA antibodiesSystemic lupus erythematosus
The present invention relates to methods and compositions for use in treatment of patients with autoantibody positive disease. In a specific embodiment, the present invention relates to a method of treating a patient that has an ANA titer of 1:80 or greater and / or greater than or equal to 30 IU / ml of anti-dsDNA antibodies in his / her blood plasma or serum comprising administering a therapeutically effective amount of an immunomodulatory agent, such as an antagonist of Neutrokine-alpha. Additionally provided is a method of reducing the frequency and / or quantity of corticosteroid administration to patients. In preferred embodiments, the patient has systemic lupus erythematosus. Methods for determining if a lupus patient is responding to medical treatment are also provided.
Owner:HUMAN GENOME SCI INC
Prevention of myocarditis, abortion and intrauterine infection associated with porcine circovirus-2
InactiveUS20050058653A1Avoid spreadingPeptide/protein ingredientsGenetic material ingredientsDiseaseStaining
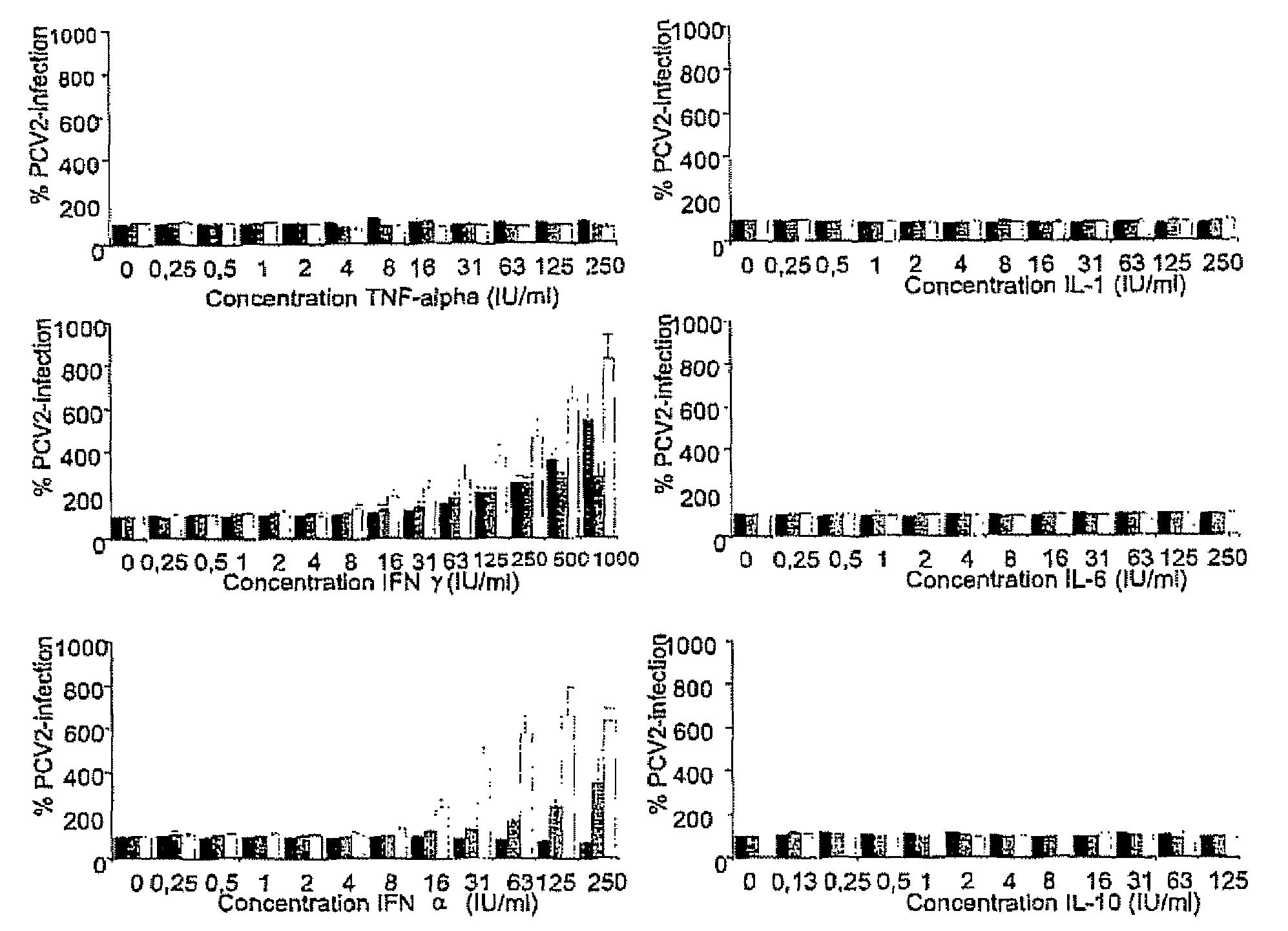
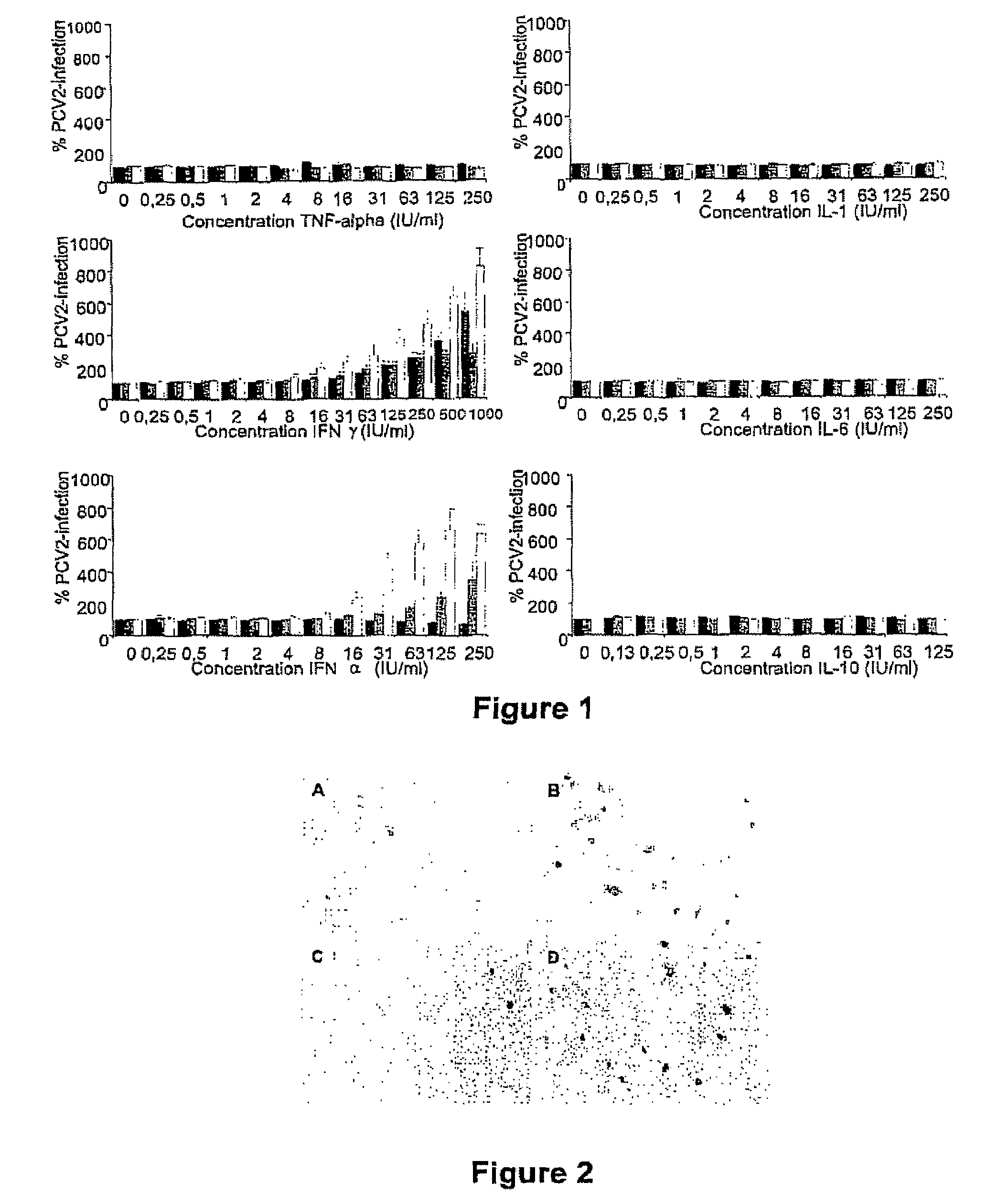
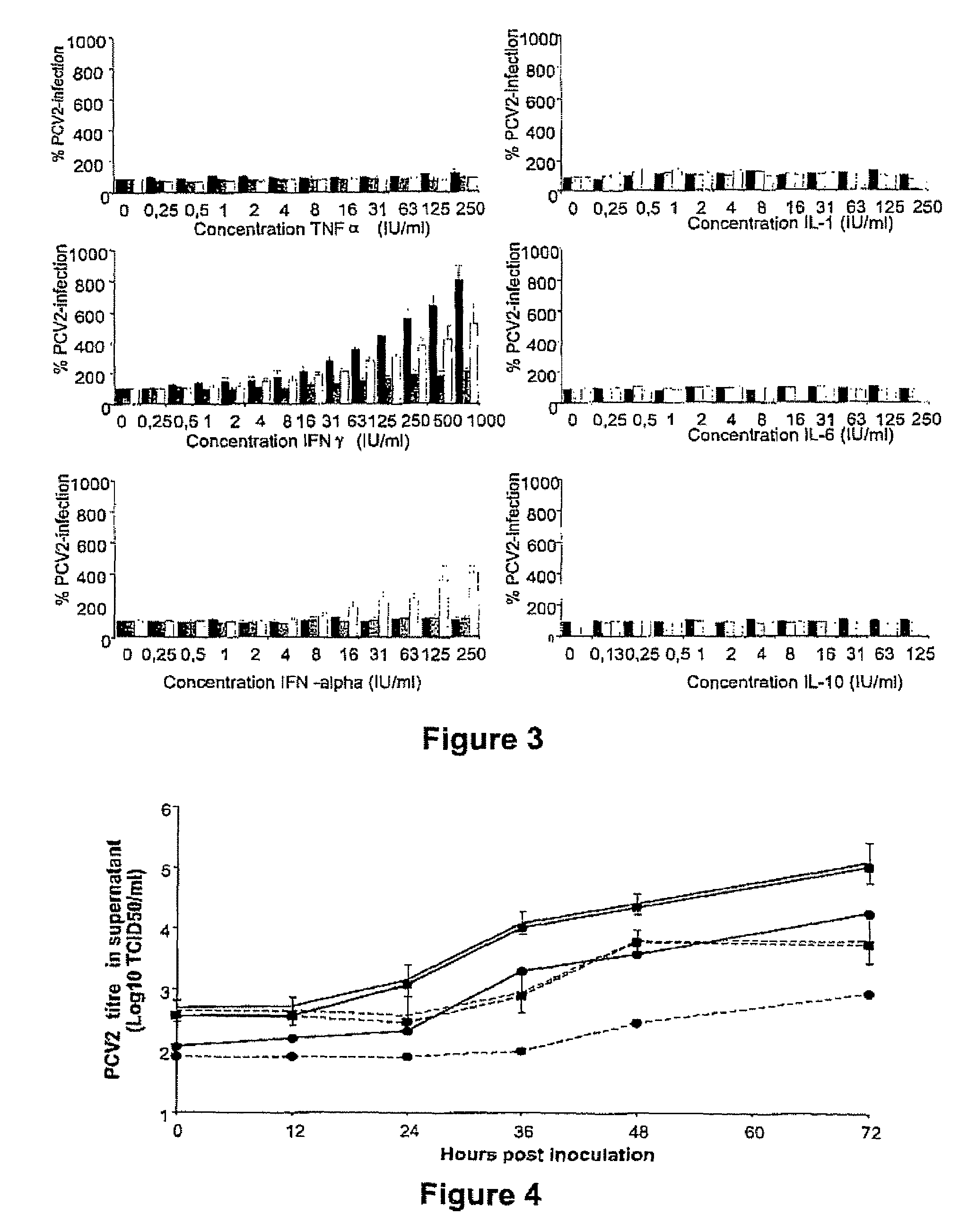
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6and 8and / or the 10and 13weeks of gestation, can lower viral titer, prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:MERIAL SAS +1
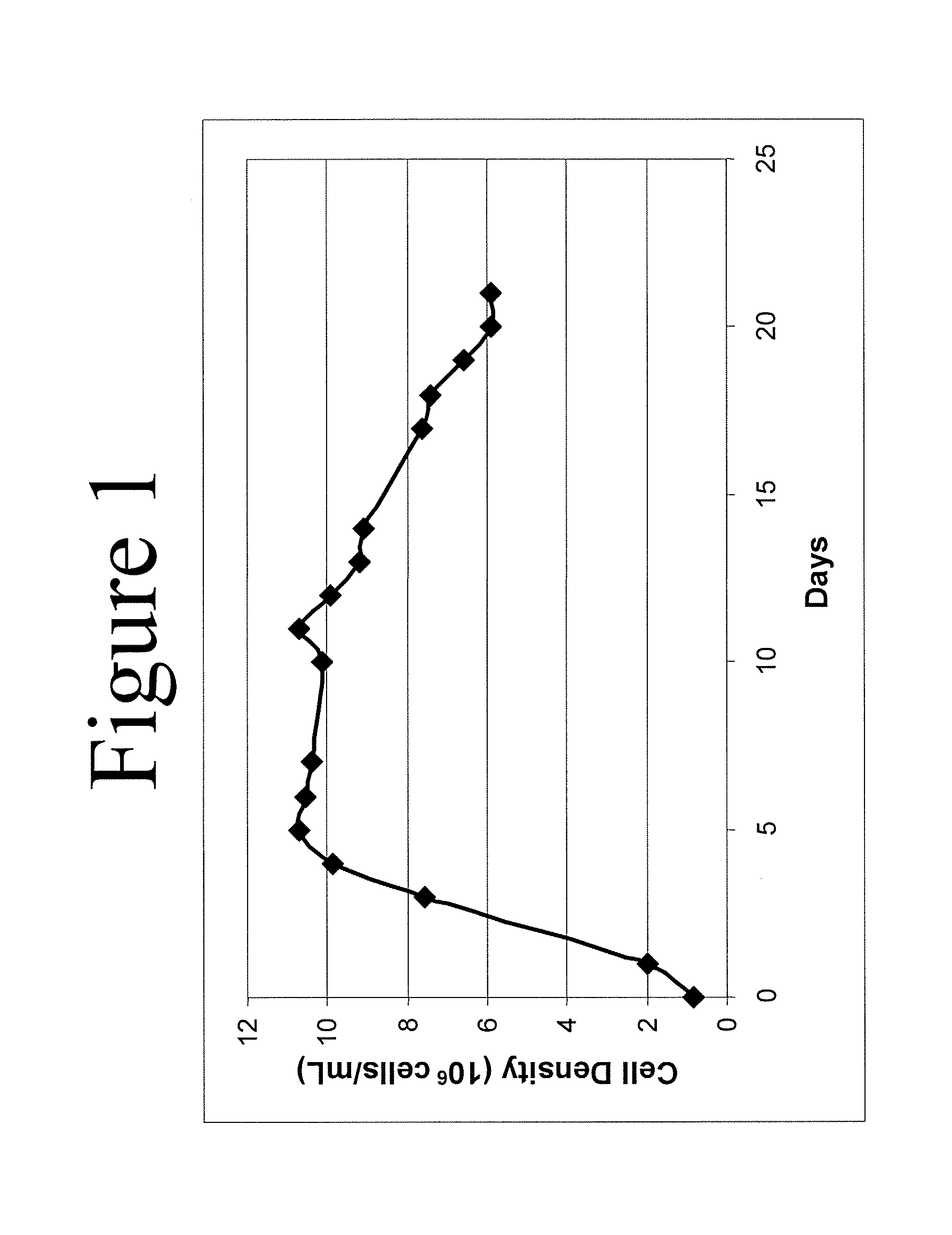
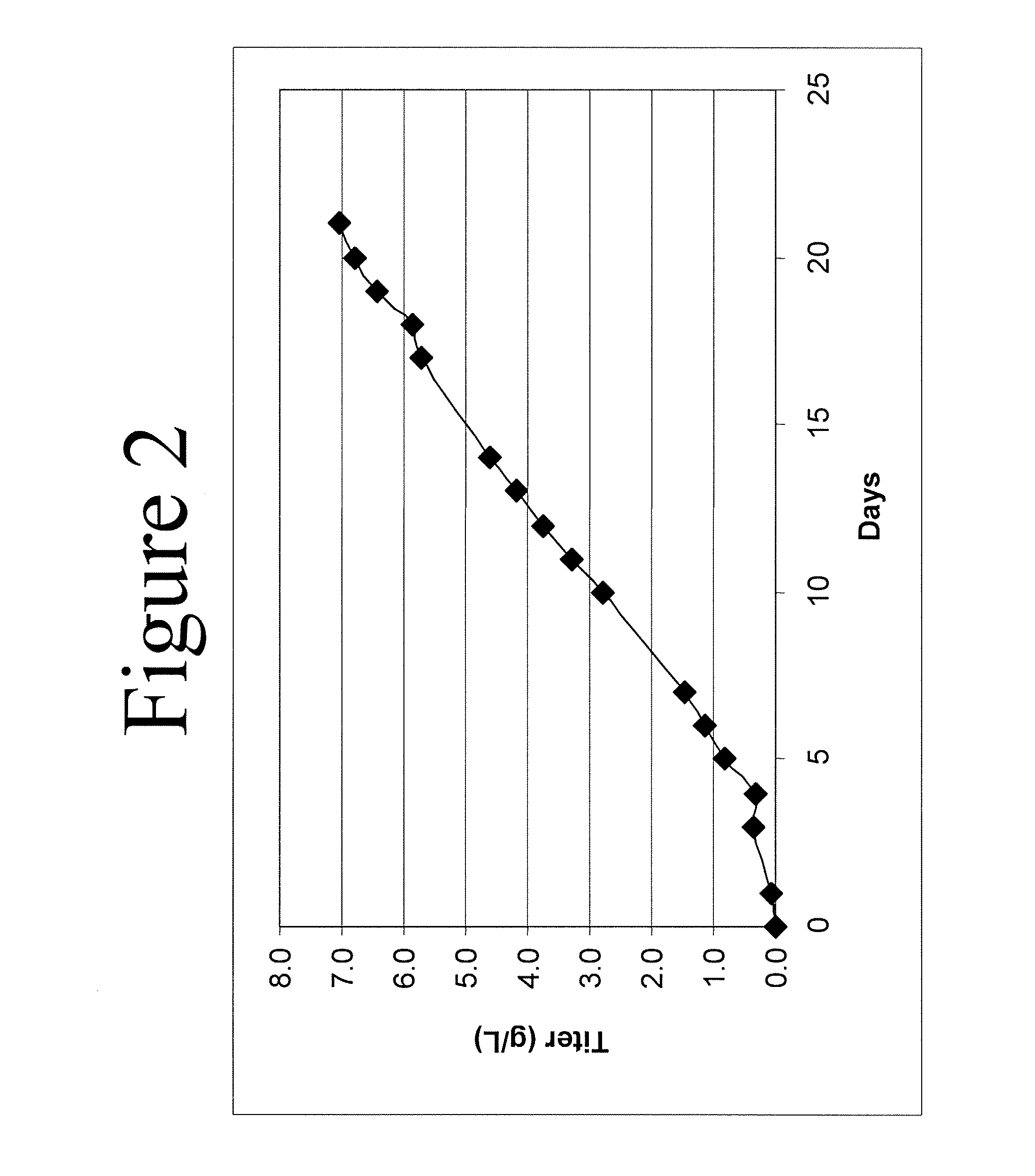
Use of perfusion to enhance production of fed-batch cell culture in bioreactors
InactiveUS20090042253A1Bioreactor/fermenter combinationsBiological substance pretreatmentsFiltrationFeed pump
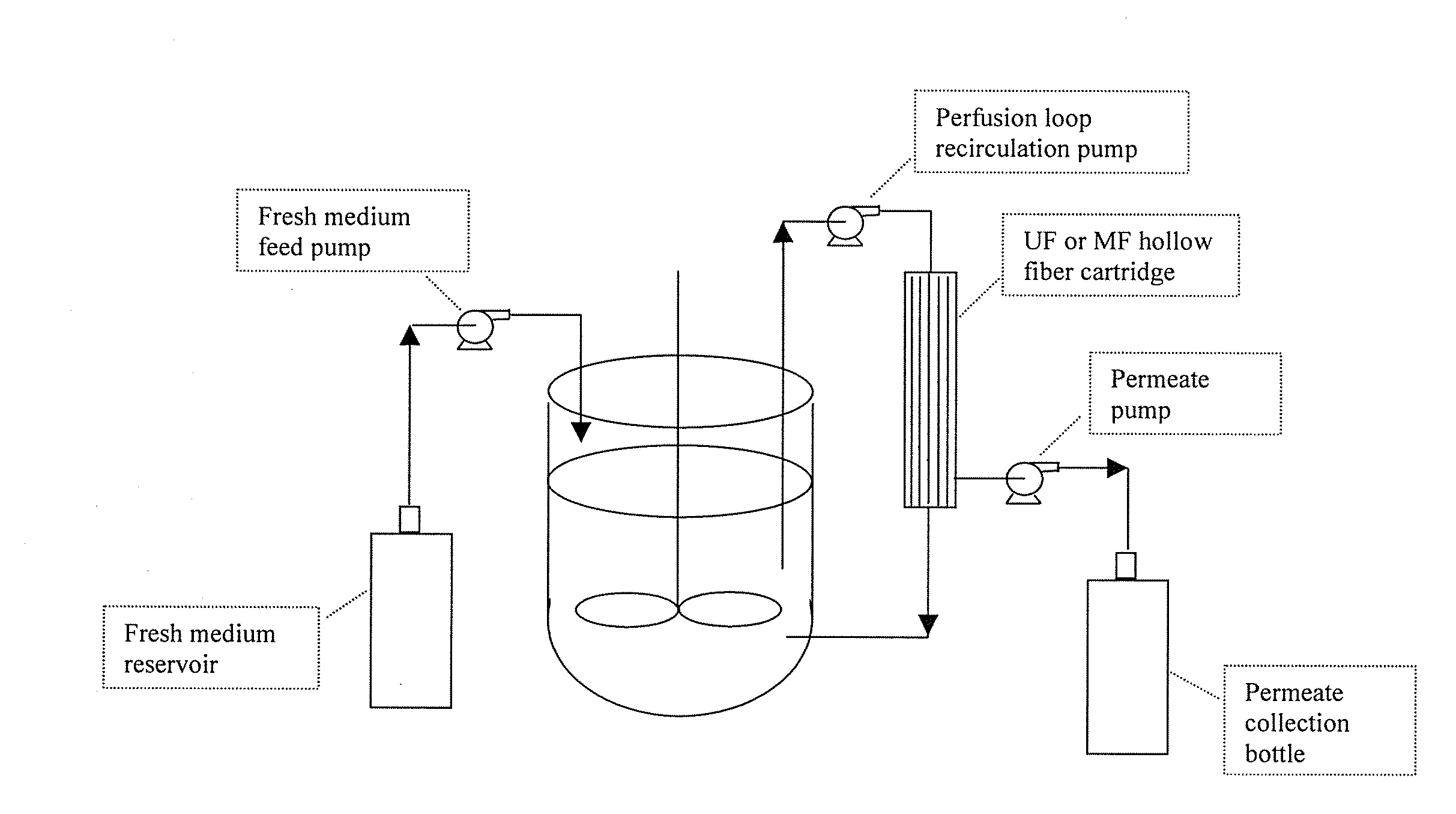
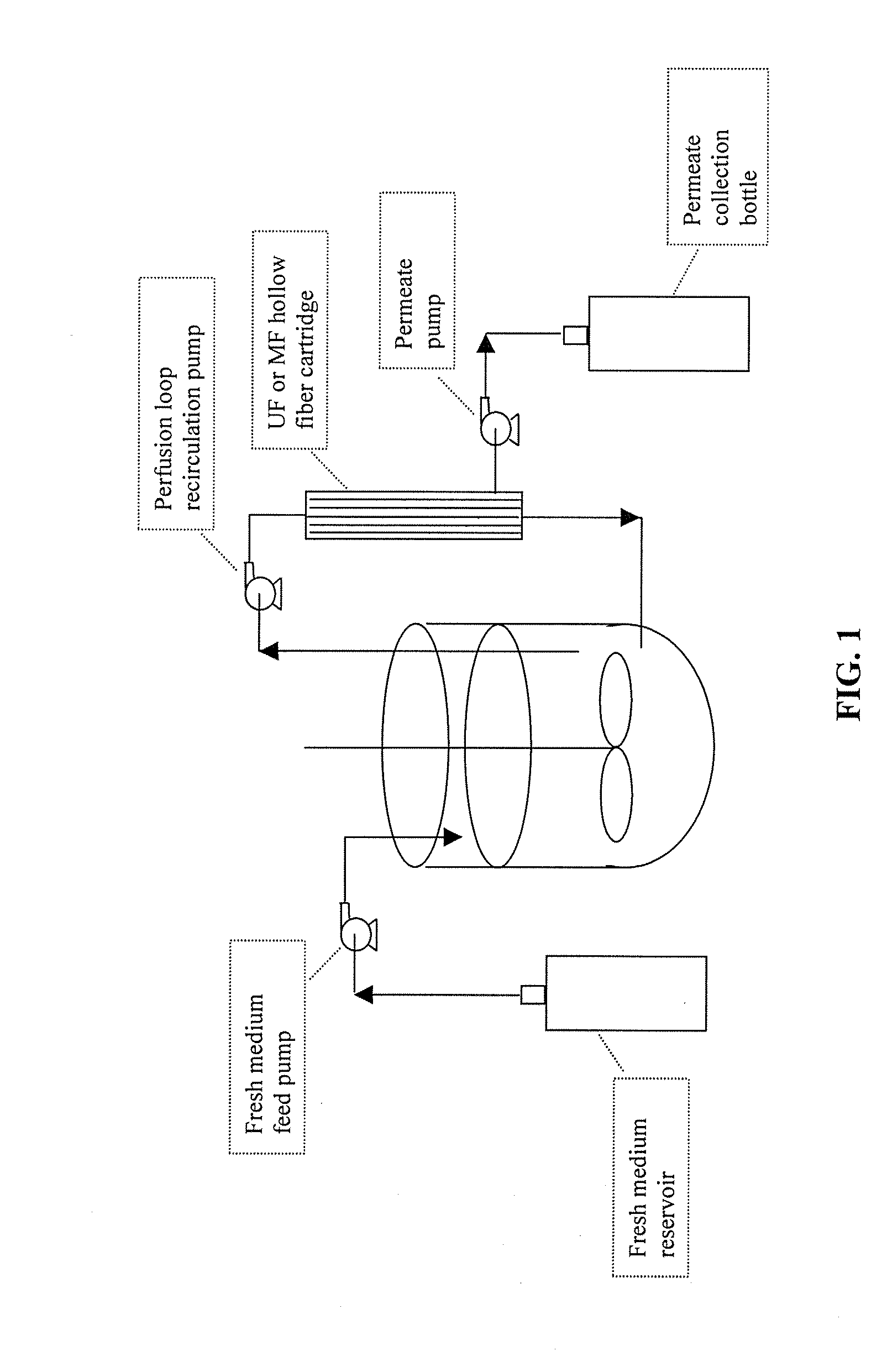
The invention relates to methods of improving protein production, e.g., large-scale commercial protein production, e.g., antibody production, utilizing a modified fed-batch cell culture method comprising a cell growth phase and a polypeptide production phase. The modified fed-batch cell culture method combines both cell culture perfusion and fed-batch methods to achieve higher titers of polypeptide products. Because the modified fed-batch cell culture method of the invention produces higher polypeptide product titers than fed-batch culture alone, it will substantially improve commercial-scale protein production. The invention also relates to a perfusion bioreactor apparatus comprising a fresh medium reservoir connected to a bioreactor by a feed pump, a recirculation loop connected to the bioreactor, wherein the recirculation loop comprises a filtration device, e.g., ultrafiltration or microfiltration, and a permeate pump connecting the filtration device to a permeate collection container.
Owner:WYETH LLC
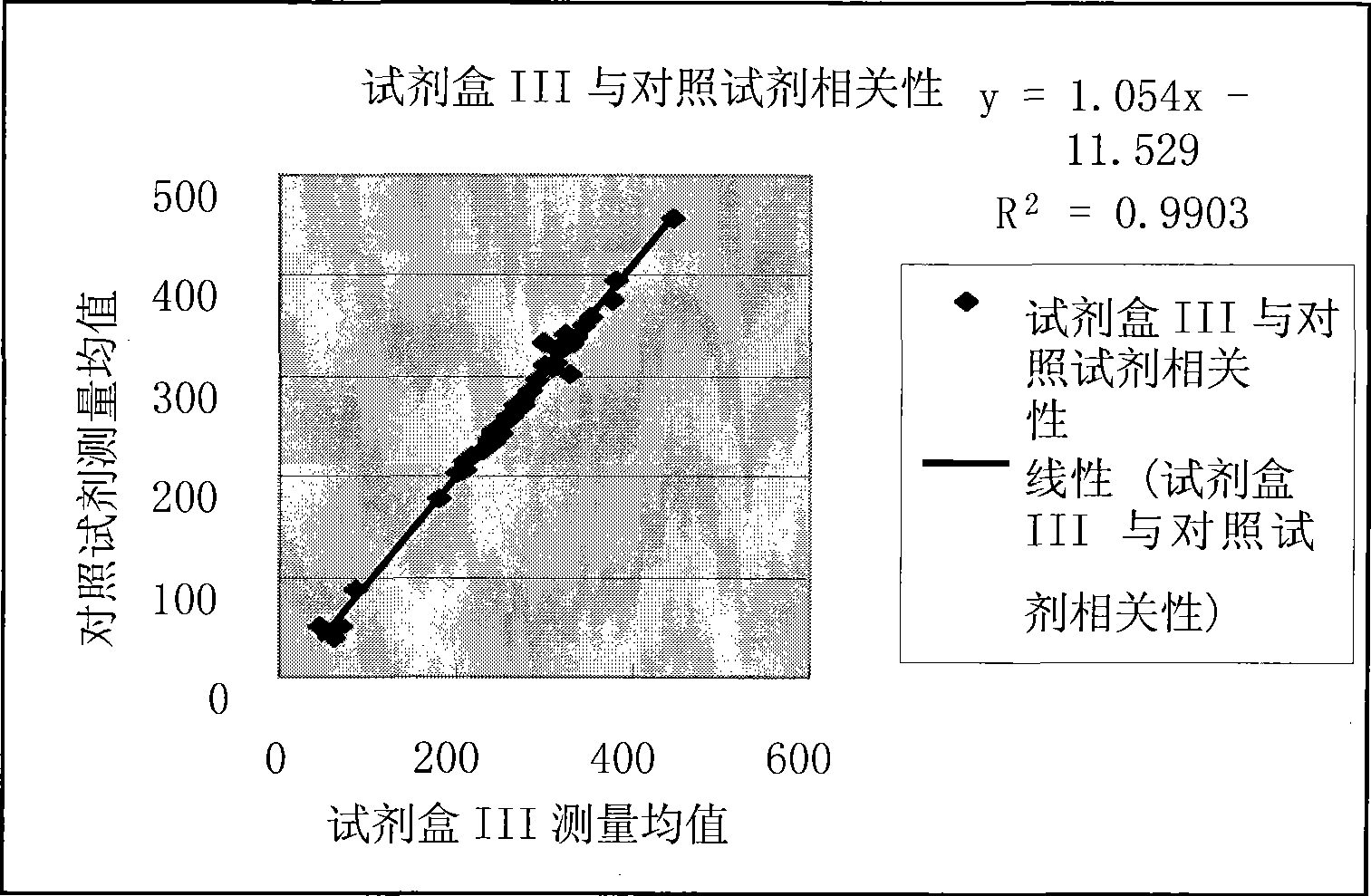
Production of pseudotyped recombinant AAV virions
InactiveUS20070015238A1Highly purified and concentratedEfficient and large-scale productionVectorsTissue culturePurification methodsSerotype
Vectors that encode Adeno-Associated Virus (AAV) Rep and Cap proteins of different serotypes and Adenovirus transcription products that provide helper functions were used to produce pseudotyped recombinant AAV (rAAV) virions. Purification methods generated pseudotyped rAAV virion stocks that were 99% pure with titers of 1×1012−1×1013 vector genomes / ml.
Owner:SNYDER RICHARD O +12
Mammalian cell culture processes for protein production
ActiveUS7541164B2Enhance cell viabilityPeptide/protein ingredientsImmunoglobulinsGrowth phaseTwo temperature
Owner:BRISTOL MYERS SQUIBB CO
Methods of administering/dosing anti-RSV antibodies for prophylaxis and treatment
InactiveUS20030091584A1Reduce dosageLess frequent administrationViral antigen ingredientsMicrobiological testing/measurementSerum igeAntibody fragments
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection
Owner:MEDIMMUNE LLC
Culturing circular ssDNA viruses for the production of vaccines
InactiveUS7300785B2Increase virus titresIncreased virus titresViral antigen ingredientsMicrobiological testing/measurementSsDNA virusesInterferon alpha
The present invention relates to the use of interferon in the in vitro cultivation of animal circular ssDNA virus such as Porcine Circovirus 2 or human TT virus in an animal cell line. Increased titres of animal circular ssDNA virus are obtained by addition of interferons or agents which ensure the production of endogenous interferons by said cell line.
Owner:UNIV GENT
Method for preparing health-benefiting fermented soybean meal
ActiveCN103283961AIncrease nutritionHas antibacterial effectAnimal feeding stuffAntibiotic YHealth benefits
The invention relates to a method for preparing health-benefiting fermented soybean meal enriched in small peptides, gamma-aminobutyric acid (GABA), reducing sugar, lactobacillus plantarum and antimicrobial peptide. By utilizing strains of aspergillus oryzae, saccharomyces cerevisiae, bacillus subtilis and lactobacillus plantarum, the small peptides, free amino acids, reducing sugar, GABA and high viable count content in the lactobacillus plantarum in the health-benefiting fermented soybean meal are obtained through the two-stage solid state fermentation method, the small peptides account for 8.01-18 percent, the free amino acids has the content of 80-300mg / g, the reducing sugar reaches 20-80mg / g, the GABA reaches 0.317-0.5mg / g, the viable count content in the lactobacillus plantarum is 2-6.5*1010cfu / g, the antibacterial titer of antimicrobial peptides is 2.0*103-7.0*104 IU / g, and the yield of the health-benefiting fermented soybean meal is 82.03-90 percent. According to the process, the nutrient effect of the soybean meal can be improved, the prepared fermented soybean meal has the antibacterial effect, partial antibiotics can be replaced, the fermented soybean meal is conveniently preserved and saved, the process is simple and feasible, and the fermented soybean meal is green and environment-friendly and has application significance and wide prospect in the feed industry and breeding industry.
Owner:ZHEJIANG UNIV OF TECH
Packaging cell
A virus-producing cell sustaining the ability to produce viruses at high titer is successfully constructed by expressing the virus structural gene under the regulation of EF1α promoter. In this virus-producing cell, the virus structural gene is ligated to a selection marker gene via IRES and domains other than the protein coding domain are eliminated from the DNA encoding virus structural proteins. Thus, reduction of the titer due to cell passages can be prevented and emergence of wild type viruses caused by unfavorable recombination of the virus genome can be inhibited.
Owner:CHUGAI PHARMA CO LTD
Mammalian cell culture processes for protein production
ActiveUS20110081679A1High densityReduce aggregationPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsBiotechnologyGlucocorticoid
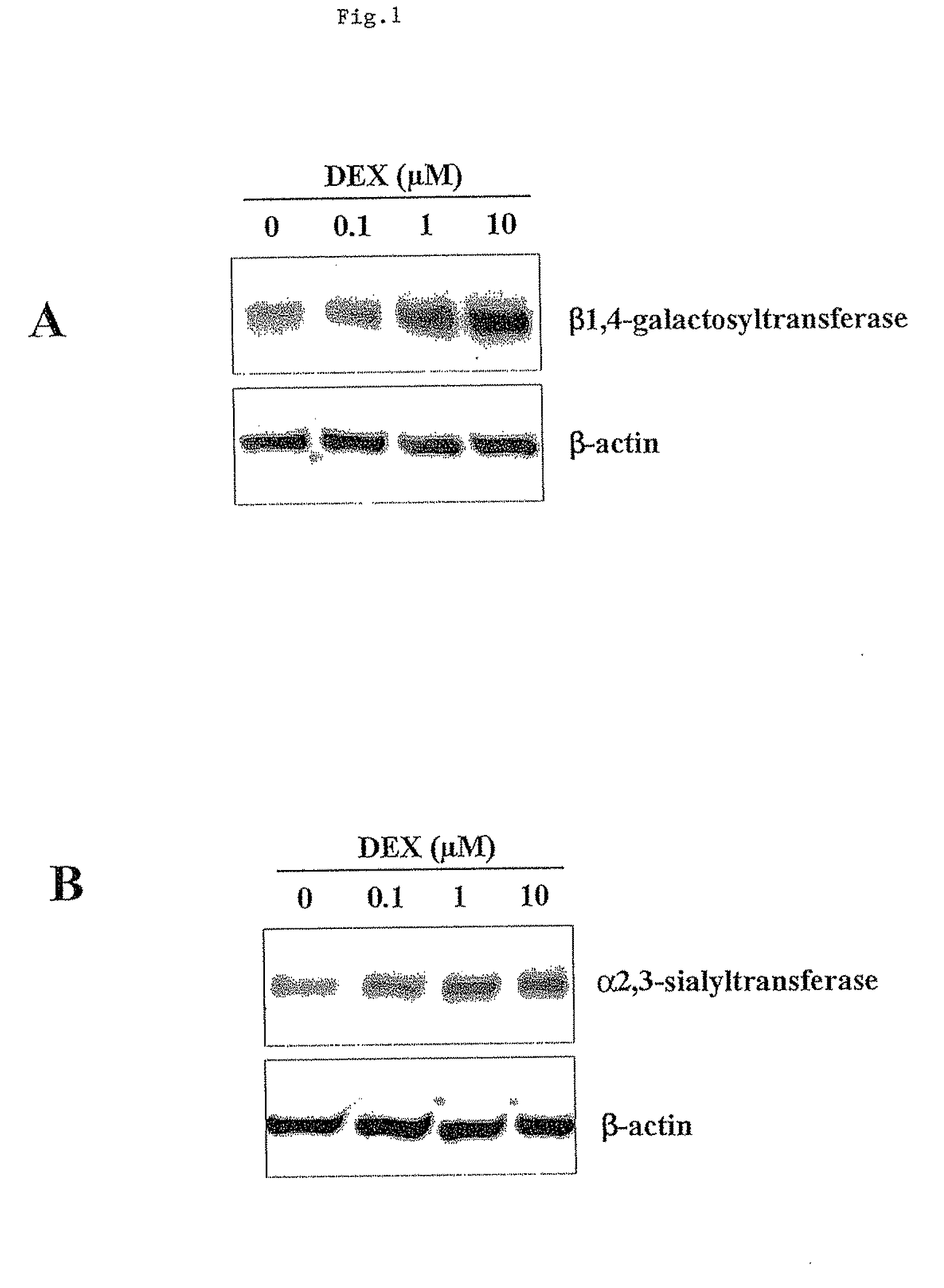
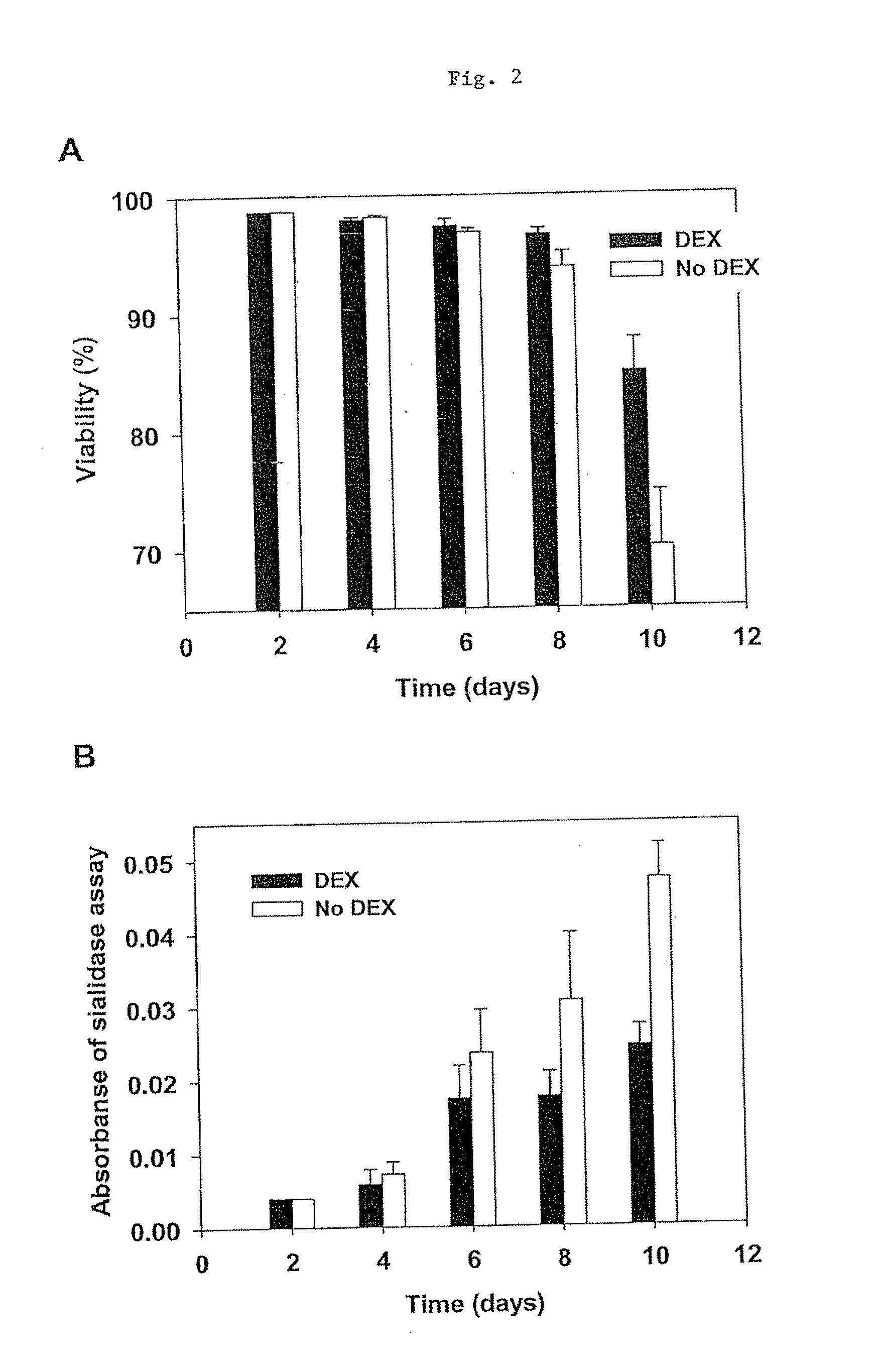
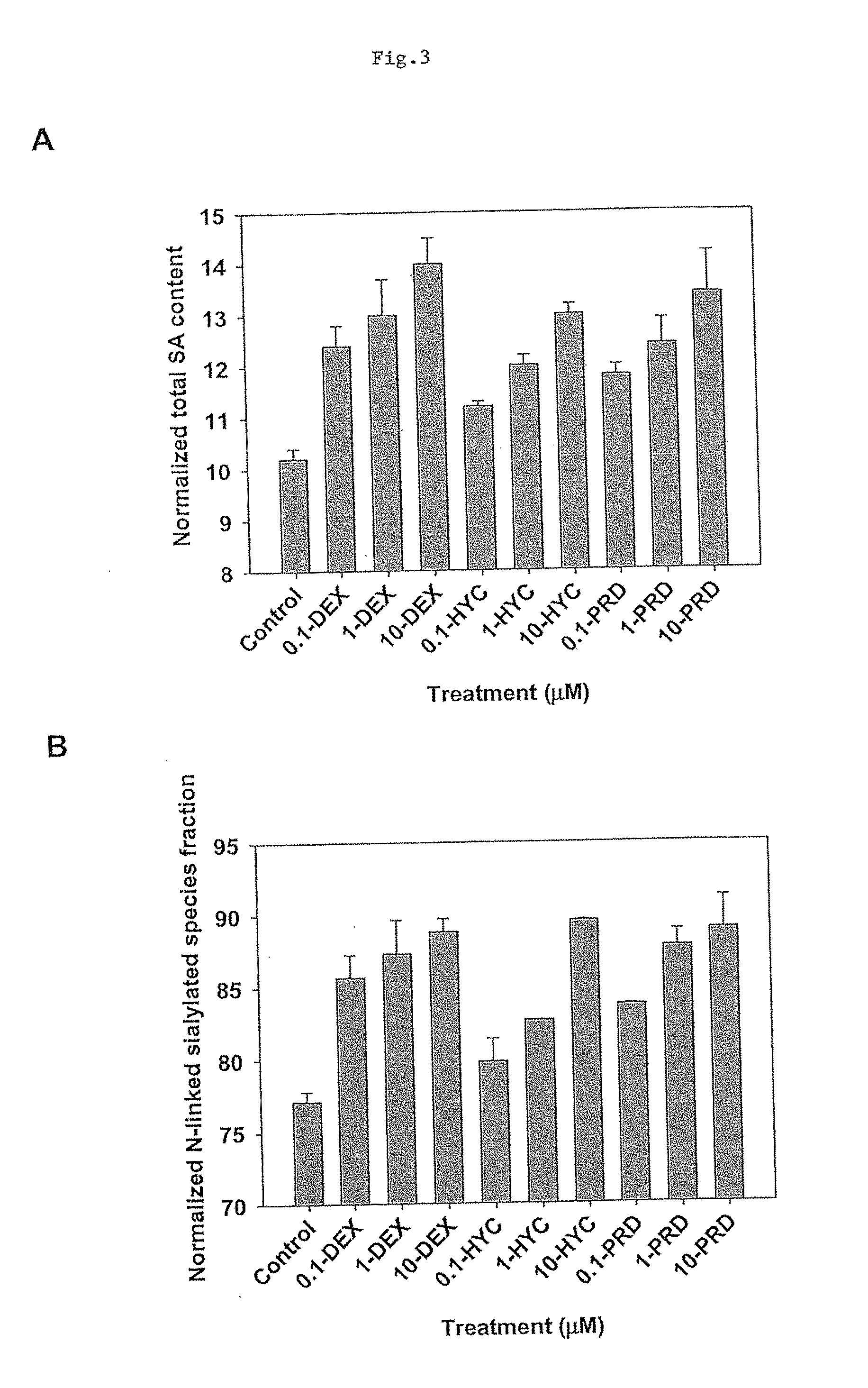
The present invention describes methods and processes for the production of proteins, particularly glycoproteins, by animal cell or mammalian cell culture, preferably, but not limited to, fed-batch cell cultures. In one aspect, the methods comprise the addition of glucocorticoid compound during the culturing period. The addition of glucocorticoid compound sustain a high viability of the cultured cells, and can yield an increased end titer of protein product, and a high quality of protein product, as determined, e.g., by sialic acid content of the produced protein.
Owner:BRISTOL MYERS SQUIBB CO
Assay for porcine circovirus production
ActiveUS7358075B2Microbiological testing/measurementBiological material analysisAssayCell culture media
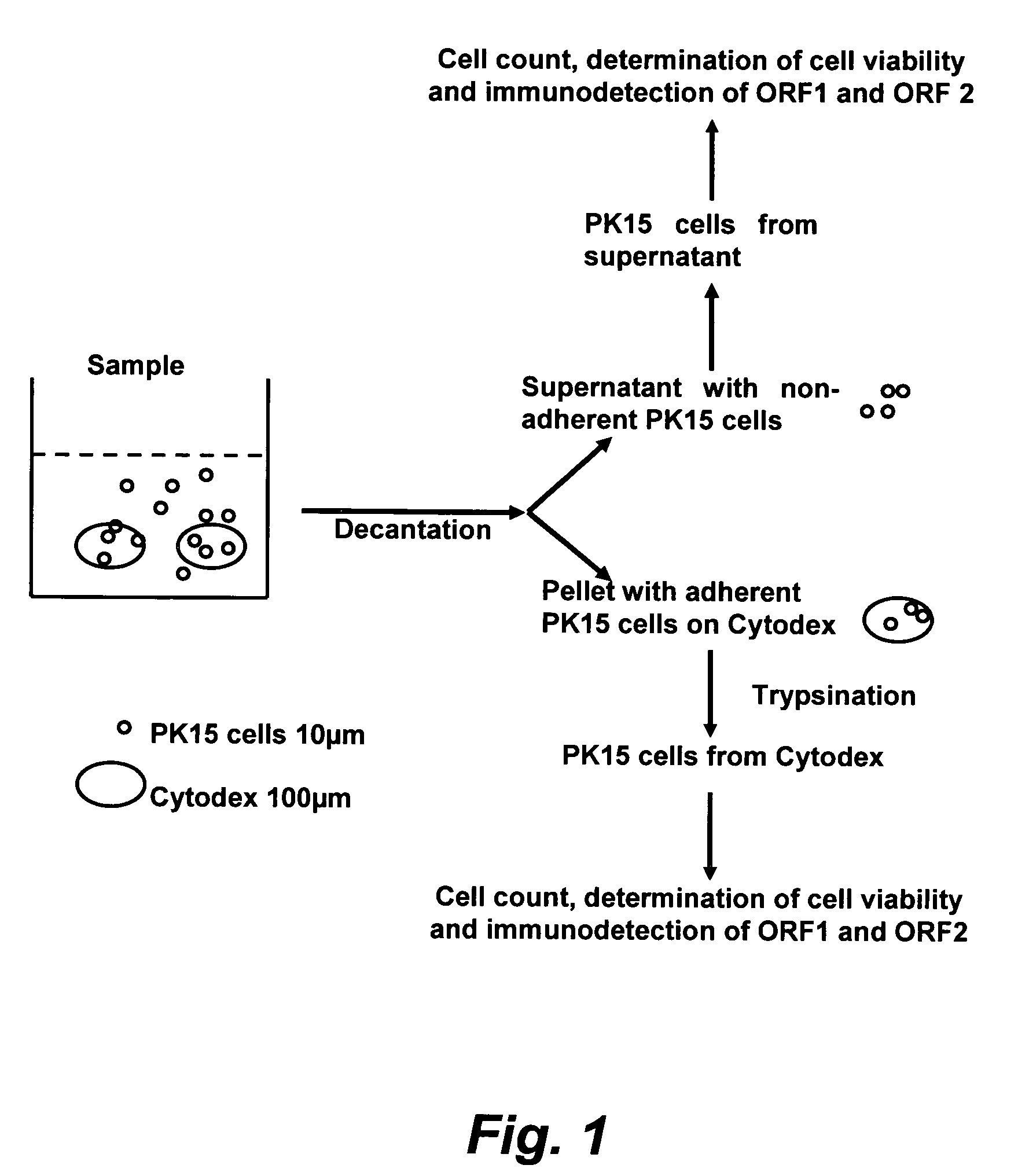
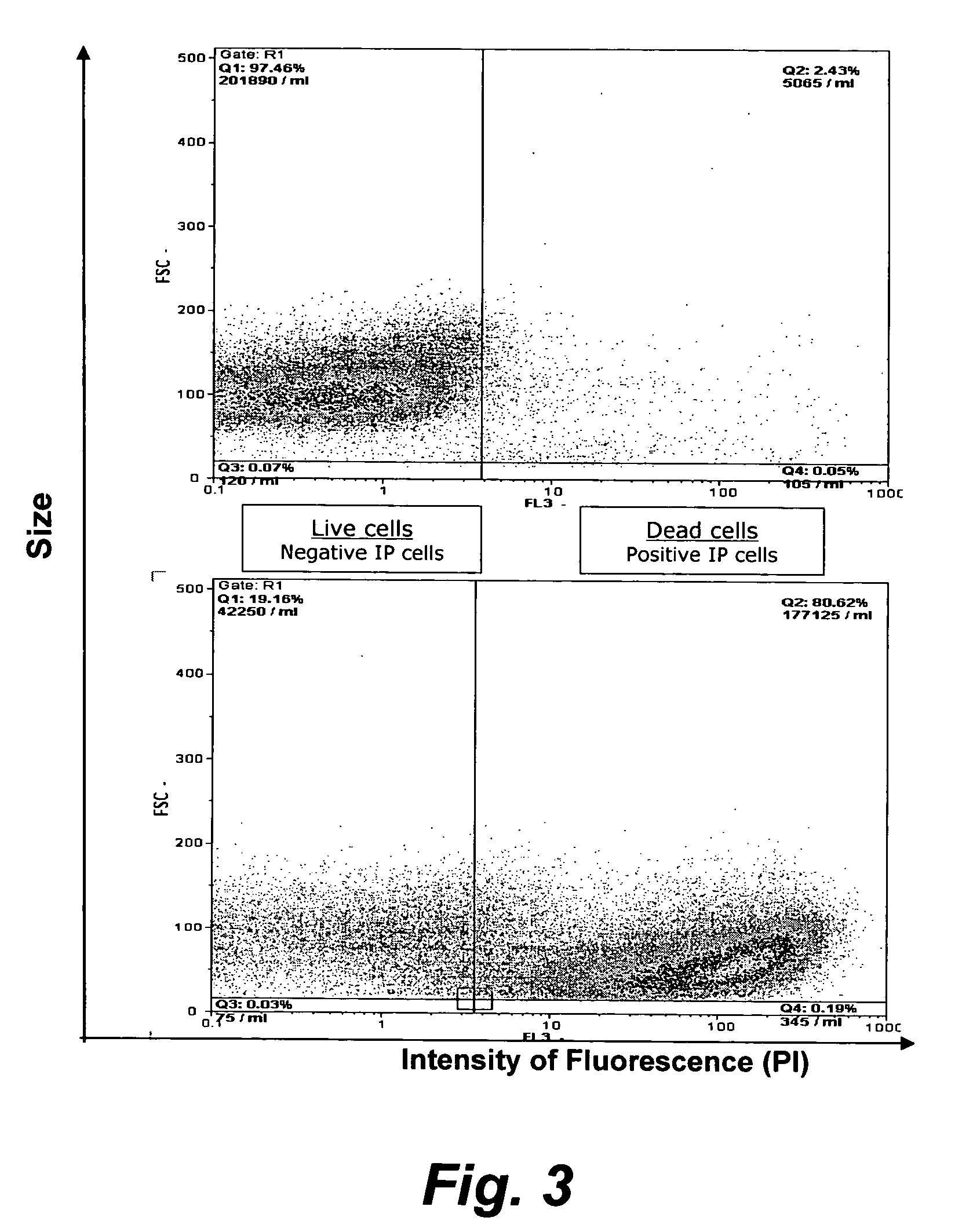
The present invention provides methods for the determination of the viral titer of a culture of host animal host cells infected with a circovirus. The FACS-based methods of the invention may include determining the viability of the host cells in a cell culture medium supernatant and of those cells that remain adherent to a solid support. Detecting and measuring the percentage of cells that expressed the viral antigens ORF1 and ORF2 may determine the viral load of the cultured host cells. The yield of the virus may be established by the detection and measurement of both antigens in supernatant cells, for example 5 to 7 days from when the host cells are transferred to a serum free medium. The methods of the invention may yield rapid quantitative data. This allows the repeated in-process monitoring of the viral production throughout the incubation period, and ready selection of the most appropriate harvesting point.
Owner:MERIAL INC
Mammallian cell culture process for protein production
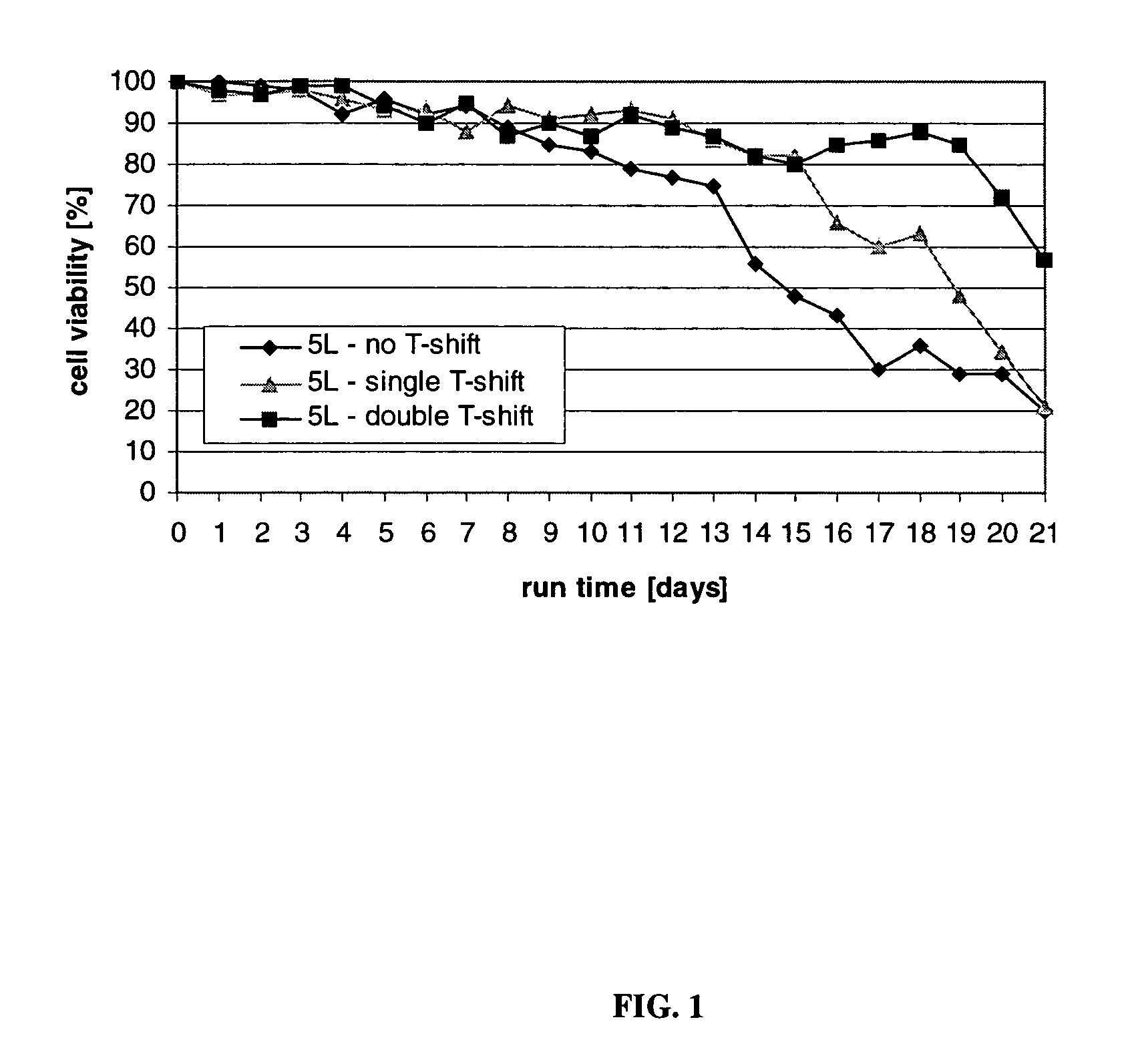
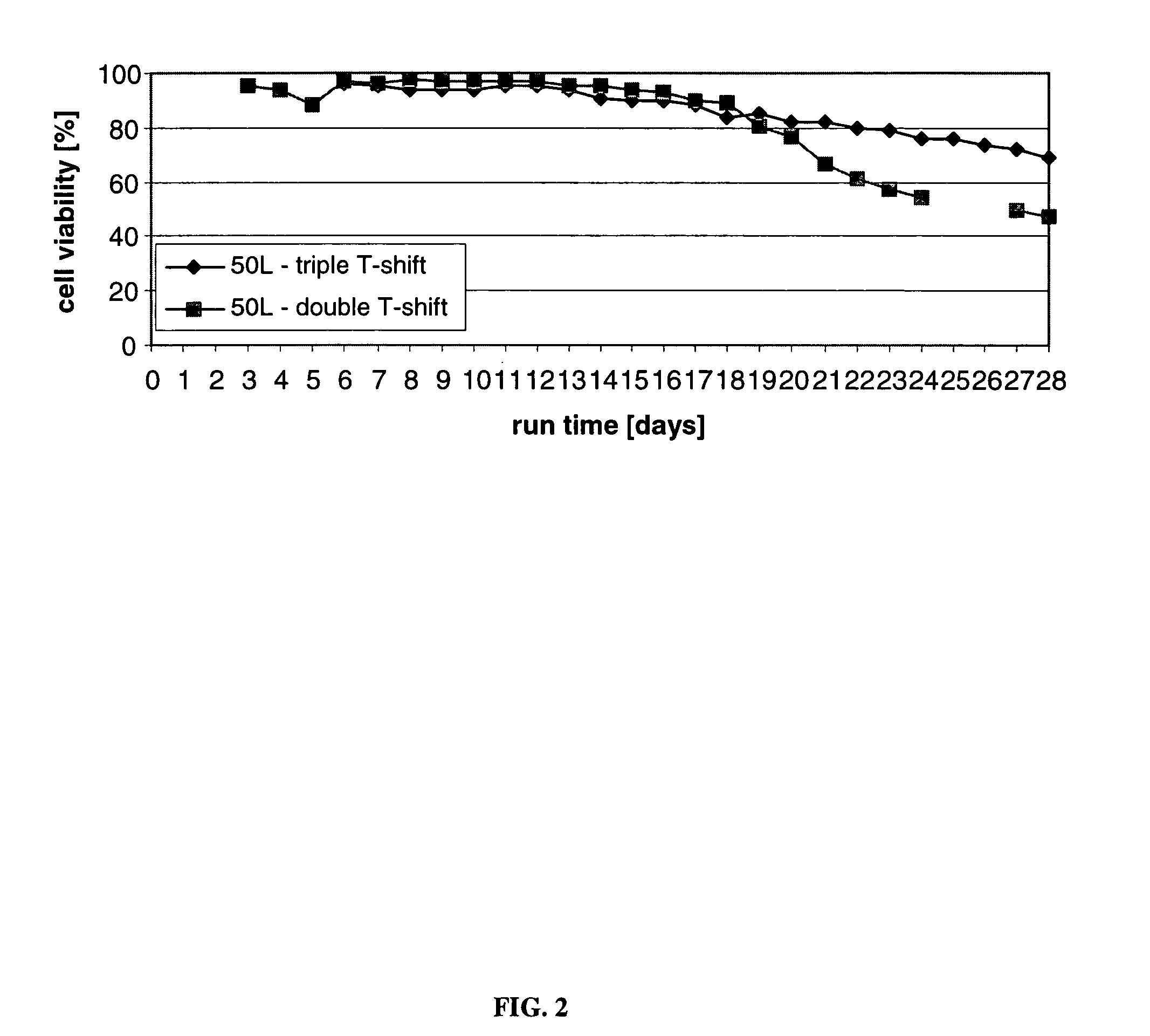
InactiveUS20120015438A1Enhance cell viabilityEnhanced final titer and concentrationAnimal cellsCell receptors/surface-antigens/surface-determinantsGrowth phaseSialic acid
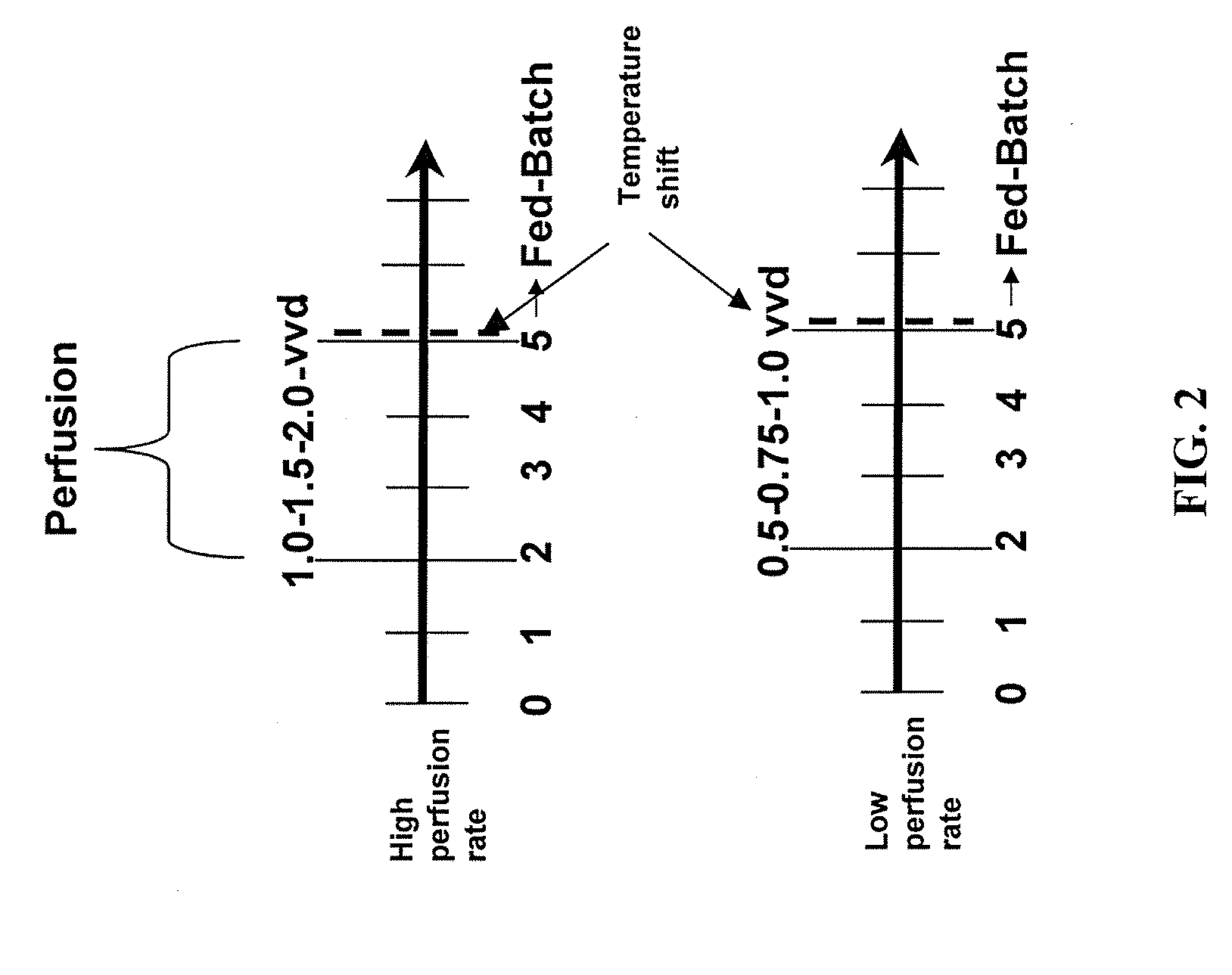
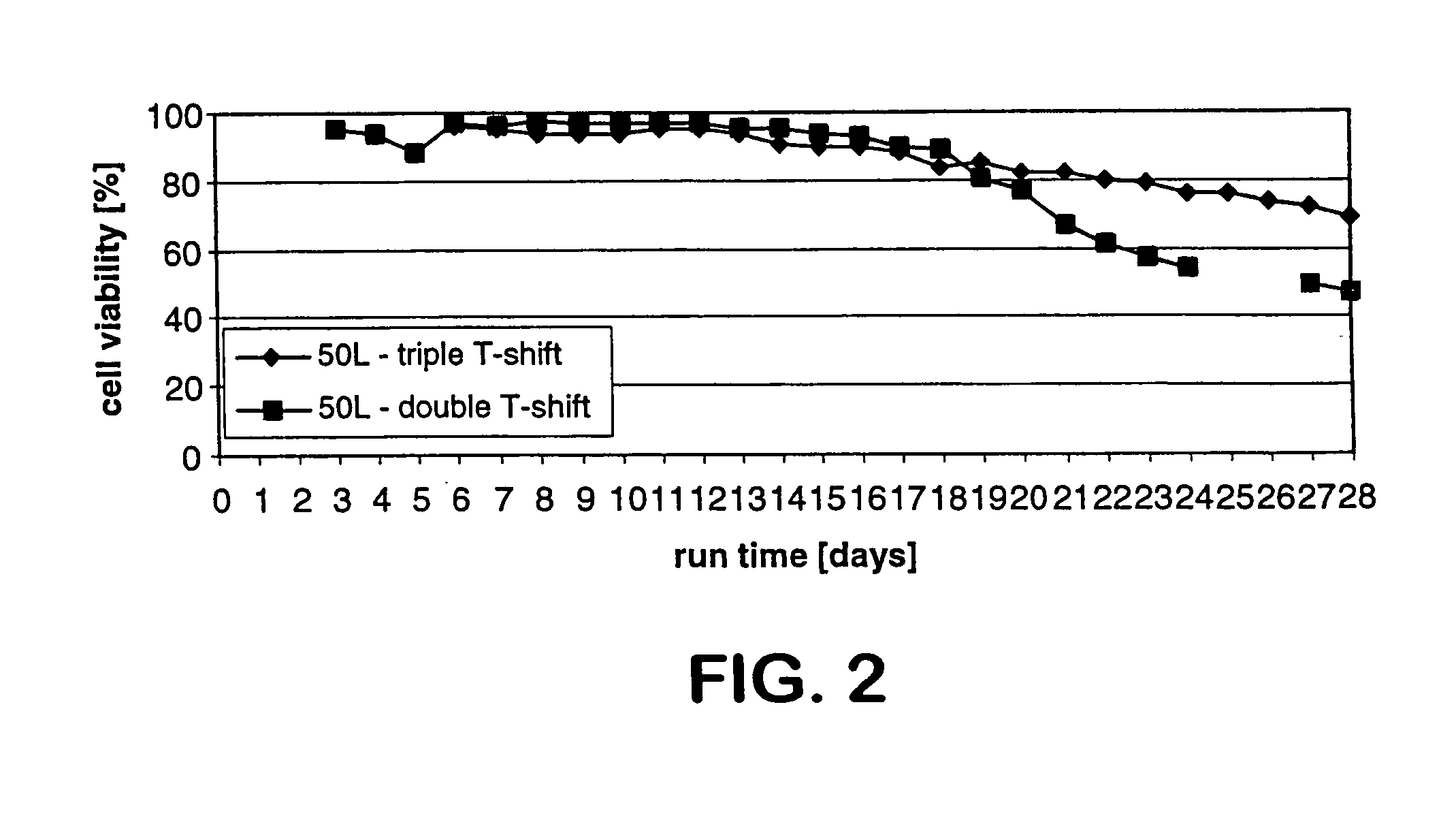
The present invention describes methods and processes for the production of proteins, particularly glycoproteins, by animal cell or mammalian cell culture, preferably, but not limited to, fed-batch cell cultures. In one aspect, the methods comprise at least two temperature shifts performed during the culturing period, in which the temperature is lower at the end of the culturing period than at the time of initial cell culture. Throughout their duration, the culturing processes of the invention involving two or more downward shifts in temperature sustain a high viability of the cultured cells, and can yield an increased end titer of protein product, and a high quality of protein product, as determined, e.g., by sialic acid content of the produced protein. In another aspect, the methods comprise the delayed addition of polyanionic compound during the culturing period. The delayed addition of polyanionic compound sustains a high viability of the cultured cells, and can extend the growth phase, delay the onset of the death phase, and arrest the death phase.
Owner:BRISTOL MYERS SQUIBB CO
Hybridoma cell line 1C11 and anti-aflatoxin general monoclonal antibody generated by same as well as applications thereof
ActiveCN101993855AHigh sensitivityHigh practical application valueMicroorganism based processesTissue cultureCell strainHybridoma cell
The invention provides a hybridoma cell line 1C11 and an anti-aflatoxin general monoclonal antibody secreted by the same as well as the applications thereof. The hybridoma cell line 1C11 can be used for preparing a high-titer aflatoxin antibody, and a mouse hydroperitoneum antibody is measured to reach 5.12*106 by using an ELISA (Enzyme-Linked Immunosorbent Assay). The anti-aflatoxin general monoclonal antibody has high sensitivity, respectively reaches the IC50 (50% inhibiting concentration) of aflatoxin B1, B2, G1 and G2 to be 1.2, 1.3, 2.2 and 18.0 pg / mL, is the antibody with highest sensitivity among currently reported four aflatoxin antibodies, is used for measuring the total aflatoxin amounts, i.e. the total amounts of the aflatoxin B1, B2, G1 and G2 and has great practical application values.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Methods and compositions for use in treatment of patients with autoantibody positive disease
ActiveUS20090148462A1Reduce frequencyReduce in quantityNervous disorderPeptide/protein ingredientsAnti-dsDNA antibodiesSystemic lupus erythematosus
The present invention relates to methods and compositions for use in treatment of patients with autoantibody positive disease. In a specific embodiment, the present invention relates to a method of treating a patient that has an ANA titer of 1:80 or greater and / or greater than or equal to 30 IU / ml of anti-dsDNA antibodies in his / her blood plasma or serum comprising administering a therapeutically effective amount of an immunomodulatory agent, such as an antagonist of Neutrokine-alpha. Additionally provided is a method of reducing the frequency and / or quantity of corticosteroid administration to patients. In preferred embodiments, the patient has systemic lupus erythematosus. Methods for determining if a lupus patient is responding to medical treatment are also provided.
Owner:HUMAN GENOME SCI INC
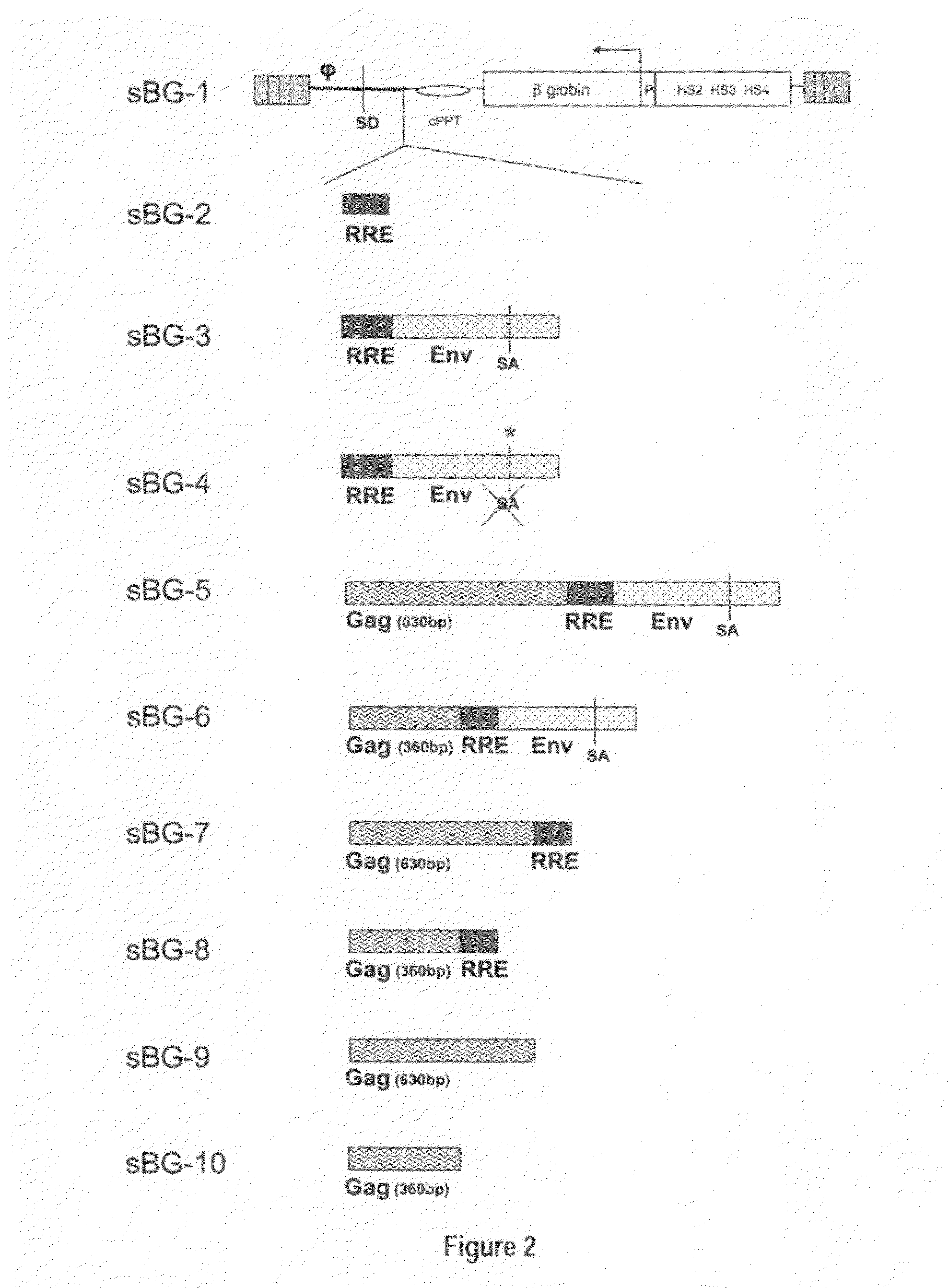
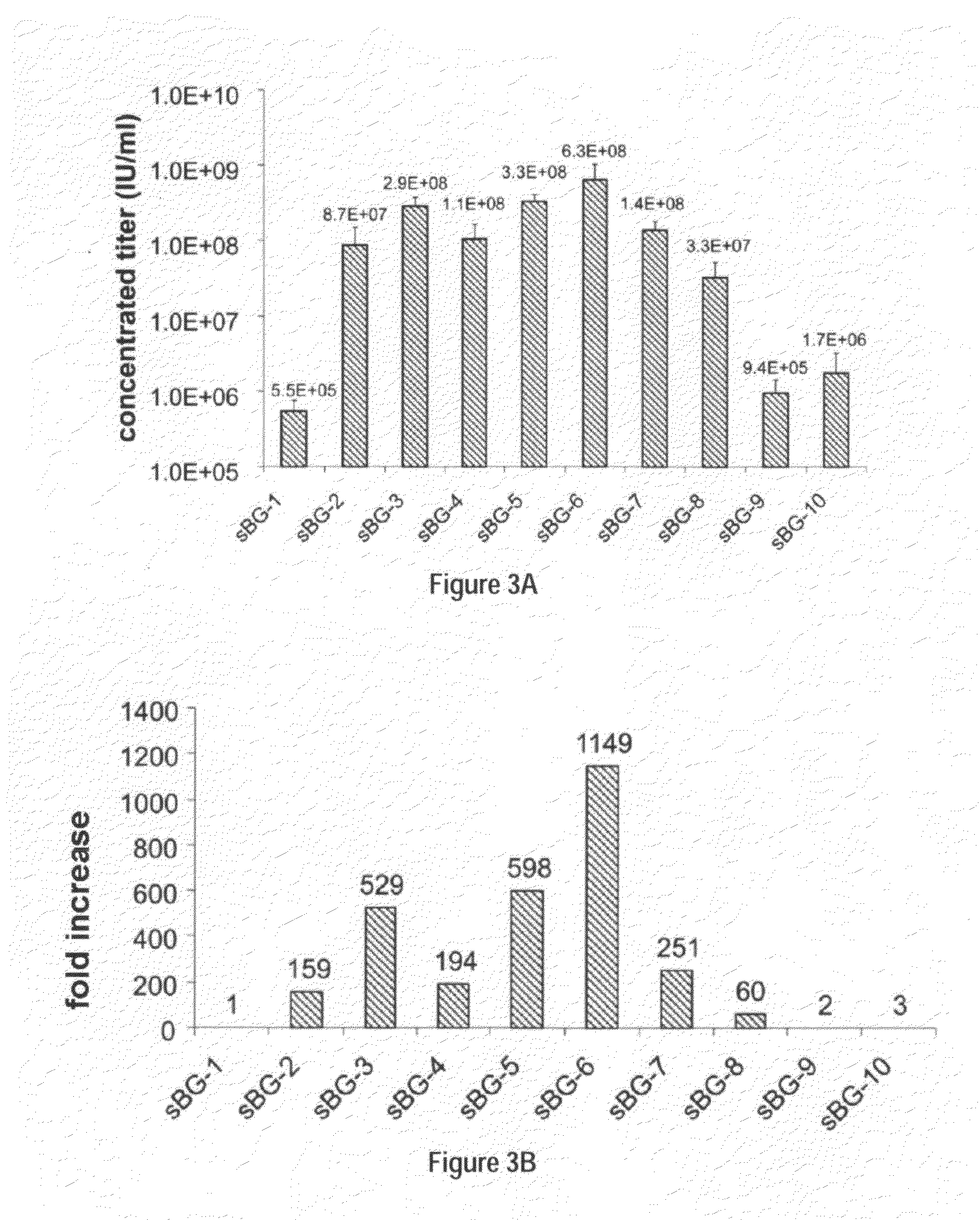
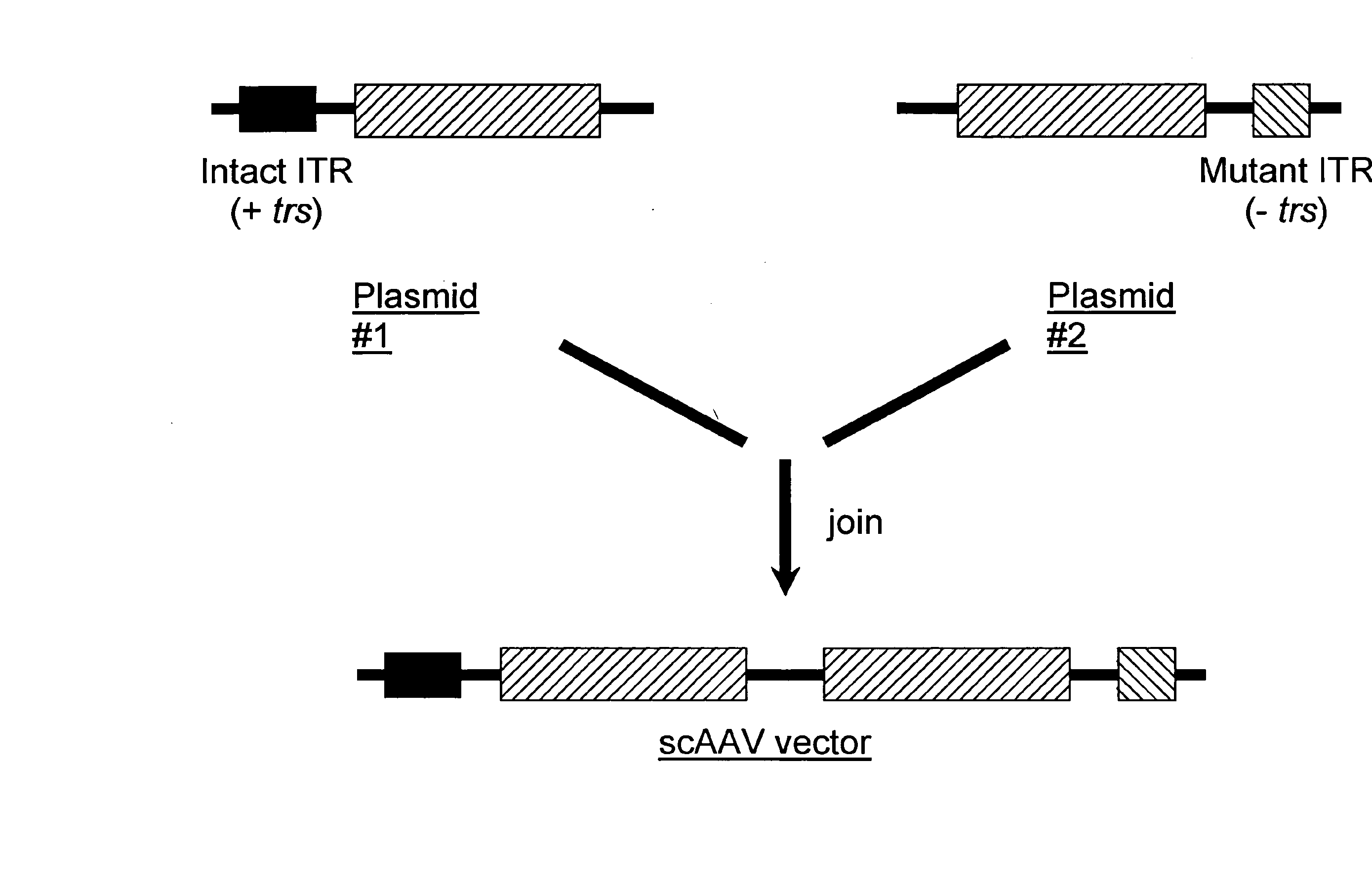
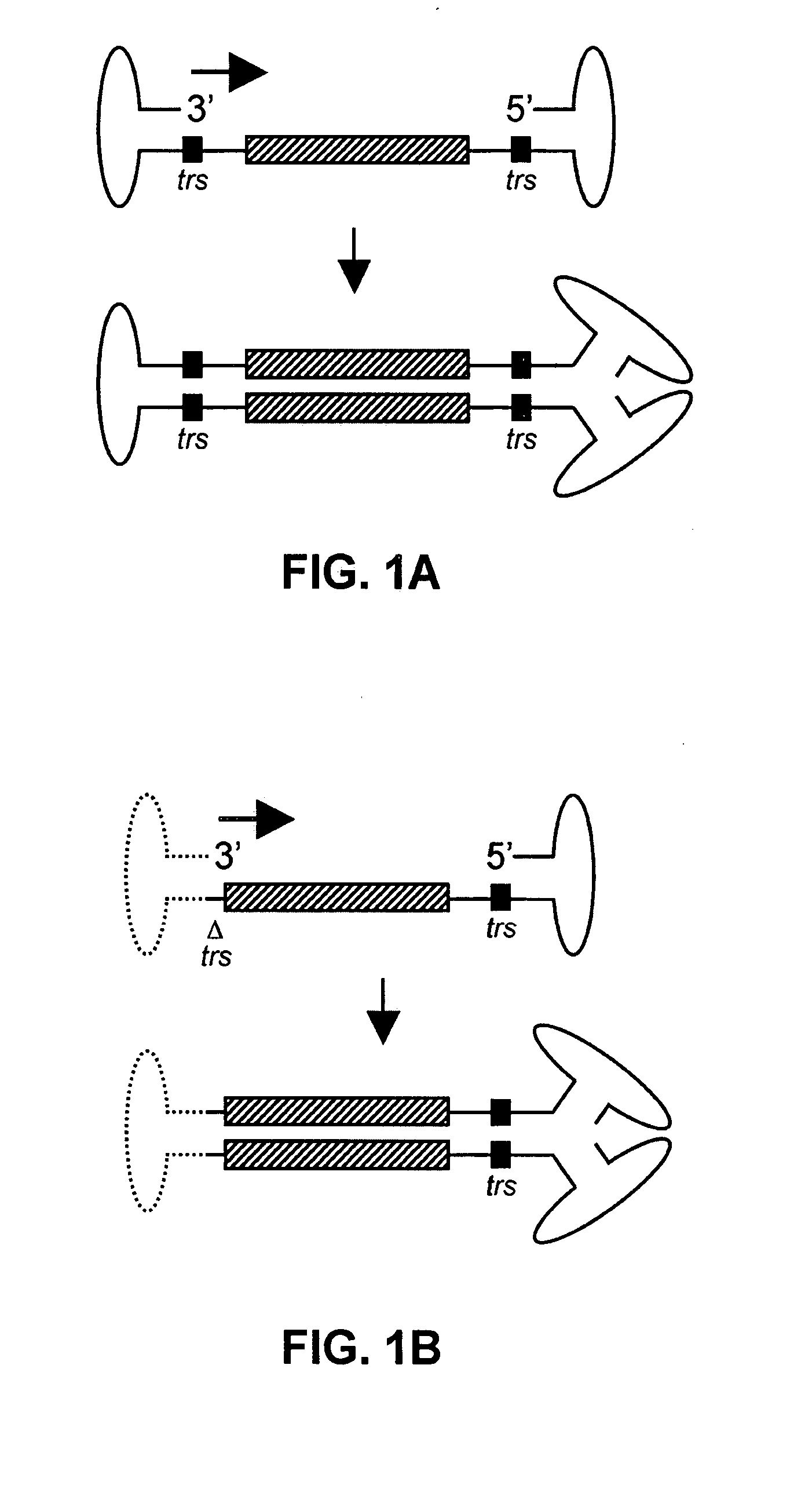
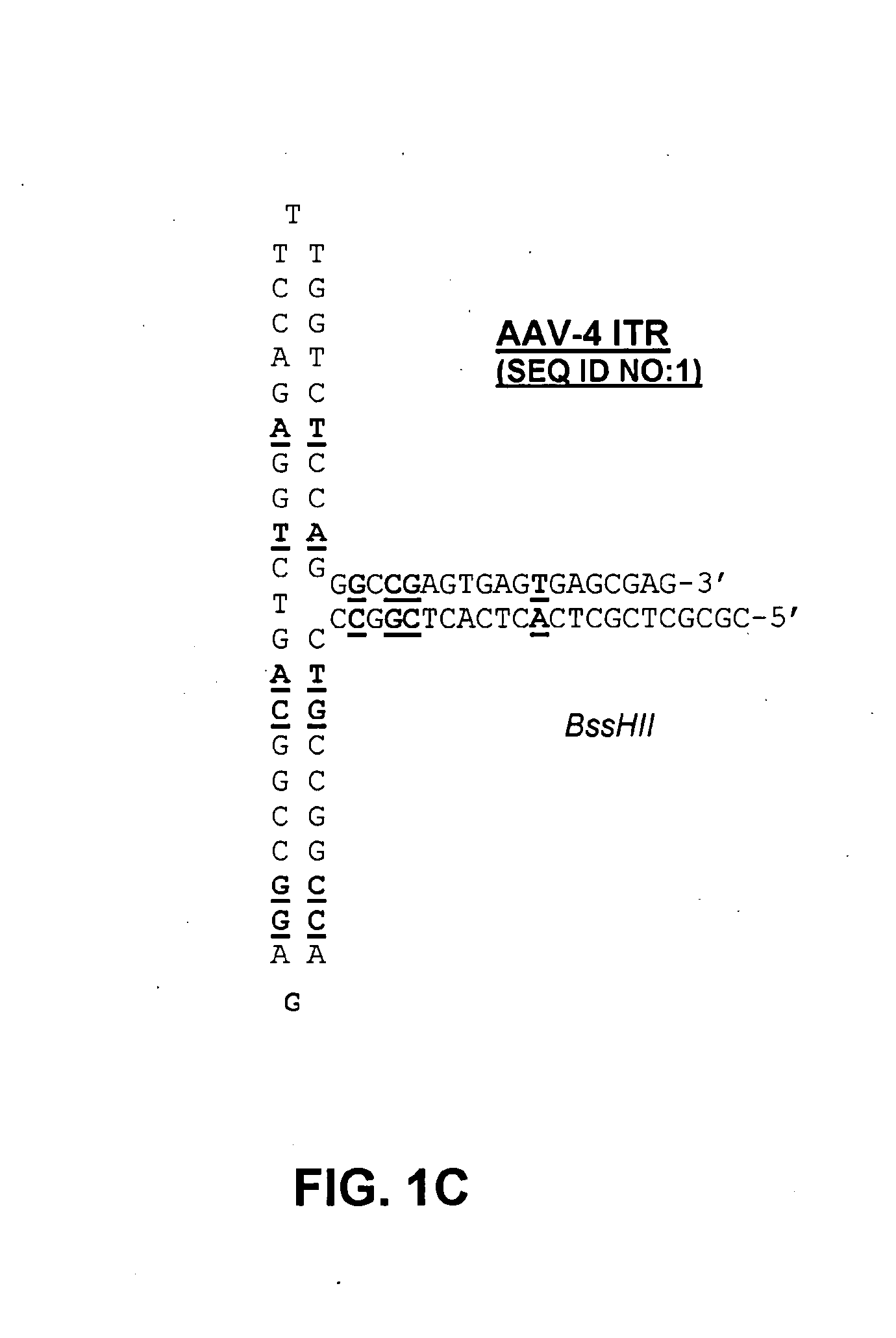
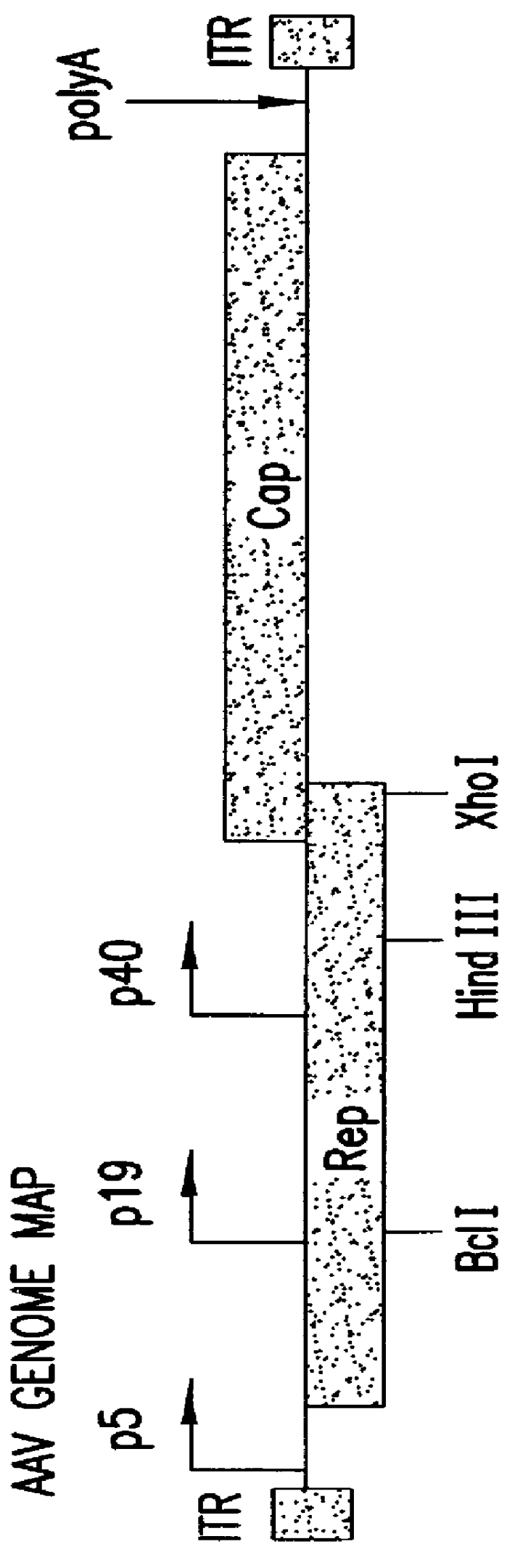
Self-complementary parvoviral vectors, and methods for making and using the same
ActiveUS20070253936A1High genetic stabilityStabilized parvovirus vector is more stableBiocideAnimal repellantsGene transferTiter
The teachings herein are generally directed to a method of enhancing the genetic stability of parvovirus vectors. The stability of conventional ss or dsAAV vector constructs can be enhanced, for example, to obtain a concurrent increase in vector titer and purity, as well as an improvement in vector safety, due at least in part to the elimination of stuffer DNA from the AAV vector. The method is broadly applicable to all gene transfer / therapy applications, such as those requiring delivery of foreign DNA containing recombinant gene expression cassettes. Such foreign DNA can range, for example, from about 0.2 up to about 5.2 kb in length. The enhanced vector constructs are highly flexible, user-friendly, and can be easily modified (via routine DNA cloning) and utilized (via standard AAV vector technology) by anyone skilled in the art.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Improved prealbumin detection kit
The invention discloses an improved immune transmission turbidity reagent kit for detecting the prealbumin content in blood serum, comprising a reagent R1, a reagent R2 and a prealbumin standard. Through the specific reaction of an antigen-antibody, the reagent kit forms a small immune compound which is smaller than 19s and forms a large visual compound which is larger than 19s under the action of a flocculant to generate a certain turbidity, thereby being suitable for measuring the transmission turbidity. The reagent can effectively prevent false turbidity from generating, has high accuracy rate, favorable reproducibility, strong capacity of resisting disturbance and simple operation and is suitable for various fully automatic biochemical analysers. The titer containing in the reagent adopts stroma with whole serum, thereby the influence of stroma effect is avoided.
Owner:BEIJING STRONG BIOTECH INC
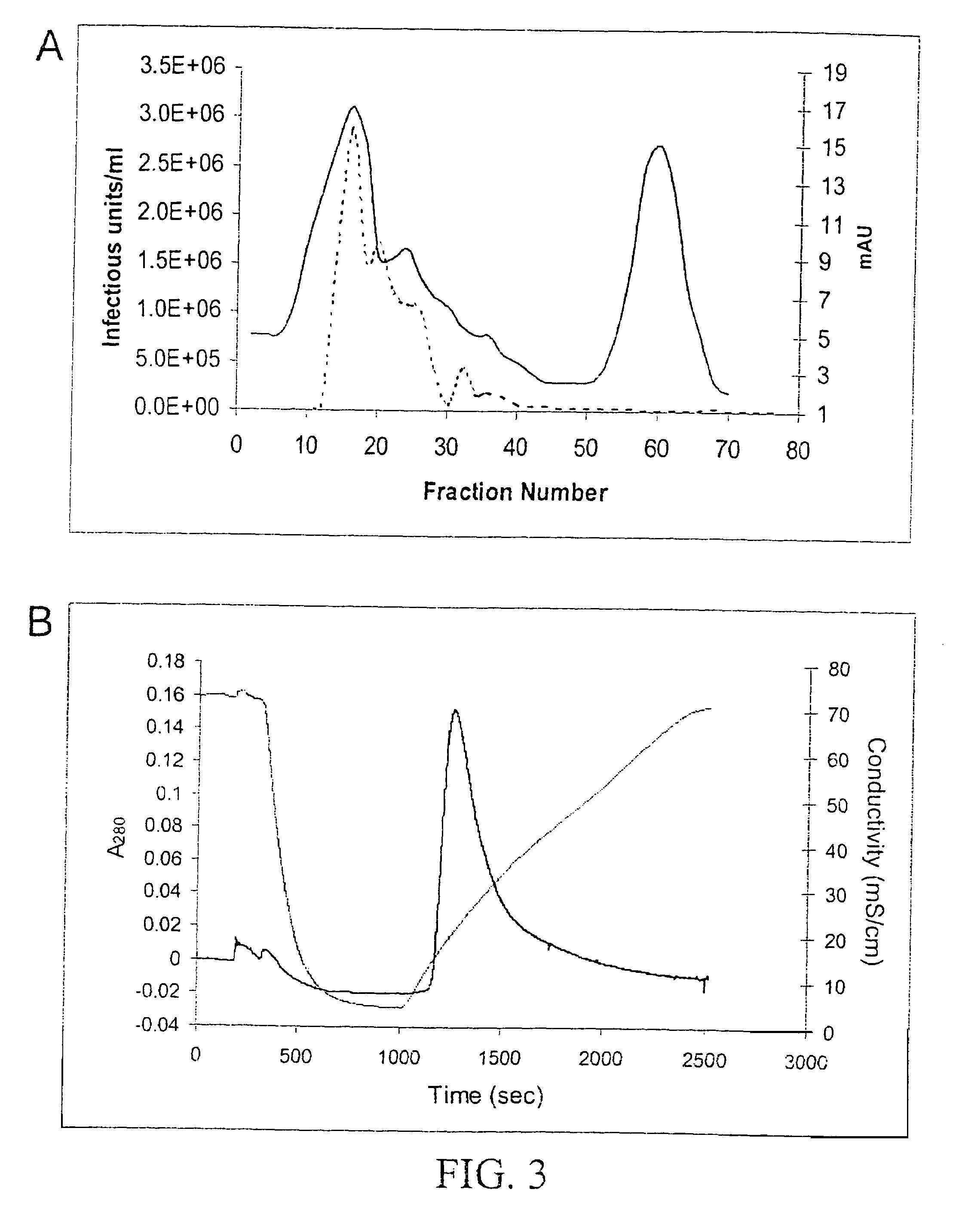
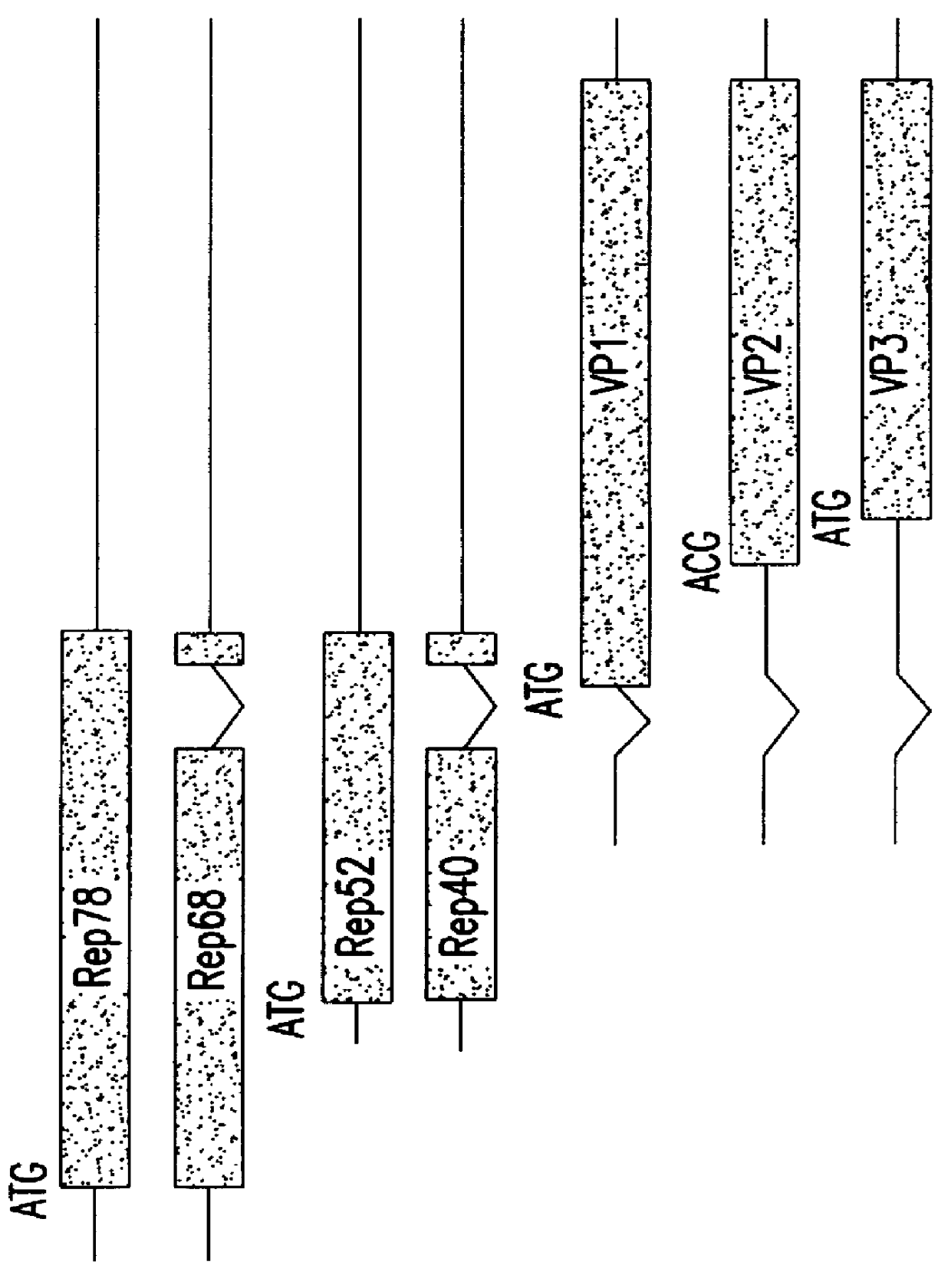
Method for increasing the efficiency of recombinant AAV production
InactiveUS6037177AReduce expressionIncreased of replicationArtificial cell constructsVertebrate cellsPost translationalTiter
The present invention relates to methods and compositions for increasing the production of high titre stocks of recombinant AAV (rAAV) through regulation of expression of the AAV REP and CAP proteins. The methods and compositions of the invention are based on the observation that the low level expression of the AAV REP 78 / 68 protein increases the production of AAV viral capsid protein and efficiency of packaging resulting in production of higher titre recombinant viral stocks. The invention encompasses recombinant AAV vectors that direct the expression of AAV REP and CAP proteins and the use of such vectors for the production of novel stable cell lines capable of generating high titre rAAV vectors. The invention provides methods for regulating the expression of the AAV REP 78 / 68, REP 40 / 52 and CAP gene at the transcriptional and post-translational level. The methods and compositions of the invention can be used to produce high titre stocks of rAAV which can be used in gene therapy for the purpose of transferring genetic information into appropriate host cells for the management and correction of human diseases including inherited and acquired disorders.
Owner:CELLS GENESYS INC
Rationally designed media for cell culture
ActiveUS20080108553A1Peptide/protein ingredientsMicrobiological testing/measurementCell culture mediaCell mass
This invention relates to methods for rationally designing cell culture media for use in cell cultures, e.g., cell cultures employed in polypeptide production; cell culture media designed with the disclosed methods; methods of producing a polypeptide of interest, e.g., an antibody, using such media; polypeptides produced using the methods and media disclosed herein; and pharmaceuticals compositions containing such polypeptides. The rationally designed media contain a concentration of an amino acid that is calculated for use in cell mass, a concentration of the amino acid that is calculated for use in cell maintenance, and a concentration of the amino acid that is calculated for incorporation into the polypeptide of interest. The rationally designed media may contain a baseline-adjusted concentration, A, of at least one amino acid that is calculated according to the formula A=[(M*X)+(N*P)+(Y*M*X)]*F, wherein X is the concentration of the amino acid that is used per unit of cell mass, P is the concentration of the amino acid that is used for incorporation into the polypeptide of interest per unit of polypeptide titer, M is the multiplier for the desired peak cell density of the cell culture, N is the multiplier for the desired concentration of the polypeptide of interest, Y is the cell maintenance factor; and F is the baseline factor. This rationally designed media may also be used to produce a starting cell culture medium comprising a concentration, B, of at least one amino acid according to the formula B=[A−(Z*V)] / (1−V), wherein Z is a concentration of the amino acid in the feeding cell culture medium, and V is a volume of the feeding culture medium as a proportion of the desired cell culture medium volume.
Owner:WYETH LLC
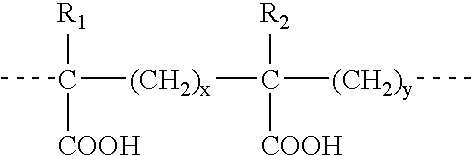
Method for preparing low-titer high-strength high-modulus polyethylene fibre
The present invention discloses a process for producing low-titer, high-strength and high-modulus polyethylene fibers, comprising the following steps: dissolving the ultra-high molecular weight polyethylene into paraffin oil with a low viscosity to form a spinning solution with a concentration of 3ˆ¼15%; extruding the spinning solution through a thin spinneret with at least 10 orifices having a diameter ¦ of 0.7ˆ¼0.8mm and a length / diameter ratio of 10ˆ¼12, by applying a high pressure in the range of 2.5±1.0MPa to the spinning solution, such that the fluid in the orifices is extruded at a shear rate of 200ˆ¼3 500sec -1 ; and then performing a jet stretch at a deformation rate of 200ˆ¼5 000min -1 within an air-gap of 10ˆ¼15mm between the spinneret and the quench bath surface; feeding the jet-stretched fluid into the quench bath to form gel filaments; extracting and drying the gel filaments; and performing a multistage ultrahigh post stretch on the dried gel filaments with a stretch ratio of 15 or less. In this process, the solution is extruded at a high rate by using a spinneret with small orifice diameter, thereby achieving a high shear rate and a high deformation rate, so the present process is a novel and effective spinning process.
Owner:HUNAN ZHONGTAI SPECIAL EQUIP
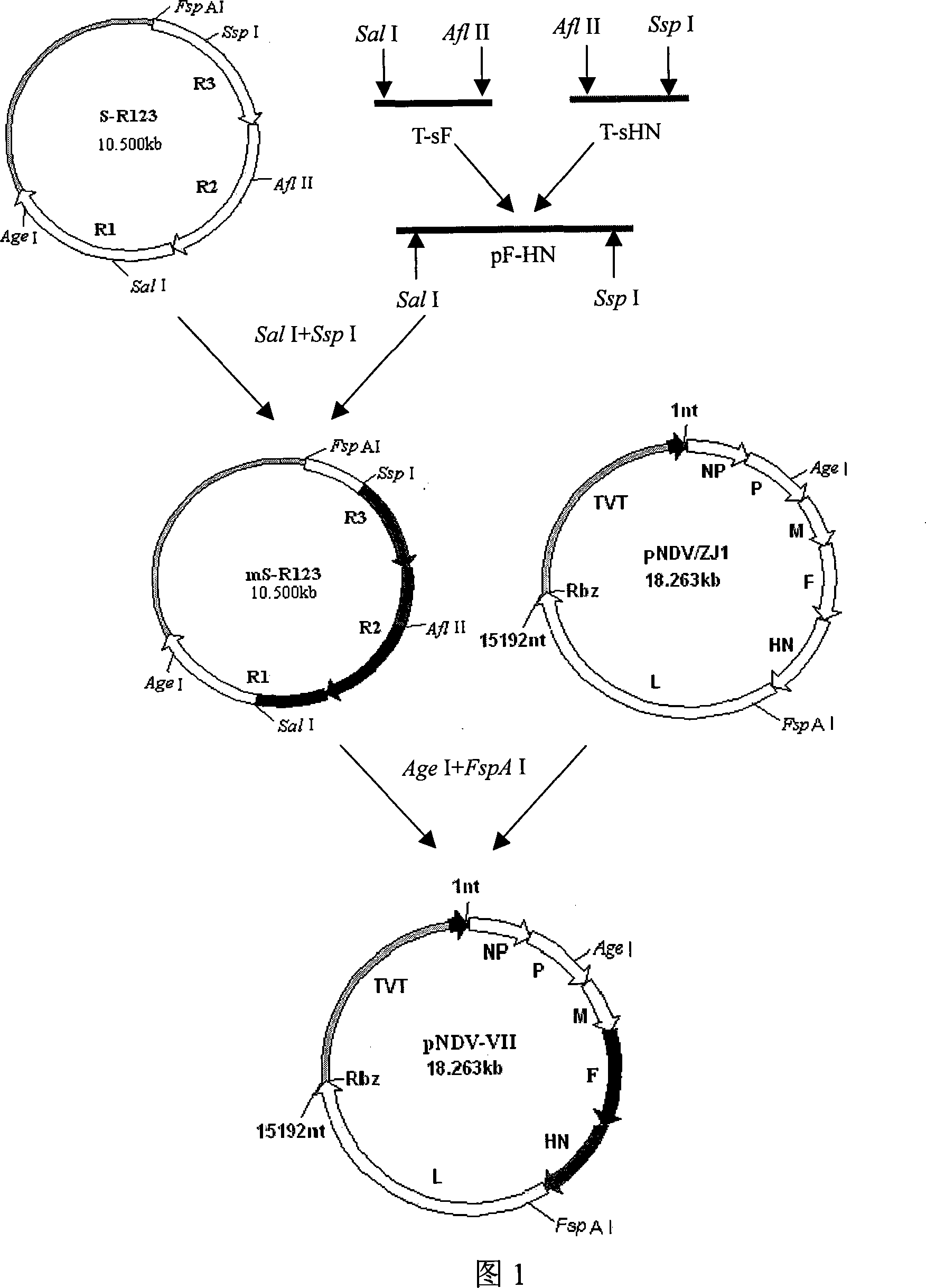
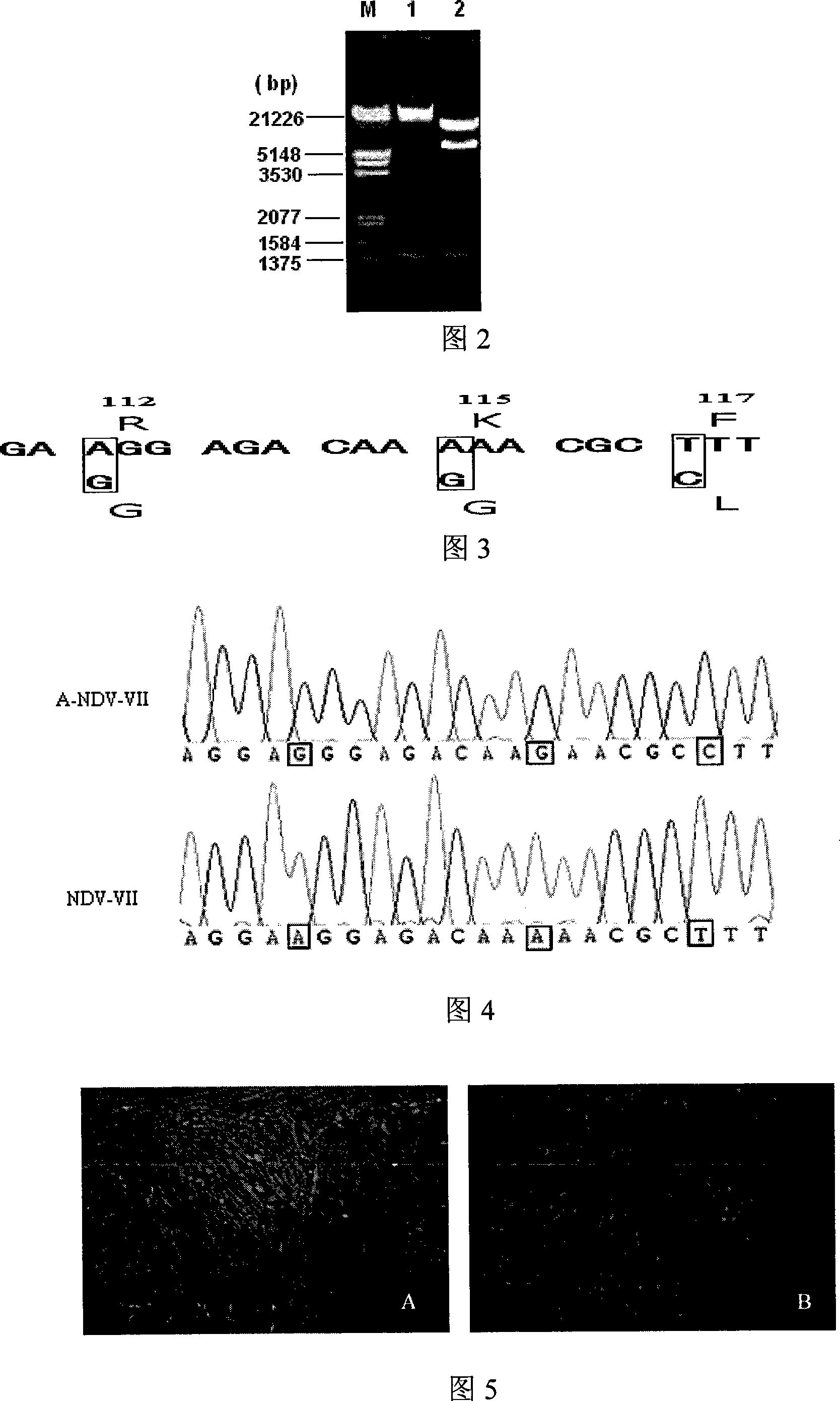
Gene ó¸ type new castle disease virus weakening strain A-NDV-ó¸ and construction method thereof
ActiveCN101182494AHigh reproductive titerSuitable for mass productionInactivation/attenuationNewcastle disease virus NDVGene type
A VII gene type of an attenuated strain of Newcastle disease virus A-NDV-VII and a construction method are disclosed. The invention relates to the application of reverse genetics technique. The invention uses the constructed reverse genetics platform of ZJ1 strain of Newcastle disease virus of goose origin. The invention replaces two envelope glycoprotein gene fragments F and HN of an isolated strain JS-5-05-Go of Newcastle disease virus with high reproductive performance with the corresponding fragments of the ZJ1 strain of Newcastle disease virus of goose origin, so that the recombinant virus NDV-VII is obtained. The VII gene type of Newcastle disease virus A-NDV-VII which is highly attenuated is rescued after the attenuated mutation of the F gene of the recombinant virus. And the virus has a higher reproduction titer on chicken embryo. The invention is suitable for a mass production of vaccine, which can be used for the manufacture of vaccine.
Owner:YANGZHOU UNIV
Quantitative determination RBP4 kit by chemiluminescence magnetic enzymoimmune method
ActiveCN101452001AExtended storage timeStable LuminescenceChemiluminescene/bioluminescenceBiological testingImmunocompetenceMagnetic bead
The invention relates to a medical testing kit for performing quantitative detection on human serum RBP4 using chemiluminescence magnetic-enzyme immunotherapy. The kit is composed of four reagent parts: specificity mouse anti-human RBP4 custodite immunomagnetic beads, enzyme labelling specificity mouse anti-human RBP4 antibody II, chemiluminescence substrate, corresponding titer and quality control liquid. The using method of the kit comprises: using bead particulates as solid phase carrier, combining specificity mouse anti-human RBP4 antibody I on the surface, forming RBP4 specificity immunocompetence beads, capturing antigen RBP4 to be detected in the enzyme labelling specificity mouse anti-human RBP4 antibody II, forming double antibody sandwich composite on the surface of the beads, wherein enzyme marked on the composite reacts with corresponding irradiance substrate in the reaction system to form stable luminous signals, thereby reaching quantitative detection and analysis on RBP4 through strength of the detection light signals. The invention has the advantages of high sensitivity, high specificity, simple and fast operation.
Owner:WUHAN EASYDIAGNOSIS BIOMEDICINE
Methods and compositions for use in treatment of patients with autoantibody positive disease
InactiveUS20090081213A1Reduce quantity and frequencyReduce frequencyPeptide/protein ingredientsAntipyreticAnti-dsDNA antibodiesSystemic lupus erythematosus
The present invention relates to methods and compositions for use in treatment of patients with autoantibody positive disease. In a specific embodiment, the present invention relates to a method of treating a patient that has an ANA titer of 1:80 or greater and / or greater than or equal to 30 IU / ml of anti-dsDNA antibodies in his / her blood plasma or serum comprising administering a therapeutically effective amount of an immunomodulatory agent, such as an antagonist of Neutrokine-alpha. Additionally provided is a method of reducing the frequency and / or quantity of corticosteroid administration to patients. In preferred embodiments, the patient has systemic lupus erythematosus. Methods for determining if a lupus patient is responding to medical treatment are also provided.
Owner:HUMAN GENOME SCI INC
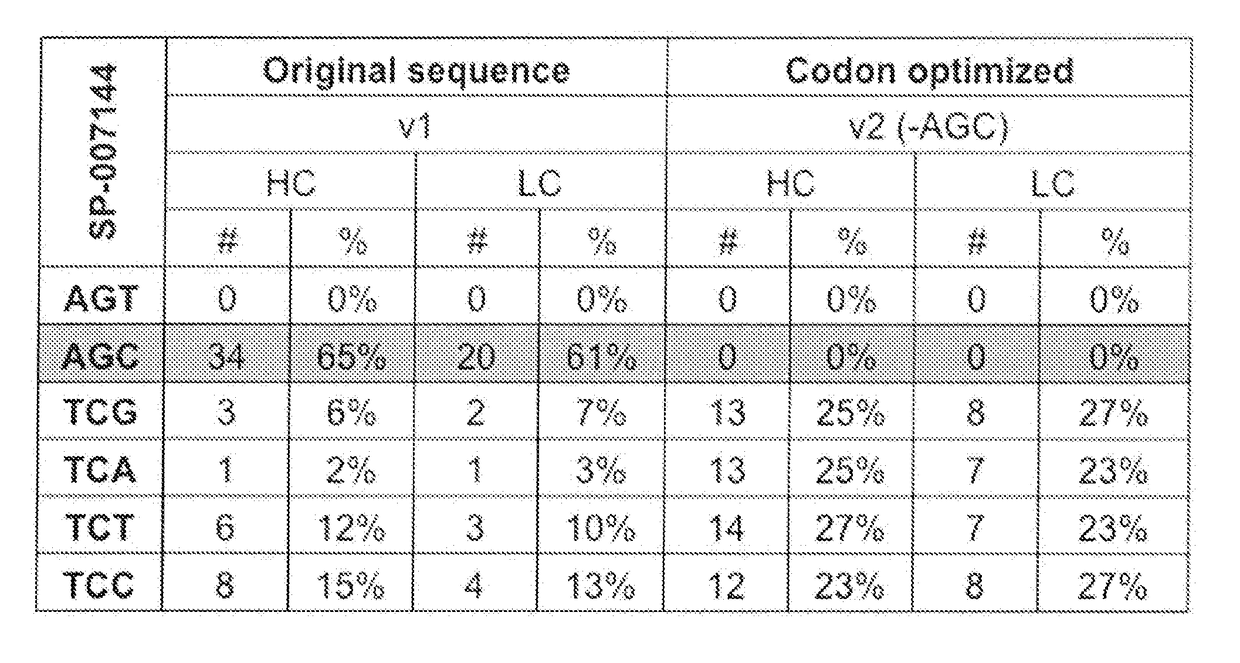
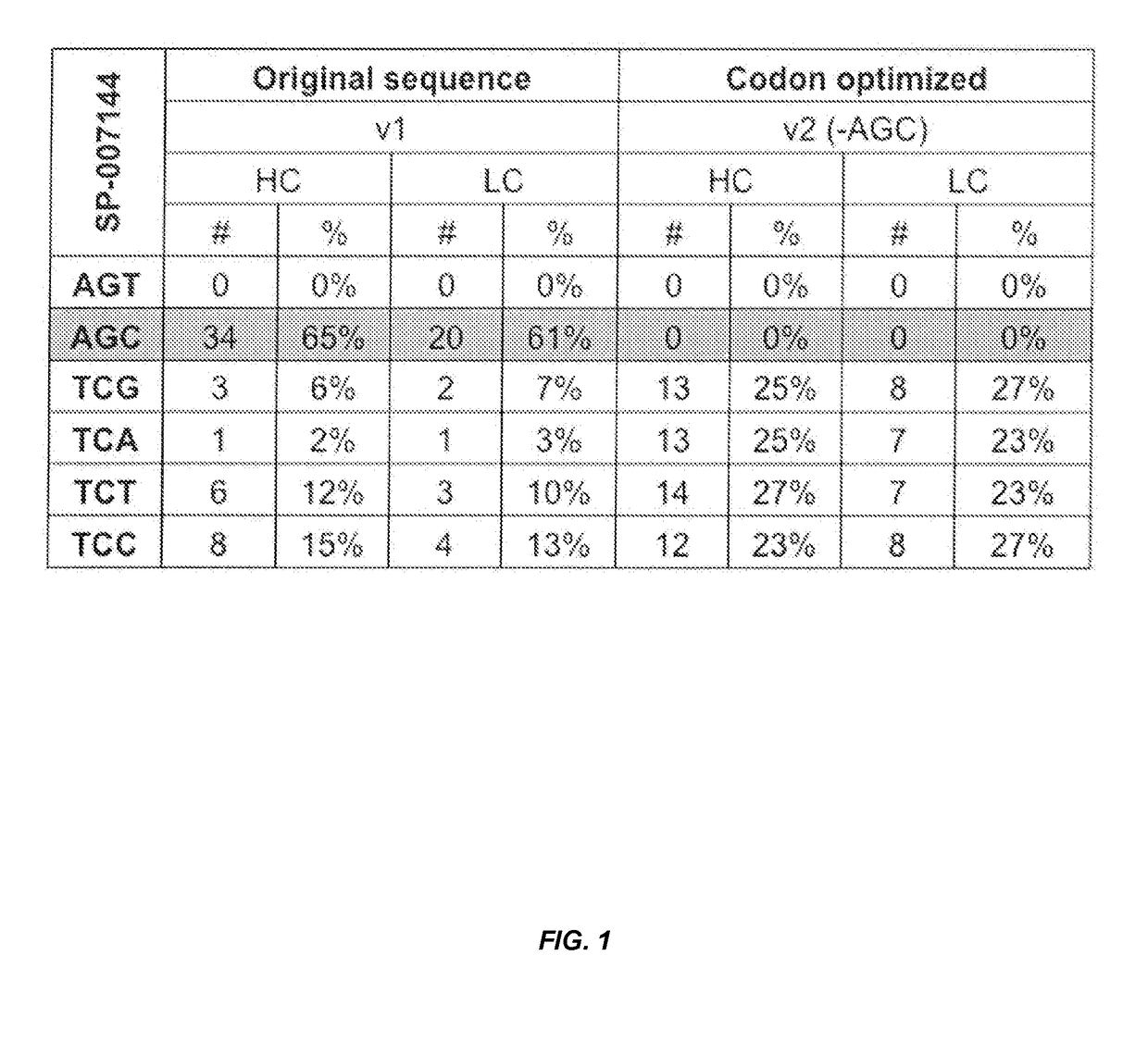
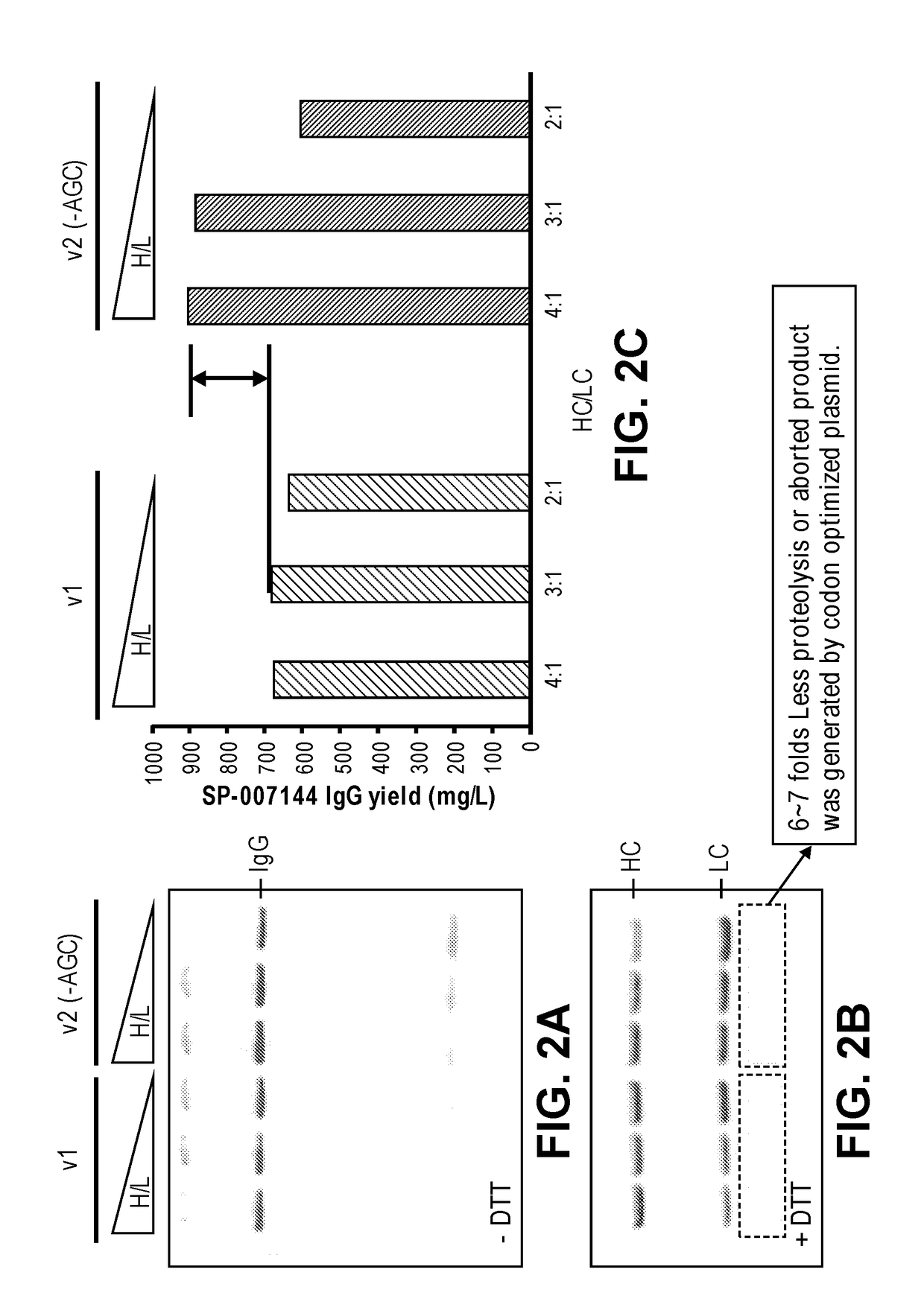
Codon optimization for titer and fidelity improvement
The invention provides methods for producing a protein in a cell free protein synthesis system such that the protein does not contain an asparagine (Asn or N) residue at serine (Ser or S) positions. Also provided are compositions and nucleic acid templates for use in the methods described herein.
Owner:SUTRO BIOPHARMA
Mycoplasma hyopneumoniae culture medium and preparation method thereof
ActiveCN102154167ARich in nutrientsIncrease the titer of live bacteriaBacteriaMicroorganism based processesPenicillinMycoplasma culture
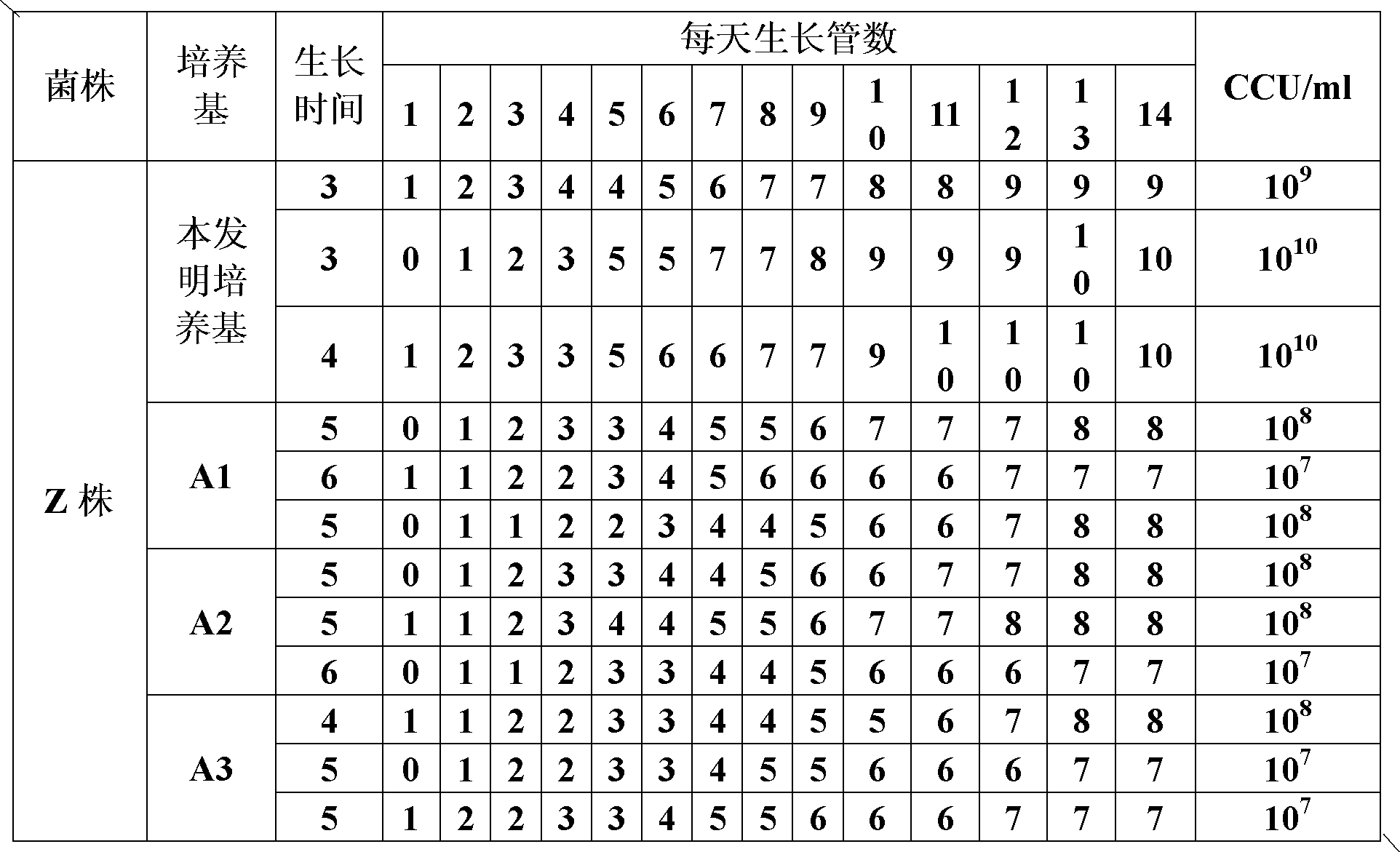
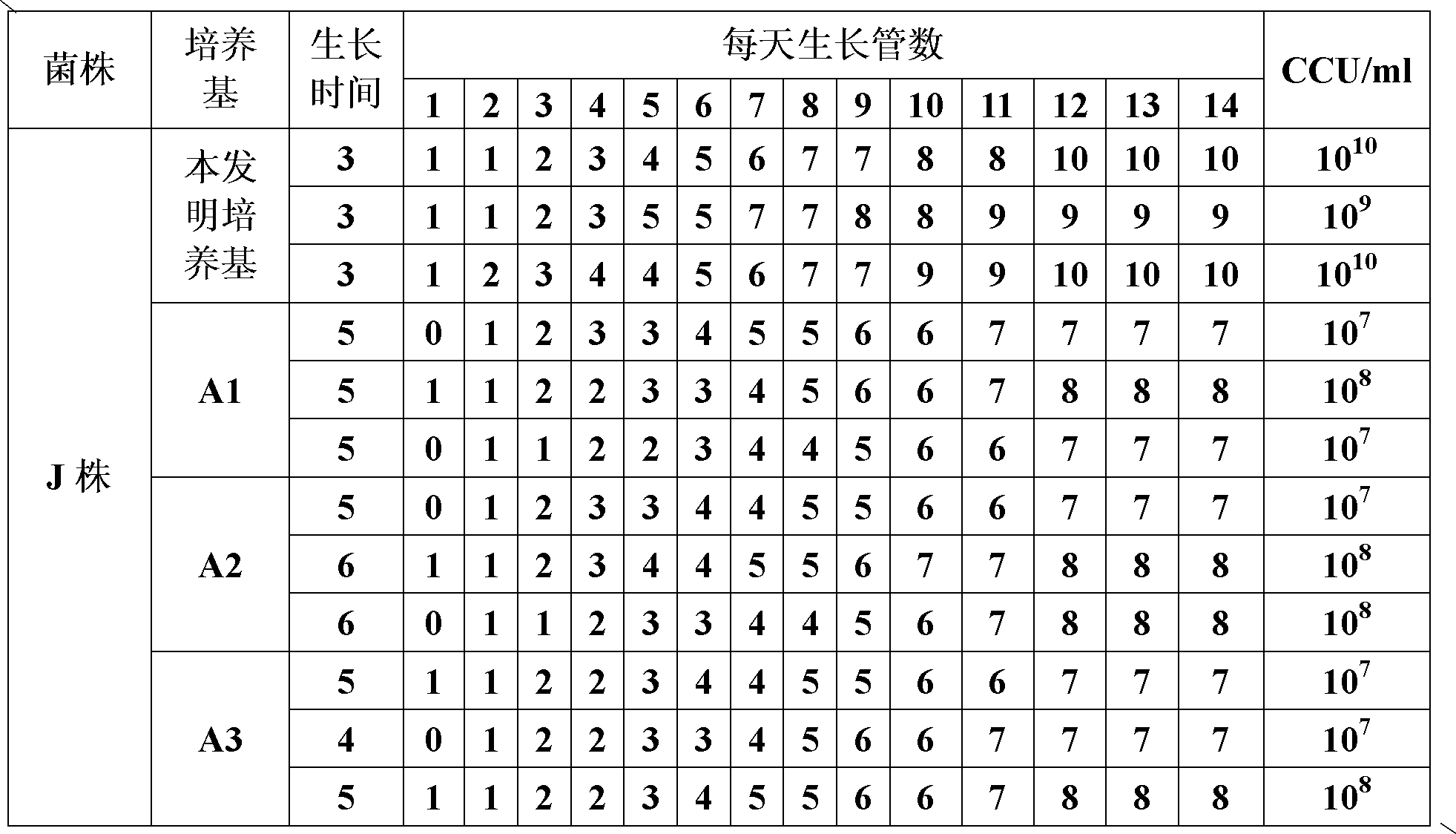
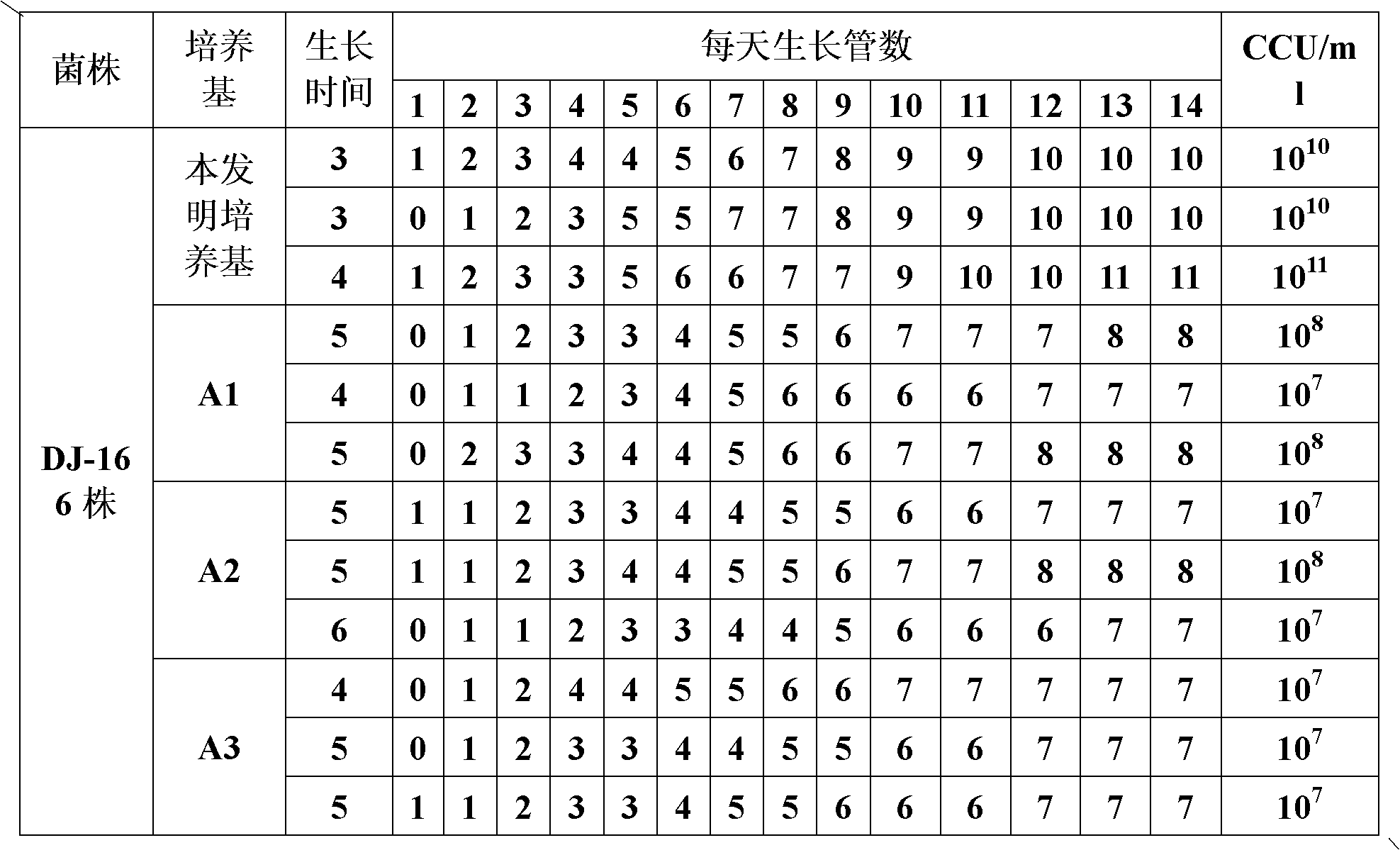
The invention provides a mycoplasma hyopneumoniae culture medium and a preparation method thereof, belonging to the technical field of veterinary biology. The mycoplasma hyopneumoniae liquid culture medium comprises the components as follows: brain heart infusion, lactalbumin hydrolysate, PPLO (pleuropneumonia-like organism) broth, yeast extract powder, proteose peptone, sodium thiosulfate, Hank's liquid, sodium pyruvate, 0.1% phenol red solution, penicillin and deionized water. The preparation method comprises the following steps of: adding health horse serum before using, and adding agar into the liquid culture medium to obtain a solid culture medium of mycoplasma hyopneumoniae. The viable bacteria titer of the mycoplasma hyopneumoniae culture medium can reach 1*109CCU / ml-1*1010CCU / ml; the viable bacteria titer and the separation sensibility are far higher than those of the existing culture medium, and the mycoplasma hyopneumoniae is fast in growth speed and high in the separation sensibility; and the preparation method of the culture medium is simple in technology, strong in operability, and suitable for industrial large-scale production.
Owner:兆丰华生物科技(南京)有限公司 +1
Intravenous injection of cytomegalovirus human immunoglobulin and its preparation method
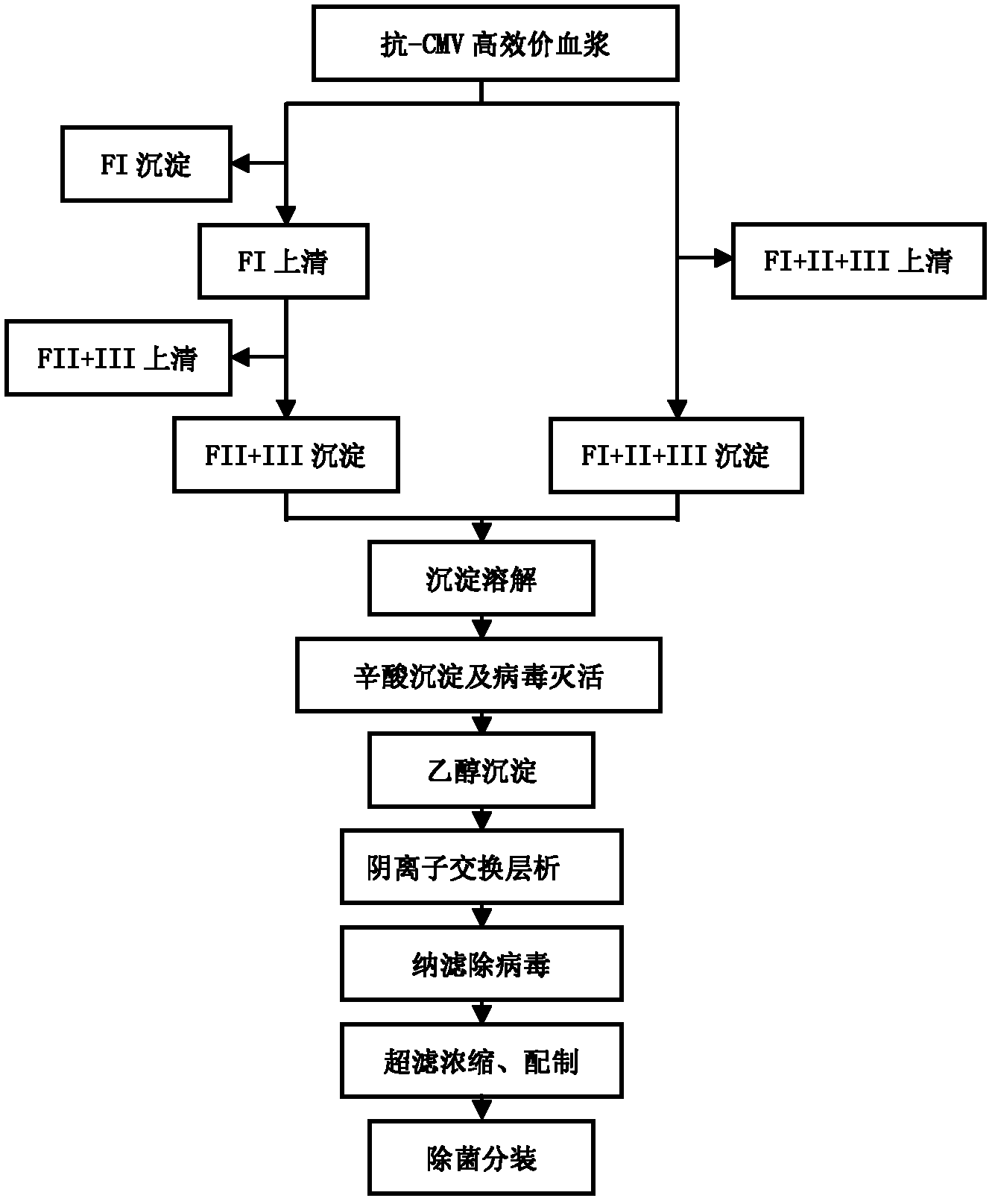
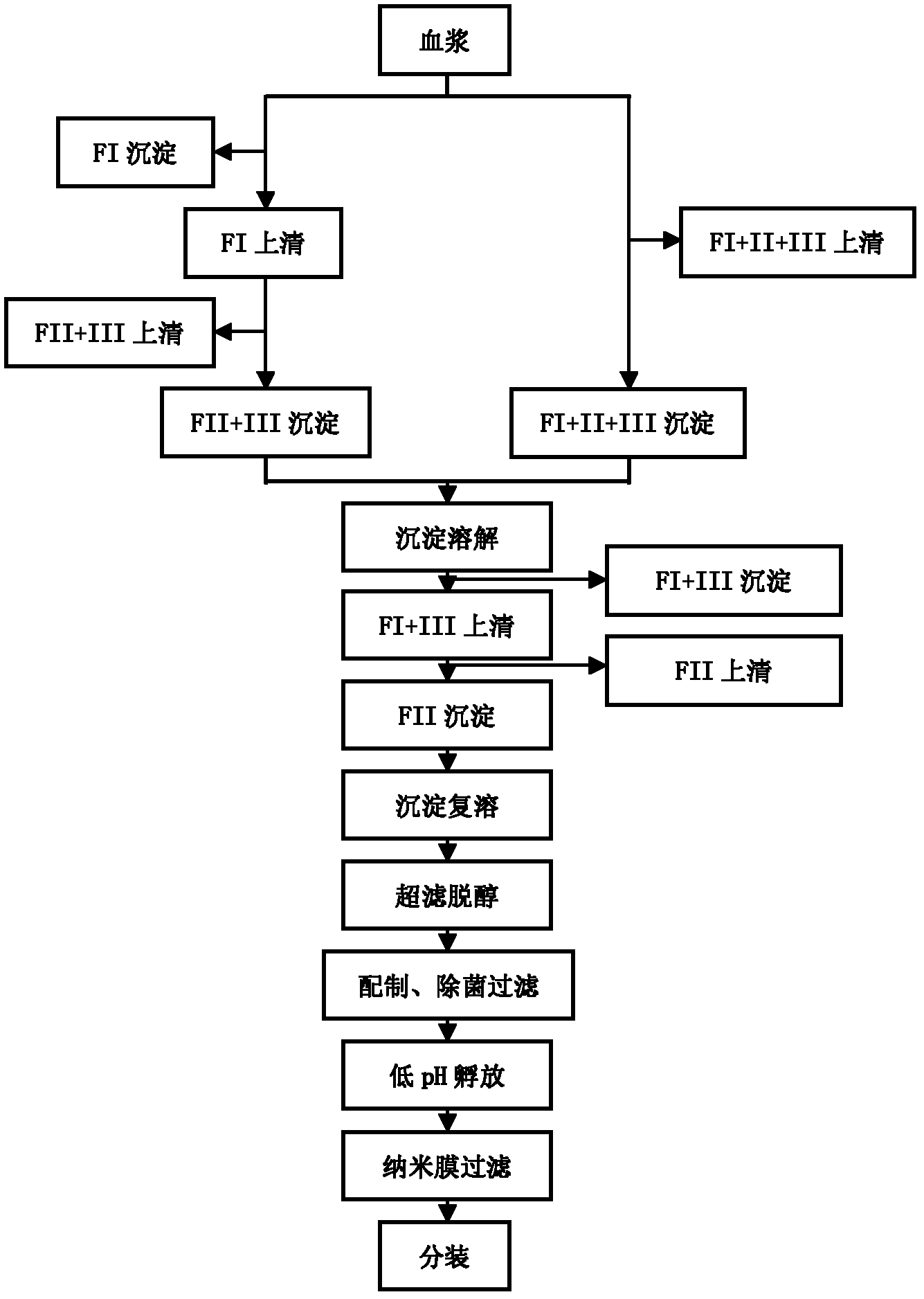
ActiveCN102286099ASteps to reduce precipitationKeep aliveImmunoglobulins against virusesAntiviralsEthanol precipitationAnion-exchange chromatography
The invention discloses a human cytomegalovirus immunoglobulin for intravenous injection and a preparation method thereof, and aims to improve the purity, yield and safety of the product. In the invention, the specific activity of the human cytomegalovirus immunoglobulin for intravenous injection is not less than 2.5 PEI-U / mg, the anti-CMV titer is not less than 100 PEI-U / ml, the purity is greater than 98.2%, and the protein content is 51-55 mg / ml. Caprylic acid precipitation and anion exchange chromatography are used instead of the partial ethanol precipitation step in the traditional low-temperature ethanol method, thereby keeping IgG in the supernate all the time so as to keep the IgG activity; and processes of caprylic acid virus inactivation and nano film virus removal are used, thereby effectively ensuring the safety of the product. Researches show that the preparation method disclosed by the invention improves the purity, yield and safety of the product, saves the energy and reduces the production cost.
Owner:SHENZHEN WEIGUANG BIOLOGICAL PROD
Methods and compositions for use in treatment of patients with autoantibody positive disease
InactiveUS20090081231A1Reduce quantity and frequencyReduce frequencyPeptide/protein ingredientsAntipyreticDiseaseAnti-dsDNA antibodies
The present invention relates to methods and compositions for use in treatment of patients with autoantibody positive disease. In a specific embodiment, the present invention relates to a method of treating a patient that has an ANA titer of 1:80 or greater and / or greater than or equal to 30 IU / ml of anti-dsDNA antibodies in his / her blood plasma or serum comprising administering a therapeutically effective amount of an immunomodulatory agent, such as an antagonist of Neutrokine-alpha. Additionally provided is a method of reducing the frequency and / or quantity of corticosteroid administration to patients. In preferred embodiments, the patient has systemic lupus erythematosus. Methods for determining if a lupus patient is responding to medical treatment are also provided.
Owner:HUMAN GENOME SCI INC
Food anaphylactogen specificity IgG4 antibody ELISA detection kit and preparation method thereof
The invention discloses an ELISA detection kit of food allergen specificity IgG4 antibody and preparation method thereof, using enzyme target rod coated by purified food allergen protein. The kit comprises: enzyme target rod coated by food allergen protein, human IgG2 titer, enzymic-lableled antibody, TMB substrate developing liquid, sample diluent, concentrated cleaning solution and termination reaction liquid. The specificity IgG4 antibodies in sample serum are bonded with the corresponding antigens fixed on the micropore surface of the enzyme target rod and the enzymic-lableled antibodies identify and bond with the IgG4 to form antigen-IgG4-antibody-enzyme composite. The absorbency is measured by the development reaction between the enzyme and substrate and the specificity IgG4 antibody concentration in sample serum is measured by standard curve. The allergen specificity IgG4 antibody concentration in sample serum is used as biology index of exposure state of food allergen and for judging the patient immune deviation and immune tolerance and monitoring the immunotherapy effectiveness of special food selected by patients.
Owner:杭州浙大生科生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com